Attached files
| file | filename |
|---|---|
| EX-99.1 - EX-99.1 - Sesen Bio, Inc. | d927999dex991.htm |
| EX-99.2 - EX-99.2 - Sesen Bio, Inc. | d927999dex992.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): May 18, 2015
ELEVEN BIOTHERAPEUTICS, INC.
(Exact Name of Registrant as Specified in Charter)
| Delaware | 001-36296 | 26-2025616 | ||
| (State or Other Jurisdiction of Incorporation |
(Commission File Number) |
(IRS Employer Identification No.) |
| 215 First Street, Suite 400 Cambridge, MA |
02142 | |
| (Address of Principal Executive Offices) | (Zip Code) |
Registrant’s telephone number, including area code: (617) 871-9911
None
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ¨ | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ¨ | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ¨ | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ¨ | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
| Item 7.01. | Regulation FD Disclosure. |
On May 18, 2015, Eleven Biotherapeutics, Inc. (the “Company”) issued a press release announcing the results from the Company’s first pivotal Phase 3 trial of its lead drug candidate, EBI-005, in moderate to severe dry eye disease. A copy of the press release is furnished as Exhibit 99.1 hereto and is hereby incorporated by reference into this Item 7.01.
On May 18, 2015, representatives of the Company will present by conference call and audio webcast information about the results of the Company’s Phase 3 trial of EBI-005 in moderate to severe dry eye disease described in the slides attached to this report as Exhibit 99.2 hereto, which are hereby incorporated by reference into this Item 7.01.
The information in this Item 7.01 (including Exhibits 99.1 and 99.2) is being furnished, not filed, pursuant to Regulation FD. Accordingly, the information in this Item 7.01 will not be incorporated by reference into any registration statement filed by the Company under the Securities Act of 1933, as amended, unless specifically identified therein as being incorporated therein by reference. The furnishing of the information in this Item 7.01 is not intended to, and does not, constitute a determination or admission by the Company that this information is material or complete, or that investors should consider this information before making an investment decision with respect to any security of the Company.
| Item 8.01. | Other Events. |
On May 18, 2015, the Company announced top-line results from the Company’s first pivotal Phase 3 trial of EBI-005 in moderate to severe dry eye disease. This trial was a double masked, randomized, placebo controlled trial in 669 patients with moderate to severe dry eye disease. Patients were randomly assigned, or randomized, to receive topical administration in each eye three times per day for 12 weeks of EBI-005 at 5 mg/ml or vehicle control beginning at randomization. We refer to the time at which we randomized a patient as baseline. The patients underwent study evaluations at weeks one, three, six, nine and 12 following randomization. The last dose of EBI-005 or vehicle was completed 12 weeks after randomization. We required patients to attend a final visit three weeks after their week 12 visit.
One co-primary endpoint of this Phase 3 trial was mean change in the total corneal fluorescein staining, or CFS, score, a sign of dry eye disease, from baseline at week 12 with EBI-005 treatment compared to mean change from baseline at week 12 with vehicle control. The other co-primary endpoint in this Phase 3 trial was mean change in pain and discomfort as measured by the painful or sore eyes question on the ocular surface disease index, or OSDI, a symptom of dry eye disease, from baseline at week 12 with EBI-005 treatment compared to mean change from baseline at week 12 with vehicle control. EBI-005 did not meet either of these two co-primary endpoints.
There was no statistically significant difference between the EBI-005 treated group and the vehicle control group on the co-primary endpoints or any secondary endpoints. Patients with dry eye disease in both the EBI-005 and vehicle treatment groups showed statistically significant improvement from baseline on the co-primary endpoints. While the change from baseline on the co-primary endpoints was greater in the vehicle group than the EBI-005 group, the differences between the two groups were not statistically significant and we believe the differences were not clinically meaningful.
EBI-005 was generally well tolerated in the Phase 3 trial with fewer than 5% of patients reporting eye irritation and no treatment related serious adverse events. We did not observe any significant imbalances between the EBI-005 treatment group and the vehicle control group in the incidence of ocular adverse events or systemic adverse events. Approximately 13% of patients in the trial reported some use of artificial tears, with no difference in artificial tear use between the EBI-005 treated and vehicle control groups. Overall, 92% of patients completed the trial, with 33 patients having dropped out of the EBI-005 group and 20 patients having dropped out of the vehicle control group.
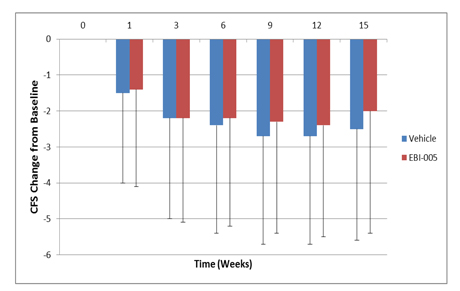
The graph below sets forth with respect to the EBI-005 treatment group and the vehicle control group the mean change in CFS scores from baseline at each evaluation visit following randomization during the trial period.
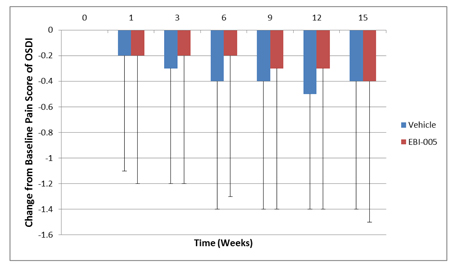
The graph below sets forth with respect to the EBI-005 treatment group and the vehicle control group the mean change in score on the OSDI question regarding painful or sore eyes from baseline at each evaluation visit following randomization during the trial period.
The table below sets forth for the EBI-005 treatment group and the vehicle control group the mean score at baseline and the mean change from baseline at week 12 on the following co-primary endpoints.
| EBI-005-3 | Baseline | Week 12 | ||||
| Total CFS |
EBI-005 | 8.5 Points | -2.4 Points | |||
| Vehicle | 8.6 Points | -2.7 Points | ||||
| OSDI Pain |
EBI-005 | 1.2 Points | -.3 Points | |||
| Vehicle | 1.3 Points | -.5 Points |
The table below sets forth for the EBI-005 treatment group and the vehicle control group the number and percentage of patients with one or more treatment emergent ocular adverse events and the number and percentage of patients with the most common ocular adverse events. All other ocular adverse events occurred in fewer than 2% of patients.
| Vehicle (N=334) |
EBI-005 (N=335) |
|||||||
| Subjects with at least one TEAE |
58 (17.4 | %) | 68 (20.3 | %) | ||||
| Eye Irritation |
15 (4.5 | %) | 15 (4.5 | %) | ||||
| Eye Pain |
8 (2.4 | %) | 9 (2.7 | %) | ||||
| Conjunctival Hyperemia |
5 (1.5 | %) | 9 (2.7 | %) | ||||
| Eyelid Itching |
5 (1.5 | %) | 9 (2.7 | %) | ||||
| Item 9.01 | Financial Statements and Exhibits |
(d) Exhibits
The following exhibits relating to Item 7.01 shall be deemed to be furnished, and not filed:
| 99.1 | Press release issued by the Company on May 18, 2015 | |
| 99.2 | EBI-005 Phase 3 Dry Eye Disease Trial Data Review dated May 18, 2015 | |
SIGNATURE
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| ELEVEN BIOTHERAPEUTICS, INC. | ||||
| Date: May 18, 2015 | By: | /s/ Abbie C. Celniker | ||
| Abbie C. Celniker, Ph.D. President & Chief Executive Officer | ||||
