Attached files
| file | filename |
|---|---|
| EXCEL - IDEA: XBRL DOCUMENT - Q Therapeutics, Inc. | Financial_Report.xls |
| EX-32.1 - EX-32.1 - Q Therapeutics, Inc. | d900336dex321.htm |
| EX-31.2 - EX-31.2 - Q Therapeutics, Inc. | d900336dex312.htm |
| EX-31.1 - EX-31.1 - Q Therapeutics, Inc. | d900336dex311.htm |
| EX-23.1 - EX-23.1 - Q Therapeutics, Inc. | d900336dex231.htm |
| EX-32.2 - EX-32.2 - Q Therapeutics, Inc. | d900336dex322.htm |
| EX-10.4.1 - EX-10.4.1 - Q Therapeutics, Inc. | d900336dex1041.htm |
| EX-21.1 - EX-21.1 - Q Therapeutics, Inc. | d900336dex211.htm |
Table of Contents
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
(Mark One)
| x | ANNUAL REPORT UNDER SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended: December 31, 2014
| ¨ | TRANSITION REPORT UNDER SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to
Commission File No. 000-52062
Q THERAPEUTICS, INC.
(Exact name of registrant as specified in its charter)
| Delaware | 20-3708500 | |
| (State or other jurisdiction of incorporation or formation) |
(I.R.S. employer identification number) |
Q Therapeutics, Inc.
615 Arapeen Drive, Suite 102
Salt Lake City, UT 84108
(Address of principal executive offices)
Issuer’s telephone number: (801) 582-5400
Securities registered under Section 12(b) of the Exchange Act:
| Title of each class |
Name of each exchange where registered | |
| Common stock, par value $0.001 per share | N/A |
Securities registered under Section 12(g) of the Exchange Act: None.
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ¨ No x
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ¨ No x
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Exchange Act during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes x No ¨
Table of Contents
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§ 229.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes x No ¨
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (§229.405 of this chapter) is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. x
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer,” “non-accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check one):
| Large accelerated filer | ¨ | Accelerated filer | ¨ | |||
| Non-accelerated filer | ¨ | Smaller reporting company | x | |||
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ¨ No x
State the aggregate market value of the voting and non-voting common equity held by non-affiliates computed by reference to the price at which the common equity was last sold, or the average bid and asked price of such common equity, as of the last business day of the registrant’s most recently completed fiscal quarter.
As of June 30, 2014, the last business day of the registrant’s most recently completed second quarter, there was no established public market for the registrant’s common stock.
As of March 31, 2015, there were 30,826,549 shares of common stock, $0.0001 par value per share, issued and outstanding.
DOCUMENTS INCORPORATED BY REFERENCE:
None
Table of Contents
| PAGE | ||||||||
| PART I | ||||||||
| Item 1. |
1 | |||||||
| Item 1A. |
12 | |||||||
| Item 1B |
27 | |||||||
| Item 2. |
27 | |||||||
| Item 3. |
27 | |||||||
| PART II | ||||||||
| Item 5. |
Market for Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities |
27 | ||||||
| Item 6 |
28 | |||||||
| Item 7. |
Management’s Discussion and Analysis of Financial Condition and Results of Operations |
28 | ||||||
| Item 7A. |
33 | |||||||
| Item 8. |
33 | |||||||
| Item 9. |
Changes in and Disagreements with Accountants on Accounting and Financial Disclosure |
33 | ||||||
| Item 9A. |
34 | |||||||
| Item 9B. |
35 | |||||||
| PART III | ||||||||
| Item 10. |
36 | |||||||
| Item 11. |
39 | |||||||
| Item 12. |
Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters |
44 | ||||||
| Item 13. |
Certain Relationships and Related Transactions, and Director Independence |
46 | ||||||
| Item 14. |
46 | |||||||
| Item 15. |
47 | |||||||
| 50 | ||||||||
| CERTIFICATIONS |
||||||||
Table of Contents
FORWARD-LOOKING STATEMENTS
Certain statements made in this Annual Report on Form 10-K are “forward-looking statements” (within the meaning of the Private Securities Litigation Reform Act of 1995) regarding the plans and objectives of management for future operations. Such statements involve known and unknown risks, uncertainties and other factors that may cause actual results, performance or achievements of the Registrant to be materially different from any future results, performance or achievements expressed or implied by such forward-looking statements. The forward-looking statements included herein are based on current expectations that involve numerous risks and uncertainties. Assumptions relating to the foregoing involve judgments with respect to, among other things, future economic, competitive and market conditions and future business decisions, all of which are difficult or impossible to predict accurately and many of which are beyond the control of the Registrant. Although the Registrant believes its assumptions underlying the forward-looking statements are reasonable, any of the assumptions could prove inaccurate and, therefore, there can be no assurance the forward-looking statements included in this Report will prove to be accurate. In light of the significant uncertainties inherent in the forward-looking statements included herein, the inclusion of such information should not be regarded as a representation by the Registrant or any other person that the objectives and plans of the Registrant will be achieved.
| Item 1. | Business |
Overview
Q Therapeutics, Inc. (hereinafter Q Therapeutics, Q, we, us, our and similar expressions) is a Salt Lake City, Utah-based biopharmaceutical company that is developing human cell-based therapies that can be sold as “off-the-shelf” pharmaceuticals intended to treat neurodegenerative diseases of the brain and spinal cord, the primary components of the central nervous system or CNS.
The technology upon which these potential therapies are based was developed by Q’s co-founder Mahendra Rao, M.D., Ph.D., a leader in glial stem cell biology, during Dr. Rao’s tenure as a Professor at the University of Utah and as Head of the Stem Cell Section in the Laboratory of Neuroscience at the National Institutes of Health (NIH) Institute of Aging. Dr. Rao was one of the first scientists to identify and seek patent coverage on stem cells and their progeny cells found in the CNS.
Every year, millions of people suffer from debilitating and often fatal neurodegenerative diseases of the brain and spinal cord. Despite much effort by the pharmaceutical/biotechnology industry, current approaches to treating these diseases have not been effective. A new approach is needed for treating the multimodal nature of neurodegenerative disease.
Q has identified and patented a new treatment modality that provides a multi-factorial approach, bypassing the need to address individual pathways. Specifically, Q is developing a cell-based therapeutic that employs a natural cell type found in the healthy CNS: human glial restricted progenitors and their progeny cells. We call them, Q-Cells®. Q-Cells produce astrocytes and oligodendrocytes, the support cells that enable the normal function of neurons. We believe that Q-Cells may provide multiple and complementary mechanisms of action in the treatment of many neurodegenerative diseases. An advantage of this approach is that one need not fully understand the mechanistic aspects of these diseases, as we are tapping into the natural cellular machinery that enables healthy systems to function. We believe this is a more realistic approach than seeking a drug that affects a single disease pathway (although the addition of Q-Cell therapy may enhance benefits seen with such single drugs). Based on data in animal models, we believe that repairing damaged neurons is a faster, more realistic and easier approach than trying to replace them.
Initially, Q is targeting orphan diseases, where the U.S. Food and Drug Administration (FDA) can allow fast-track approvals and market exclusivity, and for which smaller, less-expensive clinical trials may be warranted. This approach may result in accelerated commercialization efforts while maintaining a financing approach focused on capital efficiency. Q is advancing its initial product candidate, trademarked “Q-Cells®” as a potential treatment for Amyotrophic Lateral Sclerosis (Lou Gehrig’s disease or ALS), and eventually other indications, potentially including Multiple Sclerosis (MS), Transverse Myelitis (TM), Spinal Cord Injury (SCI), Stroke, Huntington’s disease, Parkinson’s disease and Alzheimer’s disease.
1
Table of Contents
Our Technology and Approach to the Difficult Problem of Neurodegenerative Diseases
The Problem of Neurodegenerative Diseases
Many degenerative diseases are caused by the loss of normal cellular function in a particular organ. When cells are damaged or destroyed, they no longer produce, metabolize or accurately regulate the many substances essential to life. There is no drug or technology existing today that can deliver these essential substances precisely to the sites of action, under the appropriate physiological regulation, in the appropriate quantity, or for the duration required to cure the degenerative condition. Cells, however, can do all of this naturally. Transplantation of stem or progenitor cells may therefore prevent the loss of, or even generate new, functional cells and thereby potentially maintain or restore organ function and the patient’s health.
Neurodegenerative diseases are by nature complex. For most such diseases, there is not just one “cause” or just one “disease”; there can be multiple different causes with many factors and pathways contributing to the many disease symptoms. Despite much effort by the pharmaceutical/biotechnology industry, current approaches have not been effective. Traditional drugs have focused on single disease pathways. Due to the multi-factorial nature of neurodegenerative diseases, these drugs tend to fail to treat the nerve damage caused by these diseases as they do not fully address all of the disease factors or pathways. As a result, patients suffering from these diseases can, in many cases, only hope to slow the inexorable disease progression and the associated disabilities.
Q’s Approach to the Problem
Stem cells are “building block” cells as they produce all the mature functional cell types found in normal organs. Stem cells have two defining characteristics: (i) they produce all of the mature cell types of the particular organ, and (ii) they self-renew — that is, some of the cells developed from stem cells are themselves new stem cells. Progenitor cells are cells that have already developed from stem cells, but can still produce one or more mature cell types within an organ.
Glial cells are progenitor cells found only in the brain and spine. Many neurodegenerative disease arises when glial cells are damaged or destroyed, causing neurons to malfunction and eventually die. We aim to treat neurodegenerative diseases by providing new, healthy glial cells to help maintain and/or restore neuron function to a more robust state. Glia restore function to neurons through multimodal actions in a process that is unique to stem and progenitor cells. Our research shows they can be administered into, and act locally, in the brain or spinal cord, where they differentiate into the two mature glial cell subtypes found in the brain and spinal cord:
| • | Oligodendrocytes, which provide the myelin “insulation” around the axons of neurons required for proper signal transmission by the conducting neurons. They also provide nutritional support to neurons. |
| • | Astrocytes, which provide support to neurons through production of growth factors, toxin removal and other trophic support mechanisms. |
Our therapeutic product, Q-Cells, are late-stage, lineage-restricted human glial progenitor cells. We believe that Q-Cells may have the potential to restore health and function to neurons prior to their death by using the natural support mechanisms in the CNS. No current treatment has been shown to achieve this outcome.
2
Table of Contents
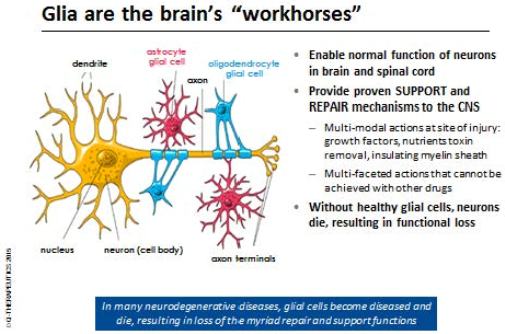
Advantages of Q-Cells, demonstrated in animal models, include:
| • | When placed in the CNS, Q-Cells predictably replicate, migrate, and terminally differentiate into physiologically relevant glia cells: oligodendrocytes and astrocytes. |
| • | Q-Cells can provide important growth factors and other trophic functions that support neuronal health, which may provide treatment options for diseases directly involving neuronal degeneration such as ALS, Huntington’s disease and Parkinson’s disease. |
| • | Q-Cells can provide myelin repair functions for diseases or injuries involving demyelination such as MS, Transverse Myelitis, Cerebral Palsy, and Spinal Cord Injury. |
| • | Q-Cells don’t give rise to appreciable numbers of neurons, reducing potential for unwanted effects due to aberrant neuronal connections. |
| • | Tissue-sourced Q-Cells spend little time in culture providing advantages in production. |
| • | The isolation process is readily scalable to good manufacturing practices (GMP). |
Due to the multifactorial nature of Q-Cells, we believe there is a significant market for our cell therapy.
Business Strategy
Our business objective is to discover, develop, and commercialize novel cell-based therapeutics products for neurodegenerative disease indications where there are both substantial unmet medical needs and commercial opportunity. Our strategy to achieve that objective involves several key elements, outlined below:
| • | Efficiently Conduct Human Trials to Establish Clinical Proof of Concept and Biological Activity with our Lead Therapeutic Product Candidate – We are preparing to submit an IND for our first-in-human clinical trial of our Q-Cells product candidate in patients with amyotrophic lateral sclerosis (ALS). This trial is intended to demonstrate both the safety of our product as well as measures of its biological activity, thereby establishing a proof of concept for our approach. We intend to submit additional INDs which, on acceptance by the FDA, would allow us to explore the potential of expanding the number of disease areas and more broadly demonstrate the scope of mechanisms of action our products are capable of producing. By initially focusing on orphan indications, we believe we will be able to achieve proof of concept in an efficient manner and lay a robust foundation for subsequent development and partnering activity, expanding into complementary areas where our product’s mechanisms of action are likely to have significant effects. |
3
Table of Contents
| • | Continue to Refine and Improve our Manufacturing and Related Processes and Deepen our Understanding of Therapeutic Mechanisms of Action – A fundamental capability of our Q-Cells product is the production of cells which provide the primary repair and support mechanisms needed to sustain and enable healthy function of neurons in the central nervous system (CNS). Our research shows that we can achieve significantly greater manufacturing yields through process improvements. These greater yields would reduce the need for manufacturing runs and enable optimization of the manufacturing-to-bedside delivery of our product. We continue to discover additional biological activity and mechanisms of action of our product, enabling us to develop additional next-generation products that can be highly targeted to specific disease indications. |
| • | Efficiently Explore New High Potential Therapeutic Applications, Leveraging Third-Party Research Collaborations and our Results - We have a demonstrated track record of successful collaboration with our academic research partners that has resulted in scientific findings that will enable us to move into additional therapeutic areas where medical needs are substantial. These collaborations have included laboratories at The Johns Hopkins University, Drexel University, the University of Wisconsin, the University of California, San Diego, and the University of Utah as well as MPI Research and have enabled us to cost-effectively explore where Q-Cells may have relevance and how they may be utilized to advance treatment for devastating neurodegenerative diseases. We believe we can leverage preclinical data from our collaborative partners as well as clinical results from our upcoming trials to support accelerated development efforts in additional indications, saving substantial development time and resources when compared to traditional drug development where generally each program is completely separately developed. |
| • | Enter into Arrangements with Business Partners to Accelerate Development and Value Creation – In addition to our internal development efforts, an important part of our development strategy is to work with collaborators and partners to accelerate product development and reduce our development costs. We have entered into licensing and product co-development arrangements with highly qualified partners to achieve these objectives. We anticipate that this strategy will help us to develop a portfolio of high quality product development opportunities, enhance our clinical development and commercialization capabilities, and increase our ability to generate value from our proprietary technologies. We have entered into arrangements with partners to develop second generation products derived from induced pluripotent stem cell (iPSC) sources and expect to move these projects forward. |
| • | Continue to Expand our Intellectual Property Portfolio - We have a broad intellectual property estate that covers our proprietary products and technologies, as well as methods of production and methods of use. Our intellectual property is important to our business and we take significant steps to protect its value. We have ongoing research and development efforts, both through internal activities and through collaborative research activities with others, which aim to develop new intellectual property and enable us to file patent applications that encompass both new applications of our existing technologies and products, including Q-Cells, as well as other products and opportunities. We currently have 21 patents related to our stem cell technologies and cell therapy applications providing protection in the United States, Canada, Japan and other markets. |
Opportunity for Q Therapeutics
Q Therapeutics aims to change the way medicine is practiced in the treatment of many debilitating, and often fatal, diseases of the brain and spinal cord by bringing to market a patented cell-based therapeutic that addresses substantial unmet needs in the CNS therapeutic market today. Q Therapeutics believes that its initial Q-Cells product could meet a variety of these needs across a number of CNS diseases. By demonstrating that Q-Cells are safe and effective first in smaller orphan indications and later in larger target markets, Q Therapeutics believes it can both augment and/or displace current therapeutic approaches as well as expand the therapeutic market in currently untreatable CNS conditions. Should this prove true, Q-Cells would address a multi-billion dollar market opportunity in treatment of neurodegenerative diseases for which there are no effective treatments.
4
Table of Contents
Q Market Development Strategy – Orphan Indications First, Larger Markets Follow
Q’s first commercial targets are orphan diseases with billion-dollar market potential. The Orphan Drug Act of 1983 (in this paragraph, the Act) defined an “orphan drug” as a therapy intended to treat rare “orphan” diseases, those affecting fewer than 200,000 Americans. The benefits provided by the Act may include more rapid regulatory timelines, tax benefits, and seven-year market exclusivity for the first product approved for an indication. Despite the smaller numbers of affected patients, orphan diseases often have highly motivated patient advocacy groups that are eager to assist companies with patient recruitment and therapeutic development. Moreover, substantial annual per-patient treatment prices effectively offset the relatively small patient populations. Due to the focused nature of marketing permitted by targeting orphan indications, Q might be able to capture a significant portion of the U.S. market for certain orphan diseases without need for a major marketing partner, provided that it has adequate financial resources. This ability to target patients suffering from orphan diseases also provides an incentive to potential Q development partners with interest in funding development costs and providing developmental expertise in exchange for marketing rights.
Q intends to bring Q-Cells to the market first to treat ALS to demonstrate the safety and efficacy of its cell-based therapeutic. Q also intends that Q-Cells will be subsequently brought to other orphan indications potentially including Transverse Myelitis, Progressive Multiple Sclerosis, Huntington’s disease, later expanding into Spinal Cord Injury, Stroke, Parkinson’s and Alzheimer’s diseases. Q estimates that the annual market opportunity for its initial orphan disease targets exceeds $1 billion worldwide. Application of its Q-Cells product to larger market diseases would substantially expand the market available to Q. By targeting orphan diseases like ALS first, Q can continue its strategy of increasing the value of the Company in a capital efficient manner by taking advantage of the benefits of the Act. Evaluation of Q-Cells in these various neurodegenerative diseases provides multiple opportunities for success utilizing this single therapeutic product. We believe that such success will be very attractive to larger pharmaceutical and biotechnology companies who will want to partner with us in the application of Q-Cells to larger markets where the development and sales capacity of those partners can be advantageous. We have broadly characterized the disease targets into three general but overlapping groups: diseases where neuronal loss is the dominant mechanism, diseases primarily of myelination deficiencies, and diseases where both of the above come into play. Evaluation of Q-Cells in these neurodegenerative disease groups provides multiple opportunities for success utilizing this single therapeutic product.
Diseases resulting from Neuronal Loss
Amyotrophic Lateral Sclerosis (ALS), also called Lou Gehrig’s Disease
ALS is a neurodegenerative disease causing progressive deterioration and loss of motor neurons, affecting both upper and lower motor neurons. The loss of nerve stimulus to specific muscles results in atrophy and progressive weakness that leads to paralysis. Death usually results in respiratory failure. ALS is diagnosed in 5,000-6,000 new individuals per year in the U.S., comparable in annual incidence to Multiple Sclerosis resulting in an estimated U.S. prevalence of 30,000 with 450,000 people living with ALS worldwide (ALS Therapy Development Institute). Patient death generally occurs two to five years after initial diagnosis.
There is presently no curative treatment for ALS. The only drug approved for treatment for the disease—riluzole (Rilutek®) - is believed to reduce (but not reverse) damage to motor neurons by decreasing the release of glutamate, and prolongs survival by only 60-90 days. ALS is always fatal, and there are no approved drugs that substantially slow or halt the progression of the disease, much less reverse its devastating effects. Costs for treating individuals with advanced ALS can cost an average of $200,000 a year.
Q has identified ALS as its first target indication.
In September 2013, we received notice from the FDA that our orphan drug request of Q-Cells had been granted for the treatment of ALS patients in the U.S.
Huntington’s Disease
Huntington’s disease is a fatal neurodegenerative disease caused by a hereditary mutation in the Huntington gene. Symptoms are normally manifested in adult life, resulting in decline in muscle coordination and cognitive function, as well as psychiatric problems, with death usually ensuing after 20 years from onset of visible symptoms. Approximately 30,000 people in the U.S. are living with Huntington’s disease (Huntington’s Disease Society of America).
5
Table of Contents
Alzheimer’s Disease
Alzheimer’s Disease (AD) is a progressive, neurodegenerative disease characterized by abnormal clumps (amyloid plaques) and tangled bundles of fibers (neurofibrillary tangles) in the brain. Symptoms include memory loss, language deterioration and confusion and eventually lead to loss of cognition and personality. Currently, there is not a way to prevent, cure or even delay its progression. It is the 6th leading cause of death in the U.S. and is estimated to affect about 5.2 million people in the U.S., with annual healthcare costs in excess of $200 billion. The number of patients is projected to reach 16 million by 2050 (Alzheimer’s Association, 2012).
Traumatic Spinal Cord Injury
Approximately 12,000 people are paralyzed each year in the U.S. due to a traumatic spinal Cord Injury (SCI) and it is estimated that over 270,000 people are living with Spinal Cord Injury in the U.S. (National Spinal Cord Injury Statistical Center, Birmingham, Alabama). The average age of occurrence for SCI is 41. This has tremendous costs both in terms of patient care, lost productivity as well as quality of life. Average medical costs are estimated at between $15,000 - $30,000 a year with an estimated lifetime cost up to $3.0 million depending on the severity of the injury and the age at which the injury occurred. It has been projected that the U.S. could save up to $400 billion in future healthcare costs if there were effective therapies to treat and prevent spinal cord injuries (Christopher and Dana Reeve Foundation, Centers for Disease Control, University of Alabama National Spinal Cord Injury Statistical Center).
Parkinson’s Disease
Parkinson’s Disease (PD) affects more than one million patients in the U.S., with approximately 60,000 new cases diagnosed each year (Parkinson’s Disease Foundation). Due to the increase in the aging population, it is anticipated that the number of individuals diagnosed with PD will continue to increase. PD involves the breakdown and death of vital nerve cells, or neurons, found in the substantia nigra of the brain. These neurons normally secrete dopamine, and lack of dopamine leads to symptoms such as shaking, rigidity, difficulty and slowness in movement, and postural instability. While the cause of PD is currently unknown and there is no cure, there are therapies available to manage its symptoms, but they become less effective as the disease progresses. Combined direct and indirect costs of treating PD in the U.S. alone are estimated to be $25 billion a year. If surgery is needed, the costs can approach up to a $100,000 per patient.
Diseases resulting from Myelination Deficiencies
Transverse Myelitis
It is estimated that approximately 1,400 new cases of Transverse Myelitis (TM) are diagnosed each year in the U.S. with approximately 33,000 individuals currently suffering the effects of the disease (National Institute of Neurological Disorders and Stroke). Lack of effective treatments can leave the individual with a disability such as partial or complete paralysis resulting from the disorder. Researchers are currently uncertain of the primary causes and there are currently no effective cures for TM. Current treatment is targeted towards minimizing inflammation and pain. Estimated costs for diagnosing and treating TM are approximately $600,000 per patient in the U.S.
Multiple clinical and pathological studies suggest that there are many common features of the inflammation and neural injury between TM and MS, with a shared primary pathology of demyelination due to immune attack resulting in destruction of the oligodendrocytes.
Multiple Sclerosis
The annual worldwide pharmacotherapy market for MS is approximately $10 billion. Diagnosis of MS typically occurs between 20 and 40 years of age with over 2.5 million people worldwide estimated to have been diagnosed
6
Table of Contents
(National MS Society). While MS is rarely fatal in the short term, individuals diagnosed with MS are usually required to implement both temporary and/or permanent modifications to their lifestyle as they experience the varying range of MS symptoms. The vast majority of all approved MS therapies seek to slow disease progression by “knocking down” the autoimmune component of the disease, which causes the damage and destruction of the myelin sheath that surrounds the neurons. However, there is no approved product that repairs this damage once it has occurred. Thus, while current therapies may slow or even halt the progression of the disease, once the damage is done, there is currently no means of repairing that damage.
Stroke
Stroke afflicts almost 800,000 people in the U.S. and is the fourth leading cause of death, with total annual direct and indirect costs over $73.7 billion (National Stroke Association Fact Sheet). Market projections are significant even if only a small portion of these patients were treated. Q-Cells may be beneficial in treating stroke both for restoring myelination, as well as the support provided by astrocytes to restoring neuron function.
Cerebral Palsy
Cerebral Palsy (CP) has a U.S. patient population of about 764,000 children and adults (United Cerebral Palsy Association) and refers to any of a number of neurological disorders that permanently affect muscle coordination and mobility. No cure currently exists, but the earlier the disease is diagnosed in an individual’s life, the higher likelihood there is of being able to improve muscle function and coordination. An initial target may be for spastic diplegia, which involves injury to the cerebral cortex and represents approximately 22% of CP.
Traumatic Brain Injury
It is estimated that 1.7 million people in the U.S. sustain a Traumatic Brain Injury (TBI) each year with approximately 5.3 million Americans living with a TBI related injury (Centers for Disease Control and Prevention). In 2010, it was estimated that direct and indirect medical costs for those with TBI were approximately $76.5 billion. Other than acute phase treatments to prevent further injury (e.g., surgery to relieve pressure build-up), treatment of headaches, seizures and physical rehabilitation, there is no treatment for and nothing to reverse TBI. The multiple support functions of Q-Cells may be beneficial in achieving recovery from TBI.
Collaborations and Grant Funding
Q Therapeutics pursues opportunities to obtain grant funding from government and private organizations when such grants are aimed at an area that augments Q’s internal development efforts. Generally, we seek opportunities that have the potential to provide funding over several years and that can advance programs into clinical development. We normally seek qualified academic collaborators to work with us on these programs to complement our internal developmental expertise. Grant funding is very competitive to obtain, and it is normal to apply for many grants for each one funded. As applying for grants requires expenditure of internal resources, Q is selective as to which grant opportunities it will pursue that are aligned with our corporate goals. To date, Q has been the beneficiary of:
1) Two SBIR Phase 1 grants (approximately $120,000 each);
2) A $4 million (in direct costs) grant from National Institutes of Health (NIH) to help fund the preclinical studies of Q-Cells to result in submission of an IND to study Q-Cells as a potential treatment for ALS patients;
3) An $850,000 grant from the Maryland Stem Cell Fund, to help fund preclinical studies with The Johns Hopkins University on Q-Cells for treatment of ALS; and
4) A $250,000 grant from the Neilsen Foundation to fund preclinical studies of Q-Cells in traumatic spinal cord injury.
Q Therapeutics works in conjunction and maintains collaborations with the highly respected laboratories of Dr. Nicholas Maragakis, Dr. Piotr Walczak and Dr. Itzhak Fischer. The collaborations with these laboratories do not involve any milestone or royalty payments or any form of agreement for compensation of any kind. Material transfer agreements cover the supply and use of cells from Q to the collaborators. To date, most of their research has been funded through grant funding from the NIH, Maryland Stem Cell Fund, and the Neilsen Foundation.
7
Table of Contents
The NIH grant that has funded the collaboration with Dr. Maragakis’ laboratory concluded in 2014. Approximately $800,000 to $900,000 in direct costs was funded in each of the four grant years. Year four funding was primarily devoted to Q-Cell production at the University of Utah, antibody production at Goodwin Biotechnology, Inc., and GLP safety studies at MPI Research to support the Company’s IND submission for ALS.
Material Terms of the NIH Grant
The NIH grant is intended to fund work leading to an IND submission for use of Q-Cells in the treatment of ALS. The grant was issued directly to our collaborators and covers costs that would otherwise be borne by Q, including our collaboration with Dr. Maragakis’ laboratory, the University of Utah, MPI Research and Goodwin Biotechnology. In 2014, the Company was notified of a sub-award of $677,864 as part of grant funding awarded to The Johns Hopkins University from the National Institute of Neurological Disorders and Stroke (NINDS) of the National Institutes of Health for the fourth and final grant plan year.
Q Therapeutics has the proprietary rights to the cells that are the subject of all of these grants.
Agreement Terms of Maragakis Collaboration
With respect to the Maragakis collaboration, Q provides technical expertise as well as product for testing and actively assists with experimental design and analysis. The Maragakis Laboratory provides its technical expertise and carries out experiments as mutually agreed upon with Q Therapeutics. Q maintains all rights to the Q-Cells product. Upon the development of any new intellectual property in which technologies outside of Q-Cells are involved, Q Therapeutics has the right of first negotiation to obtain an exclusive license on such innovation with The Johns Hopkins University.
Future Collaborations with Third Parties
We are in discussions with third parties about collaborating in the development of our Q-Cell product and may, under the right terms, enter into one or more partnership(s) to advance the program.
Manufacturing
Q Therapeutics has developed manufacturing protocols to isolate Q-Cells® directly from fetal cadaver tissue, without the need for additional differentiation in vitro. Q has developed proprietary methods to expand the cells at this stage to enable treatment of many patients from each preparation. Q’s strategy is to start with the most straightforward path: isolate and characterize unmodified cells that are already, naturally, at the desired stage of differentiation, to achieve proof of activity in clinical trials. Q believes that this strategy enables development of Q-Cells and other cellular therapeutics in the most cost and time-effective manner.
Q Therapeutics will use a centralized laboratory (contract GMP cell production manufacturer) for cell isolation and expansion and to provide the necessary quality control. The clinical transplant sites will receive frozen cells, which they will subsequently thaw, wash and inject into the target site of the patient. Use of allogeneic cells (i.e., cells derived from tissue not obtained from the patient), rather than autologous cells (cells derived from the patient’s own tissues) both enables use of what Q believes may be the most effective healthy cells as well as permits cell therapy to be an off-the-shelf treatment, promoting more widespread use. The manufacturing of the Q-Cells is done via a proprietary, patented, process developed by Q. This process can be shared with Q’s contract manufacturer of choice, or with several manufacturers to mitigate the risk of loss of any one manufacturing subcontractor.
Q Therapeutics is currently working with the GLP/GMP Cell Therapy and Regenerative Medicine Facility (CTRM) operated by the University of Utah. Q may seek other manufacturers as its development programs progress.
Manufacturing Agreement with the University of Utah
In December 2011, Q Therapeutics entered into a service and supply agreement with the University of Utah for an initial period of five years, with an option to renew the agreement on an annual basis. The basic terms of the agreement stipulate the pricing for the manufacturing and processing of batches of cells and the index to be used for any price modifications, the granting of non-exclusive rights to the CTRM work product, and a non-exclusive, non-transferable and royalty-free license to the University of Utah to manufacture Q-Cells for use by Q and by collaborators specified by Q, with payment of manufacturing costs to the University of Utah. Either party can terminate the agreement upon appropriate notice.
8
Table of Contents
Manufacturing Agreement with Goodwin Biotechnology
Q Therapeutics has an agreement with Goodwin Biotechnology (GBI) which specifies the contract research to achieve GMP production and conjugation of the antibody used in cell purification for manufacture of Q-Cells. Q maintains all rights to the products. The Agreement with GBI, which sets out obligations of GBI to provide process development and GMP manufacture and stability studies of the antibody originally provided by Q, is close to completion with stability studies ongoing. Q Therapeutics can terminate the project at any time, subject to a cancellation penalty.
Competition
The competitive landscape involves companies working on a range of alternative treatments for diseases of the CNS including traditional drug products, protein therapeutics, gene therapy and different types of cell therapy. A handful of companies are exploring the possibility of harnessing the power of stem cells to treat neurodegenerative disease, though no clear leader has emerged. Q believes that Q-Cells are different from other cell therapy products both in their novel mechanism of action/capabilities as well as the cost-effective methods of production. Q believes the cell therapy approach has many advantages over single drug therapeutics for many neurodegenerative diseases. Thus, the closest direct competition is other cell therapy companies working on the same disease targets.
Direct Competition
A limited number of companies are working on the therapeutic neurological application of stem and progenitor cells for CNS treatment, including the public companies Neuralstem (NYSE MKT: CUR), StemCells Inc. (NASDAQ: STEM), ReNeuron (LSE: RENE.L), and Brainstorm Cell Therapeutics (OB: BCLI).
StemCells Inc. has completed the first portion of their Phase 1/2 clinical trial using human fetal-derived neural stem cells for the treatment of Spinal Cord Injury. In 2014, they expanded their single country; single site trial to a multi-country, multi-site trial conducted in Canada and Switzerland and recently reported positive interim data for SCI patients. Final results of this study are expected to be released in 2015. Stem Cells Inc. has also completed a Phase 1 clinical trial for treatment of Pelizaeus-Merzbacher disease, a rare myelination disorder of the brain. ReNeuron uses human fetal-derived neural stem cells to treat patients disabled by ischaemic stroke and is mid-stage in their clinical development. Neuralstem conducted the first neural stem cell clinical trial for the treatment of ALS and has completed its Phase 2 safety and efficacy trial.
Other Potential Competitors
Another approach is the use of cells or virus-based vectors implanted in the brain as delivery vehicles for gene therapy, with the goal of achieving local production of desired proteins and growth factors. Sangamo, through its acquisition of Ceregene, is conducting clinical trials with virus-based vectors for gene therapy for Parkinson’s and Alzheimer’s diseases, with other disease targets in earlier research. Cedars Sinai is developing neural progenitors engineered to secrete glial cell-derived neurotrophic factor (GDNF) for treatment of ALS. Q’s initial approach differs significantly in that it does not rely on a single growth factor for success, and in that it does not use genetically modified cells or viruses; rather, unmodified Q-Cells produce the range of growth factors and trophic support that is typical of healthy glial cells. Q has recently acquired rights to intellectual property enabling engineering of its cells to provide augmented production of proteins, such as growth factors, which could develop into a second product platform.
Other companies working in the CNS space have focused on stimulating endogenous differentiation of nascent adult stem cells with administration of molecules to trigger their expansion and differentiation in vivo (e.g., BrainCells, Inc.). Still other pharmaceutical and biotechnology companies (including, among others Genzyme/Sanofi-Aventis, Biogen, Acorda, Merck-Serono, GlaxoSmithKline and Roche) have programs to identify therapeutics to treat neurodegenerative diseases such as MS, Parkinson’s disease and Alzheimer’ disease. Some of these programs might yield effective therapeutics that could compete with Q’s products, and which might prove less costly and easier to administer.
A noteworthy distinction between Q and other CNS cell therapy companies involves the specific properties of Q-Cells.
9
Table of Contents
These cells naturally produce mature glial cells (astrocytes and oligodendrocytes) that can both achieve myelination of neuronal axons as well as otherwise promote neuron health, while minimizing unwanted and potentially deleterious neuron formation. Q-Cells are a more mature cell type - at a more advanced stage of differentiation - than are the cells used by StemCells Inc., ReNeuron, and Neuralstem, who all use the less-differentiated CNS stem cells that produce more neurons than glial cells. Unlike ReNeuron, Q does not use genetically immortalized cells, which Q believes could potentially raise safety issues, e.g., concerns about deleterious effects such as tumor formation. Unlike Brainstorm, which uses non-CNS autologous mesenchymal stem cells (MSCs) that it modifies in vitro to make what they call “glial-like” cells, Q uses non-autologous natural CNS glial cells that are not modified in vitro and that are intended to be an “off-the shelf” product.
Pluripotent stem cells could also be a starting source. This approach requires the identification of proper signaling factors and establishing the sequence of use to correctly and safely induce differentiation into the desired CNS cell type and to ensure sufficient elimination of the undifferentiated pluripotent stem cells which can form tumors, prior to transplantation. Our current Q-Cells are not derived from undifferentiated pluripotent cells, such as embryonic stem cells. Rather, Q-Cells are isolated already at the desired final cell type, and require no in vitro differentiation protocols. Q believes that the progenitor cell therapies it is developing as its first products, generated from pre-formed cells that naturally occur, offer safety and efficacy benefits as well as a faster path to market.
In August 2014, Q entered into a fee-for-service contract with XCell, Inc. to develop a process for deriving Glial Restricted Progenitor (GRPs) from adult induced pluripotent stem cell (iPSC) lines. To date, Q has paid XCell $22,500, with the remaining $27,500 due upon completion of the final milestones. Q owns all technology generated under the contract, in addition to IP owned by Q on production of GRPs from iPSC lines.
Intellectual Property
Q Therapeutics has exclusive worldwide rights to its Q-Cells product, through an agreement with the University of Utah Research Foundation (UURF or the Foundation) and through exclusively owned, internally developed intellectual property. The Q patent portfolio encompasses five families of neural lineage progenitor or stem cell technologies. Currently, Q has rights to 23 issued patents plus 12 U.S. and international patents pending encompassing composition of matter, methods of production and methods of use of multiple cell types of the CNS (including Q-Cells) as well as some of the peripheral nervous system, giving us a strong intellectual property position for Q-Cells as well as other neural lineage cells. Some of our patents are the subject of a license agreement with the University of Utah and the NIH. This license provides for royalties on Q Therapeutics’ and sublicensees’ sales and contains due diligence obligations and related provisions; a portion of the consideration to the University of Utah was equity in Q.
Trademarks
Q also holds a U.S. Trademark to its first product name, Q-Cells (serial number: 78869175; registration number: 3,385,490), and U.S. and International Trademarks/Service marks to: Q Therapeutics® (U.S. serial number: 78-415,125; U.S. registration number: 3,280,432; international registration number: 867,474).
In addition, Q has entered into out-license agreements with Life Technologies, Molecular Transfer, Inc., and XCell, under which the licensees may develop, manufacture and sell certain cell products covered by Q’s IP for research and drug discovery use only, for which Q (or its subsidiary NeuroQ) is anticipated to receive royalty payments, and in the case of XCell, Q may provide certain payments for research activities.
Research and Development
Our research and development expenses for the years ended December 31, 2014 and 2013 were $1,960,815 and $1,827,533, respectively. Our research and development expenses to date have been primarily the result of our research and the research of our collaborative partners relating to the development of Q-Cells.
Government Regulation
Any products we may develop and our research and development activities are subject to stringent government regulation in the United States by the FDA and, in many instances, by corresponding foreign and state regulatory agencies. The European Union, or EU, has vested centralized authority in the European Medicines Agency and Committee on Proprietary Medicinal Products to standardize review and approval across EU member nations.
10
Table of Contents
These regulatory agencies enforce comprehensive statutes, regulations and guidelines governing the drug development process. This process involves several steps. Initially, a company must generate preclinical data to show safety before human testing may be initiated. In the United States, a drug company must submit an IND to the FDA prior to securing authorization for human testing. The IND must contain adequate data on product candidate chemistry, toxicology and metabolism and, where appropriate, animal research testing to support initial safety.
A Clinical Trial Agreement, or CTA, is the European equivalent of the IND. CTA requirements are issued by each competent authority within the European Union and are enacted by local laws and directives.
Any of our potential product candidates will require regulatory clearance and compliance with regulations made by United States and foreign government agencies prior to commercialization in such countries. The process of obtaining FDA or foreign regulatory agency clearance has historically been extremely costly and time consuming. The FDA regulates, among other things, the development, testing, manufacture, safety, efficacy, record keeping, labeling, storage, approval, advertising, promotion, sale, and distribution of biologics and new drugs.
The standard process required by the FDA before a pharmaceutical agent may be marketed in the United States includes:
| • | preclinical tests in animals that demonstrate a reasonable likelihood of safety and effectiveness in human patients; |
| • | submission to the FDA of an IND, which must become effective before clinical trials in humans can commence. If Phase 1 clinical trials are to be conducted initially outside the United States, a different regulatory filing is required, depending on the location of the trial; |
| • | adequate and well controlled human clinical trials to establish the safety and efficacy of the drug or biologic in the intended disease indication; |
| • | for drugs, submission of a New Drug Application, or NDA, or a Biologic License Application, or BLA, with the FDA; and |
| • | FDA clearance of the NDA or BLA before any commercial sale or shipment of the drug. |
Preclinical studies can take several years to complete, and there is no guarantee that an IND based on those studies will become effective to permit clinical trials to begin. The clinical development phase generally takes five to seven years, or longer, to complete (i.e., from the initiation of Phase 1 through completion of Phase 3 studies). After successful completion of clinical trials for a new drug or biologic product, FDA clearance of the NDA or BLA must be obtained. This process requires substantial time and effort and there is no assurance that the FDA will accept the NDA or BLA for filing and, even if filed, that the FDA will grant marketing clearance. In the past, the FDA’s clearance of an NDA or BLA has taken, on average, one to two years, but in some instances may take substantially longer. If questions regarding safety or efficacy arise, additional studies may be required and then followed by a resubmission of the NDA or BLA. Review and clearance of an NDA or BLA can take several years.
In addition to obtaining FDA clearance for each product, each drug manufacturing facility must be inspected and approved by the FDA. All manufacturing establishments are subject to inspections by the FDA and by other federal, state, and local agencies, and must comply with good manufacturing practices, or GMP, requirements. We do not currently have any GMP manufacturing capabilities, and will rely on contract manufacturers to produce material for any clinical trials that we may conduct.
We must also obtain regulatory clearance in other countries in which we intend to market any drug. The requirements governing conduct of clinical trials, product licensing, pricing, and reimbursement vary widely from country to country. FDA clearance does not ensure regulatory approval in other countries. The current approval process varies from country to country, and the time spent in gaining approval varies from that required for FDA clearance. In some countries, the sale price of the drug must also be approved. The pricing review period often begins after market approval is granted. Even if a foreign regulatory authority approves a drug product, it may not approve satisfactory prices for the product.
11
Table of Contents
In addition to regulations enforced by the FDA, we are also subject to regulation under the Occupational Safety and Health Act, the Environmental Protection Act, the Toxic Substances Control Act, the Resource Conservation and Recovery Act, and other present and potential future federal, state, or local regulations. Our research and development involves the controlled use of hazardous materials, chemicals, biological materials, and various radioactive compounds. Although we believe that our safety procedures for handling and disposing of such materials currently comply in all material respects with the standards prescribed by state and federal regulations, the risk of accidental contamination or injury from these materials cannot be completely eliminated. In the event of such an accident, we could be held liable for any damages that result and any such liability could exceed our available resources.
Our Employees
As of March 31, 2015, we had five full-time employees and five part-time employees. Approximately 60% of our employees are involved with research, development and clinical affairs and 40% are involved with finance, human resources, and administration.
We currently maintain compensation, benefits, equity participation and work environment policies intended to assist in attracting and retaining qualified personnel. We believe that the success of our business will depend to a significant extent on our ability to attract and retain such personnel.
Corporate Information
Q conducts its business and operations through its wholly owned subsidiary, Q Therapeutic Products, Inc. (Q Products) and Q Products’ wholly owned subsidiary, NeuroQ Research, Inc. Q Products was incorporated in the state of Delaware on March 28, 2002, and merged with Q Acquisition, Inc., a Delaware corporation and wholly owned subsidiary of Grace 2, Inc., on October 13, 2011. On November 2, 2011, Grace 2 changed its name to Q Holdings, Inc., and on December 10, 2012, Q Holdings changed its name to Q Therapeutics, Inc.
| Item 1A. | Risk Factors |
RISK FACTORS
In addition to the other information contained in this Annual Report, the following risk factors should be considered carefully in evaluating our company. Our business, financial conditions, liquidity or results or operations could be materially adversely affected by any of these risks.
RISKS RELATED TO THE COMPANY’S BUSINESS
Our business is at an early stage of development.
Our business is at an early stage of development, in that we do not yet have product candidates in clinical trials or on the market. Our ability to develop product candidates that progress to and through clinical trials is subject to our ability to, among other things:
| • | succeed in our research and development efforts; |
| • | select therapeutic compounds or cell therapies for development that have acceptable safety and efficacy profiles; |
| • | obtain required regulatory approvals; |
| • | finance, or obtain additional financing for, our clinical trials; |
| • | manufacture product candidates; and |
| • | collaborate successfully with clinical trial sites, academic institutions, physician investigators, clinical research organizations and other third parties; and achieve adequate enrollment of subjects. |
Any delay or failure to develop and commercialize a product could negatively impact the value of our common stock. Even if we do develop a product, we cannot assure you that we would be able to successfully commercialize such product and increase the value of our company.
12
Table of Contents
Our financial statements have been prepared on the assumption that we will continue as a going concern. Our independent registered public accounting firm has issued its report dated March 31, 2015, which includes an explanatory paragraph stating that our recurring losses, among other things, raise substantial doubt about our ability to continue as a going concern. It has been necessary to rely upon debt and the sale of our equity securities to sustain operations. Our management anticipates that we may require additional capital over the next 12 months to fund ongoing operations. There can be no guarantee that we will be able to obtain such funds, or obtain them on satisfactory terms, and that such funds would be sufficient. If such additional funding is not obtained, we may be required to scale back or discontinue operations.
We have incurred net losses since our inception and anticipate that we will continue to incur substantial net losses for the foreseeable future. We may never achieve or sustain profitability.
We have incurred operating losses every year since our incorporation in 2002. As of December 31, 2014, our accumulated deficit was $27,232,071. Losses have resulted principally from costs incurred in connection with our research and development activities and from general and administrative expenses associated with our operations. We expect to incur additional operating losses and, as our development efforts continue, our operating losses may increase in size. Substantially all of our revenues to date have been from grants or license/research agreements. We may be unsuccessful in obtaining any new grants or entering into new license agreements that result in revenues. We do not expect that the revenues generated from any of these arrangements will be sufficient alone to continue or expand our research or development activities and otherwise sustain our operations. Our ability to continue or expand our research and development activities and otherwise sustain our operations is dependent on our ability, alone or with others, to, among other things, manufacture and market therapeutic products. We also expect to experience negative cash flows from operating activities for the foreseeable future as we fund our operating losses and capital expenditures. This will result in decreases in our working capital, total assets and stockholders’ equity, which may not be offset by future financings. We will need to generate significant revenues to achieve profitability. We may not be able to generate these revenues, and we may never achieve profitability. Our failure to achieve profitability could negatively impact the market price of our common stock. Even if we do become profitable, we cannot assure you that we would be able to sustain or increase profitability on a quarterly or annual basis.
We will need additional capital to conduct our operations and develop our product candidates, and our ability to obtain the necessary funding is uncertain.
We will require substantial capital resources in order to conduct our operations and develop our product candidates, and we cannot assure you that our existing capital resources, grants, interest income and/or financing agreements will be sufficient to fund future planned operations. The timing and degree of any future capital requirements will depend on many factors, including:
| • | the accuracy of the assumptions underlying our estimates for our capital needs for 2015 and beyond; |
| • | the magnitude and scope of our research and development programs; |
| • | the progress we make in our research and development programs, preclinical development and clinical trials; |
| • | our ability to establish, enforce and maintain strategic arrangements for research, development, clinical testing, manufacturing and marketing; |
| • | the number and type of product candidates that we pursue; |
| • | the time and costs involved in obtaining regulatory approvals and clearances; and |
| • | the costs involved in preparing, filing, prosecuting, maintaining, defending and enforcing patent claims. |
We do not have any committed sources of capital. Additional financing through strategic collaborations, public or private equity financings, debt financing, capital lease transactions or other financing sources may not be available on acceptable terms, or at all. The receptivity of the public and private equity markets to proposed financings is substantially affected by the general economic, market and political climate and by other factors which are unpredictable and over which we have no control. Additional equity financings, if we obtain them, could result in significant dilution to our shareholders. Further, in the event that additional funds are obtained through arrangements with collaborative partners, these arrangements may require us to relinquish rights to some of our technologies,
13
Table of Contents
product candidates or proposed products that we would otherwise seek to develop and commercialize ourselves. If sufficient capital is not available, we may be required to delay, reduce the scope of or eliminate one or more of our programs, any of which could have a material adverse effect on our business.
Our business is dependent on limited product candidates.
At present, our ability to progress as an entity is significantly dependent on our first product candidate for neurodegenerative diseases, with the initial clinical indication for ALS, which is currently in preclinical development. Any preclinical, regulatory or other development that significantly delays or prevents us from commencing or subsequently completing any of our trials, any material safety issue or adverse side effect to any study participant in these future trials, or the failure of trials to show the results expected would likely depress our stock price significantly and could prevent us from raising the additional capital we will need to further develop our cellular technologies. Moreover, any material adverse occurrence in our first clinical trials could substantially impair our ability to initiate clinical trials to test our cell therapies in other potential indications. Also, any material adverse event in clinical trials from other companies using cell therapy products could have a material adverse impact on our ability to carry out clinical trials. This, in turn, could adversely impact our ability to raise additional capital and pursue our planned research and development efforts.
Our business relies on cell therapy technologies that we may not be able to commercially develop.
We have concentrated the majority of our research on cell therapy technologies. Our ability to generate revenue and operate profitably will depend on being able to develop these technologies for human applications. These are emerging technologies and have limited human applications. We cannot guarantee that we will be able to develop our technologies or that such development will result in products with any commercial utility or value. We anticipate that the commercial sale of such products and royalty/licensing fees related to the technology will be our future primary sources of revenues. If we are unable to develop our technologies, we may never realize significant revenues.
Our product development programs are based on novel technologies and are inherently risky.
We are subject to the risks of failure inherent in the development of products based on new technologies. The novel nature of these therapies creates significant challenges in regard to product development and optimization, manufacturing, government regulation, third-party reimbursement and market acceptance. For example, the pathway to regulatory approval for cell-based therapies, including our product candidates, may be more complex and lengthy than the pathway for conventional drugs. These challenges may prevent us from developing and commercializing products on a timely or profitable basis, or at all.
We intend to rely upon third-party manufacturers for our cells.
We currently have no internal manufacturing capability, and will rely extensively on licensees, strategic partners or third-party contract manufacturers or suppliers. Should we be forced to manufacture our cell therapy product, we cannot provide any assurance that we will be able to develop an internal manufacturing capability or procure alternative third-party suppliers. Moreover, we cannot give you any assurance that any contract manufacturers or suppliers we procure will be able to supply our product in a timely or cost effective manner or in accordance with applicable regulatory requirements or our specifications. We are presently working with the University of Utah’s Cell Therapy & Regenerative Medicine Facility (CTRM) in the production of Q-Cells. We will need to change manufacturers at some point in the future in order to manufacture a product for commercial sale, among other things. When that change to a new manufacturer is made, it will likely entail additional expense to achieve technology transfer and validation, which could cause delays in manufacturing and product development activities.
We base our research and development on the use of human cells obtained from human tissue. The U.S. federal and state governments and other jurisdictions impose restrictions on the acquisition and use of human tissue, including those incorporated in federal Good Tissue Practice (GTP) regulations. These regulatory and other constraints could prevent us from obtaining cells and other components of our products in the quantity or of the quality needed for their development or commercialization. These restrictions change from time to time and may become more onerous. Additionally, we may not be able to identify or develop reliable sources for the cells necessary for our potential products—that is, sources that follow all state and federal laws and guidelines for cell procurement. Certain components used to manufacture our stem and progenitor cell product candidates will need to be manufactured in
14
Table of Contents
compliance with the FDA’s Good Manufacturing Practices (GMP). Accordingly, we will need to enter into supply agreements with companies that manufacture these components to GMP standards. There is no assurance that we will be able to enter into any such agreements.
Noncompliance with applicable requirements both before and after approval, if any, can subject us, our third-party suppliers and manufacturers and our other collaborators to administrative and judicial sanctions, such as, among other things, warning letters, fines and other monetary payments, recall or seizure of products, criminal proceedings, suspension or withdrawal of regulatory approvals, interruption or cessation of clinical trials, total or partial suspension of production or distribution, injunctions, limitations on or the elimination of claims we can make for our products, refusal of the government to enter into supply contracts or fund research, or government delay in approving or refusal to approve new drug applications.
Our inability to complete preclinical and clinical testing and receive regulatory approvals will impair our viability.
No assurances can be given that we will be able to commence clinical trials or that any clinical trials will be completed or result in a successful outcome. If regulatory authorities do not give us marketing clearance for our products or if we fail to maintain regulatory compliance, we would be unable to commercialize our therapeutic products, and our business and results of operations would be materially harmed.
Positive results from preclinical studies should not be relied upon as evidence that our clinical trials will succeed. Even if our product candidates achieve positive results in preclinical studies, we will be required to demonstrate through clinical trials that the product candidates are safe and effective for use in our target population before we can seek regulatory approvals for their commercial sale. There is typically an extremely high rate of attrition from the failure of product candidates as they proceed through clinical trials. If any product candidate fails to demonstrate sufficient safety and efficacy in any clinical trial, then we would experience potentially significant delays in, or be required to abandon, development of that product candidate. If we delay or abandon our development efforts of any of our product candidates, then we may not be able to generate sufficient revenues to become profitable, and our operations could be materially harmed.
Potential lead drug compounds or other product candidates and technologies require significant preclinical and clinical testing prior to regulatory approval in the United States and other countries. Our product candidates may prove to have undesirable and unintended side effects or other characteristics adversely affecting their safety, efficacy or cost-effectiveness that could prevent or limit their commercial use. In addition, our product candidates may not prove to be more effective for treating disease or injury than current therapies. Accordingly, we may have to delay or abandon efforts to research, develop or obtain regulatory approvals to market our product candidates. In addition, we will need to determine whether any of our potential products can be manufactured in commercial quantities at an acceptable cost. Our research and development efforts may not result in a product that can be or will be approved by regulators or marketed successfully. Competitors may have proprietary rights that prevent us from developing and marketing our products or they may sell similar, superior or lower cost products. Adverse results in research or clinical trials of others with cell therapy products may adversely affect regulatory or financial areas that could hinder our ability to continue with our clinical development. Because of the significant scientific, regulatory and commercial milestones that must be reached for any of our development programs or product candidates to be successful, any program or product candidate may be abandoned, even after we have expended significant resources, such as our investments or prospective investments in cell therapy technologies, which could adversely affect our business and materially and adversely affect our stock price.
The science and technology of stem and progenitor cell biology are relatively new. There is no precedent for the successful commercialization of therapeutic product candidates being developed by Q Therapeutics based on these technologies. Therefore, our development programs are particularly risky and uncertain. In addition, we, and/or our collaborators must undertake significant research and development activities to develop product candidates based on these technologies. This development work may take years to accomplish and will require additional funding.
Our business is subject to ethical and social concerns and restrictions on use of stem or progenitor cells could prevent us from developing or gaining acceptance for commercially viable products based upon such technology and adversely affect the market price of our common stock.
The use of stem cells for research and therapy has been the subject of debate regarding ethical, legal and social
15
Table of Contents
issues. Negative public attitudes toward stem cell therapy could result in greater governmental regulation of such therapies, which could harm our business. For example, concerns regarding such possible regulation could impact our ability to attract collaborators and investors. Existing and potential U.S. government regulation of human tissue may lead researchers to leave the field of stem and progenitor cell research or the country altogether, in order to assure that their careers will not be impeded by restrictions on their work. Similarly, these factors may induce graduate students to choose other fields less vulnerable to changes in regulatory oversight, thus exacerbating the risk that we may not be able to attract and retain the scientific personnel we need in the face of competition among pharmaceutical, biotechnology and health care companies, universities and research institutions for what may become a shrinking class of qualified individuals.
Some of our most important programs involve the use of progenitor cells that are derived from human fetal cadaver tissue. The use of these cells can give rise to ethical and social issues regarding elective termination of pregnancy and the appropriate use of tissue derived therefrom. Some political and religious groups have voiced opposition to these technologies and practices. We use progenitor cells, and may use stem cells, derived from fetal cadaver tissue. Our research related to these derivatives may become the subject of adverse commentary or publicity or hinder partnering activities, which could significantly harm the market price of our common stock. Changes in federal or state laws regulating rights to elective terminations of pregnancy may adversely impact our ability to procure tissue for manufacturing our products.
These potential effects and others may result in a decrease in the market price of our common stock.
We operate in leased facilities and cannot be assured that these facilities will be available to us upon expiration of our current lease.
We occupy laboratory and office space in facilities leased from a commercial landlord, and we have added improvements to these facilities at our own expense to meet our laboratory needs. We cannot be assured that we will be able to remain in the same space after our lease expires. If we move to a new facility, we will incur additional expense to add the improvements needed for our operations, as well as delay our development activities until we are fully operational in a new space.
We may engage in acquisitions or strategic transactions or make investments that could result in significant changes or management disruption and fail to enhance stockholder value.
We may engage in acquisitions or strategic transactions or make investments with the goal of maximizing stockholder value. We may acquire businesses, in-license new technologies for development, enter into joint ventures or other strategic transactions, and purchase equity and debt securities, including minority interests in public and private companies, non-investment-grade debt securities, equity and debt mutual and exchange-traded funds, and corporate bonds/notes. Some of our strategic investments may entail a high degree of risk and will not become liquid until more than one year from the date of investment, if at all. Any or all of our acquisitions or strategic investments that we may undertake in the future may not generate financial returns or result in increased adoption or continued use of our technologies. In addition, our other investments may not generate financial returns or may result in losses due to market volatility, the general level of interest rates and inflation expectations. In some cases, we may be required to consolidate or record our share of the earnings or losses of those companies. Our share of any losses will adversely affect our financial results until we exit from or reduce our exposure to these investments. Achieving the anticipated benefits of acquisitions depends in part upon our ability to integrate the acquired businesses in an efficient and effective manner. The integration of companies that have previously operated independently may result in significant challenges, and we may be unable to accomplish the integration smoothly or successfully. We cannot assure you that the integration of acquired businesses with our business will result in the realization of the full benefits anticipated by us to result from the acquisition. We may not derive any commercial value from the acquired technology, products and intellectual property or from future technologies and products based on the acquired technology and/or intellectual property, and we may be subject to liabilities that are not covered by indemnification protection we may obtain.
Compliance with changing regulation of corporate governance and public disclosure may result in additional expenses.
Changing laws, regulations and standards relating to corporate governance and public disclosure may create uncertainty regarding compliance matters. New or changed laws, regulations and standards are subject to varying
16
Table of Contents
interpretations in many cases. As a result, their application in practice may evolve over time. We are committed to maintaining high standards of corporate governance and public disclosure. Complying with evolving interpretations of new or changed legal requirements may cause us to incur higher costs as we revise current practices, policies and procedures, and may divert management time and attention from product development and revenue generation to compliance activities. If our efforts to comply with new or changed laws, regulations and standards differ from the activities intended by regulatory or governing bodies due to ambiguities related to practice, our reputation might also be harmed.
We are subject to the reporting requirements of federal securities laws, which can be expensive and may divert management’s time and Company resources from other projects, thus impairing our ability to grow.
We are an SEC reporting company and, accordingly, subject to the information and reporting requirements of the Securities Exchange Act of 1934, as amended, and other federal securities laws, including compliance with the Sarbanes-Oxley Act of 2002 (the Sarbanes-Oxley Act). The costs of preparing and filing annual, quarterly and current reports, proxy statements and other information with the SEC, and furnishing audited reports to shareholders are high and will put pressure on our cash resources.
It may be time consuming, difficult and costly for us to develop and implement the internal controls and reporting procedures required by the Sarbanes-Oxley Act. We may need to hire additional financial reporting, internal controls and other finance personnel in order to develop and implement appropriate internal controls and reporting procedures. If we are unable to comply with the internal controls requirements of the Sarbanes-Oxley Act, then we may not be able to obtain the independent registered public accounting firm certifications required by such Act, which may preclude us from keeping our filings with the SEC current.
We are an “emerging growth company,” and a “smaller reporting company” and any decision on our part to comply only with certain reduced reporting and disclosure requirements applicable to emerging growth companies and “smaller reporting companies” could make our common stock less attractive to investors.
We are an “emerging growth company,” as defined in the Jumpstart Our Business Startups, or JOBS, Act enacted in April 2012, and, for as long as we continue to be an “emerging growth company,” we may choose to take advantage of exemptions from various reporting requirements applicable to other public companies including, but not limited to, not being required to have our independent registered public accounting firm audit our internal control over financial reporting under Section 404, reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements, and exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and stockholder approval of any golden parachute payments not previously approved. We could be an “emerging growth company” for up to five years, although, if we have more than $1.0 billion in annual revenue, if the market value of our common stock that is held by non-affiliates exceeds $700 million as of June 30 of any year, or we issue more than $1.0 billion of non-convertible debt over a three-year period before the end of that five-year period, we would cease to be an “emerging growth company” as of the following December 31. We cannot predict if investors will find our common stock less attractive if we choose to rely on these exemptions. If some investors find our common stock less attractive as a result of any choices to reduce future disclosure, there may be a less active trading market for our common stock and our stock price may be more volatile. As an “emerging growth company” the JOBS Act allows us to delay adoption of new or revised accounting pronouncements applicable to public companies until such pronouncements are made applicable to private companies. We have elected to use this extended transition period under the JOBS Act. As a result, our financial statements may not be comparable to the financial statements of issuers who are required to comply with the effective dates for new or revised accounting standards that are applicable to public companies, which may make our common stock less attractive to investors.
We are also currently a “smaller reporting company” as defined in the Securities Exchange Act of 1934, and in the event that we are still considered a smaller reporting company at such time as we cease being an emerging growth company, we will be required to provide additional disclosure in our SEC filings. However, similar to emerging growth companies, smaller reporting companies are able to provide simplified executive compensation disclosures in their filings, are exempt from the provisions of Section 404(b) of the Sarbanes-Oxley Act requiring that independent registered public accounting firms provide an attestation report on the effectiveness of internal control over financial reporting, and have certain other decreased disclosure obligations in their SEC filings, including, among other things, only being required to provide two years of audited financial statements in annual reports. We cannot predict whether investors will find our common stock less attractive because of our reliance on any of these exemptions. If some investors find our common stock less attractive as a result, there may be a less active trading market for our common stock and our stock price may be more volatile.
17
Table of Contents
If we fail to establish and maintain an effective system of internal control, we may not be able to report our financial results accurately or to prevent fraud. Any inability to report and file our financial results accurately and timely could harm our reputation and adversely impact the trading price of our common stock.
Effective internal control is necessary for us to provide reliable financial reports and prevent fraud. If we cannot provide reliable financial reports or prevent fraud, we may not be able to manage our business as effectively as we would if an effective control environment existed, and our business and reputation with investors may be harmed. As a result, our small size and any current internal control deficiencies may adversely affect our financial condition, results of operations and access to capital. We have not performed an in-depth analysis to determine if historical un-discovered failures of internal controls exist, and may in the future discover areas of our internal control that need improvement.
Public company compliance may make it more difficult to attract and retain officers and directors.
The Sarbanes-Oxley Act and new rules subsequently implemented by the SEC have required changes in corporate governance practices of public companies. As a public company, we expect these new rules and regulations to increase our compliance costs in 2015 and beyond and to make certain activities more time consuming and costly. As a public company, we also expect that these new rules and regulations may make it more difficult and expensive for us to obtain director and officer liability insurance in the future and we may be required to accept reduced policy limits and coverage or incur substantially higher costs to obtain the same or similar coverage. As a result, it may be more difficult for us to attract and retain qualified persons to serve on our board of directors or as executive officers.
Compliance with changing regulation of corporate governance and public disclosure, and our management’s inexperience with such regulations results in additional expenses and creates a risk of non-compliance.
Changing laws, regulations and standards relating to corporate governance and public disclosure, including the Sarbanes-Oxley Act of 2002 and related SEC regulations, have created uncertainty for public companies and significantly increased the costs and risks associated with accessing the public markets and public reporting. Our management team expects to invest significant management time and financial resources to comply with both existing and evolving standards for public companies, which will lead to increased general and administrative expenses and a diversion of management time and attention from revenue generating activities to compliance activities. Management’s inexperience may cause us to fall out of compliance with applicable regulatory requirements, which could lead to enforcement action against us and a negative impact on our stock price.
RISKS RELATING TO OUR COMMON STOCK
There is currently no trading market for our common stock and we cannot ensure that one will ever develop or be sustained.
To date there has been no trading market for our common stock. We cannot predict how liquid the market for our common stock might become. We are evaluating the possibility of having our common stock quoted for trading on the OTC Bulletin Board, or applying for listing of our common stock on either the NYSE, The NASDAQ Capital Market or other national securities exchange, assuming that we can satisfy the initial listing standards for such exchange. We currently do not satisfy the initial listing standards for such exchanges, and cannot ensure that we will be able to satisfy such listing standards or that our common stock will be accepted for listing on any such exchange. Should we fail to satisfy the initial listing standards of such exchanges, or our common stock is otherwise rejected for listing and remain listed on the OTC Bulletin Board or suspended from the OTC Bulletin Board, the trading price of our common stock could suffer, the trading market for our common stock may be less liquid and our common stock price may be subject to increased volatility.
If and when our common stock becomes publicly traded, our common stock may be deemed a “penny stock,” which would make it more difficult for our investors to sell their shares.
If and when our common stock becomes publicly traded, our common stock may be subject to the “penny stock” rules adopted under Section 15(g) of the Exchange Act. The penny stock rules generally apply to companies whose
18
Table of Contents
common stock is not listed on The NASDAQ Stock Market or other national securities exchange and trades at less than $4.00 per share, other than companies that have had average revenue of at least $6,000,000 for the last three years or that have tangible net worth of at least $5,000,000 ($2,000,000 if the company has been operating for three or more years). These rules require, among other things, that brokers who trade penny stocks to persons other than established customers complete certain documentation, make suitability inquiries of investors and provide investors with certain information concerning trading in the security, including a risk disclosure document and quote information under certain circumstances. Many brokers have decided not to trade penny stocks because of the requirements of the penny stock rules and, as a result, the number of broker-dealers willing to act as market makers in such securities is limited. If we remain subject to the penny stock rules for any significant period, it could have an adverse effect on the market, if any, for our securities. If our securities are subject to the penny stock rules, investors will find it more difficult to dispose of our securities.
Our stock price may be volatile.
The stock market in general, and the stock prices of technology-based and biotechnology / biopharmaceutical stocks in particular, have experienced volatility that often has been unrelated to the operating performance of any specific public company. The market price of our common stock is likely to be highly volatile and could fluctuate widely in price in response to various factors, many of which are beyond our control, including the following:
| • | changes in our industry; competitive pricing pressures; our ability to obtain working capital financing; additions or departures of key personnel; |
| • | limited public float in the hands of a small number of persons whose sales or lack of sales could result in positive or negative pricing pressure on the market price for our common stock; |
| • | sales of our common stock; |
| • | our ability to execute our business plan; |
| • | operating results that fall below expectations; |
| • | loss of any strategic relationship; |
| • | regulatory developments; |
| • | changes in federal or state healthcare-related laws; |
| • | economic and other external factors; |
| • | period-to-period fluctuations in our financial results; |
| • | challenges and/or invalidation of key patents; and |
| • | the inability to develop or acquire new or needed technology. |
In addition, the securities markets have from time to time experienced significant price and volume fluctuations that are unrelated to the operating performance of particular companies. These market fluctuations may also materially and adversely affect the market price of our common stock.
Offers or availability for sale of a substantial number of shares of our common stock may cause the price of our common stock to decline.
If our shareholders sell substantial amounts of our common stock in the public market, including shares issued in past private offerings, upon the effectiveness of the registration statement required to be filed, or upon the expiration of any statutory holding period, under Rule 144, or upon expiration of lock-up periods applicable to outstanding shares, or issued upon the exercise of outstanding options or warrants, it could create a circumstance commonly referred to as an overhang and in anticipation of which the market price of our common stock could fall. The existence of an overhang, whether or not sales have occurred or are occurring, also could make more difficult our ability to raise additional financing through the sale of equity or equity-related securities in the future at a time and price that we deem reasonable or appropriate.
Our undesignated preferred stock may inhibit potential acquisition bids; this may adversely affect the market price for our common stock and the voting rights of holders of our common stock.
Our certificate of incorporation provides our Board of Directors with the authority to issue up to 10,000,000 shares of undesignated preferred stock and to determine or alter the rights, preferences, privileges and restrictions granted to or imported upon these shares without further vote or action by our shareholders. As of the date of this filing, no shares of preferred stock have been designated and the Board of Directors still has authority to designate and issue
19
Table of Contents
up to 10,000,000 shares of preferred stock in one or more classes or series. The issuance of shares of preferred stock may delay or prevent a change in control transaction without further action by our shareholders. As a result, the market price of our common stock may be adversely affected. In addition, if we issue preferred stock in the future that has preference over our common stock with respect to the payment of dividends or upon our liquidation, dissolution or winding up, or if we issue preferred stock with voting rights that dilute the voting power of our common stock, the rights of holders of our common stock or the market price of our common stock could be adversely affected.
We have not paid dividends in the past and do not expect to pay dividends in the future. Any return on investment may be limited to the value of our common stock.
We have never paid cash dividends on our common stock and do not anticipate doing so in the foreseeable future. The payment of dividends on our common stock will depend on earnings, financial condition and other business and economic factors affecting us at such time as our board of directors may consider relevant. If we do not pay dividends, our common stock may be less valuable because a return on your investment will only occur if our stock price appreciates.
RISKS RELATED TO CLINICAL AND COMMERCIALIZATION ACTIVITIES
Delays in the commencement of clinical testing of our current and potential product candidates could result in increased costs to us and delay our ability to generate revenues.
The commencement of clinical trials can be delayed for a variety of reasons, including delays in:
| • | obtaining adequate financing; |
| • | demonstrating sufficient safety and efficacy to obtain regulatory clearance to commence a clinical trial; |
| • | manufacturing sufficient quantities or producing products meeting our quality standards of a product candidate; |
| • | obtaining an injection device or methodology meeting our quality standards; |
| • | obtaining clearance of an Investigational New Drug (IND) application or proposed trial design from the FDA; |
| • | reaching agreement on acceptable terms with our collaborators on all aspects of the clinical trial, including the contract research organizations (CROs) and the trial sites; and |
| • | obtaining Institutional Review Board (IRB) approval to conduct a clinical trial at a prospective site. |
In addition, clinical trials may be delayed due to insufficient patient enrollment, which is a function of many factors, including the size and nature of the patient population, the nature of the protocol, the proximity of patients to clinical sites, other competing clinical trials in the same disease indication, the availability of effective treatments for the relevant disease, and the eligibility criteria for the clinical trial. Delays in commencing clinical testing of our product candidates could prevent or delay us from obtaining approval for our potential product candidates.
We do not have experience as a company in conducting clinical trials, or in other areas required for the successful commercialization and marketing of our potential product candidates.
We have no experience as an entity in conducting either early stage or large-scale, late stage clinical trials. We cannot be certain that planned clinical trials will begin or be completed on time, if at all. Large-scale trials would require either additional financial and management resources, or reliance on third-party clinical investigators, pharmaceutical partners, CROs and/or consultants. Relying on third-party clinical investigators, pharmaceutical partners or CROs may force us to encounter delays that are outside of our control. Any such delays could have a material adverse effect on our business.
We also do not currently have marketing and distribution capabilities for our potential product candidates. Developing an internal sales and distribution capability would be an expensive and time-consuming process. We may enter into agreements with third parties that would be responsible for marketing and distribution. However, these third parties may not be capable of successfully selling any of our potential product candidates. The inability to commercialize and market our potential product candidates could materially adversely affect our business.
20
Table of Contents
Obtaining regulatory approvals to market our potential product candidates in the United States and other countries is a costly and lengthy process and we cannot predict whether or when we will be permitted to commercialize our potential product candidates.
Federal, state and local governments in the United States and governments in other countries have significant regulations in place that govern many of our activities and may prevent us from creating commercially viable products from our discoveries. The regulatory process, particularly for biopharmaceutical potential product candidates like ours, is uncertain, can take many years and requires the expenditure of substantial resources.
Our potential product candidates will require extensive preclinical and clinical testing prior to submission of any regulatory application to commence commercial sales. In particular, human pharmaceutical therapeutic potential product candidates are subject to rigorous requirements of the FDA in the United States and similar health authorities in other countries in order to demonstrate safety and efficacy. Data obtained from preclinical and clinical activities is susceptible to varying interpretations that could delay, limit or prevent regulatory agency approvals. In addition, delays or rejections may be encountered as a result of changes in regulatory agency policy during the period of product development and/or the period of review of any application for regulatory agency approval for a product candidate.
Any product candidate that we or our collaborators develop must receive all relevant regulatory agency approvals/clearances before it may be marketed in the United States or other countries. Obtaining regulatory approvals/clearances is a lengthy, expensive and uncertain process. Because certain of our potential product candidates involve the application of new technologies or are based upon a new therapeutic approach, they may be subject to substantial additional review by various government regulatory authorities, and, as a result, the process of obtaining regulatory approvals/clearances for them may proceed more slowly than for potential product candidates based upon more conventional technologies.
Delays in obtaining regulatory agency approvals/clearances could:
| • | significantly delay and harm the marketing of any product that we or our collaborators develop; |
| • | impose costly procedures upon our activities or the activities of our collaborators; |
| • | diminish any competitive advantages that we or our collaborators may attain; or |
| • | adversely affect our ability to receive royalties and generate revenues and profits. |
Even if we commit the necessary time and resources, the required regulatory agency approvals/clearances may not be obtained for any product candidates developed by us or in collaboration with us. If we obtain regulatory agency approvals/clearances for a new product, these approvals/clearances may entail limitations on the indicated uses for which it can be marketed that could limit the potential commercial use of the product.
Failure to achieve continued compliance with government regulation over approved products could delay or halt commercialization of our products.
Approved products and their manufacturers are subject to continual review, and discovery of previously unknown problems with a product or its manufacturer may result in restrictions on the product or manufacturer, including withdrawal of the product from the market. The future sale by us or our collaborators of any commercially viable product will be subject to government regulation from several standpoints, including the processes of:
| • | manufacturing; |
| • | adverse-event reporting; |
| • | advertising and promoting; |
| • | selling and marketing; |
| • | labeling; and |
| • | distribution. |
If, and to the extent that, we are unable to comply with these regulations, our ability to earn revenues will be materially and negatively impacted.
21
Table of Contents
Failure to comply with regulatory requirements can result in severe civil and criminal penalties, including but not limited to:
| • | recall or seizure of products; |
| • | injunction against the manufacture, distribution and sales and marketing of products, and |
| • | criminal prosecution. |
The imposition of any of these penalties or other commercial limitations could significantly impair our business, financial condition and results of operations.
RISKS RELATED TO PROTECTING OUR INTELLECTUAL PROPERTY
Impairment of our intellectual property rights may adversely affect the value of our technologies and product candidates and limit our ability to pursue their development.
Protection of our proprietary technology is critically important to our business. Our success will depend in part on our ability to obtain and enforce our patents and maintain trade secrets, both in the United States and in other countries. Further, our patents may be challenged, invalidated or circumvented, and our patent rights may not provide proprietary protection or competitive advantages to us. In the event that we are unsuccessful in obtaining and enforcing patents, we may not be able to further develop or commercialize our product candidates and our business would be negatively impacted.
The patent positions of pharmaceutical and biopharmaceutical companies, including ours, are highly uncertain and involve complex legal and technical questions. In particular, legal principles for biotechnology and pharmaceutical patents in the United States and in other countries are evolving, and the extent to which we will be able to obtain patent coverage to protect our technology, or enforce issued patents, is uncertain. In the United States, recent court decisions in patent cases as well as proposed legislative changes to the patent system only exacerbate this uncertainty. Furthermore, significant amendments to the regulations governing the process of obtaining patents were proposed in a new rule package by the United States Patent and Trademark Office (the Patent Office) in 2007. The proposed new rules were widely regarded as detrimental to the interests of biotechnology and pharmaceutical companies. The implementation of the rule package was blocked by a court injunction requested by a pharmaceutical company. The Patent Office challenged the court decision through an appeal to the U.S. Court of Appeals for the Federal Circuit (CAFC), but the appeal was dismissed in November 2009, after the Patent Office changed course and rescinded the proposed new rules. At this point we do not know whether the Patent Office will attempt to introduce new rules to replace those that were recently withdrawn or whether any such new rules would also be challenged.
We are uncertain of what patent coverage we will obtain for our products in Europe or Asia. At this time, we do not know whether or to what extent we will be able to obtain future patent protection for our technologies and products in Europe or Asia. If we are unable to protect our inventions related to cell therapy products in Europe or Asia, our future business opportunities could be negatively impacted.
Challenges to our patent rights can result in costly and time-consuming legal proceedings that may prevent or limit development of our product candidates.
Publication of discoveries in scientific or patent literature tends to lag behind actual discoveries by at least several months and sometimes several years. Therefore, the persons or entities that we or our licensors name as inventors in our patents and patent applications may not have been the first to file patent applications for these inventions. As a result, we may not be able to obtain patents for discoveries that we otherwise would consider patentable and that we consider to be extremely significant to our future success.
Where more than one party seeks U.S. patent protection for the same technology, the Patent Office may declare an interference proceeding in order to ascertain the party to which the patent should be issued. Patent interferences are typically complex, highly contested legal proceedings, subject to appeal. They are usually expensive and prolonged, and can cause significant delay in the issuance of patents. Moreover, parties that receive an adverse decision in an interference can lose important patent rights. Our pending patent applications, or our issued patents, may be drawn into interference proceedings which may delay or prevent the issuance of patents, or result in the loss of issued patent rights.
Outside of the United States, certain jurisdictions, such as Europe, New Zealand and Australia, permit oppositions to be filed against the granting of patents. Because our intent is to commercialize products internationally, securing
22
Table of Contents
both proprietary protection and freedom to operate outside of the United States is important to our business. We may be, in the future, involved in both opposing the grant of patents to others through such opposition proceedings and in defending our patent applications against oppositions filed by others.
European opposition and appeal proceedings can take several years to reach final decision. The potential oppositions discussed above reflect the complexity of the patent landscape in which we operate, and illustrate the risks and uncertainties.
Patent opposition proceedings are not currently available in the U.S. patent system, but legislation has been proposed to introduce them. However, issued U.S. patents can be reexamined by the Patent Office at the request of a third party. Patents owned or licensed by Q Therapeutics may therefore be subject to reexamination. As in any legal proceeding, the outcome of patent reexaminations is uncertain, and a decision adverse to our interests could result in the loss of valuable patent rights.
As more groups become engaged in scientific research and product development in the areas of stem cells, the risk of our patents being challenged through patent interferences, oppositions, reexaminations, litigation or other means will likely increase. Challenges to our patents through these procedures can be extremely expensive and time-consuming, even if the outcome is favorable to us. An adverse outcome in a patent dispute could severely harm our business by:
| • | causing us to lose patent rights in the relevant jurisdictions(s); |
| • | subjecting us to litigation, or otherwise preventing us from commercializing potential products in the relevant jurisdiction(s); |
| • | requiring us to obtain licenses to the disputed patents; |
| • | forcing us to cease using the disputed technology; or |
| • | requiring us to develop or obtain alternative technologies. |
Furthermore, if such challenges to our patent rights are not resolved promptly in our favor, our existing and future business relationships may be jeopardized and we could be delayed or prevented from entering into new collaborations or from commercializing certain products, which could materially harm our business.
If we fail to meet our obligations under license agreements, we may lose our rights to key technologies on which our business depends.
Our business depends on several critical technologies that are based in part on patents licensed from third parties. Those third-party license agreements impose obligations on us, such as payment obligations and obligations to diligently pursue development of commercial products under the licensed patents. If a licensor believes that we have failed to meet our obligations under a license agreement, the licensor could seek to limit or terminate our license rights, which could lead to costly and time-consuming litigation and, potentially, a loss of the licensed rights. During the period of any such litigation our ability to carry out the development and commercialization of potential products could be significantly and negatively affected. If our license rights were restricted or ultimately lost, our ability to continue our business based on the affected technology would be severely adversely affected.
We may be subject to litigation that will be costly to defend or pursue and uncertain in its outcome.
Our business may bring us into conflict with our licensees, licensors, or others with whom we have contractual or other business relationships, or with our competitors or others whose interests differ from ours. If we are unable to resolve those conflicts on terms that are satisfactory to all parties, we may become involved in litigation brought by or against us. That litigation is likely to be expensive and may require a significant amount of management’s time and attention, at the expense of other aspects of our business. The outcome of litigation is always uncertain, and in some cases could include judgments against us that require us to pay damages, enjoin us from certain activities, or otherwise affect our legal or contractual rights, which could have a significant adverse effect on our business.
The development, manufacturing and commercialization of cell-based therapeutic products expose us to product liability claims, which could lead to substantial liability.
By developing and, ultimately, commercializing therapeutic products, we are exposed to the risk of product liability claims. Product liability claims against us could result in substantial litigation costs and damage awards against us.
23
Table of Contents
We intend to obtain liability insurance that covers our clinical trials, and we will need to increase our insurance coverage if and when we begin commercializing products. We may not be able to obtain insurance on acceptable terms, if at all, and the policy limits on our insurance policies may be insufficient to cover our liability.
We may be subject to infringement claims that are costly to defend, and which may limit our ability to use disputed technologies and may prevent us from pursuing research and development or commercialization of potential products.
Our commercial success depends significantly on our ability to operate without infringing patents and the proprietary rights of others. Our technologies may infringe the patents or proprietary rights of others. In addition, we may become aware of discoveries and technology controlled by third parties that are advantageous to our programs. In the event our technologies infringe the rights of others or we require the use of discoveries and technology controlled by third parties, we may be prevented from pursuing research, development or commercialization of potential products or may be required to obtain licenses to those patents or other proprietary rights or develop or obtain alternative technologies. We have obtained or are negotiating licenses from universities and companies for technologies that we anticipate incorporating into our potential products, and we initiate negotiation for licenses to other technologies as the need or opportunity arises. We may not be able to obtain a license to patented technology on commercially favorable terms, or at all. If we do not obtain a necessary license, we may need to redesign our technologies or obtain rights to alternate technologies, the research and adoption of which could cause delays in product development. In cases where we are unable to license necessary technologies, we could be prevented from developing certain potential products. Our failure to obtain alternative technologies or a license to any technology that we may require to research, develop or commercialize our product candidates would significantly and negatively affect our business.
Much of the information and know-how that is critical to our business is not patentable and we may not be able to prevent others from obtaining this information and establishing competitive enterprises.
We sometimes rely on trade secrets to protect our proprietary technology, especially in circumstances in which we believe patent protection is not appropriate or available. We attempt to protect our proprietary technology in part by confidentiality agreements with our employees, consultants, collaborators and contractors. We cannot assure you that these agreements will not be breached, that we would have adequate remedies for any breach, or that our trade secrets will not otherwise become known or be independently discovered by competitors, any of which would harm our business significantly.
RISKS RELATED TO OUR RELATIONSHIPS WITH THIRD PARTIES
We depend on other parties to help us develop, manufacture and test our product candidates, and our ability to develop and commercialize potential products may be impaired or delayed if collaborations are unsuccessful.
Our strategy for the development, clinical testing and commercialization of our product candidates requires that we enter into collaborations with corporate partners, licensors, licensees and others. We are dependent upon the subsequent success of these other parties in performing their respective responsibilities and the continued cooperation of our partners. Our collaborators, contractors and licensees may not cooperate with us or perform their obligations under our agreements with them. We cannot control the amount and timing of our collaborators’ resources that will be devoted to activities related to our collaborative agreements with them. Our collaborators may choose to pursue existing or alternative technologies in preference to those being developed in collaboration with us.
Under agreements with other parties, we may rely significantly on them to, among other activities:
| • | conduct research and development activities and preclinical safety studies in conjunction with or for us; |
| • | manufacture product(s) or reagents used for such product manufacture for use by us, our contractors and our partners; |
| • | design and conduct advanced clinical trials in the event that we reach clinical trials; |
| • | fund research and development activities with us; |
| • | manage and license certain patent rights; |
| • | pay us fees upon the achievement of milestones; and |
| • | market with us any commercial products that result from our collaborations. |
24
Table of Contents
The development and commercialization of potential products will be delayed if collaborators or other partners fail to conduct these activities in a timely manner or at all. In addition, our collaborators could terminate their agreements with us and we may not receive any development or milestone payments. If we do not achieve milestones set forth in the agreements, or if our collaborators breach or terminate their collaborative agreements with us, our business may be materially harmed.
We also rely on other companies for certain reagent development, manufacturing or other technical scientific work, with respect to our cell products. We have contracts with these companies that specify the work to be done and results to be achieved, but we do not have direct control over their personnel or operations. If these companies do not perform the work that they were assigned, our ability to develop or manufacture our product candidates could be significantly harmed.
Our reliance on the activities of our consultants, research institutions, and scientific contractors, whose activities are not wholly within our control, may lead to delays in development of our product candidates.
We rely extensively upon and have relationships with scientific consultants at academic and other institutions, some of whom conduct research at our request, and other consultants who assist us in formulating our research and development and clinical strategy or other matters. These consultants are not our employees and may have commitments to, or consulting or advisory contracts with, other entities that may limit their availability to us. We have limited control over the activities of these consultants and, except as otherwise required by our collaboration and consulting agreements, can expect only limited amounts of their time to be dedicated to our activities.
In addition, we have formed research collaborations with academic and other research institutions in the U.S. These research facilities may have commitments to other commercial and noncommercial entities. We have limited control over the operations of these laboratories and can expect only limited amounts of their time to be dedicated to our research goals. If any of these third parties are unable or refuse to contribute to projects on which we need their help, our ability to generate advances in our technologies and develop our product candidates could be significantly harmed.
RISKS RELATED TO COMPETITIVE FACTORS
The loss of key personnel could slow our ability to conduct research and develop product candidates.
Our future success depends to a significant extent on the skills, experience and efforts of our executive officers and key members of our scientific staff. We face intense competition for qualified individuals from numerous pharmaceutical, biopharmaceutical and biotechnology companies, as well as academic and other research institutions. We may be unable to retain our current personnel or attract or assimilate other highly qualified management and scientific personnel in the future on acceptable terms. The loss of any or all of these individuals could harm our business and might significantly delay or prevent the achievement of research, development or business objectives.
Our product candidates are likely to be expensive to manufacture, and they may not be profitable if we are unable to significantly reduce the costs to manufacture them.
Our Q-Cells and other cell-based products could be more expensive to manufacture than other treatments currently on the market today or that will be on the market when our product is ready to be marketed. Our present manufacturing processes are conducted at a modest scale and we may be able to reduce manufacturing costs through process improvements, as well as through scale increases. If we are not able to do so, however, and, depending on the pricing of the potential product, the profit margin on our product may be significantly less than that of most drugs on the market today. If we are unable to scale our production of Q-Cells at a commercially acceptable manufacturing cost, it would impact the affordability of the therapy for patients and reduce our potential profitability.
The cell-based therapies we are developing may require large quantities of cells. We continue to develop processes to scale up production and increase yields of the cells in a cost-effective way. We may not be able to charge a high enough price for any cell therapy product we develop, even if it is safe and effective, to make a profit. If we are unable to realize significant profits from our potential product candidates, our business would be materially harmed.
25
Table of Contents
Some of our competitors may develop technologies that are superior to or more cost-effective than ours, which may impact the commercial viability of our technologies and which may significantly limit our ability to sustain operations.
The pharmaceutical and biotechnology industries are intensely competitive. Other pharmaceutical and biotechnology companies and research organizations currently engage in or have in the past engaged in efforts related to the biological mechanisms that are the focus of our programs in glial cell therapies, including the study of neural stem cells as precursors for glial cells and use of embryonic stem cells to derive glial cells. In addition, other products and therapies that could compete directly with the product candidates that we are seeking to develop and market currently exist or are being developed by pharmaceutical and biopharmaceutical companies and by academic and other research organizations.
Other companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies. Academic institutions, government agencies and other public and private research organizations may also conduct research, seek patent protection and establish collaborative arrangements for research, clinical development and marketing of products similar to ours. These companies and institutions compete with us in recruiting and retaining qualified scientific and management personnel as well as in acquiring technologies complementary to our programs.
In addition to the above factors, we expect to face competition in the following areas:
| • | product efficacy and safety; |
| • | the timing and scope of regulatory consents; |
| • | availability of resources; |
| • | reimbursement coverage; |
| • | price; and |
| • | patent position, including potentially dominant patent positions of others. |
As a result of the foregoing, our competitors may develop more effective or more affordable products, or achieve earlier patent protection or product commercialization than we do. Most significantly, competitive products may render any product candidates that we develop obsolete, which would negatively impact our business and ability to sustain operations. Although we believe that our approach holds the promise of achieving the desired safety and efficacy profiles for treatment of many neurodegenerative diseases, competitor companies may indeed demonstrate safety and efficacy of their competing products, as well as achieve a marketed product before we do.
To be successful, our product candidates must be accepted by the health care community, which can be very slow to adopt or unreceptive to new technologies and products.
Our product candidates and those developed by our collaborators, if approved for marketing, may not achieve market acceptance since hospitals, physicians, patients or the medical community in general may decide not to accept and utilize these products. The product candidates that we are attempting to develop represent substantial departures from established treatment methods and will compete with a number of conventional drugs and therapies manufactured and marketed by major pharmaceutical companies. The degree of market acceptance of any of our developed potential products will depend on a number of factors, including:
| • | our establishment and demonstration to the medical community of the clinical efficacy and safety of our product candidates; |
| • | our ability to create products that are superior to the alternatives currently on the market; |
| • | our ability to establish in the medical community the potential advantage of our treatments over alternative treatment methods, and |
| • | reimbursement policies of government and third-party payers. |
If the health care community does not accept our potential products for any of the foregoing reasons, or for any other reason, our business would be materially harmed.
Because of the uncertainty of pharmaceutical pricing, reimbursement and healthcare reform measures, we or our licensees may be unable to sell our products profitably.
26
Table of Contents
Our ability to successfully commercialize our proposed products, if developed, in the human therapeutic field depends to a significant degree on patient reimbursement of the costs of such products and related treatments. We cannot assure you that reimbursement in the U.S. or in foreign countries will be available for any products developed, or, if available, will not decrease in the future, or that reimbursement amounts will not reduce the demand for, or the price of, our products. There is considerable pressure to reduce the cost of therapeutic products. Government and other third party payors are increasingly attempting to contain health care costs by limiting both coverage and the level of reimbursement for new therapeutic products and by refusing, in some cases, to provide any coverage for uses of approved products for disease indications for which the FDA or other relevant authority has not granted marketing approval. Moreover, in some cases, government and other third party payors have refused to provide reimbursement for uses of approved products for disease indications for which the FDA or other relevant authority has granted marketing approval. Significant uncertainty exists as to the reimbursement status of newly approved health care products or novel therapies such as ours. We cannot predict what additional regulation or legislation relating to the health care industry or third-party coverage and reimbursement may be enacted in the future or what effect such regulation or legislation may have on our business. If additional regulations are overly onerous or expensive or if healthcare related legislation makes our business more expensive or burdensome than originally anticipated, we may be forced to significantly downsize our business plans or completely abandon the current business model.
Our competition has significantly greater experience and financial resources.
The biotechnology industry is characterized by intense competition. We compete against numerous companies, and many have substantially greater resources. Several of these companies have initiated cell therapy research programs and/or efforts to treat the same diseases that we target. Companies such as Neuralstem, Inc., StemCells, Inc., BioTime, Inc., and Genzyme, a Sanofi Company, as well as others, may have substantially greater resources and experience in our fields that put us at a competitive disadvantage.
| Item 1B. | Unresolved Staff Comments |
Not applicable.
| Item 2. | Properties |
Our facility in Salt Lake City, Utah is comprised of approximately 5,300 square feet of research and office space located at 615 Arapeen Drive, Suite 102, Salt Lake City, Utah, 84108. The Company leases its research and office facility under an operating lease which expires March 31, 2016. We own certain property and equipment as summarized in the accompanying financial statements.
We are not aware of any environmental issues that may affect the use of our property and equipment. We currently have no investments in real estate, real estate mortgages or real estate securities, and do not anticipate making any such investments.
| Item 3. | Legal Proceedings |
We are not currently a party to any legal proceedings nor do we have knowledge of any pending or threatened legal claims.
Item 5. Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities
Our common stock is not currently trading on any stock exchange and it is not quoted on any quotation system or traded in any other manner in the public markets. We are not aware of any market activity in our stock since inception through the date of this filing.
As of the date of this filing, there are approximately 194 record holders of 30,826,549 shares of our common stock.
27
Table of Contents
We have never paid any dividends to stockholders and presently do not intend to pay cash dividends on our common stock in the foreseeable future. We intend to retain any future earnings to fund the development and growth of our business.
Recent Sales of Unregistered Securities
None.
There is no active trading market for our common stock.
Currently, there is no active trading market for our common stock.
| Item 6. | Selected Financial Data |
N/A
| Item 7. | Management’s Discussion and Analysis of Financial Condition and Results of Operations |
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION
AND RESULTS OF OPERATIONS
The following discussion contains forward-looking statements based upon current expectations that involve risks and uncertainties, such as our plans, objectives, expectations and intentions. Our actual results and the timing of events could differ materially from those anticipated as a result of a number of factors, including those set forth under the Risk Factors, Cautionary Notice Regarding Forward-Looking Statements and Business sections in this Form 10-K. The following discussion of our financial condition and results of operations should be read with our consolidated financial statements and the related notes included elsewhere in this Form 10-K. We use words such as “anticipate,” “estimate,” “plan,” “project,” “continuing,” “ongoing,” “expect,” “believe,” “intend,” “may,” “will,” “should,” “could,” and similar expressions to identify forward-looking statements.
Company Overview
Q Therapeutics, Inc. (hereinafter Q Therapeutics, Q, we, us, our and similar expressions) is a Salt Lake City, Utah-based biopharmaceutical company that is developing human cell-based therapies that can be sold as “off-the-shelf” pharmaceuticals intended to treat neurodegenerative diseases of the brain and spinal cord, the primary components of the central nervous system or CNS.
The technology upon which these potential therapies are based was developed by Q’s co-founder Mahendra Rao, M.D., Ph.D., a leader in glial stem cell biology, during Dr. Rao’s tenure as a Professor at the University of Utah and as Head of the Stem Cell Section in the Laboratory of Neuroscience at the National Institutes of Health (NIH) Institute of Aging. Dr. Rao was one of the first scientists to identify and seek patent coverage on stem cells and their progeny cells found in the CNS.
Every year, millions of people suffer from debilitating and often fatal neurodegenerative diseases of the brain and spinal cord. Despite much effort by the pharmaceutical/biotechnology industry, current approaches to treating these diseases have not been effective. A new approach is needed for treating the multimodal nature of neurodegenerative disease.
Q has identified and patented a new treatment modality that provides a multi-factorial approach, bypassing the need to address individual pathways. Specifically, Q is developing a cell-based therapeutic that employs a natural cell type found in the healthy CNS: human glial restricted progenitors and their progeny cells. We call them, Q-Cells®. Q-Cells produce astrocytes and oligodendrocytes, the support cells that enable the normal function of neurons. We believe that Q-Cells may provide multiple and complementary mechanisms of action in the treatment of many
28
Table of Contents
neurodegenerative diseases. An advantage of this approach is that one need not fully understand the mechanistic aspects of these diseases, as we are tapping into the natural cellular machinery that enables healthy systems to function. We believe this is a more realistic approach than seeking a drug that affects a single disease pathway (although the addition of Q-Cell therapy may enhance benefits seen with such single drugs). Based on data in animal models, we believe that repairing damaged neurons is a faster, more realistic and easier approach than trying to replace them.
Initially, Q is targeting orphan diseases, where the U.S. Food and Drug Administration (FDA) can allow fast-track approvals and market exclusivity, and for which smaller, less-expensive clinical trials may be warranted. This approach may result in accelerated commercialization efforts while maintaining a financing approach focused on capital efficiency. Q is advancing its initial product candidate, trademarked “Q-Cells®” as a potential treatment for Amyotrophic Lateral Sclerosis (Lou Gehrig’s disease or ALS), and eventually other indications, potentially including Multiple Sclerosis (MS), Transverse Myelitis (TM), Spinal Cord Injury (SCI), Stroke, Huntington’s disease, Parkinson’s disease and Alzheimer’s disease.
Results of Operations for the Year Ended December 31, 2014 compared to the Year Ended December 31, 2013:
We have not generated revenues in excess of expenses and have been dependent on government grants and debt and equity raised from individual investors to sustain our operations. Our products have not yet been approved by the FDA) for commercial sale and as a result we have not yet generated revenues from product sales.
We expect to file an Investigational New Drug Application (IND) with the FDA in the first half of 2015 seeking authorization from the FDA to administer Q cells to humans in a Phase I clinical trial. If our application is authorized, we will advance towards the commencement of our first clinical trial in humans during the second half of 2015. As a result of starting our first clinical trial, we anticipate that our research and development, clinical and regulatory, and general and administrative expenses will significantly increase. We anticipate that revenues will be minimal until such time as our cell therapy is approved for commercialization.
We have incurred losses and used cash in operating activities since inception. As of December 31, 2014, the Company had an accumulated deficit of $27,232,071 and negative working capital of $322,420.
29
Table of Contents
| 2014 | 2013 | Change | % | |||||||||||||
| Grant revenues |
$ | 677,864 | $ | 14,175 | $ | 663,689 | 4682.1 | % | ||||||||
| Other revenues |
2,400 | 12,000 | (9,600 | ) | -80.0 | % | ||||||||||
|
|
|
|
|
|
|
|||||||||||
| Total operating revenues |
680,264 | 26,175 | 654,089 | 2498.9 | % | |||||||||||
| Cost of revenues |
800 | 4,800 | (4,000 | ) | -83.3 | % | ||||||||||
|
|
|
|
|
|
|
|||||||||||
| Gross profit |
679,464 | 21,375 | 658,089 | 3078.8 | % | |||||||||||
|
|
|
|
|
|
|
|||||||||||
| Operating expenses: |
||||||||||||||||
| Research and development |
1,960,815 | 1,827,533 | 133,282 | 7.3 | % | |||||||||||
| General and administrative |
2,098,851 | 1,384,712 | 714,139 | 51.6 | % | |||||||||||
|
|
|
|
|
|
|
|||||||||||
| Total operating expenses |
4,059,666 | 3,212,245 | 847,421 | 26.4 | % | |||||||||||
|
|
|
|
|
|
|
|||||||||||
| Operating loss |
(3,380,202 | ) | (3,190,870 | ) | (189,332 | ) | 5.9 | % | ||||||||
|
|
|
|
|
|
|
|||||||||||
| Other income (expense): |
||||||||||||||||
| Interest expense |
(111,022 | ) | (202,111 | ) | 91,089 | -45.1 | % | |||||||||
| Gain on derivative liability |
134,019 | — | 134,019 | — | ||||||||||||
| Other income, net |
6,504 | 3,889 | 2,615 | 67.2 | % | |||||||||||
|
|
|
|
|
|
|
|||||||||||
| Total other income (expense), net |
29,501 | (198,222 | ) | 227,723 | -114.9 | % | ||||||||||
|
|
|
|
|
|
|
|||||||||||
| Loss before provision (benefit) for income taxes |
(3,350,701 | ) | (3,389,092 | ) | 38,391 | -1.1 | % | |||||||||
| Provision (benefit) for income taxes |
— | — | — | — | ||||||||||||
|
|
|
|
|
|
|
|||||||||||
| Net loss |
$ | (3,350,701 | ) | $ | (3,389,092 | ) | $ | 38,391 | -1.1 | % | ||||||
|
|
|
|
|
|
|
|||||||||||
Grant Revenues
Grant revenues for the years ended December 31, 2014 and 2013 were $677,864 and $14,175, respectively, an increase of $663,689. The increase was primarily due to approval for the fourth and final year of an NIH grant issued to The Johns Hopkins University, of which we received a sub-award. In June 2014, we received notification for the approved budget and were awarded $677,864, all of which was billed in 2014. While we intend to apply for grants in 2015 and beyond for additional indications, there is no assurance that we will be awarded future grant opportunities.
Other Revenues
Other revenues for the year ended December 31, 2014 were $2,400 compared to $12,000 for the year ended December 31, 2013. The 2013 revenue was due to a one-time sale of non-commercial products to a collaborative research partner. We do not anticipate any other revenues in 2015.
Research and Development Expenses
We anticipate that current research and development activities and costs will remain the same until such time as additional financing is obtained. Once financing has been obtained, we anticipate a significant increase in our research and development costs as we prepare our IND submission to the FDA, and upon its authorization, commence human clinical studies. Specifically, we anticipate increases in our costs related to clinical and regulatory consulting costs, clinical start-up costs, and clinical trial and associated management costs. We may also
30
Table of Contents
increase research and development activities to evaluate use of our proprietary products in other disease indications, including working with outside collaborators. The following table details the research and development expenses we incurred for the years ended December 31, 2014 and 2013:
| 2014 | 2013 | Change | % | |||||||||||||
| Lab materials and supplies |
$ | 1,351,061 | $ | 1,279,056 | $ | 72,005 | 5.6 | % | ||||||||
| Salaries and benefits |
331,704 | 342,890 | (11,186 | ) | -3.3 | % | ||||||||||
| Consulting |
99,078 | 4,700 | 94,378 | 2008.0 | % | |||||||||||
| Facility-related expenses |
87,292 | 78,427 | 8,865 | 11.3 | % | |||||||||||
| License fees |
65,500 | 110,000 | (44,500 | ) | -40.5 | % | ||||||||||
| Travel and entertainment |
18,486 | 2,780 | 15,706 | 565.0 | % | |||||||||||
| Depreciation |
7,694 | 9,680 | (1,986 | ) | -20.5 | % | ||||||||||
|
|
|
|
|
|
|
|||||||||||
| Total research and development expenses |
$ | 1,960,815 | $ | 1,827,533 | $ | 133,282 | 7.3 | % | ||||||||
|
|
|
|
|
|
|
|||||||||||
Research and development expenses for the year ended December 31, 2014 were $1,960,815, an increase of $133,282, or 7.3%, from $1,827,533 for the year ended December 31, 2013. The increase in expense is primarily related to the preparation of our IND application for submission to the FDA, which required additional animal safety studies and the use of outside regulatory and clinical consultants, offset by a reduction in annual license fees. We anticipate research and development expenses to remain the same until such time as we commence our clinical studies, at which time we anticipate our research and development expenses to significantly increase.
General and Administrative Expenses
The following table details the general and administrative expenses incurred by the Company for the years ended 2014 and 2013:
| 2014 | 2013 | Change | % | |||||||||||||
| Salaries and benefits |
$ | 597,269 | $ | 594,033 | $ | 3,236 | 0.5 | % | ||||||||
| Stock-based compensation |
829,959 | 101,753 | 728,206 | 715.7 | % | |||||||||||
| Legal and professional fees |
421,559 | 473,923 | (52,364 | ) | -11.0 | % | ||||||||||
| General office and administrative |
132,266 | 100,211 | 32,055 | 32.0 | % | |||||||||||
| Facility-related expenses |
93,888 | 82,129 | 11,759 | 14.3 | % | |||||||||||
| Travel and entertainment |
21,939 | 30,496 | (8,557 | ) | -28.1 | % | ||||||||||
| Depreciation |
1,971 | 2,167 | (196 | ) | -9.0 | % | ||||||||||
|
|
|
|
|
|
|
|||||||||||
| Total expenses |
$ | 2,098,851 | $ | 1,384,712 | $ | 714,139 | 51.6 | % | ||||||||
|
|
|
|
|
|
|
|||||||||||
General and administrative expenses for the year ended December 31, 2014 was $2,098,851, an increase of $714,139 or 51.6%, from $1,384,712 for the year ended December 31, 2013. The increase in 2014 expense is primarily due to stock-based compensation expense resulting from annual director, officer, and employee option grants, new hire grants to our Chief Strategy Officer in lieu of cash compensation, as well as one-time grants to our officers equivalent to the amount of their deferred salaries in 2014. No stock options were granted during 2013. This increase was offset by a reduction in legal and professional fees. We anticipate general and administrative expenses to remain at approximately the same levels in 2015.
Liquidity and Capital Resources
For the year ended December 31, 2014, net cash used in operating activities totaled $1,458,653 compared to $877,873 for the year ended December 31, 2013. Cash expenditures increased in 2014 primarily due to the costs associated with the completion of animal safety studies and consulting costs related to the preparation of our IND submission to the FDA.
For the year ended December 31, 2014, net cash used in investing activities was $8,234 compared to $23,802 for the year ended December 31, 2013.
For the year ended December 31, 2014, net cash provided by financing activities was $2,031,366 compared to $250,000 for the year ended December 31, 2013.
31
Table of Contents
As of December 31, 2014, we had negative working capital of $322,420 and a stockholders’ deficit of $295,852. It is anticipated that we will continue to generate operating losses and use cash in operating activities through 2015.
Between January 26, 2015 and February 23, 2015, 327,455 warrants and 21,634 options were exercised for cash proceeds of $114,131.
In order for us to continue to meet our operational and liquidity needs, we will need to obtain additional funding. Additional funding may not be available to us on acceptable terms, or at all. Any additional equity financing, if available, may not be available on favorable terms and may be dilutive to current stockholders; and debt financing, if available, may involve more restrictive covenants that may potentially impair our ability to operate the company. Our ability to access capital when needed is not assured and, if not achieved on a timely basis, will materially harm our business, financial position and results of operations. These uncertainties create substantial doubt about our ability to continue as a going concern. No adjustment has been made to our financial statements as a result of this uncertainty. The Report of Independent Registered Public Accounting Firm included in this Annual Report on Form 10-K contains a going concern explanatory paragraph.
Recent Accounting Pronouncements
In May 2014, the Financial Accounting Standards Board (FASB) issued ASU 2014-09, Revenue from Contracts with Customers. ASU 2014-09 provides for a single, principles-based model for revenue recognition that replaces existing revenue recognition guidance. ASU 2014-09 is effective for annual and interim periods beginning on or after December 15, 2016. It permits the use of either a retrospective or cumulative effect transition method and early adoption is not permitted. We have not yet determined the impact this standard will have on our consolidated financial statements and related disclosures.
In June 2014, the FASB issued ASU 2014-10, Topic 915, Development Stage Entities, Elimination of Certain Financial Reporting Requirements. ASU 2014-10 removes all incremental financial reporting requirements for development stage entities, including but not limited to, inception-to-date financial information included in the statements of operations, statements of stockholders’ deficit and statements of cash flows. The Company elected early adoption of ASU 2014-10 beginning with the reporting period ended June 30, 2014. As a result of our early adoption, all references to the Company as a development stage entity have been removed. The adoption of this pronouncement has no impact on the Company’s financial position, results of operations or liquidity.
In August 2014, the FASB issued ASU 2014-15, Presentation of Financial Statements – Going Concern (Subtopic 310-40): Disclosure of Uncertainties about an Entity’s Ability to Continue as a Going Concern. ASU 2014-15 provides guidance in US GAAP about management’s responsibility to evaluate whether there is substantial doubt about an entity’s ability to continue as a going concern and to provide related disclosures. In doing so, this is intended to reduce diversity in the timing and content of disclosures. This is effective for the annual period ending after December 15, 2016, and for annual periods and interim periods thereafter. Early application is permitted. We are currently evaluating the potential impact of adopting this guidance on our consolidated financial statements and related disclosures.
Off-Balance Sheet Arrangements
We do not have any off-balance sheet arrangements, financings, or other relationships with unconsolidated entities or other persons, also known as “special purpose entities” (SPEs).
Significant Accounting Policies
Our discussion and analysis of financial condition and results of operations is based upon the consolidated financial statements included herein, which have been prepared in accordance with U.S. generally accepted accounting principles and the requirements and regulations of the Securities and Exchange Commission (SEC). The preparation of these financial statements requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities as of the dates of the financial statements and the reported amounts of revenues and expenses during the reporting periods. Significant accounting policies and areas where substantial judgments are made by management include:
Stock-Based Compensation – We calculate the estimated fair value of our stock options and warrants on the grant
32
Table of Contents
date using the Black-Scholes option-pricing model and recognize the estimated fair value as compensation expense on a straight-line basis over the vesting period. We recognize stock compensation expense in the period in which the employee is required to provide service, which is generally over the vesting period of the individual equity instruments. Stock options issued in lieu of cash to non-employees for services performed are recorded at the fair value of the options at the time they are issued and are expensed as service is provided.
The volatility assumption used in the Black-Scholes option-pricing model is based on the volatility of similar publicly traded companies in our industry. The expected term of the options and warrants granted represent the similar periods of time that the options granted are expected to be outstanding. The risk free rate for periods within the contractual lives of the options and warrants is based on the U.S. treasury securities constant maturity rate that corresponds to the expected term in effect at the time of grant.
Revenue Recognition and Grants Receivable – We apply for research grants generally as a sub-recipient to grants funded by government agencies through research institutions. Grant revenues are recognized as associated expenses are incurred and are billed in conjunction with the terms of the grants. Grants receivable are recorded in accordance with the provisions defined in the sub-award or grant agreement. Grants receivable are considered past due when payment has not been received within 30 days of the invoice date, although certain institutions do not pay within these terms. The amounts of the specific allowances are estimated by management based on various assumptions including the age of the individual grant receivable, as well as changes in payment schedules and histories. Receivable balances are charged off against the allowance for doubtful accounts when management determines the probablity for recovery is remote. Recoveries of receivables previously charged off are recorded when payment is received. We did not incur any losses relating to bad debts during 2014 and 2013.
Income Taxes – We record federal and state income taxes using the asset and liability method. Under the asset and liability method, deferred income tax assets or liabilities are recognized for future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases. Deferred income tax assets or liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred income tax assets and liabilities of a change in tax rates is recognized in operations in the period that includes the enactment date.
Net Loss Per Common Share – Basic net income (loss) per share (Basic EPS) is calculated by dividing income (loss) available to common stockholders by the weighted-average number of common shares outstanding during the period.
Diluted net income or loss per common share (Diluted EPS) is computed by dividing net income or loss by the sum of the weighted average number of common shares outstanding and the dilutive potential common share equivalents then outstanding. Potential dilutive common share equivalents (options and warrants) consist of shares issuable upon the exercise of outstanding stock options and warrants to acquire common stock. Shares having an antidilutive effect on periods presented are not included in the computation of Diluted EPS.
| Item 7A. | Quantitative and Qualitative Disclosures about Market Risk |
N/A
| Item 8. | Financial Statements and Supplementary Data |
The financial statements required to be filed pursuant to this Item are included in this Annual Report on Form 10-K beginning on page F-1.
| Item 9. | Changes in and Disagreements with Accountants on Accounting and Financial Disclosure |
None.
33
Table of Contents
| Item 9A. | Controls and Procedures |
Disclosure Controls and Procedures
Evaluation of disclosure controls and procedures
As of December 31, 2014, under the supervision and with the participation of management, including our Chief Executive Officer and Chief Financial Officer (the “Certifying Officers”), we conducted an evaluation of our Company’s disclosure controls and procedures. As defined in Rules 13a-15(e) and 15d-15(e) of the Exchange Act, the term “disclosure controls and procedures” means our controls and other procedures that are designed to ensure that information required to be disclosed in reports that we file or submit under the Securities Exchange Act of 1934 (the “Exchange Act”) is recorded, processed, summarized and reported, within the time periods specified in the SEC’s rules and forms. Disclosure controls and procedures include, without limitation, controls and procedures designed to ensure that information required to be disclosed in the reports we file or submit under the Exchange Act is accumulated and communicated to our Company’s management, including the Certifying Officers, to allow timely decisions regarding required disclosure. Based on this evaluation, the Certifying Officers have concluded that our Company’s disclosure controls and procedures were effective as of December 31, 2014.
Management’s Report on Internal Control over Financial Reporting
The management of the Company is responsible for establishing and maintaining adequate internal control over financial reporting, as such term is defined in Rule 13a-15(f) under the Exchange Act. The Company’s internal control over financial reporting is a process designed under the supervision of the Company’s Chief Executive Officer and Chief Financial Officer to provide reasonable assurance regarding the reliability of financial reporting and the preparation of the Company’s financial statements for external purposes in accordance with U.S. generally accepted accounting principles (US GAAP) and includes those policies and procedures that:
| • | Pertain to the maintenance of records that in reasonable detail accurately and fairly reflect the transactions and dispositions of our assets; |
| • | Provide reasonable assurance that the transactions are recorded as necessary to permit the preparation of financial statements in accordance with US GAAP, and that our receipts and expenditures are being made only in accordance with authorizations of our management and directors; and |
| • | Provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of our assets that could have a material effect on financial statements. |
Management recognizes that there are inherent limitations in the effectiveness of any system of internal control. Accordingly, even effective internal control can provide only reasonable assurance with respect to financial statement preparation and may not prevent or detect misstatements. In addition, effective internal control at a point in time may become ineffective in future periods because of changes in conditions or due to deterioration in the degree of compliance with our established policies and procedures.
Effective as of December 31, 2014, Q Therapeutics, Inc.’s management assessed the effectiveness of the Company’s internal control over financial reporting based on criteria for effective internal control over financial reporting established in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (“COSO”) and SEC guidance on conducting such assessments.
Based on that evaluation, management concluded that, during the period covered by this report, such internal controls and procedures were sufficient to detect the inappropriate application of US GAAP as more fully described below.
A material weakness is a deficiency, or combination of deficiencies, that results in more than a remote likelihood that a material misstatement of annual or interim financial statements will not be prevented or detected.
A significant deficiency is a deficiency, or a combination of deficiencies, in internal control that is less severe than a material weakness, yet important enough to merit attention by those charged with governance.
In connection with the assessment described above, management identified the following significant deficiencies in internal control as of December 31, 2014:
| • | Lack of an established audit committee comprised of outside directors who are independent of management; and |
34
Table of Contents
| • | Lack of sufficient resources to establish and operate an internal audit function. |
| • | Ineffective controls over segregation of access to the accounting information system and records. |
Management believes that the significant deficiencies set forth above do not have an adverse effect on the Company’s financial reporting for the years ended December 31, 2014 and 2013. Management is committed to improving the Company’s financial control capability. To the extent reasonably possible given our limited resources, we intend to address these weaknesses through measures that include, but are not limited to:
| • | Appoint one or more outside directors to our board of directors who are qualified and willing to perform the audit oversight function and establish a fully functioning audit committee. |
| • | Continue to evaluate the feasibility of establishing an internal audit function. |
| • | We will continue to monitor and evaluate the effectiveness of our internal control, processes and procedures over financial reporting on an ongoing basis and are committed to taking further actions and implementing additional enhancements or improvements, as necessary and as resources allow. |
Changes in Controls and Procedures
There were no significant changes made in our internal controls over financial reporting during the year ended December 31, 2014 that have materially affected or are reasonably likely to materially affect these controls.
Attestation Report of the Independent Registered Public Accounting Firm
This Form 10-K Report does not include an attestation report of our independent registered public accounting firm regarding internal control over financial reporting. Management’s report was not subject to attestation by our independent registered public accounting firm pursuant to rules of the SEC that permit us to provide only management’s report in this Form 10-K. Pursuant to the rules of the SEC, we were not required to have, nor have we, engaged our independent registered public accounting firm to perform an audit of internal control over financial reporting.
| Item 9B. | Other Information |
None.
35
Table of Contents
| Item 10. | Directors, Executive Officers and Corporate Governance |
Directors and Executive Officers
Our current directors and executive officers and their respective ages and positions are as follows:
| Name |
Age | Directors and Officers | ||
| Deborah A. Eppstein, Ph.D. |
66 | President, Chief Executive Officer and Director (Principal Executive Officer) | ||
| Steven J. Borst, M.B.A. |
57 | Chief Financial Officer and Vice President Corporate Development (Principal Financial and Accounting Officer) | ||
| Mahendra Rao, Ph.D. |
53 | Chief Strategy Officer and Chairman of the Scientific Advisory Board | ||
| Dinesh C. Patel, Ph.D. |
64 | Chairman of the Board of Directors | ||
| Peter Barton Hutt |
80 | Director | ||
| Linda F. Powers |
59 | Director | ||
| Peter Grebow, Ph.D. |
68 | Director | ||
| Diane Jorkasky, M.D. |
63 | Director | ||
| Hunter Jackson, Ph.D. |
65 | Director |
The following is a brief summary of the background of each of our current directors and executive officers.
Deborah A. Eppstein, Ph.D., has served as our President and Chief Executive Officer and member of our Board of Directors since 2006. Prior to joining Q Therapeutics, Ms. Eppstein held senior executive roles at various biopharmaceutical companies including; co-founder and President of Altea Therapeutics, Inc., a privately held clinical-stage pharmaceutical company focused on the development of transdermal delivery technology of biological drugs, and, Vice President of Corporate Development at TheraTech, Inc., a research and development company that specialized in development of technologies used to administer controlled, time-release medicines, which was acquired by Watson Pharmaceuticals Inc. in 1998 (now part of Activis). Dr. Eppstein received a B.A. from Grinnell College, received a Ph.D. in biochemistry from the University of Arkansas, and conducted research in virology and cell biology as an NIH postdoctoral fellow at the University of California, Santa Barbara. Dr. Eppstein has received the Women’s Technology Leadership and Businesswoman of the Year awards in Salt Lake City.
Steven J. Borst, M.B.A., has served as our Chief Financial Officer and Vice President of Corporate Development since October 2011. From 2002 to October 2011, Mr. Borst served as our Vice President, Finance and Corporate Development. Mr. Borst’s business experience with the Company over the last nine years encompasses seeking investors and concluding financings, working with corporate partners and managing facilities and operations. Mr. Borst also serves concurrently as one of three Managing Directors of UpStart Ventures Management, which manages a seed stage, life science focused venture capital fund in Salt Lake City. He possesses both an operational and venture capital background in healthcare and the life sciences. He has co-founded five Salt Lake biotech companies. Mr. Borst was previously associated with two Chicago-based venture funds and served in senior management positions with two venture-backed healthcare portfolio companies. Mr. Borst holds a B.S. degree in Industrial Engineering from the University of Michigan and an M.B.A. from the J.L. Kellogg Graduate School of Management at Northwestern University.
Mahendra S. Rao, M.D., Ph.D. is a scientific co-founder of the Company and has served as our Chief Strategy Officer and Chairman of the Scientific Advisory Board since April 2014. From March 2014 to the present, Dr. Rao has been engaged as a consultant with his own consulting firm Mahendra Rao, LLC. From August 2011 to March 2014, Dr. Rao served as Director - NIH Center for Regenerative Medicine at the National Institutes of Health where he was the founding director of the Center for Regenerative Medicine. Prior to joining the NIH, from January 2006 to July 2011, Dr. Rao served as Vice President Regenerative Medicine for Life Technologies Corporation. Dr. Rao is internationally known for his research involving human embryonic stem cells (hESCs) and other somatic stem cells and has worked in the stem cell field for more than 20 years. Dr. Rao has an extensive background teaching medical and graduate students, as well as postdoctoral fellows at institutions including The Johns Hopkins
36
Table of Contents
University School of Medicine, The National Centre for Biological Sciences in Bangalore, India, and the University of Utah School of Medicine. Dr. Rao has published more than 300 papers on stem cell research. Dr. Rao has also served on the Board of Directors for Cesca Therapeutics and Aastrom, companies which are involved in stem cell processing and therapy. Dr. Rao received his M.D. (MBBS) from Bombay University in India and his Ph.D. in Developmental Neurobiology from the California Institute of Technology.
Board of Directors
In addition to Dr. Eppstein, the Q Therapeutics’ Board of Directors is composed of:
Dinesh C. Patel, Ph.D., has served as Chairman of the Company’s Board of Directors since April 2004. From 2000 to present, Dr. Patel has served as a Managing Director and Founding Partner of vSpring Capital (which is now SignalPeak), an early-stage venture capital fund with over $400 million under management. From 1985-1999, Dr. Patel served as co-founder, Chairman, President and CEO of TheraTech, Inc., a Salt Lake City, Utah-based company that has been a pioneer in the development and manufacturing of innovative drug delivery products.
The Company believes that the demonstrated leadership, excellent academic credentials, numerous awards and proven track record with both TheraTech and vSpring Capital, particularly his vast experience as it specifically relates to development and manufacturing of innovative drug delivery products, renders Dr. Patel an asset to our Company and an exemplary member and a qualified Chairman of our Board of Directors.
Peter Barton Hutt, has served on our Board of Directors since July 2002. Mr. Hutt has been a partner of the Washington, D.C.-based law firm of Covington & Burling, specializing in food and drug law, since 1968, except for the period from 1971 to 1975 when he served as Chief Counsel of the FDA. He received a B.A., magna cum laude, from Yale University, a L.L.B. from Harvard Law School and a L.L.M. from New York University. Mr. Hutt has served on the boards of several publicly traded biotechnology companies and is a member of the Institute of Medicine, National Academy of Sciences. Since 1994, he has taught a full course on Food and Drug Law during Winter Term at Harvard Law School. Mr. Hutt has received numerous honors, including being named by the National Law Journal as one of the 40 best health care lawyers in the United States.
The Company believes that Mr. Hutt’s 40-plus year stellar legal and professional record, excellent academic credentials, numerous prior board memberships and experience for publicly traded biotech companies, and particularly his vast experience as it specifically relates to the biotech and medical fields in both a legal and business capacity, render Mr. Hutt an asset to our Company and a qualified member of our Board of Directors.
Linda F. Powers, has served on our Board of Directors since April 2004. Ms. Powers presently serves as CEO of Northwest Biotherapeutics, a portfolio company of Toucan Capital. Ms. Powers is also concurrently serving as a Managing Director and co-founder of Toucan Capital LLC, a Bethesda, MD venture fund formed in 2001 that invested in Q Products’ Series A-2 round. Ms. Powers has more than 10 years of experience in seed and early-stage venture investing, and more than 18 years of experience in corporate finance and restructuring, mergers and acquisitions, joint ventures and intellectual property licensing. Ms. Powers holds an A.B. degree in economics from Princeton University, magna cum laude and Phi Beta Kappa, as well as a J.D. degree, magna cum laude, from Harvard Law School.
The Company believes that the combination of Ms. Powers’ distinguished academic credentials, substantial biotech venture fund management experience, and particularly her vast knowledge and business dealings as they specifically relate to the biotherapeutics and research and development fields in both a legal and business capacity, render Ms. Powers an asset to our Company and a qualified member of our Board of Directors.
Peter Grebow Ph.D., has served as a director of the Company since December of 2011. Dr. Grebow held several key senior management positions at Cephalon Inc., a biopharmaceutical company before retiring. Dr. Grebow joined Cephalon in January 1991, where he had served in several positions including Senior Vice President, Worldwide Business Development Senior Vice President, Drug Development, Executive Vice President of Technical Operations, and most recently Executive Vice President of Cephalon Ventures. Prior to joining Cephalon, Dr. Grebow served as the Vice President, Drug Development for Rorer Central Research, a division of Rhone-Poulenc Rorer Pharmaceuticals Inc., from 1988 to 1990. Dr. Grebow is President and founder of P.E. Grebow Consulting, Inc. which he formed in 2011. He also serves as Executive Vice President of Research and Development at Eagle Pharmaceuticals, Inc. since October 2013. In addition, Dr. Grebow also served as a director for Optimer
37
Table of Contents
Pharmaceuticals, Inc. from February 2009 until October 2013 and has served as a director for GenSpera, Inc. since May 2012. Dr. Grebow received his undergraduate degree from Cornell University, a Masters of Science in Chemistry from Rutgers University and a Ph.D. in Physical Biochemistry from the University of California, Santa Barbara.
Dr. Grebow’s demonstrated leadership in his field, his knowledge of scientific matters affecting our business and his understanding of our industry render Dr. Grebow an asset to our Company and a qualified member of our Board of Directors.
Diane Jorkasky M.D., joined our Board of Directors in March 2013. Since June 2014 Dr. Jorkasky has served as Executive Vice President, Chief Medical Officer and Head of Development at Complexa Inc., a clinical-stage platform anti-inflammatory and fibrotic disease-focused biopharmaceutical company. From February 2012 to May 2014 and from January 2011 to August 2011, Dr. Jorkasky served as President of Diane K. Jorkasky Consulting. From August 2011 to February 2012 she served as Senior Vice President, Development and Chief Medical Officer for Endo Pharmaceuticals, Inc. From September 2009 to January 2011, Dr. Jorkasky served as Senior Vice President, Chief Development and Medical Officer for Aileron Therapeutics, Inc., a clinical stage biopharmaceutical company developing therapeutics based on its proprietary Stapled Peptide drug platform. Dr. Jorkasky has also served in various positions with Pfizer, Inc. and Smithkline Beecham (now GlaxoSmithkline) including as; Vice President of Development and Head of Worldwide Clinical Research Operations, Leader of the Operational Excellence Board for Development, and Vice President and Director of Clinical Pharmacology for North America. Dr. Jorkasky earned her M.D. from the University of Pennsylvania School of Medicine.
Because Dr. Jorkasky is a 25-year veteran of the pharmaceutical industry with experience in Phase 1-4 clinical trials including protocol development, conduct and reporting, site placement strategy, and regulatory interaction, the Company believes she is qualified to serve on its Board of Directors.
Hunter Jackson, Ph.D., has been a member of our Board of Directors since September 2014. Since April 2014, Dr. Jackson has served as Executive Chairman of Navigen Pharmaceuticals, Inc., a company he co-founded and led as President and Chief Executive Officer from 2006 until his appointment as Executive Chairman. From 1986 through 2006, Dr. Jackson was Chairman and Chief Executive Officer of NPS Pharmaceuticals, Inc., a company he co-founded to pioneer and deliver therapies that transform the lives of patients with rare diseases. During his tenure at NPS, two products were advanced through clinical research and development: Sensipar® for the treatment of secondary hyperparathyroidism and hypercalcemia, marketed by Amgen, Inc. (U.S. and Europe) and by Kyowa Hakko Kirin (Asia), which exceeded $1 billion in sales in 2013; and Preotact®, for treatment of osteoporosis marketed by Nycomed, Ltd. (Europe) in 2006, then repurchased by NPS in 2013. Dr. Jackson currently serves on the boards of Neuroadjuvants, Inc. and Fluorinov, Inc. Dr. Jackson was a member of the Governing Authority of the Utah Science, Technology, and Research Economic Development Initiative (USTAR) from 2006-2012 and received the 1998 Ernst & Young Utah Entrepreneur of the Year Award and the 2002 Utah Governor’s Medal for Science and Technology. Dr. Jackson has a Bachelor of Arts in American Literature from the University of Illinois, a Ph.D. in Psychobiology from Yale University, and completed postdoctoral work in the Neurosurgery Department at the University of Virginia Medical Center.
The Company believes that Dr. Jackson is qualified to serve on its Board of Directors because of his extensive record of achievement and his over 30 years’ experience in the life sciences industry
Section 16(A) Beneficial Ownership Reporting Compliance
Section 16(a) of the Securities Exchange Act of 1934, as amended, requires our executive officers and directors, and persons who beneficially own more than 10% of a registered class of our equity securities to file with the Securities and Exchange Commission initial statements of beneficial ownership, reports of changes in ownership and annual reports concerning their ownership of our common shares and other equity securities, on Forms 3, 4 and 5, respectively. Executive officers, directors and greater than 10% stockholders are required by the Securities and Exchange Commission regulations to furnish us with copies of all Section 16(a) reports they file. Based on our review of the copies of such forms received by us, and to the best of our knowledge, all executive officers, directors other than Mahendra Rao and Hunter Jackson, and greater than 10% stockholders filed the required reports in a timely manner.
38
Table of Contents
CORPORATE GOVERNANCE
During the year ended December 31, 2014, our Board of Directors held nine meetings. During the year ended December 31, 2014, all but one director, Linda Powers, attended at least 75% of the total meetings of our Board of Directors. On September 16, 2014, Dr. Hunter Jackson was appointed as a director of the Company. The independent directors met in executive sessions at the end of scheduled Board meetings.
Communications with the Board
Stockholders and other interested parties may communicate with one or more directors or the non-management directors as a group in writing by regular mail. The following address may be used by those who wish to send such communications by regular mail:
Board of Directors or Name of Individual Director(s) c/o Corporate Secretary
Q Therapeutics, Inc.
615 Arapeen Drive, Suite 102
Salt Lake City, UT 84108
Code of Ethics and Conduct
We have adopted a corporate Code of Ethics and Conduct (Code) for our directors, officers, employees, and agents. We require that all of our directors, officers, employees and consultants acknowledge and agree to comply to the Code. The Code of Ethics and Conduct is available on the Company’s website at www.qthera.com or upon written request to Q Therapeutics, Inc. 615 Arapeen Dr. Suite 102, Salt Lake City, Utah 84108, Attention: Investor Relations. All officers, directors and employees are subject to the Code of Ethics and Conduct. We have implemented a whistleblower policy which provides a means by which any conduct that is not compliant with the Code of Ethics and Conduct can be reported anonymously and confidentially.
| Item 11. | Executive Compensation |
Executive Compensation.
The following discussion relates to the compensation of our “named executive officers,” including our Chief Executive Officer and President, Deborah A. Epstein, Ph.D., and our two most highly compensated executive officers (other than our chief executive officer), Steven J. Borst, our Chief Financial Officer and Vice President of Corporate Development and Mahendra S. Rao, our Chief Strategy Officer and Chairman of our Scientific Advisory Board.
Summary Compensation Table
The following table sets forth information about certain compensation awarded or paid to our named executive officers for the 2013 and 2014 fiscal years.
39
Table of Contents
SUMMARY COMPENSATION TABLE
| Name |
Title |
Fiscal Year |
Salary ($) (1) |
Bonus ($) (2) |
Option Awards ($) (3) |
Other Comp. ($) (4) |
Total ($) | |||||||||||||||||||
| Deborah A. Eppstein, Ph.D. |
President and CEO |
2014 | 57,396 | 90,000 | 229,511 | 225 | 377,132 | |||||||||||||||||||
| 2013 | 116,510 | 177,500 | 30,902 | 225 | 325,137 | |||||||||||||||||||||
| Steven J. Borst |
CFO and VP of |
2014 | 56,500 | 51,500 | 249,239 | 225 | 357,464 | |||||||||||||||||||
| Corporate Development |
2013 | 99,064 | 110,000 | 30,902 | 225 | 240,191 | ||||||||||||||||||||
| Mahendra S. Rao (5) |
Chief Strategy Officer |
2014 | — | 51,500 | 146,437 | 9,900 | 207,837 | |||||||||||||||||||
| 2013 | — | — | — | — | — | |||||||||||||||||||||
| (1) | The amount shown represents the amount of salaries actually paid in each of 2013 and 2014. Starting in March 2013, Ms. Eppstein and Mr. Borst agreed to defer part, if not all, of their salaries until additional funding was obtained. As of December 31, 2014, Ms. Eppstein and Mr. Borst had accrued wages of $370,625 and $286,116, respectively. |
| (2) | The amounts shown represent the amount of bonus awards approved by the Board for each of the named executive officers. However, while approved, no bonuses will be paid until certain financing objectives have been met. |
| (3) | Represents the aggregate grant date fair value of options expensed, including the impact of estimated forfeitures, computed in accordance with FASB ASC Topic 718. These amounts represent the fair value of the awards on the grant date and do not correspond to the actual income that may be recognized by the officers. Ms. Eppstein and Mr. Borst received multiple grants of options in 2014. Each received annual grants which vest monthly for up to 46 months. In recognition for their willingness to defer their salaries until additional financing has been obtain, Ms. Eppstein received options of 421,294 with a grant date fair value of $131,571 and Mr. Borst received options of 315,602 with a grant date value of $98,552, both of which were considered immediately vested. Additionally, Mr. Borst received options with a grant date fair value of $76,613. The options vested equally over twelve months. |
| (4) | Represents Group Term Life insurance premiums paid. |
| (5) | Mr. Rao’s employment commenced April 1, 2014, and thus he did not receive any compensation in 2013. Mr. Rao was granted 600,000 options upon his hire, which vest equally over 48 months. Mr. Rao also received 400,000 options as salary compensation in lieu of cash. These options vest equally over twelve months. Mr. Rao also received consulting income equivalent to $9,900 in 2014. |
NARRATIVE DISCLOSURES TO SUMMARY COMPENSATION TABLE
Base Salaries. The base salaries for our named executive officers were determined by our compensation committee after reviewing a number of factors, including:
| • | the responsibilities associated with the position held by each of our executive officers and where that position fits within our overall corporate structure; |
| • | the seniority of the individual executive’s position; |
| • | the base salary level of each executive officer in prior years; |
| • | our overall financial position; and |
| • | for executive officers other than our Chief Executive Officer, recommendations made by our Chief Executive Officer. |
Annual Cash Bonuses. We have historically awarded discretionary cash bonuses to our executive officers. These bonuses are intended to reward our executive officers for the achievement of key strategic and business outcomes. Accordingly, bonuses in the amount of $90,000 for Ms. Eppstein and $60,000 for Mr. Borst were approved for both 2013 and 2014. Actual payment of approved bonus amounts is contingent on obtaining additional financing and therefore these bonuses have not yet been paid as of the date of this Annual Report on Form 10-K.
Long-term incentives. All options granted to our executive officers have been granted under the 2002 Stock Option Plan or the Amended and Restated Q Therapeutics 2011 Equity Incentive Compensation Plan (2011 Plan). It is our policy to award stock options at the fair market value of our common stock on the business day prior to the date of the grant in conjunction with the terms of the 2011Plan. The grant date is deemed to be the date on which the Board
40
Table of Contents
of Directors approves of the stock option grant. These options vest over a period of time, generally no more than four years. Our Compensation Committee, with the approval of our Board of Directors, may grant to our named executive officers under the 2011 Plan, incentive stock options, non-qualified stock options, restricted and unrestricted stock awards, or stock-based awards, including RSUs and other stock-based awards.
In December 2014, in an effort to increase employee retention and morale, the Company offered its employees the opportunity to have certain outstanding options issued in 2012 modified by reducing the grant exercise price of $1.00 to $.70, which was the fair market value of the common stock as of the modification date. No other terms of the grant were modified. As a result, the exercise price relating to 890,000 options to purchase common stock were modified and the incremental expense of $57,761 resulting from the modification is being amortized through December 31, 2015. Specifically, each of Ms. Eppstein and Mr. Borst had 250,000 options repriced from $1.00 to $.70. As of December 31, 2014, no options have been exercised under the 2011 Plan.
2013 Compensation
Our Board of Directors approved a salary of $257,500 for Ms. Eppstein and $206,000 for Mr. Borst, effective as of January 1, 2013. Mr. Rao was not employed by us at this time and as such received no compensation from us in 2013. In an effort to conserve cash, Ms. Eppstein and Mr. Borst agreed to defer part, if not all, of their compensation until such time that additional financing was obtained.
No equity grants or awards were issued to the executives in 2013.
2014 Compensation
Our Board of Directors made no change in salaries to our executives in 2014. Ms. Eppstein and Mr. Borst continued to receive limited salary payments in 2014.
On March 11, 2014, our Board of Directors approved option grants to our named executive officers in the aggregate of 1,462,596 shares, with an exercise price of $.70 per share, with a ten-year term, as detailed below:
| • | Ms. Eppstein was awarded (1) 250,000 options with 29.16% vested upon grant, with the balance vesting in equal increments monthly over the next 34 months; (2) 135,594 options which vested immediately upon grant, and (3) 275,000 options with 8.33% vested upon grant, with the balance vesting in equal increments monthly over the next 46 months. |
| • | Mr. Borst was awarded (1) 200,000 options with 29.16% vested upon grant, with the balance vesting in equal increments monthly over the next 34 months; (2) 102,002 options which vested immediately upon grant, (3) 250,000 options vesting in equal increments monthly over the next 12 months, (4) 250,000 options with 8.33% vested upon grant, with the balance vesting in equal increments over the next 46 months. |
| • | Mr. Rao was not yet employed by the Company. |
On April 1, 2014, we employed Mahendra Rao as our Chief Strategy Officer and Chairman of our Scientific Advisory Board. Mr. Rao received an initial grant of 600,000 options, vesting in equal increments monthly over 48 months. In lieu of base salary, Mr. Rao received an additional grant of 400,000 options, vesting in equal increments monthly over twelve months. Both issuances had an exercise price of $.70 per share.
On December 18, 2014, our Board of Directors approved option grants to our named executive officers in the aggregate of 1,299,300 shares, with an exercise price of $.70 per share, with a ten-year term, as detailed below:
| • | Ms. Eppstein was awarded (1) 300,000 options to vest in equal increments monthly over 48 months beginning January 1, 2015 and (2) 285,700 options which vested immediately upon grant. |
| • | Mr. Borst was awarded (1) 250,000 options to vest in equal increments monthly over 48 months beginning January 1, 2015 and (2) 213,600 options which vested immediately upon grant. |
| • | Mr. Rao was awarded 250,000 options to vest in equal increments monthly over 48 months beginning January 1, 2015. |
41
Table of Contents
Potential Payments upon Termination or Change in Control
We have entered into certain agreements and maintain certain plans that may require us to make certain payments and/or provide certain benefits to executive officers named in the Summary Compensation Table in the event employment is terminated by the Company without cause, by the employee for good reason, or there is a change of control. Should one of these three events occur, Ms. Eppstein is entitled to receive eighteen (18) months’ salary and Mr. Borst is entitled to receive twelve (12) months’ salary (plus benefits, accrued vacation earned and any bonus granted). No such employment agreement has been executed with Mr. Rao.
“Good Reason” is defined as occurring if (i) the Company materially reduces Employee’s salary and benefits; (ii) the Company materially diminishes Employee’s duties and responsibilities in a manner that is inconsistent with the provisions hereof or with her status as a senior executive officer of the Company; (iii) the Company willfully fails or refuses to perform or breaches its obligations under any other material provision of this Agreement and does not correct such failure or breach (if correctable) within thirty (30) days following notice thereof by Employee to the Company; (iv) the Company transfers Employee to a location other than Salt Lake City, Utah without the Employee’s prior written consent; or (v) there is a Change of Control (as defined below).
“Change of Control” is defined in the employment agreements as either (a) the acquisition of the Company by one or more other individuals or entities by means of any transaction or series of related transactions to which the Company is party (including, without limitation, any stock acquisition, reorganization, merger or consolidation but excluding any sale of stock for capital raising purposes) other than a transaction or series of transactions in which the holders of the voting securities of the Company outstanding immediately prior to such transaction continue to retain (either by such voting securities of the surviving entity), as a result of shares in the Company held by such holders prior to such transaction, at least fifty percent (50%) of the total voting power represented by the voting securities of the Company or such surviving entity outstanding immediately after such transaction or series of transactions or (b) a sale, lease or other conveyance of all or substantially all of the assets of the Company.
42
Table of Contents
Outstanding Equity Awards at Fiscal Year-End
The following table shows information regarding equity awards held by our named executive officers as of December 31, 2014:
EQUITY INCENTIVE PLAN: STOCK OPTION AWARDS
OUTSTANDING EQUITY AWARDS AS OF DECEMBER 31, 2014
| Name (a) |
Number of securities underlying unexercised options (#) exercisable (b) |
Number of securities underlying unexercised options (#) unexerciseable (c) |
Option Exercise Price ($) (e) |
Option Expiration Date (f) |
||||||||||||
| Deborah Eppstein |
141,377 | — | 0.1525 | (1) | 2/24/2017 | |||||||||||
| Deborah Eppstein |
28,327 | — | 0.1525 | (1) | 2/9/2019 | |||||||||||
| Deborah Eppstein |
346,141 | — | 0.1525 | (1) | 3/19/2019 | |||||||||||
| Deborah Eppstein |
448,956 | — | 0.0786 | (1) | 10/13/2019 | |||||||||||
| Deborah Eppstein |
216,338 | — | 0.1941 | (1) | 12/22/2020 | |||||||||||
| Deborah Eppstein |
216,338 | — | 0.1941 | (1) | 12/22/2020 | |||||||||||
| Deborah Eppstein |
187,500 | 62,500 | 0.7000 | (2) | 3/28/2022 | |||||||||||
| Deborah Eppstein |
125,000 | 125,000 | 0.7000 | (3) | 3/10/2024 | |||||||||||
| Deborah Eppstein |
135,594 | — | 0.7000 | (1) | 3/10/2024 | |||||||||||
| Deborah Eppstein |
206,250 | 68,750 | 0.7000 | (4) | 3/10/2024 | |||||||||||
| Deborah Eppstein |
— | 300,000 | 0.7000 | (6) | 12/17/2024 | |||||||||||
| Deborah Eppstein |
285,700 | — | 0.7000 | (1) | 12/17/2024 | |||||||||||
| Steven Borst |
18,930 | — | 0.1525 | (1) | 2/9/2019 | |||||||||||
| Steven Borst |
173,071 | — | 0.1525 | (1) | 3/19/2019 | |||||||||||
| Steven Borst |
209,995 | — | 0.0786 | (1) | 10/13/2019 | |||||||||||
| Steven Borst |
216,338 | — | 0.1941 | (1) | 12/22/2020 | |||||||||||
| Steven Borst |
216,338 | — | 0.1941 | (1) | 12/22/2020 | |||||||||||
| Steven Borst |
187,500 | 62,500 | 0.7000 | (2) | 3/28/2022 | |||||||||||
| Steven Borst |
100,000 | 100,000 | 0.7000 | (3) | 3/10/2024 | |||||||||||
| Steven Borst |
102,002 | — | 0.7000 | (1) | 3/10/2024 | |||||||||||
| Steven Borst |
62,500 | 187,500 | 0.7000 | (4) | 3/10/2024 | |||||||||||
| Steven Borst |
208,333 | 41,667 | 0.7000 | (5) | 3/10/2024 | |||||||||||
| Steven Borst |
— | 250,000 | 0.7000 | (6) | 12/17/2024 | |||||||||||
| Steven Borst |
213,600 | — | 0.7000 | (1) | 12/17/2024 | |||||||||||
| Mahendra Rao |
112,500 | 487,500 | 0.7000 | (7) | 3/31/2024 | |||||||||||
| Mahendra Rao |
300,000 | 100,000 | 0.7000 | (8) | 3/31/2024 | |||||||||||
| Mahendra Rao |
— | 250,000 | 0.7000 | (6) | 12/17/2024 | |||||||||||
| (1) | Option shares are fully vested and exercisable. |
| (2) | In December 2014, the Board approved the modification of exercise price relating to shares originally issued in 2012 from $1.00 to $.70. The shares maintain their original vesting term which provides that 6.25% of the option shares vested on March 28, 2012 with the remaining option shares vesting monthly over the following 45 months. Ms. Eppstein and Mr. Borst each had 250,000 options repriced from $1.00 to $.70. |
| (3) | 29.167% of the option shares vested on March 11, 2014 with the remaining option shares vesting monthly over the following 34 months. |
| (4) | 4.1667% of the option shares vested on March 11, 2014, with the remaining option shares vesting monthly over the following 46 months. |
| (5) | 8.33% of the option shares vest monthly over twelve months, with the first 8.33% vesting March 31, 2014. |
| (6) | 2.083% of the option shares vest monthly on the last day of each month over 48 months beginning January 1, 2015. |
| (7) | 8.33% of the option shares vest monthly on the last day of each month over twelve months beginning April 1, 2015. |
| (8) | 2.083% of the option shares vest monthly on the last day of each month over 48 months beginning April 1, 2015. |
43
Table of Contents
Director Compensation:
The following table shows the total compensation paid or accrued during the year ended December 31, 2014 to each of our non-employee directors.
| Name |
Title | Fiscal Year |
Option Awards (1) ($) |
|||||||||
| Peter Grebow, Ph.D. (2) |
Director | 2014 | 34,060 | |||||||||
| 2013 | — | |||||||||||
| Peter Barton Hutt (3) |
Director | 2014 | 34,060 | |||||||||
| 2013 | — | |||||||||||
| Diane Jorkasky, M.D. (4) |
Director | 2014 | 31,277 | |||||||||
| 2013 | — | |||||||||||
| Hunter Jackson, Ph.D. (5) |
Director | 2014 | 4,828 | |||||||||
| 2013 | — | |||||||||||
| (1) | Represents the grant date fair value of options expensed, including the impact of estimated forfeitures, computed in accordance with FASB ASC Topic 718. Options vest over twelve months and were considered fully vested as of December 31, 2014. On December 18, 2014, the Board of Directors approved the annual director grants for 2015, all of which will be expensed during 2015. These amounts represent the fair value of the awards on the grant date and do not correspond to the actual income that will be recognized by the officers. |
| (2) | Mr. Grebow had 175,000 options outstanding as of December 31, 2014. |
| (3) | Mr. Hutt had 239,902 options outstanding as of December 31, 2014. |
| (4) | Ms. Jorkasky had 141,667 options outstanding as of December 31, 2014. |
| (5) | Mr. Jackson was appointed to the Board of Directors on September 16, 2014. Dr. Jackson had 64,658 options outstanding as of December 31, 2014. |
On December 18, 2014, the Board of Directors approved the annual director option grants for 2015. Directors are eligible to receive an annual stock option grant up to 50,000 shares of common stock for each year of service thereafter. Such options vest monthly over the course of a twelve month period and are considered fully vested at year end. Aside from the above outside director compensation arrangements, our Directors receive no compensation for their service. Directors may be reimbursed for expenses incurred in attending meetings of the Board of Directors.
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
Security Ownership of Certain Beneficial Owners and Management
The following table sets forth certain information concerning the beneficial ownership of the Company’s common stock as of March 31, 2015 by (i) each person known by the Company to be the owner of more than 5% of the outstanding common stock, (ii) each director, (iii) each named executive officer, and (iv) all directors and executive officers as a group. In general, “beneficial ownership” includes those shares that a stockholder has the power to vote or the power to transfer, and stock options and other rights to acquire common stock that are exercisable currently or become exercisable within 60 days. Unless otherwise indicated, the address for each person is Q Therapeutics, Inc., 615 Arapeen Drive, Suite 102, Salt Lake City, UT 84108.
44
Table of Contents
| Name of Beneficial Owner |
Number of Shares of Voting Stock Beneficially Owned (1) |
Percentage of Class (1) |
||||||
| Cephalon, Inc., |
||||||||
| 41 Moores Road, Frazer, PA 19355 (2) |
11,056,641 | 28.9 | % | |||||
| Dinesh C. Patel, Ph.D., Chairman (3) |
6,763,643 | 21.9 | % | |||||
| MPI Research |
||||||||
| 54943 North Main Street, Mattawan, MI 49071 (4) |
5,639,660 | 16.7 | % | |||||
| UpStart Life Sciences Capital, L.P . |
||||||||
| 417 Wakara Way, Suite 3510, Salt Lake City, UT 84108 (5) |
3,740,302 | 11.3 | % | |||||
| Black Rhino, LP (9) |
||||||||
| 4203 Yoakum Blvd, Houston, TX 77006 (7) |
3,000,000 | 9.3 | % | |||||
| Deborah A. Eppstein, Ph.D., President, CEO and Director (6) |
3,013,063 | 9.1 | % | |||||
| Life Technologies Corporation, |
||||||||
| 5791 Van Allen Way, Carlsbad, CA 92008 (7) |
2,445,564 | 7.9 | % | |||||
| Steven J. Borst, CFO and Vice President of Corporate |
||||||||
| Development (8) |
2,376,159 | 7.3 | % | |||||
| Linda F. Powers, Director (9) |
1,400,916 | 4.5 | % | |||||
| Mahendra S. Rao, CSO and Chairman of the Scientific |
||||||||
| Advisory Board (10) |
989,196 | 3.1 | % | |||||
| Peter Barton Hutt, Director (11) |
275,637 | * | ||||||
| Peter Grebow Ph.D., Director (12) |
145,833 | * | ||||||
| Diane Jorkasky, Director (13) |
112,500 | * | ||||||
| Hunter Jackson, Director (14) |
35,491 | * | ||||||
| All Directors and Executive Officers as a Group (7 persons) (15) |
14,123,242 | 28.0 | % | |||||
| (1) | Percentage ownership is based on 30,477,460 shares of common stock outstanding as of March 31, 2015 and any shares of common stock that the beneficial owner has the right to acquire within 60 days. Any securities not outstanding which are subject to such options, warrants, rights or conversion privileges shall be deemed to be outstanding for the purpose of computing the percentage of outstanding securities of the class owned by such person but shall not be deemed to be outstanding for the purpose of computing the percentage of the class by any other person. Except as otherwise indicated, each of the persons and entities named in the table has sole voting and dispositive power with respect to all shares of common shares owned by them. |
| (2) | Includes 3,685,547 shares of common stock and warrants to purchase 7,371,094 shares of common stock. |
| (3) | Shares are owned by vSpring, Mr. Patel’s employer and includes 5,379,646 shares of common stock each held directly vSpring SBIC, L.P. Includes 1,099,410 shares of common stock held directly by vSpring, L.P. Includes 141,319 shares of common stock held directly by vSpring Partners, L.P. Includes 93,268 shares of common stock and a warrant to purchase 50,000 shares of common stock owned by Mr. Patel personally, for which he also has sole voting and dispositive power. |
| (4) | Includes 2,608,330 shares of common stock and warrants to purchase 3,031,330 shares of common stock. |
| (5) | Includes 1,390,247 shares of common stock and warrants to purchase 2,350,055 shares of common stock. Upstart Ventures Management, L.L.C. as the general partner of UpStart Life Sciences Capital, LP has voting and dispositive control over the shares held by UpStart Life Sciences Capital, LP. UpStart Ventures Management, LLC is managed collectively by Dennis B. Farrar, Theodore H. Stanley, and Steven J. Borst. |
| (6) | Includes 701,062 shares of common stock and 2,312,001 options exercisable for shares of common within 60 days of March 31, 2015. |
| (7) | Includes 2,445,564 shares of common stock. |
45
Table of Contents
| (8) | Includes 495,871 shares of common stock and 1,849,233 options exercisable for shares of common stock within 60 days of March 31, 2015 held directly by Mr. Borst. Mr. Borst has indirect ownership of 5,435 shares of common stock and warrants to purchase 25,620 shares of common stock which are owned by his spouse. |
| (9) | Includes 1,400,916 of common stock owned by Toucan Capital Fund III, L.P. |
| (10) | Includes 400,654 shares of common stock held by Mr. Rao’s son and 588,542 options exercisable for shares of common stock exercisable within 60 days of March 31, 2015 held directly by Mr. Rao. |
| (11) | Includes 32,451 shares of common stock and 193,185 options exercisable to purchase common stock within 60 days of March 31, 2015. |
| (12) | Includes 64,902 shares of common stock and 210,735 options exercisable to purchase common stock within 60 days of March 31, 2015. |
| (13) | Includes options 145,833 exercisable to purchase common stock within 60 days of March 31, 2015. |
| (14) | Includes options 112,500 exercisable to purchase common stock within 60 days of March 31, 2015. |
| (15) | Includes options 35,491 exercisable to purchase common stock within 60 days of March 31, 2015. |
| (16) | Includes options for an aggregate of 5,218,844 shares of common stock held by executive officers and directors as a group that are exercisable within 60 days of March 31 ,2015. |
Equity Compensation Plan Information
The following table summarizes our equity compensation plans as of December 31, 2014:
| Plan category |
(a) Number of securities to be issued upon exercise of outstanding options, warrants, and rights |
(b) Weighted- average exercise price of outstanding options, warrants and rights |
(c) Number of securities remaining available for future issuances under equity compensation plans (excluding securities reflected in column (a)) |
|||||||||
| Equity compensation plans approved by security holders |
8,952,927 | $ | .55 | 5,049,308 | ||||||||
| Equity compensation plans not approved by security holders |
17,991,161 | 1.28 | — | |||||||||
|
|
|
|
|
|||||||||
| Total |
26,944,088 | 1.04 | 5,049,308 | |||||||||
|
|
|
|
|
|||||||||
| Item 13. | Certain Relationships and Related Transactions, and Director Independence |
Related Party and Certain Transactions
Between August 12 and September 30, 2013, the Company received in aggregate $200,000 in cash proceeds from a bridge financing by an officer and an affiliate as evidenced by promissory notes. The notes were issued at 50% of face value, bore interest at a rate of 8% per annum, and matured beginning February 5, 2014. The officer transferred his ownership to the affiliate, of which the officer is a managing partner, in 2013. In February 2014, the affiliate agreed to extend the maturity date of its note for an additional 180 days, in exchange for certain call rights language being removed from warrants the affiliate had acquired in 2011. In June 2014, the affiliate converted its notes totaling $427,863, including interest, into units of one share of common stock and one warrant to purchase a share of common stock.
| Item 14. | Principal Accounting Fees and Services |
The following sets forth the fees billed to us for audit work and other services performed by Tanner LLC, our independent registered public accounting firm, for the years ended December 31, 2014 and 2013.
46
Table of Contents
| 2014 | 2013 | |||||||
| Audit fees |
$ | 84,500 | $ | 81,000 | ||||
| Audit related fees |
10,334 | 21,201 | ||||||
| Tax fees |
6,050 | 5,825 | ||||||
|
|
|
|
|
|||||
| Total |
$ | 100,884 | $ | 108,026 | ||||
|
|
|
|
|
|||||
Audit Fees
Audit fees consist of the aggregate fees billed for professional services rendered by Tanner LLC for audit of the Company’s annual financial statements and review of financial statements included in quarterly reports.
Audit Related Fees
Audit related fees consist of the aggregate fees billed for professional services rendered by Tanner LLC in the review of preliminary our 1933 Act registration statements.
Tax Fees
Tax fees consist of the aggregate fees billed for tax return preparation and compliance.
All Other Fees
None.
Pre-Approval Policies and Procedures
The Chairman of the Board of Directors has specifically approved the audit, audit related, and tax services performed by Tanner LLC (Tanner) and has determined the rendering of such was compatible with maintaining Tanner’s independence.
In 2014 and 2013, all audit fees, audit related fees, and tax fees were approved by the Chairman of the Board of Directors prior to the commencement of the services.
PART IV.
| Item 15. | Exhibits |
Index to Exhibits
| Exhibit |
Description | |
| 2.1 | Agreement and Plan of Merger by and between Grace 2, Inc., Q Merger Sub., and Q Therapeutics, Inc., dated October 13, 2011 (incorporated by reference from the Company’s Current Report on Form 8-K filed on October 18, 2011). | |
| 3.1 | Certificate of Incorporation of Grace 2, Inc. (incorporated by reference from the Company’s Registration Statement on Form 10SB filed on June 19, 2006). | |
| 3.2 | Certificate of Amendment to Certificate of Incorporation of Grace 2, Inc. dated October 18, 2011 (incorporated by reference from the Company’s Registration Statement on Form S-1 filed on June 29, 2012). | |
| 3.3 | By Laws of Q Therapeutics, Inc (incorporated by reference from the Company’s Form 10-K filed April 15, 2014.) | |
| 3.4 | Certificate of Amendment to the Certificate of Incorporation of Q Therapeutics, Inc. dated October 9, 2012 (incorporated by reference from the Company’s Form 10-Q filed November 13, 2012). | |
47
Table of Contents
| 3.5 | Certificate of Amendment to the Certificate of Incorporation of Q Holdings, Inc. dated October 30, 2012 (incorporated by reference from the Company’s Form 10-Q filed November 13, 2012). | |
| 4.1 | Form of Series A Warrant (incorporated by reference from the Company’s Form 10-Q filed on August 10, 2012.) | |
| 4.1.1 | Form of Amendment of Series A Warrant (incorporated by reference from the Company’s Form 10-K filed April 15, 2014.) | |
| 4.2 | Form of Series B Warrant (incorporated by reference from the Company’s Registration Statement on Form S-1 filed on June 29, 2012). | |
| 4.2.1 | Form of Amendment of Series B Warrant (incorporated by reference from the Company’s Form 10-K filed April 15, 2014.) | |
| 4.3 | Form of Bridge Warrant (incorporated by reference from the Company’s Registration Statement on Form S-1/A filed on August 16, 2012.) | |
| 4.4 | Form of Amendment to Bridge Warrant (incorporated by reference from the Company’s Registration Statement on Form S-1/A filed on August 16, 2012). | |
| 4.5 | Q Holdings Stock Purchase Agreement (incorporated by reference from the Company’s Current Report on Form 8-K/A filed on December 9, 2011.) | |
| 4.6 | Q Holdings Security Rights Agreement (incorporated by reference from the Company’s Current Report on Form 8-K/A filed on December 9, 2011.) | |
| 4.7 | Amended and Restated Q Therapeutics 2002 Stock Incentive Plan (incorporated by reference from the Company’s Registration Statement on Form S-1 filed on June 29, 2012). | |
| 4.8 | Amended and Restated Q Therapeutics 2011 Equity Incentive Compensation Plan (incorporated by reference from the Company’s Current Report on Form 8-K filed on March 31, 2015.) | |
| 4.9 | Form of Subscription Agreement (incorporated by reference from the Company’s Form 10-K filed April 15, 2014.) | |
| 4.10 | Form of Registration Rights Agreement (incorporated by reference from the Company’s Form 10-K filed April 15, 2014.) | |
| 4.11 | Form of Warrant to Purchase Common Stock (incorporated by reference from the Company’s Form 10-K filed April 15, 2014.) | |
| 10.1 | Exclusive License Agreement between Q Therapeutics, Inc. and the University of Utah Research Foundation dated October 5, 2005, as amended, re-filed pursuant to SEC recommendations (incorporated by reference from the Company’s Current Report on Form 8-K filed on March 13, 2012. Certain provisions have been omitted pursuant to Confidential Treatment Requests and have been filed separately with the SEC.) | |
| 10.2 | Employment Agreement between Q Holdings, Inc. and Deborah A. Eppstein Ph.D. dated October 13, 2012 (incorporated by reference from the Company’s Current Report on Form 8-K filed on October 18, 2011). | |
| 10.2.1 | Amendment to Employment & Proprietary Rights Agreement between Q Therapeutics, Inc. and Deborah A. Eppstein Ph.D. dated December 18, 2012 (incorporated by reference from the Company’s Annual Report on Form 10-K filed on March 28, 2013). | |
| 10.3 | Employment Agreement between Q Holdings, Inc. and Steven J. Borst, dated October 13, 2012 (incorporated by reference from the Company’s Current Report on Form 8-K filed on October 18, 2011). | |
| 10.3.1 | Amendment to Employment & Proprietary Rights Agreement between Q Therapeutics, Inc. and Steven J. Borst, dated December 18, 2012 (incorporated by reference from the Company’s Annual Report on Form 10-K filed on March 28, 2013). | |
| 10.4 | Lease Agreement dated January 30, 2004 between Q Therapeutics, Inc. and Paradigm Resources, LLC (incorporated by reference from the Company’s Current Report on Form 8-K/A filed on December 9, 2011). | |
| 10.4.1(1) | Eighth Amendment to Lease Agreement dated March 24, 2015 between Q Therapeutics, Inc. and Paradigm Resources, LLC. | |
| 10.5 | Form of Promissory Note (incorporated by reference from the Company’s Quarterly Report on Form 10-Q filed on November 14, 2013). | |
| 14.1 | Code of Ethics and Conduct (incorporated by reference from the Company’s Form 10-Q filed November 13, 2012). | |
| 21.1(1) | List of Subsidiaries | |
| 23.1(1) | Consent of Independent Registered Public Accounting Firm | |
48
Table of Contents
| 31.1(1) | Certification of the Company’s Principal Executive Officer pursuant to 15d-15(e), under the Securities and Exchange Act of 1934, as amended, with respect to the registrant’s Annual Report on Form 10-K for the year ended December 31, 2014. | |
| 31.2(1) | Certification of the Company’s Principal Financial Officer and Principal Accounting Officer pursuant to 15d-15(e), under the Securities and Exchange Act of 1934, as amended, with respect to the registrant’s Annual Report on Form 10-K for the year ended December 31, 2014. | |
| 32.1* | Certification pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 (Principal Executive Officer). | |
| 32.2* | Certification pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 (Principal Financial Officer and Principal Accounting Officer). | |
| XBRL Instance Document | ||
| XBRL Taxonomy Extension Schema Document | ||
| XBRL Taxonomy Extension Calculation Linkbase Document | ||
| XBRL Taxonomy Extension Definition Linkbase Document | ||
| XBRL Taxonomy Extension Label Linkbase Document | ||
| XBRL Taxonomy Extension Presentation Linkbase Document | ||
| (1) | Filed herewith. |
| * | Furnished herewith. |
49
Table of Contents
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this Report to be signed on its behalf by the undersigned, thereunto duly authorized.
| Q THERAPEUTICS, INC. | ||||||
| March 31, 2015 | By: | /s/ DEBORAH A. EPPSTEIN, PH.D. | ||||
| Deborah A. Eppstein, Ph.D. | ||||||
| President and Chief Executive Officer, Director | ||||||
| (Principal Executive Officer) | ||||||
| March 31, 2015 | By: | /s/ STEVEN J. BORST, M.B.A. | ||||
| Steven J. Borst, M.B.A. | ||||||
| Chief Financial Officer and Vice President of Corporate Development | ||||||
| (Principal Financial Officer, Principal Accounting Officer) | ||||||
Pursuant to the requirements of the Securities Exchange Act of 1934, this Report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
| Signature |
Title |
Date | ||
| /s/ DEBORAH A. EPPSTEIN, PH.D. |
President and Chief Executive Officer, Director | March 31, 2015 | ||
| Deborah A. Eppstein, Ph.D. | (Principal Executive Officer) | |||
| /s/ STEVEN J. BORST |
Chief Financial Officer and Vice President of | March 31, 2015 | ||
| Steven J. Borst | Corporate Development (Principal Financial Officer, Principal Accounting Officer) | |||
| /s/ DINESH C. PATEL, PH.D. |
Chairman of the Board of Directors | March 31, 2015 | ||
| Dinesh C. Patel, Ph.D. | ||||
| /s/ PETER BARTON HUTT |
Director | March 31, 2015 | ||
| Peter Barton Hutt | ||||
| /s/ PETER GREBOW, PH.D. |
Director | March 31, 2015 | ||
| Peter Grebow, Ph.D. | ||||
| /s/ DIANE JORKASKY, M.D. |
Director | March 31, 2015 | ||
| Diane Jorkasky, M.D. | ||||
| /s/ HUNTER JACKSON, PH.D. | Director | March 31, 2015 | ||
| Hunter Jackson, Ph.D. | ||||
50
Table of Contents
Q THERAPEUTICS, INC. & SUBSIDIERIES
| Page No. | ||||
| F-1 | ||||
| Consolidated Financial Statements: |
||||
| F-2 | ||||
| F-3 | ||||
| F-4 | ||||
| F-5 | ||||
| F-6 | ||||
Table of Contents
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders of Q Therapeutics, Inc.
We have audited the accompanying consolidated balance sheets of Q Therapeutics, Inc. and subsidiaries (collectively, the Company) as of December 31, 2014 and 2013, and the related consolidated statements of operations, stockholders’ deficit, and cash flows for the years then ended. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the financial statements are free of material misstatement. The Company is not required to have, nor were we engaged to perform, an audit of the Company’s internal control over financial reporting. Our audits included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the consolidated financial position of Q Therapeutics, Inc. and subsidiaries as of December 31, 2014 and 2013, and the consolidated results of their operations and their cash flows for the years then ended in conformity with U.S. generally accepted accounting principles.
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As more fully described in Note 2, the Company has negative working capital, has incurred operating losses and negative cash flows from operating activities, expects to incur further losses, and has an accumulated deficit and a stockholders’ deficit. These conditions raise substantial doubt about the Company’s ability to continue as a going concern. Management’s plans in regard to these matters are also described in Note 2. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
/s/ Tanner LLC
Salt Lake City, Utah
March 31, 2015
F-1
Table of Contents
Q THERAPEUTICS, INC. AND SUBSIDIARIES
Consolidated Balance Sheets
As of December 31, 2014 and 2013
| 2014 | 2013 | |||||||
| Assets |
||||||||
| Current assets: |
||||||||
| Cash |
$ | 707,011 | $ | 142,532 | ||||
| Receivables |
4,136 | 5,556 | ||||||
| Prepaid financing costs, net |
— | 63,333 | ||||||
| Prepaid expenses and other |
10,596 | 10,109 | ||||||
|
|
|
|
|
|||||
| Total current assets |
721,743 | 221,530 | ||||||
| Property and equipment, net |
26,568 | 27,999 | ||||||
| Other assets |
— | 7,513 | ||||||
|
|
|
|
|
|||||
| Total assets |
$ | 748,311 | $ | 257,042 | ||||
|
|
|
|
|
|||||
| Liabilities and Stockholders’ Deficit |
||||||||
| Current liabilities: |
||||||||
| Accounts payable |
$ | 263,728 | $ | 2,364,001 | ||||
| Accrued liabilities |
42,507 | 81,156 | ||||||
| Accrued compensation |
705,753 | 353,950 | ||||||
| Notes payable |
— | 500,000 | ||||||
| Derivative liabilities |
32,175 | — | ||||||
|
|
|
|
|
|||||
| Total current liabilities |
1,044,163 | 3,299,107 | ||||||
|
|
|
|
|
|||||
| Commitments (Note 9) |
||||||||
| Stockholders’ deficit: |
||||||||
| Common stock, $0.0001 par value: 100,000,000 shares authorized; 30,477,460 and 24,936,833 shares outstanding as of December 31, 2014 and 2013, respectively |
3,048 | 2,494 | ||||||
| Additional paid-in capital |
26,933,171 | 20,836,811 | ||||||
| Accumulated deficit |
(27,232,071 | ) | (23,881,370 | ) | ||||
|
|
|
|
|
|||||
| Total stockholders’ deficit |
(295,852 | ) | (3,042,065 | ) | ||||
|
|
|
|
|
|||||
| Total liabilities and stockholders’ deficit |
$ | 748,311 | $ | 257,042 | ||||
|
|
|
|
|
|||||
See accompanying notes to consolidated financial statements.
F-2
Table of Contents
Q THERAPEUTICS, INC. AND SUBSIDIARIES
Consolidated Statements of Operations
For the Years Ended December 31, 2014 and 2013
| 2014 | 2013 | |||||||
| Grant revenues |
$ | 677,864 | $ | 14,175 | ||||
| Other revenues |
2,400 | 12,000 | ||||||
|
|
|
|
|
|||||
| Total revenues |
680,264 | 26,175 | ||||||
| Cost of revenues |
800 | 4,800 | ||||||
|
|
|
|
|
|||||
| Gross profit |
679,464 | 21,375 | ||||||
|
|
|
|
|
|||||
| Operating expenses: |
||||||||
| Research and development |
1,960,815 | 1,827,533 | ||||||
| General and administrative |
2,098,851 | 1,384,712 | ||||||
|
|
|
|
|
|||||
| Total operating expenses |
4,059,666 | 3,212,245 | ||||||
|
|
|
|
|
|||||
| Operating loss |
(3,380,202 | ) | (3,190,870 | ) | ||||
|
|
|
|
|
|||||
| Other income (expense): |
||||||||
| Gain on derivative liability |
134,019 | — | ||||||
| Interest expense |
(111,022 | ) | (202,111 | ) | ||||
| Other income, net |
6,504 | 3,889 | ||||||
|
|
|
|
|
|||||
| Total other income (expense) |
29,501 | (198,222 | ) | |||||
|
|
|
|
|
|||||
| Loss before provision (benefit) for income taxes |
(3,350,701 | ) | (3,389,092 | ) | ||||
| Provision (benefit) for income taxes |
— | — | ||||||
|
|
|
|
|
|||||
| Net loss |
$ | (3,350,701 | ) | $ | (3,389,092 | ) | ||
|
|
|
|
|
|||||
| Weighted average number of common shares outstanding - basic and diluted |
28,944,366 | 24,839,755 | ||||||
|
|
|
|
|
|||||
| Net loss per common share - basic and diluted |
$ | (0.12 | ) | $ | (0.14 | ) | ||
|
|
|
|
|
|||||
See accompanying notes to consolidated financial statements.
F-3
Table of Contents
Q THERAPEUTICS, INC. AND SUBSIDIARIES
Consolidated Statements of Stockholders’ Deficit
For the Years Ended December 31, 2014 and 2013
| Additional | Total | |||||||||||||||||||
| Common Stock | Paid-in | Accumulated | Stockholders’ | |||||||||||||||||
| Shares | Amount | Capital | Deficit | Deficit | ||||||||||||||||
| Balance as of December 31, 2012 |
24,761,832 | $ | 2,476 | $ | 20,494,792 | $ | (20,492,278 | ) | $ | 4,990 | ||||||||||
| Common stock issued for services |
175,001 | 18 | 174,982 | — | 175,000 | |||||||||||||||
| Warrants issued for services |
— | — | 65,284 | — | 65,284 | |||||||||||||||
| Stock-based compensation expense |
— | — | 101,753 | — | 101,753 | |||||||||||||||
| Net loss |
— | — | — | (3,389,092 | ) | (3,389,092 | ) | |||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Balance as of December 31, 2013 |
24,936,833 | 2,494 | 20,836,811 | (23,881,370 | ) | (3,042,065 | ) | |||||||||||||
| Common stock issued for: |
||||||||||||||||||||
| Cash, $1.00 per unit, containing one share of common stock and one warrant |
2,016,000 | 202 | 2,015,798 | — | 2,016,000 | |||||||||||||||
| Issuance of common stock upon exercise of stock options |
100,734 | 10 | 15,356 | — | 15,366 | |||||||||||||||
| License agreements |
65,000 | 6 | 45,494 | — | 45,500 | |||||||||||||||
| Issued for settlement of accounts payable |
2,727,030 | 273 | 2,726,758 | — | 2,727,030 | |||||||||||||||
| Issued for settlement of debt |
531,863 | 53 | 531,809 | — | 531,863 | |||||||||||||||
| Issued for services |
100,000 | 10 | 69,990 | — | 70,000 | |||||||||||||||
| Warrants issued for services |
— | — | 27,390 | — | 27,390 | |||||||||||||||
| Stock-based compensation expense |
— | — | 829,959 | — | 829,959 | |||||||||||||||
| Derivatives related to common stock and warrant down-round protection rights |
— | — | (166,194 | ) | — | (166,194 | ) | |||||||||||||
| Net loss |
— | — | — | (3,350,701 | ) | (3,350,701 | ) | |||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Balance as of December 31, 2014 |
30,477,460 | $ | 3,048 | $ | 26,933,171 | $ | (27,232,071 | ) | $ | (295,852 | ) | |||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
See accompanying notes to consolidated financial statements.
F-4
Table of Contents
Q THERAPEUTICS, INC. AND SUBSIDIARIES
Consolidated Statements of Cash Flows
For the Years Ended December 31, 2014 and 2013
| 2014 | 2013 | |||||||
| Cash flows from operating activities: |
||||||||
| Net loss |
$ | (3,350,701 | ) | $ | (3,389,092 | ) | ||
| Adjustments to reconcile net loss to net cash used in operating activities: |
||||||||
| Depreciation and amortization |
9,665 | 11,847 | ||||||
| Original debt discount |
63,333 | 186,667 | ||||||
| Gain on derivative liabilities |
(134,019 | ) | — | |||||
| Stock-based compensation |
829,959 | 101,753 | ||||||
| Common stock issued for services |
115,500 | 175,000 | ||||||
| Warrants issued for services |
27,390 | 65,284 | ||||||
| Decrease in: |
||||||||
| Receivables |
1,420 | 472,246 | ||||||
| Prepaid expenses and other assets |
7,026 | 257 | ||||||
| Increase in: |
||||||||
| Accounts payable and accrued liabilities |
619,971 | 1,232,107 | ||||||
| Accrued compensation |
351,803 | 266,058 | ||||||
|
|
|
|
|
|||||
| Net cash used in operating activities |
(1,458,653 | ) | (877,873 | ) | ||||
|
|
|
|
|
|||||
| Cash flows from investing activities: |
||||||||
| Purchase of property and equipment |
(8,234 | ) | (23,802 | ) | ||||
|
|
|
|
|
|||||
| Cash flows from financing activities: |
||||||||
| Issuance of common stock for cash |
2,016,000 | — | ||||||
| Proceeds from exercise of common stock options |
15,366 | — | ||||||
| Proceeds from issuance of notes payable |
— | 250,000 | ||||||
|
|
|
|
|
|||||
| Net cash provided by financing activities |
2,031,366 | 250,000 | ||||||
|
|
|
|
|
|||||
| Net increase (decrease) in cash |
564,479 | (651,675 | ) | |||||
| Cash as of beginning of the year |
142,532 | 794,207 | ||||||
|
|
|
|
|
|||||
| Cash as of end of the year |
$ | 707,011 | $ | 142,532 | ||||
|
|
|
|
|
|||||
| Supplemental disclosure of cash flow information: |
||||||||
| Cash paid for interest |
$ | 729 | $ | 1,009 | ||||
Supplemental disclosure of noncash investing and financing activities for the years ended December 31, 2014 and 2013:
| • | Between August 12 and September 30, 2013, the Company received $250,000 in cash proceeds in exchange for notes payable of $500,000 and recorded a debt discount of $250,000. |
| • | Between March 7 and June 30, 2014, the Company settled accounts payable of $2,727,030 with the issuance of 2,727,030 shares of common stock. |
| • | Between March 7 and June 30, 2014, the Company settled notes payable and interest of $531,863 with the issuance of 531,863 shares of common stock. |
See accompanying notes to consolidated financial statements.
F-5
Table of Contents
Q THERAPEUTICS, INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements
| 1. | Organization |
Q Therapeutics, Inc. (Q Therapeutics) conducts its operations through its wholly owned subsidiary, Q Therapeutic Products, Inc. (Q Products), and its wholly owned subsidiary NeuroQ Research, Inc. (collectively, the Company). Q Therapeutics is a Salt Lake City, Utah-based biopharmaceutical company that is developing human cell-based therapies intended to treat degenerative diseases of the brain and spinal cord, the primary components of the central nervous system (CNS). Q Products was incorporated in the state of Delaware on March 28, 2002 and merged with Q Acquisition, Inc., a Delaware corporation and a wholly owned subsidiary of Grace 2, Inc., on October 13, 2011. Grace 2, Inc. was incorporated on October 27, 2005. On November 2, 2011, Grace 2 changed its name to Q Holdings, Inc. and on December 10, 2012, it changed its name to Q Therapeutics, Inc.
These potential therapies are based on technology developed by Q Products’ co-founder Mahendra Rao, M.D., Ph.D., a leader in glial stem cell biology, during his tenure at the University of Utah and as Head of the Stem Cell Section in the Laboratory of Neuroscience at the National Institutes of Health (NIH) Institute of Aging. Dr. Rao was one of the first scientists to identify and seek patent coverage on stem cells and their progeny cells found in the CNS. After licensing Dr. Rao’s technology from the University of Utah and NIH, Q Products commenced operations in the spring of 2004 to develop cell-based therapeutics that can be sold as “off-the-shelf” pharmaceuticals.
| 2. | Significant Accounting Policies |
The Company has adopted the following significant accounting policies in preparing its consolidated financial statements:
Basis of Presentation and Consolidation
The accompanying consolidated financial statements have been prepared by management in accordance with U.S. generally accepted accounting principles (US GAAP), and include all assets and liabilities of the Company and its wholly owned subsidiary, Q Therapeutic Products, Inc. All material transactions and balances have been eliminated.
Going Concern Assumption and Liquidity
The Company has not generated significant revenues and has been developing its products. Historically, the Company has been dependent on government grants and debt and equity raised from individual investors to sustain its operations. The Company’s continued operations will depend on its ability to raise funds through similar sources. There can be no assurance that such capital will be available on favorable terms or at all. If it is unable to raise additional capital, the Company will likely be forced to curtail desired development activities, which will delay the development of its product candidates. The Company’s products have not been approved by the U.S. Food and Drug Administration (FDA) for commercial sale; therefore, the Company has not generated revenues from commercial therapeutic product sales.
The Company has incurred losses and has negative cash flows from operating activities. As of December 31, 2014, the Company had an accumulated deficit of $27,232,071, a stockholders’ deficit of $295,852, and negative working capital of $322,420.
These uncertainties create substantial doubt about the Company’s ability to continue as a going concern. The Company’s financial statements have been prepared on a basis which assumes that the Company will continue as a going concern, which contemplates the realization of assets and the satisfaction of liabilities and commitments in the normal course of business. The accompanying financial statements do not include any adjustments that may result from the outcome of this uncertainty.
2014 Financing Transactions
Between March 7 and April 14, 2014, the Company issued an aggregate of 4,420,530 units, each unit consisting of one share of the Company’s common stock and one warrant to purchase one share of the Company’s common stock for which the Company received cash consideration of $2,012,500 and the settlement of indebtedness of $2,408,030 (2014 Financing
F-6
Table of Contents
Q THERAPEUTICS, INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements Continued
Transactions). The warrants have an initial exercise prices of $1.00 per share, are immediately exercisable, and expire in no more than four years. Both the shares of common stock and the warrants issued in the 2014 Financing Transactions have a “down-round” protection provision provided to the investors in the financing. With respect to the common shares and warrants issued with certain exceptions, if the Company subsequently issues or sells any shares of common stock or any common stock equivalents pursuant to which shares of common stock may be acquired at a prices less than $1.00 per share, then the Company shall promptly issue additional shares of common stock to the investor in an amount such that the subscription price paid, when divided by the total number of shares issued will result in an actual price paid per share of common stock equal to such lower price and with respect to warrants, the warrant exercise price shall be reduced to the lesser price at which the common stock or common stock equivalents were issued. The down-round provisions expire upon the earlier of (1) the effectiveness of a registration statement with the Securities Exchange Commission (SEC) registering the shares of common stock issued and the common shares underlying warrants issued in the 2014 Financing Transactions, or (2) one year after the issuance date. The down-round protection related to the March 7, 2014 round of financing has expired. The remaining down-round protection will expire by June 30, 2015.
On June 30, 2014, the Company issued 854,363 shares of common stock and warrants to purchase 1,277,363 shares of common stock resulting from an additional tranche of financing for which the Company received cash consideration of $3,500 and a settlement of indebtedness of $850,863. The common stock and warrants have terms similar to the 2014 Financing Transactions.
Between January 26, 2015 and February 23, 2015, 327,455 warrants and 21,634 options were exercised for cash proceeds of $114,131.
Use of Estimates
The preparation of financial statements in conformity with US GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosures of contingent assets and liabilities at the dates of the financial statements and the reported amounts of the revenues and expenses for the reporting periods. Accordingly, actual results could differ from those estimates. Key estimates include allowances for doubtful accounts receivable, useful lives for property and equipment, valuation allowances for net deferred income tax assets, and valuations for stock-based compensation awards. The Company bases its estimates on historical experience and on various other assumptions that are believed to be reasonable under the circumstances.
Revenue Recognition and Receivables
The Company periodically applies for research grants, generally as a sub-recipient to grants funded by government agencies through research institutions. Grant revenues are recognized as associated expenses are incurred and are billed in conjunction with the terms of the grants. The Company records its grants receivable in accordance with the provisions of the grant agreements. The Company’s grants receivable are considered past due when payment has not been received within 30 days of the invoice date, although certain institutions customarily do not pay within these terms. The amounts of the specific allowances are estimated by management based on various assumptions including the age of the individual receivable, and changes in payment schedules and histories. Receivable balances are charged off against the allowance for doubtful accounts when management determines the probability of collection is remote. Recoveries of receivables previously charged off are recorded when payment is received. Revenue earned in 2014 was derived from two customers. The Company did not incur any losses relating to bad debts associated with grant revenues for the years ended December 31, 2014 and 2013.
In June 2014, the Company was notified of a sub-award as part of the fourth and final year of grant funding awarded to The Johns Hopkins University from the National Institute of Neurological Disorders and Stroke (NINDS) of the National Institutes of Health in the amount of $677,864. As of December 31, 2014, $4,136 remains receivable from this sub-award.
Concentration of Suppliers
The Company has entered and will enter into agreements with outside research facilities to assist in the clinical research, monitoring, and reporting of its pilot and clinical studies. In some instances, the Company is dependent upon a single supplier. The loss of key suppliers could have a material adverse effect upon the Company’s operations by interrupting or delaying the progress or completion of the Company’s clinical trials.
F-7
Table of Contents
Q THERAPEUTICS, INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements Continued
For the year ended December 31, 2014, one supplier accounted for approximately 61.4% of the Company’s research and development purchases.
Property and Equipment
Property and equipment are stated at cost less accumulated depreciation and amortization. Depreciation and amortization are calculated using the straight-line method over the estimated economic useful lives of the assets as follows:
| Lab equipment | 5 years | |
| Computers and software | 3 years | |
| Leasehold improvements | 7 years | |
| Office equipment and furniture | 3 years |
Expenditures that materially increase values or capacities or extend useful lives of property and equipment are capitalized. Routine maintenance and repairs are expensed as incurred. Upon sale or other retirement of depreciable property, the cost and accumulated depreciation are removed from the related accounts and any gain or loss is reflected in the statements of operations.
Impairment of Long-Lived Assets
The Company reviews its property, equipment, and other long-lived assets for impairment whenever events or changes in circumstances indicate that the carrying amounts of the assets may be impaired. Management does not consider any of the Company’s assets to be impaired as of December 31, 2014 and 2013.
Leases
The Company leases its research and office facility under an operating lease. The current lease expires on March 31, 2016. If rent escalations in the new lease are material, the Company will record the total rent payable during the lease term on a straight-line basis over the term of the lease. The Company will record any difference between the rent paid and the straight-line rent as a deferred rent liability.
Stock-Based Compensation
The Company calculates the estimated fair value of its stock options and warrants on the grant date using the Black-Scholes option-pricing model. The Company recognizes stock-based compensation expense as services are provided, which is generally over the vesting period of the individual equity instruments. Expense related to stock options issued in lieu of cash to non-employees for services performed are measured at the fair value of the options on the date they are earned and the related expense is recognized as services are provided.
The volatility assumption used in the Black-Scholes option-pricing model is based on the volatility of publicly traded companies in the same industry segment as the Company. The expected lives of the options and warrants granted represent the periods of time that the options granted are expected to be outstanding. The risk free rates for periods within the contractual lives of the options and warrants are based on the U.S. Treasury securities constant maturity rate that corresponds to the expected terms in effect at the time of grant. Stock compensation expense recorded by the Company was $829,959 and $101,753 for the years ended December 31, 2014 and 2013, respectively, and is included in general and administrative expense in the statements of operations.
Income Taxes
The Company is a C corporation and federal and state income taxes are computed using the asset and liability method. Under the asset and liability method, deferred income tax assets and liabilities are recognized for future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their
F-8
Table of Contents
Q THERAPEUTICS, INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements Continued
respective tax bases. Deferred income tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred income tax assets and liabilities of a change in tax rates is recognized in net income (loss) in the period that includes the enactment date.
Uncertain Tax Positions
The Company recognizes the financial statement amount of an uncertain tax position only after considering the probability that a tax authority would sustain the position in an examination. For tax positions meeting a “more likely-than-not” threshold, the amount recognized in the financial statements is the amount expected to be realized upon settlement with the tax authority. For tax positions not meeting the threshold, no financial statement benefit is recognized. The Company recognizes interest and penalties, if any, related to uncertain tax positions as general and administrative expenses. The Company currently has no federal or state examinations in progress. The Company’s tax years subject to federal and state tax examination are 2011, 2012, 2013 and 2014.
Research and Development Costs
Research and development (R&D) costs, including research performed under contract by third parties, are expensed as incurred. Major components of R&D expenses consist of personnel costs including salaries and benefits, outside research services, consulting fees, lab supplies and materials, license fees, and facility-related expenses. R&D expenses recorded by the Company were $1,960,815 and $1,827,533 for the years ended December 31, 2014 and 2013, respectively.
Net Loss Per Common Share
Basic net income or loss per common share (Basic EPS) is computed by dividing net income or loss by the weighted average number of common shares outstanding. Diluted net income or loss per common share (Diluted EPS) is computed by dividing net income or loss by the sum of the weighted average number of common shares outstanding and the dilutive potential common share equivalents then outstanding. Potential dilutive common share equivalents consist of shares issuable upon the exercise of outstanding stock options and warrants to acquire common stock.
Due to the fact that for all periods presented, the Company incurred net losses, potential dilutive common share equivalents as of December 31, 2014 and 2013, totaling 26,994,088 and 16,169,658, respectively, are not included in the calculation of Diluted EPS because they are anti-dilutive. Therefore, basic loss per common share is the same as diluted loss per common share for the years ended December 31, 2014 and 2013.
Recent Accounting Pronouncements
In May 2014, the Financial Accounting Standards Board (FASB) issued ASU 2014-09, Revenue from Contracts with Customers. ASU 2014-09 provides for a single, principles-based model for revenue recognition that replaces existing revenue recognition guidance. ASU 2014-09 is effective for annual and interim periods beginning on or after December 15, 2016. It permits the use of either a retrospective or cumulative effect transition method and early adoption is not permitted. The Company has not yet determined the impact this standard will have on its consolidated financial statements and related disclosures.
In June 2014, the FASB issued ASU 2014-10, Topic 915, Development Stage Entities, Elimination of Certain Financial Reporting Requirements. ASU 2014-10 removes all incremental financial reporting requirements for development stage entities, including but not limited to, inception-to-date financial information included in the statements of operations, statements of stockholders’ deficit and statements of cash flows. The Company elected early adoption of ASU 2014-10 beginning with the reporting period ended June 30, 2014. As a result of the Company’s early adoption, all references to the Company as a development stage entity have been removed. The adoption of this pronouncement had no impact on the Company’s financial position, results of operations or liquidity.
In August 2014, the FASB issued ASU 2014-15, Presentation of Financial Statements – Going Concern (Subtopic 310-40): Disclosure of Uncertainties about an Entity’s Ability to Continue as a Going Concern. ASU 2014-15 provides guidance in US GAAP about management’s responsibility to evaluate whether there is substantial doubt about an entity’s
F-9
Table of Contents
Q THERAPEUTICS, INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements Continued
ability to continue as a going concern and to provide related disclosures. In doing so, this is intended to reduce diversity in the timing and content of disclosures. This is effective for the annual period ending after December 15, 2016, and for annual periods and interim periods thereafter. Early application is permitted. The Company is currently evaluating the potential impact of adopting this guidance on its consolidated financial statements and related disclosures.
Subsequent Events
The Company has evaluated all subsequent events through the issuance date of the financial statements.
| 3. | Property and Equipment |
Property and equipment consist of the following:
| December 31, 2014 | December 31, 2013 | |||||||
| Lab equipment |
$ | 329,406 | $ | 321,172 | ||||
| Computers and software |
67,739 | 67,739 | ||||||
| Leasehold improvements |
38,934 | 38,934 | ||||||
| Office equipment and furniture |
3,647 | 3,647 | ||||||
|
|
|
|
|
|||||
| 439,726 | 431,492 | |||||||
| Less accumulated depreciation and amortization |
(413,158 | ) | (403,493 | ) | ||||
|
|
|
|
|
|||||
| Property and equipment, net |
$ | 26,568 | $ | 27,999 | ||||
|
|
|
|
|
|||||
Depreciation and amortization expense for the years ended December 31, 2014 and 2013 was $9,665 and $11,847, respectively.
| 4. | Accounts Payable |
Between March 7, 2014 and June 30, 2014, the Company settled $2,727,030 of accounts payable through the issuance of equity.
| 5. | Accrued Compensation |
Accrued compensation consists of the following:
| December 31, 2014 | December 31, 2013 | |||||||
| Accrued wages |
$ | 621,072 | $ | 278,393 | ||||
| Accrued vacation |
84,681 | 75,557 | ||||||
|
|
|
|
|
|||||
| Total accrued compensation |
$ | 705,753 | $ | 353,950 | ||||
|
|
|
|
|
|||||
Accrued wages consists primarily of salaries and related employment taxes resulting from the decision in March 2013 by certain of the Company’s executives to defer part, or all, of their salaries until additional funding for the Company is obtained.
F-10
Table of Contents
Q THERAPEUTICS, INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements Continued
| 6. | Notes Payable |
Between August 12 and September 30, 2013, the Company received $250,000 in cash proceeds from a bridge financing from certain note holders, some of which were affiliates, as evidenced by promissory notes. The notes were issued at 50% of face value, bore interest at the rate of 8% per annum, and matured beginning February 5, 2014. As of June 30, 2014, all notes payable and associated interest had been converted into equity as part of the 2014 Financing Transactions (see Note 2).
The effective interest rate related to this financing is approximately 156%.
| 7. | Derivative Liabilities |
In connection with the 2014 Financing Transactions, the Company recorded derivative liabilities related to down-round protection provided to the stockholders in the event that the Company does another offering of units, similar to those issued in the 2014 Financing Transactions, at a price below $1.00 per share. The down-round provision expires upon the earlier of the effectiveness of a registration statement with the SEC or one year after the issuance date. With the assistance of a third-party valuation specialist, the Company valued the derivative liabilities pursuant to the accounting guidance of ASC 820-10, Fair Value Measurements.
Fair values for warrants and common stock are determined using the Monte-Carlo Simulation Model valuation technique. The Monte-Carlo Simulation Model provides for dynamic assumptions regarding volatility and risk-free interest rates within the total period to expected conversion. In addition, management assessed the probabilities of future financing assumptions.
Fair value is based on the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. In order to increase consistency and comparability in fair value measurements, US GAAP establishes a fair value hierarchy that prioritizes observable and unobservable inputs used to measure fair value into three broad levels, which are described below:
| Level 1 | Quoted prices (unadjusted) in active markets that are accessible at the measurement date for assets or liabilities. | |
| Level 2 | Other inputs that are observable directly or indirectly, such as quoted prices for similar assets and liabilities or market corroborated inputs. | |
| Level 3 | Unobservable inputs that are used when little or no market data is available, which require the Company to develop its own assumptions about how market participants would value the assets or liabilities. | |
Determining which category an asset or liability falls within the hierarchy requires significant judgment. The Company evaluates its hierarchy disclosure each quarter. Assets and liabilities measured at fair value on a recurring basis as of December 31, 2014 are summarized as follows:
| Fair Value as of December 31, 2014 | ||||||||||||||||
| Level 1 | Level 2 | Level 3 | Total | |||||||||||||
| Derivative liabilities |
$ | — | $ | — | $ | 32,175 | $ | 32,175 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
F-11
Table of Contents
Q THERAPEUTICS, INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements Continued
The following table presents the reconciliation of Level 3 liabilities measured at fair value on a recurring basis during the year ended 2014.
| Fair Value Measurements Using Significant Unobservable Inputs (Level 3) |
||||
| Derivatives | ||||
| Beginning balance, as of December 31, 2013 |
$ | — | ||
| Issuances: |
||||
| Derivative liabilities related to down-round provision of common stock and warrants |
166,194 | |||
| Gain on derivative liabilities |
(134,019 | ) | ||
|
|
|
|||
| Ending balance, as of December 31, 2014 |
$ | 32,175 | ||
|
|
|
|||
Given the nature of the derivative liabilities, the carrying amount of $32,175 as of December 31, 2014 was derived from Level 3 inputs and represents management’s best estimate of fair value.
The valuation assumptions used in the Monte-Carlo Simulation Model for the year ended December 31, 2014 are as follows:
| Threshold barrier |
$1.00 | |
| Average down-round protection value per unit |
$.06 - $.1714 | |
| Probability of down-round offer |
3% - 10% |
| 8. | Stockholders’ Equity |
Common Stock
As of December 31, 2014, the Company is authorized to issue 100,000,000 shares of common stock, of which 30,477,460 shares were outstanding. Outstanding options and warrants for 26,944,088 shares of common stock existed as of December 31, 2014.
Holders of shares of common stock are entitled to cast one vote for each share held at all stockholders’ meetings for all purposes, including the election of directors. The common stock does not have cumulative voting rights.
Preferred Stock
As of December 31, 2014, the Company was authorized to issue 10,000,000 shares of preferred stock; however, no shares of preferred stock have been issued to date.
Stock Options
2002 Stock Option Plan
In April 2002, the Board of Directors approved the Q Therapeutics 2002 Stock Incentive Plan (the 2002 Plan) and in February 2003, the stockholders approved the 2002 Plan. The 2002 Plan permits the grant of incentive stock options, non-qualified stock options and restricted stock.
F-12
Table of Contents
Q THERAPEUTICS, INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements Continued
All but 228,472 options available for award under the 2002 Plan have been granted. On December 6, 2011, the Board of Directors authorized the addition of the 228,472 shares available but unissued pursuant to the 2002 Plan to the authorized/reserved option pool for the 2011 Plan (discussed below). As of December 31, 2014, 2,874,706 options had been issued and remained outstanding under the 2002 Plan.
2011 Equity Incentive Compensation Plan
In connection with the Merger, on October 13, 2011, the Board of Directors and stockholders approved the Q Therapeutics, Inc. 2011 Equity Incentive Compensation Plan (the 2011 Plan). Subject to the provisions of the 2011 Plan, a designated committee (Committee) of the Board of Directors (or if none, the Board of Directors itself) may, from time to time, in its sole discretion select from among eligible employees, non-employee directors and consultants those to whom awards shall be granted under the 2011 Plan, and shall determine in its discretion the nature, terms, conditions and amount of each award, subject to the terms of the 2011 Plan. The term of the 2011 Plan commenced on October 13, 2011 (the Effective Date) and remains in effect, subject to the right of the Committee or the Board to amend or terminate the 2011 Plan at any time pursuant to the 2011 Plan, until the earlier of (i) the tenth anniversary of the Effective Date, or (ii) all shares subject to the 2011 Plan have been purchased or acquired according to the 2011 Plan’s provisions.
The 2011 Plan initially provided for a reservation pool of up to 1,500,000 shares of common stock reserved for issuance pursuant to the 2011 Plan. On December 6, 2011, the Board of Directors authorized the addition of the remaining 228,472 shares available but unissued pursuant to the 2002 Plan to the authorized/reserved option pool for the 2011 Plan, bringing the 2011 Plan pool to 1,728,472 shares of common stock reserved for issuance. The Board of Directors also resolved to roll over all forfeited or expired awards made under the 2002 Plan into the 2011 Plan. As of December 31, 2014, 149,057 expired options issued under the 2002 Plan had been rolled into the 2011 Plan.
On December 18, 2012, the Board of Directors approved, subject to stockholder approval, the addition of 3,000,000 shares to the 2011 Plan. On May 6, 2013, the stockholders approved the additional 3,000,000 shares under the Plan, increasing the number of shares from 1,877,529 to 4,877,529.
On December 18, 2014, the Board of Directors and stockholders approved the addition of 6,250,000 shares to the 2011 Plan, increasing the number of shares from 4,877,529 to 11,127,529. In an effort to increase employee retention and morale, the Company offered its employees the opportunity to have certain outstanding options issued in 2012 modified by reducing the grant exercise price of $1.00 to $.70, which was the fair market value of the common stock as of the modification date. No other terms of the options were modified. As a result, the exercise price of 890,000 options held in aggregate by 10 employees to purchase common stock was modified and the incremental expense of $57,761 resulting from the modification is being to be amortized through December 31, 2015.
As of December 31, 2014, 6,078,221 options had been issued under the 2011 Plan with 5,049,308 options available for future grant.
F-13
Table of Contents
Q THERAPEUTICS, INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements Continued
The following sets forth all outstanding common stock options and related activity for the years ended December 31, 2014 and 2013:
| Number of Options Outstanding |
Weighted Average Exercise Price |
Weighted Average Remaining Contractual Life (Years) |
Aggregate Intrinsic Value |
|||||||||||||
| Outstanding as of December 31, 2012 |
3,865,440 | $ | 0.34 | 6.94 | $ | 2,549,625 | ||||||||||
| Granted |
— | — | ||||||||||||||
| Exercised |
— | — | ||||||||||||||
| Forfeited |
— | — | ||||||||||||||
|
|
|
|||||||||||||||
| Outstanding as of December 31, 2013 |
3,865,440 | 0.34 | 6.06 | 2,549,625 | ||||||||||||
| Granted |
5,188,221 | 0.70 | ||||||||||||||
| Exercised |
(100,734 | ) | 0.15 | |||||||||||||
| Forfeited |
— | — | ||||||||||||||
|
|
|
|||||||||||||||
| Outstanding as of December 31, 2014 |
8,952,927 | 0.55 | 6.97 | 1,601,845 | ||||||||||||
|
|
|
|||||||||||||||
| Options vested and exercisable as of December 31, 2014 |
5,773,344 | 0.42 | 6.69 | 1,601,845 | ||||||||||||
|
|
|
|||||||||||||||
In 2014, the Company received proceeds of $15,366 from the exercise of options. The Company issues shares of common stock upon receipt of the recipient’s exercise notification and payment.
A summary of the activity for unvested option awards for the year ended December 31, 2014:
| Number of Options |
Weighted Average Grant Date Fair Value |
|||||||
| Non-vested options as of December 31, 2013 |
424,167 | $ | 0.62 | |||||
| Granted |
5,188,221 | 0.45 | ||||||
| Vested |
(2,432,805 | ) | 0.41 | |||||
| Forfeited |
— | — | ||||||
|
|
|
|||||||
| Non-vested options as of December 31, 2014 |
3,179,583 | 0.50 | ||||||
|
|
|
|||||||
The Company determines the expected term of its stock option awards by using the simplified method, which assumes each vesting tranche of the award has a term equal to the midpoint between when the award vests and when the award expires. Expected volatility is calculated by weighting the stock price of similar industry segment public companies equivalent to the expected term of each grant. The risk-free interest rate for the expected term of each option granted is based on the U.S. Treasury securities rate in effect at the time of the grant with the period that approximates the expected term of the option.
The aggregate intrinsic value of outstanding stock options and the aggregate intrinsic value of outstanding exercisable stock options as of December 31, 2014 was $1,601,845. The total fair value of equity awards that vested during fiscal year 2014 was $1,702,963. This total fair value includes equity-based awards issued in the form of stock options, stock-settled stock appreciation rights, and deferred stock units.
F-14
Table of Contents
Q THERAPEUTICS, INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements Continued
Stock-based compensation for the years ended December 31, 2014 and 2013 was $829,959 and $101,753, respectively. As of December 31, 2014, the Company had $1,282,743 of unrecognized stock-based compensation expense related to non-vested awards that will be recognized over a weighted average period of 2.95 years.
Warrants
The following sets forth outstanding warrants and related activity for the years ended December 31, 2014 and 2013, all of which are exercisable:
| Number of Warrants |
Weighted Average Exercise Price Per Share |
Weighted Average Remaining Life in Years |
Intrinsic Value |
|||||||||||||
| Outstanding as of December 31, 2012 |
11,979,518 | $ | 1.40 | 5.52 | $ | 216,710 | ||||||||||
| Granted |
325,000 | 1.77 | — | — | ||||||||||||
| Exercised |
— | — | — | — | ||||||||||||
| Forfeited |
— | — | — | — | ||||||||||||
|
|
|
|||||||||||||||
| Outstanding and exercisable as of December 31, 2013 |
12,304,518 | 1.41 | 4.44 | 216,710 | ||||||||||||
| Granted |
5,749,143 | 1.00 | — | — | ||||||||||||
| Exercised |
— | — | — | — | ||||||||||||
| Forfeited |
(62,500 | ) | 1.25 | — | — | |||||||||||
|
|
|
|||||||||||||||
| Outstanding and exercisable as of December 31, 2014 |
17,991,161 | 1.28 | 3.41 | 216,710 | ||||||||||||
|
|
|
|||||||||||||||
The following presents information related to outstanding warrants as of December 31, 2014, all of which are exercisable:
| Warrants Outstanding and Exercisable | ||||||||||
| Exercise Price | Number of Warrants | Weighted Average Remaining Life in Years |
||||||||
| $ | 0.05 | 132,797 | 0.1 | |||||||
| 0.53 | 192,242 | 0.1 | ||||||||
| 1.00 | 11,542,584 | 3.6 | ||||||||
| 1.01 | 125,000 | 3.8 | ||||||||
| 1.04 | 823,347 | 0.1 | ||||||||
| 1.20 | 43,000 | 2.0 | ||||||||
| 1.75 | 4,944,691 | 0.3 | ||||||||
| 2.00 | 62,500 | 3.9 | ||||||||
| 2.25 | 62,500 | 0.5 | ||||||||
| 2.75 | 62,500 | 0.8 | ||||||||
|
|
|
|||||||||
| 17,991,161 | ||||||||||
|
|
|
|||||||||
F-15
Table of Contents
Q THERAPEUTICS, INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements Continued
Between January 26 and February 13, 2015, 327,455 warrants were exercised for total cash proceeds of $110,831. On February 14, 2015, 820,931 warrants expired.
| 9. | Commitments |
Employee Agreements
The Company has entered into employment and proprietary rights agreements with all of its employees. These agreements stipulate that employment is on an at-will basis and outline salary, benefits, non-disclosure of confidential information, restrictions and assignment of intellectual property to the Company.
The Company has employment agreements with its CEO and CFO that specify compensation, benefits and vacation. These agreements address severance for termination of employment and have provisions if a change of control occurs.
License and Royalty Agreements
The Company has entered into an exclusive license agreement with a university, through which the Company has obtained certain intellectual property from the university to commercialize, produce, manufacture, use and sell products. The Company is required to pay the university a contractual dollar amount for each new investigational drug application filed with the FDA using the licensed intellectual property. Should the Company receive cash payments from licensing revenue during human trial research, the Company is required to pay 25% of all net licensing revenue (but not including payments for product development activities or equity purchases) to the university up to a certain maximum amount. The Company is also required to pay an amount upon New Drug Application (NDA) approval. The NDA approval occurs when the FDA has approved all prior drug testing and allows a new drug to go to market. Once the Company has an NDA, the Company must pay the university a royalty of 2% of net sales up to a certain dollar amount, 2.5% of net sales in excess of that amount of human therapeutics, and a royalty of 5% of net sales on any services. If the Company sublicenses the intellectual property, then the Company must pay the university in accordance with the provisions of the agreement.
In March 2014, the Company entered into a license agreement with a company (the licensor) whereby the Company was granted use of the licensor’s technology. Should any patents be filed in conjunction with this technology, the Company would be granted a non-exclusive, worldwide, perpetual, royalty-free fully paid license to utilize the technology. In exchange for the technology, the licensor will receive a license for manufacturing related technology from the Company and up to 110,000 shares of common stock upon the completion of certain milestones. Additionally, any sales generated from the licensor’s technology will result in a 5% royalty to the licensor. As of December 31, 2014, the licensor has been issued 65,000 shares of common stock.
Collaborative Arrangements
From time to time, the Company enters into collaborative arrangements for research and development, manufacture and/or commercialization of product and product candidates. These collaborations can provide for non-refundable, upfront license fees, R&D and commercial performance milestones, cost sharing, and royalty payments. The Company’s collaboration agreements with outside parties are performed on a “best efforts” basis with no guarantees of success.
In September 2013, the Company executed an agreement to acquire a non-exclusive, worldwide, perpetual, royalty free technology license. In consideration for this license, the Company issued 100,000 shares of the Company’s common stock. Should the Company sublicense the technology to a third party, the Company is required to compensate the licensor 10% of the upfront fees. The agreement is in place for 10 years from the effective date of the agreement or the life of the patents, whichever is longer.
Supplier Agreements
In March 2010, the Company entered into a service agreement with an outside research firm to support the Company’s submission of an investigational new drug application (IND) to the FDA. On March 7, 2014, the Company paid for certain services by issuing 2,185,330 shares of common stock and a warrant to purchase 2,185,330 shares of common stock.
F-16
Table of Contents
Q THERAPEUTICS, INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements Continued
In June 2014, the Company entered into a new agreement with the research firm in which the Company has the option to pay up to $500,000 of a pre-clinical study with equity. For each dollar in expense, the Company had the option to pay the research firm with one share of common stock and one warrant to purchase two shares of common stock. The warrants have an exercise price of $1.00, have a four-year life, and are immediately exercisable. Further, at the Company’s discretion, the Company may elect to pay for the services in cash. For each dollar paid in cash, the Company would issue the research firm a warrant to purchase one share of the Company’s common stock with similar terms as stated above. Any amount in excess of this limit, could be converted into a note payable upon completion of the study. Interest would accrue at 8% annually until August 1, 2015 when the interest rate would increase to 10% through the maturity date of the note of March 31, 2016.
Advisory Agreements
On October 1, 2012, the Company entered into an agreement with an investor relations firm. In lieu of cash, the firm received 200,000 shares of restricted stock for services rendered between October 2012 through October 2014. As of October 31, 2014, the service agreement was terminated. Additionally, 250,000 warrants were granted from January 1, 2013 through October 1, 2013 with exercise prices ranging between $1.25 and $2.75. As of December 31, 2014, 62,500 of these warrants have expired.
In July 2013, the Company entered into a business consulting services agreement effective through December 31, 2015. Under the agreement, the Company issued three warrants to purchase up to 175,000 shares of common stock at a strike price of $1.01 per share, with a five-year life and a no call provision. All warrants are immediately exercisable.
Operating Leases
The Company leases its research and office facility under an operating lease which expires March 31, 2016. Lease expense under operating leases was $160,001 and $152,098 for the years ended December 31, 2014 and 2013, respectively. Future minimum lease payments are approximately $123,891 and $41,297 for the years ended December 31, 2015 and 2016, respectively.
| 10. | Income Taxes |
The benefit for income taxes differs from the amount computed at federal statutory rates as follows for the years ended December 31:
| 2014 | 2013 | |||||||
| Federal income tax at statutory rates |
$ | (1,139,136 | ) | $ | (1,152,292 | ) | ||
| State income tax at statutory rates |
(99,401 | ) | (108,575 | ) | ||||
| Research and development credits |
(89,692 | ) | (161,935 | ) | ||||
| Change in valuation allowance |
1,143,266 | 1,389,156 | ||||||
| Other |
184,963 | 33,646 | ||||||
|
|
|
|
|
|||||
| $ | — | $ | — | |||||
|
|
|
|
|
|||||
F-17
Table of Contents
Q THERAPEUTICS, INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements Continued
Significant components of the Company’s deferred income tax assets (liabilities) are as follows as of December 31:
| 2014 | 2013 | |||||||
| Current: |
||||||||
| Accruals and reserves |
$ | 271,219 | $ | 140,274 | ||||
| Non-qualified stock options and other |
24,351 | — | ||||||
| Change in valuation allowance |
(295,570 | ) | (140,274 | ) | ||||
|
|
|
|
|
|||||
| $ | — | $ | — | |||||
|
|
|
|
|
|||||
| Long-term: |
||||||||
| Net operating loss carryforwards |
$ | 8,805,743 | $ | 8,064,635 | ||||
| Depreciation and amortization |
(339 | ) | (358 | ) | ||||
| Non-qualified stock options and other |
227,944 | 70,605 | ||||||
| Research and development credits |
884,477 | 794,750 | ||||||
| Valuation allowance |
(9,917,825 | ) | (8,929,632 | ) | ||||
|
|
|
|
|
|||||
| $ | — | $ | — | |||||
|
|
|
|
|
|||||
As of December 31, 2014, the Company had net operating loss (NOL) carryforwards available to offset future taxable income, if any, of approximately $23,608,000, which will begin to expire in 2022.
The Company has research and development credits totaling $884,477 available for offset against future federal income tax, if any. The credits begin to expire in 2022.
The utilization of the NOL carryforwards is subject to annual limitations under Section 382 of the Internal Revenue Code. Section 382 imposes limitations on a corporation’s ability to utilize its NOL carryforwards if it experiences an “ownership change.” In general terms, an ownership change results from transactions increasing the ownership of certain stockholders in the stock of a corporation by more than 50% over a three-year period.
The Company has concluded that there are no significant uncertain tax positions requiring disclosure.
| 12. | Benefit Plan |
The Company sponsors a defined contribution 401(k) retirement plan (the Plan). Employees who are 18 years of age or older are eligible to participate in the Plan. Employees may elect to contribute to the Plan up to 100% of their annual compensation, up to the maximum amount allowed by the Internal Revenue Service.
The Company may elect to match part of employee contributions, make a profit sharing contribution, or a non-qualified contribution at its discretion. The Company elected to make non–qualified contributions of $20,549 and $15,144 to the Plan for the years ended December 31, 2014 and 2013, respectively.
| 13. | Related-Party Transactions |
Between August 12 and September 30, 2013, the Company received in aggregate $200,000 in cash proceeds from a bridge financing by an officer and an affiliate as evidenced by promissory notes. The notes were issued at 50% of face value, bore interest at a rate of 8% per annum, and matured beginning February 5, 2014. The officer transferred his ownership to the affiliate, of which the officer is a managing partner, in 2013. In February 2014, the affiliate agreed to extend the maturity date of its note for an additional 180 days in exchange for certain call rights language being removed from warrants the affiliate had acquired in 2011. In June 2014, the affiliate converted its notes totaling $427,863, including interest, into units of one share of common stock and one warrant to purchase a share of common stock.
| 14. | Subsequent Events |
Between January 26, 2015 and February 23, 2015, 327,455 warrants and 21,634 options were exercised for cash proceeds of $114,131.
F-18
