Attached files
| file | filename |
|---|---|
| 8-K - 8-K - POZEN INC /NC | a15-15891_38k.htm |
Exhibit 99.1
|
|
“Strategic Rationale and Vision for Aralez Pharmaceuticals” July, 2015 |
|
|
This presentation contains forward-looking statements under applicable securities laws, including, but not limited to, statements related to the anticipated consummation of the business combination transaction among Aralez, POZEN and Tribute and the timing and benefits thereof, the anticipated equity and debt financings and the closings thereof, the combined company's strategy, plans, objectives, expectations (financial or otherwise) and intentions, future financial results and growth potential, anticipated product portfolio, development programs and management structure, the proposed listing on the NASDAQ and TSX and other statements that are not historical facts. These forward-looking statements are based on POZEN's current assumptions and expectations and inherently involve significant risks and uncertainties. Actual results and the timing of events could differ materially from those anticipated in such forward looking statements as a result of these risks and uncertainties, which include, without limitation, risks related to the parties ability to complete the combination and financings on the proposed terms and schedule; the parties ability to close the capital investment on the proposed terms and schedule; the combined company meeting the listing on the NASDAQ and TSX; risk that Aralez may be taxed as a U.S. resident corporation; risks associated with business combination transactions, such as the risk that the businesses will not be integrated successfully, that such integration may be more difficult, time-consuming or costly than expected or that the expected benefits of the transaction will not occur; risks related to future opportunities and plans for the combined company, including uncertainty of the expected financial performance and results of the combined company following completion of the proposed transaction; disruption from the proposed transaction, making it more difficult to conduct business as usual or maintain relationships with customers, employees or suppliers; the calculations of, and factors that may impact the calculations of, the acquisition price in connection with the proposed merger and the allocation of such acquisition price to the net assets acquired in accordance with applicable accounting rules and methodologies; and the possibility that if the combined company does not achieve the perceived benefits of the proposed transaction as rapidly or to the extent anticipated by financial analysts or investors, the market price of the combined company's shares could decline, as well as other risks related to POZEN's and Tribute’s business, including POZEN's inability to build, acquire or contract with a sales force of sufficient scale for the commercialization of YOSPRALA™ in a timely and cost-effective manner, the parties’ failure to successfully commercialize our product candidates; costs and delays in the development and/or FDA approval of our product candidates (including YOSPRALA™), including as a result of the need to conduct additional studies or due to issues with third-party manufacturers, or the failure to obtain such approval of POZEN’s product candidates for all expected indications, including as a result of changes in regulatory standards or the regulatory environment during the development period of any of its product candidates; the inability to maintain or enter into, and the risks resulting from POZEN’s dependence upon, collaboration or contractual arrangements necessary for the development, manufacture, commercialization, marketing, sales and distribution of any products, including its dependence on AstraZeneca and Horizon for the sales and marketing of VIMOVO®, POZEN’s dependence on Patheon for the manufacture of YOSPRALA™ 81/40 and YOSPRALA™ 325/40 ; the ability of POZEN and Tribute to protect their intellectual property and defend their patents; regulatory obligations and oversight; and those risks detailed from time-to-time under the caption "Risk Factors" and elsewhere in POZEN's SEC filings and reports, including in its Annual Report on Form 10-K for the year ended December 31, 2014 and Form 10-Q for the quarter ended March 31, 2015 and in Tribute’s SEC filings and reports, including in its Annual Report on Form 10-K for the year ended December 31, 2014 and Form 10-Q for the quarter ended March 31, 2015. We undertake no duty or obligation to update ay forward-looking statements contained in this presentation as a result of new information, future events or changes in their expectations. This communication does not constitute an offer to sell, or the solicitation of an offer to sell, or the solicitation of an offer to subscribe for or buy, any securities nor shall there be any sale, issuance or transfer of securities in any jurisdiction in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such jurisdiction. Disclaimer |
|
|
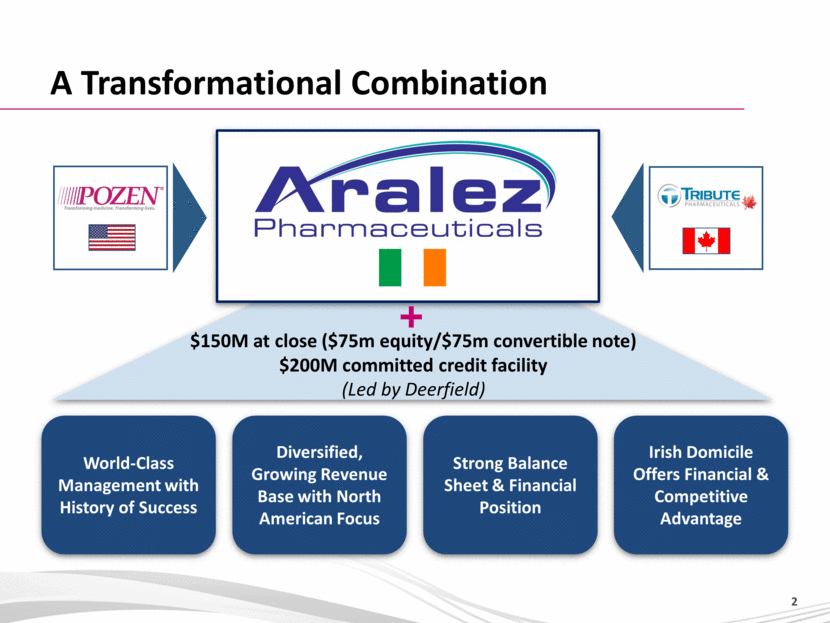
A Transformational Combination World-Class Management with History of Success Diversified, Growing Revenue Base with North American Focus Strong Balance Sheet & Financial Position Irish Domicile Offers Financial & Competitive Advantage $150M at close ($75m equity/$75m convertible note) $200M committed credit facility (Led by Deerfield) |
|
|
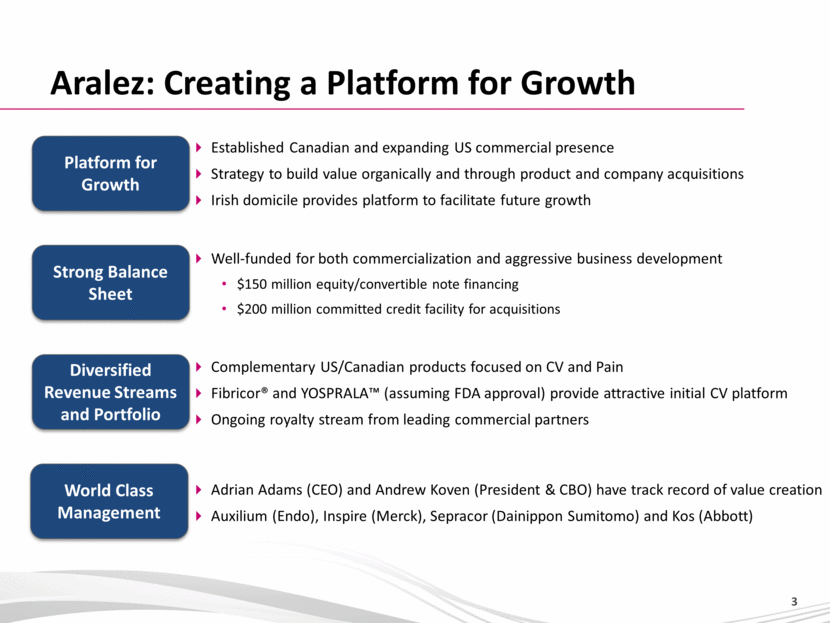
Aralez: Creating a Platform for Growth Strong Balance Sheet Platform for Growth Diversified Revenue Streams and Portfolio Adrian Adams (CEO) and Andrew Koven (President & CBO) have track record of value creation Auxilium (Endo), Inspire (Merck), Sepracor (Dainippon Sumitomo) and Kos (Abbott) World Class Management Well-funded for both commercialization and aggressive business development $150 million equity/convertible note financing $200 million committed credit facility for acquisitions Complementary US/Canadian products focused on CV and Pain Fibricor® and YOSPRALA™ (assuming FDA approval) provide attractive initial CV platform Ongoing royalty stream from leading commercial partners Established Canadian and expanding US commercial presence Strategy to build value organically and through product and company acquisitions Irish domicile provides platform to facilitate future growth |
|
|
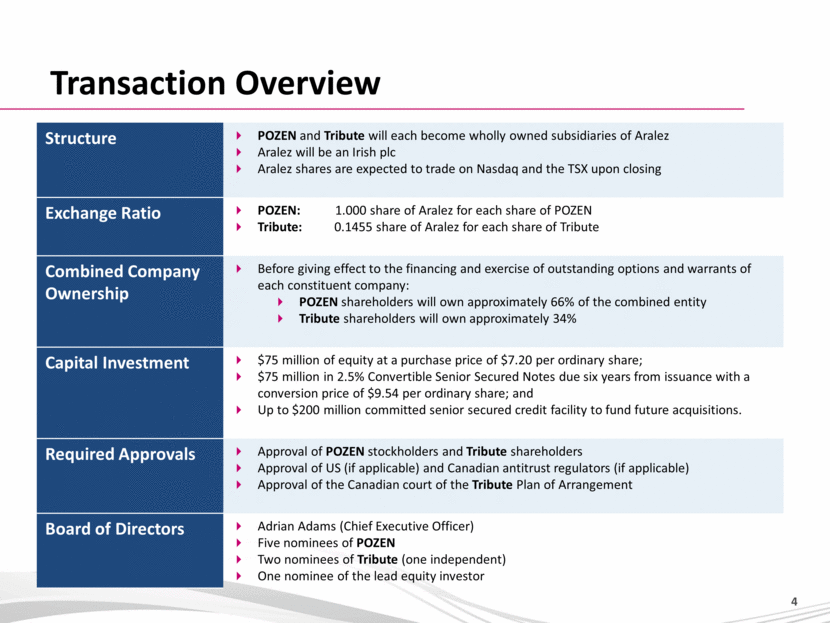
Transaction Overview Structure POZEN and Tribute will each become wholly owned subsidiaries of Aralez Aralez will be an Irish plc Aralez shares are expected to trade on Nasdaq and the TSX upon closing Exchange Ratio POZEN: 1.000 share of Aralez for each share of POZEN Tribute: 0.1455 share of Aralez for each share of Tribute Combined Company Ownership Before giving effect to the financing and exercise of outstanding options and warrants of each constituent company: POZEN shareholders will own approximately 66% of the combined entity Tribute shareholders will own approximately 34% Capital Investment $75 million of equity at a purchase price of $7.20 per ordinary share; $75 million in 2.5% Convertible Senior Secured Notes due six years from issuance with a conversion price of $9.54 per ordinary share; and Up to $200 million committed senior secured credit facility to fund future acquisitions. Required Approvals Approval of POZEN stockholders and Tribute shareholders Approval of US (if applicable) and Canadian antitrust regulators (if applicable) Approval of the Canadian court of the Tribute Plan of Arrangement Board of Directors Adrian Adams (Chief Executive Officer) Five nominees of POZEN Two nominees of Tribute (one independent) One nominee of the lead equity investor |
|
|
[LOGO] |
|
|
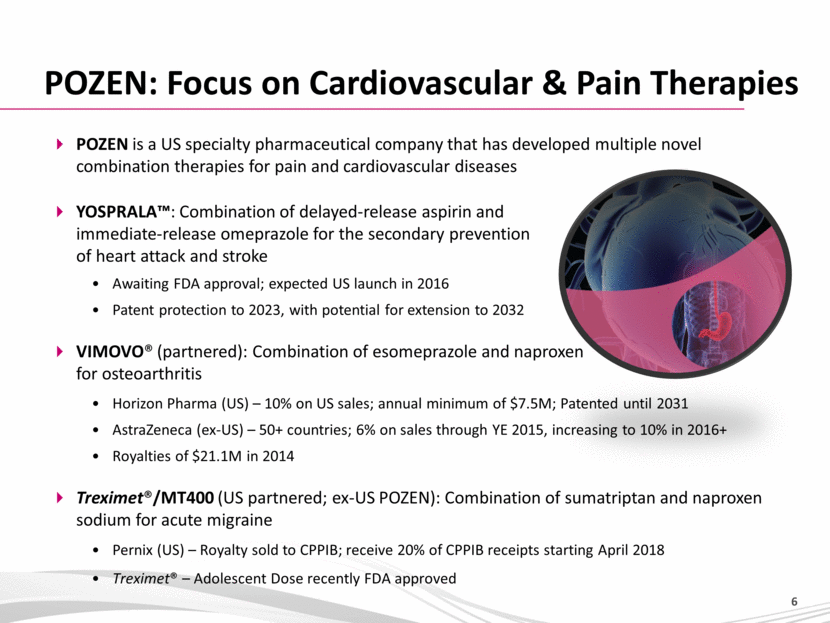
POZEN is a US specialty pharmaceutical company that has developed multiple novel combination therapies for pain and cardiovascular diseases YOSPRALA™: Combination of delayed-release aspirin and immediate-release omeprazole for the secondary prevention of heart attack and stroke Awaiting FDA approval; expected US launch in 2016 Patent protection to 2023, with potential for extension to 2032 VIMOVO® (partnered): Combination of esomeprazole and naproxen for osteoarthritis Horizon Pharma (US) – 10% on US sales; annual minimum of $7.5M; Patented until 2031 AstraZeneca (ex-US) – 50+ countries; 6% on sales through YE 2015, increasing to 10% in 2016+ Royalties of $21.1M in 2014 Treximet®/MT400 (US partnered; ex-US POZEN): Combination of sumatriptan and naproxen sodium for acute migraine Pernix (US) – Royalty sold to CPPIB; receive 20% of CPPIB receipts starting April 2018 Treximet® – Adolescent Dose recently FDA approved POZEN: Focus on Cardiovascular & Pain Therapies |
|
|
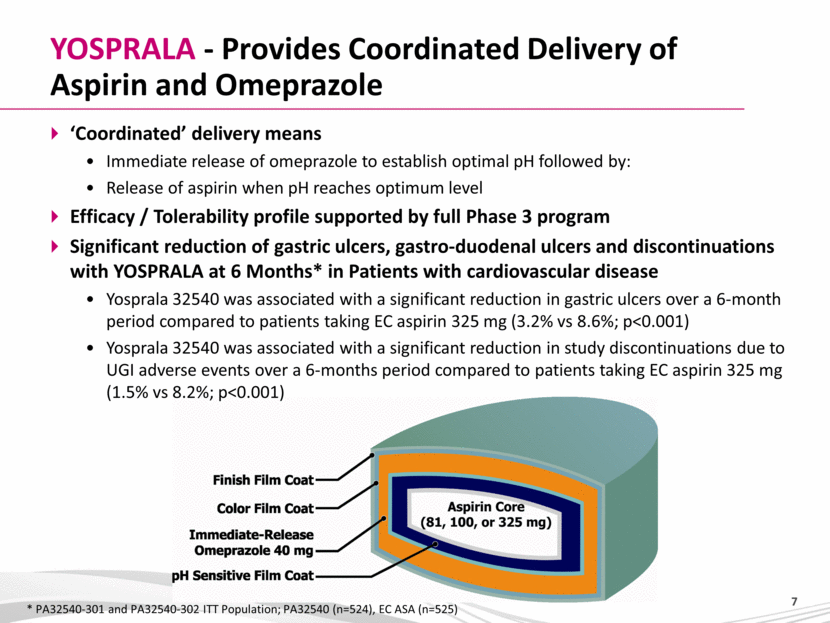
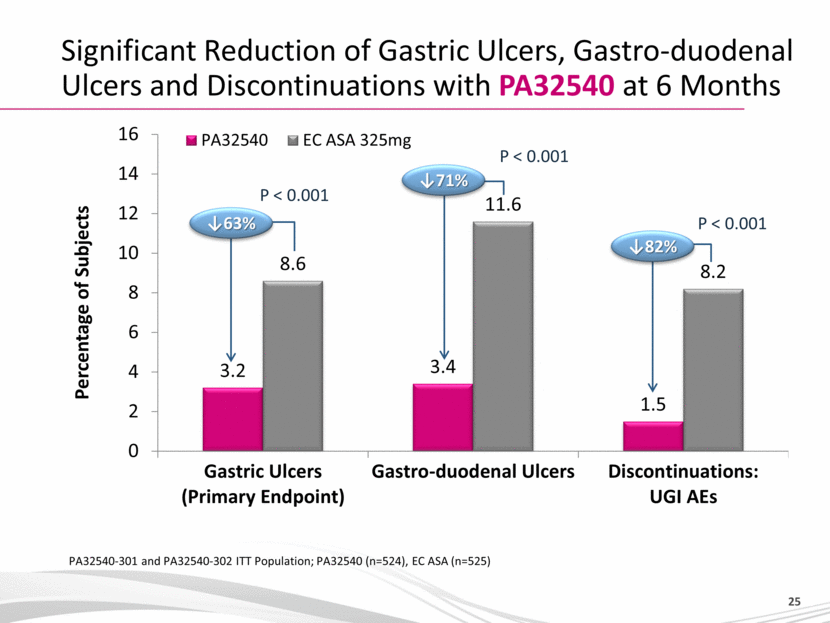
YOSPRALA - Provides Coordinated Delivery of Aspirin and Omeprazole ‘Coordinated’ delivery means Immediate release of omeprazole to establish optimal pH followed by: Release of aspirin when pH reaches optimum level Efficacy / Tolerability profile supported by full Phase 3 program Significant reduction of gastric ulcers, gastro-duodenal ulcers and discontinuations with YOSPRALA at 6 Months* in Patients with cardiovascular disease Yosprala 32540 was associated with a significant reduction in gastric ulcers over a 6-month period compared to patients taking EC aspirin 325 mg (3.2% vs 8.6%; p<0.001) Yosprala 32540 was associated with a significant reduction in study discontinuations due to UGI adverse events over a 6-months period compared to patients taking EC aspirin 325 mg (1.5% vs 8.2%; p<0.001) * PA32540-301 and PA32540-302 ITT Population; PA32540 (n=524), EC ASA (n=525) |
|
|
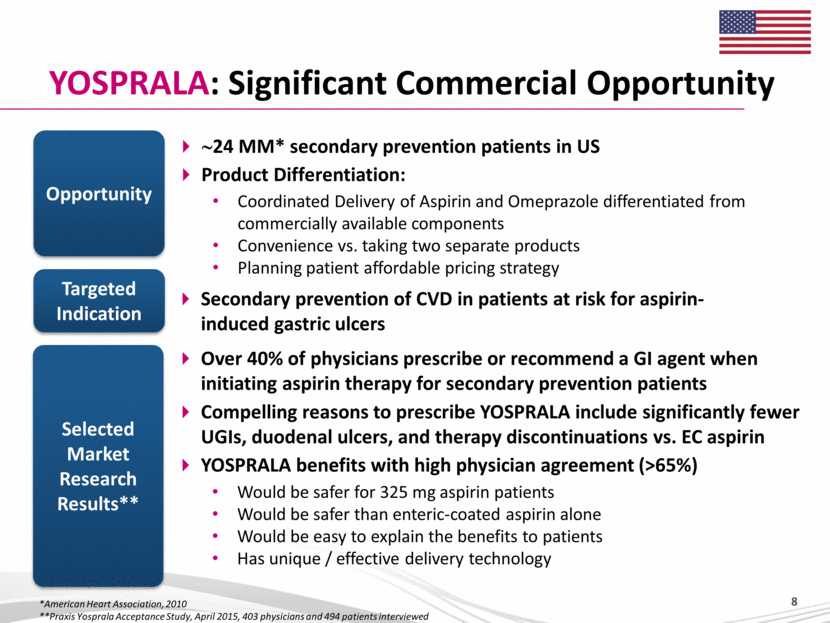
YOSPRALA: Significant Commercial Opportunity ~24 MM* secondary prevention patients in US Product Differentiation: Coordinated Delivery of Aspirin and Omeprazole differentiated from commercially available components Convenience vs. taking two separate products Planning patient affordable pricing strategy Opportunity Selected Market Research Results** Secondary prevention of CVD in patients at risk for aspirin-induced gastric ulcers Targeted Indication Over 40% of physicians prescribe or recommend a GI agent when initiating aspirin therapy for secondary prevention patients Compelling reasons to prescribe YOSPRALA include significantly fewer UGIs, duodenal ulcers, and therapy discontinuations vs. EC aspirin YOSPRALA benefits with high physician agreement (>65%) Would be safer for 325 mg aspirin patients Would be safer than enteric-coated aspirin alone Would be easy to explain the benefits to patients Has unique / effective delivery technology *American Heart Association, 2010 **Praxis Yosprala Acceptance Study, April 2015, 403 physicians and 494 patients interviewed 8 |
|
|
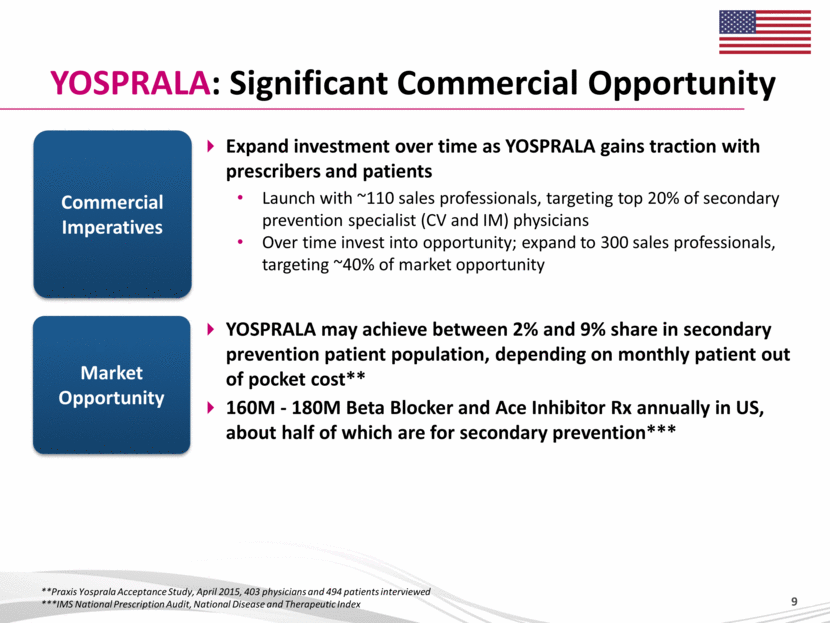
YOSPRALA: Significant Commercial Opportunity Expand investment over time as YOSPRALA gains traction with prescribers and patients Launch with ~110 sales professionals, targeting top 20% of secondary prevention specialist (CV and IM) physicians Over time invest into opportunity; expand to 300 sales professionals, targeting ~40% of market opportunity Commercial Imperatives Market Opportunity YOSPRALA may achieve between 2% and 9% share in secondary prevention patient population, depending on monthly patient out of pocket cost** 160M - 180M Beta Blocker and Ace Inhibitor Rx annually in US, about half of which are for secondary prevention*** **Praxis Yosprala Acceptance Study, April 2015, 403 physicians and 494 patients interviewed ***IMS National Prescription Audit, National Disease and Therapeutic Index |
|
|
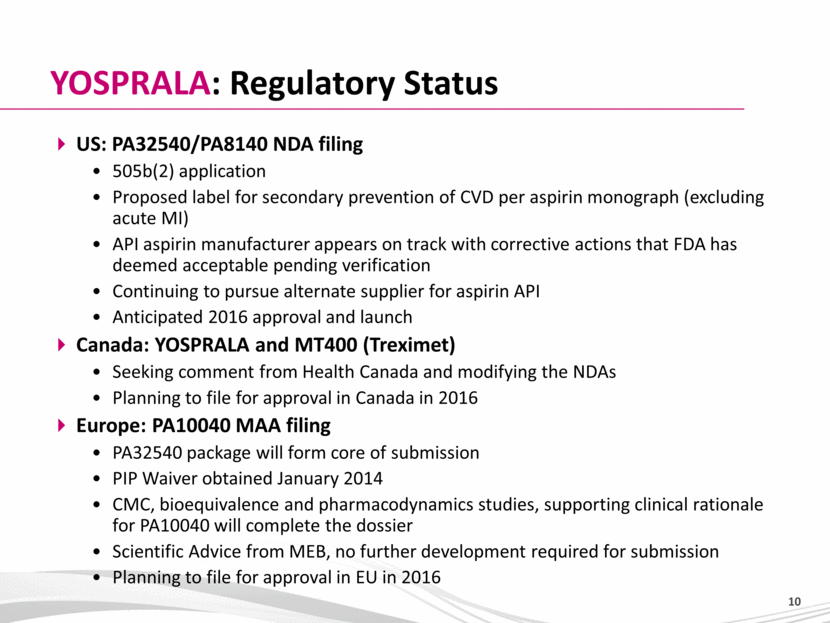
US: PA32540/PA8140 NDA filing 505b(2) application Proposed label for secondary prevention of CVD per aspirin monograph (excluding acute MI) API aspirin manufacturer appears on track with corrective actions that FDA has deemed acceptable pending verification Continuing to pursue alternate supplier for aspirin API Anticipated 2016 approval and launch Canada: YOSPRALA and MT400 (Treximet) Seeking comment from Health Canada and modifying the NDAs Planning to file for approval in Canada in 2016 Europe: PA10040 MAA filing PA32540 package will form core of submission PIP Waiver obtained January 2014 CMC, bioequivalence and pharmacodynamics studies, supporting clinical rationale for PA10040 will complete the dossier Scientific Advice from MEB, no further development required for submission Planning to file for approval in EU in 2016 YOSPRALA: Regulatory Status |
|
|
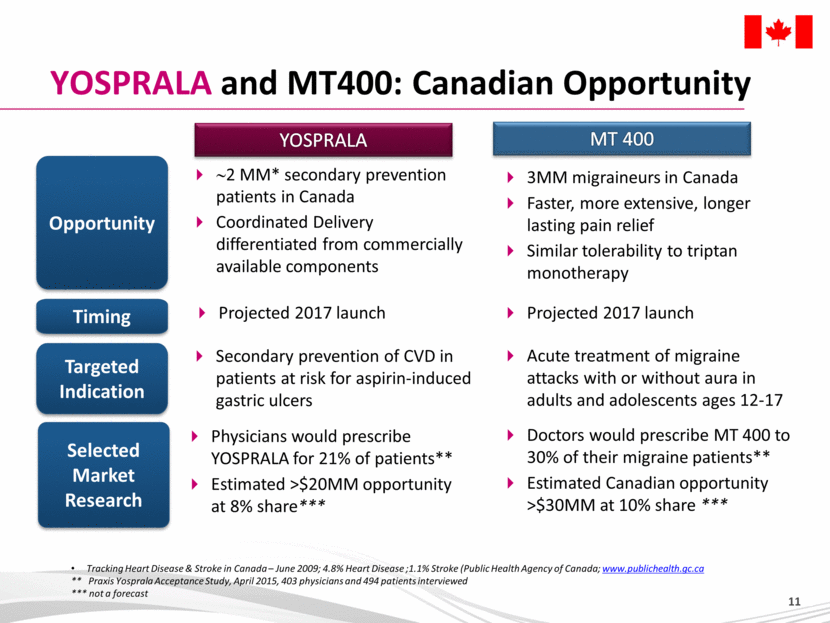
YOSPRALA and MT400: Canadian Opportunity Opportunity Selected Market Research Timing Tracking Heart Disease & Stroke in Canada – June 2009; 4.8% Heart Disease ;1.1% Stroke (Public Health Agency of Canada; www.publichealth.gc.ca ** Praxis Yosprala Acceptance Study, April 2015, 403 physicians and 494 patients interviewed *** not a forecast YOSPRALA MT 400 Targeted Indication ~2 MM* secondary prevention patients in Canada Coordinated Delivery differentiated from commercially available components Projected 2017 launch 3MM migraineurs in Canada Faster, more extensive, longer lasting pain relief Similar tolerability to triptan monotherapy Secondary prevention of CVD in patients at risk for aspirin-induced gastric ulcers Physicians would prescribe YOSPRALA for 21% of patients** Estimated >$20MM opportunity at 8% share*** Acute treatment of migraine attacks with or without aura in adults and adolescents ages 12-17 Doctors would prescribe MT 400 to 30% of their migraine patients** Estimated Canadian opportunity >$30MM at 10% share *** Projected 2017 launch |
|
|
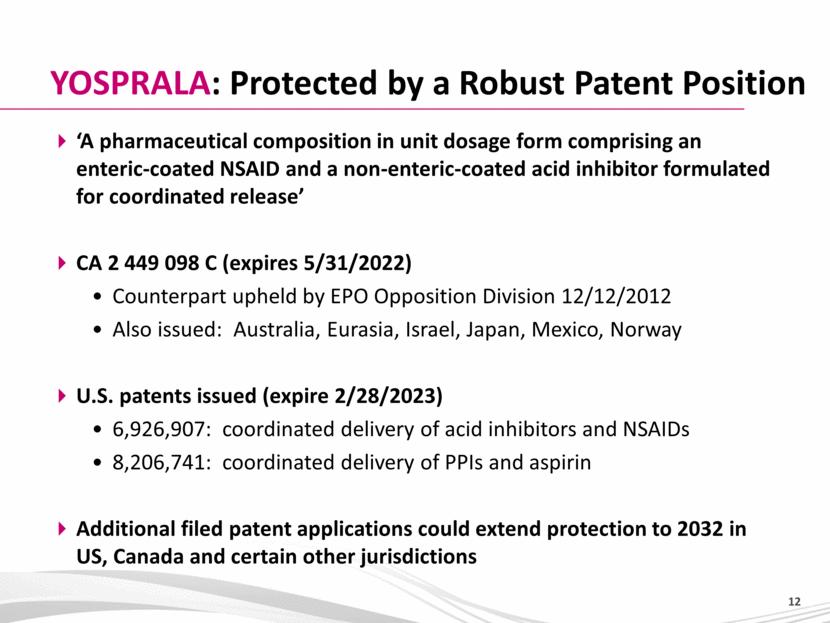
‘A pharmaceutical composition in unit dosage form comprising an enteric-coated NSAID and a non-enteric-coated acid inhibitor formulated for coordinated release’ CA 2 449 098 C (expires 5/31/2022) Counterpart upheld by EPO Opposition Division 12/12/2012 Also issued: Australia, Eurasia, Israel, Japan, Mexico, Norway U.S. patents issued (expire 2/28/2023) 6,926,907: coordinated delivery of acid inhibitors and NSAIDs 8,206,741: coordinated delivery of PPIs and aspirin Additional filed patent applications could extend protection to 2032 in US, Canada and certain other jurisdictions YOSPRALA: Protected by a Robust Patent Position |
|
|
[LOGO] |
|
|
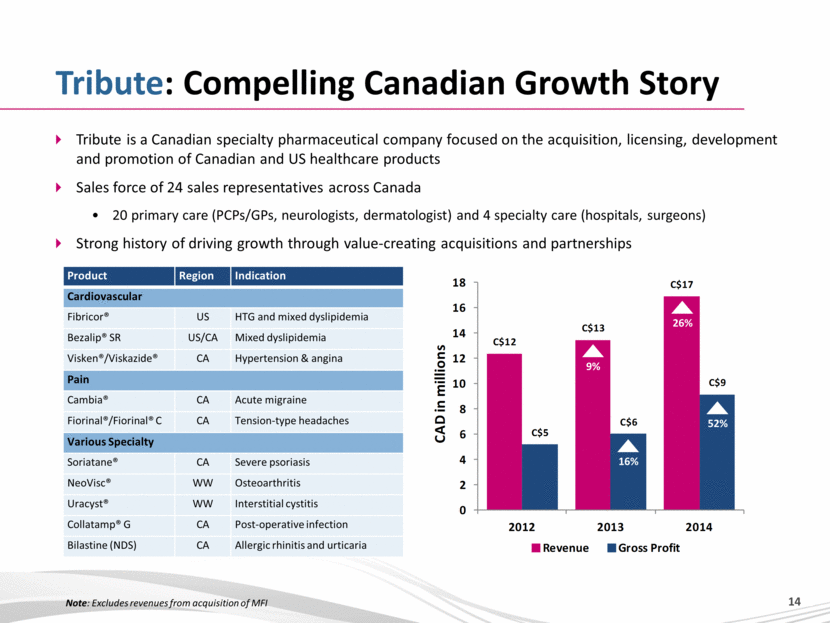
Tribute: Compelling Canadian Growth Story Tribute is a Canadian specialty pharmaceutical company focused on the acquisition, licensing, development and promotion of Canadian and US healthcare products Sales force of 24 sales representatives across Canada 20 primary care (PCPs/GPs, neurologists, dermatologist) and 4 specialty care (hospitals, surgeons) Strong history of driving growth through value-creating acquisitions and partnerships Product Region Indication Cardiovascular Fibricor® US HTG and mixed dyslipidemia Bezalip® SR US/CA Mixed dyslipidemia Visken®/Viskazide® CA Hypertension & angina Pain Cambia® CA Acute migraine Fiorinal®/Fiorinal® C CA Tension-type headaches Various Specialty Soriatane® CA Severe psoriasis NeoVisc® WW Osteoarthritis Uracyst® WW Interstitial cystitis Collatamp® G CA Post-operative infection Bilastine (NDS) CA Allergic rhinitis and urticaria 9% 26% 52% 16% Note: Excludes revenues from acquisition of MFI |
|
|
Indications Severe Hypertriglyceridemia Primary Hypercholesterolemia or Mixed Dyslipidemia Unique fenofibric acid formulation competes in the US$2.5 billion triglyceride lowering medication market Protected by 4 separate patents valid until August 20, 2027 Product sales of approximately US$4.7 million for the 12 months ending April 30, 2015* Gross margin >80% Focus will be to maximize pricing and distribution for the short-term 20-25 high quality reps Build relationships and grow product use in the U.S. and build springboard ahead of YOSPRALA Fibricor *Source: Tribute Pharmaceuticals press release dated May 21, 2016. |
|
|
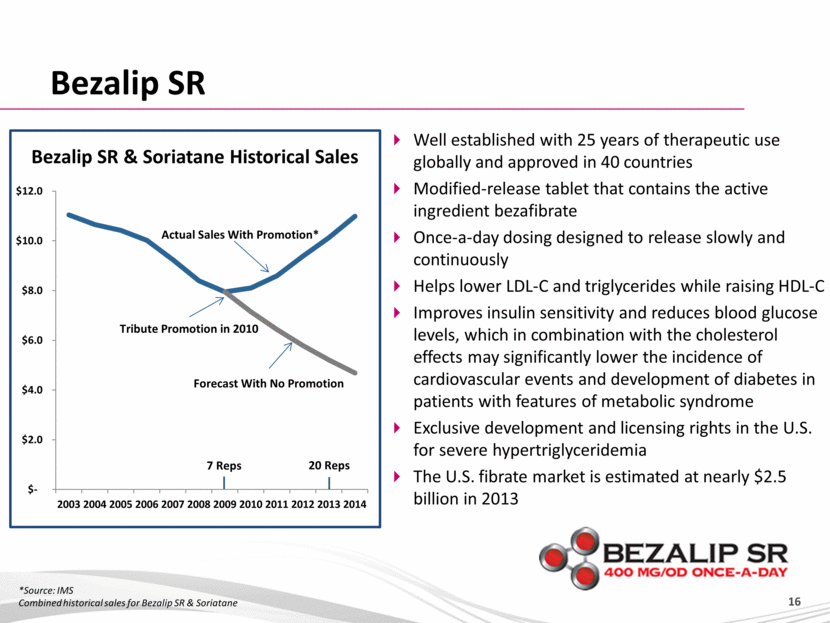
Bezalip SR Bezalip SR & Soriatane Historical Sales *Source: IMS Combined historical sales for Bezalip SR & Soriatane Well established with 25 years of therapeutic use globally and approved in 40 countries Modified-release tablet that contains the active ingredient bezafibrate Once-a-day dosing designed to release slowly and continuously Helps lower LDL-C and triglycerides while raising HDL-C Improves insulin sensitivity and reduces blood glucose levels, which in combination with the cholesterol effects may significantly lower the incidence of cardiovascular events and development of diabetes in patients with features of metabolic syndrome Exclusive development and licensing rights in the U.S. for severe hypertriglyceridemia The U.S. fibrate market is estimated at nearly $2.5 billion in 2013 $- $2.0 $4.0 $6.0 $8.0 $10.0 $12.0 2003 2004 2005 2006 2007 2008 2009 2010 2011 2012 2013 2014 20 Reps Tribute Promotion in 2010 7 Reps Actual Sales With Promotion* Forecast With No Promotion |
|
|
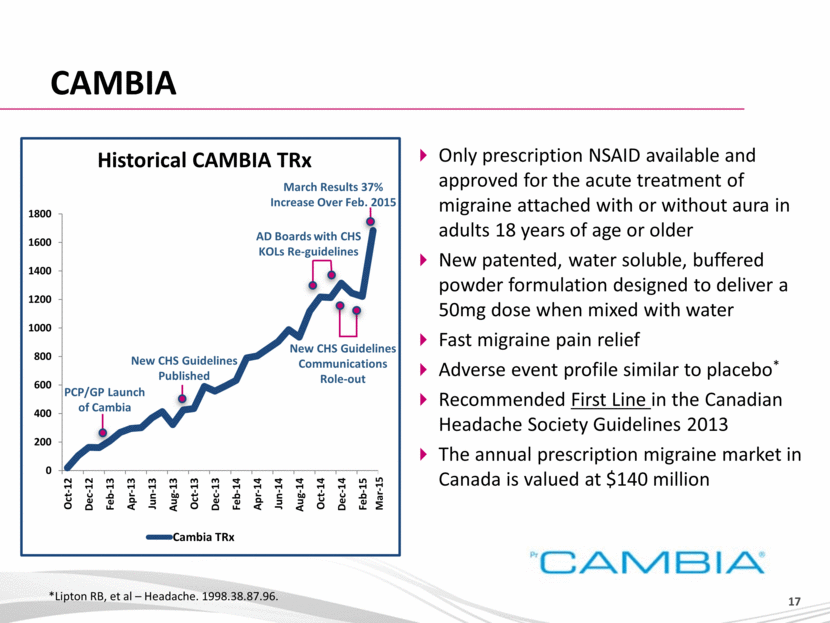
CAMBIA *Lipton RB, et al – Headache. 1998.38.87.96. Only prescription NSAID available and approved for the acute treatment of migraine attached with or without aura in adults 18 years of age or older New patented, water soluble, buffered powder formulation designed to deliver a 50mg dose when mixed with water Fast migraine pain relief Adverse event profile similar to placebo* Recommended First Line in the Canadian Headache Society Guidelines 2013 The annual prescription migraine market in Canada is valued at $140 million PCP/GP Launch of Cambia New CHS Guidelines Published New CHS Guidelines Communications Role-out AD Boards with CHS KOLs Re-guidelines March Results 37% Increase Over Feb. 2015 Historical CAMBIA TRx Mar-15 0 200 400 600 800 1000 1200 1400 1600 1800 Oct-12 Dec-12 Feb-13 Apr-13 Jun-13 Aug-13 Oct-13 Dec-13 Feb-14 Apr-14 Jun-14 Aug-14 Oct-14 Dec-14 Feb-15 Cambia TRx |
|
|
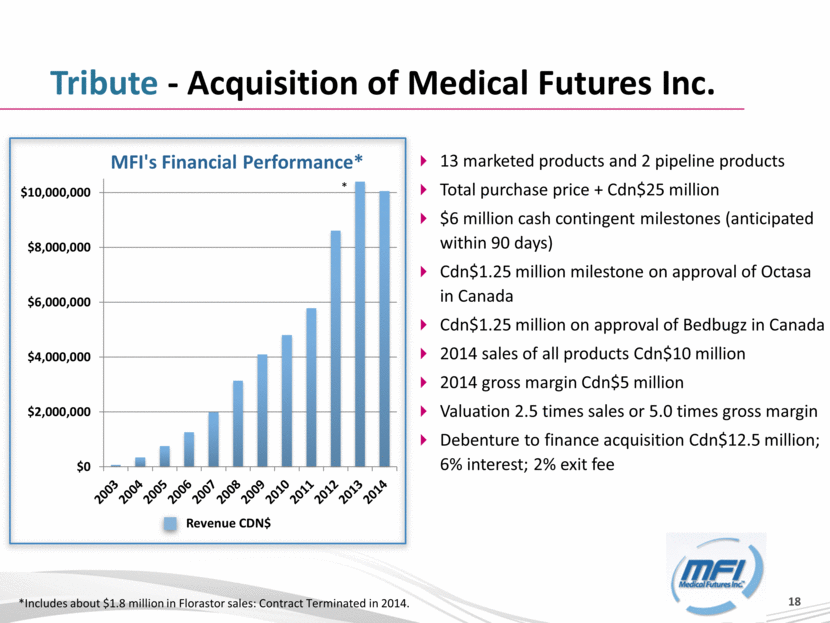
Tribute - Acquisition of Medical Futures Inc. *Includes about $1.8 million in Florastor sales: Contract Terminated in 2014. * Revenue CDN$ 13 marketed products and 2 pipeline products Total purchase price + Cdn$25 million $6 million cash contingent milestones (anticipated within 90 days) Cdn$1.25 million milestone on approval of Octasa in Canada Cdn$1.25 million on approval of Bedbugz in Canada 2014 sales of all products Cdn$10 million 2014 gross margin Cdn$5 million Valuation 2.5 times sales or 5.0 times gross margin Debenture to finance acquisition Cdn$12.5 million; 6% interest; 2% exit fee $0 $2,000,000 $4,000,000 $6,000,000 $8,000,000 $10,000,000 MFI's Financial Performance* |
|
|
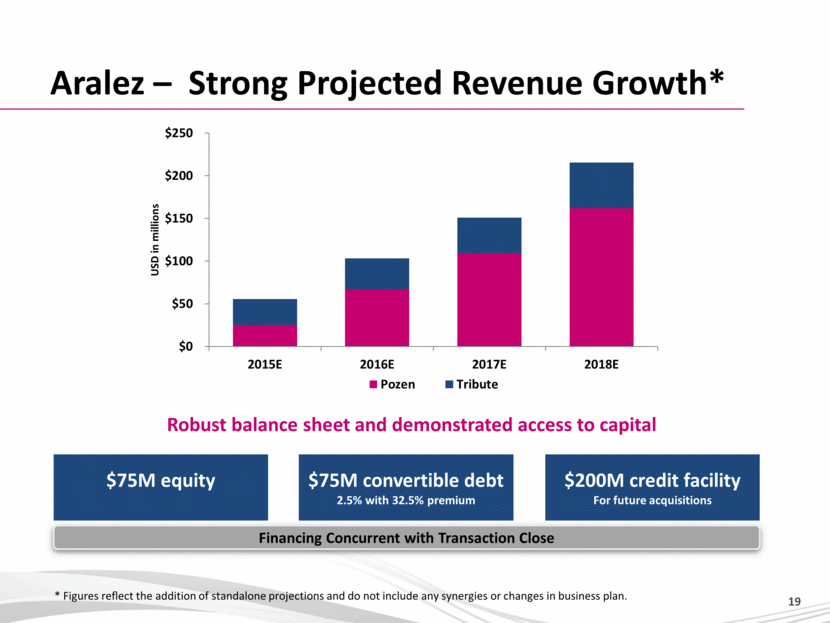
Aralez – Strong Projected Revenue Growth* $75M equity Robust balance sheet and demonstrated access to capital $75M convertible debt 2.5% with 32.5% premium $200M credit facility For future acquisitions * Figures reflect the addition of standalone projections and do not include any synergies or changes in business plan. Financing Concurrent with Transaction Close $0 $50 $100 $150 $200 $250 2015E 2016E 2017E 2018E USD in millions Pozen Tribute 19 |
|
|
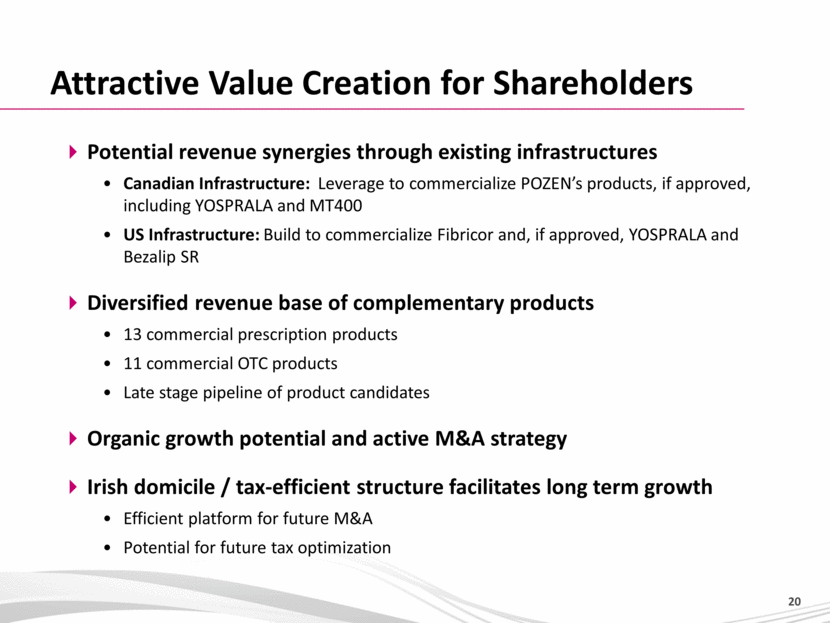
Attractive Value Creation for Shareholders Potential revenue synergies through existing infrastructures Canadian Infrastructure: Leverage to commercialize POZEN’s products, if approved, including YOSPRALA and MT400 US Infrastructure: Build to commercialize Fibricor and, if approved, YOSPRALA and Bezalip SR Diversified revenue base of complementary products 13 commercial prescription products 11 commercial OTC products Late stage pipeline of product candidates Organic growth potential and active M&A strategy Irish domicile / tax-efficient structure facilitates long term growth Efficient platform for future M&A Potential for future tax optimization 20 |
|
|
The Aralez Growth Strategy & Priorities Maximize the value of expanded portfolio & geographic footprint Build around a cardiovascular-based, North American-focused portfolio Aggressive approach to CD&L and M&A to drive growth supported by new platform Leverage platform for growth with tax-efficient structure Anchor position in Cardiovascular supported by a pain franchise Remain opportunistic for other specialty areas Acquire low-risk, high potential, revenue generating “bolt on” products in Cardiovascular or specialty orientation Strive towards closing the Tribute Pharmaceuticals merger in 4Q 2015 Maintain sound financial position through disciplined decision making Ample liquidity to commercialize YOSPRALA and explore additional acquisition opportunities Continue to grow the Canadian business Grow Fibricor use in the U.S. and build springboard ahead of YOSPRALA approval and commercial launch Successfully launch YOSPRALA in the U.S. and grow use with cardiologists and high prescribing physicians Build sales force, deploy managed care strategy and tactics to drive growth and invest into the opportunity Consider submission of YOSPRALA and TREXIMET in Canada |
|
|
“Strategic Rationale and Vision for Aralez Pharmaceuticals” July, 2015 |
|
|
World Class Management Team with Proven Track Record of Successfully Growing Companies Kos Pharmaceuticals: Licensed NIASPAN and ADVICOR to Oryx in Canada Licensed NIASPAN and ADVICOR to Merck KGa in Europe Co-Promotion with Takeda for NIASPAN in US Co-Promotion with Barr Laboratories for NIASPAN in women's Healthcare in US Acquired AZMACORT from Aventis Acquired CV portfolio comprising TEVETEN/ TEVETEN HCT and CARDIZEM LA from BIOVAIL (together with approx. 120 Biovail reps) Licensed FIRAZYR (for Hereditary Angiodema) from Jerini for US and Canada Licensed (Asthma and COPD) pipeline product from Skyepharma for US Sepracor Inc: Licensed STEDESA (anti-epileptic) from Bial Licensed ALVESCO and OMNARIS for Asthma, COPD and Allergic Rhinitis for US from Nycomed Acquired Oryx Pharmaceuticals (Canada) to give Canadian Presence Auxilium Pharmaceuticals: Licensed out XIAFLEX to Actelion for four counties Licensed out XIAFLEX in Europe to Swedish Orphan (SOBI) Co-promotion with GSK for Testim in the USA Acquired Actient Pharmaceuticals together with 10 products – immediate portfolio diversification and formation of leadership position in Men’s Healthcare Licensed in STENDRA (for Erectile Dysfunction) from VIVUS |
|
|
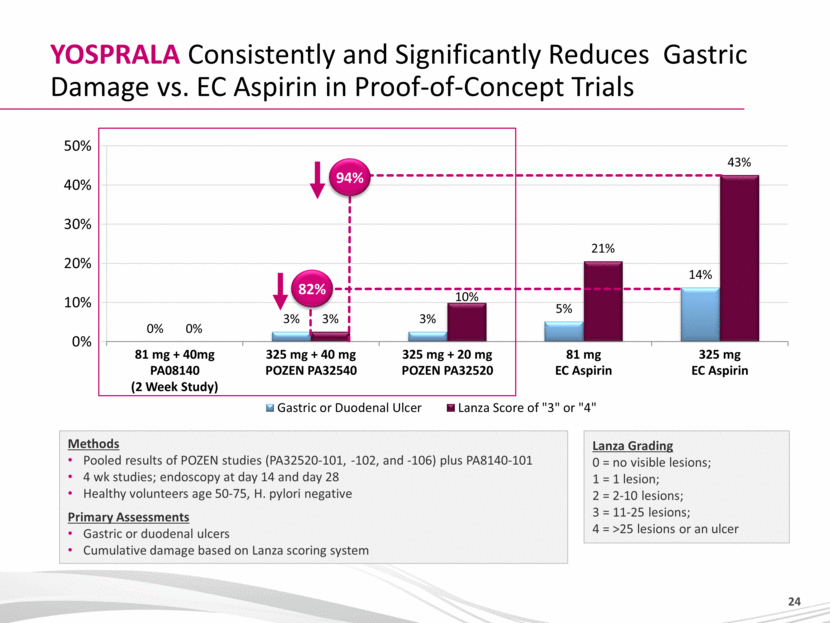
YOSPRALA Consistently and Significantly Reduces Gastric Damage vs. EC Aspirin in Proof-of-Concept Trials Lanza Grading 0 = no visible lesions; 1 = 1 lesion; 2 = 2-10 lesions; 3 = 11-25 lesions; 4 = >25 lesions or an ulcer Methods Pooled results of POZEN studies (PA32520-101, -102, and -106) plus PA8140-101 4 wk studies; endoscopy at day 14 and day 28 Healthy volunteers age 50-75, H. pylori negative Primary Assessments Gastric or duodenal ulcers Cumulative damage based on Lanza scoring system 94% 82% |
|
|
Significant Reduction of Gastric Ulcers, Gastro-duodenal Ulcers and Discontinuations with PA32540 at 6 Months PA32540-301 and PA32540-302 ITT Population; PA32540 (n=524), EC ASA (n=525) P < 0.001 P < 0.001 Percentage of Subjects 63% 71% P < 0.001 82% |
|
|
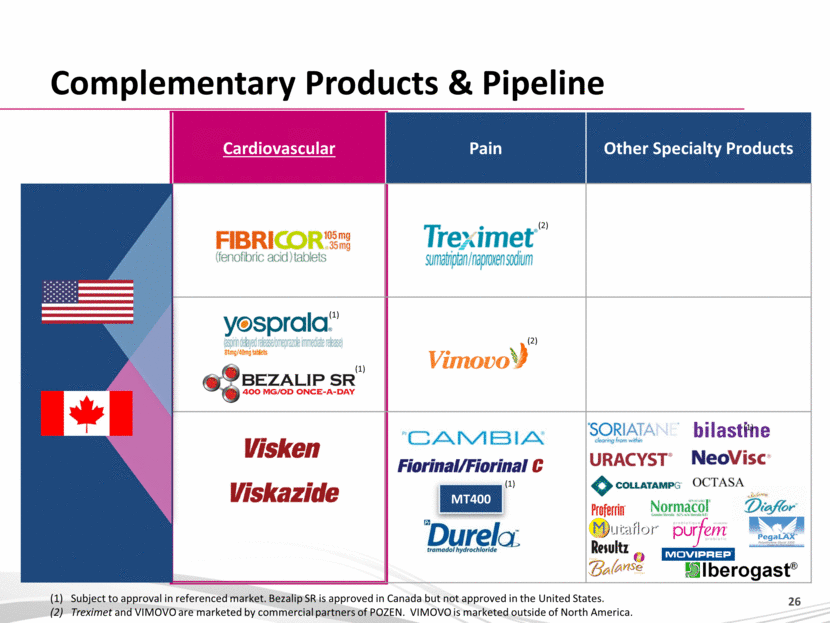
Cardiovascular Pain Other Specialty Products Complementary Products & Pipeline MT400 (1) Subject to approval in referenced market. Bezalip SR is approved in Canada but not approved in the United States. (2) Treximet and VIMOVO are marketed by commercial partners of POZEN. VIMOVO is marketed outside of North America. (2) (2) (1) (1) (1) |
|
|
Medical Futures Inc. Products |