Attached files
| file | filename |
|---|---|
| 8-K - 8-K - SCYNEXIS INC | form8kitems701and901.htm |

SCYNEXIS, Inc. Speed and Innovation in Anti-Infectives Canaccord Conference Boston, August 12-13, 2015

1 Certain statements regarding SCYNEXIS, Inc. (the “Company”) made in this presentation may constitute forward-looking statements, including, but not limited to, statements regarding our business strategies and goals, plans and prospects, market size, adoption rate, potential revenue, clinical validity and utility, growth opportunities, future products and product pipeline. Forward-looking statements are subject to risks and uncertainties that could cause actual results to differ materially from our expectations. These risk and uncertainties include but are not limited to our ability to successfully develop SCY-078, including an IV formulation of SCY-078; our expectations regarding QIDP designation; our ability to obtain FDA approval for SCY-078; the expected costs of studies and when they will begin and our reliance on third parties to conduct our clinical studies. Forward-looking statements may be identified by the use of the words “anticipates,” “expects,” “intends,” “plans,” “could,” “should,” “would,” “may,” “will,” “believes,” “estimates,” “potential,” or “continue” and variations or similar expressions. These statements are based upon the current expectations and beliefs of management and are subject to certain risks and uncertainties that could cause actual results to differ materially from those described in the forward-looking statements. These risks and uncertainties include, but are not limited to, risks and uncertainties discussed in the company's most recent reports filed with the Securities and Exchange Commission ("SEC") including the Company’s quarterly report on Form 10-Q filed with the SEC on May 15, 2015 and other risks and uncertainties detailed from time to time in the Company's filings with the SEC, which factors are incorporated herein by reference. Readers are cautioned not to place undue reliance on any of these forward-looking statements. The Company undertakes no obligation to update any of these forward-looking statements to reflect events or circumstances after the date of this presentation, or to reflect actual outcomes. Forward-looking Statements

Experienced Management Team 2 CEO effective April 2015 Former CMO of Forest Labs and President of the Forest Research Institute Former Head of R&D at Stiefel Labs and of Anti-Infectives at Schering-Plough Marco Taglietti, MD Chief Executive Officer Interim CFO from CMF Associates, LLC Previously CFO at M&F Bancorp, Community Health and Gilero Certified Public Accountant, Former Big-4 partner Jon Woodall Chief Financial Officer Some recent changes to the Board of Directors − Guy Macdonald, Tetraphase President and CEO, appointed Chairman of the Board − Steve Gilman, former Chief Scientific Officer at Cubist, appointed Member of Board Management team with significant experience in drug development − Successful track record in many therapeutic areas including antifungals and other anti- infectives products CMO effective June 2015 Previously with Brickell Biotech, Stiefel and Schering-Plough Infectious Disease Specialist with more than 10 products approved David Angulo, MD Chief Medical Officer
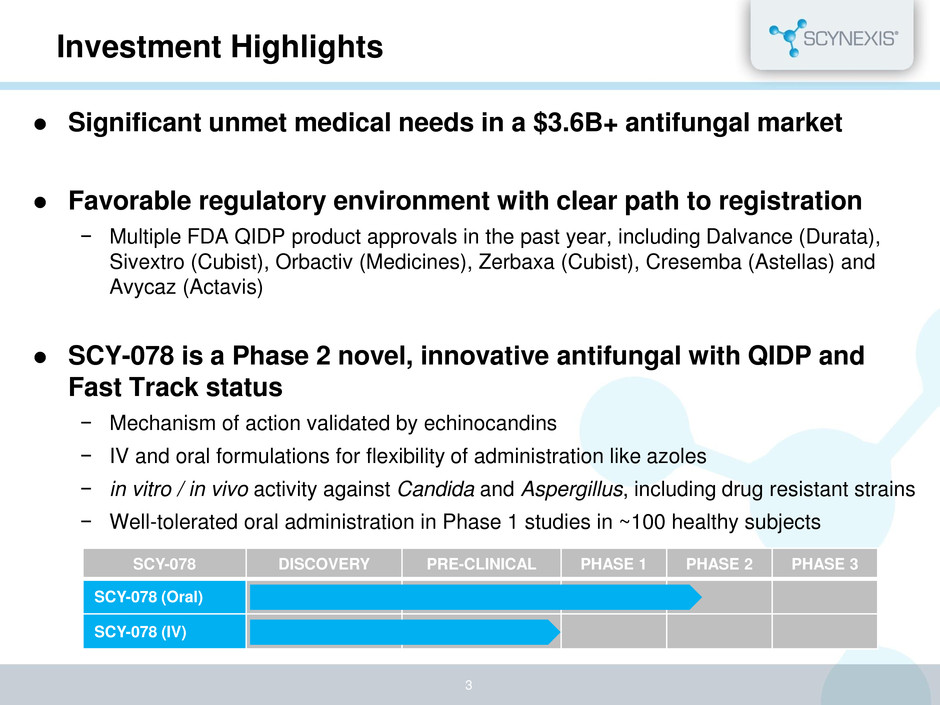
Investment Highlights Significant unmet medical needs in a $3.6B+ antifungal market Favorable regulatory environment with clear path to registration − Multiple FDA QIDP product approvals in the past year, including Dalvance (Durata), Sivextro (Cubist), Orbactiv (Medicines), Zerbaxa (Cubist), Cresemba (Astellas) and Avycaz (Actavis) SCY-078 is a Phase 2 novel, innovative antifungal with QIDP and Fast Track status − Mechanism of action validated by echinocandins − IV and oral formulations for flexibility of administration like azoles − in vitro / in vivo activity against Candida and Aspergillus, including drug resistant strains − Well-tolerated oral administration in Phase 1 studies in ~100 healthy subjects 3 SCY-078 DISCOVERY PRE-CLINICAL PHASE 1 PHASE 2 PHASE 3 SCY-078 (Oral) SCY-078 (IV)
Candidiasis and Aspergillosis are serious and life threatening fungal infections with growing resistance Invasive Candidiasis remains a serious clinical problem − 4th most common cause of hospital-acquired blood infection in US − Mortality rate of 27 to 40% despite treatment − Identified by CDC as an antimicrobial resistance threat − Increasing prevalence of azole resistant Candida spp. and Multi Drug Resistant (MDR) species, like C. glabrata − Limited therapeutic options for MDR Candida infections − Drug resistant Candida infections associated with higher mortality, hospital cost and longer length of stay − Treatment guidelines changing as result of resistance Invasive Aspergillosis − 2nd most common invasive hospital-acquired fungal infection in US − Mortality approaches 50% despite treatment 4 Significant Unmet Medical Needs

Limited number of antifungal options -- Only three classes of antifungals -- POLYENES Ambisome ($450M1), amphotericin B Effective vs a broad range of fungi Significant toxicity (renal, cardiac, infusion) Limited activity against C. glabrata Only IV administration AZOLES voriconazole ($754M2), fluconazole, posaconazole, isavuconazole Good efficacy with flexible administration (both IV and oral) Rising resistance to azoles (C. glabrata, C. krusei, Aspergillus) Liver toxicity and drug interactions ECHINOCANDINS caspofungin ($619M2), micafungin, anidulafungin Effective (drug of choice in invasive candidiasis) Good tolerability profile Some Candida strains are showing resistance Only IV administration 5 $3.6B+ Sales2 Invasive Fungal Infections Echinocandins Azoles Polyenes 1) Lipid amphotericin B sales in 2012 2) Approximate, as measured by sales in 2011
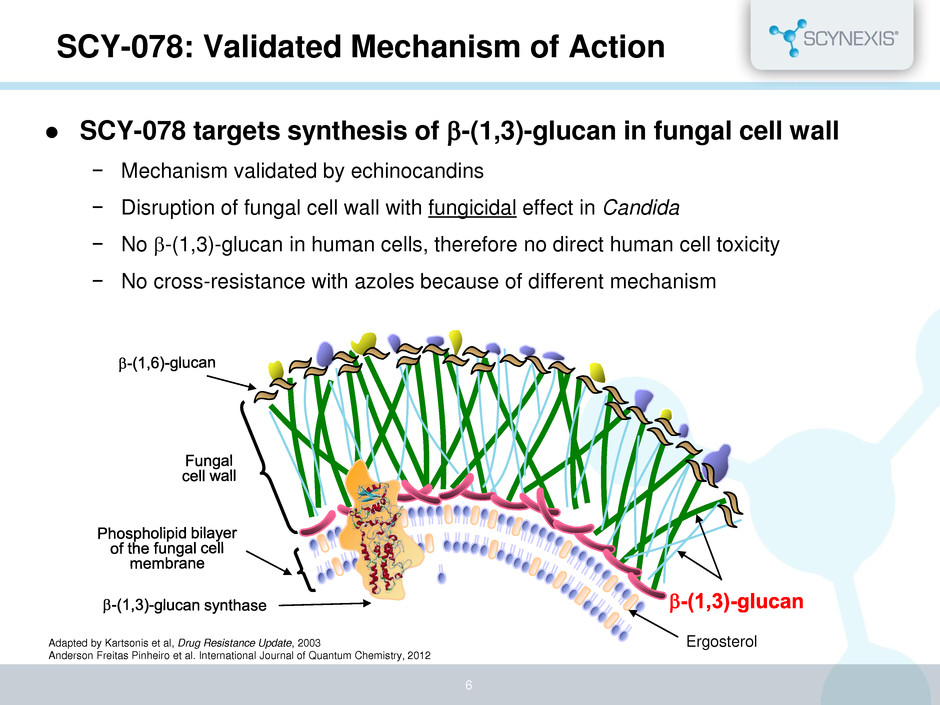
Ergosterol SCY-078: Validated Mechanism of Action Adapted by Kartsonis et al, Drug Resistance Update, 2003 Anderson Freitas Pinheiro et al. International Journal of Quantum Chemistry, 2012 6 SCY-078 targets synthesis of b-(1,3)-glucan in fungal cell wall − Mechanism validated by echinocandins − Disruption of fungal cell wall with fungicidal effect in Candida − No b-(1,3)-glucan in human cells, therefore no direct human cell toxicity − No cross-resistance with azoles because of different mechanism
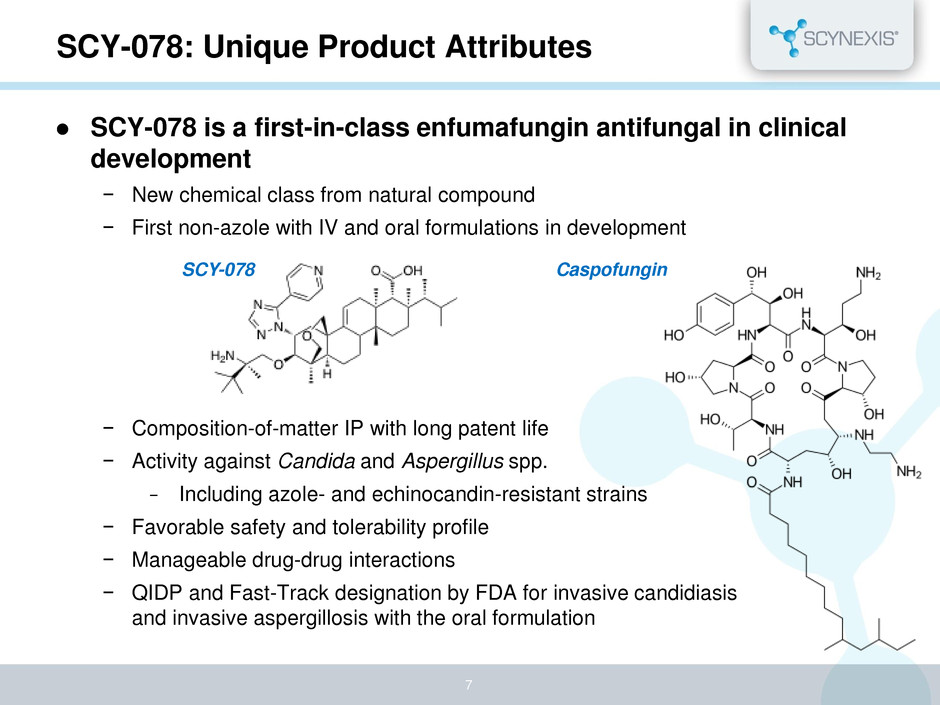
SCY-078: Unique Product Attributes SCY-078 is a first-in-class enfumafungin antifungal in clinical development − New chemical class from natural compound − First non-azole with IV and oral formulations in development − Composition-of-matter IP with long patent life − Activity against Candida and Aspergillus spp. − Including azole- and echinocandin-resistant strains − Favorable safety and tolerability profile − Manageable drug-drug interactions − QIDP and Fast-Track designation by FDA for invasive candidiasis and invasive aspergillosis with the oral formulation SCY-078 Caspofungin 7
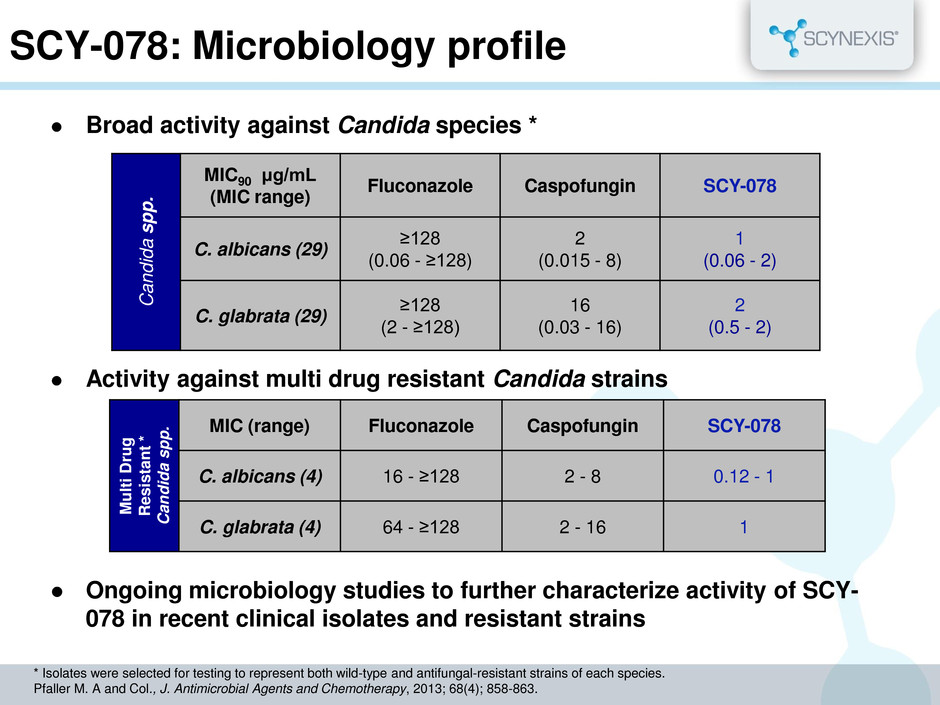
SCY-078: Microbiology profile Broad activity against Candida species * Activity against multi drug resistant Candida strains Ongoing microbiology studies to further characterize activity of SCY- 078 in recent clinical isolates and resistant strains C andid a s p p . MIC90 μg/mL (MIC range) Fluconazole Caspofungin SCY-078 C. albicans (29) ≥128 (0.06 - ≥128) 2 (0.015 - 8) 1 (0.06 - 2) C. glabrata (29) ≥128 (2 - ≥128) 16 (0.03 - 16) 2 (0.5 - 2) M ulti Dru g Re s is tant * Candid a s pp . MIC (range) Fluconazole Caspofungin SCY-078 C. albicans (4) 16 - ≥128 2 - 8 0.12 - 1 C. glabrata (4) 64 - ≥128 2 - 16 1 * Isolates were selected for testing to represent both wild-type and antifungal-resistant strains of each species. Pfaller M. A and Col., J. Antimicrobial Agents and Chemotherapy, 2013; 68(4); 858-863.

Development risks in anti-infectives are lower than in other areas − The pathogen is an external target with predictive in vitro and in vivo models − Well established PK/PD models − Significantly de-risked development projects once in the clinical stage Favorable current regulatory environment simplifies and accelerates development with significant financial incentives − GAIN Act (July 2012) − New pending regulations (DISARM, ADAPT, 21st Century Cures) − Multiple FDA approvals of QIDP products over the past year Impact of Current and Pending Legislation − Increased awareness of urgent need for new antifungals to fight resistance − Additional market exclusivity − Possibility for faster development pathway − Pending legislation may provide pricing power in resistant patient populations 9 Favorable Environment for Anti-Infectives
SCY-078 evaluated in seven Phase 1 studies in ~100 healthy subjects − Well characterized oral PK − Half-life supports once daily dosing − Predicted human efficacious oral dose of ~500-750mg daily based on murine disseminated candidiasis PK/PD studies Favorable safety and tolerability profile − Safe and well-tolerated at single oral doses up to 1600mg and multiple doses of 800mg/day for up to 28 days − Most common adverse events were gastrointestinal (nausea, diarrhea) − Majority of adverse events were mild to moderate and did not lead to discontinuation of therapy Metabolized primarily by glucuronidation and oxidative mechanisms involving CYP-3A4 10 SCY-078: Well-tolerated in Phase 1
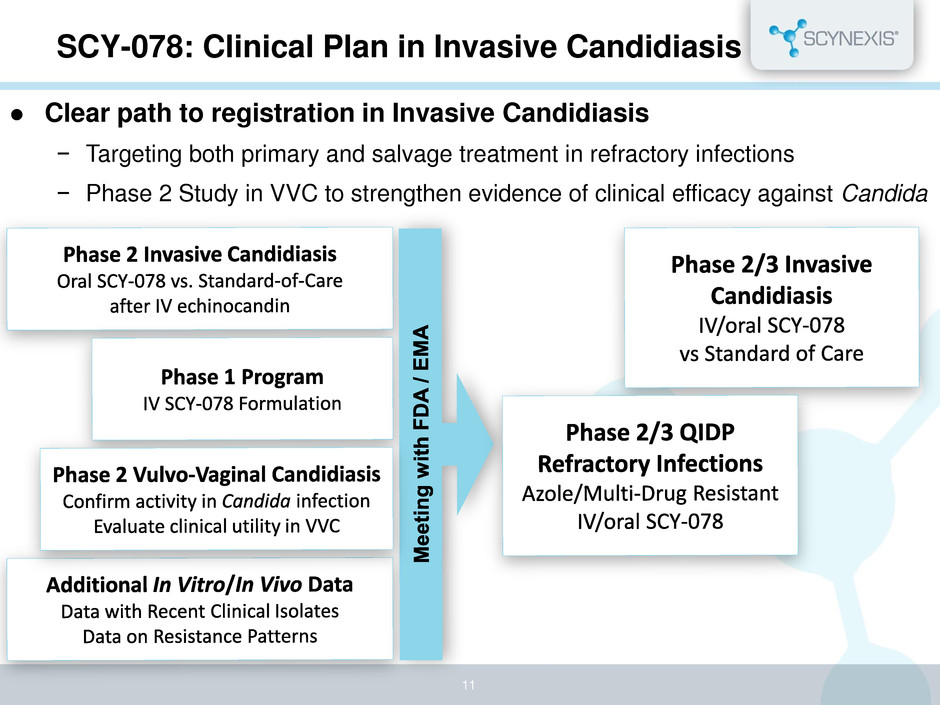
SCY-078: Clinical Plan in Invasive Candidiasis 11 Clear path to registration in Invasive Candidiasis − Targeting both primary and salvage treatment in refractory infections − Phase 2 Study in VVC to strengthen evidence of clinical efficacy against Candida
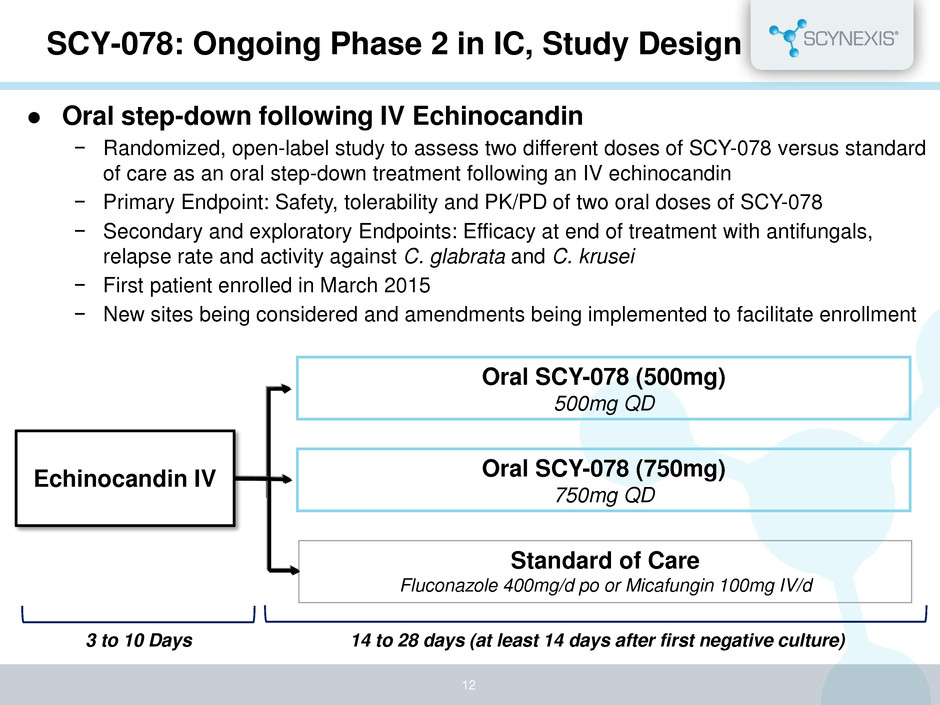
Oral step-down following IV Echinocandin − Randomized, open-label study to assess two different doses of SCY-078 versus standard of care as an oral step-down treatment following an IV echinocandin − Primary Endpoint: Safety, tolerability and PK/PD of two oral doses of SCY-078 − Secondary and exploratory Endpoints: Efficacy at end of treatment with antifungals, relapse rate and activity against C. glabrata and C. krusei − First patient enrolled in March 2015 − New sites being considered and amendments being implemented to facilitate enrollment SCY-078: Ongoing Phase 2 in IC, Study Design 14 to 28 days (at least 14 days after first negative culture) Oral SCY-078 (500mg) 500mg QD Oral SCY-078 (750mg) 750mg QD Standard of Care Fluconazole 400mg/d po or Micafungin 100mg IV/d 3 to 10 Days Echinocandin IV 12
Randomized, evaluator-blinded to assess two different dose regimens of Oral SCY-078 versus standard of care (Fluconazole) in subjects with moderate to severe VVC Two dose-regimens of oral SCY-078 (5 days or 3 days) compared to standard dose-regimen of Fluconazole. Assessment − Day 24 (Primary Test of Cure efficacy, TOC) − Week 8 and Week 16 (Secondary efficacy endpoint) Primary Endpoint: Safety and Efficacy (therapeutic outcome) at TOC Secondary Endpoints: Relapse rates, clinical & microbiological outcomes - Month 4 Approximately 30 patients per arm to be enrolled and followed for up to 4 months Initiation planned for 4Q 2015 with topline results (Day 24 Test of Cure) expected by first half of 2016 SCY-078: Planned Phase 2 in VVC 13

Invasive Candidiasis is the first target indication − Primary and salvage therapy in Invasive Candidiasis SCY-078 Positioning in Invasive Candidiasis − Treatment for refractory, multi-drug resistant pathogens − Alternative IV treatment to echinocandins allowing step-down option − Step-down options following IV dosing of any echinocandin Additional potential future indications − Invasive Aspergillosis − Prophylaxis of Invasive Fungal Infections − Vulvo-Vaginal Candidiasis, including recurrent VVC 14 IV SCY-078 Oral SCY-078 Any IV Echinocandin Oral SCY-078 SCY-078: Target Indications

SCYNEXIS Milestones Achieved Jan-2014 QIDP Status for Oral May-2014 IPO Completed Oct-2014 Waterstone Deal (SCY-635) Dec-2014 IV Formulation Selected Dec-2014 Fast Track for Oral Mar-2015 IV IND GLP Tox Started Mar-2015 First Patient in Phase 2 Oral Apr-2015 Follow-on Public Offering Jul-2015 Sale of the Service Business 15 Projected 2H-2015 Start Oral Phase 2 study in VVC Start IV Phase 1 Program 1H-2016 QIDP Status for IV Top Line Phase 2 Oral Invasive Candidiasis Complete IV Phase 1 Program Top Line Phase 2 VVC Additional In Vitro / In Vivo Data 2H-2016 FDA / EMA Meetings Fast Track for IV Initiate QIDP Refractory Trial

Financial Highlights 16 Initial Public Offering May 2014 − $62mm raised (gross proceeds) − Repaid $15mm debt − Top-tier Life Sciences Investors $41mm Follow-On Offering Completed April 2015 (gross proceeds) $57mm Cash as of June 30, 2015 − Funds company into H1-2017 − SCY-078 development − Ongoing Phase 2 study in IC − IV formulation Phase 1 studies − Phase 2 study in VVC − Pre-clinical in vitro / in vivo susceptibility and resistance data − CMC for the registration programs − Commencement of the registration clinical studies

SCYNEXIS Summary SCY-078: Innovative QIDP / Fast Track product in Phase 2 − Unique combination of attributes of safety, efficacy and flexibility of administration − Activity against resistant fungal strains, including multi-drug resistant pathogens − Well tolerated orally in ~100 subjects − IV and oral administration A clear path to registration − Favorable regulatory environment − QIDP and Fast Track status granted for the oral formulation − Significant financial legislative incentives An experienced team to execute the plan Committed to the development and commercialization of novel anti-infectives to address significant unmet therapeutic needs 17
