Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - PIERIS PHARMACEUTICALS, INC. | d928656d8k.htm |
 May
19, 2015 Pieris Pharmaceuticals, Inc.
(OTC:PIRS)
UBS Global Healthcare
Conference 2015
Stephen S. Yoder
President & CEO
Exhibit 99.1 |
 NON-CONFIDENTIAL
Forward Looking Statements
2
Statements in this presentation that are not descriptions of historical facts are
forward-looking statements that are based on management’s current
expectations and assumptions and are subject to risks and uncertainties.
In
some
cases,
you
can
identify
forward-looking
statements
by
terminology
including
“anticipates,”
“believes,”
“can,”
“continue,”
“could,”
“estimates,”
“expects,”
“intends,”
“may,”
“plans,”
“potential,”
“predicts,”
“projects,”
“should,”
“will,”
“would”
or
the
negative
of
these
terms
or
other
comparable
terminology.
Factors
that
could
cause actual results to differ materially from those currently anticipated include,
without limitation, risks relating to the results of our research and
development activities, including uncertainties relating to the discovery of
potential drug candidates and the preclinical and clinical testing of our drug
candidates; the early stage of our drug candidates presently under
development; our ability to obtain and, if obtained, maintain regulatory
approval of our current drug candidates and any of our other future drug
candidates; our need for substantial additional funds in order to continue
our operations and the uncertainty of whether we will be able to obtain the
funding
we
need;
our
ability
to
retain
or
hire
key
scientific
or
management
personnel;
our
ability
to
protect
our
intellectual property rights that are valuable to our business, including patent
and other intellectual property rights; our dependence on third-party
manufacturers, suppliers, research organizations, testing laboratories and
other potential collaborators; competition in our industry; regulatory developments in the U.S. and foreign
countries;
as
well
as
those
risks
more
fully
discussed
in
the
“Risk
Factors”
section
of
our
Current
Report
on
Form 8-K filed with the SEC on December 18, 2014, the Company’s annual
report on Form 10-K for the fiscal year ended December 31, 2014, the
Company’s quarterly reports on Form 10-Q, and the other reports we file
with the SEC. In light of these risks, uncertainties and assumptions, the
forward-looking statements regarding future
events
and
circumstances
discussed
in
this
report
may
not
occur
and
actual
results
could
differ
materially and adversely from those anticipated or implied in the
forward-looking statements. You should not rely upon forward-looking
statements as predictions of future events. The forward-looking statements included
in this presentation speak only as of the date hereof, and except as required by
law, we undertake no obligation to update publicly any forward-looking
statements for any reason after the date of this presentation to conform
these statements to actual results or to changes in our expectations. |
 NON-CONFIDENTIAL
3
Clinical-stage R&D company developing first-in-class
biologics
Proprietary Anticalin®
technology
Highly differentiated next generation therapeutic proteins
Superior drug-like properties
Strong patent position and no 3
rd
party IP identified to date for FTO
Clinical activity, lack of immunogenicity in cancer patients
Proprietary pipeline in Immuno-Oncology, Immunology,
Anemia and Respiratory
Proven track record for successful collaborations with Pharma
Pieris Pharmaceuticals, Inc. –
The Corporation (OTC:PIRS)
Strong pipeline |
 NON-CONFIDENTIAL
4
Pieris Pharmaceuticals, Inc. –
The Corporation (OTC:PIRS)
Solid Financial Position
$43.8M in licensing & milestone payments since inception
$14.1M in non-dilutive grants since inception
$83.4M total capital raised from investors
Went public in Dec 2014 through reverse merger
Raised gross proceeds of $13.6M in a private placement
transaction -
straight common stock at $2.00
$13.2M in cash as of March 31, 2015
As of 5/12/15, institutional investors collectively owned more than 65% of PIRS
common shares -
Ally Bridge Group, Forbion Capital, Gilde, GLSV, Lombard
Odier, Novo Nordisk, Sphera Funds, Zydus Cadila, and OrbiMed Advisors (23%)
CEO,
CSO,
Head
of
Discovery,
Head
of
BD
formerly
at
MorphoSys,
a
German
biotech success story with market cap in excess of $1.5 billion
Top caliber Board of Directors
Former Sanofi, Celgene and Chiron executives; Chairman from OrbiMed
Highly experienced leadership team |
 5
November 2014 through May 2015
Ph I study initiated for anti-hepcidin anemia program (PRS-080)
Private Placement ($13.6 M)
Public Listing (OTC Markets)
Dr. Holbrook Kohrt, M.D., Ph.D, joins as
IO Advisor for Translational Medicine
AUD $500k Grant with University of Melbourne
for anti-IL4Ra asthma progam (PRS-060)
Appointment of Jean-Pierre Bizzari,
M.D., to Board of Directors
Presentation of preclinical data on
CD137-based IO multispecifics
Recent Key Achievements
NON-CONFIDENTIAL |
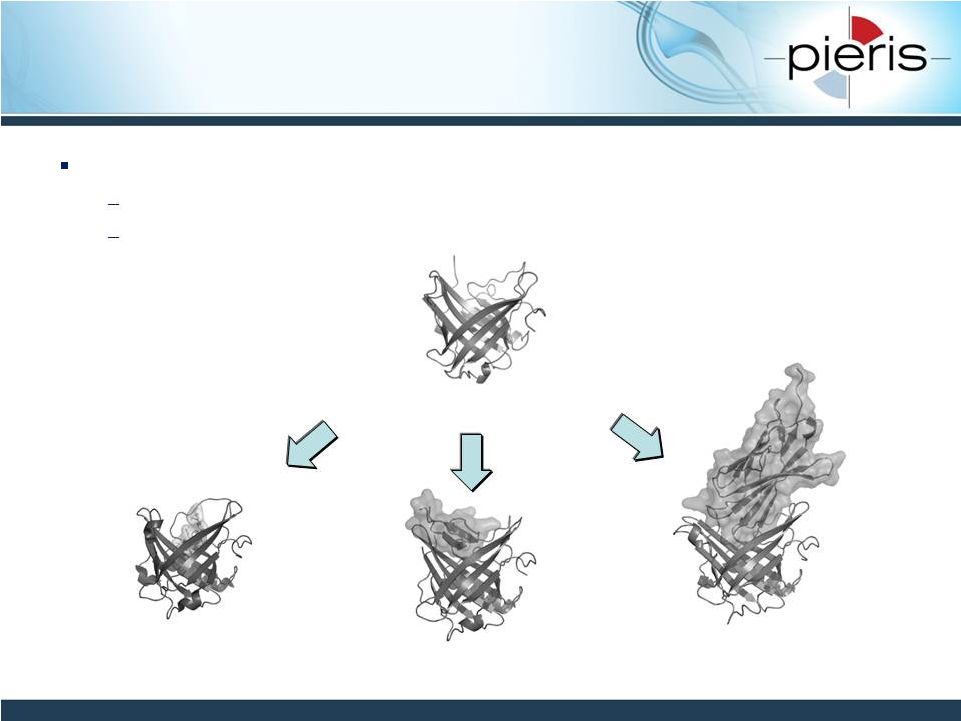 NON-CONFIDENTIAL
Anticalins are Re-purposed Human
Therapeutic Proteins
6
Anticalin in complex with a
small molecule (Y-DTPA)
Anticalin bound to the
Hepcidin peptide
Anticalin bound to the
CTLA4 protein
Anticalins®
are a novel class of therapeutic proteins, derived from lipocalins
Small and simple make-up
Individual derivatives can be generated that bind to a broad range of
targets lipocalin |
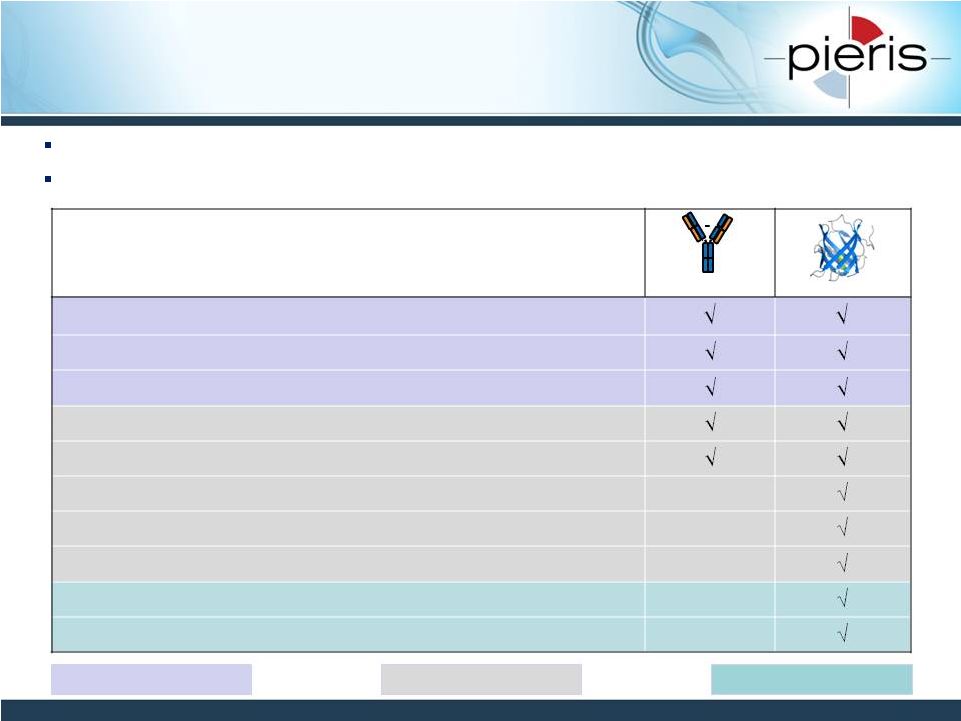 Differentiating Features
Human-derived
Natural binding molecule
Non-immunogenic
High affinity and specificity
Systemic delivery
Tunable pharmacokinetics
Local delivery (e.g., inhalation)
Versatile bispecifics & multispecifics
Protein Class Exclusivity
Positive Freedom to Operate Landscape
Anticalins Share Several Other Features
with mAbs yet are Highly Differentiated
7
Monoclonal Antibodies (mAbs) are highly successful drugs
Anticalins share many of the beneficial properties of mAbs yet are highly
differentiated Antibody
Anticalin
Safety Related
Efficacy Related
IP Related
NON-CONFIDENTIAL |
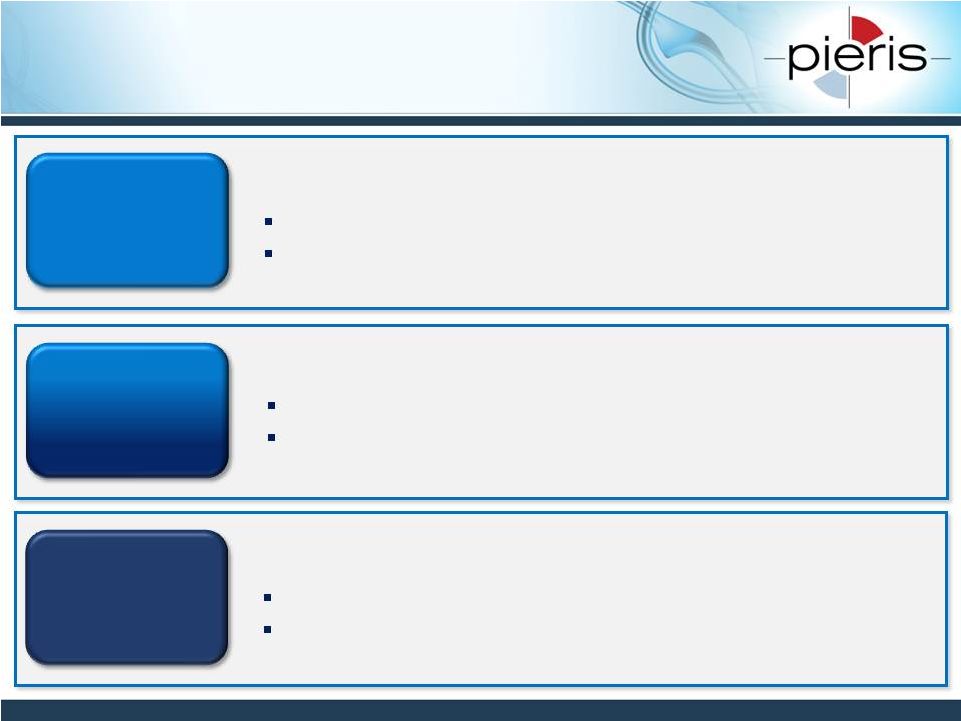 Commercialization Strategy
Multiple Shots on Goal: Partnered & Proprietary
8
Pieris selects target, funds all costs
Shared investment, shared ownership
Fully
Proprietary
Co-Dev
Partner selects target, funds all costs
Fully
Partnered
Immuno-oncology, anemia, respiratory: strong networks
High barriers to entry: e.g. IP, multispecifics, inhalation
Alternative mechanism to advance several programs
Retain commercialization rights in major markets
Industry validation
Cash flow upfront and milestone payments
NON-CONFIDENTIAL |
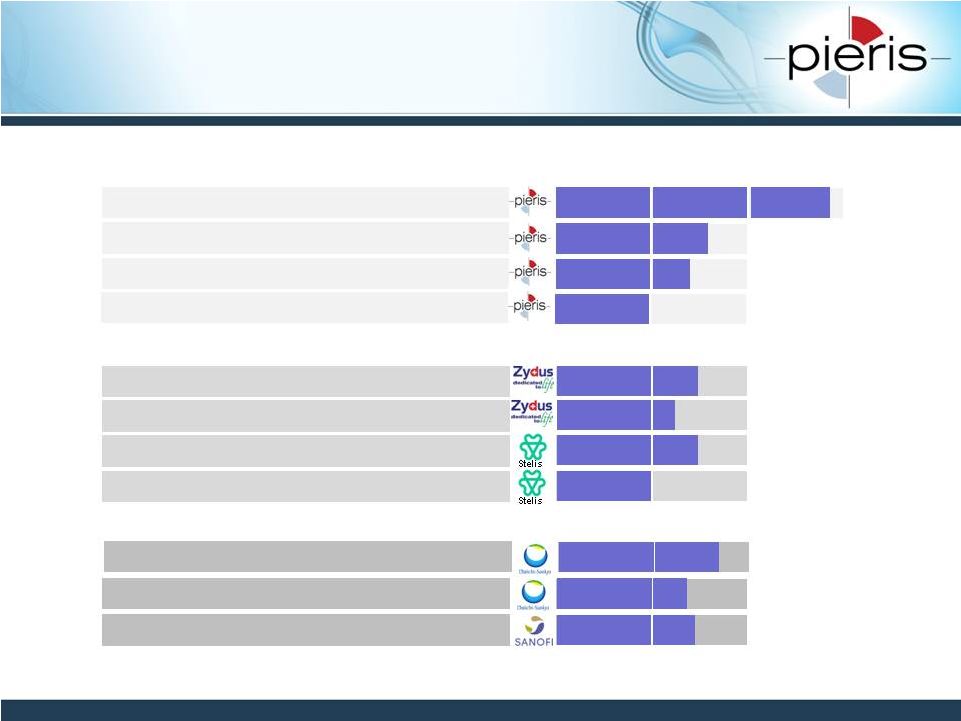 NON-CONFIDENTIAL
9
PRS-080
Hepcidin
Anemia
PRS-060
IL4Ra
Asthma
Discovery
Preclinical
Phase 1
Target(s)
1°
Indication
PRS-110
cMet
Oncology
PRS-NN
n.d.
n.d.
PRS-NN
n.d.
Ophthalmology
PRS-NN
n.d.
Ophthalmology
Sanofi Group
n.d.
Sept 2010 Initiation
Daiichi Sankyo
n.d.
April 2011 Initiation
n.d. = not disclosed
Pipeline Overview
PRS-343
CD137/HER2
IO
PRS-300 other
n.d
IO
Daiichi Sankyo
n.d.
April 2011 Initiation |
 NON-CONFIDENTIAL
Pieris Announced A Novel IO
Program on May 19
10 |
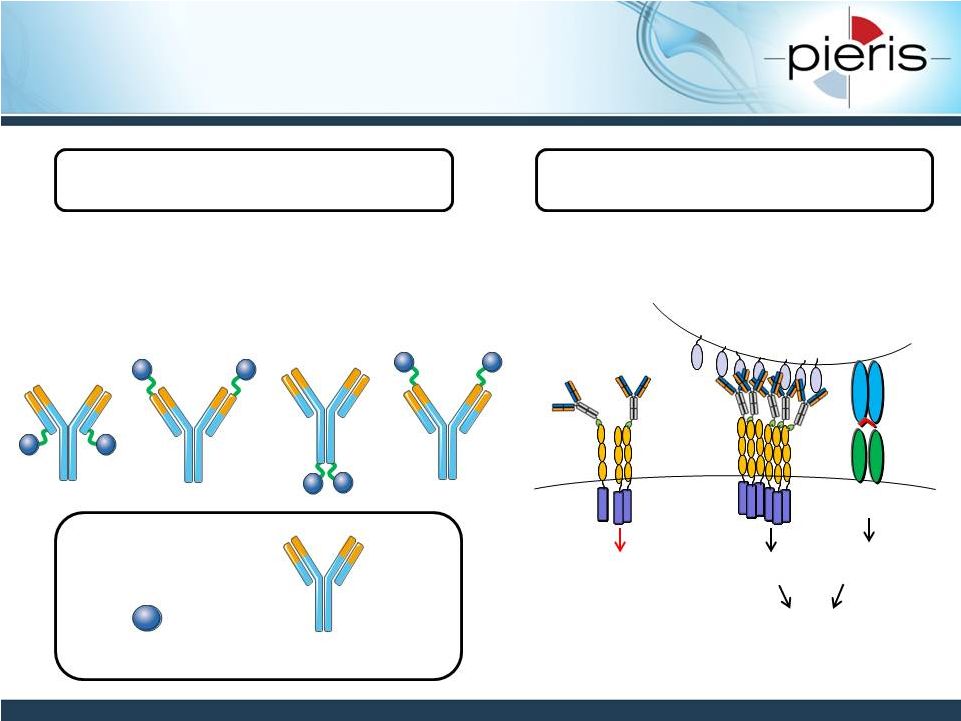 NON-CONFIDENTIAL
PRS-343: CD137-HER2 Bispecific
Member of PRS-300 Series
Several Bispecific Formats
Under Preclinical Evaluation
11
CD137 Ac
trastuz. deriv. (HER2)
Tumor Cell
T Cell
Perceived costimulatory T cell
engagement in tumor environment
Costimulatory
signal
Primary TcR
signal
Activation
Proliferation
Survival
CD137
No
signalling
Her2
Protein Engineering Aspects
Target Biology Aspects |
 NON-CONFIDENTIAL
CD137 is a TNFR Costimulatory
IO Target
Validated marker for tumor-reactive T cells in man (1)
Anti-CD137 mAbs improve the expansion of CD8+ melanoma TIL in
adoptive T-cell therapy (2)
In mouse tumor model, forced tumor expression of CD137L (3) or anti-
CD137 scFv (4,5,6) leads to tumor elimination in T-cell-
and NK-cell-
dependent manner
In clinical CAR-T, inclusion of CD137 downstream signaling has proven key
to success
–
CD137 costimulation in T-cells shown to lead to higher persistence,
proinflammatory cytokine release and more effective tumor cell killing
–
CD137 costimulation in NK-cells shown to improve therapeutic
response in mouse models (7) and currently translated to clinical trials
12
1 Ye, Q. et al., Clin Canc Res: 2014 Jan 1; 20(1):44-55.
2 Chacon, J. A. et al., PloS One 2013 8(4):e60031.
3 Melero, I. et al., Eur J Immunol 1998 Mar; 28(3):1116-1121.
4 Ye, Z. et al., Nat Med 2002 Apr; 8(4):343-348.
5 Zhang, H. et al., Mol Canc Ther 2006 Jan; 5(1):149-155.
6 Yang, Y. et al., Canc Res 2007 Mar 1; 67(5):2339-2344.
7 Kohrt, H. et al, J Clin Invest. 2012 Mar;122(3):1066-75.
|
 NON-CONFIDENTIAL
CD137-Targeting mAbs Struggling
To Find a Therapeutic Window
Several early-stage clinical trials with CD137 mAbs have been
terminated
Doses of systemic CD137 mAbs required for T cell activation have
led to
toxicity
TNFR activation requires receptor clustering
Bivalent mAbs shown to depend on Fc receptor interaction (1, 2)
Fc receptor interaction is a random process which takes place throughout
the body and not just at the tumor
Most recent trials initiated with CD137 mAbs are focusing on NK cell
activation
When used at low dose in combination with, e.g., rituximab or cetuximab,
may enhance ADCC activity through NK cell activation
While this approach may work in defined populations, it may not take full
advantage of CD137 role in T cell activation
13
1 Bulliard, Y. et al., J Exp Med 2013 Aug 26; 210(9):1685-1693
2 Bulliard, Y. et al., Immunol Cell Biol 2014 Jul; 92(6):475-480
|
 NON-CONFIDENTIAL
Current HER2-Targeted
Therapies Leave an Umet Need
Several Solid Tumors With Upregulated HER2 Expression
Not Adequately Addressed with Current Therapies
Bladder cancer
Overexpressed in 36% cases. Her2 is a poor prognostic indicator (1)
Five-year survival rate is 48% for Her2 negative versus 9.7% for
HER2-positive tumors (2, 3)
Micropapillary urothelial carcinoma subtype. Her2 amplification asscoiated with a
three-fold increased risk of death (4)
Advanced Gastric Cancer
Over-expressed in 20% of cases. Overall survival of 14 months
(trastuzumab + chemotheapy) (5)
Ovarian cancer
Overexpressed in 20-30% of cases of ovarian cancer
Her2 is a poor prognostic indicator (Median survival of 15.7 months for
HER2-high versus
32.8
months
for
HER2
normal)
(6)
1 Hansel et al, Am J clin Pathology: 2008, 130: 274-281
2 Sato K et al, Cancer: 1992, 70: 2493-9.
3 Scholl et al, Annals of Oncology: 2001, 12: S81-S87
4 Schneider et al, Modern Pathology: 2014, 27: 758-764
5 Bang et al, Lancet: 2010, 28: 687-97
6 Berchuck et al, Cancer Res: 1990, 50:4087-91
14 |
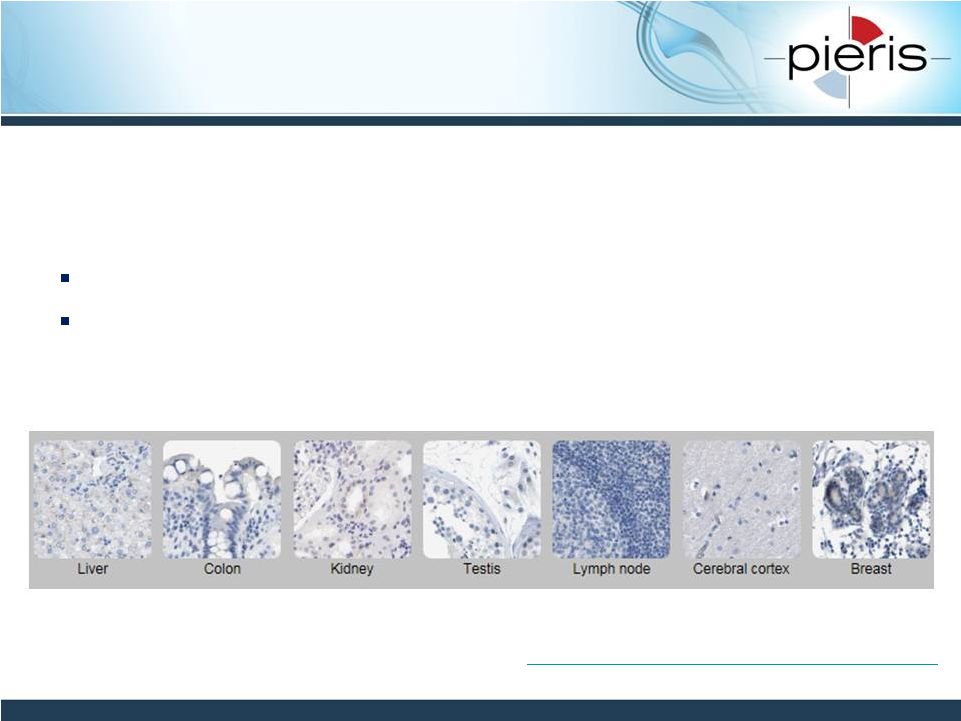 NON-CONFIDENTIAL
HER2 has Restricted Expression
in Normal Tissue
Favorable tissue expression profile for immunotherapy
approach
Low level of HER2 expression on healthy epithelial cells (1)
Receptor density range observed in tumor tissue will allow Pieris
to interrogate the level of expression required for optimal activity
15
1 Press et al 1990, Oncogene. 5: 953-962
(2)
2 Protein
Atlas
http://www.proteinatlas.org/ENSG00000141736-ERBB2/tissue
|
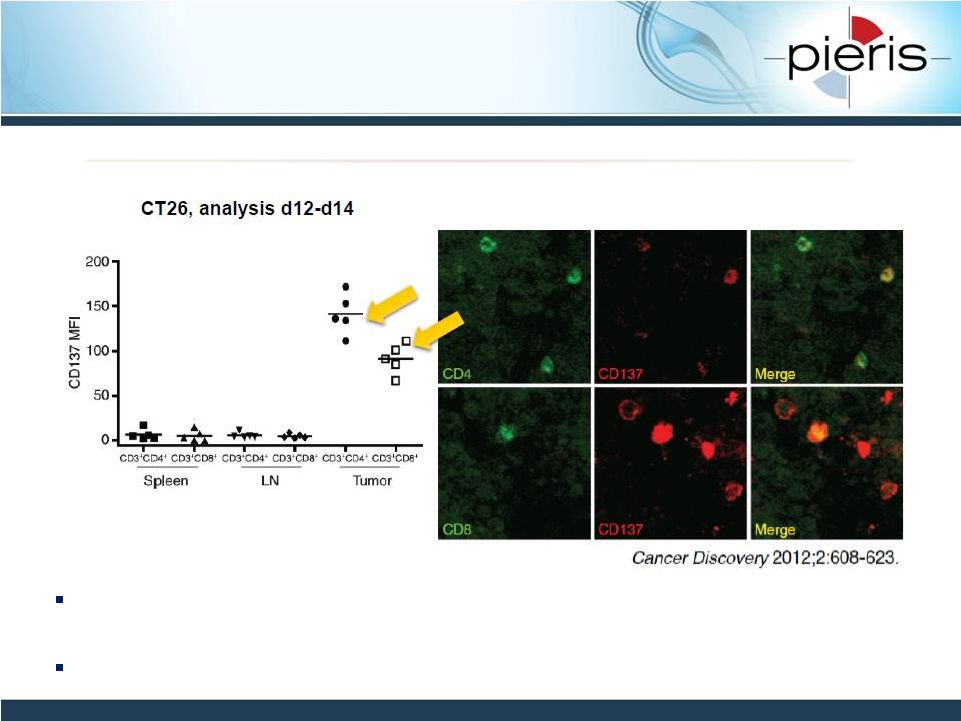 NON-CONFIDENTIAL
CD137 Expression is Localized
in Tumor Microenvironment
In tumor models there are high levels of CD137 on intratumoral CD4 and
CD8 T cells
Existing TIL population primed for response to CD137 agonist
16 |
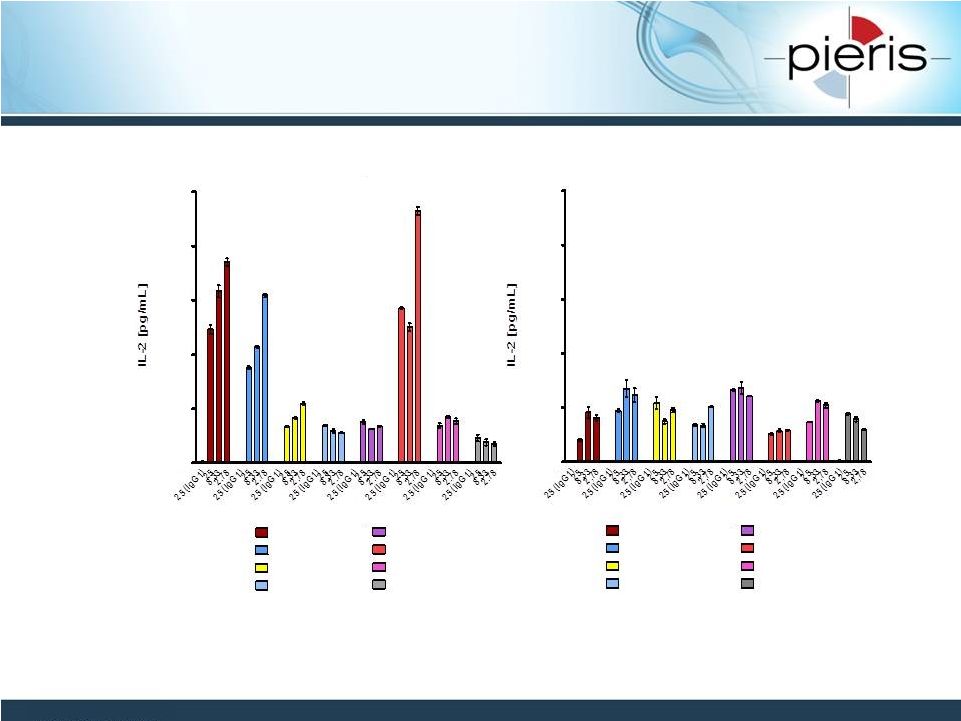 NON-CONFIDENTIAL
17
Anticalins Activate T Cells
By CD137 Cross Linking
2nM
9nM
23nM
77nM
50nM
140nM
´~1µM
Representative
CD137-specific
Anticalins
Control
Control
NON-CONFIDENTIAL
Donor A Capture
0
100
200
300
400
500
S0575.01L03
S0575.01O13
S0575.02K12
S0575.01F03
S0575.01J04
S0575.04J10
S0565.12D24
Concentration AC [µg/ml]
Donor A Solution
0
100
200
300
400
500
S0575.01O13
S0575.02K12
S0575.01F03
S0575.01J04
S0575.01L03
S0575.04J10
S0565.12D24
Concentration AC [µg/ml] |
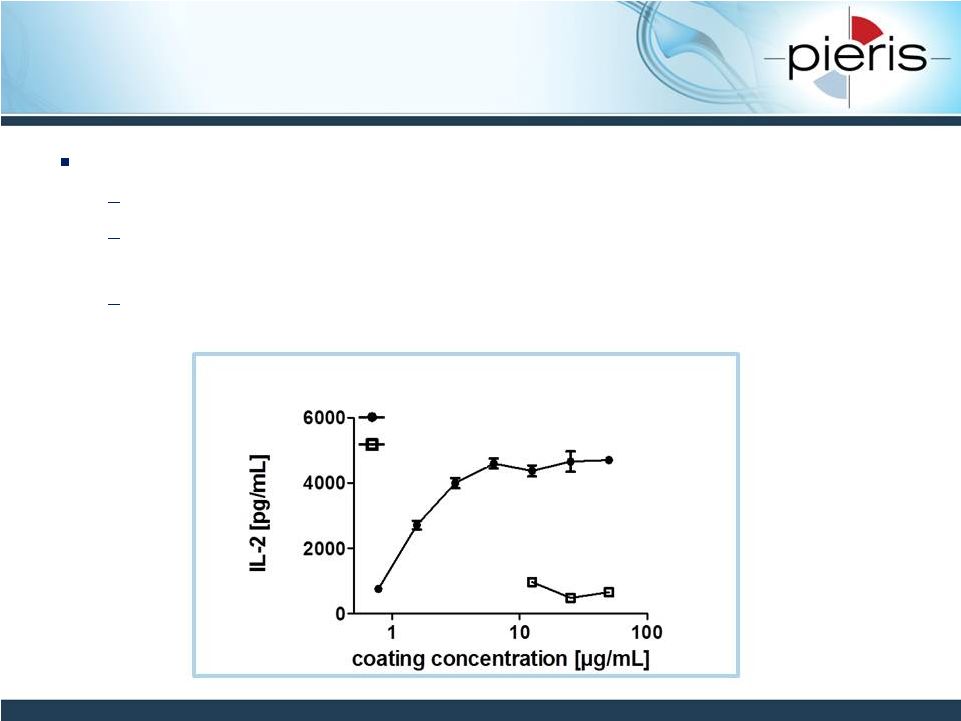 NON-CONFIDENTIAL
CD137-Targeting Anticalin Has
Demonstrated Agonistic Properties
Lead CD137-engager Anticalin identified
Affinity: KD
hCD137
= 2nM
“Non-competitive”
CD137 engagement preserves ligand-binding
capability to CD137L
Leads to T-cell activation in ex vivo human donor cell assay
18
IL-2 production Assay (3d in culture)
CD137 Ac (J10)
Control |
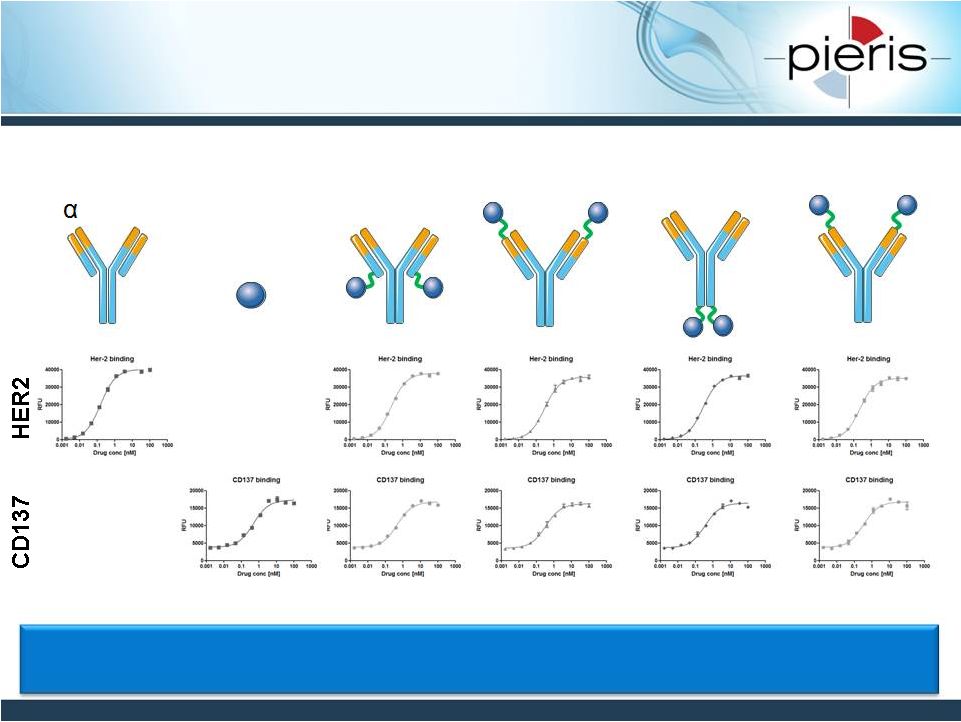 NON-CONFIDENTIAL
HER2-CD137 Bispecific Formats
Retain Target Binding Capacity
19
-HER2
CD137 Ac
•
Bispecific formats behave similarly to CD137 and HER2 building blocks
•
Simultaneous target engagement confirmed for bispecific formats
|
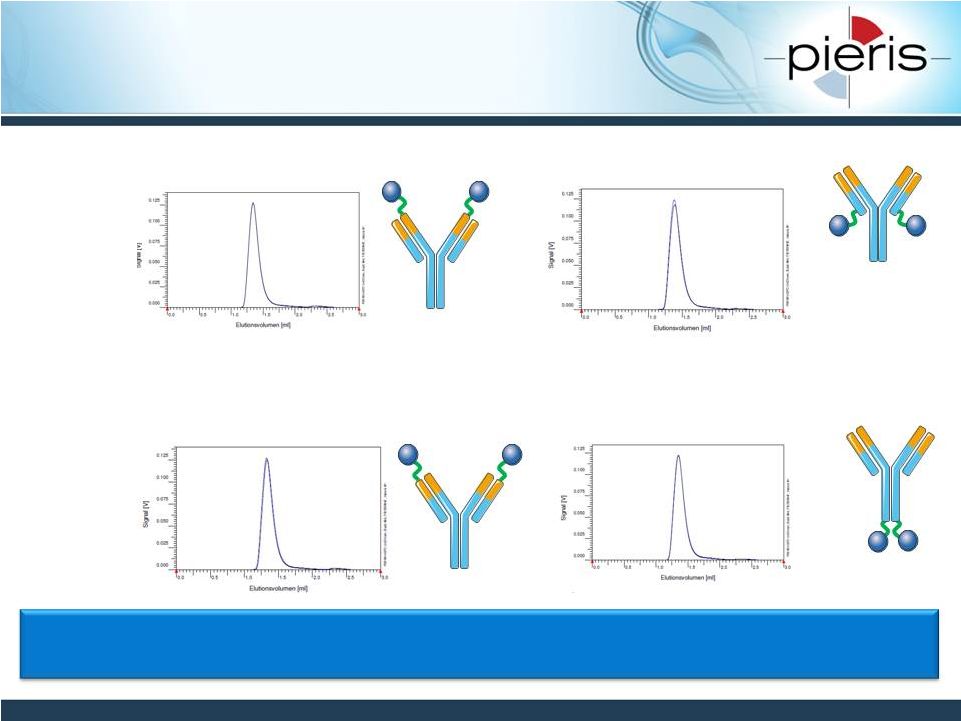 NON-CONFIDENTIAL
HER2-CD137 Bispecific Formats
Exhibit Good Biophysical Properties
20
CD137-HER2-IgG4
(HC,
N-term)
CD137-HER2-IgG4
(LC,
C-term)
CD137-HER2-IgG4
(LC,
N-term)
CD137-HER2-IgG4
(HC,
C-term)
•
Constructs
stable
after
one
week
in
PBS
at
37°C
-
no
change
in
SEC
profile
observed
•
Stability in human plasma also confirmed using a dual binding ELISA
|
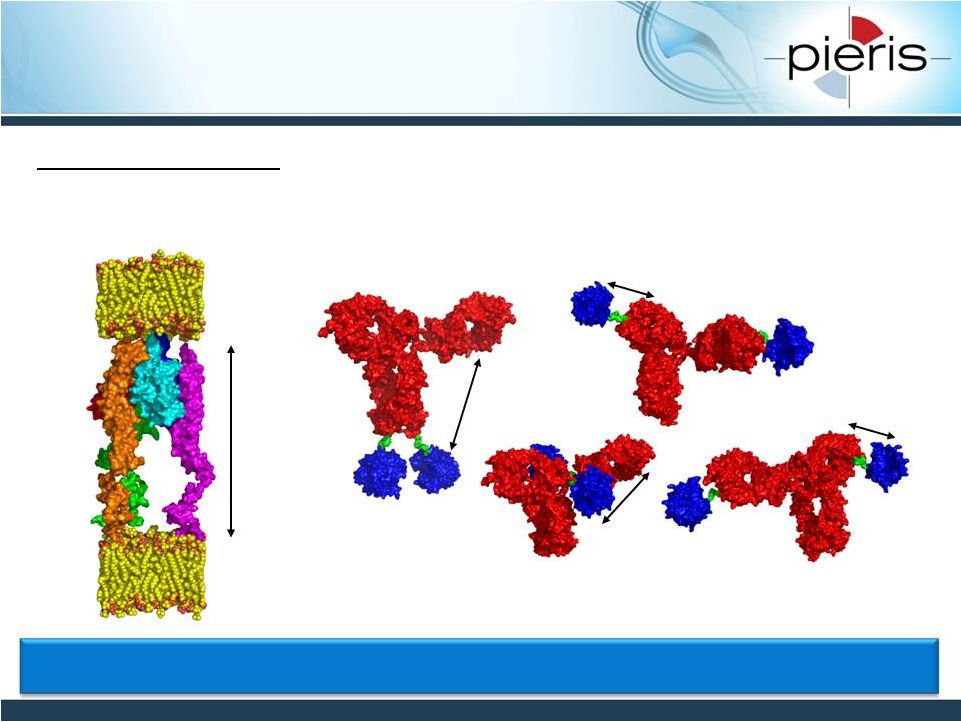 NON-CONFIDENTIAL
Bispecific Geometry May Create
Different Pharmacodynamic Effects
21
13.4
nm
e.g. TNFRS / TNFRSL
TNFRS
TNFRSL
Minmum: 8nm
Expected: 13.4nm
Stretched: 34nm
Ab-Ac(HC,
C-term): ~15nm
Ab-Ac(LC,
N-term): ~5nm Pieris
bispecific constructs result in different distances between target binding
sites Ab-Ac(HC, N-term): ~5nm Ab-Ac(LC, C-term): ~8nm Several Bispecific
Formats to Interrogate Optimal Target Synapse |
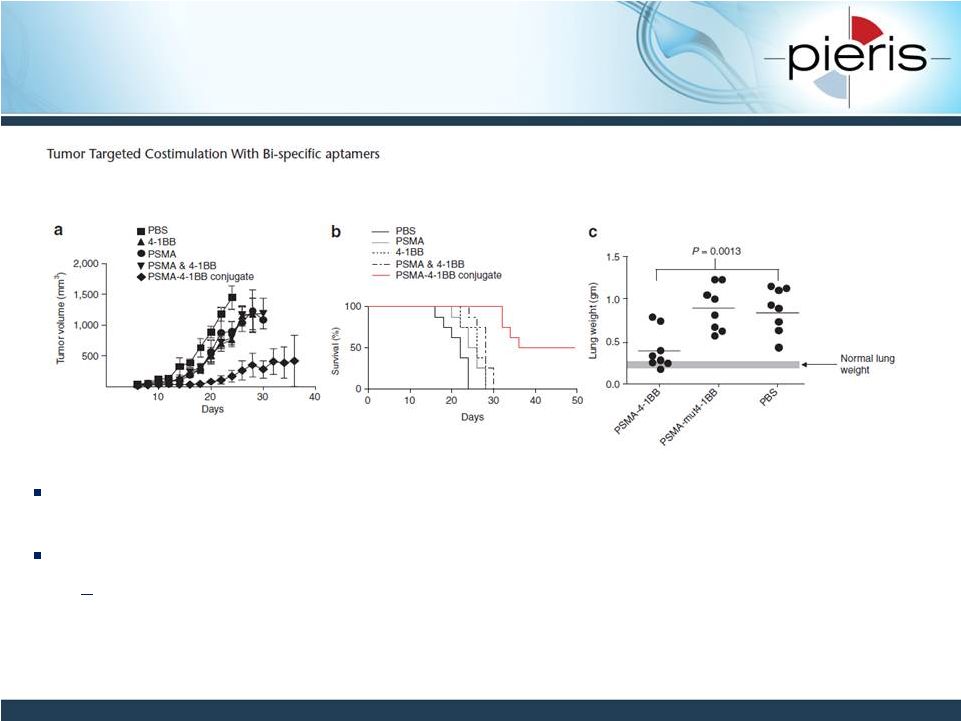 NON-CONFIDENTIAL
Preclinical Validation of Tumor-
Localized Activation of CD137 (4-1BB)
22
Pastor et al, Molecular Therapy: 2011, 10: 1878-1886
Tumor-targeting CD137 bispecific aptamer leads to tumor growth inhibition and
survival advantage in vivo compared to combination therapy
Supports Pieris’
bispecifics MoA
Tumor-specific clustering and activation of CD137 positive T cells
©
The American Society of Gene & Cell Therapy |
 NON-CONFIDENTIAL
Summary of Pieris’
Immuno-
Oncology Efforts
Focusing on multispecifics to address non-responding patients and
broaden therapeutic window compared to mAb approaches
Trafficking immunomodulation to tumor microenvironment
Varied geometry provides opportunity to test for optimal “synapse”
between tumor cell and T cell
Multispecifics can be mAb-Anticalin fusions (like PRS-343) or
Anticalin-Anticalin fusions (undisclosed)
Immunomodulatory engagers (like CD137) can be combined with
several tumor-targeting moieties, in a hub-and-spoke fashion
Prioritization
of
costimulatory
targets
(multiple
targets
beyond
CD137
actively pursued with Anticalins), but multiple checkpoint inhibitors
also being investigated
Internal and external resources (e.g. Holbrook Kohrt, M.D., Ph.D.) to
validate approach
Objective of achieving drug candidate nomination by the end of 2015
23 |
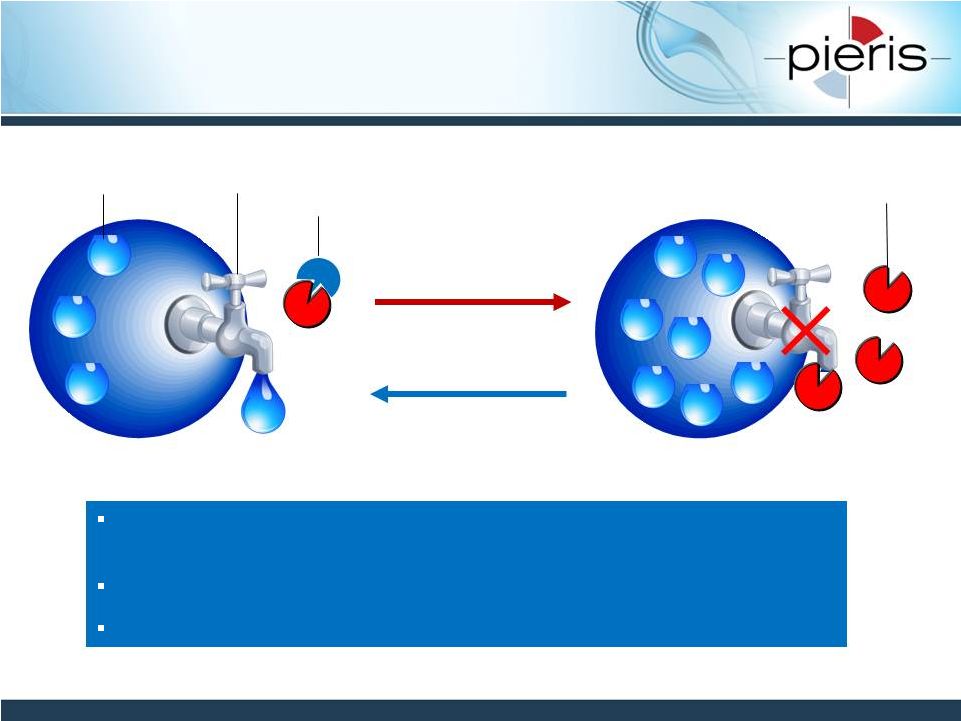 NON-CONFIDENTIAL
PRS-080: Intended to Reverse Hepcidin-
Mediated Functional Iron Deficiency
24
PRS-080 designed to reverse hepcidin-mediated anemia by
mobilizing iron trapped in the body’s iron storage cells
Addresses patients unresponsive to ESA and iron therapies
PK profile of PRS-080 designed to match hepcidin biology
Iron
Ferroportin
Inflammation
Hepcidin
PRS-080
PRS-080 |
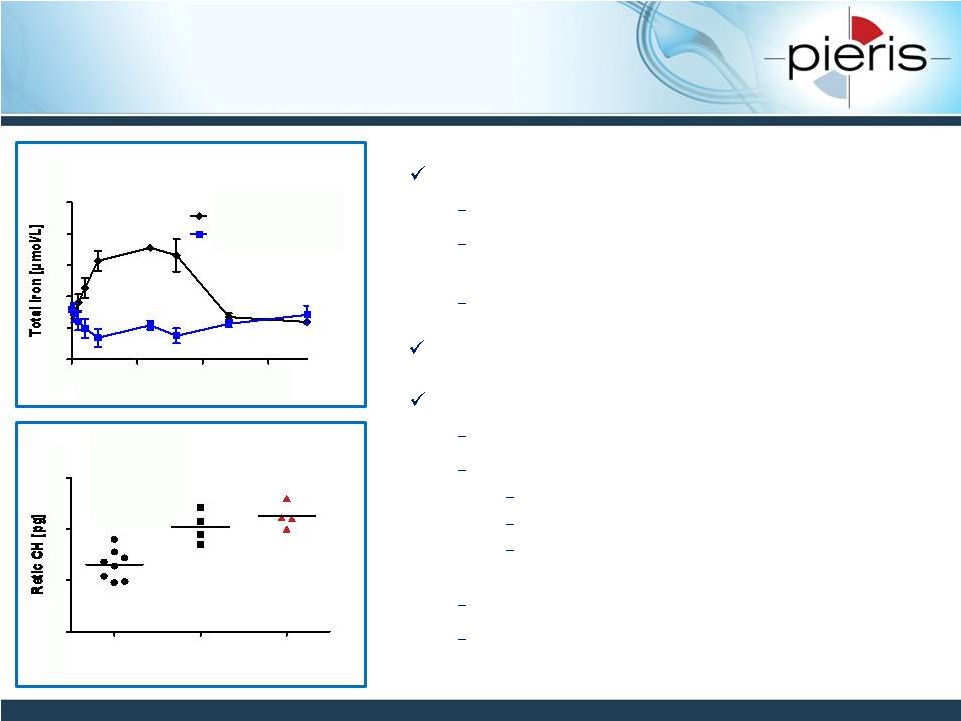 NON-CONFIDENTIAL
PRS-080: Effective in vivo –
Currently in Phase 1
25
Demonstrated efficacy and safety in cynos
Single-dose serum iron response
Increased reticulocyte hemoglobin after
multiple doses
No adverse events in GLP tox
First-in-man study initiated November 2014
Single-dose escalation in HVs (n=48)
Endpoints:
Safety, MTD, PK, immunogenicity
Target engagement
PD effects: serum iron, ferritin, transferrin
saturation, reticulocyte count, hemoglobin
Final cohort of subjects planned mid 2015
Reporting of results expected 2H 2015
Serum iron response in cyno following
single i.v. administration
Pre-dose
Day 30
i.v. 150 mg/kg
Day 30
s.c. 20 mg/kg
Elevation of reticulocyte Hg in cyno
following repeated administration
Funded through Ph I by ongoing €
6M EU grant
0
20
40
60
80
100
0
20
40
60
Time [h]
15
20
25
30
10 mg/kg PRS-080
10 mg/kg NGAL wt |
 NON-CONFIDENTIAL
PRS-080 in Chronic Kidney Disease
Market Opportunity
26
Sources:
USRDS 2014 Annual Data Report (2012 numbers): Atlas of Chronic Kidney Disease and
End-Stage Renal Disease in the U.S ESRD Patients in 2011 –
A Global Perspective, Fresenius Medical Care; Artisan Healthcare Consulting market
research study Hemodialysis
Patients
(Total 1.9M Worldwide)
No
anemia
18%
Anemic
82%
Hemodialysis Patients
with Anemia
(Total 1.6M Worldwide)
FID
24%
No FID
76%
Target Functional
Iron-Deficient (FID)
population:
U.S. 80,000
EU 61,000
JP 57,000
ROW 186,000
Estimated yearly
treatment costs:
~ $5,000 -
$10,000
We believe treating
FID anemic patients
has large commercial
potential |
 NON-CONFIDENTIAL
Anticalin Intellectual Property –
Safe & Sound
Exclusivity
Drug class protected through 2020s
Controlled patent filings and prior art
enable broad follow-on protection
Unique IP for each program
Freedom to Operate
No third party IP identified to date for
FTO on platform or therapeutic programs
27
Program
(Target)
CoM Patent
Term
cMet
2030
Hepcidin
2031
IL4Ra
2031
300 Series
(IO)
2035+ |
 NON-CONFIDENTIAL
Investment Highlights
28
Human PoC achieved with Anticalin platform
Novel therapeutic proteins
Desirable drug-like properties
Validation through strategic partnerships and collaborations
Sanofi, Daiichi Sankyo, Zydus, Stelis, Allergan
Several differentiated proprietary and partnered drug candidates
advancing towards or through clinical development
Potential for rich news flow in 2015
Potential milestone payments; expected clinical data; seeking new
partnerships
Proven management team and highly regarded Board of Directors
Unique approach to Immuno-oncology
Solid Financial Position |
 NON-CONFIDENTIAL
29
Pieris Pharmaceuticals, Inc.
Lise Meitner Strasse 30
85354 Freising
Germany
Tel.: +49 (0) 8161 1411 400
Fax: +49 (0) 8161 1411 444
info@pieris.com
www.pieris.com |
