Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - NORTHWEST BIOTHERAPEUTICS INC | v405632_8k.htm |
| EX-99.1 - EXHIBIT 99.1 - NORTHWEST BIOTHERAPEUTICS INC | v405632_ex99-1.htm |
| EX-99.2 - EXHIBIT 99.2 - NORTHWEST BIOTHERAPEUTICS INC | v405632_ex99-2.htm |
Exhibit 99.3

Autologous Dendritic Cell Therapy for Cancer 2 nd Immunotherapy of Cancer Conference, ITOC - 2 Munich, Germany March 26, 2015 Marnix L. Bosch, MBA, PhD Chief Technical Officer Northwest Biotherapeutics

Disclaimer Certain statements made in this presentation are “forward - looking statements” of NW Bio as defined by the Securities and Exchange Commission (“SEC”). All statements, other than statements of historical fact, included in this presentation that address activities, events or developments that NW Bio believes or anticipates will or may occur in the future are forward - looking statements. These statements are based on certain assumptions made based on experience, expected future developments and other factors NW Bio believes are appropriate in the circumstances. Such statements are subject to a number of assumptions, risks and uncertainties, many of which are beyond the control of NW Bio. Investors and others are cautioned that any such statements are not guarantees of future performance. These forward - looking statements could cause actual results and developments to differ materially from those expressed or implied in such statements, including our ability to raise funds for general corporate purposes and operations, including our clinical trials, the commercial feasibility and success of our technology, our ability to recruit qualified management and technical personnel, our ability to scale up the manufacturing of our product candidates for commercialization, the success of our clinical trials and our ability to obtain and maintain required regulatory approvals for our products. Furthermore, NW Bio does not intend (and is not obligated) to update publicly any forward - looking statements. The contents of this presentation should be considered in conjunction with the risk factors contained in NW Bio’s recent filings with the SEC, including its most recent Form 10K. This communication is neither an offer to sell nor a solicitation of an offer to buy any securities mentioned herein. This publication is confidential for the information of the addressee only and may not be reproduced in whole or in part; copies circulated, or disclosed to another party, without the prior written consent of Northwest Biotherapeutics (NW Bio) are strictly prohibited. 2

Glioblastoma Multiforme (GBM) • GBM, Stage IV glioma, presents a significant unmet medical need – Average time to recurrence approximately 7 months – Average time to death approximately 15 months • Standard of care, radiation therapy + temozolomide, was approved in 2005 for GBM • There is no life - extending treatment for GBM following recurrence • Average time to death from first recurrence is approximately 9 months 3

GBM – Standard Treatment and Progression 1. If possible, surgical removal of most of the tumor tissue 2. Chemo - radiation: six weeks of radiation therapy + concomitant temozolomide 3. Adjuvant temozolomide: ≥ 6 cycles of 5 days on, 23 days off 4. Following recurrence 1. NovoTTF device 2. BCNU/CCNU chemotherapy 3. Bevacizumab, mostly to combat symptoms • Disease progression is monitored through MRI imaging • Radiation necrosis and other artifacts following radiation can mask as progression and are known as pseudoprogression 4

DCVax - L: Introduction • DCVax - L is autologous dendritic cells (DC) pulsed with autologous tumor cell lysate – DC are the master cells of the immune system, and are required to induce an adaptive immune response – Tumor lysate provides the antigens against which the immune response will be directed • DCVax - L is injected into the skin, as an outpatient treatment: the DC migrate to the lymph nodes to activate anti - tumor T cells • DCVax - L treatment is administered in conjunction with standard of care therapies, such as chemo - radiation for newly diagnosed GBM • Early clinical data (UCLA) are encouraging in both newly diagnosed and recurrent GBM 5

DCVax - L Phase I/II Trials for Newly Diagnosed GBM • 20 newly diagnosed GBM; 14 recurrent GBM; 5 lower grade gliomas • Standard of care (surgery & 6 weeks radiation & chemo) + DCVax - L • Primary endpoint: safety; Secondary endpoint: p rogression f ree survival 6 Standard of Care* Matched Concurrent Controls** DCVax - L Progression (Tumor Recurrence) 6.9 mos 8.1 mos 2 years Overall Survival 14.6 mos 17 mos 3 years Long Tail of Survival 2 – 3% alive at 5 years To date: 33% alive >4 yrs 27% alive >6 yrs 2 pts alive >10 yrs ** matched for age, gender, Karnofsky score, extent of surgical resection, and same std of care treatment, at same hospital, in same time period * N Engl J Med 352: 987 - 96, 2005

7 International Phase III Trial With DCVax - L for GBM • 348 patient , randomized (2:1), double blind, placebo controlled Phase III trial ▪ Primary endpoint: PFS (progression free survival ) ▪ Secondary endpoints include OS (overall survival ) ▪ 3 DCVax - L treatments upfront (Day 0, 10, 20), then 3 boosters (months 2, 4, 8) then 4 treatments twice/year for maintenance phase (months 12, 18, 24, 30) • Newly diagnosed GBM; trial under way in both US, Europe & Canada • Trial Design Features ▪ 4 - month extension of PFS required to meet primary endpoint ▪ Trial powered to reach p value = 0.02 if 4 - month difference in PFS shown ▪ Trial also powered for secondary endpoint of Overall Survival ▪ Multiple sub - group analyses were prospectively included

Informational Arm open label, for patients with apparent PD post chemo - radiation 8 51 patients had evidence of apparent disease progression in imaging at Baseline Visit following 6 weeks’ chemo - radiation Patients re - imaged at Month 2 after Baseline Visit to confirm either actual disease progression or pseudo - progression (patients categorized by independent medical imaging review company) 20 patients had further progression at Month 2: Rapid - Progressors 25 patients had stable disease or modest progression/regression or measurements unclear at Month 2: Indeterminate 1 patient had resolution at Month 2: Pseudo - progressor 5 patients Unclassified due to lack of images

Informational Arm: Overall Results • Median overall survival for the 51 patients with evidence of disease progression post chemo - radiation = 18.3 months (range 6.7 to >40.7 months) – This compares favorably to 14.6 months mOS for newly diagnosed GBM patients receiving standard care • 15 of 51 patients (~30%) lived beyond 2 years • 12 of the 15 patients are still alive, with follow up times of 26.9 – 40.7 months 9
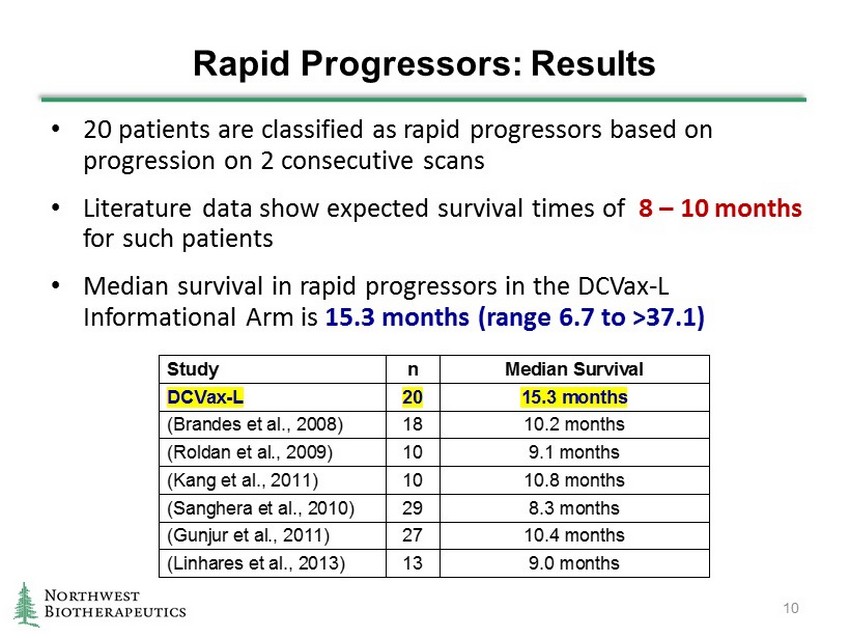
Rapid Progressors: Results • 20 patients are classified as rapid progressors based on progression on 2 consecutive scans • Literature data show expected survival times of 8 – 10 months for such patients • Median survival in rapid progressors in the DCVax - L Informational Arm is 15.3 months (range 6.7 to >37.1 ) 10 Study n Median Survival DCVax-L 20 15.3 months (Brandes et al., 2008) 18 10.2 months (Roldan et al., 2009) 10 9.1 months (Kang et al., 2011) 10 10.8 months (Sanghera et al., 2010) 29 8.3 months (Gunjur et al., 2011) 27 10.4 months (Linhares et al., 2013) 13 9.0 months

0 5 10 15 20 25 30 35 40 Recurrent GBM Median OS 8.3 – 10.8 Months In Literature 20 Patients Months 20 Rapid - Progressor Patients : Median Overall Survival 15.3 Months (Data as of February 2015) Patients Still Alive Patients Still Alive

Other Patients: Results • 25 Informational Arm patients classified as Indeterminate : i.e., they had stable disease, or modest progression/regression, or unclear measurements – mOS = 21.5 months – 9 patients are still alive, at 26.9 – 40.7 months • 1 Informational Arm patient classified as a Pseudo - Progressor: i.e., the appearance of tumor re - growth at the Baseline Visit had resolved by the Month 2 visit. – t his patient is still alive, at 30.1 months 12

25 Indeterminate Patients: Median Overall Survival 21.5 Months (Data as of February 2015) 0.0 5.0 10.0 15.0 20.0 25.0 30.0 35.0 40.0 45.0 Months 25 Patients Patients Still Alive Std of Care for New GBM: 14.6 Months mOS N Engl J Med 352: 987 - 96 , 2005

0 5 10 15 20 25 30 35 5 Patients 1 Pseudo - Progressor Patient Overall Survival To Date 30 Months (Data as of February 2015) 0 5 10 15 20 25 30 35 Months 1 Patient Patients Still Alive 5 Patients Unclassified Due to Lack of Follow - Up Images (Data as of February 2015) Informational Arm: Other Patients Patients Still Alive Months

Conclusions • Patients with evidence of disease recurrence immediately following 6 weeks of daily radiotherapy and chemotherapy after surgical resection of their brain tumors appear to survive longer than would be expected based on data in the literature, when treated with DCVax - L. • The apparent extended survival of these patients is seen in both Rapid Progressor Patients and Indeterminate Patients (as well as the Pseudo - Progressor Patient). • The combined data suggest a possible survival benefit for patients with recurrent GBM conferred by the DCVax - L treatment. • The ~30% of survivors who have lived beyond 2 years may reflect long - term tumor control. • DCVax - L treatment (vaccination of patients with autologous dendritic cells loaded with autologous tumor lysate antigens) continues to have an excellent safety profile. 15
