Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - ABEONA THERAPEUTICS INC. | v409962_8k.htm |
Exhibit 99.1

NASDAQ: PTBI Investor Presentation

This presentation contains certain statements that may be forward - looking within the meaning of Section 27 a of the Securities Act of 1933 , as amended, including statements relating to the product portfolio and pipeline and clinical programs of the company, the market opportunities for the Plasma Technologies fractionation technology, MuGard, ProctiGard, and the other mucoadhesive hydrogel products, and the company ’ s goals and objectives . These statements are subject to numerous risks and uncertainties, including but not limited to the risks detailed in the Company’s Annual Report on Form 10 - K for the year ended December 31 , 2014 , and other reports filed by the company with the Securities and Exchange Commission . This presentation does not constitute an offer or invitation for the sale or purchase of securities or to engage in any other transaction with PlasmaTech or its affiliates . The information in this presentation is not targeted at the residents of any particular country or jurisdiction and is not intended for distribution to, or use by, any person in any jurisdiction or country where such distribution or use would be contrary to local laws or regulations . 1 Safe Harbor Statement

2 1 st Generation 2 nd Generation 3 rd Generation Plasma fractionation (ethanol based) • Albumin • Platelets • IVIG New Cell Therapy Technologies 1950 - 1980s New SDF ™ plasma process enables and validates orphan proteins and targets requiring periodic treatments : • A1PI – SDF Alpha • IVIG – SDF Gamma • Ultra - Orphan Proteins 1990 - 2010s Gene therapy enables approaches for single treatment curative therapy : • MPS IIIA • MPS IIIB • Additional Rare/Orphan Disease Programs Future PTBI Strategic Imperative Progression of Cell Therapy to Gene Therapy

3 PlasmaTech & Abeona Merger Building a world class cell & gene therapy company focused on rare diseases MPS IIIA & MPS IIIB Programs ( Abeona ) • AAV - based gene therapies for MPS III & MPS IIIB • Orphan Drug and Pediatric Rare Disease Designations from FDA • Plan to file IND in MPS IIIB in June 2015, first patient in - 4Q15 Alpha - 1 Deficiency & Ultra - Orphan Proteins ( PlasmaTech ) • Proprietary SDF process expands yields significantly relative to Cohn process • Scaling up Alpha - 1 protease inhibitor (A1PI), plan to file IND in 4Q15 • Evaluating process for IVIG and ultra - orphan proteins Overview

4 Abeona Therapeutics Overview Abeona – Roman goddess, protector of children leaving home x Founded in March 2013 x Lead products are licensed gene therapy technology from Nationwide Children’s Hospital (Columbus, OH) x Lead products : AAV9 gene therapy for MPS IIIA & MPS IIIB x Received the FDA Orphan Drug Designation for both MPS IIIA and MPS IIIB clinical programs x Received Pediatric Rare Disease Designations for both programs x Conducted RAC and Pre - IND meetings x Raised $4.8M for MPS IIIA and IIIB programs with leading Sanfilippo Foundations worldwide

5 Sanfilippo Syndromes MPS III x Rare lysosomal storage disease affecting children x Deficiency in one of four cellular enzymes ( MPS IIIA, IIIB, IIIC and IIID ) x Onset between ages 2 and 6; inability to walk by 10. Progressive, severe neurological and muscular disorder x Aggressive behavior, seizures, loss of speech/vision, inability to sleep x Treatments for comparable diseases (MPS I) are $250,000 to $450,000 per year x No FDA approved treatments x Active and supportive Foundations as partners Courtesy: Chester Hembree

6 Scientific Team Nationwide Children’s Scientific Advisors Kevin Flanigan , MD Clinical Investigator Doug McCarty, Ph.D. Scientific Founder Haiyan Fu, Ph.D. Scientific Founder Joseph Muenzer , Ph.D. Univ of North Carolina Maria Escolar, MD University of Pittsburgh Center for Neurodegenerative Disorders Barry Byrne, MD, Ph.D. Univ of Florida Powell Gene Therapy Center Brian Kaspar , Ph.D. Nationwide Children’s and Ohio State

7 Global Foundation Support 2013 Global Genes “Champions of Hope” Award – Co - Recipients USA Canada USA Switzerland USA Spain Mexico Australia USA USA Spain USA

8 Abeona’s Gene Therapy Approach MPS III x AAV9 single injection gene therapies, intravenous delivery for MPS IIIA and MPS IIIB x Lysosomal Storage Diseases • Ideal candidates for gene therapy – Bystander cell uptake • Requires lower levels of delivery to cells for therapeutic benefit • Fast track therapy status decreases time to market x Established delivery system • High safety profile, strong preclinical efficacy observed • Gene therapy drug product crosses blood/brain barrier • Long - term gene expression in the CNS and peripheral tissues • Patients and families very supportive of approach x Market readiness • First approved gene therapy product - ( Glybera ) • Patient driven need – time is critical for kids • No FDA approved therapies available Normal MPS III Ref: Asokan 2012

9 Normalization of Behavior and Muscle Function in 4 - 6 Week Old MPS IIIB Mice after a Single Injection of rAAV9 - CMV - hNAGLU 5.0 - 5.5 months old (4 months post - injection) * # # # * * * * * ^ ^ & * & & 0 10 20 30 40 50 60 70 1 2 3 4 Latency to find platform (sec) Days of testing a. Hidden task in Morris water maze +/+ -/- AAV9-L AAV9-H ^ * ^ * # # 0 50 100 150 200 250 300 350 Time to fall (sec) 1 2 Days of testing b. Latency to fall from a rotarod +/+ - / - AAV9 - L AAV9 - H

10 IV Infusion of rAAV9 - hNAGLU Vector at 4 – 6 Weeks of Age Normalized The Survival in MPS IIIB Mice 0 20 40 60 80 100 120 1 4 7 10 13 16 19 22 25 28 31 Survival (%) Age (months) Survival (rAAV9 - CMV - hNAGLU) +/+ -/- AAV9-L AAV9-H Survival Increased 100% after single IV treatment

11 Critical Abeona Competitive Advantages MPS III x Pre - clinical Effectiveness : Only MPS therapy to demonstrate in cognitive , muscular and survival benefits! x Neurotropism : Only therapy in development that cross blood brain barrier (AAV9) • Single IV injection, compared to multiple injections yearly • No ports needed; no drilling into child’s skull for access • Use of self - complementary technology for MPS IIIA – improved efficacy x Lasting Treatment Effects: Pre - clinical data shows lasting treatment from months to years after delivery x Safety Profile: No significant adverse events to date x Abeona granted Orphan Drug Designation for both AAV9 MPS IIIA and IIIB drugs x Abeona received Rare Pediatric Disease Designations for both MPS IIIA and IIIB drugs x Backing of multiple international Sanfilippo Foundations; science featured on CBS’s 60 Minutes in June 2014
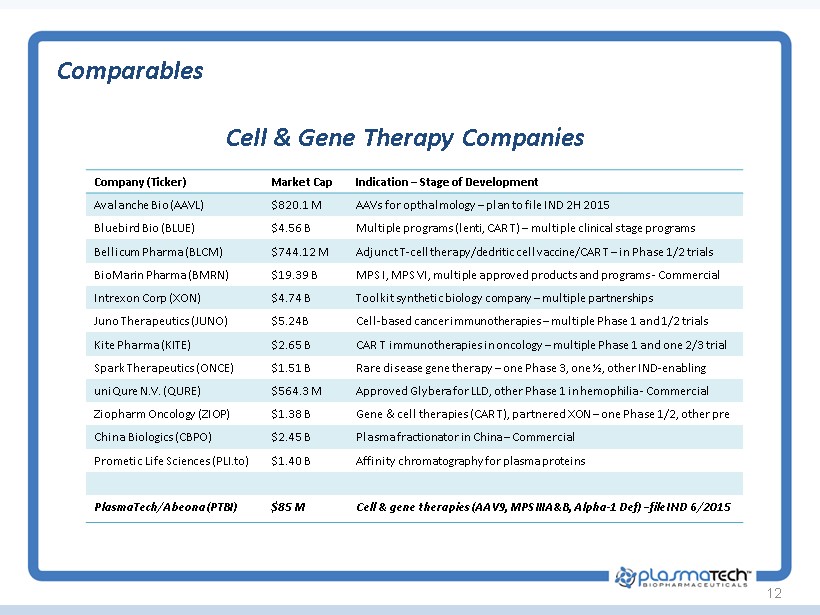
12 Cell & Gene Therapy Companies Comparables Company (Ticker) Market Cap Indication – Stage of Development Avalanche Bio (AAVL) $820.1 M AAVs for opthalmology – plan to file IND 2H 2015 Bluebird Bio (BLUE) $4.56 B Multiple programs ( lenti , CAR T) – multiple clinical stage programs Bellicum Pharma (BLCM) $744.12 M Adjunct T - cell therapy/ dedritic cell vaccine/CAR T – in Phase 1/2 trials BioMarin Pharma (BMRN) $19.39 B MPS I, MPS VI, multiple approved products and programs - Commercial Intrexon Corp (XON) $4.74 B Toolkit synthetic biology company – multiple partnerships Juno Therapeutics (JUNO) $5.24B Cell - based cancer immunotherapies – multiple Phase 1 and 1/2 trials Kite Pharma (KITE) $2.65 B CAR T immunotherapies in oncology – multiple Phase 1 and one 2/3 trial Spark Therapeutics (ONCE) $1.51 B Rare disease gene therapy – one Phase 3, one ½, other IND - enabling uniQure N.V. (QURE) $564.3 M Approved Glybera for LLD, other Phase 1 in hemophilia - Commercial Ziopharm Oncology (ZIOP) $1.38 B Gene & c ell therapies (CAR T), partnered XON – one Phase 1/2, other pre China Biologics (CBPO) $2.45 B Plasma fractionator in China – Commercial Prometic Life Sciences ( PLI.to ) $1.4 0 B Affinity chromatography for plasma proteins PlasmaTech / Abeona (PTBI) $85 M Cell & gene therapies (AAV9, MPS IIIA&B, Alpha - 1 Def ) – file IND 6/2015

13 Alpha - 1 Deficiency – Inherited COPD Alpha - 1 x AATD is an under - diagnosed hereditary condition • Genetic condition whereby insufficient AAT protein produced by liver; leads to COPD and liver disease • Roughly 1 in every 2,500 Americans have AATD; 5 times more prevalent than Cystic Fibrosis • Up to 3% of all people diagnosed with COPD may have undetected AATD x Alpha - 1 Market Drivers • Increased clinician awareness; • Early detection; newborn screening in NY and MA • Grifols ’ SPARTA study (doubling dose); new indications x New Sources of Alpha - 1 Required • Current supply based upon 75 - year old Cohn process • Cohn fractionation does not selectively target Alpha - 1; low yields • Proprietary SDF process targets high - value proteins, increasing yields

SDF Process 14 Kinder, Gentler x Simple 2 - Stage Sodium Citrate Precipitation + Diafiltration x No ethanol or PH changes like Cohn method x Less denaturing of select proteins x Roughly 2 - 3 day process, versus 6 - day for Cohn Yield Improvements x Alpha - 1 yield increase ~10X x IVIG yield increase ~ 20% x Potential for multiple ultra - orphan proteins Margin Improvements x Yield improvements could drive 80% product margins versus ~30% Cohn process margin Proprietary Intellectual Property x Three issued US and WW patents , foreign counterparts pending; additional patent filings Benefits of SDF Process over Cohn

Alpha - 1 15 Alpha - 1 Opportunity x Orphan Drug Characteristics • Up to 300,000 potential patients in US and Europe; only roughly 10,000 on replacement therapy today • A1PI replacement therapy is reimbursed at $100,000 per patient annually • Patients on therapy for 22 years (average) x High value, high growth market • A1PI global market in 2014 was ~$900 million; growing at greater than 20% annually • Current Cohn yields may be insufficient to address future demand x Abbreviated Regulatory Pathway • BLA approval pathway 351(a) – estimated $4 to 6 million per protein to regulatory approval • A1PIs have been approved on short, 50 - patient Phase 1/Pivotal safety and bioequivalency trials • Anticipate SDF Alpha approval in 24 month timeframe x Collaborative Development & Commercialization Strategy • Initiated contract manufacturing relationships; optimizing downstream chromatography, viral inactivation and lyophilization stages of process • Evaluating SDF process for additional ultra - orphan proteins, such as C - 1 esterase inhibitor, fibrinogen and plasminogen • Multiple product opportunities enhance partnering opportunities globally

SDF Gamma 16 Neurology Hematology Dermatology Other x Guillain Barre x Immune thrombo - cytopenia x Kawasaki Syndrome x Primary antibody deficiencies x Lambert Eaton Syndrome x Post BMT x Dermatomyositis x Vasculitis x Multifocal motor neuropathy x Myeloma and CLL x Toxic epidermal necrolysis x Autoimmune uveitis x Myasthenia gravis x Immune neutropenia x Atopic dermatitis x Birdshot retinochoro - idopathy x Stiff person syndrome x Parovirus B19 associated aplasia x Blistering diseases x Mucous membrane pemphigoid x SDF Process increases IVIG yield by ~20 % x ~50% of the $15 billion plasma protein market x IVIG is the high value protein with utility in patients with decreased or abolished antibody production capabilities Intravenous Immunoglobulin (IVIG)

Leaders in Alpha - 1 and Mucositis 17 Eugene Zurlo, BS, MS Pharmacy, Chairman ▪ 56 years experience ▪ Founder/Chairman/ Inventor Plasma Technologies, LLC (Licensor) ▪ Baxter Hyland, Millipore, NY Blood Center, Alpine Biologics, Ayerst Laboratories Charlie Strange, MD ▪ Professor Pulmonary, Critical Care, Allergy, and Sleep Medicine, University S Carolina, Charleston SC ▪ Director: Alpha - 1 Foundation Research Registry ▪ Clinical trial design & rare diseases expert Robert Sandhaus, MD, PhD ▪ Professor of Medicine: National Jewish Health, Denver CO ▪ Clinical Director: Alpha - 1 Foundation ▪ Medical Director, Founder: AlphaNet ▪ Extensive plasma therapeutics industry experience Charles Heldebrant, PhD ▪ Alpha Therapeutic Corporation ▪ Grifols: development, regulatory clearance, production of AAT product (sold to Baxter) ▪ Extensive experience in biological & pharmaceutical product development, regulatory, quality, validation Stephen T. Sonis, DMD, DMSc ▪ Clinical Professor of Oral Medicine, Harvard, Senior Surgeon, Brigham and Women’s Hospital and Dana - Farber Cancer Institute, Founder, CSO Biomodels ▪ Expert in epithelial injury due to cancer therapy ▪ Author >200 original publications, 9 books, 5 patents Allan Louderback, PhD ▪ Head of Biomechanical Research: Baxter Hyland ▪ Founder/President CRO served: Baxter, Dade, Amgen, Biogen, Nichols, Technion, NY Blood Center ▪ Co - inventor Plasma Technologies SDF Process SAB

Hydrogel Polymer Products 18 Two FDA - cleared Commercial Products x Patented mucoadhesive hydrogel delivery system enables extended delivery of drugs to mucosal tissue x MuGard® for Oral Mucositis (“OM”) • $ 1 billion market, underserved, few competing products with demonstrated clinical benefit • > 400 K OM patients annually in the US • Four commercial partners in 5 geographic regions • Commercial launches in Europe, Korea, China to drive royalties in 2015 / 2016 x ProctiGard™ for Rectal Mucositis/Radiation Proctitis • Filed with FDA and received marketing clearance in just 90 days (July 2014 ) • Large market opportunity with no good treatment options • Partnering discussions ongoing

Board of Directors Management Management & Board of Directors 19 Tim Miller, Ph.D. – President & CEO ▪ Extensive scientific, product development and clinical operations experience ▪ Multiple IND submissions ▪ Ph.D. in Pharmacology (Gene Therapy/Cystic Fibrosis), Case Western University Harrison Wehner - CFO ▪ 21 years healthcare & biotech IB, financial advisory, M&A ▪ Senior positions: Canaccord Genuity, CitiGroup, UBS David Nowotnik, Ph.D. - SVP R&D ▪ 41 years experience pharmaceutical R&D, quality systems, regulatory affairs ▪ Bristol - Myers Squibb, Amersham International, Guilford Pharmaceuticals Steven Rouhandeh , Executive Chairman ▪ SCO Capital Partners ▪ Founder SCO Financial Group ▪ Deutsche Bank, Cravath Mark Ahn, Ph.D. ▪ Genentech, Galena Biopharma, Bristol - Myers Squibb, Amgen Mark Alvino ▪ Bradley Woods, Griffin Securities Stephen Howell, M.D. ▪ UCSD, UCSD Cancer Center ▪ Miliken Foundation prize: cancer chemo Jeffrey Davis , COO & Director ▪ 20+ years, CEO & CFO roles ▪ Bell Laboratories, Philips Medical Systems ▪ Deutsche Bank Stephen Thompson – VP Finance, Treasurer, Sec ▪ 26 years financing and accounting ▪ Prior CFO and Controller experience

Recent events: • Does not include 3,979,761 common shares to be issued to Abeona upon closing • Does not include 577,756 “old” warrants outstanding with WAEP of $46.50 • No long term debt, no convertible preferred stock Capitalization Table 20 Capitalization Shares Outstanding WAEP Outstanding common shares (PTBI) 22,332,135 - Warrants (PTBIW, fully traded) 3,500,000 $5.00 Options 233,834 $23.60 Primary Total 22,332,135 Fully Diluted Total 26,065,969

Milestones 21 Milestones Timing Finalize Abeona acquisition May 2015 File IND for MPS IIIB June/July 2015 First Patient In, MPS IIIB in USA Sept./Oct 2015 Complete and file IND for MPS IIIA 2H 2015 First Patient in, MPS IIIB in Europe, Australia 1H 2016 In - license Complementary Gene Therapy Programs 2015 - 16 SDF Alpha™ validation, characterization 2015 SDF Alpha™ clinical study and BLA filing 2016 SDF Alpha™ regulatory approval and revenue 2016 - 17 Follow - on plasma product targets: ultra - orphan proteins, and additional hydrogel platform products 2016 - 17+

Investment Highlights 22 PlasmaTech & Abeona Merger Building a world class cell therapy company focused on rare diseases MPS IIIA & MPS IIIB Programs ( Abeona ) • AAV9 gene therapies for MPS III & MPS IIIB • Orphan Drug and Pediatric Rare Disease Designations from FDA • Plan to file IND in MPS IIIB in June 2015, first patient in - 4Q15 Alpha - 1 Deficiency & Ultra - Orphan Proteins ( PlasmaTech ) • Proprietary SDF process expands yields significantly relative to Cohn process • Scaling up Alpha - 1 protease inhibitor (A1PI), plan to file IND in 4Q15 • Evaluating process for IVIG and ultra - orphan proteins


24 Addendum 1 Abeona Pre - Clinical Data

25 Clearance of Lysosomal Storage Pathology in the Liver of MPS IIIB Mice after an IV rAAV9.NAGLU Infusion 6 months post - injection . The red arrows indicate the nuclei of liver cells . Note the decrease in “storage lesions” or holes in the cells from the liver of a treated animal on the left . This data demonstrates that an IV injection of AAV 9 can get into the liver, introduce a functioning copy of the gene that is altered in Sanfilippo syndrome and allow lysosomes to function appropriately .

1.5E13 vg/kg 26 IV Delivery of rAAV9.CMV.NAGLU Induces Clearance of Lysosomal GAG Storage in the CNS and Somatic Tissues 6 months post - injection Lower bars are better. Demonstrates that treated IIIB mice have reduced GAG content, similar to unaffected animals, in multiple tissues compared to untreated IIIB mice (with exception of kidney).

27 IV Delivered AAV9 - CMV - NAGLU Corrects Secondary Neuropathology in MPS IIIB Mice: Astrocytosis and Neurodegeneration Cerebral cortex Thalamus Striatum No treatment ( 7 month) AAV 9 treated (End) * * * * 0 20 40 60 80 100 No. astrocytes CTX ST TH BS a. MPS IIIB MPS IIIB +AAV9 * 0 5 10 15 No. cells/200 μ m Purkinje cells b. MPS IIIB MPS IIIB +AAV9 Shorter bars are better. Indicates less trauma and destruction of neurons. Longer bars are better. Indicates more neurons and less degeneration 27

28 scAAV9 Delivered by IV Demonstrates Systemic Transduction in Mice Self - complementary AAV9 virus delivered intravenously to mice. The AAV9 is delivering a gene to make the mouse glow where the AAV9 got into cells, referred to as “expression”. This demonstrates that by 7 days post - injection, the AAV9 vector administered by IV injection has been incorporated into cells all over the body and is “expressing” the gene of interest. Ref: Asokan 2012

29 IV Delivery of rAAV9.CMV.NAGLU Induces Clearance of Lysosomal GAG Storage in the CNS and Somatic Tissues rAAV9 - mediated rNAGLU expression in MPS IIIB mouse brain Vector dose (vg/kg) AOI NAGLU activity (% of wt levels) 1mo pi 3mo pi 6mo pi 12mo pi End 1x10 13 4 - 6wk 60 - 560% 80 - 440% 60 - 312% 44 - 159% 268 - 721% 1x10 13 4 - 6mo n/a n/a n/a 0 - 40%* - 2x10 13 4 - 6wk 40 - 440% 50 - 674% 40 - 440% 55 - 419% 377 - 621% 2x10 13 4 - 6mo n/a n/a n/a n/a 560 - 1,549% x NAGLU expression at or above heterozygote (and often above wild - type) x Comparison of lifetime exposure at lower levels (as in heterozygotes) versus treated patients may not be relevant – because CNS and somatic tissues have abundant storage of GAGs that have to be cleared

30 MPS IIIB – 6 Month Toxicology Study in Non - Human Primates IV delivery of rAAV9-CMV-hNAGLU in NHP NHP Age (y) *AAV9 Abs (titer) **IS Vector dose (vg/kg) Termination (pi) Group 1 NT1 10.3 1:64 - Saline 6wk S1 10.5 1:32 - 1E13 6wk S2 11.0 Neg - 1E13 3mo Group 2 NT-2 2.3 Neg - Saline 3mo M1 2.3 Neg - 2E13 3mo M2 2.1 Neg - 2E13 3mo M3 2.0 1:16 + 2E13 3mo M4 1.9 1:4 - 2E13 6mo M5 1.8 1:32 - 2E13 6mo M6 2.3 1:1000 + 2E13 6mo *: Pre-existing Abs; **IS: immunosuppression with prednisolone. x Study Complete x Single Intravenous Injection of rAAV9.CMV.Naglu x Standard clinical and toxicology assessments x No Adverse events observed x No specific abnormalities in blood chemistry, hematology or histology
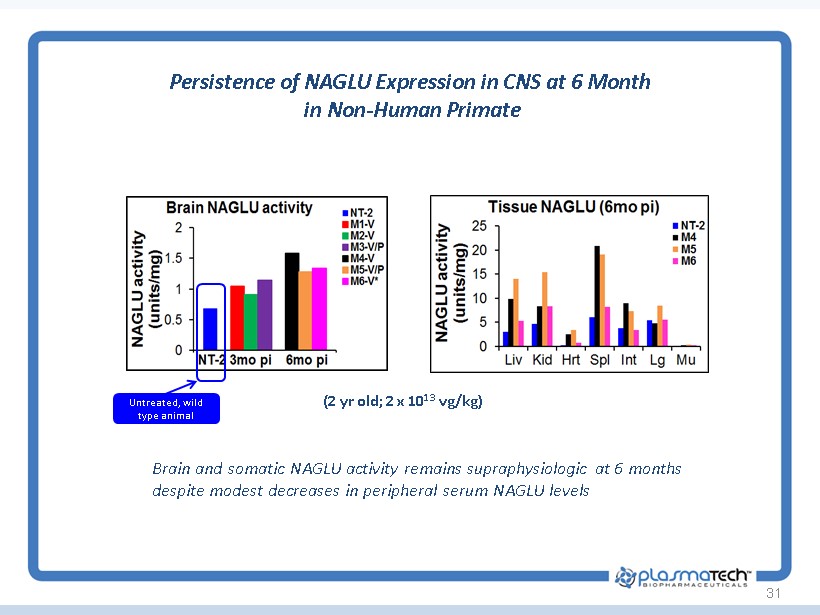
31 Persistence of NAGLU Expression in CNS at 6 Month in Non - Human Primate (2 yr old; 2 x 10 13 vg/kg) Brain and somatic NAGLU activity remains supraphysiologic at 6 months despite modest decreases in peripheral serum NAGLU levels Untreated, wild type animal

32 No Evidence for Autoimmune Responses in Non - Human Primates x Antibody responses to human NAGLU was observed in NHP However : • No evidence for autoimmune pathology in any tissues at 6 months post - injection • MPSIIIB mice – null for NAGLU – did not show evidence for clearance of transduced cells over two years despite Ab response • No CTL responses by ELISpot in NHPs

33 Systemic Delivery of scAAV9 is Broadly Distributed in the CNS of Non - Human Primates A dose of scAAV9 - CBA - GFP was injected into 3 year old cynomolgous macaque via saphenous vein. GFP expression in the brain was assayed at 3 weeks post injection by immunohistochemistry in 40 um coronal sections. Image from Foust and Kaspar , submitted Red/brown staining = expression of scAAV9 transgene

34 Intravenous Delivery of scAAV9 - U1a - SGSH Increases SGSH Activity In MPS IIIA Treated Mice 8 Months Post - Treatment MPS IIIA mice were treated at the indicated ages ( ie - “ 3 mo ” indicates a 3 month old IIIA mouse treated with an IV dose of scAAV - U 1 a - SGSH) and the indicated tissues were tested for SGSH enzyme activity at 8 months age . WT = unaffected mice . Lower bars are indicative of normalizing SGSH activity similar to unaffected mice . Data demonstrated that AAV 9 - SGSH delivered by IV are able to normalize SGSH activity in most of these tissues .

35 IV scAAV9 - U1s - SGSH Reduces GAG Content in CNS and Peripheral Organs > 9 months 0 0.5 1 1.5 2 2.5 3 3.5 4 Brain Hrt Lung Mus GAG contents ( μ g/mg tissue) Tissue GAG (endpoint) WT NT 1mo 2mo 3mo 6mo 0 1 2 3 4 5 6 7 8 LIV Kid Spl Int GAG contents ( μ g/mg tissue) Tissue GAG (endoint) WT NT 1mo 2mo 3mo 6mo Mice were treated at the indicated ages (1 - 6 mo ) with scAAV9 - U1a - SGSH vector and the indicated tissues were tested for GAG content at >9 mo age. (n=3 - 6/group). Data demonstrate that IV delivery of AAV9 vector is able to functionally normalize the GAG content in these tissues months after injection. WT = unaffected mice; NT = MPS IIIA mice that did not receive treatment.

36 IV Delivery of scAAV9 - U1a - SGSH Corrects Behavioral Deficiency in MPS IIIA Mice with Beneficial Effects lasting 1 Year Post - Treatment ^ ^ * * ^ # @ @ # ^ * ^ 15 25 35 45 55 65 1 2 3 4 Latency (sec.) Days of testing Task Acquisition (hidden ) wt IIIA 1mo 2mo 3mo 6mo # # # @ * * * * 15 25 35 45 55 65 75 1 2 3 4 Latency (sec.) Days of testing Task acquisition (hidden) 6 mo treated, re - test at 12 mo WT IIIA 5E12 A B AAV9 MPS IIIA mice and wt littermates were tested in the Morris water maze at 7.5 mo age for ability to find a hidden platform. Mice were treated with vector at 1 - 6 mo age (A). Mice treated at 6 mo age were re - tested at 12 mo age (B).

37 IV Delivery of scAAV9 - UA1 - SGSH Increases Survival of MPS IIIA Mice Groups of MPS IIIA mice were treated at 1 mo age with different doses (A), or at different ages with vector (B) of scAAV9 - U1a - SGSH vector by IV injection. Life spans were compared to wt or untreated MPS IIIA mice. A B

38 Preclinical Data Summary – IV Delivery of AAV9 Vectors For Treatment of MPS IIIA and MPS IIIB • MPS III mice were treated at ages 1mo, 2mo, 3mo, 6mo and 9mo were treated with an intravenous injection of AAV9 vector carrying correct version of gene. • The treatment led to the restoration of MPS III enzyme activity and reduction of GAG content to normal levels throughout the CNS and in somatic tissues in all treated groups. • Treatment of MPS III Mice 1 - 6 months of age resulted in significant improvement in: x Cognitive performance in finding a hidden task in Morris water maze x Neuromuscular function (swimming ability) x Survival • Results were diminished in animals treated at 9 mo of age. • All preclinical studies (MPS IIIB mice, non - human primates, wt mice) for MPS IIIB are complete • Final MPS IIIA GLP toxicology study in unaffected mice initiated x FDA will allow IND submission after 6 week data point

39 MPS IIIA and MPS IIIB Natural History Study • Study Site – Nationwide Children’s Hospital, Ohio, USA • Enrollment complete: 25 subjects, 15 MPS IIIA and 10 MPS IIIB • FDA requested Natural History study to support clinical endpoints • Study visits – assessments at Months 0, 6, and 12: x Neurocognitive ( Leiter ) and parental rating assessments (ABAS II) x Timed functional motor tests x Standard laboratory assessments x Serum/leukocyte NAGLU or SGSH activity x Quality of life ( PedsQL ) x Urine GAG levels x Overnight sleep actigraphy • Study visits – assessments at Months 0 and 12: x Brain MRI (including DTI and 1 H spectroscopy) x CSF for standard chemistries/cell counts and NAGLU or SGSH activity • 8 subjects through 6 month follow up appointments All 25 patients will be tested for AAV9 neutralizing antibodies

Contacts Jeff Davis Chief Operating Officer Phone : 212.786.6201 Email : jdavis@plasmatechbio.com Andre’a Lucca Director of Communications Phone : 212.786.6208 Email : alucca@plasmatechbio.com 40
