Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Cellectar Biosciences, Inc. | v418172_8k.htm |
| EX-99.2 - EXHIBIT 99.2 - Cellectar Biosciences, Inc. | v418172_ex99-2.htm |
| EX-99.1 - EXHIBIT 99.1 - Cellectar Biosciences, Inc. | v418172_ex99-1.htm |
| EX-99.4 - EXHIBIT 99.4 - Cellectar Biosciences, Inc. | v418172_ex99-4.htm |
Exhibit 99.3

1 Cellectar Biosciences NASDAQ: CLRB August 2015

2 Safe Harbor Statement This slide presentation contains forward - looking statements . Such statements are valid only as of today, and we disclaim any obligation to update this information . These statements are only estimates and predictions and are subject to known and unknown risks and uncertainties that may cause actual future experience and results to differ materially from the statements made . These statements are based on our current beliefs and expectations as to such future outcomes . Drug discovery and development involve a high degree of risk . Factors that might cause such a material difference include, among others, uncertainties related to the ability to raise additional capital required to complete the development programs described herein, the ability to attract and retain partners for our technologies, the identification of lead compounds, the successful preclinical development thereof, the completion of clinical trials, the FDA review process and other government regulation, our pharmaceutical collaborators’ ability to successfully develop and commercialize drug candidates, competition from other pharmaceutical companies, product pricing and third - party reimbursement . A complete description of risks and uncertainties related to our business is contained in our periodic reports filed with the Securities and Exchange Commission including our Form 10 - K/A for the year ended December 31 , 2014 . These forward looking statements are made only as of the date hereof, and we disclaim any obligation to update any such forward looking statements .

3 Investment Overview • Oncology - focused biopharmaceutical company in Madison, WI • First and best - in - class PDC delivery platform – Phospholipid ether - Drug Conjugate (PDC) – Cancer - targeting delivery of oncologic payloads • Pipeline of cancer therapeutics and diagnostics • New leadership delivering on focused plan to unlock PDC platform value – Developing wholly - owned PDC therapeutics – Expanding cytotoxic therapeutic windows – Advancing PDC platform through collaborations
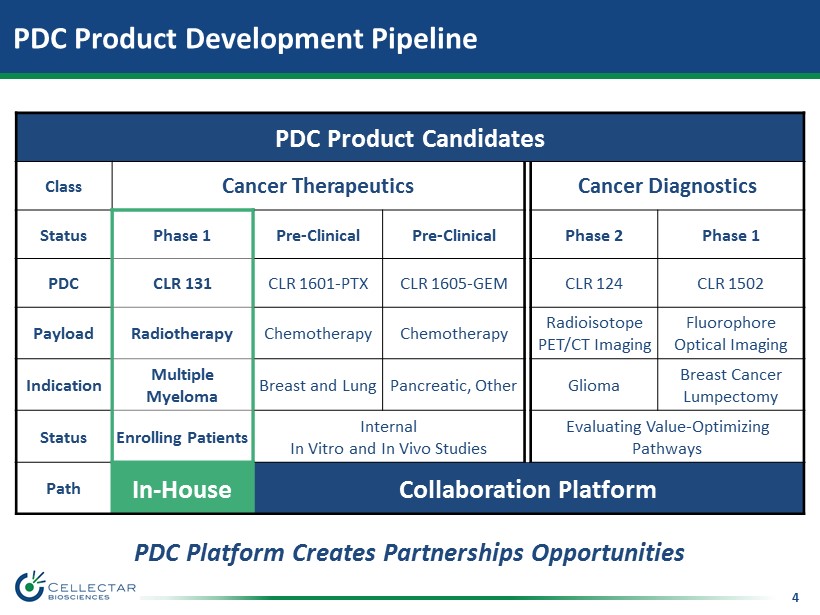
4 PDC Product Development Pipeline PDC Product Candidates Class Cancer Therapeutics Cancer Diagnostics Status Phase 1 Pre - Clinical Pre - Clinical Phase 2 Phase 1 PDC CLR 131 CLR 1601 - PTX CLR 1605 - GEM CLR 124 CLR 1502 Payload Radiotherapy Chemotherapy Chemotherapy Radioisotope PET/CT Imaging Fluorophore Optical Imaging Indication Multiple Myeloma Breast and Lung Pancreatic, Other Glioma Breast Cancer Lumpectomy Status Enrolling Patients Internal In Vitro and In Vivo Studies Evaluating Value - Optimizing Pathways Path In - House Collaboration Platform PDC Platform Creates Partnerships Opportunities

5 • Proprietary small - molecule • Highly selective cancer and CSC targeting • Uptake and prolonged retention in malignant cells – POC in broad range of cancers • Ability to attach diverse oncologic payloads • Extensive research and peer reviewed scientific validation Basis for PDC Delivery Platform Phospholipid Ether Cancer Targeting Vehicle 6/2014 2/2015

6 Proprietary Small - Molecule, Cancer - Targeting Delivery Vehicle PDC D elivery Platform Overview + + Linker = Increased Therapeutic Window Drug Linker Drug Phospholipid Ether Enabling Targeted Delivery of Diverse Oncologic Payloads PDC Cancer Selective Versatile Chemistry Payload Diversity
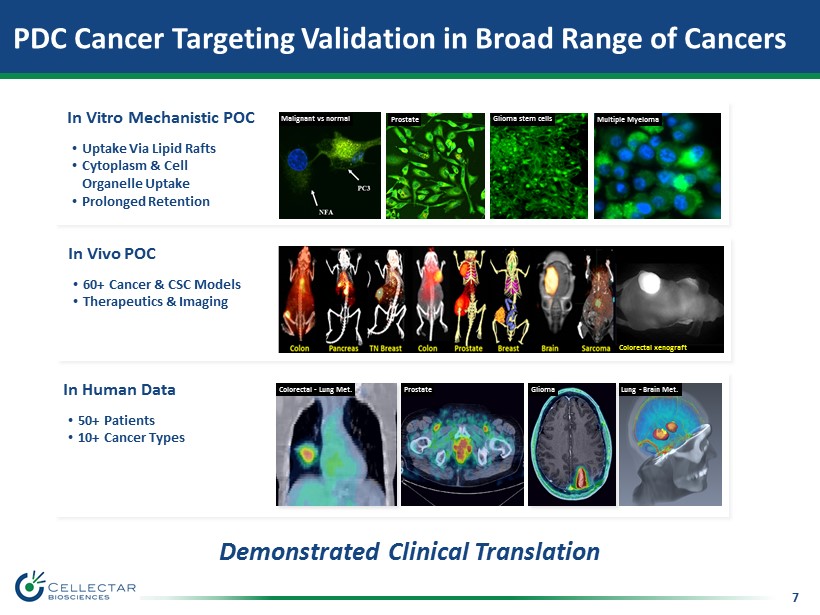
7 PDC Cancer Targeting Validation in Broad Range of Cancers Demonstrated Clinical Translation In Vitro Mechanistic POC • Uptake Via Lipid Rafts • Cytoplasm & Cell Organelle Uptake • Prolonged Retention In Vivo POC • 60+ Cancer & CSC Models • Therapeutics & Imaging In Human Data • 50+ Patients • 10+ Cancer Types Colorectal - Lung Met. Prostate Glioma Lung - Brain Met. Multiple Myeloma Malignant vs normal Glioma stem cells Colorectal xenograft Prostate

8 PDC Diverse Payload Delivery Validation Radioisotopes for Cancer Imaging • CLR 124: Glioma • Iodine - 123 Fluorophores for Imaging - Guided Surgery • CLR 1502 (near - infrared spectrum) • CLR 1501 (visible spectrum) Cytotoxins for Improved Therapeutic Index • CLR 1601 - PTX: Paclitaxel - pre - clinical • CLR 1605 - GEM: Gemcitabine - pre - clinical Other Payloads • Product development and commercialization collaborations Radioisotopes for Cancer Therapy • CLR 131: Multiple Myeloma • Iodine - 125
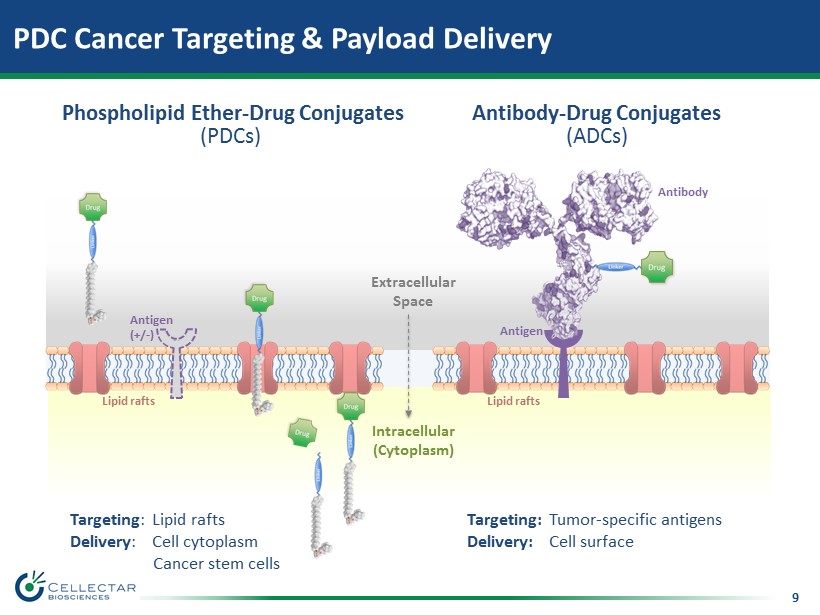
9 PDC Cancer Targeting & Payload Delivery Antibody - Drug Conjugates (ADCs) Phospholipid Ether - Drug Conjugates (PDCs) Extracellular Space Intracellular (Cytoplasm) Antigen Antibody Lipid rafts Lipid rafts Antigen (+/ - ) Targeting : Lipid rafts Delivery : Cell cytoplasm Cancer stem cells Targeting: Tumor - specific antigens Delivery: Cell s urface
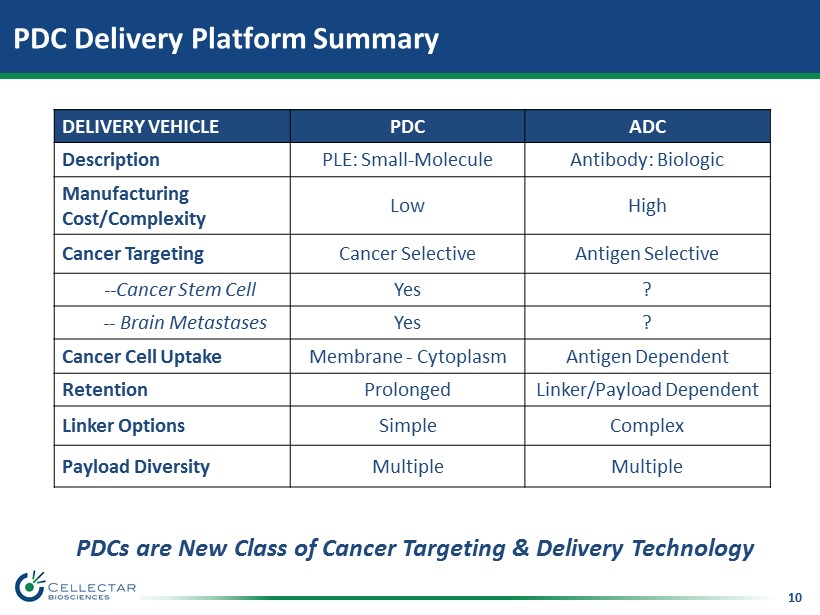
10 PDC Delivery Platform Summary DELIVERY VEHICLE PDC ADC Description PLE: Small - Molecule Antibody: Biologic Manufacturing Cost/Complexity Low High Cancer Targeting Cancer Selective Antigen Selective -- Cancer Stem Cell Yes ? -- Brain Metastases Yes ? Cancer Cell Uptake Membrane - Cytoplasm Antigen Dependent Retention Prolonged Linker/Payload Dependent Linker Options Simple Complex Payload Diversity Multiple Multiple PDCs are New Class of Cancer Targeting & Delivery Technology

11 CLR 131: Lead PDC Radiotherapeutic Product • Payload: Iodine 131 – Cytotoxic r adioisotope – Thyroid cancer • PDC: CLR 131 – Targeted cytotoxic delivery – Tumor uptake, retention and efficacy demonstrated in vivo • Liquid and solid tumors – Solid tumor MTD established and activity observed – Indications beyond thyroid cancer • Multiple Myeloma, o ther cancers Linker 131 I Opportunity for Expanded Oncology Indications
12 Market Opportunity in Multiple Myeloma • Incurable hematologic cancer - malignant plasma cells • Unmet need in relapse/refractory setting • 2 nd most common hematologic cancer – Prevalence ~ 90,000 – Incidence ~ 26,850 – Relapse/Refractory ~ 13,000 • Global MM drug m arket – 2014 - 2019 8.5% CAGR • Premium pricing for marketed products – $55k - $ 150k +

13 CLR 131: Rationale for Multiple Myeloma • Novel mechanism of action • Single dose treatment • Established radiosensitivity • In vivo MM cell uptake • Quantitative response criteria – M - protein marker • Regulatory Pathway – Unmet need in relapse/refractory patients – Orphan designation granted – Potential for fast track, breakthrough t herapy, accelerated a pproval

14 Phase 1 Study in Multiple Myeloma Underway • Multi - center, open label, dose escalation trial initiated Q2 2015 – D etermine Phase II dose • 3rd line relapse/refractory patients • Primary objective: Dose limiting toxicity • Secondary objective: Efficacy • Next Steps – Evaluate cohort 1 - 1H 2016 – Initiate cohort 2 - 1H 2016

15 PDC Chemotherapeutic Program Overview • Objective – Develop chemotherapy PDCs with improved efficacy & tolerability • Clinical Rationale – Numerous chemotherapeutics available for PDC – Highly effective yet highly toxic drugs – Improve drug therapeutic index through targeted delivery • Business Rationale – Many failed, pre - clinical, clinical and on market chemotherapeutics – New products, new patent life & life cycle management • Initial Chemotherapeutic Candidates – CLR 1601 - PTX (Paclitaxel), CLR 1605 - GEM (Gemcitabine) – Evaluating other chemotherapeutic compounds Creating Opportunities for Clinical Development Partnerships

16 CLR 1601 - PTX: Pre - Clinical PDC Chemotherapeutic Product • Payload: Paclitaxel – Well characterized chemotherapeutic – Breast, lung and ovarian cancers • PDC: CLR 1601 - PTX – Multiple derivatives developed – Established linker and Conjugation – I n vitro studies document: • Stability • Retention of cytotoxic activity • Next Steps – Pre - clinical data update - Q4 2015 Expanding Therapeutic Index Linker PTX

17 CLR 1502: Phase I PDC Cancer Diagnostic Product • Payload: Fluorophore – Fluorescent dye: Assess tissue perfusion • PDC: CLR 1502 – Cancer surgery i maging agent – Accurate visualization of tumor margins • Breast Cancer/Lumpectomy – Complete malignant tissue removal – Improved patient outcomes & QOL – Limit repeat surgeries – Healthcare system savings • Next steps – Identify optimal clinical development pathway – Assess future cancer surgery indications Evaluating Value - Optimizing Pathways

18 CLR 124: Phase II PDC Cancer Diagnostic Product • Payload: Iodine 124 – PET/CT imaging isotope – Limited cancer use • PDC: CLR 124 – More precise cancer imaging diagnostic – Early detection, staging, monitoring – Prostate , colorectal, head & neck , other – Brain cancer, glioma & metastases • Next Steps – NCI, ICTR (brain metastases/cancer) and Phase II Glioma data - collate & assess Evaluating Value - Optimizing Pathways 124

19 Intellectual Property Portfolio Patent Composition of Matter Methods of Use Methods of Manufacturing Freedom to Operate Asset CLR 131 12/2016 Orphan Drug - Multiple Myeloma 2025 - Cancer, Solid Tumors 2030 - Cancer Stem Cell 2028 CLR 124 12/2016 Orphan Drug - Glioma 2025 - Cancer TRX 2028 CLR 1502 2029 2029 2029 Phospholipid Ethers (various) 12/2016 - 2028 2028 2028 Over 28 Patents Issued or Pending
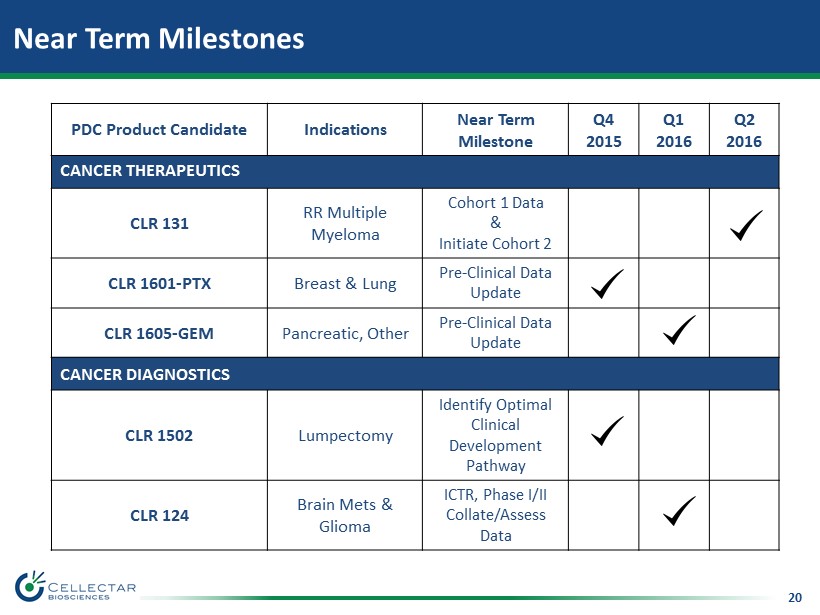
20 Near Term Milestones PDC Product Candidate Indications Near Term Milestone Q4 2015 Q1 2016 Q2 2016 CANCER THERAPEUTICS CLR 131 RR Multiple Myeloma Cohort 1 Data & Initiate Cohort 2 CLR 1601 - PTX Breast & Lung Pre - Clinical Data Update CLR 1605 - GEM Pancreatic, Other Pre - Clinical Data Update CANCER DIAGNOSTICS CLR 1502 Lumpectomy Identify Optimal Clinical Development Pathway CLR 124 Brain Mets & Glioma ICTR, Phase I/II Collate/Assess Data
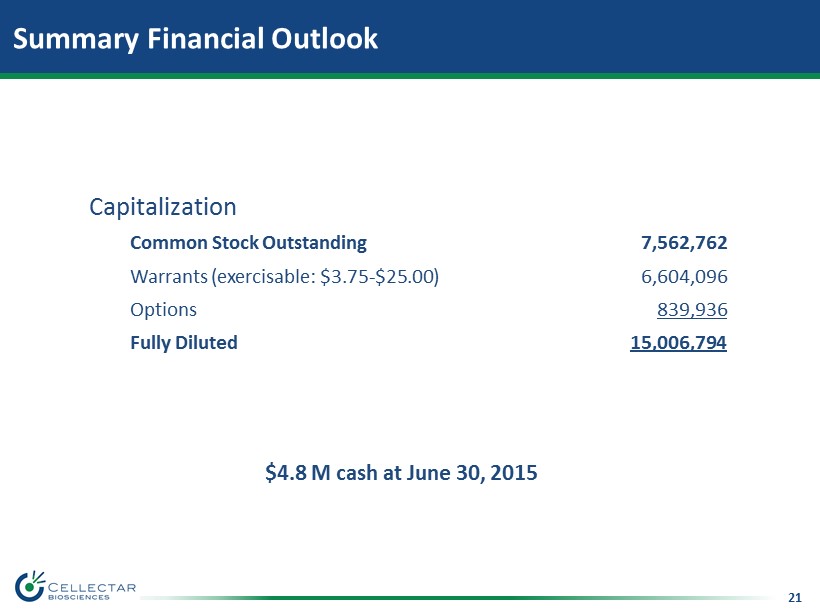
21 Summary Financial Outlook Capitalization Common Stock Outstanding 7,562,762 Warrants (exercisable: $3.75 - $25.00) 6,604,096 Options 839,936 Fully Diluted 15,006,794 $4.8 M cash at June 30, 2015
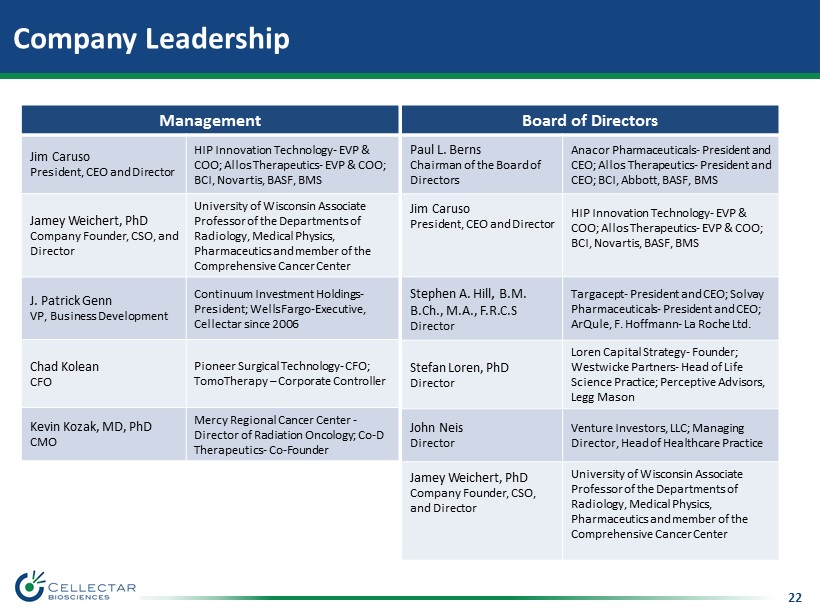
22 Company Leadership Management Jim Caruso President, CEO and Director HIP Innovation Technology - EVP & COO; Allos Therapeutics - EVP & COO; BCI, Novartis, BASF, BMS Jamey Weichert, PhD Company Founder, CSO, and Director University of Wisconsin Associate Professor of the Departments of Radiology, Medical Physics, Pharmaceutics and member of the Comprehensive Cancer Center J. Patrick Genn VP, Business Development Continuum Investment Holdings - President; Wells Fargo - Executive, Cellectar since 2006 Chad Kolean CFO Pioneer Surgical Technology - CFO; TomoTherapy – Corporate Controller Kevin Kozak, MD, PhD CMO Mercy Regional Cancer Center - Director of Radiation Oncology; Co - D Therapeutics - Co - Founder Board of Directors Paul L. Berns Chairman of the Board of Directors Anacor Pharmaceuticals - President and CEO; Allos Therapeutics - President and CEO; BCI, Abbott, BASF, BMS Jim Caruso President, CEO and Director HIP Innovation Technology - EVP & COO; Allos Therapeutics - EVP & COO; BCI, Novartis, BASF, BMS Stephen A. Hill , B.M. B.Ch., M.A., F.R.C.S Director Targacept - President and CEO; Solvay Pharmaceuticals - President and CEO; ArQule, F. Hoffmann - La Roche Ltd. Stefan Loren, PhD Director Loren Capital Strategy - Founder; Westwicke Partners - Head of Life Science Practice; Perceptive Advisors, Legg Mason John Neis Director Venture Investors, LLC; Managing Director, Head of Healthcare Practice Jamey Weichert, PhD Company Founder, CSO, and Director University of Wisconsin Associate Professor of the Departments of Radiology, Medical Physics, Pharmaceutics and member of the Comprehensive Cancer Center

23 Investment Overview • Oncology - focused biopharmaceutical company in Madison, WI • First and best - in - class PDC delivery platform – Phospholipid ether Drug Conjugate (PDC) – Cancer - targeting delivery of oncologic payloads • Pipeline of cancer therapeutics and diagnostics • New leadership delivering on focused plan to unlock PDC platform value – Developing wholly owned PDC therapeutics – Expanding cytotoxic therapeutic windows – Advancing PDC platform through collaborations
