Attached files
| file | filename |
|---|---|
| EXCEL - IDEA: XBRL DOCUMENT - Bone Biologics Corp | Financial_Report.xls |
| EX-32.2 - Bone Biologics Corp | ex32-2.htm |
| EX-31.2 - Bone Biologics Corp | ex31-2.htm |
| EX-31.1 - Bone Biologics Corp | ex31-1.htm |
| EX-32.1 - Bone Biologics Corp | ex32-1.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
| [X] | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended: December 31, 2014
| [ ] | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from _________ to _________
Commission File Number: 000-53078
Bone Biologics, Corp.
(Exact name of registrant as specified in its charter)
Delaware
(State or other jurisdiction of
incorporation or organization)
42-1743430
(I.R.S. Employer
Identification No.)
175 May Street, Suite 400, Edison, NJ, 08837
(732) 661-2224
Securities registered pursuant to Section 12(b) of the Act:
None
Securities registered pursuant to Section 12(g) of the Act:
Common Stock, $0.001 par value per share
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes [ ] No [X]
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes [ ] No [X]
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes [X] No [ ]
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate website, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes [X] No [ ]
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. [X]
Indicate by check mark whether the Company is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See definitions of “large accelerated filer,” “accelerated filer,” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check one):
| Large accelerated filer | [ ] | Accelerated filer | [ ] | |||
| Non-accelerated filer | [ ] (Do not check if a smaller reporting company) | Smaller reporting company | [X] | |||
Indicate by check mark whether the Company is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes [ ] No [X]
As of March 30, 2015, there were 24,269,047 shares of common stock, par value $.001, outstanding.
Documents Incorporated by Reference
None.
TABLE OF CONTENTS
| 2 |
Cautionary Note on Forward-Looking Statements
This annual report on form 10-K (“Annual Report”) contains forward-looking statements. Such forward-looking statements include those that express plans, anticipation, intent, contingency, goals, targets or future development and/or otherwise are not statements of historical fact. These forward-looking statements are based on our current expectations and projections about future events and they are subject to risks and uncertainties known and unknown that could cause actual results and developments to differ materially from those expressed or implied in such statements. These forward-looking statements are subject to a number of risks, uncertainties and assumptions, including those under the heading “Risk Factors” in our Current Report on Form 8-K filed with the Securities and Exchange Commission (“SEC”) on September 25, 2014.
All statements other than historical facts contained in this Annual Report, including statements regarding our future financial position, capital expenditures, cash flows, business strategy and plans and objectives of management for future operations are forward-looking statements. The words “anticipated,” “believe,” “expect,” “plan,” “intend,” “seek,” “estimate,” “project,” “could,” “may,” and similar expressions are intended to identify forward-looking statements. These statements include, among others, information regarding future operations, future capital expenditures, and future net cash flow. Such statements reflect our management’s current views with respect to future events and financial performance and involve risks and uncertainties, including, without limitation, our ability to raise additional capital to fund our operations, obtaining Food and Drug Administration (“FDA”) and other regulatory authorization to market our drug and biological products, successful completion of our clinical trials, our ability to achieve regulatory authorization to market our lead product Nell-1, our reliance on third party manufacturers for our drug products, market acceptance of our products, our dependence on licenses for certain of our products, our reliance on the expected growth in demand for our products, exposure to product liability and defect claims, development of a public trading market for our securities, and various other matters, many of which are beyond our control.
Should one or more of these risks or uncertainties occur, or should underlying assumptions prove to be incorrect, actual results may vary materially and adversely from those anticipated, believed, estimated or otherwise indicated. Consequently, all of the forward-looking statements made in this Annual Report are qualified by these cautionary statements and accordingly there can be no assurances made with respect to the actual results or developments. We undertake no obligation to revise or publicly release the results of any revision to these forward-looking statements, except as required by law. Given these risks and uncertainties, readers are cautioned not to place undue reliance on such forward-looking statements.
Unless expressly indicated or the context requires otherwise, the terms “Company,” “we,” “us,” and “our” in this document refer to Bone Biologics, Corp., a Delaware corporation, and, its wholly owned subsidiary, after giving effect to the Merger, as defined under Part I, Item 1-“Business” in this Annual Report.
| 3 |
Bone Biologics, Corp. (the “Company”) was incorporated under the laws of the State of Delaware on October 18, 2007 as AFH Acquisition X, Inc. On September 19, 2014, the Company and its wholly-owned subsidiary, Bone Biologics Acquisition Corp., a Delaware corporation (“Merger Sub”), entered into an Agreement and Plan of Merger, dated September 19, 2014 (the “Merger Agreement”), with Bone Biologics, Inc. (“Bone” or “Bone Biologics”). Pursuant to the terms of the Merger Agreement, Bone merged with Merger Sub on September 19, 2014, with Bone as the surviving entity (the “Merger”). After the Merger, the Company ceased to be a shell company, as defined in the rules of the SEC, and the Company officially changed its name to “Bone Biologics, Corp.” The 5,000,000 outstanding shares of common stock of the Company prior to the Merger were consolidated into 3,853,600 shares of common stock, par value $0.001 per share (“Common Stock”), and the remaining shares were cancelled.
Immediately following the Merger, the business of Bone Biologics, Inc. became our business. Bone Biologics was founded by University of California professors in collaboration with an Osaka University professor and a University of Southern California surgeon in 2004 as a privately-held company with proprietary, patented technology that has been validated in sheep and non-human primate models to facilitate bone growth. Our platform technology has application in delivering improved outcomes in the surgical specialties of spinal, orthopedic, general orthopedic, plastic reconstruction, neurosurgery, interventional radiology, and sports medicine. Lead product development and clinical studies are targeted on spinal fusion surgery, a growing market space. The following chart provides a segment overview of bone replacement products in the U.S.:
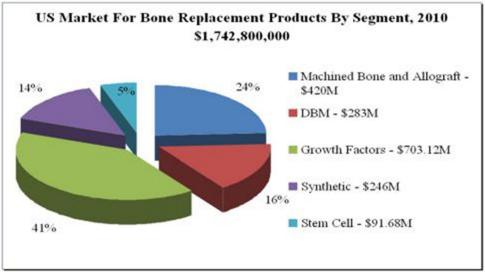 |
||
| (See O’Reilly, Sharon. Beyond INFUSE: Spine Community Searches For Answers. INVIVO. November 2011, Vol. 29, No. 10.) |
Products
We have developed a stand-alone platform technology through significant lab, small animal, large animal, and rhesus monkey research over more than ten years to generate the current applications across broad fields of use. The platform technology is UCB-1, a proprietary skeletal specific growth factor used in combination with DBX®, a proprietary demineralized bone matrix from Musculoskeletal Transplant Foundation (MTF). Together, with DBX®, or alone, Nell-1 provides regulation over skeletal tissue formation and stem cell differentiation during bone regeneration.
| 1 |
We are currently focused on bone regeneration in lumbar spinal fusion using our recombinant human protein, known as Nell-1. Nell-1 is an osteoinductive orthobiologic protein that provides control over bone regeneration. This patent protected technology has been exclusively licensed to us from the University of California, Los Angeles (“UCLA”). Leveraging the resources of investors and strategic partners, we have successfully surpassed two critical milestones:
| ● | Demonstrating a successful small laboratory scale pilot run for the recombinant manufacturing of the human Nell-1 protein in Chinese hamster ovary cells, which is a commercially established mammalian cell line for other recombinant proteins, which is well-defined and accepted by international regulatory agencies; and | |
| ● | Validation of protein dosing and efficacy in established large animal sheep models and Rhesus Monkey primate models. |
We are targeting spinal fusion as the first clinical indication of our platform technology and are currently in the pre-investigational device exemption (“IDE”) phase. The lead product, purified Nell-1, is expected to be dried onto ß-tricalcium phosphate (“TCP”) bone void filler to produce a medical device, known as Nell-1/TCP (“Nell/TCP Fusion Device”). This device will be mixed with 510(k) cleared DBX® Demineralized Bone Putty recommended for use in conjunction with a cleared intervertebral body fusion device. The Nell-1/TCP Fusion Device will be comprised of a single dose vial of NELL-1 recombinant protein freeze dried onto TCP. A vial of Nell-1/TCP will be sold in a convenience kit with a diluent and a syringe of 510(k) cleared demineralized bone (“DBX® Putty”), produced by MTF. An elegant delivery device will allow the surgeon to mix the reconstituted Nell 1/TCP with the appropriate quantity of DBX® Putty just prior to implantation.
The Nell-1/TCP Fusion Device is intended for use in lumbar spinal fusion and may have a variety of other applications such as cervical spinal fusion.
While the product is initially targeted at the lumbar spine fusion market, we believe Nell-1’s unique set of characteristics, target specific mechanism of action, efficacy, safety, and affordability, position the product well for application in a variety of procedures, including:
Spine Implants. This is the largest market for bone substitute product, representing approximately 80% of the total 2009 U.S. market. While use of the patient’s own bone, also referred to as autograft, to enhance fusion of vertebral segments remains the gold standard for this type of treatment, complications associated with use of autograft bone including pain, increased surgical time and infection limit its use.
Non-Union Trauma Cases. While the majority of fractures heal without the need for osteosynthetic products, bone substitutes are used in complicated breaks where the bone does not mend naturally. Nell-1 is expected to perform as well as high-priced growth factors in this market.
Hip & Knee Revisions. The use of bone substitutes in reconstruction surgery is generally limited to revision cases where the products are used to account for the significant bone loss that accompanies these cases. The treatment of osteoporotic patients also represents a substantial opportunity for Nell-1 use in hip and knee reconstruction.
Implant Coating. The use of Nell-1 as a direct coating on hip and knee implants could have a very significant impact on the market. A Nell-1 coating may prolong the life of primary implants and allow for differentiation in a commodity market.
Osteoporosis. The medical need to find a solution to counter a decrease in bone mass and density seen in women most frequently after menopause or a similar effect on astronauts in microgravity environments for an extend period of time is a major medical challenge. The systemic use of Nell-1 to stimulate bone regeneration throughout the body thereby increasing bone density could have a very significant impact on the treatment of osteoporosis.
| 2 |
UCLA’s initial research was funded with approximately $18 million in resources from UCLA and government grants. After licensing the exclusive worldwide intellectual property rights from UCLA, development was funded with additional grant funding and $6.5 million in strategic investment from MTF. We anticipate that it will require an additional $8.5 million for preclinical studies, $1 million for completion of the filing of an IDE application, and $4 million for initiation of human studies. An estimated $50-60 million will be required to achieve product launch.
Nell-1’s powerful specific bone and cartilage forming properties derive from the ability of Nell-1 to only target cells that exhibit an activated “master switch” to develop into bone or cartilage. Nell-1 is a function specific recombinant human protein that has been proven in lab bench models to recapitulate normal human growth and development to provide control over bone and cartilage regeneration through research and development work at UCLA and written in publications.
Nell-1 was isolated in 1996, and the first Nell-1 patent on bone regeneration was filed in 1999. Subsequent patents and continuations in part describing Nell-1 manufacturing, delivery, and cartilage regeneration were filed in 2002, 2003, 2006, 2007, 2008, and 2009 to further strengthen the patent portfolio.
Research & Publications
Our leading scientists have been published in notable scientific journals and publications in our field. These publications have served to highlight the work and achievements of the Company.
Proposed Initial Clinical Application
The Nell-1/TCP Fusion Device will be indicated for spinal fusion procedures in skeletally mature patients with degenerative disc disease (“DDD”) at one level from L4-S1. These DDD patients may also have up to Grade I spondylolisthesis at the involved level. The Nell-1/TCP Fusion Device is to be implanted via an anterior open or an anterior laparoscopic approach in conjunction with a cleared intervertebral body fusion device. Patients receiving the device should have had at least six months of non-operative treatment prior to treatment with the device. A cervical indication is currently under consideration. This indication for use would fill a current clinical gap, created by potentially dangerous inflammatory response caused by Infuse® Bone Graft, the subject of a Public Health Notification from the United States Food and Drug Administration (the “FDA”) on July 1, 2008 about life threatening complications associated with recombinant human bone morophogenic protein-2 (BMP-2) in cervical spine fusion. Bone would not expect to see the same adverse events with Nell-1/TCP as have been observed with and bone morophogenic protein-7 (OP-1). We have performed a rat femoral onlay model to compare proinflammatory response of BMP-2 (Infuse Bone Graft) and Nell-1 within Helistate collagen sponges. While Nell-1 induced normal healing, BMP-2 induced significant amounts of swelling and histological evidence of intense inflammatory response.
Description of the DBX® Putty to be used with Nell-1/TCP
The DBX® Demineralized Bone Putty provided in the convenience kit with Nell-1/TCP is a Class III device with a pre-market approval (PMA). The common name is “Bone Void Filler Containing Human Demineralized Bone Matrix.” The product is regulated under 21 C.F.R. §888.3045 Resorbable calcium salt bone void filler device, Product Codes MQV, GXP, and MBP. MTF is the manufacturer of the DBX® Putty. This product was cleared by the FDA under 510(k) number K053218 for spine indication in December 2006.
DBX® Putty is a matrix composed of processed human cortical bone. Demineralized bone granules are mixed with sodium hyaluronate to form the DBX® Putty. Every lot of final DBX® Putty product is tested in an athymic mouse model or in an alkaline phosphatase assay, which has been shown to have a positive correlation with the athymic mouse model, to ensure the osteoinductive potential of the final product.
| 3 |
Our instructions for use will recommend use of the Nell-1/TCP Fusion Device with a lumbar (or cervical) indication. The surgeon can therefore choose to use the intervertebral fusion device that he or she is most experienced with and, in their judgment, is the best option for successful treatment. We have three precedent products, which are also osteoinductive, with intended uses similar or the same as Nell-1, that have been cleared by the FDA as medical devices. These are as follow:
| ● | Infuse Bone Graft/LT-Cage Lumbar Tapered Fusion Device (PMA)-rhBMP-2 dissolved in water and applied to a collagen (bovine type I) sponge and placed in a cage; | |
| ● | GEM 21S (PMA)-rhPDGF-BB and -tricalcium phosphate (growth factor enhanced matrix); and | |
| ● | DBX® (510k)-human cortical bone (ground and demineralized) and mixed with sodium hyaluronate to form a putty. |
Based upon extensive discussions with regulatory experts and a specific communication from the FDA in response to a submission of our plan under the Exclusive License between UCLA and Bone Biologics we believe the Nell-1 TCP Fusion Device will be regulated as a Class III medical device and will therefore require submission and approval of a pre-market approval, (“PMA”). The FDA response to the submission of our plan is: “We have determined that the product is a combination product that will be regulated under Device authorities, with CDRH (Center for Devices and Radiological Health) as the lead center.”
Our Business Strategy
Our business strategy has been to develop our target specific platform technology to meet a current established market with improvement in patient outcomes and reduction in costs to the healthcare delivery system. This narrowing of our focus from the research to the development stage is to allow for the approval for use of our target specific protein exhibiting efficacy and safety by matching or exceeding current market approved products. Identifying the best future strategic partners to facilitate the development through pre IDE, clinical, and ultimate commercialization is critical as we fund the pre-IDE work and continue achieving milestones. We believe that the licensing of the distribution of the Nell-1 product in the fields of use focused upon will generate sufficient funding to provide for the ongoing development of the Platform Technology across other surgical and therapeutic fields.
Material Agreements
UCLA Exclusive License Agreement
On March 15, 2006, Bone entered into an exclusive license agreement (the “Regents’ License”) with the Regents of the University of California Los Angeles (the “Regents”). The Regents’ License provides us with an exclusive license to several of the Regents’ patents covering, among other things, enhanced Nell-1 bone mineralization. The grant of the Regents’ License is subject to any license obligations to the U.S. government, and the term of the license lasts until the last-to-expire Regent patent licensed under the agreement expires. Under the Regents’ License, we are permitted to make, have made, use, sell, offer for sale and import any products covered by the Regents’ licensed patents in a certain field of use. By a subsequent Seventh Amendment entered into on August 7, 2012, the parties modified the applicable field of use that we are permitted to use the Regents’ patents in, which generally comprises musculoskeletal repair and regeneration, plus some related methods of manufacture. We have agreed to pay an annual maintenance fee to the Regents of $10,000 as well as to pay certain royalties to the Regents under the Regents’ License at the rate of 3.0% of net sales of licensed products. We must pay the royalties to the Regents on a quarterly basis, and we also must pay a minimum annual royalty of $25,000 to the Regents once earned royalties commence. If we are required to pay any third party any royalties as a result of us making use of the Regents’ patents, then we may reduce the royalty owed to the Regents by 0.333% for every percentage point paid to a third party. If we grant sublicensing rights to a third party to use the Regent’s patent, then we shall pay to the Regents 8.0% to 10.0% of the sublicensing income we receives from such sublicense.
By a subsequent Eighth Amendment entered into on October 22, 2013, the parties agreed that we are obligated to pay a milestone fee of 2.0% of the amount raised from the Private Placement (as defined below). Additionally, if the Private Placement does not close or is less than $2.5 million, then a fee of $100,000 will be due and paid to the Regents by June 1, 2014. Furthermore, the Agreement was modified in that we shall pay the Regents $25,000 for dosing of Phase 1 clinical trial and $50,000 for dosing of Phase 3 clinical trial. This amendment also stipulates that human clinical trials will commence no later than December 31, 2015.
Management will not commence human clinical trials before the expiration of our current license. While the Company will continue to use commercially reasonable efforts to achieve this milestone, the parties are engaged in discussions to amend the license agreement but there are no assurances that an agreement can be reached.
| 4 |
We are obligated to diligently proceed with developing and commercializing licensed products under the Regents’ patents set forth in the Regents’ License. The Regents have the right to either terminate the license or reduce the license to a non-exclusive license if we do not meet certain diligence milestone deadlines set forth in the Regents’ License.
Under a Fourth Amendment to the Regents’ License, entered into on August 19, 2009, we must reimburse or pre-pay the Regents for patent prosecution and maintenance costs incurred during the term of the Regents’ License. Bone has the right to bring infringement actions against third party infringers of the Regents’ License, the Regents may join voluntarily, at its own expense, or, at our expenses, be joined involuntarily to the action. We are required to indemnify the Regents against any third party claims arising out of our exercise of the rights under the Regents’ License or any sublicense.
Milestone Side Letter Agreement
Pursuant to a letter agreement, dated September 7, 2014, by and among AFH Advisory, Bone Biologics, and MTF (the “Milestone Side Letter Agreement”), we have agreed to use its commercially reasonable efforts to achieve the following milestones (the “Milestone Targets”) by the specified times following the closing a private placement commencing upon the closing of the Merger (the “Private Placement”):
| (i) | Complete media screening studies of cell line within two (2) to three (3) months: | |
| (ii) | Initiate manufacturing of master cell bank within three (3) to four (4) months; | |
| (iii) | Initiate formulation studies for the cGMP manufacturing process once sufficient Nell-1 material is available within approximately eight (8) to ten (10) months; | |
| (iv) | Initiate a pre-clinical bioreactor production run for toxicology material within nine (9) to twelve (12) months; | |
| (v) | Initiate pre-clinical toxicology studies to include carcinogenicity and reproductive within approximately eleven (11) to thirteen (13) months; | |
| (vi) | Finalize refinement of the manufacturing process within approximately twelve (12) to fourteen (14) months; | |
| (vii) | Initiate cGMP bioreactor run within twelve (12) to fourteen (14) months or after completion of (v), and | |
| (viii) | Request an IDE meeting to review the clinical safety plan within eighteen (18) to twenty (20) months; |
AFH Advisory and MTF will each receive restricted shares pursuant to the Milestone Targets equal to and not to exceed 2.5% of the fully diluted shares of the Company at the time of the completion of all Milestone Targets.
Our first closing on the Private Placement occurred on October 24, 2014. Management believes that it is likely the milestones will not be met within the time-frame prescribed. While the Company will continue to use commercially reasonable efforts to achieve these milestones, the parties are engaged in discussions to amend this side letter agreement but there are no assurances that an agreement can be reached.
Placement Agent Agreement
On December 12, 2013, the Company and Bone Biologics entered into an engagement letter, which engagement letter was amended on September 22, 2014, with Forefront Capital Markets, LLC (“Forefront”) a registered FINRA broker-dealer, to act as placement agent for the Private Placement and a private investment in public entity offering in an amount between $8.0 million and $10.0 million at a valuation of not less than the post-money valuation of the Company at the closing of the Private Placement through the sale of securities of the Company (the “PIPE”). Forefront shall be entitled to receive (i) a cash fee of 8% of the gross proceeds of the Private Placement, (ii) a warrant to purchase shares of the Company’s Common Stock (the “Agent Warrant”) equal to 8.0% of the Company’s Common Stock underlying the securities issued in the Private Placement, (iii) a cash fee of 3% of the gross proceeds received by the Company from any financing of non-convertible debt securities, and (iv) a warrant to purchase shares of the Company’s Common Stock (the “Advisory Warrant”) equal to 2.0% of the Company’s post-merger and financing fully diluted shares outstanding upon the closing of $2.5 million of investors on which Forefront is eligible to receive compensation. Forefront shall only be entitled to receive a management fee of 4% and a 4% Agent Warrant on the gross proceeds received from the sale of securities to investors introduced to the Company by AFH Advisory, Bone Biologics or their respective officers and directors at closing. The Agent Warrant will be issued at each closing and shall provide, among other things, that the Agent Warrant shall: (i) be exercisable at the price of the securities (or the exercise price of the securities) issued to the investors in the offering, (ii) expire five (5) years from the date of issuance, (iii) include customary registration rights, including the registration rights provided to the investors, (iv) contain provisions for cashless exercise and (v) include such other terms that are normal and customary for warrants of this type. Forefront will serve as the Company’s exclusive placement agent in connection with the Private Placement through December 31, 2014, which exclusive period may be extended to 12 months at the discretion of the Company. This agreement was extended until February 15, 2015 and is expired without renewal as of the date of this report.
| 5 |
MTF Credit Agreement & Promissory Note
Bone and MTF entered into a loan agreement in 2008 and a credit agreement in 2009 (collectively, the “MTF Credit Agreements”), and accompanying promissory and convertible promissory notes in January 2008, November 2008, March 2009 and August 2009 to fund the development of Bone. On March 31, 2014, Bone and MTF entered into the Tenth Amendment to the MTF Credit Agreements and accompanying promissory notes wherein MTF and Bone agreed that the aggregate principal amount of all advances would remain the same, but the maturity date of the notes would be extended to March 31, 2015. As of September 19, 2014, $5,192,684 in principal and interest was outstanding under the MTF Credit Agreements and $117,302 in principal and interest was outstanding under the 2013 Bridge Notes. On September 19, 2014, $1,533,356 of the amounts due under the MTF Credit Agreements were converted to shares of the Company. The remaining amounts due under the MTF Credit Agreements were cancelled and the New MTF Convertible Note (as defined herein) was issued by the Company.
In 2013, Bone and MTF also entered into a bridge note in the principal amount of $100,000. On June 6, 2014, the maturity date of the 2013 Bridge Note was extended to October 14, 2014. Prior to consummation of the Merger, the 2013 Bridge Note converted outstanding principal and accrued interest into Common Stock at a conversion price of $1.00 per share.
MTF Short Term 2014 Loan
On September 15, 2014, Bone and MTF entered into a loan agreement and accompanying promissory note (the “MTF Short Term 2014 Loan”) to fund the continued operations of Bone prior to the Merger. Pursuant to the MTF Short Term 2014 Loan, MTF has agreed to advance an initial $250,000 to Bone and, at Bone’s request and subject to the terms and conditions of the MTF Short Term 2014 Loan, to advance up to an additional $250,000 to Bone. The MTF Short Term 2014 Loan has an interest rate of eight and one-half percent (8.5%) accruing annually. The MTF Short Term 2014 Loan matures on the earlier to occur of (i) the date on which at least $1 million is loaned to or invested in the Company and (ii) December 31, 2014. In further consideration of the MTF 2014 Loan, Bone granted to MTF 625,000 warrants at a strike price of $1.62. The MTF 2014 Loan was assigned to the Company on September 19, 2014. On October 27, 2014 the balance was paid in full and the line of credit was cancelled.
New MTF Convertible Note
On September 19, 2014, the MTF 2008 and 2009 Promissory Notes and any related loan agreements, credit agreements, guarantee agreements or other agreements related to the MTF 2008 and 2009 Promissory Notes were cancelled and Bone Biologics issued MTF a convertible promissory note in the face amount of $3,659,328 (the “New MTF Convertible Note”). Pursuant to the terms of the New MTF Convertible Note, 50% of all principal and accrued and unpaid interest due under the New MTF Convertible Note will be converted into Common Stock of the Company upon the closing of the PIPE. The remainder of the New MTF Convertible Note, including all accrued and unpaid interest, will be converted upon consummation of an initial public offering of the Company.
Secured Convertible Note and Warrant
On October 24, 2014, Bone issued a convertible promissory note in the amount of $5,000,000 (the “Convertible Note”) to Hankey Capital, LLC (“Hankey Capital”). The Convertible Note matures on October 24, 2017 (the “Maturity Date”) and bears interest at an annual rate of interest of the “prime rate” (as quoted in the “Money Rates” section of The Wall Street Journal) plus 4.0%, with a minimum rate of 8.5% per annum until maturity, with interest payable monthly in arrears. Prior to the Maturity Date, Hankey Capital has a right, in their sole discretion, to convert the Convertible Note into shares of the Company’s Common Stock, at a conversion rate equal to the greater of (i) $1.58 per share or (ii) 70% of the average daily price for the Common Stock as measured over the course of the 60 day period prior to the conversion.
| 6 |
The Convertible Note is secured by certain collateral shares of Common Stock issued by the Company in the name of Hankey Capital, in such amount so as to maintain a loan to value ratio of no greater than 50% (the “Collateral”). 6,329,114 shares were issued upon closing the lending. The number of shares in the Collateral shall be adjusted on a yearly basis. The shares representing the Collateral contain a restrictive legend. The Company shall seek to register the Collateral shares initially delivered on the date of the Convertible Note pursuant to the Registration Rights Agreement described below. Upon the effectiveness of such Registration Statement, the Company will remove the restrictive legends from the Collateral shares so long as Hankey Capital agrees in any event not to sell any Collateral shares if Hankey Capital is notified that the Registration Statement is no longer effective. Hankey Capital may hold the Collateral in any brokerage account of its choosing, but shall not transfer, sell or otherwise dispose of any Collateral, except during the existence of an Event of Default, as defined in the Convertible Note. The Convertible Note is further secured by collateral assignments of all the Company’s license agreements.
The principal amount of the loan is pre-payable in whole or in part at any time, without premium or penalty. Upon any voluntary partial prepayment of outstanding principal, Hankey Capital shall return Collateral shares to the Company in the amount necessary, if any, to maintain the loan to value ratio at no less than 50%. Upon a full payment of the outstanding principal, all Collateral shares shall be returned return and cancelled. Hankey Capital shall also return Collateral shares under the same terms in case of partial or full conversion of the Convertible Note.
The Company paid a commitment fee in the amount of 3.0% of the original principal amount of the loan ($150,000) to Hankey Capital. The Company intends to use the proceeds of the Convertible Note for working capital and general corporate purposes.
On October 24, 2014, the Company also issued a warrant to Hankey Capital for 3,955,697 shares of Common Stock at an exercise price per share of $1.58. The Warrant will expire on October 24, 2017. The Warrant also includes such other terms that are normal and customary for warrants of this type.
Registration Rights Agreement
On October 24, 2014, the Company entered into a Registration Rights Agreement with Hankey Capital, for certain demand registration rights and unlimited piggyback registration rights for the shares underlying the Convertible Note and the Warrant, and subject to an agreed lock up period. Pursuant to the Registration Rights Agreement, Hankey Capital may at any time request registration of their registrable shares. Within 30 days of such demand, the Company will provide written notice of such request to all other holders of registrable securities and will include in such registration all registrable shares with respect to which the Company has received written requests for inclusion within twenty-five (25) days after delivery of the Company’s notice. The Company has agreed to pay all registration expenses relating to up to three long-form registrations or short-form registrations for Hankey Capital.
Whenever the Company proposes to register any of its securities under the Securities Act (other than pursuant to a demand registration under the Registration Rights Agreement) and the registration form to be used may be used for the registration of any registrable shares, the Company will give prompt written notice to all holders of the registrable shares of its intention to effect such a registration and will include in such registration all registrable shares (in accordance with the priorities set forth in the Registration Rights Agreement) with respect to which the Company has received written requests for inclusion within fifteen (15) days after the delivery of the Company’s notice. Pursuant to Registration Rights Agreement, holders of registrable shares and the Company agree not to effect any public sale or distribution of equity securities of the Company, or any securities convertible into or exchangeable or exercisable for such securities, during the six (6) months following, the effective date of the Company’s merger with Bone Biologics, Inc. on September 19, 2014.
| 7 |
Competition
Our most significant competitor is Infuse™ Bone Graft or BMP-2 (bone morphogenic protein 2) from Medtronics. Despite Medtronics dominant market position, it is suffering from bad press related to negative off label cervical fusion outcomes due to inflammatory response. Bone believes that BMP-2 also suffers from disadvantageous margins due, to an unfavorable revenue sharing agreement with Wyeth. We believe that our product will not suffer from these same negative factors as, to date, our products have not had inflammatory response issues and we are not burdened by an unfavorable revenue sharing agreement. A second potential competitor was OP-1 or BMP-7 from Stryker and sold to Olympus, which has had significant regulatory setbacks long delaying time to market beyond humanitarian use.
Customers
The customers for the product we are developing are the acute care hospitals performing spinal fusion and long bone non-union fracture repair and regeneration. This universe of customers has been identified by Medtronic, with their bone growth product Infuse Bone Graft which is a bone morphogenic protein. Medtronic’s market share at its highest level was greater than $800 million. FDA approval pathways, reimbursement pathways, and procedure acceptance by surgeons has been established by the Medtronic product. This does not provide any assurance that the Company will be approved by the FDA on the same pathway, reimbursed by payors comparably, and accepted by hospitals and surgeons as an alternative to Medtronic or any of the less efficacious modalities of therapy. Medrontic has experienced difficulties in this market from FDA questions relative to off label use, payors on reimbursement rates, and hospitals on procedural cost which create an environment that could be unfavorable to the Company achieving current forecasts for approval, commercialization, and revenue.
Intellectual Property
We have an intellectual property portfolio that includes exclusive, worldwide licenses from UCLA which we believe constitute a formidable barrier to entry.
Additional patent applications are currently in preparation. The intellectual property is unique and comprehensively covers Nell-1 manufacture, Nell-1 compositions and Nell-1 use in wide ranging clinical and diagnostic applications. We protect our proprietary technology through all mechanisms including U.S. and foreign patent filings, trade secret protections, and collaboration agreements with domestic and international corporations, universities and research institutions. We are the exclusive licensee for the following twelve (12) UCLA issued patents:
| U.S. Patent No. | Summary | Date Issued | ||
| 7052856 | NELL-1 Enhanced Bone Mineralization | 5/20/2006 | ||
| 7544486 | NELL-1 Peptide Expression Systems | 6/9/2009 | ||
| 7687462 | Composition for promoting Cartilage | 3/30/2010 | ||
| 7691607 | Expression system of NELL peptide | 4/6/2010 | ||
| 7776361 | NELL-1 Enhanced Bone Mineralization | 8/17/2010 | ||
| 7807787 | NELL-1 Peptide | 10/5/2010 | ||
| 7833968 | Pharmaceutical compositions for treating or preventing bone conditions | 11/16/2010 | ||
| 7844066 | Nell-1 Enhanced Bone Minerilization | 2/8/2011 | ||
| 8044026 | Composition for promoting cartilage | 10/25/2011 | ||
| 8048646 | NELL-1 peptide expression systems | 11/1/2011 | ||
| 8053412 | NELL-1 Peptides | 11/8/2011 | ||
| 8207120 | Nell-1 Enhanced Bone Mineralization | 6/26/2012 |
| 8 |
Government Regulation
The manufacturing and marketing of any product which we may formulate with our technologies as well as our related research and development activities are subject to regulation for safety, efficacy and quality by governmental authorities in the U.S. and other countries. We anticipate that these regulations will apply separately to each biotechnology product. Bone believes that complying with these regulations will involve a considerable level of time, expense and uncertainty.
In the U.S., drugs are subject to rigorous federal regulation and, to a lesser extent, state regulation. The Federal Food, Drug and Cosmetic Act, as amended, and the regulations promulgated thereunder, and other federal and state statutes and regulations govern, among other things, the testing, manufacture, safety, efficacy, labeling, storage, record keeping, approval, advertising and promotion of Bone’s products. Drug development and approval within this regulatory framework is difficult to predict, requires a number of years and involves the expenditure of substantial resources. Moreover, ongoing legislation by U.S. Congress and rule making by the FDA presents an ever-changing landscape where we could be required to undertake additional activities before any governmental approval is granted allowing us to market our products. The steps required before a pharmaceutical agent may be marketed in the U.S. include:
| ● | Laboratory and non-clinical tests for safety and small scale manufacturing of the agent; | |
| ● | The submission to the FDA of an IDE which must become effective before human clinical trials can commence; | |
| ● | Clinical trials to characterize the efficacy and safety of the product in the intended patient population; | |
| ● | The submission of a New Drug Application (“NDA”) or PMA to the FDA; and | |
| ● | FDA approval of the NDA or PMA prior to any commercial sale or shipment of the product. |
In addition to obtaining FDA approval for each product, each manufacturing establishment must be registered with, and approved by, the FDA. Moreover, manufacturing establishments are subject to biennial inspections by the FDA and must comply with the FDA’s Good Manufacturing Practices for products, drugs and devices.
Non-clinical Trials
Non-clinical testing includes laboratory evaluation of chemistry and formulation as well as tissue culture and animal studies to assess the safety and potential efficacy of the product. Non-clinical safety tests must be conducted by laboratories that comply with FDA regulations regarding good laboratory practices. Non-clinical testing is inherently risky and the results can be unpredictable or difficult to interpret. The results of non-clinical testing are submitted to the FDA as part of an IDE and are reviewed by the FDA prior to the commencement of clinical trials. Unless the FDA objects to an IDE, clinical studies may begin 30 days after the IDE is submitted. We have relied and intend to continue to rely on third-party contractors to perform non-clinical trials.
Clinical Trials
Clinical trials involve the administration of the investigational product to healthy volunteers or to patients under the supervision of a qualified investigator. Clinical trials must be conducted in accordance with good clinical practices under protocols that detail the objectives of the study, the parameters to be used to monitor safety and the efficacy criteria to be evaluated. Each protocol must be submitted to the FDA prior to its conduct. Further, each clinical study must be conducted under the auspices of an independent institutional review board. The institutional review board will consider, among other things, ethical factors, the safety of human subjects and the possible liability of the institution. The drug product used in clinical trials must be manufactured according to the FDA’s Good Manufacturing Practices.
| 9 |
Clinical trials under IDE regulations are typically conducted in two sequential trials. In the Pilot trial, the initial introduction of the product into healthy human subjects, the drug is tested for safety (adverse side effects), absorption, metabolism, bio-distribution, excretion, food and drug interactions, abuse as well as limited measures of pharmacologic effect and proof of principle that involves studies in a limited patient population in order to:
| ● | assess the potential efficacy of the product for specific, targeted indications; | |
| ● | demonstrate efficacy in a limited patient population; | |
| ● | identify the range of doses likely to be effective for the indication; and | |
| ● | identify possible adverse events and safety risks. |
When there is evidence that the product may be effective and has an acceptable safety profile in Pilot evaluations, Pivotal trials are undertaken to establish and confirm the clinical efficacy and establish the safety profile of the product within a larger population at geographically dispersed clinical study sites. Pivotal trials frequently involve randomized controlled trials and, whenever possible, studies are conducted in a manner so that neither the patient nor the investigator knows what treatment is being administered. The Company, or the FDA, may suspend clinical trials at any time if it is believed that the individuals participating in such trials are being exposed to unacceptable health risks. We intend to rely upon third-party contractors to advise and assist us in the preparation of our IDEs and the conduct of clinical trials that will be conducted under the IDEs.
Premarket Approval and FDA Approval Process
The results of the manufacturing process, development work, non-clinical studies and clinical studies are submitted to the FDA in the form of a PMA prior to marketing and selling the product. The testing and approval process is likely to require substantial time and effort. In addition to the results of non-clinical and clinical testing, the PMA applicant must submit detailed information about chemistry, manufacturing and controls that will describe how the product is made and tested through the manufacturing process.
The PMA review process involves FDA investigation into the details of the manufacturing process, as well as the design and analysis of each of the non-clinical and clinical studies. This review includes inspection of the manufacturing facility, the data recording process for the clinical studies, the record keeping at a sample of clinical trial sites and a thorough review of the data collected and analyzed for each non-clinical and clinical study. Through this investigation, the FDA reaches a decision about the risk-benefit profile of a product candidate. If the benefit is worth the risk, the FDA begins negotiating with the company about the content of an acceptable package insert and associated Risk Evaluation and Mitigation Strategies (“REMS”), if required.
The approval process is affected by a number of factors, including the severity of the disease, the availability of alternative treatments and the risks and benefits demonstrated in clinical trials. Consequently, there is a risk that approval may not be granted on a timely basis, if at all. The FDA may deny a PMA if applicable regulatory criteria are not satisfied, require additional testing or information or require post-marketing testing (Phase 4) and surveillance to monitor the safety of a company’s product if it does not believe the PMA contains adequate evidence of the safety and efficacy of the product. Moreover, if regulatory approval of a product is granted, such approval may entail limitations on the indicated uses for which it may be marketed. Finally, product approvals may be withdrawn if compliance with regulatory standards is not maintained or health problems are identified that would alter the risk-benefit analysis for the product. Post-approval studies may be conducted to explore the use of the product for new indications or populations such as pediatrics.
Among the conditions for PMA approval is the requirement that any prospective manufacturer’s quality control and manufacturing procedures conform to the FDA’s Good Manufacturing Practices and the specifications approved in the PMA. In complying with standards set forth in these regulations, manufacturers must continue to expend time, money and effort in the area of product and quality control to ensure full technical compliance. Manufacturing establishments, both foreign and domestic, also are subject to inspections by or under the authority of the FDA and by other federal, state or local agencies. Additionally, in the event of non-compliance, FDA may issue warning letters and/or seek criminal and civil penalties, enjoin manufacture, seize product or revoke approval.
| 10 |
International Approval
Whether or not FDA approval has been obtained, approval of a product by regulatory authorities in foreign countries must be obtained prior to the commencement of commercial sales of the drug in such countries. The requirements governing the conduct of clinical trials and drug approvals vary widely from country to country, and the time required for approval may be longer or shorter than that required for FDA approval. Although there are some procedures for unified filings for certain European countries, in general, each country at this time has its own procedures and requirements.
Other Regulation
In addition to regulations enforced by the FDA, we are also subject to U.S. regulation under the Controlled Substances Act, the Occupational Safety and Health Act, the Environmental Protection Act, the Toxic Substances Control Act, the Resource Conservation and Recovery Act and other present and potential future federal, state, local or similar foreign regulations. Our research and development may involve the controlled use of hazardous materials, chemicals and radioactive compounds. Although we believe that its safety procedures for handling and disposing of such materials comply with the standards prescribed by state and federal regulations, the risk of accidental contamination or injury from these materials cannot be completely eliminated. In the event of any accident, we could be held liable for any damages that result and any such liability could exceed our resources.
Employees
As of the date hereof, we have one full-time employee working for us as our President and Chief Technology Officer (“CTO”) and one half-time employee working as our Chief Financial Officer.
Strategic Partners
Musculoskeletal Transplant Foundation (MTF)
We have formed a formal strategic alliance with MTF on the collaborative development of osteoinductive products that incorporate MTF’s current product line of natural bone graft substitutes with Nell-1. MTF is the exclusive allograft supplier for BIOBONE-X™. MTF has become one of the major investors of the Company. MTF is the world’s largest allograft bone supplier. It is also the country’s largest full service tissue organization dedicated to providing quality tissue through a commitment to excellence in education, research, recovery and care for recipients, donors and their families. A not-for-profit organization, MTF is a consortium of academic medical institutions and organ and tissue recovery organizations across the country. We anticipate that MTF, with its proven ISO 9001 manufacturing and packaging of FDA approved osteogenic carriers, will significantly accelerate the clinical development cycle of Nell-1 related products.
Katayama Chemical Industries Co., Ltd.
Katayama Chemical Industries Co., Ltd. (“KCI”), based in Osaka, Japan, was founded in 1918. KCI focuses on the production of OEM laboratory products for many distributors such as Amersham Biosciences, Millipore, and Sigma-Aldrich Japan, the exclusive Japanese distributor for laboratory products manufactured by KCI. Under a strategic partnership with the Company, KCI is seeking to develop clinical diagnostic reagents related to bone metabolism and regeneration. KCI produced the Nell-1 protein in an insect cell line that was utilized in development work and for proof of concept validation in rodent models and large animal (sheep) spinal fusion trials.
| 11 |
The Merger and Related Transactions
At the effective time of the Merger (“Effective Time”), all of the issued and outstanding shares of Bone Biologics’ $0.0001 par value common stock (“Bone Biologics Common Stock”) converted into a combined total of 19,897,587 shares of the Company’s Common Stock (including 2,151,926 shares issuable upon the exercise of outstanding warrants and 5,648,658 shares issuable upon the conversion of debt). In exchange, Bone Biologics agreed to pay AFH Holding & Advisory, LLC (“AFH Advisory”) the principal sum of $590,000. On July 3, 2014, Bone Biologics paid AFH Advisory $250,000 of such amount and on July 31, 2014, Bone Biologics issued that certain Promissory Note, dated July 31, 2014 (the “Note”), pursuant to which the Bone Biologics promised to pay AFH Advisory the principal sum of $340,000. MTF has granted AFH Advisory a standby letter of credit in the amount of $340,000 for the remaining amount due under the Note. On September 19, 2014, the Note was assigned to the Company. On October 27, 2014, the Company paid the remaining outstanding balance on the Note and the letter of credit was released.
MTF converted all amounts due, $1,533,356, pursuant to a convertible promissory note dated January 18, 2008 in the original face amount of $1,107,000 entered into by and between the Bone Biologics and MTF prior to consummation of the Merger to shares of Series B Preferred Stock of Bone Biologics at $1.00 per share, then to shares of Bone Biologics Common Stock at a 1:1 basis. MTF agreed to execute any additional agreements reasonably necessary to give effect to that provision. Prior to the Merger, MTF converted all of the outstanding shares of Series A Preferred Stock and Series B preferred stock of Bone Biologics that MTF held into shares of Bone Biologics Common Stock.
Bridge Financing
Bone Biologics borrowed $400,000 pursuant to the sale and issuance of convertible promissory notes (the “Bridge Notes,” or singularly each a “Bridge Note”) and warrants to purchase Common Stock of the Company, as the successor to Bone Biologics. Prior to consummation of the Merger, the 2013 Bridge Note holders converted outstanding principal and accrued interest of $455,974 into Common Stock at a conversion price of $1.00 per share. MTF has purchased $100,000 of the Notes and Warrants. Orthofix Holdings Inc. (“Orthofix”) has purchased $250,000 of the Notes and Warrants. AFH Advisory purchased $50,000 of the Bridge Notes and Bridge Warrants which was contingent upon liquidation of the securities transferred to the Company by AFH Advisory, as described in that certain Letter Agreement, dated September 26, 2013, by and between Amir F. Heshmatpour and the Company. The issuance of the Bridge Notes and Bridge Warrants are collectively referred to as the “Bridge Financing.”
Bone entered into a Security Agreement, dated March 17, 2009, wherein it pledged certain of its assets as collateral to the Bridge Note holders. Additionally, Bone and MTF entered into a Subordination Agreement, dated April 2013, wherein the parties agreed that the security interest granted to MTF pursuant to the March 17, 2009 Security Agreement between the parties wherein MTF agreed to subordinate any rights to any payment and any security interest it may have in Bone’s assets to the holders of the Bridge Notes. In addition, Benjamin Wu, Kang Ting, and Chia Soo executed a Pledge and Guarantee Agreement, dated April 18, 2013, in favor of the Bridge Note purchasers wherein Benjamin Wu, Kang Ting, and Chia Soo pledged their shares of Bone Biologics Common Stock to secure the full and punctual payment of Bone’s obligations to the Bridge Note holders and unconditionally and irrevocably guaranteed such payment. Upon consummation of the Merger, the notes converted into notes of the Company.
In addition to and subsequent to the Bridge Notes and Bridge Warrants discussed in the preceding paragraph, Orthofix, on July 1, 2014, also (A) purchased $500,000 worth of Bone Biologics Common Stock (the “Subsequent Orthofix Shares”); (B) was issued two convertible promissory notes (the “Subsequent Orthofix Convertible Promissory Notes”), each in the principal amount of $250,000 and exercisable for $333,333 worth of Bone Biologics Common Stock; and (C) was issued two warrants (the “Subsequent Orthofix Warrants”), each exercisable for 166,667 shares of Bone Biologics Common Stock at an exercise price per share of $1.50. Upon subscribing for the Subsequent Orthofix Shares, the Subsequent Orthofix Convertible Promissory Notes converted by its terms into a combined total of $666,666 worth of shares of Bone Biologics Common Stock in accordance with the terms of the Subsequent Orthofix Convertible Promissory Notes. The Subsequent Orthofix Warrants converted into warrants of the Company with substantially identical terms upon consummation of the Merger. Amounts received by Bone Biologics in connection with the Subsequent Orthofix Convertible Promissory Notes and the Subsequent Orthofix Shares will be aggregated towards the $5 million amount to be raised in the Private Placement for purposes of determining when various parties will be paid their fees in connection with the Merger and the Private Placement.
| 12 |
Hankey Capital Financing
On October 24, 2014, the Company issued a convertible promissory note in the amount of $5,000,000 (the “Convertible Note”) to Hankey Capital, LLC (“Hankey Capital”). The Convertible Note matures on October 24, 2017 (the “Maturity Date”) and bears interest at an annual rate of interest at the “prime rate” (as quoted in the “Money Rates” section of The Wall Street Journal) plus 4.0%, with a minimum rate of 8.5% per annum until maturity, with interest payable monthly in arrears. Prior to the Maturity Date, Hankey Capital has a right, in their sole discretion, to convert the Convertible Note into shares of the Company’s Common Stock, at a conversion rate equal to the greater of (i) $1.58 per share and (ii) 70% of the average daily price for the Common Stock as measured over the course of the 60 day period prior to the conversion. The Convertible Note is secured by certain collateral shares of Common Stock issued by the Company in the name of Hankey Capital, in such amount so as to maintain a loan to value ratio of no greater than 50% (the “Collateral”). The number of shares in the Collateral shall be adjusted on a yearly basis. The Company shall seek to register the Collateral shares initially delivered on the date of the Convertible Note pursuant to the Registration Rights Agreement described below. The Convertible Note is further secured by collateral assignments of all the Company’s license agreements. The principal amount of the loan is prepayable in whole or in part at any time, without premium or penalty. Upon any voluntary partial prepayment of outstanding principal. Hankey Capital shall return Collateral shares to the Company in the amount necessary, if any, to maintain the loan to value ratio at no less than 50%. Upon a full payment of the outstanding principal, all Collateral shares shall be returned and cancelled. Hankey Capital shall also return Collateral shares under the same terms in case of partial or full conversion of the Convertible Note. Simultaneously, the Company also issued a warrant to Hankey Capital for 3,955,697 shares of Common Stock at an exercise price per share of $1.58. The Warrant will expire on October 24, 2017. The Warrant includes provisions for cashless exercise and also includes such other terms that are normal and customary for warrants of this type.
In connection with issuing the Convertible Note and the Warrant, the Company entered into a Registration Rights Agreement with Hankey Capital, for certain demand registration rights and unlimited piggyback registration rights for the shares underlying the Convertible Note and the Warrant, and subject to an agreed lock up period. Pursuant to the Registration Rights Agreement, Hankey Capital may at any time request registration of their registrable shares. Pursuant to Registration Rights Agreement, holders of registrable shares and the Company agree not to effect any public sale or distribution of equity securities of the Company, or any securities convertible into or exchangeable or exercisable for such securities, during the six (6) months following, the effective date of the Company’s merger with Bone Biologics, Inc. on September 19, 2014.
Not applicable.
Item 1B. Unresolved Staff Comments
None.
Our primary office, including administrative and laboratory space, is located at 175 May Street, Suite 400, Edison, NJ 08837. We also lease office space located at 26632 Towne Centre Drive, Suite 300, Foothill Ranch, CA 92610.
In the normal course of our business, Bone may periodically become subject to various lawsuits. However, there are currently no legal actions pending against us or, to our knowledge, are any such proceedings contemplated.
On January 24, 2007, Bone entered into a Biopharmaceutic Services Agreement with Cytovance, Inc. to provide certain services including the production of NELL protein in mammallian cells. In January 2008, Bone terminated the agreement based upon Cytovance’s alleged breach of contract. On July 31, 2008, Cytovance commenced legal action in the District Court of Oklahoma County State of Oklahoma against Bone for breach of contract, but the action was subsequently dismissed as the parties had initially agreed contractually to resolve all disputes through mediation. Bone alleges that, as a result of Cytovance’s breach of contract, the company is owed more than $150,000 for damages. Bone has attempted to amicably resolve this matter with a settlement offer made on April 15, 2009 but the matter has remained unresolved without further communication.
Item 4. Mine Safety Disclosures
Not applicable.
| 13 |
Item 5. Market for Registrant’s Common Equity, Related Stockholder Matters, and Issuer Purchases of Equity Securities
Market.
There is presently no public market for our Common Stock, although we plan to apply for quotation of our Common Stock on the on the Over-the-Counter Bulletin Board.
Holders.
As of March 30, 2015, there are approximately 27 record holders of 24,269,047 shares of Common Stock.
Dividends.
To date, we have paid no cash dividends on our Common Stock. For the foreseeable future, earnings generated from our operations will be retained for use in our business and not to pay dividends.
Securities Authorized for Issuance under Equity Compensation Plans
2014 Stock Option Plan
2,642,898 shares of our Common Stock have been initially authorized and reserved for issuance under our 2014 Stock Plan as option awards. This reserve may be increased by the Board on each anniversary of Jan 1, 2015, through January 1, 2024 by up to the number of shares of stock equal to 5% of the number of shares of stock issued and outstanding on the immediately preceding December 31. Appropriate adjustments will be made in the number of authorized shares and other numerical limits in our 2014 Stock Option Plan and in outstanding awards to prevent dilution or enlargement of participants’ rights in the event of a stock split or other change in our capital structure. Shares subject to awards granted under our 2014 Stock Option Plan which expire, are repurchased or are cancelled or forfeited will again become available for issuance under our 2014 Stock Option Plan. The shares available will not be reduced by awards settled in cash. Shares withheld to satisfy tax withholding obligations will not again become available for grant. The gross number of shares issued upon the exercise of stock appreciation rights or options exercised by means of a net exercise or by tender of previously owned shares will be deducted from the shares available under our 2014 Stock Option Plan.
Awards may be granted under our 2014 Stock Option Plan to our employees, including officers, director or consultants, and our present or future affiliated entities. While we may grant incentive stock options only to employees, we may grant non-statutory stock options, stock appreciation rights, restricted stock purchase rights or bonuses, restricted stock units, performance shares, performance units and cash-based awards or other stock based awards to any eligible participant.
The 2014 Stock Option Plan is administered by our compensation committee. Subject to the provisions of our 2014 Stock Option Plan, the compensation committee determines, in its discretion, the persons to whom, and the times at which, awards are granted, as well as the size, terms and conditions of each award. All awards are evidenced by a written agreement between us and the holder of the award. The compensation committee has the authority to construe and interpret the terms of our 2014 Stock Option Plan and awards granted under our 2014 Stock Option Plan.
During the year ended December 31, 2014 and 2013, the Company had stock-based compensation expense of $323,510 and $-0-, respectively, related to issuances to the Company’s employees and directors, included in reported net loss. The total amount of stock-based compensation for the year ended December 31, 2014 and 2013, related solely to the issuance of stock options.
| 14 |
| Plan category | Number of securities to be issued upon exercise of outstanding options, warrants and rights (a) | Weighted-average exercise price of outstanding options, warrants and rights (b) | Number of securities remaining available for future issuance under equity compensation plans (excluding securities reflected in column (a)) (c) | |||||||||
| Equity compensation plans approved by security holders | ||||||||||||
| Equity compensation plans not approved by security holders | 757,977 | $ | 1.00 | 1,884,921 | ||||||||
| Total | 757,977 | $ | 1.00 | 1,884,921 | ||||||||
Purchases of Equity Securities by the Issuer and Affiliated Purchasers
None
Item 6. Selected Financial Data
Not applicable.
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations
Overview
We are a biotechnology company that is currently focused on bone regeneration in spinal fusion using the recombinant human protein, known as Nell-1. The Nell-1 protein is an osteoinductive recombinant protein that provides target specific control over bone regeneration. The protein, as part of the UCB-1 technology platform has been licensed exclusively for worldwide applications to Bone through a technology transfer from UCLA. UCLA and Bone received guidance from the FDA that Nell-1 will be classified as a combination product with a device lead.
We are a development stage entity. The production and marketing of our products and ongoing research and development activities will be subject to extensive regulation by numerous governmental authorities in the United States. Prior to marketing in the United States, any combination product developed by us must undergo rigorous preclinical (animal) and clinical (human) testing and an extensive regulatory approval process implemented by the FDA under the Food, Drug and Cosmetic Act. There can be no assurance that we will not encounter problems in clinical trials that will cause us or the FDA to delay or suspend the clinical trial.
Our success will depend in part on our ability to obtain patents and product license rights, maintain trade secrets, and operate without infringing on the proprietary rights of others, both in the United States and other countries. There can be no assurance that patents issued to or licensed by us will not be challenged, invalidated, or circumvented, or that the rights granted thereunder will provide proprietary protection or competitive advantages to us.
UCLA Exclusive License Agreement
On March 15, 2006, Bone entered into an exclusive license agreement (the “Regents’ License”) with the Regents of the University of California (the “Regents”). The Regents’ License provides us with an exclusive license to several of the Regents’ patents covering, among other things, enhanced Nell-1 bone mineralization. The grant of the Regents’ License is subject to any license obligations to the U.S. government, and the term of the license lasts until the last-to-expire Regent patent licensed under the agreement expires. Under the Regents’ License, we are permitted to make, have made, use, sell, offer for sale and import any products covered by the Regents’ licensed patents in a certain field of use. By a subsequent Seventh Amendment entered into on August 7, 2012, the parties modified the applicable field of use that we are permitted to use the Regents’ patents in, which generally comprises musculoskeletal repair and regeneration, plus some related methods of manufacture. We have agreed to pay an annual maintenance fee to the Regents of $10,000 as well as to pay certain royalties to the Regents under the Regents’ License at the rate of 3.0% of net sales of licensed products. We must pay the royalties to the Regents on a quarterly basis, and we also must pay a minimum annual royalty of $25,000 to the Regents once earned royalties commence. If we are required to pay any third party any royalties as a result of us making use of the Regents’ patents, then we may reduce the royalty owed to the Regents by 0.333% for every percentage point paid to a third party. If we grant sublicensing rights to a third party to use the Regent’s patent, then we shall pay to the Regents 8.0% to 10.0% of the sublicensing income we receives from such sublicense.
By a subsequent Eighth Amendment entered into on October 22, 2013, the parties agreed that we are obligated to pay a milestone fee of 2.0% of the amount raised from the Private Placement (as defined below). Additionally, if the Private Placement does not close or is less than $2.5 million, then a fee of $100,000 will be due and paid to the Regents by June 1, 2014. Furthermore, the Agreement was modified in that we shall pay the Regents $25,000 for dosing of Phase 1 clinical trial and $50,000 for dosing of Phase 3 clinical trial. This amendment also stipulates that human clinical trials will commence no later than December 31, 2015. Management will not commence human clinical trials before the expiration of our current license. While the Company will continue to use commercially reasonable efforts to achieve this milestone, the parties are engaged in discussions to amend the license agreement but there are no assurances that an agreement can be reached.
We are obligated to diligently proceed with developing and commercializing licensed products under the Regents’ patents set forth in the Regents’ License. The Regents have the right to either terminate the license or reduce the license to a non-exclusive license if we do not meet certain diligence milestone deadlines set forth in the Regents’ License.
Under a Fourth Amendment to the Regents’ License, entered into on August 19, 2009, we must reimburse or pre-pay the Regents for patent prosecution and maintenance costs incurred during the term of the Regents’ License. Bone has the right to bring infringement actions against third party infringers of the Regents’ License, the Regents may join voluntarily, at its own expense, or, at our expenses, be joined involuntarily to the action. We are required to indemnify the Regents against any third party claims arising out of our exercise of the rights under the Regents’ License or any sublicense.
Recapitalization
We were incorporated under the laws of the State of Delaware on October 18, 2007 as AFH Acquisition X, Inc. Through a reverse merger in September 2014 (the “Merger”), the Company acquired its operating subsidiary Bone Biologics, Inc. Upon the consummation of the Merger, the Company officially changed its name to “Bone Biologics, Corp.” to more accurately reflect the nature of its business, and Bone Biologics, Inc. became a wholly-owned subsidiary of the Company. Bone Biologics, Inc. was incorporated in California on March 9, 2004.
| 15 |
In connection with the Merger, the 5,000,000 outstanding shares of Common Stock of the Company prior to the Merger were consolidated into 3,853,600 shares of Common Stock and the remaining shares were cancelled.
Additionally, all of the issued and outstanding shares of Bone Biologics Inc.’s $0.0001 par value common stock converted into a combined total of 19,897,587 shares of the Company’s Common Stock (including 2,151,926 shares issuable upon the exercise of outstanding warrants and 5,648,658 shares issuable upon the conversion of debt). In exchange, Bone Biologics, Inc. agreed to pay AFH Holding & Advisory, LLC (“AFH Advisory”), former majority shareholder of AFH Acquisition X, Inc., the principal sum of $590,000.
Results of Operations
Since our inception, we devoted substantially all of our efforts and funding to the development of the Nell-1 protein and raising capital. We have not yet generated revenues from our planned operations.
Year ended December 31, 2014 compared to the year ended December 31, 2013
| Year ended December 31, 2014 | Year ended December 31, 2013 | % Change | ||||||||||
| Operating expenses | ||||||||||||
| Research and development | $ | 623,522 | $ | 188,236 | 231.24 | % | ||||||
| General and administrative | 1,509,306 | 483,749 | 212.00 | % | ||||||||
| Transaction costs | 877,776 | 0 | - | |||||||||
| Total operating expenses | 3,010,604 | 671,985 | 348.02 | % | ||||||||
| Loss from operations | (3,010,604 | ) | (671,985 | ) | 348.02 | % | ||||||
| Other expense | (9,624 | ) | 0 | - | ||||||||
| Interest expense, net | (1,402,585 | ) | (409,419 | ) | 242.58 | % | ||||||
| Total other income/expense | (1,412,209 | ) | (409,419 | ) | 244.93 | % | ||||||
| Loss before provision for income taxes | (4,422,813 | ) | (1,081,404 | ) | 308.99 | % | ||||||
| Provision for income taxes | 1,600 | 800 | 100.00 | % | ||||||||
| Net loss | $ | (4,424,413 | ) | $ | (1,082,204 | ) | 308.83 | % | ||||
Research and Development
Our research and development expenses increased from $188,236 during the year ended December 31, 2013 to $623,522 during the year ended December 31, 2014. The $435,286 increase was related to patent costs and development activities for our lead product Nell-1. We will continue to incur significant expenses for development activities for Nell-1. Additionally we incurred increased wages for our President/Chief Technology Officer who became full-time in September 2014. We also incurred $199,585 of stock based compensation costs related to the issuance of stock options to our President/Chief Technology Officer.
General and Administrative
Our general and administrative expenses increased from $483,749 during the year ended December 31, 2013 to $1,509,306 during the year ended December 31, 2014. The $1,025,557 increase was driven by increased expenses for legal, accounting and consulting professional services related to the merger and SEC reporting requirements and also attributable to the fair value of warrants issued to consultants and stock options issued to our CFO totaling $435,922.
Transaction Costs
Transaction costs are expenses associated with our recapitalization which include accounting, legal and other professional services and the $590,000 fee paid to AFH Holding & Advisory.
| 16 |
Interest Expense
Our net interest expense increased from $409,419 for the year ended December 31, 2013 to $1,402,585 during the year ended December 31, 2014. The increase in expenses of $993,166 was due to interest expense on our related party promissory notes, bridge financing and the amortization of debt discounts.
Liquidity and Capital Resources
| December 31, 2014 | December 31, 2013 | |||||||
| Assets | ||||||||
| Current assets | ||||||||
| Cash | $ | 2,661,396 | $ | 1,538 | ||||
| Prepaid expenses | 89,517 | 10,767 | ||||||
| Deferred transaction costs | - | 75,000 | ||||||
| Deferred financing fees | 983,857 | - | ||||||
| Other receivable – related party | 75,000 | - | ||||||
| Total current assets | 3,809,770 | 87,305 | ||||||
| Property and Equipment, net | 11,621 | - | ||||||
| Total assets | $ | 3,821,391 | $ | 87,305 | ||||
| Liabilities and Stockholders’ Deficit | ||||||||
| Current liabilities | ||||||||
| Accounts payable and accrued expenses | $ | 215,389 | $ | 1,525,604 | ||||
| Advances due to related party | - | 41,300 | ||||||
| Notes payable to related party | 3,659,328 | 3,947,817 | ||||||
| Notes payable, net of debt discount | - | 180,690 | ||||||
| Total current liabilities | 3,874,717 | 5,695,411 | ||||||
| Notes payable, net of debt discount | 3,645,194 | - | ||||||
| Total liabilities | 7,519,911 | 5,695,411 | ||||||
| Commitments and Contingencies | ||||||||
| Stockholders’ deficit | ||||||||
| Preferred Stock, $0.001 par value per share; 20,000,000 shares authorized; none issued or outstanding at December 31, 2014 and 2013 | - | - | ||||||
| Common stock, $0.001 par value per share; 100,000,000 shares authorized; 24,269,047 and 10,928,099 shares issued and outstanding at December 31, 2014 and 2013, respectively | 24,269 | 10,928 | ||||||
| Additional paid-in capital | 8,315,128 | 1,994,470 | ||||||
| Accumulated deficit | (12,037,917 | ) | (7,613,504 | ) | ||||
| Total stockholders’ deficit | (3,698,520 | ) | (5,608,106 | ) | ||||
| Total liabilities and stockholders’ deficit | $ | 3,821,391 | $ | 87,305 | ||||
We have no significant operating history and, from our inception to December 31, 2014, have generated a net loss of approximately $12 million. The financial statements for the year ended December 31, 2014 and 2013 were prepared assuming we will continue as a going concern. Operating expenditures for the next twelve months are estimated at $3.6 million. The Company has no principal payment requirements for the next 12 months
The Company has no significant operating history and, from March 9, 2004 (inception) to December 31, 2014, has generated a net loss of approximately $12 million. The Company will continue to incur significant expenses for development activities for their lead product Nell-1. The Company’s December 31, 2014 audited financial statements contained a notation by our auditors regarding the Company’s ability to continue as a going concern. The accompanying consolidated financial statements for the year ended December 31, 2014, have been prepared assuming the Company will continue as a going concern. In connection with the LOI (see financial statements Note 4), management intends to raise additional debt and/or equity financing to fund future operations and to provide additional working capital. However, there is no assurance that such financing will be consummated or obtained in sufficient amounts necessary to meet the Company’s needs.
As of December 31, 2014 and 2013, we had cash of $2,661,396 and $1,538, respectively. Management intends to raise additional debt and/or equity financing to fund future operations and to provide additional working capital. However, there is no assurance that such financing will be consummated or obtained in sufficient amounts necessary to meet our needs. We can provide no assurance that we can continue to satisfy our cash requirements for the next twelve months.
In May, 2014, the Company entered into a convertible promissory note with MTF (the “2014 Note”) for $250,000 with interest at 7.0% per annum compounded annually and a maturity date of June 15, 2015. In the event of a financing of not less than $1 million, the 2014 Note automatically converts into Equity Securities, as defined in the 2014 Note, at a 25% discount to the price paid per share in such financing. In connection with the 2014 Note, the Company issued a warrant to purchase 166,667 shares of the Company’s Common Stock at an exercise price of $1.50 per share and 4 year term. This note was assumed by Orthofix in July 2014.
On July 1, 2014, Orthofix (A) purchased $500,000 worth of Bone Biologics Common Stock; (B) was issued two convertible promissory notes, each in the principal amount of $250,000 and exercisable for $333,333 worth of Bone Biologics Common Stock; and (C) was issued two warrants, each exercisable for 166,667 shares of Bone Biologics Common Stock at an exercise price per share of $1.50. Upon subscribing for the Subsequent Orthofix Shares, the Subsequent Orthofix Convertible Promissory Notes converted by their terms into a combined total of $666,666 worth of shares of Bone Biologics’ Common Stock in accordance with the terms of the Subsequent Orthofix Convertible Promissory Notes.
At the closing of the Subsequent Orthofix Shares and Notes, AFH Advisory was entitled to receive warrants to purchase up to 500,000 shares of Common Stock of the Company at the per share price of the shares offered or $1.00 per share, with a 5 year term and a cashless exercise provision (the “Extra Warrants”).
Per our agreement dated December 31, 2013, Forefront or its designees will receive an Agent Warrant equal to 8.0% of the Common Stock underlying the securities issued in the Private Placement (4% if investors are introduced by Bone Biologics, AFH Holdings & Advisory, LLC or their respective officers and directors). Such Agent Warrant will be issued at the closing of the Private Placement and shall provide, among other things, that the Agent Warrant shall: (i) be exercisable at the price of the securities (or the exercise price of the securities) issued to the investors in the offering, (ii) expire five (5) years from the date of issuance, (iii) include customary registration rights, including the registration rights provided to the Investors, (iv) contain provisions for cashless exercise and (v) include such other terms that are normal and customary for warrants of this type. In addition, Forefront or its designees will receive an Advisory Warrant equal to 2.0% of the Company’s post-merger and financing fully diluted shares outstanding upon the closing of $2.5 million of investors on which Forefront is eligible to receive compensation. Forefront was issued a warrant to purchase 46,667 shares of Common Stock at $1.00 per share upon completion of the Orthofix Subsequent Financing. Forefront will receive a cash fee equal to 8.0% of gross proceeds received and payable upon each closing (4% if investors are introduced to the Company by either Bone Biologics, AFH Holdings and Advisory, LLC, or their respective officers and directors, or an aggregate of $40,000 on the Orthofix Subsequent Financing. Upon the closing of the Hankey Capital note, Forefront received a $200,000 cash fee and a warrant to purchase 126,582 shares of Common Stock. This agreement was extended until February 15, 2015 and has expired without renewal as of the date of this report.
| 17 |
On September 15, 2014, Bone and MTF entered into the MTF Short Term 2014 Loan pursuant to which MTF has agreed to advance an initial $250,000 to Bone and, at Bone’s request and subject to the terms and conditions of the MTF Short Term 2014 Loan, to advance up to an additional $250,000 to Bone. The MTF Short Term 2014 Loan has an interest rate of eight and one-half percent (8.5%) accruing annually. The MTF Short Term 2014 Loan matures on the earlier to occur of (i) the date on which at least $1 million is loaned to or invested in the Company and (ii) December 31, 2014. In further consideration of the MTF 2014 Loan, Bone granted to MTF 625,000 warrants at a strike price of $1.62. On October 27, 2014 the balance was paid in full and the line of credit was cancelled.
On October 24, 2014, the Company issued a convertible promissory note in the amount of $5,000,000 (the “Convertible Note”) to Hankey Capital, LLC (“Hankey Capital”). The Convertible Note matures on October 24, 2017 (the “Maturity Date”) and bears interest at an annual rate of interest at the “prime rate” (as quoted in the “Money Rates” section of The Wall Street Journal) plus 4.0%, with a minimum rate of 8.5% per annum until maturity, with interest payable monthly in arrears. Prior to the Maturity Date, Hankey Capital has a right, in their sole discretion, to convert the Convertible Note into shares of the Company’s Common Stock, par value $0.001 per share (“Common Stock”), at a conversion rate equal to the greater of (i) $1.58 per share and (ii) 70% of the average daily price for the Common Stock as measured over the course of the 60 day period prior to the conversion. Simultaneously, the Company also issued a warrant to Hankey Capital for 3,955,697 shares of Common Stock at an exercise price per share of $1.58. The Warrant will expire on October 24, 2017. In connection with the Convertible Note and Warrant issuance, the Company also issued 6,329,114 shares of Common Stock in the name of Hankey Capital to be held as collateral. The principal amount of the loan is prepayable in whole or in part at any time, without premium or penalty. Upon any voluntary partial prepayment of outstanding principal. Hankey Capital shall return Collateral shares to the Company in the amount necessary, if any, to maintain the loan to value ratio at no less than 50%. Upon a full payment of the outstanding principal, all Collateral shares shall be returned and cancelled. Hankey Capital shall also return Collateral shares under the same terms in case of partial or full conversion of the Convertible Note.
Cash Flows
The following is a summary of our cash flows provided by operating, investing and financing activities for the years ended December 31, 2014 and 2013:
| December 31, 2014 | December 31, 2013 | |||||||
| Operating activities | ||||||||
| Net loss | $ | (4,424,413 | ) | $ | (1,082,204 | ) | ||
| Adjustments to reconcile net loss to net cash provided by (used in) operating activities: | ||||||||
| Depreciation | 280 | - | ||||||
| Accrued interest expense | 318,215 | 340,268 | ||||||
| Debt discount amortization | 437,115 | 67,104 | ||||||
| Debt financing costs amortization | 60,398 | |||||||
| Warrants issued with Line of Credit | 520,487 | - | ||||||
| Stock-based compensation | 256,642 | - | ||||||
| Warrants issued to Consultants | 379,349 | - | ||||||
| Loss on sale of marketable securities | 9,624 | - | ||||||
| Transaction costs financed through notes payable | 590,000 | |||||||
| Changes in operating assets and liabilities: | ||||||||
| Prepaid expenses and other current assets | (78,750 | ) | (10,767 | ) | ||||
| Deferred financing costs | (543,401 | ) | 4,717 | |||||
| Other receivables – related party | (75,000 | ) | - | |||||
| Advances due to related party | (41,300 | ) | 41,300 | |||||
| Accrued expenses | (164,864 | ) | 114,217 | |||||
| Net cash (used in) operating activities | (2,755,618 | ) | (525,365 | ) | ||||
| Investing activities | ||||||||
| Purchase of furniture and equipment | (11,901 | ) | - | |||||
| Proceeds from sale of marketable securities | 37,377 | - | ||||||
| Net cash provided by investing activities | 25,476 | - | ||||||
| Financing activities | ||||||||
| Proceeds from the issuance of common stock | 480,000 | - | ||||||
| Repayment of debt | (590,000 | ) | - | |||||
| Proceeds from issuance of notes payable – related party | 553,371 | 524,533 | ||||||
| Repayment of notes payable – related party | (303,371 | ) | - | |||||
| Proceeds from issuance of notes payable | 5,250,000 | - | ||||||
| Net cash provided by financing activities | 5,390,000 | 524,533 | ||||||
| Net increase (decrease) in cash | 2,659,858 | (832 | ) | |||||
| Cash, beginning of period | 1,538 | 2,370 | ||||||
| Cash, end of period | $ | 2,661,396 | $ | 1,538 | ||||
Operating activities
In the years ended December 31, 2014 and 2013, cash used in operating activities was $2,755,618 and $525,365 respectively, which was driven by our net losses of $4,424,413 and $1,082,204, respectively. Cash expenditures in each of the years ended December 31, 2014 and 2013 increased primarily due to patent costs and professional fees as a result of Bone’s financing activities which included the Bridge and Private Placement Financing and matters related to the merger transactions.
During the year ended December 31, 2014, cash used in operating activities was partially offset by non-cash increases in accrued interest expense of $318,215, debt discount amortization of $437,115, debt financing costs amortization of $60,398, non-cash items of $520,487, $256,642 and $379,349 related to the issuance of warrants and stock options, and a loss of $9,624 on the sale of marketable securities received in lieu of cash on our 2013 Bridge Note with AFH. During the year ended December 31, 2013, cash used in operating activities included a decrease in prepaid expenses and other current assets of $10,767 and an increase in accrued expenses of $114,217, and were partially offset by non-cash increases in accrued interest expense of $340,268 and debt discount amortization of $67,104.
| 18 |
Investing activities
In the year ended December 31, 2014, cash provided by investing activities totaled $25,476 of which $37,377 resulted from the sale of marketable securities which were received in lieu of cash for our Bridge Note with AFH and $11,901 used for the purchase of furniture and equipment. There were no investing activities in the year ended December 31, 2013.
Financing activities
In the year ended December 31, 2014, cash provided by financing activities was $5,390,000 and resulted from the proceeds received from the issuance of notes and sale of shares. In the year ended December 31, 2013, cash provided by financing activities was $524,533 resulting from the proceeds received from the issuance of the 2013 Bridge Notes.
Off-Balance Sheet Arrangements
The Company does not have any off-balance sheet arrangements that have or are reasonably likely to have a current or future effect on the Company’s financial condition, changes in financial condition, revenues or expenses, results of operations, liquidity, capital expenditures or capital resources that is material to investors.
Item 7A. Quantitative and Qualitative Disclosures about Market Risk
Not applicable.
Item 8. Financial Statements and Supplementary Data
The financial statements and supplementary data required by Regulation S-X are included in Item 15. “Exhibits, Financial Statements Schedules” contained in Part IV, Item 15 of this Annual Report.
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure
None.
Item 9A. Controls and Procedures
Evaluation of Disclosure Controls and Procedures
Under the supervision and with the participation of our management, including our Chief Financial Officer and Chief Executive Officer, we evaluated the effectiveness of the design and operation of our disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) under the Securities Exchange Act of 1934 (Exchange Act)) as of December 31, 2014. Based upon that evaluation, our Chief Financial Officer and Chief Executive Officer concluded that as of December 31, 2014, our disclosure controls and procedures were not effective.
Management’s Annual Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting. Internal control over financial reporting is defined in Rule 13a-15(f) or 15d-15(f) promulgated under the Securities Exchange Act of 1934 as a process designed by, or under the supervision of, the company’s principal executive officers and effected by the company’s board of directors, management and other personnel, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with GAAP and includes those policies and procedures that:
| 19 |
Pertain to the maintenance of records that in reasonable detail accurately and fairly reflect the transactions and dispositions of the assets of the company;
Provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with accounting principles generally accepted in the United States of America and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and
Provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate. All internal control systems, no matter how well designed, have inherent limitations. Therefore, even those systems determined to be effective can provide only reasonable assurance with respect to financial statement preparation and presentation. Because of the inherent limitations of internal control, there is a risk that material misstatements may not be prevented or detected on a timely basis by internal control over financial reporting. However, these inherent limitations are known features of the financial reporting process. Therefore, it is possible to design into the process safeguards to reduce, though not eliminate, this risk.
As of December 31, 2014, management assessed the effectiveness of our internal control over financial reporting and based on that evaluation, they concluded that our internal controls and procedures were not effective.
Changes in Internal Control over Financial Reporting
During 2013, Bone Biologics Inc., a privately held company, had a material weakness identified by management related to having sufficient and adequate accounting resources and proper communication of non-routine transactions. We have recently hired a chief financial officer who has implemented procedures to accumulate and assess all matters and management commenced steps to remediate the material weakness identified and implemented additional controls through increased levels of accounting expertise to review and approve, among other things, the complex accounting and related calculations.
During 2014, Bone Biologics Corp identified a material weakness related to the valuation of options and warrants issued.
On September 19, 2014, we closed the Reverse Merger, which has been accounted for as a reverse acquisition. The financial statements and information relating to Bone Biologics Inc., a privately held company, now constitute the financial statements and information of the “Company.” Because Bone Biologics Inc. was a privately held company prior to the Reverse Merger, it was not required to design or maintain its controls in accordance with Exchange Act Rule 13a-15 prior to the Reverse Merger. The operations of Pre-Merger AFH Acquisition X, Inc., a publicly held company, were insignificant both before and after the Reverse Merger compared to those of the post-combination consolidated entity. As such, significant time and resources from our management and other personnel have been required and will continue to be required for the design and implementation of public company internal control over financial reporting for the post-combination consolidated Company. Except for the continuing design and implementation of new processes and procedures following the Reverse Merger, there was no change in our internal control over financial reporting that occurred during the year ended December 31, 2014 that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
None.
| 20 |
Item 10. Directors, Executive Officers and Corporate Governance
The Company’s directors are elected annually for a one year term or until their respective successors are duly elected and qualified or until their earlier resignation or removal. The following table sets forth certain information regarding the Company’s directors and executive officers:
| Name | Age | Position | ||
| Michael Schuler | 65 | Interim Chief Executive Officer | ||
| William J. Treat | 59 | President and Chief Technology Officer | ||
| Deina H. Walsh | 50 | Chief Financial Officer | ||
| Bruce Stroever | 65 | Chairman of the Board of Directors | ||
| Dr. Chia Soo | 46 | Director | ||
| William Coffin | 69 | Director | ||
| John Booth | 59 | Director | ||
| Jimmy Delshad | 74 | Director | ||
| Steve Warnecke | 58 | Director | ||
| George A. Oram | 66 | Director |
Michael Schuler: Interim Chief Executive Officer
Pursuant to a consulting agreement by and between the Company and MTF, MTF has agreed to provide the services of Mr. Schuler to serve as the Company’s interim Chief Executive Officer. Mr. Schuler has served as Vice-President, New Business Development of MTF since 2002. In addition to his work at MTF, since 2000 Mr. Schuler is the founder and partner in Tri-Medics, LLC, an early stage company developing and marketing innovative medical devices. From 1995 to 2001, Mr. Schuler served as an independent strategic planner and new business development consultant with Olympus USA and SurgiNex, Inc. From 1993 to 1995, he was President of the Surgical Products Division of Imagyn Medical Technologies, a NASDAQ listed company. From 1992 to 1996, he served as Executive Vice President, Marketing and Sales, then President and CEO of Advanced Surgical, Inc., an innovator in surgical instruments for advanced procedures and a NASDAQ listed company. Prior to his roles at Advanced Surgical, Inc. Mr. Schuler spent 18 years with Johnson & Johnson, where he progressively held positions of greater responsibility, including a position in new business development which was responsible for the assessment and development of the Palmaz-Schatz Stent technology that was utilized in cardiology and radiology. As Director and then Vice-President of Marketing of Johnson & Johnson Interventional Systems from 1987 to 1992, he was involved in the launch of the Intraluminal Stent that grew to over $1 billion in annual sales. Mr. Schuler was the only Johnson & Johnson employee to receive both the Phillip B. Hoffman award for outstanding achievement in R&D and the Johnson & Johnson Entrepreneurial Award for creating over $50 million in new business. He is the holder of 13 US patents and numerous international patents. Mr. Schuler was a part-time professor at the Rutgers University Graduate School from 1978-1993. He has also served as a member of Bone’s Board of Directors since April 7, 2006. He earned a Bachelors of Science in Industrial Engineering in 1971, a Masters in Business Administration in 1973, and a Masters of Science in Engineering in 1974, all from Rutgers University. Given Mr. Schuler’s extensive background in medical device development and marketing, as well as his previous roles with Bone Biologics, the Company believes he is well qualified to fill the role of interim Chief Executive Officer.
| 21 |
William Jay Treat, Ph.D.: President and Chief Technology Officer
Dr. Treat has served as the President and Chief Technology Officer of Bone Biologics since November 2012. Since 2005 to present, he has run his own consulting business specializing in providing senior management leadership to clients in developing their products from concept to commercial launch. During this period he was one of the founders and remains active in America Stem Cell (now Targazyme) overseeing their manufacturing and clinical supplies. He also currently serves as Advisor for Systems Operations at PBS BioTech. From 2001 to 2005 he served first as Vice President of Business Development and later as Chief Operations Officer for Avid Bioservices, a contract manufacturing organization specializing in the production of monoclonal antibodies and other recombinant non-antibody proteins. As Chief Operating Officer of Avid Bioservices he presided over a successful PAI and commercial license of a biologic for Halozyme. Prior to joining Avid Bioservices, from 1999 to 2001 he was Vice President of Research and Development at Irvine Scientific where his team developed products for human assisted reproductive medicine and for cell culture medium formulations used to produce medically important biological molecules. From 1990 to 1999 he was with BioWhittaker (acquired by Cambrex), where he held positions of progressively greater responsibility in manufacturing, technical support, new business acquisitions and marketing while they were a privately held company and later as a public company. Dr. Treat started his career at Lipogen, where he quickly moved to Vice President of Manufacturing and managed the technology transfer to BioWhittaker. During his career, Dr. Treat has also served as Chief Operating Officer for BioTork, CSO for Algenol, Vice President of Manufacturing for Attenuion, CTO for Geneve Bio and Head of Commercial Manufacturing for DianaPlantSciences. Dr. Treat has served on the Scientific Advisory Boards for the Chemical Engineering Department at Texas A&M University from 2000 to 2013 and on the Emergent Biosolutions and XOMA External Advisory Boards in support of NAIAD/DOD bioterrorism programs. He earned a Bachelors of Science in Microbiology in 1979, a Masters of Science in Microbiology in 1982 and a Ph.D. in Agricultural and Biochemical Engineering in 1988 all from Texas A&M University. Given Dr. Treat’s extensive background with biologics and his previous roles with Bone Biologics, the Company believe Dr. Treat is well qualified to serve as its President and Chief Technology Officer.
Deina H. Walsh: Chief Financial Officer
Ms. Walsh has serves as our Chief Financial Officer since November 2014. She is a certified public accountant and owner/founder of DHW CPA, PLLC a Public Companies Accounting Oversight Board (PCAOB) registered firm since 2014. Prior to forming her firm, Ms. Walsh has 13 years at a public accounting firm where as a partner she was actively responsible for leading firm audit engagements of publicly held entities in accordance with PCAOB standards and compliance with SEC regulations, including internal control requirements under section 404 of the Sarbanes-Oxley Act. Ms. Walsh had a global client base including entities throughout the United States, Canada and China. These entities encompass a diverse range of industries including manufacturing, wholesale, life sciences, pharmaceuticals, and technology. Her experience includes work with start-up companies and well-established operating entities. She has assisted many entities seeking debt and equity capital. Areas of specialty include mergers, acquisitions, reverse mergers, consolidations, complex equity structures, foreign currency translations and revenue recognition complexities. Ms. Walsh has an Associates of Science Degree in Business Administration from Monroe Community College and a Bachelor of Science Degree in Accounting from the State University of New York at Brockport.
Bruce Stroever: Chairman of the Board of Directors
Mr. Stroever has forty years of product development and general management experience in the medical device and orthobiologics fields. Mr. Stroever joined MTF in late 1988 as General Manager and is currently the President and Chief Executive Officer of MTF. He has served MTF’s President since his appointment in 1992 and as Chief Executive Officer since 1996. Under Mr. Stroever’s leadership, MTF has grown to be the largest tissue bank in the world providing over 450,000 grafts per year. From 1971 to 1988, Mr. Stroever held several positions with Ethicon, Inc., a Johnson & Johnson, Inc. subsidiary. Mr. Stroever currently serves on the advisory boards for the Department of Bioengineering at UCLA and the New Jersey Organ and Tissue Sharing Network. He was elected to the Board of Governors of the American Association of Tissue Banks for a three year term in 1999 and subsequently in 2012. Mr. Stroever has served as the Chairman of Bone’s Board of Directors since 2012. Mr. Stroever received his B.E. in Mechanical/Chemical Engineering from Stevens Institute of Technology in 1972 and a Masters of Science in Bioengineering from Columbia University in 1977. Given Mr. Stroever’s forty years of experience in the medical device and orthobiologics fields, as well as, the roles he has held at Bone, the Company believes he is well qualified to serve as the Chairman of the Board of Directors.
| 22 |
Dr. Chia Soo. MD: Director
Since 2009, Dr. Soo has served as a Professor in the Orthopedic Hospital Department of Orthopedics of UCLA. Dr. Soo and her colleagues have been studying the osteogenic potential of the Nell-1 protein for the last 13 years. In addition to currently being the PI of an NIH and a DOD translational grant to study the osteoinductive properties of Nell-1, Dr. Soo has served as PI for two NIH SBIR grants, as Co-PI on an RO1 grant, and managed two (2) University of California Discovery grants. Since 2011, Dr. Soo has served as a consultant to MTF, the world’s largest tissue bank for allograft product development. She also has consulted extensively on FDA issues related to allograft products, cGMP protein production, and FDA regulatory submissions. She is technically experienced with both large and small translational animal models of skeletal disease. Dr. Soo is certified by the American Board of Plastic Surgery and a Fellow of the American College of Surgeons. She has also served as a member of Bone’s Board of Directors since 2004. Given Dr. Soo’s background with grants, her studies of the Nell-1 protein and position on Bone’s Board of Directors, the Company thinks she is well qualified to serve as a Director of the Company.
William Coffin: Director
Founder and CEO of CCG, a national investor relations agency which during his leadership conducted business through offices in New York, Los Angeles, Beijing, Mr. Coffin was an investor relations counselor for over 25 years until his retirement in 2012. In this role, Mr. Coffin represented numerous publicly held and private companies, assisted in over 100 initial public offerings, counseled and participated in over 50 mergers and acquisitions, and worked with virtually every major investment banking firm in the country. Since 2004, Mr. Coffin has served as Chairman of the Board of the California Council on Economic Education, a nonprofit, nonpartisan organization that works towards implementing and increasing economic and financial literacy among California primary and secondary school students. Mr. Coffin is also an adjunct professor in the MBA program at Mount St. Mary’s college, a private liberal arts college in Los Angeles, where he teaches modern theories of corporate governance and corporate communications. Mr. Coffin received a B.A. in journalism from California State University, Los Angeles.
John Booth: Director
Mr. Booth has been CEO of Spineology Inc. since 2004 and has been a board member since its inception in 1998. Spineology is involved in the development and commercialization of minimally invasive spinal implants and access systems. Mr. Booth held various executive level positions at Phillips Plastics Corporation, most recently serving as CEO from June of 2001 to December 2002. Before serving as CEO of Phillips, he was CEO of Microvena Corporation, a cardiovascular device subsidiary of Phillips, from 1999 to 2001 and CEO of Phillips Origen Group Division from 1998 to 1999. Prior to Phillips, Mr. Booth was President and CEO of INCSTAR Corporation, a publicly held medical technology company involved in in-vitro diagnostics. He has held various positions in both financial and general management in the medical technology industry since 1981. Mr. Booth has also serve on the boards of directors of INCSTAR Corporation from 1994 to 1997, Microvena Corporation from 1998 to 2001, Phillips Plastics Corporation from 2000 to 2002, Imricor Medical Systems Inc. from 2007 to 2014, Spineology Inc. from 1998 to the present. Mr. Booth received a B.S. degree in accounting from Villanova University and an MBA from Seton Hall University.
Jimmy Delshad: Director
Mr. Delshad brings more than ten years of elected public service to the Company. From 2003 through 2011, Mr. Delshad served as Mayor and Councilmember of the City of Beverly Hills, California. In this role, Mr. Delshad was responsible for, among other things: the formulation of city policies and ordinances; the establishment and monitoring of a budget of approximately $500 million; and the management of more than 1000 city employees. Additionally, since his retirement as Mayor in 2011, Mr. Delshad has served as a Goodwill Ambassador for the City of Beverly Hills. Since 2012, Mr. Delshad has held the position of Chairman of Delshad Capital, which guides companies in technology, security, crowd-funding and marketing. During 2011 through 2012, Mr. Delshad was Vice Chairman at Pacific Capital Group where he evaluated and managed various projects, such as Smart City initiatives, fuel technology and software products. From 1978 through 2002, Mr. Delshad was founder and Chief Executive Officer of American International Business, Inc., a manufacturer of computer storage technologies with offices in Germany, London and Brussels. Mr. Delshad served on the board of directors of Evryx Corp from 2008 through 2010 and Dream Team Gaming from 2007 through 2009. Mr. Delshad has also served on the Boards of Directors of the Iranian American Jewish Federation from 2002 through the present, the World Affair Council from 2011 through 2012, Sheba Medical Center from 2003 through 2008, Maple Counseling Center from 2001 through 2003, Mount Sinai Mortuaries from 2001 through the present and Nessah Synagogue from 2001 through 2004. Mr. Delshad received his B.S. in Computer Science from California State University and completed additional post-graduate coursework at the University of Southern California.
| 23 |
Steve Warnecke: Director
Mr. Warnecke brings to the Company over thirty years of management experience in biotech, pharma, medical device, healthcare, software/telecom, travel/airline, construction/real estate and manufacturing/distribution. Since 2010 Mr. Warnecke has served as CEO and a member of the board of directors of Evolutionary Genomics, Inc., unique biotechnology company that is changing how relevant gene/target discovery and validation is performed during the post-genomics era. From 2003 through 2008 and from 2011 through the present, Mr. Warnecke has held several positions with Children’s Hospital Colorado Foundation, including Senior Vice President, CFO and COO. From 2012 through the present Mr. Warnecke has served as CFO and Chairman of the board of directors of VETDC, Inc., a veterinary product development company that adapts innovative, underutilized human biomedical technologies for use in companion animals. Since 2014, Mr. Warnecke has also served as a member of the board of directors of CereScan and of the Rockies Venture Club. Since 2013, Mr. Warnecke has served as a member of the board of directors of the University of Iowa Cardiovascular Research Center. Since 2004 he has also served as chairman of Children’s Partners Foundation. Mr. Warnecke has also served as member of the board of directors of the Cystic Fibrosis Foundation since 2002. From 2003 through 2011, Mr. Warnecke has held various positions, including lead independent director, chairman of the audit committee, financial expert and member of the nominating and governance committee of Evolving Systems, Inc. In 2011, he also served as a member of the board of directors, chairman of the audit committee and financial expert for Emmaus Life Sciences. Mr. Warnecke also served as the CFO of Targeted Medical Pharma, Inc. in 2011. From 2008 to 2010, Mr. Warnecke served as the CFO and a member of the board of directors of Bacterin International, Inc. From 2005 through 2008 he served as a member of the board of directors and served as a member of the compensation committee of Boppy Company. From 2001 to 2002, he served as Senior Vice President of Strategic Planning at First Data/Western Union (NYSE-FDC). From 1999 to 2001 he served as the CFO of Frontier Airlines (NASDAQ-FRNT). From 1982 to 1999 he served as the CFO of Helm Corporation and affiliates. Mr. Warnecke earned a BBA with honors from the University of Iowa in 1979. Given Mr. Warnecke’s broad base of experience in serving as an officer and director of a variety of companies and his experience in serving on audit committees and compensation committees, the Company believes he is well suited to serve as a member of the Company’s Board of Directors.
George A. Oram: Director
Mr. Oram has served as a member of our board of directors since November 2014. Mr. Oram served as Executive Vice President of Musculoskeletal Transplant Foundation (MTF) from 1997 to 2013, when he retired. From 2005 to 2013, he was also President of DIZG, MTF’s European affiliate. He served as President of MTF Sports Medicine from 2006 to 2012. From 1998 to 2001, Mr. Oram was President of Tissue Transformation Technologies, Inc., an MTF subsidiary. From 1996 to 1997, Mr. Oram served as President and Chief Operating Officer of Orthologic Corporation. From 1995 to 1996, Mr. Oram served as President and Chief Executive Officer of Fibromed Inc., a biomaterials start up. From 1987 to 1994, Mr. Oram was President and Chief Operating Officer of Biomatrix, Inc., a development stage biomaterials company, and took them public in 1981. From 1984 to 1987, Mr. Oram was employed by Electro Biology, Inc. lastly as its President. Mr. Oram served as a board member of Orthologic Corporation from 1993 to 1997, board member of Osteodyne, Inc. from 1994 to 1996 and board member of Health Industry Manufacturer’s Association (HIMA) from 1993 to 1994. Mr. Oram was a founding member of Biotechnology Council of New Jersey and a member of the New Jersey Biotechnology Task Force. Mr. Oram received his B.A. in Business Administration from Rutgers University in 1970. Given Mr. Oram’s over thirty years of large and small healthcare and biotechnology company experience, the Company thinks he is well qualified to serve as a director of the Company.
Family Relationships
Drs. Ting and Soo are husband and wife.
Board of Directors and Corporate Governance
Our Board of Directors currently consists of seven (7) members, including Bruce Stroever, Dr. Chia Soo, William Coffin, John Booth, Jimmy Delshad, Steve Warnecke, and George Oram. George Oram who was appointed to fill the vacancy resulted from the increase of the size of the Board from six (6) to seven (7) on November 24, 2014.
| 24 |
Board Independence and Committees
We are not currently listed on any national securities exchange or in an inter-dealer quotation system that has a requirement that the Board of Directors be independent. However, in evaluating the independence of our members and the composition of the committees of our Board of Directors, our Board utilizes the definition of “independence” as that term is defined by applicable listing standards of the Nasdaq Stock Market and SEC rules, including the rules relating to the independence standards of an audit committee and the non-employee director definition of Rule 16b-3 promulgated under the Exchange Act.
Our Board of Directors expects to continue to evaluate its independence standards and whether and to what extent the composition of the Board and its committees meets those standards. We ultimately intend to appoint such persons to our Board and committees of our Board as are expected to be required to meet the corporate governance requirements imposed by a national securities exchange. Therefore, we intend that a majority of our directors will be independent directors of which at least one director will qualify as an “audit committee financial expert,” within the meaning of Item 407(d)(5) of Regulation S-K, as promulgated by the SEC.
Our Board of Directors has appointed an audit committee, governance committee and compensation committee.
Audit Committee
The audit committee is responsible for overseeing: (i) our accounting and reporting practices and compliance with legal and regulatory requirements regarding such accounting and reporting practices; (ii) the quality and integrity of our financial statements; (iii) our internal control and compliance programs; (iv) our independent auditors’ qualifications and independence and (v) the performance of our independent auditors and our internal audit function. In so doing, the audit committee maintains free and open means of communication between our directors, internal auditors and management. We are not required to have an Audit Committee consisting solely of independent directors or required to have an “audit committee financial expert” as we are neither listed on NASDAQ nor the New York Stock Exchange.
Our audit committee consists of Steve Warnecke, as Chairman, John booth and Jimmy Delshad.
Compensation Committee
The compensation committee is responsible for reviewing and approving the compensation of our executive officers and directors and our performance plans and other compensation plans. The compensation committee makes recommendations to our Board of Directors in connection with such compensation and performance plans.
Our compensation committee consists of John Booth, as Chairman, George Oram and Bill Coffin.
Compensation Committee Interlocks and Insider Participation
The members of the compensation committee who served during 2014 are John Booth, George Oram and Bill Coffin. There were no interlocks or insider participation between any member of our Board of Directors or compensation committee and any member of the board of directors or compensation committee of any other company, nor has any interlocking relationship existed in the past.
Nominating and Corporate Governance Committee
The nominating and corporate governance committee is responsible for (i) identifying, screening and reviewing individuals qualified to serve as directors (consistent with criteria approved by our Board of Directors) and recommending to our Board candidates for nomination for election at the annual meeting of shareholders or to fill board vacancies or newly created directorships; (ii) developing and recommending to our Board of Directors and overseeing the implementation of our corporate governance guidelines (if any); (iii) overseeing evaluations of our Board of Directors and (iv) recommending to our Board of Directors candidates for appointment to board committees.
| 25 |
Our nominating and corporate governance committee consists of Bill Coffin, as Chairman, Jimmy Delshad and Steve Warnecke.
Code of Ethics
On February 9, 2014 the Company adopted a formal code of ethics within the meaning of Item 406 of Regulation S-K promulgated under the Securities Act, that applies to our principal executive officer, principal financial officer, principal accounting officer or controller, or persons performing similar functions and that that establishes, among other things, procedures for handling actual or apparent conflicts of interest. Our Code of Ethics is available at our website http://bonebiologics.com/investor-relations/corporate-governance/.
Section 16(a) Beneficial Ownership Reporting Compliance
In fiscal 2014, none of the Company’s officers, directors, and beneficial owners of more than ten percent of the company’s common stock were delinquent in filing of any of their Section 16(a) reports.
Indemnification Agreements
Our Board has approved a form of indemnification agreement for our directors and executive officers (“Indemnification Agreement”). Following Board approval, we entered into Indemnification Agreements with each of our current directors and executive officers.
The Indemnification Agreement provides for indemnification against expenses, judgments, fines and penalties actually and reasonably incurred by an indemnitee in connection with threatened, pending or completed actions, suits or other proceedings, subject to certain limitations. The Indemnification Agreement also provides for the advancement of expenses in connection with a proceeding prior to a final, non-appealable judgment or other adjudication, provided that the indemnitee provides an undertaking to repay to us any amounts advanced if the indemnitee is ultimately found not to be entitled to indemnification by us. The Indemnification Agreement sets forth procedures for making and responding to a request for indemnification or advancement of expenses, as well as dispute resolution procedures that will apply to any dispute between us and an indemnitee arising under the Indemnification Agreement.
The foregoing description is qualified in its entirety by reference to the form of Indemnification Agreement filed as Exhibit 10.17 to the Current Report on Form 8-K filed on September 25, 2014.
Effective as of September 19, 2014, our Board of Directors also approved the Former D&O Indemnification Agreement to be entered into between us, Don Hankey and Amir Heshmatpour. The Former D&O Indemnification Agreement requires that for a period of four (4) years from and after September 19, 2014, we will indemnify (including advancement of expenses) and hold harmless persons who were officers and directors of the Company (i) by reason of being an officer or director of the Company prior to the Merger, including through all transactions relating to the Merger, or (ii) is related to acts in connection with the Merger taken by the Former D&O Indemnified Persons, provided however, that the foregoing indemnity shall be excess of all any insurance coverage available to the Former D&O Indemnified Parties for any such loss. The accuracy of the Hankey Affidavit and Heshmatpour Affidavit in connection with the Former D&O Indemnification is a condition precedent to the foregoing indemnity (including advancement of expenses). The Company has no insurance coverage that would cover any claim asserted against the Company by any Former D&O Indemnified Person pursuant to this Former D&O Indemnification Agreement.
This description is qualified in its entirety by the Former D&O Indemnification Agreement filed as Exhibit 10.18 to the Current Report on Form 8-K filed on September 25, 2014 and incorporated herein by reference.
| 26 |
Scientific Advisory Board
Mr. Gertzman has served as a member of Bone’s Scientific Advisory Board since 2005. He is the former Executive Vice President for Research and Development from MTF since 1996 and is currently a consultant to MTF in patent prosecution. He has been engaged in industrial product development of surgical implants for forty years. From 1964 to 1993, he was employed by Ethicon, Inc., a Johnson & Johnson Company as Director of Product Engineering Johnson & Johnson USA. From 1993 to 1996, he was employed by Xomed Medical Products and served in several positions of responsibility in research and product development, after his appointment as Vice President, Research and Development in 1993. Mr. Gertzman was appointed Vice President, Research and Development for MTF in 1996 and is currently employed in the development of new tissue forms and related processes. He holds over twenty-five (25) U.S. patents, with many more pending, both in the U.S. and internationally. He completed a Bachelors of Science at CCNY in 1960 and a Masters of Science degree in Chemistry from Boston University in 1963.
Dr. Shun’ichi Kuroda has served as a member of Bone’s Scientific Advisory Board since 2005. He taught as a professor at the Department of Bio-agricultural Sciences of Nagoya University since 2009 and has served as the Chairman of the Department since 2012. Dr. Kuroda has expertise is in recombinant protein engineering and manufacturing.
Dr. Jeffrey C. Wang, MD has served as a member of Bone’s Scientific Advisory Board since 2005. Dr. Wang has been Chief of the Orthopaedic Spine Surgery Service since 1997, Fellowship Director of the UCLA Orthopaedic Spine Surgery Fellowship, and is Currently Professor of Orthopaedic Surgery and Neurosurgery. He is also the Vice Chair of Clinical Operations for the UCLA Department of Orthopaedic Surgery. He is Co-Director of the UCLA Spine Center. Dr. Wang’s research areas include the use of osteoinductive and osteoconductive materials for spinal fusion as well as novel gene therapy and minimally invasive techniques for spinal surgery. He obtained his undergraduate degree from Stanford University and his medical degree from the University of Pittsburgh. He then completed his Orthopaedic Surgery training at UCLA and his Spine Fellowship at Case Western Reserve University.
Dr. Xinli Zhang has served as a member of Bone’s Scientific Advisory Board since 2005. Since 2009, he has served as an Associate Professor at the UCLA School of Dentistry. Prior to joining UCLA, Dr. Zhang was Associate Professor in the Third Military Medical University in China from 1994 to 2000. Dr. Zhang combines his specialized training as a pathologist with a PhD in molecular biology. Dr. Zhang brings over twenty years of experience in medical and dental research in both China and the U.S. Dr. Zhang is an expert in developmental molecular biology and pathology of various bone and cartilaginous tissue related conditions.
Dr. Mark Spilker joined MTF in early 2009 and is currently managing the R&D, Project Management, Clinical Studies, Quality Assurance and Regulatory departments. Mark has been leading the continued development and expansion of MTF’s human allograft tissue portfolio. Under his leadership, the MTF team has developed new tissue forms including allograft bone designs for extremity surgery, allograft scaffolds for cartilage regeneration and a living allogeneic stem cell graft for orthopedic surgery. Over his career, Mark has held positions in Marketing, R&D and Program Management. Prior to MTF, Mark was formerly Vice President, R&D and Program Management for Integra NeuroSciences where he was responsible for development programs ranging from collagen-based scaffolds for bone and soft tissue regeneration to medical electronics and implantable fluid-handling shunts and valves. Prior to joining Integra he conducted his post-graduate studies at Massachusetts Institute of Technology, making significant contributions in the field of biomaterials technology and tissue engineering in neurosurgery. He received a B.S. degree in Mechanical Engineering from the University of Utah and an M.S. and Ph.D. in Mechanical Engineering from the Massachusetts Institute of Technology.
Dr. Ben Wu, MD as a founder and has served on served as a member of Bone’s Scientific Advisory Board since 2005. He is Professor and Chair of the Division of Advanced Prosthodontics, and the Director of the Weintraub Center for Reconstructive Biotechnology at the School of Dentistry. He also chairs the Department of Bioengineering at the School of Engineering.
| 27 |
Dr. Wu provides multidisciplinary patient care in the UCLA Faculty Group Dental Practice, where he focuses on the treatment of advanced, complex oral rehabilitation using implant, fixed, and removable prosthodontics. He is a fellow of the Academy of Prosthodontics. Dr. Wu is internationally recognized for his cutting-edge research in the formation of biomimetic apatites, development of bioinspired growth factors, and engineering of biomimetic microenvironment to deliver cells, proteins, and genes to promote repair and regeneration of hard and soft tissues. Dr. Wu has been highly prolific throughout his entire career (over 120 original research articles, 9 issued patents with more pending) and has been continuously funded by federal research grants. Professor Wu’s research group has extensively analyzed the effects of processing parameters on the formation of biomimetic apatites, and his fundamental understanding has led to applications in the areas of art conservation, drug delivery, separations, and biosensors. His research group has also shed light on the interplay between orthobiologic growth factors and adult stem cells in the area of bone repair. His experimental skills are complemented by insightful mathematical modeling of complex, moving boundary diffusion-reaction problems that have led to key design criteria for tissue engineering, material degradation, and cancer survival mechanisms. His work has impacted clinical disciplines ranging from Orthopedics, Interventional Radiology, Urology, Pediatric Surgery, Orthodontics, and Dentistry. He has work with Dr. Ting and Dr. Soo as —PI of a major UC Discovery grant to develop novel material systems to deliver Nell-1 to promote bone and cartilage regeneration. He had is residency in advanced prosthodontics at Harvard School of Dental Medicine, PhD from the Dept of Material Science and Engineering at MIT.
Dr. Eric Ting, MD as a founder and has served on served as a member of Bone’s Scientific Advisory Board since 2005. He is a UCLA (Harvard trained orthodontist) is fully endowed Department Chair in Department of Surgery and Director of UCLA Craniofacial Abnormalities Laboratory. He is an endowed professor and Director of UCLA Laboratory for Craniofacial Anomalies at the Dental and Craniofacial Research Institute and holds a faculty position in the UCLA Dept of Surgery. His research interests include the molecular mechanism of craniosynostosis, which is the premature fusion of calvarial suture line in infants and tissue engineering of bone. Craniosyntosisis requires surgeries through childhood to alleviate and open the skull. Starting in the mid 1990’s the research identified that the NELL protein (a protein whose sole purpose was to create the bone growth to close the skull and after performing its task went dormant) was over expressed in the infants, which created the premature closing. Dr. Ting received his Doctorate of Dental Medicine Degree from Harvard University, School of Dental Medicine and completed his Postdoctoral Orthodontic Residency and also received the Doctorate of Medical Sciences from Harvard.
Item 11. Executive Compensation
The table below summarizes the compensation earned for services rendered to our predecessor and us in all capacities, for the fiscal years indicated, by its named executive officers:
| Name and Principal Position | Year | Salary | Bonus ($) | Stock Awards ($) | Option Awards ($) | Non-Equity Incentive Plan Compensation ($) | Deferred Compensation ($) | All Other Compensation ($) | Total Compensation ($) | |||||||||||||||||||||||||
| Mike Schuler, CEO (1) | 2014 | $ | 47,500 | $ | 47,500 | |||||||||||||||||||||||||||||
| Dr. William Jay Treat, President, Chief Technology Officer | 2014 | $ | 195,000 | $ | 199,585 | $ | 394,585 | |||||||||||||||||||||||||||
| 2013 | $ | 60,000 | $ | 60,000 | ||||||||||||||||||||||||||||||
| Deina Walsh, Chief Financial Officer | 2014 | $ | 25,000 | $ | 56,573 | $ | 81,573 | |||||||||||||||||||||||||||
| Amir F. Heshmatpour, President, Secretary, Chief Financial Officer and Director (2) | 2014 | - | - | - | ||||||||||||||||||||||||||||||
| 2013 | - | - | - | |||||||||||||||||||||||||||||||
| Don Hankey, President, Secretary, Chief Financial Officer and Director (3) | 2014 | - | $ | 4,050 | - | $ | 4,050 | |||||||||||||||||||||||||||
| (1) | MTF is paid compensation for Mr. Schuler’s CEO services per a consulting agreement with the Company. | |
| (2) | Mr. Heshmatpour resigned as of August 6, 2014. | |
| (3) | Mr. Hankey was appointed as of August 6, 2014 and resigned on September 19, 2014 upon completion of the merger. |
| 28 |
Changes in Executive Compensation
The Company engaged a professional executive compensation expert that recommended to the Company that they institute a stock option plan. Accordingly, immediately following the Merger, our Company’s Board of Directors approved a compensation program for our named executive officers. Consistent with the size and nature of our Company, our executive compensation program is simple, consisting of a base salary, an annual performance-based cash award and an annual long-term equity award under our 2014 Stock Option Plan.
| ● | Base Salary: The Company’s base salaries are designed as a means to provide a fixed level of compensation in order to attract and retain talent. The base salaries of our named executive officers depend on their job responsibilities, the market rate of compensation paid by companies in our industry for similar positions, our financial position and the strength of our business. | |
| ● | Performance-Based Cash Awards: As part of the Company’s executive compensation program, the board intends to establish an annual performance-based cash award program for our executive officers and other key employees based upon individual performance and the Company’s performance. The award program will also be designed to reinforce the Company’s goals and then current strategic initiatives. The annual performance-based cash awards will be based on the achievement of Company and individual performance metrics established at the beginning of each fiscal year by the compensation committee and our Board of Directors. Following the end of each fiscal year, the compensation committee will be responsible for determining the bonus amount payable to the executive officer based on the achievement of the Company’s performance and the individual performance metrics established for such executive. | |
| ● | Long-Term Equity Awards: Our Board of Directors believes that equity ownership by our executive officers and key employees encourages them to create long-term value and aligns their interest with those of our stockholders. We intend to grant annual equity awards to our executive officers under our 2014 Stock Option Plan. Our Board of Directors adopted and approved the following 2014 Stock Option Plan and intends to submit it for approval by our stockholders. | |
| ● | 2014 Stock Option Plan: 2,642,898 shares of our Common Stock have been initially authorized and reserved for issuance under our 2014 Stock Plan as option awards. This reserve may be increased by the Board on January 1, 2015 and each subsequent anniversary through January 1, 2024 by up to the number of shares of stock equal to 5.0% of the number of shares of stock issued and outstanding on the immediately preceding December 31. Appropriate adjustments will be made in the number of authorized shares and other numerical limits in our 2014 Stock Option Plan and in outstanding awards to prevent dilution or enlargement of participants’ rights in the event of a stock split or other change in our capital structure. Shares subject to awards granted under our 2014 Stock Option Plan which expire, are repurchased or are cancelled or forfeited will again become available for issuance under our 2014 Stock Option Plan. The shares available will not be reduced by awards settled in cash. Shares withheld to satisfy tax withholding obligations will not again become available for grant. The gross number of shares issued upon the exercise of stock appreciation rights or options exercised by means of a net exercise or by tender of previously owned shares will be deducted from the shares available under our 2014 Stock Option Plan. |
| 29 |
| ● | Awards may be granted under our 2014 Stock Option Plan to our employees, including officers, director or consultants, and our present or future affiliated entities. While we may grant incentive stock options only to employees, we may grant non-statutory stock options, stock appreciation rights, restricted stock purchase rights or bonuses, restricted stock units, performance shares, performance units and cash-based awards or other stock based awards to any eligible participant. | |
| ● | The 2014 Stock Option Plan will be administered by our compensation committee. Subject to the provisions of our 2014 Stock Option Plan, the compensation committee determines, in its discretion, the persons to whom, and the times at which, awards are granted, as well as the size, terms and conditions of each award. All awards are evidenced by a written agreement between us and the holder of the award. The compensation committee has the authority to construe and interpret the terms of our 2014 Stock Option Plan and awards granted under our 2014 Stock Option Plan. |
Our Board of Directors approved the following compensation for our named executive officers:
Michael Schuler, Interim Chief Executive Officer:
Base Salary: The compensation provided to MTF for Mr. Schuler’s monthly services is $15,000, pro-rated based upon the actual amount of time Mr. Schuler provides services to the Company.
Warrants: For the services provide by Mr. Schuler, MTF shall receive warrants with a 10 year term to purchase 50,000 shares of the Company’s Common Stock at an exercise price of $1.00 per share upon completion of Consultant’s first year of service as the Company’s Chief Executive Officer. MTF shall also receive $50,000 worth of Common Stock upon completion of each year of service Mr. Schuler provides as the Company’s Chief Executive Officer. Such issuances of Common Stock shall be split into four installments, each valued at $12,500 and distributed quarterly on the date that the Company files its Form 10-Q or Form 10-K, as applicable, with the SEC. The Common Stock will be valued at the average of the trading price for shares of Common Stock over the 10 day period prior to the issuance.
The board of directors believes that the compensation paid to MTF for Mr. Schuler’s services is in line with the Company’s goal of rapidly increasing its profitability and helps to ensure that MTF’s and Mr. Schuler’s interests are aligned with those of the Company’s stockholders.
Deina H. Walsh, Chief Financial Officer:
Base Salary: Ms. Walsh’s base salary is $100,000.
Bonus: During each calendar year beginning in 2015, Ms. Walsh shall be eligible to earn an annual target bonus of thirty-five percent (35%) of her base salary as in-effect for the applicable calendar year, subject to the achievement of personal and corporate objectives or milestones to be established by the board of directors, or any compensation committee thereof, (after considering any input or recommendations from Ms. Walsh) within sixty (60) days following the beginning of each calendar year during Ms. Walsh’s employment. In order to earn the annual bonus under this provision, the applicable objectives must be achieved and Ms. Walsh must be employed by Company at the time the annual bonus is distributed by Company. The annual bonus, if any, shall be paid on or before March 15th of the calendar year following the year in which it is considered earned. The actual annual bonus paid may be more or less than thirty-five percent (35%) of Ms. Walsh’s base salary.
| 30 |
Stock Options: Ms. Walsh was granted an option to purchase 0.75% of the Company’s fully diluted shares of Common Stock. The option will be granted under Company’s stock plan and related stock option documents. The Option is intended to be an “incentive stock option” (within the meaning of Section 422 of the Internal Revenue Code of 1986, as amended) to the greatest extent permitted under the code. The option will have an exercise price of $1.00 per share, equal to the price of the shares awarded under the Merger Agreement in connection with the Merger. As a condition of receipt of the option, Ms. Walsh will be required to sign Company’s standard form of stock option agreement and the option will be subject to the terms and conditions of the plan, the option agreement and her employment agreement. The option will vest over a three-year period from the effective date subject to Ms. Walsh’s continued Service (as defined in the plan), with 33.33% of the shares subject to the option becoming vested and exercisable on the date that Ms. Walsh’s employment agreement is executed, 33.33% of the shares subject to the option becoming vested and exercisable on the date that is twelve (12) months after the effective date, and 33.34% of the shares subject to the option vesting and becoming exercisable on the date that is twenty four (24) months after the effective date; provided, however, that all unvested shares subject to the option (and any additional equity awards hereafter issued by Company to Ms. Walsh pursuant to the plan) shall fully vest and be exercisable if Ms. Walsh’s service ceases as a result of a “qualifying termination” occurring on or within twelve (12) months after a “change in control.”
The board of directors believes that Ms. Walsh’s compensation is in line with the Company’s goal of rapidly increasing its profitability and helps to ensure that Ms. Walsh’s interests are aligned with those of the Company’s stockholders. The board of directors also believes that the compensation awarded to Ms. Walsh provides appropriate incentives and provides for rewards appropriate for the level of difficulty in achieving the applicable metrics.
William Jay Treat, Ph.D., President and Chief Technology Officer:
Base Salary: Dr. Treat’s base salary is $300,000.
Bonus: During each calendar year beginning in 2014, Dr. Treat shall be eligible to earn an annual target bonus of thirty-five percent (35%) of his base salary as in-effect for the applicable calendar year, subject to the achievement of personal and corporate objectives or milestones to be established by the board of directors, or any compensation committee thereof, (after considering any input or recommendations from Dr. Treat) within sixty (60) days following the beginning of each calendar year during Dr. Treat’s employment. In order to earn the annual bonus under this provision, the applicable objectives must be achieved and Dr. Treat must be employed by Company at the time the annual bonus is distributed by Company. The annual bonus, if any, shall be paid on or before March 15th of the calendar year following the year in which it is considered earned. The actual annual bonus paid may be more or less than thirty-five percent (35%) of Dr. Treat’s base salary.
Stock Options: Dr. Treat was granted an option to purchase 2.5% of the Company’s fully diluted shares of Common Stock outstanding as of the date of closing of the Merger. The option will be granted under Company’s stock plan and related stock option documents. The Option is intended to be an “incentive stock option” (within the meaning of Section 422 of the Internal Revenue Code of 1986, as amended) to the greatest extent permitted under the code. The option will have an exercise price per share equal to the fair market value of one share of Company’s Common Stock on the date of grant, as determined by the board of directors. As a condition of receipt of the option, Dr. Treat will be required to sign Company’s standard form of stock option agreement and the option will be subject to the terms and conditions of the plan, the option agreement and his employment agreement. The option will vest over a two-year period from the effective date subject to Dr. Treat’s continued Service (as defined in the plan), with 33.33% of the shares subject to the option becoming vested and exercisable on the date that Dr. Treat’s employment agreement is executed, 33.33% of the shares subject to the option becoming vested and exercisable on the date that is twelve (12) months after the effective date, and 33.34% of the shares subject to the option vesting and becoming exercisable on the date that is twenty four (24) months after the effective date; provided, however, that all unvested shares subject to the option (and any additional equity awards hereafter issued by Company to Dr. Treat pursuant to the plan) shall fully vest and be exercisable if Dr. Treat’s service ceases as a result of a “qualifying termination” occurring on or within twelve (12) months after a “change in control.”
| 31 |
The board of directors believes that Dr. Treat’s compensation is in line with the Company’s goal of rapidly increasing its profitability and helps to ensure that Dr. Treat’s interests are aligned with those of the Company’s stockholders. The board of directors also believes that the compensation awarded to Dr. Treat provides appropriate incentives and provides for rewards appropriate for the level of difficulty in achieving the applicable metrics.
Potential Payments upon Termination of Change in Control
There were no payments or benefits due to any of the Company’s named executive officers upon termination of their employment or a change in control of the shell company as of the end of its fiscal year ending October 31, 2013.
There were no payments or benefits due to any of Bone Biologic Inc.’s named executive officers upon termination of their employment or a change in control of Bone Biologics at the end of its fiscal year ending December 31, 2014 and 2013.
Changes to Potential Payments upon Termination of Change in Control
William Jay Treat, Ph.D.: Pursuant to the employment agreement signed with Dr. Treat immediately following the Merger, if the Company terminates Dr. Treat’s employment for other than Cause, or if Dr. Treat terminates his employment due to a breach of his employment agreement by the Company, or if Dr. Treat terminates his employment agreement for Good Reason, Dr. Treat will receive the standard entitlements and shall be entitled to receive reimbursement of any business expenses, to the extent not previously reimbursed, in accordance with his employment agreement. In addition, Dr. Treat will receive (a) a severance payment in an amount which is equivalent to the greater of the remaining number of months left in the initial term of his employment or twelve (12) months of his base salary then in effect on the date of termination, payable in equal installments (but no less frequently than once per calendar month) for a duration equal to the greater of the remaining number of months left in the Initial Term or twelve (12) months, in accordance with Company’s regular payroll cycle, beginning on the first payroll date following the date on which the general release referenced below has become effective and (b) payment (or reimbursement) of monthly premiums for Dr. Treat and Dr. Treat’s dependents’ group health care coverage continuation pursuant to the Consolidated Omnibus Budget Reconciliation Act of 1986, for the severance period, provided Dr. Treat elects to continue and remains eligible for such benefits and does not become eligible for health coverage through another employer during the severance period. Dr. Treat will only receive the severance package and other severance benefits and payments described if Dr. Treat: (i) complies with all surviving provisions of his employment agreement; and (ii) executes a separation agreement and release of claims agreement and such release has become effective in accordance with its terms prior to the 60th day following the termination date. Except for any terms and conditions of Dr. Treat’s employment agreement that by their terms survive termination of Dr. Treat’s employment, all other Company obligations to Dr. Treat pursuant to Dr. Treat’s employment agreement will become automatically terminated and completely extinguished.
For purposes of Dr. Treat’s payments upon termination, “cause” and “good reason” shall have the following meanings:
“Cause” means (a) acts or omissions constituting gross negligence, recklessness or willful misconduct on the part of Dr. Treat with respect to Dr. Treat’s obligations or otherwise relating to the business of Company; (b) any acts or conduct by Dr. Treat that are materially adverse to Company’s interests; (c) Dr. Treat’s material breach of this Agreement; (d) Dr. Treat’s material breach of Company’s employee proprietary information and inventions agreement; (e) Dr. Treat’s conviction or entry of a plea of nolo contendere for fraud, misappropriation or embezzlement, or any felony or crime of moral turpitude or that otherwise materially negatively impacts Dr. Treat’s ability to effectively perform Dr. Treat’s duties hereunder; (f) Dr. Treat’s willful neglect of duties as determined in the good faith discretion of the board of directors (provided that poor performance and/or subpar results by themselves do not constitute “cause”); or (g) the winding down of Company’s business and/or dissolution or liquidation of Company (other than in connection with a change in control). In the event of termination of Dr. Treat’s employment based on clauses (a), (b) or (f) above, Dr. Treat will have fifteen (15) days following receipt of notice from Company to cure the issue, if curable.
| 32 |
“Good Reason” means that any one or more of the following events have occurred without Dr. Treat’s express prior written consent: (i) a material adverse change in Dr. Treat’s authority, duties and/or responsibilities such that Dr. Treat’s authority, duties and/or responsibilities are no longer commensurate with Dr. Treat being Company’s President or most senior technology officer; (ii) the relocation of the primary workplace to a location that increases Dr. Treat’s daily commute by more than thirty (30) miles from its location specified in his employment agreement; (iii) any material breach by Company of any material term of Dr. Treat’s employment agreement; or (iv) any material reduction by Company (or its successor) of (A) Dr. Treat’s base salary or (B) Dr. Treat’s target bonus, unless any such reduction is made as part of, and is generally consistent with, a general reduction of senior executive base salaries or target bonuses, respectively, in which case such a reduction shall not constitute Good Reason. In order to resign his employment for Good Reason, Dr. Treat must within 60 days of Dr. Treat’s awareness of the applicable Good Reason event(s) provide Company with written notice informing Company about Dr. Treat’s intention to resign his employment for Good Reason unless such event(s) is cured or remedied by Company. Company will have 30 days after its receipt of such notice to cure or remedy the good reason event(s). If Company does not timely cure or remedy the good reason event(s), then Dr. Treat can resign Dr. Treat’s employment for good reason at any time within 30 days following the expiration of the 30 day cure/remedy period.
Deina H. Walsh: Pursuant to the employment agreement signed with Ms. Walsh immediately following the Merger, if the Company terminates Ms. Walsh’s employment for other than Cause, or if Ms. Walsh terminates her employment due to a breach of her employment agreement by the Company, or if Ms. Walsh terminates her employment agreement for Good Reason, Ms. Walsh will receive the standard entitlements and shall be entitled to receive reimbursement of any business expenses, to the extent not previously reimbursed, in accordance with her employment agreement. In addition, Ms. Walsh will receive (a) a severance payment in an amount which is equivalent to the greater of the remaining number of months left in the initial term of her employment or six (6) months of her base salary then in effect on the date of termination, payable in equal installments (but no less frequently than once per calendar month) for a duration equal to the greater of the remaining number of months left in the Initial Term or six (6) months, in accordance with Company’s regular payroll cycle, beginning on the first payroll date following the date on which the general release referenced below has become effective and (b) payment (or reimbursement) of monthly premiums for Ms. Walsh and Ms. Walsh’s dependents’ group health care coverage continuation pursuant to the Consolidated Omnibus Budget Reconciliation Act of 1986, for the severance period, provided Ms. Walsh elects to continue and remains eligible for such benefits and does not become eligible for health coverage through another employer during the severance period. Ms. Walsh will only receive the severance package and other severance benefits and payments described if Ms. Walsh: (i) complies with all surviving provisions of her employment agreement; and (ii) executes a separation agreement and release of claims agreement and such release has become effective in accordance with its terms prior to the 60th day following the termination date. Except for any terms and conditions of Ms. Walsh’s employment agreement that by their terms survive termination of Ms. Walsh’s employment, all other Company obligations to Ms. Walsh pursuant to Ms. Walsh’s employment agreement will become automatically terminated and completely extinguished.
For purposes of Ms. Walsh’s payments upon termination, “cause” and “good reason” shall have the following meanings:
“Cause” means (a) acts or omissions constituting gross negligence, recklessness or willful misconduct on the part of Ms. Walsh with respect to Ms. Walsh’s obligations or otherwise relating to the business of Company; (b) any acts or conduct by Ms. Walsh that are materially adverse to Company’s interests; (c) Ms. Walsh’s material breach of this Agreement; (d) Ms. Walsh’s material breach of Company’s employee proprietary information and inventions agreement; (e) Ms. Walsh’s conviction or entry of a plea of nolo contendere for fraud, misappropriation or embezzlement, or any felony or crime of moral turpitude or that otherwise materially negatively impacts Ms. Walsh’s ability to effectively perform Ms. Walsh’s duties hereunder; (f) Ms. Walsh’s willful neglect of duties as determined in the good faith discretion of the board of directors (provided that poor performance and/or subpar results by themselves do not constitute “cause”); or (g) the winding down of Company’s business and/or dissolution or liquidation of Company (other than in connection with a change in control). In the event of termination of Ms. Walsh’s employment based on clauses (a), (b) or (f) above, Ms. Walsh will have fifteen (15) days following receipt of notice from Company to cure the issue, if curable.
| 33 |
“Good Reason” means that any one or more of the following events have occurred without Ms. Walsh’s express prior written consent: (i) a material adverse change in Ms. Walsh’s authority, duties and/or responsibilities such that Ms. Walsh’s authority, duties and/or responsibilities are no longer commensurate with Ms. Walsh being Company’s President or most senior technology officer; (ii) the relocation of the primary workplace to a location that increases Ms. Walsh’s daily commute by more than thirty (30) miles from its location specified in her employment agreement; (iii) any material breach by Company of any material term of Ms. Walsh’s employment agreement; or (iv) any material reduction by Company (or its successor) of (A) Ms. Walsh’s base salary or (B) Ms. Walsh’s target bonus, unless any such reduction is made as part of, and is generally consistent with, a general reduction of senior executive base salaries or target bonuses, respectively, in which case such a reduction shall not constitute Good Reason. In order to resign her employment for Good Reason, Ms. Walsh must within 60 days of Ms. Walsh’s awareness of the applicable Good Reason event(s) provide Company with written notice informing Company about Ms. Walsh’s intention to resign her employment for Good Reason unless such event(s) is cured or remedied by Company. Company will have 30 days after its receipt of such notice to cure or remedy the good reason event(s). If Company does not timely cure or remedy the good reason event(s), then Ms. Walsh can resign Ms. Walsh’s employment for good reason at any time within 30 days following the expiration of the 30 day cure/remedy period.
Employment and Consulting Agreements for Executives
The Company has entered into employment agreements with the following executives: William Jay Treat, Deina H. Walsh and MTF (for the services of Michael Schuler).
MTF (for the services of Michael Schuler): Immediately following the Merger, the Company entered into a consulting agreement with MTF, which has agreed to provide the services of Mr. Schuler to the Company as a contractor. Pursuant to the agreement, Mr. Schuler will serve as the Company’s Interim Chief Executive Officer for a period of 6 months. The agreement shall automatically renew for successive three (3) month periods unless either party provides written notice to the other party at least 10 days in advance of the renewal term of its decision not to renew the term. The agreement is intended to be temporary in nature, and will cease once the Company retains a permanent Chief Executive Officer. There are no payments due to MTF or Mr. Schuler with respect to any change in control of the Company or termination of the consulting agreement. Please see our discussion of Changes in Executive Compensation for further information regarding the compensation to be paid to MTF for Mr. Schuler’s services pursuant to the consulting agreement with the Company.
William J. Treat: Immediately following the Merger, the Company entered into an employment agreement with Dr. Treat. Pursuant to the agreement, Dr. Treat will serve as the Company’s President and Chief Technology Officer for an initial term of two years. The agreement shall automatically renew for successive one (1) year periods unless either party provides written notice to the other party at least thirty (30) days in advance of the renewal term of its decision not to renew the agreement. Please see our discussion of Changes in Executive Compensation for further information regarding Dr. Treat’s compensation pursuant to his employment agreement with the Company. Please see our discussion of Potential Payments upon Termination or Change in Control for information regarding any termination payments that may become due to Dr. Treat pursuant to his employment agreement with the Company.
Deina H. Walsh: On November 4, 2014, the Company entered into a part-time employment agreement with Ms. Walsh. Ms. Walsh will serve as the Company’s Chief Executive Officer at-will and not for any specified period and may be terminated at any time with or without cause. Please see our discussion of Changes in Executive Compensation for further information regarding Ms. Walsh’s compensation pursuant to her employment agreement with the Company. Please see our discussion of Potential Payments upon Termination or Change in Control for information regarding any termination payments that may become due to Ms. Walsh pursuant to her employment agreement with the Company.
Grants of Plan-Based Awards
The Company issued equity awards to William Jay Treat, President, and Deina Walsh, CFO, of 585.059 and 174,918 options, respectively. The options will have an exercise price per share equal to the fair market value of one share of Company’s Common Stock on the date of grant. The options vest over a two-year period and have an expiration date between 7 to 10 years.
| 34 |
Outstanding Equity Awards at Fiscal Year End
| Option awards | Stock awards | |||||||||||||||||||||||
| Name | Number of securities underlying unexercised options (#) exercisable | Number of securities underlying unexercised options (#) unexercisable | Equity incentive plan awards: Number of securities underlying unexercised unearned options (#) | Option exercise price ($) | Option expiration date | Number of shares or units of stock that
have not vested (#) | Market value of shares of units of stock
that have not vested ($) | Equity incentive plan awards: Number of
unearned shares, units or other rights that have not vested (#) | Equity incentive plan awards: Market or
payout value of unearned shares, units or other rights that have not vested ($) | |||||||||||||||
| (a) | (b) | (c) | (d) | (e) | (f) | (g) | (h) | (i) | (j) | |||||||||||||||
| Mike Schuler, CEO 2014 2013 | - - | - - | - - | - - | - - | - - | - - | - - | - - | |||||||||||||||
| Dr. William Jay Treat, President, Chief Technology Officer 2014 2013 | 198,202 - | 384,857 - | - - | $ | 1.00 - | September 18, 2020 - | - - | - - | - - | - - | ||||||||||||||
| Deina Walsh, Chief Financial Officer 2014 2013 | 58,306 - | 116,612 - | - - | $ | 1.00 - | November 3, 2024 - | - - | - - | - - | - - | ||||||||||||||
The Company had no outstanding equity awards owed to our named executive officers that were outstanding as of the end of our fiscal year on October 31, 2013. Additionally, Bone Biologics Inc. had no outstanding equity awards owed to its named executive officers that were outstanding as of the end of its fiscal year on December 31, 2013.
Director Compensation
The following table shows information regarding the compensation earned during the fiscal year ended December 31, 2014 by the members of our board of directors.
| Name | Fees
Earned or Paid in Cash |
Option Awards | Total | |||||||
| Bruce Stroever(1) | $ | 8,750 | $ | - | $ | 8,750 | ||||
| Dr. Chia Soo | 6,250 | - | 6,250 | |||||||
| William Coffin | 7,500 | - | 7,500 | |||||||
| John Booth | 7,500 | 7,500 | ||||||||
| Jimmy Delshad | 6,250 | 6,250 | ||||||||
| Steve Warnecke | 7,500 | 7,500 | ||||||||
| George A. Oram | 1,648 | 1,648 | ||||||||
| | | | | | | | | | | |
| Total | $ | 45,398 | $ | - | $ | 45,398 | ||||
| (1) | MTF is paid compensation for Mr. Stroever’s services per a consulting agreement with the Company. |
For the year ended December 31, 2014, there were no option awards for our directors outstanding pursuant to the 2014 Stock Incentive Plan.
| 35 |
Changes in Director Compensation
Since June 2014, the Company executed employment agreements with certain board of directors and all these employment agreements are effective immediately following the Merger. Following is the compensation for each of these Board of Directors.
Bruce Stroever: Pursuant to a consulting agreement by and between the Company and MTF, MTF has agreed to provide the services of Mr. Stroever to serve as the chairman of the Company’s board of directors. MTF has agreed to provide Mr. Stroever’s services as chairman of the board of directors for a one year term. For the services being provided, MTF shall receive an annual payment of $35,000, paid quarterly. In addition, MTF shall receive warrants equal to 50,000 shares of the Company’s $0.001 par value per share Common Stock upon completion of the first year of service as Company’s Chief Executive Officer. Consultant shall thereafter receive $50,000 worth of Common Stock upon completion of each year of service as the Company’s Chief Executive Officer. Such issuances of Common Stock shall be split into four installments, each valued at $12,500 and distributed quarterly on the date that the Company files its Form 10-Q or Form 10-K, as applicable, with the SEC. The Common Stock will be valued at the average of the trading price for shares of Common Stock over the 10 day period prior to the issuance.
Dr. Chia Soo, MD: Dr. Soo shall serve as a director of the Company for a one year term. Dr. Soo shall receive annual compensation of $25,000, paid quarterly, during her tenure as a board member. In addition, Dr. Soo shall receive an option to purchase 50,000 shares of the Company’s $0.001 par value per share Common Stock at an exercise price of $1.00 per share upon completion of first year of service. These options will have a term of 10 years. Dr. Soo shall also receive $50,000 worth of Common Stock upon completion of each year of service as member of the Board. Such issuances of Common Stock shall be split into four installments, each valued at $12,500 and distributed quarterly on the date that the Company files its Form 10-Q or Form 10-K, as applicable, with the SEC. The Common Stock will be valued at the average of the trading price for shares of Common Stock over the 10 day period prior to the issuance.
William Coffin: Mr. Coffin shall serve as a director of the Company and chairman of the corporate governance committee for a one year term. Mr. Coffin shall receive annual compensation of $25,000, paid quarterly, during his tenure as a board member. Mr. Coffin shall also receive $5,000 as annual compensation for his service as the chairman of the corporate governance committee. In addition, Mr. Coffin shall receive an option to purchase 50,000 shares of the Company’s $0.001 par value per share Common Stock at an exercise price of $1.00 per share upon completion of first year of service. These options will have a term of 10 years. Mr. Coffin shall also receive $50,000 worth of Common Stock upon completion of each year of service as member of the Board. Such issuances of Common Stock shall be split into four installments, each valued at $12,500 and distributed quarterly on the date that the Company files its Form 10-Q or Form 10-K, as applicable, with the SEC. The Common Stock will be valued at the average of the trading price for shares of Common Stock over the 10 day period prior to the issuance.
John Booth: Mr. Booth shall serve as a director of the Company and chairman of the compensation committee for a one year term. Mr. Booth shall receive annual compensation of $25,000, paid quarterly, during his tenure as a board member. Mr. Booth shall also receive $5,000 as annual compensation for his service as the chairman of the compensation committee. In addition, Mr. Booth shall receive an option to purchase 50,000 shares of the Company’s $0.001 par value per share Common Stock at an exercise price of $1.00 per share upon completion of first year of service. These options will have a term of 10 years. Mr. Booth shall also receive $50,000 worth of Common Stock upon completion of each year of service as member of the Board. Such issuances of Common Stock shall be split into four installments, each valued at $12,500 and distributed quarterly on the date that the Company files its Form 10-Q or Form 10-K, as applicable, with the SEC. The Common Stock will be valued at the average of the trading price for shares of Common Stock over the 10 day period prior to the issuance.
Jimmy Delshad: Mr. Delshad shall serve as a director of the Company for a one year term. Mr. Delshad shall receive annual compensation of $25,000, paid quarterly, during his tenure as a board member. In addition, Mr. Delshad shall receive an option to purchase 50,000 shares of the Company’s $0.001 par value per share Common Stock at an exercise price of $1.00 per share upon completion of first year of service. These options will have a term of 10 years. Mr. Delshad shall also receive $50,000 worth of Common Stock upon completion of each year of service as member of the Board. Such issuances of Common Stock shall be split into four installments, each valued at $12,500 and distributed quarterly on the date that the Company files its Form 10-Q or Form 10-K, as applicable, with the SEC. The Common Stock will be valued at the average of the trading price for shares of Common Stock over the 10 day period prior to the issuance.
| 36 |
Steve Warnecke: Mr. Warnecke shall serve as a director of the Company and chairman of the audit committee for a one year term. Mr. Warnecke shall receive annual compensation of $25,000, paid quarterly, during his tenure as a board member. Mr. Warnecke shall also receive $5,000 as annual compensation for his service as the chairman of the audit committee. In addition, Mr. Warnecke shall receive an option to purchase 50,000 shares of the Company’s $0.001 par value per share Common Stock at an exercise price of $1.00 per share upon completion of first year of service. These options will have a term of 10 years. Mr. Warnecke shall also receive $50,000 worth of Common Stock upon completion of each year of service as member of the Board. Such issuances of Common Stock shall be split into four installments, each valued at $12,500 and distributed quarterly on the date that the Company files its Form 10-Q or Form 10-K, as applicable, with the SEC. The Common Stock will be valued at the average of the trading price for shares of Common Stock over the 10 day period prior to the issuance.
George A. Oram: Mr. Oram shall serve as a director of the Company for a one year term. Mr. Oram shall receive annual compensation of $25,000, paid quarterly, during his tenure as a board member. In addition, Mr. Oram shall receive an option to purchase 50,000 shares of the Company’s $0.001 par value per share Common Stock at an exercise price of $1.00 per share upon completion of first year of service. These options will have a term of 10 years. Mr. Oram shall also receive $50,000 worth of Common Stock upon completion of each year of service as member of the Board. Such issuances of Common Stock shall be split into four installments, each valued at $12,500 and distributed quarterly on the date that the Company files its Form 10-Q or Form 10-K, as applicable, with the SEC. The Common Stock will be valued at the average of the trading price for shares of Common Stock over the 10 day period prior to the issuance.
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
The following table sets forth information with respect to the beneficial ownership of the Company’s Common Stock as of March 30, 2015, by each person or group of affiliated persons known to the Company to beneficially own 5% or more of its Common Stock, each director, each named executive officer, and all of its directors and named executive officers as a group.
| Name of Beneficial Owner or Identity of Group | Title of Class | Shares(1) | Percentage | |||||||
| 5% or greater stockholders: | ||||||||||
| The Musculoskeletal Transplant
Foundation, Inc. 125 May Street Edison, NJ 08837 |
Common Stock | 11,932,807 | (5) | 37.8 | % | |||||
Hankey Capital, LLC 4751 Wilshire Blvd #110 Los Angeles, CA 90010 |
Common Stock | 7,120,254 | (3) | 22.6 | % | |||||
Don R. Hankey 4751 Wilshire Blvd #110 Los Angeles, CA 90010 |
Common Stock | 7,570,254 | (4) | 24.0 | % | |||||
| AFH Holding & Advisory, LLC 10830 Massachusetts Ave., Penthouse Los Angeles, CA 90024 |
Common Stock | 2,609,602 | (6) | 8.3 | % | |||||
| Amir Heshmatpour 269 Beverly Drive, Ste. 1600 Beverly Hills, CA 90212 |
Common Stock | 3,609,602 | (2) | 11.4 | % | |||||
| Dr. Kang Ting 115 North Doheny Drive Beverly Hills, CA 90211 |
Common Stock | 2,000,000 | 6.3 | % | ||||||
| Orthofix Holdings Inc. 3451 Plana Parkway Lewisville, TX 75056 |
Common Stock | 1,909,908 | (7) | 6.0 | % | |||||
| 37 |
| Executive Officers and Directors: | ||||||||||
| Michael Schuler 175 May Street Edison, NJ 08837 |
Common Stock | - | - | |||||||
| William J. Treat 175 May Street, Suite 400 Edison, NJ 08837 |
Common Stock | 198,202 | (8) | 0.6 | % | |||||
| Deina H. Walsh 175 May Street, Suite 400 Edison, NJ 08837 |
Common Stock | 58,306 | (10) | 0.2 | % | |||||
| Bruce Stroever | ||||||||||
| 175 May Street, Suite 400 | ||||||||||
| Edison, NJ 08837 | - | - | ||||||||
| Dr. Chia Soo 1175 May Street, Suite 400
Edison, NJ 08837 |
Common Stock | 1,119,318 | (11) | 3.5 | % | |||||
| William Coffin 175 May Street, Suite 400 Edison, NJ 08837 |
- | - | ||||||||
| John Booth 175 May Street, Suite 400 Edison, NJ 08837 |
- | - | ||||||||
| Jimmy Delshad 175 May Street, Suite 400 Edison, NJ 08837 |
- | - | ||||||||
| Steve Warnecke 1026 Anaconda Drive Castle Rock, CO 80108 |
- | - | ||||||||
George Oram 12 Edgemont Lane, Bedminster, NJ 07920 |
- | - | ||||||||
| Total Officers and Directors as a Group | Common Stock | 1,375,826 | (12) | 4.4 | % | |||||
| Reserve for Future Issuance: | ||||||||||
| Option Plan | 2,386,390 | (9) |
| 38 |
| (1) | The number of shares of Common Stock issued and outstanding that was used to calculate the percentage ownership of each listed person includes the shares of Common Stock underlying convertible debt, stock options and warrants that are exercisable 60 days after the close of the Merger. | |
| (2) | These shares of Common Stock are owned by AFH Holding, H&H and Mr. Heshmatpour’s spouse and children. Mr. Heshmatpour is sole member of AFH Holding and has sole voting and investment control over the 2,609,602 shares of Common Stock owned of record by AFH Holding. Mr. Heshmatpour is the sole member of H&H and has sole voting and investment control over the 200,000 shares of Common Stock owned of record by H&H. Mr. Heshmatpour has voting and investment control over the 800,000 shares of Common Stock owned by his spouse and children. Accordingly, he may be deemed a beneficial owner with respect to these 3,609,602 shares of Common Stock. | |
| (3) | Consists of 3,955,697 shares of Common Stock underlying warrants exercisable within 60 days and 3,164,557 shares of Common Stock underlying debt convertible at any time. This does not include the 6,329,114 shares that were issued as collateral for the October 2014 convertible note to Hankey Capital. Mr. Hankey has voting and investment control over any shares held by Hankey Capital and may be deemed a beneficial owner with respect to any such shares. | |
| (4) | These shares of Common Stock are owned by Don R. Hankey, Trustee of the Don Hankey Trust and Hankey Capital. Mr. Hankey has voting and investment control over the 450,000 shares of Common Stock owned of record by Don R. Hankey, Trustee of the Don Hankey Trust and the 7,120,254 held by Hankey Capital. Accordingly, Mr. Hankey may be deemed a beneficial owner with respect to the 7,570,254 shares. | |
| (5) | Includes 3,659,328 shares of Common Stock underlying convertible debt and 793,383 shares of Common Stock underlying warrants exercisable within 60 days. | |
| (6) | Includes 525,000 shares of Common Stock underlying warrants exercisable within 60 days. | |
| (7) | Includes 458,334 shares of Common Stock underlying warrants exercisable within 60 days. | |
| (8) | Includes 198,202 shares of Common Stock underlying stock options exercisable within 60 days. | |
| (9) | Represents a reserve for a future option plan. | |
| (10) | Includes 58,306 shares of Common Stock underlying stock options exercisable within 60 days. | |
| (11) | Includes 119,318 shares of Common Stock underlying warrants exercisable within 60 days. | |
| (12) | Includes 119,318 shares of Common Stock underlying warrants exercisable within 60 days and 256,508 shares of Common Stock underlying stock options exercisable within 60 days. |
Item 13. Certain Relationships and Related Transactions, and Director Independence
Except as disclosed below, none of the following persons has any direct or indirect material interest in any transaction to which we are a party since our incorporation or in any proposed transaction to which we are proposed to be a party:
| ● | Any of our directors or officers; | |
| ● | Any proposed nominee for election as our director; | |
| ● | Any person who beneficially owns, directly or indirectly, shares carrying more than 5% of the voting rights attached to our Common Stock; or | |
| ● | Any relative or spouse of any of the foregoing persons, or any relative of such spouse, who has the same house as such person or who is a director or officer of any parent or subsidiary of our Company. |
| 39 |
Review, Approval or Ratification of Transactions with Related Persons
Due to the small size of our Company, we do not at this time have a formal written policy regarding the review of related party transactions, and rely on our full Board of Directors to review, approve or ratify such transactions and identify and prevent conflicts of interest. Our Board of Directors reviews any such transaction in light of the particular affiliation and interest of any involved director, officer or other employee or stockholder and, if applicable, any such person’s affiliates or immediate family members. Management aims to present transactions to our Board of Directors for approval before they are entered into or, if that is not possible, for ratification after the transaction has occurred. If our Board of Directors finds that a conflict of interest exists, then it will determine the appropriate action or remedial action, if any. Our Board of Directors approves or ratifies a transaction if it determines that the transaction is consistent with our best interests and the best interest of our stockholders.
Director Independence
Our Board of Directors currently consists of seven (7) members, including Bruce Stroever, Dr. Chia Soo, William Coffin, John Booth, Jimmy Delshad, Steve Warnecke and George Oram. Our Board of Directors undertook a review of the composition of our Board of Directors and the independence of each director. Based upon information requested from and provided by each director concerning his background, employment and affiliations, including family relationships, our Board of Directors has determined that William Coffin, John Booth, Jimmy Delshad, Dr. Chia Soo and Steve Warnecke (the “Independent Directors”) would qualify as “independent” as that term is defined by NASDAQ Listing Rule 5605(a) (2). Further, our Board of Directors has determined that each of the independent directors would qualify as “independent” under NASDAQ Listing Rules applicable to such board committees. Bruce Stroever would not qualify as “independent” under applicable NASDAQ Listing Rules applicable to the Board of Directors generally or to separately designated board committees because he is the Chief Executive Officer of MTF, a significant shareholder of the Company and an entity to whom the Company continues to owe obligations to pursuant to notes outstanding to MTF. George Oram would not qualify as “independent” under applicable NASDAQ Listing Rules applicable to the Board of Directors generally or to separately designated board committees because he was an executive vice president of MTF until December 2013. In making such determinations, our Board of Directors considered the relationships that each of our nonemployee directors has with the Company and all other facts and circumstances deemed relevant in determining independence, including the beneficial ownership of our capital stock by each non-employee director.
Subject to some exceptions, NASDAQ Listing Rule 5605(a)(2) provides that a director will only qualify as an “independent director” if, in the opinion of our Board of Directors, that person does not have a relationship that would interfere with the exercise of independent judgment in carrying out the responsibilities of a director, and that a director cannot be an “independent director” if (a) the director is, or in the past three years has been, an employee of ours; (b) a member of the director’s immediate family is, or in the past three years has been, an executive officer of ours; (c) the director or a member of the director’s immediate family has received more than $120,000 per year in direct compensation from us within the preceding three years, other than for service as a director or benefits under a tax-qualified retirement plan or non-discretionary compensation (or, for a family member, as a non-executive employee); (d) the director or a member of the director’s immediate family is a current partner of our independent public accounting firm, or has worked for such firm in any capacity on our audit at any time during the past three years; (e) the director or a member of the director’s immediate family is, or in the past three years has been, employed as an executive officer of a company where one of our executive officers serves on the compensation committee; or (f) the director or a member of the director’s immediate family is an executive officer, partner or controlling shareholder of a company that makes payments to, or receives payments from, us in an amount which, in any twelve-month period during our past three fiscal years, exceeds the greater of 5% of the recipient’s consolidated gross revenues for that year or $200,000 (except for payments arising solely from investments in our securities or payments under non-discretionary charitable contribution matching programs). Additionally, in order to be considered an independent member of an audit committee under Rule 10A-3 of the Exchange Act, a member of an audit committee may not, other than in his or her capacity as a member of the audit committee, the Board of Directors, or any other committee of the Board of Directors, accept, directly or indirectly, any consulting, advisory, or other compensatory fee from the applicable company or any of its subsidiaries or otherwise be an affiliated person of the applicable company or any of its subsidiaries.
| 40 |
Item 14. Accounting Fees and Services
Audit Committee Pre-Approval of Audit and Permissible Non-Audit Services of Independent Registered Public Accounting Firm
The audit committee pre-approves all audit and permissible non-audit services provided by our independent registered public accounting firm. These services may include audit services, audit-related services, tax services and other services. The audit committee has adopted policies and procedures for the pre-approval of services provided by our independent registered public accounting firm. The policies and procedures provide that management and our independent registered public accounting firm jointly submit to the audit committee a schedule of audit and non-audit services for approval as part of the annual plan for each year. In addition, the policies and procedures provide that the audit committee may also pre-approve particular services not in the annual plan on a case-by-case basis. For each proposed service, management must provide a detailed description of the service and the projected fees and costs (or a range of such fees and costs) for the service. The policies and procedures require management and our independent registered public accounting firm to provide quarterly updates to the audit committee regarding services rendered to date and services yet to be performed.
The following table sets forth the aggregate fees billed to us for the years ended December 31, 2014 and 2013.
Audit Fees
| 2014 | 2013 | |||||||
| Anton & Chia (1) | $ | 73,628 | $ | - | ||||
| BDO (2) | $ | 28,925 | $ | 13,651 | ||||
| EFP Rotenberg (3) | $ | - | $ | 2,500 | ||||
| Anton & Chia (3) | $ | 2,080 | $ | - | ||||
(1) Includes review and consent of Form S-1 registration statement and the audit of the historical financial statements of Bone Biologics Inc. included in the Company’s Form 8k filed on September 24, 2014.
(2) BDO was initially engaged to audit the historical financial statements of Bone Biologics Inc.
(3) Fees paid for services provided prior to the merger for audit of the shell entity.
Audit Related Fees
There were no fees billed to the Company by A&C for assurance and related services that are reasonably related to the performance of the audit related fees.
Tax Fees
| Foster, Griffith and Allen, Inc. | $ | 3,373 | $ | 2,350 |
Item 15. Exhibits, Financial Statement Schedules
(a) Exhibits
See Exhibit Index.
(b) Financial Statements:
The financial statements required to be included in this Annual Report are included at pages 45 to 68
| 41 |
Pursuant to the requirements of Section 13 or 15(d) of the Securities Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized on March 31, 2015.
BONE BIOLOGICS, CORP. | ||
| By: | /s/ Michael Schuler | |
| Name: | Michael Schuler | |
| Title: | Chief Executive Officer | |
KNOW ALL PERSONS BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints Michael Schuler and Deina H. Walsh, and each of them, as his true and lawful attorneys-in-fact and agents, with full power of substitution and resubstitution, for him and in his name, place, and stead, in any and all capacities, to sign any and all amendments to this Annual Report on Form 10-K and to file the same, with all exhibits thereto and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorneys-in-fact and agents and each of them, full power and authority to do and perform each and every act and thing requisite and necessary to be done in connection therewith as fully to all intents and purposes as he might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents, or any of them, or their or his substitute or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Act of 1934, this report has been signed by the following persons in the capacities and on the dates indicated.
| Signature | Title | Date | ||
| /s/ Michael Schuler | ||||
| Michael Schuler | Chief Executive Officer (Principal Executive Officer) |
March 31, 2015 | ||
| /s/ Deina H. Walsh | ||||
| Deina H. Walsh | Chief Financial Officer (Principal Financial Officer and Principal Accounting Officer) |
March 31, 2015 | ||
| /s/ Bruce Stroever | ||||
Bruce Stroever |
Director | March 31, 2015 | ||
| /s/ Dr. Chia Soo | ||||
| Dr. Chia Soo | Director | March 31, 2015 | ||
| /s/ William Coffin | ||||
| William Coffin | Director | March 31, 2015 | ||
| /s/ John Booth | ||||
John Booth |
Director | March 31, 2015 | ||
| /s/ Jimmy Delshad | ||||
Jimmy Delshad |
Director | March 31, 2015 | ||
| /s/ Steve Warnecke | ||||
Steve Warnecke |
Director | March 31, 2015 | ||
| /s/ George A. Oram | ||||
| George A. Oram | Director | March 31, 2015 |
| 42 |
| Bone Biologics, Corp. | |
Consolidated Financial Statements For the year ended December 31, 2014 |
| 43 |
Bone Biologics, Corp.
| 44 |

REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors
Bone Biologics, Corp.
26632 Towne Centre Drive Suite 300
Foothill Ranch, CA 92610
We have audited the accompanying consolidated balance sheets of Bone Biologics, Corp. (the “Company”) as of December 31, 2014 and 2013 and the related consolidated statements of operations, consolidated changes in stockholders’ deficit and consolidated cash flows for the years then ended. These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these consolidated financial statements based on our audits.
We conducted our audits in accordance with standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement. The Company was not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audits included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the consolidated financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall consolidated financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the consolidated financial position of the Company as of December 31, 2014 and 2013 and the consolidated results of its operations and its consolidated cash flows for the years then ended, in conformity with accounting principles generally accepted in the United States of America.
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 1 to the consolidated financial statements, these conditions raise substantial doubt about its ability to continue as a going concern. Management’s plans in regard to these matters are also described in Note 1. The consolidated financial statements do not include any adjustments relating to the recoverability and classification of asset carrying amounts or the amount and classification of liabilities that might result should the Company be unable to continue as a going concern.
| /s/ Anton & Chia, LLP | |
| Newport Beach, California | |
| March 26, 2015 |
| 45 |
Bone Biologics, Corp.
| December 31, 2014 | December 31, 2013 | |||||||
| Assets | ||||||||
| Current assets | ||||||||
| Cash | $ | 2,661,396 | $ | 1,538 | ||||
| Prepaid expenses | 89,517 | 10,767 | ||||||
| Deferred transaction costs | - | 75,000 | ||||||
| Deferred financing fees | 983,857 | - | ||||||
| Other receivable – related party | 75,000 | - | ||||||
| Total current assets | 3,809,770 | 87,305 | ||||||
| Property and Equipment, net | 11,621 | - | ||||||
| Total assets | $ | 3,821,391 | $ | 87,305 | ||||
| Liabilities and Stockholders’ Deficit | ||||||||
| Current liabilities | ||||||||
| Accounts payable and accrued expenses | $ | 215,389 | $ | 1,525,604 | ||||
| Advances due to related party | - | 41,300 | ||||||
| Notes payable to related party | 3,659,328 | 3,947,817 | ||||||
| Notes payable, net of debt discount | - | 180,690 | ||||||
| Total current liabilities | 3,874,717 | 5,695,411 | ||||||
| Notes payable, net of debt discount | 3,645,194 | - | ||||||
| Total liabilities | 7,519,911 | 5,695,411 | ||||||
| Commitments and Contingencies | ||||||||
| Stockholders’ deficit | ||||||||
| Preferred Stock, $0.001 par value per share; 20,000,000 shares authorized; none issued or outstanding at December 31, 2014 and 2013 | - | - | ||||||
| Common stock, $0.001 par value per share; 100,000,000 shares authorized; 24,269,047 and 10,928,099 shares issued and outstanding at December 31, 2014 and 2013, respectively | 24,269 | 10,928 | ||||||
| Additional paid-in capital | 8,315,128 | 1,994,470 | ||||||
| Accumulated deficit | (12,037,917 | ) | (7,613,504 | ) | ||||
| Total stockholders’ deficit | (3,698,520 | ) | (5,608,106 | ) | ||||
| Total liabilities and stockholders’ deficit | $ | 3,821,391 | $ | 87,305 | ||||
See accompanying notes to consolidated financial statements.
| 46 |
Bone Biologics, Corp.
Consolidated Statements of Operations
| December 31, 2014 | December 31, 2013 | |||||||
| Revenues | $ | - | $ | - | ||||
| Cost of revenues | - | - | ||||||
| Gross profit | - | - | ||||||
| Operating expenses | ||||||||
| Research and development | 623,522 | 188,236 | ||||||
| General and administrative | 1,509,306 | 483,749 | ||||||
| Transaction costs | 877,776 | - | ||||||
| Total operating expenses | 3,010,604 | 671,985 | ||||||
| Loss from operations | (3,010,604 | ) | (671,985 | ) | ||||
| Other expense | ||||||||
| Other expense | (9,624 | ) | - | |||||
| Interest expense, net | (1,402,585 | ) | (409,419 | ) | ||||
| Total other expense | (1,412,209 | ) | (409,419 | ) | ||||
| Loss before provision for income taxes | (4,422,813 | ) | (1,081,404 | ) | ||||
| Provision for income taxes | 1,600 | 800 | ||||||
| Net loss | $ | (4,424,413 | ) | $ | (1,082,204 | ) | ||
| Weighted average shares outstanding – basic and diluted | 14,952,205 | 10,928,099 | ||||||
| Loss per share – basic and diluted | $ | (0.30 | ) | $ | (0.10 | ) | ||
See accompanying notes to consolidated financial statements.
| 47 |
Bone Biologics, Corp.
Consolidated Statements of Stockholders’ Deficit
| Common Stock | Additional Paid-in | Accumulated | Total Stockholders’ | |||||||||||||||||
| Shares | Amount | Capital | Deficit | Deficit | ||||||||||||||||
| Balance at December 31, 2012 | 10,928,099 | $ | 10,928 | $ | 1,844,103 | $ | (6,531,301 | ) | $ | (4,676,270 | ) | |||||||||
| Net Loss | - | - | - | (1,082,203 | ) | (1,082,203 | ) | |||||||||||||
| Warrants issued in connection with Bridge Notes | - | - | 150,367 | - | 150,367 | |||||||||||||||
| Balance at December 31, 2013 | 10,928,099 | $ | 10,928 | $ | 1,994,470 | $ | (7,613,504 | ) | $ | (5,608,106 | ) | |||||||||
| Warrants issued in connection with Notes Payable | 2,312,755 | - | 2,312,755 | |||||||||||||||||
| Issuance of common stock, net of issuance costs | 500,000 | 500 | 479,500 | - | 480,000 | |||||||||||||||
| Shares issued to existing AFH Acquisition X, Inc. shareholders | 3,853,600 | 3,854 | (3,854 | ) | - | - | ||||||||||||||
| Debt converted into common shares | 2,658,234 | 2,658 | 2,488,350 | - | 2,491,008 | |||||||||||||||
| Warrants issued for Services | - | - | 787,266 | - | 787,266 | |||||||||||||||
| Stock Compensation | - | - | 256,641 | - | 256,641 | |||||||||||||||
| Common shares issued for collateral on note payable | 6,329,114 | 6,329 | - | - | 6,329 | |||||||||||||||
| Net Loss | - | - | - | (4,424,413 | ) | (4,424,413) | ||||||||||||||
| Balance at December 31, 2014 | 24,269,047 | $ | 24,269 | $ | 8,315,128 | $ | (12,037,917 | ) | $ | (3,698,520 | ) | |||||||||
See accompanying notes to consolidated financial statements.
| 48 |
Bone Biologics, Corp.
Consolidated Statements of Cash Flows
| December 31, 2014 | December 31, 2013 | |||||||
| Operating activities | ||||||||
| Net loss | $ | (4,424,413 | ) | $ | (1,082,204 | ) | ||
| Adjustments to reconcile net loss to net cash provided by (used in) operating activities: | ||||||||
| Depreciation | 280 | - | ||||||
| Accrued interest expense | 318,215 | 340,268 | ||||||
| Debt discount amortization | 437,115 | 67,104 | ||||||
| Debt financing costs amortization | 60,398 | |||||||
| Warrants issued with Line of Credit | 520,487 | - | ||||||
| Stock-based compensation | 256,642 | - | ||||||
| Warrants issued to Consultants | 379,349 | - | ||||||
| Loss on sale of marketable securities | 9,624 | - | ||||||
| Transaction costs financed through notes payable | 590,000 | |||||||
| Changes in operating assets and liabilities: | ||||||||
| Prepaid expenses and other current assets | (78,750 | ) | (10,767 | ) | ||||
| Deferred financing costs | (543,401 | ) | 4,717 | |||||
| Other receivables – related party | (75,000 | ) | - | |||||
| Advances due to related party | (41,300 | ) | 41,300 | |||||
| Accounts payable and accrued expenses | (164,864 | ) | 114,217 | |||||
| Net cash (used in) operating activities | (2,755,618 | ) | (525,365 | ) | ||||
| Investing activities | ||||||||
| Purchase of furniture and equipment | (11,901 | ) | - | |||||
| Proceeds from sale of marketable securities | 37,377 | - | ||||||
| Net cash provided by investing activities | 25,476 | - | ||||||
| Financing activities | ||||||||
| Proceeds from the issuance of common stock | 480,000 | - | ||||||
| Repayment of debt | (590,000 | ) | - | |||||
| Proceeds from issuance of notes payable – related party | 553,371 | 524,533 | ||||||
| Repayment of notes payable – related party | (303,371 | ) | - | |||||
| Proceeds from issuance of notes payable | 5,250,000 | - | ||||||
| Net cash provided by financing activities | 5,390,000 | 524,533 | ||||||
| Net increase (decrease) in cash | 2,659,858 | (832 | ) | |||||
| Cash, beginning of period | 1,538 | 2,370 | ||||||
| Cash, end of period | $ | 2,661,396 | $ | 1,538 | ||||
| Supplemental non-cash information | ||||||||
| Conversion of notes payable and accrued interest | $ | - | $ | 129,717 | ||||
| Accrued transaction costs | $ | - | $ | 2,047 | ||||
| Issuance of warrants in connection with Notes Payable, net of amortization included above | $ | 248,744 | $ | 150,367 | ||||
| Issuance of warrants in payment of financing fees | $ | 21,738 | $ | - | ||||
| Interest paid | $ | 72,014 | $ | - | ||||
| Taxes paid | $ | 1,600 | $ | 800 | ||||
See accompanying notes to consolidated financial statements.
| 49 |
Bone Biologics, Corp.
Notes to Consolidated Financial Statements
1. The Company
Bone Biologics, Corp. (“Bone” or the “Company”) was incorporated under the laws of the State of Delaware on October 18, 2007 as AFH Acquisition X, Inc. Pursuant to a Merger Agreement, dated September 19, 2014, Merger Sub merged with and into Bone Biologics Inc., with Bone Biologics Inc. remaining as the surviving corporation in the Merger. Upon the consummation of the Merger, the separate existence of Merger Sub ceased. On September 22, 2014 the Company officially changed its name to “Bone Biologics, Corp.” to more accurately reflect the nature of its business, and Bone Biologics, Inc. became a wholly-owned subsidiary of the Company. Bone Biologics, Inc. was incorporated in California on March 9, 2004.
Bone is a biotechnology company that is currently focused on bone regeneration in spinal fusion using the recombinant human protein, known as UCB-1 (or “Nell-1”). The Nell-1 protein is an osteoinductive recombinant protein that provides target specific control over bone regeneration. The protein has been licensed exclusively for worldwide applications to Bone Biologics through a technology transfer from the University of California, Los Angeles (“UCLA”). Bone Biologics recently received guidance from the United States Food and Drug Administration (“FDA”) that Nell-1 will be classified as a combination product with a device lead.
The Company is a development stage entity. The production and marketing of the Company’s products and its ongoing research and development activities will be subject to extensive regulation by numerous governmental authorities in the United States. Prior to marketing in the United States, any drug developed by the Company must undergo rigorous preclinical (animal) and clinical (human) testing and an extensive regulatory approval process implemented by the FDA under the Food, Drug and Cosmetic Act. The Company has limited experience in conducting and managing the preclinical and clinical testing necessary to obtain regulatory approval. There can be no assurance that the Company will not encounter problems in clinical trials that will cause the Company or the FDA to delay or suspend clinical trials.
The Company’s success will depend in part on its ability to obtain patents and product license rights, maintain trade secrets, and operate without infringing on the proprietary rights of others, both in the United States and other countries. There can be no assurance that patents issued to or licensed by the Company will not be challenged, invalidated, or circumvented, or that the rights granted thereunder will provide proprietary protection or competitive advantages to the Company.
Recapitalization
In connection with the Merger described above, the 5,000,000 outstanding shares of Common Stock of the Company prior to the Merger were consolidated into 3,853,600 shares of Common Stock and the remaining shares were cancelled.
Additionally, all of the issued and outstanding shares of Bone Biologics Inc.’s $0.0001 par value common stock converted into a combined total of 19,897,587 shares of the Company’s Common Stock (including 2,151,926 shares issuable upon the exercise of outstanding warrants and 5,648,658 shares issuable upon the conversion of debt). In exchange, Bone Biologics agreed to pay AFH Holding & Advisory, LLC (“AFH”) the principal sum of $590,000.
Going Concern and Liquidity
The Company has no significant operating history and, from March 9, 2004 (inception) to December 31, 2014, has generated a net loss of approximately $12 million. The Company will continue to incur significant expenses for development activities for their lead product Nell-1. Operating expenditures for the next twelve months are estimated at $3.6 million. The accompanying consolidated financial statements for the year ended December 31, 2014, have been prepared assuming the Company will continue as a going concern. In connection with the LOI (see Note 4), management intends to raise additional debt and/or equity financing to fund future operations and to provide additional working capital. However, there is no assurance that such financing will be consummated or obtained in sufficient amounts necessary to meet the Company’s needs.
| 50 |
Bone Biologics, Corp.
Notes to Consolidated Financial Statements
The accompanying consolidated financial statements do not include any adjustments to reflect the possible future effects on the recoverability and classification of assets or the amounts and classifications of liabilities that may result from the possible inability of the Company to continue as a going concern.
2. Summary of Significant Accounting Policies
Basis of Presentation
The accompanying consolidated financial statements and related notes include activities of the Company, and have been prepared in accordance with accounting principles generally accepted in the United States of America (“GAAP”).
Use of Estimates
The preparation of the accompanying consolidated financial statements in conformity with GAAP requires management to make certain estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the consolidated financial statements and reported amounts of expenses during the reporting period. Significant estimates include warrants, stock options and income tax valuation allowances. Actual results could differ from those estimates.
Principles of consolidation
The consolidated financial statements include the accounts of the Company (and its wholly-owned subsidiary, Bone Biologics, Inc.). All significant intercompany transactions have been eliminated.
Fair Value of Financial Instruments
The Company’s financial instruments are accounts receivable, accounts payable, and notes payable. The recorded values of accounts receivable and accounts payable approximate their values based on their short term nature. Notes payable are recorded at their issue value or if warrants are attached at their issue value less the value of the warrant.
The Company defines fair value as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. Valuation techniques used to measure fair value must maximize the use of observable inputs and minimize the use of unobservable inputs. The fair value hierarchy is based on three levels of inputs that may be used to measure fair value, of which the first two are considered observable and the last is considered unobservable:
Level 1: Quoted prices in active markets for identical assets or liabilities.
Level 2: Inputs other than Level 1 that are observable, either directly or indirectly, such as quoted prices for similar assets or liabilities; quoted prices in markets that are not active; or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities.
Level 3 assumptions: Unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities including liabilities resulting from embedded derivatives associated with certain warrants to purchase common stock.
| 51 |
Bone Biologics, Corp.
Notes to Consolidated Financial Statements
Property and Equipment
Property and equipment are stated at cost. Depreciation is calculated using the straight-line method over the estimated useful lives of the related assets, ranging from three to seven years. Expenditures for additions and improvements are capitalized, while repairs and maintenance costs are expensed as incurred. The cost and related accumulated depreciation of property and equipment sold or otherwise disposed of are removed from the accounts and any gain or loss is recorded in the year of disposal.
Impairment of Long-Lived Assets
The long-lived assets held and used by the Company are reviewed for impairment no less frequently than annually or whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. In the event that facts and circumstances indicate that the cost of any long-lived assets may be impaired, an evaluation of recoverability is performed. Management has determined that there was no impairment in the value of long-lived assets during the year ended December 31, 2014.
Research and Development Costs
Research and development costs include, but are not limited to, patents and license expenses, payroll and other personnel expenses, consultants, expenses incurred under agreements with contract research and manufacturing organizations and animal clinical investigative sites and the cost to manufacture clinical trial materials. Costs related to research, design and development of products are charged to research and development expense as incurred.
Patents and Licenses
In March 2006, the Company entered into an exclusive license agreement (“Exclusive License Agreement”), with UCLA for the worldwide application of the Nell-1 protein through a technology transfer. See Note 4 for commitments related to the Exclusive License Agreement. Patent expenses include costs to acquire the license of Nell -1, which was de minimus, and costs to file patent applications related to Nell-1.
Bone expenses the costs incurred to file patent applications, all costs related to abandoned patent applications and maintenance costs, and these costs are included in research and development expenses. Costs associated with licenses acquired to be able to use products from third parties prior to receipt of regulatory approval to market the related products are also expensed. The Company’s licensed technologies may have alternative future uses in that they are enabling (or platform) technologies that can be the basis for multiple products that would each target a specific indication. Costs of acquisition of licenses are expensed.
Deferred Financing and Transaction Costs
Deferred financing costs represent costs incurred in connection with the issuance of the convertible notes payable and private equity financing. Deferred financing costs related to the issuance of debt are being amortized over the term of the financing instrument using the effective interest method, while deferred financing costs from equity financings are netted against the gross proceeds received from the equity financings.
| 52 |
Bone Biologics, Corp.
Notes to Consolidated Financial Statements
During the year ended December 31, 2014, the Company capitalized deferred financing costs of $401,118 in connection with the Extra Warrants issued to AFH (See Note 4) and $617,018 related to issuance of notes payable. Amortization of deferred financing costs was $60,398 and $-0- for the years ended December 31, 2014 and 2013, respectively.
Deferred transaction costs represent fees associated with the merger. All costs have been expensed as of the merger date.
Other receivables – related party
Other receivables – related party represent a receivable from AFH Holding & Advisory, a shareholder, for fees paid on their behalf for legal services.
Concentration of Credit Risk and Other Risks and Uncertainties
Cash balances are maintained at financial institutions and, at times, balances may exceed federally insured limits. The Company has never experienced any losses related to these balances. As of January 1, 2013, federal insurance coverage is $250,000 per depositor at each financial institution. A substantial majority of the Company’s cash and cash equivalent bank balances exceed federally insured limits.
Stock Based Compensation
ASC 718, Compensation – Stock Compensation, prescribes accounting and reporting standards for all share-based payment transactions in which employee services are acquired. Transactions include incurring liabilities, or issuing or offering to issue shares, options, and other equity instruments such as employee stock ownership plans and stock appreciation rights. Share-based payments to employees, including grants of employee stock options, are recognized as compensation expense in the consolidated financial statements based on their fair values. That expense is recognized over the period during which an employee is required to provide services in exchange for the award, known as the requisite service period (usually the vesting period).
The Company accounts for stock-based compensation issued to non-employees and consultants in accordance with the provisions of ASC 505-50, Equity – based Payments to Non-Employees. Measurement of share-based payment transactions with non-employees is based on the fair value of whichever is more reliably measurable: (a) the goods or services received; or (b) the equity instruments issued. The fair value of the share-based payment transaction is determined at the earlier of performance commitment date or performance completion date.
Income Taxes
Income taxes are provided for the tax effects of transactions reported in the consolidated financial statements and consist of taxes currently due and deferred taxes resulting from timing differences in recording of transactions for tax purposes and financial reporting purposes.
The deferred tax assets and liabilities represent the future tax return consequences of those differences, which will either be taxable or deductible when the assets and liabilities are received or settled. Valuation allowances are established when necessary to reduce deferred tax assets to amounts expected to be realized.
The accounting provisions related to uncertain income tax positions require the Company to determine whether any tax position in all open years meets a more likely than not threshold of being sustained upon examination by the applicable taxing authority. The Company did not have any changes to its liability for uncertain tax positions as at December 31, 2014 and 2013.
| 53 |
Bone Biologics, Corp.
Notes to Consolidated Financial Statements
The Company’s policy is to recognize interest and/or penalties related to income tax matters in income tax expense. No such amounts are accrued as of December 31, 2014 and 2013.
Loss per Common Share
The Company utilizes FASB ASC Topic No. 260, Earnings per Share. Basic loss per share is computed by dividing loss available to common shareholders by the weighted-average number of common shares outstanding. Diluted loss per share is computed similar to basic loss per share except that the denominator is increased to include the number of additional common shares that would have been outstanding if the potential common shares had been issued and if the additional common shares were dilutive. Diluted loss per common share reflects the potential dilution that could occur if convertible debentures, options and warrants were to be exercised or converted or otherwise resulted in the issuance of common stock that then shared in the earnings of the entity.
Since the effects of outstanding options, warrants, and the conversion of convertible debt are anti-dilutive in all periods presented, shares of common stock underlying these instruments have been excluded from the computation of loss per common share.
The following sets forth the number of shares of common stock underlying outstanding options, warrants, and convertible debt as of December 31, 2014 and 2013:
| December 31, | ||||||||
| 2014 | 2013 | |||||||
| Warrants | 7,023,464 | 634,300 | ||||||
| Stock options | 757,977 | — | ||||||
| Convertible promissory notes | 6,911,659 | 5,095,427 | ||||||
| 14,693,100 | 5,729,727 | |||||||
New Accounting Standards
The Company has reviewed all recently issued, but not yet adopted, accounting standards in order to determine their effects, if any, on its results of operation, financial position or cash flows. Based on that review, the Company believes that none of these pronouncements will have a significant effect on its consolidated financial statements.
In June 2014, the FASB issued ASU 2014-10, Development Stage Entities (Topic 915): Elimination of Certain Financial Reporting Requirements. ASU 2014-10 eliminates the distinction of a development stage entity and certain related disclosure requirements, including the elimination of inception-to-date information on the statements of operations, cash flows and stockholders’ equity. The amendments in ASU 2014-10 will be effective prospectively for annual reporting periods beginning after December 15, 2014, and interim periods within those annual periods, however early adoption is permitted. The Company adopted ASU 2014-10 during the quarter ended June 30, 2014, thereby no longer presenting or disclosing any information required by Topic 915.
In May 2014, the FASB issued ASU 2014-09, “Revenue from Contracts with Customers (Topic 606),” which is the new comprehensive revenue recognition standard that will supersede all existing revenue recognition guidance under GAAP. The standard’s core principle is that a company will recognize revenue when it transfers promised goods or services to a customer in an amount that reflects the consideration to which the company expects to be entitled in exchange for those goods or services. This ASU is effective for annual and interim periods beginning on or after December 15, 2016, and early adoption is not permitted. Entities will have the option of using either a full retrospective approach or a modified approach to adopt the guidance in the ASU. The Company currently has no revenues and doesn’t expect any impact of adopting this guidance.
| 54 |
Bone Biologics, Corp.
Notes to Consolidated Financial Statements
In June 2014, the FASB issued ASU 2014-12, “Compensation - Stock Compensation (Topic 718): Accounting for Share-Based Payments When the Terms of an Award Provide That a Performance Target Could be Achieved after the Requisite Service Period.” This ASU provides more explicit guidance for treating share-based payment awards that require a specific performance target that affects vesting and that could be achieved after the requisite service period as a performance condition. The new guidance is effective for annual and interim reporting periods beginning after December 15, 2015. The Company does not expect the adoption of this guidance to have a material impact on the consolidated financial statements.
August 2014, the FASB issued ASU 2014-15, “Presentation of Financial Statements – Going Concern (Topic 205-40)”, which requires management to evaluate whether there is substantial doubt about an entity’s ability to continue as a going concern for each annual and interim reporting period. If substantial doubt exists, additional disclosure is required. This new standard will be effective for the Company for annual and interim periods beginning after December 15, 2016. Early adoption is permitted. The Company adopted this new standard for the year ending December 31, 2014.
3. Property and Equipment
Property and equipment consist of the following at:
| December 31, 2014 | December 31, 2013 | |||||||
| Furniture and equipment | $ | 11,901 | $ | - | ||||
| Less accumulated depreciation | (280 | ) | - | |||||
| $ | 11,621 | $ | - | |||||
Depreciation expense for the years December 31, 2014 and 2013 was $280 and $-0-, respectively.
4. Accounts Payable and Accrued Expenses
Accounts payable and accrued expenses consist of the following:
| December 31, 2014 | December 31, 2013 | |||||||
| Interest expense | $ | 87,774 | $ | 1,158,465 | ||||
| Professional services | 119,776 | 114,849 | ||||||
| Patents | - | 85,412 | ||||||
| Deferred compensation | - | 90,199 | ||||||
| Transaction costs | - | 75,000 | ||||||
| Payroll liabilities | 7,839 | 1,679 | ||||||
| $ | 215,389 | $ | 1,525,604 | |||||
| 55 |
Bone Biologics, Corp.
Notes to Consolidated Financial Statements
5. Commitments and Contingencies
Letter of Intent
In August of 2012, the Bone Biologics, Inc., along with its then majority owner and debt holder, MTF, entered into a Letter of Intent (“LOI”) with AFH to consummate a business combination through a share exchange, reverse merger, or other similar transactions resulting in the Company becoming a public entity (“The Transaction”). In August, 2013, the LOI was amended and restated, and on May 7, 2014, the LOI was again amended and restated. The Amended and Restated Letter of Intent dated May 7, 2014 (the “Amended LOI”) contemplates and defines the following events:
Consummation of Bridge Financings (“Closing I”)
In April 2013 and September 2013, the Company’s Board approved the Company to borrow up to an aggregate principal amount of $300,000 (April Bridge Financing) and $250,000 (September Bridge Financing) pursuant to the sale and issuance of convertible promissory notes and warrants to purchase common stock of the Company (collectively, the “Bridge Financings”). The note accrues interest at a rate of 12% per year and is payable each quarter. A warrant to purchase the Company’s common stock equal to 50% of the original principal amount at $1.00 per share was issued to each Bridge Financing participant. Principal and unpaid accrued interest may be converted into equity securities issued in the Company’s next equity financing in an aggregate amount of at least $2.5 million at a price equal to the price paid by investors in the next equity financing. On April 29, 2013 and on June 5, 2013, the Company borrowed $100,000 from MTF and $100,000 from Orthofix, Corp., respectively, under the April Bridge Financing. In September 2013, the Company borrowed $50,000 from AFH under the April Bridge Financing. In October 2013, the Company borrowed an additional $150,000 from Orthofix under the September Bridge Financing.
Consummation of Business Combination (“Closing II”)
Under the amended LOI, it was contemplated that the Company and its equity holders will consummate a share exchange, reverse merger, or other business combination, with a Delaware corporation publicly reporting pursuant to United States Securities Exchange Act of 1934, as amended (the “Exchange Act”), or a private Delaware corporation (“Acquisition Co.”), either directly or indirectly through an affiliate. If the post-business combination entity is not already a corporation publicly reporting pursuant to the Exchange Act, AFH will assist the post business combination entity with the filing of an appropriate registration statement resulting in the Company becoming a public company (“PubCo”). The Company affected a merger on September 19, 2014 (See Note 1 Recapitalization). AFH received $590,000 in connection with the Business Combination.
Consummation of the Private Placement (“Closing III”)
Subsequent to Closing II, AFH will use its best efforts to assist PubCo in procuring one or more investors for a private financing, whether debt or equity, of up to $10.0 million. Such transaction is to include an over-allotment option of 15% at AFH’s discretion (the “Private Placement”). The Company closed on $1,000,000 in July 2014 and $5,000,000 of convertible debt in October 2014. At the consummation of Closing III, AFH Group shall be entitled to receive warrants to purchase up to 500,000 share of common stock of PubCo at the per share price of the shares offered in the Private Placement with a 5 year term and a cashless exercise provision (the “Extra Warrants”).
| 56 |
Bone Biologics, Corp.
Notes to Consolidated Financial Statements
Consummation of the PIPE Transaction (“Closing IV”)
Subsequent to Closing III, AFH Advisory will use its best efforts to assist PubCo in procuring an investment bank (the “Bank”) to facilitate a private investment in public equity transaction in an amount between $8.0 million and $10.0 million through the sale of securities of PubCo (the “PIPE”). Such transaction will include a 15% over allotment at AFH and/or the Bank’s discretion. Such transaction is contingent upon the appointment of a Bank and filing appropriate forms with the Financial Industry Regulatory Authority, Corp. (“FINRA”).
Consummation of Initial Public Offering (“Closing V”)
Subsequent to Closing IV, AFH will assist PubCo in procuring a Bank to act as underwriter for an initial public offering in an amount of up to $40.0 million (the “Initial Public Offering”). The Initial Public Offering shall include a 15% over allotment option at AFH and/or the Bank’s discretion. Such a transaction is contingent upon the appointment of the Bank.
License Commitment
In connection with the Exclusive License Agreement, the Company is required to pay a royalty fee beginning in the first year of commercial sale of the licensed product equal to 3% of net sales on a quarterly basis with an annual minimum royalty of $25,000 for the life of the patent rights. In addition to the royalty fees, the Company is also required to pay UCLA a $10,000 annual maintenance fee, $50,000 upon FDA marketing approval and $25,000 upon first commercial sale.
On October 22, 2013, the Exclusive License Agreement was amended. The following additional fees will be due to UCLA i) 2% of the amount raised in the Private Placement or if the Private Placement did not close or was less than $2.5 million then a fee of $100,000 was due and payable by June 1, 2014, ii) $25,000 due upon closing of Phase 1 clinical trial and iii) $50,000 due upon closing of Phase 3 clinical trial. The Company paid the fee of $100,000 in June 2014.
Contingencies
The Company is subject to claims and assessments from time to time in the ordinary course of business. The Company’s management does not believe that any such matters, individually or in the aggregate, will have a material adverse effect on the Company’s business, financial condition, results of operations or cash flows.
Indemnification
In the normal course of business, the Company enters into contracts and agreements that contain a variety of representations and warranties and provide for general indemnifications. The Company’s exposure under these agreements is unknown because it involves claims that may be made against the Company in the future, but have not yet been made. To date, the Company has not paid any claims or been required to defend any action related to its indemnification obligations. However, the Company may record charges in the future as a result of these indemnification obligations.
In accordance with its amended and restated certificate of incorporation and amended and restated bylaws, the Company has indemnification obligations to its officers and directors for certain events or occurrences, subject to certain limits, while they are serving at the Company’s request in such capacity. There have been no claims to date and the Company has a director and officer insurance policy that enables it to recover a portion of any amounts paid for future potential claims.
| 57 |
Bone Biologics, Corp.
Notes to Consolidated Financial Statements
6. Notes Payable to Related Party
As of December 31, 2014 and 2013, the Company had a total of $3,747,102 and $5,095,427, respectively, of notes outstanding (principal and interest) including unamortized discount, with MTF a related party, which consisted of the following:
| Note Type | Issue Date | Maturity Date(1) | Interest Rate | December 31, 2014 | December 31, 2013 | |||||||||
| Convertible Promissory Note | 1/18/08 | 3/31/15 | PRIME + 1 ½% | $ | - | $ | 1,479,654 | |||||||
| Promissory Note | 11/4/08 | 3/31/15 | PRIME + 3% | - | 343,429 | |||||||||
| Promissory Note | 3/17/09 | 3/31/15 | PRIME + 8% | - | 584,745 | |||||||||
| Promissory Note | 8/24/09 | 3/31/15 | LIBOR + 8% | - | 23,193 | |||||||||
| Tranched Promissory Note | 9/30/09 | 3/31/15 | LIBOR + 8% | - | 2,570,126 | |||||||||
| Bridge Note, net of discount | 4/29/13 | 10/14/14 | 12% | - | 94,280 | |||||||||
| New MTF Convertible Promissory Note | 9/19/14 | 3/31/15 | 8.5% | 3,747,102 | ||||||||||
| 3,747,102 | 5,095,427 | |||||||||||||
| Less: Accrued interest expense | 87,774 | 1,147,610 | ||||||||||||
| Notes payable to related party, net of debt discount | $ | 3,659,328 | $ | 3,947,817 | ||||||||||
| (1) | As amended. |
Accrued interest on the notes payable to related party of $87,774 and $1,147,610 is recorded in accrued expenses at December 31, 2014 and 2013, respectively.
Convertible Related Party Promissory Notes
The related party convertible promissory notes are considered hybrid instruments, which consist of a debt host instrument together with a conversion feature, thus giving the holder of a convertible note an option to convert into an equity instrument providing the holder a residual interest in the Company. The holder of a convertible promissory note also has the option to present its convertible promissory note to the Company and demand payment under the terms of the note after the maturity date or upon the occurrence of certain events such as the failure of the Company to make a payment on the note when due, bankruptcy or certain other liquidation events. The Company concluded that the convertible promissory notes would be accounted for as a typical debt instrument with related interest expense recorded in the Company’s statements of operations. The Company concluded that there is no beneficial conversion feature as of the date of issuance of the convertible notes. However, the note contains a contingent feature whereby the conversion rate may be lowered if a financing occurs at a lower rate than the note’s conversion rate. If the contingency is met and the conversion feature is determined to be “beneficial” in a future accounting period, an additional financing cost would be recorded for the beneficial conversion feature in the Company’s statements of operations at that time.
In January 2008, the Company issued a $1,107,000 convertible promissory note (“January 2008 Note”) to MTF in accordance with the Convertible Promissory Note dated January 18, 2008, as amended. This note’s principal and accrued interest was converted into Common Stock on September 19, 2014.
The Company issued promissory notes to MTF in November 2008 of $250,000 (“November 2008 Note”), in March 2009 of $400,000 (“March 2009 Note) and in August 2009 of $16,400 (August 2009 Note”). On September 19, 2014 these notes’ principal and accrued interest were consolidated into the New MTF Convertible Note.
| 58 |
Bone Biologics, Corp.
Notes to Consolidated Financial Statements
In September 2009, the Company entered into a tranched promissory note with MTF (“Tranched Note”), allowing the Company to initially borrow up to $445,000 in a series of one or more tranches. The Tranched Note was subsequently amended which, among other things, increased the maximum advance amount to $2,090,000. The Company borrowed a total of $2,088,350 under the Tranched Note through September 19, 2014. This note’s principal and accrued interest was consolidated into the New MTF Convertible Note.
In May, 2014, the Company entered into a convertible promissory note with MTF (the “2014 Note”) for $250,000 with interest at 7% per annum compounded annually and a maturity date of June 15, 2015. In the event of a financing of not less than $1 million, the 2014 Note automatically converts into Equity Securities, as defined in the 2014 Note, at a 25% discount to the price paid per share in such financing. In connection with the 2014 Note, the Company issued a warrant to purchase 166,667 shares of the Company’s common stock at an exercise price of $1.50 per share and 4 year term (See Note 7). In July 2014, the 2014 Note and related warrants were assigned to Orthofix.
Upon consummation of the merger, the 2008 January Convertible Note was converted into 1,533,356 shares of common stock of the Company. Upon consummation of the merger, MTF also converted all their outstanding Series A and B Preferred Stock, 5,829,438 shares, into common stock.
Bridge Note
In April 2013 the Company borrowed $100,000 from MTF under the April Bridge Financing. The convertible promissory note accrued interest at a rate of 12% per year and payable per quarter. A warrant to purchase the Company’s common stock equal to 50% of the original principal amount divided by $1.00 was issued to the Bridge Financing participant. Principal and unpaid accrued interest may be converted into equity securities issued in the Company’s next equity financing in an aggregate amount of at least $2.5 million at a price equal to the price paid by investors in the next equity financing. In June 2014, the note held by MTF under the April Bridge Financing was amended to extend the maturity date to October 14, 2014. The note was converted into Common Stock on September 19, 2014.
MTF Short Term 2014 Loan
On September 15, 2014, Bone and MTF entered into a loan agreement and accompanying promissory note to fund the continued operations of Bone prior to the Merger. Pursuant to the MTF Short Term 2014 Loan, MTF has agreed to advance an initial $250,000 to Bone and, at Bone’s request and subject to the terms and conditions of the MTF Short Term 2014 Loan, to advance up to an additional $250,000 to Bone. The MTF Short Term 2014 Loan has an interest rate of eight and one-half percent (8.5%) accruing annually. The MTF Short Term 2014 Loan matures on the earlier to occur of (i) the date on which at least $1 million is loaned to or invested in the Company and (ii) December 31, 2014. In further consideration of the MTF 2014 Loan, Bone granted to MTF 625,000 warrants at a strike price of $1.62. On October 27, 2014 the balance was paid in full and the line of credit was cancelled.
New MTF Convertible Note
On September 19, 2014, the MTF 2008 and 2009 Promissory Notes and any related loan agreements, credit agreements, guarantee agreements or other agreements related to the MTF 2008 and 2009 Promissory Notes were cancelled and the Company issued MTF a convertible promissory note in the face amount of $3,659,328 (the “New MTF Convertible Note”). Pursuant to the terms of the New MTF Convertible Note, 50% of all principal and accrued and unpaid interest due under the New MTF Convertible Note will be converted into common stock of the Company upon the closing of the PIPE. The remainder of the New MTF Convertible Note, including all accrued and unpaid interest, will be converted upon consummation of the Initial Public Offering.
| 59 |
Bone Biologics, Corp.
Notes to Consolidated Financial Statements
7. Notes Payable
Convertible Notes Payable
The convertible promissory notes are considered hybrid instruments, which consist of a debt host instrument together with a conversion feature, thus giving the holder of a convertible note an option to convert into an equity instrument providing the holder a residual interest in the Company. The holder of a convertible promissory note also has the option to present its convertible promissory note to the Company and demand payment under the terms of the note after the maturity date or upon the occurrence of certain events such as the failure of the Company to make a payment on the note when due, bankruptcy or certain other liquidation events. The Company concluded that the convertible promissory notes would be accounted for as a typical debt instrument with related interest expense recorded in the Company’s statements of operations. The Company concluded that there is no beneficial conversion feature as of the date of issuance of the convertible notes.
Bridge Notes
In June 2013, the Company borrowed $100,000 from Orthofix, Corp. under the April Bridge Financing, and in September 2013 and October 2013 the Company borrowed $50,000 from AFH and an additional $150,000 from Orthofix, Corp. under the September Bridge Financing. The convertible promissory notes accrue interest at a rate of 12% per year and payable per quarter. A warrant to purchase the Company’s common stock equal to 50% of the original principal amount divided by $1.00 was issued to the Bridge Financing participant. Principal and unpaid accrued interest may be converted into equity securities issued in the Company’s next equity financing in an aggregate amount of at least $2.5 million at a price equal to the price paid by investors in the next equity financing. These notes were converted into Common Stock on September 19, 2014.
Orthofix Subsequent Financing
On July 1, 2014, (i) Orthofix purchased $500,000 worth of Bone Biologics Common Stock or the Subsequent Orthofix Shares; (ii) was issued the Subsequent Orthofix Convertible Promissory Notes in the principal amount of $500,000 (which includes the assignment of a $250,000 2014 note from MTF) and convertible into 666,666 worth of the Company’s Common Stock at $0.75 per share; and (iii) was issued the Subsequent Orthofix Warrants (including the assignment of warrants by MTF issued in connection with a 2014 note) which were exercisable for 333,334 shares of Bone Biologics Common Stock at an exercise price per share of $1.50 (the “Orthofix Subsequent Financing”). Upon subscribing for the Subsequent Orthofix Shares, the Subsequent Orthofix Convertible Promissory Notes and accrued interest converted into a combined total of 668,904 shares of Bone Biologics Common Stock in accordance with the terms of the Subsequent Orthofix Convertible Promissory Notes.
At the closing of the Subsequent Orthofix Shares and Notes, AFH Advisory was entitled to receive warrants to purchase up to 500,000 shares of Common Stock of the Company (See Note 7).
Secured Convertible Note and Warrant
On October 24, 2014, Bone issued a convertible promissory note in the amount of $5,000,000 (the “Convertible Note”) to Hankey Capital, LLC (“Hankey Capital”). The Convertible Note matures on October 24, 2017 (the “Maturity Date”) and bears interest at an annual rate of interest of the “prime rate” (as quoted in the “Money Rates” section of The Wall Street Journal) plus 4.0%, with a minimum rate of 8.5% per annum until maturity, with interest payable monthly in arrears. Prior to the Maturity Date, Hankey Capital has a right, in their sole discretion, to convert the Convertible Note into shares of the Company’s common stock, par value $0.001 per share (“Common Stock”), at a conversion rate equal to the greater of (i) $1.58 per share and (ii) 70% of the average daily price for the Common Stock as measured over the course of the 60 day period prior to the conversion.
| 60 |
Bone Biologics, Corp.
Notes to Consolidated Financial Statements
The Convertible Note is secured by certain collateral shares of Common Stock issued by the Company in the name of Hankey Capital, in such amount so as to maintain a loan to value ratio of no greater than 50% (the “Collateral”). 6,329,114 shares were issued upon closing the Convertible Note. The number of shares in the Collateral shall be adjusted on a yearly basis. The shares representing the Collateral contain a restrictive legend. The Company shall seek to register the Collateral shares initially delivered on the date of the Convertible Note pursuant to the Registration Rights Agreement described below. Upon the effectiveness of such Registration Statement, the Company will remove the restrictive legends from the Collateral shares so long as Hankey Capital agrees in any event not to sell any Collateral shares if Hankey Capital is notified that the Registration Statement is no longer effective. Hankey Capital may hold the Collateral in any brokerage account of its choosing, but shall not transfer, sell or otherwise dispose of any Collateral, except during the existence of an Event of Default, as defined in the Convertible Note. The Convertible Note is further secured by collateral assignments of all the Company’s license agreements.
The principal amount of the loan is pre-payable in whole or in part at any time, without premium or penalty. Upon any voluntary partial prepayment of outstanding principal, Hankey Capital shall return Collateral shares to the Company in the amount necessary, if any, to maintain the loan to value ratio at no less than 50%. Upon a full payment of the outstanding principal, all Collateral shares shall be returned return and cancelled. Hankey Capital shall also return Collateral shares under the same terms in case of partial or full conversion of the Convertible Note.
The Company paid a commitment fee in the amount of 3% of the original principal amount of the loan ($150,000) to Hankey Capital. The Company intends to use the proceeds of the Convertible Note for working capital and general corporate purposes.
On October 24, 2014, the Company also issued a warrant to Hankey Capital for 3,955,697 shares of Common Stock at an exercise price per share of $1.58. The Warrant will expire on October 24, 2017. The Warrant also includes such other terms that are normal and customary for warrants of this type.
Registration Rights Agreement
On October 24, 2014, the Company entered into a Registration Rights Agreement with Hankey Capital, for certain demand registration rights and unlimited piggyback registration rights for the shares underlying the Convertible Note and the Warrant, and subject to an agreed lock up period. Pursuant to the Registration Rights Agreement, Hankey Capital may at any time request registration of their registrable shares. Within 30 days of such demand, the Company will provide written notice of such request to all other holders of registrable securities and will include in such registration all registrable shares with respect to which the Company has received written requests for inclusion within twenty-five (25) days after delivery of the Company’s notice. The Company has agreed to pay all registration expenses relating to up to three long-form registrations or short-form registrations for Hankey Capital.
| 61 |
Bone Biologics, Corp.
Notes to Consolidated Financial Statements
Whenever the Company proposes to register any of its securities under the Securities Act (other than pursuant to a demand registration under the Registration Rights Agreement) and the registration form to be used may be used for the registration of any registrable shares, the Company will give prompt written notice to all holders of the registrable shares of its intention to effect such a registration and will include in such registration all registrable shares (in accordance with the priorities set forth in the Registration Rights Agreement) with respect to which the Company has received written requests for inclusion within fifteen (15) days after the delivery of the Company’s notice. Pursuant to Registration Rights Agreement, holders of registrable shares and the Company agree not to effect any public sale or distribution of equity securities of the Company, or any securities convertible into or exchangeable or exercisable for such securities, during the six (6) months following, the effective date of the Company’s merger with Bone Biologics, Inc. on September 19, 2014.
On October 24, 2014, Forefront Capital was issued a warrant to purchase 126,582 shares of Common Stock upon completion of the Hankey Capital Convertible Note.
8. Stockholders’ Equity
Preferred Stock
The Company’s amended and restated certificate of incorporation authorizes the Company to issue a total of 20,000,000 shares of preferred stock. No shares have been issued.
Common Stock
The Company’s amended and restated certificate of incorporation authorizes the Company to issue a total of 100,000,000 shares of common stock. As of December 31, 2014, the Company had an aggregate of 24,269,047 shares of common stock outstanding.
In connection with the Convertible Note to Hankey Capital, Bone issued 6,329,114 common shares as collateral. (See Note 7)
Each share of common stock has the right to one vote. The holders of common stock are also entitled to receive dividends whenever funds are legally available and when declared by the Board of Directors, subject to the prior rights of holders of all classes of stock outstanding having priority rights as to dividends. No dividends have been declared by the Board from inception through December 31, 2014.
Common Stock Warrants
As of December 31, 2014, the Company had outstanding unexercised common stock warrants as follows:
| Date Issued | Exercise Price | Number of Shares | ||||
| 2006 | $0.17 | 60,920 | ||||
| 2009 | $0.44 | 118,383 | ||||
| 2010 | $0.44 | 254,997 | ||||
| 2013 | $1.00 | 200,000 | ||||
| 2014 | $1.00 - $1.62 | 6,389,164 | ||||
| Total warrants at December 31, 2014 | 7,023,464 | |||||
In connection with the Bridge Financings (see Notes 6 and 7), warrants were issued to purchase 200,000 shares of the Company’s common stock at an exercise price of $1.00 per share. The warrants expire in seven years from issuance date and may be exercised for cash or, if the current market price of the Company’s common stock is greater than the per share exercise price, by surrender of a portion of the warrant in a cashless exercise. The initial fair value of the warrant was estimated at an aggregate value of $171,143 using the Black-Scholes option pricing model with a volatility of 109%, a risk free interest rate of 1.10% to 2.11%. The fair value on the warrants was recorded as a debt issuance cost and is being amortized to interest expense over the term of the note.
| 62 |
Bone Biologics, Corp.
Notes to Consolidated Financial Statements
In connection with a 2014 MTF note, assigned to Orthofix in July 2014, the Company issued a warrant to purchase 166,667 shares of the Company’s common stock at an exercise price of $1.50 per share and 4 year term (See Note 7). The warrants had a fair value of $111,804, calculated using the Black-Scholes option pricing model with a volatility of 109%, a risk free interest rate of 0.79%.
In connection with the Orthofix Subsequent Financing, the Company issued a warrant to purchase 166,667 shares of the Company’s common stock at an exercise price of $1.50 per share and 4 year term (See Note 7). The warrants had a fair value of $116,164, calculated using the Black-Scholes option pricing model with a volatility of 100.83%, a risk free interest rate of 1.66%. The fair value on the warrants was recorded as a debt issuance cost and is being amortized to interest expense over the term of the note.
Extra Warrants
At the closing of the Subsequent Orthofix Shares and Notes, AFH Advisory was entitled to receive the 500,000 Extra Warrants. AFH Advisory has normal and customary piggyback registration rights with respect to the shares of Common Stock issuable upon exercise of the Extra Warrants. The warrants expire on February 2, 2020 and may be exercised for cash or, if the current market price of the Company’s common stock is greater than the per share exercise price, by surrender of a portion of the warrant in a cashless exercise. The initial fair value of the warrants was estimated at an aggregate value of $407,917, using the Black-Scholes option pricing model with the following assumptions at the date of issuance: expected volatility of 109.42%, risk-free interest rate of 2.17%, contractual term of 5 years and dividend yield of 0%. The warrants are classified as permanent equity.
Agent Warrants
Forefront or its designees will receive the Agent Warrant. Such Agent Warrant will be issued at the closing of the Private Placement and shall provide, among other things, that the Agent Warrant shall: (i) be exercisable at the price of the securities (or the exercise price of the securities) issued to the investors in the offering, (ii) expire five (5) years from the date of issuance, (iii) include customary registration rights, including the registration rights provided to the Investors, (iv) contain provisions for cashless exercise and (v) include such other terms that are normal and customary for warrants of this type. In addition, Forefront or its designees will receive an Advisory Warrant equal to 2.0% of the Company’s post-merger and financing fully diluted shares outstanding upon the closing of $2.5 million of investors on which Forefront is eligible to receive compensation.
Forefront was issued a warrant to purchase 46,667 shares of Common Stock at $1.00 per share upon completion of the Orthofix Subsequent Financing. The warrants expire in five years from issuance date and may be exercised for cash or, if the current market price of the Company’s common stock is greater than the per share exercise price, by surrender of a portion of the warrant in a cashless exercise. The initial fair value of the warrants was estimated at an aggregate value of $28,629, using the Black-Scholes option pricing model with the following assumptions at the date of issuance: expected volatility of 109.1%, risk-free interest rate of 0.39%, contractual term of 2.5 years and dividend yield of 0%.
Forefront was issued a warrant to purchase 126,582 shares of Common Stock at $1.00 per share upon completion of the Hankey Capital Secured Term Note. The warrants expire in five years from issuance date and may be exercised for cash or, if the current market price of the Company’s common stock is greater than the per share exercise price, by surrender of a portion of the warrant in a cashless exercise. The initial fair value of the warrants was estimated at an aggregate value of $197,441, using the Black-Scholes option pricing model with the following assumptions at the date of issuance: expected volatility of 101.05%, risk-free interest rate of 1.52%, contractual term of 5 years and dividend yield of 0%.
| 63 |
Bone Biologics, Corp.
Notes to Consolidated Financial Statements
MTF Short Term 2014 Loan
In further consideration of the MTF 2014 Loan, Bone granted to MTF 625,000 warrants at a strike price of $1.62. The warrants expire in seven years from issuance date and may be exercised for cash or, if the current market price of the Company’s common stock is greater than the per share exercise price, by surrender of a portion of the warrant in a cashless exercise. The initial fair value of the warrants was estimated at an aggregate value of $520,487, using the Black-Scholes option pricing model with the following assumptions at the date of issuance: expected volatility of 113.7%, risk-free interest rate of 0.0117%, contractual term of seven years and dividend yield of 0%. The fair value on the warrants was recorded as a debt issuance cost and amortized to interest expense over the term of the note. For the year ended December 31, 2014, $520,487 of the debt issuance costs was amortized to interest expense as the Note is payable on demand.
Warrants issued to Consultants
On July 11, 2014, the Company granted warrants to purchase up to 12,625 shares of Common Stock of at a strike price of $0.00 per share, with a 4 year term to a consultant. The initial fair value of the warrant was estimated at an aggregate value of $12,625, using the Black-Scholes option pricing model with the following assumptions at the date of issuance: expected volatility of 100.77%, risk-free interest rate of 1.28%, contractual term of 4 years and dividend yield of 0%. The fair value on the warrant was recorded as general and administrative expense upon issuance.
On September 19, 2014, the Company granted warrants to purchase up to 3% of the Company’s fully diluted shares of common stock outstanding as of the date of closing of the Merger totaling 699,671 shares of Common Stock of at a strike price of $1.00 per share, with a 7 year term to a consultant. The warrant will vest over a two-year period from the effective date, with 33.33% of the shares subject to the warrant becoming vested and exercisable on the date that the consulting agreement is executed, 33.33% of the shares subject to the option becoming vested and exercisable on the date that is twelve (12) months after the effective date, and 33.34% of the shares subject to the warrant vesting and becoming exercisable on the date that is twenty four (24) months after the effective date. The initial fair value of the warrant was estimated at an aggregate value of $614,049, using the Black-Scholes option pricing model with the following assumptions at the date of issuance: expected volatility of 113.7%, risk-free interest rate of 2.29%, contractual term of 7 years and dividend yield of 0%. The fair value on the warrant was recorded as general and administrative expense and amortized over the term of the agreement. As of December 31, 2014, total unrecognized consulting cost related to unvested warrants was $324,532. The cost is expected to be recognized over a weighted average period of 1.75 years.
On September 30, 2014, the Company granted warrants to purchase up to 89,588 shares of Common Stock of at a strike price of $1.00 per share, with a 7 year term to a consultant. The initial fair value of the warrants was estimated at an aggregate value of $77,207, using the Black-Scholes option pricing model with the following assumptions at the date of issuance: expected volatility of 108.9%, risk-free interest rate of 2.20%, contractual term of 7 years and dividend yield of 0%. The fair value on the warrant was recorded as general and administrative expense upon issuance.
| 64 |
Bone Biologics, Corp.
Notes to Consolidated Financial Statements
Secured Term Note and Warrant
In connection with the Hankey Capital Secured Term Note, the Company issued a warrant for 3,955,697 shares of Common Stock at an exercise price per share of $1.58. The Warrant will expire on October 24, 2017. The initial relative fair value of the warrants was estimated at a relative value of $1,434,000, using the Black-Scholes option pricing model with the following assumptions at the date of issuance: expected volatility of 96.77%, risk-free interest rate of 0.82%, contractual term of 3 years and dividend yield of 0%. The fair value on the warrants was recorded as a debt discount and amortized to interest expense over the term of the note.
The total debt discount costs related to our outstanding debt was for the years ended December 31, 2014 and 2013, $437,115 and $67,104, respectively. These costs were amortized to interest expense. The unamortized debt discount at December 31, 2014 was $1,354,806. The cost is expected to be recognized over a period of 2.75 years. The unamortized debt discount at December 31, 2013 was $83,263.
9. Stock-based Compensation
2014 Stock Option Plan
2,642,898 shares of our common stock have been initially authorized and reserved for issuance under our 2014 Stock Plan as option awards. This reserve may be increased by the Board on January 1, 2015 and each subsequent anniversary through January 1, 2024 by up to the number of shares of stock equal to 5% of the number of shares of stock issued and outstanding on the immediately preceding December 31. Appropriate adjustments will be made in the number of authorized shares and other numerical limits in our 2014 Stock Option Plan and in outstanding awards to prevent dilution or enlargement of participants’ rights in the event of a stock split or other change in our capital structure. Shares subject to awards granted under our 2014 Stock Option Plan which expire, are repurchased or are cancelled or forfeited will again become available for issuance under our 2014 Stock Option Plan. The shares available will not be reduced by awards settled in cash. Shares withheld to satisfy tax withholding obligations will not again become available for grant. The gross number of shares issued upon the exercise of stock appreciation rights or options exercised by means of a net exercise or by tender of previously owned shares will be deducted from the shares available under our 2014 Stock Option Plan.
Awards may be granted under our 2014 Stock Option Plan to our employees, including officers, director or consultants, and our present or future affiliated entities. While we may grant incentive stock options only to employees, we may grant non-statutory stock options, stock appreciation rights, restricted stock purchase rights or bonuses, restricted stock units, performance shares, performance units and cash-based awards or other stock based awards to any eligible participant.
The 2014 Stock Option Plan will be administered by our compensation committee. Subject to the provisions of our 2014 Stock Option Plan, the compensation committee determines, in its discretion, the persons to whom, and the times at which, awards are granted, as well as the size, terms and conditions of each award. All awards are evidenced by a written agreement between us and the holder of the award. The compensation committee has the authority to construe and interpret the terms of our 2014 Stock Option Plan and awards granted under our 2014 Stock Option Plan.
During the year ended December 31, 2014 and 2013, the Company had stock-based compensation expense of $323,510 and $-0-, respectively, related to issuances to the Company’s employees and directors, included in reported net loss. The total amount of stock-based compensation for the year ended December 31, 2014 and 2013, related solely to the issuance of stock options.
| 65 |
Bone Biologics, Corp.
Notes to Consolidated Financial Statements
A summary of stock option activity for the year ended December 31, 2014, is presented below:
| Number of Shares | Weighted Average | Weighted | ||||||||||||||
| Remaining | Exercise | Average | Intrinsic | |||||||||||||
| Subject to Exercise | Options | Price | Life (Years) | Value | ||||||||||||
| Outstanding as of January 1, 2014 | - | $ | - | - | - | |||||||||||
| Granted – 2014 | 757,977 | 1.00 | 7.69 | |||||||||||||
| Forfeited – 2014 | - | $ | - | - | - | |||||||||||
| Exercised – 2014 | - | $ | - | - | - | |||||||||||
| Outstanding as of December 31, 2014 | 757,977 | $ | 1.00 | 7.69 | - | |||||||||||
The aggregate intrinsic value in the table above represents the total pretax intrinsic value (i.e., the difference between our closing stock price on the respective date and the exercise price, times the number of shares) that would have been received by the option holders had all option holders exercised their options. There have not been any options exercised during the years ended December 31, 2014 and 2013.
All options that the Company granted during the year ended December 31, 2014, were granted at the per share fair value on the grant date. Vesting of options differs based on the terms of each option. The Company has valued the options at their date of grant utilizing the Black Scholes option pricing model. As of the issuance of these consolidated financial statements, there was not an active public market for the Company’s shares. Accordingly, the fair value of the underlying options was determined based on the historical volatility data of similar companies, considering the industry, products and market capitalization of such other entities. The risk-free interest rate used in the calculations is based on the implied yield available on U.S. Treasury issues with an equivalent term approximating the expected life of the options as calculated using the simplified method. The expected life of the options used was based on the contractual life of the option granted. Stock-based compensation is a non-cash expense because we settle these obligations by issuing shares of our common stock from our authorized shares instead of settling such obligations with cash payments.
The Company utilized the Black-Scholes option pricing model. The assumptions used for the year ended December 31, 2014 are as follows:
| December 31, 2014 | |||
| Risk free interest rate | 1.83%-1.84% | ||
| Expected life (in years) | 4.5 to 6 | ||
| Expected Volatility | 96.64%-98.7% | ||
| Expected dividend yield | 0% |
A summary of the changes in the Company’s non-vested options during the year ended December 31, 2014, is as follows:
| Number of Non-vested Options | Weighted Average Fair Value at Grant Date | Intrinsic Value | ||||||||||
| Non-vested at January 1, 2014 | - | $ | - | - | ||||||||
| Vested in year ended December 31, 2014 | 256,508 | $ | 0.73 | - | ||||||||
| Non-vested at December 31, 2014 | 501,469 | $ | 0.73 | - | ||||||||
| Exercisable at December 31, 2014 | 256,508 | $ | 0.73 | - | ||||||||
| Outstanding at December 31, 2014 | 757,977 | $ | 0.73 | - | ||||||||
As of December 31, 2014, total unrecognized compensation cost related to unvested stock options was $297,148. The cost is expected to be recognized over a weighted average period of 1.75 years.
| 66 |
Bone Biologics, Corp.
Notes to Consolidated Financial Statements
10. Income Taxes
The provision for income taxes consists of the following:
| Year Ended December 31, | 2014 | 2013 | ||||||
| Current: | ||||||||
| Federal | $ | - | $ | - | ||||
| State | 1,600 | 800 | ||||||
| Total current | 1,600 | 800 | ||||||
| Deferred: | ||||||||
| Federal | - | - | ||||||
| State | - | - | ||||||
| Total deferred | - | - | ||||||
| Provision for income taxes | $ | 1,600 | $ | 800 | ||||
The components of deferred tax assets and liabilities consist of the following:
| December 31, | 2014 | 2013 | ||||||
| Deferred tax assets | ||||||||
| Net operating losses | $ | 2,631,000 | $ | 1,866,000 | ||||
| Patents | 326,000 | 560,000 | ||||||
| Accrued expenses | 952,000 | 550,000 | ||||||
| R&D credits | 85,000 | 57,000 | ||||||
| Warrants | 765,000 | 45,000 | ||||||
| Total | 4,759,000 | 3,078,000 | ||||||
| Less: Valuation allowance | (4,759,000 | ) | (3,078,000 | ) | ||||
| $ | - | $ | - | |||||
The Company’s federal and state net operating loss carryfowards at December 31, 2014 and 2013 were approximately $6,638,000 and $4,681, 000, respectively, and will begin to expire in 2020 if not utilized.
The Company reviews its deferred tax assets for realization based upon historical taxable income, prudent and feasible tax planning strategies, the expected timing of the reversals of existing temporary differences and expected future taxable income. The Company has concluded that it is more likely than not that the deferred tax assets will not be realized. Accordingly, the Company has recorded a valuation allowance against the net deferred tax assets in the amount of $4,759,000 at December 31, 2014. The net change in the valuation allowance for the year ended December 31, 2014 was $1,680,000.
| 67 |
Bone Biologics, Corp.
Notes to Consolidated Financial Statements
The effective tax rate differs from the statutory tax rate principally due to the change in valuation allowance, nondeductible permanent differences, credits, and state income taxes.
The Company’s effective tax rate is 0% for income tax for the years ended December 31, 2014 and 2013. Based on the weight of available evidence, including cumulative losses since inception and expected future losses, the Company has determined that it is more likely than not that the deferred tax asset amount will not be realized and therefore a valuation allowance has been provided on net deferred tax assets.
The Company files tax returns for U.S. Federal and State of California. The Company is not currently subject to any income tax examinations. Since the Company’s inception, the Company had incurred losses from operations, which generally allows all tax years to remain open.
Uncertain Tax Positions
The Company recognizes the consolidated financial statement effects of a tax position when it becomes more likely than not, based upon the technical merits, that the position will be sustained upon examination.
The Company recognizes interest and/or penalties related to uncertain tax positions. To the extent accrued interest and penalties do not ultimately become payable, amounts accrued will be reduced and reflected in the period that such determination is made. The interest and penalties are recognized as other expense and not tax expense. The Company currently has no interest and penalties related to uncertain tax positions.
11. Related Party Transactions
In September 2006, the Company entered into a consulting agreement with one of its stockholders whom previously served as chairman, president and CEO of the Company. The Company paid $178,597 and $120,000, respectively, for the years ended December 31, 2014 and 2013 in consulting fees to this related party. Additionally, on September 19, 2014 the Company issued a warrant to purchase 699,671 shares of the Company’s common stock. (See Note 8)
One of the Company’s co-founders had previously provided research and development consulting services to the Company and earned an aggregate of $320,000 of fees from inception to January 2010. Of the $320,000, $52,500 has been deferred for payment until the Company’s next equity financing. As of December 31, 2013, the $52,500 deferred payment was included in the accrued expenses. On October 27, 2014 the deferred payment was fully paid.
In September 2013 the Company entered into a consulting agreement with MTF for the services of Michael Schuler. Immediately following the Merger, the Company entered into a consulting agreement with MTF, which has agreed to provide the services of Mr. Schuler to the Company as a contractor. Pursuant to the agreement, Mr. Schuler will serve as the Company’s Interim Chief Executive Officer for a period of 6 months. The agreement shall automatically renew for successive three (3) month periods unless either party provides written notice to the other party at least 10 days in advance of the renewal term of its decision not to renew the term. The agreement is intended to be temporary in nature, and will cease once the Company retains a permanent Chief Executive Officer. There are no payments due to MTF or Mr. Schuler with respect to any change in control of the Company or termination of the consulting agreement. For the year ended December 31, 2014, the Company recognized $47,500 of expense related to this contract.
See Note 6 for related party notes payable to MTF.
See Note 12 for related party transactions with AFH
| 68 |
Bone Biologics, Corp.
Notes to Consolidated Financial Statements
12. Transaction Costs
The Company paid (i) AFH $500,000 (the “Shell Cost”) to allow Bone Biologic Inc. stockholders to acquire shares of common stock of the Company and become the majority owners in the aggregate of the Company and to achieve the desired post-merger capitalization of the Company and to (ii) reimbursed AFH Advisory for its advancement of expenses on behalf of the Company related to the Merger and a public offering of $90,000. These Transaction Costs have been recorded as an expense in the accompanying statement of operations in the period in which they were incurred. Additional transaction costs included accounting, legal and other professional services. Other receivables – related party represent a $75,000 receivable from AFH, a shareholder, for fees paid on their behalf for legal services.
13. Subsequent Events
On February 29, 2015, the Company terminated its consulting contract with T.O. Medical. As per the contract they were provided a 90 day notice and all warrants issued became fully vested.
On February 15, 2015 our agreement with Forefront Capital expired without renewal.
The Company has evaluated subsequent events through March 30, 2015, the date which the consolidated financial statements were available to be issued. There were no additional subsequent events noted that would require adjustment to or disclosure in these consolidated financial statements.
| 69 |
| Exhibit No. | Description | |
| 2.1 | Agreement and Plan of Merger, dated as of September 19, 2014, by and among AFH Acquisition X, Inc., Bone Biologics Acquisition Corp., and Bone Biologics, Inc. (incorporated herein by reference to Exhibit 2.1 to current report on Form 8-K, File No. 000-53078, filed September 25, 2014) | |
| 2.2 | Certificate of Merger as filed with the California Secretary of State effective September 19, 2014 (incorporated herein by reference to Exhibit 2.2 to current report on Form 8-K, File No. 000-53078, filed September 25, 2014) | |
| 3.1(i) | Amended and Restated Articles of Incorporation, of Bone Biologics, Corp., as filed with the Delaware Secretary of State on July 28, 2014 (incorporated herein by reference to Exhibit 3.1(i) to current report on Form 8-K, File No. 000-53078, filed September 25, 2014) | |
| 3.1(ii) | Amended and Restated Bylaws of Bone Biologics, Corp. (incorporated herein by reference to Exhibit 3.1(ii) to current report on Form 8-K, File No. 000-53078, filed September 25, 2014) | |
| 4.1 | Bone Biologics, Corp. September 2013 Warrant issued to AFH (incorporated herein by reference to Exhibit 4.1 to current report on Form 8-K, File No. 000-53078, filed September 25, 2014) | |
| 4.2 | Bone Biologics, Corp. June 2013 Warrant issued to Orthofix (incorporated herein by reference to Exhibit 4.2 to current report on Form 8-K, File No. 000-53078, filed September 25, 2014) | |
| 4.3 | Bone Biologics, Corp. April 2013 Warrant issued to MTF (incorporated herein by reference to Exhibit 4.3 to current report on Form 8-K, File No. 000-53078, filed September 25, 2014) | |
| 4.4 | Amendment to Bone Biologics, Corp. April 2013 Warrant issued to MTF, June 2013 Warrant issued to Orthofix and September 2013 Warrant issued to AFH (incorporated herein by reference to Exhibit 4.4 to current report on Form 8-K, File No. 000-53078, filed September 25, 2014) | |
| 4.5 | Bone Biologics, Corp. Warrant issued to Marie Antonia Gray (incorporated herein by reference to Exhibit 4.5 to current report on Form 8-K, File No. 000-53078, filed September 25, 2014) | |
| 4.6 | Bone Biologics, Corp. March 2009 Warrant issued to MTF (incorporated herein by reference to Exhibit 4.6 to current report on Form 8-K, File No. 000-53078, filed September 25, 2014) | |
| 4.7 | Bone Biologics, Corp. Warrant issued to T.O. Medical Development Inc. (incorporated herein by reference to Exhibit 4.7 to current report on Form 8-K, File No. 000-53078, filed September 25, 2014) | |
| 4.8 | Bone Biologics, Corp. Warrant issued to Chia Soo (incorporated herein by reference to Exhibit 4.8 to current report on Form 8-K, File No. 000-53078, filed September 25, 2014) | |
| 4.9 | Bone Biologics, Corp. Warrant issued to Aragen Bioscience, Inc. (incorporated herein by reference to Exhibit 4.9 to current report on Form 8-K, File No. 000-53078, filed September 25, 2014) | |
| 4.10 | Bone Biologics, Corp. Warrant issued to Alquest, Inc. (incorporated herein by reference to Exhibit 4.10 to current report on Form 8-K, File No. 000-53078, filed September 25, 2014) | |
| 4.11 | Bone Biologics, Corp. October 2013 Warrant issued to Orthofix (incorporated herein by reference to Exhibit 4.11 to current report on Form 8-K, File No. 000-53078, filed September 25, 2014) |
| 70 |
| 4.12 | Bone Biologics, Corp. June 2014 Warrant issued to MTF, as thereafter assigned to Orthofix (incorporated herein by reference to Exhibit 4.12 to current report on Form 8-K, File No. 000-53078, filed September 25, 2014) | |
| 4.13 | Bone Biologics, Corp. July 2014 Warrant issued to Orthofix (incorporated herein by reference to Exhibit 4.13 to current report on Form 8-K, File No. 000-53078, filed September 25, 2014) | |
| 4.14 | Bone Biologics, Corp. July 2014 Warrant issued to AFH (incorporated herein by reference to Exhibit 4.14 to current report on Form 8-K, File No. 000-53078, filed September 25, 2014) | |
| 4.15 | Bone Biologics, Corp. Warrant issued to Catherine Doll (incorporated herein by reference to Exhibit 4.15 to current report on Form 8-K, File No. 000-53078, filed September 25, 2014) | |
| 4.16 | Bone Biologics, Corp. Warrant issued to Forefront Capital Markets, LLC (incorporated herein by reference to Exhibit 4.16 to current report on Form 8-K, File No. 000-53078, filed September 25, 2014) | |
| 4.17 | Bone Biologics, Corp. September 2014 Warrant issued to MTF(incorporated herein by reference to Exhibit 4.17 to current report on Form 8-K, File No. 000-53078, filed September 25, 2014) | |
| 4.18 | Form of Registration Rights Agreement, by and between Bone Biologics, Corp., AFH, HIC and MTF (incorporated herein by reference to Exhibit 4.18 to current report on Form 8-K, File No. 000-53078, filed September 25, 2014) | |
| 4.19 | Bone Biologics, Corp. Convertible Note Purchase Agreement, dated September 19, 2014 by and Between Bone Biologics, Corp. and MTF (incorporated herein by reference to Exhibit 4.19 to current report on Form 8-K, File No. 000-53078, filed September 25, 2014) | |
| 4.20 | Bone Biologics, Corp. Convertible Promissory Note, dated September 19, 2014, issued to MTF (incorporated herein by reference to Exhibit 4.20 to current report on Form 8-K, File No. 000-53078, filed September 25, 2014) | |
| 4.21 | $340,000 Note issued in Favor of AFH Advisory (incorporated herein by reference to Exhibit 4.21 to current report on Form 8-K, File No. 000-53078, filed September 25, 2014) | |
| 4.22 | Loan Agreement dated September 15, 2014, by and Between MTF and Bone Biologics, Inc. and Assignment, Assumption and Consent Agreement (incorporated herein by reference to Exhibit 4.22 to current report on Form 8-K, File No. 000-53078, filed September 25, 2014) | |
| 4.23 | Note dated as of September 15, 2014, issued in Favor of MTF (incorporated herein by reference to Exhibit 4.23 to current report on Form 8-K, File No. 000-53078, filed September 25, 2014) | |
| 4.24 | Letter of Credit granted by MTF to AFH Advisory (incorporated herein by reference to Exhibit 4.24 to current report on Form 8-K, File No. 000-53078, filed September 25, 2014) | |
| 4.25 | Return to Treasury Agreement of Bone Biologics, Corp. with AFH Advisory (incorporated herein by reference to Exhibit 4.25 to current report on Form 8-K, File No. 000-53078, filed September 25, 2014) | |
| 10.1 | Director Offer Letter, dated November 24, 2014, by and between George A. Oram and Bone Biologics, Corp. ((incorporated herein by reference to Exhibit 10.1 to current report on Form 8-K, File No. 000-53078, filed December 1, 2014)+ | |
| 10.2 | Letter Agreement, dated September 7, 2014, by and among AFH Holding & Advisory, LLC, Bone Biologics, Inc. and Musculoskeletal Transplant Foundation, Inc. (incorporated herein by reference to Exhibit 10.1 to current report on Form 8-K, File No. 000-53078, filed September 25, 2014) |
| 71 |
| 10.3 | Director Offer Letter, dated July 2, 2014, by and between Chia Soo and Bone Biologics, Corp. (incorporated herein by reference to Exhibit 10.3 to current report on Form 8-K, File No. 000-53078, filed September 25, 2014)+ | |
| 10.4 | Director Offer Letter, dated July 1, 2014, by and between Bruce Stroever and Bone Biologics, Corp. (incorporated herein by reference to Exhibit 10.4 to current report on Form 8-K, File No. 000-53078, filed September 25, 2014) + | |
| 10.5 | Director Offer Letter, dated August 22, 2014, by and between John Booth and Bone Biologics, Corp. (incorporated herein by reference to Exhibit 10.5 to current report on Form 8-K, File No. 000-53078, filed September 25, 2014)+ | |
| 10.6 | Director Offer Letter, dated June 23, 2014, by and between Jimmy Delshad and Bone Biologics, Corp. (incorporated herein by reference to Exhibit 10.6 to current report on Form 8-K, File No. 000-53078, filed September 25, 2014)+ | |
| 10.7 | Director Offer Letter, dated, June 25, 2014, by and between Steve Warnecke and Bone Biologics, Corp. (incorporated herein by reference to Exhibit 10.7 to current report on Form 8-K, File No. 000-53078, filed September 25, 2014)+ | |
| 10.8 | Director Offer Letter, dated, June 25, 2014, by and between William Coffin and Bone Biologics, Corp. (incorporated herein by reference to Exhibit 10.8 to current report on Form 8-K, File No. 000-53078, filed September 25, 2014)+ | |
| 10.9 | Management Consulting Agreement, dated September 19, 2014, by and between the Musculoskeletal Transplant Foundation, Inc. and Bone Biologics, Corp. (Mike Schuler) (incorporated herein by reference to Exhibit 10.9 to current report on Form 8-K, File No. 000-53078, filed September 25, 2014) + | |
| 10.10 | Management Consulting Agreement, dated September 19, 2014, by and between the Musculoskeletal Transplant Foundation, Inc. and Bone Biologics, Corp. (Bruce Stroever) (incorporated herein by reference to Exhibit 10.10 to current report on Form 8-K, File No. 000-53078, filed September 25, 2014)+ | |
| 10.11 | President and Chief Technology Officer Employment agreement, dated September 19, 2014, by and between Bone Biologics, Corp. and William Jay Treat (incorporated herein by reference to Exhibit 10.11 to current report on Form 8-K, File No. 000-53078, filed September 25, 2014)+ | |
| 10.12 | Management Consulting Agreement, dated July 1, 2014, by and between The Gilson Group, LLC and Bone Biologics, Corp. (incorporated herein by reference to Exhibit 10.12 to current report on Form 8-K, File No. 000-53078, filed September 25, 2014)+ | |
| 10.13 | Consulting Agreement, dated September 19, 2014, by and between Bone Biologics, Corp. and T.O. Medical Development Inc. (incorporated herein by reference to Exhibit 10.13 to current report on Form 8-K, File No. 000-53078, filed September 25, 2014)+ | |
| 10.14 | Bone Biologics, Corp. 2014 Stock Plan (incorporated herein by reference to Exhibit 10.14 to current report on Form 8-K, File No. 000-53078, filed September 25, 2014)+ | |
| 10.15 | Bone Biologics, Corp. Convertible Secured Term Note issued to Hankey Capital, LLC on October 24, 2014 (incorporated herein by reference to Exhibit 10.1 to current report on Form 8-K, File No. 000-53078, filed October 30, 2014) |
| 72 |
| 10.16 | Bone Biologics, Corp. Warrant issued to Hankey Capital, LLC on October 24, 2014 (incorporated herein by reference to Exhibit 10.2 to current report on Form 8-K, File No. 000-53078, filed October 30, 2014) | |
| 10.17 | Registration Rights Agreement by and between Bone Biologics, Corp. and Hankey Capital, LLC, dated October 24, 2014 (incorporated herein by reference to Exhibit 10.3 to current report on Form 8-K, File No. 000-53078, filed October 30, 2014) | |
| 10.18 | Chief Financial Officer Employment agreement, dated November 4, 2014, by and between Bone Biologics, Corp. and Deina H. Walsh (incorporated herein by reference to Exhibit 10.1 to current report on Form 8-K, File No. 000-53078, filed November 12, 2014)+ | |
| 21.1 | Subsidiaries (incorporated herein by reference to Exhibit 21.1 to current report on Form 8-K, File No. 000-53078, filed September 25, 2014) | |
| 23.1 | Consent of Anton & Chia, LLP * | |
| 24.1 | Power of Attorney (included on signature page of this Form 10-K) | |
| 31.1 | Certification of Chief Executive Officer Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002*
| |
| 31.2 | Certification of Chief Financial Officer Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002*
| |
| 32.1 | Certification of Chief Executive Officer Pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002* | |
| 32.2 | Certification of Chief Financial Officer Pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002* | |
| 101.INS* | XBRL Instance Document. | |
| 101.SCH* | XBRL Taxonomy Extension Schema Document. | |
| 101.CAL* | XBRL Taxonomy Extension Calculation Linkbase Document. | |
| 101.DEF* | XBRL Taxonomy Extension Definition Linkbase Document | |
| 101.LAB* | XBRL Taxonomy Extension Label Linkbase Document. | |
| 101.PRE* | XBRL Taxonomy Extension Presentation Linkbase Document. | |
| * | Filed herewith. |
| + | Designates management contracts and compensation plans. |
| 73 |
