Attached files
| file | filename |
|---|---|
| EXCEL - IDEA: XBRL DOCUMENT - SKINVISIBLE, INC. | Financial_Report.xls |
| EX-31.2 - EX31_2 - SKINVISIBLE, INC. | ex31_2.htm |
| EX-31.1 - EX31_1 - SKINVISIBLE, INC. | ex31_1.htm |
| EX-32.1 - EX32_1 - SKINVISIBLE, INC. | ex32_1.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 10-K
| [X] | ANNUAL REPORT UNDER SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
| For the fiscal year ended December 31, 2014 | |
| [ ] | TRANSITION REPORT UNDER SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT |
For the transition period from _________ to ________
| |
| Commission file number: 000-25911 |
| Skinvisible, Inc. | |
| (Exact
name of registrant as specified in its charter) | |
| Nevada | 88-0344219 |
(State or other jurisdiction of incorporation or organization)
|
(I.R.S. Employer Identification No.) |
| 6320 South Sandhill Road, Suite 10, Las Vegas, NV | 89120 |
| (Address of principal executive offices) | (Zip Code) |
Registrant’s telephone number: 702.433.7154
|
|
| |
| Title of each class | Name of each exchange on which registered |
| None | not applicable |
Securities registered under Section 12(g) of the Exchange Act:
| |
| Title of each class | |
| Common Stock, par value $0.001 | |
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes [ ] No [X]
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes [ ] No [X]
Indicate by checkmark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes [X] No [ ]
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes [X] No [ ]
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (§ 229.405 of this chapter) is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. Yes [ ] No [X]
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company.
| [ ] Large accelerated filer | [ ] Accelerated filer |
| [ ] Non-accelerated filer | [X] Smaller reporting company |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act).
Yes [ ] No [X]
State the aggregate market value of the voting and non-voting common equity held by non-affiliates computed by reference to the price at which the common equity was last sold, or the average bid and asked price of such common equity, as of the last business day of the registrant’s most recently completed second fiscal quarter. $3,902,182
Indicate the number of shares outstanding of each of the registrant’s classes of common stock, as of the latest practicable date. 113,813,969 common shares as of February 17, 2015

| 2 |
PART I
Company Overview
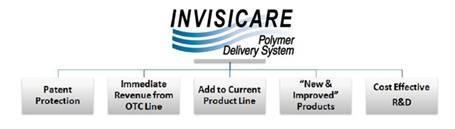
We, through our wholly owned subsidiary Skinvisible Pharmaceuticals Inc., are a pharmaceutical research and development (“R&D”) company that has developed and patented an innovative polymer delivery system, Invisicare® and formulated over forty topical skin products, which we out-license globally. We were incorporated in 1998, and target an estimated $80 billion global skincare and dermatology market and a $30 billion global over-the-counter market as well as other healthcare / medical and consumer goods markets.
With the research and development complete on forty products and numerous patents issued (technology and product patents), we are ready to monetize our investment. Our business model will continue to be to out-license our patented prescription and over-the-counter (“OTC”) products featuring Invisicare to established manufacturers and marketers of brands internationally and to maximize profits from the products we have already out-licensed. We have also formed a commercial subsidiary, Kintari USA Inc., in order to take our cosmeceutical and select OTC products with Invisicare to market.
The opportunity for us to license our products continues to be a viable model as the need for pharmaceutical companies to access external R&D companies for new products due to their own down-sizing or elimination of internal R&D departments. The demand for our products is enhanced due to the granting of key US and international patents and the completed development of a number of unique products.
The Products
Pivotal to our success is our patented polymer delivery system technology Invisicare. The advantage of products formulated with Invisicare is (1) Invisicare’s ability to bind active ingredients (the drug) to the skin, forming a protective bond on the skin, for extended periods of time - some up to eight hours or more; (2) Invisicare can deliver targeted levels (high or low) of therapeutic or cosmetic ingredients to the skin in a controlled release; (3) Invisicare can help to reduce the irritation of some active ingredients due to how it controls the slower release of that active ingredient; and (4) Invisicare science proves that it provides a protective skin barrier which helps retain the natural moisture content of the skin, while still allowing it to breathe. These benefits present an excellent opportunity for clear scientific advantages and marketing messages which resonate with physicians and consumers.
The Market
The dermatology market is large, with over 80% of Americans affected by some kind of skin condition in their lifetime. The worldwide market for dermatology products including prescription, OTC and cosmeceuticals is estimated at $80 billion.
Company History
We formed Skinvisible Pharmaceuticals, Inc. (“Skinvisible”), in March 1998 and purchased the exclusive worldwide manufacturing and marketing rights for a polymer delivery system invention now called Invisicare® from the inventor for $2 million. We have continued to develop the Invisicare technology and subsequent product development resulting in over seven series of Invisicare and over forty unique, patented formulations offering distinctive benefits that differentiate them significantly from other leading products in the marketplace.
| 3 |
What We Do
We have positioned ourselves in the $80 billion worldwide prescription and over-the-counter dermatology and skincare market. We generate revenue by:
- LICENSING: We develop topical prescription and over-the-counter products enhanced with Invisicare to license to pharmaceutical and consumer goods companies around the world for an upfront fee and ongoing royalties;
- DIRECT SALES: We develop topical over-the-counter products enhanced with Invisicare to sell directly into the market through our wholly-owned subsidiary, Kintari USA Inc.
- CO-DEVELOPMENT: We assist pharmaceutical clients in the early development of the most optimal formulation, which they then take forward into clinical testing;
- LIFE CYCLE MANAGEMENT: We provide cost-effective solutions to global pharmaceutical companies by reformulating their products coming off patent with a new Invisicare patent and new product benefits and line extensions. Pharmaceutical companies are under a lot of pressure to develop innovative strategies to counteract the revenue loss from their drugs coming off patent.
Corporate Ownership
We are a publicly traded company under the symbol SKVI, listed on the OTC Bulletin Board since February 1999 and currently trading on the OTCQB in the US.
We carry on business primarily through our wholly owned subsidiaries Skinvisible Pharmaceuticals, Inc. and Kintari USA Inc., both Nevada corporations.
Patents
We continue to expand our patent protection in the US for Invisicare®, our unique topical drug delivery technology as well as internationally for various products formulated with Invisicare®. Invisicare® patents protect Invisicare® in three areas: composition, manufacturing and use. Product patents include UVA stabilization in sunscreens (Invisicare® stabilizes avobenzone sunscreen for eight hours), retinoid stabilization, dermal barrier product protection and cationic activities (i.e. imiquimod). Patents pending include acne, anti-fungal, ichthyosis and others.
Our patent portfolio includes a total of fifteen US and international patents specifically protecting Invisicare and the products formulated with it.
Trademarks
When developing new products using Invisicare, we file for both patent and trademark protection. We have been granted trademarks in the U.S. and Canada for the following names:
- Skinvisible® w Invisicare® w JUSTCARE® w Work Gluv® w Bare Sunless Tanning® w Kintari® w Skinbrella®
Revenue generation: We receive a combination of five revenue streams including:
|
| 4 |
Strategic Growth Opportunities
Our growth strategy is to:
| 1. | Generate revenue from direct sales of our cosmeceutical/OTC product line; |
| 2. | Capitalize on the success of current licensees; |
| 3. | Increase the value of our current pipeline; and |
| 4. | Boost licensing revenues by securing additional licensees globally and develop a robust royalty revenue stream that will finance our future growth. |
| 1. | Kintari USA Inc.: |

On September 9, 2014, we formed Kinatri USA Inc., a new wholly-owned subsidiary of Kintari International Inc., wholly-owned subsidiary of Skinvisible Inc., to market a premium line of scientifically formulated skincare products powered by our patented Invisicare® technology. As part of our strategic focus on revenue generation and creating shareholder value, Kintari USA Inc. products will be sold via network marketing. The products will be sold initially in the US and then in Canada in Q3, 2015.
The Kintari product portfolio consists of 2 anti-aging products to help fight the signs of aging. These products have been developed using proven anti-aging ingredients with scientific evidence of their effectiveness at reducing the look of fine lines and wrinkles resulting in youthful looking skin. These potent ingredients will be powered by patented Invisicare technology, providing consumers with unique, effective products which we believe cannot be duplicated. Additional products will be added to enhance this product line as the company grows and expands with our Skinbrella® SPF 30 sunscreen to be launched in April 2015.
| 5 |
| 2. | Capitalize On Current Licensees: |
We have licensees around the globe. Two of these licensees are currently in the marketplace: Avon Products globally and Women’s Choice Pharmaceuticals in the United States.
We continue to work diligently with our licensees to ensure they have a smooth manufacturing process, ongoing R&D support and marketing feedback.
Avon Products, Inc: Product: We have a long-term contract with Avon globally for over ten years to provide Invisicare polymer for their long-lasting lipsticks.
Sales: Invisicare polymers are purchased directly from Skinvisible.
|
|
Women’s Choice Pharmaceuticals: Product: ProCort®, long lasting prescription hemorrhoid cream launched in the United States August 2011.
Sales and Royalties: Skinvisible receives a royalty based on net sales of ProCort. This past year Women's Choice Pharmaceuticals LLC partnered with Advanced Medical Enterprises, LLC to market ProCort® in Puerto Rico. With over thirty pharmaceutical sales reps calling on OBGYNs in the US, Women’s Choice has been successfully growing their sales of ProCort® and we look forward to increased growth in 2015. Women’s Choice is seeking to form other strategic alliances in order to increase its sales efforts by targeting new territories and targeting medical specialists which previously were not called upon. |
|
Product Updates:
We have additional information on specific products which add value to Skinvisible’s product pipeline.
DermSafe® Hand Sanitizer Skinvisible’s hand sanitizer formulated with Invisicare® and chlorhexidine gluconate has received registration in Belgium on behalf of Skinvisible. This registration allows Skinvisible to make DermSafe® available in most of Europe through a simple registration process. The Company is currently seeking licensees and/or distributors to begin the sale of DermSafe in the EU. This registration has recently been granted for a ten year term to expire in 2024. The product will be sold through Kintari Canada Inc. when it launches in Q3 2015. |
 |
| 6 |
Sunless Tanning Products: We have developed a new sunless tanning mousse / foam which uses a unique foam with Invisicare®, developed specifically for its foaming properties. This adds to Skinvisible’s line of sunless tanning products which includes sunless tanning lotions (light, medium and dark), pre-sun moisturizer and after-sun moisturizer along with sunless tanning spray products for commercial use. The addition of a sunless tanning mousse enhances this line of products which will be available through Kintari both in Canada and the US in 2016.
Sunscreen Products: We have developed 3 broad spectrum sunscreens, with SPF 15, 30 and 50 (the highest SPF allowed by the FDA). All are formulated with Avobenzone, the only UVA sun filter allowed under the US FDA monograph. This UVA/UVB sunscreen was granted a patent from the United States patent office in 2013. Avobenzone is known for breaking down in the sun after only two hours – thus the requirement to reapply every 2 hours. Skinvisible’s patent was granted based on Invisicare's® minimum 8 hour photo stability. For countries outside the United States, Skinvisible has additionally patented UVA/UVB sunscreens formulated with Tinosorb S.
In March 25, 2014, we announced that we have successfully completed independent testing to validate our broad spectrum sunscreen claims according to new labeling guidelines by the FDA which are designed to help reduce the incidents of skin cancer in the U.S.
Skinvisible sunscreens can be labeled with the following claims:
Claim # 1 – Broad-Spectrum: According to the FDA, in order for a sunscreen to be labeled “broad spectrum” it must prove it protects against both UVA and UVB rays by having an SPF (Sun Protection Factor) of at least 15 and a critical wave length of at least 370 nm. Skinvisible’s sunscreen has surpassed both of these criteria, allowing Skinvisible’s broad spectrum sunscreen label to also state “prevents sunburn, skin cancer and aging due to the sun.”
Claim # 2 – Water-Resistant 80 Minutes: The FDA sunscreen water resistant claim requires that a sunscreen must have the same SPF after being in water or sweating for 40 or 80 minutes. Skinvisible’s testing was conducted at an independent laboratory specializing in sunscreen testing. The test involved human subjects that applied sunscreen to their arm, followed by the immersion of the arm into a Jacuzzi for 80 minutes (10 minutes in / 10 minutes out). Skinvisible’s sunscreen successfully completed this testing and is allowed to use “Water-resistant for 80 Minutes” on its sunscreen label, the longest length of time allowed by the FDA.
Claim # 3 – Unique Patented Technology / Eight-Hour Photostability: As previously announced, Skinvisible was granted a patent from the United States Patent and Trademark Office entitled “Sunscreen Composition with Enhanced UVA Absorber Stability and Methods”, which provides protection until November 2029. Skinvisible successfully formulated a unique Invisicare® delivery system specifically for stabilizing avobenzone; the key sunscreen used in the USA. Data submitted to the US patent office proved that Skinvisible’s sunscreen provides a minimum of eight hours of photostability.
| 3. | Increasing The Value Of Skinvisible’s Pipeline: Clinical Enhancement Of Pipeline |
We have a pipeline of over forty products which are available for licensing. Testing is conducted in-house generating proof of concept including release of the active ingredient as well as long term shelf life (stability). Additional studies conducted on specific products including skin sensitivity, toxicity and product efficacy are outsourced to FDA compliant laboratories. These studies are critical in attracting potential licensees. Our clinical strategy is to:
- Add new studies for our prescription products. Our clinical strategy is to increase the amount of outsourced studies, specifically for our prescription products. Additional studies including skin penetration and skin irritation studies will add to the integrity and value of our products available for licensing.
- Add new long-term efficacy studies for our DermSafe® hand sanitizer. In 2013, we commissioned an independent laboratory to further analyze the long-term effectiveness of DermSafe® when put in contact with two bacteria; the “super bug” MRSA and E. coli, the “restaurant bug” since it is often transmitted by food and food handlers. The long-term effectiveness of two bacteria; Methicillin-resistant Staphylococcus aureus or MRSA (ATCC #33591) and Escherichia coli or E. coli (ATCC #43888") were tested up to four hours after application. The results showed that the individual arms of subjects which had DermSafe® applied and were even rinsed prior to each bacteria challenge, showed a 95.83% reduction at the 4 hour time point for MRSA and 99.38% for E. coli. In 2013, we obtained the registration rights for DermSafe® in Belgium. This designation allows for the sale and/ or registration of DermSafe in most EU countries. A strategy is being developed along with a larger global strategy to bring DermSafe to the EU and Asia in 2015. Skinvisible has also commissioned further testing of DermSafe against the (Middle East Respiratory Syndrome Coronavirus (MERS-CoV); a SARS-like virus and the avian influenza A virus, H7N9 which is presently a major health issue in China .
- We continue to look for avenues to obtain orphan drug status for our Netherton syndrome product. Netherton syndrome. is a disease caused by a genetic defect which causes the skin to continually exfoliate, never forming a skin bond. This leaves the patient highly susceptible to infection and dealing with a life-long condition that has no cure. Our product has shown excellent results in lab studies blocking the enzyme that breaks down the skin and we are seeking “Orphan Drug” designation in both the US (FDA) and Europe (EMA).
The advantages of obtaining Orphan Drug designation is that it provides various incentives including a reduction or elimination of registration and market authorization fees, protocol assistance, and seven years of market exclusivity for the product in the US and ten years in Europe. There can be no assurances that our project will be successful.
| 7 |
| 4. | Secure Additional Licensees: |
We are in discussions and undergoing internal discussions with various pharmaceutical companies for licenses.
To facilitate further expansion, we are seeking an exclusive license with a proven US or global based Pharmaceutical Company for our existing Rx product formulations. The licensee would be expected to pay all costs in getting FDA approval. The licensee would pay Skinvisible for the license in milestone payments as Clinical Phases are proven.
Invisicare – The Technology and Products
 |
At the heart of our product line is our patented technology and trademarked Invisicare family of polymer delivery vehicles. Invisicare has a unique formula and process for combining hydrophilic and hydrophobic polymers into stable formulations with almost any type of active ingredient. The Invisicare technology delivers drugs on, in or through the skin with a controlled release and can be tailored to almost any type of molecule and the needs of our licensees. |
Key Benefits of the Invisicare Technology
Invisicare enhances topical products with the following advantages:
- Independent studies have proven that products utilizing Invisicare will bond active ingredients to the skin for up to four hours or more even after washing. - Invisicare is non-occlusive and allows for normal skin respiration and perspiration while holding the body’s natural moisture in the skin as well as protecting against exposure from a wide variety of environmental irritants. - Invisicare adheres to the skin's outer layers, forming a protective bond and delivering targeted levels of therapeutic or cosmetic skincare agents to the skin. The "invisible" polymer compositions wear off as part of the natural exfoliation process of the skin’s outer layer. - Invisicare allows enhanced delivery performance for a variety of skincare agents resulting in improved efficacy, longer duration of action, reduced irritation and often requires a lower dosage of the active. - Invisicare has a patented process for combining water-soluble and water-insoluble polymers to hold water insoluble and certain cationic active ingredients on the skin without the use of alcohol, waxes, or other organic solvents; a key advantage over similar product formulas. - When Invisicare is formulated into a new product, a patent can be applied for, giving the product a unique position in the marketplace that cannot be duplicated. It also provides a cost-effective solution to pharmaceutical companies looking for life cycle management for their existing products coming off patent or requiring market revitalization. |
 |
Some product specific benefits illustrated in independent studies:
| - | DermSafe kills bacteria for up to four hours – studies conclude that DermSafe hand sanitizer kills the “Super Bug” methicillin-resistant staphylococcus (MRSA) up to 94% and Escherichia coli (E.coli), a major cause of food borne illnesses in humans, up to 99% after four hours, even when hands are rinsed and towel dried at hours 1, 2 and 4. (Bioscience Labs, Montana) |
| - | DermSafe kills viruses for up to four hours – studies conclude that DermSafe kills the influenza A virus’s including H1N1 (“bird flu”), H5N1 (“swine flu”) and H3N2. |
(RetroScreen Virology, Queen Mary School of Medicine, London England)
| - | Sunless Tanner lasts 73% longer than similar products – studies conducted by the largest supplier of DHA; the active that makes skin brown, showed significant advantages over leading branded products. (EMD Merck, Germany) |
| - | Sunscreens meet FDA critical wave length requirements – conducted by one of the most trusted names in sunscreen testing, our sunscreens, SPF 15, 30 and 50 exceed the new FDA regulations for sunscreens in both critical wavelength and “broad spectrum” rating. (Suncare Research Laboratories, LLC, Winston Salem, NC ) |
| 8 |
Invisicare Formulations
Our forty products have been successfully tested in-house to show proof of concept and are ready to be licensed. We continue to develop other prescription, OTC and cosmeceutical products in response to the needs of the marketplace.
| Product patent applications are immediately filed on newly developed products. FDA regulatory approvals are required for prescription products while OTC products have limited requirements. Cosmetic-type products, products without therapeutic claims and OTC products that follow the FDA monograph, are immediately available for marketing. In Canada, OTC products follow the Health Canada monograph requiring only the submission of a DIN registration. | 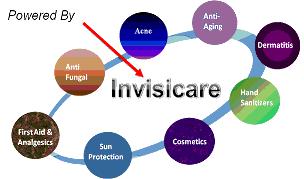 |
We have over 40 products developed and available for licensing:
| CONDITION / USE | PRESCRIPTION (PRE-CLINICAL) | OTC / COSMECEUTICAL | ||||||
| Acne | 3 | 2 | ||||||
| Actinic Keratosis | 1 | |||||||
| Analgesics | 1 | 6 | ||||||
| Anti-Fungal | 2 | 2 | ||||||
| Anti-Inflammatory | 4 | 1 | ||||||
| Antimicrobial | 1 | 4 | ||||||
| Pre-Operative Skin Prep | 1 | |||||||
| Dermatitis / Dry Skin | 1 | 5 | ||||||
| Netherton Syndrome | 1 | |||||||
| Anti- Aging | 4 | |||||||
| Suncare | 6 | |||||||
| Sunless Tanning | 3 | |||||||
| TOTAL | 15 | 33 |
Skinvisible Introduces Complete Product Lines
Invisicare® adds many benefits to products including skin barrier properties to aid skin healing by retaining moisture, superior adherence, and improved delivery of actives. Through our research and development efforts over the past year, Skinvisible has formulated complete product lines that are available for immediate licensing and launch. The product lines include: Acne, Anti-Aging, Sunless Tanning, Sun Care and Leg & Foot Care.
| 9 |


| 10 |

Research and Development
Our facilities include a research and development laboratory, headed by James Roszell PhD, where we continue to enhance our current product offerings and to develop a variety of new product formulations with Invisicare for out-licensing.
Our R&D focus is centered on the following initiatives:
| - | We continue to expand our product development beyond the dermatology market into other areas including women’s health, orphan drugs, pain management and surgical; |
| - | To increase the value of our prescription products, with additional testing on our most lucrative prescription products in order to provide independent validation and verification of our product claims. We utilize FDA compliant, independent laboratories with extensive qualifications for carrying out investigative product studies, utilizing protocols incorporating Good Lab Practice and Good Clinical Practice ("GLP/GCP") standards; |
| - | We have successfully developed a unique product for Netherton syndrome and are seeking orphan drug status for this product. Additional studies are required in order to receive approval as an orphan drug in the United States and Europe. |
| - | We have also completed preliminary development of a new Invisicare technology which will provide transdermal delivery of drugs. This new transdermal delivery system will allow us to enter the very lucrative markets of hormone replacement therapy, neurological treatment, nicotine cessation and others. |
| 11 |
Sales and Marketing Plan
Our Licensing Strategy
As stated above, we are seeking a proven US based Pharmaceutical company to license all our prescription products and for them to seek FDA approval. This would be an exclusive licensing agreement and allow management to focus on developing Kintari while our research department continues to support the Pharmaceutical Company.
Competition
Market research indicates there is reasonably limited direct competition for Invisicare and patented products in terms of performance capabilities for topically administered skin products. Many companies are seeking unique delivery systems to enhance their portfolio and purchasing companies that have delivery technology.
Some of the companies involved in developing delivery technology are listed below. However, none of these competitors offer the same advantages of Invisicare principally the “long-term staying power” and the ability to control the release of active ingredients on the skin.
- GSK through its subsidiary, Stiefel Laboratories Inc., purchased Connetics Corporation for approximately $640 million in the fall of 2006. (Subsequently in 2009, Stiefel, with $900 million in sales, was purchased by GlaxoSmithKline for $3.9 billion – at 4 times revenue). Connetics has a patented foam delivery technology.
- Foamix Ltd is a drug development company with its head office in Israel. It has developed five platforms which use a foam delivery technology and is used in products like Rogaine®.
- A.P. Pharma sold its acne and actinic keratosis products made with its patented Microsponge® delivery system to two companies for a reported $30 million; Johnson & Johnson purchased the Retin-A Micro® product line, with revenues of $110 million in the US in 2006 and sanofi-aventis purchased Carac®, a product used to treat actinic keratosis, with $11 million in sales in 2001.
Government Regulation
We are not subject to any significant or material federal or state government regulation in connection with the research and development and licensing of our innovative topical polymer-based delivery systems and technologies.
With respect to our products under development, our licensing agreements require the licensee to seek all required approvals for marketing, distribution, and sale in the jurisdictions for which it is desired to make the product available should we succeed in developing a successful product.
We are not subject to any significant or material environmental regulation in the normal operation of our business.
Orphan Drug Designation
We are seeking “Orphan Drug” designation in both the US and Europe for a product to treat a rare skin condition called Netherton syndrome. The FDA may grant orphan drug designation to drugs intended to treat a rare disease or condition, which generally is a disease or condition that affects fewer than 200,000 individuals in the United States. Orphan drug designation must be requested before submitting a New Drug Application, or NDA. If the FDA grants orphan drug designation, which it may not, the identity of the therapeutic agent and its potential orphan use are publicly disclosed by the FDA. Orphan drug designation does not convey an advantage in, or shorten the duration of, the review and approval process. If a product which has an orphan drug designation subsequently receives the first FDA approval for the indication for which it has such designation, the product is entitled to seven years of orphan drug exclusivity, meaning that the FDA may not approve any other applications to market the same drug for the same indication for a period of seven years, except in limited circumstances, such as a showing of clinical superiority to the product with orphan exclusivity (superior efficacy, safety, or a major contribution to patient care). Orphan drug designation does not prevent competitors from developing or marketing different drugs for that indication. We have not received orphan drug status for any of our products.
Under European Union medicines laws, the criteria for designating a product as an “orphan medicine” are similar but somewhat different from those in the United States. A drug is designated as an orphan drug if the sponsor can establish that the drug is intended for a life-threatening or chronically debilitating condition affecting no more than five in 10,000 persons in the European Union or that is unlikely to be profitable, and if there is no approved satisfactory treatment or if the drug would be a significant benefit to those persons with the condition. Orphan medicines are entitled to ten years of marketing exclusivity, except under certain limited circumstances comparable to United States law. During this period of marketing exclusivity, no “similar” product, whether or not supported by full safety and efficacy data, will be approved unless a second applicant can establish that its product is safer, more effective or otherwise clinically superior. This period may be reduced to six years if the conditions that originally justified orphan designation change or the sponsor makes excessive profits.
| 12 |
Employees
We currently have five employees, including our sole officer Terry Howlett.
Subsidiaries
We conduct our operations through our wholly-owned subsidiaries, Skinvisible Pharmaceuticals, Inc. and Kintari USA Inc.
Currently, we do not own any real estate. We are leasing our executive offices and research facility. We are located at 6320 South Sandhill Road, Suite 10, Las Vegas, Nevada 89120. We signed an addendum to our lease on February 21, 2013, which extends the term until February 28, 2015. Rent is $2,858 per month plus all applicable CAM charges.
Skinvisible Pharmaceuticals, Inc., our wholly-owned subsidiary, owns the manufacturing and laboratory equipment at this location.
We are not a party to any pending legal proceeding. We are not aware of any pending legal proceeding to which any of our officers, directors, or any beneficial holders of 5% or more of our voting securities are adverse to us or have a material interest adverse to us.
Item 4. Mine Safety Disclosures
Not Applicable
| 13 |
PART II
Item 5. Market for Registrant’s Common Equity and Related Stockholder Matters and Issuer Purchases of Equity Securities
Market Information
Our common stock is quoted under the symbol “SKVI” on the OTCBB operated by the Financial Industry Regulatory Authority, Inc. (“FINRA”) and the OTCQB operated by OTC Markets Group, Inc. Few market makers continue to participate in the OTCBB system because of high fees charged by FINRA. Consequently, market makers that once quoted our shares on the OTCBB system may no longer be posting a quotation for our shares. As of the date of this report, however, our shares are quoted by several market makers on the OTCQB. The criteria for listing on either the OTCBB or OTCQB are similar and include that we remain current in our SEC reporting. Our reporting is presently current and, since inception, we have filed our SEC reports on time.
Only a limited market exists for our securities. There is no assurance that a regular trading market will develop, or if developed, that it will be sustained. Therefore, a shareholder may be unable to resell his securities in our company.
The following table sets forth the range of high and low bid quotations for our common stock for each of the periods indicated as reported by the OTCQB. These quotations reflect inter-dealer prices, without retail mark-up, mark-down or commission and may not necessarily represent actual transactions.
| Fiscal Year Ending December 31, 2013 | ||||||||||
| Quarter Ended | High $ | Low $ | ||||||||
| December 31, 2013 | 0.026 | 0.011 | ||||||||
| September 30, 2013 | 0.025 | 0.013 | ||||||||
| June 30, 2013 | 0.033 | 0.01 | ||||||||
| March 31, 2013 | 0.035 | 0.0225 | ||||||||
| Fiscal Year Ending December 31, 2014 | ||||||||||
| Quarter Ended | High $ | Low $ | ||||||||
| December 31, 2014 | 0.09 | 0.035 | ||||||||
| September 30, 2014 | 0.0524 | 0.0337 | ||||||||
| June 30, 2014 | 0.045 | 0.0155 | ||||||||
| March 31, 2014 | 0.022 | 0.0135 | ||||||||
On March 24, 2015, the last sales price per share of our common stock on the OTCQB was $0.052.
Penny Stock
The SEC has adopted rules that regulate broker-dealer practices in connection with transactions in penny stocks. Penny stocks are generally equity securities with a market price of less than $5.00, other than securities registered on certain national securities exchanges or quoted on the NASDAQ system, provided that current price and volume information with respect to transactions in such securities is provided by the exchange or system. The penny stock rules require a broker-dealer, prior to a transaction in a penny stock, to deliver a standardized risk disclosure document prepared by the SEC, that: (a) contains a description of the nature and level of risk in the market for penny stocks in both public offerings and secondary trading; (b) contains a description of the broker’s or dealer’s duties to the customer and of the rights and remedies available to the customer with respect to a violation of such duties or other requirements of the securities laws; (c) contains a brief, clear, narrative description of a dealer market, including bid and ask prices for penny stocks and the significance of the spread between the bid and ask price; (d) contains a toll-free telephone number for inquiries on disciplinary actions; (e) defines significant terms in the disclosure document or in the conduct of trading in penny stocks; and (f) contains such other information and is in such form, including language, type size and format, as the SEC shall require by rule or regulation.
The broker-dealer also must provide, prior to effecting any transaction in a penny stock, the customer with (a) bid and offer quotations for the penny stock; (b) the compensation of the broker-dealer and its salesperson in the transaction; (c) the number of shares to which such bid and ask prices apply, or other comparable information relating to the depth and liquidity of the market for such stock; and (d) a monthly account statement showing the market value of each penny stock held in the customer’s account.
In addition, the penny stock rules require that prior to a transaction in a penny stock not otherwise exempt from those rules, the broker-dealer must make a special written determination that the penny stock is a suitable investment for the purchaser and receive the purchaser’s written acknowledgment of the receipt of a risk disclosure statement, a written agreement as to transactions involving penny stocks, and a signed and dated copy of a written suitability statement.
These disclosure requirements may have the effect of reducing the trading activity for our common stock. Therefore, stockholders may have difficulty selling our securities.
| 14 |
Holders of Our Common Stock
As of January 6, 2015, we had 111,741,969 shares of our common stock issued and outstanding, held by 184 shareholders of record, other than those held in street name.
Dividends
There are no restrictions in our articles of incorporation or bylaws that prevent us from declaring dividends. The Nevada Revised Statutes, however, do prohibit us from declaring dividends where after giving effect to the distribution of the dividend:
| 1. | we would not be able to pay our debts as they become due in the usual course of business, or; |
| 2. | our total assets would be less than the sum of our total liabilities plus the amount that would be needed to satisfy the rights of shareholders who have preferential rights superior to those receiving the distribution. |
We have not declared any dividends and we do not plan to declare any dividends in the foreseeable future.
Recent Sales of Unregistered Securities
The information set forth below relates to our issuances of securities without registration under the Securities Act of 1933 during the reporting period which were not previously included in a Quarterly Report on Form 10-Q or Current Report on Form 8-K.
On May 19, 2014, we approved a financing plan to offer accredited investors up to $1,000,000 in secured promissory notes. For the period from May 19, 2014 to December 31, 2014 the Company entered into twenty-four 9% notes payable to investors and received total proceeds of $940,000. The notes are due two years from the anniversary date of execution. The Notes are secured by the US Patent rights granted for the Company's Sunscreen Products: US patent number #8,128,913: "Sunscreen Composition with Enhanced UV-A Absorber Stability and Methods".
From January 1, 2015 through the date of filing, we entered into four 9% notes payable to investors and received total proceeds of $110,000. The notes are, due two years from the anniversary date of execution. The Notes are secured by the US Patent rights granted for the Company's Sunscreen Products: US patent number #8,128,913: "Sunscreen Composition with Enhanced UV-A Absorber Stability and Methods".
On October 17, 2014, we granted stock options for 450,000 shares of our common stock to three employees and one consultant. The options have a strike price of $0.05. The stock options were exercisable upon grant and have a life of 5 years.
On December 31, 2014, we issued 72,000 shares with a fair value of $3,312 to settle $3,600 in outstanding accounts payable due to a consultant for research and development services.
On January 27, 2015, we sold 2,000,000 shares of our common stock to two individuals and received proceeds of $80,000.
These securities were issued pursuant to Section 4(2) of the Securities Act and/or Rule 506 promulgated thereunder. The holders represented their intention to acquire the securities for investment only and not with a view towards distribution. The investors were given adequate information about us to make an informed investment decision. We did not engage in any general solicitation or advertising. We directed our transfer agent to issue the stock certificates with the appropriate restrictive legend affixed to the restricted stock.
Securities Authorized for Issuance under Equity Compensation Plans
The following table provides information about our compensation plans under which shares of common stock may be issued upon the exercise of options as of December 31, 2014.
In July 2006, we adopted the 2006 Skinvisible, Inc. Stock Option Plan, which provides for the grant of incentive stock options, non-qualified stock options, stock appreciation rights, restricted stock, performance shares and performance units, and stock awards our officers, directors or employees of, as well as advisers and consultants. This plan was confirmed by our stockholders on August 7, 2006 at the annual shareholders meeting.
Under the 2006 Skinvisible, Inc. Stock Option Plan, we reserved 10,000,000 shares of common stock for the granting of options and rights.
| 15 |
Equity
Compensation Plans as of December 31, 2014
| A | B | C | ||||||||||
| Plan Category | Number of securities to be issued upon exercise of outstanding options, warrants and rights | Weighted-average exercise price of outstanding options, warrants and right | Number of securities remaining available for future issuance under equity compensation plans (excluding securities reflected in column (A)) | |||||||||
| Equity compensation plans approved by security holders | 9,750,00 | $ | 0.05 | 250,000 | ||||||||
| Equity compensation plans not approved by security holders | 2,541,030 | $ | 0.05 | — | ||||||||
| Total | 12,291,030 | $ | 0.05 | 250,000 |
Item 6. Selected Financial Data
A smaller reporting company is not required to provide the information required by this Item.
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations
Forward-Looking Statements
Certain statements, other than purely historical information, including estimates, projections, statements relating to our business plans, objectives, and expected operating results, and the assumptions upon which those statements are based, are “forward-looking statements.” These forward-looking statements generally are identified by the words “believes,” “project,” “expects,” “anticipates,” “estimates,” “intends,” “strategy,” “plan,” “may,” “will,” “would,” “will be,” “will continue,” “will likely result,” and similar expressions. Forward-looking statements are based on current expectations and assumptions that are subject to risks and uncertainties which may cause actual results to differ materially from the forward-looking statements. Our ability to predict results or the actual effect of future plans or strategies is inherently uncertain. Factors which could have a material adverse affect on our operations and future prospects on a consolidated basis include, but are not limited to: changes in economic conditions, legislative/regulatory changes, availability of capital, interest rates, competition, and generally accepted accounting principles. These risks and uncertainties should also be considered in evaluating forward-looking statements and undue reliance should not be placed on such statements.
Results of Operations for the Years Ended December 31, 2014 and 2013
Revenues
Our total revenue from product sales, royalties on patent licenses and license fees for the year ended December 31, 2014 was $54,923, a slight decrease from $66,696 for the year ended December 31, 2013.
The decrease in revenue for the year ended December 31, 2014 was mainly due to a decrease in product sales. With the launch of Kintari USA Inc., we expect increased revenue from our line of anti-aging products and select over-the-counter products.
| 16 |
Cost of Revenues
Our cost of revenues for the year ended December 31, 2014 increased to $26,485 from the prior year when cost of revenues was 2,173. The increase in cost of revenues was attributable to increased cost of goods sold in 2014 of $20,012 as compared with no cost of goods sold for 2013.
Gross Profit
Gross profit for the year ended December 31, 2014 was $28,438, or approximately 51% of sales. Gross profit for the year ended December 31, 2013 was $64,523, or approximately 96% of sales.
Operating Expenses
Operating expenses increased to $1,194,673 for the year ended December 31, 2014 from $824,050 for the year ended December 31, 2013. Our operating expenses for the year ended December 31, 2014 consisted mainly of salaries and wages of $214,223, accrued salaries and wages of $224,336, consulting fees of $167,685, commissions of $94,690, research and development of $56,517, legal fees of $44,205, rent of $41,868, depreciation and amortization expenses of $40,421, accounting and audit expenses of $37,069, payroll tax expenses of $32,418, travel expenses of $26,618, office expenses of $18,548, medical expenses of $18,516, dues and subscriptions of $16,780, director fees of $14,400 and meals and entertainment of $13,767. In comparison, our operating expenses for the year ended December 31, 2013 consisted mainly of salaries and wages of $146,563, accrued salaries and wages of $215,032, commissions of $100,000, consulting fees of $64,185, accounting and audit expenses of $42,280, depreciation and amortization expenses of $37,198, rent of $25,349, edgar fees of $25,853 and travel expenses of $21,450.
Other Expenses
We had other expenses of $760,098 for the year ended December 31, 2014, compared with other expenses of $583,916 for the year ended December 31, 2013. This was largely the result of $755,488 we paid in interest expenses for the year ended December 31, 2014 from $588,098 in the prior period ended December 31, 2013.
We expect to continue to experience high interest payments in the future as a result of our outstanding liabilities. If we are unable to generate sufficient revenues and/or additional financing to service this debt, there is a risk the lenders will call the notes and we will be unable to repay the loans. If this happens, we could go out of business.
Net Loss
Net loss for the year ended December 31, 2014 was $1,926,333 compared to net loss of $1,343,443 for the year ended December 31, 2013.
Liquidity and Capital Resources
As of December 31, 2014, we had total current assets of $290,359 and total assets in the amount of $646,054. Our total current liabilities as of December 31, 2014 were $4,996,725. We had a working capital deficit of $4,706,366 as of December 31, 2014.
Operating activities used $1,167,723 in cash for the year ended December 31, 2014. Our net loss of $1,926,333 was the main component of our negative operating cash flow, offset mainly by amortization of debt discount of $340,972, an increase of accrued interest of $182,914 and an increase in accounts payable and accrued liabilities of $192,919.
Cash flows used by investing activities during the year ended December 31, 2014 was $71,173 as a result of the purchase of fixed and intangible assets.
Cash flows provided by financing activities during the year ended December 31, 2014 amounted to $922,078 and mainly consisted of $940,000 in proceeds on notes payable, offset by $18,000 in payments on convertible promissory notes.
On May 19, 2014, we approved a financing plan to offer accredited investors up to $1,000,000 in secured promissory notes. For the period from May 19, 2014 to December 31, 2014 we entered into twenty-four 9% notes payable to investors and received total proceeds of $940,000. The notes are due two years from the anniversary date of execution. The Notes are secured by the US Patent rights granted for the Company's Sunscreen Products: US patent number #8,128,913: "Sunscreen Composition with Enhanced UV-A Absorber Stability and Methods."
Based upon our current financial condition, we do not have sufficient cash to operate our business at the current level for the next twelve months. We intend to fund operations through increased sales and debt and/or equity financing arrangements, which may be insufficient to fund expenditures or other cash requirements. We plan to seek additional financing in a private equity offering to secure funding for operations. There can be no assurance that we will be successful in raising additional funding. If we are not able to secure additional funding, the implementation of our business plan will be impaired. There can be no assurance that such additional financing will be available to us on acceptable terms or at all.
| 17 |
Off Balance Sheet Arrangements
As of December 31, 2014, there were no off balance sheet arrangements.
Critical Accounting Policies
In December 2001, the SEC requested that all registrants list their most “critical accounting polices” in the Management Discussion and Analysis. The SEC indicated that a “critical accounting policy” is one which is both important to the portrayal of a company’s financial condition and results, and requires management’s most difficult, subjective or complex judgments, often as a result of the need to make estimates about the effect of matters that are inherently uncertain.
Going concern – The accompanying financial statements have been prepared on a going concern basis, which contemplates the realization of assets and the satisfaction of liabilities in the normal course of business. The Company has incurred cumulative net losses of $25,481,141 since its inception and requires capital for its contemplated operational and marketing activities to take place. The Company’s ability to raise additional capital through the future issuances of common stock is unknown. The obtainment of additional financing, the successful development of the Company’s contemplated plan of operations, and its transition, ultimately, to the attainment of profitable operations are necessary for the Company to continue operations. The ability to successfully resolve these factors raise substantial doubt about the Company’s ability to continue as a going concern. The consolidated financial statements of the Company do not include any adjustments that may result from the outcome of these aforementioned uncertainties.
Revenue recognition
Product sales – Revenues from the sale of products (Invisicare® polymers) are recognized when title to the products are transferred to the customer and only when no further contingencies or material performance obligations are warranted, and thereby have earned the right to receive reasonably assured payments for products sold and delivered.
Royalty sales – The Company also recognizes royalty revenue from licensing its patented product formulations only when earned, when no further contingencies or material performance obligations are warranted, and thereby have earned the right to receive and retain reasonably assured payments.
Distribution and license rights sales – The Company also recognizes revenue from distribution and license rights only when earned (and are amortized over a five year period), when no further contingencies or material performance obligations are warranted, and thereby have earned the right to receive and retain reasonably assured payments.
Costs of Revenue – Cost of revenue includes raw materials, component parts, and shipping supplies. Shipping and handling costs is not a significant portion of the cost of revenue.
Accounts Receivable – Accounts receivable is comprised of uncollateralized customer obligations due under normal trade terms requiring payment within 30 days from the invoice date. The carrying amount of accounts receivable is reviewed periodically for collectability. If management determines that collection is unlikely, an allowance that reflects management’s best estimate of the amounts that will not be collected is recorded. Management reviews each accounts receivable balance that exceeds 30 days from the invoice date and, based on an assessment of creditworthiness, estimates the portion, if any, of the balance that will not be collected. As of December 31, 2014, the Company had not recorded a reserve for doubtful accounts. The Company has $1,000,000 in convertible notes payable which are secured by the accounts receivable of a license agreement the Company has with Women's Choice Pharmaceuticals, LLC on its proprietary prescription product, ProCort®.
Recently Issued Accounting Pronouncements
We do not expect the adoption of recently issued accounting pronouncements to have a significant impact on our results of operations, financial position or cash flow.
Item 7A. Quantitative and Qualitative Disclosures About Market Risk
A smaller reporting company is not required to provide the information required by this Item.
| 18 |
Item 8. Financial Statements and Supplementary Data
Index to Financial Statements Required by Article 8 of Regulation S-X:
Audited Financial Statements:
| 19 |

PCAOB Registered Auditors – www.sealebeers.com
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders of
Skinvisible, Inc.
We have audited the accompanying balance sheets of Skinvisible, Inc. as of December 31, 2014 and 2013, and the related statements of income, stockholders’ equity (deficit), and cash flows for periods ended December 31, 2014 and 2013. Skinvisible, Inc.’s management is responsible for these financial statements. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. The company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audit included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of Skinvisible, Inc. as of December 31, 2014 and 2013, and the related statements of income, stockholders’ equity (deficit), and cash flows for the periods ended December 31, 2014 and 2013 in conformity with accounting principles generally accepted in the United States of America.
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 1 to the financial statements, the Company has minimal revenues, has negative working capital at December 31, 2014, has incurred recurring losses and recurring negative cash flow from operating activities, and has an accumulated deficit which raises substantial doubt about its ability to continue as a going concern. Management’s plans concerning these matters are also described in Note 1. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
/s/ Seale and Beers, CPAs
Seale and Beers, CPAs
Las Vegas, Nevada
March 30, 2015
| F-1 |
SKINVISIBLE,
INC.
CONSOLIDATED BALANCE SHEETS
(AUDITED)
| Years Ended | ||||||||
| December 31, 2014 | December 31, 2013 | |||||||
| ASSETS | ||||||||
| Current assets | ||||||||
| Cash | $ | 196,602 | $ | 513,420 | ||||
| Accounts receivable | 9,457 | 11,591 | ||||||
| Inventory | 83,155 | 22,437 | ||||||
| Due from related party | 1,145 | 1,145 | ||||||
| Prepaid expense and other current assets | — | 515 | ||||||
| Total current assets | 290,359 | 549,108 | ||||||
| Fixed assets, net of accumulated depreciation of $324,275 and $322,813, respectively | 3,275 | 3,555 | ||||||
| Intangible and other assets: | ||||||||
| Patents and trademarks, net of accumulated amortization of $288,203 and $249,064, respectively | 352,420 | 321,388 | ||||||
| Total assets | $ | 646,054 | $ | 874,051 | ||||
| LIABILITIES AND STOCKHOLDERS' DEFICIT | ||||||||
| Current liabilities | ||||||||
| Accounts payable and accrued liabilities | $ | 420,762 | $ | 479,622 | ||||
| Accrued interest payable | 348,111 | 165,197 | ||||||
| Loans from related party | 1,910 | 1,832 | ||||||
| Loans payable | 1,940,000 | 1,000,000 | ||||||
| Convertible notes payable, net of unamortized debt discount of $26,822 and $66,490, respectively | 1,112,764 | 1,091,097 | ||||||
| Convertible notes payable related party, net of unamortized discount of $874,163 and $949,672, respectively | 1,173,178 | 873,689 | ||||||
| Total current liabilities | 4,996,725 | 3,611,437 | ||||||
| Total liabilities | 4,996,725 | 3,611,437 | ||||||
| Stockholders' deficit | ||||||||
| Common stock; $0.001 par value; 200,000,000 shares authorized; 111,813,969 and 110,909,969 shares issued and outstanding at December 31, 2014 and December 31, 2013, respectively | 111,814 | 110,910 | ||||||
| Additional paid-in capital | 21,378,656 | 21,066,512 | ||||||
| Stock payable | — | — | ||||||
| Accumulated deficit | (25,841,141 | ) | (23,914,808 | ) | ||||
| Total stockholders' deficit | (4,350,671 | ) | (2,737,386 | ) | ||||
| Total liabilities and stockholders' deficit | $ | 646,054 | $ | 874,051 | ||||
See Accompanying Notes to Consolidated Financial Statements.
| F-2 |
SKINVISIBLE,
INC.
CONSOLIDATED STATEMENTS OF OPERATIONS
(AUDITED)
| Year Ended | ||||||||
| December 31, 2014 | December 31, 2013 | |||||||
| Revenues | $ | 54,923 | $ | 66,696 | ||||
| Cost of revenues | 26,485 | 2,173 | ||||||
| Gross profit | 28,438 | 64,523 | ||||||
| Operating expenses | ||||||||
| Depreciation and amortization | 40,421 | 37,198 | ||||||
| Selling general and administrative | 1,154,252 | 786,852 | ||||||
| Total operating expenses | 1,194,673 | 824,050 | ||||||
| Loss from operations | (1,166,235 | ) | (759,527 | ) | ||||
| Other income and (expense) | ||||||||
| Other income | 303 | 272 | ||||||
| Interest expense | (755,488 | ) | (588,098 | ) | ||||
| Gain on extinguishment of debt | (4,913 | ) | 3,910 | |||||
| Total other expense | (760,098 | ) | (583,916 | ) | ||||
| Net loss | $ | (1,926,333 | ) | $ | (1,343,443 | ) | ||
| Basic loss per common share | $ | (0.02 | ) | $ | (0.01 | ) | ||
| Basic weighted average common shares outstanding | 111,399,613 | 110,508,469 | ||||||
See Accompanying Notes to Consolidated Financial Statements.
| F-3 |
SKINVISIBLE,
INC.
CONSOLIDATED STATEMENT OF STOCKHOLDERS' DEFICIT
| Total | ||||||||||||||||||||||||
| Common Stock | Additional | Stock | Accumulated | Stockholders' | ||||||||||||||||||||
| Shares | Amount | Paid-in Capital | Payable | Deficit | Deficit | |||||||||||||||||||
| Balance, December 31, 2012 (audited) | 109,507,409 | 109,507 | 20,841,670 | 1,800 | (22,571,365 | ) | $ | (1,618,388 | ) | |||||||||||||||
| Issuance of stock for conversion of debts | 1,402,560 | 1,403 | 45,241 | (1,800 | ) | — | 44,844 | |||||||||||||||||
| Issuance of warrants for services | — | — | 13,923 | — | — | 13,923 | ||||||||||||||||||
| Financing costs related to convertible notes payable | — | — | 165,678 | — | — | 165,678 | ||||||||||||||||||
| Net loss | — | — | — | — | (1,343,443 | ) | (1,343,443 | ) | ||||||||||||||||
| Balance, December 31, 2013 (audited) | 110,909,969 | 110,910 | 21,066,512 | — | (23,914,808 | ) | $ | (2,737,386 | ) | |||||||||||||||
| Issuance of stock for conversion of debts | 904,000 | 904 | 31,809 | — | — | 32,713 | ||||||||||||||||||
| Issuance of warrants and options for services | — | — | 54,450 | — | — | 54,450 | ||||||||||||||||||
| Financing costs related to convertible notes payable | — | — | 225,885 | — | — | 225,885 | ||||||||||||||||||
| Net loss | — | — | — | — | (1,926,333 | ) | (1,926,333 | ) | ||||||||||||||||
| Balance, December 31, 2014 (audited) | 111,813,969 | 111,814 | 21,378,656 | — | (25,841,141 | ) | $ | (4,350,671 | ) | |||||||||||||||
See Accompanying Notes to Consolidated Financial Statements.
| F-4 |
SKINVISIBLE,
INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS
(AUDITED)
| Years Ended | ||||||||
| December 31, 2014 | December 31, 2013 | |||||||
| Cash flows from operating activities: | ||||||||
| Net loss | $ | (1,926,333 | ) | $ | (1,343,443 | ) | ||
| Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||
| Depreciation and amortization | 40,421 | 37,198 | ||||||
| Stock-based compensation | 54,540 | 21,200 | ||||||
| Amortization of debt discount | 340,972 | 288,671 | ||||||
| Stock issued for interest expense | — | 422 | ||||||
| Gain on extinguishment of debt | 4,913 | 3,910 | ||||||
| Gain of sale of assets | ||||||||
| Changes in operating assets and liabilities: | ||||||||
| (Increase) decrease in inventory | (60,718 | ) | (3,668 | ) | ||||
| Decrease in accounts receivable | 2,134 | 15,708 | ||||||
| Decrease in prepaid assets | 515 | — | ||||||
| Increase in accounts payable and accrued liabilities | 192,919 | 109,343 | ||||||
| Increase in accrued interest | 182,914 | 138,797 | ||||||
| Decrease in unearned revenue | — | (19,792 | ) | |||||
| Net cash used in operating activities | (1,167,723 | ) | (751,654 | ) | ||||
| Cash flows from investing activities: | ||||||||
| Purchase of fixed and intangible assets | (71,173 | ) | (106,129 | ) | ||||
| Net cash used in investing activities | (71,173 | ) | (106,129 | ) | ||||
| Cash flows from financing activities: | ||||||||
| Proceeds from related party loans, net of payments | 78 | (5,829 | ) | |||||
| Payments on related parties convertible notes payable | — | (55,475 | ) | |||||
| Proceeds from notes payable | 940,000 | 1,000,000 | ||||||
| Payments on convertible notes payable | (18,000 | ) | (18,000 | ) | ||||
| Net cash provided by (used in) financing activities | 922,078 | 920,696 | ||||||
| Net change in cash | (316,818 | ) | 62,913 | |||||
| Cash, beginning of period | 513,420 | 450,507 | ||||||
| Cash, end of period | $ | 196,602 | $ | 513,420 | ||||
| Supplemental disclosure of cash flow information: | ||||||||
| Cash paid for interest | $ | 206,383 | $ | 144,763 | ||||
| Cash paid for tax | $ | — | $ | — | ||||
| SUPPLEMENTAL DISCLOSURE OF CASH FLOW INFORMATION: | ||||||||
| Non-cash investing and financing activities: | ||||||||
| Common stock payable on conversion of debts | $ | — | $ | (1,800 | ) | |||
| Common stock issued on conversion of debts | $ | — | $ | 37,567 | ||||
| Common stock issued on extinguishment of debts | $ | 32,713 | $ | — | ||||
| Beneficial conversion feature | $ | 225,885 | $ | 165,678 | ||||
| Common stock issued on conversion of interest | $ | — | $ | 422 | ||||
| Accrued expenses converted to notes | $ | 223,979 | $ | 422 | ||||
See Accompanying Notes to Consolidated Financial Statements.
| F-5 |
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(AUDITED)
1. DESCRIPTION OF BUSINESS, HISTORY AND SUMMARY OF SIGNIFICANT POLICIES
Description of business – Skinvisible, Inc., (referred to as the “Company”) is focused on the development and manufacture and sales of innovative topical, transdermal and mucosal polymer-based delivery system technologies and formulations incorporating its patent-pending formula/process for combining hydrophilic and hydrophobic polymer emulsions. The technologies and formulations have broad industry applications within the pharmaceutical, over-the-counter, personal skincare and cosmetic arenas. Additionally, the Company’s non-dermatological formulations, offer solutions for a broad spectrum of markets women’s health, pain management, and others. The Company maintains executive and sales offices in Las Vegas, Nevada.
History – Skinvisible, Inc. (referred to as the “Company”) was incorporated in Nevada on March 6, 1998, under the name of Microbial Solutions, Inc. The Company underwent a name change on February 26, 1999, when it changed its name to Skinvisible, Inc. The Company’s subsidiary’s name of Manloe Labs, Inc. was also changed to Skinvisible Pharmaceuticals, Inc.
On September 9, 2014, the Company formed Kinatri USA Inc., a new wholly-owned subsidiary, to market a premium line of scientifically formulated skincare products powered by our patented Invisicare® technology. As part of our strategic focus on revenue generation and creating shareholder value, Kintari USA Inc. products will be sold via network marketing.
The Kintari product portfolio consists of anti-aging products to help fight the signs of aging. These products have been developed using proven anti-aging ingredients with scientific evidence of their effectiveness at reducing the look of fine lines and wrinkles resulting in youthful looking skin. These potent ingredients will be powered by patented Invisicare technology, providing consumers with unique, effective products which we believe cannot be duplicated. Additional products will be added to enhance this product line as the company grows and expands.
Skinvisible, Inc. together with its subsidiary shall herein be collectively referred to as the “Company”.
Going concern – The accompanying financial statements have been prepared on a going concern basis, which contemplates the realization of assets and the satisfaction of liabilities in the normal course of business. The Company has incurred cumulative net losses of $25,481,141 since its inception and requires capital for its contemplated operational and marketing activities to take place. The Company’s ability to raise additional capital through the future issuances of common stock is unknown. The obtainment of additional financing, the successful development of the Company’s contemplated plan of operations, and its transition, ultimately, to the attainment of profitable operations are necessary for the Company to continue operations. The ability to successfully resolve these factors raise substantial doubt about the Company’s ability to continue as a going concern. The consolidated financial statements of the Company do not include any adjustments that may result from the outcome of these aforementioned uncertainties.
Principles of consolidation – The consolidated financial statements include the accounts of the Company and its subsidiaries. All significant intercompany balances and transactions have been eliminated.
Use of estimates – The preparation of consolidated financial statements in conformity with accounting principles generally accepted in the United States requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the consolidated financial statements and the reported amounts of revenue and expenses during the reporting period. Actual results could differ from those estimates.
Cash and cash equivalents – For purposes of the statement of cash flows, the Company considers all highly liquid investments and short-term debt instruments with original maturities of three months or less to be cash equivalents. There are $196,602 and $513,420 in cash and cash equivalents as of December 31, 2014 and December 31, 2013, respectively.
| F-6 |
Fair Value of Financial Instruments
The carrying amounts reflected in the balance sheets for cash, accounts payable and accrued expenses approximate the respective fair values due to the short maturities of these items.
As required by the Fair Value Measurements and Disclosures Topic of the FASB ASC, fair value is measured based on a three-tier fair value hierarchy, which prioritizes the inputs used in measuring fair value as follows: (Level 1) observable inputs such as quoted prices in active markets; (Level 2) inputs, other than the quoted prices in active markets, that are observable either directly or indirectly; and (Level 3) unobservable inputs in which there is little or no market data, which require the reporting entity to develop its own assumptions.
The three levels of the fair value hierarchy are described below:
Level 1: Unadjusted quoted prices in active markets that are accessible at the measurement date for identical, unrestricted assets or liabilities;
Level 2: Quoted prices in markets that are not active, or inputs that are observable, either directly or indirectly, for substantially the full term of the asset or liability;
Level 3: Prices or valuation techniques that require inputs that are both significant to the fair value measurement and unobservable (supported by little or no market activity).
Revenue recognition
Product sales – Revenues from the sale of products (Invisicare® polymers) are recognized when title to the products are transferred to the customer and only when no further contingencies or material performance obligations are warranted, and thereby have earned the right to receive reasonably assured payments for products sold and delivered.
Royalty sales – The Company also recognizes royalty revenue from licensing its patented product formulations only when earned, when no further contingencies or material performance obligations are warranted, and thereby have earned the right to receive and retain reasonably assured payments.
Distribution and license rights sales – The Company also recognizes revenue from distribution and license rights only when earned (and are amortized over a five year period), when no further contingencies or material performance obligations are warranted, and thereby have earned the right to receive and retain reasonably assured payments.
Costs of Revenue – Cost of revenue includes raw materials, component parts, and shipping supplies. Shipping and handling costs is not a significant portion of the cost of revenue.
Accounts Receivable – Accounts receivable is comprised of uncollateralized customer obligations due under normal trade terms requiring payment within 30 days from the invoice date. The carrying amount of accounts receivable is reviewed periodically for collectability. If management determines that collection is unlikely, an allowance that reflects management’s best estimate of the amounts that will not be collected is recorded. Management reviews each accounts receivable balance that exceeds 30 days from the invoice date and, based on an assessment of creditworthiness, estimates the portion, if any, of the balance that will not be collected. As of December 31, 2014, the Company had not recorded a reserve for doubtful accounts. The Company has $1,000,000 in convertible notes payable which are secured by the accounts receivable of a license agreement the Company has with Women's Choice Pharmaceuticals, LLC on its proprietary prescription product, ProCort®.
Inventory – Substantially all inventory consists of finished goods and are valued based upon first-in first-out ("FIFO") cost, not in excess of market. The determination of whether the carrying amount of inventory requires a write-down is based on an evaluation of inventory.
Goodwill and intangible assets – The Company follows Financial Accounting Standard Board’s (FASB) Codification Topic 350-10 (“ASC 350-10”), “Intangibles – Goodwill and Other”. According to this statement, goodwill and intangible assets with indefinite lives are no longer subject to amortization, but rather an annual assessment of impairment by applying a fair-value based test. Fair value for goodwill is based on discounted cash flows, market multiples and/or appraised values as appropriate. Under ASC 350-10, the carrying value of assets are calculated at the lowest level for which there are identifiable cash flows.
Income taxes – The Company accounts for its income taxes in accordance with FASB Codification Topic ASC 740-10, “Income Taxes”, which requires recognition of deferred tax assets and liabilities for future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases and tax credit carry-forwards. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period that includes the enactment date.
| F-7 |
Stock-based compensation – The Company follows the guidelines in FASB Codification Topic ASC 718-10 “Compensation-Stock Compensation”, which requires the measurement and recognition of compensation expense for all share-based payment awards made to employees and directors including employee stock options and employee stock purchases related to a Employee Stock Purchase Plan based on the estimated fair values.
Stock based compensation expense recognized under ASC 718-10 for the year ended December 31, 2014 and 2013 totaled $54,450 and $21,200, respectively.
Earnings (loss) per share – The Company reports earnings (loss) per share in accordance with FASB Codification Topic ASC 260-10 “Earnings Per Share”, Basic earnings (loss) per share is computed by dividing income (loss) available to common shareholders by the weighted average number of common shares available. Diluted earnings (loss) per share is computed similar to basic earnings (loss) per share except that the denominator is increased to include the number of additional common shares that would have been outstanding if the potential common shares had been issued and if the additional common shares were dilutive. Diluted earnings (loss) per share has not been presented since the effect of the assumed exercise of options and warrants to purchase common shares (common stock equivalents) would have an anti-dilutive effect.
2. FIXED ASSETS
Fixed assets consist of the following as of December 31, 2014 and December 31, 2013:
| December 31, 2014 | December 31, 2013 | |||||||
| Machinery and equipment | $ | 48,163 | $ | 48,163 | ||||
| Furniture and fixtures | 113,635 | 113,635 | ||||||
| Computers, equipment and software | 39,722 | 38,540 | ||||||
| Leasehold improvements | 12,569 | 12,569 | ||||||
| Lab equipment | 113,461 | 113,461 | ||||||
| Total | 327,550 | 326,368 | ||||||
| Less: accumulated depreciation | (324,275 | ) | (322,813 | ) | ||||
| Fixed assets, net of accumulated depreciation | $ | 3,275 | $ | 3,555 | ||||
Depreciation expense for the years ended December 31, 2014 and 2013 was $1,462 and $1,339, respectively.
3. INVENTORY
Inventory consist of the following as of December 31, 2014 and December 31, 2013:
| December 31, 2014 | December 31, 2013 | |||||||
| Shipping and Packing materials | $ | 14,758 | — | |||||
| Finished Goods | 51,756 | $ | 5,955 | |||||
| Raw Materials | 16,641 | 16,482 | ||||||
| Total | 83,155 | 22,437 | ||||||
4. INTANGIBLE AND OTHER ASSETS
Patents and trademarks are capitalized at their historical cost and are amortized over their estimated useful lives. As of December 31, 2014, patents and trademarks total $587,944, net of $288,023 of accumulated amortization. Amortization expense for the years ended December 31, 2014 and 2013 was $38,959 and $35,859 respectively.
License and distributor rights (“agreement”) were acquired by the Company in January 1999 and provide exclusive use distribution of polymers and polymer based products. The Company has a non-expiring term on the license and distribution rights. Accordingly, the Company annually assesses this license and distribution rights for impairment and has determined that no impairment write-down is considered necessary as of December 31, 2014.
| F-8 |
5. UNEARNED REVENUE
On January 16, 2013, the company terminated its licensing agreement with Panalab dated January 23, 2008. The agreement provided Panalab the right to distribute, market, sell and promote Skinvisible’s proprietary formulas made with Invisicare and Adapalene through-out Panalabs assigned territory. Panalab had failed to sell or sub-license the products in the territory, thereby not fulfilling the conditions as set forth in the agreement and allowing for immediate termination of the agreement. As a result of this cancelation, unearned revenue of $19,792 was recognized as revenue during the year ended December 31, 2013.
6. STOCK OPTIONS AND WARRANTS
The following is a summary of option activity during the year ended December 31, 2014.
| Number of Shares | Weighted Average Exercise Price | |||||||
| Balance, December 31, 2013 | 9,300,000 | $ | 0.05 | |||||
| Options granted and assumed | 450,000 | $ | 0.05 | |||||
| Options expired | — | — | ||||||
| Options canceled | — | — | ||||||
| Options exercised | — | — | ||||||
| Balance, December 31, 2014 | 9,750,000 | $ | 0.05 | |||||
As of December 31, 2014, 9,750,000 stock options are exercisable.
On September 23, 2013, the Company granted stock options for 300,000 shares of its common stock with a strike price of $0.03. The stock options were exercisable upon grant and have a life of 5 years. The stock options were valued at $8,400 using the Black-Scholes option pricing model based upon the following assumptions: term of 5 years, risk free interest rate of 1.33%, a dividend yield of 0% and volatility rates of 443%. The Company recorded an expense of $8,400 for the year ended December 31, 2013.
On October 17, 2014, the Company granted stock options for 450,000 shares of its common stock to three employees of the Company and one Consultant. The options have a strike price of $0.05. The stock options were exercisable upon grant and have a life of 5 years. The stock options were valued at $23,400 using the Black-Scholes option pricing model based upon the following assumptions: term of 5 years, risk free interest rate of 1.44%, a dividend yield of 0% and volatility rates of 470%. The Company recorded an expense of $23,400 for the year ended December 31, 2014.
| F-9 |
Stock warrants -
The following is a summary of warrants activity during the year ended December 31, 2014.
| Number of Shares | Weighted Average Exercise Price | |||||||
| Balance, December 31, 2013 | 3,818,780 | $ | 0.05 | |||||
| Warrants granted and assumed | 700,000 | 0.04 | ||||||
| Warrants expired | 1,977,750 | 0.06 | ||||||
| Warrants canceled | — | — | ||||||
| Warrants exercised | — | — | ||||||
| Balance, December 31, 2014 | 2,541,030 | $ | 0.05 | |||||
All warrants outstanding as of December 31, 2014 are exercisable.
On February 11, 2013, the Company issued a warrant for 180,915 shares of common stock to the note holder with a exercise price of $0.05. The vesting period on these grant was immediate. The value of these warrants were estimated by using the Black-Scholes option pricing model with the following assumptions: expected life of 2 years; risk free interest rate of 0.27%; dividend yield of 0% and expected volatility of 178%. To account for such grants to non-employees, we recorded the issuance as consulting expense in the amount of $1,683.
On February 11, 2013, the Company issued a warrant for 90,365 shares of common stock to a note holder with a exercise price of $0.05. The vesting period on these grant was immediate. The value of these warrants were estimated by using the Black-Scholes option pricing model with the following assumptions: expected life of 2 years; risk free interest rate of 0.27%; dividend yield of 0% and expected volatility of 178%. To account for such grants to non-employees, we recorded the issuance as consulting expense in the amount of $840.
On May 22, 2013, the Company issued a warrant for 200,000 shares of common stock to Retire Happy, LLC for Consulting services. The warrants have an exercise price of $0.04 and the vesting period on these grants was immediate. The value of these warrants were estimated by using the Black-Scholes option pricing model with the following assumptions: expected life of 1 years; risk free interest rate of 0.11%; dividend yield of 0% and expected volatility of 167%. To account for such grants to non-employees, we recorded the issuance as consulting expense in the amount of $3,000.
On May 20, 2014, the Company issued a warrant for 200,000 shares of common stock to Retire Happy, LLC for Consulting services. The warrants have an exercise price of $0.02 and the vesting period on these grants was immediate. The value of these warrants were estimated by using the Black-Scholes option pricing model with the following assumptions: expected life of 1 years; risk free interest rate of 0.09%; dividend yield of 0% and expected volatility of 292%. To account for such grants to non-employees, we recorded the issuance as consulting expense in the amount of $4,600.
On August 20, 2014, the Company issued a warrant for 500,000 shares of common stock to Retire Happy, LLC for Consulting services. The warrants have an exercise price of $0.05 and the vesting period on these grants was immediate. The value of these warrants were estimated by using the Black-Scholes option pricing model with the following assumptions: expected life of 1 years; risk free interest rate of 0.012%; dividend yield of 0% and expected volatility of 217%. To account for such grants to non-employees, we recorded the issuance as consulting expense in the amount of $26,450.
| F-10 |
7. NOTES PAYABLE
On May 22, 2013 the Company approved a financing plan to offer accredited investors up to $1,000,000 in secured promissory notes. During the year ended December 31, 2013 the Company entered into twenty-four 9% notes payable to investors and received total proceeds of $1,000,000. The notes are due two years from the anniversary date of execution. The Notes are secured by the US Patent rights granted for the Company's Sunscreen Products: US patent number #8,128,913: "Sunscreen Composition with Enhanced UV-A Absorber Stability and Methods".
On May 19, 2014 the Company approved a financing plan to offer accredited investors up to an additional $1,000,000 in secured promissory notes. For the period from May 19, 2014 to December 31, 2014 the Company entered into twenty-four 9% notes payable to investors and received total proceeds of $940,000. The notes are due two years from the anniversary date of execution. The Notes are secured by the US Patent rights granted for the Company's Sunscreen Products: US patent number #8,128,913: "Sunscreen Composition with Enhanced UV-A Absorber Stability and Methods".
8. RELATED PARTY TRANSACTIONS
During the year ended 2013 various officers advanced funds to support the daily operations of the company. As of December 31, 2014, $1,145 remained due to related parties as repayment for advanced monies, all related other party notes have been extinguished or re-negotiated as convertible notes. See note 10.
| F-11 |
9. CONVERTIBLE NOTES PAYABLE
| Convertible Notes Payable at consists of the following: | December 31, | December 31, | ||||||
| 2014 | 2013 | |||||||
| $52,476 face value,10% unsecured note payable to an investor, note interest and payment are due on demand. The note could be converted to option rights for Skinvisible, Inc. shares at ten cents per share ($0.10), these rights expired January 12, 2010. Note is currently in default, no penalties occur due to default. | $ | 28,476 | $ | 36,476 | ||||
| $27,000 face value 10% unsecured notes payable to investors, due October, 2012. At the written request of the investor’s until the repayment date, the note may be converted at the investors option to shares of the Company’s common stock at a fixed price of $0.05 per share along with additional warrants to purchase one share for every two shares issued at the exercise price of $0.07 per share for two years after the conversion date. The Company has determined the value associated with the beneficial conversion feature in connection with the notes to be $19,385. The aggregate beneficial conversion feature was accreted and charged to interest expenses as a financing expense in the amount of $19,385 in the year ending December 31, 2012. The beneficial conversion feature is valued under the intrinsic value method. Interest due to lender can also be converted at a rate of ($0.05) per share into warrants. | — | 10,000 | ||||||
| $1,000,000 face value 9% unsecured notes payable to investors, due in 2015. At the investor’s option until the repayment date, the note and related interest may be converted to shares of the Company’s common stock a discount of 90% of the current share price after the first anniversary of the note. The Notes are secured by the accounts receivable of a license agreement the Company has with Womens Choice Pharmaceuticals, LLC on its proprietary prescription product, ProCort®. The Company has determined the value associated with the beneficial conversion feature in connection with the notes and interest to be $111,110. The aggregate original issue discount feature has been accreted and charged to interest expenses as a financing expense in the amount of $36,776 during the year ended December 31, 2014. The original issue discount feature is valued under the intrinsic value method. | 1,000,000 | 1,000,000 | ||||||
| Original issue discount | 111,110 | 111,110 | ||||||
| Unamortized debt discount | (26,822 | ) | (66,490 | ) | ||||
| $ | 1,112,764 | $ | 1,091,097 | |||||
| F-12 |
10. CONVERTIBLE NOTES PAYABLE RELATED PARTY
| Convertible Notes Payable Related Party at consists of the following: | December 31, | December 31, | ||||||
| 2014 | 2013 | |||||||
| On December 31, 2011, the Company re-negotiated accrued salaries and interest for three employees. Under the terms of the agreements, the notes dated before December 31, 2010, and all salaries not previously converted were converted to promissory notes convertible into common stock with a warrant feature. The promissory notes are unsecured, due five years from issuance, and bear an interest rate of 10%. At the investor’s option until the repayment date, the note may be converted to shares of the Company’s common stock at a fixed price of $0.04 per share along with additional warrants to purchase one share for every two shares issued at the exercise price of $0.06 per share for three years after the conversion date. The Company has determined the value associated with the beneficial conversion feature in connection with the notes negotiated on December 31, 2011 to be $1,123,078. The aggregate beneficial conversion feature has been accreted and charged to interest expenses as a financing expense in the amount of $166,510 during the year ending December 31, 2014. The beneficial conversion feature is valued under the intrinsic value method. In the year ending December 2013, the Company made $51,485 in cash payments to reduce the note balance. | 1,071,593 | 1,071,593 | ||||||
| Unamortized debt discount | (333,483 | ) | (499,993 | ) | ||||
| On June 30, 2012, the Company re-negotiated accrued salaries and interest for three employees. Under the terms of the agreements, the notes dated before July 1, 2011, and all salaries not previously converted were converted to promissory notes convertible into common stock with a warrant feature. The promissory notes are unsecured, due five years from issuance, and bear an interest rate of 10%. At the investor’s option until the repayment date, the note may be converted to shares of the Company’s common stock at a fixed price of $0.04 per share along with additional warrants to purchase one share for every two shares issued at the exercise price of $0.06 per share for three years after the conversion date. The Company has determined the value associated with the beneficial conversion feature in connection with the notes to be $209,809. The aggregate beneficial conversion feature has been accreted and charged to interest expenses as a financing expense in the amount of $41,576 during the year ending December 31, 2014. The beneficial conversion feature is valued under the intrinsic value method. On January 18, 2013, the Company made a $3,990 cash payment to reduce the note balance. | 321,032 | 321,032 | ||||||
| Unamortized debt discount | (103,886 | ) | (145,462 | ) | ||||
| F-13 |
| On December 30 and 31, 2012, the Company re-negotiated accrued salaries and interest for three employees. Under the terms of the agreements, $182,083 of related party notes accrued interest and salaries not previously converted were converted to promissory notes convertible into common stock with a warrant feature. The $182,083 face value promissory notes are unsecured, due five years from issuance, and bear an interest rate of 10%. At the investor’s option until the repayment date, the note may be converted to shares of the Company’s common stock at a fixed price of $0.03 per share along with additional warrants to purchase one share for every two shares issued at the exercise price of $0.04 per share for three years after the conversion date. The Company has determined the value associated with the beneficial conversion feature in connection with the notes to be $182,083. The aggregate beneficial conversion feature has been accreted and charged to interest expenses as a financing expense in the amount of $36,395 during the year ending December 31, 2014. The beneficial conversion feature is valued under the intrinsic value method. | 182,083 | 182,083 | ||||||
| Unamortized debt discount | (109,277 | ) | (145,672 | ) | ||||
| On June 30, 2013, the Company re-negotiated accrued salaries and interest for two employees. Under the terms of the agreements, $106,153 of accrued interest and salaries were converted to promissory notes convertible into common stock with a warrant feature. The $106,153 face value promissory notes are unsecured, due five years from issuance, and bear an interest rate of 10%. At the investor’s option until the repayment date, the note may be converted to shares of the Company’s common stock at a fixed price of $0.03 per share along with additional warrants to purchase one share for every two shares issued at the exercise price of $0.04 per share for three years after the conversion date. The Company has determined the value associated with the beneficial conversion feature in connection with the notes to be $70,768. The aggregate beneficial conversion feature has been accreted and charged to interest expenses as a financing expense in the amount of $14,145 during the year ending December 31, 2014. The beneficial conversion feature is valued under the intrinsic value method. | 106,152 | 106,152 | ||||||
| Unamortized debt discount | (49,491 | ) | (63,636 | ) |
| F-14 |
On December 31, 2013, the Company re-negotiated accrued salaries and interest for three employees. Under the terms of the agreements, $142,501 of accrued interest and salaries not previously converted were converted to promissory notes convertible into common stock with a warrant feature. The $142,501 face value promissory notes are unsecured, due five years from issuance, and bear an interest rate of 10%. At the investor’s option until the repayment date, the note may be converted to shares of the Company’s common stock at a fixed price of $0.03 per share along with additional warrants to purchase one share for every two shares issued at the exercise price of $0.04 per share for three years after the conversion date. The Company has determined the value associated with the beneficial conversion feature in connection with the notes to be $94,909. The aggregate beneficial conversion feature has been accreted and charged to interest expenses as a financing expense in the amount of $18,972 during the year ending December 31, 2014. The beneficial conversion feature is valued under the intrinsic value method. | 142,501 | 142,501 | ||||||
| Unamortized debt discount | (75,937 | ) | (94,909 | ) | ||||
| On June 30, 2014, the Company re-negotiated accrued salaries and interest for three employees. Under the terms of the agreements, $118,126 of accrued salaries not previously converted were converted to promissory notes convertible into common stock with a warrant feature. The $118,126 face value promissory notes are unsecured, due five years from issuance, and bear an interest rate of 10%. At the investor’s option until the repayment date, the note may be converted to shares of the Company’s common stock at a fixed price of $0.025 per share along with additional warrants to purchase one share for every two shares issued at the exercise price of $0.03 per share for three years after the conversion date. The Company has determined the value associated with the beneficial conversion feature in connection with the notes to be $118,126. The aggregate beneficial conversion feature has been accreted and charged to interest expenses as a financing expense in the amount of $11,903 during the year ending December 31, 2014. The beneficial conversion feature is valued under the intrinsic value method. | 118,126 | — | ||||||
| Unamortized debt discount | (106,223 | ) | — | |||||
| On September 30, 2014, the Company re-negotiated accrued salaries and interest for two employees. Under the terms of the agreements, $40,558 of accrued salaries not previously converted were converted to promissory notes convertible into common stock with a warrant feature. The $40,558 face value promissory notes are unsecured, due five years from issuance, and bear an interest rate of 10%. At the investor’s option until the repayment date, the note may be converted to shares of the Company’s common stock at a fixed price of $0.04 per share along with additional warrants to purchase one share for every two shares issued at the exercise price of $0.05 per share for three years after the conversion date. The Company has determined the value associated with the beneficial conversion feature in connection with the notes to be $40,466. The aggregate beneficial conversion feature has been accreted and charged to interest expenses as a financing expense in the amount of $2,039 during the year ending December 31, 2014. The beneficial conversion feature is valued under the intrinsic value method. | 40,558 | — | ||||||
| Unamortized debt discount | (38,427 | ) | — |
| F-15 |
| On December 31, 2014, the Company re-negotiated accrued salaries and interest for two employees. Under the terms of the agreements, $65,295 of accrued salaries not previously converted were converted to promissory notes convertible into common stock with a warrant feature. The $65,295 face value promissory notes are unsecured, due five years from issuance, and bear an interest rate of 10%. At the investor’s option until the repayment date, the note may be converted to shares of the Company’s common stock at a fixed price of $0.04 per share along with additional warrants to purchase one share for every two shares issued at the exercise price of $0.05 per share for three years after the conversion date. The Company has determined the value associated with the beneficial conversion feature in connection with the notes to be $57,439. The aggregate beneficial conversion feature will be accreted and charged to interest expenses as a financing expense. The beneficial conversion feature is valued under the intrinsic value method. | 65,295 | — | ||||||
| Unamortized debt discount | (57,438 | ) | — | |||||
| $ | 1,173.178 | $ | 873,689 |
11. STOCKHOLDERS’ DEFICIT
The Company is authorized to issue 200,000,000 shares of $0.001 par value common stock. The Company had 111,813,969 and 110,909,969 issued and outstanding shares of common stock as of December 31, 2014 and December 31, 2013, respectively.
During the year ended December 31, 2013, the Company issued a total of 1,402,560 shares of common stock, with a fair value of $44,554 for the conversion of outstanding debts of $37,567, related interest of $1,277 and settlement of stock payable $1,800. The Company recorded a gain of $3,910 on extinguishment of debts.
On January 7, 2014 the Company issued 180,000 shares with a fair value of $5,400 to settle $3,600 in outstanding accounts payable. As a result of this transaction a loss on extinguishment of debt of $1,800 was recognized.
On April 3, 2014 the Company issued 180,000 shares with a fair value of $2,790 to settle $3,600 in outstanding accounts payable. As a result of this transaction a gain on extinguishment of debt of $810 was recognized.
On July 1, 2014 the Company issued 140,000 shares with a fair value of $5,614 to settle $3,600 in outstanding accounts payable. As a result of this transaction a loss on extinguishment of debt of $2,014 was recognized.
On July 7, 2014 the Company issued 80,000 shares with a fair value of $3,400 to settle $2,400 in outstanding accounts payable due to a consultant for research and development services. As a result of this transaction a loss on extinguishment of debt of $1,000 was recognized.
On August 4, 2014 the Company issued 80,000 shares with a fair value of $3,440 to settle $2,400 in outstanding accounts payable due to a consultant for research and development services. As a result of this transaction a loss on extinguishment of debt of $1,040 was recognized.
On August 4, 2014 the Company issued 80,000 shares with a fair value of $3,440 to settle $2,400 in outstanding accounts payable due to a consultant for research and development services. As a result of this transaction a loss on extinguishment of debt of $1,040 was recognized.
On October 6, 2014 the Company issued 72,000 shares with a fair value of $3,557 to settle $3,600 in outstanding accounts payable due to a consultant for research and development services. As a result of this transaction a gain on extinguishment of debt of $43 was recognized.
On October 17, 2014 the Company issued 50,000 shares with a fair value of $2,600 to settle $2,500 in outstanding accounts payable due to a consultant for research and development services. As a result of this transaction a loss on extinguishment of debt of $100 was recognized.
| F-16 |
On October 17, 2014 the Company issued 50,000 shares with a fair value of $2,600 to settle $2,500 in outstanding accounts payable due to a consultant for research and development services. As a result of this transaction a loss on extinguishment of debt of $100 was recognized.
On December 31, 2014 the Company issued 72,000 shares with a fair value of $3,312 to settle $3,600 in outstanding accounts payable due to a consultant for research and development services. As a result of this transaction a gain on extinguishment of debt of $288 was recognized.
12. COMMITMENTS AND CONTINGENCIES
Lease obligations – The Company has operating leases for its offices. Future minimum lease payments under the operating leases for the facilities as of December 31, 2014, are as follows:
2015 32,084
2016 5,717
Rental expense, resulting from operating lease agreements, approximated $41,868 and $25,349 for the years ended December 31, 2014 and 2013, respectively.
13. DEFINITIVE AGREEMENTS
On January 16, 2013, the company terminated its licensing agreement with Panalab dated January 23, 2008. The agreement provided Panalab the right to distribute, market, sell and promote the Skinvisible’s proprietary formulas made with Invisicare and Adapalene through-out Panalabs assigned territory. Panalab had failed to sell or sub-license the products in the territory, thereby not fulfilling the conditions as set forth in the agreement and allowing for immediate termination of the agreement. . As a result of this cancelation the deferred revenue of $19,792 was recognized as revenue in the in the year ended December 31, 2013.
14. SUBSEQUENT EVENTS
From January 1, 2015 through the date of filing the Company entered into four 9% notes payable to investors and received total proceeds of $110,000. The notes are, due two years from the anniversary date of execution. The Notes are secured by the US Patent rights granted for the Company's Sunscreen Products: US patent number #8,128,913: "Sunscreen Composition with Enhanced UV-A Absorber Stability and Methods".
On January 27, 2015 the company sold 2,000,000 shares of it's $0.001 par value common stock to two individuals and received proceeds of $80,000.
On March 3, 2015 the Company executed an extension agreement with a note holder who's note had reached maturity. The note was extended for an additional 12 months and is now due in December of 2015.
On March 11, 2015 the Company executed an extension agreement with a note holder who's note had reached maturity. The note was extended for an additional 24 months and is now due in October of 2016.
| F-17 |
Item 9. Changes In and Disagreements with Accountants on Accounting and Financial Disclosure
On February 4, 2014, Sarna & Company resigned as our accountant. We have engaged Seale and Beers, CPAs as our principal accountants effective February 4, 2014. The decision to change accountants was approved by our board of directors. For more information on the change in accountants, please see our Form 8-K filed with the Securities and Exchange Commission on February 5, 2014.
Item 9A. Controls and Procedures
As required by Rule 13a-15 under the Securities Exchange Act of 1934, we have carried out an evaluation of the effectiveness of our disclosure controls and procedures as of the end of the period covered by this annual report, being December 31, 2014. This evaluation was carried out under the supervision and with the participation of our management, including our Chief Executive Officer and Chief Financial Officer.
Disclosure controls and procedures are controls and other procedures that are designed to ensure that information required to be disclosed in our reports filed or submitted under the Securities Exchange Act of 1934 is recorded, processed, summarized and reported, within the time periods specified in the Securities and Exchange Commission’s rules and forms. Disclosure controls and procedures include controls and procedures designed to ensure that information required to be disclosed in our company’s reports filed under the Securities Exchange Act of 1934 is accumulated and communicated to management, including our Chief Executive Officer and Chief Financial Officer, to allow timely decisions regarding required disclosure.
Based upon that evaluation, including our Chief Executive Officer and Chief Financial Officer, we have concluded that our disclosure controls and procedures were ineffective as of the end of the period covered by this annual report.
Management’s Annual Report on Internal Control over Financing Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting (as defined in Rule 13a-15(f) under the Securities Exchange Act of 1934). Management has assessed the effectiveness of our internal control over financial reporting as of December 31, 2013 based on criteria established in Internal Control-Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission. As a result of this assessment, management concluded that, as of December 31, 2014, our internal control over financial reporting was not effective. Our management identified the following material weaknesses in our internal control over financial reporting, which are indicative of many small companies with small staff: (i) inadequate segregation of duties and effective risk assessment; and (ii) insufficient written policies and procedures for accounting and financial reporting with respect to the requirements and application of both US GAAP and SEC guidelines.
We plan to take steps to enhance and improve the design of our internal control over financial reporting. During the period covered by this annual report on Form 10-K, we have not been able to remediate the material weaknesses identified above. To remediate such weaknesses, we hope to implement the following changes during our fiscal year ending December 31, 2015 1: (i) appoint additional qualified personnel to address inadequate segregation of duties and ineffective risk management; and (ii) adopt sufficient written policies and procedures for accounting and financial reporting. The remediation efforts set out in (i) and (ii) are largely dependent upon our securing additional financing to cover the costs of implementing the changes required. If we are unsuccessful in securing such funds, remediation efforts may be adversely affected in a material manner.
This annual report does not include an attestation report of our registered public accounting firm regarding internal control over financial reporting. Management’s report was not subject to attestation by our registered public accounting firm pursuant to an exemption for non-accelerated filers set forth in Section 989G of the Dodd-Frank Wall Street Reform and Consumer Protection Act.
None
| 20 |
PART III
Item 10. Directors, Executive Officers and Corporate Governance
The following information sets forth the names, ages, and positions of our current directors and executive officers as of December 31, 2014.
| Name | Age | Position(s) and Office(s) Held | ||||
| Terry Howlett | 67 | Chief Executive Officer, Chief Financial Officer, & Director | ||||
| Greg McCartney | 63 | Director |
Set forth below is a brief description of the background and business experience of each of our current executive officers and directors.
Mr. Terry H. Howlett, has been our Chief Executive Officer and Director since March 5, 1998. Mr. Howlett has a diversified background in market initialization and development, sales and venture capital financing for emerging growth companies. He has held senior management, marketing and sales positions with various companies, including the Canadian Federation of Independent Business, Family Life Insurance, and Avacare of Canada and founded Presley Laboratories, Inc., which marketed cosmetic and skin, care products on a direct sales basis. For the ten years prior to becoming President of the Company, Mr. Howlett was the President and CEO of Voice-it Solutions, Inc., a publicly traded company on the Vancouver Stock exchange that made voice response software for order entry systems.
Mr. Greg McCartney has been a member of our board of directors since January 10, 2005. Mr. McCartney is Managing Director of Taylor, Butterfield & Worth Asset Management Corporation, a management consulting services firm assisting clients in becoming fully reporting public companies. Previously Mr. McCartney was the Chairman of the Board for Genesis Bioventures (formerly BioLabs) and also formerly served as their CEO. Mr. McCartney has over 20 years’ experience serving as officer and director of both private and public companies in various manufacturing and technology industries. Prior to founding BioLabs in 1997, Mr. McCartney was the founder and director of Aspenwood Holdings Corporation, a business consulting firm specializing in financing, public relations and venture capital in the technology and manufacturing industries. From 1986 to 1995 he was the President of an emerging high technology company and also served as officer and director of other companies. Previously, he was involved with international real estate and land development.
Directors
Our bylaws authorize no less than one (1) and more than twelve (12) directors. We currently have two directors.
Term of Office
Our Directors are appointed for a one-year term to hold office until the next annual general meeting of our shareholders or until removed from office in accordance with our bylaws. Our officers are appointed by our board of directors and hold office until removed by the board.
Significant Employees
Ms. Doreen McMorran, is head of Business Development. Ms. McMorran brings to the Company almost 20 years of experience in the medical and pharmaceutical industry, specifically in the areas of strategic planning, sales and marketing. She has spent the last seven years selling to international dermatology and skincare focused companies like Procter and Gamble, Johnson & Johnson, Stiefel, Galderma, Novartis and Graceway, to name a few. Ms. McMorran, who holds a Bachelor of Commerce (Honors) degree, spent six years in the pharmaceutical industry with Astra Pharma. Additionally she has held senior management level positions with a number of healthcare companies, focusing on business development, sales, marketing and operations.
Dr. James A. Roszell, Ph.D, is a doctoral chemist with over 35 years’ experience in product formulation, experimental design, analysis, and method validation. Since joining Skinvisible in 1998, he has been responsible for research and development of our patented technology, related polymer delivery vehicles, product formulations and compositions. Dr. Roszell is a joint contributor to Skinvisible’s first Patent Number 6.756.059 and responsible for all of our patents in the US and internationally. Prior to joining Skinvisible, he worked as a chemist for Supertech Products, Inc. in Florida where his responsibilities included ensuring compliance with OSHA, EPA and other standards and regulations, maintenance of quality control, research and development for new products. Dr. Roszell’s background includes work in chemical, pharmaceutical, environmental and clinical laboratory arenas. His chemical and scientific expertise makes a significant contribution to our business.
| 21 |
Family Relationships
There are no family relationships between or among the directors, executive officers or persons nominated or chosen by us to become directors or executive officers.
Involvement in Certain Legal Proceedings
To the best of our knowledge, during the past ten years, none of the following occurred with respect to a present or former director, executive officer, or employee: (1) any bankruptcy petition filed by or against any business of which such person was a general partner or executive officer either at the time of the bankruptcy or within two years prior to that time; (2) any conviction in a criminal proceeding or being subject to a pending criminal proceeding (excluding traffic violations and other minor offenses); (3) being subject to any order, judgment or decree, not subsequently reversed, suspended or vacated, of any court of competent jurisdiction, permanently or temporarily enjoining, barring, suspending or otherwise limiting his or her involvement in any type of business, securities or banking activities; and (4) being found by a court of competent jurisdiction (in a civil action), the SEC or the Commodities Futures Trading Commission to have violated a federal or state securities or commodities law, and the judgment has not been reversed, suspended or vacated.
Audit Committee
We do not have a separately-designated standing audit committee. The entire board of directors performs the functions of an audit committee, but no written charter governs the actions of the board of directors when performing the functions of that would generally be performed by an audit committee. The board of directors approves the selection of our independent accountants and meets and interacts with the independent accountants to discuss issues related to financial reporting. In addition, the board of directors reviews the scope and results of the audit with the independent accountants, reviews with management and the independent accountants our annual operating results, considers the adequacy of our internal accounting procedures and considers other auditing and accounting matters including fees to be paid to the independent auditor and the performance of the independent auditor.
We do not have an audit committee financial expert because of the size of our company and our board of directors at this time. We believe that we do not require an audit committee financial expert at this time because we retain outside consultants who possess these attributes as needed.
For the fiscal year ending December 31, 2014, the board of directors:
- Reviewed and discussed the audited financial statements with management, and
- Reviewed and discussed the written disclosures and the letter from our independent auditors on the matters relating to the auditor’s independence.
| Based upon the board of directors’ review and discussion of the matters above, the board of directors authorized inclusion of the audited financial statements for the year ended December 31, 2014 to be included in this Annual Report on Form 10-K and filed with the Securities and Exchange Commission. |
| 22 |
Section 16(a) Beneficial Ownership Reporting Compliance
Section 16(a) of the Exchange Act requires our directors and executive officers and persons who beneficially own more than ten percent of a registered class of the Company’s equity securities to file with the SEC initial reports of ownership and reports of changes in ownership of common stock and other equity securities of the Company. Officers, directors and greater than ten percent beneficial shareholders are required by SEC regulations to furnish us with copies of all Section 16(a) forms they file. To the best of our knowledge based solely on a review of Forms 3, 4, and 5 (and any amendments thereof) received by us during or with respect to the year ended December 31, 2014, the following persons have failed to file, on a timely basis, the identified reports required by Section 16(a) of the Exchange Act during fiscal year ended December 31, 2014:
| Name and principal position | Number of late reports | Transactions not timely reported | Known failures to file a required form | |||||||||
| Terry
Howlett CEO, CFO & Director | 0 | 3 | 0 | |||||||||
| Greg McCartney
Director | 0 | 2 | 0 | |||||||||
| Lutz Family Trust | 0 | 0 | 0 | |||||||||
| Doreen McMorran | 0 | 2 | 0 | |||||||||
Code of Ethics
We adopted a Code of Ethics for Financial Executives, which include our principal executive officer, principal financial officer, principal accounting officer or controller, or persons performing similar functions. The Code of Ethics was filed as an exhibit to the annual report on Form 10KSB for the fiscal year ended December 31, 2004 and filed with the SEC on April 14, 2005.
Item 11. Executive Compensation
Compensation Discussion and Analysis
Currently, the objective of the cash compensation paid by the company is to provide fair reimbursement for the time spent by our executive officer and independent directors to the extent feasible within the financial constraints faced by our developing business. The stock options granted to our executive officer and to our independent directors are intended to provide these individuals with incentives to pursue the growth and development of the company’s operations and business opportunities. Although the options awarded to our executive and directors are typically exercisable immediately, they also remain valid and exercisable for terms of several years. We believe this provides the proper balance of short-term and long-term incentives to increase the value of the company. Although an immediate increase in share price following the issuance of the options would obviously result in a profit if those options were exercised, the longer exercisable period of the options also provides an incentive to increase value over the long term and gives our executive officer and directors the opportunity to realize gains based on the sustained growth of our operations and revenues.
In addition, our sole executive officer holds substantial ownership in the company and is generally motivated by a strong entrepreneurial interest in expanding our operations and revenue base to the best of his ability.
| 23 |
Summary Compensation Table
The table below summarizes all compensation awarded to, earned by, or paid to our former or current executive officers for the fiscal years ended December 31, 2014 and 2013.
| SUMMARY COMPENSATION TABLE | ||||||||||||||||||||||||||
| Name and principal position | Year | Salary
($) | Bonus ($) | Stock Awards ($) | Option
Awards ($) | Non-Equity
Incentive Plan Compensation ($) | Nonqualified
Deferred Compensation Earnings ($) | All
Other Compensation ($) | Total ($) | |||||||||||||||||
| Terry Howlett CEO & CFO | 2014 2013
| 180,000 180,000 | 9,941 6,121 | - - | - - | - - | - - | - - | 189,941(2) 186,121(1) | |||||||||||||||||
(1) Due to financial constraints, however, the total paid to Mr. Howlett during the fiscal year ended December 31,
2013 was $65,160.
(2) Due to financial constraints, however, the total paid to Mr. Howlett during the fiscal year ended December 31, 2014 was $71,584
| 24 |
Narrative Disclosure to the Summary Compensation Table
On January 29, 2009, we entered into an employment agreement with our sole executive officer, Terry Howlett. The agreement is effective retroactively to January 1, 2009, and term of the agreement is until December 31, 2013, and may be renewed for an additional 36 months unless terminated. Under the agreement, Mr. Howlett earns a cash stipend of $13,333.33 per month ($160,000 per year). Effective January 1, 2010, his cash stipend increased to $15,000 per month ($180,000 per year). He will also receive bonuses based on a percentage of license fees, royalty fees, and financings; paid vacation or the election to receive vacation benefits in payment; and reimbursements of expenses, including automobile and limited living expenses. In addition, the agreement provides for Mr. Howlett to be awarded stock options at the discretion of the board of directors.
On December 31, 2011, we executed a convertible promissory note in favor of Mr. Howlett, our CEO and member of our board of directors. The aggregate principal amount of his note is $415,657.03 and includes accrued salary through 2010 and the last six months of 2011. The interest rate on his note is 10% per annum, matures on December 31, 2016, is convertible into shares of our common stock at $0.04 per share, and includes a warrant for the right to purchase further shares at $0.06 per share if exercised within 3 years following the conversion date. The warrant agreement will give Mr. Howlett the right to purchase one share for every two shares acquired by him in the conversion.
On June 30, 2012, we issued a promissory note to Mr. Howlett totaling $105,642.86. Under the terms of the promissory note, Mr. Howlett has the right to convert all or any part of the principal sum of the loan into common stock at $0.04 per share as well as a warrant agreement to purchase one share for every two shares acquired. The expiration date on the warrant is 3 years from the date of conversion.
On January 30, 2013, we issued a convertible promissory note to Terry Howlett to convert his accrued compensation of $46,056 as of December 31, 2012 into our common stock at $0.03 per share at any time until December 31, 2017. If exercised, we also agreed to issue a three year warrant to Mr. Howlett to purchase an aggregate amount of 767,600 shares of common shares at a strike price of $0.04 per share.
On January 30, 2013, we issued a convertible promissory note to Terry Howlett to convert debt of $46,352 as of December 31, 2012 into our common stock at $0.03 per share at any time until December 31, 2017. If exercised, we also agreed to issue a three year warrant to Mr. Howlett to purchase an aggregate amount of 772,533 shares of common shares at a strike price of $0.04 per share.
On June 30, 2013, we issued a convertible promissory note to Terry Howlett to convert debt of $62,490 as of June 30, 2013 into our common stock at $0.03 per share at any time until June 30, 2018. If exercised, we also agreed to issue a three year warrant to Mr. Howlett to purchase an aggregate amount of 1,041,500 shares of common shares at a strike price of $0.04 per share.
On December 31, 2013, we issued a convertible promissory note to Terry Howlett to convert debt of $77,514 as of December 31, 2013 into our common stock at $0.02 per share at any time until December 31, 2018. If exercised, we also agreed to issue a three year warrant to Mr. Howlett to purchase an aggregate amount of 1,937,873 shares of common shares at a strike price of $0.03 per share.
On June 30, 2014, we issued a convertible promissory note to Terry Howlett to convert debt of $65,115 as of June 30, 2014 into our common stock at $0.025 per share at any time until June 30, 2019. If exercised, we also agreed to issue a three year warrant to Mr. Howlett to purchase an aggregate amount of 1,262,300 shares of common shares at a strike price of $0.03 per share.
On September 30, 2014, we issued a convertible promissory note to Terry Howlett to convert debt of $36,150 as of September 30, 2014 into our common stock at $0.04 per share at any time until September 30, 2019. If exercised, we also agreed to issue a three year warrant to Mr. Howlett to purchase an aggregate amount of 451,875 shares of common shares at a strike price of $0.05 per share.
On December 31, 2014, we issued a convertible promissory note to Terry Howlett to convert debt of $34,092 as of December 31, 2014 into our common stock at $0.04 per share at any time until December 31, 2019. If exercised, we also agreed to issue a three year warrant to Mr. Howlett to purchase an aggregate amount of 426,154 shares of common shares at a strike price of $0.05 per share.
| 25 |
Outstanding Equity Awards at Fiscal Year-End
The table below summarizes all unexercised options, stock that has not vested, and equity incentive plan awards for each named executive officer as of December 31, 2014.
| OUTSTANDING EQUITY AWARDS AT FISCAL YEAR-END | ||||||||||||||||||||||
| OPTION AWARDS | STOCK AWARDS | |||||||||||||||||||||
| Name | Number of Securities Underlying Unexercised Options (#)Exercisable | Number of Securities Underlying Unexercised Options (#) Unexercisable | Equity Incentive Plan Awards: Number of Securities Underlying Unexercised Unearned Options (#) | Option Exercise Price ($)(1) | Option Expiration Date | Number of Shares or Units of Stock That Have Not Vested(#) | Market Value of Shares or Units of Stock That Have Not Vested ($) | Equity Incentive Plan Awards: Number of Unearned Shares, Units or Other Rights That Have Not Vested (#) | Equity Incentive Plan Awards: Market or Payout Value of Unearned Shares, Units or Other Rights That Have Not Vested (#) | |||||||||||||
| Terry Howlett | 1,000,000 400,000 200,000 1,000,000 1,000,000 1,000,000 | - - - - - - | - - - - - - | 0.04 0.04 0.04 0.04 0.05 0.06 | 10/19/2018(5) 1/30/2018(2) 1/3/2016(3) 1/20/2019(4) 12/7/2019(6) 11/14/2015 | - - - - - | - - - - - | - - - - - | - - - - - - | |||||||||||||
| (1) | On April 21, 2009, we modified the exercise price on all of our outstanding options issued prior to March 31, 2009 to $0.04 per share, which included all options issued to Mr. Howlett aside from the option issued on December 7, 2009 of 1,000,000 shares at $0.08 per share and the option issued on November 15, 2010 at $0.06 per share. On October 17, 2014, we modified the exercise price to $0.05 per share on the option issued on December 7, 2009 of 1,000,000 shares. Aside from this modification, during the last fiscal year there was not any outstanding option re-priced or otherwise modified. There was no tandem feature, reload feature, or tax-reimbursement feature associated with any of the stock options we granted to our executive officers or otherwise. | |
| (2) | On January 3, 2013, our Board of Directors approved to extend the expiration date 5 years. | |
| (3) | On January 10, 2010, our Board of Directors approved to extend the expiration date 5 years. | |
| (4) | On January 19, 2014, our Board of Directors approved to extend the expiration date 5 years. | |
| (5) | On January 19, 2014, our Board of Directors approved to extend the expiration date 5 years. | |
| (6) | On October 17, 2014, our Board of Directors approved to extend the expiration date 5 years. |
| 26 |
Director Compensation
The table below summarizes all compensation of our directors as of December 31, 2014.
| DIRECTOR COMPENSATION | ||||||||||||||||||
| Name | Fees Earned or Paid in Cash ($) | Stock
Awards ($) | Option Awards ($) | Non-Equity Incentive Plan Compensation ($) | Non-Qualified Deferred Compensation Earnings ($) | All Other Compensation ($) | Total ($) | |||||||||||
| Greg McCartney | 14,400 | 14,400 | ||||||||||||||||
Narrative Disclosure to the Director Compensation Table
All the fees earned or paid in cash and stock options awards granted to Terry Howlett were earned in connection with his service as an executive officer. Mr. Howlett received no compensation for his service as a member of our board of directors.
We pay our independent directors a monthly fee of $1,200.
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
The following table sets forth, as of January 6, 2014, the beneficial ownership of our common stock by each executive officer and director, by each person known by us to beneficially own more than 5% of the our common stock and by the executive officers and directors as a group.
Title of class | Name and address of beneficial owner (1) | Amount of beneficial ownership(2) | Percent of class(3) | |||||||
| Executive Officers & Directors: | ||||||||||
| Common | Terry Howlett(4) | 51,979,475 shares | 33.3 | % | ||||||
| Common | Greg McCartney(5) | 2,298,500 shares | 2 | % | ||||||
| Total of All Directors and Executive Officers: | 54,277,975 shares | 34.5 | % | |||||||
| More Than 5% Beneficial Owners: | ||||||||||
Lutz Family Trust(6) 8322 West Tonto Lane, Peoria, AZ 85382 | 10,998,300 shares | 9.9 | % | |||||||
| Doreen McMorran(7) | 52,176,848 shares | 32.1 | % | |||||||
| (1) | Except as otherwise indicated, the address of each person named in this table is c/o Skinvisible, Inc., 6320 South Sandhill Road, Suite 10, Las Vegas, Nevada 89120. |
| (2) | As used in this table, "beneficial ownership" means the sole or shared power to vote, or to direct the voting of, a security, or the sole or shared investment power with respect to a security (i.e., the power to dispose of, or to direct the disposition of, a security). In addition, for purposes of this table, a person is deemed, as of any date, to have "beneficial ownership" of any security that such person has the right to acquire within 60 days after such date. |
| (3) | Except as otherwise indicated, all shares are owned directly and the percentage shown is based on 111,741,969 shares of common stock issued and outstanding on January 6, 2014. |
| (4) | Includes 7,723,248 shares held in his name as indicated on our shareholder list, 26,437,485 shares of common stock that may be converted under the principal balance and accrued interest under debt conversion agreements with conversion prices ranging from $0.025 to $0.04 per share, warrants that may be exercised upon the above conversions to purchase one shares for every two shares acquired in the conversions amounting to 13,218,742 shares at prices ranging from $0.03 to $0.06 per share, options that may be exercised immediately to purchase 2,600,000 shares at a price of $0.04 per share, options that may be exercised immediately to purchase 1,000,000 shares at a price of $0.08 per share, and options that may be exercised immediately to purchase 1,000,000 shares at a price of $0.06 per share. |
| (5) | Includes 998,500 shares held in his name, options that may be exercised immediately to purchase 800,000 shares at a price of $0.04 per share, options that may be exercised immediately to purchase 250,000 shares at a price of $0.05 per share, and options that may be exercised immediately to purchase 250,000 shares at a price of $0.06 per share. |
| (6) | As stated in the reporting person’s Form 4 filed with the Securities and Exchange Commission on January 25, 2010. |
| (7) | Includes 1,800,000 shares held in her name as indicated on our shareholder list, 32,913,899 shares of common stock that may be converted under the principal balance and accrued interest of debt conversion agreements with conversion prices ranging from $0.02 to $0.04 per share, warrants that may be exercised upon the above conversions to purchase one shares for every two shares acquired in the conversions amounting to 16,456,949 shares at prices ranging from $0.03 to $0.06 per share, options that may be exercised immediately to purchase 600,000 shares at a price of $0.04 per share, options that may be exercised immediately to purchase 500,000 shares at a price of $0.05 per share, and options that may be exercised immediately to purchase 500,000 shares at a price of $0.06 per share |
| 27 |
Item 13. Certain Relationships and Related Transactions, and Director Independence
Aside from that which follows and in “Executive Compensation,” none of our directors or executive officers, nor any proposed nominee for election as a director, nor any person who beneficially owns, directly or indirectly, shares carrying more than 5% of the voting rights attached to all of our outstanding shares, nor any members of the immediate family (including spouse, parents, children, siblings, and in-laws) of any of the foregoing persons has any material interest, direct or indirect, in any transaction for the last two fiscal years or in any presently proposed transaction which, in either case, has or will materially affect us.
Doreen McMorran
On January 30, 2013, we issued and convertible promissory note to Doreen McMorran to convert her accrued compensation of $66,281 as of December 31, 2012 into our common stock at $0.03 per share at any time until December 31, 2017. If exercised, we also agreed to issue a three year warrant to Ms. McMorran to purchase an aggregate amount of 1,104,685 shares of common shares at a strike price of $0.04 per share.
On June 30, 2013, we issued and convertible promissory note to Doreen McMorran to convert her accrued compensation of $43,661 as of June 30, 2013 into our common stock at $0.03 per share at any time until December 31, 2017. If exercised, we also agreed to issue a three year warrant to Ms. McMorran to purchase an aggregate amount of 727,700 shares of common shares at a strike price of $0.04 per share.
On December 31, 2013, we issued and convertible promissory note to Doreen McMorran to convert her accrued compensation of $60,725 as of December 31, 2013 into our common stock at $0.02 per share at any time until December 31, 2018. If exercised, we also agreed to issue a three year warrant to Ms. McMorran to purchase an aggregate amount of 1,518,125 shares of common shares at a strike price of $0.03 per share.
On June 30, 2014, we issued and convertible promissory note to Doreen McMorran to convert her accrued compensation of $78,384 as of June 30, 2014 into our common stock at $0.025 per share at any time until June 30, 2019. If exercised, we also agreed to issue a three year warrant to Ms. McMorran to purchase an aggregate amount of 979,800 shares of common shares at a strike price of $0.03 per share.
On December 7, 2009, Doreen McMorran was granted an option to purchase 500,000 shares of common stock under the Issuers Stock Option Plan dated July 17, 2006. On October 17, 2014 our board of directors approved to modify the options granted with a new expiry date of December 7, 2019 and a new exercise price of $0.05 per share.
On December 31, 2014, we issued and convertible promissory note to Doreen McMorran to convert her accrued compensation of $31,203 as of December 31, 2014 into our common stock at $0.04 per share at any time until December 31, 2019. If exercised, we also agreed to issue a three year warrant to Ms. McMorran to purchase an aggregate amount of 390,043 shares of common shares at a strike price of $0.05 per share.
Greg McCartney
On October 20, 2013, Greg McCartney was granted an option to purchase 250,000 shares of common stock under our Stock Option Plan dated July 17, 2006. The option expires on October 20, 2018 and has an exercise price of $0.04 per share.
On December 7, 2009, Greg McCartney was granted an option to purchase 250,000 shares of common stock under our Stock Option Plan dated July 17, 2006. On October 17, 2014 our board of directors approved to modify the options granted with a new expiry date of December 7, 2019 and a new exercise price of $0.05 per share.
Item 14. Principal Accounting Fees and Services
Below is the table of Audit Fees (amounts in US$) billed by our auditor in connection with the audit of the Company’s annual financial statements for the years ended:
| Financial
Statements for the Year Ended December 31 | Audit Services | Audit Related Fees | Tax Fees | Other Fees | ||||||||||||||
| 2014 | $ | 25,000 | $ | 0 | $ | 0 | $ | 0 | ||||||||||
| 2013 | $ | 13,000 | $ | 0 | $ | 0 | $ | 0 | ||||||||||
| 28 |
PART IV
Item 15. Exhibits, Financial Statements Schedules
| (a) | Financial Statements and Schedules |
The following financial statements and schedules listed below are included in this Form 10-K.
Financial Statements (See Item 8)
| (b) | Exhibits |
| Exhibit Number | Description |
| 3.1 | Articles of Incorporation, as amended (1) |
| 3.2 | Bylaws, as amended (1) |
| 3.3 | Certificate of Amendment to The Company’s Articles of Incorporation(2) |
| 14.1 | Code of Ethics (3) |
| 31.1 | Certification of Chief Executive Officer pursuant to Securities Exchange Act Rule 13a-14(a)/15d-14(a), as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 |
| 31.2 | Certification of Chief Financial Officer pursuant to Securities Exchange Act Rule 13a-14(a)/15d-14(a), as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 |
| 32.1 | Certification of Chief Executive Officer and Chief Financial Officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 |
| 1 | Incorporated by reference to the Registration Statement on Form 10SB12G filed on April; 30, 1999. |
| 2 | Incorporated by reference to the Report on Form 8-K filed on September 12, 2008. |
| 3 | Incorporated by reference to Current report on Form 10-KSB filed with the Securities and Exchange Commission on April 14, 2005. |
| 29 |
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| Skinvisible, Inc. | |
| By: | /s/ Terry Howlett |
Terry Howlett President, Chief Executive Officer, Principal Executive Officer, Chief Financial Officer, Principal Financial Officer, Principal Accounting Officer and Director
| |
| March 31, 2015 |
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
| By: | /s/ Terry Howlett |
| Terry Howlett | |
President, Chief Executive Officer, Principal Executive Officer, Chief Financial Officer, Principal Financial Officer, Principal Accounting Officer and Director | |
| March 31, 2015 | |
| By: | /s / Greg McCartney |
| Greg McCartney | |
| Director | |
| March 31, 2015 |
| 30 |


