Attached files
| file | filename |
|---|---|
| 8-K - 8-K - EAGLE PHARMACEUTICALS, INC. | a8-k060315.htm |

Eagle Pharmaceuticals NASDAQ: EGRX Scott Tarriff, CEO David Riggs, CFO June 2015

JUNE 2015 2 Forward Looking Statements This presentation contains certain forward-looking information about Eagle Pharmaceuticals that is intended to be covered by the safe harbor for "forward-looking statements" provided by the Private Securities Litigation Reform Act of 1995, as amended. Forward-looking statements are statements that are not historical facts. Words such as “anticipate,” “believe,” “estimate,” “expect,” “intend,” “may,” “plan,” “predict,” “project,” “target,” “potential,” “will,” “would,” “could,” “should,” “continue,” “strong,” “upcoming,” “prepared” and similar expressions are intended to identify forward-looking statements. These statements include, but are not limited to, statements regarding our ability to successfully develop and commercialize our therapeutic products; our ability to expand our long-term business opportunities; financial projections and estimates and their underlying assumptions; and future performance. All such statements are subject to certain risks and uncertainties, many of which are difficult to predict and generally beyond the Company’s control, that could cause actual results to differ materially from those expressed in, or implied or projected by, the forward-looking information and statements. These risks and uncertainties include, but are not limited to: whether the FDA will accept for filing and approve our NDA for our bendamustine HCl rapid infusion product and other pharmaceutical products in development and when such approvals might be granted; the timing of future events, including but not limited to clinical development of our products and regulatory submissions; whether we will successfully market our products; our ability to successfully compete with other pharmaceutical and biotechnology companies; the success of our commercial partnerships; the development of, and our ability to take advantage of, the market for our therapeutic products; the strength and enforceability of our intellectual property rights; general economic conditions; and the other risk factors included in our annual report on form 10-K filed with the U.S. Securities and Exchange Commission on December 22, 2014. Our audience is cautioned not to place undue reliance on these forward-looking statements that speak only as of the date hereof, and we do not undertake any obligation to revise and disseminate forward-looking statements to reflect events or circumstances after the date hereof, or to reflect the occurrence of or non- occurrence of any events.
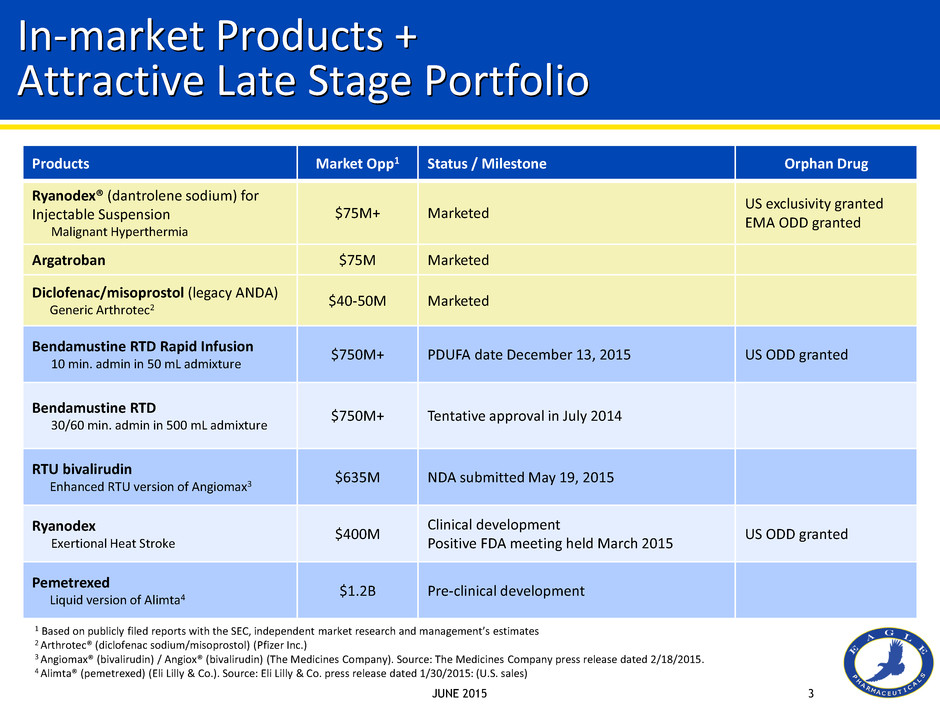
JUNE 2015 3 Products Market Opp1 Status / Milestone Orphan Drug Ryanodex® (dantrolene sodium) for Injectable Suspension Malignant Hyperthermia $75M+ Marketed US exclusivity granted EMA ODD granted Argatroban $75M Marketed Diclofenac/misoprostol (legacy ANDA) Generic Arthrotec2 $40-50M Marketed Bendamustine RTD Rapid Infusion 10 min. admin in 50 mL admixture $750M+ PDUFA date December 13, 2015 US ODD granted Bendamustine RTD 30/60 min. admin in 500 mL admixture $750M+ Tentative approval in July 2014 RTU bivalirudin Enhanced RTU version of Angiomax3 $635M NDA submitted May 19, 2015 Ryanodex Exertional Heat Stroke $400M Clinical development Positive FDA meeting held March 2015 US ODD granted Pemetrexed Liquid version of Alimta4 $1.2B Pre-clinical development In-market Products + Attractive Late Stage Portfolio 1 Based on publicly filed reports with the SEC, independent market research and management’s estimates 2 Arthrotec® (diclofenac sodium/misoprostol) (Pfizer Inc.) 3 Angiomax® (bivalirudin) / Angiox® (bivalirudin) (The Medicines Company). Source: The Medicines Company press release dated 2/18/2015. 4 Alimta® (pemetrexed) (Eli Lilly & Co.). Source: Eli Lilly & Co. press release dated 1/30/2015: (U.S. sales)

JUNE 2015 4 Bendamustine HCl RTD NDA 1: in 500 mL admixture, 30- or 60-minute infusion time (same as Treanda) – Tentative approval received July 2014 – One patent infringement lawsuit dismissed, one settled NDA 2: in 50 mL admixture, 10-minute infusion time (Rapid Infusion Product) – Bioequivalence to Treanda + comparable safety & AE profile established by 81 patient clinical trial – Entered into exclusive license agreement with Teva – NDA accepted in Apr:15 for CLL & NHL -- Dec:15 PDUFA date ODD granted in Jul:14 for use in both indications – May afford 7 years exclusivity upon approval – Three patents issued to June 2031 and March 2033; five pending 1 Treanda® (bendamustine HCl) (Cephalon, Inc., a wholly-owned subsidiary of Teva Pharmaceutical Industries Ltd.) 2 CLL: chronic lymphocytic leukemia 3 NHL: indolent B-cell non-Hodgkin lymphoma Improved formulation of Treanda1

JUNE 2015 5 Responsibilities TEVA • U.S. regulatory approvals • Supply product to Teva for a specified period while Teva or a third party second-source supplier is qualified • Any post-approval clinical studies, if required • Market Rapid Infusion Bendamustine for CLL1 & indolent B-cell NHL2 in U.S. • All U.S. commercial activities, including minimum promotional & detailing efforts • IP covering Rapid Infusion Bendamustine Exclusive Agreement with Teva for Bendamustine RTD Rapid Infusion 1 CLL: chronic lymphocytic leukemia 2 NHL: indolent B-cell non-Hodgkin lymphoma 3 Assuming FDA approval Summary Financial Terms $30M upfront cash payment from Teva Up to $90M in additional milestone payments: – Timing of FDA approval – Achievement of certain sales levels – Achievement of certain reimbursement criteria Royalty payments consisting of double-digit percentage of Teva’s net sales of Rapid3

JUNE 2015 6 Multiple Milestones in 2015 to Drive Value Apr:15 Acceptance for filing of Rapid NDA May:15 Submitted NDA for Bivalirudin RTU 3Q:15 Acceptance for filing of Bivalirudin RTU NDA Mid:15 Decision expected: Hospira vs. MDCO patent litigation (bivalirudin) Dec:15 PDUFA date for Rapid with Standard Review* 4Q:15 File for J-Code for Rapid 2H:15 Decision on ODD Exclusivity for Rapid Additional opportunities: product line expansion internally or through product or company acquisitions * Followed by Teva launch of Rapid, assuming approval

JUNE 2015 7 Teva Agreement: Other Material Terms Agreed to settle all patent litigation Eagle has the option to terminate agreement and commercialize Rapid: – If, by a specified date, certain milestones for Rapid have not been obtained/achieved and certain generic Bendamustine products are launched in the U.S. – If Teva challenges Eagle’s patents on either Rapid or the Bendamustine RTD product Eagle has the option to launch our tentatively approved Bendamustine RTD product in the U.S. after May 1, 2016

JUNE 2015 8 Multiple Benefits of Rapid Infusion Bendamustine RTD • Less chair time: 30 or 60 min. reduced to 10 min. • Less volume & issues related to 50mL vs. 500mL admixture Patient • Less nursing time required Nurse • 90% reduction in NaCl – renal suppressed population Medical/ Patient • Additional patients treated in the cancer clinic enabled by shorter infusion time Economic

JUNE 2015 9 Large Near-Term Market Opportunity Treanda sales over $760M1 Teva expected to launch shortly after approval Eagle is preparing for a near complete conversion Plan to file for unique J-code in 4Q:15 1 Treanda® (bendamustine HCl) sales in 2014 of $767 million, source: Teva Pharmaceuticals press release dated 2/5/2015

JUNE 2015 10 Ryanodex® (dantrolene sodium) for Malignant Hyperthermia (MH) No change to SOC for MH treatment in over 30 years – Often fatal if untreated Ryanodex approved in July 2014 – Optimized formulation of dantrolene sodium – Reduces to 5mL (1 vial) vs. 720 mL (12 vials) for old product Breakthrough change Protected market position – Three patents issued + one filed – Orphan drug designation for MH (U.S. & Europe) Granted seven years market exclusivity in US EU regulatory submission to be filed in 4Q:15

JUNE 2015 11 U.S. Commercial Landscape All U.S. hospitals required to stock dantrolene – ~9,000 outlets – MHAUS1 recommends a minimum 36 vials = 3 vials Ryanodex Repurchase required every 2 years – Premium pricing supported by Ryanodex advantages Faster administration Reduced volume Significant reduction of Mannitol (diuretic) 1 Joint Commission; MHAUS: Malignant Hyperthermia Association of the United States

JUNE 2015 12 U.S. Launch Update Launched Aug:14 15 field force representatives Premium pricing to old formulation expands addressable market from $20M1 to $75M (over two years) Conversions to date: multiple top-tier hospitals, Mayo Clinic, NIH, VAs Sales Growth Since Launch Trending as expected Ongoing sequential growth expected as market converts to Ryanodex Commercialization Snapshot 1 Branded dantrolene sales for MH 2013 $- $0.5 $1.0 $1.5 $2.0 Q3 '14 Q4 '14 Q1 '15

JUNE 2015 13 Initial Label Expansion Opportunity: EHS Exertional Heat Stroke (EHS) – Est. 23,000-38,000 cases/year1 – A leading cause of student athlete death (US) & non-combat military deaths – Similarities to MH Potential for Ryanodex to be first to market for EHS Orphan drug designation for EHS – Potential for 7 years of exclusivity Market estimated at $400M 1 Estimate based on 2010 population projections

JUNE 2015 14 EHS: Development Update Held Type C meeting with FDA on March 11th Productive discussion regarding regulatory pathway and required development program to support label expansion We believe the Agency recognizes the importance of this rare, life- threatening condition and the potential benefit Ryanodex may afford patients Path forward: a ‘hybrid’ development program including human and animal efficacy and safety data Eagle clinical plans in development
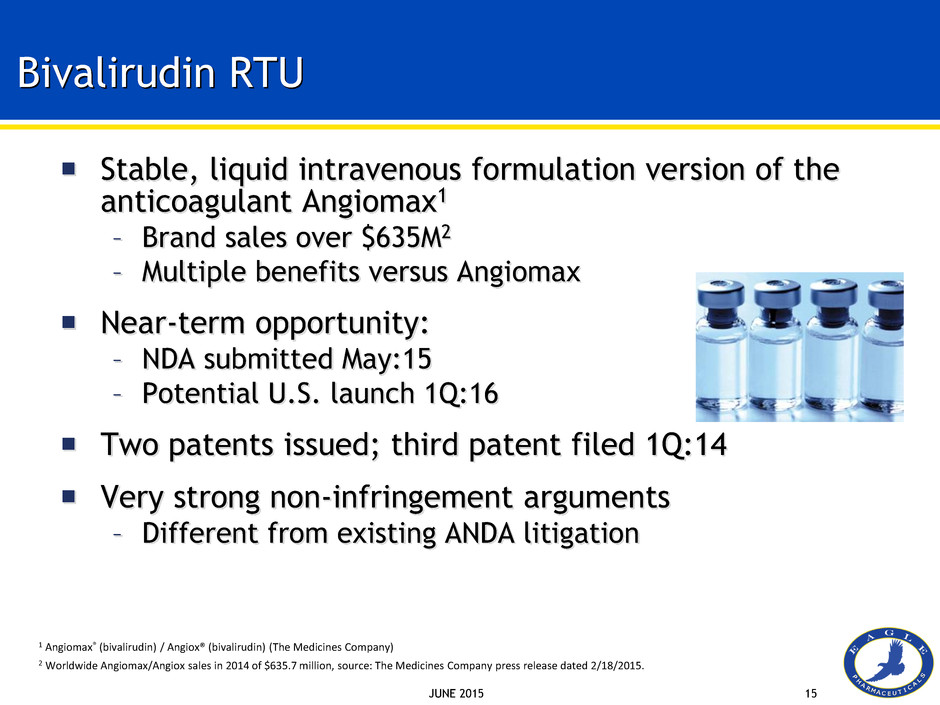
JUNE 2015 15 Bivalirudin RTU Stable, liquid intravenous formulation version of the anticoagulant Angiomax1 – Brand sales over $635M2 – Multiple benefits versus Angiomax Near-term opportunity: – NDA submitted May:15 – Potential U.S. launch 1Q:16 Two patents issued; third patent filed 1Q:14 Very strong non-infringement arguments – Different from existing ANDA litigation 1 Angiomax® (bivalirudin) / Angiox® (bivalirudin) (The Medicines Company) 2 Worldwide Angiomax/Angiox sales in 2014 of $635.7 million, source: The Medicines Company press release dated 2/18/2015.
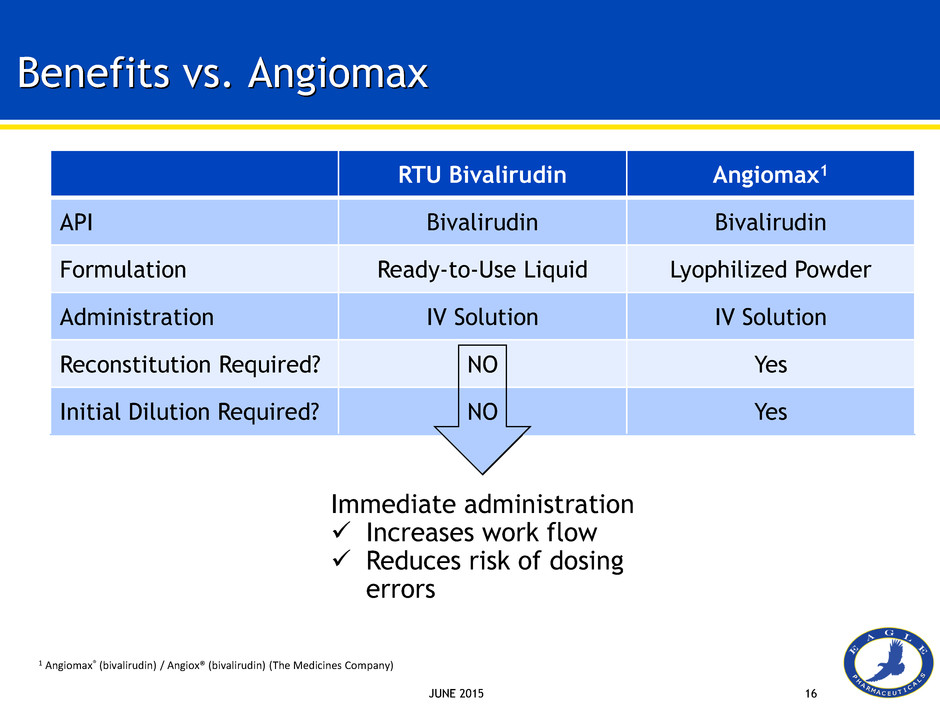
JUNE 2015 16 Benefits vs. Angiomax RTU Bivalirudin Angiomax1 API Bivalirudin Bivalirudin Formulation Ready-to-Use Liquid Lyophilized Powder Administration IV Solution IV Solution Reconstitution Required? NO Yes Initial Dilution Required? NO Yes 1 Angiomax® (bivalirudin) / Angiox® (bivalirudin) (The Medicines Company) Immediate administration Increases work flow Reduces risk of dosing errors

JUNE 2015 17 Why Eagle? Why now? Transformative partnership with Teva for Bendamustine RTD Rapid Infusion drives profitability in 2015 Significant near-term R&D opportunities • Two product approvals anticipated • Rapid infusion bendamustine by YE 2015 $1.4 billion market • RTU bivalirudin in mid-2016 opp. (aggregate) • New clarity on likely clinical path for Ryanodex in EHS Executing strategy to leverage assets to build critical mass, increase profitability, and create long-term, sustainable value Well capitalized to execute growth strategy • $113M cash • Ongoing cash flow from products • Up to $90M in additional milestone payments from Teva

JUNE 2015 18 June 2015
