Attached files
| file | filename |
|---|---|
| EXCEL - IDEA: XBRL DOCUMENT - EMMAUS LIFE SCIENCES, INC. | Financial_Report.xls |
| EX-32.1 - EX-32.1 - EMMAUS LIFE SCIENCES, INC. | a2223903zex-32_1.htm |
| EX-31.2 - EX-31.2 - EMMAUS LIFE SCIENCES, INC. | a2223903zex-31_2.htm |
| EX-4.35 - EX-4.35 - EMMAUS LIFE SCIENCES, INC. | a2223903zex-4_35.htm |
| EX-31.1 - EX-31.1 - EMMAUS LIFE SCIENCES, INC. | a2223903zex-31_1.htm |
| EX-10.23(F) - EX-10.23(F) - EMMAUS LIFE SCIENCES, INC. | a2223903zex-10_23f.htm |
| EX-10.23(D) - EX-10.23(D) - EMMAUS LIFE SCIENCES, INC. | a2223903zex-10_23d.htm |
| EX-10.23(E) - EX-10.23(E) - EMMAUS LIFE SCIENCES, INC. | a2223903zex-10_23e.htm |
| EX-10.23(B) - EX-10.23(B) - EMMAUS LIFE SCIENCES, INC. | a2223903zex-10_23b.htm |
| EX-10.23(C) - EX-10.23(C) - EMMAUS LIFE SCIENCES, INC. | a2223903zex-10_23c.htm |
Use these links to rapidly review the document
TABLE OF CONTENTS
INDEX TO FINANCIAL STATEMENTS
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 10-K
ý |
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
|
For the fiscal year ended December 31, 2014 |
||
OR |
||
o |
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
|
For the transition period from to |
||
Commission File Number: 000-53072
Emmaus Life Sciences, Inc.
(Exact name of Registrant as specified in its charter)
| Delaware (State or Other Jurisdiction of Incorporation or Organization) |
2834 (Primary Standard Industrial Classification Code Number) |
41-2254389 (I.R.S. Employer Identification No.) |
21250 Hawthorne Boulevard, Suite 800, Torrance, California 90503
(Address of principal executive offices, including zip code)
(310) 214-0065
(Registrant's telephone number, including area code)
SECURITIES
REGISTERED PURSUANT TO SECTION 12(b) OF THE ACT:
None.
SECURITIES
REGISTERED PURSUANT TO SECTION 12(g) OF THE ACT:
Common Stock, $0.001 par value
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes o No ý
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes o No ý
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ý No o
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes ý No o
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant's knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ý
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer or a smaller reporting company. See the definitions of "large accelerated filer," "accelerated filer" and "smaller reporting company" in Rule 12b-2 of the Exchange Act:
| Large accelerated filer o | Accelerated filer o | Non-accelerated filer o (Do not check if smaller reporting company) |
Smaller reporting company ý |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes o No ý
There was no aggregate market value of shares of common stock held by non-affiliates of the registrant as of June 30, 2014, the last business day of the registrant's most recently completed second fiscal quarter, because the registrant's common stock was not trading on any exchange on that date.
There were 28,093,848 shares outstanding of the registrant's common stock, par value $0.001 per share, as of March 20, 2015. The registrant's common stock is not traded or listed on any exchange.
EMMAUS LIFE SCIENCES, INC.
TABLE OF CONTENTS TO ANNUAL REPORT ON FORM 10-K
FOR THE FISCAL YEAR ENDED DECEMBER 31, 2014
2
CAUTIONARY STATEMENT REGARDING FORWARD-LOOKING STATEMENTS
The information contained in this Annual Report on Form 10-K contains some statements that are not purely historical and that are considered "forward-looking statements" within the meaning of Section 27A if the Securities Act of 1933, as amended, which we refer to as the Securities Act, and Section 21E of the Securities Exchange Act of 1934, as amended, which we refer to as the Exchange Act. Such forward-looking statements include, but are not limited to, statements regarding our plans for our business and products; clinical studies and regulatory reviews of our products under development; our strategies and business outlook; our financial condition, results of operations and business prospects; the positioning of our products in relation to demographic and pricing trends in the relevant markets; and various other matters (including contingent liabilities and obligations and changes in accounting policies, standards and interpretations). These forward-looking statements express our management's expectations, hopes, beliefs, and intentions regarding the future. In addition, without limiting the foregoing, any statements that refer to projections, forecasts or other characterizations of future events or circumstances, including any underlying assumptions, are forward-looking statements. The words "anticipates," "believes," "continue," "could," "estimates," "expects," "intends," "may," "might," "plans," "possible," "potential," "predicts," "projects," "seeks," "should," "will," "would" and similar expressions and variations, or comparable terminology, or the negatives of any of the foregoing, may identify forward-looking statements, but the absence of these words does not mean that a statement is not forward-looking.
The forward-looking statements contained in this Annual Report on Form 10-K are based on current expectations and beliefs concerning future developments that are difficult to predict. We cannot guarantee future performance, or that future developments affecting our company will be those currently anticipated. These forward-looking statements involve a number of risks, uncertainties (some of which are beyond our control) or other assumptions that may cause actual results or performance to be materially different from those expressed or implied by these forward-looking statements, including the following:
- •
- the acceptance of our NDA by the FDA based on our single Phase 3 clinical trial;
- •
- completion of clinical trials or confirmatory study for our product candidates;
- •
- our ability to obtain and maintain U.S. Food and Drug Administration, or FDA, and other regulatory approvals to market our drug
products under development, including our pharmaceutical grade L-glutamine treatment for sickle cell disease, or SCD;
- •
- our ability to manage our business despite continuing operating losses and cash outflows;
- •
- our ability to raise additional capital or enter into strategic relationships, or do both, in order to fund our operations and product
development plans, including meeting our financial obligations under our existing agreements and any future licensing and related arrangements;
- •
- our ability to build and maintain the management and human resources and infrastructure necessary to support our business strategy and
product development and commercialization plans;
- •
- our ability to find and obtain strategic partners on acceptable terms;
- •
- following approval, if any, of each of our product candidates by the FDA and other government regulators outside the United States, our ability to comply with any applicable pharmacovigilance (drug safety) regulatory requirements, including without limitation, implementation and operation of any Risk Evaluation Mitigation Strategies and successful completion of any post-approval safety studies, and the absence of any safety issues that would require withdrawal of such products from the market or any warning or other material limitation on their prescription for or use by patients or consumers;
3
- •
- our reliance on third party manufacturers for our product candidates and products;
- •
- the ability of our third party manufacturers to meet regulatory and supply requirements;
- •
- the approval and market entry of competitors' products and developments in science and medicine beyond our control;
- •
- market acceptance of our product candidates, including our pharmaceutical grade L-glutamine treatment for SCD, and our ability to
commercialize the resulting products;
- •
- our reliance on the expected growth in demand for our products
- •
- our dependence on licenses for certain of our product candidates and products, including our pharmaceutical grade L-glutamine
treatment for SCD, and our ability to obtain, maintain and, if necessary, enforce against third parties additional intellectual property rights, through patents or otherwise, to technology required or
desirable for the conduct of our business;
- •
- exposure to product liability and defect claims;
- •
- the costs and uncertain outcome of remaining issues subject to litigation relating to our 2011 merger transaction;
- •
- exposure to intellectual property claims from third parties;
- •
- the lack of a current public trading market for our securities;
- •
- the cost of complying with current and future governmental regulations, our ability to comply with applicable governmental
regulations, and the impact of any changes in governmental regulations upon our operations; and
- •
- such other factors referenced in this Annual Report, including, without limitation, under the sections entitled "Risk Factors," "Management's Discussion and Analysis of Financial Condition and Results of Operations," and "Business."
All forward-looking statements attributable to us are expressly qualified in their entirety by these risks and uncertainties. These risks and uncertainties, along with others, are also described below under the heading "Risk Factors." Should one or more of these risks or uncertainties materialize, or should any of the parties' assumptions prove incorrect, actual results may vary in material respects from those projected in these forward-looking statements. You should not place undue reliance on any forward-looking statements and should not make an investment decision based solely on these forward-looking statements. We undertake no obligation to update or revise any forward-looking statements, whether as a result of new information, future events or otherwise, except as may be required under applicable securities laws.
4
With respect to this discussion, the terms, "we," "us," "our" or the "Company" refer to Emmaus Life Sciences, Inc., and its wholly-owned subsidiary Emmaus Medical, Inc., a Delaware corporation which we refer to as Emmaus Medical, and Emmaus Medical's wholly-owned subsidiaries, Newfield Nutrition Corporation, a Delaware corporation which we refer to as Newfield Nutrition; Emmaus Medical Japan, Inc., a Japanese corporation which we refer to as EM Japan, and Emmaus Medical Europe Ltd., a U.K. corporation which we refer to as EM Europe.
Overview
We are a biopharmaceutical company engaged in the discovery, development and commercialization of innovative treatments and therapies primarily for rare and orphan diseases. We are initially focusing our product development efforts on sickle cell disease, or SCD, a genetic disorder and a significant unmet medical need. Our lead product candidate is an oral pharmaceutical grade L-glutamine treatment that demonstrated positive clinical results in our completed Phase 3 clinical trial for sickle cell anemia and sickle ß0-thalassemia, two of the most common forms of SCD.
We are in the process of preparing a new drug application, or NDA, for submission to the U.S. Food and Drug Administration, or FDA, with respect to this product candidate. If the FDA accepts our submission and approves this NDA, we may be authorized to market in the United States our pharmaceutical grade L-glutamine treatment for SCD patients who are at least five years old. L-glutamine for the treatment of SCD has received Fast Track designation from the U.S. Food and Drug Administration, or FDA, as well as Orphan Drug designation from both the FDA and the European Commission, or EC.
We plan to market our L-glutamine treatment in the United States, if approved, by either strategic partnership or by building our own targeted sales force of approximately 30 sales representatives. We intend to utilize strategic partnerships to market our treatment in the rest of the world.
SCD is an inherited blood disorder characterized by the production of an altered form of hemoglobin which polymerizes and becomes fıbrous, causing red blood cells to become rigid and change form so that they appear sickle-shaped instead of soft and rounded. Patients with SCD suffer from debilitating episodes of sickle cell crisis, which occur when the rigid, adhesive and inflexible red blood cells occlude blood vessels. Sickle cell crisis causes excruciating pain as a result of insufficient oxygen being delivered to tissue, referred to as tissue ischemia, and inflammation. These events may lead to organ damage, stroke, pulmonary complications, skin ulceration, infection and a variety of other adverse outcomes.
We have completed a 230 patient randomized, double-blind, placebo-controlled, parallel-group, multi-center Phase 3 clinical trial which enrolled adult and pediatric patients as young as five years of age, involving 31 sites in the United States. Our pharmaceutical grade L-glutamine treatment and the placebo were randomized by site and by patients using hydroxyurea, a chemotherapeutic agent first approved for SCD by the U.S. Food and Drug Administration, or FDA, in 1998. All participants other than those who received a placebo, including children, received up to 30 grams of pharmaceutical grade L-glutamine treatment daily, dissolved in liquid, split between morning and evening—the same dosage as our Phase 2 clinical trial completed in 2009.
We interpret the results of our Phase 3 clinical trial to indicate that our oral pharmaceutical grade L-glutamine treatment for SCD, when taken on a daily basis by a patient with SCD, can potentially decrease the incidence of sickle cell crisis by restoring the flexibility and function of red blood cells in patients with SCD. Further, we interpret these results to indicate that our prescription grade L-glutamine product candidate can reduce the number of costly hospitalizations as well as unexpected
5
emergency room and urgent care visits from patients with SCD. L-glutamine enhances nicotinamide adenine dinucleotide, or NAD, synthesis to reduce excessive oxidative stress in sickle red blood cells that induces much of the damage leading to characteristic symptoms of SCD.
Although non-prescription L-glutamine supplements are available, we are not aware of any reports in peer reviewed literature of any demonstrations of their clinical safety and effectiveness for the treatment of SCD in controlled clinical trials and they have not been approved by the FDA as a prescription drug for SCD. Our pharmaceutical grade, consistent formulation of L-glutamine may be able to meet the rigorous safety and effectiveness requirements of regulatory agencies for approval, as a prescription drug, and if so, may be preferred by treating physicians and payors as compared to non-prescription L-glutamine supplements.
We have extensive experience in the field of SCD, including the development, outsourced manufacturing and conduct of clinical trials of our L-glutamine product candidate for the treatment of SCD. Our chief executive officer, Yutaka Niihara, M.D., MPH, is a leading hematologist in the field of SCD. Dr. Niihara is licensed to practice medicine in both the United States and Japan and has been actively engaged in SCD research and the care of patients with SCD for over 20 years, primarily at the University of California Los Angeles and the Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center, or LA BioMed, a nonprofit biomedical research institute.
To a lesser extent, we are also engaged in the marketing and sale of NutreStore L-glutamine powder for oral solution, which has received FDA approval, as a treatment for short bowel syndrome ("SBS"), in patients receiving specialized nutritional support when used in conjunction with a recombinant human growth hormone that is approved for this indication. Our indirect wholly owned subsidiary, Newfield Nutrition, sells L-glutamine as a nutritional supplement under the brand name AminoPure through retail stores in multiple states and via importers and distributors in Japan, Taiwan and South Korea. Since inception, we have generated minimal revenues from the sale and promotion of NutreStore and AminoPure.
Sickle Cell Disease—Market Overview
Sickle cell disease is a genetic blood disorder that affects 20-25 million people worldwide, and occurs with increasing frequency among those whose ancestors are from regions including sub-Saharan Africa, South America, the Caribbean, Central America, the Middle East, India and Mediterranean regions such as Turkey, Greece and Italy. The CDC estimates that there are as many as 100,000 patients with SCD in the United States, and we estimate there are approximately 80,000 patients in the European Union. In regions such as Central Africa, 90% of patients with SCD die by age five and 99% of patients die by age 20. In all regions, SCD requires ongoing physician care and considerable medical intervention. The overall survival of patients in the United States with sickle cell anemia correlates with the severity of their disease state, especially the number of crises per year.
SCD is characterized by the production of an altered form of hemoglobin which polymerizes and becomes fıbrous, causing the red blood cells of patients with SCD to become sickle-shaped, inflexible and adhesive rather than round, smooth and flexible. The complications associated with SCD occur when these inflexible and sticky cells block, or occlude, small blood vessels, which can then cause severe and chronic pain throughout the body due to insufficient oxygen being delivered to tissue, or ischemia, and inflammation. According to an article in Annals of Internal Medicine, "In the Clinic: Sickle Cell Disease" by M.H. Steinberg (September 2011), which we refer to as the Steinberg Article, this leads to long-term organ damage, diminished exercise tolerance, increased risk of stroke and infection and decreased lifespan.
6
Sickle cell crisis, a broad term covering a range of disorders, is one of the most devastating complications of SCD. Types of sickle cell crisis include:
- •
- Vaso-occlusive crisis, characterized by obstructed blood flow to organs such as the
bones, liver, kidney, eye, or central nervous system;
- •
- Aplastic crisis, characterized by acute anemia typically due to viral infection;
- •
- Hemolytic crisis, characterized by accelerated red blood cell death and hemoglobin
loss;
- •
- Splenic sequestration crisis, characterized by painful enlargement of the spleen due
to trapped red blood cells; and
- •
- Acute chest syndrome, a potentially life-threatening obstruction of blood supply to the lungs characterized by fever, chest pain, cough and lung infiltrates.
According to the Steinberg Article, acute chest syndrome affects more than half of all patients with SCD and is a common reason for hospitalization. Other symptoms and complications of SCD include swelling of the hands and feet, infections, pneumonia, vision loss, leg ulcers, gallstones and stroke.
A crisis is characterized by excruciating musculoskeletal pain, visceral pain and pain in other locations. These crises occur periodically throughout the life of a person with SCD. In adults, the acute pain typically persists for five to ten days or longer, followed by a dull, aching pain generally ending only after several weeks and sometimes persisting between crises. According to the Steinberg Article, frequency of sickle cell crises varies within patients with SCD from rare occurrences to occurrences several times a month. Approximately 30% of patients have rare crises, 50% have occasional crises, and 20% have weekly or monthly crises. Crisis frequency tends to increase late in the second decade of life and to decrease after the fourth decade. The overall survival of patients in the United States with sickle cell anemia correlates with the severity of their disease state, especially the number of crises per year.
Patients with more than 3 sickle cell crises per year will experience fatal complications during the fourth and fifth decades of life whereas patients who experience between 1 and 3 crises per year have a median survival of nearly 50 years (Hematology in Clinical Practice by Robert S. Hillman et. al. (5th ed. 2011)).
Treatment of sickle cell crisis is burdensome and expensive for patients and payors, as it encompasses costs for hospitalization, emergency room visits, urgent care visits, and prescription pain medication. According to an article in American Journal of Hematology, "The Burden of Emergency Department Use for Sickle Cell Disease: An Analysis of the National Emergency Department Sample Database" by S. Lanzkron (October 2010), there were approximately 70,000 hospitalizations and 230,000 emergency room visits with a combined emergency room and hospital inpatient charges for these SCD patients estimated to be $2.4 billion in 2006.
Limitations of the Current Standard of Care
The only approved pharmaceutical targeting sickle cell crisis is hydroxyurea, which is available in both generic and branded formulations. Hydroxyurea, a drug originally developed as an anticancer chemotherapeutic agent, has been approved as a once-daily oral treatment for reducing the frequency of sickle cell crisis and the need for blood transfusions in adult patients with recurrent moderate-to-severe sickle cell crisis. While hydroxyurea has been shown to reduce the frequency of sickle cell crisis in some patient groups, it is not suitable for many patients due to significant toxicities and side effects and is not approved by the FDA for pediatric use. In particular, hydroxyurea can cause a severe decrease in the number of blood cells in a patient's bone marrow, which may increase the risk that the patient will develop a serious infection or bleeding, or that the patient will develop certain cancers. Another potential treatment option for SCD, bone marrow transplant, is limited in its use due
7
to the lack of availability of matched donors and the risk of serious complications, including graft versus host disease, infection and potentially death, as well as by its high cost.
Upon onset of sickle cell crisis, the current standard of care is focused on symptom management. Narcotics are typically used for the management of acute pain associated with sickle cell crisis. Pain management often starts with oral medications taken at home at the onset of pain. However, if the pain is not relieved, or if it progresses, patients may seek medical attention in a clinic setting or emergency department. Pain that is not controlled in these settings may require hospitalization for more potent pain medications, typically opioids administered intravenously. The patient must stay in the hospital to receive these intravenous pain medications until the sickle cell crisis resolves and the pain subsides. Other supportive measures during hospitalization include hydration, supplemental oxygen and treatment of any concurrent infections or other conditions.
According to Hematology in Clinical Practice, by Robert S. Hillman et. al. (5th ed. 2011), sickle cell crisis, once it has started, almost always results in tissue damage at the affected site in the body, increasing the importance of preventative measures. While pain medications can be effective in managing pain during sickle cell crisis, they do not affect or resolve the underlying vascular occlusion, tissue ischemia or potential tissue damage. Additionally, opioid narcotics that are generally prescribed to treat pain can also lead to tissue or organ damage and resulting complications and morbidities, prolonged hospital stays and associated continuation of pain and suffering. Given the duration and frequency of sickle cell crises, addiction to these opioid narcotics is also a significant concern.
Our Solution of a Pharmaceutical Grade L-glutamine Treatment for SCD
Our pharmaceutical grade L-glutamine treatment, if approved, may provide a safe and effective means for reducing the frequency of sickle cell crisis in patients with SCD and reducing the need for costly hospital stays or treatment with opioid narcotics. Published academic research identifies L-glutamine as a precursor to NAD and its reduced form known as NADH. NAD is the major molecule that regulates and prevents oxidative damage in red blood cells. Several published studies have identified that sickle red blood cells have a significantly increased rate of transport of glutamine, which appears to be driven by the cells' need to promote NAD synthesis, protecting against the oxidative damage and thereby leading to further improvement in their regulation of oxidative stress. In turn this made sickle red blood cells less adhesive to cells of the interior wall of blood vessels. This implied that there is decreased chance of blockage of blood vessels especially the small ones. In summary, improved regulation of oxidative stress appears to lead to less obstruction or blockage of small blood vessels, thereby alleviating a major cause of the problems that patients with SCD face.
Several of the studies in which our chief executive officer has participated have been published in peer-reviewed academic research journals. These publications include, "L-glutamine Therapy Reduces Endothelial Adhesion of Sickle Red Blood Cells to Human Umbilical Vein Endothelial Cells" by Yutaka Niihara et al., published in BMC Blood Disorders (2005), "Oral L-glutamine Therapy for Sickle Cell Anemia: I. Subjective Clinical Improvement and Favorable Change in Red Cell NAD Redox Potential" by Yutaka Niihara et al., published in the American Journal of Hematology (1998) and "Increased Red Cell Glutamine Availability in Sickle Cell Anemia: Demonstration of Increased Active Transport, Affinity, and Increased Glutamate Level in Intact Red Cells" by Yutaka Niihara et al., published in the Journal of Laboratory and Clinical Medicine (1997).
In December 2013, we completed a Phase 3 prospective, randomized, double-blind, placebo controlled, parallel-group multicenter clinical trial to measure, over a 48-week time frame, as its primary outcome, the reduction in the number of occurrences of painful sickle cell crises experienced by patients in the trial. This Phase 3 clinical trial enrolled a total of 230 patients across 31 clinical trial sites in the United States. Study participants included adults and children as young as five years of age. All participants other than those who received placebo, including children, received up to 30 grams of
8
pharmaceutical grade L-glutamine treatment daily, dissolved in liquid, split between morning and evening—the same dosage as our Phase 2 clinical trial completed in 2009. Patients were randomized to the study treatment using a 2:1 ratio of L-glutamine to placebo. The randomization was stratified by investigational site and hydroxyurea usage.
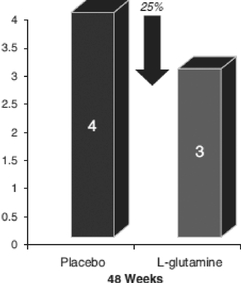
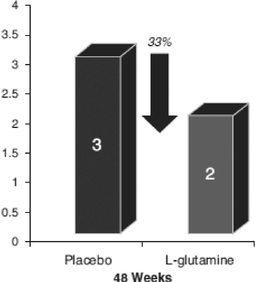
The following charts summarize the results of this Phase 3 clinical trial.
| Median Frequency of Sickle Cell Painful Crises | Median Frequency of Hospitalizations | |
|
|
Primary Endpoint—Reduction in the Frequency of Sickle Cell Crises
For the primary efficacy endpoint of number of sickle cell crises through 48 weeks, there was a trend favoring L-glutamine in the intent-to-treat population with 25% reduction in the median frequency of sickle cell crises (median 3 vs. 4; p=0.063). These results utilized the Cochran-Mantel-Haenszel, or CMH test, controlling for region and hydroxyurea use. The pre-specified p-value for this analysis was 0.045.
Additional sensitivity analyses of the number of sickle cell crises through Week 48 in which the statistical control for region was omitted showed a statistically significantly lower number of sickle cell crises through Week 48 in the L-glutamine group compared to the placebo group (p=0.008). Similarly, when region was included but not hydroxyurea use, a significant effect was seen (p=0.014), and when no covariates were included, a significant effect was also seen (p=0.005) as shown in the table below.
Sensitivity Analysis of Number of Sickle Cell Crises and Impact of Stratification Factors
| |
Statistical Controls Used in Test(1) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Analysis
|
Hydroxyurea use and Region |
Hydroxyurea use only |
Region only | None | |||||||||
Number of Sickle Cell Crises |
0.063 | 0.008 | 0.014 | 0.005 | |||||||||
- (1)
- All p-values are from the CMH test controlling for stratification variables noted.
The above results include imputation of values for patients who dropped out of the trial before reaching 48 weeks. In order to evaluate results without the imputation of values we have performed further sensitivity analyses using a Poisson distribution which showed a statistically significant lower number of sickle cell crises at all of the above levels of stratification.
9
In addition, the severity of a patient's sickle cell crises was observed in our trial. The analyses of such data from the trial showed a statistically significant lower level in the severity of crises (p=0.0167 using the CMH test). Further, the data from the trial showed a statistically significant reduction in the severity of such incidence of ACS.
Another indication of the reduction in the frequency and severity of crises is the reduction in acute chest syndrome, or ACS, which was also part of our sickle cell crises definition. In the study, the incidence of ACS, was found to be significantly lower with the L-glutamine treatment compared to the placebo group experiencing ACS (26.9% of placebo patients compared to 11.9% of patients in the L-glutamine group) (P=0.006, Fisher's Exact test).
Secondary Endpoint—Reduction in the Frequency of Hospitalization
We also assessed the effect of our prescription grade L-glutamine treatment for SCD by evaluating the frequency of hospitalizations for sickle cell pain as a secondary efficacy endpoint. The number of such hospitalizations, a key secondary endpoint, was significantly less, demonstrating a benefit from our prescription grade L-glutamine treatment as compared to placebo through Week 48 (a lower median number of hospitalizations of 2 vs. 3; p=0.041). These results utilized the same CMH test controlling for region and hydroxyurea use as for the primary endpoint.
Sensitivity Analysis of Number of Hospitalizations and Impact of Stratification Factors
| |
Statistical Controls Used in Test(a) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Analysis
|
Hydroxyurea use and Region |
Hydroxyurea use only |
Region only | None | |||||||||
Number of Hospitalizations |
0.041 | 0.005 | 0.012 | 0.006 | |||||||||
- (a)
- All p-values are from the CMH test controlling for stratification variables noted.
Further analyses
Further analyses revealed that the observed treatment effect was consistent across regions, prior hydroxyurea use, sex and age group.
Additional analyses were conducted to look at two key indicators which support the relevance of the efficacy findings. All such results demonstrated a benefit from our prescription grade L-glutamine treatment as compared to placebo:
- •
- the median number of days before the first sickle cell crisis was longer in the L-glutamine group (87 days) compared to the
placebo group (54 days), a difference that was statistically significant (p=0.010); and
- •
- the median number of days in hospital was statistically significantly shorter in the L-glutamine group (6.5 days) compared to the placebo group (11 days) (p=0.022).
The analysis of subgroups within the trial for efficacy showed consistent results demonstrating the benefit of our prescription grade L-glutamine treatment as compared to placebo as described in the paragraphs below.
Efficacy across region: Subgroup analyses for efficacy as measured by the primary endpoint within regions were significant favoring L-glutamine for the number of sickle cell crises through Week 48 in two of the five regions: Northeast (median of 3 vs. 4) and West (median of 3 vs. 5). These two regions represented 48% of the patients. Two other regions favored L-glutamine, but the between-group differences were not statistically significant: South Atlantic (median of 3 vs. 4) and South Central (median of 3 vs. 4). These two regions represented 41% of the patients. The Midwest region, representing only 11% of the patients, showed no difference (median of 4 vs. 4). The number of hospitalizations for sickle cell pain showed very similar findings in both the between-group differences and statistical significance.
10
Efficacy across hydroxyurea use: Subgroup analysis for efficacy across prior hydroxyurea use showed a trend favoring L-glutamine in the number of sickle cell crises through Week 48. The number of hospitalizations for sickle cell pain showed similar results with significance found in the prior hydroxyurea subgroup (median of 2 vs. 3). The no prior hydroxyurea subgroup showed a trend favoring L-glutamine in both the number of sickle cell crises (median of 3 vs. 4) and the number of such hospitalizations (median of 2 vs. 3) through Week 48.
Efficacy across sex: Subgroup analysis for efficacy across sex showed a lower median number of sickle cell crises through Week 48 in the L-glutamine group as compared to the placebo group for both males and females (medians of 3 vs. 4 for both groups). The median number of hospitalizations for sickle cell pain was also lower in the L-glutamine group for both males and females (2 vs. 3 for both groups).
Efficacy across age: Subgroup analysis for efficacy across age (adults vs. children) showed a lower median number of sickle cell crises through Week 48 in the L-glutamine group as compared to the placebo group (3 vs. 4 for both groups). The median number of hospitalizations for sickle cell pain showed similar results in favor of L-glutamine (2 vs. 3 for both groups).
Regarding safety, adverse events, or AEs, were reported for 98% of patients in the L-glutamine group and 100% of the placebo group. Most AEs were mild or moderate (73.5% in the L-glutamine group, 83.4% in the placebo group). As expected in this disease population, the most common AE was sickle cell anemia with crisis, which was reported for 81.5% of patients in the L-glutamine group and 91.0% of patients in the placebo group. Other notable differences between the treatment groups (difference of about 10 percentage points or more) all favored L-glutamine treatment and included acute chest syndrome (11.9% L-glutamine, 26.9% placebo). Patients in both groups had AEs that were considered possibly related to the study medication (21.2% L-glutamine, 15.4% placebo). The majority of the possibly related AEs were due to gastrointestinal disorders.
Serious adverse events, or SAE, were reported for 78.1% of patients in the L-glutamine group and 87.2% of patients in the placebo group. The most common SAE was sickle cell crisis which was reported in fewer patients treated with L-glutamine (67.5% L-glutamine, 80.8% placebo).
In summary our trial data indicates that our pharmaceutical grade L-glutamine treatment achieved:
- •
- a 25% reduction in the median number of sickle cell crises;
- •
- a 33% reduction in the median number of sickle cell-related hospitalizations;
- •
- a 41% reduction in the median number of sickle cell-related cumulative hospital days;
- •
- a 56% lower reported incidence of sickle cell-related acute chest syndrome; and
- •
- and increase in the median number of days before the first sickle cell crisis from 54 to 87 days
Regulatory Status of L-glutamine for SCD
On June 11, 2014, we met with the FDA to discuss the preliminary data from our Phase 3 clinical trial and our plans to submit to the FDA a NDA, based on our single Phase 3 clinical trial. In minutes of the meeting that the FDA has provided to us, based on its review of preliminary data from our Phase 3 clinical trial, the FDA expressed concern that the primary endpoint of painful sickle cell crisis of 0.063 (controlling for both region and hydroxyurea use) did not reach the pre-specified p-value significance level of 0.045.
The FDA commented that the primary efficacy results were inconsistent among regions, as shown by the large difference in results observed based on the stratified analyses adjusted for both region and hydroxyurea use and the stratified analysis adjusted for hydroxyurea use only. The FDA also commented that a reduction in the median number of painful sickle cell crises by one (from four to three) is not clinically meaningful and is not consistent across regions. The FDA asked us to provide
11
explanations for the differences in results observed based on the stratified analysis adjusted for region and hydroxyurea.
The FDA also commented on the number of patients who discontinued the study, which was greater in the L-glutamine group, and the FDA would need to understand what impact the imputation of such data, which is a method of accounting for missing information, would have on the interpretation of the results.
Based on preliminary data, the FDA recommended a second Phase 3 study be conducted to support the indication for SCD and suggested enrolling patients with a higher baseline level of sickle cell crises to help demonstrate a larger difference in the mean results.
Based on other questions we submitted to the FDA regarding the previous pre-clinical work and evidence gathered for the L-glutamine treatment related to pharmacology, toxicology, and clinical pharmacology, the FDA noted that the information provided appeared to be acceptable; however, final affirmations regarding such matters would be made during the FDA's review of a NDA. The FDA also made certain recommendations to the chemistry, manufacturing and controls of our pharmaceutical grade L-glutamine treatment for SCD which we believe we will be able to successfully incorporate or address.
In September 2014 we submitted to the FDA the Clinical Study Report for our Phase 3 clinical trial, supplemental analyses and a new meeting request. Consequently, we met again with the FDA on October 15, 2014. In the minutes of the October 15 meeting, the FDA noted that it continued to be concerned that the primary endpoint did not meet the pre-specified p-value and observed that our efficacy finding would be more persuasive if supported by an additional, confirmatory study. We communicated to the FDA that over-stratification in our statistical analysis plan led to the pre-specified p-value not being met. We also provided sensitivity analyses and results to address the over stratification which demonstrated a statistically significant p-value for the primary endpoint.
At the October 15 meeting, in response to a question raised by the FDA at the June 11 meeting, we provided evidence of the clinical meaningfulness of a reduction in the median number of painful sickle cell crises by one (from four to three). In the October 15 minutes the FDA expressed the view that the reduction of one sickle cell crises may not be clinically significant and advised us to provide all information and data to support this clinical meaningfulness and the FDA stated they will evaluate the overall clinical benefit and risk of L-glutamine based on results from our Phase 3 trial and all available safety data.
In the October 15 minutes the FDA reiterated certain concerns outlined in the June 11 minutes. Other questions that we posed for discussion at the October 15 meeting related to the safety of L-glutamine use, the benefit-risk margin for L-glutamine use, the sufficiency of our Phase 2 and 3 trials to support a NDA submission and whether a confirmatory study could be conducted as a post-marketing commitment. The FDA noted that its decisions regarding these questions would be made after the submission of a NDA.
Based on our discussions with the FDA, we intend to conduct a confirmatory study of our L-glutamine treatment for SCD. We submitted a draft protocol outline to the FDA. We plan to work with the FDA to finalize the study design.
In addition to planning and conducting a confirmatory study, we plan to submit a NDA for our L-glutamine treatment for SCD during 2015 containing all efficacy and safety data through our completed Phase 3 trial and additional analyses and information in accord with the feedback the FDA provided in the context of the June 11 and October 15 meetings. We will request that our planned confirmatory study be accepted by the FDA as a post-approval study that is, a study to be completed after approval of the NDA.
12
There can be no assurance that the FDA will accept our submission for filing before the completion of a confirmatory study or that the information and data in the NDA will satisfy the concerns identified by the FDA.
Regarding the submission of NDAs that include only one Phase 3 clinical trial, the FDA has in some cases accepted evidence from one clinical trial to support a finding of substantial evidence of effectiveness. A change in the law under the U.S. Food and Drug Administration Modernization Act of 1997, or Modernization Act, made clear that the FDA may consider data from only one adequate and well controlled clinical investigation and confirmatory evidence if the FDA determines that such evidence is sufficient to establish effectiveness of the medicine under study.
In a guidance document titled "Providing Clinical Evidence of Effectiveness for Human Drug and Biological Products" (May 1998), the FDA stated that reliance on a single clinical trial is generally limited to situations in which a trial has demonstrated a clinically meaningful effect on mortality, irreversible morbidity, or prevention of a disease with a potential serious outcome, and where confirmation of the result in a second trial would be impractical or unethical. The factors the FDA considers for accepting a single clinical trial include, but are not limited to, having large multi centered studies, consistent data across subgroups, multiple endpoints, and statistically very persuasive findings.
Sales and Marketing Plans for our SCD Product Candidate
We plan to work with strategic partners to market our SCD product candidate on a worldwide basis. This effort could lead to a global strategic partner or a group of regional partners. This would permit us to access established sales, marketing and regulatory organizations to sell, distribute and provide regulatory compliance for our product sales. This would also permit us to focus our resources on the development of future pipeline products.
Although we have started a formal process, if we are not able to find a strategic partner on acceptable terms for the United States market, subject to FDA approval of our pharmaceutical grade L-glutamine treatment for SCD, we intend to build a focused sales and marketing force of approximately 30 sales representatives to commercialize this product in the United States. We intend to focus our sales and marketing efforts across several different groups, including patients, physicians, health care providers, hospitals, treatment centers, insurance carriers, non-profit associations, and potentially, collaborating pharmaceutical or biotechnology companies. Our in-house product specialists and sales representatives will focus on the following tasks as part of our marketing strategy:
- •
- promote our pharmaceutical grade L-glutamine treatment for SCD to SCD specialist physicians and key opinion leaders;
- •
- starting with our 31 clinical trial sites, promote awareness of our pharmaceutical grade L-glutamine treatment for SCD at all U.S.
community-based treatment centers;
- •
- develop collateral materials and informational packets about our pharmaceutical grade L-glutamine treatment for SCD to educate
patients and physicians and garner industry support;
- •
- establish collaborative relationships with non-profit organizations and patient advocacy groups that focus on SCD; and
- •
- identify license partners and other international opportunities to commercialize our pharmaceutical grade L-glutamine treatment for SCD, if approved by international regulatory authorities.
Currently Marketed Products
We currently market two L-glutamine-based products, NutreStore and AminoPure, in the United States and certain other territories. We generate limited revenues from the sale of these products, which we consider to be non-core operations.
13
NutreStore is our FDA-approved prescription L-glutamine powder for oral solution for the treatment of SBS in conjunction with an approved recombinant human growth hormone and other customary SBS management. Patients with SBS have had half or more of their small intestine surgically removed or have a poorly functioning small intestine due to inflammatory bowel disease. These patients cannot adequately absorb nutrition through their small intestine and thus require long-term intravenous nutrition, which is expensive, inconvenient, and poses significant infection risk. As cited in the NutreStore label, after four weeks of treatment with NutreStore, the patients enrolled in the Phase 3 trial showed:
- •
- Reduced mean frequency of intravenous nutrition in days per week from 5.4 to 1.2;
- •
- Reduced mean intravenous nutritional volume in liters per week from 10.5 to 2.9; and
- •
- Reduced mean intravenous nutritional calories per week from 7,895 to 2,144.
NutreStore is distributed through local treating medical centers and physicians. We also provide the product to the U.S. Department of Veterans Affairs, U.S. Department of Defense, U.S. Coast Guard and Public Health Service (Indian Health Service).
AminoPure is our dietary supplement L-glutamine, sold through our indirect wholly-owned subsidiary, Newfield Nutrition Corporation. AminoPure is currently sold in several U.S. states, and we export the product to Japan, Taiwan and South Korea. AminoPure is subject to regulation under the Dietary Supplement Health and Education Act of 1994.
CellSeed Collaboration
In April 2011, the Company entered into a Research Agreement and an Individual Agreement with the Japanese company CellSeed, Inc., or CellSeed, and, in August 2011, entered into an addendum to the Research Agreement. Pursuant to the Individual Agreement, CellSeed granted the Company the exclusive right to manufacture, sell, market and distribute Cultured Autologous Oral Mucosal Epithelial Cell Sheet, or CAOMECS, for the treatment of corneal impairments in the United States, which we would be able to exercise only after receiving FDA marketing approval for the product, and agreed to disclose to the Company its accumulated information package for the joint development of CAOMECS. Under the Individual Agreement, the Company agreed to pay CellSeed $1.5 million, which it paid in February 2012. The technology acquired under the Individual Agreement is being used to support an ongoing research and development project and management believes the technology has alternative future uses in other future development initiatives.
Pursuant to the Research Agreement, the Company and CellSeed formed a relationship regarding the future research and development of cell sheet engineering regenerative medicine products, and the future commercialization of such products. Under the Research Agreement, as supplemented by the addendum, the Company agreed to pay CellSeed $8.5 million within 30 days of the completion of all of the following: (i) the execution of the Research Agreement; (ii) the execution of the Individual Agreement; and (iii) CellSeed's delivery of the accumulated information package, as defined in the Research Agreement, to the Company and the Company providing written confirmation of its acceptance of the complete Package, which has not yet been completed as of March 31, 2015.
A cell sheet is a composite of cells grown and harvested in an intact sheet, rather than as individual cells. These cell sheets can be used for tissue transplantation. CellSeed's technology involves culturing cells on a surface coated with a temperature-responsive polymer. The thinness of this polymer coating is measured at the nanometer scale. The cells cultured on this polymer can be harvested intact as a composite cell sheet. Using a patient's own oral mucosal cells, we are working toward being able to grow and harvest a cell sheet for directly transplanting onto the cornea of the patient's affected eye to repair the damaged cornea.
14
Our lead CAOMECS program is for treatment of corneal diseases. CAOMECS products are in preclinical development and have not been approved for marketing in the United States or any jurisdiction. The development of therapeutic products based on this cell sheet technology is in its early stages. We are not aware that cell sheets of the type that CellSeed and we are developing for treating corneal and other diseases are currently being used or sold by any third parties. The potential market for the corneal cell sheet products that CellSeed and we are developing includes patients with damaged corneas, which we believe represents a small percentage of the approximately 40,000 corneal transplants in the United States performed each year. The principal steps to development of a corneal cell sheet product include engaging a manufacturer compliant with applicable current cGMP regulations and sufficient manufacturing capacity, conducting preclinical studies and human clinical trials, obtaining FDA approval of the product, training physicians who will use the product and perform procedures with the product, and marketing the product.
Under the Individual Agreement with CellSeed, we have the exclusive rights to manufacture, sell, market and distribute cell sheets for treating corneal disease in the United States. Since 2011, an Emmaus-led team at LA BioMed has been conducting preclinical studies on corneal cell sheet technology. Subject to filing an investigational new drug application, or IND, that the FDA allows to become effective, we are preparing to begin our first clinical studies with human participants. We currently intend, if our clinical studies are successful, to file with the FDA a Biological License Application, or BLA, for this product. Based on the current status of our research and development efforts relating to this technology, we anticipate it will be four to five years or longer before we would be able to submit and obtain FDA approval of a BLA that would allow us to begin to commercialize this product in the United States. If the product is approved for marketing, we plan to build a cGMP level facility as part of our U.S. commercialization plan for this technology.
We estimate that the cost to develop products based on corneal cell sheet technology in the United States will be approximately $3.0 million, in addition to the $8.5 million fee payable to CellSeed under the Research Agreement. This estimate includes the anticipated cost of obtaining FDA approval for the corneal cell sheets and assumes that we will need the FDA to approve a BLA for the corneal cell sheets, rather than a NDA. We estimate that we will need another $2.0 million to commercialize any approved products based on corneal cell sheet technology.
Raw Materials and Manufacturing
Our SCD treatment uses pharmaceutical grade L-glutamine. This differs from non-pharmaceutical grade L-glutamine available as a nutritional supplement. The manufacturing of large quantities of pharmaceutical grade L-glutamine is a complex and expensive undertaking, which we believe discourages entry of third parties into the market. As a result of these challenges, there are limited alternative suppliers from which we can obtain the pharmaceutical grade L-glutamine required to manufacture our current products and our SCD treatment product under development.
We currently obtain, and plan to continue to obtain, our pharmaceutical grade L-glutamine from Ajinomoto North America, Inc., a subsidiary of Ajinomoto U.S.A. referred to as Ajinomoto, a Japanese food, amino acid and pharmaceutical company, and from Kyowa Hakko Bio Co., Ltd., a Japanese pharmaceutical company referred to as Kyowa. Ajinomoto and Kyowa together produce the majority of pharmaceutical grade L-glutamine approved for sale in the United States.
Ajinomoto has provided pharmaceutical grade L-glutamine to us free of charge for our clinical work, including our completed Phase 2 and Phase 3 clinical trials. Pursuant to a letter of intent between Emmaus Medical and Ajinomoto, we agreed to purchase or cause relevant third party purchasers to purchase from Ajinomoto all of the L-glutamine that we will need for our commercial products. Pursuant to the letter of intent, we will be permitted to source pharmaceutical grade L-glutamine from third party suppliers for up to 10% of our requirement for L-glutamine on a back-up
15
basis. We also currently source pharmaceutical grade L-glutamine from Kyowa for our NutreStore product.
Eventually we plan to enter into exclusive long term supply contracts with these manufacturers for pharmaceutical grade L-glutamine for our SCD treatment that will require these companies to agree not to sell L-glutamine as a nutritional supplement or pharmaceutical for SCD applications. We do not currently have long term supply contracts with these manufacturers for L-glutamine or any other compound. As such, there is no assurance we will be able to obtain agreements for obtaining pharmaceutical grade L-glutamine from these proposed suppliers on terms acceptable to us, or on an exclusive basis, or that these suppliers will not experience an interruption in supply that could materially and adversely affect our business.
Our commercial products must be packaged by a facility that meets FDA requirements for cGMP. Packaging Coordinators, Inc., or PCI, of Rockville, Illinois, has handled the packaging for our Phase 2 and Phase 3 clinical trials of our pharmaceutical grade L-glutamine treatment for SCD, and we plan to use the same company for commercial packaging of the product, if approved. PCI packaged L-glutamine for the clinical trials that resulted in the FDA's marketing approval for L-glutamine for SBS using the same dosage and packaging protocol as we expect to use for the treatment of SCD. Previous compliance with cGMP requirements for the packaging of pharmaceutical products, however, does not guarantee the ability to maintain cGMP compliance for the packaging of pharmaceutical products in the future.
Facilities
We lease office space under operating leases from unrelated entities. The rent expense during the years ended December 31, 2014 and 2013 amounted to $277,866 and $137,147, respectively.
We lease approximately 13,329 square feet of headquarters offices in Torrance, California, at a base rental of $32,505 per month. The lease relating to this space expires on February 28, 2019. We lease an additional office suite in Torrance, California at a base rent of $1,750 per month for 1,400 square feet. The lease relating to this space expires on February 19, 2016.
In addition, EM Japan leases 1,322 total square feet of office space in Tokyo, Japan and 1,313 square feet of office space in Osaka, Japan. The leases relating to this space will expire on September 30, 2016 and February 19, 2016, respectively. Our existing facilities are adequate for our operations at this time and we expect to be able to renew our headquarters office lease on commercially reasonable terms. In the event we determine that we require additional space to accommodate expansion of our operations, we believe suitable facilities will be available in the future on commercially reasonable terms as needed.
Employees
As of December 31, 2014, we had 22 employees, 17 of whom are full time, and we retained three consultants. We have not experienced any work stoppages and we consider our relations with our employees to be good.
Competition
The biopharmaceutical industry is highly competitive and subject to rapid and significant technological change. While we believe that our development experience and scientific knowledge provide us with competitive advantages, we face potential competition from both large and small pharmaceutical and biotechnology companies, academic institutions, governmental agencies (such as the National Institutes of Health) and public and private research institutions. In comparison to us, many of the entities against whom we are competing, or against whom we may compete in the future, have significantly greater financial resources and expertise in research and development, manufacturing,
16
preclinical testing, conducting clinical trials, obtaining regulatory approvals, and marketing approved products. Smaller or early stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies.
Mergers and acquisitions in the pharmaceutical and biotechnology industries may result in increasing concentration of resources among a smaller number of our competitors. These competitors compete with us in recruiting and retaining qualified scientific and management personnel and establishing clinical trial sites and patient registration for clinical trials, as well as in acquiring technologies complementary to, or necessary for, our product development programs.
Any product candidates that we successfully develop and commercialize will compete with existing therapies and new therapies that may become available in the future. The key competitive factors affecting the success of each of our product candidates, if approved, are likely to be their safety, efficacy, convenience, price, the level of proprietary and generic competition, and the availability of coverage and reimbursement from government and other third-party payors. Our commercial opportunity could be reduced or eliminated if our competitors develop and commercialize products that are safer or more effective, have fewer or less severe side effects, or are more convenient or less expensive than any products that we may develop. Our competitors may also obtain FDA or other regulatory approval for their products more rapidly than we may obtain approval for ours, which could result in their establishing a strong market position before we are able to enter the market.
Sickle Cell Disease
Our pharmaceutical grade L-glutamine treatment for SCD is being developed as a therapy to reduce the frequency of sickle cell crisis in patients with SCD. The only approved drug targeting a reduction in the frequency of sickle cell crisis is hydroxyurea, which is available in both generic and branded formulations. While hydroxyurea has been shown to reduce the frequency of sickle cell crisis in some patient groups, it is not suitable for all patients because it can have significant toxicities and side effects. Additionally, hydroxyurea has not been approved by the FDA for pediatric use in SCD patients.
There is a high level of interest in SCD and we understand several academic centers and pharmaceutical companies are researching new treatments and therapies for SCD. There are studies underway testing different compounds that target various aspects of SCD pathophysiology. We are aware of two studies targeting the reduction or duration of vaso occlusive crisis events in patients with SCD which are currently in Phase 3 clinical trials sponsored by Eli Lilly and Mast Therapeutics, Inc. In addition, GlycoMimetics Inc. has announced completion of its Phase 2 clinical trial evaluating GMI-1070, a pan-selectin inhibitor that it is developing in collaboration with Pfizer, Inc.
We are also aware of efforts to develop cures for SCD through approaches such as bone marrow transplant and gene therapy. Although bone marrow transplant is currently available for SCD patients, its use is limited by the lack of availability of matched donors and by the risk of serious complications, including graft versus host disease and infection. Attempts to develop a cure through gene therapy remain at an early stage, but if these attempts were to succeed and receive regulatory approval, this could limit the market for a product such as our L-glutamine product candidate.
L-glutamine is marketed and sold without a prescription as a nutritional supplement. Although our L-glutamine treatment for SCD requires pharmaceutical grade L-glutamine, which we believe offers a more consistent quality and purity profile than non-pharmaceutical grade L-glutamine sold as a nutritional supplement, our L-glutamine product candidate for the treatment for SCD may compete with non-pharmaceutical grade alternative sources of L-glutamine. If our L-glutamine treatment for SCD is approved, we expect that it will be priced at a significant premium over non-prescription L-glutamine products.
17
Oral Mucosa Epithelial Cell Sheet
The development of regenerative medicine products using cell sheet technology is in the early stages. Although there are many academic centers and biotechnology companies working on regenerative medicine in various fields, we are not aware of any treatments using cell sheet technology that have been approved by the FDA. Additionally, we are not aware of any other biotechnology companies in the United States who are currently working to develop products based on cell sheet technology. We are, however, aware of academic centers and biotechnology companies that are researching stem cells in various forms, including in cell sheets, with potential applications for the treatment of limbal stem cell deficiency, or LSCD, a disease of the cornea.
For example, two academic centers outside the United States researching the transplantation of cells as a treatment for LSCD are the Centre Hospitalier National d'Ophtalmologie des Quinze-Vingts located in Paris, France, who we believe is conducting Phase 2 clinical trials evaluating the survivability of transplanted epithelium, and the Instituto Universitario de Oftalmobiología Aplicada located in Valladolid, Spain, who we believe has completed Phase 3 clinical trials looking at the viability and safety of mesenchymal stem cell transplants but the study results are not published. We are also aware of Holostem Terapie Avanzate, an Italian biotechnological company, who we believe is working with autologous cultures of limbal stem cells for corneal regeneration and restoration of visual acuity in patients with severe corneal chemical and thermal burns associated with total unilateral or severe bilateral LSCD. Holostem Terapie Avanzate has received conditional marketing approval from the European Commission for its therapy based on autologous stem cells for patients with severe cornea damage.
Currently, the standard of care for LSCD patients is the treatment of symptoms. This treatment may include use of artificial tears, topical cyclosporine or topical steroids. In more advanced cases, the treatment plan will likely include surgery. The initial surgical interventions may include management of eyelid positioning, insertion of small plugs into the openings in the eye that allows tears to drain or partially sew the eyelids together to protect the cornea prior to considering transplantation of healthy limbal tissue using either cultured cells or whole tissue grafts. The source of the transplanted tissue can be from the patient's own cells from their healthy eye, matched living donors, or cadavers. Similar to other transplantations, there is the risk of serious complications, including graft versus host disease when not using the patient's own tissue.
In regenerative medicine and cell-based therapy, cell transplantation success not only depends on the cells, but also on the carrier/scaffold used, as it is not possible to graft separate cells in a suspension. Biodegradable polymers were the key technology for the first generation of cell therapy. Tremendous efforts have been made since to develop biofilms, acellular matrix (blood products e.g.; fibrin gel, amniotic membranes, etc.) to carry and deliver the cells to the target site. However, the risks of infection due to blood products or biomaterials cannot be completely denied. It has been repeatedly reported that decomposition of biodegradable transplanted scaffolds used for cell transplantations caused inflammation, foreign body reaction and cell damage. The use of an innovative temperature-responsive culture surface vessel technology eliminates these issues and, for the first time, offers the possibility to culture and engineer any type of cells to safely transplant cell-sheets to target organs for regenerative purpose, drug delivery, or tissue replacement.
This cell-sheet-based regenerative medicine technology is advanced, simple and has already shown dramatic results in pilot studies in Japan and Europe. An important feature of this novel and innovative cell sheet therapy is that harvested cell-sheets retain intact basal membranes and intact extracellular matrix (fibronectin, laminin, collagen type IV), eliminating the inherent risks of suture during transplantation. Another innovative feature this cell sheet therapy is that the construction of multiple layered cell sheets is made possible during the culture process and that cell sheet harvesting is
18
achieved without harmful enzymes use (trypsin or dispase) that may damage the cell-based therapeutic potential.
This technology has the unique potential to construct in vitro stratified tissue equivalents by alternately layering different harvested cell sheets to provide regenerated tissue architectures. This novel technique thus holds promise for the study of cell-cell communications and angiogenesis in reconstructed, three-dimensional environments, as well as for tissues engineering with complex, multicellular architectures.
Government Regulation
In 2001, the FDA granted Orphan Drug designation to L-glutamine for the treatment of SCD. In 2005, the FDA granted Fast Track designation to our clinical study program of L-glutamine for treating SCD. The FDA also approved, in 2004, our NDA for our L-glutamine product for the treatment of SBS. In addition, in July 2012, the European Commission, or EC, granted Orphan Drug designation to L-glutamine for the treatment of SCD. We describe below the significance of these designations and of data exclusivity under the Hatch/Waxman Act.
Orphan Drug Designation. The FDA has authority under the U.S. Orphan Drug Act to grant Orphan Drug designation to a drug or biological product intended to treat a rare disease or condition. This law defines a rare disease or condition generally as one that affects fewer than 200,000 individuals in the United States, or more than 200,000 individuals in the United States and for which there is no reasonable expectation that the cost of the development and distribution of the orphan product in the United States will be recovered from sales of the product. Being granted Orphan Drug designation provides tax benefits to mitigate expenses of developing the orphan product. More importantly, Orphan Drug designation provides seven years of market exclusivity if the product receives the first FDA approval for the disease or condition for which it was granted such designation and the indication for which approval is granted matches the indication for which Orphan Drug designation was granted. During the seven year exclusivity period, Orphan Drug exclusivity precludes FDA approval of a marketing application for the same product for the same indication. Orphan Drug exclusivity is limited and will not preclude the FDA from approving the same product for the same indication if the same product is shown to be clinically superior to the product previously granted exclusivity. For example, if the same product for the same indication is shown to have significantly fewer side effects, the FDA may approve the second product despite the Orphan Drug exclusivity granted to the first product. In addition, a product that is the same as the orphan product may receive approval for a different indication (whether orphan or not) during the exclusivity period of the orphan product. Also, Orphan Drug market exclusivity will not bar a different product intended to treat the same orphan disease or condition from obtaining its own Orphan Drug designation and Orphan Drug exclusivity. Orphan Drug status in the European Union has similar, but not identical, benefits, which includes a ten year Orphan Drug exclusivity period.
Fast Track Designation/Priority Review. The FDA has authority under the U.S. Food, Drug, and Cosmetic Act, or the FD&C Act, to designate for "Fast Track" review new drugs and biologics that are intended to treat a serious or life-threatening condition and demonstrate the potential to meet an unmet medical need for the condition. Fast Track designation applies to the combination of the product and the specific indication for which it is being studied. A product candidate that receives Fast Track designation is eligible for some or all of the following: more frequent meetings with the FDA to discuss the development plan for the product candidate and ensure collection of appropriate data needed to support marketing approval; more frequent written correspondence from the FDA about the development of the product candidate; the ability, if agreed to by the FDA, to submit a NDA on a rolling basis; and, if certain criteria are met, eligibility for Priority Review. These criteria for Priority Review include whether, if approved, the resulting product would provide significant improvements in the safety or effectiveness of the treatment, diagnosis, or prevention of serious conditions caused by a
19
disease when compared to standard treatment, diagnosis, or prevention of those conditions. Under Priority Review of a NDA, assuming that there are no requests from the FDA for additional information, the FDA's goal is to take action on the NDA within six months (compared to 10 months under standard review) after it is accepted for review, with the review clock starting at the time of submission. Requests for Priority Review of a NDA for the applicable product candidate must be submitted to the FDA when the NDA is submitted.
505(b)(2) Applications. Under Section 505(b)(2) of the FD&C Act, a person may submit a NDA for which one or more of the clinical studies relied upon by the applicant for approval were not conducted by or for the applicant and for which the applicant does not have a right of reference or use from the person by or for whom the clinical studies were conducted. Instead, a 505(b)(2) applicant may rely on published literature containing the specific information (e.g., clinical trials, animal studies) necessary to obtain approval of the application. The 505(b)(2) applicant may also rely on the FDA's finding of safety and/or effectiveness of a drug previously approved by the FDA when the applicant does not own or otherwise have the right to access the data in that previously approved application. The 505(b)(2) pathway to market thus allows an applicant to submit to the FDA a NDA without having to conduct its own studies to obtain data that are already documented in published reports or previously submitted NDAs. In addition to relying on safety data from our previously approved drug product, NutreStore, we intend to take advantage of the 505(b)(2) pathway to the extent published literature will further support our new drug marketing application.
Hatch/Waxman Data Exclusivity. Under the Drug Price Competition and Patent Term Restoration Act of 1984, or the Hatch/Waxman Act, a three-year period of data exclusivity is granted for a drug product that contains an active moiety that has been previously approved, when the application contains reports of new clinical investigations (other than bioavailability studies) conducted or sponsored by the sponsor that were essential to approval of the application. When granted to a sponsor, data exclusivity under the Hatch/Waxman Act prevents any third parties, such as generic drug manufacturers, from relying on or using the sponsor's data from the sponsor's new clinical investigations in order to obtain marketing approval for the same active moiety and indication for which the sponsor's NDA was approved. The FDA defines "new clinical investigation" as a "clinical study in humans the results of which have not been relied upon by FDA to demonstrate substantial evidence of effectiveness of a previously approved drug product for any indication or of safety for a new patient population and do not duplicate the results of another investigation that was relied upon by the agency to demonstrate the effectiveness or safety in a new patient population of a previously approved drug product." Our pharmaceutical grade L-glutamine drug product contains the same active moiety contained in our previously approved NutreStore drug product and we believe our Phase 3 clinical study meets the definition of a new clinical investigation for purposes of satisfying the requirements for Hatch/Waxman data exclusivity to be granted. Therefore, if approved, we anticipate receiving three years of data exclusivity under the Hatch/Waxman Act for our pharmaceutical grade L-glutamine treatment for SCD. These three years of data exclusivity would run concurrently with any other market exclusivity we may receive, as well as concurrently with any remaining patent term protection. However, the three-year exclusivity provided under the Hatch/Waxman Act would bar the approval of the same product for the same indication, even if the same product demonstrated clinical superiority. Thus, when running concurrently with Orphan Drug exclusivity, clinical superiority would not be sufficient to allow the FDA to approve a third party product during the first three years of Orphan Drug exclusivity. Similar to Orphan Drug exclusivity, the three-year exclusivity provided under Hatch/Waxman would not bar the FDA from approving another L-glutamine product for another indication, nor would it bar the FDA from approving a different active moiety to treat the same indication.
Regulation by United States and foreign governmental authorities is a significant factor in the development, manufacture and expected marketing of our product candidates and in our ongoing
20
research and development activities. The nature and extent to which such regulation will apply to us will vary depending on the nature of the product candidates we seek to develop.
In particular, human therapeutic products, such as drugs, biologics and cell-based therapies, are subject to rigorous preclinical and clinical testing and other preapproval requirements of the FDA and similar regulatory authorities in other countries. Various federal and state statutes and regulations govern and influence pre- and post-approval requirements related to research, testing, manufacturing, labeling, packaging, storage, distribution and record-keeping of such products to ensure the safety and effectiveness for their intended uses. The process of obtaining marketing approval and ensuring post-approval compliance with the FD&C Act for drugs and biologics (and applicable provisions of the Public Health Service Act for biologics), and the regulations promulgated thereunder, and other applicable federal and state statutes and regulations, requires substantial time and financial resources. Any failure by us or our collaborators to obtain, or any delay in obtaining, marketing approval could adversely affect the marketing of any of our product candidates, our ability to receive product revenues, and our liquidity and capital resources.
The manufacture of these products is subject to cGMP regulations. The FDA inspects manufacturing facilities for compliance with cGMP regulations before deciding whether to approve a product candidate for marketing. If the facility in which L-glutamine is manufactured is not ready for inspection at the time the FDA seeks to inspect it, or cGMP deficiencies are found upon such inspection, the FDA's approval of our NDA for our L-glutamine treatment for SCD could be delayed unless and until the facility is inspected and no deficiencies in need of correction prior to approval are identified or, if so identified, such deficiencies are corrected.
The steps required by the FDA before a new product, such as a drug, biologic, or cell-based therapy, may be marketed in the United States include:
- •
- completion of preclinical studies (during this stage, the treatment is called a development candidate);
- •
- the submission to the FDA of a proposal for the design of a clinical trial program for studying in humans the safety and effectiveness
of the product candidate. This submission is referred to as an investigational new drug application, or IND. The FDA reviews the IND to ensure it adequately protects the safety and rights of trial
participants and that the design of the studies are adequate to permit an evaluation of the product candidate's safety and effectiveness. The IND becomes effective within thirty days after the FDA
receives the IND, unless the FDA notifies the sponsor that the investigations described in the IND are deficient and cannot begin;
- •
- the conduct of adequate and well-controlled clinical trials, usually completed in three phases, to demonstrate the safety and
effectiveness of the product candidate for its intended use;
- •
- the submission to the FDA of a marketing application, a NDA, if the product candidate is a drug, that provides data and other
information to demonstrate the product is safe and effective for its intended use, or a BLA, if the product candidate is a biologic that provides data and other information to demonstrate that the
product candidate is safe, pure, and potent; and
- •
- the review and approval of the NDA or BLA by the FDA before the product candidate may be distributed commercially as a product.
In addition to obtaining FDA approval for each product candidate before we can market it as a product, the manufacturing establishment from which we obtain it must be registered and is subject to periodic FDA post-approval inspections to ensure continued compliance with cGMP requirements. If, as a result of these inspections, the FDA determines that any equipment, facilities, laboratories, procedures, or processes do not comply with applicable FDA regulations and the conditions of the product approval, the FDA may seek civil, criminal, or administrative sanctions and/or remedies against
21
us, including the suspension of the manufacturing operations, recalls, the withdrawal of approval and debarment. Manufacturers must expend substantial time, money and effort in the area of production, quality assurance and quality control to ensure compliance with these standards.
Preclinical testing includes laboratory evaluation of the safety of a product candidate and characterization of its formulation. Preclinical testing is subject to Good Laboratory Practice, or GLP, regulations. Preclinical testing results are submitted to the FDA as a part of an IND which must become effective prior to commencement of clinical trials. Clinical trials are typically conducted in three sequential phases following submission of an IND. In Phase 1, the product candidate under investigation (and therefore often called an investigational product) is initially administered to a small group of humans, either patients or healthy volunteers, primarily to test for safety (e.g., to identify any adverse effects), dosage tolerance, absorption, distribution, metabolism, excretion and clinical pharmacology, and, if possible, to gain early evidence of effectiveness. In Phase 2, a slightly larger sample of patients who have the condition or disease for which the investigational product is being studied receive the investigational product to assess the effectiveness of the investigational product, to determine dose tolerance and the optimal dose range, and to gather additional information relating to safety and potential adverse effects. If the data show the investigational product may be effective and has an acceptable safety profile in the targeted patient population, Phase 3 studies, also referred to as pivotal studies or enabling studies, are initiated to further establish clinical safety and provide substantial evidence of the effectiveness of the investigational product in a broader sample of the general patient population, to determine the overall risk-benefit ratio of the investigational product, and provide an adequate basis for physician and patient labeling. During all clinical studies, Good Clinical Practice, or GCP, standards and applicable human subject protection requirements must be followed. The results of the research and product development, manufacturing, preclinical studies, clinical studies, and related information are submitted in a NDA or BLA to the FDA.
The process of completing clinical testing and obtaining FDA approval for a new therapeutic product, such as a drug, biologic, or cell based product, is likely to take a number of years and require the expenditure of substantial resources. If a NDA or BLA is submitted, there can be no assurance that the FDA will file, review, and approve it. Even after initial FDA approval has been obtained, post-market studies could be required to provide additional data on safety or effectiveness. Additional pivotal studies would be required to support adding other indications to the labeling. Also, the FDA will require post-market reporting and could require specific surveillance or risk mitigation programs to monitor for known and unknown side effects of the product. Results of post-marketing programs could limit or expand the continued marketing of the product. Further, if there are any modifications to the product, including changes in indication, manufacturing process, labeling, or the location of the manufacturing facility, a NDA or BLA supplement would generally be required to be submitted to the FDA prior to or corresponding with that change, or for minor changes in the periodic safety update report that must be submitted annually to the FDA.
The rate of completion of any clinical trial depends upon, among other factors, sufficient patient enrollment and retention. Patient enrollment is a function of many factors, including the size of the patient population, the nature of the trial, the number of clinical sites, the availability of alternative therapies, the proximity of patients to clinical sites, and the eligibility and exclusion criteria for the trial. Delays in planned patient enrollment might result in increased costs and delays. Patient retention could be affected by patient non-compliance, adverse events, or any change in circumstances making the patient no longer eligible to remain in the trial.
Failure to adhere to regulatory requirements for the protection of human subjects, to ensure the integrity of data, other IND requirements, and GCP standards in conducting clinical trials could cause the FDA to place a "clinical hold" on one or more studies of a product candidate, which would stop the studies and delay or preclude further data collection necessary for product approval. Noncompliance with GCP standards would also have a negative impact on the FDA's evaluation of a
22
NDA or BLA. If at any time the FDA finds that a serious question regarding data integrity has been raised due to the appearance of a wrongful act, such as fraud, bribery or gross negligence, the FDA may invoke its Application Integrity Policy, or AIP, under which it could immediately suspend review of any pending NDA or BLA or refuse to accept the submission of a NDA or BLA as filed, require the sponsor to validate data, require additional clinical studies, disapprove a pending NDA or BLA or withdraw approval of marketed products, as well as require corrective and preventive action to ensure data integrity in future submissions. Significant noncompliance with IND regulations could result in the FDA not only refusing to accept a NDA or BLA as filed but could also result in enforcement actions, including civil and administrative actions, civil money penalties, criminal prosecution, criminal fines and debarment. Whether or not FDA approval has been obtained, approval of a product by regulatory authorities in foreign countries must be obtained prior to the commencement of marketing the product in those countries.
The requirements governing the conduct of clinical trials and product approvals vary widely from country to country, and the time required for approval might be longer or shorter than that required for FDA approval. Although there are some procedures for unified filings for some European countries, in general, each country at this time has its own procedures and requirements.
In most cases, if the FDA has not approved a product candidate for sale in the United States, the unapproved product may be exported to any country in the world for clinical trial or sale if it meets U.S. export requirements and has marketing authorization in any one of the following listed countries: Canada, Australia, New Zealand, Japan, Israel, Switzerland, South Africa, or any member nation in the European Union or the European Economic Area, without submitting an export request to the FDA or receiving FDA approval to export the product, as long as the product meets the regulatory requirements of the country to which the product is being exported. If an unapproved product is not approved in one of the listed countries, the unapproved product may be exported directly to an unlisted country if the product meets the requirements of the regulatory authority of that country, and the FDA determines that the foreign country has statutory or regulatory requirements similar or equivalent to the United States.
In addition to the regulatory framework for product approvals, we and our collaborative partners must comply with federal, state and local laws and regulations regarding occupational safety, laboratory practices, the use, handling and disposition of radioactive materials, environmental protection and hazardous substance control, and other local, state, federal and foreign regulation. All facilities and manufacturing processes used by third parties to produce our product candidates for clinical use in the United States and our products for commercialization must be in compliance with cGMP requirements and are subject to periodic regulatory inspections. The failure of third party manufacturers to comply with applicable regulations could extend, delay, or cause the termination of clinical trials conducted for our product candidates or the withdrawal of our products from the market. The impact of government regulation upon us cannot be predicted and could be material and adverse. We cannot accurately predict the extent of government regulation that might result from future legislation or administrative action.
With respect to our L-glutamine product candidate for the treatment of SCD, we have completed preclinical studies, submitted an IND to the FDA which has become effective and conducted Phase 1, Phase 2 and Phase 3 clinical trials. We expect to proceed with submitting a NDA to the FDA. If the FDA accepts the NDA for filing based on our single Phase 3 clinical trial, the review officially begins from the date the FDA received the original submission. The FDA can refuse to file an application for review if it is incomplete in the view of the FDA. Because our product has obtained Orphan Drug designation and Fast Track designation, we expect that the FDA will prioritize the review of our pharmaceutical grade L-glutamine treatment for SCD with the intent of rendering a decision within six months of the date that the NDA is treated as submitted to the FDA.
23
After reviewing the NDA, the FDA will make a determination as to whether data and other information demonstrate the safety and provide substantial evidence of effectiveness of the product candidate for its intended use(s), which includes an analysis of whether its benefits outweigh its risks; whether its proposed labeling is adequate and complete; and whether the methods used in, and the facilities or controls used for, its manufacture, processing, packing, and storage conform to and are operated or administered in conformity with cGMP regulations. Upon completion of the NDA review, the FDA will either approve or not approve the application, or issue a Complete Response Letter (a letter from the FDA indicating that it cannot approve the application in its present form and informing the applicant of changes that should be made before the application could be approved). If the FDA approves the NDA, we will be able to commercialize the product. If the FDA does not approve our NDA, we will not be able to commercialize our SCD product candidate, which may have a material adverse impact on our business and financial condition.
If the FDA issues a Complete Response Letter, the FDA would be required to provide the basis for its decision and we would have an opportunity to meet with FDA officials to discuss any deficiencies noted in the letter. At that point, we could choose to request a hearing and appeal the agency's decision, or address deficiencies and, if necessary, submit additional data or information, or withdraw the application. Common problems which may delay or prevent the FDA from approving a NDA include, but are not limited to, unexpected safety issues, inadequate data analysis, or the failure, in the FDA's judgment, to provide substantial evidence of a product candidate's effectiveness. If we receive a Complete Response Letter, we may need to conduct additional studies, perhaps studies of more people or, different types of people, or conduct studies for a longer period of time. If we must conduct additional clinical studies in order to address any deficiencies identified in a Complete Response Letter, we may not have sufficient funding to conduct such additional trials or studies.
Outside the United States, we sell AminoPure in Japan, Taiwan and South Korea. There are no regulatory requirements to sell AminoPure in Japan because it is classified as a nutritional supplement product. To sell AminoPure in Taiwan, we are required to obtain a Certificate of Free Sale from the FDA, which we provide to our distributor. The FDA issues a Certificate of Free Sale upon request for products that either meet the applicable requirements of the FD&C Act and may be legally marketed in the United States or may be legally exported under the FD&C Act although they may not be legally marketed in the United States. Once the Certificate of Free Sale is furnished to our distributor in Taiwan, it is the distributor's responsibility to comply with local regulations, including but not limited to, obtaining the proper import license. The Certificate of Free Sale for the sale of AminoPure in Taiwan expires in 2015. In South Korea, AminoPure is imported and sold as a dietary supplement, which does not require any regulatory approval but is subject to dietary supplement cGMP regulations. In Ghana, AminoPure is a registered product with the Food and Drug Board of the Ministry of Health.
Patents, Proprietary Rights and Know-How
Our success will depend in part on our ability to obtain patents and otherwise preserve the intellectual property rights relating to the design, operation, sale and distribution of our products. We intend to seek patents on our products when we deem it commercially appropriate. The process of seeking patent protection can be lengthy and expensive, and there can be no assurance that patents will be issued for currently pending or future applications or that our existing patents or any new patents issued will be of sufficient scope or strength or provide meaningful protection or any commercial advantage to us. We may be subject to, or may initiate, litigation or patent office interference proceedings, which may require significant financial and management resources. The failure to obtain necessary licenses or other rights or the advent of litigation arising out of any such intellectual property claims could have a material adverse effect on our operations.
We have relied to date on a combination of patent licenses, trademark and trade secret protection and other unpatented proprietary information to protect our intellectual property rights and to
24
maintain and enhance our competitiveness in the pharmaceutical industry. While we do not currently own any issued patents, we have two patent licenses with third parties. We have also submitted patent applications which have yet to be published. If our pharmaceutical grade L-glutamine treatment for SCD is approved by the FDA, we will seek seven years of Orphan Drug market exclusivity based on the FDA's previous grant to us of Orphan Drug designation for this product candidate. In addition, we will also seek three years of data exclusivity under Title I of the Hatch/Waxman Act, as described above under the caption "Government Regulation". These additional periods of exclusivity, if any are granted, would run concurrently.
We also rely on unpatented technologies to protect the proprietary nature of our products. We require that our officers and key employees enter into confidentiality agreements that require these officers and employees to assign to us the rights to any inventions developed by them during the course of their employment with us. All of the confidentiality agreements include non-solicitation provisions that remain effective during the course of employment and for periods following termination of employment.
Licenses and Promotional Rights Agreements
On March 1, 2001, Emmaus Medical became the exclusive worldwide licensee under U.S. Patent No. 5,693,671, entitled "L-glutamine Therapy for SCD and Thalassemia" issued on December 2, 1997 to Niihara et al., which we refer to as the SCD Patent, to develop a treatment approach for SCD and thalassemia using L-glutamine pursuant to a license agreement. The license agreement is effective until the expiration of the SCD Patent in 2016. Pursuant to the license agreement, we acquired the exclusive right to test, gain governmental approval of, make, have made, use, distribute and sell products designed for use in carrying out methods covered under the SCD Patent, which we refer to as the Licensed Methods, and/or incorporating technical information provided by the licensor or by any of certain doctors affiliated with the licensor, which we refer to as the Licensed Products. Pursuant to an addendum to the license agreement, we agreed to pay royalties to the licensor during the term of the agreement equal to 4.5% of net sales of Licensed Products in the United States until lifetime royalty payments made to the licensor total $100,000, at which time the royalty rate will decrease to 2.5% of net sales of the Licensed Products. No royalties will be paid to the licensor for Licensed Products sold or distributed, or Licensed Methods practiced, on a non-profit basis. Royalty payments are due within 45 days after the end of each fiscal quarter, with the last payment due 45 days after the termination of the agreement. Any payments not made when due accrue interest on and after the due date at a rate equal to the prime interest rate quoted by the Bank of America on the date the payment is due, with interest being compounded on the last day of each calendar quarter.
We are also responsible for paying all fees and costs relating to the maintenance of the SCD Patent. Before any Licensed Products are commercially sold or Licensed Methods are practiced on humans, we are required to obtain comprehensive general liability insurance policies, including product liability insurance coverage in the minimum amount of $0.5 million. If we fail to obtain the required insurance policies, the licensor may terminate the agreement or obtain such insurance at our sole cost and expense. We currently have product liability insurance for NutreStore and also have clinical trial insurance for the SCD trial.
The license agreement will terminate upon the expiration of the patent in 2016, or earlier upon a court's determination that the patent is invalid or unenforceable. If we fail to pay royalties when due and payable, the licensor may terminate the agreement upon 90 days' written notice, unless we pay all outstanding royalties and interest due, during such 90-day period. The licensor may also terminate the agreement upon our material breach of the agreement upon providing us with 90 days' written notice. The agreement will automatically terminate at the end of such 90-day period unless we cure the breach or default during such period.
25
In October 2007, under a sublicense granted by CATO, Emmaus Medical became the exclusive sublicensee of US Patent No. 5,288,703, which we refer to as the SBS Patent, for the U.S. market, including the rights to distribute the L-glutamine treatment for SBS under the trademark NutreStore in the United States, which we refer to as the SBS License. CATO granted Emmaus Medical the SBS License under a sublicense that CATO held from Ares Trading S.A. We commercially launched NutreStore in June 2008. In February 2011, we entered into an assignment and transfer agreement with CATO. Under this agreement, CATO assigned and transferred to us ownership of the NutreStore NDA, Drug Master Files, or DMFs, No. 16633 and 16639, NutreStore-related trademarks, and the know-how represented by the NutreStore NDA and DMFs. The two DMFs contain the proprietary information relating to the manufacturing and packaging specifications of the NutreStore product. In consideration of this assignment and transfer, we agreed to pay to CATO royalties following the expiration of the SBS License on October 7, 2011, when the SBS Patent expired, in the following amounts: 10% of adjusted gross sales of NutreStore from 2012 through 2016; and 1% of gross sales of L-glutamine as a treatment for SCD and thalassemia for a period of five years from the date of first commercial sale of the treatment. We do not anticipate that the expiration of the SBS License will have a significant impact on us and we intend to sell NutreStore in accordance with the terms of the assignment and transfer agreement. We currently do not believe there should be significant competition in the marketplace for a generic version of NutreStore given the small population of SBS patients (<10,000 adults).
In April 2011, we entered into the Research Agreement and the Individual Agreement with CellSeed and, in August 2011, entered into an addendum to the Research Agreement. Pursuant to the Individual Agreement, CellSeed granted us the exclusive right to manufacture, sell, market and distribute CAOMECS for the cornea in the United States and agreed to disclose to us its accumulated information package for the joint development of CAOMECS. In 2012, we paid CellSeed $1.5 million under the Individual Agreement. Under the Research Agreement, as supplemented by the addendum, we agreed to pay CellSeed $8.5 million within 30 days of the completion of all of the following: (i) the execution of the Research Agreement; (ii) the execution of the Individual Agreement; and (iii) CellSeed's delivery of the accumulated information package, as defined in the Research Agreement, to us and our providing written confirmation of its acceptance of the complete package, which has not yet been completed. Pursuant to the Research Agreement, we and CellSeed formed a relationship regarding the future research and development of cell sheet engineering regenerative medicine products and the future commercialization of such products in the United States.
All intellectual property rights created in the course of the Research Agreement, the Individual Agreement and any future individual agreement, including rights made jointly by our employees and CellSeed employees or made solely by the employees of one party based on confidential information or intellectual property rights exchanged between the parties, will be owned jointly by us and CellSeed. Intellectual property rights related to the products that are developed solely by one party's employees independently from confidential information and intellectual property rights of the other party, will be owned by the party whose employees made such invention, but that party must grant a worldwide, perpetual, irrevocable, non-exclusive, royalty free, fully paid up, sub-licensable, transferable license of such rights to the other party.
Trademarks
We currently own three federal U.S. trademark registrations, including for "Emmaus Medical," "NutreStore" and "AminoPure," a Japanese trademark for "AminoPure," a South Korean trademark for "AminoPure," a Taiwanese trademark for "AminoPure," and a Philippines trademark for "AminoPure." We are also pursuing trademark registrations for "AminoPure" in Ghana and Kenya.
26
Corporate Information
Orphan Drugs International, LLC was organized on December 20, 2000 and its name was changed to Emmaus Medical, LLC in March 2002. In October 2003, Emmaus Medical, LLC undertook a reorganization and merged with Emmaus Medical, Inc., which was originally incorporated in September 2003. Through this merger of Emmaus Medical, LLC into Emmaus Medical, Emmaus Medical acquired the exclusive patent rights for a treatment for SCD.
AFH Acquisition IV, Inc. was incorporated in the state of Delaware on September 24, 2007 and was originally organized as a "blank check" shell company. On May 3, 2011, pursuant to an Agreement and Plan of Merger dated April 21, 2011, Emmaus Medical merged with and into AFH Merger Sub, Inc., a wholly-owned subsidiary of AFH Acquisition IV, with Emmaus Medical continuing as the surviving entity, which we refer to as the Merger. Upon the closing of the Merger, we (i) became the 100% parent of Emmaus Medical, (ii) assumed the operations of Emmaus Medical and its subsidiaries, and (iii) changed our name from "AFH Acquisition IV, Inc." to "Emmaus Holdings, Inc." On September 14, 2011, we changed our name from "Emmaus Holdings, Inc." to "Emmaus Life Sciences, Inc."
In May 2006, we formed Newfield Nutrition Corporation, referred to as Newfield Nutrition, a wholly-owned subsidiary of Emmaus Medical that distributes L-glutamine as a nutritional supplement under the brand name AminoPure.
In October 2010, we formed Emmaus Medical Japan, Inc., referred to as EM Japan, a wholly-owned subsidiary of Emmaus Medical that markets and sells AminoPure in Japan and other countries in Asia. EM Japan also manages our distributors in Japan and may also import other medical products and drugs in the future.
In November 2011, we formed Emmaus Medical Europe, Ltd., referred to as EM Europe, a wholly-owned subsidiary of Emmaus Medical whose primary focus is expanding our business in Europe. L-glutamine for the treatment of SCD has received Orphan Drug designation from the EC.
Our principal executive offices and corporate offices are located at 21250 Hawthorne Boulevard, Suite 800, Torrance, California and our telephone number at that address is (310) 214-0065. We maintain an Internet website at the following address: www.emmausmedical.com. The information on our website is not incorporated by reference in this Annual Report on Form 10-K or in any other filings we make with the Securities and Exchange Commission, or SEC.
We have registered "Emmaus Medical," "NutreStore" and "AminoPure" as federal U.S. trademarks, and have registered Japanese, Taiwanese, South Korean and Philippines trademarks for "AminoPure," which are protected under applicable intellectual property laws and are the property of Emmaus. This Annual Report on Form 10-K also contains trademarks, service marks, trade names and copyrights of other companies, which are the property of their respective owners. Solely for convenience, trademarks and trade names referred to in this Annual Report on Form 10-K may appear without the® or TM symbols, but such references are not intended to indicate, in any way, that we will not assert, to the fullest extent under applicable law, our rights or the right of the applicable licensor to these trademarks and trade names.
27
The following risk factors should be considered in conjunction with the other information included in this Annual Report on Form 10-K. This report may include forward-looking statements that involve risks and uncertainties. In addition to those risk factors discussed elsewhere in this report, we identify the following risk factors, which could affect our actual results and cause actual results to differ materially from those in the forward-looking statements.
RISKS RELATED TO OUR FINANCIAL CONDITION AND CAPITAL REQUIREMENTS
We have incurred losses since inception, have limited cash resources and anticipate that we will continue to incur substantial losses for the foreseeable future, and we may never become profitable.
As of December 31, 2014, we had an accumulated deficit of $70.6 million since our inception in December 2000. Our net losses were $20.8 million and $14.1 million for the years ended December 31, 2014 and 2013, respectively. These losses resulted principally from costs incurred in our research and development programs and from our general and administrative expenses. We have had limited revenue and have sustained significant operating losses since inception, and we are likely to sustain operating losses in the foreseeable future. Since inception, we have funded our operations through the private placement of equity securities and convertible notes and loans. We expect that we will continue to fund our operations primarily through the issuance of public or private equity or debt securities, or other sources, such as strategic partnerships. Such financings or other sources may not be available in amounts or on terms acceptable to us, if at all. Our failure to raise capital as and when needed would inhibit our ability to continue operations and implement our business strategy.
We expect to continue to incur significant and increasing negative cash flow and operating losses as we continue our research activities, conduct potential future clinical trials, seek regulatory approvals for our pharmaceutical grade L-glutamine treatment for SCD and prepare for commercialization of our SCD product. These losses, among other things, have had and will continue to have an adverse effect on our stockholders' equity, total assets and working capital. Because of the numerous risks and uncertainties associated with pharmaceutical development, we are unable to predict the extent of any future losses, whether or when we will be able to commercialize our pharmaceutical grade L-glutamine treatment for SCD, or when we will become profitable, if ever. Our strategy depends heavily on the success of our pharmaceutical grade L-glutamine treatment for SCD. If we are unable to commercialize our pharmaceutical grade L-glutamine treatment for SCD or any of our other product candidates, or if we experience significant delays in doing so, our business may fail. Even if we do achieve profitability, we may not be able to sustain or increase profitability on a quarterly or annual basis.
We will require substantial additional funding and may be unable to raise capital when needed, which could force us to delay, reduce or eliminate planned activities or result in our inability to continue as a going concern.
We will require additional capital to pursue potential future clinical trials and regulatory approvals, as well as further research and development and marketing efforts for our products and potential products. We have no committed sources of additional capital and our access to capital funding is uncertain. If we are not able to secure financing, we may no longer be a going concern and may be forced to curtail operations, delay or stop ongoing clinical trials, or cease operations altogether, file for bankruptcy, or undertake any combination of the foregoing. In such event, our stockholders may lose their entire investment in our company. Our future capital requirements will depend on, and could increase significantly as a result of, many factors, including:
- •
- the duration and results of the clinical trials for our various products going forward;
- •
- unexpected delays or complications in seeking regulatory approvals;
28
- •
- the time and cost in preparing, filing, prosecuting, maintaining and enforcing patent claims;
- •
- adverse developments encountered in implementing our business development and commercialization strategies;
- •
- our undertaking of collaborations with strategic partners and the outcome of those collaborations; and
- •
- any need to engage in litigation relating to protecting our intellectual property rights or other commercial or regulatory matters and the outcome of any such litigation.
We may attempt to raise additional funds through public or private financings, collaborations with other companies or financing from other sources. Additional funding may not be available in amounts or on terms which are acceptable to us, if at all. If adequate funding is not available to us, or on reasonable terms, we may need to delay, reduce or eliminate one or more of our product development programs or obtain funds on terms less favorable than we would otherwise accept.
In addition, if we do not meet our payment obligations to third parties as they become due, we may be subject to litigation claims and our credit worthiness would be adversely affected. Even if we are successful in defending against these claims, litigation could result in substantial costs and would be a distraction to management, and may result in unfavorable results that could further adversely impact our financial condition.
Raising additional capital may cause dilution to our stockholders, restrict our operations or require us to relinquish rights.
We may seek additional capital through a combination of private and public equity offerings, debt financings and collaborations and strategic and licensing arrangements. To the extent that additional capital is raised through the sale of equity securities or securities convertible into or exchangeable for equity securities, the issuance of those securities could result in dilution to our stockholders. The terms of such securities may include liquidation or other preferences that could adversely affect the rights of our existing stockholders. Moreover, the incurrence of debt financing could result in a substantial portion of our future operating cash flow, if any, being dedicated to the payment of principal and interest on such indebtedness and could impose restrictions on our operations. This could render us more vulnerable to competitive pressures and economic downturns.
Debt financing, if available, would result in increased fixed payment obligations and may involve agreements that include grants of security interests or covenants limiting or restricting our ability to take specific actions such as incurring debt, making capital expenditures or declaring dividends. If we raise additional funds through collaboration, strategic alliance and licensing arrangements with third parties, we may have to relinquish valuable rights to our technologies, future revenue streams or product candidates, or grant licenses on terms that are not favorable to us.
Our recurring operating losses and significant short-term indebtedness have raised substantial doubt regarding our ability to continue as a going concern.
We have incurred recurring operating losses. In addition, we have a significant amount of notes payable and other obligations due within the next year and are projecting that our operating losses and expected capital needs will exceed our existing cash balances and cash expected to be generated from operations for the foreseeable future, including the expected costs relating to seeking FDA approval for, and the commercialization of, our pharmaceutical grade L-glutamine treatment for SCD. These factors raise substantial doubt about our ability to continue as a going concern. As a result, our independent registered public accounting firm included an explanatory paragraph in its report on our financial statements as of and for the year ended December 31, 2014 with respect to this uncertainty. The continuation and expansion of our business is dependent upon obtaining further financing,
29
successful and sufficient market acceptance of our products, and, finally, achieving a profitable level of operations. The perception of our ability to continue as a going concern may make it more difficult for us to obtain financing for the continuation of our operations and could result in the loss of confidence by investors, suppliers and employees.
We have adopted an equity incentive plan under which we may grant securities to compensate employees and other services providers, which could result in increased share-based compensation expenses and, therefore, reduced net income.
Under current accounting rules, we are required to recognize share-based compensation as compensation expense in our statement of comprehensive loss, based on the fair value of equity awards on the date of the grant, and recognize the compensation expense over the period in which the recipient is required to provide service in exchange for the equity award. We made grants of equity awards in 2013 and 2014, and accordingly our results of operations for the years ended December 31, 2013 and 2014 contain share-based compensation charges. Additional expenses associated with share-based compensation may reduce the attractiveness of issuing stock options under our existing equity incentive plan or any equity incentive plan that we may adopt in the future. If we grant equity compensation to attract and retain key personnel, the expenses associated with share-based compensation may adversely affect our net income. However, if we do not grant equity compensation, we may not be able to attract and retain key personnel or be forced to expend cash or other compensation instead. Furthermore, the issuance of equity awards would dilute existing stockholders' ownership interests in our company. In February 2013 our board of directors amended our equity incentive plan to increase the number of shares of our common stock that may be subject to awards granted under the plan from 3 million shares to 6 million shares. In July 2014, our board of directors amended our equity incentive plan again to increase the number of shares of our common stock that may be subject to awards granted under the plan from 6 million shares to 9 million shares.
We have issued and may continue to issue debt instruments that are convertible and/or may include warrant features, which could result in the calculation of discounts on the debt issued and increased amortization of discount expenses and, therefore, reduced net income.
Under current accounting rules, we are required to recognize discounts on debt issued with stock conversion features or with attached or accompanying warrants which can result in amortization of the discount as an expense in our statement of consolidated comprehensive loss, based on the fair value of conversion feature or warrant on the date of issue. If we grant stock conversion features or warrants with debt instruments to attract capital, the expenses associated with amortization of the calculated discount may adversely affect our net income. However, if we do not grant stock conversion features or warrants with debt instruments, we may not be able to attract debt capital.
We obtained a summary judgment and court order in June 2013 allowing us to cancel approximately 2.5 million shares of our common stock issued to AFH Advisory and certain others in connection with our Merger. Following the litigation of certain claims that were not adjudicated in the summary judgment, the summary judgment and court order would be subject to appeal for a thirty-day period. If the summary judgment and court order are appealed, and our defense of such appeal is unsuccessful, we may have to re-issue the cancelled shares, resulting in dilution to our stockholders.
Since July 2012, we have been engaged in significant litigation with Amir Heshmatpour and AFH Holding and Advisory, LLC, or AFH Advisory. Mr. Heshmatpour, one of our former directors, owns AFH Advisory, which was our majority stockholder until our combination with Emmaus Medical, Inc. pursuant to the Merger in May 2011. In September 2012, AFH Advisory, Griffin Ventures, Ltd., or Griffin, and The Amir & Kathy Heshmatpour Family Foundation, or the Foundation, filed a complaint against us in the Superior Court of Delaware. The complaint alleged that we did not have the right to
30
cancel the plaintiffs' shares, a right that we had previously asserted under a Letter of Intent between us and AFH Advisory, and asked the court to issue a declaratory judgment to that effect. In October 2012 we filed counterclaims against the plaintiffs and third-party claims against Mr. Heshmatpour. We asked the court for an order declaring that the plaintiffs' shares were canceled and that, because of fraudulent inducement on their part, the letter of intent between us and AFH Advisory was void and of no further effect. In addition, we asked the court to award compensatory and punitive damages, in amounts to be determined, for breach of contract, unjust enrichment and fraud.
On June 27, 2013, the court issued an order implementing a partial summary judgment in our favor. The order, among other things, (i) stated that the letter of intent between us and AFH Advisory was properly terminated as of July 19, 2012, and (ii) ordered our transfer agent to effect the cancellation of 2,504,249 shares of our common stock held by AFH Advisory, Griffin and the Foundation. The cancellation of such shares, which represented approximately 10 percent of our common stock, was effected by our transfer agent on June 28, 2013. The cancellation of such shares will be subject to appeal for 30 days after the completion of trial court proceedings. While the partial summary judgment in favor of the Company led to the cancellation of 2,504,249 shares of the Company's common stock by the transfer agent on June 28, 2013, we have determined that gain contingency accounting, which would result in derecognition of these shares and the related liability amount, is not appropriate under the accounting principles generally accepted in the United States, or GAAP, until the right of appeal has lapsed and no further contingency remains. Hence, the Company will continue to present these shares in its financial statements as outstanding until the right of appeal has lapsed and all contingencies have been resolved.
On April 16, 2014, the court granted our motion for attorneys' fees, costs and expenses and denied a competing motion for attorneys' fees, costs and expenses brought by AFH Advisory and Mr. Heshmatpour. The court ruled that under the letter of intent between us and AFH Advisory, we are entitled to an award of $700,000 in attorneys' fees, costs and expenses from AFH Advisory. Our cause of action for fraud has not yet been litigated or settled.
Conduct of the litigation has entailed significant expense to us and will continue to do so until it is resolved. It has also required significant amounts of time and attention from our management. If the court's summary judgment ruling is appealed, it could result in our incurring liabilities and expenses that may have a material adverse effect on our financial condition and cash flows and result in dilution to our stockholders.
We may be required to make an $8.5 million payment to CellSeed under our Research Agreement with CellSeed relating to the delivery to and acceptance by us of data relating to CellSeed's technology, and if this condition is satisfied and we do not make this payment, CellSeed has the right to terminate one or both of the Research Agreement and Individual Agreement with us, which will negatively impact our ability to implement our business strategies related to CAOMECS.
In April 2011, Emmaus Medical, Inc. entered into the Research Agreement and the Individual Agreement with CellSeed and, in August 2011, entered into an addendum to the Research Agreement. Pursuant to the Individual Agreement, CellSeed granted us the exclusive right to manufacture, sell, market and distribute CAOMECS for the treatment of corneal impairments in the United States, which we would be able to exercise only after receiving FDA marketing approval for the product, and agreed to disclose to us its accumulated information package for the joint development of CAOMECS. Under the Individual Agreement, we agreed to pay CellSeed $1.5 million, which we paid in February 2012. Pursuant to the Research Agreement, we formed a relationship with CellSeed regarding the future research and development of cell sheet engineering regenerative medicine products, and the future commercialization of such products. Under the Research Agreement, as supplemented by the addendum, we agreed to pay CellSeed $8.5 million within 30 days of the completion of all of the following: (i) the execution of the Research Agreement; (ii) the execution of the Individual Agreement;
31
and (iii) CellSeed's delivery of the accumulated information package, as defined in the Research Agreement, to us and our providing written confirmation of our acceptance of the complete accumulated information package. The third of these conditions has not yet been satisfied. At the time we entered into our agreements with CellSeed, we left for further negotiation provisions covering how we and CellSeed will share any financial results of commercializing any cell sheet engineering regenerative medicine products that we are seeking to develop in collaboration with CellSeed. If we are not able to successfully negotiate these terms, our current development and commercialization plans with respect to any of these products would be materially adversely affected.
If our payment obligation under the Research Agreement becomes due and payable prior to our generation of revenue from the commercialization of our pharmaceutical grade L-glutamine treatment for SCD under development, we will need to use available cash or seek other funding sources, including the sale of additional equity or debt securities, in order to fund that payment. CellSeed may terminate either or both of the Research Agreement and the Individual Agreement if we are unable to timely make this payment. If CellSeed terminates one or both of these agreements, our strategy to develop and commercialize regenerative medicine products would be adversely affected and we would become more reliant on our pharmaceutical grade L-glutamine treatment for SCD, making it more difficult to raise external funding.
RISKS RELATED TO DEVELOPMENT OF OUR PRODUCT CANDIDATES
We may not be able to receive regulatory approval for our pharmaceutical grade L-glutamine treatment for SCD, or, even if approved, we may not be able to successfully commercialize our pharmaceutical grade L-glutamine treatment for SCD or any other product candidates, which would adversely affect our financial and operating condition.
Our current efforts are, and a substantial portion of our efforts for the foreseeable future will be, focused primarily on our lead product candidate, our pharmaceutical grade L-glutamine treatment for SCD, for which we have recently completed a single Phase 3 clinical trial. All of our other product candidates are still in preclinical development. Regulatory approval is required to market our pharmaceutical grade L-glutamine treatment for SCD, or other product candidates we may develop. There are many reasons that we may fail in our efforts to commercialize our pharmaceutical grade L-glutamine treatment for SCD or that such efforts will be delayed, including:
- •
- the chance that the FDA will conclude that our clinical trial does not demonstrate substantial evidence that our pharmaceutical grade
L-glutamine treatment for SCD is effective and safe;
- •
- the chance that the FDA will not accept for filing the NDA that we intend to submit based on the results of only one Phase 3
clinical trial, instead of two such studies, to demonstrate substantial evidence of effectiveness of our pharmaceutical grade L-glutamine treatment for SCD;
- •
- the failure of our pharmaceutical grade L-glutamine treatment for SCD to receive necessary regulatory approvals from the FDA or
foreign regulatory authorities in a timely manner, or at all;
- •
- the failure of our pharmaceutical grade L-glutamine treatment for SCD, once approved, to be produced in commercial quantities or at
reasonable costs;
- •
- physicians' reluctance to switch to our pharmaceutical grade L-glutamine treatment for SCD from existing SCD treatment methods,
including traditional therapy agents;
- •
- the failure of our L-glutamine product candidate, once approved, to achieve commercial acceptance for the treatment of SCD;
32
- •
- the introduction of products by our competitors that are or are perceived to be more effective or have or are perceived to have a
better safety profile than our pharmaceutical grade L-glutamine treatment for SCD;
- •
- the application of restrictions to our pharmaceutical grade L-glutamine treatment for SCD by regulatory or governmental authorities;
- •
- the possibility that we may not be able to maintain Orphan Drug designation prior to or after our submission to the FDA of a NDA for
our pharmaceutical grade L-glutamine treatment for SCD, as well as the possibility that we may not be able to obtain and maintain Orphan Drug market exclusivity for our pharmaceutical grade
L-glutamine treatment for SCD even if the FDA approves that NDA because the approved indication differs from the indication for which the FDA granted us Orphan Drug designation;
- •
- the possibility, even if we obtain regulatory approval and Orphan Drug market exclusivity for our pharmaceutical grade L-glutamine
treatment for SCD, that another company with the same drug for the same indication could demonstrate clinical superiority over our product, thus making that third party drug eligible for approval and
Orphan Drug market exclusivity despite our market exclusivity;
- •
- the possibility that another product, different from L-glutamine, receives Orphan Drug designation and is approved for the treatment
of SCD, which our Orphan Drug exclusivity, should we receive it, would not bar;
- •
- the possibility, if we obtain Orphan Drug market exclusivity for our pharmaceutical grade L-glutamine treatment for SCD, that we are
unable to ensure an adequate supply of it, thus allowing the FDA to approve another L-glutamine product for Orphan Drug exclusivity; and
- •
- the possibility that our Fast Track designation for our pharmaceutical grade L-glutamine treatment for SCD may be rescinded or may not actually lead to a faster regulatory review and approval of the NDA that we intend to submit in 2015.
Even if the FDA and other regulatory authorities approve our pharmaceutical grade L-glutamine treatment for SCD, or any of our other product candidates, the manufacture, packaging, labeling, distribution, marketing and sale of such products will be subject to strict and ongoing post-approval regulations. Compliance with such regulations will be expensive and consume substantial financial and management resources.
The FDA has the authority to regulate the claims we make in marketing our prescription products to ensure that such claims are true, not misleading, supported by scientific evidence, and consistent with the approved labeling of those products. The FDA also has the authority to regulate the claims we make in marketing our dietary supplement AminoPure. Failure to comply with FDA requirements in this regard could result in, among other things, warning letters, withdrawal of approvals, seizures, recalls, injunctions prohibiting a product's manufacture and distribution, restricting promotional activities, requiring corrective actions regarding sales and marketing activities, other operating restrictions, civil money penalties, and prosecution under the False Claims Act, which imposes liability on individuals and companies who defraud governmental programs and may lead to criminal prosecutions. Any of these government enforcement actions, if taken against us, could negatively impact our product sales and profitability.
Additionally, regulatory approval of any of our prescription products may be conditioned on our agreement to conduct costly post-marketing follow-up studies to monitor the safety or effectiveness of such products or to implement specific risk mitigation strategies. In addition, as clinical experience with any of our products following such approval, if any, expands after approval because the product is used by a greater number and more diverse group of patients than during clinical trials, unknown side
33
effects or other problems may be observed that were not observed or anticipated during pre-approval clinical trials. In any such case, one or more regulatory authorities could restrict the indications for which the product may be sold or restrict the distribution channels, or revoke the product's regulatory approval, which could hinder our ability to generate revenues from that product. If we fail to develop and commercialize our product candidates as planned, our financial results and financial condition will be adversely affected, we will have to delay or terminate some or all of our research product development programs, and we may be forced to cease operations.
If the FDA does not accept for filing a NDA with only one Phase 3 clinical trial, instead of two such studies, to demonstrate substantial evidence of effectiveness of our pharmaceutical grade L-glutamine treatment for SCD, we may be forced to incur the time, expense, and risk of undertaking a confirmatory study prior to obtaining marketing approval of such product, which could have a material adverse effect on our business, financial condition and operating results.
Historically, the FDA has generally required applicants to submit as part of a NDA in respect of a drug two adequate and well-controlled clinical trials to demonstrate substantial evidence of effectiveness of that drug. The Modernization Act, however, makes clear that the FDA may consider data from only one adequate and well-controlled clinical investigation and confirmatory evidence (obtained prior to or after such investigation) if the FDA determines that such evidence is sufficient to establish effectiveness. In a guidance document titled "Providing Clinical Evidence of Effectiveness for Human Drug and Biological Products" (May 1998), the FDA stated that reliance on a single study is generally limited to situations in which a trial has demonstrated a clinically meaningful effect on mortality, irreversible morbidity, or prevention of a disease with a potential serious outcome, and where confirmation of the result in a second trial would be impractical or unethical. The factors the FDA considers for accepting a single study include, but are not limited to, having large multi-centered studies, consistent data across subgroups, multiple endpoints, and statistically very persuasive findings. Our single Phase 3 clinical trial was multi-centered with multiple endpoints. However, there can be no assurance that the FDA will accept this single study to be sufficient to demonstrate substantial evidence of effectiveness.
On June 11, 2014, we met with the FDA to discuss the preliminary data from our Phase 3 clinical trial and our plans to submit to the FDA a NDA, based on our single Phase 3 clinical trial. In minutes of the meeting that the FDA has provided to us, based on its review of preliminary data from our Phase 3 clinical trial, the FDA expressed concern that the primary endpoint of painful sickle cell crisis (controlling for both region and hydroxyurea use) did not reach the pre-specified p-value significance level of 0.045.
The FDA commented that the primary efficacy results were inconsistent among regions, as shown by the large difference in results observed based on the stratified analyses adjusted for both region and hydroxyurea use and the stratified analysis adjusted for hydroxyurea use only. The FDA also commented that a reduction in the median number of painful sickle cell crises by one (from four to three) is not clinically meaningful and is not consistent across regions. The FDA asked us to provide explanations for the differences in results observed based on the stratified analysis adjusted for region and hydroxyurea.
The FDA also commented on the number of patients who discontinued the study, which was greater in the L-glutamine group, and the FDA would need to understand what impact the imputation of such data, which is a method of accounting for missing information, would have on the interpretation of the results.
Based on preliminary data, the FDA recommended a second Phase 3 study be conducted to support the indication for SCD and suggested enrolling patients with a higher baseline level of sickle cell crises to help demonstrate a larger difference in the mean results.
34
Based on other questions we submitted to the FDA regarding the previous pre-clinical work and evidence gathered for the L-glutamine treatment related to pharmacology, toxicology, and clinical pharmacology, the FDA noted that the information provided appeared to be acceptable; however, final affirmations regarding such matters would be made during the FDA's review of a NDA. The FDA also made certain recommendations to the chemistry, manufacturing and controls of our pharmaceutical grade L-glutamine treatment for SCD which we intend to incorporate or address.
In September 2014 we submitted to the FDA the Clinical Study Report for our Phase 3 clinical trial, supplemental analyses and a new meeting request. Consequently, we met again with the FDA on October 15, 2014. In the minutes of the October 15 meeting, the FDA noted that it continued to be concerned that the primary endpoint did not meet the pre-specified p-value and observed that our efficacy finding would be more persuasive if supported by an additional, confirmatory study. We communicated to the FDA that over-stratification in our statistical analysis plan led to the pre-specified p-value not being met. We also provided sensitivity analyses and results to address the over-stratification which demonstrated a statistically significant p-value for the primary endpoint.
At the October 15 meeting, in response to a question raised by the FDA at the June 11 meeting, we provided evidence of the clinical meaningfulness of a reduction in the median number of painful sickle cell crises by one (from four to three). In the October 15 minutes the FDA expressed the view that the reduction of one sickle cell crises may not be clinically significant and advised us to provide all information and data to support this clinical meaningfulness and the FDA stated they will evaluate the overall clinical benefit and risk of L-glutamine based on results from our Phase 3 trial and all available safety data.
In the October 15 minutes the FDA reiterated certain concerns outlined in the June 11 minutes. Other questions that we posed for discussion at the October 15 meeting related to the safety of L-glutamine use, the benefit-risk margin for L-glutamine use, the sufficiency of our Phase 2 and 3 trials to support a NDA submission and whether a confirmatory study could be conducted as a post-marketing commitment. The FDA noted that its decisions regarding these questions would be made after the submission of a NDA.
Based on our discussions with the FDA, we intend to conduct a confirmatory study of our L-glutamine treatment for SCD. We submitted a draft protocol outline to the FDA. We plan to work with the FDA to finalize the study design. In addition to planning and conducting a confirmatory study, we plan to submit a NDA for our L-glutamine treatment for SCD during 2015 containing all efficacy and safety data through our completed Phase 3 trial and additional analyses and information in accord with the feedback the FDA provided in the context of the June 11 and October 15 meetings. We will request that our planned confirmatory study be accepted by the FDA as a post-approval study that is, a study to be completed after approval of the NDA. There can be no assurance that the FDA will accept our submission for filing before the completion of a confirmatory study or that the information and data in the NDA will satisfy concerns identified by the FDA.
Regarding the submission of NDAs that include only one Phase 3 clinical trial, the FDA has in some cases accepted evidence from one clinical trial to support a finding of substantial evidence of effectiveness. A change in the law under the Modernization Act made clear that the FDA may consider data from only one adequate and well controlled clinical investigation and confirmatory evidence if the FDA determines that such evidence is sufficient to establish effectiveness of the medicine under study.
In a guidance document titled "Providing Clinical Evidence of Effectiveness for Human Drug and Biological Products" (May 1998), the FDA stated that reliance on a single clinical trial is generally limited to situations in which a trial has demonstrated a clinically meaningful effect on mortality, irreversible morbidity, or prevention of a disease with a potential serious outcome, and where confirmation of the result in a second trial would be impractical or unethical. The factors the FDA
35
considers for accepting a single clinical trial include, but are not limited to, having large multi centered studies, consistent data across subgroups, multiple endpoints, and statistically very persuasive findings.
The development process to obtain FDA approval for new drugs, biologics and cell-based therapies is very costly and time consuming and if we cannot complete our clinical trials in a cost-effective manner, our operations may be adversely affected.
Before obtaining marketing approval from regulatory authorities for the sale of any product candidate, we or a collaborator must complete preclinical development and then complete one or more extensive clinical trials to demonstrate the safety and effectiveness of the product candidate in humans. Clinical testing is expensive, difficult to design and implement, can take many years to complete and is uncertain as to outcome. Costs of clinical trials may vary significantly over the life of a development project owing, but not limited to, the following:
- •
- the number of patients that participate in the trials;
- •
- the per patient trial costs;
- •
- the number of sites included in the trials;
- •
- the length of time required to enroll eligible patients;
- •
- the duration of the clinical trials;
- •
- the countries in which the trials are conducted;
- •
- the number of doses that patients receive;
- •
- adverse events experienced by trial participants;
- •
- the drop-out or discontinuation rates of patients;
- •
- potential additional safety monitoring or other studies requested by regulatory agencies;
- •
- the extent and duration of patient follow-up;
- •
- difficulties that could arise in analyzing and reporting to regulators the results of clinical trials; and
- •
- the efficacy and safety profile of the product candidate.
If we are unable to control the timing and costs of our clinical trials and conduct our trials and apply for regulatory approvals in a timely and cost-effective manner, our operations may be adversely affected.
Our product development costs will also increase if any regulatory agencies impose a clinical hold on any of our clinical studies or we experience delays in obtaining marketing approvals, particularly if we are required to conduct additional clinical studies beyond those that we submit in any NDA. We do not know whether any of our preclinical studies or clinical trials will begin as planned, will need to be restructured or will be completed on schedule, or at all. Significant preclinical study or clinical trial delays also could shorten any periods during which we may have the exclusive right to commercialize our approved product candidates or allow our competitors to bring products to market before we do, and thereby impair our ability to successfully commercialize our product candidates.
36
We may not be able to complete clinical trial programs for any of our product candidates successfully within any specific time period or at all, and if such clinical trials take longer to complete than we project, our ability to execute our current business strategy will be adversely affected.
Clinical testing is expensive, difficult to design and implement, can take many years to complete, and is uncertain as to outcome. A failure of one or more clinical trials can occur at any stage of development. The outcome of preclinical testing and early clinical trials may not be predictive of the success of later clinical trials, and interim results of a clinical trial do not necessarily predict final results. Moreover, clinical data are often susceptible to varying interpretations and analyses, and many companies that have believed their product candidates performed satisfactorily in preclinical studies and clinical trials have nonetheless failed to obtain marketing approval of them.
Generally speaking, whether we complete our clinical trials in a timely manner, or at all, for any product candidate is dependent in part upon: (i) the date the applicable investigational new drug application, or IND, becomes effective enabling us to commence the applicable clinical studies (which, under U.S. law, occurs no more than thirty days after the FDA receives the IND, unless the FDA places the IND on clinical hold, in which case the FDA may request us to provide additional data from completed preclinical studies or undertake additional preclinical studies, the latter of which could materially delay the clinical and regulatory development of the applicable product candidate); (ii) the engagement of clinical trial sites and clinical investigators; (iii) reaching an agreement with clinical investigators on acceptable clinical trial agreement terms, clinical trial protocols or informed consent forms; (iv) obtaining approval from the institutional review boards used by the clinical trial sites we seek to engage; (v) the rate of patient enrollment and retention; and (vi) the rate to collect, clean, lock and analyze the clinical trial database.
Patient enrollment in trials is a function of many factors, including the design of the protocol, the size of the patient population, the proximity of patients to and availability of clinical sites, the eligibility criteria for the trial, the perceived risks and benefits of the product candidate under trial and of the control product, if any, the clinical investigator's efforts to facilitate timely enrollment in clinical trials, the patient referral practices of local physicians, the existence of competitive clinical trials, and whether other investigational or new therapies are available for the indication. If we experience delays in identifying and contracting with appropriate clinical investigators and sites, in obtaining approval of the applicable institutional review boards, in enrolling and retaining patients and/or in completing our clinical trial programs, we may incur additional costs and delays in our development programs, and may not be able to complete our clinical trials on a cost-effective or timely basis, if at all. If we or any third party have difficulty obtaining sufficient clinical drug materials or enrolling a sufficient number of patients in a timely or cost-effective manner to conduct clinical trials as planned, or if enrolled patients do not complete the trial as planned, we or a third party may need to delay or terminate ongoing clinical trials, which could negatively affect our business.
Clinical trials required for demonstration of substantial evidence of efficacy and safety often require the enrollment of large numbers of patients, and suitable patients may be difficult to identify and recruit. Our ability to enroll sufficient numbers of patients in our clinical trials, especially when the disease or condition being studied is rare, depends on many factors, including the size of the relevant patient population, the nature and design of the protocol, the proximity of patients to clinical sites, the eligibility and exclusion criteria applicable for the trial, existence of competing clinical trials and the availability of already approved therapeutics for the indications being studied (whether or not such therapeutics are less safe or less effective than our product candidate under trial). In addition, patients may withdraw from a clinical trial or be unwilling to follow our clinical trial protocols for a variety of reasons, such as adverse events or noncompliance with trial requirements. If we fail to enroll and maintain the number of patients for which the clinical trial was designed, the statistical significance and/or statistical power of that clinical trial may be reduced which would make it harder to
37
demonstrate that the product candidate being tested in such clinical trial is safe and effective for its intended use.
We may be required to suspend, repeat or terminate our clinical trials if they do not meet regulatory requirements, the results are negative or inconclusive, human subject protections are inadequate, the trials are not well designed, or clinical investigators fail to comply with all requirements for the conduct of trials under the applicable IND, any of which may result in significant negative repercussions on our business and financial condition.
Our pharmaceutical grade L-glutamine treatment for SCD, which is currently our only product candidate in clinical development, has not yet received marketing approval in any jurisdiction. We cannot market a pharmaceutical product in any jurisdiction until we have completed rigorous preclinical testing and clinical trials for that product, demonstrated the product's safety and substantial evidence of effectiveness for its intended use, obtained the approval of the applicable regulatory authority for our proposed labeling of the product, and met the other requirements of such jurisdiction's extensive regulatory approval process. Preclinical testing and the conduct of clinical trials are long, expensive and uncertain processes. Data obtained from preclinical and clinical tests can be interpreted in different ways and could ultimately be deemed by regulatory authorities to be insufficient with respect to providing substantial evidence of safety and effectiveness required for regulatory approval, which could delay, limit or prevent regulatory approval. It may take us many years to complete the required testing of our product candidates to support an application for marketing approval and failure can occur at any stage during this process.
We cannot provide assurance that our preclinical testing and clinical trials will be completed successfully within any time period specified by us, or without significant additional resources or expertise provided by third parties to conduct such testing. We cannot provide assurance that any such testing will demonstrate that our product candidates meet regulatory approval requirements for safety and effectiveness or that any such product will be approved for a specific indication. Results from early clinical trials may not be indicative of the results that will be obtained in later-stage clinical trials or in the population of patients for whom the applicable product is prescribed following any approval. In addition, negative or inconclusive results from the clinical trials we conduct or adverse events experienced by the patients in such clinical trials could cause us to have to suspend, repeat or terminate the clinical trials. Clinical trials are subject to continuing oversight by governmental regulatory authorities and institutional review boards and must meet the requirements of these authorities including but not limited to requirements for informed consent, human subject protection and good clinical practices; and we cannot guarantee that we will be able to comply, or that a regulatory authority will agree that we have complied, with such requirements. We rely on third parties, such as contract research organizations and/or contract laboratories, regulatory consultants and data management companies to assist us in overseeing and monitoring clinical trials as well as to process the clinical data and manage test requests, which may result in delays or failure to complete trials, if the third parties fail to perform or meet applicable regulatory requirements and standards. A failure by us or any such third parties to comply with the terms and conditions of the protocol for any clinical study or the regulatory requirements for any particular product candidate or to complete the clinical trials for a product candidate in the projected time frame could have a significant negative effect on our business and financial condition.
There are significant requirements imposed on us and on clinical investigators who conduct clinical trials under an IND. Although we are responsible for selecting qualified clinical investigators, providing them with the information they need to conduct an investigation properly, ensuring proper monitoring of the investigation(s), and ensuring that the investigation(s) is conducted in accordance with the general investigational plan and protocols contained in the IND, we cannot ensure the clinical investigators will maintain compliance with all regulatory requirements at all times. The pharmaceutical
38
industry has experienced cases where clinical investigators have been found to incorrectly record data, omit data, or even falsify data. We cannot ensure that the clinical investigators in our trials will not make mistakes or otherwise compromise the integrity or validity of data, any of which would have a significant negative effect on our ability to obtain marketing approval, our business, and our financial condition.
Changes in regulatory requirements and guidance or unanticipated events during our clinical trials may occur, which may result in necessary changes to clinical trial protocols, informed consents and clinical trial budgets, any of which changes could result in increased costs to us, delay our development timeline or reduce the likelihood of successful completion of the clinical trial.
Changes in regulatory requirements or the FDA's interpretation of those requirements, which may be provided through guidance documents, or the occurrence of unanticipated events during our clinical trials could require us to amend clinical trial protocols, informed consent forms and trial budgets. If we experience delays in initiation, conduct or completion of any of our clinical trials, or if we terminate any of our clinical trials due to changes in regulatory requirements or guidance documents, unexpected and serious adverse events, or other unanticipated events, we may incur additional costs and have difficulty enrolling subjects or achieving clinical investigator or institutional review board acceptance of the changes and successfully completing the trial. Any such additional costs and difficulties could potentially materially harm the commercial prospects for our product candidates and delay our ability to generate product revenue.
There are various uncertainties related to the research, development and commercialization of the cell sheet engineering regenerative medicine products we are developing in collaboration with a strategic partner which could negatively affect our ability to commercialize such products.
We have historically focused on the research and development of our pharmaceutical grade L-glutamine treatment for SCD and have limited experience in the research, development or commercialization of cell sheet regenerative medicine products or any other biological product. No clinical trials of cell sheet regenerative products have been conducted in the United States and no biological products based on cell sheet engineering have been approved by regulatory authorities in any jurisdiction. Such products must be manufactured in conformance with current Good Manufacturing Practices, or cGMP, requirements as well as Good Tissue Practice, or GTP, requirements and demonstrate that they are safe, pure and potent to be effective for their intended uses to obtain FDA approval. The GTP requirements, which are specifically applicable to all cellular-based products, are intended to prevent communicable disease transmission. It is uncertain what type and quantity of scientific data would be required to support initiation of clinical studies or to sufficiently demonstrate the safety, purity and potency of cell sheet regenerative medicine products for their intended uses. Such uncertainties could delay our ability to obtain FDA approval for and to commercialize such products. In addition, the research and commercialization of cell sheet regenerative medicine products could be hindered if third party manufacturers of such products are not compliant with cGMP, GTP, and any other applicable regulations. Any delay in the development of, obtaining FDA approval for, or the occurrence of any problems with third party manufacturers of cell sheet regenerative medicine products would negatively affect our ability to commercialize such products.
The use of any of our product candidates in clinical trials and in the market may expose us to liability claims.
The nature of our business exposes us to potential liability risks inherent in the testing, manufacturing and marketing of our product candidates. While we are in clinical stage testing, our product candidates could potentially harm people or allegedly harm people and we may be subject to costly and damaging product liability claims. Some of the patients who participate in clinical trials are
39
already critically ill when they enter a trial. Informed consent and contractual limitations on payments for subject injury or waivers we obtain may not be enforceable and may not protect us from liability or the costs of product liability litigation. Although we currently carry a $5 million clinical product liability insurance policy, it may not be sufficient to cover future claims. In addition, in some cases the contractors on which we rely for manufacturing our product candidates may indemnify us for third party claims brought against us arising from matters for which these contractors are responsible. We could be materially and adversely affected if we were required to pay damages or incur defense costs in connection with a claim outside the scope of indemnity or insurance coverage, if the indemnity is not performed or enforced in accordance with its terms, or if our liability exceeds the amount of applicable insurance or indemnity. In addition, there can be no assurance that insurance will continue to be available on terms acceptable to us, if at all, or that if obtained, the insurance coverage will be sufficient to cover any potential claims or liabilities. Similar risks would exist upon the commercialization or marketing of any products by us or our partners. We currently do not have any clinical or product liability claims or threats of claims filed against us.
We may expend our limited resources to pursue a particular product candidate or indication and fail to capitalize on product candidates or indications that may be more profitable or for which there is a greater likelihood of success.
Because we have limited financial and management resources, we focus on a limited number of research programs and product candidates. As a result, we may forego or delay pursuit of opportunities with other product candidates or for other indications that later prove to have greater commercial potential. Our resource allocation decisions may cause us to fail to capitalize on viable product candidates or profitable market opportunities. Our spending on current and future research and development programs and product candidates for the specific indications we have selected may not yield any commercially viable products. If we do not accurately evaluate the commercial potential or target market for a particular product candidate, we may relinquish valuable rights to that product candidate through collaboration, licensing or other arrangements in cases in which it would have been more advantageous for us to retain sole development and commercialization rights.
RISKS RELATED TO COMMERCIALIZATION OF OUR PRODUCT CANDIDATES
The failure of our products to gain market acceptance will hinder our ability to generate revenues from the sale of our products.
Even if our product candidates are approved for commercialization, they may be too expensive to manufacture or market or they may not otherwise be successful in the marketplace. Market acceptance of any of our product candidates, if approved for commercial sale, will depend on a number of factors including, but not limited to:
- •
- demonstration of significant clinical effectiveness and safety of our product;
- •
- the prevalence and severity of any adverse side effects experienced by patients to whom our product is administered;
- •
- limitations or warnings contained in the approved labeling or package insert for our product;
- •
- the availability of alternative treatments for the indications approved for our product;
- •
- the availability of acceptable pricing and adequate third-party reimbursement for our product;
- •
- the effectiveness of marketing and distribution methods for our product; and
- •
- comparison of the above factors to those associated with treatments with which our product competes.
40
If our products do not gain market acceptance among physicians, patients, treatment centers, healthcare payors and others in the health care community, any of which may not accept or utilize our products, our ability to generate significant revenues from our products would be limited and our financial condition will be materially adversely affected. In addition, if we fail to successfully penetrate our core markets and successfully expand our business into new markets, the growth in sales of our products, along with our business's operating results, could be negatively impacted.
Our ability to penetrate the SCD treatment market and other markets in which we compete, or to expand our business into additional countries in Africa, Europe, Asia and the Americas, is subject to numerous factors, many of which are beyond our control. Our products, if successfully developed, may compete with a number of therapeutic products currently manufactured and marketed by major pharmaceutical companies. Our products may also compete with new products currently under development by others or with products which may be less expensive than our products. There is no assurance that our efforts to increase market penetration in our core markets and existing geographic markets will be successful. Our failure to do so could have an adverse effect on our operating results.
We may need to meet some or all of our commercialization needs through strategic partnerships and alliances which may not yield anticipated benefits and may adversely affect our operating results, financial condition and existing business.
We may seek to meet some or all of our commercialization needs through strategic partnerships and alliances. The success of this approach will depend on, among other things:
- •
- the availability of suitable strategic partners and alliance candidates;
- •
- competition from other companies for strategic partners or forming alliances with available strategic partner and alliance candidates;
- •
- our ability to value any potential strategic partnerships or alliances accurately and negotiate favorable terms for any strategic
partnerships or alliances;
- •
- the ability to establish new informational, operational and financial systems to meet the needs of such strategic partnerships or
alliances;
- •
- the ability to achieve anticipated synergies with such strategic partnerships or alliances; and
- •
- the availability of management resources to oversee the integration and operation of such strategic partnerships or alliances.
If we are not successful in finding, negotiating and operating with such strategic partnerships or alliances in the future, we may be required to reevaluate our strategic partnerships and alliance strategy. We also may incur substantial expenses and devote significant management time and resources in seeking to complete such strategic partnerships and alliances. Strategic partnerships and alliances may fail to meet our performance expectations. If we do not achieve the anticipated benefits of such strategic partnerships and alliances as rapidly as expected, or at all, investors or analysts may not perceive the same benefits of the acquisition or alliance as we do. If these risks materialize, our operating results, financial condition and existing business could be materially adversely affected.
We lack experience in commercializing products, and we may not be able to establish the sales and marketing infrastructure necessary to support the commercialization of our product candidates if and when they are approved.
With the conclusion of our Phase 3 clinical trial with respect to our pharmaceutical grade L-glutamine treatment for SCD, we are transitioning from a company with a development focus to a company capable of supporting commercial activities. We may not be successful in such a transition. We have not yet demonstrated an ability to obtain marketing approval for product candidates and have
41
limited experience in commercializing products. As a result, we may not be as successful as companies that have previously obtained marketing approval for product candidates and have more experience commercially launching them as products.
To achieve commercial success for any of our product candidates for which we may obtain marketing approval, including our pharmaceutical grade L-glutamine treatment for SCD, we will need to establish a sales and marketing infrastructure to market them as products. This will require us to attract and retain key personnel with commercial experience to lead the commercialization of our pharmaceutical grade L-glutamine treatment for SCD if approved by the FDA and other product candidates that we may develop. We have not yet identified or retained these key personnel and have no assurance that we will be able to do so on commercially acceptable terms, or at all. There are risks involved with establishing our own sales, marketing and distribution capabilities. For example, recruiting and training a sales force is expensive and time consuming and could delay any product launch. If the commercial launch of a product for which we recruit a sales force and establish marketing capabilities is delayed or does not occur for any reason, we would have prematurely or unnecessarily incurred these commercialization expenses. This may be costly, and our investment would be lost if we cannot retain or reposition our sales and marketing personnel.
Factors that may inhibit our efforts to commercialize our products on our own include:
- •
- our inability to recruit, train and retain adequate numbers of effective sales and marketing personnel;
- •
- the inability of sales personnel to obtain access to physicians or persuade adequate numbers of physicians to prescribe any of our
products;
- •
- the lack of complementary or sample products to be offered by sales personnel, which may put us at a competitive disadvantage relative
to companies with more products;
- •
- unforeseen costs and expenses associated with creating an independent sales and marketing organization; and
- •
- the inability of sales and marketing personnel to maintain compliance with federal and state regulations and our inability to adequately monitor and ensure such compliance.
If we are unable to establish our own sales, marketing and distribution capabilities and instead enter into arrangements with third parties to perform these services, our revenue and our profitability, if any, are likely to be lower than if we were to sell, market and distribute any products that we develop ourselves. In addition, we may not be successful in entering into arrangements with third parties to sell, market and distribute our product candidates or may be unable to do so on terms that are favorable to us. We likely will have little control over such third parties, and any of them may fail to devote the necessary resources and attention to sell and market our products effectively. If we do not establish sales, marketing and distribution capabilities successfully, either on our own or in collaboration with third parties, we will not be successful in commercializing any of our product candidates for which we obtain marketing approval.
Failure to obtain acceptable prices or adequate reimbursement for our products may cause an adverse impact on our results of operations.
Our ability to successfully commercialize our products will depend significantly on our ability to obtain acceptable prices and the availability of reimbursement to the patient from third-party payors, such as governmental and private insurance plans. Our products may not be considered medically necessary or cost-effective, may not compare favorably on price with other L-glutamine products, and reimbursement to the patient may not be available or sufficient to allow us or our partners to sell our
42
products on a competitive basis. It may not be possible to negotiate favorable reimbursement rates for our products.
Significant uncertainty exists as to the reimbursement status of newly approved healthcare products. Third-party payors frequently require companies to provide predetermined discounts from list prices, and they are increasingly challenging the prices charged for pharmaceuticals and other medical products. In addition, the continuing efforts of third-party payors to contain or reduce the costs of healthcare through various means may limit our commercial opportunity and reduce any associated revenue and profits. For example, in some foreign markets, the pricing or profitability of healthcare products is subject to government control. In other foreign markets, including Africa where the largest population of patients with SCD exists, there is a limited number of third-party payors from which reimbursement can be sought. In the United States, there have been, and we expect there will continue to be, a number of federal and state proposals to implement similar government control, as evidenced by the passing of the Patient Protection and Affordable Care Act and its amendment, the Health Care and Education Reconciliation Act. Such government-adopted reform measures may adversely impact the pricing of healthcare products and services in the United States or internationally and the amount of reimbursement available from governmental agencies or other third-party payors. In addition, increasing emphasis on managed care will continue to put pressure on the pricing of pharmaceutical products. Cost control initiatives could decrease the price that we or any current or potential collaborators could receive for any of our products and could adversely affect our profitability. If we fail to obtain acceptable prices or an adequate level of reimbursement for our products, the sales of our products would be adversely affected or there may be no commercially viable market for our products.
If the market opportunities for our product candidates are smaller than we believe they are, our revenues may be adversely affected and our business may suffer. Because the target patient populations of our product candidates are small, we must be able to successfully identify patients and achieve a significant market share to maintain profitability and growth.
We focus our research and product development on treatments for rare diseases and conditions. Our projections of both the number of people who have these diseases or conditions, as well as the subset of people with these diseases or conditions who have the potential to benefit from treatment with our product candidates, are based on estimates. These estimates may prove to be incorrect and new studies may change the estimated incidence or prevalence of these diseases or conditions. The number of patients in the United States, Europe and elsewhere may turn out to be lower than expected, may not be otherwise amenable to treatment with our product candidates (if approved), or new patients may become increasingly difficult to identify or gain access to, all of which would adversely affect our results of operations and our business.
The intellectual property that we have licensed relating to our pharmaceutical grade L-glutamine treatment for SCD is limited, which could adversely affect our ability to compete in the market and adversely affect the value of our product candidate.
The patent and exclusivity protections that we expect to protect our pharmaceutical grade L-glutamine product for treatment of SCD from competition is a combination of (i) rights under a license of U.S. Patent No. 5,693,671, entitled "L-glutamine Therapy for SCD and Thalassemia" issued on December 2, 1997 to Niihara et al., which we refer to as the SCD Patent, (ii) Orphan Drug market exclusivity under FDA, European Union and similar foreign regulations, and (iii) data exclusivity in the United States under the Drug Price Competition and Patent Restoration Act of 1984, called the Hatch/Waxman Act. These protections are limited in ways that may affect our ability to effectively
43
exclude third parties from competing against us if we obtain regulatory approval to market our pharmaceutical grade L-glutamine treatment for SCD. In particular:
- •
- each of the protections run concurrently, resulting in protection from competition that will diminish over time as the protections
expire;
- •
- protection under the SCD Patent is scheduled to expire in 2016;
- •
- the FDA may not agree that our product is entitled to data exclusivity under the Hatch/Waxman Act and, if granted, data exclusivity
protection under the Hatch/Waxman Act will expire three years after our product is approved;
- •
- Orphan Drug market exclusivity in the United States will only be granted if our product receives the first FDA approval for an
L-glutamine treatment for SCD and, if granted, Orphan Drug market exclusivity protection will expire seven years after our product is approved. To be eligible for Orphan Drug market exclusivity in the
United States requires that the indication approved by the FDA for our product (if it is approved) matches the indication for which the FDA originally granted us Orphan Drug designation. Orphan Drug
designation will not preclude the FDA from approving a clinically superior product, approving a product that is the same as our product for a different indication, or approving a different product
intended to treat SCD;
- •
- Orphan Drug status in the European Union is subject to exclusions similar to those in the United States; and
- •
- there are many countries, including some key markets for our product, in which we do not have intellectual property coverage and where neither orphan drug nor data exclusivity is available.
If we obtain marketing approval for our pharmaceutical grade L-glutamine treatment for SCD, these limitations and any reductions in our expected protection resulting from any failure to obtain exclusivity under Orphan Drug protections or the Hatch/Waxman Act, or the approval of competing products based on the exceptions to Orphan Drug protections, may subject our product to greater competition than we expect and reduce our ability to generate revenue from our product candidate, possibly materially. These circumstances may also impair our ability to obtain license partners or other international commercialization opportunities on terms acceptable to us, if at all.
If our competitors succeed in developing products and technologies that are more effective than our own, or if scientific developments change our understanding of the potential scope and utility of our product candidates and products, then our technologies and future product candidates and products may be rendered less competitive.
We face significant competition from industry participants, both pharmaceutical and nutritional, that are pursuing similar technologies to those we are pursuing and are developing pharmaceutical products that are competitive with our product candidates and any resulting products. Nearly all of our industry competitors have greater capital resources, larger research and development staffs and facilities, and a longer history in drug discovery and development, obtaining regulatory approval and pharmaceutical product manufacturing and marketing than we do. With these additional resources and experience, our competitors may be able to respond to the rapid and significant technological changes in the biotechnology and pharmaceutical industries faster than we can. Our future success will depend in large part on our ability to maintain a competitive position with respect to these technologies and to respond effectively and timely to competition. We may not be able to do this successfully. Rapid technological development, as well as new scientific developments, may result in our lead compounds, development compounds, product candidates or products becoming obsolete or less effective or otherwise clinically inferior for some or all patients before we can recover any of the expenses incurred to develop them. For example, changes in our understanding of the appropriate population of patients who should be treated with a targeted therapy of the type that we are developing may limit the market
44
potential of our treatment if it is subsequently demonstrated that only certain subsets of the overall patient population should be treated with our treatment. If a competitor develops the same product candidate as ours for the same indication but is able to demonstrate that its product candidate is clinically superior to ours, or if the same product candidate is intended for a different indication, or if we are unable to ensure adequate supplies of our product candidate following any approval by the FDA, the FDA may approve our competitor's marketing application regardless of any marketing exclusivity we may obtain. Any of these circumstances could have a material adverse effect on our ability to successfully commercialize our product candidate if it is approved and thus on our business and financial condition.
We may face potential competition for our pharmaceutical grade L-glutamine treatment for SCD from manufacturers and resellers of L-glutamine that may or may not be manufactured with the same purity and quality as our pharmaceutical grade L-glutamine.
The amino acid L-glutamine is manufactured in large quantities, primarily by a few large chemical companies, and processed and sold as nutritional supplements. These quantities may not evidence the same level of quality and purity as our pharmaceutical grade L-glutamine. The sale of either pharmaceutical grade or non-pharmaceutical grade supplies of L-glutamine at prices lower than the minimum that we must charge to become and remain profitable could have a material adverse effect on our results.
There are known adverse side effects to our NutreStore product.
We market and sell NutreStore L-glutamine powder for oral solution, a prescription pharmaceutical product that has received FDA approval for the treatment of SBS when used in combination with a recombinant human growth hormone approved for SBS, and a specialized nutritional support. Patients with SBS receiving intravenous parenteral nutrition, or IPN, and NutreStore should be routinely monitored for kidney and liver function, particularly patients with kidney or liver impairment. Common reported side effects of NutreStore for patients with SBS include, but are not limited to, nausea or vomiting, feeling the need or urge to empty bowels, gas, abdominal pain, hemorrhoids, constipation, and aggravation of Crohn's disease, gastric ulcer or gastric fistula. The approved indication of the product in combination with recombinant human growth hormone and specialized diets, and any of these known side effects or any associated warning statements or labeling requirements may limit the commercial profile of this product and prevent us from achieving or maintaining market acceptance of such product.
RISKS RELATED TO OUR RELIANCE ON THIRD PARTIES
If the L-glutamine manufacturers upon which we rely fail to produce in the volumes and quality that we require on a timely basis, or fail to comply with stringent regulations applicable to pharmaceutical manufacturers, we may face delays in the development and commercialization of, or be unable to meet demand for, our L-glutamine-based products, if any, and may lose any marketing exclusivity and potential revenues.
We do not currently have our own manufacturing capabilities. We therefore depend heavily upon third party manufacturers for supplies of both our product candidates under development and the products we sell. The manufacture of pharmaceutical products requires significant expertise and capital investment, including the development of advanced manufacturing techniques and process controls. Our third party manufacturers and key suppliers may experience manufacturing difficulties due to resource constraints or as a result of labor disputes, unstable political environments at foreign facilities, financial difficulties or other problems beyond their control and may not be able to expand their capacity or to produce additional product requirements for us in the event that demand for our products increases. If these manufacturers or key suppliers were to encounter any of these difficulties, or otherwise fail to
45
comply with their regulatory and contractual obligations, our ability to timely launch any potential product candidate, if approved, would be jeopardized. If we are unable to ensure adequate supply of an orphan drug for which we have obtained marketing exclusivity, the FDA may approve another drug for marketing, which could have a material adverse effect on our business and financial condition.
We currently obtain our pharmaceutical grade L-glutamine from two Japanese companies, which together produce the vast majority of pharmaceutical grade L-glutamine approved for sale in the United States, and obtain all of our L-glutamine for our NutreStore product from one of these companies. We intend to continue to rely on these manufacturers to produce our pharmaceutical grade L-glutamine, but we have not entered into, and may not be able to establish, long-term supply agreements with these key suppliers on acceptable terms. If these suppliers were to experience any manufacturing or production difficulties producing pharmaceutical grade L-glutamine, or we were unable to purchase sufficient quantities of pharmaceutical grade L-glutamine on acceptable terms, our ability to conduct additional clinical trials, to commercialize pharmaceutical grade L-glutamine for the treatment of SCD, to maintain marketing exclusivity if granted, and to continue to sell NutreStore would be harmed.
In addition, all manufacturers, packers, distributors, and suppliers of pharmaceutical products must comply with applicable cGMP regulations for the manufacture of pharmaceutical products, which are enforced by the FDA through its facilities inspection program. The FDA is likely to conduct inspections of our third party manufacturer and key supplier facilities as part of the FDA's pre-approval review of any of our NDAs and post-approval, ongoing compliance programs. If our third party manufacturers and key suppliers are not in compliance with cGMP requirements, it may result in a delay of approval for products undergoing regulatory review or the inability to meet market demands for any approved products, particularly if these sites are supplying single source ingredients required for the manufacture of any potential product. These cGMP requirements include quality control, quality assurance and the maintenance of records and documentation, among other items. Furthermore, each manufacturing facility used to manufacture drug or biological products is subject to FDA inspection and must meet cGMP requirements. As a result, if one of the manufacturers that we rely on shifts production from one facility to another, the new facility must undergo a preapproval inspection and, for biological products, must be licensed by regulatory authorities prior to being used for commercial supply. Our manufacturers may be unable or fail to comply with these cGMP requirements and with other FDA, state and foreign regulatory requirements. A failure to comply with any applicable manufacturing requirements may result in warning or untitled letters, fines, product recalls, seizures, corrective actions involving public notifications, injunctions, total or partial suspension of production, civil money penalties, suspension or withdrawals of previously granted regulatory approvals, refusal to approve new applications or supplements to applications for marketing of new products, import or export bans or restrictions, disgorgement of profits, debarment, and criminal prosecution and criminal penalties. Any of these penalties could delay or prevent the promotion, marketing or sale of our products. If the safety of any quantities supplied is compromised due to a third party manufacturer's failure to comply with or adhere to applicable laws or for other reasons, we may be liable for injuries suffered by patients who have taken such products and we may not be able to obtain regulatory approval for or successfully commercialize our products.
We expect to rely on third parties to conduct future clinical trials of our product candidates and those third parties may not perform satisfactorily, including failing to meet deadlines for the conduct of such trials.
We engaged a third-party contract research organization, or CRO, to conduct our clinical trials for our pharmaceutical grade L-glutamine treatment for SCD and expect to engage a CRO to conduct any further required clinical trials with respect to such product and any clinical trials with respect to any of our other product candidates that may progress to clinical development. We expect to continue to rely on third parties, such as CROs, clinical data management organizations, medical institutions and
46
clinical investigators, to conduct those clinical trials. Agreements with such third parties might terminate for a variety of reasons, including a failure to perform by the third parties. If we need to enter into alternative arrangements, that would delay our product development activities.
Our reliance on these third parties for research and development activities will reduce our control over these activities, but will not relieve us of our responsibilities. For example, we will remain responsible for ensuring that each of our clinical trials is conducted in accordance with the general investigational plan and protocols for the trial. Moreover, the FDA requires us to comply with standards, commonly referred to as good clinical practices, or GCPs, for conducting, recording and reporting the results of clinical trials to ensure that data and reported results are credible and accurate and that the rights, integrity and confidentiality of trial participants are protected. We also are required to register ongoing clinical trials and post the results of completed clinical trials on a government-sponsored database, www.ClinicalTrials.gov, within specified timeframes. Failure to do so can result in the FDA refusing to accept a NDA for the product candidate under study, fines, adverse publicity and civil and criminal sanctions.
Furthermore, these third parties may also have relationships with other entities, some of which may be our competitors. If these third parties do not successfully carry out their contractual duties, meet expected deadlines or conduct our clinical trials in accordance with regulatory requirements and our stated protocols, we will not be able to obtain, or may be delayed in obtaining, marketing approvals for our product candidates and will not be able to, or may be delayed in our efforts to, successfully commercialize them as products.
We also expect to rely on other third parties to store and distribute supplies of our product candidates for clinical trials of them. Any performance failure on the part of our distributors could delay clinical development or marketing approval of our product candidates or commercialization of them as products, producing additional losses and depriving us of potential revenue.
If we do not obtain the support of new, and maintain the support of existing, key scientific collaborators, it may be difficult to research medical indications for L-glutamine other than SCD and to expand our product offerings, which may limit our revenue growth and profitability and could have a material adverse effect on our business, financial condition and operating results.
We will need to establish relationships with additional leading scientists and research institutions in order to develop new products and expand our product offerings and to explore other medical indications for L-glutamine-based products. Although we have established research collaborations, we cannot assure you that our relationships with our research collaborators will continue or that we will be able to attract additional research partners. If we are not able to maintain existing or establish new scientific relationships to assist in our research and development, we may not be able to successfully develop our product candidates or expand our product offerings.
RISKS RELATED TO OUR INTELLECTUAL PROPERTY
We depend on licenses and sublicenses of certain patents for our existing L-glutamine products and our pharmaceutical grade L-glutamine treatment for SCD product candidate. If any of these licenses, sublicenses, or the licenses under which we have been sublicensed terminates, or if any of the patents that have been licensed or sublicensed to us are challenged and we are limited in our ability to utilize any of those patents, we may be unable to develop, out-license, market and sell our products, which would cause a material adverse effect on our business, prospects, financial condition, and operating results.
Our ability to develop products depends on licenses and sublicenses we have obtained to patents that claim the use of L-glutamine to treat SCD.
47
We face the risk that any of these licenses and sublicenses could be terminated if we fail to satisfy our obligations under them. In addition, even if we satisfy our obligations as sublicensee under any sublicense, if the license under which we have been sublicensed terminates, our sublicense could also terminate. In the event any claims in the patents that we have been licensed or sublicensed are challenged, the court or patent authority to which such challenge is presented could determine that such patent claims are invalid or unenforceable or not sufficiently broad in scope to protect our proprietary rights. In addition, as the licensee or sublicensee of such patents, our ability to participate in the defense or enforcement of such patents could be limited.
If we are unable to protect proprietary technology that we invent and develop, we may not be able to compete effectively and our business and financial prospects may be harmed.
Where appropriate, we seek patent protection for inventions we conceive and reduce to practice. Patent protection, however, is not available for all of these inventions and for some of these patent protection may be limited. In addition, in developing some of our inventions, we may have to design around patents held by others. If we must spend significant time and money protecting our patents, designing around patents held by others or in-licensing patent or other proprietary rights held by others, potentially for large fees, our business and financial prospects may be harmed.
The patent prosecution process is expensive and time consuming, and we may not be able to file and prosecute all necessary or desirable patent applications at a reasonable cost or in a timely manner. It is also possible that we will fail to identify patentable aspects of our research and development output before it is too late to obtain patent protection. We also may have to relinquish to strategic partners or other third parties to whom we license our technology the right to control the preparation, filing and prosecution of patent applications claiming our inventions and to maintain any resulting patents. Therefore, patent applications and patents claiming our inventions may not be prosecuted and enforced in a manner consistent with the best interests of our business.
The patent position of biotechnology and pharmaceutical companies generally is highly uncertain, involves complex legal and factual questions and has in recent years been the subject of much litigation. In addition, the laws of foreign countries may not protect our rights to the same extent as the laws of the United States, or vice versa. For example, European patent law restricts the patentability of methods of treatment of the human body more than U.S. law does. Publications of discoveries in the scientific literature often lag behind the actual discoveries, and patent applications in the United States and other jurisdictions are typically not published until 18 months after filing or, in some cases, not at all. Therefore, we cannot know with certainty whether we were the first to make the inventions claimed in our patents or pending patent applications, or that we were the first to file for patent protection of such inventions. As a result, the issuance, scope, validity, enforceability and commercial value of our patent rights are highly uncertain. Our pending and future patent applications may not result in patents being issued that protect our product candidates, in whole or in part, or which effectively prevent others from commercializing competitive product candidates. Changes in either the patent laws or interpretation of the patent laws in the United States and other countries may diminish the value of our patents or narrow the scope of our patent protection.
Recent patent reform legislation could increase the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of our issued patents. On September 16, 2011, the Leahy-Smith America Invents Act, or the Leahy-Smith Act, was signed into law. The Leahy-Smith Act includes a number of significant changes to U.S. patent law. These include provisions that affect the way patent applications are prosecuted and may also affect patent litigation. The U.S. Patent and Trademark Office recently developed new regulations and procedures to govern administration of the Leahy-Smith Act, and many of the substantive changes to patent law associated with the Leahy-Smith Act, and in particular, the first to file provisions, only became effective on March 16, 2013. Accordingly, it is not clear what, if any, impact the Leahy-Smith Act will have on the
48
operation of our business. However, the Leahy-Smith Act and its implementation could increase the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of our licensed patents, all of which could have a material adverse effect on our business and financial condition.
Even if our patent applications issue as patents, they may not issue in a form that will provide us with any meaningful protection, prevent competitors from competing with us or otherwise provide us with any competitive advantage. Our competitors may be able to circumvent our patents by developing similar or alternative treatments in a non-infringing manner.
In addition, the issuance of a patent is not conclusive as to its inventorship, scope, validity or enforceability, and our patents may be challenged in the courts or patent offices in the United States and abroad. Such challenges may result in loss of exclusivity or freedom to operate or in patent claims being narrowed, invalidated or held unenforceable, in whole or in part, which could limit our ability to stop others from using or commercializing products similar or identical to our product candidates or products, or limit the duration of the patent protection of our product candidates or products. Given the amount of time required for the development, testing and regulatory review of new therapeutics, patents protecting our product candidates might expire before or shortly after such candidates are commercialized as products. Patent protection for our pharmaceutical grade L-glutamine treatment for SCD expires in 2016. As a result, our patent portfolio may not provide us with sufficient rights to exclude others from commercializing products similar or identical to ours.
We will incur significant ongoing expenses in maintaining our patent portfolio. Should we lack the funds to maintain our patent portfolio or to enforce our rights against infringers, we could be adversely impacted.
Third parties may initiate legal proceedings alleging that we are infringing their intellectual property rights, the outcome of which would be uncertain and could have a material adverse effect on the success of our business.
Our commercial success depends upon our ability, and the ability of our collaborators, to develop, manufacture, market and sell our products without infringing the proprietary rights of third parties. There is considerable intellectual property litigation in the biotechnology and pharmaceutical industries. We may become party to, or threatened with, future adversarial proceedings or litigation regarding intellectual property rights with respect to our product candidates and products. In these proceedings or litigation, third parties may assert infringement claims against us based on existing patents or patents that may be granted in the future. The party making such claims could secure a judgment awarding substantial damages, as well as injunctive or other equitable relief. Such relief could effectively block our ability to make, use, sell, distribute or market our products in such jurisdiction. Even if claims of infringement are without merit, any such action could divert the time and attention of management and impair our ability to access additional capital and/or cost us significant funds to defend.
If we are found to infringe a third party's intellectual property rights, we could be required to obtain a license from such third party to continue developing our product candidates and manufacturing and marketing any of our products. However, we may not be able to obtain any required license on commercially reasonable terms or at all. Even if we were able to obtain a license, it could be non-exclusive, thereby giving our competitors access to the same technologies licensed to us. Alternatively, we could be ordered to cease commercializing any of our products that is found to infringe a third party's intellectual property rights. In addition to being forced to cease commercialization of such a product, we could be found liable for monetary damages, including treble damages and attorneys' fees if we are found to have willfully infringed a third party's patent. A finding of infringement could prevent us from developing our product candidates and commercializing our products or force us to cease some of our business operations. Claims that we have misappropriated
49
the confidential information or trade secrets of third parties could have a similar negative impact on our business.
We may be subject to claims by third parties asserting that we or our employees have misappropriated their intellectual property, or claiming ownership of what we regard as our own intellectual property.
Although we try to ensure that our employees do not use the proprietary information or know-how of others in their work for us, we may be subject to claims that these employees or we have used or disclosed intellectual property, including trade secrets or other proprietary information, of any such employee's former employer. Litigation may be necessary to defend against these claims.
Moreover, we may be subject to a third-party preissuance submission of prior art to the U.S. Patent and Trademark Office, or become involved in opposition, derivation, reexamination, inter partes review, post-grant review or interference proceedings challenging our patent rights or the patent rights of others. An adverse determination in any such submission, proceeding or litigation could reduce the scope of or invalidate our patent rights, allow third parties to commercialize products similar or identical to our product candidates or products and compete directly with us, without payment to us, or result in our inability to manufacture or commercialize products without infringing third-party patent rights. In addition, if the breadth or strength of protection provided by our patents and patent applications is threatened, it could dissuade companies from collaborating with us to license, develop or commercialize current or future product candidates.
We may become involved in lawsuits to protect or enforce our patents or other intellectual property, which could be expensive, time consuming and unsuccessful.
Competitors may infringe our issued patents, trade secrets, know-how or other intellectual property. To counter infringement or unauthorized use or to determine the scope and validity of our intellectual property rights, we may be required to file infringement claims or pursue other proceedings, which can be expensive and time consuming. Any claims we assert against perceived infringers could provoke these parties to assert counterclaims against us alleging that we infringe their patents. In addition, in a patent infringement proceeding, a court may decide that a patent of ours is invalid or unenforceable, in whole or in part, construe the patent's claims narrowly or refuse to stop the other party from using the technology at issue on the grounds that our patents do not cover the technology. An adverse result in any litigation or other proceeding could put one or more of our patents at risk of being invalidated or interpreted narrowly, subject us to significant liabilities, require us to cease using the subject technology or require us to license the subject technology from the third party, any or all of which could have a material adverse effect on our business.
Intellectual property litigation could cause us to spend substantial resources and distract our personnel from their normal responsibilities.
Even if resolved in our favor, litigation or other legal proceedings relating to intellectual property claims may cause us to incur significant expenses, and could distract our technical and management personnel from their normal responsibilities. In addition, there could be public announcements of the results of hearings, motions or other interim proceedings or developments, and if securities analysts or investors perceive these results to be negative it could have a substantial adverse effect on the price of our common stock. Such litigation or proceedings could substantially increase our operating losses and reduce the resources available for development activities or any future sales, marketing or distribution activities. We may not have sufficient financial or other resources to conduct such litigation or proceedings adequately. Some of our competitors may be able to sustain the costs of such litigation or proceedings more effectively than we can because of their greater financial resources. Uncertainties resulting from the initiation and continuation of patent litigation or other proceedings could compromise our ability to compete in the marketplace.
50
If we are unable to protect the confidentiality of our trade secrets, our business and competitive position would be harmed.
In addition to licensing patent rights and seeking patents for our intellectual property, we also rely on trade secrets, including unpatented know-how, technology and other proprietary information, to maintain our competitive position. Our competitors may use our methods, or acquire similar expertise, in order to develop L-glutamine-based treatments and progress these through clinical development and commercialization, which could impair our ability to successfully develop our product candidates and commercialize them as products.
We seek to protect our trade secrets, in part, by entering into non-disclosure and confidentiality agreements with parties who have access to them, such as our employees, corporate collaborators, outside scientific collaborators, contract manufacturers, consultants, advisors and other third parties. Despite these efforts, any of these parties may breach the agreements and disclose our proprietary information, including our trade secrets, and we may not be able to obtain adequate remedies for such breaches. Enforcing a claim that a party illegally disclosed or misappropriated a trade secret is difficult, expensive and time consuming, and the outcome is unpredictable. In addition, some courts inside and outside the United States are less willing or unwilling to protect trade secrets. If any of our trade secrets were to be lawfully obtained or independently developed by a competitor, we would have no right to prevent them, or those to whom they communicate it, from using that technology or information to compete with us. If any of our trade secrets were to be disclosed to or independently developed by a competitor, our competitive position would be harmed.
We may not be able to protect our intellectual property rights throughout the world.
Filing, prosecuting and defending patents in all countries throughout the world would be prohibitively expensive, and our intellectual property rights in some countries outside the United States can be less extensive than those in the United States. In addition, the laws of some foreign countries do not protect intellectual property rights to the same extent as federal and state laws in the United States. Consequently, we may not be able to prevent third parties from practicing our inventions in all countries outside the United States, or from selling or importing products made using our inventions in and into the United States or other jurisdictions. Competitors may use our technologies in jurisdictions where we have not obtained patent protection to develop their own products and further, may export otherwise infringing products to territories where we have patent protection, but enforcement is not as strong as that in the United States. These products may compete with our products and our patents or other intellectual property rights may not be effective or sufficient to prevent them from competing.
Many companies have encountered significant problems in protecting and defending intellectual property rights in foreign jurisdictions. The legal systems of certain countries, particularly certain developing countries, do not favor the enforcement of patents, trade secrets and other intellectual property protection, particularly those relating to biotechnology products, which could make it difficult for us to stop the infringement of our patents or marketing of competing products in violation of our proprietary rights generally. Proceedings to enforce our patent rights in foreign jurisdictions could result in substantial costs and divert our efforts and attention from other aspects of our business, could put our patents at risk of being invalidated or interpreted narrowly and our patent applications at risk of not issuing and could provoke third parties to assert claims against us. We may not prevail in any lawsuits that we initiate and the damages or other remedies awarded, if any, may not be commercially meaningful. Accordingly, our efforts to enforce our intellectual property rights around the world may be inadequate to obtain a significant commercial advantage from the intellectual property that we develop or license.
51
Companies and universities that have licensed product candidates to us for research, clinical development and marketing are sophisticated competitors that could develop similar products to compete with our products, which could reduce our future revenues.
Licensing our product candidates from other companies, universities or individuals does not always prevent them from developing non-identical but competitive products for their own commercial purposes, nor from pursuing patent protection in areas that are competitive with us. While we seek patent protection for all of our owned and in-licensed product candidates, the entities and individuals who have assigned or licensed to us these product candidates employ or are, as applicable, experienced scientists who may continue to do research and development relevant to our product candidates, and any of them may seek patent protection in the same areas that led to the discovery of the product candidates that they have assigned or licensed to us. By virtue of the previous research that led to the discovery of the inventions that they licensed or assigned to us, these companies, universities, and individuals may be able to develop and market competitive products in less time than might be required to develop a product with which they have no prior experience and may reduce our future revenues from products resulting from successful development and approval of our product candidates.
RISKS RELATED TO REGULATORY APPROVAL OF OUR PRODUCT CANDIDATES AND OTHER LEGAL COMPLIANCE MATTERS
Our business is subject to extensive government regulation, which could cause delays in the development of our product candidates and commercialization of any resulting products, impose significant costs on us or provide advantages to our larger competitors.
The FDA and similar regulatory authorities in foreign countries impose substantial requirements upon the development, manufacture and marketing of therapeutic products, such as drugs, biologics, and cell-based therapies. Failure to obtain marketing approval for any of our product candidates in any jurisdiction will prevent us from commercializing it as a product in that jurisdiction. The FDA and most other regulatory authorities impose requirements for laboratory and clinical testing, manufacturing, labeling, registration, marketing, storage, distribution, recordkeeping, reporting, and advertising and promotion, and other costly and time-consuming processes and procedures applicable to therapeutic products. In some cases, as a condition for approval to market any of our product candidates, the FDA or other regulatory authorities may impose commitments that we must satisfy following any such approval. These post-approval commitments could vary substantially from country to country depending upon the type, complexity and novelty of the applicable therapeutic product. Satisfaction of any such post-approval commitments (including the requirement to conduct additional clinical studies), if imposed by the FDA or other regulatory authorities, could take several years or more. In addition, post-approval requirements regarding safety surveillance, cGMP compliance, advertising and promotion, adverse event reporting, and recordkeeping must be satisfied at all times.
The effect of government regulation may be to delay marketing approval of our product candidates for a considerable or indefinite period of time, to impose costly processes and procedures upon our activities and to furnish a competitive advantage to companies that compete with us. There can be no assurance that marketing approval for any of our product candidates would be granted by the FDA or other regulatory authority on a timely basis, if at all, or, once granted, that the marketing authorization would not be withdrawn or other regulatory actions taken which might limit our ability to market our proposed products. Any such delay in obtaining or failing to obtain such approvals or imposition of regulatory actions would adversely affect us, the manufacturing and marketing of the products resulting from marketing approval of any of our product candidates, and our ability to generate product revenue.
52
Even though we have obtained Orphan Drug designation for our most advanced product candidate, our pharmaceutical grade L-glutamine treatment for SCD, we may not be able to obtain or maintain Orphan Drug marketing exclusivity for this product candidate or any of our other product candidates.
Regulatory authorities in some jurisdictions, including the United States and the European Union, or EU, may designate therapeutic products under development for relatively small patient populations as "orphan drugs". Under the Orphan Drug Act, the FDA may designate a therapeutic product as an Orphan Drug if it is intended to treat a rare disease or condition, which is generally defined as a patient population of fewer than 200,000 individuals in the United States. We have obtained Orphan Drug designation from the FDA and European Commission, or EC, for L-glutamine treatment for SCD, and we may seek Orphan Drug designation for our other product candidates. Generally, if a product candidate with an Orphan Drug designation subsequently receives the first marketing approval for the indication for which it has been granted such designation, the drug is entitled to a period of marketing exclusivity, which precludes the FDA or European Medicines Agency, or EMA, as applicable, from approving another marketing application for the same product candidate prior to the expiration of that time period. The applicable period is seven years in the United States and 10 years in the European Union. The exclusivity period in the European Union can be reduced to six years if the product no longer meets the criteria for Orphan Drug designation or if its commercialization is sufficiently profitable so that market exclusivity is no longer justified. Orphan Drug exclusivity may be lost if the FDA or EMA determines that the request for designation was materially defective or if the manufacturer is unable to ensure sufficient quantity of the product to meet the needs of patients with the rare disease or condition. In the United States, Orphan Drug exclusivity may be lost if another L-glutamine product for the same indication demonstrates clinical superiority, such as a better safety or efficacy profile, in which case the FDA would be permitted to approve the third party product. Orphan Drug exclusivity does not bar the FDA from approving another L-glutamine product for any other indication. Nor does Orphan Drug designation bar the FDA from granting Orphan Drug designation and approving another product for the same orphan disease or condition.
Even if we receive three years of data exclusivity upon approval of our pharmaceutical grade L-glutamine treatment for SCD under the Hatch-Waxman Act, that exclusivity will run concurrently with Orphan Drug exclusivity and will not prevent the FDA from approving other products intended to treat SCD or other L-glutamine products intended to treat other diseases or conditions.
Under the Hatch/Waxman Act, a three-year period of data exclusivity is granted for a drug product that contains an active pharmaceutical ingredient, or active moiety, that has been previously approved, when the NDA for that drug product contains reports of new clinical investigations (other than bioavailability studies) conducted or sponsored by the sponsor of the NDA that were essential to approval of the NDA. When granted to a sponsor, data exclusivity under the Hatch/Waxman Act prevents any third parties, such as generic drug manufacturers, from relying on or using the sponsor's data from the sponsor's new clinical investigations in order to obtain marketing approval for the same active moiety and indication for which the sponsor's NDA was approved.
The FDA defines "new clinical investigation" as "a clinical study in humans the results of which have not been relied upon by FDA to demonstrate substantial evidence of effectiveness of a previously approved drug product for any indication or of safety for a new patient population and do not duplicate the results of another investigation that was relied upon by the agency to demonstrate the effectiveness or safety in a new patient population of a previously approved drug product." Our pharmaceutical grade L-glutamine treatment for SCD contains the same active moiety contained in our previously approved NutreStore product and we believe our Phase 3 clinical study meets the definition of a new clinical investigation for purposes of satisfying the requirements for Hatch/Waxman data exclusivity to be granted.
53
Although we anticipate receiving three years of data exclusivity if our pharmaceutical grade L-glutamine treatment for SCD is approved, we may not be able to take advantage of all or any of the benefits of such exclusivity if Orphan Drug exclusivity is granted, because both exclusivities would run concurrently. However, Hatch/Waxman three-year exclusivity would bar the approval of the same product for the same indication even if the same product demonstrated clinical superiority. Similar to Orphan Drug exclusivity, the three-year exclusivity provided under the Hatch/Waxman Act would not bar the FDA from approving another L-glutamine product for an indication other than SCD; nor would it bar the FDA from approving a different active moiety to treat SCD.
The FDA Fast Track designation for our pharmaceutical grade L-glutamine treatment for SCD may not actually lead to a faster development or regulatory review or approval process.
If a product candidate is intended for the treatment of a serious or life-threatening disease or condition and it demonstrates the potential to address unmet medical needs for this disease or condition, its sponsor may apply for FDA Fast Track designation. If Fast Track designation is obtained, the FDA may initiate review of completed individual sections of a NDA before all sections of the application are complete and the application can be submitted for filing by the FDA. This "rolling review" is available if the applicant provides, and the FDA approves, a schedule for submission of the individual sections of the application.
Although we have obtained a Fast Track designation from the FDA for our pharmaceutical grade L-glutamine treatment for SCD, we may not experience a faster development process, review or approval compared to conventional FDA procedures. Our Fast Track designation may be withdrawn by the FDA if it believes that the designation is no longer supported by data from our clinical development program. Our Fast Track designation does not guarantee that we will qualify for or be able to take advantage of the expedited review procedures or that we will ultimately obtain regulatory approval of our pharmaceutical grade L-glutamine treatment for SCD.
A product candidate that receives Fast Track designation is also eligible for, among other benefits, Priority Review, if certain criteria are met. These criteria for Priority Review include whether, if approved, the resulting product would provide significant improvements in the safety or effectiveness of the treatment, diagnosis, or prevention of serious conditions caused by a disease when compared to standard treatment, diagnosis, or prevention of those conditions. Under Priority Review of a NDA, assuming that there are no requests for additional information, the FDA's goal is to take action on the NDA within six months (compared to 10 months under standard review) after it is accepted as filed. Requests for Priority Review of a NDA must be submitted to the FDA when the NDA is submitted. Our current plan is to request Priority Review for the NDA for our pharmaceutical grade L-glutamine treatment for SCD that we plan to file this year. We cannot predict whether the FDA will grant our request for Priority Review for any such NDA or, if the request is granted, that the Priority Review status will be maintained and the NDA approved in a timely manner.
Any product candidate for which we obtain marketing approval would be subject to post-marketing regulatory requirements and limitations and could be subject to recall or withdrawal from the market, and we may be subject to penalties if we fail to comply with such regulatory requirements or if we experience unanticipated problems in commercializing any of our product candidates, when and if any of them are approved by regulators.
Any product candidate for which we obtain marketing approval, along with the collection and reporting of post-approval clinical data, manufacturing processes, labeling, advertising and promotional activities for the resulting product, will be subject to continual requirements of and review by the FDA and other regulatory authorities. These requirements include submissions of safety and other post-marketing information and reports, establishment registration and product listing requirements, cGMP requirements relating to manufacturing, quality control, quality assurance and corresponding
54
maintenance of records and documents, requirements regarding the distribution of samples to physicians and recordkeeping. Even if the FDA or other regulators outside the United States grant marketing approval to any of our product candidates, the approval may be subject to limitations on the indicated uses for which it may be marketed as a product or to the conditions of approval, including the requirement to implement a risk evaluation and mitigation strategy, or REMS. If any of our product candidates receives marketing approval, the labeling (including the package insert) that must accompany its distribution as a product may limit its approved use, which could limit the total number of prescriptions written for such products.
The FDA may also impose requirements for costly post-marketing studies or clinical trials and surveillance to monitor the safety or effectiveness of any approved product. The FDA closely regulates the post-approval marketing and promotion of therapeutic products to ensure they are marketed only for the approved indications and in accordance with the provisions of the approved labeling. The FDA imposes stringent restrictions on manufacturers' communications regarding off-label use, and if we market any of our products for indications that have not been approved, we may be subject to enforcement action for off-label marketing. Violations of the FD&C Act relating to the promotion of prescription products may lead to investigations alleging violations of federal and state healthcare fraud and abuse laws, as well as state consumer protection laws. In recent years, several pharmaceutical companies have been fined significant amounts for such violations.
In addition, later discovery of previously unknown adverse events or other problems with any of our product candidates that are approved for marketing as products, the contract manufacturers from which we obtain supplies of these products, the manufacturing processes they use to manufacture these products, or our or their failure to comply with regulatory requirements, may have negative consequences, including:
- •
- restrictions on the manufacturers or manufacturing processes for such products;
- •
- restrictions on the labeling or marketing of such products;
- •
- restrictions on distribution or use of such products;
- •
- requirements to conduct post-marketing studies or clinical trials;
- •
- warning letters;
- •
- recall or withdrawal of such products from the market;
- •
- refusal to approve pending applications or supplements to approved marketing applications that we submit;
- •
- clinical holds on clinical studies of such products;
- •
- fines, restitution or disgorgement of revenue or profit generated by sales of such products;
- •
- suspension or withdrawal of the marketing approvals of such products;
- •
- refusal to permit the import or export of such products;
- •
- seizure of such products;
- •
- injunctions prohibiting the manufacture, marketing, sale, distribution, or related action in respect of such products;
- •
- the imposition of civil or criminal penalties; or
- •
- debarment of our company and any of our officers or other employees responsible for such problems from future dealings with the FDA.
55
Non-compliance with applicable regulatory requirements regarding safety monitoring, also called pharmacovigilance, and with requirements related to the development of therapeutics for the pediatric population, can also result in significant financial penalties. Similarly, failure to comply with applicable regulatory requirements regarding the protection of personal information can also lead to significant penalties and sanctions.
Our current and future relationships with customers and third-party payors in the United States and elsewhere may be subject, directly or indirectly, to applicable anti-kickback, fraud and abuse, false claims, transparency, health information privacy and security and other healthcare laws and regulations, which could expose us to criminal sanctions, civil penalties, contractual damages, reputational harm, administrative burdens and diminished profits and future earnings.
We are subject to various federal and state laws pertaining to healthcare fraud and abuse, including inducing, facilitating or encouraging submission of false claims to government programs and prohibitions on the offer or payment or acceptance of kickbacks or other remuneration for the purchase of our products. Healthcare providers, physicians and third-party payors in the United States and elsewhere will play a primary role in the recommendation and prescription of any product candidates for which we obtain marketing approval. Our future arrangements with customers and third-party payors may expose us to broadly applicable fraud and abuse and other healthcare laws and regulations, which may constrain the business or financial arrangements and relationships through which we sell, market and distribute any products. In addition, we may be subject to transparency laws aimed at controlling healthcare costs and patient privacy regulation by the U.S. federal and state governments and by governments in foreign jurisdictions in which we conduct our business. The applicable federal, state and foreign healthcare laws and regulations that may affect our ability to operate include:
- •
- the federal Anti-Kickback Statute, which prohibits, among other things, persons from knowingly and willfully soliciting, offering,
receiving or providing remuneration, directly or indirectly, in cash or in kind, to induce or reward, or in return for, either the referral of an individual for, or the purchase, order or
recommendation of, any good or service, for which payment may be made under federal and state healthcare programs, such as Medicare and Medicaid;
- •
- federal civil and criminal false claims laws and civil monetary penalty laws, including the federal False Claims Act, which impose
criminal and civil penalties, including civil qui tam actions (commonly referred to as "whistleblower actions"), against individuals or entities for knowingly presenting, or causing to be presented,
to the federal government, including the Medicare and Medicaid programs, claims for payment that are false or fraudulent or making a false statement to avoid, decrease or conceal an obligation to pay
money to the federal government;
- •
- provisions under the federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, that impose criminal and civil
liability for executing a scheme to defraud any healthcare benefit program or making false statements relating to healthcare matters;
- •
- provisions under HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act of 2009, or HITECH, and
their respective implementing regulations, that impose obligations on covered healthcare providers, health plans, and healthcare clearinghouses, as well as their business associates that create,
receive, maintain or transmit individually identifiable health information for or on behalf of a covered entity, with respect to safeguarding the privacy, security and transmission of individually
identifiable health information;
- •
- the federal Open Payments program under the federal Physician Payments Sunshine Act, which requires manufacturers of drugs, biologics, cell-based therapies, medical devices and medical supplies for which payment is available under Medicare, Medicaid or the Children's Health Insurance Program, with specific exceptions, to report annually to the Centers for Medicare &
56
- •
- state and foreign laws and regulations analogous to those described above, such as state anti-kickback and false claims laws, which
may apply to sales or marketing arrangements and claims involving healthcare items or services reimbursed by non-governmental third-party payors, including private insurers;
- •
- state and foreign laws that require pharmaceutical companies to comply with the pharmaceutical industry's voluntary compliance
guidelines and the relevant compliance guidance promulgated by the federal government or otherwise restrict payments that may be made to healthcare providers;
- •
- state and foreign laws that require pharmaceutical and biopharmaceutical manufacturers to report information related to payments and
other transfers of value to physicians and other healthcare providers or marketing expenditures; and
- •
- state and foreign laws governing the privacy and security of health information in certain circumstances, many of which differ from each other in significant ways and often are not preempted by HIPAA, thus complicating compliance efforts.
Medicaid Services, or CMS, information related to "payments or other transfers of value" made to physicians, which is defined to include doctors, dentists, optometrists, podiatrists and chiropractors, and teaching hospitals and applicable manufacturers and applicable group purchasing organizations to report annually to CMS ownership and investment interests held by the physicians and their immediate family members;
Because of the sweeping language of the federal Anti-Kickback Statute, many potentially beneficial business arrangements would be prohibited if the statute were strictly applied. To avoid this outcome, the Department of Health and Human Services has published regulations, known as "safe harbors," that identify exceptions to or exemptions from the statute's prohibitions. Arrangements that do not fit within the safe harbors are not automatically deemed to be illegal, but must be evaluated on a case by case basis for compliance with the statute. We seek to comply with the Anti-Kickback Statute and, if necessary, to fit within one of the defined safe harbors. We are unaware of any violations of these laws. However, due to the breadth of the statutory provisions and the absence of uniform guidance in the form of regulations or court decisions, there can be no assurance that our practices will not be challenged under anti-kickback or similar laws. Violations of such restrictions may be punishable by civil or criminal sanctions, including fines and civil monetary penalties, as well as the possibility of exclusion from U.S. federal healthcare programs (including Medicaid and Medicare). Any such violations could have a material adverse effect on our business, financial condition, results or operations and cash flows.
Under the False Claims Act, drug manufacturers have been held responsible for claims filed by physicians for reimbursement of the cost of medical services related to uses of a pharmaceutical product that are not on the approved labeling, known as "off-label use," if the manufacturer promoted the product for such off-label use. Violations of the False Claims Act may result in treble damages based on the amount of overpayment and additional civil fines of $5,000 to $10,000 for each false claim. Drug manufacturers could also be held responsible for reimbursement claims submitted by any physician for pharmaceutical products that were knowingly not manufactured in compliance with cGMP regulations.
Efforts to ensure that our business arrangements with third parties will comply with applicable healthcare laws and regulations may involve substantial costs. It is possible that governmental authorities will conclude that our business practices may not comply with current or future statutes, regulations or case law involving applicable fraud and abuse or other healthcare laws and regulations. If our operations are found to be in violation of any of these laws or any other governmental regulations that may apply to us, we, our employees, officers, or directors may be subject to significant civil,
57
criminal and administrative penalties, including, without limitation, damages, fines, imprisonment, exclusion from participation in government healthcare programs, such as Medicare and Medicaid, and the curtailment or restructuring of our operations, which could have a material adverse effect on our business. If any of the physicians or other healthcare providers or entities with whom we expect to do business, including our collaborators, is found not to be in compliance with applicable laws, such person or entity may be subject to criminal, civil or administrative sanctions, including exclusions from participation in government healthcare programs, which could also materially affect our business.
Recently enacted and future legislation may increase the difficulty and cost for us to obtain marketing approval of our product candidates and then commercialize them as products and affect the prices we may obtain.
In the United States and some foreign jurisdictions, there have been a number of legislative and regulatory changes and proposed changes regarding the healthcare system that could prevent or delay marketing approval of our product candidates, restrict or regulate post-approval activities and affect our ability to profitably sell any product candidates for which we obtain marketing approval.
Among policy makers and payors in the United States and elsewhere, there is significant interest in promoting changes in healthcare systems with the stated goals of containing healthcare costs, improving quality and expanding access. In the United States, the pharmaceutical industry has been a particular focus of these efforts and has been significantly affected by major legislative initiatives. In March 2010, President Obama signed into law the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Affordability Reconciliation Act, or, collectively, the PPACA (often commonly referred to as the "Affordable Care Act"), a law intended to broaden access to health insurance, reduce or constrain the growth of healthcare spending, enhance remedies against fraud and abuse, add new transparency requirements for the healthcare and health insurance industries, impose new taxes and fees on the health industry and impose additional health policy reforms.
Among the provisions of the PPACA of importance to our potential product candidates are:
- •
- an annual, nondeductible fee on any entity that manufactures or imports specified branded prescription drugs and biologic agents,
apportioned among these entities according to their market share in certain government healthcare programs;
- •
- an increase in the statutory minimum rebates a manufacturer must pay under the Medicaid Drug Rebate Program to 23.1% and 13.0% of the
average manufacturer price for branded and generic drugs, respectively;
- •
- expansion of healthcare fraud and abuse laws, including the False Claims Act and the Anti-Kickback Statute, new government
investigative powers and enhanced penalties for non-compliance;
- •
- a new Medicare Part D coverage gap discount program, in which manufacturers must agree to offer 50% point-of-sale discounts off
negotiated prices of applicable brand medicines to eligible beneficiaries during their coverage gap period, as a condition for a manufacturer's medicines purchased outside a hospital setting to be
covered under Medicare Part D;
- •
- extension of a manufacturer's Medicaid rebate liability to covered medicines dispensed to individuals who are enrolled in Medicaid
managed care organizations;
- •
- expansion of eligibility criteria for Medicaid programs by, among other things, allowing states to offer Medicaid coverage to additional individuals and by adding new mandatory eligibility categories for certain individuals with income at or below 133% of the federal poverty level beginning in 2014, thereby potentially increasing a manufacturer's Medicaid rebate liability;
58
- •
- expansion of the entities eligible for discounts under the Public Health Service pharmaceutical pricing program;
- •
- the new requirements under the federal Open Payments program and its implementing regulations;
- •
- a new requirement to annually report samples of medicines that manufacturers and distributors provide to physicians; and
- •
- a new Patient-Centered Outcomes Research Institute to oversee, identify priorities in, and conduct comparative clinical effectiveness research, along with funding for such research.
In addition, other legislative changes have been proposed and adopted since the PPACA was enacted. These changes include aggregate reductions to Medicare payments to providers of up to 2% per fiscal year, starting in 2013. On March 1, 2013, the President signed an executive order implementing the 2% Medicare payment reductions, and on April 1, 2013, these reductions went into effect. In January 2013, President Obama signed into law the American Taxpayer Relief Act of 2012, which, among other things, reduced Medicare payments to several providers and increased the statute of limitations period for the government to recover overpayments to providers from three to five years. These new laws may result in additional reductions in Medicare and other healthcare funding, which could have a material adverse effect on customers for any of our products, and, accordingly, our financial operations.
We expect that the PPACA, as well as other healthcare reform measures that may be adopted in the future, may result in more rigorous coverage criteria and in additional downward pressure on the price that we receive for any of our products. Any reduction in reimbursement from Medicare or other government-funded programs may result in a similar reduction in payments from private payors. The implementation of cost containment measures or other healthcare reforms may prevent us from being able to generate revenue, attain profitability or commercialize any of our products.
Legislative and regulatory proposals have been made to expand post-approval requirements and restrict sales and promotional activities for prescription medicines. We cannot be sure whether additional legislative changes will be enacted, or whether the FDA regulations, guidance or interpretations will be changed, or what the impact of such changes on the marketing approvals of our product candidates, if any, may be. In addition, increased scrutiny by the U.S. Congress of the FDA's approval process may significantly delay or prevent marketing approval, as well as subject us to more stringent product labeling and post-marketing testing and other requirements.
A variety of risks associated with marketing any of our products internationally could hurt our business.
We may seek regulatory approval for our pharmaceutical grade L-glutamine treatment for SCD and our other product candidates outside of the United States and, accordingly, we expect that we will be subject to additional risks related to operating in foreign countries if we obtain the necessary approvals, including:
- •
- differing regulatory requirements in foreign countries;
- •
- the potential for so-called parallel importing, which is what happens when a local seller, faced with high or higher local prices,
opts to import goods from a foreign market with low or lower prices rather than buying them locally;
- •
- unexpected changes in tariffs, trade barriers, price and exchange controls and other regulatory requirements;
- •
- economic weakness, including inflation or political instability in particular foreign economies and markets;
59
- •
- compliance with tax, employment, immigration and labor laws for employees living or traveling abroad;
- •
- foreign taxes, including withholding of payroll taxes;
- •
- foreign currency fluctuations, which could result in increased operating expenses and reduced revenues, and other obligations related
to doing business in another country;
- •
- difficulties staffing and managing foreign operations;
- •
- workforce uncertainty in countries where labor unrest is more common than in the United States;
- •
- potential liability under the U.S. Foreign Corrupt Practices Act or comparable foreign regulations;
- •
- challenges enforcing our contractual and intellectual property rights, especially in foreign countries that do not respect and protect
intellectual property rights to the same extent as the United States;
- •
- production shortages resulting from any events affecting raw material supply or manufacturing capabilities abroad; and
- •
- business interruptions resulting from geo-political actions, including war and terrorism.
These and other risks associated with our potential international operations may compromise our ability to achieve or maintain profitability.
Governments outside the United States tend to impose strict price controls, which may adversely affect our revenue, if any.
In some countries, particularly in the European Union, the pricing of prescription pharmaceuticals is subject to governmental control. In these countries, pricing negotiations with governmental authorities can take considerable time after the receipt of marketing approval for a product candidate. To obtain coverage and reimbursement or pricing approval in some countries, we may be required to conduct a clinical trial that compares the cost-effectiveness of any of our products to other available therapies. If reimbursement of any of our products is unavailable or limited in scope or amount, or if pricing is set at unsatisfactory levels, our business could be harmed, possibly materially.
If we fail to comply with environmental, health and safety laws and regulations, we could become subject to fines or penalties or incur costs that could harm our business.
We are subject to numerous environmental, health and safety laws and regulations, including those governing laboratory procedures and the handling, use, storage, treatment and disposal of hazardous materials and wastes. Our operations involve the use of hazardous and flammable materials, including chemicals and biological materials. Our operations also produce hazardous waste products. We generally contract with third parties for the disposal of these materials and wastes. We cannot eliminate the risk of contamination or injury from these materials. In the event of contamination or injury resulting from our use of hazardous materials, we could be held liable for any resulting damages, and any liability could exceed our resources. We also could incur significant costs associated with civil or criminal fines and penalties for failure to comply with such laws and regulations.
Although we maintain workers' compensation insurance to cover us for costs and expenses we may incur due to injuries to our employees resulting from the use of hazardous materials, this insurance may not provide adequate coverage against potential liabilities. We do not maintain insurance for environmental liability or toxic tort claims that may be asserted against us in connection with our storage or disposal of biological, hazardous or radioactive materials.
60
In addition, we may incur substantial costs in order to comply with current or future environmental, health and safety laws and regulations. These current or future laws and regulations may impair our research, development or production efforts. Our failure to comply with these laws and regulations also may result in substantial fines, penalties or other sanctions.
RISKS RELATED TO EMPLOYEE MATTERS AND MANAGING OUR GROWTH
We rely heavily on the founder of Emmaus Medical, Yutaka Niihara, M.D., MPH, our President and Chief Executive Officer. The loss of his services would have a material adverse effect upon us and our business and prospects.
Our success depends, to a significant extent, upon the continued services of Yutaka Niihara, M.D., MPH, founder of Emmaus Medical and our President and Chief Executive Officer. Since inception, we have been dependent upon Dr. Niihara, who was one of the initial patentees for the method we are utilizing in our pharmaceutical grade L-glutamine treatment for SCD. While Dr. Niihara and the rest of our executive officers are parties to confidentiality agreements that prevent them from soliciting our existing customers or disclosing information deemed confidential to us, we do not have any agreement with Dr. Niihara or any key members of management that would prohibit them from joining our competitors or forming competing companies. In addition, we do not maintain key man life insurance policies on any of our executive officers. If Dr. Niihara or any key management personnel resign to join a competitor or form a competing company, the loss of such personnel, together with the loss of any customers or potential customers due to such executive's departure, could materially and adversely affect our business and results of operations.
We are dependent on a technically trained workforce and an inability to retain or effectively recruit such employees could have a material adverse effect on our business, financial condition and results of operations.
Our ability to compete effectively depends largely on our ability to attract and retain certain key personnel, including our clinical, regulatory and scientific staff members. Industry demand for such skilled employees, however, exceeds the number of personnel available, and the competition for attracting and retaining these employees is intense. Because of this intense competition for skilled employees, we may be unable to retain our existing personnel or attract additional qualified employees to keep up with future business needs. If this should happen, our business, operating results and financial condition could be adversely affected.
In addition, we intend to hire in-house marketing personnel to promote and market and sell our SCD and SBS treatment products to patients, physicians and treatment centers, and obtain the approval of insurance companies and healthcare payors for reimbursement of the cost of these treatments. We cannot assure you that we will be able to recruit and retain qualified personnel to perform these marketing functions. Our inability to hire and then retain such personnel and scientists could have a materially adverse effect on our business, financial condition and results of operations.
The pharmaceutical and biotechnology industries are subject to rapid technological change, and if we fail to keep up with such change, our results of operations and financial condition could be adversely impacted.
Biotechnology and related pharmaceutical technology have undergone and are subject to rapid and significant change. We expect that the technologies associated with biotechnology research and development will continue to develop rapidly. Our failure to keep pace with such rapid change could result in our pharmaceutical grade L-glutamine treatment for SCD and other product candidates becoming obsolete and we may be unable to recoup any expenses incurred with developing such products, which may adversely affect our future revenues and financial condition.
61
We expect to expand our product development, regulatory and marketing capabilities, and, as a result, we may encounter difficulties managing our growth, which could disrupt our operations.
We expect to continue to grow, which could strain our managerial, operational, financial and other resources. With the completion of our Phase 3 clinical trial of our pharmaceutical grade L-glutamine treatment for SCD and the potential expansion of clinical-stage programs and in-licensing and acquisition of additional clinical-stage product candidates, we will be required to retain experienced personnel in the regulatory, clinical and medical areas over the next several years. Also, as our preclinical pipeline diversifies through the acquisition or in-licensing of new product candidates, we will need to hire additional scientific and other personnel in order to supplement our existing scientific expertise over the next several years.
Our staff, financial resources, systems, procedures or controls may be inadequate to support our operations, and our management may be unable to take advantage of future market opportunities or manage successfully our relationships with third parties if we are unable to adequately manage our anticipated growth and the integration of new personnel. We may not be able on a timely and cost effective basis to identify, hire and retain any needed additional management, scientific, or sales and marketing personnel to develop and implement our product development plans, conduct preclinical and clinical testing of our product candidates and, if they are approved by the FDA and other government regulators, commercialize them as products. In addition, we may not be able to successfully manage potential rapid growth with our current limited managerial, operational, and financial resources.
We may pursue future growth through strategic acquisitions and alliances which may not yield anticipated benefits and may adversely affect our operating results, financial condition and existing business.
We may seek to grow in the future through strategic acquisitions and alliances in order to complement and expand our business. The success of our acquisition strategy will depend on, among other things:
- •
- the availability of suitable acquisition and alliance candidates;
- •
- competition from other companies for acquiring or forming alliances with available acquisition and alliance candidates;
- •
- our ability to value any acquisition candidates or alliances accurately and negotiate favorable terms for any prospective acquisitions
or alliances;
- •
- the availability of funds to finance any such acquisitions or alliances;
- •
- the ability to establish new informational, operational and financial systems to meet the needs of our business;
- •
- the ability to achieve anticipated synergies, including with respect to complementary products; and
- •
- the availability of management resources to oversee the integration and operation of the acquired businesses.
If we are not successful in integrating acquired businesses and completing acquisitions in the future, we may be required to reevaluate our acquisition and alliance strategy. We also may incur substantial expenses and devote significant management time and resources in seeking to complete acquisitions and alliances. Acquired businesses and alliances may fail to meet our performance expectations. If we do not achieve the anticipated benefits of an acquisition or alliance as rapidly as expected, or at all, investors or analysts may not perceive the same benefits of the acquisition or alliance as we do. If these risks materialize, our operating results, financial condition and existing business could be materially adversely affected.
62
RISKS RELATED TO OWNERSHIP OF OUR COMMON STOCK
There is no current trading market for our common stock, and we cannot predict when or if an established public trading market will develop.
There is no market for shares of our common stock. An active trading market for our shares may never develop. The lack of an active market may impair your ability to sell your shares at the time you wish to sell them or at a price that you consider reasonable. The lack of an active market may also reduce the fair market value of your shares. Further, an inactive market may also impair our ability to raise capital by selling shares of our common stock and may impair our ability to enter into collaborations or acquire companies or products by using our shares of common stock as consideration.
The market price and trading volume of shares of our common stock may be volatile and you could lose all of your investment.
When and if a market develops for our securities, the market price of our common stock could fluctuate significantly for many reasons, including reasons unrelated to our specific performance, such as reports by industry analysts, investor perceptions, or announcements by our competitors regarding their own performance, as well as general economic and industry conditions. For example, to the extent that other companies, whether large or small, within our industry experience declines in their share price, our share price may decline as well. Fluctuations in operating results or the failure of operating results to meet the expectations of public market analysts and investors may negatively impact the price of our securities. Quarterly operating results may fluctuate in the future due to a variety of factors that could negatively affect revenues or expenses in any particular quarter, including vulnerability of our business to a general economic downturn; changes in the laws that affect our products or operations; competition; compensation related expenses; application of accounting standards; and our ability to obtain and maintain all necessary government certifications and/or licenses to conduct our business. In addition, in situations where the market price of a company's shares may drop significantly, stockholders could institute securities class action lawsuits against the company. A lawsuit against us could cause us to incur substantial costs and could divert the time and attention of our management and other resources. In addition, the market price for securities of pharmaceutical and biotechnology companies historically has been volatile, and the securities markets have from time to time experienced significant price and volume fluctuations that are unrelated to the operating performance of particular companies. These broad market fluctuations may cause the market price of our common stock to decline substantially. Market price and volume volatility could cause you to lose part or all of your investment.
If securities or industry analysts do not publish research or reports or publish unfavorable research about our business, the price and trading volume of our common stock could decline.
In the event a public trading market develops for our common stock, the price and trading volume of our common stock will depend in part on the research and reports that securities or industry analysts publish about us or our business. We do not currently have and may never obtain research coverage by securities and industry analysts. If no securities or industry analysts commence coverage of us, the trading price for our common stock and other securities would be negatively affected. In the event we obtain securities or industry analyst coverage, if one or more of the analysts who covers us downgrades our securities, the price of our securities would likely decline. If one or more of these analysts ceases to cover us or fails to publish regular reports on us, interest in the purchase of our common stock could decrease, which could cause the price and trading volume of our common stock to decline.
63
Members of our management team have significant influence over us.
As of March 20, 2015, our officers and directors beneficially owned approximately 43.5% of the outstanding shares of our common stock on an undiluted basis. These stockholders, therefore, have a controlling influence in determining the outcome of any corporate transaction or other matters submitted to our stockholders for approval, including mergers, consolidations and the sale of all or substantially all of our assets, election of directors, and other significant corporate actions. These stockholders may also have the power to prevent or cause a change in control. In addition, without the consent of these stockholders, we could be prevented from entering into transactions that could be beneficial to us. The interests of these stockholders may differ from the interests of our other stockholders.
We have identified a material weakness in our internal control over financial reporting and we may be unable to develop, implement and maintain appropriate controls in future periods. If the material weakness is not remediated, or if after remediation we are unable to maintain appropriate controls, the accuracy and timing of our financial reporting may be adversely affected.
We have identified a material weakness in our internal control over financial reporting and, as a result of such weakness, our management, with the participation of our Chief Executive Officer and Chief Financial Officer, concluded that our disclosure controls and procedures and internal control over financial reporting were not effective as of December 31, 2014. The material weakness related to our not having an adequate level of resources with the appropriate level of training and experience regarding the application of generally accepted accounting principles for certain complex transactions. In addition, we did not maintain effective controls over the completeness and accuracy of financial reporting for complex or significant unusual transactions. With respect to our controls over the reporting of complex or significant unusual transactions, we did not have controls designed and in place to ensure the accuracy of our accounting treatment of such transactions, including, without limitation, our reporting of liabilities and costs associated with the issuance and sale of units consisting of common stock and common stock purchase warrants during the third quarter of fiscal 2013, the recording of share based compensation only after establishing the grant date of the awards, and the proper reporting of the amounts relating to gain contingencies. This and related events contributed to a delay in the filing of our Annual Report on Form 10-K for the fiscal year ended December 31, 2013 and the restatement of our unaudited condensed consolidated financial statements contained in our Quarterly Report on Form 10-Q for the quarterly period ended September 30, 2013.
Unless and until remediated, this material weakness could result in additional material misstatements to our interim or annual consolidated financial statements and disclosures that may not be prevented or detected on a timely basis. In addition, we may experience delay or be unable to meet our reporting obligations or to comply with SEC rules and regulations, which could result in investigation and sanctions by regulatory authorities. Management's assessment of internal controls over financial reporting may in the future identify additional weaknesses and conditions that need to be addressed in our internal control over financial reporting. Any failure to improve our disclosure controls and procedures and our internal control over financial reporting or to address identified weaknesses in the future, if they were to occur, could prevent us from maintaining accurate accounting records and discovering accounting errors and financial frauds. Any of these results could adversely affect our business and the value of our common stock.
We incur significant costs as a result of operating as a public company, and our management is required to devote substantial time to compliance efforts.
As a public company, we incur significant legal, accounting and other expenses that we did not incur as a private company. The Sarbanes-Oxley Act of 2002, the Dodd-Frank Wall Street Reform and Consumer Protection Act, and related SEC regulations have created uncertainty for public companies
64
and significantly increased the costs and risks associated with accessing the public markets and public reporting. We are required to comply with these rules. Our management and other personnel need to devote a substantial amount of time and financial resources to comply with these requirements, as well any new requirements implemented by the SEC. Moreover, these rules and regulations increase our legal and financial compliance costs and will make some activities more time-consuming and costly and could lead to a diversion of management time and attention from revenue generating activities to compliance activities. We are currently unable to estimate these costs with any degree of certainty. These rules and regulations could also make it more difficult for us to attract and retain qualified persons to serve on our board of directors and board committees or as executive officers and more expensive for us to obtain director and officer liability insurance.
Our certificate of incorporation and bylaws and Delaware law may have anti-takeover effects that could discourage, delay or prevent a change in control, which may cause our stock price to decline.
Our certificate of incorporation and bylaws and Delaware law could make it more difficult for a third party to acquire us, even if closing such a transaction would be beneficial to our stockholders. We are authorized to issue up to 20,000,000 shares of preferred stock. This preferred stock may be issued in one or more series, the terms of which may be determined at the time of issuance by our board of directors without further action by stockholders. The terms of any series of preferred stock may include voting rights (including the right to vote as a series on particular matters), preferences as to dividend, liquidation, conversion and redemption rights and sinking fund provisions. No preferred stock is currently outstanding. The issuance of any preferred stock could materially adversely affect the rights of the holders of our common stock, and therefore, reduce the value of our common stock. In particular, specific rights granted to future holders of preferred stock could be used to restrict our ability to merge with, or sell our assets to, a third party and thereby preserve control by the present management.
Provisions of our certificate of incorporation and bylaws and Delaware law also could have the effect of discouraging potential acquisition proposals or making a tender offer or delaying or preventing a change in control, including changes a stockholder might consider favorable. Such provisions may also prevent or frustrate attempts by our stockholders to replace or remove our management. In particular, the certificate of incorporation and bylaws and Delaware law, as applicable, among other things:
- •
- provide the board of directors with the ability to alter the bylaws without stockholder approval;
- •
- place limitations on the removal of directors;
- •
- provide that vacancies on the board of directors may be filled by a majority of directors in office, although less than a quorum; and
- •
- provide that stockholders must provide advance notice to nominate directors or submit proposals for consideration at stockholder meetings.
These provisions are expected to discourage certain types of coercive takeover practices and inadequate takeover bids and to encourage persons seeking to acquire control of our company to first negotiate with our board of directors. These provisions may delay or prevent someone from acquiring or merging with us, which may cause the market price of our common stock to decline.
We are an "emerging growth company" and we may take advantage of reduced disclosure and governance requirements applicable to emerging growth companies, which could result in our common stock being less attractive to investors.
We are an "emerging growth company," as defined in the Jumpstart Our Business Startups Act of 2012, or the JOBS Act, and we may choose to take advantage of certain exemptions from various reporting requirements that are applicable to other public companies that are not emerging growth companies including, but not limited to, not being required to comply with the auditor attestation
65
requirements of Section 404 of the Sarbanes-Oxley Act, reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements, and exemptions from the requirements of holding a non-binding advisory vote on executive compensation and stockholder approval of any golden parachute payments not previously approved. We cannot predict if investors will find our common stock less attractive if we choose to rely on these exemptions. If some investors find our common stock less attractive as a result, there may be a less active trading market for our common stock if one develops and our stock price may be more volatile. We may take advantage of these reporting exemptions until we are no longer an emerging growth company, which in certain circumstances could be for up to five years.
Our status as an "emerging growth company" under the JOBS Act may make it more difficult to raise capital as and when we need it.
Because of the exemptions from various reporting requirements provided to us as an "emerging growth company" we may be less attractive to investors and it may be difficult for us to raise additional capital as and when we need it. Investors may be unable to compare our business with other companies in our industry if they believe that our financial accounting is not as transparent as other companies in our industry. If we are unable to raise additional capital as and when we need it, our financial condition and results of operations may be materially and adversely affected.
We will remain an "emerging growth company" until the earliest of (a) the last day of the first fiscal year in which our annual gross revenues exceed $1.0 billion, (b) the date that we become a "large accelerated filer" as defined in Rule 12b-2 under the Exchange Act, which would occur if the market value of our shares that are held by non-affiliates exceeds $700 million as of the last business day of our most recently completed second fiscal quarter, (c) the date on which we have issued more than $1.0 billion in nonconvertible debt during the preceding three-year period and (d) the last day of our fiscal year containing the fifth anniversary of the date on which shares of our common stock become publicly traded in the U.S.
We do not foresee paying cash dividends in the foreseeable future and, as a result, our investors' sole source of gain, if any, will depend on capital appreciation, if any.
We do not plan to declare or pay any cash dividends on our shares of common stock in the foreseeable future and currently intend to retain any future earnings for funding growth. As a result, investors should not rely on an investment in our securities if they require the investment to produce dividend income. Capital appreciation, if any, of our shares may be investors' sole source of gain for the foreseeable future. Moreover, investors may not be able to resell their shares of our common stock at or above the price they paid for them.
ITEM 1B. UNRESOLVED STAFF COMMENTS
Not applicable.
We lease office space under operating leases from unrelated entities. The rent expense during the years ended December 31, 2014 and 2013 amounted to $277,866 and $137,147, respectively.
We lease approximately 13,329 square feet of headquarters offices in Torrance, California, at a base rental of $32,505 per month. The lease relating to this space expires on February 28, 2019. We lease an additional office suite in Torrance, California at a base rent of $1,750 per month for 1,400 square feet. The lease relating to this space expires on February 19, 2016.
66
In addition, EM Japan leases 1,322 total square feet of office space in Tokyo, Japan and 1,313 square feet of office space in Osaka Japan. Our existing facilities are adequate for our operations at this time and we expect to be able to renew our headquarters office lease on commercially reasonable terms. In the event we determine that we require additional space to accommodate expansion of our operations, we believe suitable facilities will be available in the future on commercially reasonable terms as needed.
On June 27, 2013, the Superior Court of the State of Delaware issued an order implementing a partial summary judgment in our favor in our litigation against AFH Advisory, Griffin Ventures, Ltd., or Griffin, The Amir & Kathy Heshmatpour Family Foundation, or the Foundation, and Amir Heshmatpour. The order, among other things, (i) stated that a letter of intent, referred to as the Letter of Intent, between us and AFH Advisory was properly terminated as of July 19, 2012, and (ii) ordered our transfer agent to effect the cancellation of 2,504,249 shares of our common stock held by AFH Advisory, Griffin and the Foundation. The cancellation of such shares, which represented approximately 10% of our common stock, was effected by our transfer agent on June 28, 2013.
The Letter of Intent was signed on November 10, 2010 and we entered into amended and restated versions on April 21, 2011, and on or around October 4, 2011. The Letter of Intent sets forth the basic terms of the Merger, and the issuance of the now-canceled shares to AFH Advisory and its related parties. It provided that following the Merger, AFH Advisory would assist us in conducting a public offering of our shares, and that if such public offering could not be consummated to provide for minimum gross proceeds to us of at least $5 million, we would have the right to terminate such public offering at our sole discretion, in which case we would have the right to cancel all shares issued to AFH Advisory and certain other parties in connection with the Merger, which we refer to as the Advisory Shares. The Letter of Intent also included provisions regarding the payment of transaction costs, AFH Advisory's right to appoint members of our board and its right to serve as our financial advisor.
On July 19, 2012, we provided written notice to AFH Advisory and Mr. Heshmatpour that we were exercising our right under the Letter of Intent to terminate such public offering and cancel the Advisory Shares. On September 7, 2012, AFH Advisory, Griffin and the Foundation filed a complaint against us in the Superior Court of Delaware. The complaint alleged that we did not have the right to cancel the plaintiffs' shares and asked the court to issue a declaratory judgment to that effect. On October 12, 2012, we filed counterclaims against the plaintiffs and third-party claims against Mr. Heshmatpour. We asked the court for an order declaring that the plaintiffs' shares are canceled and that, because of fraudulent inducement on their part, the Letter of Intent was void and of no further effect. In addition, we asked the court to award compensatory and punitive damages, in amounts to be determined, for breach of contract, unjust enrichment and fraud.
On May 15, 2013, the court issued an opinion declaring as a matter of law that we had properly canceled all shares of our common stock held by AFH Advisory, Griffin and the Foundation and that the Letter of Intent had properly terminated. An order issued by the court on June 27, 2013 implemented these holdings. We do not know if any of the defendants will appeal the court's judgment and related order. The cancellation of the Advisory Shares will be subject to appeal for 30 days after the completion of trial court proceedings.
On April 16, 2014, the court granted our motion for attorneys' fees, costs and expenses and denied a competing motion for attorneys' fees, costs and expenses brought by AFH Advisory and Mr. Heshmatpour. The court ruled that under the Letter of Intent, we are entitled to an award of $700,000 in attorneys' fees, costs and expenses from AFH Advisory. Our cause of action for fraud has not yet been litigated or settled.
67
On October 31, 2013, we filed suit against Timothy Brasel in the state District Court in Arapahoe County, Colorado. Mr. Brasel purchased 500,000 shares of our common stock from AFH Advisory in connection with the Merger. Our complaint against Mr. Brasel sets forth causes of action for conversion, civil theft, imposition of constructive trust and unjust enrichment based on Mr. Brasel's refusal to return the certificates representing those shares of our common stock, which we believe are cancelable under the terms of the Letter of Intent. Our complaint asked the court to order Mr. Brasel to return those certificates, and in addition requested an award of actual damages, statutory damages and other relief. On April 29, 2014, we entered into a settlement agreement with Mr. Brasel pursuant to which Mr. Brasel has surrendered for cancellation the 500,000 shares of our common stock in his possession in exchange for $377,500 that we have paid to him.
Except as described above, we are not aware of any legal proceedings in which any of our directors, nominees, officers or affiliates, any owner of record or beneficially of more than five percent of any class of our voting securities, or any associate of any such director, nominee, officer, affiliate, or securityholder is a party adverse to us or any of our subsidiaries or has a material interest adverse to us or any of our subsidiaries.
ITEM 4. MINE SAFETY DISCLOSURES
Not applicable.
68
ITEM 5. MARKET FOR REGISTRANT'S COMMON STOCK, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
Market Information
There is no established public trading market for our common stock, and our shares of common stock are not currently listed or quoted for trading on any national securities exchange or national quotation system.
Holders
As of March 20, 2015, we had 424 stockholders of record and 28,093,848 shares of our common stock were issued and outstanding. This excludes 2,504,249 shares previously owned by AFH Advisory canceled by our transfer agent on June 28, 2013 pursuant to a court order. See Part I, Item 3. "Legal Proceedings" of this Annual Report for additional information regarding the share cancellation and related legal proceedings.
Dividends
We do not expect to declare or pay any cash dividends on our common stock in the foreseeable future, and we currently intend to retain future earnings, if any, to finance the expansion of our business. The decision whether to pay cash dividends on our common stock will be made by our board of directors in its discretion and will depend on our financial condition, operating results, capital requirements and other factors that the board of directors considers relevant. We did not pay cash dividends in the years ended December 31, 2014 and 2013.
Recent Sales of Unregistered Securities
During the quarter ended December 31, 2014, a note issued in October 2013 to the Shitabata Family Trust, a third party, with an original principal amount of $2,136,146 reached maturity pursuant to the original terms of the note. Upon maturity, we retired the note and issued a new convertible note in the principal amount of $2,136,146 which bears interest at 10% per annum. The term of this note is two years from the date of issuance. The principal amount plus the unpaid accrued interest due under the convertible note is convertible into shares of our common stock at $3.60 per share.
Also during the quarter ended December 31, 2014, a note issued in December 2012 to Alison Brown, a third party, with an original principal amount of $100,800 reached maturity pursuant to the original terms of the note. Upon maturity, we retired the note and issued a new convertible note in the principal amount of $100,800 which bears interest at 10% per annum. The term of this note is two years from the date of issuance. The principal amount plus the unpaid accrued interest due under the convertible note is convertible into shares of our common stock at $3.60 per share.
All such securities were issued in reliance upon the exemption from registration provided by Section 4(a)(2) of the Securities Act and Rule 506 promulgated thereunder. These securities qualified for exemption under Rule 506 promulgated under Section 4(a)(2) of the Securities Act because the issuance of securities by the Company did not involve a "public offering." The issuance was not a public offering based upon the following factors: (i) a limited number of securities were issued to a limited number of offerees; (ii) there was no public solicitation; (iii) each offeree was an "accredited investor" as such term is defined by Rule 501 under the Securities Act; and (iv) the investment intent of the offerees. No underwriters were used in connection with such sales of unregistered securities.
69
Issuer Purchases of Equity Securities
In relation to a suit filed on October 31, 2013 against Timothy Brasel in the state District Court in Arapahoe County, Colorado, on April 29, 2014 we entered into a settlement agreement with Mr. Brasel pursuant to which Mr. Brasel has surrendered for cancellation the 500,000 shares of our common stock in his possession in exchange for $377,500 that we have paid to him.
Additional Information
Copies of our annual reports, quarterly reports, current reports, and any amendments to those reports, are available free of charge on the Internet at www.sec.gov. All statements made in any of our filings, including all forward-looking statements, are made as of the date of the document in which the statement is included, and we do not assume or undertake any obligation to update any of those statements or documents unless we are required to do so by law.
ITEM 6. SELECTED CONSOLIDATED FINANCIAL DATA
Not required for a smaller reporting company.
ITEM 7. MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
Forward-Looking Statements
The following discussion of our financial condition and results of operations should be read in conjunction with our financial statements and the related notes, and the other financial information included in this Annual Report on Form 10-K. This discussion contains forward-looking statements based upon current expectations that involve risks and uncertainties. Our actual results may differ materially from those anticipated in these forward looking statements as a result of various factors, including those set forth under "Risk Factors" and "Cautionary Statement Regarding Forward-Looking Statements" or in other parts of this Annual Report on Form 10-K.
Company Overview
We are a biopharmaceutical company engaged in the discovery, development and commercialization of innovative treatments and therapies primarily for rare and orphan diseases. We are initially focusing our product development efforts on sickle cell disease, or SCD, a genetic disorder and a significant unmet medical need. Our lead product candidate is an oral pharmaceutical grade L-glutamine treatment that demonstrated positive clinical results in our completed Phase 3 clinical trial for sickle cell anemia and sickle ß0-thalassemia, two of the most common forms of SCD.
We are in the process of preparing a new drug application, or NDA, for submission to the U.S. Food and Drug Administration, or FDA, with respect to this product candidate. If the FDA accepts our submission and approves this NDA, we will be authorized to market in the United States our pharmaceutical grade L-glutamine treatment for SCD patients who are at least five years old.
We plan to market our L-glutamine treatment in the United States, if approved, by either strategic partnership or by building our own targeted sales force of approximately 30 sales representatives. We intend to utilize strategic partnerships to market our treatment in the rest of the world. L-glutamine for the treatment of SCD has received Fast Track designation from the FDA as well as Orphan Drug designation from both the FDA and the European Commission, or EC.
We have extensive experience in the field of SCD, including the development, outsourced manufacturing and conduct of clinical trials of our prescription grade L-glutamine product candidate for the treatment of SCD. Our chief executive officer, Yutaka Niihara, M.D., MPH, is a leading
70
hematologist in the field of SCD. Dr. Niihara is licensed to practice medicine in both the United States and Japan and has been actively engaged in SCD research and the care of patients with SCD for over 20 years, primarily at the University of California Los Angeles and the Los Angeles Biomedical Research Institute, or LA BioMed, a nonprofit biomedical research institute at Harbor UCLA Medical Center.
To a lesser extent, we are also engaged in the marketing and sale of NutreStore L-glutamine powder for oral solution, which has received FDA approval, as a treatment for short bowel syndrome, or SBS, in patients receiving specialized nutritional support when used in conjunction with a recombinant human growth hormone that is approved for this indication. Our indirect wholly owned subsidiary, Newfield Nutrition Corporation, referred to as Newfield Nutrition, sells L-glutamine as a nutritional supplement under the brand name AminoPure through retail stores in multiple states and via importers and distributors in Japan, Taiwan and South Korea. Since inception, we have generated minimal revenues from the sale and promotion of NutreStore and AminoPure.
In May 2006, we formed Newfield Nutrition, a wholly-owned subsidiary of Emmaus Medical, Inc., referred to as Emmaus Medical, that distributes L-glutamine as a nutritional supplement under the brand name AminoPure.
In October 2010, we formed Emmaus Medical Japan, Inc., referred to as EM Japan, a wholly-owned subsidiary of Emmaus Medical, that markets and sells AminoPure in Japan and other countries in Asia. EM Japan also manages our distributors in Japan and may also import other medical products in the future.
In November 2011, we formed Emmaus Medical Europe, Ltd., referred to as EM Europe, a wholly-owned subsidiary of Emmaus Medical, whose primary focus is expanding our business in Europe.
Our corporate structure is illustrated as follows:
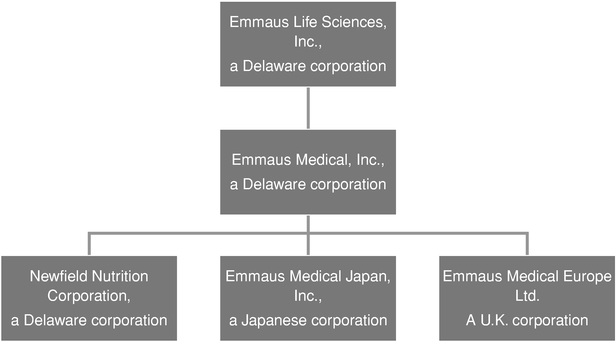
Emmaus Medical, LLC was organized on December 20, 2000. In October 2003, Emmaus Medical, LLC undertook a reorganization and merged with Emmaus Medical, which was originally incorporated in September 2003.
71
Pursuant to an Agreement and Plan of Merger dated April 21, 2011, which we refer to as the Merger Agreement, by and among us, AFH Merger Sub, Inc., our wholly-owned subsidiary, which we refer to as AFH Merger Sub, AFH Advisory and Emmaus Medical, Emmaus Medical merged with and into AFH Merger Sub on May 3, 2011 with Emmaus Medical continuing as the surviving entity, which we refer to as the Merger. Upon the closing of the Merger, we changed our name from "AFH Acquisition IV, Inc." to "Emmaus Holdings, Inc." Subsequently, on September 14, 2011, we changed our name from "Emmaus Holdings, Inc." to "Emmaus Life Sciences, Inc."
Our future capital requirements are substantial and may increase beyond our current expectations depending on many factors, including, but not limited to: the duration and results of the clinical trials for our various product candidates going forward; unexpected delays or developments when seeking regulatory approvals; the time and cost in preparing, filing, prosecuting, maintaining and enforcing patent claims; current and future unexpected developments encountered in implementing our business development and commercialization strategies; the outcome of litigation in which we are currently engaged or may become engaged in the future; and further arrangements, if any, with collaborators.
Until we can generate a sufficient amount of product revenue, future cash requirements are expected to be financed through public or private equity offerings, debt financings or corporate collaboration and licensing arrangements. As of December 31, 2014, our accumulated deficit is $70.6 million and we had cash and cash equivalents of $0.6 million. Since inception we have had minimal revenues and have been required to rely on funding from sales of equity securities and borrowings from officers and stockholders. Currently, we estimate we will need approximately $2.1 million to submit a NDA to the FDA for our pharmaceutical grade L-glutamine treatment for SCD. We also intend to conduct a confirmatory study of our pharmaceutical grade L-glutamine treatment for SCD and are in the process of finalizing the study design.
We also own a minority interest of less than 1% in CellSeed, Inc., a Japanese company listed on the Tokyo Stock Exchange, which is engaged in research and development of regenerative medicine products and the manufacture and sale of temperature-responsive cell culture equipment. In collaboration with CellSeed, we are engaged in research and development of cell sheet engineering regenerative medicine products.
Financial Overview
Revenue
Since our inception in 2000, we have had limited revenue from the sale of NutreStore, an FDA approved prescription drug to treat short bowel syndrome, or SBS, and AminoPure, a nutritional supplement. We have funded operations principally through the private placement of equity securities and debt financings. Our operations to date have been primarily limited to staffing, licensing and promoting products for SBS, outsourcing distribution and sales activities, developing and sponsoring clinical trials of our pharmaceutical grade L-glutamine treatment for SCD, manufacturing products and maintaining and improving our patent portfolio.
Currently, we generate revenue through the sale of NutreStore L-glutamine powder for oral solution as a treatment for SBS as well as AminoPure, a nutritional supplement. Pursuant to the exclusive sublicense agreement for US Patent No. 5,288,703, or SBS Patent, we are required to pay an annual royalty equal to 10% of adjusted gross sales of NutreStore to CATO Holding Company, or CATO. We made royalty payments to CATO in the amount of $6,870 in October 2013 and $6,501 in June 2014. Management expects that any revenues generated from the sale of NutreStore and AminoPure will fluctuate from quarter to quarter based on the timing of orders and the amount of product sold.
72
Cost of Goods Sold
Cost of goods sold includes the raw materials, packaging, shipping and distribution costs of NutreStore and AminoPure.
Research and Development Expenses
Research and development costs consist of expenditures for new products and technologies, which primarily involve fees paid to the contract research organization, or CRO that conducts the clinical trials of our product candidates, payroll-related expenses, study site payments, consultant fees, and activities related to regulatory filings, manufacturing development costs and other related supplies. The costs of later stage clinical studies, such as Phase 2 and 3 trials, are generally higher than those of earlier stages of development, such as preclinical studies and Phase 1 trials. This is primarily due to the increased size, expanded scope, patient related healthcare and regulatory compliance costs, and generally longer duration of later stage clinical studies.
The most significant clinical trial expenditures are related to the CRO costs and the payments to study sites. The contract with the CRO is based on time and material expended, whereas the study site agreements are based on per patient costs as well as other pass-through costs, including, but not limited to, start-up costs and institutional review board fees. The financial terms of these agreements are subject to negotiation and vary from contract to contract and may result in uneven payment flows. Payments under some of these contracts depend on factors such as the successful enrollment of patients and the completion of clinical trial milestones.
Future research and development expenses will depend on any new product candidates or technologies that we may introduce into our research and development pipeline. In addition, we cannot forecast with any degree of certainty which product candidates may be subject to future collaborations, when such arrangements will be secured, if at all, and to what degree, if any, such arrangements would affect our development plans and capital requirements. We currently estimate that we will need an additional $2.1 million to submit a NDA to the FDA for our pharmaceutical grade L-glutamine treatment for SCD. Our current cash burn rate is approximately $0.7 million per month. We also intend to conduct a confirmatory study of our pharmaceutical grade L-glutamine treatment for SCD and are in the process of finalizing the study design.
At this time, due to the inherently unpredictable nature of the process for developing drugs, biologics and cell-based therapies and the interpretation of the regulatory requirements, we are unable to estimate with any degree of certainty the amount of costs which will be incurred in obtaining FDA approval of our pharmaceutical grade L-glutamine treatment for SCD and the continued development of our other preclinical and clinical programs. Clinical development timelines, the probability of success and development costs can differ materially from expectations and can vary widely. These and other risks and uncertainties relating to product development are described in this Annual Report on Form 10-K under the headings "Risk Factors—Risks Related to Development of our Product Candidates," "Risk Factors—Risks Related to our Reliance on Third Parties," and "Risk Factors—Risks Related to Regulatory Approval of our Product Candidates and Other Legal Compliance Matters."
We estimate that the cost to us to develop in the United States corneal cell sheet products based on Cultured Autologous Oral Mucosal Epithelial Cell-Sheets, or CAOMECS, technology will be approximately $3.0 million, in addition to the $8.5 million fee payable to CellSeed under the Research Agreement. This estimate includes the anticipated cost of obtaining FDA approval for the corneal cell sheets and assumes that we will need the FDA to approve a Biologic License Application, or BLA, for the corneal cell sheets, rather than a NDA. We estimate that we will need another $2.0 million to commercialize any approved products based on corneal cell sheet technology.
73
In addition, we estimate that we will need $2.5 million for research related to other cell sheet applications and to build a current Good Manufacturing Practices, or cGMP, laboratory to establish the infrastructure and production capabilities related to regenerative medicine products. At this time, we do not plan to incur any research and development costs for our NutreStore and AminoPure products.
General and Administrative Expenses
General and administrative expenses consist principally of salaries and related costs for personnel in executive, finance, business development, information technology, marketing, and legal functions. Other general and administrative expenses include share-based compensation, facility costs, patent filing costs, and professional fees for legal, consulting, auditing and tax services. Inflation has not had a material impact on our general and administrative expenses over the past two years.
Environmental Expenses
The cost of compliance with environmental laws has not been material over the past two years and any such costs are included in general and administrative costs.
Inventories
Inventories consist of finished goods and work-in-process and are valued based on a first-in, first-out basis and at the lower of cost or market value. All of the purchases of raw materials during 2014 were from one vendor and during 2013 were from two vendors.
Critical Accounting Policies
Management's discussion and analysis of financial condition and results of operations is based on our financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States, or GAAP. The preparation of these financial statements requires us to make estimates and judgments that affect the reported amounts of assets, liabilities and expenses. On an ongoing basis, we evaluate these estimates and judgments, including those described below. We base our estimates on our historical experience and on various other assumptions that we believe to be reasonable under the present circumstances. These estimates and assumptions form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Actual results may differ materially from these estimates.
While our significant accounting policies are more fully described in Note 2 to our consolidated financial statements which are provided at the end of this Annual Report on Form 10-K, we believe that the following accounting policies are the most critical to assist you in fully understanding and evaluating our reported financial results and affect the more significant judgments and estimates that we use in the preparation of our financial statements.
Notes Payable, Convertible Notes Payable and Warrants
From time to time, we obtain financing in the form of notes payable and/or notes with detachable warrants, some of which are convertible into shares of our common stock and some of which are issued to related parties. We analyze all of the terms of our convertible notes and notes issued with warrants to determine the appropriate accounting treatment, including determining whether conversion features are required to be bifurcated and treated as a discount, allocation of fair value of the issuance to the debt instrument, detachable stock purchase warrant, and any beneficial conversion features, and the applicable classification of the convertible notes payable and warrants as debt, equity or temporary equity (mezzanine).
74
We allocate the proceeds from the issuance of a debt instrument with detachable stock purchase warrants to the two elements based on the relative fair values of the debt instrument without the warrants and of the warrants themselves at the time of issuance. We account for the portion of the proceeds allocated to warrants in additional paid-in capital and the remaining proceeds are allocated to the debt instrument. The allocation to warrants results in a discount to notes payable which is amortized using the effective interest method to interest expense over the expected term of the note. We also include the intrinsic value of the embedded conversion feature of convertible notes payable in the discount to notes payable, which is amortized and charged to interest expense over the expected term of the note.
We also estimate the total fair value of any beneficial conversion feature and accompanying warrants in allocating debt proceeds. The proceeds allocated to the beneficial conversion feature are determined by taking the estimated fair value of shares issuable under the convertible notes less the fair value of the number of shares that would be issued if the conversion rate equaled the fair value of our common stock as of the date of issuance. In situations where the debt includes both a beneficial conversion feature and a warrant, the proceeds are allocated to the warrants and beneficial conversion feature based on the pro-rata fair value. We use the Black Scholes-Merton option pricing model to determine the fair value of our warrants.
Notes payable to related parties and interest expense and accrued interest to related parties are separately identified in our consolidated financial statements. We also disclose significant terms of all transactions with related parties.
Share-based Compensation
We recognize compensation cost for share-based compensation awards during the service term of the recipients of the share-based awards. The fair value of share-based awards is calculated using the Black-Scholes-Merton pricing model. The Black-Scholes-Merton model requires subjective assumptions regarding future stock price volatility and expected time to exercise, which greatly affect the calculated values. The expected term of awards granted is derived from historical data on awards exercised and post-vesting employment termination behavior. The risk-free rate selected to value any particular grant is based on the U.S. Treasury rate that corresponds to the vesting period of the grant effective as of the date of the grant. The expected volatility is based on the historical volatility of the common stock of comparable publicly traded companies. These factors could change, affecting the determination of stock-based awards expense in future periods.
Fair Value of Common Stock
We are required to periodically estimate the fair value of our common stock when issuing share-based awards and accounting for share-based compensation expense. The fair value of the common stock underlying our share based awards was determined on each grant date by our board of directors, with the assistance of management and an independent third-party valuation expert. We considered multiple approaches to valuing our common stock. The Probability Weighted Expected Return Method, or PWERM, and the option pricing method were selected as the methods to allocate the enterprise value to the common stock. PWERM requires the projection of several likely scenarios and the determination of the future value of equity within different scenarios. For the scenarios analyzed, a Market Approach was used to derive the value of equity for two liquidity event scenarios and for a staying private scenario. In applying the Market Approach we relied on data of actual transactions that have occurred in our industry, more specifically orphan drug companies and pharmaceutical companies in Phase 3. Using PWERM, shares were valued upon the probability-weighted present value of expected future net cash flows, considering various future outcomes available to the company, discount rate as determined using the capital asset pricing model, as well as the rights of each share class. We then applied a probability weight to the value per share under each scenario and summed the resulting
75
weighted values per share to estimate the fair value per share of our common stock. The majority of the weighting was applied to the liquidity event scenarios which relied on market transactions with similar type companies. We also apply a discount for lack of marketability of our common stock, as it is not freely traded in public markets.
The assumptions underlying these valuations represent management's best estimates, which involve inherent uncertainties and the application of significant levels of management judgment. As a result, if factors or expected outcomes change and we use significantly different assumptions or estimates, our accounting for share-based awards could be materially different.
In estimating the fair value of our common stock, our board of directors exercised reasonable judgment and considered a range of objective and subjective factors, including:
- •
- independent third-party valuation;
- •
- recent arm's length transactions involving the sale of common stock, if any;
- •
- progress of our research and development efforts;
- •
- our operating results and financial condition, including our levels of available capital resources;
- •
- our stages of development and material risks related to our business;
- •
- the achievement of enterprise milestones, including entering into collaboration or license agreements and our progress in clinical
trials;
- •
- the valuation of publicly-traded companies in the life sciences and biotechnology sectors, as well as recently completed mergers and
acquisitions of peer companies;
- •
- equity market conditions affecting comparable public companies;
- •
- issuance of new convertible notes, although not grandfathered refinanced notes;
- •
- the likelihood of achieving a liquidity event for the shares of common stock, such as an initial public offering, sale, licensing or
other strategic transaction, given prevailing market and biotechnology sector conditions; and
- •
- the illiquid nature of our common stock.
Fair Value Measurements
We define fair value as the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. We measure fair value under a framework that provides a fair value hierarchy that prioritizes the inputs to valuation techniques used to measure fair value. The hierarchy gives the highest priority to unadjusted quoted prices in active markets for identical assets or liabilities (Level 1 measurements) and the lowest priority to unobservable inputs (Level 3 measurements). The three levels of the fair value hierarchy are described as follows:
- •
- Quoted prices for similar assets or liabilities in active markets;
- •
- Quoted prices for identical or similar assets or liabilities in inactive markets;
- •
- Inputs other than quoted prices that are observable for the asset or liability; and
- •
- Inputs that are derived principally from or corroborated by observable market data by correlation or other means.
Level 1: Inputs to the valuation methodology are unadjusted quoted prices for identical assets or liabilities in active markets that we have the ability to access.
Level 2: Inputs to the valuation methodology include:
If the asset or liability has a specified (contractual) term, the Level 2 input must be observable for substantially the full term of the asset or liability.
Level 3: Inputs to the valuation methodology are unobservable and significant to the fair value measurement.
76
The asset's or liability's fair value measurement level within the fair value hierarchy is based on the lowest level of any input that is significant to the fair value measurement. Valuation techniques used need to maximize the use of observable inputs and minimize the use of unobservable inputs. The fair value assigned to marketable securities is determined by obtaining quoted prices on nationally recognized securities exchanges, and are classified as Level 1 investments at December 31, 2014 and 2013. The fair value of our debt instruments is not materially different from their carrying values as presented. The fair value of our convertible debt instruments was determined based on Level 2 inputs. The carrying value of the debt was discounted based on allocating proceeds to other financial instruments within the arrangement as discussed in Note 6 to our consolidated financial statements.
The Company issued stock purchase warrants in conjunction with its September 2013 private placement and issued replacement warrants upon the exercise of certain of such warrants in June 2014 (see Note 7 to our consolidated financial statements). Warrants issued in September 2013 that were not exercised became exercisable on a cashless exercise basis in accordance with their terms on September 11, 2014 and due to the exercisability of the cashless exercise feature, are classified as warrant derivative liabilities as of December 31, 2014. Such warrants and replacement warrants are accounted for as liability classified warrants or warrant derivative liabilities whose fair market value is determined using Level 3 inputs. These inputs include expected term and expected volatility.
Marketable Securities
Securities available-for-sale are recorded at cost and any increases or decreases in fair market value are recorded as unrealized gains or losses, net of taxes in accumulated other comprehensive income. We monitor these investments for impairment and make appropriate reductions in carrying values when necessary.
Revenue Recognition
Revenue is recognized when there is persuasive evidence that an arrangement exists, delivery has occurred, the price is fixed and determinable and collection is reasonably assured. With our prior written approval, in certain situations, product is returnable only by our direct customers for a returned goods credit, for product that is expired, damaged in transit, or which is discontinued, withdrawn or recalled. We estimate our sales returns based upon our prior sales and return history and accrue a Sales Return Allowance at the time of sale. We pay royalties on an annual basis based on existing license arrangements. These royalties are recognized as cost of goods sold upon sale of the products.
77
Financial Overview
| |
Year Ended December 31, | ||||||
|---|---|---|---|---|---|---|---|
| |
2014 | 2013 | |||||
REVENUES, Net |
$ | 500,679 | $ | 390,911 | |||
COST OF GOODS SOLD |
267,040 | 196,395 | |||||
| | | | | | | | |
GROSS PROFIT |
233,639 | 194,516 | |||||
| | | | | | | | |
OPERATING EXPENSES |
|||||||
Research and development |
1,966,254 | 2,436,585 | |||||
Selling |
668,862 | 516,599 | |||||
General and administrative |
11,904,092 | 10,078,954 | |||||
Transaction costs |
— | 1,014,019 | |||||
| | | | | | | | |
|
14,539,208 | 14,046,157 | |||||
| | | | | | | | |
LOSS FROM OPERATIONS |
(14,305,569 | ) | (13,851,641 | ) | |||
| | | | | | | | |
OTHER INCOME (EXPENSE) |
|||||||
Realized gain (loss) on securities available-for-sale |
— | 399,068 | |||||
Gain on derecognition of accounts payable |
— | 341,361 | |||||
Change in fair value of liability classified warrants |
(1,622,744 | ) | 932,000 | ||||
Change in fair value of warrant derivative liabilities |
474,000 | — | |||||
Warrant exercise inducement expense |
(3,523,000 | ) | — | ||||
Interest and other income |
246,379 | 103,987 | |||||
Interest expense |
(2,082,541 | ) | (2,173,561 | ) | |||
| | | | | | | | |
|
(6,507,906 | ) | (397,145 | ) | |||
| | | | | | | | |
LOSS BEFORE INCOME TAXES |
(20,813,475 | ) | (14,248,786 | ) | |||
INCOME TAXES (BENEFIT) |
3,665 | (182,438 | ) | ||||
| | | | | | | | |
NET LOSS |
(20,817,140 | ) | (14,066,348 | ) | |||
NET LOSS PER COMMON SHARE |
$ | (0.70 | ) | $ | (0.53 | ) | |
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
WEIGHTED AVERAGE COMMON SHARES OUTSTANDING |
29,846,962 | 26,585,578 | |||||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
Years ended December 31, 2014 and 2013
Net Losses. Net losses were $20.8 million for the year ended December 31, 2014 compared to $14.1 million for the year ended December 31, 2013, representing an increase of $6.7 million, or 48%. The increase in losses is primarily a result of a $3.5 million increase in warrant exercise inducement expense, a $2.6 million decrease in change in fair value of liability classified warrants and a $1.8 million increase in general and administrative expenses as discussed below, partially offset by a $1.0 million decrease in non-recurring transaction costs and a $0.5 million increase in change in fair value of warrant derivative liabilities. As of December 31, 2014, we had an accumulated deficit of approximately $70.6 million. Losses will continue as we advance our pharmaceutical grade L-glutamine treatment for SCD toward regulatory approval and potential commercialization. As a result, we anticipate that we will continue to incur net losses and be unprofitable for the foreseeable future. There can be no assurance that we will ever operate at a profit, even if all of our products are commercialized.
Revenues, Net. Net revenues increased $0.1 million, or 28%, to $0.5 million from $0.4 million for the years ended December 31, 2014 and 2013, respectively. This was primarily due to a $0.2 million increase in our AminoPure® L-glutamine nutritional supplement product revenues attributable to an increase in sales volume from expansion of markets in Taiwan and South Korea, which was partially
78
offset by a slight decline in net revenues of $50,000 from our NutreStore® L-glutamine powder for oral solution for treatment of SBS.
Cost of Goods Sold. Cost of goods sold increased $0.1 million, or 36%, to $0.3 million for the year ended December 31, 2014 from $0.2 million for the year ended December 31, 2013. Cost of goods sold includes costs for raw material, packaging, testing, shipping and costs related to scrapped inventory. All purchases of raw materials during the year ended December 31, 2014 were from one vendor and all purchases of raw materials during the year ended December 31, 2013 were from two vendors, with one of such vendors representing 79% of such purchases and the other vendor representing 21% of such purchases. The increase in cost of goods sold in the year ended December 31, 2014 as compared to the year ended December 31, 2013 was primarily due to an increase in sales volumes of our AminoPure® L-glutamine nutritional supplement product from expansion of international markets.
Research and Development Expenses. Research and development expenses decreased $0.5 million, or 19%, to $1.9 million from $2.4 million for the years ended December 31, 2014 and 2013, respectively. This decrease was primarily due to the completion of our Phase 3 clinical trial of our pharmaceutical grade L-glutamine treatment for SCD in 2013. Costs in 2013 were primarily CRO costs and payments to sites. Costs in 2014 were primarily related to the cost of compiling and analyzing data from our Phase 3 clinical trial and other end of study costs. We expect such costs to continue in 2015 to support our submission of a NDA and to potentially increase depending upon future clinical trial activity.
Selling Expenses. Selling expenses increased $0.2 million, or 29%, to $0.7 million from $0.5 million for the years ended December 31, 2014 and 2013, respectively. Selling expenses included the costs for distribution, promotion, travel, tradeshows and exhibits related to NutreStore and AminoPure as well as the marketing expense for our pharmaceutical grade L-glutamine treatment for SCD.
General and Administrative Expenses. General and administrative expenses increased $1.8 million, or 18%, to $11.9 million from $10.1 million for the years ended December 31, 2014 and December 31, 2013, respectively. The increase was primarily due to a $0.8 million increase in share-based compensation expenses, a $0.4 million increase in professional fees, a $0.2 million increase in payroll related costs, a $0.1 million increase each in travel expenses, office rent expenses, publications expenses, and insurance expenses.
Transaction Expenses. Transaction costs decreased $1.0 million or 100% to $0 from $1.0 million due to costs associated with the Company's 2013 private placement of units that were allocated to the liability classified warrants that did not recur in 2014.
Other Income and Expense. Total other expense has increased by $6.1 million, or 1,539%, to a $6.5 million expense from a $0.4 million expense for the years ended December 31, 2014 and 2013, respectively. The increase was primarily due to an increase in warrant exercise inducement expense of $3.5 million, a decrease in change in fair value of liability classified warrants of $2.6 million, a decrease in realized gain on securities available-for-sale of $0.4 million and decrease in non-recurring gain on derecognition of accounts payable of $0.3 million, partially offset by a $0.5 million increase in change in fair value of warrant derivative liabilities.
We anticipate that our operating expenses will increase for, among others, the following reasons:
- •
- as a result of increased payroll, expanded infrastructure and higher consulting, legal, accounting and investor relations costs, and director and officer insurance premiums associated with being a public company;
79
- •
- to support research and development activities, which we expect to expand as development of our product candidates continues; and
- •
- to build a sales and marketing team before we receive regulatory approval of our pharmaceutical grade L-glutamine treatment for SCD in anticipation of commercial launch.
Liquidity and Capital Resources
Based on our losses to date, anticipated future revenue and operating expenses, debt repayment obligations and cash and cash equivalents balance of $0.6 million as of December 31, 2014, we do not have sufficient operating capital for our business without raising additional capital. We incurred losses of $20.8 million and $14.1 million for each of the years ended December 31, 2014 and 2013. We had an accumulated deficit at December 31, 2014 of $70.6 million. We anticipate that we will continue to incur net losses for the foreseeable future as we incur expenses for the commercialization of our pharmaceutical grade L-glutamine treatment for SCD, research costs for corneal cell sheets using CAOMECS technology and the expansion of corporate infrastructure, including costs associated with being a public reporting company. We have previously relied on private equity offerings, debt financings, and loans, including loans from related parties. As part of this effort, we have received various loans from officers, stockholders and other investors as discussed below. As of December 31, 2014, we had outstanding notes payable in an aggregate principal amount of $12.0 million, consisting of $3.4 million of non-convertible promissory notes and $8.6 million of convertible notes. Of the $12.0 million aggregate principal amount of notes outstanding as of December 31, 2014, approximately $6.3 million is either due on demand or will become due and payable within the next twelve months. Our failure to raise capital as and when needed would have a negative impact on our financial condition and our ability to pursue our business strategies, including the commercialization of our pharmaceutical grade L-glutamine treatment for SCD and the development in the United States of CAOMECS-based cell sheet technology.
As described in Note 2 to the consolidated financial statements, we have had recurring operating losses, have a significant amount of notes payable and other obligations due within the next year and projected operating losses including the expected costs relating to the commercialization of our pharmaceutical grade L-glutamine treatment for SCD that exceed both the existing cash balances and cash expected to be generated from operations for at least the next year. In order to meet our expected obligations, management intends to raise additional funds through equity and debt financings and partnership agreements. In addition, we may seek to raise additional funds through collaborations with other companies or financing from other sources. As previously reported, the Company has filed a draft registration statement with the SEC with respect to an initial public offering. Additional funding may not be available in amounts or on terms which are acceptable to us, if at all. Due to the uncertainty of our ability to meet our current operating and capital expenses, there is substantial doubt about our ability to continue as a going concern.
On April 8, 2011, pursuant to the Research Agreement with CellSeed, we agreed to pay CellSeed $8.5 million within 30 days after the completion of all of the following: (i) the execution of the Research Agreement; (ii) the execution of the Individual Agreement; and (iii) CellSeed's delivery to us of the accumulated information package, as defined in the Research Agreement, which is not completed yet. Pursuant to the Individual Agreement with CellSeed, we agreed to pay $1.5 million to CellSeed and a royalty to be agreed upon by the parties. We paid the $1.5 million due to CellSeed pursuant to the Individual Agreement in February 2012. We currently anticipate that the additional $8.5 million payment obligation under the Research Agreement will become due and payable after we begin to generate revenues from the commercialization of our pharmaceutical grade L-glutamine treatment for SCD. If the payment obligation becomes due and payable prior to our generation of revenue from the commercialization of our pharmaceutical grade L-glutamine treatment for SCD, we will need to seek other funding sources, including the sale of additional equity or debt securities, and
80
partnership arrangements in order to make the payment. CellSeed may terminate these agreements if we are unable to make timely payments as required under the agreements.
In addition, we currently estimate that we will need an additional $2.1 million to submit a NDA to the FDA for our pharmaceutical grade L-glutamine treatment for SCD. Our current cash burn rate is approximately $0.7 million per month. We also intend to conduct a confirmatory study of our pharmaceutical grade L-glutamine treatment for SCD and are in the process of finalizing the study design.
As discussed in this Annual Report on Form 10-K under the headings "Risk Factors—Risks Related to Development of our Product Candidates" above, if the FDA does not accept for filing our NDA for our pharmaceutical grade L-glutamine treatment or does not approve our NDA based on a single Phase 3 clinical trial, in each case unless we conduct a second Phase 3 clinical trial or confirmatory study, the potential approval of our product candidate will be delayed. Under these circumstances, we will incur additional costs to seek to convince the FDA that a confirmatory study is unnecessary for filing or approval, or to design and conduct a second Phase 3 clinical trial or confirmatory study, or both. If we conduct a second Phase 3 clinical trial or confirmatory study prior to the approval of our NDA, it is possible that the results of that trial will be less favorable than the results of our completed Phase 3 trial and further delay or complicate the approval process. The incurrence of additional costs may require us to raise additional capital, and any delay in obtaining approval of our product candidate will reduce the period during which we can market and sell our product with patent protection and may affect our ability to obtain other protections against competition.
Our cash flow from operations is not adequate and our future capital requirements will be substantial and may increase beyond our current expectations depending on many factors including, but not limited to: the number, duration and results of the clinical trials for our various product candidates going forward; unexpected delays or developments in seeking regulatory approvals; the time and cost in preparing, filing, prosecuting, maintaining and enforcing patent claims; other unexpected developments encountered in implementing our business and commercialization strategies; the outcome of existing and any future litigation; and further arrangements, if any, with collaborators. We will rely, in part, on sales of AminoPure for revenues, which we expect will increase due to the expected growth in its export volume as we have added additional distributors and expanded retail markets outside of the United States. Revenues from NutreStore are currently not significant and we are unsure whether sales of NutreStore will increase.
Until we can generate a sufficient amount of product revenue, future cash needs are expected to be financed through public or private equity offerings, debt financings, loans, including loans from related parties, or other sources, such as strategic partnership agreements and corporate collaboration and licensing arrangements.
Until we can generate a sufficient amount of product revenue, there can be no assurance of the availability of such capital on terms acceptable to us (or at all).
For the years ended December 31, 2014 and 2013, we borrowed varying amounts pursuant to convertible notes and non-convertible promissory notes, the majority of which have been issued to our officers and stockholders. As of December 31, 2014 and 2013, the aggregate principal amounts outstanding under convertible notes and non-convertible promissory notes totaled $12.0 million and $11.5 million, respectively. The convertible notes and non-convertible promissory notes bear interest at rates ranging from 0% to 11% and, except for the 2011 convertible note listed below in the principal amount of $0.5 million, are unsecured. Interest on 0% loans was imputed at the incremental borrowing rate of 6.25% per annum. The net proceeds of the loans were used for working capital.
81
The table below lists our outstanding notes payable as of December 31, 2014 and 2013, and the material terms of our outstanding borrowings:
Year Issued
|
Interest rate range |
Term of Notes | Conv. Price | Principal Outstanding December 31, 2014 |
Discount Amount December 31, 2014 |
Carrying Amount December 31, 2014 |
Shares Underlying Principal as of December 31, 2014 |
Principal Outstanding December 31, 2013 |
Discount Amount December 31, 2013 |
Carrying Amount December 31, 2013 |
Shares Underlying Principal as of December 31, 2013 |
||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Convertible notes payable |
|||||||||||||||||||||||||||||||
2009 |
6.50% | 5 years | $3.05 | $ | — | $ | — | $ | — | — | $ | 254,460 | $ | — | $ | 254,460 | 83,366 | ||||||||||||||
2010 |
0 ~ 6.0% | 5 years | $3.05 | 74,000 | 3,195 | 70,805 | 24,248 | 74,000 | 8,308 | 65,692 | 24,248 | ||||||||||||||||||||
2011 |
10% | 5 years | $3.05 | 500,000 | — | 500,000 | 163,809 | 500,000 | — | 500,000 | 163,809 | ||||||||||||||||||||
2012 |
10% | 2 years | $3.30 ~ $3.60 | — | — | — | — | 251,100 | — | 251,100 | 71,000 | ||||||||||||||||||||
2013 |
10% | 2 years | $3.30 ~ $3.60 | 2,463,299 | 18,750 | 2,444,549 | 834,667 | 6,913,606 | 215,798 | 6,697,808 | 1,998,215 | ||||||||||||||||||||
2014 |
10% | Due on demand ~ 2 years | $3.05 ~ $7.00 | 4,939,773 | 846,613 | 4,093,160 | 1,241,241 | — | — | — | — | ||||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
|
$ | 7,977,072 | $ | 868,558 | $ | 7,108,514 | 2,263,965 | $ | 7,993,166 | $ | 224,106 | $ | 7,769,060 | 2,340,638 | |||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
|
Current | $ | 3,478,904 | $ | 21,945 | $ | 3,456,959 | 1,156,050 | $ | 4,955,868 | $ | 153,396 | $ | 4,802,472 | 1,438,033 | ||||||||||||||||
|
Non-current | $ | 4,498,168 | $ | 846,613 | $ | 3,651,555 | 1,107,915 | $ | 3,037,298 | $ | 70,710 | $ | 2,966,588 | 902,605 | ||||||||||||||||
Convertible notes payable—related party |
|||||||||||||||||||||||||||||||
2012 |
10% | Due on demand | $3.30 | $ | 373,000 | $ | — | $ | 373,000 | 121,461 | $ | 373,000 | $ | — | $ | 373,000 | 113,030 | ||||||||||||||
2013 |
10% | 1 year | $3.60 | — | — | — | — | 187,706 | — | 187,706 | 52,141 | ||||||||||||||||||||
2014 |
10% | 2 years | $7.00 | 200,000 | — | 200,000 | 30,187 | — | — | — | — | ||||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
|
$ | 573,000 | $ | — | $ | 573,000 | 151,648 | $ | 560,706 | $ | — | $ | 560,706 | 165,171 | |||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
|
Current | $ | 373,000 | $ | — | $ | 373,000 | 121,461 | $ | 560,706 | $ | — | $ | 560,706 | 165,171 | ||||||||||||||||
|
Non-current | $ | 200,000 | $ | — | $ | 200,000 | 30,187 | $ | — | $ | — | $ | — | — | ||||||||||||||||
Notes payable |
|||||||||||||||||||||||||||||||
2012 |
11% | 2 years | NA | $ | — | $ | — | $ | — | — | $ | 833,335 | $ | 18,265 | $ | 815,070 | — | ||||||||||||||
2013 |
2% ~ 10% | Due on demand ~ 2 years | NA | 1,030,000 | — | 1,030,000 | — | 1,150,000 | — | 1,150,000 | — | ||||||||||||||||||||
2014 |
11% | Due on demand ~ 2 years | NA | 1,446,950 | — | 1,446,950 | — | — | — | — | — | ||||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
|
$ | 2,476,950 | $ | — | $ | 2,476,950 | — | $ | 1,983,335 | $ | 18,265 | $ | 1,965,070 | — | |||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
|
Current | $ | 1,643,615 | $ | — | $ | 1,643,615 | — | $ | 1,783,335 | $ | 18,265 | $ | 1,765,070 | — | ||||||||||||||||
|
Non-current | $ | 833,335 | $ | — | $ | 833,335 | — | $ | 200,000 | $ | — | $ | 200,000 | — | ||||||||||||||||
Notes payable—related party |
|||||||||||||||||||||||||||||||
2012 |
8% ~ 10% | Due on demand | NA | $ | 656,730 | $ | — | $ | 656,730 | — | $ | 880,062 | $ | 4,421 | $ | 875,641 | — | ||||||||||||||
2013 |
8% | Due on demand | NA | 50,000 | — | 50,000 | — | 50,000 | — | 50,000 | — | ||||||||||||||||||||
2014 |
11% | Due on demand ~ 2 years | NA | 252,165 | — | 252,165 | — | — | — | — | — | ||||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
|
$ | 958,895 | $ | — | $ | 958,895 | — | $ | 930,062 | $ | 4,421 | $ | 925,641 | — | |||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
|
Current | $ | 825,562 | $ | — | $ | 825,562 | — | $ | 930,062 | $ | 4,421 | $ | 925,641 | — | ||||||||||||||||
|
Non-current | $ | 133,333 | $ | — | $ | 133,333 | — | $ | — | $ | — | $ | — | — | ||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
|
Grand Total | $ | 11,985,917 | $ | 868,558 | $ | 11,117,359 | 2,415,613 | $ | 11,467,269 | $ | 246,792 | $ | 11,220,477 | 2,505,809 | ||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
82
Also, between June 6, 2014 and June 10, 2014 certain holders of warrants exercised their existing warrants to purchase an aggregate of 1,095,465 shares of common stock, the Exercised Warrants, for the cash exercise price of $3.50 per share, as a result of which we received aggregate exercise proceeds of $3.8 million. On June 10, 2014, based on an offer made to holders in connection with such exercises, we issued an aggregate of 1,095,465 replacement warrants to holders exercising private placement warrants, which replacement warrants have terms that are generally the same as the exercised warrants, including an expiration date of September 11, 2018 and an exercise price of $3.50 per share.
Subsequent to December 31, 2014, we entered into financing arrangements as set forth below. The total amount of these loans amounted to $2.5 million.
Notes issued after December 31, 2014
|
Principal Amounts |
Annual Interest Rate |
Term of Notes | Conversion Price |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
Convertible note(1) |
$ | 196,026 | 10 | % | On Demand up to 1 Year | $ | 3.60 | |||||
Convertible note(1) |
656,052 | 10 | % | Approximately 7 months | $ | 3.50 | ||||||
Convertible note(1) |
100,800 | 10 | % | 1 Year | $ | 3.60 | ||||||
Convertible note(1) |
120,960 | 10 | % | 2 Years | $ | 3.60 | ||||||
Convertible note(1) |
504,614 | 10 | % | 2 Years | $ | 3.50 | ||||||
Convertible note(2) |
200,000 | 10 | % | 2 Years | $ | 7.00 | ||||||
Promissory note(3) |
464,800 | 11 | % | On Demand up to 2 years | N/A | |||||||
Promissory notes-related party |
230,000 | 10 | % | On Demand up to 2 years | N/A | |||||||
Promissory notes-related party(1) |
13,161 | 11 | % | 1 Year | N/A | |||||||
| | | | | | | | | | | | | |
Total |
$ | 2,486,413 | ||||||||||
| | | | | | | | | | | | | |
| | | | | | | | | | | | | |
| | | | | | | | | | | | | |
- (1)
- Refinance
of prior note.
- (2)
- Includes
mandatory conversion at the time of an initial public offering at a conversion price equal to 80% of the initial public offering price.
- (3)
- Principal amount of note is JPY56, 000,000 which was converted into US dollars using an exchange rate of 0.0083 as of December 31, 2014.
We are required to pay royalties which are recognized as an expense upon sale of the related products. We are required to pay a royalty to CATO equivalent to 10% of our adjusted gross sales of NutreStore calculated on an annual basis. The 10% royalty is calculated at the end of the year and accrued on an annual basis. Once commercialized and pursuant to an addendum to the license agreement with LA BioMed, we agreed to pay royalties to LA BioMed during the term of the agreement equal to 4.5% of net sales of Licensed Products in the United States until lifetime royalty payments made to LA BioMed total $100,000, at which time the royalty rate will decrease to 2.5% of net sales of the Licensed Products. No royalties will be paid to LA BioMed for Licensed Products sold or distributed, or Licensed Methods practiced, on a non-profit basis. Royalty payments are due within 45 days of the end of each fiscal quarter, with the last payment due 45 days after the termination of the agreement. Any payments not made when due accrue interest on and after the due date at a rate equal to the prime interest rate quoted by the Bank of America on the date the payment is due, with interest being compounded on the last day of each calendar quarter.
Cash Flows
Net cash used in operating activities
Net cash flows used in operating activities increased by $0.3 million, or 4%, to $8.7 million from $8.4 million for the years ended December 31, 2014 and 2013, respectively. The increase in cash flows used in operating activities of $0.3 million was due primarily to an increase of $6.8 million in net loss
83
and a decrease of $1.0 million in non-recurring transaction costs included in net income, offset by a $7.2 million increase in non-cash components of net loss and a $0.2 million decrease in working capital. The increase in non-cash components of net loss is primarily comprised of a $3.5 million warrant exercise inducement expense, a $2.6 million higher change in fair value of liability classified warrants and an increase of $0.8 million in share-based compensation, partially offset by a $0.5 million increase in change in fair value of warrant derivative liabilities. During 2013, we incurred certain transaction costs associated with the issuance of common stock and warrants to purchase shares of our common stock in the September 2013 private placement. To arrive at net cash flows used in operating activities for the year ended December 31, 2013, we added back transaction costs of $1.0 million that were included in net loss as a reconciling item in cash flows from operating activities. We also incurred an additional $0.2 million in transaction costs that were not included in net loss for accounting purposes since it was offset against the equity component of the private placement.
Net cash from (used in) investing activities
Net cash flows from (used in) investing activities decreased by $0.6 million, or 111%, to $(64,000) used in investing activities from $0.6 million from investing activities for the years ended December 31, 2014 and 2013, respectively. The decrease was due to the absence of proceeds from the sale of marketable securities of $0.6 million and purchases of property and equipment of $0.1 million. No other investing activities occurred in the years ended December 31, 2014 and 2013.
Net cash from financing activities
Net cash flows from financing activities decreased by $5.3 million, or 48%, to $5.7 million from $11.0 million for the years ended December 31, 2014 and 2013, respectively, primarily as a result of a $6.4 million decrease in proceeds received from the private placement units net of transaction costs and a $4.5 million decrease in proceeds from issuance of notes and common stock from $6.6 million to $2.1 million, which was partially offset by a $4.3 million increase in proceeds from the exercise of warrants and a decrease of $1.7 million in payments of notes payable and convertible notes payable. During 2013, we received gross proceeds of $7.6 million from our September 2013 private placement, from which we deducted total transaction costs of $1.2 million to arrive at proceeds from private placement units, net of transaction costs of $6.4 million included in net cash flows from financing activities for the year ended December 31, 2013. The $1.2 million in transaction costs deducted include $0.9 million in payments we made to T.R. Winston & Company, LLC, or TRW, plus $0.3 million in attorney fees and other transaction-related costs.
Off-Balance-Sheet Arrangements
Since our inception, we have not engaged in any off-balance sheet arrangements, including the use of structured finance, special purpose entities or variable interest entities.
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
Not required for a smaller reporting company.
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
The information required by this Item 8 is incorporated by reference to the information that begins on Page F-1 of this Annual Report on Form 10-K.
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
None.
84
ITEM 9A. CONTROLS AND PROCEDURES
Evaluation of Disclosure Controls and Procedures
Disclosure controls and procedures are controls and other procedures that are designed to ensure that information required to be disclosed by us in the reports that we file or submit under the Exchange Act is recorded, processed, summarized and reported within the time periods specified in the Securities and Exchange Commission's rules and forms. Disclosure controls and procedures include, without limitation, controls and procedures designed to ensure that information required to be disclosed by us in the reports that we file under the Exchange Act is accumulated and communicated to our management, including our principal executive and financial officers, as appropriate to allow timely decisions regarding required disclosure.
As of the end of the period covered by this Annual Report on Form 10-K, we conducted an evaluation, under the supervision and with the participation of our Chief Executive Officer and Chief Financial Officer, of the effectiveness of our disclosure controls and procedures (as defined in Rule 13a-15(e) of the Exchange Act). Based upon this evaluation and due to the material weakness in our internal control over financial reporting as of December 31, 2014 described below, our Chief Executive Officer and Chief Financial Officer concluded that the Company's disclosure controls and procedures are not effective.
Internal Control over Financial Reporting
(a) Management's Annual Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting as defined in Rules 13a-15(f) under the Exchange Act. Our internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles in the United States of America. Our internal control over financial reporting includes those policies and procedures that (i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect our transactions and our dispositions of the assets; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that our receipts and expenditures are being made only in accordance with authorizations of our management and directors; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of our assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risks that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate. Under the supervision and with the participation of our management, including our Chief Executive Officer and Chief Financial Officer, we conducted an evaluation of the effectiveness of our internal control over financial reporting based on the criteria set forth in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission. Based on this evaluation, our management concluded that our internal control over financial reporting was not effective as of December 31, 2014.
A material weakness is a deficiency, or combination of deficiencies, in internal control over financial reporting such that there is a reasonable possibility that a material misstatement of a company's annual or interim financial statements will not be prevented or detected on a timely basis. Material weaknesses would permit information required to be disclosed by a company in the reports that it files or submits to not be recorded, processed, summarized and reported, within the time periods
85
specified in the SEC's rules and forms. In conducting our review of our internal control over financial reporting, we identified a continuing material weakness in our internal control over financial reporting initially identified in our evaluation of the effectiveness of our internal control over financial reporting as of December 31, 2013. This material weakness relates to the adequacy of resources and controls resulting from us not having an adequate level of resources with the appropriate level of training and experience in regards to the application of generally accepted accounting principles for certain complex transactions. In addition, we did not maintain effective controls over the completeness and accuracy of financial reporting for complex or significant unusual transactions.
The material weakness described above resulted in a material misstatement of our liabilities, shareholders' deficit, net loss, non-cash expenses and related financial disclosures for the three month period ended September 30, 2013 that was not prevented or detected on a timely basis and, consequently, a restatement of the unaudited condensed consolidated financial statements contained in our Quarterly Report on Form 10-Q for the quarter ended September 30, 2013. Additionally, other prior period adjustments related to recording share based compensation are attributable to us not having an adequate level of resources in regard to these transactions. As a result of these matters, we were unable to timely file our Annual Report on Form 10-K for the year ended December 31, 2013.
To assist with the accounting for complex and/or significant unusual transactions, we have retained a consulting professional with greater knowledge and experience of U.S. GAAP and related regulatory requirements to supplement our internal resources and added to our accounting staff. We also have implemented procedures to evaluate and improve our existing internal control documentation and procedures to develop clear identification of key financial and reporting controls over complex or significant unusual transactions. We continue to strengthen our procedures and staffing levels in order to fully address this material weakness.
(b) Attestation Report
This Annual Report on Form 10-K does not include an attestation report of our independent registered public accounting firm regarding internal control over financial reporting. We are not subject to the attestation requirement because we are a smaller reporting company.
Changes in Internal Control over Financial Reporting
Except as described above, based on the evaluation of our management as required by paragraph (d) of Rule 13a-15 of the Exchange Act, we believe that there were no changes in our internal control over financial reporting that occurred during the quarter ended December 31, 2014 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
None.
86
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
Executive Officers, Directors and Key Employees
The following individuals constitute our board of directors and executive management:
Name
|
Age | Position | |||
|---|---|---|---|---|---|
Yutaka Niihara, M.D., MPH |
55 | President, Chief Executive Officer and Director | |||
Willis C. Lee |
54 | Chief Operating Officer | |||
Peter B. Ludlum |
59 | Executive Vice President and Chief Financial Officer | |||
Lan T. Tran, MPH |
39 | Chief Administrative Officer and Corporate Secretary | |||
Yasushi Nagasaki |
47 | Senior Vice President, Finance | |||
Charles Stark, Pharm.D. |
59 | Senior Vice President, Research and Development | |||
Henry A. McKinnell, Jr., Ph.D. |
72 | Chairman of the Board | |||
Tracey C. Doi |
54 | Director | |||
Akiko Moni Miyashita |
58 | Director | |||
Phillip M. Satow |
72 | Director | |||
Mayuran Sriskandarajah |
34 | Director | |||
All of our officers devote at least forty (40) hours per week, the equivalent of a full-time employee, to their positions with us.
Background of Officers and Directors
The following is a brief summary of the background of each of our directors, executive officers and executive management:
Yutaka Niihara, M.D., MPH has served as our President and Chief Executive Officer since May 2011 and has served as the President, Chief Executive Officer and Chairman of the Board of Emmaus Medical since 2003. Since May 2005, Dr. Niihara has also served as the President, Chief Executive Officer and Medical Director of Hope International Hospice, Inc., or Hope Hospice, a Medicare-certified hospice program. From June 1992 to October 2009, Dr. Niihara served as a physician specialist for Los Angeles County. Dr. Niihara is the principal inventor of the patented L-glutamine treatment for SCD. Dr. Niihara has been involved in patient care and research for sickle cell disease during most of his career and is a widely published author in the area of sickle cell disease. Dr. Niihara is board-certified by the American Board of Internal Medicine and the American Board of Internal Medicine/Hematology. He is licensed to practice medicine in both the United States and Japan. Dr. Niihara is a Professor of Medicine at the David Geffen School of Medicine at UCLA. Dr. Niihara received his B.A. in Religion from Loma Linda University in 1982, obtained his MD degree from the Loma Linda University School of Medicine in 1986 and received his MPH from Harvard School of Public Health in 2006. Dr. Niihara is qualified to serve on our board of directors due to his knowledge and experience.
Henry A. McKinnell, Jr., Ph.D. has served as our Chairman of the Board since May 2011 and as a director of Emmaus Medical since April 2010. Dr. McKinnell served as the Chairman of the Board of Pfizer Inc. (NYSE: PFE), a pharmaceutical company, from May 2001 until December 2006. He retired from Pfizer, Inc. and its board in February 2007. He also served as Chief Executive Officer of Pfizer, Inc. from January 2001 to July 2006. He served as President of Pfizer, Inc. from May 1999 to May 2001, and as President of Pfizer Pharmaceuticals Group from January 1997 to April 2001. Dr. McKinnell served as Chief Operating Officer of Pfizer, Inc. from May 1999 to December 2000 and as Executive Vice President from 1992 to 1999. Since October 1997, Dr. McKinnell has served as a member of the board of directors of Moody's Corporation (NYSE: MCO), where he serves as Chairman and a member of the audit committee, the governance and compensation committee and
87
Moody's investor services committee. Dr. McKinnell served as a director of Optimer Pharmaceuticals, Inc. (NasdaqGM: OPTR), a global biopharmaceutical company, from January 2011 to March 2012. Dr. McKinnell served as Optimer's Chief Executive Officer from February 2013 to October 2013 and as Chairman of the Optimer Board from April 2012 to October 2013. He also served as Optimer's lead independent director from March 2012 to February 2013. Dr. McKinnell serves as Chairman of the Board of the Accordia Global Health Foundation. He is Chairman Emeritus of the Connecticut Science Center, and is a member of the Academic Alliance for AIDS Care and Prevention in Africa. He served as director of ExxonMobil Corporation from 2002 to 2007 and John Wiley & Sons from 1996 to 2005. Dr. McKinnell holds a Bachelor's Degree in business from the University of British Columbia, and M.B.A. and Ph.D. degrees from the Stanford University Graduate School of Business. We believe that Dr. McKinnell is qualified to serve on our board of directors due to his extensive experience and leadership in the pharmaceutical industry, in addition to his substantial involvement with business and civic organizations and years of experience as an officer and director of publicly traded companies.
Tracey C. Doi has served as a director since August 2011. Since 2003, Ms. Doi has served as Group Vice President and Chief Financial Officer of Toyota Motor Sales, U.S.A., Inc., an automotive company and sales, marketing and distribution arm for Toyota, Lexus and Scion in the United States. Ms. Doi is responsible for corporate finance, tax, customs, treasury, accounting, procurement and administrative services. Ms. Doi serves on Toyota Motor Sales' audit, risk and benefit committees, as well as an officer or director of several Toyota Motor Sales subsidiaries. Ms. Doi joined Toyota in 2000 as Vice President, Corporate Controller and was promoted to Chief Financial Officer in 2003. Prior to that, she held financial executive positions with AT&T Wireless, a wireless telecommunications company, L.A. Cellular Telephone Company, a wireless telecommunications company, and L.A. Gear, Inc., a footwear company. Ms. Doi received a Bachelor's Degree in Business Economics from UCLA in 1983 and is a Certified Public Accountant (inactive). Ms. Doi served on the Federal Reserve Bank of San Francisco's Economic Advisory Council from 2009-2014. Ms. Doi serves on the board of governors for the Japanese American National Museum, and the board of directors for the US-Japan Council. We believe that Ms. Doi is qualified to serve as a director due to her business and financial management experience, including her position as a chief financial officer, and her knowledge of audit committee functions.
Akiko Moni Miyashita has served as a director since May 2014. Ms. Miyashita is an independent advisor on mergers and acquisitions. She previously was employed for more than 30 years by IBM, where she served as Vice President, Corporate Development, including 15 years in executive M&A roles as Vice President, M&A Strategy and Investments; Managing Director of M&A Integration; and Director of Corporate Development Asia Pacific, headquartered in Tokyo. Ms. Miyashita serves on the Board of Trustees of the Children's Hospital of Philadelphia; the Executive Advisory Board of the University of Denver's Daniels School of Business; the International Executive Advisory Council of The Navigators; and the Board of Directors of the US-Japan Council. Ms. Miyashita holds a B.S. degree in Marketing from the University of Colorado, Leeds School of Business, and a M.B.A. from the University of Denver, Daniels School of Business. We believe that Ms. Miyashita is qualified to serve as a director of the Company due to her business and financial management experience, including her significant experience with M&A.
Phillip M. Satow has served as a director since May 2014. Mr. Satow is currently Chairman of the Board of Directors of JDS Therapeutics, LLC, which markets proprietary healthcare products to physicians, healthcare-delivery networks and other healthcare professionals. He was co-founder, Chairman and CEO of JDS Pharmaceuticals, LLC, a specialty pharmaceutical company focused in the fields of psychiatric and women's health treatments. JDS Pharmaceuticals was sold to Noven Pharmaceuticals, Inc. in 2008. Prior to founding JDS Pharmaceuticals, Mr. Satow served as Executive Vice President of Forest Laboratories, Inc., President of Forest Pharmaceuticals, Inc., and as a member
88
of Forest's Board of Directors. Prior to joining Forest, Mr. Satow served as Vice President and General Manager of the Wallace Laboratories Division of Carter—Wallace, Inc. In addition, he has held executive positions at Pfizer, including Director of Marketing with Pfizer Laboratories and Vice President of Pfizer Europe. Mr. Satow holds a B.A. degree from Columbia College and an M.A. degree in Economics from Georgetown University. We believe that Mr. Satow is qualified to serve on our Board of Directors due to his extensive experience and leadership in the pharmaceutical industry, in addition to his involvement with business and civic organizations and experience as an officer and director of publicly traded companies.
Mayuran Sriskandarajah ("Mayu Sris") has served as a director since May 2014. Mr. Sriskandarajah, or Mr. Sris, is a Founding Partner and Managing Director of Sarissa Capital Management LP, or Sarissa, a hedge-fund firm. He previously served as an Investment Analyst at Icahn Capital, an investment company, from 2005 to 2010 and as a consultant in 2011, where he contributed to all aspects of the activist investing process with the goal of proactively creating substantial value. Prior to Icahn Capital, Mr. Sris served as a consultant at Bain & Company, a management consulting firm, from 2002 to 2005, working with both corporate and private equity clients. Previously, he served as an investment banker at Wasserstein Perella & Company, an investment bank, where he advised technology clients on M&A transactions. In addition, from 2006 to 2010, Mr. Sris was a member of the Board of Directors of publicly traded Viskase Companies, Inc. Mr. Sris received his A.B. degree from Brown University. We believe that Mr. Sris is qualified to serve as a director of the Company due to his business and financial management experience, including his investment banking experience.
Willis C. Lee, MS has served as our Chief Operating Officer since May 2011. Mr. Lee served as a director of Emmaus Life Sciences, Inc. from May 2011 to May 2014 and also served as the Co-Chief Operating Officer and Chief Financial Officer and as a director of Emmaus Medical from March 2010 to May 2011. Prior to that, he was the Controller at Emmaus from February 2009 to February 2010. From 2004 to 2010, Mr. Lee led worldwide sales and business development of Yield Dynamics product group at MKS Instruments, Inc., a provider of instruments, subsystems, and process control solutions for the semiconductor, flat panel display, solar cell, data storage media, medical equipment, pharmaceutical manufacturing, and energy generation and environmental monitoring industries. Mr. Lee also served as President and Managing Director of Kenos Inc., a private service provider that provides funeral services to individuals, from January 2004 to December 2008. Mr. Lee held various managerial and senior positions at semiconductor companies such as MicroUnity Systems Engineering, Inc., a private semiconductor company that designs and develops new generation multimedia processors for computers and cable control boxes, from August 1995 to July 1996, HPL, Inc., a public semiconductor company that provides a software platform that enables data-driven decisions by gathering, managing and analyzing semiconductor manufacturing data, from June 2000 to October 2002, Syntricity, Inc., a private semiconductor software company that provides a software platform that enables data-driven decisions by gathering, managing and analyzing semiconductor manufacturing data, from November 2002 to April 2004 and also at Reden & Anders, a subsidiary of United Healthcare that provides actuarial services including capitation and risk assessment analyses for healthcare insurance carriers, from September 1996 to June 2000. Mr. Lee received his B.S. and M.S. in Physics from University of Hawaii (1984) and University of South Carolina (1986), respectively.
Peter B. Ludlum has served as our Executive Vice President and Chief Financial Officer since April 2012. Mr. Ludlum previously served as the Chief Financial Officer of Energy and Power Solutions, Inc., an energy intelligence company, from April 2008 to March 2012. Mr. Ludlum also served as the Financial Compliance Officer from April 2006 to March 2008 and the Chief Financial Officer from April 2005 to April 2006 of Applied Medical Resources Corporation, a medical device company. Mr. Ludlum also served as Group Controller for IsoTis S.A., a biomedical company, from October 2003 to April 2005. IsoTis S.A. acquired GenSci Regeneration Sciences Inc., an orthobiologics company, in October 2003 where Mr. Ludlum served as Vice President and Chief Financial Officer
89
from December 1999 to October 2003. Prior to his position at GenSci, Mr. Ludlum had served as the Vice President Finance and Chief Financial Officer from November 1996 to December 1999 of Pacific Biometrics, Inc., a diagnostic products and reference laboratory company. Earlier in his career, Mr. Ludlum had worked for Derlan Industries, Ltd., a diversified manufacturing company, as Corporate Controller and Treasurer and in various positions at Mobil Oil Corporation, an international oil and gas company, and for subsidiaries of Bechtel Corporation, an engineering, procurement, construction, and project management services provider, and PacifiCorp, a diversified utility holding company. He received a B.S. in Business and Economics with a major in accounting from Lehigh University in 1977 and a Masters in Business Administration with a concentration in Finance from California State University, Fullerton in 1991.
On September 23, 2011, Mr. Ludlum was serving as the Chief Financial Officer of Energy and Power Solutions, Inc., or EPS, when EPS filed for bankruptcy protection under Chapter 11 of the U.S. Bankruptcy Code. The bankruptcy filing was made in part to effect a sale of EPS' primary assets pursuant to Section 363 of the U.S. Bankruptcy Code, and the EPS bankruptcy case has been dismissed.
Lan T. Tran, MPH has served as our Chief Administrative Officer and Corporate Secretary since May 2011. She has served as the Co-Chief Operating Officer and Corporate Secretary of Emmaus Medical since April 2010 and as the Chief Compliance Officer of Emmaus Medical since May 2008. Prior to joining Emmaus Medical, Ms. Tran was with LA BioMed, a non-profit medical research and education company, from September 1999 to April 2008 and held positions of increasing responsibility including Grants and Contracts Trainee from September 1999 to March 2000, Grants and Contracts Officer from April 2000 to August 2004, Associate Director, Pre-Clinical/Clinical Trials Unit from September 2004 to June 2005, Director, Pre-Clinical/Clinical Trials Unit from July 2005 to June 2007, and Assistant Vice President, Research Administration from June 2007 to April 2008. In her position as Director, Pre-Clinical/Clinical Trials Unit and Assistant Vice President, Research Administration, Ms. Tran was part of the executive management team of LA BioMed and responsible for all administrative aspects of research in her assigned area at LA BioMed, which had a research budget of $61,000,000 in 2008. Ms. Tran holds a B.S. in Psychobiology from UCLA, which was awarded in 1999, and a Masters of Public Health from UCLA which was awarded in 2002.
Yasushi Nagasaki was appointed as our Senior Vice President, Finance effective April, 2012. From May 2011 to April 2012, Mr. Nagasaki served as our Chief Financial Officer. From September 2005 until joining us, Mr. Nagasaki was the Chief Financial Officer of Hexadyne Corporation, an aerospace and defense supplier. Mr. Nagasaki also served on the board of directors at Hexadyne Corporation from September 2005 to April 2011. From May 2003 to August 2005, Mr. Nagasaki was the Controller at Upsilon Intertech Corporation, an international distributor of defense and aerospace parts and sub systems. Mr. Nagasaki received a B.A. in Commerce in 1992 from Waseda University and an M.A. in International Policy Studies in 1994 from the Monterey Institute of International Studies.
Charles Stark, Pharm.D. was appointed Senior Vice President of Research and Development of the Company effective November 2013. Dr. Stark previously served as Director of Clinical Development at Bavarian Nordic, Inc., an immunotherapeutic company, from March 2013 to November 2013. Dr. Stark had also served as Associate Director of Medical Affairs from July 2010 to March 2013 for the Dendreon Corporation, an immunotherapeutic company, and prior to his position at Dendreon, he was the Director, Medical Science Liaisons (cardiovascular, metabolic and oncology) from July 1999 to July 2010 at Pfizer, Inc., a pharmaceutical company. Dr. Stark served as the Director of Investigational Drug Services and Clinical Research at LA BioMed at Harbor-UCLA from 1989 to 2003 and at the Health Research Association at USC Medical Center from 1995 to 1999. Dr. Stark has also served as a faculty member at the University of Southern California School of Pharmacy since 1997. Dr. Stark received his Pharm.D. from the University of Southern California in 1982 and completed his residency at the Veteran's Affairs Medical Center in West Los Angeles in 1983.
90
Family Relationships
There are no family relationships among any of the officers and directors.
Board of Directors and Committees and Director Independence
Our board of directors currently consists of six members. Our board of directors has determined that each of Henry A. McKinnell, Jr., Tracey C. Doi, Akiko Moni Miyashita, Phillip M. Satow and Mayu Sris is an "independent" director as defined by the NASDAQ Marketplace Rules currently in effect and all applicable rules and regulations of the SEC. All members of the Audit and Compensation, Nominating and Corporate Governance Committees satisfy the "independence" standards applicable to members of each such committee. The board of directors made this affirmative determination regarding these directors' independence based on discussions with the directors and on its review of the directors' responses to a standard questionnaire regarding employment and compensation history; affiliations, family and other relationships; and transactions with the Company. The board of directors considered relationships and transactions between each director or any member of his immediate family and the Company and its subsidiaries and affiliates. Mr. Satow was designated to serve as a director by T.R. Winston & Company, LLC, or TRW, pursuant to its rights under the Director Designation Agreement described on page 92 of this Annual Report on Form 10-K, and Mr. Sris was designated to serve as a director by Sarissa Capital Management LP, or Sarissa, pursuant to its rights under such Director Designation Agreement. Sarissa also has the right to designate an additional individual to be appointed to our board of directors pursuant to such Director Designation Agreement.
Audit Committee
We established our Audit Committee on May 3, 2011. The Audit Committee consists of Ms. Doi, Dr. McKinnell, Ms. Miyashita, Mr. Sris and Mr. Satow, each of whom is an independent director as defined by the NASDAQ Marketplace Rules. Ms. Doi serves as Chairman of the Audit Committee and qualifies as an "audit committee financial expert" as defined under Item 407(d) of Regulation S-K. The purpose of the Audit Committee is to represent and assist our board of directors in its general oversight of our accounting and financial reporting processes, audits of the financial statements and internal control and audit functions. The Audit Committee's responsibilities include:
- •
- The appointment, replacement, compensation, and oversight of work of the independent registered public accounting firm, including
resolution of disagreements between management and the independent registered public accounting firm regarding financial reporting, for the purpose of preparing or issuing an audit report or
performing other audit, review or attest services.
- •
- Reviewing and discussing with management and the independent registered public accounting firm various topics and events that may have significant financial impact on our company or that are the subject of discussions between management and the independent registered public accounting firm.
The board of directors has adopted a written charter for the Audit Committee. A copy of the Audit Committee Charter is available on our website at www.emmausmedical.com.
Compensation, Nominating and Corporate Governance Committee
We established our Compensation Committee and Nominating and Corporate Governance Committee on May 3, 2011. In May 2013, we combined these two committees into one. The Compensation, Nominating and Corporate Governance Committee consists of Ms. Miyashita, Ms. Doi, Dr. McKinnell, Mr. Satow and Mr. Sris, each of whom is an independent director as defined by the
91
NASDAQ Marketplace Rules. Ms. Miyashita serves as Chairman of the Compensation, Nominating and Corporate Governance Committee. The Compensation, Nominating and Corporate Governance Committee is responsible for the design, review, recommendation and approval of compensation arrangements for our directors, executive officers and key employees, and for the administration of our equity incentive plans, including the approval of grants under such plans to our employees, consultants and directors, assists in the selection of director nominees, approves director nominations to be presented for stockholder approval at our annual general meeting, fills any vacancies on our board of directors, considers any nominations of director candidates validly made by stockholders, and reviews and considers developments in corporate governance practices. The Compensation, Nominating and Corporate Governance Committee also reviews and determines compensation of our executive officers, including our Chief Executive Officer. The board of directors has adopted a written charter for the Compensation, Nominating and Corporate Governance Committee. A copy of the Compensation, Nominating and Corporate Governance Committee Charter is available on our website at www.emmausmedical.com.
Director Designation Agreement
In connection with the private placement of securities we completed on September 11, 2013, we entered into an agreement with Dr. Niihara, TRW, who served as our placement agent, and Sarissa, an investor in the private placement. Pursuant to the agreement, we agreed to expand the size of our board of directors by three members to a total of eight directors, with one member to be designated by TRW and two members to be designated by Sarissa to fill the newly-created vacancies.
Mr. Satow was designated to serve as a member of the board by TRW pursuant to its rights under the agreement, and Mr. Sris was designated to serve as a member of the board by Sarissa pursuant to its rights under the agreement. Sarissa has the right to designate another individual to be appointed to the board, and at the time of such appointment we expect to further expand the size of the board by one member.
The agreement provides that, prior to the date such designations first become effective, without the prior approval of TRW and Sarissa, we will not approve, effect, amend or enter into any agreements or transactions, or create or assume any obligations, other than those expressly contemplated in the agreement or undertaken in the ordinary course of business consistent with past practice.
The agreement also provides that each of Dr. Niihara, TRW and Sarissa agree to vote all shares of our common stock and any other class of our voting securities owned or controlled by them, and otherwise to use their respective commercially reasonable best efforts as stockholders, to elect as directors each of the TRW and Sarissa designees and every other candidate nominated for election to the board of directors by the Compensation, Nominating and Corporate Governance Committee of the board.
Pursuant to the agreement, until the closing of a qualified initial public offering (as defined in the subscription agreements for the private placement), we are required to obtain unanimous approval of the board of directors for a detailed annual budget, certain related party transactions, and certain other corporate transactions.
Section 16(a) Beneficial Ownership Reporting Compliance
Our common stock is currently registered under Section 12 of the Securities Exchange Act of 1934, as amended. As a result, and pursuant to Rule 16a-2, our directors and officers and holders of 10% or more of our common stock are currently required to file statements of beneficial ownership with respect to their ownership of our equity securities under Sections 13 or 16 of the Exchange Act. Based on a review of written representations from our executive officers and directors and a review of Forms 3, 4 and 5 furnished to us, we believe that during the fiscal year ended December 31, 2014 the directors, officers and owners of more than 10% of our common stock filed, on a timely basis, all reports required by Section 16(a) of the Exchange Act.
92
Code of Business Conduct and Ethics
On May 3, 2011, our board of directors approved a Code of Conduct and Ethics, which we refer to as the Code of Ethics, which applies to all of our directors, officers and employees. The Code of Ethics addresses, among other things, honesty and ethical conduct, conflicts of interest, compliance with laws, regulations and policies, including disclosure requirements under the federal securities laws, confidentiality, trading on inside information, and reporting of violations of the code. A copy of the Code of Ethics is available on our website at www.emmausmedical.com. Requests for copies of the Code of Ethics should be sent in writing to Emmaus Medical, Inc., Attention: Secretary, 21250 Hawthorne Boulevard, Suite 800, Torrance, California 90503.
ITEM 11. EXECUTIVE COMPENSATION
Summary Compensation Table
The following table sets forth information concerning the compensation earned by our chief executive officer, chief financial officer and our next three most highly compensated executive officers for the two fiscal years ended December 31, 2014 and 2013.
Name and Position
|
Year | Salary | Bonus | Option Awards(1) |
Total | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Yutaka Niihara, M.D., MPH |
2014 | $ | 250,000 | $ | — | $ | — | $ | 250,000 | |||||||
President, Chief Executive Officer and Director |
2013 | 250,000 | — | 1,583,035 | 1,833,035 | |||||||||||
Willis C. Lee |
2014 | 180,000 | — | — | 180,000 | |||||||||||
Chief Operating Officer |
2013 | 180,000 | 20,000 | 1,583,035 | 1,783,035 | |||||||||||
Peter B. Ludlum |
2014 | 180,000 | — | — | 180,000 | |||||||||||
Executive Vice President and Chief Financial Officer |
2013 | 180,000 | 50,000 | 1,583,035 | 1,813,035 | |||||||||||
Lan T. Tran, MPH |
2014 | 180,000 | — | — | 180,000 | |||||||||||
Chief Administrative Officer and Corporate Secretary |
2013 | 180,000 | 20,000 | 1,583,035 | 1,783,035 | |||||||||||
Yasushi Nagasaki |
2014 | 180,000 | — | — | 180,000 | |||||||||||
Senior Vice President, Finance |
2013 | 180,000 | 20,000 | 1,583,035 | 1,783,035 | |||||||||||
The Company has calculated the grant date fair value of the option grants calculated in accordance with the provisions of Financial Accounting Standards Board, or FASB, Accounting Standards Codification, or ASC, Topic 718, "Stock Compensation". For a description of the assumptions used to calculate these amounts, see Note 7 to our consolidated financial statements included in this Annual Report on Form 10-K. The actual value of any option award will depend on the excess of our stock price over the exercise price on the date the option award is exercised. The actual value realized upon exercise of the option awards may be higher or lower than the value shown in this column.
Total compensation for Dr. Niihara, Mr. Lee and Ms. Tran for the years ended December 31, 2014 and 2013 did not include an amount for the annual performance bonus pursuant to their respective employment agreements. We did not grant performance-based cash bonuses in 2014 and 2013 in part because of management's emphasis on preserving available capital to fund operating expenses. Additionally, specific performance criteria were not established for our executive officers for 2014 or 2013. Each of our executive officers waived any right under their respective employment agreement to an annual performance bonus for 2014 and 2013 and we did not incur any liability as a result of not granting such performance-based cash bonuses.
In December 2013, the Compensation, Nominating and Corporate Governance Committee approved discretionary bonuses for each of Messrs. Lee, Ludlum, and Nagasaki and Ms. Tran as shown in the table above under the heading "Bonuses."
93
On February 28, 2013, the board of directors of the Company authorized and approved option grants to the named executive officers in the amounts set forth below, with a term of 10 years and an exercise price of $3.60 per share, the fair market value of a share of Common Stock as of the grant date, as determined by the board of directors.
Name
|
Title | Number of Options |
||||
|---|---|---|---|---|---|---|
Yutaka Niihara, M.D., MPH |
President, Chief Executive Officer and Director | 500,000 | ||||
Willis C. Lee |
Chief Operating Officer | 500,000 | ||||
Peter B. Ludlum |
Executive Vice President and Chief Financial Officer | 500,000 | ||||
Yasushi Nagasaki |
Senior Vice President, Finance | 500,000 | ||||
Lan T. Tran, MPH |
Chief Administrative Officer and Corporate Secretary | 500,000 | ||||
The options granted to each named executive officer will vest as follows: one-third (1/3) will vest on the first anniversary of the grant date, and the remaining two-thirds (2/3) will vest in twenty-four approximately equal monthly installments over a period of two years thereafter.
There were no option grants to management in 2014.
Outstanding Equity Awards at 2014 Fiscal Year End
The following table sets forth information regarding outstanding equity awards held by our named executive officers at the end of fiscal 2014.
Name
|
Number of Securities Underlying Unexercised Options (#) Exercisable |
Number of Securities Underlying Unexercised Options (#) Unexercisable(1)(2) |
Option Exercise Price |
Option Expiration Date |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Yutaka Niihara, M.D., MPH |
166,667 | 83,333 | (1) | $ | 3.60 | 4/1/2022 | |||||||
|
305,556 | 194,444 | (2) | $ | 3.60 | 2/28/2023 | |||||||
Willis C. Lee |
166,667 | 83,333 | (1) | $ | 3.60 | 4/1/2022 | |||||||
|
305,556 | 194,444 | (2) | $ | 3.60 | 2/28/2023 | |||||||
Peter B. Ludlum |
305,556 | 194,444 | (2) | $ | 3.60 | 2/28/2023 | |||||||
Lan T. Tran, MPH |
166,667 | 83,333 | (1) | $ | 3.60 | 4/1/2022 | |||||||
|
305,556 | 194,444 | (2) | $ | 3.60 | 2/28/2023 | |||||||
Yasushi Nagasaki |
166,667 | 83,333 | (1) | $ | 3.60 | 4/1/2022 | |||||||
|
305,556 | 194,444 | (2) | $ | 3.60 | 2/28/2023 | |||||||
- (1)
- Options
will vest annually in equal amounts (or as close to an equal amount as possible) over a period of three years, on each of April 1, 2013, 2014
and 2015.
- (2)
- One-third (1/3) of the options vested on February 28, 2014, the first anniversary of the grant date. The remaining two-thirds (2/3) vest in approximately equal monthly installments over a period of two years thereafter.
Employment Agreements
On April 5, 2011, we entered into employment agreements with Yutaka Niihara, M.D., MPH, our Chief Executive Officer, Willis C. Lee, our Chief Operating Officer, and Lan T. Tran, MPH, our Chief Administrative Officer. On April 8, 2011, we entered into an employment agreement with Yasushi Nagasaki, our former Chief Financial Officer and current Senior Vice President, Finance. On April 2, 2012, we entered into an employment agreement with Peter B. Ludlum, our Executive Vice President and Chief Financial Officer (the employment agreements, collectively, are referred to as the "Employment Agreements"). Each of the Employment Agreements has an initial 2-year term, unless
94
terminated earlier. Mr. Nagasaki's employment agreement expired in April 2013, and Mr. Ludlum's employment agreement expired in April 2014. The Employment Agreements for Dr. Niihara, Mr. Lee and Ms. Tran automatically renew for additional one year periods unless the Company or the officer provides notice of non-renewal at least sixty (60) days prior to the expiration of the then current term.
Base Salary, Bonus and Other Compensation. Dr. Niihara's, Mr. Lee's, and Ms. Tran's base salary is $250,000, $180,000, and $180,000 per year, respectively, which will be reviewed at least annually. In addition to base salary, the officers may be entitled to receive an annual performance bonus based on the officer's performance for the previous year. The Employment Agreements each provide that the respective officer's performance for the previous year will be measured against a set of targets and goals as mutually established by us and the officer. To date, our board of directors and the Compensation, Nominating and Corporate Governance Committee have evaluated an officer's performance on an overall basis related to the Company's progress on major milestones, without reliance on specific position by position pre-established targets and goals. The officers are also eligible to receive paid vacation, and participate in health and other benefit plans and will be reimbursed for reasonable and necessary business expenses.
Equity Compensation. The Employment Agreements state that at the end of each calendar year on December 31, or as soon as reasonably practicable after such date (each a "Grant Date"), the Company will grant non-qualified ten-year stock options with a Black Scholes value of $100,000 to Dr. Niihara, $50,000 to Mr. Lee, and $40,000 to Ms. Tran, in each case with an exercise price per share equal to the "Fair Market Value" (as such term is defined in the Company's 2011 Stock Incentive Plan) on the applicable Grant Date. One-third of the options granted to each officer will vest on the first anniversary of the applicable Grant Date, one-third will vest on the second anniversary of the applicable Grant Date and the final one-third will vest on the third anniversary of the applicable Grant Date. Any unvested options will vest immediately upon a change in control (as defined below), termination of the officer's employment other than a voluntary termination by the officer or a termination of the officer for cause. In the event that the officer is terminated for any reason other than cause, death or disability or retirement, each option, to the extent that it is exercisable at the time of such termination, shall remain exercisable for the 90 day period following such termination, but in no event following the expiration of its term. In the event that the officer's employment terminates on account of death, disability or, with respect to any non-qualified stock option, retirement, each option granted that is outstanding and vested as of the date of such termination shall remain exercisable by such officer (or the officer's legal representatives, heirs or legatees) for the one year period following such termination, but in no event following the expiration of its term.
Severance Compensation. If Dr. Niihara's, Mr. Lee's, or Ms. Tran's employment is terminated for any reason during the term of his or her respective employment agreement, other than without cause or good reason, each will be entitled to receive his or her base salary prorated through the termination date, any expense reimbursement due and owing for reasonable and necessary business expenses, and unpaid vacation benefits (the "Voluntary Termination Benefits"). If Dr. Niihara's, Mr. Lee's, or Ms. Tran's employment is terminated due to death or disability of such officer during the term of his or her respective employment agreement, then such officer will also receive an amount equal to his or her target annual performance bonus, and for a termination due to disability only, 6 additional months of his or her base salary to be paid out over a 6-month period and payment of COBRA benefits of up to 6 months following the termination. If Dr. Niihara's employment is terminated without cause or Dr. Niihara resigns with good reason (not within 2 years following a change in control), he will receive the Voluntary Termination Benefits and, provided he signs a Release, a severance package equal to one year of his base salary to be paid out over a 12-month period, a pro rata amount of the annual performance bonus for the calendar year in which the termination date occurs based on the achievement of any applicable performance terms or goals for the year, and payment of COBRA benefits of up to 12 months following the termination. If the employment of any of Mr. Lee, or
95
Ms. Tran is terminated without cause or any of them resigns with good reason (not within 2 years following a change in control) during the term of his or her respective employment agreement, he or she will receive the Voluntary Termination Benefits and, provided he or she signs a Release, a severance package equal to 6 months of his or her base salary to be paid out over a 6-month period, an amount equal to half of the targeted annual performance bonus, and payment of COBRA benefits of up to 6 months following the termination.
Termination with cause includes a proven act of dishonesty, fraud, embezzlement or misappropriation of Company proprietary information; a conviction of, or plea of nolo contendere to, a felony or a crime involving moral turpitude; willful misconduct which cannot be cured on reasonable notice to the officer; or the officer's habitual failure or refusal to perform his duties if such failure or refusal is not cured within 20 days after receiving written notice thereof from the board of directors. Good reason includes a reduction of more than 10% to the officer's base salary or other compensation (except as part of a general reduction for all executive employees); a material diminution of the officer's authority, responsibilities, reporting or job duties (except for any reduction for cause); the Company's material breach of the Employment Agreement; or, in the case of Dr. Niihara, Mr. Lee and Ms. Tran, a relocation of the business requiring the officer to move or drive to work more than 40 miles from its current location. The officer may terminate the Employment Agreement for good reason if he or she provides written notice to the Company within ninety (90) days of the event constituting good reason and the Company fails to cure the good reason within thirty (30) days after receiving such notice.
Change of Control. The Employment Agreements will not be terminated upon a change of control. A change of control means any merger or reorganization where the holders of the Company's capital stock prior the transaction own fewer than 50% of the shares of capital stock after the transaction, an acquisition of 50% of the voting power of the Company's outstanding securities by another entity, or a transfer of at least 50% of the fair market value of the Company's assets. Upon Dr. Niihara's termination without cause or good reason that occurs within 2 years after a change of control, he will receive the Voluntary Termination Benefits and, provided he signs a release of all claims relating to his employment, a severance package equal to 2 years of his base salary to be paid out over a 12-month period, an amount equal to double the targeted annual performance bonus, payment of COBRA benefits of up to 18 months following the termination; and a one-time cash payment of $3.0 million. Upon Mr. Lee's or Ms. Tran's termination without cause or good reason that occurs within 2 years after a change of control, he or she will receive the Voluntary Termination Benefits and, provided he or she signs a Release, a severance package equal to 1 year of his or her base salary to be paid out over a 12-month period, an amount equal to the full year targeted annual performance bonus, payment of COBRA benefits of up to 12 months following the termination. Mr. Lee and Ms. Tran will also receive a one-time cash payment of $200,000. In addition, each officer's unvested equity awards shall vest upon such termination and the officer will have 36 months in which to sell or exercise such awards (subject to expiration of the term of such options). The officer will also be free from all lock-up or other contractual restrictions upon the free sale of shares that are subject to waiver by the Company upon such termination.
Director Compensation
Since February 26, 2014, the compensation program for non-employee directors has consisted of:
- •
- a cash retainer of $500 per month;
- •
- cash compensation of $700 for each in-person board meeting attended, $400 for each telephonic board meeting attended, $400 for each
committee meeting attended, and $200 for each meeting chaired by such non-employee director;
- •
- an initial grant of 100,000 options upon election and 50,000 stock options annually thereafter;
96
- •
- a one-time grant of 2,000 options to the chairman of each committee; and
- •
- a one-time grant of 10,000 options to the Chairman of the Board.
Options granted under this program are expected to vest over 3 years. Pursuant to the 2011 Stock Incentive Plan, non-officer directors receive at a minimum an annual grant of 10,000 options. Additionally, the Chairman of the Board receives a one-time retainer grant of 10,000 options and each committee chair receives a one-time retainer grant of 2,000 options. These provisions of the 2011 Stock Incentive Plan have effectively been superseded by the current non-employee director compensation program.
The following table shows information regarding the compensation earned during the fiscal year ended December 31, 2014 by the non-employee members of our board of directors. Compensation earned by our current and former employee directors, Yutaka Niihara, M.D., MPH and Willis C. Lee, who do not receive compensation for their services as a director, is reported above under the heading "Summary Compensation Table."
Name
|
Fees Earned or Paid in Cash |
Option Awards(1)(2) |
Total | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
Henry A. McKinnell, Jr., Ph.D. |
$ | 12,800 | $ | 146,699 | $ | 159,499 | ||||
Tracey C. Doi |
13,900 | 127,139 | 141,039 | |||||||
Maurice J. DeWald(3) |
9,100 | 127,139 | 136,239 | |||||||
Duane K. Kurisu(4) |
5,500 | 329,382 | 334,882 | |||||||
Akiko Moni Miyashita |
5,500 | 335,970 | 341,470 | |||||||
Phillip M. Satow |
4,700 | 329,382 | 334,082 | |||||||
Mayuran Sriskandarajah |
4,000 | 349,215 | 353,215 | |||||||
| | | | | | | | | | | |
Total |
$ | 55,500 | $ | 1,744,926 | $ | 1,800,426 | ||||
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
- (1)
- The
amounts in this column represent the aggregate grant date fair value of option awards granted to the directors in 2014, calculated in accordance with
the provisions of FASB ASC Topic 718, "Stock Compensation". For a description of the assumptions used to calculate these amounts, see Note 7 to our audited consolidated financial statements
included in this Annual Report on Form 10-K. The actual value of any option award to a director, if any, will depend on the excess of our stock price over the exercise price on the date the
option award is exercised. The actual value realized by the director upon exercise of the option awards may be higher or lower than the value shown in this column.
- (2)
- As of December 31, 2014, our non-employee directors had option awards outstanding pursuant to the 2011 Stock Incentive Plan to purchase the following number of shares of our common stock in the table directly below:
97
Name
|
Number of Securities Underlying Unexercised Options ($) Exercisable (Vested) |
Number of Securities Underlying Unexercised Options ($) Unexercisable (Unvested) |
Option Exercise Price |
Option Expiration Date |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Henry A. McKinnell, Jr., Ph.D. |
11,795 | (a) | — | $ | 3.05 | 6/30/2015 | |||||||
|
27,000 | — | $ | 3.60 | 12/19/2021 | ||||||||
|
83,333 | 166,667 | (c) | $ | 3.60 | 2/28/2023 | |||||||
|
10,000 | — | $ | 3.60 | 2/28/2023 | ||||||||
|
— | 60,000 | $ | 3.60 | 2/26/2024 | ||||||||
Tracey C. Doi |
10,000 | — | $ | 3.60 | 12/19/2021 | ||||||||
|
50,000 | (b) | 25,000 | (b) | $ | 3.60 | 4/2/2022 | ||||||
|
83,333 | 166,667 | (c) | $ | 3.60 | 2/28/2023 | |||||||
|
10,000 | — | $ | 3.60 | 2/28/2023 | ||||||||
|
— | 52,000 | $ | 3.60 | 2/26/2024 | ||||||||
Maurice J. DeWald(3) |
12,000 | — | $ | 3.60 | 12/19/2021 | ||||||||
|
50,000 | (b) | 25,000 | (b) | $ | 3.60 | 4/2/2022 | ||||||
|
83,333 | 166,667 | (c) | $ | 3.60 | 2/28/2023 | |||||||
|
10,000 | — | $ | 3.60 | 2/28/2023 | ||||||||
|
— | 52,000 | $ | 3.60 | 2/26/2024 | ||||||||
Duane K. Kurisu(4) |
— | 100,000 | $ | 4.90 | 5/8/2024 | ||||||||
Akiko Moni Miyashita |
— | 102,000 | $ | 4.90 | 5/8/2024 | ||||||||
Phillip M. Satow |
— | 100,000 | $ | 4.90 | 5/8/2024 | ||||||||
Mayuran Sriskandarajah |
— | 100,000 | $ | 5.10 | 7/17/2024 | ||||||||
- (a)
- Includes
options to purchase 11,795 shares that vested as of December 31, 2012 and are exercisable at $3.05 per share through 2015, which award was
issued prior to the commencement of the 2011 Stock Incentive Plan.
- (b)
- Options
vest in equal annual amounts (or as close to an equal amount as possible) over a period of three years with the first one-third (1/3)
vesting on April 1, 2013.
- (c)
- Options vest annually in equal amounts (or as close to an equal amount as possible) over a period of three years with the first one-third (1/3) vesting on February 28, 2014.
- (3)
- Mr. DeWald
resigned from the board of directors on May 8, 2014.
- (4)
- Mr. Kurisu resigned from the board of directors on January 9, 2015.
On March 19, 2015, the board of directors authorized and approved the annual option grants to non-employee directors contemplated by our director compensation program, which options have a term of 10 years and an exercise price of $3.60 per share, the fair market value of a share of common stock as of the grant date as determined by the board of directors.
98
2011 Stock Incentive Plan
Background
On May 3, 2011, the board of directors and stockholders adopted the Emmaus Life Sciences, Inc. 2011 Stock Incentive Plan, or the Plan. Stockholder approval of the Plan enables us to satisfy stock exchange listing requirements, if and when we become subject to such requirements, and to make awards that qualify as performance-based compensation that is exempt from the deduction limitation set forth under Section 162(m) of the Internal Revenue Code of 1986, as amended, or the Code. Subject to certain exceptions, Section 162(m) generally limits the corporate income tax deductions to $1,000,000 annually for compensation paid to each of the Chief Executive Officer and our other three highest paid executive officers required to be reported under the proxy disclosure rules.
The Plan permits grants of stock options, stock appreciation rights, or SARs, restricted stock, stock units, stock bonus and unrestricted stock awards, which we collectively refer to as Awards. Our board of directors believes that the Plan is an important factor in attracting, retaining and motivating our employees, consultants, agents, and directors and our affiliates, who are collectively referred to as Eligible Persons. Our board of directors believes that we need the flexibility both to have an ongoing reserve of common stock available for future equity-based awards, and to make future awards in a variety of forms.
On February 28, 2013, our board of directors authorized and adopted an amendment to Section 1.5(a) of the Plan. The amendment increased the total number of shares of our common stock with respect to which awards may be granted pursuant to the Plan from 3,000,000 to 6,000,000 (subject to adjustment as set forth in the Plan), which we refer to as the Cap. Except for this increase in the Cap, the terms of the Plan, including the adjustment mechanisms set forth therein, were unchanged by the amendment. The amendment was subsequently ratified by our stockholders at our 2013 Annual Meeting on September 23, 2013. On July 17, 2014, our board of directors authorized and adopted an amendment to Section 1.5(a) of the Plan. The amendment increased the total number of shares of our common stock with respect to which awards may be granted pursuant to the Plan from 6,000,000 to 9,000,000 (subject to adjustment as set forth in the Plan) and was subsequently ratified by our stockholders at our 2014 Annual Meeting on October 23, 2014.
Purpose
The purpose of the Plan is to attract, retain and motivate select employees, consultants and directors for us and our affiliates and to provide incentives and rewards for superior performance. As of December 31, 2014, there were approximately 25 Eligible Persons who were our directors or employees of one of our subsidiaries.
Shares Subject to the Plan
The Plan provides that no more than 9,000,000 shares of common stock may be issued pursuant to Awards under the Plan. The number of shares available for Awards, as well as the terms of outstanding Awards, is subject to adjustment as provided in the Plan for stock splits, stock dividends, recapitalizations and other similar events.
The maximum awards that can be granted under the Plan to a single participant in any calendar year shall be 500,000 shares of common stock in the form of options or SARs, and 500,000 shares of common stock in the form of restricted shares, restricted stock units, stock bonus and other stock-based awards.
99
Administration
The Compensation, Nominating and Corporate Governance Committee of the board of directors or another committee appointed by our board of directors administer the Plan. The Compensation, Nominating and Corporate Governance Committee of our board of directors and any other committee exercising discretion under the Plan from time to time are referred to below as the Committee.
Subject to the terms of the Plan, the Committee has express authority to determine the Eligible Persons who will receive Awards, the number of shares of common stock, units or dollars to be covered by each Award, and the terms and conditions of Awards. The Committee has broad discretion to prescribe, amend, and rescind rules relating to the Plan and its administration, to interpret and construe the terms of the Plan and the terms of all Award agreements, and to take all actions necessary or advisable to administer the Plan.
Stock awards granted under the Plan (other than performance-based awards, awards involving an aggregate number of shares equal to less than 5% of shares available for awards and annual director stock grants described below) will have a minimum vesting period of three years.
The Plan provides that we will indemnify members of the Committee and their delegates against any claims, liabilities or costs arising from the good faith performance of their duties under the Plan. The Plan releases these individuals from liability for good faith actions associated with the Plan's administration.
Eligibility
The Committee may grant options that are intended to qualify as incentive stock options, or ISOs, only to our and our subsidiaries' employees, and may grant all other Awards to Eligible Persons. The Plan and the discussion below use the term "Participant" to refer to an Eligible Person who has received an Award.
Types of Awards
Options. Options granted under the Plan provide Participants with the right to purchase shares of common stock at a predetermined exercise price. The Committee may grant options that are intended to qualify as ISOs or options that are not intended to so qualify, referred to as Non-ISOs. The Plan also provides that ISO treatment may not be available for options that become first exercisable in any calendar year to the extent the value of the underlying shares that are the subject of the option exceeds $100,000 (based upon the fair market value of the shares of common stock on the option grant date).
Stock Appreciation Rights (SARs). A stock appreciation right generally permits a Participant to receive, upon exercise, cash and/or shares of common stock equal in value to an amount determined by multiplying (a) the excess of the fair market value, on the date of exercise, of the shares of common stock with respect to which the SAR is being exercised, over the exercise price of the SAR for such shares by (b) the number of shares with respect to which the SARs are being exercised. The Committee may grant SARs in tandem with options or independently of them.
Exercise Price for Options and SARs. The exercise price of ISOs, Non- ISOs, and SARs may not be less than 100% of the fair market value on the grant date of the shares of common stock subject to the Award (110% of fair market value for ISOs granted to employees who, on the grant date, own stock representing more than 10% of the combined voting power of all classes of our stock).
Exercise of Options and SARs. To the extent exercisable in accordance with the agreement granting them, an option or SAR may be exercised in whole or in part, during the term established by the Committee, subject to earlier termination relating to a holder's termination of employment or service. The expiration date of options and SARs may not exceed ten years from the date of grant (five
100
years in the case of ISOs granted to employees who, on the grant date, own more than 10% of the combined voting power of all classes of our stock).
Unless otherwise provided under the terms of the agreement evidencing a grant, options and SARs that have vested may be exercised during the one-year period after the optionee's termination of service due to death or permanent disability or after the optionee retires (with respect to SARs and Non-ISOs only) and during the 90-day period after the optionee's termination of employment other than for cause (but in no case later than the termination date of the option or SAR). Each option or SAR that remains unexercisable at the time of termination shall be terminated at the time of termination.
Restricted Stock, Stock Units, Stock Bonus, and Other Stock-Based Awards. Under the Incentive Plan, the Committee may grant restricted stock that is forfeitable until certain vesting requirements are met, may grant restricted stock units, or RSUs, which represent the right to receive shares of common stock after certain vesting requirements are met, and may grant unrestricted stock as to which the Participant's interest is immediately vested (subject to the exceptions to the minimum vesting requirements described above). The Plan provides the Committee with discretion to determine the terms and conditions under which a Participant's interests in such Awards becomes vested, which may include the achievement of financial or other objective performance goals or other objectives.
Annual Non-Employee Director Grants. The Plan provides for annual grants of 10,000 options to non-employee directors, which we refer to as the Annual Director Award. Each Annual Director Award will vest in four substantially equal quarterly installments. These grants have been superseded by, and are not in addition to, the compensation program for non-employee directors.
Clawback of Awards
Unless otherwise provided in an agreement granting an Award, we may terminate any outstanding, unexercised, unexpired or unpaid Award, rescind any exercise, payment or delivery pursuant to the Award, or recapture any common stock (whether restricted or unrestricted) or proceeds from the Participant's sale of shares issued pursuant to the Award in the event of the discovery of the Participant's fraud or misconduct, or otherwise in connection with a financial restatement.
Income Tax Withholding
As a condition for the issuance of shares pursuant to Awards, the Plan requires satisfaction of any applicable federal, state, local, or foreign withholding tax obligations that may arise in connection with the award or the issuance of shares.
Transferability
Awards may not be sold, pledged, assigned, hypothecated, transferred or disposed of other than by will or the laws of descent and distribution, except to the extent the Committee permits.
Certain Corporate Transactions
The Committee shall equitably adjust, as it deems necessary and appropriate, the number of shares covered by each outstanding Award, the number of shares that have been authorized for issuance under the Plan but have not yet been granted or that have been returned to the Plan upon cancellation, forfeiture or expiration of an Award, and the maximum number of shares that may be granted in any calendar year to individual participants, as well as the price per share covered by each outstanding Award, to reflect any increase or decrease in the number of issued shares resulting from a stock split, reverse stock split, stock dividend, combination, recapitalization or reclassification of the
101
shares, or any other increase or decrease in the number of issued shares effected without receipt of consideration by us.
The Committee has the authority, in the event of a merger, sale of assets, reorganization or similar change in control transaction, to cause us to accelerate the vesting of Awards. To the extent that an Award is not exercised prior to consummation of a transaction in which the Award is not being assumed or substituted, such Award shall terminate upon such consummation.
Term of the Plan; Amendments or Termination
The term of the Plan is ten years from the date of adoption by the board of directors. Our board of directors may from time to time, amend, alter, suspend, discontinue or terminate the Plan; provided that no amendment, suspension or termination of the Plan shall materially and adversely affect Awards already granted. Furthermore, the Plan specifically prohibits the repricing of stock options or SARs without stockholder approval. For this purpose, a "repricing" means any of the following (or any other action that has the same effect as any of the following): (i) changing the terms of a stock option or SAR to lower its exercise price; (ii) any other action that is treated as a "repricing" under generally accepted accounting principles; and (iii) repurchasing for cash or canceling a stock option or SAR at a time when its exercise price is greater than the fair market value of the underlying stock in exchange for another award, unless the cancellation and exchange occurs in connection with a change in capitalization or similar change. Such cancellation and exchange would be considered a "repricing" regardless of whether it is treated as a "repricing" under generally accepted accounting principles and regardless of whether it is voluntary on the part of the participant.
U.S. Federal Income Tax Consequences
The U.S. federal income tax rules applicable to awards under the Plan under the Code are summarized below. This summary omits the tax laws of any municipality, state, or foreign country in which a participant resides. Stock option grants under the Plan may be intended to qualify as incentive stock options under Section 422 of the Code or may be non-qualified stock options governed by Section 83 of the Code. Generally, federal income tax is not due from a participant upon the grant of a stock option, and a deduction is not taken by us. Under current tax laws, if a participant exercises a non-qualified stock option, he or she will have taxable income equal to the difference between the market price of the common stock on the exercise date and the stock option grant price. We are entitled to a corresponding deduction on our income tax return. A participant will not have any taxable income upon exercising an incentive stock option after the applicable holding periods have been satisfied (except that the alternative minimum tax may apply), and we will not receive a deduction when an incentive stock option is exercised. The treatment for a participant of a disposition of shares acquired through the exercise of a stock option depends on how long the shares were held and whether the shares were acquired by exercising an incentive stock option or a non-qualified stock option. We may be entitled to a deduction in the case of a disposition of shares acquired under an incentive stock option before the applicable holding periods have been satisfied.
Generally, taxes are not due when a restricted stock or RSU award is initially granted, but the award becomes taxable when it is no longer subject to a "substantial risk of forfeiture" (generally, when it becomes vested or transferable), in the case of restricted stock, or when shares are issued in connection with vesting, in the case of an RSU. Income tax is calculated on the value of the stock at ordinary rates at that time, and then at capital gain rates when the shares are sold. However, no later than 30 days after a participant receives an award of restricted stock, pursuant to Section 83(b) of the Code, the participant may elect to recognize taxable ordinary income in an amount equal to the fair market value of the stock at the time of receipt. Provided that the election is made in a timely manner, the participant will not recognize any additional income when the award is no longer transferable or subject to a "substantial risk of forfeiture."
102
Section 409A of the Code provides additional tax rules governing non-qualified deferred compensation. Generally, Section 409A will not apply to awards granted under the Plan, but may apply in some cases to RSUs. For such awards subject to Section 409A, certain of our officers may experience a delay of up to six months in the settlement of the awards in shares of our stock.
Awards granted under the Plan may be structured to qualify as performance-based compensation under Section 162(m) of the Code. To qualify, the Plan must satisfy the conditions set forth in Section 162(m) of the Code, and stock options and other awards must be granted under the Plan by a committee consisting solely of two or more outside directors (as defined under Section 162 regulations) and must satisfy the Plan's limit on the total number of shares that may be awarded to any one participant during any calendar year. For awards other than stock options and SARs to qualify, the grant, issuance, vesting, or retention of the award must be contingent upon satisfying one or more of the performance criteria set forth in the Plan, as established and certified by a committee consisting solely of two or more outside directors. The rules and regulations promulgated under Section 162(m) are complicated and subject to change from time to time, sometimes with retroactive effect. In addition, a number of requirements must be met in order for particular compensation to so qualify. As such, there can be no assurance that any compensation awarded or paid under the Plan will be deductible under all circumstances.
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
Beneficial ownership is determined in accordance with the rules of the SEC. In computing the number of shares beneficially owned by a person and the percentage of ownership of that person, shares of common stock subject to options, warrants and convertible notes held by that person that are currently exercisable or become exercisable within 60 days of March 31, 2015 are deemed outstanding even if they have not actually been exercised. Those shares, however, are not deemed outstanding for the purpose of computing the percentage ownership of any other person.
The following table sets forth certain information with respect to beneficial ownership of our common stock based on issued and outstanding shares of common stock owned by:
- •
- Each person known to be the beneficial owner of 5% or more of our outstanding common stock;
- •
- Each named executive officer;
- •
- Each director; and
- •
- All of our executive officers and directors as a group.
The number of shares of our common stock outstanding as of March 20, 2015 excludes 5,930,795 shares of common stock underlying outstanding options, 4,708,072 shares of common stock underlying outstanding warrants and 2,462,792 shares of common stock underlying outstanding principal and accrued interest amount on the convertible notes as of March 20, 2015. Unless otherwise indicated, the persons and entities named in the table have sole voting and sole investment power with respect to the shares set forth opposite the stockholder's name, subject to community property laws, where applicable. Unless otherwise indicated in the table or its footnotes, the address of each stockholder listed in the
103
table is c/o Emmaus Life Sciences, Inc., 21250 Hawthorne Boulevard, Suite 800, Torrance, California 90503.
Name of Beneficial Owner
|
Title | Amount and Nature of Beneficial Ownership of Shares of Common Stock |
Percent of Class(1) |
||||||
|---|---|---|---|---|---|---|---|---|---|
Directors and Executive Officers |
|||||||||
Yutaka Niihara, M.D., MPH |
President, Chief Executive Officer and Director | 11,177,274 | (2)(13) | 37.7 | % | ||||
Peter B. Ludlum |
Executive Vice President and Chief Financial Officer | 364,111 | (3) | 1.3 | % | ||||
Yasushi Nagasaki |
Senior Vice President, Finance | 651,686 | (4) | 2.3 | % | ||||
Willis C. Lee |
Chief Operating Officer | 776,835 | (5) | 2.7 | % | ||||
Lan T. Tran, MPH |
Chief Administrative Officer and Corporate Secretary | 553,072 | (6) | 1.9 | % | ||||
Henry A. McKinnell, Jr., Ph.D. |
Chairman of the Board | 235,462 | (7) | * | |||||
Tracey C. Doi |
Director | 270,000 | (8) | 1.0 | % | ||||
Akiko Moni Miyashita |
Director | — | * | ||||||
Phillip M. Satow |
Director | 15,412 | (9) | * | |||||
Mayu Sris |
Director | — | * | ||||||
Officers and Directors as a Group (total of 10 persons) |
14,043,852 | (10) | 43.5 | % | |||||
5% or More Owners |
|||||||||
Daniel R. and Yuka I. Kimbell |
2,434,028 | (11) | 8.7 | % | |||||
Sarissa Capital Management LP |
1,997,657 | (12)(13) | 6.9 | % | |||||
- *
- Less
than 1%.
- (1)
- Based
on 28,093,848 shares of common stock issued and outstanding as of March 20, 2015. This excludes 2,504,249 shares previously owned by AFH
Advisory cancelled by the Company's transfer agent on June 28, 2013 pursuant to a court order. See Part I Item 3. "Legal Proceedings" of this Annual Report on Form 10-K for
additional information regarding the share cancellation and related legal proceedings. The Company continues to present these shares in its financial statements as outstanding until the right of
appeal has lapsed and all contingencies have been resolved.
- (2)
- Includes
9,529,711 shares that are held jointly by Yutaka and Soomi Niihara, his wife. Also includes 44,229 shares of common stock for which
Dr. Niihara is custodian. Dr. Niihara may be deemed the indirect beneficial owner of these securities since he has sole voting and investment control over the securities. Also includes
55,556 shares owned by Hope Hospice. Dr. Niihara is the chief executive officer of Hope Hospice and has voting and investment power over such shares. Also includes 1,000,000 shares underlying
warrants to purchase shares of common stock and 527,778 shares underlying stock options.
- (3)
- Includes
361,111 shares underlying options to purchase shares of common stock.
- (4)
- Includes
123,908 shares of common stock underlying outstanding convertible notes (as of March 20, 2015), and 527,778 shares underlying options to
purchase shares of common stock.
- (5)
- Includes
527,778 shares of common stock underlying outstanding options to purchase shares of common stock.
- (6)
- Includes
527,778 shares underlying options to purchase shares of common stock.
- (7)
- Includes
options to purchase 235,462 shares of common stock.
- (8)
- Includes
8,000 shares that are held jointly by Tracey & Mark Doi, her husband. Also, includes 254,000 shares underlying stock options and 8,000
shares underlying warrants to purchase shares of common stock owned by Tracey and Mark Doi.
- (9)
- Includes
15,412 shares of common stock underlying outstanding convertible notes (as of March 20, 2015)
- (10)
- Includes options to purchase 2,961,685 shares of common stock, warrants to purchase 1,008,000 shares of common stock and 139,320 (as of March 20, 2015) shares of common stock underlying outstanding convertible notes.
104
- (11)
- Includes
44,229 shares of common stock held by the holder as custodian. Daniel and Yuka Kimbell may be deemed the indirect beneficial owner of these
securities since they have sole voting and investment control over the securities. The address for these stockholders is 16 N. Marengo Street, Suite 307, Pasadena, CA 91101.
- (12)
- Includes
(i) 1,192,801 shares of common stock held by Sarissa Capital Domestic Fund LP ("Sarissa Domestic"), consisting of 715,121 shares of
common stock and 477,680 shares underlying warrants to purchase shares of common stock and (ii) 804,856 shares of common stock held by Sarissa Capital Offshore Master Fund LP ("Sarissa
Offshore"), consisting of 482,536 shares of common stock and 322,320 shares underlying warrants to purchase shares of common stock. Sarissa Capital Management LP ("Sarissa Capital"), as the
investment advisor to Sarissa Domestic and Sarissa Offshore (collectively, the "Sarissa Funds"), may be deemed to beneficially own the 1,997,657 aggregate shares beneficially owned by the Sarissa
Funds on the basis of its sole or shared power to vote or direct the vote of such shares and/or its sole or shared power to dispose or direct the disposition of such shares. By virtue of his position
as the Chief Investment Officer of Sarissa Capital and as the managing member of Sarissa Capital's general partner, Dr. Alexander J. Denner may also be deemed to have the shared power to vote
or direct the vote of (and the shared power to dispose or direct the disposition of) the 1,997,657 shares beneficially owned by the Sarissa Funds. The address for this stockholder is 660 Steamboat
Road Greenwich, CT 06830.
- (13)
- Dr. Niihara, Sarissa and TRW are parties to the Director Designation Agreement described in Part III, Item 10 of this Annual Report on Form 10-K under the heading "Director Designation Agreement." These parties beneficially own, in the aggregate, 14,242,931 shares of common stock, including 2,484,000 shares underlying warrants to purchase shares of common stock and 527,778 shares underlying stock options, representing aggregate beneficial ownership of 45.8%.
Securities Authorized for Issuance under Equity Compensation Plans
The following table provides information as of December 31, 2014 regarding compensation plans, including any individual compensation arrangements, under which equity securities of the Company are authorized for issuance. Please see Part III. Item 11 "2011 Stock Incentive Plan" for a description of the terms of the 2011 Stock Incentive Plan as amended.
Plan Category
|
Number of Securities to be issued upon exercise of outstanding options, warrants and rights |
Weighted-average exercise price of outstanding options, warrants and rights |
Number of securities remaining available for future issuance under equity compensation plans |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
Equity compensation plans approved by securityholders |
5,669,000 | $ | 3.68 | 3,331,000 | ||||||
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
Emmaus Medical, Inc.
Emmaus Life Sciences, Inc., Emmaus Medical Inc., or Emmaus Medical, Newfield Nutrition Corporation, or Newfield Nutrition, Emmaus Medical Japan, Inc., or EM Japan and Emmaus Medical Europe Ltd., or EM Europe, which are either our directly or indirectly wholly-owned subsidiaries, each have interlocking executive and director positions with us and with each other. Dr. Niihara and Mr. Lee are directors of Newfield Nutrition. Dr. Niihara is a director of EM Japan. Dr. Niihara and Ms. Tran are directors of EM Europe. The officers of Emmaus Life Sciences are also the officers of Emmaus Medical and Newfield Nutrition and hold the same officer positions in Emmaus Medical and Newfield Nutrition as they do in Emmaus Life Sciences.
The Merger
Pursuant to the Merger Agreement, AFH Merger Sub merged with and into Emmaus Medical with Emmaus Medical continuing as the surviving entity. As a result of the Merger, Emmaus Medical became our wholly-owned subsidiary. In connection with the Merger, we changed our corporate name from "AFH Acquisition IV, Inc." to "Emmaus Holdings, Inc." Subsequently, on September 14, 2011, we changed our name from "Emmaus Holdings, Inc." to "Emmaus Life Sciences, Inc."
105
Upon consummation of the Merger, (i) each outstanding share of Emmaus Medical common stock was exchanged for 29.48548924976 shares of our common stock, (ii) each outstanding Emmaus Medical option and warrant, which was exercisable for one share of Emmaus Medical common stock, was exchanged for an option or warrant, as applicable, exercisable for 29.48548924976 shares of our common stock and (iii) each outstanding convertible note of Emmaus Medical, which was convertible for one share of Emmaus Medical common stock, was exchanged for a convertible note exercisable for 29.48548924976 shares of our common stock. As a result of the Merger, Emmaus Medical securityholders received 20,673,714 shares of our common stock, options and warrants to purchase an aggregate of 326,507 shares of common stock, and convertible notes to purchase an aggregate of 271,305 shares of our common stock, totaling 85% of our issued and outstanding common stock on a fully diluted basis. Immediately after the closing of the Merger, we had 24,423,714 shares of common stock, no shares of preferred stock, options to purchase 23,590 shares of common stock, warrants to purchase 302,918 shares of common stock and convertible notes convertible into 271,305 shares of our common stock issued and outstanding.
The Merger resulted in a change in control of our company from AFH Advisory, which is owned by Mr. Heshmatpour, to the former securityholders of Emmaus Medical. In connection with the change in control, we appointed new persons to our board of directors and elected new officers. Mr. Heshmatpour, an officer and director of the Company prior to the consummation of the Merger, resigned from all of his officer positions with us at the time the transaction was consummated. Mr. Heshmatpour resigned from the board of directors on April 24, 2012.
Since July 2012 we have been engaged in significant litigation with Mr. Heshmatpour and AFH Advisory relating to the Merger. For a detailed description of this litigation please see "Item 3. Legal Proceedings" above in Part I of this Annual Report on Form 10-K.
Loans by Related Persons
The following table sets forth information relating to our loans from related persons outstanding at any time during the year ended December 31, 2014.
Class
|
Lender | Annual Interest Rate |
Date of loan |
Term of Loan |
Principal Amount Outstanding at December 31, 2014 |
Highest Principal outstanding |
Amount of Principal Repaid During the Year Ended December 31, 2014 |
Amount of Interest Paid |
Conv. Rate |
Shares underlying principal at December 31, 2014 |
|||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Current, Promissory note payable to related parties: |
|||||||||||||||||||||||||||||
|
Hope Hospice(1) | 8 | % | 1/17/2012 | Due on demand | $ | 200,000 | $ | 200,000 | $ | — | $ | 16,000 | — | — | ||||||||||||||
|
Hope Hospice(1) | 8 | % | 6/14/2012 | Due on demand | 200,000 | 200,000 | — | 20,000 | — | — | ||||||||||||||||||
|
Hope Hospice(1) | 8 | % | 6/21/2012 | Due on demand | 100,000 | 100,000 | — | 10,000 | — | — | ||||||||||||||||||
|
Yutaka Niihara(2)(4) | 10 | % | 12/5/2012 | Due on demand | 156,730 | 1,213,700 | 1,056,970 | 60,851 | — | — | ||||||||||||||||||
|
Hope Hospice(1) | 8 | % | 2/11/2013 | Due on demand | 50,000 | 50,000 | — | 4,000 | — | — | ||||||||||||||||||
|
Lan T. Tran(2) | 11 | % | 2/10/2014 | 2 years(3) | 106,976 | 106,976 | — | — | — | — | ||||||||||||||||||
|
Cuc T. Tran(5) | 11 | % | 3/5/2014 | 1 year | 11,856 | 11,856 | — | — | — | — | ||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
|
Sub total | $ | 825,562 | $ | 1,882,532 | $ | 1,056,970 | $ | 110,851 | — | — | ||||||||||||||||||
Current, Convertible notes payable to related parties: |
|||||||||||||||||||||||||||||
|
Yasushi Nagasaki(2) | 10 | % | 6/29/2012 | Due on demand | $ | 373,000 | $ | 388,800 | $ | 15,800 | $ | 67,680 | $ | 3.30 | 121,461 | |||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
|
Sub total | $ | 373,000 | $ | 388,800 | $ | 15,800 | $ | 67,680 | — | 121,461 | ||||||||||||||||||
Long-term, Promissory note payable to related parties: |
|||||||||||||||||||||||||||||
|
Hideki & Eiko Uehara(5) | 11 | % | 2/15/2014 | 2 years | $ | 133,333 | $ | 133,333 | $ | — | $ | 14,697 | — | — | ||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
|
Sub total | $ | 133,333 | $ | 133,333 | $ | — | $ | 14,697 | — | — | ||||||||||||||||||
Long-term, Convertible notes payable to related parties: |
|||||||||||||||||||||||||||||
|
Phillip M. Satow(4) | 10 | % | 6/6/2014 | 2 years | $ | 100,000 | $ | 100,000 | $ | — | $ | — | $ | 7.00 | 15,103 | |||||||||||||
|
Richard S. Pechter(5) | 10 | % | 6/11/2014 | 2 years | 100,000 | 100,000 | — | — | $ | 7.00 | 15,084 | |||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
|
Sub total | $ | 200,000 | $ | 200,000 | $ | — | $ | — | — | 30,187 | ||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
|
Total | $ | 1,531,895 | $ | 2,604,665 | $ | 1,072,770 | $ | 193,228 | — | 151,648 | ||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
- (1)
- Dr. Niihara, who is our CEO, is also the CEO of Hope Hospice.
106
- (2)
- Officer
- (3)
- Due
on Demand
- (4)
- Director
- (5)
- Family of Officer/Director
The above loans were made to provide us with needed working capital.
In February 2014, we refinanced a promissory note payable to Ms. Tran, one of our officers, in the amount of $106,976. The new note bears interest at 11% per annum is due on demand and matures on the two year anniversary date of the note.
In February 2014, we refinanced a two-year promissory note payable to Hideki and Eiko Uehara in the amount of $133,333. The new note bears interest at 11% per annum and all unpaid principal and interest are due upon demand at the lender's option. Hideki and Eiko Uehara are family members of Dr. Niihara, one of our officers.
In March 2014, we refinanced a one year promissory note payable to Ms. Cuc T. Tran, in the amount of $11,856. The new note bears interest at 11% per annum and matures on the anniversary date of the note. Ms. Tran is a family member of Ms. Lan T. Tran, one of our officers.
In June 2014, we received $200,000 of subscription proceeds received from Phillip M. Satow and one of his relatives and the related notes were issued after June 30, 2014. The convertible notes to these related parties totaling $200,000 in aggregate principal amount, bear interest at 10% per annum and mature on the respective two-year anniversary dates of the notes. If we complete a qualified public offering, the principal amount of the notes will automatically convert into our common shares at a conversion price equal to 80% of the initial public offering price. At or after the first anniversary of the loan date, the holders may convert some or all of the unpaid principal amount, including unpaid accrued interest amount into shares of our common stock at $7.00 per share.
The loans detailed below from related persons have been fully settled as of December 31, 2014.
In September 2013, we issued a one-year convertible note to Hideki and Eiko Uehara in the amount of $35,640, which bears interest at 10% per annum and matures on the anniversary date of the note. The principal amount plus the unpaid accrued interest due under the convertible note is convertible into shares of our common stock at $3.60 per share. Hideki and Eiko Uehara are family members of Dr. Niihara, one of our officers. We repaid this note in full as of September 8, 2014.
In October 2013, we refinanced a convertible note payable to MLPF&S Cust. FBO Willis C. Lee, one of our officers, in the amount of $138,242 with a new convertible note in the amount of $152,066 which bears interest at 10% per annum and matures on the one-year anniversary date of the note. The principal amount and any unpaid interest due under the note are convertible into shares of our common stock at $3.60 per share. The convertible note was originally effective in October 2011. The outstanding principal amount and accrued interest payable as of December 31, 2013 was $152,066 and $3,666, respectively. At maturity, Mr. Lee partially converted the note into our common stock and exercised all of the warrants attached to the same note.
Guarantees by Officers
On May 1, 2014, we issued a promissory note to Mr. Terasaki in the amount of $605,000. Dr. Niihara provided a personal guarantee of the promissory note issued to Mr. Terasaki.
On October 10, 2014, we refinanced a promissory note payable to the Shitabata Family Trust for $2,136,146. Dr. Niihara and Mr. Lee each provided a personal guarantee of the new promissory note issued to the Shitabata Family Trust.
107
On October 10, 2014, we issued a promissory note payable to The Shitabata Family Trust for $613,615. Dr. Niihara and Mr. Lee each provided a personal guarantee of the new promissory note issued to The Shitabata Family Trust.
Policy for Approval of Related Party Transactions
All related party transactions during the year ended December 31, 2014 have been reviewed and approved by the independent directors of our board of directors. On May 9, 2013, our board of directors approved a new written policy, or the Policy, for the review, approval and ratification of related-party transactions required to be disclosed pursuant to Item 404(a) of Regulation S-K. It is the responsibility of the board to administer the Policy. From time to time, however, the board may delegate responsibility for administration of the Policy to the Compensation, Nominating and Corporate Governance Committee of the board. Whichever body is responsible for administering the Policy at any given time or in regard to a particular related-party transaction is referred to as the "Reviewing Body."
Our management is responsible for determining whether a transaction requires review under the Policy, based on a review of all facts and circumstances. Upon a determination by management that a transaction requires review under this Policy, the material facts concerning the transaction and the related person's interest in the transaction must be disclosed to the Reviewing Body.
At each of its meetings, the Reviewing Body is provided with the details of each new, existing or proposed related-party transaction, including the terms of the transaction, the business purpose of the transaction, and the benefits to us and to the relevant related party. In determining whether to approve a related-party transaction, the Reviewing Body considers, among other factors, the following factors to the extent relevant to the related-party transaction: (i) whether the terms of the related-party transaction are fair to us and on the same basis as would apply if the transaction did not involve a related party; (ii) whether there are business reasons for us to enter into the related-party transaction; (iii) whether the related-party transaction would impair the independence of an outside director; and (iv) whether the related-party transaction would present an improper conflict of interests for any of our directors or executive officers, taking into account the size of the transaction, the overall financial position of the director, executive officer or related party, the direct or indirect nature of the director's, executive officer's or related party's interest in the transaction and the ongoing nature of any proposed relationship, and any other factors the Reviewing Body deems relevant.
Any member of the Reviewing Body who has an interest in the transaction under discussion must abstain from voting on its approval but may, if so requested by the Chairperson of the Reviewing Body, participate in some or all of the Reviewing Body's discussions of the transaction. Upon completion of its review of the transaction, the Reviewing Body may determine to permit or to prohibit the transaction.
A related-party transaction entered into without pre-approval of the Reviewing Body is not deemed to violate the Policy, or be invalid or unenforceable, so long as the transaction is brought to the Reviewing Body as promptly as reasonably practical after it is entered into or after it becomes reasonably apparent that the transaction is covered by the Policy.
Director Independence
Currently, our board of directors has determined that each of Henry A. McKinnell, Jr., Tracey C. Doi, Akiko Moni Miyashita, Phillip M. Satow and Mayu Sris is an "independent" director as defined by the NASDAQ Marketplace Rules, currently in effect and all applicable rules and regulations of the SEC. All members of the Audit and Compensation, Nominating and Corporate Governance Committees satisfy the "independence" standards applicable to members of each such committee. The board of directors made this affirmative determination regarding these directors' independence based on discussions with the directors and on its review of the directors' responses to a standard
108
questionnaire regarding employment and compensation history; affiliations, family and other relationships; and transactions with the Company. The board of directors considered relationships and transactions between each director or any member of his immediate family and the Company and its subsidiaries and affiliates.
Dr. McKinnell, Ms. Doi, Ms. Miyashita, Mr. Satow and Mr. Sris, each of whom is an independent director as defined by the NASDAQ Marketplace Rules, are the sole members of the Audit Committee and the Compensation, Nominating and Corporate Governance Committee.
ITEM 14. PRINCIPAL ACCOUNTING FEES AND SERVICES
KPMG LLP ("KPMG") was engaged by the Company to serve as its independent registered public accounting firm on January 10, 2014. Prior to such engagement, EFP Rotenberg LLP ("EFP Rotenberg") was serving as the Company's independent registered public accounting firm. The following table presents fees billed or to be billed, including reimbursements for expenses, for professional audit services rendered by KPMG for the audits of the Company's consolidated financial statements for the years ended December 31, 2014, 2013 and re-audit of 2012, and reviews of the Company's interim financial statements for the year ended December 31, 2014 and certain interim financial statements for the year ended December 31, 2013. It also includes fees for services rendered by EFP Rotenberg for the interim reviews of the Company's quarterly financial statements for the years ended December 31, 2013. The schedule includes fees billed for other services rendered by EFP Rotenberg during those periods.
| |
Year ended December 31, | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
2014 | 2013 | ||||||||
| |
KPMG | KPMG | EFP | |||||||
Audit Fees (including fees for interim reviews) |
$ | 228,000 | $ | 305,000 | $ | 34,713 | ||||
Audit-Related Fees |
— | — | 7,500 | |||||||
Tax Fees |
— | — | — | |||||||
All Other Fees |
50,500 | — | — | |||||||
| | | | | | | | | | | |
Total |
$ | 278,500 | $ | 305,000 | $ | 42,213 | ||||
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
Pre-Approval Policy
The Audit Committee on an annual basis reviews audit and non-audit services performed by the independent registered public accounting firm for such services. The audit committee pre-approves (i) auditing services (including those performed for purposes of providing comfort letters and statutory audits) and (ii) non-auditing services that exceed a de minimis standard established by the committee, which are rendered to the Company by its outside auditors (including fees).
109
ITEM 15. EXHIBITS AND FINANCIAL STATEMENT SCHEDULES
- 1.
- Financial
Statements: See "Index to Consolidated Financial Statements" on page F-1 of this Annual Report on Form 10-K.
- 2.
- Financial
Statement Schedule: See Notes to Consolidated Financial Statements starting on page F-8 of this Annual Report on Form 10-K.
- 3.
- Exhibits: The exhibits listed in the accompanying "Exhibit Index" are filed or incorporated by reference as part of this Annual Report on Form 10-K.
Exhibit Index
| Exhibit No. | Exhibit Description | ||
|---|---|---|---|
| 2.1 | Merger Agreement dated as of April 21, 2011 by and among the registrant, AFH Merger Sub, Inc., AFH Holding and Advisory, LLC, and Emmaus Medical, Inc. (incorporated by reference from Exhibit 2.1 to the registrant's Current Report on Form 8-K filed with the Securities and Exchange Commission on April 25, 2011). | ||
3.1 |
Certificate of Incorporation (incorporated by reference from Exhibit 3.1 to the registrant's Form 10-SB filed with the Securities and Exchange Commission on February 1, 2008). |
||
3.2 |
Bylaws (incorporated by reference from Exhibit 3.2 to the registrant's Form 10-SB filed with the Securities and Exchange Commission on February 1, 2008). |
||
3.2 |
(a) |
Amendment No. 1 to the Company's Bylaws (incorporated by reference from Exhibit 3.1 to the registrant's Current Report on Form 8-K filed with the Securities and Exchange Commission on March 6, 2013). |
|
3.3 |
Certificate of Ownership and Merger filed with the Office of Secretary of State of Delaware on May 3, 2011 (incorporated by reference from Exhibit 3.3 to the registrant's Current Report on Form 8-K filed with the Securities and Exchange Commission on May 4, 2011). |
||
3.4 |
Certificate of Ownership and Merger effecting the name change to Emmaus Life Sciences, Inc. filed with the Delaware Secretary of State on September 14, 2011 (incorporated by reference from Exhibit 3.1 to the registrant's Current Report on Form 8-K filed with the Securities and Exchange Commission on September 15, 2011). |
||
4.1 |
Convertible Promissory Note (Cash Interest) dated March 14, 2011 (incorporated by reference from Exhibit 4.2 to the registrant's Current Report on Form 8-K filed with the Securities and Exchange Commission on May 4, 2011). |
||
4.2 |
Form of Convertible Note (No Interest) entered into with the persons indicated in Schedule A attached to the Form of Convertible Note (incorporated by reference from Exhibit 4.3 to the registrant's Current Report on Form 8-K filed with the Securities and Exchange Commission on May 4, 2011). |
||
4.3 |
Convertible Promissory Note (Cash Interest) dated November 23, 2010 (incorporated by reference from Exhibit 4.5 to the registrant's Current Report on Form 8-K/A filed with the Securities and Exchange Commission on July 5, 2011). |
||
110
| Exhibit No. | Exhibit Description | ||
|---|---|---|---|
| 4.4 | Specimen Certificate of Common Stock (incorporated by reference from Exhibit 4.6 to the registrant's Annual Report on Form 10-K filed with the Securities and Exchange Commission on May 8, 2014). | ||
4.5 |
Warrant dated January 17, 2012 issued by the registrant to Hope International Hospice, Inc (incorporated by reference from Exhibit 4.11 to the registrant's Annual Report on Form 10-K filed with the Securities and Exchange Commission on May 8, 2014). |
||
4.6 |
Form of Warrant issued by the registrant to the persons indicated in Schedule A attached to the Form of Warrant (incorporated by reference from Exhibit 4.12 to the registrant's Annual Report on Form 10-K filed with the Securities and Exchange Commission on May 8, 2014). |
||
4.7 |
Warrant issued by the registrant to the persons indicated in Schedule A attached to the Warrant (incorporated by reference from Exhibit 4.13 to the registrant's Annual Report on Form 10-K filed with the Securities and Exchange Commission on May 8, 2014). |
||
4.8 |
Form of Warrant dated February 29, 2012 issued to Henry McKinnell in the amounts indicated on Schedule A attached to the Form of Warrant (incorporated by reference from Exhibit 4.15 to the registrant's Annual Report on Form 10-K filed with the Securities and Exchange Commission on May 8, 2014). |
||
4.9 |
Form of Warrant issued by the registrant to Yutaka Niihara (incorporated by reference to Exhibit 4.9 to the registrant's Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission on November 14, 2012). |
||
4.10 |
Form of Convertible Promissory Note issued by the registrant to the persons indicated in Schedule A attached to the Form of Convertible Promissory Note (incorporated by reference to Exhibit 4.1 to the registrant's Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission on May 15, 2013) |
||
4.11 |
Form of Convertible Promissory Note issued by the registrant to the persons indicated in Schedule A attached to the Form of Convertible Promissory Note (incorporated by reference to Exhibit 4.3 to the registrant's Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission on May 15, 2013). |
||
4.12 |
Form of Convertible Promissory Note issued by the registrant to the persons indicated in Schedule A attached to the Form of Convertible Promissory Note (incorporated by reference to Exhibit 4.4 to the registrant's Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission on May 15, 2013). |
||
4.13 |
Form of Convertible Promissory Note issued by the registrant to the persons indicated in Schedule A attached to the Form of Convertible Promissory Note (incorporated by reference to Exhibit 4.5 to the registrant's Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission on May 15, 2013). |
||
4.14 |
Form of Convertible Promissory Note issued by the registrant to the persons indicated in Schedule A attached to the Form of Convertible Promissory Note (incorporated by reference to Exhibit 4.1 to the registrant's Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission on August 14, 2013). |
||
4.15 |
Convertible Promissory Note, dated April 3, 2013, issued by the registrant to Yoshiko & Yuki Takemoto (incorporated by reference to Exhibit 4.2 to the registrant's Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission on August 14, 2013). |
||
111
| Exhibit No. | Exhibit Description | ||
|---|---|---|---|
| 4.16 | Form of Convertible Promissory Note issued by the registrant to the persons indicated in Schedule A attached to the Form of Convertible Promissory Note (incorporated by reference to Exhibit 4.3 to the registrant's Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission on August 14, 2013). | ||
4.17 |
Convertible Promissory Note, dated May 1, 2013, issued by the registrant to Paul Terasaki (incorporated by reference to Exhibit 4.4 to the registrant's Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission on August 14, 2013). |
||
4.18 |
Form of Warrant issued by the registrant to Paul Terasaki (incorporated by reference to Exhibit 4.5 to the registrant's Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission on August 14, 2013). |
||
4.19 |
Form of Convertible Promissory Note issued by the registrant to the persons indicated in Schedule A attached to the Form of Convertible Promissory Note (incorporated by reference to Exhibit 4.1 to the registrant's Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission on November 14, 2013). |
||
4.20 |
Form of Convertible Promissory Note issued by the registrant to the persons indicated in Schedule A attached to the Form of Convertible Promissory Note (incorporated by reference to Exhibit 4.2 to the registrant's Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission on November 14, 2013). |
||
4.21 |
Convertible Promissory Note, dated July 11, 2013, issued by the registrant to Yung Ming Suh (incorporated by reference to Exhibit 4.3 to the registrant's Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission on November 14, 2013). |
||
4.22 |
Convertible Promissory Note, dated July 18, 2013, issued by the registrant to the Takemoto Family (incorporated by reference to Exhibit 4.4 to the registrant's Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission on November 14, 2013). |
||
4.23 |
Convertible Promissory Note, dated October 5, 2013, issued by the registrant to MLPF&S Cust FBO Willis C. Lee (incorporated by reference to Exhibit 4.5 to the registrant's Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission on November 14, 2013). |
||
4.24 |
Convertible Promissory Note, dated October 10, 2013, issued by the registrant to The Shitabata Family Trust (incorporated by reference to Exhibit 4.6 to the registrant's Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission on November 14, 2013). |
||
4.25 |
Form of Convertible Promissory Note issued by the registrant to the persons indicated in Schedule A (incorporated by reference from Exhibit 4.22 to the registrant's Annual Report on Form 10-K filed with the Securities and Exchange Commission on March 29, 2013). |
||
4.26 |
Promissory Note dated December 5, 2012 issued by the registrant to Dr. Yutaka Niihara (incorporated by reference from Exhibit 4.23 to the registrant's Annual Report on Form 10-K filed with the Securities and Exchange Commission on March 29, 2013). |
||
4.27 |
Promissory Note dated December 26, 2012 issued by the registrant to For Days Co. Ltd. (incorporated by reference from Exhibit 4.24 to the registrant's Annual Report on Form 10-K filed with the Securities and Exchange Commission on March 29, 2013). |
||
112
| Exhibit No. | Exhibit Description | ||
|---|---|---|---|
| 4.28 | Convertible Promissory Note dated January 12, 2014 issued by the registrant to Shigeru Matsuda (incorporated by reference from Exhibit 4.47 to the registrant's Annual Report on Form 10-K filed with the Securities and Exchange Commission on May 8, 2014). | ||
4.29 |
Form of Promissory Note issued by the registrant to the persons (Matsuda and Uehara) indicated in Schedule A (incorporated by reference from Exhibit 4.48 to the registrant's Annual Report on Form 10-K filed with the Securities and Exchange Commission on May 8, 2014). |
||
4.30 |
Form of Convertible Promissory Note issued by the registrant to the persons indicated in Schedule A attached to the Form of Convertible Promissory Note (incorporated by reference from Exhibit 4.1 to the registrant's Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission on August 14, 2014). |
||
4.31 |
Convertible Promissory Note, dated May 1, 2014, issued by the registrant to Paul Terasaki (incorporated by reference from Exhibit 4.2 to the registrant's Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission on August 14, 2014). |
||
4.32 |
Form of Warrant issued by the registrant to Paul Terasaki (incorporated by reference from Exhibit 4.3 to the registrant's Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission on August 14, 2014). |
||
4.33 |
Form of Warrant issued by the registrant on June 10, 2014 to holders of warrants issued by the registrant in its September 11, 2013 private placement who exercised such warrants (incorporated by reference from Exhibit 4.4 to the registrant's Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission on August 14, 2014). |
||
4.34 |
Convertible Promissory Note, dated October 10, 2014, issued by the registrant to The Shitabata Family Trust (incorporated by reference from Exhibit 4.1 to the registrant's Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission on November 13, 2014). |
||
4.35 |
Convertible Promissory Note, Dated December 21, 2014, issued by the registrant to Alison Brown-Carvalho |
||
10.1 |
+ |
2011 Stock Incentive Plan (incorporated by reference from Exhibit 10.3 to the registrant's Current Report on Form 8-K filed with the Securities and Exchange Commission on May 4, 2011). |
|
10.1 |
(a)+ |
Form of Incentive Stock Option Agreement (Time-based and Performance-based Vesting) under the Emmaus Life Sciences, Inc. 2011 Stock Incentive Plan (incorporated by reference from Exhibit 10.3(a) to the registrant's Current Report on Form 8-K filed with the Securities and Exchange Commission on May 4, 2011). |
|
10.1 |
(b)+ |
Form of Incentive Stock Option Agreement (Time-based Vesting) under the Emmaus Life Sciences, Inc. 2011 Stock Incentive Plan (incorporated by reference from Exhibit 10.3(b) to the registrant's Current Report on Form 8-K filed with the Securities and Exchange Commission on May 4, 2011). |
|
10.1 |
(c)+ |
Form of Non-Qualified Stock Option Agreement (Time-based and Performance-based Vesting) under the Emmaus Life Sciences, Inc. 2011 Stock Incentive Plan (incorporated by reference from Exhibit 10.3(c) to the registrant's Current Report on Form 8-K filed with the Securities and Exchange Commission on May 4, 2011). |
|
113
| Exhibit No. | Exhibit Description | ||
|---|---|---|---|
| 10.1 | (d)+ | Form of Non-Qualified Stock Option Agreement (Time-based Vesting) under the Emmaus Life Sciences, Inc. 2011 Stock Incentive Plan (incorporated by reference from Exhibit 10.3(d) to the registrant's Current Report on Form 8-K filed with the Securities and Exchange Commission on May 4, 2011). | |
10.1 |
(e)+ |
Form of Restricted Stock Agreement (Time-based and Performance-based Vesting) under the Emmaus Life Sciences, Inc. 2011 Stock Incentive Plan (incorporated by reference from Exhibit 10.3(e) to the registrant's Current Report on Form 8-K filed with the Securities and Exchange Commission on May 4, 2011). |
|
10.1 |
(f)+ |
Form of Restricted Stock Agreement (Time-based Vesting) under the Emmaus Life Sciences, Inc. 2011 Stock Incentive Plan (incorporated by reference from Exhibit 10.3(f) to the registrant's Current Report on Form 8-K filed with the Securities and Exchange Commission on May 4, 2011). |
|
10.1 |
(g)+ |
Amended and Restated 2011 Stock Incentive Plan (incorporated by reference from Annex A to the registrant's Definitive Proxy Statement on Schedule 14A filed with the Securities and Exchange Commission on August 21, 2013). |
|
10.1 |
(h)+ |
Amended and Restated 2011 Stock Incentive Plan (incorporated by reference from Annex A to the registrant's Definitive Proxy Statement on Schedule 14A filed with the Securities and Exchange Commission on September 19, 2014). |
|
10.2 |
License Agreement dated as of March 7, 2001 by and between Harbor-UCLA Research and Education Institute and Orphan Drug International, L.L.C (i.e. Emmaus Medical, Inc.) and corresponding Addendum to the License Agreement entered into December 19, 2003 between Harbor-UCLA Research and Education Institute and Emmaus Medical, Inc. (incorporated by reference from Exhibit 10.4 to the registrant's Current Report on Form 8-K/A filed with the Securities and Exchange Commission on July 5, 2011). |
||
10.3 |
Assignment and Transfer Agreement dated as of February 1, 2011 by and among CATO Holding Company, Nutritional Restart Pharmaceutical Limited Partnership and Emmaus Medical, Inc. (incorporated by reference from Exhibit 10.8 to the registrant's Current Report on Form 8-K filed with the Securities and Exchange Commission on May 4, 2011). |
||
10.4 |
Joint Research and Development Agreement dated as of April 8, 2011 by and between Emmaus Medical, Inc. and CellSeed, Inc. (incorporated by reference from Exhibit 10.10 to the registrant's Current Report on Form 8-K filed with the Securities and Exchange Commission on May 4, 2011). |
||
10.4 |
(a) |
Addendum to the Joint Research and Development Agreement effective August 8, 2011 (incorporated by reference from Exhibit 10.4(a) to the registrant's Annual Report on Form 10-K filed with the Securities and Exchange Commission on May 8, 2014). |
|
10.5 |
Individual Agreement dated as of April 8, 2011 by and between Emmaus Medical, Inc. and CellSeed, Inc. (incorporated by reference from Exhibit 10.11 to the registrant's Current Report on Form 8-K filed with the Securities and Exchange Commission on May 4, 2011). |
||
10.6 |
+ |
Employment Agreement dated as of April 5, 2011 by and between Emmaus Medical, Inc. and Yutaka Niihara, M.D., MPH (incorporated by reference from Exhibit 10.12 to the registrant's Current Report on Form 8-K filed with the Securities and Exchange Commission on May 4, 2011). |
|
114
| Exhibit No. | Exhibit Description | ||
|---|---|---|---|
| 10.7 | + | Employment Agreement dated as of April 5, 2011 by and between Emmaus Medical, Inc. and Willis C. Lee (incorporated by reference from Exhibit 10.13 to the registrant's Current Report on Form 8-K filed with the Securities and Exchange Commission on May 4, 2011). | |
10.8 |
+ |
Employment Agreement dated as of April 5, 2011 by and between Emmaus Medical, Inc. and Lan T. Tran (incorporated by reference from Exhibit 10.14 to the registrant's Current Report on Form 8-K filed with the Securities and Exchange Commission on May 4, 2011). |
|
10.9 |
+ |
Form of Indemnification Agreement and List of Officers and Directors (incorporated by reference from Exhibit 10.20 to the registrant's Current Report on Form 8-K filed with the Securities and Exchange Commission on May 4, 2011). |
|
10.10 |
Trademark Assignment dated as of February 14, 2011 by and between Cato Research Ltd. and Emmaus Medical, Inc. (incorporated by reference from Exhibit 10.23 to the registrant's Current Report on Form 8-K/A filed with the Securities and Exchange Commission on July 5, 2011). |
||
10.11 |
Letter of Intent by and between Ajinomoto Company and Emmaus Medical, Inc. (incorporated by reference from Exhibit 10.24 to the registrant's Current Report on Form 8-K/A filed with the Securities and Exchange Commission on July 5, 2011). |
||
10.12 |
Promissory Note dated January 17, 2012 issued by the registrant to Hope International Hospice, Inc. (incorporated by reference from Exhibit 10.13 to the registrant's Annual Report on Form 10-K filed with the Securities and Exchange Commission on May 8, 2014) |
||
10.13 |
Form of Subscription Agreement dated September 11, 2013 (incorporated by reference to Exhibit 10.1 to the registrant's Current Report on Form 8-K filed with the Securities and Exchange Commission on September 17, 2013). |
||
10.14 |
Form of Warrant issued to the Investors (incorporated by reference to Exhibit 10.2 to the registrant's Current Report on Form 8-K filed with the Securities and Exchange Commission on September 17, 2013). |
||
10.15 |
Agreement dated September 11, 2013 by and among the Company, Dr. Yutaka Niihara, TRW and Sarissa (incorporated by reference to Exhibit 10.3 to the registrant's Current Report on Form 8-K filed with the Securities and Exchange Commission on September 17, 2013). |
||
10.16 |
Form of Placement Agent Warrant (incorporated by reference to Exhibit 10.4 to the registrant's Current Report on Form 8-K filed with the Securities and Exchange Commission on September 17, 2013). |
||
10.17 |
Form of Promissory Note issued by the registrant on the dates and to the persons and in the amounts indicated on Schedule A attached to the Form of Promissory Note (incorporated by reference to Exhibit 10.1 to the registrant's Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission on August 14, 2012). |
||
10.18 |
Form of Promissory Note issued by the registrant to Hope International Hospice, Inc. in the amounts indicated in Schedule A attached to the Form of Promissory Note (incorporated by reference to Exhibit 10.2 to the registrant's Current Report on Form 10-Q filed with the Securities and Exchange Commission on May 15, 2013). |
||
115
| Exhibit No. | Exhibit Description | ||
|---|---|---|---|
| 10.19 | Promissory Note, dated June 28, 2013, issued by the registrant to For Days Co., Ltd. (incorporated by reference to Exhibit 10.1 to the registrant's Current Report on Form 10-Q filed with the Securities and Exchange Commission on August 14, 2013). | ||
10.20 |
Share Cancellation Agreement dated as of April 21, 2011 by and between the registrant and AFH Holding and Advisory, LLC (incorporated by reference from Exhibit 10.1 to the registrant's Current Report on Form 8-K filed with the Securities and Exchange Commission on May 4, 2011). |
||
10.21 |
Registration Rights Agreement dated as of May 3, 2011 by and among the registrant and the individuals listed on Schedule A thereto (incorporated by reference from Exhibit 10.2 to the registrant's Current Report on Form 8-K filed with the Securities and Exchange Commission on May 4, 2011). |
||
10.21 |
(a) |
Amendment and Waiver No. 1 to Registration Rights Agreement dated as of March 6, 2012 by and among the registrant and the individuals listed on Schedule A thereto (incorporated by reference from Exhibit 10.26(a) to the registrant's Annual Report on Form 10-K filed with the Securities and Exchange Commission on May 8, 2014). |
|
10.22 |
Amended and Restated Letter of Intent by and between the registrant and AFH Holding & Advisory LLC dated September 30, 2011 (incorporated by reference from Exhibit 10.27 to the registrant's Annual Report on Form 10-K filed with the Securities and Exchange Commission on May 8, 2014). |
||
10.23 |
Office Lease, dated March 12, 2008, by and between Emmaus Medical, Inc. and 20655 S. Western Avenue, LLC (incorporated by reference from Exhibit 10.6 to the registrant's Current Report on Form 8-K/A filed with the Securities and Exchange Commission on July 5, 2011). |
||
10.23 |
(a) |
Office Lease extension, dated November 4, 2013, by and between Emmaus Medical, Inc. and 20655 S. Western Avenue, LLC (incorporated by reference from Exhibit 10.28(a) to the registrant's Annual Report on Form 10-K filed with the Securities and Exchange Commission on May 8, 2014). |
|
10.23 |
(b) |
Office Lease extension, dated July 30, 2014, by and between Emmaus Medical, Inc. and 20655 S. Western Avenue, LLC. |
|
10.23 |
(c) |
Office Lease extension, dated July 30, 2014, by and between Emmaus Medical, Inc. and 3830 Del Amo Blvd, LLC. |
|
10.23 |
(d) |
Consent to Subletting Agreement, dated October 20, 2014, by and between Bixby Torrance LLC, Flipswap, Inc. and Emmaus Life Sciences, Inc. |
|
10.23 |
(e) |
Office Sublease, dated October 20, 2014, by and between Emmaus Life Sciences, Inc. and Flipswap, Inc. |
|
10.23 |
(f) |
Office Lease, dated October 20, 2014, by and between Emmaus Life Sciences, Inc. and Bixby Torrance LLC. |
|
10.24 |
Form of Warrant issued by the registrant to the persons indicated in Schedule A attached to the Form of Warrant (incorporated by reference from Exhibit 10.33 to the registrant's Annual Report on Form 10-K filed with the Securities and Exchange Commission on May 8, 2014). |
||
116
| Exhibit No. | Exhibit Description | ||
|---|---|---|---|
| 10.25 | Federal Supply Schedule Contract Award dated December 8, 2010 by and between the United States Department of Veterans Affairs and Emmaus Medical, Inc. (incorporated by reference from Exhibit 10.5 to the registrant's Current Report on Form 8-K/A filed with the Securities and Exchange Commission on July 5, 2011). | ||
10.26 |
Promissory Note dated as of October 10, 2014 by and between Emmaus Medical, Inc. and The Shitabata Family Trust (incorporated by reference from Exhibit 10.1 to the registrant's Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission on November 13, 2014). |
||
21.1 |
List of Subsidiaries (incorporated by reference from Exhibit 21.1 to the registrant's Annual Report on Form 10-K filed with the Securities and Exchange Commission on May 8, 2014). |
||
31.1 |
Certification of Chief Executive Officer pursuant to Item 601(b)(31) of Regulation S-K, as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. |
||
31.2 |
Certification of Chief Financial Officer pursuant to Item 601(b)(31) of Regulation S-K, as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. |
||
32.1 |
Certification of Chief Executive Officer and Chief Financial Officer Pursuant to 18 U.S.C. Section 1350, as Adopted Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. |
||
101.INS |
XBRL Instance Document |
||
101.SCH |
XBRL Taxonomy Extension Schema Document |
||
101.CAL |
XBRL Taxonomy Extension Calculation Linkbase Document |
||
101.DEF |
XBRL Taxonomy Extension Definition Linkbase Document |
||
101.LAB |
XBRL Taxonomy Extension Label Linkbase Document |
||
101.PRE |
XBRL Taxonomy Extension Presentation Linkbase Document |
||
- +
- Designates management contract or compensatory plan or arrangement.
117
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Torrance, California, on March 31, 2015.
| Emmaus Life Sciences, Inc. | ||||||
By: |
/s/ YUTAKA NIIHARA |
|||||
| Name: | Yutaka Niihara, MD, MPH | |||||
| Title: | President and Chief Executive Officer (principal executive officer and duly authorized officer) | |||||
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the Company in the capacities and on the dates indicated.
SIGNATURE
|
TITLE
|
DATE
|
||
|---|---|---|---|---|
| /s/ YUTAKA NIIHARA Yutaka Niihara, M.D., MPH |
President and Chief Executive Officer (Principal Executive Officer) and Director | March 31, 2015 | ||
/s/ PETER B. LUDLUM Peter B. Ludlum |
Executive Vice President and Chief Financial Officer (Principal Financial and Accounting Officer) |
March 31, 2015 |
||
/s/ HENRY A. MCKINNELL, JR. Dr. Henry A. McKinnell, Jr. |
Chairman of the Board |
March 31, 2015 |
||
/s/ TRACEY C. DOI Tracey C. Doi |
Director |
March 31, 2015 |
||
/s/ AKIKO MONI MIYASHITA Akiko Moni Miyashita |
Director |
March 31, 2015 |
||
/s/ PHILLIP M. SATOW Phillip M. Satow |
Director |
March 31, 2015 |
||
/s/ MAYURAN SRISKANDARAJAH Mayuran Sriskandarajah |
Director |
March 31, 2015 |
118
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
F-1
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
The
Board of Directors and Stockholders
Emmaus Life Sciences, Inc.:
We have audited the accompanying consolidated balance sheets of Emmaus Life Sciences, Inc. and subsidiaries as of December 31, 2014 and 2013, and the related consolidated statements of comprehensive loss, changes in stockholders' deficit, and cash flows for each of the years in the two-year period ended December 31, 2014. These consolidated financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on these consolidated financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of Emmaus Life Sciences, Inc. and subsidiaries as of December 31, 2014 and 2013, and the results of their operations and their cash flows for each of the years in the two-year period ended December 31, 2014, in conformity with U.S. generally accepted accounting principles.
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in note 2 to the consolidated financial statements, the Company has suffered recurring losses from operations, has significant amounts of debt due within a year, and has a net capital deficiency that raise substantial doubt about its ability to continue as a going concern. Management's plans in regard to these matters are also described in note 2. The consolidated financial statements and financial statement schedules do not include any adjustments that might result from the outcome of this uncertainty.
|
(signed) KPMG LLP |
San
Diego, California
March 31, 2015
F-2
EMMAUS LIFE SCIENCES, INC.
CONSOLIDATED BALANCE SHEETS
| |
As of | ||||||
|---|---|---|---|---|---|---|---|
| |
December 31, 2014 |
December 31, 2013 |
|||||
ASSETS |
|||||||
CURRENT ASSETS |
|||||||
Cash and cash equivalents |
$ | 556,318 | $ | 3,638,600 | |||
Accounts receivable |
42,734 | 35,237 | |||||
Inventories, net |
250,413 | 239,009 | |||||
Marketable securities |
79,236 | 162,564 | |||||
Prepaid expenses and other current assets |
129,554 | 81,046 | |||||
| | | | | | | | |
Total current assets |
1,058,255 | 4,156,456 | |||||
| | | | | | | | |
PROPERTY AND EQUIPMENT, net |
72,391 | 26,120 | |||||
| | | | | | | | |
OTHER ASSETS |
|||||||
Marketable securities, pledged to creditor |
334,410 | 686,090 | |||||
Intangibles, net |
892,857 | 1,107,143 | |||||
Deposits |
306,379 | 137,900 | |||||
| | | | | | | | |
Total other assets |
1,533,646 | 1,931,133 | |||||
| | | | | | | | |
Total Assets |
$ | 2,664,292 | $ | 6,113,709 | |||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
LIABILITIES AND STOCKHOLDERS' EQUITY (DEFICIT) |
|||||||
CURRENT LIABILITIES |
|||||||
Accounts payable and accrued expenses |
$ | 2,601,420 | $ | 2,283,446 | |||
Due to related party |
394,446 | 394,446 | |||||
Dissenting stockholders payable |
— | 125,000 | |||||
Notes payable, net |
1,643,615 | 1,765,070 | |||||
Notes payable to related parties, net |
825,562 | 925,641 | |||||
Convertible notes payable, net |
3,456,959 | 4,802,472 | |||||
Convertible notes payable to related parties, net |
373,000 | 560,706 | |||||
| | | | | | | | |
Total current liabilities |
9,295,002 | 10,856,781 | |||||
| | | | | | | | |
LONG-TERM LIABILITIES |
|||||||
Deferred rent |
3,729 | — | |||||
Liability classified warrants |
3,206,000 | 5,928,000 | |||||
Warrant derivative liabilities |
5,641,000 | — | |||||
Notes payable, net |
833,335 | 200,000 | |||||
Notes payable to related parties, net |
133,333 | — | |||||
Convertible notes payable, net |
3,651,555 | 2,966,588 | |||||
Convertible notes payable to related parties, net |
200,000 | — | |||||
| | | | | | | | |
Total long-term liabilities |
13,668,952 | 9,094,588 | |||||
| | | | | | | | |
Total Liabilities |
22,963,954 | 19,951,369 | |||||
| | | | | | | | |
COMMITMENTS AND CONTINGENCIES |
|||||||
STOCKHOLDERS' EQUITY (DEFICIT) |
|||||||
Preferred stock—par value $0.001 per share, 20,000,000 shares authorized, none issued and outstanding |
— | — | |||||
Common stock—par value $0.001 per share, 100,000,000 shares authorized, 30,511,573 and 29,228,306 shares issued and outstanding at December 31, 2014 and December 31, 2013, respectively |
30,512 | 29,228 | |||||
Additional paid-in capital |
50,479,843 | 35,669,291 | |||||
Accumulated other comprehensive (loss) income |
(194,015 | ) | 262,683 | ||||
Accumulated deficit |
(70,616,002 | ) | (49,798,862 | ) | |||
| | | | | | | | |
Total Stockholders' Deficit |
(20,299,662 | ) | (13,837,660 | ) | |||
| | | | | | | | |
Total Liabilities & Stockholders' Deficit |
$ | 2,664,292 | $ | 6,113,709 | |||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
The accompanying notes are an integral part of these financial statements.
F-3
EMMAUS LIFE SCIENCES, INC.
CONSOLIDATED STATEMENTS OF COMPREHENSIVE LOSS
| |
Year Ended December 31, | ||||||
|---|---|---|---|---|---|---|---|
| |
2014 | 2013 | |||||
REVENUES, Net |
$ | 500,679 | $ | 390,911 | |||
COST OF GOODS SOLD |
267,040 | 196,395 | |||||
| | | | | | | | |
GROSS PROFIT |
233,639 | 194,516 | |||||
| | | | | | | | |
OPERATING EXPENSES |
|||||||
Research and development |
1,966,254 | 2,436,585 | |||||
Selling |
668,862 | 516,599 | |||||
General and administrative |
11,904,092 | 10,078,954 | |||||
Transaction costs |
— | 1,014,019 | |||||
| | | | | | | | |
|
14,539,208 | 14,046,157 | |||||
| | | | | | | | |
LOSS FROM OPERATIONS |
(14,305,569 | ) | (13,851,641 | ) | |||
| | | | | | | | |
OTHER INCOME (EXPENSE) |
|||||||
Realized gain on securities available-for-sale |
— | 399,068 | |||||
Gain on derecognition of accounts payable |
— | 341,361 | |||||
Change in fair value of liability classified warrants |
(1,622,744 | ) | 932,000 | ||||
Change in fair value of warrant derivative liabilities |
474,000 | — | |||||
Warrant exercise inducement expense |
(3,523,000 | ) | — | ||||
Interest and other income |
246,379 | 103,987 | |||||
Interest expense |
(2,082,541 | ) | (2,173,561 | ) | |||
| | | | | | | | |
|
(6,507,906 | ) | (397,145 | ) | |||
| | | | | | | | |
LOSS BEFORE INCOME TAXES |
(20,813,475 | ) | (14,248,786 | ) | |||
INCOME TAXES (BENEFIT) |
3,665 | (182,438 | ) | ||||
| | | | | | | | |
NET LOSS |
(20,817,140 | ) | (14,066,348 | ) | |||
COMPONENTS OF OTHER COMPREHENSIVE INCOME (LOSS) |
|||||||
Unrealized holding gain (loss) on securities available-for-sale |
(435,010 | ) | 293,774 | ||||
Unrealized foreign translation |
(21,688 | ) | (18,660 | ) | |||
| | | | | | | | |
COMPREHENSIVE LOSS |
$ | (21,273,838 | ) | $ | (13,791,234 | ) | |
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
NET LOSS PER COMMON SHARE |
$ | (0.70 | ) | $ | (0.53 | ) | |
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
WEIGHTED AVERAGE COMMON SHARES OUTSTANDING |
29,846,962 | 26,585,578 | |||||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
The accompanying notes are an integral part of these financial statements.
F-4
CONSOLIDATED STATEMENT OF CHANGES IN STOCKHOLDERS' DEFICIT
FOR THE PERIOD FROM DECEMBER 31, 2012 TO DECEMBER 31, 2013
| |
Common stock—par value $0.001 per share, 100,000,000 shares authorized |
|
|
|
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
|
Accumulated Other Comprehensive Income (Loss) |
|
|
|||||||||||||||
| |
Shares | Common Stock |
Additional Paid-in Capital |
Accumulated Deficit |
Total | ||||||||||||||
Balance, December 31, 2012 |
24,878,436 | $ | 24,878 | $ | 25,076,813 | $ | (12,432 | ) | $ | (35,732,514 | ) | $ | (10,643,255 | ) | |||||
Warrants issued in conjunction with convertible note |
— | — | 116,831 | — | — | 116,831 | |||||||||||||
Beneficial conversion feature related to convertible and promissory notes payable |
— | — | 396,801 | — | — | 396,801 | |||||||||||||
Equity components of stock and warrant units issued for cash |
3,020,501 | 3,021 | 586,053 | — | — | 589,074 | |||||||||||||
Share-based compensation |
— | — | 5,108,666 | — | — | 5,108,666 | |||||||||||||
Stock issued as a payment of professional fee |
32,840 | 33 | 118,191 | — | — | 118,224 | |||||||||||||
Stock issued for cash |
527,284 | 527 | 1,889,595 | — | — | 1,890,122 | |||||||||||||
Conversion of notes payable to common stock |
769,245 | 769 | 2,626,203 | — | — | 2,626,972 | |||||||||||||
Loss on extinguishment of debt with related party |
— | — | (249,862 | ) | — | — | (249,862 | ) | |||||||||||
Realized gain on marketable securities |
— | — | — | (399,068 | ) | — | (399,068 | ) | |||||||||||
Unrealized gain on marketable securities net of tax |
— | — | — | 692,843 | — | 692,843 | |||||||||||||
Foreign currency translation effect |
— | — | — | (18,660 | ) | — | (18,660 | ) | |||||||||||
Net loss |
— | — | — | — | (14,066,348 | ) | (14,066,348 | ) | |||||||||||
| | | | | | | | | | | | | | | | | | | | |
Balance, December 31, 2013 |
29,228,306 | $ | 29,228 | $ | 35,669,291 | $ | 262,683 | $ | (49,798,862 | ) | $ | (13,837,660 | ) | ||||||
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
The accompanying notes are an integral part of these financial statements.
F-5
EMMAUS LIFE SCIENCES, INC.
CONSOLIDATED STATEMENT OF CHANGES IN STOCKHOLDERS' DEFICIT (Continued)
FOR THE PERIOD FROM DECEMBER 31, 2013 TO DECEMBER 31, 2014
| |
Common stock—par value $0.001 per share, 100,000,000 shares authorized |
|
|
|
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
|
Accumulated Other Comprehensive Income (Loss) |
|
|
|||||||||||||||
| |
Shares | Common Stock |
Additional Paid-in Capital |
Accumulated Deficit |
Total | ||||||||||||||
Balance, December 31, 2013 |
29,228,306 | $ | 29,228 | $ | 35,669,291 | $ | 262,683 | $ | (49,798,862 | ) | $ | (13,837,660 | ) | ||||||
Warrant issued in conjunction with convertible note |
— | — | 126,732 | — | — | 126,732 | |||||||||||||
Beneficial conversion feature related to convertible and promissory notes payable |
— | — | 1,392,387 | — | — | 1,392,387 | |||||||||||||
Proceeds from exercise of warrants |
1,254,621 | 1,255 | 4,276,380 | — | — | 4,277,635 | |||||||||||||
Share-based compensation |
— | — | 5,870,638 | — | — | 5,870,638 | |||||||||||||
Conversion of notes payable to common stock |
528,646 | 529 | 1,768,671 | — | — | 1,769,200 | |||||||||||||
Excess value of liability classified warrants upon exercise |
— | — | 1,752,744 | — | — | 1,752,744 | |||||||||||||
Common stock repurchased and cancelled |
(500,000 | ) | (500 | ) | (377,000 | ) | — | — | (377,500 | ) | |||||||||
Unrealized gain (loss) on marketable securities net of tax |
— | — | — | (435,010 | ) | — | (435,010 | ) | |||||||||||
Foreign currency translation effect |
— | — | — | (21,688 | ) | — | (21,688 | ) | |||||||||||
Net loss |
— | — | — | — | (20,817,140 | ) | (20,817,140 | ) | |||||||||||
| | | | | | | | | | | | | | | | | | | | |
Balance, December 31, 2014 |
30,511,573 | $ | 30,512 | $ | 50,479,843 | $ | (194,015 | ) | $ | (70,616,002 | ) | $ | (20,299,662 | ) | |||||
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
The accompanying notes are an integral part of these financial statements.
F-6
EMMAUS LIFE SCIENCES, INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS
| |
Year ended December 31, | ||||||
|---|---|---|---|---|---|---|---|
| |
2014 | 2013 | |||||
CASH FLOWS FROM OPERATING ACTIVITIES |
|||||||
Net loss |
$ | (20,817,140 | ) | $ | (14,066,348 | ) | |
Adjustments to reconcile net loss to net cash flows from operating activities |
|||||||
Depreciation and amortization |
230,906 | 235,260 | |||||
Interest expense accrued from discount of convertible notes |
897,353 | 949,103 | |||||
Realized gain on marketable securities available-for-sale |
— | (399,068 | ) | ||||
Tax benefit recognized on unrealized gain on marketable securities available-for-sale |
— | (185,713 | ) | ||||
Gain on derecognition of accounts payable |
— | (341,361 | ) | ||||
Share-based compensation |
5,870,638 | 5,108,666 | |||||
Warrant exercise inducement expense |
3,523,000 | — | |||||
Change in fair value of liability classified warrants |
1,622,744 | (932,000 | ) | ||||
Change in fair value of warrant derivative liabilities |
(474,000 | ) | — | ||||
Transaction costs related to private placement |
— | 1,014,019 | |||||
Net changes in operating assets and liabilities |
|||||||
Accounts receivable |
(9,259 | ) | 43,884 | ||||
Inventory |
(19,233 | ) | (50,675 | ) | |||
Prepaid expenses and other current assets |
(69,178 | ) | (50,078 | ) | |||
Deposits |
(172,903 | ) | 55,942 | ||||
Accounts payable and accrued expenses |
677,518 | 229,331 | |||||
Deferred rent |
4,075 | — | |||||
| | | | | | | | |
Net cash flows used in operating activities |
(8,735,479 | ) | (8,389,038 | ) | |||
| | | | | | | | |
CASH FLOWS FROM INVESTING ACTIVITIES |
|||||||
Proceeds from sales of investment securities available-for-sale |
— | 591,422 | |||||
Purchases of property and equipment |
(64,192 | ) | (8,508 | ) | |||
| | | | | | | | |
Net cash flows from (used in) investing activities |
(64,192 | ) | 582,914 | ||||
| | | | | | | | |
CASH FLOWS FROM FINANCING ACTIVITIES |
|||||||
Proceeds from notes payable issued |
400,000 | 1,541,000 | |||||
Proceeds from convertible notes payable issued |
1,720,000 | 3,139,941 | |||||
Due to dissenters |
(125,000 | ) | (75,000 | ) | |||
Repurchase of common stock |
(377,500 | ) | — | ||||
Payments of notes payable |
— | (1,505,696 | ) | ||||
Payments of convertible notes payable |
(174,300 | ) | (365,773 | ) | |||
Proceeds from private placement units, net of transaction costs |
— | 6,435,055 | |||||
Proceeds from exercise of warrants |
4,277,635 | — | |||||
Proceeds from issuance of common stock |
— | 1,890,122 | |||||
| | | | | | | | |
Net cash flows from financing activities |
5,720,835 | 11,059,649 | |||||
| | | | | | | | |
Effect of exchange rate changes on cash |
(3,446 | ) | (17,748 | ) | |||
| | | | | | | | |
Net increase (decrease) in cash and cash equivalents |
(3,082,282 | ) | 3,235,777 | ||||
Cash and cash equivalents, beginning of period |
3,638,600 | 402,823 | |||||
| | | | | | | | |
Cash and cash equivalents, end of period |
$ | 556,318 | $ | 3,638,600 | |||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
SUPPLEMENTAL DISCLOSURES OF CASH FLOW ACTIVITIES |
|||||||
Interest paid |
$ | 434,115 | $ | 470,525 | |||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
Income taxes paid |
$ | 3,665 | $ | 3,275 | |||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
Non-cash financing activities: |
|||||||
Stock issued as a payment of professional fee |
$ | — | $ | 118,224 | |||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
Conversion of notes payable to common stock |
$ | 1,621,862 | $ | 2,606,100 | |||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
Conversion of accrued interest payable to common stock |
$ | 147,338 | $ | 20,872 | |||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
The accompanying notes are an integral part of these financial statements.
F-7
Emmaus Life Sciences, Inc.
Notes to Consolidated Financial Statements
NOTE 1—DESCRIPTION OF BUSINESS
Organization—Emmaus Life Sciences, Inc. (the "Company" or "Emmaus"), which is engaged in the discovery, development, and commercialization of innovative treatments and therapies primarily for rare and orphan diseases, was incorporated in the state of Delaware on September 24, 2007. Pursuant to an Agreement and Plan of Merger, dated April 21, 2011 (the "Merger Agreement"), by and among the Company, AFH Merger Sub, Inc., a wholly-owned subsidiary of the Company ("AFH Merger Sub"), AFH Holding and Advisory, LLC ("AFH Advisory"), and Emmaus Medical, Inc. ("Emmaus Medical"), Emmaus Medical merged with and into AFH Merger Sub with Emmaus Medical continuing as the surviving entity (the "Merger"). Upon the closing of the Merger, the Company changed its name from "AFH Acquisition IV, Inc." to "Emmaus Holdings, Inc." and became the parent company of Emmaus Medical. The Company changed its name from "Emmaus Holdings, Inc." to "Emmaus Life Sciences, Inc." on September 14, 2011.
Emmaus Medical is a Delaware corporation originally incorporated on September 12, 2003. Emmaus Medical, LLC was organized on December 20, 2000. In October 2003, Emmaus Medical, LLC conducted a reorganization and merged with Emmaus Medical. As a result of the merger, Emmaus Medical acquired the exclusive patent rights for a treatment for sickle cell disease ("SCD").
In October 2010, the Company established Emmaus Medical Japan, Inc., a Japanese corporation ("EM Japan") by funding 97% of the initial capital. EM Japan is engaged in the business of trading in nutritional supplements and other medical products and drugs. The results of EM Japan have been included in the consolidated financial statements of the Company since the date of formation. The aggregate formation cost was $52,500. Emmaus Medical acquired the additional 3% of the outstanding shares of EM Japan during the three months ended March 31, 2011 and is now the 100% owner of the outstanding share capital.
In November 2011, the Company formed Emmaus Medical Europe, Ltd. ("EM Europe"), a wholly owned subsidiary of Emmaus Medical. EM Europe's primary focus is expanding the business of Emmaus Medical in Europe.
Emmaus, its wholly-owned subsidiary, Emmaus Medical, and Emmaus Medical's wholly-owned subsidiaries, Newfield Nutrition Corporation ("Newfield Nutrition"), EM Japan and EM Europe, are collectively referred to herein as the "Company."
Nature of Business—The Company has undertaken the business of developing and commercializing cost-effective treatments and therapies for rare diseases. The Company's primary business purpose is to commercialize its treatment for SCD.
To a lesser extent, the Company is also engaged in the marketing and sale of NutreStore®, which has received approval from the U.S. Food and Drug Administration ("FDA"), as a treatment for short bowel syndrome ("SBS") in patients receiving specialized nutritional support when used in conjunction with a recombinant human growth hormone that is approved for this indication. The Company's indirect wholly-owned subsidiary, Newfield Nutrition, sells L-glutamine as a nutritional supplement under the brand name AminoPure® through retail stores in multiple states and via importers and distributors in Japan, Taiwan and South Korea. The Company also owns a minority interest of less than 1% in CellSeed, Inc., a Japanese company listed on the Tokyo Stock Exchange ("CellSeed"), which is engaged in research and development of regenerative medicine products and the manufacture and sale of temperature- responsive cell culture equipment.
F-8
Emmaus Life Sciences, Inc.
Notes to Consolidated Financial Statements (Continued)
NOTE 1—DESCRIPTION OF BUSINESS (Continued)
The Company also has certain rights to regenerative medicine products owned by CellSeed and is involved in research focused on providing innovative solutions for tissue-engineering through the development of novel cell harvest methods and 3-dimensional living tissue replacement products for "cell sheet therapy" and regenerative medicine and the commercialization of such products.
NOTE 2—SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Basis of presentation—The accompanying consolidated financial statements have been prepared in accordance with accounting principles generally accepted in the United States of America ("GAAP") codified in the Accounting Standards Codification ("ASC") and Accounting Standards Updates ("ASU") of the Financial Accounting Standards Board ("FASB"). Certain prior period amounts have been reclassified to conform to the current period presentation.
Going concern—The accompanying consolidated financial statements have been prepared on the basis that the Company will continue as a going concern. The Company had recurring net losses of $20.8 million and $14.1 million in 2014 and 2013, respectively. In addition, the Company has a significant amount of notes payable and other obligations due within the next twelve months and is projecting that its operating losses and expected capital needs, including the expected costs relating to the commercialization of the Company's pharmaceutical grade L-glutamine treatment for SCD, will exceed its existing cash balances and cash expected to be generated from operations for the foreseeable future. In order to meet the Company's expected obligations, management intends to raise additional funds through equity and debt financings and partnership agreements. However, there can be no assurance that the Company will be able to complete any additional equity or debt financings or enter into partnership agreements. Therefore, due to the uncertainty of the Company's ability to meet its current operating and capital expenses, there is substantial doubt about the Company's ability to continue as a going concern, as the continuation and expansion of its business is dependent upon obtaining further financing, successful and sufficient market acceptance of its products, and achieving a profitable level of operations. The consolidated financial statements do not include any adjustments that might result from the outcome of these uncertainties.
Principles of consolidation—The consolidated financial statements include the accounts of the Company (and its wholly-owned subsidiary, Emmaus Medical, and Emmaus Medical's wholly-owned subsidiaries, Newfield Nutrition, EM Japan and EM Europe). All significant intercompany transactions have been eliminated.
Estimates—Financial statements prepared in accordance with GAAP require management to make estimates and assumptions that affect the reported amounts of assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Among other things, management has estimated the useful lives of equipment and other assets, along with the variables used to calculate the valuation of stock options and warrants using the Black-Scholes-Merton option valuation model.
The warrants issued by the Company in a private placement in September 2013 and replacement warrants issued in June 2014 contain non-standard anti-dilution protection and, consequently, are being accounted for as liabilities that are remeasured to fair market value at each reporting period (Note 7). In addition, the remaining initial private placement warrants may now utilize a cashless exercise feature since the shares associated with them were not registered by the one-year anniversary of their issue.
F-9
Emmaus Life Sciences, Inc.
Notes to Consolidated Financial Statements (Continued)
NOTE 2—SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
These warrants have now been reclassified from liability classified warrants to warrant derivative liabilities and to continue to be remeasured at fair value each reporting period. The initial value as well as the fair value of all such warrants were determined using a Binomial Monte-Carlo Cliquet (aka Ratchet) Option Pricing Model. The model is similar to traditional Black-Scholes-type option pricing models except that the exercise price resets at certain dates in the future. Actual results could differ from those estimates.
Cash and cash equivalents—Cash and cash equivalents include short-term securities with original maturities of less than ninety days. The Company maintains its cash and cash equivalents at insured financial institutions, the balances of which may, at times, exceed federally insured limits. Management believes that the risk of loss due to the concentrations is minimal.
Inventories—Inventories are valued based on first-in, first-out and at the lesser of cost or market value. Work-in-process inventories consist of raw material L-glutamine for the Company's AminoPure and NutreStore products that has not yet been packaged and labeled for sale.
All of the raw material purchases during the year ended December 31, 2014 were from one vendor and in 2013 were from two vendors.
| |
December 31, | ||||||
|---|---|---|---|---|---|---|---|
Inventory by category
|
2014 | 2013 | |||||
Raw materials and components |
$ | 43,700 | $ | 20,700 | |||
Work-in-process |
27,665 | 68,887 | |||||
Finished goods |
179,048 | 149,422 | |||||
| | | | | | | | |
Total |
$ | 250,413 | $ | 239,009 | |||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
Deposits—Carrying value of amounts transferred to third parties for security purposes that are expected to be returned or applied towards payment after one year or beyond the operating cycle, if longer, are recorded as deposits. Deposit amounts consist of retainer payments for professional services and security deposits for its offices.
Revenue recognition—Revenue is recognized when there is persuasive evidence that an arrangement exists, delivery has occurred, the price is fixed and determinable and collection is reasonably assured.
With prior written approval of the Company, in certain situations, product is returnable only by its direct customers for a returned goods credit, for product that is expired, damaged in transit, or which is discontinued, withdrawn or recalled.
The Company estimates its sales returns based upon its prior sales and return history and accrues a sales return allowance at the time of sale. Historically, sales returns have been immaterial. The Company pays royalties on an annual basis based on existing license arrangements. These royalties are recognized as cost of goods sold upon sale of the products.
Allowance for doubtful accounts—The Company provides an allowance for uncollectible accounts based upon prior experience and management's assessment of the collectability of existing specific accounts.
F-10
Emmaus Life Sciences, Inc.
Notes to Consolidated Financial Statements (Continued)
NOTE 2—SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
Advertising cost—Advertising costs are expensed as incurred. Advertising costs for the years ended December 31, 2014 and 2013 were $222,883 and $240,743, respectively.
Property and equipment—Leaseholds, furniture, and fixtures are recorded at historical cost and depreciated on a straight-line basis over their estimated useful lives of 5 to 7 years. Maintenance and repairs are expensed as incurred, while major additions and improvements are capitalized. Gains and losses on disposition are included in current operations.
Intangibles—The Company's intangible assets include license issue fees and patent costs relating to a license agreement (Note 4). These intangible assets are amortized over a period of 3 to 7 years, the estimated legal life of the patents and economic life of the license agreements. The intangible assets are assessed by management for potential impairment on an annual basis. No impairment existed as of December 31, 2014 and December 31, 2013.
Impairment of long-lived assets—The Company evaluates the carrying value of its long-lived assets for impairment whenever events or changes in circumstances indicate that such carrying values may not be recoverable. The Company uses its best judgment based on the current facts and circumstances relating to its business when determining whether any significant impairment factors exist.
If the Company determines that the carrying values of long-lived assets may not be recoverable based upon the existence of one or more indicators of impairment, the Company performs an undiscounted cash flow analysis to determine if impairment exists. If impairment exists, the Company measures the impairment based on the difference between the asset's carrying amount and its fair value, and the impairment is charged to consolidated statement of comprehensive loss in the period in which the long-lived asset impairment is determined to have occurred.
The Company has determined that no impairment of the carrying value of its long-lived assets existed at December 31, 2014 and 2013.
There can be no assurance, however, that market conditions will not change or demand for the Company's products will continue or allow the Company to realize the value of its long-lived assets and prevent future impairment.
Research and development—Research and development consists of expenditures for the research and development of new products and technologies, which primarily involve contract research, payroll-related expenses, and other related supplies. Research and development costs are expensed as incurred. Intangible assets acquired for research and development purposes are capitalized if they have alternative future use.
Share-based compensation—The Company recognizes compensation cost for share-based compensation awards over the service term of the recipients of the share-based awards. The fair value of share-based compensation is calculated using the Black-Scholes-Merton pricing model. The Black-Scholes-Merton model requires subjective assumptions regarding future stock price volatility and expected time to exercise, which greatly affect the calculated values. The expected term of awards granted is derived from historical data on awards exercised and post-vesting employment termination behavior. The risk-free rate selected to value any particular grant is based on the U.S. Treasury rate on the grant date that corresponds to the expected term of the award. The expected volatility is based on
F-11
Emmaus Life Sciences, Inc.
Notes to Consolidated Financial Statements (Continued)
NOTE 2—SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
the historical volatility of the common stock of comparable publicly traded companies. These factors could change, affecting the determination of stock-based compensation expense in future periods.
Income taxes—The Company accounts for income taxes under the asset and liability method, wherein deferred tax assets and liabilities are recognized for the future tax consequences attributable to the differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period the enactment occurs. A valuation allowance is provided for certain deferred tax assets if it is more likely than not that the Company will not realize tax assets through the generation of future taxable income for the related jurisdictions.
For balance sheet presentation, current deferred tax assets and liabilities within each tax jurisdiction have been offset and presented as a single amount and non-current deferred tax assets and liabilities within each tax jurisdiction have been offset and presented as a single amount.
When tax returns are filed, it is highly probable that some positions taken would be sustained upon examination by the taxing authorities, while others are subject to uncertainty about the merits of the position taken or the amount of the position that would be ultimately sustained. The benefit of a tax position is recognized in the financial statements in the period during which, based on all available evidence, management believes it is more likely than not that the position will be sustained upon examination, including the resolution of appeals or litigation processes, if any. Tax positions taken are not offset or aggregated with other positions. Tax positions that meet the more-likely-than-not recognition threshold are measured as the largest amount of tax benefit that is more than 50 percent likely of being realized upon settlement with the applicable taxing authority. The portion of the benefits associated with tax positions taken that exceeds the amount measured as described above is reflected as a liability for unrecognized tax benefits along with any associated interest and penalties that would be payable to the taxing authorities upon examination. As of December 31, 2014, the Company had no unrecognized tax benefits, and the Company had no positions which, in the opinion of management, would be reversed if challenged by a taxing authority. In the event the Company is assessed interest and/or penalties, such amounts will be classified as income tax expense in the financial statements.
As of December 31, 2014, all federal tax returns since 2011 and state tax returns since 2010 are still subject to adjustment upon audit. No tax returns are currently being examined by taxing authorities.
Comprehensive income (loss)—Comprehensive income (loss) includes net loss and other comprehensive income (loss). The items of other comprehensive income (loss) for the Company are unrealized gains and losses on marketable securities classified as available-for-sale and foreign translation adjustments relating to its subsidiaries. When the Company realizes a gain or loss on securities available-for-sale for which an unrealized gain or loss was previously recognized, a corresponding reclassification adjustment is made to remove the unrealized gain or loss from accumulated other comprehensive income and reflect the realized gain or loss in current operations.
Marketable securities—Investment securities as of December 31, 2014 and December 31, 2013 are classified as available-for-sale. Available-for-sale securities are recorded at cost and any increases or decreases in fair market value are recorded as unrealized gain or loss, net of taxes in accumulated
F-12
Emmaus Life Sciences, Inc.
Notes to Consolidated Financial Statements (Continued)
NOTE 2—SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
other comprehensive income. The Company monitors these investments for impairment and makes appropriate reductions in carrying values when necessary. CellSeed, Inc. securities are the only marketable security the Company currently carries on its books. The Company's marketable securities consist of 48,550 shares of CellSeed stock which are part of 147,100 shares acquired in January 2009 for 100,028,000 Japanese Yen (equivalent to $1,109,819), at 680 Yen per share. CellSeed's IPO (Tokyo Stock Exchange symbol 7776) was completed on March 16, 2010. As of December 31, 2014 and December 31, 2013, the closing price per share was 1,027 Yen and 1,840 Yen, respectively.
In July 2013, based on an increase in market value of CellSeed shares, Mitsubishi UFJ Capital III Limited Partnership (Mitsubishi) released to the Company 34,300 shares of CellSeed stock. This was part of the 73,550 shares of CellSeed stock held by Mitsubishi as collateral on a $500,000 convertible note issued to Mitsubishi which is due in 2016 ("the Mitsubishi note"). The Mitsubishi note is now secured by the remaining 39,250 shares of CellSeed stock held by Mitsubishi as collateral.
During the fourth quarter of 2013, the Company sold 25,000 of the shares released from Mitsubishi in open market transactions for $591,422. As of December 31, 2014 and 2013, 9,300 shares of CellSeed stock are classified as a current asset, as they are available for sale by the Company. The remaining 39,250 shares of CellSeed stock are pledged to secure the Mitsubishi note and are classified as marketable securities, pledged to creditor.
Gain on derecognition of accounts payable—The Company derecognizes accounts payable and records gain when the related contractual obligation is discharged, cancelled or expired. During the year ended December 31, 2013, the Company recorded a total of $341,361 as a gain on derecognition of accounts payable due to reaching a settlement with a creditor in regards to the amounts owed relating to services provided to the Company.
Foreign Currency Translation—The Company's reporting currency is the U.S. dollar. The yen and the euro are the functional currencies of its subsidiaries, EM Japan and EM Europe, respectively, as they are the primary currencies within the economic environments in which EM Japan and EM Europe operate. Assets and liabilities of their operations are translated into U.S. dollars at period-end exchange rates, and revenues and expenses are translated into U.S. dollars at average exchange rates in effect during each reporting period. Adjustments resulting from the translation are reported in other comprehensive income or loss.
Financial Instruments—Financial instruments included in the financial statements are comprised of cash and cash equivalents, short-term and long-term available-for-sale investments, accounts receivable, liability classified warrants, warrant derivative liabilities, accounts payable, certain accrued liabilities, convertible notes, promissory notes, due to related party, dissenting stockholders payable, contingent consideration and other contingent liabilities. The carrying amounts of cash and cash equivalents, accounts receivable, accounts payable and accrued liabilities, due to related party, and dissenting stockholders payable approximate their fair values due to the short-term nature of those instruments.
Fair value measurements—The Company defines fair value as the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. The Company measures fair value under a framework that provides a fair value hierarchy that prioritizes the inputs to valuation techniques used to measure fair value. The hierarchy
F-13
Emmaus Life Sciences, Inc.
Notes to Consolidated Financial Statements (Continued)
NOTE 2—SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
gives the highest priority to unadjusted quoted prices in active markets for identical assets or liabilities (Level 1 measurements) and the lowest priority to unobservable inputs (Level 3 measurements). The three levels of the fair value hierarchy are described as follows:
- •
- Quoted prices for similar assets or liabilities in active markets;
- •
- Quoted prices for identical or similar assets or liabilities in inactive markets;
- •
- Inputs other than quoted prices that are observable for the asset or liability;
- •
- Inputs that are derived principally from or corroborated by observable market data by correlation or other means.
Level 1: Inputs to the valuation methodology are unadjusted quoted prices for identical assets or liabilities in active markets that the Company has the ability to access.
Level 2: Inputs to the valuation methodology include:
If the asset or liability has a specified (contractual) term, the Level 2 inputs must be observable for substantially the full term of the asset or liability.
Level 3: Inputs to the valuation methodology are unobservable and significant to the fair value measurement.
The asset's or liability's fair value measurement level within the fair value hierarchy is based on the lowest level of any input that is significant to the fair value measurement. Valuation techniques used need to maximize the use of observable inputs and minimize the use of unobservable inputs. The fair value assigned to marketable securities is determined by obtaining quoted prices on nationally recognized securities exchanges, and are classified as Level 1 investments at December 31, 2014 and 2013. The fair value of the Company's debt instruments is not materially different from their carrying values as presented. The fair value of the Company's convertible debt instruments was determined based on Level 2 inputs. The carrying value of the debt was discounted based on allocating proceeds to other financial instruments within the arrangement as discussed in Note 6.
The Company issued stock purchase warrants in conjunction with its September 2013 private placement and issued replacement warrants upon the exercise of certain of such warrants in June 2014 (see Note 7). Such warrants and replacement warrants contain non-standard anti-dilution protection, and consequently, are accounted for as liabilities measured at fair value on a recurring basis, whose fair value is determined using Level 3 inputs. In addition, the remaining initial private placement warrants may now utilize a cashless exercise feature since the shares associated with them were not registered by the one-year anniversary of their issue. These warrants have now been reclassified from liability classified warrants to warrant derivative liabilities and to continue to be remeasured at fair value each reporting period.
These inputs include expected term and expected volatility.
F-14
Emmaus Life Sciences, Inc.
Notes to Consolidated Financial Statements (Continued)
NOTE 2—SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
The following tables present the activity for those items measured at fair value on a recurring basis using Level 3 inputs during 2014 and 2013:
| |
Year ended December 31, |
||||||
|---|---|---|---|---|---|---|---|
Liability Classified Warrants—Stock Purchase Warrants
|
2014 | 2013 | |||||
Balance, beginning of period |
$ | 5,928,000 | $ | — | |||
Fair value at issuance date |
3,523,000 | 6,860,000 | |||||
Settlement of liability associated with warrants exercised |
(1,752,744 | ) | — | ||||
Reclassification to warrant derivative liabilities |
(6,115,000 | ) | — | ||||
Reduction of the warrants exercised to intrinsic value included in the statement of comprehensive loss |
(1,770,256 | ) | — | ||||
Change in fair value included in the statement of comprehensive loss |
3,393,000 | (932,000 | ) | ||||
| | | | | | | | |
Balance, end of period |
$ | 3,206,000 | $ | 5,928,000 | |||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
| |
Year ended December 31, |
||||||
|---|---|---|---|---|---|---|---|
Warrant Derivative Liabilities—Stock Purchase Warrants
|
2014 | 2013 | |||||
Balance, beginning of period |
$ | — | $ | — | |||
Reclassification from liability classified warrants |
6,115,000 | — | |||||
Change in fair value included in the statement of comprehensive loss |
(474,000 | ) | — | ||||
| | | | | | | | |
Balance, end of period |
$ | 5,641,000 | $ | — | |||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
The initial value of the liability classified warrants as of September 11, 2013, value of warrant derivative liability as of September 11, 2014, and the change in fair value of the liability classified warrants and warrant derivative liabilities as of December 31, 2014, September 11, 2014, June 10, 2014 (the date of exercise and issuance), and December 31, 2013 were determined using a Binomial Monte-Carlo Cliquet (aka Ratchet) Option Pricing Model. The model is similar to traditional Black-Scholes-type option pricing models except that the exercise price resets at certain dates in the future. The values as of December 31, 2014, September 11, 2014, June 10, 2014, December 31, 2013, and the initial value as of September 11, 2013 were calculated based on the following assumptions:
| |
December 31, 2014 |
September 11, 2014 |
June 10, 2014 |
December 31, 2013 |
Initial Value |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Stock price |
$ | 4.90 | $ | 5.10 | $ | 5.10 | $ | 3.60 | $ | 3.60 | ||||||
Risk-free interest rate |
1.38 | % | 1.43 | % | 1.32 | % | 1.75 | % | 1.72 | % | ||||||
Expected volatility (peer group) |
71.50 | % | 69.50 | % | 68.20 | % | 63.20 | % | 72.40 | % | ||||||
Expected life (in years) |
3.70 | 3.95 | 4.26 | 4.70 | 5.00 | |||||||||||
Expected dividend yield |
— | — | — | — | — | |||||||||||
Number outstanding |
3,020,501 | 3,020,501 | 3,020,501 | 3,020,501 | 3,020,501 | |||||||||||
Balance, end of period: |
||||||||||||||||
Liability classified warrants |
$ | 3,206,000 | $ | 3,479,000 | $ | 9,714,000 | $ | 5,928,000 | $ | 6,860,000 | ||||||
Warrant derivative liabilities |
$ | 5,641,000 | $ | 6,115,000 | $ | — | $ | — | $ | — | ||||||
F-15
Emmaus Life Sciences, Inc.
Notes to Consolidated Financial Statements (Continued)
NOTE 2—SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
Debt and Related Party Debt—The Company accounts for the proceeds from the issuance of convertible notes payable with detachable stock purchase warrants and embedded conversion features in accordance with FASB ASC 470-20, Debt with Conversion and Other Options. Under FASB ASC 470-20, the proceeds from the issuance of a debt instrument with detachable stock purchase warrants shall be allocated to the two elements based on the relative fair values of the debt instrument without the warrants and of the warrants themselves at the time of issuance. The portion of the proceeds allocated to the warrants is accounted for as additional paid-in capital and the remaining proceeds are allocated to the debt instrument, which results in a discount to debt that is amortized and charged as interest expense over the term of the note agreement. Additionally, pursuant to FASB ASC 470-20, the intrinsic value of the embedded conversion feature of the convertible notes payable is included in the discount to debt and amortized and charged to interest expense over the term of the note agreement. The following table presents the effective interest rates on the original loan principal amount for loans originated in the respective periods that either had a beneficial conversion interest or an attached warrant:
Type of Loan
|
Term of Loan |
Stated Annual Interest Rate |
Original Loan Principal Amount |
Conversion Rate |
Beneficial Conversion Discount Amount |
Warrants Issued with Notes |
Exercise Price |
Warrant FMV Discount Amount |
Effective Interest Rate Including Discounts |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
2013 convertible notes payable |
1 ~ 2 years | 10 | % | $ | 3,079,666 | $3.30 | $ | 396,801 | 50,000 | — | $ | 116,831 | 19% ~ 62% | |||||||||||
2014 convertible notes payable |
6 mo. ~ 2 years | 10 | % | 3,096,266 | $3.05 ~ $3.60 | 1,392,387 | 50,000 | $ | 3.50 | $ | 126,732 | 28% ~ 100% | ||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | |
Total |
$ | 6,175,932 | $ | 1,789,188 | 100,000 | $ | 243,563 | |||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
Related party notes are disclosed as separate line items in the Company's balance sheet presentation.
Net loss per share—In accordance with FASB ASC Topic 260, "Earnings per Share," the basic loss per common share is computed by dividing net loss available to common stockholders by the weighted average number of common shares outstanding. Dilutive loss per share is computed in a manner similar to the basic loss per common share except that the denominator is increased to include the number of additional common shares that would have been outstanding if the potential common shares had been issued and if the additional common shares were dilutive. As of December 31, 2014 and 2013, there were 13,197,858 and 13,416,768 shares of potentially dilutive securities outstanding, respectively. As the Company reported a net loss, none of the potentially dilutive securities were included in the calculation of diluted loss per share since their effect would be anti-dilutive for all periods presented.
Recent accounting pronouncements
In July 2013, the FASB issued ASU No. 2013-11, Presentation of an Unrecognized Tax Benefit When a Net Operating Loss Carryforward, a Similar Tax Loss, or a Tax Credit Carryforward Exists. The amendments in this update aim to eliminate diversity in the presentation of unrecognized tax benefits when a net operating loss carryforward ("NOL carryforward"), a similar tax loss, or a tax credit carryforward exists. Under the amendments, an unrecognized tax benefit, or a portion of an unrecognized tax benefit, should be presented in the financial statements as a reduction to a deferred tax asset for a NOL carryforward, a similar tax loss, or a tax credit carryforward, except if a NOL carryforward, similar tax loss, or tax credit carryforward is not available at the reporting date
F-16
Emmaus Life Sciences, Inc.
Notes to Consolidated Financial Statements (Continued)
NOTE 2—SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
under the tax law of the applicable jurisdiction to settle any additional income taxes that would result from the disallowance of a tax position or the tax law of the applicable jurisdiction does not require the entity to use, and the entity does not intend to use, the deferred tax asset for such purpose, then the unrecognized tax benefit should be presented in the financial statements as a liability and should not be combined with deferred tax assets. The assessment of whether a deferred tax asset is available is based on the unrecognized tax benefit and deferred tax asset that exist at the reporting date and should be made presuming disallowance of the tax position at the reporting date. For example, an entity should not evaluate whether the deferred tax asset expires before the statute of limitations on the tax position or whether the deferred tax asset may be used prior to the unrecognized benefit being settled. The amendments in this update are effective for fiscal years and interim periods within those years beginning after December 15, 2013, with early adoption permitted. In May 2014, the FASB issued ASU No. 2014-09, Revenue from Contracts with Customers. This amendment will eliminate transaction-and industry-specific revenue recognition guidance under current GAAP and replace it with a principle-based approach for determining revenue recognition. The core principle of the revenue recognition guidance is that an entity recognizes revenue to depict the transfer of promised goods or services to customers in an amount that reflects the consideration to which the entity expects to be entitled in exchange for those goods or services. To achieve that core principle, an entity should apply the following steps: (1) identify the contract(s) with a customer; (2) identify the performance obligations in the contract(s); (3) determine the transaction price; (4) allocate the transaction price to the performance obligations in the contract(s); and (5) recognize revenue when (or as) the entity satisfies a performance obligation. Entities should also disclose qualitative and quantitative information about (1) contracts with customers, including revenue and impairments recognized, disaggregation of revenue, and information about contract balances and performance obligations and related transaction price allocation to remaining performance obligations, (2) significant judgments and changes thereof in determining the timing of performance obligations over time or at a point in time and the transaction price and amounts allocated to performance obligations, and (3) assets recognized from the costs to obtain or fulfill a contract. For public companies, these amendments are effective for annual reporting periods beginning after December 15, 2016, including interim periods within that reporting period (early adoption prohibited). The standard permits the use of either the retrospective or cumulative effect transition method. Currently, the Company is assessing the impact of adoption of the amendment on its financial statements and accompanying notes.
In August 2014, the FASB issued ASU No. 2014-15, Presentation of Financial Statements—Going Concern (Sub-topic 205-40): Disclosure of Uncertainties about an Entity's Ability to Continue as a Going Concern. The new guidance addresses management's responsibility to evaluate whether there is substantial doubt about an entity's ability to continue as a going concern and to provide related footnote disclosures. This update is effective for interim and annual periods beginning on or after December 15, 2016, and early adoption is permitted. The Company does not expect the adoption of this guidance to have a material impact on its financial position or results of operations.
In November 2014, the FASB issued ASU No. 2014-16, Derivatives and Hedging (Topic 815): Determining Whether the Host Contract in a Hybrid Financial Instrument Issued in the Form of a Share Is More Akin to Debt or to Equity. The new guidance requires an entity to determine the nature of the host contract by considering all stated and implied substantive terms and features of the hybrid financial instrument, weighing each term and feature on the basis of the relevant facts and circumstances. Additionally, the amendments state that no one term or feature would define the host
F-17
Emmaus Life Sciences, Inc.
Notes to Consolidated Financial Statements (Continued)
NOTE 2—SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
contract's economic characteristics and risks. Instead, the economic characteristics and risks of the hybrid financial instrument as a whole would determine the nature of the host contract. This update applies to all entities and is effective for annual periods beginning after December 15, 2015, and interim periods thereafter. Early adoption is permitted. Currently, the Company is assessing the impact of adoption of the new guidance on its financial statements and accompanying notes.
In January 2015, the FASB issued a new update ASU No. 2015-01, Income Statement—Extraordinary and Unusual Items (Subtopic 225-20): Simplifying Income Statement Presentation by Eliminating the Concept of Extraordinary Items, as part of the simplification initiative to identify, evaluate, and improve areas of GAAP for which cost and complexity can be reduced while maintaining or improving the usefulness of the information provided to users of financial statements. The new guidance eliminates the concept of extraordinary items and will align more closely GAAP income statement presentation guidance with IAS 1, Presentation of Financial Statements, which prohibits the presentation and disclosure of extraordinary items. The amendments in this update are effective for fiscal years, and interim periods within those fiscal years, beginning after December 15, 2015. A reporting entity may apply the amendments prospectively and also may apply the amendments retrospectively to all prior periods presented in the financial statements. Early adoption is permitted provided that the guidance is applied from the beginning of the fiscal year of adoption. The adoption of the amendments in this update is not expected to have a material impact on the Company's consolidated results of operations or financial position.
NOTE 3—PROPERTY AND EQUIPMENT
Property and equipment consisted of the following at:
| |
December 31, 2014 |
December 31, 2013 |
|||||
|---|---|---|---|---|---|---|---|
Equipment |
$ | 160,201 | $ | 128,343 | |||
Leasehold improvements |
30,579 | 23,054 | |||||
Furniture and fixtures |
74,683 | 52,269 | |||||
| | | | | | | | |
|
265,463 | 203,666 | |||||
Less: accumulated depreciation |
(193,072 | ) | (177,546 | ) | |||
| | | | | | | | |
Total |
$ | 72,391 | $ | 26,120 | |||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
During the years ended December 31, 2014 and 2013, depreciation expense was $16,620 and $20,974, respectively.
NOTE 4—INTANGIBLE ASSETS
The Company is licensed to market and sell NutreStore® L-glutamine powder for oral solution as a treatment for SBS.
In April 2011, the Company entered into a Research Agreement and an Individual Agreement with CellSeed and, in August 2011, entered into an addendum to the Research Agreement. Pursuant to the Individual Agreement, CellSeed granted the Company the exclusive right to manufacture, sell, market and distribute Cultured Autologous Oral Mucosal Epithelial Cell-Sheet ("CAOMECS") for the cornea in the United States and agreed to disclose to the Company its accumulated information package for the joint development of CAOMECS. Under the Individual Agreement, the Company
F-18
Emmaus Life Sciences, Inc.
Notes to Consolidated Financial Statements (Continued)
NOTE 4—INTANGIBLE ASSETS (Continued)
agreed to pay CellSeed $1.5 million, which it paid in February 2012. The technology acquired under the Individual Agreement is being used to support an ongoing research and development project and management believes the technology has alternative future uses in other future development initiatives.
Pursuant to the Research Agreement, the Company and CellSeed formed a relationship regarding the future research and development of cell sheet engineering regenerative medicine products. Under the Research Agreement, as supplemented by the addendum, the Company agreed to pay CellSeed $8.5 million within 30 days after the completion of all of the following: (i) the execution of the Research Agreement; (ii) the execution of the Individual Agreement; and (iii) CellSeed's delivery of the accumulated information package, as defined in the Research Agreement, to the Company and the Company providing written confirmation of its acceptance of the complete Package, which has not yet been completed as of December 31, 2014.
The Company has estimated the economic life of the CAOMECS produced in connection with the CellSeed Research and Individual Agreement at seven years. The determination of this life is based in part on the Company's estimate of economic useful life and the time period in which the Company may enjoy an advantage over competing technologies and techniques. Key reasons for a useful life shorter than the life of a patent include: (i) the patents related to this technology are yet to be approved, (ii) potential redundancy with similar medication/device due to changes in market preferences, (iii) uncertainty of regulatory approval and (iv) potential development of new treatments for the same disease.
Intangible assets consisted of the following at:
| |
December 31, 2014 |
December 31, 2013 |
|||||
|---|---|---|---|---|---|---|---|
License fees and patent filing costs |
$ | 2,250,000 | $ | 2,250,000 | |||
Less: accumulated amortization |
(1,357,143 | ) | (1,142,857 | ) | |||
| | | | | | | | |
Total |
$ | 892,857 | $ | 1,107,143 | |||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
During the years ended December 31, 2014 and 2013, amortization expense was $214,286 in each period.
As of December 31, 2014 estimated aggregate amortization expense for the next five years is as follows:
Year ending December 31
|
Amount | |||
|---|---|---|---|---|
2015 |
$ | 214,286 | ||
2016 |
214,286 | |||
2017 |
214,286 | |||
2018 |
214,286 | |||
2019 |
35,713 | |||
| | | | | |
Total |
$ | 892,857 | ||
| | | | | |
| | | | | |
| | | | | |
F-19
Emmaus Life Sciences, Inc.
Notes to Consolidated Financial Statements (Continued)
NOTE 5—ACCOUNTS PAYABLE AND ACCRUED EXPENSES
Accounts payable and accrued expenses consisted of the following at:
| |
December 31, 2014 |
December 31, 2013 |
|||||
|---|---|---|---|---|---|---|---|
Accounts payable |
|||||||
Clinical and regulatory expenses |
$ | 266,537 | $ | 270,694 | |||
Legal expenses |
176,691 | 178,119 | |||||
Other vendors |
774,444 | 386,847 | |||||
| | | | | | | | |
Subtotal |
1,217,672 | 835,660 | |||||
Accrued interest payable, related parties |
110,200 | 195,051 | |||||
Accrued interest payable |
761,682 | 473,356 | |||||
Accrued expenses |
220,200 | 294,379 | |||||
Deferred salary |
291,666 | 485,000 | |||||
| | | | | | | | |
Total accounts payable and accrued expenses |
$ | 2,601,420 | $ | 2,283,446 | |||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
F-20
Emmaus Life Sciences, Inc.
Notes to Consolidated Financial Statements (Continued)
NOTE 6—NOTES PAYABLE
Notes payable consisted of the following at December 31, 2014:
Year Issued
|
Interest rate range |
Term of Notes | Conv. Price | Principal Outstanding December 31, 2014 |
Discount Amount December 31, 2014 |
Carrying Amount December 31, 2014 |
Shares Underlying Principal as of December 31, 2014 |
Principal Outstanding December 31, 2013 |
Discount Amount December 31, 2013 |
Carrying Amount December 31, 2013 |
Shares Underlying Principal as of December 31, 2013 |
||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Convertible notes payable |
|||||||||||||||||||||||||||||||
2009 |
6.50% | 5 years | $3.05 | $ | — | $ | — | $ | — | — | $ | 254,460 | $ | — | $ | 254,460 | 83,366 | ||||||||||||||
2010 |
0 ~ 6.0% | 5 years | $3.05 | 74,000 | 3,195 | 70,805 | 24,248 | 74,000 | 8,308 | 65,692 | 24,248 | ||||||||||||||||||||
2011 |
10% | 5 years | $3.05 | 500,000 | — | 500,000 | 163,809 | 500,000 | — | 500,000 | 163,809 | ||||||||||||||||||||
2012 |
10% | 2 years | $3.30 ~ $3.60 | — | — | — | — | 251,100 | — | 251,100 | 71,000 | ||||||||||||||||||||
2013 |
10% | 2 years | $3.30 ~ $3.60 | 2,463,299 | 18,750 | 2,444,549 | 834,667 | 6,913,606 | 215,798 | 6,697,808 | 1,998,215 | ||||||||||||||||||||
2014 |
10% | Due on demand ~ 2 years | $3.05 ~ $7.00 | 4,939,773 | 846,613 | 4,093,160 | 1,241,241 | — | — | — | — | ||||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
|
$ | 7,977,072 | $ | 868,558 | $ | 7,108,514 | 2,263,965 | $ | 7,993,166 | $ | 224,106 | $ | 7,769,060 | 2,340,638 | |||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
|
Current | $ | 3,478,904 | $ | 21,945 | $ | 3,456,959 | 1,156,050 | $ | 4,955,868 | $ | 153,396 | $ | 4,802,472 | 1,438,033 | ||||||||||||||||
|
Non-current | $ | 4,498,168 | $ | 846,613 | $ | 3,651,555 | 1,107,915 | $ | 3,037,298 | $ | 70,710 | $ | 2,966,588 | 902,605 | ||||||||||||||||
Convertible notes payable—related party |
|||||||||||||||||||||||||||||||
2012 |
10% | Due on demand | $3.30 | $ | 373,000 | $ | — | $ | 373,000 | 121,461 | $ | 373,000 | $ | — | $ | 373,000 | 113,030 | ||||||||||||||
2013 |
10% | 1 year | $3.60 | — | — | — | — | 187,706 | — | 187,706 | 52,141 | ||||||||||||||||||||
2014 |
10% | 2 years | $7.00 | 200,000 | — | 200,000 | 30,187 | — | — | — | — | ||||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
|
$ | 573,000 | $ | — | $ | 573,000 | 151,648 | $ | 560,706 | $ | — | $ | 560,706 | 165,171 | |||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
|
Current | $ | 373,000 | $ | — | $ | 373,000 | 121,461 | $ | 560,706 | $ | — | $ | 560,706 | 165,171 | ||||||||||||||||
|
Non-current | $ | 200,000 | $ | — | $ | 200,000 | 30,187 | $ | — | $ | — | $ | — | — | ||||||||||||||||
Notes payable |
|||||||||||||||||||||||||||||||
2012 |
11% | 2 years | NA | $ | — | $ | — | $ | — | — | $ | 833,335 | $ | 18,265 | $ | 815,070 | — | ||||||||||||||
2013 |
2% ~ 10% | Due on demand ~ 2 years | NA | 1,030,000 | — | 1,030,000 | — | 1,150,000 | — | 1,150,000 | — | ||||||||||||||||||||
2014 |
11% | Due on demand ~ 2 years | NA | 1,446,950 | — | 1,446,950 | — | — | — | — | — | ||||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
|
$ | 2,476,950 | $ | — | $ | 2,476,950 | — | $ | 1,983,335 | $ | 18,265 | $ | 1,965,070 | — | |||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
|
Current | $ | 1,643,615 | $ | — | $ | 1,643,615 | — | $ | 1,783,335 | $ | 18,265 | $ | 1,765,070 | — | ||||||||||||||||
|
Non-current | $ | 833,335 | $ | — | $ | 833,335 | — | $ | 200,000 | $ | — | $ | 200,000 | — | ||||||||||||||||
Notes payable—related party |
|||||||||||||||||||||||||||||||
2012 |
8% ~ 10% | Due on demand | NA | $ | 656,730 | $ | — | $ | 656,730 | — | $ | 880,062 | $ | 4,421 | $ | 875,641 | — | ||||||||||||||
2013 |
8% | Due on demand | NA | 50,000 | — | 50,000 | — | 50,000 | — | 50,000 | — | ||||||||||||||||||||
2014 |
11% | Due on demand ~ 2 years | NA | 252,165 | — | 252,165 | — | — | — | — | — | ||||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
|
$ | 958,895 | $ | — | $ | 958,895 | — | $ | 930,062 | $ | 4,421 | $ | 925,641 | — | |||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
|
Current | $ | 825,562 | $ | — | $ | 825,562 | — | $ | 930,062 | $ | 4,421 | $ | 925,641 | — | ||||||||||||||||
|
Non-current | $ | 133,333 | $ | — | $ | 133,333 | — | $ | — | $ | — | $ | — | — | ||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
|
Grand Total | $ | 11,985,917 | $ | 868,558 | $ | 11,117,359 | 2,415,613 | $ | 11,467,269 | $ | 246,792 | $ | 11,220,477 | 2,505,809 | ||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
F-21
Emmaus Life Sciences, Inc.
Notes to Consolidated Financial Statements (Continued)
NOTE 6—NOTES PAYABLE (Continued)
The average stated interest rate of notes payable as of December 31, 2014 and 2013 was 10% in each period. The average effective interest rate of notes payable as of December 31, 2014 and 2013 was 23% and 15%, respectively, after giving effect to discounts relating to beneficial conversion features and the fair value of warrants issued in connection with these notes. The notes payable and convertible notes payable do not have restrictive financial covenants or acceleration clauses associated with a material adverse change event. The holders of the convertible notes have the option to convert their notes into the Company's common stock at the stated conversion price during the term of their convertible notes. Conversion prices on these convertible notes payable range from $3.05 to $3.60 per share. Certain notes with a $7.00 stated conversion price in the second year of their two-year term are subject to automatic conversion into shares of our common stock at a conversion price equal to 80% of the initial public offering price at the time of a qualified public offering. All due on demand notes are treated as current liabilities.
Contractual principal payments due on notes payable are as follows:
Year Ending
|
at December 31, 2014 | |||
|---|---|---|---|---|
2015 |
$ | 6,321,081 | ||
2016 |
5,664,836 | |||
| | | | | |
Total |
$ | 11,985,917 | ||
| | | | | |
| | | | | |
| | | | | |
The Company estimated the total fair value of any beneficial conversion feature and accompanying warrants in allocating the debt proceeds. The proceeds allocated to the beneficial conversion feature were determined by taking the estimated fair value of shares issuable under the convertible notes less the fair value of the number of shares that would be issued if the conversion rate equaled the fair value of the Company's common stock as of the date of issuance (see Note 2). The fair value of the warrants issued in conjunction with notes was determined using the Black-Scholes-Merton option pricing model with the following inputs for the years ended:
| |
December 31, 2014 | December 31, 2013 | |||||
|---|---|---|---|---|---|---|---|
Stock price |
$4.90 | $3.60 | |||||
Exercise price |
$3.50 | $3.30 | |||||
Term |
5 years | 5 years | |||||
Risk-free interest rate |
1.66% | 0.65% | |||||
Expected dividend yield |
— | — | |||||
Expected volatility |
70.10% | 118.06% | |||||
In situations where the debt included both a beneficial conversion feature and a warrant, the proceeds were allocated to the warrants and beneficial conversion feature based on the pro-rata fair value.
NOTE 7—STOCKHOLDERS' DEFICIT
Private Placement—On September 11, 2013, the Company issued an aggregate of 3,020,501 units at a price of $2.50 per unit (the "Private Placement"). Each unit consisted of one share of common
F-22
Emmaus Life Sciences, Inc.
Notes to Consolidated Financial Statements (Continued)
NOTE 7—STOCKHOLDERS' DEFICIT (Continued)
stock and one common stock warrant for the purchase of an additional share of common stock. The aggregate purchase price for the units was $7,551,253.
The warrants issued in the Private Placement entitle the holders thereof to purchase, at any time on or prior to September 11, 2018, shares of common stock of the Company at an exercise price of $3.50 per share. The warrants contain non-standard anti-dilution protection and, consequently, are being accounted for as liabilities, were originally recorded at fair value, and are adjusted to fair market value each reporting period. Because the shares of common stock underlying the Private Placement warrants were not effectively registered for resale by September 11, 2014, the warrant holders have an option to exercise the warrants using a cashless exercise feature. The shares have not been registered for resale as of December 31, 2014 and the availability to warrant holders of the cashless exercise feature as of September 11, 2014 caused the then outstanding 2,225,036 Private Placement warrants with fair value of $6,115,000 to be reclassified from liability classified warrants to warrant derivative liabilities and to continue to be remeasured at fair value each reporting period. As of December 31, 2014, the fair value of these 2,225,036 Private Placement warrant derivative liabilities was $5,641,000 (Note 2). For further details regarding registration rights associated with the Private Placement warrants, see Registration Rights, below.
Stock warrants—In addition to the warrants issued in connection with the Private Placement discussed above, during the year ended December 31, 2013, the Company issued warrants in connection with the issuance of a convertible note to purchase an aggregate of 50,000 shares of common stock at a per share exercise price of $3.30 per share. Also, in each of December 2014 and December 2013, warrants to purchase 500,000 shares of common stock, totaling 1,000,000 shares of common stock, at an exercise price of $1.00 per share that had previously been issued to a director expired. On May 1, 2014, in connection with the issuance of a convertible note, the Company issued a five year warrant to purchase 50,000 shares of common stock at an exercise price of $3.50 per share.
Warrant exercises and issuance—On June 10, 2014, certain warrant holders exercised 1,095,465 warrants issued in the Private Placement for the exercise price of $3.50 per share, resulting in the Company receiving aggregate exercise proceeds of $3.8 million and issuing 1,095,465 shares of common stock. Prior to exercise, these Private Placement warrants were accounted for at fair value as liability classified warrants. As of June 10, 2014, immediately prior to exercise, the carrying value of these Private Placement warrants was reduced to their fair value immediately prior to exercise of $1.8 million, representing their intrinsic value, with this adjusted carrying value of $1.8 million being transferred to additional paid in capital. Also on June 10, 2014, based on an offer made to holders of Private Placement warrants in connection with such exercises, the Company issued an aggregate of 1,095,465 replacement warrants to holders exercising Private Placement warrants, which replacement warrants have terms that are generally the same as the exercised warrants, including an expiration date of September 11, 2018 and an exercise price of $3.50 per share. The replacement warrants are treated for accounting purposes as liability classified warrants, and their issuance gave rise to a $3.5 million warrant exercise inducement expense based on their fair value as of issuance as determined using a Binomial Monte-Carlo Cliquet (aka Ratchet) Option Pricing Model. If the shares of common stock underlying the replacement warrants are not effectively registered for resale by June 10, 2015, the warrant holders have an option to exercise the replacement warrants using a cashless exercise feature. For more details regarding registration rights associated with the replacement warrants, see Registration Rights, below.
F-23
Emmaus Life Sciences, Inc.
Notes to Consolidated Financial Statements (Continued)
NOTE 7—STOCKHOLDERS' DEFICIT (Continued)
In addition to the above, during the year ended December 31, 2014, the Company issued 159,156 shares of common stock upon the exercise of warrants at exercise prices ranging from $1.00 to $3.825 per share, including 7,735 warrants exercised on a cashless basis.
A summary of outstanding warrants as of December 31, 2014 and 2013 is presented below.
| |
Year ended December 31, 2014 |
Year ended December 31, 2013 |
|||||
|---|---|---|---|---|---|---|---|
Warrants outstanding, beginning of period |
6,279,296 | 3,408,795 | |||||
Granted |
1,145,465 | 3,370,501 | |||||
Exercised |
(1,254,621 | ) | — | ||||
Cancelled, forfeited and expired |
(1,068,690 | ) | (500,000 | ) | |||
| | | | | | | | |
Warrants outstanding, end of period |
5,101,450 | 6,279,296 | |||||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
A summary of outstanding warrants by year issued and exercise price as of December 31, 2014 is presented below.
| |
Outstanding | Exercisable | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Year issued and Exercise Price
|
Number of Warrants Issued |
Weighted Average Remaining Contractual Life (Years) |
Weighted Average Exercise Price |
Total | Weighted Average Exercise Price |
|||||||||||
Balance 2012 |
||||||||||||||||
$1.00 |
411,020 | 0.11 | $ | 1.00 | 411,020 | $ | 1.00 | |||||||||
$2.50 |
1,000,000 | 0.66 | $ | 2.50 | 1,000,000 | $ | 2.50 | |||||||||
$3.05 |
227,106 | 0.56 | $ | 3.05 | 227,106 | $ | 3.05 | |||||||||
75% of FMV |
42,823 | 0.14 | 75% of FMV | 42,823 | 75% of FMV | |||||||||||
| | | | | | | | | | | | | | | | | |
2012 total |
1,680,949 | 1,680,949 | ||||||||||||||
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
During 2013 |
||||||||||||||||
$3.30 |
50,000 | 3.33 | $ | 3.30 | 50,000 | $ | 3.30 | |||||||||
$3.50 |
2,225,036 | 3.70 | $ | 3.50 | 2,225,036 | $ | 3.50 | |||||||||
| | | | | | | | | | | | | | | | | |
2013 total |
2,275,036 | 2,275,036 | ||||||||||||||
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
During 2014 |
||||||||||||||||
$3.50 |
1,145,465 | 3.73 | $ | 3.50 | 1,145,465 | $ | 3.50 | |||||||||
| | | | | | | | | | | | | | | | | |
Total |
5,101,450 | 5,101,450 | ||||||||||||||
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
Stock options—The 2011 Stock Incentive Plan, which is shareholder-approved, permits grants of incentive stock options to employees, including executive officers, and other share-based awards such as stock appreciation rights, restricted stock, stock units, stock bonus and unrestricted stock awards to employees, directors, and consultants for up to 9,000,000 shares of common stock. On February 28, 2013, the number of shares of common stock authorized for issuance under the 2011 Stock Incentive Plan was increased from 3,000,000 shares to 6,000,000 shares. On July 14, 2014, the number of shares of common stock authorized for issuance under the 2011 Stock Incentive Plan was increased from 6,000,000 shares to 9,000,000 shares. Options granted under the 2011 Stock Incentive Plan expire
F-24
Emmaus Life Sciences, Inc.
Notes to Consolidated Financial Statements (Continued)
NOTE 7—STOCKHOLDERS' DEFICIT (Continued)
10 years after grant. Options granted to directors vest in quarterly installments, and all other option grants vest over a minimum period of three years, all based on continuous service with the Company.
Management has valued stock options at their date of grant utilizing the Black-Scholes-Merton Option pricing model. The fair value of the underlying shares was determined by the market value of stock of similar companies and recent arm's length transactions involving the sale of the Company's common stock. The expected volatility was calculated using the historical volatility of a similar public entity in the industry through August 2013 and a group of similar public entities thereafter. The following table presents the assumptions used on recent dates on which options were granted by the Board of Directors.
| |
July 17, 2014 | May 8, 2014 | February 26, 2014 | July 18, 2013 | February 28, 2013 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Stock price |
$ | 5.10 | $ | 4.90 | $ | 3.60 | $ | 3.60 | $ | 3.60 | ||||||
Exercise price |
$ | 5.10 | $ | 4.90 | $ | 3.60 | $ | 3.60 | $ | 3.60 | ||||||
Term |
10 years | 10 years | 10 years | 10 years | 10 years | |||||||||||
Risk-Free Interest Rate |
2.47 | % | 2.61 | % | 2.67 | % | 2.56 | % | 1.89 | % | ||||||
Dividend Yield |
0.00 | % | 0.00 | % | 0.00 | % | 0.00 | % | 0.00 | % | ||||||
Volatility |
77.90 | % | 75.50 | % | 76.60 | % | 113.60 | % | 119.30 | % | ||||||
In making the determination of fair value and finding similar companies, the Company considered the industry, stage of life cycle, size and financial leverage of such other entities. While the Company was initially able to identify only one similar public company using these criteria, based on the more advanced stage of development of the Company additional similar companies with enough historical data that met the industry criterion have now been identified. Accordingly, the Company has based its expected volatility on the historical stock prices of a group of companies since September 2013.
The risk-free interest rate is based on the implied yield available on U.S. Treasury issues with an equivalent term approximating the expected life of the options depending on the date of the grant and expected life of the options. The expected life of options used was based on the contractual life of the option granted.
During the year ended December 31, 2014, the Company's Board of Directors granted 840,000 options to its directors, employees and consultants. In addition, 340,000 options that were approved by the Company's Board of Directors in April 2012 were deemed issued during the year ended December 31, 2014. The aggregate fair value of these tranches of options, including options granted or deemed issued in 2014 was approximately $3.0 million. As of December 31, 2014, there were 5,669,000 options outstanding under the 2011 Stock Incentive Plan and 11,795 options outstanding that were issued prior to the 2011 Stock Incentive Plan.
F-25
Emmaus Life Sciences, Inc.
Notes to Consolidated Financial Statements (Continued)
NOTE 7—STOCKHOLDERS' DEFICIT (Continued)
A summary of the Company's stock option activity for the years ended December 31, 2014 and 2013 is presented below.
| |
Prior Plan | December 31, 2014 | December 31, 2013 | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Number of Options |
Weighted- Average Exercise Price |
Number of Options |
Weighted- Average Exercise Price |
Number of Options |
Weighted- Average Exercise Price |
|||||||||||||
Options outstanding, beginning of period |
11,795 | $ | 3.05 | 4,504,000 | $ | 3.58 | 1,199,000 | $ | 3.60 | ||||||||||
Granted or deemed issued |
— | — | 1,180,000 | $ | 4.06 | 3,305,000 | $ | 3.57 | |||||||||||
Exercised |
— | — | — | — | — | — | |||||||||||||
Cancelled, forfeited and expired |
— | — | (15,000 | ) | $ | 3.60 | — | — | |||||||||||
| | | | | | | | | | | | | | | | | | | | |
Options outstanding, end of period |
11,795 | $ | 3.05 | 5,669,000 | $ | 3.68 | 4,504,000 | $ | 3.58 | ||||||||||
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
Options exercisable at end of year |
11,795 | $ | 3.05 | 2,855,251 | $ | 3.55 | 962,332 | $ | 2.14 | ||||||||||
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
Options available for future grant |
— | N/A | 3,331,000 | N/A | 1,496,000 | N/A | |||||||||||||
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
The weighted average grant-date fair value of options granted or deemed issued during the years ended December 31, 2014 and 2013 was $2.58 and $3.17, respectively. The weighted average grant-date fair value of common shares underlying stock options granted or deemed issued during the years ended December 31, 2014 and 2013 was $4.06 and $3.60, respectively.
During the years ended December 31, 2014 and 2013, the Company recognized $5.9 million and $5.1 million of share-based compensation cost arising from stock option grants. As of December 31, 2014, there was $6.2 million of total unrecognized compensation cost related to unvested share-based compensation arrangements granted under the 2011 Stock Incentive Plan. That cost is expected to be recognized over the weighted average remaining period of 1.4 years.
Registration Rights—Pursuant to the Subscription Agreements relating to the Private Placement and certain warrants, the Company agreed to use its commercially reasonable best efforts to have on file with the SEC, by September 11, 2014 and at the Company's sole expense, a registration statement to permit the public resale of 4,115,966 shares of the Company's common stock and 3,320,501 shares of common stock underlying warrants (collectively, the "Registrable Securities"). In the event such registration statement includes securities to be offered and sold by the Company in a fully underwritten primary public offering pursuant to an effective registration under the Securities Act, and the Company is advised in good faith by any managing underwriter of securities being offered pursuant to such registration statement that the number of Registrable Securities proposed to be sold in such offering is greater than the number of such securities which can be included in such offering without materially adversely affecting such offering, the Company will include in such registration the following securities in the following order of priority: (i) any securities the Company proposes to sell; and (ii) the Registrable Securities, with any reductions in the number of Registrable Securities actually included in
F-26
Emmaus Life Sciences, Inc.
Notes to Consolidated Financial Statements (Continued)
NOTE 7—STOCKHOLDERS' DEFICIT (Continued)
such registration to be allocated on a pro rata basis among the holders thereof. The registration rights described above apply until all Registrable Securities have been sold pursuant to Rule 144 under the Securities Act or may be sold without registration in reliance on Rule 144 under the Securities Act without limitation as to volume and without the requirement of any notice filing.
If the shares of common stock underlying warrants to purchase 2,225,036 shares are not registered for resale at the time of exercise or if the shares of common stock underlying warrants to purchase 1,095,465 shares are not registered for resale at the time of exercise on or after June 10, 2015, and in each such case the registration rights described above then apply with respect to the holder of such warrants, such holder may exercise such warrants on a cashless basis. In such a cashless exercise of all the shares covered by the warrant, the warrant holder would receive a number of shares equal to the quotient of (1) the difference between the fair market value of the common stock, as defined, and the $3.50 exercise price, as adjusted, multiplied by the number of shares exercisable under the warrant, divided by (2) the fair market value of the common stock, as defined. As of December 31, 2014, based on a fair market value of a share of the Company's common stock of $4.90 and 2,225,036 Private Placement warrants issued and outstanding and eligible for cashless exercise, the maximum number of shares the Company would be required to issue, if the warrant holders elected to exercise the cashless exercise feature with respect to all then eligible warrants, is 635,725 shares. If the fair market value of a share of the Company's common stock were to increase by $1.00 from $4.90 to $5.90, the maximum number of shares the Company would be required to issue, if the warrant holders elected to exercise the cashless exercise feature with respect to all then eligible warrants, would increase to 905,099 shares as of December 31, 2014. The maximum number of shares issuable under cashless exercises if the warrant holders elected to exercise the cashless exercise feature with respect to all then eligible warrants would increase to 863,000 on June 10, 2015 based on a fair market value of a share of our common stock of $4.90 and 3,020,501 Private Placement warrants issued and outstanding and eligible for cashless exercise. If the fair market value of a share of the Company's common stock were to increase by $1.00 from $4.90 to $5.90, the maximum number of shares the Company would be required to issue, if the warrant holders elected to exercise the cashless exercise feature with respect to all then eligible warrants would increase to 1,228,678 shares as of June 10, 2015.
The Company has not yet filed a registration statement with respect to the resale of the Registrable Securities because doing so is not feasible prior to the completion by the Company of its initial public offering. As previously reported, the Company has filed a draft registration statement with the SEC with respect to its initial public offering. The Company believes that it has used commercially reasonable efforts to pursue an initial public offering and, accordingly, considers itself to be in compliance with its registration rights obligations notwithstanding that it has not filed a registration statement with respect to the resale of the Registrable Securities and the deadline for doing so has passed without extension.
F-27
Emmaus Life Sciences, Inc.
Notes to Consolidated Financial Statements (Continued)
NOTE 8—INCOME TAXES
The provision (benefit) for income taxes consists of the following for the years ended December 31:
| |
|
2014 | 2013 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Current | U.S. | $ | 2,500 | $ | 2,550 | ||||
| International | 1,165 | 725 | |||||||
| Deferred | U.S. | — | (185,713 | ) | |||||
| International | — | — | |||||||
| | | | | | | | | | |
| $ | 3,665 | $ | (182,438 | ) | |||||
| | | | | | | | | | |
| | | | | | | | | | |
| | | | | | | | | | |
A valuation allowance for the net deferred tax assets has been recorded as it is more likely than not that these benefits will not be realized through future operations.
Deferred tax assets consist of the following as of December 31, 2014 and 2013:
| |
2014 | 2013 | |||||
|---|---|---|---|---|---|---|---|
Net operating loss carryforward |
$ | 14,354,064 | $ | 11,406,675 | |||
General business tax credit |
5,720,604 | 4,737,477 | |||||
Stock options |
4,843,871 | 2,966,286 | |||||
Charitable contribution |
81,434 | 77,659 | |||||
Accrued expenses |
171,325 | 236,159 | |||||
Deferred compensation |
— | 130,633 | |||||
Other |
126,983 | 120,236 | |||||
| | | | | | | | |
Total gross deferred tax assets |
25,298,281 | 19,675,125 | |||||
Less valuation allowance |
(25,184,004 | ) | (19,461,748 | ) | |||
| | | | | | | | |
Net deferred tax assets |
$ | 114,277 | $ | 213,377 | |||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
Deferred tax liabilities consist of the following as of December 31, 2014 and 2013:
| |
2014 | 2013 | |||||
|---|---|---|---|---|---|---|---|
Unrealized gain on foreign exchange translation and others |
$ | (98,825 | ) | $ | (29,154 | ) | |
Unrealized gain on securities available-for-sale |
(15,452 | ) | (184,223 | ) | |||
| | | | | | | | |
Total deferred tax liability |
$ | (114,277 | ) | $ | (213,377 | ) | |
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
During 2014 and 2013, the valuation allowance increased by $5,722,255 and $5,434,885, respectively.
As of December 31, 2014 and 2013, the Company had net operating loss carryforwards ("NOL") for federal reporting purposes of approximately $36,673,000 and $29,107,000 which are available to offset future federal taxable income, if any, through 2034. In addition, the Company had net operating loss carryforwards for state income tax purposes of approximately $35,884,000 and $27,602,000 respectively, which expire in various years through 2034. The utilization of our net operating losses could be subject to an annual limitation as a result of certain past and future events, such as acquisition or other significant equity events, which may be deemed as a "change in ownership" under the
F-28
Emmaus Life Sciences, Inc.
Notes to Consolidated Financial Statements (Continued)
NOTE 8—INCOME TAXES (Continued)
provisions of the Internal Revenue Code of 1986, as amended, and similar state provisions. The annual limitations could result in the expiration of net operating losses and tax credits before utilization. As of December 31, 2014 and 2013, the Company has general business tax credits of $5,721,000 and $4,737,000, respectively, for federal tax purposes. The tax credits are available to offset future tax liabilities, if any, through 2024.
The income tax provision differs from that computed using the statutory federal tax rate of 34%, due to the following:
| |
2014 | 2013 | |||||
|---|---|---|---|---|---|---|---|
Tax benefit at statutory federal rate |
$ | (7,022,409 | ) | $ | (4,844,588 | ) | |
State taxes, net of federal tax benefit |
(601,078 | ) | (532,099 | ) | |||
Increase in valuation allowance |
5,553,486 | 5,434,885 | |||||
Permanent items |
2,842,996 | 997,820 | |||||
General business tax credit |
(983,127 | ) | (1,218,292 | ) | |||
Other |
213,797 | (20,164 | ) | ||||
| | | | | | | | |
|
$ | 3,665 | $ | (182,438 | ) | ||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
As of December 31, 2014 and 2013, the Company had no unrecognized tax benefits, and the Company had no positions which, in the opinion of management, would be reversed if challenged by a taxing authority. In the event the Company is assessed interest and/or penalties, such amounts will be classified as income tax expense in the financial statements. As of December 31, 2014, all federal tax returns since 2011 and state tax returns since 2010 are still subject to adjustment upon audit. No tax returns are currently being examined by taxing authorities.
NOTE 9—COMMITMENTS AND CONTINGENCIES
Distribution contract—Cardinal Health Specialty Pharmacy Services has been contracted to distribute NutreStore to other wholesale distributors and some independent pharmacies since April 2008. For these services, the Company pays a monthly commercialization management fee of $5,000.
Operating leases—The Company leases its office space under operating leases with unrelated entities. The rent expense during the years ended December 31, 2014 and 2013 amounted to $277,866 and $137,147, respectively.
Future minimum lease payments under the agreements are as follows:
Year
|
Amount | |||
|---|---|---|---|---|
2015 |
$ | 410,474 | ||
2016 |
495,599 | |||
2017 |
468,977 | |||
2018 |
483,047 | |||
2019 |
82,510 | |||
| | | | | |
Total |
$ | 1,940,607 | ||
| | | | | |
| | | | | |
| | | | | |
F-29
Emmaus Life Sciences, Inc.
Notes to Consolidated Financial Statements (Continued)
NOTE 9—COMMITMENTS AND CONTINGENCIES (Continued)
Licensing agreement—The Company licensed certain current and future technology from CellSeed (see Note 4 for further discussion). CellSeed may terminate these agreements with the Company if the Company is unable to make timely payments required under the agreements. At the time the Company entered into the agreements with CellSeed, it left for further negotiation provisions covering how the Company and CellSeed will share any financial results of commercializing any cell sheet engineering regenerative medicine products that it is seeking to develop in collaboration with CellSeed. If the Company is not able to successfully negotiate these terms, its current development and commercialization plans with respect to any of these products would be materially adversely affected.
NOTE 10—AMOUNTS RECLASSIFIED OUT OF ACCUMULATED OTHER COMPREHENSIVE INCOME
| |
Unrealized holding gain (loss) on securities available-for-sale |
Unrealized foreign translation |
Total | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
Balance—December 31, 2012 |
$ | (4,386 | ) | $ | (8,046 | ) | $ | (12,432 | ) | |
| | | | | | | | | | | |
Other comprehensive income before reclassifications |
692,843 | (18,660 | ) | 674,183 | ||||||
Amounts reclassified from accumulated other comprehensive income |
(399,068 | ) | — | (399,068 | ) | |||||
| | | | | | | | | | | |
Net current period other comprehensive income |
293,775 | (18,660 | ) | 275,115 | ||||||
| | | | | | | | | | | |
Balance—December 31, 2013 |
289,389 | (26,706 | ) | 262,683 | ||||||
Other comprehensive income before reclassifications |
(435,010 | ) | (21,688 | ) | (456,698 | ) | ||||
Amounts reclassified from accumulated other comprehensive income |
||||||||||
| | | | | | | | | | | |
Net current period other comprehensive income |
(435,010 | ) | (21,688 | ) | (456,698 | ) | ||||
| | | | | | | | | | | |
Balance—December 31, 2014 |
$ | (145,621 | ) | $ | (48,394 | ) | $ | (194,015 | ) | |
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
Amounts in parentheses indicate debits. |
||||||||||
All amounts are net of tax. |
||||||||||
F-30
Emmaus Life Sciences, Inc.
Notes to Consolidated Financial Statements (Continued)
NOTE 11—RELATED PARTY TRANSACTIONS
The following table sets forth information relating to the Company's loans from related persons outstanding as of December 31, 2014.
Class
|
Lender | Annual Interest Rate |
Date of loan |
Term of Loan | Principal Amount Outstanding at December 31, 2014 |
Highest Principal outstanding |
Amount of Principal Repaid |
Amount of Interest Paid |
Conv. Rate |
Shares underlying principal at December 31, 2014 |
|||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Current, Promissory note payable to related parties: |
|||||||||||||||||||||||||||||
|
Hope Hospice(1) | 8 | % | 1/17/2012 | Due on demand | $ | 200,000 | $ | 200,000 | $ | — | $ | 16,000 | — | — | ||||||||||||||
|
Hope Hospice(1) | 8 | % | 6/14/2012 | Due on demand | 200,000 | 200,000 | — | 20,000 | — | — | ||||||||||||||||||
|
Hope Hospice(1) | 8 | % | 6/21/2012 | Due on demand | 100,000 | 100,000 | — | 10,000 | — | — | ||||||||||||||||||
|
Yutaka Niihara(2)(4) | 10 | % | 12/5/2012 | Due on demand | 156,730 | 1,213,700 | 1,056,970 | 60,851 | — | — | ||||||||||||||||||
|
Hope Hospice(1) | 8 | % | 2/11/2013 | Due on demand | 50,000 | 50,000 | — | 4,000 | — | — | ||||||||||||||||||
|
Lan T. Tran(2) | 11 | % | 2/10/2014 | 2 years(3) | 106,976 | 106,976 | — | — | — | — | ||||||||||||||||||
|
Cuc T. Tran(5) | 11 | % | 3/5/2014 | 1 year | 11,856 | 11,856 | — | — | — | — | ||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
|
Sub total | $ | 825,562 | $ | 1,882,532 | $ | 1,056,970 | $ | 110,851 | — | — | ||||||||||||||||||
Current, Convertible notes payable to related parties: |
|
||||||||||||||||||||||||||||
|
Yasushi Nagasaki(2) | 10 | % | 6/29/2012 | Due on demand | $ | 373,000 | $ | 388,800 | $ | 15,800 | $ | 67,680 | $ | 3.30 | 121,461 | |||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
|
Sub total | $ | 373,000 | $ | 388,800 | $ | 15,800 | $ | 67,680 | — | 121,461 | ||||||||||||||||||
Long-term, Promissory note payable to related parties: |
|
||||||||||||||||||||||||||||
|
Hideki & Eiko Uehara(5) | 11 | % | 2/15/2014 | 2 years | $ | 133,333 | $ | 133,333 | $ | — | $ | 14,697 | — | — | ||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
|
Sub total | $ | 133,333 | $ | 133,333 | $ | — | $ | 14,697 | — | — | ||||||||||||||||||
Long-term, Convertible notes payable to related parties: |
|
||||||||||||||||||||||||||||
|
Phillip M. Satow(4) | 10 | % | 6/6/2014 | 2 years | $ | 100,000 | $ | 100,000 | $ | — | $ | — | $ | 7.00 | 15,103 | |||||||||||||
|
Richard S. Pechter(5) | 10 | % | 6/11/2014 | 2 years | 100,000 | 100,000 | — | — | $ | 7.00 | 15,084 | |||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
|
Sub total | $ | 200,000 | $ | 200,000 | $ | — | $ | — | — | 30,187 | ||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
|
Total | $ | 1,531,895 | $ | 2,604,665 | $ | 1,072,770 | $ | 193,228 | — | 151,648 | ||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
- (1)
- Dr. Niihara,
who is the Company's CEO, is also the CEO of Hope International Hospice, Inc ("Hope Hospice").
- (2)
- Officer
- (3)
- Due
on Demand
- (4)
- Director
- (5)
- Family of Officer/Director
F-31
Emmaus Life Sciences, Inc.
Notes to Consolidated Financial Statements (Continued)
NOTE 11—RELATED PARTY TRANSACTIONS (Continued)
The following table sets forth information relating to the Company's loans from related persons outstanding as of December 31, 2013.
Class
|
Lender | Interest rate |
Date of loan |
Term of Loan | Principal Amount Outstanding as of December 31, 2013 |
Highest Principal outstanding |
Amount of Principal Repaid |
Amount of Interest Paid |
Conv. Rate |
Shares Underlying Principal as of December 31, 2013 |
|||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Current, Promissory note payable to related parties: |
|||||||||||||||||||||||||||||
|
Hope Hospice(1) | 8 | % | 1/17/2012 | Due on demand | $ | 200,000 | $ | 200,000 | $ | — | $ | 20,000 | — | — | ||||||||||||||
|
Lan T. Tran(2) | 11 | % | 2/10/2012 | 2 years(3) | 80,000 | 205,000 | 125,000 | — | — | — | ||||||||||||||||||
|
Hideki & Eiko Uehara(5) | 11 | % | 2/15/2012 | 2 years(3) | 133,333 | 133,333 | — | 14,433 | — | — | ||||||||||||||||||
|
Hope Hospice(1) | 8 | % | 6/14/2012 | Due on demand | 200,000 | 200,000 | — | 20,000 | — | — | ||||||||||||||||||
|
Hope Hospice(1) | 8 | % | 6/21/2012 | Due on demand | 100,000 | 100,000 | — | 10,000 | — | — | ||||||||||||||||||
|
Cuc T. Tran(5) | 11 | % | 6/27/2012 | 1 year | 10,000 | 10,000 | — | — | — | — | ||||||||||||||||||
|
Yutaka Niihara(2)(4) | 10 | % | 12/5/2012 | Due on demand | 156,730 | 1,213,700 | 1,056,970 | — | — | — | ||||||||||||||||||
|
Hope Hospice(1) | 8 | % | 2/11/2013 | Due on demand | 50,000 | 50,000 | — | 3,000 | — | — | ||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
|
Sub total | $ | 930,063 | $ | 2,112,033 | $ | 1,181,970 | $ | 67,433 | — | — | ||||||||||||||||||
|
Less discount | $ | (4,422 | ) | |||||||||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
|
Total | $ | 925,641 | ||||||||||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Current, Convertible notes payable to related parties: |
|||||||||||||||||||||||||||||
|
Yasushi Nagasaki(2) | 10 | % | 6/29/2012 | Due on demand | $ | 373,000 | $ | 388,800 | $ | 15,800 | $ | — | $ | 3.30 | 113,030 | |||||||||||||
|
Hideki & Eiko Uehara(5) | 10 | % | 9/7/2013 | 1 year | 35,640 | 35,640 | — | — | 3.60 | 9,900 | ||||||||||||||||||
|
MLPF&S Cust. FBO Willis C. Lee(2)(4) | 10 | % | 10/5/2013 | 1 year | 152,066 | 152,066 | — | — | $ | 3.60 | 42,240 | |||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
|
Sub total | $ | 560,706 | $ | 576,506 | $ | 15,800 | $ | — | — | 165,170 | ||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
|
Total | $ | 1,486,347 | $ | 2,688,539 | $ | 1,197,770 | $ | 67,433 | — | 165,170 | ||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
- (1)
- Dr. Niihara
is also the CEO of Hope Hospice.
- (2)
- Officer
- (3)
- Due
on Demand
- (4)
- Director
- (5)
- Family of Officer/Director
Since July 2012 the Company has been engaged in litigation with AFH Advisory, which was the sole stockholder of AFH Acquisition IV immediately prior to its combination with Emmaus Medical pursuant to the Merger in May 2011. In September 2012 AFH Advisory and related parties filed a complaint against the Company in the Superior Court of Delaware. In October 2012 the Company filed counterclaims against the plaintiffs and third-party claims against Amir Heshmatpour. Mr. Heshmatpour is a former officer of AFH Acquisition IV, Inc. (prior to the Merger) and former director of the Company and is the Managing Partner of AFH Advisory. On June 27, 2013, the Superior Court of the State of Delaware issued an order implementing a partial summary judgment in favor of the Company in its litigation against AFH Advisory, Griffin Ventures, Ltd. ("Griffin"), The Amir & Kathy Heshmatpour Family Foundation (the "Foundation") and Amir Heshmatpour. The order, among other things, (i) stated that a letter of intent previously entered into between the Company and AFH Advisory (the "Letter of Intent") was properly terminated as of July 19, 2012 by the Company, and (ii) ordered the transfer agent for the Company to effect the cancellation of 2,504,249 shares of the Company's common stock held by AFH Advisory, Griffin and the Foundation. The cancellation of such shares was effected by the Company's transfer agent on June 28, 2013. While no appeal of this ruling has been pursued, if the court's ruling is appealed, it could result in the Company's incurring liabilities and expenses that may have a material adverse effect on its financial
F-32
Emmaus Life Sciences, Inc.
Notes to Consolidated Financial Statements (Continued)
NOTE 11—RELATED PARTY TRANSACTIONS (Continued)
condition and cash flows. The cancellation of such shares is subject to appeal until 30 days after the completion of trial court proceedings. While the partial summary judgment in favor of the Company in this litigation noted above led to the cancellation of 2,504,249 shares of the Company by the transfer agent on June 28, 2013, the Company will continue to present these shares in its financial statements as outstanding until the right of appeal has lapsed and all contingencies have been resolved. The Company's cause of action for fraud, which was not part of the summary judgment, has not yet been litigated or settled.
NOTE 12—GEOGRAPHIC INFORMATION
For the years ended December 31, 2014 and 2013, the Company earned revenue from countries outside of the United States as outlined in the table below. The Company did not have any significant currency translation or foreign transaction adjustments during the years ended December 31, 2014 and 2013.
Country
|
Sales year ended December 31, 2014 |
% of Total Revenue year ended December 31, 2014 |
Sales year ended December 31, 2013 |
% of Total Revenue year ended December 31, 2013 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Japan |
$ | 179,570 | 36 | % | $ | 171,054 | 44 | % | |||||
Taiwan |
193,552 | 39 | % | 63,254 | 16 | % | |||||||
South Korea |
45,312 | 9 | % | 12,000 | 3 | % | |||||||
NOTE 13—SUBSEQUENT EVENTS
Subsequent to the year ended December 31, 2014, the Company issued the following:
Notes issued after December 31, 2014
|
Principal Amounts |
Annual Interest Rate |
Term of Notes | Conversion Price |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
Convertible note(1) |
$ | 196,026 | 10 | % | On Demand up to 1 Year | $ | 3.60 | |||||
Convertible note(1) |
656,052 | 10 | % | Approximately 7 months | $ | 3.50 | ||||||
Convertible note(1) |
100,800 | 10 | % | 1 Year | $ | 3.60 | ||||||
Convertible note(1) |
120,960 | 10 | % | 2 Years | $ | 3.60 | ||||||
Convertible note(1) |
504,614 | 10 | % | 2 Years | $ | 3.50 | ||||||
Convertible note(2) |
200,000 | 10 | % | 2 Years | $ | 7.00 | ||||||
Promissory note(3) |
464,800 | 11 | % | On Demand up to 2 years | N/A | |||||||
Promissory notes-related party |
230,000 | 10 | % | On Demand up to 2 years | N/A | |||||||
Promissory notes-related party(1) |
13,161 | 11 | % | 1 Year | N/A | |||||||
| | | | | | | | | | | | | |
Total |
$ | 2,486,413 | ||||||||||
| | | | | | | | | | | | | |
| | | | | | | | | | | | | |
| | | | | | | | | | | | | |
- (1)
- Refinance
of prior note.
- (2)
- Includes
mandatory conversion at the time of an initial public offering at a conversion price equal to 80% of the initial public offering price.
- (3)
- Principal amount of note is JPY56, 000,000 which was converted into US dollars using an exchange rate of 0.0083 as of December 31, 2014.
F-33
