Attached files
| file | filename |
|---|---|
| 8-K/A - 8-K/A - EAGLE PHARMACEUTICALS, INC. | a15-19936_28ka.htm |
Exhibit 99.1
|
|
September 2015 Corporate Overview |
|
|
Forward Looking Statements This presentation contains certain forward-looking information about Eagle Pharmaceuticals that is intended to be covered by the safe harbor for "forward-looking statements" provided by the Private Securities Litigation Reform Act of 1995, as amended. Forward-looking statements are statements that are not historical facts. Words such as “anticipate,” “believe,” “estimate,” “expect,” “intend,” “may,” “plan,” “predict,” “project,” “target,” “potential,” “will,” “would,” “could,” “should,” “continue,” “strong,” “upcoming,” “prepared” and similar expressions are intended to identify forward-looking statements. These statements include, but are not limited to, statements regarding our ability to successfully develop and commercialize our therapeutic products; our ability to expand our long-term business opportunities; financial projections and estimates and their underlying assumptions; and future performance. All such statements are subject to certain risks and uncertainties, many of which are difficult to predict and generally beyond the Company’s control, that could cause actual results to differ materially from those expressed in, or implied or projected by, the forward-looking information and statements. These risks and uncertainties include, but are not limited to: whether the FDA will accept for filing and approve our NDA for our bendamustine HCl rapid infusion product and other pharmaceutical products in development and when such approvals might be granted; the timing of future events, including but not limited to clinical development of our products and regulatory submissions; whether we will successfully market our products; our ability to successfully compete with other pharmaceutical and biotechnology companies; the success of our commercial partnerships; the development of, and our ability to take advantage of, the market for our therapeutic products; the strength and enforceability of our intellectual property rights; general economic conditions; and the other risk factors included in our annual report on form 10-K and other periodic reports filed with the U.S. Securities and Exchange Commission. Our audience is cautioned not to place undue reliance on these forward-looking statements that speak only as of the date hereof, and we do not undertake any obligation to revise and disseminate forward-looking statements to reflect events or circumstances after the date hereof, or to reflect the occurrence of or non-occurrence of any events. |
|
|
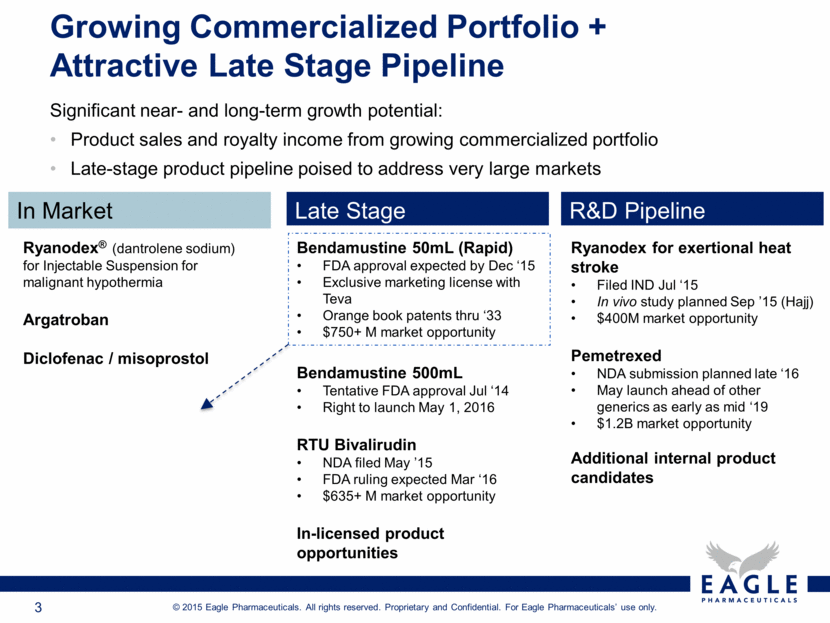
Growing Commercialized Portfolio + Attractive Late Stage Pipeline Significant near- and long-term growth potential: Product sales and royalty income from growing commercialized portfolio Late-stage product pipeline poised to address very large markets In Market Late Stage R&D Pipeline Ryanodex® (dantrolene sodium) for Injectable Suspension for malignant hypothermia Argatroban Diclofenac / misoprostol Bendamustine 500mL Tentative FDA approval Jul ‘14 Right to launch May 1, 2016 RTU Bivalirudin NDA filed May ’15 FDA ruling expected Mar ‘16 $635+ M market opportunity In-licensed product opportunities Bendamustine 50mL (Rapid) FDA approval expected by Dec ‘15 Exclusive marketing license with Teva Orange book patents thru ‘33 $750+ M market opportunity Ryanodex for exertional heat stroke Filed IND Jul ‘15 In vivo study planned Sep ’15 (Hajj) $400M market opportunity Pemetrexed NDA submission planned late ‘16 May launch ahead of other generics as early as mid ‘19 $1.2B market opportunity Additional internal product candidates |
|
|
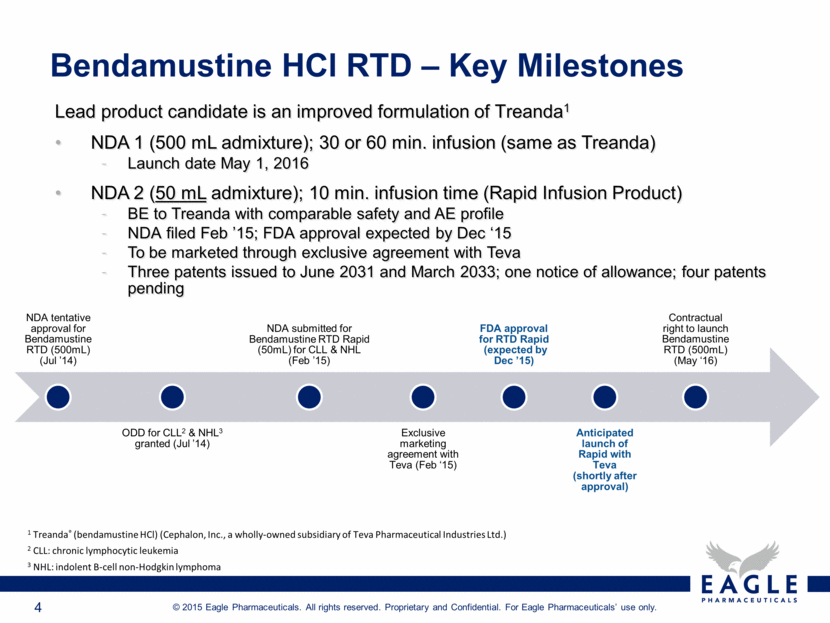
Bendamustine HCl RTD – Key Milestones 1 Treanda® (bendamustine HCl) (Cephalon, Inc., a wholly-owned subsidiary of Teva Pharmaceutical Industries Ltd.) 2 CLL: chronic lymphocytic leukemia 3 NHL: indolent B-cell non-Hodgkin lymphoma Lead product candidate is an improved formulation of Treanda1 NDA 1 (500 mL admixture); 30 or 60 min. infusion (same as Treanda) Launch date May 1, 2016 NDA 2 (50 mL admixture); 10 min. infusion time (Rapid Infusion Product) BE to Treanda with comparable safety and AE profile NDA filed Feb ’15; FDA approval expected by Dec ‘15 To be marketed through exclusive agreement with Teva Three patents issued to June 2031 and March 2033; one notice of allowance; four patents pending NDA tentative approval for Bendamustine RTD (500mL) (Jul ’14) ODD for CLL2 & NHL3 granted (Jul ’14) NDA submitted for Bendamustine RTD Rapid (50mL) for CLL & NHL (Feb ’15) Exclusive marketing agreement with Teva (Feb ‘15) FDA approval for RTD Rapid (expected by Dec ’15) Anticipated launch of Rapid with Teva (shortly after approval) Contractual right to launch Bendamustine RTD (500mL) (May ‘16) |
|
|
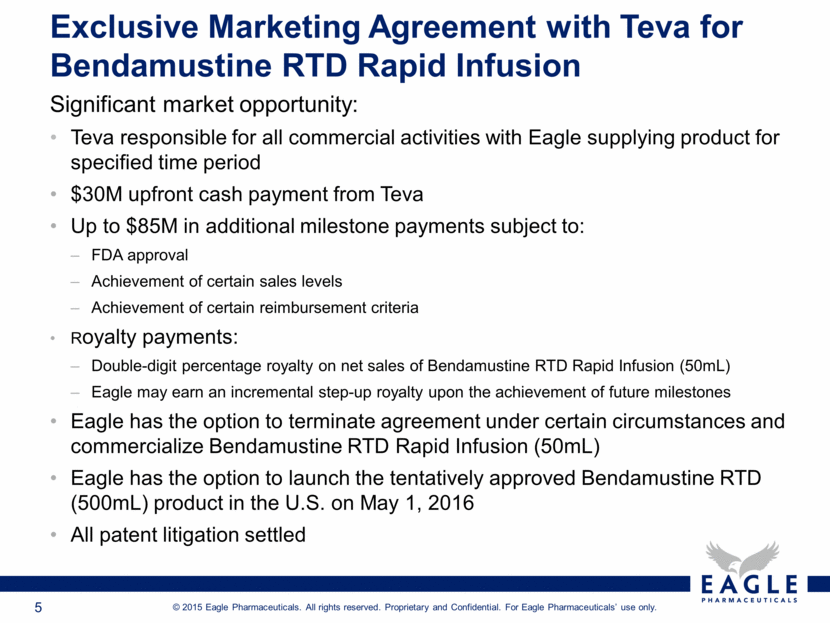
Significant market opportunity: Teva responsible for all commercial activities with Eagle supplying product for specified time period $30M upfront cash payment from Teva Up to $85M in additional milestone payments subject to: FDA approval Achievement of certain sales levels Achievement of certain reimbursement criteria Royalty payments: Double-digit percentage royalty on net sales of Bendamustine RTD Rapid Infusion (50mL) Eagle may earn an incremental step-up royalty upon the achievement of future milestones Eagle has the option to terminate agreement under certain circumstances and commercialize Bendamustine RTD Rapid Infusion (50mL) Eagle has the option to launch the tentatively approved Bendamustine RTD (500mL) product in the U.S. on May 1, 2016 All patent litigation settled Exclusive Marketing Agreement with Teva for Bendamustine RTD Rapid Infusion |
|
|
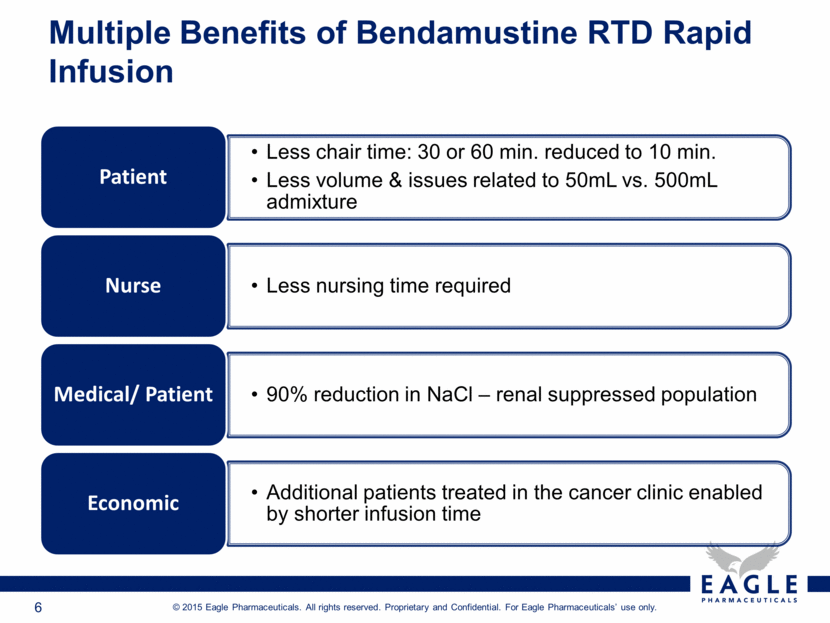
Multiple Benefits of Bendamustine RTD Rapid Infusion Less chair time: 30 or 60 min. reduced to 10 min. Less volume & issues related to 50mL vs. 500mL admixture Patient Less nursing time required Nurse 90% reduction in NaCl – renal suppressed population Medical/ Patient Additional patients treated in the cancer clinic enabled by shorter infusion time Economic |
|
|
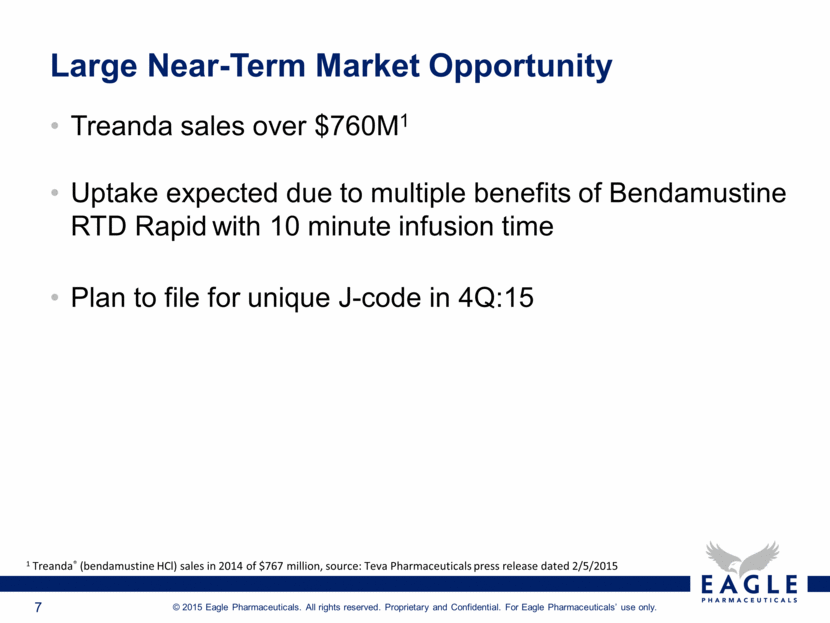
Large Near-Term Market Opportunity Treanda sales over $760M1 Uptake expected due to multiple benefits of Bendamustine RTD Rapid with 10 minute infusion time Plan to file for unique J-code in 4Q:15 1 Treanda® (bendamustine HCl) sales in 2014 of $767 million, source: Teva Pharmaceuticals press release dated 2/5/2015 |
|
|
Bivalirudin RTU Stable, liquid intravenous formulation version of the anticoagulant Angiomax1 Brand sales over $635M2 Multiple benefits versus Angiomax Near-term opportunity: NDA submitted May ‘15 Potential U.S. launch March ‘16 Reduced licensing payment to SciDose to 15% of net profit, increasing sales margin, assuming approval Two patents issued; third patent filed 1Q ‘14 1 Angiomax® (bivalirudin) / Angiox® (bivalirudin) (The Medicines Company) 2 Worldwide Angiomax/Angiox sales in 2014 of $635.7 million, source: The Medicines Company press release dated 2/18/2015. |
|
|
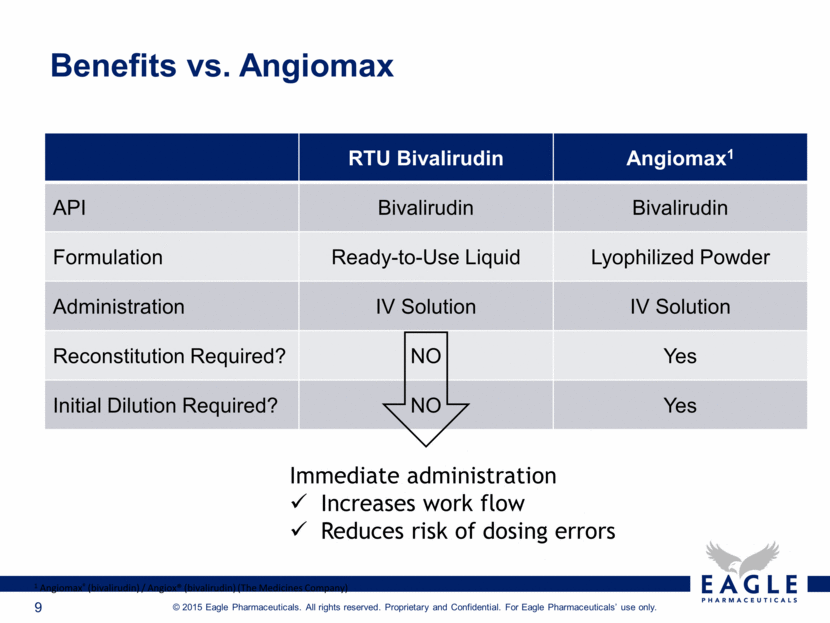
Benefits vs. Angiomax RTU Bivalirudin Angiomax1 API Bivalirudin Bivalirudin Formulation Ready-to-Use Liquid Lyophilized Powder Administration IV Solution IV Solution Reconstitution Required? NO Yes Initial Dilution Required? NO Yes 1 Angiomax® (bivalirudin) / Angiox® (bivalirudin) (The Medicines Company) Immediate administration Increases work flow Reduces risk of dosing errors |
|
|
No change to SOC for MH treatment in over 30 years Often fatal if untreated Ryanodex approved in July 2014 Optimized formulation of dantrolene sodium Reduces to 5mL (1 vial) vs. 720 mL (12 vials) for old product Breakthrough change Protected market position Three patents issued + one filed Orphan drug designation for MH (U.S. & Europe) Granted seven years market exclusivity in US Ryanodex® (dantrolene sodium) for Malignant Hyperthermia (MH) |
|
|
All U.S. hospitals required to stock dantrolene ~9,000 outlets MHAUS1 recommends a minimum 36 vials = 3 vials Ryanodex Repurchase required every 2 years Premium pricing supported by Ryanodex advantages Faster administration Reduced volume Significant reduction of Mannitol (diuretic) U.S. Commercial Landscape 1 Joint Commission; MHAUS: Malignant Hyperthermia Association of the United States |
|
|
Launched Aug ‘14 Premium pricing to old formulation expands addressable market from $20M1 to $75M (over two years) 95% of unit vial volume sold to hospitals and medical centers in Q2 ‘15 Multiple top-tier hospital conversions: Mayo Clinic, NIH, VAs, among others Sales force realigned Q2 ‘15 U.S. Launch Update 1 Branded dantrolene sales for MH 2013 |
|
|
Exertional Heat Stroke (EHS) Sudden, unpredictable and life threatening condition Patient population impacted: military, student athletes, athletes, construction workers, migrant workers, and firemen A leading cause of student athlete death (US) & non-combat military deaths Similarities to MH Potential for Ryanodex to be first to market for EHS Orphan drug designation for EHS Potential for 7 years of exclusivity Market estimated at $400M Initial Label Expansion Opportunity |
|
|
Held Type C meeting with FDA on Mar ’15 We believe the Agency recognizes the potential benefit Ryanodex may afford patients Path forward: a ‘hybrid’ development program including human and animal efficacy and safety data IND filed Jul ‘15 Human study planned for Sep ‘15 in Saudi Arabia during Hajj Conducted at 4 hospitals in the Mecca region Top emergency medicine experts as investigators Patients 18-45 who meet the criteria for EHS Randomized 1:1 to receive standard of care or standard of care plus Ryanodex EHS: Development Update |
|
|
Lilly’s Alimta patent infringement lawsuit win should prevent current ANDA filers from launching until May 24, 2022 Eagle plans to file Pemetrexed RTU late 2016 We believe our patent position may enable us to bring the product to market as early as Q4 2017 30 month stay to expire 1st half of 2019 $1.2B market opportunity1 Pemetrexed Update 1 Alimta® (pemetrexed) (Eli Lilly & Co.). Source: Eli Lilly & Co. press release dated 1/30/2015: (U.S. sales) |
|
|
Growing portfolio of commercialized products delivering top line growth 3 potential product launches in the next 12 months Significant near-term opportunities Bendamustine RTD Rapid approval expected by December 2015 RTU bivalirudin approval anticipated in March 2016 Potential for label expansion for Ryanodex in EHS increases longevity of the product Transformative partnership with Teva for Bendamustine RTD Rapid will drive profitability Successfully leveraging assets to build critical mass, increase profitability, and create long-term, sustainable value Marketing at least 4 products by Q2 2016 Royalty stream from Teva on Bendamustine RTD Rapid (50mL) Well capitalized to execute growth strategy Why Eagle? Why Now? |