Attached files
| file | filename |
|---|---|
| EX-31.2 - EX-31.2 - ZS Pharma, Inc. | d846181dex312.htm |
| EX-14 - EX-14 - ZS Pharma, Inc. | d846181dex14.htm |
| EX-32.1 - EX-32.1 - ZS Pharma, Inc. | d846181dex321.htm |
| EX-31.1 - EX-31.1 - ZS Pharma, Inc. | d846181dex311.htm |
| EX-23.1 - EX-23.1 - ZS Pharma, Inc. | d846181dex231.htm |
| EX-32.2 - EX-32.2 - ZS Pharma, Inc. | d846181dex322.htm |
| EXCEL - IDEA: XBRL DOCUMENT - ZS Pharma, Inc. | Financial_Report.xls |
| EX-10.17 - EX-10.17 - ZS Pharma, Inc. | d846181dex1017.htm |
Table of Contents
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
| x | Annual Report Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934 |
For the fiscal year ended December 31, 2014.
or
| ¨ | Transition Report Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934 |
For the transition period from to
Commission File Number 001-36489
ZS PHARMA, INC.
(Exact name of registrant as specified in its charter)
| Delaware | 26-3305698 | |
| (State or other jurisdiction of incorporation or organization) |
(I.R.S. Employer Identification No.) | |
| 508 Wrangler Drive, Suite 100 Coppell, Texas |
75019 | |
| (Address of principal executive offices) | (Zip Code) | |
(877) 700-0240
(Registrant’s telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class |
Name of each exchange on which registered | |
| Common Stock, $0.001 par value | The Nasdaq Global Market |
Securities registered pursuant to Section 12(g) of the Act:
None
(Title of class)
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ¨ No x
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ¨ No x
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports) and (2) has been subject to such filing requirements for the past 90 days. Yes x No ¨
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes x No ¨
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (§ 229.405 of this chapter) is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. x
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See definitions of “large accelerated filer”, “accelerated filer”, and “smaller reporting company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer | ¨ | Accelerated filer | ¨ | |||
| Non-accelerated filer | x (Do not check if a smaller reporting company) | Smaller reporting company | ¨ | |||
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ¨ No x
The aggregate market value of the voting common equity held by non-affiliates of the registrant on June 30, 2014 (the last business day of the registrant’s most recently completed second fiscal quarter) was $65,017,780.
As of March 3, 2015, the registrant had 20,902,312 shares of common stock outstanding, with a par value of $0.001.
DOCUMENTS INCORPORATED BY REFERENCE
No items are incorporated by reference into this Form 10-K.
Table of Contents
i
Table of Contents
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
This Annual Report on Form 10-K contains forward-looking statements that involve risks and uncertainties. We make such forward-looking statements pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995 and other federal securities laws. Any statements contained herein that are not statements of historical facts may be deemed to be forward-looking statements. In some cases, you can identify forward-looking statements by terminology such as “aim,” “anticipate,” “assume,” “believe,” “contemplate,” “continue,” “could,” “due,” “estimate,” “expect,” “goal,” “intend,” “may,” “objective,” “plan,” “predict,” “potential,” “positioned,” “seek,” “should,” “target,” “will,” “would,” and other similar expressions that are predictions of or indicate future events and future trends, or the negative of these terms or other comparable terminology. These forward-looking statements include, but are not limited to, statements about:
| • | our expectations regarding the timing of reporting primary endpoint results from our ongoing clinical trials of sodium zirconium cyclosilicate (ZS-9); |
| • | our expectations regarding the timing of submitting an NDA to the FDA and MAA to the EMA and the likelihood of regulatory approval for ZS-9; |
| • | our expectations regarding the potential market size and the size of the patient populations for ZS-9, if approved for commercial use; |
| • | projections relating to the ability and timing of competitive products to enter the market; |
| • | our expectations regarding our ability to successfully commercialize ZS-9, if approved; |
| • | estimates of our expenses, future revenue, capital requirements and needs for additional financing; |
| • | our expectations regarding the number of nephrologists and cardiologists that we plan to target; |
| • | our ability to develop, acquire and advance product candidates into, and successfully complete, clinical trials; |
| • | our ability to produce ZS-9 in commercial quantities in higher capacity reactors; |
| • | the scope of protection we are able to establish and maintain for intellectual property rights covering ZS-9 and our other potential product candidates; |
| • | the expected results and timing related to our competitors’ efforts to obtain intellectual property protection for their compounds; |
| • | the implementation of our business model and strategic plans for our business and technology; |
| • | our expectations regarding our future costs of goods; |
| • | the initiation, timing, progress and results of future nonclinical studies, clinical trials and research and development programs; |
| • | our ability to maintain and establish collaborations or obtain additional funding; |
| • | our expectations regarding the time during which we will be an emerging growth company under the JOBS Act; |
| • | our financial performance; and |
| • | developments and projections relating to our competitors and our industry. |
Any forward-looking statements in this Annual Report on Form 10-K are based on management’s current expectations, estimates, forecasts, and projections about our business and the industry in which we operate and management’s beliefs and assumptions and are not guarantees of future performance or development and involve known and unknown risks, uncertainties, and other factors that are in some cases beyond our control. As a result, any or all of our forward-looking statements in this Annual Report on Form 10-K may turn out to be inaccurate. Factors that may cause actual results to differ materially from current expectations include, among other things, those listed under Part I, Item 1A. Risk Factors and elsewhere in this Annual Report on Form 10-K. You are urged to consider these factors carefully in evaluating the forward-looking statements. These forward-looking statements speak only as of the date of this Annual Report on Form 10-K. Except as required by law, we assume
Table of Contents
no obligation to update or revise these forward-looking statements for any reason, even if new information becomes available in the future. You should, however, review the factors and risks we describe in the reports we will file from time to time with the SEC after the date of this Annual Report on Form 10-K.
EXPLANATORY NOTE
Throughout this Annual Report on Form 10-K (“Form 10-K”), we refer to ZS Pharma, Inc. as “we”, “us”, “our” or the “Company”. We were incorporated in Delaware in February 2008 under the name ZS Pharma, Inc. We commenced operations in November 2008. Our principal executive offices are located at 508 Wrangler Drive, Suite 100, Coppell, Texas 75019, and our telephone number is (877) 700-0240. Our website address is www.zspharma.com.
2
Table of Contents
PART I. — BUSINESS FACTORS
| ITEM 1. | BUSINESS |
Overview
We are a biopharmaceutical company focused on the development and commercialization of highly selective, non-absorbed drugs to treat renal, cardiovascular, liver and metabolic diseases. Our proprietary zirconium silicate technology allows us to create highly selective ion traps that can reduce toxic levels of specific electrolytes without disturbing the balance of other electrolytes. Our initial focus is on the development of sodium zirconium cyclosilicate (ZS-9), our product candidate for which we recently completed two Phase III clinical trials for the treatment of hyperkalemia, a life-threatening condition in which elevated levels of potassium in the blood (greater than 5.0 mEq/L) increase the risk of muscle dysfunction, including cardiac arrhythmias and sudden cardiac death. We have designed our development program based on input from the United States Food and Drug Administration, or FDA, including a recent pre-NDA meeting, and European Medicines Agency, or EMA, and plan to submit our New Drug Application, or NDA, in the United States in the second quarter of 2015 and our Marketing Authorization Application, or MAA, in Europe in the second half of 2015 with the goal of obtaining approval for the treatment of acute and chronic hyperkalemia, regardless of the underlying disease state. If we receive regulatory approval, we intend to commercialize ZS-9 for the treatment of hyperkalemia in the United States with our own specialty sales force targeting nephrologists and cardiologists and intend to seek one or more partners for commercialization in markets outside of the United States.
As of March 1, 2015, we have enrolled over 1,474 patients in our clinical studies. In our ongoing long-term studies, patients have safely received once-daily ZS-9 for over 10 months. We have completed three, double-blind, randomized placebo controlled clinical studies with ZS-9 that together enrolled 1,101 patients with hyperkalemia, including patients with chronic kidney disease (CKD), heart failure (HF), type 2 diabetes and those on renin-angiotensin aldosterone system (RAAS) inhibitor therapy. Our first in-man study, ZS002, was completed in May 2012 and our first Phase III study, ZS003, was completed in November 2013. Our second Phase III study, ZS004, was completed in September 2014. All three trials met their pre-specified primary efficacy endpoints with clinically meaningful and statistically significant results. We announced the top-line results for ZS004 in September 2014, and presented detailed primary and secondary endpoint results for ZS004 at the American Heart Association Scientific Sessions on November 17, 2014. The ZS004 study results were simultaneously published in the Journal of the American Medical Association, and we announced the publication of detailed results from the ZS003 study in the New England Journal of Medicine on November 21, 2014. We initiated an extension to the ZS004 study, or ZS004e, and a long-term safety study, ZS005, in the second quarter of 2014. We expect to file our NDA with the FDA in the second quarter of 2015 and our MAA with the EMA in the second half of 2015.
ZS-9 is an insoluble, non-absorbed zirconium silicate with a clearly defined three-dimensional crystalline lattice structure that was designed and engineered to preferentially trap potassium ions. Sodium Polystyrene Sulfonate, or SPS (e.g., Kayexalate), the current standard of care for hyperkalemia in the United States, is a nonselective polymer resin. In contrast to nonselective polymer resins, the potassium selectivity of ZS-9 enables high in-vitro binding capacity for potassium ions even in the presence of other competing ions. Head-to-head in-vitro experiments demonstrate that ZS-9 has roughly ten times the potassium binding capacity of SPS. In our clinical studies to date, this selectivity has resulted in the following important differentiating characteristics for ZS-9 that we believe support its potential use to treat acute and chronic hyperkalemia:
| • | high efficacy, with 98% of patients from our completed clinical trials receiving at our top dose returning to a normal level of potassium (between 3.5 and 5.0 mEq/L) in their blood, which is referred to as normokalemia, within 48 hours; |
| • | rapid onset of action, with statistically significant reductions in potassium observed at one hour and a median time to normalization of serum potassium levels of 2.2 hours in our most recently completed clinical trial following a single 10g dose; |
3
Table of Contents
| • | ability in our studies to date to safely and effectively maintain normokalemia over 28 days, with 80%, 90%, and 94% of patients on once-daily 5g, 10g, and 15g of ZS-9, respectively, controlled in ZS004; |
| • | normalization of bicarbonate levels in patients with metabolic acidosis (low blood pH); |
| • | reduction in the levels of serum aldosterone, high levels of aldosterone have been implicated in the progression of cardiovascular and kidney diseases; |
| • | potential suitability for chronic, once daily administration; |
| • | non-absorbed, with no evidence of systemic absorption in our human and animal studies to date; |
| • | well-tolerated in our studies to date, including our long-term studies where patients have been receiving once-daily ZS-9 for over 10 months, with a low rate of hypokalemia and an incidence of adverse events similar to placebo; |
| • | administered as a convenient oral suspension powder or dissolvable tablets; |
| • | no clinically significant effects on other electrolytes that are critical for normal physiological functioning, including sodium, calcium and magnesium; and |
| • | stability at room temperature with a long shelf-life, which we expect will simplify distribution, physician sampling and storage for both physicians and patients. |
Hyperkalemia occurs frequently in patients with CKD, HF and type 2 diabetes, each of which has a high and increasing incidence worldwide. We estimate the number of patients in the United States with CKD will increase from approximately 26 million in 2009 to approximately 46 million by 2022. Hyperkalemia can also arise from the use of RAAS inhibitors, which cause the kidney to retain potassium, thereby increasing serum potassium levels. RAAS inhibitors (such as Angiotensin Converting Enzyme inhibitors (ACEs), or Angiotensin Receptor Blockers (ARBs)) are commonly prescribed for patients with hypertension, HF and CKD, and have been proven in large outcomes studies to reduce morbidity, mortality and progression of disease in these patients. As a result of these studies, RAAS inhibitors are the third most commonly prescribed class of medication in the United States. However, RAAS inhibitors’ mechanism of action results in the retention of potassium, often forcing physicians to choose between the risk of hyperkalemia and the benefits of RAAS therapy.
The current standard of care for hyperkalemia is SPS, which was approved in 1958 as a drug efficacy study implementation, or DESI, drug. The safety and efficacy of SPS have never been proven in randomized, controlled trials. SPS is poorly tolerated by most patients and causes a high incidence of gastrointestinal, or GI, side effects, including nausea, vomiting, constipation, intestinal necrosis and diarrhea, which leads to poor compliance and renders the drug unsuitable for chronic use. For patients with hyperkalemia, we believe ZS-9 represents a well-tolerated alternative to SPS that can rapidly lower and maintain serum potassium at normal levels and may allow physicians to avoid reducing the use of RAAS inhibitors.
We believe a significant commercial opportunity exists for ZS-9 in the United States and internationally. If approved, we plan to commercialize ZS-9 in the United States with a specialty sales force focused on a subset of the approximately 5,000 practicing nephrologists and approximately 15,000 practicing non-interventional cardiologists currently treating our target patient populations. In addition, we intend to make ZS-9 available as a safe and effective therapy in the hospital setting for the rapid treatment of acute hyperkalemia. We believe that ZS-9 will be a useful therapy to treat hyperkalemia, regardless of the underlying cause, and will allow patients to benefit from the cardio-renal protective effect of RAAS therapies. We plan to commercialize ZS-9 internationally with one or more partners. Over time, we intend to build a pipeline of product candidates using our proprietary zirconium silicate technology, which provides the opportunity to target indications susceptible to treatment by non-absorbed binders in the GI tract. We are in nonclinical development with an ammonium binding candidate, ZS-1, which is currently being evaluated as a treatment for patients suffering from disorders associated with high blood ammonia levels, or hyperammonemia.
We have assembled an experienced management team with extensive pharmaceutical development, manufacturing, reimbursement and commercialization expertise from pharmaceutical and biotechnology
4
Table of Contents
companies, including, among others, Merck and Co., Affymax, Genentech, Adams Respiratory Therapeutics, Bone Care International, Novo Nordisk, Encysive, Genzyme and Amgen. Members of our team have led the discovery, development, manufacturing and commercialization of several therapeutics in the renal field, including Omontys, Hectorol and Phoslo.
Our Strategy
Our strategy is to develop and commercialize a product portfolio of novel therapeutics to treat renal, cardiovascular, liver and metabolic diseases. The key elements of our strategy are to:
| • | Obtain FDA, EMA and other international regulatory approvals to market our lead product candidate, ZS-9, for the treatment of hyperkalemia. We have completed our Phase II study and two Phase III studies and are also conducting a long-term safety and efficacy study. Based on the feedback from our recently completed pre-NDA meeting, we believe these studies will serve as the basis for demonstrating safety and efficacy in our NDA and MAA submissions, which we plan to file in the second quarter and second half of 2015, respectively. The FDA has informed us that it does not plan at this time to conduct an advisory committee meeting to review ZS-9, but such decisions are subject to potential revision. |
| • | Commercialize ZS-9 in the United States. If approved, we plan to commercialize ZS-9 in the United States with our own specialty sales force focused on a subset of the approximately 5,000 practicing nephrologists and approximately 15,000 practicing non-interventional cardiologists treating our target patient populations. Based on average annual disease prevalence rates from multi-year surveys conducted between 1999 and 2004 for CKD and between 2005 and 2008 for HF, when applied to 2010 U.S. Census Data, we estimate there are approximately 19 million stage 3 or 4 CKD patients and 5.7 million HF patients in the United States. Based on the medical literature, nearly 4 million patients have hyperkalemia due to their compromised kidney function, type 2 diabetes, and/or use of cardio-renal protective RAAS inhibitors. We believe that, if approved, ZS-9 will address an unmet need for a well-tolerated, effective treatment for hyperkalemia and believe a significant commercial opportunity exists for ZS-9 in the United States. |
| • | Commercialize ZS-9 outside of the United States with one or more partners. We believe the prevalence of CKD and HF in Europe and Japan is similar to that of the United States and is growing rapidly. As a result of the increasing global prevalence of CKD, HF and type 2 diabetes, and wide use of RAAS inhibitors, we believe attractive opportunities exist for ZS-9 for the treatment of hyperkalemia in international markets. Outside of the United States, we may seek partners with international sales expertise who can sell ZS-9 in target markets. These partnerships could be structured to provide us with an opportunity to receive milestone or other one-time payments as well as royalties on product sales. |
| • | Manufacture in-house. We believe that the proprietary manufacturing processes and know-how that we have developed to manufacture zirconium silicates will provide us with a competitive advantage. We currently manufacture ZS-9 in-house in two facilities from readily available starting materials under carefully optimized conditions using specialized equipment. We are in the process of increasing our manufacturing capacity to support anticipated commercial scale quantities, and installed a 2,000 liter commercial scale reactor during the second quarter of 2014 and received delivery of a second 2,000 liter commercial scale reactor in the fourth quarter of 2014. We believe our in-house manufacturing capabilities will better ensure our ability to meet market demand and will allow us to achieve attractive cost of goods if ZS-9 is approved. |
| • | Leverage our proprietary technology to build our pipeline over time. Over time, we intend to build a pipeline of products using our proprietary zirconium silicate technology, which provides the opportunity to target indications susceptible to treatment by non-absorbed binders in the GI tract. We are in nonclinical development with an ammonium binding candidate, ZS-1, which is currently being evaluated as a treatment for patients suffering from disorders associated with hyperammonemia, and we plan to continue to evaluate our zirconium silicate technology for other indications. |
5
Table of Contents
| • | Acquire or in-license additional specialty products. If we are able to successfully launch ZS-9, we intend to leverage our commercial infrastructure by acquiring or licensing rights to products prescribed by the physicians targeted by our specialty sales force. |
Hyperkalemia Background
Potassium’s Role in the Body
Potassium plays a critical role in human physiology. It is needed for membrane activation (in order to propagate electrochemical signals between neurons), ion and solute transport and the regulation of cell volume. Potassium is absorbed from the diet passively, while excretion of potassium is mainly a regulated active process through the kidneys. Due to fluctuations in dietary potassium intake, the body has developed methods to retain potassium when intake is low and to increase potassium excretion when potassium intake is high. Under normal conditions, serum potassium levels are maintained in the range of 3.5 to 5.0 mEq/L by hormones such as insulin, which acts to shift potassium into cells, and aldosterone, which acts on the kidneys and the colon to stimulate the secretion of excess potassium. In healthy individuals, the kidneys excrete 90% to 95% of the absorbed dietary potassium, with the remaining 5% to 10% excreted in the colon. Elevated potassium levels in the body can disrupt the membrane activation in cardiac cells that regulate the electrical impulses that cause the heart muscles to contract. Decreased contraction of the cardiac tissue may cause fatal heart arrhythmias and is the main risk for patients with hyperkalemia.
Causes of Hyperkalemia
There are several possible causes of hyperkalemia, including:
| • | decreased excretion of potassium by the kidneys, which is common in patients with CKD and HF because the kidney is the primary organ involved in potassium removal; |
| • | imbalances of potassium between the extracellular and intracellular fluid compartments, which is common in patients with type 2 diabetes, due to insulin’s role in shifting potassium into the intracellular compartments; and |
| • | use of a number of commonly used drugs that cause elevated potassium levels, including RAAS inhibitors, transplant medicines and nonsteroidal anti-inflammatory drugs. |
Consequences of Hyperkalemia
The impact of hyperkalemia on mortality is well documented and is a result of potassium’s importance in regulating electrochemical signals in nerve and muscle cells. Cardiac arrhythmias are the leading cause of mortality in hyperkalemia patients, and the condition can develop with few symptoms until cardiac arrhythmias manifest and hospitalization is required. Several studies have independently documented the risk of death or cardiac events associated with hyperkalemia in many different patient populations, including those with previous acute myocardial infarctions, previously hospitalized veterans, intensive care patients, dialysis patients and veterans with HF on medication for hypertension.
The morbidity and mortality impact of hyperkalemia is significant. Hyperkalemia is detected in between 1% and 10% of all hospitalized patients. In a 2009 study published in the Archives of Internal Medicine, the one-day mortality rate was up to 17 times higher for admitted patients with serum potassium above 6.0 mEq/L versus those with serum potassium less than 5.5 mEq/L. Even patients with between 5.5 and 6.0 mEq/L of serum potassium had a one-day mortality rate six times higher than those with less than 5.5 mEq/L.
Another study published in the Journal of the American Medical Association in 2012 showed that sudden death and serious cardiac events increased as serum potassium levels rose above 4.5 mEq/L. This retrospective study was the largest of its kind and examined 38,689 patients from 67 hospitals over a nine-year period. The percentage of patients with in-hospital mortality or in-hospital ventricular fibrillation or cardiac arrest was determined across several ranges of post-admission serum potassium. Although hyperkalemia patients
6
Table of Contents
represented only 3.2% of the total patient population, they accounted for 16% of the total hospital deaths. The results demonstrate that high serum potassium levels are a risk factor for cardiac events and mortality. We believe that a well-tolerated treatment that effectively lowers and maintains normal levels of serum potassium would provide significant clinical benefits to these patients and reduce their risk of mortality and cardiac events.
Additional studies have provided further evidence that reducing excess serum potassium levels in hyperkalemia patients reduces mortality risk. One study found reduced serum potassium levels resulting from common therapies led to a statistically significant increase in survival rates. Another study, in hospitalized patients receiving critical care, showed that the reduction of serum potassium by more than 1 mEq/L 48 hours after hospitalization decreases the risk of patient mortality. These studies suggest that treating hyperkalemia in the acute and chronic settings can have a significant impact on patient outcomes by reducing the risk of death.
Cardio-Renal Protective RAAS Inhibitor Therapy is Limited by Hyperkalemia
Maximum doses of RAAS inhibitors are widely recommended for patients with hypertension, HF, CKD and type 2 diabetes in order to regulate blood pressure. Large outcomes studies have shown that RAAS inhibitors can significantly decrease hospitalization, morbidity and mortality in these patients. However, inhibition of the RAAS pathway also promotes potassium retention by reducing aldosterone, a hormone involved in potassium secretion, leading to hyperkalemia. As a consequence, physicians are often forced to make the choice between the risk of hyperkalemia and the benefits of RAAS inhibitor therapy.
Outcomes Studies Demonstrate the Importance of RAAS Inhibitor Therapy
Large outcomes studies have demonstrated that RAAS inhibitors significantly improve the health of patients. RAAS inhibitors reduce mortality and hospitalization rates in HF patients by improving heart function. The inhibitors also reduce all-cause mortality by minimizing damage to secondary organs such as the kidneys and delaying the progression of type 2 diabetes. A prominent study, the Randomized Aldactone Evaluation Study, or RALES, in HF patients showed a significant reduction in death and hospitalization with the aldosterone inhibitor spironolactone and demonstrated that dose optimization plays a significant role in the efficacy of these drugs. Patients receiving spironolactone had a much higher probability of survival, especially at later time points after treatment began. Overall the risk of death was 30% lower in the spironolactone group compared to the placebo group.
Both the rate of admission for hyperkalemia and the rate of in-hospital death attributed to hyperkalemia increased following the publication of RALES. The rate of hospital admission due to hyperkalemia increased dramatically from a rate of 2.4 per 1000 patients prior to RALES to approximately 11 per 1000 in the two years following RALES publication (p<0.001). Furthermore, the rate of in-hospital death from hyperkalemia increased from approximately 0.3 per 1000 patients to 2 per 1000 patients after RALES (p<0.001). These data are not surprising given the effect of RAAS inhibitors, such as spironolactone, on potassium retention and the association of hyperkalemia with mortality.
The Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study, or EPHESUS, a 6,632 patient randomized, placebo-controlled study published in 2003 described the benefit of eplerenone, an aldosterone blocker, on mortality and morbidity in patients with HF who had a previous myocardial infarction. Another study, the Reduction of Endpoints in Non-Insulin-Dependent Diabetes Mellitus (or adult-onset or type 2 diabetes) with the Angiotension II Antagonist Losartan, which is referred to as the RENAAL study, demonstrated that the use of the angiotensin receptor blocker losartan resulted in a 28% reduction in progression to end-stage renal disease, corresponding to a two year delay in reaching end-stage renal disease and reduced hospitalization in type 2 diabetics. This trial specifically highlights the benefits of RAAS inhibition on non-heart conditions.
As a result of these compelling outcomes studies, high dose RAAS therapy is guideline recommended by the American Heart Association, the Kidney Disease Outcomes Quality Initiative, American Association of Family Physicians, and the American Diabetes Association, making it the third most commonly prescribed class
7
Table of Contents
of medication in the United States. The guidelines recommend monitoring serum potassium levels while utilizing RAAS therapy and reducing use of RAAS therapy if serum potassium levels near or exceed 5 mEq/L.
Hyperkalemia Predicts Mortality for Patients on RAAS Inhibitors
A 2012 study published in the American Journal of Cardiology in 2012 found that hyperkalemia is the top predictor of all-cause mortality in cardiovascular patients with CKD on anti-hypertensive drugs. Historically, hyperkalemia has been seen as secondary to more serious conditions such as CKD or HF. However, as illustrated in the graph below, hyperkalemia was found to be more likely than factors such as age, hospitalization or CKD stage to predict mortality in cardiovascular disease patients with CKD receiving antihypertensive drugs, indicating that hyperkalemia is an independent predictor of mortality separate from other conditions such as CKD or HF. In the graph below, an odds ratio in excess of 1 illustrates a positive correlation between the underlying risk factor and the resulting co-morbidity, or mortality in this case. Generally, a higher odds ratio signifies an increased risk of mortality due to the corresponding underlying risk.
Predictors of All-cause Mortality in Cardiovascular Disease Patients with CKD Receiving Antihypertensive Drugs
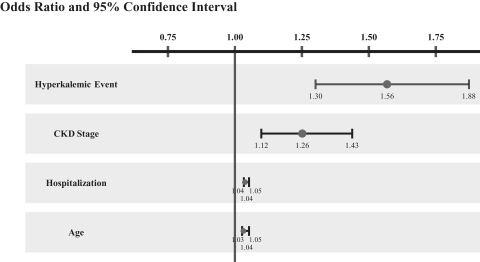
Source: Jain et al. American Journal Cardiology. 2012 May 15;109(10):1510-3
Full Benefits of RAAS Inhibitors are Not Realized Due to Hyperkalemia
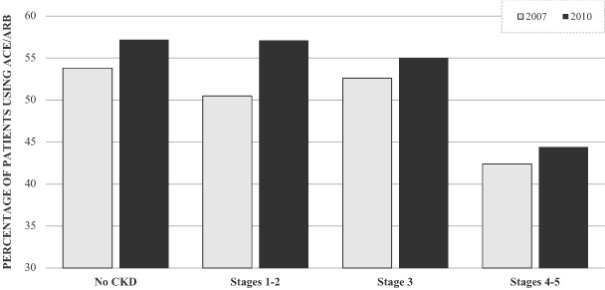
Due to the risk of hyperkalemia, a large percentage of patients are either not treated with RAAS inhibitor therapy, or are undertreated, despite clear guideline recommendations and proven patient benefit. According to articles in the American Heart Journal and the European Heart Journal, over 50% of HF patients are on suboptimal RAAS inhibitor doses and up to 15% of HF patients are not on RAAS inhibitor treatment at all. In CKD patients with type 2 diabetes, over 70% are not treated or are being treated below the recommended dose. The 2012 U.S. Renal Data Survey shows that RAAS inhibitor usage in the Medicare population declines as CKD stage progresses and suggests this decline is likely due to the impact of hyperkalemia. The graph below shows the percentage of patients on ACE inhibitors or ARBs for 2007 and 2010. Although the percentage of patients using ACE inhibitors or ARBs, a RAAS inhibitor, increased from 2007 to 2010, there was an approximate 20% decline in the patients with stage 4 or 5 CKD who were taking ACE inhibitors or ARBs as compared to patients in earlier stages of the disease. We believe a well-tolerated and effective potassium-reducing agent would allow patients to avoid the hyperkalemia associated with RAAS therapy while receiving the full benefit of these medications, including reduced mortality and slowed progression to end stage renal disease.
8
Table of Contents
ACE and ARB Inhibitor Utilization in Patients with Advancing Cardiovascular Disease
Current Treatments for Hyperkalemia
In the hospital setting, hyperkalemia is currently treated with drugs that support heart function and temporarily reduce serum potassium by shifting potassium into the cells. However, no treatment is currently available to effectively and safely remove excess potassium and maintain normal levels of serum potassium. SPS (e.g., Kayexalate), which was approved in 1958 as a DESI drug, is the current standard of care for potassium reduction, but its safety and efficacy have never been proven in randomized, controlled trials. SPS is poorly tolerated by most patients and causes a high incidence of GI side effects, including nausea, vomiting, constipation, intestinal necrosis and diarrhea, which leads to poor compliance and renders the drug unsuitable for chronic use. In addition, in 2009, the FDA issued a warning for colonic necrosis that sparked a debate in the medical community on whether SPS should ever be used. Low potassium diets are widely prescribed, but enforcing compliance is difficult and, as the underlying disease biology progresses, dietary restrictions become ineffective. For patients on RAAS inhibitors, limiting or removing RAAS medications decreases potassium, but puts patients at a higher risk of mortality and/or more rapid disease progression due to uncontrolled hypertension. Given the widely recognized limitations of existing therapies, we believe that there is a significant unmet need and market for a well-tolerated, effective treatment for hyperkalemia.
Key Properties of ZS-9
ZS-9 is a highly stable, non-absorbed zirconium silicate with a defined three-dimensional crystalline lattice that was designed and engineered to preferentially trap potassium ions throughout the GI tract. We are developing ZS-9 to be administered orally at doses of 10g given three times per day to treat acute hyperkalemia and at doses of 5g to 15g given once per day to prevent the reoccurrence of hyperkalemia. The compound is made via a two-step, proprietary manufacturing process using readily available starting materials. ZS-9 is formulated as a room temperature, stable, dry, odorless, tasteless powder that is filled into packets or bottles or pressed into a dissolvable tablet and is easily suspendable in small amounts of water (40-120 mL). Another formulation of ZS-9 that we are developing is an enema for pediatric and hospital use. In our clinical studies to date, we have observed that ZS-9 acts as soon as one hour after administration of a 10g dose and that once daily administration maintains normal potassium levels for 28 days in our ZS004 Phase III study and over 10 months in our long-term safety studies.
9
Table of Contents
ZS-9 Crystal Structure is Specific for Potassium
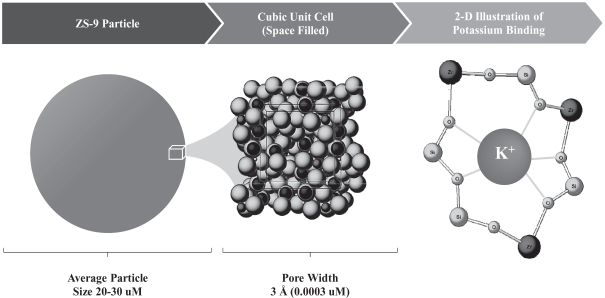
We have designed ZS-9 to efficiently bind and remove potassium from the body thereby reducing serum potassium levels and treating hyperkalemia. ZS-9 is a microporous zirconium silicate compound comprised of zirconium, silicon and oxygen atoms covalently bound to one another and arranged to form ion-binding pores that exchange hydrogen and sodium for potassium. The figure below shows an atomic depiction of the ion binding pores as well as the ionic diameter of some common ions, with each image in the figure depicting a reduction in scale.
Cubic Lattice Structure is Comprised of Pores
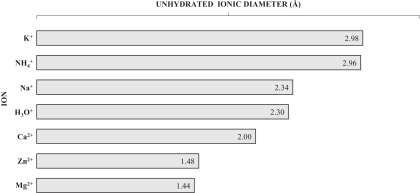
The oxygen atoms in the crystal structure are arranged in a way that disfavors the binding of smaller and larger ions such as magnesium, calcium and sodium, and thus the compound can preferentially trap potassium even in the presence of other positive ions. In contrast, organic polymers like SPS are non-selective and bind potassium only in areas of high potassium concentration like the colon, thus the onset of activity is dependent on gastrointestinal transit time and begins hours to days after administration. This inherent difference in selectivity was demonstrated in-vitro by comparing the binding of ZS-9 and SPS in the presence of potassium (K+), calcium (Ca2+) and magnesium (Mg2+) ions at a 1:1:1 ratio. Whereas SPS shows a preference for Ca2+ over K+ or Mg2+, ZS-9 is nearly 100% selective for K+ (depicted in the figure below). Based on these in vitro studies, ZS-9 has approximately ten times the total potassium binding capacity of SPS.
10
Table of Contents
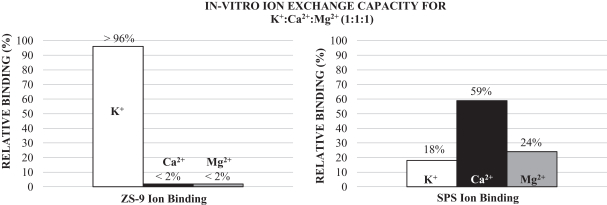
We believe ZS-9’s high selectivity for potassium enables ZS-9 to absorb potassium immediately on ingestion and throughout the GI tract, even in locations where potassium is not the most abundant ion. In our clinical studies to date this selectivity has resulted in the following important differentiating characteristics for ZS-9: high efficacy in patients treated in our studies to date at modest dose amounts; a rapid onset of action as soon as one hour after a 10g dose by acting early in the GI tract where potassium is not the most abundant ion; and well tolerated with no material effect on other important electrolytes that are critical for normal human function.
Absorption, Distribution, Metabolism and Excretion, or ADME, and Physical Properties of ZS-9
ZS-9 is a non-absorbed, odorless, tasteless substance that has been formulated as a powder and tablet. ZS-9 is insoluble in typical solvents and passes through the GI tract without degradation. The compound does not swell in the presence of water, enabling it to be effectively eliminated from the body without the need for a laxative to avoid constipation caused by organic polymers such as SPS. ZS-9 is thermally stable and does not require refrigeration or any special handling.
To date, we have not observed evidence of ZS-9 absorption in animals or humans. ZS-9 is substantially in a spherical form, having an average diameter of approximately 20 to 30 micrometers, which is too large to be absorbed into the body. We conducted in-vivo recovery studies in rats which recovered greater than 98% of the dosage of ZS-9 administered, similar to the level of recovery of the control compound. In addition, high sensitivity analysis of samples collected from 6 month rat, 9 month dog, and 28 day human studies have shown no sign of ZS-9 absorption with a limit of detection of 10 parts per 1,000,000,000. We believe these studies provide evidence that ZS-9 is excreted in feces and is not systemically absorbed. In addition, we believe ZS-9 is essentially insoluble under physiologic conditions. Exposure of the powder form of ZS-9 to simulated gastric and intestinal fluids for an extended period of time resulted in negligible increases in the levels of zirconium, silicon and oxygen in solution. These experiments suggest that the amount of zirconium available for absorption from a 10g dose of ZS-9 would be 0.00028 mg. Compared to the average range of between 1.0 and 9.0 mg of zirconium found in the average daily human diet, ZS-9 is not expected to be a significant source of dietary zirconium intake.
In addition, zirconium has a long safety history in biomedical applications including hip and knee implants and nephrology applications such as hemofiltration, hemodialysis, peritoneal dialysis, and in the design and construction of wearable artificial kidneys. Notably zirconium containing sorbent columns have been used in over 6,000,000 dialysis sessions since the 1970’s without safety issues and zirconium containing sorbent columns are currently being developed by Fresenius, a leading dialysis provider. In sorbent hemodialysis sessions, patients with CKD with little or no kidney function have their blood exposed directly to zirconium containing columns. The amount of zirconium directly released into the blood of patients during each session has been quantified to be 0.758 mg, or almost 3,000 times that which could be absorbed from a 10g dose of ZS-9. Zirconium is commonly found in quantities much higher than the theoretical amount of zirconium released from
11
Table of Contents
a 10g dose of ZS-9 as shown in the table below. Given experience with zirconium compounds in biomedical applications for over 40 years and the non-absorbed nature of ZS-9, and other zirconium compounds, we expect ZS-9 will be well tolerated and safe in man.
Relative Zirconium Levels
| Product |
Amount | Relative to ZS-9 | ||||||
| 85g Antiperspirant Stick |
2,295 mg | 8,196,429x | ||||||
| Soil |
300 mg/L | 1,071,429x | ||||||
| Human Body Content |
300 mg | 1,071,429x | ||||||
| Daily Food Content |
3.65 mg | 13,036x | ||||||
| Zirconium from 4 Hour Sorbent Hemodialysis |
0.758 mg | 2,707x | ||||||
| Daily Drinking Water Content |
0.65 mg | 2,321x | ||||||
| Sea Water |
0.004 mg/L | 14x | ||||||
| Soluble Zirconium from 10g of ZS-9* |
0.00028 mg | 1.0x | ||||||
| * | Amount of zirconium in solution after exposing to simulated gastric and simulated intestinal fluids for a period of 24 hours (calculations assume all of the soluble Zr from ZS-9 is absorbed). |
ZS-9 Clinical Development Program and Regulatory Pathway
Overview
We are advancing ZS-9 through a clinical development program with the goal of obtaining approval for the treatment of acute and chronic hyperkalemia, regardless of the underlying cause. We submitted an Investigational New Drug, or IND, application for ZS-9 as a treatment for hyperkalemia with, and such application was acknowledged by, the FDA in December 2010. As of March 1, 2015, our clinical program has enrolled 1,474 patients, which is representative of the hyperkalemia population, including patients with CKD, HF, type 2 diabetes and those on RAAS inhibitor therapy. We have completed ZS002, ZS003 and ZS004, which resulted in positive Phase II and Phase III data. We initiated an 11 month extension to the ZS004 study, ZS004e, and a 12 month long-term safety study, ZS005, in the second quarter of 2014. We received guidance from the FDA on the non clinical requirements and clinical program for the development of ZS-9, including a recent pre NDA meeting, and we believe that we have incorporated this guidance into our development program. As is typically the case, the FDA may provide additional guidance or change its guidance as our drug candidates move through development, clinical trials and the regulatory approval process. We plan to submit our NDA in the second quarter of 2015 and MAA in the second half of 2015.
To date, daily administration of ZS-9 has been observed to lower, and maintain control of, serum potassium levels while maintaining a favorable tolerability profile. The acute portion of our most recent pivotal Phase III trial, ZS004, showed clinically meaningful reductions in serum potassium that were statistically significant, with a median time to normalization of 2.2 hours, and 84% and 98% of subjects becoming normokalemic after receiving 10g of ZS-9 three times a day for 24 and 48 hours, respectively. In addition, the extension portion of this trial demonstrated that the reductions achieved in the acute phase could be maintained in 80%, 90%, and 94% of patients using 5g, 10g, or 15g of ZS-9 administered once daily, respectively. These results were statistically significant when compared to patients randomized to placebo who had their potassium level revert to near pretreatment levels. These positive results are supported by results from our Phase II study, ZS002, and our first Phase III study, ZS003, which also showed statistically significant reductions in serum potassium levels, with 99% of patients becoming normokalemic after receiving 10g of ZS-9 given three times a day for 48 hours, and sustained potassium reductions in patients using 5g and 10g of ZS-9 administered once daily.
To date, ZS-9 was observed to have a favorable safety profile and was well-tolerated across trial populations, including CKD, HF and type 2 diabetes subjects and patients that are taking RAAS therapy. In the
12
Table of Contents
completed trials, the most common adverse events were mild-to-moderate GI symptoms, including nausea, vomiting, constipation and diarrhea. These events occurred at a similar rate and severity as placebo treated patients. No serious adverse events were deemed attributable to ZS-9. In contrast, the product label of SPS, the only approved potassium-binding treatment, includes warnings of severe GI side effects which can be fatal, including GI necrosis, bleeding and perforation.
We expect that the NDA and MAA will include the results from the Phase II trial (ZS002), the Phase III trial (ZS003) and the Phase III one month randomized withdrawal study, ZS004. We expect to include safety data from the long-term studies ZS004e and ZS005 as part of our submission package and provide updates at regular intervals during FDA review. The ZS002 study and both Phase III studies were published in peer reviewed journals, the ZS002 study was published in Kidney International, the ZS003 study was published in the New England Journal of Medicine (NEJM) and the ZS004 study was published in the Journal of the American Medical Association (JAMA). If all of the planned studies are completed (ZS002, ZS003, ZS004 and ZS005), approximately 1,500 patients will have been exposed to ZS-9. We discussed the design of these studies and the adequacy of our proposed clinical package with the FDA in our recent pre NDA meeting.
Clinical Program
The table below summarizes our clinical development program for ZS-9, which began with ZS002, a dose escalating, double-blind, randomized, placebo-controlled Phase II study in hyperkalemic patients with CKD, including those on RAAS inhibitor therapy, in November 2011. Following the positive results of this trial, we conducted ZS003, a double-blind, randomized, placebo-controlled Phase III study in a representative population of hyperkalemic patients including those with CKD, HF, type 2 diabetes and those on RAAS therapy. The goal of this study was to confirm the acute findings observed in our ZS002 Phase II study and explore ZS-9’s ability to maintain normal levels of serum potassium for an extended period regardless of etiology. Following the positive results of this trial, we conducted ZS004, a double-blind, randomized, placebo-controlled Phase III study in patients with higher baseline potassium levels, including those with CKD, HF, type 2 diabetes and those on RAAS therapy. The goal of this study was to establish the dose for once daily maintenance treatment and explore ZS-9’s ability to maintain normal levels of serum potassium in patients with higher baseline potassium levels over a longer time period. We recently reported clinically meaningful and statistically significant results from ZS004 and have initiated two additional studies to explore long-term treatment with ZS-9. The first study, ZS004e, is an 11 month open label extension study for subjects completing ZS004 and the second study, ZS005, is a 12 month exposure study designed to establish long-term safety and efficacy.
| Trial (Phase) |
Trial Design |
Patient Population |
Treatment Duration |
Objective |
Key Results | |||||
| ZS002 (II) |
Double-blind RCT | N=90 5.0 to 6.0 mEq/L |
48 hours | Proof of concept for ZS-9 rapidly lowering K+ levels |
Met primary endpoint for the 3g and 10g doses | |||||
| ZS003 (III) |
Double-blind RCT | N=753 Hyperkalemia, regardless of etiology, 5.0 to 6.5 mEq/L |
14 days | Confirm dose for rapid K+ control and proof of concept for maintenance dosing |
Met primary endpoint for the 2.5g, 5g and 10g doses, and met secondary endpoint for 5g and 10g doses in the maintenance phase | |||||
13
Table of Contents
| Trial (Phase) |
Trial Design |
Patient Population |
Treatment Duration |
Objective |
Key Results | |||||
| ZS004 (III) |
Double-blind RCT | N=258 Hyperkalemia, regardless of etiology, >5.0 mEq/L |
1 month | Establish a maintenance dose |
Met primary endpoint at all doses with 80%, 90%, and 94% normokalemic at 5g, 10g and 15g QD doses, respectively | |||||
| ZS004/e (III) |
Double-blind RCT | N=258 Hyperkalemia, regardless of etiology, >5.0 mEq/L |
11 months | Establish a maintenance dose |
Ongoing; initiated March 2014 | |||||
| ZS005 (III) |
Open label safety | N=750 Hyperkalemia, regardless of etiology, >5.0 mEq/L |
Up to 12 months |
Establish long-term safety and efficacy |
Ongoing; initiated second quarter of 2014 | |||||
Safety and tolerability data from the completed trials (2 days, 14 days, and 28 days of treatment) suggests that ZS-9 is safe and well-tolerated. Adverse events potentially related to ZS-9 have primarily been mild-to-moderate transient GI symptoms in approximately 5% of subjects, a rate comparable to placebo. In one study, the ZS004 study, mild-to-moderate cases of edema were observed in approximately 2% to 14% of subjects; however rates of edema from our ongoing 12 month long-term studies are lower and comparable to placebo rates observed in previous studies. Only one serious adverse event was assessed as being possibly related to study drug (a case of gastroenteritis in ZS003), but when the study was unblinded, the patient who experienced the serious adverse event was found to have received placebo. All other serious adverse events reported to date have been assessed as unrelated to ZS-9.
Phase II Study
ZS002 First-In-Man Proof-of-Concept Trial (Completed)
In this first trial, we conducted a double-blind, randomized, placebo-controlled study that tested three doses of ZS-9 in 90 patients with CKD (as measured by estimated glomerular filtration rate (a measurement of overall renal function), or eGFR, of 30-60 mL/min, which corresponds to Stage 3 CKD) and with mild to moderate hyperkalemia (5.0-6.0 mEq/L). For patient safety reasons, patients with serum potassium levels over 6.0 mEq/L were not allowed to participate in the study due to the potential that such patients would receive placebo. The study was designed to explore ZS-9’s ability to rapidly treat hyperkalemia. Patients received 0.3, 3 or 10g of ZS-9 or placebo three times daily with meals for 48 hours. Potassium levels were measured at multiple time points during the 48 hour treatment phase as well as once daily during the five day follow up period. The primary endpoint of the study was the rate of change in serum potassium over 48 hours. The study design is illustrated in the figure below.
14
Table of Contents
Study ZS002: Trial Design
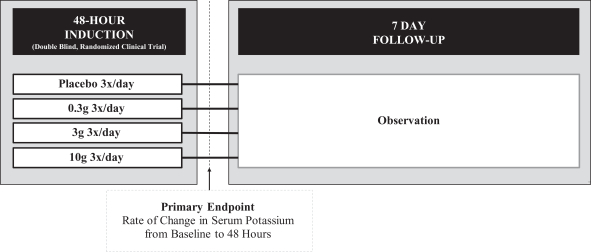
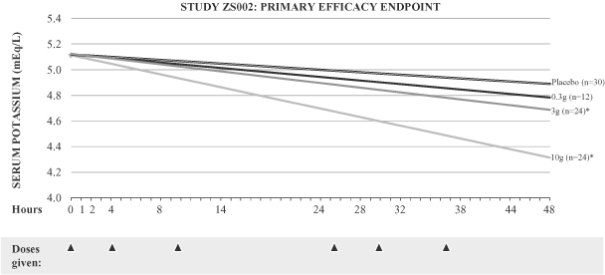
As shown below, we observed a dose dependent relationship between ZS-9 and serum potassium levels, with the study meeting the primary endpoint for the 3g (p=0.048) and 10g (p<0.0001) doses of ZS-9. Of particular note, the 10g dose produced a significant decrease in serum potassium, reducing levels by a mean of 0.92 mEq/L in only 38 hours. The onset of action was rapid, and patients receiving the 10g dose achieved statistically significant efficacy one hour after the first dose of ZS-9.
Study ZS002: Results
| Placebo | 0.3g 3x/day | 3g 3x/day | 10g 3x/day | |||||
| Primary Endpoint (p-value) |
N/A | 0.4203 | 0.0480 | <0.0001 | ||||
| Mean K+ Change at 48h mEq/L (14h post last dose) |
-0.20 | -0.30 | -0.35 | -0.68 | ||||
| Max K+ Change at 38h mEq/L (4h post last dose) |
-0.26 | -0.39 | -0.42 | -0.92 |
15
Table of Contents
Study ZS002: ZS-9 Demonstrates Dose Dependent Efficacy Over 48 Hours
| * | p-value <0.05 |
Statistically significant results at various levels are denoted by p-values in the figures above and below. The p-value is the probability that the reported result was achieved purely by chance (e.g., a p-value of less than or equal to 0.01 means that there is a 1% chance that the difference between the placebo group and the treatment group is purely due to chance). A p-value of less than or equal to 0.05 is a commonly used criterion for statistical significance. The symbol “n” is used to denote sample size per group.
Within 38 hours, 100% of patients receiving the 10g dose had serum potassium levels less than 5.0 mEq/L and approximately 88% had serum potassium levels less than or equal to 4.5 mEq/L. The percentage of patients with serum potassium levels less than or equal to 4.5 mEq/L during the 48 hour treatment period is displayed in the graph below. Furthermore, patients on RAAS inhibitor therapy showed similar responses to those not on RAAS inhibitor therapy. The data suggests that ZS-9 can treat hyperkalemia caused by RAAS inhibitor therapy.
16
Table of Contents
Study ZS002: Percentage of Patients Below 4.5 mEq/L
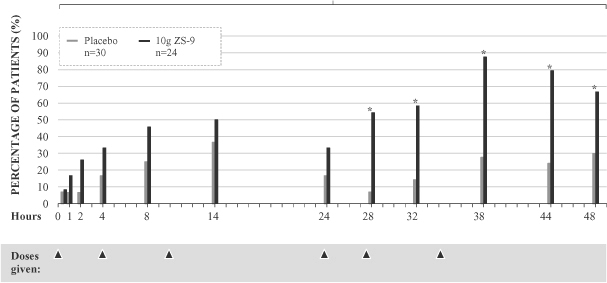
| * | p-value <0.05 |
In this study, potassium levels rose in patients after ZS-9 therapy was halted, reverting to near baseline levels after only a few days without therapy. This rapid rebound shows that patients with a hyperkalemic episode will rapidly return to an elevated risk state if therapy is not maintained.
The adverse event profile of ZS-9 was favorable. There were no discontinuations and no treatment related serious adverse events. Transient minimal to mild GI adverse events, including nausea, vomiting, constipation and diarrhea, were the most frequent adverse events reported. Consistent with ZS-9’s mechanism of action, the amount of potassium excreted in 24 hour urine collections decreased in ZS-9 treated subjects relative to placebo treated subjects. Importantly, urinary sodium excretion was similar in ZS-9 and placebo treated subjects and no clinically significant changes in non-potassium serum electrolytes or cases of significant hypokalemia were observed.
17
Table of Contents
Phase III Studies
ZS003 (Complete)
ZS003 was a two part double-blind, randomized, placebo-controlled, pivotal Phase III clinical trial that enrolled 753 patients with hyperkalemia (indicated by potassium levels between 5.0 and 6.5 mEq/L), and included patients with CKD, HF, type 2 diabetes and those on RAAS inhibitor therapy. Results of the study were initially presented at a symposium at the American Society of Nephrology and were later published in the New England Journal of Medicine. The study was designed to confirm ZS-9’s ability to rapidly treat hyperkalemia in the induction phase and to maintain normokalemia in the maintenance phase. Patients were randomized to receive one of four doses of ZS-9 (1.25g, 2.5g, 5g or 10g) or placebo, administered three times daily for the initial 48 hours, or the induction phase. The primary endpoint was the rate of change in serum potassium from baseline throughout the 48 hour induction phase, which was the same primary endpoint used in the ZS002 study. Patients that normalized at a potassium level between 3.5 and 5.0 mEq/L in the induction phase were then randomized to active drug (1.25g, 2.5g, 5g or 10g) or placebo administered once-daily for 12 days during the maintenance phase. The endpoint for the randomized withdrawal maintenance phase was the rate of change in serum potassium during the 12-day dosing period. The study design is illustrated in the figure below.
Study ZS003: Trial Design
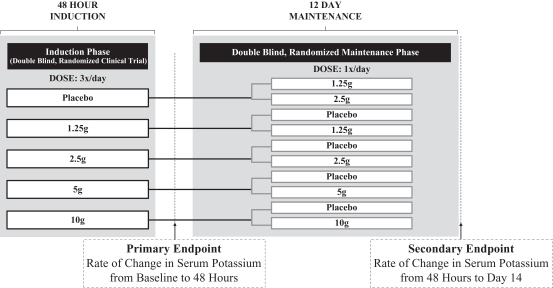
ZS003 Phase III Demographics
Demographics of the patient population enrolled in the pivotal Phase III trial were substantially the same as that studied in the Phase II trial and the patient population being enrolled in our ongoing studies. We have tested a representative population of patients with hyperkalemia, representative of the patient population we are seeking to treat. Patients were eligible for enrollment if they had elevated potassium levels, defined as serum potassium greater than 5.0 mEq/L. Although not a criteria for entry, most patients had one of the following conditions which predisposed them to hyperkalemia: CKD, HF, type 2 diabetes and/or were taking RAAS inhibitor therapy. The pivotal Phase III trial was conducted with clinical sites in the United States, Australia and South Africa. Enrollment was completed within 10 months, suggesting the high prevalence of hyperkalemia, and approximately 94% of patients were from the United States.
18
Table of Contents
Study ZS003: Patient Demographics
| Placebo (n=158) |
1.25g (n=154) |
2.5g (n=141) |
5g (n=157) |
10g (n=143) |
||||||||||||||||
| Age (years) at Screening |
65.6 | 65.4 | 65.9 | 65.2 | 66.2 | |||||||||||||||
| Male (%) |
62.0 | 53.9 | 64.5 | 61.1 | 55.9 | |||||||||||||||
| Female (%) |
38.0 | 46.1 | 35.5 | 38.9 | 44.1 | |||||||||||||||
| White (%) |
86.1 | 85.1 | 88.7 | 84.1 | 83.9 | |||||||||||||||
| Black or African American (%) |
10.8 | 13.0 | 7.8 | 12.7 | 13.3 | |||||||||||||||
| Asian (%) |
1.3 | 0.0 | 3.5 | 1.9 | 1.4 | |||||||||||||||
| American Indian or Alaska Native (%) |
1.3 | 0.0 | 0.0 | 0.6 | 0.7 | |||||||||||||||
| Native Hawaiian or other Pacific Islander (%) |
0.6 | 1.9 | 0.0 | 0.0 | 0.7 | |||||||||||||||
| Other (%) |
0.0 | 0.0 | 0.7 | 0.6 | 0.0 | |||||||||||||||
| Multiple Races Checked (%) |
0.0 | 0.0 | 0.7 | 0.0 | 0.0 | |||||||||||||||
| Weight (kg) at Baseline |
87.6 | 87.6 | 83.1 | 89.5 | 87.0 | |||||||||||||||
ZS003 Phase III Induction Phase Results
The trial met the primary efficacy endpoint for the induction phase at the 2.5g, 5g and 10g doses compared to placebo. ZS-9 showed significant, rapid and dose-dependent reductions in serum potassium at the 2.5g, 5g and 10g doses within hours of administration of the first dose. At the highest dose tested, 10g, mean serum potassium reduction was -0.73 mEq/L and 99% of patients had normal potassium values within 48 hours. Similar to the results seen in our Phase II study, ZS002, statistically significant reductions in potassium were observed one hour after the first 10g dose of ZS-9.
Study ZS003: Primary Endpoint Rate of
Change of Serum Potassium Over 48 Hours
| 1.25g | ||||||||||||||||||||
| p-Value of Primary Endpoint |
N/A | 0.5 | 0.0009 | <0.0001 | <0.0001 | |||||||||||||||
| Mean K+ Change at 48 hours (14 hours post last dose) (mEq/L) |
-0.25 | -0.30 | -0.46 | -0.54 | -0.73 | |||||||||||||||
Study ZS003: Primary Efficacy Endpoint
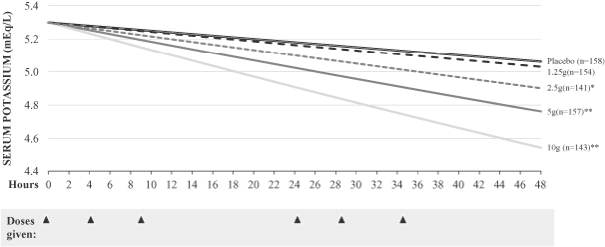
| * | p-value <0.05 |
| ** | p-value <0.0001 |
19
Table of Contents
ZS-9 was equally efficacious across patient subsets. Patients with CKD, HF, type 2 diabetes and those taking RAAS inhibitor therapy all received the same magnitude of potassium lowering as the entire intent to treat, or ITT, population. In contrast, subjects with higher starting potassium levels had a higher response to ZS-9. Patients with pretreatment potassium levels in excess of 5.5 mEq/L (average baseline 5.84 mEq/L) saw an average decrease of 1.1 mEq/L at 48 hours while those with starting potassium levels at or below 5.3 mEq/L had an average decrease of 0.6 mEq/L at the highest dose. We believe that ZS-9 works in conjunction with normal potassium excretion mechanisms to remove potassium when it is above 5.0 mEq/L, but as serum potassium drops below 5.0 mEq/L, the body lowers physiologic excretion to avoid hypokalemia, defined as serum potassium level below 3.5 mEq/L. This may explain why hypokalemia has been rare within the dose range administered despite ZS-9’s rapid and potent ability to remove potassium. In study ZS003, hypokalemia occurred in only two patients (0.3% of all enrolled patients) in the induction and maintenance phases. Even in those two cases, the hypokalemia was mild (3.1 and 3.4 mEq/L, respectively), transient and did not require any treatment.
Study ZS003: Reduction in Serum Potassium Levels Across Patient Subgroups
| Placebo | 1.25g | 2.5g | 5.0g | 10.0g | ||||||||||||||||
| Overall ITT Populations |
-0.25 | -0.30 | -0.46 | -0.54 | -0.73 | |||||||||||||||
| Disease Subsets (n; %) |
||||||||||||||||||||
| CKD (463; 61.5%) |
-0.22 | -0.31 | -0.43 | -0.58 | -0.83 | |||||||||||||||
| Diabetes (451; 60%) |
-0.25 | -0.25 | -0.47 | -0.52 | -0.74 | |||||||||||||||
| HF (300; 39.8%) |
-0.24 | -0.27 | -0.46 | -0.52 | -0.78 | |||||||||||||||
| RAAS inhibitors (491; 65.2%) |
-0.24 | -0.28 | -0.48 | -0.53 | -0.73 | |||||||||||||||
| Starting Serum K+ (or S-K) |
||||||||||||||||||||
| Baseline S-K < 5.3 (n=427) |
-0.15 | -0.23 | -0.39 | -0.39 | -0.57 | |||||||||||||||
| Baseline S-K 5.4-5.5 (n=152) |
-0.37 | -0.37 | -0.49 | -0.65 | -0.99 | |||||||||||||||
| Baseline S-K > 5.5 (n=174) |
-0.42 | -0.34 | -0.55 | -0.87 | -1.10 | |||||||||||||||
| eGFR (mL/min/1.73m2) eGFR <15 (n=56) |
-0.17 | -0.15 | -0.49 | -0.70 | -1.03 | |||||||||||||||
| eGFR 15-29 (n=209) |
-0.25 | -0.27 | -0.41 | -0.63 | -0.87 | |||||||||||||||
| eGFR 30-60 (n=296) |
-0.22 | -0.30 | -0.43 | -0.54 | -0.79 | |||||||||||||||
| eGFR >60 (n=183) |
-0.34 | -0.40 | -0.53 | -0.44 | -0.47 | |||||||||||||||
ZS003 Phase III Extended Phase Results
The maintenance portion of our Phase III study was a randomized withdrawal design. Patients that became normokalemic after receiving ZS-9 during the induction phase were re-randomized to receive once daily placebo or once daily ZS-9 at the same dose level as they had received three times daily during the induction phase. Thus, patients who had received 10g three times per day in the induction phase were randomized to receive 10g of ZS-9 once per day or 10g of placebo once per day. Results from the maintenance portion of the Phase III study were released in January 2014. The results from this portion of the study met the predefined efficacy endpoints at the 5g and 10g doses when compared with their respective placebo groups (p=0.0075 and <0.0001). Patients who received 5g of ZS-9 once daily during the maintenance portion had an increase of 0.11 mEq/L compared to a placebo increase of serum potassium levels of 0.25 mEq/L. Patients who received 10g of ZS-9 once daily during the maintenance portion had an increase in average potassium levels of 0.06 mEq/L compared to an average increase in placebo patients of 0.58 mEq/L. At the end of the maintenance period, when ZS-9 was no longer administered, average potassium levels increased to near baseline levels, similar to the levels in those patients that received placebo during the maintenance portion.
20
Table of Contents
Normokalemia Maintained in Extended Phase at 5g and 10g Once Daily
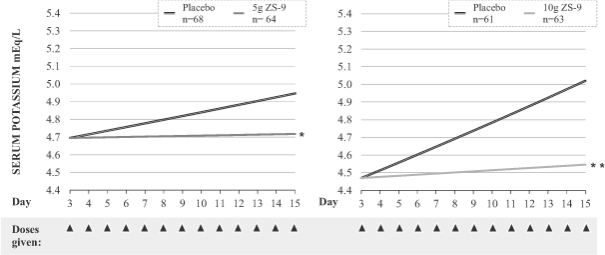
| * | p <0.05 |
| ** | p <0.0001 |
Study ZS003: 10g ZS-9 Maintains Potassium Levels
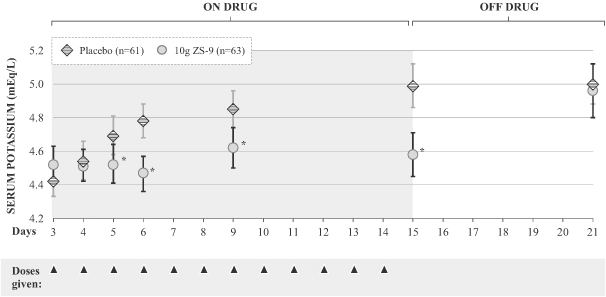
| * | p-value <0.05 |
Bars indicate 95% confidence level
ZS003 Phase III Adverse Events
In study ZS003, our largest clinical study, the incidence of adverse events in patients taking ZS-9 was similar to placebo. Given the high rate of GI adverse events seen with SPS, we believe the low incidence of reported GI adverse events in the study is of particular note. For example, the highest dose tested during the dose intensive induction phase, 10g of ZS-9 given three times per day (30g per day), displayed a GI event rate of 3.5%
21
Table of Contents
relative to a placebo rate of 5%. Urinary tract infections, or UTIs, were observed more frequently in ZS-9 treated patients than those receiving placebo, however the UTIs did not appear to be dose related, the overall incidence was consistent with what would be expected in a patient population with significant co-morbidities such as CKD, type 2 diabetes, and HF, and more ZS-9 treated patients had urinary tract infections at the initiation of the study than the patients receiving placebo which may explain this finding. In our most recent completed study, ZS004, UTIs occurred at the same rate as placebo.
Study ZS003: Percentage (Number) of Patients with Treatment Emergent Adverse Events
| Induction Phase |
Maintenance Phase |
|||||||||||||||
| Placebo (n=158) |
ZS-9 (n=595) |
Placebo (n=216) |
ZS-9 (n=327) |
|||||||||||||
| All Adverse Events |
10.8 | % | 12.9 | % | 24.5 | % | 25.1 | % | ||||||||
| (17 | ) | (77 | ) | (53 | ) | (82 | ) | |||||||||
| Gastrointestinal Events |
5.2 | % | 3.5 | % | 3.7 | % | 5.5 | % | ||||||||
| (8 | ) | (21 | ) | (8 | ) | (18 | ) | |||||||||
In general, there were no clinically meaningful treatment-related changes in most laboratory parameters. Hypokalemia (defined in this trial as a serum potassium level less than 3.5 mEq/L) occurred in two subjects out of the 753 subjects in the study (0.3% of all enrolled patients), none of whom had any complications related to hypokalemia. In both cases, hypokalemia was mild (3.4 and 3.1 mEq/L, respectively), transient and did not require any treatment. No statistically significant changes in serum sodium or magnesium levels were observed. There was a small decrease in the average serum calcium levels in the ZS-9 group that was statistically different to the mean change from baseline in the placebo group at the highest doses tested. These changes were deemed not to be clinically significant and all patients remained in the normal range.
Adverse events were generally mild or moderate and transient in nature. There were a total of 16 serious adverse events reported in the study, 15 of which were assessed by the study investigators and by us as not related to ZS-9. Only one serious adverse event, a case of gastroenteritis, was classified as being possibly related to study drug; however, when the data was unblinded, this event occurred in a patient treated with placebo. The remaining 15 non-related serious adverse events were well distributed between ZS-9 and placebo, and there was no dose dependent relationship within the four ZS treatment groups.
ZS004 (Complete)
ZS004, also known as the HARMONIZE study, was a two part double-blind, randomized, placebo controlled, pivotal Phase III withdrawal study designed to confirm the dosing for chronic administration of ZS-9. The results of the trial were presented on November 17, 2014 at a symposium at the American Heart Association meeting and simultaneously published in the Journal of the American Medical Association. The trial consisted of an open label induction phase followed by a month long randomized, double-blind, placebo-controlled withdrawal phase. In the open-label acute phase of the study, 258 patients received 10g of ZS-9 administered three times daily with meals for 48 hours and were monitored to establish the speed and magnitude of serum K+ reduction. Then, patients who achieved normokalemia (K+ levels between 3.5 and 5.0 mEq/L) were randomized in a double-blind fashion to one of three doses of ZS-9 (5g (N=45), 10g (N=51), or 15g (N=56)) or placebo (N=85) administered once-daily for 28 days (the double-blind randomized withdrawal phase). The primary efficacy endpoint compared the mean serum K+ level of each ZS-9 treatment group to that of placebo over the interval between day 8 and day 29 of the double-blind randomized withdrawal phase. Key secondary efficacy endpoints included the proportion of patients with mean K+ <5.1 mEq/L between day 8 and day 29, median time to normalization, change from baseline in serum K+ and the proportion of patients achieving normokalemia. Patients who completed this initial 28-day randomized phase were eligible to enroll in an ongoing 11 month open-label extension study, ZS004e.
22
Table of Contents
Study ZS004: Trial Design

ZS004 Phase III Demographics
Demographics of the patient population enrolled in the pivotal Phase III trial were substantially the same as that studied in the previous Phase II and Phase III trials, however given the efficacy observed in previous studies, patients were permitted entry into the study even with potassium levels in excess of 6.5 mEq/L, the range of baseline potassium levels was 5.1-7.4 mEq/L. We believe the ZS004 trial tested a representative population of hyperkalemic patients, which included those with CKD (eGFR<60mL/min/1.73m2; 69%), HF (NYHA Class I-IV; 36%), type 2 diabetes (66%) and those on RAAS inhibitor therapy (70%) from a range of ethnic backgrounds (white (83%); African American (14%); Asian (2%); other (1%)). The demographic and other baseline characteristics of subjects treated in the Maintenance Phase were generally similar among the Maintenance Phase treatment groups. The pivotal Phase III trial was conducted at 49 clinical sites in the United States, Australia and South Africa. Enrollment was completed within four months, suggesting the high prevalence of hyperkalemia, with approximately 80% of patients from the United States.
23
Table of Contents
Study ZS004 Patient Demographics
Baseline Characteristics
| Open Label |
Randomized Phase |
|||||||||||||||||||
| 10g (n=258) |
Placebo (n=85) |
5g (n=45) |
10g (n=51) |
15g (n=56) |
||||||||||||||||
| Median Age years |
65 | 66 | 64 | 65 | 65 | |||||||||||||||
| Male % |
58 | 52 | 60 | 53 | 71 | |||||||||||||||
| White % |
83 | 86 | 80 | 86 | 82 | |||||||||||||||
| Black % |
14 | 12 | 18 | 10 | 16 | |||||||||||||||
| BL Serum K+ <5.5 mEq/ L % |
46 | 51 | 51 | 37 | 43 | |||||||||||||||
| BL Serum K+ 5.5-<6.0 mEq/ L % |
39 | 35 | 38 | 45 | 46 | |||||||||||||||
| BL Serum K+ ³6.0 mEq/ L % |
15 | 14 | 11 | 18 | 11 | |||||||||||||||
| RAAS inhibitors % |
70 | 72 | 73 | 71 | 59 | |||||||||||||||
| Heart Failure % |
36 | 31 | 40 | 35 | 45 | |||||||||||||||
| Diabetes Mellitus % |
66 | 64 | 58 | 75 | 70 | |||||||||||||||
| Baseline eGFR <60 % |
69 | 61 | 69 | 75 | 73 | |||||||||||||||
| Brain Natriuretic Peptide pg/mL |
126 | 101 | 175 | 101 | 152 | |||||||||||||||
ZS004 Phase III Open Label Induction Phase Results
Similar to the results seen in our previous studies, statistically significant reductions in potassium were observed one hour after the first 10g dose of ZS-9. Median time to normokalemia was 2.2 hours with 84% of patients achieving normokalemia within 24 hours and 98% within 48 hours. Mean potassium decreased from a baseline level of 5.6 mEq/L to 5.4 mEq/L at one hour, 5.2 mEq/L (-0.4 mEq/L) at two hours, 5.1 mEq/L (-0.5 mEq/L) at four hours, and 4.5 mEq/L (-1.1 mEq/L) at 48 hours (p-value <0.05 at all time points). In the induction phase, there were no reports of edema, and adverse events were reported in 7.8% of patients and GI adverse events were reported in only 3.9% of patients. All events were considered mild or moderate in severity and the most common adverse events were diarrhea (1.2%), as well as constipation, dizziness and nausea (all 0.8%).
24
Table of Contents
Study ZS004: Open Label Induction Phase Serum Potassium Over 48 Hours
Results: Open-Label Phase
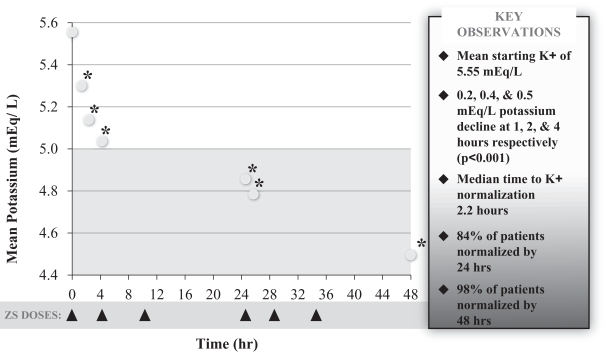
| * | p-value <0.001 |
ZS-9 was equally efficacious across patient subsets. Patients with CKD, HF, type 2 diabetes and those taking RAAS inhibitor therapy all showed the same magnitude of potassium lowering as the entire population. In contrast, responses were larger in patients with higher levels of hyperkalemia; patients with baseline serum K+ <5.5 mEq/L had a change of -0.8 mEq/L at 48 hours, compared to those with baseline serum K+ in the range of 5.5-5.9 mEq/L and those with a baseline >6 mEq/L which had changes of -1.2 and -1.5 mEq/L respectively. Notably, in patients with severe hyperkalemia (K+ ³6.0 mEq/L), ZS-9 produced mean changes of -0.5 mEq/L at one hour and -0.7 mEq/L at two hours suggesting a potential for use in emergency and acute settings.
25
Table of Contents
Study ZS004: Reduction in Serum Potassium Levels Across Patient Subgroups
Open Label Phase: Consistent Results in All Subgroups
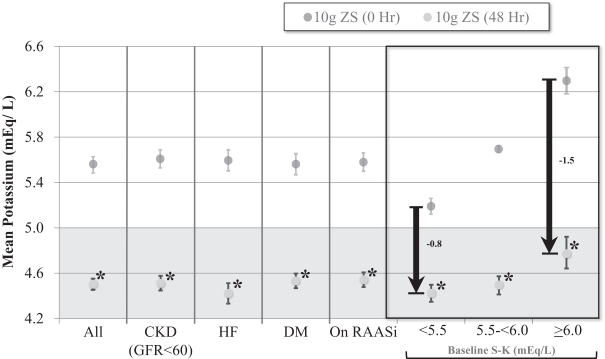
| * | P value<0.0001 |
Error bars represent ±95 confidence intervals
ZS004 Phase III Extended Phase Results
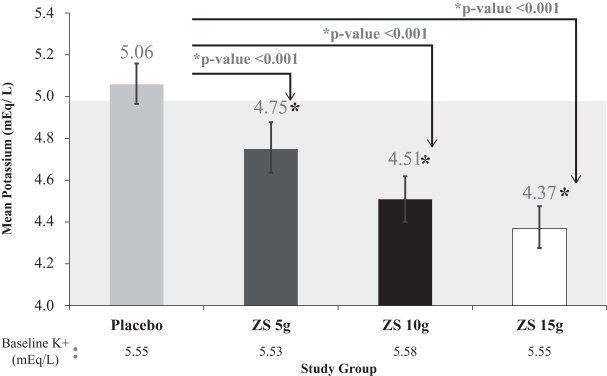
The primary endpoint was assessed in the second phase of the Phase III study, which was a double-blind, placebo controlled, randomized withdrawal design. Patients that became normokalemic after receiving ZS-9 during the induction phase were randomized to receive once daily placebo or 5g, 10g, or 15g of once daily ZS-9. The ZS004 trial met the primary efficacy endpoint by demonstrating that all three doses (5g, 10g, and 15g) of once daily ZS-9 maintained mean potassium at lower levels than placebo over the day 8 to 28-day period (placebo average was 5.1 mEq/L vs. 4.8, 4.5, and 4.4 mEq/L for 5g, 10g, and 15g, respectively, p-value £0.001 for all doses). Significantly higher proportions of patients had mean serum potassium (K+) levels in the normal range (K more than 3.4mEq/L and less than 5.1mEq/L) while on ZS-9 than placebo (80%, 90%, and 94% at the 5g, 10g and 15g dose, respectively, compared with 46% on placebo).
26
Table of Contents
ZS004 Meets Primary Endpoint for all Doses Tested in Extended Phase
Achieves Primary Endpoint, Mean K+ Maintenance on Days 8-29 for All Doses
| * | Error bars represent ±95 confidence intervals |
27
Table of Contents
Study ZS004: Proportion of Patients with Mean Serum in the Normal Range in the Extended Phase
Randomized Phase: Proportion of Patients with Mean K <5.1 mEq/L during Days 8-29
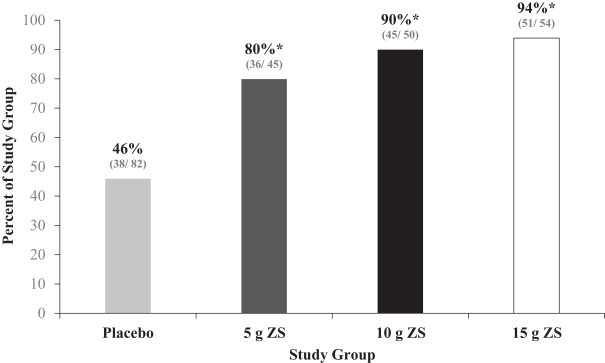
| * | P value<0.001 |
ZS004 Phase III Adverse Events
In the ZS004 study, ZS-9 appeared to be well tolerated. The rate of adverse events and rate of GI adverse events were generally similar across placebo and ZS-9 treated groups with no dose dependent effects on overall rates. There were a total of 10 serious adverse events reported in patients receiving ZS-9 during the study, all of which were assessed by the study investigators and by us as not related to ZS-9. The serious adverse events were distributed across ZS-9 groups, and there was no dose dependent relationship.
Study ZS004: Percentage (Number) of Patients with Treatment Emergent Adverse Events
| Randomized Phase |
Maintenance Phase |
|||||||||||||||
| Placebo (n=85) |
5 g (n=45) |
10 g (n=51) |
15 g (n=56) |
|||||||||||||
| All Adverse Events |
31.8 | % | 53.3 | % | 29.4 | % | 44.6 | % | ||||||||
| (27 | ) | (24 | ) | (15 | ) | (25 | ) | |||||||||
| Gastrointestinal Events |
14.1 | % | 6.7 | % | 2 | % | 8.9 | % | ||||||||
| (12 | ) | (3 | ) | (1 | ) | (5 | ) | |||||||||
The adverse events with the highest rates in ZS004 were anemia, constipation, edema, nasopharyngitis, and upper respiratory tract infections. The incidence of urinary tract infections was low with one case reported in each of the placebo (1.2%), 5g (2.2%), and 15g (1.8%) groups and no reported cases in the 10g group. Constipation was more frequent on placebo, whereas peripheral edema, mostly foot and ankle swelling, was
28
Table of Contents
more frequent on ZS-9. There were six cases of constipation on placebo (7.1%) compared to no cases on 5g, one case on 10g (2.0%), and one case on 15g (1.8%). There were 14 cases of edema: two cases of edema on placebo (2.4%), one case on 5g (2.2%), three cases on 10g (5.9%), and eight cases on 15g (14.3%). Seven cases of edema resolved or did not require treatment during study (one case on 5g, all three cases on 10g, and three cases on 15g). Of the 14 patients with edema, 13 completed the study. Each of the subjects who had edema events were receiving multiple types of medications for treatment of hypertension, of which edema is a common adverse effect. The majority of the subjects also had CKD and most had a medical history of edema. None of the edema events were considered related to ZS-9, and most were mild or moderate in severity. This conclusion is supported by data from the long term ZS004e and ZS005 extension studies which to date do not show an increased rate of edema at 15g, or any other dose, after ten months of treatment.
In general, there were no clinically meaningful treatment-related changes in most laboratory parameters. There were five cases of hypokalemia (defined in this trial as a serum potassium level less than 3.5 mEq/L) on 10g (9.8%) and six cases on 15g (10.7%), all of which occurred in the extended phase, compared to no cases on both placebo and 5g. All cases of hypokalemia were transient, mild (3.0 to 3.4 mEq/L), and resolved after the dose of ZS-9 was reduced from daily to every other day (per protocol) for the remainder of the study. There were dose-related mean increases from baseline in serum bicarbonate after treatment with 10g and 15g of ZS-9 that were statistically significantly different from the placebo group. The restoration of normal bicarbonate levels may provide additional benefit to this population, which often suffer from metabolic acidosis, and will be monitored in ongoing trials. No patients had bicarbonate levels that went out of the normal range. At the end of treatment in the Maintenance Phase, statistically significant larger mean decreases from baseline in serum aldosterone were observed in each of the ZS dose groups (range: -3.68 to -6.12 ng/dL) compared with the placebo group (-0.83 ng/dL). No statistically significant changes in serum sodium, serum calcium, or serum magnesium levels were observed, and none of the subjects developed clinically significantly lower values for calcium or magnesium or high values for sodium. No clinically important changes in other laboratory values were observed. In addition, there were no dose-related changes in urinary excretion of sodium between the treatment groups, despite significant reductions in urinary potassium, suggesting that there was little or no sodium absorbed from ZS-9.
Ongoing Studies
ZS004e (Ongoing)
ZS004e (ongoing) is an eleven month open label safety exposure extension study for patients that completed the ZS004 trial. Patients that were normokalemic at the end of the ZS004 study were eligible to enroll in the open label ZS004e treatment phase where they will receive once daily dosing of ZS-9. Regardless of dose received during the randomized phase of ZS004, patients will initially receive 10g once daily of ZS-9 and will be allowed to increase or decrease their dose in 5g increments to a maximum of 15g once daily or minimum of 5g every other day. The primary goal of the eleven month portion of the study will be to demonstrate the safety and tolerability of ZS-9, but control of serum potassium levels will be an important secondary endpoint. To date, with patients entering the 11th month of treatment, the rate of edema in ZS004e is comparable to the rate of edema in placebo treated patients from our completed studies.
ZS005 (Ongoing)
ZS005 is an open label safety exposure study designed to demonstrate that chronic administration of ZS-9 is safe and well-tolerated and maintains control of serum potassium levels over 12 months. ZS005 was initiated in the second quarter of 2014. The trial consists of an open label 24-72 hour induction phase and twelve month open-label treatment phase. In the open label induction portion, patients with hyperkalemia (defined as potassium levels greater than 5.0 mEq/L) regardless of cause will be given 10g of ZS-9 three times per day for 24-72 hours, depending on serum potassium response. Patients that become normokalemic at the end of the induction phase will be eligible to enroll in the open label treatment phase where they will receive once daily dosing of ZS-9. Patients will initially receive 5g once daily of ZS-9 but will be allowed to increase or decrease their dose in 5g
29
Table of Contents
increments to a maximum of 15g once daily or minimum of 5g every other day. The primary goal of the 12 month portion of the study will be to demonstrate the safety and tolerability of ZS-9, but control of serum potassium levels will be an important secondary endpoint. To date, with patients entering the 9 month of treatment, the rate of edema in ZS005 is comparable to the rate of edema in placebo treated patients from our completed studies.
Study ZS005: Trial Design
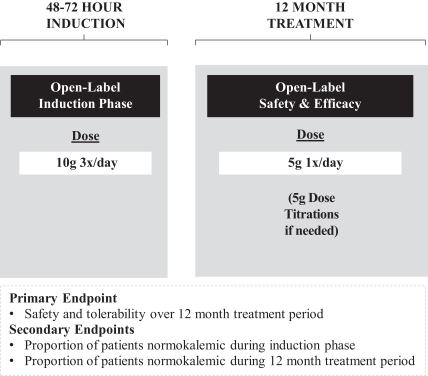
Nonclinical and Toxicology Studies
To date, we have not observed evidence of ZS-9 absorption in animals or humans. We conducted in-vivo recovery studies in rats which recovered greater than 98% of the dosage of ZS-9 administered, similar to the level of recovery of the control compound. In addition, high sensitivity analysis of samples collected from 6 month rat, 9 month dog, and 28 day human studies have shown no sign of ZS-9 absorption with a limit of detection of 10 parts per 1,000,000,000. We believe these studies provide evidence that ZS-9 is excreted in feces and is not systemically absorbed. In addition, we believe ZS-9 is essentially insoluble under physiologic conditions. Exposure of the powder form of ZS-9 to simulated gastric and intestinal fluids for an extended period of time resulted in negligible increases in the levels of zirconium, silicon and oxygen in solution. These experiments suggest that the amount of zirconium available for absorption from a 10g dose of ZS-9 would be 0.00028 mg. Compared to the average range of between 1.0 and 9.0 mg of zirconium found in the average daily human diet, ZS-9 is not expected to be a significant source of dietary zirconium intake.
Nonclinical pharmacology studies have shown that ZS-9 binds potassium in solution as well as in complex environments, such as in-vivo animal models. Nonclinical safety data showed that ZS-9 was not genotoxic and was not associated with adverse effects in cardiovascular, central nervous system or gastrointestinal motility safety pharmacology studies. Results of preliminary nonclinical drug-to-drug interaction studies indicated that ZS-9 did not interfere with the absorption of common drugs used in our target patient population when evaluated
30
Table of Contents
in-vitro. To date, the only drug that interacted with ZS-9 is lithium. We have completed 6 month rat and 9 month dog toxicology studies, which demonstrated that daily administration ZS-9, at levels as high as 7 times the human equivalent dose, did not result in toxicity or histological changes.
ZS-1
Utilizing our proprietary drug discovery technology, we have a non-absorbed product candidate, ZS-1, in the nonclinical stage of development. ZS-1 has been designed to trap ammonium ions and is currently being evaluated as a treatment for disorders where elevated ammonium levels are thought to play a role. We have developed a nonclinical and clinical plan, but we have not yet initiated IND-enabling clinical studies for ZS-1. We intend to focus substantially all of our resources on the advancement of ZS-9 through NDA submission and commercialization.
Commercial Opportunity
Individuals with CKD and HF are the major populations at risk for chronic hyperkalemia due to poor renal function and/or the need for treatment with RAAS inhibitors. Together, there are over 30 million CKD and HF patients in the United States, which number is expected to grow to nearly 50 million patients by 2022. We believe a significant commercial opportunity exists for ZS-9, and, subject to FDA approval, we plan to initially market ZS-9 in the United States to a subset of the approximately 5,000 practicing nephrologists and approximately 15,000 practicing non-interventional cardiologists, who treat patients in one or more of the following categories:
| • | CKD patients with moderate to severe hyperkalemia. We believe the prevalence of moderate-to-severe hyperkalemia (serum potassium level greater than or equal to 5.5 mEq/L) is 20% and 40% in stage 3 and stage 4 CKD patients, respectively. Therefore, we estimate that there are approximately 2.4 million CKD patients in the United States with moderate-to-severe hyperkalemia being treated by a nephrologist or cardiologist. Based on our market research, approximately 80% of physicians surveyed indicated that they are likely to intervene with some form of treatment for hyperkalemia when a patient’s serum potassium level reaches 5.5 mEq/L. Accordingly, we view these patients as a readily identifiable initial patient population likely to be prescribed ZS-9. We believe that a safe, effective and well-tolerated daily use chronic therapy such as ZS-9 would be useful for patients in this category, in particular for those patients who have a history of recurrent episodes of hyperkalemia. |
| • | CKD and HF patients not currently taking a RAAS inhibitor or who have had their RAAS inhibitor dose reduced to address their hyperkalemia. Based on average annual disease prevalence rates from multi-year surveys conducted between 1999 and 2004 for CKD and between 2005 and 2008 for HF, when applied to 2010 U.S. Census Data, we estimate there are approximately 19 million stage 3 or 4 CKD patients and approximately 5.7 million HF patients in the United States. Based on our market research and medical literature, 80% of these patients who are under the care of a nephrologist or cardiologist are indicated for RAAS therapy, yet nearly half of these patients are not receiving RAAS inhibitors or are receiving sub-optimal RAAS inhibitor dosing, in large part because they have developed, or physicians fear that they will develop, hyperkalemia from these treatments. Current CKD and HF treatment guidelines recommend the use of RAAS inhibitors to preserve kidney function and delay time to dialysis in CKD patients and decrease morbidity and mortality in HF patients. We believe that, with the introduction of a safe, effective and well-tolerated daily use chronic treatment option for hyperkalemia, some physicians may increase their RAAS inhibitor usage in patients who have hyperkalemia by either maintaining their RAAS inhibitor therapy, increasing the doses of RAAS inhibitor where indicated or by reinitiating RAAS inhibitor medications. We believe that ZS-9 would provide physicians with an important tool to treat such hyperkalemia in this patient population. |
| • | Patients with acute or episodic hyperkalemia. According to medical literature, between 1% and 10% of all patients hospitalized in the United States are hyperkalemic at admission. The medical literature suggests that many end-stage renal disease patients have potassium levels that are not adequately |
31
Table of Contents
| controlled between dialysis sessions, often resulting in hospitalization. Approximately 200 million grams of SPS (e.g., Kayexalate) are prescribed annually in the hospital setting to manage hyperkalemia, despite its poor tolerability and unproven efficacy. Given the predictable efficacy and rapid-acting nature of ZS-9 suggested by our clinical trials, we believe ZS-9 would be a desirable alternative to existing therapy in these acute and episodic use settings. |
We believe there are opportunities for expanding ZS-9 usage beyond physician specialists in the United States, including as follows:
| • | Treating patients in the United States who are outside of our currently targeted specialty care market. Although our marketing effort will be focused initially on a nephrology and cardiology market, we believe that a significant number of patients with hyperkalemia are under the care of endocrinologists, diabetologists and/or primary care physicians. We may address these patients in the future by expanding our sales and marketing efforts or initiating a partnership to address hyperkalemia in the type 2 diabetes and primary care markets. |
| • | Clinical need outside of the United States. The need for an improved hyperkalemia treatment also exists outside of the United States. We are currently in the process of evaluating the opportunities for ZS-9 outside the United States. We intend to seek one or more partners to commercialize ZS-9 in these markets. |
Competition
Other companies are developing drugs for the treatment of hyperkalemia. We are aware of one other company, Relypsa, which is developing a hyperkalemia treatment, Patiromer, for which it has announced that it has completed its Phase III clinical development program and it has submitted an NDA to the FDA that has been accepted. If approved by the FDA, this treatment could reach the US market before ZS-9, potentially giving Relypsa an advantage over us in marketing and gaining market acceptance. In order to compete successfully in this market, we will have to demonstrate that the treatment of hyperkalemia with ZS-9 is a superior alternative to existing or new therapies for hyperkalemia, including Patiromer. Even if we are able to demonstrate the superiority of our product, Relypsa or other competitors may attempt to lock us out of the market by entering into exclusive or preferred arrangements with insurers and other third party payors before our product is approved for sale, which may make it difficult for us to successfully gain market acceptance for ZS-9. In addition, our competitors may attempt to undercut our pricing for ZS-9 once it is introduced, or we may need to undercut the pricing of Relypsa’s drug to gain market acceptance, which could also harm our results of operations.
In the United States, the current treatment options for the chronic management of hyperkalemia are limited. These options include dietary potassium restriction, potassium-wasting diuretics or SPS. Dietary potassium restriction is difficult due to the ubiquitous presence of potassium in foods. Because fat, carbohydrates (in diabetics), sodium and phosphorus tend to be restricted in CKD patients, the addition of potassium restriction severely limits food options for these patients, which results in significant patient compliance issues. Diuretics, a mainstay for managing sodium, water balance and hypertension, are highly efficient at removing potassium in patients with normal renal function; however, the effectiveness of these drugs is greatly diminished in patients with CKD. SPS’ efficacy is unproven in large randomized controlled trials and its product labeling includes warnings of serious and potentially fatal GI side effects, and therefore, we believe that its use as a daily treatment option over the long-term is limited.
We compete in the segments of the pharmaceutical, biotechnology and other related markets that address CKD, HF and metabolic diseases. While we are not aware of any direct competition from pharmaceutical and biotechnology companies that are actively engaged in development of drugs to treat hyperkalemia, other than Relypsa, we face significant competition from many pharmaceutical and biotechnology companies that are researching and selling products to treat CKD, HF and metabolic diseases. Many of our competitors have materially greater financial, manufacturing, marketing, research and drug development resources than we do.
32
Table of Contents
Large pharmaceutical companies in particular have extensive expertise in nonclinical and clinical testing and in obtaining regulatory approvals for drugs and commercializing drugs upon approval. In addition, academic institutions, government agencies and other public and private organizations conducting research may seek patent protection with respect to potentially competitive products or technologies. These organizations may also establish exclusive collaborative or licensing relationships with our competitors.
Manufacturing and Distribution
ZS-9 is stable at room temperature and does not absorb or attract high levels of moisture from the air. ZS-9 does not require any special handling. The ongoing 36-month stability studies supporting our powder formulation are expected to provide 24 months of data to support the NDA filing. We expect that these data will support the storage of ZS-9 for 36 months at 25°C. ZS-9 tablets are also being tested in 36 month stability studies, and we believe that these studies will support storage of ZS-9 tablets for 24 to 36 months at 25°C.
ZS-9 is manufactured at our two manufacturing facilities located in Coppell, Texas and in Denver, Colorado. ZS-9, the drug product, is a dry, odorless powder or tablet formulation of the active pharmaceutical ingredient, or API. The API is manufactured in a straightforward two-step process from readily available starting materials. The initial phase involves mixing silicate solution, zirconium acetate and sodium hydroxide in water at high temperature and at elevated pressures. By carefully selecting the reaction conditions we are able to form ZS-9 with the appropriate particle size and particle size distribution. The resulting crystalline material is then washed with an acidic solution to improve the potassium exchange kinetics and reduce the API’s pH. For the powder drug product, the resulting API is filled into packets, a well-established configuration for oral suspension. For the tablet formulation, the API is blended with binding agents and pressed into tablets.
We completed manufacturing of registration batches of ZS-9 with a reactor that has approximately one fourth the capacity of our anticipated commercial scale reactor and are producing commercial grade ZS-9 from our 2,000 liter (commercial scale) reactor. We received delivery of a second 2,000 liter commercial scale reactor in the fourth quarter of 2014. Registration batches were used for our pivotal Phase III trials and ongoing stability studies. We have completed development of most analytical methods for impurities and commercial release testing, and we are now validating those methods. We have completed testing for impurities in drug substance registration stability batches and clinical batches using analytical procedures developed in-house and have found that the purity of our drug substance is high. We believe the levels of impurities are below the suggested guidance recommendations. Control of impurities during API and drug product manufacturing will be confirmed through process validation; we are using the maximum daily dose to establish the allowable levels of genotoxic impurities, impurities of special toxicological concern and residual solvents.
To meet our strategic objectives, which contemplate internally manufacturing all of our drug substance to support anticipated commercial demand if ZS-9 is approved, we plan to install additional reactors, dryers and other equipment, add manufacturing personnel and ensure that validated processes are consistently implemented in our facilities. In particular, we currently intend to eventually transition to use a 5,000 liter reactor to produce ZS-9 and there can be no assurance we will be able to successfully do so. Should we encounter unexpected difficulties in transitioning to these larger reactors, we may be forced to rely on 2,000 liter reactors, which could both increase our expected costs and impact the timing of our ability to expand our production.
We plan to continue to manufacture ZS-9 API in our two manufacturing sites to establish a diverse and volume appropriate supply. This material will then be sent to finished product manufacturers for further processing and drug product completion. Our current manufacturing capacity is insufficient to meet peak anticipated market demand; however we plan to produce quantities sufficient for launch and increase production capacity post approval. We believe our strategy to build in-house API manufacturing will allow us to ensure adequate supply of API and attain an attractive cost of goods structure from the time of launch if ZS-9 is approved.
33
Table of Contents
Currently, packaging and drug product formation is performed by Sharp. We plan to enter into in a long term agreement with Sharp, and/or another drug product manufacturer, to produce ZS-9 once approved by the FDA. A number of contractors are available, and we may establish alternate drug product manufacturers to reduce supply risk for ZS-9.
Our manufacturing facilities, as well as third-party drug product manufacturers, their facilities and all lots of drug substance and drug products used in our clinical trials, are required to be in compliance with cGMP. The cGMP regulations include requirements relating to organization of personnel, buildings and facilities, equipment, control of components and drug product containers and closures, production and process controls, packaging and labeling controls, holding and distribution, laboratory controls, records and reports, and returned or salvaged products. The manufacturing facilities for our products must meet cGMP requirements and FDA satisfaction before any product is approved and we can manufacture commercial products. We and our third-party manufacturers are also subject to periodic inspections of facilities by the FDA and other authorities, including procedures and operations used in the testing and manufacture of our products to assess our compliance with applicable regulations. Failure to comply with statutory and regulatory requirements subjects a manufacturer to possible legal or regulatory action, including warning letters, the seizure or recall of products, injunctions, consent decrees placing significant restrictions on or suspending manufacturing operations and civil and criminal penalties. These actions could have a material impact on the availability of our products. Contract manufacturers at times encounter difficulties involving production yields, quality control and quality assurance, as well as shortages of qualified personnel.
Intellectual Property
ZS-9 has been developed utilizing our proprietary ion trap drug discovery technology, which targets indications susceptible to treatment by non-absorbed ion binders in the GI tract. The initial zirconium silicates were discovered by UOP. We have licensed certain patent rights relating to zirconium silicates from UOP. These patents include claims covering ZS-9 and methods of oral administration of ZS-9. During our development of ZS-9, we have made certain improvements to the ZS-9 composition. For example, we found that reducing the level of ZS-9 particles with a particle size below 3 microns that are contained in a composition and protonating the compound was advantageous for avoiding systemic exposure during oral administration. We also found that the ZS-9 production method can be controlled to reduce the levels of more soluble forms of zirconium silicate impurities in the composition. We have been issued three U.S. patents based on improvements with composition of matter and method of use claims covering ZS-9, which are currently expected to expire in 2032. We have also filed several non-provisional and provisional patent applications directed to these improvements and other novel zirconium silicate compositions, methods of treating various indications with ZS-9, and methods of manufacturing ZS-9.
UOP License Agreement
In December 2011, we entered into a license agreement with UOP. Under the agreement, UOP granted us a worldwide, exclusive license, with the right to sublicense, under certain UOP patent rights to develop, make and sell products in the field of the removal of toxins from bodily fluids and the gastrointestinal tract of humans and animals. We are required to pay UOP a 5% royalty based on annual worldwide net sales of licensed products made by us and by our sublicensees, provided that we are obligated to pay UOP an annual minimum royalty payment of $100,000. We are obligated to indemnify UOP against all claims arising out of the use of the licensed products, processes and the suitability of the licensed patents in connection therewith, as well as claims of intellectual property infringement in connection therewith, and must carry certain levels of insurance to cover our indemnity obligations under the agreement. Our license agreement with UOP, unless earlier terminated by us or UOP, will continue until the expiration or invalidation of all licensed UOP patent rights. Either party may terminate the agreement if the other party materially breaches the agreement and the breach remains uncured for a specified period. UOP may terminate the agreement at any time if it determines that our financial condition materially changes in such a way that materially impairs our ability to meet our indemnification and insurance
34
Table of Contents
obligations, or if the number of claims that are subject to our indemnification obligations that are reasonably anticipated by UOP materially impairs our ability to meet our indemnification obligations. We may also terminate the agreement at the end of a specified period following written notice to UOP if we determine that the use of the licensed patents is not technically or commercially feasible, or if we determine that we no longer desire to use the licensed patent rights in the licensed field.
In December 2011, we also entered into a sublicense agreement with HemoCleanse, Inc., or HemoCleanse. Under the sublicense agreement, we granted HemoCleanse a worldwide, exclusive sub-license under the licensed UOP patent rights to research, develop, make, have made, use, sell, have sold, offer for sale, import, export, market, commercialize and to otherwise exploit products and to practice processes covered by the UOP patent rights within the sub-field of removing toxins from bodily fluids outside the body of humans and animals. HemoCleanse is required to pay us a 5% royalty based on worldwide annual net sales of licensed products, which we must in turn pay to UOP. The sublicense agreement, unless earlier terminated by us or HemoCleanse, will continue until the expiration or invalidation of all of the sublicensed UOP patent rights. The sublicense agreement will terminate automatically upon termination of the UOP license agreement with us. Either party may terminate the agreement if the other party materially breaches the agreement and the breach remains uncured for a specified period. In addition, either party may terminate the sublicense agreement immediately if the other party is dissolved or undergoes certain insolvency events. We have the right to terminate the sub-license on a country-by-country basis if HemoCleanse fails to use commercially reasonable efforts to develop, make, market and sell the licensed products. The sublicense to HemoCleanse does not include rights under three U.S. patents granted to us based on improvements with composition of matter and method of use claims covering the commercial form of ZS-9 for which we are seeking approval, nor do we have any obligation to grant a license under our patents or know-how or to otherwise transfer any technology to HemoCleanse.
Proprietary Protection
Our commercial success depends in part on our ability to obtain and maintain proprietary protection for our drug candidates, manufacturing and process discoveries, and other know-how, to operate without infringing the proprietary rights of others and to prevent others from infringing our proprietary rights. Our policy is to seek to protect our proprietary position by, among other methods, filing United States and certain foreign patent applications related to our proprietary technology, inventions and improvements that are important to the development and implementation of our business. We also intend to rely on trade secrets, know-how, continuing technological innovation and potential in-licensing opportunities to develop and maintain our proprietary position.
Other Patent Rights and Strategy
With regard to the pharmaceutical products we may develop, we intend to pursue composition-of-matter patents, where possible, and dosage and formulation patents, as well as method-of-use patents on novel indications for known compounds. We also intend to seek patent protection for manufacturing discoveries, including new in-process controls and starting materials.
The patents under license from UOP include claims directed to compositions and methods for oral administration of ZS-9. If ZS-9 receives FDA approval, we intend to list patents licensed from UOP in the Orange Book, including two composition of matter patents that, if the appropriate maintenance fees are paid, are expected to expire in 2017, and a method of treatment patent that, if the appropriate maintenance fees are paid, is expected to expire in 2019. The UOP license grants worldwide rights; however, UOP does not have any issued patents or pending patent applications outside the United States that are subject to the license agreement. The term of individual patents depends upon the legal term for patents in the countries in which they are granted. In most countries, including the United States, the patent term is generally 20 years from the earliest claimed filing date of a non-provisional patent application in the applicable country. In the United States, a patent’s term may, in certain cases, be lengthened by patent term adjustment, which compensates a patentee for administrative delays by the U.S. Patent and Trademark Office in examining and granting a patent, or may be shortened if a
35
Table of Contents
patent is terminally disclaimed over a commonly owned patent or a patent naming a common inventor and having an earlier expiration date. In the United States, we believe that a patent term extension will be available under the Drug Price Competition and Patent Term Restoration Act of 1984, or the Hatch-Waxman Act, for one of the patents we intend to list in the Orange Book if ZS-9 receives FDA approval and if certain eligibility requirements are met, as ZS-9 is a new chemical entity (NCE) that the FDA has not previously approved. The Hatch-Waxman Act allows for a maximum patent term extension of five years from the expiration date of the subject patent as compensation for patent term lost in the product development and regulatory review process. A patent term extension cannot extend the remaining term of a patent beyond 14-years from the approval date and only one patent applicable to a regulatory review period for a product may be extended. If all regulatory requirements are met and maintenance fees are paid, the maximum patent term extension available for the method of treatment patent would result in a term expiring in 2024.
We have filed several patent applications directed to improved zirconium silicate compositions, methods of treatment, including treatment of hyperkalemia and other conditions, and methods of manufacturing zirconium silicate compositions. These patent applications include United States applications, and corresponding foreign national and regional patent applications in Australia, Brazil, Canada, China, Chile, Colombia, Europe, Hong Kong, India, Israel, Japan, Mexico, Philippines, South Africa, and South Korea. If patents issue from these applications, and if the appropriate maintenance fees are paid, these patents are currently expected to begin to expire in 2031.
Trade Secrets and Other Protection
We also rely on trade secrets to protect certain aspects of our technology. However, trade secrets can be difficult to protect. In addition, our trade secrets may otherwise become known or be independently discovered by competitors. We also protect our proprietary technology and processes, in part, by entering into confidentiality and invention assignment agreements with our employees, consultants, scientific advisors and other contractors. These agreements may be breached, and we may not have adequate remedies for any breach. To the extent that our employees, consultants, scientific advisors or other contractors use intellectual property owned by others in their work for us, disputes may arise as to the rights in related or resulting know-how and inventions.
Our commercial success will also depend in part on not infringing the proprietary rights of third parties. It is uncertain whether the issuance of any third-party patent would require us to alter our development or commercial strategies, alter our drugs or processes, obtain licenses or cease certain activities. Our breach of any license agreements or failure to obtain a license to proprietary rights that we may require to develop or commercialize our future drugs may have a material adverse impact on us. If third parties prepare and file patent applications in the United States or in other jurisdictions that also claim technology to which we have rights, we may have to participate in interference or derivation proceedings in the United States Patent and Trademark Office determine priority of invention or inventorship and may also become involved in similar proceedings in other jurisdictions. Please see “Risk Factors—Risks Related to Intellectual Property” for a further description of risks relating to intellectual property.
Third-Party Reimbursement
Sales of pharmaceutical products depend significantly on the availability of coverage and adequate reimbursement by third-party payors, such as state and federal governments, including Medicare and Medicaid, and commercial managed care providers. Decisions regarding the extent of coverage and amount of reimbursement to be provided for ZS-9 will be made on a plan by plan basis. We anticipate that a significant proportion of patients eligible for ZS-9 will be covered by Medicare. Within the Medicare program, as a self-administered drug, we expect that ZS-9 will be reimbursed under the expanded prescription drug benefit, known as Medicare Part D.
36
Table of Contents
Government Regulation
The FDA and comparable regulatory authorities in state and local jurisdictions and in other countries impose substantial and burdensome requirements upon companies involved in the clinical development, manufacture, marketing and distribution of drugs. These agencies and other federal, state and local entities regulate research and development activities and the testing, manufacture, quality control, safety, effectiveness, labeling, storage, record keeping, approval, advertising and promotion, distribution, post-approval monitoring and reporting, sampling and export and import of our product candidates.
In the United States, the FDA regulates drug products under the Federal Food, Drug, and Cosmetic Act, or FFDCA, and the FDA’s implementing regulations. If we fail to comply with applicable FDA or other foreign cGMP requirements at any time during the drug development process, clinical testing, the approval process or after approval, we may become subject to administrative or judicial sanctions. These sanctions could include the FDA’s refusal to approve pending applications, license suspension or revocation, withdrawal of an approval, warning letters, product recalls, product seizures, total or partial suspension of production or distribution, injunctions, fines, civil penalties or criminal prosecution. Any FDA enforcement action could have a material adverse effect on us. FDA approval is required before any new unapproved drug or dosage form, including a new use of a previously approved drug, can be marketed in the United States.
The process required by the FDA before a drug may be marketed in the United States generally involves:
| • | completion of extensive nonclinical laboratory tests, nonclinical animal studies and formulation studies some performed in accordance with the FDA’s current Good Laboratory Practice, or cGLP, regulations; |
| • | submission to the FDA of an IND application which must become effective before human clinical trials in the United States may begin; |
| • | approval by an independent institutional review board, or IRB, or ethics committee at each clinical trial site before each trial may be initiated; |
| • | performance of adequate and well-controlled human clinical trials to establish the safety and efficacy of the drug candidate for each proposed indication; |
| • | submission to the FDA of an NDA; |
| • | satisfactory completion of an FDA inspection of the manufacturing facility or facilities at which the drug is produced to assess compliance with cGMP regulations; |
| • | satisfactory completion of a potential review by an FDA advisory committee, if applicable; and |
| • | FDA review and approval of the NDA prior to any commercial marketing, sale or commercial shipment of the drug. |
The nonclinical and clinical testing and approval process requires substantial time, effort and financial resources, and we cannot be certain that any approvals for our product candidates will be granted on a timely basis, if at all. Nonclinical tests include laboratory evaluation of product chemistry, formulation, stability and toxicity, as well as animal studies to assess the characteristics and potential safety and efficacy of the product. The results of nonclinical tests, together with manufacturing information, analytical data and a proposed clinical trial protocol and other information, are submitted as part of an IND to the FDA. Some nonclinical testing may continue even after the IND is submitted. The IND automatically becomes effective 30 days after receipt by the FDA, unless the FDA, within the 30-day time period, raises concerns or questions relating to one or more proposed clinical trials and places the clinical trial on a clinical hold, including concerns that human research subjects will be exposed to unreasonable health risks. In such a case, the IND sponsor and the FDA must resolve any outstanding concerns before the clinical trial can begin. A separate submission to an existing IND must also be made for each successive clinical trial conducted during product development.
37
Table of Contents
Clinical trials involve the administration of the investigational drug to human subjects under the supervision of qualified investigators. Clinical trials are conducted under protocols detailing, among other things, the objectives of the clinical trial, the parameters to be used in monitoring safety and the effectiveness criteria to be used. Each protocol must be submitted to the FDA as part of the IND. An IRB or ethics committee for each medical center proposing to conduct a clinical trial must also review and approve a plan for any clinical trial before it can begin at that center and the IRB must monitor the clinical trial until it is completed. The FDA, the IRB, or the sponsor may suspend or discontinue a clinical trial at any time on various grounds, including a finding that the subjects are being exposed to an unacceptable health risk. Clinical testing also must satisfy extensive Good Clinical Practice, or GCP, requirements, including the requirements for informed consent.
All clinical research performed in the United States in support of an NDA must be authorized in advance by the FDA under the IND regulations and procedures described above. However, a sponsor who wishes to conduct a clinical trial outside the United States may, but need not, obtain FDA authorization to conduct the clinical trial under an IND. If a foreign clinical trial is not conducted under an IND, the sponsor may submit data from the clinical trial to the FDA in support of an NDA so long as the clinical trial is conducted in compliance with an international guideline for the ethical conduct of clinical research known as the Declaration of Helsinki and/or the laws and regulations of the country or countries in which the clinical trial is performed, whichever provides the greater protection to the participants in the clinical trial.
Within the European Union, approval of clinical trials as well as marketing authorization of all new drugs is regulated by the EMA. The requirements for granting approval of a MAA, the European equivalent of an NDA, are very similar to the FDA requirements and include demonstration of efficacy and safety in a sufficiently large number of subjects. We have been in contact with the EMA throughout the development process and have incorporated European requirements into our clinical development programs.
Clinical Trials
For purposes of NDA and MAA submission and approval, clinical trials are typically conducted in three or four phases, which may overlap or be combined.
| • | Phase I: Clinical trials are initially conducted in a limited population of subjects to test the drug candidate for safety, dose tolerance, absorption, metabolism, distribution and excretion in healthy humans or, on occasion, in patients with severe problems or life-threatening diseases to gain an early indication of its effectiveness. |
| • | Phase II: Clinical trials are generally conducted in a limited patient population to evaluate dosage tolerance and appropriate dosage, identify possible adverse effects and safety risks and evaluate preliminarily the efficacy of the drug for specific targeted indications in patients with the disease or condition under study. |
| • | Phase III: Clinical trials are typically conducted when Phase II clinical trials demonstrate that a dose range of the product candidate is effective and has an acceptable safety profile. Phase III clinical trials are commonly referred to as “pivotal” studies, which typically denotes a study which presents the data that the FDA, EMA or other relevant regulatory agency will use to determine whether or not to approve a drug. Phase III clinical trials are generally undertaken with large numbers of patients, such as groups of several hundred to several thousand, to further evaluate dosage, to provide substantial evidence of clinical efficacy and to further test for safety in an expanded and diverse patient population at multiple, geographically-dispersed clinical trial sites. |
| • | Phase IV: In some cases, FDA may condition approval of an NDA for a product candidate on the sponsor’s agreement to conduct additional clinical trials after NDA approval. In other cases, a sponsor may voluntarily conduct additional clinical trials post approval to gain more information about the drug. Such post approval trials are typically referred to as Phase IV clinical trials. |
38
Table of Contents
Concurrent with clinical trials, companies usually complete additional animal trials and must also develop additional information about the chemistry and physical characteristics of the drug and finalize a process for manufacturing the drug in commercial quantities in accordance with cGMP requirements. The manufacturing process must be capable of consistently producing quality batches of the drug candidate and, among other things, the manufacturer must develop methods for testing the identity, strength, quality and purity of the final drug product. Additionally, appropriate packaging must be selected and tested and stability studies must be conducted to demonstrate that the drug candidate does not undergo unacceptable deterioration over its shelf life.
New Drug Applications
The results of nonclinical studies and of the clinical trials, together with other detailed information, including extensive manufacturing information and information on the composition of the drug, are submitted to the FDA in the form of an NDA requesting approval to market the drug for one or more specified indications. The FDA reviews an NDA to determine, among other things, whether a drug is safe and effective for its intended use.
Under the Prescription Drug User Fee Act, the FDA has a goal of responding to standard review NDAs within ten months after the 60 day filing review period, but this timeframe is often extended. The first indication of the FDA’s review progress is provided at the mid-cycle review. This typically occurs five months after the NDA is submitted. However, the review process is often significantly extended by FDA requests for additional information or clarification. The FDA may refer the application to an advisory committee for review, evaluation and recommendation as to whether the application should be approved. The FDA is not bound by the recommendation of an advisory committee, but it generally follows such recommendations. The FDA may deny approval of an NDA if the applicable statutory and regulatory criteria are not satisfied, or it may require additional clinical data or an additional Phase III clinical trial. Even if such data are submitted, the FDA may ultimately decide that the NDA does not satisfy the criteria for approval. Data from clinical trials are not always conclusive and the FDA may interpret data differently than we interpret data. Once the FDA approves an NDA, or supplement thereto, the FDA may withdraw the approval if ongoing regulatory requirements are not met or if safety problems are identified after the drug reaches the market. Where a withdrawal may not be appropriate, the FDA still may seize existing inventory of such drug or require a recall of any drug already on the market. In addition, the FDA may require testing, including Phase IV clinical trials and surveillance programs to monitor the effect of approved drugs which have been commercialized. The FDA has the authority to prevent or limit further marketing of a drug based on the results of these post-marketing programs.
Drugs may be marketed only for the FDA approved indications and in accordance with the provisions of the approved labeling. Further, if there are any modifications to the drug, including changes in indications, labeling or manufacturing processes or facilities, the applicant may be required to submit and obtain FDA approval of a new NDA or NDA supplement, which may require the applicant to develop additional data or conduct additional nonclinical studies and clinical trials.
Before approving an application, the FDA will inspect the facility or the facilities at which the finished drug product, and sometimes the active drug ingredient, is manufactured and will not approve the drug unless cGMP compliance is satisfactory. The FDA may also inspect the sites at which the clinical trials were conducted to assess their compliance and will not approve the drug unless compliance with GCP requirements is satisfactory.
The testing and approval processes require substantial time, effort and financial resources, and each may take several years to complete. The FDA may not grant approval on a timely basis, or at all. Even if we believe a clinical trial has demonstrated safety and efficacy of one of our drug candidates for the treatment of a disease, the results may not be satisfactory to the FDA. Nonclinical and clinical data may be interpreted by the FDA in different ways, which could delay, limit or prevent regulatory approval. We may encounter difficulties or unanticipated costs in our efforts to secure necessary governmental approvals which could delay or preclude us from marketing drugs. The FDA may limit the indications for use or place other conditions on any approvals that could restrict the commercial application of the drugs. After approval, certain changes to the approved drug, such
39
Table of Contents
as adding new indications, manufacturing changes or additional labeling claims are subject to further FDA review and approval. Depending on the nature of the change proposed, an NDA supplement must be filed and approved before the change may be implemented. For many proposed post-approval changes to an NDA, but excluding efficacy supplements to an NDA, the FDA has up to 180 days to review the application. As with new NDAs, the review process is often significantly extended by the FDA requests for additional information or clarification.
Other Regulatory Requirements
Any drugs manufactured or distributed by us or our collaborators pursuant to FDA approvals would be subject to continuing regulation by the FDA, including recordkeeping requirements and reporting of adverse experiences associated with the drug. Drug manufacturers and their subcontractors are required to register their establishments with the FDA and certain state agencies, and are subject to periodic unannounced inspections by the FDA and certain state agencies for compliance with ongoing regulatory requirements, including cGMP, which impose certain procedural and documentation requirements upon us and our third-party manufacturers. Failure to comply with the statutory and regulatory requirements can subject a manufacturer to possible legal or regulatory action, such as warning letters, suspension of manufacturing, seizure of product, injunctive action or possible civil penalties. We cannot be certain that we or our present or future third-party manufacturers or suppliers will be able to comply with the cGMP regulations and other ongoing FDA regulatory requirements. If we or our present or future third-party manufacturers or suppliers are not able to comply with these requirements, the FDA may halt our clinical trials, require us to recall a drug from distribution or withdraw approval of the NDA for that drug.
The FDA closely regulates the post-approval marketing and promotion of drugs, including standards and regulations for direct-to-consumer advertising, off-label promotion, industry-sponsored scientific and educational activities and promotional activities involving the Internet. A company can make only those claims relating to safety and efficacy that are approved by the FDA. Failure to comply with these requirements can result in adverse publicity, warning letters, corrective advertising and potential civil and criminal penalties. Physicians may prescribe legally available drugs for uses that are not described in the product’s labeling and that differ from those tested by us and approved by the FDA. Such off-label uses are common across medical specialties. Physicians may believe that such off-label uses are the best treatment for many patients in varied circumstances. The FDA does not regulate the behavior of physicians in their choice of treatments. The FDA does, however, impose stringent restrictions on manufacturers’ communications regarding off-label use.
Healthcare Reform
In March 2010, the President signed one of the most significant healthcare reform measures in decades. The Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act, collectively known as the Affordable Care Act, substantially changes the way healthcare will be financed by both governmental and private insurers, and significantly impacts the pharmaceutical industry. The comprehensive $940 billion dollar overhaul is expected to extend coverage to approximately 32 million previously uninsured Americans. The Affordable Care Act contains a number of provisions, including those governing enrollment in federal healthcare programs, reimbursement changes and fraud and abuse, which will impact existing government healthcare programs and will result in the development of new programs, including Medicare payment for performance initiatives and improvements to the physician quality reporting system and feedback program. Additionally, the Affordable Care Act:
| • | mandates a further shift in the burden of Medicaid payments to the states; |
| • | increases the minimum level of Medicaid rebates payable by manufacturers of brand-name drugs from 15.1% to 23.1%; |
| • | requires collection of rebates for drugs paid by Medicaid managed care organizations; |
40
Table of Contents
| • | requires manufacturers to participate in a coverage gap discount program, under which they must agree to offer 50% point-of-sale discounts off negotiated prices of applicable brand drugs to eligible beneficiaries during their coverage gap period, as a condition for the manufacturer’s outpatient drugs to be covered under Medicare Part D, beginning January 2011; and |
| • | imposes a non-deductible annual fee on pharmaceutical manufacturers or importers who sell “branded prescription drugs” to specified federal government programs. |
Anti-Kickback and False Claims Laws
In the United States, the research, manufacturing, distribution, sale and promotion of drug products and medical devices are potentially subject to regulation by various federal, state and local authorities in addition to the FDA, including the Centers for Medicare & Medicaid Services, other divisions of the United States Department of Health and Human Services (e.g., the Office of Inspector General), the United States Department of Justice, state Attorneys General and other state and local government agencies. For example, sales, marketing and scientific/educational grant programs must comply with the Medicare-Medicaid Anti-Fraud and Abuse Act, as amended, or the Anti-Kickback Statute, the False Claims Act, as amended, the privacy regulations promulgated under the Health Insurance Portability and Accountability Act, or HIPAA and similar state laws. Pricing and rebate programs must comply with the Medicaid Drug Rebate Program requirements of the Omnibus Budget Reconciliation Act of 1990, as amended, and the Veterans Health Care Act of 1992, as amended. If products are made available to authorized users of the Federal Supply Schedule of the General Services Administration, additional laws and requirements apply. All of these activities are also potentially subject to federal and state consumer protection and unfair competition laws.
As noted above, in the United States, we are subject to complex laws and regulations pertaining to healthcare “fraud and abuse,” including, but not limited to, the Anti-Kickback Statute, the federal False Claims Act and other state and federal laws and regulations. The Anti-Kickback Statute makes it illegal for any person, including a prescription drug manufacturer (or a party acting on its behalf) to knowingly and willfully solicit, receive, offer or pay any remuneration that is intended to induce the referral of business, including the purchase, order or prescription of a particular drug, for which payment may be made under a federal healthcare program, such as Medicare or Medicaid. Violations of this law are punishable by up to five years in prison, criminal fines, administrative civil money penalties and exclusion from participation in federal healthcare programs. In addition, many states have adopted laws similar to the Anti-Kickback Statute. Some of these state prohibitions apply to the referral of patients for healthcare services reimbursed by any insurer, not just federal healthcare programs such as Medicare and Medicaid. Due to the breadth of these federal and state anti-kickback laws, the absence of guidance in the form of regulations or court decisions, and the potential for additional legal or regulatory change in this area, it is possible that our future sales and marketing practices and/or our future relationships with physicians might be challenged under anti-kickback laws, which could harm us. Because we intend to commercialize products that could be reimbursed under a federal healthcare program and other governmental healthcare programs, we plan to develop a comprehensive compliance program that establishes internal controls to facilitate adherence to the rules and program requirements to which we will or may become subject.
The federal False Claims Act prohibits anyone from knowingly presenting, or causing to be presented, for payment to federal programs (including Medicare and Medicaid) claims for items or services, including drugs, that are false or fraudulent, claims for items or services not provided as claimed, or claims for medically unnecessary items or services. Although we would not submit claims directly to payors, manufacturers can be held liable under these laws if they are deemed to “cause” the submission of false or fraudulent claims by, for example, providing inaccurate billing or coding information to customers or promoting a product off-label. In addition, our future activities relating to the reporting of wholesaler or estimated retail prices for our products, the reporting of prices used to calculate Medicaid rebate information and other information affecting federal, state and third-party reimbursement for our products, and the sale and marketing of our products, are subject to scrutiny under this law. For example, pharmaceutical companies have been prosecuted under the federal False Claims Act in connection with their off-label promotion of drugs. Penalties for a False Claims Act violation
41
Table of Contents
include three times the actual damages sustained by the government, plus mandatory civil penalties of between $5,500 and $11,000 for each separate false claim, the potential for exclusion from participation in federal healthcare programs, and, although the federal False Claims Act is a civil statute, conduct that results in a False Claims Act violation may also implicate various federal criminal statutes. If the government were to allege that we were, or convict us of, violating these false claims laws, we could be subject to a substantial fine and may suffer a decline in our stock price. In addition, private individuals have the ability to bring actions under the federal False Claims Act and certain states have enacted laws modeled after the federal False Claims Act.
There are also an increasing number of state laws that require manufacturers to make reports to states on pricing and marketing information. Many of these laws contain ambiguities as to what is required to comply with the laws. In addition, as discussed below, a similar federal requirement requires manufacturers to track and report to the federal government certain payments made to physicians and teaching hospitals made in the previous calendar year. These laws may affect our sales, marketing, and other promotional activities by imposing administrative and compliance burdens on us. In addition, given the lack of clarity with respect to these laws and their implementation, our reporting actions could be subject to the penalty provisions of the pertinent state, and soon federal, authorities.
Patient Protection and Affordable Health Care Act
In March 2010, the Patient Protection and Affordable Health Care Act, as amended by the Health Care and Education Reconciliation Act of 2010, or collectively PPACA, was enacted, which includes measures that have or will significantly change the way health care is financed by both governmental and private insurers. Among the provisions of PPACA of greatest importance to the pharmaceutical industry are the following:
| • | The Medicaid Drug Rebate Program requires pharmaceutical manufacturers to enter into and have in effect a national rebate agreement with the Secretary of the Department of Health and Human Services a condition for states to receive federal matching funds for the manufacturer’s outpatient drugs furnished to Medicaid patients. Effective in 2010, PPACA made several changes to the Medicaid Drug Rebate Program, including increasing pharmaceutical manufacturers’ rebate liability by raising the minimum basic Medicaid rebate on most branded prescription drugs and biologic agents from 15.1% of average manufacturer price, or AMP, to 23.1% of AMP and adding a new rebate calculation for “line extensions” (i.e., new formulations, such as extended release formulations) of solid oral dosage forms of branded products, as well as potentially impacting their rebate liability by modifying the statutory definition of AMP. PPACA raised the methodology by which rebates owed by manufacturers to the state and federal government for outpatient drugs are calculated under the Medicaid Drug Rebate Program and extended the Medicaid Drug Rebate Program to utilization of prescriptions of individuals enrolled in Medicaid managed care organizations. The Centers for Medicare and Medicaid Services, or CMS, have proposed to expand Medicaid rebate liability to the territories of the United States as well. In addition, PPACA provides for the public availability of retail survey prices and certain weighted average AMPs under the Medicaid program. The implementation of this requirement by the CMS may also provide for the public availability of pharmacy acquisition cost data, which could negatively impact our sales. |
| • | In order for a pharmaceutical product to receive federal reimbursement under the Medicare and Medicaid programs or to be sold directly to United States government agencies, the manufacturer must extend discounts to entities eligible to participate in the 340B drug pricing program. The required 340B discount on a given product is calculated based on the AMP and Medicaid rebate amounts reported by the manufacturer. Effective in 2010, PPACA expanded the types of entities eligible to receive discounted 340B pricing, although, under the current state of the law, with the exception of children’s hospitals, these newly eligible entities will not be eligible to receive discounted 340B pricing on orphan drugs when used for the orphan indication. In addition, as 340B drug pricing is determined based on AMP and Medicaid rebate data, the revisions to the Medicaid rebate formula and AMP definition described above could cause the required 340B discount to increase. |
42
Table of Contents
| • | Effective in 2011, PPACA imposed a requirement on manufacturers of branded drugs and biologic agents to provide a 50% discount off the negotiated price of branded drugs dispensed to Medicare Part D patients in the coverage gap (i.e., “donut hole”). |
| • | Effective in 2011, PPACA imposed an annual, nondeductible fee on any entity that manufactures or imports certain branded prescription drugs and biologic agents, apportioned among these entities according to their market share in certain government healthcare programs, although this fee would not apply to sales of certain products approved exclusively for orphan indications. |
| • | Effective in 2012, PPACA required pharmaceutical manufacturers to track certain financial arrangements with physicians and teaching hospitals, including any “transfer of value” made or distributed to such entities, as well as any investment interests held by physicians and their immediate family members. Manufacturers were required to report this information beginning in 2013. |
| • | As of 2010, a new Patient-Centered Outcomes Research Institute was established pursuant to PPACA to oversee, identify priorities in, and conduct comparative clinical effectiveness research, along with funding for such research. The research conducted by the Patient-Centered Outcomes Research Institute may affect the market for certain pharmaceutical products. |
| • | PPACA created the Independent Payment Advisory Board which, beginning in 2014, has authority to recommend certain changes to the Medicare program to reduce expenditures by the program that could result in reduced payments for prescription drugs. Under certain circumstances, these recommendations will become law unless Congress enacts legislation that will achieve the same or greater Medicare cost savings. |
| • | PPACA established the Center for Medicare and Medicaid Innovation within CMS to test innovative payment and service delivery models to lower Medicare and Medicaid spending, potentially including prescription drug spending. Funding has been allocated to support the mission of the Center for Medicare and Medicaid Innovation from 2011 to 2019. |
Many of the details regarding the implementation of PPACA are yet to be determined, and at this time, it remains unclear the full effect that PPACA would have on our business.
Other Regulations
We are also subject to numerous federal, state and local laws relating to such matters as safe working conditions, manufacturing practices, environmental protection, fire hazard control, and disposal of hazardous or potentially hazardous substances. We may incur significant costs to comply with such laws and regulations now or in the future.
Employees
As of December 31, 2014, we had 71 full-time employees, including a total of eight employees with M.D. or Ph.D. degrees. Within our workforce, 54 employees are engaged in research and development and the remaining 17 in general management and administration, including finance, legal, human resources, facilities and information technology. None of our employees is represented by a labor union or covered by a collective bargaining agreement.
We believe that we maintain good relations with our employees.
Available Information
We are subject to the information and periodic reporting requirements of the Exchange Act and, in accordance therewith, file periodic reports, proxy statements and other information with the SEC. Such periodic reports, proxy statements and other information are available for inspection and copying at the public reference
43
Table of Contents
room and website of the SEC referred to above. We maintain a website at www.zspharma.com. You may access our annual reports on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K, and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Exchange Act with the SEC free of charge at our website as soon as reasonably practicable after such material is electronically filed with, or furnished to, the SEC. The reference to our website address does not constitute incorporation by reference of the information contained on our website, and you should not consider the contents of our website in making an investment decision with respect to our common stock.
Copies of any materials we file with the SEC can be obtained at www.sec.gov or at the SEC’s public reference room at 100 F Street, N.E., Washington, D.C. 20549. Information on the operation of the public reference room is available by calling the SEC at 1-800-SEC-0330. The SEC also maintains a website that contains reports, proxy and information statements and other information regarding registrants that file electronically with the SEC. The address is www.sec.gov.
| ITEM 1A. | RISK FACTORS |
Risks Related to our Business
We have a limited operating history, have incurred significant losses since our inception and anticipate that we will continue to incur losses for the foreseeable future. We have only one product candidate in clinical trials and have had no commercial sales, which, together with our limited operating history, make it difficult to assess our future viability.
We are a clinical-stage biopharmaceutical company with a limited operating history. Pharmaceutical product development is a highly speculative undertaking and involves a substantial degree of risk. To date, we have focused principally on developing ZS-9, which is our only product candidate in clinical development. We have no approved drug product and as a result have not generated any revenue from product sales to date and have incurred losses in each year since our inception in February 2008. We have only a limited operating history upon which you can evaluate our business and prospects. In addition, we have limited experience and have not yet demonstrated an ability to successfully overcome many of the risks and uncertainties frequently encountered by companies in new and rapidly evolving fields, particularly in the pharmaceutical industry. We continue to incur significant research and development and other expenses related to our ongoing operations. Our research and development costs for the fiscal years ended December 31, 2014, 2013 and 2012 were $45.6 million, $24.8 million and $7.0 million, respectively. Our net loss for the fiscal years ended December 31, 2014, 2013 and 2012 was approximately $64.0 million, $33.6 million and $10.3 million, respectively. As of December 31, 2014, we had an accumulated deficit of $114.3 million. We expect to continue to incur losses for the foreseeable future, and we anticipate these losses will increase as we continue our development of, seek regulatory approval for and begin commercialization of ZS-9. Accordingly, our ability to continue to develop and commercialize ZS-9 and our other product candidates depends on our ability to obtain additional financing to fund our operations and there can be no assurance that additional financing will be available to us or that such financing, if available, will be available on favorable terms. Even if we achieve profitability in the future, we may not be able to sustain profitability in subsequent periods.
We are substantially dependent on the success of our only product candidate in clinical development, ZS-9.
We have completed three clinical studies with ZS-9 that together enrolled 1,101 patients with hyperkalemia, including patients with CKD, HF, type 2 diabetes and those on RAAS inhibitor therapy. Our first in-man study, ZS002, was completed in May 2012 and our first Phase III study, ZS003, was completed in November 2013. Our second Phase III study, ZS004, was completed in September 2014. All three trials met their pre-specified primary efficacy endpoints with clinically meaningful and statistically significant results; ZS002 and ZS003 also met their pre-specified secondary endpoints and ZS004 met all but one pre-specified secondary endpoints. We presented the top-line results for ZS004 in September 2014 and detailed primary and secondary endpoint results for ZS004 during a session at the American Heart Association Scientific Sessions on November 17, 2014. The ZS004 study results were simultaneously published in the Journal of the American Medical Association, and we announced
44
Table of Contents
the publication of detailed results from the ZS003 study in the New England Journal of Medicine on November 21, 2014. We initiated an extension to the ZS004 study, or ZS004e, and a long-term safety study, ZS005, in the second quarter of 2014. We expect to file our NDA with the FDA in the second quarter of 2015 and our MAA with the EMA in the second half of 2015.
Our near-term prospects, including our ability to finance our operations and generate revenue, will depend heavily on the successful development and commercialization of ZS-9. The clinical and commercial success of ZS-9 will depend on a number of factors, including the following:
| • | our ability to demonstrate ZS-9’s safety and efficacy to the satisfaction of the FDA and regulatory bodies outside of the United States; |
| • | the timely completion of our ongoing clinical trials, which may be significantly slower than we currently anticipate and will depend substantially upon the satisfactory performance of third-party contractors; |
| • | our ability to obtain, and the timing of, regulatory approval of ZS-9 in the United States and outside the United States; |
| • | whether we are required by the FDA or similar foreign regulatory agencies to conduct clinical trials beyond those that we are presently conducting; |
| • | the prevalence and severity of adverse side effects of ZS-9; |
| • | our ability to successfully commercialize ZS-9, if approved for marketing and sale by the FDA or foreign regulatory authorities, either by ourselves in the United States with a specialty sales force or in collaboration with others outside the United States; |
| • | our ability to manufacture clinical trial and commercial supplies of ZS-9 at a scale sufficient to meet anticipated demand and reduce our cost of manufacturing, to maintain regulatory approval of our manufacturing process, and to develop, validate and maintain commercially viable manufacturing processes that are compliant with current Good Manufacturing Practice, or cGMP, regulations; |
| • | our success in educating physicians and patients about the benefits, administration and use of ZS-9; |
| • | achieving and maintaining compliance with all regulatory requirements applicable to ZS-9; |
| • | acceptance of ZS-9 as safe and effective by patients and the medical community; |
| • | the availability, perceived advantages, relative cost, relative safety and relative efficacy of alternative and competing treatments; |
| • | our ability to avoid and defend against third-party patent interference or patent infringement claims; |
| • | our ability to obtain and sustain an adequate level of reimbursement for ZS-9 by third-party payors; |
| • | our ability to obtain, maintain, enforce and defend our intellectual property rights relating to ZS-9 and to comply with our obligations under, and otherwise maintain, our intellectual property licenses with third parties; and |
| • | a continued acceptable safety profile of ZS-9 following approval. |
If we or any future partners are not successful in commercializing ZS-9, or are significantly delayed in doing so, our business will be materially harmed.
45
Table of Contents
We may be unable to obtain regulatory approval for ZS-9. The failure to obtain, or any delay in obtaining, any such approval would adversely impact our ability to generate revenue, our business and our results of operations.
To gain approval to market a drug product, we must provide the FDA and foreign regulatory authorities with clinical and nonclinical data that adequately demonstrates the safety and efficacy of the product for the intended indication applied for in the NDA or other regulatory filings. Drug development is a long, expensive and uncertain process, and delay or failure can occur at any stage of any of our clinical or nonclinical trials. A number of companies in the pharmaceutical industry, including biotechnology companies, have suffered significant setbacks in clinical trials even after promising results in earlier nonclinical or clinical trials. These setbacks have been caused by, among other things, nonclinical findings made while clinical trials were underway and safety or efficacy observations made in clinical trials, including previously unreported adverse events. Success in nonclinical testing and early clinical trials does not ensure that later clinical trials will be successful, and the results of clinical trials by other parties may not be indicative of the results in trials we may conduct.
We completed our second Phase III clinical trial for our lead product candidate, ZS-9, in September 2014, and our business currently depends entirely on its successful development, regulatory approval and commercialization. We currently have no drug products approved for sale, and we may never obtain regulatory approval to commercialize ZS-9. The research, testing, manufacturing, labeling, approval, sale, marketing and distribution of drug products are subject to extensive regulation by the FDA and other regulatory authorities in the United States and other countries, and such regulations differ from country to country. We are not permitted to market ZS-9 in the United States or in other countries until we receive approval of an NDA from the FDA or similar applicable foreign regulatory bodies, respectively.
The FDA or any foreign regulatory bodies can delay, limit or deny approval to market ZS-9 for many reasons, including:
| • | inability to demonstrate to the satisfaction of the FDA or the applicable foreign regulatory body that ZS-9 is safe and effective for the requested indication; |
| • | the FDA’s or the applicable foreign regulatory agency’s disagreement with the interpretation of data from nonclinical studies or clinical trials; |
| • | inability to demonstrate that the clinical and other benefits of ZS-9 outweigh any safety or other perceived risks; |
| • | the FDA’s or the applicable foreign regulatory agency’s requirement for additional nonclinical studies or clinical trials; |
| • | the FDA’s or the applicable foreign regulatory agency’s failure to approve the formulation of ZS-9 or the imposition of undesirable labeling limitations or requirements; |
| • | the FDA’s or the applicable foreign regulatory agency’s failure to approve our manufacturing processes; |
| • | the FDA and other applicable regulatory bodies having differing requirements for the trial protocols used in our clinical trials; or |
| • | the potential for approval policies or regulations of the FDA or the applicable foreign regulatory agencies to significantly change in a manner rendering our clinical data or regulatory filings insufficient for approval. |
Of the large number of drugs in development, only a small percentage successfully complete the FDA or other regulatory approval processes and are commercialized.
Even if we eventually receive approval of an NDA or foreign marketing authorization for ZS-9, the FDA or the applicable foreign regulatory agency may grant approval contingent on the performance of costly additional clinical trials, which may be required after approval. The FDA or the applicable foreign regulatory agency may
46
Table of Contents
also approve ZS-9 for a more limited indication and/or a narrower patient population than we originally request, and the FDA, or applicable foreign regulatory agency, may not approve the labeling that we believe is necessary or desirable for the successful commercialization of ZS-9. Any delay in obtaining, or inability to obtain, applicable regulatory approval would delay or prevent commercialization of ZS-9 and would materially adversely impact our business and prospects.
ZS-9, if approved, may face significant competition and our failure to effectively compete may prevent us from achieving significant market penetration.
The pharmaceutical market is highly competitive and dynamic, and is characterized by rapid and substantial technological development and product innovations. We intend to seek regulatory approval of ZS-9 for the treatment of hyperkalemia. While current options for the chronic management of hyperkalemia are limited, we expect to compete against well-known treatment options, including sodium polystyrene sulfonate, which is generically available. In addition, Relypsa is developing a hyperkalemia treatment, Patiromer, for which it has announced that it has completed its Phase III development program and for which it has submitted an NDA to the FDA that the FDA has accepted. If approved by the FDA, this treatment will likely reach the U.S. market before ZS-9, potentially giving Relypsa an advantage over us in marketing and gaining market acceptance. In order to compete successfully in this market, we will have to demonstrate that the treatment of hyperkalemia with ZS-9 is a superior alternative to existing or new therapies for hyperkalemia, including Patiromer. Even if we are able to demonstrate the superiority of our product, Relypsa or other competitors may attempt to lock us out of the market by entering into exclusive or preferred arrangements with insurers and other third party payors before our product is approved for sale, which may make it difficult for us to successfully gain market penetration for ZS-9. In addition, our competitors may attempt to undercut our pricing for ZS-9 once it is introduced, or we may need to undercut the pricing of Relypsa’s drug to gain market acceptance, which could also harm our results of operations.
We may face significant competition from pharmaceutical and biotechnology companies that are also researching and selling products designed to address these markets. Many of our potential competitors have materially greater financial, manufacturing, marketing, research and drug development resources than we do. Large pharmaceutical companies, in particular, have extensive expertise in nonclinical testing and clinical trials, in obtaining regulatory approvals for drugs and in commercializing drugs following approval. In addition, academic institutions, government agencies and other public and private organizations conducting research may seek patent protection with respect to potentially competitive products or technologies. These organizations may also establish exclusive collaborative or licensing relationships with our competitors. Failure to effectively compete against established or future treatment options for hyperkalemia would harm our business, financial condition and results of operations.
Clinical drug development is a lengthy and expensive process with an uncertain outcome, and results of earlier studies and trials may not be predictive of later trial results.
Clinical testing is expensive and can take many years to complete, and its outcome is inherently uncertain. Furthermore, we rely on clinical trial sites and, in connection with some of our clinical trials outside the United States, contract research organizations, or CROs, to ensure the proper and timely conduct of our clinical trials and, while we have agreements governing their committed activities, we have limited influence over their actual performance. Failure can occur at any time during the clinical trial process. The results of nonclinical studies and clinical trials of our product candidates may not be predictive of the results of later-stage clinical trials. For example, the positive results generated to date in clinical trials for ZS-9 do not ensure that our ongoing clinical trials will demonstrate similar results. Product candidates in later stages of clinical trials may fail to show the desired safety and efficacy, despite having progressed through nonclinical studies and earlier clinical trials. A number of companies in the pharmaceutical industry have suffered significant setbacks in advanced clinical trials due to inadequate efficacy or adverse safety profiles, notwithstanding promising results in earlier studies, and we cannot be certain that we will not face similar setbacks. Even if our clinical trials are completed, the results may not be sufficient to obtain regulatory approval for our product candidates.
47
Table of Contents
We may experience delays in our ongoing clinical trials, and we do not know whether future clinical trials, if any, will begin on time, require redesign, enroll an adequate number of patients on time or be completed on schedule, if at all. Clinical trials can be delayed or aborted for a variety of reasons, including delay or failure to:
| • | obtain regulatory approval to commence a trial, if applicable; |
| • | reach agreement on acceptable terms with prospective CROs and clinical trial sites, the terms of which can be subject to extensive negotiation and may vary significantly among different CROs and trial sites; |
| • | obtain institutional review board, or IRB, approval at each site; |
| • | recruit suitable patients to participate in a trial and have such patients complete the trial or return for post-treatment follow-up; |
| • | ensure that clinical sites observe trial protocol or continue to participate in a trial; |
| • | address any patient safety concerns that arise during the course of a trial; |
| • | address any conflicts with new or existing laws or regulations; |
| • | initiate or add a sufficient number of clinical trial sites; |
| • | manufacture sufficient quantities of product candidate or placebo for use in clinical trials; or |
| • | obtain regulatory approval of the statistical analysis plan to be used to evaluate our forthcoming clinical trial data. |
Patient enrollment is a significant factor in the timing of clinical trials and is affected by many factors, including the size and nature of the patient population, the proximity of patients to clinical sites, the eligibility criteria for the trial, the design of the clinical trial, competing clinical trials and physicians’ and patients’ perceptions as to the potential advantages of the drug being studied in relation to other available therapies, including any new drugs or treatments that may be approved for the indications we are investigating.
We could also encounter delays if a clinical trial is suspended or terminated by us, by the IRBs of the institutions in which such trials are being conducted, by an independent Data Monitoring Committee, or DMC, for such trial or by the FDA or other regulatory authorities. Such authorities may suspend or terminate a clinical trial due to a number of factors, including failure to conduct the clinical trial in accordance with regulatory requirements or our clinical protocols, inspection of the clinical trial operations or trial site by the FDA or other regulatory authorities resulting in the imposition of a clinical hold, unforeseen safety issues or adverse side effects, failure to demonstrate a benefit from using a drug, changes in governmental regulations or administrative actions or lack of adequate funding to continue the clinical trial. For example, in February 2011, the FDA issued a clinical hold, which remained in place for 11 months, on the basis that a No Observed Adverse Effect Level (NOAEL) had not been adequately established in our nonclinical studies.
Further, conducting clinical trials in foreign countries presents additional risks that may delay completion of our clinical trials. These risks include the failure of enrolled patients in foreign countries to adhere to clinical protocol as a result of differences in healthcare services or cultural customs, managing additional administrative burdens associated with foreign regulatory schemes, as well as political and economic risks relevant to such foreign countries.
If we experience delays in the completion of any clinical trial of ZS-9 or any future product candidates or such trials are terminated, the commercial prospects of ZS-9 or any future product candidates may be harmed, and our ability to generate revenues from any of these product candidates will be delayed. In addition, any delays in completing our clinical trials will increase our costs, decelerate our product candidate development and approval process and jeopardize our ability to commence product sales and generate revenues. Any of these occurrences may significantly harm our business, financial condition and prospects. In addition, many of the factors that cause, or lead to, a delay in the commencement or completion of clinical trials may also ultimately lead to the denial of regulatory approval of our product candidates.
48
Table of Contents
We will require substantial additional financing to achieve our goals, and a failure to obtain this necessary capital when needed, on acceptable terms, or at all, could force us to delay, limit, reduce or terminate our product development, other operations or commercialization efforts.
Since our inception, most of our resources have been dedicated to the nonclinical and clinical development of our lead product candidate, ZS-9. As of December 31, 2014, we had an accumulated deficit of $114.3 million and capital resources consisting of cash and cash equivalents of $47.4 million and short-term investments of $54.9 million. In addition, we also had $10.0 million available to us under the MidCap Credit Facility, as defined in the section entitled “Management’s Discussion and Analysis of Financial Condition and Results of Operations — Liquidity and Capital Resources.” We believe that we will continue to expend substantial resources for the foreseeable future as we continue clinical development, seek regulatory approval, expand our manufacturing capability, prepare for the commercialization of ZS-9 and develop any other product candidates we may choose to pursue. These expenditures will include costs associated with research and development, sales and marketing, conducting nonclinical studies and clinical trials, seeking regulatory approvals, and developing our manufacturing facility and supply arrangements. In addition, other unanticipated costs may arise. Because the outcome of any clinical trial and/or regulatory approval process is highly uncertain, we cannot reasonably estimate the actual amounts necessary to successfully complete the development, regulatory approval process and commercialization of ZS-9 or any future product candidates.
We believe that our existing cash, cash equivalents, short-term investments and available borrowings under our MidCap Credit Facility will allow us to fund our operating plan through at least December 31, 2015. However, our operating plan may change as a result of many factors currently unknown to us, and we may need to seek additional funds sooner than planned, through public or private equity, debt financings or other sources, such as strategic collaborations. Such financing may result in dilution to stockholders, imposition of debt covenants and repayment obligations, or other restrictions that may affect our business. In addition, we may seek additional capital due to favorable market conditions or strategic considerations even if we believe we have sufficient funds for our current or future operating plans.
Our future funding requirements will depend on many factors, including, but not limited to:
| • | the time and cost necessary to obtain regulatory approvals for ZS-9 and the costs of post-marketing studies that could be required by regulatory authorities; |
| • | whether, where and when we receive regulatory approval for ZS-9 and our ability to successfully commercialize ZS-9 if approved by regulatory authorities; |
| • | the time and cost necessary to respond to technological and market developments; |
| • | the costs of preparing, filing and prosecuting patent applications, maintaining and enforcing our intellectual property rights and defending intellectual property-related claims; |
| • | the manufacturing, selling and marketing costs associated with ZS-9, including the cost and timing of expanding our manufacturing and sales and marketing capabilities; |
| • | the amount of sales and other revenues from ZS-9, including the sales price and the availability of adequate third-party reimbursement; |
| • | the cash requirements of any future acquisitions or discovery of product candidates; and |
| • | the progress, timing, scope and costs of our nonclinical studies and clinical trials, including the ability to enroll patients in a timely manner for ongoing or potential future clinical trials. |
Additional funds may not be available when we need them, on terms that are acceptable to us, or at all. If adequate funds are not available to us on a timely basis, we may be required to delay, limit, reduce or terminate:
| • | clinical trials or other development activities for ZS-9 or any future product candidate; |
| • | our research and development activities; or |
49
Table of Contents
| • | our establishment of sales and marketing capabilities, expansion of our manufacturing capacity or other activities that may be necessary to commercialize ZS-9 or any future product candidate. |
Our clinical drug development program may not uncover all possible adverse events that patients who take ZS-9 may experience. The number of patients exposed to ZS-9 treatment and the average exposure time in the clinical development program may be inadequate to detect rare adverse events, or chance findings, that may only be detected once ZS-9 is administered to more patients and for greater periods of time.
Clinical trials by their nature utilize a sample of the potential patient population. However, given the relatively limited longer-term exposure of patients to ZS-9 to date, rare and severe side effects of ZS-9 may be uncovered when a significantly larger number of patients are exposed to the drug or exposure over a longer period of time. Further, we have not designed our clinical trials to determine the effect and safety consequences of lowering serum potassium on total body potassium levels over a multi-year period, nor have we designed our clinical trials to measure the effect of introducing patients to, or increasing a patient’s dosage of, RAAS inhibitors while taking ZS-9.
Although we have monitored the patients in our studies for certain safety concerns and we have not seen evidence of significant safety concerns in our clinical trials, patients treated with ZS-9, if approved, may experience adverse reactions. For example, we have seen some GI events and other adverse events, such as nausea, vomiting, constipation and diarrhea, in our clinical trials. It is possible that the FDA may ask for additional data regarding such matters. If safety problems occur or are identified after ZS-9 reaches the market, the FDA may require that we amend the labeling of, recall, or even withdraw approval for, ZS-9.
Even if ZS-9 or any future product candidates obtain regulatory approval, they may never achieve market acceptance or commercial success, which will depend, in part, upon the degree of acceptance among physicians, patients, patient advocacy groups, health care payors and the medical community.
Even if we obtain FDA or other regulatory approvals, ZS-9 or any future product candidates may not achieve market acceptance among physicians and patients, and may not be commercially successful. ZS-9 may not gain market acceptance among physicians, patients, patient advocacy groups, health care payors and the medical community. Market acceptance of ZS-9 or any future product candidates for which we receive regulatory approval depends on a number of factors, including:
| • | the efficacy of the product as demonstrated in clinical trials; |
| • | the prevalence and severity of any side effects and overall safety profile of the product; |
| • | the clinical indications for which the product is approved; |
| • | advantages over existing therapies or therapies under development, such as, in the case of ZS-9, SPS and Patiromer; |
| • | acceptance by physicians, operators of clinics and patients of the product as safe and effective; |
| • | relative convenience and ease of administration of ZS-9; |
| • | the potential and perceived advantages of ZS-9 over current treatment options or alternative treatments, including future alternative treatments; |
| • | the cost of treatment in relation to alternative treatments and willingness to pay for our products, if approved, on the part of third-party payors and patients; |
| • | the availability of ZS-9, if approved, or any future products and their ability to meet market demand, including a reliable supply for long-term daily treatment; |
| • | the strength of our marketing and distribution organizations; and |
| • | the quality of our relationships with patient advocacy groups. |
Any failure by ZS-9 or any future product candidates to achieve market acceptance or commercial success upon receiving regulatory approval would adversely affect our results of operations.
50
Table of Contents
We plan to manufacture all of our clinical and commercial drug supply of ZS-9, which could be costly and subject to regulatory or other delays.
Although we have the infrastructure and internal capability required to produce our clinical drug supply of ZS-9 in our two manufacturing facilities, we are currently in the process of expanding the infrastructure and internal capability required to produce our commercial supply of ZS-9. We must obtain approval from the FDA and other comparable foreign regulatory agencies, pursuant to inspections that will be conducted after we submit our NDA or relevant foreign regulatory submission, to manufacture the active pharmaceutical ingredient and final drug for ZS-9.
We must comply with cGMP regulations for manufacture of both active drug substances and finished drug products. If we cannot successfully manufacture material that conforms to our specifications and the strict regulatory requirements of the FDA or foreign regulatory agencies, we will not be able to secure and/or maintain regulatory approval for our manufacturing facilities. In addition, we must maintain adequate quality control, quality assurance and qualified personnel and our manufacturing equipment must meet certain qualifications. If the FDA or a comparable foreign regulatory agency does not approve our facilities for the manufacture of our product candidates or if it withdraws its approval in the future, we may need to modify our manufacturing process or facilities or find alternative manufacturing facilities, which would be costly and could negatively impact our ability to develop, obtain regulatory approval for and market ZS-9, if approved.
We continue to refine and improve the manufacturing process for ZS-9, certain aspects of which are complex and unique, and we may encounter difficulties with new or existing processes, particularly as we seek to significantly increase our capacity to commercialize ZS-9.
Additionally, if our manufacturing facilities were to be damaged, destroyed or otherwise unable to operate, whether due to earthquakes, fire, floods, hurricanes, storms, tornadoes, other natural disasters, employee malfeasance, terrorist acts, power outages or otherwise, or if performance of our manufacturing facility is disrupted for any other reason, such an event could delay our clinical trials or, if ZS-9 or any future product candidates are approved, jeopardize our ability to manufacture our products as promptly as our customers expect or at all. If we are unable to manufacture a product candidate or approved product within a timeframe that meets our clinical development needs or customers’ expectations, our business, prospects, results of operations and reputation could be materially harmed.
We have limited experience manufacturing ZS-9 at our anticipated commercial scale. We will face certain risks associated with scaling up our manufacturing capabilities to support anticipated commercial demand.
We have developed two manufacturing facilities to manufacture drug substance, which we use for research and development purposes and for clinical trials of ZS-9. We installed a 2,000 liter commercial scale reactor during the second quarter of 2014 and have successfully manufactured drug substances in this reactor. We also received delivery of a second 2,000 liter commercial scale reactor in the fourth quarter of 2014. To meet our strategic objectives, which contemplate internally manufacturing all of our drug substance to support anticipated commercial demand if ZS-9 is approved, we will need to install additional reactors, dryers and other equipment, add manufacturing personnel and ensure that validated processes are consistently implemented in our facilities. In particular, we currently intend to eventually transition to 5,000 liter reactors for the commercial production of ZS-9. We have not yet attempted to use a 5,000 liter reactor to produce ZS-9 and there can be no assurance we will be able to successfully do so. Should we encounter unexpected difficulties in transitioning to these larger reactors, we may be forced to rely on smaller 2,000 liter reactors, which could increase our expected costs.
Any future additions of reactors and further upgrade and expansion of our facilities may require additional regulatory approvals. In addition, it will be costly and time-consuming to install additional equipment, expand our facilities and recruit necessary additional personnel. For example, we are currently dependent on one supplier for our reactors. If we were unable to obtain additional reactors from our current supplier, we would incur additional costs and experience delays in connection with securing a new supplier. If we are unable to add
51
Table of Contents
reactors and expand our manufacturing capabilities in compliance with regulatory requirements or to hire additional necessary manufacturing personnel, we may encounter delays or additional costs in achieving our research, development and commercialization objectives, including obtaining regulatory approvals of ZS-9, which could materially damage our business and financial position.
Manufacture and supply of drug substance, drug product and finished drug product is a complex and technically challenging undertaking, and there is potential for failure at many points in the manufacturing, testing, quality assurance and distribution supply chain, as well as the potential for latent defects after a product has been manufactured and distributed.
Manufacture and supply of drug substance, drug product and finished drug product is technically challenging. Changes that may be made outside the purview of our direct control can have an impact on the success of our processes, on quality, and on successful delivery of product. Mistakes and mishandling could affect successful production and supply. Some of these risks include:
| • | failure to follow cGMP requirements including adequate recordkeeping, or mishandling of our product while in production or in preparation for transit; |
| • | delays in analytical results or failure of analytical techniques that we depend on for quality control and release of drug product; |
| • | natural disasters, labor disputes, lack of raw material supply, issues with facilities and equipment or other forms of disruption to business operations at our manufacturing facilities; and |
| • | latent defects that may become apparent after drug product has been released and which may result in recall or required destruction of drug product. |
If any of these risks materialize it would have a material and adverse impact on our ability to develop, obtain regulatory approval for and market ZS-9, if approved.
We rely to some extent on third parties to conduct some of our nonclinical studies and our clinical trials. If these third parties do not successfully carry out their contractual duties or meet expected deadlines, we may be unable to obtain regulatory approval for or commercialize ZS-9 or any future product candidates.
We do not have the ability to independently conduct clinical trials and, in some cases, nonclinical studies. We rely on medical institutions, clinical investigators, contract laboratories and other third parties, such as CROs, to conduct clinical trials on our drug candidates. The third parties with whom we contract for execution of our clinical trials play a significant role in the conduct of these trials and the subsequent collection and analysis of data. However, these third parties are not our employees, and except for contractual duties and obligations, we have limited ability to control the amount or timing of resources that they devote to our programs. Although we rely on these third parties to conduct some of our nonclinical studies and all of our clinical trials, we remain responsible for ensuring that each of our nonclinical studies and clinical trials is conducted in accordance with its investigational plan and protocol. Moreover, the FDA and foreign regulatory authorities require us to comply with regulations and standards, including some regulations commonly referred to as good clinical practices, or cGCPs, for conducting, monitoring, recording and reporting the results of clinical trials to ensure that the data and results are scientifically credible and accurate, and that the trial patients are adequately informed of the potential risks of participating in our clinical trials.
In addition, the execution of nonclinical studies and clinical trials, and the subsequent compilation and analysis of the data produced, requires coordination among various parties. In order for these functions to be carried out effectively and efficiently, it is imperative that these parties communicate and coordinate with one another. Moreover, these third parties may also have relationships with other commercial entities, some of which may compete with us. These third parties may terminate their agreements with us upon as little as 30 days’ prior written notice. Some of these agreements may also be terminated by such third parties under certain other
52
Table of Contents
circumstances. If the third parties conducting our clinical trials do not perform their contractual duties or obligations, experience work stoppages, do not meet expected deadlines, terminate their agreements with us or need to be replaced, or if the quality or accuracy of the clinical data they obtain is compromised due to the failure to adhere to our clinical trial protocols or GCPs, or for any other reason, we may need to enter into new arrangements with alternative third parties, which could be difficult, costly or impossible, and our clinical trials may be extended, delayed or terminated or may need to be repeated. If any of the foregoing were to occur, we may not be able to obtain regulatory approval for or commercialize ZS-9 or any future product candidate being tested in such trials.
We currently have no sales organization. If we are unable to establish sales capabilities on our own or through third parties, we may not be able to market and sell ZS-9, if approved, or any future product candidates or generate product revenue.
We currently do not have a sales organization. In order to commercialize ZS-9 in the United States, we intend to build our marketing, sales, distribution, managerial and other non-technical capabilities or make arrangements with third parties to perform these services, and we may not be successful in doing so. We expect to establish a specialty sales organization with technical expertise and distribution capabilities to commercialize ZS-9, if approved, which will be expensive and time consuming. There are significant risks involved in building and managing a sales organization, including our ability to hire, retain, and incentivize qualified individuals, generate sufficient sales leads, provide adequate training to sales and marketing personnel, and effectively manage a geographically dispersed sales and marketing team. Any failure or delay in the development of our internal sales, marketing and distribution capabilities would adversely impact the commercialization of these products.
In both the United States and internationally, we may choose to collaborate with third parties that have direct sales forces and established distribution systems, either to augment our own sales force and distribution systems or in lieu of our own sales force and distribution systems. If we are unable to enter into such arrangements on acceptable terms or at all, we may not be able to successfully commercialize ZS-9. If we are not successful in commercializing ZS-9 or any future product candidates, either on our own or through collaborations with one or more third parties, our future product revenue will suffer and we would incur significant additional losses.
If we fail to establish an effective packaging and distribution process for ZS-9, our business may be adversely affected.
Packaging and final drug product formation is currently performed by Sharp. We do not have a long term agreement with Sharp, or any other final drug product producer, to produce ZS-9 once approved by the FDA. Failure to secure an effective arrangement with Sharp or another final drug product producer, or the failure of Sharp or any other final drug product producer we may work with to perform as expected, could negatively impact the availability of ZS-9. If we are unable to effectively establish and manage the packaging process, the commercial launch and sale of ZS-9, if approved, will be delayed or severely compromised and our results of operations may be harmed.
Similarly, we do not currently have the infrastructure necessary for distributing pharmaceutical products to patients. We intend to contract with a third-party logistics company to warehouse these products and distribute them. This distribution network will require significant coordination with our manufacturing, sales and marketing and finance teams. Failure to secure an effective arrangement with a logistics company, or the failure of that logistics company to perform as expected, could negatively impact the distribution of ZS-9, and failure to coordinate financial systems could negatively impact our ability to accurately report product revenue. If we are unable to effectively establish and manage the distribution process, the commercial launch and sales of ZS-9, if approved, will be delayed or severely compromised and our results of operations may be harmed.
53
Table of Contents
If we fail to obtain and sustain an adequate level of reimbursement for our products by third-party payors, sales would be adversely affected.
We expect patients who have hyperkalemia to need treatment throughout their lifetimes but anticipate that most patients would not be capable of paying for this treatment themselves absent reimbursement by third-party payors, including Medicare. Additionally, if the level of reimbursement is below our expectations, our revenue and gross margins would be adversely affected.
Obtaining formulary approval from third-party payors can be an expensive and time-consuming process. We cannot be certain if and when we will obtain formulary approval to allow us to sell ZS-9 or any future products into our target markets. Even if we do obtain formulary approval, third-party payors, such as government or private health care insurers, carefully review and increasingly question the coverage of, and challenge the prices charged for, drugs. Reimbursement rates from private health insurance companies vary depending on the company, the insurance plan and other factors. Reimbursement systems in international markets vary significantly by country and by region, and reimbursement approvals must be obtained on a country-by-country basis. Cost containment has been an increasing trend in the U.S. health care industry. Large public and private payors, managed care organizations, group purchasing organizations and similar organizations are exerting increasing influence on decisions regarding the use of, and reimbursement levels for, particular treatments. Such third-party payors are questioning the coverage of, and challenging the prices charged for medical products and services, and many third-party payors limit coverage of, or reimbursement for, newly approved health care products.
Cost-control initiatives could decrease the price we might establish for products, which could result in product revenues being lower than anticipated. If the prices for our products decrease or if governmental and other third-party payors do not provide adequate coverage and reimbursement levels, our revenue and prospects for profitability will suffer.
If product liability lawsuits are brought against us, we may incur substantial liabilities and may be required to limit or cease commercialization of ZS-9 or any future product candidates.
We face an inherent risk of product liability as a result of the clinical testing of our product candidates and will face an even greater risk if we commercialize any products. For example, we may be sued if any product we develop allegedly causes injury or is found to be otherwise unsuitable during product testing, manufacturing, marketing or sale. Any such product liability claims may include allegations of defects in manufacturing, defects in design, a failure to warn of dangers inherent in the product, negligence, strict liability, or breach of warranty. Claims could also be asserted under state consumer protection acts. If we cannot successfully defend ourselves against product liability claims, we may incur substantial liabilities or be required to limit or cease commercialization of ZS-9 or any future product candidates.
Even successful defense would require significant financial and management resources. Regardless of the merits or eventual outcome, liability claims may result in:
| • | decreased demand for ZS-9 or any future product candidates; |
| • | injury to our reputation; |
| • | withdrawal of clinical trial participants; |
| • | costs to defend the related litigation; |
| • | a diversion of management’s time and our resources; |
| • | substantial monetary awards to trial participants or patients; |
| • | regulatory investigations, product recalls or withdrawals, or labeling, marketing or promotional restrictions; |
54
Table of Contents
| • | loss of revenue; and |
| • | the inability to commercialize ZS-9 or any future product candidates. |
Our inability to obtain and maintain sufficient product liability insurance at an acceptable cost and scope of coverage to protect against potential product liability claims could prevent or inhibit the commercialization of ZS-9 or any future products we develop. We currently carry product liability insurance covering use of ZS-9 in our clinical trials in the amount of $3.0 million in the aggregate. Although we maintain such insurance, any claim that may be brought against us could result in a court judgment or settlement in an amount that is not covered, in whole or in part, by our insurance or that is in excess of the limits of our insurance coverage. Our insurance policies also have various exclusions and deductibles, and we may be subject to a product liability claim for which we have no coverage. We will have to pay any amounts awarded by a court or negotiated in a settlement that exceed our coverage limitations or that are not covered by our insurance, and we may not have, or be able to obtain, sufficient capital to pay such amounts. Moreover, in the future, we may not be able to maintain insurance coverage at a reasonable cost or in sufficient amounts to protect us against losses. If and when we obtain approval for marketing ZS-9, we intend to expand our insurance coverage to include the sale of ZS-9. However, we may be unable to obtain this liability insurance on commercially reasonable terms.
We will need to significantly increase the size of our organization, and we may experience difficulties in managing growth.
As of December 31, 2014, we had 71 full-time employees. We will need to continue to expand our managerial, operational, finance and other resources in order to manage our operations, regulatory filings, manufacturing and supply activities, marketing and commercialization activities, and clinical trials, and commercialize ZS-9 or any future product candidates. Our management, personnel, systems and facilities currently in place may not be adequate to support this future growth. Our need to effectively execute our growth strategy requires that we:
| • | expand our general and administrative, medical, manufacturing and sales and marketing organizations; |
| • | identify, recruit, retain, incentivize and integrate additional employees; |
| • | manage our internal development efforts effectively while carrying out our contractual obligations to third parties; and |
| • | continue to improve our operational, regulatory, legal, financial and management controls, reporting systems and procedures. |
If we fail to attract and retain senior management, we may be unable to successfully develop ZS-9 or any future product candidates, conduct our clinical trials and commercialize ZS-9 or any future product candidates.
Our success depends in part on our continued ability to attract, retain and motivate highly qualified personnel. In particular, we are highly dependent on our senior management, including Dr. Alvaro Guillem and Dr. Jeffrey Keyser, who have substantial experience with our operations, clinical development programs and manufacturing processes. The loss of services of any of our senior management could delay or prevent the successful development of our product pipeline, completion of our planned clinical trials or the commercialization of ZS-9 or any future product candidates.
Competition for qualified personnel in the biotechnology and pharmaceuticals field is intense due to the limited number of individuals who possess the skills and experience required by our industry. We will need to hire additional personnel as we expand our clinical development, manufacturing and commercial activities. We may not be able to attract and retain quality personnel on acceptable terms, or at all. In addition, to the extent we hire personnel from competitors, we may be subject to allegations that they have been improperly solicited or that they have divulged proprietary or other confidential information, or that their former employers own their research output.
55
Table of Contents
We recently began incurring significant costs as a result of becoming a public company, which will likely increase in the future, and our management is now required to devote substantial time to new compliance initiatives and corporate governance practices.
We recently began incurring significant legal, accounting and other expenses as a public company, including costs resulting from public company reporting obligations under the Securities Exchange Act of 1934, as amended, or the Exchange Act, and regulations regarding corporate governance practices. The listing requirements of The Nasdaq Global Market require that we satisfy certain corporate governance requirements relating to director independence, distributing annual and interim reports, stockholder meetings, approvals and voting, soliciting proxies, conflicts of interest and a code of conduct. Our management and other personnel are devoting a substantial amount of time to ensure that we comply with all of these requirements. Any further changes we make to comply with these obligations may not be sufficient to allow us to satisfy our obligations as a public company on a timely basis, or at all. These reporting requirements, rules and regulations, coupled with an increase in potential litigation exposure associated with being a public company, could also make it more difficult for us to attract and retain qualified persons to serve on our board of directors or board committees or to serve as executive officers, or to obtain certain types of insurance, including directors’ and officers’ insurance, on acceptable terms, in the future.
We are subject to Section 404 of the Sarbanes-Oxley Act of 2002, or Section 404, and the related rules of the Securities and Exchange Commission, or SEC, which generally require our management and independent registered public accounting firm to report on the effectiveness of our internal controls over financial reporting. Beginning with the second annual report that we will be required to file with the SEC, Section 404 requires an annual management assessment of the effectiveness of our internal controls over financial reporting. However, for so long as we remain an emerging growth company as defined in the Jumpstart Our Business Startups Act, or JOBS Act, we intend to take advantage of certain exemptions from various reporting requirements that are applicable to public companies that are not emerging growth companies, including, but not limited to, not being required to comply with the auditor attestation requirements of Section 404. Once we are no longer an emerging growth company or, if prior to such date, we opt to no longer take advantage of the applicable exemption, we will be required to include an opinion from our independent registered public accounting firm on the effectiveness of our internal controls over financial reporting. Obtaining this opinion will likely force us to incur significant expenses and consume significant management resources. We will remain an emerging growth company until the earlier of (1) December 31, 2019, (2) the last day of the fiscal year in which we have total annual gross revenue of at least $1.0 billion, (3) the date on which we are deemed to be a large accelerated filer, which means the market value of our common stock that is held by non-affiliates exceeds $700 million as of the prior June 30th, and (4) the date on which we have issued more than $1.0 billion in non-convertible debt during the prior three-year period.
If we have a material weakness in our internal controls over financial reporting, we may not detect errors on a timely basis and our financial statements may be materially misstated. We or our independent registered public accounting firm may not be able to conclude on an ongoing basis that we have effective internal controls over financial reporting, which could harm our operating results, cause investors to lose confidence in our reported financial information and cause the trading price of our stock to fall. In addition, as a public company we are required to file accurate and timely quarterly and annual reports with the SEC under the Exchange Act. Any failure to report our results of operations on an accurate and timely basis could result in sanctions, lawsuits, delisting of our shares from The Nasdaq Global Market or other adverse consequences that would materially harm our business.
If we are not successful in discovering, developing, acquiring or commercializing additional product candidates, our ability to expand our business and achieve our strategic objectives would be impaired.
Although a substantial amount of our effort will focus on the continued clinical testing, potential approval and commercialization of ZS-9, a key element of our strategy is to discover, develop and commercialize a portfolio of products utilizing proprietary zirconium silicate technology. We are seeking to do so through our
56
Table of Contents
internal research programs and/or by selectively pursuing commercially synergistic in-licensing or acquisition of additional compounds that would be compatible with ZS-9 or any other future product candidates we may have. Research programs to identify product candidates require substantial technical, financial and human resources, whether or not any product candidates are ultimately identified. Our research programs may initially show promise in identifying potential product candidates, yet fail to yield product candidates for clinical development for many reasons, including the following:
| • | the research methodology used may not be successful in identifying potential product candidates; |
| • | competitors may develop alternatives that render our product candidates obsolete or less attractive; |
| • | product candidates we develop may be covered by third parties’ patents or other exclusive rights; |
| • | the market for a product candidate may change during our program so that it may become impractical or uneconomical to continue to develop such a product candidate; |
| • | a product candidate may on further study be shown to have harmful side effects or other characteristics that indicate it is unlikely to be effective or otherwise does not meet applicable regulatory criteria; |
| • | a product candidate may not be capable of being produced in commercial quantities at an acceptable cost, or at all; and |
| • | a product candidate may not be accepted as safe and effective by patients, the medical community or third-party payors, if applicable. |
If we fail to develop and successfully commercialize other product candidates, our business and future prospects may be harmed and our business will be more vulnerable to any problems that we encounter in developing and commercializing ZS-9.
Any partnership arrangements that we may enter into in the future may not be successful, which could adversely affect our ability to commercialize ZS-9, and potential future product candidates, outside the United States.
We may seek partnerships for the commercialization of ZS-9 or potential future product candidates outside the United States. We may enter into these arrangements on a selective basis depending on the merits of retaining commercialization rights for ourselves as compared to entering into selective partnership arrangements for our product candidates internationally. We will face significant competition in seeking appropriate partners. Moreover, partnership arrangements are complex and time consuming to negotiate, document and implement. We may not be successful in our efforts to establish and implement partnerships or other alternative arrangements should we choose to pursue such arrangements. The terms of any partnerships or other arrangements that we may establish may not be favorable to us. For example, we could grant exclusive rights to our partners which could prevent us from partnering with others.
Any future partnerships that we enter into may not be successful. The success of our partnership arrangements will depend heavily on the efforts and activities of our partners, who may have significant discretion in determining the efforts and resources that they apply to these partnerships. In addition, partners may not properly maintain or defend our intellectual property rights, may infringe third-party intellectual property rights, may misappropriate our trade secrets or may otherwise use our proprietary information in such a way as to invite litigation that could jeopardize or invalidate our intellectual property or proprietary information or expose us to litigation and potential liability.
Disagreements between parties to a partnership arrangement regarding commercialization matters and proprietary rights can lead to delays in commercialization of the applicable product candidate and, in some cases, termination of the partnership arrangement. These disagreements can be difficult to resolve if neither party has final decision-making authority.
57
Table of Contents
Partnerships with third parties often are terminated or allowed to expire by the other party. Any such termination or expiration would adversely affect us financially and could harm our business reputation.
If we fail to comply with our obligations in the agreements under which we in-license or acquire development or commercialization rights to products or technology from third parties, we could lose commercial rights that are important to our business and could be prevented from commercializing our product candidates.
We are party to a license agreement with UOP that we depend on for certain rights related to ZS-9. Under our license agreement with UOP, we are subject to various obligations, including royalty payment obligations on net sales of ZS-9 or other product candidates or related technologies made by us or our sublicensees to the extent they are covered by the agreement and indemnification obligations. In addition, we may, in the future, enter into other agreements, including license agreements, that may impose diligence, milestone payment, royalty, insurance and other obligations on us. If we fail to comply with these obligations or otherwise breach our license or other agreements, the counterparty may have the right to terminate the applicable agreement in whole or part, in which event we might not be able to market any product that is covered by these agreements, which could materially adversely affect the value of the product candidate being developed under any such license agreement. The loss of our license agreement with UOP could materially affect our ability to proceed with the development and commercialization of ZS-9, which could have a material adverse effect on our operating results and overall financial condition. Termination of these license agreements, or the reduction or elimination of our licensed rights thereunder, may result in our having to negotiate new or restated agreements that may contain less favorable terms or may not be available at all, or cause us to lose rights in important intellectual property or technology.
If we engage in acquisitions or in-licensing, we will incur a variety of costs and we may never realize the anticipated benefits of such acquisitions or in-licensing.
We may attempt to acquire businesses, technologies, services, products or product candidates that we believe are a strategic fit with our business. If we do undertake any acquisitions or in-licensing, the process of integrating an acquired business, technology, service, product or product candidates into our business may result in unforeseen operating difficulties and expenditures, including diversion of resources and management’s attention from our core business. In addition, we may fail to retain key executives and employees of the companies we acquire, which may reduce the value of the acquisition or give rise to additional integration costs. Future acquisitions could result in additional issuances of equity securities that would dilute the ownership of existing stockholders. Acquisitions and in-licensing could also result in the incurrence of debt, contingent liabilities or the amortization of expenses related to other intangible assets, any of which could adversely affect our operating results. In addition, we may fail to realize the anticipated benefits of any acquisition or in-license.
If we obtain approval to commercialize ZS-9 outside of the United States, a variety of risks associated with international operations could materially and adversely affect our business.
If ZS-9 is approved for commercialization outside the United States, we will be subject to additional risks related to entering into these international business relationships, including:
| • | differing U.S. and foreign drug import and export rules and regulatory requirements; |
| • | reduced protection for intellectual property rights in certain foreign countries; |
| • | unexpected changes in tariffs, trade barriers and regulatory requirements; |
| • | different reimbursement systems, and different competitive drugs indicated to treat hyperkalemia; |
| • | economic weakness, including inflation, or political instability in particular foreign economies and markets; |
| • | compliance with tax, employment, immigration and labor laws for employees living or traveling abroad; |
58
Table of Contents
| • | foreign taxes, including income taxes, indirect taxes, payroll taxes and withholding taxes; |
| • | foreign currency fluctuations, which could result in increased operating expenses and reduced revenues, and other obligations incident to doing business in another country; |
| • | workforce uncertainty in countries where labor unrest is more common than in the United States; |
| • | production shortages resulting from any events affecting raw material supply or manufacturing capabilities abroad; |
| • | potential liability resulting from development work conducted by these distributors; and |
| • | business interruptions resulting from geopolitical actions, including war and terrorism, or natural disasters. |
Our business involves the use of hazardous materials and we and our third-party suppliers must comply with environmental laws and regulations, which can be expensive and restrict how we do business.
Our research and development and manufacturing activities, as well as the activities of our third-party suppliers, involve the controlled storage, use and disposal of hazardous materials, including the components of our product and product candidates and other hazardous compounds. We and our suppliers are subject to laws and regulations governing the use, manufacture, storage, handling and disposal of these hazardous materials. In some cases, these hazardous materials and various wastes resulting from their use are stored at our manufacturing facilities pending their use and disposal. We cannot eliminate the risk of contamination, which could cause an interruption of our commercialization efforts, research and development efforts and business operations or environmental damage resulting in costly clean-up and liabilities under applicable laws and regulations governing the use, storage, handling and disposal of these materials and specified waste products. Although we believe that the safety procedures utilized by us and our third-party suppliers for handling and disposing of these materials generally comply with the standards prescribed by these laws and regulations, we cannot guarantee that this is the case or eliminate the risk of accidental contamination or injury from these materials. In such an event, we may be held liable for any resulting damages and such liability could exceed our resources and state or federal or other applicable authorities may curtail our use of certain materials and/or interrupt our business operations. Furthermore, environmental laws and regulations are complex, change frequently and have tended to become more stringent. We cannot predict the impact of such changes and cannot be certain of our future compliance.
We or the third parties upon whom we depend may be adversely affected by earthquakes, tornadoes, hurricanes or other natural disasters and our business continuity and disaster recovery plans may not adequately protect us from a serious disaster.
Natural disasters could severely disrupt our operations, and have a material adverse effect on our business, results of operations, financial condition and prospects. If a natural disaster, power outage or other event occurred that prevented us from using all or a significant portion of our offices, damaged critical infrastructure or disrupted operations, it may be difficult or, in certain cases, impossible for us to continue our business for a substantial period of time. The disaster recovery and business continuity plans we have in place currently are limited and are unlikely to prove adequate in the event of a serious disaster or similar event. We may incur substantial expenses as a result of the limited nature of our disaster recovery and business continuity plans, which could have a material adverse effect on our business.
Furthermore, integral parties in our supply chain are operating from single sites, increasing their vulnerability to natural disasters or other sudden, unforeseen and severe adverse events. If such an event were to affect our supply chain, it could have a material adverse effect on our business.
59
Table of Contents
We are currently expanding and improving our information technology systems. If these implementations are not successful, our business and operations could be disrupted and our operating results could be harmed.
We are currently expanding and improving our information technology systems to assist us in the management of our business. The implementation of new software management platforms requires significant time, support and cost. We cannot be sure that these expanded systems will be fully or effectively implemented on a timely basis, if at all. Although our existing systems may be satisfactory in the short term, we do not believe these systems are adequate to support our long-term growth. Thus, if we are not able to implement these new systems successfully, our business, prospects, financial condition and results of operations could be harmed. In addition, the new systems may not operate as we expect them to, and we may be required to expend significant resources to correct problems or find alternative sources for performing these functions.
Our information technology systems, or those of our CROs or other contractors or consultants, may fail or suffer security breaches, which could result in a material disruption of our drug development programs.
Despite the implementation of security measures, our information technology systems and those of our CROs and other contractors and consultants are vulnerable to damage from computer viruses, unauthorized access, natural disasters, terrorism, war and telecommunication and electrical failures. While we have not experienced any such system failure, accident or security breach to date, if such an event were to occur and cause interruptions in our operations, it could result in a material disruption of our drug development programs. For example, the loss of clinical trial data from completed or ongoing clinical trials for any of our product candidates could result in delays in our regulatory approval efforts and significantly increase our costs to recover or reproduce the data. To the extent that any disruption or security breach were to result in a loss of or damage to our data or applications, or inappropriate disclosure of confidential or proprietary information, we could incur liability, our competitive position could be adversely affected, our reputation could be harmed and the further development of our product candidates could be delayed.
Risks Related to Government Regulation
The regulatory approval process is highly uncertain and we may not obtain regulatory approval for the commercialization of ZS-9 or any future product candidates.
The research, testing, manufacturing, labeling, approval, selling, import, export, marketing and distribution of drug products are subject to extensive regulation by the FDA and other regulatory authorities in the United States and other countries, which regulations differ from country to country.
Neither we nor any future collaboration partner is permitted to market ZS-9 or any future product candidate in the United States until we receive approval of an NDA from the FDA. We have not submitted an application or obtained marketing approval for ZS-9 anywhere in the world. Obtaining regulatory approval of an NDA can be a lengthy, expensive and uncertain process. In addition, failure to comply with FDA and other applicable U.S. and foreign regulatory requirements may subject us to administrative or judicially imposed sanctions or other actions, including:
| • | warning letters or an injunction; |
| • | civil and criminal penalties; |
| • | injunctions; |
| • | withdrawal of regulatory approval of products; |
| • | product recalls; |
| • | total or partial suspension of production; |
| • | refusal to approve pending NDAs or supplements to approved NDAs; and |
| • | restrictions on operations, including costly new manufacturing requirements. |
60
Table of Contents
Prior to obtaining approval to commercialize a drug candidate in the United States or abroad, we or our partners must demonstrate with substantial evidence from well-controlled clinical trials, and to the satisfaction of the FDA or other foreign regulatory agencies, that such drug candidates are safe and effective for their intended uses. The number of nonclinical studies and clinical trials that will be required for FDA approval varies depending on the drug candidate, the disease or condition that the drug candidate is designed to address, and the regulations applicable to any particular drug candidate. Results from nonclinical studies and clinical trials can be interpreted in different ways. Even if we believe the nonclinical or clinical data for our drug candidates are promising, such data may not be sufficient to support approval by the FDA and other regulatory authorities. Administering drug candidates to humans may produce undesirable side effects, which could interrupt, delay or halt clinical trials and result in the FDA or other regulatory authorities denying approval of a drug candidate for any or all targeted indications.
Regulatory approval of an NDA or NDA supplement is not guaranteed, and the approval process is expensive and may take several years. The FDA also has substantial discretion in the approval process and we may encounter matters with the FDA that require us to expend additional time and resources and delay or prevent the approval of our product candidates. For example, the FDA may require us to conduct additional studies or trials for ZS-9 either prior to or post-approval, such as additional drug-drug interaction studies or safety or efficacy studies or trials, or it may object to elements of our clinical development program such as the number of patients in our current clinical trials from the United States. Further, there have been subject deaths in our clinical programs. While the incidence of subject deaths is not unexpected in view of the morbidity and mortality for the patient populations in our trials and have been determined by the study investigators and by us as unrelated to ZS-9, the FDA may require us to perform additional studies or otherwise delay regulatory approval of ZS-9. Despite the time and expense exerted, failure can occur at any stage. The FDA can delay, limit or deny approval of a drug candidate for many reasons, including, but not limited to, the following:
| • | a drug candidate may not be deemed safe or effective; |
| • | FDA officials may not find the data from nonclinical studies and clinical trials sufficient; |
| • | the FDA might not approve our third-party manufacturers’ processes or facilities; or |
| • | the FDA may change its approval policies or adopt new regulations. |
If ZS-9 or any future product candidate fails to demonstrate safety and efficacy in clinical trials or does not gain regulatory approval, our business and results of operations will be materially and adversely harmed. Additionally, if the FDA requires that we conduct additional clinical trials, places limitations on ZS-9 in our label, delays approval to market ZS-9 or limits the use of ZS-9, our business and results of operations may be harmed.
Even if we receive regulatory approval for ZS-9 or any future product candidates, we will be subject to ongoing regulatory obligations and continued regulatory review, which may result in significant additional expense. Additionally, ZS-9 or any future product candidates, if approved, could be subject to labeling and other restrictions and market withdrawal, and we may be subject to penalties if we fail to comply with regulatory requirements or experience unanticipated problems with our products.
Even if a drug is approved by the FDA and/or non-U.S. regulatory authorities, regulatory authorities may still impose significant restrictions on a product’s indicated uses or marketing or impose ongoing requirements for potentially costly post-marketing studies. Furthermore, any new legislation addressing drug safety issues could result in delays or increased costs to assure compliance.
If ZS-9 is approved it will be subject to ongoing regulatory requirements for labeling, packaging, storage, advertising, promotion, sampling, record-keeping and submission of safety and other post-market information, including both federal and state requirements in the United States and those of non-U.S. regulatory authorities. In addition, our manufacturing facilities are required to comply with extensive FDA requirements, including ensuring that quality control and manufacturing procedures conform to cGMP. As such, we are subject to
61
Table of Contents
continual review and periodic inspections to assess compliance with cGMP. Accordingly, we and others with whom we work must continue to expend time, money, and effort in all areas of regulatory compliance, including manufacturing, production, and quality control. We will also be required to report certain adverse reactions and production problems, if any, to the FDA, and to comply with requirements concerning advertising and promotion for our products. Promotional communications with respect to prescription drugs are subject to a variety of legal and regulatory restrictions and must be consistent with the information in the product’s approved label. As such, we may not promote our products for indications or uses for which they do not have FDA approval.
If a regulatory agency discovers previously unknown problems with a product, such as adverse events of unanticipated severity or frequency, or problems at our manufacturing facilities, or disagrees with the promotion, marketing, or labeling of a product, a regulatory agency may impose restrictions on that product or us, including requiring withdrawal of the product from the market. If we fail to comply with applicable regulatory requirements, a regulatory agency or enforcement authority may:
| • | issue warning letters; |
| • | impose civil or criminal penalties; |
| • | suspend or withdraw regulatory approval; |
| • | suspend any of our ongoing clinical trials; |
| • | refuse to approve pending applications or supplements to approved applications submitted by us; |
| • | impose restrictions on our operations, including closing our contract manufacturers’ facilities; or |
| • | seize or detain products or require a product recall. |
Any government investigation of alleged violations of law could require us to expend significant time and resources in response, and could generate negative publicity. Any failure to comply with ongoing regulatory requirements may significantly and adversely affect our ability to commercialize and generate revenues from ZS-9. If regulatory sanctions are applied or if regulatory approval is withdrawn, the value of our company and our operating results will be adversely affected. Additionally, if we are unable to generate revenues from the sale of ZS-9 our potential for achieving profitability will be diminished and the capital necessary to fund our operations will be increased.
Currently we plan to seek regulatory approval to market ZS-9 solely for the treatment of hyperkalemia and, unless we seek regulatory approval for additional indications, we will be prohibited from marketing ZS-9 for any other indication.
We intend to seek approval to market ZS-9 for the treatment of hyperkalemia. We do not have plans to seek approval of ZS-9 for any other indication at this time. Even if we obtain regulatory approval to market ZS-9 with an indication statement for the treatment of hyperkalemia, we will likely be prohibited from marketing ZS-9 using any promotional claims relating to maintaining more patients on, or enabling the introduction, increased or optimized usage of, RAAS inhibitors. The FDA strictly regulates the promotional claims that may be made about prescription products. While ZS-9 has not been studied in patients who are on RAAS inhibitors, ZS-9 may not be promoted for uses that are not approved by the FDA as reflected in its approved labeling. Under applicable regulations, the ability of a company to make marketing statements about the effectiveness of its drug outside of the statements made in the label, referred to as “off-label” marketing, is prohibited. If we are found to have promoted such off-label uses, we may become subject to significant liability.
If we fail to comply or are found to have failed to comply with FDA and other regulations related to the promotion of ZS-9 for unapproved uses, we could be subject to criminal penalties, substantial fines or other sanctions and damage awards.
The regulations relating to the promotion of products for unapproved uses are complex and subject to substantial interpretation by the FDA and other government agencies. If we receive marketing approval for ZS-9,
62
Table of Contents
physicians may nevertheless prescribe it to their patients in a manner that is inconsistent with the approved label. We intend to implement compliance and training programs designed to ensure that our sales and marketing practices comply with applicable regulations. Notwithstanding these programs, the FDA or other government agencies may allege or find or private parties may allege that our practices constitute prohibited promotion of ZS-9 for unapproved uses. We also cannot be sure that our employees will comply with company policies and applicable regulations regarding the promotion of products for unapproved uses.
Over the past several years, a significant number of pharmaceutical and biotechnology companies have been the target of inquiries and investigations by various federal and state regulatory, investigative, prosecutorial and administrative entities in connection with the promotion of products for unapproved uses and other sales practices, including the Department of Justice and various U.S. Attorneys’ Offices, the Office of Inspector General of the Department of Health and Human Services, the FDA, the Federal Trade Commission and various state Attorneys General offices. These investigations have alleged violations of various federal and state laws and regulations, including claims asserting antitrust violations, violations of the Food, Drug and Cosmetic Act, the False Claims Act, the Prescription Drug Marketing Act, anti-kickback laws, and other alleged violations in connection with the promotion of products for unapproved uses, pricing and Medicare and/or Medicaid reimbursement. Many of these investigations originate as “qui tam” actions under the False Claims Act. Under the False Claims Act, any individual can bring a claim on behalf of the government alleging that a person or entity has presented a false claim, or caused a false claim to be submitted, to the government for payment. The person bringing a qui tam suit is entitled to a share of any recovery or settlement. Qui tam suits, also commonly referred to as “whistleblower suits,” are often brought by current or former employees. In a qui tam suit, the government must decide whether to intervene and prosecute the case. If it declines, the individual may pursue the case alone.
If the FDA or any other governmental agency initiates an enforcement action against us or if we are the subject of a qui tam suit and it is determined that we violated prohibitions relating to the promotion of products for unapproved uses, we could be subject to substantial civil or criminal fines or damage awards and other sanctions such as consent decrees and corporate integrity agreements pursuant to which our activities would be subject to ongoing scrutiny and monitoring to ensure compliance with applicable laws and regulations. Any such fines, awards or other sanctions would have an adverse effect on our revenue, business, financial prospects and reputation.
If approved, ZS-9 or any future products may cause or contribute to adverse medical events that we are required to report to regulatory agencies and if we fail to do so we could be subject to sanctions that would materially harm our business.
Some participants in our trials have reported adverse effects after being treated with ZS-9. If we are successful in commercializing ZS-9 or any other products, the FDA and foreign regulatory agency regulations require that we report certain information about adverse medical events if those products may have caused or contributed to those adverse events. The timing of our obligation to report would be triggered by the date we become aware of the adverse event as well as the nature of the event. We may fail to report adverse events we become aware of within the prescribed timeframe. We may also fail to appreciate that we have become aware of a reportable adverse event, especially if it is not reported to us as an adverse event or if it is an adverse event that is unexpected or removed in time from the use of our products. If we fail to comply with our reporting obligations, the FDA or a foreign regulatory agency could take action, including criminal prosecution, the imposition of civil monetary penalties, seizure of our products or delay in approval or clearance of future products.
If we fail to comply with manufacturing regulations, our results of operations and financial condition will be adversely affected.
Before we can begin commercial manufacture of ZS-9, we must obtain regulatory approval of our manufacturing facilities, processes and quality systems. In addition, pharmaceutical manufacturing facilities are
63
Table of Contents
continuously subject to inspection by the FDA and foreign regulatory authorities, before and after product approval. Due to the complexity of the processes used to manufacture pharmaceutical products and product candidates, we may be unable to continue to pass or initially pass federal, state or foreign regulatory inspections in a cost-effective manner.
If we are unable to comply with manufacturing regulations, ZS-9 may not be approved, or we may be subject to fines, unanticipated compliance expenses, recall or seizure of our products, total or partial suspension of production and/or enforcement actions, including injunctions, and criminal or civil prosecution. These possible sanctions would adversely affect our results of operations and financial condition.
Our failure to obtain regulatory approvals in foreign jurisdictions for ZS-9 would prevent us from marketing our products internationally.
In order to market any product in the European Economic Area, or EEA (which is composed of the 27 member states of the European Union plus Norway, Iceland and Liechtenstein), and many other foreign jurisdictions, separate regulatory approvals are required. In the EEA, medicinal products can only be commercialized after obtaining a MAA. Before granting a MAA, the EMA or the competent authorities of the member states of the EEA make an assessment of the risk-benefit balance of the product on the basis of scientific criteria concerning its quality, safety and efficacy.
The approval procedures vary among countries and can involve additional clinical testing, and the time required to obtain approval may differ from that required to obtain FDA approval. Clinical trials conducted in one country may not be accepted by regulatory authorities in other countries. Approval by the FDA does not ensure approval by regulatory authorities in other countries, and approval by one or more foreign regulatory authorities does not ensure approval by regulatory authorities in other foreign countries or by the FDA. However, a failure or delay in obtaining regulatory approval in one country may have a negative effect on the regulatory process in others. The foreign regulatory approval process may include all of the risks associated with obtaining FDA approval as well as other unknown risks. We may not be able to file for regulatory approvals or to do so on a timely basis, and even if we do file we may not receive necessary approvals to commercialize our products in any market.
We may be subject to healthcare laws, regulation and enforcement and our failure to comply with these laws could have a material adverse effect on our results of operations and financial condition.
Although we do not currently have any products on the market, once we begin commercializing our products, we may be subject to additional healthcare statutory and regulatory requirements and enforcement by the federal government and the state and foreign governments in which we conduct our business. The laws that may affect our ability to operate as a commercial organization include:
| • | the federal healthcare programs’ Anti-Kickback Statute, which prohibits, among other things, persons from knowingly and willfully soliciting, receiving, offering or paying remuneration, directly or indirectly, in exchange for or to induce either the referral of an individual for, or the purchase, order or recommendation of, any good or service for which payment may be made under federal healthcare programs such as the Medicare and Medicaid programs; |
| • | federal false claims laws which prohibit, among other things, individuals or entities from knowingly presenting, or causing to be presented, claims for payment from Medicare, Medicaid, or other third-party payors that are false or fraudulent; |
| • | federal criminal laws that prohibit executing a scheme to defraud any healthcare benefit program or making false statements relating to healthcare matters; |
| • | the federal Health Insurance Portability and Accountability Act of 1996, as amended by the Health Information Technology for Economic and Clinical Health Act, which governs the conduct of certain electronic healthcare transactions and protects the security and privacy of protected health information; |
64
Table of Contents
| • | state law equivalents of each of the above federal laws, such as anti-kickback and false claims laws which may apply to items or services reimbursed by any third-party payor, including commercial insurers; and |
| • | U.S. and European reporting requirements detailing interactions with and payments to healthcare providers. |
If our operations are found to be in violation of any of the laws described above or any other governmental laws and regulations that apply to us, we may be subject to penalties, including civil and criminal penalties, damages, fines, the curtailment or restructuring of our operations, the exclusion from participation in federal and state healthcare programs and imprisonment, any of which could adversely affect our ability to market our products and adversely impact our results of operations. The risk of our being found in violation of these laws is increased by the fact that many of them have not been fully interpreted by the regulatory authorities or the courts, and their provisions are open to a variety of interpretations. Further, the PPACA, among other things, amends the intent requirement of the federal anti-kickback and criminal health care fraud statutes. A person or entity no longer needs to have actual knowledge of this statute or specific intent to violate it. In addition, the PPACA provides that the government may assert that a claim including items or services resulting from a violation of the federal anti-kickback statute constitutes a false or fraudulent claim for purposes of the false claims statutes. Any action against us for violation of these laws, even if we successfully defend against it, could cause us to incur significant legal expenses and divert our management’s attention from the operation of our business.
The PPACA also imposes new reporting and disclosure requirements on drug manufacturers for any “transfer of value” made or distributed to prescribers and other healthcare providers. In addition, drug manufacturers will also be required to report and disclose any investment interests held by physicians and their immediate family members during the preceding calendar year. Failure to submit required information may result in civil monetary penalties of up to an aggregate of $150,000 per year (and up to an aggregate of $1 million per year for “knowing failures”), for all payments, transfers of value or ownership or investment interests not reported in an annual submission. Manufacturers were required to begin data collection on August 1, 2013 and to report such data to the government by March 31, 2014 and by the 90th calendar day of each year thereafter.
In addition, there has been a recent trend of increased federal and state regulation of payments made to physicians for marketing. Some states mandate implementation of compliance programs and/or the tracking and reporting of gifts, compensation, and other remuneration to physicians. The shifting compliance environment and the need to build and maintain robust and expandable systems to comply with multiple jurisdictions with different compliance and/or reporting requirements increases the possibility that a healthcare company may run afoul of one or more of the requirements.
Healthcare reform measures could hinder or prevent our product candidates’ commercial success.
In the United States, there have been and we expect there will continue to be a number of legislative and regulatory changes to the healthcare system that could affect our future revenue and profitability and the future revenue and profitability of our potential customers. Federal and state lawmakers regularly propose and, at times, enact legislation that would result in significant changes to the healthcare system, some of which are intended to contain or reduce the costs of medical products and services. For example, the PPACA contains a number of provisions, including those governing enrollment in federal healthcare programs, reimbursement changes and fraud and abuse measures, all of which will impact existing government healthcare programs and will result in the development of new programs. The PPACA, among other things:
| • | imposes a non-deductible annual fee on pharmaceutical manufacturers or importers who sell “branded prescription drugs,” effective 2011; |
| • | increases the minimum level of Medicaid rebates payable by manufacturers of brand-name drugs from 15.1% to 23.1%, effective 2011; |
| • | could result in the imposition of injunctions; |
65
Table of Contents
| • | requires collection of rebates for drugs paid by Medicaid managed care organizations; |
| • | requires manufacturers to participate in a coverage gap discount program, under which they must agree to offer 50% point-of-sale discounts off negotiated prices of applicable branded drugs to eligible beneficiaries during their coverage gap period, as a condition for the manufacturer’s outpatient drugs to be covered under Medicare Part D; and |
| • | creates a process for approval of biologic therapies that are similar or identical to approved biologics. |
While the U.S. Supreme Court upheld the constitutionality of most elements of the PPACA in June 2012, other legal challenges are still pending final adjudication in several jurisdictions. In addition, Congress has also proposed a number of legislative initiatives, including possible repeal of the PPACA. At this time, it remains unclear whether there will be any changes made to the PPACA, whether to certain provisions or its entirety. We cannot assure you that the PPACA, as currently enacted or as amended in the future, will not adversely affect our business and results of operations and we cannot predict how future federal or state legislative or administrative changes relating to healthcare reform will affect our business.
In addition, other legislative changes have been proposed and adopted since the PPACA was enacted. For example, the Budget Control Act of 2011, among other things, created the Joint Select Committee on Deficit Reduction to recommend proposals for spending reductions to Congress. The Joint Select Committee did not achieve a targeted deficit reduction of at least $1.2 trillion for the years 2013 through 2021, which triggered the legislation’s automatic reduction to several government programs, including aggregate reductions to Medicare payments to providers of up to 2% per fiscal year, starting in 2013. In January 2013, President Obama signed into law the American Taxpayer Relief Act of 2012, or the ATRA, which delayed for another two months the budget cuts mandated by the sequestration provisions of the Budget Control Act of 2011. The ATRA, among other things, also reduced Medicare payments to several providers, including hospitals, and increased the statute of limitations period for the government to recover overpayments to providers from three to five years. In March 2013, the President signed an executive order implementing sequestration, and in April 2013, the 2% Medicare reductions went into effect. We cannot predict whether any additional legislative changes will affect our business.
There likely will continue to be legislative and regulatory proposals at the federal and state levels directed at containing or lowering the cost of health care. We cannot predict the initiatives that may be adopted in the future or their full impact. The continuing efforts of the government, insurance companies, managed care organizations and other payors of healthcare services to contain or reduce costs of health care may adversely affect:
| • | our ability to set a price that we believe is fair for our products; |
| • | our ability to generate revenue and achieve or maintain profitability; and |
| • | the availability of capital. |
Legislative or regulatory healthcare reforms in the United States may make it more difficult and costly for us to obtain regulatory clearance or approval of ZS-9 or any future product candidates and to produce, market and distribute our products after clearance or approval is obtained.
From time to time, legislation is drafted and introduced in Congress that could significantly change the statutory provisions governing the regulatory clearance or approval, manufacture, and marketing of regulated products or the reimbursement thereof. In addition, FDA regulations and guidance are often revised or reinterpreted by the FDA in ways that may significantly affect our business and our products. Any new regulations or revisions or reinterpretations of existing regulations may impose additional costs or lengthen review times of ZS-9 or any future product candidates. We cannot determine what effect changes in regulations, statutes, legal interpretation or policies, when and if promulgated, enacted or adopted may have on our business in the future. Such changes could, among other things, require:
| • | additional clinical trials to be conducted prior to obtaining approval; |
| • | changes to manufacturing methods; |
66
Table of Contents
| • | recall, replacement, or discontinuance of one or more of our products; and |
| • | additional record keeping. |
Each of these would likely entail substantial time and cost and could materially harm our business and our results of operations. In addition, delays in receipt of or failure to receive regulatory clearances or approvals for any future products would harm our business, financial condition and results of operations.
Risks Related to Intellectual Property
We may become subject to claims alleging infringement of third parties’ patents or proprietary rights and/or claims seeking to invalidate our patents or other proprietary rights, which would be costly, time consuming and could delay or prevent the development and commercialization of ZS-9 or any future product candidates.
There is a significant amount of intellectual property litigation in the pharmaceutical and biotechnology industries, and we may become a party to, or threatened with litigation or other adversarial proceedings regarding intellectual property rights with respect to our technology or product candidates. Third parties may assert infringement claims against us based on existing or future intellectual property rights. We cannot assure you that ZS-9 or any future product candidates will not infringe existing or future third-party patents. Because patent applications can take many years to issue and may be confidential for 18 months or more after filing, there may be applications now pending of which we are unaware and which may later result in issued patents that we may infringe by commercializing ZS-9 or future product candidates. Moreover, we may face claims from non-practicing entities that have no relevant product revenue and against whom our own patent portfolio may have no deterrent effect. In addition, ZS-9 has a complex structure that makes it difficult to conduct a thorough search and review of all potentially relevant third-party patents that may impact our freedom to operate. We may be unaware of one or more issued patents that would be infringed by the manufacture, sale or use of ZS-9 or our other product candidates. There also may be prior art of which we are aware, but which we do not believe affects the validity or enforceability of our owned or licensed patents or patent applications, which may, nonetheless, ultimately be found to affect the validity or enforceability of such patents or patent applications.
In October 2013, one of our competitors, Relypsa, filed a patent application with the United States Patent and Trademark Office, or USPTO, seeking broad claims directed to removing potassium from a patient. Prosecution of this patent application is ongoing and we expect the next office action from the USPTO to occur in the third quarter of 2015, although it could happen earlier. Such action could include a Notice of Allowance, a Non-Final Rejection or a Final Rejection. If a patent were to issue with the currently pending or similarly broad claims pursuant to a Notice of Allowance, it would potentially cover the use of ZS-9 in the treatment of hyperkalemia. We believe that such a patent should not issue, and if it does, it should be found to be invalid and unenforceable. Nevertheless, challenging this patent would be costly and time consuming, and if such challenge were unsuccessful, it could materially impact or prevent the commercialization of ZS-9 for the treatment of hyperkalemia, which could have a material adverse impact on our business, financial condition and results of operations. In the event that a patent with the current or similarly broad claims is issued, we intend to vigorously challenge it, although there is no assurance that a court would find any challenged claims to be invalid.
We may be subject to third-party infringement claims in the future against us or our partners that would cause us to incur substantial expenses and, if successful against us, could cause us to pay substantial damages, including treble damages and attorney’s fees if we are found to be willfully infringing a third-party’s patent or other intellectual property right. We may be required to indemnify present and future partners against such claims. We are currently required to indemnify UOP against such claims pursuant to the terms of our license agreement. If a patent infringement suit were brought against us or our partners, we or they could be forced to stop or delay research, development, manufacturing or sales of the product or product candidate that is the subject of the suit. As a result of patent infringement claims, or in order to avoid potential claims, we or our partners may choose to seek, or be required to seek, a license from the third-party and would most likely be
67
Table of Contents
required to pay license fees or royalties or both. These licenses may not be available on acceptable terms, or at all. Even if we or our partners were able to obtain a license, the rights may be nonexclusive, which would give our competitors access to the same intellectual property, and we may be required to make substantial licensing and royalty payments. Ultimately, we could be prevented from commercializing a product, or forced to redesign it, or to cease some aspect of our business operations if, as a result of actual or threatened patent infringement claims, we or our partners are unable to enter into licenses on acceptable terms. Even if we are successful in defending against such claims, such litigation can be expensive and time consuming and would divert management’s attention from our core business. Any of these events could harm our business and competitive position significantly. Claims that we have misappropriated the confidential information or trade secrets of third parties could expose us to similar liabilities and have a similar negative impact on our business.
In addition to infringement claims against us, if third parties prepare and file patent applications in the United States that also claim technology similar or identical to ours, we may have to participate in interference or derivation proceedings in the USPTO, to determine which party is entitled to a patent on the disputed invention. We may also become involved in similar opposition proceedings in the European Patent Office or corresponding offices in other jurisdictions regarding our intellectual property rights with respect to our products and technology. Since patent applications are confidential for a period of time after filing, we cannot be certain that we were the first to file any patent application related to our product candidates. We may additionally become involved in additional proceedings regarding intellectual property rights with respect to our products and technology including re-examination, inter partes review, post-grant review, cancellation or similar proceedings before the USPTO or its foreign counterparts. The risks of being involved in such litigation or other proceedings may also increase as ZS-9 or our other product candidates near commercialization and as we gain greater visibility associated with being a public company.
We may be involved in lawsuits to protect or enforce our intellectual property rights, which could be expensive, time consuming and unsuccessful.
Competitors or other third parties may infringe or otherwise violate our patents, the patents of our licensors, or our other intellectual property rights. Even where laws provide protection, to counter such infringement or other violations, costly and time-consuming litigation could be necessary to enforce and determine the scope of our proprietary rights, and the outcome of such litigation would be uncertain. If we or one of our present or future partners were to initiate legal proceedings against a third-party to enforce a patent covering ZS-9 or one of our future products or product candidates, the defendant could counterclaim that our patent is invalid and/or unenforceable. In patent litigation in the United States, counterclaims by defendants alleging invalidity and/or unenforceability are commonplace. Grounds for a validity challenge could be an alleged failure to meet any of several statutory requirements, including lack of novelty, obviousness or non-enablement. Grounds for an unenforceability assertion could be an allegation that someone connected with prosecution of the patent withheld relevant information from the USPTO, or made a misleading statement, during prosecution of the relevant patent. The outcome following legal assertions of invalidity and unenforceability is unpredictable. With respect to validity, for example, we cannot be certain that there is no invalidating prior art, of which we and the patent examiner were unaware during prosecution. An adverse result in any litigation or defense proceeding could put one or more of our patents at risk of being invalidated, held unenforceable or interpreted narrowly, and could put any of our patent applications at risk of not yielding an issued patent. If a defendant were to prevail on a legal assertion of invalidity and/or unenforceability against our intellectual property related to ZS-9, we would lose at least part, and perhaps all, of the patent protection on ZS-9. Such a loss of patent protection would have a material adverse impact on our business. In addition, in an infringement proceeding a court may refuse to prevent the other party from using the technology at issue on the grounds that our patents do not cover the technology in question. Moreover, our competitors or other third parties could counterclaim that we infringe their intellectual property, and some of our competitors have substantially greater intellectual property portfolios than we do. In any intellectual property litigation, even if we are successful, any award of monetary damages or other remedy that we may receive may not be commercially valuable. In addition, due to the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of our confidential information could be compromised by disclosure during such litigation.
68
Table of Contents
The patent prosecution process is expensive and time-consuming, is highly uncertain and involves complex legal and factual questions. Recent patent reform legislation could increase the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of our issued patents.
Our success depends in large part on our and our licensors’ ability to obtain and maintain patent protection in the United States and other countries with respect to our proprietary technology and product candidates. We seek to protect our proprietary position by filing in the United States and in certain foreign jurisdictions patent applications related to our novel technologies and product candidates that are important to our business.
The patent prosecution process is expensive and time-consuming, and we may not be able to file and prosecute all necessary or desirable patent applications at a reasonable cost or in a timely manner. It is also possible that we will fail to identify patentable aspects of our research and development output before it is too late to obtain patent protection. In addition, we may not pursue or obtain patent protection in all major markets. Moreover, in some circumstances, we may not have the right to control the preparation, filing or prosecution of patent applications, or to maintain the patents, covering technology that we license from third parties. In some circumstances, our licensors may have the right to enforce the licensed patents without our involvement or consent, or to decide not to enforce or to allow us to enforce the licensed patents. Therefore, these patents and patent applications may not be prosecuted and enforced in a manner consistent with the best interests of our business. For example, pursuant to our license agreement with UOP, UOP has retained responsibility for prosecuting and maintaining the patent rights they have licensed to us and has the first right to enforce the licensed patent rights against third-party infringement. If any of our licensors fail to maintain such patents, or lose rights to those patents, the rights that we have licensed may be reduced or eliminated and our right to develop and commercialize any of our product candidates that are the subject of such licensed rights could be adversely affected.
In addition, the laws of foreign jurisdictions may not protect our rights to the same extent as the laws of the United States. For example, European patent law restricts the patentability of methods of treatment of the human body more than United States law does. Publications of discoveries in scientific literature often lag behind the actual discoveries, and patent applications in the United States and other jurisdictions are typically not published until 18 months after filing, or in some cases not at all. Therefore, we cannot be certain that we or our licensors were the first to make the inventions claimed in our owned or licensed patents or pending patent applications, or that we or our licensors were the first to file for patent protection of such inventions. Moreover, the USPTO might require that the term of a patent issuing from a pending patent application be disclaimed and limited to the term of another patent that is commonly owned or names a common inventor. As a result, the issuance, scope, validity, term, enforceability and commercial value of our patent rights are highly uncertain.
Our pending and future patent applications may not result in patents being issued which protect our technology or products, in whole or in part, or which effectively prevent others from commercializing competitive technologies and products. In particular, during prosecution of any patent application, the issuance of any patents based on the application may depend upon our ability to generate additional nonclinical or clinical data that support the patentability of our proposed claims. We may not be able to generate sufficient additional data on a timely basis, or at all. Moreover, changes in either the patent laws or interpretation of the patent laws in the United States or other countries may diminish the value of our patents or narrow the scope of our patent protection.
Recent patent reform legislation could increase the uncertainties and costs surrounding the prosecution of patent applications and the enforcement or defense of issued patents that we own or license. On September 16, 2011, the Leahy-Smith America Invents Act, or the Leahy-Smith Act, was signed into law. The Leahy-Smith Act includes a number of significant changes to United States patent law. These include provisions that affect the way patent applications are prosecuted, redefine prior art, may affect patent litigation and switch the United States patent system from a “first-to-invent” system to a “first-to-file” system. Under a first-to-file system, assuming the other requirements for patentability are met, the first inventor to file a patent application generally will be entitled to the patent on an invention regardless of whether another inventor had made the invention
69
Table of Contents
earlier. The USPTO recently developed new regulations and procedures to govern administration of the Leahy-Smith Act, and many of the substantive changes to patent law associated with the Leahy-Smith Act, and in particular, the first-to-file provisions, only became effective on March 16, 2013. Accordingly, it is not clear what, if any, impact the Leahy-Smith Act will have on the operation of our business. However, the Leahy-Smith Act and its implementation could increase the uncertainties and costs surrounding the prosecution of our owned and licensed patent applications and the enforcement or defense of issued patents that we own or license, all of which could have a material adverse effect on our business and financial condition.
Moreover, we may be subject to a third-party pre-issuance submission of prior art to the USPTO, or become involved in opposition, derivation, reexamination, inter partes review, post-grant review or interference proceedings or other patent office proceedings or litigation, in the United States or elsewhere, challenging our patent rights or the patent rights of others. An adverse determination in any such submission or proceeding could reduce the scope of, or invalidate, our patent rights; allow third parties to commercialize our technology or products and compete directly with us, without payment to us; or result in our inability to manufacture or commercialize products without infringing third-party patent rights. In addition, if the breadth or strength of protection provided by our owned and licensed patents and patent applications is threatened, it could dissuade companies from collaborating with us to license, develop or commercialize current or future product candidates.
If we are unable to obtain and maintain sufficient intellectual property protection for ZS-9 or any future product candidates or if the scope of the intellectual property protection is not sufficiently broad, our competitors could develop and commercialize products similar or identical to ours, and our ability to successfully commercialize our product candidates may be adversely affected.
The patentability of inventions, and the validity, enforcement and scope of patents in the biotechnology and pharmaceutical field involves complex legal and scientific questions and can be uncertain. Consequently, the patent applications that we own or license may fail to result in issued patents in the United States or in foreign countries for many reasons. For example, there is no assurance that we were the first to invent or the first to file patent applications in respect of the inventions claimed in our patent applications. Even if patents have issued or do successfully issue, third parties may challenge the validity, enforceability or scope thereof, which may result in such patents being narrowed, invalidated or held unenforceable. For example, patents granted by the European Patent Office may be opposed by any person within nine months from the publication of the grant. Similar proceedings are available in other jurisdictions, and in some jurisdictions third parties can raise questions of validity with a patent office even before a patent has granted. Furthermore, even if they are unchallenged, our patents and patent applications may not adequately protect our intellectual property or prevent others from designing around our claims. For example, a third-party may develop a competitive product that provides therapeutic benefits similar to ZS-9 but has a sufficiently different composition to fall outside the scope of our patent protection. If the breadth or strength of protection provided by the patents and patent applications we hold, license or pursue with respect to ZS-9 or any future product candidates is successfully challenged, it could negatively affect our ability to commercialize ZS-9 or any future product candidates. As a result, we may also face unexpected competition that could have a material adverse impact on our business. Further, if we encounter delays in our clinical trials, the period of time during which we could market ZS-9 or any future product candidates under patent protection, if approved, would be reduced. The scope of our owned and licensed intellectual property rights may not be sufficient to prevent others from manufacturing or selling competing products. For example, our intellectual property position depends in part on patent rights that we have licensed from UOP. Certain of these licensed patents, including patents with composition-of-matter claims covering ZS-9 are expected to expire by 2017. Such expiration date is only shortly after the date by which we expect ZS-9 to be commercialized in the United States, assuming we obtain marketing approval, and may even be prior to such date. We have also licensed rights to a United States patent claiming a method of using ZS-9 to remove toxins from a fluid, including in the treatment of hyperkalemia, from UOP that is currently expected to expire in 2019, without taking into account any potential patent term extension that may be available to us under the Drug Price Competition and Patent Term Restoration Act of 1984, or the Hatch-Waxman Act. As a result, we may not be able to prevent competitors from commercializing products similar or identical to ZS-9 after these licensed patents expire.
70
Table of Contents
Obtaining and maintaining our patent protection depends on compliance with various procedural, document submission, fee payment and other requirements imposed by governmental patent agencies, and our patent protection could be reduced or eliminated for non-compliance with these requirements.
The USPTO and various foreign governmental patent agencies require compliance with a number of procedural, documentary, fee payment and other provisions to maintain patent applications and issued patents. We and our licensors may fail to take the necessary actions and to pay the applicable fees to obtain or maintain our patents. Noncompliance with these requirements can result in abandonment or lapse of a patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction. In such an event, competitors might be able to use our technologies and enter the market earlier than would otherwise have been the case.
Changes in U.S. patent law could diminish the value of patents in general, thereby impairing our ability to protect our products.
As is the case with other biopharmaceutical companies, our success is heavily dependent on intellectual property, particularly patents. Obtaining and enforcing patents in the biopharmaceutical industry involves both technological and legal complexity. Therefore, obtaining and enforcing biopharmaceutical patents is costly, time consuming and inherently uncertain. In addition, the United States has recently enacted the Leahy-Smith Act and is currently implementing wide-ranging patent reform legislation. The United States Supreme Court has ruled on several patent cases in recent years, either narrowing the scope of patent protection available in certain circumstances or weakening the rights of patent owners in certain situations. In addition to increasing uncertainty with regard to our ability to obtain patents in the future, this combination of events has created uncertainty with respect to the value of patents once obtained. Depending on future actions by the United States Congress, the federal courts, and the USPTO, the laws and regulations governing patents could change in unpredictable ways that would weaken our ability to obtain new patents or to enforce our existing patents and patents that we might obtain in the future.
We have not yet registered trademarks for a commercial trade name for ZS-9 in the United States or elsewhere and failure to secure such registrations could adversely affect our business.
We have not yet registered trademarks for a commercial trade name for ZS-9 in the United States or in any other foreign jurisdictions. During trademark registration proceedings, our trademark application may be rejected. Although we are given an opportunity to respond, we may be unable to overcome such rejections. In addition, in the USPTO and in comparable agencies in many foreign jurisdictions, third parties can oppose pending trademark applications and seek to cancel registered trademarks. Opposition or cancellation proceedings may be filed against our trademarks, and our trademarks may not survive such proceedings. If we fail to secure trademark registration for ZS-9 and our future product candidates, our ability to market such product offerings and our business could be adversely affected. Moreover, any name we propose to use with our product candidates in the United States must be approved by the FDA, regardless of whether we have registered it, or applied to register it, as a trademark. The FDA typically conducts a review of proposed product names, including an evaluation of potential for confusion with other product names. If the FDA objects to any of our proposed proprietary product names, we may be required to expend significant additional resources in an effort to identify a suitable substitute name that would qualify under applicable trademark laws, not infringe the existing rights of third parties and be acceptable to the FDA. The EMA will conduct a similar review process of the names we propose to use with our product candidates.
We may not be able to enforce our intellectual property rights throughout the world.
Filing, prosecuting and defending patents on ZS-9 and all of our future product candidates throughout the world would be prohibitively expensive. Competitors may use our technologies in jurisdictions where we have not obtained patent protection to develop their own products and, further, may export otherwise infringing products to territories where we have patent protection but where enforcement is not as strong as in the United
71
Table of Contents
States. The laws of certain foreign countries do not protect intellectual property rights to the same extent as the laws of the United States. Many companies have encountered significant problems in protecting and defending intellectual property rights in certain foreign jurisdictions. The legal systems of certain countries, particularly certain developing countries, do not favor the enforcement of patents and other forms of intellectual property protection, especially those relating to biopharmaceuticals. This could make it difficult for us to stop the infringement of our patents or the misappropriation of our other intellectual property rights. For example, many foreign countries have compulsory licensing laws under which a patent owner must grant licenses to third parties. In addition, many countries limit the enforceability of patents against third parties, including government agencies or government contractors. In these countries, patents may provide limited or no benefit.
Proceedings to enforce our patent rights in foreign jurisdictions, whether or not successful, could result in substantial costs and divert our efforts and attention from other aspects of our business. Furthermore, while we intend to protect our intellectual property rights in our expected significant markets, we do not yet own or license rights to any issued patents covering ZS-9 in any jurisdiction other than the United States, and we cannot guarantee that we will be able to initiate or maintain similar efforts in all jurisdictions in which we may wish to market ZS-9 or any future products. Accordingly, our efforts to protect our intellectual property rights in such countries may be inadequate. In addition, changes in the law and legal decisions by courts in the United States and foreign countries may affect our ability to obtain and enforce adequate intellectual property protection for our technology.
We may be subject to claims by third parties asserting that we or our employees have misappropriated their intellectual property, or claiming ownership of what we regard as our own intellectual property.
Many of our employees were previously employed at other biotechnology or pharmaceutical companies, including our competitors or potential competitors. Although we try to ensure that our employees and consultants do not use the proprietary information or know-how of others in their work for us, we may be subject to claims that we or these employees or consultants have used or disclosed intellectual property, including trade secrets or other proprietary information, of any such employee’s or consultant’s former employer. We are not aware of any material threatened or pending claims related to these matters, but in the future, litigation may be necessary to defend against such claims.
In addition, while we typically require our employees, consultants and contractors who may be involved in the development of intellectual property to execute agreements assigning such intellectual property to us, we may be unsuccessful in executing such an agreement with each party who in fact develops intellectual property that we regard as our own. Moreover, because we acquired certain rights to our lead product candidate under our license with UOP, we must rely on UOP’s practices, and those of its predecessors, with regard to obtaining necessary assignments of intellectual property from UOP’s employees to UOP. Such assignment agreements may not be self-executing, may be insufficient in scope or may be breached, and we may be forced to bring claims against third parties, or defend claims they may bring against us, to determine the ownership of what we regard as our intellectual property.
If we fail in prosecuting or defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights or personnel. Even if we are successful in prosecuting or defending against such claims, litigation could result in substantial costs and be a distraction to management.
If we are unable to protect the confidentiality of our trade secrets, the value of our technology could be materially adversely affected and our business could be harmed.
In addition to seeking patent protection, we also rely on trade secret protection and confidentiality agreements to protect proprietary know-how that may not be patentable, processes for which patents may be difficult to obtain and/or enforce, and other elements of our product development processes that involve proprietary know-how, information or technology that is not covered by patents. Any disclosure to or misappropriation by third parties of our confidential proprietary information could enable competitors to quickly
72
Table of Contents
duplicate or surpass our technological achievements, thus eroding our competitive position in our market. We seek to protect our confidential proprietary information, in part, by entering into confidentiality agreements and invention assignment agreements with all of our employees, consultants, advisors and any third parties who have access to our proprietary know-how, information or technology. However, we cannot be certain that we have executed such agreements with all parties who may have helped to develop our intellectual property or who had access to our proprietary information, nor can we be certain that our agreements will not be breached. Any party with whom we have executed such an agreement may breach that agreement and disclose our proprietary information, including our trade secrets, and we may not be able to obtain adequate remedies for such breaches. We cannot guarantee that our trade secrets and other confidential proprietary information will not be disclosed or that competitors will not otherwise gain access to our trade secrets or independently develop substantially equivalent information and techniques. Detecting the disclosure or misappropriation of a trade secret and enforcing a claim that a party illegally disclosed or misappropriated a trade secret is difficult, time-consuming and could result in substantial costs and the outcome of such a claim is unpredictable. Further, the laws of certain foreign countries do not protect proprietary rights to the same extent or in the same manner as the laws of the United States. As a result, we may encounter significant problems in protecting and defending our intellectual property both in the United States and abroad. If we are unable to prevent material disclosure of the intellectual property related to our technologies to third parties, or if our competitors independently develop any of our trade secrets we will not be able to establish or maintain a competitive advantage in our market, which could materially adversely affect our business, results of operations and financial condition.
Risks Related to Our Common Stock
Our stock price may be volatile and investors in our common stock could incur substantial losses.
The trading price of our common stock has been, and is likely to continue to be, highly volatile. We priced our IPO at $18.00 per share on June 17, 2014 and, as of March 9, 2015, our common stock has reached a high of $52.80 per share. The trading price of our common stock could be subject to wide fluctuations in response to various factors, some of which are beyond our control, including those discussed in this “Risk Factors” section and others such as:
| • | announcements of regulatory approval or a complete response letter relating to ZS-9, specific label indications or patient populations for its use, or changes or delays in the regulatory review process; |
| • | announcements of therapeutic innovations, new product candidates or approved products by us or our competitors; |
| • | adverse actions taken by regulatory agencies with respect to our clinical trials, our manufacturing facilities, suppliers or sales and marketing activities; |
| • | changes or developments in laws or regulations applicable to ZS-9; |
| • | changes in existing tax laws, treaties or regulations or the interpretation or enforcement thereof, or the enactment or adoption of new tax laws, regulations or policies; |
| • | any adverse changes to our relationship with suppliers; |
| • | the success of our nonclinical studies and clinical trials; |
| • | the success of our efforts to acquire or license or discover additional product candidates; |
| • | any intellectual property infringement actions in which we may become involved; |
| • | announcements concerning our competitors or the pharmaceutical industry in general; |
| • | achievement of expected product sales and profitability; |
| • | manufacture, supply or distribution shortages; |
| • | actual or anticipated fluctuations in our operating results; |
| • | changes in financial estimates or recommendations by securities analysts; |
73
Table of Contents
| • | trading volume of our common stock; |
| • | sales of our common stock by us, our executive officers and directors or our stockholders in the future; |
| • | general economic and market conditions and overall fluctuations in the U.S. equity markets; and |
| • | the loss of any of our key scientific or management personnel. |
In addition, the stock markets in general, and the markets for pharmaceutical, biopharmaceutical and biotechnology stocks in particular, have experienced extreme volatility that may have been unrelated to the operating performance of the issuer. These broad market fluctuations may adversely affect the trading price or liquidity of our common stock. In the past, when the market price of a stock has been volatile, holders of that stock have sometimes instituted securities class action litigation against the issuer. If any of our stockholders were to bring such a lawsuit against us, we could incur substantial costs defending the lawsuit and the attention of our management would be diverted from the operation of our business, which could seriously harm our financial position. Any adverse determination in litigation could also subject us to significant liabilities.
If securities or industry analysts do not publish research or reports about our business, or if they issue an adverse or misleading opinion regarding our stock, our stock price and trading volume could decline.
The trading market for our common stock is influenced by the research and reports that industry or securities analysts publish about us or our business. If one or more of the analysts currently covering our stock ceases coverage of us, the trading price for our stock would be negatively impacted. If any of the analysts who cover us issue an adverse or misleading opinion regarding us, our business model, our intellectual property or our stock performance, or if our clinical trials and operating results fail to meet the expectations of analysts, our stock price would likely decline. If one or more of these analysts cease coverage of us or fail to publish reports on us regularly, we could lose visibility in the financial markets, which in turn could cause our stock price or trading volume to decline.
We are an “emerging growth company” and as a result of the reduced disclosure and governance requirements applicable to emerging growth companies, our common stock may be less attractive to investors.
We are an “emerging growth company,” as defined in the JOBS Act, and we intend to take advantage of certain exemptions from various reporting requirements that are applicable to other public companies that are not emerging growth companies including, but not limited to, not being required to comply with the auditor attestation requirements of Section 404, reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements and exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and stockholder approval of any golden parachute payments not previously approved. We cannot predict if investors will find our common stock less attractive because we will rely on these exemptions. If some investors find our common stock less attractive as a result, there may be a less active trading market for our common stock and our stock price may be more volatile. We may take advantage of these reporting exemptions until we are no longer an emerging growth company. We will remain an emerging growth company until the earlier of (1) December 31, 2019, (2) the last day of the fiscal year in which we have total annual gross revenue of at least $1.0 billion, (3) the date on which we are deemed to be a large accelerated filer, which means the market value of our common stock that is held by non-affiliates exceeds $700 million as of the prior June 30th, and (4) the date on which we have issued more than $1.0 billion in non-convertible debt during the prior three-year period.
Raising additional capital may restrict our operations or require us to relinquish rights to our technologies or product candidates, and if we sell shares of our common stock in future financings, stockholders may experience immediate dilution and, as a result, our stock price may decline.
Until such time, if ever, as we can generate substantial product revenues, we expect to finance our cash needs through a combination of equity offerings, debt financings, partnerships and marketing, distribution or licensing arrangements. We do not have any committed external source of funds. We may also from time to time
74
Table of Contents
issue additional shares of common stock at a discount from the then current trading price of our common stock. As a result, our stockholders would experience immediate dilution upon the purchase of any shares of our common stock sold at such discount. In addition, as opportunities present themselves, we may enter into financing or similar arrangements in the future, including the issuance of debt securities, preferred stock or common stock. If we issue common stock or securities convertible into common stock, our common stockholders would experience additional dilution and, as a result, our stock price may decline. Additional debt financing, if available, may involve agreements that include covenants limiting or restricting our ability to take specific actions, such as incurring additional debt, making capital expenditures or declaring dividends.
If we raise additional funds through partnerships or marketing, distribution or licensing arrangements with third parties, we may have to relinquish valuable rights to our technologies, future revenue streams or product candidates or to grant licenses on terms that may not be favorable to us. If we are unable to raise additional funds through equity or debt financings when needed, we may be required to delay, limit, reduce or terminate our product development or future commercialization efforts or grant rights to develop and market product candidates that we would otherwise prefer to develop and market ourselves.
Our principal stockholders and management own a significant percentage of our stock and will be able to exert significant control over matters subject to stockholder approval.
As of December 31, 2014, our executive officers, directors, holders of 5% or more of our capital stock and their respective affiliates will beneficially own approximately 71% of our outstanding voting stock. Therefore, these stockholders will have the ability to influence us through this ownership position. These stockholders may be able to determine all matters requiring stockholder approval. For example, these stockholders may be able to control elections of directors, amendments of our organizational documents, or approval of any merger, sale of assets, or other major corporate transaction. This may prevent or discourage unsolicited acquisition proposals or offers for our common stock that you may feel are in your best interest as one of our stockholders.
Provisions in our charter documents and under Delaware law could discourage a takeover that stockholders may consider favorable and may lead to entrenchment of management.
Our seventh amended and restated certificate of incorporation and amended and restated bylaws contain provisions that could significantly reduce the value of our shares to a potential acquirer or delay or prevent changes in control or changes in our management without the consent of our board of directors. The provisions in our charter documents include the following:
| • | a classified board of directors with three-year staggered terms, which may delay the ability of stockholders to change the membership of a majority of our board of directors; |
| • | no cumulative voting in the election of directors, which limits the ability of minority stockholders to elect director candidates; |
| • | the exclusive right of our board of directors to elect a director to fill a vacancy created by the expansion of the board of directors or the resignation, death or removal of a director, which prevents stockholders from being able to fill vacancies on our board of directors; |
| • | the ability of our board of directors to authorize the issuance of shares of preferred stock and to determine the price and other terms of those shares, including preferences and voting rights, without stockholder approval, which could be used to significantly dilute the ownership of a hostile acquiror; |
| • | the ability of our board of directors to alter our amended and restated bylaws without obtaining stockholder approval; |
| • | the required approval of at least 66 2/3% of the shares entitled to vote at an election of directors to adopt, amend or repeal our amended and restated bylaws or repeal the provisions of our amended and restated certificate of incorporation regarding the election and removal of directors; |
75
Table of Contents
| • | a prohibition on stockholder action by written consent, which forces stockholder action to be taken at an annual or special meeting of our stockholders; |
| • | the requirement that a special meeting of stockholders may be called only by the chairman of the board of directors, the chief executive officer, the president or the board of directors, which may delay the ability of our stockholders to force consideration of a proposal or to take action, including the removal of directors; and |
| • | advance notice procedures that stockholders must comply with in order to nominate candidates to our board of directors or to propose matters to be acted upon at a stockholders’ meeting, which may discourage or deter a potential acquiror from conducting a solicitation of proxies to elect the acquiror’s own slate of directors or otherwise attempting to obtain control of us. |
In addition, these provisions would apply even if we were to receive an offer that some stockholders may consider beneficial.
We are also subject to the anti-takeover provisions contained in Section 203 of the Delaware General Corporation Law. Under Section 203, a corporation may not, in general, engage in a business combination with any holder of 15% or more of its capital stock unless the holder has held the stock for three years or, among other exceptions, the board of directors has approved the transaction.
Claims for indemnification by our directors and officers may reduce our available funds to satisfy successful third-party claims against us and may reduce the amount of money available to us.
Our seventh amended and restated certificate of incorporation and amended and restated bylaws provide that we will indemnify our directors and officers, in each case to the fullest extent permitted by Delaware law.
In addition, as permitted by Section 145 of the Delaware General Corporation Law, our amended and restated bylaws and our indemnification agreements that we have entered into with our directors and officers provide that:
| • | we will indemnify our directors and officers for serving us in those capacities or for serving other business enterprises at our request, to the fullest extent permitted by Delaware law. Delaware law provides that a corporation may indemnify such person if such person acted in good faith and in a manner such person reasonably believed to be in or not opposed to the best interests of the registrant and, with respect to any criminal proceeding, had no reasonable cause to believe such person’s conduct was unlawful; |
| • | we may, in our discretion, indemnify employees and agents in those circumstances where indemnification is permitted by applicable law; |
| • | we are required to advance expenses, as incurred, to our directors and officers in connection with defending a proceeding, except that such directors or officers shall undertake to repay such advances if it is ultimately determined that such person is not entitled to indemnification; |
| • | we will not be obligated pursuant to our amended and restated bylaws to indemnify a person with respect to proceedings initiated by that person against us or our other indemnitees, except with respect to proceedings authorized by our board of directors or brought to enforce a right to indemnification; |
| • | the rights conferred in our amended and restated bylaws are not exclusive, and we are authorized to enter into indemnification agreements with our directors, officers, employees and agents and to obtain insurance to indemnify such persons; and |
| • | we may not retroactively amend our bylaw provisions to reduce or eliminate our indemnification obligations to directors, officers, or employees and agents serving at the request of such persons, after the occurrence of the civil, criminal, administrative or investigative act or omission for which indemnification is sought. |
76
Table of Contents
Our ability to use our net operating loss carryforwards and certain other tax attributes is limited and may be further limited.
As of December 31, 2014, we had gross federal income tax net operating loss, or NOL, carryforwards of approximately $93.7 million and federal research tax credit carryforwards of approximately $5.2 million, net of limitations pursuant to Section 382 of the Internal Revenue Code of 1986, as amended, or the Code, discussed below. These NOL carryforwards and research tax credit carryforwards, if not previously used, will begin to expire in 2029. The future utilization of our NOL carryforwards and research tax credit carryforwards will be subject to certain annual limitations, pursuant to Section 382 of the Code, as a result of several ownership changes since the Company was founded in 2008, with the most recent Section 382 change of ownership occurring on June 23, 2014. We estimate the ownership changes will result in $1.5 million of net operating loss carryforwards expiring unused and $0.1 million of federal research tax credit carryforwards expiring unused. Moreover, we may experience ownership changes in the future as a result of subsequent shifts in our stock ownership. As a result, if we earn net taxable income in the future, the limitations on our ability to use our NOL carryforwards and research tax credit carryforwards to reduce U.S. federal tax liability could potentially result in higher future tax liability to us compared to what we would have incurred had we been able to fully take advantage of these assets.
| ITEM 1B. | UNRESOLVED STAFF COMMENTS |
None.
| ITEM 2. | PROPERTIES |
Our headquarters is currently located in Coppell, Texas, and consists of 25,682 square feet of leased office, manufacturing and laboratory space under a lease that expires on September 30, 2023. We also have 19,145 square feet of manufacturing space in Denver, Colorado and 6,370 square feet of office space in Redwood City, California. We believe that our existing facilities are adequate for our current needs; however, we may require additional space and facilities as our business expands.
| ITEM 3. | LEGAL PROCEEDINGS |
We are not currently subject to any material legal proceedings.
| ITEM 4. | MINE SAFETY DISCLOSURES |
Not applicable.
77
Table of Contents
PART II.—FINANCIAL INFORMATION
| ITEM 5. | MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF COMMON EQUITY |
Our common stock has been publicly traded on The Nasdaq Global Market under the symbol “ZSPH” since June 18, 2014. Prior to that time, there was no public market for our common stock. Our IPO was priced at $18.00 per share on June 17, 2014. The following table sets forth the high and low sale prices per share for our common stock on The Nasdaq Global Market for the periods indicated:
| Year ended December 31, 2014 | High | Low | ||||||
| Second Quarter (beginning June 18, 2014) |
$ | 30.67 | $ | 26.10 | ||||
| Third Quarter |
$ | 43.00 | $ | 25.51 | ||||
| Fourth Quarter |
$ | 49.77 | $ | 31.07 | ||||
On March 12, 2015, the last reported sale price of our common stock on The Nasdaq Global Market was $47.62 per share. As of March 1, 2015, we had approximately 56 holders of record of our common stock. This number does not include beneficial owners whose shares are held by nominees in street name.
Dividend Policy
We have never declared or paid cash dividends on our capital stock. We intend to retain all available funds and any future earnings, if any, to fund the development and expansion of our business and we do not anticipate paying any cash dividends in the foreseeable future. Any future determination related to dividend policy will be made at the discretion of our board of directors.
Issuer Purchases of Equity Securities
During the quarter and year ended December 31, 2014, we did not purchase any of our equity securities that are registered under Section 12(b) of the Exchange Act.
Securities Authorized for Issuance Under Equity Compensation Plans
Set forth below is certain information about the Company’s common stock authorized for issuance under the Company’s equity compensation plan.
| Plan category |
Number of securities to be issued upon exercise of outstanding options, warrants and rights |
Weighted-average exercise price of outstanding options, warrants and rights |
Number of securities remaining available for future issuance under equity compensation plans (excluding securities reflected in column (a)) |
|||||||||
| (a) | (b) | (c) | ||||||||||
| Equity compensation plans approved by security holders |
4,732,564 | $ | 7.52 | 1,215,385 | ||||||||
| Equity compensation plans not approved by security holders |
— | — | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Total |
4,732,564 | $ | 7.52 | 1,215,385 | ||||||||
78
Table of Contents
Stock Performance Graph
The following graph shows a comparison from June 18, 2014 (the date our common stock commenced trading on the NASDAQ) through December 31, 2014 of the total cumulative return of our common stock with the total cumulative return of the S&P 500 Index (“S&P 500”), the NASDAQ Biotechnology Total Return Index (“NBI”) and the NSYE Arca Biotechnology Total Return Index (“BTK”). The figures below assume an investment of $100 of our common stock at the closing price of $28.38 on June 18, 2014 and in the S&P 500, NBI and BTK. Data for the S&P 500, NBI and BTK assume reinvestment of dividends. The comparisons in the graph are historical and are not intended to forecast or be indicative of possible future performance of our common stock.
This performance graph is not deemed filed with the SEC and is not to be incorporated by reference into any of our filings under the Securities Act of 1933, as amended, or the Securities Exchange Act of 1934, as amended, unless such filings specifically incorporate the performance graph by reference therein.
Recent Sales of Unregistered Securities
The following sets forth information regarding all unregistered securities sold since January 1, 2012.
Convertible Notes, Warrants and Preferred Stock
In November 2011, December 2011, and July 2012, we issued an aggregate of $5.3 million of bridge notes to 19 accredited investors. The bridge notes were issued pursuant to Section 4(a)(2) of the Securities Act. In November 2011, Chigwell Consulting, Inc. converted its note into warrants to purchase 11,766 shares of Series B preferred stock at an exercise price of $3.72 per share, which were fully exercised in June 2014. The warrants were issued to Chigwell Consulting Inc. pursuant to Section 4(a)(2) of the Securities Act. In October 2012, upon the consummation of our Series C preferred stock financing, the remaining bridge notes were converted into shares of our Series B preferred stock at a price per share of $3.72. In connection with the conversion of the bridge notes, warrants to purchase shares of our Series B preferred stock were issued pursuant to the terms of the bridge notes.
79
Table of Contents
The warrants were fully exercised in May 2014 for 365,986 shares of our Series B preferred stock at an exercise price of $3.72 per share.
In November 2011, December 2011, and July 2012, we issued warrants to purchase 51,166 shares, 6,723 shares, and 13,447 shares of our common stock, respectively, at a price per share of $3.72 to Salem Partners, LLC, in connection with the Engagement Agreements, dated September 27, 2010 and June 29, 2012 with Salem Partners, LLC, whereby we committed to engage Salem Partners, LLC on an exclusive basis as our financial advisor pursuant to the terms therein. In July 2013, Salem Partners, LLC transferred these warrants to Salem Capital Partners, LLC. Salem Capital Partners, LLC transferred these warrants to five accredited investors in October 2013. In November 2013, we issued an aggregate of 71,336 shares of our common stock at a price per share of $3.72 upon the exercise of the Common Stock Warrants for an aggregate gross consideration of $0.3 million to such accredited investors. The shares of common stock were issued to such accredited investors pursuant to Section 4(a)(2) of the Securities Act.
In October 2012, November 2012, and August 2013, we issued an aggregate of 6,899,281 shares of our Series C preferred stock at a price per share of $6.67 for aggregate gross consideration of $46.0 million to 7 accredited investors. The shares of Series C preferred stock were issued to such accredited investors pursuant to Section 4(a)(2) of the Securities Act.
In January 2012, we issued warrants to purchase an aggregate of 29,247 shares of our common stock at a price per share of $2.56 (9,749 shares each) to Miles B. Davis, Marc Ostro, and Robert Truitt, as non-cash compensation for those individuals’ continued participation as outside Board members for 2012. In April 2014, Marc Ostro transferred his warrants to Devon Park Associates, L.P. In May 2014, Devon Park Associates, L.P. exercised the warrant for 9,749 shares of our common stock at an exercise price of $2.56 per share. In May 2014, all remaining Common Stock Warrants were fully exercised. The warrants were issued to such individuals pursuant to Section 4(a)(2) of the Securities Act.
In August 2013, we issued warrants to purchase 344,964 shares of our common stock, at a price of $6.67 per share to Salem Capital Partners, LLC, in connection with that certain Engagement Agreement, dated June 29, 2012 with Salem Partners, LLC. In May 2014, Salem Capital Partners, LLC transferred these warrants to five accredited investors, and we issued an aggregate of 344,964 shares of our common stock upon the exercise of these warrants for an aggregate gross consideration of $2.3 million. The shares of common stock were issued to such accredited investors pursuant to Section 4(a)(2) of the Securities Act.
In February 2014, we issued an aggregate of 1,858,012 shares of our Series D preferred stock at a price per share of $13.455 for aggregate gross consideration of $25.0 million to 12 accredited investors. The shares of Series D preferred stock were issued to such accredited investors pursuant to Section 4(a)(2) of the Securities Act.
Stock Options
From January 1, 2011 through the filing our registration statement on Form S-8 on July 3, 2014, we granted stock options and stock awards to employees, directors and consultants under our 2008 Stock Incentive Plan, as amended, and our 2014 Incentive Plan, covering an aggregate of 4,067,579 shares of common stock, at a weighted-average exercise price of $4.40 per share. Of these, options covering an aggregate of 19,498 shares were cancelled without being exercised and we sold an aggregate of 23,493 shares of common stock to employees and consultants for cash consideration in the aggregate amount of $24,198 upon the exercise of stock options. We claimed exemption from registration under the Securities Act for those sales and issuances under Section 4(a)(2) of the Securities Act in that such sales and issuances did not involve a public offering or under Rule 701 promulgated under the Securities Act, in that they were offered and sold either pursuant to written compensatory plans or pursuant to a written contract relating to compensation, as provided by Rule 701.
80
Table of Contents
Use of Proceeds
On June 17, 2014, our registration statement on Form S-1 (File No. 333-195961) relating to the IPO of our common stock was declared effective by the SEC. J.P. Morgan Securities LLC and Credit Suisse Securities (USA) LLC acted as joint book-running managers and representatives of the underwriters in the offering.
The shares began trading on The NASDAQ Global Select Market on June 18, 2014. The sale of the shares in our IPO closed on June 23, 2014 and we sold 6,836,111 shares of common stock, which included 891,667 shares of common stock issued pursuant to the option to purchase additional shares granted to the underwriters. The total proceeds of our IPO, based on the public offering price of $18.00 per share, were approximately $123.0 million. There were no purchases of our securities by us or any “affiliated purchaser” as defined in Rule 10b-18(a)(3) of the Exchange Act. We received net proceeds from the offering of $114.4 million, after deducting underwriting discounts and commissions of $8.6 million. After deducting offering expenses of approximately $2.3 million, remaining proceeds were $112.1 million. Upon the closing of the IPO, all shares of convertible preferred stock then outstanding converted into 11,979,479 shares of common stock. No underwriting discounts or expenses resulted in direct or indirect payments to any of our directors, officers or their associates, to persons owning 10% or more of our common stock or to any of our affiliates.
The net proceeds from our IPO have been used and will be used, together with our cash, cash equivalents and short- term investments, to fund continued advancement of our Phase III ZS004e clinical trial, long-term safety trial ZS005, manufacturing scale-up activities and pre-clinical programs, with the balance to be used to fund working capital, capital expenditures and other general corporate purposes, which may include in-licenses, acquiring, or investing in additional businesses, technologies, products, or assets the acquisition or licensing of other products, businesses or technologies.
There has been no material change in the planned use of proceeds from our initial public offering as described in our prospectus dated June 17, 2014, filed with the SEC pursuant to Rule 424(b) (4) of the Securities Act.
81
Table of Contents
| ITEM 6. | SELECTED FINANCIAL DATA |
In the table below, we provide you with summary historical financial data of ZS Pharma. We have prepared this information using the financial statements of ZS Pharma for the three years ended December 31, 2014, 2013 and 2012. The financial statements for the three years ended December, 31 2014, 2013 and 2012 have been audited by Ernst & Young LLP, independent registered public accounting firm.
When you read this summary historical financial data, it is important that you read it together with the historical financial statements and related notes in our annual and quarterly reports filed with the SEC, as well as the section of our annual and quarterly reports titled “Management’s Discussion and Analysis of Financial Condition and Results of Operations” which are included in this form 10-K.
| Fiscal Years Ended December 31, | ||||||||||||
| 2014 | 2013 | 2012 | ||||||||||
| Statement of Operations Data: |
||||||||||||
| Operating expenses: |
||||||||||||
| Research and development |
$ | 45,618 | $ | 24,762 | $ | 6,989 | ||||||
| General and administrative |
14,919 | 7,432 | 1,148 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total operating expenses |
60,537 | 32,194 | 8,137 | |||||||||
|
|
|
|
|
|
|
|||||||
| Loss from operations |
(60,537 | ) | (32,194 | ) | (8,137 | ) | ||||||
| Interest income |
(94 | ) | (30 | ) | (17 | ) | ||||||
| Interest expense |
530 | 9 | 2,099 | |||||||||
| Expense to mark warrants to market |
3,071 | 1,424 | 62 | |||||||||
|
|
|
|
|
|
|
|||||||
| Net loss |
$ | (64,044 | ) | $ | (33,597 | ) | $ | (10,281 | ) | |||
| Preferred stock accretion |
(310 | ) | (689 | ) | (174 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Net loss attributable to common stockholders |
$ | (64,354 | ) | $ | (34,286 | ) | $ | (10,455 | ) | |||
|
|
|
|
|
|
|
|||||||
| Per share information: |
||||||||||||
| Net loss attributable to common stockholders, basic and diluted |
$ | (5.47 | ) | $ | (21.84 | ) | $ | (6.74 | ) | |||
|
|
|
|
|
|
|
|||||||
| Weighted-average common shares used to compute net loss per share attributable to common stockholders, basic and diluted(1) |
11,768,267 | 1,569,583 | 1,552,276 | |||||||||
|
|
|
|
|
|
|
|||||||
| (1) | The net loss per share of common stock, basic and diluted, does not give effect to potential dilutive securities where the impact would be anti-dilutive. |
| As of December 31, | ||||||||
| 2014 | 2013 | |||||||
| Balance Sheet Data: |
||||||||
| Cash, cash equivalents and short-term investments |
$ | 102,280 | $ | 9,170 | ||||
| Working capital |
94,117 | 4,571 | ||||||
| Total assets |
116,162 | 14,046 | ||||||
| Convertible preferred stock warrant liability |
— | 2,667 | ||||||
| Convertible preferred stock |
— | 52,830 | ||||||
| Accumulated deficit |
(114,278 | ) | (50,234 | ) | ||||
| Total stockholders’ equity (deficit) |
96,772 | (45,182 | ) | |||||
82
Table of Contents
| ITEM 7. | MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS |
You should read the following discussion and analysis of our financial condition and results of operations together with the section entitled “Item 8. Financial Statements and Supplementary Data” and our financial statements and related notes included elsewhere in this report. This discussion and other parts of this report contain forward-looking statements that involve risks and uncertainties, such as our plans, objectives, expectations, intentions and beliefs. Our actual results could differ materially from those discussed in these forward-looking statements. Factors that could cause or contribute to such differences include, but are not limited to, those discussed in under “Item 1A. Risk Factors” or included elsewhere in this report.
Overview
We are a biopharmaceutical company focused on the development and commercialization of highly selective, non-absorbed drugs to treat renal, cardiovascular, liver and metabolic diseases. Our proprietary zirconium silicate technology allows us to create highly selective ion traps that can reduce toxic levels of specific electrolytes without disturbing the balance of other electrolytes. Our initial focus is on the development of sodium zirconium cyclosilicate (ZS-9), our product candidate for which we recently completed two Phase III clinical trials for the treatment of hyperkalemia, a life-threatening condition in which elevated levels of potassium in the blood (greater than 5.0 mEq/L) increase the risk of muscle dysfunction, including cardiac arrhythmias and sudden cardiac death. We have designed our development program based on input from the United States Food and Drug Administration, or FDA, including a recent pre-NDA meeting, and European Medicines Agency, or EMA, and plan to submit our New Drug Application, or NDA, in the United States in the second quarter of 2015 and our Marketing Authorization Application, or MAA, in Europe in the second half of 2015 with the goal of obtaining approval for the treatment of acute and chronic hyperkalemia, regardless of the underlying disease state. If we receive regulatory approval, we intend to commercialize ZS-9 for the treatment of hyperkalemia in the United States with our own specialty sales force targeting nephrologists and cardiologists and intend to seek one or more partners for commercialization in markets outside of the United States.
As of March 1, 2015, we have enrolled over 1,474 patients in our clinical studies. In our ongoing long-term studies, patients have safely received once daily ZS-9 for over 10 months. We have completed three, double-blind, randomized placebo controlled clinical studies with ZS-9 that together enrolled 1,101 patients with hyperkalemia, including patients with chronic kidney disease (CKD), heart failure (HF), type 2 diabetes and those on renin-angiotensin aldosterone system (RAAS) inhibitor therapy. Our first in-man study, ZS002, was completed in May 2012 and our first Phase III study, ZS003, was completed in November 2013. Our second Phase III study, ZS004, was completed in September 2014. All three trials met their pre-specified primary efficacy endpoints with clinically meaningful and statistically significant results. We announced the top-line results for ZS004 in September 2014 and presented detailed primary and secondary endpoint results for ZS004 at the American Heart Association Scientific Sessions on November 17, 2014. The ZS004 study results were simultaneously published in the Journal of the American Medical Association, and we announced the publication of detailed results from the ZS003 study in the New England Journal of Medicine on November 21, 2014. We initiated an extension to the ZS004 study, or ZS004e, and a long-term safety study, ZS005, in the second quarter of 2014. We expect to file our NDA with the FDA in the second quarter of 2015 and our MAA with the EMA in the second half of 2015.
ZS-9 was developed utilizing proprietary zirconium silicate technology, the rights to which we currently have pursuant to a 2011 license agreement with UOP, which grants us an exclusive license under specified patent rights held by UOP to develop and commercialize pharmaceutical products for use in the field of removing toxins from bodily fluids and the gastrointestinal (GI) tract of humans and animals, which includes ZS-9 and any other product covered by the terms of the license agreement. Under the terms of the license agreement, we will owe UOP royalties equal to 5% of worldwide net sales of ZS-9 made by us or our sublicensees, and we are obligated to make a minimum annual royalty payment to UOP, which commenced with payments of $25,000 and $50,000 in 2010 and 2011, respectively, increasing to $100,000 for 2012 and years thereafter.
83
Table of Contents
We have never been profitable and, as of December 31, 2014, had an accumulated deficit of $114.3 million. We incurred net losses of approximately $64.0 million, $33.6 million and $10.3 million in the fiscal years ended December 31, 2014, 2013 and 2012, respectively. We expect to continue to incur net losses as we advance ZS-9 through clinical development, seek regulatory approval, expand our manufacturing capabilities and prepare for and, if approved, proceed to commercialization. We manufacture clinical trial quantities of ZS-9 in-house in two facilities from readily available starting materials using specialized equipment. We are currently in the process of increasing our manufacturing capabilities to support anticipated commercial demand. Additionally, we have established a supply chain to provide us with the materials required to manufacture ZS-9. We expect to significantly increase our investment in our commercial manufacturing process and inventory of ZS-9, and in our commercialization and marketing related activities as we prepare for a possible commercial launch of ZS-9. We do not have a sales organization, and we will need substantial additional funding to support our operating activities. Adequate funding may not be available to us on acceptable terms, or at all. Our failure to obtain sufficient funds on acceptable terms when needed could have a material adverse effect on our business, results of operations and financial condition.
Financial Overview
On June 23, 2014, we completed our IPO of 6,836,111 shares of our common stock for net proceeds of $112.1 million after deducting underwriting discounts and commissions and offering expenses. We have invested substantially all of our efforts and financial resources to identify, acquire, and develop our product candidates, and in particular ZS-9, including conducting clinical studies and providing general and administrative support for these operations. To date, we have funded our operations primarily from the sale of convertible preferred stock and equity securities and a small amount of secured debt. We have never been profitable and have incurred net losses in each year since inception. Our net losses were $64.0 million, $33.6 million and $10.3 million for the fiscal years ended December 31, 2014, 2013 and 2012. As of December 31, 2014 we had an accumulated deficit of $114.3 million. Substantially all of our net losses have resulted from costs incurred in connection with our research and development programs and from general and administrative costs associated with our operations.
Revenue
To date, we have not generated any revenues. Our ability to generate product revenues, which we do not expect will occur before 2016, at the earliest, will depend heavily on our obtaining marketing approval from the FDA and EMA for, and, subsequent to that, our successful commercialization of ZS-9. If we fail to complete the development of ZS-9 in a timely manner or to obtain regulatory approval, our ability to generate future revenue, and our results of operations and financial position, would be materially adversely effected.
Research and Development Expenses
Our research and development expenses consist primarily of:
| • | salaries and related costs, including stock-based compensation expense, for personnel in our research and development functions; |
| • | costs related to nonclinical studies in animal models; |
| • | fees paid to clinical consultants, clinical trial sites and vendors in conjunction with implementing and monitoring our clinical trials and acquiring and evaluating clinical trial data, including all related fees, such as for investigator grants, patient screening fees, laboratory work and statistical compilation and analysis; |
| • | costs related to production of clinical supplies, including fees paid to contract packagers; |
| • | costs related to compliance with drug development regulatory requirements; |
| • | annual minimum royalty payments to UOP pursuant to a license agreement; and |
| • | depreciation and other allocated facility-related and overhead expenses. |
84
Table of Contents
We expense both internal and external research and development costs in the periods in which they are incurred. To date, we have focused substantially all of our resources and development efforts on the development of ZS-9 and we incurred research and development costs of $45.6 million, $24.8 million and $7.0 million in the years 2014, 2013 and 2012, respectively.
We expect our research and development expenses to increase during the next few years as we seek to complete our clinical program, pursue regulatory approval of ZS-9 in the United States and internationally and prepare for a possible commercial launch of ZS-9, which, if approved, will require significant investment to increase our manufacturing capabilities to support anticipated commercial demand. Product candidates in later stages of clinical development generally have higher development costs than those in earlier stages, primarily due to increased size and greater duration of the related clinical trials, which we expect to be the case for our ZS004e and ZS005 clinical trials. Predicting the timing or the final cost to complete our clinical program and/or validation of our commercial manufacturing and supply processes is difficult and delays or unexpected costs may occur because of many factors, including factors outside of our control. Furthermore, we are unable to predict when or if ZS-9 will receive regulatory approval in the United States or in any other countries with any certainty.
General and Administrative Expenses
Our general and administrative expenses consist primarily of personnel costs, including salaries, benefits and stock-based compensation expense, associated with our executive, finance, business and corporate development and other administrative functions. Other general and administrative expenses include allocated depreciation and facility-related costs, legal costs of pursuing patent protection of our intellectual property and professional fees for consulting, auditing, tax and legal services. We incurred general and administrative expenses of $14.9 million, $7.4 million and $1.1 million in the years 2014, 2013 and 2012, respectively.
We expect our general and administrative expenses will increase as we expand our operating activities and increase our headcount as we begin to prepare for a potential commercial launch of ZS-9 and to support our operations as a public company. Additionally, we anticipate increased expenses as compared to the expenses we incurred prior to our IPO in June of 2014 related to audit, legal, regulatory and tax-related services associated with maintaining compliance with exchange listing and SEC requirements, director and officer insurance premiums and investor relations costs.
Interest Income
Interest income consists primarily of interest received or earned on our cash, cash equivalents and short-term investments. We expect interest income to vary each reporting period depending on our average cash, cash equivalents and short-term investments balances during the period and applicable interest rates.
Interest Expense
Interest expense consists of cash and noncash interest costs related to our borrowings. The noncash interest costs through October 2012 consisted of the fair value of shares issued for interest earned on convertible notes during the period the notes were outstanding, the amortization of the beneficial conversion feature of the convertible notes over the period the notes were outstanding, the amortization of the fair value of warrants that were issued in connection with our borrowings, with the initial fair value of the warrants being amortized to interest expense over the term of the governing agreements, and since November 2012 consists of the amortization of debt issuance costs, primarily legal and banker fees, and debt discount in connection with the notes issued under the MidCap Credit Facility (as defined in “—Liquidity and Capital Resources”) over the period the notes are expected to be outstanding.
85
Table of Contents
Expense to Mark Warrants to Market
Expense to mark warrants to market includes gains and losses from the re-measurement of our liabilities related to our warrants to purchase shares of our Series B preferred stock issued in November 2011 and October 2012, or the Series B Warrants. We recorded adjustments to the estimated fair value of the convertible preferred stock warrants until such time as these instruments were exercised. At that time, the convertible preferred stock warrant liability was reclassified to additional paid- in capital, a component of stockholders’ equity (deficit), and we no longer recorded any related periodic fair value adjustments.
Results of Operations
Comparison of the Years Ended December 31, 2014 and 2013
| Year Ended December 31, | Change | |||||||||||||||
| 2014 | 2013 | $ | % | |||||||||||||
| (in thousands, except percentages) |
||||||||||||||||
| Operating expenses: |
||||||||||||||||
| Research and development |
$ | 45,618 | $ | 24,762 | $ | 20,856 | 84 | % | ||||||||
| General and administrative |
14,919 | 7,432 | 7,487 | 101 | % | |||||||||||
|
|
|
|
|
|
|
|||||||||||
| Total operating expenses |
60,537 | 32,194 | 28,343 | 88 | % | |||||||||||
|
|
|
|
|
|
|
|||||||||||
| Loss from operations |
(60,537 | ) | (32,194 | ) | (28,343 | ) | 88 | % | ||||||||
| Interest income |
(94 | ) | (30 | ) | (64 | ) | ** | |||||||||
| Interest expense |
530 | 9 | 521 | ** | ||||||||||||
| Expense to mark warrants to market |
3,071 | 1,424 | 1,647 | 116 | % | |||||||||||
|
|
|
|
|
|
|
|||||||||||
| Net loss |
$ | (64,044 | ) | $ | (33,597 | ) | $ | (30,447 | ) | 91 | % | |||||
|
|
|
|
|
|
|
|||||||||||
| ** | Percentage not meaningful |
Research and Development. Research and development expenses increased $20.9 million, or 84%, from $24.8 million for the year ended December 31, 2013 to $45.6 million for the year ended December 31, 2014. The increase was primarily due to increases in medical affairs activities of $7.2 million and clinical trial related expenses of $4.2 million in support of our product candidate ZS-9. In addition, personnel costs increased by $3.5 million, non-cash, stock-based compensation costs increased $3.0 million and certain other costs increased by $2.9 million in connection with our expanded clinical and manufacturing activities.
General and Administrative. General and administrative expenses increased $7.5 million, or 101%, from $7.4 million for the year ended December 31, 2013 to $14.9 million for the year ended December 31, 2014. The increase was primarily due to $3.1 million of non-cash, stock-based compensation expense related to stock options granted, $2.3 million in legal and accounting professional fees and other miscellaneous costs associated with our increased activity and increased complexity as a public company, increases of $1.4 million in personnel costs as a result of an increase in headcount to support growth in our operations and $0.7 million in expenses related to medical education.
Expense to mark warrants to market. Expense to mark warrants to market increased $1.6 million in 2014 to $3.1 million for the year ended December 31, 2014 from $1.4 million for the year ended December 31, 2013. The increase was due to the increased value of warrants to acquire Series B preferred stock as we approached our IPO.
Interest Expense. Interest expense increased $0.5 million in 2014, primarily due to amortization of debt discount and debt issuance costs during the year.
86
Table of Contents
Comparison of the Years Ended December 31, 2013 and 2012
| Year Ended December 31, | Change | |||||||||||||||
| 2013 | 2012 | $ | % | |||||||||||||
| (in thousands, except percentages) |
||||||||||||||||
| Operating expenses: |
||||||||||||||||
| Research and development |
$ | 24,762 | $ | 6,989 | $ | 17,773 | 254 | % | ||||||||
| General and administrative |
7,432 | 1,148 | 6,284 | 547 | % | |||||||||||
|
|
|
|
|
|
|
|||||||||||
| Total operating expenses |
32,194 | 8,137 | 24,057 | 296 | % | |||||||||||
|
|
|
|
|
|
|
|||||||||||
| Loss from operations |
(32,194 | ) | (8,137 | ) | (24,057 | ) | 296 | % | ||||||||
| Interest income |
(30 | ) | (17 | ) | (13 | ) | 76 | % | ||||||||
| Interest expense |
9 | 2,099 | (2,090 | ) | (100 | %) | ||||||||||
| Expense to mark warrants to market |
1,424 | 62 | 1,362 | ** | ||||||||||||
|
|
|
|
|
|
|
|||||||||||
| Net loss |
$ | (33,597 | ) | $ | (10,281 | ) | $ | (23,316 | ) | 227 | % | |||||
|
|
|
|
|
|
|
|||||||||||
| ** | Percentage not meaningful |
Research and Development. Research and development expenses increased $17.8 million, or 254%, from $7.0 million for the year ended December 31, 2012 to $24.8 million for the year ended December 31, 2013. The increase was primarily due to increases in clinical trial related expenses of $11.1 million attributable to conducting and supporting our Phase III pivotal trial. In addition, personnel costs increased by $3.5 million and certain other costs increased by $3.2 million in connection with our expanded clinical and manufacturing activities.
General and Administrative. General and administrative expenses increased $6.3 million, or 547%, from $1.1 million for the year ended December 31, 2012 to $7.4 million for the year ended December 31, 2013. The increase was primarily due to increases of $2.4 million in personnel costs as a result of an increase in headcount to support growth in our operations, $2.1 million in legal and accounting professional fees and other miscellaneous costs associated with our increased activity and $1.8 million in expenses related to medical education.
Expense to mark warrants to market. Expense to mark warrants to market increased $1.4 million in 2013 to $1.4 million for the year ended December 31, 2013 from $62,000 for the year ended December 31, 2012. The increase was due to the increased value of warrants to acquire Series B preferred stock as we approached our IPO.
Interest Expense. Interest expense decreased $2.1 million for the year ended December 31, 2013 due to the amortization of debt discount and deferred debt issuance costs in 2012 related to the convertible note financing, which were fully amortized in October 2012 when the notes were converted into Series B preferred stock.
Liquidity and Capital Resources
Due to our significant research and development expenditures, we have generated significant operating losses since our inception. We have funded our operations primarily through our IPO, sales of our preferred shares of stock and a small amount of secured debt. Our expenditures are primarily related to research and development activities. At December 31, 2014, we had available cash and cash equivalents of $47.4 million and short-term investments of $54.9 million. Our cash, cash equivalents and short-term investments are currently held in a variety of interest and non-interest bearing accounts at financial institutions and invested in a variety of short-term interest-bearing instruments. Cash in excess of immediate requirements is invested with a view toward liquidity and capital preservation, and we seek to minimize the potential effects of concentration and degrees of risk.
87
Table of Contents
In July 2014, we entered into a Credit and Security Agreement (Credit Agreement) with MidCap Financial SBIC, LP (MidCap) for a $20 million credit facility, secured by all of our assets, with a negative pledge against our intellectual property (with all amendments thereto, the MidCap Credit Facility). The MidCap Credit Facility is structured in two $10 million tranches, with the first tranche previously drawn at closing and the second tranche to be drawn after we provide evidence to MidCap of positive primary endpoint results from our ZS004 clinical study, which has occurred, but before its expiration on October 31, 2014. In September 2014, we entered into the First Amendment to Credit and Security Agreement, which extended the second tranche commitment termination date from October 31, 2014 to March 31, 2015. Notes issued under the terms of the Credit Agreement bear interest at the rate of 7.5% per annum, payable monthly, have principal payable in 36 monthly installments commencing on January 1, 2016 and are subject to a $50,000 fee which we paid to MidCap upon closing. On January 1, 2019, the maturity date of the notes, we will owe MidCap an exit fee equal to 6.5% of the amount of notes funded under the Credit Agreement. We issued a note payable to MidCap in the amount of $10 million for the first tranche on July 14, 2014. The 6.5% exit fee is being recognized as additional interest expense over the term of the note. Also in September 2014, MidCap assigned $2.5 million of the credit facility to Silicon Valley Bank.
On June 23, 2014, we closed our IPO of 6,836,111 shares of our common stock, which included 891,667 shares of common stock issued pursuant to the option to purchase additional shares granted to the underwriters. The public offering price of the shares sold in the offering was $18.00 per share. The total proceeds from the offering to us, net of underwriting discounts and commissions of approximately $8.6 million, were approximately $114.4 million. After deducting offering expenses payable by us of approximately $2.3 million, our net proceeds were approximately $112.1 million. Upon the closing of the IPO, 11,979,479 shares of Preferred Stock and Redeemable Preferred Stock then outstanding automatically converted into 11,979,479 shares of our common stock.
Summary Statement of Cash Flows
The following table shows a summary of our cash flows for each of the years ended December 31, 2014, 2013 and 2012.
| Years Ended December 31, | ||||||||||||
| 2014 | 2013 | 2012 | ||||||||||
| (in thousands) | ||||||||||||
| Net cash (used in) provided by: |
||||||||||||
| Operating activities |
$ | (49,021 | ) | $ | (26,529 | ) | $ | (7,174 | ) | |||
| Investing activities |
(62,888 | ) | (3,771 | ) | (706 | ) | ||||||
| Financing activities |
150,141 | 15,069 | 28,633 | |||||||||
|
|
|
|
|
|
|
|||||||
| Net increase (decrease) in cash and cash equivalents |
$ | 38,232 | $ | (15,231 | ) | 20,753 | ||||||
|
|
|
|
|
|
|
|||||||
Cash Flows from Operating Activities.
Net cash used in operating activities was $49.0 million for the year ended December 31, 2014 and consisted primarily of our net loss of $64.0 million, less noncash charges such as stock-based compensation expense of $7.7 million and $3.1 million related to the revaluation of warrants to purchase convertible preferred stock. The significant items in the change in operating assets and liabilities include an increase in accounts payable of $2.5 million and a decrease in prepaid and other expenses of $1.1 million.
Net cash used in operating activities was $26.5 million for the year ended December 31, 2013 and consisted primarily of our net loss of $33.6 million, less noncash charges such as stock-based compensation expense of $2.1 million and $1.4 million related to the revaluation of warrants to purchase convertible preferred stock. The significant items in the change in operating assets and liabilities include increases in accrued liabilities of $2.3 million and accounts payable of $0.5 million.
88
Table of Contents
Net cash used in operating activities was $7.2 million for the year ended December 31, 2012 and consisted primarily of our net loss of $10.3 million, less noncash charges such as stock-based compensation expense of $0.2 million, depreciation expense and amortization of certain debt related costs of $1.9 million and interest expense relating to our Series B preferred stock of $0.3 million. The significant items in the change in operating assets and liabilities include an increase in accounts payable of $0.5 million and an increase in accrued liabilities of $0.1 million.
Cash Flows from Investing Activities. Net cash used in investing activities for the fiscal years ended December 31, 2014, 2013 and 2012 was $62.9 million, $3.8 million and $0.7 million respectively, with the use in the year ended December 31, 2014 consisting primarily of investment in marketable securities of $54.9 million and purchases of property and equipment and build-out of our production facility of $8.0 million. For the fiscal years ended December 31, 2013 and 2012 net cash used in investing activities consisted primarily of purchases of property and equipment of $3.8 million and $0.7 million, respectively.
Cash Flows from Financing Activities. Net cash provided by financing activities for the fiscal years ended December 2014, 2013 and 2012 was $150.1 million, $15.1 million and $28.6 million, respectively. For the year ended December 31, 2014, net cash provided by financing activities consisted mainly of proceeds of $112.1 million from the issuance of common stock, proceeds from the issuance of debt of $9.8 million, and proceeds from the issuance of Series D preferred stock of $24.8 million. For the year ended December 31, 2013, net cash provided by financing activities consisted mainly of the net proceeds from the issuance of the second tranche of our Series C preferred stock of $15.1 million. For the year ended December 31, 2012, net cash provided by financing activities consisted mainly of the net proceeds from the issuance of the second tranche of our convertible notes of $0.9 million and the net proceeds from the issuance of the first tranche of our Series C preferred stock of $27.8 million.
Operating and Capital Expenditure Requirements
We have not achieved profitability on a quarterly or annual basis since our inception and we expect to continue to incur net losses for the foreseeable future. We expect our cash expenditures to increase in the near term as we work to complete our clinical program for ZS-9, pursue regulatory approval in the United States and prepare for a possible commercial launch of ZS-9. In particular, our pre-commercialization activities for ZS-9, including development costs relating to the expansion and validation of our commercial manufacturing process and inventory supply and the hiring and training of a sales force, will require significant investment in our manufacturing facilities, personnel and other commercial launch related costs. We believe that our existing cash, cash equivalents, short-term investments and available borrowings under our MidCap Credit Facility will allow us to fund our operating plan through at least December 31, 2015. However, we anticipate that we may need to raise additional financing in the future. In order to meet these additional cash requirements, we may seek to sell additional equity or convertible debt securities that may result in dilution to our stockholders or enter into additional debt financing arrangements. If we raise additional funds through the issuance of convertible debt securities, these securities could have rights senior to those of our common stock and could contain covenants that restrict our operations. Debt financing, if available, would result in increased fixed payment obligations and may involve arrangements that include covenants limiting or restricting our ability to take specific actions such as incurring debt, making capital expenditures or declaring dividends. There can be no assurance that we will be able to obtain additional equity or debt financing on terms acceptable to us, if at all. Our failure to obtain sufficient funds on acceptable terms when needed could have a material adverse effect on our business, results of operations and financial condition.
89
Table of Contents
Our future capital requirements will depend on many factors, including:
| • | the results of our remaining clinical trials for ZS-9; |
| • | the time and cost necessary to obtain regulatory approvals for ZS-9 and the costs of post-marketing studies that could be required by regulatory authorities; |
| • | the progress, timing, scope and costs of our nonclinical studies and clinical trials for ZS-9 and any other product candidates we may have, including the ability to enroll patients in a timely manner for potential future clinical trials; |
| • | the costs of commercialization activities for ZS-9 if we receive marketing approval, including the costs and timing of expanding our manufacturing activities and other costs of manufacturing ZS-9 and establishing product sales, marketing and distribution capabilities; |
| • | subject to receipt of marketing approval, the amount of sales and other revenues from ZS-9, including the sales price and the availability of adequate third-party reimbursement; |
| • | the expenses needed to attract and retain skilled personnel; |
| • | the costs associated with being a public company; and |
| • | the costs involved in preparing, filing, prosecuting, maintaining, defending and enforcing any patent claims and other intellectual property rights. |
Please see “Item 1A. Risk Factors” for additional risks associated with our substantial capital requirements.
Contractual Obligations and Other Commitments
The following table summarizes our contractual obligations as of December 31, 2014:
| Payments Due by Period | ||||||||||||||||||||
| Less than One Year |
1-3 Years | 3-5 Years | More than 5 Years |
Total | ||||||||||||||||
| (in thousands) | ||||||||||||||||||||
| Operating lease obligations(1) |
$ | 688 | $ | 1,172 | $ | 326 | $ | 464 | $ | 2,650 | ||||||||||
| Capital lease obligations(2) |
304 | 127 | — | — | 431 | |||||||||||||||
| Minimum royalty due to UOP(3) |
100 | 300 | 200 | — | 600 | |||||||||||||||
| MidCap Financial Credit Facility(4) |
— | 6,667 | 3,983 | — | 10,650 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total contractual obligations(5) |
$ | 1,092 | $ | 8,266 | $ | 4,509 | $ | 464 | $ | 14,331 | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| (1) | Operating leases include total future minimum rent payments under non-cancelable operating lease agreements. |
| (2) | Capital leases equal the total minimum lease payments due under non-cancelable capital lease agreements. As of December 31, 2014, the total amount of these capital lease obligations equaled $0.4 million. |
| (3) | Represents minimum annual royalty payment due to UOP under a license agreement with UOP. This table does not include minimum royalty amounts due to UOP beyond the minimum amount, which is equal to 5% of worldwide net sales, as these amounts are not currently determinable. This table reflects no annual minimum royalty payments after five years because this amount is not currently determinable. |
| (4) | The MidCap Credit Facility is structured in two $10 million tranches, with the first tranche drawn at closing in July 2014 and the second tranche to be drawn after we provide evidence to MidCap of positive primary endpoint results from our ZS004 clinical study, which has occurred, but before its expiration on October 31, 2014. The First Amendment to Credit and Security Agreement, entered into in September 2014, extended the second tranche commitment termination date from October 31, 2014 to March 31, 2015. |
| (5) | This table does not include (a) contracts that are entered into in the ordinary course of business which are not material in the aggregate in any period presented above and (b) the royalty payments due to UOP of 5% of worldwide net sales of ZS-9 in excess of the minimum royalty of $100,000 per year, as these amounts are not currently determinable. |
90
Table of Contents
Capital and Operating Leases
In July 2012, we entered into a capital lease agreement with GL Filtration, Ltd. to lease production equipment, which was recorded on our balance sheet at $0.3 million. The term of the lease was for 24 months, with equal monthly installments in the amount of $13,185. Expense related to the amortization of this asset is included in depreciation expense.
In August 2012, we entered into a lease agreement with the University of North Texas Health Science Center at Fort Worth for the lease of laboratory space within the Health Science Center in Fort Worth, Texas. The term of the lease was for 12 months, commencing on August 1, 2012, with the provision that either party may cancel the lease upon providing 30 days’ advance notice. In August 2013, we renewed the lease under substantially the same lease terms for an additional 12 months. In August 2014, we renewed the lease under substantially the same terms for an additional 6 months. This lease was terminated by us, effective December 31, 2014, after we provided appropriate notice.
In February 2013, we entered into a lease agreement with PW Commerce Center LP for the lease of 25,682 square feet of space to house our corporate offices and production facility in Coppell, Texas. The term of the lease is for 125 months, commencing on April 15, 2013, with an option to renew the lease for one five-year period at the then market rate. Under the terms of the lease, rent was abated for the first five months of the lease term and the rent for the sixth month was paid at the time the lease was executed. Thereafter, rent is due and payable on the first day of each month of the lease term. Monthly rent expense under this lease will approximate $12,758 on a straight-line basis.
In April 2013, we entered into a lease agreement with Renault & Handley Oakmead Solar Joint Venture LP for the lease of 1,172 square feet of office space in Menlo Park, California for executive use. The term of the lease is for 24 months, commencing on May 1, 2013, with an option to extend the lease for an additional period of 24 months at a rental rate equal to 95% of the then fair market rental value. Rent is due and payable on the first day of each month of the lease term. In October 2014, we vacated this space and with appropriate notice terminated the lease effective as of November 30, 2014.
In October 2013, we entered into a sublease agreement with InB: Hauser Pharmaceutical Services, Inc., or Hauser, to sublease Hauser’s production facility in Denver, Colorado and assume operation of the facility and ownership of certain equipment and fixtures located in the facility. At the same time, we entered into an additional agreement with Hauser to allow us to hire the Hauser employees at the facility and obtain all of the documentation and permits necessary for us to operate the facility. The term of the sublease is for 38.4 months, commencing on October 18, 2013, with an option to extend the lease for an additional period of 36 months at a rental rate equal to the amount Hauser is obligated to pay to its landlord. Rent is due and payable on the first day of each month of the lease term. Monthly rent expense under this lease will approximate $15,399 on a straight-line basis.
In June 2014, we entered into a capital lease agreement with GL Filtration, Ltd. to lease production equipment, which was recorded on our balance sheet at $0.6 million. The term of the lease is for 24 months, with equal monthly installments in the amount of $27,885. Expense related to the amortization of this asset is included in depreciation expense.
In July 2014, we entered into a lease agreement with Harvard Investment Company for the lease of 6,370 square feet of space in Redwood City, CA for executive use. The term of the lease is for 36 months, commencing on September 1, 2014, with an option to extend the lease for one three-year period at the then market rate. Rent is due and payable on the first day of each month of the lease term. Monthly rent expense under this lease will approximate $25,062 on a straight-line basis.
In October 2014, we entered into a one month lease agreement with Regus Management Group, LLC for 9 individual offices in Grapevine, TX commencing November 1, 2014 and a five month lease agreement for 15 individual offices in Grapevine, TX commencing December 1, 2014. Rent is due and payable on the first day of each month of the lease term.
91
Table of Contents
Indemnification
In the normal course of business, we enter into contracts and agreements that contain a variety of representations and warranties and provide for general indemnifications. Our exposure under these agreements is unknown because it involves claims that may be made against us in the future, but have not yet been made. To date, we have not paid any claims or been required to defend any action related to our indemnification obligations. However, we may record charges in the future as a result of these indemnification obligations.
In accordance with our seventh amended and restated certificate of incorporation and amended and restated bylaws, we have indemnification obligations to our officers and directors for specified events or occurrences, subject to some limits, while they are serving at our request in such capacities. There have been no claims to date, and we have director and officer insurance that may enable us to recover a portion of any amounts paid for future potential claims.
Off-Balance Sheet Arrangements
We do not have any off-balance sheet arrangements.
Critical Accounting Polices and Significant Estimates
Our financial statements are prepared in accordance with U.S. generally accepted accounting principles, or U.S. GAAP. The preparation of these financial statements requires us to make estimates and assumptions that affect the reported amounts of assets, liabilities, revenues, costs and expenses and related disclosures. We base our estimates on historical experience and on various other assumptions that we believe to be reasonable under the circumstances. In many instances, we could have reasonably used different accounting estimates, and in other instances changes in the accounting estimates are reasonably likely to occur from period to period. Accordingly, actual results could differ significantly from management’s estimates. To the extent that there are material differences between these estimates and actual results, our future financial statement presentation, financial condition, results of operations and cash flows will be affected. While our significant accounting policies are described in the Notes to our financial statements, we believe that the accounting policies discussed below are critical to understanding our historical and future performance, as these policies relate to the more significant areas involving management’s judgments and estimates.
Clinical Trial Accruals
Clinical trial costs are a component of research and development expenses. We accrue and expense clinical trial activities performed by third parties based upon actual patient enrollment in accordance with agreements established with third-party vendors and clinical sites. We determine the actual expense accrual through review of patient enrollment databases and the agreed-upon fee to be paid for each patient enrolled less any payments made. During the course of a clinical trial, we may adjust our rate of clinical expense recognition if actual results differ from our estimates. We make estimates of our accrued expenses as of each balance sheet date in our financial statements based on the facts and circumstances known to us at that time. Although we do not expect our estimates to be materially different from amounts actually incurred, our understanding of the status and timing of services performed relative to the actual status and timing of services performed may vary and may result in us reporting amounts that are too high or too low for any particular period. Our clinical trial accrual is dependent, in part, upon the receipt of timely and accurate reporting from clinical sites and other third-party vendors. Through December 31, 2014, there have been no material adjustments to our prior period estimates of accrued expenses for clinical trials.
Stock-Based Compensation
We measure and recognize compensation expense for stock options granted to our employees and directors, based on the estimated fair value of the award on the grant date. Historically, for all periods prior to our IPO, the fair values of the shares of common stock underlying our stock-based awards were estimated on each grant date
92
Table of Contents
by our board of directors. In order to determine the fair value of our common stock underlying option grants, our board of directors considered, among other things, contemporaneous valuations of our common stock prepared by an unrelated third-party valuation firm.
We use the Black-Scholes valuation model to estimate the fair value of stock option awards. The fair value is recognized as expense, net of estimated forfeitures, over the requisite service period, which is generally the vesting period of the respective award on a straight-line basis. Vesting terms for certain employee grant agreements did not initially match the terms approved by our board of directors. Accordingly, these options were not considered granted for accounting purposes until a mutual understanding of the terms was obtained through amendment of the grant agreements in January 2014. Certain of these options were considered to have service inception dates in 2013, which resulted in the vested portion of these options being re-measured to fair value throughout 2013.
Prior to the public trading of our common stock, our board of directors exercised reasonable judgment and considered a number of objective and subjective factors to determine the best estimate of the fair value of our common stock, including:
| • | contemporaneous valuations of our common stock performed by unrelated third-party valuation firms; |
| • | our stage of development; |
| • | our operational and financial performance; |
| • | the nature of our services and our competitive position in the marketplace; |
| • | the value of companies that we consider peers based on a number of factors, including similarity to us with respect to industry and business model; |
| • | the likelihood of achieving a liquidity event, such as an IPO or sale given prevailing market conditions, and the nature and history of our business; |
| • | issuances of preferred stock and the rights, preferences and privileges of our preferred stock relative to those of our common stock; |
| • | business conditions and projections; |
| • | the history of our company and progress of our research and development efforts and clinical trials; and |
| • | the lack of marketability of our common stock. |
For valuations after the completion of our IPO, our board of directors determines the fair value of each share of underlying common stock based on the closing price of our common stock as reported by the Nasdaq Global Market on the date of grant.
Net Operating Loss Carryforwards and Research Tax Credit Carryforwards
As of December 31, 2014, we had federal income tax net operating loss, or NOL, carryforwards of approximately $93.7 million and federal research tax credit carryforwards of approximately $5.2 million, net of limitations pursuant to Section 382 of the Code discussed below. These NOL carryforwards and research tax credit carryforwards, if not previously used, will begin to expire in 2029. The future utilization of our NOL carryforwards and research tax credit carryforwards will be subject to an annual limitation, pursuant to Section 382 of the Code, as a result of several ownership changes since the Company was founded in 2008, with the most recent Section 382 change of ownership occurring on June 23, 2014. We estimate the ownership changes will result in $1.5 million of net operating loss carryforwards expiring unused and $0.1 million of federal research tax credit carryforwards expiring unused. Moreover, we may experience ownership changes in the future as a result of subsequent shift in stock ownership. As a result, if we earn net taxable income in the future, the limitations on our ability to use our NOL carryforwards and research tax credit carryforwards to reduce U.S. federal tax liability could potentially result in higher future tax liability to us compared to what we would have incurred had we been able to fully take advantage of these assets.
93
Table of Contents
| ITEM 7A. | QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK |
Quantitative and Qualitative Disclosures about Market Risk
Our exposure to market risk for changes in interest rates relates primarily to interest earned on our cash equivalents and short-term investments. The primary objective of our investment activities is to preserve our capital to fund operations. A secondary objective is to maximize income from our investments without assuming significant risk. Our investment policy provides for investments in low-risk, investment-grade debt instruments. As of December 31, 2014, we had cash, cash equivalents and short-term investments totaling $102.3 million consisting of bank deposits, investments in short-term interest-bearing instruments and investments in short-term marketable securities. A hypothetical 10% change in interest rates during any of the periods presented would not have had a material impact on our financial statements.
We face foreign exchange risk as a result of entering into transactions denominated in currencies other than U.S. dollars. In particular, we pay our research sites outside of the United States in the currencies of their respective jurisdictions. Due to the uncertain timing of expected payments in foreign currencies, we do not utilize any forward exchange contracts. All foreign transactions settle on the applicable spot exchange basis at the time such payments are made. An adverse movement in foreign exchange rates could have a material effect on payments made to foreign suppliers and for license agreements. A hypothetical 10% change in foreign exchange rates or interest rates during any of the periods presented would not have had a material impact on our financial statements.
| ITEM 8. | FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA |
See “Index to Financial Statements,” which is located on page F-1 appearing at the end of this annual report on Form 10-K.
| ITEM 9. | CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE |
None.
| ITEM 9A. | CONTROLS AND PROCEDURES |
Included in this Form 10-K are certifications of our Chief Executive Officer and our Chief Financial Officer, which are required in accordance with Rule 15d-14 of the Securities Exchange Act of 1934, as amended (“Exchange Act”). This section includes information concerning the controls and controls evaluation referred to in the certifications.
Evaluation of Disclosure Controls and Procedures
We carried out an evaluation, under the supervision and with the participation of our management, including our Chief Executive Officer and our Chief Financial Officer, of the effectiveness of our “disclosure controls and procedures” as of the end of the period covered by this report, pursuant to Rules 13a-15(b) and 15d-15(b) under the Securities Exchange Act of 1934, as amended, or the Exchange Act. In connection with that evaluation, our Chief Executive Officer and our Chief Financial Officer concluded that our disclosure controls and procedures were effective to provide reasonable assurance that the information required to be disclosed is recorded, processed, summarized and reported within the time periods specified in the SEC rules and forms as of December 31, 2014. For the purpose of this review, disclosure controls and procedures means controls and procedures designed to ensure that information required to be disclosed by us in the reports that we file or submit is recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms. These disclosure controls and procedures include, without limitation, controls and procedures designed to ensure that information required to be disclosed by us in the reports that we file or submit is accumulated and communicated to management, including our principal executive officer and principal financial officer, as appropriate to allow timely decisions regarding required disclosure.
94
Table of Contents
Management’s Report on Internal Control Over Financial Reporting
This annual report does not include a report of management’s assessment regarding internal control over financial reporting or an attestation report of our independent registered public accounting firm due to a transition period established by the rules of the Securities and Exchange Commission for newly public companies.
Change in Internal Control over Financial Reporting
There has been no change in our internal control over financial reporting (as defined in Rule 13a-15(f) under the Exchange Act) during the fiscal year ended December 31, 2014, that has materially affected, or is reasonably likely to materially affect our internal control over financial reporting.
Limitations on Effectiveness of Controls
Our management, including our Chief Executive Officer and the Chief Financial Officer, does not expect that our disclosure controls and procedures or internal control over financial reporting will prevent all errors and all fraud. A control system, no matter how well conceived and operated, can only provide reasonable, not absolute, assurances that the objectives of the control system are met. The design of a control system reflects resource constraints, and the benefits of controls must be considered relative to their costs. Because there are inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that all control issues and instances of error or fraud, if any, have been or will be detected.
| ITEM 9B. | OTHER INFORMATION |
None.
95
Table of Contents
PART III. — OTHER INFORMATION
| ITEM 10. | DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE |
Executive Officers and Directors
The following table sets forth information regarding our executive officers and directors as of March 10, 2015:
| Name |
Age | Position(s) | ||||
| Executive Officers |
||||||
| Robert Alexander |
45 | Chief Executive Officer, Director | ||||
| Alvaro Guillem |
57 | President | ||||
| D. Jeffrey Keyser |
61 | Chief Operating Officer and Secretary, Director | ||||
| Todd A. Creech |
47 | Chief Financial Officer and Treasurer | ||||
| Henrik S. Rasmussen |
56 | Chief Medical Officer | ||||
| Cynthia Smith |
46 | Chief Commercial Officer | ||||
| Adam Tomasi |
45 | Chief Scientific Officer and Head of Corporate Development | ||||
| Mark Asbury |
51 | Senior Vice President and General Counsel | ||||
| Non-Employee Directors |
||||||
| Marc Ostro |
65 | Director | ||||
| Guy Nohra |
54 | Director | ||||
| John Whiting |
60 | Director | ||||
| Srinivas Akkaraju |
46 | Director | ||||
| Martin Babler |
50 | Director | ||||
Executive Officers
Robert Alexander, Ph.D., has served as a member of our board of directors since October 2012 and has served as our Chief Executive Officer since December 2013. From March 2013 to March 2014, Dr. Alexander served as Chairman of our board of directors. From November 2005 to March 2013, Dr. Alexander served as a Director at Alta Partners, a venture capital firm in life sciences. In addition, he acted as Executive Chairman and interim Chief Executive Officer of SARcode Biosciences (acquired by Shire plc in April 2013), a biopharmaceutical company. During his time at Alta, he led investments in SARcode Biosciences, Sonexa Therapeutics, Allakos, Lumena Pharmaceuticals, and ZS Pharma. From April 2004 to November 2005, Dr. Alexander was a Principal in MPM Capital’s BioEquities fund where he sourced opportunities and led due diligence efforts for both public and private investments. From December 2000 to April 2004, Dr. Alexander worked in the Business Development group at Genentech, Inc. (now a member of the Roche Group), a biotechnology company, where he was responsible for sourcing and screening product opportunities based on scientific merit and strategic fit, leading diligence teams and negotiating terms and definitive agreements. Dr. Alexander joined Genentech after completing his post-doctoral fellowship at Stanford University in the Pathology department. He also holds a Ph.D. in Immunology from the University of North Carolina and a B.A. in Zoology from Miami University of Ohio. We believe Dr. Alexander is qualified to serve on our board of directors based on his background and experience in the life sciences sector.
Alvaro Guillem, Ph.D., is our co-founder and has served as our President since 2008. From February 2008 to December 2013, Dr. Guillem served as our Chief Executive Officer and from February 2008 to February 2015 he served as a member of our board of directors. Dr. Guillem is a veteran of the pharmaceutical industry with over thirty years of leadership experience in bringing new therapies to market at both well-established and start-up companies. From September 2006 to December 2009, Dr. Guillem held the role of Vice President of Quality and Scientific Affairs at Ash Access Technology and helped develop a catheter lock for patients with end stage renal disease on dialysis. From October 2005 to November 2006, Dr. Guillem held senior positions at Genzyme/
96
Table of Contents
Bone Care. From October 1998 to September 2005, Dr. Guillem worked at Adams Respiratory Therapeutics. Dr. Guillem holds a B.S. in Chemistry from Mary Washington University and a Ph.D. in Chemistry from Virginia Commonwealth University. We believe Dr. Guillem is qualified to serve on our board of directors based on his extensive experience in senior positions at various pharmaceutical companies and his tremendous experience in the life sciences field for over thirty years.
D. Jeffrey Keyser, RPh, J.D., MPA, Ph.D., is our co-founder and has served as our Chief Operating Officer, Secretary, and member of our board of directors since 2008. Dr. Keyser has over thirty years of experience in the pharmaceutical industry. From 2004 to 2008, Dr. Keyser served as the Chief Compliance Officer and Vice President of Regulatory Affairs at Encysive Pharmaceuticals. In addition, he served as Vice President of Development and Regulatory Affairs at Adams Respiratory Therapeutics from 1998 to 2004, and previously held senior management positions at Medeva Americas, Marion Merrell Dow, Marion Laboratories and Abbott Laboratories. Dr. Keyser has experience in regulatory, medical, clinical and product development and has directed efforts to develop, prepare and secure approvals of numerous INDs and NDAs in the United States, Canada, Australia and Europe. Dr. Keyser received his B.S. in Pharmacy and J.D. from Creighton University. He also holds an MPA from the University of Missouri at Kansas City and a Ph.D. in Economics from the University of Texas at Dallas. We believe Dr. Keyser is qualified to serve on our board of directors based on the expertise he holds from over thirty years in the pharmaceutical industry and extensive senior management experience in the life sciences industry.
Todd A. Creech, MBA, has served as our Chief Financial Officer since August 2013 and as our Treasurer since February 2014. From October 2010 to April 2013, Mr. Creech was Chief Financial Officer and Vice-President of Business Development at SARcode Biosciences (acquired by Shire plc in April 2013), where he led all financing, legal, accounting and corporate development activities. From September 2006 to September 2010, Mr. Creech was Chief Financial Officer of Sirion Therapeutics, an ophthalmic pharmaceutical company. During his tenure there, he raised $100 million in debt and equity financing to support the development of six late-stage clinical programs and two NDA approvals and led the sale of Sirion’s drug assets to Alcon and Bausch and Lomb. From May 2005 to September 2006, Mr. Creech worked with NovaQuest, the investment group within Quintiles, Inc., where he structured, placed and managed capital investments in emerging U.S. biotechnology and specialty pharmaceutical companies. In 2003, Mr. Creech co-founded Centice, an optical sensor spin out from Duke University. Mr. Creech also brings an additional ten years of biotech- and high-tech-specific consulting experience from his time at SRI International and Anderson Consulting. Mr. Creech holds undergraduate degrees in Finance and Accounting from Miami University of Ohio and an MBA from Duke University.
Henrik Sandvad Rasmussen, M.D., Ph.D., has served as our Chief Medical Officer since October 2012. From August 2009 to October 2012, Dr. Rasmussen served as President and Chief Executive Officer of Rasmussen Biotech and Pharma Consulting. From March 2007 to July 2009, Dr. Rasmussen held the positions of Corporate Vice President and Head of Clinical Development and Medical and Regulatory Affairs at Novo Nordisk. He also served as Chief Medical Officer for Nabi Biopharmaceuticals from March 2003 to February 2007 and for Genvec from March 1999 to February 2003. He was Vice President for Clinical Research and Senior Vice President for Clinical Research and Regulatory Affairs at British Biotech from January 1995 to February 1999, and International Clinical Project Manager and Global Study Director for cardiovascular drug development at Pfizer Central Research from January 1989 to December 1994. Dr. Rasmussen has led numerous global development programs and regulatory filings worldwide, including NDAs. He has over 150 published peer-reviewed papers in therapeutic areas including nephrology, cardiology and diabetes. Dr. Rasmussen received his M.D. and Ph.D. from the University of Copenhagen in Denmark and is trained in internal medicine and cardiology.
Cynthia Smith, MS, MBA, has served as our Chief Commercial Officer since June 2013. From October 2008 to March 2013, Ms. Smith was at Affymax Inc., a biotechnology company focused on the development and commercialization of novel renal therapies, most recently in the position of Vice President of Market Access and Commercial Development. From July 2000 to October 2008, Ms. Smith was at Merck and Co., Inc., where she
97
Table of Contents
held various leadership positions in managed markets, corporate strategy, and public affairs, most recently as Executive Director of Healthcare System and Medicare Strategy. From June 1995 to July 2000, Ms. Smith served in the White House Office of Management and Budget under the Clinton Administration. Ms. Smith earned her B.A. from the University of North Carolina at Chapel Hill, her M.S. in Public Policy from the Eagleton Institute at Rutgers University and her MBA from the Wharton School at the University of Pennsylvania.
Adam Tomasi, MBA, Ph.D., has served as our Senior Vice President, Corporate Development since August 2013 and Chief Scientific Officer and Head of Corporate Development since February 2015. From June 2006 to August 2013, Mr. Tomasi was a Principal at Alta Partners, where he was involved in the funding and development of notable medical technology and life science companies including Chemgenex, Excaliard, Lumena Pharmaceuticals, Achaogen, Immune Design and Allakos. Prior to joining Alta Partners, Mr. Tomasi was in the Harvard-MIT Biomedical Enterprise Program where he completed fellowships in venture capital at MPM Capital and as an equity analyst at Lehman Brothers. From January 1992 to June 1994, Mr. Tomasi worked as an organic chemist with Gilead Sciences and from June 2000 to July 2004 at Cytokinetics, where he played a key role in the creation of the cardiovascular drug CK-1827452, which was licensed to Amgen. Mr. Tomasi holds a B.S. in Chemistry from the University of California, Berkeley, an MBA from the Massachusetts Institute of Technology Sloan School of Management and a Ph.D. in Chemistry from the University of California, Irvine.
Mark Asbury, JD, has served as Senior Vice President and General Counsel since July 2014. From June 2011 to June 2012, Mr. Asbury was the Vice President and General Counsel of Pharmacyclics, a biopharmaceutical company. From October 1996 to June 2011, Mr. Asbury was at Genentech where he held a variety of positions, most recently as Associate General Counsel and Senior Director of Transactional Law. From October 1992 to September 1996, Mr. Asbury worked for the law firm of Shearman & Sterling, where he specialized in corporate finance, mergers and acquisitions and commercial debt financings. Mr. Asbury earned his B.A. from Vanderbilt University and his JD from Stanford University.
Non-Employee Directors
Marc Ostro, Ph.D., has served as a member of our board of directors since November 2011. Dr. Ostro has been a General Partner at Devon Park Bioventures, a venture capital fund targeting investments in therapeutics companies and, in certain cases, medical device, diagnostic and drug discovery technology companies, since February 2006. Previously, from January 2002 to February 2006, Dr. Ostro was a managing partner at TL Ventures, L.P., a Pennsylvania-based venture capital firm. From 1997 to 2002, he was Senior Managing Director and Head of KPMG’s Life Science Group (Mergers and Acquisitions) and he was Senior Vice President of Ross Financial Corporation from August 1997 to December 1997. Dr. Ostro was a Managing Director of UBS Securities from 1994 to 1997, where he was involved with numerous IPOs and secondary offerings and was with Mabon Securities from 1993 to 1994, where he initiated and grew the firm’s biotechnology practice. In 1981, Dr. Ostro co-founded The Liposome Company, a biotechnology company, and served as President, Vice Chairman, and Chief Science Officer. He also founded the Journal of Liposome Research. To date, Dr. Ostro has served on the boards of directors of seventeen biotechnology companies. Dr. Ostro received a B.S. in Biology from Lehigh University, a Ph.D. in Biochemistry from Syracuse University, and was a Postdoctoral Fellow and Assistant Professor at the University of Illinois Medical School. We believe Dr. Ostro is qualified to serve on our board of directors due to his investment and industry experience and extensive service on other biotechnology companies’ boards of directors.
Guy Nohra, MBA, has served as a member of our board of directors since June 2013. Mr. Nohra is a co-founder of and has been a managing director at Alta Partners since 1996, and was also a partner at Burr, Egan, Deleage & Co. from 1989 to 1997. He has been involved in the funding and development of notable medical technology and life science companies including AcelRx Pharmaceuticals, ATS Medical, Cutera, Innerdyne, R2 Technology, deCODE genetics, and Vesica. From November 1983 to June 1987, Mr. Nohra was Product Manager of Medical Products with Security Pacific Trading Corporation. He was responsible for a multi-million
98
Table of Contents
dollar product line and traveled extensively in Korea, Taiwan, Hong Kong, China and Southeast Asia. Currently, Mr. Nohra serves on the board of directors of several companies, including Bioventus, Carbylan Biosurgery, Cerenis Therapeutics, PneumRx and Vertiflex, and is the Chairman of the Board of USGI Medical. He also served on the board of directors of the Medical Device Manufacturing Association from June 2003 to June 2013. Mr. Nohra served as the President of the Silicon Valley chapter of The Leukemia and Lymphoma Society from April 2010 to December 2013. He has a B.A. in History from Stanford University and an MBA from the University of Chicago. We believe Mr. Nohra is qualified to serve on our board of directors due to his longtime involvement in the development of life science companies and extensive service on other boards of directors for similar pharmaceutical companies.
John Whiting, MBA, has served as a member of our board of directors since June 2014. From October 2010 to present, Mr. Whiting has been at Gladstone Institutes, most recently in the position of Executive Vice President, Chief Financial and Administrative Officer. Mr. Whiting provides overall direction and leadership to a wide range of Gladstone’s finance and administrative functions, including finance and accounting, extramural funding and grants and contracts, human resources, information technology, operations and facilities management, and purchasing. Prior to joining Gladstone Institutes, Mr. Whiting was at Genentech, Inc. (now a member of the Roche Group) from March 1989 to July 2009, most recently as Deputy Chief Financial Officer from February 2009 to July 2009. Mr. Whiting’s responsibilities at Genentech, included accounting and financial reporting, financial planning, treasury, divisional controllerships, mergers and acquisitions and strategic planning. Mr. Whiting has served on the board of directors for the non-profit Larkin Street Youth Services since October 2010. Mr. Whiting has also served on the board of directors for MacPherson’s, an art supply and distribution company, since August 2011. He has served as chair of MacPherson’s audit committee since August 2011. Mr. Whiting earned both a bachelor’s degree in biology and an MBA at the University of Oregon. He is an inactive certified public accountant licensed in California. We believe Mr. Whiting is qualified to serve on our board of directors due to his longtime experience in the industry and strong record of service on other companies’ boards of directors as chair of both a finance and an audit committee.
Srinivas Akkaraju, M.D., Ph.D., has served as a member of our board of directors since June 2014. Dr. Akkaraju has over 16 years of investment and operational experience in the life sciences sector. Dr. Akkaraju has served as a General Partner, concentrating on biopharmaceutical investments, of Sofinnova Ventures since April 2013. Prior to Sofinnova Ventures, Dr. Akkaraju served as a Managing Director at New Leaf Venture Partners from January 2009 to April 2013. Prior to New Leaf Venture Partners, Dr. Akkaraju was a founding Managing Director at Panorama Capital, the venture spin-out from the arm of J.P. Morgan Partners that specialized in biotech and information technology investments. Before forming Panorama Capital, he was a part of the biotech investment team of J.P. Morgan Partners from April 2001 to July 2006, most recently serving as Partner. Prior to J.P. Morgan Partners, Dr. Akkaraju held business and corporate development positions at Genentech, Inc. (now a member of the Roche Group), from October 1998 to March 2001. Dr. Akkaraju’s past board memberships have included Piramed Ltd. (acquired by Roche), Eyetech Pharmaceuticals, Inc. and Synageva BioPharma Corp. He currently serves on the boards of Seattle Genetics, Inc., Intercept Pharmaceuticals, Inc., and Versartis, Inc. Dr. Akkaraju received his B.A. degrees in both Biochemistry and Computer Science from Rice University and M.D. and Ph.D. degrees in Immunology from Stanford University School of Medicine. We believe Dr. Akkaraju is qualified to serve on our board of directors due to his investment and industry experience and service on other boards of directors of life science companies.
Martin Babler, has served as a member of our board of directors since February 2015. Mr. Babler has been the Chief Executive Officer of Principia Biopharma since April 2011. From December 2007 to March 2011, Mr. Babler was President and Chief Executive Officer of Talima Therapeutics. From July 1998 to January 2007, Mr. Babler was a senior executive at Genentech in various roles, including Vice President, Immunology Sales and Marketing, where he oversaw the successful product launches of Xolair and Rituxan for Rheumatoid Arthritis. During his tenure at Genentech, he also helped prepare for the launches of additional novel products such as Avastin, Tarceva, and Lucentis. Mr. Babler began his pharmaceutical industry career at Eli Lilly and Company with positions in sales management, global marketing and business development. Mr. Babler has been
99
Table of Contents
a Guest Lecturer for the BioExec Institute at the Haas School of Business at the University of California, Berkeley. From 2008 to 2014, Mr. Babler served on the board of directors of Infinity Pharmaceuticals, Inc. He also serves on the BIO Emerging Companies Section Governing Board. Mr. Babler holds a Swiss Federal Diploma in Pharmacy from the Federal Institute of Technology in Zurich. Mr. Babler also graduated from the Executive Development Program at the Kellogg Graduate School of Management at Northwestern University. We believe Mr. Babler is qualified to serve on our board of directors due to his significant industry knowledge and expertise in the commercialization of biopharmaceutical products, including several recent successful product launches.
Board Composition
We currently have seven directors. Our directors hold office until their successors have been elected and qualified or, if earlier, until their death, resignation or removal.
Director Independence
Under applicable rules of The Nasdaq Global Market, or the Nasdaq rules, a director will only qualify as an “independent director” if, in the opinion of our board of directors, that person does not have a relationship that would interfere with the exercise of independent judgment in carrying out the responsibilities of a director. Our board of directors has determined that all of our directors, other than Dr. Alexander and Dr. Keyser, are independent directors, as defined by the applicable Nasdaq rules. In making such determination, the board of directors considered the relationships that each such non-employee director has with our company and all other facts and circumstances that the board of directors deemed relevant in determining their independence. In particular, in considering the independence of our directors, our board of directors considered the association of certain of our directors with the holders of more than 5% of our common stock as well as the effect of each of the transactions described in the “Item 13. Certain Relationships and Related Party Transactions” section of this report.
There are no family relationships among any of our directors or executive officers.
Classified Board of Directors
In accordance with our seventh amended and restated certificate of incorporation, our board of directors is divided into three classes with staggered, three-year terms. At each annual meeting of stockholders, the successors to directors whose terms then expire will be elected to serve from the time of election and qualification until the third annual meeting following election. Our directors are divided among the three classes as follows:
| • | the Class I directors are Mr. Babler and Dr. Keyser, and their terms expire at the annual meeting of stockholders to be held in 2015; |
| • | the Class II directors are Mr. Nohra and Dr. Ostro, and their terms expire at the annual meeting of stockholders to be held in 2016; and |
| • | the Class III directors are Dr. Alexander, Mr. Whiting and Dr. Akkaraju, and their terms expire at the annual meeting of stockholders to be held in 2017. |
Our seventh amended and restated certificate of incorporation provides that the authorized number of directors may be changed only by resolution of the board of directors. Any additional directorships resulting from an increase in the number of directors will be distributed among the three classes so that, as nearly as possible, each class will consist of one-third of the directors. The division of our board of directors into three classes with staggered three-year terms may delay or prevent a change of our management or a change in control of our company.
100
Table of Contents
Leadership Structure of the Board
Our amended and restated bylaws and corporate governance guidelines provide our board of directors with flexibility to combine or separate the positions of Chairman of the Board and Chief Executive Officer and/or the implementation of a lead director in accordance with its determination that utilizing one or the other structure would be in the best interests of our company.
Our board of directors has concluded that our current leadership structure is appropriate at this time. However, our board of directors will continue to periodically review our leadership structure and may make such changes in the future as it deems appropriate.
Role of Board in Risk Oversight Process
Risk assessment and oversight are an integral part of our governance and management processes. Our board of directors encourages management to promote a culture that incorporates risk management into our corporate strategy and day-to-day business operations. Management discusses strategic and operational risks at regular management meetings, and conducts specific strategic planning and review sessions during the year that include a focused discussion and analysis of the risks facing us. Throughout the year, senior management reviews these risks with the board of directors at regular board meetings as part of management presentations that focus on particular business functions, operations or strategies, and presents the steps taken by management to mitigate or eliminate such risks.
Our board of directors does not have a standing risk management committee, but rather administers this oversight function directly through our board of directors as a whole, as well as through various standing committees of our board of directors that address risks inherent in their respective areas of oversight. In particular, our board of directors is responsible for monitoring and assessing strategic risk exposure and our audit committee is responsible for overseeing our major financial risk exposures and the steps our management has taken to monitor and control these exposures. The audit committee also monitors compliance with legal and regulatory requirements. Our nominating and governance committee monitors the effectiveness of our corporate governance guidelines and considers and approves or disapproves any related-persons transactions. Our compensation committee assesses and monitors whether any of our compensation policies and programs has the potential to encourage excessive risk-taking.
Board Committees
Audit Committee
Our audit committee consists of Dr. Ostro, Mr. Whiting and Dr. Akkaraju, with Mr. Whiting serving as chairman of the committee. Our board of directors has determined that Mr. Whiting and Dr. Akkaraju meet the independence requirements of Rule 10A-3 under the Exchange Act and the applicable listing standards of The Nasdaq Global Market while Dr. Ostro does not meet such requirements and standards. Dr. Ostro will serve as a member of the audit committee and will be replaced by a director meeting the requirements under the Exchange Act and the standards of The Nasdaq Global Market in accordance with the phase-in exception schedule found in Rule 10A-3 of the Exchange Act, with Dr. Ostro being replaced within one year of the completion of our IPO. Our board of directors has determined that Mr. Whiting is an “audit committee financial expert” within the meaning of the SEC regulations and applicable listing standards of The Nasdaq Global Market. The audit committee’s responsibilities include:
| • | appointing our independent registered public accounting firm; |
| • | evaluating the independent registered public accounting firm’s qualifications, independence and performance; |
| • | determining the engagement of the independent registered public accounting firm; |
| • | reviewing and approving the scope of the annual audit and the audit fee; |
101
Table of Contents
| • | discussing with management and the independent registered public accounting firm the results of the annual audit and the review of our quarterly financial statements; |
| • | approving the retention of the independent registered public accounting firm to perform any proposed permissible non-audit services; |
| • | monitoring the rotation of partners of the independent registered public accounting firm on our engagement team as required by law; |
| • | reviewing our financial statements and our management’s discussion and analysis of financial condition and results of operations to be included in our annual and quarterly reports to be filed with the SEC; |
| • | reviewing our critical accounting policies and estimates; and |
| • | annually reviewing the audit committee charter and the committee’s performance. |
Compensation Committee
Our compensation committee consists of Mr. Nohra, Dr. Ostro and Dr. Akkaraju, with Dr. Ostro serving as chairman of the committee. Our board of directors has determined each member of the compensation committee is “independent” as defined under the applicable listing standards of The Nasdaq Global Market. The compensation committee’s responsibilities include:
| • | annually reviewing and approving individual and corporate goals and objectives relevant to the compensation of our executive officers; |
| • | evaluating the performance of our executive officers in light of such individual and corporate goals and objectives and determining the compensation of our executive officers; |
| • | appointing, compensating and overseeing the work of any compensation consultant, legal counsel or other advisor retained by the compensation committee; |
| • | conducting the independence assessment outlined in Nasdaq rules with respect to any compensation consultant, legal counsel, or other advisor retained by the compensation committee; |
| • | annually reviewing and reassessing the adequacy of the committee charter in its compliance with the listing requirements of The Nasdaq Global Market; |
| • | overseeing and administering our compensation and similar plans; |
| • | reviewing and approving our policies and procedures for the grant of equity-based awards; |
| • | reviewing and making recommendations to the board of directors with respect to director compensation; |
| • | reviewing and discussing with management the compensation discussion and analysis to be included in our annual proxy statement or Annual Report on Form 10-K; |
| • | preparing the compensation committee report required by the rules of the SEC to be included in our annual proxy statement; |
| • | reviewing and discussing with the board of directors corporate succession plans for the chief executive officer and other senior management positions; and |
| • | periodically reviewing our Company’s overall policies, practices, and plans. |
102
Table of Contents
Nominating and Corporate Governance Committee
Our nominating and corporate governance committee consists of Mr. Nohra and Dr. Ostro, with Mr. Nohra serving as chairman of the committee. Our board of directors has determined that each member of the nominating and corporate governance committee is “independent” as defined under the applicable listing standards of The Nasdaq Global Market. The nominating and corporate governance committee’s responsibilities include:
| • | developing and recommending to the board of directors criteria for board and committee membership; |
| • | establishing procedures for identifying and evaluating board of director candidates, including nominees recommended by stockholders; |
| • | identifying individuals qualified to become members of the board of directors; |
| • | recommending to the board of directors the persons to be nominated for election as directors and to each of the board’s committees; and |
| • | developing and recommending to the board of directors a set of corporate governance principles and guidelines. |
Our board of directors may establish other committees from time to time.
Compensation Committee Interlocks and Insider Participation
Since our IPO closed on June 23, 2014, our compensation committee has consisted of Dr. Ostro, Mr. Nohra and Dr. Akkaraju. From December 2012 through April 2013, our compensation committee consisted of Dr. McKearn, Dr. Ostro, Mr. Truitt and Dr. Alexander, our current Chief Executive Officer, who prior to March 2013, was not employed by us. From April 2013 through May 2014, our compensation committee consisted of Dr. McKearn, Dr. Ostro and Mr. Nohra. None of the members of our compensation committee has at any time been one of our officers or employees other than Dr. Alexander. None of our executive officers currently serves, or in the past fiscal year has served, as a member of the board of directors or compensation committee of any entity that has one or more executive officers on our board of directors or compensation committee.
Board Diversity
Our nominating and corporate governance committee is responsible for reviewing with the board of directors, on an annual basis, the appropriate characteristics, skills and experience required for the board of directors as a whole and its individual members. In evaluating the suitability of individual candidates (both new candidates and current members), the nominating and corporate governance committee, in recommending candidates for election, and the board of directors, in approving (and, in the case of vacancies, appointing) such candidates, takes into account many factors, including the following:
| • | personal and professional integrity; |
| • | ethics and values; |
| • | experience in corporate management, such as serving as an officer or former officer of a publicly held company; |
| • | experience in the industries in which we compete; |
| • | experience as a board member or executive officer of another publicly held company; |
| • | diversity of expertise and experience in substantive matters pertaining to our business relative to other board members; |
| • | conflicts of interest; and |
| • | practical and mature business judgment. |
103
Table of Contents
Our board of directors evaluates each individual in the context of the board of directors as a whole, with the objective of assembling a group that can best maximize the success of the business and represent stockholder interests through the exercise of sound judgment using its diversity of experience in these various areas.
Code of Business Conduct and Ethics
We have a code of business conduct and ethics that applies to all of our employees, officers and directors, including those officers responsible for financial reporting. The code of business conduct and ethics is available on our website at investors.zspharma.com/corporate-governance.cfm. We expect that any amendments to the code, or any waivers of its requirements, will be promptly disclosed on our website. The inclusion of our website address in this report does not incorporate by reference the information on or accessible through our website into this report.
Limitation on Liability and Indemnification Matters
Our seventh amended and restated certificate of incorporation contains provisions that limit the liability of our directors for monetary damages to the fullest extent permitted by Delaware law. Consequently, our directors are not personally liable to us or our stockholders for monetary damages for any breach of fiduciary duties as directors, except liability for:
| • | any breach of the director’s duty of loyalty to us or our stockholders; |
| • | any act or omission not in good faith or that involves intentional misconduct or a knowing violation of law; |
| • | unlawful payments of dividends or unlawful stock repurchases or redemptions as provided in Section 174 of the Delaware General Corporation Law; or |
| • | any transaction from which the director derived an improper personal benefit. |
Our seventh amended and restated certificate of incorporation and amended and restated bylaws provide that we are required to indemnify our directors and officers, in each case to the fullest extent permitted by Delaware law. Our amended and restated bylaws also provide that we are obligated to advance expenses incurred by a director or officer in advance of the final disposition of any action or proceeding, and permit us to secure insurance on behalf of any officer, director, employee or other agent for any liability arising out of his or her actions in that capacity regardless of whether we would otherwise be permitted to indemnify him or her under Delaware law. We have entered and expect to continue to enter into agreements to indemnify our directors as determined by our board of directors. With specified exceptions, these agreements provide for indemnification for related expenses including, among other things, attorneys’ fees, judgments, fines and settlement amounts incurred by any of these individuals in any action or proceeding. We believe that these bylaw provisions and indemnification agreements are necessary to attract and retain qualified persons as directors. We will also maintain directors’ and officers’ liability insurance.
The limitation of liability and indemnification provisions in our seventh amended and restated certificate of incorporation and amended and restated bylaws may discourage stockholders from bringing a lawsuit against our directors and officers for breach of their fiduciary duty. They may also reduce the likelihood of derivative litigation against our directors and officers, even though an action, if successful, might benefit us and our stockholders. Further, a stockholder’s investment may be adversely affected to the extent that we pay the costs of settlement or damages.
| ITEM 11. | EXECUTIVE COMPENSATION |
This section describes the material elements of compensation of our named executive officers, or NEOs. Our NEOs for the year ended December 31, 2014 were Robert Alexander, Ph.D., our Chief Executive Officer, Mark Asbury, our Senior Vice President and General Counsel and Alvaro Guillem, Ph.D., our President.
104
Table of Contents
This discussion contains forward-looking statements that are based on our current plans, considerations, expectations and determinations regarding future compensation programs. Actual compensation programs that we adopt may differ materially from currently planned programs as summarized in this discussion. As an “emerging growth company” as defined in the JOBS Act, we are not required to include a Compensation Discussion and Analysis section and have elected to comply with the scaled disclosure requirements applicable to emerging growth companies.
Summary Compensation Table
The following table sets forth information regarding the compensation awarded to or earned by our NEOs during the years ended December 31, 2013 and December 31, 2014.
| Name and Principal Position |
Year | Salary ($) | Bonus ($)(1) | Option Awards ($)(2) |
All Other Compensation ($)(3) |
Total ($) | ||||||||||||||||||
| Robert Alexander |
2014 | 435,231 | (4) | 217,733 | 2,430,888 | 41,105 | 3,124,957 | |||||||||||||||||
| Chief Executive Officer |
2013 | 291,667 | (4) | 87,740 | 799,710 | 26,397 | 1,205,514 | |||||||||||||||||
| Mark Asbury |
2014 | 151,250 | 57,980 | 2,016,319 | 19,474 | 2,245,023 | ||||||||||||||||||
| Senior Vice President and General Counsel |
2013 | — | — | — | — | — | ||||||||||||||||||
| Alvaro Guillem |
2014 | 347,500 | 121,647 | 1,443,923 | 36,161 | 1,949,231 | ||||||||||||||||||
| President |
2013 | 325,000 | 113,750 | 278,160 | 27,065 | 743,975 | ||||||||||||||||||
| (1) | The amounts reported in the Bonus column represent annual cash bonus payments awarded upon the achievement of certain Company and individual performance targets, as established by the board of directors, which the NEOs earned during 2014. |
| (2) | The amounts reported in the Option Awards column represent the grant date fair value of the stock options granted to our NEOs during 2014 as computed in accordance with ASC 718. The assumptions used in calculating the grant date fair value of the stock options reported in the Option Awards column are set forth in Note 1 to the audited financial statements included in this prospectus. The amounts reported in this column exclude the impact of estimated forfeitures related to service-based vesting conditions. Note that the amounts reported in this column reflect the accounting cost for these stock options, and do not correspond to the actual economic value that may be received by the NEOs from the options. |
| (3) | The amounts reported in the All Other Compensation column consist of premiums we paid with respect to each of our NEOs for medical, dental, vision, and term life insurance, pursuant to the terms of each NEO’s employment agreement with us and 401(k) contributions made by the company. |
| (4) | We accepted Dr. Alexander’s voluntary resignation as our Chairman on March 21, 2014. Dr. Alexander continues to serve as the Chief Executive Officer and as a member of our board of directors. |
105
Table of Contents
Narrative Disclosure to 2014 Summary Compensation Table
Employment Agreements with our NEOs
We have entered into agreements with each of our NEOs in connection with his employment with us. With the exception of his own arrangement, each of these employment agreements was negotiated on our behalf by Dr. Guillem, with the oversight and approval of our board of directors. Dr. Guillem’s agreement was negotiated directly with the Chairman of our compensation committee.
These agreements provide for “at will” employment and set forth the terms and conditions of employment of each NEO, including base salary, target annual bonus opportunity, stock option grants, welfare and fringe benefits, non-competition, non-solicitation, confidentiality, invention assignment and severance. Our board of directors or the compensation committee reviews each NEO’s base salary and target bonus opportunity from time to time to ensure compensation adequately reflects the NEO’s qualifications, experience, role and responsibilities. For fiscal year 2013, Dr. Alexander’s base salary was $350,000 and Dr. Guillem’s base salary was $325,000. In addition, for fiscal year 2013, Dr. Alexander and Dr. Guillem each had an annual bonus target of up to 30% and 35%, respectively, of base salary awarded based on the achievement of annual performance targets established by the respective NEO and the board of directors. For fiscal year 2014, Dr. Alexander’s base salary was $520,463, Mr. Asbury’s base salary was $330,000 and Dr. Guillem’s base salary was $370,000. In addition, for fiscal year 2014, Dr. Alexander, Mr. Asbury and Dr. Guillem each had an annual bonus target of up to 50%, 30% and 35%, respectively, of base salary awarded based on the achievement of annual performance targets established by the respective NEO and the board of directors. Please see “Terms and Conditions of Annual Bonuses” for a further description of the annual bonuses awarded to our NEOs.
Additionally, Dr. Alexander and Dr. Guillem were granted options to purchase 448,453 and 155,983 shares of our common stock, respectively, in 2013 and Dr. Alexander, Mr. Asbury and Dr. Guillem were granted options to purchase 267,458, 85,000 and 114,506 shares of our common stock, respectively, in 2014. Please see “Terms and Conditions of Equity Award Grants” for a further description of the options awarded to our NEOs.
In addition, pursuant to the terms of each NEO’s employment agreement, we agreed to reimburse each NEO for his share of any medical, dental, vision and term life benefits available to our officers. The company also provides a 3% 401(k) contribution to all employees, including officers. For the fiscal year 2013, we paid the following amounts for Dr. Alexander and Dr. Guillem, respectively, for such benefits: $26,397 and $27,065. For fiscal year 2014, we paid the following amounts for Dr. Alexander, Mr. Asbury and Dr. Guillem, respectively, for such benefits: $41,105, $19,474 and $36,161. Pursuant to the terms of each employment agreement with our NEOs, each NEO is restricted while he is employed by us and for a period of one year after his termination from directly or indirectly competing with the business of ZS or recruiting or soliciting any employee of ZS. Each employment agreement with our NEOs contains standard confidential information and invention assignment provisions.
We may terminate the employment of our NEOs under these employment agreements at any time and for any reason; provided, each NEO must be given 10 days’ notice of a termination because of disability. Each NEO may terminate his employment under his employment agreement at any time and for any reason. In certain circumstances, the employment agreements with our NEOs provide for a lump sum payment of severance. Please see “Terms and Conditions of Executive Severance” for a further description of our severance obligations to our NEOs.
Terms and Conditions of Executive Severance
Each of our NEO’s employment agreements provides for certain severance benefits. Under these agreements, in the event that the executive is terminated without cause or resigns for good reason (each as
106
Table of Contents
defined below) and subject to such executive executing and not revoking a general release of all claims against us, then such executive is entitled to receive (i) his base salary earned through his last day of employment, (ii) on the first business day following the 75-day period following the executive’s termination, a lump sum cash severance payment equal to twelve months of the executive’s base salary and (iii) payment by us of an amount equal to the product of (a) 24 times (b) the amount that was paid by, allocated to or otherwise attributed to us as our employer premium contribution of our welfare and fringe benefit plans with respect to such executive and his dependents. In the event the executive is terminated without cause or resigns for good reason, such executive’s stock options will become fully vested and exercisable upon the date of his termination.
Under the NEO employment agreements, in the event the executive is terminated for cause, resigns without good reason or leaves because of death or disability (as defined below), then such executive is entitled to receive (i) his base salary earned through his last day of employment and (ii) benefits under the stock option plan and welfare and fringe benefit plans in accordance with the respective terms of those plans.
For purposes of the agreements, “cause” means the occurrence of any of the following events, as determined by our board of directors, in its sole discretion: (i) the NEO’s willful and continued failure to perform substantially the duties of the NEO’s position or to follow lawful instructions of a senior executive or the board of directors, if such failure continues for a period of five days after we delivered such NEO written notice identifying such failure; (ii) the NEO’s conviction of a felony or of another crime that reflects adversely on us; (iii) the NEO’s engaging in fraudulent or dishonest conduct, gross misconduct that is injurious to us, or any misconduct that involves moral turpitude; (iv) the NEO’s material breach of his obligations under his employment agreement or under his nondisclosure or noncompete agreement with us; (v) alcohol or substance abuse that has impaired or could reasonably be expected to impair the ability of the NEO to perform his duties to us; (vi) failure to comply with our policies in any material respect, including those regarding harassment or discrimination in employment; or (vii) excessive absenteeism, willful or persistent neglect of, or abandonment of the NEO’s duties (other than due to illness or any other physical condition that could reasonably be expected to result in disability), which has not been cured after reasonable notice from us. “Good reason” means the occurrence of any of the following events: (i) a material adverse change in the nature or scope of the NEO’s responsibilities; (ii) a reduction in the NEO’s salary rate or target bonus; (iii) a reduction of ten percent or more in the aggregate benefits provided to the NEO and his dependents under our employee benefit plans; or (iv) failure by us to obtain any assumption agreement from any successor as contemplated in the NEO’s employment agreement. “Disability” means when the NEO is unable to perform the essential functions of his job, with or without reasonable accommodation in accordance with the Americans with Disabilities Act, as determined by the board of directors in good faith for (i) a period of 120 consecutive days or (ii) periods greater than 150 days in the aggregate during any 12-month period.
Pension Benefits and Nonqualified Preferred Compensation
We do not and have not historically provided a pension plan for our employees, and none of our named executive officers participated in a nonqualified deferred compensation plan in 2013 or 2014.
107
Table of Contents
Outstanding Equity Awards at 2014 Fiscal Year-End
The following table sets forth specified information concerning unexercised stock options for each of the named executive officers outstanding as of December 31, 2014.
| Option Awards | ||||||||||||||||||||
| Name |
Vesting Commencement Date(1) |
Number of Securities Underlying Unexercised Options (#) Unexercisable |
Number of Securities Underlying Unexercised Options (#) Exercisable |
Option Exercise Price(2) |
Option Expiration Date |
|||||||||||||||
| Robert Alexander |
3/1/2013 | 252,249 | 196,204 | $ | 2.13 | 2/28/2023 | ||||||||||||||
| 1/25/2014 | 189,846 | — | $ | 5.62 | 1/24/2024 | |||||||||||||||
| 7/8/2014 | 77,612 | — | $ | 27.16 | 7/7/2024 | |||||||||||||||
| Alvaro Guillem |
6/5/2009 | (3) | — | 37,046 | $ | 1.03 | 6/4/2019 | |||||||||||||
| 1/1/2012 | (4) | 16,898 | 33,796 | $ | 2.56 | 12/31/2021 | ||||||||||||||
| 10/15/2012 | 84,894 | 100,336 | $ | 2.13 | 10/14/2022 | |||||||||||||||
| 3/1/2013 | 87,737 | 68,246 | $ | 2.13 | 2/28/2023 | |||||||||||||||
| 4/24/2014 | 81,469 | — | $ | 10.92 | 4/23/2024 | |||||||||||||||
| 7/8/2014 | 33,037 | — | $ | 27.16 | 7/7/2024 | |||||||||||||||
| Mark Asbury |
6/30/2014 | 85,000 | — | $ | 28.75 | 6/29/2024 | ||||||||||||||
| (1) | Except as otherwise indicated, the options vest as to 25% of the shares subject to such option on the first anniversary of the vesting commencement date with the remaining 75% of the shares vesting monthly in substantially equal installments over the following 36 months, subject to the holder continuing to provide services to us through each vesting date. |
| (2) | This column represents the fair value of our common stock on the date of grant, as determined by our board of directors. |
| (3) | The shares subject to this option are fully vested. |
| (4) | These options vest annually over three years in substantially equal installments, subject to the holder continuing to provide services to us through each vesting date. |
Equity Compensation Plans and Other Benefit Plans
2014 Incentive Plan
Our board of directors and stockholders approved an incentive plan, the 2014 Incentive Plan or the 2014 Plan, for the benefit of the employees, non-employee directors and consultants who perform services for us. The 2014 Plan became effective upon the completion of our IPO. The principal purpose of the 2014 Plan is to attract, retain and motivate selected employees, consultants and non-employee directors through the granting of stock-based compensation and cash-based performance bonus awards. The material terms of the 2014 Plan are summarized below.
Share Reserve. Under the Fourth Amended Stock Incentive Plan, or Amended Plan, and the 2014 Plan as of December 31, 2014, there were options to purchase 4,732,564 shares of our common stock outstanding at a weighted-average exercise price per share of $7.52. From the effective date of the 2014 Plan through December 31, 2014, we granted options to purchase 734,412 shares of our common stock with a weighted-average exercise price of $29.87 and after December 31, 2014, we granted options to purchase 98,024 shares of our common stock with a weighted-average exercise price of $45.49. Under the 2014 Plan, 1,949,797 shares of our common stock were initially reserved for issuance pursuant to a variety of stock-based compensation awards, including stock options, stock appreciation rights, or SARs, restricted stock awards,
108
Table of Contents
restricted stock unit awards, dividend equivalent awards, stock payment awards, performance awards and other stock-based awards. On the first day of each calendar year beginning in 2015, an additional number of shares of our common stock shall be reserved under the 2014 Plan in an amount equal to the lesser of (A) a number of shares equal to the excess of four percent (4%) of the shares of stock outstanding (on an as converted basis) on December 31st of the immediately preceding calendar year less the aggregate number of shares available for awards under the 2014 Plan as of such date (but not less than zero), (B) a number of shares of stock determined by our board of directors or (C) 1,169,878 shares. The aggregate of the shares initially reserved hereunder and the annual increases from time to time are referred to as the Maximum Share Limit, all of which will be available for incentive stock options or ISOs. The shares of our common stock to be delivered under the 2014 Plan are made available from authorized but unissued shares of stock, shares held in treasury, or previously issued shares reacquired by us, including by purchase on the open market or by private purchase.
Awards under the 2014 Plan settled in cash do not reduce the Maximum Share Limit. If an award under the 2014 Plan expires or is terminated, cancelled or forfeited, the shares of our common stock associated with such expired, terminated, cancelled or forfeited award will again be available for future awards under the 2014 Plan. The following shares of our common stock will also become available again for awards under the 2014 Plan other than awards of incentive stock options:
(i) shares that are tendered by a participant or withheld as full or partial payment of minimum withholding taxes or as payment for the exercise price of an award under the 2014 Plan; and
(ii) shares reserved for issuance upon grant of a SAR issued under the 2014 Plan, to the extent the number of reserved shares of our common stock exceeds the number of shares actually issued upon exercise or settlement of such SAR.
The foregoing notwithstanding, subject to The Nasdaq Global Market listing requirements, the Maximum Share Limit is not be reduced by (x) shares of our common stock issued under awards granted in the assumption of, or substitution or exchange for, previously granted awards of a company acquired by us and (y) available shares under a stockholder approved plan of an acquired company (as appropriately adjusted to reflect the transaction) and such shares are available for awards under the 2014 Plan.
Administration. The compensation committee of our board of directors administers the 2014 Plan. The compensation committee must consist of at least two members of our board of directors, each of whom is intended to qualify as an “outside director,” within the meaning of Section 162(m) of the Code, a “non-employee director” for purposes of Rule 16b-3 under the Exchange Act and an “independent director” within the meaning of the rules of the applicable stock exchange, or other principal securities market on which shares of our common stock are traded. The 2014 Plan provides that the compensation committee may delegate its authority to grant awards to employees other than executive officers and certain senior executives to a committee consisting of one or more members of our board of directors, other than awards made to our non-employee directors, which must be approved by our full board of directors.
Subject to the terms and conditions of the 2014 Plan, the compensation committee, or the administrator, has the authority to select the persons to whom awards are to be made, to determine the number of shares to be subject to awards and the terms and conditions of awards, and to make all other determinations and to take all other actions necessary or advisable for the administration of the 2014 Plan. The compensation committee is also authorized to adopt, amend or rescind rules relating to administration of the 2014 Plan. The full board of directors administers the 2014 Plan with respect to awards to non-employee directors, and the compensation committee administers the 2014 Plan with respect to awards to employees and consultants.
Eligibility. Options, SARs, restricted stock and all other stock-based and cash-based awards under the 2014 Plan may be granted to individuals who are then our officers, employees or consultants or are the officers, employees or consultants of certain of our subsidiaries. Such awards (other than incentive stock options) also may be granted to our non-employee directors. Only employees or those of certain of our subsidiaries may be granted incentive stock options.
109
Table of Contents
Awards. The 2014 Plan provides that the administrator may grant or issue stock options, SARs, restricted stock, restricted stock units, dividend equivalents, performance awards, stock payments and other stock-based and cash-based awards, or any combination thereof. Each award will be set forth in a separate agreement with the person receiving the award and will indicate the type, terms and conditions of the award.
| • | Nonqualified stock options, or NSOs, will provide for the right to purchase shares of our common stock at a specified price which may not be less than fair market value on the date of grant. NSOs may be granted for any term specified by the administrator that does not exceed ten years. |
| • | ISOs will be designed in a manner intended to comply with the provisions of Section 422 of the Code and will be subject to specified restrictions contained in the Code. Among such restrictions, ISOs must have an exercise price of not less than the fair market value of a share of common stock on the date of grant, may only be granted to employees, and must not be exercisable after a period of ten years measured from the date of grant. In the case of an ISO granted to an individual who owns (or is deemed to own) at least 10% of the total combined voting power of all classes of our capital stock, the 2014 Plan provides that the exercise price must be at least 110% of the fair market value of a share of common stock on the date of grant and the ISO must not be exercisable after a period of five years measured from the date of grant. |
| • | Restricted stock may be granted to any eligible individual, typically without payment of consideration, and made subject to such restrictions as may be determined by the administrator. Restricted stock, typically, may be forfeited for no consideration or repurchased by us at the original purchase price if the conditions or restrictions on vesting are not met. In general, restricted stock may not be sold or otherwise transferred until restrictions are removed or expire. Recipients of restricted stock, unlike recipients of options, will have voting rights and will have the right to receive dividends, if any, prior to the time when the restrictions lapse. |
| • | Restricted stock units may be awarded to any eligible individual, typically without payment of consideration, but subject to vesting conditions based on continued employment or service or on performance criteria established by the administrator. Like restricted stock, restricted stock units may not be sold, or otherwise transferred or hypothecated, until vesting conditions are removed or expire. Unlike restricted stock, stock underlying restricted stock units will not be issued until the restricted stock units have vested, and recipients of restricted stock units generally will have no voting or dividend rights prior to the time when vesting conditions are satisfied. |
| • | SARs may be granted in connection with stock options or other awards, or separately. SARs typically will provide for payments to the holder based upon increases in the price of our common stock over a set exercise price. The exercise price of any SAR granted under the 2014 Plan must be at least 100% of the fair market value of a share of our common stock on the date of grant. SARs under the 2014 Plan will be settled in cash or shares of our common stock, or in a combination of both, at the election of the administrator. |
| • | Dividend equivalents represent the value of the dividends, if any, per share paid by us, calculated with reference to the number of shares covered by an award of restricted stock units. Dividend equivalents are not paid with respect to unvested awards but may be accumulated and paid when the award vests. Dividend equivalents may be settled in cash or shares and at such times as determined by the administrator. |
| • | Performance awards are a right to receive all or part of an award based upon performance goals set forth in the 2014 Plan or other criteria specified by the administrator. The administrator will determine the period over which certain specified company or individual goals or objectives must be met. The performance award may be paid in cash, shares of our common stock or other awards or property, in the discretion of the administrator. Performance awards may be structured as “qualified performance-based compensation” under Section 162(m) of the Code, which we refer to as Qualified Awards. For Qualified Awards, performance goals must be established by the plan administrator prior to the earlier of (i) 90 days after the commencement of the period of service to which the performance goals relate or |
110
Table of Contents
| (ii) the lapse of 25% of the period of service. For Qualified Awards, a performance goal may be based upon one or more business criteria that apply to the participant or the performance of one or more of our business units or the company as a whole, and must be based on one or more of the criteria set forth under the 2014 Plan. |
| • | Stock awards represent the right to receive shares of our common stock as authorized by the administrator and may be fully vested on the grant date. |
| • | Cash awards represent the right to receive a cash payment as authorized by the administrator, which payment may be paid under a performance award that meets the requirements of a Qualified Award. |
Certain Limitations. With respect to employee awards made under the 2014 Plan, no employee may be granted during a single calendar year (i) stock options or stock appreciation rights that are exercisable for more than 389,959 shares of our common stock; (ii) Qualified Awards that are in the form of our common stock covering or relating to more than 389,959 shares of our common stock; and (iii) Qualified Awards in the form of awards that may be settled solely in cash having a grant date value in excess of $5,000,000. No non-employee director may be granted during a single calendar year awards having a value determined on the grant date in excess of $500,000.
Change in Control. In the event of a change in control, the administrator may, in its discretion, accelerate the vesting or exercisability of an award, eliminate or make less restrictive any restrictions contained in an award, waive any restriction or other provision of the 2014 Plan or an award or otherwise amend or modify an award in any manner that is, in either case, (i) not materially adverse to the participant to whom such award was granted, (ii) consented to by such participant or (iii) as otherwise authorized under the 2014 Plan, provided, however, the term of any option or SAR cannot be extended to greater than ten years from its original grant date. The administrator may also make appropriate adjustments to awards under the 2014 Plan and is authorized to provide for the acceleration, cash-out, termination, assumption, substitution or conversion of such awards in the event of a change in control or certain other unusual or nonrecurring events or transactions. Under the 2014 Plan, a change in control is generally defined as:
| • | the transfer or exchange in a single transaction or series of related transactions by our stockholders of more than 50% of our voting stock to a person or group; |
| • | a merger, consolidation, reorganization or business combination in which we are involved, directly or indirectly, other than a merger, consolidation, reorganization or business combination which results in our outstanding voting securities immediately before the transaction continuing to represent a majority of the voting power of the acquiring company’s outstanding voting securities and after which no person or group beneficially owns 50% or more of the outstanding voting securities of the surviving entity immediately after the transaction; |
| • | the sale, exchange or transfer of all or substantially all of our assets; or |
| • | stockholder approval of our liquidation or dissolution. |
Adjustments of Awards. In the event of any stock dividend, stock split, subdivision of shares, combination or exchange of shares, merger, consolidation, spin-off, recapitalization, distribution of our assets to stockholders (other than normal cash dividends) or any other corporate event affecting the number of outstanding shares of our common stock or the share price of our common stock that would require adjustments to the 2014 Plan or any awards under the 2014 Plan in order to prevent the dilution or enlargement of the potential benefits intended to be made available thereunder, the administrator will make appropriate, proportionate adjustments to:
| • | the aggregate number and type of shares subject to the 2014 Plan; |
| • | the number and kind of shares subject to outstanding awards and terms and conditions of outstanding awards (including, without limitation, any applicable performance targets or criteria with respect to such awards); |
111
Table of Contents
| • | the grant or exercise price per share of any outstanding awards under the 2014 Plan; and |
| • | the stock-based award limitations under the 2014 Plan. |
Amendment. Our board of directors may amend or modify the 2014 Plan at any time and from time to time provided that no amendment may adversely affect the rights of an award holder without consent. However, we must generally obtain stockholder approval:
| • | to increase the number of shares available under the 2014 Plan (other than in connection with certain corporate events, as described above); |
| • | to grant options with an exercise price that is below 100% of the fair market value of shares of our common stock on the grant date; |
| • | to extend the exercise period for an option beyond ten years from the date of grant; or |
| • | to the extent required by applicable law, rule or regulation (including any applicable stock exchange rule). |
Notwithstanding the foregoing, an option may be amended to reduce the per share exercise price below the per share exercise price of such option on the grant date and options may be granted in exchange for, or in connection with, the cancellation or surrender of options having a higher per share exercise price without receiving additional stockholder approval.
Termination. The board of directors may terminate the 2014 Plan at any time. Unless earlier terminated by our board of directors, the 2014 will terminate on the tenth anniversary of the 2014 Plan’s effective date and no further awards shall be granted after the 2014 Plan’s termination date. Any award that is outstanding on the termination date of the 2014 Plan will remain in force according to the terms of the 2014 Plan and the applicable award agreement.
Restrictions. No payment or delivery of shares of our common stock may be made unless we are satisfied that payment or delivery will comply with applicable laws and regulations. Certificates (if any) evidencing shares of our common stock delivered under the 2014 Plan may be subject to stop transfer orders and other restrictions that the administrator deems advisable. The administrator may cause a legend or legends to be placed upon the certificates to make appropriate reference to these restrictions.
Clawback. Any award under the 2014 Plan will be subject to recovery or clawback by us under any clawback policy adopted by us. Moreover, any award which is subject to recovery under any law, government regulation, or stock exchange listing requirement will be subject to the deductions and clawback that are required to be made pursuant to such law, government regulation, stock exchange listing requirement and any policy adopted by us pursuant to any such law, government regulation or stock exchange listing requirement.
Tax Withholding. We have the right to deduct taxes at the applicable rate from any award payment and withhold, at the time of delivery or vesting of an award, an appropriate amount of cash or number of shares of our common stock for the payment of taxes. The administrator may also permit withholding to be satisfied by the transfer of shares of our common stock previously owned by the holder of the award.
Unfunded Plan. The 2014 Plan is unfunded. Bookkeeping accounts that may be established for purposes of the 2014 Plan are used merely as a bookkeeping convenience. We are not required to segregate any assets or purposes of the long-term incentive plan, and neither us, our board of directors nor the administrator will be deemed to be a trustee of any benefit granted under the 2014 Plan. Our obligations under the 2014 Plan are based solely on any contractual obligations that may be created by the 2014 Plan and the award agreements, and no such obligation will be deemed to be secured by any pledge or other encumbrance on our property. None of us, our board of directors or the administrator will be required to give any security or bond for the performance of any obligation that may be created by the 2014 Plan.
112
Table of Contents
Securities Laws and U.S. Federal Income Taxes. The 2014 Plan is designed to comply with various securities and U.S. federal tax laws as follows:
| • | Securities Laws. The 2014 Plan is intended to conform to all provisions of the Securities Act and the Exchange Act and any and all regulations and rules promulgated by the SEC thereunder, including without limitation, Rule 16b-3. The 2014 Plan will be administered, and options will be granted and may be exercised, only in such a manner as to conform to such laws, rules and regulations. |
| • | Section 409A of the Code. Certain awards under the 2014 Plan may be considered “nonqualified deferred compensation” for purposes of Section 409A of the Code, which imposes certain additional requirements regarding the payment of deferred compensation. Generally, if at any time during a taxable year a nonqualified deferred compensation plan fails to meet the requirements of Section 409A of the Code, or is not operated in accordance with those requirements, all amounts deferred under the 2014 Plan and all other equity incentive plans for the taxable year and all preceding taxable years by any participant with respect to whom the failure relates are includible in gross income for the taxable year to the extent not subject to a substantial risk of forfeiture and not previously included in gross income. If a deferred amount is required to be included in income under Section 409A of the Code, the amount also is subject to interest and an additional income tax. The interest imposed is equal to the interest at the underpayment rate plus one percentage point, imposed on the underpayments that would have occurred had the compensation been includible in income for the taxable year when first deferred, or if later, when not subject to a substantial risk of forfeiture. The additional U.S. federal income tax is equal to 20% of the compensation required to be included in gross income. In addition, certain states, including California, have laws similar to Section 409A of the Code, which impose additional state penalty taxes on such compensation. |
| • | Section 162(m) of the Code. In general, under Section 162(m) of the Code, income tax deductions of publicly held corporations may be limited to the extent total compensation (including, but not limited to, base salary, annual bonus, and other non-qualified benefits) for certain executive officers exceeds $1,000,000 (less the amount of any “excess parachute payments” as defined in Section 280G of the Code) in any taxable year of the corporation. However, under Section 162(m) of the Code, the deduction limit does not apply to certain “performance-based compensation” established by an independent compensation committee that is adequately disclosed to and approved by stockholders. In particular, stock options and SARs will satisfy the “performance-based compensation” exception if the awards are made by a qualifying compensation committee, the 2014 Plan sets the maximum number of shares that can be granted to any person within a specified period and the compensation is based solely on an increase in the stock price after the grant date. Specifically, the option exercise price must be equal to or greater than the fair market value of the stock subject to the award on the grant date. Under a Code Section 162(m) transition rule for compensation plans of corporations which are privately held and which become publicly held in an initial public offering, the 2014 Plan will not be subject to Section 162(m) of the Code until a specified transition date, which is the earlier of: |
| • | the material modification of the 2014 Plan; |
| • | the issuance of all of the shares of our common stock reserved for issuance under the 2014 Plan; or |
| • | the first meeting of our stockholders at which members of our board of directors are to be elected that occurs after the close of the third calendar year following the calendar year in which this offering occurs. |
After the transition date, rights or awards granted under the 2014 Plan, other than options and SARs, will not qualify as “performance-based compensation” for purposes of Section 162(m) of the Code unless such rights or awards are granted or vest upon pre-established objective performance goals, the material terms of which are disclosed to and approved by our stockholders.
We filed with the SEC a registration statement on Form S-8 covering the shares of our common stock issuable under the 2014 Plan.
113
Table of Contents
Fourth Amended Stock Incentive Plan
Our board of directors adopted, and our stockholders approved, our Amended Plan on February 28, 2014. The Amended Plan provides for the grant of stock options and restricted stock awards to our employees, non-employee directors and consultants.
As of December 31, 2014, options to purchase 4,732,564 shares of our common stock at a weighted average exercise price per share of $7.52 remained outstanding under the Amended Plan. Other than stock options, no other equity-based awards have been granted under the Amended Plan.
Following our IPO and in connection with the effectiveness of the 2014 Plan, we have not granted, and we expect no further grants will be made, under the Amended Plan. However, all outstanding awards under the Amended Plan will continue to be governed by their existing terms.
Administration. Our board of directors, a committee thereof appointed by our board of directors, or a combination thereof, has the authority to administer the Amended Plan and the awards granted under it. The administrator has the authority to determine the fair market value of our common stock, the employees to whom options will be granted, the number of shares to be subject to the awards, the terms and conditions of the awards granted, including the exercise or purchase price, vesting conditions and any restrictions or limitations, and the forms of consideration. In addition, the administrator has the authority to amend any outstanding awards or agreements, provided that such amendment does not materially impair the existing rights of any participant without such participant’s consent, construe and interpret the Amended Plan and to adopt rules for the administration, interpretation and application of the Amended Plan that are consistent with the terms of the Amended Plan and are final and binding on all participants of the Amended Plan.
Capitalization Adjustments. In the event of certain corporate adjustments, the administrator of the Amended Plan will adjust the class and maximum number of shares of common stock that may be delivered under the Amended Plan, the class and maximum number of shares of common stock that may be issued pursuant to the exercise of ISOs and/or the number, class and price of shares of common stock covered by each outstanding award.
Dissolution or Liquidation. In the event of a dissolution or liquidation, all outstanding awards will terminate immediately prior to the consummation of such action, unless otherwise determined by the administrator.
Corporate Transaction. In the event of a triggering event, as defined in the Amended Plan, and except as otherwise provided in the applicable option agreement, (i) the administrator shall have the discretion to accelerate the vesting of each outstanding option and (ii) each outstanding option, as may be determined by the administrator in its discretion, shall either be assumed or an equivalent option or right shall be substituted by such successor corporation or a parent or subsidiary of such successor corporation, or terminated in exchange for a payment of cash, securities and/or other property equal to the excess of the fair market value of the optioned stock that is vested and exercisable immediately prior to the consummation of the triggering event over the per share exercise price thereof. In the event the successor corporation does not agree to such assumption, substitution or exchange, each option shall terminate upon the consummation of the triggering event.
Amendment; Termination. Our board of directors may amend or terminate the Amended Plan or any portion thereof at any time, but no amendment will impair the rights of a holder of an outstanding award without the holder’s consent. An amendment of the Amended Plan shall be subject to the approval of our stockholders, where such approval by our stockholders of an amendment is required by applicable law. No awards may be granted under our Amended Plan after it is terminated.
401(k) Plan
Effective March 1, 2014, we adopted our 401(k) plan for our employees. The 401(k) plan is intended to qualify under Section 401(k) of the Code so that contributions to the 401(k) plan by employees or by us, and the
114
Table of Contents
investment earnings thereon, are not taxable to the employees until withdrawn from the 401(k) plan, and so that contributions by us, if any, will be deductible by us when made. Under the 401(k) plan, employees may elect to reduce their current compensation by up to the statutorily prescribed annual limit and to have the amount of such reduction contributed to the 401(k) plan. The 401(k) plan permits us to make contributions up to the limits allowed by law on behalf of all eligible employees.
Rule 10b5-1 Sales Plans
Our directors and executive officers may adopt written plans, known as Rule 10b5-1 plans, in which they will contract with a broker to buy or sell shares of our common stock on a periodic basis. Under a Rule 10b5-1 plan, a broker executes trades pursuant to parameters established by the director or officer when entering into the plan, without further direction from the director or officer. The director or officer may amend or terminate the plan in limited circumstances. Our non-employee directors and executive officers may also buy or sell additional shares of our common stock outside of a Rule 10b5-1 plan when they are not in possession of material, nonpublic information.
Director Compensation
Dr. Alexander, our Chief Executive Officer and Dr. Keyser, our Chief Operating Officer and Secretary, receive no compensation for their service as directors. As of December 31, 2014, Dr. Alexander, Dr. Guillem and Dr. Keyser held options to purchase 715,911, 543,459 and 506,338 shares of our common stock, respectively. As of December 31, 2014, Mr. Nohra, Dr. Akkaraju, Mr. Whiting and Dr. Ostro each held options to purchase 12,000 shares of our common stock. Mr. Babler joined our board after December 31, 2014 and accordingly held no options to purchase shares of our common stock at that time.
Our board of directors adopted a non-employee director compensation policy that is designed to provide a total compensation package that enables us to attract and retain, on a long-term basis, high caliber non-employee directors. Under the policy, all non-employee directors are paid as set forth below:
| Annual Retainer |
||||
| Board of Directors: |
||||
| All non-employee members |
$ | 35,000 | (1) | |
| Additional retainer for Non-Executive Chairmen of the Board |
$ | 20,000 | (1) | |
| Audit Committee: |
||||
| Chairman |
$ | 7,500 | (1) | |
| Compensation Committee: |
||||
| Chairman |
$ | 5,000 | (1) | |
| Nominating and Corporate Governance Committee: |
||||
| Chairman |
$ | 5,000 | (1) | |
| Stock Options |
||||
| All non-employee members |
12,000 | (2)(3) | ||
| (1) | Payable quarterly in arrears. |
| (2) | Options for shares of our common stock under the 2014 Incentive Plan. |
| (3) | On the date of the annual meeting of stockholders, each continuing non-employee director will be eligible to receive an annual option grant to purchase up to 12,000 shares of our common stock, which terms will be determined by our compensation committee at the time of the grant. All options will be granted at fair market value on the date of grant. |
115
Table of Contents
The compensation earned during fiscal year 2014 by Messrs. Nohra and Whiting and Drs. Akkaraju and Ostro for serving as a member of our board of directors is set forth in the following table.
| Name |
Fees earned or paid in cash |
Option awards |
All other compensation |
Total | ||||||||||||
| Guy Nohra |
$ | 60,000 | $ | 282,620 | — | $ | 342,620 | |||||||||
| Srinivas Akkaraju |
$ | 18,306 | $ | 282,620 | — | $ | 300,926 | |||||||||
| Marc Ostro |
$ | 40,000 | $ | 282,620 | — | $ | 322,620 | |||||||||
| John Whiting |
$ | 22,227 | $ | 282,620 | — | $ | 304,847 | |||||||||
| ITEM 12. | SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS |
The following table sets forth information relating to the beneficial ownership of our common stock as of December 31, 2014 for:
| • | each person, or group of affiliated persons, known by us to beneficially own more than 5% of our outstanding shares of common stock; |
| • | each of our directors and named executive officers; and |
| • | all of our current directors and executive officers as a group. |
The number of shares beneficially owned by each entity, person, director or executive officer is determined in accordance with the rules of the SEC and the information is not necessarily indicative of beneficial ownership for any other purpose. Under such rules, beneficial ownership includes any shares over which the individual or entity has sole or shared voting power or investment power as well as any shares that the individual or entity has the right to acquire within 60 days of December 31, 2014 through the exercise of any stock option, warrants or other rights. Except as otherwise indicated, and subject to applicable community property laws, the persons named in the table have sole voting and investment power with respect to all shares of common stock held by that person or entity. This information does not necessarily indicate beneficial ownership for any other purpose, including for purposes of Section 13(d) and 13(g) of the Exchange Act.
The percentage of shares beneficially owned is computed on the basis of 20,898,212 shares of our common stock outstanding as of December 31, 2014. Unless otherwise indicated below, the address for each beneficial owner listed is c/o ZS Pharma, Inc., 508 Wrangler Drive, Suite 100, Coppell, Texas 75019.
| Shares of Common Stock Beneficially Owned |
||||||||
| Name and Address of Beneficial Owner |
Number | % | ||||||
| 5% Stockholders |
||||||||
| Alta Partners VIII, L.P.(1) |
3,230,089 | 15.46 | ||||||
| FMR LLC(2) |
2,862,361 | 13.75 | ||||||
| Entities affiliated with Devon Park Bioventures, L.P.(3) |
2,586,471 | 12.38 | ||||||
| Entities affiliated with 3x5 Special Opportunity Fund, L.P.(4) |
1,503,333 | 7.19 | ||||||
| Named Executive Officers and Directors |
||||||||
| Robert Alexander(5) |
275,653 | 1.30 | ||||||
| Alvaro Guillem(6) |
460,712 | 2.18 | ||||||
| Mark Asbury(7) |
— | — | ||||||
| D. Jeffrey Keyser(8) |
472,576 | 2.23 | ||||||
| Guy Nohra(9) |
3,230,089 | 15.46 | ||||||
| Marc Ostro(10) |
2,586,471 | 12.38 | ||||||
| John Whiting(11) |
— | — | ||||||
| Srinivas Akkaraju(12) |
609,455 | 2.92 | ||||||
| Martin Babler(13) |
— | — | ||||||
| All directors and executive officers as a group (13 persons)(14) |
8,074,165 | 36.46 | ||||||
116
Table of Contents
| (1) | Consists of 3,230,089 shares of common stock held by Alta Partners VIII, L.P. Alta Partners VIII, L.P. is a Delaware limited partnership, whose general partner is Alta Partners Management VIII, LLC, a Delaware limited partnership. Guy Nohra is a managing director of Alta Partners Management VIII, LLC. The two other managing directors of Alta Partners Management VIII, LLC are Daniel Janney and Farah Champsi. Such individuals share voting and investment power over the shares held by the fund and expressly disclaim beneficial ownership over such shares except to the extent of their pecuniary interest therein. The address of Alta Partners VIII, L.P. is One Embarcadero Center, Suite 3700, San Francisco, CA 94111. |
| (2) | Consists of 2,862,361 shares of common stock beneficially owned by FMR LLC, Edward C. Johnson 3d and Abigail P. Johnson, including 1,202,575 shares of common stock held by FMR Co., Inc. FMR LLC is a Delaware limited liability company and a parent holding company. FMR Co., Inc. is a Massachusetts corporation and a subsidiary of FMR LLC. FMR LLC has sole power to dispose, or to direct the disposition, of all 2,862,361 shares of common stock. Edward C. Johnson 3d is a Director and the Chairman of FMR LLC, and Abigail P. Johnson is a Director, the Vice Chairman, the Chief Executive Officer and the President of FMR LLC. Members of the family of Edward C. Johnson, 3d, including Abigail P. Johnson, are the predominant owners, directly or through trusts, of Series B voting common shares of FMR LLC, representing 49% of the voting power of FMR LLC. The Johnson family group and all other Series B shareholders have entered into a shareholders’ voting agreement under which all Series B voting common shares will be voted in accordance with the majority vote of Series B voting common shares. Accordingly, through their ownership of voting common shares and the execution of the shareholders’ voting agreement, members of the Johnson family may be deemed, under the Investment Company Act of 1940, to form a controlling group with respect to FMR LLC. The address of FMR LLC is 245 Summer Street, Boston, MA 02210. |
| (3) | Consists of (a) 9,748 shares of common stock held by Devon Park Associates, L.P. and (b) 2,576,723 shares of common stock held by Devon Park Bioventures, L.P. Devon Park Associates, L.P. is the general partner of Devon Park Bioventures, L.P., and Devon Park Associates, LLC is the general partner of Devon Park Associates, L.P. Dr. Marc Ostro, a member of our board since November 2011, and Messrs. Christopher Moller and Devang Kantesaria are the founding members and managing members (each a “Founding Member”) of Devon Park Associates, LLC. Each Founding Member may be deemed to have shared voting and investment power over the shares held by the funds as described above. Each managing director disclaims beneficial ownership of such shares except to the extent of their pecuniary interest therein. The address of Devon Park Bioventures, L.P., Devon Park Associates, L.P. and Devon Park Associates, LLC is 1400 Liberty Ridge Drive, Suite 103, Wayne, PA 19087. |
| (4) | Consists of (a) 1,319,077 shares of common stock, held by 3x5 Special Opportunity Fund, L.P., (b) 144,901 shares of common stock held by RiverVest Venture Fund II, L.P., and (c) 39,355 shares of common stock held by RiverVest Venture Fund II (Ohio), L.P. The address of 3x5 Special Opportunity Fund, L.P., RiverVest Venture Fund II, L.P., and RiverVest Venture Fund II (Ohio), L.P. is 7733 Forsyth Blvd., Suite 1650, St. Louis, MO 63105. |
| (5) | Consists of 275,653 shares of common stock issuable pursuant to stock options exercisable within 60 days of December 31, 2014. |
| (6) | Consists of (a) 186,923 shares of common stock and (b) 273,789 shares of common stock issuable pursuant to stock options exercisable within 60 days of December 31, 2014. |
| (7) | Mr. Asbury was granted 85,000 options pursuant to the Company’s 2014 incentive plan. No options will have vested within 60 days of December 31, 2014. |
| (8) | Consists of (a) 167,425 shares of common stock, (b) 256,407 shares of common stock issuable pursuant to stock options exercisable within 60 days of December 31, 2014 held by Dr. Keyser, and (c) 48,744 shares of common stock held by Private Trust Company FBO Donald Jeffrey Keyser IRA. |
| (9) | Consists of 3,230,089 shares of common stock held by Alta Partners VIII, L.P., as referenced in footnote 1 above. Mr. Nohra was granted 12,000 options pursuant to the Company’s Non-Employee Director Compensation Policy on August 22, 2014, which vest in accordance with the default vesting schedule detailed in “Executive Compensation — Outstanding Equity Awards at 2014 Fiscal Year-End.” No options will have vested within 60 days of December 31, 2014. Mr. Nohra has served as a member of our board since June 2013, and is a Managing Director of Alta Partners Management VIII, LLC (which is the general |
117
Table of Contents
| partner of Alta Partners VIII, L.P.). The two other Managing Directors of Alta Partners Management VIII, LLC are Daniel Janney and Farah Champsi. Such individuals share voting and investment power over the shares held by the fund and disclaim beneficial ownership over such shares except to the extent of their pecuniary interest therein. |
| (10) | Consists of (a) 9,748 shares of common stock held by Devon Park Associates, L.P., as referenced in footnote 2 above and (b) 2,576,723 shares of common stock held by Devon Park Bioventures, L.P. Dr. Ostro was granted 12,000 options pursuant to the Company’s Non-Employee Director Compensation Policy on August 22, 2014, which vest in accordance with the default vesting schedule detailed in “Executive Compensation — Outstanding Equity Awards at 2014 Fiscal Year-End.” No options will have vested within 60 days of December 31, 2014. The Founding Members are managing members of Devon Park Associates, LLC, which is the general partner of Devon Park Bioventures, L.P. Each Founding Member may be deemed to have shared voting and investment power over the shares held by the funds as described above. Each Founding Member disclaims beneficial ownership of such shares except to the extent of their pecuniary interest therein. |
| (11) | Mr. Whiting was granted 12,000 options pursuant to the Company’s Non-Employee Director Compensation Policy on August 22, 2014, which vest in accordance with the default vesting schedule detailed in “Executive Compensation — Outstanding Equity Awards at 2014 Fiscal Year-End.” No options will have vested within 60 days of December 31, 2014. |
| (12) | Consists of 609,455 shares of common stock held by Sofinnova Venture Partners VIII, L.P. Sofinnova Management VIII, L.L.C. (“SM VIII”) is the general partner of Sofinnova Venture Partners VIII, L.P. Dr. Akkaraju, James I. Healy, Michael F. Powell and Anand Mehra are the managing members of SM VIII and may be deemed to have shared voting and investment power over the shares held by Sofinnova Venture Partners VIII, L.P. Each of the reporting persons disclaims beneficial ownership of such securities, except to the extent of their proportionate pecuniary interest therein. Dr. Akkaraju was granted 12,000 options pursuant to the Company’s Non-Employee Director Compensation Policy on August 22, 2014, which vest in accordance with the default vesting schedule detailed in “Executive Compensation — Outstanding Equity Awards at 2014 Fiscal Year-End.” No options will have vested within 60 days of December 31, 2014. |
| (13) | Was appointed to our board of directors on February 4, 2015. |
| (14) | Consists of (a) 6,829,107 shares of common stock held by our directors and officers and entities affiliated with certain of our directors and (b) 1,245,052 shares of common stock issuable pursuant to stock options exercisable within 60 days of December 31, 2014 held by our directors and officers. |
| ITEM 13. | CERTAIN RELATIONSHIPS AND RELATED PARTY TRANSACTIONS, AND DIRECTOR INDEPENDENCE |
The following is a description of transactions since January 1, 2012 to which we have been a party, in which the amount involved exceeds $120,000, and in which any of our directors, executive officers or holders of more than 5% of our capital stock, or an affiliate or immediate family member thereof, had or will have a direct or indirect material interest.
Sales and Purchases of Securities
Bridge Financing
In November 2011, December 2011, and July 2012, we issued an aggregate of $5.3 million of bridge notes.
The bridge notes were convertible into shares of our equity securities issued pursuant to a subsequent qualified financing. In October 2012, the bridge notes were converted into shares of our Series B preferred stock on substantially the same terms as the other investors in our Series B preferred stock financing.
118
Table of Contents
The table below sets forth the number of shares of Series B preferred stock issued to our directors, executive officers or owners of more than 5% of a class of our capital stock, or an affiliate or immediate family member thereof, upon conversion of all of our outstanding bridge notes:
| Name |
Number of Shares of Series B Preferred Stock |
Purchase Price | ||||||
| Devon Park Bioventures, L.P. |
1,138,321 | $ | 4,232,660 | |||||
| The Dyett Family Trust |
7,204 | $ | 26,787 | |||||
| Patricia P. Truitt |
7,204 | $ | 26,787 | |||||
| D. Jeffrey Keyser |
7,204 | $ | 26,787 | |||||
| Alvaro Guillem |
7,204 | $ | 26,787 | |||||
| HemoCleanse, Inc. |
21,612 | $ | 80,359 | |||||
In connection with the conversion of the bridge notes, warrants to purchase shares of our Series B preferred stock were issued pursuant to the terms of the bridge notes. The warrants were exercised in May 2014 for 365,986 shares of our Series B preferred stock at an exercise price of $3.72 per share. We also issued warrants to purchase 11,766 shares of Series B preferred stock at an exercise price of $3.72 per share in connection with the convertible note financing that closed in November 2011, which were exercised in June 2014.
The table below sets forth the number of Series B Warrants issued to our directors, executive officers or owners of more than 5% of a class of our capital stock, or an affiliate or immediate family member thereof, all of which were fully exercised in May and June 2014:
| Name |
Number of Shares Underlying Series B Warrants |
|||
| HemoCleanse, Inc. |
5,403 | |||
| Patricia P. Truitt |
1,801 | |||
| D. Jeffrey Keyser |
1,801 | |||
| Alvaro Guillem |
1,801 | |||
| Devon Park Bioventures, L.P. |
284,580 | |||
| Chigwell Consulting, Inc. |
11,766 | |||
Series C Preferred Stock
In October 2012, November 2012 and August 2013, we issued an aggregate of 6,899,281 shares of our Series C preferred stock at a price per share of $6.67. The aggregate gross consideration received for these issuances was $46.0 million.
The table below sets forth the number of shares of Series C preferred stock sold to our directors, executive officers or owners of more than 5% of a class of our capital stock, or an affiliate or immediate family member thereof:
| Name |
Number of Shares of Series C Preferred Stock |
Aggregate Purchase Price |
||||||
| Alta Partners VIII, L.P. |
2,849,703 | $ | 19,000,000 | |||||
| Entities affiliated with 3x5 Special Opportunity Fund, L.P.(1) |
1,987,293 | $ | 13,250,003 | |||||
| Salem ZS Investors, LLC |
1,237,371 | $ | 8,249,998 | |||||
| Devon Park Bioventures, L.P. |
824,914 | $ | 5,499,998 | |||||
| (1) | Consists of (a) 1,687,324 shares held by 3x5 Special Opportunity Fund, L.P., (b) 235,896 shares held by RiverVest Venture Fund II, L.P., and (c) 64,073 shares held by RiverVest Venture Fund II (Ohio), L.P. |
119
Table of Contents
Series D Preferred Stock
In February 2014, we issued an aggregate of 1,858,012 shares of our Series D preferred stock at a price per share of $13.455. The aggregate gross consideration received for these issuances was $25.0 million.
The table below sets forth the number of shares of Series D preferred stock sold, to our directors, executive officers or owners of more than 5% of a class of our capital stock, or an affiliate or immediate family member thereof:
| Name |
Number of Shares of Series D Preferred Stock |
Aggregate Purchase Price | ||||||
| Alta Partners VIII, L.P. |
180,387 | $ | 2,427,157 | |||||
| Entities affiliated with 3x5 Special Opportunity Fund, L.P.(1) |
202,692 | $ | 2,727,275 | |||||
| Salem ZS Investors, LLC |
123,651 | $ | 1,663,755 | |||||
| Devon Park Bioventures, L.P. |
168,910 | $ | 2,272,728 | |||||
| Novo A/S |
506,730 | $ | 6,818,183 | |||||
| RA Capital Healthcare Fund, L.P. |
253,365 | $ | 3,409,091 | |||||
| Adage Capital Partners, L.P. |
168,910 | $ | 2,272,728 | |||||
| Entities affiliated with PFM Healthcare(2) |
168,910 | $ | 2,272,728 | |||||
| (1) | Consists of (a) 172,097 shares held by 3x5 Special Opportunity Fund, L.P., (b) 24,060 shares held by RiverVest Venture Fund II, L.P., and (c) 6,535 shares held by RiverVest Venture Fund II (Ohio), L.P. |
| (2) | Consists of (a) 9,684 shares held by PFM Healthcare Principals Fund, L.P. and (b) 159,226 shares held by PFM Healthcare Master Fund, L.P. |
Issuances of Additional Warrants to Purchase Common Stock
In November 2011, December 2011, and July 2012, we issued warrants to purchase 51,166 shares, 6,723 shares, and 13,447 shares of our common stock, respectively, at a price per share of $3.72 to Salem Partners, LLC (the “Initial Salem Warrants”). In October 2012, we granted Salem Capital Partners, LLC the right to receive warrants, which were issued in August 2013, to purchase 344,964 shares of our common stock, at a price of $6.67 per share (the “Additional Salem Warrants”).
The November 2011 and December 2011 warrants were issued in connection with that certain Engagement Agreement, dated September 27, 2010, by and between us and Salem Partners, LLC, whereby we committed to engage Salem Partners, LLC on an exclusive basis as our financial advisor and agreed to compensate Salem Partners, LLC with (i) a non-refundable expense advance of $10,000 and (ii) (a) a cash fee of 6% of the gross amount of any of our debt or equity securities issued after November 13, 2010 and (b) warrants to purchase our common stock with an initial value equal to 5% of the gross amount of debt or equity sold or otherwise issued, in each case in a transaction involving the acquisition, directly or indirectly, by a purchaser of any of our debt, equity or equity-linked securities. The July 2012 and October 2012 warrants were issued in connection with that certain Engagement Agreement, dated June 29, 2012, by and between us and Salem Partners, LLC, whereby we committed to pay Salem Partners, LLC (x) a cash fee of 6% of the gross amount of any of our equity securities issued and (y) warrants to purchase our common stock with an initial value equal to 5% of the gross amount of equity sold or otherwise issued, in each case in a transaction involving the acquisition, directly or indirectly, by a purchaser of any of our debt, equity or equity-linked securities. The Initial Salem Warrants and the Additional Salem Warrants have customary weighted-average anti-dilution provisions and bear terms of 10 years and a strike price equivalent to the price per share implied in the transaction pursuant to which the warrants were granted.
In July 2013, Salem Partners, LLC transferred the Initial Salem Warrants to Salem Capital Partners, LLC. In October 2013, Salem Capital Partners, LLC transferred the right to purchase 45,330 shares under the Initial Salem Warrants to The Dyett Family Trust and the right to purchase the remaining shares under the Initial Salem Warrants to four other accredited investors. In November 2013, The Dyett Family Trust and the four accredited investors referenced above exercised the right to purchase all the shares under the Initial Salem Warrants.
120
Table of Contents
In May 2014, Salem Capital Partners, LLC transferred the right to purchase 217,202 shares of the Additional Salem Warrants to The Dyett Family Trust and the right to purchase the remaining shares under the Additional Salem Warrants to four other accredited investors. The Dyett Family Trust and the four accredited investors referenced above exercised the right to purchase all the shares under the Additional Salem Warrants immediately thereafter.
In January 2012, we issued warrants to purchase an aggregate of 29,247 shares of our common stock at a price per share of $2.56 (9,749 shares each) to Miles B. Davis, Marc Ostro, and Robert Truitt, as non-cash compensation for those individuals’ continued participation as outside board members for 2012. In April 2014, Marc Ostro transferred his warrant to Devon Park Associates, L.P. In May 2014, Devon Park Associates, L.P. exercised the warrant for 9,749 shares of our common stock at an exercise price of $2.56 per share. In May 2014, all remaining warrants to purchase shares of our common stock issued in November and December 2011, January and July 2012 and August 2013, or the Common Stock Warrants, were fully exercised.
Process Development Agreement with Sovereign Pharmaceuticals, LLC
In October 2010, we began utilizing Sovereign Pharmaceuticals, LLC, or Sovereign, as a provider of various analytical testing, packaging, and formulation and process development services. In December 2010, Miles B. Davis, RPh., Ph.D., the President and Chief Executive Officer of Sovereign joined our board of directors. He served as a Director until October 2012. We accepted various project quotes with Sovereign for its services, and the total expense charged to operations for services provided by Sovereign was $0.2 million for the year ended December 31, 2012.
Consulting Agreement with Rasmussen Biotech and Pharma Consulting
On January 1, 2010, we entered into a Consulting Agreement with Rasmussen Biotech and Pharma Consulting, LLC, an entity of which Dr. Rasmussen is the sole member, for his services as our contracting Chief Scientific Officer. In October 2012, the Consulting Agreement terminated because Dr. Rasmussen joined us as a full-time employee and began serving as our Chief Scientific Officer and Chief Medical Officer. For Dr. Rasmussen’s consulting services during his engagement with us until his full-time employment in October 2012, we paid Dr. Rasmussen approximately $0.3 million.
Indemnification Agreements and Directors’ and Officers’ Liability Insurance
Our seventh amended and restated certificate of incorporation contains provisions that limit the liability of directors. In addition, we have entered into indemnification agreements with each of our directors. These agreements, among other things, require us to indemnify each director to the fullest extent permitted by Delaware law, including indemnification of expenses such as attorneys’ fees, judgments, fines and settlement amounts incurred by the director in any action or proceeding, including any action or proceeding by or in right of us, arising out of the person’s services as a director. We have directors’ and officers’ liability insurance policies covering each of our directors and executive officers. For more information regarding these agreements, please see “Management — Limitation on Liability and Indemnification Matters.”
Fourth Amended and Restated Investors’ Rights Agreements
We have entered into a fourth amended and restated investors’ rights agreement with the purchasers of our outstanding convertible preferred stock and certain holders of common stock, including entities with which certain of our directors are affiliated. As of December 31, 2014, the holders of up to 5,456,562 shares of our common stock, including the shares of common stock issuable upon the conversion of our convertible preferred stock and shares of common stock issued upon exercise of warrants, are entitled to rights with respect to the registration of their shares under the Securities Act. For a more detailed description of these registration rights, see “Description of Capital Stock — Registration Rights.”
121
Table of Contents
Second Amended and Restated Voting Agreement
We have entered into a second amended and restated voting agreement with certain holders of our common stock and convertible preferred stock. The second amended and restated voting agreement terminated upon the completion of our IPO.
Amended and Restated Right of First Refusal and Co-Sale Agreement
We have entered into an amended and restated right of first refusal and co-sale agreement with certain holders of our common stock and convertible preferred stock. This agreement provides for rights of first refusal and co-sale relating to the shares of our common stock and common stock issuable upon conversion of the shares of convertible preferred stock held by the parties thereto. The amended and restated right of first refusal and co-sale agreement terminated upon the completion of our IPO.
Participation in our IPO
Entities affiliated with certain of our directors and an entity affiliated with one of our directors purchased an aggregate of approximately $15.9 million in shares of our common stock in our IPO at the IPO price.
Policies and Procedures for Related Party Transactions
Our board of directors has adopted a written related person transaction policy setting forth the policies and procedures for the review and approval or ratification of related person transactions. This policy covers, with certain exceptions set forth in Item 404 of Regulation S-K under the Securities Act, any transaction, arrangement or relationship, or any series of similar transactions, arrangements or relationships in which we were or are to be a participant, where the amount involved exceeds $120,000 and a related person had or will have a direct or indirect material interest, including, without limitation, purchases of goods or services by or from the related person or entities in which the related person has a material interest, indebtedness, guarantees of indebtedness and employment by us of a related person. In reviewing and approving any such transactions, our audit committee is tasked to consider all relevant facts and circumstances, including, but not limited to, whether the transaction is on terms comparable to those that could be obtained in an arm’s length transaction and the extent of the related person’s interest in the transaction. All of the transactions described in this section occurred prior to the adoption of this policy.
| ITEM 14. | PRINCIPAL ACCOUNTANT FEES AND SERVICES |
Independent Registered Public Accounting Firm’s Fees
The aggregate fees, including expenses, of Ernst & Young LLP and their respective affiliates for the fiscal years ended December 31, 2014 and December 31, 2013, are as follows:
| 2014 | 2013 | |||||||
| (in thousands) | ||||||||
| Audit Fees |
$ | 976 | $ | 110 | ||||
| Audit-Related Fees |
— | — | ||||||
| Tax Fees |
50 | — | ||||||
| All Other Fees |
— | — | ||||||
Fees for audit services include fees associated with the annual audit, the reviews of the Company’s quarterly reports on Form 10-Q and services related to the Company’s registration statement on Form S-1. Tax fees include fees associated with tax advice and tax planning.
122
Table of Contents
PART IV.
| ITEM 15. | EXHIBITS |
A.1. Financial Statements
| F-2 | ||||
| F-3 | ||||
| F-4 | ||||
| F-5 | ||||
| Statements of Redeemable and Convertible Preferred Stock and Shareholders’ Equity (Deficit) |
F-6 | |||
| F-7 | ||||
| F-9 |
A.2. Exhibits
See “Index to Exhibits” set forth on page 125.
123
Table of Contents
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
| ZS Pharma, Inc. | ||||
| (registrant) | ||||
| Date: March 13, 2015 |
By: | /s/ Todd A. Creech | ||
| Name: | Todd A. Creech | |||
| Title: | Chief Financial Officer | |||
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
| Signature |
Date |
Capacity | ||
| /s/ Robert Alexander Robert Alexander |
March 13, 2015 | Director and Chief Executive Officer (Principal Executive Officer) | ||
| /s/ Todd Creech Todd Creech |
March 13, 2015 | Chief Financial Officer (Principal Financial and Accounting Officer) | ||
| /s/ Srinivas Akkaraju Srinivas Akkaraju |
March 13, 2015 | Director | ||
| /s/ Martin Babler Martin Babler |
March 13, 2015 | Director | ||
| /s/ D. Jeffrey Keyser D. Jeffrey Keyser |
March 13, 2015 | Director | ||
| /s/ Guy Nohra Guy Nohra |
March 13, 2015 | Director | ||
| /s/ Marc Ostro Marc Ostro |
March 13, 2015 | Director | ||
| /s/ John Whiting John Whiting |
March 13, 2015 | Director | ||
124
Table of Contents
INDEX TO EXHIBITS
| Exhibit |
Exhibit Description | |
| 3.1 | Seventh Amended and Restated Certificate of Incorporation (incorporated by reference herein to Exhibit 3.1 of the Form 8-K, filed by the registrant on June 23, 2014). | |
| 3.2 | Amended and Restated Bylaws (incorporated by reference herein to Exhibit 3.2 of the Form 8-K, filed by the registrant on June 23, 2014). | |
| 4.1 | Form of Common Stock certificate (incorporated by reference herein to Exhibit 4.1 of the Form S-1, filed by the registrant on May 14, 2014). | |
| 10.1 | Fourth Amended and Restated Investors’ Rights Agreement (incorporated by reference herein to Exhibit 4.1 of the Form 10-Q, filed by the registrant on August 14, 2014). | |
| 10.2 | Form of Indemnification Agreement for directors (incorporated by reference herein to Exhibit 10.2 of the Form S-1, filed by the registrant on May 14, 2014). | |
| 10.3 | Fourth Amended Stock Incentive Plan (incorporated by reference herein to Exhibit 10.3 of the Form S-1, filed by the registrant on May 14, 2014). | |
| 10.4 | Form of Stock Option Grant Notice and Incentive Stock Option Agreement under the Fourth Amended Stock Incentive Plan (incorporated by reference herein to Exhibit 10.4 of the Form S-1, filed by the registrant on May 14, 2014). | |
| 10.5 | Form of Stock Option Grant Notice and Nonstatutory Stock Option Agreement under the Fourth Amended Stock Incentive Plan (incorporated by reference herein to Exhibit 10.5 of the Form S-1, filed by the registrant on May 14, 2014). | |
| 10.6 | ZS Pharma, Inc. 2014 Incentive Plan (incorporated by reference herein to Exhibit 10.6 of the Form 8-K, filed by the registrant on June 23, 20140). | |
| 10.7 | Form of Stock Option Grant Notice and Incentive Stock Option Agreement under the 2014 Incentive Plan (incorporated by reference herein to Exhibit 4.6 of the Form S-8, filed by the registrant on July 3, 2014). | |
| 10.8 | Form of Stock Option Grant Notice and Nonqualified Stock Option Agreement under the 2014 Incentive Plan (incorporated by reference herein to Exhibit 4.7 of the Form S-8, filed by the registrant on July 3, 2014). | |
| 10.9 | Form of Employment Agreement (incorporated by reference herein to Exhibit 10.9 of the Form S-1, filed by the registrant on May 14, 2014). | |
| 10.10 | Form of Invention Assignment and Confidentiality Agreement (incorporated by reference herein to Exhibit 10.10 of the Form S-1, filed by the registrant on May 14, 2014). | |
| 10.11 | License Restructuring Agreement, dated December 19, 2011, by and between ZS Pharma, Inc., HemoCleanse, Inc. and UOP LLC (incorporated by reference herein to Exhibit 10.11 of the Form S-1, filed by the registrant on May 14, 2014). | |
| 10.12 | License Agreement, dated December 19, 2011, by and between ZS Pharma, Inc. and UOP LLC (incorporated by reference herein to Exhibit 10.12 of the Form S-1, filed by the registrant on May 14, 2014). | |
| 10.13 | Lease Agreement, dated August 1, 2013, by and between ZS Pharma, Inc. and University of North Texas Health Science Center at Fort Worth (incorporated by reference herein to Exhibit 10.13 of the Form S-1, filed by the registrant on May 14, 2014). | |
| 10.14 | Park West Commerce Center Lease, dated February 13, 2013, by and between ZS Pharma, Inc. and PW Commerce Center LP (incorporated by reference herein to Exhibit 10.14 of the Form S-1, filed by the registrant on May 14, 2014). | |
125
Table of Contents
| Exhibit |
Exhibit Description | |
| 10.15 | Lease, dated April 26, 2013, by and between ZS Pharma, Inc. and Renault & Handley Oakmead Solar Joint Venture LP (incorporated by reference herein to Exhibit 10.15 of the Form S-1, filed by the registrant on May 14, 2014). | |
| 10.16 | Sublease, dated October 18, 2013, by and between ZS Pharma, Inc. and InB: Hauser Pharmaceutical Services, Inc. (incorporated by reference herein to Exhibit 10.16 of the Form S-1, filed by the registrant on May 14, 2014). | |
| 10.17 | Lease, dated September 1, 2014, by and between ZS Pharma, Inc. and Harvard Investment Company. | |
| 10.18 | Engagement Agreement, dated September 27, 2010, by and between ZS Pharma, Inc. and Salem Partners LLC (incorporated by reference herein to Exhibit 10.17 of the Form S-1, filed by the registrant on May 14, 2014). | |
| 10.19 | Engagement Agreement, dated June 29, 2012, by and between ZS Pharma, Inc. and Salem Partners LLC (incorporated by reference herein to Exhibit 10.18 of the Form S-1, filed by the registrant on May 14, 2014). | |
| 10.20 | Form of Amendment to Employment Agreement (incorporated by reference herein to Exhibit 10.19 of the Form S-1/A, filed by the registrant on May 30, 2014). | |
| 10.21 | Credit and Security Agreement, dated July 14, 2014, by and among MidCap Financial SBIC LP, MidCap Financial, LLC and ZS Pharma, Inc. (incorporated by reference herein to Exhibit 10.1 of the Form 8-K, filed by the registrant on July 17, 2014). | |
| 14 | Code of Business Conduct and Ethics | |
| 23.1 | Consent of independent registered public accounting firm. | |
| 31.1* | Certification of Chief Executive Officer pursuant to Rule 13a-14(a) under the Securities Exchange Act of 1934, as adopted pursuant to §302 of the Sarbanes-Oxley Act of 2002 | |
| 31.2* | Certification of Chief Financial Officer pursuant to Rule 13a-14(a) under the Securities Exchange Act of 1934, as adopted pursuant to §302 of the Sarbanes-Oxley Act of 2002 | |
| 32.1 | Certifications of Chief Executive Officer pursuant to 18 U.S.C. §1350 | |
| 32.2 | Certifications of Chief Financial Officer pursuant to 18 U.S.C. §1350 | |
| 101 | The following information from the Company’s Annual Report on Form 10-K for the fiscal year ended December 31, 2014 formatted in eXtensible Business Reporting Language: (i) Consolidated statements of operations and comprehensive loss as of December 31, 2014, December 31, 2013 and December 31, 2012; (ii) Consolidated balance sheets as of December 31, 2014, December 31, 2013 and December 31, 2012; (iii) Consolidated statements of cash flows as of December 31, 2014, December 31, 2013 and December 31, 2012; (iv) Consolidated statements of changes in equity as of December 31, 2014, December 31, 2013 and December 31, 2012 and (v) Notes to the consolidated financial statements. | |
| * | Exhibit is furnished rather than filed, and shall not be deemed incorporated by reference into any filing, in accordance with Item 601 of Regulation S-K. |
| ** | In accordance with Rule 406T of Regulation S-T, the information in these exhibits is furnished and is deemed not filed or a part of a registration statement or prospectus for purposes of Sections 11 or 12 of the Securities Act of 1933, is deemed not filed for purposes of Section 18 of the Securities Exchange Act of 1934, and otherwise is not subject to liability under these sections and shall not be incorporated by reference into any registration statement or other document filed under the Securities Act of 1933, as amended, except as expressly set forth by specific reference in such filing. |
126
Table of Contents
INDEX TO THE AUDITED FINANCIAL STATEMENTS
| Financial Statements |
||||
| F-2 | ||||
| F-3 | ||||
| F-4 | ||||
| F-5 | ||||
| Statements of Redeemable and Convertible Preferred Stock and Shareholders’ Equity (Deficit) |
F-6 | |||
| F-7 | ||||
| F-9 |
F-1
Table of Contents
Report of Independent Registered Public Accounting Firm
The Board of Directors and Shareholders of ZS Pharma, Inc.
We have audited the accompanying balance sheets of ZS Pharma, Inc. as of December 31, 2014 and 2013, and the related statements of operations, comprehensive loss, redeemable and convertible preferred stock and shareholders’ equity (deficit) and cash flows for each of the three years in the period ended December 31, 2014. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. We were not engaged to perform an audit of the Company’s internal control over financial reporting. Our audits included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of ZS Pharma at December 31, 2014 and 2013, and the results of its operations and its cash flows for each of the three years in the period ended December 31, 2014, in conformity with U.S. generally accepted accounting principles.
/s/ Ernst & Young LLP
Dallas, Texas
March 13, 2015
F-2
Table of Contents
ZS Pharma, Inc.
(In Thousands, Except Share and per Share amounts)
| December 31, | ||||||||
| 2014 | 2013 | |||||||
| Assets |
||||||||
| Current assets: |
||||||||
| Cash and cash equivalents |
$ | 47,402 | $ | 9,170 | ||||
| Short-term investments |
54,878 | — | ||||||
| Prepaid expenses |
556 | 71 | ||||||
| Other current assets |
419 | 6 | ||||||
|
|
|
|
|
|||||
| Total current assets |
103,255 | 9,247 | ||||||
| Property and equipment, net |
12,425 | 4,625 | ||||||
| Restricted cash |
301 | 150 | ||||||
| Other assets |
181 | 24 | ||||||
|
|
|
|
|
|||||
| Total assets |
$ | 116,162 | $ | 14,046 | ||||
|
|
|
|
|
|||||
| Liabilities and shareholders’ equity (deficit) |
||||||||
| Current liabilities: |
||||||||
| Accounts payable |
$ | 4,565 | $ | 1,489 | ||||
| Clinical trial accrual |
1,244 | 534 | ||||||
| Accrued liabilities |
3,025 | 2,580 | ||||||
| Current portion of capital lease obligation |
304 | 73 | ||||||
|
|
|
|
|
|||||
| Total current liabilities |
9,138 | 4,676 | ||||||
| Deferred rent |
74 | 67 | ||||||
| Lease incentive |
92 | 184 | ||||||
| Capital lease obligation |
127 | — | ||||||
| Long-term debt, net of discount |
9,959 | — | ||||||
| Series B redeemable preferred stock warrant liability |
— | 2,667 | ||||||
|
|
|
|
|
|||||
| Total liabilities |
19,390 | 7,594 | ||||||
| Commitments and contingencies (Note 12) |
||||||||
| Preferred stock whose redemption is outside the control of the issuer: |
||||||||
| Series B convertible, redeemable preferred stock, par $0.001; nil and 6,768,033 shares authorized at December 31, 2014 and 2013, respectively; nil and 2,261,506 shares issued and outstanding at December 31, 2014 and 2013, respectively; liquidation value of $8,409 at December 31, 2013 |
— | 8,387 | ||||||
| Series C convertible, redeemable preferred stock, par $0.001; nil and 17,692,308 shares authorized at December 31, 2014 and 2013, respectively; nil and 6,899,281 shares issued and outstanding at December 31, 2014 and 2013, respectively; liquidation value of $46,000 at December 31, 2013 |
— | 43,247 | ||||||
| Shareholders’ equity (deficit): |
||||||||
| Series A convertible preferred stock, par $0.001; nil and 1,494,966 shares authorized at December 31, 2014 and 2013, respectively; nil and 582,976 shares issued and outstanding at December 31, 2014 and 2013, respectively |
— | 1,196 | ||||||
| Preferred Stock, par $0.001; 5,000,000 and nil shares authorized at December 31, 2014 and 2013, respectively; nil shares issued and outstanding at December 31, 2014 and 2013 |
— | — | ||||||
| Common stock, par $0.001; 250,000,000 and 49,574,865 shares authorized December 31, 2014 and 2013, respectively; 20,898,212 and 1,628,997 shares issued and outstanding at December 31, 2014 and 2013, respectively |
21 | 2 | ||||||
| Additional paid-in capital |
211,059 | 3,854 | ||||||
| Accumulated deficit |
(114,278 | ) | (50,234 | ) | ||||
| Accumulated other comprehensive loss |
(30 | ) | — | |||||
|
|
|
|
|
|||||
| Total shareholders’ equity (deficit) |
96,772 | (45,182 | ) | |||||
|
|
|
|
|
|||||
| Total liabilities and shareholders’ equity (deficit) |
$ | 116,162 | $ | 14,046 | ||||
|
|
|
|
|
|||||
See accompanying notes.
F-3
Table of Contents
ZS Pharma, Inc.
(In Thousands, Except Share and per Share amounts)
| Year Ended December 31, | ||||||||||||
| 2014 | 2013 | 2012 | ||||||||||
| Costs and expenses: |
||||||||||||
| Research and development |
$ | 45,618 | $ | 24,762 | $ | 6,989 | ||||||
| General and administrative |
14,919 | 7,432 | 1,148 | |||||||||
|
|
|
|
|
|
|
|||||||
| 60,537 | 32,194 | 8,137 | ||||||||||
|
|
|
|
|
|
|
|||||||
| Loss from operations |
(60,537 | ) | (32,194 | ) | (8,137 | ) | ||||||
| Other (income) expense: |
||||||||||||
| Interest income |
(94 | ) | (30 | ) | (17 | ) | ||||||
| Interest expense |
530 | 9 | 2,099 | |||||||||
| Expense to mark warrants to market |
3,071 | 1,424 | 62 | |||||||||
|
|
|
|
|
|
|
|||||||
| Net loss |
$ | (64,044 | ) | $ | (33,597 | ) | $ | (10,281 | ) | |||
|
|
|
|
|
|
|
|||||||
| Preferred stock accretion |
(310 | ) | (689 | ) | (174 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Net loss attributable to common shareholders |
$ | (64,354 | ) | $ | (34,286 | ) | $ | (10,455 | ) | |||
|
|
|
|
|
|
|
|||||||
| Net loss per share attributable to common shareholders, basic and diluted |
$ | (5.47 | ) | $ | (21.84 | ) | $ | (6.74 | ) | |||
|
|
|
|
|
|
|
|||||||
| Weighted-average common shares used to compute net loss per share attributable to common shareholders, basic and diluted |
11,768,267 | 1,569,583 | 1,552,276 | |||||||||
|
|
|
|
|
|
|
|||||||
F-4
Table of Contents
ZS Pharma, Inc.
Statements of Comprehensive Loss
(In Thousands)
| Year Ended December 31, | ||||||||||||
| 2014 | 2013 | 2012 | ||||||||||
| Net loss |
$ | (64,044 | ) | $ | (33,597 | ) | $ | (10,281 | ) | |||
| Other comprehensive loss: |
||||||||||||
| Unrealized loss on available-for-sale securities, net of tax |
(30 | ) | — | — | ||||||||
|
|
|
|
|
|
|
|||||||
| Total comprehensive loss |
$ | (64,074 | ) | $ | (33,597 | ) | $ | (10,281 | ) | |||
|
|
|
|
|
|
|
|||||||
See accompanying notes.
F-5
Table of Contents
ZS Pharma, Inc.
Statements of Redeemable and Convertible Preferred Stock and Shareholders’ Equity (Deficit)
(In Thousands, Except Share amounts)
| Redeemable Preferred Stock | Preferred Stock | Additional Paid-In Capital |
Accumulated Other Comprehensive Income/(Loss) |
Accumulated Deficit |
Total | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Series B | Series C | Series D | Series A | Common Stock | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | Shares | Amount | Shares | Amount | Shares | Amount | |||||||||||||||||||||||||||||||||||||||||||||||
| Balances, December 31, 2011 |
797,569 | $ | 2,931 | — | $ | — | — | $ | — | 582,976 | $ | 1,196 | 1,534,263 | $ | 2 | $ | 1,237 | $ | — | $ | (6,356 | ) | $ | (3,921 | ) | |||||||||||||||||||||||||||||||
| Beneficial conversion feature of convertible notes |
— | — | — | — | — | — | — | — | — | — | 261 | — | — | 261 | ||||||||||||||||||||||||||||||||||||||||||
| Option exercise |
— | — | — | — | — | — | — | — | 23,398 | — | 24 | — | — | 24 | ||||||||||||||||||||||||||||||||||||||||||
| Issuance of Series B preferred stock, net (2012) |
1,463,937 | 5,447 | — | — | — | — | — | — | — | — | — | — | — | — | ||||||||||||||||||||||||||||||||||||||||||
| Issuance of Series C preferred stock, net (2012) |
— | — | 4,634,516 | 27,298 | — | — | — | — | — | — | — | — | — | — | ||||||||||||||||||||||||||||||||||||||||||
| Stock-based compensation (options) |
— | — | — | — | — | — | — | — | — | — | 359 | — | — | 359 | ||||||||||||||||||||||||||||||||||||||||||
| Stock-based compensation (warrants) |
— | — | — | — | — | — | — | — | — | — | 33 | — | — | 33 | ||||||||||||||||||||||||||||||||||||||||||
| Warrants issued for banking services |
— | — | — | — | — | — | — | — | — | — | 553 | — | — | 553 | ||||||||||||||||||||||||||||||||||||||||||
| Accretion of Series B |
— | 6 | — | — | — | — | — | — | — | — | (6 | ) | — | — | (6 | ) | ||||||||||||||||||||||||||||||||||||||||
| Accretion of Series C |
— | — | — | 167 | — | — | — | — | — | — | (167 | ) | — | — | (167 | ) | ||||||||||||||||||||||||||||||||||||||||
| Net loss |
— | — | — | — | — | — | — | — | — | — | — | — | (10,281 | ) | (10,281 | ) | ||||||||||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||||||
| Balances, December 31, 2012 |
2,261,506 | $ | 8,384 | 4,634,516 | $ | 27,465 | — | $ | — | 582,976 | $ | 1,196 | 1,557,661 | $ | 2 | $ | 2,294 | $ | — | $ | (16,637 | ) | $ | (13,145 | ) | |||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||||||
| Write off balance of beneficial conversion feature |
— | (3 | ) | — | — | — | — | — | — | — | — | — | — | — | ||||||||||||||||||||||||||||||||||||||||||
| Accretion of Series B |
— | 6 | — | — | — | — | — | — | — | — | (6 | ) | — | — | (6 | ) | ||||||||||||||||||||||||||||||||||||||||
| Issuance of Series C preferred Stock |
— | — | 2,264,765 | 15,100 | — | — | — | — | — | — | — | — | — | |||||||||||||||||||||||||||||||||||||||||||
| Accretion of Series C |
— | — | — | 682 | — | — | — | — | — | — | (682 | ) | — | — | (682 | ) | ||||||||||||||||||||||||||||||||||||||||
| Warrant exercise |
— | — | — | — | — | — | — | — | 71,336 | — | 265 | — | — | 265 | ||||||||||||||||||||||||||||||||||||||||||
| Stock-based compensation (options) |
— | — | — | — | — | — | — | — | — | — | 1,983 | — | — | 1,983 | ||||||||||||||||||||||||||||||||||||||||||
| Net loss |
— | — | — | — | — | — | — | — | — | — | — | — | (33,597 | ) | (33,597 | ) | ||||||||||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||||||
| Balances, December 31, 2013 |
2,261,506 | $ | 8,387 | 6,899,281 | $ | 43,247 | — | $ | — | 582,976 | $ | 1,196 | 1,628,997 | $ | 2 | $ | 3,854 | $ | — | $ | (50,234 | ) | $ | (45,182 | ) | |||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||||||
| Issuance of Series D preferred stock |
— | — | — | — | 1,858,012 | 24,756 | — | — | — | — | (5 | ) | — | — | (5 | ) | ||||||||||||||||||||||||||||||||||||||||
| Issuance costs for Series C preferred stock |
— | — | — | 25 | — | — | — | — | — | — | — | — | — | |||||||||||||||||||||||||||||||||||||||||||
| Accretion of Series B |
2 | (2 | ) | — | — | (2 | ) | |||||||||||||||||||||||||||||||||||||||||||||||||
| Accretion of Series C |
291 | (291 | ) | — | — | (291 | ) | |||||||||||||||||||||||||||||||||||||||||||||||||
| Accretion of Series D |
17 | (17 | ) | — | — | (17 | ) | |||||||||||||||||||||||||||||||||||||||||||||||||
| Exercise of Series B Warrants |
377,752 | 1,405 | — | — | — | — | — | — | — | — | 5,737 | — | — | 5,737 | ||||||||||||||||||||||||||||||||||||||||||
| Exercise of Common Stock Warrants |
— | — | — | — | — | — | — | — | 374,210 | — | 2,374 | — | — | 2,374 | ||||||||||||||||||||||||||||||||||||||||||
| Option Exercises |
— | — | — | — | — | — | — | — | 79,441 | — | 157 | — | — | 157 | ||||||||||||||||||||||||||||||||||||||||||
| Proceeds from sale of common stock, net of issuance costs of $10.9 million |
— | — | — | — | — | — | — | — | 6,836,111 | 7 | 112,112 | — | — | 112,119 | ||||||||||||||||||||||||||||||||||||||||||
| Elimination of fractional shares created by reverse stock split |
(27 | ) | — | (6 | ) | — | (4 | ) | — | (11 | ) | — | (26 | ) | — | — | — | — | — | |||||||||||||||||||||||||||||||||||||
| Conversion of Preferred shares to Common |
(2,639,231 | ) | (9,794 | ) | (6,899,275 | ) | (43,563 | ) | (1,858,008 | ) | (24,773 | ) | (582,965 | ) | (1,196 | ) | 11,979,479 | 12 | 79,314 | — | — | 78,130 | ||||||||||||||||||||||||||||||||||
| Stock-based compensation |
— | — | — | — | — | — | — | — | — | — | 7,668 | — | — | 7,668 | ||||||||||||||||||||||||||||||||||||||||||
| Reclass of Liability options to Equity options |
158 | — | — | 158 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Net loss |
— | — | — | — | — | — | — | — | — | — | — | — | (64,044 | ) | (64,044 | ) | ||||||||||||||||||||||||||||||||||||||||
| Other comprehensive loss |
— | — | — | — | — | — | — | — | — | — | — | (30 | ) | — | (30 | ) | ||||||||||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||||||
| Balances, December 31, 2014 |
— | — | — | — | — | — | — | — | 20,898,212 | $ | 21 | $ | 211,059 | $ | (30 | ) | $ | (114,278 | ) | $ | 96,772 | |||||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||||||
See accompanying notes.
F-6
Table of Contents
ZS Pharma, Inc.
(In Thousands)
| Years Ended December 31, | ||||||||||||
| 2014 | 2013 | 2012 | ||||||||||
| Operating activities |
||||||||||||
| Net loss |
$ | (64,044 | ) | $ | (33,597 | ) | $ | (10,281 | ) | |||
| Adjustments to reconcile net loss to net cash used in operating activities: |
||||||||||||
| Depreciation and amortization |
1,340 | 705 | 49 | |||||||||
| Amortization of lease incentive |
(92 | ) | (18 | ) | — | |||||||
| Stock-based compensation |
7,668 | 2,142 | 233 | |||||||||
| Warrant expense |
— | — | 33 | |||||||||
| Series B warrant mark-to-market expense |
3,071 | 1,424 | 76 | |||||||||
| Other |
(1 | ) | — | — | ||||||||
| Amortization of deferred debt issuance costs |
19 | — | 1,814 | |||||||||
| Amortization of debt discount |
131 | — | — | |||||||||
| Amortization of premium on marketable securities |
107 | — | — | |||||||||
| Interest expense repaid in Series B preferred stock upon conversion of convertible notes |
— | — | 278 | |||||||||
| Loss on disposal of assets and other noncash income |
— | (2 | ) | — | ||||||||
| Changes in operating assets and liabilities: |
||||||||||||
| Prepaid expenses and other assets |
(1,083 | ) | (84 | ) | (6 | ) | ||||||
| Accounts payable |
2,543 | 505 | 488 | |||||||||
| Clinical trial accrual |
839 | 67 | ||||||||||
| Accrued expenses |
474 | 2,262 | 142 | |||||||||
| Deferred rent |
7 | 67 | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Net cash used in operating activities |
(49,021 | ) | (26,529 | ) | (7,174 | ) | ||||||
| Investing activities |
||||||||||||
| Purchases of property and equipment |
(7,999 | ) | (3,771 | ) | (706 | ) | ||||||
| Purchase of short-term investments |
(54,889 | ) | — | — | ||||||||
|
|
|
|
|
|
|
|||||||
| Net cash used in investing activities |
(62,888 | ) | (3,771 | ) | (706 | ) | ||||||
F-7
Table of Contents
ZS Pharma, Inc.
Statements of Cash Flows (continued)
| Years Ended December 31, | ||||||||||||
| 2014 | 2013 | 2012 | ||||||||||
| Financing activities |
||||||||||||
| Proceeds from issuance of note payable, net of issuance costs |
$ | 9,828 | $ | — | $ | 925 | ||||||
| Payment of debt issuance costs for long-term debt |
(115 | ) | — | 24 | ||||||||
| Proceeds from exercise of stock options |
157 | — | — | |||||||||
| Proceeds from exercise of warrants |
3,780 | 265 | — | |||||||||
| Proceeds from issuance of Series C preferred stock, net of issuance costs |
25 | 15,100 | 27,757 | |||||||||
| Proceeds from issuance of Series D preferred stock, net of issuance costs |
24,751 | — | — | |||||||||
| Proceeds from issuance of common stock, net of issuance costs |
112,117 | — | — | |||||||||
| Principal payments on capital lease |
(251 | ) | (146 | ) | (73 | ) | ||||||
| Restricted cash |
(151 | ) | (150 | ) | — | |||||||
|
|
|
|
|
|
|
|||||||
| Net cash provided by financing activities |
150,141 | 15,069 | 28,633 | |||||||||
|
|
|
|
|
|
|
|||||||
| Net increase (decrease) in cash and cash equivalents |
38,232 | (15,231 | ) | 20,753 | ||||||||
| Cash and cash equivalents at beginning of period |
9,170 | 24,401 | 3,648 | |||||||||
|
|
|
|
|
|
|
|||||||
| Cash and cash equivalents at end of period |
$ | 47,402 | $ | 9,170 | $ | 24,401 | ||||||
|
|
|
|
|
|
|
|||||||
| Supplemental disclosures of cash flow information |
||||||||||||
| Cash paid for interest |
$ | 318 | $ | 12 | $ | 6 | ||||||
|
|
|
|
|
|
|
|||||||
| Cash paid for taxes |
$ | 44 | $ | 7 | $ | 21 | ||||||
|
|
|
|
|
|
|
|||||||
| Non-cash investing and financing activities: |
||||||||||||
| Non-cash additions to property and equipment |
$ | 1,142 | $ | 363 | $ | 317 | ||||||
|
|
|
|
|
|
|
|||||||
See accompanying notes.
F-8
Table of Contents
ZS Pharma, Inc. Notes to Financial Statements
December 31, 2014
1. Nature of Business and Summary of Significant Accounting Policies
Description of Organization
ZS Pharma, Inc. is a biopharmaceutical company focused on the development and commercialization of highly selective, non-absorbed drugs to treat renal, cardiovascular, liver and metabolic diseases. Our proprietary zirconium silicate technology allows us to create highly selective ion traps that can reduce toxic levels of specific electrolytes without disturbing the balance of other electrolytes. Our lead product candidate, sodium zirconium cyclosilicate, (or ZS-9), recently completed Phase III development for the treatment of hyperkalemia, a life-threatening condition in which elevated levels of potassium increase the risk of muscle dysfunction, including cardiac arrhythmias and sudden cardiac death. We expect to file a New Drug Application (NDA) for ZS-9 for the treatment of hyperkalemia within the first half of 2015.
On June 17, 2014, our registration statement on Form S-1 (File No. 333-195961) relating to our initial public offering (IPO) of our common stock was declared effective by the Securities and Exchange Commission (SEC). Our shares began trading on The NASDAQ Global Select Market on June 18, 2014. The public offering price of the shares sold in the offering was $18.00 per share. The IPO closed on June 23, 2014 and included 6,836,111 shares of common stock, which included 891,667 shares of common stock issued pursuant to the option granted to the underwriters to purchase additional shares. We received total proceeds from the offering to us, net of underwriting discounts and commissions of $8.6 million, of approximately $114.4 million. After deducting offering expenses of approximately $2.3 million, net proceeds were approximately $112.1 million. Upon the closing of the IPO, all shares of convertible preferred stock then outstanding converted into 11,979,479 shares of common stock.
Upon the effectiveness of the Amended and Restated Certificate of Incorporation of the Company on June 23, 2014, the number of shares of capital stock the Company is authorized to issue was increased to 255,000,000 shares, of which 250,000,000 shares are common stock and 5,000,000 shares are preferred stock. Both the common stock and preferred stock have a par value of $0.001 per share. There were no shares of preferred stock outstanding at September 30, 2014.
Basis of Presentation Including Liquidity
The accompanying financial statements have been prepared in accordance with U.S. generally accepted accounting principles (GAAP), which contemplate continuation of the Company as a going concern. The Company has financed its operations from inception primarily through sales of common and preferred stock, and financing under debt arrangements. The future success of the Company is dependent on its ability to develop its product candidate and ultimately upon its ability to attain profitable operations. The Company is subject to a number of risks similar to other life science companies, including, but not limited to, successful discovery and development of its drug candidates, raising additional capital, development by its competitors of new technological innovations, protection of proprietary technology, and market acceptance of the Company’s products. As a result of the aforementioned factors and the related uncertainties, there can be no assurance of the Company’s future success. As of December 31, 2014, the Company has an accumulated deficit of $114.3 million and may require substantial additional capital for research, product development and commercialization of ZS-9. The Company believes its cash, cash equivalents and short-term investments at December 31, 2014, totaling $102.3 million as well as its ability to draw $10 million on its MidCap Credit Facility (see Note 6) are sufficient to fund operations for a period of at least 12 months from the balance sheet date based on projected 2015 cash needs. The accompanying financial statements do not include any adjustments that might be necessary if the Company is unable to continue as a going concern.
F-9
Table of Contents
Reverse Stock Split
In June 2014, our board of directors and our stockholders approved an amendment to our amended and restated certificate of incorporation to effect a reverse split of shares of our common stock on a 1-for-2.56437 basis (the Reverse Stock Split). The par values and the authorized shares of the common and convertible preferred stock were not adjusted as a result of the Reverse Stock Split. All issued and outstanding common stock, convertible preferred stock, options for common stock, warrants for common and preferred stock, and per share amounts contained in the financial statements have been retroactively adjusted to reflect this Reverse Stock Split for all periods presented. The Reverse Stock Split was made effective on June 11, 2014.
Cash, Cash Equivalents and Short-Term Investments
Our cash equivalents consist of highly liquid investments with maturities of 90 days or less at the date of purchase. Our marketable debt and equity securities have been classified and accounted for as available-for-sale. We determine the appropriate classification of our investments at the time of purchase and reevaluate the designations at each balance sheet date. We invest in highly-rated securities, and our investment policy limits the amount of credit exposure to any one issuer, industry group and currency. The policy requires investments to be investment grade, with the primary objective of minimizing the potential risk of principal loss and providing liquidity of investments sufficient to meet our operating and capital spending requirements and debt repayments. No security in our portfolio shall have a maturity date greater than 12 months at the time of purchase.
We classify our marketable securities as either short-term or long-term based on each instrument’s underlying contractual maturity date and as to whether and when we intend to sell a particular security prior to its maturity date. Marketable securities with maturities greater than 90 days at the date of purchase and 12 months or less remaining at the balance sheet date will be classified as short-term. Our marketable securities are carried at fair value, with the unrealized gains and losses, net of taxes, reported in accumulated other comprehensive loss as a component of stockholders’ equity. Fair values are determined for each individual security in the investment portfolio.
Realized gains and losses on the sale of securities are determined by specific identification of each security’s cost basis. We may sell certain of our marketable securities prior to their stated maturities for strategic reasons including, but not limited to, anticipation of credit deterioration and liquidity and duration management.
We continually review our available for sale securities to determine whether a decline in fair value below the carrying value is other than temporary. When evaluating an investment for other-than-temporary impairment, we review factors such as the length of time and extent to which fair value has been below its cost basis, the financial condition of the issuer and any changes thereto, and our intent to sell, or whether it is more likely than not it will be required to sell the investment before recovery of the investment’s cost basis. Once a decline in fair value is determined to be other than temporary, an impairment charge is recorded and a new cost basis in the investment is established. If we do not intend to sell the debt security, but it is probable that we will not collect all amounts due, then only the impairment due to the credit risk would be recognized in earnings and the remaining amount of the impairment would be recognized in accumulated other comprehensive loss within stockholders’ equity.
The Company places its cash with institutions with high credit quality. However, at certain times such cash may be in excess of Federal Deposit Insurance Corporation and Securities Investor Protection Corporation insurance limits.
Restricted Cash
On November 22, 2013, the Company executed an agreement to pledge, assign, transfer, and grant a security interest to J.P. Morgan Bank to secure the payment and performance of the Company’s credit card program. The Company agreed to maintain $150,000 in a restricted cash account, given it is a pre-revenue company. In August 2014, the credit limit of the program was increased from $150,000 to $300,000 and at that time we increased the balance in the restricted cash account by $150,000.
F-10
Table of Contents
Property and Equipment
Property and equipment are recorded at cost. Equipment under a capital lease is stated at the present value of minimum lease payments at lease inception. Depreciation is computed on a straight-line basis over the estimated useful lives of the respected assets. The useful lives of property and equipment are as follows:
| Production equipment |
7 years | |||
| Office equipment |
3–8 years | |||
| Lab equipment |
3–8 years |
Impairment of Long-Lived Assets
The Company reviews long-lived assets, including property and equipment, for impairment when events or changes in business conditions indicate that their carrying value may not be recoverable. Recoverability of assets to be held and used is measured by a comparison of the carrying amount of an asset to the estimated undiscounted future cash flows expected to be generated by the asset. If the carrying amount of an asset exceeds its estimated future cash flows, an impairment charge is recognized in the amount by which the carrying amount of the asset exceeds the fair value of the asset. There have been no impairment charges related to long-lived assets from inception through December 31, 2014.
Licenses and Patents
Licenses and patent costs are expensed as incurred. Licenses are classified as research and development expenses, and patents are classified as general and administrative expenses in the statements of operations.
Research and Development Expenses
Research and development expenses are expensed as incurred. Research and development expenses represent costs associated with the ongoing development of ZS-9, the Company’s lead development compound, and include salaries, supplies, stock compensation and expenses for clinical trials and preclinical research.
Redeemable Preferred Stock Warrant Liability
Freestanding warrants for shares that are either puttable or redeemable are classified as liabilities on the balance sheet and are carried at their estimated fair value. As further discussed in Note 7, the Series B Redeemable Preferred Stock, which was able to be purchased with the warrants, was redeemable at a future date in 2017. As such, these warrants were classified as liabilities and at the end of each reporting period, changes in the estimated fair value during the period were recorded in other income (expense). Prior to June 17, 2014, the warrants were all exercised into Series B Redeemable Preferred Stock, which was converted into Common Stock upon the IPO, and the amount of warrant liability was reclassified to shareholders’ equity.
Fair Value Measurements
ASC 820, Fair Value Measurement, provides a comprehensive framework for measuring the fair value of assets and liabilities, which provides for consistency in how fair value determinations are made under various existing accounting standards that permit, or in some cases require, estimates of fair market value. Certain financial assets and liabilities are measured at fair value on a recurring basis in the financial statements. The hierarchy ranks the quality and reliability of inputs, or assumptions, used in the determination of fair value and requires financial assets and liabilities carried at fair value to be classified and disclosed in one of the following three categories:
| • | Level 1 – quoted prices in active markets for identical assets and liabilities; |
| • | Level 2 – inputs other than Level 1 quoted prices that are directly or indirectly observable; and |
| • | Level 3 – unobservable inputs that are not corroborated by market data. |
F-11
Table of Contents
Financial assets and liabilities that have recurring fair value measurements are shown below as of December 31 (in thousands):
| Fair Value Measurements at December 31, 2014 Using |
||||||||||||||||
| Quoted Prices in Active Markets for Identical Assets |
Significant Other Observable Inputs |
Significant Unobservable Inputs |
||||||||||||||
| Description |
Total | Level 1 | Level 2 | Level 3 | ||||||||||||
| Financial Assets: |
||||||||||||||||
| Corporate Bonds |
$ | 42,768 | $ | — | $ | 42,768 | $ | — | ||||||||
| Commercial Paper |
7,699 | — | 7,699 | — | ||||||||||||
| Government Securities |
4,411 | — | 4,411 | — | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total financial assets |
$ | 54,878 | $ | — | $ | 54,878 | $ | — | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Fair Value Measurements at December 31, 2013 Using |
||||||||||||||||
| Quoted Prices in Active Markets for Identical Assets |
Significant Other Observable Inputs |
Significant Unobservable Inputs |
||||||||||||||
| Description |
Total | Level 1 | Level 2 | Level 3 | ||||||||||||
| Financial liabilities: |
||||||||||||||||
| Series B Redeemable Preferred Stock warrants |
$ | 2,667 | $ | — | $ | — | $ | 2,667 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total financial liabilities |
$ | 2,667 | $ | — | $ | — | $ | 2,667 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
Level 3 liabilities comprise redeemable preferred stock warrant liabilities. These warrants were classified as liabilities because the Series B Redeemable Preferred shares that were able to be purchased by these warrants were redeemable and, therefore, were classified as temporary equity. The following table sets forth a summary of the changes in the estimated fair value of the Company’s redeemable preferred stock warrants, which were measured at fair value on a recurring basis since inception (in thousands):
| Balance as of December 31, 2011 |
$ | 968 | ||
| Issuance of redeemable preferred stock warrants |
213 | |||
| Net increase in fair value included in other (income) expense |
62 | |||
|
|
|
|||
| Balance as of December 31, 2012 |
1,243 | |||
| Net increase in fair value included in other (income) expense |
1,424 | |||
|
|
|
|||
| Balance as of December 31, 2013 |
$ | 2,667 | ||
| Net increase in fair value |
3,071 | |||
| Amount reclassified to shareholders’ equity upon exercise |
(5,738 | ) | ||
|
|
|
|||
| Balance as of December 31, 2014 |
$ | — | ||
|
|
|
Level 2 available-for-sale securities primarily consisted of: (i) bonds and notes issued by the United States government and its agencies, domestic and foreign corporations and foreign governments; and (ii) preferred securities issued by domestic and foreign corporations. The estimated fair values of these securities are determined using various valuation techniques, including a multi-dimensional relational model that incorporates standard observable inputs and assumptions such as benchmark yields, reported trades, broker/dealer quotes, issuer spreads, benchmark securities, bids/offers and other pertinent reference data.
F-12
Table of Contents
The fair value of the outstanding Series B Redeemable Preferred Stock warrants was measured using the Black-Scholes option-pricing model. Inputs used to determine estimated fair value included the estimated fair value of the warrant, as determined by the fair value of the underlying stock relative to the warrant exercise price at the valuation measurement date, volatility of the price of the underlying stock, the remaining contractual term of the warrants, risk-free interest rates, and expected dividends.
The Company believes the recorded values of cash and cash equivalents, other current assets, accounts payable, and accrued expenses approximate fair value because of the short maturity of these financial instruments.
Share-Based Compensation
The Company accounts for its equity-based compensation in accordance with ASC 718, Compensation — Stock Compensation, which requires the measurement and recognition of compensation expense for all share-based payment awards made to employees and directors to be recognized in the financial statements, based on their fair value. The value of awards ultimately expected to vest is recognized as expense over the requisite service periods in the Company’s statements of operations.
The Company measures equity-based compensation to non-employees in accordance with ASC 505-50, Equity-Based Payments to Non-Employees, and recognizes the fair value of the award over the period the services are rendered or goods are provided.
The Company has one share-based compensation plan, the 2014 Incentive Plan, as amended from time to time, which is more fully described in Note 8. Share-based compensation expense recognized in the statements of operations was $7.7 million and $2.1 million for the fiscal years ended December 31, 2014 and 2013, respectively. These amounts include $0.2 million of share-based compensation related to options issued to non-employees, which are recorded as liabilities at December 31, 2013.
The Company records the expense attributed to nonemployee services paid with share-based awards based on the estimated fair value of the awards determined using the Black-Scholes option pricing model. The measurement of stock-based compensation for nonemployees is subject to re-measurement as the options vest, and the expense is recognized over the period during which services are received.
In addition to stock options, the Company provided restricted stock to certain employees and consultants in 2008 that vested through 2010, resulting in a cumulative compensation expense of $69,976 that the Company classified as stock compensation expense during these periods.
Income Taxes
The Company accounts for income taxes using the asset and liability approach in accordance with the provision of ASC 740, Income Taxes. Under this approach, deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases and operating loss and tax credit carryforwards. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which these temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period that includes the enactment date. Valuation allowances are provided if, based upon the weight of available evidence, it is more likely than not that some or all of the deferred tax assets will not be realized. The Company continues to record a valuation allowance for the full amount of deferred tax assets, which would otherwise be recorded for tax benefits relating to the operating loss and tax credit carryforwards, as realization of such deferred tax assets cannot be determined to be more likely than not.
The Company accounts for uncertain tax positions in accordance with the provisions of ASC 740. When uncertain tax positions exist, the Company recognizes the tax benefit of tax positions to the extent that the benefit will more likely than not be realized. The determination as to whether the tax benefit will more likely than not be
F-13
Table of Contents
realized is based upon the technical merits of the tax position as well as consideration of the available facts and circumstances. As of December 31, 2014, the Company had no uncertain tax positions and no interest or penalties have been charged to the Company from inception through December 31, 2014. If incurred, the Company will classify any interest and penalties as a component of tax expense.
We have determined that we have had several changes of ownership, as defined under Internal Revenue Code Section 382 (Section 382), since the company was founded in 2008, with the most recent Section 382 change of ownership occurring on June 23, 2014. According to the provisions of Section 382, our net operating losses and research credits will be subject to certain annual limitations. As of December 31, 2014, we had federal income tax net operating loss, or NOL, carryforwards of approximately $93.7 million and federal research tax credit carryforwards of approximately $5.2 million, net of limitations pursuant to Section 382 of the Code.
Use of Estimates
The preparation of the financial statements in accordance with U.S. generally accepted accounting principles requires management to make certain estimates and judgments that affect the reported amounts of assets, liabilities, and expenses. Actual results could differ from those estimates.
The Company estimates its clinical trial expense accrual for a given period based on the number of patients enrolled at each site and the length of time each patient has been in the trial, less amounts previously billed.
Prior to its IPO, the Company utilized significant estimates and assumptions in determining the fair value of its Common Stock. Management determined the estimated fair value of the Company’s Common Stock based on a number of objective and subjective factors, including external market conditions affecting the biotechnology industry sector and the prices at which the Company sold shares of convertible preferred stock; the superior rights and preferences of securities senior to the Company’s Common Stock at the time; and the likelihood of achieving a liquidity event, such as an IPO or sale of the Company.
The Company engaged third-party valuation specialists to assist in estimating the fair value of its Common Stock and in performing valuation analyses as of the grant or other valuation dates in 2013 and prior periods. The valuation specialists utilized various valuation methodologies in accordance with the framework of the American Institute of Certified Public Accountants, or AICPA, Audit and Accounting Practice Aid Series: Valuation of Privately-Held Company Equity Securities Issued as Compensation, or the AICPA Practice Aid. The methodologies included the Option Pricing Method utilizing the Backsolve Method (a form of the market approach defined in the AICPA Practice Aid) and the Probability-Weighted Expected Return Method based upon the probability of occurrence of certain future liquidity events such as an initial public offering or sale of the Company. Each valuation methodology included estimates and assumptions that required the Company’s judgment. Significant changes to the key assumptions used in the valuations could result in different fair values of Common Stock at each valuation date.
Reclassifications
During 2014, prior-period amounts for medical affairs expenses previously presented as administrative expenses are now presented as research and development expenses in the consolidated statements of income to conform to the current year’s presentation. Medical Affairs is a subset of clinical development focused on interfacing with key physician researchers to help refine clinical trial design, review and analyze results of our clinical trials, and work with key clinical investigators to help them disseminate trial results to other physicians and patients through publications and presentations. The amount of Medical Affairs expenses reclassified for 2013 and 2012 were $254,000 and $0, respectively.
During 2014, the Company determined that the holding period for restricted cash used to secure the payment and performance of the Company’s credit card program would be longer than 12 months. Accordingly, the Company reclassified restricted cash on its balance sheet from current assets to non-current assets. The amount of restricted cash reclassified for 2013 was $150,000.
F-14
Table of Contents
Comprehensive Loss
Comprehensive loss is defined as the change in equity of a business enterprise during a period from transactions, and other events and circumstances from non-owner sources. For the year ended December 31, 2013, net loss equaled comprehensive loss. In 2014, the Company had short-term investments which included unrealized gains and losses which were reported in other comprehensive loss.
Net Loss per Common Share Attributable to Common Shareholders
Basic net loss per share attributable to common shareholders is calculated by dividing the net loss attributable to common shareholders by the weighted-average number of common shares outstanding during the period without consideration for common stock equivalents. Diluted net loss per share of common stock is the same as basic net loss per share of common stock, since the effects of potentially dilutive securities are antidilutive. The net loss per share of common stock attributable to common shareholders was computed using the two-class method required for participating securities. All series of our convertible preferred stock were considered to be participating securities as they were entitled to participate in undistributed earnings with shares of common stock. Due to our net loss, there was no impact on the earnings per share calculation in applying the two-class method since the participating securities had no legal requirement to share in any losses.
The following outstanding shares of common stock equivalents were excluded from the computations of diluted net loss per common share attributable to common shareholders for the periods presented as the effect of including such securities would be antidilutive:
| Year Ended December 30 | ||||||||||||
| 2014 | 2013 | 2012 | ||||||||||
| Convertible preferred stock – as converted to common stock |
— | 9,743,762 | 7,478,998 | |||||||||
| Warrants to purchase convertible preferred stock – as converted to common stock |
— | 377,752 | 377,752 | |||||||||
| Warrants to purchase common stock |
— | 374,210 | 445,546 | |||||||||
| Options to purchase common stock |
4,732,564 | 3,286,870 | 1,522,304 | |||||||||
|
|
|
|
|
|
|
|||||||
| 4,732,564 | 13,782,594 | 9,824,600 | ||||||||||
|
|
|
|
|
|
|
|||||||
New Accounting Pronouncements
In May 2014, FASB issued ASU 2014-09, Revenue from Contracts with Customers. ASU 2014-09 is effective for annual periods beginning after December 15, 2016 and interim periods within those annual periods. The revenue standard’s core principle is built on the contract between a vendor and a customer for the provision of goods and services. It attempts to depict the exchange of rights and obligations between the parties in the pattern of revenue recognition based on the consideration to which the vendor is entitled. To accomplish this objective, the standard requires five basic steps: (1) identify the contract with the customer, (2) identify the performance obligations in the contract, (3) determine the transaction price, (4) allocate the transaction price to the performance obligations in the contract, (5) recognize revenue when (or as) the entity satisfies a performance obligation. Management is currently evaluating the effect the adoption of this standard will have on our financial statements.
In June 2014, FASB issued ASU 2014-12, Accounting for Share-Based Payments When the Terms of an Award Provide That a Performance Target Could Be Achieved after the Requisite Service Period. The amendments in this update require that a performance target that affects vesting and that could be achieved after the requisite service period be treated as a performance condition. A reporting entity should apply existing guidance in ASC 718, Compensation—Stock Compensation, as it relates to awards with performance conditions that affect vesting to account for such awards. The amendments in this update will be effective for the Company as of January 1, 2016. Earlier adoption is permitted. Entities may apply the amendments in this update either: (1) prospectively to all awards granted or modified after the effective date; or (2) retrospectively to all awards with performance
F-15
Table of Contents
targets that are outstanding as of the beginning of the earliest annual period presented in the financial statements and to all new or modified awards thereafter. If a retrospective transition is adopted, the cumulative effect of applying this update as of the beginning of the earliest annual period presented in the financial statements should be recognized as an adjustment to the opening retained earnings balance at that date. In addition, if a retrospective transition is adopted, an entity may use hindsight in measuring and recognizing the compensation cost. Management is currently assessing the impact of this update, and believes that its adoption on January 1, 2016 will not have a material impact on our consolidated financial statements.
In August 2014, FASB issued ASU 2014-15, Presentation of Financial Statements—Going Concern. The amendments in this update require management to assess an entity’s ability to continue as a going concern by incorporating and expanding upon certain principles that are currently in U.S. auditing standards. Specifically, the amendments (1) provide a definition of the term substantial doubt, (2) require an evaluation every reporting period, including interim periods, (3) provide principles for considering the mitigating effect of management’s plans, (4) require certain disclosures when substantial doubt is alleviated as a result of consideration of management’s plans, (5) require an express statement and other disclosures when substantial doubt is not alleviated, and (6) require an assessment for a period of one year after the date that the financial statements are issued (or available to be issued). The amendments in this update are effective for us as of January 1, 2017. Early application is permitted. Management is currently assessing the impact of this update.
2. Cash, Cash Equivalents and Short-Term Investments
The following is a summary of cash, cash equivalents and short-term investments as of December 31, 2014 (in thousands):
| Amortized Cost |
Gross Unrealized Gains |
Gross Unrealized Losses |
Estimated Fair Value |
|||||||||||||
| Cash and cash equivalents: |
||||||||||||||||
| Cash and money market funds |
$ | 36,534 | $ | — | $ | — | $ | 36,534 | ||||||||
| Corporate bonds |
10,869 | — | (1 | ) | 10,868 | |||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total cash and cash equivalents |
$ | 47,403 | $ | — | $ | (1 | ) | $ | 47,402 | |||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Short-term investments: |
||||||||||||||||
| Corporate bonds |
$ | 42,810 | $ | — | $ | (42 | ) | $ | 42,768 | |||||||
| Commercial paper |
7,686 | 13 | — | 7,699 | ||||||||||||
| Government securities |
4,411 | — | — | 4,411 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total short-term investments |
$ | 54,907 | $ | 13 | $ | (42 | ) | $ | 54,878 | |||||||
|
|
|
|
|
|
|
|
|
|||||||||
For the year ended December 31, 2014, there were no realized gains or losses on the available-for-sale securities. All available-for-sale marketable securities held at December 31, 2014 had maturity dates less than one year. Unrealized losses are recognized when a decline in fair value is determined to be other-than-temporary. As of December 31, 2014, no investment was in a continuous unrealized loss position for more than one year, the unrealized losses were not due to changes in credit risk and we believe that it is more likely than not that the investments will be held to maturity or a forecasted recovery of fair value.
F-16
Table of Contents
3. Property and Equipment
Property and equipment consisted of the following (in thousands):
| December 31, | ||||||||
| 2014 | 2013 | |||||||
| Production equipment |
$ | 6,082 | $ | 2,148 | ||||
| Office equipment |
620 | 345 | ||||||
| Lab equipment |
1,963 | 1,601 | ||||||
| Leasehold improvements |
4,009 | 890 | ||||||
| Construction in progress |
1,850 | 400 | ||||||
|
|
|
|
|
|||||
| 14,524 | 5,384 | |||||||
| Less accumulated depreciation |
(2,099 | ) | (759 | ) | ||||
|
|
|
|
|
|||||
| $ | 12,425 | $ | 4,625 | |||||
|
|
|
|
|
|||||
Depreciation and amortization expense for the years ended December 31, 2014 and 2013, was $2.1 million and $0.8 million, respectively.
4. Accrued Liabilities
Accrued Liabilities consisted of the following (in thousands):
| December 31, | ||||||||
| 2014 | 2013 | |||||||
| Accrued compensation and Benefits |
$ | 509 | $ | 1,184 | ||||
| Accrued accounts payable |
967 | 902 | ||||||
| Accrued payroll tax withholding |
1,082 | — | ||||||
| Other accruals |
467 | 494 | ||||||
|
|
|
|
|
|||||
| $ | 3,025 | $ | 2,580 | |||||
|
|
|
|
|
|||||
5. Leases
In July 2012, the Company entered into a capital lease agreement with GL Filtration, Ltd. to lease production equipment, which was recorded on the Company’s balance sheet at $293,000. The term of the lease is for 24 months, with equal monthly installments in the amount of $13,185. Expense related to the amortization of this asset is included in depreciation expense.
In August 2012, the Company entered into a lease agreement with the University of North Texas Health Science Center at Fort Worth for the lease of laboratory space within the Health Science Center in Fort Worth, TX. The term of the lease was for 12 months, commencing on August 1, 2012, with the provision that either party may cancel the lease upon providing 30 days’ advance notice. In August 2013, the Company renewed the lease under substantially the same lease terms for an additional 12 months. In August 2014, the Company renewed the lease under substantially the same lease terms for an additional 6 months. This lease was terminated by the Company, effective December 31, 2014, after the Company provided appropriate notice.
In February 2013, the Company entered into a lease agreement with PW Commerce Center, LP for the lease of 25,682 square feet of space to house the Company’s corporate offices and production facility in Coppell, TX. The term of the lease is for 125 months, commencing on April 15, 2013, with an option to renew the lease for one five-year period at the then-market rate. Under the terms of the lease, rent was abated for the first five months of the lease term and the rent for the sixth month was paid at the time the lease was executed. Thereafter, rent is due and payable on the first day of each month of the lease term. Monthly rent expense under this lease will approximate $12,758 on a straight-line basis.
F-17
Table of Contents
In April 2013, the Company entered into a lease agreement with Renault & Handley Oakmead Solar Joint Venture LP for the lease of 1,172 square feet of office space in Menlo Park, CA, for executive use. The term of the lease is for 24 months, commencing on May 1, 2013, with an option to extend the lease for an additional period of 24 months at a rental rate equal to 95% of the then fair market rental value. Rent is due and payable on the first day of each month of the lease term. In October 2014, the Company vacated this space and with appropriate notice terminated the lease effective as of November 30, 2014.
In October 2013, the Company entered into a sublease agreement with InB: Hauser Pharmaceutical Services, Inc. to sublease 19,415 square feet of production and office space in Denver, CO, for production and research and development purposes. The term of the sublease is for 38.4 months, commencing on October 18, 2013, with an option to extend the lease for an additional period of 36 months at a rental rate equal to the amounts the sublessor is required to pay its landlord. Rent is due and payable on the first day of each month of the lease term. Monthly rent expense under this lease will approximate $15,399 on a straight-line basis. In addition to the sublet space, the Company also received title to certain production, laboratory, and office equipment and furniture located in the facility. The Company utilized a third-party consultant to appraise the fair value of the equipment at the time of the commencement of the lease, and the Company recorded the fair value of the assets and a corresponding lease incentive amount at the lease commencement date. The lease incentive is being amortized over the life of the sublease and the Company recognized $92,000 and $19,000 of amortization in 2014 and 2013, respectively.
In June 2014, the Company entered into a capital lease agreement with GL Filtration, Ltd. to lease production equipment, which was recorded on its balance sheet at $0.6 million. The term of the lease is for 24 months, with equal monthly installments in the amount of $27,885. Expense related to the amortization of this asset is included in depreciation expense. As of December 31, 2014, accumulated amortization related to this asset totaled $43,000.
In July 2014, the Company entered into a lease agreement with Harvard Investment Company for the lease of 6,370 square feet of space in Redwood City, CA for executive use. The term of the lease is for 36 months, commencing on September 1, 2014, with an option to extend the lease for one three-year period at the then market rate. Rent is due and payable on the first day of each month of the lease term. Monthly rent expense under this lease will approximate $25,062 on a straight-line basis.
In October 2014, the Company entered into a one month lease agreement with Regus Management Group, LLC for 9 individual offices in Grapevine, TX commencing November 1, 2014 and a five month lease agreement for 15 individual offices in Grapevine, TX commencing December 1, 2014. Rent is due and payable on the first day of each month of the lease term.
Minimum annual lease payments under noncancelable lease arrangements at December 31, 2014, are as follows (in thousands):
| Capital Leases |
Operating Leases |
|||||||
| Year ending December 31: |
||||||||
| 2015 |
$ | 335 | $ | 688 | ||||
| 2016 |
139 | 649 | ||||||
| 2017 |
— | 365 | ||||||
| 2018 |
— | 158 | ||||||
| 2019 |
— | 163 | ||||||
| Thereafter |
627 | |||||||
|
|
|
|
|
|||||
| Total minimum lease payments |
474 | $ | 2,650 | |||||
|
|
|
|||||||
| Less amount representing interest |
43 | |||||||
|
|
|
|||||||
| Present value of future minimum lease payments |
431 | |||||||
| Less current installments of obligations under capital leases |
304 | |||||||
|
|
|
|||||||
| Long-term capital lease obligations |
$ | 127 | ||||||
|
|
|
|||||||
F-18
Table of Contents
Total rent expense charged to operations under terms of these operating leases was $0.5 million and $0.2 million for the years ended December 31, 2014 and 2013, respectively.
6. Borrowings
Convertible Notes
In November 2011, the Company issued a $3.0 million mandatorily convertible note payable to Devon Park Bioventures, L.P. (Devon Park), with $2.5 million received at closing and $0.5 million received in December 2011. At the same time, the Company issued identical notes payable to 18 other investors and received an additional $1.3 million. One investor immediately converted its $175,000 note into 47,064 shares of Series B Redeemable Preferred Stock. In July 2012, the Company issued an additional identical $1.0 million note payable to Devon Park. The notes were payable at the earlier of (i) the sale of the Company or (ii) the liquidation of the Company and bore interest at the rate of 8.0% simple interest per annum, to be accrued but not payable unless and until the principal was repaid. The notes also included a requirement for mandatory conversion, upon the closing of a future equity financing of at least $15.0 million from new investors, into either Series B Redeemable Preferred Stock or the new equity security issued in the financing transaction. Upon the conversion of the notes, the noteholders were entitled to receive warrants exercisable for shares of the same security into which the notes were converted, at an amount equal to 25% of the shares received upon conversion. In October 2012, the Company consummated its Series C redeemable preferred equity offering, which triggered the mandatory conversion of the notes payable. The notes, plus accrued interest of $0.3 million, were converted into 1,463,937 shares of Series B Redeemable Preferred Stock. At the same time, the Company issued warrants exercisable for 365,986 shares of Series B Redeemable Preferred Stock.
The contingent repayment feature of the notes was determined to be an embedded derivative to be accounted for separately from the notes payable and subsequently marked to market. Given the unlikelihood of the events requiring repayment, the value of the repayment feature was determined to be inconsequential. The right to receive the warrants was accounted for as warrants issued at the date the notes were issued. The fair value of these warrants was recorded separately from the value of the notes, and the warrants are classified as a liability and are being marked to market each period (see Note 7). The warrants to be received by the note holders upon conversion of the notes into preferred stock resulted in a beneficial conversion feature associated with the notes payable of $1.0 million on the notes issued in November 2011 and $0.3 million on the additional note issued in 2012.
In connection with the convertible note transactions, the Company received gross proceeds of $5.3 million, which were offset by issuance costs for legal and banking services of $0.5 million. Warrants were also issued to bankers totaling $0.1 million for services provided relating to the convertible note transactions, which were recorded as deferred debt issuance costs, similar to the other legal and banking services paid for in cash. These costs were amortized over the period the notes were outstanding. The fair value of the warrants issued to the noteholders of $1.1 million and the beneficial conversion feature relating to the notes, including accrued interest, of $1.3 million were recorded as discounts to the notes and accreted through interest expense in 2011 and 2012. Amortization of the deferred financing costs and accretion of the discount on the notes to the date the notes were converted, including the effect of the beneficial conversion feature noted above, resulted in a charge recognized in interest expense of $1.8 million for the year ended December 31, 2012 ($0 for each of the years ended December 31, 2014 and 2013).
Credit Facility
In July 2014, we entered into a Credit and Security Agreement (Credit Agreement) with MidCap Financial SBIC, LP (MidCap) for a $20 million credit facility, secured by all of our assets, with a negative pledge against our intellectual property. The credit facility is structured in two $10 million tranches, with the first tranche drawn at closing and the second tranche to be drawn after we provide evidence to MidCap of positive primary endpoint results from our ZS004 clinical study, which has occurred, but before its expiration on October 31, 2014. Notes
F-19
Table of Contents
issued under the terms of the Credit Agreement will bear interest at the rate of 7.5% per annum, payable monthly, have principal payable in 36 monthly installments commencing on January 1, 2016 and are subject to a $50,000 fee payable to MidCap upon closing. On January 1, 2019, the maturity date of the notes, we will owe MidCap an exit fee equal to 6.5% of the amount of notes funded under the Credit Agreement. We issued a note payable to MidCap in the amount of $10 million for the first tranche on July 14, 2014. The 6.5% exit fee is being recognized as additional interest expense over the term of the note.
In September 2014, we entered into the First Amendment to Credit and Security Agreement, which extended the second tranche commitment termination date from October 31, 2014 to March 31, 2015. Also in September 2014, MidCap assigned $2.5 million of the credit facility to Silicon Valley Bank (SVB).
The estimated fair value of the MidCap debt is $10.1 million. The fair value was estimated based upon management’s use of an income approach utilizing a present value technique (Level 3 measurement).
7. Shareholders’ Equity and Redeemable Preferred Stock
Preferred Stock
Prior to its IPO, the Company had issued four classes of preferred stock: Series A, Series B, Series C and Series D (collectively, Pre-IPO Preferred Stock), each with a par value of $0.001. The shares of Series A Preferred Stock were issued from February 2009 through July 2010 at an Original Issue Price of $2.05 per share. The shares of Series B Redeemable Preferred Stock were issued from November 2010 through October 2012 at an Original Issue Price of $3.72 per share. The shares of Series C Redeemable Preferred Stock were issued from October 2012 through August 2013 at an Original Issue Price of $6.67 per share. On February 28, 2014 the Company issued 1,858,012 shares of Series D Redeemable Preferred Stock with an Original Issue Price of $13.455 per share, and total proceeds, net of offering costs, of $24.8 million.
As of December 31, 2013, the Company’s total outstanding convertible Pre-IPO Preferred Stock was as follows (in thousands, except for share and price per share amounts):
| Pre-IPO Preferred Stock |
Shares Authorized |
Shares Issued |
Price Per Share |
Total Proceeds (Net of Offering Costs) |
||||||||||||
| Series A |
1,494,966 | 582,976 | $ | 2.05 | $ | 1,196 | ||||||||||
| Series B |
6,768,033 | 2,261,506 | 3.72 | 8,371 | ||||||||||||
| Series C |
17,692,308 | 6,899,281 | 6.67 | 42,398 | ||||||||||||
|
|
|
|||||||||||||||
| $ | 51,965 | |||||||||||||||
|
|
|
|||||||||||||||
On June 23, 2014, upon the closing of the IPO and the filing of our Seventh Amended and Restated Certificate of Incorporation, each one of the outstanding shares of Preferred Stock and Redeemable Preferred Stock was converted to a share of common stock, with a total of 11,979,479 shares converted to common stock. In addition, 5,000,000 shares of new Preferred Stock, par value $0.001 per share, were authorized. The voting powers, preferences and other rights of these Preferred Shares will be determined by our Board of Directors prior to their issuance.
Warrants
During 2011 and 2012 the Company issued warrants to directors, bankers, and investors that were exercisable into Common Stock or Series B Redeemable Preferred Stock. The Company issued 57,889 warrants to Salem Partners (Salem) for banking services related to the convertible notes transaction entered into in November 2011 (see Note 6). In July 2012, the Company issued 13,447 warrants to Salem for the final tranche of the convertible debt transaction. In October 2012, the Company granted Salem Capital Partners, LLC (Salem Capital) the rights to receive 344,964 warrants for banking services related to the Series C Redeemable Preferred Stock issuance.
F-20
Table of Contents
These warrants were issued to Salem Capital in August 2013. All of the warrants issued to Salem and Salem Capital were exercisable for Common Stock and were recorded at fair value as equity on the Company’s balance sheet. For those warrants issued in connection with the notes payable transaction, the fair value of the warrants was recognized as a debt issuance cost and amortized over the period the notes payable was outstanding. For those warrants issued in connection with the Series C Redeemable Preferred Stock, the warrants were recorded as a reduction of the proceeds received as an equity issuance cost. On November 1, 2013, Salem, exercised all 71,336 of its warrants, which resulted in proceeds of $0.3 million. On May 13, 2014, Salem Capital exercised all 344,964 of its warrants, which resulted in proceeds of $2.3 million.
In January 2012, the Company issued 29,247 warrants to three non-executive members of its Board of Directors as compensation for their service to the Company as board members. These warrants are exercisable for Common Stock and were recognized as compensation expense in the Company’s statements of operations.
In November 2011, the Company issued 11,766 warrants to Chigwell Consulting, Inc. (Chigwell) as part of a Series B subscription by Chigwell. These warrants are exercisable for Series B Redeemable Preferred Stock. In October 2012, the Company issued 365,986 warrants to investors in the convertible debt transaction upon the conversion of the notes into Series B Redeemable Preferred Stock. These warrants are exercisable for Series B Redeemable Preferred Stock. Warrants exercisable for Series Redeemable B Preferred Stock are recorded as liabilities, based on the classification of Series B Redeemable Preferred Stock as temporary equity, and as such are marked to market through the income statement. The Company recorded $3.1 million and $1.4 million of other expense for mark-to-market adjustments related to these warrants for the years ended December 31, 2014 and 2013, respectively.
Below is a summary of the warrants exercisable for Common Stock that were outstanding as of December 31, 2013 and 2014:
| Date |
Number of Shares |
Exercise Price per Share |
||||||
| Outstanding as of January 1, 2013 |
445,546 | |||||||
| Exercised November 1, 2013 |
(71,336 | ) | $ | 3.72 | ||||
|
|
|
|||||||
| Outstanding as of December 31, 2013 |
374,210 | |||||||
| Exercised May 6, 2014 |
(9,749 | ) | 2.56 | |||||
| Exercised May 13, 2014 |
(344,964 | ) | 6.67 | |||||
| Exercised May 31, 2014 |
(19,497 | ) | 2.56 | |||||
|
|
|
|||||||
| Outstanding as of December 31, 2014 |
— | |||||||
|
|
|
|||||||
Below is a summary of the warrants exercisable for Series B Redeemable Preferred Stock that were outstanding as of December 31, 2013 and 2014:
| Date |
Expiration Date |
Number of Shares |
Exercise Price per Share |
|||||||
| Outstanding as of January 1, 2013 and 2014 |
377,752 | |||||||||
| Exercised May 31, 2014 |
(340,772 | ) | $ | 3.72 | ||||||
| Exercised June 5, 2014 |
(36,980 | ) | 3.72 | |||||||
|
|
|
|||||||||
| — | ||||||||||
|
|
|
|||||||||
Common Stock
At inception, the Company entered into an agreement with the founders to issue shares of restricted Common Stock, which had a two-year vesting period for services that would be provided to the Company. Under the terms of the agreement, the Company has the right, but not the obligation, to repurchase the shares of Common Stock at the original purchase price, $0.03 per share, if the holders of the restricted common shares do not meet certain service conditions. In November 2008, the Company issued 779,919 shares of restricted Common Stock to the
F-21
Table of Contents
founders. In November 2009, the Company exercised its right to repurchase 103,663 shares of unvested restricted stock from certain non-employee founders who were no longer providing services to the Company. The Company repurchased these shares at $0.03 per share.
In November 2008, the Company also issued 779,919 shares of Common Stock to HemoCleanse, Inc. in exchange for a sublicense of certain intellectual property rights held by HemoCleanse, Inc., valued at $20,000, or $0.03 per share (see Note 9 below).
As of December 31, 2014, the Company has authorized the issuance of 250,000,000 shares of Common Stock.
Voting Rights
On any matter presented to the shareholders of the Company for their action at any duly authorized meeting of shareholders of the Company, each holder of outstanding shares of Common Stock will be entitled to case the number of votes equal to the number of whole shares of Common Stock held of record by such holder. Except as provided by law or by the other provisions of the Certificate of Incorporation, holders of Common Stock shall vote together as a single class on all matters.
8. Share-Based Compensation
The Company has an employee stock option plan (the Plan), which authorizes the granting of 3,841,099 shares of Common Stock to employees and directors of the Company, as well as non-employees, and allows the holder of the option to purchase Common Stock at a stated exercise price. The number of shares authorized by the plan may be increased each January 1st following the effective date of the plan by the lesser of: a) 4% of the total number of shares outstanding (on an as-converted basis) on December 31st of the preceding year, less the aggregate number of shares available under the plan as of that date (if the result is negative, then this clause equals zero); b) a number of shares determined by the Board of Directors, or c) 1,169,878 (subject to adjustment for any subdivision or consolidation of outstanding shares of Common Stock, declaration of the dividend payable in shares of Common Stock or other stock split). Options vest according to the terms of the grant, which may be immediate or based on the passage of time or the achievement of specified milestones (which had been achieved by December 31, 2014) and have a term of up to 10 years. Unexercised stock options terminate on the expiration date of the grant. The Company recognizes the stock-based compensation expense over the requisite service period of the individual grantees, which generally equals the vesting period.
F-22
Table of Contents
The valuation of the share-based compensation awards is a significant accounting estimate that requires the use of judgment and assumptions that are likely to have a material impact on the financial statements. The fair value of option grants is determined using the Black-Scholes option-pricing model. Expected volatilities utilized in the model are based on implied volatilities from traded stocks of peer companies. Similarly, the dividend yield is based on historical experience and the estimate of future dividend yields. The risk-free interest rate is derived from the U.S. Treasury yield curve in effect at the time of grant. The expected lives of the grants are based on the average period the stock options are expected to remain outstanding. As the Company does not have sufficient historical information to develop reasonable expectations about future exercise patterns and post-vesting employment termination behavior, the expected term is calculated as the midpoint between the weighted-average vesting term and the contractual expiration periods also known as the simplified method. The Company assumed no awards would be forfeited during the vesting period, as actual forfeitures have been minimal through December 31, 2014, and the remaining weighted-average vesting term for nonvested options is for a relatively brief time (less than two years). The fair value of the option grants have been estimated, with the following weighted-average assumptions:
| Stock Options | ||||||||||||
| Year Ended December 31, 2014 |
Year Ended December 31, 2013 |
Year Ended December 31, 2012 |
||||||||||
| Expected dividend yield |
— | % | — | % | — | % | ||||||
| Risk-free interest rate |
2.13 | % | 1.87 | % | 1.84 | % | ||||||
| Volatility |
96.9 | % | 102.2 | % | 95.9 | % | ||||||
| Expected life |
6.45 years | 7 years | 5 years | |||||||||
The Company’s stock option activity and related information are summarized as follows:
| Outstanding Options | ||||||||||||
| Number of Shares |
Weighted- Average Exercise Price |
Weighted- Average Remaining Contractual Life in Years |
||||||||||
| Options outstanding at December 31, 2011 |
183,281 | $ | 1.03 | 7.75 | ||||||||
| Options granted |
1,358,520 | 2.08 | 9.39 | |||||||||
| Options exercised |
(19,498 | ) | 1.03 | 7.97 | ||||||||
|
|
|
|
|
|
|
|||||||
| Options outstanding at December 31, 2012 |
1,522,304 | 1.95 | 9.10 | |||||||||
| Options granted |
1,764,566 | 2.13 | 9.34 | |||||||||
|
|
|
|
|
|
|
|||||||
| Options outstanding at December 31, 2013 |
3,286,870 | 2.05 | 8.77 | |||||||||
| Options granted |
1,525,135 | 19.00 | 9.41 | |||||||||
| Options exercised |
(79,441 | ) | 1.98 | 7.16 | ||||||||
|
|
|
|
|
|
|
|||||||
| Options outstanding at December 31, 2014 |
4,732,564 | $ | 7.52 | 8.31 | ||||||||
|
|
|
|
|
|
|
|||||||
| Exercisable at December 31, 2014 |
1,668,413 | $ | 2.07 | 7.47 | ||||||||
|
|
|
|
|
|
|
|||||||
The aggregate intrinsic value of options exercised in the twelve months ended December 31, 2014 was $2.4 million. The aggregate intrinsic value of options exercisable as of December 31, 2014, was $65.9 million. The Company will issue new shares of Common Stock upon the exercise of vested options.
F-23
Table of Contents
The following is a rollforward of the Company’s nonvested stock options for 2014:
| Options | Weighted- Average Grant Date Fair Value |
|||||||
| Nonvested stock options at December 31, 2011 |
26,517 | $ | 0.56 | |||||
| Granted during the year |
1,358,520 | 1.62 | ||||||
| Vested during the year |
(183,962 | ) | 1.36 | |||||
| Exercised or forfeited during the year |
— | — | ||||||
|
|
|
|
|
|||||
| Nonvested stock options at December 31, 2012 |
1,201,075 | 1.64 | ||||||
| Granted during the year |
1,764,566 | 2.05 | ||||||
| Vested during the year |
(393,144 | ) | 1.62 | |||||
| Forfeited during the year |
— | — | ||||||
|
|
|
|
|
|||||
| Nonvested stock options at December 31, 2013 |
2,572,497 | 1.92 | ||||||
| Granted during the year |
1,525,135 | 15.31 | ||||||
| Vested during the year |
(1,033,481 | ) | 2.07 | |||||
| Forfeited during the year |
— | — | ||||||
|
|
|
|
|
|||||
| Nonvested stock options at December 31, 2014 |
3,064,151 | $ | 7.45 | |||||
|
|
|
|
|
|||||
The weighted-average grant date fair value of options granted in the year ended December 31, 2014, is $15.31. Vesting terms for certain employee grant agreements did not initially match the terms approved by the Company’s Board of Directors for 2,017,650 of the options reflected as granted in 2012 and 2013 in the tables above. Accordingly, these options were not considered granted for accounting purposes until a mutual understanding of the terms was obtained through amendment of the grant agreements on January 10, 2014. Certain of these options were considered to have service inception dates in 2013, which resulted in the vested portion of these options being remeasured throughout 2013. Share-based compensation expense of $1.9 million was recorded for the vested portion of these options for the year ended December 31, 2013.
At December 31, 2014, total compensation cost related to nonvested awards not yet recognized was $25.4 million, and the weighted-average period over which this amount is expected to be recognized was 2.74 years. This amount includes the fair value of the unvested portion of those awards that did not have an accounting grant date until January 10, 2014 based on fair value of those options at December 31, 2013.
9. License Agreement
In July 2008, the Company entered into a sublicense agreement with HemoCleanse, Inc. (HemoCleanse) for an exclusive worldwide license under certain patent rights held by HemoCleanse for the zirconium silicate compound that HemoCleanse had licensed from UOP, LLC (UOP). In exchange for the sublicense rights, the Company issued 779,919 shares of Common Stock to HemoCleanse, Inc. (see Note 7). The sublicense allowed the Company to research, develop, and commercialize the zirconium silicate compound within specified therapeutic fields and to practice certain licensed processes with those therapeutic fields. In December 2011, the Company entered into a license restructuring agreement with HemoCleanse and UOP that terminated the license and sublicense agreements between the parties and created a license agreement between the Company and UOP, whereby the Company received the same exclusive worldwide rights directly from UOP, and a sublicense agreement between the Company and HemoCleanse, whereby HemoCleanse received an exclusive sublicense from the Company for a specified subfield of the UOP rights. The license agreement with UOP expires when the UOP patent rights expire. Under the terms of the license agreement, the Company will pay to UOP a 5% royalty on the Company’s net sales subject to a minimum annual royalty. Total royalty expense charged to operations under terms of these licensing agreements was $100,000 for each of the years ended December 31, 2014 and 2013. As of December 31, 2014, there was an outstanding balance payable to UOP of $100,000 related to these agreements. The Company has received no royalty income under the sublicensing agreement with HemoCleanse since the inception of the sublicense agreement with HemoCleanse.
F-24
Table of Contents
10. Income Taxes
The Company did not recognize tax expense during 2014, 2013 or 2012. The reconciliation between U.S. federal income taxes at the statutory rate and the Company’s income tax expense for the year is as follows (in thousands):
| Year Ended December 31, 2014 |
Year Ended December 31, 2013 |
Year Ended December 31, 2012 |
||||||||||
| U.S. tax benefit at statutory rate |
$ | (21,785 | ) | $ | (11,423 | ) | $ | (3,495 | ) | |||
| U.S. state income tax |
(303 | ) | (383 | ) | (105 | ) | ||||||
| State effective rate changes |
333 | — | — | |||||||||
| Permanent differences |
1,044 | 541 | 748 | |||||||||
| Deferred tax valuation allowances |
22,097 | 12,758 | 2,848 | |||||||||
| Research and development credit |
(1,885 | ) | (1,073 | ) | — | |||||||
| Other |
499 | (420 | ) | 4 | ||||||||
|
|
|
|
|
|
|
|||||||
| Income tax expense |
$ | — | $ | — | $ | — | ||||||
|
|
|
|
|
|
|
|||||||
Net deferred tax assets and liabilities consisted of the following at December 31 (in thousands):
| 2014 | 2013 | 2012 | ||||||||||
| Deferred income tax assets: |
||||||||||||
| Net operating loss carryforwards |
$ | 31,224 | $ | 14,325 | $ | 4,519 | ||||||
| Research and development credit carryforwards |
5,196 | 2,403 | 114 | |||||||||
| Stock compensation |
3,425 | 860 | 160 | |||||||||
| Other |
190 | 52 | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Total deferred tax assets |
40,035 | 17,640 | 4,793 | |||||||||
| Valuation allowance |
(39,554 | ) | (17,457 | ) | (4,718 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Total deferred tax assets, net |
481 | 183 | 75 | |||||||||
| Deferred income tax liabilities: |
||||||||||||
| Depreciation and amortization |
(481 | ) | (183 | ) | (75 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Total deferred tax assets, net |
$ | — | — | — | ||||||||
|
|
|
|
|
|
|
|||||||
Income taxes are computed using the asset and liability method and current income taxes are recorded based on amounts refundable or payable in the current year. Deferred income taxes are recorded based on the estimated future tax effects of loss carryforwards and temporary differences between financial statement carrying amounts of existing assets and liabilities and their respective tax basis. Deferred tax assets and liabilities are measured using enacted tax rates that are expected to apply to taxable income in the years in which we expect those carryforwards and temporary differences to be recovered or settled. Management regularly evaluates the future realization of deferred tax assets and provides a valuation allowance, if considered necessary, based on such evaluation. As part of the evaluation, management has evaluated taxable income in carryback years, future reversals of taxable temporary differences, feasible tax planning strategies, and future expectations of income. Based upon this evaluation, as of December 31, 2014, the company has recorded a valuation allowance of $39.6 million to reduce the Company’s net deferred tax assets to the amount that is more likely than not to be realized. In 2014, the total valuation allowance increased by $22.1 million primarily due to the increase in net operating loss and federal credit carryforwards which the Company does not believe are more likely than not to be utilized.
As of December 31, 2014, the Company has federal and state income tax net operating loss (NOL) carryforwards, net of the 382 limitation discussed below, of approximately $93.7 million and $0.5 million, respectively, and federal research tax credit carryforwards, net of the 382 limitation discussed below, of approximately $5.2 million. Additionally, the Company has a Federal NOL carryforward of $3.3 million related to the excess tax benefits associated with stock-based compensation and stock option exercises. The benefit of this NOL will be recognized as an increase to the additional paid-in capital at the point when such NOL provides cash benefit to the Company.
F-25
Table of Contents
The NOL carryforwards and research tax credit carryforwards begin to expire in 2029. The Company has experienced multiple changes in ownership as defined under Internal Revenue Code Section 382, prior to the year ending December 31, 2014. The ownership changes have subjected our net operating loss carryforwards and federal research tax credit carryforwards to an annual limitation. In general, the annual use limitation equals the aggregate value of our stock at the time of the ownership change multiplied by a specified tax-exempt interest rate. We estimate the ownership changes will result in $1.5 million of net operating loss carryforwards expiring unused and $0.1 million of federal research tax credit carryforwards expiring unused. Such unusable net operating loss carryforwards and federal tax credit carryforwards are therefore not included in the amount disclosed in the paragraph above.
The Company had no accrued income taxes payable at December 31, 2014. The federal and state income tax returns of the Company for 2014 are subject to examination by the taxing authority, generally for a period of three years for federal and four years for states from the date the tax returns were filed.
The Company recognizes uncertain tax positions when it is more-likely-than-not, based on the technical merits, that the position will not be sustained upon examination. At December 31, 2014 and 2013, the Company had no uncertain tax positions.
11. Defined Contribution Plan
In March, 2014, we began to sponsor a 401(k) retirement plan, in which substantially all of our full-time employees are eligible to participate. Eligible participants may contribute a percentage of their annual compensation to this plan, subject to statutory limitations. Irrespective of the amount of any participant contributions in a plan year, we will contribute an amount equal to 3% of each participant’s annual salary into their account in the plan each plan year, subject to federal limits per employee for safe harbor plans such as the plan we have offered. For the year ended December 31, 2014, we made safe harbor contributions into the plan totaling $0.2 million.
12. Commitments and Contingencies
Commitments
We have various manufacturing, clinical, research and other contracts with vendors in the conduct of the normal course of business. As of December 31, 2014, we had purchase commitments for approximately $1.5 million with contract manufacturers to produce clinical materials and $1.3 million with equipment vendors for the manufacture of equipment to produce our product. All other significant contracts as of December 31, 2014 were terminable, with varying provisions regarding termination. If a contract with a specific vendor were to be terminated, we would only be obligated for the product or services that we had received at the time the termination became effective.
Contingencies
While there are no legal proceedings we are aware of, we may become party to various claims and complaints arising from the ordinary course of business. We do not believe that any ultimate liability resulting from any such claims will have a material adverse effect on our results of operations, financial position or liquidity.
13. Subsequent Events
Management has evaluated subsequent events through March 13, 2015, the date that the financial statements were available to be issued.
F-26
Table of Contents
14. Selected Quarterly Financial Data (unaudited)
The following table contains quarterly financial information for 2014 and 2013. The Company believes that the following information reflects all normal recurring adjustments necessary for a fair statement of the information for the periods presented. The operating results for any quarter are not necessarily indicative of results for any future period.
| Quarter Ended (In Thousands, Except Share and per Share amounts) |
||||||||||||||||||||||||||||||||
| December 31, 2014 |
September 30, 2014 |
June 30, 2014 |
March 31, 2014 |
December 31, 2013 |
September 30, 2013 |
June 30, 2013 |
March 31, 2013 |
|||||||||||||||||||||||||
| Costs and expenses: |
||||||||||||||||||||||||||||||||
| Research and development(1) |
$ | 14,574 | $ | 12,541 | $ | 11,649 | $ | 6,854 | $ | 6,880 | $ | 7,138 | $ | 6,335 | $ | 4,409 | ||||||||||||||||
| General and administrative(2) |
5,535 | 4,081 | 2,881 | 2,422 | 3,521 | 2,224 | 972 | 715 | ||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Total costs and expenses |
20,109 | 16,622 | 14,530 | 9,276 | 10,401 | 9,362 | 7,307 | 5,124 | ||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Loss from operations |
(20,109 | ) | (16,622 | ) | (14,530 | ) | (9,276 | ) | (10,401 | ) | (9,362 | ) | (7,307 | ) | (5,124 | ) | ||||||||||||||||
| Other (income) expense: |
||||||||||||||||||||||||||||||||
| Interest/other income(3) |
(43 | ) | (31 | ) | (11 | ) | (9 | ) | (5 | ) | (5 | ) | (8 | ) | (12 | ) | ||||||||||||||||
| Interest expense(4) |
249 | 272 | 6 | 3 | (4 | ) | 3 | 5 | 5 | |||||||||||||||||||||||
| Expense to mark warrants to market |
— | — | 1,774 | 1,297 | 1,272 | 32 | 11 | 109 | ||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Net loss |
$ | (20,315 | ) | $ | (16,863 | ) | $ | (16,299 | ) | $ | (10,567 | ) | $ | (11,664 | ) | $ | (9,392 | ) | $ | (7,315 | ) | $ | (5,226 | ) | ||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Preferred Stock Accretion |
— | — | (129 | ) | (181 | ) | (174 | ) | (173 | ) | (172 | ) | (170 | ) | ||||||||||||||||||
| Net loss attributable to common shareholders |
$ | (20,315 | ) | $ | (16,863 | ) | $ | (16,428 | ) | $ | (10,748 | ) | $ | (11,838 | ) | $ | (9,565 | ) | $ | (7,487 | ) | $ | (5,396 | ) | ||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Net loss per share attributable to common shareholders, basic and diluted |
$ | (0.98 | ) | $ | (0.81 | ) | $ | (4.72 | ) | $ | (6.60 | ) | $ | (7.38 | ) | $ | (6.14 | ) | $ | (4.81 | ) | $ | (3.46 | ) | ||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Weighted-average common shares used to compute net loss per share attributable to common shareholders, basic and diluted |
20,832,475 | 20,818,794 | 3,482,340 | 1,628,976 | 1,604,490 | 1,557,643 | 1,557,643 | 1,557,643 | ||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| (1) | Research and development expenses in the quarters ending December 31, 2013, March 31, 2014, June 30, 2014 and September 30, 2014 include medicalaffairs expenses previously reported as administrative expenses in the amounts of $254, $1,595, $1,673 and $1,618, respectively. |
| (2) | Administrative expenses in the quarter ended December 31, 2013 include $650 of additional legal and professional fees, incurred primarily in support of our product development, $219 of additional consulting fees in support of medical communications and $174 of additional non-cash option expense to mark to market certain stock options recorded as liabilities. |
| (3) | Interest income in the quarters ended September 30, 2014 and December 31, 2014 include interest income on invested surplus cash. |
| (4) | Interest expense in the quarters ended September 30, 2014 and December 31, 2014 include interest expense related to the MidCap Credit Facility initiated in July 2014 (see note 6 above). |
F-27
