Attached files
| file | filename |
|---|---|
| EXCEL - IDEA: XBRL DOCUMENT - VACCINOGEN INC | Financial_Report.xls |
| EX-23 - EXHIBIT 23 - VACCINOGEN INC | v407000_ex23.htm |
| EX-21 - EXHIBIT 21 - VACCINOGEN INC | v407000_ex21.htm |
| EX-31.1 - EXHIBIT 31.1 - VACCINOGEN INC | v407000_ex31-1.htm |
| EX-32.1 - EXHIBIT 32.1 - VACCINOGEN INC | v407000_ex32-1.htm |
UNITED STATES SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 10-K
(Mark One)
| x | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2014
| ¨ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from ___________ to _____________
Commission File Number 000-54997
VACCINOGEN, inc.
(Name of Registrant as Specified in its Charter)
| Maryland | 14-1997223 |
|
(State or other jurisdiction of incorporation or organization) |
(I.R.S. Employer Identification No.) |
| 949 Fell Street, Baltimore, MD | 21231 |
| (Address of principal executive offices) | (Zip Code) |
Registrant’s telephone number, including area code: (410) 387-4000
Securities registered pursuant to Section 12(b) of the Act: None
Securities registered pursuant to Section 12(g) of the Act: Common Stock, $.0001 par value
___________________
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. ¨ Yes No x
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Act: ¨ Yes No x
Indicate by check mark whether the registrant(1) has filed all reports required by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 day. x Yes ¨ No
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files).
x Yes ¨ No
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulations S-K is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 if the Exchange Act.
| Large accelerated filter ¨ | Accelerated filter ¨ |
| Non-accelerated filter ¨(Do not check if a smaller reporting company) | Smaller reporting company x |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act. Yes ¨ No x
The aggregate market value of the voting and non-voting common stock held by non-affiliates of the registrant at June 30, 2014 was approximately $46,880,000.
As of March 24, 2015, 35,986,752 shares of our common stock were issued and outstanding.
PART I
Item 1. DESCRIPTION OF BUSINESS
Overview
Vaccinogen, Inc. (referred to as “Vaccinogen”, “the Company”, “we”, “us”, or “our”), a Maryland corporation, is a biotechnology company focused on the development and commercialization of cancer vaccines and immunotherapeutic products for cancers. Our primary product is OncoVAX®, an autologous cancer vaccine currently indicated for the post-surgical treatment of stage II colon cancer. We believe that OncoVAX® will be the first successful immunotherapy for stage II colon cancer.
We currently employ 24 persons. Our executive offices are located at 949 Fell Street, Baltimore, MD 21231. Our telephone number is (410) 387-4000 and our Internet address is www.vaccinogeninc.com.
Company History
We are a Maryland corporation that was originally formed as a Delaware corporation on May 2, 2007. On November 23, 2010, the Company changed its domicile from Delaware to Maryland.
License Agreements
For a description of the Company’s important license arrangements, see “Item 13, Certain Relationships and related Transactions.”
Operating Strategy
We will maintain a cGMP facility and outsource clinical activities to contract research organizations (“CROs”) in an effort to achieve the following:
| 1. | Complete our Autologous Colon Tumor Immunotherapy Validation Experiment (“ACTIVE”) in our pivotal phase IIIb clinical trial of OncoVAX®, an autologous cancer vaccine which prevents the recurrence of stage II colon cancer. The planned trial will closely replicate a successful prior phase IIIa study which study we believe demonstrated a 61% improvement in relative risk of recurrence and a 54% reduction in the relative risk of death in stage II patients as compared to surgery alone. The Company began its preparations for the planned phase IIIb clinical trial in September 2014. The Company also selected RXTRIALS, INC d/b/a OnPoint CRO as its Clinical Research Organization (“CRO”) to provide services of site selection, clinical operations, project management and trial enrollment for our phase IIIb clinical trial. |
| 2. | Develop revenues by establishing licensing arrangements for distribution of OncoVAX® in certain territories. |
| 3. | Investigate the role of OncoVAX® treatment in additional carcinomas with high post-surgical recurrence rates. |
| 4. | Develop diagnostic and therapeutic drug products as well as revenues by exploiting our distinctive Human Monoclonal Antibody (“HuMab”) technology. |
| 2 |
Active Immunotherapy of Cancer
Most phase III trials evaluating cancer vaccines have failed to achieve meaningful clinical results.1 In 2007, a major review of the active cancer immunotherapy space outlined the various scientific and economic reasons which account for the disappointing results in the field.2 One of the first considerations enumerated by the authors was to "select the most informative targets" for vaccine development. They point out that the ideal targets should be tumor-specific and that "it is important to use the intended study population to assess the proportion of tumors that express the target of choice and the proportion of cells within each tumor that express it”. However, the word “select” has important implications. This presupposes we have the ability to identify targets which are potently identified by the immune system and simultaneously displayed within and across different tumors to a sufficient degree that clinical benefit can be achieved. This requires an assumption that tumors have an inherent degree of inter-and intra-tumor homogeneity, rather than the extreme heterogeneity which has been well-understood for some time.3 Furthermore, omission of key immunogens in vaccine development can actually result in lower adaptive immune responses. The biology of cancer needs to be recognized, not ignored, if we intend to exploit it.
DNA sequencing data has provided indisputable evidence of the extreme genetic diversity, or heterogeneity, both within individual tumors and across tumor subtypes (Vogelstein ref from above). Despite the visual similarities of these cells under the microscope, when analyzed with the correct tools, they display a shocking level of genetic and proteomic variation. Due to the genetic basis of cancer, these differences have far-reaching clinical ramifications. We believe that ignoring this critical facet of cancer evolution led to the development of "one size fits all" therapies. However, these strategies employ homogeneous tools to solve a heterogeneous problem. The laboratory process of antigen “discovery” is not required if the entire tumor is used as the source material for personalized treatment; each patient’s immune system is tasked with determining which mutations, gene alterations, post-translational modifications, and protein-protein interactions are truly cancer-specific.
An additional complication of cancer vaccine development is deploying immune-based therapies in patients with advanced disease. Hanahan and Weinberg recently updated their famous “Hallmarks of Cancer” to include “evading immune destruction” as a defining feature of malignant cells.4 It is now well-understood that cancer, and particularly advanced and disseminated disease, has multiple avenues for local suppression of the immune system within their malignant microenvironments.5 Thus, systemic cancer vaccine therapies may engender cancer-specific immune cells only to have these therapeutic responses inhibited once they reach their intended target. Focusing on advanced disease may seem logical from a clinical standpoint; however, this dire population also provides an economic advantage.
Patients with recurrent and metastatic cancer have few therapeutic options and their overall survival is often measured in months, not years. Therefore, if a vaccine is able to demonstrate any clinical efficacy over this shorter period of time, that product can be expedited to market, and begin generating revenue. We believe the immune system, if trained and deployed within the appropriate indication, can be mobilized for effective anti-tumor responses.
1Kudrin A and Hanna MG Jr. (Guest Editors) Special Focus Cancer Commentaries Series, Human Vaccines &Immunotherapeutics, Vol 8 Issue 8, August 2012.
2Finke LH, Wentworth K, Blumenstein B, Rudolph NS, Levitsky H, Hoos A. Lessons from randomized phase III studies with active cancer immunotherapies—outcomes from the 2006 meeting of the Cancer Vaccine Consortium (CVC). Vaccine 2007;25 (Suppl 2):B97-109.
3 Vogelstein B, Papadopoulos N, Velculescu VE, Zhou S, Diaz LA Jr, Kinzler KW. Cancer genome landscape. Science 2013; 339(6127):1546-1558.
4 Hanahan D and Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011; 144(5):646-674.
5 Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012; 12(4):252-264
| 3 |
We believe the immune system, if trained and deployed within the appropriate indication, can be mobilized for effective anti-tumor responses.
It is well recognized that surgical removal is the best treatment for reducing bulky tumors. However, surgery can fail to remove all the cancerous cells, leaving microscopic pockets of the original tumor behind. In fact, 54% of stage II colon cancer patients have PCR-detectable micrometastases in at least one lymph node.6 The five-year survival rates between stage II patients with PCR-positive and –negative lymph nodes is illustrative of the problem (36% vs. 75%, p=0.03). To find and destroy these cells, active specific immunotherapy uses the excised tumor as the source material for mobilizing the immune system against the residual disease. By definition, the original tumor contains all the relevant antigens which define the individual patient’s remaining cells, which can be leveraged to create a robust and comprehensive immune response. Autologous tumor cell vaccines are more demanding to create on a population-wide basis; however, the realities of cancer have dictated the requirement of this method.
Larger pharmaceutical companies continue to advocate for more targeted therapies to be utilized in smaller patient populations. However, the heterogeneity of cancer as described above provides two logistical hurdles for this treatment paradigm: 1.) the complication of accurate mutational diagnosis with DNA, RNA, or proteomic analysis and 2.) the increases probability of developing treatment resistance. Due to the extreme diversity inherent to a tumor, a simple biopsy which is sequenced for mutational analysis is not adequate to fully represent the diversity of the entire lesion. Consequently, while a given mutation may be evident in the biopsy, diverse clones within the remaining tumor could harbor mutations or gene expression differences which render these cells resistant to the drug of choice (de novo resistance). Additionally, due to the genetic flexibility inherent to malignant disease, these cells can alter their gene expression to compensate for targeted agent selective pressure (acquired resistance). In either instance, the more cells which exist, the more advanced disease becomes, and the probability increases targeted agent use will be effective.
However, evolution has provided the tools to address a clinical foe with untold diversity: the immune system. This molecular armament was designed to tackle the extreme diversity of disease. With the exception of safe drinking water and antibiotic development, in our opinion, no other clinical modality can compare with vaccination for reducing mortality and increasing population survival and growth. By correctly harnessing and deploying the most recent advancements in molecular biology within the appropriate indication, we can now apply vaccination strategies to effectively treat malignant disease.
OncoVAX® Overview
OncoVAX® is a cancer vaccine designed for the post-surgical treatment of minimal residual disease. Effectively eradicating these small pockets of cells greatly decreases the risk of recurrence. Currently, Vaccinogen is planning a pivotal phase IIIb trial for the treatment of stage II colon cancer under the auspices of an FDA-granted Special Protocol Assessment (SPA) with Fast Track designation. OncoVAX® uses a patient’s live, metabolically active cells to create a sterile, non-tumorigenic vaccine capable of mobilizing the immune system to prevent high-risk, early-stage disease recurrence. Embracing the heterogeneity of cancer, OncoVAX® uses a patient's own tumor to stimulate a broad and effective immune response against the diverse array of cancer cells which can remain after surgery.
6 Liefers G-J, Cleton-Jansen A-M, van de Velde CJH, Hermans J, van Krieken JHJM, Cornelisse CJ, Tollenaar RAEM. Micrometastases and survival in stage II colorectal cancer. New England Journal of Medicine 2004; 339: 223-228.
| 4 |
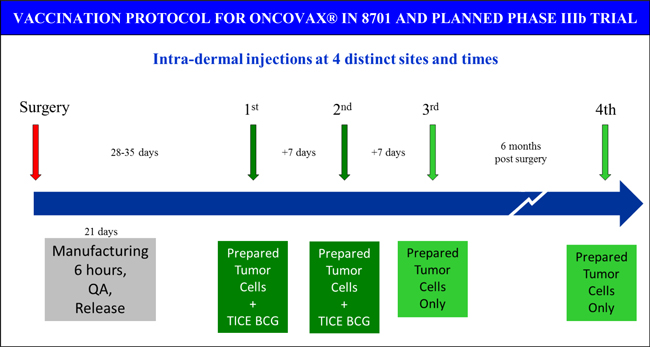
After surgery, the tumor is dissociated and sterilized using a patented enzymatic process. The cells are then frozen and gamma-irradiated to preserve viability, but prevent further tumorigenicity. A full course of OncoVAX® consists of four inoculations administered over a six month period. The first inoculation is provided approximately four to five weeks after surgery and the second inoculation is provided one week later. This involves thawing the frozen cells and compounding them with a proprietary formulation of Bacillus Calmette-Guerin (“BCG”), an adjuvant which serves to enhance the immune response against the tumor material previously defined as “self”. The third inoculation (given one week after the second inoculation) and the final booster (given six months after the initial inoculation) consist of the tumor cells alone. BCG was originally developed as a vaccine against tuberculosis prepared from a strain of live bovine tuberculosis bacillus. However, it has since been used as a therapy for the treatment of bladder cancer due to its ability to stimulate a robust immune response. BCG-containing vaccines create an induration, or welt, at the site of injection indicating a potent immune response is occurring against the tumor cells and bacteria. If the appropriate immunological training has occurred during the first two treatments, subsequent OncoVAX® injections, which only contain tumor cells, also create an induration. Previous studies have determined the diameter of the induration serves a prognostic biomarker capable of identifying patients at higher risk of recurrence.
OncoVAX® represents a flexible process, not a single therapeutic agent. However, the upcoming phase IIIb study will focus on stage II colon cancer patients, the most optimal population identified in the previous phase IIIa trial. The global incidence of colon cancer is 900,000 patients per year, of which 269,000 are stage II. In recent years, the prevalence of stage II colon cancer has actually increased with the emergence of more rigorous screening practices and endoscopic examinations. The Company estimates expect stage II colon cancer to represent about 46% of the US and EU colon cancer at diagnoses by 2020.
OncoVAX® Clinical Trials in Colon Cancer Patients
Since the 1970s, investigations by Dr. Michael G. Hanna, Jr. and colleagues7, 8, 9, 10 have translated the lessons learned with syngeneic guinea pig models into successful phase I, II, and III clinical trials in patients with colon cancer. These trials were based on preclinical evidence that the immune system could be educated to control a limited tumor burden after surgical excision of solid tumors. The reliance on patient-derived material was identified in 1977 by Fidler and Kripke11 when these researchers described the phenotypic heterogeneity of cancer in Science. This implied “off-the-shelf” treatments or homogenous tools would not be appropriate to treat this diverse disease. Their work demonstrated that different clones of murine melanoma cells, derived in vitro from a parent culture, varied greatly in their ability to produce metastatic colonies in the lungs of mice upon intravenous injection. This suggested that the parent tumor was heterogeneous and that highly metastatic tumor cell variants pre-exist in the parental population. As the Director of the National Cancer Institute (“NCI”), Dr. Hanna, recognized that if intra- and inter-tumoral heterogeneity for metastasis exists, it was also probable this diversity extended to the antigens which defined these lesions from the perspective of the immune system. Therefore, it was hypothesized the use of autologous tumor vaccines would obviate the need to screen for all the possible antigenic clones. After almost forty years and five clinical trials, we believe this paradigm has been validated. Vaccinogen's unbiased approach creates a patient-specific vaccine which employs the heterogeneity of cancer.
7Hanna MG Jr, Peters LC. Immunotherapy of established micrometastases with Bacillus Calmette-Guerin (BCG) tumor cell vaccine. Cancer Res 38:204, 1978.
8Hanna MG Jr, Peters LC. BCG immunotherapy: Efficacy of BCTG-induced tumor immunity in guinea pigs with regional tumor and/or visceral micrometastases. In Immunotherapy of Human Cancer. Raven Press, 1978, pp 11-129.
9Hanna MG Jr, Active specific immunotherapy of residual micrometastases: A comparison of postoperative treatment with BCG-tumor cell vaccine to preoperative intratumoral BCG injection. In Immunobiology and Immunotherapy of Cancer. WD Terry, Y Yamamura, eds, Elsevier/North Holland, 1979, pp 331-350
10Hanna MG Jr, Brandhorst JS, Peters LC. Active specific immunotherapy of residual micrometastasis: An evaluation of sources, doses and ratios of BCG with tumor cells. Cancer Immunol Immunother 7:165, 1979
11Fidler IJ, Kripke ML. Metastasis results from pre-existing variant cells within a malignant tumor. Science 197:4306 893-895, August 1977.
| 5 |
Clinical testing of OncoVAX® as a post-surgical treatment began in 1980 with a single-center pilot study of 5 subjects with colon cancer. To date 757 subjects with colorectal cancer, of which 720 had colon cancer, have been enrolled in OncoVAX® trials (see Table 1 for summary). In addition, a bioequivalency study (2002-01) enrolled 15 patients, also with colon cancer. Typical vaccine development involves a series of dose and regimen optimizations. For the final development of OncoVAX®, the FDA required an additional process change which involved creating a sterile drug product with an improved Potency and Identity Quality Control procedure. Both of these changes were included and we believe achieved within the latest bioequivalency study (2002-01). This study was conducted to compare the immunogenicity (i.e. the ability to promote an immune response) of the new sterile product compared to historical data from the phase III clinical study (8701). This was determined by the magnitude of delayed type hypersensitivity (DTH) responses to tumor cells alone. We believe the results of this study support the premise that the immunogenicity of vaccines produced by either process are comparable.
Excluding the bioequivalency study, 385 subjects have been randomized to receive OncoVAX®, of which 353 received at least one vaccination. 372 subjects were treated with surgery alone. In addition, subjects with colorectal cancer were enrolled in three separate trials that assessed the effects of ASI with OncoVAX® in combination with chemotherapy as adjuvant therapy to surgical resection. Lastly, two trials have been performed using autologous tumor cells/BCG in melanoma (n=86) and renal cell carcinoma (n=14).
Table 1: OncoVAX® Clinical Trials for Colon Cancer
| Protocol Number |
Dates of Trial |
Trial Phase |
Number of Patients |
Country in Which Trial Conducted |
Number of Vaccine Doses |
Disease and Stage |
Manufacturing* | ||||||||
| 8101 | 1980-1984 | I | 5 Treated | United States (US) | 3 | Colon Cancer Stage III/IV | Centralized, Non Validated, Non cGMP | ||||||||
| 8102 | 1980-1984 | II/III |
47 Control 50 Treated |
United States (US) | 3 | Colorectal Cancer Stage I-IV | Centralized, Non Validated, Non cGMP | ||||||||
| 5283 | 1984-1995 | III |
207 Control 205 Treated |
United States (US) | 3 | Colon Cancer Stage II/III | Decentralized, Non Validated, Non cGMP | ||||||||
| 8701 | 1986-1996 | III |
128 Control 126 Treated |
Netherlands (NL) | 4 | Colon Cancer Stage I-III | Centralized, Validated, cGMP | ||||||||
| ASI-2002-01 | 2002-2003 | I/II | 15 Treated | United States (US) | 4 | Colon Cancer Stage II/III | Centralized, Validated, cGMP |
| 6 |
*Centralized = Manufacture at a single, centralized location; Decentralized = manufacture at each investigational site.
As outlined in Table 1, OncoVAX® underwent significant optimization across the various clinical trials. For example, studies 8102 and 5283 used a three-vaccine regimen. In 8102, vaccine production was conducted at a single institution under the supervisor of Dr. Herbert Hoover,12 while in 5283, vaccine processing was decentralized. In this study, each clinical site manufactured the vaccine, without Quality Assurance (QA) or adequate Quality Control (QC). While both of these studies provided encouraging clinical results, neither demonstrated statistically significant clinical benefit in an intent-to-treat analysis of recurrence-free survival or overall survival. The lessons learned from these trials with respect to manufacturing and dosing informed the later, more successful clinical trials.
Specifically, in the Eastern Cooperative Oncology Group (ECOG) study 5283,13 a statistical analysis determined OncoVAX® clinical benefit correlated with the quality of the vaccine produced. Thus, centralized, quality controlled processing became a requirement for clinical efficacy moving forward. Additionally, in 8102, a subset of patients underwent DTH testing that revealed the vaccine-induced immune response had deteriorated 6 months post-surgery. Therefore, it was determined that a fourth booster immunization at 6 months would enhance the waning immune response to autologous tumor cells. Following these guidelines, a four vaccine regimen was planned with manufacturing centralized at the Free University of Amsterdam (8701).14 This study also featured patient stratification by tumor class in order allow for prospective evaluation of stage-specific clinical benefit. Ultimately, 8701 demonstrated that patients with stage II disease had clinically meaningful and statistically significant outcomes in both recurrence-free interval and recurrence-free survival. A statistically significant reduction in relative risk of death was also observed when stage II colon cancer patients completed all four inoculations. Thus, we believe that OncoVAX®, at an optimum dose and regimen, is the first vaccine to demonstrate effectiveness in preventing colon cancer recurrence after surgical resection.
Phase IIIa Trial 8701 (1986-1996, NL)
8701 was a phase IIIa trial conducted with 254 stage II or III colon cancer patients. As outlined above, this study was stratified by tumor stage, location, and institution to accurately determine with a prospective analysis the specific indications for OncoVAX® clinical benefit. Patients were randomized 1:1 to receive surgery and OncoVAX® treatment or surgery alone with follow-up observation. Treated patients received OncoVAX® according to the protocol Vaccinogen plans to use in the upcoming confirmatory phase IIIb trial (schedule depicted below). Patients were given one month to recover from surgery and regain their full immune function before receiving their personalized OncoVAX® injections. Processed, irradiated tumor cells were compounded with BCG and provided as the first two injections a week apart. A week later, a third injection of irradiated tumor cells alone was provided. A fourth injection of tumor cells alone was given as a booster six months after surgery. This was the first clinical trial where the manufacturing process was optimized in a central processing facility and a four dose regimen was executed. Due to the encouraging results observed in 8701, this is the optimal regimen established for OncoVAX® investigation in the upcoming phase IIIb clinical trial.
12Hoover HC Jr, Brandhorst J, Peters C, Surdyke MG, et al. Adjuvant active specific immunotherapy for human colorectal cancer – 6.5-year median follow-up of a phase III prospectively randomized trial. J Clin Oncology, 11:3 390-399, 1993
13Vermorken JB, Claessen AME, van Tinteren H, Gall HE, et al. Active specific immunotherapy for stage II and III human colon cancer. A randomized trial. The Lancet 353 345-350, January 1999
14Harris JE, Ryan L, Hoover HC Jr, Stuart RK, Oken MN, Benson AB, et al. Adjuvant active specific immunotherapy of stage II and III colon cancer with an autologous tumor cell vaccine: ECOG study E5283. Journal of Clinical Oncology Vol 18 148-157, January 2000
| 7 |
| 8 |
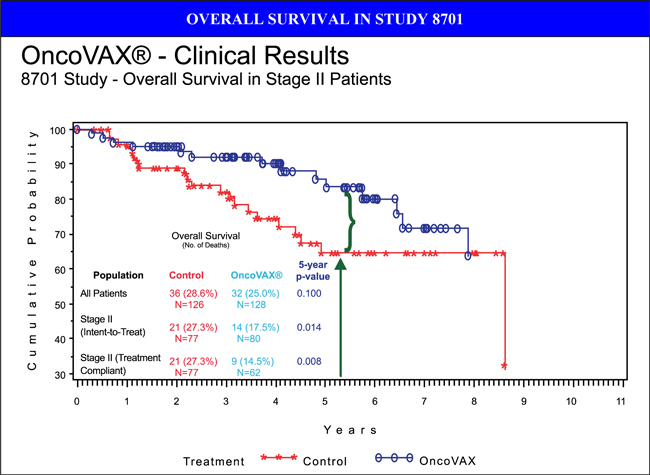
In the unstratified, intent-to-treat population (all patients), 8701 demonstrated a trend towards clinical efficacy with respect to recurrence-free and overall survival. However, when patients were stratified by tumor stage, it was determined that stage II patients had a statistically significant improvement in recurrence-free interval, recurrence-free survival and overall survival. In fact, stage II OncoVAX® patients had an 88% 4-year recurrence-free survival rate compared to control patients who recurred more frequently (74%, p=0.032).
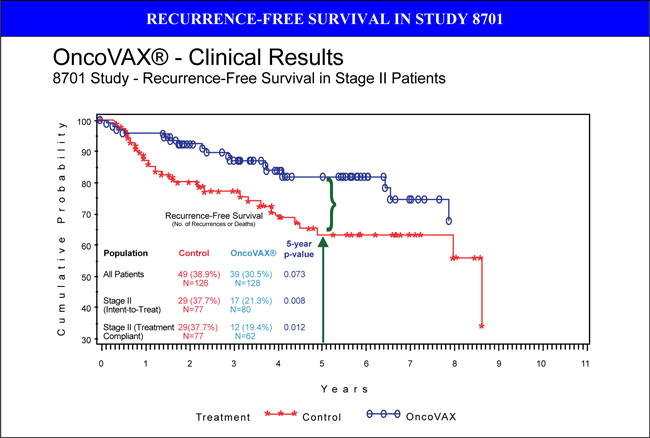
This published, randomized phase IIIa clinical trial (1986-1996, NL) was stratified by tumor stage for prospective analysis. Stage II colon cancer patients derived the most benefit from OncoVAX® treatment. This study was accepted by the FDA as supportive data for the next phase IIIb clinical trial to be conducted under an FDA granted SPA with Fast Track designation. Based on these results, recurrence-free survival is the accepted endpoint for the interim analysis. The initial results of this study were published in The Lancet January 30, 1999; 353: 345-350.
| 9 |
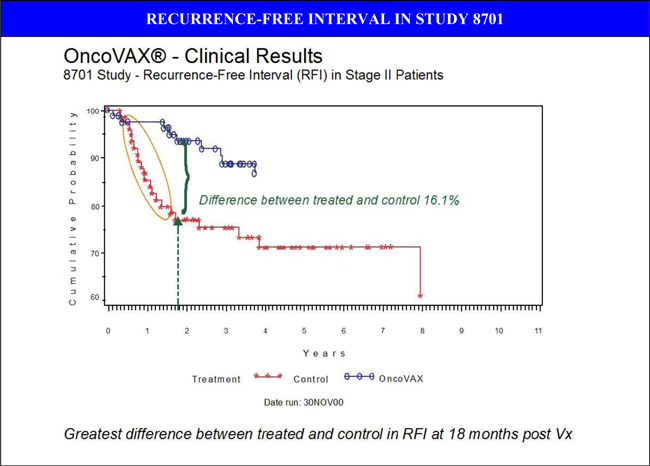
Patients with Stage II disease who received all four inoculations had superior clinical outcomes to those who received fewer than four inoculations. In stage II colon cancer patients who received all four inoculations, relative risk of death or recurrence decreased by 61% (RR: 0.46, p = 0.046). Consequently, with OncoVAX® treatment, the risk of recurrence in stage II colon cancer decreases from one in three patients to one in ten. Additionally, OncoVAX® treatment was safe and well-tolerated, as control patients had a higher rate of non-fatal serious adverse events compared to the treated group15. Consequently, adverse events were minimal and no treatment-related deaths were observed.
To further investigate the long-term benefits of OncoVAX® treatment, a 15-year follow-up of the 8701 patients was recently conducted by Vermorken and colleagues16. The results demonstrated that OncoVAX®-treated patients still had improved recurrence-free interval compared to the control group [HR=0.62, (95%, CI:34-0.96), log-rank p-value = 0.03). This robust long-term benefit is the hallmark of a successful therapy.
In establishing the upcoming phase IIIb trial, the FDA in a June 2, 2010 letter confirmed recurrence- or disease-free survival, defined as the time from randomization to the first objective test confirming tumor progression or death due to any cause, will be an acceptable primary endpoint for OncoVAX® in stage II colon cancer patients (ACTIVE).
15 Hanna et al., Active specific immunotherapy with autologous tumor cell vaccines. Human Vaccines, 2006, 2:4, 185-191.
16Weger de VA, Turksma AW, Voorham, QJM, Euler Z, et al. Clinical effects of adjuvant active specific immunotherapy differ between patients with microsatellite stable and microsatellite instable colon cancer. Clin Cancer Res Feb 1, 2012, 18;882
| 10 |
Based on the previous clinical trial results, some patients will still recur. Patients with recurrent disease will receive treatment with other modalities and their overall survival will consequently depend in part on the efficacy of other therapies. The stage-specific prospective analysis of 8701 has been particularly informative for planning the upcoming confirmatory phase IIIb clinical trial.
Planned Trials - Stage II Colon Cancer, Phase IIIb Trial
The FDA has requested a second confirmatory, randomized, controlled phase IIIb trial of OncoVAX® in stage II colon cancer patients. The principal objective of this pivotal study is to achieve required registration of OncoVAX® as a therapeutic drug product for an unmet medical need. To accomplish this objective, Vaccinogen needs to recruit and organize a team of expert consultants and in-house managerial staff to meet all of the necessary requirements. The conduct of a complex phase III clinical trial involves many disciplines including but not restricted too, regulatory affairs, clinical training and site management, clinical data administrative functions, logistics of in-house manufacturing, transport of tumor and final drug product, and clinical quality control and assurance. The Vaccinogen management team and Medical Advisory Board members interviewed several contractors for each discipline and selected, based on experience and documented successes, three top groups as consultants. Then working with these selected contractors, Vaccinogen developed a chart of action accountability to which all of the selected groups agreed. We have preliminary agreements in principal with each of the consultant contractors. Formal contracts will be entered into and executed upon receipt of additional funds.
Vaccinogen filed an investigational new drug (IND) application for OncoVAX® and was granted BB-IND 4561 by the FDA in 2006. On June 2, 2010, based on a revision of the statistical analytical plan negotiated with the FDA, Vaccinogen submitted a revised Special Protocol Assessment (SPA) request for BB-IND 4561 No 102 and this was granted by the FDA. Further on February 18, 2011 changes in the protocol and the addition of three chartered committees, Monitoring, Adjudication and Radiology Review, were added and filed with BB-IND 4561, Serial No. 103.
The protocol of this trial, including endpoints and the statistical analysis plan, is the subject of an SPA and Fast Track review agreement granted by the FDA. An SPA granted by the FDA provides a mechanism for the sponsors and the FDA to reach agreement on size, execution and analysis of a clinical trial that is intended to form the primary basis for regulatory approval.
This study will be carried out under an SPA that was negotiated with the FDA in 2010. The primary endpoint of this pivotal phase IIIb trial is recurrence-free survival (RFS) with an interim analysis after 70 “events,” and a final primary analysis three years following completed enrollment. “Events” are incidents of either recurrent disease or death. The study is powered at 90% to detect a 50% improvement in RFS versus resection only for final analysis with an adjustment for interim analysis. The power calculations in the study assume an event rate and distribution that match those observed in the 8701 phase IIIa study. If a robust p-value is achieved at the interim analysis, we believe this may be sufficient to support filing of a Biologics License Application (BLA) with the FDA’s center for Biologics Evaluation and Research (CBER) at that point. However, determination of sufficient results to file for a BLA is a review issue that will be addressed upon completion of the study. In order for a single trial to support registration for an indication, the trial must be well conducted and the results of the trial must be internally consistent, clinically meaningful and statistically persuasive. It is possible that the FDA could request additional information prior to submission of a BLA. However, past clinical trials using the optimal regimen with four inoculations will be accepted as supportive studies during the FDA review of the BLA.
| 11 |
The phase IIIb study will enroll 550 patients, randomized 1:1 to receive surgery alone or surgery plus OncoVAX®. Patients will be followed on a regular schedule to determine whether and when their cancer might recur. The experience with OncoVAX® in 8701 demonstrated disease recurrence in stage II patients happened more quickly and more frequently in those who received surgery alone. If the phase IIIb trial replicates the experience of the 8701, an interim analysis after 2/3 of the expected events occur should give a clear and statistically significant separation between the Kaplan-Meier curves at that point, and would form the basis for a BLA seeking marketing approval from the FDA. The planned phase IIIb clinical trial will commence within 60 days of obtaining adequate funding. Management currently estimates that approximately $30 million in funding will be required to commence the phase IIIb clinical trial.
It is important to emphasize that the FDA has agreed that the primary endpoint of the planned phase IIIb trial is a statistically significant improvement in recurrence-free survival (RFS). Based on the average age of the population at the time of tumor presentation (65 years) this is the most appropriate endpoint to evaluate a trial in stage II colon cancer. The alternative in cancer studies is often OS and is the secondary endpoint in our planned phase III clinical trial. The average age of patients presenting with colon cancer is 65 years, so deaths from causes other than the tumor will dilute the outcome in both arms. Patients in either arm whose disease recurs will receive chemotherapy and other treatments for their metastatic disease, and the success of these treatments will dilute the impact of earlier therapies such as OncoVAX® on OS. From a patient perspective, being disease free is clearly a meaningful endpoint.
Planned Trial - Phase I/II Trial in Stage III Colon Cancer
When patients present for colon cancer surgery, it is not immediately clear whether they have stage II or stage III disease. Vaccinogen’s planned phase IIIb trial will only enroll stage II patients based on the 8701 results, but the clinical sites will perform surgery and process a number of stage III tumors. Due to the reliance on metabolically-active cells, these tumors must be processed immediately irrespective of staging, which is determined later by the pathologist. Any vaccination protocol also needs to accommodate the schedule of adjuvant chemotherapy, which is the current standard of care for stage III colon cancer patients. There is a risk the common immunosuppression which accompanies cytotoxic chemotherapy treatment could interfere with the effect of the fourth booster vaccination. Nonetheless, there is a considerable unmet need in stage III colon cancer morbidity and mortality, as well as a significant market opportunity. Consequently, we believe appropriately optimizing an OncoVAX® dosing regimen within the bounds of adjuvant chemotherapy could provide added clinical benefit for stage III colon cancer patients.
The expense of conducting a parallel registration program in stage III patients is beyond our current means, with our primary clinical and financial focus on the stage II patients in the ACTIVE trial. However, with relatively modest additional funds, a small pilot program in parallel with ACTIVE is possible to demonstrate the ability to accommodate OncoVAX® and adjuvant chemotherapy combination treatments. This pilot trial will have DTH and safety endpoints to demonstrate whether cytotoxic chemotherapy interferes with the known immunogenicity of the vaccine. This trial should enroll 30 patients and is planned to start one month after the ACTIVE trial. DTH data will be available for each patient after the third immunization, which will be prior to chemotherapy treatment. Full data from this trial could be available within eighteen months after beginning ACTIVE. A successful outcome of this study would pave the way for larger trials to establish OncoVAX® combination therapies as the standard of care for reducing stage III risk of recurrence.
Additional Applications of Technology
Our technology may also have applications in other tumor types, notably melanoma and renal cell carcinoma (RCC). Clinical development in both these tumor types is more challenging than in stage II colon cancer because of the limited tumor starting material in early-stage disease and number of approved chemotherapies for each indication. However, due to the potent baseline immunogenicity of these tumors, OncoVAX® treatment could be positioned to provide enhanced therapeutic benefit beyond the current standard of care.
| 12 |
Human Monoclonal Antibodies (“HuMabs”) Program
Monoclonal antibodies (Mab) are immunological agents secreted by a single clone of B-cells which recognize a unique biological target, or epitope. The exquisite affinity and specificity these molecules exhibit make them excellent candidates to create unique drug products. Mab technologies evolved rapidly over the last 20 years. Antibody-based therapeutics now make up a significant and growing portion of total pharmaceutical sales.
Mabs recognize and bind to specific epitopes evident on various cells, viruses, and other molecules in a very targeted manner. This makes them very effective agents for targeted approaches to fight or detect many diseases. Mabs are a part of the body's own natural defense mechanism. Consequently, combined with their specificity, they have the potential for lower side-effects compared to small molecule based treatments. Also, it is possible to patent the composition of an epitope/antibody target, which provides significant intellectual property protection within this clinical space. We expect the interest in Mab technology will only continue to grow, particularly in the oncology field. Last year alone, over 400 random mutational events were discovered yielding 400 new markers. This number is expected to rise into the thousands (MR Stratton, Science, 331 pp 1553-1558). Furthermore, most major pharmaceutical companies now have Mab projects in their R&D plans. Due to our unique access to patient-derived, cancer-specific B-cells, we believe that we are optimally positioned to exploit these developments by building completely novel libraries of cancer-specific, fully human monoclonal antibodies. As outlined above, we intend to leverage our planned phase III trial to participate and innovate within this ever-growing market.
Our previous phase III OncoVAX® trials demonstrated that immunized patients produced circulating B-cells, which were able to generate tumor-specific, fully human monoclonal antibodies (HuMabs). A B-cell is a specialized blood cell which secretes antibodies to help guide the immune system recognize foreign invaders. Due to the long-term efficacy observed in stage II colon cancer patients, it is very likely cancer-specific memory B-cells represent a significant portion of this protection. We plan to collect this rare set of B-cells generated from the treated patients and screen new B-cells with more advanced technology not available during the original OncoVAX® clinical trials. We intend to perpetuate these cells and select the most relevant antibodies for in-house pipeline development, or further drug development with strategic partnerships to create new therapeutics, vaccines, diagnostics, or imaging agents.
We believe access to this highly novel asset (patient-derived, cancer-specific B-cells), and similar materials developed during predecessor company efforts, represents a valuable asset that can generate future revenue stream including upfront fees, development milestones, and royalties via collaborations and licensing activities that will complement the OncoVAX® investment opportunity.
History of HuMabs Program
Michael G. Hanna, Jr., Ph.D., Vaccinogen’s Founder,a member of the Company’s Board of Directors and Chairman Emeritus, led a team of scientists during the initial OncoVAX® investigations which discovered that patients treated with autologous colon tumor vaccines produced circulating B-cells which were capable of being perpetuated into tumor-specific, human monoclonal antibody (HuMab) producing cells. A research program was initiated which ultimately assembled a large array of fully human monoclonal antibodies. HuMabs may be effective in the diagnosis, treatment, and prevention of disease, overcoming many of the limitations of cytotoxic drugs. The powerful combination of specificity and patentability has made monoclonal antibodies an attractive source for development in the biopharmaceutical marketplace.
| 13 |
Competitive Advantage
During the course of the first OncoVAX® trials, Dr. Hanna’s team recognized that tumor cell eradication was mediated by cytotoxic, immunized T-cells. However, evidence also existed of a humeral (antibody) mediated response. It was discovered that during a specific window in the OncoVAX® treatment protocol, patients produced tumor-specific B-cells which secreted fully human monoclonal antibodies.
We believe our competitive advantage is the unique access to and ownership of a valuable educated B-cell repertoire to be collected from immunized patients in the upcoming phase III trial, as well as the insight and experience from legacy efforts to build a HuMab-based business unit. From this institutional knowledge, we should be able to leverage a plethora of new development and production strategies as well as technologies to efficiently and effectively exploit this opportunity. Selected contractors and collaborators will collect blood from patients over the first year of the trial and develop libraries from these unique HuMabs, building on the legacy material from developed during the previous trials.
Due to our strategic intellectual property protection, we believe Vaccinogen has a unique proprietary source for generating various types of tumor-specific antibodies which cannot be produced by similar competitors from alternate production sources.
Fully Human Monoclonal Antibodies
Initial attempts to generate therapeutic antibody products involved development in mice, chimeric antibody engineering, or similar “humanizing”, resulting in significant drug development challenges as these agents:
• Do not interact efficiently with the human immune system,
• Have rapid clearance, potency issues,
• Can cause allergic reactions,
• Often trigger a human anti-mouse antibody (HAMA) response, and
• Often require royalty payments to third party owners of the tools and methods to optimize the drug product.
By contrast, Vaccinogen’s HuMabs are fully human (not humanized) and carcinoma-specific. Consequently, they can be administered safely to humans in large quantities with potential applications in chronic and acute disease settings. This is in contrast to mouse Mabs which have been engineered with human portions (i.e. humanized Mabs) to minimize, but not eliminate antigenic potential. These HuMabs are also expected to be of interest in the vaccine, imaging, and diagnostics markets. Furthermore, they may have applications in biomedical research beyond the cancer therapeutic market.
Immune Libraries
We believe our repertoire of educated B-cells from OncoVAX® immunized patients will allow the creation of a unique library comprised solely of human products from immunized patients. Due to the natural development of these antibodies, they demonstrate potent target identification with minimal recognition by the innate human immune system.
These two characteristics, “fully human” and “immune-based”, provide us with what we believe is a valuable competitive advantage and an asset that can complement the OncoVAX® investment opportunity.
| 14 |
As outlined above, we intend to launch a pivotal phase IIIb trial of OncoVAX® which will confirm the clinical benefit of this autologous tumor vaccine for the prevention of stage II colon cancer recurrence. The 275 treated patients will yield “cancer educated” B-cells from which lead antibody candidates can be identified. Our intended approach to exploit this market opportunity is to execute strategic partnerships with a select group of qualified pharma and genomics companies, allowing them licensed access to select monoclonal antibodies. Our partners could “mine” these targets using proprietary antigens or target molecules to identify potential antibodies for further development. Ultimately, we expect these antibodies will be developed and commercialized into products for use in the detection, treatment, and prevention of malignant diseases. The utility of safe and effective HuMabs with high affinity and avidity to newly defined therapeutic biomarkers would be a powerful application of our exclusive reagents.
This business model may provide us with multiple potential funding events as our partners explore, discover, qualify, and ultimately commercialize a product using one of our antibodies. In general terms, the potential funding events for each single antibody targeted for a specific indication will follow a pattern similar to the following.
| Development Stage | Agreement Stage | Timing | |||
| Exploration | Technology Access | Lump-sum on signing, and annual renewal fees | |||
| Discovery | Technology Development | Lump-sum on identification of target antibody | |||
| Qualification | Developmental Collaboration | Payments at various stages throughout the FDA approval process | |||
| Commercialization | Royalty | Quarterly payments |
The landscape of the biopharmaceutical industry is rapidly transforming. We expect the entities within this space to constantly search for novel ways to develop effective pharmaceutical products in a timely manner to minimize cost and maximize economic potential.
The legacy material generated during previous trials already provided an intriguing lead candidate. On September 25, 1998, the European Medicines Agency (EMA) granted marketing authorization for one of these HuMabs (88BV59) called HumaSPECT. The EMA concluded that as a tumor imaging agent HumaSPECT did not elicit aberrant or unwanted immunogenic reactions, such as anti-human antibody responses. This is significant because mouse monoclonal antibodies have limited utility due to the robust human anti-mouse response and similar humanized monoclonal antibodies often required co-treatment with immunosuppressive therapies.
We believe that our fully human immune-based repertoire is unique, extensive, and has previously generated a lead pharmaceutical candidate. Therefore, this asset can serve as an attractive option for strategic partnership.
| 15 |
Colon Cancer Incidence by Stage
Colon cancer represents the third most common form of cancer in both the U.S. and Europe. American Cancer Society statistics suggest there will be approximately 97,000 new cases of colon cancer diagnosed within the U.S. in 2014, resulting in approximately 50,000 deaths.
Following diagnosis, colon cancer can be categorized into four stages. Stage I is confined to the mucosa and sub-mucosa of the colon. Stage II tumors have penetrated farther into the muscles around the colon, but the tumor has not visibly spread to lymph nodes of more distant sites. Stage III tumors have either locally advanced disease or demonstrate regional lymph node involvement. Stage IV tumors have distant metastases at diagnosis.
Vaccinogen’s analysis of available colon cancer data suggests that the proportion of stage I and IV patients has remained relatively constant over the past decade. However, there has been an on-going increase in the proportion of patients being diagnosed with earlier-stage, localized disease (stage II), largely resulting from more consistent screening practices in the US and improved diagnostic technologies (fecal occult blood tests, sigmoidoscopy, colonoscopy, CEA screening). Available data suggests that stage III is still a significant, but declining, percentage of reported colon cancers.
The table below highlights some the shifts in historical and anticipated incidence of stage I-IV colon cancer in the US.
| COLON CANCER INCIDENCE BY STAGE | ||||||||||||
| 1990s1 | 20042 | 2020E3 | ||||||||||
| Stage I | 15 | % | 8 | % | 10 | % | ||||||
| Stage II | 36 | % | 39 | % | 46 | % | ||||||
| Stage III | 28 | % | 39 | % | 31 | % | ||||||
| Stage IV | 22 | % | 14 | % | 13 | % | ||||||
| 1 Journal of the National Cancer Institute, Vol. 96, No. 19 |
| 2 SEER Data |
| 3 Company Estimates |
Epidemiology to Market Characteristics
In stage I colon cancer, the disease is adequately treated with surgical intervention. However, there is an unmet medical need with respect to more effective stage II colon cancer therapies. Current standard of care does not adequately address the micro-metastases which lead to disease recurrence in approximately 30% of these patients. The use of adjuvant chemotherapy in stage II colon cancer is controversial, with no clear consensus in the literature regarding its use. This is in stark contrast to stage III colon cancer where the indicate for combined surgery and cytotoxic chemotherapy is clear. Advanced stage IV patients are also treated with a combination of chemotherapeutics and surgical intervention, depending on the site of metastasis.
| 16 |
Stage II Tumor Colon Cancer Market
While the stage II colon cancer market is considerable, Vaccinogen’s OncoVAX® production methodology requires a total of about 3.5 grams of tumor to provide enough material for a complete course of therapy. Based on prior clinical trial experience, Vaccinogen estimates that about 30% of surgically resected stage II colon tumors will not meet minimum size requirements for vaccine production. Vaccinogen’s colon cancer market models and forecasts reduce the stage II total market to reflect this minimum tumor size constraint. Vaccinogen further adjusted stage II colon cancer figures by an additional 10% to account for potential logistical problems inherent with its centralized manufacturing requirements, damages, etc.
A review of available data on estimated annual colon cancer incidence is produced by the American Cancer Society, European Cancer organizations, SEER and the Journal of the National Cancer Institute. This statistical work and related assumptions provides the foundation for determining the scope and magnitude of colon cancer across major global regions, and was further stratified to evaluate distinct stage II colon cancer market opportunities for OncoVAX®.
United States Trend
The overall incidence of colorectal cancer has actually been modestly declining in the US, from a total of about 112,000 cases in 2007 to approximately 97,000 cases in 2014. The reason for this decline is not completely understood, but may include factors such as reduced smoking, higher aspirin use, changes in diet, etc. For the purposes of forecasting, the Company has assumed a modest decline in the net number of new colon cancers over the next ten years. This reduction in overall colon cancer cases in the US is largely offset by a greater proportion of disease detected at stage II.
More rigorous screening for colon cancer has led to improvement in earlier detection, giving a significant redistribution of the TNM (tumor, node, metastases) staging of the disease. Over the past decade, there has been a gradual shift toward earlier detection, and therefore more localized disease, as well as relative declines in advanced disease. Vaccinogen estimates that stage IV disease has declined significantly over the past 20 years and now accounts for only about 13% of cases in the US. Stage III disease has also been gradually declining. Vaccinogen projects that the overall proportion of colon cancer represented by stage II disease should gradually increase to 46% in 2020 and beyond.
European Trends
The European market for colon cancer is considerably larger than the US and still growing, owing to sheer population demographics. Vaccinogen estimates that many of the more wealthy major European markets (France, Germany, Italy, Spain, UK) have characteristics closely mirroring US colon cancer staging, but with a slightly higher proportion of stage III and stage IV disease. The trend toward these stages is even more pronounced in Eastern European countries with less comprehensive screening. For Eastern Europe (and other less developed healthcare economies), stage II is estimated to represent about 20%-25% of total colon cancer diagnosis. Similar to the shift in the US, Vaccinogen projects that stage II colon cancer should gradually increase to about 45% of all cases in major EU economies and to 35% in emerging European countries by 2020.
Rest of World (ROW)
In contrast to the United States, colon cancer rates are actually rising in Japan, and changes in diet and higher rates of obesity are seen as key drivers for increasing colon cancer incidence throughout major emerging economies such as China. While sheer population lends to a significant number of colon cancer cases in the region, the lack of rigorous screening protocols leads to later diagnosis and more advanced disease at presentation than in the US or Europe. Vaccinogen forecasts assume that approximately 25% of colon cancer is stage II across the collective world markets. Vaccinogen forecasts that this proportion of stage II cases will gradually increase, to approximately 35% by 2020.
| 17 |
Global Totals
These factors point to a considerable and growing global market for OncoVAX® in stage II colon cancer patients. In the US, Vaccinogen estimates that the overall number of stage II colon cancer cases will range between 41,000 to 46,000 over the next decade. For the top five major European countries, Vaccinogen forecasts the stage II colon cancer market will increase from about 61,000 in 2010 to 74,000 by 2020. In the remaining global markets, the cases are collectively estimated to grow from about 201,000 in 2010 to about 252,000 by 2020, albeit largely confined within less developed healthcare economies.
Stage III Colon Cancer Opportunity
The shift in colon cancer diagnosis to earlier stage II disease is expected to lead to a gradual reduction in the proportion of patients diagnosed with later stage III disease. We believe that OncoVAX® has significant potential for treating stage III patients and plans to conduct an inexpensive pilot study with a surrogate endpoint to test combining this treatment with adjuvant chemotherapy. Larger studies in stage III patients could begin after successful data stage II clinical trials. This timeline could result in a label indication for stage III disease around 2019. Although stage III cases are modestly declining, it still represents an attractive commercial target. Our modeling suggests a US market for stage III colon cancer of about 35,000 cases in 2020. Europe Top 5 stage III colon cancer cases should collectively represent over 57,000 patients, while ROW regions will account for over 241,000 cases in 2020. Due to the potential uncertainty in combining OncoVAX® with a cytotoxic chemotherapeutic, our current revenue forecast does not contain stage III colon cancer revenue.
Intellectual Property
Intellectual property protection is important for our ability to successfully commercialize innovative technology. We have broad patents covering OncoVAX® technology in the U.S. and eight other countries (Australia, Canada, Switzerland, Germany, France, Great Britain, Ireland, and Italy). These patents and applications provide broad coverage for the production of autologous cancer vaccines. The key protection of OncoVAX®, in addition to considerable expertise and trade secrets, is the broad, issued patent protection around the production of autologous, sterile, metabolically active cancer vaccines developed by Vaccinogen. This patent, entitled “Sterile Immunogenic Non-Tumorigenic Tumor Cell Composition and Methods” was issued in 2009 and expires no sooner than 2025. This patent covers the entire OncoVAX® technology platform, but emphasizes the manufacturing process that results in a sterile vaccine. The FDA has mandated that sterility of vaccines is required for any drug product to reach the US market. The standard pharmaceutical procedures for sterility are not possible for drugs consisting of live tumor cells. Thus, a patented procedure that creates a sterile cellular drug product could result in a regulatory barrier to entry for other competitors. Our intellectual property is pledged as collateral under certain financing arrangements described below.
Outside of the United States, we have, in certain territories, corresponding issued patents related to OncoVAX®. Patent expiration dates may be subject to patent term extension depending on certain factors. In addition, following expiration of a basic product patent or loss of patent protection resulting from a legal challenge, it may be possible to continue to obtain commercial benefits from other characteristics such as clinical trial data, product manufacturing trade secrets, uses for products, and special formulations of the product or delivery mechanisms.
We intend to continue using our scientific experience to pursue and patent new developments to enhance our position in the cancer field. Patents, if issued, may be challenged, invalidated, declared unenforceable, circumvented or may not cover all applications we desire. Thus, any patent that we own or license from third parties may not provide adequate protection against competitors. Our pending patent applications, those we may file in the future, or those we may license from third parties may not result in issued patents. Also, patents may not provide us with adequate proprietary protection or advantages against competitors with, or who could develop, similar or competing technologies, or who could design around our patents. In addition, future legislation may impact our competitive position in the event brand-name and follow-on biologics do not receive adequate patent protection. From time to time, we have received invitations to license third-party patents.
| 18 |
We also rely upon unpatented proprietary know-how and continuing technological innovation and other trade secrets to develop and maintain our competitive position, in part by using confidentiality agreements. Our policy is to require our officers, employees, consultants, contractors, manufacturers, outside scientific collaborators and sponsored researchers and other advisors to execute confidentiality agreements. These agreements provide that all confidential information developed or made known to the individual during the course of the individual’s relationship with us must be kept confidential and not disclosed to third parties except in specific limited circumstances. We also require signed confidentiality agreements from companies that receive our confidential data. For employees, consultants, and contractors, we require agreements providing that all inventions conceived while rendering services to us shall be assigned to us as our exclusive property. We hold considerable proprietary expertise and trade secrets related to the OncoVAX® technology, including the production of autologous cancer vaccines.
We have brand names for our OncoVAX® products and related technologies, and anticipate filing 5 trademark applications for these and related marks.
OncoVAX® Patents Pledged As Collateral under Certain Financing Arrangements.
Agreements with Organon Teknika
The patent related to our OncoVAX® technology was pledged as collateral under a New Security Agreement dated October 31, 2007 between Intracel Holdings Corporation and Organon Teknika Corporation and secures payments due Organon under that certain October 31, 2007 letter agreement with Intracel, including the remaining $3.5 million in settlement payments due Organon thereunder (plus any accrued interest from the date of the October 31, 2007 letter agreement based on the prime lending rate in effect on the anniversary of such letter agreement), of which $500,000 is past due and has not been paid and the remaining $3,000,000 is due in equal annual payments of $1 million commencing the first year after the first marketing approval of OncoVAX® by the FDA or EMA (the “Settled Amount”). We assumed these liabilities in connection with our October 24, 2010 Asset Transfer Agreement with Organon. Under this letter agreement, failure to make a payment with respect to the Settled Amount when due is an event of default, which if unremedied for a period of 45 days after written notice of such default has been received will be a default under the New Security Agreement. To date, we have not received any notice of default from Organon in respect of our past due $500,000 payment, and as of November 1, 2014 the statute of limitations has expired on the collectability of the past due amount.
The Abell Foundation Financing
The patent related to our OncoVAX® technology was pledged as collateral under a Patent Security Agreement to secure payment of amounts due under a Fourth Amended and Restated Promissory Note in the original principal amount of $1,800,000. Abell’s security interest under the Patent Security Agreement is granted in conjunction with our October 26, 2011 Security Agreement with Abell. The promissory note was paid in full on August 25, 2014 and Abell released its security interest in Vaccinogen’s patents via the filing of an UCC-3 termination statement on September 3, 2014.
Competition
The biotechnology and biopharmaceutical industries are characterized by rapidly advancing technologies, intense competition, and a strong emphasis on proprietary products. Pharmaceutical and biotechnology companies, academic institutions, and other research organizations are actively engaged in the discovery, research, and development of products designed to address prostate cancer and other indications. There are products currently under development by other companies and organizations that could compete with OncoVAX® or other products that we are developing.
| 19 |
Our competitors include major pharmaceutical companies. These companies may have significantly greater financial resources and expertise in research and development, manufacturing, pre-clinical testing, conducting clinical trials, obtaining regulatory approvals and marketing. In addition, smaller competitors may collaborate with these large established companies to obtain access to their resources. However, to our knowledge there are no products in development that are competing with OncoVAX® in stage II colon cancer or have achieved the development of stage III clinical trials. FOLFOX is a chemotherapeutic regimen for the treatment of colorectal cancer, consisting of the drugs: (i) FOL– Folinic acid (leucovorin); (ii) F – Fluorouracil (5-FU) and (iii) OX – Oxaliplatin (Eloxatin). FOLFOX is currently the approved adjuvant treatment for stage III colon cancer. We know of no other autologous tumor cell vaccines being developed to treat stage II or III colon cancer.
Competition among products approved for sale will be based upon, among other things, efficacy, reliability, product safety, price-value analysis, and patent position. Our competitiveness will also depend on our ability to advance our product candidates, license additional technology, maintain a proprietary position in our technologies and products, obtain required government and other approvals on a timely basis, attract and retain key personnel and enter into corporate relationships that enable us and our collaborators to develop effective products that can be manufactured cost-effectively and marketed successfully.
Regulatory
General
Government authorities in the United States and other countries extensively regulate, among other things, the pre-clinical and clinical testing, manufacturing, labeling, storage, record-keeping, advertising, promotion, export, marketing and distribution of biologic products. In the United States, the FDA subjects pharmaceutical and biologic products to rigorous review under the Federal Food, Drug, and Cosmetic Act, the Public Health Service Act and other federal statutes and regulations.
FDA Approval Process
To obtain approval of our product candidates from the FDA, we must, among other requirements, submit data supporting safety and efficacy as well as detailed information on the manufacture and composition of the product candidate, otherwise known as an Investigational New Drug (“IND”) application. In most cases, this entails extensive laboratory tests and pre-clinical and clinical trials. The collection of these data, as well as the preparation of the IND applications for review by the FDA, are costly in time and effort, and may require significant capital investment. We may encounter significant difficulties or costs in our efforts to obtain
FDA approvals that could delay or preclude us from marketing any products we may develop. If the IND is accepted by the FDA, we would then start clinical trials to determine, among other things, the proper dosage, safety, and efficacy of the drug candidate. A company typically conducts human clinical trials in three sequential phases, but the phases may overlap. Phase I trials consist of testing the product in a small number of patients or healthy volunteers for safety at one or more doses. Phase I trials in cancer are often conducted with patients who are not healthy and who have end-stage or metastatic cancer. Phase II trials, in addition to safety, evaluate the efficacy of the product in a patient population somewhat larger than phase I trials. Phase III trials typically involve additional testing for safety and clinical efficacy in an expanded population at geographically dispersed test sites. Prior to commencement of each clinical trial, a company must submit to the FDA a clinical plan, or “protocol,” which must also be approved by the Institutional Review Boards (IRBs) at the institutions participating in the trials. The trials must be conducted in accordance with the FDA’s good clinical practices. The FDA may order the temporary or permanent discontinuation of a clinical trial at any time. Clinical trials can be conducted under a Special Protocol Assessment (SPA) which is an agreement between the sponsor and the FDA that the design, size, execution, and analysis of a clinical trial that is intended to form the primary basis for regulatory approval.
| 20 |
To obtain marketing authorization, a company must submit to the FDA the results of the pre-clinical and clinical testing, together with, and among other things, detailed information on the manufacture and composition of the product, in the form of a new drug application or, in the case of a biologic such as OncoVAX®, a BLA. The FDA may deny approval to a BLA if applicable regulatory criteria are not satisfied. Moreover, even if regulatory approval is granted, such approval may be subject to limitations on the indicated uses for which we may market our product. In addition, under a BLA, the manufacturer of the product continues to be subject to facility inspections.
We are also subject to a variety of regulations governing clinical trials and sales of our products outside the United States. Whether or not FDA approval has been obtained, approval of conduct of a clinical trial or authorization of a product by the comparable regulatory authorities of foreign countries and regions must be obtained prior to the commencement of marketing the product in those countries. The approval process varies from one regulatory authority to another and the time may be longer or shorter than that required for FDA approval. In the E.U., Canada and Australia, regulatory requirements and approval processes are similar, in principle, to those in the United States.
Fast Track Designation/Priority Review
Congress enacted the Food and Drug Administration Modernization Act of 1997 (the “Modernization Act”) in part to ensure the availability of safe and effective drugs, biologics, and medical devices by expediting the development and review for certain new products. The Modernization Act establishes a statutory program for the review of Fast Track products, including biologics. A Fast Track product is defined as a new drug or biologic intended for the treatment of a serious or life-threatening condition that demonstrates the potential to address unmet medical needs for this condition. Under the Fast Track program, the sponsor of a new drug or biologic may request that the FDA designate the drug or biologic as a Fast Track product at any time during the development of the product, prior to a new drug application submission.
Post-Marketing Obligations
The Food and Drug Administration Amendments Act of 2007 expanded FDA authority over drug products after approval. All approved drug products are subject to continuing regulation by the FDA, including record-keeping requirements, reporting of adverse experiences with the product, sampling and distribution requirements, notifying the FDA and gaining approval for certain manufacturing or labeling changes, complying with certain electronic records and signature requirements, submitting periodic reports to the FDA, maintaining and providing updated safety and efficacy information to the FDA, and complying with FDA promotion and advertising requirements. Failure to comply with the statutory and regulatory requirements can subject a manufacturer to possible legal or regulatory action, such as warning letters, suspension of manufacturing, seizure of product, injunctive action, criminal prosecution, or civil penalties.
The FDA may require post-marketing studies or clinical trials to develop additional information regarding the safety of a product. These studies or trials may involve continued testing of a product and development of data, including clinical data, about the product’s effects in various populations and any side-effects associated with long-term use. The FDA may require post-marketing studies or trials to investigate known serious risks or signals of serious risks or identify unexpected serious risks and may require periodic status reports if new safety information develops. Failure to conduct these studies in a timely manner may result in substantial civil fines.
| 21 |
Drug and biologics manufacturers and their subcontractors are required to register their establishments with the FDA and certain state agencies, and to list their products with the FDA. The FDA periodically inspects manufacturing facilities in the United States and abroad in order to assure compliance with the applicable cGMP regulations and other requirements. Facilities also are subject to inspections by other federal, foreign, state or local agencies. In complying with the cGMP regulations, manufacturers must continue to assure that the product meets applicable specifications, regulations and other post-marketing requirements. We must ensure that any third-party manufacturers continue to ensure full compliance with all applicable regulations and requirements. Failure to comply with these requirements subjects the manufacturer to possible legal or regulatory action, such as suspension of manufacturing or recall or seizure of product.
Also, newly discovered or developed safety or efficacy data may require changes to a product’s approved labeling, including the addition of new warnings and contraindications, additional pre-clinical or clinical studies, or even in some instances, revocation or withdrawal of the approval. Violations of regulatory requirements at any stage, including after approval, may result in various adverse consequences, including the FDA’s withdrawal of an approved product from the market, other voluntary or FDA-initiated action that could delay or restrict further marketing, and the imposition of civil fines and criminal penalties against the manufacturer and Biologics License Applications (“BLA”) holder. In addition, later discovery of previously unknown problems may result in restrictions on the product, manufacturer or BLA holder, including withdrawal of the product from the market. Furthermore, new government requirements may be established that could delay or prevent regulatory approval of our products under development, or affect the conditions under which approved products are marketed.
Federal Anti-Kickback, False Claims Laws & The Federal Physician Payment Sunshine Act
In addition to FDA restrictions on marketing of pharmaceutical products, several other types of state and federal laws are relevant to certain marketing practices in the pharmaceutical industry. These laws include anti-kickback statutes, false claims statutes, and the federal Physician Payment Sunshine Act. The federal anti-kickback statute prohibits, among other things, persons from knowingly and willfully soliciting, receiving, offering or paying remuneration, directly or indirectly, in exchange for or to induce either the referral of an individual for, or the purchase, lease, order or recommendation of, any good or service for which payment may be made under federal health care programs such as Medicare and Medicaid. For example, this statute has been interpreted to apply to arrangements between pharmaceutical manufacturers on the one hand and prescribers, purchasers and formulary managers on the other. Violations of the federal anti-kickback statute are punishable by imprisonment, criminal fines, civil monetary penalties and exclusion from participation in federal healthcare programs. There are a number of statutory exceptions and regulatory safe harbors protecting certain common activities from prosecution or other regulatory sanctions, however, the exceptions and safe harbors are drawn narrowly, and practices that do not fit squarely within an exception or safe harbor may be subject to scrutiny.
Federal false claims laws prohibit, among other things, any person from knowingly presenting, or causing to be presented, a false or fraudulent claim for payment, or knowingly making, or causing to be made, a false record or statement material to a false or fraudulent claim. For example, several pharmaceutical and other healthcare companies have faced enforcement actions under these laws for allegedly inflating drug prices they report to pricing services, which in turn were used by the government to set Medicare and Medicaid reimbursement rates, and for allegedly providing free product to customers with the expectation that the customers would bill federal programs for the product. In addition, anti-kickback statute violations and certain marketing practices, including off-label promotion, may also implicate false claims laws. Federal false claims laws violations may result in imprisonment, criminal fines, civil monetary damages and penalties and exclusion from participation in federal healthcare programs. The majority of states also have statutes or regulations similar to the federal anti-kickback law and false claims laws, which apply to items and services reimbursed under Medicaid and other state programs. A number of states have anti-kickback laws that apply regardless of the payor.
| 22 |
In addition, the federal Physician Payment Sunshine Act, as implemented in 2010 will require the reporting by drug manufacturers of “payments or transfers of value” made or distributed to physicians and teaching hospitals, with limited exceptions. Failure to comply with the reporting obligations may result in civil monetary penalties.
State Laws
Marketing Restrictions and Disclosure Requirements. A number of states, such as Minnesota, Massachusetts and Vermont, have requirements that restrict pharmaceutical marketing activities that go beyond commitments made related to adhering to the Pharmaceutical Research and Manufacturers of America Code for Interactions with Healthcare Professionals. These state requirements limit the types of interactions we may have with healthcare providers licensed in these jurisdictions. In addition, a number of states have laws that require pharmaceutical companies to track and report payments, gifts and other benefits provided to physicians and other health care professionals and entities. Still other state laws mandate implementation of specific compliance policies to regulate interactions with health care professionals.
Healthcare Reform. Certain states, such as Massachusetts, are pursuing their own programs for health reform. These programs may include cost containment measures that could affect state healthcare benefits, particularly for higher priced drugs. Under the Federal Patient Protection and Affordable Care Act, states will have the authority to define packages of “essential health benefits”, that health plans in the individual and small group markets must offer beginning in 2014. The definition of these packages could affect coverage of our products by those plans.
Sale of Pharmaceutical Products. In addition, in the United States, many states have enacted their own laws and statutes applicable to the sale of pharmaceutical products within the state, with which we must comply. We are also subject to certain state privacy and data protection laws and regulations.
Data Privacy
Numerous federal and state laws, including state security breach notification laws, state health information privacy laws and federal and state consumer protection laws, govern the collection, use and disclosure of personal information. Other countries also have, or are developing, laws governing the collection, use and transmission of personal information. In addition, most healthcare providers who prescribe our product and from whom we obtain patient health information are subject to privacy and security requirements under the Health Insurance Portability and Accountability Act of 1996 (“HIPAA”). We are not a HIPAA-covered entity and therefore, these privacy and security requirements do not apply to us. However, we could be subject to criminal penalties if we knowingly obtain individually identifiable health information from a covered entity in a manner that is not authorized or permitted by HIPAA or for aiding and abetting the violation of HIPAA. We are unable to predict whether our actions could be subject to prosecution in the event of an impermissible disclosure of health information to us. The legislative and regulatory landscape for privacy and data protection continues to evolve, and there has been an increasing amount of focus on privacy and data protection issues with the potential to affect our business, including recently enacted laws in a majority of states requiring security breach notification. These laws could create liability for us or increase our cost of doing business.
Commercial Product Pricing
In the United States and some foreign jurisdictions, many of the markets in which we may do business in the future, the prices of pharmaceutical products are subject to direct price controls (by law) and to drug reimbursement programs with varying price control mechanisms.
| 23 |
In the United States, the Medicare Prescription Drug, Improvement, and Modernization Act of 2003, or Medicare Modernization Act, changed the way Medicare covers and pays for pharmaceutical products. The legislation expanded Medicare coverage for drug purchases by the elderly and introduced a new reimbursement methodology based on average sales prices for physician administered drugs. In addition, this legislation provided authority for limiting the number of drugs that will be covered in any therapeutic class in certain cases. Cost reduction initiatives and other provisions of this and other more recent legislation could decrease the coverage and reimbursement that is provided for any approved products. While the Medicare Modernization Act applies only to drug benefits for Medicare beneficiaries, private payors often follow Medicare coverage policy and payment limitations in setting their own reimbursement rates. Therefore, any reduction in reimbursement that results from the Medicare Modernization Act or other more recent legislation may result in a similar reduction in payments from private payors.
In March 2010, President Obama signed into law the Health Care Reform Law, a sweeping law intended to broaden access to health insurance, reduce or constrain the growth of healthcare spending, enhance remedies against fraud and abuse, add new transparency requirements for health care and health insurance industries, impose new taxes and fees on the health industry and impose additional health policy reforms. Effective October 1, 2010, the Health Care Reform Law revises the definition of “average manufacturer price” for reporting purposes, which could increase the amount of Medicaid drug rebates to states. Further, the new law imposes a significant annual fee on companies that manufacture or import branded prescription drug products. Substantial new provisions affecting compliance have also been enacted, which may affect our business practices with health care practitioners. We will not know the full effects of the Health Care Reform Law until applicable federal and state agencies issue regulations or guidance under the new law. Although it is too early to determine the effect of the Health Care Reform Law, the new law appears likely to continue the pressure on pharmaceutical pricing, especially under the Medicare program, and may also increase our regulatory burdens and operating costs.
Legislative and regulatory proposals have been made to expand post-approval requirements and restrict sales and promotional activities for pharmaceutical products. We cannot be sure whether additional legislative changes will be enacted, or whether the FDA regulations, guidance or interpretations will be changed, or what the impact of such changes on the marketing approvals of our product candidates, if any, may be. In addition, increased scrutiny by the U.S. Congress of the FDA’s approval process may significantly delay or prevent marketing approval, as well as subject us to more stringent product labeling and post-marketing testing and other requirements.
With the enactment of the Biologics Price Competition and Innovation Act of 2009, or BPCIA, as part of the Health Care Reform Law, an abbreviated pathway for the approval of biosimilar and interchangeable biological products was created. The new abbreviated regulatory pathway establishes legal authority for the FDA to review and approve biosimilar biologics, including the possible designation of a biosimilar as “interchangeable” based on its similarity to an existing brand product. Under the BPCIA, an application for a biosimilar product cannot be submitted to the FDA until four years, or approved by the FDA until 12 years, after the original brand product identified as the reference product was approved under a BLA. The new law is complex and is only beginning to be interpreted and implemented by the FDA. As a result, its ultimate impact, implementation and meaning is subject to uncertainty. While it is uncertain when any such processes may be fully adopted by the FDA, any such processes could have a material adverse effect on the future commercial prospects for our biological products.
| 24 |
We believe that if any of our product candidates were to be approved as biological products under a BLA, such approved products should qualify for the four-year and 12-year periods of exclusivity. However, there is a risk that the U.S. Congress could amend the BPCIA to significantly shorten these exclusivity periods as proposed by President Obama, or that the FDA will not consider our product candidates to be reference products for competing products, potentially creating the opportunity for generic competition sooner than anticipated. Moreover, the extent to which a biosimilar, once approved, will be substituted for any one of our reference products in a way that is similar to traditional generic substitution for non-biological products is not yet clear, and will depend on a number of marketplace and regulatory factors that are still developing.
European Regulatory Authorities
In the European Union, governments influence the price of pharmaceutical products through their pricing and reimbursement rules and control of national healthcare systems that fund a large part of the cost of such products to consumers. The approach taken varies from member state to member state. Some jurisdictions operate positive and/or negative list systems under which products may be marketed only once a reimbursement price has been agreed. Other member states allow companies to fix their own prices for medicines, but monitor and control company profits. The downward pressure on healthcare costs in general, particularly prescription drugs, has become very intense. As a result, increasingly high barriers are being erected to the entry of new products, as exemplified by the role of the National Institute for Health and Clinical Excellence in the United Kingdom, which evaluates the data supporting new medicines and passes reimbursement recommendations to the government. In addition, in some countries cross-border imports from low-priced markets (parallel imports) exert commercial pressure on pricing within a country.
Environmental and Safety Laws
We are subject to a variety of federal, state and local regulations relating to the use, handling, storage and disposal of hazardous materials, including chemicals and radioactive and biological materials. Our operations produce such hazardous waste products. Although we believe that our safety procedures for handling and disposing of these materials comply with the standards prescribed by federal, state and local regulations, the risk of accidental contamination or injury from these materials cannot be eliminated. We generally contract with third parties for the disposal of such substances. We are also subject to various laws and regulations governing laboratory practices and the experimental use of animals.
We are also subject to regulation by the Occupational Safety and Health Administration (“OSHA”), and the Environmental Protection Agency (the “EPA”), and to regulation under the Toxic Substances Control Act, the Resource Conservation and Recovery Act and other regulatory statutes, and may in the future be subject to other federal, state or local regulations. OSHA and/or the EPA may promulgate regulations that may affect our research and development programs.
Taxes
We are current on all of our known tax filings. Prior to September 2013 we were delinquent in filing our U.S. tax returns. Our 2008 and 2009 tax returns were filed in August 2013, our 2010, 2011and 2012 tax returns were filed in September 2013, and our 2013 tax return was filed in September 2014. We do not owe any income taxes for any of those years as we have not shown any income in such years.
There is an issue with penalties regarding the U.S. information reporting Form 5471 for Switzerland, the Netherlands, and the United Kingdom. The Company failed to timely file, including extension, all applicable U.S. information reporting Forms 5471 for annual periods 2007 through 2011. The Company did timely file all applicable U.S. information reporting Forms 5471 for the 2012 annual period.
| 25 |
The IRS has been imposing penalties on a regular basis for the failure of U.S. taxpayers to file Form 5471. The penalty is $10,000 per entity for each year. A U.S. income tax return is not considered complete without the filing of the report.
The penalties are being appealed and to date $30,000 of penalties have been abated. In accordance with statutory filing requirements, the Company’s penalty accrual related to failure to file and late filings of Forms 5471 is $100,000 as of December 31, 2014.
Employees
As of April 10, 2015, we had 24 full time employees. None of our employees are subject to a collective bargaining agreement or represented by a labor or trade union, and we believe that our relations with our employees are good. In addition, we also have 8 technical and administrative consultants that we may hire as additional full time employees subject to improvement in our financial condition.
Item 1A. RISK FACTORS AND CAUTIONARY STATEMENT REGARDING FORWARD-LOOKING INFORMATION
The statements in this section describe the most significant risks to our business and should be considered carefully in conjunction with “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and the “Notes to Consolidated Financial Statements” to this Form 10-K. In addition, the statements in this section and other sections of this Form 10-K include “forward-looking statements” as that term is defined in the Private Securities Litigation Reform Act of 1995 and involve uncertainties that could significantly impact results. Forward-looking statements give current expectations or forecasts of future events about the Company or our outlook. You can identify forward-looking statements by the fact they do not relate to historical or current facts and by the use of words such as “believe,” “expect,” “estimate,” “anticipate,” “will be,” “should,” “plan,” “project,” “intend,” “could” and similar words or expressions.
Forward-looking statements are based on assumptions and on known risks and uncertainties. Although we believe we have been prudent in our assumptions, any or all of our forward-looking statements may prove to be inaccurate, and we can make no guarantees about our future performance. Should known or unknown risks or uncertainties materialize or underlying assumptions prove inaccurate, actual results could materially differ from past results and/or those anticipated, estimated or projected.
We undertake no obligation to publicly update forward-looking statements, whether as a result of new information, future events or otherwise. You should, however, consult any subsequent disclosures we make in our filings with the SEC on Form 10-Q or Form 8-K.
The following is a cautionary discussion of risks, uncertainties and assumptions that we believe are significant to our business. In addition to the factors discussed elsewhere in this report, the following are some of the important factors that, individually or in the aggregate, we believe could make our actual results differ materially from those described in any forward-looking statements. It is impossible to predict or identify all such factors and, as a result, you should not consider the following factors to be a complete discussion of risks, uncertainties and assumptions.
| 26 |
RISKS RELATED TO OUR BUSINESS
We are subject to credit risks relating to our cash and cash equivalents deposits which may exceed insured limits.
Financial instruments that potentially subject the Company to concentrations of credit risk consist primarily of cash and cash equivalents. The Company maintains its cash and cash equivalents with high-credit-quality financial institutions in the United States and the Netherlands.
Cash and cash equivalents in the United States are maintained at financial institutions and, at times, balances may exceed federally insured limits. All non-interest bearing cash balances were fully insured to $250,000 per depositor at each financial institution, and noninterest bearing cash balances may again exceed federally insured limits.
Cash and cash equivalents in The Netherlands are maintained at a financial institution and, at times, balances may exceed insured limits. Insurance coverage is limited to 100.000€ for all company accounts at each financial institution.
RISKS RELATED TO OUR FINANCIAL POSITION AND NEED FOR ADDITIONAL CAPITAL
We have incurred significant losses since our inception. We expect to incur losses for at least the next several years and may never achieve or maintain profitability.
Since inception, we have incurred significant operating losses. As a result of our operating losses and negative cash flows from operations, the report of our independent registered public accounting firm, BDO USA, LLP, on our December 31, 2014 and 2013 financial statements included an explanatory paragraph indicating that there is substantial doubt about our ability to continue as a going concern. To date, we have financed our operations primarily through private placements of our common stock. We have devoted substantially all of our efforts to research and development, including clinical trials. We have not completed development of any product candidates. We expect to continue to incur significant expenses and increasing operating losses for at least the next several years. We anticipate that our expenses will increase substantially if and as we:
| • | continue our ongoing phase III clinical trial of OncoVAX® for the treatment of stage II colon cancer; |
| • | seek regulatory approvals for OncoVAX® upon successful completion of its clinical trial; |
| • | lease, build out and equip a new commercial facility for the manufacturing of approved products; |
| • | establish a sales, marketing and distribution infrastructure to commercialize products for which we may obtain regulatory approval; |
| • | maintain, expand and protect our intellectual property portfolio; |
| • | continue our other research and development efforts; |
| • | hire additional clinical, quality control, scientific and management personnel; and |
| • | add operational, financial and management information systems and personnel, including personnel to support our product development and planned commercialization efforts. |
| 27 |
To become and remain profitable, we must develop and eventually commercialize a product or products with significant market potential. This development and commercialization will require us to be successful in a range of challenging activities, including successfully completing preclinical testing and clinical trials of our product candidates, obtaining regulatory approval for these product candidates, leasing, building out and equipping a new commercial manufacturing facility and manufacturing, marketing and selling those products for which we may obtain regulatory approval. We are only in the preliminary stages of some of these activities. We may never succeed in these activities and may never generate revenues that are significant or large enough to achieve profitability.
Even if we do achieve profitability, we may not be able to sustain or increase profitability on a quarterly or annual basis. Our failure to become and remain profitable would decrease the value of the Company and could impair our ability to raise capital, expand our business, maintain our research and development efforts or continue our operations. A decline in the value of our Company could also cause you to lose all or part of your investment.
We will need substantial additional funding and our current funding arrangements may not provide timely and adequate funding as needed. If we are unable to raise capital when needed, we would be forced to delay, reduce, terminate or eliminate our product development programs, including our phase III clinical trial of OncoVAX®, our plans to lease, build out and equip a new commercial manufacturing facility or our commercialization efforts.
We expect our expenses to increase in connection with our ongoing activities, particularly as we continue our phase III clinical trial of OncoVAX®, seek regulatory approval for our product candidates and lease, build out and equip a new commercial manufacturing facility. In addition, if we obtain regulatory approval of any of our product candidates, we expect to incur significant commercialization expenses for product sales, marketing, manufacturing and distribution. Furthermore, we expect to incur additional costs associated with operating as a public company. Accordingly, we will need to obtain substantial additional funding in connection with our continuing operations. If we are unable to raise capital when needed or on attractive terms, we would be forced to delay, reduce, terminate or eliminate our product development programs, our plans to lease, build out and equip a new commercial manufacturing facility or our commercialization efforts.
Our future capital requirements will depend on many factors, including:
| • | the progress and results of our ongoing pivotal phase III clinical trial of OncoVAX®; |
| • | the scope, progress, results and costs of preclinical development, laboratory testing and clinical trials for our other research and development activities; |
| • | the costs and timing of our planned leasing, build-out and equipping of a new commercial manufacturing facility; |
| • | the costs, timing and outcome of regulatory review of our product candidates; |
| • | the costs of commercialization activities, including product sales, marketing, manufacturing and distribution, for any of our product candidates for which we receive regulatory approval; |
| • | revenue, if any, received from commercial sales of our product candidates, should any of our product candidates be approved by the FDA or a similar regulatory authority outside the United States; |
| 28 |
| • | the costs of preparing, filing and prosecuting patent applications, maintaining and enforcing our intellectual property rights and defending intellectual property-related claims; |
| • | the extent to which we acquire or invest in other businesses, products and technologies; |
| • | our ability to obtain government or other third party funding for the development of our product candidates; and |
| • | our ability to establish collaborations on favorable terms, if at all, particularly arrangements to develop, market and distribute OncoVAX® outside North America. |
Conducting preclinical testing and clinical trials is a time-consuming, expensive and uncertain process that takes years to complete, and we may never generate the necessary data or results required to obtain regulatory approval and achieve product sales. In addition, our product candidates, if approved, may not achieve commercial success. Our commercial revenues, if any, will be derived from sales of products that we do not expect to be commercially available for several years, if at all. Accordingly, we will need to continue to rely on additional financing to achieve our business objectives. Additional financing may not be available to us on acceptable terms, or at all.
Raising additional capital may cause dilution to our existing stockholders, restrict our operations or require us to relinquish rights to our technologies or product candidates.
Until such time, if ever, as we can generate substantial product revenues, we expect to finance our cash needs through a combination of equity offerings, debt financings, and third party grants or other third party funding, marketing and distribution arrangements and other collaborations, strategic alliances and licensing arrangements. To the extent that we raise additional capital through the sale of equity or convertible debt securities, your ownership interest will be diluted, and the terms of these securities may include liquidation or other preferences that adversely affect your rights as a stockholder. Debt financing, if available, may involve agreements that include covenants limiting or restricting our ability to take specific actions, such as incurring additional debt, making capital expenditures or declaring dividends.
We currently intend to collaborate with third parties for the development and commercialization of OncoVAX® outside of North America. We plan to seek government or other third party funding for the continued development and to collaborate with third parties for the development and commercialization of other potential products. If we raise additional funds through government or other third party funding, marketing and distribution arrangements or other collaborations, strategic alliances or licensing arrangements with third parties, we may have to relinquish valuable rights to our technologies, future revenue streams, research programs or product candidates or to grant licenses on terms that may not be favorable to us.
If we do not raise and receive additional capital in a timely manner, we may not be able to complete the development, testing and commercialization of our treatment systems or continue as a going concern.
To complete the development and commercialization of our product and to continue as a going concern, we will need to raise substantial amounts of additional capital. On April 24, 2014 we entered into, and on August 22, 2014 we amended, a binding agreement (the “TIS Agreement”) with a Stockholm-based investor group known as The Investment Syndicate (“TIS”), pursuant to which TIS agreed to purchase up to $80,000,000 of our common stock and warrants, all in up to five closings, subject to certain terms and conditions. On August 22, 2014, we consummated the first closing under the TIS Agreement under which we issued and sold $10,000,001 of our common stock and warrants and the second closing was consummated in two equal and separate transactions on December 26, 2014 and January 26, 2015 for which we issued and sold $10,000,001 of our common stock and warrants. The remaining $60,000,000 is expected to be paid in three separate closings upon the completion of certain milestones.
| 29 |
The Company has received repeated assurances from a TIS representative that TIS fully intends to meet its obligations under the TIS Agreement. Specifically, on November 11, 2014, the Company received a letter from TIS indicating a desire on the part of TIS to invest the remaining $60,000,000 shortly after consummation of the second closing (with the final $20,000,000 to be held in escrow pending fulfillment of the milestone contained in the TIS Agreement relating to the first patient receiving the first three doses of OncoVax®, with positive results). Since receipt of that letter, an additional $10,000,000 investment has been consummated by a TIS member, bringing the total TIS investment to $20 million, without any further investments from TIS members. In more recent communications from TIS, a TIS representative acknowledged that the Company has met the conditions for funding of at least an additional $20,000,000 and re-confirmed that TIS fully intends to meet its obligations under the TIS Agreement to fund the remaining TIS committed investment notwithstanding any conditions to closing. However, the TIS representative has informed the Company that the timing of TIS funding continues to be dependent upon funds TIS members expect to receive from a third party relating to investments made and services provided by TIS members. Notwithstanding these verbal and written assurances from TIS, the Company has been unable to verify the validity of the TIS assurances, the availability of the funds TIS members anticipate using to satisfy their obligations, the timing of the receipt of the funds, or the reliability of the third party from which TIS members expect to receive funds. As of the date hereof, the TIS members' option to invest an additional $30,000,000 (on top of the $80,000,000 committed investment) has expired
Other than the TIS Agreement, the Company does not have any committed sources of financing and we cannot offer any assurances that additional funding will be available in a timely manner, on acceptable terms or at all. In the event we cannot raise sufficient capital (including, without limitation, in the event TIS fails to meet its obligations under the TIS Agreement), we may be required to delay, scale back or eliminate certain aspects of our operations or attempt to obtain funds through unfavorable arrangements with partners or others that may force us to relinquish rights to certain of our technologies, products or potential markets or that could impose onerous financial or other terms. In addition, if we do not receive the funds from TIS, we may be unable to pay our existing debts or any other obligations as they come due. Furthermore, if we cannot fund our ongoing development and other operating requirements we may no longer be able to continue as a going concern.
As of December 31, 2014 the Company had cash and cash equivalents of approximately $3.0 million, and with a $5.0 million investment through TIS in January, was maintaining approximately $3.0 million in cash and cash equivalents as of April 6, 2015.
RISKS IN PROTECTING OUR INTELLECTUAL PROPERTY
If we are unable to obtain and maintain patent protection for our technology and products, or if the scope of the patent protection obtained is not sufficiently broad, our competitors could develop and commercialize technology and products similar or identical to ours, and our ability to successfully commercialize our technology and products may be adversely affected.
| 30 |
We have sought to protect our proprietary position by filing patent applications in the United States and abroad related to our novel technologies and products that are important to our business. This process is expensive and time-consuming, and we may not be able to file and prosecute all necessary or desirable patent applications at a reasonable cost or in a timely manner. It is also possible that we will fail to identify patentable aspects of our research and development efforts before it is too late to obtain patent protection.
The patent position of biotechnology and pharmaceutical companies generally is highly uncertain, involves complex legal and factual questions and has in recent years been the subject of much litigation. As a result, the issuance, scope, validity, enforceability and commercial value of our patent rights are highly uncertain. Our pending and future patent applications may not result in patents being issued which protect our technology or products or which effectively prevent others from commercializing competitive technologies and products. Changes in either the patent laws or interpretation of the patent laws in the United States and other countries may diminish the value of our patents or narrow the scope of our patent protection.
Third parties could practice our inventions in territories where we do not have patent protection. Furthermore, the laws of foreign countries may not protect our rights to the same extent as the laws of the United States. In foreign countries, however, importation of a product made by a process patented in that country may not constitute an infringing activity, which would limit our ability to enforce our process patents against importers in that country. Furthermore, the legal systems of certain countries, particularly certain developing countries, do not favor the enforcement of patents and other intellectual property protection. This could make it difficult for us to stop the infringement of our patents or the misappropriation of our other intellectual property rights. If competitors are able to use our technologies, our ability to compete effectively or profitably could be harmed.
Assuming the other requirements for patentability are met, in the United States, the first to invent the claimed invention was previously entitled to the patent, while outside the United States, the first to file a patent application is generally entitled to the patent. Under the America Invents Act, or AIA, enacted in September 2011, the United States moved to a first inventor to file system in March 2013. The United States Patent and Trademark Office only recently finalized the rules relating to these changes and courts have yet to address the new provisions. These changes could increase the costs and uncertainties surrounding the prosecution of our patent applications and the enforcement or defense of our patent rights. Furthermore, we may become involved in interference proceedings, opposition proceedings, or other post-grant proceedings, such as reexamination or inter partes review proceedings, challenging our patent rights or the patent rights of others. An adverse determination in any such proceeding or litigation could reduce the scope of, or invalidate, our patent rights, allow third parties to commercialize our technology or products and compete directly with us, without payment to us, or result in our inability to manufacture or commercialize products without infringing third party patent rights.
| 31 |
Even if our owned and licensed patent applications issue as patents, they may not issue in a form that will provide us with any meaningful protection, prevent competitors from competing with us or otherwise provide us with any competitive advantage. Our competitors may be able to circumvent our owned or licensed patents by developing similar or alternative technologies or products in a non-infringing manner. The issuance of a patent is not conclusive as to its scope, validity or enforceability, and our owned and licensed patents may be challenged in the courts or patent offices in the United States and abroad. Such challenges may result in patent claims being narrowed, invalidated or held unenforceable, which could limit our ability to or stop or prevent us from stopping others from using or commercializing similar or identical technology and products, or limit the duration of the patent protection of our technology and products. Given the amount of time required for the development, testing and regulatory review of new product candidates, patents protecting such candidates might expire before or shortly after such candidates are commercialized. Our owned and licensed patent portfolio may not provide us with sufficient rights to exclude others from commercializing products similar or identical to ours.
In addition to our OncoVAX® patent, which expires in 2025, we have a patent covering the methodology for making tumor-specific human monoclonal antibodies from the B-cells of OncoVAX® immunized patients and describe the relevant epitopes (tumor-associated antigens) which with the antibodies react. This patent was issued in 1999 and expires in 2015. Patents for individual monoclonal antibodies require molecular identification of the target (epitope) and reactive (variable) region of the antibody. Consequently, these individual patents have been made for existing antibodies (legacy antibodies) and will be made for new antibodies discovered in our Human Monoclonal Antibody technology platform. We do not expect the loss of this patent related to legacy antibodies to affect the future of the technology platform.
We may become involved in lawsuits to protect or enforce our patents, which could be expensive, time consuming and unsuccessful.
Competitors may infringe our patents. To counter such infringement or unauthorized use, we may be required to file infringement claims against third parties, which can be expensive and time consuming. In addition, during an infringement proceeding, a court may decide that the patent rights we are asserting are invalid or unenforceable, or may refuse to stop the other party from using the technology at issue on the grounds that our patents do not cover the technology in question. An adverse result in any litigation proceeding could put one or more of our patents at risk of being invalidated or interpreted narrowly. Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of our confidential information could be compromised by disclosure during this type of litigation. In addition, our licensors may have rights to file and prosecute such claims and we are reliant on them.
Third parties may initiate legal proceedings alleging that we are infringing their intellectual property rights, the outcome of which would be uncertain and could have a material adverse effect on the success of our business.
Our commercial success depends upon our ability to develop, manufacture, market and sell our product candidates and use our proprietary technologies without infringing the proprietary rights of third parties. We cannot ensure that third parties do not have, or will not in the future obtain, intellectual property rights such as granted patents that could block our ability to operate as we would like. There may be patents in the United States or abroad owned by third parties that, if valid, may block our ability to make, use or sell our products in the United States or certain countries outside the United States, or block our ability to import our products into the United States or into certain countries outside the United States.
| 32 |
We may become party to, or threatened with, future adversarial proceedings or litigation regarding intellectual property rights with respect to our products and technology. For example, third parties may assert infringement claims against us based on existing patents or patents that may be granted in the future. If we are found to infringe a third party’s intellectual property rights, we could be required to obtain a license from such third party to continue developing and marketing our products and technology. However, we may be unable to obtain any required license on commercially reasonable terms or even obtain a license at all. Even if we were able to obtain a license, it could be non-exclusive, thereby giving our competitors access to the same technologies licensed to us. We could be forced, including by court order, to cease commercializing the infringing technology or product. In addition, we could be found liable for monetary damages. A finding of infringement could prevent us from commercializing our product candidates or force us to cease some of our business operations, which could materially harm our business. Claims that we have misappropriated the confidential information or trade secrets of third parties could have a similar negative impact on our business.
We may be subject to claims that our employees have wrongfully used or disclosed alleged trade secrets of their former employers.
Many of our employees were previously employed at universities or other biotechnology or pharmaceutical companies, including our competitors or potential competitors. Although we try to ensure that our employees do not use the proprietary information or know-how of others in their work for us, we may be subject to claims that we or these employees have used or disclosed intellectual property, including trade secrets or other proprietary information, of any such employee’s former employer. Litigation may be necessary to defend against these claims. If we fail in defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights or personnel. Even if we are successful in defending against such claims, litigation could result in substantial costs and be a distraction to management.
Intellectual property litigation could cause us to spend substantial resources and distract our personnel from their normal responsibilities.
Even if resolved in our favor, litigation or other legal proceedings relating to intellectual property claims may cause us to incur significant expenses, and could distract our technical and management personnel from other responsibilities. In addition, there could be public announcements of the results of hearings, motions or other interim proceedings or developments and if securities analysts or investors perceive these results to be negative, it could have a substantial adverse effect on the price of our common stock. Such litigation or proceedings could substantially increase our operating losses and reduce the resources available for development activities. We may not have sufficient financial or other resources to adequately conduct such litigation or proceedings. Some of our competitors may be able to sustain the costs of such litigation or proceedings more effectively than we can because of their greater financial resources. Uncertainties resulting from the initiation and continuation of patent litigation or other proceedings could have a material adverse effect on our ability to compete in the marketplace.
| 33 |
If we are unable to protect the confidentiality of our trade secrets, our business and competitive position would be harmed.
In addition to seeking patents for some of our technology and products, we also rely on trade secrets, including unpatented know-how, technology and other proprietary information, to maintain our competitive position. The types of protections available for trade secrets are particularly important with respect to our OncoVAX® platform’s manufacturing capabilities, which involve significant unpatented know-how. We seek to protect these trade secrets, in part, by entering into non-disclosure and confidentiality agreements with parties who have access to them, such as our officers, employees, corporate collaborators, outside scientific collaborators, sponsored researchers, contract manufacturers, consultants, advisors and other third parties. We also enter into confidentiality and invention or patent assignment agreements with our employees and consultants. Despite these efforts, any of these parties may breach the agreements and disclose our proprietary information, including our trade secrets, and we may not be able to obtain adequate remedies for such breaches. Enforcing a claim that a party illegally disclosed or misappropriated a trade secret is difficult, expensive and time-consuming, and the outcome is unpredictable. In addition, some courts inside and outside the United States are less willing or unwilling to protect trade secrets. If any of our trade secrets were to be lawfully obtained or independently developed by a competitor, we would have no right to prevent them from using that technology or information to compete with us. If any of our trade secrets were to be disclosed to or independently developed by a competitor, our competitive position would be harmed.
If an event of default is declared under agreements with Organon Teknika, including the security agreement, we could lose possession of the OncoVAX® intellectual property.
In connection with certain prior agreements between Intracel Holdings Corporation and Organon Teknika (now Merck), Intracel entered into a security agreement granting Organon Teknika a first priority security interest in all of intellectual property and patents related to OncoVAX®. The OncoVAX® patent secures payments due Organon under that certain October 31, 2007 letter agreement with Intracel, including the remaining $3.5 million in settlement payments due Organon thereunder, (plus any accrued interest from the date of the October 31, 2007 letter agreement based on the prime lending rate in effect on the anniversary of such letter agreement), of which $500,000 was past due and as of November 1, 2014 was written off as the payment had passed the statute of limitations. The remaining $3 million is due in equal annual payments of $1 million commencing the first year after the first marketing approval of OncoVAX® by the FDA or EMA (the “Settled Amount”) and a royalty of 10% of the net sales of OncoVAX® until the $3.0 million (and accrued interest) settlement payment is paid. We assumed these liabilities in connection with our October 24, 2010 Asset Transfer Agreement with Organon. Under this letter agreement, failure to make a payment with respect to the Settled Amount when due is an event of default, which if unremediated for a period of 45 days after written notice of such default has been received will be a default under the New Security Agreement under which our intellectual property was pledged as collateral. Should we lose our OncoVAX® patent to Organon, we would be required to seek a license from Organon or cease using certain technologies related to OncoVAX® or delay commercialization of OncoVAX®, and we could be required to pay substantial damages if it is found we infringed on the patents assigned to Organon, which could materially harm our business.
| 34 |
RISKS RELATED TO LEGAL AND COMPLIANCE MATTERS
Any product candidate for which we obtain marketing approval could be subject to restrictions or withdrawal from the market and we may be subject to penalties if we fail to comply with regulatory requirements or if we experience unanticipated problems with our products, when and if any of them are approved.
Any product candidate for which we obtain marketing approval, along with the manufacturing processes, post-approval clinical data, labeling, advertising and promotional activities for such product, will be subject to continual requirements of and review by the FDA and other regulatory authorities. These requirements include submissions of safety and other post-marketing information and reports, registration and listing requirements, cGMP requirements relating to quality control, quality assurance and corresponding maintenance of records and documents. Even if regulatory approval of a product candidate is granted, the approval may be subject to limitations on the indicated uses for which the product may be marketed or to the conditions of approval, or contain requirements for costly post-marketing testing and surveillance to monitor the safety or efficacy of the product. The FDA closely regulates the post-approval marketing and promotion of drugs to ensure drugs are marketed only for the approved indications and in accordance with the provisions of the approved label. The FDA imposes stringent restrictions on manufacturers’ communications regarding off-label use and if we do not market our products for their approved indications, we may be subject to enforcement action for off-label marketing.
In addition, later discovery of previously unknown problems with our products, manufacturers or manufacturing processes, or failure to comply with regulatory requirements, may yield various results, including:
• restrictions on such products, manufacturers or manufacturing processes;
• restrictions on the marketing of a product;
• restrictions on product distribution;
• requirements to conduct post-marketing clinical trials;
• warning or untitled letters;
• withdrawal of the products from the market;
• refusal to approve pending applications or supplements to approved applications that we submit;
• recall of products;
• fines, restitution or disgorgement of profits or revenue;
• suspension or withdrawal of regulatory approvals;
• refusal to permit the import or export of our products;
• product seizure; or
• injunctions or the imposition of civil or criminal penalties.
Our relationships with customers and third party payors will be subject to applicable anti-kickback, fraud and abuse and other healthcare laws and regulations, which could expose us to criminal sanctions, civil penalties, program exclusion, contractual damages, reputational harm and diminished profits and future earnings.
| 35 |
Healthcare providers, physicians and third party payors play a primary role in the recommendation and prescription of any product candidates for which we obtain marketing approval. Our future arrangements with third party payors and customers may expose us to broadly applicable fraud and abuse and other healthcare laws and regulations that may constrain the business or financial arrangements and relationships through which we market, sell and distribute our products for which we obtain marketing approval. Restrictions under applicable federal and state healthcare laws and regulations, include the following:
| • | the federal healthcare anti-kickback statute prohibits, among other things, persons from knowingly and willfully soliciting, offering, receiving or providing remuneration, directly or indirectly, in cash or in kind, to induce or reward either the referral of an individual for, or the purchase, order or recommendation of, any good or service, for which payment may be made under federal and state healthcare programs such as Medicare and Medicaid; |
| • | the federal False Claims Act imposes civil penalties, including civil whistleblower or qui tam actions, against individuals or entities for knowingly presenting, or causing to be presented, to the federal government, claims for payment that are false or fraudulent or making a false statement to avoid, decrease or conceal an obligation to pay money to the federal government; |
| • | the federal Health Insurance Portability and Accountability Act of 1996, as amended by the Health Information Technology for Economic and Clinical Health Act, imposes criminal and civil liability for executing a scheme to defraud any healthcare benefit program and also imposes obligations, including mandatory contractual terms, with respect to safeguarding the privacy, security and transmission of individually identifiable health information; |
| • | the federal false statements statute prohibits knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false statement in connection with the delivery of or payment for healthcare benefits, items or services; |
| • | the federal transparency requirements under the Health Care Reform Law will require manufacturers of drugs, devices, biologics and medical supplies to report to the Department of Health and Human Services information related to physician payments and other transfers of value and physician ownership and investment interests; and |
| • | analogous state laws and regulations, such as state anti-kickback and false claims laws, may apply to sales or marketing arrangements and claims involving healthcare items or services reimbursed by non-governmental third party payors, including private insurers, and some state laws require pharmaceutical companies to comply with the pharmaceutical industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government in addition to requiring drug manufacturers to report information related to payments to physicians and other health care providers or marketing expenditures. |
Some state laws require pharmaceutical companies to comply with the pharmaceutical industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government and may require drug manufacturers to report information related to payments and other transfers of value to physicians and other healthcare providers or marketing expenditures. State and foreign laws also govern the privacy and security of health information in some circumstances, many of which differ from each other in significant ways and often are not preempted by the federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, thus complicating compliance efforts.
Efforts to ensure that our business arrangements with third parties will comply with applicable healthcare laws and regulations will involve substantial costs. It is possible that governmental authorities will conclude that our business practices may not comply with current or future statutes, regulations or case law involving applicable fraud and abuse or other healthcare laws and regulations. If our operations are found to be in violation of any of these laws or any other governmental regulations that may apply to us, we may be subject to significant civil, criminal and administrative penalties, damages, fines, exclusion from government funded healthcare programs, such as Medicare and Medicaid, and the curtailment or restructuring of our operations. If any of the physicians or other providers or entities with whom we expect to do business are found to be not in compliance with applicable laws, they may be subject to criminal, civil or administrative sanctions, including exclusions from government funded healthcare programs.
| 36 |
Recently enacted and future legislation may increase the difficulty and cost for us to obtain marketing approval of and commercialize our product candidates and affect the prices we may obtain.
In the United States and some foreign jurisdictions, there have been a number of legislative and regulatory changes and proposed changes regarding the healthcare system that could prevent or delay marketing approval of our product candidates, restrict or regulate post-approval activities and affect our ability to profitably sell any product candidates for which we obtain marketing approval.
In the United States, the Medicare Prescription Drug, Improvement, and Modernization Act of 2003, or Medicare Modernization Act, changed the way Medicare covers and pays for pharmaceutical products. The legislation expanded Medicare coverage for drug purchases by the elderly and introduced a new reimbursement methodology based on average sales prices for physician administered drugs. In addition, this legislation provided authority for limiting the number of drugs that will be covered in any therapeutic class in certain cases. Cost reduction initiatives and other provisions of this and other more recent legislation could decrease the coverage and reimbursement that is provided for any approved products. While the Medicare Modernization Act applies only to drug benefits for Medicare beneficiaries, private payors often follow Medicare coverage policy and payment limitations in setting their own reimbursement rates. Therefore, any reduction in reimbursement that results from the Medicare Modernization Act or other more recent legislation may result in a similar reduction in payments from private payors.
In March 2010, President Obama signed into law the Health Care Reform Law, a sweeping law intended to broaden access to health insurance, reduce or constrain the growth of healthcare spending, enhance remedies against fraud and abuse, add new transparency requirements for health care and health insurance industries, impose new taxes and fees on the health industry and impose additional health policy reforms. Effective October 1, 2010, the Health Care Reform Law revises the definition of “average manufacturer price” for reporting purposes, which could increase the amount of Medicaid drug rebates to states. Further, the new law imposes a significant annual fee on companies that manufacture or import branded prescription drug products. Substantial new provisions affecting compliance have also been enacted, which may affect our business practices with health care practitioners. We will not know the full effects of the Health Care Reform Law until applicable federal and state agencies issue regulations or guidance under the new law. Although it is too early to determine the effect of the Health Care Reform Law, the new law appears likely to continue the pressure on pharmaceutical pricing, especially under the Medicare program, and may also increase our regulatory burdens and operating costs.
| 37 |
Legislative and regulatory proposals have been made to expand post-approval requirements and restrict sales and promotional activities for pharmaceutical products. We cannot be sure whether additional legislative changes will be enacted, or whether the FDA regulations, guidance or interpretations will be changed, or what the impact of such changes on the marketing approvals of our product candidates, if any, may be. In addition, increased scrutiny by the U.S. Congress of the FDA’s approval process may significantly delay or prevent marketing approval, as well as subject us to more stringent product labeling and post-marketing testing and other requirements.
With the enactment of the Biologics Price Competition and Innovation Act of 2009, or BPCIA, as part of the Health Care Reform Law, an abbreviated pathway for the approval of biosimilar and interchangeable biological products was created. The new abbreviated regulatory pathway establishes legal authority for the FDA to review and approve biosimilar biologics, including the possible designation of a biosimilar as “interchangeable” based on its similarity to an existing brand product. Under the BPCIA, an application for a biosimilar product cannot be submitted to the FDA until four years, or approved by the FDA until 12 years, after the original brand product identified as the reference product was approved under a BLA. The new law is complex and is only beginning to be interpreted and implemented by the FDA. As a result, its ultimate impact, implementation and meaning is subject to uncertainty. While it is uncertain when any such processes may be fully adopted by the FDA, any such processes could have a material adverse effect on the future commercial prospects for our biological products.
We believe that if any of our product candidates were to be approved as biological products under a BLA, such approved products should qualify for the four-year and 12-year periods of exclusivity. However, there is a risk that the U.S. Congress could amend the BPCIA to significantly shorten these exclusivity periods as proposed by President Obama, or that the FDA will not consider our product candidates to be reference products for competing products, potentially creating the opportunity for generic competition sooner than anticipated. Moreover, the extent to which a biosimilar, once approved, will be substituted for any one of our reference products in a way that is similar to traditional generic substitution for non-biological products is not yet clear, and will depend on a number of marketplace and regulatory factors that are still developing.
If we fail to comply with environmental, health and safety laws and regulations, we could become subject to fines or penalties or incur costs that could have a material adverse effect on the success of our business.
We are subject to numerous environmental, health and safety laws and regulations, including those governing laboratory procedures and the handling, use, storage, treatment and disposal of hazardous materials and wastes. Our operations involve the use of hazardous and flammable materials, including chemicals and radioactive and biological materials. Our operations also produce hazardous waste products. We generally contract with third parties for the disposal of these materials and wastes. We cannot eliminate the risk of contamination or injury from these materials. In the event of contamination or injury resulting from our use of hazardous materials, we could be held liable for any resulting damages, and any liability could exceed our resources. We also could incur significant costs associated with civil or criminal fines and penalties.
| 38 |
Although we maintain workers’ compensation insurance to cover us for costs and expenses we may incur due to injuries to our employees resulting from the use of hazardous materials, this insurance may not provide adequate coverage against potential liabilities. We do not maintain insurance for environmental liability or toxic tort claims that may be asserted against us in connection with our storage or disposal of biological, hazardous or radioactive materials.
In addition, we may incur substantial costs in order to comply with current or future environmental, health and safety laws and regulations. These current or future laws and regulations may impair our research, development or production efforts. Failure to comply with these laws and regulations also may result in substantial fines, penalties or other sanctions.
RISKS RELATED TO THE DEVELOPMENT AND REGULATORY APPROVAL OF OUR PRODUCT CANDIDATE(S)
If we experience any of a number of possible unforeseen events in connection with our clinical trials, potential marketing or commercialization of our product candidates could be delayed or prevented.
We may experience numerous unforeseen events during, or as a result of, clinical trials that could delay or prevent our ability to receive regulatory approval or commercialize our product candidates. Unforeseen events that could delay or prevent our ability to receive regulatory approval or commercialize our product candidates include:
| • | regulators or institutional review boards may not authorize us or our investigators to commence a clinical trial or conduct a clinical trial at a prospective trial site; |
| • | we may have delays in reaching or fail to reach agreement on acceptable clinical trial contracts or clinical trial protocols with prospective trial sites; |
| • | clinical trials of our product candidates may produce negative or inconclusive results, and we may decide, or regulators may require us, to conduct additional clinical trials or abandon product development programs; |
| • | the number of patients required for clinical trials of our product candidates may be larger than we anticipate; |
| • | enrollment in these clinical trials may be slower than we anticipate or participants may drop out of these clinical trials at a higher rate than we anticipate; |
| • | our third party contractors may fail to comply with regulatory requirements or meet their contractual obligations to us in a timely manner, or at all; |
| • | we may decide, or regulators or institutional review boards may require us or our investigators to, suspend or terminate clinical research for various reasons, including noncompliance with regulatory requirements or a finding that the participants are being exposed to unacceptable health risks; |
| • | the cost of clinical trials of our product candidates may be greater than we anticipate; and |
| • | the supply or quality of our product candidates or other materials necessary to conduct clinical trials of our product candidates may be insufficient or inadequate. |
| • | Our product development costs will also increase if we experience delays in testing or obtaining marketing approvals. We do not know whether clinical trials will begin as planned, will need to be restructured or will be completed on schedule, or at all. |
| 39 |
The FDA has reviewed the protocol for our OncoVAX® clinical trial under the SPA process. However, agreement by the FDA with the protocol under the SPA process does not guarantee that the trial will be successful or that, if successful, OncoVAX® will receive marketing approval.
The SPA process is designed to facilitate the FDA’s review and approval of drug and biological products by allowing the FDA to evaluate the proposed design and size of phase III clinical trials that are intended to form the primary basis for determining a drug candidate’s efficacy. In June 2010, we received a letter from the FDA advising us that the FDA had completed its review of our protocol for the pivotal phase III clinical trial under the SPA process. In the letter, the FDA stated that it had determined that the protocol sufficiently addressed the trial’s objectives and that the trial was adequately designed to provide the necessary data to support a submission for marketing approval.
An SPA does not guarantee that OncoVAX® will receive marketing approval. The FDA may raise issues related to safety, study conduct, bias, deviation from the protocol, statistical power, patient completion rates, changes in scientific or medical parameters or internal inconsistencies in the data prior to making its final decision. The FDA may also seek the guidance of an outside advisory committee prior to making its final decision. In addition,
OncoVAX® may not achieve the primary endpoint of the trial. Even if the primary endpoint in our pivotal phase III clinical trial is achieved, OncoVAX® may not be approved. Many companies which have been granted SPAs have ultimately failed to obtain final approval to market their products.
In its June 2010 letter, the FDA informed us that in order for a single trial to support approval of an indication, the trial must be well conducted, and the results of the trial must be internally consistent, clinically meaningful and statistically very persuasive. If the results for the primary endpoint are not robust, are subject to confounding factors, or are not adequately supported by other study endpoints, the FDA may refuse to approve our BLA based upon a single clinical trial. There can be no guarantee that the FDA will not require additional pivotal clinical trials as a condition for approving OncoVAX®.
If we experience delays or difficulties in the enrollment of patients in our clinical trials, our receipt of necessary regulatory approvals could be delayed or prevented.
We may not be able to initiate or continue the clinical trial OncoVAX® if we are unable to locate and enroll a sufficient number of eligible patients to participate in these trials as required by the FDA or similar regulatory authorities outside the United States. Our competitors may have ongoing clinical trials for product candidates that could be competitive with our product candidate, and patients who would otherwise be eligible for our clinical trials may instead enroll in clinical trials of our competitors’ product candidates. Patient enrollment is affected by other factors including:
| • | stage of the disease under investigation; |
| • | eligibility criteria for the study in question; |
| • | perceived risks and benefits of the product candidate under study; |
| • | efforts to facilitate timely enrollment in clinical trials; |
| • | patient referral practices of physicians; |
| • | the ability to monitor patients adequately during and after treatment; and |
| • | proximity and availability of clinical trial sites for prospective patients. |
| 40 |
| • | design of the trial protocol; |
| • | the size of the patient population; |
| • | eligibility criteria for the study in question; |
| • | perceived risks and benefits of the product candidate under study; |
| • | availability of competing therapies and clinical trials; |
| • | efforts to facilitate timely enrollment in clinical trials; |
| • | patient referral practices of physicians; |
| • | the ability to monitor patients adequately during and after treatment; and |
| • | geographic proximity and availability of clinical trial sites for prospective patients. |
| • | safety and efficacy results from human clinical trials may show the product candidate to be less effective or safe than desired or earlier results may not be replicated in later clinical trials; |
| • | the results of pre-clinical studies may be inconclusive or they may not be indicative of results that will be obtained in human clinical trials; |
| • | after reviewing relevant information, including pre-clinical testing or human clinical trial results, we may abandon or substantially restructure programs that we might previously have believed to be promising; |
| • | we, the FDA, an IRB, an EC, or similar regulatory authorities in other countries may suspend or terminate clinical trials if the participating patients are being exposed to unacceptable health risks or for other reasons; and |
| • | the effects of our product candidates may not be the desired effects or may include undesirable side effects or other characteristics that interrupt, delay or cause us or the FDA, or equivalent governmental authorities in other countries, to halt clinical trials or cause the FDA or non-United States regulatory authorities to deny approval of the product candidate for any or all target indications. |
The actual amount of time for full enrollment could be longer than planned. Enrollment delays in this phase III clinical trial may result in increased development costs for our product candidates, which would cause the value of the company to decline and limit our ability to obtain additional financing. Our inability to enroll a sufficient number of patients for this ongoing phase III clinical trial or any of our other clinical trials would result in significant delays or may require us to abandon one or more clinical trials altogether. In addition, any significant delays or increases in costs of our ongoing phase 3 clinical trial of AGS-003 could result in the need for us to obtain additional funding to complete the trial.
Our OncoVAX® product candidate and our immunotherapies are based on a novel technology utilizing a patient’s own tissue. This may raise development issues that we may not be able to resolve, regulatory issues that could delay or prevent approval or personnel issues that may prevent us from further developing and commercializing our product candidates.
Regulatory approval of novel product candidates such as OncoVAX® product candidate manufactured using novel manufacturing processes such as ours can be more expensive and take longer than for other, more well-known or extensively studied pharmaceutical or biopharmaceutical products, due to our and regulatory agencies’ lack of experience with them. The FDA has only approved one personalized immunotherapy product to date. This lack of experience may lengthen the regulatory review process, require us to conduct additional studies or clinical trials, increase our development costs, lead to changes in regulatory positions and interpretations, delay or prevent approval and commercialization of these product candidates or lead to significant post-approval limitations or restrictions.
| 41 |
The novel nature of our product candidates also means that fewer people are trained in or experienced with product candidates of this type, which may make it difficult to find, hire and retain capable personnel for research, development and manufacturing positions.
A fast track designation by the FDA may not actually lead to a faster development, regulatory review or approval process.
If a product is intended for the treatment of a serious or life-threatening condition and the product demonstrates the potential to address unmet needs for this condition, the treatment sponsor may apply for FDA fast track designation. In September 2006, the FDA notified us that we obtained fast track designation for OncoVAX® for the treatment of stage II colon cancer. Fast track designation does not ensure that we will experience a faster development, regulatory review or approval process compared to conventional FDA procedures. Additionally, the FDA may withdraw fast track designation if it believes that the designation is no longer supported by data from our clinical development program.
The FDA, other non-United States regulatory authorities, or an Advisory Committee may determine our clinical trials data regarding safety or efficacy are insufficient for regulatory approval.
Although we obtain guidance from regulatory authorities on certain aspects of our clinical development activities, these discussions are not binding obligations on regulatory authorities. Regulatory authorities may revise or retract previous guidance or may disqualify a clinical trial in whole or in part from consideration in support of approval of a potential product for commercial sale or otherwise deny approval of that product. Even if we obtain successful clinical safety and efficacy data, we may be required to conduct additional, expensive trials to obtain regulatory approval. FDA, or equivalent other country authorities, may elect to obtain advice from outside experts regarding scientific issues and/or marketing applications under FDA or other country authority review through the FDA’s Advisory Committee process or other country procedures. Views of the Advisory Committee or other experts may differ from those of the FDA, or equivalent other country authority, and may impact our ability to commercialize a product candidate.
Our OncoVAX® product candidates are immunotherapies that are based on a novel technology utilizing a patient’s own tissue. This may raise development issues that we may not be able to resolve, regulatory issues that could delay or prevent approval or personnel issues that may prevent us from further developing and commercializing our product candidates.
Regulatory approval of novel product candidates such as our OncoVAX® product candidates manufactured using novel manufacturing processes such as ours can be more expensive and take longer than for other, more well-known or extensively studied pharmaceutical or biopharmaceutical products, due to our and regulatory agencies’ lack of experience with them. The FDA has only approved one personalized immunotherapy product to date. This lack of experience may lengthen the regulatory review process, require us to conduct additional studies or clinical trials, increase our development costs, lead to changes in regulatory positions and interpretations, delay or prevent approval and commercialization of these product candidates or lead to significant post-approval limitations or restrictions.
The novel nature of our product candidates also means that fewer people are trained in or experienced with product candidates of this type, which may make it difficult to find, hire and retain capable personnel for research, development and manufacturing positions.
| 42 |
OncoVAX® and our other products in development cannot be sold if we do not maintain or gain required regulatory approvals.
Our business is subject to extensive regulation by numerous state and federal governmental authorities in the United States, including the FDA, and potentially by foreign regulatory authorities, with regulations differing from country to country. In the United States, the FDA regulates, among other things, the pre-clinical testing, clinical trials, manufacturing, safety, efficacy, potency, labeling, storage, record keeping, quality systems, advertising, promotion, sale and distribution of therapeutic products. Other applicable non-United States regulatory authorities have equivalent powers. Failure to comply with applicable requirements could result in, among other things, one or more of the following actions: withdrawal of product approval, notices of violation, untitled letters, warning letters, fines and other monetary penalties, unanticipated expenditures, delays in approval or refusal to approve a product candidate; product recall or seizure; interruption of manufacturing or clinical trials; operating restrictions; injunctions; and criminal prosecution. We are required in the United States and in foreign countries to obtain approval from regulatory authorities before we can manufacture, market and sell our products.
Obtaining regulatory approval for marketing of a product candidate in one country does not assure we will be able to obtain regulatory approval in other countries. However, a failure or delay in obtaining regulatory approval in one country may have a negative effect on the regulatory process in other countries. Once approved, the FDA and other United States and non-United States regulatory authorities have substantial authority to limit the uses or indications for which a product may be marketed, restrict distribution of the product, require additional testing, change product labeling or mandate withdrawal of our products. The marketing of our approved products will be subject to extensive regulatory requirements administered by the FDA and other regulatory bodies, including: the manufacturing, testing, distribution, labeling, packaging, storage, reporting and record-keeping related to the product, advertising, promotion, and adverse event reporting requirements. In addition, incidents of adverse drug reactions, unintended side effects or misuse relating to our products could result in required post-marketing studies, additional regulatory controls or restrictions, or even lead to withdrawal of a product from the market.
In general, the FDA and equivalent authorities in other countries require labeling, advertising and promotional materials to be truthful and not misleading, and marketed only for the approved indications and in accordance with the provisions of the approved label. If the FDA or other regulatory authorities were to challenge our promotional materials or activities, they may bring enforcement action.
Our failure to obtain approval, significant delays in the approval process, or our failure to maintain approval in any jurisdiction will prevent us from selling a product in that jurisdiction. Any product and its manufacturer will continue to be subject to strict regulations after approval, including but not limited to, manufacturing, quality control, labeling, packaging, adverse event reporting, advertising, promotion and record-keeping requirements. Any problems with an approved product, including the later exhibition of adverse effects or any violation of regulations could result in restrictions on the product, including its withdrawal from the market, which could materially harm our business. The process of obtaining approvals in foreign countries is subject to delay and failure for many of the same reasons.
| 43 |
Failure to comply with foreign regulatory requirements governing human clinical trials and failure to obtain marketing approval for product candidates could prevent us from selling our products in foreign markets, which may adversely affect our operating results and financial condition.
The requirements governing the conduct of clinical trials, manufacturing, testing, product approvals, pricing and reimbursement outside the United States vary greatly from country to country. In addition, the time required to obtain approvals outside the United States may differ significantly from that required to obtain FDA approval. We may not obtain foreign regulatory approvals on the timeframe we may desire, if at all. Approval by the FDA does not assure approval by regulatory authorities in other countries, and foreign regulatory authorities could require additional testing. Failure to comply with these regulatory requirements or obtain required approvals could impair our ability to develop foreign markets for our products and may have a material adverse effect on our business and future prospects.
RISKS RELATED TO THE COMMERCIALIZATION OF OUR PRODUCT CANDIDATE(S)
We have no history of commercializing pharmaceutical products, which may make it difficult to evaluate the prospects for our future viability.
Our operations to date have been limited to financing and staffing our company, developing our technology and product candidates and establishing collaborations. We have not yet demonstrated an ability to successfully complete a pivotal clinical trial, obtain marketing approvals, manufacture a commercial scale product or conduct sales and marketing activities necessary for successful product commercialization. Consequently, predictions about our future success or viability may not be as accurate as they could be if we had a history of successfully developing and commercializing pharmaceutical products.
In addition, we may encounter unforeseen expenses, difficulties, complications, delays and other known and unknown factors. We will need to transition at some point from a company with research and development focus to a company capable of supporting commercial activities. We may not be successful in such a transition.
Our success is dependent upon OncoVAX® achieving regulatory approval and acceptance in the marketplace, the failure of either will have a negative effect on our business, financial condition and results of operations.
Our future growth and profitability will depend on its ability to introduce and market OncoVAX®. There can be no assurance that we will be able to obtain necessary regulatory approvals for OncoVAX® in a timely manner, if at all. Our failure to introduce and market OncoVAX® in a timely manner would have a material adverse effect on our business, financial condition and results of operations.
Even if OncoVAX® is approved for marketing by the FDA and other regulatory authorities, there can be no assurance that it will be commercially successful or that we will be successful producing OncoVAX® on a commercial scale at a cost that will enable us to realize a profit. Despite the results of the clinical trials of OncoVAX®, there can be no assurance that oncologists and other physicians will refer patients for treatment with OncoVAX®. Market acceptance also could be affected by the availability of third-party reimbursement. Failure of OncoVAX® to achieve significant market acceptance could have a material adverse effect on our business, financial condition and results of operations.
| 44 |
We have no internal sales or marketing capability and must enter into alliances with others possessing such capabilities to commercialize our products successfully.
We intend to market our products, if and when such products are approved for commercialization by the FDA, either directly or through other strategic alliances and distribution arrangements with third parties. There can be no assurance that we will be able to enter into third-party marketing or distribution arrangements on advantageous terms or at all. To the extent that we do enter into such arrangements, we will be dependent on our marketing and distribution partners. In entering into third-party marketing or distribution arrangements, we expect to incur significant additional expense. There can be no assurance that, to the extent that we sell products directly or we enter into any commercialization arrangements with third parties, such third parties will establish adequate sales and distribution capabilities or be successful in gaining market acceptance for our products and services.
Our business is subject to numerous and evolving state, federal and foreign regulations and we may not be able to secure the government approvals needed to develop and market our products.
Our research and development activities, pre-clinical tests and clinical trials, and ultimately the manufacturing, marketing and labeling of our products, all are subject to extensive regulation by the FDA and foreign regulatory agencies. Pre-clinical testing and clinical trial requirements and the regulatory approval process typically take years and require the expenditure of substantial resources. Additional government regulation may be established that could prevent or delay regulatory approval of our product candidates. Delays or rejections in obtaining regulatory approvals would adversely affect our ability to commercialize any product candidates and our ability to generate product revenues or royalties.
The FDA and foreign regulatory agencies require that the safety and efficacy of product candidates be supported through adequate and well-controlled clinical trials. If the results of pivotal clinical trials do not establish the safety and efficacy of our product candidates to the satisfaction of the FDA and other foreign regulatory agencies, we will not receive the approvals necessary to market such product candidates. Even if regulatory approval of a product candidate is granted, the approval may include significant limitations on the indicated uses for which the product may be marketed.
Many states in which we do, or in the future may do, business, or in which our products may be sold, impose licensing, labeling or certification requirements that are in addition to those imposed by the FDA. There can be no assurance that one or more states will not impose regulations or requirements that have a material adverse effect on our ability to sell our products.
In many of the foreign countries in which we may do business or in which our products may be sold, we will be subject to regulation by national governments and supranational agencies as well as by local agencies affecting, among other things, product standards, packaging requirements, labeling requirements, import restrictions, tariff regulations, duties and tax requirements. There can be no assurance that one or more countries or agencies will not impose regulations or requirements that could have a material adverse effect on our ability to sell our products.
Legislative and regulatory changes affecting the health care industry could adversely affect our business.
Political, economic and regulatory influences are subjecting the healthcare industry to potential fundamental changes that could substantially affect our results of operations. There have been a number of government and private sector initiatives during the last few years to limit the growth of healthcare costs, including price regulation, competitive pricing, coverage and payment policies, comparative effectiveness of therapies, technology assessments and managed-care arrangements. It is uncertain which legislative proposals, if any, will be adopted (or when) or what actions federal, state, or private payors for health care treatment and services may take in response to any health care reform proposals or legislation. We cannot predict the effect health care reforms may have on our business and we can offer no assurances that any of these reforms will not have a material adverse effect on our business. These actual and potential changes are causing the marketplace to put increased emphasis on the delivery of more cost-effective treatments. In addition, uncertainty remains regarding proposed significant reforms to the U.S. healthcare system.
| 45 |
The success of our products may be harmed if the government, private health insurers and other third-party payors do not provide sufficient coverage or reimbursement.
Our ability to commercialize our products successfully will depend in part on the extent to which reimbursement for the costs of such products and related treatments will be available from government health administration authorities, private health insurers and other third-party payors. The reimbursement status of newly approved medical products is subject to significant uncertainty. We cannot guarantee that adequate third-party insurance coverage will be available for us to establish and maintain price levels sufficient for us to realize an appropriate return on our investment in developing new therapies. Government, private health insurers and other third-party payors are increasingly attempting to contain health care costs by limiting both coverage and the level of reimbursement for new therapeutic products approved for marketing by the FDA. Accordingly, even if coverage and reimbursement are provided by government, private health insurers and third-party payors for uses of our products, market acceptance of these products would be adversely affected if the reimbursement available proves to be unprofitable for health care providers.
We face intense competition and the failure to compete effectively could adversely affect our ability to develop and market our products.
There are many companies and other institutions engaged in research and development of various technologies for cancer treatment products. We believe that the level of interest by others in investigating the potential of possible competitive treatments and alternative technologies will continue and may increase. Potential competitors engaged in all areas of cancer treatment research in the United States and other countries include, among others, major pharmaceutical companies, specialized technology companies, and universities and other research institutions. Most of our current and potential competitors have substantially greater financial, technical, human and other resources, and may also have far greater experience than do we, both in pre-clinical testing and human clinical trials of new products and in obtaining FDA and other regulatory approvals. One or more of these companies or institutions could succeed in developing products or other technologies that are more effective than the products and technologies that we have been or are developing, or which would render our technology and products obsolete and non-competitive. Furthermore, if we are permitted to commence commercial sales of any of our products, we will also be competing, with respect to manufacturing efficiency and marketing, with companies having substantially greater resources and experience in these areas.
Our products may not be accepted in the marketplace; therefore we may not be able to generate significant revenue, if any.
Even if OncoVAX® or any of our other potential products is approved and sold, physicians and the medical community may not ultimately use them or may use them only in applications more restricted than we expect. Our products, if successfully developed, will compete with a number of traditional products and immunotherapies manufactured and marketed by major pharmaceutical and other biotechnology companies. Our products will also compete with new products currently under development by such companies and others. Physicians will only prescribe a product if they determine, based on experience, clinical data, side effect profiles and other factors, that it is beneficial and preferable to other products currently in use. Many other factors influence the adoption of new products, including marketing and distribution restrictions, course of treatment, adverse publicity, product pricing, the views of thought leaders in the medical community, and reimbursement by government and private third party payers.
| 46 |
Technologies for the treatment of cancer are subject to rapid change, and the development of treatment strategies that are more effective than our technologies could render our technologies obsolete.
Various methods for treating cancer currently are, and in the future are expected to be, the subject of extensive research and development. Many possible treatments that are being researched, if successfully developed, may not require, or may supplant, the use of our technologies. The successful development and acceptance of any one or more of these alternative forms of treatment could render our technology obsolete as a cancer treatment method.
Products we may potentially commercialize and market may be subject to promotional limitations.
The FDA has the authority to impose significant restrictions on an approved product through the product label and allowed advertising, promotional and distribution activities. The FDA also may require us to undertake post-marketing clinical trials. If the results of such post-marketing studies are not satisfactory, the FDA may withdraw marketing authorization or may condition continued marketing on commitments from us that may be expensive and/or time consuming to fulfill. Even if we receive FDA and other regulatory approvals, if we or others identify adverse side effects after any of our products are on the market, or if manufacturing problems occur, regulatory approval may be suspended or withdrawn and reformulation of our products, additional clinical trials, changes in labeling of our products and additional marketing applications may be required, any of which could harm our business and cause our stock price to decline.
Our competitors may develop and market products that are less expensive, more effective, and safer or reach the market sooner, which may diminish or eliminate the commercial success of any products we may commercialize.
Competition in the cancer therapeutics field is intense and is accentuated by the rapid pace of advancements in product development. In addition, we compete with other clinical-stage companies and institutions for clinical trial participants, which could reduce our ability to recruit participants for our clinical trials. Delay in recruiting clinical trial participants could adversely affect our ability to bring a product to market prior to our competitors. Further, research and discoveries by others may result in breakthroughs that render potential products obsolete before they generate revenue.
Some of our competitors in the cancer therapeutics field have substantially greater research and development capabilities and manufacturing, marketing, financial and managerial resources than we do. Acquisitions of competing companies by large pharmaceutical and biotechnology companies could enhance our competitors’ resources. In addition, our competitors may obtain patent protection or FDA approval and commercialize products more rapidly than we do, which may impact future sales of our products. We expect that competition among products approved for sale will be based, among other things, on product efficacy, price, safety, reliability, availability, patent protection, and sales, marketing and distribution capabilities. Our profitability and financial position will suffer if our products receive regulatory approval, but cannot compete effectively in the marketplace.
| 47 |
RISKS RELATING TO MANUFACTURING ACTIVITIES
Lack of coordination internally among our employees and externally with physicians, hospitals and third-party suppliers and carriers, could cause manufacturing difficulties, disruptions or delays and cause us to not have sufficient product to meet our expected clinical trial requirements or potential commercial requirements.
Manufacturing our OncoVAX® product requires coordination internally among our employees and externally with physicians, hospitals and third party suppliers and carriers. For example, a patient’s physician or clinical site will need to coordinate with us for the shipping of a patient’s disease sample to our manufacturing facility, and we will need to coordinate with them for the shipping of the manufactured product to them. Such coordination involves a number of risks that may lead to failures or delays in manufacturing including:
| • | failure to obtain a sufficient supply of key raw materials of suitable quality; |
| • | difficulties in manufacturing OncoVAX® product for multiple patients simultaneously; |
| • | difficulties in obtaining adequate patient-specific material, such as tumor samples, from physicians; |
| • | difficulties in completing the development and validation of the specialized assays required to ensure the consistency of our product candidates; |
| • | failure to ensure adequate quality control and assurances in the manufacturing process as we increase production quantities; |
| • | difficulties in the timely shipping of patient-specific materials to us or in the shipping of our OncoVAX® vaccine to the treating physicians due to errors by third party carriers, transportation restrictions or other reasons; |
| • | destruction of, or damage to, patient-specific materials during the shipping process due to improper handling by third party carriers, hospitals, physicians or us; |
| • | destruction of, or damage to, patient-specific materials during storage at our facilities; and |
| • | destruction of, or damage to, patient-specific materials or our product candidates stored at clinical and future commercial sites due to improper handling or holding by clinicians, hospitals or physicians. |
If we are unable to coordinate appropriately, we may encounter delays or additional costs in achieving our clinical and commercialization objectives or we may not be able to complete these objectives, which may also delay or make it unable for us to obtain regulatory approvals of our product candidates and supplying product, which could materially damage our business and financial position.
| 48 |
We and our contract manufacturers are subject to significant regulation with respect to manufacturing of our products.
All of those involved in the preparation of a therapeutic drug for clinical trials or commercial sale, including our existing (and any future) supply contract manufacturers and clinical trial investigators, are subject to extensive regulation. Components of a finished therapeutic product approved for commercial sale or used in late-stage clinical trials must be manufactured in accordance with the FDA’s current Good Manufacturing Practices, or equivalent cGMP regulations developed by authorities in other countries. These regulations govern manufacturing processes and procedures and the implementation and operation of quality systems to control and assure the quality of investigational products and products approved for sale. Our facilities and quality systems and the facilities and quality systems of some or all of our third-party contractors must be inspected and audited routinely for compliance with applicable United States and other country regulations. Our current Emmen facility is operating under cGMP protocols and had its cGMP recertification inspection in July 2013. On August 5, 2013, the Dutch inspector issued its official report on the July 2013 inspection under which they concluded that the cGMP levels for the Emmen Facility were sufficient. We received official recertificated cGMP status in October 2013. If any future inspection or audit identifies a failure to comply with applicable regulations or if a violation of our product specifications or applicable regulation occurs independent of such an inspection or audit, we, the FDA, or governmental authorities in other countries may require remedial measures that may be costly and/or time consuming for us or a third-party to implement and that may include the temporary or permanent suspension or change of a clinical trial or commercial sales, recalls, market withdrawals, seizures or the temporary or permanent closure of a facility. Any such remedial measures imposed upon us or third parties with whom we contract could materially harm our business.
RISKS RELATED TO RELIANCE ON THIRD PARTIES
We rely on third parties to conduct our clinical trials, and those third parties may not perform satisfactorily, including failing to meet deadlines for the completion of such trials.
We do not independently conduct clinical trials of our product candidates. We rely on third parties, such as contract research organizations, clinical data management organizations, medical institutions and clinical investigators, to perform this function. Our reliance on these third parties for clinical development activities reduces our control over these activities but does not relieve us of our responsibilities. We remain responsible for ensuring that each of our clinical trials is conducted in accordance with the general investigational plan and protocols for the trial. Moreover, the FDA requires us to comply with standards, commonly referred to as Good Clinical Practices, for conducting, recording and reporting the results of clinical trials to assure that data and reported results are credible and accurate and that the rights, integrity and confidentiality of trial participants are protected. We also are required to register ongoing clinical trials and post the results of completed clinical trials on a government-sponsored database, ClinicalTrials.gov, within certain timeframes. Failure to do so can result in fines, adverse publicity and civil and criminal sanctions. Furthermore, these third parties may also have relationships with other entities, some of which may be our competitors. If these third parties do not successfully carry out their contractual duties, meet expected deadlines or conduct our clinical trials in accordance with regulatory requirements or our stated protocols, we will not be able to obtain, or may be delayed in obtaining, regulatory approvals for our product candidates and will not be able to, or may be delayed in our efforts to, successfully commercialize our product candidates.
We also rely on other third parties to store and distribute product supplies for our clinical trials. Any performance failure on the part of our existing or future distributors could delay clinical development or regulatory approval of our product candidates or commercialization of our products, producing additional losses and depriving us of potential product revenue.
| 49 |
RISKS RELATED TO EMPLOYEE MATTERS AND MANAGING GROWTH
Our future success depends on our ability to retain our chief executive officer (who currently also serves as our principal financial officer, secretary and treasurer) and other key executives and to attract, retain and motivate qualified personnel.
We are highly dependent on the principal members of our management and scientific teams. Although we have formal employment agreements with our executive officer, these agreements do not prevent our executives from terminating their employment with us at any time. We do not maintain “key person” insurance for any of our executives or other employees. The loss of the services of any of these persons could impede the achievement of our research, development and commercialization objectives.
Recruiting and retaining qualified scientific, clinical, manufacturing and sales and marketing personnel will also be critical to our success. We may not be able to attract and retain these personnel on acceptable terms given the competition among numerous pharmaceutical and biotechnology companies for similar personnel. We also experience competition for the hiring of scientific and clinical personnel from universities and research institutions. In addition, we rely on consultants and advisors, including scientific and clinical advisors, to assist us in formulating our research and development and commercialization strategy. Our consultants and advisors may be employed by employers other than us and may have commitments under consulting or advisory contracts with other entities that may limit their availability to us.
We expect to expand our development, regulatory, manufacturing and sales and marketing capabilities, and as a result, we may encounter difficulties in managing our growth, which could disrupt our operations.
We expect to experience significant growth in the number of our employees and the scope of our operations, particularly in the areas of research and development, regulatory affairs, manufacturing and sales and marketing. To manage our anticipated future growth, we must continue to implement and improve our managerial, operational and financial systems, expand our facilities and continue to recruit and train additional qualified personnel. Due to our limited financial resources and the limited experience of our management team in managing a company with such anticipated growth, we may not be able to effectively manage the expansion of our operations or recruit and train additional qualified personnel. The physical expansion of our operations may lead to significant costs and may divert our management and business development resources. Any inability to manage growth could delay the execution of our business plans or disrupt our operations.
We may not be able to hire or retain key officers or employees that we need to implement our business strategy and develop our products and business.
Our success depends significantly on the continued contributions of our executive officers, scientific and technical personnel and consultants, and on our ability to attract additional personnel as we seek to implement our business strategy and develop our products and businesses. During our operating history, we have assigned many essential responsibilities to a relatively small number of individuals. However, as our business and the demands on our key employees expand, we have been, and will continue to be, required to recruit additional qualified employees. The competition for such qualified personnel is intense, and the loss of services of certain key personnel or our inability to attract additional personnel to fill critical positions could adversely affect our business.
| 50 |
Our Success Will Depend In Part On Our Ability To Grow And Diversify, Which In Turn Will Require That We Manage And Control Our Growth Effectively.
Our business strategy contemplates growth and diversification. Our ability to manage growth effectively will require that we continue to expend funds to improve our operational, financial and management controls, reporting systems and procedures. In addition, we must effectively expand, train and manage our employees. We will be unable to manage our businesses effectively if we are unable to alleviate the strain on resources caused by growth in a timely and successful manner. There can be no assurance that we will be able to manage our growth and a failure to do so could have a material adverse effect on our business.
RISKS RELATED TO OUR COMMON STOCK
Provisions in our corporate charter documents and under Maryland law could make an acquisition of us, which may be beneficial to our stockholders, more difficult and may prevent attempts by our stockholders to replace or remove our current management.
Provisions in our corporate charter and our bylaws may discourage, delay or prevent a merger, acquisition or other change in control of us that stockholders may consider favorable, including transactions in which you might otherwise receive a premium for your shares. These provisions could also limit the price that investors might be willing to pay in the future for shares of our common stock, thereby depressing the market price of our common stock. In addition, because our board of directors is responsible for appointing the members of our management team, these provisions may frustrate or prevent any attempts by our stockholders to replace or remove our current management by making it more difficult for stockholders to replace members of our board of directors. Among other things, these provisions:
| • | permit us to establish a classified board of directors such that not all members of the board are elected at one time; |
| • | establish advance notice requirements for stockholder proposals that can be acted on at stockholder meetings and nominations to our board of directors; |
| • | limit who may call stockholder meetings; and |
| • | authorize our board of directors to issue preferred stock without stockholder approval, which could be used to institute a “poison pill” that would work to dilute the stock ownership of a potential hostile acquirer, effectively preventing acquisitions that have not been approved by our board of directors. |
If our stock price is volatile, purchasers of our common stock could incur substantial losses.
Our stock price is likely to be volatile. The stock market in general and the market for biotechnology companies in particular have experienced extreme volatility that has often been unrelated to the operating performance of particular companies. As a result of this volatility, you may not be able to sell your common stock at or above your purchase price. The market price for our common stock may be influenced by many factors, including:
| 51 |
| • | results of clinical trials of our product candidates or those of our competitors; |
| • | the success of competitive products or technologies; |
| • | potential approvals of our product candidates for marketing by the FDA or equivalent foreign regulatory authorities or our failure to obtain such approvals; |
| • | regulatory or legal developments in the United States and other countries; |
| • | the results of our efforts to commercialize our product candidates; |
| • | developments or disputes concerning patents or other proprietary rights; |
| • | the recruitment or departure of key personnel; |
| • | the level of expenses related to any of our product candidates or clinical development programs; |
| • | the results of our efforts to discover, develop, acquire or in-license additional product candidates or products; |
| • | actual or anticipated changes in estimates as to financial results, development timelines or recommendations by securities analysts; |
| • | variations in our financial results or those of companies that are perceived to be similar to us; |
| • | market conditions in the pharmaceutical and biotechnology sectors and issuance of new or changed securities analysts’ reports or recommendations; |
| • | general economic, industry and market conditions; and |
| • | the other factors described in this “Risk Factors” section. |
We will incur increased costs as a result of operating as a public company, and our management will be required to devote substantial time to new compliance initiatives and corporate governance practices.
As a public company, and particularly after we are no longer an “emerging growth company,” we will incur significant legal, accounting and other expenses that we did not incur as a private company. The Sarbanes-Oxley Act of 2002, the Dodd-Frank Wall Street Reform and Consumer Protection Act, the listing requirements of a national exchange and other applicable securities rules and regulations impose various requirements on public companies, including establishment and maintenance of effective disclosure and financial controls and corporate governance practices. We expect that we will need to hire additional accounting, finance and other personnel in connection with our becoming, and our efforts to comply with the requirements of being, a public company and our management and other personnel will need to devote a substantial amount of time towards maintaining compliance with these requirements. These requirements will increase our legal and financial compliance costs and will make some activities more time-consuming and costly. For example, we expect that the rules and regulations applicable to us as a public company may make it more difficult and more expensive for us to obtain director and officer liability insurance, which could make it more difficult for us to attract and retain qualified members of our board of directors. We are currently evaluating these rules and regulations, and cannot predict or estimate the amount of additional costs we may incur or the timing of such costs. These rules and regulations are often subject to varying interpretations, in many cases due to their lack of specificity, and, as a result, their application in practice may evolve over time as new guidance is provided by regulatory and governing bodies. This could result in continuing uncertainty regarding compliance matters and higher costs necessitated by ongoing revisions to disclosure and governance practices.
| 52 |
Pursuant to Section 404 of the Sarbanes-Oxley Act of 2002, we are required to furnish a report to our management on our internal control over financial reporting. However, while we remain an emerging growth company and/or a smaller reporting company, we will not be required to include an attestation report on internal control over financial reporting issued by our independent registered public accounting firm. To achieve compliance with Section 404 of the Sarbanes-Oxley Act of 2002, we have been engaged in a process to document and evaluate our internal control over financial reporting, which is both costly and challenging. In this regard, we will need to continue to dedicate internal resources, potentially engage outside consultants and adopt a detailed work plan to assess and document the adequacy of internal control over financial reporting, continue steps to improve control processes as appropriate, validate through testing that controls are functioning as documented and implement a continuous reporting and improvement process for internal control over financial reporting. Despite our efforts, there is a risk that we will not be able to conclude, that our internal control over financial reporting is effective as required by Section 404 of the Sarbanes-Oxley Act of 2002. If we identify one or more material weaknesses, it could result in an adverse reaction in the financial markets due to a loss of confidence in the reliability of our financial statements.
We are an “emerging growth company,” and the reduced disclosure requirements applicable to emerging growth companies may make our common stock less attractive to investors.
We are an “emerging growth company,” as defined in the JOBS Act For so long as we remain an emerging growth company, we are permitted and plan to rely on exemptions from certain disclosure requirements that are applicable to other public companies that are not emerging growth companies. These exemptions include not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act of 2002, not being required to comply with any requirement that may be adopted by the Public Company Accounting Oversight Board regarding mandatory audit firm rotation or a supplement to the auditor’s report providing additional information about the audit and the financial statements, reduced disclosure obligations regarding executive compensation and exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and shareholder approval of any golden parachute payments not previously approved. In this 10-K, we have not included all of the executive compensation related information that would be required if we were not an emerging growth company. We cannot predict whether investors will find our common stock less attractive if we rely on these exemptions. If some investors find our common stock less attractive as a result, there may be a less active trading market for our common stock and our stock price may be more volatile.
In addition, the JOBS Act provides that an emerging growth company can take advantage of an extended transition period for complying with new or revised accounting standards. This allows an emerging growth company to delay the adoption of certain accounting standards until those standards would otherwise apply to private companies. We have irrevocably elected not to avail ourselves of this exemption from new or revised accounting standards and, therefore, we will be subject to the same new or revised accounting standards as other public companies that are not emerging growth companies.
Because we do not anticipate paying any cash dividends on our capital stock in the foreseeable future, capital appreciation, if any, will be your sole source of gain.
We have never declared or paid cash dividends on our capital stock. We currently intend to retain all of our future earnings, if any, to finance the growth and development of our business. In addition, the terms of existing or any future debt agreements may preclude us from paying dividends. As a result, capital appreciation, if any, of our common stock will be your sole source of gain for the foreseeable future.
| 53 |
If securities or industry analysts do not publish research or publish inaccurate or unfavorable research about our business, our share price and trading volume could decline.
The trading market for our common stock will depend on the research and reports that securities or industry analysts publish about us or our business. We do not have any control over these analysts. There can be no assurance that analysts will cover us, or provide favorable coverage. If one or more analysts downgrade our stock or change their opinion of our stock to be less favorable, our share price would likely decline. In addition, if one or more analysts cease coverage of our company or fail to regularly publish reports on us, we could lose visibility in the financial markets, which could cause our share price or trading volume to decline.
Issuance of stock to fund our operations may dilute your investment and reduce your equity interest.
We may need to raise capital in the future to fund the development of our product candidates or for other purposes. Any equity financing may have a significant dilutive effect to stockholders and a material decrease in our existing stockholders’ equity interest in us. Equity financing, if obtained, could result in substantial dilution to our existing stockholders. At its sole discretion, our board of directors may issue additional securities without seeking stockholder approval, and we do not know when we will need additional capital or, if we do, whether it will be available to us.
Our Articles of Incorporation authorizes the Board of Directors to issue up to 200,000,000 shares of common stock and up to 50,000,000 shares of preferred stock. The power of the Board of Directors to issue shares of common stock, preferred stock or warrants or options to purchase shares of common stock or preferred stock is generally not subject to stockholder approval. Accordingly, any additional issuance of our common stock, or preferred stock that may be convertible into common stock, may have the effect of diluting your investment.
Upon the exercise of our outstanding warrants, holders of our common stock may experience immediate dilution and the market price of our common stock may be adversely affected.
The Company has raised capital through the private placements some of which were sold with warrants. Additionally warrants have been issued to service providers, founders, as finder’s fees, to broker dealers and as contingent interest payments. At December 31, 2104 there were 34,962,172 shares of common stock issued and outstanding and 4,759,065 warrants for the purchase of shares of common stock. If any portion of the outstanding warrants are exercised for shares of our common stock, our stockholders may experience immediate dilution and the market price of our common stock may be adversely affected.
| 54 |
Our ability to use our net operating losses and research and development credit carryforwards to offset future taxable income may be subject to certain limitations.
In general, under Sections 382 and 383 of the Internal Revenue Code of 1986, as amended, or the Code, a corporation that undergoes an “ownership change,” generally defined as a greater than 50% change (by value) in its equity ownership over a three year period, is subject to limitations on its ability to utilize its pre-change net operating losses, or NOLs, and its research and development credit carryforwards to offset future taxable income. Our existing NOLs and research and development credit carryforwards may be subject to limitations arising from previous ownership changes, and if we undergo an ownership change, our ability to utilize NOLs and research and development credit carryforwards could be further limited by Sections 382 and 383 of the Code. Future changes in our stock ownership, some of which might be beyond our control, could result in an ownership change under Sections 382 and 383 of the Code. Furthermore, our ability to utilize NOLs and research and development credit carryforwards of any companies we may acquire in the future may be subject to limitations, in accordance with Sections 382 and 383 of the Code. For these reasons, in the event we experience a change of control, we may not be able to utilize a material portion of the NOLs and research and development credit carryforwards, even if we attain profitability.
You may not be able to liquidate your investment since there is no assurance that an active public market will develop for our common stock.
There is no established public trading market for our securities. Although our common stock is actively trading on the OTC Link (OTC.QB Tier) under the symbol VGEN, there has been limited public trading of our common stock and there can be no assurance that a regular trading market will develop or that if developed, will be sustained. In the absence of a trading market, an investor may be unable to liquidate its investment, which will result in the loss of your investment.
Transfers of our securities may be restricted by virtue of state securities “blue sky” laws which prohibit trading absent compliance with individual state laws. These restrictions may make it difficult or impossible to sell shares in those states.
Transfers of our securities may be restricted under the securities regulations laws promulgated by various states and foreign jurisdictions, commonly referred to as “blue sky” laws. Absent compliance with such individual state laws, our common stock may not be traded in such jurisdictions. Because the securities registered hereunder have not been registered for resale under the blue sky laws of any state, the holders of such shares and persons who desire to purchase them should be aware that there may be significant state blue sky law restrictions upon the ability of investors to sell the securities and of purchasers to purchase the securities. These restrictions may prohibit the trading of our securities. Investors should consider the secondary market for our securities to be a limited one.
Our officers, directors and principal stockholders collectively own a substantial portion of our outstanding common stock, and as long as they do, they may be able to control the outcome of stockholder voting.
Our officers and directors are collectively the beneficial owners of approximately 43% of the outstanding shares of our common stock as of December 31, 2014. In addition, our principal stockholder is the beneficial owner of approximately 41% of the outstanding shares of our common stock as of the date of this filing. Accordingly, these stockholders, individually and as a group, may be able to control us and direct our affairs and business, including any determination with respect to a change in control, future issuances of common stock or other securities, declaration of dividends on the common stock and the election of directors.
| 55 |
By issuing preferred stock, we may be able to delay, defer, or prevent a change of control.
Our Articles of Incorporation permits us to issue, without approval from our stockholders, a total of 50,000,000 shares of preferred stock. Our Board of Directors can determine the rights, preferences, privileges and restrictions granted to, or imposed upon, the shares of preferred stock and to fix the number of shares constituting any series and the designation of such series. It is possible that our Board of Directors, in determining the rights, preferences and privileges to be granted when the preferred stock is issued, may include provisions that have the effect of delaying, deferring or preventing a change in control, discouraging bids for our common stock at a premium over the market price, or that adversely affect the market price of and the voting and other rights of the holders of our common stock.
Once publicly trading, the application of the “penny stock” rules could adversely affect the market price of our common shares and increase your transaction costs to sell those shares.
The Securities and Exchange Commission has adopted Rule 15g-9 which establishes the definition of a “penny stock,” for the purposes relevant to us, as any equity security that has a market price of less than $5.00 per share or with an exercise price of less than $5.00 per share, subject to certain exceptions. For any transaction involving a penny stock, unless exempt, the rules require:
| • | that a broker or dealer approve a person's account for transactions in penny stocks; and |
| • | the broker or dealer receives from the investor a written agreement to the transaction, setting forth the identity and quantity of the penny stock to be purchased. |
In order to approve a person's account for transactions in penny stocks, the broker or dealer must:
| • | obtain financial information and investment experience objectives of the person; and |
| • | make a reasonable determination that the transactions in penny stocks are suitable for that person and the person has sufficient knowledge and experience in financial matters to be capable of evaluating the risks of transactions in penny stocks. |
The broker or dealer must also deliver, prior to any transaction in a penny stock, a disclosure schedule prescribed by the Commission relating to the penny stock market, which, in highlight form:
| • | sets forth the basis on which the broker or dealer made the suitability determination; and |
| • | that the broker or dealer received a signed, written agreement from the investor prior to the transaction. |
Generally, brokers may be less willing to execute transactions in securities subject to the “penny stock” rules. This may make it more difficult for investors to dispose of our common stock and cause a decline in the market value of our stock.
Disclosure also has to be made about the risks of investing in penny stocks in both public offerings and in secondary trading and about the commissions payable to both the broker-dealer and the registered representative, current quotations for the securities and the rights and remedies available to an investor in cases of fraud in penny stock transactions. Finally, monthly statements have to be sent disclosing recent price information for the penny stock held in the account and information on the limited market in penny stocks
| 56 |
If we fail to remain current on our reporting requirements, we could be removed from the OTC bulletin board which would limit the ability of broker-dealers to sell our securities and the ability of stockholders to sell their securities in the secondary market.
Companies quoted on the Over-The-Counter Bulletin Board must be reporting issuers under Section 12 of the Securities Exchange Act of 1934, as amended, and must be current in their reports under Section 13, in order to maintain price quotation privileges on the OTC Bulletin Board. If we fail to remain current on our reporting requirements, we could be removed from the OTC Bulletin Board. As a result, the market liquidity for our securities could be severely adversely affected by limiting the ability of broker-dealers to sell our securities and the ability of stockholders to sell their securities in the secondary market. In addition, we may be unable to get re-quoted on the OTC Bulletin Board, which may have an adverse material effect on our Company.
We were delinquent in filing our U.S. tax returns including the form regarding our international subsidiaries which will cause us to incur penalties.
We are current on all of our known tax filings. Prior to September 2013 we were delinquent in filing our U.S. tax returns. Our 2008 and 2009 tax returns were filed in August 2013 and our 2010, 2011 and 2012 tax returns were filed in September 2013. Our 2013 tax return was filed in September 2014. We do not owe any income taxes for any of those years as we have not shown any income in such years.
There is an issue with penalties regarding the U.S. information reporting Form 5471 for Switzerland, the Netherlands, and the United Kingdom. The Company failed to timely file, including extension, all applicable U.S. information reporting Forms 5471 for annual periods 2007 through 2011. The Company did timely file all applicable U.S. information reporting Forms 5471 for the 2012 and 2013 annual period.
The IRS has been imposing penalties on a regular basis for the failure of U.S. taxpayers to file Form 5471. The penalty is $10,000 per entity for each year. A U.S. income tax return is not considered complete without the filing of the report.
In accordance with statutory filing requirements, the Company’s penalty accrual related to failure to file and late filings of Forms 5471 is $100,000 as of December 31, 2014.
All income tax returns for our foreign subsidiary in the Netherlands have been timely filed.
The requirements of being a public company may strain our resources, divert management’s attention and affect our ability to attract and retain qualified board members.
As a public company, we incur significant legal, accounting and other expenses that we would not incur as a private company, including costs associated with public company reporting requirements. We also incur costs associated with the Sarbanes-Oxley Act of 2002, as amended, the Dodd-Frank Wall Street Reform and Consumer Protection Act and related rules implemented or to be implemented by the SEC and the NYSE AMEX. The expenses incurred by public companies generally for reporting and corporate governance purposes have been increasing. We expect these rules and regulations to increase our legal and financial compliance costs and to make some activities more time-consuming and costly, although we are currently unable to estimate these costs with any degree of certainty. These laws and regulations could also make it more difficult or costly for us to obtain certain types of insurance, including director and officer liability insurance, and we may be forced to accept reduced policy limits and coverage or incur substantially higher costs to obtain the same or similar coverage. These laws and regulations could also make it more difficult for us to attract and retain qualified persons to serve on our board of directors, our board committees or as our executive officers and may divert management’s attention. Furthermore, if we are unable to satisfy our obligations as a public company, we could be subject to delisting of our common stock, fines, sanctions and other regulatory action and potentially civil litigation.
| 57 |
Cautionary Statement Concerning Forward-Looking Information.
This annual report and the documents to which we may refer you and incorporate into this annual report by reference contain forward-looking statements. In addition, from time to time, we, or our representatives, may make forward-looking statements orally or in writing. These are statements that relate to future periods and include statements regarding our future strategic, operational and financial plans, potential acquisitions, anticipated or projected revenues, expenses and operational growth, markets and potential customers for our products and services, plans related to sales strategies and efforts, the anticipated benefits of our relationships with strategic partners, growth of our competition, our ability to compete, the adequacy of our current facilities and our ability to obtain additional space, use of future earnings, and the feature, benefits and performance of our current and future products and services.
You can identify forward-looking statements by those that are not historical in nature, particularly those that use terminology such as “may,” “should,” “expects,” “anticipates,” “contemplates,” “estimates,” “believes,” “plans,” “projected,” “predicts,” “potential,” “seek” or “continue” or the negative of these or similar terms. In evaluating these forward-looking statements, you should consider various factors, including those described in this annual report under the heading “Risk Factors.” These and other factors may cause our actual results to differ materially from any forward-looking statement. We caution you not to place undue reliance on these forward-looking statements.
We base these forward-looking statements on our expectations and projections about future events, which we derive from the information currently available to us. Such forward-looking statements relate to future events or our future performance. Forward-looking statements are only predictions. The forward-looking events discussed in this annual report, the documents to which we may refer you and other statements made from time to time by us or our representatives, may not occur, and actual events and results may differ materially and are subject to risks, uncertainties and assumptions about us. For these statements, we claim the protection of the “bespeaks caution” doctrine. The forward-looking statements speak only as of the date hereof, and we expressly disclaim any obligation to publicly release the results of any revisions to these forward-looking statements to reflect events or circumstances after the date of this filing.
| Item 1B. | UNRESOLVED STAFF COMMENTS |
Not applicable.
| Item 2. | PROPERTIES |
Our executive officers are located at 949 Fell Street, Baltimore, MD 21231 and comprise approximately 10,000 square feet. We lease our executive offices at a rate of $17,292 per month, which lease term ends on June 30, 2018, with an optional additional twelve month extension period.
We also maintain offices, laboratory and scientific manufacturing space at Marco Polostraat 27. 7825 VM, Emmen, The Netherlands which comprises approximately 9,600 square feet. We lease this space at a price of approximately $4,780 per month, which lease term ends on November 30, 2015. We have the right to extend the term for successive one year periods.
A satellite office has been established at Science Park 408, 1098 XH Amsterdam, The Netherlands. We lease this space at a price of approximately $1,150 per month, which lease term ends on October 31, 2015. We have the right to extend the term for an additional twelve months.
| 58 |
| Item 3. | LEGAL PROCEEDINGS |
We are currently in a dispute with a vendor regarding amounts owed for services performed. A demand for payment under a written agreement has been made in the amount of approximately $150,000. Management believes the vendor did not perform under the terms of the contract and contends that no amounts are due. Settlement discussions between the parties are continuing. We had reserved $75,000 under our financial statements for resolution of this matter and the matter was settled in November 2014 for $65,000.
We are party to claims and litigation that arise in the normal course of business. Management believes that the ultimate outcome of these claims and litigation will not have a material impact on our financial position or results of operations.
| Item 4. | MINE SAFETY DISCLOSURES. |
Not applicable.
PART II
Item 5. MARKET FOR COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER REPURCHASES OF EQUITY SECURITIES
Market information
On March 8, 2013, FINRA assigned our common stock the trading symbol “VACC.” Effective March 14, 2013, the trading symbol for our common stock was changed to “VGEN”. Our stock has been approved for quotation on the OTC Link (OTC.QB Tier). The table below sets forth the high and low bid prices for our common stock for each quarter since March 8, 2013, as reported on the OTC Link.
We consider our stock to be “thinly traded” and any reported sale prices may not be a true market-based valuation of our stock. Some of the bid quotations from the OTC Markets set forth below may reflect inter-dealer prices, without retail mark-up, mark-down or commission and may not represent actual transactions.
| 2013 (OTC Link) | High Bid | Low Bid | ||||||
| First quarter | - | - | ||||||
| Second quarter | 5.50 | - | ||||||
| Third quarter | 4.75 | 1.01 | ||||||
| Fourth quarter | 7.91 | 1.02 | ||||||
| 2014 (OTC Link) | High Bid | Low Bid | ||||||
| First quarter | 10.85 | 6.50 | ||||||
| Second quarter | 6.50 | 4.00 | ||||||
| Third quarter | 5.00 | 2.52 | ||||||
| Fourth quarter | 3.45 | 2.00 | ||||||
As of April 10, 2015, there were 35,896,752 shares of common stock outstanding, which were held by approximately 400 record stockholders. In addition, we have reserved 5,077,247 shares of common stock for issuance upon exercise of outstanding warrants.
| 59 |
Dividend Policy
We have not paid cash dividends since our inception and we do not contemplate paying dividends in the foreseeable future.
Recent Sales of Unregistered Securities
| 1. | From September 10, 2014 through April 6, 2015, we issued 1,869,160 units (“Units”). Each Unit consists of 1 share of common stock and a warrant to purchase 0.3 shares of common stock resulting in the issuance of 1,818,182 shares of common stock, 50,978 adjustment shares and warrants to purchase 545,454 shares of our common stock. The purchase price for each unit was $5.50 per unit and the exercise price for each warrant was $6.05 per shares. We received gross proceeds of $10,000,000 from the sale of these Units for cash. The proceeds were used for working capital and general corporate purposes. These issuances were exempt under Rule 506 of the Securities Act of 1933, as amended. All investors in Unit offering were accredited investors and were solicited by officer Andrew Tussing of Vaccinogen and all investors had a substantial pre-existing relationship with such officer of Vaccinogen. The shares of common stock and warrants received were issued as restricted securities and all certificates issued contained a legend stating the shares have not been registered under the Securities Act and setting forth or referring to the restrictions on transferability and the sale of the shares under the Securities Act. |
| 2. | The Units referred to in paragraph 1. above contained anti-dilution rights such that if the market price of our Common Stock on the effective date of our first S-1 registration statement filed with the SEC (the “Effectiveness Date Market Price”) was less than $5.50 per share, then we agreed to each subscriber of the Units, for no additional consideration, such number of shares (“Adjustment Shares”) of Common Stock equal to the difference between (x) the number of shares of our Common Stock that would have been issued to purchaser if the per-Unit purchase price for such shares had been equal to either (1) the Effectiveness Date Market Price or (2) $5.00, whichever is greater and (y) the number of shares of the Common Stock originally issued to purchaser upon the closing of the sale of Units. The Effectiveness Date Market Price was $5.35 on October 31, 2013. Accordingly, we issued 15,397 Adjustment Shares to the Unit subscribers under paragraph 1 above. The Adjustment Shares were issued to all accredited investors and were issued as restricted securities and all certificates for such shares contained stating that the shares have not been registered under the Securities Act of 1933, as amended, and setting forth or referring to the restrictions on transferability and the sale of the shares under the Securities Act of 1933, as amended. We relied on the exemption provided by Rule 506 of the Securities Act of 1933, as amended for issuance of the Adjustment Shares. We did not receive any proceeds for issuance of the Adjustment Shares. |
| Item 6. | SELECTED FINANCIAL DATA |
We are a smaller reporting company as defined by Rule 12b-2 of the Exchange Act and are not required to provide the information required under this item.
| 60 |
Item 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATION
The following discussion should be read in conjunction with the consolidated financial statements and notes. In addition to historical information, this discussion and analysis contains forward-looking statements that involve risks, uncertainties and assumptions, which could cause actual results to differ materially from management's expectations. Our Management’s Discussion and Analysis of Financial Condition and Results of Operations (“MD&A”) is provided in addition to the accompanying financial statements and notes to assist readers in understanding our results of operations, financial condition and cash flows. Our MD&A is organized as follows:
| • | Overview — Discussion of our business and overall analysis of financial and other highlights affecting the Company in order to provide context for the remainder of MD&A. | |
| • | Trends & Outlook — Discussion of what we view as the overall trends affecting our business and the strategy for 2015 and beyond. | |
| • | Critical Accounting Policies— Accounting policies that we believe are important to understanding the assumptions and judgments incorporated in our reported financial results and forecasts. | |
| • | Results of Operations— Analysis of our financial results comparing 2014 and 2013. | |
| • | Liquidity and Capital Resources— An analysis of changes in our balance sheet and cash flows and discussion of our financial condition and future liquidity needs. |
The various sections of this MD&A contain a number of forward-looking statements. Words such as “expects,” “goals,” “plans,” “believes,” “continues,” “may,” and variations of such words and similar expressions are intended to identify such forward-looking statements. In addition, any statements that refer to projections of our future financial performance, our anticipated growth and trends in our businesses, and other characterizations of future events or circumstances are forward-looking statements. Such statements are based on our current expectations and could be affected by the uncertainties and risk factors described throughout this Form 10-K and particularly in the “Risk Factors”, “Overview” and “Trends & Outlook” section. Our actual results may differ materially.
Overview
We are a biotechnology company founded in 2007 relying on almost four decades of prior research by Michael G. Hanna, Jr., a board member and his colleagues into combating cancer by employing the body’s own immune system. Vaccinogen is the developer of OncoVAX®, which we believe is the only patient specific immunotherapy for stage II colon cancer and our technology may also have application in other tumor types, notably melanoma and renal cell carcinoma.
Colon cancer represents the third most common form of cancer in both the U.S. and Europe. American Cancer Society statistics suggest there will be approximately 97,000 new cases of colon cancer diagnosed within the U.S. in 2014, resulting in approximately 50,000 deaths
| 61 |
We have broad patents covering the OncoVAX® technology in the U.S. and eight other countries (Australia, Canada, Switzerland, Germany, France, Great Britain, Ireland and Italy). These patents and applications provide broad coverage for the production of autologous cancer vaccines. The key protection of OncoVAX®, in addition to considerable expertise protected as trade secrets, is the broad, issued patent protection around the production of autologous, sterile, metabolically active cancer vaccines developed by us. This patent, entitled “Sterile Immunogenic Non-Tumorigenic Tumor Cell Composition and Methods” was issued in 2009 and expires no sooner than 2025. We believe that sterility, with the process we have developed, will be required for any product to reach the US market and likely in any other market. This could result in a regulatory barrier to entry to competitors.
We hold 1 U.S. patent to related technologies and including the sterility patent referred to above, 2 U.S. patents total.
We also rely upon unpatented proprietary know-how and continuing technological innovation and other trade secrets to develop and maintain our competitive position. We hold considerable proprietary expertise related to the OncoVAX® technology, including the production of autologous cancer vaccines. We have brand names for our OncoVAX® products and related technologies, and anticipate filing 5 trademark applications for these and related marks.
All of our research efforts to date are at the pre-clinical or clinical stage of development. We are focused on leveraging our other key assets, including our intellectual property, our scientific team and our facilities, to advance our technologies.
We are an “emerging growth company,” as defined in the Jumpstart Our Business Startups Act, or the JOBS Act. For as long as we continue to be an emerging growth company, we may take advantage of exemptions from various reporting requirements that are applicable to other public companies that are not emerging growth companies, including not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act, reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements and exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and shareholder approval of any golden parachute payments not previously approved. We could be an emerging growth company for up to five years after we make a sale of equity securities pursuant to an effective Securities Act registration statement, although circumstances could cause us to lose that status earlier.
Under the JOBS Act, emerging growth companies can also delay adopting new or revised accounting standards until such time as those standards apply to private companies. We have irrevocably elected not to avail ourselves of this exemption from new or revised accounting standards and, therefore, will be subject to the same new or revised accounting standards as other public companies.
Operating Strategy
We will maintain a cGMP facility and outsource clinical activities to contract research organizations (“CROs”) in an effort to achieve the following:
| 1. | Complete our Autologous Colon Tumor Immunotherapy Validation Experiment (“ACTIVE”) in our pivotal phase IIIb clinical trial of OncoVAX®, an autologous cancer vaccine which prevents the recurrence of stage II colon cancer. The planned trial will closely replicate a successful prior phase IIIa study which study we believe demonstrated a 61% improvement in relative risk of recurrence and a 54% reduction in the relative risk of death in stage II patients as compared to surgery alone. The Company began its preparations for the planned phase IIIb clinical trial in September 2014. The Company also selected RXTRIALS, INC d/b/a OnPoint CRO as its Clinical Research Organization (“CRO”) to provide services of site selection, clinical operations, project management and trial enrollment for our phase IIIb clinical trial. |
| 62 |
| 2. | Develop revenues by establishing licensing arrangements for distribution of OncoVAX® in certain territories. |
| 3. | Investigate the role of OncoVAX® treatment in additional carcinomas with high post-surgical recurrence rates. |
| 4. | Develop diagnostic and therapeutic drug products as well as revenues by exploiting our distinctive Human Monoclonal Antibody (“HuMab”) technology. |
Trends & Outlook
Revenue
To date, the Company has not earned any revenues as the use of OncoVAX® to create cancer related vaccines is still undergoing clinical trials and has not yet received regulatory approval for commercialization and sale.
We are mainly focused on preparing for the initiation of phase IIIb clinical trials relating to OncoVAX® and our research regarding human monoclonal antibodies (HuMabs).
On a long-term basis, we anticipate that our revenue will be derived primarily from global sales of our OncoVAX® product and licensing fees. Commercialization of the OncoVAX® product is dependent on successful completion of a phase IIIb clinical trial and subsequent approval by the FDA.
Research & Development Expenses
Our research and development costs consist of expenses incurred in activities connected with the development of a stage II colon cancer vaccine. These expenses consist primarily of the amortization of intangible assets, cost of conducting clinical trials, salaries and related expenses for personnel, fees paid to professional service providers and, costs of a manufacturing facility in The Netherlands. We expect that research and development expenses will increase in the near term with the onset of the phase IIIb clinical trial.
Clinical Trials
The FDA has requested a second confirmatory, randomized, controlled phase IIIb trial of OncoVAX® in stage II colon cancer and the principal objective of Vaccinogen is to organize a team to conduct this trial. The protocol for this trial, including endpoints and the statistical analysis plan, has been approved by the FDA and is the subject of a Special Protocol Assessment (“SPA”) agreement.
This study will be carried out under a SPA that was negotiated with the FDA in 2010. An SPA granted by the FDA provides a mechanism for the sponsors and the FDA to reach agreement on size, execution and analysis of a clinical trial that is intended to form the primary basis for regulatory approval. The primary endpoint of this pivotal phase IIIb trial is recurrence-free survival (“RFS”) with an interim analysis after 70 “events,” and a final primary analysis three years following completed enrollment. “Events” are incidents of either recurrent disease or death. The study is powered at 90% to detect a 50% improvement in RFS versus resection only for final analysis with an adjustment for interim analysis. The power calculations in the study assume an event rate and distribution that match those observed in the 8701 phase IIIa study. If a robust p-value is achieved at the interim analysis, the Biologics License Application (“BLA”) can be filed with the FDA’s center for Biologics Evaluation and Research (“CBER”). Past clinical trials using the optimal regimen of four inoculations will be accepted as supportive studies during the FDA review of the BLA.
| 63 |
The phase IIIb study will enroll 550 patients, randomized 1:1 to receive surgery alone or surgery plus OncoVAX®. Patients will be followed on a regular schedule to determine whether and when their cancer might recur. The experience with OncoVAX® in the 8701 study demonstrated disease recurrence in stage II patients happened more quickly and more frequently in those who received surgery alone. If the phase IIIb trial replicates the experience of 8701, an interim analysis after 2/3 of the expected events occur should give a clear and statistically significant separation between the Kaplan-Meier curves at that point, and would form the basis for a BLA seeking marketing approval from the FDA.
It is important to emphasize that the FDA has agreed that RFS is the most appropriate endpoint to evaluate a trial in stage II colon cancer. The alternative in cancer studies is often overall survival (“OS”) and is a secondary endpoint in our planned phase III clinical trial. An OS endpoint has several drawbacks in this case. The average age of patients presenting with colon cancer is 65 years. Therefore, deaths from causes other than tumor recurrence will dilute the outcome in both arms. Patients in either arm who experience disease recurrence will receive chemotherapy and other treatments for their metastatic disease. The success of these treatments will dilute the impact of earlier therapies such as OncoVAX® on OS. From a patient perspective, being disease free is clearly a meaningful endpoint.
General and Administrative Expenses
Our general and administrative (“G&A”) expenses consist of the general costs, expenses and salaries for the operation and maintenance of our business. We anticipate that general and administrative expenses will increase as we progress through the clinical phase of development into commercialization.
Critical Accounting Policies
Our consolidated financial statements have been prepared in accordance with generally accepted accounting principles in the United States of America. The preparation of these financial statements requires management to make estimates and assumptions that affect the reported amounts of assets, liabilities, revenues and expenses. Note 3 of the Notes to consolidated financial statements describes the significant accounting policies used in the preparation of the consolidated financial statements. Certain of these significant accounting policies are considered to be critical accounting policies, as defined below.
A critical accounting policy is defined as one that is both material to the presentation of our financial statements and requires management to make difficult, subjective or complex judgments that could have a material effect on our financial condition and results of operations. Specifically, critical accounting estimates have the following attributes: (1) we are required to make assumptions about matters that are highly uncertain at the time of the estimate; and (2) different estimates we could reasonably have used, or changes in the estimate that are reasonably likely to occur, would have a material effect on our financial condition or results of operations.
Estimates and assumptions about future events and their effects cannot be determined with certainty. We base our estimates on historical experience and on various other assumptions believed to be applicable and reasonable under the circumstances. These estimates may change as new events occur, as additional information is obtained and as our operating environment changes. These changes have historically been minor and have been included in the financial statements as soon as they became known. Based on a critical assessment of our accounting policies and the underlying judgments and uncertainties affecting the application of those policies, management believes that our financial statements are fairly stated in accordance with accounting principles generally accepted in the United States, and present a meaningful presentation of our financial condition and results of operations. We believe the following critical accounting policies reflect our more significant estimates and assumptions used in the preparation of our financial statements:
| 64 |
Use of Estimates - Our financial statements have been prepared in accordance with accounting principles generally accepted in the United States and, accordingly, require management to make estimates and assumptions that affect the reported amounts of assets, liabilities, revenues and expenses and the disclosure of contingent assets and liabilities in the financial statements. On an ongoing basis, the Company evaluates the estimates used in recording common stock warrant related liabilities, derivative financial instruments, stock based compensation, and where applicable, the fair value of assets. The Company may base such estimates on various assumptions including information received from valuation consultants which it believes to be reasonable under the circumstances. Actual results could differ from those estimates.
Intangible Assets- Intangible assets consist primarily of the cost of the acquired patents associated with OncoVAX® to be used in research and development and the commercialization cancer related vaccines. The Company has capitalized the cost of OncoVAX® because the Company has identified alternative future research and development efforts for numerous forms of cancer which it intends to pursue and for which management believes will result in commercialization of related vaccines. Acquired patents are carried at cost less accumulated amortization. Amortization is calculated on a straight-line basis, over the estimated useful economic life of the patent, which is 12.6 years for OncoVAX®.
The carrying value of the Company’s intangible assets is $56,022,289 and $62,725,559 as of December 31, 2014 and December 31, 2013. Long-lived assets, including identifiable intangible assets with finite lives, are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of such assets may not be recoverable. When events or circumstances indicate that assets may not be recoverable, the Company prepares cash flows, undiscounted, for each asset or asset group. The Company groups assets for this purpose at the lowest level for which identifiable cash flows exist or are projected to exist. If the sum of the undiscounted cash flows exceeds the carrying value of the asset or asset group, no impairment is deemed present. If the sum of undiscounted cash flows is less than the carrying amount of the asset or asset group, the Company records an impairment charge, if any, for the amount by which the carrying value exceeds the estimated sum of undiscounted cash flows of the asset or asset group.
The Company considers various factors when evaluating whether there are indications that the carrying value of its intangible assets may not be recoverable. Among others, those factors include, adverse changes in marketplace conditions or in the regulatory or legal environment impacting the anticipated use of OncoVAX® and related patents for their intended purpose in research and development and eventual commercialization, significant increases as compared to budget in the level of investment necessary to conduct research and development, clinical trials, and eventual commercialization of OncoVAX®, significant negative variances to budget for operating losses and operating cash flow deficits, and indications of adverse changes in the value of the Company’s equity.
The Company considers these factors no less frequently than the end of each reporting period, including as of December 31, 2014. The Company has noted no adverse change in the demand for the development and commercialization of cancer related vaccines indicated in part by the continuing interest of new and existing investors to fund the Company’s ongoing research and development. The Company has considered the progress of its research and development activities against its operating plan noting the timing for the conduct of its clinical trials and recertification of its production facilities remains consistent with its operating plan. The Company has also evaluated its financial performance and cash flow results noting that the level of investment necessary to conduct its research and development, complete necessary testing for regulatory approval, and begin eventual commercialization of cancer related vaccines remains consistent with its operating plan and financial budgets. The Company also has noted no adverse change in the estimated fair value of the Company based upon values indicated in its ongoing capital raising activities.
| 65 |
Through December 31, 2014, the Company has determined that there have been no events or circumstances that would indicate that the carrying value of its intangible assets is not recoverable.
Fair Value of Financial Instruments - Fair value is defined as the price at which an asset could be exchanged or a liability transferred (an exit price) in an orderly transaction between knowledgeable, willing parties in the principal or most advantageous market for the asset or liability. Where available, fair value is based on observable market prices or parameters or derived from such prices or parameters.
Financial assets recorded at fair value in the accompanying financial statements are categorized based upon the level of judgment associated with the inputs used to measure their fair value. Hierarchical levels, as defined by the new guidance related to fair value measurements and disclosures, and directly related to the amount of subjectivity associated with the inputs to fair valuation of these assets and liabilities, are as follows:
| Level 1- | Inputs are unadjusted, quoted prices in active markets for identical assets at the reporting date. Active markets are those in which transactions for the asset or liability occur in sufficient frequency and volume to provide pricing information on an ongoing basis. | |
| We carry no investments classified as Level 1. | ||
| Level 2 - | Inputs are other than quoted prices included in Level 1, which are either directly or indirectly observable for the asset or liability through correlation with market data at the reporting date and for the duration of the instrument's anticipated life. | |
|
We carry no investments classified as Level 2. | ||
| Level 3- |
Unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities and which reflect management's best estimate of what market participants would use in pricing the asset or liability at the reporting date. Consideration is given to the risk inherent in the valuation technique and the risk inherent in the inputs to the model.
Our Abell Warrants, Round C Warrants, Bridge Loan and Abell Investment Option obligations are considered Level 3. |
For a further discussion regarding fair value measurements, see Note 9 on Fair Value in the Notes to consolidated financial statements.
Accounting for Warrants – We have adopted FASB guidance related to determining whether an instrument or embedded feature is indexed to an entity’s own stock. This guidance applies to any freestanding financial instruments or embedded features that have the characteristics of a derivative, as defined in ASC Topic 815, Derivatives and hedging, and to any freestanding financial instruments that are potentially settled in an entity’s own common stock. As a result, certain of our warrants were considered to be derivatives and were valued using various assumptions as they are recorded as liabilities.
| 66 |
Research and Development Costs – Research and development costs that do not have alternative future use are expensed as incurred. Research and development expenses primarily include the amortization of intangible assets, cost of conducting clinical trials, compensation and related overhead for employees, consultants, facilities costs and the cost of materials purchased for research and development.
Stock-Based Compensation – The Company measures the cost of employee services received in exchange for stock options or restricted stock awards based upon the fair value of the award on the date of the grant. The Company recognizes the estimated grant date fair value of the award as stock-based compensation expense on a straight-line basis over the requisite service period, which is generally the vesting period.
The Company initially measures the cost of awards granted to non-employees based on the fair value of the award on the date of grant however such cost is re-measured at the end of each reporting period until performance is fully satisfied or services are rendered by the non-employee.
The fair value of stock options granted is calculated using the Black-Scholes option-pricing model, which requires the use of subjective assumptions including volatility, expected term, risk-free rate, and the fair value of the underlying common stock. The fair value of non-vested stock awards is determined based upon the estimated fair value of the Company's common stock.
Results of Operations
Consolidated Statements of Operations Data
| Year ended December 31, | ||||||||
| 2014 | 2013 | |||||||
| Revenues | $ | - | $ | - | ||||
| Operating Expenses: | ||||||||
| Research and development | 9,092,072 | 8,687,406 | ||||||
| General and administrative expenses | 5,739,370 | 10,756,150 | ||||||
| Total Operating Expenses | 14,831,442 | 19,443,556 | ||||||
| Loss From Operations | (14,831,442 | ) | (19,443,556 | ) | ||||
| Gain (loss) on Financial Instruments | 7,753,694 | (3,074,600 | ) | |||||
| Interest Expense and Other Expenses | (1,210,840 | ) | (2,062,385 | ) | ||||
| Loss Before Income Taxes | (8,288,588 | ) | (24,580,541 | ) | ||||
| Provision for Income Taxes | (238,217 | ) | — | |||||
| Net Loss | $ | (8,526,805 | ) | $ | (24,580,541 | ) | ||
| 67 |
Revenue
Since inception, we have not earned any revenues as the use of OncoVax® to create cancer related vaccines is still undergoing clinical trials and has not yet received regulatory approval for commercialization and sale.
Operating Expenses – Years Ended December 31, 2014 and 2013
Our operating expenses totaled $14,831,442 and $19,443,556 in the years ended December 31, 2014 and December 31, 2013 respectively. The operating expenses are discussed in further detail below.
Research and Development Expenses
| Year Ended December 31, | Change in 2014 v. 2013 | |||||||||||||||
| 2014 | 2013 | $ | % | |||||||||||||
| Research & Development | $ | 9,092,072 | $ | 8,687,406 | $ | 404,666 | 4.66 | % | ||||||||
Research and development expenses increased to $9,092,072 in the year ended December 31, 2014, or approximately an increase of 4.66%, from $8,687,406 in the year ended December 31, 2013. These expenses include approximately $6,700,000 for amortization of intangibles, related to OncoVax® intellectual property, for the years ended December 31, 2014 and 2013. The increase was primarily attributable to start up expenses associated with the initiation of the phase IIIb clinical trial.
General and Administrative Expenses
| Year Ended December 31, | Change in 2014 v. 2013 | |||||||||||||||
| 2014 | 2013 | $ | % | |||||||||||||
| General & Administrative | $ | 5,739,370 | $ | 10,756,150 | $ | (5,016,780 | ) | -46.64 | % | |||||||
General and administrative expenses decreased to $5,739,370 in the year ended December 31, 2014 from $10,756,150 in the comparable period in 2013. The decrease of $5,016,780, from 2013 to 2014 was attributable to stock based compensation.
The non-cash items are comprised of the estimated value ($5,954,545) of the Abell Option issued with respect to the Abell Investment Agreement in January 2013 as discussed in Note 3 of the consolidated financial statements and the charges ($1,347,318) related to the termination of the Kodiak Capital Group, LLC Equity Line of Credit. These charges were partially offset by a decrease in depreciation of ($54,000).
The non-cash item decreases were partially offset by an increases in cash items such as: public/investor relations ($400,000); legal expenses ($1,800,000); bonuses ($312,000); and corporate governance ($140,000).
| 68 |
Non-operating Income (Expense) – Years Ended December 31 2014 and 2013
Nonoperating income (expense) were $6,542,894 and $5,136,985 in the years ended December 31, 2014 and 2013 respectively. The nonoperating income (expense) are discussed below.
(Gain) Loss on Financial Instruments
| Year Ended December 31, | Change in 2014 v. 2013 | |||||||||||||||
| 2014 | 2013 | $ | % | |||||||||||||
| (Gain) Loss on Financial Instruments | $ | (7,753,694 | ) | $ | 3,074,600 | $ | (10,828,294 | ) | -352.19 | % | ||||||
Recorded gains or losses on Financial Instruments may fluctuate from period to period as determination of fair value is based on Vaccinogen stock values and management’s assessment of the probability of qualifying events which may affect the fair value of the Financial Instrument.
Gains on Financial Instruments were generated due to changes in the estimated fair value during the year ended December 31, 2014. Gains were recorded for the Round C Warrants in the amount of approximately $2,462,000, The Abell Option for $4,224,000, the Abell Warrants of $1,177,000, and losses of ($110,000) on the repayment of 2:1 Bridge loans during the calendar year 2014.
Loss on Financial Instruments was generated due to changes in the estimated fair value during the year ended December 31, 2013. Losses were recorded for the Round C Warrants in the amount of approximately ($453,000), The Abell Option of approximately ($1,438,000), The Bridge Loan ($400,000) and the Abell Warrants ($508,000).
The Company issued additional warrants associated with the January 2013 amendment as a result of the deemed extinguishment of the Abell Loan. The fair value of the warrants ($275,813) is included in the Loss on Financial Instruments. Further discussion of this transaction can be found in Note 9 to the accompanying consolidated financial statements.
Interest and Other Expenses
| Year Ended December 31, | Change in 2014 v. 2013 | |||||||||||||||
| 2014 | 2013 | $ | % | |||||||||||||
| Interest & Other Expenses | $ | 1,210,840 | $ | 2,062,385 | $ | (851,545 | ) | -41.29 | % | |||||||
Interest & Other expense was $1,210,840 for the year ended December 31, 2014, and interest & other expense was $2,062,385 for the comparable period in 2013. The decrease of $851,545 is primarily due to the increase of other income of $500,000 attributable to the extinguishment of a portion of the Organon loan and associated accrued interest of $140,000, as the payment had surpassed the statute of limitations.
Liquidity and Capital Resources
Since our inception, we have financed our operations through the private placement of our securities. During the years ended December 31, 2014 and 2013 we raised gross proceeds of approximately $17,800,000 and $4,200,000, respectively.
| 69 |
| Year Ended December 31, | Change in 2014 v. 2013 | |||||||||||||||
| 2014 | 2013 | $ | % | |||||||||||||
| Net cash used in operating activities | $ | (11,624,987 | ) | $ | (3,718,110 | ) | $ | (7,906,877 | ) | 212.66 | % | |||||
| Net cash used in investing activities | (241,827 | ) | (116,087 | ) | (125,740 | ) | 108.32 | % | ||||||||
| Net cash provided by financing activities | 14,968,623 | 3,731,110 | 11,237,513 | 301.18 | % | |||||||||||
| Effect of foreign exchange rate changes on cash and equivalents | (133,963 | ) | 62,343 | (196,306 | ) | -314.88 | % | |||||||||
| Net increase (decrease) in cash and cash equivalents | $ | 2,967,846 | $ | (40,744 | ) | $ | 3,008,590 | * | ||||||||
Cash & Cash Equivalents
Total cash and cash equivalents were $3,040,942 at December 31, 2014, compared with $73,096 at December 31, 2013, for an increase of $2,967,846. Cash balances will fluctuate from one reporting period to another based on the amount and timing of the issuance of our common stock and warrants for cash.
Net Cash Used in Operating Activities
The net cash used in operating activities for the year ended December 31, 2014 consisted of our net loss of $8,526,805 which was increased by our non-cash adjustments and changes in our operating assets and liabilities of $3,098,182. The net cash used in operating activities for the year ended December 31, 2013 consisted of our net loss of $24,580,541 which was offset by our non-cash adjustments and changes in our operating assets and liabilities of $20,862,431.
The decrease of approximately $23,961,000 in non-cash adjustments and changes in our assets and liabilities for the year ended December 31, 2014 is primarily attributable to the decrease in stock based compensation ($5,552,000); changes in Gain or Loss on Financial Instruments ($10,828,000) and an increase in prepaid accounts of $4,150,000.
Net Cash Used in Investing Activities
We invested $241,827 and $116,087 in the years ended December 31, 2014 and 2013 respectively in purchases of property and equipment. Purchases for both 2014 and 2013 were primarily for improvements to the Emmen facility clean room in preparation for the upcoming phase III clinical trial.
Net Cash Provided by Financing Activities.
Listed below are key financing transactions entered into by us during 2014:
| · | During the year ended December 31, 2014 we raised additional capital of approximately $17,800,000 through the issuance of 3,246,417 Units Round C common stock (each Unit consisting of 1 share of common stock and a common stock purchase warrant to purchase 0.3 shares of common stock). |
| · | The December 31, 2013 principal balance for the Abell Loan of $1,331,217 was fully paid during 2014. |
| · | The remaining obligation for the 2:1 Investors in the amount of $800,000 was paid in December 2014. |
| 70 |
Listed below are key financing transactions entered into by us during 2013:
| · | During the year ended December 31, 2013 we raised additional capital of approximately $4,200,000 through the issuance of 764,578 Units Round C common stock (each Unit consisting of 1 share of common stock and a common stock purchase warrant to purchase 0.3 shares of common stock). |
| · | A principal reduction in the Abell Loan was made in the amount of approximately $469,000. |
Future Liquidity & Needs
We have incurred significant operating losses and negative cash flows since inception. We do not expect to be profitable in the next several years, but rather expect to incur additional operating losses. We have limited liquidity and capital resources and must obtain significant additional capital resources in a timely manner, in order to sustain our product development efforts, for our phase IIIb clinical trial, pursuit of regulatory approvals, acquisition of capital equipment, costs to maintain office and manufacturing facilities, for general and administrative expenses and other working capital requirements. We rely on cash balances and the proceeds from the offering of our securities to fund our operations. As of December 31, 2014 we had approximately $3.0 million in cash which is less than twelve month’s cash required to fund operations. Our current monthly cash requirement is approximately $1,000,000.
We have recurring losses and negative cash flows from operating activities and have significant future commitments, and as a result substantial doubt exists regarding our ability to remain in operation at our current level. The report of BDO USA, LLP, our independent registered accounting firm, with respect to our financial statements at December 31, 2014 and December 31, 2013 contain an explanatory paragraph as to our potential inability to continue as a going concern. Additionally, this may adversely affect our ability to obtain new financing on reasonable terms, in a timely manner, or at all.
Our Unit (Round C) offering of common stock and warrants has been completed as of January 31, 2015. Our only anticipated current source of funding is through our agreement with TIS (as described below), for a committed funding of $80 million. To date, the Company has received $20 million of this funding.
In April 2014, the Company entered into a binding agreement (as amended in August 2014, the “TIS Agreement”) with a Stockholm-based investor group known as The Investment Syndicate (“TIS”) under which TIS members agreed to purchase up to $80 million of the Company’s common stock and warrants, all in up to five closings, subject to certain terms and conditions. To date, a total of $20 million in equity financing has closed following Vaccinogen’s satisfaction of certain closing conditions. The TIS Agreement calls for the remaining $60 million to be paid in three separate closings upon the completion of certain milestones.
| 71 |
The Company has received repeated assurances from a TIS representative that TIS fully intends to meet its obligations under the TIS Agreement. Specifically, on November 11, 2014, the Company received a letter from TIS indicating a desire on the part of TIS to invest the remaining $60,000,000 shortly after consummation of the second closing (with the final $20,000,000 to be held in escrow pending fulfillment of the milestone contained in the TIS Agreement relating to the first patient receiving the first three doses of OncoVax®, with positive results). Since receipt of that letter, an additional $10,000,000 investment has been consummated by a TIS member, bringing the total TIS investment to $20 million, without any further investments from TIS members. In more recent communications from TIS, a TIS representative acknowledged that the Company has met the conditions for funding of at least an additional $20,000,000 and re-confirmed that TIS fully intends to meet its obligations under the TIS Agreement to fund the remaining TIS committed investment notwithstanding any conditions to closing. However, the TIS representative has informed the Company that the timing of TIS funding continues to be dependent upon funds TIS members expect to receive from a third party relating to investments made and services provided by TIS members. Notwithstanding these verbal and written assurances from TIS, the Company has been unable to verify the validity of the TIS assurances, the availability of the funds TIS members anticipate using to satisfy their obligations, the timing of the receipt of the funds, or the reliability of the third party from which TIS members expect to receive funds. As of the date hereof, the TIS members' option to invest an additional $30,000,000 (on top of the $80,000,000 committed investment) has expired.
We do not have sufficient capital to fund our plan of operations over the next twelve months. In order to address our capital needs, including our planned phase IIIb clinical trial, we are actively pursuing additional equity or debt financing, in the form of either a private placement or a public offering. We have been in ongoing discussions with strategic institutional investors and investment banks with respect to such possible offerings. Such additional financing opportunities might not be available to us, when and if needed, on acceptable terms or at all. If we are unable to obtain additional financing in sufficient amounts or on acceptable terms under such circumstances, our operating results and prospects will be adversely affected.
Off-Balance Sheet Arrangements
We do not have any relationships with unconsolidated entities or financial partnerships, such as entities often referred to as structured finance or special purpose entities that would have been established for the purpose of facilitating off-balance sheet arrangements or other contractually narrow or limited purposes. As such, we are not exposed to any financing, liquidity, market or credit risk that could arise if we had engaged in those types of relationships.
Item 7A QUANTITATIVE AND QUALITATIVE DISLOSURES ABOUT MARKET RISK
We are a smaller reporting company as defined by Rule 12b-2 of the Exchange Act and are not required to provide the information required under this item.
| 72 |
Item 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
Please refer to the pages specified hereunder for financial statements and supplementary data.
| 73 |
Item 9.CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
Not applicable.
Item 9A. CONTROLS AND PROCEDURES
Evaluation of Disclosure Controls and Procedures
Our management, with the participation of our chief executive officer and principal financial officer, evaluated the effectiveness of our disclosure controls and procedures pursuant to Rule 13a-15 under the Exchange Act, as of the end of the period covered by this Annual Report on Form 10-K.
Based on this evaluation, our chief executive officer and principal financial officer concluded that, as of December 31, 2014, our disclosure controls and procedures are effective to ensure that information we are required to disclose in reports that we file or submit under the Exchange Act is recorded, processed, summarized, and reported within the time periods specified in the SEC’s rules and forms, and that such information is accumulated and communicated to our management, including our chief executive officer and chief financial officer, as appropriate, to allow timely decisions regarding required disclosure.
Changes in Internal Control over Financial Reporting
There were no changes in our internal control over financial reporting that occurred during the quarter ended December 31, 2014 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
Management’s Report on Internal Control over Financial Reporting
Our chief executive officer and chief financial officer is responsible for establishing and maintaining adequate internal control over financial reporting, as defined in Rule 13a-15(f) of the Exchange Act. Our management conducted an evaluation of the effectiveness of our internal control over financial reporting based on the framework in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission. Based on this evaluation, chief executive officer and chief financial officer concluded that our internal control over financial reporting was effective as of December 31, 2014.
This annual report on Form 10-K does not include an attestation report of our registered public accounting firm regarding internal control over financial reporting. The chief executive officer and chief financial officer’s report is not subject to attestation of our registered public accounting firm pursuant to the Smaller Reporting Company and Emerging Growth Company rules of the Securities and Exchange Commission that permits us to provide only management’s report in this annual report.
Limitations on Effectiveness of Controls and Procedures
In designing and evaluating the disclosure controls and procedures, management recognizes that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving the desired control objectives. In addition, the design of disclosure controls and procedures must reflect the fact that there are resource constraints and that management is required to apply its judgment in evaluating the benefits of possible controls and procedures relative to their costs.
| 74 |
Item 9B. OTHER INFORMATION.
None.
PART III
Item 10. DIRECTORS, EXECUTIVE OFFICERS, AND CORPORATE GOVERNANCE
Directors and Executive Officers
Set forth below is certain information concerning our directors and executive officers:
| Name | Age | Position | ||
| Andrew L. Tussing | [50] | President, Chief Executive Officer, Secretary, Chief Operating Officer, acting Chief Financial Officer, Treasurer and Director | ||
| Michael G. Hanna, Jr., Ph.D. | [78] | Director (Chairman Emeritus) | ||
| Benjamin S. Carson, Sr., MD. | [62] | Director (Chairman) | ||
| Anders Halldin | [56] | Director (Vice Chairman) | ||
| Ronald Kaiser | [61] | Director | ||
| Hakan Edstrom | [65] | Director |
Andrew Tussing is our President, Chief Executive Officer, Secretary, Treasurer, Chief Operating Officer and acting Chief Financial Officer. Mr. Tussing also serves a director of the Company. Mr. Tussing has served as an officer of Vaccinogen since its founding in 2007 Mr. Tussing was appointed Chief Executive Officer and elected to serve on our board of directors in 2014 in connection with the “first closing” of a $10 million dollar investment pursuant to the agreement with The Investment Syndicate (the “TIS Agreement”), as further described in our Forms 8-K filed with the SEC on April 28, 2014 and August 25, 2014.
Mr. Tussing has a track record of building companies and co-founded Vaccinogen along with Dr. Hanna in 2007. In his various roles with the Company, he oversaw the financial and operational management of the Company and led several initiatives; including establishing and maintaining the Dutch manufacturing facility that will make patient-specific doses of OncoVAX® necessary to support the Company’s phase IIIb study.
Previously, Mr. Tussing was active in investment banking activities at 1st BridgeHouse Securities and as Managing Director of Growth5 – a venture capital marketing strategy firm. Prior to that, Mr. Tussing was a co-founder of Milestone International Asset Management, which developed hedge-fund-related solutions for financial advisors. He also served in senior roles at Pershing, a division of the Bank of New York, and at Deutsche Bank’s Alex Brown division where he was Director of Strategic Development, Marketing & Sales for its Correspondent Services business. Mr. Tussing has a BS in Business Administration from the University of Baltimore.
Mr. Tussing’s qualifications to serve on our Board of Directors include his experience as a senior executive at our Company with significant leadership responsibilities, his pivotal role in the founding of our Company, and his vast experience in the financial sector.
| 75 |
Michael G. Hanna, Jr., Ph.D., co-founded the Company in 2007 and has served as Chairman and Chief Executive Officer of the Company. Dr. Hanna is currently a member of our Board of Directors (Chairman Emeritus) since he stepped down as our Chief Executive Officer and Chairman in 2014 in connection with the “first closing” of a $10 million dollar investment pursuant to the TIS Agreement, as further described in our Forms 8-K filed with the SEC on April 28, 2014 and August 25, 2014.
Dr. Hanna is the discoverer and developer of Vaccinogen’s lead product, OncoVAX®, an autologous vaccine designed to elicit a specific immune response against colon cancer cells. Prior to co-founding Vaccinogen, Dr. Hanna founded PerImmune Inc. in 1994, for which he served as President and Chief Executive Officer before it merged with Intracel Corp. in 1998. He continued to work as Chief Scientific Officer and Chairman for Intracel Resources until February 2007, which is an affiliate of Intracel Holdings Corporation, our largest stockholder and affiliate. From 1984 to 1994, he served as Chief Operating Officer of Organon Teknika/Biotechnology Research Institute and Senior Vice President of Organon Teknika Corporation, a subsidiary of Akzo Nobel, The Netherlands. During his tenure there, he developed and obtained approvals for TICE BCG for the treatment of carcinoma in situ (CIS) bladder cancer, which remains the standard of care for prophylaxis of recurrence of superficial bladder cancer and therapy of CIS. Dr. Hanna’s research resulted in over 225 publications in international peer-reviewed journals, and book chapters, and he holds 10 patents related to immunotherapy. Dr. Hanna has been the recipient of numerous honors and awards and has served on many editorial boards, including for Human Vaccines & Immunotherapeutics. Dr. Hanna received his PhD in experimental pathology and immunology from the University of Tennessee.
Dr. Hanna’s qualifications to serve on our Board of Directors include his pivotal role in the founding of our Company and his role in the development OncoVAX®, Vaccinogen’s lead project, as well as his experience and leadership within the biopharmaceutical industry.
Benjamin S. Carson, Sr., MD, is Chairman of the Board of Directors and a member of Vaccinogen’s Medical Advisory Board. Dr. Carson was elected Chairman of the Board of Directors of the Company in connection with the “first closing” of a $10 million dollar investment pursuant to the TIS Agreement, as further described in our Forms 8-K filed with the SEC on April 28, 2014 and August 25, 2014.
Dr. Carson is an Emeritus Professor of Neurosurgery, Oncology, Plastic Surgery and Pediatrics at the Johns Hopkins School of Medicine. In 1984, he was named Director of Pediatric Neurosurgery at Johns Hopkins Children’s Center, a position he retired from in June 2013. Dr. Carson is lauded for major innovations in surgery, including the first intrauterine procedure to relieve pressure on the brain of a hydrocephalic fetal twin, and resurrected the procedure for cerebral hemispherectomy (the removal of half the brain) to alleviate uncontrollable seizures. He made medical history in 1987 when he became the first surgeon to successfully separate occipital craniopagus twins, and was involved in several other subsequent separations. He was appointed by President George W. Bush to serve on the President’s Council on Bioethics in 2004 and in 2008, was awarded the Presidential Medal of Freedom, the nation's highest civilian honor. He has also authored more than 100 neurosurgical publications. He has been awarded more than 60 honorary doctorate degrees and dozens of national merit citations.Dr. Carson sits on the board of directors of numerous organizations, including Kellogg Company, Costco Wholesale Corporation, the Academy of Achievement, and is an Emeritus Fellow of the Yale Corporation, the governing body of Yale University.
Dr. Carson’s qualifications to serve on our Board of Directors include his experience serving on the board of other public companies and his experience and training as a practicing physician and a nationally recognized leader in medicine, which enable him to bring valuable insights to the our board, including through his understanding of the scientific nature of our business and the ability to assist us in prioritizing opportunities for drug development.
| 76 |
Anders Halldin was elected Vice Chairman of the Board of Directors in 2014 in connection with the “first closing” of a $10 million dollar investment pursuant to the TIS Agreement, as further described in our Forms 8-K filed with the SEC on April 28, 2014 and August 25, 2014. In 2005 Mr. Halldin co-founded Malchor AB, a Swedish company that provides business and corporate development along with financing services to internationally-focused companies or projects with potential high return on investment IDuring August 2014, Mr Halldin became a director of a small family company, Swedish Express Financial Services AB, providing financial services to the consumer market primarily in Scandinavia and restarted his private firm, Adakon a consultancy firm.
Mr. Halldin has more than 25 years of experience in IT-development and more than 15 years in building and running businesses. He has developed and delivered systems providing services to retail, institutional and corporate customers worldwide. Mr. Halldin has also overseen market research and planning initiatives for companies looking to enter new global markets.
He has extensive leadership experience as founder and CEO of several Scandinavian technology firms, including the Interbizz Group, a software solution company providing advanced trading in securities software package for the global financial industry; AnyX Systems Integration AB, a sister Company to Upnet, the distributor of Cisco products in Scandinavia; and Telia, the Swedish telecommunication company. Mr. Halldin also designed hardware and software communication systems for Telia, Televerket Radio and Ericsson. He has also completed extensive work in the life science and health care sector in Sweden through research and various business development projects, particularly for the African and Chinese markets.
Mr. Halldin’s qualifications to serve on our Board of Directors include his valuable business, leadership and management experience in a wide range of industries and his unique perspectives on the issues facing our Company.
Ronald W.Kaiser - has served as a member of our Board of Directors since his appointment to our Board effective February 6, 2015. Mr. Kaiser has been a member of the board of directors of Tangoe, Inc.(NASDAQ: TNGO) since January 2009, and has been a member of the board of directors of Acetylon Pharmaceuticals, Inc., a private pharmaceutical company focused on drug development for treating certain cancers, including multiple myeloma, since November, 2012. Mr. Kaiser has served on the board of directors of the Neat Company, a private provider of digital filing systems, since November, 2012 and, from October 2013 through October 2014, he served as its interim CEO. From November 2009 to March 2011, Mr. Kaiser served as Chief Executive Officer and Chairman of the Board of MobileAccess Networks, Inc., a provider of in-building wireless communications equipment. From January 2008 to November 2009 and from March 2011 to October 2013, Mr. Kaiser served as an independent consultant. From January 2007 to December 2007, Mr. Kaiser served as Chief Financial Officer of Sucampo Pharmaceuticals, Inc., a pharmaceutical research and development company. From March 2005 to December 2006, Mr. Kaiser served as Chief Financial Officer of PharmAthene, Inc., a provider of medical products to counter biological and chemical weapons. From April 2003 to January 2005, Mr. Kaiser served as Chief Financial Officer of Air Cargo, Inc., a freight logistics and bill processing provider. Mr. Kaiser also served on the board of directors of Vocus, Inc., a publicly traded provider of software for public relations management, from 2005 through June, 2014, when the company was sold. From September 2003 until its sale in December 2012, he served on the board of directors and as chairman of the audit committee of OPNET Technologies, Inc., a publicly traded provider of solutions for managing applications networks.
| 77 |
Mr. Kaiser’s qualifications to serve on our Board of Directors include his detailed knowledge of accounting issues faced by companies, his experience in corporate finance and executive management and his service as Chief Financial Officer for nine different technology companies.
Håkan Edström is a member of our Board of Directors. Mr. Edstrom has been the President and Chief Operating Officer of Mannkind Corporation since April 2001 and has served as one of its directors since December 2001. Mr. Edstrom was with Bausch &Lomb, Inc., a health care product company, from January 1998 to April 2001, advancing to the position of Senior Corporate Vice President and President of Bausch &Lomb, Inc. Americas Region. From 1981 to 1997, Mr. Edstrom was with Pharmacia Corporation, where he held various executive positions, including President and Chief Executive Officer of Pharmacia Ophthalmics Inc. Mr. Edstrom was educated in Sweden and holds a master's degree in Business Administration from the Stockholm School of Economics.
Mr. Edstrom’s qualifications to serve on our Board of Directors include his business knowledge and experience, including his extensive experience as an executive officer of health care product companies, combined with his business acumen and judgment.
Information about the Board of Directors, Board Committees, Medical Advisory Board and Director Independence
Our Board of Directors currently has six (6) members. Among our current directors, our Board of Directors has determined that Messrs. Carson, Kaiser and Edstrom are “independent” directors as defined under the standards set forth by the NYSE MKT LLC (the “NYSE MKT”). In making this determination, the Board of Directors considered all transactions set forth under “Certain Relationships and Related Transactions” below. During fiscal year 2014 (and until August 22, 2014), Alan Cohen, Daniel Fitzgerald, and Daniel Kane served on our Board of Directors. During fiscal year 2014 (and until August 25, 2014), John Nichols served on our Board of Directors. During their tenure as director during fiscal year 2014, each of Messrs. Nicolis, Fitzgerald, Cohen and Kane were “independent” as defined under the standards set forth by the NYSE MKT.
We have standing Audit, Nominating and Corporate Governance, and Compensation committees. Messrs. Kaiser (Chairman), Halldin and Edstrom are current members of our Audit Committee. We have determined that Mr. Kaiser is an “audit committee financial expert” as such term is defined under the rules and regulations of the Securities Exchange Act of 1934, as amended.
Our Board of Directors has determined that each of Messrs. Kaiser and Edstrom are independent under NYSE MKT listing standards for audit committee members, although Mr. Halldin is not independent under NYSE MKT listing standards for audit committee members. Until his resignation on August 22, 2014, Mr. Kane was the sole member of our Audit Committee and was independent as defined under the standards set forth by the NYSE MKT.
Messrs. Edstrom (Chairman), Carson, and Halldin are members of our Compensation Committee. Our Board of Directors has determined that each of Messrs. Carson and Edstrom are independent under NYSE MKT listing standards for compensation committee members, although Mr. Halldin is not independent under NYSE MKT listing standards for compensation committee members.
Messrs. Halldin (Chairman), Carson, and Kaiser are members of our Nominating and Corporate Governance Committee. Our Board of Directors has determined that each of Messrs. Kaiser and Carson are independent under NYSE MKT listing standards for nominating committee members, although Mr. Halldin is not independent under NYSE MKT listing standards for nominating committee members.
| 78 |
Family Relationships
Andrew Tussing, our President and Chief Operating Officer is married to the daughter of Michael G. Hanna, Ph.D., a Director, Chairman Emertius and Special Advisor to the Chief Executive Officer.
The Medical Advisory Board
Our Medical Advisory Board is comprised of internationally recognized individuals in the fields of oncology and cancer surgery. Our Medical Advisory Board advises and assists our management by reviewing and evaluating our research strategy and research, development and clinical programs. To accomplish this purpose, the Medical Advisory Board will review and monitor the processes and procedures, infrastructure underlying our major discovery and clinical development programs and consists of the following individuals:
Benjamin S. Carson, Sr., M.D. (see above for information on Dr. Carson)
Michael G. Hanna, Jr., Ph.D. (see above for information on Dr. Hanna)
Herbert C. Hoover, M.D., Dr. Hoover serves as a Consulting Medical Advisor for Vaccinogen and has been the one clinician involved in all of the clinical trials of OncoVAX®. Dr. Hoover initiated the original pilot trial of active specific immunotherapy in patients with colon cancer at Johns Hopkins Hospital in 1981. He served in a medical advisory role with Organon Teknika/Biotechnology Research, PerImmune and Intracel.
Dr. Hoover has had numerous honors and awards throughout his career including the Charles B. Thornton Advanced Technology Achievement Award (for the development of tumor-specific human monoclonal antibodies) as well as the Best Doctors in America recognition. He shares multiple patents for the development of monoclonal antibodies and active-specific immunotherapy and has published nearly 100 scientific articles.
Dr. Hoover received his M.D. from the University of Kansas School of Medicine in 1970. His surgical residency was served at the Massachusetts General Hospital and Harvard Medical School from 1970 to 1977 with a Chief Residency in 1978. He was a Clinical Associate in the Surgery Branch of the NCI, NIH in Bethesda from 1972 to 1974.
John S. Macdonald, M.D., professor of medicine, New York Medical College. Previously, Dr. Macdonald has served on the medical staffs at Georgetown University Medical School, University of Kentucky, Temple University New York Medical College and Saint Vincent's Hospital’s Comprehensive Cancer Center where he was chief of medical oncology.
| 79 |
Dr. Macdonald graduated from Harvard University Medical School and is board certified in internal medicine and medical oncology as well as a specialist in gastrointestinal cancer and cancer immunotherapy. Dr. Macdonald is the recipient of the distinguished American Cancer Society Junior Faculty Fellowship as well as having received numerous awards and distinctions including being named in the Best Doctors in America listing and the Good Housekeeping "Best 300 Doctors in America."
Jan Vermorken, M.D., Ph.D., Dr. Vermorken served on the medical staff of oncology, at the University Hospital Vrije Universiteit in Amsterdam, The Netherlands, and is Emeritus Professor of Oncology at the University of Antwerp and past head of the Department of Medical Oncology at the Antwerp University Hospital in Edegem, Belgium, where he still works as a consultant. Dr. Vermorken was principal investigator of the OncoVAX® 8701 phase IIIa clinical trial. Dr. Vermorken’s main field of interest is in gynecological oncology, and head and neck oncology. In addition, he coordinated large clinical trials in breast and colon cancer, including the OncoVAX®’s Phase III trial (8701). His main research areas concern early clinical and pharmacological studies with new drugs, studies on the interaction of chemotherapy and radiation therapy, HPV in various malignancies and immunological approaches.
Dr. Vermorken is a member of the European Organization for Research and Treatment of Cancer (EORTC) Gynecological Cancer Group (GCG) and the EORTC Head and Neck Cancer Group (HNCG). He chaired the EORTC-GCG from 1983 to 1989 and the EORTC-HNCG from 2006 to 2009. He is still an active member with both groups.
He is an officer for ESMO, was a member of the ESMO executive committee during the time that he chaired the ESMO National Representative Committee (1991 – 1996) and the ESMO Education Committee (1996 – 2002) and is a prominent member of the ESMO faculty. He is also a member of the International Gynecological Cancer Society (IGCS), was founding chair of the Gynecological Cancer Intergroup (GCIG) and was Chairman of the Belgian Association of Cancer Research (BACR) from 2003 to 2011, now still being part of its board.
Dr. Vermorken is a member of six editorial boards of international journals, reviewer of 13 cancer journals and author or co-author of more than 500 publications in international journals. Since January 2009, Dr. Vermorken has been Editor-in-Chief of Annals of Oncology. On March 1, 2013 he received the title of Commander in the Order of Leopold for his contributions to oncology.
Code of Ethics
We have adopted a code of ethics that applies to the principal executive officer and principal financial and other persons performing similar functions. Our code of ethics is posted on our website at http://vaccinogeninc.investorroom.com/code-of conduct.
Section 16(a) Beneficial Ownership Reporting Compliance
Section 16(a) of the Securities Exchange Act of 1934, as amended, requires our directors, officers, and stockholders holding more than 10% of our outstanding common stock, to file with the Securities and Exchange Commission initial reports of ownership and changes in beneficial ownership of our common stock. Officers, directors and greater than 10% stockholders are required by SEC regulations to furnish us with copies of all Section 16(a) reports they file. To our knowledge, based solely on review of the copies of such reports furnished to us during the fiscal year ended December 31, 2014, all of the Section 16(a) reports required to be filed by our officers, directors, and greater than 10% stockholders were filed on a timely basis, except that Stephen W. Robinson and Jeanne M. Robinson filed a Form 4 late on August 27, 2014; Daniel H. Fitzgerald filed a Form 4 late on May 1, 2014; John Nicolis filed a Form 4 late on April 30, 2014; Alan Cohen filed a Form 4 late on April 29, 2014; Daniel Kane filed a Form 4 late on April 29, 2014; and Intracel Holdings Corp. filed a Form 4 late on February 14, 2014.
| 80 |
Item 11. EXECUTIVE COMPENSATION
Our compensation program is generally designed to pay all of our employees, including our named executive officers, in ways that support our business objectives to attract and retain talented employees to help grow our business and increase stockholder value.
The following table sets forth the compensation paid to the Chief Executive Officer and our other executive officers for services rendered during the fiscal years ended December 31, 2014, and 2013.
| SUMMARY COMPENSATION TABLE | ||||||||||||||||||||||||||||||||||||
| Name and principle
position | Year | Salary ($) | Bonus ($) | Stock
Awards ($) | Option
Awards ($) | Non-equity
incentive plan compensation ($) | Change
in Pension value And non- Qualified deferred Compensation earnings ($) | All
other compensation ($) | Total ($) | |||||||||||||||||||||||||||
| Andrew Tussing | 2014 | 284,815 | (1) | 250,000 | — | — | — | — | 10,143 | (5) | 544,958 | |||||||||||||||||||||||||
| Chief Executive Officer, Chief Operating Officer, President, Acting Chief Financial Officer, Treasurer | 2013 | 250,000 | (2) | — | — | — | — | — | — | 250,000 | ||||||||||||||||||||||||||
| Michael G. Hanna, | 2014 | 350,041 | (3) | — | — | — | — | — | 29,236 | (6) | 379,277 | |||||||||||||||||||||||||
| Jr., Ph.D. Former Chief Executive Officer (Resigned August 2014) | 2013 | 350,000 | (4) | — | — | — | — | — | — | 350,000 | ||||||||||||||||||||||||||
| (1) | $130,209 was accrued for 2014 and not yet paid; to date a total of $182,015 has been accrued but not paid. |
| (2) | $177,162 was accrued for 2013 and only $1 has been paid to date. |
| (3) | $182,292 was accrued for 2014 and not yet paid; to date a total of $302,490 has been accrued but not paid. |
| (4) | $248,189 was accrued for 2013 and only $127,991 has been paid to date. |
| (5) | Consists of $10,143 legal fee reimbursements. |
| (6) | Consists of $29,236 legal fee reimbursements. |
Outstanding Equity Awards
There were no outstanding equity awards, unexercised options, unvested stock, or equity incentive plan awards as of December 31, 2014 for any of the executive officers named in the Summary Compensation Table above.
| 81 |
Employment Agreements
Prior Employment Agreement with Andrew L. Tussing
Until September 19, 2014, Andrew L. Tussing and the Company were parties to that certain Employment Agreement dated February 1, 2010 (and as previously amended by that certain side letter dated December 21, 2010, the “Prior Tussing Employment Agreement”) under which he served as President and Chief Operating Officer. Under the Prior Tussing Employment Agreement, the Company agreed to pay Mr. Tussing an annual base salary of $250,000, which base salary may be increased (but not decreased) at the discretion of the Board. The base salary (less all amounts required to be withheld under applicable law) will be paid in equal periodic installments in accordance with the Company’s practices, but at least monthly. In addition, Mr. Tussing may receive an annual bonus of up to seventy-five percent (75%) of his annual base salary depending on the satisfaction of performance goals for the calendar year. Fifty-percent (50%) of the bonus will be paid on personal goal achievements and the other fifty percent (50%) will be a function of the Company’s performance for the calendar year. No performance goals were set for Mr. Tussing in 2014.
The Prior Tussing Employment Agreement also provides for the following benefits: (i) the same employee benefits that the Company may provide to similarly-situated executives of the Company; (ii) Pinnacle Care membership (or similar medical concierge membership selected by the Company) for Mr. Tussing and his family at the top membership level as well as health club memberships; (iii) reimbursement for expenses incurred in a calendar year for tax, financial, family office, and legal planning services up to Ten Thousand Dollars ($10,000) per calendar month; and (iv) reimbursement for all necessary business expenses reasonably incurred in connection with his employment.
Current Employment Agreement with Andrew L. Tussing
The Company entered into a written employment agreement with Mr. Tussing, effective September 19, 2014 (the “Tussing Employment Agreement”), under which he will serve as President and Chief Executive Officer of the Company. The Tussing Employment Agreement replaces the Prior Tussing Employment Agreement. Mr. Tussing also currently serves as our acting Chief Financial Officer, Treasurer, Secretary.
The term of Mr. Tussing’s employment under the Tussing Employment Agreement is for an initial period of three (3) years, and may thereafter be extended for additional one (1) year periods (the “Tussing Term”). Pursuant to the Tussing Employment Agreement, Mr. Tussing is entitled to an annual base salary of Three Hundred Seventy Thousand Dollars ($370,000), which base salary may be increased (but not decreased) at the discretion of the Compensation Committee. The base salary (less all amounts required to be withheld under applicable law) will be paid in equal periodic installments in accordance with the Company’s practices, but at least monthly. In addition, Mr. Tussing may receive an annual bonus of up to one hundred percent (100%) of his annual base salary at the discretion of the Compensation Committee, or an annual bonus under a written bonus plan intended to qualify as “performance based compensation” under Section 162(m) of the Internal Revenue Code. The Tussing Employment Agreement provides that the Company will pay Mr. Tussing a one-time bonus of Two Hundred Fifty Thousand Dollars ($250,000) upon the effective date of the Tussing Employment Agreement in consideration of past services. Additionally, the Company shall provide Mr. Tussing with equity-based compensation (a one-time equity catch up grant to equalize Mr. Tussing with the percentage of ownership typical among other founder/CEOs in the industry, plus annual equity grants in the types and amounts that are industry standards, in each case determined by an independent compensation consultant mutually agreeable to the Compensation Committee and Mr. Tussing). In the event of a “change in control”, the Tussing Employment Agreement provides that Mr. Tussing will vest in any outstanding equity based awards granted under the Tussing Employment Agreement.
| 82 |
The Tussing Employment Agreement also provides for the following benefits: (i) the same employee benefits as are provided to the Company’s rank-and-file employees and such enhanced benefits as the Compensation Committee determines to be appropriate; (ii) Pinnacle Care membership (or similar medical concierge membership selected by the Company) for Mr. Tussing and his family at the top membership level as well as health club memberships; (iii) reimbursement for expenses incurred in a calendar year for tax, financial, and legal planning services up to Twenty Thousand Dollars ($20,000) per calendar year (although for 2014, Mr. Tussing will be entitled to additional reimbursement for reasonable legal fees incurred in negotiating and drafting the Tussing Employment Agreement up to a maximum of Ten Thousand Dollars ($10,000)); and (iv) reimbursement for all reasonable business and travel expenses incurred in connection with his employment, including business or first class air travel.
The Tussing Employment Agreement provides Mr. Tussing with the following payments upon termination whereby, if (i) Mr. Tussing resigns with “good reason,” or if we terminate Mr. Tussing’s employment without “cause”, Mr. Tussing shall receive continued payments for the greater of (A) the remainder of the Tussing Term or (B) one (1) year, with each payment equal to two hundred percent (200%) of his Base Salary Periodic Installment (as defined in the Tussing Employment Agreement), at the times set forth in the Tussing Employment Agreement, and the Company shall pay Mr. Tussing that portion, if any, of his base salary and any bonus amounts due that remain unpaid for the period prior to termination, or (ii) if Tussing employment is terminated due to death or disability, Mr. Tussing (or his estate) will be paid that portion of the base salary that remains unpaid for the period prior to the date of death or disability and a lump sum cash payment equal to two (2) times his base salary, and he shall vest in any outstanding equity awards granted under the Tussing Employment Agreement; (iii) if Mr. Tussing is terminated for “cause”, he shall be entitled to that portion, if any, of the base salary and any prior year bonus that remains unpaid for the period to the date of termination; or (iv) if Mr. Tussing terminates employment other than for “good reason”, he shall be entitled to that portion, if any, of the base salary and any bonus amounts due that remain unpaid for the period prior to the date of termination. The Tussing Employment Agreement also contains non-compete/non-solicitation provisions as well confidentiality provisions and provisions relating to the Company’s intellectual property rights.
Prior Employment Agreement with Michael G. Hanna, Jr., Ph.D.
Until September 19, 2014, Michael G. Hanna, Jr., Ph.D. and the Company were parties to that certain Employment Agreement dated February 1, 2010 (and as previously amended by that certain side letter dated December 21, 2010, the “Prior Hanna Employment Agreement”) under which he had served as Chief Executive Officer and Chairman of the Company. Under the Prior Hanna Employment Agreement, the Company agreed to pay Dr. Hanna an annual base salary of $350,000, which base salary may be increased (but not decreased) at the discretion of the Board. The base salary (less all amounts required to be withheld under applicable law) will be paid in equal periodic installments in accordance with the Company’s practices, but at least monthly. In addition, Dr. Hanna may receive an annual bonus of up to seventy-five percent (75%) of his annual base salary depending on the satisfaction of performance goals for the calendar year. No performance goals were set for Dr. Hanna in 2014.
| 83 |
The Prior Hanna Employment Agreement also provides for the following benefits: (i) the same employee benefits that the Company may provide to similarly-situated executives of the Company; (ii) Pinnacle Care membership (or similar medical concierge membership selected by the Company) for Dr. Hanna and his family at the top membership level as well as health club memberships; (iii) reimbursement for expenses incurred in a calendar year for tax, financial, family office, and legal planning services up to Ten Thousand Dollars ($10,000) per calendar month; and (iv) reimbursement for all necessary business expenses reasonably incurred in connection with his employment.
Current Employment Agreement with Michael G. Hanna, Jr., Ph.D.
On September 19, 2014, the Company entered into a written employment agreement with Dr. Hanna (the “Hanna Employment Agreement”), under which he will serve as Special Advisor to the Chief Executive Officer of the Company and a member of the Company’s Medical Advisory Committee. Dr. Hanna will also serve as a director of the Company until immediately prior to TIS funding Eighty Million Dollars ($80,000,000) as contemplated by its agreement with the Company. Upon such funding, Dr. Hanna has agreed to resign from the Board. Notwithstanding the foregoing, Dr. Hanna will serve as Chairman Emeritus of the Board throughout the Hanna Term (as defined below). The Hanna Employment Agreement replaces the Prior Hanna Employment Agreement.
The term of Dr. Hanna’s employment under the Hanna Employment Agreement is for an initial period of three (3) years, and may thereafter be extended for additional one (1) year periods (the “Hanna Term”). Pursuant to the Hanna Employment Agreement, Dr. Hanna is entitled to an annual base salary of Three Hundred Fifty Thousand Dollars ($350,000), which base salary may be increased (but not decreased) at the discretion of the Board of Directors. The base salary (less all amounts required to be withheld under applicable law) will be paid in equal periodic installments in accordance with the Company’s practices, but at least monthly. In addition, Dr. Hanna may receive an annual bonus of up to seventy-five percent (75%) of his base salary at the discretion of the Board of Directors, or an annual bonus under a written bonus plan intended to qualify as “performance based compensation” under Section 162(m) of the Internal Revenue Code.
The Hanna Employment Agreement provides for the following benefits: (i) paid leave, medical leave and other benefits as set forth in the Company’s employee handbook and as otherwise offered to other employees; (ii) Pinnacle Care membership (or similar medical concierge membership selected by the Company) for Dr. Hanna and his family at the top membership level; (iii) reimbursement for expenses incurred in a calendar year for tax, financial, and legal planning services up to Twenty Thousand Dollars ($20,000) per calendar year; and (iv) reimbursement for all reasonable business and travel expenses incurred in connection with his employment, including business or first class air travel.
The Hanna Employment Agreement provides Dr. Hanna with the following payments upon termination whereby, if (i) Dr. Hanna resigns with “good reason,” or if we terminate Dr. Hanna’s employment without “cause”, Dr. Hanna shall receive continued payments for the greater of (A) the remainder of the Hanna Term or (B) one (1) year, with each payment equal to one hundred seventy five percent (175%) of his per-pay period base salary, at the times set forth in the Hanna Employment Agreement, (ii) if Dr. Hanna’s employment is terminated due to death or disability, Dr. Hanna (or his estate) will be paid that portion of the base salary that remains unpaid for the period prior to the date of death or disability and a lump sum cash payment equal to one (1) year of his base salary plus seventy five percent (75%) of his base salary; (iii) if Dr. Hanna is terminated for “cause”, he shall be entitled to that portion, if any, of the base salary and any prior year bonus that remains unpaid for the period to the date of termination; or (iv) if Dr. Hanna terminates employment other than for “good reason”, he shall be entitled to that portion, if any, of the base salary that remains unpaid for the period prior to the date of termination. The Hanna Employment Agreement also contains non-compete/non-solicitation provisions as well confidentiality provisions and provisions relating to the Company’s intellectual property rights.
| 84 |
Compensation of Directors for Fiscal 2014
| Name (1) | Fees Earned or Paid in Cash ($) | Stock Awards (S) | Option Awards ($) | Non-Equity Incentive Plan Compensation ($) | Nonqualified Deferred Compensation Earnings ($) | All Other Compensation ($)(d) | Total ($) | |||||||||||||||||||||
| Dr. Benjamin Carson Sr., MD (2) | $ | 25,000 | - | - | - | - | - | $ | 25,000 | |||||||||||||||||||
| Anders Halldin (2) | $ | 21,000 | - | - | - | - | - | $ | 21,000 | |||||||||||||||||||
| Alan Cohen (3) | $ | 16,667 | - | - | - | - | - | $ | 16,667 | |||||||||||||||||||
| Daniel Fitzgerald (3) | $ | 16,667 | - | - | - | - | - | $ | 16,667 | |||||||||||||||||||
| Daniel Kane (3) | $ | 16,667 | - | - | - | - | - | $ | 16,667 | |||||||||||||||||||
| John Nicolos (3) | $ | 16,667 | - | - | - | - | - | $ | 16,667 | |||||||||||||||||||
| (1) | Mr. Edstrom and Mr. Kaiser were elected to the board in fiscal year 2015, and are therefore not included in this table. |
| (2) | Elected to the board in August 2014; Fees earned may be paid in cash or stock during 2015. |
| (3) | Resigned from the board in August 2014; In lieu of fees earned, stock will be issued in 2015. |
We intend to award each of Messers. Cohen, Fitzgerald, Kane and Nicolis stock under our 2015 Stock Incentive Plan in lieu of cash payments. These stock awards are contingent on each former director completing a release agreement with the Company, as well as an accredited investor questionnaire.
The Board of Directors has set the following cash retainer arrangement for non-employee directors for fiscal 2015 (although payments to Mr. Halldin and Dr. Carson were payable retroactively to September 2014):
| Annual Retainer for each member of the Board | $ | 35,000 | ||
| Annual Retainer for Committee Members | ||||
| Audit Committee | $ | 8,000 | ||
| Compensation Committee | $ | 5,000 | ||
| Nominating and Corporate Governance Committee | $ | 4,000 | ||
| Annual Retainer for Committee Chairs | ||||
| Audit Committee | $ | 15,000 | ||
| Compensation Committee | $ | 10,000 | ||
| Nominating and Corporate Governance Committee | $ | 7,500 | ||
| Annual Retainer for Chairman of the Board | $ | 30,000 | ||
| Annual Retainer for Lead Director | $ | 20,000 |
| 85 |
Retainers shall be pro-rated for the first and last year of board service. Retainers for Anders Halldin and Dr. Benjamin Carson shall be payable retroactively to September 1, 2014, and for Ronald Kaiser and Hakin Edstrom, shall be payable retroactively to their respective Board service inception dates. For the retainerfees earned between September 1, 2014 and April 13, 2015, each of Messrs. Halldin, Carson, Kaiser and Edstrom may elect to the retainer fees to which he is entitled in the form of one of the following: (i) cash at the time such award is earned and payable; or (ii) fully vested shares of common stock, with such shares having a value equivalent to the cash value of such award. For the 2015 retainer fees earned beginning April14, 2015, each director may elect to receive retainer fees to which he is entitled in the form of one of the following: (i) cash at the time such award is earned and payable; (ii) cash deferred in accordance with a deferred compensation plan; (iii) fully vested options to purchase shares of common stock, with such options having a value equivalent to the cash value of such award; (iv) fully vested shares of common stock with such shares having a value equivalent to the cash value of such award; or (v) restricted shares of common stock equal in value to 115% of the cash value of such award (with such shares of common stock subject to two year cliff vesting). The Board of Directors has also authorized the following option grants for director in respect of 2015 service, although as of the date hereof, awards have not yet been granted.
| • | One-time award of options to purchase 30,000 shares of Common Stock, with three-year annual graded vesting for each member of the board. |
| • | Additional one time award of options to purchase 8,750 shares of common stock, with three year annual graded vesting, for each of Anders Halldin and Dr. Benjamin Carson. |
| • | Additional one time award of options to purchase 3,750 shares of common stock, with three year annual graded vesting, for each of Ronald Kaiser and Hakan Edstrom. |
| • | Annual award of options to purchase 15,000 shares of Common Stock, with one-year cliff vesting, for each member of the Board. |
From time to time we may engage certain members of the Board of Directors to perform services on our behalf. In such cases, we compensate the members for their services at rate no more favorable than could be obtained from unaffiliated parties.
Item 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATERS
The following tables sets forth information as of April 3, 2015 as to each person or group who is known to us to be the beneficial owner of more than 5% of our common stock and as to the common stock ownership of each of our named executive officers and directors and of all of our executive officers and directors as a group. As of April 3, 2015 we had 35,896,752 shares of common stock outstanding.
| 86 |
Beneficial ownership is determined under Rule 13d-3 of Exchange Act and generally includes voting and/or investment power over securities. Except in cases where community property laws apply or as indicated in the footnotes to this table, we believe that each stockholder identified in the table below possesses sole voting and investment power over all shares of common stock shown as beneficially owned by the stockholder.
Shares of common stock subject to options or warrants that are currently exercisable or exercisable within 60 days of April 3, 2015 are considered outstanding and beneficially owned by the person holding the options or warrants for the purpose of computing the percentage ownership of that person but are not treated as outstanding for the purpose of computing the percentage ownership of any other person.
As disclosed in prior filings with the SEC, the acquisition by members of The Investment Syndicate may result in a change of control of the Company.
| Name And Address | Number Of Shares of Common Stock Beneficially Owned | Percentage of Common Stock Beneficially Owned | ||||||
| 5% Stockholders: | ||||||||
| Intracel Holdings Corporation (2) 340 N. Westlake Blvd., Suite 260 Westlake Village, CA 91362 | 13,324,864 | 37.12 | % | |||||
| John Nicolis (3) 3203/368 Stkilda Rd. Melbourne 3004 Australia | 3,953,829 | 10.65 | % | |||||
| Alan Cohen(4) 10 Reuten Drive Closter, NJ 07624 | 2,511,362 | 6.99 | % | |||||
| Daniel Kane (5) 340 N. Westlake Blvd., Suite 260 Westlake Village, CA 91362 | 2,511,362 | 6.99 | % | |||||
| Stephen W. Robinson (6) 2555 N. Pearl St. #1709 Dallas, TX 75201 | 3,131,918 | 8.58 | % | |||||
| Executive Officers and Directors:(1) | ||||||||
| Andrew L. Tussing (7) | 421,002 | 1.17 | % | |||||
| Dr. Benjamin Carson, Sr. MD | — | — | ||||||
| Michael G. Hanna, Jr., PhD (8) | 3,926,101 | 10.88 | % | |||||
| Anders Halldin (9) | 13,323,704 | 28.13 | % | |||||
| Donald Kaiser | — | — | ||||||
| Hakan Edstrom | — | — | ||||||
| All directors and executive officers as a group (6 persons) | 17,670,817 | 37.15 | % | |||||
| 87 |
| (1) | The beneficial owners’ address is c/o Vaccinogen, Inc., 949 Fell Street, Baltimore, MD 21231. |
| (2) | As disclosed in Schedule 13G filed with the Securities and Exchange Commission on February 14, 2014. |
| (3) | As disclosed in Schedule 13G filed with the Securities and Exchange Commission on February 14, 2014. Includes presently exercisable warrants to purchase 1,194,748 shares of common stock and 42,336 shares that Mr. Nicolis may acquire pursuant to restricted stock grants. |
| (4) | As disclosed in Schedule 13G filed with the Securities and Exchange Commission on February 14, 2014. Includes 1,729,581 shares of common stock held of record by Intracel Holdings Corporation in respect of Mr. Cohen’s indirect ownership of Intracel Holdings Corporation, and over which Mr. Cohen has shared voting and investment power. Includes 7,272 shares that Mr. Cohen may acquire pursuant to restricted stock grants. |
| (5) | As disclosed in Schedule 13G filed with the Securities and Exchange Commission on February 14, 2014. Includes 1,729,581 shares of common stock held of record by Intracel Holdings Corporation in respect of Mr. Kane’s indirect ownership of Intracel Holdings Corporation, and over which Mr. Kane has shared voting and investment power. Includes 7,272 shares that Mr. Kane may acquire pursuant to restricted stock grants. |
| (6) | As disclosed in the Schedule 13D, Mr. Robinson shares voting and investment power with his wife, Jeanne M. Robinson, and made the Schedule 13D filing as member of The Investment Syndicate, which constitutes a “group” within the meaning of Section 13(d)(3) of the Exchange Act. As disclosed in the Schedule 13D, the total number of shares beneficially owned includes 1,869,159 shares of common stock and warrants to purchase 545,454 shares of common stock purchased in connection with The Investment Syndicate agreement, and 536,007 shares of common stock from prior holdings. The Company’s records indicate that of the 536,007 shares from prior holdings, The Cleaning Authority LLC 401(k) Profit Sharing Plan FBO Stephen W. Robinson (the “Plan”) owns 37,383 shares. The Company’s records also indicate that the plan owns a warrant to purchase 10,908 shares of common stock and Mr. and Mrs. Robinson own warrants to purchase an additional 62,838 shares of common stock. The Company is also aware that Mr. Robinson owns an additional 107,552 Shares of common stock, which he purchased on the open market in 2015. |
| (7) | Includes 20,575 shares that Mr. Tussing may acquire pursuant to exercisable warrants. |
| (8) | Includes 3,642,018 shares which Dr. Hanna shares voting and investment power with his wife, Barbara, and 200,000 shares that Dr. Hanna may acquire pursuant to exercisable warrants (over which he also shares voting and investment power with his wife). |
| (9) | Mr. Halldin currently owns 1,869,160 shares of common stock and exercisable warrants to purchase 545,454 shares of common stock, purchased pursuant to the agreement with The Investment Syndicate. As leader of The Investment Syndicate, Mr. Halldin has an option to purchase an additional 10,909,090 shares of common stock of the Company at any time for a purchase price of $5.50 per share. |
| 88 |
Item 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS
Intracel Holdings Corporation
Prior to our formation, Dr. Hanna, a member of our Board of Directors, was an employee and minority shareholder of Intracel Holdings Corporation (“Intracel”) and until April 23, 2014, had a de-minimis ownership in Intracel. Intracel is our largest stockholder holding approximately 37% of our outstanding stock
On October 10, 2007, we entered into a License Agreement with Intracel (the “Original License Agreement”), for exclusive rights to use the OncoVAX® technology platform. In consideration of the license, we: (i) agreed to issue equity equal to 10% of the fully diluted capitalization of the Company; (ii) assumed $4 million (plus any accrued interest) of liabilities of Intracel to Organon Teknika Corporation (“Organon”) under an October 30, 2007 Letter Agreement between Intacel and Organon, of which $3.0 million remains outstanding as $500,000 which had been past due and payable, has as of November 1, 2014 been written off as the payment had passed the statute of limitations. The outstanding $3 million is due in annual installments of $1 million commencing the first year after first marketing approval of OncoVAX® by the FDA or European Medicines Agency (EMA); (iii) paid $450,000 in cash for settling trade payable related to the OncoVAX® intellectual property; and (iv) granted royalty payments based on future sales of OncoVAX® to Intracel and Organon.
On June 24, 2010, we entered into an Asset Transfer Agreement (the “Transfer Agreement”) with Intracel pursuant to which we acquired all of the intellectual property associated with OncoVAX®. In connection with the Transfer Agreement, we entered into an Amendment to License Agreement (the “License Amendment, and together with the Original License Agreement, the “License Agreement”) upon which significant terms of the License Agreement were terminated except the provision for royalties on future sales and we agreed to certain additional licensing payments. Generally, the royalty provisions on future sales includes an agreement to pay Intracel a running royalty on net sales of the OncoVAX® Program vaccine and related products according to the following schedule: (x) 3% of net sales on the first $350 million of net sales occurring in a calendar year; (y) 4% of that portion of net sales in the calendar year in excess of $350 million and up to and including $750 million and (z) 5% of that portion of net sales in the calendar year in excess of $750 million. The royalty provisions are in effect for as long as the OncoVAX® Program vaccine and related products are being sold by the Company. Additionally, to the extent the Company receives prepayments on future royalties, or other payments that are credible against or in lieu of future royalties (the “Prepaid Royalties), made in respect of OncoVAX® Program vaccine and related products, the Company shall pay a royalty to Intracel at the percentages set forth in the immediately preceding sentence, with net sales being equal to the net sales level that would result in payment of royalties to the Company equal to the Prepaid Royalties. Additionally, the Company agreed to pay Intracel 25% of any and all compensation received by the Company in consideration for licensing or other transfer of rights with respect to certain licensed technology and know-how, but excluding any royalties received on sales that are included under net sales and any Prepaid Royalties.
In consideration of the asset transfer, we also agreed to assume all of Intracel’s obligations to Organon and agreed to enter into a stock exchange agreement with Intracel. The liabilities due Organon assumed by us were (A) the remaining $3.5 million (out of $4 million) in settlement payments due Organon from Intracel (plus any accrued interest from the date of the October 31, 2007 letter agreement based on the prime lending rate in effect on the anniversary of such letter agreement), of which $500,000 had been past due and payable, but as of November 1, 2014 was written off as the payment had passed the statute of limitations. The remaining $3,000,000 is due in equal annual payments of $1 million commencing the first year after the first marketing approval of OncoVAX by the FDA or EMA and (B) a royalty of (1) 10% of the net sales of OncoVAX® until the $3.5 million (and accrued interest) settlement payment is paid and (2) 3% for five years thereafter.
| 89 |
In connection The Investment Syndicate (“TIS”) funding Eighty Million Dollars ($80,000,000) as contemplated by the TIS Agreement (as further described below), the Company and Intracel entered into Letter Agreement, dated August 22, 2014, pursuant to which both parties agreed that effective automatically at the time TIS pays to the Company the aggregate amount of $80,000,000 in accordance with the TIS Agreement, the License Agreement shall terminate and no longer be of any force or effect, and the Company shall no longer have any obligation to pay Intracel any royalties or other payments under the License Agreement. Additionally, in connection with the TIS Agreement, the Company and Intracel entered into that certain Rights of First Refusal Agreement (the “ROFR Agreement”), dated August 22, 2014, under which the Company agreed to offer any new securities (as defined therein) to Intracel prior to offering them to any new investor. This right provides Intracel with the right to purchase a pro-rata amount of such new securities based on the amount of securities held by Intracel prior to the offering of such new securities. These rights do not apply to the sale of certain exempted securities (as defined therein), or any securities being sold to TIS under the TIS Agreement, unless the terms of the TIS investment are revised to lower the price or in a manner materially adverse to Intracel. The rights under the ROFR Agreement expire upon the first to occur of: (a) an initial public offering (as defined therein); (b) a change of control (as defined therein); (c) liquidation, dissolution or winding up of the business affairs of Intracel; or (d) the listing of any capital stock of the Company on an exchange.
TIS Transaction
On April 24, 2014, we entered into a binding agreement (the “Original TIS Agreement”) with TIS pursuant to which TIS agreed to purchase (a) up to 3,636,364 units (“Units”), each Unit consisting of one share of our common stock and one warrant, exercisable for five years, to purchase three tenths (0.3) of a share of common stock at an exercise price of $6.05 per whole share, at $5.50 per Unit, for a total of up to $20,000,000, in our current Unit (Round C) offering, and (b) up to 10,909,091 shares of our common stock at $5.50 per share, for a total of up to an additional $60,000,000, all in up to five closings. Each Unit contains anti-dilution rights providing for the issuance of additional shares. In addition, TIS has the option, but not the obligation, to purchase up to an additional 5,454,545 shares of our common stock at $5.50 per share, for a total of up to $30,000,000 (the “TIS Option”). To date, TIS has consummated the first two closings and the TIS Option has expired. Anders Halldin, a member of our Board of Directors, is a representative of TIS and has purchase securities in the second closing.
TIS’ obligation to purchase any Units (or shares of common stock) under the Original TIS Agreement is subject to the satisfaction of various closing conditions (including board re-constitution and amendments to our agreements with Intracel and the Abell Foundation). On August 22, 2014, we entered into an amendment to the Original TIS Agreement (as amended, the “TIS Agreement”) with TIS, pursuant to which certain material terms of the Original TIS Agreement were amended. Our agreement with TIS is further and fully described in our Forms 8-K filed with the SEC on April 28, 2014 and August 25, 2014.
| 90 |
Director Independence
For a discussion of director independence, pleae refer to “Information about the Board of Directors, Board Committees, Medical Advisory Board, and Director Independence” under “Item 10. Directors, executive Officers and Corporate Governance.”
Item 14. PRINCIPAL ACCOUNTANT FEES AND SERVICES.
Audit Fees
BDO USA, LLP billed us $130,500 in fees for 2014 for annual audit and quarterly reviews and $356,300 in fees during 2013 for the annual audits, quarterly reviews, Form 10 and S-1 filing.
Audit-Related Fees
We did not pay any fees to BDO USA, LLP for audit related services that are not reported under Audit Fees above in 2014 or 2013.
Tax Fees
BDO USA, LLP billed us $28,798 in fees for 2014 with no in fees for 2013 for tax advice, tax compliance and tax planning, related to Section 382, regarding change(s) in ownership.
All Other Fees
We did not pay any fees to BDO USA, LLP for other fees that were not reported under Audit Fees above in 2014 or 2013.
Pre-Approval Policies and Procedures
We have implemented pre-approval policies and procedures related to the provision of audit and non-audit services. Under these procedures, our audit committee pre-approves all services to be provided by BDO USA, LLP and the estimated fees related to these services. The Audit Committee may delegate to one or more designated members of the Audit Committee the authority to grant pre-approvals, provided such approvals are presented to the Audit Committee at its next scheduled meeting. At least annually, the Audit Committee reviews and evaluates the performance, independence and quality control procedures of BDO USA, LLP.
All audit, audit related, and tax services were pre-approved by the audit committee, which concluded that the provision of such services by BDO USA, LLP was compatible with the maintenance of that firm’s independence in the conduct of its auditing functions.
| 91 |
PART IV
Item 15. Exhibits, Financial Statement Schedules
| 1. | FINANICAL STATEMENTS |
| Report of Independent Registered Public Accounting Firm | F-1 |
| Consolidated Financial Statements | |
| Consolidated Balance Sheets | F-2 |
| Consolidated Statements of Operations | F-3 |
| Consolidated Statements of Comprehensive Loss | F-4 |
| Consolidated Statements of Changes in Stockholders’ Equity | F-5 |
| Consolidated Statements of Cash Flows | F-6 |
| Notes to Consolidated Financial Statements | F-7 – F-38 |
| 2. | FINANCIAL STATEMENT SCHEDULES |
All financial statement schedules are omitted because the information is inapplicable and presented in the notes to the financial statements.
| 3. | EXHIBITS |
| Exhibit No. | Description | |
| 2.1 | Asset Transfer Agreement dated June 24, 2012 by and among Vaccinogen, Inc., Intracel Holdings Corporation and the other parties set forth therein.(1) | |
| 3.1 | Articles of Amendment and Restated of Vaccinogen, Inc. dated August 1, 2012.(1) | |
| 3.2 | Bylaws.(1) | |
| 4.1 | Investor Rights Agreement dated June 24, 2010 by and among Vaccinogen, Inc., Intracel Holdings Corporation and the other parties set forth therein.(1) |
| 92 |
| 4.2 | Seventh Amended and Restated Promissory Note dated February 1, 2014 in the amount of $1,038,957.94 made by Vaccinogen, Inc. in favor of The Abell Foundation, Inc.(4) | |
| 4.3 | Common Stock Purchase Warrant issued to The Abell Foundation.(2) | |
| 4.4 | Form of Promissory Note (2012 bridge note offering).(1) | |
| 4.5 | Form of Warrant (2012 Unit Offering).(1) | |
| 4.6 | Warrant, dated December 26, 2014, in favor of Anders Halldin (10) | |
| 4.7 | Warrant, dated January 28, 2015, in favor of Anders Halldin (12) | |
| 10.1 | License Agreement dated October 10, 2007 between Vaccinogen, Inc. and Intracel Holdings Corporation.(1) | |
| 10.2 | Letter Agreement dated October 31, 2007 by and among Intracel Holdings Corporation, Intracel Acquisition Holding Company LLC, OrganonTeknika Corp. and Organon Biosciences International BV.(1) | |
| 10.3 | New Security Agreement dated October 31, 2007 by and among Intracel Holdings Corporation, OrganonTeknika Corp. and Organon Biosciences International BV.(1) | |
| 10.4 | Extension and Second Amendment to Lease dated October 23, 2012 by and between Martens Properties, LLLP and Vaccinogen.(1) | |
| 10.5 | Security Agreement dated October 26, 2011 by and between Vaccinogen, Inc. and The Abell Foundation, Inc.(1) | |
| 10.6 | Stock Exchange Agreement dated as of June 24, 2010 by and between Vaccinogen, Inc. Intracel Holdings Corporation and the other parties thereto.(1) | |
| 10.7 | Agreement dated April 16, 2012 between the Company and Oncology Trials Insight, Inc.(1) | |
| 10.8 | Investment Agreement dated July 18, 2012 between Kodiak Capital Group, LLC and Vaccinogen, Inc.(1) | |
| 10.9 | Investment Agreement dated January 16, 2013 between The Abell Foundation and Vaccinogen, Inc.(1) | |
| 10.10 | Registration Rights Agreement dated July 18, 2012 between Kodiak Capital Group, LLC and Vaccinogen.(1) | |
| 10.11 | Registration Rights Agreement dated June 24, 2010 by and between Vaccinogen, Inc. Intracel Holdings Corporation and the other parties thereto (series B preferred stockholders). (1) | |
| 10.12 | Amended and Restated Registration Rights Agreement by and between Vaccinogen, Inc., and the other parties thereto (series AA preferred stockholders).(1) |
| 93 |
| 10.13 | Advisory Agreement dated May 1, 2009 between Alms and Associates, Inc. and Vaccinogen, Inc.(1) | |
| 10.14 | Agreement dated October 10, 2012 between Marquant Partners and Vaccinogen, Inc.(1) | |
| 10.15 | Employment Agreement dated September 19, 2014, between Vaccinogen, Inc. and Andrew L. Tussing (6)** | |
| 10.16 | Employment Agreement, effective July 9, 2014, between Vaccinogen, Inc. and Michael G. Hanna, Jr.(6)** | |
| 10.17 | Amendment No. 2 to Note and Warrant Purchase Agreement dated January 16, 2013 between Vaccinogen and The Abell Foundation.(1) | |
| 10.18 | Patent Security Agreement dated April 2013 by and between Vaccinogen and The Abell Foundation, Inc.(1) | |
| 10.19 | Amendment No. 3 to Note and Warrant Purchase Agreement dated April 18, 2013 between Vaccinogen and The Abell Foundation.(1) | |
| 10.20 | Placement Agent Agreement with First Liberties Financial. (1) | |
| 10.21 | Extension and Third Amendment to Lease dated April 2013 by and between Martens Properties LLLP and Vaccinogen.(1) | |
| 10.22 | Form of Subscription Agreement (2012-2013 Unit Offering)(1) | |
| 10.23 | First Amendment to Investment Agreement dated July 8, 2013 between Vaccinogen, Inc. and Kodiak Capital Group, LLC.(3) | |
| 10.24 | Amendment No. 7 to Note and Warrant Purchase Agreement between Vaccinogen and The Abell Foundation (4) | |
| 10.25 | Agreement, dated April 23, 2014, between Vaccinogen, Inc. and The Investment Syndicate (7) | |
| 10.26 | Amendment, dated August 22, 2014, to Agreement between Vaccinogen, Inc. and The Investment Syndicate (8) | |
| 10.27 | Termination Agreement, dated August 22, 2014, between Vaccinogen, Inc. and Intracel Holdings Corporation (8) | |
| 10.28 | Agreement, dated August 22, 2014, between Vaccinogen, Inc. and The Abell Foundation (8) | |
| 10.29 | Right of First Refusal Agreement, dated August 22, 2014, between Vaccinogen, Inc. and Intracel Holdings Corporation (8) | |
| 10.30 | Master Services Agreement, dated September 10, 2014, between Vaccinogen, Inc. and RxTrial, Inc. (9) |
| 94 |
| 10.31 | Subscription Agreement, dated December 26, 2014, between Vaccinogen, Inc. and Anders Halldin (10) | |
| 10.32 | Lease Agreement, dated January 6,2015, between Vaccinogen, Inc. and 1001 Fell Street Limited Partnership LLLP (11) | |
| 10.33 | Subscription Agreement, dated January 28, 2015, between Vaccinogen, Inc. and Anders Halldin (12) | |
| 10.34 | Vaccinogen, Inc. 2015 Stock Incentive Plan (13)** | |
| 10.35 | Form of Restricted Stock Agreement (13)** | |
| 10.36 | Form of Non statutory Stock Option Agreement (13)** | |
| 21. | Subsidaries (5) | |
| 23. | Consent of BDO USA, LLP (5) | |
| 31 | Certification of Principal Executive Officer and Principal Financial Officer Required Under Rule 13a-14(a) and 15d-14(a) of the Securities Exchange Act of 1934, as amended. (5) | |
| 32 | Certification of Primcipal Executive Officer and Principal Financial Officer under 18 U.S.C. Section 1350. (5) | |
| 101 | The following financial statements from the Company’s 10-K for the fiscal year ended December 31, 2014 formatted in XBRL: (i) consolidated balance sheet; (ii) consolidated statement of operations; (iii) consolidated statements of comprehensive loss; (iv) consolidated statements of stockholder’s equity; (v) consolidated statements of cash flows; (vi) notes to consolidated financial statements. |
(1) Filed as an exhibit to our Registration on Form 10 filed with the SEC on July 5, 2013 (File No. 000-54997) and incorporated herein by reference.
(2) Filed as an exhibit to our Amendment No. 2 to Registration Statement on Form 10 filed with the SEC on September 12, 2013 (File No. 000-54997) and incorporate herein by reference.
(3) Filed as an exhibit to our Registration Statement on Form S-1 filed with the SEC on July 12, 2013 (File No. 333-189927) and incorporated herein by reference.
(4) Filed as an exhibit to our Current Report on Form 8-K filed with the SEC on March 4, 2014.
(5) Filed herewith.
(6) Filed as an exhibit to our Current Report on Form 8-K filed with the SEC on September 22, 2014
| 95 |
(7) Filed as an exhibit to our Current Report on Form 8-K filed with the SEC on April 28, 2014
(8) Filed as an exhibit to our Current Report on Form 8-K filed with the SEC on August 25, 2014
(9) Filed as an exhibit to our Current Report on Form 8-K filed with the SEC on September 16, 2014
(10) Filed as an exhibit to our Current Report on Form 8-K filed with the SEC on December 31, 2014
(11) Filed as an exhibit to our Current Report on Form 8-K filed with the SEC on January 12, 2015
(12) Filed as an exhibit to our Current Report on Form 8-K filed with the SEC on January 30, 2015
(13) Filed as an exhibit to our Current Report on Form 8-K filed with the SEC on February 20, 2015
** Denotes a management contract or compensatory plan or arrangement.
| 96 |
Report of Independent Registered Public Accounting Firm
Board of Directors and Stockholders
Vaccinogen, Inc.
Baltimore, Maryland
We have audited the accompanying consolidated balance sheets of Vaccinogen, Inc. and subsidiaries as of December 31, 2014 and 2013 and the related consolidated statements of operations, comprehensive loss, changes in stockholders’ equity, and cash flows for the years then ended. These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these consolidated financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audits included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the consolidated financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall consolidated financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of Vaccinogen, Inc. and subsidiaries at December 31, 2014 and 2013, and the results of their operations and their cash flows for the years then ended, in conformity with accounting principles generally accepted in the United States of America.
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 2 to the consolidated financial statements, the Company has incurred recurring losses from operations, and losses are expected to continue in the future. These factors raise substantial doubt about its ability to continue as a going concern. Management’s plans in regards to these matters are also described in Note 2. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
| /s/ BDO USA, LLP |
| Bethesda, Maryland |
| April 15, 2015 |
| F-1 |
| VACCINOGEN INC. AND SUBSIDIARIES |
| Consolidated Balance Sheets |
| December 31, | 2014 | 2013 | ||||||
| Assets | ||||||||
| Current Assets | ||||||||
| Cash and cash equivalents | $ | 3,040,942 | $ | 73,096 | ||||
| Restricted cash | 39,563 | 44,394 | ||||||
| Inventory | 99,250 | 100,150 | ||||||
| Prepaid expenses and other current assets | 2,910,476 | 115,522 | ||||||
| Total Current Assets | 6,090,231 | 333,162 | ||||||
| Long-Term Assets | ||||||||
| Prepaid income taxes | 8,575,810 | - | ||||||
| Property and equipment, net | 393,046 | 198,790 | ||||||
| Intangible assets, net | 56,022,289 | 62,725,559 | ||||||
| Total Long-Term Assets | 64,991,145 | 62,924,349 | ||||||
| Total Assets | $ | 71,081,376 | $ | 63,257,511 | ||||
| Liabilities and Stockholders' Equity | ||||||||
| Current Liabilities | ||||||||
| Accounts payable | $ | 2,709,551 | $ | 3,357,287 | ||||
| Financial instruments | 1,798,184 | 11,794,790 | ||||||
| Accrued compensation | 653,294 | 1,108,541 | ||||||
| Accrued expenses and other liabilities | 374,106 | 639,448 | ||||||
| Notes payable | - | 4,831,217 | ||||||
| Accrued interest | - | 863,637 | ||||||
| Related party payable | - | 54,099 | ||||||
| Total Current Liabilities | 5,535,135 | 22,649,019 | ||||||
| Long-Term Liabilities | ||||||||
| Income tax payable | 8,814,027 | - | ||||||
| Notes payable | 3,000,000 | - | ||||||
| Accrued interest | 856,666 | - | ||||||
| Total Long-Term Liabilities | 12,670,693 | - | ||||||
| Total Liabilities | 18,205,828 | 22,649,019 | ||||||
| Commitments and Contigencies | ||||||||
| Stockholders' Equity | ||||||||
| Preferred stock, $0.0001 par value: 50,000,000 shares authorized; 0 shares issued and oustanding | - | - | ||||||
| Common stock, $0.0001 par value; 200,000,000 shares authorized; 34,962,172 and 31,568,629 shares issued and outstanding at December 31, 2014 and December 31, 2013, respectively. | 3,496 | 3,157 | ||||||
| Additional paid-in capital | 164,844,066 | 143,920,855 | ||||||
| Accumulated other comprehensive loss | (165,888 | ) | (36,199 | ) | ||||
| Accumulated deficit | (111,806,126 | ) | (103,279,321 | ) | ||||
| Total Stockholders' Equity | 52,875,548 | 40,608,492 | ||||||
| Total Liabilities and Stockholders' Equity | $ | 71,081,376 | $ | 63,257,511 | ||||
See accompanying notes to consolidated financial statements.
| F-2 |
| VACCINOGEN INC. AND SUBSIDIARIES |
| Consolidated Statements of Operations |
| Years ended December 31, | 2014 | 2013 | ||||||
| Revenues | $ | - | $ | - | ||||
| Operating Expenses: | ||||||||
| Research and development | 9,092,072 | 8,687,406 | ||||||
| General and administrative expenses | 5,739,370 | 10,756,150 | ||||||
| Total Operating Expenses | 14,831,442 | 19,443,556 | ||||||
| Loss From Operations | (14,831,442 | ) | (19,443,556 | ) | ||||
| Gain (loss) on Financial Instruments | 7,753,694 | (3,074,600 | ) | |||||
| Interest Expense and Other Expenses | (1,210,840 | ) | (2,062,385 | ) | ||||
| Net Loss Before Income Taxes | (8,288,588 | ) | (24,580,541 | ) | ||||
| Provision for income taxes | (238,217 | ) | - | |||||
| Net Loss | $ | (8,526,805 | ) | $ | (24,580,541 | ) | ||
| Net loss available to commmon stockholders | $ | (8,526,805 | ) | $ | (24,580,541 | ) | ||
| Net loss per common share | ||||||||
| Basic | $ | (0.26 | ) | $ | (0.79 | ) | ||
| Diluted | $ | (0.50 | ) | $ | (0.79 | ) | ||
| Weighted average shares | ||||||||
| Basic | 32,760,479 | 31,089,148 | ||||||
| Diluted | 32,760,479 | 31,089,148 | ||||||
See accompanying notes to consolidated financial statements.
| F-3 |
| VACCINOGEN INC. AND SUBSIDIARIES |
| Consolidated Statements of Comprehensive Loss |
| Years ended December 31, | 2014 | 2013 | ||||||
| Comprehensive Loss | ||||||||
| Net loss | $ | (8,526,805 | ) | $ | (24,580,541 | ) | ||
| Foreign currency translation adjustments | (129,689 | ) | 46,953 | |||||
| Total Comprehensive Loss | $ | (8,656,494 | ) | $ | (24,533,588 | ) | ||
See accompanying notes to consolidated financial statements.
| F-4 |
| VACCINOGEN INC. AND SUBSIDIARIES |
| Consolidated Statements of Changes in Stockholders’ Equity |
| Stockholders' Equity | ||||||||||||||||||||||||
| Common Stock | Additional Paid- | Accumulated | Accumulated Other | Total
Stockholders' | ||||||||||||||||||||
| Shares | Amount | In Capital | Deficit | Comprehensive Loss | Equity | |||||||||||||||||||
| Balance, January 1, 2013 | 30,601,700 | $ | 3,060 | $ | 138,118,424 | $ | (78,698,780 | ) | $ | (83,152 | ) | $ | 59,339,552 | |||||||||||
| Issuance of common stock for cash | 796,389 | 80 | 3,253,236 | - | - | 3,253,316 | ||||||||||||||||||
| Conversion of 2012 Bridge Loan for common stock | 170,540 | 17 | 751,544 | - | - | 751,561 | ||||||||||||||||||
| Stock-based compensation | - | - | 46,543 | - | - | 46,543 | ||||||||||||||||||
| Contingent warrants | - | - | 1,751,108 | - | - | 1,751,108 | ||||||||||||||||||
| Other comprehensive income | - | - | - | - | 46,953 | 46,953 | ||||||||||||||||||
| Net loss | - | - | - | (24,580,541 | ) | - | (24,580,541 | ) | ||||||||||||||||
| Balance, December 31, 2013 | 31,568,629 | $ | 3,157 | $ | 143,920,855 | $ | (103,279,321 | ) | $ | (36,199 | ) | $ | 40,608,492 | |||||||||||
| Issuance of common stock for cash | 3,332,795 | 333 | 15,200,356 | - | - | 15,200,689 | ||||||||||||||||||
| Investment option reclassification | - | - | 3,168,224 | - | - | 3,168,224 | ||||||||||||||||||
| Contingent Warrants | - | - | 1,818,642 | - | - | 1,818,642 | ||||||||||||||||||
| Conversion of 2012 Bridge Loan for common stock | 56,075 | 6 | 227,933 | - | - | 227,939 | ||||||||||||||||||
| Stock-based compensation | - | - | 86,943 | - | - | 86,943 | ||||||||||||||||||
| Stock-based compensation warrants and modifications | - | - | 402,147 | - | - | 402,147 | ||||||||||||||||||
| Conversion of payable to common stock | 4,673 | - | 18,966 | - | - | 18,966 | ||||||||||||||||||
| Other comprehensive loss | - | - | - | - | (129,689 | ) | (129,689 | ) | ||||||||||||||||
| Net Loss | - | - | - | (8,526,805 | ) | - | (8,526,805 | ) | ||||||||||||||||
| Balance, December 31, 2014 | 34,962,172 | $ | 3,496 | $ | 164,844,066 | $ | (111,806,126 | ) | $ | (165,888 | ) | $ | 52,875,548 | |||||||||||
See accompanying notes to consolidated financial statements.
| F-5 |
| VACCINOGEN INC. AND SUBSIDIARIES |
| Consolidated Statements of Cash Flows |
| Years ended December 31, | 2014 | 2013 | ||||||
| Cash Flows From Operating Activities | ||||||||
| Net loss | $ | (8,526,805 | ) | $ | (24,580,541 | ) | ||
| Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||
| Depreciation | 51,723 | 24,165 | ||||||
| Gain on extinguishment of debt | (500,000 | ) | — | |||||
| Amortization of intangible assets | 6,703,270 | 6,703,272 | ||||||
| (Gain) Loss on financial instruments | (7,753,694 | ) | 3,074,600 | |||||
| Other stock based expense | 402,147 | 5,954,545 | ||||||
| Stock based compensation - employees | 86,943 | 46,543 | ||||||
| Non-cash interest expense | 1,818,642 | 1,751,108 | ||||||
| Changes in accrued and prepaid taxes | 238,217 | — | ||||||
| Changes in operating assets and liabilities, net: | ||||||||
| Accrued interest | (6,971 | ) | (66,278 | ) | ||||
| Changes in restricted cash | 4,831 | (576 | ) | |||||
| Prepaid expenses and other assets | (2,793,932 | ) | 1,356,113 | |||||
| Accounts payable and accrued expenses and liabilities | (1,349,358 | ) | 2,018,939 | |||||
| Net Cash Used In Operating Activities | (11,624,987 | ) | (3,718,110 | ) | ||||
| Cash Flows From Investing Activities | ||||||||
| Purchases of property and equipment | (241,827 | ) | (116,087 | ) | ||||
| Net Cash Used In Investing Activities | (241,827 | ) | (116,087 | ) | ||||
| Cash Flows From Financing Activities: | ||||||||
| Proceeds from issuance of common stock and warrants net of issuance costs | 17,153,939 | 4,179,893 | ||||||
| Repayments of related party notes payable | (54,099 | ) | 20,000 | |||||
| Repayments of Abell Loan and Bridge Loan | (2,131,217 | ) | (468,783 | ) | ||||
| Net Cash Provided by Financing Activities | 14,968,623 | 3,731,110 | ||||||
| Impact of foreign currency translation on cash and cash equivalents | (133,963 | ) | 62,343 | |||||
| Net Increase (Decrease) in Cash and Cash Equivalents | 2,967,846 | (40,744 | ) | |||||
| Cash and Cash Equivalents, beginning of period | 73,096 | 113,840 | ||||||
| Cash and Cash Equivalents, end of period | $ | 3,040,942 | $ | 73,096 | ||||
See accompanying notes to consolidated financial statements.
| F-6 |
| VACCINOGEN INC. AND SUBSIDIARIES |
| Notes to Consolidated Financial Statements |
1. Organization
The Business
Vaccinogen, Inc. (the “Company” or “Vaccinogen”), a biotechnology Company headquartered in Baltimore, Maryland, was incorporated in the State of Delaware during 2007 for the purpose of developing therapies and vaccines to combat cancer by using the body’s own immune system. On November 23, 2010, the Company changed its domicile from Delaware to Maryland by means of a merger of the Company with and into its wholly owned subsidiary Vaccinogen I, Inc., a Maryland corporation.
On October 10, 2007, the Company entered into a license agreement with Intracel Holdings Corporation (“Intracel”), a related party, for the exclusive and indefinite rights to use the OncoVAX® technology platform. OncoVAX® is an active specific immunotherapy (“ASI”) that uses the patient’s own cancer cells to create a vaccine that in turn is used to block the return of cancer following surgery. On October 23, 2007, Vaccinogen acquired out of bankruptcy, certain tangible assets that had been previously owned and used by Intracel’s wholly owned subsidiary in the Netherlands. These assets will be used to conduct research and development and in the commercialization of OncoVAX® to produce vaccines. In connection with the acquisition of these assets, the Company formed, Vaccinogen BV, for the purposes of continuing development of OncoVAX®. In June 2010, the Company entered into an agreement with Intracel (the “Asset Transfer Agreement”) whereby the Company acquired title to the patents associated with the OncoVAX® (See Note 4).
During September 2014 three new-wholly owned entities were formed by Vaccinogen. The three entities are Vaccinogen (US) R&D, Inc. a Delaware corporation; Vaccinogen International Partners, LP, a Bermuda partnership and Vaccinogen Bermuda, Ltd., a Bermuda company. The purpose of the entities is to facilitate the global commercialization of OncoVAX® and other products as they are developed and marketed by the Company.
| 2. | Going Concern |
The accompanying consolidated financial statements have been prepared assuming the Company will continue as a going concern. The Company has recurring losses and as of December 31, 2014, the Company had an accumulated deficit of approximately $112 million and nominal working capital of less than $1 million. The Company expects to incur additional losses in the future in connection with research and development activities. Since inception, the Company has financed its activities principally from the issuance of equity and debt securities and loans from officers.
The Company’s ability to continue as a going concern is dependent upon the Company’s ability to raise additional debt and equity capital. There can be no assurance that such capital will be available in sufficient amounts or on terms acceptable to the Company. These factors raise substantial doubt about the Company's ability to continue as a going concern. The accompanying consolidated financial statements do not include any adjustments relating to the recoverability of the recorded assets or the classification of liabilities that may be necessary should the Company be unable to continue as a going concern.
| F-7 |
| VACCINOGEN INC. AND SUBSIDIARIES |
| Notes to Consolidated Financial Statements |
The Company does not have sufficient capital to fund its plan of operations over the next twelve months. In order to address its capital needs, including its planned phase IIIb clinical trial, the Company is actively pursuing additional equity financing, in the form of either a private placement or a public offering. The Company has been in ongoing discussions with strategic institutional investors and investment banks with respect to such possible offerings.
In April 2014, the Company entered into a binding agreement (as amended, the “TIS Agreement”) with a Stockholm-based investor group known as The Investment Syndicate (“TIS”) under which TIS agreed to purchase up to $80 million of the Company’s common stock. A total of $20 million in equity financing has closed since April 2014 following Vaccinogen’s satisfaction of certain closing conditions. The remaining $60 million will be paid in three separate closings upon completion of certain milestones.
The Company has received repeated assurances from a TIS representative that TIS fully intends to meet its obligations under the TIS Agreement. Specifically, on November 11, 2014, the Company received a letter from TIS indicating a desire on the part of TIS to invest the remaining $60,000,000 shortly after consummation of the second closing (with the final $20,000,000 to be held in escrow pending fulfillment of the milestone contained in the TIS Agreement relating to the first patient receiving the first three doses of OncoVax, with positive results). Since receipt of that letter, an additional $10,000,000 investment has been consummated by a TIS member without any further investments from TIS members. In more recent communications from TIS, a TIS representative acknowledged that the Company has met the conditions for funding of at least $20,000,000 and re-confirmed that TIS fully intends to meet its obligations under the TIS Agreement to fund the remaining TIS committed investment notwithstanding any conditions to closing. However, the TIS representative has informed the Company that the timing of TIS funding continues to be dependent upon funds TIS members expect to receive from a third party relating to investments made and services provided by TIS members. Notwithstanding these verbal and written assurances from TIS, the Company has been unable to verify the validity of the TIS assurances, the availability of the funds TIS members anticipate using to satisfy their obligations, the timing of the receipt of the funds, or the reliability of the third party from which TIS members expect to receive funds. As of the date hereof, the TIS members' option to invest an additional $30,000,000 (on top of the $80,000,000 committed investment) has expired.
Adequate financing opportunities might not be available to the Company, when and if needed, on acceptable terms or at all. If the Company is unable to obtain additional financing in sufficient amounts or on acceptable terms under such circumstances or if the Company fails to consummate the additional closings under the TIS Agreement, the Company’s operating results and prospects will be adversely affected.
| F-8 |
| VACCINOGEN INC. AND SUBSIDIARIES |
| Notes to Consolidated Financial Statements |
3. Summary of Significant Accounting Policies
Principles of Consolidation
The consolidated financial statements include the accounts of Vaccinogen, Inc. and its direct or indirect wholly owned subsidiaries, Vaccinogen BV (a company incorporated in the Netherlands); Vaccinogen (US) R&D, Inc.; Vaccinogen International Partners, LP; and Vaccinogen Bermuda, Ltd. All intercompany balances and transactions have been eliminated in consolidation.
New Accounting Standards
In September 2014, the Financial Accounting Standards Board (FASB) issued Accounting Standards Update (ASU) 2014-15, Presentation of Financial Statements – Going Concern (Subtopic 205-40): Disclosure of Uncertainties about an Entity’s Ability to Continue as a Going Concern. The amendments in this Update define when and how companies are required to disclose going concern uncertainties, which must be evaluated each interim and annual period. Specifically, the ASU requires management to determine whether substantial doubt exists regarding the entity’s going concern presumption. Substantial doubt about an entity’s ability to continue as a going concern exists when relevant conditions and events, considered in the aggregate, indicate that it is probable that the entity will be unable to meet its obligations as they become due within one year after the date that the financial statements are issued (or available to be issued). If substantial doubt exists, certain disclosures are required; the extent of those disclosures depends on an evaluation of management’s plans (if any) to mitigate the going concern uncertainty. The new standard applies prospectively, to annual periods ending after December 15, 2016, and to annual and interim periods thereafter. Early adoption is permitted. The Company has evaluated the new standard and it is not expected to have an effect on its consolidated financial statements.
Use of Estimates
The preparation of consolidated financial statements in conformity with generally accepted accounting principles in the United States requires management to make estimates and assumptions that affect the reported amounts of assets, liabilities, revenues and expenses and the disclosure of contingent assets and liabilities in its financial statements. On an ongoing basis, the Company evaluates the estimates used in recording common stock warrant related liabilities, derivative financial instruments, stock-based compensation, and where applicable, the fair value of assets. The Company may base such estimates on various assumptions which it believes to be reasonable under the circumstances. Actual results could differ from those estimates.
Cash and Cash Equivalents
The Company considers all highly liquid securities with a maturity of three months or less at acquisition to be cash equivalents. Cash and cash equivalents include demand deposits with financial institutions and at times the amounts may exceed federally insured deposit limits. The Company has not experienced any losses and does not believe it is exposed to any significant credit risk related to demand deposits.
| F-9 |
| VACCINOGEN INC. AND SUBSIDIARIES |
| Notes to Consolidated Financial Statements |
Restricted Cash
Restricted cash represents monies pledged by the Company’s foreign subsidiary for a lease obligation related to the manufacturing facility and to the Dutch government as required for companies with irradiator equipment.
Concentrations of Credit Risk
Financial instruments that potentially subject the Company to concentrations of credit risk consist primarily of cash and cash equivalents. The Company maintains its cash and cash equivalents with high-credit-quality financial institutions in the United States and the Netherlands.
Cash and cash equivalents in the United States are maintained at financial institutions and, at times, balances may exceed federally insured limits. All non-interest bearing cash balances were fully insured to $250,000 per depositor at each financial institution, and noninterest bearing cash balances may again exceed federally insured limits.
Cash and cash equivalents in The Netherlands are maintained at a financial institution and, at times, balances may exceed insured limits. Insurance coverage is limited to €100.000 for all Company accounts at each financial institution.
The Company has not experienced any losses with respect to cash and cash equivalents.
Inventory
Inventory is reported at the lower of cost or market value. The Company analyzes its inventory and writes down inventory that has become obsolete, or has a cost basis in excess of its expected net realizable value and inventory quantities in excess of expected requirements. Inventory primarily consists of a product used in creating vaccines using the OncoVAX® technology platform to be utilized in the planned phase IIIb clinical trial and for research and development activities.
The Company has determined that an obsolescence reserve is not required for the years ended December 31, 2014 and 2013.
| F-10 |
| VACCINOGEN INC. AND SUBSIDIARIES |
| Notes to Consolidated Financial Statements |
Property and Equipment
Property and equipment are recorded at cost and are depreciated or amortized over their estimated useful lives using the straight-line method. Estimated useful lives are as follows:
| Machinery and equipment | 3 – 5 years | |
| Automobile | 3 – 5 years | |
| Furniture and fixtures | 5 years | |
| Computers and software | 3 years | |
| Leasehold improvements | Shorter of lease term or useful life |
Maintenance and repairs are charged to expense as incurred. Major betterments and improvements, which extend the useful life of the underlying assets, are capitalized and depreciated.
Intangible Assets
Intangible assets consist primarily of the cost of acquired patents associated with OncoVAX® to be used in research and development and the commercialization of cancer related vaccines. The Company has capitalized the cost of the acquired patents because the Company has identified alternative future research and development efforts for numerous forms of cancer which it intends to pursue and for which management believes will result in commercialization of related vaccines. Acquired patents are carried at cost less accumulated amortization. Amortization is calculated on a straight-line basis, over the estimated useful economic life of the patent, which is 12.6 years for OncoVAX®.
Impairment of Long-Lived Assets
The Company accounts for the impairment of long-lived assets in accordance with ASC No. 360, Property, Plant and Equipment, which requires that long-lived assets and certain identifiable intangibles are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. Recoverability of assets to be held and used is measured by a comparison of the carrying amount of an asset to future net cash flows expected to be generated by the asset. If such assets are considered to be impaired, the impairment to be recognized is measured by the amount by which the carrying amounts of the assets exceed the fair value of the assets. Assets to be disposed of are reported at the lower of the carrying amount or fair value less costs to sell. No impairment losses have been recorded in the accompanying consolidated statements of operations.
Foreign Currency Translation
The financial statements of foreign subsidiaries are maintained in their functional currency, which generally is the local currency. The assets and liabilities are translated to U.S. dollars using the exchange rate in effect at the balance sheet date. Revenues, expenses and cash flows of these operations are translated using average exchange rates during the reporting period which they occur. The resulting translation adjustments are reflected in other comprehensive loss.
| F-11 |
| VACCINOGEN INC. AND SUBSIDIARIES |
| Notes to Consolidated Financial Statements |
Revenue Recognition
To date, the Company has not earned any revenues as the use of OncoVAX® to create cancer related vaccines still requires additional clinical trials and has not received regulatory approval for commercialization and sale.
Research and Development Expense
Research and development costs are expensed as incurred. Research and development expenses primarily include the amortization of intangible assets, cost of conducting clinical trials, compensation and related overhead for employees, consultants, facilities costs and the cost of materials purchased for research and development.
Stock-Based Compensation
The Company measures the cost of employee services received in exchange for stock options or restricted stock awards based upon the fair value of the award on the date of the grant. The Company recognizes the estimated grant date fair value of the award as stock-based compensation expense on a straight-line basis over the requisite service period, which is generally the vesting period.
The Company initially measures the cost of awards granted to non-employees based on the fair value of the award on the date of grant however such cost is re-measured at the end of each reporting period until performance is fully satisfied or services are rendered by the non-employee.
The fair value of stock options granted is calculated using the Black-Scholes option-pricing model, which requires the use of subjective assumptions including volatility, expected term, risk-free rate, and the fair value of the underlying common stock. The fair value of non-vested stock awards is determined based upon the estimated fair value of the Company's common stock.
Income Taxes
Deferred income tax assets and liabilities are determined based on differences between the financial statements and tax basis of assets and liabilities, as measured using the enacted tax rates, which are expected to be in effect when the differences reverse. Valuation allowances are established when necessary to reduce deferred tax assets to the amount expected to be realized. A full valuation allowance was recorded against its deferred tax assets for the years ended December 31, 2014 and 2013, respectively.
| F-12 |
| VACCINOGEN INC. AND SUBSIDIARIES |
| Notes to Consolidated Financial Statements |
The tax effects of uncertain tax positions are recognized in the financial statements only if the position is more likely than not to be sustained on audit, based on the technical merits of the position. For tax positions meeting the more likely than not threshold, the amount recognized in the consolidated financial statements is the largest benefit that had greater than 50% likelihood of being realized. Management has not identified any uncertain tax positions with the exception of income tax return filing penalties and accordingly has established a liability of $100,000 and $130,000 under ASC 740-10 as of December 31, 2014 and 2013, respectively. It is the Company’s accounting policy to account for ASC 740-10 related penalties and interest by including those items in other liabilities/expenses and not in the income tax provision in the consolidated statements of operations. The Company has identified its U.S. Federal income tax return, its state return in Maryland and Netherlands corporate income tax returns as its major tax jurisdictions. Tax returns for fiscal years 2007 and forward are still open for examination.
Financial Instruments
Warrants Accounted for as Liabilities
Abell Warrants
In October 2011, the Company entered into a borrowing arrangement with The Abell Foundation (“Abell”). In connection with that arrangement, the Company also issued warrants (the “Abell Warrants”) exercisable into common stock of the Company. In February 2012, the Company and Abell amended the agreement to provide for additional borrowings (the “Abell Loan”). Between January 2013 and August 2014, the maturity of the Abell Loan was extended on various occasions and additional warrants were issued. The accounting treatment of these extensions is described within Note 7 to these consolidated financial statements.
In connection with the promissory note issued to The Abell Foundation, the Company granted The Abell Foundation a security interest in its patents related to OncoVAX®. The promissory note was paid in full on August 25, 2014 and Abell released its security interest in Vaccinogen’s patents.
The number of shares issuable pursuant to the Abell Warrants is based upon a fixed amount of $1.1 million divided by 85% of the per share price of stock sold in the next qualifying round of venture capital financing where the total proceeds are at least $35 million. The Abell Warrants have a contractual term of 10 years and were fully vested upon issuance.
As of December 31, 2014 and 2013, the estimated fair value of the Abell Warrants was $458,537 and $1,615,835, respectively. The Company recorded a gain of approximately $1.2 million and a loss of $784,030 for the years ended December 31, 2014 and 2013, respectively.
| F-13 |
| VACCINOGEN INC. AND SUBSIDIARIES |
| Notes to Consolidated Financial Statements |
The Abell Warrants represent a fixed obligation that is to be settled through the issuance of a variable number of shares of the Company’s common stock. Consistent with the provisions of ASC Topic 480, Distinguishing Liabilities from Equity, the Company has concluded that the Abell Warrants should be accounted for as a liability. The Company is required to record the Abell Warrants at their estimated fair value at the end of each reporting period, with changes in the estimated fair value recorded in the consolidated statements of operations as a component of Loss on Financial Instruments.
Abell Investment Option
Accounting for Warrants – The Company has adopted FASB guidance related to determining whether an instrument or embedded feature is indexed to an entity’s own stock. This guidance applies to any freestanding financial instruments or embedded features that have the characteristics of a derivative, as defined in ASC Topic 815, Derivatives and hedging, and to any freestanding financial instruments that are potentially settled in an entity’s own common stock. As a result, certain warrants were considered to be derivatives and were valued using various assumptions as they are recorded as liabilities.
On January 16, 2013, the Company entered into an investment agreement with Abell under which Abell was granted an option to acquire up to $5.0 million of common stock of the Company (the “Abell Option”). On August 22, 2014 the Company entered into a letter agreement with Abell (the “Abell Amendment”), pursuant to which Abell agreed to either (a) purchase up to 909,091 shares of the Company’s common stock at $5.50 per share, for a total of up to $5,000,000, on or before the 14th day following Abell’s receipt of written notice (together with such further related information and documentation as Abell may reasonably require) that both (i) the first patient has been given three doses of OncoVAX, with positive results (Delayed-Type Hypersensitivity), in the next phase IIIb trial and (ii) the aggregate investment by TIS of at least $40,000,000 for the purchase of our securities in accordance with the TIS Agreement has occurred or (b) surrender its investment option if the conditions above have occurred and Abell chooses not to invest within such 14 day period. TIS executed the Abell Letter Agreement for the sole purpose of affirming our commitments to (x) relocate our offices to Baltimore, Maryland within 12 months of the closing of a financing of at least $35 million and (y) to construct and maintain our next and principal manufacturing facility in Baltimore, Maryland.
As of December 31, 2014 and 2013, the estimated fair value of the Abell Option was $0 and $7,392,528, which the Company has recorded a liability. The Company recorded a gain of $4,224,304 and a loss of $1,437,983 for the years ended December 31, 2014 and 2013, respectively.
The Abell Investment Agreement was determined to no longer be classified as a liability as of August 22, 2014, due to changes in terms and conditions of the instrument and was reclassified to permanent equity in the amount of approximately $3.2 million.
| F-14 |
| VACCINOGEN INC. AND SUBSIDIARIES |
| Notes to Consolidated Financial Statements |
Derivative Financial Instruments
The Company may enter into transactions that represent free-standing or embedded derivative financial instruments as those terms are defined in ASC Topic 815 Derivatives and Hedging (“Topic 815”). The Company records the estimated fair value of derivative financial instruments in its consolidated balance sheets and records changes in the estimated fair value of derivative financial instruments as income or expense in its consolidated statements of operations.
Round C Warrants
From October 2012 through December 2013 and then again from January 2014 through December 31, 2014, the Company issued warrants to certain investors in the common stock of the Company (the “Round C Warrants”). Round C Warrants to acquire 339,966 shares of common stock were issued through December 2013 and Round C Warrants to acquire 990,281 shares of common stock were issued during the year ended December 31, 2014. The Round C Warrants have an exercise price of $6.05, a contractual term of 5 years and were fully vested upon issuance.
As of December 31, 2014 and 2013, the estimated fair value of the Round C Warrants was $1,359,647 and $1,796,427, respectively. The Company recorded a gain of $2,462,093 and a loss of $453,088 for the years ended December 31, 2014 and 2013, respectively.
2012 Bridge Loan
Between April 2012 and October 2012, the Company entered into transactions with various investors which resulted in the Company raising $1,019,000 from the issuance of unsecured notes payable (collectively the “Bridge Loan”). The Bridge Loan has no contractual maturity date, and is repayable only in the event that the Company closes on a future round of equity financing which results in gross proceeds of at least $20 million. If the Company fails to raise sufficient additional capital, there is no obligation to pay interest or repay any amount borrowed under the Bridge Loan. The Company did meet the $20 million threshold upon receipt of the second tranche of funding under the TIS Agreement and at that time the Company repaid an amount to the investors equal to 2 times the amount originally raised.
The Company has classified the Bridge Loan as a derivative financial instrument, as it met the qualifying criteria of ASC Topic 815 Derivatives and Hedging (“Topic 815”) including the contractual terms whereby the Company can be required to settle its obligation under the Bridge Loan by transferring cash to investors if and only when sufficient additional capital is raised. As of December 31, 2014 and 2013, the estimated fair value of the liability associated with the Bridge Loan was $0 and $990,000 respectively, which has been recorded and included in financial instruments in the accompanying consolidated balance sheets. The Company recorded a loss of $80,000 and $389,500 for the years ended December 31, 2014 and 2013, respectively.
| F-15 |
| VACCINOGEN INC. AND SUBSIDIARIES |
| Notes to Consolidated Financial Statements |
The changes in the estimated fair value have been classified in Gain (Loss) on Financial Instruments in the accompanying consolidated statements of operations for the years ended December 31, 2014 and 2013.
Net Loss Per Share
Basic loss per share is determined by dividing the loss attributable to common stockholders by the weighted-average number of common shares outstanding during the period, without consideration of common stock equivalents. The following common stock equivalents were excluded in the calculation of diluted loss per share because their effect would be anti-dilutive:
| 2014 | 2013 | |||||||
| Stock Option Pool | 177,000 | 77,000 | ||||||
| Abell Investment Option | 909,091 | 934,579 | ||||||
| Convertible debt | - | 73,421 | ||||||
| Restricted stock awards | 179,969 | 129,846 | ||||||
| Warrants | 4,759,065 | 3,310,378 | ||||||
Dilutive loss per share is determined by dividing loss attributable to common stockholders by the weighted-average number of common shares outstanding during the period, without consideration of common stock equivalents. The net loss available to common shareholders is adjusted for gains on financial instruments, as its effect would be anti-dilutive.
| F-16 |
| VACCINOGEN INC. AND SUBSIDIARIES |
| Notes to Consolidated Financial Statements |
| Year ended December 31, 2014 | ||||||||||||
| Weighted | Loss | |||||||||||
| Average | Per | |||||||||||
| Net Loss | Shares | Share | ||||||||||
| Basic loss per share | $ | (8,526,805 | ) | 32,760,479 | $ | (0.26 | ) | |||||
| Gain on derivatives | (7,753,694 | ) | ||||||||||
| Dilutive loss per share | $ | (16,280,499 | ) | 32,760,479 | $ | (0.50 | ) | |||||
| Year ended December 31, 2013 | ||||||||||||
| Weighted | Loss | |||||||||||
| Average | Per | |||||||||||
| Net Loss | Shares | Share | ||||||||||
| Basic loss per share | $ | (24,580,541 | ) | 31,089,148 | $ | (0.79 | ) | |||||
| Gain on derivatives | 0 | |||||||||||
| Dilutive loss per share | $ | (24,580,541 | ) | 31,089,148 | $ | (0.79 | ) | |||||
4. Agreements with Intracel
License Agreement
On October 10, 2007, the Company entered into an agreement (the “License Agreement”) with Intracel Holdings Corporation (“Intracel”), for the exclusive and indefinite rights to license and use the OncoVAX® technology platform. OncoVAX® is an active specific immunotherapy (“ASI”) that uses the patient’s own cancer cells to block the return of cancer following surgery. In exchange for the rights to OncoVAX®, the Company (i) agreed to issue equity securities equal to 10% of the fully diluted capitalization of the Company, (ii) assumed liabilities of Intracel to Organon Teknika Corporation (“Organon”) totaling $4.0 million under an October 31, 2007 Letter Agreement between Intracel and Organon, (iii) agreed to pay $450,000 in cash for settling trade payable related to the OncoVAX intellectual property, and (iv) agreed to make royalty payments to Intracel based on future sales of OncoVAX®. The terms of the securities issued to Intracel provided Intracel with anti-dilution rights with respect to its 10% ownership interest. The License Agreement also contained a provision such that if the Company obtained specified levels of financing in a specified time period, that title to OncoVAX® would transfer to the Company without further consideration. If the Company did not reach the specified levels of financing in the specified period of time, Intracel could cancel the License Agreement and could re-purchase the rights to OncoVAX®. The Company did not obtain the necessary financing in the period specified.
| F-17 |
| VACCINOGEN INC. AND SUBSIDIARIES |
| Notes to Consolidated Financial Statements |
In connection with the License Agreement, the Company issued 1,506,750 shares of common stock valued at approximately $984,000 and assumed liabilities with an estimated value of $4,450,000. The Company estimated the fair value of the liabilities assumed based upon their face amount as these liabilities were due currently and on demand.
On August 22, 2014 Intracel and Vaccinogen entered into an Agreement, whereby the License Agreement will terminate and no longer be of any force or effect once TIS invests in Vaccinogen the aggregate amount of $80,000,000. Vaccinogen shall no longer, from that point, have any obligation to pay to Intracel any royalties or other payments under the Original License Agreement and/or the License Amendment.
Asset Transfer Agreement and Stock Exchange Agreement
As a result of the Company’s inability to raise the necessary capital under the License Agreement, the Company and Intracel negotiated amended terms to the License Agreement. On June 24, 2010, the Company and Intracel entered into the Asset Transfer Agreement pursuant to which the title to all of the intellectual property associated with OncoVAX® was transferred to the Company. Under the Asset Transfer Agreement, the Company also agreed to exchange the previously issued common stock and Series AA preferred stock representing a 10% interest in the Company for shares of its Series B preferred stock equal to a 20% interest in the Company on a fully diluted basis.
The terms and conditions of the Series B preferred stock provided Intracel with anti-dilution rights with respect to its 20% ownership interest. In addition, the Company agreed that Intracel’s ownership position (and corresponding anti-dilution rights) would increase to 50% upon failure of the Company to meet certain defined milestones, which included but were not limited to, the Company attaining specified levels of additional financing.
The Company accounted for the acquisition of the rights to the OncoVAX® technology platform under the License Agreement in 2007 and the Asset Transfer Agreement in 2010 as an asset acquisition in accordance with ASC Topic 805, Business Combinations. Furthermore, as described in Note 1 to these consolidated financial statements, and in accordance with ASC Topic 730, Research and Development, the Company has capitalized the cost of acquiring the rights to OncoVAX® technology platform as these rights represent intangible assets to be used in research and development activities and for which future alternative uses exist.
In June 2010, in connection with the Asset Transfer Agreement, the Company issued 3,452,766 shares of Series B preferred stock, with an estimated value of approximately $16.8 million. The Company estimated the fair value of the common stock issued pursuant to the License Agreement and the Series B Preferred Stock issued pursuant to the Asset Transfer Agreement by considering various commonly accepted valuation techniques, including the income and market approaches.
| F-18 |
| VACCINOGEN INC. AND SUBSIDIARIES |
| Notes to Consolidated Financial Statements |
The Company ultimately relied on the income approach, specifically, the discounted cash flow method, to estimate the value of the Company’s equity. The Company further utilized commonly used option pricing techniques to estimate the fair value of the various equity classes. The use of the income approach, and specifically the discounted cash flow method, requires management to make significant assumptions about the future level and timing of revenues, direct and indirect costs associated with continued research and development, the conduct of clinical trials, and the production and commercialization of the intended cancer vaccines. The discounted cash flow method also requires the estimation of discount rates used to reflect the risk inherent in the projected cash flows, the terminal growth rate, among other factors.
The Company did not meet these milestones and consequently, in December 2010, was required to increase Intracel’s total ownership interest in the Company to 50% through the issuance of additional shares of Series B preferred stock.
In December 2010, the Company issued 10,973,612 additional shares of Series B preferred stock to Intracel when the Company failed to meet certain conditions of the Asset Transfer Agreement. The estimated value of those additional shares of approximately $63.1 million was accounted for as additional consideration and is included in the total cost of acquiring the OncoVAX® technology platform.
All shares of Series B preferred stock were converted to common stock in 2012.
As of April 10, 2015 Intracel directly owns approximately 37% of the Company on a fully diluted basis, and all directors and executive officers of Vaccinogen collectively own approximately 37% of the Company on a fully diluted basis.
5. Property and Equipment
Property and equipment consisted of the following:
| December 31, | 2014 | 2013 | ||||||
| Machinery and equipment | $ | 740,042 | $ | 903,964 | ||||
| Leasehold improvements | 258,505 | - | ||||||
| Furniture and fixtures | 31,764 | 35,690 | ||||||
| Computers and software | 119,473 | 1,929 | ||||||
| 1,149,784 | 941,583 | |||||||
| Less accumulated depreciation | $ | (756,738 | ) | $ | (742,793 | ) | ||
| $ | 393,046 | $ | 198,790 | |||||
Depreciation expense was $51,837 and $24,165 for the years ended December 31, 2014 and 2013, respectively.
| F-19 |
| VACCINOGEN INC. AND SUBSIDIARIES |
| Notes to Consolidated Financial Statements |
6. Intangible Assets
Intangible assets consist of the capitalized costs associated with the acquisition of patents related to OncoVAX® (the “Intellectual Property”) and the costs associated with website development and domain names.
Intangible assets by major asset class were as follows at December 31, 2014:
| Gross Carrying Amount | Accumulated Amortization | Net Carrying Amount | ||||||||||
| Intellectual Property | $ | 84,481,856 | (28,490,967 | ) | $ | 55,990,889 | ||||||
| Other Intangible Assets | 121,944 | (90,544 | ) | $ | 31,400 | |||||||
| $ | 84,603,800 | (28,581,511 | ) | $ | 56,022,289 | |||||||
Intangible assets by major asset class were as follows at December 31, 2013:
| Gross Carrying Amount | Accumulated Amortization | Net Carrying Amount | ||||||||||
| Intellectual Property | $ | 84,481,856 | (21,792,439 | ) | $ | 62,689,417 | ||||||
| Other Intangible Assets | 121,944 | (85,802 | ) | 36,142 | ||||||||
| $ | 84,603,800 | (21,878,241 | ) | $ | 62,725,559 | |||||||
The amortization expense for intangible assets was approximately $6.7 million for the years ended December 31, 2014 and 2013. The weighted average amortization period for intangible assets was 12.6 years.
The estimated future amortization relating to all intangible assets that are recorded in the consolidated balance sheets as of December 31, 2014 is as follows:
| F-20 |
| VACCINOGEN INC. AND SUBSIDIARIES |
| Notes to Consolidated Financial Statements |
| Years ending December 31, | ||||
| 2015 | $ | 6,702,516 | ||
| 2016 | 6,702,516 | |||
| 2017 | 6,702,516 | |||
| 2018 | 6,702,516 | |||
| 2019 | 6,702,516 | |||
| Thereafter | 22,509,709 | |||
| $ | 56,022,289 | |||
7. Prepaid Expenses and Other Current Assets
Prepaid expenses and other current assets consisted of the following:
| December 31, | 2014 | 2013 | ||||||
| Prepaid Clinical Trial expense | $ | 2,477,571 | $ | - | ||||
| Prepaid Other | 432,905 | 115,522 | ||||||
| $ | 2,910,476 | $ | 115,522 | |||||
The prepaid balance for 2014 consists primarily of payments made under the Company’s contract with RXTRIALS, Inc. d/b/a OnPoint CRO of approximately $2.4 million.
8. Notes Payable
| December 31, | 2014 | 2013 | ||||||
| Organon Obligation | $ | 3,000,000 | $ | 3,500,000 | ||||
| Abell Loan | - | 1,331,217 | ||||||
| $ | 3,000,000 | $ | 4,831,217 | |||||
Organon Obligation
Organon, currently owned by Merck & Co, Inc., manufactures a key component used with the OncoVAX® technology. In 2007, in conjunction with the Agreement with Intracel, the Company assumed $4.0 million of related liabilities from Intracel due to Organon (“Organon Obligation”). Of the $4.0 million due to Organon, $500,000 was paid at the time of the Agreement. The remaining $3.5 million was due in installments with an additional $500,000 (plus accrued interest) payable the first year but no later than one year after the agreement date of October 31, 2007. Organon may elect to receive this first $500,000 installment in stock. Commencing one year after the earlier of the first marketing approval of OncoVAX® by the United States Food and Drug Administration or the European Medicines Agency or October 31, 2007, Vaccinogen would make an annual payment of $1.0 million to Organon until repayment of the entire liability amount. The obligation accrued interest based on a simple annual interest rate based on the US prime lending rate in effect on the anniversary date of the agreement, or October 31, 2008, which was 4.00%. Interest expense under this agreement was approximately $136,666 and $96,314 for the year ended December 31, 2014 and 2013, respectively. The accrued interest on this obligation is shown on the consolidated Balance Sheet as accrued interest.This obligation was secured by the OncoVAX® Intellectual Property.
| F-21 |
| VACCINOGEN INC. AND SUBSIDIARIES |
| Notes to Consolidated Financial Statements |
While the Company did not pay the installment due one year after the Agreement, no event of default has been declared by Organon or its successors including Merck & Co, Inc. and the statute of limitations expired on November 1, 2014. As of that date, the $500,000 installment due was included in Other Income and the related accrued interest of $140,000 was recorded as a reduction in interest expense. Since payments are not subject to future events which will not occur within the next twelve months, the remaining principal and accrued interest has been classified as a long term liability in the accompanying consolidated balance sheets.
Abell Loan
On October 26, 2011, the Company obtained a $1.5 million working capital loan from The Abell Foundation Inc. (“Abell”). The Abell Loan was originally due on April 26, 2012, with 8% simple interest accruing and payable on the maturity date. On February 16, 2012, the Company received an additional $300,000, thereby increasing the amount outstanding to $1.8 million.
The Abell Loan was repaid in full on August 25, 2014.
Debt, Modifications and Extinguishments
The Abell Loan underwent several amendments which were treated either as debt modifications or debt extinguishments, with the most recent being the extension of the maturity date of the loan to August 26, 2014. No costs or expenses were incurred by the Company in connection with the August 2014 extension.
The July 2013 amendment required the issuance of a fixed number of shares at various points in time known as Contingent Share Warrants. The Contingent Share Warrants are not considered a derivative instrument as they are considered indexed to the Company’s own stock as defined by ASC 815-40. As a result, the value assigned to the Contingent Share Warrants has been classified within stockholders’ equity. The outstanding debt was not repaid in full December 31, 2013 and 300,000 warrants were issued to Abell. The warrants were valued using the Black-Scholes method, and for the year ending December 31, 2014 approximately $0.4 million was included in interest expense.
| F-22 |
| VACCINOGEN INC. AND SUBSIDIARIES |
| Notes to Consolidated Financial Statements |
Contingent Share Warrants were issued and valued using the Black-Scholes method, and for the years ended December 31, 2014 and 2013, $1,818,642 and $1,751,108 was included in interest expense and other expenses, respectively.
The Company recorded interest expense related to the Abell Loan of approximately $48,000 and $133,000 for the years ended December 31, 2014 and 2013, respectively.
9. Fair Value Measurements
Fair value is the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction occurring in the most advantageous market. The Company determines fair value based on a hierarchy that priorities valuation techniques used to measure fair value based on observable and unobservable inputs. Observable inputs reflect market data obtained from independent sources. Unobservable inputs reflect assumptions based on the best information available.
The three levels of the fair value hierarchy are:
| Level 1 — | Inputs are quoted prices for identical assets or liabilities in an active market |
| Level 2 — | Inputs include quoted prices in active markets for similar assets and liabilities, quoted prices for identical or similar assets or liabilities in markets that are not active, inputs other than quoted prices that are observable for the asset or liability (interest rates and yield curves), and inputs that are derived principally from or corroborated by observable market data correlation or other means |
| Level 3 — | Inputs that are unobservable and significant to the fair value measurement |
The Company is required to record or disclose the fair value of certain assets and liabilities. The fair value guidance described above is used in measuring and recording the fair value of the liability associated with the Abell Warrants, and the fair value of the financial derivatives including the Round C Warrants and the Bridge Financing. This fair value guidance also applies to the disclosure of the fair value of financial instruments not otherwise recorded in the Company’s consolidated balance sheet at fair value.
The Company’s financial instruments measured on a recurring basis using fair value estimates are as follows:
| F-23 |
| VACCINOGEN INC. AND SUBSIDIARIES |
| Notes to Consolidated Financial Statements |
| December 31, 2014 | ||||||||||||||||
| Description | Total | Level 1 | Level 2 | Level 3 | ||||||||||||
| Abell Warrants | $ | 438,537 | $ | - | $ | - | $ | 438,537 | ||||||||
| Round C Warrants | 1,359,647 | - | - | 1,359,647 | ||||||||||||
| $ | 1,798,184 | $ | - | $ | - | $ | 1,798,184 | |||||||||
| December 31, 2013 | ||||||||||||||||
| Description | Total | Level 1 | Level 2 | Level 3 | ||||||||||||
| Abell Warrants | $ | 1,615,835 | $ | - | $ | - | $ | 1,615,835 | ||||||||
| Round C Warrants | 1,796,427 | - | - | 1,796,427 | ||||||||||||
| Bridge Loan | 990,000 | - | - | 990,000 | ||||||||||||
| Abell Investment Option | 7,392,528 | - | - | 7,392,528 | ||||||||||||
| $ | 11,794,790 | $ | - | $ | - | $ | 11,794,790 | |||||||||
The following is a reconciliation of the fair value measurements from January 1, 2013 to December 31, 2014.
| Abell Warrants | Round C Warrants | Bridge Loan | Abell Option | |||||||||||||
| Balance, January 1, 2013 | $ | 831,806 | $ | 230,349 | $ | 1,528,500 | $ | - | ||||||||
| Issuance of securities | 275,813 | 1,112,990 | (938,000 | ) | 5,954,545 | |||||||||||
| Fair value change included in earnings | 508,216 | 453,088 | 399,500 | 1,437,983 | ||||||||||||
| Balance, December 31, 2013 | 1,615,835 | 1,796,427 | 990,000 | 7,392,528 | ||||||||||||
| Issuance of securities | 37,529 | 2,025,313 | - | - | ||||||||||||
| Repayment/extinguishment of debt | - | - | (1,100,000 | ) | (3,168,224 | ) | ||||||||||
| Fair value change included in earnings | (1,214,827 | ) | (2,462,093 | ) | 110,000 | (4,224,304 | ) | |||||||||
| Balance, December 31, 2014 | $ | 438,537 | $ | 1,359,647 | $ | - | $ | - | ||||||||
| F-24 |
| VACCINOGEN INC. AND SUBSIDIARIES |
| Notes to Consolidated Financial Statements |
Quantitative Information
Quantitative information as of December 31, 2014 with respect to financial instruments measured and carried at fair value on a recurring basis with the use of significant unobservable inputs (Level 3) are as follows:
| Level 3 - Significant Unobservable Inputs | ||||||||||
| Description | Fair Value | Principal Valuation Techniques | Unobservable Inputs | Range (Weighted Average) | ||||||
| Abell Warrants | $ | 438,537 | Black-Scholes | Strike price Equity volatility | N/A | |||||
| Round C Warrants | $ | 1,359,647 | Black-Scholes | Strike price Equity volatility | N/A | |||||
Abell Warrants and Round C Warrants
The fair value of the Abell Warrants and the Round C Warrants are estimated at the end of each reporting period using an option pricing model. More specifically, the Black-Scholes option pricing model was utilized in the valuation of both the Abell Warrants and the Round C Warrants. The following assumptions were used:
| Abell Warrants | Round C Warrants | |||||||||||||||
| 2014 | 2013 | 2014 | 2013 | |||||||||||||
| Volatility | 94 | % | 80 | % | 87 | % | 80 | % | ||||||||
| Exercise price | $ | 4.55 | $ | 4.68 | $ | 5.88 | $ | 5.88 | ||||||||
| Stock price at December 31 | $ | 2.20 | $ | 7.91 | $ | 2.20 | $ | 7.91 | ||||||||
| Risk free interest rate | 2.17 | % | 3.04 | % | 1.02% - 1.65 | % | 1.13% - 1.75 | % | ||||||||
| Dividend yield | 0 | % | 0 | % | 0 | % | 0 | % | ||||||||
| Expected life | 10 | 10 | 2.8 -5.0 | 3.7 -5.0 | ||||||||||||
The exercise price of the Abell Warrants is ultimately dependent upon the per share price and size of future rounds of equity financing. The Black-Scholes option pricing model was used to value the Abell Warrants as management believes that it can reasonably estimate the terms and conditions of future equity offerings that would impact the valuation of the Abell Warrants. Management’s ability to estimate these terms is based in part upon the terms and conditions of binding agreements to raise future equity capital in place at the time of each valuation.
| F-25 |
| VACCINOGEN INC. AND SUBSIDIARIES |
| Notes to Consolidated Financial Statements |
The Round C Warrants include a form of anti-dilution protection that may result in future adjustments to the terms of the warrants.
The fair value calculations include the use of both observable and estimated inputs. There remains an inherent subjectivity in the development of the strike price used for both the Abell and Round C Warrants. Therefore, the Company considers the derived fair value to have been determined using Level 3 inputs.
Significant changes to the assumptions used in the Company’s model would result in changes in the fair value of the Abell Warrants and the Round C Warrants.
Abell Option
Management has valued the Abell Option based upon an estimate of the fair value of the Company’s underlying stock because management believes it can reasonably estimate the occurrence and the terms of the future equity offering necessary to trigger Abell’s right to exercise the option.
As of January 16, 2013, the date of issuance for the Abell Option, the Company reasonably expected the undiscounted common stock price issuable to be $5.50 per share, discounted to $4.40 per share. Therefore, the $5 million investment divided by the discounted share price of $4.40 yielded an option for 1,136,364 shares. At December 31, 2014, the estimated number of shares is 934,580 in recognition of the expected Venture Capital Financing price per share of $5.35, the effective price of the Round C issuances.
In August 2014, the Abell Investment Option was modified, including changes to the terms of the optional investment (now a fixed number of shares (909,091) and a fixed purchase price ($5.50 per share), certain defined operating conditions, and a set expiry period; whereas the original investment terms were $5 million worth of common stock valued at the lowest sales price in effect at the time, and no expiry period (i.e., perpetual option).
Historically, the Company had determined that the Investment Option was a liability pursuant to the guidance contained in ASC 480, and as such should be marked to its fair value at each balance sheet date since they were determined to be probable of occurrence. Since the modification eliminated the variability in the Investment Option (the number of shares and purchase price are now fixed), in effect the Investment Agreement is now similar to a contingent “written call” or warrant where the investor can buy a fixed number of shares at a fixed price; as such the Investment Option is no longer within the scope of ASC 480.
The Company accounted the new terms of the Abell option under EITF 00-19 (ASC 815-40-25-1 through 25-43 and related interpretative guidance in ASC 815-40-55-1 through 55-18). Since the written call are fixed (fixed number of shares at a fixed price), it is considered indexed to the Company’s own stock and treated as an equity instrument and should be classified under stockholders’ equity. The value of the Abell option as of the effective date, August 22, 2014, was $3,168,224. The Company removed this amount from financial liabilities and included the same to additional paid-in capital under the stockholder’s equity section in the unaudited condensed consolidated balance sheets.
| F-26 |
| VACCINOGEN INC. AND SUBSIDIARIES |
| Notes to Consolidated Financial Statements |
Bridge Loan
The estimated fair value of the Bridge Loan was determined based upon the present value of probability weighted cash flows, using assumptions about the timing and amount of future cash flows and discount rates that management considers to be appropriate in the circumstances.
Because of the inherent subjectivity in management’s assumptions, the Company considers the derived fair value to have been determined using Level 3 inputs.
Disclosure of the Fair Value of Financial Instruments
Cash and cash equivalents and accounts payable, are carried at amounts that approximate their fair values due to the short term nature of these financial instruments. The fair value of the Abell Loan approximates its carrying value due to the short term nature of the Abell Loan’s maturity. The fair value of the Organon Obligation approximates its carrying value as the note is due on demand.
10. Stockholders’ Equity
In August 2012, the Company amended and restated its Certificate of Incorporation to increase the number of shares authorized for issuance to 200,000,000 shares of common stock with a par value $.0001 and 50,000,000 shares of preferred stock with a par value of $.0001 per share.
During 2014, the Company raised additional capital of $17,830,303 through the issuance of 3,246,417 shares of common stock (Round C), 90,923 adjustment shares and common stock warrants (Round C Warrants) to purchase 972,525 shares of common stock. $15,200,689 was allocated to the common stock; $1,947,224 was allocated to the common stock warrants; and $682,390 represents placement agent commissions paid. The Round C common stock warrants were determined to be a derivative financial instrument.
In addition, during 2014, an investor in the Bridge Loan opted to convert its loan to equity in the amount of $300,000 and the Company issued 54,545 shares of common stock (Round C), 1,530 adjustment shares and common stock warrants (Round C Warrants) exercisable into 16,363 shares of common stock. $227,939 was allocated to the common stock and $72,061 was allocated to the common stock warrants. Also in 2014, a payable in the amount of $25,000 was converted into 4,545 shares of the Company’s common stock (Round C), 128 adjustment shares and common stock warrants (Round C Warrants) to purchase 1,363 shares of common stock $18,966 was allocated to common stock and $6,034 was allocated to the common stock warrants. The common stock warrants were determined to be a derivative financial instrument. The terms and conditions of the Round C Warrants are described in Note 3 to the consolidated financial statements.
| F-27 |
| VACCINOGEN INC. AND SUBSIDIARIES |
| Notes to Consolidated Financial Statements |
During 2013, the Company raised additional capital of $4,205,105 through the issuance of 764,578 shares of common stock (Round C), 21,465 adjustment shares and common stock warrants (Round C Warrants) to purchase 229,368 shares of common stock. $3,253,316 was allocated to the common stock; $926,581 was allocated to the common stock warrants; and $25,288 represents placement agent commissions paid. The Round C common stock warrants were determined to be a derivative financial instrument.
In addition, during 2013, several investors in the Bridge Loan opted to convert their loan to equity in the amount of $937,970 and the Company issued 170,540 shares of common stock (Round C), 4.787 adjustment shares and common stock warrants (Round C Warrants) exercisable into 51,159 shares of common stock. $751,561 was allocated to the common stock and $186,409 was allocated to the common stock warrants. The common stock warrants were determined to be a derivative financial instrument. The terms and conditions of the Round C Warrants are described in Note 3 to the consolidated financial statements.
Round C Common Stock
The subscription agreement for the Company’s most recent financing, beginning October 2012, through the issuance of common stock (“Round C”) provides a form of anti-dilution protection to subscribers. The anti-dilution rights are determined to be clearly and closely related to the Round C common stock as that term is defined in Topic 815 and as a result these rights are not required to be accounted for as a free standing derivative financial instrument.
The Company filed a Form S-1 Registration Statement with the SEC, which became effective on October 31, 2013. The market price of Vaccinogen stock as of that date was $5.35. Pursuant to the protection provided to subscribers prior to October 31, 2013, 27,213 adjustment shares were issued. All subscriptions subsequent to the October 31, 2013 effective date through December 31, 2013 have been issued adjustment shares, totaling 92,581 adjustment shares, for the year ended December 31, 2014. All of the adjustment shares are included in the calculation of total shares issued or outstanding for the years ended December 31, 2014 and 2013.
The Company has increased the size of the Round C Private Placement Offering to $30,800,000 (5,600,000 Units) and has approved a Final Closing Date to January 31, 2015.
| F-28 |
| VACCINOGEN INC. AND SUBSIDIARIES |
| Notes to Consolidated Financial Statements |
11. Stock-Based Compensation
Restricted Stock
The Company from time to time has authorized shares of restricted common shares as director compensation. From August 2010 through December 31, 2014, the Company authorized 179,969 shares of restricted common stock to directors whose vesting will be determined by the approval of a 2015 Stock Incentive Plan.
The Company records compensation expense for the award of restricted stock based upon the awards fair value determined as the difference in the estimated fair value of the Company’s common stock and the price paid by the employee, if any, generally on the date of grant. The fair value of restricted stock awards is recognized as compensation expense over the service period which is generally the same as the vesting period. No compensation cost has been or will be recognized for the restricted stock awards whose vesting is contingent upon a approval of a 2015 Stock Incentive Plan. No compensation expense related to awards with service based vesting was recorded for the years ended December 31, 2014 and 2013.
The following table summarizes activity related to restricted-stock awards for 2014 and 2013. The weighted average fair values for the awards below are based on the fair value at the grant date of the respective awards, which is equal to the value of the Company’s common stock on such date.
| 2014 | 2013 | |||||||||||||||
| Shares | Weighted Average Fair Value | Shares | Weighted Average Fair Value | |||||||||||||
| Balance, beginning of year | 129,846 | $ | 3.77 | 119,734 | $ | 3.42 | ||||||||||
| Granted | 50,123 | $ | 2.20 | 10,112 | $ | 7.91 | ||||||||||
| Vested | - | $ | - | - | $ | - | ||||||||||
| Forfeitures | - | $ | - | - | $ | - | ||||||||||
| Balance, end of year | 179,969 | $ | 3.35 | 129,846 | $ | 3.77 | ||||||||||
As of December 31, 2014 and 2013, total unrecognized compensation costs related to nonvested restricted stock awards to purchase 179,969 and 134,278 shares of common stock was approximately $604,000 and $489,000 respectively, which will be recognized upon the approval and implementation of the 2015 Stock Incentive Plan. No awards vested during the years ended December 31, 2014 and 2013.
| F-29 |
| VACCINOGEN INC. AND SUBSIDIARIES |
| Notes to Consolidated Financial Statements |
Stock Option Pool
The Company recorded stock based compensation in the amount of $86,943 and $46,543 for the years ended December 31, 2014 and 2013, respectively. For the years ended December 31, 2014 and 2013, $27,019 and $10,777 was allocated to General & Administrative expense with $59,924 and $35,766 was allocated to Research & Development, respectively.
Total compensation costs for unvested stock option awards outstanding at December 31, 2014 was approximately $375,000 to be recognized over approximately 3.0 years.
The Company uses the Black-Scholes option pricing model to calculate the fair value of options. The significant assumptions for options issued in 2014, used in this model include:
| Annual Dividend | - | |
| Expected life (in years) | 6.25 – 10.00 | |
| Risk free interest rate | 1.35% - 2.17% | |
| Expected volatility | 80% |
The following table summarizes the Stock Option pool activity for 2014.
| Shares | Weighted Average Exercise Price | Weighted Average Remaining Life | ||||||||||
| Balance, beginning of year | 77,000 | $ | 7.00 | - | ||||||||
| Granted | 100,000 | $ | 3.64 | - | ||||||||
| Forfeited | - | $ | - | - | ||||||||
| Balance, end of year | 177,000 | $ | 6.44 | 8.72 | ||||||||
Stock Purchase Warrants
From time to time the Company has issued stock purchase warrants to non-employees in exchange for services. As of December 31, 2014 and 2013 there are 820,575 and 785,575, respectively, warrants issued and outstanding with exercise prices ranging from $1.00 to $7.50. To date, all warrants have been issued with the exercise price at least equal to the then estimated fair value of the underlying security, and had contractual terms ranging from 2.5 to 7.5 years.
| F-30 |
| VACCINOGEN INC. AND SUBSIDIARIES |
| Notes to Consolidated Financial Statements |
The following table summarizes the stock purchase warrant activity for 2014 and 2013.
| 2014 | 2013 | |||||||||||||||
| Shares | Weighted Average Fair Value | Shares | Weighted Average Fair Value | |||||||||||||
| Balance, beginning of year | 785,575 | $ | 1.48 | 785,575 | $ | 1.48 | ||||||||||
| Granted | 35,000 | $ | 4.22 | - | $ | - | ||||||||||
| Balance, end of year | 820,575 | $ | 1.60 | 785,575 | $ | 1.48 | ||||||||||
The following table summarizes information on stock purchase warrants outstanding as of December 31, 2014:
| Exercise price | Shares | Weighted Average Remaining Life | Weighted Average Exercise Price | |||||||||||
| $ | 1.00 | 705,575 | 1.05 | $ | 1.00 | |||||||||
| $ | 5.50 | 80,000 | 3.74 | $ | 5.50 | |||||||||
| $ | 7.50 | 35,000 | 4.34 | $ | 7.50 | |||||||||
| Total | 820,575 | 2.35 | $ | 1.72 | ||||||||||
Common Stock Warrants
The following table summarizes the warrant activity for 2013 and 2014 and is inclusive of the Abell Warrants, the Round C Warrants, the Stock Purchase Warrants and represents the total outstanding warrants.
| F-31 |
| VACCINOGEN INC. AND SUBSIDIARIES |
| Notes to Consolidated Financial Statements |
| Shares | Weighted Average Exercise Price | Weighted Average Remaining Life | ||||||||||
| Outstanding at January 1, 2013 | 2,663,648 | $ | 1.48 | 1.81 | ||||||||
| Granted | 646,730 | $ | 5.20 | 7.66 | ||||||||
| Exercised | - | $ | - | - | ||||||||
| Expired | - | $ | - | - | ||||||||
| Outstanding at December 31, 2013 | 3,310,378 | $ | 2.20 | 2.95 | ||||||||
| Granted | 1,448,687 | $ | 5.69 | 5.91 | ||||||||
| Exercised | - | $ | - | - | ||||||||
| Expired | - | $ | - | - | ||||||||
| Outstanding at December 31, 2014 | 4,759,065 | $ | 1.73 | 3.77 | ||||||||
The warrants granted in the years ended December 31, 2014 and 2013 had a weighted average fair value at grant date of $4.25 and $4.76 respectively.
The following table summarizes the outstanding warrants by year of expiration:
| Shares | ||||
| 2015 | 1,151,690 | |||
| 2016 | 1,201,475 | |||
| 2017 | 59,439 | |||
| 2018 | 360,527 | |||
| 2019 | 765,861 | |||
| Thereafter | 1,220,073 | |||
| Total | 4,759,065 | |||
12. Commitments and Contingencies
Leases
The Company leases office space, a manufacturing facility, and equipment under operating leases expiring in 2018. In addition, the Company leases storage facilities on a month to month basis. Rent expense was approximately $162,000 and $160,000 for the years ended December 31, 2014 and 2013, respectively.
| F-32 |
| VACCINOGEN INC. AND SUBSIDIARIES |
| Notes to Consolidated Financial Statements |
Minimum future lease payments under non-cancelable operating leases, at December 31, 2014 were as follows:
| For year ended December 31, | Amount | |||
| 2015 | $ | 210,887 | ||
| 2016 | 210,170 | |||
| 2017 | 214,297 | |||
| 2018 | 89,950 | |||
| Total | $ | 725,304 | ||
Royalty Agreement with Intracel
Pursuant to the Agreement (see Note 7), the Company agreed to pay Intracel the following royalties on the Net Sales of Royalty Bearing Products (as defined): (i) 3% of net sales on the first $350.0 million of Net Sales of Colon Cancer Products occurring in the calendar year; (ii) 4% of net sales of Net Sales of Royalty Bearing Products occurring in the calendar year in excess of $350.0 million and up to and including $750.0 million and (iii) 5% of net sales of Net Sales of Royalty Bearing Products occurring in the calendar year in excess of $750.0 million.
On August 22, 2014 Intracel and Vaccinogen entered into an Agreement, whereby the License Agreement will terminate and no longer be of any force or effect once TIS invests in Vaccinogen the aggregate amount of $80,000,000. Vaccinogen shall no longer, from that point, have any obligation to pay to Intracel any royalties or other payments under the original License Agreement and/or License Amendment.
Royalty Agreement with Organon
The Company has agreed to pay Organon a royalty of 10% of the Net Sales of OncoVAX® (and all other TICE BCG related products, if any) until the Organon Obligation is paid in full, including interest, and 3% for 5 years thereafter.
Litigation
The Company may be subject to certain claims arising in the ordinary course of business. The Company and a vendor were in dispute over amounts owed for services performed. A demand for payment under a written agreement was made against the Company in the amount of approximately $150,000 and was settled for $65,000 in November 2014.
VAT Taxes
The foreign subsidiary of the Company, located in The Netherlands, was presented with a VAT tax bill for the years 2010 and 2011 in the amount of €65,666. The Company is appealing the decision as we believe that we should be VAT exempt. There has not yet been a decision made by the Dutch authorities and there is no estimate of when a decision will be made. In the event an unfavorable decision is made, the Company will be required to pay the full amount plus interest. The original bill at the December 31, 2014 exchange rate would have a USD equivalent of approximately $79,800. There has been no expense accrued on the accompanying consolidated financial statements.
| F-33 |
| VACCINOGEN INC. AND SUBSIDIARIES |
| Notes to Consolidated Financial Statements |
13. Related Party Transactions
During April 2014, an executive of the Company loaned the Company $20,000. As of December 31, 2014, the loan was fully paid.
In 2012, an executive of the Company loaned the Company $10,000. As of December 31, 2013, the balance due of $4,099 is past due and in default. The carrying value of this amount due to the Company executive is included in related party payable in the accompanying consolidated balance sheets as of December 31, 2013. As of December 31, 2014, the loan was fully paid.
In December 2013, an executive of the Company loaned the Company $50,000. As of December 31, 2013, the balance due of $20,000 is past due. The carrying value of this amount due to the Company executive is included in related party payable in the accompanying consolidated balance sheets as of December 31, 2013. As of December 31, 2014, the loan was fully paid.
14. Income Taxes
The Company is subject to U.S. Federal, state and foreign income taxes. The provision/(benefit) for income taxes for the tax years ended December 31, 2014 and 2013 are as follows:
| December 31, | ||||||||
| 2014 | 2013 | |||||||
| Current: | ||||||||
| Federal | $ | 206,568 | $ | - | ||||
| State | 31,649 | - | ||||||
| Foreign | - | - | ||||||
| Total Current | 238,217 | - | ||||||
| Deferred: | ||||||||
| Federal | (3,932,476 | ) | (4,466,979 | ) | ||||
| State | (629,774 | ) | (715,374 | ) | ||||
| Foreign | (223,923 | ) | (165,845 | ) | ||||
| Total Deferred (Provision)/Benefit | (4,786,173 | ) | (5,348,198 | ) | ||||
| Valuation Allowance | 4,786,173 | 5,348,198 | ||||||
| Total Tax Provision/(Benefit) | $ | 238,217 | $ | - | ||||
The Company has approximately $10.1 million of net operating loss (“NOL”) available for foreign purposes as of December 31, 2014, which will expire between 2016 and 2023.
| F-34 |
| VACCINOGEN INC. AND SUBSIDIARIES |
| Notes to Consolidated Financial Statements |
A reconciliation of the U.S. federal statutory income tax rate to the Company’s consolidated effective income tax rate for the years ended December 31, 2014 and 2013 is as follows:
| Effective Rate Reconciliation | ||||||||
| Years ended | 12/31/2014 | 12/31/2013 | ||||||
| Income tax at statutory rate | 34% | 34% | ||||||
| Financial instruments | 32% | -12% | ||||||
| State taxes - (net of fed) | 5% | 3% | ||||||
| International rate difference | -10% | -1% | ||||||
| Amortization of prepaid | -3% | 0% | ||||||
| Change-valuation allowance - fed | -64% | -22% | ||||||
| All other items | 3% | -2% | ||||||
| Effective income tax rate | -3% | 0% | ||||||
The temporary differences that arise between financial statement reporting and income tax basis carrying amounts of assets and liabilities give rise to deferred income taxes. The Company’s deferred income tax assets and liabilities as of December 31, 2014 and 2013 were as follows:
| Start-up expenditures and amortization | 8,036,569 | 5,910,419 | ||||||
| Amortization of intangibles | 4,996,993 | 8,631,105 | ||||||
| Financial instruments | 1,408,087 | 705,008 | ||||||
| Nonqualified stock options | 478,061 | 343,372 | ||||||
| U.S. net operating loss | - | 2,576,756 | ||||||
| Foreign net operating loss | 2,020,627 | 1,796,704 | ||||||
| Total long-term deferred income taxes: | 16,940,337 | 19,963,364 | ||||||
| Valuation allowance | (16,940,337 | ) | (19,963,364 | ) | ||||
| Total Current and Deferred Income Tax Assets/(Liabilities) | $ | - | $ | - |
Total long-term deferred income tax assets represent $2.0 and $1.8 million related to non-U.S. operations for years December 31, 2014 and December 31, 2013, respectively. A valuation allowance is maintained against all amounts.
Management assesses the available positive and negative evidence to estimate if sufficient future taxable income will be generated to utilize the existing deferred tax assets. A significant piece of the objective negative evidence evaluated was the cumulative loss incurred over the three year period ended December 31, 2014. Such objective evidence limits the ability to consider other subjective evidence such as our projection for future growth.
| F-35 |
| VACCINOGEN INC. AND SUBSIDIARIES |
| Notes to Consolidated Financial Statements |
On the basis of this evaluation, as of December 31, 2014 and 2013, a valuation allowance of $16.9 million and $20.0 million has been recorded to record the portion of the deferred tax asset that is more likely than not to be realized. The amount of the deferred tax asset considered realizable, however, could be adjusted if estimates of future taxable income during the carryforward period are reduced or increased or if objective negative evidence in the form of cumulative losses is no longer present and additional weight may be given to subjective evidence such as our projections for growth.
The Company is subject to income taxes in the U.S. as well as certain state and foreign jurisdictions. Tax regulations within each jurisdiction are subject to interpretation and require significant judgment to apply.
U.S. federal income taxes have not been provided on undistributed earnings of our international subsidiaries as it is our intention to reinvest these earnings into respective subsidiaries. At December 31, 2014 the Company has not provided for U.S. federal income and non-U.S. withholding taxes as the international subsidiaries do not have undistributed earnings, only deficits to date.
As of December 31, 2014 and 2013 there were $8,914,027 and $130,000 of unrecognized tax benefits that if recognized would be recorded as a component of continuing operations. The remaining unrecognized benefits represent federal and state taxes on intercompany transactions between the Company and its international subsidiaries.
| 2014 | 2013 | |||||||
| Unrecognized tax benefits, beginning of the year | $ | 130,000 | $ | 90,000 | ||||
| Decreases for tax positions related to prior years | (30,000 | ) | - | |||||
| Increases for tax positions taken during the year | 8,814,027 | 40,000 | ||||||
| Unrecognized tax benefits, end of the year | $ | 8,914,027 | $ | 130,000 | ||||
As a result of an intercompany sale of intellectual property during 2014, for ASC 740-10 purposes, the Company recorded an unrecognized tax benefit of $8.8 million. Under ASC 810-10-45-1 (formerly known as “ARB 51”), the GAAP and tax gains were eliminated in consolidation. ASC 810-10-45 guidance provides for a prepaid tax asset to be recorded for the same amount as the unrecognized tax benefit. The prepaid tax asset is being amortized as tax expense over the life of the intellectual property. The ASC 740-10 reserve recorded for this transaction will reverse in the future as the gain is reported on the Company’s US tax returns. Income tax expense represents the amortization of the Prepaid Income Tax for the period of October 1, 2014 through December 31, 2014.
It is the Company’s accounting policy to account for ASC 740-10 related penalties and interest in other liabilities/expenses and not to include them within he income tax expense line in the consolidated statement of operations.
As of December 31, 2014 the statutes of limitation, for U.S. purposes, are open for the 2007 through 2013 tax years and we are not expected to close within the next twelve months. The Company is not currently under examination by the taxing authority.
The Company's various tax positions could be challenged by the Internal Revenue Service ("IRS") at such time the Company becomes profitable. The Company has treated the acquisition of the OncoVAX® technology as the acquisition of a portfolio of assets and not as a trade or business. The Company has taken the position that the purchase price paid in stock represents a taxable acquisition under the Internal Revenue Code ("IRC") with a taxable basis in those assets equal to the fair market value of the stock paid.
| F-36 |
| VACCINOGEN INC. AND SUBSIDIARIES |
| Notes to Consolidated Financial Statements |
In the event the Company is challenged upon receiving a tax benefit, the Company could be subject to income tax understatement penalties.
The Company has a small §382 NOL limitation due to a change in ownership. A detailed review was performed and it was determined that the §382 NOL limitation is immaterial.
15. Supplemental Disclosure of Cash Flow Information
For the years ended December 31, 2014 and December 31, 2013 the Company paid interest costs of $53,279 and $308,506, respectively.
In January 2014, the Company issued 56,075 shares of common stock, including 1,530 adjustment shares to certain holders of the 2012 Bridge Loan that had elected to convert their rights, with a value of $300,000, into common stock of the Company. The Company also issued additional Round C Warrants exercisable into 16,822 shares of common stock of the Company, with an exercise price of $6.05 per share.
During 2014, $25,000 was converted into 4,545 shares of the Company’s common stock (Round C), 128 adjustment shares and common stock warrants (Round C Warrants) to purchase 1,363 shares of common stock (See Note 10).
During 2013, the Company issued 170,540 shares of common stock and common stock warrants (Round C Warrants) exercisable into 51,159 shares of common stock of the Company to certain investors in the Bridge Loan who elected to convert their rights to receive cash under the Bridge Loan into shares of common stock and common stock warrants. The Company also issued an additional 4,787 adjustment shares.
16. Subsequent Events
In accordance with ASC 855-50, “Subsequent Events,” the Company has reviewed events through the date of the filing.
| F-37 |
| VACCINOGEN INC. AND SUBSIDIARIES |
| Notes to Consolidated Financial Statements |
January 1, 2015 through April 3, 2015, the Company raised additional capital totaling approximately $4.2 million (net of issuance costs) through the issuance of 909,091 shares of Common Stock, 25,489 adjustment shares and warrants to purchase 272,727 shares of Common Stock.
Vaccinogen established Vaccinogen Ireland R&D Company, Limited on January 7, 2015. The entity was established to undertake, take part in, promote, protect and encourage research, training, development, design, manufacture and production in human monoclonal antibodies, and subjects relative thereto, and to promote scientific and industrial training therein.
| F-38 |
Signatures
In accordance with Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant caused this report to be signed on its behalf by the undersigned, thereunto duly authorized on April 15, 2015
| VACCINOGEN, INC. | ||
| By: | /s/ Andrew L. Tussing | |
| Andrew L. Tussing | ||
President, Chief Executive Officer and acting Chief Financial Officer (Principal Executive Officer and Principal Financial Officer) | ||
Pursuant to the requirements of the Securities Act of 1933, as amended, this report has been signed by the following person in the capacities and on the date indicated.
| Signatures | Title | Date | ||
| /s/ Benjamin S. Carson, Sr., M.D. | Chairman of the Board | April 15, 2015 | ||
| Benjamin S. Carson, Sr., M.D. | ||||
| /s/ Andrew L. Tussing | President, Chief Executive Officer, | April 15, 2015 | ||
| Andrew L. Tussing | acting Chief Financial Officer and Director | |||
| (Principal Executive Officer and Principal | ||||
| Financial Officer) | ||||
| /s/ Michael G. Hanna, Jr., Ph.D. | Director | April 15, 2015 | ||
| Michael G. Hanna, Jr., Ph.D. | ||||
| /s/ Roanald W. Kaiser | Director | April 15, 2015 | ||
| Ronald W. Kaiser | ||||
| /s/ Anders Halldin | Director | April 15, 2015 | ||
| Anders Halldin | ||||
| /s/ Håkan Edström | Director | April 15, 2015 | ||
| Håkan Edström |
