Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Inspyr Therapeutics, Inc. | v408639_8k.htm |
| EX-99.02 - EXHIBIT 99.02 - Inspyr Therapeutics, Inc. | v408639_ex99-02.htm |
Exhibit 99.01

1 Investor Presentation April 2015 OTC QB : GNSZ Craig A. Dionne, PhD President & CEO

2 Safe Harbor Statement Safe Harbor Statement Under the Private Securities Litigation Reform Act of 1995: Any statements that are not historical facts are forward - looking statements that involve risks and uncertainties that could cause actual results to differ materially from those in the forward - looking statements, which may include, but are not limited to, factors related to GenSpera's anticipated growth strategies, the outcome of its clinical trials, future business development, ability to develop new products, expand to other related industries or markets in other geographical locations, and other information detailed from time to time in the Company's filings and future filings made with the United States Securities and Exchange Commission. Readers are advised that this information is intended for the use of investment professionals. Anyone interested in obtaining information on GenSpera should contact GenSpera directly. This presentation was developed by GenSpera and is intended solely for informational purposes and is not to be construed as an offer to sell or the solicitation of an offer to buy the Company's stock. This presentation is based upon information available to the public, as well as other information from sources which management believes to be reliable, but is not guaranteed by the Company as being accurate nor does it purport to be complete. Opinions expressed herein are those of management as of the date of the presentation and are subject to change without notice.

3 • An innovation driven biotech company that unlocks conventional thinking • Deep experience in cancer drug discovery and development • A leader in prodrug therapeutics for the treatment of cancer • Robust global IP platform • Global barrier to generic entry via exclusive manufacturing and farming controls of raw goods • World class strategic partners • 2015 is tipping point for value creation GenSpera Profile

4 Milestones + Future Opportunity Identified 2003 Our Journey Begins POC in Animals 2006 First Financing 2007 IND Approved (10 weeks) 2009 Phase I Trial Begins 2010 Phase II HCC Trial 2013 Global Partnership Outreach 2015 / 2016 Global Mipsagargin Development 2015 / 2017 Next Generation GenSpera 2018 GenSpera 2015 - 2017 Entertain Partnerships Expand Trials Innovation - Driven Platform To Exploit Technologies Proof of Concept Validated 1/15/2015 Phase II Trials 2015 Prostate Brain Kidney

5 Current Financials Quick Facts Symbol: GNSZ Current Share Price (As of 4/3/2015): $0.82 Shares Outstanding (As of 12/31/2014): 33.2 million Diluted Shares Outstanding (As of 12/31/2014): 61.8 million Market Cap: $27.2 million 52 - Week Trading Range : $0.54 - $1.30 Cash (As of 12/31/2014 ) : $2.3 million Total Assets (As of 12/31/2014): $2.6 million

6 • World Class pedigree - technology developed at John Hopkins University and the University of Copenhagen • Our patents provide REAL value • Future company acquisition/partnerships • Expansive by design – much broader than mipsagargin alone • Development of commercial scale thapsigargin production is a barrier to generic intrusion • Current portfolio • Patent coverage in U.S. to 2023 • Orphan Drug Designation and patent restoration add significant coverage • Data exclusivity outside of U.S. - up to 10 years after drug approval in EU • Portfolio expansion strategy • New composition of matter PCT - Worldwide exclusivity expected through 2033 • Other applications filed for methods of use, formulations, process improvements Intellectual Property

7 • Unique, naturally derived, cancer killing drug has advantages in potency and significantly reduced side - effects • Mipsagargin , GenSpera’s lead candidate, is showing excellent clinical results • Outstanding clinical safety profile • Data strongly suggestive of clinical activity in HCC • Positive proof of concept in patients at level of tumor physiology • Will be evaluated in 4 different cancer indications by Q3 2015 • Multiple development opportunities based on our prodrug platform Clinical Activity

8 Initial Market Focus Tremendous unmet needs in numerous types of cancer Mipsagargin attacks a universal attribute of all solid tumors Liver Cancer • 3rd largest cancer - killer worldwide • $10 billion by 2020 Brain Cancer • 13,000 deaths annually in the U.S. • Avastin expected to generate $460 million in revenue by 2017 Prostate Cancer • 240,000 new cases diagnosed annually in the U.S. • $6.7 billion by 2020 Kidney Cancer • 64,000 new cases diagnosed annually in the U.S. • 14,000 deaths annually in the U.S .

9 Mechanism of Action 1. Blood vessels feed living tumor 2. Drug circulates in bloodstream in benign fashion 3. Enzyme within tumor blood vessels activates drug 4. Activated drug kills blood vessels and tumor cells 5. Death of t umor As a result: • Potential for Complete Tumor Kill • Fewer Side Effects Precision Targeting by Design

10 Impressive clinical activity • Highly differentiated from other anti - cancer approaches • Outstanding clinical safety profile • Minimal and manageable side effects • Fatigue, nausea, rash, reversible creatinine increase • No apparent effect on bone marrow • Data strongly suggestive of clinical activity in HCC • Prolonged disease stabilization in a significant percentage of patients • TTP=4.2 months • 35 % with stable disease > 7 months • Will be evaluated in 4 different cancer indications by Q3 2015 Mipsagargin Phase II Results
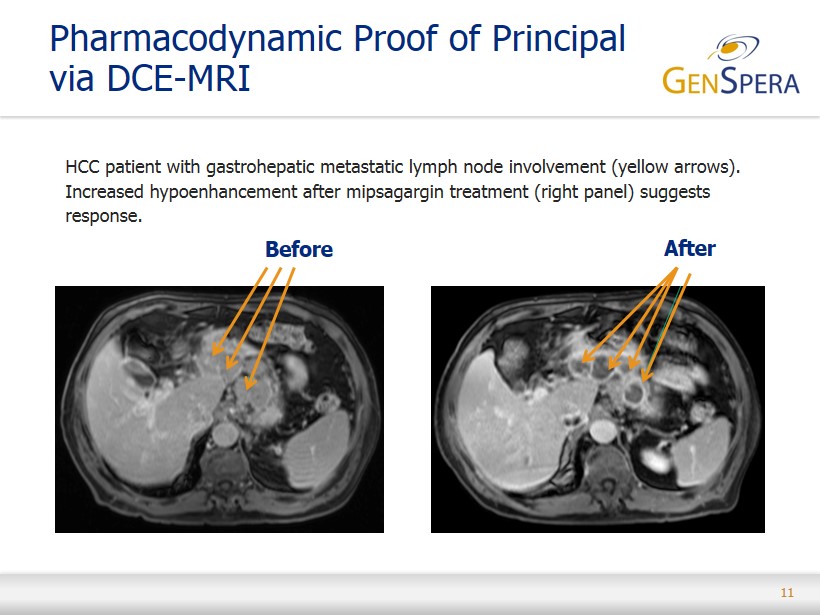
11 HCC patient with gastrohepatic metastatic lymph node involvement (yellow arrows). Increased hypoenhancement after mipsagargin treatment (right panel) suggests response. Pharmacodynamic Proof of Principal via DCE - MRI Before After

12 GenSpera Clinical Programs – Evaluating Multiple Oncology Indications RESEARCH PRE - CLINICAL PHASE 1 PHASE 2 Liver Cancer (HCC) TARGET SITE DRUG INDICATION Brain Cancer (GBM) Prostate Cancer (PCa) Mipsagargin Blood vessels of most solid tumors DEVELOPMENT STAGE HUMAN CLINICAL TRIALS PHASE 3 25 patients treated in Ph2 study at 5 sites – Study Closed – Results presented 12 patients enrolled in Ph2 study at UCSD* Ph2 to begin Q2 2015 at UTHSC Houston* * Ph2 costs primarily supported by investigator funds Kidney Cancer (RCC) Ph2 to begin Q3 2015 at UTHSC Houston* 2015 Partner Outreach • Three Phase II trials ongoing 2015 • Data with IP = Increased corporate valuation • Provides expanded options for partnering • Global market = partnership opportunities driven by disease type

13 Utilize world class strategic partnerships for global development of platform technology • Expanded global clinical trials – heavy focus Asia/ China - Japan • Manufacture at commercial scale • Collaboration with, and adoption by, leading physicians, scientists and institutions worldwide • Seek strategic Pharma /Biotech relationships Partner Strategy

14 Experienced Scientific and Development Team Management, Directors, Advisors Craig A. Dionne, PhD Chairman & CEO Over 25 years of experience in pharmaceutical industry, SVP Drug Discovery at Cephalon, Inc. (acquired by Teva Pharmaceuticals), EVP Prostate Cancer Foundation Russell Richerson, PhD COO Over 25 years of experience in biotechnology industry, Previously COO Molecular Profiling Institute, VP Diagnostic R&D Prometheus, Abbott Labs Peter E. Grebow, PhD Director Over 37 years of experience in pharmaceutical industry, EVP Drug Development Cephalon, Inc., Board member Optimer Pharmaceuticals, Q Holdings, Complexa. Bo Jesper Hansen, MD, PhD Director Executive Chairman of Swedish Orphan Biovitrum AB, Board member CMC AB, Orphazyme ApS, Newron, Onxeo SAS, Hyperion Therapeutics and Ablynx NV Scott Ogilvie Director President and CEO AFIN International, Inc., Board member Research Solutions, Inc., and Neuralstem, Inc. Søren Brøgger Christensen, PhD Scientific Advisor Professor at University of Copenhagen, Expert in chemistry of thapsigargin, Exploring further derivatives and manufacturing efficiencies for thapsigargin Samuel R. Denmeade, MD Chief Clinical Advisor Professor at Johns Hopkins and Board Certified Medical Oncologist, Co - Inventor of GenSpera’s technologies John T. Isaacs, PhD Chief Scientific Advisor Professor at Johns Hopkins School of Medicine, Co - Inventor of GenSpera’s technologies Melanie Thomas, MD, MS Medical Advisor Medical Director of Clinical Research, Gibbs Cancer Center and Research Institute, Principal Investigator on 29 separate clinical trials, primarily in gastrointestinal cancers.

15 GenSpera operates by hiring the best talent anywhere in the world. Beyond science and clinical expertise this also includes business and operational expertise. • North American / European Union /Asia Pacific – Mergers and Acquisition, product development partnerships, regulatory expertise and financial structures. • World class Brand building strategist across multi - channel communication protocols • Specialist in Healthcare public relations specific to oncology and FDA guidelines • Board of Directors who have bought/sold/ partnered biotech companies, products and clinical stage development assets • Worldwide intellectual property prosecution and strategy Specialist by Design

16 Proof of Concept/Data create future value • Shareholders • Company options 2015 Tipping Point for growth • Additional Phase II studies • Design next liver cancer studies • World Class Partnership outreach Undervalued • $ 27.2mm Market Cap, comparable between $200mm and $600mm • Share price at $ 0.82 vs $3.00 or higher GenSpera in the News • Public Relations • Social Media Channels • 3rd Party Endorsements Summary Recent Events March 30, 2015 – GenSpera Launches National Investor Relations Program March 17, 2015 – GenSpera presents at BIO Asia International Conference February 9, 2015 – GenSpera Announces Partnership with PHYTON Biotech January 16, 2015 – ASCO GI Conference Poster Presentation – Proof of Concept January 8, 2015 – GenSpera Announces New Digital Channels for Corporate Communications October 16, 2014 – Melanie Thomas MD, MS, will serve Company as Medical Advisor October 9, 2014 – Completion of Enrollment in Mipsagargin Phase II Liver Cancer Trial

17 GenSpera, Inc. ( OTC QB: GNSZ) 2511 N Loop 1604 W, Suite 204 San Antonio, TX 78258 Craig Dionne, PhD President, CEO and Chairman (210) 479 - 8112 info@genspera.com Investor Relations Jeff Ramson PCG Advisory Group (646) 863 - 6893 Jramson@pcgadvisory.com Connect with GenSpera https://www.facebook.com/GenSpera https://twitter.com/GenSperaNews https://www.linkedin.com/company/genspera - inc - https://plus.google.com/u/0/b/111021404 725787491742/ 111021404725787491742 / posts https://www.youtube.com/ channel/UCdvATHFsc6Z LVgVMTJtPq3A http://www.thechairmansblog.com/genspera
