Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Hepion Pharmaceuticals, Inc. | a15-12940_18k.htm |
Exhibit 99.1
|
|
Investor Presentation May 2015 James Sapirstein, CEO Nasdaq: CTRV |
|
|
This presentation may contain forward-looking statements within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934. Such forward-looking statements are characterized by future or conditional verbs such as “may,” “will,” “expect,” “intend,” “anticipate,” believe,” “estimate” and “continue” or similar words. You should read statements that contain these words carefully because they discuss future expectations and plans, which contain projections of future results of operations or financial condition or state other forward-looking information. Such statements are only predictions and our actual results may differ materially from those anticipated in these forward-looking statements. We believe that it is important to communicate future expectations to investors. However, there may be events in the future that we are not able to accurately predict or control. Factors that may cause such differences include, but are not limited to, those discussed under Risk Factors in our periodic reports filed with the Securities and Exchange Commission, including the uncertainties associated with product development, the risk that products that appeared promising in early clinical trials do not demonstrate safety and efficacy in larger-scale clinical trials, the risk that we will not obtain approval to market our products, the risks associated with dependence upon key personnel and the need for additional financing. We do not assume any obligation to update forward-looking statements as circumstances change. This presentation does not constitute an offer or invitation for the sale or purchase of securities or to engage in any other transaction with ContraVir or its affiliates. The information in this presentation is not targeted at the residents of any particular country or jurisdiction and is not intended for distribution to, or use by, any person in any jurisdiction or country where such distribution or use would be contrary to local law or regulation. Safe Harbor Statement |
|
|
Corporate Overview Company Profile Advanced clinical stage drug development company with a Virology focus Value Drivers FV-100: Reduced shingles-associated, post-herpetic neuralgia (PHN) in Phase 2 trials Initiating Phase 3 trial CMX157: Novel, high potency lipid conjugate of tenofovir Phase 2-ready for HBV Seeking to create a diversified virology pipeline Experienced Leadership Significant background in drug development and commercialization of targeted antiviral agents |
|
|
Leadership History Experienced Leadership James Sapirstein, R.Ph. Chief Executive Officer 30+ years of biotech/pharma experience, including leading 6 product launches Key in the development and launch of tenofovir Seasoned public and private co. executive John Sullivan-Bolyai, M.D., MPH Chief Medical Officer Former Exec. Dir. of Clinical Research at Merck Renowned global Hepatitis expert Extensive antiviral development experience in all 3 clinical phases at Idenix and responsible for transition of Idenix’s clinical team to Merck William Hornung Chief Financial Officer 22+ years in biopharma finance Prior IPO experience |
|
|
Scientific Advisory Board Nathaniel Katz, M.D. (Chair) Global PHN Expert Renu Gupta, M.D. Renowned Antiviral Academic Carol S. Brosgart, M.D. Tenofovir Developer Richard Whitley, M.D. Herpes Virus Expert Experienced Leadership Board of Directors Gary Jacob, Ph.D. (Chair) CEO, Synergy Pharmaceuticals Chris McGuigan, Ph.D. FV-100 Inventor John P. Brancaccio, CPA CFO, Accelerated Technologies Timothy M. Block, Ph.D. Hepatitis B Foundation, Co-Founder and President |
|
|
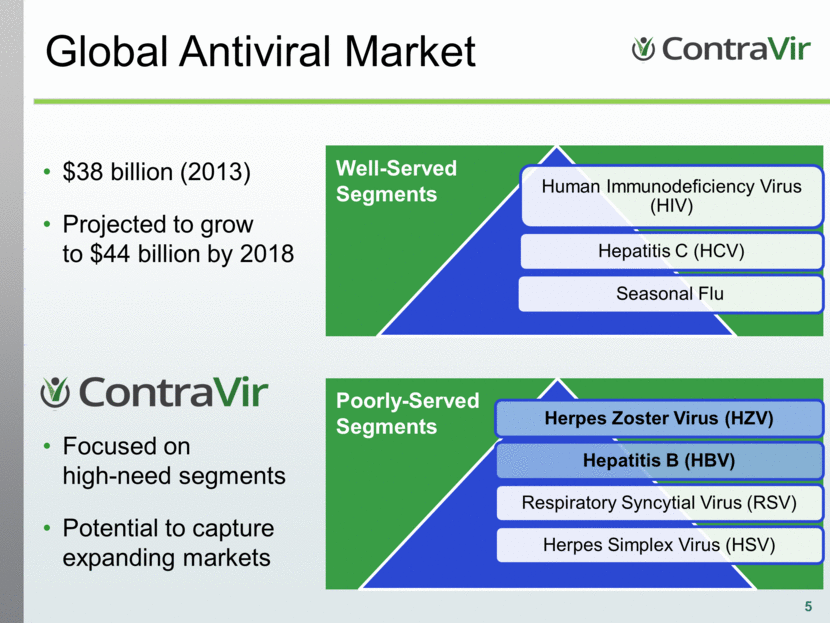
Global Antiviral Market 5 $38 billion (2013) Projected to grow to $44 billion by 2018 Well-Served Segments Poorly-Served Segments Focused on high-need segments Potential to capture expanding markets Human Immunodeficiency Virus (HIV) Hepatitis C (HCV) Seasonal Flu Herpes Zoster Virus (HZV) Hepatitis B (HBV) Respiratory Syncytial Virus (RSV) Herpes Simplex Virus (HSV) |
|
|
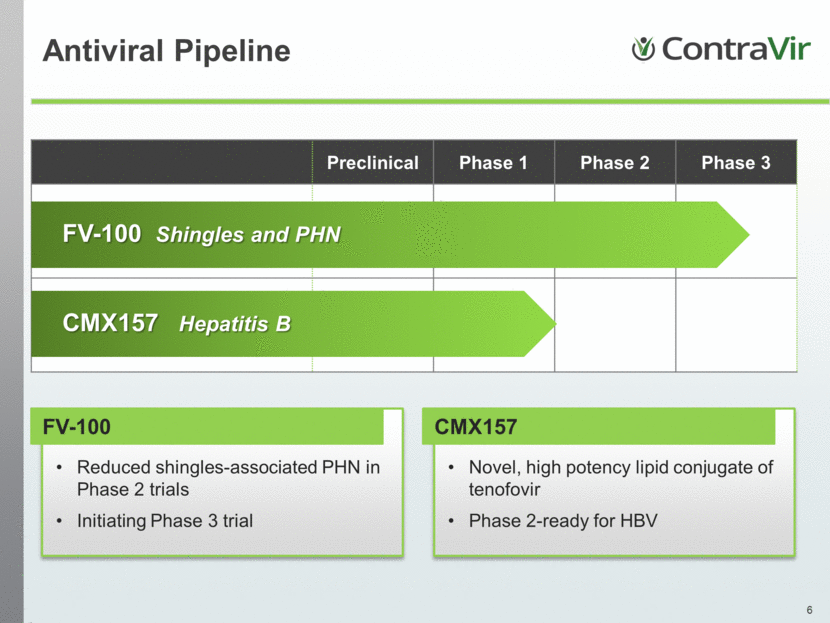
Antiviral Pipeline FV-100 Reduced shingles-associated PHN in Phase 2 trials Initiating Phase 3 trial CMX157 Novel, high potency lipid conjugate of tenofovir Phase 2-ready for HBV Preclinical Phase 1 Phase 2 Phase 3 FV-100 Shingles and PHN CMX157 Hepatitis B |
|
|
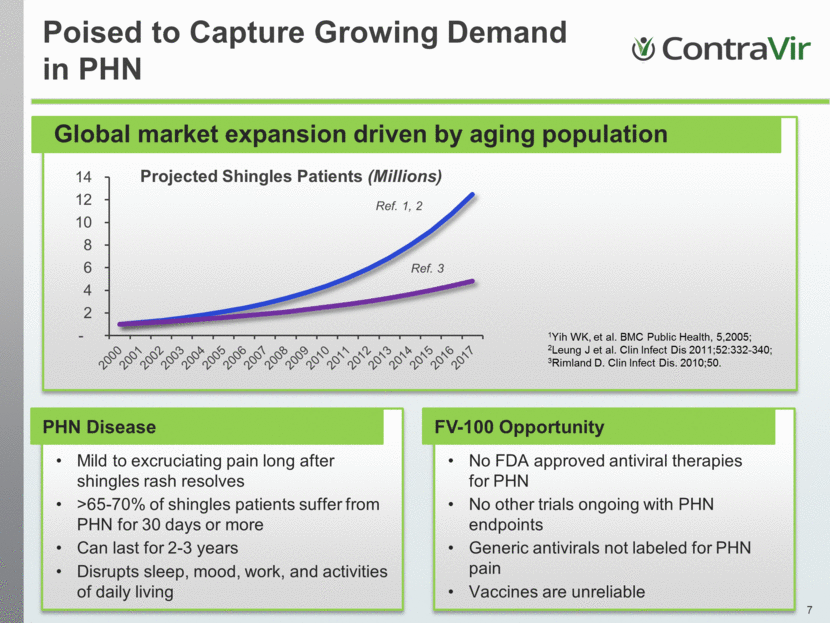
1Yih WK, et al. BMC Public Health, 5,2005; 2Leung J et al. Clin Infect Dis 2011;52:332-340; 3Rimland D. Clin Infect Dis. 2010;50. Ref. 1, 2 Ref. 3 Poised to Capture Growing Demand in PHN Global market expansion driven by aging population PHN Disease Mild to excruciating pain long after shingles rash resolves >65-70% of shingles patients suffer from PHN for 30 days or more Can last for 2-3 years Disrupts sleep, mood, work, and activities of daily living FV-100 Opportunity No FDA approved antiviral therapies for PHN No other trials ongoing with PHN endpoints Generic antivirals not labeled for PHN pain Vaccines are unreliable - 2 4 6 8 10 12 14 Projected Shingles Patients (Millions ) |
|
|
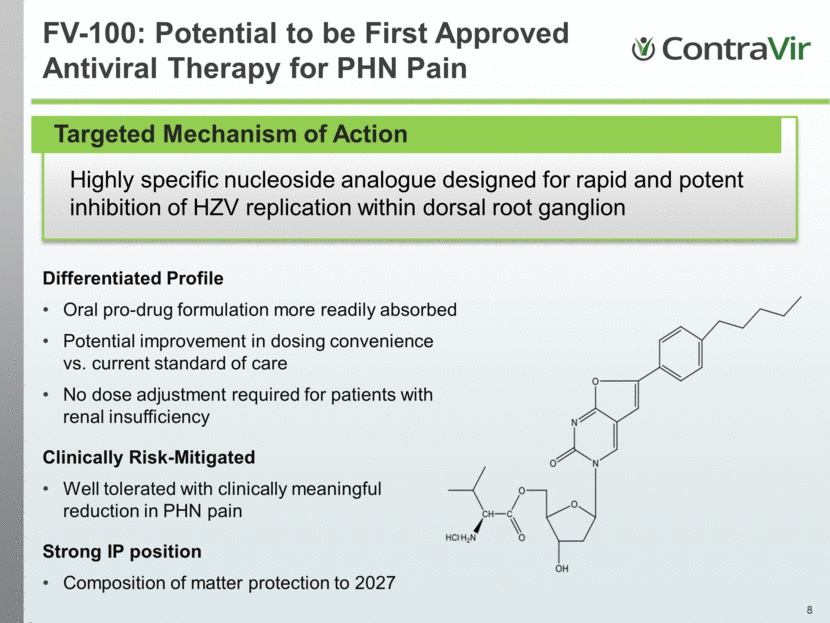
Highly specific nucleoside analogue designed for rapid and potent inhibition of HZV replication within dorsal root ganglion FV-100: Potential to be First Approved Antiviral Therapy for PHN Pain Differentiated Profile Oral pro-drug formulation more readily absorbed Potential improvement in dosing convenience vs. current standard of care No dose adjustment required for patients with renal insufficiency Clinically Risk-Mitigated Well tolerated with clinically meaningful reduction in PHN pain Strong IP position Composition of matter protection to 2027 Targeted Mechanism of Action |
|
|
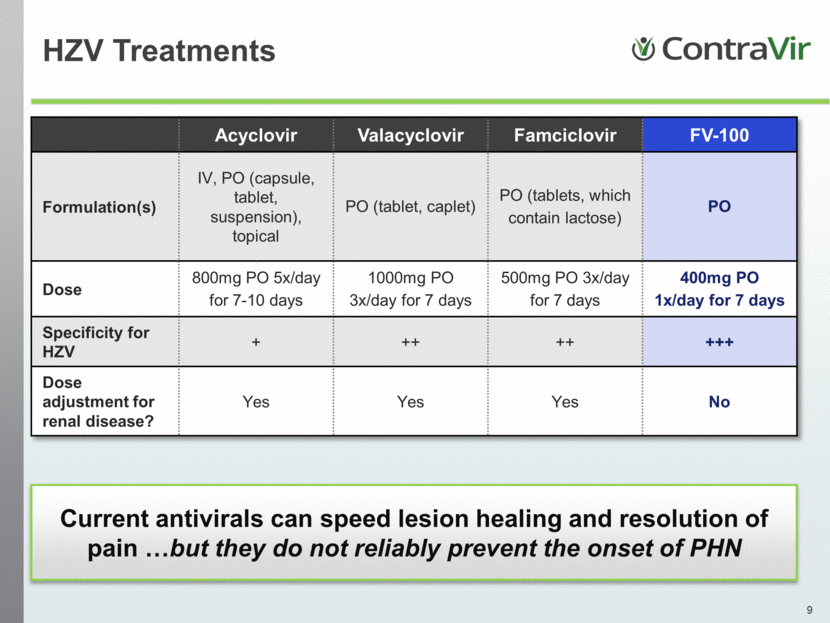
Acyclovir Valacyclovir Famciclovir FV-100 Formulation(s) IV, PO (capsule, tablet, suspension), topical PO (tablet, caplet) PO (tablets, which contain lactose) PO Dose 800mg PO 5x/day for 7-10 days 1000mg PO 3x/day for 7 days 500mg PO 3x/day for 7 days 400mg PO 1x/day for 7 days Specificity for HZV + ++ ++ +++ Dose adjustment for renal disease? Yes Yes Yes No Current antivirals can speed lesion healing and resolution of pain but they do not reliably prevent the onset of PHN HZV Treatments |
|
|
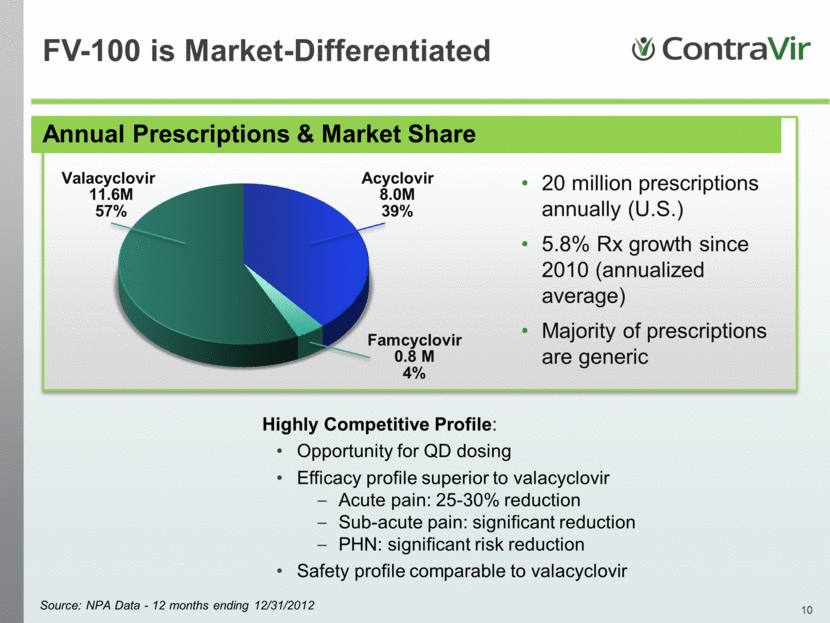
Annual Prescriptions & Market Share 20 million prescriptions annually (U.S.) 5.8% Rx growth since 2010 (annualized average) Majority of prescriptions are generic Source: NPA Data - 12 months ending 12/31/2012 Highly Competitive Profile: Opportunity for QD dosing Efficacy profile superior to valacyclovir Acute pain: 25-30% reduction Sub-acute pain: significant reduction PHN: significant risk reduction Safety profile comparable to valacyclovir FV-100 is Market-Differentiated Valacyclovir 11.6M 57% Acyclovir 8.0M 39% Famcyclovir 0.8 M 4% |
|
|
FV-100 Clinical Highlights Demonstrated Safety and Efficacy >350 patients treated with FV-100 Phase 1 (N=3) and Phase 2 (N=1) studies completed Clinically meaningful reduction in PHN occurrence vs. valacyclovir (Valtrex) Meaningful reduction in time to resolution of clinically significant pain 8-10% reduction in narcotic use for pain control Safety similar to other antivirals Proprietary & Differentiated Rapid onset of action for quick pain relief Higher potency vs. approved agents against HZV Potential for QD dosing vs. 3-5x/day for Valtrex No dose adjustments needed for patients with renal insufficiency Proceeding to Phase 3 Pivotal Phase 3 (Study 007) 3-arm study with PHN reduction endpoint (vs. Valtrex) |
|
|
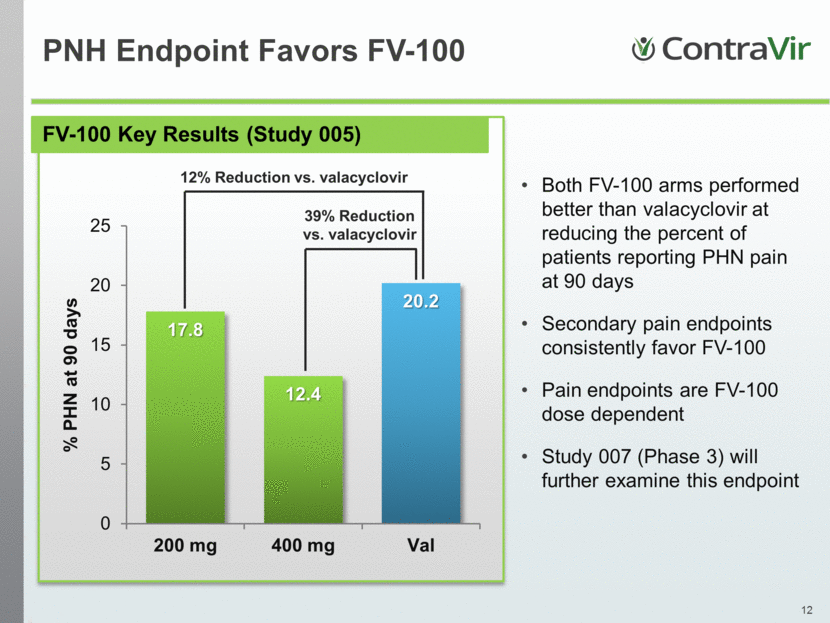
FV-100 Key Results (Study 005) PNH Endpoint Favors FV-100 Both FV-100 arms performed better than valacyclovir at reducing the percent of patients reporting PHN pain at 90 days Secondary pain endpoints consistently favor FV-100 Pain endpoints are FV-100 dose dependent Study 007 (Phase 3) will further examine this endpoint 12% Reduction vs. valacyclovir 39% Reduction vs. valacyclovir 17.8 12.4 20.2 0 5 10 15 20 25 200 mg 400 mg Val % PHN at 90 days |
|
|
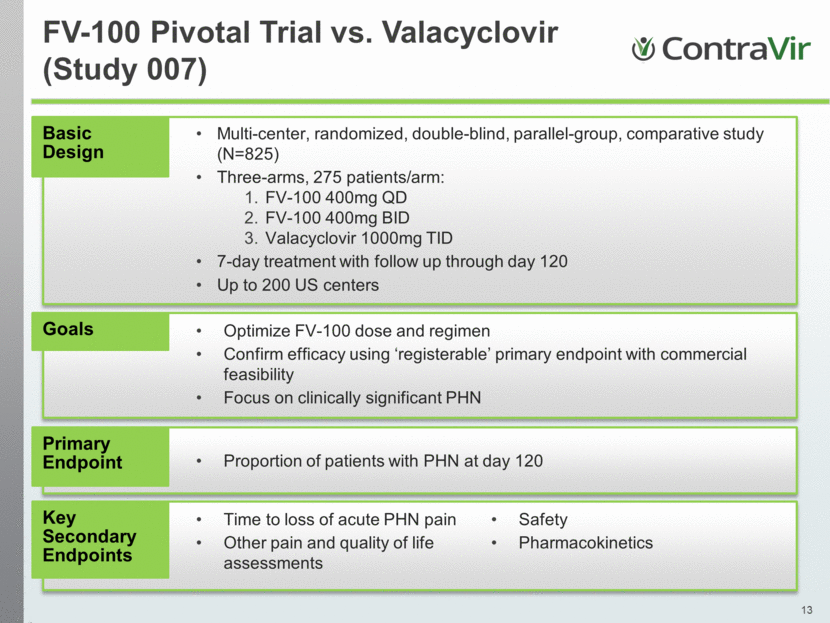
FV-100 Pivotal Trial vs. Valacyclovir (Study 007) Basic Design Multi-center, randomized, double-blind, parallel-group, comparative study (N=825) Three-arms, 275 patients/arm: FV-100 400mg QD FV-100 400mg BID Valacyclovir 1000mg TID 7-day treatment with follow up through day 120 Up to 200 US centers Goals Optimize FV-100 dose and regimen Confirm efficacy using ‘registerable’ primary endpoint with commercial feasibility Focus on clinically significant PHN Primary Endpoint Proportion of patients with PHN at day 120 Key Secondary Endpoints Time to loss of acute PHN pain Other pain and quality of life assessments Safety Pharmacokinetics |
|
|
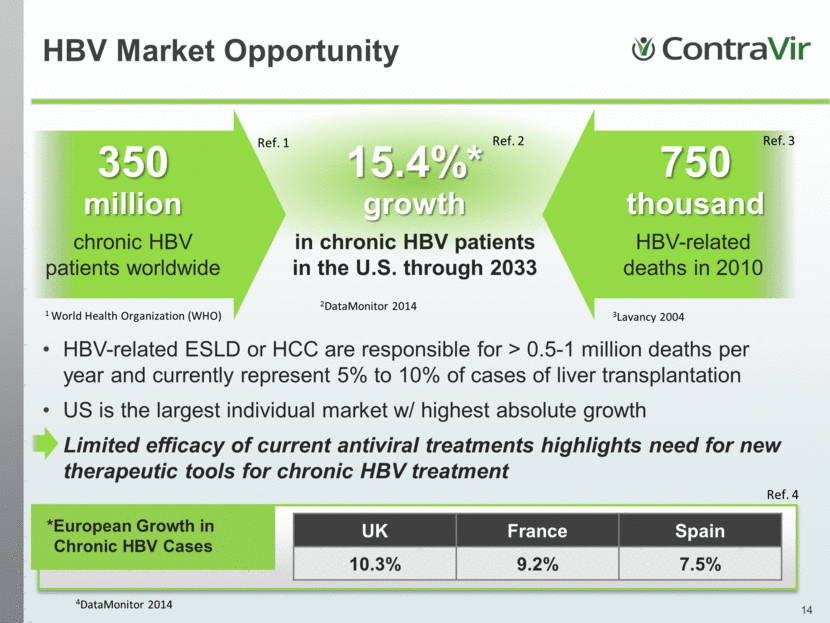
15.4%* growth in chronic HBV patients in the U.S. through 2033 350 million chronic HBV patients worldwide 750 thousand HBV-related deaths in 2010 UK France Spain 10.3% 9.2% 7.5% *European Growth in Chronic HBV Cases HBV Market Opportunity HBV-related ESLD or HCC are responsible for > 0.5-1 million deaths per year and currently represent 5% to 10% of cases of liver transplantation US is the largest individual market w/ highest absolute growth Limited efficacy of current antiviral treatments highlights need for new therapeutic tools for chronic HBV treatment Ref. 1 Ref. 2 Ref. 3 1 World Health Organization (WHO) 2DataMonitor 2014 3Lavancy 2004 Ref. 4 4DataMonitor 2014 |
|
|
CMX157 CMX157 Polymerase inhibitor Lipid conjugate of tenofovir (TFV) In-licensed from Chimerix in stock-only transaction Enhanced Absorption Technology Lipid side chains facilitate more efficient uptake and significantly enhances tissue penetration Phase 2-ready Will be developed for HBV New patents extend composition of matter coverage to 2031 |
|
|
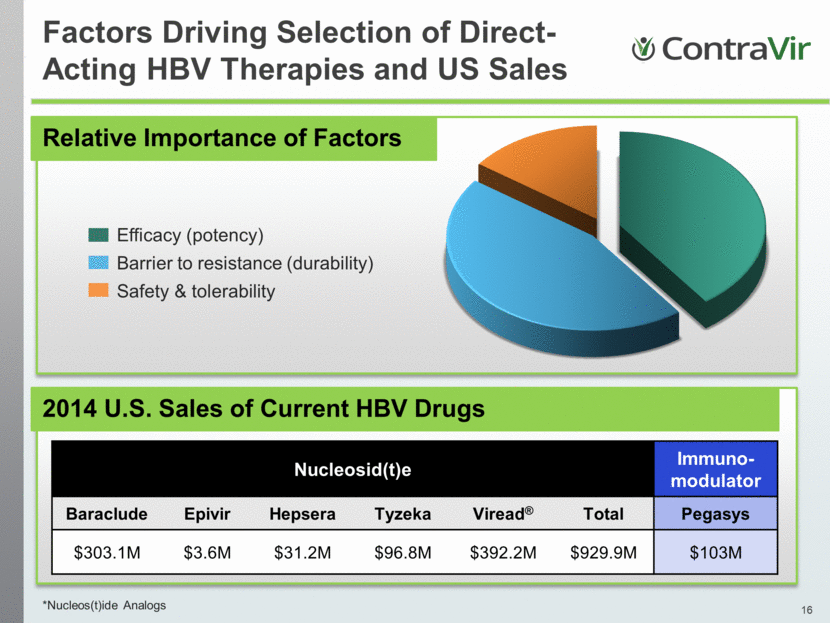
Factors Driving Selection of Direct-Acting HBV Therapies and US Sales *Nucleos(t)ide Analogs 2014 U.S. Sales of Current HBV Drugs Nucleosid(t)e Immuno-modulator Baraclude Epivir Hepsera Tyzeka Viread® Total Pegasys $303.1M $3.6M $31.2M $96.8M $392.2M $929.9M $103M Relative Importance of Factors Efficacy (potency) Barrier to resistance (durability) Safety & tolerability |
|
|
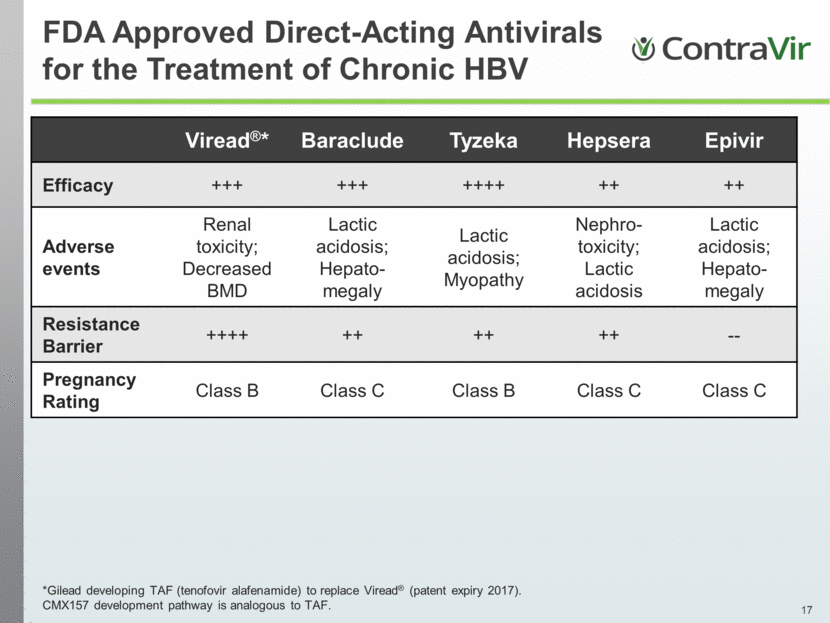
FDA Approved Direct-Acting Antivirals for the Treatment of Chronic HBV Viread®* Baraclude Tyzeka Hepsera Epivir Efficacy +++ +++ ++++ ++ ++ Adverse events Renal toxicity; Decreased BMD Lactic acidosis; Hepato-megaly Lactic acidosis; Myopathy Nephro-toxicity; Lactic acidosis Lactic acidosis; Hepato-megaly Resistance Barrier ++++ ++ ++ ++ -- Pregnancy Rating Class B Class C Class B Class C Class C *Gilead developing TAF (tenofovir alafenamide) to replace Viread® (patent expiry 2017). CMX157 development pathway is analogous to TAF. |
|
|
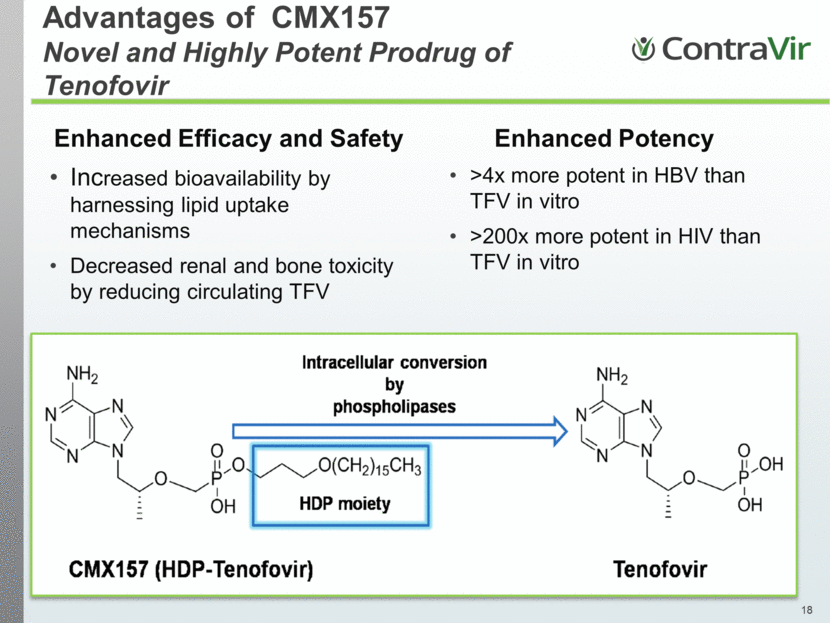
Advantages of CMX157 Novel and Highly Potent Prodrug of Tenofovir Enhanced Efficacy and Safety Increased bioavailability by harnessing lipid uptake mechanisms Decreased renal and bone toxicity by reducing circulating TFV Enhanced Potency >4x more potent in HBV than TFV in vitro >200x more potent in HIV than TFV in vitro |
|
|
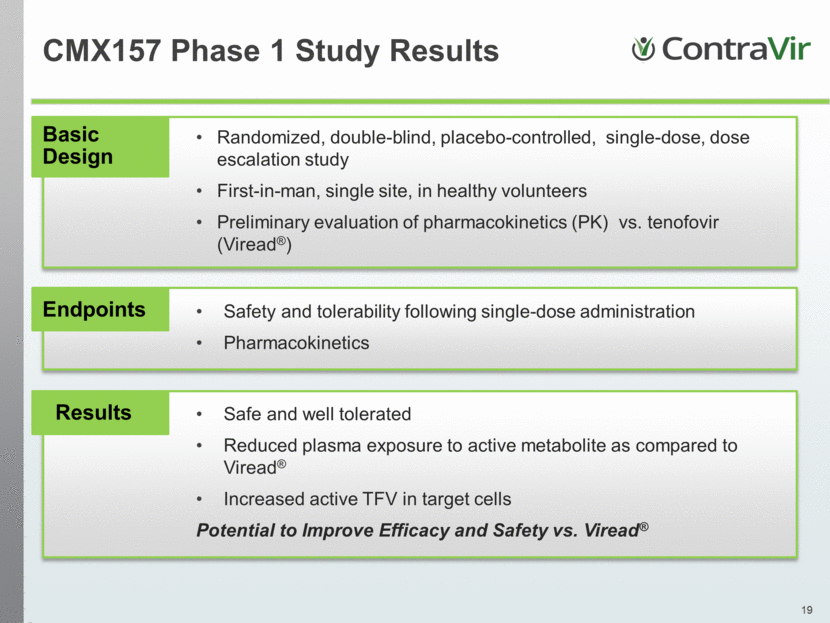
CMX157 Phase 1 Study Results Basic Design Randomized, double-blind, placebo-controlled, single-dose, dose escalation study First-in-man, single site, in healthy volunteers Preliminary evaluation of pharmacokinetics (PK) vs. tenofovir (Viread®) Endpoints Safety and tolerability following single-dose administration Pharmacokinetics Results Safe and well tolerated Reduced plasma exposure to active metabolite as compared to Viread® Increased active TFV in target cells Potential to Improve Efficacy and Safety vs. Viread® |
|
|
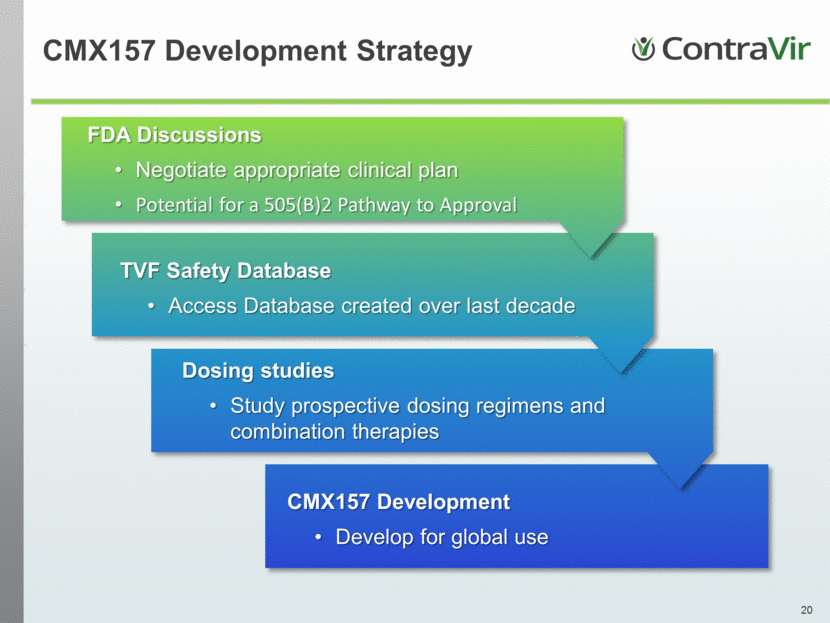
CMX157 Development Strategy CMX157 Development Develop for global use Dosing studies Study prospective dosing regimens and combination therapies TVF Safety Database Access Database created over last decade FDA Discussions Negotiate appropriate clinical plan Potential for a 505(B)2 Pathway to Approval |
|
|
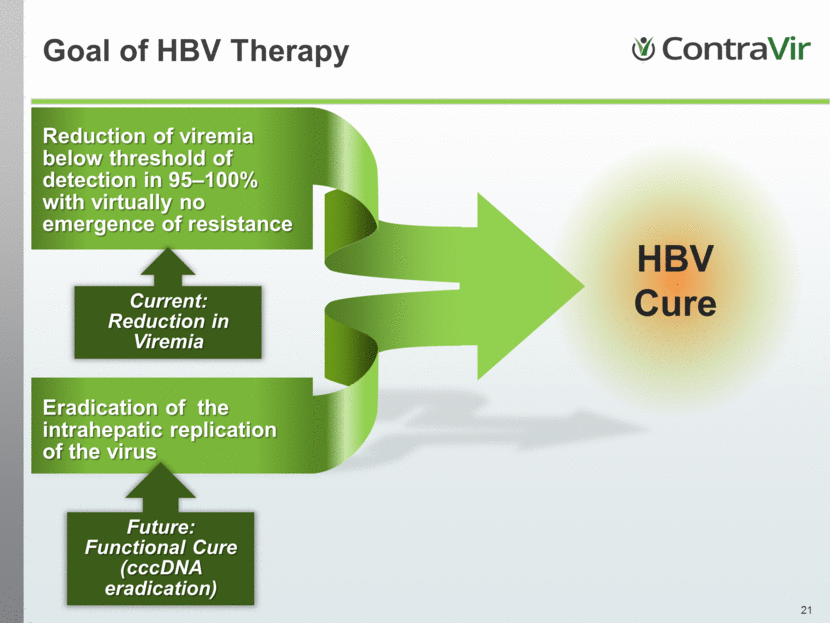
Reduction of viremia below threshold of detection in 95–100% with virtually no emergence of resistance Goal of HBV Therapy HBV Cure Eradication of the intrahepatic replication of the virus Current: Reduction in Viremia Future: Functional Cure (cccDNA eradication) |
|
|
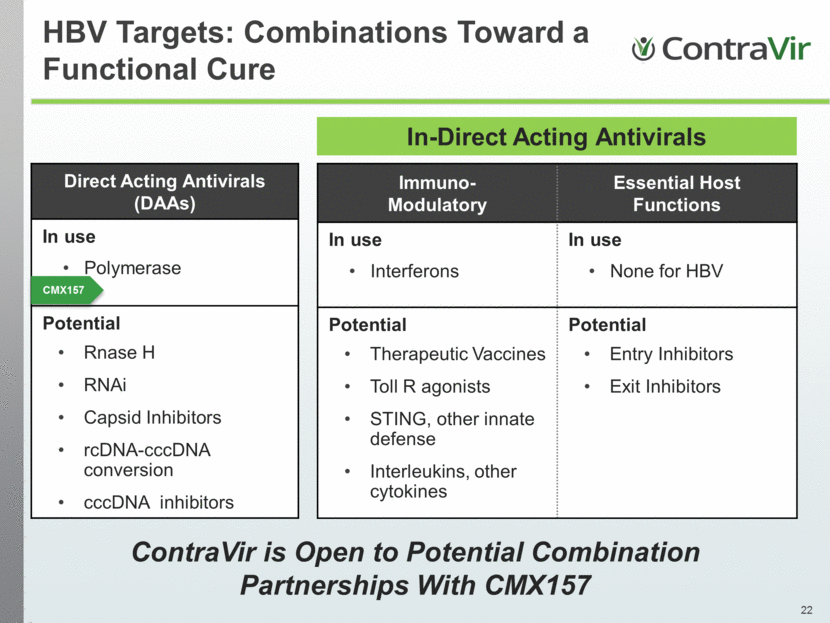
In-Direct Acting Antivirals Direct Acting Antivirals (DAAs) In use Polymerase Potential Rnase H RNAi Capsid Inhibitors rcDNA-cccDNA conversion cccDNA inhibitors CMX157 ContraVir is Open to Potential Combination Partnerships With CMX157 HBV Targets: Combinations Toward a Functional Cure Immuno-Modulatory Essential Host Functions In use Interferons In use None for HBV Potential Therapeutic Vaccines Toll R agonists STING, other innate defense Interleukins, other cytokines Potential Entry Inhibitors Exit Inhibitors |
|
|
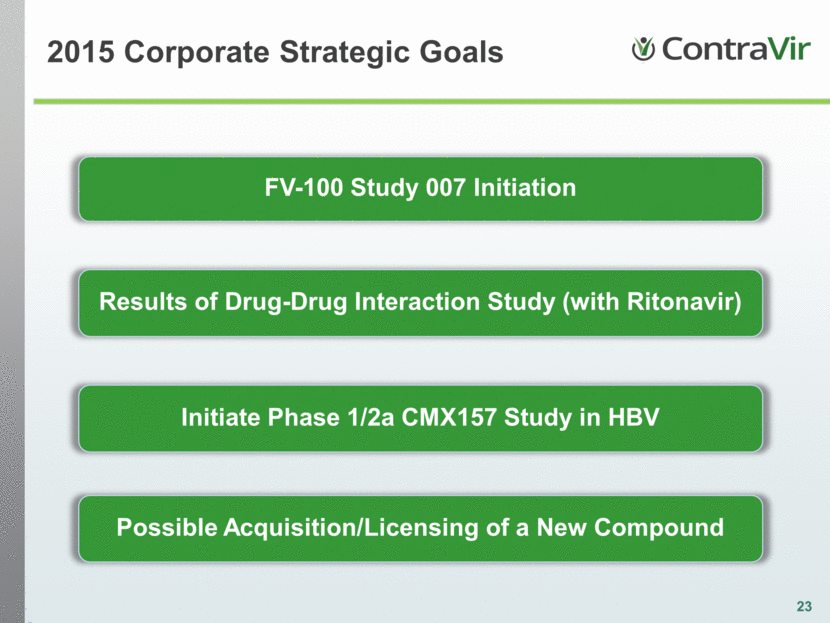
c 23 FV-100 Study 007 Initiation Results of Drug-Drug Interaction Study (with Ritonavir) Initiate Phase 1/2a CMX157 Study in HBV 2015 Corporate Strategic Goals Possible Acquisition/Licensing of a New Compound |
|
|
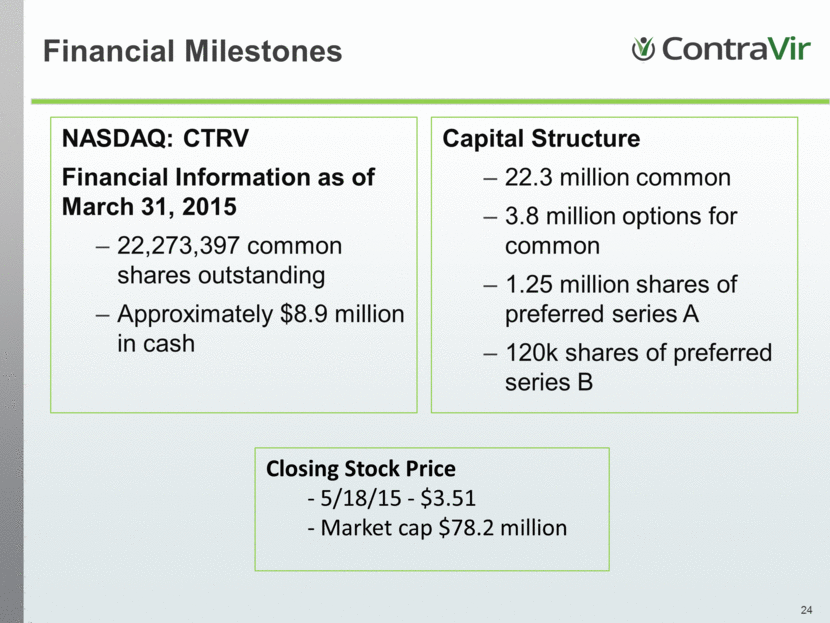
Financial Milestones NASDAQ: CTRV Financial Information as of March 31, 2015 22,273,397 common shares outstanding Approximately $8.9 million in cash Capital Structure 22.3 million common 3.8 million options for common 1.25 million shares of preferred series A 120k shares of preferred series B Closing Stock Price - 5/18/15 - $3.51 - Market cap $78.2 million |
|
|
Corporate Summary Strategic Focus Develop and commercialize novel drug treatments to improve the lives of patients suffering from chronic viral infections Mid-to-Late Stage Pipeline FV-100: Reduced shingles-associated post-herpetic neuralgia (PHN) in clinical trials; Initiating Phase 3 trial CMX157: Novel, high potency lipid conjugate of tenofovir; Phase 2-ready for HBV Experienced Leadership Significant background in drug development and commercialization of targeted antiviral agents Value Drivers Ongoing Phase 3 and upcoming Phase 2 studies Seeking to create a diversified pipeline in virology |
|
|
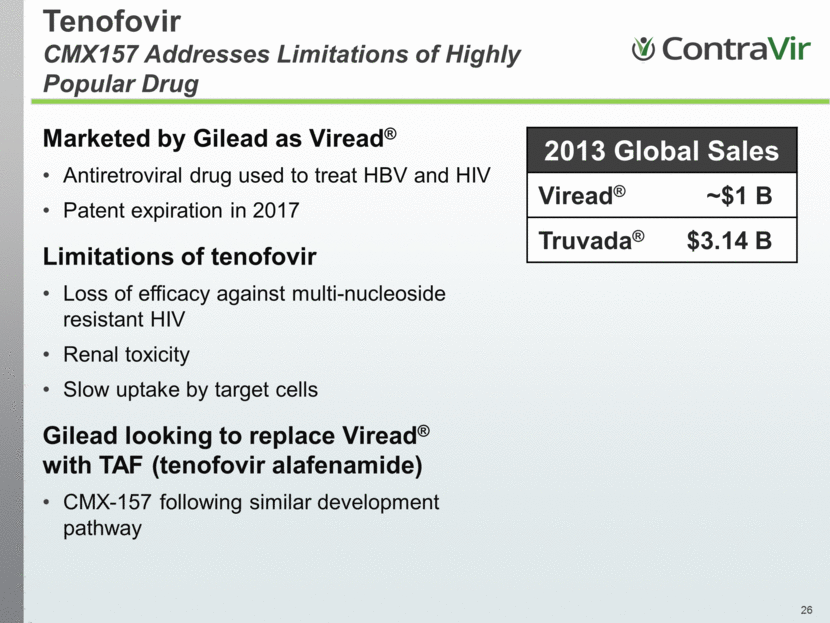
Tenofovir CMX157 Addresses Limitations of Highly Popular Drug Marketed by Gilead as Viread® Antiretroviral drug used to treat HBV and HIV Patent expiration in 2017 Limitations of tenofovir Loss of efficacy against multi-nucleoside resistant HIV Renal toxicity Slow uptake by target cells Gilead looking to replace Viread® with TAF (tenofovir alafenamide) CMX-157 following similar development pathway 2013 Global Sales Viread® ~$1 B Truvada® $3.14 B |
|
|
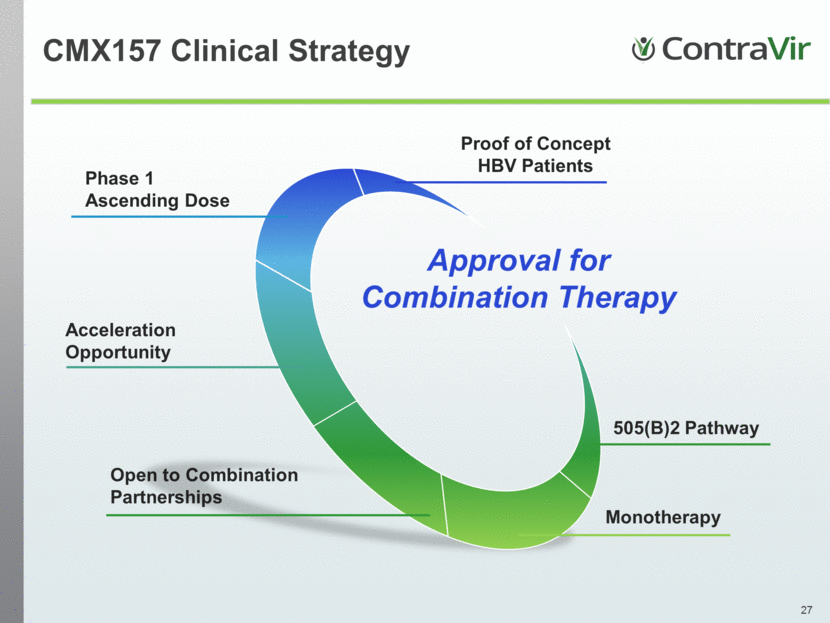
CMX157 Clinical Strategy Phase 1 Ascending Dose Proof of Concept HBV Patients Open to Combination Partnerships Monotherapy Acceleration Opportunity 505(B)2 Pathway Approval for Combination Therapy |