Attached files
| file | filename |
|---|---|
| EX-32.1 - EX-32.1 - EMISPHERE TECHNOLOGIES INC | d868964dex321.htm |
| EX-23.1 - EX-23.1 - EMISPHERE TECHNOLOGIES INC | d868964dex231.htm |
| EX-31.1 - EX-31.1 - EMISPHERE TECHNOLOGIES INC | d868964dex311.htm |
| EX-31.2 - EX-31.2 - EMISPHERE TECHNOLOGIES INC | d868964dex312.htm |
| EX-21.1 - EX-21.1 - EMISPHERE TECHNOLOGIES INC | d868964dex211.htm |
| EXCEL - IDEA: XBRL DOCUMENT - EMISPHERE TECHNOLOGIES INC | Financial_Report.xls |
Table of Contents
UNITED STATES SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form 10-K
(Mark One)
| þ | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2014
OR
| ¨ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to
Commission file number 0-17758
EMISPHERE TECHNOLOGIES, INC.
(Exact name of registrant as specified in its charter)
| Delaware | 13-3306985 | |
| (State or other jurisdiction of incorporation or organization) |
(I.R.S. Employer Identification Number) | |
| 4 Becker Farm Road, Suite 103 Roseland, NJ |
07068 | |
| (Address of principal executive offices) | (Zip Code) |
(973) 532-8000
(Registrant’s telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act:
None
Securities registered pursuant to Section 12(g) of the Act:
Common Stock — $.01 par value
Preferred Stock Purchase Rights
Indicate by check mark if the Registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ¨ No þ
Indicate by check mark if the Registrant is not required to file reports pursuant to Section 13 or 15(d) of the Act. Yes ¨ No þ
Indicate by check mark whether the Registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that Registrant was required to file such reports) and (2) has been subject to such filing requirements for at least the past 90 days. Yes þ No ¨
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes þ No ¨
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (§ 229.405 of this chapter) is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check one):
| Large accelerated filer ¨ | Accelerated filer ¨ | Non-accelerated filer ¨ | Smaller reporting company þ | |||
| (Do not check if a smaller reporting company) | ||||||
Indicate by check mark whether the Registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes ¨ No þ
As of June 30, 2014 (the last business day of the registrant’s most recently completed second quarter), the aggregate market value of the common stock held by non-affiliates of the Registrant (i.e. excluding shares held by executive officers, directors, and control persons) was $12,882,005 computed at the closing price on that date.
The number of shares of the Registrant’s common stock, $.01 par value, outstanding as of March 1, 2015 was 60,687,478.
Table of Contents
Table of Contents
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This Annual Report on Form 10-K and the documents incorporated by reference herein contain forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 and Section 21E of the Securities Exchange Act of 1934, as amended, that involve substantial risks and uncertainties. These forward-looking statements include, without limitation, statements regarding the success of our commercialization initiatives; the sufficiency of our cash position; our ability to enter into strategic partnerships; our ability, and that of our partners, to develop, manufacture and commercialize products using our Eligen® technology; planned or expected studies and trials of oral formulations that utilize our Eligen® Technology; the potential market size, advantages or therapeutic uses of our potential products and the sufficiency of our available capital resources to meet our funding needs. You should read this Annual Report on Form 10-K completely and with the understanding that our actual future results may be materially different from what we expect. We do not undertake any obligation to publicly update any forward-looking statement, whether as a result of new information, future events, or otherwise, except as required by law. Such forward-looking statements involve known and unknown risks, uncertainties and other factors which may cause our actual results or achievements to be materially different from any future results or achievements expressed or implied by such forward-looking statements. Such factors include the factors described in Part 1, Item 1A. “Risk Factors” and the other factors discussed in connection with any forward-looking statements.
| ITEM 1. | BUSINESS |
Overview of Emisphere
Introduction and History
Emisphere Technologies, Inc. (“Emisphere,” “the Company,” “our,” “us,” or “we”) is a specialty pharmaceutical company that has recently commenced commercial operations. The Company launched its first prescription product, oral Eligen B12™ Rx (1000 mcg.), in the U.S. during March 2015. Oral Eligen B12™ Rx meets significant unmet patient and medical needs by combining vitamin B12 with our proprietary delivery system technology to provide a therapeutic equivalent to injectable B12, which is the current medical standard of care. Eligen B12™ is a prescription food product for use by B12 deficient individuals and the first oral B12 product to be supported by published clinical data (Clin Ther. 2011 Jul;33(7):934-45) demonstrating that oral Eligen B12™ Rx restores normal vitamin B12 blood levels in deficient patients as effectively as injectable B12. The proprietary Eligen B12™ Rx formulation is covered by a newly issued U.S. Patent (Patent No. 8,022,048) which provides protection through 2029. Beyond oral Eligen B12™ Rx, the Company utilizes its proprietary Eligen® Technology to create new oral formulations of therapeutic agents. Emisphere is currently partnered with global pharmaceutical companies for the development of such new orally delivered therapeutics.
In addition to launching oral Eligen B12™ Rx in the United States during March 2015, the Company is currently engaged in multiple ex-US licensing discussions with the intention of offering oral Eligen B12™ Rx for sale in global markets.
By building on the oral Eligen B12™ Rx product, the Company intends to establish a sound product portfolio platform on which to expand its B12 therapeutic franchise as well as expand internal new product development with new therapeutic agents. The Company will also continue to develop its existing drug delivery carrier partnerships and expand its carrier business by seeking out and engaging in new global licensing opportunities.
As it focuses on building a commercial platform based on the oral Eligen B12™ Rx product, Emisphere will continue to develop and expand upon the unique and improved delivery of therapeutic molecules using its Eligen® Technology. These molecules could be currently available or are under development. Such molecules are usually delivered by injection; in many cases, their benefits are limited due to poor bioavailability, slow on-set of action or variable absorption. In those cases, our technology may increase the benefit of the therapy by improving bioavailability or absorption or by decreasing time to onset of action. The Eligen® Technology can be applied to the oral route of administration as well as other delivery pathways, such as buccal, rectal, inhalation,
1
Table of Contents
intra-vaginal or transdermal. The Eligen® Technology can make it possible to deliver certain therapeutic molecules orally without altering their chemical form or biological activity. Eligen® delivery agents, or “carriers”, facilitate or enable the transport of therapeutic molecules across the mucous membranes of the gastrointestinal tract, to reach the tissues of the body where they can exert their intended pharmacological effect. Our development efforts are conducted internally or in collaboration with corporate development partners. Typically, the drugs that we target are at an advanced stage of development, or have already received regulatory approval, and are currently available on the market. Our website is www.emisphere.com. The contents of that website are not incorporated herein by reference. Investor related questions should be directed to info@emisphere.com.
Emisphere was originally founded as Clinical Technologies Associates, Inc. in 1986 and is listed for trading on the Over-the-Counter Bulletin Board (the “OTCBB”), an electronic quotation service maintained by the Financial Industry Regulatory Authority, and is trading under the symbol EMIS (or EMIS.OB for certain stock quote publication websites).
The Eligen® Technology
The Eligen® Technology is a broadly applicable proprietary oral drug delivery technology based on the use of proprietary synthetic chemical compounds known as Eligen® delivery agents, or carriers. These delivery agents facilitate and enable the transport of therapeutic macromolecules (such as proteins, peptides, and polysaccharides) and poorly absorbed small molecules across biological membranes. The Eligen® Technology not only facilitates absorption, but it acts rapidly in the upper sections of the gastrointestinal tract where absorption is thought to occur. Using Eligen® Technology, most therapeutic macromolecules reach the general circulation in less than an hour post-dose. Rapid absorption can limit enzymatic degradation that typically affects macromolecules and may be advantageous in cases where time to onset of action is important (i.e. analgesics). Eligen® is distinguished from the competition in that absorption takes place through a transcellular pathway, as opposed to passing between cells. This underscores the safety of Eligen® as the passage of the Eligen® carrier and the molecule preserve the integrity of the tight junctions within the cell and reduces any likelihood of inflammatory processes and autoimmune gastrointestinal diseases. Furthermore, Eligen® Technology carriers are rapidly absorbed, distributed, metabolized and eliminated from the body, they do not accumulate in the organs and tissues and are considered safe at anticipated doses and dosing regimens.
Results from two clinical studies published by F. Hoffmann-La Roche Ltd illustrate important safety characteristics of Emisphere’s Eligen® Technology. These studies were performed with a novel oral formulation of ibandronate (a drug used to prevent and treat osteoporosis) with Emisphere’s SNAC carrier, an Eligen® Technology compound. The first study (J Drug Del Technol 2011; 21: 521-5) showed that the SNAC carrier must be co-formulated, and not simply co-dosed, with ibandronate in order to increase ibandronate bioavailability. The second study (Arnzelmittelforschung 2011; 61:707-13) demonstrated that co-dosing of a SNAC/ibandronate formulation with metformin, a drug widely used in Type 2 Diabetes patients, did not influence the absorption of metformin. Together, these studies support the hypothesis that Eligen® Technology facilitates oral absorption only when co-formulated with the intended active ingredient, and that co-dosing with other ingredients should not result in accidental or incidental absorption of unintended ingredients.
Another important safety characteristic of the Eligen® Technology was recently demonstrated by the results of three clinical safety studies conducted by Novartis International AG with the former osteoporosis and osteoarthritis treatment candidate SMC021. SMC021 used Emisphere’s permeation enhancer 5-CNAC, an Eligen® Technology compound, in combination with salmon calcitonin (“SCT”). These studies addressed the potential for SMC021 drug interaction with several widely used drugs and found, in each case, no evidence to indicate a safety concern for drug interaction. Scientific posters describing the results of these clinical studies were presented at the annual meeting of the American Society of Clinical Pharmacology and Therapeutics on March 17, 2012. The first study (The effect of esomeprazole on the pharmacokinetics and pharmacodynamics of SMC021 in healthy volunteers. Choi L et al.) concluded that pre-treatment with a proton pump inhibitor, esomeprazole, decreased SCT exposure by approximately 30% without impacting the pharmacodynamic response to SCT. The second study (Pharmacokinetic interaction assessment between SMC021 and ibuprofen and between SMC021 and acetaminophen. Choi L et al.) concluded that ibuprofen and acetaminophen did not
2
Table of Contents
significantly alter the pharmacokinetics of SMC021 when used jointly with either of these analgesics. The third study (Pharmacokinetic interaction assessment between SMC021 and rosiglitazone. Choi L et al.) concluded that SMC021 did not inhibit the drug metabolizing enzyme CYP2C8 when SMC021 and rosiglitazone, a Type II diabetes drug metabolized by CYP2C8, were administered together at expected clinical doses. Together, these studies support the hypothesis that Eligen® Technology does not pose a safety risk for drug interaction.
Based on extensive study by our scientists, senior management and expert consultants, we believe that our technology can enhance overall healthcare, including patient accessibility and compliance, while benefiting the commercial pharmaceutical marketplace and driving company valuation. The application of the Eligen® Technology is potentially broad and may provide for a number of opportunities across a spectrum of therapeutic modalities.
Implementing the Eligen® Technology requires co-formulating a drug or nutritional supplement and an Eligen® carrier to produce an effective formulation. The carrier does not alter the chemical properties of the drug nor its biological activity. Drugs or nutritional supplements whose bioavailability is limited by poor membrane permeability or chemical or biological degradation, and which have a moderate-to-wide therapeutic index, appear to be the best candidates for use with the Eligen® Technology. Drugs with a narrow therapeutic window or high molecular weight may not be favorable with the technology.
We believe that our Eligen® Technology makes it possible to safely deliver a therapeutic macromolecule orally or increase the absorption of a poorly absorbed small molecule without altering its chemical composition or compromising the integrity of biological membranes. We believe that the key benefit of our Eligen® Technology is that it improves the ability of the body to absorb small and large molecules.
Emisphere Today
Mr. Alan L. Rubino, the Company’s President and Chief Executive Officer, and Mr. Timothy G. Rothwell, its Chairman of the Board of Directors, are seasoned industry executives with major and emerging pharmaceutical company experience who form the core of a leadership team that will implement the Company’s strategic plans. To that end, we have sought to expand opportunities with existing partners and will continue to work to expand and explore new efforts to attract new delivery system, product development, and licensing partnerships. After evaluating the Company’s operations and strategy, the leadership team determined the Company should refocus its corporate strategy to reemphasize the commercialization of oral Eligen B12™ Rx, build new high-value partnerships, evaluate new prescription commercial product opportunities, reprioritize the product pipeline, and promote new uses for the Eligen® Technology.
In furtherance of this new strategic direction, spending has been redirected and aggressive cost control initiatives, including the elimination of certain research and development positions, have been implemented in order to allow investment in commercialization resources. To accelerate the commercialization of oral Eligen B12™ Rx and evaluate new opportunities for prescription medical foods and other prescription products under development, the Company hired Mr. Carl V. Sailer in October 2012 to head its commercial efforts. Mr. Sailer has extensive experience in pharmaceuticals products marketing and supply chain management. He has a proven track record of launching new, and enhancing the financial performance of, existing pharmaceutical products by implementing progressive commercial marketing and distribution models. The Company engaged the consulting services of Dr. Carlos de Lecea, M.D., Ph.D., to expand its business development efforts globally. Dr. de Lecea has over 20 years’ experience in business development, including in and out licensing pharmaceutical products and delivery technologies in global markets. Dr. de Lecea also works with Mr. Rubino to expand the application of the Eligen® Technology by taking advantage of its suitability to facilitate oral absorption of emerging peptides and biologic products that are typically only available as injectables or are currently under development. We believe that these products represent tremendous promise for realizing improvements in healthcare and growth in the industry, and that the Eligen® Technology is well suited to deliver many of these molecules safely and efficiently. More recently the Company continues to strengthen its commercial operations team by engaging the consulting services of Mr. Nicholas G. Rothwell, CPIM, as Vice President, Global Supply, and Dr. Peter J. Shaw, M.D., as Chief Medical Officer. Mr. Rothwell has an exceptional background in commercial operations for life sciences and will play an integral role in supporting Emisphere’s new commercial operations building on the launch of Eligen B12™ Rx. With more than 30 years of experience developing, implementing and managing
3
Table of Contents
end-to-end integrated supply chains, Mr. Rothwell is a senior healthcare executive with extensive commercial experience in operations management and strong expertise in leading strategic planning, product launch and growth initiatives of leading pharmaceutical products. Mr. Rothwell will be responsible for global supply chain, logistics, manufacturing and quality assurance. Dr. Shaw brings significant industry and medical experiences as a practicing physician and in progressive pharmaceutical sales representative training. His expertise and vision will facilitate the launch and growth of our oral Eligen B12™ Rx product, and strengthen our development efforts supporting the advancement of the Company’s other pipeline product candidates. Dr. Shaw will have responsibility for global clinical development programs, medical affairs and other related functions. Mr. Rothwell and Dr. Shaw report to Mr. Rubino.
These actions support the Company’s decision to transition and reposition Emisphere into a viable commercial-stage entity, anchored by the oral Eligen B12™ Rx product. As it transitions to this strategy, the Company remains dedicated to further realizing the full potential and commercial value of its platform Eligen® Technology. As a result of our recent steps to refocus and prioritize our commercial opportunities, and promising trends with peptides, pegylated peptides and proteins in the industry that should provide new growth opportunities, we believe that Emisphere’s new business strategy will present opportunities for growth and value creation for the Company and its shareholders.
The application of the Eligen® Technology is potentially broad and may provide for a number of opportunities across a spectrum of therapeutic modalities or nutritional supplements. During the remainder of 2015 we plan to continue to develop our product pipeline utilizing the Eligen® Technology with prescription and medical foods product candidates and prioritized our development efforts based on overall potential returns on investment, likelihood of success, and market and medical needs. Medical foods are a distinct product category defined by the Orphan Drug Act of 1988 and an FDA regulation, and encompass foods which are formulated to be consumed or administered enterally under the supervision of a physician and which are intended for the specific dietary management of a disease or condition for which distinctive nutritional requirements, based on recognized scientific principles, are established by medical evaluation. Our goal is to implement our Eligen® Technology to enhance overall healthcare, including patient accessibility and compliance, while benefiting the commercial pharmaceutical and healthcare marketplace and driving company valuation.
In March 2015, Emisphere announced the U.S. commercial availability of oral Eligen B12™ Rx, the first and only once-daily oral prescription medical food tablet shown to normalize B12 levels without the need for an injection. Oral Eligen B12™ Rx (1000 mcg) is indicated for the dietary management of patients who have a medically-diagnosed vitamin B12 deficiency, associated with a disease or condition that cannot be managed by a modification of the normal diet alone. Eligen B12 is the first product to market using the Eligen® Technology, which utilizes a carrier, salcaprozate sodium (SNAC), to chaperone B12 through the gastric lining and directly into the bloodstream independent of intrinsic factor, a protein made in the stomach that normally facilitates B12 absorption.
To accelerate commercialization of the Eligen® Technology, Emisphere will continue to focus on its two-pronged strategy. First, we will focus on commercializing oral Eligen B12™ Rx (1000 mcg) as a medical food for use by documented B12 deficient individuals in the United States and globally. During the fourth quarter of 2010, the Company completed a clinical trial which demonstrated that both oral Eligen B12™ Rx (1000 mcg) and injectable B12 (current standard of care) can efficiently and quickly restore normal Vitamin B12 levels in deficient individuals. The manuscript summarizing the results from that clinical trial was published in the July 2011 edition of the journal Clinical Therapeutics (Volume 22, pages 934 — 945). We also conducted market research to help assess the potential commercial opportunity for our oral Eligen B12™ Rx (1000 mcg) product. On August 5, 2011, we received notice from the United States Patent Office that the U.S. patent application directed to the oral Eligen B12™ Rx formulation was allowed. This new patent (US 8,022,048) provides intellectual property protection for Eligen B12™ Rx through approximately October 2029. Second, we will concentrate on expanding our Eligen® drug delivery technology business by seeking applications with prescription molecules obtained through partnerships with other pharmaceutical companies for molecules with poor oral absorption yet substantially beneficial when absorbed effectively. We are also working to generate new interest in the Eligen® Technology with potential partners and attempting to expand our current collaborative relationships to take advantage of the critical knowledge that others have gained by working with our technology.
4
Table of Contents
Second, we continue to pursue commercialization of product candidates developed internally. We believe that these internal candidates need to be developed with reasonable investment in an acceptable time period and with a reasonable risk-benefit profile.
To support our internal development programs, the Company implemented its new commercialization strategy for the Eligen® Technology. Using extensive safety data available for its Sodium N-[8-(2-hydroxybenzoyl) Amino] Caprylate (“SNAC”) carrier, the Company obtained GRAS (“Generally Recognized as Safe”) status for its SNAC carrier, and then applied the Eligen® Technology with B12, another GRAS substance where bioavailability and absorption is difficult and improving such absorption would yield substantial benefit and value. Given sufficient time and resources, the Company intends to apply this strategy to develop other products. Examples of other GRAS substances that may be developed into additional commercial products using this strategy would include vitamins such as other B Vitamins, minerals such as iron, and other supplements such as the polyphenols and catechins, among others.
Our product pipeline includes prescription and medical food product candidates that are being developed in partnership or internally. During 2014, we continued to make progress on plans to commercialize our internally developed oral Eligen B12™ Rx product, having launched in the U.S. during the first quarter, 2015. Additionally, our development partner, Novo Nordisk A/S (“Novo Nordisk”), continues its development programs.
Novo Nordisk is using our Eligen® drug delivery technology in combination with its proprietary GLP-1 receptor agonists and insulins. During December 2010, the Company entered into a license agreement with Novo Nordisk to develop and commercialize oral formulations of Novo Nordisk’s insulins using Emisphere’s Eligen® Technology. This was the second license agreement between the two companies. The GLP-1 License Agreement, entered into in June 2008, and amended for the second time on April 26, 2013, provides for the development of oral formulations of GLP-1 receptor agonists, with a potential drug for the treatment of type 2 diabetes which recently completed a Phase II clinical trial. The Amendment provided for a payment of $10 million from Novo Nordisk to the Company as a prepayment of certain development milestone payments that would have otherwise become payable to the Company under the Development Agreement in exchange for a reduction in the rate of potential future royalty payments as provided in the Development Agreement.
On February 20, 2015 Novo Nordisk highlighted positive Phase 2 data pertaining to OG217SC, the oral formulation of semaglutide, a long-acting human GLP-1 analogue that stimulates insulin and suppresses glucagon secretion in a glucose-dependent manner. OG217SC is provided in a tablet formulation with the absorption-enhancing excipient, SNAC. SNAC is an Eligen® Carrier. Novo Nordisk announced that it has successfully completed the phase 2 trial for OG217SC, investigating dose range, escalation, efficacy and safety of once-daily oral semaglutide compared with oral placebo or once-weekly subcutaneously administered semaglutide in around 600 people with type 2 diabetes treated for 26 weeks. Based on these results, Novo Nordisk announced that it will initiate consultations with regulatory authorities subsequent to which a decision of whether to progress OG217SC into phase 3 development will be made.
Under its GLP-1 License Agreement, Novo Nordisk is working to develop and commercialize oral formulations of its proprietary GLP-1 receptor agonists in combination with Emisphere carriers. Under the GLP-1 License Agreement, Emisphere could receive additional contingent product development and sales milestone payments and would also be entitled to receive royalties in the event Novo Nordisk commercializes products developed under such Agreement. Under the GLP-1 License Agreement, Novo Nordisk is responsible for the development and commercialization of the products.
We continue to assess therapeutic molecules for their potential compatibility with our technology and market need. Our intent is to continue to expand our pipeline with product candidates that demonstrate significant opportunities for growth. Our focus is on molecules that meet the criteria for success based on our increased understanding of our Eligen® Technology. Depending on the molecule, market potential and interest, we intend to pursue potential product development opportunities through development alliances or internal development.
We have collaborated with Novartis in connection with the development and testing of oral formulations of several drug candidates. Novartis has the right to evaluate the feasibility of using Emisphere’s Eligen®
5
Table of Contents
Technology with two new compounds to assess the potential for new product development opportunities. Novartis is considering its options accordingly. If Novartis chooses to develop oral formulations of these new compounds using the Eligen® Technology, the parties will negotiate additional agreements. In that case, Emisphere could be entitled to receive development milestone and royalty payments in connection with the development and commercialization of these potentially new products.
Our other product candidates in development are in earlier or preclinical research phases, and we continue to assess them for their compatibility with our technology and market need. Our intent is to seek partnerships with pharmaceutical and biotechnology companies for certain of these products as we continue to expand our pipeline with product candidates that demonstrate significant opportunities for growth. Our focus is on molecules that meet the criteria for success based on our increased understanding of our Eligen® Technology and prescription medical foods. Our preclinical programs focus on the development of oral formulations of potentially new treatments for diabetes and products in the areas of cardiovascular, appetite suppression and pain and on the development and potential expansion of nutritional supplement products.
We recognize, however, that further development, exploration and commercialization of our technology entails substantial risk and requires significant operational expenditures. We continue to refocus our efforts on strategic development initiatives to reduce non-strategic spending aggressively, and seek to obtain the funding necessary to implement our new corporate strategy. There can be no assurances, however, that the Company will be able to secure adequate funding to meet its current obligations and successfully pursue its strategic direction. Furthermore, despite our optimism regarding the Eligen® Technology and the commercialization of oral Eligen B12™ Rx, even in the event that the Company is adequately funded, there is no guarantee that any of our products or product candidates will perform as hoped or that such products can be successfully commercialized. For further discussion, see Part II, Item 1A “Risk Factors.”
Overall Product Pipeline
Substantial efforts and resources have been devoted to understanding the Eligen® Technology and establishing a product development pipeline that incorporates this technology with selected molecules. Our core business strategy had been to develop oral forms of drugs or nutrients that are not currently available or have poor bioavailability in oral form by combining the Eligen® Technology to those drugs or nutrients, and to commercialize the Company’s Oral Eligen B12™ Rx product. Emisphere’s product pipeline includes prescription drugs and medical food product candidates in varying stages of development. Novo Nordisk recently completed Phase 2 Testing of OG217SC with Emisphere’s SNAC carrier. Additionally, Emisphere has a number of pre-clinical (research stage) projects, which we are pursuing on our own and with partners. We continue to assess therapeutic molecules for their potential compatibility with our technology and market need. Our intent is to continue to expand our pipeline with product candidates that demonstrate significant opportunities for growth. Our focus is on molecules that meet the criteria for success based on our increased understanding of our Eligen® Technology. Depending on the molecule, market potential and interest, we intend to pursue potential product development opportunities through development alliances or internal development.
Vitamin B12
The Company has developed an oral formulation of Eligen B12™ Rx (1000 mcg) which is marketed as a medical food for use by B12 deficient individuals. During the fourth quarter 2010, the Company completed a clinical trial which demonstrated that both oral Eligen B12™ Rx (1000 mcg) and injectable B12 (current standard of care) can efficiently and quickly restore normal Vitamin B12 levels in deficient individuals. The manuscript summarizing the results from that clinical trial has been published in the July 2011 edition of the journal Clinical Therapeutics (Volume 33, pages 934 — 945). We also conducted market research to help assess the potential commercial opportunity for our Eligen B12™ Rx (1000 mcg) product candidate. On August 5, 2011, we received notice from the United States Patent Office that the U.S. patent application directed to the oral Eligen B12™ Rx formulation (US Patent 8,022,048) was allowed. This new patent provides intellectual property protection for Eligen B12™ Rx through approximately October 2029.
On March 2, 2015, the Company offered oral Eligen B12™ Rx for commercial sale in major markets throughout the United States. We continue to evaluate the results of our clinical trials and market research and
6
Table of Contents
are exploring alternative development options with the purpose of maximizing the commercial and health benefits potential of our Eligen B12™ Rx (1000 mcg.) product.
Vitamin B12 is an important nutrient that is poorly absorbed in the oral form. In most healthy people, Vitamin B12 is absorbed in a receptor-mediated pathway in the presence of an Intrinsic Factor. A large number of people take oral B12 supplements, many in megadoses, and by injection. Currently, it is estimated that at least five million people in the U.S. are taking 40 million injections of Vitamin B12 per year to treat a variety of debilitating medical conditions. Another estimated five million people are consuming more than 600 million tablets of Vitamin B12 orally. The international market is larger than the U.S. market. Many B12 deficient patients suffer from pernicious anemia and neurological disorders and many of them are infirm or elderly. Vitamin B12 deficiency can cause severe and irreversible damage, especially to the brain and nervous system. At levels only slightly lower than normal, a variety of symptoms such as fatigue, depression, and poor memory may occur.
The data from our first pharmacokinetic study of our new Vitamin B12 formulation showed mean Vitamin B12 peak blood levels were more than 10 times higher for the Eligen B12™ Rx 5mg formulation than for the 5mg commercial formulation. The mean time to reach peak concentration (T max) was reduced by over 90%, to 0.5 hours for the Eligen B12™ Rx 5mg from 6.8 hours for the commercial 5mg product. Improvement in bioavailability, the fraction of an administered dose of unchanged drug that reaches systemic circulation, was approximately 240%, with absorption time at 30 minutes and a mean bioavailability of 5%. The study was conducted with a single administration of Eligen® B12. There were no reported adverse reactions, and Eligen B12™ Rx was well-tolerated.
In May 2009, the Company was informed by an independent expert panel of scientists that its SNAC carrier had been provisionally designated as GRAS (generally recognized as safe) for its intended application in combination with nutrients added to food and dietary supplements. Following a comprehensive evaluation of research and toxicology data, Emisphere’s SNAC was found to be safe at a dosage up to 250 mg per day when used in combination with nutrients to improve their dietary availability. In July 2009, concurrent with the publication of two papers in the July/August issue of the peer reviewed journal, International Journal of Toxicology, which describes the toxicology of its SNAC carrier, SNAC achieved GRAS status for its intended use in combination with nutrients added to food and dietary supplements. The publication of those two papers in the International Journal of Toxicology was the final, necessary step in the process of obtaining GRAS status for its SNAC carrier. Since SNAC achieved GRAS status, it is exempt from pre-market approval for its intended use in combination with nutrients added to food and dietary supplements. This opens the way for the potential commercialization of the Eligen® Technology with other substances such as other vitamins, minerals and supplements.
We have obtained patents for the carrier we are using in the oral B12 formulation, the oral Eligen B12™ Rx formulation (as described above), and have filed applications covering the combination of the carrier and many other compounds.
Phase II Programs
Emisphere has one Eligen® carrier product that has recently completed Phase II clinical development and a number of pre-clinical (research stage) projects. Some of the pre-clinical projects are partnered and others were initiated by the Company.
For the treatment of diabetes, research using the Eligen® Technology and GLP-1 (Glucagon-Like Peptide-1), a potential treatment for Type 2 diabetes, is being conducted by Novo Nordisk. GLP-1 is a natural hormone involved in controlling blood sugar levels. It stimulates the release of insulin only when blood sugar levels become too high. GLP-1 secretion is often impaired in people with Type 2 diabetes. Emisphere had previously conducted extensive tests on native insulin and native GLP-1which demonstrated that both macromolecules can be effectively delivered using the Eligen® Technology. With the progress that has been made in the development of second generation proteins, we concluded that a more productive pathway is to move forward with GLP-1 analogs, an oral form of which might be used to treat Type 2 diabetes and related conditions.
7
Table of Contents
Our research indicated that the development of oral formulations of Novo Nordisk proprietary GLP-1 receptor agonists may represent an opportunity for Emisphere.
During December 2013, Novo Nordisk announced that it had initiated its first Phase II clinical trial with a long-acting oral GLP-1 analog (NN9924). Emisphere’s proprietary Eligen® Technology is used in the formulation of NN9924. There are many challenges in developing an oral formulation of GLP-1, in particular obtaining adequate bioavailability. NN9924 addresses some of these key challenges by utilizing Emisphere’s Eligen® Technology to facilitate absorption from the gastrointestinal tract.
The Phase II trial was designed to examine the dose range, escalation and efficacy of oral semaglutide dosed once daily in subjects with Type 2 diabetes. This Phase II trial followed the successful completion of Phase 1 with Oral GLP-1, OG217SC (NN9924). On February 20, 2015, Novo Nordisk reported positive Phase 2 data pertaining to OG217SC, the oral formulation of semaglutide, a long-acting human GLP-1 analogue that stimulates insulin and suppresses glucagon secretion in a glucose-dependent manner. OG217SC is provided in a tablet formulation with the absorption-enhancing excipient, SNAC. SNAC is an Eligen® Carrier. Novo Nordisk announced that it has successfully completed the phase 2 trial for OG217SC, investigating dose range, escalation, efficacy and safety of once-daily oral semaglutide compared with oral placebo or once-weekly subcutaneously administered semaglutide in around 600 people with type 2 diabetes treated for 26 weeks. Based on these results, Novo Nordisk announced that it will initiate consultations with regulatory authorities subsequent to which a decision of whether to progress OG217SC into phase 3 development will be made.
Under its GLP-1 License Agreement, Novo Nordisk is working to develop and commercialize oral formulations of its proprietary GLP-1 receptor agonists in combination with Emisphere carriers. Novo Nordisk is responsible for the development and commercialization of the products.
During January 2010, we announced that Novo Nordisk had initiated its first Phase I clinical trial with a long-acting oral GLP-1 analog (NN9924). The first Phase I trial investigated the safety, tolerability and bioavailability of NN9924 in healthy volunteers. The trial enrolled 155 individuals and was completed in May 2010. Novo Nordisk also conducted a multiple-dose Phase I trial. This multiple-dose trial investigated safety, tolerability, pharmacokinetics and pharmacodynamics of NN9924 in healthy male subjects. The trial enrolled 96 individuals and was completed in July 2011. In May of 2013, Novo Nordisk completed the last of five clinical pharmacology trials investigating the safety, tolerability as well as pharmacokinetic and pharmacodynamic profiles of oral administration of semaglutide tablets, OG217SC. The Phase I program in total comprised 400 healthy volunteers and 10 people with type 2 diabetes. In the trials, oral semaglutide treatment appeared to be safe and was well-tolerated. The most frequent reported adverse events were mild or moderate in severity and in line with observations from other GLP-1 class treatments with type 2 diabetes. In a 10-week multiple-dosing trial, oral administration of semaglutide was associated with a statistically significantly larger weight loss than placebo in healthy volunteers and people with Type 2 diabetes. Further, a statistically significant improvement in HbA1c was observed when compared to placebo treatment in the low number of people with Type 2 diabetes participating in the trial.
Preclinical Programs
Our other product candidates in development are in earlier or preclinical research phases, and we continue to assess them for their compatibility with our technology and market need. Some of these pre-clinical projects are partnered and others were initiated and are being pursued internally by the Company. Our intent is to seek partnerships with pharmaceutical and biotechnology companies for certain of these products as we continue to expand our pipeline with product candidates that demonstrate significant opportunities for growth. Our focus is on molecules that meet the criteria for success based on our increased understanding of our Eligen® Technology. Our preclinical programs focus on the development of oral formulations of potentially new treatments for diabetes and products in the areas of cardiovascular, appetite suppression and pain and on the development and potential expansion of nutritional supplement products.
8
Table of Contents
Overview of the Drug Delivery Industry
The drug delivery industry develops technologies for the improved administration of therapeutic molecules with the goal of expanding markets for existing products and extending drug franchises. Drug delivery companies also seek to develop products on their own that would be patent-protected by applying proprietary technologies to off-patent pharmaceutical products. Primarily, drug delivery technologies are focused on improving safety, efficacy, ease of patient use and/or patient compliance. Pharmaceutical and biotechnology companies consider improved drug delivery as a means of gaining competitive advantage over their peers.
Therapeutic macromolecules, of which proteins are the largest sub-class, are prime targets for the drug delivery industry for a number of reasons. Most therapeutic macromolecules must currently be administered by injection (most common) or other device such as an inhaler or nasal spray system. Many of these compounds address large markets for which there is an established medical need. These drugs are widely used, as physicians are familiar with them and accustomed to prescribing them. However, therapeutic macromolecules could be significantly enhanced through alternative delivery. These medicines are comprised of proteins and other large or highly charged molecules (carbohydrates, peptides, ribonucleic acids) that, if orally administered using traditional oral delivery methods, would degrade in the stomach or intestine before they are absorbed into the bloodstream. Also, these molecules are typically not absorbed following oral administration due to their poor permeability. Therefore, the vast majority are administered parenterally (other than orally or rectally). However, for many reasons, parenteral administration is undesirable, including patient discomfort, inconvenience and risk of infection. Poor patient acceptance of parenteral therapies can lead to medical complications. In addition, parenteral therapies can often require incremental costs associated with administration in hospitals or doctors’ offices.
Previously published research indicates that patient acceptance of and adherence to a dosing regimen is higher for orally delivered medications than it is for non-orally delivered medications. Our business strategy is partly based upon our belief that the development of an efficient and safe oral delivery system for therapeutic macromolecules represents a significant commercial opportunity. We believe that more patients will take orally delivered drugs more often, spurring market expansion.
Leading Current Approaches to Drug Delivery
Transdermal (via the skin) and “Needleless” Injection
The size of most macromolecules makes penetration into or through the skin inefficient or ineffective. Some peptides and proteins can be transported across the skin barrier into the bloodstream using high-pressure “needleless” injection devices. Needleless devices, which inject proteins through the skin into the body, have been in development for many years. We believe these devices have not been well accepted due to patient discomfort, relatively high cost, and the inconvenience of placing the drugs into the device.
Nasal (via the nose)
The nasal route (through the membranes of the nasal passage) of drug administration has been limited by low and variable bioavailability for proteins and peptides. As a result, penetration enhancers often are used with nasal delivery to increase bioavailability. These enhancers may cause local irritation to the nasal tissue and may result in safety concerns with long-term use. A limited number of peptides delivered nasally have been approved for marketing in the U.S., including MIACALCIN®, developed by Novartis as an osteoporosis therapy, a therapeutic area we have targeted.
Pulmonary (via the lung)
Pulmonary delivery (through the membranes of the lungs) of drugs is emerging as a delivery route for large molecules. Although local delivery of respiratory drugs to the lungs is common, the systemic delivery (i.e., delivery of the drugs to the peripheral vasculature) of macromolecular drugs is less common because it requires new formulations and delivery technologies to achieve efficient, safe and reproducible dosing. Only one protein using pulmonary delivery has been approved for marketing in the U.S., which is EXUBERA®, an insulin product
9
Table of Contents
developed by Pfizer and Nektar, as a diabetes therapy, a therapeutic area we have targeted. However after market acceptance of EXUBERA® was demonstrated to be limited, Pfizer withdrew from further commercialization of, and terminated its license with Nektar for, EXUBERA®.
Intraoral (via the membranes in the mouth)
Intraoral delivery is also emerging as a delivery route for large molecules. Buccal delivery (through the membrane of the cheek) and sublingual delivery (through the membrane under the tongue) are forms of intraoral delivery. Some Vitamin B12 manufacturers sell and distribute sublingual versions of their product.
Oral (via the mouth)
We believe that the oral method of administration is the most patient-friendly option, in that it offers convenience, is a familiar method of administration that enables increased compliance and, for some therapies, may be considered the most physiologically appropriate. We, and other drug delivery and pharmaceutical companies, have developed or are developing technologies for oral delivery of drugs. We believe that our Eligen® Technology provides an important competitive advantage in the oral route of administration because it does not alter the chemical composition of the therapeutic macromolecules. We have conducted over 140,000 human dosings and have witnessed no serious adverse events that can be attributed to the EMISPHERE® delivery agents dosed or the mechanism of action of the Eligen® Technology.
In general, we believe that oral administration will be preferred to other methods of administration. However, such preference may be offset by possible negative attributes of orally administered drugs such as the quantity or frequency of the dosage, the physical size of the capsule or tablet being swallowed or the taste. For example, in our previous Phase III trial with heparin as an oral liquid formulation, patient compliance was hindered by patients’ distaste for the liquid being administered. In addition, patients and the marketplace will more likely respond favorably to improvements in absorption, efficacy, safety, or other attributes of therapeutic molecules. It is possible that greater convenience alone may not lead to success.
Business Financing
Since our inception in 1986, we have generated significant losses from operations and we anticipate that we will continue to generate significant losses from operations for the foreseeable future. We have limited capital resources and operations to date have been funded with the proceeds from collaborative research agreements, public and private equity and debt financings and income earned on investments.
As of December 31, 2014, our accumulated deficit was approximately $514.1 million. Our loss from operations was $9.3 million, $7.6 million and $6.8 million for the years ended December 31, 2014, 2013 and 2012, respectively. Our net loss was $25.4 million, $20.9 million and $1.9 million for the years ended December 31, 2014, 2013 and 2012, respectively. Our net cash provided (outlays) from operations and capital expenditures were ($8.4) million, $3.1 million and ($3.0) million for the years ended December 31, 2014, 2013 and 2012, respectively. Net cash provided (outlays) include receipts of deferred revenue of $0.0 million, $10.0 million, and $0.02 million, for 2014, 2013, and 2012, respectively. Our stockholders’ deficit was $111.9 million and $86.8 million as of December 31, 2014 and 2013, respectively.
As of December 31, 2014, the Company’s obligations included approximately $40.9 million (face value) under its Convertible Notes (the “Convertible Notes”), approximately $8.3 million (face value) under a loan agreement entered into on August 20, 2014 (the “Loan Agreement”), approximately $1.9 million (face value) under its Bridge Notes (the “Bridge Notes”), and approximately $0.7 million (face value) under its Reimbursement Notes (the “Reimbursement Notes”). Additional borrowings under the Loan Agreement are subject to various sales, operating and manufacturing performance criteria.
Under the terms of the Loan Agreement, described in Note 7 to the Financial Statements, Emisphere may borrow, at specified times and based on the attainment of specified performance milestones, up to an aggregate of $20.0 million to finance the development, manufacturing, marketing and sales of its oral Eligen B12™ Rx Product. The new loan facility will mature on December 31, 2019, and bear interest at a rate of 13% per year.
10
Table of Contents
The first borrowing under the Loan Agreement occurred on August 20, 2014 in an original principal amount of $5.0 million, the second occurred on November 4, 2014, in an original principal amount of $3.0 million, and the third borrowing occurred on January 6, 2015, in an original principal amount of $5.0 million. Subject to achieving certain operational milestones relating to the timely manufacture and commencement of sales of Eligen B12™, of which there can be no assurance, the Company may request two additional borrowings under the Loan Agreement as follows: up to $5.0 million in the second quarter of 2015, and up to $2.0 million in the third quarter of 2015. In addition to funding available through the Loan Agreement, the Company received approximately $1.7 million and $0.3 million on January 14, 2014, and December 9, 2014, respectively, from the sale of unused net operating losses by participating in the Technology Business Tax Certificate Transfer Program, sponsored by the New Jersey Economic Development Authority.
We believe the Company’s current cash balance, in addition to cash available through additional borrowings under the Loan Agreement, assuming attainment of the milestones, will provide sufficient capital to support the commercial launch of oral Eligen B12™ Rx in the U.S. market and to continue operations through the end of 2015. The Company’s future capital requirements beyond 2015 and financial success depend largely on the commercial success of the oral Eligen B12™ Rx product and our ability to leverage existing as well as securing new partnering opportunities. There is no assurance that our plans will be successful. If we fail to raise sufficient capital from commercial operations or partnerships, we will need to seek capital from other sources. There can be no assurance that financing will be available on favorable terms or at all. If we fail to generate sufficient additional capital from sales of oral Eligen B12™ Rx product, or to obtain substantial cash inflows from existing or new partners or other sources prior to the end 2015, we could be forced to cease operations. Additionally, if additional capital is raised through the sale of equity or convertible debt securities, the issuance of such securities would result in dilution to our existing stockholders. These conditions raise substantial doubt about our ability to continue as a going concern. Consequently, the audit reports prepared by our independent registered public accounting firm relating to our financial statements for the years ended December 31, 2014, 2013 and 2012 include an explanatory paragraph expressing substantial doubt about our ability to continue as a going concern.
Furthermore, despite our optimism regarding the Eligen® Technology, even in the event that the Company is adequately funded, there is no guarantee that any of our products or product candidates will perform as hoped or that such products can be successfully commercialized. For further discussion, see Part I, Item 1A “Risk Factors.”
Collaborative and Commercial Agreements
We are a party to certain collaborative and commercial agreements with corporate partners to provide development and commercialization services relating to our products and technology. Our collaborative agreements are in the form of research and development collaborations and licensing agreements. Under these agreements, we have granted licenses or the rights to obtain licenses to our oral drug delivery technology. In return, we are entitled to receive certain payments upon the achievement of milestones and royalties on the sales of the products should a product ultimately be commercialized. We also are entitled to be reimbursed for certain research and development costs that we incur.
All of our collaborative agreements are subject to termination by our corporate partners, without significant financial penalty to them. Under the terms of these agreements, upon a termination we are entitled to reacquire all rights in our technology at no cost and are free to re-license the technology to other collaborative partners.
inVentiv
Contract Sales Force
During October 2013, we entered into a Master Services Agreement with inVentiv Health Inc. (the “inVentiv MSA”), which was subsequently amended, for, among other things, a contract sales force that will promote our oral Eligen™ B12 Rx product and a general manager who will coordinate the provision of sales force and other marketing services. The inVentiv MSA provides for a fixed monthly fee for the services of the general manager, and for monthly payments based on the number of individuals comprising the sales force, as well as fixed fees for the maintenance of the sales force. The agreement also provides for performance based fees
11
Table of Contents
based on the achievement by the sales force of predefined metrics. Either party may terminate the agreement with respect to the sales force or the general manager with 60 days-notice, provided that the actual termination date of the sales force may not be any earlier than March 2016.
Novo Nordisk A/S
GLP-1 Receptor Agonists Agreement
During June 2008, we entered into the GLP-1 License Agreement with Novo Nordisk, pursuant to which Novo Nordisk will develop and commercialize oral formulations of its proprietary GLP-1 receptor agonists in combination with Emisphere carriers. Under the GLP-1 License Agreement, Emisphere could receive more than $87 million in contingent product development and sales milestone payments, including a $10 million non-refundable license fee which was received in June 2008. To date, the Company has received a total of $23.6 million under the terms of this agreement. Emisphere would also be entitled to receive royalties on sales in the event Novo Nordisk commercializes products developed under such Agreement. Under the GLP-1 License Agreement, Novo Nordisk is responsible for the development and commercialization of the products. See “Phase II Program” above for a description of development activity conducted in connection with the GLP-1 License Agreement, and certain payments made to Emisphere as a result thereof. The agreement remains in effect, on a country-by-country basis, for the longer of 10 years from the date of first sale of a licensed product in such country, or the date of expiration of the last-to-expire patent covered by the agreement in such country, after which time Novo Nordisk will have a fully paid, exclusive license to the licensed product. Novo Nordisk may terminate this agreement with 90 days prior notice. We may terminate this agreement in the event that Novo Nordisk challenges the validity of any licensed patent under the agreement, but only with respect to the patents belonging to the patent family of the challenged patent. Either party may also terminate the agreement upon the other party’s material breach, if not cured within a specified period of time. Upon a termination of the agreement by Emisphere for Novo Nordisk’s breach, all intellectual property rights conveyed under the agreement shall revert back to us; upon a termination by Novo Nordisk for our breach, the licenses granted under the agreement shall remain in effect, subject to Novo Nordisk’s payment obligations under the agreement.
On April 26, 2013, the Company entered into an Amendment No. 2 (the “Amendment”) to a Development and License Agreement, The Amendment provides, among other things, for a payment of $10 million from Novo Nordisk to the Company as a prepayment for the achievement of certain development milestones that would have otherwise become payable to the Company under the Development Agreement in exchange for a reduction in the rate of potential future royalty payments as provided in the Development Agreement. The $10 million payment from Novo Nordisk was received by the Company on May 6, 2013, and recorded as deferred revenue.
During December 2013, Novo Nordisk announced that it had initiated its first Phase II clinical trial with a long-acting oral GLP-1 analog (NN9924). Emisphere’s proprietary Eligen® Technology is used in the formulation of NN9924. The achievement of this milestone would have triggered a milestone payment to Emisphere, but, instead, that payment was part of $10 million payment received from Novo Nordisk on April 26, 2013. There are many challenges in developing an oral formulation of GLP-1, in particular obtaining adequate bioavailability. NN9924 addresses some of these key challenges by utilizing Emisphere’s Eligen® Technology to facilitate absorption from the gastrointestinal tract.
On February 20, 2015 Novo Nordisk announced positive Phase 2 data pertaining to OG217SC, the oral formulation of semaglutide, a long-acting human GLP-1 analogue that stimulates insulin and suppresses glucagon secretion in a glucose-dependent manner. OG217SC is provided in a tablet formulation with the absorption-enhancing excipient, SNAC. SNAC is an Eligen® Carrier. Novo Nordisk announced that it has successfully completed the phase 2 trial for OG217SC, investigating dose range, escalation, efficacy and safety of once-daily oral semaglutide compared with oral placebo or once-weekly subcutaneously administered semaglutide in around 600 people with type 2 diabetes treated for 26 weeks. Based on these results, Novo Nordisk announced that it will initiate consultations with regulatory authorities subsequent to which a decision of whether to progress OG217SC into phase 3 development will be made.
Under its GLP-1 License Agreement, Novo Nordisk is working to develop and commercialize oral formulations of its proprietary GLP-1 receptor agonists in combination with Emisphere carriers. Under the
12
Table of Contents
GLP-1 License Agreement, Emisphere could receive additional contingent product development and sales milestone payments and would also be entitled to receive royalties in the event Novo Nordisk commercializes products developed under such Agreement. Under the GLP-1 License Agreement, Novo Nordisk is responsible for the development and commercialization of the products.
The Phase II trial was designed to examine the dose range, escalation and efficacy of oral semaglutide dosed once daily over 26 weeks in subjects with Type 2 diabetes. Phase I development successfully completed with Oral GLP-1, OG217SC (NN9924). During January 2010, we announced that Novo Nordisk had initiated its first Phase I clinical trial with a long-acting oral GLP-1 analog (NN9924). This milestone released a $2 million payment to Emisphere. The first Phase I Trial investigated the safety, tolerability and bioavailability of NN9924 in healthy volunteers. The trial enrolled 155 individuals and was completed in May 2010. Novo Nordisk also conducted a multiple-dose Phase I trial. This multiple-dose trial investigated safety, tolerability, pharmacokinetics and pharmacodynamics of NN9924 in healthy male subjects. The trial enrolled 96 individuals and was completed in July 2011. In May of 2013, Novo Nordisk completed the last of five clinical pharmacology trials investigating the safety, tolerability as well as pharmacokinetic and pharmacodynamic profiles of oral administration of semaglutide tablets, OG217SC. The Phase I program in total comprised 400 healthy volunteers and 10 people with Type 2 diabetes. In the trials, oral semaglutide treatment appeared to be safe and was well-tolerated. The most frequent reported adverse events were mild or moderate in severity and in line with observations from other GLP-1 class treatments with Type 2 diabetes. In a 10-week multiple-dosing trial, oral administration of semaglutide was associated with a statistically significantly larger weight loss than placebo in healthy volunteers and people with Type 2 diabetes. Further, a statistically significant improvement in HbA1c was observed when compared to placebo treatment in the low number of people with Type 2 diabetes participating in the trial.
Insulins License Agreement
During December 2010, the Company entered into an exclusive license agreement with Novo Nordisk to develop and commercialize oral formulations of Novo Nordisk’s insulins using Emisphere’s Eligen® Technology (the “Insulins License Agreement”). The Insulins License Agreement includes $57.5 million in potential product development and sales milestone payments to Emisphere, of which $5 million was paid upon signing, as well as royalties on sales. The agreement remains in effect, on a country-by-country basis, for the longer of 10 years from the date of first sale of a licensed product in such country, or the date of expiration of the last-to-expire patent covered by the agreement in such country. Novo Nordisk may terminate this agreement with 90 days prior notice. We may terminate this agreement in the event that Novo Nordisk challenges the validity of any licensed patent under the agreement, but only with respect to the patents belonging to the patent family of the challenged patent. Either party may also terminate the agreement upon the other party’s material breach, if not cured within a specified period of time. Upon a termination of the agreement by Emisphere for Novo Nordisk’s breach, all intellectual property rights conveyed under the agreement shall revert back to us; upon a termination by Novo Nordisk for our breach, the licenses granted under the agreement shall remain in effect, subject to Novo Nordisk’s payment obligations under the agreement.
This extended partnership with Novo Nordisk has the potential to offer significant new solutions to millions of people with diabetes worldwide and it also serves to further validate our Eligen® Technology.
Novartis Pharma AG
Discontinued Oral Salmon Calcitonin Program for Osteoporosis and Osteoarthritis
We have collaborated with Novartis in connection with the development and testing of oral formulations of salmon calcitonin (“sCT”) to treat osteoarthritis and osteoporosis (the “Salmon Calcitonin Program”). We entered into a Research Collaboration and Option Agreement, dated as of December 3, 1997, as amended on October 20, 2000 (the “Salmon Calcitonin Option Agreement”) with Novartis to develop an oral form of sCT, which is a hormone that inhibits the bone-tissue resorbing activity of specialized bone cells called osteoclasts, enabling the bone to retain more of its mass and functionality. Pursuant to the Salmon Calcitonin Option Agreement, the Company granted Novartis the option to acquire from the Company a license to develop and commercialize oral
13
Table of Contents
sCT utilizing Emisphere’s Eligen® Technology and the right to commence research collaboration with the Company with respect to a second compound, in exchange for certain option exercise payments. Novartis also agreed to reimburse the Company with respect to certain research and development costs incurred by the Company in connection with the sCT Program. Furthermore, under the Salmon Calcitonin Option Agreement, the Company is obligated to help to manage this program through a joint “steering committee” with Novartis. The Salmon Calcitonin Option Agreement expires upon the expiration of the last to expire of the patents of the Company described therein, subject to certain early termination rights, including termination by either party for material breach of the other party and termination by Novartis in favor of a license executed thereunder.
In May 2007, Novartis and Nordic Bioscience notified the Company that they were initiating a Phase III clinical study of SMC021 for the treatment of osteoarthritis (“OA”) using the Company’s Eligen® Technology. A second Phase III study of SMC021 for the treatment of OA, designed to meet FDA requirements for U.S. registration, was initiated by Novartis and Nordic Bioscience in October 2008.
On December 14, 2011, the Company announced that Novartis had informed the Company that it would not pursue further clinical development of the investigational drug SMC021 (oral calcitonin) as a treatment option in osteoarthritis and for post-menopausal osteoporosis and that it would not seek regulatory submission for SMC021in either indication. Novartis advised the Company that its decision to stop the clinical program of SMC021 in both indications was based on analysis and evaluation of data from three Phase III clinical trials (two in osteoarthritis and one in osteoporosis) conducted by Nordic Bioscience showed that SMC021 failed to meet key efficacy endpoints in all three trials, despite displaying a favorable safety profile.
Although Novartis has not informed Emisphere of its intention to terminate the Salmon Calcitonin Option Agreement and the Salmon Calcitonin License Agreement, in the event that Novartis determines to terminate these agreements, we will reacquire the rights to our technology licensed to Novartis thereunder.
Oral PTH-1-34 Program
We have collaborated with Novartis in connection with the development and testing of oral formulations of PTH-1-34 (“PTH”) to treat osteoarthritis and osteoporosis (the “PTH Program”). On December 1, 2004, we entered into a Research Collaboration Option and License Agreement with Novartis whereby Novartis obtained an option to license our existing technology to develop oral forms of PTH 1-34 (the “PTH Option Agreement”). On March 7, 2006, Novartis exercised its option to the license. During April 2010, we announced that Novartis initiated a second Phase I trial for an oral PTH-1-34 which uses Emisphere’s Eligen® Technology, and was in development for the treatment of postmenopausal osteoporosis. On June 17, 2011, the Company announced that Novartis informed Emisphere of the results of its recently completed Proof of Concept study for an oral PTH1-34 using Emisphere’s Eligen® Technology in post-menopausal women with osteoporosis or osteopenia. Novartis informed Emisphere that, although the study confirmed that oral PTH1-34 was both safe and well-tolerated, several clinical endpoints were not met. Based on the data analyzed, Novartis has terminated the study and anticipates no further work on the oral formulation of PTH1-34. The Company has requested additional information from Novartis in order to further analyze and evaluate the results of this trial. Although Novartis has not informed Emisphere of its intention to terminate the PTH Option Agreement in accordance with relevant terms thereunder, Emisphere would reacquire the rights to develop and/or commercialize the product should Novartis so terminate the Agreement.
Terminated Oral Recombinant Human Growth Hormone Program
From 1998 through August 2003, we developed oral rhGH in collaboration with Eli Lilly and Company (“Lilly”). As of August 2003, Lilly returned to us all rights to the oral rhGH program pursuant to the terms of our license agreement. On September 23, 2004, we announced a new partnership with Novartis to develop our oral rhGH program (the “Oral HGH Program”). We entered into a Research and Collaboration Agreement with Novartis, dated September 22, 2004, whereby Novartis licensed the right to develop a convenient oral human growth hormone product using the Eligen® Technology (the “Oral HGH Agreement”). Under this agreement, Novartis had an exclusive worldwide license to develop, make, have made, use and sell products developed
14
Table of Contents
under this program. On May 1, 2006, we announced that Novartis initiated the development of an oral rhGH product using Emisphere’s Eligen® Technology.
On August 3, 2011, the Company received notification from Novartis that Novartis terminated the Oral HGH Agreement. In connection with this termination, Emisphere has reacquired the rights to develop and/or commercialize the product. Emisphere has requested that Novartis provide the data generated from the collaboration that would be necessary for the Company to continue to develop and commercialize an oral human growth hormone product using the Eligen® Technology. The Company did not incurred any penalties in connection with the termination of the Oral HGH Agreement.
Research and Development Costs
We have devoted substantially all of our efforts and resources to research and development conducted on our own behalf (self-funded) and in collaborations with corporate partners (partnered). Generally, research and development expenditures are allocated to specific research projects. Due to various uncertainties and risks, including those described in Part 1, Item 1A. “Risk Factors” below, relating to the progress of our product candidates through development stages, clinical trials, regulatory approval, commercialization and market acceptance, it is not possible to accurately predict future spending or time to completion by project or project category.
The following table summarizes research and development spending to date by project category:
| Year Ended December 31, | Cumulative Spending 2014(1) |
|||||||||||||||
| 2014 | 2013 | 2012 | ||||||||||||||
| (In thousands) | ||||||||||||||||
| Research(2) |
$ | 492 | $ | 51 | $ | 81 | $ | 52,681 | ||||||||
| Feasibility projects |
||||||||||||||||
| Self-funded |
201 | 230 | 679 | 14,262 | ||||||||||||
| Partnered |
— | 8 | 17 | 4,324 | ||||||||||||
| Development projects |
||||||||||||||||
| Oral heparin (self-funded) |
— | — | 1 | 99,592 | ||||||||||||
| Oral insulin (self-funded) |
— | — | 4 | 21,292 | ||||||||||||
| Partnered |
— | — | — | 12,157 | ||||||||||||
| Other(3) |
435 | 547 | 1,085 | 107,992 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total all projects |
$ | 1,128 | $ | 836 | $ | 1,867 | $ | 312,300 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| (1) | Cumulative spending from August 1, 1995 through December 31, 2014. |
| (2) | Research is classified as resources expended to expand the ability to create new carriers, to ascertain the mechanisms of action of carriers, and to establish computer based modeling capabilities, prototype formulations, animal models, and in vitro testing capabilities. |
| (3) | Other includes indirect costs such as rent, utilities, training, standard supplies and management salaries and benefits. |
Patents and Other Forms of Intellectual Property
Our success depends, in part, on our ability to obtain patents, maintain trade secret protection, and operate without infringing the proprietary rights of others (please refer to Part I, Item 1A “Risk Factors” for further discussion of how our business will suffer if we cannot adequately protect our patent and proprietary rights”). We seek patent protection on various aspects of our proprietary chemical and pharmaceutical delivery technologies, including the delivery agent compounds and the structures which encompass Emisphere’s delivery agents, their method of preparation, the combination of our compounds with a pharmaceutical, and use of our compounds with therapeutic molecules to treat various disease states. We have patents and patent applications in the U.S. and
15
Table of Contents
certain foreign countries. As of March 1, 2013, Emisphere had been granted more than 110 U.S. patents and more than 200 foreign patents. Emisphere also has more than 50 pending U.S. patent applications as well as more than 200 counterpart applications pending in foreign countries.
We intend to file additional patent applications when appropriate and to aggressively prosecute, enforce, and defend our patents and other proprietary technology.
We have five trademarks registered with the U.S. Patent and Trademark Office. They include three registrations for Emisphere® in connection with drug delivery agents and research and development in the field of drug delivery systems, and two registrations for ELIGEN® in connection with drug delivery agents and research and development in the field of drug delivery systems.
We also rely on trade secrets, know-how, and continuing innovation in an effort to develop and maintain our competitive position. Patent law relating to the patentability and scope of claims in the biotechnology and pharmaceutical fields is evolving and our patent rights are subject to this additional uncertainty. Others may independently develop similar product candidates or technologies or, if patents are issued to us, design around any products or processes covered by our patents. We expect to continue, when appropriate, to file product and other patent applications with respect to our inventions. However, we may not file any such applications or, if filed, the patents may not be issued. Patents issued to or licensed by us may be infringed by the products or processes of others.
Defense and enforcement of our intellectual property rights can be expensive and time consuming, even if the outcome is favorable to us. It is possible that the patents issued to or licensed to us will be successfully challenged, that a court may find that we are infringing validly issued patents of third parties, or that we may have to alter or discontinue the development of our products or pay licensing fees to take into account patent rights of third parties.
The primary raw materials used in making the delivery agents for our product candidates are readily available in large quantities from multiple sources. In the past we manufactured delivery agents internally using our own facilities on a small scale for research and development purposes and for early stage clinical supplies. We believe that our manufacturing capabilities complied with the FDA’s current Good Manufacturing Practice (“GMP”).
Currently, EMISPHERE® delivery agents are manufactured by third parties in accordance with GMP regulations. We have identified other commercial manufacturers meeting the FDA’s GMP regulations that have the capability of producing EMISPHERE® delivery agents and we do not rely on any particular manufacturer to supply us with needed quantities.
Competition
Our success depends in part upon maintaining a competitive position in the development of product candidates and technologies in an evolving field in which developments are expected to continue at a rapid pace. We compete with other drug delivery, biotechnology and pharmaceutical companies, research organizations, individual scientists and non-profit organizations engaged in the development of alternative drug delivery technologies or new drug research and testing, and with entities developing new drugs that may be orally active. Our product candidates compete against alternative therapies or alternative delivery systems for each of the medical conditions our product candidates address, independent of the means of delivery. Many of our competitors have substantially greater research and development capabilities, experience, marketing, financial and managerial resources than we have. In many cases we rely on our development partners to develop and market our product candidates.
Oral Vitamin B12 Competition
Emisphere’s potential competition in the Vitamin B12 market will depend on the direction the company takes in the development and commercialization of the product. In the event that Emisphere pursues the nutritional supplements market, competition would include a number of companies selling generic Vitamin B12 in a variety of dosage strengths and methods of delivery (e.g., oral, transdermal, nasal, sublingual) many of which have substantial distribution and marketing capabilities that exceed and will likely continue to exceed our
16
Table of Contents
own. In addition, our competition is likely to include many sellers, distributors, and others who are in the business of marketing, selling, and promoting multiple vitamins, vitamin-mineral, and specialized vitamin combinations. Many of these competitors are engaged in low cost, high volume operations that could provide substantial market barriers or other obstacles for a higher cost, potentially superior product that has no prior market history.
In order to successfully penetrate the Vitamin B12 medical food market, the Company will need to successfully demonstrate to physicians, nurse-practitioners and payers that an oral dose would be safe, efficacious, readily-accessible and improve compliance. These factors will likely require the Company to engage in a substantial educational and promotional product launch and a marketing outreach initiative, the time, cost, and outcome of which are uncertain.
Oral Diabetes Competition — Type 2 Diabetes
In diabetes, there are a number of unmet needs which amplify the need for further product development in the area. There are three main areas of drug therapy, oral anti-diabetes, insulin, and injectable in which companies are attempting to develop innovative products for the treatment of patients.
There are four leading classes for new product development in the area of diabetes. All four seek to take advantage of the potential to improve upon currently available products:
1. GLP-1 Agonists
2. Pulmonary Insulin
3. DPP-IV Inhibitors
4. PPAR modulators.
The objective of our collaboration with Novo Nordisk is to develop an orally available GLP-1 agonist for the treatment of Type 2 diabetes and potentially obesity. A product with the benefits of glucose control, promotion of weight loss, low risk of hypoglycemia, and other benefits is expected to significantly improve therapeutic options and can be expected to perform as well as or better than the existing competition.
Competition Summary
Although we believe that our oral formulations, if successful, will likely compete with well-established injectable versions of the same drugs, we believe that we will enjoy a competitive advantage because physicians and patients prefer orally delivered forms of products over injectable forms. Oral forms of products enable improved compliance, and for many programs, the oral form of products enable improved therapeutic regimens.
Government Regulation
Our operations and product candidates under development are subject to extensive regulation by the FDA, other governmental authorities in the U.S. and governmental authorities in other countries.
The duration of the governmental approval process for marketing new pharmaceutical substances, from the commencement of pre-clinical testing to receipt of governmental approval for marketing a new product, varies with the nature of the product and with the country in which such approval is sought. The approval process for new chemical entities could take eight to ten years or more. The process for reformulations of existing drugs is typically shorter, although a combination of an existing drug with a currently unapproved carrier could require extensive testing. In either case, the procedures required to obtain governmental approval to market new drug products will be costly and time-consuming to us, requiring rigorous testing of the new drug product. Even after such time and effort, regulatory approval may not be obtained for our products.
The steps required before we can market or ship a new human pharmaceutical product commercially in the U.S. include pre-clinical testing, the filing of an Investigational New Drug Application (“IND”), the conduct of clinical trials and the filing with the FDA of either a New Drug Application (“NDA”) for drugs or a Biologic License Application (“BLA”) for biologics.
17
Table of Contents
In order to conduct the clinical investigations necessary to obtain regulatory approval of marketing of new drugs in the U.S., we must file an IND with the FDA to permit the shipment and use of the drug for investigational purposes. The IND sets forth, in part, the results of pre-clinical (laboratory and animal) toxicology testing and the applicant’s initial Phase I plans for clinical (human) testing. Unless notified that testing may not begin, the clinical testing may commence 30 days after filing an IND.
Under FDA regulations, the clinical testing program required for marketing approval of a new drug typically involves three clinical phases. In Phase I, safety studies are generally conducted on normal, healthy human volunteers to determine the maximum dosages and side effects associated with increasing doses of the substance being tested. Phase II studies are conducted on small groups of patients afflicted with a specific disease to gain preliminary evidence of efficacy, including the range of effective doses, and to determine common short-term side effects and risks associated with the substance being tested. Phase III involves large-scale trials conducted on disease-afflicted patients to provide statistically significant evidence of efficacy and safety and to provide an adequate basis for product labeling. Frequent reports are required in each phase and if unwarranted hazards to patients are found, the FDA may request modification or discontinuance of clinical testing until further studies have been conducted. The FDA may also require post-approval Phase IV testing either to meet FDA requirements for additional information as a condition of approval. Our drug product candidates are and will be subjected to each step of this lengthy process from conception to market and many of those candidates are still in the early phases of testing.
Once clinical testing has been completed pursuant to an IND, the applicant files an NDA or BLA with the FDA seeking approval for marketing the drug product. The FDA reviews the NDA or BLA to determine whether the drug is safe, effective, and adequately labeled, and whether the applicant can demonstrate proper and consistent manufacture of the drug. The time required for initial FDA action on an NDA or BLA is set on the basis of user fee goals; for most NDA or BLAs the action date is 10 months from receipt of the NDA or BLA at the FDA. The initial FDA action at the end of the review period may be approval or a request for additional information that will be needed for approval depending on the characteristics of the drug and whether the FDA has concerns with the evidence submitted. Once our product candidates reach this stage, we will be subjected to these additional costs of time and money.
The FDA has different regulations and processes governing and regulating food products, including vitamin supplements and nutraceuticals. These products are variously referred to as “dietary supplements”, “food additives”, “dietary ingredients”, “medical foods”, and, most broadly, “food”. These foods products do not require the IND, NDA or BLA process outlined above. Medical foods, which are defined under the FDA’s 1988 Orphan Drug Act Amendments and are subject to the general food and safety labeling requirements of the Federal Food, Drug, and Cosmetic Act, are specially formulated and intended for the dietary management of a disease that has distinctive nutritional needs that cannot be met by normal diet alone. Medical foods are distinct from the broader category of foods for special dietary use and from traditional foods that bear a health claim. In order to be considered a medical food the product must, at a minimum:
| Ÿ | be a food for oral ingestion or tube feeding (nasogastric tube); |
| Ÿ | be labeled for the dietary management of a specific medical disorder, disease or condition for which there are distinctive nutritional requirements; and |
| Ÿ | be intended to be used under medical supervision. Medical foods require a prescription from a physician. |
The facilities of each company involved in the commercial manufacturing, processing, testing, control and labeling of pharmaceutical products must be registered with and approved by the FDA. Continued registration requires compliance with GMP regulations and the FDA conducts periodic establishment inspections to confirm continued compliance with its regulations. We are subject to various federal, state and local laws, regulations and recommendations relating to such matters as laboratory and manufacturing practices and the use, handling and disposal of hazardous or potentially hazardous substances used in connection with our research and development work.
While we do not currently manufacture any commercial products ourselves, if we did, we would bear additional cost of FDA compliance.
18
Table of Contents
In addition, the distribution of prescription pharmaceutical products in the United States is subject to the Prescription Drug Marketing Act, or PDMA, which regulates the distribution and recordkeeping requirements for drugs and drug samples at the federal level, and sets minimum standards for the registration and regulation of drug distributors by the states. Both the PDMA and state laws limit the distribution of prescription pharmaceutical product samples and impose requirements to ensure accountability in distribution.
We are also subject to various federal and state laws pertaining to health care “fraud and abuse” issues, including anti-kickback laws and false claims laws. Anti-kickback laws make it illegal for a prescription drug manufacturer to solicit, offer, receive, or pay any remuneration in exchange for, or to induce, the referral of business, including the purchase or prescribing of a particular drug. False claims laws prohibit anyone from knowingly and willfully presenting, or causing to be presented for payment to the United States government, including Medicare and Medicaid, claims for reimbursed drugs or services that are false or fraudulent, claims for items or services not provided as claimed, or claims for medically unnecessary items or services. We have adopted the Pharmaceutical Research and Manufacturers of America Code on Interactions with Healthcare Professionals, which is a voluntary industry code developed to establish standards for interactions with and communications to healthcare professionals and we have adopted processes that we believe enhance compliance with this code and applicable federal and state laws.
Employees
As of December 31, 2014, we had 10 employees, one of whom is engaged in scientific research and technical functions and nine of whom are performing product management, sales and market planning, logistics and supply chain planning, accounting, information technology, engineering, facilities maintenance, legal and regulatory and administrative functions. Our scientific employee holds a Ph.D. degree. We believe our relations with our employees are good.
Corporate Information
We were incorporated as Clinical Technologies Associates, Inc. under the laws of Delaware in 1986 and changed our name to Emisphere Technologies, Inc. in 1991. Our principal executive offices are located at 4 Becker Farm Road, Suite 103, Roseland, NJ 07068 and our telephone number is (973) 532-8000.
Available Information
Emisphere files annual, quarterly, and current reports, proxy statements, and other documents with the Securities and Exchange Commission, (the “SEC”) under the Securities Exchange Act of 1934 as amended (the “Exchange Act”). The public may read and copy any materials that we file with the SEC at the SEC’s Public Reference Room at 100 F Street, NE, Washington, D.C. 20549. The public may obtain information on the operation of the Public Reference Room by calling the SEC at 1-800-SEC-0330. Also, the SEC maintains an internet website that contains reports, proxy and information statements, and other information regarding issuers, including Emisphere, that file electronically with the SEC. The public can obtain any documents that Emisphere files with the SEC at www.sec.gov.
We also make available free of charge on or through our internet website (www.emisphere.com) our Annual Report on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K, Section 16 filings, and, if applicable, amendments to those reports filed or furnished pursuant to Section 13(a) or Section 16 of the Exchange Act as soon as reasonably practicable after we or the reporting person electronically files such material with, or furnishes it to, the SEC. Our internet website and the information contained therein or connected thereto are not intended to be incorporated into the Annual Report or this Form 10-K.
Our Board of Directors has adopted a Code of Business Conduct and Ethics which is posted on our website at http://ir.emisphere.com/documentdisplay.cfm?DocumentID=4947.
19
Table of Contents
| ITEM 1A. | RISK FACTORS |
An investment in our common stock involves a number of very significant risks. You should carefully consider the following risks and uncertainties in addition to other information in this report in evaluating our company and its business before purchasing shares of our company’s common stock. Our business, operating results and financial condition could be seriously harmed due to any of the following risks. You could lose all or part of your investment due to any of these risks.
Risks Related to the Company
We have limited capital resources and significant commitments and obligations.
We have limited capital resources and operations to date have been funded with the proceeds from collaborative research agreements, public and private equity and debt financings and income earned on investments. We anticipate that we will continue to generate significant losses from operations for the foreseeable future, and that our business will require substantial additional investment that we have not yet secured. As such, we anticipate that our existing capital resources will enable us to continue operations through approximately December 2015, or earlier if unforeseen events or circumstances arise that negatively affect our liquidity.
As of December 31, 2014, our accumulated deficit was approximately $514.1 million. Our loss from operations was $9.3 million, $7.6 million and $6.8 million for the years ended December 31, 2014, 2013 and 2012, respectively. Our net loss was $25.4 million, $20.9 million and $1.9 million for the years ended December 31, 2014, 2013 and 2012, respectively. Our net cash provided (outlays) from operations and capital expenditures were ($8.4) million, $2.9 million and ($3.0) million for the years ended December 31, 2014, 2013 and 2012, respectively. Net cash provided (outlays) include receipts of deferred revenue of $0.0 million, $10.0 million, and $0.02 million, for 2014, 2013, and 2012, respectively. Our stockholders’ deficit was $111.9 million and $86.8 million as of December 31, 2014 and 2013, respectively.
As of December 31, 2014, the Company’s obligations included approximately $40.9 million (face value) under its Convertible Notes (the “Convertible Notes”), approximately $8.3 million (face value) under a loan agreement entered into on August 20, 2014 (the “Loan Agreement”), approximately $1.9 million (face value) under its Bridge Notes (the “Bridge Notes”), and approximately $0.7 million (face value) under its Reimbursement Notes (the “Reimbursement Notes”). The Convertible Notes and the Loan Agreement are subject to various sales, operating and manufacturing performance criteria.
Under the terms of the Loan Agreement, described in Note 7 to the Financial Statements, Emisphere may borrow, at specified times and based on the attainment of specified performance milestones, up to an aggregate of $20.0 million to finance the development, manufacturing, marketing and sales of its oral Eligen B12™ Rx Product. The new loan facility will mature on December 31, 2019, and bear interest at a rate of 13% per year. The first borrowing under the Loan Agreement occurred on August 20, 2014 in an original principal amount of $5.0 million, the second occurred on November 4, 2014 in an original principal amount of $3.0 million, and the third borrowing occurred on January 6, 2015, in an original principal amount of $5.0 million. Subject to achieving certain operational milestones relating to the timely manufacture and commencement of sales of Eligen B12™, of which there can be no assurance, the Company may request two additional borrowings under the Loan Agreement as follows: up to $5.0 million in the second quarter of 2015, and up to $2.0 million in the third quarter of 2015.
We believe the Company’s current cash balance in addition to cash available through additional borrowings under the Loan Agreement, assuming attainment of the milestones, will provide sufficient capital to support the commercial launch of oral Eligen B12™ Rx in the U.S. market and to continue operations through the end of 2015. The Company’s future capital requirements beyond 2015 and future financial success depend largely on the commercial success of the oral Eligen B12™ Rx product and our ability to leverage existing as well as securing new partnering opportunities. There is no assurance that our plans will be successful. If we fail to raise sufficient capital from commercial operations or partnerships, we will need to seek capital from other sources. We cannot assure you that financing will be available on favorable terms or at all. If we fail to generate sufficient
20
Table of Contents
additional capital from sales of oral Eligen B12™ Rx product, or to obtain substantial cash inflows from existing or new partners or other sources prior to the end 2015, we could be forced to cease operations. Furthermore, despite our optimism regarding the Eligen® Technology, even in the event that the Company is adequately funded, there is no guarantee that any of our products or product candidates will perform as hoped or that such products can be successfully commercialized. Additionally, if additional capital is raised through the sale of equity or convertible debt securities, the issuance of such securities would result in dilution to our existing stockholders. These conditions raise substantial doubt about our ability to continue as a going concern. Consequently, the audit reports prepared by our independent registered public accounting firm relating to our financial statements for the years ended December 31, 2014, 2013 and 2012 include an explanatory paragraph expressing substantial doubt about our ability to continue as a going concern.
We have a history of operating losses and we may never achieve profitability.
As of December 31, 2014, we had approximately $3.7 million in cash and cash equivalents, approximately $1.7 million in working capital deficiency, a stockholders’ deficit of approximately $111.9 million and an accumulated deficit of approximately $514.1 million. Our operating loss for the twelve months ended December 31, 2014 was approximately $9.3 million. Since our inception in 1986, we have generated significant losses from operations. We anticipate that we will continue to generate significant losses from operations for the foreseeable future, and that our business will require substantial additional investment that we have not yet secured. These conditions raise substantial doubt about our ability to continue as a going concern.
We anticipate that our existing capital resources will enable us to continue operations through approximately December 2015, or earlier if unforeseen events or circumstances arise that negatively affect our liquidity. While our plan is to raise capital and/or to pursue product partnering opportunities to address our capital deficiencies, we cannot be sure how much we will need to spend in order to develop, market, and manufacture new products and technologies in the future. We expect to continue to spend substantial amounts on research and development, including amounts spent on conducting clinical trials for our product candidates. Further, we will not have sufficient resources to develop fully any new products or technologies unless we are able to raise substantial additional financing or to secure funds from new or existing partners. We cannot assure you that financing will be available when needed, or on favorable terms or at all. The current economic environment combined with a number of other factors pose additional challenges to the Company in securing adequate financing under acceptable terms. If additional capital is raised through the sale of equity or convertible debt securities, the issuance of such securities would result in dilution to our existing stockholders.
Additionally, these conditions may increase the costs to raise capital. Our failure to raise capital when needed would adversely affect our business, financial condition, and results of operations, and could force us to reduce or discontinue operations.
We are highly dependent upon the commercial success of oral Eligen™ B12 and cannot be sure that our plans will be successful.
We expended substantial resources on the development of an oral dosage form of Vitamin B12 which can be marketed as a medical food for use by B12 deficient individuals. We completed a clinical trial which demonstrated that both oral Eligen B12™ (1000 mcg) and injectable B12 (current standard of care) can efficiently and quickly restore normal Vitamin B12 levels in deficient individuals. During November 2009, the Company launched its first commercially available product, oral Eligen B12™ (100 mcg), which had been specifically developed to help improve Vitamin B12 absorption and bioavailability with a patented formulation. During the third quarter 2010, we terminated our distributor agreement for the marketing, distribution and sale of oral Eligen B12™ (100mcg) with Quality Vitamins and Supplements, Inc. to allow us to focus on the development of a higher dose, oral formulation of Eligen B12™ (1000 mcg) to be offered for B12 deficient patients. Our inability to meet our targets for commercializing the B12 product could have a significant material adverse effect on our business.
Our ability to make additional borrowings or meet future payment obligations under the terms of the Loan Agreement; fund continuing operations and business expansion are highly dependent upon our ability to meet our
21
Table of Contents
commercial targets for the oral Eligen B12™ product in the U.S. and global markets during 2015 and in future years. We cannot assure you that we will succeed in these efforts as these involve activities (or portions of activities) that we have not previously completed. In addition, if we succeed in these activities, Vitamin B12 is available at reasonably low prices both in injections and tablet forms (as well as other forms) through a variety of distributors, sellers, and other sources. We have newly established commercial capabilities. Therefore, we would be entering a highly competitive market with an untested, newly-established commercial capability. This outline of risks involved in the commercialization of our B12 product candidate is not exhaustive, but illustrative. For example, it does not include additional competitive, intellectual property, commercial, product liability, and commercial risks involved in a launch of the B12 product candidate outside the U.S. or certain of such risks in the U.S.
We may not be able to meet covenants or financial obligations detailed in our Loan Agreement, Convertible Notes, Reimbursement Notes, and Bridge Notes issued to MHR in August 2014 (collectively, the “MHR Notes”), or the Royalty Agreement
If we fail to meet any of the terms or covenants contained in the Loan Agreement, Convertible Notes, Reimbursement Notes, and Bridge Notes issued to MHR in August 2014, or the Royalty Agreement it could result in an increase in the interest rate on the MHR Notes and/or accelerated maturity of the MHR Notes, which we might not be able to satisfy. The MHR Notes are secured by a first priority lien in favor of MHR on substantially all of our assets, and if we default on our obligations under the MHR Notes, MHR may elect to foreclose on such assets, in which event we would be required to cease operations.
We are highly dependent on third parties to manufacture, distribute, and sell our products.
We have hired expert commercial trade, professional sales, manufacturing, and logistics and customer services vendors to support the commercialization of our oral Eligen B12™ (1000mcg) product. The success of our commercial operations is dependent upon the ability of these vendors to provide a high level of service and support at an economical price. If we fail to attract and retain such professions or services at a reasonable price, or if third parties do not successfully carry out their contractual obligations, meet expected deadlines or conduct our activities in accordance with applicable regulatory requirements or our stated specifications, we may not be able to, or may be delayed in our efforts to, successfully execute upon our commercial strategy.
We are highly dependent upon collaborative partners to develop and commercialize compounds using our delivery agents.
A key part of our strategy is to form collaborations with pharmaceutical companies that will assist us in developing, testing, obtaining government approval for and commercializing oral forms of therapeutic macromolecules using the Eligen® Technology. We currently have collaborative agreements for candidates in clinical development with Novartis and Novo Nordisk, although Novartis has indicated that it has ceased work on all of the programs it had entered into with us.
We negotiate specific ownership rights with respect to the intellectual property developed as a result of the collaboration with each partner. While ownership rights vary from program to program, in general we retain ownership rights to developments relating to our carrier and the collaborator retains rights related to the drug product developed.
Despite our existing agreements, we cannot make any assurances that:
| Ÿ | we will be able to enter into additional collaborative arrangements to develop products utilizing our drug delivery technology; |
| Ÿ | any existing or future collaborative arrangements will be sustainable or successful; |
| Ÿ | the product candidates in collaborative arrangements will be further developed by partners in a timely fashion; |
| Ÿ | any collaborative partner will not infringe upon our intellectual property position in violation of the terms of the collaboration contract; or |
| Ÿ | milestones in collaborative agreements will be met and milestone payments will be received. |
22
Table of Contents
If we are unable to obtain development assistance and funds from other pharmaceutical companies to fund a portion of our product development costs and to commercialize our product candidates, we may be unable to issue equity to allow us to raise sufficient capital to fund clinical development of our product candidates. Lack of funding would cause us to delay, curtail, or stop clinical development of one or more of our projects. The determination of the specific project to curtail would depend upon the relative future economic value to us of each program.
Our collaborative partners control the clinical development of the drug candidates and may terminate their efforts at will.
Novo Nordisk controls the clinical development of oral GLP-1 analogs. Novartis and Novo Nordisk control the decision-making for the design and timing of their clinical studies.
Moreover, the agreements with Novartis and Novo Nordisk provide that they may terminate their programs at will for any reason and without any financial penalty or requirement to fund any further clinical studies. Novartis has discontinued all active clinical programs with us, and it is likely that it will terminate all remaining collaboration and license agreements with us in connection with those programs. We cannot make any assurance that Novartis or Novo Nordisk will continue to advance the clinical development of the drug candidates subject to collaboration.
Our collaborative partners are free to develop competing products.
Aside from provisions preventing the unauthorized use of our intellectual property by our collaborative partners, there is nothing in our collaborative agreements that prevent our partners from developing competing products. If one of our partners were to develop a competing product, our collaboration could be substantially jeopardized.
Our product candidates are in various stages of development, and we cannot be certain that any will be suitable for commercial purposes.
To be profitable, we must successfully research, develop, obtain regulatory approval for, manufacture, introduce, market, and distribute our products under development, or secure a partner to provide financial and other assistance with these steps. The time necessary to achieve these goals for any individual pharmaceutical product is long and can be uncertain. Before we or a potential partner can sell any of the pharmaceutical products currently under development, pre-clinical (animal) studies and clinical (human) trials must demonstrate that the product is safe and effective for human use for each targeted indication. We have never successfully commercialized a drug or a nonprescription candidate and we cannot be certain that we or our current or future partners will be able to begin, or continue, planned clinical trials for our product candidates, or if we are able, that the product candidates will prove to be safe and will produce their intended effects.
Even if our products are safe and effective, the size of the solid dosage form, taste, and frequency of dosage may impede their acceptance by patients.
A number of companies in the drug delivery, biotechnology, and pharmaceutical industries have suffered significant setbacks in clinical trials, even after showing promising results in earlier studies or trials. Only a small number of research and development programs ultimately result in commercially successful drugs. Favorable results in any pre-clinical study or early clinical trial do not imply that favorable results will ultimately be obtained in future clinical trials. We cannot make any assurance that results of limited animal and human studies are indicative of results that would be achieved in future animal studies or human clinical studies, all or some of which will be required in order to have our product candidates obtain regulatory approval. Similarly, we cannot assure you that any of our product candidates will be approved by the FDA. Even if clinical trials or other studies demonstrate safety and effectiveness of any of our product candidates for a specific disease or condition and the necessary regulatory approvals are obtained, the commercial success of any of our product candidates will depend upon their acceptance by patients, the medical community, and third-party payers and on our partners’ ability to successfully manufacture and commercialize our product candidates.
23
Table of Contents
Our future business success depends heavily upon regulatory approvals, which can be difficult and expensive to obtain.
Pre-clinical studies and clinical trials of prescription drugs and biologic product candidates, as well as the manufacturing and marketing of our product candidates, are subject to extensive, costly and rigorous regulation by governmental authorities in the U.S. and other countries. The process of obtaining required approvals from the FDA and other regulatory authorities often takes many years, is expensive, and can vary significantly based on the type, complexity, and novelty of the product candidates. We cannot assure you that we, either independently or in collaboration with others, will meet the applicable regulatory criteria in order to receive the required approvals for manufacturing and marketing. Delays in obtaining U.S. or foreign approvals for our self-developed projects could result in substantial additional costs to us, and, therefore, could adversely affect our ability to compete with other companies. Additionally, delays in obtaining regulatory approvals encountered by others with whom we collaborate also could adversely affect our business and prospects. Even if regulatory approval of a product is obtained, the approval may place limitations on the intended uses of the product, and may restrict the way in which we or our partner may market the product.
The regulatory approval process for our prescription drug product candidates presents several risks to us:
| Ÿ | In general, pre-clinical tests and clinical trials can take many years, and require the expenditure of substantial resources. The data obtained from these tests and trials can be susceptible to varying interpretation that could delay, limit or prevent regulatory approval |
| Ÿ | Delays or rejections may be encountered during any stage of the regulatory process based upon the failure of the clinical or other data to demonstrate compliance with, or upon the failure of the product to meet, a regulatory agency’s requirements for safety, efficacy, and quality or, in the case of a product seeking an orphan drug indication, because another designee received approval first |
| Ÿ | Requirements for approval may become more stringent due to changes in regulatory agency policy or the adoption of new regulations or guidelines |
| Ÿ | New guidelines can have an effect on the regulatory decisions made in previous years |
| Ÿ | The scope of any regulatory approval, when obtained, may significantly limit the indicated uses for which a product may be marketed and may impose significant limitations in the nature of warnings, precautions, and contraindications that could materially affect the profitability of the drug |
| Ÿ | Approved drugs, as well as their manufacturers, are subject to continuing and ongoing review, and discovery of problems with these products or the failure to adhere to manufacturing or quality control requirements may result in restrictions on their manufacture, sale or use or in their withdrawal from the market |
| Ÿ | Regulatory authorities and agencies may promulgate additional regulations restricting the sale of our existing and proposed products |
| Ÿ | Once a product receives marketing approval, the FDA may not permit us to market that product for broader or different applications, or may not grant us clearance with respect to separate product applications that represent extensions of our basic technology. In addition, the FDA may withdraw or modify existing clearances in a significant manner or promulgate additional regulations restricting the sale of our present or proposed products |
Additionally, we face the risk that our competitors may gain FDA approval for a product before we do. Having a competitor reach the market before we do would impede the future commercial success for our competing product because we believe that the FDA uses heightened standards of approval for products once approval has been granted to a competing product in a particular product area. We believe that this standard generally limits new approvals to only those products that meet or exceed the standards set by the previously approved product.
The regulatory approval process for nonprescription product candidates will likely vary by the nature of therapeutic molecule being delivered.
24
Table of Contents
In particular, the European Medical Agency (“EMA”) announced in January 2011 that its committee for Medicinal Products for Human Use has begun to review available data relevant to the potential for increased risk of prostate cancer progression and other types of malignancies in patients taking calcitonin-containing medicines for the prevention of acute bone loss. The announcement indicated that the decision to review followed review of two clinical trials which suggested an increased frequency of malignancies. The EMA indicated it intended to assess the data obtained in the balance of risks and benefits of calcitonin-containing medicines.
Our collaboration partner Novartis has indicated to us that it has responded to the EMA’s request for information. Novartis notified us that it has informed the FDA of the EMA request, and has provided the FDA with relevant data regarding calcitonin at its request. Subsequent to these actions, Novartis announced that it is discontinuing the oral salmon calcitonin program.
On July 20, 2012, the European Medicines Agency’s Committee for Medicinal Products for Human Use issued a press release in which it recommended that calcitonin-containing medicines should only be used for short-term treatment, because of evidence that long-term use of these medicines is associated with an increased risk of cancer.
Our business will suffer if we cannot adequately protect our patent and proprietary rights.
Although we have patents for some of our product candidates and have applied for additional patents, there can be no assurance that patents applied for will be granted, that patents granted to or acquired by us now or in the future will be valid and enforceable and provide us with meaningful protection from competition, or that we will possess the financial resources necessary to enforce any of our patents. Also, we cannot be certain that any products that we (or a licensee) develop will not infringe upon any patent or other intellectual property right of a third party.
We also rely upon trade secrets, know-how, and continuing technological advances to develop and maintain our competitive position. We maintain a policy of requiring employees, scientific advisors, consultants, and collaborators to execute confidentiality and invention assignment agreements upon commencement of a relationship with us. We cannot assure you that these agreements will provide meaningful protection for our trade secrets in the event of unauthorized use or disclosure of such information.
Part of our strategy involves collaborative arrangements with other pharmaceutical companies for the development of new formulations of drugs developed by others and, ultimately, the receipt of royalties on sales of the new formulations of those drugs. These drugs are generally the property of the pharmaceutical companies and may be the subject of patents or patent applications and other rights of protection owned by the pharmaceutical companies. To the extent those patents or other forms of rights expire, become invalid or otherwise ineffective, or to the extent those drugs are covered by patents or other forms of protection owned by third parties, sales of those drugs by the collaborating pharmaceutical company may be restricted, limited, enjoined, or may cease. Accordingly, the potential for royalty revenues to us may be adversely affected.
We may be at risk of having to obtain a license from third parties making proprietary improvements to our technology.
There is a possibility that third parties may make improvements or innovations to our technology in a more expeditious manner than we do. Although we are not aware of any such circumstance related to our product portfolio, should such circumstances arise, we may need to obtain a license from such third party to obtain the benefit of the improvement or innovation. Royalties payable under such a license would reduce our share of total revenue. Such a license may not be available to us at all or on commercially reasonable terms. Although we currently do not know of any circumstances related to our product portfolio which would lead us to believe that a third party has developed any improvements or innovation with respect to our technology, we cannot assure you that such circumstances will not arise in the future. We cannot reasonably determine the cost to us of the effect of being unable to obtain any such license.
We are dependent on third parties to manufacture and test our products.
Currently, we have no long term manufacturing agreements in place and no manufacturing facilities for production of our carriers or any therapeutic compounds being commercialized or under consideration as
25
Table of Contents
products. We have no facilities for clinical testing. The success of our self-developed programs is dependent upon securing manufacturing capabilities and contracting with clinical service and other service providers.
The availability of manufacturers is limited by both the capacity of such manufacturers and their regulatory compliance. Among the conditions for FDA approval is the requirement that the prospective manufacturer’s quality control and manufacturing procedures continually conform with the FDA’s current GMP (GMP are regulations established by the FDA that govern the manufacture, processing, packing, storage and testing of drugs intended for human use). In complying with GMP, manufacturers must devote extensive time, money, and effort in the area of production and quality control and quality assurance to maintain full technical compliance. Manufacturing facilities and company records are subject to periodic inspections by the FDA to ensure compliance. If a manufacturing facility is not in substantial compliance with these requirements, regulatory enforcement action may be taken by the FDA, which may include seeking an injunction against shipment of products from the facility and recall of products previously shipped from the facility. Such actions could severely delay our ability to obtain product from that particular source.
The success of our clinical trials and our partnerships is dependent on the proposed or current partner’s capacity and ability to adequately manufacture drug products to meet the proposed demand of each respective market. Any significant delay in obtaining a supply source (which could result from, for example, an FDA determination that such manufacturer does not comply with current GMP) could harm our potential for success. Additionally, if a current manufacturer were to lose its ability to meet our supply demands during a clinical trial, the trial may be delayed or may even need to be abandoned.
The availability and amount of reimbursement for oral Eligen B12™ and our product candidates, and the manner in which government and private payers may reimburse for our products, are uncertain.
Sales of Eligen B12™ or any of our product candidates will depend in part on the availability of reimbursement from third-party payers such as government health administration authorities, private health insurers and other organizations. The future magnitude of our revenues and profitability may be affected by the continuing efforts of governmental and third-party payers to contain or reduce the costs of health care. We cannot predict the effect that private sector or governmental health care reforms may have on our business, and there can be no assurance that any such reforms will not have a material adverse effect on our business, financial condition and results of operations.
In addition, in both the United States and elsewhere, sales of prescription drugs are dependent in part on the availability of reimbursement to the consumer from third-party payers, such as government and private insurance plans. The ability to obtain reimbursement of our products from these parties is a critical factor in the commercial success for any of our products. Failure to obtain reimbursement could result in reduced or no sales of our products.
Third-party payers are increasingly challenging the price and cost-effectiveness of medical products and services. Significant uncertainty exists as to the reimbursement status of newly-approved health care products. There can be no assurance that our products will be considered cost-effective or that adequate third-party reimbursement will be available to enable us to maintain price levels sufficient to realize an appropriate return on our investment in product development. Legislation and regulations affecting the pricing of pharmaceuticals may change before any of our products are approved for marketing. Adoption of such legislation could further limit reimbursement for medical products and services.
Current and future legislation may increase the difficulty and cost of commercializing oral Eligen B12™ and our products candidates, affect the prices we may obtain and limit reimbursement amounts.
In the United States and some foreign jurisdictions, there have been a number of legislative and regulatory changes and proposed changes regarding the healthcare system that could restrict or regulate post-approval activities and affect our revenues from future sales of our products.
The Medicare Modernization Act, or MMA, enacted in December 2003, has altered the way in which some physician-administered drugs and biologics are reimbursed by Medicare Part B. Under this reimbursement methodology, physicians are reimbursed based on a product’s “average sales price.” This reimbursement
26
Table of Contents
methodology has generally led to lower reimbursement levels. This legislation also added an outpatient prescription drug benefit to Medicare, which went into effect in January 2006. These benefits are provided primarily through private entities, which we expect will attempt to negotiate price concessions from pharmaceutical manufacturers.
The Patient Protection and Affordable Care Act of 2010, or the PPACA, may have a significant impact on the healthcare system. As part of this legislative initiative, Congress enacted a number of provisions that are intended to reduce or limit the growth of healthcare costs, which could significantly change the market for pharmaceuticals and biological products. The provisions of the PPACA could, among other things, increase pressure on drug pricing or make it more costly for patients to gain access to prescription drugs like our product candidates at affordable prices. This could ultimately lead to fewer prescriptions for our product candidates and could force individuals who are prescribed our products to pay significant out-of-pocket costs or pay for the prescription entirely by themselves. As a result of such initiatives, market acceptance and commercial success of our products, once approved, may be limited and our business may be harmed.
We may face product liability claims related to participation in clinical trials or future products.
We have product liability insurance with a policy limit of $7.0 million per occurrence and in the aggregate. The testing, manufacture, and marketing of products for humans utilizing our drug delivery technology may expose us to potential product liability and other claims. These may be claims directly by consumers or by pharmaceutical companies or others selling our future products. We seek to structure development programs with pharmaceutical companies that would complete the development, manufacturing and marketing of the finished product in a manner that would protect us from such liability, but the indemnity undertakings for product liability claims that we secure from the pharmaceutical companies may prove to be insufficient.
We face rapid technological change and intense competition.
Our success depends, in part, upon maintaining a competitive position in the development of products and technologies in an evolving field in which developments are expected to continue at a rapid pace. We compete with other drug delivery, biotechnology and pharmaceutical companies, research organizations, individual scientists, and non-profit organizations engaged in the development of alternative drug delivery technologies or new drug research and testing, as well as with entities developing new drugs that may be orally active. Many of these competitors have greater research and development capabilities, experience, and marketing, financial, and managerial resources than we have, and, therefore, represent significant competition.
Our products, when developed and marketed, may compete with existing parenteral or other versions of the same drug, some of which are well established in the marketplace and manufactured by formidable competitors, as well as other existing drugs. For example, our salmon calcitonin product candidate, if developed and marketed, would compete with a wide array of existing osteoporosis therapies, including a nasal dosage form of salmon calcitonin, estrogen replacement therapy, selective estrogen receptor modulators, bisphosphonates, and other compounds in development.
Our competitors may succeed in developing competing technologies or obtaining government approval for products before we do. Developments by others may render our product candidates, or the therapeutic macromolecules used in combination with our product candidates, noncompetitive or obsolete. At least one competitor has notified the FDA that it is developing a competing formulation of salmon calcitonin. If our products are marketed, we cannot assure you that they will be preferred to existing drugs or that they will be preferred to or available before other products in development.
If a competitor announces a successful clinical study involving a product that may be competitive with one of our product candidates or an approval by a regulatory agency of the marketing of a competitive product, such announcement may have a material adverse effect on our operations or future prospects resulting from reduced sales of future products that we may wish to bring to market or from an adverse impact on the price of our common stock or our ability to obtain regulatory approval for our product candidates.
27
Table of Contents
We are dependent on our key personnel and if we cannot recruit and retain leaders in our research, development, manufacturing, and commercial organizations, our business will be harmed.
We are dependent on our executive officers. The loss of one or more members of our executive officers or key employees could have an adverse effect on our business, financial condition and results of operations, given their specific knowledge related to our proprietary technology and personal relationships with our pharmaceutical company partners. If we are not able to retain our executive officers, our business may suffer. We do not maintain “key-man” life insurance policies for any of our executive officers.
There is intense competition in the biotechnology industry for qualified scientists and managerial personnel in the development, manufacture, and commercialization of drugs. We may not be able to continue to attract and retain the qualified personnel necessary for developing our business. Additionally, because of the knowledge and experience of our scientific personnel and their specific knowledge with respect to our drug carriers the continued development of our product candidates could be adversely affected by the loss of any significant number of such personnel.
Provisions of our corporate charter documents, Delaware law, and our stockholder rights plan may dissuade potential acquirers, prevent the replacement or removal of our current management and may thereby affect the price of our common stock.
Our Board of Directors has the authority to issue up to 4,000,000 shares of preferred stock and to determine the rights, preferences and privileges of those shares without any further vote or action by our stockholders. Of these 4,000,000 shares, the Board of Directors has the authority to designate that number of shares of Series A Junior Participating Cumulative Preferred Stock (“A Preferred Stock”) as is required under our stockholders rights plan described below. Those shares of preferred stock not designated as A Preferred Stock remain available for future issuance. Rights of holders of common stock may be adversely affected by the rights of the holders of any preferred stock that may be issued in the future.
We also have a stockholders rights plan, commonly referred to as a “poison pill,” in which A Preferred Stock purchase rights (the “Rights”) have been granted at the rate of one one-hundredth of a share of A Preferred Stock at an exercise price of $80 for each share of our common stock. The Rights are not exercisable or transferable apart from the common stock, until the earlier of (i) ten days following a public announcement that a person or group of affiliated or associated persons have acquired beneficial ownership of 20% or more of our outstanding common stock or (ii) ten business days (or such later date, as defined) following the commencement of, or announcement of an intention to make a tender offer or exchange offer, the consummation of which would result in the beneficial ownership by a person, or group, of 20% or more of our outstanding common stock. If we enter into consolidation, merger, or other business combination, as defined in the stockholders rights plan, each Right would entitle the holder upon exercise to receive, in lieu of shares of A Preferred Stock, a number of shares of common stock of the acquiring company having a value of two times the exercise price of the Right, as defined in the stockholders rights plan. By potentially diluting the ownership of the acquiring company, our rights plan may dissuade prospective acquirers of our company. MHR is specifically excluded from the provisions of the plan.
The holders of A Preferred Stock would be entitled to a preferential cumulative quarterly dividend of the greater of $1.00 per share or 100 times the per-share dividend declared on our stock and are also entitled to a liquidation preference, thereby hindering an acquirer’s ability to freely pay dividends or to liquidate the company following an acquisition. Each A Preferred Stock share will have 100 votes and will vote together with the common shares, effectively preventing an acquirer from removing existing management. The Rights contain anti-dilutive provisions and are redeemable at our option, subject to certain defined restrictions for $.01 per Right. The Rights expire on April 7, 2016.
Provisions of our corporate charter documents, Delaware law and financing agreements may prevent the replacement or removal of our current management and members of our Board of Directors and may thereby affect the price of our common stock.
In connection with the MHR financing transaction in 2005, and after approval by our Board of Directors, Dr. Mark H. Rachesky was appointed to the Board of Directors by MHR (the “MHR Nominee”) and Dr. Michael
28
Table of Contents
Weiser was appointed to the Board of Directors by both the majority of our Board of Directors and MHR (the “Mutual Director”), as contemplated by our bylaws and certificate of incorporation. Our certificate of incorporation provides that the MHR Nominee and the Mutual Director may be removed only by the affirmative vote of at least 85% of the shares of common stock outstanding and entitled to vote at an election of directors. Our certificate of incorporation also provides that the MHR Nominee may be replaced only by an individual designated by MHR unless the MHR Nominee has been removed for cause, in which case the MHR Nominee may be replaced only by an individual approved by both a majority of our Board of Directors and MHR. Furthermore, certain amendments to the bylaws and the certificate of incorporation provide that the rights granted to MHR by these amendments may not be amended or repealed without the unanimous vote or unanimous written consent of the Board of Directors or the affirmative vote of the holders of at least 85% of the shares of Common Stock outstanding and entitled to vote at the election of directors. The amendments to the bylaws and the certificate of incorporation will remain in effect as long as MHR holds at least 2% of the shares of fully diluted Common Stock. The amendments to the bylaws and the certificate of incorporation will have the effect of making it more difficult for a third party to gain control of our Board of Directors.
Additional provisions of our certificate of incorporation and bylaws could have the effect of making it more difficult for a third party to acquire a majority of our outstanding voting common stock. These include provisions that classify our Board of Directors, limit the ability of stockholders to take action by written consent, call special meetings, remove a director for cause, amend the bylaws or approve a merger with another company. We are subject to the provisions of Section 203 of the Delaware General Corporation Law which prohibits a publicly-held Delaware corporation from engaging in a “business combination” with an “interested stockholder” for a period of three years after the date of the transaction in which the person became an interested stockholder, unless the business combination is approved in a prescribed manner. For purposes of Section 203, a “business combination” includes a merger, asset sale or other transaction resulting in a financial benefit to the interested stockholder, and an “interested stockholder” is a person who, either alone or together with affiliates and associates, owns (or within the past three years, did own) 15% or more of the corporation’s voting stock.
Our stock price has been and may continue to be volatile.
The trading price for our common stock has been and is likely to continue to be highly volatile. The market prices for securities of drug delivery, biotechnology and pharmaceutical companies have historically been highly volatile.
Factors that could adversely affect our stock price include:
| Ÿ | fluctuations in our operating results; |
| Ÿ | announcements of partnerships or technological collaborations and announcements of the results or further actions in respect of any partnerships or collaborations, including termination of same; |
| Ÿ | innovations or new products by us or our competitors; |
| Ÿ | governmental regulation; |
| Ÿ | developments in patent or other proprietary rights; |
| Ÿ | public concern as to the safety of drugs developed by us or others; |
| Ÿ | the results of pre-clinical testing and clinical studies or trials by us, our partners or our competitors; |
| Ÿ | litigation; |
| Ÿ | general stock market and economic conditions; |
| Ÿ | number of shares available for trading (float); and |
| Ÿ | inclusion in or dropping from stock indexes. |
As of December 31, 2014, our 52-week high and low closing market price for our common stock was $0.45 and $0.17, respectively.
29
Table of Contents
Future sales of common stock or warrants, or the prospect of future sales, may depress our stock price.
Sales of a substantial number of shares of common stock or warrants, or the perception that sales could occur, could adversely affect the market price of our common stock. Additionally, as of December 31, 2014, there were outstanding options to purchase up to 3,174,094 shares of our common stock that are currently exercisable, and additional outstanding options to purchase up to 1,396,933 shares of common stock that are exercisable over the next several years. As of December 31, 2014, the Convertible Notes were convertible into 32,717,484 shares of our common stock; the Bridge Notes were convertible into 3,710,158 shares of our common stock; and the Reimbursement Notes were convertible into 1,365,606 shares of our common stock. As of December 31, 2014, there were outstanding warrants to purchase 27,443,727 shares of our stock. The holders of these options have an opportunity to profit from a rise in the market price of our common stock with a resulting dilution in the interests of the other shareholders. The existence of these options may adversely affect the terms on which we may be able to obtain additional financing. The weighted average exercise price of issued and outstanding options is $0.84 and the weighted average exercise price of warrants is $0.63, which compares to the $0.28 market price at closing on December 31, 2014. Additionally, there may be additional shares available on the market if we are required to file additional re-sale registration statements on Form S-1, including if MHR exercises its registration rights under its Registration Rights Agreement with the Company dated September 26, 2005.
Because the Company’s common stock is on the OTCQB tier of the OTC Markets, the volume of shares traded and the prices at which such shares trade may result in lower prices than might otherwise exist if our common stock was traded on a national securities exchange.
The Company’s shares are traded on the OTCQB tier of the OTC Markets. Stock traded on the OTCQB tier of the OTC Markets is often less liquid than stock traded on national securities exchanges, not only in terms of the number of shares that can be bought and sold at a given price, but also in terms of delays in the timing of transactions and reduced coverage of the Company by security analysts and media. This may result in lower prices for the Company’s common stock than might otherwise be obtained if the common stock were traded on a national securities exchange, and may also result in a larger spread between the bid and asked prices for the Company’s common stock. There is no guarantee that the Company will ever be able to re-list its common stock on the NASDAQ Capital Market or any other market.
| ITEM 1B. | UNRESOLVED STAFF COMMENTS |
None.
| ITEM 2. | PROPERTIES |
In November 2012, we entered into a sub-lease agreement with New American Therapeutics, Inc. to lease approximately 4,100 square feet of office space at 4 Becker Farm Road, Suite 103, Roseland, New Jersey, for use as our corporate office beginning February 1, 2013. The sub lease for this corporate office expired on June 30, 2014.
In December 2012, we entered into a lease agreement with 4 Becker SPE LLC to initially lease approximately 2,000 square feet adjacent to the sub-lease office space beginning in December 2012. Upon expiration of the above referenced sub-lease on June 30, 2014, that approximately 4,100 square feet will become “additional premises” included in this lease agreement. This lease for our corporate office is set to expire on June 30, 2017.
| ITEM 3. | LEGAL PROCEEDINGS |
None.
| ITEM 4. | MINE SAFETY DISCLOSURES |
Not applicable.
30
Table of Contents
| ITEM 5. | MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES |
The Company’s securities began trading on the OTCQB, an electronic quotation service maintained by the Financial Industry Regulatory Authority, effective with the open of business on Tuesday, June 9, 2009. The Company’s trading symbol has remained EMIS, however, it is our understanding that, for certain stock quote publication websites, investors may be required to key EMIS.QB to obtain quotes.
The following table sets forth the range of high and low intra-day sale prices as reported by the OTCQB, electronic quotation service for each period indicated:
| High | Low | |||||||
| 2013 |
||||||||
| First quarter |
0.18 | 0.11 | ||||||
| Second quarter |
0.25 | 0.11 | ||||||
| Third quarter |
0.25 | 0.16 | ||||||
| Fourth quarter |
0.23 | 0.12 | ||||||
| 2014 |
||||||||
| First quarter |
0.33 | 0.15 | ||||||
| Second quarter |
0.36 | 0.21 | ||||||
| Third quarter |
0.46 | 0.24 | ||||||
| Fourth quarter |
0.46 | 0.20 | ||||||
| 2015 |
||||||||
| First quarter (through March 3, 2015) |
0.72 | 0.22 | ||||||
As of March 3, 2015 there were 209 stockholders of record, including record owners holding shares on behalf of an indeterminate number of beneficial owners, and 60,687,478 shares of common stock outstanding. The closing price of our common stock on March 3, 2015, was $0.58. We have never paid cash dividends and do not intend to pay cash dividends in the foreseeable future. We intend to retain earnings, if any, to finance the growth of our business.
31
Table of Contents
Equity Compensation Plan Information
The following table provides information as of December 31, 2014, about the common stock that may be issued upon the exercise of options granted to employees, consultants or members of our board of directors under all of our existing equity compensation plans, including the 2000 Stock Option Plan, the 2002 Broad Based Plan, the 2007 Stock Award and Incentive Plan, (collectively the “Plans”), the Stock Incentive Plan for Outside Directors, and the Directors Deferred Compensation Plan:
| Plan Category |
(a) Number of Securities to be Issued Upon Exercise of Outstanding Options |
(b) Weighted Average Exercise Price of Outstanding Options |
(c) Number of Securities Remaining Available for Future Issuance Under Equity Compensation Plans (Excluding Securities Reflected in Column (a)) |
|||||||||
| Equity Compensation Plans Approved by Security Holders |
||||||||||||
| The Plans |
4,799,750 | $ | 0.80 | 4,483,766 | ||||||||
| Stock Incentive Plan for Outside Directors |
21,000 | 8.97 | — | |||||||||
| Equity Compensation Plans not approved by Security Holders(1) |
0 | 0 | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Total |
4,820,750 | $ | 0.84 | 4,483,766 | ||||||||
| (1) | Our Board of Directors has granted options which are currently outstanding for a former consultant. The Board of Directors determines the number and terms of each grant (option exercise price, vesting and expiration date). This grant was made on July 14, 2003. |
32
Table of Contents
Comparative Stock Performance Graph
The graph below compares the cumulative total stockholder return through December 31, 2014, on Emisphere’s common stock with the cumulative total stockholder return of the NASDAQ Composite Index, the NASDAQ Pharmaceutical Index, the RDG MicroCap Pharmaceutical Index, the Dow Jones U.S. Pharmaceuticals and SIC Code: 2834 — Pharmaceutical Preparations, assuming an investment of $100 on December 31, 2009, in the Company’s common stock, and in the stocks comprising each index (with all dividends reinvested).
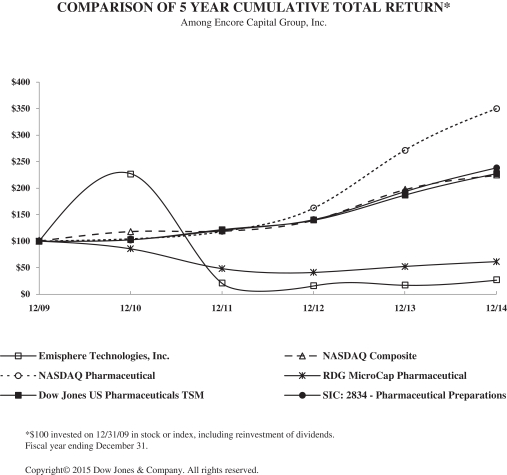
33
Table of Contents
| ITEM 6. | SELECTED FINANCIAL DATA |
The following selected financial data for the years ended December 31, 2014, 2013, 2012, 2011, and 2010 have been derived from the financial statements of Emisphere and notes thereto, which have been audited by our independent registered public accounting firm.
| Year Ended December 31, | ||||||||||||||||||||
| 2014 | 2013 | 2012 | 2011 | 2010 | ||||||||||||||||
| (in thousands, except per share data) | ||||||||||||||||||||
| Revenue |
$ | — | $ | — | $ | — | $ | — | $ | 100 | ||||||||||
| Cost of goods sold |
— | — | — | — | 22 | |||||||||||||||
| Costs and expenses |
||||||||||||||||||||
| Research and development expenses |
1,128 | 836 | 1,867 | 1,951 | 2,495 | |||||||||||||||
| General and administrative expenses |
8,162 | 6,749 | 4,935 | 5,310 | 7,963 | |||||||||||||||
| Other costs and expenses |
15 | 19 | 19 | 277 | 835 | |||||||||||||||
| Impairment of intangible asset |
— | — | — | 598 | — | |||||||||||||||
| Restructuring charge |
— | — | — | — | 50 | |||||||||||||||
| (Income) expense from lawsuit, net |
— | — | — | — | 278 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total costs and expenses |
9,305 | 7,604 | 6,821 | 8,136 | 11,621 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Operating loss |
(9,305 | ) | (7,604 | ) | (6,821 | ) | (8,136 | ) | (11,543 | ) | ||||||||||
| Sale of patent |
— | — | — | — | 500 | |||||||||||||||
| Other Income |
10 | 81 | 45 | — | — | |||||||||||||||
| Research and development tax credit |
— | — | — | 137 | 252 | |||||||||||||||
| Change in fair value of derivative instruments |
(11,872 | ) | (8,433 | ) | 8,110 | 28,696 | (23,651 | ) | ||||||||||||
| Interest expense |
(6,232 | ) | (4,955 | ) | (6,236 | ) | (5,646 | ) | (3,595 | ) | ||||||||||
| Loss on extinguishment of debt |
— | — | — | — | (17,014 | ) | ||||||||||||||
| Financing fees |
— | — | — | — | (1,858 | ) | ||||||||||||||
| Income (loss) before income tax benefit |
(27,399 | ) | (20,911 | ) | (4,902 | ) | 15,051 | (56,909 | ) | |||||||||||
| Income tax benefit (expense) |
2,019 | (28 | ) | 2,974 | — | — | ||||||||||||||
| Net income (loss) |
(25,380 | ) | (20,939 | ) | (1,928 | ) | 15,051 | (56,909 | ) | |||||||||||
| Net income (loss) per share — basic |
(0.42 | ) | (0.35 | ) | (0.03 | ) | 0.27 | (1.23 | ) | |||||||||||
| Net income (loss) per share — diluted |
(0.42 | ) | (0.35 | ) | (0.03 | ) | 0.25 | (1.23 | ) | |||||||||||
| December 31, | ||||||||||||||||||||
| 2014 | 2013 | 2012 | 2011 | 2010 | ||||||||||||||||
| (In thousands) | ||||||||||||||||||||
| Balance Sheet Data: |
||||||||||||||||||||
| Cash, cash equivalents, restricted cash and investments |
$ | 3,683 | $ | 4,053 | $ | 1,484 | $ | 3,069 | $ | 5,326 | ||||||||||
| Working capital (deficit) |
(1,694 | ) | (1,398 | ) | (34,745 | ) | (33,221 | ) | (20,568 | ) | ||||||||||
| Total assets |
5,988 | 4,979 | 2,176 | 4,221 | 7,276 | |||||||||||||||
| Derivative instruments |
29,920 | 15,509 | 2,089 | 10,199 | 34,106 | |||||||||||||||
| Long-term liabilities and deferrals |
86,172 | 74,146 | 31,614 | 31,597 | 51,966 | |||||||||||||||
| Accumulated deficit |
(514,139 | ) | (488,759 | ) | (467,820 | ) | (465,892 | ) | (480,943 | ) | ||||||||||
| Stockholders’ deficit |
(111,950 | ) | (86,801 | ) | (66,066 | ) | (64,527 | ) | (82,520 | ) | ||||||||||
34
Table of Contents
| ITEM 7. | MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS |
Management’s Discussion and Analysis of Financial Conditions and Results of Operations (MD&A) is provided to supplement the accompanying financial statements and notes incorporated herein to help provide an understanding of our financial condition, changes in our financial condition and results of operations. To supplement its audited financial statements presented in accordance with US GAAP, the company is providing a comparison of operating results describing net income and operating expenses which removed certain non-cash and one-time or nonrecurring charges and receipts. The Company believes that this presentation of net income and operating expense provides useful information to both management and investors concerning the approximate impact of the items above. The Company also believes that considering the effect of these items allows management and investors to better compare the Company’s financial performance from period to period and to better compare the Company’s financial performance with that of its competitors. The presentation of this additional information is not meant to be considered in isolation of, or as a substitute for, results prepared in accordance with US GAAP.
CAUTION CONCERNING FORWARD-LOOKING STATEMENTS
The following discussion and analysis contain forward-looking statements that involve risks and uncertainties. When used in this Report, the words, “intend,” “anticipate,” “believe,” “estimate,” “plan,” “expect” and similar expressions as they relate to us are included to identify forward-looking statements. Our actual results could differ materially from those anticipated in these forward-looking statements as a result of factors, including those set forth under Item 1A. “Risk Factors” (above) and elsewhere in this Report. This discussion and analysis should be read in conjunction with the “Selected Financial Data” and the Financial Statements and notes thereto included in this Report.
Overview
Emisphere Technologies, Inc. is a specialty pharmaceutical company that has recently commenced commercial operations. The Company launched its first prescription product, oral Eligen B12™ Rx (1000 mcg.), in the U.S. during March 2015. Oral Eligen B12™ Rx meets significant unmet patient and medical needs by combining vitamin B12 with our proprietary delivery system technology to provide a therapeutic equivalent to injectable B12, which is the current medical standard of care. It is a prescription product for use by B12 deficient individuals; and it is the first oral B12 to be supported by published clinical data ( Clin Ther . 2011 Jul;33(7):934-45) demonstrating that oral Eligen B12™ Rx restores normal vitamin B12 blood levels in deficient patients as effectively as injectable B12. The proprietary Eligen B12™ Rx formulation is covered by a newly issued U.S. Patent (Patent No. 8,022,048) which provides protection through 2029. Beyond oral Eligen B12™ Rx, the Company utilizes its proprietary Eligen® Technology to create new oral formulations of therapeutic agents. Emisphere is currently partnered with global pharmaceutical companies for the development of such new orally delivered therapeutics.
Oral Eligen B12™ Rx was introduced in the United States during March 2015. Additionally, the Company is currently engaged in multiple ex-US oral Eligen™ B12 Rx licensing discussions.
By building on the oral Eligen B12™ Rx product, the Company intends to establish a sound product portfolio platform on which to expand its B12 therapeutic franchise as well as expand internal new product development with new therapeutic agents. The Company will also continue to develop its existing drug delivery carrier partnerships and expand its carrier business by seeking out and engaging in new global licensing opportunities.
As it focuses on building a commercial platform based on the oral Eligen B12™ Rx product, Emisphere will continue to develop and expand upon the unique and improved delivery of therapeutic molecules using its Eligen® Technology. These molecules could be currently available or are under development. Such molecules are usually delivered by injection; in many cases, their benefits are limited due to poor bioavailability, slow on-set of action or variable absorption. In those cases, our technology may increase the benefit of the therapy by
35
Table of Contents
improving bioavailability or absorption or by decreasing time to onset of action. The Eligen® Technology can be applied to the oral route of administration as well as other delivery pathways, such as buccal, rectal, inhalation, intra-vaginal or transdermal. The Eligen® Technology can make it possible to deliver certain therapeutic molecules orally without altering their chemical form or biological activity. Eligen® delivery agents, or “carriers”, facilitate or enable the transport of therapeutic molecules across the mucous membranes of the gastrointestinal tract, to reach the tissues of the body where they can exert their intended pharmacological effect. Our development efforts are conducted internally or in collaboration with corporate development partners. Typically, the drugs that we target are at an advanced stage of development, or have already received regulatory approval, and are currently available on the market.
Our website is www.emisphere.com. The contents of that website are not incorporated herein by reference. Investor related questions should be directed to info@emisphere.com.
Mr. Alan L. Rubino, the Company’s President and Chief Executive Officer, and Mr. Timothy G. Rothwell, its Chairman of the Board of Director, are seasoned industry executives with major and emerging pharmaceutical company experience who form the core of a leadership team that will implement the Company’s strategic plans. To that end, we have sought to expand opportunities with existing partners and will continue to work to expand and explore new efforts to attract new delivery system, product development, and licensing partnerships. After evaluating the Company’s operations and strategy, the leadership team determined the Company should refocus its corporate strategy to reemphasize the commercialization of oral Eligen B12™Rx, build new high-value partnerships, evaluate new prescription medical foods commercial opportunities, reprioritize the product pipeline, and promote new uses for the Eligen® Technology.
To accelerate the commercialization of oral Eligen B12™ Rx and evaluate new opportunities for prescription medical foods and other prescription products under development, the Company hired Mr. Carl V. Sailer in October 2012 to head its commercial efforts. Mr. Sailer has extensive experience in pharmaceuticals products marketing and supply chain management. He has a proven track record of launching new, and enhancing the financial performance of, existing pharmaceutical products by implementing progressive commercial marketing and distribution models. The Company engaged the consulting services of Dr. Carlos de Lecea, M.D., Ph.D., to expand its business development efforts globally. Dr. de Lecea has over 20 years’ experience in business development, including in and out licensing pharmaceutical products and delivery technologies in global markets. Dr. de Lecea also works with Mr. Rubino to expand the application of the Eligen® Technology by taking advantage of its suitability to facilitate oral absorption of emerging peptides and biologic products that are typically only available as injectables or are currently under development. We believe that these products represent tremendous promise for realizing improvements in healthcare and growth in the industry, and that the Eligen® Technology is well suited to deliver many of these molecules safely and efficiently. More recently the Company continues to strengthen its commercial operations team by engaging the consulting services of Mr. Nicholas G. Rothwell, CPIM, as Vice President, Global Supply, and Dr. Peter J. Shaw, M.D., as Chief Medical Officer. Mr. Rothwell has an exceptional background in commercial operations for life sciences and will play an integral role in supporting Emisphere’s new commercial operations building on the launch of Eligen B12™ Rx. With more than 30 years of experience developing, implementing and managing end-to-end integrated supply chains Mr. Rothwell is a senior healthcare executive with extensive commercial experience in operations management and strong expertise in leading strategic planning, product launch and growth initiatives of leading pharmaceutical products. Mr. Rothwell will be responsible for global supply chain, logistics, manufacturing and quality assurance. Dr. Shaw brings significant industry and medical experiences as a practicing physician and in progressive pharmaceutical sales representative training. His expertise and vision will facilitate the launch and growth of our oral Eligen B12™ product, and strengthen our development efforts supporting the advancement of the Company’s other pipeline product candidates. Dr. Shaw will have responsibility for global clinical development programs, medical affairs and other related functions. Mr. Rothwell and Dr. Shaw report to Mr. Rubino.
The application of the Eligen® Technology is potentially broad and may provide for a number of opportunities across a spectrum of therapeutic modalities or nutritional supplements. During the remainder of 2015 we plan to continue to develop our product pipeline utilizing the Eligen® Technology with prescription and medical foods product candidates and prioritized our development efforts based on overall potential returns on
36
Table of Contents
investment, likelihood of success, and market and medical needs. Medical foods are a distinct product category defined by the Orphan Drug Act of 1988 and an FDA regulation, and encompass foods which are formulated to be consumed or administered enterally under the supervision of a physician and which are intended for the specific dietary management of a disease or condition for which distinctive nutritional requirements, based on recognized scientific principles, are established by medical evaluation. Our goal is to implement our Eligen® Technology to enhance overall healthcare, including patient accessibility and compliance, while benefiting the commercial pharmaceutical and healthcare marketplace and driving company valuation.
To accelerate commercialization of the Eligen® Technology, Emisphere will continue to focus on its two-pronged strategy. First, we will focus on commercializing oral Eligen B12™ Rx (1000 mcg) as a medical food for use by documented B12 deficient individuals in the United States and globally. During the fourth quarter of 2010, the Company completed a clinical trial which demonstrated that both oral Eligen B12™ (1000 mcg) and injectable B12 (current standard of care) can efficiently and quickly restore normal Vitamin B12 levels in deficient individuals. The manuscript summarizing the results from that clinical trial was published in the July 2011 edition of the journal Clinical Therapeutics (Volume 22, pages 934 — 945). We also conducted market research to help assess the potential commercial opportunity for our oral Eligen B12™ Rx (1000 mcg) product. On August 5, 2011, we received notice from the United States Patent Office that the U.S. patent application directed to the oral Eligen B12™ formulation was allowed. This new patent (US 8,022,048) provides intellectual property protection for Eligen B12™ through approximately October 2029. Second, we will concentrate on expanding our Eligen® drug delivery technology business, by seeking applications with prescription molecules obtained through partnerships with other pharmaceutical companies for molecules where oral absorption is difficult yet substantially beneficial if proven. We are also working to generate new interest in the Eligen ® Technology with potential partners and attempting to expand our current collaborative relationships to take advantage of the critical knowledge that others have gained by working with our technology. Second, we continue to pursue commercialization of product candidates developed internally. We believe that these internal candidates need to be developed with reasonable investment in an acceptable time period and with a reasonable risk-benefit profile.
To support our internal development programs, the Company implemented its new commercialization strategy for the Eligen ® Technology. Using extensive safety data available for its Sodium N-[8-(2-hydroxybenzoyl) Amino] Caprylate (“SNAC”) carrier, the Company obtained GRAS (“Generally Recognized as Safe”) status for its SNAC carrier, and then applied the Eligen® Technology with B12, another GRAS substance where bioavailability and absorption is difficult and improving such absorption would yield substantial benefit and value. Given sufficient time and resources, the Company intends to apply this strategy to develop other products. Examples of other GRAS substances that may be developed into additional commercial products using this strategy would include vitamins such as other B Vitamins, minerals such as iron, and other supplements such as the polyphenols and catechins, among others.
Funding required to continue developing our product pipeline may be partially paid by income-generated from sales of Eligen B12™ in the U.S., and from license arrangements whose value tends to increase as product candidates move from pre-clinical into clinical development. It is our intention that investments that may be required to fund our research and development will be approached incrementally in order to minimize disruption or dilution. The Company also continues to focus on improving operational efficiency. Annual non-commercial operating costs have been reduced by approximately 80% from 2008 levels. Its cash burn rate to support continuing operations is less than $6 million per year. Additionally, we have accelerated the commercialization of the Eligen® Technology in a cost effective way and to gain operational efficiencies by tapping into advanced scientific processes offered by independent contractors.
Our product pipeline includes prescription and medical food product candidates that are being developed in partnership or internally. During 2014, we continued to make progress on plans to commercialize our internally developed oral Eligen B12™ Rx product, having launched in the U.S. during the first quarter, 2015. Additionally, our development partner, Novo Nordisk A/S (“Novo Nordisk”), continues its development programs.
37
Table of Contents
Novo Nordisk is using our Eligen® drug delivery technology in combination with its proprietary GLP-1 receptor agonists and insulins. During December 2010, the Company entered into a license agreement with Novo Nordisk to develop and commercialize oral formulations of Novo Nordisk’s insulins using Emisphere’s Eligen® Technology. This was the second license agreement between the two companies. The GLP-1 License Agreement, entered into in June 2008, and amended for the second time on April 26, 2013 provides for the development of oral formulations of GLP-1 receptor agonists, with a potential drug for the treatment of type 2 diabetes currently in a Phase II clinical trial. The Amendment provided for a payment of $10 million from Novo Nordisk to the Company as a prepayment of certain development milestone payments that would have otherwise become payable to the Company under the Development Agreement in exchange for a reduction in the rate of potential future royalty payments as provided in the Development Agreement.
On February 20, 2015 Novo Nordisk announced positive Phase 2 data pertaining to OG217SC, the oral formulation of semaglutide, a long-acting human GLP-1 analogue that stimulates insulin and suppresses glucagon secretion in a glucose-dependent manner. OG217SC is provided in a tablet formulation with the absorption-enhancing excipient, SNAC. SNAC is an Eligen® Carrier. Novo Nordisk announced that it has successfully completed the phase 2 trial for OG217SC, investigating dose range, escalation, efficacy and safety of once-daily oral semaglutide compared with oral placebo or once-weekly subcutaneously administered semaglutide in around 600 people with type 2 diabetes treated for 26 weeks. Based on these results, Novo Nordisk announced that it will initiate consultations with regulatory authorities subsequent to which a decision of whether to progress OG217SC into phase 3 development will be made.
Under its GLP-1 License Agreement, Novo Nordisk is working to develop and commercialize oral formulations of its proprietary GLP-1 receptor agonists in combination with Emisphere carriers. Under the GLP-1 License Agreement, Emisphere could receive additional contingent product development and sales milestone payments and would also be entitled to receive royalties in the event Novo Nordisk commercializes products developed under such Agreement. Under the GLP-1 License Agreement, Novo Nordisk is responsible for the development and commercialization of the products.
We continue to assess therapeutic molecules for their potential compatibility with our technology and market need. Our intent is to continue to expand our pipeline with product candidates that demonstrate significant opportunities for growth. Our focus is on molecules that meet the criteria for success based on our increased understanding of our Eligen® Technology. Depending on the molecule, market potential and interest, we intend to pursue potential product development opportunities through development alliances or internal development.
We have collaborated with Novartis in connection with the development and testing of oral formulations of several drug candidates. Novartis has the right to evaluate the feasibility of using Emisphere’s Eligen® Technology with two new compounds to assess the potential for new product development opportunities. Novartis is considering its options accordingly. If Novartis chooses to develop oral formulations of these new compounds using the Eligen® Technology, the parties will negotiate additional agreements. In that case, Emisphere could be entitled to receive development milestone and royalty payments in connection with the development and commercialization of these potentially new products.
Our other product candidates in development are in earlier or preclinical research phases, and we continue to assess them for their compatibility with our technology and market need. Our intent is to seek partnerships with pharmaceutical and biotechnology companies for certain of these products as we continue to expand our pipeline with product candidates that demonstrate significant opportunities for growth. Our focus is on molecules that meet the criteria for success based on our increased understanding of our Eligen® Technology and prescription medical foods. Our preclinical programs focus on the development of oral formulations of potentially new treatments for diabetes and products in the areas of cardiovascular, appetite suppression and pain and on the development and potential expansion of nutritional supplement products.
Liquidity and Capital Resources
Since our inception in 1986, we have generated significant losses from operations and we anticipate that we will continue to generate significant losses from operations for the foreseeable future.
38
Table of Contents
As of December 31, 2014, our accumulated deficit was approximately $514.1 million. Our loss from operations was $9.3 million, $7.6 million and $6.8 million for the years ended December 31, 2014, 2013 and 2012, respectively. Our net loss was $25.4 million, $20.9 million and $1.9 million for the years ended December 31, 2014, 2013 and 2012, respectively. Our net cash provided (outlays) from operations and capital expenditures were ($8.4) million, $3.1 million and ($3.0) million for the years ended December 31, 2014, 2013 and 2012, respectively. Net cash provided (outlays) include receipts of deferred revenue of $0.0 million, $10.0 million, and $0.02 million for 2014, 2013, and 2012, respectively. Our stockholders’ deficit was $111.9 million and $86.8 million as of December 31, 2014 and 2013, respectively. On December 31, 2014 we had approximately $3.7 million cash.
As of December 31, 2014, the Company’s obligations included approximately $40.9 million (face value) under its Amended and Restated Convertible Notes (the “Convertible Notes”), approximately $8.3 million (face value) under a loan agreement entered into on August 20, 2014 (the “Loan Agreement”), approximately $0.7 million (face value) under its Amended and Restated Reimbursement Notes (the “Reimbursement Notes”), and approximately $1.9 million (face value) under its Amended and Restated Bridge Notes (the “Bridge Notes”). Additional borrowings under the Loan Agreement are subject to various sales, operating and manufacturing performance criteria.
Under the terms of the Loan Agreement, described in Note 7 to the Financial Statements, Emisphere may borrow, at specified times and based on the attainment of specified performance milestones, up to an aggregate of $20.0 million to finance the development, manufacturing, marketing and sales of its oral Eligen B12™ Rx Product. The new loan facility will mature on December 31, 2019 and bear interest at a rate of 13% per year. The first borrowing under the Loan Agreement occurred on August 20, 2014 in an original principal amount of $5.0 million, and the second occurred on November 4, 2014 in an original principal amount of $3.0 million. Subject to achieving certain operational milestones relating to the timely manufacture and commencement of sales of oral Eligen B12™ Rx, of which there can be no assurance, the Company may request three additional borrowings under the Loan Agreement as follows: up to $5.0 million in the first quarter of 2015, up to $5.0 million in the second quarter of 2015, and up to $2.0 million in the third quarter of 2015. In addition to funding available through the Loan Agreement, the Company received approximately $1.7 million and $0.3 million on January 14, 2014 and December 9, 2014, respectively, from the sale of unused net operating losses by participating in the Technology Business Tax Certificate Transfer Program, sponsored by the New Jersey Economic Development Authority.
We believe the Company’s current cash balance, in addition to cash available through additional borrowings under the Loan Agreement, assuming attainment of the milestones, will provide sufficient capital to support the commercial launch of oral Eligen B12™ Rx in the U.S. market and to continue operations through the end of 2015. The Company’s future capital requirements beyond 2015 and financial success depend largely on the commercial success of our oral Eligen B12™ Rx product and our ability to leverage existing as well as securing new partnering opportunities. This is no assurance that our plans will be successful. If we fail to raise sufficient capital from commercial operations or partnerships, we will need to seek capital from other sources. We cannot assure you that financing will be available on favorable terms or at all. If we fail to generate sufficient additional capital from sales of oral Eligen B12™ Rx or obtain substantial cash inflows from existing or new partners or other sources prior to the end 2015, we could be forced to cease operations. Additionally, if additional capital is raised through the sale of equity or convertible debt securities, the issuance of such securities would result in dilution to our existing stockholders. These conditions raise substantial doubt about our ability to continue as a going concern. Consequently, the audit reports prepared by our independent registered public accounting firm relating to our financial statements for the years ended December 31, 2014, 2013 and 2012 include an explanatory paragraph expressing substantial doubt about our ability to continue as a going concern.
Furthermore, despite our optimism regarding the Eligen® Technology, even in the event that the Company is adequately funded, there is no guarantee that any of our products or product candidates will perform as hoped or that such products can be successfully commercialized.
39
Table of Contents
During the year ended December 31, 2014, our cash liquidity (consisting of $3.7 million cash at December 31, 2014) increased as follows:
Cash and Cash Equivalents:
| (In thousands) | ||||
| At December 31, 2013 |
$ | 4,100 | ||
| At December 31, 2014 |
3,700 | |||
|
|
|
|||
| Decrease in cash and cash equivalents |
$ | (400 | ) | |
|
|
|
|||
The increase (decrease) in cash and cash equivalents is comprised of the following components for the years ended December 31, 2014 and 2013:
| 2014 | 2013 | |||||||
| (In thousands) | ||||||||
| Proceeds from loan |
$ | 8,000 | $ | — | ||||
| Proceeds from collaboration, technology business tax certificate transfer program and other projects |
2,000 | 10,300 | ||||||
|
|
|
|
|
|||||
| Sources of cash and cash equivalents |
10,000 | 10,300 | ||||||
|
|
|
|
|
|||||
| Uses of cash and cash equivalents |
(10,400 | ) | (7,700 | ) | ||||
|
|
|
|
|
|||||
| (Decrease) Increase in cash and cash equivalents |
$ | (400 | ) | $ | 2,600 | |||
|
|
|
|
|
|||||
During the year ended December 31, 2014, our working capital deficiency increased by $0.3 million as follows:
| December 31, | Change | |||||||||||
| 2014 | 2013 | |||||||||||
| (In thousands) | ||||||||||||
| Current assets |
$ | 5,939 | $ | 4,905 | $ | 1,063 | ||||||
| Current liabilities |
7,633 | 6,303 | 1,329 | |||||||||
|
|
|
|
|
|
|
|||||||
| Working capital (deficiency) |
$ | (1,694 | ) | $ | (1,398 | ) | $ | (266 | ) | |||
|
|
|
|
|
|
|
|||||||
The increase in current assets is driven primarily by the increase in inventory. The increase in current liabilities is driven primarily by the increase in fair value of derivative instruments.
Primary Sources of Cash
On January 21, 2014, and December 8, 2014, the Company received approximately $1.7 million and $0.3 million, respectively by participating in the Technology Business Tax Certificate Transfer Program, sponsored by the New Jersey Economic Development Authority. On August 20, 2014, and November 4, 2014, the Company received $5.0 million and $3.0 million in proceeds pursuant to a loan agreement entered into with MHR.
During 2013, the Company received $10.0 million when it entered into Amendment No. 2 to the Development and License Agreement, dated June 21, 2008, between it and Novo Nordisk. The Amendment provided, among other things, for a payment of $10.0 million from Novo Nordisk to the Company as a prepayment for the achievement of certain development milestones that would have otherwise become payable to the Company under the Development Agreement in exchange for a reduction in the rate of potential future royalty payments as provided in the Development Agreement.
During 2012, we received approximately $3.0 million from the sale of NJ State Net Operating Losses from prior periods through the 2011 and 2012 Technology Business Tax Certificate Transfer Program, sponsored by the New Jersey Economic Development Authority. Additional funding of $1.4 million was provided by MHR pursuant to the Bridge Note.
40
Table of Contents
Results of Operations
Year Ended December 31, 2014, Compared to Year Ended December 31, 2013
| Year Ended December 31, |
||||||||||||
| (In thousands) | ||||||||||||
| 2014 | 2013 | Change | ||||||||||
| Revenue |
$ | — | $ | — | $ | — | ||||||
| Operating expenses |
$ | 9,305 | $ | 7,604 | $ | 1,701 | ||||||
| Operating loss |
$ | (9,305 | ) | $ | (7,604 | ) | $ | (1,701 | ) | |||
| Change in fair value of derivative instruments |
$ | (11,872 | ) | $ | (8,433 | ) | $ | (3,439 | ) | |||
| Interest expense |
$ | (6,232 | ) | $ | (4,955 | ) | $ | (1,277 | ) | |||
| Other non-operating income |
$ | 10 | $ | 81 | $ | (71 | ) | |||||
| Income tax benefit |
$ | 2,019 | $ | (28 | ) | $ | 2,047 | |||||
| Net loss |
$ | (25,380 | ) | $ | (20,939 | ) | $ | (4,441 | ) | |||
Our principal operating costs include the following items as a percentage of total expense:
| Year Ended | ||||||||
| December 31, 2014 | December 31, 2013 | |||||||
| Human resource costs, including benefits |
32 | % | 40 | % | ||||
| Professional fees for legal, intellectual property, accounting and consulting |
50 | % | 44 | % | ||||
| Product development costs |
7 | % | 2 | % | ||||
| Occupancy costs |
2 | % | 3 | % | ||||
| Depreciation and amortization |
0 | % | 0 | % | ||||
| Other |
9 | % | 11 | % | ||||
Operating expenses, increased by $1.7 million or 22% as a result of the following items:
| (In thousands) | ||||
| Decrease in human resource costs |
$ | (100 | ) | |
| Increase in professional and consulting fees |
1,300 | |||
| Increase in product development costs |
400 | |||
| All other |
100 | |||
|
|
|
|||
| Net increase |
$ | 1,700 | ||
|
|
|
|||
Human resource costs decreased approximately $0.1 million due primarily to reductions in headcount.
Professional and consulting fees increased approximately $1.3 million due to an increase in sales and marketing expenses and advertising expenses related to the launch of Eligen B-12 of approximately $1.2 million, and, and an increase in legal fees related to the August 2014 restructuring of approximately $0.1 million.
Product development costs increased approximately $0.4 million primarily due to costs associated with developing and optimizing manufacturing production of oral Eligen B12™ Rx.
Occupancy costs were substantially unchanged in 2014 compared to 2013.
Depreciation and amortization expense were substantially unchanged in 2014 compared to 2013.
All other operating costs increased approximately $0.1 million.
As a result of the factors above, Emisphere’s operating expenses were $9.3 million for the year ended December 31, 2014, which represents an increase of $1.7 million (22%) compared to operating expenses for the year ended December 31, 2013.
41
Table of Contents
Other non-operating expense increased by approximately $4.8 million for the year ended December 31, 2014, in comparison to the same period last year due primarily to a $3.4 million increase in the change in the value of derivative instruments, and a $1.3 million increase in interest expense due primarily to the additional accrued interest on the restatement of the Convertible Notes following the August 2014 restructuring described above. The change in the fair value of derivative instruments for 2014 and 2013 is the result of a higher fair value of the Company’s stock price and a higher estimated future volatility at December 31, 2014 compared to December 31, 2013. Future gains and losses recognized in the Company’s operating results from changes in value of the derivative instrument liability are based in part on the fair value of the Company’s common stock which is outside the control of the Company. These potential future gains and losses could be material.
During 2014, we recognized a state income tax benefit of approximately $2.0 million as a result of proceeds from the sale of $24.6 million of New Jersey net operating losses through the Technology Business Certificate Transfer Program, sponsored by the New Jersey Economic Development Authority.
As a result of the above factors, we reported a net loss of $25.4 million, which was $4.4 million (21%) higher than the net loss of $20.9 million for the year ended December 31, 2013.
Year Ended December 31, 2013, Compared to Year Ended December 31, 2012
| Year Ended December 31, |
||||||||||||
| (In thousands) | ||||||||||||
| 2013 | 2012 | Change | ||||||||||
| Revenue |
$ | — | $ | — | $ | — | ||||||
| Operating expenses |
$ | 7,604 | $ | 6,821 | $ | 811 | ||||||
| Operating loss |
$ | (7,604 | ) | $ | (6,821 | ) | $ | (811 | ) | |||
| Change in fair value of derivative instruments |
$ | (8,433 | ) | $ | 8,110 | $ | (16,543 | ) | ||||
| Interest expense |
$ | (4,955 | ) | $ | (6,236 | ) | $ | 1,281 | ||||
| Other non-operating income |
$ | 81 | $ | 45 | $ | 36 | ||||||
| Income tax benefit (expense) |
$ | (28 | ) | $ | 2,974 | $ | (3,002 | ) | ||||
| Net loss |
$ | (20,939 | ) | $ | (1,928 | ) | $ | (19,011 | ) | |||
Our principal operating costs include the following items as a percentage of total expense:
| Year Ended | ||||||||
| December 31, 2013 | December 31, 2012 | |||||||
| Human resource costs, including benefits |
40 | % | 38 | % | ||||
| Professional fees for legal, intellectual property, accounting and consulting |
44 | % | 40 | % | ||||
| Clinical and laboratory costs |
2 | % | 8 | % | ||||
| Occupancy costs |
3 | % | 5 | % | ||||
| Depreciation and amortization |
0 | % | 0 | % | ||||
| Other |
11 | % | 9 | % | ||||
Operating expenses, increased by $0.8 million or 12% as a result of the following items:
| (In thousands) | ||||
| Increase in human resource costs |
$ | 500 | ||
| Increase in professional and consulting fees |
600 | |||
| Decrease in occupancy costs |
(200 | ) | ||
| Decrease in laboratory costs |
(300 | ) | ||
| Increase in all other operating costs |
200 | |||
|
|
|
|||
| Net increase |
$ | 800 | ||
|
|
|
|||
42
Table of Contents
Human resource costs increased approximately $0.5 million due primarily to the award of bonuses and related employer taxes.
Professional and consulting fees increased approximately $0.6 million due to an increase in consulting fees of approximately $0.5 million (primarily from new consulting contracts for business development services, scientific services and medical consulting services); $0.3 million in other professional fees (primarily from restructuring costs, oral Eligen B-12™ Rx launch costs, and upgrades to our information technology); offset by a $0.2 million reduction in legal and accounting fees.
Occupancy costs decreased approximately $0.2 million due to the lease expiration at the former Cedar Knolls Offices and the reduction in square footage at the Company’s Roseland Offices.
Laboratory fees decreased approximately $0.3 million due primarily to a 2012 charge for the reduction of a prepaid asset.
Depreciation expense was substantially unchanged in 2013 compared to 2012.
All other operating costs increased $0.2 million due to investment in commercial preparations to launch the oral Eligen B-12™ Rx product.
As a result of the factors above, Emisphere’s operating expenses were $7.6 million for the year ended December 31, 2013, which represents an increase of $0.8 million or 11% compared to operating expenses for the year ended December 31, 2012.
Other non-operating expense increased by approximately $15.2 million for the year ended December 31, 2013 in comparison to the same period last year due primarily to a $16.5 million increase in the change in the value of derivative instruments, offset by a $1.3 million decrease in interest expense due to the restructuring of the MHR notes. The change in the fair value of derivative instruments for 2013 and 2012 is the result of the revaluation of derivative instruments from the restructuring of the Convertible notes payable, bridge notes and reimbursement notes. Future gains and losses recognized in the Company’s operating results from changes in value of the derivative instrument liability are based in part on the fair value of the Company’s common stock which is outside the control of the Company. These potential future gains and losses could be material.
In 2012, we recognized a state income tax benefit of approximately $3.0 million as a result of proceeds from the sale of $36 million of New Jersey net operating losses through the Technology Business Certificate Transfer Program, sponsored by the New Jersey Economic Development Authority. A state income tax benefit for 2013 of $1.7 million was received and recognized during the first quarter of 2014.
As a result of the above factors, we reported a net loss of $20.9 million, which was $19.0 million (985%) greater than the net loss of $1.9 million for the year ended December 31, 2012.
Critical Accounting Estimates and New Accounting Pronouncements
Critical Accounting Estimates
The preparation of financial statements in accordance with accounting principles generally accepted in the U.S. requires management to make estimates and assumptions that affect reported amounts and related disclosures in the financial statements. Management considers an accounting estimate to be critical if:
| Ÿ | It requires assumptions to be made that were uncertain at the time the estimate was made, and |
| Ÿ | Changes in the estimate or different estimates that could have been selected could have a material impact on our results of operations or financial condition. |
Share-Based Payments — We recognize expense for our share-based compensation in accordance with FASB ASC 718, “Compensation-Stock Compensation”, which establishes standards for share-based transactions in which an entity receives employee’s services for (a) equity instruments of the entity, such as stock options, or (b) liabilities that are based on the fair value of the entity’s equity instruments or that may be settled by the issuance of such equity instruments. FASB ASC 718 requires that companies expense the fair value of stock options and similar awards, as measured on the awards’ grant date. FASB ASC 718 applies to all awards granted after the date of adoption, and to awards modified, repurchased or cancelled after that date.
43
Table of Contents
We estimate the value of stock option awards on the date of grant using the Black-Scholes-Merton (“Black-Scholes”) option-pricing model. The determination of the fair value of share-based payment awards on the date of grant is affected by our stock price as well as assumptions regarding a number of complex and subjective variables. These variables include our expected stock price volatility over the term of the awards, expected term, risk-free interest rate, expected dividends and expected forfeiture rates.
If factors change and we employ different assumptions in the application of FASB ASC 718 in future periods, the compensation expense that we record under FASB ASC 718 may differ significantly from what we have recorded in the current period. There is a high degree of subjectivity involved when using option pricing models to estimate share-based compensation under FASB ASC 718. Consequently, there is a risk that our estimates of the fair values of our share-based compensation awards on the grant dates may bear little resemblance to the actual values realized upon the exercise, expiration, early termination or forfeiture of those share-based payments in the future. Employee stock options may expire worthless or otherwise result in zero intrinsic value as compared to the fair values originally estimated on the grant date and reported in our financial statements. Alternatively, value may be realized from these instruments that are significantly in excess of the fair values originally estimated on the grant date and reported in our financial statements. During the year ended December 31, 2011, we do not believe that reasonable changes in the projections would have had a material effect on share-based compensation expense.
Revenue Recognition — Revenue includes amounts earned from sales of our oral Eligen® B12 (100 mcg) product, collaborative agreements and feasibility studies. Revenue earned from the sale of oral Eligen® B12 (100 mcg) was recognized when the product was shipped, when all revenue recognition criteria were met in accordance with Staff Accounting Bulletin No. 104 , “Revenue Recognition” (codified under ASC 605 “Revenue Recognition”). Our Distributor Agreement for the marketing, distribution and sale of oral Eligen® B12 100 mcg) with Quality Vitamins and Supplements, Inc. was terminated during the third quarter, 2010. Revenue from feasibility studies, which are typically short term in nature, is recognized upon delivery of the study, provided that all other revenue recognition criteria are met. Revenue from collaboration agreements are recognized using the proportional performance method provided that we can reasonably estimate the level of effort required to complete our performance obligations under an arrangement and such performance obligations are provided on a best effort basis and based on “expected payments.” Under the proportional performance method, periodic revenue related to nonrefundable cash payments is recognized as the percentage of actual effort expended to date as of that period to the total effort expected for all of our performance obligations under the arrangement. Actual effort is generally determined based upon actual hours incurred and include research and development (“R&D”) activities performed by us and time spent for joint steering committee (“JSC”) activities. Total expected effort is generally based upon the total R&D and JSC hours incorporated into the project plan that is agreed to by both parties to the collaboration. Significant management judgments and estimates are required in determining the level of effort required under an arrangement and the period over which we expect to complete the related performance obligations. Estimates of the total expected effort included in each project plan are based on historical experience of similar efforts and expectations based on the knowledge of scientists for both the Company and its collaboration partners. The Company periodically reviews and updates the project plan for each collaborative agreement. The most recent reviews took place in January 2013. In the event that a change in estimate occurs, the change will be accounted for using the cumulative catch-up method which provides for an adjustment to revenue in the current period. Estimates of our level of effort may change in the future, resulting in a material change in the amount of revenue recognized in future periods.
Generally under collaboration arrangements, nonrefundable payments received during the period of performance may include time- or performance-based milestones. The proportion of actual performance to total expected performance is applied to the “expected payments” in determining periodic revenue. However, revenue is limited to the sum of (1) the amount of nonrefundable cash payments received and (2) the payments that are contractually due but have not yet been paid.
With regard to revenue recognition from collaboration agreements, the Company previously interpreted expected payments to equate to total payments subject to each collaboration agreement. On a prospective basis, the Company has revised its application of expected payments to equate to a “best estimate” of payments. Under this application, expected payments typically include (i) payments already received and (ii) those milestone
44
Table of Contents
payments not yet received but that the Company believes are “more likely than not” of receiving. Our support for the assertion that the next milestone is likely to be met is based on the (a) project status updates discussed at JSC meetings; (b) clinical trial/development results of prior phases; (c) progress of current clinical trial/development phases; (d) directional input of collaboration partners and (e) knowledge and experience of the Company’s scientific staff. After considering the above factors, the Company believes those payments included in “expected payments” are more likely than not of being received. While this interpretation differs from that used previously by the Company, it does not result in any change to previously recognized revenues in either timing or amount for periods through December 31, 2013.
With regard to revenue recognition in connection with the Insulins License Agreement and the GLP-1 License Agreements with Novo Nordisk, such agreements include multiple deliverables including license grants, several versions of the Company’s Eligen® Technology (or carriers), support services and manufacturing. Emisphere’s management reviewed the relevant terms of the Novo Nordisk agreements and determined such deliverables should be accounted for as a single unit of accounting in accordance with FASB ASC 605-25, “Multiple-Element Arrangements” since the delivered license and Eligen® Technology do not have stand-alone value and Emisphere does not have objective evidence of fair value of the undelivered Eligen® Technology or the manufacturing value of all the undelivered items. Such conclusion will be reevaluated as each item in the arrangement is delivered. Consequently, any payments received from Novo Nordisk pursuant to such agreements, including the initial $10 million upfront payment and any payments received for support services in connection with the GLP-1 License Agreement and the $5 million upfront payment from the Insulins License Agreement will be deferred and included in Deferred Revenue within our balance sheet. Management cannot currently estimate when all of such deliverables will be delivered nor can they estimate when, if ever, Emisphere will have objective evidence of the fair value for all of the undelivered items, therefore all payments from Novo Nordisk are expected to be deferred for the foreseeable future.
As of December 31, 2014, total deferred revenue from the GLP-1 License Agreement was $23.6 million, comprised of the $10.0 million April 26, 2013, prepayment, the $10.0 million non-refundable license fee, $2 million milestone payment and $1.6 million in support services.
With regard to revenue recognition in connection with Novartis’ discontinued oral salmon calcitonin program for osteoporosis and osteoarthritis, discontinued oral PTH-1-34 program for osteoporosis, and terminated oral recombinant human growth hormone program: all such agreements include(d) multiple deliverables including license grants, several versions of the Company’s Eligen® Technology (or carriers) and support services. Emisphere’s management reviewed the relevant terms of each development license agreement with Novartis and determined such deliverables should be accounted for as a single unit of accounting in accordance with FASB ASC 605-25, “Multiple-Element Arrangements” since the delivered license and Eligen® Technology do not have stand-alone value and Emisphere does not have objective evidence of fair value of the undelivered Eligen® Technology. Such conclusion will be reevaluated as each item in the arrangement is delivered or the status of each agreement changes. Consequently, any payments received from Novartis pursuant to such agreements have been deferred and included in Deferred Revenue within our balance sheet.
During 2011, Novartis terminated its oral human growth hormone program and informed the Company of its intention not to continue development of its oral calcitonin and oral PTH programs involving Emisphere’s Eligen® Technology. However, Novartis did not terminate its development license agreements in calcitonin or PTH. At such time that Novartis terminates its oral calcitonin and oral PTH agreements, or does not demonstrate reasonable commercial effort to continue developing oral calcitonin or oral PTH products, then the Company will recognize revenue in connection with past receipts of payments from Novartis derived from those agreements which are currently included in Deferred Revenue within our balance sheet. Management will pay close attention to Novartis actions and reevaluate circumstances that influence this determination in future.
As of December 31, 2014 total deferred revenue from all Novartis development license programs was approximately $13.0 million, comprised of the principal value ($10 million) plus interest ($3.0 million) we recorded on June 4, 2010, upon executing the Novartis Agreement, pursuant to which the Company was released and discharged from its obligations under the Novartis Note described in Note 7 to the Financial Statements included herein.
45
Table of Contents
Purchased Technology — Purchased technology represents the value assigned to patents and the rights to use, sell or license certain technology in conjunction with our proprietary carrier technology. These assets underlie our research and development projects related to various research and development projects. In December 2011, the Company reviewed its purchased technology in light of industry trends and advances in reformulating and stabilizing active pharmaceutical ingredients through the development of fractions and analogs, and determined that its technology is no longer applicable in the development of a potential future oral formulation of heparin. As a result the net book value of the purchased technology was not deemed recoverable and the Company realized an impairment charge of $0.6 million.
Warrants and Conversion Feature of Amended and Restated Convertible Note — Warrants issued in connection with various equity financings and embedded conversion feature of the Amended and Restated Convertible Notes, Bridge Notes and Reimbursement Notes described in Note 7 to the Financial Statements have been classified as liabilities due to certain provisions that may require cash settlement in certain circumstances. At each balance sheet date, we adjust the warrants to reflect their current fair value. For derivatives other than the Amended and Restated Convertible Notes, Bridge Notes, Reimbursement Notes, and June 2010 Warrants, we estimate the fair value of these instruments using the Black-Scholes model which takes into account a variety of factors, including historical stock price volatility, risk-free interest rates, remaining term and the closing price of our common stock. Changes in the assumptions used to estimate the fair value of these derivative instruments could result in a material change in the fair value of the instruments. The fair value of the embedded conversion feature of the Amended and Restated Convertible Notes, Amended and Restated June 2010 Warrants, Amended and Restated Bridge Notes and Amended and Restated Reimbursement Notes contain anti-dilution protection provisions, which are triggered by potentially dilutive events (including subsequent common share offerings meeting certain criteria). Due to these additional protective provisions within the instruments, additional value has been provided to the holders, which has not been provided to other equity investors. In order to estimate the value of this protection, the Company uses the Monte Carlo valuation model which assesses the probability of the occurrence of potential triggering events, such as the probability of the Company’s engaging in a capital or debt markets offering to calculate the value of the derivative at the reporting date. We believe the assumptions used to estimate the fair values of the warrants and convertible shares are reasonable. For a more complete discussion on the volatility in market value of derivative instruments, see Part I, Item 7A “Quantitative and Qualitative Disclosures about Market Risk.”
Clinical Trial Accrual Methodology — Clinical trial expenses represent obligations resulting from our contracts with various research organizations in connection with conducting clinical trials for our product candidates. We account for those expenses on an accrual basis according to the progress of the trial as measured by patient enrollment and the timing of the various aspects of the trial. Accruals are recorded in accordance with the following methodology: (i) the costs for period expenses, such as investigator meetings and initial start-up costs, are expensed as incurred based on management’s estimates, which are impacted by any change in the number of sites, number of patients and patient start dates; (ii) direct service costs, which are primarily on-going monitoring costs, are recognized on a straight-line basis over the life of the contract; and (iii) principal investigator expenses that are directly associated with recruitment are recognized based on actual patient recruitment. All changes to the contract amounts due to change orders are analyzed and recognized in accordance with the above methodology. Change orders are triggered by changes in the scope, time to completion and the number of sites. During the course of a trial, we adjust our rate of clinical expense recognition if actual results differ from our estimates.
New Accounting Pronouncements
In May 2014, the FASB issued ASU No. 2014-09, “Revenue from Contracts with Customers” (“ASU 2014-09”), which requires entities to recognize revenue in a way that depicts the transfer of promised goods or services to customers in an amount that reflects the consideration to which the entity expects to be entitled to in exchange for those goods or services. The new guidance also requires additional disclosure about the nature, amount, timing and uncertainty of revenue and cash flows arising from customer contracts, including significant judgments and changes in judgments and assets recognized from costs incurred to obtain or fulfill a contract. ASU 2014-09 is effective for fiscal years, and interim periods within those years, beginning after December 15, 2016. The Company is currently evaluating the impact of the new standards.
46
Table of Contents
In August 2014, the FASB issued ASU No. 2014-15, “Disclosure of Uncertainties About an Entity’s Ability to Continue as a Going Concern” (“ASU 2014-15”), which provides guidance on management’s responsibility in evaluating whether there is substantial doubt about an entity’s ability to continue as a going concern and to provide related footnote disclosures. ASU 2014-15 is effective for the annual period ending after December 15, 2016, and for annual and interim periods thereafter. The adoption of ASU 2014-15 is not expected to have a material impact on our financial position, results of operations or cash flows.
Management does not believe there would have been a material effect on the accompanying financial statements had any other recently issued, but not yet effective, accounting standards been adopted in the current period.
Off-Balance Sheet Arrangements
As of December 31, 2014, we had no material off-balance sheet arrangements.
In the ordinary course of business, we enter into agreements with third parties that include indemnification provisions which, in our judgment, are normal and customary for companies in our industry sector. These agreements are typically with business partners, clinical sites, and suppliers. Pursuant to these agreements, we generally agree to indemnify, hold harmless, and reimburse indemnified parties for losses suffered or incurred by the indemnified parties with respect to our product candidates, use of such product candidates, or other actions taken or omitted by us. The maximum potential amount of future payments we could be required to make under these indemnification provisions is unlimited. We have not incurred material costs to defend lawsuits or settle claims related to these indemnification provisions. As a result, the estimated fair value of liabilities relating to these provisions is minimal. Accordingly, we have no liabilities recorded for these provisions as of December 31, 2014.
In the normal course of business, we may be confronted with issues or events that may result in a contingent liability. These generally relate to lawsuits, claims, environmental actions or the actions of various regulatory agencies. We consult with counsel and other appropriate experts to assess the claim. If, in our opinion, we have incurred a probable loss as set forth by accounting principles generally accepted in the U.S., an estimate is made of the loss and the appropriate accounting entries are reflected in our financial statements.
Contractual Arrangements
Significant contractual obligations as of December 31, 2014 are as follows:
| Amount Due in | ||||||||||||||||||||
| Type of Obligation |
Total | Less than 1 Year |
1 to 3 Years |
3 to 5 Years |
More than 5 Years |
|||||||||||||||
| (In thousands) | ||||||||||||||||||||
| Notes Payable(1) |
$ | 51,741 | $ | — | $ | — | $ | — | $ | 51,741 | ||||||||||
| Derivative liabilities(2) |
29,920 | 5,787 | — | — | 24,133 | |||||||||||||||
| Operating lease obligations |
358 | 136 | 222 | — | — | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total |
$ | 82,019 | $ | 5,923 | $ | 222 | $ | — | $ | 75,874 | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| (1) | Amounts include both principal and related interest payments. |
| (2) | We have issued warrants to purchase shares of our common stock which contain provisions requiring us to make a cash payment to the holders of the warrant for any gain that could have been realized if the holders exercise the warrants and we subsequently fail to deliver a certificate representing the shares to be issued upon such exercise by the third trading day after such warrants have been exercised. As a result, these warrants have been recorded at their fair value and are classified as current liabilities. The value and timing of the actual cash payments, if any, related to these derivative instruments could differ materially from the amounts and periods shown. |
47
Table of Contents
| ITEM 7A. QUANTITATIVE | AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK |
Fair Value of Warrants and Derivative Liabilities. At December 31, 2014, the value of derivative instruments was $29.9 million. We estimate the fair values of these instruments using the Black-Scholes option pricing model which takes into account a variety of factors, including historical stock price volatility, risk-free interest rates, remaining maturity and the closing price of our common stock. Furthermore, the estimated fair values of the conversion features embedded in our Convertible Notes, Bridge Notes, Reimbursement Notes, and Amended and Restated June 2010 Warrants, which contain reset provisions, were measured using the Monte Carlo valuation model. In using the Monte Carlo model, we estimate the probability and timing of potential future financing and fundamental transactions as applicable. We are required to revalue this liability each quarter. We believe that the assumption that has the greatest impact on the determination of fair value is the closing price of our common stock. The following table illustrates the potential effect of changes in the assumptions used to calculate fair value:
| Increase/(Decrease) | ||||
| (In thousands) | ||||
| 25% increase in stock price |
$ | 3,596 | ||
| 50% increase in stock price |
$ | 8,691 | ||
| 5% increase in assumed volatility |
$ | 650 | ||
| 25% decrease in stock price |
$ | (3,517 | ) | |
| 50% decrease in stock price |
$ | (6,021 | ) | |
| 5% decrease in assumed volatility |
$ | (116 | ) | |
48
Table of Contents
| ITEM 8. FINANCIAL | STATEMENTS AND SUPPLEMENTARY DATA |
EMISPHERE TECHNOLOGIES, INC.
FINANCIAL STATEMENTS
Index
49
Table of Contents
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders
Emisphere Technologies, Inc.
We have audited the accompanying balance sheets of Emisphere Technologies, Inc. as of December 31, 2014 and 2013, and the related statements of operations, stockholders’ deficit, and cash flows for each of the three years in the period ended December 31, 2014. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of Emisphere Technologies, Inc. as of December 31, 2014 and 2013, and the results of its operations and its cash flows for each of the three years in the period ended December 31, 2014, in conformity with U.S. generally accepted accounting principles.
We have also audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), Emisphere Technologies, Inc.‘s internal control over financial reporting as of December 31, 2014, based on criteria established in Internal Control — Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission in 2013, and our report dated March 31, 2015 expressed an unqualified opinion on the effectiveness of Emisphere Technologies, Inc.’s internal control over financial reporting.
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 1 to the financial statements, the Company has suffered recurring losses from operations, has a working capital deficiency and a significant stockholders’ deficit, and has limited cash availability. This raises substantial doubt about the Company’s ability to continue as a going concern. Management’s plans in regard to these matters are also described in Note 1. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
/s/ McGladrey LLP
New York, NY
March 31, 2015
50
Table of Contents
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders
Emisphere Technologies, Inc.
We have audited Emisphere Technologies, Inc.‘s internal control over financial reporting as of December 31, 2014, based on criteria established in Internal Control — Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission in 2013. Emisphere Technologies, Inc’s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting included in the accompanying Management’s Report on Internal Control over Financial Reporting. Our responsibility is to express an opinion on the company’s internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audit also included performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (a) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (b) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (c) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, Emisphere Technologies, Inc. maintained, in all material respects, effective internal control over financial reporting as of December 31, 2014, based on criteria established in Internal Control — Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission in 2013.
We have also audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the balance sheets of Emisphere Technologies, Inc. as of December 31, 2014 and 2013, and the related statements of operations, stockholder’s deficit and cash flows for each of the three years in the period ended December 31, 2014 and our report dated March 31, 2015 expressed an unqualified opinion.
/s/ McGladrey LLP
New York, NY
March 31, 2015
51
Table of Contents
BALANCE SHEETS
| December 31, | ||||||||
| 2014 | 2013 | |||||||
| (In thousands, except share data) |
||||||||
| ASSETS | ||||||||
| Current assets: |
||||||||
| Cash and cash equivalents |
$ | 3,683 | $ | 4,053 | ||||
| Inventories |
2,068 | 230 | ||||||
| Prepaid expenses and other current assets |
188 | 622 | ||||||
|
|
|
|
|
|||||
| Total current assets |
5,939 | 4,905 | ||||||
| Equipment and leasehold improvements, net |
25 | 40 | ||||||
| Other assets |
24 | 34 | ||||||
|
|
|
|
|
|||||
| Total assets |
$ | 5,988 | $ | 4,979 | ||||
|
|
|
|
|
|||||
| LIABILITIES AND STOCKHOLDERS’ DEFICIT | ||||||||
| Current liabilities: |
||||||||
| Notes payable, related party, net of related discount |
$ | — | $ | 556 | ||||
| Accounts payable and accrued expenses |
1,846 | 1,539 | ||||||
| Derivative instruments: |
||||||||
| Related party |
5,548 | 3,638 | ||||||
| Others |
239 | 540 | ||||||
| Other current liabilities |
— | 30 | ||||||
|
|
|
|
|
|||||
| Total current liabilities |
7,633 | 6,303 | ||||||
| Notes payable, related party net of related discount |
44,546 | 32,523 | ||||||
| Derivative instruments — Related party |
24,133 | 11,331 | ||||||
| Deferred revenue |
41,616 | 41,616 | ||||||
| Deferred lease liability and other liabilities |
10 | 7 | ||||||
|
|
|
|
|
|||||
| Total liabilities |
117,938 | 91,780 | ||||||
|
|
|
|
|
|||||
| Commitments and contingencies |
— | — | ||||||
| Stockholders’ deficit: |
||||||||
| Preferred stock, $.01 par value; authorized 4,000,000 shares at December 31, 2014; and authorized 2,000,000 shares at December 31, 2013; issued and outstanding at December 31, 2014 — none |
— | — | ||||||
| Common stock, $.01 par value; authorized 400,000,000 shares at December 31, 2014; and authorized 200,000,000 at December 31, 2013; issued 60,977,210 shares (60,687,478 outstanding) at December 31, 2014 and 2013 |
610 | 610 | ||||||
| Additional paid-in capital |
405,531 | 405,300 | ||||||
| Accumulated deficit |
(514,139 | ) | (488,759 | ) | ||||
| Common stock held in treasury, at cost; 289,732 shares |
(3,952 | ) | (3,952 | ) | ||||
|
|
|
|
|
|||||
| Total stockholders’ deficit |
(111,950 | ) | (86,801 | ) | ||||
|
|
|
|
|
|||||
| Total liabilities and stockholders’ deficit |
$ | 5,988 | $ | 4,979 | ||||
|
|
|
|
|
|||||
(See accompanying Notes to the Financials)
52
Table of Contents
STATEMENTS OF OPERATIONS
| Year Ended December 31, | ||||||||||||
| 2014 | 2013 | 2012 | ||||||||||
| (In thousands, except share and per share data) | ||||||||||||
| Revenue |
$ | — | $ | — | $ | — | ||||||
| Cost of goods sold |
— | — | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Gross profit |
— | — | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Costs and expenses: |
||||||||||||
| Research and development |
1,128 | 836 | 1,867 | |||||||||
| General and administrative |
8,162 | 6,749 | 4,935 | |||||||||
| Loss (gain) on disposal of fixed assets |
— | 10 | (10 | ) | ||||||||
| Depreciation and amortization |
15 | 9 | 29 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total costs and expenses |
9,305 | 7,604 | 6,821 | |||||||||
|
|
|
|
|
|
|
|||||||
| Operating loss |
(9,305 | ) | (7,604 | ) | (6,821 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Other non-operating income (expense): |
||||||||||||
| Investment and other income |
10 | 81 | 45 | |||||||||
| Change in fair value of derivative instruments: |
||||||||||||
| Related party |
(12,172 | ) | (8,491 | ) | 7,880 | |||||||
| Others |
300 | 58 | 230 | |||||||||
| Interest expense — related party |
(6,232 | ) | (4,955 | ) | (6,236 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Total other non-operating income (expense) |
(18,094 | ) | (13,307 | ) | 1,919 | |||||||
|
|
|
|
|
|
|
|||||||
| Loss before income tax benefit (expense) |
(27,399 | ) | (20,911 | ) | (4,902 | ) | ||||||
| Income tax benefit (expense) |
2,019 | (28 | ) | 2,974 | ||||||||
|
|
|
|
|
|
|
|||||||
| Net loss |
$ | (25,380 | ) | $ | (20,939 | ) | $ | (1,928 | ) | |||
|
|
|
|
|
|
|
|||||||
| Net loss per share, basic |
$ | (0.42 | ) | $ | (0.35 | ) | $ | (0.03 | ) | |||
|
|
|
|
|
|
|
|||||||
| Net loss per share, diluted |
$ | (0.42 | ) | $ | (0.35 | ) | $ | (0.03 | ) | |||
|
|
|
|
|
|
|
|||||||
| Weighted average shares outstanding, basic |
60,687,478 | 60,687,478 | 60,687,478 | |||||||||
|
|
|
|
|
|
|
|||||||
| Weighted average shares outstanding, diluted |
60,687,478 | 60,687,478 | 60,687,478 | |||||||||
|
|
|
|
|
|
|
|||||||
(See accompanying Notes to the Financials)
53
Table of Contents
STATEMENTS OF CASH FLOWS
| Year Ended December 31, | ||||||||||||
| 2014 | 2013 | 2012 | ||||||||||
| (In thousands) | ||||||||||||
| Cash flows from operating activities: |
||||||||||||
| Net loss |
$ | (25,380 | ) | $ | (20,939 | ) | $ | (1,928 | ) | |||
|
|
|
|
|
|
|
|||||||
| Adjustments to reconcile net loss to net cash provided by (used in) operating activities: |
||||||||||||
| Depreciation and amortization |
15 | 9 | 29 | |||||||||
| Non-cash interest expense: |
||||||||||||
| Related party |
6,007 | 4,955 | 6,236 | |||||||||
| Others |
— | — | — | |||||||||
| Changes in the fair value of derivative instruments: |
||||||||||||
| Related party |
12,172 | 8,491 | (7,880 | ) | ||||||||
| Others |
(300 | ) | (58 | ) | (230 | ) | ||||||
| Non-cash compensation |
231 | 205 | 344 | |||||||||
| Loss (gain) on disposal of fixed assets |
— | 10 | (10 | ) | ||||||||
| Provision for bad debts |
— | — | 31 | |||||||||
| Changes in assets and liabilities excluding non-cash charges: |
||||||||||||
| Decrease (increase) in accounts receivable |
— | 1 | (10 | ) | ||||||||
| (Increase) decrease inventories |
(1,361 | ) | 19 | 9 | ||||||||
| (Increase) decrease in prepaid expenses and other current assets |
(43 | ) | (473 | ) | 432 | |||||||
| Decrease (increase) in security deposits |
10 | — | (34 | ) | ||||||||
| Increase in accounts payable, accrued expenses and other |
306 | 615 | 29 | |||||||||
| (Decrease) increase in other current liabilities |
(30 | ) | 30 | (33 | ) | |||||||
| Increase in deferred revenue |
— | 10,002 | 21 | |||||||||
| (Increase) decrease in deferred lease and other liabilities |
3 | (2 | ) | (4 | ) | |||||||
|
|
|
|
|
|
|
|||||||
| Total adjustments |
17,010 | 23,804 | (1,070 | ) | ||||||||
|
|
|
|
|
|
|
|||||||
| Net cash (used in) provided by operating activities |
(8,370 | ) | 2,865 | (2,998 | ) | |||||||
|
|
|
|
|
|
|
|||||||
| Cash flows from investing activities: |
||||||||||||
| Decrease in restricted cash |
— | 247 | — | |||||||||
| Purchase of fixed assets |
— | (46 | ) | — | ||||||||
| Proceeds from sale of fixed assets |
— | — | 13 | |||||||||
|
|
|
|
|
|
|
|||||||
| Net cash provided by investing activities |
— | 201 | 13 | |||||||||
|
|
|
|
|
|
|
|||||||
| Cash flows from financing activities: |
||||||||||||
| Proceeds from notes payable |
8,000 | — | 1,400 | |||||||||
| Payments for debt issue costs |
— | (497 | ) | — | ||||||||
|
|
|
|
|
|
|
|||||||
| Net cash (used in) provided by financing activities |
8,000 | (497 | ) | 1,400 | ||||||||
|
|
|
|
|
|
|
|||||||
| Net increase (decrease) in cash and cash equivalents |
(370 | ) | 2,569 | (1,585 | ) | |||||||
| Cash and cash equivalents, beginning of year |
4,053 | 1,484 | 3,069 | |||||||||
|
|
|
|
|
|
|
|||||||
| Cash and cash equivalents, end of year |
$ | 3,683 | $ | 4,053 | $ | 1,484 | ||||||
|
|
|
|
|
|
|
|||||||
| Non-cash investing and financing activities: |
||||||||||||
| Debt discounts issued in debt modifications |
$ | — | $ | 4,041 | $ | — | ||||||
| Conversion of accrued interest to notes payable |
$ | 5,542 | $ | 3,031 | $ | — | ||||||
(See accompanying Notes to the Financials)
54
Table of Contents
STATEMENTS OF STOCKHOLDERS’ DEFICIT
For the years ended December 31, 2014, 2013 and 2012
| Common Stock | Additional Paid-in Capital |
Accumulated Deficit |
Common Stock Held in Treasury Shares Amount |
Total | ||||||||||||||||||||||||
| Shares | Amount | |||||||||||||||||||||||||||
| (In thousands except share data) | ||||||||||||||||||||||||||||
| Balance, December 31, 2011 |
60,977,210 | $ | 610 | $ | 404,707 | $ | (465,892 | ) | 289,732 | $ | (3,952 | ) | $ | (64,527 | ) | |||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Net Loss |
(1,928 | ) | (1,928 | ) | ||||||||||||||||||||||||
| Capital contributed from imputed interest |
45 | 45 | ||||||||||||||||||||||||||
| Stock based compensation for employees |
176 | 176 | ||||||||||||||||||||||||||
| Stock based compensation for directors |
168 | 168 | ||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Balance, December 31, 2012 |
60,977,210 | $ | 610 | $ | 405,096 | $ | (467,820 | ) | 289,732 | $ | (3,952 | ) | $ | (66,066 | ) | |||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Net Loss |
(20,939 | ) | (20,939 | ) | ||||||||||||||||||||||||
| Stock based compensation for employees |
47 | 47 | ||||||||||||||||||||||||||
| Stock based compensation for directors |
157 | 157 | ||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Balance, December 31, 2013 |
60,977,210 | $ | 610 | $ | 405,300 | $ | (488,759 | ) | 289,732 | $ | (3,952 | ) | $ | (86,801 | ) | |||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Net Loss |
(25,380 | ) | (25,380 | ) | ||||||||||||||||||||||||
| Stock based compensation for employees |
71 | 71 | ||||||||||||||||||||||||||
| Stock based compensation for directors |
160 | 160 | ||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Balance, December 31, 2014 |
60,977,210 | $ | 610 | $ | 405,531 | $ | (514,139 | ) | 289,732 | $ | (3,952 | ) | $ | (111,950 | ) | |||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
55
Table of Contents
NOTES TO FINANCIAL STATEMENTS
1. Nature of Operations, Risks and Uncertainties and Liquidity
Nature of Operations. Emisphere Technologies, Inc. (“Emisphere,” “the Company,” “our,” “us,” or “we”) is a commercial stage, specialty pharmaceutical company that has recently commenced commercial operations. The Company launched its first prescription product, oral Eligen B12™ Rx, in the U.S. during March 2015. Additionally, the Company is currently engaged in multiple ex-US licensing discussions with the intention of offering oral Eligen B12™ Rx for sale in global markets. Beyond Eligen B12™ Rx, the Company utilizes its proprietary Eligen® Technology to create new oral formulations of therapeutic agents. Emisphere is currently partnered with global pharmaceutical companies for the development of new orally delivered therapeutics
By building on the oral Eligen B12™ Rx product, the Company intends to establish a sound product portfolio platform on which to expand its B12 therapeutic franchise as well as expand internal new product development with new therapeutic agents. The Company will also continue to develop its existing drug delivery carrier partnerships and expand its carrier business by seeking out and engaging in new global licensing opportunities.
Our core business strategy is to pursue the commercialization of oral Eligen B12™ Rx, build new, high-value partnerships and continue to expand upon existing partnerships, evaluate commercial opportunities for new prescription medical foods, and promote new uses for our Eligen® Technology, a broad-based proprietary oral drug delivery platform which makes it possible to avoid injections for drug administration through the use of delivery agents, or “carriers,” which facilitate or enable transport of therapeutic molecules, including large peptides and proteins, across biological membranes such as those of the gastrointestinal tract. Our delivery agents have no known pharmacological activity in the amounts used to enhance oral drug delivery and therefore may be considered excipients.
Risks and Uncertainties. We have no prescription products currently approved for sale by the U.S. FDA. There can be no assurance that our research and development will be successfully completed, that any products developed will obtain necessary government regulatory approval or that any approved products will be commercially viable. In addition, we operate in an environment of rapid change in technology and are dependent upon the continued services of our current employees, consultants and subcontractors. We are highly dependent upon the commercial success of oral Eligen B12™ Rx and cannot be sure that our plans will be successful. We have limited capital resources and significant commitments and obligations.
Since our inception in 1986, we have generated significant losses from operations and we anticipate that we will continue to generate significant losses from operations for the foreseeable future, and that in order to continue as a going concern, our business will require substantial additional investment that we have not yet secured.
As of December 31, 2014, our accumulated deficit was approximately $514.1 million. Our loss from operations was $9.3 million, $7.6 million and $6.8 million for the years ended December 31, 2014, 2013 and 2012, respectively. Our net loss was $25.4 million, $20.9 million and $1.9 million for the years ended December 31, 2014, 2013 and 2012, respectively. Our net cash provided (outlays) from operations and capital expenditures were ($8.4) million, $3.1 million and ($3.0) million for the years ended December 31, 2014, 2013 and 2012, respectively. Net cash provided (outlays) include receipts of deferred revenue of $0.0 million, $10.0 million, and $0.02 million for 2014, 2013, and 2012, respectively. Our stockholders’ deficit was $111.9 million and $86.8 million as of December 31, 2014 and 2013, respectively. On December 31, 2014 we had approximately $3.7 million cash.
As of December 31, 2014, the Company’s obligations included approximately $40.9 million (face value) under its Second Amended and Restated Convertible Notes (the “Convertible Notes”), approximately $8.3 million (face value) under a loan agreement entered into on August 20, 2014 (the “Loan Agreement”), approximately $0.7 million (face value) under its Second Amended and Restated Reimbursement Notes (the “Reimbursement Notes”), and approximately $1.9 million (face value) under its Second Amended and Restated
56
Table of Contents
EMISPHERE TECHNOLOGIES, INC.
NOTES TO FINANCIAL STATEMENTS — (Continued)
Bridge Notes (the “Bridge Notes”). The Convertible Notes and the Loan Agreement are subject to various sales, operating and manufacturing performance criteria.
Under the terms of the Loan Agreement, described in Note 7 to the Financial Statements, Emisphere may borrow, at specified times and based on the attainment of specified performance milestones, up to an aggregate of $20.0 million to finance the development, manufacturing, marketing and sales of its oral Eligen B12™ Rx Product. The new loan facility will mature on December 31, 2019 and bear interest at a rate of 13% per year. The first borrowing under the Loan Agreement occurred on August 20, 2014, in an original principal amount of $5.0 million, and the second occurred on November 4, 2014 in an original principal amount of $3.0 million. Subject to achieving certain operational milestones relating to the timely manufacture and commencement of sales of oral Eligen B12™ Rx, of which there can be no assurance, the Company may request three additional borrowings under the Loan Agreement as follows: up to $5.0 million in the first quarter of 2015, up to $5.0 million in the second quarter of 2015, and up to $2.0 million in the third quarter of 2015. In addition to funding available through the Loan Agreement, the Company received approximately $1.7 million and $0.3 million on January 14, 2014, and December 9, 2014, respectively, from the sale of unused net operating losses by participating in the Technology Business Tax Certificate Transfer Program, sponsored by the New Jersey Economic Development Authority.
We believe the Company’s current cash balance, in addition to cash available through additional borrowings under the Loan Agreement, assuming attainment of the milestones, will provide sufficient capital to support the commercial launch of oral Eligen B12™ Rx in the U.S. market and to continue operations through the end of 2015. The Company’s future capital requirements beyond 2015 and financial success depend largely on the commercial success of our oral Eligen B12™ Rx product and our ability to leverage existing as well as securing new partnering opportunities. There is no assurance that our plans will be successful. If we fail to raise sufficient capital from commercial operations or partnerships, we will need to seek capital from other sources. We cannot assure you that financing will be available on favorable terms or at all. If we fail to generate sufficient additional capital from sales of oral Eligen B12™ Rx or obtain substantial cash inflows from existing or new partners or other sources prior to the end 2015, we could be forced to cease operations. Additionally, if additional capital is raised through the sale of equity or convertible debt securities, the issuance of such securities would result in dilution to our existing stockholders. These conditions raise substantial doubt about our ability to continue as a going concern. Consequently, the audit reports prepared by our independent registered public accounting firm relating to our financial statements for the years ended December 31, 2014, 2013 and 2012 include an explanatory paragraph expressing substantial doubt about our ability to continue as a going concern.
Furthermore, despite our optimism regarding the Eligen® Technology, even in the event that the Company is adequately funded, there is no guarantee that any of our products or product candidates will perform as hoped or that such products can be successfully commercialized.
2. Summary of Significant Accounting Policies
Use of Estimates. The preparation of financial statements in accordance with accounting principles generally accepted in the U.S. involves the use of estimates and assumptions that affect the recorded amounts of assets and liabilities as of the date of the financial statements and the reported amounts of revenue and expenses and performance period for revenue recognition. Actual results may differ substantially from these estimates. Significant estimates include accrued expenses, the variables and method used to calculate stock-based compensation, derivative instruments and deferred taxes.
Concentration of Credit Risk. Financial instruments, which potentially subject us to concentrations of credit risk, consist of cash, cash equivalents, restricted cash and investments. We invest excess funds in accordance with a policy objective seeking to preserve both liquidity and safety of principal. We generally invest our excess funds in obligations of the U.S. government and its agencies, bank deposits, money market funds, and investment grade debt securities issued by corporations and financial institutions. We hold no collateral for these financial instruments.
57
Table of Contents
EMISPHERE TECHNOLOGIES, INC.
NOTES TO FINANCIAL STATEMENTS — (Continued)
Cash, Cash Equivalents, and Investments. We consider all highly liquid, interest-bearing instruments with original maturity of three months or less when purchased to be cash equivalents. Cash and cash equivalents may include demand deposits held in banks and interest bearing money market funds. Our investment policy requires that commercial paper be rated A-1, P-1 or better by either Standard and Poor’s Corporation or Moody’s Investor Services or another nationally recognized agency and that securities of issuers with a long-term credit rating must be rated at least “A” (or equivalent). As of December 31, 2014, we held no investments.
Inventory. Inventories are stated at the lower of cost or market determined by the first in, first out method.
Impairment of Long-Lived Assets. In accordance with FASB ASC 360-10-35, we review our long-lived assets for impairment whenever events and circumstances indicate that the carrying value of an asset might not be recoverable. An impairment loss, measured as the amount by which the carrying value exceeds the fair value, is recognized if the carrying amount exceeds estimated undiscounted future cash flows.
Equipment and Leasehold Improvements. Equipment and leasehold improvements are stated at cost. Depreciation and amortization are provided on a straight-line basis over the estimated useful life of the asset. Leasehold improvements are amortized over the term of the lease or useful life of the improvements, whichever is shorter. Expenditures for maintenance and repairs that do not materially extend the useful lives of the respective assets are charged to expense as incurred. The cost and accumulated depreciation or amortization of assets retired or sold are removed from the respective accounts and any gain or loss is recognized in operations.
Deferred Lease Liability. Our leases provide for rental holidays and escalations of the minimum rent during the lease term, as well as additional rent based upon increases in real estate taxes and common maintenance charges. We record rent expense from leases with rental holidays and escalations using the straight-line method, thereby prorating the total rental commitment over the term of the lease. Under this method, the deferred lease liability represents the difference between the minimum cash rental payments and the rent expense computed on a straight-line basis.
Revenue Recognition. We recognize revenue in accordance with FASB ASC 605-10-S99, Revenue Recognition.
Oral Eligen B12™ Rx Product
We will sell our Oral Eligen B12™ Rx product through drug wholesalers and retail pharmacies. We will recognize revenue from prescription product sales, net of sales discounts, chargebacks, and rebates. We will accept returns of unsalable product from customers within a return period of six months prior to and 12 months following product expiration. Our Oral Eligen B12™ Rx product currently has a shelf life of 24 months from the date of manufacture. Given the limited history of our Oral Eligen B12™ Rx product, we currently cannot reliably estimate expected returns of the prescription products at the time of shipment. Accordingly, we will defer recognition of revenue on prescription products until the right of return no longer exists, which occurs at the earlier of the time the Oral Eligen B12™ Rx product is dispensed through patient prescriptions or expiration of the right of return.
Collaborative Agreements and Feasibility Studies
Revenue earned from collaborative agreements and feasibility studies is comprised of reimbursed research and development costs, as well as upfront and research and development milestone payments. Deferred revenue represents payments received which are related to future performance. Revenue from feasibility studies, which are typically short term in nature, is recognized upon delivery of the study, provided that all other revenue recognition criteria are met.
Revenue from collaboration agreements are recognized using the proportional performance method provided that we can reasonably estimate the level of effort required to complete our performance obligations under an arrangement and such performance obligations are provided on a best effort basis and based on
58
Table of Contents
EMISPHERE TECHNOLOGIES, INC.
NOTES TO FINANCIAL STATEMENTS — (Continued)
“expected payments.” Under the proportional performance method, periodic revenue related to nonrefundable cash payments is recognized as the percentage of actual effort expended to date as of that period to the total effort expected for all of our performance obligations under the arrangement. Actual effort is generally determined based upon actual hours incurred and include research and development (“R&D”) activities performed by us and time spent for Joint Steering Committee (“JSC”) activities. Total expected effort is generally based upon the total R&D and JSC hours incorporated into the project plan that is agreed to by both parties to the collaboration. Significant management judgments and estimates are required in determining the level of effort required under an arrangement and the period over which we expect to complete the related performance obligations. Estimates of the total expected effort included in each project plan are based on historical experience of similar efforts and expectations based on the knowledge of scientists for both the Company and its collaboration partners. The Company periodically reviews and updates the project plan for each collaborative agreement. The most recent reviews took place in January 2014. In the event that a change in estimate occurs, the change will be accounted for using the cumulative catch-up method which provides for an adjustment to revenue in the current period. Estimates of our level of effort may change in the future, resulting in a material change in the amount of revenue recognized in future periods.
Generally under collaboration arrangements, nonrefundable payments received during the period of performance may include time- or performance-based milestones. The proportion of actual performance to total expected performance is applied to the “expected payments” in determining periodic revenue. However, revenue is limited to the sum of (i) the amount of nonrefundable cash payments received and (ii) the payments that are contractually due but have not yet been paid.
With regard to revenue recognition in connection with development and license agreements that include multiple deliverables, Emisphere’s management reviews the relevant terms of the agreements and determines whether such deliverables should be accounted for as a single unit of accounting in accordance with FASB ASC 605-25, Multiple-Element Arrangements. If it is determined that a delivered license and Eligen® Technology do not have stand-alone value and Emisphere does not have objective evidence of fair value of the undelivered Eligen® Technology or the manufacturing value of all the undelivered items, then such deliverables are accounted for as a single unit of accounting and any payments received pursuant to such agreement, including any upfront or development milestone payments and any payments received for support services, will be deferred and included in deferred revenue within our balance sheet until such time as management can estimate when all of such deliverables will be delivered, if ever. Management reviews and reevaluates such conclusions as each item in the arrangement is delivered and circumstances of the development arrangement change. See Note 12 for more information about the Company’s accounting for revenue from specific development and license agreements.
Research and Development and Clinical Trial Expenses. Research and development expenses include costs directly attributable to the conduct of research and development programs, including the cost of salaries, payroll taxes, employee benefits, materials, supplies, maintenance of research equipment, costs related to research collaboration and licensing agreements, the cost of services provided by outside contractors, including services related to our clinical trials, clinical trial expenses, the full cost of manufacturing drug for use in research, pre-clinical development, and clinical trials. All costs associated with research and development are expensed as incurred.
Clinical research expenses represent obligations resulting from our contracts with various research organizations in connection with conducting clinical trials for our product candidates. We account for those expenses on an accrual basis according to the progress of the trial as measured by patient enrollment and the timing of the various aspects of the trial. Accruals are recorded in accordance with the following methodology: (i) the costs for period expenses, such as investigator meetings and initial start-up costs, are expensed as incurred based on management’s estimates, which are impacted by any change in the number of sites, number of patients and patient start dates; (ii) direct service costs, which are primarily ongoing monitoring costs, are recognized on a straight-line basis over the life of the contract; and (iii) principal investigator expenses that are directly associated
59
Table of Contents
EMISPHERE TECHNOLOGIES, INC.
NOTES TO FINANCIAL STATEMENTS — (Continued)
with recruitment are recognized based on actual patient recruitment. All changes to the contract amounts due to change orders are analyzed and recognized in accordance with the above methodology. Change orders are triggered by changes in the scope, time to completion and the number of sites. During the course of a trial, we adjust our rate of clinical expense recognition if actual results differ from our estimates.
Income Taxes. Deferred tax liabilities and assets are recognized for the expected future tax consequences of events that have been included in the financial statements or tax returns. These liabilities and assets are determined based on differences between the financial reporting and tax basis of assets and liabilities measured using the enacted tax rates and laws that will be in effect when the differences are expected to reverse. A valuation allowance is recognized to reduce deferred tax assets to the amount that is more likely than not to be realized. In assessing the likelihood of realization, management considered estimates of future taxable income.
Stock-Based Employee Compensation. We recognize expense for our share-based compensation based on the fair value of the awards at the time they are granted. We estimate the value of stock option awards on the date of grant using the Black-Scholes model. The determination of the fair value of share-based payment awards on the date of grant is affected by our stock price as well as assumptions regarding a number of complex and subjective variables. These variables include our expected stock price volatility over the term of the awards, expected term, risk-free interest rate, expected dividends and expected forfeiture rates. The forfeiture rate is estimated using historical option cancellation information, adjusted for anticipated changes in expected exercise and employment termination behavior. Our outstanding awards do not contain market or performance conditions therefore we have elected to recognize share-based employee compensation expense on a straight-line basis over the requisite service period.
Fair Value of Financial Instruments. The carrying amounts for cash, cash equivalents, accounts payable, and accrued expenses approximate fair value because of their short-term nature. At December 31, 2014, the carrying value of the Second Amended and Restated Convertible Notes, Second Amended and Restated Reimbursement Notes, Second Amended and Restated Bridge Notes and Loan Agreement was $44.5 million, which reflects its original cost plus accrued interest. See Note 7 for further discussion of the notes payable.
Derivative Instruments. Derivative instruments consist of common stock warrants, and certain instruments embedded in certain notes payable and related agreements. These financial instruments are recorded in the balance sheets at fair value as liabilities. Changes in fair value are recognized in earnings in the period of change.
Fair Value Measurements. The authoritative guidance for fair value measurements defines fair value as the price that would be received if an asset were to be sold or paid to transfer a liability in an orderly transaction between market participants on the measurement date. Market participants are buyers and sellers in the principal market that are (i) independent, (ii) knowledgeable, (iii) able to transact, and (iv) willing to transact. The guidance describes a fair value hierarchy based on the levels of inputs, of which the first two are considered observable and the last unobservable, that may be used to measure fair value which are the following:
| Ÿ | Level 1 — Quoted prices in active markets for identical assets or liabilities |
| Ÿ | Level 2 — Inputs other than Level 1 that are observable, either directly or indirectly, such as quoted prices for similar assets or liabilities; quoted prices in markets that are not active; or other inputs that are observable or corroborated by observable market data for substantially the full term of the assets or liabilities |
| Ÿ | Level 3 — Unobservable inputs that are supported by little or no market activity and that are significant to the value of the assets or liabilities |
60
Table of Contents
EMISPHERE TECHNOLOGIES, INC.
NOTES TO FINANCIAL STATEMENTS — (Continued)
Future Impact of Recently Issued Accounting Standards
New Accounting Pronouncements
In May 2014, the FASB issued ASU No. 2014-09, “Revenue from Contracts with Customers” (“ASU 2014-09”), which requires entities to recognize revenue in a way that depicts the transfer of promised goods or services to customers in an amount that reflects the consideration to which the entity expects to be entitled to in exchange for those goods or services. The new guidance also requires additional disclosure about the nature, amount, timing and uncertainty of revenue and cash flows arising from customer contracts, including significant judgments and changes in judgments and assets recognized from costs incurred to obtain or fulfill a contract. ASU 2014-09 is effective for fiscal years, and interim periods within those years, beginning after December 15, 2016. The Company is currently evaluating the impact of the new standards.
In August 2014, the FASB issued ASU No. 2014-15, “Disclosure of Uncertainties About an Entity’s Ability to Continue as a Going Concern” (“ASU 2014-15”), which provides guidance on management’s responsibility in evaluating whether there is substantial doubt about an entity’s ability to continue as a going concern and to provide related footnote disclosures. ASU 2014-15 is effective for the annual period ending after December 15, 2016, and for annual and interim periods thereafter. The adoption of ASU 2014-15 is not expected to have a material impact on our financial position, results of operations or cash flows.
Management does not believe there would have been a material effect on the accompanying financial statements had any other recently issued, but not yet effective, accounting standards been adopted in the current period.
3. Inventory
Inventory consists of the following:
| December 31, | ||||||||
| 2014 | 2013 | |||||||
| (In thousands) | ||||||||
| Raw Materials |
$ | 1,350 | ||||||
| Work-in-process |
718 | 230 | ||||||
|
|
|
|
|
|||||
| Total |
$ | 2,068 | $ | 230 | ||||
|
|
|
|
|
|||||
4. Prepaid Expenses and Other Current Assets
Prepaid expenses and other current assets consist of the following:
| December 31, | ||||||||
| 2014 | 2013 | |||||||
| (In thousands) | ||||||||
| Prepaid corporate insurance |
$ | 53 | $ | 92 | ||||
| Deposit on inventory |
— | 477 | ||||||
| Prepaid expenses and other current assets |
135 | 53 | ||||||
|
|
|
|
|
|||||
| $ | 188 | $ | 622 | |||||
|
|
|
|
|
|||||
61
Table of Contents
EMISPHERE TECHNOLOGIES, INC.
NOTES TO FINANCIAL STATEMENTS — (Continued)
5. Fixed Assets
Equipment and leasehold improvements, net, consists of the following:
| December 31, |
||||||||||
| Useful Lives In Years |
2014 | 2013 | ||||||||
| (In thousands) | ||||||||||
| Equipment |
3-7 | $ | 601 | $ | 601 | |||||
| Leasehold improvements |
Term of lease | 27 | 27 | |||||||
|
|
|
|
|
|||||||
| 628 | 628 | |||||||||
| Less, accumulated depreciation and amortization |
603 | 588 | ||||||||
|
|
|
|
|
|||||||
| $ | 25 | $ | 40 | |||||||
|
|
|
|
|
|||||||
Depreciation expense for the years ended December 31, 2014, 2013 and 2012, was $15 thousand, $9 thousand and $29 thousand, respectively.
6. Accounts Payable and Accrued Expenses
Accounts payable and accrued expenses consist of the following:
| December 31, | ||||||||
| 2014 | 2013 | |||||||
| (In thousands) | ||||||||
| Accounts payable |
$ | 530 | $ | 525 | ||||
| Accrued legal, professional fees and other |
1,262 | 967 | ||||||
| Accrued vacation |
54 | 47 | ||||||
|
|
|
|
|
|||||
| $ | 1,846 | $ | 1,539 | |||||
|
|
|
|
|
|||||
7. Notes Payable
Notes payable, net of related discounts, consists of the following:
| December 31, 2014 |
2013 | |||||||
| (in thousands) | ||||||||
| Convertible Notes |
$ | 35,332 | $ | 32,230 | ||||
| Loan Agreement |
8,307 | — | ||||||
| Reimbursement Notes |
636 | 556 | ||||||
| Bridge Notes |
271 | 293 | ||||||
|
|
|
|
|
|||||
| 44,546 | 33,079 | |||||||
| Less: Current portion |
— | 556 | ||||||
|
|
|
|
|
|||||
| Non-current Notes payable, net of related discounts |
$ | 44,546 | $ | 32,523 | ||||
|
|
|
|
|
|||||
On August 20, 2014, the Company entered into a series of agreements (the “Transaction Documents”) with MHR Capital Partners Master Account LP, MHR Capital Partners (100) LP, MHR Institutional Partners II LP, and MHR Institutional Partners IIA LP, (collectively, “MHR” or the “Lenders”), for a new loan facility, an extension of the Company’s existing obligations under various promissory notes previously issued to the Lenders, and for payment by the Company of certain royalties to MHR (the “Transaction”).
62
Table of Contents
EMISPHERE TECHNOLOGIES, INC.
NOTES TO FINANCIAL STATEMENTS — (Continued)
The Loan Agreement provides for, among other things, a commitment (the “Commitment”) of the Lenders to loan the Company up to $20 million to finance the development, manufacturing, marketing and sale of oral Eligen B12™ Rx (the “B12 Product”). The Company may make five borrowings (each, a “Borrowing”, and collectively, the “Loan”) under the Loan Agreement. The first Borrowing occurred on August 20, 2014 in an original principal amount of $5 million, and the second occurred on November 4, 2014, in an original principal amount of $3 million. Subject to achieving certain operational milestones relating to the timely manufacture and commencement of sales of the B12 Product, of which there can be no assurance, the Company may request three additional Borrowings as follows: up to $5.0 million in the first quarter of 2015, up to $5.0 million in the second quarter of 2015, and up to $2.0 million in the third quarter of 2015.
In addition, as described below, if the Company does not have sufficient cash in excess of the Minimum Cash Balance, as defined below, to pay any Royalties that become due under the Royalty Agreement, as described below, in cash, such Royalties will be paid as an additional Loan under the Loan Agreement by increasing the principal amount outstanding under the Loan Agreement (any such Loan, “Paid-In-Kind Royalties”). The “Minimum Cash Balance” generally means cash on hand (plus certain cash expenditures during such fiscal year that are unrelated to the B12 Product or related products) of at least $10 million (or $15 million, under certain circumstances beginning as early as October 1, 2015), subject to certain permitted deductions.
Except with respect to Paid-In-Kind Royalties incurred under the Loan Agreement after all amounts of principal and interest have previously been paid in full, the Loan will mature on the earlier of (a) December 31, 2019, and, (b) 30 days after the end of any fiscal year in which the Company’s cash (plus certain cash expenditures during such fiscal year that are unrelated to the B12 Product or related products) as of the end of such fiscal year (subject to certain permitted deductions) is more than three times the principal amount of the Loan as of the end of such fiscal year. Paid-In-Kind Royalties incurred under the Loan Agreement after all amounts of principal and interest have previously been paid in full mature one year following the date of incurrence. The Loan bears interest at a rate of 13% per annum (the “Interest Rate”), compounded monthly, and will be payable in kind and in arrears on June 30 and December 31 of each year up to and including the maturity date by increasing the outstanding principal amount of the Loan by the amount of each such interest payment. So long as an event of default under the Loan Agreement (an “Event of Default”) has occurred and is continuing, at the election of MHR, interest shall accrue on the Loan at a rate equal to 2% per annum above the Interest Rate (“Default Rate”). Interest at the Default Rate shall accrue from the initial date of such Event of Default until that Event of Default is cured or waived in writing and shall be payable upon demand and, if not paid when due, shall itself bear interest at the Default Rate. The Loan must be repaid from time to time prior to maturity pursuant to (a) a cash sweep of 50% of the Company’s adjusted consolidated free cash flow, or 75% of the Company’s adjusted consolidated free cash flow in any year in which the adjusted consolidated free cash flow exceeds $50 million, to the extent such cash sweep does not cause the Company’s cash as of the end of such year to be less than the Minimum Cash Balance, (b) a cash sweep of 50% of any cash proceeds received from any third party in connection with the license, distribution or sale of any of the Company’s products other than the B12 Product or related products (the “Non-B12 Products”), subject to the priority described below, and (c) a Royalty Match (as described below), to the extent such Royalty Match does not cause the Company’s cash as of the end of such year to be less than the Minimum Cash Balance and subject to the priority described below. The Loan Agreement provides for certain representations and warranties, conditions precedent to the Lenders’ obligation to lend, affirmative and negative covenants of the Company (including, but not limited to, certain milestones in the development of its B12 Products) and Events of Default.
In connection with the entry into the Loan Agreement, on August 20, 2014, the Lenders and the Company further amended and restated (i) the Convertible Notes issued by the Company to certain of the Lenders, (ii) the Bridge Notes issued by the Company to certain of the Lenders, and (iii) the Reimbursement Notes (and, together with the Convertible Notes and Bridge Notes, the “MHR Notes”). Also, in connection with the entry into the Loan Agreement and the amendment and restatement of the MHR Notes, Institutional Partners IIA and the Company have amended the Pledge and Security Agreement, dated September 26, 2005, as amended, by and
63
Table of Contents
EMISPHERE TECHNOLOGIES, INC.
NOTES TO FINANCIAL STATEMENTS — (Continued)
between the Company and Institutional Partners IIA to, among other things, secure the Reimbursement Notes and payments due under the Loan Agreement with substantially all of the Company’s assets, and secure the payments due under the Royalty Agreement and Paid-In-Kind Royalties due under the Loan Agreement with the Company’s intellectual property relating to the B12 Products and related products.
As of December 31, 2014, the principal balance of the Loan agreement was $8.3 million including approximately $0.3 million interest payable in kind.
Convertible Notes. On September 26, 2005, we received net proceeds of approximately $12.9 million under a $15 million secured loan agreement (the “2005 Loan Agreement”) executed with MHR. Under the 2005 Loan Agreement, MHR requested, and on May 16, 2006, we effected, the exchange of the loan from MHR for the predecessor of the Convertible Notes, which were 11% senior secured convertible notes with substantially the same terms as the 2005 Loan Agreement, except that the original Convertible Notes were convertible, at the sole discretion of MHR, into shares of our common stock at a price per share of $3.78. In connection with the original Convertible Notes exchange, the Company agreed to appoint a representative of MHR (the “MHR Nominee”) and another person (the “Mutual Director”) to the Board. Further, the Company agreed to amend, and in January 2006 did amend, its certificate of incorporation to provide for continuity of the MHR Nominee and the Mutual Nominee on the Board so long as MHR holds at least 2% of the outstanding common stock of the Company. The original Convertible Notes were amended and restated on May 7, 2013 and amended and restated a second time on August 20, 2014 as described below.
The Convertible Notes now provide for a new maturity date of March 31, 2022 (subject to acceleration upon the occurrence of certain specified events of default, including the failure to meet certain sales, performance, and manufacturing milestones specified in the Convertible Notes). The interest rate is 13% per annum, compounded monthly, which interest will be payable in the form of additional Convertible Notes. The Convertible Notes are collateralized by a first priority lien in favor of the Lenders on substantially all of the Company’s assets. After all principal and interest under the Loan Agreement and Reimbursement Notes are repaid, the remaining Convertible Notes must be redeemed from time to time prior to maturity pursuant to a cash sweep of 50% of the Company’s adjusted consolidated free cash flow (75% of the Company’s adjusted consolidated free cash flow in any year in which the Company’s adjusted consolidated free cash flow exceeds $50 million) to the extent such cash sweep does not cause the Company’s cash as of the end of such year to be less than the Minimum Cash Balance. The Convertible Notes are convertible, at the option of the holders, at a conversion price of $1.25 per share of common stock, which conversion price is subject to adjustment upon the occurrence of specified events, including stock dividends, stock splits, certain fundamental corporate transactions, and certain issuances of common stock by the Company. The Convertible Notes must also be redeemed from time to time prior to maturity pursuant to (a) a cash sweep of 50% of any cash proceeds received from any third party in connection with the license, distribution or sale of any Non-B12 Product, subject to the priority described below and (b) a Royalty Match (as described below), to the extent such Royalty Match does not cause the Company’s cash as of the end of such year to be less than the Minimum Cash Balance and subject to the priority described below. If we fail to meet our obligations under the terms of the Convertible Notes, or fail to meet any of the sales, operating or manufacturing performance criteria included in the Convertible Notes, we would be in default under these notes, which would give MHR the option of foreclosing on substantially all of our assets. As of December 31, 2014, the principal balance of the Convertible Notes was $40.9 million; and the Convertible Notes were convertible into 32,717,484 shares of our common stock.
Reimbursement Notes. On June 8, 2010, the Company issued the predecessor to the Reimbursement Notes to MHR in the form of certain non-interest bearing promissory notes in the aggregate principal amount of $600,000 in reimbursement for legal expenses incurred by MHR in connection with MHR’s agreement to, among other things, waive certain rights as a senior secured party of the Company and enter into a non-disturbance agreement with the Company’s collaboration partner Novartis Pharma AG, and, if necessary, to enter into a comparable agreement in connection with another potential Company transaction. The original Reimbursement Notes were amended and restated on May 7, 2013 and amended and restated again on August 20, 2014 as described below.
64
Table of Contents
EMISPHERE TECHNOLOGIES, INC.
NOTES TO FINANCIAL STATEMENTS — (Continued)
The Reimbursement Notes provide for a maturity date of the earlier of (a) March 31, 2022 and (b) immediately prior to the time that any amounts outstanding under the Loan Agreement are repaid (subject to acceleration upon the occurrence of certain events of default specified in the Reimbursement Notes), and bear interest at the rate of 10% per annum, compounded monthly, which interest is payable in the form of additional Reimbursement Notes. The Reimbursement Notes are collateralized by a first priority lien in favor of the Lenders on substantially all of the Company’s assets. The Reimbursement Notes are convertible, at the option of the holders, at a conversion price of $0.50 per share of common stock, which conversion price is subject to adjustment upon the occurrence of specified events, including stock dividends, stock splits, certain fundamental corporate transactions, and certain issuances of common stock by the Company. The Reimbursement Notes must also be redeemed from time to time prior to maturity pursuant to a cash sweep of 50% of any cash proceeds received from any third party in connection with the license, distribution or sale of any Non-B12 Product, subject to the priority described below. As of December 31, 2014, the principal balance of the Reimbursement Notes was $0.68 million; and the Reimbursement Notes were convertible into 1,365,606 shares of our common stock.
Bridge Notes. On October 17, 2012, the Company issued to MHR the predecessor to the Bridge Notes in the aggregate principal amount of $1,400,000. The original Bridge Notes provided for an interest rate of 13% per annum and were payable on demand. The Bridge Notes were amended and restated on May 7, 2013 and restated again on August 20, 2014 as described below.
The Bridge Notes provide for a maturity date of March 31, 2022 (subject to acceleration upon the occurrence of certain events of default specified) and bear interest at 13% per year, compounded monthly and payable in the form of additional Bridge Notes. The Bridge Notes are collateralized by a first priority lien in favor of the Lenders on substantially all of the Company’s assets. The Bridge Notes are convertible, at the option of the holders, at a conversion price of $0.50 per share of common stock, which conversion price is subject to adjustment upon the occurrence of specified events, including stock dividends, stock splits, certain fundamental corporate transactions, and certain issuances of common stock by the Company. The Bridge Notes must also be redeemed from time to time prior to maturity pursuant to (a) a cash sweep of 50% of any cash proceeds received from any third party in connection with the license, distribution or sale of any Non-B12 Product, subject to the priority described below and (b) a Royalty Match (as described below), to the extent such Royalty Match does not cause the Company’s cash as of the end of such year to be less than the Minimum Cash Balance and subject to the priority described below. As of December 31, 2014, the principal balance of the Bridge Notes was $1.86 million; and the Reimbursement Notes were convertible into 3,710,158 shares of our common stock.
The priority of the cash sweep for Non-B12 Products is as follows: (i) to redeem the Reimbursement Notes, (ii) to prepay principal and interest outstanding under the Loan Agreement; (ii) to reduce the Commitment; (iv) to redeem the Convertible Notes; and (v) to redeem the Bridge Notes.
As a condition to MHR entering into the Loan Agreement and amending and restating the MHR Notes, the Company and MHR entered into a Royalty Agreement (the “Royalty Agreement”) on August 20, 2014, pursuant to which the Company agreed to pay to MHR, subject to specified terms and conditions, royalties in perpetuity (the “Royalties”), commencing as of the date of the Royalty Agreement, in an amount equal to: twenty percent (20%) of all Net Product Sales (as defined in the Royalty Agreement) and any third party payments arising in connection with the sale of the B12 Product and related products, during any fiscal year; provided that, from and after October 1, 2015, if no amount of indebtedness is outstanding under the Loan Agreement (the “Indebtedness Repayment Condition”), such amount shall be reduced to (i) five percent (5%) of all Net Sales and third party payments commencing with the first quarter immediately following the quarter in which the Indebtedness Repayment Condition is satisfied, or (ii) two and one half percent (2.5%) of all Net Sales commencing with the quarter immediately following the quarter in which the Indebtedness Repayment Condition is satisfied, but only with respect to the Net Sales made in any country in which there was not a Valid Patent Claim (as defined in the Royalty Agreement) and where generic entry of a competitive product not by the Company or its affiliates that does not infringe a Valid Patent Claim in such country has occurred, in each case as of the last day of such Fiscal Quarter. Once the royalty rate has been reduced to 5%, the rate shall not be reinstated to 20% even if amounts
65
Table of Contents
EMISPHERE TECHNOLOGIES, INC.
NOTES TO FINANCIAL STATEMENTS — (Continued)
become outstanding under the Loan Agreement as a result of Paid-In-Kind Royalties. Payments of Royalties shall be made in cash to the extent such Royalties do not cause the Company’s cash as of the end of any year to be less than the Minimum Cash Balance, and otherwise shall be paid as Paid-In-Kind Royalties.
If any Royalties become due under the Royalty Agreement when the royalty rate is 5% or 2.5%, the amount outstanding under the Loan Agreement, Convertible Notes and Bridge Notes shall be reduced in an amount equal to such royalty payment, to the extent such payment does not cause the Company’s cash as of the end of such year to be less than the Minimum Cash Balance (the “Royalty Match”), in the following priority: (i) first, to prepay the Loan; (ii) second, to redeem the Convertible Notes; and (iii) finally, to redeem the Bridge Notes.
Additional fees paid by Emisphere in connection with the Loan Agreement, MHR Notes and the Royalty Agreement included the reimbursement of $225 thousand of MHR’s professional fees associated with the transaction, which was recorded as interest expense.
We accounted for the modifications to the Company’s obligations to MHR evidenced by the MHR Notes as a troubled debt restructuring under FASC ASC 470-60. As there was only a modification of terms to the existing debt and we did not transfer any assets or equity in a settlement to MHR no gain or loss was recorded on the transaction. The change in cash outflows resulting from the modification of terms are accounted for on a prospective basis. In accordance with FASB ASC 470-60, the $225 thousand of fees were accounted for as a financing fee and included in interest expense on the accompanying statements of operations.
On April 26, 2013, the Company entered into a restructuring agreement (the “Restructuring Agreement”) with MHR regarding the restructuring of the terms of the Company’s obligations under the Convertible Notes, the Reimbursement Notes, and the Bridge Notes. As of April 26, 2013, these obligations, which were amended and restated in conjunction with the restructuring, included approximately $32.9 million due and payable under the Convertible Notes, approximately $0.6 million due and payable under the Reimbursement Notes, and approximately $1.5 million due and payable under the Bridge Notes. All of these obligations were either past due or payable on demand prior to the Restructuring Agreement. After restructuring, as of December 31, 2013, these obligations included approximately $35.9 million (face value) under the Amended and Restated Convertible Notes, approximately $0.6 million (face value) under the Amended and Restated Reimbursement Notes, and approximately $1.6 million (face value) under the Amended and Restated Bridge Notes.
Pursuant to the Restructuring, the Company (i) amended and restated its August 2009 Warrants entitling MHR to purchase, in the aggregate, 3,729,323 shares of the Company’s common stock (collectively, the “Amended and Restated 2009 Warrants”); (ii) amended and restated its June 2010 Warrants described in Note 8 entitling MHR to purchase, in the aggregate, 865,000 shares of the Company’s common stock (the “Amended and Restated June 2010 Warrants”); (iii) amended and restated its August 2010 Warrants and August 2010 Waiver Warrants entitling MHR to purchase, in the aggregate, 3,598,146 shares of the Company’s common stock (the “Amended and Restated August 2010 Warrants”); (iv) amended and restated the July 2011 Warrants and July 2011 Waiver Warrants entitling MHR to purchase, in the aggregate, 3,805,307 shares of the Company’s common stock (the “Amended and Restated 2011 Warrants” and, together with the Amended and Restated 2009 Warrants, the Amended and Restated June 2010 Warrants, and the Amended and Restated August 2010 Warrants, the “Amended and Restated Warrants”); and (v) issued new warrants to MHR to purchase 10,000,000 shares of the Company’s common stock (the “2013 Restructuring Warrants” and, together with the Amended and Restated Warrants, the “MHR Restructuring Warrants”). The MHR Restructuring Warrants entitle MHR to purchase, in the aggregate, 21,997,776 shares of the Company’s common stock (the “Warrant Shares”) at an exercise price of $0.50 per share, and will expire on July 8, 2019. The exercise price of the MHR Restructuring Warrants and number of Warrant Shares issuable upon exercise of the MHR Restructuring Warrants are subject to adjustment upon the occurrence of specified events, including stock dividends, stock splits, combinations of shares, and certain fundamental corporate transactions.
66
Table of Contents
EMISPHERE TECHNOLOGIES, INC.
NOTES TO FINANCIAL STATEMENTS — (Continued)
Additional fees paid by Emisphere in connection with the consummation of the transactions contemplated by the Restructuring Agreement included the reimbursement of $497 thousand of MHR’s legal fees associated with the transaction.
The Company determined that the modifications to the Company’s obligations to MHR evidenced by the Convertible Notes, Reimbursement Notes, and Bridge Notes (collectively, the “MHR Obligations”) were not substantial in accordance with ASC 470-50, “Modifications and Extinguishments”. The amendments to the MHR Obligations were accounted for as modifications rather than extinguishments. As such, each of (i) the $497 thousand of MHR’s legal fees, (ii) the fair value of the 10,000,000 MHR Restructuring Warrants, and (iii) the incremental value from the modification of the August 2009 Warrants, June 2010 Warrants, August 2010 MHR Warrants, August 2010 Waiver Warrants, July 2011 MHR Warrants, and July 2011 Waiver Warrants, (collectively, the “Modification Fees”) were accounted for as discounts to the MHR Obligations as of May 7, 2013, the date that the transactions contemplated by the Restructuring Agreement were consummated. The Modification Fees were allocated to the Amended and Restated Convertible Notes, Amended and Restated Bridge Notes and Amended and Restated Reimbursement Notes based on their weighted average. The Company calculated the incremental value of the modification to the August 2009 Warrants, June 2010 Warrants, August 2010 Warrants, August 2010 Waiver Warrants, July 2011 Warrants, and July 2011 Waiver Warrants as the difference between the value of their fair value immediately before and after the consummation of Restructuring.
The estimated fair value of the Amended and Restated June 2010 Warrants, which contain reset provisions, were calculated using the Monte Carlo valuation model, while the estimated fair value of the other warrants were calculated using the Black-Scholes valuation model. Inherent in both of these models are assumptions related to expected volatility, remaining life, risk-free rate and expected dividend yield. For the Amended and Restated June 2010 Warrants using a Monte Carlo model, we estimate the probability and timing of potential future financing and fundamental transactions as applicable. The assumptions used by the Company are summarized below:
Amended and Restated August 2009 Warrants
| Immediately Before |
Immediately After |
|||||||
| Closing stock price |
$ | 0.24 | $ | 0.23 | ||||
| Conversion price |
$ | 0.70 | $ | 0.50 | ||||
| Expected volatility |
175.71 | % | 143.31 | % | ||||
| Remaining term (years) |
1.29 | 6.17 | ||||||
| Risk-free rate |
0.11 | % | 1.21 | % | ||||
| Expected dividend yield |
0 | % | 0 | % | ||||
Amended and Restated June 2010 Warrants
| Immediately Before |
Immediately After |
|||||||
| Closing stock price |
$ | 0.24 | $ | 0.23 | ||||
| Conversion price |
$ | 2.90 | $ | 0.50 | ||||
| Expected volatility |
185.0 | % | 145.0 | % | ||||
| Remaining term (years) |
1.29 | 6.17 | ||||||
| Risk-free rate |
0.14 | % | 1.01 | % | ||||
| Expected dividend yield |
0 | % | 0 | % | ||||
67
Table of Contents
EMISPHERE TECHNOLOGIES, INC.
NOTES TO FINANCIAL STATEMENTS — (Continued)
Amended and Restated August 2010 Warrants
| Immediately Before |
Immediately After |
|||||||
| Closing stock price |
$ | 0.24 | $ | 0.23 | ||||
| Conversion price |
$ | 1.26 | $ | 0.50 | ||||
| Expected volatility |
182.34 | % | 143.31 | % | ||||
| Remaining term (years) |
2.31 | 6.17 | ||||||
| Risk-free rate |
0.22 | % | 1.21 | % | ||||
| Expected dividend yield |
0 | % | 0 | % | ||||
Amended and Restated July 2011 Warrants
| Immediately Before |
Immediately After |
|||||||
| Closing stock price |
$ | 0.24 | $ | 0.23 | ||||
| Conversion price |
$ | 1.09 | $ | 0.50 | ||||
| Expected volatility |
174.11 | 143.31 | % | |||||
| Remaining term (years) |
3.17 | 6.17 | ||||||
| Risk-free rate |
0.36 | % | 1.21 | % | ||||
| Expected dividend yield |
0 | % | 0 | % | ||||
MHR Restructuring Warrants
| Immediately Before |
Immediately After |
|||||||
| Closing stock price |
$ | — | $ | 0.23 | ||||
| Conversion price |
$ | — | $ | 0.50 | ||||
| Expected volatility |
— | 143.31 | % | |||||
| Remaining term (years) |
— | 6.17 | ||||||
| Risk-free rate |
— | 1.21 | % | |||||
| Expected dividend yield |
— | 0 | % | |||||
The estimated fair value of the warrants immediately before and after the modification is as follows:
| Immediately Before |
Immediately After |
|||||||
| (in thousands) | ||||||||
| Amended and Restated August 2009 Warrants |
$ | 445 | $ | 768 | ||||
| Amended and Restated June 2010 Warrants |
$ | 152 | $ | 294 | ||||
| Amended and Restated August 2010 Warrants |
$ | 570 | $ | 741 | ||||
| Amended and Restated July 2011 Warrants |
$ | 696 | $ | 783 | ||||
| MHR Restructuring Warrants |
$ | — | $ | 2,058 | ||||
The Company determined that due to the adjustment of the conversion price of the Amended and Restated Convertible Notes, Amended and Restated Bridge Notes, and Amended and Restated Reimbursement Notes upon the occurrence of certain events, the embedded conversion features are not considered indexed to the Company’s own stock and, therefore, does not meet the scope exception in FASB ASC 815-10-15, requiring the embedded
68
Table of Contents
EMISPHERE TECHNOLOGIES, INC.
NOTES TO FINANCIAL STATEMENTS — (Continued)
conversion features to be accounted for as derivative liabilities. Because the modification of the bifurcated conversion option of the Amended and Restated Convertible Notes was accounted for at fair value both before and after the modification, the change in the fair value of the conversion options was reflected in the accompanying statements of operations. The estimated fair value of the embedded conversion feature of the Amended and Restated Convertible Notes was $0 and $12,810,557 immediately before and after the modification, respectively. Since there were no conversion terms to the Bridge Notes or the Reimbursement Notes prior to their amendment and restatement, the addition of the embedded conversion option in the Amended and Restated Bridge Notes and Amended and Restated Reimbursement Notes was recorded as a discount to the respective notes. The fair value of the embedded conversion feature of the Amended and Restated Bridge Notes and the Amended and Restated Reimbursement Notes on May 7, 2013, was $1,104,767 and $156,041, respectively.
The estimated fair values of the conversion features embedded in the Amended and Restated Convertible Notes, Amended and Restated Reimbursement Notes, and the Amended and Restated Bridge Notes which contain reset provisions were measured using the Monte Carlo valuation model. In using the Monte Carlo model, we estimate the probability and timing of potential future financing and fundamental transactions as applicable. Assumptions used by the Company are summarized below:
Amended and Restated Convertible Notes
| May 7, 2013 |
||||
| Closing stock price |
$ | 0.23 | ||
| Conversion price |
$ | 1.25 | ||
| Expected volatility |
160 | % | ||
| Remaining term (years) |
4.39 | |||
| Risk-free rate |
0.63 | % | ||
| Expected dividend yield |
0 | % | ||
Amended and Restated Reimbursement Notes
| May 7, 2013 |
||||
| Closing stock price |
$ | 0.23 | ||
| Conversion price |
$ | 0.50 | ||
| Expected volatility |
184 | % | ||
| Remaining term (years) |
0.97 | |||
| Risk-free rate |
0.15 | % | ||
| Expected dividend yield |
0 | % | ||
Amended and Restated Bridge Notes
| May 7, 2013 |
||||
| Closing stock price |
$ | 0.23 | ||
| Conversion price |
$ | 0.50 | ||
| Expected volatility |
160 | % | ||
| Remaining term (years) |
4.39 | |||
| Risk-free rate |
0.63 | % | ||
| Expected dividend yield |
0 | % | ||
69
Table of Contents
EMISPHERE TECHNOLOGIES, INC.
NOTES TO FINANCIAL STATEMENTS — (Continued)
The carrying value of the MHR Obligations is comprised of the following:
| December 31, | ||||||||
| 2014 | 2013 | |||||||
| (in thousands) | ||||||||
| Amended and Restated Convertible Notes |
$ | 40,897 | $ | 35,935 | ||||
| Loan Agreement |
8,307 | — | ||||||
| Amended and Restated Reimbursement Notes |
683 | 637 | ||||||
| Amended and Restated Bridge Notes |
1,855 | 1,627 | ||||||
| Unamortized discounts |
(7,196 | ) | (5,120 | ) | ||||
|
|
|
|
|
|||||
| $ | 44,546 | $ | 33,079 | |||||
|
|
|
|
|
|||||
8. Derivative Instruments
Derivative instruments consist of the following:
| December 31, | ||||||||
| 2014 | 2013 | |||||||
| (in thousands) | ||||||||
| Convertible Notes |
$ | 21,501 | $ | 10,371 | ||||
| Reimbursement Notes |
705 | 7 | ||||||
| Bridge Notes |
1,926 | 960 | ||||||
| Amended and Restated August 2009 Warrants |
930 | 597 | ||||||
| Amended and Restated June 2010 MHR Warrants |
282 | 249 | ||||||
| Amended and Restated August 2010 Warrants |
654 | 420 | ||||||
| August 2010 Investor Warrants |
29 | 171 | ||||||
| Amended and Restated August 2010 MHR Waiver Warrants |
243 | 156 | ||||||
| Amended and Restated July 2011 Warrants |
750 | 482 | ||||||
| July 2011 Investor Warrants |
210 | 369 | ||||||
| Amended and Restated July 2011 MHR Waiver Warrants |
198 | 127 | ||||||
| May 2013 MHR Modification Warrants |
2,492 | 1,600 | ||||||
|
|
|
|
|
|||||
| $ | 29,920 | $ | 15,509 | |||||
|
|
|
|
|
|||||
Some of the Company’s outstanding derivative instruments have an exercise price reset feature. The estimated fair value of warrants and embedded conversion features that have an exercise price reset feature is estimated using the Monte Carlo valuation model. The estimated fair value of warrants that do not contain an exercise price reset feature is measured using the Black-Scholes valuation model. Inherent in both of these models are assumptions related to expected volatility, remaining life, risk-free rate and expected dividend yield. For the Monte Carlo model, we estimate the probability and timing of potential future financing and fundamental transactions as applicable.
Embedded Conversion Feature of MHR Notes. The Convertible Notes, the Reimbursement Notes, and the Bridge Notes (collectively, the “MHR Notes”) contain a provision whereby the conversion price is adjustable upon the occurrence of certain events, including the issuance by Emisphere of common stock or common stock equivalents at a price which is lower than the current conversion price of each of the MHR Notes and lower than the then-current market price. Under FASB ASC 815-40-15-5, the embedded conversion feature of the MHR Notes is not considered indexed to the Company’s own stock and, therefore, does not meet the scope exception in
70
Table of Contents
EMISPHERE TECHNOLOGIES, INC.
NOTES TO FINANCIAL STATEMENTS — (Continued)
FASB ASC 815-10-15 and thus needs to be accounted for as a derivative liability. The liability associated with the Convertible Notes and the Bridge Notes has been presented as a non-current liability as of December 31, 2014 and 2013, to correspond to its host contract. The liability associated with the Reimbursement Notes has been presented as a current liability as of December 31, 2013 and a non-current liability as of December 31, 2014, to correspond to its host contract.
Convertible Notes. In addition to the foregoing, the adjustment provision of the Convertible Notes does not become effective unless and until the Company were to raise $10 million through the issuance of common stock or common stock equivalents during any consecutive 24 month period. The fair value of the embedded conversion feature of the Convertible Notes is estimated at the end of each quarterly reporting period using the Monte Carlo model. The assumptions used in computing the fair values as December 31, 2014 and 2013, are as follows:
| December 31, 2014 |
December 31, 2013 |
|||||||
| Closing stock price |
$ | 0.28 | $ | 0.18 | ||||
| Conversion price |
$ | 1.25 | $ | 1.25 | ||||
| Expected volatility |
140 | % | 160 | % | ||||
| Remaining term (years) |
7.25 | 3.75 | ||||||
| Risk-free rate |
1.97 | % | 1.13 | % | ||||
| Expected dividend yield |
0 | % | 0 | % | ||||
The fair value of the embedded conversion feature of the Convertible Notes increased $8.9 million and $10.1 million for the years ended December 31, 2014 and 2013, respectively, and decreased $7.1 million for the year ended December 31, 2012 which amounts have been recognized in the accompanying statements of operations.
Reimbursement Notes. The fair value of the embedded conversion feature of the Reimbursement Notes is estimated at the end of each quarterly reporting period using the Monte Carlo model. The assumptions used in computing the fair value as of December 31, 2014 and 2013 are as follows:
| December 31, 2014 |
December 31, 2013 |
|||||||
| Closing stock price |
$ | 0.28 | $ | 0.18 | ||||
| Conversion price |
$ | 0.50 | $ | 0.50 | ||||
| Expected volatility |
140 | % | 99 | % | ||||
| Remaining term (years) |
7.25 | 0.33 | ||||||
| Risk-free rate |
1.97 | % | 0.07 | % | ||||
| Expected dividend yield |
0 | % | 0 | % | ||||
The fair value of the embedded conversion of the Reimbursement Notes increased $0.7 million for the year ended December 31, 2014 and decreased $0.1 million from its inception date of May 7, 2013 through December 31, 2013, which has been recognized in the accompanying statements of operations.
71
Table of Contents
EMISPHERE TECHNOLOGIES, INC.
NOTES TO FINANCIAL STATEMENTS — (Continued)
Bridge Notes. The fair value of the embedded conversion feature of the Bridge Notes is estimated at the end of each quarterly reporting period using the Monte Carlo model. The assumptions used in computing the fair value as of December 31, 2014 and 2013 are as follows:
| December 31, 2014 |
December 31, 2013 |
|||||||
| Closing stock price |
$ | 0.28 | $ | 0.18 | ||||
| Conversion price |
$ | 0.50 | $ | 0.50 | ||||
| Expected volatility |
140 | % | 160 | % | ||||
| Remaining term (years) |
7.25 | 3.75 | ||||||
| Risk-free rate |
1.97 | % | 1.13 | % | ||||
| Expected dividend yield |
0 | % | 0 | % | ||||
The fair value of the embedded conversion feature of the Bridge Notes increased $0.7 million for the year ended December 31, 2014 and decreased $0.1 million from its inception date of May 7, 2013 through December 31, 2013, which has been recognized in the accompanying statements of operations.
Amended and Restated June 2010 Warrants. In June 2010, the Company granted MHR warrants to purchase 865,000 shares of its common stock (the “June 2010 Warrants”). In connection with the Restructuring, on May 7, 2013, the Company amended and restated the Original Warrants such that the expiration date of the Original Warrant was extended to July 8, 2019, and the exercise price was reduced to $0.50 per share (as amended and restated, the “Amended and Restated August 2010 Warrants”, The exercise price of the Amended and Restated June 2010 Warrants is adjustable upon the occurrence of certain events, including the issuance by Emisphere of common stock or common stock equivalents at a price which is lower than the current exercise price of these warrants and lower than the current market price. However, the adjustment provision does not become effective unless the Company were to raise $10 million through the issuance of common stock or common stock equivalents at a price which is lower than the current conversion price of these warrants and lower than the current market price during any consecutive 24 month period. The fair value of the Amended and Restated June 2010 Warrants is estimated at the end of each quarterly reporting period using the Monte Carlo model. The assumptions used in computing the fair value of the Amended and Restated June 2010 Warrants as of December 31, 2014 and 2013, are as follows:
| December 31, 2014 |
December 31, 2013 |
|||||||
| Closing stock price |
$ | 0.28 | $ | 0.18 | ||||
| Conversion price |
$ | 0.50 | $ | 0.50 | ||||
| Expected volatility |
160 | % | 150 | % | ||||
| Remaining term (years) |
4.52 | 5.5 | ||||||
| Risk-free rate |
1.51 | % | 1.90 | % | ||||
| Expected dividend yield |
0 | % | 0 | % | ||||
The fair value of the Amended and Restated June 2010 MHR Warrants increased $32 thousand and $0.2 million for the years ended December 31, 2014 and 2013, respectively, and decreased $0.3 million for the year ended December 31, 2012. These fluctuations have been recognized in the accompanying statements of operations.
Amended and Restated Warrants. Prior to the Restructuring, the Company issued to MHR warrants to purchase varying amounts of its common stocks at various times from 2009 through 2011, as described more fully below (the August 2009 Warrants, August 2010 Warrants, August 2010 MHR Waiver Warrants, July 2011 Warrants, July 2011 MHR Waiver Warrants, and collectively, the “Original Warrants”). In connection with the
72
Table of Contents
EMISPHERE TECHNOLOGIES, INC.
NOTES TO FINANCIAL STATEMENTS — (Continued)
Restructuring, on May 7, 2013, the Company amended and restated each of the Original Warrants such that the expiration date of each Original Warrant was extended to July 8, 2019, and the exercise price was reduced to $0.50 per share (as amended and restated, the “Amended and Restated August 2009 Warrants”, “Amended and Restated August 2010 Warrants”, “Amended and Restated August 2010 MHR Waiver Warrants”, “Amended and Restated July 2011 Warrants”, “Amended and Restated July 2011 MHR Waiver Warrants”, and collectively, the “Amended and Restated Warrants”) . Under the terms of each of the Amended and Restated Warrants, as well as the August 2010 Investor Warrants, July 2011 Investor Warrants and 2013 Restructuring Warrants (collectively, the Investor Warrants, and together with the Original Warrants, the “Warrants”), the Company has an obligation to make a cash payment to the holders of each of the Warrants for any gain that could have been realized if such holder exercised the warrants and we subsequently failed to deliver a certificate representing the shares to be issued upon such exercise by the third trading day after the Warrants were exercised. Accordingly, the Warrants have been accounted for as a liability. The fair value of each of the Warrants is estimated, at the end of each quarterly reporting period, using the Black-Scholes model. The assumptions used in computing the fair value the Original Warrants as of December 31, 2014 and 2013, are as follows:
| December 31, 2014 |
December 31, 2013 |
|||||||
| Closing stock price |
$ | 0.28 | $ | 0.18 | ||||
| Conversion price |
$ | 0.50 | $ | 0.50 | ||||
| Expected volatility |
161 | % | 153 | % | ||||
| Remaining term (years) |
4.52 | 5.5 | ||||||
| Risk-free rate |
1.65 | % | 1.75 | % | ||||
| Expected dividend yield |
0 | % | 0 | % | ||||
The assumptions used in computing the fair value of the Investor Warrants, as well as the fair value of each of the Warrants and any other relevant terms, are described below.
Amended and Restated August 2009 Warrants. In connection with an equity financing in August 2009 (the “August 2009 Financing”), Emisphere sold warrants to purchase 3.7 million shares of common stock to MHR (the “August 2009 Warrants”, and as amended and restated, the “Amended and Restated August 2009 Warrants”). The fair value of the Amended and Restated August 2009 Warrants increased $0.3 million and $0.2 million for the years ended December 31, 2013, respectively, and decreased $0.2 million for the year ended December 31, 2012, which has been recognized in the accompanying statements of operations.
Amended and Restated August 2010 Warrants. In connection with an equity financing conducted in August 2010 (the “August 2010 Financing”), Emisphere sold warrants to purchase 2.6 million shares of common stock to MHR (the “August 2010 MHR Warrants”). The fair value of the Amended and Restated August 2010 Warrants increased $0.2 million and $0.1 million for the years ended December 31, 2013, respectively, and decreased $0.1 million for the year ended December 31, 2012 which has been recognized in the accompanying statements of operations.
73
Table of Contents
EMISPHERE TECHNOLOGIES, INC.
NOTES TO FINANCIAL STATEMENTS — (Continued)
August 2010 Investor Warrants. Also in connection with the August 2010 Financing, Emisphere sold warrants to purchase 2.6 million shares of common stock to unrelated investors (the “August 2010 Warrants”). On January 12, 2011, one of the unrelated investors notified the Company of its intention to exercise 0.2 million warrants. The assumptions used in computing the fair value of the remaining August 2010 Warrants as of December 31, 2014 and 2013, are as follows:
| December 31, 2014 |
December 31, 2013 |
|||||||
| Closing stock price |
$ | 0.28 | $ | 0.18 | ||||
| Conversion price |
$ | 1.26 | $ | 1.26 | ||||
| Expected volatility |
116 | % | 165 | % | ||||
| Remaining term (years) |
0.65 | 1.66 | ||||||
| Risk-free rate |
0.12 | % | 0.38 | % | ||||
| Expected dividend yield |
0 | % | 0 | % | ||||
The fair value of the August 2010 Investor Warrants decreased by $0.1 million, in each of the years ended December 31, 2014 and 2013, 2012 which has been recognized in the accompanying statements of operations.
Amended and Restated August 2010 MHR Waiver Warrants. Also in connection with the August 2010 Financing, the Company entered into a waiver agreement with MHR, pursuant to which MHR waived certain anti-dilution adjustment rights under the Convertible Notes and certain warrants issued by the Company to MHR that would otherwise have been triggered by the August 2010 Financing. As consideration for such waiver, the Company issued to MHR warrants to purchase 975,000 shares of its common stock (the “August 2010 Waiver Warrants”). The fair value of the Amended and Restated August 2010 Waiver Warrants increased by $0.1 million, and decreased by $0.1 million, and $0.4 million for the years ended December 31, 2014, 2013, 2012, respectively, which has been recognized in the accompanying statements of operations.
Amended and Restated July 2011 MHR Warrants. In connection with an equity financing conducted in July 2011 (the “July 2011 Financing”), Emisphere sold warrants to purchase 3.01 million shares of common stock to MHR (the “July 2011 MHR Warrants”. The fair value of the Amended and Restated July 2011 MHR Warrants increased $0.3 million and $0.1 million for the years ended December 31, 2014 and 2013, respectively, and decreased $0.01 million for the year ended December 31, 2012 which has been recorded in the accompanying statements of operations.
July 2011 Investor Warrants. Also in connection with the July 2011 Financing, Emisphere sold warrants to purchase 3.01 million shares of common stock to unrelated investors (the “July 2011 Warrants”). The July 2011 Warrants are exercisable at $1.09 per share and expire July 6, 2016. The assumptions used in computing the fair value of the July 2011 Warrants as of December 31, 2014 and 2013, are as follows:
| December 31, 2014 |
December 31, 2013 |
|||||||
| Closing stock price |
$ | 0.28 | $ | 0.18 | ||||
| Conversion price |
$ | 1.09 | $ | 1.09 | ||||
| Expected volatility |
122 | % | 183 | % | ||||
| Remaining term (years) |
1.51 | 2.5 | ||||||
| Risk-free rate |
0.67 | % | 0.63 | % | ||||
| Expected dividend yield |
0 | % | 0 | % | ||||
The fair value of the July 2011 Investor Warrants decreased $0.2 million for the year ended December 31, 2014, increased $28 thousand for the year ended December 31, 2013, and decreased $0.03 million for the year ended December 31, 2012, which has been recorded in the statements of operations.
74
Table of Contents
EMISPHERE TECHNOLOGIES, INC.
NOTES TO FINANCIAL STATEMENTS — (Continued)
Amended and Restated July 2011 MHR Waiver Warrants. Also in connection with the July 2011 Financing, the Company entered into a waiver agreement with MHR, pursuant to which MHR waived certain anti-dilution adjustment rights under the Convertible Notes and certain warrants issued by the Company to MHR that would otherwise have been triggered by the July 2011 Financing. As consideration for such waiver, the Company issued to MHR warrants to purchase 795,000 shares of its common stock (the “July 2011 Waiver Warrants”). The fair value of the Amended and Restated July 2011 MHR Waiver Warrants increased $71 thousand and $37 thousand for the years ended December 31, 2014, and 2013, respectively, and decreased $33 thousand for the year ended December 31, 2012 which has been recorded in the statements of operations.
2013 Restructuring Warrants. The Company issued to MHR warrants to purchase 10 million shares of its common stock (the “2013 Restructuring Warrants”) as part of the Restructuring. The assumptions used in computing the fair value of the 2013 Restructuring Warrants as of December 31, 2014 and 2013, are as follows:
| December 31, 2014 |
December 31, 2013 |
|||||||
| Closing stock price |
$ | 0.28 | $ | 0.18 | ||||
| Conversion price |
$ | 0.50 | $ | 0.50 | ||||
| Expected volatility |
161 | % | 153 | % | ||||
| Remaining term (years) |
4.52 | 5.5 | ||||||
| Risk-free rate |
1.65 | % | 1.75 | % | ||||
| Expected dividend yield |
0 | % | 0 | % | ||||
The fair value of the 2013 Restructuring Warrants increased $0.9 million for the year ended December 31, 2014 and decreased by $0.5 million from its inception date of May 7, 2013, through December 31, 2013, which has been recognized in the accompanying statements of operations.
9. Income Taxes
The components of our income tax expense (benefit) in 2014 and 2013 are as follows:
| 2014 | 2013 | |||||||
| Current Tax Expense (Benefit) |
||||||||
| Federal |
— | 28 | ||||||
| State |
(2,019 | ) | — | |||||
|
|
|
|
|
|||||
| (2,019 | ) | 28 | ||||||
|
|
|
|
|
|||||
| Deferred Tax Expense (Benefit) |
||||||||
| Federal |
— | — | ||||||
| State |
— | — | ||||||
|
|
|
|
|
|||||
| — | — | |||||||
|
|
|
|
|
|||||
| Total Tax Expense (Benefit) |
(2,019 | ) | 28 | |||||
|
|
|
|
|
|||||
As of December 31, 2014, we have available unused federal net operating loss (NOL) carry-forwards of $355 million, which will expire in various years from 2018 to 2034. We have New York NOL carry-forwards of $296 with the remainder expiring in various years from 2018 through 2034. We have New Jersey NOL carry-forwards of $7.2 million, which will expire in various years from 2023 through 2034.
As of December 31, 2014, we have Research and Development tax credit carryforwards of $11 million which will expire in various years from 2018 to 2032.
75
Table of Contents
EMISPHERE TECHNOLOGIES, INC.
NOTES TO FINANCIAL STATEMENTS — (Continued)
The effective rate differs from the statutory rate of 34% for 2014, 2013 and 2012 primarily due to the following:
| 2014 | 2013 | 2012 | ||||||||||
| Statutory Rate on pre-tax book loss |
(34.00 | %) | (34.00 | %) | (34.00 | %) | ||||||
| Stock option issuance |
0.09 | % | 0.08 | % | 1.22 | % | ||||||
| Sale of NJ NOL’s |
2.51 | % | 0.00 | % | 0.00 | % | ||||||
| Disallowed interest |
0.51 | % | 3.57 | % | 12.05 | % | ||||||
| Derivatives |
14.75 | % | 13.71 | % | (56.26 | %) | ||||||
| Expired net operating losses and credits |
2.74 | % | 41.83 | % | 61.64 | % | ||||||
| Utilization of net operating loss |
0.00 | % | (2.86 | )% | 0.00 | % | ||||||
| State Tax benefit of Sale of NJ NOL |
(7.37 | %) | 0.00 | % | (60.67 | %) | ||||||
| State Tax benefit — other |
(2.82 | %) | 6.21 | % | 0.00 | % | ||||||
| True-ups and adjustments |
0.02 | % | 0.44 | % | 5.29 | % | ||||||
| Change in federal valuation allowance |
16.22 | % | (28.85 | %) | 10.06 | % | ||||||
|
|
|
|
|
|
|
|||||||
| (7.35 | %) | 0.13 | % | (60.67 | %) | |||||||
|
|
|
|
|
|
|
|||||||
The tax effect of temporary differences, net operating loss carry-forwards, and research and experimental tax credit carryforwards as of December 31, 2014 and 2013 is as follows:
Deferred tax assets and valuation allowance:
| December 31, | ||||||||
| 2014 | 2013 | |||||||
| (in thousands) | ||||||||
| Current deferred tax asset: |
||||||||
| Accrued liabilities |
23 | 16 | ||||||
| Valuation allowance |
(23 | ) | (16 | ) | ||||
|
|
|
|
|
|||||
| Net current deferred tax asset |
— | — | ||||||
|
|
|
|
|
|||||
| Non-current deferred tax assets: |
||||||||
| Fixed and intangible assets |
9 | 54 | ||||||
| Net operation loss carry-forwards |
121,180 | 119,797 | ||||||
| AMT credit carry-forwards |
74 | 74 | ||||||
| Capital loss and charitable carry-forwards |
9 | 3 | ||||||
| Research and experimental tax credits |
11,021 | 10,307 | ||||||
| Stock compensation |
486 | 423 | ||||||
| Deferred revenue |
16,621 | 16,621 | ||||||
| Interest |
3,414 | 1,101 | ||||||
| Valuation allowance |
(152,814 | ) | (148,380 | ) | ||||
|
|
|
|
|
|||||
| Net non-current deferred tax asset |
— | — | ||||||
|
|
|
|
|
|||||
Future ownership changes may limit the future utilization of these net operating loss and research and development tax credit carry-forwards as defined by the Internal Revenue Code. We performed an in-depth analysis and determined that the net operating losses and research and development expenses are not limited
76
Table of Contents
EMISPHERE TECHNOLOGIES, INC.
NOTES TO FINANCIAL STATEMENTS — (Continued)
under Section 382. The net deferred tax asset has been fully offset by a valuation allowance due to our history of taxable losses and uncertainty regarding our ability to generate sufficient taxable income in the future to utilize these deferred tax assets.
We apply the provisions of ASC 740-10-25 which provides recognition criteria and a related measurement model for uncertain tax positions taken or expected to be taken in income tax returns. ASC 740-10-25 requires that a position taken or expected to be taken in a tax return be recognized in the financial statements when it is more likely than not that the position would be sustained upon examination by tax authorities. Tax positions that meet the more likely than not threshold are then measured using a probability weighted approach recognizing the largest amount of tax benefit that is greater than 50% likely of being realized upon ultimate settlement. The Company had no tax positions relating to open income tax returns that were considered to be uncertain. Accordingly, we have not recorded a liability for unrecognized tax benefits upon adoption of ASC 740-10-25. There continues to be no liability related to unrecognized tax benefits at December 31, 2014.
The Company’s 2011, 2012 and 2013 Federal, New York and New Jersey tax returns remain subject to examination by the respective taxing authorities. In addition, net operating losses and research tax credits arising from prior years are also subject to examination at the time that they are utilized in future years. Neither the Company’s federal or state tax returns are currently under examination.
10. Stockholders’ Deficit
Our certificate of incorporation provides for the issuance of 4,000,000 shares of preferred stock with the rights, preferences, qualifications, and terms to be determined by our Board of Directors. As of December 31, 2014 and 2013, there were no shares of preferred stock outstanding.
We have a stockholder rights plan in which Preferred Stock Purchase Rights (the “Rights”) have been granted at the rate of one one-hundredth of a share of Series A Junior Participating Cumulative Preferred Stock (“Series A Preferred Stock”) at an exercise price of $80 for each share of our common stock. The Rights expire on April 7, 2016.
The Rights are not exercisable, or transferable apart from the common stock, until the earlier of (i) ten days following a public announcement that a person or group of affiliated or associated persons have acquired beneficial ownership of 20% or more of our outstanding common stock or (ii) ten business days (or such later date, as defined) following the commencement of, or announcement of an intention to make a tender offer or exchange offer, the consummation of which would result in the beneficial ownership by a person, or group, of 20% or more of our outstanding common stock. MHR is specifically excluded from the provisions of the plan.
Furthermore, if we enter into consolidation, merger, or other business combinations, as defined, each Right would entitle the holder upon exercise to receive, in lieu of shares of Series A Preferred Stock, a number of shares of common stock of the acquiring company having a value of two times the exercise price of the Right, as defined. The Rights contain anti-dilutive provisions and are redeemable at our option, subject to certain defined restrictions for $.01 per Right.
As a result of the Rights dividend, the Board of Directors designated 200,000 shares of preferred stock as Series A Preferred Stock and on June 5, 2012, the Company filed a Certificate of Increase of Series A Preferred Stock, increasing the number of shares of the Company’s Series A Preferred Stock from 200,000 to 1,000,000. Holders of Series A Preferred Stock will be entitled to a preferential cumulative quarterly dividend of the greater of $1.00 per share or 100 times the per share dividend declared on our common stock. Shares of Series A Preferred Stock have a liquidation preference, as defined, and each share will have 100 votes and will vote together with the common shares.
On June 5, 2014, the Company filed with the Secretary of the State of Delaware a Certificate of Amendment (the “Certificate of Amendment”) to its Amended and Restated Certificate of Incorporation, increasing the
77
Table of Contents
EMISPHERE TECHNOLOGIES, INC.
NOTES TO FINANCIAL STATEMENTS — (Continued)
number of authorized shares of common stock from 200,000,000 to 400,000,000 shares and increasing the number of authorized shares of preferred stock from 2,000,000 to 4,000,000 shares.
11. Stock-Based Compensation Plans
Total compensation expense recorded during the years ended December 31, 2014, 2013 and 2012 for share-based payment awards was $0.2 million, $0.2 million and $0.3 million, respectively. At December 31, 2014, total unrecognized estimated compensation expense related to non-vested stock options granted prior to that date was approximately $0.1 million, which is expected to be recognized over a weighted-average period of 1.6 years. No tax benefit was realized due to a continued pattern of operating losses. We have a policy of issuing new shares to satisfy share option exercises. No options were exercised during the years ended December 31, 2014 and 2013.
During the year ended December 31, 2014, the Company granted 495,000 options which included 215,000 options to Timothy Rothwell, Chairman of the Board, 40,000 options to each of the Company’s outside directors, and 40,000 options to Michael Garone, Chief Financial Officer. The options were valued on the grant date at $144 thousand using the Black Scholes pricing model.
Using the Black-Scholes model, we have estimated our stock price volatility using the historical volatility in the market price of our common stock for the expected term of the option. The risk-free interest rate is based on the yield curve of U.S. Treasury STRIP securities for the expected term of the option. We have never paid cash dividends and do not intend to pay cash dividends in the foreseeable future. Accordingly, we assumed a 0% dividend yield. The forfeiture rate is estimated using historical option cancellation information, adjusted for anticipated changes in expected exercise and employment termination behavior. Forfeiture rates and the expected term of options are estimated separately for groups of employees that have similar historical exercise behavior. The ranges presented below are the result of certain groups of employees displaying different behavior.
The following weighted-average assumptions were used for grants made under the stock option plans for the years ended December 31, 2014, 2013 and 2012:
| 2014 | ||||||||||||
| Directors | Executives | Employees | ||||||||||
| Expected volatility |
143.09-145.11 | % | 142.69 | % | 145.03 | % | ||||||
| Expected term |
6.8 years | 6.8 years | 6.8 years | |||||||||
| Risk-free interest rate |
1.97-2.20 | % | 2.22 | % | 1.75 | % | ||||||
| Dividend yield |
0 | % | 0 | % | 0 | % | ||||||
| Annual forfeiture rate |
14.5 | % | 14.5 | % | 14.5 | % | ||||||
| 2013 | ||||||||||||
| Directors | Executives | Employees | ||||||||||
| Expected volatility |
138.29-141.54 | % | 134.9 | % | 134.9-141.9 | % | ||||||
| Expected term |
6.8 years | 6.8 years | 6.8 years | |||||||||
| Risk-free interest rate |
1.45-2.24 | % | 1.21 | % | 1.21 -2.03 | % | ||||||
| Dividend yield |
0 | % | 0 | % | 0 | % | ||||||
| Annual forfeiture rate |
14.5 | % | 14.5 | % | 14.5 | % | ||||||
78
Table of Contents
EMISPHERE TECHNOLOGIES, INC.
NOTES TO FINANCIAL STATEMENTS — (Continued)
| 2012 | ||||||||||||
| Directors | Executives | Employees | ||||||||||
| Expected volatility |
120.0-125.6 | % | 121.9-131.1 | % | 121.9 | % | ||||||
| Expected term |
6.8 years | 6.8 years | 6.8 years | |||||||||
| Risk-free interest rate |
1.04-1.38 | % | 0.99-1.07 | % | 0.99 | % | ||||||
| Dividend yield |
0 | % | 0 | % | 0 | % | ||||||
| Annual forfeiture rate |
14.5 | % | 14.5 | % | 14.5 | % | ||||||
Stock Option Plans. On April 20, 2007, the stockholders approved the 2007 Stock Award and Incentive Plan (the “2007 Plan”). The 2007 Plan provides for grants of options, stock appreciation rights, restricted stock, deferred stock, bonus stock and awards in lieu of obligations, dividend equivalents, other stock based awards and performance awards to executive officers and other employees of the Company, and non-employee directors, consultants and others who provide substantial service to us. The 2007 Plan provides for the issuance of 9,195,376 shares as follows: 7,500,000 new shares, 1,294,306 shares remaining and transferred from the Company’s 2000 Stock Option Plan (the “2000 Plan”) (which was then replaced by the 2007 Plan) and 401,070 shares remaining and transferred from the Company’s Stock Option Plan for Outside Directors (the “Directors Stock Plan”). In addition, shares cancelled, expired, forfeited, settled in cash, settled by delivery of fewer shares than the number underlying the award, or otherwise terminated under the 2000 Plan will become available for issuance under the 2007 Plan, once registered. As of December 31, 2014 4,483,766 shares remain available for issuance under the 2007 Plan. Generally, the options vest at the rate of 20% per year and expire within a five-to-ten-year period, as determined by the compensation committee of the Board of Directors and as defined by the Plans.
The Company also has grants outstanding under its expired and terminated 2000 Stock Option Plan (the “2000 Plan”). Under our 2000 Plan a maximum of 1,945,236 shares of our common stock were available for issuance. The 2000 Plan was available to employees, directors and consultants. The 2000 Plan provides for the grant of either ISOs, as defined by the Internal Revenue Code, or non-qualified stock options, which do not qualify as ISOs. Generally, the options vest at the rate of 20% per year and expire within a five- to ten-year period, as determined by the compensation committee of the Board of Directors and as defined by the Plans.
79
Table of Contents
EMISPHERE TECHNOLOGIES, INC.
NOTES TO FINANCIAL STATEMENTS — (Continued)
Transactions involving stock options awarded under the Plans described above during the years ended December 31, 2014, 2013 and 2012 are summarized as follows:
| Number of Shares |
Weighted Average Exercise Price |
Weighted Average Remaining Contractual Term in Years |
Aggregate Intrinsic Value |
|||||||||||||
| (In thousands) | ||||||||||||||||
| Outstanding at December 31, 2011 |
3,168,630 | $ | 3.03 | 3.4 | $ | 18 | ||||||||||
| Granted |
2,736,750 | $ | 0.43 | |||||||||||||
| Expired |
(1,709,020 | ) | $ | 3.67 | ||||||||||||
| Forfeited |
(45,950 | ) | $ | 1.30 | ||||||||||||
|
|
|
|||||||||||||||
| Outstanding at December 31, 2012 |
4,150,410 | $ | 1.07 | 8.4 | $ | 1,076 | ||||||||||
| Granted |
505,000 | $ | 0.20 | |||||||||||||
| Expired |
(88,000 | ) | $ | 3.03 | ||||||||||||
| Forfeited |
(226,660 | ) | $ | 1.61 | ||||||||||||
|
|
|
|||||||||||||||
| Outstanding at December 31, 2013 |
4,340,750 | $ | 0.90 | 7.8 | $ | 56 | ||||||||||
| Granted |
495,000 | $ | 0.31 | |||||||||||||
| Expired |
(2,000 | ) | $ | 5.20 | ||||||||||||
| Forfeited |
(13,000 | ) | $ | 0.67 | ||||||||||||
|
|
|
|||||||||||||||
| Outstanding at December 31, 2014 |
4,820,750 | $ | 0.84 | 7.1 | $ | 223 | ||||||||||
|
|
|
|||||||||||||||
| Vested and exercisable at December 31, 2014 |
3,174,094 | $ | 0.95 | 6.7 | $ | 198 | ||||||||||
|
|
|
|||||||||||||||
| Vested and expected to vest at December 31, 2014 |
4,571,027 | $ | 0.85 | 7.1 | $ | 221 | ||||||||||
|
|
|
|||||||||||||||
The weighted-average grant date fair value of options granted during the years ended December 31, 2014, 2013 and 2012 was $0.29, $0.19 and $0.10, respectively.
Outside Directors’ Plan. We previously issued options to outside directors who are neither officers nor employees of Emisphere nor holders of more than 5% of our common stock under the Directors Stock Plan. As amended, a maximum of 725,000 shares of our common stock were available for issuance under the Outside Directors’ Plan in the form of options and restricted stock. The Directors Stock Plan expired on January 29, 2007. Options and restricted stock are now granted to directors under the 2007 Plan discussed above.
80
Table of Contents
EMISPHERE TECHNOLOGIES, INC.
NOTES TO FINANCIAL STATEMENTS — (Continued)
Transactions involving stock options awarded under the Directors Stock Plan during the years ended December 31, 2014, 2013 and 2012 are summarized as follows:
| Number of Shares |
Weighted Average Exercise Price |
Weighted Average Remaining Contractual Term in Years |
Aggregate Intrinsic Value |
|||||||||||||
| (In thousands) | ||||||||||||||||
| Outstanding at December 31, 2011 |
79,000 | $ | 9.27 | 1.7 | ||||||||||||
| Expired |
(37,000 | ) | $ | 13.06 | ||||||||||||
|
|
|
|||||||||||||||
| Outstanding at December 31, 2012 |
42,000 | $ | 5.93 | 1.9 | ||||||||||||
| Expired |
(21,000 | ) | $ | 2.89 | ||||||||||||
|
|
|
|||||||||||||||
| Outstanding at December 31, 2013 |
21,000 | $ | 8.97 | 2.7 | ||||||||||||
|
|
|
|||||||||||||||
| Outstanding at December 31, 2014 |
21,000 | $ | 8.97 | 1.4 | ||||||||||||
|
|
|
|||||||||||||||
| Vested and Exercisable at December 31, 2014 |
21,000 | $ | 8.97 | 1.4 | $ | — | ||||||||||
|
|
|
|||||||||||||||
Non-Plan Options. Our Board of Directors previously granted options (“Non-Plan Options”) to two consultants. The Board of Directors determines the number and terms of each grant (option exercise price, vesting, and expiration date).
Transactions involving awards of Non-Plan Options during the years ended December 31, 2014, 2013 and 2012 are summarized as follows:
| Number of Shares |
Weighted Average Exercise Price |
Weighted Average Remaining Contractual Term in Years |
Aggregate Intrinsic Value |
|||||||||||||
| (In thousands) | ||||||||||||||||
| Outstanding at December 31, 2011 |
10,000 | $ | 3.64 | 2.0 | ||||||||||||
| Expired |
(5,000 | ) | 3.15 | |||||||||||||
|
|
|
|||||||||||||||
| Outstanding at December 31, 2012 |
5,000 | $ | 4.12 | 0.5 | ||||||||||||
| Expired |
(5,000 | ) | 4.12 | |||||||||||||
|
|
|
|||||||||||||||
| Outstanding at December 31, 2013 |
— | $ | — | — | ||||||||||||
| Outstanding at December 31, 2014 |
— | $ | — | — | ||||||||||||
|
|
|
|||||||||||||||
| Vested and Exercisable at December 31, 2014 |
— | $ | — | — | $ | — | ||||||||||
|
|
|
|||||||||||||||
12. Collaborative Research Agreements
We are a party to collaborative agreements with corporate partners to provide development and commercialization services relating to the collaborative products. These agreements are in the form of research and development collaboration and licensing agreements. In connection with these agreements, we have granted licenses or the rights to obtain licenses to our oral drug delivery technology. In return, we are entitled to receive certain payments upon the achievement of milestones and will receive royalties on sales of products should they be commercialized. Under these agreements, we are entitled to also be reimbursed for research and development costs. We also have the right to manufacture and supply delivery agents developed under these agreements to our corporate partners.
81
Table of Contents
EMISPHERE TECHNOLOGIES, INC.
NOTES TO FINANCIAL STATEMENTS — (Continued)
We also perform research and development for others pursuant to feasibility agreements, which are of short duration and are designed to evaluate the applicability of our drug delivery agents to specific drugs. Under the feasibility agreements, we are generally reimbursed for the cost of work performed.
All of our collaborative agreements are subject to termination by our corporate partners without significant financial penalty to them. Milestone and upfront payments received in connection with these agreements was $0.0 million, $10.0 million, and $0.0 million in the years ended December 31, 2014, 2013 and, 2012, respectively. Expense reimbursements received in connection with these agreements was $0.0 million, $0.00 million and $0.02 million for the years ended December 31, 2014, 2013 and 2012, respectively. There were no expenses incurred in connection with these agreements in the years ended December 31, 2014, 2013 and 2012, respectively. Significant agreements are described below.
Novo Nordisk Agreements
GLP-1 License Agreement
On June 21, 2008, we entered into an exclusive Development and License Agreement with Novo Nordisk pursuant to which Novo Nordisk will develop and commercialize oral formulations of Novo Nordisk proprietary products in combination with Emisphere carriers (the “GLP-1 License Agreement”). Under such the GLP-1 License Agreement, Emisphere could receive more than $87.0 million in contingent product development and sales milestone payments including a $10.0 million non-refundable license fee which was received during June 2008. Emisphere would also be entitled to receive royalties in the event Novo Nordisk commercializes products developed under such agreement. Under the terms of the GLP-1 License Agreement, Novo Nordisk is responsible for the development and commercialization of the products. Initially Novo Nordisk is focusing on the development of oral formulations of its proprietary GLP-1 receptor agonists. In January 2010, Novo Nordisk had its first Phase I clinical trial with a long acting oral GLP-1 receptor agonist. This milestone released a $2 million payment to Emisphere.
The GLP-1 License Agreement includes multiple deliverables including the license grant, several versions of the Company’s Eligen® Technology (or carriers), support services and manufacturing. Emisphere management reviewed the relevant terms of the GLP-1 License Agreement and determined that such deliverables should be accounted for as a single unit of accounting in accordance with FASB ASC 605-25, Multiple-Element Arrangements, since the delivered license and Eligen® Technology do not have stand-alone value and Emisphere does not have objective evidence of fair value of the undelivered Eligen® Technology or the manufacturing value of all the undelivered items. Such conclusion will be reevaluated as each item in the arrangement is delivered. Consequently, any payments received from Novo Nordisk pursuant to such agreement, including the initial $10 million upfront payment and any payments received for support services, will be deferred and included in Deferred Revenue within our balance sheet. Management cannot currently estimate when all of such deliverables will be delivered nor can they estimate when, if ever, Emisphere will have objective evidence of the fair value for all of the undelivered items, therefore all payments from Novo Nordisk are expected to be deferred for the foreseeable future.
On April 26, 2013, the Company entered into an Amendment No. 2 (the “Amendment”) to the GLP-1 License Agreement. The Amendment provides, among other things, for a payment of $10 million from Novo Nordisk to the Company as a prepayment for the achievement of certain development milestones that would have otherwise become payable to the Company under the GLP-1 License Agreement in exchange for a reduction in the rate of potential future royalty payments as provided in the GLP-1 License Agreement. The $10 million payment from Novo Nordisk was received by the Company on May 6, 2013, and recorded as deferred revenue.
As of December 31, 2014 and 2013, total deferred revenue from the GLP-1 License Agreement was $23.6 million, comprised of the $10.0 million April 26, 2013 prepayment, the $10.0 million non-refundable license fee, $2 million milestone payment and $1.6 million in support services.
82
Table of Contents
EMISPHERE TECHNOLOGIES, INC.
NOTES TO FINANCIAL STATEMENTS — (Continued)
Insulins License Agreement
On December 20, 2010, we entered into an exclusive Development and License Agreement with Novo Nordisk, pursuant to which we granted to Novo Nordisk an exclusive license to develop and commercialize oral formulations of Novo Nordisk’s insulins, using the Company’s proprietary delivery agents (the “Insulins License Agreement”). The Insulins License Agreement includes $57.5 million in potential product development and sales milestone payments including a $5.0 million non- refundable, non-creditable license fee. Emisphere would also be entitled to receive royalties in the event Novo Nordisk commercializes products developed under such the Insulins License Agreement.
The Insulins License Agreement includes multiple deliverables including the license grant, several versions of the Company’s Eligen® Technology (or carriers), support services and manufacturing. Emisphere management reviewed the relevant terms of the Novo Nordisk agreement and determined that such deliverables should be accounted for as a single unit of accounting in accordance with FASB ASC 605-25, Multiple-Element Arrangements, since the delivered license and Eligen® Technology do not have stand-alone value and Emisphere does not have objective evidence of fair value of the undelivered Eligen® Technology or the manufacturing value of all the undelivered items. Such conclusion will be reevaluated as each item in the arrangement is delivered. Consequently any payments received from Novo Nordisk pursuant to such agreement, including the initial $5.0 million upfront payment and any payments received for support services, will be deferred and included in Deferred Revenue within our balance sheet. Management cannot currently estimate when all of such deliverables will be delivered nor can they estimate when, if ever, Emisphere will have objective evidence of the fair value for all of the undelivered items, therefore all payments from Novo Nordisk are expected to be deferred for the foreseeable future.
As of December 31, 2014 and 2013, total deferred revenue from the Insulins License Agreement was $5.0 million, comprised of the non-refundable, non-creditable license fee.
Novartis Agreements
Salmon Calcitonin Agreements
We have collaborated with Novartis in connection with the development and testing of oral formulations of salmon calcitonin (“sCT”) to treat osteoarthritis and osteoporosis (the “Salmon Calcitonin Program”). We entered into a Research Collaboration and Option Agreement, dated as of December 3, 1997, as amended on October 20, 2000 (the “Salmon Calcitonin Option Agreement”) with Novartis to develop an oral form of sCT, which is a hormone that inhibits the bone-tissue resorbing activity of specialized bone cells called osteoclasts, enabling the bone to retain more of its mass and functionality. Pursuant to the Salmon Calcitonin Option Agreement, the Company granted Novartis the option to acquire from the Company a license to develop and commercialize oral sCT utilizing Emisphere’s Eligen® Technology and the right to commence research collaboration with the Company with respect to a second compound, in exchange for certain option exercise payments. Novartis also agreed to reimburse the Company with respect to certain research and development costs incurred by the Company in connection with the sCT Program. Furthermore, under the Salmon Calcitonin Option Agreement, the Company is obligated to help to manage this program through a joint “steering committee” with Novartis. The Salmon Calcitonin Option Agreement expires upon the expiration of the last to expire of the patents of the Company described therein, subject to certain early termination rights, including termination by either party for material breach of the other party and termination by Novartis in favor of a license executed thereunder.
In May 2007, Novartis and Nordic Bioscience notified the Company that they were initiating a Phase III clinical study of SMC021 for the treatment of osteoarthritis (“OA”) using the Company’s Eligen® Technology. A second Phase III study of SMC021 for the treatment of OA, designed to meet FDA requirements for U.S. registration, was initiated by Novartis and Nordic Bioscience in October 2008.
83
Table of Contents
EMISPHERE TECHNOLOGIES, INC.
NOTES TO FINANCIAL STATEMENTS — (Continued)
On December 14, 2011, the Company announced that Novartis had informed the Company that it will not pursue further clinical development of the investigational drug SMC021 (oral calcitonin) as a treatment option in osteoarthritis and for post-menopausal osteoporosis and that it will not seek regulatory submission for SMC021 in either indication. Novartis advised the Company that its decision to stop the clinical program of SMC021 in both indications was based on analysis and evaluation of data from three Phase III clinical trials (two in osteoarthritis and one in osteoporosis) conducted by Nordic Bioscience showed that SMC021 failed to meet key efficacy endpoints in all three trials, despite displaying a favorable safety profile.
Although Novartis has not informed Emisphere of its intention to terminate the Salmon Calcitonin Option Agreement and the Salmon Calcitonin License Agreement, in the likely event that Novartis determines to terminate these agreements, we will reacquire the rights to our technology licensed to Novartis thereunder.
Oral PTH-1-34 Agreements
We have collaborated with Novartis in connection with the development and testing of oral formulations of PTH-1-34 (“PTH”) to treat osteoarthritis and osteoporosis (the “PTH Program”). On December 1, 2004, we entered into a Research Collaboration Option and License Agreement with Novartis whereby Novartis obtained an option to license our existing technology to develop oral forms of PTH 1-34 (the “PTH Option Agreement”). On March 7, 2006, Novartis exercised its option to the license. PTH is produced by the parathyroid glands to regulate the amount of calcium and phosphorus in the body. Recombinant PTH, currently approved for the treatment of osteoporosis, is available only by injection. When used therapeutically, it increases bone density and bone strength to help prevent fractures. It is approved to treat osteoporosis, a disease associated with a gradual thinning and weakening of the bones that occurs most frequently in women after menopause. Untreated postmenopausal osteoporosis can lead to chronic back pain, disabling fractures, and lost mobility. During April 2010, we announced that Novartis initiated a second Phase I trial for an oral PTH-1-34 which uses Emisphere’s Eligen® Technology, and was in development for the treatment of postmenopausal osteoporosis. On June 17, 2011, the Company announced that Novartis informed Emisphere of the results of its recently completed Proof of Concept study for an oral PTH1-34 using Emisphere’s Eligen® Technology in post-menopausal women with osteoporosis or osteopenia. Novartis informed Emisphere that, although the study confirmed that oral PTH1-34 was both safe and well-tolerated, several clinical endpoints were not met. Based on the data analyzed, Novartis has terminated the study and anticipates no further work on the oral formulation of PTH1-34. The Company has requested additional information from Novartis in order to further analyze and evaluate the results of this trial. Although Novartis has not informed Emisphere of its intention to terminate the PTH Option Agreement in accordance with relevant terms thereunder, Emisphere would reacquire the rights to develop and/or commercialize the product should Novartis so terminate the Agreement.
Genta Agreement
In March 2006, we entered into a collaborative agreement with Genta to develop an oral formulation of a gallium-containing compound. Under the terms of the agreement, we would be eligible for future milestone payments totaling up to a maximum of $24.3 million. On August 2, 2012, Genta Incorporated filed a voluntary petition for relief under Chapter 7 of the United States Bankruptcy Code in the United States Bankruptcy Court for the District of Delaware. The collaborative agreement was subsequently rejected during the bankruptcy. In connection with the bankruptcy case, Genta’s secured creditor, Heron Therapeutics (now GFV LLC), purchased substantially all of Genta’s assets including patents directed to oral gallium formulations.
Total deferred revenue as of December 31, 2014 and 2013 related to Novartis agreements was $13.0 million.
13. Defined Contribution Retirement Plan
We have a defined contribution retirement plan (the “Retirement Plan”), the terms of which, as amended, allow eligible employees who have met certain age and service requirements to participate by electing to contribute a percentage of their compensation to be set aside to pay their future retirement benefits, as defined by
84
Table of Contents
EMISPHERE TECHNOLOGIES, INC.
NOTES TO FINANCIAL STATEMENTS — (Continued)
the Retirement Plan. We have agreed to make discretionary contributions to the Retirement Plan. For the years ended December 31, 2014, 2013 and 2012, we made contributions to the Retirement Plan totaling approximately $0.04 million, $0.03 million, $0.04 million, respectively.
14. Net Loss Per Share
The following table sets forth the information needed to compute basic and diluted earnings per share for the years ended December 31, 2014, 2013 and 2012:
| Year Ended December 31, | ||||||||||||
| 2014 | 2013 | 2012 | ||||||||||
| (In thousands, except per share amounts) | ||||||||||||
| Net loss |
$ | (25,380 | ) | $ | (20,939 | ) | $ | (1,928 | ) | |||
|
|
|
|
|
|
|
|||||||
| Net loss per common share, basic and diluted: |
||||||||||||
| Weighted average common shares outstanding, basic |
60,687,478 | 60,687,478 | 60,687,478 | |||||||||
|
|
|
|
|
|
|
|||||||
| Net loss per share, basic and diluted |
$ | (0.42 | ) | $ | (0.35 | ) | $ | (0.03 | ) | |||
|
|
|
|
|
|
|
|||||||
The following table sets forth the number of potential shares of common stock that have been excluded from diluted net loss per share because their effect was anti-dilutive:
| Year Ended December 31, | ||||||||||||
| 2014 | 2013 | 2012 | ||||||||||
| Options to purchase common shares |
4,820,750 | 4,340,750 | 1,524,160 | |||||||||
| Outstanding warrants and options to purchase warrants |
27,443,728 | 27,443,727 | 17,443,727 | |||||||||
| Amended and Restated Convertible notes |
32,717,484 | 28,748,424 | 8,353,518 | |||||||||
| Amended and Restated Reimbursement notes |
1,365,606 | 1,274,334 | — | |||||||||
| Amended and Restated Bridge notes |
3,710,158 | 3,254,246 | — | |||||||||
|
|
|
|
|
|
|
|||||||
| 70,057,726 | 65,061,481 | 27,321,407 | ||||||||||
|
|
|
|
|
|
|
|||||||
15. Commitments and Contingencies
Commitments.
We lease office space at 4 Becker Farm Road, Roseland, NJ under a non-cancellable operating lease expiring in 2017.
As of December 31, 2014, future minimum rental payments are as follows:
| Years Ending December 31, |
||||
| (In thousands) | ||||
| 2015 |
136 | |||
| 2016 |
148 | |||
| 2017 |
74 | |||
|
|
|
|||
| Total |
$ | 358 | ||
|
|
|
|||
Rent expense for the years ended December 31, 2014, 2013 and 2012 was $0.1 million, $0.1 million and $0.3 million, respectively. Additional charges under this lease for real estate taxes and common maintenance charges for the years ended December 31, 2014, 2013 and 2012, were $0.04 million, $0.02 million and $0.01 million, respectively.
85
Table of Contents
EMISPHERE TECHNOLOGIES, INC.
NOTES TO FINANCIAL STATEMENTS — (Continued)
The Company evaluates the financial consequences of legal actions periodically or as facts present themselves and records accruals to account for its best estimate of future costs accordingly.
Contingencies. In the ordinary course of business, we enter into agreements with third parties that include indemnification provisions which, in our judgment, are normal and customary for companies in our industry sector. These agreements are typically with business partners, clinical sites, and suppliers. Pursuant to these agreements, we generally agree to indemnify, hold harmless, and reimburse indemnified parties for losses suffered or incurred by the indemnified parties with respect to our product candidates, use of such product candidates, or other actions taken or omitted by us. The maximum potential amount of future payments we could be required to make under these indemnification provisions is unlimited. We have not incurred material costs to defend lawsuits or settle claims related to these indemnification provisions. As a result, the estimated fair value of liabilities relating to these provisions is minimal. Accordingly, we have no liabilities recorded for these provisions as of December 31, 2014.
As a condition to MHR entering into the Loan Agreement and amending and restating the MHR Notes, the Company and MHR entered into a Royalty Agreement (the “Royalty Agreement”) on August 20, 2014, providing for the payment by the Company to MHR of certain royalties on the terms and conditions set forth therein (see Note 7). Under the terms of the Royalty Agreement, the Company agreed to pay to MHR, subject to the terms and conditions of the Royalty Agreement, royalties in perpetuity (the “Royalties”), commencing as of the date of the Royalty Agreement, in an amount equal to: twenty percent (20%) of all Net Product Sales (as defined in the Royalty Agreement) and any third party payments arising in connection with the sale of the B12 Product and related products, during any fiscal year; provided that, from and after October 1, 2015, if no amount of indebtedness is outstanding under the Loan 13 Table of Contents Agreement (the “Indebtedness Repayment Condition”), such amount shall be reduced to (i) five percent (5%) of all Net Sales and third party payments commencing with the first quarter immediately following the quarter in which the Indebtedness Repayment Condition is satisfied, or (ii) two and one half percent (2.5%) of all Net Sales commencing with the quarter immediately following the quarter in which the Indebtedness Repayment Condition is satisfied, but only with respect to the Net Sales made in any country in which there was not a Valid Patent Claim (as defined in the Royalty Agreement) and where generic entry of a competitive product not by the Company or its affiliates that does not infringe a Valid Patent Claim in such country has occurred, in each case as of the last day of such Fiscal Quarter. Once the royalty rate has been reduced to 5%, the rate shall not be reinstated to 20% even if amounts become outstanding under the Loan Agreement as a result of Paid-In-Kind Royalties. Payments of Royalties shall be made in cash to the extent such Royalties do not cause the Company’s cash as of the end of any year to be less than the Minimum Cash Balance, and otherwise shall be paid as Paid-In-Kind Royalties.
In the normal course of business, we may be confronted with issues or events that may result in a contingent liability. These generally relate to lawsuits, claims, environmental actions or the action of various regulatory agencies. If necessary, management consults with counsel and other appropriate experts to assess any matters that arise. If, in our opinion, we have incurred a probable loss as set forth by accounting principles generally accepted in the U.S., an estimate is made of the loss and the appropriate accounting entries are reflected in our financial statements.
86
Table of Contents
EMISPHERE TECHNOLOGIES, INC.
NOTES TO FINANCIAL STATEMENTS — (Continued)
16. Summarized Quarterly Financial Data (Unaudited)
Following are summarized quarterly financial data (unaudited) for the years ended December 31, 2014 and 2013:
| 2014 | ||||||||||||||||
| March 31 | June 30 | September 30 | December 31 | |||||||||||||
| (In thousands) | ||||||||||||||||
| Total revenue |
$ | — | $ | — | $ | — | $ | — | ||||||||
| Operating loss |
(2,345 | ) | (1,539 | ) | (2,120 | ) | (3,301 | ) | ||||||||
| Net income (loss) |
(3,358 | ) | (8,069 | ) | (14,373 | ) | 420 | |||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net income (loss) per share, basic |
$ | (0.06 | ) | $ | (0.13 | ) | $ | (0.24 | ) | $ | 0.01 | |||||
| Net income (loss) per share, diluted |
$ | (0.06 | ) | $ | (0.13 | ) | $ | (0.24 | ) | $ | 0.01 | |||||
| 2013 | ||||||||||||||||
| March 31 | June 30 | September 30 | December 31 | |||||||||||||
| (In thousands) | ||||||||||||||||
| Total revenue |
$ | — | $ | — | $ | — | $ | — | ||||||||
| Operating loss |
(1,712 | ) | (1,788 | ) | (1,761 | ) | (2,343 | ) | ||||||||
| Net income loss |
(2,424 | ) | (13,982 | ) | (1,720 | ) | (2,813 | ) | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net loss per share, basic |
$ | (0.04 | ) | $ | (0.23 | ) | $ | (0.03 | ) | $ | (0.05 | ) | ||||
| Net (loss) per share, diluted |
$ | (0.04 | ) | $ | (0.23 | ) | $ | (0.03 | ) | $ | (0.05 | ) | ||||
17. Fair Value
In accordance with FASB ASC 820, Fair Value Measurements and Disclosures, the following table represents the Company’s fair value hierarchy for its financial liabilities measured at fair value on a recurring basis as of December 31, 2014 and 2013:
| December 31, 2014 |
Level 2 | Level 3 | Total | |||||||||
| (in thousands) | (in thousands) | (in thousands) | ||||||||||
| Derivative instruments |
$ | 5,506 | $ | 24,414 | $ | 29,920 | ||||||
| December 31, 2013: |
Level 2 | Level 3 | Total | |||||||||
| (in thousands) | (in thousands) | (in thousands) | ||||||||||
| Derivative instruments |
$ | 3,922 | $ | 11,587 | $ | 15,509 | ||||||
Level 3 financial instruments consist of common stock warrants and embedded conversion features. The fair value of these warrants and embedded conversion features that have exercise reset features are estimated using a Monte Carlo valuation model. The unobservable input used by the Company was the estimation of the likelihood of a reset occurring on the embedded conversion feature of the Convertible Notes, the embedded conversion feature of the Reimbursement Notes, the embedded conversion feature of the Bridge Notes, and the embedded feature of Amended and Restated June 2010 Warrants. These estimates of the likelihood of completing an equity raise that would meet the criteria to trigger the reset provisions are based on numerous factors, including the remaining term of the financial statements and the Company’s overall financial condition.
87
Table of Contents
EMISPHERE TECHNOLOGIES, INC.
NOTES TO FINANCIAL STATEMENTS — (Continued)
The following table summarizes the changes in fair value of the Company’s Level 3 financial instruments for the years ended December 31, 2014 and 2013:
| Year Ended December 31, | ||||||||
| 2014 | 2013 | |||||||
| Beginning Balance |
$ | 11,587 | $ | 309 | ||||
| Derivative liability of embedded conversion feature of the Bridge Notes |
221 | 1,187 | ||||||
| Derivative liability of embedded conversion feature of the Reimbursement Notes |
47 | 156 | ||||||
| Derivative liability of embedded conversion feature of the Convertible Notes |
2,272 | 862 | ||||||
| Change in fair value |
10,287 | 9,073 | ||||||
|
|
|
|
|
|||||
| Ending Balance |
$ | 24,414 | $ | 11,587 | ||||
|
|
|
|
|
|||||
Changes in the unobservable input values would likely cause material changes in the fair value of the Company’s Level 3 financial instruments. The significant unobservable input used in the fair value measurement is the estimation of the likelihood of the occurrence of a change to the contractual terms of the financial instruments. A significant increase (decrease) in this likelihood would result in a higher (lower) fair value measurement.
18. Subsequent Events
On January 6, 2015, the Company borrowed $5.0 million in original principal amount on its $20,0 million secured credit facility governed by (i) the Loan Agreement, dated as of August 20, 2014, by and between the Company and MHR Capital Partners Master Account LP and affiliated funds, (ii) the Amended and Restated Pledge and Security Agreement, dated as of August 20, 2014, by and between the Company and MHR Institutional Partners IIA LP, and (iii) the Royalty Agreement, dated as of August 20, 2014, by and between Emisphere Technologies, Inc. and MHR Capital Master Account LP and affiliated funds.
88
Table of Contents
| ITEM 9. | CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE |
None.
| ITEM 9A. | CONTROLS AND PROCEDURES |
Evaluation of Disclosure Controls and Procedure
The Company’s senior management is responsible for establishing and maintaining a system of disclosure controls and procedures (as defined in Rule 13a-15 and 15d-15 under the Securities Exchange Act of 1934 (the “Exchange Act”)) designed to ensure that information required to be disclosed by the Company in the reports that it files or submits under the Exchange Act is recorded, processed, summarized and reported within the time periods specified in the Securities and Exchange Commission’s rules and forms. Disclosure controls and procedures include, without limitation, controls and procedures designed to ensure that information required to be disclosed by an issuer in the reports that it files or submits under the Exchange Act is accumulated and communicated to the issuer’s management, including its principal executive officer or officers and principal financial officer or officers, or persons performing similar functions, as appropriate to allow timely decisions regarding required disclosure.
The Company has evaluated the effectiveness of the design and operation of its disclosure controls and procedures under the supervision of and with the participation of management, including its Chief Executive Officer and Chief Financial Officer, as of the end of December 31, 2014. Based on that evaluation, our Chief Executive Officer and Chief Financial Officer have concluded that our disclosure controls and procedures are effective.
Changes in Internal Control over Financial Reporting
There have been no changes in the Company’s system of internal controls over financial reporting during the three month period ended December 31, 2014 that materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
Our management does not expect that our disclosure controls and procedures or internal controls over financial reporting will prevent all errors and all fraud. A control system, no matter how well conceived and operated, can provide only reasonable, not absolute, assurance that the objectives of the system are met and cannot detect all deviations. Because of the inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that all control issues and instances of fraud or deviations, if any, within the company have been detected. Projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Management’s Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting, as such term is defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act. Our management has conducted an evaluation of the effectiveness of our internal control over financial reporting based on the framework established in Internal Control — Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 framework). Based on that evaluation, our management has concluded that our internal control over financial reporting was effective as of December 31, 2014.
McGladrey LLP, our independent registered public accounting firm, has issued a report on the effectiveness of internal over financial reporting as of December 31, 2014, which report is included herein at page 49.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
| ITEM 9B. | OTHER INFORMATION |
None.
89
Table of Contents
| ITEM 10. | DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE |
Director and Executive Officer Information
Information regarding those directors serving unexpired terms and our current Executive Officers, as such term is defined in Regulation S-K under the Exchange Act, all of whom are currently serving open-ended terms, including their respective ages, the year in which each first joined the Company and their principal occupations or employment during the past five years, is provided below:
| Name |
Age | Year Joined Emisphere |
Position with the Company | |||||
| Alan L. Rubino |
60 | 2012 | President and Chief Executive Officer, Class II Director | |||||
| Michael R. Garone |
56 | 2007 | Vice President, Chief Financial Officer and Corporate Secretary | |||||
| John D. Harkey, Jr. |
54 | 2006 | Class I Director | |||||
| Timothy McInerney |
54 | 2012 | Class II Director | |||||
| Jacob M. Plotsker |
47 | 2012 | Class II Director | |||||
| Mark H. Rachesky, M.D. |
56 | 2005 | Class III Director | |||||
| Timothy G. Rothwell |
64 | 2009 | Chairman of the Board of Directors, Class I Director | |||||
| Michael Weiser, M.D., Ph.D. |
52 | 2005 | Class III Director | |||||
Alan L. Rubino joined Emisphere on September 13, 2012, as President and Chief Executive Officer and, in connection therewith, was appointed as a Class II Director of the Company. His career spans over 30 years at every level of the biopharmaceutical industry. From October 2010 until July 2012, he served as Chief Executive Officer and President of New American Therapeutics, Inc., where he and his team presided over a venture that was focused on the acquisition, marketing, and ultimate sale of Denavir, a leading Rx topical therapeutic for HSV-1 cold sore treatment. From February 2008 to September 2010, Mr. Rubino was CEO and President of Akrimax Pharmaceuticals, where he acquired two Rx launch products, NitroMist and Tirosint, which are actively marketed and in growth phases today. Prior to 2008, he was President and Chief Operating Officer of the Pharmos Corporation, which was a development-stage publicly-held firm, where he led the transformation of the company through the acquisition of Vela Pharmaceuticals. Mr. Rubino also spent four years in senior executive leadership positions on the strategic services side at both Cardinal Health and PDI, Inc., both public companies that provided high-level outsourcing offerings to the pharmaceutical industry. A major portion of Mr. Rubino’s career includes twenty-four years spent at Hoffmann-La Roche, where he served as a corporate officer and member of the US Executive Committee and held a variety of key senior executive positions with broad general management responsibilities leading major business units and operations, marketing, business development, alliance management, human resources, and supply chain/manufacturing. At Hoffmann-La Roche, Mr. Rubino led many key top level executive initiatives and presided over numerous commercial product launches across a spectrum of therapeutic areas, including the introduction of the world’s first biological product in Roferon-A [alfa-interferon 2a]. Currently, Mr. Rubino serves on the Boards of Directors of Aastrom Biosciences (NASDAQ: ASTM) and is Chairman of the Compensation Committee, and on the Board of Directors of SANUWAVE Health, Inc. (SNWV: OTC BB), and Genisphere, Inc., and serves on the Rutgers Business School Board of Advisors.
Timothy G. Rothwell has been a director of the Company since November 2009 and Chairman of the Board of Directors since September 2012. Mr. Rothwell is the former Chairman of Sanofi-Aventis U.S. From February 2007 to October 2009, Mr. Rothwell served as Chairman of Sanofi-Aventis U.S. From September 2004 to February 2007, Mr. Rothwell was President and Chief Executive Officer of that company, overseeing all domestic commercial operations as well as coordination of Industrial Affairs and Research and Development activities. From May 2003 to September 2004, Mr. Rothwell was President and Chief Executive Officer of
90
Table of Contents
Sanofi-Synthelabo, Inc. and was instrumental in the formation of Sanofi-Aventis U.S. in 2004. Prior to that, from January 1998 to May 2003, he served in various capacities at Pharmacia, including as President of the company’s Global Prescription Business. From January 1995 to January 1998, Mr. Rothwell served as worldwide President of Rhone-Poulenc Rorer Pharmaceuticals and President of the company’s Global Pharmaceutical Operations. In his long career, Mr. Rothwell has also served as Chief Executive Officer of Sandoz Pharmaceuticals, Vice President, Global Marketing and Sales at Burroughs Wellcome, and Senior Vice President of Marketing and Sales for the U.S. for Squibb Corporation. Mr. Rothwell holds a Bachelor of Arts from Drew University and earned his J.D. from Seton Hall University. He formerly served on the PhRMA Board of Directors, as well as the Institute of Medicine’s Evidence-Based Medicine roundtable, the CEO Roundtable on Cancer, the Healthcare Businesswomen’s Association Advisory Board, the Board of Trustees for the Somerset Medical Center Foundation, the Board of Trustees for the HealthCare Institute of New Jersey, as a Trustee of the Corporate Council for America’s Children at the Children’s Health Fund, the Board of Directors of Agenus (NASDAQ: AGEN), the Board of Directors of New American Therapeutics, on the Board of Visitors for Seton Hall Law School, and was Chairman of the Board of Directors of Archimedes Pharma Ltd. Presently, he is Chairman Emeritus of the Board of Directors of the PheoPara Alliance, a nonprofit 501(c)3 organization. Mr. Rothwell’s broad business and leadership experiences in the pharmaceutical industry and his affiliations with industry, educational and healthcare related organizations make him an asset to our Board of Directors. As Chairman of the Board, Mr. Rothwell is extremely conscientious and diligent in keeping the other directors abreast of current operational and oversight issues we face.
Michael R. Garone joined Emisphere in 2007 as Vice President and Chief Financial Officer. Mr. Garone has also served as the Company’s Corporate Secretary since October 2008. Mr. Garone previously served as Interim Chief Executive Officer and Chief Financial Officer of Astralis, Ltd. (OTCBB: ASTR.OB). Prior to that, Mr. Garone was with AT&T (NYSE: T) for 20 years, where he held several positions, including Chief Financial Officer of AT&T Alascom. Mr. Garone received an MBA from Columbia University and a BA in Mathematics from Colgate University. From February 28, 2011 until September 13, 2012, Michael R. Garone served as Interim Chief Executive Officer of the Company.
John D. Harkey, Jr. has over 25 years of experience as a private investor concentrating in the acquisition, consolidation and management of both public and private companies and has served on our Board of Directors since 2006. He has merged, acquired and/or operated companies in a variety of industries including oil and gas, petrochemical services, telecommunications, restaurants, real estate, and software development. He is formerly the Chairman of the Board of Regency Gas Partners, L.P. (NYSE:RGP) has served on the Board of Directors of Energy Transfer Equity, LP (NYSE: ETE), which specializes in the storage and transportation of natural gas, and Energy Transfer Partners, LP (NYSE: ETP), which operates energy assets. He currently serves on the Board of Directors and Audit Committee of Loral Space & Communications, Inc. (NASDAQ:LORL), a satellite communications company, United Development Funding III, LP, a real estate investment trust, and on the Board of Directors of the Baylor Health Care System Foundation. He formerly served on Board of Directors of Leap Wireless International, Inc. (NASDAQ:LEAP), which was recently acquired by AT&T for $4.1 billion. He is also Chairman and Chief Executive Officer of Consolidated Restaurant Companies, Inc., a restaurant operating company. He also serves on the President’s Development Council of Howard Payne University, the Executive Board of Circle Ten Council of the Boy Scouts of America, the CEO Advisory Board of Dallas Arboretum and is a member of the World President’s Organization. Mr. Harkey obtained a B.B.A. in Business Honors and a J.D. from the University of Texas at Austin, and a M.B.A. from Stanford University School of Business. Mr. Harkey’s entrepreneurial background and his business and leadership experiences in a range of different industries make him an asset to our Board of Directors.
Timothy McInerney has been a Director of the Company since March 2012. Mr. McInerney is a principal at Two River and a Partner of Riverbank Capital Securities, Inc. From 1992 to March 2007, Mr. McInerney was a Managing Director of Paramount BioCapital, Inc. where he oversaw the overall distribution of Paramount’s private equity product. Prior to 1992, Mr. McInerney was a research analyst focusing on the biotechnology industry at Ladenburg, Thalman & Co. Prior to that, Mr. McInerney held equity sales positions at Bear Stearns & Co. and Shearson Lehman Brothers, Inc. Mr. McInerney also worked in sales and marketing for Bristol-Myers Squibb. Mr. McInerney is currently Chairman of the Board of Directors of Insite Vision, Inc., (OTCBB: INSV), and is a member of the Board of Directors of ZIOPHARM, Inc., (NASDAQ: ZIOP), and Edgemont
91
Table of Contents
Pharmaceuticals, LLC. He formerly served on the Board of Directors of Manhattan Pharmaceuticals, Inc., (OTCBB: TGTX). Mr. McInerney received his B.S. in pharmacy from St. John’s University at New York. He also completed a post-graduate residency at the New York University Medical Center in drug information systems. Mr. McInerney’s knowledge of the pharmaceutical industry and capital markets, and affiliations with the financial community make him an asset to our Board of Directors.
Jacob Plotsker has been a director of the Company since March 2012. Mr. Plotsker is currently President of Cambridge Sage Group, LLC, a strategic consulting firm focused on the pharmaceutical industry. Mr. Plotsker was previously Director of IUS Strategy and Life Cycle Management at Bayer HealthCare. Prior to joining Bayer in 2013 he served from 2009 through 2012 as Senior Director, Commercial Operations and Head of Marketing for Teva Pharmaceuticals Women’s Health Division. Prior to joining Teva, Mr. Plotsker was Senior Director, US and Global Marketing at Schering-Plough Corp (previously Organon BioSciences prior to being acquired by Schering-Plough Corp, which was subsequently acquired by Merck & Co., Inc) where he was responsible for commercialization of marketed brands and launch strategy for brands in development. From 1990 to 2006, Mr. Plotsker served in various Finance and Marketing roles at Pfizer, Inc. including Director/Team Leader of the company’s Antifungal Franchise. From 1989 to 1990, Mr. Plotsker was an Accountant at Deloitte & Touche. Mr. Plotsker holds a Bachelor of Arts degree in Accounting & Information Systems from Queens College of the City University of New York, a Master of Business Administration in Marketing and Finance from New York University — Stern School of Business, and completed the Executive Development Program in General Management at the University of Chicago — Booth School of Business. From 2008 to 2014 Mr. Plotsker served on the Board of Directors of Sharsheret, a nonprofit 501(c)(3) organization providing support and resources to young women living with breast cancer, and served as President from 2009 through 2012. Mr. Plotsker’s experiences in marketing and product commercialization in the pharmaceutical industry, and his affiliations with industry and healthcare related organizations make him an asset to our Board of Directors.
Mark H. Rachesky, M.D. has been a director of the Company since 2005. Dr. Rachesky is the President of MHR Fund Management LLC and investment manager of various private investment funds that invest in inefficient market sectors, including special situation equities and distressed investments. Dr. Rachesky is currently the Non-Executive Chairman of the Board of Directors of Loral Space & Communications Inc. (NASDAQ:LORL), Lions Gate Entertainment Corp. (NYSE: LGF), and Telesat Canada, and also serves on the Board of Directors of Navistar International Corporation (NYSE:NAV), and Titan International, Inc. (NYSE:TWI). He formerly served on the Board of Directors of Neose Technologies, Inc (NASDAQ: NTEC) and of Nationshealth, Inc. (formerly quoted on OTCBB:NHRX). Dr. Rachesky was a Director of Leap Wireless International, Inc. (NASDAQ: LEAP) until Leap Wireless International, Inc. merged with AT&T. Dr. Rachesky is a graduate of Stanford University School of Medicine and Stanford University School of Business. Dr. Rachesky graduated from the University of Pennsylvania with a major in Molecular Aspects of Cancer. Dr. Rachesky’s extensive investing and financial background, his thorough knowledge of capital markets and his training as a physician, make him an asset to our Board of Directors.
Michael Weiser, M.D., Ph.D. has been a director of the Company since 2005. Dr. Weiser is currently founder and co-chairman of Actin Biomed, a New York based healthcare investment firm advancing the discovery and development of novel treatments for unmet medical needs. Prior to joining Actin Biomed, Dr. Weiser was the Director of Research at Paramount BioCapital where he was responsible for the scientific, medical and financial evaluation of biomedical technologies and pharmaceutical products under consideration for development. Dr. Weiser completed his Ph.D. in Molecular Neurobiology at Cornell University Medical College and received his M.D. from New York University School of Medicine. He performed his post-graduate medical training in the Department of Obstetrics and Gynecology at New York University Medical Center. Dr. Weiser also completed a Postdoctoral Fellowship in the Department of Physiology and Neuroscience at New York University School of Medicine and received his B.A. in Psychology from University of Vermont. Dr. Weiser is a member of The National Medical Honor Society, Alpha Omega Alpha, American Society of Clinical Oncology, American Society of Hematology and Association for Research in Vision and Ophthalmology. In addition, Dr. Weiser has received awards for both academic and professional excellence and is published extensively in both medical and scientific journals. Dr. Weiser currently serves on the board of directors of Chelsea Therapeutics International, (NASDAQ: CHTP), and Ziopharm Oncology, Inc., (NASDAQ: ZIOP), as well as
92
Table of Contents
several privately held companies. Dr. Weiser formerly served on the Board of Directors of Manhattan Pharmaceuticals, Inc., (OTCBB: TGTX), Hana Biosciences, Inc., (currently known as Talon Therapeutics, Inc., OTCBB: TLON.OB), and Vioquest Pharmaceuticals, Inc., (VOQP: OTC US). Dr. Weiser has an M.D. and a Ph.D., and his scientific, business and financial experiences, as well as his knowledge of the healthcare industry, capital markets, pharmaceutical products and biomedical technology development make him an asset to our Board of Directors.
Section 16(a) Beneficial Ownership Reporting Compliance
Section 16(a) of the Exchange Act, and the rules of the SEC require our directors, Executive Officers and persons who own more than 10% of common stock to file reports of their ownership and changes in ownership of common stock with the SEC. Our employees sometimes prepare these reports on the basis of information obtained from each director and Executive Officer. Based on written representations of the Company’s directors and Executive Officers and on confirmation that no Form 5 was required to be filed, we believe that all reports required by Section 16(a) of the Exchange Act to be filed by its directors, Executive Officers and greater than ten (10%) percent owners during the last fiscal year were filed on time.
Code of Conduct for Officers and Employees and Code of Business Conduct and Ethics for Directors
The Company has a Code of Conduct that applies to all of our officers (including our principal executive officer, principal financial officer, and principal accounting officer or controller, or persons performing similar functions) and employees as well as a Code of Business Conduct and Ethics that applies specifically to the members of the Board of Directors. The directors are surveyed annually regarding their compliance with the policies as set forth in the Code of Business Conduct and Ethics for Directors. The Code of Conduct and the Code of Ethics for Directors are available on the Corporate Governance section of our website at www.emisphere.com. The contents of our website are not incorporated herein by reference and the website address provided in this annual report is intended to be an inactive textual reference only. The Company intends to disclose on its website any amendment to, or waiver of, a provision of the Code of Conduct that applies to the Chief Executive Officer, Chief Financial Officer, or Controller. Our Code of Conduct contains provisions that apply to our Chief Executive Officer, Chief Financial Officer and all other finance and accounting personnel. These provisions comply with the requirements of a company code of ethics for financial officers that were promulgated by the SEC pursuant to the Exchange Act.
Stockholder Communications
We have an Investor Relations Office for all stockholder inquiries and communications. The Investor Relations Office facilitates the dissemination of accurate and timely information to our stockholders. In addition, the Investor Relations Office ensures that outgoing information is in compliance with applicable securities laws and regulations. All investor queries should be directed to our internal Director of Corporate Communications or our Corporate Secretary.
Election of Directors
The Governance and Nominating Committee identifies director nominees by reviewing the desired experience, mix of skills and other qualities to assure appropriate Board composition, taking into consideration the current Board members and the specific needs of the Company and the Board. Among the qualifications to be considered in the selection of candidates, the Committee considers the following attributes and criteria of candidates: experience, knowledge, skills, expertise, diversity, personal and professional integrity, character, business judgment and independence. Although it has no formal policy, our Board recognizes that nominees for the Board should reflect a reasonable diversity of backgrounds and perspectives, including those backgrounds and perspectives with respect to business experience, professional expertise, age, gender and ethnic background.
Our Board is comprised of accomplished professionals who represent diverse and key areas of expertise including national and international business, operations, manufacturing, finance and investing, management, entrepreneurship, higher education and science, research and technology. We believe our directors’ wide range of professional experiences and backgrounds, education and skills has proven invaluable to the Company and we intend to continue leveraging this strength.
93
Table of Contents
Nominations for the election of directors may be made by the Board of Directors or the Governance and Nominating Committee. The committee did not reject any candidates recommended within the preceding year by a beneficial owner of, or from a group of security holders that beneficially owned, in the aggregate, more than five percent (5%) of the Company’s voting stock.
Although it has no formal policy regarding stockholder nominees, the Governance and Nominating Committee believes that stockholder nominees should be viewed in substantially the same manner as other nominees. Stockholders may make a recommendation for a nominee by complying with the notice procedures set forth in our bylaws. The Governance and Nominating Committee will give nominees recommended by stockholders in compliance with these procedures the same consideration that it gives to any board recommendations. To date, we have not received any recommendation from stockholders requesting that the Governance and Nominating Committee (or any predecessor) consider a candidate for inclusion among the committee’s slate of nominees in the Company’s proxy statement.
To be considered by the committee, a director nominee must have broad experience at the strategy/policy-making level in a business, government, education, technology or public interest environment, high-level managerial experience in a relatively complex organization or experience dealing with complex problems. In addition, the nominee must be able to exercise sound business judgment and provide insights and practical wisdom based on experience and expertise, possess proven ethical character, be independent of any particular constituency, and be able to represent all stockholders of the Company.
The committee will also evaluate whether the nominee’s skills are complementary to the existing Board members’ skills; the board’s needs for operational, management, financial, technological or other expertise; and whether the individual has sufficient time to devote to the interests of Emisphere. The prospective board member cannot be a board member or officer at a competing company nor have relationships with a competing company. He/she must be clear of any investigation or violations that would be perceived as affecting the duties and performance of a director.
The Governance and Nominating Committee identifies nominees by first evaluating the current members of the Board of Directors willing to continue in service. Current members of the Board with skills and experience that are relevant to the business and who are willing to continue in service are considered for re-nomination, balancing the value of continuity of service by existing members of the board with that of obtaining a new perspective. If any member of the board does not wish to continue in service, or if the Governance and Nominating Committee or the board decides not to nominate a member for re-election, the Governance and Nominating Committee identifies the desired skills and experience of a new nominee and discusses with the board suggestions as to individuals that meet the criteria.
The Audit Committee
The Company has a standing Audit Committee established in accordance with Section 3(a)(58)(A) of the Exchange Act. The Audit Committee operates under a written charter adopted by the Board of Directors. The Audit Committee has reviewed the relevant standards of the Sarbanes-Oxley Act of 2002, the rules of the SEC, and the corporate governance listing standards of the NASDAQ regarding committee policies. The committee intends to further amend its charter, if necessary, as the applicable rules and standards evolve to reflect any additional requirements or changes. The updated Audit Committee charter can be found on our website at www.emisphere.com. The contents of our website are not incorporated herein by reference and the website address provided in this Report is intended to be an inactive textual reference only.
The Audit Committee is currently comprised of Timothy McInerney, (chairman), Jacob M. Plotsker, and Michael Weiser, M.D., Ph.D. All of the members of the Audit Committee meet the independence requirements under the applicable provisions of the Exchange Act and regulations promulgated thereunder and the relevant NASDAQ Listing Rules. The Board of Directors has determined that the Company does not currently have an “audit committee financial expert,” as that term is defined in Item 407(d)(5)(ii) of Regulation S-K, serving on the Audit Committee, as a result of the resignation of the previously designated audit committee financial expert from the Audit Committee.
94
Table of Contents
| ITEM 11. | EXECUTIVE COMPENSATION |
Summary Compensation Table — 2014, 2013 and 2012
The following table sets forth information regarding the aggregate compensation Emisphere paid during 2014, 2013 and 2012 to our Principal Executive Officer and the two other highest paid Executive Officers:
| Name and Principal Position(1) |
Year | Salary ($) |
Bonus ($) |
Stock Awards ($) |
Option Awards ($)(2) |
All Other Compensation($) |
Total ($) | |||||||||||||||||||||
| Alan L. Rubino(3), |
2014 | 400,000 | 250,000 | 0 | 0 | 12,000 | (5) | 662,000 | ||||||||||||||||||||
| President and CEO |
2013 | 400,000 | 291,667 | (4) | 0 | 0 | 12,000 | (5) | 703,667 | |||||||||||||||||||
| 2012 | 120,000 | 0 | 0 | 144,885 | 3,600 | (5) | 268,485 | |||||||||||||||||||||
| Michael R. Garone, Chief Financial Officer and Corporate Secretary |
2014 | 265,000 | 65,000 | 0 | 7,533 | 0 | 337,533 | |||||||||||||||||||||
| 2013 | 266,196 | 106,500 | (6) | 0 | 5,177 | 0 | 377,873 | |||||||||||||||||||||
| 2012 | 244,785 | 0 | 0 | 7,982 | 0 | 252,767 | ||||||||||||||||||||||
| Carl V. Sailer(7), Vice President Sales and Marketing |
2014 | 255,000 | 125,000 | 0 | 12,091 | 0 | 392,091 | |||||||||||||||||||||
| 2013 | 255,000 | 143,688 | (8) | 0 | 7,142 | 0 | 405,830 | |||||||||||||||||||||
| 2012 | 53,125 | 0 | 0 | 6,628 | 0 | 59,753 | ||||||||||||||||||||||
| (1) | The named executive officers, as defined in Regulation S-K, Item 402(a)(3), of the Company for the year ended December 31, 2014 were as follows: Mr. Rubino and Mr. Garone. |
| (2) | Amounts shown in this column represent the aggregate grant date fair value of stock option awards granted during the respective year computed in accordance with Financial Accounting Standards Board ASC Topic 718. This compares to prior years, during which amounts in these columns have represented the expensed accounting value of such awards. For assumptions used in the valuation of these awards please see Note 12 to our Financial Statements for the fiscal year ended December 31, 2012. |
| (3) | On September 13, 2012 Mr. Rubino joined the company as President and Chief Executive Officer. |
| (4) | Bonus payment of $66,667 paid in May 2013 related to 2012 performance, $225,000 accrued at December 31, 2013, paid in January 31, 2014 related to 2013 performance. |
| (5) | All other compensation for Mr. Rubino represents an allowance for the use of a personal automobile in accordance with the terms of his employment contract. |
| (6) | Bonus payment of $26,500 paid in May 2013 related to 2012 performance, $80,000 accrued at December 31, 2013, paid in January 31, 2014 related to 2013 performance. |
| (7) | Mr. Sailer joined the Company on October 15, 2012. |
| (8) | Bonus payment of $28,688 paid in May 2013 related to 2012 performance, $115,000 accrued at December 31, 2013, paid in January 31, 2014 related to 2013 performance. |
Compensation Discussion and Analysis
Executive Summary—
The discussion that follows outlines the compensation awarded to, earned by or paid to the named executive officers of the Company including a review of the principal elements of compensation, the objectives of the Company’s compensation program, what the program is designed to reward and why and how each element of compensation is determined.
In general, the Company operates in a marketplace where competition for talented executives is significant. The Company is engaged in the long-term development of its technology and of drug candidates, without the benefit of significant current revenues, and therefore its operations require it to raise capital in order to continue its activities. Our operations entail special needs and risks and require that the Company attempt to implement programs that promote strong individual and group performance and retention of excellent employees. The Company’s compensation program for named executive officers consists of cash compensation as base salary, medical, basic life insurance, long term disability, flexible spending accounts, paid time off, and defined
95
Table of Contents
contribution retirement plans as well as long term equity incentives offered through stock option plans. This program is developed in part by benchmarking against other companies in the biotechnology/pharmaceutical sectors, as well as by the judgment and discretion of our Board of Directors.
Employee salaries are benchmarked against Radford survey information.
Discussion and Analysis —
Objectives of the compensation and reward program — The biopharmaceutical marketplace is highly competitive and includes companies with far greater resources than ours. Our work involves the difficult, unpredictable, and often slow development of our technology and of drug candidates. Continuity of scientific knowledge, management skills, and relationships are often critical success factors to our business. The objectives of our compensation program for named executive officers is to provide competitive cash compensation, competitive health, welfare and defined contribution retirement benefits as well as long-term equity incentives that offer significant reward potential for the risks assumed and for each individual’s contribution to the long-term performance of the Company. Individual performance is measured against long-term strategic goals, short-term business goals, scientific innovation, regulatory compliance, new business development, development of employees, fostering of teamwork and other Emisphere values designed to build a culture of high performance. These policies and practices are based on the principle that total compensation should serve to attract and retain those executives critical to the overall success of Emisphere and are designed to reward executives for their contributions toward business performance that is designed to build and enhance stockholder value.
Elements of compensation and how they are determined — The key elements of the executive compensation package are base salary (as determined by the competitive market and individual performance), cash bonuses (bonus terms are specified in employment agreements of Executive Officers. Bonus payment criteria are based on business performance objectives established by the Board and Leadership Team. Bonus payment awards are based on achievement of business performance objectives as evaluated by the Compensation Committee with input from the Chairman of the Board and paid at the discretion of the Compensation Committee), the executive long term disability plan and other health and welfare benefits and long-term incentive compensation in the form of periodic stock option grants. The base salary (excluding payment for accrued but unused vacation) for the named Executive Officers for 2013 ranged from $265,000 for its Vice President and Chief Financial Officer to $400,000 for its President and Chief Executive Officer. In determining the compensation for each named Executive Officer, the Company generally considers (i) data from outside studies and proxy materials regarding compensation of executive officers at companies believed to be comparable, (ii) the input of non-executive directors, the Chairman of the Board, and the President and Chief Executive Officer (other than for his own compensation) regarding individual performance of each named executive officer and (iii) qualitative measures of Emisphere’s performance, such as progress in the development of the Company’s technology, the engagement of corporate partners for the commercial development and marketing of products, effective corporate governance, fiscal responsibility, the success of Emisphere in raising funds necessary to conduct research and development, and the pace at which the Company continues to advance its technologies in various clinical trials. Our board of directors and Compensation Committee’s consideration of these factors is subjective and informal. However, in general, it has determined that the compensation for executive officers should be competitive with market data reflected within the 50th-75th percentile of biotechnology companies for corresponding senior executive positions. Compensation levels were derived from the compensation plan set in 2006 and were based in part by information received from executive compensation consultants, Pearl Myer and Partners, based in New York, N.Y. Compensable factors benchmarked include market capitalization, head count and location. When considering the compensation of the Company’s President and Chief Executive Officer, the Company receives information and analysis prepared or secured by the Company’s outside executive compensation experts and survey data prepared by human resources management personnel as well as any additional outside information it may have available. In addition, the board of directors and Compensation Committee of the Company considered the approval by our stockholders, on an advisory basis, of the compensation of our named executive officers at our most recent annual meeting of stockholders on May 31, 2013, in determining that our executive compensation is in line with our competitive position in the marketplace and appropriately designed to reward executives for their contributions toward overall business performance that ultimately enhances stockholder value.
96
Table of Contents
The compensation program also includes periodic awards of stock options. The stock option element is considered a long-term incentive that further aligns the interests of executives with those of our stockholders and rewards long-term performance and the element of risk. Stock option awards are made at the discretion of the Board of Directors based on its subjective assessment of the individual contribution of the executive to the attainment of short and long-term Company goals, such as collaborations with partners, attainment of successful milestones under such collaborations and other corporate developments which advance the progress of our technology and drug candidates. Option grants, including unvested grants, for our named executive officers range from 230,000 for our current Vice President, Chief Financial Officer and Corporate Secretary, to 2,000,000 for our President and Chief Executive Officer, as indicated in the accompanying tables. Stock option grants to named executive officers in 2013 were made in accordance with the terms of their Employment Agreements described below in “Employment Agreements and Potential Payments Upon Termination or Change-in-Control”, the Company’s policy with respect to stock options granted to executives is that grant prices should be equal to the fair market value of the common stock on the date of grant, that employee stock options should generally vest over a three to five-year period and expire in ten years from date of grant, and that options previously granted at exercise prices higher than the current fair market value should not be re-priced. Once performance bonuses or awards are issued, there are currently no policies in place to reduce, restate or otherwise adjust awards if the relevant performance measures on which they are based are restated or adjusted. The Company has no policy to require its named executive officers to hold any specific equity interest in the Company. The Company does not offer its named executive officers any nonqualified deferred compensation, a defined benefit pension program or any post-retirement medical or other benefits.
Section 162(m) of the Internal Revenue Code of 1986, as amended, provides that compensation in excess of $1,000,000 paid to the Chief Executive Officer or to any of the other four most highly compensated executive officers of a publicly held company will not be deductible for federal income tax purposes, unless such compensation is paid pursuant to one of the enumerated exceptions set forth in Section 162(m). The Company’s primary objective in designing and administering its compensation policies is to support and encourage the achievement of the Company’s long-term strategic goals and to enhance stockholder value. In general, stock options granted under the Company’s 2000 Plan and 2007 Plan are intended to qualify under and comply with the “performance based compensation” exemption provided under Section 162(m) thus excluding from the Section 162(m) compensation limitation any income recognized by executives at the time of exercise of such stock options. Because salary and bonuses paid to our Chief Executive Officer and four most highly compensated executive officers have been below the $1,000,000 threshold, the Compensation Committee has elected, at this time, to retain discretion over bonus payments, rather than to ensure that payments of salary and bonus in excess of $1,000,000 are deductible. The Compensation Committee intends to review periodically the potential impacts of Section 162(m) in structuring and administering the Company’s compensation programs.
Grants of Plan-Based Awards — 2014
The following table sets forth information regarding grants of plan-based awards in 2014:
| All Other | ||||||||||||||||
| Name |
Grant Date |
Option Awards: Number of Securities Underlying Options (#) |
Exercise or Base Price of Option Awards ($/Sh) |
Grant Date Fair Value of Option Awards |
||||||||||||
| Michael R. Garone, |
1/14/2014 | 40,000 | (1) | $ | 0.20 | 8,000 | ||||||||||
| Vice President, |
||||||||||||||||
| Carl V. Sailer, |
10/15/2014 | 40,000 | (1) | $ | 0.32 | 12,800 | ||||||||||
| Vice President, Sales and Marketing |
||||||||||||||||
| (1) | Vests upon the 1-year anniversary of the grant date. |
97
Table of Contents
Outstanding Equity Awards at Fiscal Year-End — 2014
The following table sets forth information as to the number and value of unexercised options held by the Executive Officers as of December 31, 2014. There are no outstanding stock awards with executive officers:
| Name |
Number of Shares Underlying Unexercised Options (#) Exercisable |
Number of Securities Underlying Unexercised Unearned Options (#) Unexercisable |
Equity Incentive Plan Awards: Number of Securities Underlying Unexercised Unearned Options (#) |
Option Exercise Price ($) |
Option Expiration Date |
|||||||||||||||
| Alan L Rubino |
500,000 | 0 | 0 | $ | 0.09 | 9/13/2022 | ||||||||||||||
| President and |
500,000 | 0 | 0 | $ | 0.25 | 9/13/2022 | ||||||||||||||
| Chief Executive Officer |
0 | 500,000 | (1) | 0 | $ | 0.75 | 9/13/2022 | |||||||||||||
| 0 | 500,000 | (2) | 0 | $ | 1.00 | 9/13/2022 | ||||||||||||||
| Michael R. Garone, |
75,000 | 0 | 0 | $ | 4.03 | 8/29/2017 | ||||||||||||||
| Vice President, |
20,000 | 0 | 0 | $ | 0.62 | 4/12/2019 | ||||||||||||||
| Chief Financial Officer, |
20,000 | 0 | 0 | $ | 1.25 | 1/19/2020 | ||||||||||||||
| and Corporate Secretary |
30,000 | 0 | 0 | $ | 0.92 | 7/15/2021 | ||||||||||||||
| 22,500 | 22,500 | (3) | 0 | $ | 0.199 | 5/31/2022 | ||||||||||||||
| 40,000 | 0 | 0 | $ | 0.14 | 1/14/2023 | |||||||||||||||
| 40,000 | 0 | 0 | $ | 0.20 | 1/14/2024 | |||||||||||||||
| Carl V. Sailer, |
40,000 | 0 | 0 | $ | 0.17 | 10/15/2022 | ||||||||||||||
| Vice President, |
40,000 | 0 | 0 | $ | 0.18 | 10/15/2023 | ||||||||||||||
| Sales and Marketing |
40,000 | 40,000 | (4) | 0 | $ | 0.32 | 10/15/2024 | |||||||||||||
| (1) | 500,000 exercisable as of 9/13/2015 |
| (2) | 500,000 exercisable as of 9/13/2016 |
| (3) | 22,500 exercisable as of 5/31/2015. |
| (4) | 40,000 exercisable as of 10/15/2015. |
Option Exercises and Stock Vested — 2014
There were no stock options exercised by named executive officers during 2014. The option for Mr. Rubino’s to purchase up to an additional 500,000 shares of the Company’s common stock at $0.25 became fully vested and exercisable on September 13, 2014. Options for Mr. Garone to purchase up to an additional 40,000 and 11,250 shares of the Company’s common stock at exercise prices of $0.14 and $0.199, respectively, became fully vested on January 14, 2014, and May 31, 2014, respectively.
Employment Agreements and Potential Payments Upon Termination or Change-in-Control
Employment Agreement with Alan L. Rubino, President and Chief Executive Officer
On September 17, 2012, in connection with his appointment to the position of President and Chief Executive Officer of the Company, effective September 13, 2012, Mr. Rubino entered into an Employment Agreement with the Company dated September 13, 2012 (the “Rubino Employment Agreement”), which provides as follows:
| Ÿ | The initial term of the Rubino Employment Agreement is three years, and the agreement will automatically renew for additional one-year terms unless either party provides notice of non-renewal to the other party at least six months prior to the end of the initial term or any renewal terms. |
| Ÿ | The Rubino Employment Agreement provides for an annual base salary of $400,000, with eligibility to receive an annual bonus of up to 50% of his base salary. |
98
Table of Contents
| Ÿ | Pursuant to the Rubino Employment Agreement, upon termination by the Company without Cause or by Mr. Rubino for Good Reason (as such terms are defined in the Rubino Employment Agreement), subject to the delivery by Mr. Rubino’s of a general release of claims in favor of the Company, Mr. Rubino is entitled to (i) severance payments equal to his base salary for 12 months, except in the case of termination by the Company without Cause or termination by Mr. Rubino for Good Reason within 12 months following a Change of Control (as such terms are defined in the Rubino Employment Agreement), in which case Mr. Rubino is entitled to severance payments equal to his base salary for 18 months, (ii) prorated annual bonus payments that Mr. Rubino would have received but for his termination, (iii) prorated equity compensation that Mr. Rubino would have received but for his termination, and (iv) the cost of family health insurance coverage at the same rate as contributed by the Company prior to the termination until the earlier of twelve (12) months or loss of COBRA entitlement. In addition, in the case of termination by the Company without Cause or termination by Mr. Rubino for Good Reason within 12 months following a Change of Control (as such terms are defined in the Rubino Employment Agreement), Mr. Rubino is entitled to the vesting of all 2,000,000 stock option grants awarded pursuant to the terms of the Rubino Employment, regardless of date or condition of vesting. |
Employment Agreement with Michael R. Garone, Chief Financial Officer and Corporate Secretary.
On January 14, 2013, the Company entered into an Employment Agreement (the “Garone Employment Agreement”) with Michael R. Garone, the Company’s Vice President, Chief Financial Officer and Corporate Secretary. The Garone Employment Agreement provides as follows:
| Ÿ | The effective date of the Garone Employment Agreement is January 14, 2013. The initial term of the Garone Employment Agreement is three years, and the agreement will automatically renew for additional one-year terms unless either party provides notice of non-renewal to the other party at least six months prior to the commencement of any renewal terms. |
| Ÿ | The Garone Employment Agreement provides for an annual base salary of $265,000, with eligibility to receive an annual bonus of up to 30% of his base salary. |
| Ÿ | Pursuant to the Garone Employment Agreement, upon termination by the Company without Cause, or by Mr. Garone for Good Reason (as such terms are defined in the Garone Employment Agreement), subject to the delivery by Mr. Garone of a general release of claims in favor of the Company, Mr. Garone is entitled to (i) severance payments equal to his base salary for 6 months, except in the case of termination by the Company without Cause or termination by Mr. Garone for Good Reason within 12 months following a Change of Control (as defined in the Garone Employment Agreement), in which case Mr. Garone is entitled to severance payments equal to his base salary for 12 months, (ii) prorated annual bonus payments that Mr. Garone would have received but for his termination, (iii) prorated equity compensation that Mr. Garone would have received but for his termination, and (iv) the cost of family health insurance coverage at the same rate as contributed by the Company prior to the termination until the earlier of twelve (12) months or loss of COBRA entitlement. In addition, in the case of termination by the Company without Cause or termination by Mr. Garone for Good Reason within 12 months following a Change of Control (as such terms are defined in the Garone Employment Agreement), Mr. Garone is entitled to the vesting of all stock option grants awarded pursuant to the terms of the Rubino Employment, regardless of date or condition of vesting. |
Employment Agreement with Carl V. Sailer, Vice President, Sales and Marketing.
On October 15, 2012, the Company entered into an Employment Agreement (the “Sailer Employment Agreement”) with Carl V. Sailer, the Company’s Vice President, Marketing and Sales. The Sailer Employment Agreement provides as follows:
| Ÿ | The effective date of the Sailer Employment Agreement is October 15, 2012. The initial term of the Sailer Employment Agreement is three years, and the agreement will automatically renew for additional one-year terms unless either party provides notice of non-renewal to the other party at least six months prior to the commencement of any renewal terms. |
99
Table of Contents
| Ÿ | The Sailer Employment Agreement provides for an annual base salary of $255,000, with eligibility to receive an annual bonus of up to 45% of his base salary. |
| Ÿ | Pursuant to the Sailer Employment Agreement, upon termination by the Company without Cause, or by Mr. Sailer for Good Reason (as such terms are defined in the Sailer Employment Agreement), subject to the delivery by Mr. Sailer of a general release of claims in favor of the Company, Mr. Sailer is entitled to (i) severance payments equal to his base salary for 6 months, except in the case of termination by the Company without Cause or termination by Mr. Sailer for Good Reason within 12 months following a Change of Control (as defined in the Sailer Employment Agreement), in which case Mr. Sailer is entitled to severance payments equal to his base salary for 12 months, (ii) prorated annual bonus payments that Mr. Sailer would have received but for his termination, (iii) prorated equity compensation that Mr. Sailer would have received but for his termination, and (iv) the cost of family health insurance coverage at the same rate as contributed by the Company prior to the termination until the earlier of twelve (12) months or loss of COBRA entitlement. In addition, in the case of termination by the Company without Cause or termination by Mr. Sailer for Good Reason within 12 months following a Change of Control (as such terms are defined in the Sailer Employment Agreement), Mr. Sailer is entitled to the vesting of all stock option grants awarded pursuant to the terms of the Rubino Employment, regardless of date or condition of vesting. |
Compensation Committee Interlocks and Insider Participation.
The current members of the Compensation Committee are Mr. McInerney, Dr. Rachesky and Dr. Weiser. No member of the Compensation Committee is or has ever been an executive officer or employee of our company (or any of its subsidiaries) and no “compensation committee interlocks” existed during fiscal year 2014. For further information about our processes and procedures for the consideration and determination of executive and director compensation, please see “Executive Compensation — Compensation Discussion and Analysis.”
Compensation Committee Report
The Compensation Committee operates under a written charter adopted by the Board of Directors. The Compensation Committee charter can be found on our website at www.emisphere.com. The contents of our website are not incorporated herein by reference and the website address provided in this annual report is intended to be an inactive textual reference only.
The Compensation Committee is responsible for the consideration of stock plans, performance goals and incentive awards, and the overall coverage and composition of the compensation arrangements related to executive officers. The Compensation Committee may delegate any of the foregoing duties and responsibilities to a subcommittee of the Compensation Committee consisting of not less than two members of the committee. The Compensation Committee has the authority to retain, at the expense of the Company, such outside counsel, experts and other advisors as deemed appropriate to assist it in the full performance of its functions. The Company’s Chief Executive Officer is involved in making recommendations to the Compensation Committee for compensation of Executive Officers (except for himself) as well as recommending compensation levels for directors.
Our executive compensation program is administered by the Compensation Committee of the Board of Directors. The Compensation Committee, which is composed of non-employee independent directors, is responsible for reviewing with Company management and approving compensation policy and all forms of compensation for executive officers and directors in light of the Company’s current business environment and the Company’s strategic objectives. In addition, the Compensation Committee acts as the administrator of the Company’s stock option plans. The Compensation Committee’s practices include reviewing and establishing executive officers’ compensation to ensure that base pay and incentive compensation are competitive to attract and retain qualified executive officers, and to provide incentive systems reflecting both financial and operating performance, as well as an alignment with stockholder interests. These policies are based on the principle that total compensation should serve to attract and retain those executives critical to the overall success of Emisphere and should reward executives for their contributions to the enhancement of stockholder value.
100
Table of Contents
The Compensation Committee oversees risk management as it relates to our compensation plans, policies and practices in connection with structuring our executive compensation programs and reviewing our incentive compensation programs for other employees. The committee considered risk when developing our compensation programs and believes that the design of our current compensation programs do not encourage excessive or inappropriate risk taking. Our base salaries provide competitive fixed compensation, while annual cash bonuses and equity-based awards encourage long-term consideration rather than short-term risk taking.
The Compensation Committee has reviewed and discussed the Compensation Discussion and Analysis presented herein with the management of the Company. Based on that review and discussion, the Compensation Committee recommended to the Board of Directors that the Compensation Discussion and Analysis be included in the Form 10-K and Proxy Statement of the Company.
The Members of the Compensation Committee
Dr. Michael Weiser, M.D., Ph.D. (Chairman)
Mr. Timothy McInerney
Dr. Mark H. Rachesky, M.D.
Audit Committee Report
The Audit Committee operates under a written charter adopted by the Board of Directors. The Audit Committee has reviewed the relevant standards of the Sarbanes-Oxley Act of 2002, the rules of the SEC, and the corporate governance listing standards of the NASDAQ Listing Rules regarding committee policies. The committee intends to further amend its charter, if necessary, as the applicable rules and standards evolve to reflect any additional requirements or changes. The updated Audit Committee charter can be found on our website at www.emisphere.com. The contents of our website are not incorporated herein by reference and the website address provided in this Proxy Statement is intended to be an inactive textual reference only.
The Audit Committee is currently comprised of Mr. Timothy McInerney, (chairman), Jacob M. Plotsker and Michael Weiser, M.D., Ph.D.
All of the members of the Audit Committee meet the independence requirements under the applicable provisions of the Exchange Act and regulations promulgated thereunder and the relevant NASDAQ Listing Rules. The Board of Directors has determined that the Company does not currently have an “audit committee financial expert,” as that term is defined in Item 407(d)(5)(ii) of Regulation S-K, serving on the Audit Committee, as a result of the resignation of the previously designated audit committee financial expert from the Audit Committee.
On January 6, 2010, with the approval of the Audit Committee of the Company, the Company engaged McGladrey, LLP (“McGladrey”) to act as its independent registered public accounting firm. During the year ended December 2009, and in the subsequent interim periods through December 31, 2014, neither the Company nor anyone acting on its behalf had consulted with McGladrey on any of the matters or events set forth in Item 304(a)(2) of Regulation S-K.
Management has primary responsibility for the Company’s financial statements and the overall reporting process, including the Company’s system of internal control over financial reporting. McGladrey, the Company’s independent registered public accountants, audit the annual financial statements prepared by management, express an opinion as to whether those financial statements fairly present the financial position, results of operations and cash flows of the Company in conformity with accounting principles generally accepted in the United States, and report on internal control over financial reporting. McGladrey reports to the Audit Committee as members of the Board of Directors and as representatives of the Company’s stockholders.
The Audit Committee meets with management periodically to consider the adequacy of the Company’s internal control over financial reporting and the objectivity of its financial reporting. The Audit Committee discusses these matters with the appropriate Company financial personnel. In addition, the Audit Committee has discussions with management concerning the process used to support certifications by the Company’s Chief Executive Officer and Chief Financial Officer that are required by the SEC and the Sarbanes-Oxley Act to accompany the Company’s periodic filings with the SEC.
101
Table of Contents
On an as needed basis, the Audit Committee meets privately with McGladrey. The Audit Committee also appoints the independent registered public accounting firm, approves in advance their engagements to perform audit and any non-audit services and the fee for such services, and periodically reviews their performance and independence from management. In addition, when appropriate, the Audit Committee discusses with McGladrey plans for the audit partner rotation required by the Sarbanes-Oxley Act.
Pursuant to its charter, the Audit Committee assists the board in, among other things, monitoring and reviewing (i) our financial statements, (ii) our compliance with legal and regulatory requirements and (iii) the independence, performance and oversight of our independent registered public accounting firm. Under the Audit Committee charter, the Audit Committee is required to make regular reports to the board.
During the 2014 Fiscal Year, the Audit Committee of the Board of Directors reviewed and assessed:
| Ÿ | the quality and integrity of the annual audited financial statements with management, including issues relating to accounting and auditing principles and practices, as well as the adequacy of internal controls, and compliance with regulatory and legal requirements; |
| Ÿ | the qualifications and independence of the independent registered public accounting firm; and |
| Ÿ | management’s, as well as the independent auditor’s, analysis regarding financial reporting issues and judgments made in connection with the preparation of our financial statements, including those prepared quarterly and annually, prior to filing our quarterly reports on Form 10-Q and annual report on Form 10-K. |
The Audit Committee has reviewed the audited financial statements and has discussed them with both management and McGladrey, the independent registered public accounting firm. The Audit Committee has discussed with the independent auditors matters required to be discussed by the applicable Auditing Standards as periodically amended (including significant accounting policies, alternative accounting treatments and estimates, judgments and uncertainties). In addition, the independent auditors provided to the Audit Committee the written disclosures required by the applicable requirements of the Public Company Accounting Oversight Board regarding the independent auditors’ communications with the Audit Committee concerning independence, and the Audit Committee and the independent auditors have discussed the auditors’ independence from the Company and its management, including the matters in those written disclosures. The Audit Committee also received reports from McGladrey regarding all critical accounting policies and practices used by the Company, any alternative treatments of financial information used, generally accepted accounting principles that have been discussed with management, ramifications of the use of alternative treatments and the treatment preferred by McGladrey and other material written communications between McGladrey and management, including management letters and schedules of adjusted differences.
In making its decision to select McGladrey as Emisphere’s independent registered public accounting firm for 2014, the Audit Committee considered whether the non-audit services provided by McGladrey are compatible with maintaining the independence of McGladrey.
Based upon the review and discussions referenced above, the Audit Committee, as comprised at the time of the review and with the assistance of the Company’s Chief Financial Officer, recommended to the Board of Directors that the audited financial statements be included in our Annual Report on Form 10-K for the fiscal year ended December 31, 2014, and be filed with the SEC.
The Members of the Audit Committee
Mr. Timothy McInerney (Chairman)
Mr. Jacob M. Plotsker
Dr. Michael Weiser, M.D., Ph.D.
Compensation of Non-Employee Directors
A director who is a full-time employee of the Company receives no additional compensation for services provided as a director. It is the Company’s policy to provide competitive compensation and benefits necessary to
102
Table of Contents
attract and retain high quality non-employee directors and to encourage ownership of Company stock to further align their interests with those of stockholders. The following represents the compensation of the non-employee members of the Board of Directors:
| Ÿ | All newly appointed directors shall receive an initial stock option grant on the date of appointment of 50,000 options to purchase shares of common stock. The options subject to such initial stock option grant vest over three years in equal amounts on each anniversary of the grant date provided the director continuously serves as a director from the grant date through such vesting date, subject to accelerated vesting upon a change in control of Emisphere. Such options, once vested, remain exercisable through the period of the option term. |
All non-employee directors receive an annual retainer of $35,000, payable quarterly in cash, and an annual stock option grant of 40,000 options to purchase shares of common stock. The annual stock option grants are granted each year on the date of the annual meeting of stockholders of the Company. The director must be an eligible director on the dates the retainers are paid and the stock options are granted. Options vest over three years in equal amounts on each anniversary of the grant date provided the director continuously serves as a director from the grant date through such vesting date, subject to accelerated vesting upon a change in control of Emisphere. Such options, once vested, remain exercisable through the period of the option term.
| Ÿ | On September 13, 2012, in connection with the appointment of Timothy Rothwell as the Chairman of the Board of Directors, and upon the recommendation of the Compensation Committee, the Board of Directors approved the following compensation for Mr. Rothwell for his service as Chairman of the Board: |
| Ÿ | An annual fee of $180,000, to be paid in twelve equal monthly installments of $15,000 each. |
| Ÿ | A grant on September 13, 2012, of a non-qualified option (the “Initial Rothwell Option”) to purchase 175,000 shares of the Company’s common stock (the “Common Stock”) in accordance with the 2007 Plan at a purchase price equal to the market price of the Common Stock on the date of said grant, such options to vest on January 1, 2013. |
| Ÿ | On each of the first, second, and third anniversary of the grant of the Initial Rothwell Option, an additional grant of a non-qualified option to purchase 175,000 shares of the Common Stock in accordance with the 2007 Plan, at a price equal to the market price of the Common Stock on the date of said grant, such options to vest immediately on such date. |
| Ÿ | Additional committee and chairperson fees are paid as follows: |
| Ÿ | $10,000 audit committee chairperson fee; |
| Ÿ | $2,500 audit committee member fee; |
| Ÿ | $5,000 compensation committee chairperson fee; |
| Ÿ | $1,000 compensation committee member fee; |
| Ÿ | $2,500 governance and nominating committee chairperson fee; and |
| Ÿ | $500 governance and nominating committee member fee. |
The director must be an eligible director on the dates such fees are paid.
103
Table of Contents
Director Compensation Table — 2014
The table below represents the compensation paid to our non-employee directors during the year ended December 31, 2014:
| Name |
Fees Earned or Paid in Cash ($) |
Stock Awards ($)(1) |
Option Awards ($)(1) |
All Other Compensation ($) |
Total ($) |
|||||||||||||||
| John D. Harkey, Jr. |
35,000 | 0 | 10,096 | 0 | 45,096 | |||||||||||||||
| Timothy McInerney |
46,000 | 0 | 10,096 | 0 | 56,096 | |||||||||||||||
| Jacob M. Plotsker |
38,000 | 0 | 10,096 | 0 | 48,096 | |||||||||||||||
| Mark H. Rachesky, M.D. |
36,500 | 0 | 10,096 | 0 | 46,596 | |||||||||||||||
| Timothy G. Rothwell |
215,000 | 0 | 74,300 | 0 | 289,300 | |||||||||||||||
| Michael Weiser, M.D., Ph.D. |
45,000 | 0 | 10,096 | 0 | 55,096 | |||||||||||||||
| (1) | The value listed in the above table represents the fair value of the options recognized as expense under FASB ASC Topic 718 during 2014, including unvested options granted before 2014 and those granted in 2014. Fair value is calculated as of the grant date using the Black-Scholes Model. The determination of the fair value of share-based payment awards made on the date of grant is affected by our stock price as well as assumptions regarding a number of complex and subjective variables. Our assumptions in determining fair value are described in note 12 to our audited financial statements for the year ended December 31, 2014. |
104
Table of Contents
The following table summarizes the aggregate number of option awards and stock awards held by each non-employee director at December 31, 2014.
| Option Awards | Stock Awards | |||||||||||||||||||||||||||
| Name |
Number of Securities Underlying Unexercised Options (#) Exercisable |
Number of Securities Underlying Unexercised Unearned Options (#) Unexercisable |
Equity Incentive Plan Awards: Number of Securities Underlying Unexercised Unearned Options (#) |
Option Exercise Price ($) |
Option Expiration Date |
Number of Shares of Units of Stock That Have not Vested (#) |
Market Value of Shares or Units of Stock That Have not Vested ($) |
|||||||||||||||||||||
| John D. Harkey, Jr. |
7,000 | 0 | 0 | 8.97 | 5/26/2016 | 0 | 0 | |||||||||||||||||||||
| 7,000 | 0 | 0 | 3.76 | 4/20/2017 | ||||||||||||||||||||||||
| 7,000 | 0 | 0 | 3.79 | 8/08/2018 | ||||||||||||||||||||||||
| 75,000 | 0 | 0 | 0.93 | 5/15/2019 | ||||||||||||||||||||||||
| 40,000 | 0 | 0 | 1.20 | 9/16/2020 | ||||||||||||||||||||||||
| 40,000 | 0 | 0 | 1.53 | 9/19/2021 | ||||||||||||||||||||||||
| 26,666 | 13,334 | (1) | 0 | 0.199 | 5/31/2022 | |||||||||||||||||||||||
| 13,333 | 26,667 | (2) | 0 | 0.23 | 5/30/2023 | |||||||||||||||||||||||
| 0 | 40,000 | (3) | 0 | 0.268 | 5/29/2024 | |||||||||||||||||||||||
| Timothy McInerney |
50,000 | 0 | 0 | 0.27 | 3/01/2022 | 0 | 0 | |||||||||||||||||||||
| 26,666 | 13,334 | (1) | 0 | 0.199 | 5/31/2022 | |||||||||||||||||||||||
| 13,333 | 26,667 | (2) | 0 | 0.23 | 5/30/2023 | |||||||||||||||||||||||
| 0 | 40,000 | (3) | 0 | 0.268 | 5/29/2024 | |||||||||||||||||||||||
| Jacob M. Plotsker |
50,000 | 0 | 0 | 0.27 | 3/01/2022 | 0 | 0 | |||||||||||||||||||||
| 26,666 | 13,334 | (1) | 0 | 0.199 | 5/31/2022 | |||||||||||||||||||||||
| 13,333 | 26,667 | (2) | 0 | 0.23 | 5/30/2023 | |||||||||||||||||||||||
| 0 | 40,000 | (3) | 0 | 0.268 | 5/29/2024 | |||||||||||||||||||||||
| Mark H. Rachesky, M.D. |
7,000 | 0 | 0 | 3.76 | 4/20/2017 | 0 | 0 | |||||||||||||||||||||
| 7,000 | 0 | 0 | 3.79 | 8/08/2018 | ||||||||||||||||||||||||
| 75,000 | 0 | 0 | 0.93 | 5/15/2019 | ||||||||||||||||||||||||
| 40,000 | 0 | 0 | 1.20 | 9/16/2020 | ||||||||||||||||||||||||
| 40,000 | 0 | 0 | 1.53 | 9/19/2021 | ||||||||||||||||||||||||
| 26,666 | 13,334 | (1) | 0 | 0.199 | 5/31/2022 | |||||||||||||||||||||||
| 13,333 | 26,667 | (2) | 0 | 0.23 | 5/30/2023 | |||||||||||||||||||||||
| 0 | 40,000 | (3) | 0 | 0.268 | 5/29/2024 | |||||||||||||||||||||||
| Timothy G. Rothwell |
50,000 | 0 | 0 | 0.70 | 11/5/2019 | 0 | 0 | |||||||||||||||||||||
| 40,000 | 0 | 0 | 1.20 | 9/16/2020 | ||||||||||||||||||||||||
| 40,000 | 0 | 0 | 1.53 | 9/19/2021 | ||||||||||||||||||||||||
| 26,666 | 13,334 | (1) | 0 | 0.199 | 5/31/2022 | |||||||||||||||||||||||
| 175,000 | 0 | 0 | 0.09 | 9/13/2022 | ||||||||||||||||||||||||
| 13,333 | 26,667 | (2) | 0.23 | 5/30/2023 | ||||||||||||||||||||||||
| 175,000 | 0 | 0.175 | 9/13/2023 | |||||||||||||||||||||||||
| 0 | 40,000 | (3) | 0 | 0.268 | 5/29/2024 | |||||||||||||||||||||||
| 175,000 | 0 | 0 | 0.388 | 9/15/2024 | ||||||||||||||||||||||||
| Michael Weiser, M.D., Ph.D. |
7,000 | 0 | 0 | 8.97 | 5/26/2016 | 0 | 0 | |||||||||||||||||||||
| 7,000 | 0 | 0 | 3.76 | 4/20/2017 | ||||||||||||||||||||||||
| 7,000 | 0 | 0 | 3.79 | 8/08/2018 | ||||||||||||||||||||||||
| 75,000 | 0 | 0 | 0.93 | 5/15/2019 | ||||||||||||||||||||||||
| 40,000 | 0 | 0 | 1.20 | 9/16/2020 | ||||||||||||||||||||||||
| 40,000 | 0 | 0 | 1.53 | 9/19/2021 | ||||||||||||||||||||||||
| 26,666 | 13,334 | (1) | 0 | 0.199 | 5/31/2022 | |||||||||||||||||||||||
| 13,333 | 26,667 | (2) | 0 | 0.23 | 5/30/2023 | |||||||||||||||||||||||
| 0 | 40,000 | (3) | 0 | 0.268 | 5/29/2024 | |||||||||||||||||||||||
105
Table of Contents
| (1) | 13,334 exercisable as of 5/31/2015. |
| (2) | 13,333 exercisable as of 5/30/2015 and 13,334 exercisable as of 5/30/2016. |
| (3) | 13,333 exercisable as of 5/29/2015 and 5/29/2016, respectively and 13,334 exercisable as of 5/29/2017 |
| ITEM 12. | SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS |
Securities Available For Future Issuance Under Equity Plans
The following table provides information as of December 31, 2014, about the common stock that may be issued upon the exercise of options granted to employees, consultants or members of our Board of Directors under our existing equity compensation plans, including the 2000 Stock Option Plan, the 2002 Broad Based Plan, the 2007 Stock Award and Incentive Plan (collectively the “Plans”) the Stock Incentive Plan for Outside Directors and the Directors Deferred Compensation Plan. For a discussion of the material features of the Plans, please see Note 11 to the Financial Statements included in this Report.
| Plan Category |
(a) Number of Securities to be Issued Upon Exercise of Outstanding Options |
(b) Weighted Average Exercise Price of Outstanding Options |
(c) Number of Securities Remaining Available for Future Issuance Under Equity Compensation Plans (Excluding Securities Reflected in Column (a)) |
|||||||||
| Equity Compensation Plans Approved by Security Holders |
||||||||||||
| The Plans |
4,799,750 | $ | 0.90 | 4,483,766 | ||||||||
| Stock Incentive Plan for Outside Directors |
21,000 | 8.97 | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Total |
4,820,750 | $ | 1.20 | 4,483,766 | ||||||||
106
Table of Contents
Common Stock Ownership by Directors and Executive Officers and Principal Holders
Directors and Executive Officers
The following table sets forth certain information, as of March 1, 2015, regarding the beneficial ownership of the common stock by (i) each director; (ii) each named executive officer; (iii) all of our directors and named executive officers as a group. The number of shares beneficially owned by each director or Executive Officer is determined under the rules of the SEC, and the information is not necessarily indicative of beneficial ownership for any other purpose. Under these rules, beneficial ownership includes any shares as to which the individual has the sole or shared voting power (which includes power to vote, or direct the voting of, such security) or investment power (which includes power to dispose of, or direct the disposition of, such security). In computing the number of shares beneficially owned by a person and the percentage ownership of that person, shares of common stock subject to options, warrants or convertible notes held by that person that are currently exercisable or convertible into Common Stock or will become exercisable or convertible into common stock within 60 days after March 1, 2015 are deemed outstanding, while such shares are not deemed outstanding for purposes of computing percentage ownership of any other person. Unless otherwise indicated, all persons named as beneficial owners of common stock have sole voting power and sole investment power with respect to the shares indicated as beneficially owned:
| Name and Address(a) |
Common Shares Beneficially Owned (b) |
Common Shares Underlying Options |
Percent Of Class |
|||||||||
| Alan L. Rubino (e) |
1,000,000 | 1,000,000 | 1.6 | % | ||||||||
| Michael R. Garone |
347,500 | 247,500 | * | |||||||||
| Carl V. Sailer |
80,000 | 80,000 | * | |||||||||
| Mark H. Rachesky, M.D. |
78,483,687 | (c) | 60,000,024 | (d) | 65.0 | % | ||||||
| Timothy G. Rothwell (f) |
694,999 | 694,999 | 1.1 | % | ||||||||
| Michael Weiser, M.D., Ph.D. |
222,412 | 215,999 | * | |||||||||
| John D. Harkey, Jr. |
222,412 | 215,999 | * | |||||||||
| Timothy McInerney |
79,999 | 79,999 | * | |||||||||
| Jacob M. Plotsker |
79,999 | 79,999 | * | |||||||||
|
|
|
|
|
|||||||||
| All directors and executive officers as a group |
81,211,008 | 62,614,519 | 65.9 | % | ||||||||
| * | Less than 1% |
| (a) | Unless otherwise specified, the address of each beneficial owner is c/o Emisphere Technologies, Inc., 4 Becker Farm Road, Suite 103, Roseland, New Jersey, |
| (b) | The number of shares set forth for each Director and Executive Officer consists of direct and indirect ownership of shares, including stock options, deferred common share units, restricted stock and, in the case of Dr. Rachesky, shares of common stock that can be obtained upon conversion of convertible notes and exercise of warrants, as further described in footnotes (c) and (d) below. |
| (c) | This number consists of: |
| Ÿ | 18,483,663 shares of common stock. |
| Ÿ | 37,793,249 shares of common stock that can be obtained upon conversion of notes convertible into the common stock of the Company. |
| Ÿ | 21,997,776 shares of common stock that can be obtained upon the exercise of warrants to purchase shares of common stock of the Company. |
| Ÿ | 208,999 shares of common stock that can be obtained by Dr. Rachesky upon the exercise of currently vested stock options and options that will vest within 60 days of March 1, 2015. |
Dr. Rachesky is the managing member of MHRC LLC (“MHRC”), a Delaware limited liability company, and MHR Advisors LLC (“Advisors”), a Delaware limited liability company. Advisors is the general
107
Table of Contents
partner of each of MHR Capital Partners Master Account LP (“Master Account”), an Anguilla, British West Indies limited partnership, and MHR Capital Partners (100) LP (“Capital Partners (100)”), a Delaware limited partnership, and, in such capacity, may be deemed to beneficially own the shares of common stock held for the accounts of, or beneficially owned by, each of Master Account and Capital Partners (100). MHRC II LLC (“MHRC II”), a Delaware limited liability company, is the managing member of MHRC Institutional Advisors II, (“Institutional Advisors II”), a Delaware limited liability company, and, as such, may be deemed to beneficially own the shares of common stock held for the account of, or beneficially owned by, Institutional Advisors II. Institutional Advisors II is the general partner of each of MHR Institutional Partners II LP (“Institutional Partners II”), a Delaware limited partnership, and MHR Institutional Partners IIA LP (“Institutional Partners IIA”), a Delaware limited partnership, and, in such capacity, may be deemed to beneficially own the shares of common stock held for the accounts of, or beneficially owned by, each of Institutional Partners II and Institutional Partners IIA. MHR Holding LLC (“Holdings”), a Delaware limited liability company, is the managing member of MHR Fund Management LLC (“Fund Management”), a Delaware limited liability company, and as such, may be deemed to beneficially own the shares of common stock held for the account of, or beneficially owned by, Fund Management. Fund Management is an affiliate of and has an investment management agreement with each of Master Account, Capital Partners (100), Institutional Partners II and Institutional Partners IIA, and other affiliated entities, pursuant to which it has the power to vote or direct the vote and to dispose or to direct the disposition of the shares of common stock held by such entities and, accordingly, Fund Management may be deemed to beneficially own the shares of common stock held for the account of each of Master Account, Capital Partners (100), Institutional Partners II and Institutional Partners IIA.
| (d) | This number consists of (i) 37,793,249 shares of common stock that can be obtained by Master Account, Capital Partners (100), Institutional Partners II and Institutional Partners IIA upon the conversion of notes convertible into common stock of the Company, (ii) 21,997,776 shares of common stock that can be obtained by Master Account, Capital Partners (100), Institutional Partners II and Institutional Partners IIA upon exercise of warrants to purchase shares of common stock of the Company, (iii) 208,999 shares of common stock that can be obtained by Dr. Rachesky upon the exercise of currently vested stock options and options that will vest within 60 days of March 1, 2015. |
| (e) | On September 13, 2012, Alan L. Rubino joined the Company as President and Chief Executive Officer, and was appointed as a Class II Director. |
| (f) | On September 13, 2012, Timothy Rothwell was elected Chairman of the Board of Directors of the Company. |
Principal Holders of Common Stock
The following table sets forth information regarding beneficial owners of more than five (5%) percent of the outstanding shares of Common Stock as of March 1, 2015:
| Name and Address |
Number of Shares Beneficially Owned |
Percent Of Class(a) |
||||||
| Bai Ye Feng |
6,184,389 | (b) | 9.87 | % | ||||
| Mark H. Rachesky, M.D. |
78,483,687 | (c) | 65.0 | % | ||||
| (a) | Applicable percentage ownership is based on 60,687,478 shares of Common Stock outstanding as of March 1, 2015. In computing the number of shares beneficially owned by a person and the percentage ownership of that person, shares of Common Stock subject to options, warrants or convertible notes held by that person that are currently exercisable or convertible into Common Stock or will become exercisable or convertible into Common Stock within 60 days after March 1, 2015, are deemed outstanding, while such shares are not deemed outstanding for purposes of computing percentage ownership of any other person. |
| (b) | Based solely on a Schedule 13D/A filed on February 14, 2012 by Bai Ye Feng. Mr. Feng beneficially owns an aggregate of 6,184,389 shares of common stock, consisting of 3,908,738 shares of common stock held by |
108
Table of Contents
| Mr. Feng, warrants to purchase up to 1,981,651 shares of common stock held by Mr. Feng, and 294,000 shares of common stock owned of record by Lighthouse Consulting Limited, a Hong Kong company of which Mr. Feng is a principal and therefore may be deemed to be a beneficial holder of such shares. Mr. Feng’s address is 16A Li Dong Building, No.9 Li Yuen Street East, Central, Hong Kong |
| (c) | The address of Dr Rachesky, Master Account, Advisors, Institutional Partners II, Institutional Partners IIA, Institutional Advisors II, MHRC LLC, MHRC II LLC, Fund Management, Capital Partners (100) and Holdings, to which we refer collectively as the MHR Investors, is 40 West 57th Street, 24th Floor, New York, NY 10019. For a description of the relationships between the MHR Investors, please refer to footnote “c” in the table under “Directors and Executive Officers” above. |
109
Table of Contents
| ITEM 13. | CERTAIN RELATIONSHIPS, RELATED TRANSACTIONS AND DIRECTOR INDEPENDENCE |
Related Party Transaction Approval Policy
In February 2007, our Board of Directors adopted a written related party transaction approval policy, which sets forth our Company’s policies and procedures for the review, approval or ratification of any transaction required to be reported in our filings with the SEC. The Company’s policy with regard to related party transactions is that all material transactions non-compensation related are to be reviewed by the Audit Committee for any possible conflicts of interest. The Compensation Committee will review all material transactions that are related to compensation. All related party transactions approved by either the Audit Committee or Compensation Committee shall be disclosed to the Board of Directors at the next meeting.
Transactions with MHR
Mark H. Rachesky, M.D. is a director and member of the Company’s Compensation Committee and its Governance and Nominating Committee. Dr. Rachesky is also the managing member of (i) MHRC, the managing member of Advisors, which in turn is the general partner of Master Account and Capital Partners (100); (ii) MHRC II , the managing member of Institutional Advisors II, which is in turn the general partner of Institutional Partners II and Institutional Partners IIA; and (iii) MHR Holdings, the managing member of Fund Management (and, together with MHRC, MHRC II, MHR Holdings, Advisors, Institutional Advisors II, Master Account, Capital Partners 100, Institutional Partners II, and Institutional Partners IIA, “MHR”) which is an affiliate of and has an investment management agreement with Master Account, Capital Partners 100, Institutional Partners II, and Institutional Partners IIA.
On August 20, 2014, the Company entered into a series of agreements (the “Transaction Documents”) with Master Account, Capital Partners (100), Institutional Partners II, and Institutional Partners IIA, (collectively, the “Lenders”), for a new loan facility, an extension of the Company’s existing obligations under various promissory notes previously issued to the Lenders, and for payment by the Company of certain royalties to the Lenders (the “Transaction”). Currently, MHR owns approximately 30.5% of the common stock of the Company and has $57.1 million in outstanding indebtedness.
A special committee of the Company’s board of directors (the “Board”), composed of independent directors, negotiated the terms of the Loan Agreement and restructuring with MHR and the transactions contemplated thereby with the advice of its legal and financial advisors, and the Loan Agreement and restructuring was unanimously approved by the disinterested members of the Board with the unanimous affirmative recommendation of the special committee. For a more detailed description of the Loan and restructuring transactions see Liquidity & Capital Resources and Note 7 of the Financial Statements.
Information about Board of Directors
Our business is overseen by the Board of Directors. It is the duty of the Board of Directors to oversee the Chief Executive Officer and other senior management in the competent and ethical operation of the Company on a day-to-day basis and to assure that the long-term interests of the stockholders are being served. To satisfy this duty, our directors take a proactive, focused approach to their position, and set standards to ensure that the Company is committed to business success through maintenance of the highest standards of responsibility and ethics. The Board of Directors is kept advised of our business through regular verbal or written reports, Board of Directors meetings, and analysis and discussions with the Chief Executive Officer and other officers of the Company.
Members of the Board of Directors bring to us a wide range of experience, knowledge and judgment. Our governance organization is designed to be a working structure for principled actions, effective decision-making and appropriate monitoring of both compliance and performance.
The Board of Directors has affirmatively determined that Mr. John D. Harkey, Jr., Mr. Timothy McInerney, Mr. Jacob M. Plotsker, Dr. Mark H. Rachesky, Mr. Timothy G. Rothwell, and Dr. Michael Weiser are independent directors within the meaning of Rule 4200 of the NASDAQ Marketplace Rules. Mr. Rubino is the
110
Table of Contents
sole member of the Board of Directors who is not independent. The independent directors meet in separate sessions at the conclusion of board meetings and at other times as deemed necessary by the independent directors, in the absence of Mr. Rubino. Mr. Rothwell currently serves as Chairman. Matters are explored in Committee and brought to the full Board for discussion or action.
Committees of the Board of Directors
The Board of Directors has established an Audit Committee, a Compensation Committee and a Governance and Nominating Committee. Each of the committees of the Board of Directors acts pursuant to a separate written charter adopted by the Board of Directors.
The Audit Committee is currently comprised of Mr. McInerney (Chairman), who became a member of the Committee on March 23, 2012, and was appointed Chairman on September 13, 2012, Jacob M. Plotsker, and Dr. Weiser. All of the members of the Audit Committee meet the independence requirements under the applicable provisions of the Exchange Act and regulations promulgated thereunder and the relevant NASDAQ Listing Rules. The Board of Directors has determined that the Company does not currently have an “audit committee financial expert,” as that term is defined in Item 407(d)(5)(ii) of Regulation S-K, serving on the Audit Committee as a result of the recent resignation of the previously designated audit committee financial expert from the Audit Committee.
The Compensation Committee is currently comprised of Dr. Weiser (Chairman), Dr. Rachesky, and Mr. McInerney. All members of the Compensation Committee are independent within the meaning of Rule 4200 of the NASDAQ Marketplace Rules, non-employee directors within the meaning of the rules of the Securities and Exchange Commission and “outside” directors within the meaning set forth under Internal Revenue Code Section 162(m). The Compensation Committee’s responsibilities and duties are summarized in the report of the Compensation Committee and in the Compensation Committee charter also available on our website.
The Governance and Nominating Committee is currently comprised of Dr. Weiser (chairman), Dr. Rachesky, and Mr. Plotsker. All members of the Governance and Nominating Committee are independent within the meaning of Rule 4200 of the NASDAQ Marketplace Rules. The Governance and Nominating Committee’s responsibilities and duties are set forth in the Governance and Nominating Committee charter on our website. Among other things, the Governance and Nominating Committee is responsible for recommending to the board the nominees for election to our Board of Directors and the identification and recommendation of candidates to fill vacancies occurring between annual stockholder meetings.
The table below provides membership information for each committee of the Board of Directors as of March 15, 2015:
| Name |
Board | Audit | Compensation | Governance and Nominating |
||||||||||||
| Alan L. Rubino(1)(4) |
X | |||||||||||||||
| Mark H. Rachesky, M.D.(2) |
X | X | X | |||||||||||||
| Michael Weiser, M.D., Ph.D.(2) |
X | X | X | * | X | * | ||||||||||
| John D. Harkey, Jr.(3) |
X | |||||||||||||||
| Timothy G. Rothwell(3) |
X | * | ||||||||||||||
| Timothy McInerney(4) |
X | X | * | X | ||||||||||||
| Jacob M. Plotsker(4) |
X | X | X | |||||||||||||
| * | Chair |
| (1) | Joined the company as President, Chief Executive Officer and Class II Director as of September 13, 2012. |
| (2) | Class III directors: Term as director is expected to expire in 2017. |
| (3) | Class I directors: Term as director is expected to expire in 2015. |
| (4) | Class II directors. Term as director is expected to expire in 2016. |
111
Table of Contents
Board Involvement in Risk Oversight
Our Board of Directors is responsible for oversight of the Company’s risk assessment and management process. We believe risk can arise in every decision and action taken by the Company, whether strategic or operational. Our comprehensive approach is reflected in the reporting processes by which our management provides timely and fulsome information to the Board of Directors to support its role in oversight, approval and decision-making.
The Board of Directors closely monitors the information it receives from management and provides oversight and guidance to our management team concerning the assessment and management of risk. The Board of Directors approves the Company’s high level goals, strategies and policies to set the tone and direction for appropriate risk taking within the business.
The Board of Directors delegated to the Compensation Committee basic responsibility for oversight of management’s compensation risk assessment, and that committee reports to the board on its review. Our Board of Directors also delegated tasks related to risk process oversight to our Audit Committee, which reports the results of its review process to the Board of Directors. The Audit Committee’s process includes a review, at least annually, of our internal audit process, including the organizational structure, as well as the scope and methodology of the internal audit process. The Governance and Nominating Committee oversees risks related to our corporate governance, including director performance, director succession, director education and governance documents.
In addition to the reports from the Board committees, our board periodically discusses risk oversight.
Meetings Attendance
During the 2014 fiscal year, our Board of Directors held 5 meetings. With the exception of Mr. Rothwell, and Mr. Harkey who each attended all except one Special Meeting of the Board, each director attended 100 percent of the aggregate number of Board of Directors meetings and committee meetings of which he was a member that were held during the period of his service as a director. Mr. Rothwell and Mr. Harkey were briefed on the subject of the Special Meeting of the Board of Directors which they did not attend; provided their input to Management before the Meeting was held; and reviewed Minutes of the Meeting with the Corporate Secretary afterwards.
The Audit Committee met 4 times during the 2014 fiscal year.
The Compensation Committee met 1 time during the 2014 fiscal year.
The Governance and Nominating Committee met 1 times during the 2014 fiscal year.
The Company does not have a formal policy regarding attendance by members of the Board of Directors at the Company’s annual meeting of stockholders, although it does encourage attendance by the directors.
| ITEM 14. | PRINCIPAL ACCOUNTANT FEES AND SERVICES |
The following table presents fees for professional audit services rendered by McGladrey for the audit of our annual financial statements for the years ended December 31, 2014, and December 31, 2013, respectively, and fees billed for other services rendered by McGladrey during the respective periods.
| 2014 | 2013 | |||||||
| Type of Fees |
||||||||
| Audit Fees(1) |
255.000 | $ | 255,000 | |||||
| Audit-Related Fees(2) |
8,900 | $ | 7,500 | |||||
|
|
|
|
|
|||||
| 263,900 | $ | 262,500 | ||||||
| (1) | Audit fees for 2014 and 2013 were for professional services rendered for the audit of the Company’s financial statements for the fiscal year, including attestation services required under Section 404 of the Sarbanes-Oxley Act of 2002, and reviews of the Company’s quarterly financial statements included in its Form 10-Q filings. |
112
Table of Contents
| (2) | Audit related fees are for services related to registration statements. |
The Audit Committee has determined that the non- audit services provided by McGladrey during 2014 and 2013 did not impair their independence. All decisions regarding selection of independent registered public accounting firm and approval of accounting services and fees are made by our Audit Committee in accordance with the provisions of the Sarbanes-Oxley Act of 2002 and related SEC rules.
The Audit Committee pre-approves all audit and permissible non-audit services provided by the independent registered public accounting firm. These services may include audit services, audit related services, tax services and other services. The committee has adopted a policy for the pre-approval of services provided by the independent registered public accounting firm, where pre-approval is generally provided for up to one year and any pre-approval is detailed as to the particular service or category of services and is subject to a specific budget. For each proposed service, the independent auditor is required to provide detailed communication at the time of approval. The committee may delegate pre-approval authority to one or more of its members, who must report same to the Committee members at the next meeting. The Audit Committee, after discussion with McGladrey, agreed that any additional audit fees could be paid by us, subject to the pre-approval of the Audit Committee chairman.
The Audit Committee intends to select McGladrey to serve as independent registered public accounting firm for the fiscal year ending December 31, 2015.
| ITEM 15. | EXHIBITS AND FINANCIAL STATEMENT SCHEDULES |
(a) (1) Financial Statements
A list of the financial statements filed as a part of this report appears on page .
(2) Financial Statement Schedules
Schedules have been omitted because the information required is not applicable or is shown in the Financial Statements or the corresponding Notes to the Financial Statements.
(3) Exhibits
A list of the exhibits filed as a part of this report appears on pages thru.
(b) See Exhibits listed under the heading “Exhibit Index” beginning on page .
(c) Schedules have been omitted because the information required is not applicable or is shown in the Financial Statements or the corresponding Notes to the Financial Statements.
113
Table of Contents
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| EMISPHERE TECHNOLOGIES, INC. | ||
| By: | /s/ Alan L. Rubino | |
| Alan L. Rubino | ||
| President and Chief Executive Officer | ||
| Date: | March 31, 2015 | |
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
| Name and Signature |
Title |
Date | ||
| /s/ Alan L. Rubino Alan L. Rubino |
President and Chief Executive Officer and Director (principal executive officer) |
March 31, 2015 | ||
| /s/ Timothy G. Rothwell Timothy G. Rothwell |
Chairman of the Board | March 31, 2015 | ||
| /s/ John D. Harkey, Jr. John D. Harkey, Jr. |
Director | March 31, 2015 | ||
| /s/ Timothy McInerney Timothy McInerney |
Director | March 31, 2015 | ||
| /s/ Jacob M. Plotsker Jacob M. Plotsker |
Director | March 31, 2015 | ||
| /s/ Mark H. Rachesky, M.D. Mark H. Rachesky, M.D. |
Director | March 31, 2015 | ||
| /s/ Michael Weiser, M.D., Ph.D. Michael Weiser, M.D., Ph.D. |
Director | March 31, 2015 | ||
| /s/ Michael R. Garone Michael R. Garone |
Chief Financial Officer (principal financial and accounting officer) |
March 31, 2015 | ||
114
Table of Contents
| Exhibit |
Incorporated by Reference (1) |
|||||||||
| 3.1 | Amended and Restated Certificate of Incorporation of Emisphere Technologies, Inc., as amended by the Certificate of Amendment of Amended and Restated Certificate of Incorporation of Emisphere Technologies, Inc., dated April 20, 2007 | R | ||||||||
| 3.2 | Certificate of Increase of Series A Junior Participating Cumulative Preferred Stock of Emisphere Technologies, Inc., dated June 4, 2012 | OO | ||||||||
| 3.3 | By-Laws of Emisphere Technologies, Inc., as amended December 7, 1998, and September 23, 2005 | A, L | ||||||||
| 3.4 | Amendment to the Amended By-Laws of Emisphere Technologies, Inc., effective as of September 11, 2007 | V | ||||||||
| 4.1 | Amended and Restated Rights Agreement dated as of April 7, 2006 between Emisphere Technologies, Inc. and Mellon Investor Services, LLC | P | ||||||||
| 4.2 | Loan Agreement, dated as of August 20, 2014, by and between Emisphere Technologies, Inc. and the Lenders named therein | UU | ||||||||
| 4.3 | Form of Second Amended and Restated 13% Senior Secured Convertible Note | UU | ||||||||
| 4.4 | Form of Second Amended and Restated Senior Secured Reimbursement Promissory Note | UU | ||||||||
| 4.5 | Form of Second Amended and Restated Senior Secured Bridge Promissory Notes | UU | ||||||||
| 4.6 | Amended and Restated Pledge and Security Agreement by and between Emisphere Technologies, Inc. and MHR Institutional Partners IIA LP | UU | ||||||||
| 10.1 | Emisphere Technologies, Inc. 2007 Stock Award and Incentive Plan | R | (2 | ) | ||||||
| 10.2 | Form of Nonqualified Stock Option Agreement | R | (2 | ) | ||||||
| 10.3 | Form of Incentive Stock Option Agreement | R | (2 | ) | ||||||
| 10.4 | Form of Restricted Stock Option Agreement | R | (2 | ) | ||||||
| 10.5 | Research Collaboration and Option Agreement dated as of December 3, 1997 between Emisphere Technologies, Inc. and Novartis Pharma AG | D | (3 | ) | ||||||
| 10.6 | Research Collaboration and License Agreement dated as of September 23, 2004 between Emisphere Technologies, Inc. and Novartis Pharma AG, as amended on November 4, 2005 | J | (3 | ) | ||||||
| 10.7 | Research Collaboration Option and License Agreement dated December 1, 2004 by and between Emisphere Technologies, Inc. and Novartis Pharma AG | J | (3 | ) | ||||||
| 10.8 | Convertible Promissory Note due December 1, 2009 issued to Novartis Pharma AG | J | (3 | ) | ||||||
| 10.9 | Senior Secured Term Loan Agreement between Emisphere Technologies, Inc. and MHR Institutional Partners IIA LP, dated September 26, 2005, as amended on November 11, 2005 | L | ||||||||
| 10.10 | Pledge and Security Agreement between Emisphere Technologies, Inc. and MHR Institutional Partners IIA LP, dated September 26, 2005 | L | ||||||||
| 10.11 | Registration Rights Agreement between Emisphere Technologies, Inc. and MHR, dated September 26, 2005 | L | ||||||||
| 10.12 | Amendment No. 1 to the Senior Secured Term Loan Agreement, dated November 11, 2005 | M | ||||||||
| 10.13 | Form of 11% Senior Secured Convertible Note | L | ||||||||
| 10.14 | Form of Amendment to 11% Senior Secured Convertible Note | R | ||||||||
115
Table of Contents
| Exhibit |
Incorporated by Reference (1) |
|||||||||
| 10.15 | Warrant dated as of September 21, 2006, between Emisphere Technologies, Inc. and MHR Institutional Partners IIA LP | Q | ||||||||
| 10.16 | Warrant dated as of September 21, 2006 between Emisphere Technologies, Inc. and MHR Institutional Partners II LP | Q | ||||||||
| 10.17 | Warrant adjustment notice between Emisphere Technologies, Inc. and MHR Capital Partners (100) LP, MHR Capital Partners Master Account, LP (formerly MHR Capital Partners (500) LP), MHR Institutional Partners IIA LP, MHR Institutional Partners II LP, MHR Capital Partners (100) LP and MHR Capital Partners Master Account LP | W | ||||||||
| 10.18 | Warrant dated as of August 22, 2007 between Emisphere Technologies, Inc. and SF Capital Partners, Ltd. | W | ||||||||
| 10.19 | Warrant dated as of August 22, 2007 between Emisphere Technologies, Inc. and Option Opportunities Corp. | W | ||||||||
| 10.20 | Warrant dated as of August 22, 2007 between Emisphere Technologies, Inc. and Option Opportunities Corp. | W | ||||||||
| 10.21 | Warrant dated as of August 22, 2007 between Emisphere Technologies, Inc. and Montaur Capital/Platinum Life Montaur Life Sciences Fund I LLC | W | ||||||||
| 10.22 | Warrant dated as of August 22, 2007, between Emisphere Technologies, Inc. and MHR Institutional Partners II LP | W | ||||||||
| 10.23 | Warrant dated as of August 22, 2007, between Emisphere Technologies, Inc. and MHR Institutional Partners IIA LP | W | ||||||||
| 10.24 | Development and License Agreement, dated as of June 21, 2008, between Emisphere Technologies, Inc. and Novo Nordisk AS. | Y | (3 | ) | ||||||
| 10.25 | Form of Non-Employee Director Non-Qualified Stock Option Agreement | AA | (2 | ) | ||||||
| 10.26 | Securities Purchase Agreement dated as of August 19, 2009, between Emisphere Technologies and the Purchasers named therein | BB | ||||||||
| 10.27 | Securities Purchase Agreement dated as of August 19, 2009, between Emisphere Technologies and MHR Fund Management, LLC | BB | ||||||||
| 10.28 | Warrant dated as of August 21, 2009, between Emisphere Technologies, Inc. and MHR Capital Partners Master Account LP | CC | ||||||||
| 10.29 | Warrant dated as of August 21, 2009, between Emisphere Technologies, Inc. and MHR Capital Partners (100) LP | CC | ||||||||
| 10.30 | Warrant dated as of August 21, 2009, between Emisphere Technologies, Inc. and MHR Institutional Partners II LP | CC | ||||||||
| 10.31 | Warrant dated as of August 21, 2009, between Emisphere Technologies, Inc. and MHR Institutional Partners IIA LP | CC | ||||||||
| 10.32 | Warrant dated as of August 21, 2009, between Emisphere Technologies, Inc. and Rodman & Renshaw, LLC | CC | ||||||||
| 10.33 | Warrant dated as of August 21, 2009, between Emisphere Technologies, Inc. and Benjamin Bowen | CC | ||||||||
| 10.34 | Warrant dated as of August 21, 2009, between Emisphere Technologies, Inc. and Noam Rubinstein | CC | ||||||||
| 10.35 | Warrant adjustment notice between Emisphere Technologies, Inc. and Elan International Services, Ltd. dated October 20, 2009 | CC | ||||||||
116
Table of Contents
| Exhibit |
Incorporated by Reference (1) |
|||||||||
| 10.36 | Agreement to Extend the Maturity Date of the Convertible Promissory Note Due December 1, 2009, between Emisphere Technologies and Novartis Pharma AG dated November 25, 2009 | EE | ||||||||
| 10.37 | Agreement to Extend the Maturity Date of the Convertible Promissory Note Due December 1, 2009, between Emisphere Technologies and Novartis Pharma AG dated February 23, 2010 | EE | ||||||||
| 10.38 | Form of Incentive Stock Option Agreement under the Emisphere Technologies, Inc. 2007 Stock Award and Incentive Plan | FF | ||||||||
| 10.39 | Form of Non-Qualified Stock Option Agreement under the Emisphere Technologies, Inc. 2007 Stock Award and Incentive Plan | FF | ||||||||
| 10.40 | Letter Agreement by and between Emisphere Technologies, Inc. and MHR Institutional Partners IIA LP, dated June 8, 2010 | GG | ||||||||
| 10.41 | Form of Emisphere Technologies, Inc. Reimbursement Note | GG | ||||||||
| 10.42 | Form of Emisphere Technologies, Inc. Second Reimbursement Note | GG | ||||||||
| 10.43 | Research Master Agreement and Amendment by and between Emisphere Technologies, Inc. and Novartis Pharma AG, effective as of June 4, 2010 | HH | (3 | ) | ||||||
| 10.44 | Securities Purchase Agreement by and among Emisphere Technologies, Inc. and the Buyers named therein, dated August 25, 2010 | II | ||||||||
| 10.45 | Securities Purchase Agreement by and among Emisphere Technologies, Inc. and the MHR Buyers named therein, dated August 25, 2010 | II | ||||||||
| 10.46 | Waiver Agreement, by and among Emisphere Technologies, Inc. and MHR, dated August 25, 2010 | II | ||||||||
| 10.47 | Registration Rights Agreement by and among Emisphere Technologies, Inc. and the Buyers named therein, dated August 26, 2010 | JJ | ||||||||
| 10.48 | Warrant dated as of August 26, 2010, between Emisphere Technologies, Inc. and Bai Ye Feng | JJ | ||||||||
| 10.49 | Warrant dated as of August 26, 2010, between Emisphere Technologies, Inc. and Anson Investments Master Fund LP | JJ | ||||||||
| 10.50 | Warrant dated as of August 26, 2010, between Emisphere Technologies, Inc. and Iroquois Master Fund, Ltd. | JJ | ||||||||
| 10.51 | Warrant dated as of August 26, 2010, between Emisphere Technologies, Inc. and Hudson Bay Master Fund Ltd. | JJ | ||||||||
| 10.52 | Warrant dated as of August 26, 2010, between Emisphere Technologies, Inc. and Cranshire Capital, L.P. | JJ | ||||||||
| 10.53 | Warrant dated as of August 26, 2010, between Emisphere Technologies, Inc. and Freestone Advantage Partners, LP | JJ | ||||||||
| 10.54 | Warrant dated as of August 26, 2010, between Emisphere Technologies, Inc. and MHR Capital Partners Master Account LP | JJ | ||||||||
| 10.55 | Warrant dated as of August 26, 2010, between Emisphere Technologies, Inc. and MHR Capital Partners (100) LP | JJ | ||||||||
| 10.56 | Warrant dated as of August 26, 2010, between Emisphere Technologies, Inc. and MHR Institutional Partners II LP | JJ | ||||||||
| 10.57 | Warrant dated as of August 26, 2010, between Emisphere Technologies, Inc. and MHR Institutional Partners IIA LP | JJ | ||||||||
117
Table of Contents
| Exhibit |
Incorporated by Reference (1) |
|||||||||
| 10.58 | Warrant dated as of August 26, 2010, between Emisphere Technologies, Inc. and MHR Capital Partners Master Account LP | JJ | ||||||||
| 10.59 | Warrant dated as of August 26, 2010, between Emisphere Technologies, Inc. and MHR Capital Partners (100) LP | JJ | ||||||||
| 10.60 | Warrant dated as of August 26, 2010, between Emisphere Technologies, Inc. and MHR Institutional Partners II LP | JJ | ||||||||
| 10.61 | Warrant dated as of August 26, 2010, between Emisphere Technologies, Inc. and MHR Institutional Partners IIA LP | JJ | ||||||||
| 10.62 | Development and License Agreement, dated December 20, 2010, between Emisphere Technologies, Inc. and Novo Nordisk A/S | KK | (3 | ) | ||||||
| 10.63 | Securities Purchase Agreement, dated June 30, 2011, by and among Emisphere Technologies, Inc. and the Buyers named therein. | LL | ||||||||
| 10.64 | Securities Purchase Agreement, dated June 30, 2011, by and among Emisphere Technologies, Inc. and the MHR Fund Management LLC. | LL | ||||||||
| 10.65 | Waiver Agreement, dated June 30, 2011, by and among Emisphere Technologies, Inc. and MHR. | LL | ||||||||
| 10.66 | Registration Rights Agreement by and among Emisphere Technologies, Inc. and the Buyers named therein, dated July 6, 2011 | MM | ||||||||
| 10.67 | Warrant A-54 dated as of July 6, 2011, between Emisphere Technologies, Inc. and EOS Holdings LLC | MM | ||||||||
| 10.68 | Warrant A-55 dated as of July 6, 2011, between Emisphere Technologies, Inc. and Kingsbrook Opportunities Master Fund LP | MM | ||||||||
| 10.69 | Warrant A-56 dated as of July 6, 2011, between Emisphere Technologies, Inc. and Bai Ye Feng | MM | ||||||||
| 10.70 | Warrant A-57 dated as of July 6, 2011, between Emisphere Technologies, Inc. and Cranshire Capital, L.P. | MM | ||||||||
| 10.71 | Warrant A-58 dated as of July 6, 2011, between Emisphere Technologies, Inc. and HF H VICTOR UW VICTOR ART 7 | MM | ||||||||
| 10.72 | Warrant A-59 dated as of July 6, 2011, between Emisphere Technologies, Inc. and Freestone Advantage Partners, LP | MM | ||||||||
| 10.73 | Warrant A-60 dated as of July 6, 2011, between Emisphere Technologies, Inc. and Iroquois Master Fund Ltd. | MM | ||||||||
| 10.74 | Warrant A-61 dated as of July 6, 2011, between Emisphere Technologies, Inc. and Shipman & Goodwin LLP Profit Sharing Trust FBO James T. Betts | MM | ||||||||
| 10.75 | Warrant A-62 dated as of July 6, 2011, between Emisphere Technologies, Inc. and Son Nam Nguyen | MM | ||||||||
| 10.76 | Warrant A-63 dated as of July 6, 2011, between Emisphere Technologies, Inc. and Pine Lodge Capital Company Ltd. | MM | ||||||||
| 10.77 | Warrant A-64 dated as of July 6, 2011, between Emisphere Technologies, Inc. and Huaidong Wang | MM | ||||||||
| 10.78 | Warrant A-65 dated as of July 6, 2011, between Emisphere Technologies, Inc. and Anson Investments Master Fund LP | MM | ||||||||
| 10.79 | Warrant A-66 dated as of July 6, 2011, between Emisphere Technologies, Inc. and MHR Capital Partners Master Account LP | MM | ||||||||
118
Table of Contents
| Exhibit |
Incorporated by Reference (1) |
|||||||||
| 10.80 | Warrant A-67 dated as of July 6, 2011, between Emisphere Technologies, Inc. and MHR Capital Partners (100) LP | MM | ||||||||
| 10.81 | Warrant A-68 dated as of July 6, 2011, between Emisphere Technologies, Inc. and MHR Institutional Partners II LP | MM | ||||||||
| 10.82 | Warrant A-69 dated as of July 6, 2011, between Emisphere Technologies, Inc. and MHR Institutional Partners IIA LP | MM | ||||||||
| 10.83 | Warrant A-70 dated as of July 6, 2011, between Emisphere Technologies, Inc. and MHR Capital Partners Master Account LP | MM | ||||||||
| 10.84 | Warrant A-71 dated as of July 6, 2011, between Emisphere Technologies, Inc. and MHR Capital Partners (100) LP | MM | ||||||||
| 10.85 | Warrant A-72 dated as of July 6, 2011, between Emisphere Technologies, Inc. and MHR Institutional Partners II LP | MM | ||||||||
| 10.86 | Warrant A-73 dated as of July 6, 2011, between Emisphere Technologies, Inc. and MHR Institutional Partners IIA LP | MM | ||||||||
| 10.87 | License Agreement, dated March 8, 2000, by and between Emisphere Technologies, Inc. and Novartis Pharma AG | NN | (3 | ) | ||||||
| 10.88 | Form of Novartis Promissory Note Extension, between the Company and MHR | PP | ||||||||
| 10.89 | Form of Promissory Reimbursement Note Extension, between the Company and MHR | PP | (3 | ) | ||||||
| 10.90 | Employment Agreement, dated September 13, 2012, between Alan L. Rubino and the Company | (2 | ) | |||||||
| 10.91 | Senior Secured Promissory Note of Emisphere Technologies, Inc., dated October 17, 2012 | RR | ||||||||
| 10.92 | Amendment to Pledge and Security Agreement, by and among Emisphere Technologies, Inc. and MHR Institutional Partners IIA LP, dated October 17, 2012 | RR | ||||||||
| 10.93 | Employment Agreement, dated October 15, 2012, between Carl V. Sailer and Emisphere Technologies, Inc. | RR | (2 | ) | ||||||
| 10.94 | Employment Agreement, dated January 14, 2013, between Michael R. Garone and Emisphere Technologies, Inc. | SS | (2 | ) | ||||||
| 10.95 | Sublease Agreement, dated November 27, 2012, between New American Therapeutics, Inc. and Emisphere Technologies, Inc. | TT | ||||||||
| 10.96 | Lease Agreement, dated December 11, 2012, between 4 Becker SPE LLC and Emisphere Technologies, Inc. | TT | ||||||||
| 10.97 | Amendment to Emisphere Technologies, Inc. Amended and Restated 13% Senior Secured Convertible Note, dated March 28, 2014 | VV | ||||||||
| 10.98 | Royalty Agreement, dated as of August 20, 2014, by and between Emisphere Technologies, Inc. and the other parties named therein | UU | ||||||||
| 14.1 | Emisphere Technologies, Inc. Code of Business Conduct and Ethics for Directors | I | ||||||||
| 21.1 | Subsidiaries* | |||||||||
| 23.1 | Consent of Independent Registered Public Accounting Firm — McGladrey, LLP* | |||||||||
| 31.1 | Certification of the Chief Executive Officer Pursuant to Rule 13a-14(a) and 15d-14(a), as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002* | |||||||||
| 31.2 | Certification of the Chief Financial Officer Pursuant to Rule 13a-14(a) and 15d-14(a), as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002.* | |||||||||
119
Table of Contents
| Exhibit |
Incorporated by Reference (1) | |||||||
| 32.1 | Certification Pursuant to 18 U.S.C. Section 1350, As Adopted Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002* | |||||||
| 101 | Interactive Data File. | * | ||||||
| * | Filed herewith |
| (1) | If not filed herewith, filed as an exhibit to the document referred to by letter as follows: |
| A. | Quarterly Report on Form 10-Q for the quarterly period ended January 31, 1999 (SEC File No. 000-17758) |
| B. | Annual Report on Form 10-K for the fiscal year ended July 31, 1995 (SEC File No. 000-17758) |
| C. | [Reserved] |
| D. | Quarterly Report on Form 10-Q for the quarterly period ended October 31, 1997 (SEC File No. 000-17758) |
| E. | [Reserved] |
| F. | Annual Report on Form 10-K for the fiscal year ended July 31, 1999 (SEC File No. 000-17758) |
| G. | [Reserved] |
| H. | [Reserved] |
| I. | Annual Report on Form 10-K for the year ended December 31, 2003 (SEC File No. 000-17758) |
| J. | Registration on Form S-3/A dated and filed February 1, 2005 (SEC File No. 333-117230) |
| K. | Quarterly Report on Form 10-Q for the quarterly period ended March 31, 2005 (SEC File No. 000-17758) |
| L. | Current Report on Form 8-K, filed September 30, 2005 (SEC File No. 000-17758) |
| M. | Current Report on Form 8-K, filed November 14, 2005 (SEC File No. 000-17758) |
| N. | [Reserved] |
| O. | Quarterly Report on Form 10-Q for the quarterly period ended March 31, 2006 (SEC File No. 000-17758) |
| P. | Current Report on Form 8-K, filed April 10, 2006 (SEC File No. 000-17758) |
| Q. | Annual Report on Form 10-K for the fiscal year ended December 31, 2006 (SEC File No. 000-17758) |
| R. | Quarterly Report on Form 10-Q for the quarterly period ended March 31, 2007 |
| S. | Current Report on Form 8-K, filed April 11, 2007 |
| T. | Quarterly Report on Form 10-Q for the quarterly period ended June 30, 2007 |
| U. | Current Report on Form 8-K, filed June 29, 2007 |
| V. | Current Report on Form 8-K, filed September 14, 2007 |
| W. | Quarterly Report on Form 10-Q for the quarterly period ended September 30, 2007 |
120
Table of Contents
| X. | Annual Report on Form 10-K for the fiscal year ended December 31, 2007 |
| Y. | Current Report on Form 10-Q, filed August 11, 2008 |
| Z. | Current Report on Form 8-K, filed May 5, 2009 |
| AA. | Current Report on Form 8-K, filed May 21, 2009 |
| BB. | Current Report on Form 8-K, filed August 20, 2009 |
| CC. | Quarterly Report on Form 10-Q for the quarterly period ended September 30, 2009 |
| DD. | Current Report on Form 8-K, filed January 12, 2010 |
| EE. | Annual Report on Form 10-K for the fiscal year ended December 31, 2009 |
| FF. | Quarterly Report on Form 10-Q for the quarterly period ended March 31, 2010 |
| GG. | Current Report on Form 8-K, filed June 9, 2010 |
| HH. | Quarterly Report on Form 10-Q for the quarterly period ended June 30, 2010 |
| II. | Current Report on Form 8-K, filed August 25, 2010 |
| JJ. | Registration Statement on Form S-1, filed on September 15, 2010 |
| KK. | Current Report on Form 8-K, filed on December 21, 2010 |
| LL. | Current Report on Form 8-K, filed on June 30, 2011 (SEC File No. 000-17758) |
| MM. | Registration Statement on Form S-1, filed on July 26, 2011 (SEC File No. 333-175794). |
| NN. | Amendment No. 1 on Form 10-K/A, filed January 19, 2012, to Annual Report on Form 10-K for the fiscal year ended December 31, 2010, originally filed on March 31, 2011 |
| OO | Current Report on Form 8-K, filed on June 5, 2012 (SEC File No. 000-17758) |
| PP | Current Report on Form 8-K, filed on June 4, 2012 (SEC File No. 000-17758) |
| Current Report on Form 8-K, filed on September 17, 2012 (SEC File No. 000-17758) |
| RR | Current Report on Form 8-K, filed on October 19, 2012 (SEC File No. 000-17758) |
| SS | Current Report on Form 8-K, filed on January 17, 2013 (SEC File No. 000-17758) |
| TT | Annual Report on Form 10-K, filed on March 28, 2013 (SEC File No. 000-17758) |
| UU | Current Report on Form 8-K, filed on August 21, 2014 (SEC File No. 000-17758) |
| VV | Annual Report on Form 10-K for the fiscal year ended December 31, 2014 (SEC File No. 000-17758) |
| (2) | Management contract or compensatory plan or arrangement |
| (3) | Confidential treatment has been granted for the redacted portions of this agreement. A complete copy of this agreement, including the redacted portions, has been filed separately with the Securities and Exchange Commission. |
121
