Attached files
| file | filename |
|---|---|
| 10-Q - 10-Q - SANGAMO THERAPEUTICS, INC | sgmo-10q_20150331.htm |
| EX-31.2 - EX-31.2 - SANGAMO THERAPEUTICS, INC | sgmo-ex312_201503316.htm |
| EX-31.1 - EX-31.1 - SANGAMO THERAPEUTICS, INC | sgmo-ex311_201503319.htm |
| EX-32.1 - EX-32.1 - SANGAMO THERAPEUTICS, INC | sgmo-ex321_2015033110.htm |
| EX-10.1 - EX-10.1 - SANGAMO THERAPEUTICS, INC | sgmo-ex101_20150331717.htm |
| EXCEL - IDEA: XBRL DOCUMENT - SANGAMO THERAPEUTICS, INC | Financial_Report.xls |
Exhibit 10.2
FIRST AMENDMENT TO
RESEARCH, DEVLEOPMENT AND COMMERCIALIZATION AGREEMENT
BETYWEEN SANGAMO BIOSCIENCES, INC. (“SANGAMO”) AND JUVENILE
DIABETES RESEARCH FOUNDATION INTERNATIONAL (“JDRF”)
This First Amendment (this “First Amendment”) to the Agreement of October 24, 2006 is made as of this 8th day of January 2010 (the “First Amendment Effective Date”) by and between Sangamo and JDRF. Capitalized terms used but not defined herein shall have the definition provided in this Agreement.
WHEREAS, the Parties entered into the Agreement; and
WHEREAS, the Parties now desire to amend the Agreement so that JDRF may support Sangamo’s SB-509-901 clinical trial which is entitled “A Phase 2b Repeat Dosing Clinical Trial of SB-509 in Subjects with Moderately Severe Diabetic Neuropathy”.
NOW THEREFORE, in consideration of the foregoing, the receipt and sufficiency of which is hereby acknowledged, the Parties agree as follows:
|
1. |
Confirmation of Terms. |
Except as provided in this First Amendment, the Agreement shall remain in full force and effect.
|
2. |
Acknowledgement. |
The Parties hereby acknowledge that, prior to the First Amendment Effective Date, Sangamo fulfilled its obligations under the Agreement to perform the Research Plan as described in Exhibit A and to achieve the Milestones set forth in Exhibit B as it existed prior to the First Amendment Effective Date, and JDRF fulfilled its obligations under the Agreement to pay to Sangamo the amounts set forth in Exhibit B as it existed prior to the First Amendment Effective Date.
|
3. |
Amendment to Agreement. |
The following amendments shall be made to the Agreement:
(a) The term “Award” as defined in Section 1.5 of the Agreement shall be amended to substitute “Six Million Dollars ($6,000,000”)” in lieu of “Three Million Dollars ($3,000,000)” previously specified therein:
(b) The definiton of “Research Plan” as set forth in Section 1.54 of the Agreement shall be replaced with the following: “Research Plan” means (a) the written protocol for Sangamo’s Phase II Repeat Dosing Clinical Trial of SB-509, that shall be attached to this Agreement as Exhibit A upon approval by the FDA, which protocol was based on the Application, includes the JDRF Studies, and has been accepted ’y the FDA, as modified from time to time by Sangamo in consultation with the FDA and in accordance with Section 2.4 and (b) the written protocol for Sangamo’s SB-509-901 clinical trial that is attached to the First Amendment as Exhibit C, which Exhibit will be updated to reflect any amendments to such protocol that are agreed upon by Sangamo and the FDA.”
(c) Section 3.1.2(a) shall be amended by increasing “Three Million Dollars ($3,000,000)” to “Six Million Dollars ($6,000,000)”;
(d) Section 7. 7 (Insurance) of the Agreement is hereby deleted in its entirety and is replaced with the following provision:
Insurance. Sangamo shall maintain at its own expense, with a reputable insurance carrier reasonably acceptable to JDRF, coverage for Sangamo, its Affiliates, and their respective employees written on a per occurrence basis commensurate with a reasonable assessment of the risks associated with the research efforts being conducted by Sangamo, the following policies:
(a) Comprehensive general liability insurance for claims relating to the performance and lack of performance of Sangamo’s obligations under this Agreement;
(b) Comprehensive general liability insurance for claims for damages, including, damages as a result of bodily injury (including death) and damages to property, arising out of acts or omissions of a Sangamo Party;
1
(c) Products liability insurance for claims for damages, including, damages as a result of bodily injury (including death) and damages to property, arising out of the acts or omissions of a Sangamo Party; and
(d) Clinical trials liability insurance for damages, including, damages as a result of bodily injury (including death) and damages to property, arising out of any clinical trials conducted by Sangamo in connection with its obligations under the Agreement, or arising out of the acts or omissions of a Sangamo Party. This insurance shall specifically include coverage for obligations and liabilities of Sangamo under any clinical trial agreement or protocol that is a part of clinical trials conducted by Sangamo under the Agreement and for liability arising as a result of allegations of the insufficiency or other defects in the informed consent provided to participants in the clinical trials.
All insurance policies required hereunder shall name JDRF as an additional insured, be specifically endorsed to cover Sangamo’s indemnification obligations under this Article VII and be written with coverage limits approved by JDRF. Maintenance of such insurance coverage will not relieve Sangamo of any responsibility under this Agreement for damage in excess of insurance limits or otherwise. On or prior to the First Amendment Effective Date, Sangamo shall provide JDRF with an insurance certificate from the insurer(s) evidencing each insurance coverage and the insurer’s agreement to notify JDRF at least sixty (60) days in advance of any cancellation or material modification of such insurance coverage. At its request, JDRF may review Sangamo’s insurance coverage with relevant Sangamo officials from time to time.
In the event that the lnterruption License becomes effective pursuant to Section 9.5, JDRF shall comply with the foregoing insurance requirements and shall maintain such insurance for as long as necessary to cover any claims that may arise from JDRF’s activities during the effectiveness of the Interruption License.
(e) Section 11 .10 shall be amended to replace the notice address for JDRF with the following:
Richard Insel, MD
Chief Scientific Officer
Executive Vice President, Research
Juvenile Diabetes Research Foundation International
26 Broadway, 14th Floor
New York, NY 10004
Tel.: 212-479-7604
Email: rinsel@ jdrf.org
(f) Exhibit B shall be replaced with the Exhibit B attached to this First Amendment; and
(g) Exhibit C attached to this First Amendment shall be added to the Agreement.
[Signatures on next page]
2
IN WITNESS WHEREOF, the undersigned have executed this First Amendment as of the First Amendment Effective Date.
|
Sangamo BioSciences, Inc. |
|
|
By: |
/s/ Edward O. Lanphier |
|
Name: |
Edward O. Lanphier |
|
Title: |
President and Chief Executive Officer |
|
Juvenile Diabetes Research Foundation International |
|
|
By: |
/s/ Alan J. Lewis, Ph.D. |
|
Name: |
Alan J. Lewis, Ph.D. |
|
Title: |
President and Chief Executive Officer |
3
EXHIBIT B
RESEARCH FUNDING AND MILESTONES
I. Payment Schedule for Research Funding: Up to an aggregate amount of Six Million Dollars ($6,000,000), payable as follows:
|
Payment Amount ($) |
|
|
1. Upon the Effective Date, if FDA acceptance of Phase 2 plan (102 patients in two treatment groups) has occurred; or upon FDA acceptance of such Phase 2 plan, if such acceptance has not yet occurred as of the Effective Date |
$500,000 |
|
2. Enrollment of the first Qualified Subject |
$500,000 |
|
3. Enrollment of the 25th Qualified Subject |
$500,000 |
|
4. Enrollment of the 50th Qualified Subject |
$500,000 |
|
5. Enrollment of the 100th Qualified Subject |
$500,000 |
|
6. Receipt by JDRF of the final report of the Primary Statistical Analysis |
$500,000 |
|
7. Initiation of patient screening for SB-509-901 |
$250,000 |
|
8. First patient for SB-509-901 randomized by assignment of randomization number |
$250,000 |
|
9. 76th patient, or 50% of the then planned number of patients for SB-509-901, whichever occurs first, randomized by assignment of randomization number |
$500,000 |
|
10. 150th patient, or the last planned patient for SB-509-901, whichever occurs first, randomized by assignment of randomization number |
$500,000 |
|
11. Submission to JDRF of day 180 data analysis for SB-509-901 |
$500,000 |
|
12. Submission to JDRF of day 360 data analysis for SB-509-901 |
$500,000 |
|
13. Submission to JDRF of draft clinical study report for SB-509-901 |
$500,000 |
II Payments pursuant to this Exhibit B shall be paid by JDRF to Sangamo within forty-five (45) days following JDRF’s receipt from Sangamo of a written certification setting forth Sangamo’s achievement of the applicable Milestone unless the JRAC determines, in a meeting held within such forty-five (45) day period, that such Milestone was not achieved. If JRAC’s vote on such matter during such meeting is not unanimous, then JDRF shall make the applicable payment within forty-five (45) days following resolution of such dispute in Sangamo’s favor.
|
Sangamo BioSciences, Inc. |
|
|
Protocol SB-509-0901 for Diabetic Neuropathy
|
Confidential
|
Clinical Study Protocol
A Phase 2b Repeat Dosing Clinical Trial
of SB-509 in Subjects with Moderately
Severe Diabetic Neuropathy
|
|
Protocol Number |
|
SB-509-0901 |
|
|
|
|
|
|
|
|
|
BB-IND |
|
12189 |
|
|
|
|
|
|
|
|
|
Sponsor |
|
Sangamo BioSciences, Inc. |
|
|
|
|
|
Point Richmond Tech Center II |
|
|
|
|
|
501 Canal Blvd., Suite A100 |
|
|
|
|
|
Richmond, CA 94804 |
|
|
|
|
|
Phone: (510) 970-6000 |
|
|
|
|
|
Fax: (510) 970-6009 |
|
|
|
|
|
|
|
|
|
Medical Monitor |
|
Ely Benaim, M.D. |
|
|
|
|
|
|
|
|
|
|
|
Phone: (510) 970-7868 |
|
|
|
|
|
Fax: (510) 970-6009 |
|
|
|
|
|
|
|
|
|
Original Date |
|
August 18, 2009 |
|
|
|
|
|
|
|
|
|
Amendment 1 Date |
|
October 9, 2009 |
|
Confidentiality Statement
This document contains confidential information. It is intended solely for the use of the principal investigator, co-investigators, staff, appropriate institutional review boards or ethical committees, and other required regulatory bodies.
|
Sangamo BioSciences, Inc. |
|
|
Protocol SB-509-0901 for Diabetic Neuropathy
|
Confidential
|
Sangamo BioSciences, Inc.
Clinical Approval Signature Page
|
Protocol Number: |
SB-509-0901 |
|
|
|
|
Protocol Title: |
A Phase 2b Repeat Dosing ClinicalTrial of SB-509 in Subjects with Moderately Severe Diabetic Neuropathy |
|
|
|
|
Version: |
Amendment 1 |
|
|
|
|
Date: |
October 9, 2009 |
|
/s/Ely Benaim, M.D. |
|
10/09/2009 |
|
Ely Benaim, M.D. |
|
Date |
|
Vice Preseident, Clinical Affairs |
|
|
|
|
|
|
|
/s/Shelley Wang, MS. M.D. |
|
10/09/2009 |
|
Shelley Wang, MS. M.D. |
|
Date |
|
Associate Director, Clinical Development |
|
|
|
Page ii of 66 |
Version date: October 9, 2009 |
|
Sangamo BioSciences, Inc. |
|
|
Protocol SB-509-0901 for Diabetic Neuropathy
|
Confidential
|
TABLE OF CONTENTS
|
PROTOCOL SYNOPSIS |
VI |
|||
|
|
|
|||
|
SCHEMA |
VIII |
|||
|
|
|
|||
|
ABBREVIATIONS |
IX |
|||
|
|
|
|||
|
1. |
INTRODUCTION |
11 |
||
|
|
1.1. |
Background |
11 |
|
|
|
1.2. |
Rationale |
11 |
|
|
|
|
|
||
|
2. |
OBJECTIVES |
11 |
||
|
|
|
|
||
|
3. |
STUDY DESIGN |
13 |
||
|
|
|
|
||
|
4. |
SUBJECT SELECTION |
13 |
||
|
|
4.1. |
Inclusion Criteria |
13 |
|
|
|
4.2. |
Exclusion Criteria |
14 |
|
|
|
|
|
||
|
5. |
INFORMED CONSENT |
15 |
||
|
|
|
|
||
|
6. |
AUTHORIZATION TO USE AND DISCLOSE MEDICAL INFORMATION |
15 |
||
|
|
|
|
||
|
7. |
STUDY METHODOLOGY |
16 |
||
|
|
7.1. |
Screening Visit |
16 |
|
|
|
7.2. |
Subject Enrollment Procedures |
18 |
|
|
|
7.3. |
Baseline Duplicate Assessments |
18 |
|
|
|
7.4. |
Schedule for Treatment Period |
18 |
|
|
|
7.5. |
Schedule for Follow-Up Period |
20 |
|
|
|
|
|
||
|
8. |
CRITERIA FOR WITHDRAWAL FROM THE STUDY |
23 |
||
|
|
8.1. |
Discontinuation from Study Treatment and Follow-up of Subjects |
23 |
|
|
|
|
|
|
|
|
9. |
ADMINISTRATION OF STUDY DRUG |
23 |
||
|
|
9.1. |
Product Description |
23 |
|
|
|
9.2. |
Description and Manufacturer of Drug Substance |
23 |
|
|
|
9.3. |
SB-509 and Placebo Study Drug Composition |
24 |
|
|
|
9.4. |
Inventory, Storage, and Handling of the Drug Product |
24 |
|
|
|
9.5. |
SB-509 Administration |
31 |
|
|
|
9.6. |
Precautions |
31 |
|
|
|
9.7. |
Premedications |
31 |
|
|
|
9.8. |
Dose Modifications |
31 |
|
|
|
|
|
|
|
|
10. |
SAFETY |
31 |
||
|
|
10.1. |
Potential Risks |
31 |
|
|
|
|
|
|
|
|
11. |
EFFICACY |
32 |
||
|
|
|
|
|
|
|
|
11.1. |
Potential Benefits |
32 |
|
|
|
11.2. |
Efficacy Criteria |
32 |
|
|
|
|
|
|
|
|
12. |
CONCOMITANT MEDICATIONS |
33 |
||
|
|
|
|
|
|
|
13. |
ADVERSE EVENTS |
33 |
||
|
|
13.1. |
Adverse Event Reporting Period |
33 |
|
|
|
13.2. |
Definitions |
33 |
|
|
|
13.3. |
Recording of an Adverse Event |
34 |
|
|
Page iii of 66 |
Version date: October 9, 2009 |
|
Sangamo BioSciences, Inc. |
|
|
Protocol SB-509-0901 for Diabetic Neuropathy
|
Confidential
|
|
14. |
SERIOUS ADVERSE EVENT |
35 |
||
|
|
14.1. |
Serious Adverse Event Reporting |
35 |
|
|
|
14.2. |
Definitions |
35 |
|
|
|
14.3. |
Recording of a Serious Adverse Event |
35 |
|
|
|
|
|
|
|
|
15. |
STATISTICAL METHODS AND DATA ANALYSIS |
35 |
||
|
|
15.1. |
Sample Size |
35 |
|
|
|
15.2. |
Randomization |
36 |
|
|
|
15.3. |
Statistical Methods/Data Analysis |
36 |
|
|
|
15.4. |
Intent-to-Treat Population |
36 |
|
|
|
15.5. |
Subject Disposition |
36 |
|
|
|
15.6. |
Demographics |
36 |
|
|
|
15.7. |
Efficacy |
36 |
|
|
|
15.8. |
Baseline Values |
37 |
|
|
|
15.9. |
Missing Data |
37 |
|
|
|
15.10. |
Safety |
37 |
|
|
|
15.11. |
Laboratory Data |
38 |
|
|
|
|
|
|
|
|
16. |
STUDY ADMINISTRATION AND INVESTIGATOR OBLIGATIONS |
38 |
||
|
|
16.1. |
Institutional Review Board/Institutional Ethics Committee |
38 |
|
|
|
16.2. |
Informed Consent |
38 |
|
|
|
16.3. |
Study Amendments |
38 |
|
|
|
16.4. |
Study Drug Accountability |
39 |
|
|
|
16.5 |
Study Personnel |
39 |
|
|
|
16.6 |
Monitoring the Study |
39 |
|
|
|
16.7 |
Completion and Return of Case Report Forms |
40 |
|
|
|
16.8 |
Deviation from Protocol for Individual Subjects |
40 |
|
|
|
16.9 |
Quality Assurance Procedures |
40 |
|
|
|
16.10 |
Sangamo BioSciences Policy on Fraud in Clinical Studies |
41 |
|
|
|
16.11 |
Termination of the Study |
41 |
|
|
|
16.12 |
Record Retention |
25 |
|
|
|
16.13 |
Confidentiality |
25 |
|
|
|
16.14 |
Publication Statement |
26 |
|
|
|
|
|
|
|
|
17 |
STUDY FUNDING |
26 |
||
|
|
|
|
|
|
|
18 |
REFERENCES |
26 |
||
|
|
|
|
||
|
APPENDIX 1: |
SCHEDULE OF EVENTS |
29 |
||
|
|
|
|
||
|
APPENDIX 2: |
CTCAE |
42 |
||
|
|
|
|
||
|
APPENDIX 3: |
AMERICAN CANCER SOCIETY (ACS) CANCER DETECTION GUIDELINES |
43 |
||
|
|
|
|
||
|
APPENDIX 4: |
SB-509 DRUG PRODUCT AND PLACEBO DESCRIPTION AND INSTRUCTIONS FOR STORAGE, HANDLING, ADMINISTRATION AND DISPOSAL |
46 |
||
|
|
|
|
||
|
APPENDIX 5: |
NEUROPATHY IMPAIRMENT SCORE- LOWER LIMB (NIS-LL) |
49 |
||
|
|
|
|
||
|
APPENDIX 6: |
LOWER EXTREMITY NEUROLOGICAL SENSORY EXAM |
51 |
||
|
|
|
|
||
|
APPENDIX 7: |
ELECTROPHYSIOLOGICAL STUDIES |
52 |
||
|
|
|
|
||
|
APPENDIX 8: |
QUANTITATIVE SENSORY TESTING (QST) |
53 |
||
|
|
|
|
||
|
APPENDIX 9: |
SKIN BIOPSY |
55 |
||
|
|
|
|
||
|
APPENDIX 10: |
NEUROPATHY TOTAL SYMPTOM SCORE (NTSS-6) |
56 |
||
|
Page iv of 66 |
Version date: October 9, 2009 |
|
Sangamo BioSciences, Inc. |
|
|
Protocol SB-509-0901 for Diabetic Neuropathy
|
Confidential
|
|
APPENDIX 11: |
VISUAL ANALOG SCALE FOR PAIN INTENSITY (VASPI) |
58 |
|
|
|
|
|
APPENDIX 12: |
NEURO-QOL |
59 |
|
|
|
|
|
APPENDIX 13: |
SF-36 |
63 |
|
|
|
|
|
APPENDIX 14: |
GLOBAL ASSESSMENT |
66 |
|
Page v of 66 |
Version date: October 9, 2009 |
|
Sangamo BioSciences, Inc. |
|
|
Protocol SB-509-0901 for Diabetic Neuropathy
|
Confidential
|
PROTOCOL SYNOPSIS
|
Title |
A Phase 2b Repeat Dosing Clinical Trial of SB-509 in Subjects with Moderately Severe Diabetic Neuropathy |
|
|
|
|
|
|
Sponsor |
Sangamo BioSciences, Inc. |
|
|
|
|
|
|
Investigational Products |
SB-509 |
|
|
|
|
|
|
Objectives |
Primary: |
|
|
|
To compare the effect of SB-509 versus placebo in subjects with moderately severe diabetic neuropathy (DN) on sural Nerve Conduction Velocity (NCV) at six-months |
|
|
|
|
|
|
|
Secondary: |
|
|
|
· |
To compare in subjects with moderately severe diabetic neuropathy the effect of SB-509 versus placebo on the following endpoints: |
|
|
· Neuropathy Impairment Score - Lower Limb (NIS-LL) · Motor Nerve Conduction Velocity (NCV) · Quantitative Sensory Testing (QST) · lntraepidermal Nerve Fiber Density (IENFD) · Lower Extremity Neurological Sensory Exam · Visual Analog Scale for Pain Intensity (VASPI) · Neuropathy Total Symptom Score (NTSS-6) · Quality of Life: NeuroQoL and SF-36 · Global Assessment |
|
|
|
|
|
|
|
· |
To compare the effect of SB-509 versus placebo in subjects with moderately severe diabetic neuropathy using a multi-endpoint analysis that includes Neuropathy Impairment Score - Lower Limb (NIS-LL), Sural Nerve Conduction Velocity (NCV) and lntraepidermal Nerve Fiber Density (IENFD), as detailed by O’Brien (1984) |
|
|
· |
To evaluate the safety of SB-509 as compared to placebo in subjects with moderately severe diabetic neuropathy |
|
|
|
|
|
Subject Population |
A total of 150 subjects with moderately severe diabetic neuropathy as defined by mean sural NCV ≤ 45 m/s,IENFD ≤ 18 fibers/mm, NIS-LL ≥ 3 points, sICAM ≥ 200 ng/ML, with measurable lower extremity nerves (sural NCV amplitude must be > 1.0 µV) will be enrolled in this trial. |
|
|
|
|
|
|
Study Design |
Phase 2b, randomized, double-blind, placebo-controlled, multicenter study. |
|
|
|
|
|
|
Treatment |
150 subjects will be randomized in a 1:1 ratio to treatment with |
|
|
|
|
|
|
Plan |
SB-509 or placebo: 1) SB-509 treatment: 60 mg of SB-509 on Day 0, Day 60 and Day 120. 2) Placebo on Day 0, Day 60 and Day 120. |
|
|
|
|
|
|
|
30 mg of SB-509 or an equal volume of placebo will be injected intramuscularly (IM) into each lower limb for a total dose of 60 mg. Each subject will receive a total of three treatments, two months apart. |
|
|
Page vi of 66 |
Version date: October 9, 2009 |
|
Sangamo BioSciences, Inc. |
|
|
Protocol SB-509-0901 for Diabetic Neuropathy
|
Confidential
|
|
|
|
|
Duration of Participation |
The duration of participation will be approximately 15 months: 12 weeks for screening, 5 months for treatment (includes one month after the last dose), and 7 months for follow-up after the treatment period. |
|
|
|
|
Randomization |
Subjects will be randomized in a 1:1 ratio to SB-509 or placebo stratified by study site and screening IENFD (< 9, ≥ 9). |
|
|
|
|
Sample Size and Analyses |
A sample size of 150 subjects provides 93% power to detect a mean improvement of 2.2 units on the sNCV in a comparison between the SB-509 and placebo arms, assuming a standard deviation of 4. The statistical test used will be a two-sided Wilcoxon Rank-Sum test, with a Type I error rate of 5%. |
|
|
|
|
|
Primary Efficacy Analyses The Day 180 change from baseline sNCV will be compared by treatment group using the Wilcoxon Rank-Sum test at an alpha level of 0.05 |
|
|
|
|
|
Secondary Efficacy Analyses The Day 180 change from baseline sNCV will be compared by treatment group using the Cochran-Mantel-Haenszel test stratified by IENFD < 9 and ≥ 9 at an alpha level of 0.05 |
|
|
|
|
|
For the following measures, the Day 180 change from baseline measures will be compared by treatment group using the Wilcoxon Rank-Sum test. · Neuropathy Impairment Score - Lower Limb (NIS-LL) · Lower Extremity Neurological Sensory Exam · Motor Nerve Conduction Velocity (NCV) · Quantitative Sensory Testing (QST) · lntraepidermal Nerve Fiber Density (IENFD) · Neuropathy Total Symptom Score (NTSS-6) · Visual Analog Scale for Pain Intensity (VASPI) · Quality of Life (QOL)- NeuroQoL and SF-36 · Global assessment |
|
|
|
|
|
The effect of SB-509 versus placebo in subjects with moderately severe diabetic neuropathy will be compared using a multi-endpoint analysis that includes Neuropathy Impairment Score - Lower Limb (NIS-LL), Sural Nerve Conduction Velocity (NCV) and lntraepidermal Nerve Fiber Density (IENFD), as detailed by O’Brien (1984). |
|
|
|
|
|
Safety Safety assessment will occur on all subjects who received any study medication. Terminations/premature withdrawals, adverse events, concomitant medications, and laboratory data will be tabulated. Adverse events will be coded to a standard set of terms using the MedDRA dictionary. Frequency of adverse events will be compared using Fisher’s exact test. |
|
Page vii of 66 |
Version date: October 9, 2009 |
|
Sangamo BioSciences, Inc. |
|
|
Protocol SB-509-0901 for Diabetic Neuropathy
|
Confidential
|
SCHEMA
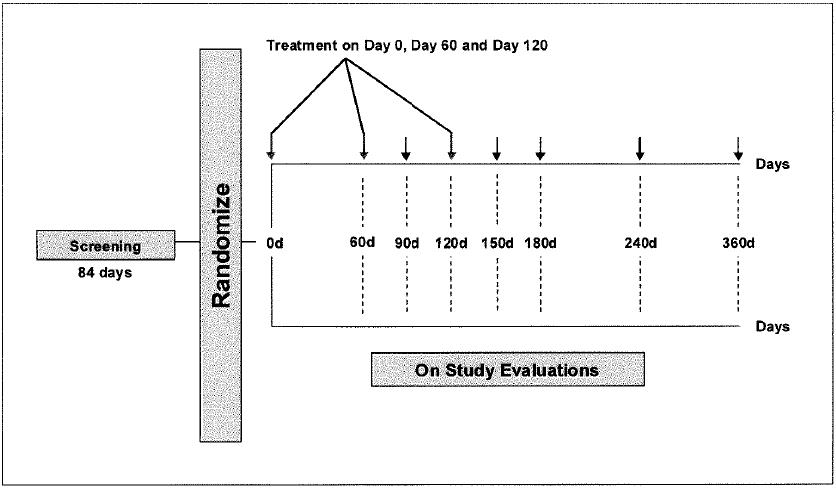
|
Page viii of 66 |
Version date: October 9, 2009 |
|
Sangamo BioSciences, Inc. |
|
|
Protocol SB-509-0901 for Diabetic Neuropathy
|
Confidential
|
ABBREVIATIONS
|
32Ep65 |
|
Engineered ZFP-TF |
|
ACS |
|
American Cancer Society |
|
ADA |
|
American Diabetes Association |
|
ADR |
|
Adverse drug reaction |
|
AE |
|
Adverse event |
|
ALP |
|
Alkaline phosphatase |
|
ALT |
|
Alanine aminotransferase |
|
ARI |
|
Aldose reductase inhibitors |
|
AST |
|
Aspartate aminotransferase |
|
BMI |
|
Body mass index |
|
BOCF |
|
Baseline observation carried forward |
|
BUN |
|
Blood urea nitrogen |
|
CBC |
|
Complete blood count |
|
CFR |
|
Code of Federal Regulations |
|
cm |
|
Centimeter |
|
CPK |
|
Creatinine phosphokinase |
|
CPKmb |
|
Creatinine phosphokinase, mb fraction |
|
CRF |
|
Case report form |
|
CTCAE |
|
Common terminology criteria for adverse events |
|
DN |
|
Diabetic neuropathy |
|
DNA |
|
Deoxyribonucleic acid |
|
ECG |
|
Electrocardiogram |
|
ENFD |
|
Epidermal nerve fiber density |
|
ENFDR |
|
Epidermal nerve fiber density regeneration |
|
FDA |
|
Food and Drug Administration |
|
FOBT |
|
Fecal occult blood test |
|
HBV |
|
Hepatitis B virus |
|
HCV |
|
Hepatitis C virus |
|
Hg |
|
Mercury |
|
HIV |
|
Human Immunodeficiency Virus |
|
HPV |
|
Human papillomaviurs |
|
ICH |
|
International Conference on Harmonization |
|
IBC |
|
Institutional Biosafety Committee |
|
ICAM |
|
Intracellular Adhesion Molecules |
|
IEC |
|
Institutional Ethics Committee |
|
IENFD |
|
lntraepidermal Nerve Fiber Density |
|
IM |
|
Intramuscular |
|
INR |
|
International normalized ratio |
|
IRB |
|
Institutional Review Board |
|
Kb |
|
Kilobase |
|
kg |
|
Kilogram |
|
L |
|
Liter |
|
LDH |
|
Lactate dehydrogenase |
|
LDL |
|
Low-density lipoprotein |
|
LOCF |
|
Last observation carried forward |
|
mg |
|
Milligram |
|
mL |
|
Milliliter |
|
mm |
|
Millimeter |
|
mM |
|
Millimolar |
|
MRI |
|
Magnetic resonance imaging |
|
NCI |
|
National Cancer Institute |
|
NCS |
|
Nerve conduction studies |
|
NCV |
|
Nerve conduction velocity |
|
NF |
|
National Formulary |
|
NTSS-6 |
|
Neuropathy Total Symptom Score |
|
ng |
|
Nanogram |
|
NGF |
|
Nerve growth factor |
|
Page ix of 66 |
Version date: October 9, 2009 |
|
Sangamo BioSciences, Inc. |
|
|
Protocol SB-509-0901 for Diabetic Neuropathy
|
Confidential
|
|
NIH |
|
National Institute of Health |
|
NIS-LL |
|
Neuropathy Impairment Score - Lower Limb |
|
NLS |
|
Nuclear localization sequence |
|
NOAEL |
|
No observed adverse effect level |
|
NSAIDs |
|
Non-steroidal anti-inflammatory drugs |
|
P188 |
|
Poloxamer 188 |
|
PCR |
|
Polymerase chain reaction |
|
PSA |
|
Prostate-specific antigen |
|
PT |
|
Prothrombin time |
|
PTT |
|
Partial prothrombin time |
|
pV-32Ep65 |
|
Plasmid; the active drug substance in SB-509 |
|
QOL |
|
Quality of Life |
|
QST |
|
Quantitative sensory testing |
|
RNA |
|
Ribonucleic acid |
|
SAE |
|
Serious adverse event |
|
SB-509 |
|
Plasmid formulation containing the 32Ep65 expression cassette, an engineered transcription factor that induces expression of VEGF-A |
|
sNCV |
|
Sural Nerve Conduction Velocity |
|
STZ |
|
Streptozotocin |
|
SV40 |
|
Simian Virus 40 |
|
TF |
|
Transcription factor |
|
TSH |
|
Thyroid Stimulating Hormone |
|
Tris-HCI |
|
Tris-(hydroxymethyl)-aminomethane hydrochloride |
|
U |
|
Unit |
|
µm |
|
Micrometer (micron) |
|
USP |
|
United States Pharmacopoeia |
|
VPT |
|
Vibration Perception Threshold |
|
VASPI |
|
Visual Analog Scale for Pain Intensity |
|
VEGF |
|
Vascular endothelial growth factor |
|
VEGF-A |
|
Vascular endothelial growth factor-A |
|
WBC |
|
White blood cell |
|
WHO |
|
World Health Organization |
|
ZFP |
|
Zinc finger protein |
|
ZFP-TF |
|
Zinc finger protein transcription factor |
|
Page x of 66 |
Version date: October 9, 2009 |
|
Sangamo BioSciences, Inc. |
|
|
Protocol SB-509-0901 for Diabetic Neuropathy
|
Confidential
|
|
1. |
INTRODUCTION |
VEGF-A is well known as a potent angiogenic factor, but it has also been shown to be a potent neurotrophic and neuroprotective factor. Studies have shown that plasmid-mediated delivery of hVEGF-A can reverse neuropathy in diabetic animals (Schratzberger et al. 2001). Another study showed that gene transfer of an engineered transcription factor promoting expression of VEGF-A protects against experimental diabetic neuropathy (Price et al. 2006). A Phase 1 clinical trial testing hVEGF-A165 cDNA suggests that this approach may be beneficial (Simovic et al.2001).
The VEGF-A mRNA is alternatively spliced to express three major protein isoforms, VEGF-A189, VEGF-A165, and VEGF-A121 Recent studies suggest that the combination of all three isoforms provides a more potent angiogenic response than any of the isoforms alone (Whitlock et al. 2004). The same may be true for the neurotrophic and neuroprotective effects of VEGF-A.
Sangamo BioSciences has developed a plasmid SB-509 that expresses an engineered transcription factor (TF) that binds the endogenous VEGF-A promoter and activates transcription of the endogenous VEGF-A gene. The transcript produced undergoes alternative splicing, naturally producing the three major protein isoforms. This TF is based on the Cys2-His2 zinc finger DNA binding domain. Treatment of disease via localized gene delivery may mimic local autocrine/paracrine mechanisms by which VEGF-A works. It has been shown, in side-by-side comparisons, that activation of the endogenous VEGF-A gene, using engineered TFs, produces non-leaky vasculature with normal vessel architecture (Rebar et al. 2002) in contrast to vessels produced in response to a single isoform, which leak and have defective architecture (Elson et al. 2001).
SB-509 is an investigational product that has not been approved or marketed in any country.
|
1.1. |
Background |
Epidemiology and Treatment of Diabetic Neuropathy
It is estimated that diabetes affects nearly 23.6 million patients in the United States, of which 5.7 million are undiagnosed (CDC 2007 National Diabetes Fact Sheet). The incidence of diabetic peripheral neuropathy ranges from 10% at the time of diagnosis of diabetes to 50% after 25 years of living with diabetes (Poncelet 2003). The severity of neuropathy in diabetes is related to the duration of disease, glucose control, hypertension, and hyperlipidemia (Feldman et al. 1999; Stratton et al. 2000; Malik 2000). In addition, neuropathy is an independent risk factor for further morbidity in diabetes due to foot ulcerations and limb amputation (McNeely et al. 1995; Potter et al. 1998). The pathogenesis is related to hyperglycemia, which affects both small and large nerve fibers. The WHO definition of diabetic neuropathy (DN) is “A disease characterized as a progressive loss of nerve fibers leading to sensation loss, foot ulceration, and amputation.” Symptoms and signs are directly related to the type of nerve fiber damage. Thinly myelinated small nerve fibers mediate pain and altered cold, heat, and light touch. Large myelinated fibers mediate vibration and proprioception. Symptoms and signs include the stocking and glove distribution of sensory loss, pain and eventually motor loss resulting in weakness of the muscles in the feet and are directly related to the type of nerve fiber damage (Thomas and Tomlinson 1993).
There is no treatment for diabetic neuropathy that has been shown to modify disease onset or progression; current treatments are limited to the relief of select symptoms (i.e. pain). Intensive glucose control can stabilize or improve diabetic neuropathy (DN) (UKPDS 33 1998). Medical treatment for pain symptoms associated with diabetic neuropathy includes anti-depressants, anti-convulsants, opiates, anti-arrhythmics, and topical agents (Poncelet 2003). Duloxetine and pregabalin have recently received FDA approval for the symptomatic treatment on Diabetic Neuropathy pain (Argoff et al. 2006). Additional treatment strategies have included attempts to protect or regenerate neurons and thus interfere with the primary disease process in neuropathy. Aldose reductase inhibitors (ARI) and growth factors such as Nerve Growth Factor (NGF) have been evaluated in clinical trials. Phase 3 trials with these agents have proved unsuccessful in detecting significant improvement in neurologic endpoints and have been limited by side effects (Boulton et al. 2004). These studies did provide significant insight into the natural course of progression of DN and the need for a composite endpoint combining measurements of the signs and symptoms of DN, neurological examination, and electrophysiological testing (Feldman 2002). Epalrestat (Kinedak™, Ono Pharmaceuticals, Osaka, Japan), approved in Japan in 1992, is the only ARI currently available commercially.
|
1.2. |
Rationale |
1.2.1. Pre-clinical Data
The biological activity of the 32Ep65 transcriptional activator (also known as VZ+434) was evaluated in both in vitro multiple cell lines and in vivo in multiple species. In vitro studies demonstrated that the transcriptional activator can 1)upregulate the major
|
Page 11 of 66 |
Version date: October 9, 2009 |
|
Sangamo BioSciences, Inc. |
|
|
Protocol SB-509-0901 for Diabetic Neuropathy
|
Confidential
|
isoforms VEGF-A mRNA and conserve their relative proportions and 2) increase VEGF-A protein and 3) the secreted protein can protect neuronal-derived cells from growth arrest in response to serum starvation. The therapeutic potential of SB-509 was assessed in a validated experimental model of diabetic neuropathy in streptozotocin (STZ)-induced diabetic rats (Schratzberger et al. 2001; Calcutt et al. 2003; Biessels et al. 1999, Price et al 2006). Single and repeat administration of the formulated plasmid encoding 32Ep65 (SB-509) showed a significant and dose-related protection against losses in both motor and sensory nerve conduction velocities. Please refer to the SB-509 DN Clinical Investigator’s Brochure.
Single administration pre-clinical toxicology and biodistribution studies of SB-509 were designed to support the Phase 1 dose-escalation clinical trial of a single treatment of SB-509 in subjects with diabetic neuropathy. Single administration toxicology studies included both toxicology and biodistribution studies in rats and rabbits. The single intramuscular injection treatment of SB-509 was, in general, well-tolerated, with no treatment-related effects or biologically significant differences among treatment groups for body weights, clinical observations, laboratory tests, and microscopic histological evaluation. SB-509 plasmid persisted up to 30 days and was localized to the injection site. A NOAEL of 1.0 mg/kg SB-509 was determined from the study
A repeat SB-509 administration study in rats was designed to support the Phase 2 repeat administration clinical trial in subjects with diabetic neuropathy. Rats received monthly SB-509 intramuscular administration for three months. In general, rats tolerated three doses of SB-509 given at monthly intervals at up to 1.0 mg/kg/dose SB-509 without any toxic effects. Specific ocular histopathology revealed no evidence of retinal arterial proliferation or other lesions related to SB-509 treatment. Proteinuria was observed at 5.0 mg/kg/dose SB-509 at Study Day 60 in 1/5 males and at Study Day 90 in 1/5 females. Proteinuria was also observed at a similar incidence at Study Day 120 for both the low (1.0 mg/kg) and high (5.0 mg/kg) dose groups in both male (1/5) and female (1/5) groups. This finding was not supported by protein serum changes in creatinine and blood urea nitrogen levels or by histological changes in the kidney. The only microscopic histology findings were local inflammation and muscle damage at the injection site. The incidence of these findings generally correlated with the volume administered and was a transient effect. No significant changes in body weight gain were observed during dosing (Study Days 0-60); however, during the recovery period (Study Days 60-120), males and females in the 5.0 mg/kg dose groups showed significant changes in body weight gains and were considered to insignificant and were not toxicologically relevant. One mortality was observed in a PCR (biodistribution) satellite group (5 mg/kg/dose SB-509), and the cause of death was undetermined. At all study time points (Study Days 30, 60, 90, and 120), plasmid DNA was detected at the injection sites in all rats given SB-509 at 5 mg/kg/dose. SB-509 plasmid was not found in heart or gonads at Study Days 30, 60, 90, and 120. The NOAEL was 1.0 mg/kg/dose for repeat administration of SB-509. For additional information, please refer to the SB-509 DN Clinical Investigator’s Brochure.
1.2.2. Clinical Rationale
Clinical trials of SB-509 in subjects with mild, moderate, or severe diabetic peripheral neuropathy have identified the following effects of SB-509: improvement in sensory and motor nerve conduction velocity, improvement in quantitative sensory testing, decrease in the neuropathy impairment score-lower limb, and a strong trend for re growth of nerve fibers in the skin. Subgroup analyses by baseline disease severity show that subjects with more advanced disease (i.e., low nerve conduction velocity, low intraepidermal nerve fiber density and poor vibration perception) tend to respond better to SB-509, as indicated, for example, by a greater decrease in the neuropathy impairment score. Thus, this protocol will be conducted in subjects with advanced diabetic neuropathy who have a mean sural nerve conduction velocity of ≤ 45 m/s, NIS-LL ≥ 3, an intraepidermal nerve fiber density ≤ 18 fibers/mm, and serum Intracellular Adhesion Molecule (ICAM) ≥ 200 ng/ml.
Further information is provided in the SB-509 DN Clinical Investigator’s Brochure.
|
2. |
OBJECTIVES |
The primary objective is:
To compare the effect of SB-509 versus placebo in subjects with moderately severe diabetic neuropathy on the sural Nerve Conduction Velocity (sNCV) endpoint at sixmonths
The secondary objective is:
|
· |
To compare in subjects with moderately severe diabetic neuropathy the effect of SB-509 versus placebo on the following endpoints: Neuropathy Impairment Score - Lower Limb (NIS-LL), motor Nerve Conduction Velocity (NCV), Quantitative Sensory Testing (QST), lntraepidermal Nerve Fiber Density (IENFD), Lower Extremity Neurological Sensory Exam, Visual |
|
Page 12 of 66 |
Version date: October 9, 2009 |
|
Sangamo BioSciences, Inc. |
|
|
Protocol SB-509-0901 for Diabetic Neuropathy
|
Confidential
|
|
Analog Scale for Pain Intensity (VASPI), Neuropathy Total Symptom Score (NTSS-6), NeuroQoL, SF-36, and Global Assessment |
|
· |
To compare the effect of SB-509 versus placebo in subjects with moderately severe diabetic neuropathy using a multi-endpoint analysis that includes Neuropathy Impairment Score - Lower Limb (NIS-LL), Sural Nerve Conduction Velocity (NCV) and lntraepidermal Nerve Fiber Density (IENFD), as detailed by O’Brien (1984) |
|
· |
To evaluate the safety of SB-509 as compared to placebo in subjects with moderately severe diabetic neuropathy |
|
3. |
STUDY DESIGN |
The Phase 2 trial is a randomized, double-blind, placebo-controlled, multi-center study of SB-509 given by intramuscular injections into the lower limbs in 150 subjects with moderately severe diabetic neuropathy.
150 subjects will be randomized in a 1:1 ratio to treatment with SB-509 or placebo stratified by study site and screening IENFD (<9 fiber/mm, ≥ 9 fiber/mm):
1) SB-509 treatment: 60 mg of SB-509 on Day 0, Day 60 and Day 120.
2) Placebo on Day 0, Day 60 and Day 120.
30 mg of SB-509 or an equal volume of placebo will be injected (IM) into each lower limb for a total dose of 60 mg. Each subject will receive a total of three treatments.
The duration of participation will be approximately 15 months: 12 weeks for screening, 5 months for treatment (includes one month after the last dose), and 7 months for follow-up after the treatment period.
|
4. |
SUBJECT SELECTION |
|
4.1. |
Inclusion Criteria |
|
1. |
Written informed consent signed and dated by study subject |
|
2. |
Male or female between the ages of 18 and 70, inclusive |
|
3. |
Clinical diagnosis of Diabetes Mellitus Type I or II for at least 12 months. A past history of Diabetes Mellitus and/or the use of anti-diabetic medications for the treatment of Diabetes Mellitus are sufficient. |
|
4. |
Clinical signs and symptoms of moderate to severe diabetic sensori motor polyneuropathy of the lower extremities for at least 6 months that are not otherwise attributed to an etiology other than diabetes, as determined by a an internist with neuropathy experience, neurologist or endocrinologist and excluding subjects with only diabetic autonomic neuropathy or mononeuropathy. |
|
5. |
Mean sural nerve conduction velocity ≤ 45 m/s as confirmed by the Neurological Core Laboratory |
|
6. |
Measurable sural response (amplitude equal or greater than 1.0 µv) and a measurable peroneal response (amplitude equal to or greater than 500 µV) bilaterally |
|
7. |
Neuropathy Impairment Score Lower Limb (NIS-LL) must be ≥ 3 points |
|
8. |
lntraepidermal Nerve Fiber Density ≤ 18 fibers per mm |
|
9. |
Serum Intracellular Adhesion Molecule level (ICAM) ≥ 200 ng/mL |
|
10. |
Hemoglobin level ≥ 10 g/dL |
|
11. |
HgbA1c level ≤ 9%. Subjects should be treated according to ADA guidelines and goals for dietary intervention and/or glucose control therapy and have stable glycemic control for 3 months, as determined by the investigator |
|
12. |
WBC count ≥ 3,000/mm3, an absolute granulocyte count ≥ 1,500/mm3, and a platelet count ≥ 100,000/mm3 |
|
13. |
Serum creatinine < 1.5 mg/dL |
|
14. |
Total bilirubin ≤ 1.5 times the upper limit of normal |
|
15. |
AST and ALT ≤ 2 times the upper limit of normal |
|
Page 13 of 66 |
Version date: October 9, 2009 |
|
Sangamo BioSciences, Inc. |
|
|
Protocol SB-509-0901 for Diabetic Neuropathy
|
Confidential
|
|
16. |
INR < 1.5 times the upper limit of normal or PTT < 1.5 times the upper limit of normal |
|
17. |
Normal or elevated serum levels of vitamin B12 |
|
18. |
Normal thyroid function (as determined by normal TSH and free T4 levels). For subjects on thyroid hormone replacement therapy, an elevated TSH level that is no higher than 7 mU/L is acceptable if the investigator feels the subject is clinically euthyroid. |
|
19. |
Random urine sample albumin/creatinine ratio ≤ 300 µg/mg creatinine. Normal urinalysis, with the exception of glucose and protein. If other abnormalities are present, the urinalysis may be repeated within the screening period at the discretion of the investigator. |
|
20. |
If subject is female and of childbearing potential, she agrees to use a medically acceptable physical barrier method contraceptive during the treatment phase through 30 days after the last dose and have a negative serum pregnancy test prior to study entry. |
A female subject is considered to be of childbearing potential if she is postmenarchial, has an intact uterus and at least 1 ovary, and is less than 2 years postmenopausal. A male subject must agree to use a medically acceptable physical barrier method contraceptive during the treatment phase through 30 days after the last dose. The following are acceptable physical barrier methods: Male condom, Female condom, Diaphragm, Cervical cap.
|
21. |
Subject must not be breastfeeding. Subjects who become pregnant during treatment must inform the investigator of their pregnancy, be withdrawn from treatment, and agree to provide follow-up information at time of delivery. |
|
22. |
Complete screening tests for malignancies of the colon, breast (females only), cervix/uterus (females only), and prostate (males only) at the time of screening based on the American Cancer Society’s current recommended guidelines (see Appendix 3). Documented evidence of negative screening tests is sufficient. Any subject found to have cancer by these screening tests is excluded from the trial. |
|
23. |
Negative mammogram, if female and over age 40 |
|
24. |
Have a PSA (<4 ng/mL), if male and over age 45 |
|
25. |
Normal Pap smear documented within a year of screening, if female, unless the subject has had a hysterectomy |
|
26. |
Be willing and able to participate in the study as an outpatient, make the required visits to the study center during the treatment and post treatment periods, and comply with study requirements |
|
27. |
LDL cholesterol ≤ 130 mg/dL. Subjects with hyperlipidemia should be treated by diet or medications according to ADA guidelines and goals. Modification of treatment during the screening period is allowed at the discretion of the investigator. Subjects with elevated LDL in spite of adequate stalin therapy (at least 3 months) or intolerant to statins, will be discussed with the FDA for eligibility approval. |
|
28. |
Blood pressure ≤ 140/90 mm Hg. Subjects with hypertension should be treated according to ADA guidelines and goals, as determined by the investigator. |
|
29. |
Subjects should have a body mass index (BMI) ≤ 38 and should be receiving medical care, education, and counseling for obesity according to ADA guidelines and goals, as determined by the investigator. BMI is obtained by dividing the body weight (in kilograms) by the height squared (in meters squared). |
|
BMI |
= |
weight (kg) |
|
|
|
height (m) x height (m) |
|
4.2. |
Exclusion Criteria |
|
1. |
Unable to comply with the protocol evaluation requirements |
|
2. |
Require surgical intervention within 4 weeks of treatment |
|
3. |
Moderate to severe ischemic heart disease or any history of congestive heart failure, or have had a myocardial infarction within the previous 6 months |
|
4. |
Evidence of cardiac enlargement and/or congestive heart failure. |
|
5. |
Current diabetic foot or leg ulcer, gangrene in the lower extremity, or any amputation of the lower extremity |
|
Page 14 of 66 |
Version date: October 9, 2009 |
|
Sangamo BioSciences, Inc. |
|
|
Protocol SB-509-0901 for Diabetic Neuropathy
|
Confidential
|
|
5. |
Bleeding diathesis (e.g., hemophilia due to Factor VIII or IX deficiency) or require treatment with warfarin 2 weeks prior to and including the dosing period (up to 24 hours after dosing) |
|
6. |
Hemorrhagic stroke |
|
7. |
Gastrointestinal hemorrhage |
|
8. |
Any other condition that, in the opinion of the clinical investigator or sponsor, might compromise any aspect of this trial |
|
9. |
Participation in another clinical trial concurrently or have participated in such a trial within 30 days of screening |
|
10. |
Received gene transfer agents within 6 months of screening |
|
11. |
History of malignancy, except for the following: adequately treated basal cell or squamous cell skin cancer, superficial bladder cancer, adequately treated Stage 1 or 2 cancer currently in complete remission, or any other cancer that has been in complete remission for at least 5 years |
|
12. |
Inflammatory angiopathy (e.g., Buerger’s disease, etc.) |
|
13. |
Have an active infection requiring systemic or oral antibiotics. Subjects with prior infection must have discontinued such treatments at least 2 weeks prior to administration of the investigational agent. |
|
14. |
Be expected to require immunosuppressants (such as methotrexate, cyclophosphamide, or cyclosporine) for 30 days prior to, during, and for 30 days following administration of the investigational drug product |
|
15. |
Known immune or immunodeficiency disorders (e.g., HIV positive, sarcoidosis, tuberculosis, rheumatoid arthritis, autoimmune disorders – e.g. psoriasis) |
|
16. |
Chronic active viral hepatitis (HBV, HCV) or other active liver disease |
|
17. |
History of clinically significant hypersensitivity reactions to any component of SB-509 |
|
18. |
Current history (within 12 months of start of study) of alcohol or chemical dependency (excluding nicotine), as assessed by the investigator. |
|
19. |
History of or current proliferative retinopathy, macular edema or retinal neovascularization based on a dilated retinal examination with fluorescein angiography and retinal photographs performed by an ophthalmologist |
|
20. |
Pre-cancerous conditions (e.g. Barrett’s Esophagus, dysplasias) or benign tumors which have the potential for clinically significant growth due to VEGF stimulation. |
|
21. |
History of or current benign colon polyps that have been removed that meet the following criteria: 3 or more adenomas, any adenoma ≥ 1cm, any adenoma with villous features, high-grade dysplasia or sessile adenomas. |
|
22. |
Family history of inherited neuropathy (e.g. Charcot Marie Tooth, Hereditary Predisposition to Pressure Palsy). |
|
23. |
Known or suspected spinal pathology such as spinal stenosis, or a history suspicious of claudication (neurogenic and/or vascular). |
|
5. |
INFORMED CONSENT |
Prior to entering the study, the investigator or designated assistant will explain to each subject the nature of the study, its purpose, the procedures, the expected duration, alternative therapies available, and the benefits and risks involved in study participation. Subjects will be given an information and consent document, will have the opportunity to ask questions, and will be informed of their right to withdraw from the study at any time without prejudice. After this explanation and before any studyspecific procedures have been performed, the subject will voluntarily sign and date the informed consent document.
If a subject is re-screened for study participation, the subject should be re-consented if outside of the acceptable screening window. The subject will receive a copy of the re-signed and dated written informed consent form and any other written information provided to the subject.
|
6. |
AUTHORIZATION TO USE AND DISCLOSE MEDICAL INFORMATION |
Under federal law, subject study records cannot be used or disclosed for research purposes unless an authorization to use and disclose medical information is signed by each subject prior to participation in the study. The investigator or designated assistant will explain
|
Page 15 of 66 |
Version date: October 9, 2009 |
|
Sangamo BioSciences, Inc. |
|
|
Protocol SB-509-0901 for Diabetic Neuropathy
|
Confidential
|
to each subject the purpose of the subject authorization, and the disclosures will be agreed to by signing the authorization document. Subjects will be given an authorization document and will have the opportunity to ask questions. The subject must also be informed of the following:
|
1. |
They may not participate in the study unless the authorization is signed; however, they have the right to revoke this authorization (in writing) at any time. |
|
2. |
If subject discontinues from the study, they need not revoke the authorization to use and disclose their medical information. |
|
3. |
If subject discontinues from the study and does decide to revoke their authorization to use and disclose their medical information, the information that has already been collected in their study records may be used and disclosed as necessary to protect the integrity of the research project. |
After this explanation and before any study-specific procedures have been performed, the subject will voluntarily sign and date an authorization document.
Prior to participation in the study, the subject will receive a copy of the signed and dated written authorization.
|
7. |
STUDY METHODOLOGY |
The following sections describe in detail all study procedures. A detailed flow chart of all study procedures is presented in the Schedule of Events (Appendix 1).
STUDY VISIT PROCEDURES
|
7.1. |
Screening Visit |
The objective of the screening visit procedures is to identify subjects who meet the stated inclusion and exclusion criteria and who are willing and able to participate in the study. The following screening information and procedures must be obtained and completed within 12 weeks prior to administering the first treatment.
Blood tests including CBC with WBC differential and platelet count, Coagulation tests PT/INR and PTT and Serum chemistry: electrolytes (Na, K, CO2 , Cl), CPK, CPKmb, troponin, creatinine, BUN, glucose, uric acid, total bilirubin, ALP, ALT (or SGPT), AST (or SGOT), LDH, albumin, calcium, vitamin B12, T4/TSH, direct LDL, and total protein must be performed and reviewed for eligibility within 12 weeks prior to administering the first treatment.
Procedures that were performed as standard care (e.g., chest X-ray and ECG) may be done prior to written informed consent and may be used for screening eligibility, but they must be completed within 12 weeks prior to administering the first treatment. Sigmoidoscopies or colonoscopies and pap smears may be used for screening eligibility if these procedures have been completed within a year of screening. Mammograms may also be used for screening eligibility if the procedure has been completed within 3 months of screening.
A summary table of the study procedures can be found in the Schedule of Events, Appendix 1. The following study procedures will be performed at the screening visit:
|
1. |
Obtain a signed and dated subject informed consent form and authorization document to use and disclose medical information prior to performing any study-specific procedures |
|
2. |
Assign a subject number |
|
3. |
Review the inclusion and exclusion criteria |
|
4. |
Collect subject demographic information |
|
5. |
A complete medical history; and perform a general physical examination, including evaluation of lower extremity edema, height, weight, vital sign measurements (temperature, blood pressure [systolic and diastolic], and pulse rate), assessment of concomitant medications and a digital rectal exam for male subjects only. If the subject is not normally seen at the study center and reports having a condition listed in the exclusion criteria, it may be necessary to obtain medical records to confirm study eligibility (i.e., document stability of a clinically significant abnormality or disease) |
|
Page 16 of 66 |
Version date: October 9, 2009 |
|
Sangamo BioSciences, Inc. |
|
|
Protocol SB-509-0901 for Diabetic Neuropathy
|
Confidential
|
|
6. |
CBC with WBC differential and platelet count |
|
7. |
Coagulation tests PT/INR and PTT |
|
8. |
Serum chemistry: electrolytes (Na, K, C02 , Cl), CPK, CPKmb, troponin, creatinine, BUN, glucose, uric acid, total bilirubin, ALP, ALT (or SGPT), AST (or SGOT), LDH, albumin, calcium, total protein, and vitamin B12 |
|
9. |
T4/TSH |
|
10. |
Direct LDL cholesterol (subject should be fasting from midnight on) |
|
11. |
HgbA1c levels |
|
12. |
HIV serology |
|
13. |
Serum pregnancy test if female subject is of childbearing potential |
|
14. |
Urinalysis - test for presence of glucose, protein, bilirubin, blood, pH, and specific gravity |
|
15. |
Spot urine test for microalbumin/creatinine |
|
16. |
Blood sample collection: 6 mL blood (3 mL plasma) sample for PCR assay and 10 mL blood (5 mL serum) sample for serum Intracellular Adhesion Molecule (ICAM) level, cytokine assay, immunogenicity testing and detection of inflammatory markers, which should be collected and stored frozen until ready to be shipped to the laboratory. Collection, handling, and shipping instructions will be provided in the Study Reference Manual. |
|
17. |
Lower extremity neurological examination for NIS-LL and Lower Extremity Neurological Sensory Exam testing. (See Appendix 5 and Appendix 6 for details). |
|
18. |
Electrophysiological testing of the sural and peroneal nerves (See Appendix 7 and the Study Reference Manual for details of testing requirements) |
|
19. |
Review of waveforms for nerve conduction velocity by the Neurological Core Laboratory. |
|
20. |
Quantitative sensory testing (QST) of bilateral lower extremities (Appendix 8) |
|
21. |
3 mm skin biopsy to determine lntraepidermal Nerve Fiber Density (IENFD) (See Appendix 9) |
|
22. |
Neuropathy Total Symptom Score (NTSS-6) (See Appendix 10) |
|
23. |
Visual Analog Scale for Pain Intensity (VASPI) (See Appendix 11) |
|
24. |
Neuro-QoL(lSee Appendix 12) |
|
25. |
SF-36 (See Appendix 13) |
|
26. |
Electrocardiogram (ECG)- standard 12-lead |
|
27. |
Chest X-ray (2 views anterior-posterior and lateral) |
|
28. |
Retinal examination with fluorescein angiography and fundus photos, performed by an ophthalmologist |
|
29. |
PSA blood test for male subjects 45 years of age and older |
|
30. |
Mammogram for female subjects 40 years of age and older; A mammogram performed within 3 months of screening can be used for screening eligibility. |
|
31. |
Pap smear for females who have not had a procedure within a year of screening |
|
32. |
Flexible sigmoidoscopy for subjects 50 years of age and older; A sigmoidoscopy or colonoscopy performed within a year of screening can be used for screening eligibility. Subjects with a history of benign colon polyps removed that do not meet exclusion criteria, must have documented evidence of a normal colonoscopy within the last 12 months. Subjects who have benign colon polyps removed during a sigmoidoscopy performed in the screening period that do not meet exclusion criteria need to have a colonoscopy performed and any additional polyps removed. |
|
33. |
Fecal Occult Blood Test (FOBT) for subjects 50 years of age and older; (Note: It is acceptable to perform the FOBT test without altering the subject’s anti-platelet medication. If the subject tests positive, anti-platelet medications should be stopped for 3 days and the test should be repeated.) Note: Only a sigmoidoscopy is required at screening, however if a colonoscopy is performed during screening, the FOBT test is not required. |
|
Page 17 of 66 |
Version date: October 9, 2009 |
|
Sangamo BioSciences, Inc. |
|
|
Protocol SB-509-0901 for Diabetic Neuropathy
|
Confidential
|
|
7.2. |
Subject Enrollment Procedures |
For the subject to be enrolled, the clinical site must fax all required documentation, signed by the investigator, to Sangamo BioSciences at (510) 970-6009. The Medical Monitor will review the enrollment form and supportive clinical documentation to confirm eligibility. If the subject meets the enrollment criteria, the subject will be approved for entry into the treatment phase of the protocol and will be randomized to one of the two treatment groups. A product kit number will be assigned to the subject. Please refer to the Study Reference Manual for additional details.
|
7.3. |
Baseline Duplicate Assessments |
After a subject is enrolled and randomized into the study, baseline duplicate measurements will be performed within 14 days prior to the Day 0 visit.
NOTE: Duplicate assessments cannot be performed on the same day (there must be a minimum of 1 day and a maximum of 14 days from the other assessment).
The following duplicate assessments must be performed in enrolled and randomized subjects within 14 days prior to the Day 0 visit.
|
1. |
Electrophysiological testing of the sural and peroneal nerves |
|
2. |
Quantitative sensory testing (QST) |
|
3. |
Lower extremity neurological examination |
|
7.4. |
Schedule for Treatment Period |
|
7.4.1 |
Day 0 - Baseline Evaluations (to be completed prior to treatment) |
|
1. |
Review eligibility (inclusion and exclusion criteria) |
|
2. |
Vital signs: blood pressure (systolic and diastolic), pulse, and temperature |
|
3. |
Weight |
|
4. |
Assessment for ischemic ulcers (present, absent, location, number, size), gangrene (present, absent), and lower extremity edema |
|
5. |
CBC with WBC differential and platelet count |
|
6. |
Coagulation tests PT/INR and PTT |
|
7. |
Serum chemistry: electrolytes (Na, K, CO2, Cl), CPK, CPKmb, troponin, creatinine, BUN, glucose, uric acid, total bilirubin, ALP, ALT (or SGPT), AST (or SGOT), LDH, albumin, calcium, and total protein |
|
8. |
Urine pregnancy test – must be negative prior to drug administration |
|
9. |
Spot urine test for microalbumin/creatinine |
|
10. |
Blood sample collection - 6 mL blood (3 mL plasma) sample for PCR assay and 10 mL blood (5 mL serum) sample for serum Intracellular Adhesion Molecule (ICAM) level, cytokine assay, immunogenicity testing and detection of inflammatory markers |
|
11. |
Electrophysiological testing of the sural and peroneal nerves |
|
12. |
Quantitative sensory testing |
|
13. |
Lower extremity neurological examination, including gait |
|
14. |
3 mm skin biopsy |
|
15. |
Neuropathy Total Symptom Score (NTSS-6) |
|
16. |
Visual Analog Scale for Pain Intensity (VASPI) |
|
17. |
NeuroQoL |
|
18. |
SF-36 |
|
Page 18 of 66 |
Version date: October 9, 2009 |
|
Sangamo BioSciences, Inc. |
|
|
Protocol SB-509-0901 for Diabetic Neuropathy
|
Confidential
|
|
19. |
Assessment of concomitant medications |
|
20. |
Subjects counseled to remain compliant with ACS guidelines and followup |
|
21. |
Drug administration |
|
7.4.2 |
Day 0 – 2 hours post-treatment |
|
1. |
AE query and appropriate medical evaluation if positive |
|
2. |
Blood sample collection. 6 mL blood (3 mL plasma) sample for PCR assay and 10 mL blood (5 mL serum) sample for cytokine assay, immunogenicity testing and detection of inflammatory markers. |
|
7.4.3 |
Day 60 - Evaluations to be completed prior to treatment (visit window may be ± 7 days) |
|
1. |
Vital signs: blood pressure (systolic and diastolic), pulse, and temperature |
|
2. |
Weight |
|
3. |
Assessment for ischemic ulcers (present, absent, location, number, size), gangrene (present, absent), and lower extremity edema. |
|
4. |
CBC with WBC differential and platelet count |
|
5. |
Serum chemistry: electrolytes (Na, K, CO2, Cl), CPK, CPKmb, troponin, creatinine, BUN, glucose, uric acid, total bilirubin, ALP, ALT (or SGPT), AST (or SGOT), LDH, albumin, calcium, and total protein |
|
6. |
Spot urine test for microalbumin/creatinine |
|
7. |
Blood sample collection: 6 mL blood (3 mL plasma) sample for PCR assay and 10 mL blood (5 mL serum) sample for cytokine assay, immunogenicity testing and detection of inflammatory markers. |
|
8. |
Global assessment |
|
9. |
AE query and appropriate medical evaluation if positive |
|
10. |
Assessment of concomitant medications |
|
11. |
Drug administration |
|
7.4.4 |
Day 60 – 2 hours post-treatment |
|
1. |
AE query and appropriate medical evaluation if positive |
|
2. |
Blood sample collection. 6 mL blood (3 mL plasma) sample for PCR assay and 10 mL blood (5 mL serum) sample for cytokine assay, immunogenicity testing and detection of inflammatory markers. |
|
7.4.5 |
Day 90 (visit window may be ± 7 days) |
|
1. |
Vital signs: blood pressure (systolic and diastolic), pulse, and temperature |
|
2. |
Weight |
|
3. |
Assessment for ischemic ulcers (present, absent, location, number, size), gangrene (present, absent), and lower extremity edema. |
|
4. |
CBC with WBC differential and platelet count |
|
5. |
Serum chemistry: electrolytes (Na, K, CO2 , Cl), CPK, CPKmb, troponin, creatinine, BUN, glucose, uric acid, total bilirubin, ALP, ALT (or SGPT), AST (or SGOT), LDH, albumin, calcium, and total protein |
|
6. |
HgbA1c |
|
7. |
Spot urine test for microalbumin/creatinine |
|
8. |
Blood Sample collection: 6 mL blood (3 mL plasma) sample for PCR assay and 10mL blood (5 mL serum) sample for cytokine assay, immunogenicity testing and detection of inflammatory markers. |
|
9. |
Electrophysiological testing of the sural and peroneal nerves |
|
Page 19 of 66 |
Version date: October 9, 2009 |
|
Sangamo BioSciences, Inc. |
|
|
Protocol SB-509-0901 for Diabetic Neuropathy
|
Confidential
|
|
10. |
Quantitative sensory testing (QST) of bilateral lower extremities |
|
11. |
Lower extremity neurological examination |
|
12. |
Neuropathy Total Symptom Score (NTSS-6) |
|
13. |
Visual Analog Scale for Pain Intensity (VASPI) |
|
14. |
NeuroQoL |
|
15. |
Global assessment |
|
16. |
AE query and appropriate medical evaluation if positive |
|
17. |
Assessment of concomitant medications |
|
7.4.6 |
Day 120 - Evaluations to be completed prior to treatment (visit window may be ± 7 days) |
|
1. |
Vital signs: blood pressure (systolic and diastolic), pulse, and temperature |
|
2. |
Weight |
|
3. |
Assessment for ischemic ulcers (present, absent, location, number, size), gangrene (present, absent), and lower extremity edema. |
|
4. |
CBC with WBC differential and platelet count |
|
5. |
Serum chemistry: electrolytes (Na, K, CO2 , Cl), CPK, CPKmb, troponin, creatinine, BUN, glucose, uric acid, total bilirubin, ALP, ALT (or SGPT), AST (or SGOT), LDH, albumin, calcium, and total protein |
|
6. |
HgbA1c |
|
7. |
Spot urine test for microalbumin/creatinine |
|
8. |
Blood sample collection: 6 mL blood (3 mL plasma) sample for PCR assay and 10 mL blood (5 mL serum) sample for cytokine assay, immunogenicity testing and detection of inflammatory markers. |
|
9. |
Global assessment |
|
10. |
AE query and appropriate medical evaluation if positive |
|
11. |
Assessment of concomitant medications |
|
12. |
Drug administration |
|
7.4.7 |
Day 120 – 2 hours post-treatment |
|
1. |
AE query and appropriate medical evaluation if positive |
|
2. |
Blood sample collection. 6 ML blood (3 mL plasma) sample for PCR assay and 10 mL blood (5 mL serum) sample for cytokine assay, immunogenicity testing and detection of inflammatory markers. |
|
7.4.8 |
Day 150 (visit window may be ± 7 days) |
|
1. |
3 mm skin biopsy |
|
2. |
Global assessment |
|
3. |
AE query and appropriate medical evaluation if positive |
|
4. |
Assessment of concomitant medications |
|
7.5. |
Schedule for Follow-Up Period |
|
7.5.1 |
Day 180 (visit window may be ± 7 days) |
|
1. |
Vital signs: blood pressure (systolic and diastolic), pulse, and temperature |
|
2. |
Weight |
|
Page 20 of 66 |
Version date: October 9, 2009 |
|
Sangamo BioSciences, Inc. |
|
|
Protocol SB-509-0901 for Diabetic Neuropathy
|
Confidential
|
|
3. |
Ischemic ulcers (present, absent, location, number, size) |
|
4. |
Gangrene (present, absent) |
|
5. |
Assessments for lower extremity edema. |
|
6. |
CBC with WBC differential and platelet count |
|
7. |
Serum chemistry: electrolytes (Na, K, CO2, Cl), CPK, CPKmb, troponin, creatinine, BUN, glucose, uric acid, total bilirubin, ALP, ALT (or SGPT), AST (or SGOT), LDH, albumin, calcium, and total protein |
|
8. |
HgbA1c |
|
9. |
Spot urine test for microalbumin/creatinine |
|
10. |
Urine pregnancy test |
|
11. |
Blood sample collection: 6 mL blood (3 mL plasma) sample for PCR assay and 10 mL blood (5 mL serum) sample for serum Intracellular Adhesion Molecule (ICAM) level, cytokine assay, immunogenicity testing and detection of inflammatory markers. |
|
12. |
Electrophysiological testing of the sural and peroneal nerves |
|
13. |
Quantitative sensory testing (QST) of bilateral lower extremities |
|
14. |
Lower extremity neurological examination, including gait |
|
15. |
3 mm skin biopsy |
|
16. |
Neuropathy Total Symptom Score (NTSS-6) |
|
17. |
Visual Analog Scale for Pain Intensity (VASPI) |
|
18. |
NeuroQoL |
|
19. |
SF-36 |
|
20. |
Global assessment |
|
21. |
Retinal examination with fluorescein angiography and fundus photos, performed by an ophthalmologist (Visit window for the retinal examination is ±14 days) |
|
22. |
AE query and appropriate medical evaluation if positive |
|
23. |
Assessment of concomitant medications |
|
7.5.2 |
Day 180 ± 14 days: The following duplicate assessments must be performed ± 14 days of the Day 180 visit. Duplicate assessments cannot be performed on the same day (there must be a minimum of 1 day and a maximum of 14 days from the other assessment). |
|
1. |
Electrophysiological testing of the sural and peroneal nerves |
|
2. |
Quantitative sensory testing |
|
3. |
Lower extremity neurological examination |
|
7.5.3 |
Day 240 (visit window may be ± 7 days) |
|
1. |
Vital signs: blood pressure (systolic and diastolic), pulse, and temperature |
|
2. |
Weight |
|
3. |
Assessment for ischemic ulcers (present, absent, location, number, size), gangrene (present, absent), and lower extremity edema. |
|
4. |
CBC with WBC differential and platelet count |
|
5. |
Serum chemistry: electrolytes (Na, K, CO2, Cl), CPK, CPKmb, troponin, creatinine, BUN, glucose, uric acid, total bilirubin, ALP, ALT (or SGPT), AST (or SGOT), LDH, albumin, calcium, and total protein |
|
6. |
HgbA1c |
|
Page 21 of 66 |
Version date: October 9, 2009 |
|
Sangamo BioSciences, Inc. |
|
|
Protocol SB-509-0901 for Diabetic Neuropathy
|
Confidential
|
|
7. |
Spot urine test for microalbumin/creatinine |
|
8. |
Blood sample collection: 6 mL blood (3 mL plasma) sample for PCR assay and 10 mL blood (5 mL serum) sample for cytokine assay, immunogenicity testing and detection of inflammatory markers. |
|
9. |
Global assessment |
|
10. |
AE query and appropriate medical evaluation if positive |
|
11. |
Assessment of concomitant medications |
|
7.5.4 |
Day 360 (visit window may be ± 14 days) |
|
1. |
General physical examination for all subjects, including a digital rectal exam for male subjects only. |
|
2. |
Vital signs: blood pressure (systolic and diastolic), pulse, and temperature |
|
3. |
Weight |
|
4. |
Assessment for ischemic ulcers (present, absent, location, number, size), gangrene (present, absent), and lower extremity edema. |
|
5. |
CBC with WBC differential and platelet count |
|
6. |
Coagulation tests PT/INR and PTT |
|
7. |
Serum chemistry: electrolytes (Na, K, CO2, Cl), CPK, CPKmb, troponin, creatinine, BUN, glucose, uric acid, total bilirubin, ALP, ALT (or SGPT), AST (or SGOT), LDH, albumin, calcium, and total protein |
|
8. |
HgbA1c |
|
9. |
Direct LDL cholesterol (subject should be fasting from midnight on) |
|
10. |
Spot urine test for microalbumin/creatinine |
|
11. |
Urine pregnancy test |
|
12. |
Blood sample collection: 6 mL blood (3 mL plasma) sample for PCR assay and 10 mL blood (5 mL serum) sample for serum Intracellular Adhesion Molecule (ICAM) level, cytokine assay, immunogenicity testing and detection of inflammatory markers. |
|
13. |
Electrophysiological testing of the sural and peroneal nerves |
|
14. |
Quantitative sensory testing (QST) of bilateral lower extremities |
|
15. |
Lower extremity neurological examination, including gait |
|
16. |
3 mm skin biopsy |
|
17. |
Neuropathy Total Symptom Score (NTSS-6) |
|
18. |
Visual Analog Scale for Pain Intensity (VASPI) |
|
19. |
NeuroQoL |
|
20. |
SF-36 |
|
21. |
Global assessment |
|
22. |
ECG – standard 12-lead |
|
23. |
Retinal examination with fluorescein angiography and fundus photos, performed by an ophthalmologist |
|
24. |
PSA blood test for male subjects 45 years of age and older |
|
25. |
Mammogram for female subjects 40 years of age and older |
|
26. |
FOBT for subjects 50 years of age and older |
|
27. |
Subject counseled to remain compliant with ACS guidelines and followup |
|
28. |
AE query and appropriate medical evaluation if positive |
|
Page 22 of 66 |
Version date: October 9, 2009 |
|
Sangamo BioSciences, Inc. |
|
|
Protocol SB-509-0901 for Diabetic Neuropathy
|
Confidential
|
|
29. |
Assessment of concomitant medications |
|
7.5.5 |
Day 360 ± 14 days: The following duplicate assessments must be performed ± 14 days of the Day 360 visit. Duplicate assessments cannot be performed on the same day (there must be a minimum of 1 day and a maximum of 14 days from the other assessment). |
|
1. |
Electrophysiological testing of the sural and peroneal nerves |
|
2. |
Quantitative sensory testing |
|
3. |
Lower extremity neurological examination |
|
8. |
CRITERIA FOR WITHDRAWAL FROM THE STUDY |
|
8.1. |
Discontinuation from Study Treatment and Follow-up of Subjects |
Subjects will be strongly encouraged to continue with follow-up safety evaluations if they withdraw consent from the study. If a subject discontinues from the study a conference between the investigator and medical monitor will take place to ensure that all subjects will comply with the follow-up safety evaluations of the protocol. Participation in this study will not prejudice the subject’s future medical care.
Subjects will be assessed for treatment-related adverse events and disease status.
Subjects who discontinue during the post-treatment follow-up period will not be replaced; therefore, study centers are encouraged to carefully select subjects who would be likely to complete all study procedures.
|
9. |
ADMINISTRATION OF STUDY DRUG |
|
9.1. |
Product Description |
The SB-509 drug product is a clear, colorless, and sterile liquid supplied in single use glass vials. Each 3 mL glass vial is filled with 2.2 mL of drug product that contains 2 mg/mL of pV-32Ep65 DNA plasmid, 2 mM Tris, 150 mM sodium chloride and 5% (w/v) poloxamer 188 at pH 8.0. It is intended for intramuscular administration.
|
9.2. |
Description and Manufacturer of Drug Substance |
The drug substance containing the active ingredient, plasmid pV-32Ep65, is manufactured under cGMP at Althea Technologies, Inc., San Diego, CA.
pV-32Ep65 is a typical eukaryotic expression vector bearing the CMV immediate early enhancer/promoter and a polyadenylation site from the bovine growth hormone gene. The vector backbone, pVAX-1 (3.0 kb), has been specifically designed for use in the development of DNA vaccines.
pV-32Ep65 is a 4106 base-pair DNA plasmid that encodes an engineered ZFP-TF. The therapeutic gene encodes a 3-finger DNA binding ZFP with a transactivation domain and nuclear localization signal (NLS) and was cloned into a pVAX-1 vector backbone. The ZFP-TF is a 378 amino acid protein that is composed of:
|
a) |
the NLS of the long T antigen of SV40 |
|
b) |
a designed 3-finger ZFP (32E) that binds to a 9 base pair target present in the human VEGF-A promoter region (GGGGGTGAC) |
|
c) |
the transactivation domain from the p65 subunit of the human transcription factor NF-KB |
|
Page 23 of 66 |
Version date: October 9, 2009 |
|
Sangamo BioSciences, Inc. |
|
|
Protocol SB-509-0901 for Diabetic Neuropathy
|
Confidential
|
The key features of pV-32Ep65 are illustrated in the plasmid map shown in Figure 5.
Figure 5. Circular Plasmid Map of pV-32Ep65

|
9.3. |
SB-509 and Placebo Study Drug Composition |
SB-509 is a purified, double-stranded bacterial DNA plasmid. It is formulated as a sterile, injectable solution and is filled 2.2 mlin 3-mL vials. The drug product composition is described in Table 1. It consists of pV-32Ep65 DNA plasmid (2 mg/ml), Poloxamer 188 (5% w/v), NaCI (150 mM), 2 mM Tris-HCI, pH 8.0, and sterile water for injection.
Table 1. Composition of SB-509
|
Component |
Unit Formula (mg/mL) |
|
pV-32Ep65 (plasmid DNA) |
2 mg |
|
Sodium chloride, USP (150mM NaCI) |
8.76 mg |
|
Poloxamer 188, NF (5%w/v) |
50 mg |
|
Tromethamine, pH 8.0, USP (2 mM Tris-HCI) |
0.242 mg |
|
Sterile Water for Injection, USP |
q.s. to 1.0 mL |
The final drug product is tested for appearance, identity, potency, concentration, purity, pH, conductivity, endotoxin, and sterility.
Normal saline (0.9% NaCI) will be provided in identical 3-mL vials and will serve as the placebo.
|
9.4. |
Inventory, Storage, and Handling of the Drug Product |
Labeled product will be stored at Fisher Clinical Services for distribution to the clinical sites. Sangamo BioSciences requires its sponsored investigators to maintain adequate drug inventory and security at all times (Appendix 4). Therefore, SB-509 and placebo will be supplied as a frozen liquid "to deliver"
2.0 ml. Upon receipt of labeled product, the investigator or designated individual (e.g., pharmacist) will check the details of the supplies and document receipt. As a double-blind controlled trial, the clinical supplies will be in a blinded packaging configuration containing 18 vials per kit. One kit will be assigned for each dose.
|
Page 24 of 66 |
Version date: October 9, 2009 |
|
Sangamo BioSciences, Inc. |
|
|
Protocol SB-509-0901 for Diabetic Neuropathy
|
Confidential
|
The investigator will store both SB-509 and the placebo in a locked –20°C freezer and will monitor and maintain a log of the temperature. Immediately before use, vials will be thawed at room temperature and allowed to equilibrate to room temperature for at least 10 minutes. Once thawed, vials will not be reused on subsequent days.
Empty vials and any remaining product in the used vials will be stored at room temperature at the clinical sites and must be kept with original carton. Vials should not be discarded until the Sangamo BioSciences monitor reviews the drug accountability log.
Accessibility to labeled product should only be to those individuals authorized by the investigator to dispense this study drug.
The investigator or designated individual will maintain an inventory. A drug accountability log will be provided to the pharmacist. This inventory will include the description and quantity of labeled product received during the course of this study, as well as a record of the labeled product that is dispensed. This inventory record shall indicate the quantity and description of labeled product on hand at any time during the course of this study. A Sangamo BioSciences monitor will review this inventory during interim monitoring visits.
At the conclusion or termination of this study, return or destruction of all drug supplies must be coordinated with Sangamo BioSciences. Please see the Study Reference Manual for additional details.
The investigator agrees not to supply labeled product to any person other than study personnel and subjects in this study.
In accordance with Good Clinical Practice, it is Sangamo BioSciences policy always to investigate suspected cases of fraud.
16.11 Termination of the Study
Sangamo BioSciences retains the right to terminate the study and remove all study materials at any time. Specific instances that may precipitate such termination are as follows:
|
● |
Completion of the study at an investigational site |
|
● |
Unanticipated adverse medical experiences in this or other studies indicating a potential health hazard caused by the investigational drug |
|
● |
Significant protocol deviation and/or lack of compliance and cooperation on the part of the investigator; such as failure to obtain signed informed consent prior to initiating study-related procedures, unsatisfactory subject enrollment with regard to quality or quantity, deviation from protocol requirements without prior approval from Sangamo BioSciences, or inaccurate and/or incomplete data recording on a recurrent basis |
|
● |
Investigator withdrawal from participation in the study |
|
● |
Withdrawal of investigational drug from investigational use |
|
● |
Termination of this study by Sangamo BioSciences |
16.12 Record Retention
The investigator should retain essential documents according to 21 CFR 312.62(c) and ICH Guidelines for Good Clinical Practices (E6), until at least 2 years after the last approval of a marketing application in an ICH region and until there are no pending or contemplated marketing applications in an ICH region or at least 2 years have elapsed since the formal discontinuation of clinical development of the investigational product. These documents should be retained for a longer period, however, if required by the applicable regulatory requirements or by an agreement with Sangamo BioSciences. It is the responsibility of Sangamo BioSciences to inform the investigator as to when these documents no longer need to be retained.
Records to be retained by the investigator include, but are not restricted to, protocols; amendments; investigator’s brochure; Investigator agreement (including financial agreement); IRB/IEC/IBC applications; approvals and composition; copies of the Form FDA 1572; pre-study and follow-up financial disclosure; completed, signed, and dated informed consents; subject medical records; case report forms;
monitoring log; serious adverse event reports; IRB notifications; subject screening/enrollment log; study personnel signature log; drug accountability record and logs; clinical laboratory normal ranges and accreditation certificate; and all correspondence between study monitor, study sponsor, and IRB/IEC/IBC.
|
Page 25 of 66 |
Version date: October 9, 2009 |
|
Sangamo BioSciences, Inc. |
|
|
Protocol SB-509-0901 for Diabetic Neuropathy
|
Confidential
|
Subject files and other source data must be kept for the maximum period of time permitted by the hospital, institution, or private practice, but not less than 15 years.
16.13 Confidentiality
Subject medical information obtained for the purpose of this trial is confidential, and disclosure to third parties, other than those noted below, is prohibited. Upon the subject’s request and receipt of written permission, medical information may be given to his/her personal physician or other appropriate medical personnel responsible for the subject’s welfare.
Data generated for this study must be available for inspection upon request to representatives of the FDA, other national or local health authorities, Sangamo BioSciences, and the associated IRB/IEC/IBC.
Release of research results or data that reveal subject names or other identifiers, such as photographs, audiotapes, or videotapes, must be carried out in accordance with the Department of Health and Human Services’ proposed Standards for Privacy of Individual Health information, 45 CFR 164.508. Written authorization must be obtained from the subject and the IRB/IEC/IBC prior to the release of such information. Identifiable subject data may not be used for the purpose of promoting the study drug.
16.14 Publication Statement
It is intended that the results of the study be published in scientific literature. Results may also be used in submissions to regulatory authorities. The following conditions are to protect commercial confidential materials (e.g., patents), not to restrict publication.
All information concerning the labeled product under study (such as patent applications, formulae, basic scientific data, or formulation information supplied to the investigator and not previously published) is considered confidential by Sangamo BioSciences and shall remain the sole property of Sangamo BioSciences.
It is understood by the investigator that the results of this clinical trial may be used by Sangamo BioSciences in registration documents for regulatory authorities in the U.S. or abroad, or for public dissemination in the form of papers, abstracts, posters, or other informational materials to be presented at scientific meetings, or published in professional journals, or as a part of an academic thesis by an investigator.
All proposed publications, papers, abstracts, or other written materials related to the study, or an outline of any proposed oral presentations, shall be submitted to
Sangamo BioSciences for approval at least 45 days prior to (1) submission for publication or (2) any proposed oral disclosure to a third party. Sangamo BioSciences shall have the right to review and comment on such written material or outline, and to confirm the accuracy of the data described therein by comparison with that collected during the course of this study. In the event that Sangamo BioSciences determines that an enabling description of patentable subject matter is contained in such written material or outline, it shall notify the clinical site(s) within 1 month after receipt by Sangamo BioSciences, and Sangamo BioSciences will have an additional 90 days for review.
In the event of publication of multi-center data, the number of subjects enrolled by each investigator will usually determine the order of participation, unless otherwise agreed upon by the investigators and Sangamo BioSciences.
17STUDY FUNDING
The costs necessary to perform the study will be agreed to by the investigator and/or the management of the study facility and will be documented in a separate financial agreement. All financial agreements will be signed by the investigator and Sangamo BioSciences.
18REFERENCES
American Cancer Society (2009). Cancer Facts & Figures 2009. Atlanta, Ga: American Cancer Society.
Argoff, C.E., Backonja, M.M., Belgrade, M.J., Bennett, G.J., Clark, M.R., Cole, B.E., Fishbain, D.A., Irving, G.A., McCarberg, B.H., and McLean, M.J. (2006). Consensus guidelines: treatment planning and options. Mayo Clin. Proc. 81, S12–S25.
|
Page 26 of 66 |
Version date: October 9, 2009 |
|
Sangamo BioSciences, Inc. |
|
|
Protocol SB-509-0901 for Diabetic Neuropathy
|
Confidential
|
Bastyr, E., Price, K., Bril, V., et al. (2005). Development and Validity Testing of the Neuropathy Total Symptom Score-6: Questionnaire for the Study of Sensory Symptoms of Diabetic Peripheral Neuropathy. Clinical Therapeutics. 27:1278-1294
Biessels, G.-J., Cristino, N.A., Rutten, G.-J., Hamers, F.P.T., Erkelens, D.W., and Gispen, W.H. (1999). Neurophysiological changes in the central and peripheral nervous system of streptozotocin-diabetic rats. Course of development and effects of insulin treatment. Brain 122, 757–768.
Bril, V. (1999). NIS-LL: The primary measurement scale for clinical trial endpoints in diabetic peripheral neuropathy. European Neurology. 41(Suppl 1):8-13
Boulton, A.J.M., Malik, R.A.M., Arezzo, J.C., and Sosenko, J.M. (2004). Diabetic somatic neuropathies. Diabetes Care. 27, 1458-1486.
Calcutt, N.A., Allendoerfer, K.L., Mizisin, A.P., Middlemas, A., Freshwater, J.D., Burgers, M., Ranciato, R., Delcroix, J.-D., Taylor, F.R., Shapiro, R., et al. (2003). Therapeutic efficacy of sonic hedgehog protein in experimental diabetic neuropathy. J. Clin. Invest. 111,507–514.
Elson, D.A., Thurston, G., Huang, L.E., Ginzinger, D.G., McDonald, D.M., Johnson, R.S., and Arbeit, J.M. (2001). Induction of hypervascularity without leakage or inflammation in transgenic mice overexpressing hypoxia-inducible factor-1alpha. Genes Dev. 15, 2520–2532.
Feldman, E.L. (2002). Diabetic Neuropathy, Presented at May 16, 2002 Anesthetic and Life Support Drugs Advisory Committee, FDA. (http://www.fda.gov/ohrms/dockets/ac/02/slides/3864S1_01_Hertz.ppt).
Feldman, E.L., Russell, J.W., Sullivan, K.A., and Golovoy, D. (1999). New insights into the pathogenesis of diabetic neuropathy. Curr. Opin. Neurol. 12, 553–563.
Levin, B., Lieberman, D.A., McFarland, et al. (2008). Screening and Surveillance for the Early Detection of Colorectal Cancer and Adenomatous Polyps, 2008: A Joint Guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology . Published online March 5, 2008. CA Cancer J Clin. 58.
Malik, R.A. (2000). Can diabetic neuropathy be prevented by angioensin-converting enzyme inhibitors? Ann. Med. 32, 1–5.
McNeely, M., Boyko, E., Ahroni, J., Stensel, V.L., Reiber, G.E., Smith, D.G., and Pecoraro, R.F. (1995). The independent contributions of diabetic neuropathy and vasculopathy in foot ulceration. How great are the risks? Diabetes Care 18, 216–219.
O’Brien, P.C. (1984). Procedures for comparing samples with multiple endpoints, Biometrics, 40, 1079–1087.
Poncelet, A.N. (2003). Diabetic polyneuropathy: Risk factors, patterns of presentation, diagnosis and treatment. Geriatrics 58, 16–30.
Potter, P., Maryniak, O., Yaworsky, R., and Jones, I. (1998). Incidence of peripheral neuropathy in the contralateral limb of persons with unilateral amputation due to diabetes. Rehabil. Res. Dev. 25, 335–339.
Price, S.A., Dent, C., Duran-Jimenez, B., Liang, Y., Zhang, L., Rebar, E.J., Case, C.C., Gregory, P.O., Martin, T.J., Spratt, S.K., Tomlinson, D.R. (2006). Gene Transfer of an Engineered Transcription Factor Promoting Expression of VEGF-A Protects Against Experimental Diabetic Neuroapthy. Diabetes. 55, 1847-1854.
Quattrini, C et al. Reduced vascular endothelial growth factor expression and intraepidermal nerve fiber loss in human diabetic neuropathy. (2008). Diabetes Care.31(1):140-145
Rebar, E.J., Huang, Y., Hickey, R., Nath, A.K., Meoli, D., Nath, S., Chen, B., Xu, L., Liang, Y., Jamieson, A.C., et al. (2002). Induction of angiogenesis in a mouse model using engineered transcription factors. Nat. Med. 8, 1427–1432.
Saslow, D., Boetes, C., Burke, W., et. al. for the American Cancer Society Breast Cancer Advisory Group (2007). American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 57:75-89.
|
Page 27 of 66 |
Version date: October 9, 2009 |
|
Sangamo BioSciences, Inc. |
|
|
Protocol SB-509-0901 for Diabetic Neuropathy
|
Confidential
|
Schratzberger, P., Walter, D.H., Rittig, K., Bahlmann, F.H., Pola, R., Curry, C., Silver, M., Krainin, J.G., Weinberg, D.H., Ropper, A.H., et al. (2001). Reversal of experimental diabetic neuropathy by VEGF gene transfer. J. Clin. Invest. 107, 1083–1092.
Simovic, D., Isner, J.M., Ropper, A.H., Pieczek, A., and Weinberg, D.H. (2001). Improvement in chronic ischemic neuropathy after intramuscular phVEGF165 gene transfer in patients with critical limb ischemia. Arch. Neurol. 58, 761–768.
Skyler, J. (1997). Diabetes Mellitus, Types I and II. In Textbook of Internal Medicine, 3rd Edition, Kelley, W.N., ed. (Philadelphia: Lippincott-Raven Publishers), pp. 2238–2252.
Stratton, I., Adler, A., Neil, H., Matthews, D.R., Manley, S.E., Cull, C.A., Hadden, D., Turner, R.C., and Homan, R.R. (2000). Association of glycaemia with macrovasclar and microvasclar complications of type 2 diabetes (UKPDS 35): Prospective observational study. BMJ 321, 405–412.
Thomas, P. and Tomlinson, D. (1993). Diabetic and hypoglycemic neuropathy. In Peripheral Neuropathy, 3rd Edition, Dyck, P. Thomas, P., eds. (Philadelphia: W.B. Saunders Company), pp. 1219–1250.
UK Prospective Diabetes Study Group. (1998). Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 352, 837–853.
Vileikyte, L, Peyrot, M, Bundy, C, Rubin, RR, Leventhal, H, Mora, P, Shaw, JE, Baker, P, and Boulton, AJ. (2003) The development and validation of a neuropathy- and foot ulcer-specific quality of life instrument. Diabetes Care. 26(9):2549-2555.
Whitlock, P.R., Hackett, N.R., Leopold, P.L., Rosengart, T.K., and Crystal, RG. (2004). Adenovirus-mediated transfer of a minigene expressing multiple isoforms of VEGF is more effective at inducing angiogenesis than comparable vectors expressing individual VEGF cDNAs. Mol. Ther. 9, 67–75.
|
Page 28 of 66 |
Version date: October 9, 2009 |
|
Sangamo BioSciences, Inc. |
|
|
Protocol SB-509-0901 for Diabetic Neuropathy
|
Confidential
|
APPENDIX 1:SCHEDULE OF EVENTS
|
Visit Number |
VO |
VI/V2 |
|
V3 |
|
V4 |
V5 |
|
V6 |
V7/V8 |
V9 |
V10/11 |
|
|
|
Dose 1 |
|
Dose 2 |
|
|
Dose 3 |
|
|
|
|
|
|
Visit |
Day-84 Screening |
Day 0 |
Day 0 (2 hours post-tx) |
60 |
Day 60 (2 hours post-tx) |
90 |
120 |
Day120 (2 hours post-tx) |
150 |
180 |
240 |
360n |
|
Informed consent |
X |
|
|
|
|
|
|
|
|
|
|
|
|
Eligibility |
X |
X |
|
|
|
|
|
|
|
|
|
|
|
Medical history |
X |
|
|
|
|
|
|
|
|
|
|
|
|
Physical exama |
X |
|
|
|
|
|
|
|
|
|
|
X |
|
Vital signsb |
X |
X |
|
X |
|
X |
X |
|
|
X |
X |
X |
|
Height |
X |
|
|
|
|
|
|
|
|
|
|
|
|
Weight |
X |
X |
|
X |
|
X |
X |
|
|
X |
X |
X |
|
Ischemic ulcers/Gangrene |
|
X |
|
X |
|
X |
X |
|
|
X |
X |
X |
|
Lower extremity edema |
X |
X |
|
X |
|
X |
X |
|
|
X |
X |
X |
|
CBC with WBC differential and platelet count |
X |
X |
|
|
|
|
|
|
|
|
|
X |
|
PT/INR and PTT |
X |
X |
|
X |
|
X |
X |
|
|
X |
X |
X |
|
Serum chemistryc |
X |
|
|
|
|
X |
X |
|
|
X |
X |
X |
|
HgbA1c |
X |
|
|
|
|
|
|
|
|
|
|
|
|
HIV serology |
X |
|
|
|
|
|
|
|
|
|
|
|
|
Serum pregnancy test |
X |
|
|
|
|
|
|
|
|
|
|
|
|
Urine pregnancy testd |
|
X |
|
|
|
|
|
|
|
X |
|
X |
|
Urinalysise |
X |
|
|
|
|
|
|
|
|
|
|
|
|
Spot urine test for microalbumin/creatinine |
X |
X |
|
X |
|
X |
X |
|
|
X |
X |
X |
|
Blood samplef |
X |
X |
X |
X |
X |
X |
X |
X |
|
X |
X |
X |
|
Drug administration |
|
X |
|
X |
|
|
X |
|
|
|
|
|
|
Electrophysiological testing (sural and peroneal nerves)g |
X |
XX* |
|
|
|
X |
|
|
|
XX* |
|
XX* |
|
QSTg |
X |
XX* |
|
|
|
X |
|
|
|
XX* |
|
XX* |
|
Lower extremity neurological examination |
X |
XX* |
|
|
|
X |
|
|
|
XX* |
|
XX* |
|
3 mm skin biopsy (IENFD) |
X |
X |
|
|
|
|
|
|
X |
X |
|
X |
|
NTSS-6 |
X |
X |
|
|
|
X |
|
|
|
X |
|
X |
|
VASPI |
X |
X |
|
|
|
X |
|
|
|
X |
|
X |
|
Neuro-QoL |
X |
X |
|
|
|
X |
|
|
|
X |
|
X |
|
SF-36 |
X |
X |
|
|
|
|
|
|
|
X |
|
X |
|
Global assessment |
|
|
|
X |
|
X |
X |
|
X |
X |
X |
X |
|
12-lead ECGh |
X |
|
|
|
|
|
|
|
|
|
|
X |
|
Chest X-rayh |
X |
|
|
|
|
|
|
|
|
|
|
|
|
Retinal examination and photos |
X |
|
|
|
|
|
|
|
|
X |
|
X |
|
PSAi |
X |
|
|
|
|
|
|
|
|
|
|
X |
|
Mammogrami |
X |
|
|
|
|
|
|
|
|
|
|
X |
|
Pap Smeark |
X |
|
|
|
|
|
|
|
|
|
|
|
|
Page 29 of 66 |
Version date: October 9, 2009 |
|
Sangamo BioSciences, Inc. |
|
|
Protocol SB-509-0901 for Diabetic Neuropathy
|
Confidential
|
|
Visit Number |
VO |
VI/V2 |
|
V3 |
|
V4 |
V5 |
|
V6 |
V7/V8 |
V9 |
V10/11 |
|
|
|
Dose 1 |
|
Dose 2 |
|
|
Dose 3 |
|
|
|
|
|
|
Visit |
Day-84 Screening |
Day 0 |
Day 0 (2 hours post-tx) |
60 |
Day 60 (2 hours post-tx) |
90 |
120 |
Day120 (2 hours post-tx) |
150 |
180 |
240 |
360n |
|
FOBTl |
X |
|
|
|
|
|
|
|
|
|
|
X |
|
Sigmoidoscopyl |
X |
|
|
|
|
|
|
|
|
|
|
|
|
AEsm |
|
|
X |
X |
X |
X |
X |
X |
X |
X |
X |
X |
|
Assessment of concomitant medications |
X |
X |
|
X |
|
X |
X |
|
X |
X |
X |
X |
|
Subject counseled to remain compliant with ACS guidelines and follow-up |
|
X |
|
|
|
|
|
|
|
|
|
X |
* Duplicate assessments (XX) must be performed in enrolled and randomized subjects within14 days prior to the Day 0 visit, or performed within ± 14 days of the projected visit day (Day 180 and Day 360).
a Physical examination for all subjects to include a digital rectal examination for male subjects only.
b Vital signs to include systolic/diastolic pressure, pulse, and temperature.
c Serum chemistry: electrolytes (Na, K, C02, Cl), CPK, CPKmb, troponin, creatinine, BUN, glucose, uric acid, total bilirubin, ALP, ALT (or SGPT), AST (or SGOT), LDH, albumin, calcium, and total protein; In addition: On screening day: vitamin B12, T4/TSH, direct LDL; On Day 360: direct LDL
d Urine pregnancy test: result must be negative prior to drug administration on Day 0.
e Urinalysis: test for the presence of glucose, protein, bilirubin, blood, pH, and specific gravity.
f Blood samples will be used for PCR assay, cytokine assay, immunogenicity testing, ICAM assay, and detection of inflammatory markers.
g The screening (Day -84) disease activity assessments may be done in stages, but they must be completed prior to Day 0.
h If none available in the preceding 12 weeks.
I PSA blood test for male subjects age 45 years or older.
j Mammogram: for females older than 40 years of age. A mammogram performed within 3 months of screening can be used for screening eligibility.
k Pap smear for females who have not had a procedure within a year of screening.
l FOBT for subjects 50 years of age or older. Only a sigmoidoscopy is required at screening, however if a colonoscopy is performed during screening, an FOBT is not required. Sigmoidoscopy for subjects 50 years of age or older. A sigmoidoscopy or colonoscopy performed within a year of screening may be used for screening eligibility.
m Query for AEs and appropriate medical evaluation if positive.
n Day 360 or early termination.
|
Page 30 of 66 |
Version date: October 9, 2009 |
|
Sangamo BioSciences, Inc. |
|
|
Protocol SB-509-0901 for Diabetic Neuropathy
|
Confidential
|
|
9.5. |
SB-509 Administration |
9.5.1. Overview
150 subjects will be randomized in a 1:1 ratio stratified by study site and screening IENFD (<9, ≥9) to treatment with SB-509 or placebo:
1) SB-509 treatment: 60 mg of SB-509 on Day 0, Day 60 and Day 120.
2) Placebo on Day 0, Day 60 and Day 120.
30 mg of SB-509 or an equal volume of placebo will be injected (IM) into each lower limb for a total dose of 60 mg. Each subject will receive a total of three treatments. The drug will be supplied in 3-mL vials and will be injected in 0.5 or 1 mL volumes each injection in the lower leg and thigh, respectively. See Appendix 4 for detailed pharmacy preparation and subject injection instructions.
Injection Procedure
The injection site in the muscle will allow the deposit of 0.5 mL and 1.0 mL doses of study drug by inserting a needle along the long axis of the muscle fiber (i.e., parallel with the femoral artery) up to its hub (the needle’s full insertion point) and depositin 0.5 mL doses to the lower leg injection sites or 1.0 mL doses to the thigh injection sites.
9.5.2. Injection Needles and Volumes for Each Injection Site
Needles (25 gauge; 1- to 1.5-inch or 27 gauge; 1.25 inch) with 1 mL syringes are to be used. Care should be taken to avoid injection into any area of skin ulceration.
|
9.6. |
Precautions |
SB-509 is an investigational drug, and there is a possible risk of anaphylaxis. SB-509 should be administered in a setting where emergency treatment is available for anaphylaxis. If any serious allergic or anaphylactoid reaction occurs, administration should be immediately discontinued and appropriate therapy initiated. Subjects must remain in the clinic for 2 hours after administration of SB-509.
|
9.7. |
Premedications |
NSAIDs or acetaminophen may be taken prior to or after injections for pain. Diphenhydramine, oral or topical, may be used for pruritus.
|
9.8. |
Dose Modifications |
No dose modifications will be allowed.
|
10. |
SAFETY |
|
10.1. |
Potential Risks |
10.1.1. SB-509
SB-509 has been administered to a total of 164 subjects with diabetic peripheral neuropathy in four clinical trials. Twenty-seven subjects received SB-509 as a single treatment; 75 subjects as a repeat dosing (Days 0, 60, and 120), and 30 subjects as a repeat dosing (Days 0 and 90). For the single treatment, separate groups received increasing doses of 1 mg, 5 mg, 15 mg, 30 mg, or 60 mg. For the repeat dosing, the dose was 60 mg. The length of follow-up was 180 days after the single dose, and 360 days after the first dose of the repeat dosing schedule.
SB-509 was well-tolerated. Most of the AEs were injection site reactions, which were mild to moderate in severity. There were no drug-related SAEs, dose-limiting toxicities, deaths, or discontinuations of study drug because of AEs. Immunogenicity was not triggered by SB-509. There was no evidence of retinal neovascularization or tumorigenesis.
Further information is provided in the SB-509 DN Clinical Investigator’s Brochure.
|
Page 31 of 66 |
Version date: October 9, 2009 |
|
Sangamo BioSciences, Inc. |
|
|
Protocol SB-509-0901 for Diabetic Neuropathy
|
Confidential
|
10.1.2. Vascular Endothelial Growth Factor
SB-509 induces the production of all isoforms of VEGF. Clinical studies have been performed with recombinant human VEGF and VEGF plasmid DNA gene therapy. A review of the previous human experience with VEGF is in the SB-509 DN Clinical Investigator’s Brochure. The major potential risk is the augmentation of growth of tumors and a reversible leg edema with VEGF plasmid DNA gene therapy.
10.1.3. Poloxamer 188
Poloxamer 188 is being used as a delivery enhancer in SB-509. It is a complex polymer that is commonly used in stool softeners and foods. It has an effect on red blood cell elasticity and has been used parenterally for the treatment of Sickle Cell Crisis. A review of the previous human experience is in the SB-509 DN Clinical Investigator’s Brochure. The major risk is an idiosyncratic allergic reaction that would be associated with any component of the formulation.
10.1.4. Gene Transfer
There is a risk that people who receive gene transfer may develop new tumors. This risk is primarily associated with viral gene transfer vectors that integrate into the cellular DNA and may induce carcinogenesis. This risk with plasmid DNA gene transfer is extremely low.
10.1.5. Skin Biopsy
Skin biopsies will be obtained during the trial for the analysis of intraepidermal nerve fiber density to see if treatment with SB-509 will affect the number of epidermal nerve fibers in the skin. The size of the biopsies is 3 mm in diameter. Side effects include pain, bleeding, low rates of infection (1/500 biopsies), and, rarely, depigmented skin at the healed biopsy site.
|
11. |
EFFICACY |
|
11.1. |
Potential Benefits |
It is likely that the subjects receiving this treatment will not derive any benefit from their participation in this trial. However, based on the results to date, subjects receiving SB-509 treatment may have improvements in nerve conduction velocities and vibration perception threshold.
|
11.2. |
Efficacy Criteria |
11.2.1. Clinical Evaluation
A neurological evaluation, electrophysiological test, and quantitative sensory testing will be performed at screening, on Day 0 pre-treatment, on Days 90, 180, and 360. Duplicate measurements will be completed within 14 days prior to the Day 0 visit, and ±14 days of Day180 and Day 360 visits. Note: Neurological evaluation must be performed by a qualified medical doctor
11.2.2. Disease Assessment
Diabetic peripheral neuropathy will be evaluated by using the following scales and modalities based on the neurological examination data, electrophysiological testing data, skin biopsy data, subject neurological questionnaire, subject pain assessment, and Quality of Life questionnaires.
|
● |
Electrophysiological testing using nerve conduction velocity (NCV) studies (Appendix 7) |
|
● |
Signs using Neuropathy Impairment Score – Lower Limb (NIS-LL) (Appendix 5) |
|
● |
Lower Extremity Neurological Sensory Exam (Appendix 6) |
|
● |
Quantitative sensory testing (QST) with the Vibratron II instrument (Appendix 8) |
|
● |
lntraepidermal Nerve Fiber Density (IENFD) (Appendix 9) |
|
● |
Neuropathy Total Symptom Score (NTSS-6) (Appendix 10) |
|
● |
Visual analog scale for pain intensity (VASPI) (Appendix 11) |
|
Page 32 of 66 |
Version date: October 9, 2009 |
|
Sangamo BioSciences, Inc. |
|
|
Protocol SB-509-0901 for Diabetic Neuropathy
|
Confidential
|
|
● |
Neuro-Qo(Appendix 12) |
|
● |
SF-36 (Appendix 13) |
|
● |
Global Assessment (Appendix 14) |
|
12. |
CONCOMITANT MEDICATIONS |
The investigator will record all concomitant medications, including those given for treatment of adverse events on the concomitant medication page in the subject's case report form. Any medication taken by the subject from screening throughout the course of the study, including over-the-counter medicinal products, dietary supplements, and herbal medications, should be recorded on this form. Medications may be adjusted as needed throughout the course of the study.
Subjects will be evaluated for pain, and use of pain medications will be recorded from the time of the first treatment throughout the course of the study.
If required after Day 0, antibiotics must be completed at least 2 weeks prior to the dose and the subject must be asymptomatic prior to dosing.
|
13. |
ADVERSE EVENTS |
|
13.1. |
Adverse Event Reporting Period |
The screening period is defined as starting with screen procedures and ending with the first treatment. The treatment period is defined as starting with the first treatment and ending 4 weeks after the last treatment. The follow-up period is defined as starting 4 weeks after the last treatment until the Day 360 visit.
During the screening period, study procedural related adverse events will be reported. During both the treatment and follow-up periods, subjects will be queried and events will be assessed at each clinic visit. All adverse events will be reported. Subjects will be reminded to immediately report any Serious Adverse Event to the investigator.
|
13.2. |
Definitions |
An adverse event is any untoward medical occurrence in a patient or clinical investigation subject that is temporally related to protocol procedures, including the administration of a labeled product at any dose, but which does not necessarily have a causal relationship with the treatment.
The term adverse event also applies to laboratory findings or results of other diagnostic procedures that are considered to be clinically relevant (e.g., that required unscheduled diagnostic procedures or treatment measures, or resulted in withdrawal from the study).
An adverse drug reaction (ADR) occurring in a clinical study is an untoward medical occurrence in a patient or clinical investigation subject that is possibly or probably causally related to the administration of a labeled product. Such ADRs are a subset of the adverse events defined above.
The term “adverse event” could include any of the following events which develop or increase in severity during the course of the study. Examples include:
|
● |
Any sign, symptom, or physical examination finding that worsens in nature, severity, or frequency compared to baseline. Whether thought to be related or unrelated to the condition under study |
|
● |
Any clinically significant laboratory abnormality or laboratory abnormality that requires medication or hospitalization |
|
● |
All reactions from study drug, including those occurring as a result of an overdose, abuse, withdrawal phenomena, sensitivity, or toxicity to study drug |
|
● |
Concurrent illness |
|
● |
Injury or accident |
|
Page 33 of 66 |
Version date: October 9, 2009 |
|
Sangamo BioSciences, Inc. |
|
|
Protocol SB-509-0901 for Diabetic Neuropathy
|
Confidential
|
A pre-existing condition is one that is present prior to or at the start of the study and is to be reported as part of the subject’s medical history. It should be reported as an adverse event only if the frequency, intensity, or the character of the condition worsen during study treatment.
An unexpected adverse event is one not identified in nature, severity, or frequency in the current protocol or the SB-509 DN Clinical Investigator’s Brochure.
|
13.3. |
Recording of an Adverse Event |
The principal investigator is responsible for evaluating all adverse events, obtaining supporting documents, and determining that documentation of the event is adequate. He/she is responsible for determining the severity and relationship to the investigational drug. The principal investigator may delegate these duties to sub investigators and must assure that these sub-investigators are qualified to perform these duties under the supervision of the principal investigator.
All adverse events will be recorded in the subject’s case report form (CRF). The detailed description of the event will include appropriately graded severity of the adverse event and its relationship to the study drug.
Severity will be categorized by toxicity grade according to the revised NCI Common Terminology Criteria for Adverse Events v4.0 (CTCAE) (Appendix 2).
Adverse events not listed in the NCI CTCAE will be evaluated by using the following criteria:
|
● |
Grade 1, Mild: Subject is aware of signs and symptoms, are easily tolerated; usually transient and requiring no special treatment; does not interfere with usual daily activities |
|
● |
Grade 2, Moderate: May be ameliorated by simple therapeutic measures; sufficient to restrict; should not prevent usual daily activities |
|
● |
Grade 3, Severe: Incapacitating; inability to perform usual daily activities |
|
● |
Grade 4, Life-threatening/Disabling: Subject was at risk of death or significant disability at the time of the event |
The relationship of the adverse event to the investigational drug will be determined by the principal investigator and will be categorized as:
|
● |
Not Related: The adverse event is clearly related to other factors, such as the subject’s clinical state, environmental factors, or other modes of therapy or concomitant drugs administered to the subject. |
|
● |
Related: The adverse event is temporally associated with the use of the study drug, and/or a causal relationship between the study drug and the adverse event is at least a reasonable possibility, i.e., the relationship cannot be ruled out. |
All Grade 3 and 4 clinical laboratory results that represent an increase in severity from baseline will be reported as adverse events. A Grade 1 or 2 clinical laboratory abnormality should be reported as an adverse event only if it is considered clinically significant by the investigator.
In the event of death, the cause of death should be recorded as the adverse event and reported as an SAE (see Section 14). “Death” is not the adverse event; “death” is an outcome. A copy of the death certificate should be obtained. Because the long-term effects of gene transfer are not known, the NIH would like an autopsy in the event of death. If an autopsy is performed, a copy of the autopsy report should be obtained.
|
Page 34 of 66 |
Version date: October 9, 2009 |
|
Sangamo BioSciences, Inc. |
|
|
Protocol SB-509-0901 for Diabetic Neuropathy
|
Confidential
|
|
14. |
SERIOUS ADVERSE EVENT |
|
14.1. |
Serious Adverse Event Reporting |
“Serious” events, whether or not unexpected or considered to be associated with the use of the labeled product, must be communicated to Sangamo BioSciences upon discovery of the event, either by telephone or fax within 24 hours.
|
Medical Monitor: |
Ely Benaim, M.D |
|
Phone Number: |
(510) 970-7868 |
|
|
(510) 621-8533, mobile |
|
Fax Number: |
(510) 970-6009 |
The investigator is responsible for promptly notifying the Institutional Review Board (IRB) or Independent Ethics Committee (IEC), in accordance with local regulations, of all serious adverse events.
The National Institutes of Health (NIH) requires that all investigators participating in gene transfer research report all drug related serious adverse events immediately to the FDA, NIH, and Institutional Biosafety Committee (IBC). Sangamo BioSciences will assume responsibility for NIH and FDA reporting.
All “serious” events must be followed with appropriate medical management until resolved or stabilized.
|
14.2. |
Definitions |
A "Serious" Adverse Event (SAE) is defined as any event that suggests a significant hazard, contraindication, side effect, or precaution. An SAE is also any adverse event or adverse drug reaction that, at any dose, results in the following outcomes:
|
· |
death |
|
· |
life-threatening condition |
|
· |
in-patient hospitalization or prolongation of an existing hospitalization |
|
· |
persistent or significant disability/incapacity |
|
· |
congenital anomaly/birth defect in the offspring of an exposed subject |
An important medical event that may not result in death, be life-threatening, or require hospitalization may be considered a serious adverse drug experience when, based upon appropriate medical judgment, it jeopardizes the subject and may require medical or surgical intervention to prevent one of the outcomes listed in this definition.
A life-threatening adverse event is defined as any adverse experience that places the subject, in the view of the investigator, at immediate risk of death from the reaction as it occurred, i.e., it does not include a reaction that, had it occurred in a more severe form, might have caused death.
|
14.3. |
Recording of a Serious Adverse Event |
Serious Adverse Events reported by telephone must be recorded on a written Serious Adverse Event Report Form, provided by Sangamo BioSciences. The SAE report form must be faxed to the medical monitor within 24 hours.
The medical monitor will then advise the investigator regarding the nature of any further information or documentation that is required. Follow-up reports must be submitted in a timely fashion as additional information becomes available.
|
15. |
STATISTICAL METHODS AND DATA ANALYSIS |
|
15.1. |
Sample Size |
The results from SB-509-601 showed a treatment effect of SB-509 in patients with baseline serum ICAM > 200 ng/mL. The change from baseline in sural NCV for treated patients showed an increase in m/s of 1.06 compared to a decrease in placebo of -1.18. With 150 patients (75 per group) the power to detect a similar difference with a standard deviation of 4 and alpha=0.05 is 93%, using Wilcoxon’s rank-sum test.
|
Page 35 of 66 |
Version date: October 9, 2009 |
|
Sangamo BioSciences, Inc. |
|
|
Protocol SB-509-0901 for Diabetic Neuropathy
|
Confidential
|
|
15.2. |
Randomization |
150 subjects will be randomized in a 1:1 ratio to treatment with SB-509 or placebo stratified by study site and screening IENFD (<9, ≥9):
|
1) |
SB-509 treatment: 60 mg of SB-509 on Day 0, Day 60 and Day 120. |
|
2) |
Placebo on Day 0, Day 60 and Day 120. |
|
15.3. |
Statistical Methods/Data Analysis |
The statistical analyses will be reported using summary tables, figures, and data listings. Statistical tests will be two-sided at the alpha =0.05 significance level. All analyses and tabulations will be performed using SAS® or SPLUS. Continuous variables will be summarized with means, standard deviations, medians, minimums, and maximums. Categorical variables will be summarized by counts and by percentages of subjects in corresponding categories. All raw data obtained from the case report forms as well as any derived data will be included in data listings.
All efficacy analyses will be performed on the intent-to-treat population. All safety analyses will be performed on all subjects that received any study medication.
All tables will report summary results by treatment and dose group.
|
15.4. |
Intent-to-Treat Population |
All subjects enrolled and randomized will be included in the intent-to-treat population and analyzed by the treatment received at Day 0.
|
15.5. |
Subject Disposition |
Summaries will include the number of randomized subjects, the number of subjects receiving study medication, the number of subjects completing the study, and the reasons for discontinuation.
|
15.6. |
Demographics |
Demographic variables include age, sex, and race. Other baseline characteristics include medical history, physical exam, duration of diabetes, duration of neuropathic pain, resting ankle-brachial systolic pressure index, and baseline assessments of diabetic neuropathy by symptoms, by neurological examination, by lower extremity electrophysiological testing and by quantitative sensory testing.
|
15.7. |
Efficacy |
The primary measurement of efficacy is:
|
· |
Sural Nerve Conduction Velocity (NCV) |
The secondary measurements of efficacy are:
|
· |
Neuropathy Impairment Score-Lower Limb (NIS-LL) |
|
· |
Lower Extremity Neurological Sensory Exam |
|
· |
Motor Nerve Conduction Velocity (NCV) |
|
· |
Quantitative Sensory Testing (QST) |
|
· |
lntraepidermal Nerve Fiber Density (IENFD) |
|
· |
Neuropathy Total Symptom Score (NTSS-6) |
|
· |
Visual Analog Scale for Pain Intensity (VASPI) |
|
· |
Quality of Life (QOL)- NeuroQoL and SF-36 |
|
· |
Global assessment |
|
Page 36 of 66 |
Version date: October 9, 2009 |
|
Sangamo BioSciences, Inc. |
|
|
Protocol SB-509-0901 for Diabetic Neuropathy
|
Confidential
|
Primary Efficacy Analyses
The Day 180 change from baseline sural NCV measurements will be compared by treatment group using the Wilcoxon Rank-Sum test at an alpha level of 0.05
Secondary Efficacy Analyses
The Day 180 change from baseline sNCV will be compared by treatment group using the Cochran-Mantel-Haenszel test stratified by IENFD < 9 fiber/mm and ≥ 9 fiber /mm at an alpha level of 0.05.
For the following measures, the Day 180 change from baseline measures will be compared by treatment group using the Wilcoxon Rank-Sum test.
|
· |
Neuropathy Impairment Score-Lower Limb (NIS-LL) |
|
· |
Lower Extremity Neurological Sensory Exam |
|
· |
Motor Nerve conduction velocity (NCV) |
|
· |
Quantitative Sensory Testing (QST) |
|
· |
lntraepidermal Nerve Fiber Density (IENFD) |
|
· |
Neuropathy Total Symptom Score (NTSS-6) |
|
· |
Visual Analog Scale for Pain Intensity (VASPI) |
|
· |
Quality of Life (QOL)- NeuroQoL and SF-36 |
|
· |
Global assessment |
The effect of SB-509 versus placebo in subjects with moderately severe diabetic neuropathy will be compared using a multi-endpoint analysis that includes Neuropathy Impairment Score - Lower Limb (NIS-LL), Sural Nerve Conduction Velocity (NCV) and lntraepidermal Nerve Fiber Density (IENFD), as detailed by O’Brien (1984).
Exploratory
The efficacy variables will be organized by clinically meaningful groups of 4 or fewer endpoints and analyzed by treatment and dose group with a multiple endpoint analysis defined by O’Brien (1984). For each grouping of endpoints, subjects will be ranked separately by each component of the multiple endpoint and the sum of the ranks will be obtained for each subject. The sums of ranks by treatment group will be compared with a Wilcoxon Rank-Sum test.
|
15.8. |
Baseline Values |
The baseline value for each variable is the last value recorded before the start of dosing. For NCV, QST, NIS-LL, and the lower extremity neurological sensory exam, the two measurements prior to dosing will be averaged for the baseline value.
|
15.9. |
Missing Data |
Physically undetectable NCV measures will be imputed with the 1st percentile value of all measurable observations by nerve. For all other measures, no imputations for missing data will be made.
|
15.10. |
Safety |
Safety assessment will occur on all subjects who received any study medication. Terminations/premature withdrawals, adverse events, concomitant medications, and laboratory data will be tabulated. Adverse events will be coded to a standard set of terms using the MedDRA dictionary. Frequency of adverse events will be compared using Fisher’s exact test.
|
Page 37 of 66 |
Version date: October 9, 2009 |
|
Sangamo BioSciences, Inc. |
|
|
Protocol SB-509-0901 for Diabetic Neuropathy
|
Confidential
|
|
15.11. |
Laboratory Data |
Laboratory data will be summarized for each time-point that specimens are collected. Change-from-baseline values may be calculated for selected laboratory parameters. (Baseline refers to blood drawn prior to the first study treatment.) Shift tables (change-from-baseline relative to the normal range) may be constructed for selected laboratory parameters.
|
16. |
STUDY ADMINISTRATION AND INVESTIGATOR OBLIGATIONS |
The investigator will ensure that the study is conducted in compliance with the protocol and according to ICH Guidelines for Good Clinical Practices (E6), the Declaration of Helsinki, and all regulatory and institutional requirements, including those for subject privacy, informed consent, IRB or IEC approval, and record retention.
A final study report will be prepared as part of Sangamo BioSciences’ commitment to Good Clinical Practice.
|
16.1. |
Institutional Review Board/Institutional Ethics Committee |
An Institutional Review Board should safeguard the rights, safety, and well being of all study subjects. In performing this study, both the investigator and sponsor endorse, as a minimum, the standards for conduct of clinical research activities as set forth in the Declaration of Helsinki and local country regulations. As such, this study must have the approval of a properly constituted IRB or IEC, with the investigator responsible for providing the IRB or IEC with all necessary documents for review.
This study will only be undertaken or investigational drug supplies shipped when full approval has been obtained from the appropriate IRB or IEC and a copy of the IRB or IEC approval letter has been received by Sangamo BioSciences. The approval letter must contain sufficient information to identify the version of both the protocol and subject information/informed consent, the date of the committee’s approval, the chairperson’s signature, and identify all study documents reviewed.
Protocol amendments must also be reviewed and approved by the IRB or IEC and must be received by Sangamo BioSciences before implementation.
Until written approval by the IRB or IEC has been received by the investigator, no subject may undergo any procedures solely for the purpose of determining eligibility for this study.
|
16.2. |
Informed Consent |
According to 21 CFR Part 50.20, no investigator may involve a human being as a subject in research covered by these regulations unless the investigator has obtained the legally effective informed consent of the subject or the subject's legally authorized representative. An investigator shall seek such consent only under circumstances that provide the prospective subject or the subject's legally authorized representative sufficient opportunity to consider whether or not to participate and that minimize the possibility of coercion or undue influence. The information that is given to the subject or the representative shall be in language understandable to the subject or the representative.
Sangamo BioSciences will provide the investigator with a template for the consent form. State and local laws and/or institutional requirements may require the disclosure of additional information in the informed consent. The proposed consent form must be submitted to Sangamo BioSciences prior to submission to the IRB or IEC to ensure that it meets Sangamo BioSciences’ standards for consent forms.
The IRB or IEC must approve the consent form. A copy of the approved form must be submitted to Sangamo BioSciences prior to the initiation of the study. Prior to the initiation of any procedures or treatment relating to the study, informed consent shall be documented by the use of a written consent form approved by the IRB and signed and dated by the subject or the subject's legally authorized representative at the time of consent. A copy of the signed informed consent will be given to the person signing the form. The investigator must keep each subject's signed consent form on file for inspection by a regulatory authority at any time.
|
Page 38 of 66 |
Version date: October 9, 2009 |
|
Sangamo BioSciences, Inc. |
|
|
Protocol SB-509-0901 for Diabetic Neuropathy
|
Confidential
|
|
16.3. |
Study Amendments |
Any changes to this protocol will be initiated by Sangamo BioSciences. Approval of the amendment by the investigator’s IRB must be obtained before implementation, with the following exception:
|
· |
When necessary to eliminate apparent immediate hazard to the subject |
All amendments must be signed and dated by Sangamo BioSciences. Sangamo BioSciences will notify other investigators using this protocol.
|
16.4. |
Study Drug Accountability |
The investigator must ensure that subjects receive study drug only from personnel who fully understand the procedures for dosing and administering the drug.
The investigator or designated individual will maintain an inventory. A drug accountability log will be provided to the pharmacist or designated personnel. This inventory will include the description and quantity of labeled product received during the course of this study, as well as a record of the labeled product that is dispensed. This inventory record shall indicate the quantity and description of labeled product on hand at any time during the course of this study. Empty vials and any remaining product in the used vials will be stored at room temperature at the clinical sites and will be kept in the original carton. Vials should not be discarded until the Sangamo BioSciences monitor reviews the drug accountability log. A Sangamo BioSciences monitor will review this log during interim monitoring visits.
Drug accountability records must be readily available for inspection by representatives of Sangamo BioSciences and are open to inspection by regulatory authorities at any time.
Return or destruction of all drug supplies must be coordinated with Sangamo BioSciences. Please see the Study Reference Manual for additional details.
|
16.5 |
Study Personnel |
The investigator should maintain a list of appropriately qualified persons who are delegated to perform significant study-related studies. In addition, the investigator should maintain a signature sheet to document signatures and initials of all persons authorized to make entries and/or corrections on the case report forms.
The investigator is responsible for informing the subject's primary care physician that the subject is participating in a clinical study. This should only be done after agreement from the subject.
|
16.6 |
Monitoring the Study |
Sangamo BioSciences, as sponsor of this study, is responsible to regulatory authorities for ensuring the proper conduct of the study as regards protocol adherence and validity of the data recorded on the case report forms presented to the regulatory authorities. Sangamo BioSciences has therefore assigned a clinical monitor and a medical monitor to this study. Their duties are to aid the investigator and, at the same time, Sangamo BioSciences in the maintenance of complete, legible, well-organized, and easily retrievable data. In addition, a Sangamo BioSciences study monitor will explain, interpret, and ensure the investigator’s understanding of all applicable regulations concerning the clinical evaluation of a labeled product (whether licensed or unlicensed) and ensure an understanding of the protocol, reporting responsibilities, and the validity of the data.
In order to perforrn their roles well, the Sangamo BioSciences monitors must be given direct access to primary subject data (source documents) that support data entered onto the case report forms (e.g., hospital and general practice charts, appointment books, original laboratory records). The investigator must exercise judgment regarding information in a subject's chart that is not relevant to the performance, observation, or conduct of this study. The investigator must make available such records to Sangamo BioSciences, Quality Assurance, Institutional Review Board, and Regulatory personnel for inspection and copying. Because this enters into the realm of subject confidentiality, this fact must be included in the information signed by the subject.
|
Page 39 of 66 |
Version date: October 9, 2009 |
|
Sangamo BioSciences, Inc. |
|
|
Protocol SB-509-0901 for Diabetic Neuropathy
|
Confidential
|
The investigator or designated person should agree, as a minimum requirement, to record the following information in the subject notes:
|
· |
Protocol identification number, brief description or title of study |
|
· |
Date and statement that subject has given written informed consent |
|
· |
All visit dates |
|
· |
All Adverse Events |
|
· |
All concomitant medications |
|
· |
Primary efficacy details (as defined by the protocol) |
|
· |
All labeled product accountability information |
Entries in the subject notes must contain the signature or initials of the person making the entries.
The clinical study monitor will perform source data verification at each monitoring visit.
A Sangamo BioSciences representative will monitor individual sites at appropriate intervals. The frequency of interim monitoring visits may vary depending on enrollment rate and the quality of data collected. Telephone contact will be made with each site Study Coordinator to keep abreast of subject status and to answer study-related questions.
|
16.7 |
Completion and Return of Case Report Forms |
The investigator is responsible for the quality of the data recorded on the case report form. The data recorded should be a complete and accurate account of the subject's record collected during the study.
The investigator and Sangamo BioSciences study monitor will identify any data that will be recorded directly on the case report form and considered as source data (i.e., no prior written or electronic record of the data). The Sangamo BioSciences monitor will document this on the study initiation report and document the use of case report forms as source documents as necessary during the course of the study.
Detailed instructions for case report form completion will be provided in the Study Reference Manual.
The investigator agrees to complete case report forms in a timely fashion, and make them available to the Sangamo BioSciences study monitor for full inspection. In addition, all data queries should be resolved promptly.
Before acceptance, the Sangamo BioSciences study monitor will review the case report forms for completeness and adherence to the protocol.
|
16.8 |
Deviation from Protocol for Individual Subjects |
Digressions from a written protocol for individual subjects are inherent to clinical research and will be categorized by Sangamo BioSciences as deviations.
Examples of protocol deviations include the following:
|
· |
Informed consent improperly obtained |
|
· |
Violation of inclusion/exclusion criteria |
|
· |
Error in product randomization |
|
· |
Major drug injection errors that may compromise subject safety or efficacy assessments |
|
· |
Administration of an excluded concomitant medication or treatment during the course of the study |
|
· |
Vital signs were not measured at study visit |
|
Page 40 of 66 |
Version date: October 9, 2009 |
|
Sangamo BioSciences, Inc. |
|
|
Protocol SB-509-0901 for Diabetic Neuropathy
|
Confidential
|
|
· |
Missed, delayed, or rescheduled visit |
|
· |
Missing laboratory results |
Investigators are encouraged to adhere to protocol procedures in that the deviation does not affect the evaluation of the data for the subject or compromise statistical analysis.
When a significant deviation occurs, the investigator should contact the study or medical monitor by telephone. The medical monitor will decide whether the subject is to continue on study.
|
16.9 |
Quality Assurance Procedures |
Quality Assurance activities undertaken during this study include monitoring and source data verification by the study monitor. It is possible that Sangamo BioSciences personnel or their agents may audit this study center.
|
16.10 |
Sangamo BioSciences Policy on Fraud in Clinical Studies |
|
Page 41 of 66 |
Version date: October 9, 2009 |
|
Sangamo BioSciences, Inc. |
|
|
Protocol SB-509-0901 for Diabetic Neuropathy
|
Confidential
|
APPENDIX 2: CTCAE
Sangamo BioSciences, Inc. will provide the Common Terminology Criteria for Adverse Events (CTCAE) v.4.0 to the clinical site.
|
Page 42 of 66 |
Version date: October 9, 2009 |
|
Sangamo BioSciences, Inc. |
|
|
Protocol SB-509-0901 for Diabetic Neuropathy
|
Confidential
|
APPENDIX 3: AMERICAN CANCER SOCIETY (ACS) CANCER DETECTION GUIDELINES
The following cancer screening guidelines are recommended for those people at average risk for cancer (unless otherwise specified) and without any specific symptoms.
People who are at increased risk for certain cancers may need to follow a different screening schedule, such as starting at an earlier age or being screened more often. Those with symptoms that could be related to cancer should see their doctor right away.
Cancer-related Checkup
For people aged 20 or older having periodic health exams, a cancer-related checkup should include health counseling, and depending on a person’s age and gender, might include exams for cancers of the thyroid, oral cavity, skin, lymph nodes, testes, and ovaries, as well as for some non-malignant (non-cancerous) diseases.
Special tests for certain cancer sites are recommended as outlined below.
Breast Cancer
|
· |
Yearly mammograms are recommended starting at age 40 and continuing for as long as a woman is in good health. |
|
· |
Clinical breast exam (CBE) should be part of a periodic health exam, about every 3 years for women in their 20s and 30s and every year for women 40 and over. |
|
· |
Women should know how their breasts normally feel and report any breast change promptly to their health care providers. Breast self-exam (BSE) is an option for women starting in their 20s. |
|
· |
Women at high risk (greater than 20% lifetime risk) should get an MRI and amammogram every year. Women at moderately increased risk (15% to 20% lifetime risk) should talk with their doctors about the benefits and limitations of adding MRI screening to their yearly mammogram. Yearly MRI screening is not recommended for women whose lifetime risk of breast cancer is less than 15%. |
Colon and Rectal Cancer
Beginning at age 50, both men and women at average risk for developing colorectal cancer should use one of the screening tests below. The tests that are designed to find both early cancer and polyps are preferred if these tests are available to you and you are willing to have one of these more invasive tests. Talk to your doctor about which test is best for you.
Tests that find polyps and cancer
|
· |
flexible sigmoidoscopy every 5 years* |
|
· |
colonoscopy every 10 years |
|
· |
double contrast barium enema every 5 years* |
|
· |
CT colonography (virtual colonoscopy) every 5 years* |
Tests that mainly find cancer
|
· |
fecal occult blood test (FOBT) every year*,** |
|
· |
fecal immunochemical test (FIT) every year*,** |
|
· |
stool DNA test (sDNA), interval uncertain* |
*Colonoscopy should be done if test results are positive.
**For FOBT or FIT used as a screening test, the take-home multiple sample method should be used. A FOBT or FIT done during a digital rectal exam in the doctor’s office is not adequate for screening.
|
Page 43 of 66 |
Version date: October 9, 2009 |
|
Sangamo BioSciences, Inc. |
|
|
Protocol SB-509-0901 for Diabetic Neuropathy
|
Confidential
|
People should talk to their doctor about starting colorectal cancer screening earlier and/or being screened more often if they have any of the following colorectal cancer risk factors:
|
· |
a personal history of colorectal cancer or adenomatous polyps |
|
· |
a personal history of chronic inflammatory bowel disease (Crohns disease or ulcerative colitis) |
|
· |
a strong family history of colorectal cancer or polyps (cancer or polyps in a first degree relative [parent, sibling, or child] younger than 60 or in 2 first-degree relatives of any age) |
|
· |
a known family history of hereditary colorectal cancer syndromes such as familial adenomatous polyposis (FAP) or hereditary non-polyposis colon cancer (HNPCC) |
Cervical Cancer
|
· |
All women should begin cervical cancer screening about 3 years after they begin having vaginal intercourse, but no later than when they are 21 years old. Screening should be done every year with the regular Pap test or every 2 years using the newer liquid-based Pap test. |
|
· |
Beginning at age 30, women who have had 3 normal Pap test results in a row may get screened every 2 to 3 years. Another reasonable option for women over 30 is to get screened every 3 years (but not more frequently) with either the conventional or liquid-based Pap test, plus the HPV DNA test. Women who have certain risk factors such as diethylstilbestrol (DES) exposure before birth, HIV infection, or a weakened immune system due to organ transplant, chemotherapy, or chronic steroid use should continue to be screened annually. |
|
· |
Women 70 years of age or older who have had 3 or more normal Pap tests in a row and no abnormal Pap test results in the last 10 years may choose to stop having cervical cancer screening. Women with a history of cervical cancer, DES exposure before birth, HIV infection or a weakened immune system should continue to have screening as long as they are in good health. |
|
· |
Women who have had a total hysterectomy (removal of the uterus and cervix) may also choose to stop having cervical cancer screening, unless the surgery was done as a treatment for cervical cancer or precancer. Women who have had a hysterectomy without removal of the cervix should continue to follow the guidelines above. |
Endometrial (Uterine) Cancer
The American Cancer Society recommends that at the time of menopause, all women should be informed about the risks and symptoms of endometrial cancer, and strongly encouraged to report any unexpected bleeding or spotting to their doctors. For women with or at high risk for hereditary non-polyposis colon cancer (HNPCC), annual screening should be offered for endometrial cancer with endometrial biopsy beginning at age 35.
Prostate Cancer
The American Cancer Society (ACS) does not support routine testing for prostate cancer at this time. ACS does believe that health care professionals should discuss the potential benefits and limitations of prostate cancer early detection testing with men before any testing begins. This discussion should include an offer for testing with the prostatespecific antigen (PSA) blood test and digital rectal exam (DRE) yearly, beginning at age 50, to men who are at average risk of prostate cancer and have at least a 10-year life expectancy. Following this discussion, those men who favor testing should be tested. Men should actively take part in this decision by learning about prostate cancer and the pros and cons of early detection and treatment of prostate cancer.
This discussion should take place starting at age 45 for men at high risk of developing prostate cancer. This includes African American men and men who have a first-degree relative (father, brother, or son) diagnosed with prostate cancer at an early age (younger than age 65).
This discussion should take place at age 40 for men at even higher risk (those with several first-degree relatives who had prostate cancer at an early age).
If, after this discussion, a man asks his health care professional to make the decision for him, he should be tested (unless there is a specific reason not to test).
|
Page 44 of 66 |
Version date: October 9, 2009 |
|
Sangamo BioSciences, Inc. |
|
|
Protocol SB-509-0901 for Diabetic Neuropathy
|
Confidential
|
References
American Cancer Society. Cancer Facts & Figures 2009. Atlanta, Ga: American Cancer Society; 2009.
Levin B, Lieberman DA, McFarland, et al. Screening and Surveillance for the Early Detection of Colorectal Cancer and Adenomatous Polyps, 2008: A Joint Guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Published online March 5, 2008. CA Cancer J Clin. 2008;58.
Saslow D, Boetes C, Burke W, et al for the American Cancer Society Breast Cancer Advisory Group. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57:75-89.
Last Revised: 05/21/2009
|
Page 45 of 66 |
Version date: October 9, 2009 |
|
Sangamo BioSciences, Inc. |
|
|
Protocol SB-509-0901 for Diabetic Neuropathy
|
Confidential
|
APPENDIX 4: SB-509 DRUG PRODUCT AND PLACEBO DESCRIPTION AND INSTRUCTIONS FOR STORAGE, HANDLING, ADMINISTRATION AND DISPOSAL
Product Description
SB-509 is a bacterial DNA plasmid (pV-32Ep65) that encodes an engineered zinc finger protein transcription factor (32Ep65) that up-regulates endogenous VEGF-A gene transcription. SB-509 is formulated as a liquid in 5% poloxamer 188. Sterile injectable saline (0.9% NaCl) serves as the placebo.
They are supplied in 3 mL glass vials filled with 2.2 mL of product, to deliver 2.0 mL. The SB-509 drug product is supplied at a concentration of 2.0 mg/mL. Each vial is prepared as a direct injectable.
Refer to the SB-509 DN Clinical Investigator’s Brochure for additional information on SB-509 and the placebo.
Storage and Administration
SB-509 and placebo are shipped to the clinical site by air courier on dry ice. Upon receipt at the clinical site, the vials should be immediately transferred to a locked -20°C or below freezer. Accurate accounting logs of all investigational drug received, dispensed, destroyed, and/or returned to Sangamo should be maintained at the clinical site.
Injection Preparation
At the time of injection, the vials are removed from the freezer, allowed to thaw at room temperature at least 10 minutes. First draw the study drug into 1 mL syringes with 19 gauge, 1½ inch needles. Syringes will be filled with either 0.5 mL or 1.0 mL according to the table shown on the next page. Once the syringes are filled, replace the 19 gauge needle with a 25 gauge needle, with either 1 inch or 1½ inch needles, as indicated in the table. A 27 gauge 1¼ inch needle may also be used for the lower leg and thigh injections. Follow the same injection technique as described on the following page. Additional syringes may be filled as back-up if necessary. The syringes are then released to the appropriate medical personnel for intramuscular injection and kept at room temperature until the drug is administered. All injections should be given the same day the study drug is thawed.
Injection Volumes
Lower leg (injection 1-14): 0.5 mL
Thigh (injections 15-22): 1.0 mL
Injection Technique
Use standard aseptic technique for preparation of the skin at the injection site. Cleaning of the sites with alcohol wipes, with a second alcohol swipe at the site prior to injection is recommended. Administer 0.5 mL and 1.0 mL doses of study drug by inserting a needle along the long axis of the muscle fiber (i.e., parallel with the femoral artery) up to its hub (the needle’s full insertion point) and depositing 0.5 mL for the 0.5 mL volume in the lower leg or 1.0 mL volume in the anterior and lateral thigh. The needle will be pointed toward the foot and inserted at an approximate 30° angle to the surface of the skin. Needles (25 gauge) with 1 mL syringes are to be used as follows: 1-inch needle for the lower leg and a 1½-inch needle for the thigh. If a 27 gauge needle is used, use the 1 ¼ inch needle for both the lower leg and thigh injections.
|
Page 46 of 66 |
Version date: October 9, 2009 |
|
Sangamo BioSciences, Inc. |
|
|
Protocol SB-509-0901 for Diabetic Neuropathy
|
Confidential
|
Injection Site Summary
|
· |
Dose level: 60 mg (30 mg per leg) |
|
· |
Total volume: 30 mL(15 mL per leg) |
|
|
Syringe Fill |
Needle Gauge & Length |
Number of Syringes per Leg* |
Total Number of Syringes |
Muscle Injection Sites |
Peripheral Nerve |
|
Lower leg |
0.5 mL |
25 gauge: 1 inch or 27 gauge: 1 ¼ inches |
14 |
28 |
1-14 |
peroneal, sural, tibial |
|
Thigh |
1.0 mL |
25 gauge: 1 ½ inches or 27 gauge: 1 ¼ inches |
8 |
16 |
15-22 |
sciatic and femoral |

|
Page 47 of 66 |
Version date: October 9, 2009 |
|
Sangamo BioSciences, Inc. |
|
|
Protocol SB-509-0901 for Diabetic Neuropathy
|
Confidential
|
Dose Level Injection Instructions
Lower leg injections
|
● |
Anterior leg injections |
Identify the bony landmarks of the anterior tibia and the fibula in the anterior lower leg. The muscle between these bones is the tibialis anterior. Identify Injection site 1 as the distal tibialis anterior muscle, 2-3 inches above the lateral malleolus of the ankle.
Beginning at site 1 as described above, identify 5 ascending injection sites in the tibialis anterior separated by at least one inch. Inject 0.5 mL into injections 1-5 (see figure).
|
● |
Lateral leg injections |
Palpate the lateral fibular head and then extending inferiorly to the lateral malleolus, the peroneus longus muscle. Inject five, 0.5 mL at least one inch apart, moving inferiorly along the leg for injections 6-10 (see figure).
|
● |
Medial leg injections |
Palpate the medial gastrocnemius muscle. At the top of the medial muscular portion of the muscle inject 4, 0.5 mL injections at least one inch apart moving inferiorly along the medial curve of the gastrocnemius for injections 11-14 (see figure).
Thigh injections
The upper leg or thigh injections will use syringes filled with 1.0 mL and will deliver one dose of 1.0 mL at maximum needle length (needle’s full insertion point).
|
● |
Anterior thigh |
Palpate the body of the rectus femoris muscle above the patella approximately 5-6 inches above the knee. Injections 15-18 (see figure) should be placed at least one inch apart, extending superiorly along the rectus femoris.
|
● |
Lateral thigh |
Palpate the body of the muscles of the lateral quadriceps, anterior and superior to the iliotibial band at approximately 5-6 inches above the knee. Injections 19-22 (see figure) should be placed at least one inch apart, extending superiorly along the lateral quadriceps.
|
Page 48 of 66 |
Version date: October 9, 2009 |
|
Sangamo BioSciences, Inc. |
|
|
Protocol SB-509-0901 for Diabetic Neuropathy
|
Confidential
|
APPENDIX 5: NEUROPATHY IMPAIRMENT SCORE- LOWER LIMB
(NIS-LL)
Adapted from Bril, Eur. Neural. (1999). 41 (supp. 1):8-13
A. Muscle Group Testing
Groups Tested
The following muscle groups are tested for power (bilaterally):
|
· |
Hip Flexion |
|
· |
Hip Extension |
|
· |
Knee Flexion |
|
· |
Knee Extension |
|
· |
Ankle Dorsiflexion |
|
· |
Ankle Plantar Flexion |
|
· |
Toe Extension |
|
· |
Toe Flexion |
Scoring
Each muscle group is scored according to the following scale:
|
Muscle Power |
NIS-LL Score |
|
Normal |
0 |
|
25% Weak |
1 |
|
50% Weak |
2 |
|
75% Weak |
3 |
|
Paralysis |
4 |
Muscles groups are scored between 0 and 4 points per side per group (bilaterally) for a maximum total of 64 points.
B. Sensory and Reflex Testing
Sensory Testing
The following sensory modalities are tested:
|
· |
Touch pressure |
|
· |
Pin Prick |
|
· |
Vibration |
|
· |
Joint position |
Reflex Testing
Deep tendon reflex testing is done at the following sites (bilaterally):
|
· |
Quadriceps (patellar) |
|
· |
Ankle (Achilles) |
|
Page 49 of 66 |
Version date: October 9, 2009 |
|
Sangamo BioSciences, Inc. |
|
|
Protocol SB-509-0901 for Diabetic Neuropathy
|
Confidential
|
Scoring
Sensory testing and reflex testing is scored according to the following scale:
|
Sensory/Reflex Activity Grading |
NIS-LL Score |
|
Normal |
0 |
|
Decreased |
1 |
|
Absent |
2 |
Sensory modalities are scored between 0 and 2 points per modality (bilaterally) for a maximum total of 16 points. Reflexes are scored between 0 and 2 points per reflex (bilaterally) for a maximum total of 8 points.
|
Page 50 of 66 |
Version date: October 9, 2009 |
|
Sangamo BioSciences, Inc. |
|
|
Protocol SB-509-0901 for Diabetic Neuropathy
|
Confidential
|
APPENDIX 6: LOWER EXTREMITY NEUROLOGICAL SENSORY EXAM
The Lower Extremity Neurological Sensory Exam includes distal to proximal examinations to evaluate sensory changes.
Pin Prick, Vibration and Touch Pressure will be evaluated at the Toe, Mid-Foot, Ankle, Mid-calf, Knee and Above Knee locations. At each location and for each leg, the sensory testing is scored according to the following scale:
|
Sensory Grading |
Score |
|
Normal |
0 |
|
Decreased |
1 |
|
Absent |
2 |
Sensory modalities are scored between 0 and 2 points per modality (bilaterally) for a maximum total of 24 points per modality and 72 points overall.
|
Page 51 of 66 |
Version date: October 9, 2009 |
|
Sangamo BioSciences, Inc. |
|
|
Protocol SB-509-0901 for Diabetic Neuropathy
|
Confidential
|
APPENDIX 7: ELECTROPHYSIOLOGICAL STUDIES
Nerve conduction studies (NCS) will be done at the sural and peroneal motor nerves. All NCS studies will be done bilaterally using standard surface recording techniques. Procedures for the recording of NCS are briefly outlined below and presented in full detail in an accompanying Study Reference Manual for NCS Studies.
The temperatures at the beginning and the end of the NCS will be recorded. Hard copies of all nerve conduction studies will be printed and saved (see Study Reference Manual for details).
Sural Sensory Nerve
Conduction velocity and sensory amplitudes will be measured using antidromic procedures. Surface electrodes will be placed adjacent to the lateral malleolus of the ankle (active recording electrode); the stimulating cathode will be applied to a more proximal segment of the nerve (12-14 cm distance).
Peroneal Motor Nerve
Motor nerve conduction velocity, distal and proximal baseline-to-peak compound muscle action potential amplitudes will be measured using orthodromic procedures. The active recording electrode will be placed over the extensor digitorum brevis muscle; the distal stimulation cathode will be placed at the ankle. The fibular head will be used as a proximal stimulation point.
|
Page 52 of 66 |
Version date: October 9, 2009 |
|
Sangamo BioSciences, Inc. |
|
|
Protocol SB-509-0901 for Diabetic Neuropathy
|
Confidential
|
APPENDIX 8: QUANTITATIVE SENSORY TESTING (QST)
|
1.0 |
SCHEDULE |
See Appendix 1, Schedule of Events.
|
2.0 |
DEFINITION OF TERMS |
THRESHOLD - minimal energy required before a subject can just detect a stimulus - in the present study, the last correctly identified intensity.
TWO ALTERNATIVE FORCED CHOICE PROCEDURE - VPT testing algorithm in which two potential stimuli are presented in each trial and the subject must select which stimulus is actually vibrating.
|
3.0 |
EQUIPMENT |
The Vibratron II, manufactured by Physitemp Instruments, Inc. (Clifton N.J), is a QST device to quantify the ability of human subjects to detect vibratory stimuli at the distal extremes of their upper and lower limbs. It consists of a controller and two vibrating posts. The frequency of vibration is fixed at 120 Hz. The power supply, switches and digital meter are encased as one unit, while the vibrating rods are located in separate units with adjoining cables. Each vibrating rod protrudes through a metal case and can be contacted by either the hands or the feet. The tandem vibrating surfaces are manufactured from hardened rubber and are identical in appearance. Vibration is achieved by driving the transducers with a variable voltage source. A dual position switch connected in series with the vibrating units, controls which rod vibrates, while a "dummy" switch is used to imitate the sounds and motions of switching. The amplitude of vibration is proportional to the square of the applied voltage and is continuously available on a digital display accurate to the nearest 0.1 units. A switch sets the maximal level of the vibration, which ranges from 0- 6.5 vibration units or 6.5 - 20 vibration units.
|
4.0 |
TESTING PROCEDURE |
|
4.1 |
Methodology |
Thresholds should be measured bilaterally, on the great toes. The methodology of testing is a "two alternative forced choice procedure." For each trial the subject is required to determine which of the two rods is actually vibrating. The position of vibration is under experimental control, determined by a randomization sequence. The intensity sequence is similarly under the control of the experimenter and is determined by a testing algorithm (see Section 4.6).
|
4.2 |
Temperature Control |
Prior to testing, each subject should be seated in a comfortable chair and allowed an adaptation period of approximately 10 minutes, during which time they can become acclimated to room temperature. Subjects should then remove all footwear, including pantyhose. A surface thermometer should be used to determine skin temperature at the arch of the foot. Skin temperature at this site must be at least 30°C, otherwise the subject should be warmed using a circulating water bath, an insulated thermal wrap or a heating pad. Radiant heaters should not be used.
|
4.3 |
Adaptation Period |
After the subject is warmed, they should be given an opportunity to become familiar with the testing apparatus and with the expected vibratory sensations. During this period, the experimenter can instruct the subject as to the appropriate length and force with which to contact the vibrating rod. An ideal duration for contact is approximately one second and the force should be the minimum necessary to detect vibration. This adaptation period will also allow the experimenter to determine the appropriate voltage level at which to begin testing. A number of vibration intensities should be set and sampled by the subject. For the initial trial, the experimenter should set the intensity at a level detectable by that subject 100% of the time. For many subjects in the 20
|
Page 53 of 66 |
Version date: October 9, 2009 |
|
Sangamo BioSciences, Inc. |
|
|
Protocol SB-509-0901 for Diabetic Neuropathy
|
Confidential
|
to 50 year age range, an initial intensity setting of 10-12 units is sufficient. This level should be increased for older subjects. For each trial, both the intensity setting and the subject's choice should be recorded in the appropriate columns on the data sheet.
|
4.4 |
Foot Support |
Throughout testing, the subject should rest his/her feet on the elevated platform provided by Physitemp. Thus, the subject should only be required to pivot his/her foot to contact each vibrating rod.
|
4.5 |
Instructions |
At the beginning of each testing session, the subject should be issued the following instructions:
"Please press your toe lightly against each rod in sequence for approximately one second. During each trial you will be allowed to touch the rods only once. Only one of the rods will be vibrating; please decided which rod (A or B) is vibrating or please let us know if you no longer can tell the difference. The task will become increasingly more difficult."
|
4.6 |
Testing Algorithm |
The starting intensity, in vibration units (vu), should be entered on the left hand column of the worksheet. The location of the stimulus (A or B rod) is indicated by the first column in the A/B matrix. If the subject correctly identifies which rod is vibrating, circle the "A" or "B" - then decrease the intensity for the next presentation by approximately 10%. If the subject incorrectly identifies the vibrating rod, cross out the "A" or "B" on the sheet with an "X" and increase the intensity for the next presentation by approximately 10% (ie., return to the level immediately prior to the error). The A/B matrix should be followed diagonally to the right (up one level and to the right one column).Testing is complete when a subject responds that there is "no difference" (mark with a single line on the A/B matrix) OR if the subject makes two errors before responding "no difference." The final lowest "correct" intensity is entered as the VPT value. Ideally, the subject should have at least 10 correct responses before they report "no difference" or make their first error. If the subject reports "no difference" or if there is an error in the first 10 trials, the test must be restarted at a higher initial intensity level. If the test was started at 20 vibration units (vu), simply report the last score correctly identified. If the subject reports "no difference" or makes an error at the 20 vu level, check the circle "intensity >20" on the worksheet. For sample worksheet see Study Reference Manual.
|
5.0 |
TECHNICAL CONCERNS |
To determine accurate vibration thresholds, the experimenter must be concerned with the following details:
|
5.1 |
The subject should be consistent in the location and duration of touch, as well as in the approximate force applied to the vibrating surface. Instructions such as “please don’t press so hard” can be issued during testing to ensure trial-to-trial consistency. |
|
5.2 |
Throughout testing, the sounds and motions associated with changing the stimulus position should be presented between each trial. For the conditions where the stimulus position remains unaltered, the "dummy" switch must be used. Both the active and "dummy" switches can be used between trials to mask the actual positioning of the stimulus. |
|
5.3 |
The rods should be visually inspected prior to every test to ensure that they are "free-standing" and not in contact with the metal covering. |
|
5.4 |
The subject must take care not to contact the metal casing around the vibrating rods during the trials. |
|
5.5 |
The subject should be carefully screened from viewing the instrument settings or the data sheet. |
|
5.6 |
All testing should be conducted in a quiet room with minimal distractions. |
|
5.7 |
Each Vibratron II should be factory calibrated at an interval appropriate for the study. |
|
Page 54 of 66 |
Version date: October 9, 2009 |
|
Sangamo BioSciences, Inc. |
|
|
Protocol SB-509-0901 for Diabetic Neuropathy
|
Confidential
|
APPENDIX 9: SKIN BIOPSY
1.0INTRODUCTION
Rationale
3 mm skin biopsy punches will be obtained for the evaluation of intra-epidermal nerve fiber density (IENFD). Epidermal nerve fibers are C and A-sigma small caliber unmyelinated nerve fibers that are relevant to diabetic neuropathy. These fibers are not directly assessed by conventional testing measures such as nerve conduction velocity, amplitude, or most forms of quantitative sensory testing.
In the figure below, epidermal nerve fiber axons can be seen extending to the dermis in a normal subject (panel A). Decreased numbers of epidermal nerve fiber axons are evident in a skin biopsy from a diabetic subject (panel B).
Skin section (50 µm) stained for PGP9.5+ IENFDs in a nondiabetic subject (A) and in a subject with established neuropathy (B). Original magnification X600 (Quattrini et al. 2008).
3.0SCHEDULE AND GENERAL PROCEDURES
The schedule for skin biopsy collection is located in Appendix 1: Schedule of Events. The method for obtaining this analysis requires a 3 mm skin biopsy on the distal lateral thigh. The sample will then be processed with fixative and shipped to Johns Hopkins University. The samples will then be further processed by the laboratory and analyzed to determine intraepidermal nerve fiber density.
A manual with detailed instructions will be provided to you in the Study Reference Manual for obtaining, processing and shipping the biopsy samples. In addition, each investigator will be provided with a video clip presentation on CD that will further illustrate the specific procedures.
|
Page 55 of 66 |
Version date: October 9, 2009 |
|
Sangamo BioSciences, Inc. |
|
|
Protocol SB-509-0901 for Diabetic Neuropathy
|
Confidential
|
APPENDIX 10: NEUROPATHY TOTAL SYMPTOM SCORE (NTSS-6)
Bastyr, Clinical Therapeutics (2005) 27:1278-1294
Instructions for the Health Care Professional (HCP)
|
1. |
Each of the 6 questions is scored by marking an X in the box on the grid corresponding to the symptom frequency and intensity. |
|
2. |
Grading of the symptom frequency and intensity should be according to the definitions provided below for each category level. |
|
3. |
The symptom evaluated should be due to diabetic peripheral neuropathy in the feet or legs, in the opinion of the administering HCP. The symptom question should be evaluated taking into consideration what would be normal for a healthy person of comparable age and sex. |
|
4. |
Symptoms are graded based on the experience of the patient during the past 24 hours. |
|
5. |
Each symptom question can be assigned a maximum score of 3.66 points. The NTSS-6 total score is the sum of the 6 individual symptom scores and has a maximum of 21.96 points. |
|
6. |
The HCP scores the symptom question based on the patient's response to the question and the definitions of symptom frequency and intensity provided to the HCP (see below). |
Definitions for Grading
Frequency
|
· |
Never or occasional means that the symptom does not occur; occurs a normal amount; not beyond normal; not noticeable. |
|
· |
Occasional, but abnormal means that the symptom occurs or is present less than a third of the time, either in total frequency or duration. It also applies to symptoms that may have a duration of only seconds and occur only during a portion of the day and/or night. |
|
· |
Often means that the symptom occurs for one third to two thirds of the time, either in total frequency or duration. It may also apply to symptoms that may have a duration of only seconds and occur throughout the day and/or night. |
|
· |
Almost continuous means that the symptom occurs for more than two thirds of the time, either in total frequency or duration. It may also apply to symptoms that may have a duration of only seconds and occur throughout the day and/or night. |
Intensity
|
· |
Not present means no symptoms noted. |
|
· |
Mild means that the patient notices the pain or other symptom but it does not interfere with or restrict daily activities, and the patient does not need any treatment to control it. |
|
· |
Moderate means that the pain or other symptom sometimes interferes with or restricts ≥1 of the patient's sleep, work, social, or family activities, or the patient needs treatment to control the pain or symptom. |
|
· |
Severe means that the pain or other symptom usually interferes with or restricts ≥1 of the patient's sleep, work, social, or family activities, even if the pain or symptom is treated. |
Symptoms and Questions Evaluated by the NTSS-6
|
· |
Aching pain: "Do you experience a deep, aching, tightness, boring, pulling, or squeezing pain in your feet or legs?" |
|
· |
Allodynia: "Do you experience unusual sensitivity or tenderness when your feet are touched or are used in activities such as walking?" |
|
· |
Burning pain: "Do you experience burning pain in your feet or legs?" |
|
· |
Lancinating pain: "Do you experience sharp, stabbing, or shooting pain, electrical shock-like pain, or surges of pain that last seconds to minutes in your feet or legs?" |
|
Page 56 of 66 |
Version date: October 9, 2009 |
|
Sangamo BioSciences, Inc. |
|
|
Protocol SB-509-0901 for Diabetic Neuropathy
|
Confidential
|
|
· |
Numbness: "Do you experience numbness, lost sensation, or a ‘dead feeling’ like an anesthetic, without prickling in your feet or legs?" |
|
· |
Prickling sensation: "Do you experience a prickling or tingling feeling, with or without an ‘asleep’ feeling, in your feet or legs?" |
NTSS-6 Sample Scoring Sheet
|
|
|
Symptom Intensity |
|
|||||||||||||
|
Symptom Frequency |
|
Not Present |
|
|
Mild |
|
|
Moderate |
|
|
Severe |
|
||||
|
Never or occasional (normal amount) |
|
|
0.00 |
|
|
|
0.00 |
|
|
|
0.00 |
|
|
|
0.00 |
|
|
Occasional but abnormal (less than 1/3 of the time) |
|
|
0.00 |
|
|
|
1.00 |
|
|
|
2.00 |
|
|
|
3.00 |
|
|
Often (1/3 to 2/3 of the time) |
|
|
0.00 |
|
|
|
1.33 |
|
|
|
2.33 |
|
|
|
3.33 |
|
|
Almost continuous (more than 2/3 of the time) |
|
|
0.00 |
|
|
|
1.66 |
|
|
|
2.66 |
|
|
|
3.66 |
|
|
Page 57 of 66 |
Version date: October 9, 2009 |
|
Sangamo BioSciences, Inc. |
|
|
Protocol SB-509-0901 for Diabetic Neuropathy
|
Confidential
|
APPENDIX 11: VISUAL ANALOG SCALE FOR PAIN INTENSITY (VASPI)
Please rate your pain by circling the one number that best describes your pain from diabetic neuropathy in the last 24 hours.
|
0 |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
10 |
|
No |
|
|
|
|
|
|
|
|
|
Worst Possible Pain |
|
Page 58 of 66 |
Version date: October 9, 2009 |
|
Sangamo BioSciences, Inc. |
|
|
Protocol SB-509-0901 for Diabetic Neuropathy
|
Confidential
|
APPENDIX 12: NEURO-QOL
Vileikyte, Diabetes Care (2003) 26:2549-2555
NEUROPATHY-SPECIFIC QUALITY OF LIFE
Questionnaire Instructions (UK Version)
|
· |
These questions ask about the effect your FOOT PROBLEMS may have on your daily life and well-being. By foot problems we mean lost or reduced feeling in your extremities, pain, discomfort and/or ulcers (open sores) on your feet and, in some cases unsteadiness while walking or standing. |
|
· |
Please note that many questions have two parts. Answer every question by ticking one box for each part (tick two boxes per line). Please make sure you answer all questions. |
|
· |
Please concentrate on how you have felt IN THE PAST 4 WEEKS for all of the questions. |
|
· |
There are no right or wrong answers. If you are unsure about how to answer a question, you can ask the person who gave you the questionnaire. Please DO NOT ask a relative or friend to help you. |
|
· |
All of your responses will be held strictly confidential. |
|
In the past 4 weeks how often have you experienced the following symptoms? |
All |
Most |
Some |
Occa- sionally |
Never |
How much bother did this cause you? |
||||||||||||||||||||||||||||
|
Very much |
Some bother |
None |
||||||||||||||||||||||||||||||||
|
1. Burning in your legs or feet |
|
|
|
|
|
|
|
|
||||||||||||||||||||||||||
|
2. Excessive heat or cold in your legs or feet |
|
|
|
|
|
|
|
|
||||||||||||||||||||||||||
|
3. Pins and needles in your legs or feet |
|
|
|
|
|
|
|
|
||||||||||||||||||||||||||
|
4. Shooting or stabbing pain in your legs or feet |
|
|
|
|
|
|
|
|
||||||||||||||||||||||||||
|
5. Throbbing in your legs or feet |
|
|
|
|
|
|
|
|
||||||||||||||||||||||||||
|
6. Sensations in your legs or feet that make them jump |
|
|
|
|
|
|
|
|
||||||||||||||||||||||||||
|
7. Irritation of the skin caused by something touching your feel, such as bedsheets or socks |
|
|
|
|
|
|
|
|
||||||||||||||||||||||||||
|
A. Have these painful symptoms reduced your quality of life? |
Very |
Quite a |
Some |
A little |
Not at all |
|||||||||||||||||||||||||||||
|
|
|
|
|
|
||||||||||||||||||||||||||||||
|
In the past 4 weeks how often have you experienced the following symptoms? |
All |
Most |
Some of the time |
Occa- sionally |
Never |
How much bother did this cause you? |
||||||||||||||||||||||||||||
|
Very much |
Some bother |
None |
||||||||||||||||||||||||||||||||
|
8. Numbness in your feet |
|
|
|
|
|
|
|
|
||||||||||||||||||||||||||
|
9. Inability to feel the difference between hot and cold with your feet |
|
|
|
|
|
|
|
|
||||||||||||||||||||||||||
|
10. Inability to feel objects with your feet |
|
|
|
|
|
|
|
|
||||||||||||||||||||||||||
|
Page 59 of 66 |
Version date: October 9, 2009 |
|
Sangamo BioSciences, Inc. |
|
|
Protocol SB-509-0901 for Diabetic Neuropathy
|
Confidential
|
|
B. Have these last three symptoms reduced your quality of life? |
Very |
Quite a |
Some |
A little |
Not at all |
|||||||||||||||||||||||||||||
|
|
|
|
|
|
||||||||||||||||||||||||||||||
|
In the past 4 weeks how often have you experienced the following symptoms? |
All the time |
Most of the time |
Some of the time |
Occa- sionally |
Never |
How much bother did this cause you? |
||||||||||||||||||||||||||||
|
Very much |
Some bother |
None |
||||||||||||||||||||||||||||||||
|
11. Weakness in your hands |
|
|
|
|
|
|
|
|
||||||||||||||||||||||||||
|
12. Problems with balance or unsteadiness while walking |
|
|
|
|
|
|
|
|
||||||||||||||||||||||||||
|
13. Problems with balance or unsteadiness while standing |
|
|
|
|
|
|
|
|
||||||||||||||||||||||||||
|
C. Have these last three symptoms reduced your quality of life? |
Very |
Quite a |
Some |
A little |
Not at all |
|||||||||||||||||||||||||||||
|
|
|
|
|
|
||||||||||||||||||||||||||||||
|
The following questions ask about how your FOOT PROBLEMS affect your daily activities, relationships and feelings. |
||||||||||||||||||||||||||||||||||
|
Are you in PAID WORK? |
Yes |
No |
If YES please go to Question 14. If NO please go to Question 15. |
|||||||||||||||||||||||||||||||
|
In the past 4 weeks, HOW MUCH have your foot problems interfered with your: |
Very |
Quite |
Some |
A |
Not |
How important is this aspect of your life to you? |
||||||||||||||||||||||||||||
|
Very |
Some what |
Not |
||||||||||||||||||||||||||||||||
|
14. Ability to perform your paid work? |
|
|
|
|
|
|
|
|
||||||||||||||||||||||||||
|
15. Ability to perform tasks around the house or garden? |
|
|
|
|
|
|
|
|
||||||||||||||||||||||||||
|
16. Ability to take part in leisure activities? |
|
|
|
|
|
|
|
|
||||||||||||||||||||||||||
|
D. Have these changes in daily activities as a result of your foot problems reduced your quality of life? |
Very |
Quite |
Some |
A little |
Not at all |
|||||||||||||||||||||||||||||
|
|
|
|
|
|
||||||||||||||||||||||||||||||
|
|
|
|
||||||||||||||||||||||||||||||||
|
In the past 4 weeks: |
How important is this aspect of your life to you? |
|||||||||||||||||||||||||||||||||
|
Page 60 of 66 |
Version date: October 9, 2009 |
|
Sangamo BioSciences, Inc. |
|
|
Protocol SB-509-0901 for Diabetic Neuropathy
|
Confidential
|
|
|
Very |
Quite |
Some |
A |
Not |
Very much |
Some |
Not at all |
|||||||||||||||||||||||||
|
17. How much have your foot problems interfered with your relationships with people close to you? |
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||
|
18. Have you felt more physically dependent than you would like to be on people close to you as a result of your foot problems? |
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||
|
19. Have you felt more emotionally dependent than you would like to be on people close to you as a result of your foot problems? |
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||
|
20. Has your role in the family changed as a result of your foot problems? |
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||
|
E. Have these changes in relationships with other people as a result of your foot problems reduced your quality of life? |
Very |
Quite a lot |
Some |
A little |
Not at all |
||||||||||||||||||||||||||||
|
|
|
|
|
|
|||||||||||||||||||||||||||||
|
|
How much bother did this cause you? |
||||||||||||||||||||||||||||||||
|
How much do you agree with the following statements: |
Completely |
Partly |
Neither agree or |
Partly |
Completely |
Very |
Some |
None |
|||||||||||||||||||||||||
|
21. People treat me differently from other people as a result of my foot problems. |
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||
|
22. I feel older than my years as a result of my foot problems. |
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||
|
23. My self - confidence is affected as a result of my foot problems. |
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||
|
24. My foot problems make my life a struggle. |
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||
|
25. I generally feel frustrated because of my foot problems. |
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||
|
26. My foot problems cause me embarrassment. |
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||
|
Page 61 of 66 |
Version date: October 9, 2009 |
|
Sangamo BioSciences, Inc. |
|
|
Protocol SB-509-0901 for Diabetic Neuropathy
|
Confidential
|
|
27. I feel depressed because of my foot problems |
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||
|
F. Have these feelings about yourself as a result of your foot problems reduced your quality of life? |
Very |
Quite a lot |
Some |
A little |
Not at all |
||||||||||||||||||||||||||||
|
|
|
|
|
|
|||||||||||||||||||||||||||||
|
|
Very |
Quite a lot |
Some |
A little |
Not at all |
||||||||||||||||||||||||||||
|
28. Overall, I would say problems with my feet reduced my Quality of Life: |
|
|
|
|
|
||||||||||||||||||||||||||||
|
|
Excellent |
Very good |
Good |
Fair |
Poor |
||||||||||||||||||||||||||||
|
29. Overall, I would rate my quality of life as: |
|
|
|
|
|
||||||||||||||||||||||||||||
|
Page 62 of 66 |
Version date: October 9, 2009 |
|
Sangamo BioSciences, Inc. |
|
|
Protocol SB-509-0901 for Diabetic Neuropathy
|
Confidential
|
APPENDIX 13: SF-36
This survey asks for your views about your health. This information will help keep track of how you feel and how well you are able to do your usual activities. Thank you for completing this survey!
For each of the following questions, please mark an x in the one box that best describes your answer.
|
1. |
In general, would you say your health is: |
|
Excellent |
Very good |
Good |
Fair |
Poor |
|
q |
q |
q |
q |
q |
|
£1 |
£2 |
£3 |
£4 |
£5 |
|
2. |
Compared to one year ago, how would you rate your health in general now? |
|
Much better now than one year ago |
Somewhat better now than one year ago |
About the same as one year ago |
Somewhat worse now than one year ago |
Much worse now than one year ago |
|
q |
q |
q |
q |
q |
|
£1 |
£2 |
£3 |
£4 |
£5 |
SF-36v2TM Health Survey ©1996, 2000 by Quality Metric Incorporated and Medical Outcomes Trust. All Rights Reserved.
SF-36 ® is a registered trademark of Medical Outcomes Trust.
(SF-36v2 Standard, US Version 2.0)
|
3. |
The following questions are about activities you might do during a typical day. Does your health now limit you in these activities? If so, how much? |
|
|
|
Yes, Limited a lot q |
Yes, Limited a little q |
No, not Limited at all q |
|
a |
Vigorous activities, such as running, lifting heavy objects, participating in strenuous |
……….£1………. |
……….£2………. |
……….£3 |
|
b |
Moderate activities, such as moving a table, pushing a vacuum cleaner, bowling, or playing golf |
……….£1……… |
……….£2………. |
……….£3 |
|
c |
Lifting or carrying groceries |
……….£1……… |
……….£2………. |
……….£3 |
|
d |
Climbing several flights of stair |
……….£1……… |
……….£2………. |
……….£3 |
|
e |
Climbing one flight of stairs |
……….£1……… |
……….£2………. |
……….£3 |
|
f |
Bending, kneeling, or stooping |
……….£1……… |
……….£2………. |
……….£3 |
|
g |
Walking more than a mile |
……….£1……… |
……….£2………. |
……….£3 |
|
h |
Walking several hundred yards |
……….£1……… |
……….£2………. |
……….£3 |
|
i |
Walking one hundred yards |
……….£1……… |
……….£2………. |
……….£3 |
|
j |
Bathing or dressing yourself |
……….£1……… |
……….£2………. |
……….£3 |
|
Page 63 of 66 |
Version date: October 9, 2009 |
|
Sangamo BioSciences, Inc. |
|
|
Protocol SB-509-0901 for Diabetic Neuropathy
|
Confidential
|
|
4. |
During the past 4 weeks, how much of the time have you had any of the following problems with your work or other regular daily activities as a result of your physical health? |
|
|
|
All of q |
Most of q |
Some of q |
A little q |
None of q |
|
a |
Cut down on the amount of time you spent on work or other activities |
…..£1….. |
…..£2….. |
…..£3….. |
…..£4….. |
…...£5 |
|
b |
Accomplished less than you would |
…..£1….. |
…..£2….. |
…..£3….. |
…..£4….. |
…...£5 |
|
c |
Were limited in the kind of work or other Activities |
…..£1….. |
…..£2….. |
…..£3….. |
…..£4….. |
…...£5 |
|
d |
Had difficulty performing the work or other activities (for example, it took extra effort) |
…..£1….. |
…..£2….. |
…..£3….. |
…..£4….. |
…...£5 |
|
5. |
During the past 4 weeks, how much of the time have you had any of the following problems with your work or other regular daily activities as a result of any emotional problems (such as feeling depressed or anxious)? |
|
|
|
All of q |
Most of q |
Some of q |
A little q |
None of q |
|
a |
Cut down on the amount of time you spent on work or other activities |
…..£1….. |
…..£2….. |
…..£3….. |
…..£4….. |
…...£5 |
|
b |
Accomplished less than you would like |
…..£1….. |
…..£2….. |
…..£3….. |
…..£4….. |
…...£5 |
|
c |
Did work or other activities less carefully than usual |
…..£1….. |
…..£2….. |
…..£3….. |
…..£4….. |
…...£5 |
|
6. |
During the past 4 weeks, to what extent has your physical health or emotional problems interfered with your normal social activities with family, friends, neighbors, or groups? |
|
Not at all
|
Slightly
|
Moderately
|
Quite a bit
|
Extremely
|
|
q |
q |
q |
q |
q |
|
£1 |
£2 |
£3 |
£4 |
£5 |
|
7. |
How much bodilv pain have you had during the past 4 weeks? |
|
None
|
Very mild
|
Mild
|
Moderate
|
Severe
|
Very Severe
|
|
q |
q |
q |
q |
q |
q |
|
£1 |
£2 |
£3 |
£4 |
£5 |
£6 |
|
Page 64 of 66 |
Version date: October 9, 2009 |
|
Sangamo BioSciences, Inc. |
|
|
Protocol SB-509-0901 for Diabetic Neuropathy
|
Confidential
|
8.During the past 4 weeks, how much did pain interfere with your normal work
(including both work outside the home and housework)?
|
Not at all
|
A little bit
|
Moderate
|
Quite a bit
|
Extremely
|
|
q |
q |
q |
q |
q |
|
£1 |
£2 |
£3 |
£4 |
£5 |
|
9. |
These questions are about how you feel and how things have been with you during the past 4 weeks. For each question, please give the one answer that comes closest to the way you have been feeling. How much of the time during the past 4 weeks…. |
|
|
|
All of q |
Most of q |
Some of q |
A little q |
None of q |
|
a |
Did you feel full of life? |
…..£1….. |
…..£2….. |
…..£3….. |
…..£4….. |
…...£5 |
|
b |
Have you been very nervous? |
…..£1….. |
…..£2….. |
…..£3….. |
…..£4….. |
…...£5 |
|
c |
Have you felt so down in the dumps that nothing could cheer you up? |
…..£1….. |
…..£2….. |
…..£3….. |
…..£4….. |
…...£5 |
|
d |
Have you felt calm and peaceful? |
…..£1….. |
…..£2….. |
…..£3….. |
…..£4….. |
…...£5 |
|
e |
Did you have a lot of energy? |
…..£1….. |
…..£2….. |
…..£3….. |
…..£4….. |
…...£5 |
|
f |
Have you felt downhearted and depressed? |
…..£1….. |
…..£2….. |
…..£3….. |
…..£4….. |
…...£5 |
|
g |
Did you feel worn out? |
…..£1….. |
…..£2….. |
…..£3….. |
…..£4….. |
…...£5 |
|
h |
Have you been happy? |
…..£1….. |
…..£2….. |
…..£3….. |
…..£4….. |
…...£5 |
|
i |
Did you feel tried? |
…..£1….. |
…..£2….. |
…..£3….. |
…..£4….. |
…...£5 |
|
10. |
During the past 4 weeks, how much of the time has your physical health or emotional problems interfered with your social activities (like visiting friends, relatives, etc.)? |
|
All of the
|
Most of the
|
Some of the
|
A little of the
|
None of the
|
|
q |
q |
q |
q |
q |
|
£1 |
£2 |
£3 |
£4 |
£5 |
11.How TRUE or FALSE is each of the following statements for you?
|
|
|
Definitely true q |
Mostly q |
Don’t q |
Mostly q |
Definitely q |
|
a |
I seem to get sick a little easier than other people |
…..£1….. |
…..£2….. |
…..£3….. |
…..£4….. |
…...£5 |
|
b |
I am as healthy as anybody I know |
…..£1….. |
…..£2….. |
…..£3….. |
…..£4….. |
…...£5 |
|
c |
I expect my health to get worse |
…..£1….. |
…..£2….. |
…..£3….. |
…..£4….. |
…...£5 |
|
d |
My health is excellent |
…..£1….. |
…..£2….. |
…..£3….. |
…..£4….. |
…...£5 |
|
Page 65 of 66 |
Version date: October 9, 2009 |
|
Sangamo BioSciences, Inc. |
|
|
Protocol SB-509-0901 for Diabetic Neuropathy
|
Confidential
|
APPENDIX 14: GLOBAL ASSESSMENT
The following question will be asked of the subject according to the schedule outlined in Appendix 1: Schedule of Events:
|
How have you felt since your last visit? |
£ |
Better |
|
|
£ |
Same |
|
|
£ |
Worse |
|
Page 66 of 66 |
Version date: October 9, 2009 |
