Attached files
| file | filename |
|---|---|
| EXCEL - IDEA: XBRL DOCUMENT - Aspira Women's Health Inc. | Financial_Report.xls |
| EX-31.1 - EX-31.1 - Aspira Women's Health Inc. | c617-20150331xex311.htm |
| EX-31.2 - EX-31.2 - Aspira Women's Health Inc. | c617-20150331xex312.htm |
| EX-32.1 - EX-32.1 - Aspira Women's Health Inc. | c617-20150331xex321.htm |
| EX-10.7 - EX-10.7 - Aspira Women's Health Inc. | c617-20150331ex1076acd19.htm |
| EX-10.6 - EX-10.6 - Aspira Women's Health Inc. | c617-20150331ex10695cec9.htm |
| EX-10.4 - EX-10.4 - Aspira Women's Health Inc. | c617-20150331ex1042e85cf.htm |
| 10-Q - 10-Q - Aspira Women's Health Inc. | c617-20150331x10q.htm |
CONFIDENTIAL MATERIAL APPEARING IN THIS DOCUMENT HAS BEEN OMITTED AND FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION IN ACCORDANCE WITH THE SECURITIES ACT OF 1933, AS AMENDED, AND RULE 24B-2 PROMULGATED THEREUNDER. OMITTED INFORMATION HAS BEEN REPLACED WITH ASTERISKS.
|
Exhibit 10.5 |
TESTING AND SERVICES AGREEMENT
THIS TESTING AND SERVICES AGREEMENT (“Agreement”) is made and entered into as of March 11, 2015 (the “Effective Date”), by and between Vermillion, Inc., a Delaware corporation (formerly known as Ciphergen Biosystems, Inc.), and ASPiRA Labs, a Delaware corporation and wholly owned subsidiary of Vermillion, Inc. (collectively “Vermillion”) on the one hand and Quest Diagnostics Incorporated, a Delaware corporation, (“Quest” or “Quest Diagnostics”) on the other hand. Vermillion and Quest Diagnostics are sometimes individually referred to herein as a “Party” and collectively as the “Parties.”
WITNESSETH:
WHEREAS, Vermillion, Inc. and Quest Diagnostics entered into a Strategic Alliance Agreement dated as of July 22, 2005, for the development and commercialization of clinical laboratory tests and test kits, including the OVA1 test for ovarian cancer (The Strategic Alliance Agreement as amended shall be referenced herein as the “SAA”); and
WHEREAS, certain disputes arose between the Parties with respect to the SAA and related agreements, among other things; and
WHEREAS, the Parties have agreed to enter into a Global Settlement Agreement and Mutual Release (the “Settlement Agreement”) and a Non-Exclusive License Agreement concurrently with this Agreement; and
WHEREAS, the SAA and all past agreements between the Parties shall terminate as of the effective date of the Settlement Agreement; and
WHEREAS, under the SAA Quest Diagnostics had exclusive rights to perform OVA1 testing in the United States, Canada, Mexico, UK, and India and the Parties have now agreed to cancel Quest Diagnostics’ exclusive rights and work together to transition the OVA1 testing for Quest Diagnostics’ Accounts to Vermillion as Vermillion is able to perform such tests with Quest Diagnostics providing certain Specimen and collection services as more fully set forth herein; and
WHEREAS, Quest Diagnostics shall perform OVA1 testing that originates in states where Vermillion is not able to perform OVA1 tests or in states where the Parties agree Quest Diagnostics should perform OVA1 tests as more fully set forth herein;
WHEREAS, Quest Diagnostics shall continue to perform OVA1 testing on Specimens that originate outside the United States, except those countries where Vermillion satisfies the requirements set forth in this Agreement; and
WHEREAS, Quest Diagnostics is licensed, as required by applicable local, state and federal laws to perform Specimen collection and courier services.
1
NOW THEREFORE, in consideration of the foregoing premises and the terms and conditions set forth below, the parties agree as follows:
1.OVA1 TESTING
1.1Subject to the terms and conditions of this Agreement:
1.1.1Vermillion shall deliver up-to-date information regarding all OVA1 test kits, test kit components, OVA1 test kit materials, and other items and services as they have supplied to Quest Diagnostics in the past to enable Quest Diagnostics to perform OVA1 testing including, but not limited to, versions and updates of the OVACALC Algorithm, qualifying batches of reagents for use in OVA1 test kits, access to the website that identifies the manufacturer and qualified lot numbers for each of the five component kits to be purchased by Quest Diagnostics directly from the manufacturer of such kits (“OVA1 Materials”) as required by Quest Diagnostics.
1.1.2Vermillion also grants to Quest Diagnostics on a worldwide non-exclusive basis all rights necessary and desirable for Quest Diagnostics to (i) perform OVA1 testing including, but not limited to, the rights to use OVA1 Materials that are needed to perform OVA1 tests; the rights to use the OVACALC Algorithm; and all updates relating to OVA1 Materials and the OVACALC Algorithm; and (ii) market and sell testing services for the OVA1 test under Vermillion’s marks relating to the OVA1 test, such as OVA1 and the OVACALC Algorithm.
1.2Vermillion shall not (i) directly or indirectly sell or transfer any OVA1 Materials or (ii) authorize the performance of OVA1 testing to any person or entity whose gross annual revenue exceeds $2,000,000,000 in the United States.
1.3Quest Diagnostics shall cooperate with Vermillion to facilitate a transition to ASPiRA Labs providing the OVA1 testing now done by Quest Diagnostics for its Accounts in the United States as follows:
1.3.1Vermillion shall deliver to Quest Diagnostics a written certification that Vermillion is legally authorized to perform OVA1 testing in specified states (a) in volumes consistent with Quest Diagnostics’ past testing experience and (b) consistently with the following standards: (i) ASPiRA Labs’ conformance to FDA labeling; (ii) CLIA certification and validation and state licensure if required; (iii) test result turnaround time of no more than 48 hours (2 business days) from receipt of Specimen at ASPiRA Labs; (iv) communications capability for test ordering, test reporting and all related communications satisfactory to Quest Diagnostics Accounts; and (v) customer-support capability that includes, but is not limited to, a toll-free customer-support telephone number with waiting times averaging less than three minutes over any 10-day time period, a website that provides on-line access for all reported OVA1 test results performed by ASPiRA Labs, and printing and delivery of Vermillion OVA Test Requisition Forms substantially identical to the requisition form attached hereto as Attachment 5 within 24 hours of a request from a Quest Diagnostics Account. The written certification shall include the documentation on which Vermillion bases their certification (“Certification Documents”). Vermillion may deliver written certifications with Certification Documents for additional states at any subsequent time. Subject to the terms of this Agreement the Parties anticipate that the 39 states listed on Attachment 1 shall be the first Certified States to go through the Transition Process and that the remaining 11 states
2
will become Certified States and subject to a second Transition Process after Vermillion becomes licensed to perform OVA1 testing on Specimens that originate in those states.
1.3.2Quest Diagnostics shall notify Vermillion in writing within 10 business days of its actual receipt of Certification Documents whether it agrees that the Certification Documents are sufficient, and, if they are not sufficient, include in its notification a reasonably detailed description of the deficiencies identified by Quest Diagnostics. Vermillion shall be entitled to supplement the Certification Documents to address any deficiencies. Quest Diagnostics will respond within 10 business days from actual receipt of any supplements. This process may continue until the Parties reach agreement or Vermillion stops supplementing their submission. States where the parties agree Vermillion may perform OVA1 testing on Specimens originating from those states are described as “Certified States.”
1.3.3On the date the Parties agree Vermillion have met the requirements of Section 1.3.1 (“Certification Date”) for one or more Certified States, the Parties shall begin the transition to ASPiRA Labs performing, reporting and billing OVA1 testing on Quest Diagnostics’ Accounts and Quest Diagnostics performing the services described in Section 3 for all Certified States, as more fully set forth herein (“Transition Process”). As part of the Transition Process, Quest Diagnostics shall make commercially reasonable efforts to deliver a list of Accounts who ordered an OVA1 test from Quest Diagnostics in the last 24 months immediately preceding the Effective Date (excluding Accounts whose test was performed at a laboratory owned or operated by a joint venture to which Quest Diagnostics is a part owner) and a separate spreadsheet which provides a description of the type of payer (i.e. fee for service, Medicare, etc.) for Accounts in each Certified State. Quest Diagnostics shall run these two reports for all 50 states at the same time and deliver the relevant portion promptly after the applicable Certification Date. Quest Diagnostics will update these reports if requested by Vermillion. Quest Diagnostics shall not be obligated to run these reports for any foreign countries. To the extent readily available from computer generated reports with Quest Diagnostics’ current software, the Account and payer reports will include the information listed on Attachment 7. Quest Diagnostics has no obligation to use other software, make any calculations or summaries or devote any time or money to collecting information beyond running these readily available computer generated reports. The Parties shall work in good faith and make commercially reasonable efforts to complete the transition of OVA1 testing from Quest Diagnostics to Vermillion in each Certified State within 40 days after the applicable Certification Date. During any Transition Process, Quest Diagnostics shall continue to perform OVA1 tests in Certified States subject to the applicable Transition Process until the Parties unconditionally agree in writing that the Transition Process is 100% complete and Vermillion is ready to start performing OVA1 tests. Quest Diagnostics shall then stop performing OVA1 tests in the Certified States where the Transition Process is complete but continue to perform OVA1 tests originating outside of those Certified States.
(a)The Parties shall each designate one or more representatives who will be responsible for implementing each Transition Process. The target date for the first meeting of these representatives is within 7 business days of the Effective Date.
(b)For the first Transition Process the Parties shall make commercially reasonable efforts to operate a test program to develop reasonable procedures for the handling of the Specimens under this Agreement. The Parties contemplate that this test program will include
3
two facilities designated by Quest Diagnostics that will send sample Specimens to ASPiRA Labs over a period of approximately two weeks. The target date for Quest Diagnostics to identify the two facilities is April 1, 2015.
1.3.4Notwithstanding any other provision in this Agreement, Quest Diagnostics may also perform OVA1 tests in Certified States on a temporary basis in the place of Vermillion with respect to the affected or applicable Quest Diagnostics Accounts in the event and for only so long as: (a) Vermillion requests and Quest Diagnostics agrees to perform OVA1 tests on such terms as the Parties agree; (b) Vermillion directly or indirectly offers to or performs any tests in violation of Section 2.4 or announces their intent to do so and does not stop within 30 days after receipt of written notice from Quest Diagnostics; (c) Quest Diagnostics determines Vermillion is no longer meeting the standards set forth in Section 1.3.1 and Vermillion fails to meet such standards within 60 days after notice from Quest Diagnostics; (d) Vermillion authorizes any Third Party to perform OVA1 testing in violation of Section 1.2; or (e) if Vermillion is forbidden by law from performing OVA1 tests (for example, Vermillion loses a required license). For clarity, the foregoing rights for Quest Diagnostics to perform OVA1 tests in Certified States in the place of Vermillion with respect to the affected or applicable Quest Diagnostics Accounts shall continue only for so long as the corresponding breach or condition under clauses (a), (b), (c) (d) or (e) remains uncured or in place, and such rights shall terminate 10 days after Vermillion cures the applicable breach or condition and provides Quest Diagnostics with written notice of said cure.
1.3.5In the event Quest Diagnostics determines it is entitled to perform OVA1 testing pursuant to Section 1.3.4 Quest Diagnostics shall send written notice to Vermillion at least 30 days (or 1 day to the extent Vermillion stops performing OVA1 tests in one or more states for a period of at least 5 business days) before it starts performing OVA1 tests and said notice will set forth in reasonable detail the basis for invoking Section 1.3.4.
1.4Quest Diagnostics shall continue to perform OVA1 testing on Specimens that originate outside of the 50 United States, including on Specimens that originate in any of the U.S. territories, until Vermillion makes a written request to start performing OVA1 testing in one or more other countries or U.S. territories for Quest Diagnostics’ Accounts. Upon Quest Diagnostics’ receipt of such notice, the Parties shall cooperate in the transition of the requested testing to ASPiRA Labs as follows:
1.4.1The Parties shall attempt to negotiate an agreement whereby Quest Diagnostics would provide specimen collection and courier services, directly or through a subcontractor of Quest Diagnostics choosing, for Vermillion in each country or territory identified by Vermillion. This section 1.4.1 is merely a statement of intent. The Parties are not obligated to reach an agreement. Any failure to reach such an agreement shall not constitute a breach of any agreement.
1.4.2In the event the Parties do not reach an agreement with respect to any particular country or territory after 90 days of Quest Diagnostics’ receipt of said notice, Vermillion may provide for any services it may need in the noticed country or territory with any Third Party at its sole and absolute discretion.
4
1.5Quest Diagnostics shall promptly notify Vermillion of its receipt of any legal action, regulatory or compliance notices it receives regarding any OVA1 test.
1.6Within 30 days of the termination or expiration of this Agreement, Vermillion shall purchase any Quest Diagnostics OVA1 or Other OVA Test reagent inventory with at least 6 months of remaining shelf-life at the same price paid by Quest Diagnostics.
2.PROCEDURES
2.1Vermillion shall deliver to Quest Diagnostics all updates relating to the OVA1 test including, but not limited to, updates to the OVACALC Algorithm within three (3) business days after final development or Vermillion’s use in their own OVA1 test, whichever first occurs.
2.2Vermillion shall provide to Quest Diagnostics all requested OVA1 Materials in sufficient quantities to supply all of Quest Diagnostics’ orders. The OVA1 Materials shall meet all regulatory and legal requirements with at least the same quality that Vermillion have provided in the past.
2.3Vermillion shall use the information it receives from Quest Diagnostics in connection with this Agreement (“Quest Information”) for the sole purpose of performing OVA1 testing. It shall not disclose Quest Information to Third Parties.
2.4Vermillion cannot directly or indirectly offer to or perform tests that are on Quest Diagnostics’ Directory of Services now or in the future or which are substantially similar to those tests to an Account on the Master Account Transfer List or other Accounts disclosed by Quest Diagnostics to Vermillion, except for tests that can only be offered by Vermillion or a Vermillion Affiliate (i.e. are proprietary to Vermillion or a Vermillion Affiliate). This Section 2.4 shall apply to Vermillion and their controlled Affiliates. It shall not apply to the products and services of any acquirer of Vermillion involved in a Change of Control with respect to Vermillion.
2.5Vermillion shall perform each OVA1 test for a Quest Diagnostics Account in a manner that (a) meets or exceeds the standards set forth in Section 1.3.1, (b) is consistent with the applicable standards in the industry and (c) meets all legal and regulatory requirements.
2.6To the extent permissible under applicable federal and state laws and regulations, Vermillion shall maintain a similar type and level of patient support services as it currently performs including, but not limited to, services to assist patients with respect to insurance coverage, reimbursement, claim denials by Third Party payors or appeals from denials, adjudication of coverage decisions by health plans and the like relating to OVA1 testing for Quest Diagnostics Accounts in states that have not become Certified States.
2.7Vermillion shall require that Quest Diagnostics Accounts use a separate Vermillion OVA Test Requisition Form for each Specimen to be tested and each such form shall contain a unique number sufficient to enable the tracking of each sample Specimen in the form attached hereto as Attachment 5.
5
2.8Before an Other OVA Test becomes subject to this Agreement (including the procedures described in this Article 2), Quest Diagnostics must consent, which consent may not be unreasonably withheld.
3.QUEST DIAGNOSTICS RIGHTS AND SERVICES IN CERTIFIED STATES
3.1Vermillion shall have completed the customer communications requirements in Section 4 prior to Quest Diagnostics being obligated to perform any of services under this Section 3.
3.2The Specimen collection and courier services set forth in this Agreement shall apply solely with regard to biological Specimens for OVA1 tests and any Other OVA Tests to be performed, reported and billed by Vermillion that originate in any Certified State. With respect to Specimens originating in the other U.S. states, Quest Diagnostics shall continue to collect or pick up such Specimens for the performance, reporting and billing of OVA1 tests and any Other OVA Tests at its own facilities.
3.3For all OVA1 tests and any Other OVA Tests originating in a Certified State Quest Diagnostics agrees to collect Specimens from patients who present at its Patient Service Centers (“PSCs”) with a valid Vermillion OVA Test Requisition Form for each Specimen for the OVA1 test or any Other OVA Test from a health care provider authorized under federal and state laws to order clinical laboratory tests, for delivery, either directly by Quest Diagnostics or through a subcontractor engaged by Quest Diagnostics, to Vermillion’s ASPiRA Labs facility located at 101 Cooperative Way, Suite 220, Georgetown, TX 78626 or such other address specified by Vermillion after 30 days written notice. As promptly as practicable but no later than the end of the first Transition Process, (a) Vermillion will develop a solution so that Quest Diagnostics staff at PSCs can print out Vermillion OVA Test Requisition Forms at the PSCs, and (b) Vermillion will train PSC staff with regard to the information that such personnel must enter into Quest Diagnostics’ Care360 system in order to facilitate the logging and tracking of the receipt and shipment of the Specimens to ASPiRA Labs and as reasonably requested by Quest Diagnostics after completion of the first Transition Process.
3.4Quest Diagnostics’ couriers shall pick up biological Specimens from the offices or other facilities of physicians or health care providers who order the OVA1 test or any Other OVA Test using a Vermillion OVA Test Requisition Form for each Specimen and who place such order and the accompanying Specimens in a Quest Diagnostics Specimen lockbox at such offices or facilities. Quest Diagnostics shall make arrangements for the delivery of Specimens to Vermillion’s ASPiRA Labs for testing. Quest Diagnostics shall not be obligated to process any Vermillion OVA Test Requisition Form that includes more than one Specimen.
3.5Upon receipt of Specimens at the appropriate Quest Diagnostics facility, Quest Diagnostics’ staff shall scan the barcode from each Vermillion OVA Test Requisition Form so as to enter the information related to the Specimens that Quest Diagnostics collects or picks up pursuant to this Agreement into its electronic systems, solely for Specimen tracking purposes and to be able to answer certain questions that Vermillion may have with regard to the delivery of the Specimens to Vermillion’s ASPiRA Labs.
6
3.6Without limiting Sections 3.7 and 4.1, Vermillion shall communicate to all of their customers and potential customers the requirement to order OVA1 tests and any Other OVA Tests using the Vermillion OVA Test Requisition Form and will provide such forms to their customers at least 30 days prior to the start of Quest Diagnostics providing the services set forth in this Section 3.
3.7With regard to any OVA1 tests and any Other OVA Tests ordered using a test requisition form other than a properly prepared Vermillion OVA Test Requisition Form by any health care provider or facility listed in the Master Account Transfer List attached to this Agreement as Attachment 4, Quest Diagnostics will contact the ordering provider or facility to require the use and submission by the Account of a properly prepared Vermillion OVA Test Requisition Form and notify ASPiRA. ASPiRA shall use reasonable efforts to ensure that each ordering provider or facility uses the correct form thereafter.
3.8Quest Diagnostics’ referrals staff at the various Quest Diagnostics facilities will create a log of OVA1 test and any Other OVA Test Specimens collected or picked up by Quest Diagnostics and forwarded to ASPiRA Labs and will send such log electronically to ASPiRA Labs for tracking purposes within the time period agreed upon by the parties in writing.
3.9Quest Diagnostics will be the sole provider of the services described in this Article 3 with respect to Quest Diagnostics Accounts. By mutual agreement of the Parties Quest Diagnostics may provide such services with respect to other Vermillion customers.
3.10Vermillion will perform all testing on Specimens collected or picked up by Quest Diagnostics in any Certified State under this Agreement and Vermillion will be solely responsible for reporting test results to the ordering care providers, answering any queries from such providers, and for billing the responsible payers. Quest Diagnostics is not responsible for testing, result reporting, or billing for any Vermillion OVA1 testing or any Other OVA Test testing under this Agreement.
3.11For the avoidance of doubt, except as expressly provided in Section 3.3, Quest Diagnostics’ communications systems including, but not limited to, its electronic ordering and reporting system shall not be used by Vermillion in their communications with Quest Diagnostics Accounts.
3.12Quest Diagnostics and its Affiliates shall be able to offer any test that competes directly or indirectly with OVA1 or any Other OVA Test on a worldwide basis (including, but not limited to, in Certified States); however, Quest Diagnostics and its Affiliates shall not (directly or indirectly) designate as an Anchor Test during the term of this Agreement the ROMA assay or any other test approved or cleared by the FDA after the Effective Date whose FDA label indication or the indication listed in Quest Diagnostics’ directory of services is substantially the same as the FDA label indication of OVA1. If Vermillion believes Quest Diagnostics or any of its Affiliates is violating this Section 3.12, it must notify Quest Diagnostics in a writing that specifically identifies the marketing or promotional activity that allegedly breaches this section. Then Quest Diagnostics has the right to cure the alleged breach within 60 days of receiving said notice by stopping the specific activity identified in the written notice and making reasonable efforts to withdraw any materials identified in the notice from the public domain.
7
3.13In the event that Vermillion develops or obtains rights to commercialize any additional clinical laboratory tests and/or test kits for which Quest Diagnostics does not already offer (or have the right to offer) the same test, the Parties may discuss the terms and conditions under which said test might be added to this Agreement. Vermillion is not contractually obligated to initiate such discussions and Quest Diagnostics is not contractually obligated to provide any additional services beyond the terms of this Agreement.
3.14The Quest Diagnostics services set forth in this Article 3 shall be performed in a manner consistent with Quest Diagnostics then existing practices and procedures. Quest Diagnostics is under no obligation to modify any of its practices and procedures.
4.CLIENT COMMUNICATIONS
4.1Vermillion will obtain written approval from Quest Diagnostics for all communications to Quest Diagnostics’ Accounts, which approval shall not be unreasonably withheld. At least 10 days prior to the start of Quest Diagnostics providing the services set forth in Section 3 of this Agreement for each Account in each Certified State, Vermillion with the assistance of Quest Diagnostics shall have completed at least three written communications to each Quest Diagnostics Account informing each Quest Diagnostics Account of the transition of OVA1 testing to Vermillion and providing all reasonably necessary information and instructions to those Accounts including, but not limited to, a copy of the Vermillion OVA Test Requisition Form and instructions on its use. Said communications will be in substantially the same form as those attached hereto as Attachment 6.
4.2Vermillion shall not use Quest Diagnostics’ trade name, trademarks or service marks with respect to anything that may enter the public domain without the express written consent of Quest Diagnostics. Except as necessary to perform its obligations under this Agreement, Quest Diagnostics shall not use Vermillion’s trade name, trademarks or service marks with respect to anything that may enter the public domain without the express written consent of Vermillion.
4.3With respect to Quest Diagnostics Accounts in Certified States where Vermillion is responsible for OVA1 testing, Vermillion shall be solely responsible for handling all matters relating to providing OVA1 testing services independently of Quest Diagnostics. For the avoidance of doubt, Vermillion is solely responsible for marketing, sales, customer service, operations, billing, and test reporting for Quest Diagnostics Accounts relating to the delivery of OVA1 testing services it performs in each Certified State.
5.TERM AND TERMINATION
The Term of this Agreement shall commence on the Effective Date and continue until terminated as set forth below.
5.1At any time by the mutual written agreement of all the Parties to this Agreement or as provided in Section 16.11.
5.2If there is a determination that this Agreement is not in compliance with any applicable law, regulation, or government requirement, and the Agreement is not amended to
8
correct such compliance, either Party may terminate this agreement immediately upon written notice to the other Party.
5.3This Agreement shall terminate in two years from the Effective Date unless extended by the written agreement of the Parties.
5.4If any Party materially violates, breaches or fails to perform any term or covenant of this Agreement, then the other Party may give written notice of such default to such Party. If such Party does not cure such default within ninety (90) days of the date of a Notice of Default, the other Party will have the right to terminate this Agreement by a second written notice to such Party. Such right to terminate is in addition to all other remedies available to such Party in equity or at law, but it also subject to the limitations in Article 11 and any other applicable provisions of this Agreement.
5.5In the event of a Change of Control of any Party any other Party may terminate this Agreement immediately by delivery of written notice.
5.6In the event of an assignment for the benefit of creditors or a filing of a petition in bankruptcy by or against Vermillion or Quest Diagnostics that is not cancelled, terminated or dismissed within 60 days, any other Party may terminate this Agreement immediately by delivery of written notice.
5.7Except as expressly provided in this Agreement, following the termination or expiration of this Agreement, all rights granted to any Party herein shall immediately terminate and each Party shall return or destroy all records and materials in its possession or control containing any other Party’s Confidential Information and destroy all electronic copies thereof within 60 days of termination. Each Party shall send a written notice to each other Party certifying its compliance with this Section 5.6 within the same 60 day time period.
6.VERMILLION REPRESENTATIONS AND WARRANTIES
Vermillion represents and warrants that:
6.1Vermillion shall perform all of their OVA1 testing to the performance standards set forth in Section 1.3.1.
6.2Vermillion have all the rights to sell OVA1 Materials, use the OVACALC Algorithm and grant the rights Vermillion is granting to Quest Diagnostics under this Agreement including, but not limited to, all intellectual property rights necessary for Quest Diagnostics to perform OVA1 testing on a worldwide basis under Vermillion’s applicable marks.
6.3Vermillion shall maintain sufficient equipment and skilled personnel to perform OVA1 testing services in a manner consistent with the applicable industry standards in all Certified States.
6.4Vermillion shall develop and maintain a quality management system that meets the applicable industry standards and regulatory requirements including, but not limited to, the FDA’s current Good Manufacturing Practice regulations (cGMP, Title 21 CFR, Part 820) and ISO 13485
9
standard (for Products shipped internationally). In addition, Vermillion shall require that their own suppliers of raw materials and any other products used by Vermillion or their designees to manufacture the OVA1 Materials or materials for Other OVA Tests sold or provided hereunder to Quest Diagnostics also maintain a quality management system that meets applicable sections of GMP (domestic) or ISO 13485 (international).
6.5Vermillion shall continue to support the Roche Elecsys 2010 instrument for the performance of the CA-125 II assay to the same extent Vermillion have supported this instrument in the past.
7.VERMILLION AND QUEST DIAGNOSTICS REPRESENTATIONS AND WARRANTIES
7.1Each entity is duly organized and validly existing under the laws of the state where it is domiciled, and (a) has the complete and unrestricted power and right to enter into this Agreement, perform its obligations hereunder, and (b) has taken all necessary action on its part required to authorize the execution and delivery of this Agreement and the performance of its obligations hereunder.
7.2This Agreement has been duly authorized, executed and delivered by such Party and constitutes a legal, valid and binding obligation of such Party enforceable against such Party in accordance with its terms except as enforceability may be limited by law or principles of equity.
7.3The execution, delivery and performance of this Agreement by such Party do not conflict with any agreement, instrument or understanding, oral or written, to which such Party is a party or by which such Party may be bound, nor violate any law or regulation of any court, governmental body or administrative or other agency having authority over such Party.
7.4All consents, approvals and authorizations of any kind required to be obtained in connection with the execution, delivery and performance of this Agreement have been obtained.
7.5As of the Effective Date, there are no actions, suits, proceedings or other forms of litigation pending or, to the best of such Party’s knowledge, threatened against such Party of any kind or nature relating to the transactions contemplated by this Agreement or that could reasonably be expected to materially affect the ability of such Party to enter into this Agreement or to perform its obligations hereunder.
8.COMPENSATION
8.1In addition to any other fees set forth elsewhere in this Agreement, including Attachment 2, Vermillion shall pay Quest Diagnostics *** (*** dollars) per OVA1 Specimen collected and shipped to ASPiRA Labs. Quest Diagnostics will not bill any other party for the services it provides hereunder. The same *** price shall apply for Other OVA Tests, if Quest Diagnostics’ projected costs are the same or less than its costs relating to OVA1 tests. If Quest Diagnostics projected costs for collecting and shipping Other OVA Tests are higher than its costs relating to OVA1 tests, Quest Diagnostics shall have no obligation to provide any services under this Agreement with respect to Other OVA Tests, unless the Parties agree on a higher price.
10
8.2Attachment 2 sets forth the description and amounts of deliverables that Vermillion will pay to Quest Diagnostics as consideration for the performance of the services under this Agreement.
8.3Vermillion agrees to compensate Quest Diagnostics within sixty (60) days of the date of Quest Diagnostics invoices for services and deliverables set forth in this Agreement as specified in the invoice.
8.4With respect to OVA1 tests performed by Quest Diagnostics after the Effective Date, as evidenced by a test report from which all protected health information (as such term is defined under the privacy and security regulations of the Federal Health Insurance Portability and Accountability Act of 1996) has been removed, Quest Diagnostics shall pay Vermillion a fixed fee of *** (*** dollars) for each OVA1 test. This fee is considered earned at the time each OVA1 test is reported. Quest Diagnostics shall provide Vermillion with a monthly report of the number of OVA1 tests reported by Quest Diagnostics during such calendar month, including the 3 digit-level zip code of Specimens tested by Quest Diagnostics, within 30 days after the end of said month. This monthly report shall also state the total amount payable by Quest Diagnostics to Vermillion in connection therewith. Quest Diagnostics will pay Vermillion the amount due for each calendar month, as reported in each report, within 60 days of the date of said report. Such payments shall be by wire transfer in accordance with the following wire transfer instructions:
|
FOR CREDIT OF: |
Vermillion, Inc. |
|
CREDIT ACCOUNT #: |
*** |
|
BANK NAME: |
Compass Bank |
|
ABA #: |
062001186 |
|
SWIFT CODE: |
CPASUS44 |
|
BY ORDER OF: |
Quest Diagnostics |
8.5The Parties understand and acknowledge that prior to the Effective Date, Quest Diagnostics has been reporting to Vermillion the number of monthly OVA1 tests performed by Quest Diagnostics on approximately the 8th day following the end of that month and has made payments to Vermillion of amounts owed to Vermillion in accordance with the terms of the SAA the following day via wire transfer. Vermillion agrees that Quest Diagnostics has fully paid Vermillion for all test results through January 2015. For OVA1 test result reports performed by Quest Diagnostics between February 1, 2015 and the Effective Date, Quest Diagnostics shall pay Vermillion *** for each reported test result instead of the formula used under the SAA and on the same terms as provided in this Agreement.
8.6For each instance where Quest Diagnostics is required to notify an Account of the need to use the correct Vermillion OVA Test Requisition Form as more fully described in Section 3.7, or for each time Quest Diagnostics services an Account that was on the Master Account Transfer List and that has not complied with the information and instructions contained in the communications referred to in Section 4.1 or for each instance Quest Diagnostics is required to notify an Account that the Specimen submitted for OVA1 testing or Other OVA Test testing does not meet the specifications of a Specimen, Quest Diagnostics will charge Vermillion and Vermillion shall pay to Quest Diagnostics *** (*** dollars). For any Accounts that were erroneously omitted from the Master Account Transfer List, the foregoing charges shall only apply
11
to a second or subsequent offense that occurs more than 60 days after Quest Diagnostics has provided notice to Vermillion of the initial offense.
8.7In the event Quest Diagnostics makes an erroneous shipment to Vermillion and Vermillion is required or asked to return the shipment, Quest Diagnostics shall reimburse Vermillion for the return shipping costs.
8.8Any payment obligations set forth in this Agreement shall survive termination or expiration of this Agreement.
9.INDEPENDENT CONTRACTOR
9.1It is understood that each Party is performing under this Agreement in the capacity of an independent contractor and not in any respect or under any circumstances as an employee, representative, agent or partner of any other Party. No Party has authority to enter into contracts or assume any obligations for or on behalf of any other Party or to speak for or on behalf of any other Party. No Party shall make any public representations to the contrary.
10.COMPLIANCE WITH LAWS
10.1In performing the Specimen collection services under this Agreement as provided in Section 3, and for that function alone, the Parties agree that Quest Diagnostics is performing a function on behalf of Vermillion and is acting as Vermillion’s Business Associate for purposes of HIPAA. Otherwise, Quest Diagnostics is a clinical laboratory that is a covered entity under HIPAA. With regard to Quest Diagnostics in its role as Vermillion’s Business Associate, the parties agree to and shall comply with the Business Associate terms and conditions set forth in Attachment 3, which is hereby made a part of this Agreement.
10.2Without limiting the above, the Parties further agree that Quest Diagnostics shall comply with Bloodborne Pathogen and Universal Precautions Standards issued by the federal Occupational Safety and Health Administration (and equivalent state agency), or other requirements applicable to the collection and handling of Specimens.
10.3It is the intent of the Parties to comply with the Federal Anti-Kickback (42 USC 1320a-7b) and the “Stark” Physician Anti-Self-Referral (42 USC 1395nn) Statutes and any related regulations (including amendments and any similar state requirements). In the event of a determination that this Agreement is not in compliance with these laws, then the Parties shall negotiate in good faith to conform this Agreement.
10.4Each Party represents and warrants that it has not been excluded, debarred or suspended from participating in any federal or state health care program. Each Party shall notify the other immediately if its status for participating in such health care programs changes.
11.INDEMNIFICATION AND LIMITATION OF LIABILITY
11.1Vermillion and Quest Diagnostics shall indemnify, defend and save the other harmless (including the respective Affiliates, employees, officers and directors of each Party) against any and all losses, claims, suits, damages, liabilities and expenses (including without
12
limitation, reasonable attorney’s fees) based upon, arising out of or attributable to the acts and/or omissions of such Party, such Party’s Affiliates and their respective employees, officers, directors, subcontractors and/or agents that result in a claim from a Third Party. In the event two or more Parties contributed to the claim, each will indemnify the other to the extent each contributed to the claim brought by a Third Party. For the avoidance of doubt Vermillion have a duty to indemnify Quest Diagnostics in the event it is the subject of patent infringement claim based on its performing OVA1 or Other OVA Test testing or related services. Each Party has no obligation to indemnify the other except as provided in this Article 11.
11.2Any Party with a Third Party claim (“Third Party Claim”) for which it seeks indemnity (the “Indemnitee”) shall promptly notify the alleged “Indemnitor” (including multiple Indemnitors) of the existence of such a claim (“Claim Notice”), however, any delay in giving the Claim Notice shall not prejudice the Indemnitee’s rights except to the extent such delay materially prejudices any defense or other right with respect to the defense of the underlying claim. The Indemnitor shall respond to the Claim Notice in writing within 15 days and specify whether the Indemnitor will take over the defense of the underlying claim and whether it will indemnify the Indemnitee from any liability arising from the Third Party Claim. The Parties shall use commercially reasonable efforts to agree on the choice of counsel for any Third Party Claim. The Parties shall agree on settlement terms for any Third Party Claim, such agreement not to be unreasonably withheld or delayed. If the Indemnitor unconditionally agrees to defend and indemnify the Indemnitee, the Indemnitor shall have the sole power to direct the defense at its expense except as follows: (i) where the Third Party Claim involves criminal liability on the part of the Indemnitee or its employees, (ii) the Third Party Claim includes injunctive relief against the Indemnitee, (iii) the Indemnitee determines the Indemnitor does not have the financial strength to adequately protect the Indemnitee, or (iv) the Indemnitor is not defending the Third Party Claim in good faith. If the Indemnitor assumes the Indemnitee’s defense, the Indemnitee may participate in the defense through its own counsel at its own cost. If the Indemnitee does not cooperate in the defense of the Third Party Claim or refuses to enter into a reasonable settlement of the Third Party Claim, the Indemnitor may withdraw its defense of the Indemnitee subject to the Indemnitee’s right to collect the fees and costs incurred as a result of such withdrawal, if it later establishes its cooperation in the defense of the Third Party Claim or that the proposed settlement was not reasonable for the Indemnitee, as applicable.
11.3The amount of each Party’s liability for Third Party indemnity claims is limited to $3,000,000.
11.4EACH PARTY AND THEIR RESPECTIVE OFFICERS, DIRECTORS, EMPLOYEES, AGENTS OR AFFILIATES SPECIFICALLY DISCLAIMS ALL LIABILITY FOR AND WILL IN NO EVENT BE LIABLE FOR ANY INCIDENTAL, SPECIAL, INDIRECT, OR CONSEQUENTIAL DAMAGES, EXPENSES, LOST PROFITS, LOST SAVINGS, INTERRUPTIONS OF BUSINESS OR PUNITIVE DAMAGES OF ANY KIND OR CHARACTER WHATSOEVER ARISING OUT OF OR RELATING TO THIS AGREEMENT OR RESULTING FROM THE DEVELOPMENT, PROVIDING, MANUFACTURE, HANDLING, MARKETING, SALE DISTRIBUTION OR USE OF TESTS OR TEST KITS.
11.5IN NO EVENT SHALL QUEST DIAGNOSTICS’ TOTAL AGGREGATE LIABILITY FOR ALL CLAIMS ARISING OUT OF OR RELATED TO THIS AGREEMENT
13
EXCEED THE GREATER OF (i) $1,000,000 AND (ii) AMOUNTS PAID BY QUEST DIAGNOSTICS TO VERMILLION PURSUANT TO THIS AGREEMENT AFTER THE EFFECTIVE DATE AND DURING THE TWELVE (12) MONTH PERIOD IMMEDIATELY PRECEDING THE EVENT GIVING RISE TO SUCH LIABILITY.
11.6IN NO EVENT SHALL VERMILLION’S TOTAL AGGREGATE LIABILITY FOR ALL CLAIMS ARISING OUT OF OR RELATED TO THIS AGREEMENT EXCEED THE GREATER OF (i) $1,000,000 AND (ii) AMOUNTS PAID BY VERMILLION TO QUEST DIAGNOSTICS PURSUANT TO THIS AGREEMENT AFTER THE EFFECTIVE DATE AND DURING THE TWELVE (12) MONTH PERIOD IMMEDIATELY PRECEDING THE EVENT GIVING RISE TO SUCH LIABILITY.
11.7NO ACTION REGARDLESS OF FORM, ARISING OUT OF OR RELATED TO BREACH OF ANY REPRESENTATION, WARRANTY OR COVENANT UNDER THIS AGREEMENT MAY BE BROUGHT BY EITHER PARTY MORE THAN TWO (2) YEARS AFTER SUCH PARTY HAS KNOWLEDGE OF THE OCCURRENCE THAT GAVE RISE TO THE CAUSE OF SUCH ACTION. THE FOREGOING LIMITATIONS SHALL APPLY REGARDLESS OF THE FORM OF ACTION, WHETHER IN CONTRACT, TORT, STRICT LIABILITY, OR OTHERWISE EVEN IF SUCH PARTY WAS ADVISED OF THE POSSIBILITY OF SUCH DAMAGES AND NOTWITHSTANDING THE FAILURE OF ANY REMEDY OF ITS ESSENTIAL PURPOSE.
12.INSURANCE
12.1Vermillion and Quest Diagnostics agree to maintain general and professional liability insurance in amounts adequate to cover their respective acts and omissions. The parties agree that such coverage shall be, at a minimum, $1,000,000 per claim and $3,000,000 aggregate. Quest Diagnostics may comply with the insurance obligations hereunder through self-insured retention.
12.2In the event that any insurance referred to herein is of the “claims made” type, each Party with such insurance coverage agrees that the insurance shall be continued for a period of at least four (4) years after the termination of this Agreement, or the Party shall purchase extended reporting period insurance (also known as “tail coverage”) to extend the insurance for a minimum of four (4) years after the termination of this Agreement. The provisions of this Section 12.2 shall survive termination of this Agreement.
12.3Vermillion and Quest Diagnostics agree to furnish each other with a current and valid Certificate of Insurance, or proof of adequate self-insurance, evidencing their general liability and professional liability insurance coverage. Any material modification or alteration in such coverage shall be promptly communicated to the other Party.
13.CONFIDENTIALITY
13.1“Confidential Information” means any information or material, in whatever form or manner relating to the business of a party (a “Disclosing Party”) and disclosed to the other Party (the “Receiving Party”) which (a) is not generally known other than by the Disclosing Party, and (b) which Receiving Party may obtain knowledge of through or as a result of the relationship
14
established hereunder with the Disclosing Party, access to the Disclosing Party’s premises, or communications with the Disclosing Party’s employees or independent contractors. Confidential Information includes but is not limited to the following types of information, and other information of a similar nature: processes, standard operating procedures (SOPs), protocols and procedures; algorithms; software (including source code); systems; equipment; designs; drawings; formulas; data; reports; memoranda; notes; records; research; experiments; business plans and strategies; marketing techniques and materials; marketing plans; account names and other information related to accounts; patient information; pricing information; cost information, sales volumes and sales projections; commercial opportunities; and organizational, technical (including without limitation know-how, patent applications, invention disclosures, trade secrets and technology that are not fully developed, patented or patentable) and financial information. Confidential Information also includes the existence of this Agreement and the terms hereof, both written and oral. The term “Confidential Information” does not include (a) information that is in the possession of the Receiving Party without obligation of confidence; (b) information that is now or later becomes publicly available without violation of this Agreement by Receiving Party; and (c) developments by Receiving Party independent of its receipt of information from Disclosing Party.
13.2The Parties recognize and acknowledge that, by virtue of entering into this Agreement, the Parties may have access to Confidential Information of the other party. The Parties warrant and covenant to each other that neither Receiving Party will at any time, either during or subsequent to the term of this Agreement, disclose to others, use, copy or permit to be copied, without the Disclosing Party’s express prior written consent, except pursuant to the performance of services duties hereunder, any Confidential Information of the Disclosing Party which is not otherwise available to the public. By way of example, no Party will disclose any other Party’s Confidential Information to potential investors or acquirers.
13.3Vermillion shall not use Quest Diagnostics Confidential Information, including without limitation those Account and payer lists provided pursuant to Section 1.3.3, outside of the performance of this Agreement, including without limitation to solicit any Accounts of Quest Diagnostics for services that are not covered by this Agreement. Quest Diagnostics shall not use any Vermillion Confidential Information for any purpose outside of the performance of this Agreement.
13.4Vermillion and Quest Diagnostics agree to use, maintain, and transfer patient health data in accordance with all applicable laws, regulations, and government requirements concerning the confidentiality or privacy of such data.
13.5If any Party receives a subpoena or other legal process purporting to require the disclosure of any other Party’s Confidential Information, that Party shall immediately notify the affected Party and fully cooperate in any efforts by that Party to prevent or limit the disclosure of its Confidential Information. No party will produce any other Party’s Confidential Information pursuant to any subpoena or other legal process before providing the notice set forth herein.
14.NOTICES
14.1Any notice required to be given hereunder will be deemed to have been served properly, if mailed by certified or registered mail, postage prepaid (or Federal Express or
15
equivalent courier), properly addressed and posted in a United States depository to the respective parties hereto at the following addresses:
|
To Vermillion: |
Vermillion, Inc. |
|
With copy to: |
Goodwin Procter LLP |
|
|
Exchange Place |
|
|
Boston, MA 02109 |
|
|
Attn: Christopher J. Denn |
|
|
Telephone: (617) 570-1000 |
|
|
Facsimile: (617) 523-1232 |
|
|
Email: cdenn@goodwinprocter.com |
|
To Quest Diagnostics: |
Quest Diagnostics Incorporated |
|
|
3 Giralda Farms |
|
|
Madison, NJ 07940 |
|
|
Attn: Executive Director, Business Development |
|
|
Telephone: (973) 520-2163 |
|
|
Facsimile: (973) 520-2005 |
|
|
Email: Nicholas.j.conti@questdiagnostics.com |
|
With a copy to: |
Quest Diagnostics Incorporated |
|
|
3 Giralda Farms |
|
|
Madison, NJ 07940 |
|
|
Attn: General Counsel |
|
|
Telephone: (973) 520-2177 |
|
|
Facsimile: (610) 271-8719 |
|
|
Email: Michael.e.prevoznik@questdiagnostics.com |
15.Legislative/Regulatory Modification
15.1In the event any applicable laws, rules, regulations or payment policies, or any rules or policies of any Third Party payer, or any other federal, state or local law, rule, regulation, policy, or any interpretation thereof, at any time during the term of this Agreement, is modified, implemented, threatened to be implemented, or determined to prohibit, restrict or in any way materially change the services to be provided under this Agreement, including the method or amount of reimbursement or compensation, then the parties to this Agreement shall negotiate in good faith to amend this Agreement to conform to the changed requirements.
16
15.2It is the intent of the parties to comply with the Federal Anti-Kickback (42 USC 1320a-7b) and the “Stark” Physician Anti-Self-Referral (42 USC 1395nn) Statutes and any related regulations (including amendments and any similar state requirements). In the event of a determination that this Agreement is not in compliance with these laws, then the parties shall negotiate in good faith to conform this Agreement.
15.3If a circumstance set forth in Sections 15.1 or 15.2 arises, and this Agreement is not amended as set forth in this Article 15, then this Agreement shall be terminated in accordance with Section 5.2, unless otherwise agreed upon by the parties in writing.
16.MISCELLANEOUS
16.1Definitions. Capitalized terms used in this Agreement have the meaning respectively ascribed to them when first used in this Agreement or as set forth in Schedule A hereto.
16.2Assignment. No Party may assign its rights or delegate its obligations under this Agreement, in whole or in part, to any Third Party without the prior written consent of the other Parties, which consent shall not be unreasonably withheld. Notwithstanding the foregoing, each Party may assign all of its rights and obligations under this Agreement to its subsidiary, successor, or parent corporation. As a condition of such an assignment, the assignor must unconditionally guarantee the full performance by the assignee of all applicable terms and conditions of this Agreement for the benefit of the other Parties to this Agreement. Notwithstanding anything to the contrary in this Section, nothing contained in this Section shall release the assigning Party from any liabilities or obligations it may have under this Agreement.
16.3Waiver. The Parties covenant and agree that if a Party fails or neglects for any reason to take advantage of any of the terms provided for the termination of this Agreement or if a Party, having the right to declare this Agreement terminated, shall fail to do so, any such failure or neglect by such Party shall not be a waiver or be deemed or be construed to be a waiver of any cause for the termination of this Agreement subsequently arising, or as a waiver of any of the terms, covenants or conditions of this Agreement or of the performance thereof. None of the terms, covenants and conditions of this Agreement may be waived by a Party except by its written consent.16.4Severability. It is the intention of the Parties that the provisions of this Agreement will be enforceable to the fullest extent permissible under all applicable laws, regulations, and government requirements, and that the unenforceability of any provisions under such laws or requirements will not render unenforceable, or impair, the remainder of the Agreement. If any provisions hereof are deemed invalid or unenforceable, either in whole or in part, this Agreement will be deemed amended to delete or to modify, as necessary, the offending provisions and to alter the bounds thereof in order to render it valid and enforceable.16.5Entire Agreement. This Agreement, the Global Settlement Agreement and Mutual Release and the Non-Exclusive License Agreement all being executed concurrently herewith constitute the entire Agreement between Vermillion and Quest Diagnostics with respect to the subject matter hereof. As provided in Section 22 of the Settlement Agreement, this Agreement, the Settlement Agreement and the Non-Exclusive License Agreement supersedes any prior understandings or agreements between the Parties. No modification of this Agreement will have any force or effect unless such modification specifically indicates it is a modification of this Agreement, is in writing and signed by authorized
17
16.6Survival. The provisions of Articles 9, 11, 13, 14, 16 and Sections 1.6, 4.2, 5.6, 8.8 and 12.2 shall survive termination of this Agreement.
16.7Arbitration and Governing Law. This Agreement shall be construed and enforced in accordance with the laws of New Jersey without regard to the conflict of law provisions thereof. Any dispute, controversy or claim arising out of or under this Agreement, or its performance, shall first be negotiated in good faith by the parties, and if an acceptable resolution does not result, shall be submitted to arbitration which shall be exclusive, final, binding, and conducted by one arbitrator in accordance with the rules of the American Arbitration Association (“AAA”) applicable to commercial disputes. The decision of the arbitrator shall be final and in writing, setting forth the award and the reasons therefor. All hearings in the arbitration shall be held in Bergen County, New Jersey. Each Party shall bear its own fees and expenses, including attorneys’ fees. The fees and expenses of the arbitrator and the cost of the arbitration shall be borne equally by the parties. Any decision of the arbitrator may be entered as a judgment in any court of competent jurisdiction and may be enforced as such in accordance with the provisions of the award. This agreement to arbitrate shall be specifically enforceable by the parties.
16.8No Third Party Rights. No provision of this Agreement shall be deemed or construed in any way to result in the creation of any rights or obligations in any Third Party not a Party to this Agreement.
16.9Press Release. Neither Party shall issue any press releases relating to this Agreement or the activities to be conducted hereunder without submitting a draft copy of such press release to the other Parties for approval at least ten business days prior to issuance, except for press releases that are reasonably required for compliance with applicable laws and regulations and the rules of any stock exchange which must be submitted for approval one business day in advance. Press release approvals shall not be unreasonably withheld by any Party. Notwithstanding the foregoing, the Parties will agree upon and release a mutual press release in the form attached to the Settlement Agreement as Exhibit G; thereafter, each Party may each disclose the information contained in such press release or any other subsequently approved press release without the need for further approval by the other Party.
16.10Headings. The descriptive headings of this Agreement are for convenience only, and will be of no force or effect in construing or interpreting any of the provisions of this Agreement.
16.11Force Majeure. No Party to this Agreement shall be liable for failure to perform any duty or obligation that said Party may have under the Agreement where such failure has been caused by any event, foreseen or unforeseen, outside the reasonable control of the Party who had the duty to perform and that renders performance impossible or impracticable including, but not limited to, acts of God, terrorist acts, fire, strike, inevitable accident, war, or any other event, like or unlike those listed above (collectively, “Force Majeure Event”), but only to the extent prevented by the Force Majeure Event. Any Party may terminate this Agreement if a Force Majeure event results in a Party suspending performance of any obligation for more than six months.
16.12Counterparts. This Agreement may be executed in two counterparts, each of which will constitute an original document, but both of which will constitute one and the same instrument.
18
IN WITNESS WHEREOF, the parties intending to be legally bound, have set their hands the date and year first above written.
|
QUEST DIAGNOSTICS INCORPORATED |
|
VERMILLION, INC. |
|||||||
|
By: |
/s/ Wilson R. Conde |
By: |
/s/ Valerie B. Palmieri |
||||||
|
Print Name: |
Wilson R. Conde |
Print Name: |
Valerie B. Palmieri |
||||||
|
Title: |
Vice President, Strategic Alliances & Clinical Franchise Business Development |
Title: |
President and CEO |
||||||
|
Date: |
3/11/15 |
Date: |
3/11/15 |
||||||
|
|
ASPIRA LABS, INC. |
|||
|
|
By: |
/s/ Eric Schoen |
||
|
|
Print Name: |
Eric Schoen |
||
|
|
Title: |
Secretary and Treasurer |
||
|
|
Date: |
3/11/15 |
||
19
SCHEDULE A
DEFINITIONS
“Account” or “Accounts” means the Third Party ordering an OVA1 test or Other OVA Test through Quest Diagnostics.
“Affiliate” means, with respect to any Party, any entity controlling, controlled by, or under common control with such Party, during and for such time as such control exists. For these purposes, “control” will refer to the ownership, directly or indirectly, of at least fifty percent (50%) of the voting securities or other ownership interest of the relevant entity.
“Anchor Test” means a test offered by Quest Diagnostics that is the subject of a specific and unique compensation program for salespeople for selling such a test or is part of a senior management level directed promotional campaign that includes the preparation of marketing materials and sales scripts prepared specifically for that campaign. For the sake of clarity all other promotional activities associated with a particular test including, but not limited to: distributing a data sheet describing the test, including the test in any list of tests performed by Quest Diagnostics, answering questions about a particular test, including the test in sales presentations; making statements (verbal or written) about the test, or agreeing to specific financial terms, such as a volume discount, do not make that test an Anchor Test.
“Change of Control” means the sale of all or substantially all the assets of a Party; any merger, consolidation or acquisition of a Party with, by or into another Third Party; or any change in the ownership of more than fifty percent (50%) of the voting capital stock of a Party in one or more related transactions.
“Other OVA Test” means a Vermillion test (i) developed and marketed as a replacement, next generation or successor version of OVA1, (ii) whose indication is the same or substantially the same as the FDA label indication of OVA1, and (iii) which has received all required approvals, clearances and/or licenses by the FDA or other federal or state body or administrative or other agency having authority over such matters.
“OVA1” means a Vermillion test that includes the following biomarkers: CA 125, Beta 2 microglobulin, Transferrin, Apolipoprotein A1 and Prealbumin (Transthyretin), and that is intended for assessing the likelihood an ovarian mass is malignant prior to a planned surgery. OVA1, as of the Effective Date, was developed pursuant to the SAA between Vermillion, Inc. and Quest Diagnostics, Inc.
“OVACALC Algorithm” is the algorithm Vermillion provides to Quest Diagnostics for scoring OVA1 tests and Other OVA Tests.
“Third Party” means any person or entity other than Vermillion, Inc., ASPiRA Labs, Quest Diagnostics or any of their respective Affiliates and employees.
“Transition Process” is defined in Section 1.3.3.
20
“Specimen” means, with respect to an OVA1 Test, one Specimen collection tube containing 6.5ml of separated serum to be used to perform one OVA1 test transported in a single Serum Separation Tube (Tiger Top Tube), and with respect to any Other OVA Test, the Parties will agree on an appropriate definition and execute a written amendment to this Agreement pursuant to Section 16.5.
“Vermillion OVA Test Requisition Form” means a test ordering form created by Vermillion and approved in writing by Quest Diagnostics for use by Accounts for each Specimen that is the subject of an OVA1 test or Other OVA Test order. This form shall contain a unique number for each Specimen that will enable the tracking of each Specimen sample and be substantially identical to the form attached hereto as Attachment 5.
21
ATTACHMENT 1 TO
TESTING AND SERVICES AGREEMENT BETWEEN
QUEST DIAGNOSTICS INCORPORATED, VERMILLION, INC. AND ASPIRA LABS
DATED MARCH 11, 2015
LIST OF INITIAL 39 CERTIFIED STATES WHOSE SPECIMENS QUEST DIAGNOSTICS WILL FORWARD TO VERMILLION FOR OVA1 TESTING
|
1. |
Alabama |
16. |
Michigan |
29. |
Oregon |
|
2. |
Alaska |
17. |
Minnesota |
30. |
South Carolina |
|
3. |
Arizona |
18. |
Mississippi |
31. |
South Dakota |
|
4. |
Arkansas |
19. |
Missouri |
32. |
Texas |
|
5. |
Connecticut |
20. |
Montana |
33. |
Utah |
|
6. |
Georgia |
21. |
Nebraska |
34. |
Vermont |
|
7. |
Hawaii |
22. |
Nevada |
35. |
Virginia |
|
8. |
Idaho |
23. |
New Hampshire |
36. |
Washington |
|
9. |
Indiana |
24. |
New Mexico |
37. |
West Virginia |
|
10. |
Iowa |
25. |
North Carolina |
38. |
Wisconsin |
|
11. |
Kansas |
26. |
North Dakota |
39. |
Wyoming |
|
12. |
Kentucky |
27. |
Ohio |
||
|
13. |
Louisiana |
28. |
Oklahoma |
||
|
14. |
Maine |
||||
|
15. |
Massachusetts |
LIST OF 11 STATES WHOSE SPECIMENS QUEST DIAGNOSTICS WILL CONTINUE TO TEST FOR OVA1 UNTIL THEY BECOME CERTIFIED STATES
|
1. |
California |
|
2. |
Colorado |
|
3. |
Florida |
|
4. |
Delaware |
|
5. |
Illinois |
|
6. |
Maryland |
|
7. |
New York |
|
8. |
New Jersey |
|
9. |
Pennsylvania |
|
10. |
Rhode Island |
|
11. |
Tennessee |
22
ATTACHMENT 2 TO
TESTING AND SERVICES AGREEMENT BETWEEN
QUEST DIAGNOSTICS INCORPORATED, VERMILLION, INC. AND ASPIRA LABS
DATED MARCH 11, 2015
Agreed upon cost of services and deliverables to Vermillion/ASPIRA
|
One Time Transition Expenses to be Paid by Vermillion 25-Jan-15 |
||
|
Description |
Final Cost |
|
|
Data Pulls |
customer list for 39 states |
*** |
|
payer list for 39 states |
*** |
|
|
customer list for remaining 11 states to be delivered at time of the applicable transition |
*** |
|
|
payer list for remaining 11 states to be delivered at time of the applicable transition |
*** |
|
|
each updated report for customer lists or payer lists |
*** |
|
|
CARE 360 |
care360 announcement |
*** |
|
care360 field adjustments for PSCs |
*** |
|
|
Total for Care360 (estimates) |
*** |
|
|
TIGER TEAM: 1st year |
5 full time months project manager |
*** |
|
25% FTE for Referral team - 25% |
*** |
|
|
Marketing -10% |
*** |
|
|
Chantilly representative - 10% |
*** |
|
|
customer service - 10% |
*** |
|
|
MML C |
3 MMLC reviews for letters |
*** |
|
Internal training |
Compliance reviews |
*** |
|
PSC training |
*** |
|
|
Accessioning and referrals training |
*** |
|
|
Customer service training |
*** |
|
|
Sales rep training |
*** |
|
|
Medical Director/other training |
*** |
|
|
Uploads to Qforce and other internal dissemination |
*** |
|
1
|
interviews |
One hour interview with Laure Park, launch leader for OVA-1 |
*** |
|
One hour interview with John McLaughlin, CMS health plan coverage |
*** |
|
|
Interviews with other staff involved in OVA-1, not to exceed 5 hours |
*** |
|
|
Total up front |
*** |
|
|
IT accessioning / tracking |
IT fixes to allow trackability for a pass-through - standard sites IT fixes to allow trackability for a pass-through - non-standard sites |
*** |
|
TOTAL COST |
*** |
2
ATTACHMENT 3 TO
TESTING AND SERVICES AGREEMENT BETWEEN
QUEST DIAGNOSTICS INCORPORATED, VERMILLION, INC. AND ASPIRA LABS
DATED MARCH 11, 2015
Business Associate Terms and Conditions
1.AUTHORIZED USES OR DISCLOSURES OF PHI. Quest Diagnostics shall use or disclose PHI only for performing the Services set forth in this Agreement, for its proper management and administration, or as required by law.
2.DEFINITIONS...”HIPAA” means the Health Insurance Portability and Accountability Act of 1996, and any amendments thereto as amended by the Health Information Technology for Economic and Clinical Health Act (the “HITECH Act”). The term “HIPAA Regulations” refers to all of the regulations in effect from time to time issued pursuant to HIPAA and applicable to the privacy or the security of Individually Identifiable Health Information found at Title 45, Code of Federal Regulations (CFR) Parts 160, 162, and 164. “PHI” means protected health information received, transmitted, maintained or created by Quest Diagnostics for or on behalf of Client. “Business Associate” shall generally have the same meaning as the term “business associate” at 45 CFR 160.103. ”Covered Entity” shall generally have the same meaning as the term “covered entity” at 45 CFR 160.103. All other capitalized terms used but not otherwise defined in this Agreement shall have the same meaning as those terms defined in the HIPAA Regulations or any successor law.
3.HIPAA COMPLIANCE. To the extent Quest Diagnostics is acting as a Business Associate of Client in performing the Services, the provisions of this Agreement shall apply, and Quest Diagnostics shall be subject to the penalty provisions of HIPAA as specified in 45 CFR Part 160. To the extent Quest Diagnostics is to carry out an obligation of Client under the HIPAA Regulations, Quest Diagnostics shall comply with the requirements of the HIPAA Regulations that apply to a Covered Entity in the performance of such obligation.
4.DUTIES RELATED TO PHI
a.Use and Disclosure. Quest Diagnostics agrees not to use or disclose any PHI, other than as permitted by this Agreement, for its proper management and administration or as required by applicable law or regulations. Quest Diagnostics may use and disclose PHI as necessary to perform the Services, provided that such uses and disclosures would not violate the HIPAA Regulations if done by Client.
b.Minimum Necessary. Quest Diagnostics shall limit its uses, disclosures and requests for PHI, when practical, to the information making up a Limited Data Set (as set forth at 45 CFR § 164.514), and in all other cases to the minimum necessary PHI to accomplish the intended purpose of the use, disclosure or request.
c.Patient Rights. Quest Diagnostics shall:
i)forward to Client any requests it receives from an Individual where the Individual
1
requests access to the Individual’s PHI held by Quest Diagnostics, which request shall be responded to by Client; and
ii)maintain a record of accountable disclosures of PHI by Quest Diagnostics as required for Client to make an accounting to the Individual as required by the HIPAA Regulations.
d.Access to Books and Records. Quest Diagnostics shall make its internal practices, books, and records relating to the receipt, transmission, creation, maintenance, use and disclosure of an Individual’s PHI available to the Secretary of Health and Human Services (“HHS”) to the extent required for determining compliance with this Agreement and the HIPAA Regulations.
e.Reporting. Quest Diagnostics shall promptly report to Client any use or disclosure of PHI not provided for by this Agreement and any Security Incident (as that term is defined in the HIPAA Regulations) of which Quest Diagnostics becomes aware, including breaches of unsecured PHI as required by § 164.410.
f.Subcontractors. In accordance with 45 CFR §§ 164.308(b)(2) and 164.502(e)(1)(i), in the event Quest Diagnostics contracts with any subcontractor or agent that creates, receives, maintains or transmits PHI on behalf of Quest Diagnostics, Quest Diagnostics shall ensure that such subcontractor or agent agrees in writing to be bound by the same restrictions and conditions that apply to Quest Diagnostics with respect to PHI.
g.Security. Quest Diagnostics shall use appropriate safeguards to prevent the use or disclosure of PHI other than as provided for by this Agreement (“Quest Diagnostics Safeguards”). In addition, Quest Diagnostics agrees to comply with the applicable requirements of 45 CFR Part 164, Subpart C of the HIPAA Regulations with respect to electronic PHI and any guidance issued by the Secretary of HHS.
5.NOTIFICATION IN CASE OF BREACH
a.In the event that Quest Diagnostics discovers that a Breach has occurred, Quest Diagnostics shall promptly notify the Client Privacy Officer, but in no event later than the time required by applicable state law or thirty (30) days, whichever is shorter, after discovering the Breach. Such notifications shall be sent to Privacy Officer, Vermillion, Inc., 12117 Bee Caves Rd., Building III, Suite 100 Austin, TX 78738, telephone: (512) 519-0400. The notification shall include information regarding the nature of the Breach, including a description of what happened, the date of the Breach and the date the Breach was discovered; specific elements of PHI that were subject to the Breach; and identification of each Individual who has been, or is reasonably believed by Quest Diagnostics to have been, affected by the Breach.
b.Quest Diagnostics shall work with Client, promptly and as reasonably required by Client, to identify all individuals whose PHI has been breached, to gather any other information required to be reported under 45 CFR 164.404 and to ensure that the cause giving rise to the Breach has been remediated.
6.CLIENT’S OBLIGATIONS. Client shall notify Quest Diagnostics of: (i) any limitations in its Notice of Privacy Practices (ii) any changes in, or revocation of permission by Individuals to
2
use or disclose PHI, and (iii) any restriction to the use or disclosure of PHI that Client has agreed to, to the extent such actions may affect Quest Diagnostics’ obligations hereunder.
7.TERM AND TERMINATION.
a.This Agreement shall become effective upon signature by the Quest Diagnostics.
b.This Agreement shall terminate when the arrangement for Quest Diagnostics to provide the Services terminates or if Client determines that Quest Diagnostics has violated a material term of this Agreement, or applicable law, that is not cured within thirty (30) calendar days after delivery of notice of the specific violation(s) to Quest Diagnostics. In the event of such a violation, Client, in its sole discretion, may report the breach to the Secretary.
c.Upon termination of this Agreement for any reason, Quest Diagnostics and its subcontractors and agents agree to return or to destroy all PHI and retain no copies unless Quest Diagnostics determines that returning or destroying such PHI is infeasible. If Quest Diagnostics retains PHI due to infeasibility, for as long as Quest Diagnostics retains any PHI, Quest Diagnostics shall extend the protections of this Agreement to the PHI that Quest Diagnostics maintains and shall limit its further use or disclosure of such PHI to those purposes that make return or destruction of the PHI infeasible. If such reasons of infeasibility are removed, Quest Diagnostics agrees to promptly comply with the first part of this paragraph.
8.BENEFIT. This Agreement is not intended to create any right in, or obligations to any person or entity that is not a party to this Agreement, including Individuals and shall not create any agency relationship between the parties.
9.AMENDMENT. This Agreement may only be amended in a writing signed by Quest Diagnostics and Client. Client and Quest Diagnostics agree to amend this Agreement in such manner as is necessary for Client to comply with any amendment of 1) HIPAA or other applicable law, or 2) the HIPAA Regulations, or other applicable regulations. If the parties are unable to agree on an amendment within 30 days of notice from Client to Quest Diagnostics of the requirement to amend the Agreement, Client may, at its option, terminate this Agreement.
10.NO WAIVER. No waiver of any term of this Agreement shall be construed as a waiver of any other term. In addition, no failure to exercise any right or demand performance of any obligation under this Agreement shall be deemed a waiver of such right or obligation.
11.SURVIVAL. To the extent Quest Diagnostics and/or its subcontractors and agents retain any PHI after the termination of this Agreement, Quest Diagnostics’ obligations under Sections 3, 4, 5, 6 and 7 shall survive termination of this Agreement.
12.INTERPRETATION. Any ambiguity in this Agreement shall be resolved in favor of a meaning that permits the parties to comply with the HIPAA Regulations.
3
ATTACHMENT 4 TO
TESTING AND SERVICES AGREEMENT BETWEEN
QUEST DIAGNOSTICS INCORPORATED, VERMILLION, INC. AND ASPIRA LABS
DATED MARCH 11, 2015
MASTER ACCOUNT TRANSFER LIST
[TO BE PROVIDED WITHIN 24 HOURS OF EFFECTIVE DATE]
1
ATTACHMENT 5 TO
TESTING AND SERVICES AGREEMENT BETWEEN
QUEST DIAGNOSTICS INCORPORATED, VERMILLION, INC. AND ASPIRA LABS
DATED MARCH 11, 2015
VERMILLION OVA TEST REQUISITION FORM
1

2

3


ATTACHMENT 6 TO
TESTING AND SERVICES AGREEMENT BETWEEN
QUEST DIAGNOSTICS INCORPORATED, VERMILLION, INC. AND ASPIRA LABS DATED MARCH 11, 2015
CUSTOMER COMMUNICATIONS


[Enter Date]
[Enter MD Name]
[Enter Client Name]
[Enter address]
[Enter City, State & Zip]
Dear [Enter MD Name],
Thank you for being a valued user of OVA1®, the FDA-cleared test used to evaluate an ovarian mass for malignancy prior to planned surgery. We are pleased to inform you that Vermillion Inc., the developer of OVA1®, has established a wholly-owned, specialized women’s health reference laboratory, ASPiRA LABS™. Effective [give date], ASPiRA LABS™ will be providing your OVA1® testing services in concert with Quest Diagnostics.
We have designed the transition to Aspira Labs to minimize the impact of this change on your practice. As you can see in the attached diagram, you will order OVA1 on a paper requisition specific to OVA1. Specimens drawn in your office will continue to be picked up by the Quest Diagnostics driver. You may also send patients with an OVA1 requisition to a Quest Diagnostic Patient Service Center (PSC) for a specimen to be drawn. ASPiRA LABS™ will be delivering the same rapid turn-around and quality results you have come to expect with OVA1®, along with a personalized approach to managing your ovarian mass patients. A sample copy of the ASPiRA LABS™ OVA1® test report is also attached for your review.
If you have any questions or billing concerns, please do not hesitate to call ASPiRA LABS™ customer service at 1.844.277.4721.
Thank you for your loyalty and we look forward to working with you in the future
Sincerely,
Add Electronic Signatures
|
Dr. Kenneth L. Sisco, MD |
Dr. Herbert A. Fritsche, PhD |
|
Laboratory Director |
Laboratory Director |
|
Quest Diagnostics |
ASPiRA LABS |


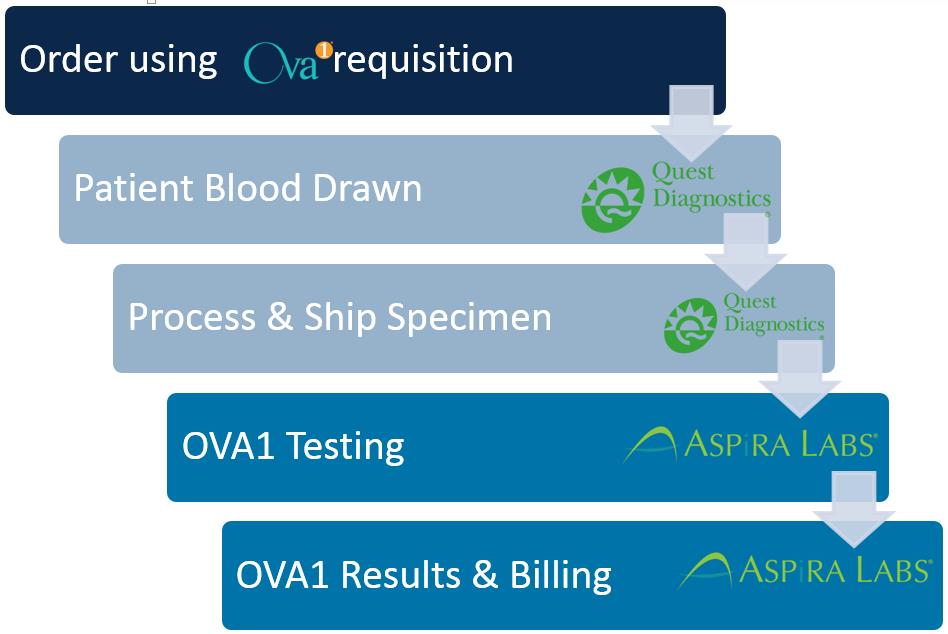
Contact ASPiRA LABS™ (1.844.ASPiRA1, 1.844.277.4721) if you have any questions regarding OVA1 test results or billing inquiries.
Contact Quest Diagnostics (1.866.MYQUEST, 1.866.697.8378) with questions regarding testing supplies or scheduling a specimen draw, pick up, or drop box location.
ATTACHMENT 7 TO
TESTING AND SERVICES AGREEMENT BETWEEN
QUEST DIAGNOSTICS INCORPORATED, VERMILLION, INC. AND ASPIRA LABS
DATED MARCH 11, 2015
NEW ACCOUNT AND PAYER VARIABLES TO THE EXTENT QUEST DIAGNOSTICS CAN RUN COMPUTER GENERATED REPORTS WITH ITS EXISTING SOFTWARE THAT CONTAIN THIS INFORMATION, AND TO THE EXTENT QUEST DIAGNOSTICS CAN PROVIDE SUCH INFORMATION WITHOUT VIOLATING CONTRACTUAL CONFIDENTIALITY OBLIGATIONS. QUEST DIAGNOSTICS IS NOT REQUIRED TO ANYTHING MORE THAN RUN CURRENTLY AVAILABLE COMPUTER GENERATED REPORTS.
Account List Variables
1.Account Name
2.Contact name
3.Account Address on File
4.Account phone and fax
5.NPI# if possible
6.Test Volume per month for the period of the report
7.Billing types (3rd party insurance, Patient, client bill)
8.Payer type per account
9.Payer type per account
10.Test volumes and revenue per account per month during the period of the report
11.Reporting Method (Fax, hard copy, LIS, portal)
12.Multiple physicians at same location, Yes/No, Name/NPI# for each
Payer Variables:
1.Volume by Payer
2.Average Unit Price by payer
3.Denial volume per payer
4.Denial codes per payer
5.Payer volume by State where aggregated
1
