Attached files
| file | filename |
|---|---|
| EX-23.1 - EXHIBIT 23.1 - InspireMD, Inc. | v401329_ex23-1.htm |
| EX-31.2 - EXHIBIT 31.2 - InspireMD, Inc. | v401329_ex31-2.htm |
| EX-32.2 - EXHIBIT 32.2 - InspireMD, Inc. | v401329_ex32-2.htm |
| EX-32.1 - EXHIBIT 32.1 - InspireMD, Inc. | v401329_ex32-1.htm |
| EX-31.1 - EXHIBIT 31.1 - InspireMD, Inc. | v401329_ex31-1.htm |
| EX-10.73 - EXHIBIT 10.73 - InspireMD, Inc. | v401329_ex10-73.htm |
| EXCEL - IDEA: XBRL DOCUMENT - InspireMD, Inc. | Financial_Report.xls |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON D.C. 20549
FORM 10-K
(Mark One)
| x | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2014
OR
| o | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
COMMISSION FILE NUMBER: 001-35731
InspireMD, Inc.
(Exact name of registrant as specified in its charter)
| Delaware | 26-2123838 |
| (State or other jurisdiction of | (I.R.S. Employer Identification Number) |
| incorporation or organization) | |
| 321 Columbus Avenue | |
| Boston, Massachusetts | 02116 |
| (Address of principal executive offices) | (Zip Code) |
Registrant’s telephone number, including area code: (857) 453-6553
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class | Name of each exchange on which registered |
| Common Stock, $0.0001 par value | NYSE MKT |
Securities registered pursuant to Section 12(g) of the Act: none
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes o No x
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes o No x
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes x No o
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Website, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes x No o
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. o
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer o | Accelerated filer x |
| Non-accelerated filer o | Smaller reporting company o |
| (Do not check if a smaller reporting company) |
Indicate by check mark whether the registrant is a shell company (as defined by Rule 12b-2 of the Act). Yes o No x
The aggregate market value of the voting and non-voting stock held by non-affiliates of the registrant as of June 30, 2014, based on the price at which the common equity was last sold on the NYSE MKT on such date, was approximately $90,786,938. For purposes of this computation only, all officers, directors and 10% or greater stockholders of the registrant are deemed to be affiliates.
Indicate the number of shares outstanding of each of the registrant’s classes of common stock as of the latest practicable date.
| Class | Outstanding at March 11, 2015 | |
| Common Stock, $0.0001 par value | 78,152,015 |
Documents incorporated by reference:
None
TABLE OF CONTENTS
| 2 |
In this Annual Report on Form 10-K, unless the context requires otherwise, the terms “we,” “our,” “us,” or “the Company” refer to InspireMD, Inc., a Delaware corporation, and its subsidiaries, including InspireMD Ltd., taken as a whole.
| Item 1. | Business. |
Overview
We are a medical device company focusing on the development and commercialization of our proprietary MicroNet stent platform technology for the treatment of complex coronary and vascular disease. A stent is an expandable “scaffold-like” device, usually constructed of a metallic material, that is inserted into an artery to expand the inside passage and improve blood flow. Our MicroNet, a micron mesh sleeve, is wrapped over a stent to provide embolic protection in stenting procedures. Our initial MGuard coronary products (MGuard and MGuard Prime Embolic Protection Stent (EPS)) are marketed for use in patients with acute coronary syndromes, notably acute myocardial infarction (heart attack) and saphenous vein graft coronary interventions (bypass surgery).
MGuard Sleeve – Microscopic View
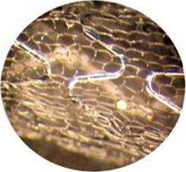
We market and sell our bare-metal MGuard products in the European Union, Southeast Asia, India, Latin America and Israel. In October 2007, our first generation MGuard stent combining the MicroNet with a stainless steel stent received CE mark approval for the treatment of coronary artery disease in the European Union. We subsequently replaced the stainless steel stent with a more advanced cobalt-chromium based stent. Our cobalt-chromium based MGuard coronary product is referred to as the MGuard Prime EPS and, unless otherwise indicated, in this Annual Report on Form 10-K, references to bare-metal MGuard coronary stents are to both our initial stainless steel based MGuard coronary product and our more current cobalt-chromium based MGuard Prime EPS. MGuard Prime EPS received CE mark approval in the European Union in October 2010 for improving luminal diameter and providing embolic protection.
In October 2014, we launched a limited market release of our second product CGuard carotid embolic prevention system (“EPS”) in certain European countries. CGuard EPS combines MicroNet and a self-expandable nitinol stent in a single device to treat carotid artery disease. CGuard EPS received CE mark approval in the European Union in March 2013. In January 2015, we received CE mark approval for our CGuard RX rapid exchange delivery system for its Micronet covered embolic prevention system. The new RX delivery system will enable clinicians to place the CGuard technology using an easy-to-use, and familiar, delivery system. The CGuard MicroNet mesh covered carotid stent remains unchanged.
We are also developing a pipeline of other products and additional applications by leveraging our MicroNet technology. Among the products in development is a coronary stent product incorporating drug-eluting (drug-coated) stents with MicroNet, for which in vivo pre-clinical testing began in the fourth calendar quarter of 2014 and will continue through 2015. We also intend to explore possible new applications of our technology in other vascular procedures and interventional medical specialties, specifically peripheral and neurovascular procedures.
| 3 |
Presently, none of our products may be sold or marketed in the U.S.
We make available, free of charge, our Annual Report on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K and amendments to these reports on our website at www.inspire-md.com as soon as reasonably practicable after those reports and other information is electronically filed with, or furnished to, the SEC.
Voluntary Field Action
On April 30, 2014, we initiated a voluntary field corrective action of our MGuard Prime EPS to address the issue of stent retention following reports of MGuard Prime EPS stent dislodgements. These reported dislodgements primarily occurred during the preparation of the MGuard Prime EPS, upon removal of the protective sleeve or during withdrawal of the MGuard Prime EPS into the guide catheter. To address this problem, we subsequently modified our manufacturing process of MGuard Prime EPS stents in order to improve stent retention and performance. We received approvals from the European regulatory agency and the U.S. Food and Drug Administration to resume the manufacturing of the MGuard Prime EPS stent with a modified stent securement process on June 18, 2014 and October 23, 2014, respectively. We also received approval to modify and re-deploy existing MGuard Prime EPS stents that had been returned to us by clinical and commercial sites worldwide. All returned inventory has been modified and returned to direct hospital customers and the majority of our distributor partners, who have begun shipping modified product back into hospital accounts. We began shipping products to new customers in our direct markets in Western Europe in late September 2014 and intend to complete the full re-launch of MGuard Prime EPS in 2015.
Business Segment and Geographic Areas
Prior to October 2014, all revenue was derived from sales of our MGuard bare-metal stent. For the twelve months ended December 31, 2014, 99% of our revenue was derived from sales of this product. For financial information about our one operating and reportable segment and geographic areas, refer to “Part II—Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations” and “Part II—Financial Statements and Supplementary Data—Note 12 - Entity Wide Disclosures.”
Our Industry
Coronary
Physicians and patients may select from among a variety of treatments to address coronary artery disease, including pharmaceutical therapy, balloon angioplasty, stenting with bare metal or drug-eluting stents, and coronary artery bypass graft procedures, with the selection often depending upon the stage of the disease.
The global market value of coronary products is estimated at $5.9 billion, of which $4.2 billion is for stable angina and $1.7 billion is for acute myocardial infarctions according to Health Research International (June 2011). According to the 2014 MEDTECH OUTLOOK produced in December 2013 by BMO Capital Markets (“MEDTECH OUTLOOK”), revenues from the global coronary stent market are predicted to slightly decline, although in volume of stents the market is predicted to continue to grow. We believe the growth in volume is due to the appeal for less invasive percutaneous coronary intervention (“PCI”) procedures and advances in technology coupled with the increase in the elderly population, obesity rates and advances in technology.
Coronary artery disease is one of the leading causes of death worldwide. According to Fact Sheet No. 310/updated May 2014 of the World Health Organization, approximately 7.4 million people worldwide died of ischaemic heart disease in 2012. The treatment of coronary artery disease includes alternative treatment methodologies, that is, coronary artery bypass grafting or angioplasty (a therapeutic procedure to treat narrowed coronary arteries of the heart found in patients with heart disease) with or without stenting. According to the MEDTECH OUTLOOK, the PCI procedures involving stents used to treat coronary artery diseases had an estimated 68% market penetration rate in 2013.
Carotid
Carotid arteries are located on each side of the neck and provide the primary blood supply to the brain. Carotid artery disease, also called carotid artery stenosis, is a type of atherosclerosis (hardening of the arteries) that is one of the major risk factors for ischemic stroke. In carotid artery disease, plaque accumulates in the artery walls, narrowing the artery and disrupting the blood supply to the brain. This disruption in blood supply, together with plaque debris breaking off the artery walls and traveling to the brain, are the primary causes of stroke. According to Fact Sheet No. 310, approximately 6.7 million people worldwide died of stroke in 2012.
The global market value of carotid stents is approximately $500 million, approximately $300 million of which consists of the U.S. market and approximately $200 million of which consists of the rest of the world. Carotid artery stenting is a minimally invasive treatment option for carotid artery disease and an alternative to carotid endarterectomy, where a surgeon accesses the blocked carotid artery though an incision in the neck, and then surgically removes the plaque. Endovascular techniques using stents and EPS protect against plaque and debris traveling downstream, blocking off the vessel and disrupting blood flow. The use of a stent with an embolic protection system avoids open surgery and we believe will increase the number of patients being treated.
| 4 |
Peripheral
Peripheral vascular diseases (PVD) are caused by the formation of atherosclerotic plaques in arteries, which carry blood to organs, limbs and head. It is also known as peripheral artery occlusive disease (PAOD) or peripheral artery disease (PAD). It comprises diseases pertaining to both peripheral veins and peripheral arteries, affecting the peripheral and cardiac circulation in the body. PVD includes diseases outside of the heart and brain, but most times refers to the leg and foot.
The overall peripheral vascular devices market consists of nine different product segments: peripheral vascular stents, chronic total occlusion devices, peripheral transluminal angioplasty balloon catheters, atherectomy devices, PTA guidewires, aortic stents, embolic protection devices, synthetic surgical grafts and inferior vena cava filters (source: Grand View Research 2014).Treatment modalities and methods have considerably improved during the last several years, and this trend is expected to continue (source: Global Data 2011). Stents and balloons hold the majority of the share in the peripheral vascular devices market. Peripheral stents are more often used in combination with balloon angioplasty to open the veins, so that blood can flow through the blocked veins in the body.
The growing prevalence of PVD is expected to cause increased demand for treatment options. The expansion of the elderly population is contributing to increasing incidence rates of PVD. The percentage of the global population above the age of 50 is expected to reach 17% by 2030. As the risk of developing PVD increases with age, a growing elderly population translates into a growing incidence of PVD (source: Global Data 2011). The growing global geriatric population base also triggers increasing demand for minimally invasive endovascular procedures on account of their shorter recovery time, lesser scaring and lesser chances of post surgery infections. In addition, a growing prevalence of disease causing lifestyle factors and eating habits such as high consumption of alcohols and tobacco products is expected to boost peripheral vascular devices market demand by triggering the incidence rates of cardiac arrest, blood clotting and other vascular diseases (source: Grand View Research 2014).
Our Products
Below is a summary of our current products and products under development, and their intended applications.
MicroNet
MicroNet is our proprietary circular knitted mesh which wraps around a stent to protect patients from plaque debris flowing downstream upon deployment. MicroNet is made of a single fiber from a biocompatible polymer widely used in medical implantations. The size, or aperture, of the current MicroNet ‘pore’ is only 150-180 microns in order to maximize protection against the potentially dangerous plaque and thrombus.
The MGuard stent is an embolic protection device based on a protective sleeve, which is constructed out of an ultra-thin polymer mesh and wrapped around the stent. The protective sleeve is comprised of a micron level fiber-knitted mesh, engineered in an optimal geometric configuration and designed for utmost flexibility while retaining strength characteristics of the fiber material (see illustration below). The sleeve expands seamlessly when the stent is deployed, without affecting the structural integrity of the stent, and can be securely mounted on any type of stent.
MGuard Deployed in Artery

The protective sleeve is designed to provide several clinical benefits:
| · | the mesh diffuses the pressure and the impact of deployment exerted by the stent on the arterial wall and reduces the injury to the vessel; |
| · | the protective sleeve reduces plaque dislodgement and blocks debris from entering the bloodstream during and post procedure (called embolic showers); |
| · | in future products, when drug coated, the mesh is expected to deliver better coverage and uniform drug distribution on the arterial wall and therefore potentially reduce the dosage of the active ingredient when compared to approved drug-eluting stents on the market; and |
| · | the protective sleeve maintains the standards of a conventional stent and therefore should require little to no additional training by physicians. |
| 5 |
MGuard Products– Coronary Applications
Our MGuard Coronary with a bio-stable mesh and our planned MGuard Coronary with a drug-eluting mesh are aimed at the treatment of coronary arterial disease.
Bare-Metal Stent MGuard Products. Our MGuard stent and MGuard Prime EPS are comprised of MicroNet wrapped around a bare-metal stent. In comparison to a conventional bare-metal stent, we believe our MGuard coronary products with MicroNet mesh provide protection from dangerous embolic showers in patients experiencing ST-segment elevation myocardial infarction (the most severe form of a heart attack, referred to as “STEMI”), the most severe type of heart attack. Standard stents were not engineered for heart attack patients. Rather, they were designed for treating stable angina patients whose occlusion is different from that of an occlusion in a heart attack patient. In acute heart attack patients, the plaque or thrombus is unstable and often breaks up as the stent is implanted causing downstream blockages in a significant portion of heart attack patients. Our MGuard Prime EPS is integrated with a precisely engineered micro net mesh that is designed to prevent the unstable arterial plaque and thrombus that caused the heart attack blockage from breaking off.
We have studied over 1,200 patients who were treated with our MGuard products. In the second calendar quarter of 2011, we conducted the MGuard for Acute ST Elevation Reperfusion trial, which we refer to as our “MASTER I trial.” The Master I trial was a prospective, randomized study in Europe, South America and Israel to compare the MGuard stent with commercially-approved bare-metal and drug-eluting stents in achieving superior myocardial reperfusion (the restoration of blood flow) in primary angioplasty for the treatment of acute STEMI. The MASTER I trial enrolled 433 subjects, 50% of whom were treated with an MGuard stent and 50% of whom were treated with a commercially-approved bare-metal or drug-eluting stent. The MASTER I trial demonstrated that among patients with acute STEMI undergoing emergency PCI, or angioplasty, use of the MGuard stent resulted in superior rates of epicardial coronary flow, or blood flow within the vessels that run along the outer surface of the heart, and complete ST-segment resolution, or restoration of blood flow to the heart muscle after a heart attack, compared to commercially-approved bare-metal or drug-eluting stents. Although each of MGuard stents and commercially-approved bare-metal or drug-eluting stents showed statistically similar rates of major adverse cardiac events 30 days following the procedure, the mortality rate was 0% for the subjects treated with the MGuard stent as opposed to 1.8% for the subjects treated with commercially-approved bare-metal or drug-eluting stents 30 days following the procedure.
In connection with our efforts to seek approval of our MGuard Prime EPS by the U.S. Food and Drug Administration, we filed an IDE application with the U.S. Food and Drug Administration during the summer of 2012 in order to conduct a pivotal trial. On April 19, 2013, we received an approval with conditions from the U.S. Food and Drug Administration for our IDE application, which allowed us to initiate enrollment in the trial. This trial, which we refer to as the “MASTER II trial,” was expected to be a multi-center, randomized study, consisting of up to 1,114 patients suffering from STEMI throughout 35 sites in the U.S. and an additional 35 sites in Europe. The MASTER II trial was designed to have two co-primary end points: superiority in complete ST-resolution and non-inferiority in death and target vessel myocardial infarction. In addition, a sub-study was planned to assess the effect of MGuard Prime EPS on infarct size, as measured by magnetic resonance imaging, and an additional sub-study was to be conducted to assess the late lumen loss, measured at 13 months. We successfully enrolled 310 patients in the trial prior to suspending enrollment in April 2014 due to manufacturing process changes in connection with the voluntary field correction action, pending a review by the U.S. Food and Drug Administration of the manufacturing improvements to the MGuard Prime EPS. The U.S. Food and Drug Administration approved the re-commencement of the MASTER II trial in October 2014. However, we elected to discontinue enrollment in the MASTER II trial in its current form, in light of current market conditions moving toward the use of drug-eluting stents over bare-metal stents, and MASTER II will no longer be a U.S. Food and Drug registration trial. Notwithstanding the discontinuance of the enrollment for the MASTER II trial, the preliminary analysis of the 30-day end point data from the 310 patients enrolled prior to the suspension of the enrollment is encouraging. We intend to continue to follow these 310 MASTER II trial patients for one year from time of enrollment. The 30 day results from the MASTER II Trial were presented at the ICI meeting in Tel-Aviv, Israel in 2014. There were no significant differences in procedural and clinical endpoints, most likely due to the small group size which is too small to find any statistical differences.
A 30 day pooled analysis of MASTER I and MASTER II trial results was presented at the ICI meeting in Tel-Aviv, Israel in 2014 and the results clearly showed that MGuard demonstrated a significant reduction in all-cause and cardiac mortality at 30 days (MGuard 0.3% vs. Control 1.9%; p=0.04) compared to conventional bare metal or drug eluting stents.
The 30 day and six month results from the International MGuard Prime Observational Study (“iMos”) were also presented at the ICI meeting in December 2014. The iMOS registry seeks to evaluate the ‘real world’ clinical performance of the MGuard Prime EPS in STEMI patients undergoing percutaneous coronary intervention. The 30 day and six month results indicate that MGuard Prime EPS is feasible, based on 100% device and lesion success rates, and safe, based on no deaths at 30 days follow-up, two deaths at six month follow-up and very low MACE rates at 30 days and six month follow-up. The use of the MGuard Prime EPS seemed also highly effective in achieving myocardial reperfusion, as suggested by the high rates of TIMI-3 flow (91.8%) and partial or complete STR (87%).
Recently we began enrollment in a multi-center, single-arm post-market registry of 500 patients with STEMI to collect post-CE mark trial clinical data on patients treated with MGuard Prime EPS from 50 planned sites across Europe, which we refer to as our “eMASTER study.” We plan to evaluate the safety and efficacy of the MGuard Prime EPS in the treatment of de novo stenotic lesions in coronary arteries in patients undergoing PCI due to STEMI, based on patients with complete ST-segment resolution and rates of all-cause death or myocardial infarction at 30 days.
| 6 |
Drug-Eluting Stent (or “DES”) MicroNet Product. We recently entered the second phase of development work for our MGuard DES, which is expected to incorporate our MicroNet with a drug-eluting stent, through a strategic partnership with a third party drug-eluting stent candidate manufacturer. We intend to develop a total of two strategic partnerships with manufactures of U.S. Food and Drug Administration-approved or CE-marked drug-eluting stents and bring two viable drug-eluting stent products with our MicroNet mesh into the in vivo pre-clinical testing phase which, if successful, should lead to submission for CE registration of a DES-MicroNet platform. The initial testing of drug-eluting stent candidates for technical feasibility testing with our MicroNet mesh was 100% successful. We believe that a drug-eluting stent with MicroNet has the potential to improve certain performance metrics over the MGuard Prime and attract a broader portion of the cardiologists in the worldwide stent market who are more accustomed to using drug-eluting stents.
CGuard – Carotid Applications
In October 2014, we initiated a limited market release of CGuard EPS, which is comprised of our MicroNet mesh and a self-expandable stent (a stent that expands without balloon dilation pressure or need of an inflation balloon) for use in carotid artery applications. We launched CGuard EPS in Germany, Poland, Switzerland, Belgium, Italy and Spain. MicroNet is placed over and attached to an open cell nitinol metal stent platform which is designed to trap debris and emboli that can dislodge from the diseased carotid artery and potentially to travel to the brain and cause a stroke. This danger is one of the greatest limitations of carotid artery stenting with conventional carotid stents and stenting methods. The CGuard technology is a highly flexible stent system that easily conforms to the carotid anatomy.
In September 2014, we reported the results of the CARENET trial at the Transcatheter Cardiovascular Therapeutics (TCT) meeting in Washington D.C. In the CARENET trial, the CGuard system demonstrated better results over historical data using conventional commercially available carotid stents.
We believe that our CGuard EPS design will provide substantial advantages over existing therapies in treating carotid artery stenosis, such as conventional carotid stenting and surgical endarterectomy, given the superior embolic protection characteristics provided by the MicroNet. We believe the MicroNet will provide acute embolic protection at the time of the procedure, but more importantly, we believe that CGuard EPS will provide post-procedure protection against embolic dislodgement, which can occur up to 48 hours post procedure. It is in this post procedure time frame that embolization is the source of post-procedural strokes in the brain. Schofer, et al. (“Late cerebral embolization after emboli-protected carotid artery stenting assessed by sequential diffusion-weighted magnetic resonance imaging,” Journal of American College of Cardiology Cardiovascular Interventions, Volume 1, 2008) have shown that the majority of the incidents of embolic showers associated with carotid stenting occur post-procedure.
The full launch of the CGuard EPS will occur concurrently with the introduction of the new rapid exchange delivery system for CGuard EPS. Since July 2014, we have been working on our next generation rapid exchange delivery system, which is the type of delivery system the majority of physicians that place carotid stents prefer. Our CGuard EPS is currently sold with the over-the-wire delivery system. An over-the-wire delivery system has two lumens and ports. The guide wire lumen and port exists independent of the other lumen for stent delivery and thus two operators must perform the procedure. A rapid exchange delivery system, on the other hand, has a guide wire that passes through the delivery system, running through the guiding catheter. It has one port and thus can be operated by one operator, and as such, can require less time to complete the procedure. The length of the guide wire required for the rapid exchange delivery system is significantly shorter than for the over-the-wire delivery system, and as such, an ordinary guiding wire can be used without adding an extension wire. Our rapid exchange delivery system recently received CE mark approval in January 2015. We plan to focus our full launch of the CGuard on countries in the European Union and Latin America. We will primarily target high volume centers in core European markets. We intend to market and sell our CGuard EPS for use in multiple medical specialties that perform carotid artery stenting. These customers would include interventional cardiologists, vascular surgeons, interventional neuroradiologists and interventional radiologists. The full launch of our CGuard EPS will not include the U.S. We are preparing materials required to conduct a clinical trial in the U.S. Once complete, we will request a pre-submission guidance meeting with the U.S. Food and Drug Administration.
PVGuard — Peripheral Vascular Applications
We intend to develop our MicroNet mesh sleeve and a self-expandable stent for use in peripheral vascular applications. Peripheral artery disease, also known as peripheral vascular disease, is usually characterized by the accumulation of plaque in arteries in the legs. This accumulation can lead to the need for amputation or even death, when untreated. Peripheral artery disease is treated either by trying to clear the artery of the blockage, or by implanting a stent in the affected area to push the blockage out of the way of normal blood flow.
As in carotid procedures, peripheral procedures are characterized by the necessity of controlling embolic showers both during and post-procedure. Controlling embolic showers is so important in these indications that physicians often use fully covered stents, at the risk of blocking branching vessels, to ensure that emboli do not fall into the bloodstream and move to the brain. We believe that our MicroNet design will provide substantial advantages over existing therapies in treating peripheral artery stenosis.
| 7 |
Product Development and Critical Milestones
Below is a list of the products described
above and our projected critical milestones with respect to each. As used below, “CQ” stands for calendar quarter (e.g.,
“CQ1-2015” means January 1, 2015 through March 31, 2015). The use of the term “to be determined” in the
table below with regard to certain milestones indicates that the achievements of such milestones is unable to be accurately predicted
as such milestones are too far in the future.
| Product | Indication | CE Mark | European Union Sales |
FDA Approval(1) |
U.S. Sales | |||||
| MGuard stent (bare-metal stent) | Bypass/ Coronary | Oct. 2007 | CQ1-2008 | To be determined | To be determined | |||||
| Drug-Eluting Stent with MicroNet |
Bypass/ Coronary |
To be determined | To be determined | To be determined | To be determined | |||||
| CGuard Carotid | Carotid Arteries | March 2013 | Oct. 2014 | To be determined | To be determined |
| (1) | We anticipate that the MGuard and CGuard products will be classified as Class III medical devices by the U.S. Food and Drug Administration. |
Pre-Clinical Studies
We performed laboratory and in vivo pre-clinical testing prior to submitting an application for CE Mark approval for our MGuard Coronary stent with MicroNet. We also performed all CE Mark-required mechanical testing of the stent and delivery system. We conducted in vivo pre-clinical studies at the CBSET lab (Lexington, MA) in July 2006 and August 2007. In these studies, on average, the performance of the MGuard Coronary stent with MicroNet was comparable with the performance of control commercially available bare-metal stents. Analysis also indicated that in these studies, the MGuard with MicroNet had comparable biological responses to those of control commercially available bare-metal stents. No human trials were conducted as part of these pre-clinical studies.
The table below describes our completed and planned pre-clinical in vivo studies. The use of the term “To be determined” in the table below with regard to milestone dates in our in vivo pre-clinical studies indicates that we have not yet decided when to schedule such milestones.
| Product | Stent Platform |
Approval Requirement |
Start of Study | End of Study | ||||
| MGuard stent |
Bare-Metal Stent Plus Bio-Stable MicroNet Mesh |
CE Mark (European Union + Rest of World) | CQ4-2006 | CQ3-2007 | ||||
| Drug-Eluting Stent with MicroNet | CE Mark (European Union + Rest of World) | To be determined | To be determined | |||||
| FDA (U.S.) | To be determined | To be determined | ||||||
| MGuard Prime EPS |
Cobalt-Chromium Stent Plus MicroNet Mesh |
FDA (U.S.) | CQ2-2011 | CQ2-2012 | ||||
| MGuard Peripheral/Carotid | Self-Expanding Stent System Plus MicroNet | CE Mark (European Union + Rest of World) | CQ4-2012 | CQ2-2013 |
With respect to the preclinical studies for MGuard Coronary with a drug eluting bio-absorbable mesh, the trials have been indefinitely suspended due to our determination to focus our time and resources on other trials at this time.
Clinical Trials
The table below describes our completed and planned clinical trials. The use of the term “To be determined” in the table below with regard to milestone dates in our clinical trials indicates that we have not yet decided when to schedule such milestones. All milestone dates set forth in the table below are our best estimates based upon the current status of each clinical trial.
| 8 |
| Study Status | ||||||||||||||||||
| Product | Stent Platform |
Clinical Trial Sites |
Follow-up Requirement |
Objective | No. of Patients |
Start Enrollment |
End Enrollment |
End of Study | ||||||||||
| MGuard Coronary |
Bare-Metal Stent Plus MicroNet |
Germany – two sites |
12 months | Study to evaluate safety and
performance of MGuard system |
41 | CQ4-2006 | CQ4-2007 | CQ2-2008 | ||||||||||
| Brazil – one site |
12 months | See above | 30 | CQ4-2007 | CQ1-2008 | CQ2-2009 | ||||||||||||
| Poland – four sites |
3 years | See above | 60 | CQ2-2008 | CQ3-2008 | CQ3-2012 | ||||||||||||
| International MGuard Observational Study – worldwide –19 sites |
12 months | See above | 550 | CQ1-2009 | CQ1-2013 | CQ1-2014 | ||||||||||||
| Israeli MGuard Observational Study – Israel – 9 sites |
6 months | See above | 87 | CQ4-2009 | CQ1-2013 | CQ3-2013 | ||||||||||||
| Master randomized control trial – 9 countries, 50 centers in South America, Europe and Israel |
13 months | See above | 433 | CQ2-2011 | CQ2-2012 | CQ3-2013 | ||||||||||||
| MASTER-II – 70 sites, U.S. and out of U.S. |
13 months | Pivotal study to evaluate safety and performance of MGuard Prime EPS system for FDA approval |
1,114 | CQ3-2013 | Enrollment discontinued | N/A | ||||||||||||
|
iMOS Prime 2 sites in the Netherlands |
12 months | Post-market registry of MGuard Prime EPS | 97 | CQ4-2012 | CQ1-2014 | CQ1-2015 | ||||||||||||
| eMASTER | 12 months | Post-market registry of MGuard Prime EPS | 500 | CQ2-2014 | To be determined |
To be determined | ||||||||||||
| CGuard Carotid | Self-Expanding Carotid Stent Plus MicroNet |
CARENET Post approval registry study 4 sites in Europe |
12 months | Evaluation of safety and efficacy for specific indications post- marketing |
30 | CQ2-2014 | CQ3-2014 | CQ3-2015 | ||||||||||
| MGuard Peripheral | Self-Expanding Stent System Plus MicroNet |
Possibly South America and Europe – |
Study to evaluate safety and performance of MGuard system for CE Mark approval |
To be determined |
To be determined |
To be determined |
To be determined | |||||||||||
| 9 |
Each of the patient numbers and study dates set forth in the tables above are management’s best estimate of the timing and scope of each referenced trial. Actual dates and patient numbers may vary depending on a number of factors, including, without limitation, feedback from reviewing regulatory authorities, unanticipated delays by us, regulatory authorities or third party contractors, actual funding for the trials at the time of trial initiation and initial trial results.
With respect to the MASTER II trial, we successfully enrolled 310 patients in the trial prior to suspending enrollment in April 2014 due to manufacturing process changes in connection with the voluntary field correction action, pending a review by the U.S. Food and Drug Administration of the manufacturing improvements to the MGuard Prime EPS. The U.S. Food and Drug Administration approved the re-commencement of the MASTER II trial in October 2014. However, we elected to discontinue enrollment in the MASTER II trial in its current form, in light of current market conditions moving toward the use of drug-eluting stents over bare-metal stents, and MASTER II will no longer be a U.S. Food and Drug registration trial. Notwithstanding the discontinuance of the enrollment for the MASTER II trial, we intend to continue to follow these 310 MASTER II trial patients for one year from time of enrollment. This follow-up is expected to be completed in June 2015.
The drug eluting stent with MicroNet’s clinical trials have been delayed from our previously announced target until additional funding is secured through potential strategic partnerships.
With respect to the MGuard Peripheral clinical trial for the self-expanding system plus MicroNet, the start date has been delayed from our previously announced start date until additional funding is secured.
Completed Clinical Trials for MGuard Bare-Metal Coronary Stent Plus MicroNet
As shown in the table above, we have completed six clinical trials with respect to our MGuard coronary stent. Our first study, conducted at two centers in Germany, included 41 patients requiring either saphenous vein graft interventions or having native coronary lesions that could be treated by a stenting procedure (blockages where no bypass procedure was performed). The MGuard rate of device success, meaning the stent was successfully deployed in the target lesion, was 100% and the rate of procedural success, meaning there were no major adverse cardiac events prior to hospital discharge, was 95.1%. At six months, only one patient (2.4% of participants) had major Q-wave myocardial infarction (QWMI) and 19.5% of participants had target vessel revascularization (an invasive procedure required due to a stenosis in the same vessel treated in the study). This data supported MGuard’s safety in the treatment of vein grafts and native coronary legions.
Our 2007 study in Brazil included 30 patients who were candidates for a PCI (angioplasty) due to narrowing of a native coronary artery or a narrowed bypass graft. In all patients, the stent was successfully deployed with perfect blood flow parameters (the blood flow parameter is a measurement of how fast the blood flows in the arteries and the micro circulation system in the heart). Except for a single case of a major adverse cardiac event (3% of participants) that was non-QWMI, there were no major cardiac events at the time of the follow-up 30 days after the deployment of the MGuard.
The MAGICAL study, which was conducted in Poland, included 60 patients with STEMI. The purpose of the study was to evaluate the clinical performance of MGuard when used in STEMI patients where PCI is the standard treatment. Perfect blood flow in the target artery was achieved in 90% of patients treated. Perfect blood flow into the heart muscle was also achieved in 73% of patients and complete (>70%) restoration of electrocardiogram normality was achieved in 61.4% of patients. The total major adverse cardiac events rate during the six-month period following the deployment of the stents was 1.7% and after a three-year period was 8.8%.
Our observational study in Europe was an open registry launched in the first calendar quarter of 2009. This registry enrolled 550 patients in 19 sites, primarily in Austria, Czech Republic and Hungary, and was aimed at evaluating the performance of MGuard in a “real world” population. Based upon the number of patients enrolled, we decided to close enrollment on January 10, 2013 and concentrate on clinical follow-up for this study. The primary endpoint of this registry was the occurrence of major adverse cardiac events at 30 days and six months following deployment of the MGuard. The clinical follow-up continued for a period of up to one year.
Our observational study in Israel was an open registry launched in the fourth calendar quarter of 2009. This registry enrolled 87 patients. Based upon the number of patients enrolled, we decided to close enrollment on February 6, 2013 and concentrate on clinical follow-up for this study. The primary endpoint of this registry was the occurrence of major adverse cardiac events at 30 days following deployment of the MGuard Prime stent. The clinical follow-up was conducted six months following deployment of the MGuard Prime.
| 10 |
In the second calendar quarter of 2011, we began the MGuard for Acute ST Elevation Reperfusion Trial (which we refer to as our “MASTER I trial”), a prospective, randomized study in Europe, South America and Israel to compare the MGuard with commercially-approved bare metal and drug-eluting stents in achieving superior myocardial reperfusion (the restoration of blood flow) in primary angioplasty for the treatment of acute STEMI, the most severe form of heart attack. The MASTER I trial enrolled 433 subjects, 50% of whom were treated with an MGuard stent and 50% of whom were treated with a commercially-approved bare metal or drug-eluting stent. The detailed acute and 30 days results from the trial were presented at the Transcatheter Cardiovascular Therapeutics (TCT) conference on October 24, 2012 and published (Prospective, Randomized, Multicenter Evaluation of a Polyethylene Terephthalate Micronet Mesh–Covered Stent (MGuard) in ST-Segment Elevation Myocardial Infarction, Stone et. Al, JACC, 60; 2012). The results were as follows:
| · | The primary endpoint of post-procedure complete ST-segment resolution (restoration of blood flow to the heart muscle after a heart attack) was statistically significantly improved in patients randomized to the MGuard stent compared to patients receiving a commercially-approved bare metal or drug-eluting stent (57.8% vs. 44.7%). |
| · | Patients receiving the MGuard Coronary stent exhibited superior rates of thrombolysis in myocardial infarction (TIMI) 3 flow, which evidences normal coronary blood flow that fills the distal coronary bed completely, as compared to patients receiving a commercially-approved bare metal or drug-eluting stent (91.7% vs. 82.9%), with comparable rates of myocardial blush grade 2 or 3 (83.9% vs. 84.7%) and corrected TIMI frame count (cTFC) (17.0 vs. 18.1), all markers of optimal blood flow to the heart. |
| · | Angiographic success rates (attainment of <50% final residual stenosis of the target lesion and final TIMI 3 flow) were higher in the MGuard group compared to commercially-approved bare metal or drug-eluting stents (91.7% vs 82.4%). |
| · | Mortality (0% vs. 1.9%) and major adverse cardiac events (1.8% vs. 2.3%) at 30 days post procedure were not statistically significantly different between patients randomized to the MGuard Coronary stent as opposed to patients randomized to commercially-approved bare metal or drug-eluting stents. All other major adverse cardiac event components, as well as stent thrombosis, were comparable between the MGuard Coronary and commercially-approved bare metal or drug-eluting stents. |
The six month results from the MASTER I trial, which were presented at the 2013 EuroPCR Meeting, the official annual meeting of the European Association for Percutaneous Cardiovascular Interventions, on May 23, 2013 in Paris, France. The results were as follows:
| · | Mortality (0.5% vs. 2.8%) and major adverse cardiac events (5.2% vs. 3.4%) at 6 months post procedure were not statistically significantly different between patients randomized to the MGuard as compared to patients randomized to commercially-approved bare metal or drug-eluting stents. All other major adverse cardiac event components, as well as stent thrombosis, were comparable between patients treated with MGuard and those treated with commercially-approved bare metal or drug-eluting stents. |
The twelve month results from the MASTER I trial were presented at the Transcatheter Cardiovascular Therapeutics (TCT) conference on October 29, 2013 and published (Mesh-Covered Embolic Protection Stent Implantation in ST-Segment–Elevation Myocardial Infarction Final 1-Year Clinical and Angiographic Results From the MGUARD for Acute ST Elevation Reperfusion Trial, Dudek e. el, Coronary Interventions, 2014. The results were as follows:
| · | Mortality (1.0% vs. 3.3%) and major adverse cardiac events (9.1% vs. 3.3%) at 12 months post procedure were not statistically significantly different between patients randomized to the MGuard stent as opposed to those randomized to commercially-approved bare metal or drug-eluting stents. All other major adverse cardiac events, as well as stent thrombosis, were comparable between the MGuard stent and commercially-approved bare metal or drug-eluting stents. |
In summary, the MASTER I trial demonstrated that among patients with acute STEMI undergoing emergency PCI, or angioplasty, patients treated with MGuard had superior rates of epicardial coronary flow (blood flow within the vessels that run along the outer surface of the heart) and complete ST-segment resolution compared to those treated with commercially-approved bare metal or drug-eluting stents. In addition, patients treated with MGuard showed a slightly lower mortality rate and a slightly higher major adverse cardiac event rate as compared to patients treated with commercially-approved bare metal or drug-eluting stents six and twelve months post procedure.
A detailed table with the results from the MASTER I trial is set forth below. The “p-Value” refers to the probability of obtaining a given test result. Any p value less than 0.05 is considered statistically significant.
| MGuard Coronary |
Bare Metal Stents/Drug Eluting Stents |
p-Value | ||||||||||
| Number of Patients | 217 | 216 | — | |||||||||
| TIMI 0-1 | 1.8 | 5.6 | 0.01 | |||||||||
| TIMI 3 | 91.7 | 82.9 | 0.006 | |||||||||
| Myocardial blush grade 0-1 | 16.1 | 14.8 | 0.71 | |||||||||
| Myocardial blush grade 3 | 74.2 | 72.1 | 0.62 | |||||||||
| ST segment resolution >70 | 57.8 | 44.7 | 0.008 | |||||||||
| 30 day major adverse cardiac event | 1.8 | 2.3 | 0.75 | |||||||||
| 6 month major adverse cardiac event | 5.2 | 3.4 | 0.34 | |||||||||
| 12 month major adverse cardiac event | 9.1 | 3.3 | 0.02 | |||||||||
| 11 |
Ongoing Clinical Trials for MGuard
MASTER II trial
Presently, none of our products may be sold or marketed in the U.S. In our efforts to seek approval of our MGuard Prime by the U.S. Food and Drug Administration, we filed an investigational device exemption application (IDE) with the U.S. Food and Drug Administration in 2012 to conduct a pivotal trial. On April 19, 2013, we received a conditional approval, which allowed us to initiate enrollment in the trial. This trial, which we refer to as our “MASTER II trial,” was to be a multi-center, randomized study, consisting of up to 1,114 patients suffering from STEMI, throughout 35 sites in the U.S. and an additional 35 sites in Europe. The MASTER II trial had two co-primary endpoints: superiority in complete ST resolution and non-inferiority in death and target vessel myocardial infarction. In addition, a 356 patient sub-study was to be conducted to assess the effect of the MGuard Coronary on infarct size, as measured by magnetic resonance imaging, and an additional 200 patient sub-study was to be conducted to assess the late lumen loss, measured at 13 months. Clinical follow-up for the subjects was planned for 30 days, six months and 12 months. We began enrollment in the MASTER II trial on July 29, 2013.
Enrollment was voluntarily suspended on April 30th, 2014 after 310 patients had been randomized at 46 international sites because of a higher than expected rate of stent dislodgement with the MGuard Prime. No patient in MASTER II experienced an endpoint event due to a stent dislodgement. The issue has been addressed with a manufacturing change and device re-approval has been granted in the U.S. for the investigational device exemption application and in Europe for commercial use. On October 14, 2014, enrollment in the MASTER II trial was discontinued due to a commercial decision by the Company as part of the plan to redirect resources to fund development of a drug-eluting version of the MGuard Prime stent. 12 month follow-up of the patients is on-going and is expected to be completed in June 2015.
The 30 day results from the MASTER II trial were presented at the ICI meeting in Tel-Aviv, Israel in 2014. The results were as follows:
| MGuard Prime (n=155) | Bare Metal Stents/Drug Eluting Stents (n=155) | p-Value | ||||||||||
| TIMI 0-1 | 0.7 | % | 1.9 | % | 0.62 | |||||||
| TIMI 3 | 91.4 | % | 89.0 | % | 0.46 | |||||||
| ST segment resolution >70 | 56.9 | % | 59.3 | % | 0.68 | |||||||
| 30 day major adverse cardiac event | 4 (2.6 | )% | 7 (4.5 | )% | 0.36 | |||||||
| – Cardiac mortality | 1 (0.6 | )% | 3 (1.9 | )% | 0.62 | |||||||
| – Reinfarction | 2 (1.3 | )% | 2 (1.3 | )% | 1.00 | |||||||
| – TLR, ischemia-driven | 4 (2.6 | )% | 4 (2.6 | )% | 1.00 | |||||||
In this small group of 310 patients, there were no significant differences in procedural and clinical endpoints. However, 310 patients is too small to find any statistical differences. Therefore, the investigators decided to analyze both MASTER I and MASTER II data to create a pooled data set.
MASTER-I and MASTER-II pooled analysis
The 30 day pooled analysis of MASTER-I and MASTER II trial was presented at the ICI meeting in Tel-Aviv, Israel in 2014. The results were as follows:
| MGuard Prime (n=372) | Bare Metal Stents/Drug Eluting Stents (n=371) | p-Value | ||||||||||
| TIMI 0-1 | 1.4 | % | 4.1 | % | 0.02 | |||||||
| TIMI 3 | 91.6 | % | 85.4 | % | 0.008 | |||||||
| ST segment resolution >70 | 57.5 | % | 50.7 | % | 0.68 | |||||||
| MACE | 8 (2.2 | )% | 12 (3.2 | )% | 0.36 | |||||||
| – Cardiac mortality | 1 (0.3 | )% | 7 (1.9 | )% | 0.04 | |||||||
| – Reinfarction | 5 (1.3 | )% | 4 (1.1 | )% | 1.00 | |||||||
| – TLR, ischemia-driven | 8 (2.2 | )% | 5 (1.3 | )% | 0.40 | |||||||
MGuard demonstrated a significant reduction in all-cause and cardiac mortality at 30 days (MGuard 0.3% vs. Control 1.9%; p=0.04).
iMOS Prime registry
The objective of the iMOS Prime Registry is to evaluate the ‘Real World’ Clinical Performance of the MGuard Prime Coronary Stent System in patients with STEMI undergoing PCI. A total of 97 patients with STEMI were enrolled at 2 sites in the Netherlands. Patients with a clear indication for PCI and with vessel diameter at the infarct lesion either known or expected to be 2.75-4.0 mm, without excessive tortuosity or calcification, were eligible for enrollment. Clinical follow-up was performed at 30 days and 6 months, and patients will be followed through 12 months.
| 12 |
The 30 day and 6 months results were presented at ICI 2014 in Tel-Aviv, Israel and were as follows:
| 30 days | 6 months | |||||||
| MACE | 2 (2.2 | )% | 5 (5.3 | )% | ||||
| Mortality | 0 (0.0 | )% | 2 (2.1 | )% | ||||
| Reinfarction | 2 (2.2 | ) | 4 (4.3 | )% | ||||
| TLR, ischemia-driven | 1 (1.1 | )% | 1 (1.1 | )% |
The MGuard stent was able to reach or cross the lesion in all 97 cases. TIMI-3 flow was restored in 91.8% of cases, and ST resolution (≥70%) in 74.5% of cases.
eMASTER registry
The eMASTER registry commenced at the end of 2014 and its objective is to evaluate the safety and efficacy of the MGuard Prime stent in the treatment of de novo stenotic lesions in coronary arteries in patients undergoing primary PCI due to STEMI in a real-world setting. This is a single arm registry which will enroll up to 500 patients at 50 sites in Europe. Enrollment has begun. The primary endpoints are the rate of complete ST-segment resolution in the first 12-lead ECG done after the stent procedure, and all-cause death or myocardial infarction at 30 days.
CARENET
The CARENET (CARotid Embolic protection using microNET) trial was the first multi-center study of CGuard following the CE Mark of this device in March 2013. The trial was designed to evaluate the feasibility and safety of the CGuard system in the treatment of carotid lesions, in consecutive patients suitable for Coronary Artery Stenting (“CAS”) in a multi-operator, real-life setting. The acute, 30 day, MRI, ultrasound and 6 month clinical event results were presented at the LINC conference in Leipzig, Germany in February, 2015.
MACCE (MI, stroke or death) was 0.0% at 30 days. At 6 months there was one case of death, which was not stent or procedure-related, and MACCE was increased to 3.6%.
| 30 days (n=30) | 6 months (n=28) | |||||||
| MACCE (MI, stroke, death) | (0) 0.0 | % | (1) 3.6 | % | ||||
| MI | (0) 0.0 | % | (0) 0.0 | % | ||||
| stroke | (0) 0.0 | % | (0) 0.0 | % | ||||
| death | (0) 0.0 | % | (1) 3.6 | % |
CAS
carries the risk of cerebral embolization during and following the procedure, leading to life-threatening complications, mainly
cerebral ischemic events. Diffusion-weighted magnetic resonance imaging (DW-MRI) is a sensitive tool used to identify cerebral
emboli during CAS by measuring ‘lesions’ within the brain which are areas that are ischemic and do not receive oxygenated
blood due to cerebral emboli. In the CARENET trial, 37.0% of patients treated with CGuard had new ischemic lesions at 48 hours
after the procedure, with an average volume of 0.039 cm3. Of these lesions, there was only one that remained at 30 days
and all others had resolved. Complete details appear in the following table. Where there is a second number shown below after a
±,,it indicates the rate of error.
| 48 hours n=27 |
30 days n=26 | ||
| Subjects with new Acute Ischemic Lesions (“AIL”) | 10 | 1 | |
| Incidence of new lesions | 37.0% | 4.0% | |
| Total number new AIL | 83 | 1 | |
| Avg. number new AIL per patient | 3.19 ± 10.33 | 0.04 ± 0.20 | |
| Average lesion volume (cm3) | 0.039 ± 0.08 | 0.08 ± 0.00 | |
| Maximum lesion volume (cm3) | 0.445 | 0.116 | |
| Permanent AIL at 30 days | - | 1 |
The healing process of the tissue and in-stent restenosis can be measured by a non-invasive form of ultrasound called duplex ultrasound. This type of ultrasound measures the velocity of the blood that flows within the carotid arteries, which increases exponentially as the lumen of the internal carotid artery narrows and the percent stenosis increases. One of the measurements is called PSV (Peak systolic volume) and is known to be highly correlated to the degree of in-stent restenosis; PSV values higher than 300 cm/sec are indicative of >70% stenosis, while PSV values lower than 104 cm/sec are indicative of <30% restenosis and healthy healing. In the CARENET trial, duplex ultrasound measurements done at 30 days and 6 months following the stenting procedure both attest to healthy normal healing without restenosis concerns, as the PSV values were 60.96 cm/sec ± 22.31 and 85.24 cm/sec ± 39.56, respectively. The internal carotid artery was patent in all patients (100%).
| 13 |
The conclusions were:
| • | CARENET trial demonstrated safety of the CGuard stent, with 30 day MACCE of 0%. |
| • | Incidence of new ipsilateral lesions (percent of patients with new lesions on the ipsilateral side (same side where the stent was employed) at 48 hours was reduced by almost half compared to published data, and volume was reduced almost tenfold. |
| • | All but one lesion had resolved completely by 30 days. |
| • | Six month ultrasound analysis is indicative of healthy healing without restenosis concern. |
| • | CGuard offers unique clinical benefits for patients undergoing CAS with unprecedented safety. |
Comparison of Clinical Trial Results to Date with Results Achieved Using Bare Metal or Drug-Eluting Stents in the STEMI Population
We conducted a meta-analysis of data from four clinical trials in which MGuard was used:
| · | the MAGICAL study, a single arm study in which 60 patients with STEMI with less than 12 hours symptom onset were enrolled, as reported in the journal article “Mesh Covered Stent in ST-segment Elevation Myocardial Infarction” in EuroIntervention, 2010 and presented by D. Dudek, “Extended Follow-up of the MAGICAL Trial”, at the EuroPCR meeting in 2012; |
| · | the PISCIONE study, a single arm study in which 100 STEMI patients were enrolled. The results were reported in the journal article “Multicentre Experience with MGuard Net Protective Stent in ST-elevation Myocardial Infarction: Safety, Feasibility, and Impact on Myocardial Reperfusion” published in Catheter Cardiovasc Interv , 2009 and presented by F. Piscione, “Multicentre Experience MGuard with MGuard net Protective Stent in ST-elevation Myocardial Infarction: Long-term Results”, at the Transcatheter Cardiovascular Therapeutics (TCT) Conference 2010 and F. Piscione, “MGuard in Acute MI: Three-Year Follow-up”, at the TCT Conference in 2011; |
| · | the iMOS study, a registry using MGuard Prime in the “real-world” setting with all patients presenting for PCI population, presented at the 2014 Euro PCR meeting in Paris, France; and |
| · | the Jain study, which looks at a small group of 51 STEMI patients treated with MGuard, as reported in the journal article “Prevention of Thrombus Embolization during Primary Percutaneous Intervention Using a Novel Mesh Covered Stent” published in Catheter Cardiovasc Interv, 2009 and presented by R. Weermckody, “A Mesh Covered Stent Effectively Reduces the Risk of Digital Embolisation During Primary Percutaneous Intervention for ST Elevation Myocardial Infarction,” at the EuroPCR meeting in 2010. |
Our meta-analysis included data from the following trials:
| · | The CADILLAC (Controlled Abciximab and Device Investigation to Lower Late Angioplasty Complications) study, which found that primary stent implantation is a preferred strategy for the treatment of acute myocardial infarction, as reported in the journal article “A Prospective, Multicenter, International Randomized Trial Comparing Four Reperfusion Strategies in Acute Myocardial Infarction: Principal Report of the Controlled Abciximab and Device Investigation to Lower Late Angioplasty Complications (CADILLAC) Trial” published in the Journal of American College of Cardiology, 2001, and the journal article “Comparison of Angioplasty with Stenting, with or without Abciximab, in Acute Myocardial Infarction” published in the New England Journal of Medicine, 2002, “Frequency, Correlates, and Clinical Implications of Myocardial Perfusion After Primary Angioplasty and Stenting, With and Without Glycoprotein IIb/IIIa Inhibition, in Acute Myocardial Infarction” published in the Journal of the American College of Cardiology, 2004 and the journal article “Combined Prognostic Utility of ST-segment Recovery and Myocardial Blush After Primary Percutaneous Coronary Intervention in Acute Myocardial Infarction” published in the European Heart Journal, 2005; |
| · | The EXPORT trial which was a randomized open-label study whose primary endpoint was to evaluate flow improvement in AMI patients using either conventional stenting or aspiration followed by stenting, as reported in the journal article “Systematic Primary Aspiration in Acute Myocardial Percutaneous Intervention: A Multicentre Randomised Controlled Trial of the Export Aspiration Catheter” published in EuroIntervention, 2008; |
| · | The EXPIRA trial which was a single-center study aimed to explore pre-treatment with manual thrombectomy as compared to conventional stenting, as reported in the journal article “Thrombus Aspiration During Primary Percutaneous Coronary Intervention Improves Myocardial Reperfusion and Reduces Infarct Size: The EXPIRA (Thrombectomy with Export Catheter in Infarct-related Artery During Primary Percutaneous Coronary Intervention) Prospective, Randomized Trial” published in the Journal of American College of Cardiology, 2009; |
| · | The REMEDIA trial, whose objective was to assess the safety and efficacy of the EXPORT catheter for thrombus aspiration in STEMI patients, as reported in the journal article “Manual Thrombus-Aspiration Improves Myocardial Reperfusion: The Randomized Evaluation of the Effect of Mechanical Reduction of Distal Embolization by Thrombus-Aspiration in Primary and Rescue Angioplasty (REMEDIA) Trial” published in the Journal of American College of Cardiology, 2005; |
| 14 |
| · | The Horizons-AMI (Harmonizing Outcomes with RevascularIZatiON and Stents in Acute MI) trial, which is the largest randomized trial which compared conventional drug-eluting stents to conventional bare metal stents in myocardial infarction patients, as reported in the journal article “Paclitaxel-Eluting Stents Versus Bare-Metal Stents in Acute Myocardial Infarction” published in the New England Journal of Medicine, 2009, the journal article “Bivalirudin in Patients Undergoing Primary Angioplasty for Acute Myocardial Infarction (HORIZONS-AMI): 1-Year Results of a Randomised Controlled Trial” published in the Lancet, 2009, and the journal article “Heparin Plus a Glycoprotein IIb/IIIa Inhibitor Versus Bivalirudin Monotherapy and Paclitaxel-eluting Stents Versus Bare-metal Stents in Acute Myocardial Infarction (HORIZONS-AMI): Final 3-year Results from a Multicentre, Randomised Controlled Trial” published in the Lancet, 2011; and |
| · | The TAPAS Trial which showed that thrombus aspiration before stenting benefits myocardial infarction patients, as reported in the journal article “Thrombus Aspiration During Primary Percutaneous Coronary Intervention” published in the New England Journal of Medicine, 2009 and the journal article “Cardiac Death and Reinfarction After 1 Year in the Thrombus Aspiration During Percutaneous Coronary Intervention in Acute Myocardial Infarction Study (TAPAS): A 1-year Follow-up Study” published in the Lancet, 2008. |
The non-randomized, pooled data analysis of MGuard outcomes in a STEMI population show comparable rates of thrombolysis in myocardial infarction (TIMI 3 flow) compared to the historical control (91.7% and 87.8%, respectively), while the rates of myocardial blush grade score 3 (37.3% for the historical control and 81.6% for MGuard) and ST segment resolution >70% (53.6% for the historical control and 79.1% for MGuard) are significantly better in the MGuard group. MGuard is also consistently superior at the 30 day follow-up in terms of major adverse cardiac event (8.4% for the historical control and 2.4% for MGuard) and 1 year major adverse cardiac event (12.8% for the historical control and 5.9% for MGuard). The data appears in the following tables.
| NAME OF STUDY | ||||||||||||||||||||
| MAGICAL | PISCIONE | iMOS | Jain | Average | ||||||||||||||||
| Number of Patients | 60 | 100 | 203 | 51 | 414 (Total) | |||||||||||||||
| Thrombolysis in myocardial infarction 0-1,% | 0 | 0 | 1.2 | 0 | 0.6 | |||||||||||||||
| Thrombolysis in myocardial infarction 3,% | 90 | 85 | 93.5 | 100 | 91.7 | |||||||||||||||
| Myocardial blush grade 0-1,% | 3.3 | 0 | — | — | 1.2 | |||||||||||||||
| Myocardial blush grade 3,% | 73 | 90 | 80 | — | 81.6 | |||||||||||||||
| ST segment resolution>70%,% | 61 | 90 | — | — | 79.1 | |||||||||||||||
| ST segment resolution>50%,% | 88 | — | 85.4 | 96 | 87.6 | |||||||||||||||
| 30 day major adverse cardiac event,% | 0 | 2.2 | 3.2 | — | 2.4 | |||||||||||||||
| 6 month major adverse cardiac events,% | 0 | 4.5 | 6.0 | — | 4.6 | |||||||||||||||
| 1 year major adverse cardiac events,% | — | 5.6 | 6.0 | 6.0 | 5.9 | |||||||||||||||
| 1 year target vessel revascularization | — | 2.3 | 2.3 | 6.0 | 2.8 | |||||||||||||||
| Acute Binary Restenosis 6M,% | — | — | 19.0 | * | — | 19.0 | ||||||||||||||
| THREE YEAR FOLLOW UP STUDIES NAME OF STUDY |
||||||||||||||||||||
| MAGICAL | PISCIONE | iMOS | Jain | Average | ||||||||||||||||
| Number of Patients | 57 out of 60 | 89 | — | — | — | |||||||||||||||
| Cardiac death at 3Y | 7 | % | 2.2 | % | — | — | — | |||||||||||||
| Non Cardiac death at 3Y | 1.8 | % | 6.8 | % | — | — | — | |||||||||||||
| Re-MI at 3Y | 0 | % | 7.9 | % | — | — | — | |||||||||||||
| TLR at 3Y | 1.8 | % | Not Reported | — | — | — | ||||||||||||||
| TVR at 3Y Include TLR | 3.5 | % | 4.5 | % | — | — | — | |||||||||||||
| Stroke | 1.8 | % | Not Reported | — | — | — | ||||||||||||||
| Stent thrombosis Definite / Probable | 0 | % | 2.2 | % | — | — | — | |||||||||||||
| MACE (Cardiac death, RE-MI, TLR) | 8.8 | % | 10.1 | % | — | — | — | |||||||||||||
| MACCE (All death, target vessel MI, TVR, Stroke) | 10.5 | % | Not Reported | — | — | — | ||||||||||||||
| 15 |
| Trial | CADILLAC | Horizons- AMI | Horizons- AMI | TAPAS | TAPAS | EXPORT | EXPORT | EXPIRA | EXPIRA | REMEDIA | REMEDIA | Historical comparison | MGuard | Level
of Significance | ||||||||||||||||||||||||||||||||||||||||||
| Group | Stent
+ Abciximab | BMS | DES | Thrombus aspiration | control | control | TA | control | Thrombus aspiration | Thrombus aspiration | control | Average | Average | |||||||||||||||||||||||||||||||||||||||||||
| Number of Patients | 524 | 749 | 2257 | 535 | 536 | 129 | 120 | 87 | 88 | 50 | 49 | 5124 (total) | 414 (total) | |||||||||||||||||||||||||||||||||||||||||||
| Thrombolysis in myocardial infarction 0-1,% | — | — | — | — | — | 3.9 | 2.4 | 1.1 | 0 | — | — | 2.1 | 0.6 | |||||||||||||||||||||||||||||||||||||||||||
| Thrombolysis in myocardial infarction 3,% | 96.9 | 89.8 | 87.6 | 86 | 82.5 | 76.9 | 82 | — | — | — | — | 87.8 | 91.7 | |||||||||||||||||||||||||||||||||||||||||||
| Myocardial blush grade 0-1,% | 48.7 | — | — | 17.1 | 26.3 | 31.6 | 27.6 | 40.2 | 11.4 | 32 | 55.1 | 35.2 | 1.2 | * | ||||||||||||||||||||||||||||||||||||||||||
| Myocardial blush grade 3,% | 17.4 | — | — | 45.7 | 32.2 | 25.4 | 35.8 | — | — | — | — | 37.3 | 81.6 | ** | ||||||||||||||||||||||||||||||||||||||||||
| ST segment resolution>70%,% | 62.1 | — | — | 56.6 | 44.2 | — | — | 39.1 | 63.6 | 58 | 36.7 | 53.6 | 79.1 | |||||||||||||||||||||||||||||||||||||||||||
| ST segment resolution>50%,% | — | — | — | — | — | 71.9 | 85 | — | — | — | — | 78.2 | 87.6 | |||||||||||||||||||||||||||||||||||||||||||
| 30 day major adverse cardiac event,% | 4.4 | — | — | 6.8 | 9.4 | — | — | — | — | 10 | 10.2 | 8.4 | 2.4 | ** | ||||||||||||||||||||||||||||||||||||||||||
| 6 month major adverse cardiac events,% | 10.2 | — | — | — | — | — | — | — | — | — | — | 10.2 | 4.6 | |||||||||||||||||||||||||||||||||||||||||||
| 1 year major adverse cardiac events,% | — | 11.9 | 10.5 | 16.6 | 20.3 | — | — | — | — | — | — | 12.8 | 5.9 | * | ||||||||||||||||||||||||||||||||||||||||||
| Acute Binary Resteonosis 6 month,% | 20.8 | — | — | — | — | — | — | — | — | — | — | 20.8 | 19.0 | |||||||||||||||||||||||||||||||||||||||||||
| 1 year target vessel revascularization | — | 8.7 | 5.8 | 12.9 | 11.2 | — | — | — | — | — | — | 8.0 | — | |||||||||||||||||||||||||||||||||||||||||||
| Acute Binary Resteonosis 1 year,% | — | 21 | 8.2 | — | — | — | — | — | — | — | — | 11.5 | — | |||||||||||||||||||||||||||||||||||||||||||
Future Clinical Trials for MGuard Coronary and CGuard Carotid
Post-marketing clinical trials (outside the United States) could be conducted to further evaluate the safety and efficacy of the MGuard and CGuard stents in specific indications. These trials would be designed to facilitate market acceptance and expand the use of the product. We should be able to rely upon CE Mark approval of the product and other supporting clinical data to obtain local approvals.
Growth Strategy
Our primary business objective is to utilize our proprietary technology to become the industry standard for treatment of acute coronary syndromes and to provide a superior solution to the common acute problems caused by current stenting procedures, such as restenosis, embolic showers and late thrombosis. We are pursuing the following business strategies in order to achieve this objective.
| · | Successfully commercialize CGuard EPS. We have launched limited market release of CGuard through direct sales organization in select European countries. The initial commercial phase of our launch will be through our direct sales team in Europe and is expected to focus on high volume, key opinion leaders in the carotid space. By the time we convert to full market release, we expect to have generated usage and a broader awareness of the CGuard in key European markets, as well as a fully developed the rapid exchange delivery system for CGuard EPS. |
| · | Successfully develop and commercialize the next generation of drug-eluting stent incorporating MicroNet. While we market our MGuard products with bare-metal stents, we are developing a drug-eluting stent that incorporates MicroNet and expect to proceed with the in vivo pre-clinical testing of the product with a CE-marked drug-eluting stent candidate. If successful, and if no CE mark trial is required due to the fact that each of MicroNet and the drug-eluting stent is CE-marked, this work is expected to lead to submission by us of a DES-MicroNet platform for CE mark approval in the second half of 2015. We intend to develop two strategic partnerships with manufactures of U.S. Food and Drug Administration-approved or CE-marked drug-eluting stents and bring two viable drug-eluting stent products with our MicroNet mesh into the in vivo pre-clinical testing phase. |
| · | Grow our presence in existing and new markets for MGuard coronary products. We have commercialized bare-metal based MGuard products in Europe, Russia, Asia and Latin America through our distributor network, and we are pursuing additional registrations and contracts in other countries such as Canada, Australia, South Korea and certain smaller countries in Latin America. We have completed the modification of our stent securement process on inventory and are back to full commercial activities in direct markets in Western Europe and sales are under way, and we believe that the eMASTER study will reinforce this positive momentum. We intend to complete the full re-launch of MGuard Prime in 2015, and we have implemented a hybrid sales strategy with direct sales representatives in key European markets to support the full re-launch. We intend to re-evaluate our commercialization strategies for MGuard coronary products in the U.S. and Japan in the future following future development of the DES-MicroNet product and future clinical trial results. |
| · | Continue to leverage MicroNet technology to develop additional applications for interventional cardiologists and vascular surgeons. In addition to the applications described above, we believe that we will eventually be able to utilize our proprietary technology to address imminent market needs for new product innovations to significantly improve patients’ care. We continue to broadly develop and file intellectual property using our mesh technology. Examples of some areas include peripheral vascular disease, neurovascular disease, renal artery disease, and bifurcation disease. |
| · | We work closely with leading physicians to evaluate and ensure the efficacy and safety of our products. Some of these prominent physicians serve on our Scientific Advisory Board, which is our advisory committee that advises our board of directors and advises and participates in the operation of our clinical trials. These physicians have and will continue to generate and publish scientific data on the use of our products, and to present their findings at various key clinical conferences. |
| 16 |
| · | Establish relationships with collaborative and development partners to fully develop and market our existing and future products. We are seeking strategic partners for collaborative research, development, marketing, distribution, or other agreements, which could assist with our development and commercialization efforts for MGuard, DES with MicroNet, CGuard EPS and other potential products that are based on our MicroNet technology. We are in discussions with multiple potential partners and may enter into an arrangement to pursue further development and commercialization of these products. |
| · | Continue to protect and expand our portfolio of patents. Our MicroNet technology and the use of patents to protect it are critical to our success. We own numerous patents for our MicroNet technology. Twelve separate patent applications have been filed in the U.S. and corresponding patent applications in Canada, China, Europe, Israel, India, and South Africa. We believe these patents and patent applications collectively cover all of our existing products, and may be useful for protecting our future technology developments. We intend to aggressively continue patenting new technology, and to actively pursue any infringement covered by any of our patents. We believe that our patents, and patent applications once allowed, are important for maintaining the competitive differentiation of our products and maximizing our return on research and development investments. |
Competition
The markets in which we compete are highly competitive, subject to change and impacted by new product introductions and other activities of industry participants. The bare-metal stent and the drug-eluting stent markets in the U.S. and Europe are dominated by Abbott Laboratories, Boston Scientific Corporation, and Medtronic, Inc. The carotid stent market in the U.S. and Europe are dominated by Abbott Laboratories, Boston Scientific Corporation, Covidien Ltd., and Cordis Corporation. Gore Medical and Terumo produce mesh-covered carotid stents. All of these larger companies have substantially greater capital resources, larger customer bases, broader product lines, larger sales forces, greater marketing and management resources, larger research and development staffs and larger facilities than ours and have established reputations and relationships with our target customers, as well as worldwide distribution channels that are more effective than ours. Due to ongoing consolidation in the industry, there are high barriers to entry for small manufacturers in both the European and the U.S. markets. However, we believe that the European market is somewhat more fragmented, and small competitors appear able to gain market share with greater ease.
In the future, we believe that physicians will look to next-generation stent technology to compete with existing therapies. These new technologies will likely include bio-absorbable stents, stents that focus on treating bifurcated lesions, and stents with superior polymer and drug coatings, and many industry participants are working to improve stenting procedures in the future as the portfolio of available stent technologies rapidly increases. As the market moves towards next-generation stenting technologies, minimally invasive procedures should become more effective, driving the growth of the market in the future. We plan to continue our research and development efforts in order to be at the forefront of the acute myocardial infarction solutions.
According to the MEDTECH OUTLOOK, the worldwide stent market is dominated by three major players, with a combined total market share of approximately 92%. Within the bare-metal stent market and drug-eluting stent market, the top three companies have approximately 71% and 97% of the market share, respectively. These three companies are Abbott Laboratories, Boston Scientific Corporation and Medtronic, Inc. To date, our sales are not significant enough to register in market share. As such, one of the challenges we face to the further growth of our products is the competition from numerous pharmaceutical and biotechnology companies in the therapeutics area, as well as competition from academic institutions, government agencies and research institutions. Most of our current and potential competitors, including but not limited to those listed above, have, and will continue to have, substantially greater financial, technological, research and development, regulatory and clinical, manufacturing, marketing and sales, distribution and personnel resources than we do.
In addition to the challenges from our competitors, we face challenges related specifically to our products. None of our products is currently approved by the U.S. Food and Drug Administration. Clinical trials necessary to support a pre-market approval application to the U.S. Food and Drug Administration for our MicroNet products will be expensive and will require the enrollment of a large number of patients. Suitable patients may be difficult to identify and recruit, which may cause a delay in the development and commercialization of our product candidates. Furthermore, our rights to our intellectual property with respect to our products could be challenged. Based on the prolific litigation that has occurred in the stent industry and the fact that we may pose a competitive threat to some large and well-capitalized companies that own or control patents relating to stents and their use, manufacture and delivery, we believe that it is possible that one or more third parties will assert a patent infringement claim against the manufacture, use or sale of our MicroNet products based on one or more of these patents, and/or will allege misappropriation of their proprietary confidential information or other intellectual property.
Research and Development Expenses
During each of the twelve months ended December 31, 2014, the six months ended December 31, 2013 and the twelve months ended June 30, 2013 and 2012, we spent $8.7 million, $3.3 million, $4.2 million and $4.0 million, respectively, on research and development.
| 17 |
Sales and Marketing
Sales and Marketing
In October 2007, the MGuard Coronary stent system with a bio-stable MicroNet received CE Mark approval in the European Union, and shortly thereafter was commercially launched in Europe through local distributors. We are also in negotiations with additional distributors in Europe, Asia and Latin America and are actively selling our MGuard Coronary stent system with a bio-stable MicroNet in more than 20 countries.
We plan to focus our marketing efforts primarily on Europe, Asia and Latin America. Within Europe, we have focused on markets with established healthcare reimbursement from local governments such as Russia, Italy, Germany, France, Austria, Poland, Czech Republic, Denmark, Holland, Belgium, Spain, Sweden, Switzerland and the United Kingdom.
In addition to utilizing local and regional distributor networks, we are using international trade shows and industry conferences to gain market exposure and brand recognition. We plan to work with leading physicians to enhance our marketing efforts. As sales volume has increased, we have engaged in direct sales in certain geographic markets.
Product Positioning
The MGuard Coronary stent system has initially penetrated the market by entering segments with indications that present high risks of embolic dislodgement, notably acute myocardial infarction and saphenous vein graft coronary interventions. The market penetration of the MGuard Coronary stent system for each of the twelve months ended December 31, 2014, as well as June 30, 2013 and June 30, 2012, was minimal, with total sales of $2.8 million, $4.9 million and $5.3 million, respectively, each representing less than 1% of the total sales of the acute myocardial infarction solutions market. The market penetration for the six months ended December 31, 2013 was also minimal, with total sales in the six months ended December 31, 2013 of $3.1 million representing less than 1% of the total sales of the acute myocardial infarction solutions market.
When performing stenting procedures in patients with acute coronary symptoms, interventional cardiologists face a difficult dilemma in choosing between bare-metal stents, which have a high rate of restenosis, and drug-eluting stents, which have a high rate of late stent thrombosis, require administration of anti-platelet drugs for at least one year post procedure and are more costly than bare-metal stents. We are marketing our platform technology, MGuard Coronary stent system, as a superior and cost effective solution to these currently unmet needs of interventional cardiologists. We believe our MGuard Coronary technology is clinically superior to bare-metal stents because it provides embolic protection during and post-procedure. We believe our MGuard Coronary stent technology is clinically superior to drug-eluting stents, due to its lower stent thrombosis rate and protection from embolic showers during and post-procedure.
In addition to the advantages of the MGuard Coronary stent technology that we believe to exist, the MGuard Coronary technology maintains the deliverability, crossing profile, and dilatation pressure of a conventional stent, and interventional cardiologists do not have to undergo any training before utilizing the product.
Insurance Reimbursement
In most countries, a significant portion of a patient’s medical expenses is covered by third-party payers. Third-party payers can include both government funded insurance programs and private insurance programs. While each payer develops and maintains its own coverage and reimbursement policies, the vast majority of payers have similarly established policies. All of the MGuard products sold to date have been designed and labeled in such a way as to facilitate the utilization of existing reimbursement codes, and we intend to continue to design and label our products in a manner consistent with this goal.
While most countries have established reimbursement codes for stenting procedures, certain countries may require additional clinical data before recognizing coverage and reimbursement for the MGuard products or in order to obtain a higher reimbursement price. In these situations, we intend to complete the required clinical studies to obtain reimbursement approval in countries where it makes economic sense to do so.
Intellectual Property
Patents
We have filed fourteen patent applications that are pending in the U.S. covering aspects of our MGuard and CGuard technology. We have filed corresponding patent applications in Canada, China, Europe, Israel, India and South Africa, for an aggregate total of 42 patents and pending applications including two issued U.S. patents. These patent rights are directed to cover percutaneous therapy, knitted stent jackets, stent and filter assemblies, in vivo filter assembly, optimized stent jackets, stent apparatuses for treatment via body lumens and methods of use, stent apparatuses for treatment via body lumens and methods of manufacture and use, among others. In lay terms, these patent applications generally cover three aspects of our products: the mesh sleeve with and without a drug, the product and the delivery mechanism of the stent. On October 27, 2010, our patent application pertaining to “Stent Apparatus for Treatment via Body Lumens and Method of Use,” South African patent application 2007/10751, was issued as South African Patent No. 2007/10751. On October 25, 2011, our patent application pertaining to “In Vivo Filter Assembly,” U.S. Patent Application 11/582,354, was issued as U.S. Patent 8,043,323. On June 13, 2012, our patent application pertaining to “Filter Assemblies,” Chinese Patent Application No. 200780046659.9, was issued as Chinese Patent No. ZL200780046659.9. On September 26, 2012, our patent application pertaining to “Bifurcated Stent Assemblies,” Chinese Patent Application No. 200780046676.2, was issued as Chinese Patent No. ZL200780046676.2. On October 10, 2012, our patent application pertaining to “Knitted Stent Jackets,” Chinese Patent Application No. 200780046697.4, was issued as Chinese Patent No. ZL200780046697.4. On January 2, 2013, our patent application pertaining to “Optimized Stent Jacket,” Chinese Patent Application No. 200780043259.2, was issued as Chinese Patent No. ZL200780043259.2. We have also had Israeli Patent No. 198189 entitled “Filter Assemblies” issued March 27, 2014, and Patent No. 198190, entitled “Knitted Stent Jackets” issued Feb. 1, 2014, and Canadian Patent No. 2609687 entitled “Stent Apparatuses For Treatment Via Body Lumens” issued April 22, 2014. U.S. Patent Application No. 11/797,168, filed May 1, 2007, was issued as U.S. Patent No. 8,961,586 on February 24, 2015. We also believe that one or more pending patent applications, upon issuance, will cover our existing products. We also believe that the patent applications we have filed, in particular those covering the use of a knitted micron-level mesh sleeve over a stent for various indications, if issued as patents with claims substantially in their present form, would likely create a significant barrier for another company seeking to use similar technology.
| 18 |
Trademarks
We use the InspireMD® and MGuard® trademarks in connection with our products. We have registered these trademarks in the European Union. The trademarks are renewable indefinitely, so long as we make the appropriate filings when required. We also have a registration for the MNP Micronet Protection Logo in the European Union. We have also applied to register the names MicroNet™, Carenet™, CGuard™ and MGuard Prime™ as trademarks in the U.S. and the names Carenet™, CGuard™ and MGuard Prime™ in the European Union. We also use and may have common law rights to various trademarks, trade names, and service marks including the following: PVGuardTM , NGuardTM , and RGuardTM.
Government Regulation
The manufacture and sale of our products are subject to regulation by numerous governmental authorities, principally the European Union CE Mark, the U.S. Food and Drug Administration and other corresponding foreign agencies.
Sales of medical devices outside the U.S. are subject to foreign regulatory requirements that vary widely from country to country. These laws and regulations range from simple product registration requirements in some countries to complex clearance and production controls in others. As a result, the processes and time periods required to obtain foreign marketing approval may be longer or shorter than those necessary to obtain U.S. Food and Drug Administration market authorization. These differences may affect the efficiency and timeliness of international market introduction of our products. For countries in the European Union, medical devices must display a CE Mark before they may be imported or sold. In order to obtain and maintain the CE Mark, we must comply with the Medical Device Directive 93/42/EEC and pass initial and annual facilities audit inspections to ISO 13485 standards by an European Union inspection agency. We have obtained ISO 13485 quality system certification and the products we currently distribute into the European Union display the required CE Mark. In order to maintain certification, we are required to pass annual facilities audit inspections conducted by European Union inspectors.
As noted below, we currently have distribution agreements for our products with distributors, or are directly selling our products, in the following countries: Italy, Austria, Slovenia, Spain, Hungary, Estonia, Ukraine, Holland, Russia, Latvia, Brazil, Mexico, Argentina, Colombia, India, Sri Lanka, South Africa, Pakistan, Belarus, Croatia, Ireland, Lithuania, Malta, Malaysia, Venezuela, Australia, Belgium, the Czech Republic, Finland, Slovakia, Sweden, Denmark, Norway, Switzerland, Poland, Germany, Cyprus, France, Finland, Romania, the United Kingdom, Saudi Arabia, New Zealand, Taiwan and Israel. We are subject to governmental regulation in each of these countries and we are not permitted to sell all of our products in each of these countries. In addition, we have distribution agreements for our products in Uzbekistan, South Korea, Canada, Kazakhstan, Kuwait and Armenia, although we have not yet obtained regulatory approval to sell our products in those countries. While each of the European Union member countries accepts the CE Mark as its sole requirement for marketing approval, some of these countries still require us to take additional steps in order to gain reimbursement rights for our products. Furthermore, while we believe that each of the above-listed countries that is not a member of the European Union accepts the CE Mark as its primary requirement for marketing approval, each such country requires additional regulatory requirements for final marketing approval of the MGuard Prime version of the MGuard Coronary product. Additionally, in Canada, we are required to pass annual facilities audit inspections performed by Canadian inspectors. Furthermore, we are currently targeting additional countries in Europe, Asia, and Latin America. We believe that each country that we are targeting also accepts the CE Mark as its primary requirement for marketing approval. We expect that the results of the MASTER I trial will enhance our ability to satisfy any additional governmental regulatory requirements in each of the countries where we currently distribute or directly sell our products and in any countries that we are currently targeting for expansion. However, even if all governmental regulatory requirements are satisfied in each such country, we anticipate that obtaining marketing approval in each country could take as few as three months or as many as twelve months, due to the nature of the approval process in each individual country, including typical wait times for application processing and review, as discussed in greater detail below.
| 19 |
The MGuard Prime version of the MGuard Coronary product received CE Mark approval in the European Union in October 2010 and marketing approval in those countries listed in the table below. We are currently seeking marketing approval for the MGuard Prime version of the MGuard Coronary product in Brazil, Mexico, Argentina, South Korea, Taiwan, Australia, Belarus, Malaysia, Saudi Arabia, the U.S. and Canada. We are focused on seeking marketing approval in these countries because we believe that these countries represent the strongest opportunities for us to grow with respect to our sales. We have determined that other countries with better organized and capitalized healthcare systems may not present us the same opportunities for growth due to the lack of use of stents in treatment of cardiac episodes and less advantageous healthcare reimbursement policies, among other reasons. While we understand that each of the countries in which we are seeking marketing approval for the MGuard Prime version of the MGuard Coronary product accepts the CE Mark as its primary requirement for marketing approval and does not to our understanding require any additional tests, each country does have some additional regulatory requirements for marketing approval. More specifically, for example, for the approval process in Mexico, where we already have approval for and sales of MGuard Coronary, we need to submit an application for regulatory approval for MGuard Prime, which we anticipate will be granted at least thirty months later. For the approval process in South Korea, we need to submit an application for regulatory approval and have in-house quality audit, which we anticipate will be granted in approximately two years. For the approval process in Canada, we need to submit an application for regulatory approval, which we anticipate will be granted approximately twelve months later.
The CGuard Carotid product received CE Mark approval in the European Union on March 14, 2013 and marketing approval in those countries listed in the table below. We are currently seeking marketing approval for the CGuard Carotid product in Israel and Belarus.
For the approval process in Brazil for MGuard Prime, where we already have approval for and sales of MGuard Coronary, we must comply with Brazilian Good Manufacturing Practice, or GMP, quality system requirements. ANVISA, Brazil’s regulatory agency, must conduct an inspection of the manufacturing of the MGuard Prime version of the MGuard Coronary product to determine compliance with Brazil GMP regulations. Upon successful completion of an audit, ANVISA will then issue the GMP certificate necessary to register a medical device in Brazil, which can take approximately one year to complete. Based upon new legislation in Brazil, we intend to apply for regulatory approval while we await the results of the audit necessary to receive our GMP certificate. We anticipate that the approval process in Brazil will be completed in 2015.
Please refer to the table below setting forth the approvals and sales for original stainless steel based MGuard Coronary product and the cobalt-chromium based MGuard Prime version of the MGuard Coronary product on a country-by-country basis.
Approvals and Sales of the Original MGuard Coronary, the MGuard Prime version of the MGuard Coronary and the CGuard on a Country-by-Country Basis
| Countries | Original MGuard Approval |
Original MGuard Sales |
MGuard Prime Approval |
MGuard Prime Sales |
CGuard Approval |
CGuard Sales |
||||||||||||||||
| Argentina | Y | Y | Y | Y | N | N | ||||||||||||||||
| Armenia | N | N | N | N | N | N | ||||||||||||||||
| Australia | N | (1) | Y | Y | Y | N | N | |||||||||||||||
| Austria | Y | Y | Y | Y | Y | N | ||||||||||||||||
| Belarus | Y | Y | Y | Y | N | N | ||||||||||||||||
| Belgium | Y | N | Y | Y | Y | N | ||||||||||||||||
| Brazil | Y | Y | N | N | N | N | ||||||||||||||||
| Chile | N | (1) | Y | N | Y | (2) | N | N | ||||||||||||||
| Colombia | Y | Y | N | N | N | N | ||||||||||||||||
| Croatia | Y | Y | Y | Y | Y | N | ||||||||||||||||
| Cyprus | Y | Y | Y | Y | Y | N | ||||||||||||||||
| Czech Republic | Y | Y | Y | Y | Y | N | ||||||||||||||||
| Denmark | Y | Y | Y | N | Y | N | ||||||||||||||||
| Egypt | Y | N | N | N | N | N | ||||||||||||||||
| Estonia | Y | Y | Y | Y | Y | N | ||||||||||||||||
| Finland | Y | N | Y | Y | Y | N | ||||||||||||||||
| France | Y | Y | Y | Y | Y | N | ||||||||||||||||
| Germany | Y | Y | Y | Y | Y | Y | ||||||||||||||||
| Greece | Y | Y | Y | N | Y | N | ||||||||||||||||
| Holland (Netherlands) | Y | Y | Y | Y | Y | N | ||||||||||||||||
| Hungary | Y | Y | Y | Y | Y | N | ||||||||||||||||
| India | Y | Y | Y | N | N | N | ||||||||||||||||
| Ireland | Y | Y | Y | Y | Y | N | ||||||||||||||||
| Israel | Y | Y | Y | Y | Y | N | ||||||||||||||||
| Italy | Y | Y | Y | Y | Y | Y | ||||||||||||||||
| Kazakhstan | N | (3) | Y | N | N | N | N | |||||||||||||||
| Latvia | Y | Y | Y | Y | Y | N | ||||||||||||||||
| Lithuania | Y | Y | Y | Y | Y | N | ||||||||||||||||
| Malaysia | N | N | N | Y | (2) | N | N | |||||||||||||||
| Malta | Y | N | Y | Y | Y | N | ||||||||||||||||
| Mexico | Y | Y | N | N | N | N | ||||||||||||||||
| Norway | Y | N | Y | Y | N | N | ||||||||||||||||
| Pakistan | Y | (4) | Y | N | N | N | N | |||||||||||||||
| Poland | Y | Y | Y | Y | Y | Y | ||||||||||||||||
| Portugal | Y | Y | Y | N | Y | N | ||||||||||||||||
| Romania | Y | Y | Y | Y | Y | N | ||||||||||||||||
| Russia | Y | Y | Y | Y | N | N | ||||||||||||||||
| Saudi Arabia | N | N | Y | Y | N | N | ||||||||||||||||
| Singapore | N | Y | (5) | N | N | N | N | |||||||||||||||
| Slovakia | Y | Y | Y | Y | Y | N | ||||||||||||||||
| Slovenia | Y | Y | Y | Y | Y | N | ||||||||||||||||
| South Africa | Y | (4) | Y | Y | Y | N | N | |||||||||||||||
| Spain | Y | Y | Y | Y | Y | N | ||||||||||||||||
| Sri Lanka | Y | (4) | Y | N | N | N | N | |||||||||||||||
| Sweden | Y | Y | Y | Y | Y | N | ||||||||||||||||
| Switzerland | Y | Y | Y | Y | Y | N | ||||||||||||||||
| Ukraine | Y | Y | N | N | N | N | ||||||||||||||||
| United Kingdom | Y | N | Y | Y | Y | N | ||||||||||||||||
| Uzbekistan | N | N | N | N | N | N | ||||||||||||||||
| Venezuela | N | (1) | Y | N | N | N | N |
| 20 |
(1) We terminated our relationship with our previous distributor in this country, through which our product had market approval. As a result of such termination, we will be required to obtain regulatory approval upon our selection of a new distributor in such country.
(2) We have made sales to distributors in this country, but based upon information from such distributors, we believe that the product has not been sold to customers in this country.
(3) Our regulatory approval for sales in Kazakhstan expired in January 2014. We intend to renew our regulatory approval for Kazakhstan in the future.
(4) We believe that we have regulatory approval for the MGuard Coronary product in this country, based upon information from our distributor in such country, who was responsible for obtaining the regulatory approval for the MGuard Coronary product. However, the certificate evidencing regulatory approval is held by our distributor and we cannot guarantee that it is in full force and effect.
(5) At time the sales were made, we satisfied the regulatory requirements in Singapore. The regulatory requirements in Singapore were subsequently changed and we no longer meet these requirements.
In the U.S., the medical devices that will be manufactured and sold by us will be subject to laws and regulations administered by the U.S. Food and Drug Administration, including regulations concerning the prerequisites to commercial marketing, the conduct of clinical investigations, compliance with the Quality System Regulation and labeling. We anticipate that our MGuard Prime Coronary product with bio-stable mesh product will be classified as a Class III medical device by the U.S. Food and Drug Administration.
A manufacturer may seek market authorization for a new medical device through the rigorous Premarket Approval application process, which first requires that the U.S. Food and Drug Administration determine that the device is safe and effective for the purposes intended.
We will also be required to register with the U.S. Food and Drug Administration as a medical device manufacturer. As such, our manufacturing facilities will be subject to U.S. Food and Drug Administration inspections for compliance with Quality System Regulation. These regulations will require that we manufacture our products and maintain our documents in a prescribed manner with respect to design, manufacturing, testing and quality control activities. As a medical device manufacturer, we will further be required to comply with U.S. Food and Drug Administration requirements regarding the reporting of adverse events associated with the use of our medical devices, as well as product malfunctions that would likely cause or contribute to death or serious injury if the malfunction were to recur. U.S. Food and Drug Administration regulations also govern product labeling and prohibit a manufacturer from marketing a medical device for unapproved applications. If the U.S. Food and Drug Administration believes that a manufacturer is not in compliance with the law, it can institute enforcement proceedings to detain or seize products, issue a recall, enjoin future violations and assess civil and criminal penalties against the manufacturer, its officers and employees.
Customers
Our customer base is varied. We began shipping our product to customers in Europe in January 2008 and have since expanded our global distribution network to Southeast Asia, India, Latin America and Israel. Unless otherwise indicated below, all of the distribution agreements described under “Customers” are subject to automatic annual extensions unless affirmatively terminated. For the twelve months ended December 31, 2014, 54% of our revenue was generated in Europe, 28% of our revenue was generated in the Middle East and 11% of our revenue was generated in Latin America, with the remaining 7% of our revenue generated in the rest of the world. Our major customers in the twelve months ended December 31, 2014 were a distributor in Saudi Arabia that accounted for 21% of our revenues, and Cardio Medical Sales L.P, a distributor in Belarus that accounted for 10% of our revenues. Our agreement with the distributor in Saudi Arabia grants them the right to be a distributor of MGuard products in Saudi Arabia until November 2016 subject to the achievement of certain order minimums. Under our agreement with them, they are required to purchase 300 stents from us in 2013, 1,700 stents in 2014 and 2,500 stents in 2015. Although they did not adhere to their order minimum for 2014, we did not terminate their right to be our distributor of MGuard products in Saudi Arabia. Our agreement with Cardio Medical Sales L.P grants Cardio Medical Sales L.P the right to be the exclusive distributor of MGuard products in Belarus until December 2016, subject to the achievement of certain order minimums. Under our agreement with Cardio Medical Sales L.P, Cardio Medical Sales L.P is required to purchase 200 stents from us in 2014, 450 stents in 2015 and 490 stents in 2016. Cardio Medical Sales L.P met their order minimum in 2014.
| 21 |
Manufacturing and Suppliers
We manufacture our stainless steel stents through a combination of outsourcing and assembly at our own facility. Third parties in Germany manufacture the base stent and catheter materials, and we add our proprietary mesh sleeve to the stent. Our current exclusive product supplier is QualiMed Innovative Medizinprodukte GmbH. QualiMed Innovative Medizinprodukte GmbH is a specialized German stent manufacturer that electro polishes and crimps the stent onto a balloon catheter that creates the base for our stainless steel MGuard stents. QualiMed Innovative Medizinprodukte GmbH has agreed to take responsibility for verifying and validating the entire stent system by performing the necessary bench test and biocompatibility testing. During the production process, QualiMed Innovative Medizinprodukte GmbH is responsible for integrating the mesh covered stent with the delivery system, sterilization, packaging and labeling. Our manufacturing agreement with QualiMed Innovative Medizinprodukte GmbH expires in September 2017, unless earlier terminated by either party in the event of breach of material terms of the agreement, liquidation of the other party, our failure to receive requested products for more than 60 days, a substantiated intellectual property claim is brought against the other party or the development agreement between the parties is terminated. The manufacturing agreement provides for a rebate program that rewards us for increases in sales of our products.
The polymer fiber for MicroNet is supplied by Biogeneral, Inc., a San Diego, California-based specialty polymer manufacturer for medical and engineering applications.
Natec Medical Ltd. supplies us with catheters that help create the base for our MGuard stents. Our agreement with Natec Medical Ltd., which may be terminated by either party upon six months’ notice, calls for non-binding minimum orders and discounted catheters upon reaching certain purchasing thresholds.
Creganna-Tactx Medical, Ireland supplies us with over the wire catheters for CGuard EPS.
Vention Medical Advance Components, Boston, Massachusetts supplies us with rapid exchange catheters for CGuard EPS.
Our MGuard Prime cobalt-chromium stent was designed by Svelte Medical Systems Inc. We have an agreement with Svelte Medical Systems Inc. that grants us a non-exclusive, worldwide license for production and use of the MGuard Prime cobalt-chromium stent for the life of the stent’s patent, subject to the earlier termination of the agreement upon the bankruptcy of either party or the uncured default by either party under any material provision of the agreement. Our royalty payments to Svelte Medical Systems Inc. are determined by the sales volume of MGuard Prime stents. Until October 20, 2012, we paid a royalty of 7% for all product sales outside of the U.S. and, for products sales within the U.S., a rate of 7% for the first $10.0 million of sales and a rate of 10% for all sales exceeding $10.0 million. We also shared with Svelte Medical Systems Inc. in the cost of obtaining the CE mark approval, with its costs not to exceed $85,000, and the cost of obtaining U.S. Food and Drug Administration approval, with its costs not to exceed $200,000. On October 20, 2012, we amended our agreement with Svelte Medical Systems Inc., pursuant to which Svelte Medical Systems Inc. reduced the royalty rate to 2.9% of all net sales both inside and outside the U.S. in exchange for (i) us waiving the $85,000 in regulatory fees for the CE mark that were owed to us by Svelte Medical Systems Inc., (ii) us making full payment of royalties in the amount of $205,587 due to Svelte Medical Systems, Inc. as of September 30, 2012, and (iii) $1,763,000, payable in 215,000 shares of our common stock (as adjusted for the one-for-four reverse stock split of our common stock that occurred on December 21, 2012), that were valued at the closing price of our common stock on October 19, 2012 of $8.20 per share (as adjusted for the one-for-four reverse stock split of our common stock that occurred on December 21, 2012). On August 22, 2013, we further amended our agreement with Svelte Medical Systems Inc., pursuant to which (i) we agreed to pay Svelte Medical Systems Inc. an advanced payment of $192,000, representing a royalty rate of 2.0% of all net sales for the period from July 1, 2013 to June 30, 2015, assuming net sales of $1.2 million per quarter, (ii) we agreed to pay a royalty rate of 2.5% on any net sales exceeding $10.56 million for the period from July 1, 2013 to June 30, 2015 and (iii) the royalty rate was increased to 2.9% of all net sales beginning July 1, 2015. We have mutual indemnification obligations with Svelte Medical Systems Inc. for any damages suffered as a result of third party actions based upon breaches of representations and warranties or the failure to perform certain covenants in the license agreement, and Svelte Medical Systems Inc. will also indemnify us for any damages suffered as a result of third party actions based upon intellectual property or design claims against the MGuard Prime cobalt-chromium stent.
Our MGuard Prime cobalt-chromium stent and our CGuard carotid stents are being manufactured and supplied by MeKo Laserstrahl-Materialbearbeitung. Our agreement with MeKo Laserstrahl-Materialbearbeitung for the production of electro polished L605 bare-metal stents for MGuard Prime and CGuard EPS is priced on a per-stent basis, subject to the quantity of stents ordered. The complete assembly process for MGuard Prime and CGuard EPS, including knitting and securing the sleeve to the stent and the crimping of the sleeve stent on to a balloon catheter, is done at our Israel manufacturing site. Once MGuard Prime and CGuard EPS have been assembled, they are sent for sterilization in Germany and then back to Israel for final packaging.
| 22 |
Drug-eluting stents for our DES-MicroNet product will be supplied by existing drug-eluting stent manufacturers. We plan to develop two strategic partnerships with drug-eluting stent manufacturers who would supply U.S. Food and Drug Administration-approved or CE-marked stents.
Each MGuard stent is manufactured from two main components, the stent and the mesh polymer. The stent is made out of stainless steel or cobalt chromium. Both of these materials are readily available and we acquire them in the open market. The mesh is made from polyethylene terephthalate. This material is readily available in the market as well, because it is used for many medical applications. In the event that our supplier can no longer supply this material in fiber form, we would need to qualify another supplier, which could take several months. In addition, in order to retain the approval of the CE mark, we are required to perform periodic audits of the quality control systems of our key suppliers in order to insure that their products meet our predetermined specifications
A CGuard EPS consists of a CGuard stent and the delivery system. Each CGuard stent is manufactured from two main components, a self-expending stent and the mesh polymer. The stent is made out of nitinol. This material is readily available and we acquire it in the open market. The mesh is made from polyethylene terephthalate. We have pending patent rights that cover the proposed CGuard stent with mesh. This material is readily available in the market as well, because it is used for many medical applications. In the event that our supplier can no longer supply this material in fiber form, we would need to qualify another supplier, which could take several months. The delivery system for CGuard is made out of polymer tubes we acquire from an original equipment manufacturer. In the event that our supplier can no longer supply this material, we would need to qualify another supplier, which could take several months. In addition, in order to retain the approval of the CE mark, we are required to perform periodic audits of the quality control systems of our key suppliers in order to insure that their products meet our predetermined specifications.
Distributors
We currently have distribution agreements for our CE Mark-approved MGuard Coronary with bio stable mesh with medical product distributors based in Europe, the Middle East, Asia Pacific, Australia, South Africa and Latin America. We are currently in discussions with multiple distribution companies in Europe, Asia, and Latin America.
We are in the process of replacing certain third party distributors with direct sales channels in key countries where end user average selling prices and the lack of strong distributors are limiting factors. While we believe that this transition to direct selling will ultimately lead to greater sales in these markets, the transition away from certain distributors adversely impacted revenue for the twelve months ended December 31, 2014, the six months ended December 31, 2013 and the twelve months ended June 30, 2013, as we had fewer parties selling our products. In addition, we are in the process of appointing new distributors in certain territories, and believe that new incentives and broader responsibilities have strengthened arrangements with our partners in those territories.
Current and future agreements with distributors stipulate that, while we are responsible for training, providing marketing guidance, marketing materials, and technical guidance, distributors will be responsible for carrying out local registration, sales and marketing activities. In addition, in most cases, all sales costs, including sales representatives, incentive programs, and marketing trials, will be borne by the distributor. Under current agreements, distributors purchase stents from us at a fixed price. Our current agreements with distributors are generally for a term of approximately three years.
Employees
As of March 11, 2015, we had 55 full-time employees. Except for some of our employees in Europe, our employees are not party to any collective bargaining agreements. We do not expect the collective bargaining agreements to which our employees are party to have a material effect on our business or results of operations. We consider our relations with our employees to be good. We believe that our future success will depend, in part, on our continued ability to attract, hire and retain qualified personnel.
There are numerous and varied risks, known and unknown, that may prevent us from achieving our goals. You should carefully consider the risks described below and the other information included in this Annual Report on Form 10-K, including the consolidated financial statements and related notes. If any of the following risks, or any other risks not described below, actually occur, it is likely that our business, financial condition, and/or operating results could be materially adversely affected. The risks and uncertainties described below include forward-looking statements and our actual results may differ from those discussed in these forward-looking statements.
Risks Related to Our Business
We have a history of net losses and may experience future losses.
To date, we have experienced net losses. A substantial portion of the expenses associated with our manufacturing facilities are fixed in nature (i.e., depreciation) and will reduce our operating margin until such time, if ever, as we are able to increase utilization of our capacity through increased sales of our products. The clinical trials necessary to support our anticipated growth will be expensive and lengthy. In addition, our strategic plan will require a significant investment in clinical trials, product development and sales and marketing programs, which may not result in the accelerated revenue growth that we anticipate. Because we expect to continue incurring negative cash flows from operations, there can be no assurance that we will ever generate sufficient revenues to become profitable.
| 23 |
We may need to raise additional capital to meet our business requirements in the future and such capital raising may be costly or difficult to obtain and could dilute our stockholders’ ownership interests.
Because we have had recurring losses and negative cash flows from operating activities and have significant future commitments, our financial statements for the quarters ended September 30, 2014, June 30, 2014 and March 31, 2014 contained an explanatory paragraph in the footnotes, as to our ability to continue as a going concern. Our financial statements for the twelve months ended December 31, 2014 did not contain such disclosure following the offering closed on March 9, 2015 and cost reduction measures, however, in order to fully realize all of our business objectives, absent any non-dilutive funding from a strategic partner or some other strategic transaction, we may need to raise additional capital in the second quarter of 2016, which may not be available on reasonable terms or at all. For instance, we will need to raise additional funds to accomplish the following:
| · | developing CGuard, a drug-eluting stent with MicroNet, PVGuard and any additional products; |
| · | pursuing growth opportunities, including more rapid expansion; |
| · | acquiring complementary businesses; |
| · | making capital improvements to improve our infrastructure; |
| · | hiring qualified management and key employees; |
| · | developing new services, programming or products; |
| · | responding to competitive pressures; |
| · | complying with regulatory requirements such as licensing and registration; and |
| · | maintaining compliance with applicable laws. |
Any additional capital raised through the sale of equity or equity backed securities may dilute our stockholders’ ownership percentages and could also result in a decrease in the market value of our equity securities.
The terms of any securities issued by us in future capital transactions may be more favorable to new investors, and may include preferences, superior voting rights and the issuance of warrants or other derivative securities, which may have a further dilutive effect on the holders of any of our securities then outstanding.
Furthermore, any additional debt or equity financing that we may need may not be available on terms favorable to us, or at all. If we are unable to obtain such additional financing on a timely basis, we may have to curtail our development activities and growth plans and/or be forced to sell assets, perhaps on unfavorable terms, which would have a material adverse effect on our business, financial condition and results of operations, and ultimately could be forced to discontinue our operations and liquidate, in which event it is unlikely that stockholders would receive any distribution on their shares. Further, we may not be able to continue operating if we do not generate sufficient revenues from operations needed to stay in business.
In addition, we may incur substantial costs in pursuing future capital financing, including investment banking fees, legal fees, accounting fees, securities law compliance fees, printing and distribution expenses and other costs. We may also be required to recognize non-cash expenses in connection with certain securities we issue, such as convertible notes and warrants, which may adversely impact our financial condition.
The voluntary field action of our MGuard Prime EPS and any future recalls and/or product withdrawals due to product defects or product enhancements and modifications , could have a significant adverse impact on us.
The manufacturing and marketing of medical devices involves an inherent risk that our products may prove to be defective and cause a health risk even after regulatory clearances have been obtained. Medical devices may also be modified after regulatory clearance is obtained to such an extent that additional regulatory clearance is necessary before the device can be further marketed. In these events, we may voluntarily implement a recall or market withdrawal or may be required to do so by a regulatory authority.
| 24 |
On April 30, 2014 we initiated a voluntary field corrective action of our MGuard Prime EPS to address the issue of stent retention following reports of MGuard Prime EPS stent dislodgements in patients. Although there have been no reports of death or serious injury as a result of such dislodgements, we decided to suspend shipments of the MGuard Prime EPS and implement a field corrective action to enhance the reliability and performance of the affected product units in the field. As a result of our voluntary field action, we are subject to numerous risks and uncertainties, including the following:
| · | although we received European regulatory approval to resume manufacturing and distribution of our MGuard Prime EPS stent with a modified stent securement process, our suspension of shipments has and will continue to adversely impact revenue ; |
| · | we are more susceptible to claims such as products liability claims, distributor claims and class action lawsuits as a result of the reported product malfunction and voluntary field action, which could significantly increase our costs and may have a material adverse effect on our business, financial condition and results of operations; |
| · | the indirect costs associated with the voluntary field action and re-launch of our product are difficult to predict and will likely divert significant managerial, financial and other resources, which could have an adverse effect on our financial condition and operating results and could hinder our ability to carry out initiatives relating to other new products or product enhancements ; and |
| · | our decision to implement the voluntary field action and discontinue shipments, and any future action, may harm our reputation or the market’s perception of our products, which could have a negative impact on our future sales and our ability to generate profits. |
In the European Economic Area, we must comply with the EU Medical Device Vigilance System. Under this system, manufacturers are required to take Field Safety Corrective Actions (“FSCAs”) to reduce a risk of death or serious deterioration in the state of health associated with the use of a medical device that is already placed on the market. A FSCA may include the recall, modification, exchange, destruction or retrofitting of the device. FSCAs must be communicated by the manufacturer or its legal representative to its customers and/or to the end users of the device through Field Safety Notices.
Any adverse event involving our products could result in other future voluntary corrective actions, such as recalls or customer notifications, or agency action, such as inspection or enforcement action. Adverse events, such as the MGuard Prime EPS stent dislodgements, have been reported to us in the past, and we cannot guarantee that they will not occur in the future. Any corrective action, whether voluntary or involuntary, as well as defending ourselves in a lawsuit, would require the dedication of our time and capital, distract management from operating our business and could harm our reputation and financial results.
In addition to the foregoing, since we initiated our voluntary field action we have received a demand from one distributor that we refund approximately $160,000 in lieu of receiving refitted product and a demand from a second distributor to provide unspecified compensation for pre-paid goods subject to the voluntary field action, related costs and any third claims. We rejected such claims in a detailed response letter sent to both distributors. Following exchanges between the first distributor and our attorney, we have been negotiating a possible settlement of the dispute, which is currently still being discussed. We do not believe that these distributors are entitled to any compensation or refunds due to the voluntary field action and we intend to defend ourselves against any such claims.
We expect to derive our revenue from sales of our MGuard and CGuard stent products and other products we may develop. If we fail to generate revenue from these sources, our results of operations and the value of our business would be materially and adversely affected.
We expect our revenue to be generated from sales of our MGuard and CGuard stent products and other products we may develop. Future sales of these products, if any, will be subject to the receipt of regulatory approvals and commercial and market uncertainties that may be outside our control. Even if we are successful in development of a DES-MicroNet product or any other products we may develop, there can be no assurance that the product will gain market acceptance or prove to be commercially successful. If we fail to generate such revenues, our results of operations and the value of our business and securities would be materially and adversely affected.
If we are unable to obtain and maintain intellectual property protection covering our products, others may be able to make, use or sell our products, which would adversely affect our revenue.
Our ability to protect our products from unauthorized or infringing use by third parties depends substantially on our ability to obtain and maintain valid and enforceable patents. Similarly, the ability to protect our trademark rights might be important to prevent third party counterfeiters from selling poor quality goods using our designated trademarks/trade names. Due to evolving legal standards relating to the patentability, validity and enforceability of patents covering medical devices and pharmaceutical inventions and the scope of claims made under these patents, our ability to enforce patents is uncertain and involves complex legal and factual questions. Accordingly, rights under any of our pending patent applications and patents may not provide us with commercially meaningful protection for our products or may not afford a commercial advantage against our competitors or their competitive products or processes. In addition, patents may not be issued from any pending or future patent applications owned by or licensed to us, and moreover, patents that may be issued to us now or in the future may not be valid or enforceable. Further, even if valid and enforceable, our patents may not be sufficiently broad to prevent others from marketing products like ours, despite our patent rights.
| 25 |
The validity of our patent claims depends, in part, on whether prior art references exist that describe or render obvious our inventions as of the filing date of our patent applications. We may not have identified all prior art, such as U.S. and foreign patents or published applications or published scientific literature, that could adversely affect the patentability of our pending patent applications. For example, some material references may be in a foreign language and may not be uncovered during examination of our patent applications. Additionally, patent applications in the U.S. are maintained in confidence for up to 18 months after their filing. In some cases, however, patent applications remain confidential in the U.S. Patent and Trademark Office for the entire time prior to issuance as a U.S. patent. Patent applications filed in countries outside the U.S. are not typically published until at least 18 months from their first filing date. Similarly, publication of discoveries in the scientific or patent literature often lags behind actual discoveries. Therefore, we cannot be certain that we were the first to invent, or the first to file patent applications relating to, our stent technologies. In the event that a third party has also filed a U.S. patent application covering our stents or a similar invention, we may have to participate in an adversarial proceeding, known as an interference, declared by the U.S. Patent and Trademark Office to determine priority of invention in the U.S. It is possible that we may be unsuccessful in the interference, resulting in a loss of some portion or all of our position in the U.S.
In addition, statutory differences in patentable subject matter depending on the jurisdiction may limit the protection we obtain on certain of the technologies we develop. The laws of some foreign jurisdictions do not offer the same protection to, or may make it more difficult to effect the enforcement of, proprietary rights as in the U.S., risk that may be exacerbated if we move our manufacturing to certain countries in Asia. If we encounter such difficulties or are otherwise precluded from effectively protecting our intellectual property rights in any foreign jurisdictions, our business prospects could be substantially harmed.
We may initiate litigation to enforce our patent rights on any patents issued on pending patent applications, which may prompt adversaries in such litigation to challenge the validity, scope, ownership, or enforceability of our patents. Third parties can sometimes bring challenges against a patent holder to resolve these issues, as well. If a court decides that any such patents are not valid, not enforceable, not wholly owned by us, or are of a limited scope, we may not have the right to stop others from using our inventions. Also, even if our patent rights are determined by a court to be valid and enforceable, they may not be sufficiently broad to prevent others from marketing products similar to ours or designing around our patents, despite our patent rights, nor do they provide us with freedom to operate unimpeded by the patent and other intellectual property rights of others that may cover our products. We may be forced into litigation to uphold the validity of the claims in our patent portfolio, as well as our ownership rights to such intellectual property, and litigation is often an uncertain and costly process.
We also rely on trade secret protection to protect our interests in proprietary know-how and for processes for which patents are difficult to obtain or enforce. We may not be able to protect our trade secrets adequately. In addition, we rely on non-disclosure and confidentiality agreements with employees, consultants and other parties to protect, in part, trade secrets and other proprietary technology. These agreements may be breached and we may not have adequate remedies for any breach. Moreover, others may independently develop equivalent proprietary information, and third parties may otherwise gain access to our trade secrets and proprietary knowledge. Any disclosure of confidential data into the public domain or to third parties could allow competitors to learn our trade secrets and use the information in competition against us.
If our manufacturing facilities are unable to provide an adequate supply of products, our growth could be limited and our business could be harmed.
We currently manufacture our MGuard and CGuard products at our facility in Tel Aviv, Israel, and we have contracted with QualiMed Innovative Medizinprodukte GmbH, a German manufacturer, to assist in production of MGuard. If there were a disruption to our existing manufacturing facility, we would have no other means of manufacturing our MGuard or CGuard stents until we were able to restore the manufacturing capability at our facility or develop alternative manufacturing facilities. If we were unable to produce sufficient quantities of our MGuard or CGuard stents to meet market demand or for use in our current and planned clinical trials, or if our manufacturing process yields substandard stents, our development and commercialization efforts would be delayed.
Additionally, any damage to or destruction of our Tel Aviv facility or its equipment, prolonged power outage or contamination at our facility would significantly impair our ability to produce either MGuard or Cguard stents.
Finally, the production of our stents must occur in a highly controlled, clean environment to minimize particles and other yield and quality-limiting contaminants. In spite of stringent quality controls, weaknesses in process control or minute impurities in materials may cause a substantial percentage of defective products in a lot. If we are unable to maintain stringent quality controls, or if contamination problems arise, our clinical development and commercialization efforts could be delayed, which would harm our business and results of operations.
| 26 |
Pre-clinical and clinical trials will be lengthy and expensive, and any delay or failure of clinical trials could prevent us from commercializing our stent products, which would materially and adversely affect our results of operations and the value of our business.
As part of the regulatory process, we must conduct clinical trials for each product candidate to demonstrate safety and efficacy to the satisfaction of the regulatory authorities, including, if we seek in the future to sell our products in the United States, the U.S. Food and Drug Administration. Clinical trials are subject to rigorous regulatory requirements and are expensive and time-consuming to design and implement. They require the enrollment of a large number of patients, and suitable patients may be difficult to identify and recruit, which may cause a delay in the development and commercialization of our product candidates. In some trials, a greater number of patients and a longer follow-up period may be required. Patient enrollment in clinical trials and the ability to successfully complete patient follow-up depends on many factors, including the size of the patient population, the nature of the trial protocol, the proximity of patients to clinical sites, the eligibility criteria for the clinical trial and patient compliance. For example, patients may be discouraged from enrolling in our clinical trials if the trial protocol requires them to undergo extensive post-treatment procedures or follow-up to assess the safety and efficacy of our products, or they may be persuaded to participate in contemporaneous clinical trials of competitive products. In addition, patients participating in our clinical trials may die before completion of the trial or suffer adverse medical events unrelated to or related to our products. Delays in patient enrollment or failure of patients to continue to participate in a clinical trial may cause an increase in costs and delays or result in the failure of the clinical trial.
In addition, the length of time required to complete clinical trials for pharmaceutical and medical device products varies substantially according to the degree of regulation and the type, complexity, novelty and intended use of a product, and can continue for several years and cost millions of dollars. The commencement and completion of clinical trials for our existing products and those under development may be delayed by many factors, including governmental or regulatory delays and changes in regulatory requirements, policy and guidelines or our inability or the inability of any potential licensee to manufacture or obtain from third parties materials sufficient for use in preclinical studies and clinical trials.
For example, we decided to discontinue our MASTER II trial notwithstanding the resources we had spent on the trial due to the change in market demand and the delay in the U.S. Food and Drug Administration review process following the voluntary field corrective action. With respect to the drug-eluting stent incorporating MicroNet, it will take more than a year to complete the clinical trials, if required for CE mark approval, and submit the DES-MicroNet product for CE mark approval and begin to commercialize the product, even if the trials are successful.
Physicians may not widely adopt our stents unless they determine, based on experience, long-term clinical data and published peer reviewed journal articles, that the use of our stents provides a safe and effective alternative to other existing treatments for coronary artery disease.
We believe that physicians will not widely adopt our stents unless they determine, based on experience, long-term clinical data and published peer reviewed journal articles, that the use of our stents provides a safe and effective alternative to other existing treatments for coronary artery disease, including coronary artery bypass grafting balloon angioplasty, bare-metal stents and other drug-eluting stents, provided by Boston Scientific Corporation, Medtronic Inc., Abbott Laboratories and others, or to carotid endarterectomy or using conventional stenting for carotid artery disease.
We cannot provide any assurance that the data collected from our current and planned clinical trials will be sufficient to demonstrate that our stents are an attractive alternative to other procedures. If we fail to demonstrate safety and efficacy that is at least comparable to existing and future therapies available on the market, our ability to successfully market our stents will be significantly limited. Even if the data collected from clinical studies or clinical experience indicate positive results, each physician’s actual experience with our stents will vary. Clinical trials conducted with our stents have involved procedures performed by physicians who are technically proficient and are high-volume stent users. Consequently, both short-term and long-term results reported in these clinical trials may be significantly more favorable than typical results of practicing physicians, which could negatively affect rates of adoptions of our products. We also believe that published peer-reviewed journal articles and recommendations and support by influential physicians regarding our stents will be important for market acceptance and adoption, and we cannot assure you that we will receive these recommendations and support, or that supportive articles will be published.
Physicians currently consider drug-eluting stents to be the industry standard for treatment of coronary artery disease. None of our current products is a drug-eluting stent, and this may adversely affect our business.
Our ability to attract customers depends to a large extent on our ability to provide goods that meet the customers’ and the market’s demands and expectations. If we do not have a product that is expected by the market, we may lose customers. While physicians currently consider drug-eluting stents to be the industry standard for treatment of coronary artery disease, none of our stent products incorporates drug-eluting stents. Although we are in the process of developing a product incorporating a drug-eluting stent and MicroNet, there is no assurance that we will complete the development and commercialize the DES-MicroNet product. Our failure to provide industry standard devices could adversely affect our business, financial condition and results of operations.
Our products are based on a new technology, and we have only limited experience in regulatory affairs, which may affect our ability or the time required to navigate complex regulatory requirements and obtain necessary regulatory approvals, if such approvals are received at all. Regulatory delays or denials may increase our costs, cause us to lose revenue and materially and adversely affect our results of operations and the value of our business.
Because our products are new and long-term success measures have not been completely validated, regulatory agencies, including the U.S. Food and Drug Administration if we seek in the future to sell our products in the United States, may take a significant amount of time in evaluating product approval applications. For example, there are currently several methods of measuring restenosis and we do not know which of these metrics, or combination of these metrics, will be considered appropriate by applicable regulators for evaluating the clinical efficacy of stents. Treatments may exhibit a favorable measure using one of these metrics and an unfavorable measure using another metric. Any change in the accepted metrics may result in reconfiguration of, and delays in, our clinical trials. Additionally, we have only limited experience in filing and prosecuting the applications necessary to gain regulatory approvals, and our clinical, regulatory and quality assurance personnel are currently composed of only six employees. As a result, we may experience delays in connection with obtaining regulatory approvals for our products.
| 27 |
In addition, the products we and any potential licensees license, develop, manufacture and market are subject to complex regulatory requirements, particularly in the U.S., Europe and Asia, which can be costly and time-consuming. There can be no assurance that such approvals will be granted on a timely basis, if at all. Furthermore, there can be no assurance of continued compliance with all regulatory requirements necessary for the manufacture, marketing and sale of the products we will offer in each market where such products are expected to be sold, or that products we have commercialized will continue to comply with applicable regulatory requirements. If a government regulatory agency were to conclude that we were not in compliance with applicable laws or regulations, the agency could institute proceedings to detain or seize our products, issue a recall, impose operating restrictions, enjoin future violations and assess civil and criminal penalties against us, our officers or employees and could recommend criminal prosecution. Furthermore, regulators may proceed to ban, or request the recall, repair, replacement or refund of the cost of, any device manufactured or sold by us. Furthermore, there can be no assurance that all necessary regulatory approvals will be obtained for the manufacture, marketing and sale in any market of any new product developed or that any potential licensee will develop using our licensed technology.
Even if our products are approved by regulatory authorities, if we or our suppliers fail to comply with ongoing regulatory requirements, or if we experience unanticipated problems with our products, these products could be subject to restrictions or withdrawal from the market.
Any product for which we obtain marketing approval in the U.S., along with the manufacturing processes, post-approval clinical data and promotional activities for such product, will be subject to continual review and periodic inspections by the U.S. Food and Drug Administration and other regulatory bodies. In particular, we and our suppliers will be required to comply with the U.S. Food and Drug Administration’s Quality System Regulation, which covers the methods and documentation of the design, testing, production, control, quality assurance, labeling, packaging, storage and shipping of, any product for which we obtain marketing approval in the U.S. The U.S. Food and Drug Administration enforces the Quality System Regulation through unannounced inspections. We and our third-party manufacturers and suppliers have not yet been inspected by the U.S. Food and Drug Administration and will have to successfully complete such inspections before we receive U.S. regulatory approval for our products. Failure by us or one of our suppliers to comply with statutes and regulations administered by the U.S. Food and Drug Administration and other regulatory bodies, or failure to take adequate response to any observations, could result in, among other things, any of the following enforcement actions:
| · | warning letters or untitled letters; | |
| · | fines and civil penalties; | |
| · | unanticipated expenditures; | |
| · | delays in approving, or refusal to approve, our products; | |
| · | withdrawal or suspension of approval by the U.S. Food and Drug Administration or other regulatory bodies; | |
| · | product recall or seizure; | |
| · | orders for physician notification or device repair, replacement or refund; | |
| · | interruption of production; | |
| · | operating restrictions; | |
| · | injunctions; and | |
| · | criminal prosecution. |
If any of these actions were to occur, it could harm our reputation and could cause our product sales and profitability to suffer. Furthermore, key component suppliers may not currently be or may not continue to be in compliance with applicable regulatory requirements.
Even if regulatory approval of a product is granted in the U.S., the approval may be subject to limitations on the indicated uses for which the product may be marketed. If the U.S. Food and Drug Administration determines that our promotional materials, training or other activities constitute promotion of an unapproved use, it could request that we cease or modify our training or promotional materials or subject us to regulatory enforcement actions. It is also possible that other federal, state or foreign enforcement authorities might take action if they consider our training or other promotional materials to constitute promotion of an unapproved use, which could result in significant fines or penalties under other statutory authorities, such as laws prohibiting false claims for reimbursement.
Moreover, any modification to a device that has received U.S. Food and Drug Administration approval that could significantly affect its safety or effectiveness, or that would constitute a major change in its intended use, design or manufacture, requires a new approval from the U.S. Food and Drug Administration. If the U.S. Food and Drug Administration disagrees with any determination by us that new approval is not required, we may be required to cease marketing or to recall the modified product until approval is obtained. In addition, we could also be subject to significant regulatory fines or penalties.
Additionally, we may be required to conduct costly post-market testing and surveillance to monitor the safety or efficacy of our products, and we will be required to report adverse events and malfunctions related to our products. Later discovery of previously unknown problems with our products, including unanticipated adverse events or adverse events of unanticipated severity or frequency, manufacturing problems, or failure to comply with regulatory requirements, such as Quality System Regulation, may result in restrictions on such products or manufacturing processes, withdrawal of the products from the market, voluntary or mandatory recalls, fines, suspension of regulatory approvals, product seizures, injunctions or the imposition of civil or criminal penalties.
| 28 |
Further, healthcare laws and regulations may change significantly in the future. Any new healthcare laws or regulations may adversely affect our business. A review of our business by courts or regulatory authorities may result in a determination that could adversely affect our operations. In addition, the healthcare regulatory environment may change in a way that restricts our operations.
Failure to obtain regulatory approval in foreign jurisdictions will prevent us from marketing our products in such jurisdictions.
We market our products in international markets. In order to market our products in other foreign jurisdictions, we must obtain separate regulatory approvals from those obtained in the U.S. and Europe. The approval procedure varies among countries and can involve additional testing, and the time required to obtain approval may differ from that required to obtain CE Mark or U.S. Food and Drug Administration approval. Foreign regulatory approval processes may include all of the risks associated with obtaining CE Mark or U.S. Food and Drug Administration approval in addition to other risks. We may not obtain foreign regulatory approvals on a timely basis, if at all. CE Mark approval does not ensure approval by regulatory authorities in other countries. We may not be able to file for regulatory approvals and may not receive necessary approvals to commercialize our products in certain markets.
We operate in an intensely competitive and rapidly changing business environment, and there is a substantial risk our products could become obsolete or uncompetitive.
The medical device market is highly competitive. We compete with many medical device companies in the U.S. and internationally in connection with our current product and products under development. We face competition from numerous pharmaceutical and biotechnology companies in the therapeutics area, as well as competition from academic institutions, government agencies and research institutions. When we commercialize our products, we expect to face intense competition from Boston Scientific Corporation, Guidant Corporation, Medtronic, Inc., Abbott Vascular Devices, Johnson & Johnson, Terumo Medical Corporation, Covidien Ltd., Cordis Corporation and others. Most of our current and potential competitors, including but not limited to those listed above, have, and will continue to have, substantially greater financial, technological, research and development, regulatory and clinical, manufacturing, marketing and sales, distribution and personnel resources than we do. There can be no assurance that we will have sufficient resources to successfully commercialize our products, if and when they are approved for sale. The worldwide market for stent products is characterized by intensive development efforts and rapidly advancing technology. Our future success will depend largely upon our ability to anticipate and keep pace with those developments and advances. Current or future competitors could develop alternative technologies, products or materials that are more effective, easier to use or more economical than what we or any potential licensee develop. If our technologies or products become obsolete or uncompetitive, our related product sales and licensing revenue would decrease. This would have a material adverse effect on our business, financial condition and results of operations.
We may become subject to claims by much larger and better capitalized competitors seeking to invalidate our intellectual property or our rights thereto.
Based on the prolific litigation that has occurred in the stent industry and the fact that we may pose a competitive threat to some large and well-capitalized companies that own or control patents relating to stents and their use, manufacture and delivery, we believe that it is possible that one or more third parties will assert a patent infringement claim against the manufacture, use or sale of our stents based on one or more of these patents. These companies also own patents relating to the use of drugs to treat restenosis, stent architecture, catheters to deliver stents, and stent manufacturing and coating processes and compositions, as well as general delivery mechanism patents like rapid exchange that might be alleged to cover one or more of our products. A number of stent-related patents are owned by very large and well-capitalized companies that are active participants in the stent market. For example, we are aware of one public company that is pursuing patent protection directed to layered materials disposed over a particular stent configuration. In addition, it is possible that a lawsuit asserting patent infringement, misappropriation of intellectual property, or related claims may have already been filed against us of which we are not aware. As the number of competitors in the stent market grows, the possibility of patent infringement by us, and/or a patent infringement or misappropriation claim against us, increases.
These companies have maintained their position in the market by, among other things, establishing intellectual property rights relating to their products and enforcing these rights aggressively against their competitors and new entrants into the market. All of the major companies in the stent and related markets, including Boston Scientific Corporation and Medtronic, Inc., have been repeatedly involved in patent litigation relating to stents since at least 1997. The stent and related markets have experienced rapid technological change and obsolescence in the past, and our competitors have strong incentives to stop or delay the introduction of new products and technologies. We may pose a competitive threat to many of the companies in the stent and related markets. Accordingly, many of these companies will have a strong incentive to take steps, through patent litigation or otherwise, to prevent us from commercializing our products. Such litigation or claims would divert attention and resources away from the development and/or commercialization of our product and product development, and could result in an adverse court judgment that would make it impossible or impractical to sell our products in one or more territories.
| 29 |
If we fail to maintain or establish satisfactory agreements with suppliers or if we experience an interruption of the supply of materials from suppliers, we may not be able to obtain materials that are necessary to develop our products.
We depend on outside suppliers for certain raw materials. These raw materials or components may not always be available at our standards or on acceptable terms, if at all, and we may be unable to locate alternative suppliers or produce necessary materials or components on our own.
Some of the components of our products are currently provided by only one vendor, or a single-source supplier. For MGuard, we depend on QualiMed Innovative Medizinprodukte GmbH, which manufactures the body of the stent, MeKo Laserstrahl-Materialbearbeitung for the laser cutting of the stent, Natec Medical Ltd. and Creganna-Tactx Medical, Ireland for the supply of catheters, and Biogeneral Inc. for the fiber. For CGuard EPS, we depend on Vention Medical Advance Components for the supply of rapid exchange catheters. We may have difficulty obtaining similar components from other suppliers that are acceptable to the U.S. Food and Drug Administration or foreign regulatory authorities if it becomes necessary.
If we have to switch to a replacement supplier, we will face additional regulatory delays and the interruption of the manufacture and delivery of our stents for an extended period of time, which would delay completion of our clinical trials or commercialization of our products. In addition, we will be required to obtain prior regulatory approval from the U.S. Food and Drug Administration or foreign regulatory authorities to use different suppliers or components that may not be as safe or as effective. As a result, regulatory approval of our products may not be received on a timely basis or at all.
Our relationship with our strategic partners in connection with the DES-MicroNet product development may not prove successful.
We plan to develop the DES-MicroNet product with two strategic partners who would supply U.S. Food and Drug Administration-approved or CE-marked drug-eluting stents. Our successful development of the DES-MicroNet product will depend, among other things, on our partners’ ability to supply drug-eluting stents that we may require. Our partners may not be able to supply us with drug-eluting stents due to bankruptcy, insolvency, liquidation, or reorganization; a lawsuit asserting patent infringement, misappropriation of intellectual property, or related claims filed against them; or failure to comply with ongoing regulatory requirements. If our partners are unable to produce sufficient quantities of drug-eluting stents for use in our current and planned clinical trials, or if their manufacturing process yields substandard stents, our development and commercialization efforts would be delayed and could increase our costs.
We may be exposed to product liability claims and insurance may not be sufficient to cover these claims.
We may be exposed to product liability claims based on the use of any of our products, or products incorporating our licensed technology, in clinical trials. We may also be exposed to product liability claims based on the sale of any such products following the receipt of regulatory approval. Product liability claims could be asserted directly by consumers, health-care providers or others. We have obtained product liability insurance coverage; however such insurance may not provide full coverage for our future clinical trials, products to be sold, and other aspects of our business. We also have liability insurance for our ongoing clinical trials. Insurance coverage is becoming increasingly expensive and we may not be able to maintain current coverage, or expand our insurance coverage to include future clinical trials or the sale of products incorporating our licensed technology if marketing approval is obtained for such products, at a reasonable cost or in sufficient amounts to protect against losses due to product liability or at all. A successful product liability claim or series of claims brought against us could result in judgments, fines, damages and liabilities that could have a material adverse effect on our business, financial condition and results of operations. We may incur significant expense investigating and defending these claims, even if they do not result in liability. Moreover, even if no judgments, fines, damages or liabilities are imposed on us, our reputation could suffer, which could have a material adverse effect on our business, financial condition and results of operations.
The successful management of operations depends on our ability to attract and retain talented personnel.
We depend on the expertise of our senior management and research personnel, which would be difficult to replace. The loss of the services of any of our senior management could compromise our ability to achieve our objectives. Furthermore, recruiting and retaining qualified personnel will be crucial to future success. There can be no assurance that we will be able to attract and retain necessary personnel on acceptable terms given the competition among medical device, biotechnology, pharmaceutical and healthcare companies, universities and non-profit research institutions for experienced management, scientists, researchers, sales and marketing and manufacturing personnel. If we are unable to attract, retain and motivate our key personnel, our operations may be jeopardized and our results of operations may be materially and adversely affected.
We are an international business, and we are exposed to various global and local risks that could have a material adverse effect on our financial condition and results of operations.
We operate globally and develop and manufacture products in multiple countries. Consequently, we face complex legal and regulatory requirements in multiple jurisdictions, which may expose us to certain financial and other risks. International sales and operations are subject to a variety of risks, including:
| · | foreign currency exchange rate fluctuations; | |
| · | greater difficulty in staffing and managing foreign operations; |
| 30 |
| · | greater risk of uncollectible accounts; |
| · | longer collection cycles; |
| · | logistical and communications challenges; |
| · | potential adverse changes in laws and regulatory practices, including export license requirements, trade barriers, tariffs and tax laws; |
| · | changes in labor conditions; |
| · | burdens and costs of compliance with a variety of foreign laws; |
| · | political and economic instability; |
| · | the escalation of hostilities in Israel, which could impair our ability to manufacture our products |
| · | increases in duties and taxation; |
| · | foreign tax laws and potential increased costs associated with overlapping tax structures; |
| · | greater difficulty in protecting intellectual property; |
| · | the risk of third party disputes over ownership of intellectual property and infringement of third party intellectual property by our products; and |
| · | general economic and political conditions in these foreign markets. |
Further, in the past, the State of Israel and Israeli companies have been subjected to an economic boycott. Several countries still restrict business and trade activity with the State of Israel and with Israeli companies. These restrictive laws and policies may have an adverse impact on our operating results, financial condition or the expansion of our business.
International markets are also affected by economic pressure to contain reimbursement levels and healthcare costs. Profitability from international operations may be limited by risks and uncertainties related to regional economic conditions, regulatory and reimbursement approvals, competing products, infrastructure development, intellectual property rights protection and our ability to implement our overall business strategy. We expect these risks will increase as we pursue our strategy to expand operations into new geographic markets. We may not succeed in developing and implementing effective policies and strategies in each location where we conduct business. Any failure to do so may harm our business, results of operations and financial condition.
If we fail to obtain an adequate level of reimbursement for our products by third party payors, there may be no commercially viable markets for our product candidates or the markets may be much smaller than expected.
The availability and levels of reimbursement by governmental and other third party payors affect the market for our product candidates. The efficacy, safety, performance and cost-effectiveness of our product candidates and of any competing products will determine the availability and level of reimbursement. Reimbursement and healthcare payment systems in international markets vary significantly by country, and include both government sponsored healthcare and private insurance. To obtain reimbursement or pricing approval in some countries, we may be required to produce clinical data, which may involve one or more clinical trials, that compares the cost-effectiveness of our products to other available therapies. We may not obtain international reimbursement or pricing approvals in a timely manner, if at all. Our failure to receive international reimbursement or pricing approvals would negatively impact market acceptance of our products in the international markets in which those approvals are sought.
We believe that future reimbursement may be subject to increased restrictions both in the U.S. and in international markets. There is increasing pressure by governments worldwide to contain health care costs by limiting both the coverage and the level of reimbursement for therapeutic products and by refusing, in some cases, to provide any coverage for products that have not been approved by the relevant regulatory agency. Future legislation, regulation or reimbursement policies of third party payors may adversely affect the demand for our products currently under development and limit our ability to sell our product candidates on a profitable basis. In addition, third party payors continually attempt to contain or reduce the costs of healthcare by challenging the prices charged for healthcare products and services. If reimbursement for our products is unavailable or limited in scope or amount or if pricing is set at unsatisfactory levels, market acceptance of our products would be impaired and future revenues, if any, would be adversely affected.
In the U.S. and in the European Union, our business could be significantly and adversely affected by recent healthcare reform legislation and other administration and legislative proposals.
The Patient Protection and Affordable Care Act and the Health Care and Education Reconciliation Act were enacted into law in the U.S. in March 2010. Certain provisions of these acts will not be fully implemented until 2018 for a number of years and there are many programs and requirements for which the details have not yet been fully established or consequences not fully understood, and it is unclear what the full impacts will be from the legislation. The legislation levies a 2.3% excise tax, that began on January 1, 2013, on all sales of any U.S. medical device listed with the U.S. Food and Drug Administration under Section 510(j) of the Federal Food, Drug, and Cosmetic Act and 21 C.F.R. Part 807, unless the device falls within an exemption from the tax, such as the exemption governing direct retail sale of devices to consumers or for foreign sales of these devices. If we commence sales of our MGuard or CGuard stent in the U.S., this new tax may materially and adversely affect our business and results of operations. The legislation also focuses on a number of Medicare provisions aimed at improving quality and decreasing costs. It is uncertain at this point what negative unintended consequences these provisions will have on patient access to new technologies. The Medicare provisions include value-based payment programs, increased funding of comparative effectiveness research, reduced hospital payments for avoidable readmissions and hospital acquired conditions, and pilot programs to evaluate alternative payment methodologies that promote care coordination (such as bundled physician and hospital payments). Additionally, the provisions include a reduction in the annual rate of inflation for hospitals which started in 2011 and the establishment of an independent payment advisory board to recommend ways of reducing the rate of growth in Medicare spending. We cannot predict what healthcare programs and regulations will be ultimately implemented at the federal or state level in the U.S., or the effect of any future legislation or regulation. However, any changes that lower reimbursements for our products or reduce medical procedure volumes could adversely affect our business plan to introduce our products in the U.S.
| 31 |
On September 26, 2012, the European Commission adopted a package of legislative proposals designed to replace the existing regulatory framework governing medical devices in the European Union. These proposals are currently being reviewed by the European Parliament and the Council and may undergo significant amendments as part of the legislative process. If adopted by the European Parliament and the Council in their present form, these proposed revisions would, among other things, impose stricter requirements on medical device manufacturers and strengthen the supervising competences of the competent authorities of European Union Member States and the notified bodies. As a result, if and when adopted, the proposed new legislation could prevent or delay the CE marking of our products under development or impact our ability to modify our currently CE marked products on a timely basis. The regulation of advanced therapy medicinal products is also in continued development in the European Union, with the European Medicines Agency publishing new clinical or safety guidelines concerning advanced therapy medicinal products on a regular basis. Any of these regulatory changes and events could limit our ability to form collaborations and our ability to continue to commercialize our products, and if we fail to comply with any such new or modified regulations and requirements it could adversely affect our business, operating results and prospects.
Our strategic business plan may not produce the intended growth in revenue and operating income.
Our strategies include making significant investments in sales and marketing programs to achieve revenue growth and margin improvement targets. If we do not achieve the expected benefits from these investments or otherwise fail to execute on our strategic initiatives, we may not achieve the growth improvement we are targeting and our results of operations may be adversely affected.
| 32 |
In addition, as part of our strategy for growth, we may make acquisitions and enter into strategic alliances such as joint ventures and joint development agreements. However, we may not be able to identify suitable acquisition candidates, complete acquisitions or integrate acquisitions successfully, and our strategic alliances may not prove to be successful. In this regard, acquisitions involve numerous risks, including difficulties in the integration of the operations, technologies, services and products of the acquired companies and the diversion of management’s attention from other business concerns. Although we will endeavor to evaluate the risks inherent in any particular transaction, there can be no assurance that we will properly ascertain all such risks. In addition, acquisitions could result in the incurrence of substantial additional indebtedness and other expenses or in potentially dilutive issuances of equity securities. There can be no assurance that difficulties encountered with acquisitions will not have a material adverse effect on our business, financial condition and results of operations.
We may have violated Israeli securities law.
We may have violated section 15 of the Israeli Securities Law of 1968. Section 15 of the Israeli Securities Law of 1968 requires the filing of a prospectus with the Israel Securities Authority and the delivery thereof to offerees in connection with an offer or sale of securities to more than 35 offerees (where for the purpose of calculating such number, offerees of the type listed on the First Addendum of the Israeli Securities Law of 1968 shall not be taken into account) during any 12-month period. We allegedly issued securities to more than 35 investors during certain 12-month periods, ending in October 2008. Our wholly-owned subsidiary, InspireMD Ltd., a private company incorporated under the laws of the State of Israel, applied for a no-action determination from the Israel Security Authority on February 14, 2011 in connection with the foregoing. To date, the Israel Securities Authority has not responded to InspireMD Ltd.’s application for no-action determination and we are unable to predict when a response will be received. The maximum penalties for violating section 15 of the Israeli Securities Law of 1968 are as follows: imprisonment of five years; a fine of up to approximately $317,000 to be paid by management of the violating company; and a fine of up to approximately $1,590,000 to be paid by the violating company, any of which penalties could result in a material adverse effect on our operations. We believe that it is unlikely that either we or any individual will be subject to fines or other penalties as a result of these alleged violations.
Risks Related to Operating in Israel
We anticipate being subject to fluctuations in currency exchange rates because we expect a substantial portion of our revenues will be generated in Euros and U.S. dollars, while a significant portion of our expenses will be incurred in New Israeli Shekels.
We expect a substantial portion of our revenues will be generated in U.S. dollars and Euros, while a significant portion of our expenses, principally salaries and related personnel expenses, is paid in New Israeli Shekels, or NIS. As a result, we are exposed to the risk that the rate of inflation in Israel will exceed the rate of devaluation of the NIS in relation to the Euro or the U.S. dollar, or that the timing of this devaluation will lag behind inflation in Israel. Because inflation has the effect of increasing the dollar and Euro costs of our operations, it would therefore have an adverse effect on our dollar-measured results of operations. The value of the NIS, against the Euro, the U.S. dollar, and other currencies may fluctuate and is affected by, among other things, changes in Israel’s political and economic conditions. Any significant revaluation of the NIS may materially and adversely affect our cash flows, revenues and financial condition. Fluctuations in the NIS exchange rate, or even the appearance of instability in such exchange rate, could adversely affect our ability to operate our business.
If there are significant shifts in the political, economic and military conditions in Israel and its neighbors, it could have a material adverse effect on our business relationships and profitability.
Our sole manufacturing facility and certain of our key personnel are located in Israel. Our business is directly affected by the political, economic and military conditions in Israel and its neighbors. Since the establishment of the State of Israel in 1948, a number of armed conflicts have occurred between Israel and its Arab neighbors. A state of hostility, varying in degree and intensity, has caused security and economic problems in Israel. Although Israel has entered into peace treaties with Egypt and Jordan, and various agreements with the Palestinian Authority, there has been a marked increase in violence, civil unrest and hostility, including armed clashes, between the State of Israel and the Palestinians since September 2000. The establishment in 2006 of a government in the Gaza Strip by representatives of the Hamas militant group has created heightened unrest and uncertainty in the region. In mid-2006, Israel engaged in an armed conflict with Hezbollah, a Shiite Islamist militia group based in Lebanon, and in June 2007, there was an escalation in violence in the Gaza Strip. From December 2008 through January 2009 and again in November and December 2012, Israel engaged in an armed conflict with Hamas, which involved missile strikes against civilian targets in various parts of Israel and negatively affected business conditions in Israel. In July 2014, Israel launched an additional operation against Hamas operatives in the Gaza strip in response to Palestinian groups launching rockets at Israel. Recent political uprisings and social unrest in Syria are affecting its political stability, which has led to the deterioration of the political relationship between Syria and Israel and have raised new concerns regarding security in the region and the potential for armed conflict. Similar civil unrest and political turbulence is currently ongoing in many countries in the region. The continued political instability and hostilities between Israel and its neighbors and any future armed conflict, terrorist activity or political instability in the region could adversely affect our operations in Israel and adversely affect the market price of our shares of common stock. In addition, several countries restrict doing business with Israel and Israeli companies have been and are today subjected to economic boycotts. The interruption or curtailment of trade between Israel and its present trading partners could adversely affect our business, financial condition and results of operations.
| 33 |
In addition, some of our officers or key employees may be called to active duty at any time under emergency circumstances for extended periods of time. See “—Our operations could be disrupted as a result of the obligation of certain of our personnel residing in Israel to perform military service.”
Our operations could be disrupted as a result of the obligation of certain of our personnel residing in Israel to perform military service.
Some of our officers and employees reside in Israel and may be required to perform annual military reserve duty. Currently, all male adult citizens and permanent residents of Israel under the age of 40 (or older, depending on their position with the Israeli Defense Forces reserves), unless exempt, are obligated to perform military reserve duty annually and are subject to being called to active duty at any time under emergency circumstances. Our operations could be disrupted by the absence for a significant period of one or more of our key officers and employees due to military service. Any such disruption could have a material adverse effect on our business, results of operations and financial condition.
We may not be able to enforce covenants not-to-compete under current Israeli law.
We have non-competition agreements with most of our employees, many of which are governed by Israeli law. These agreements generally prohibit our employees from competing with us or working for our competitors for a specified period following termination of their employment. However, Israeli courts are reluctant to enforce non-compete undertakings of former employees and tend, if at all, to enforce those provisions for relatively brief periods of time in restricted geographical areas and only when the employee has unique value specific to that employer’s business and not just regarding the professional development of the employee. Any such inability to enforce non-compete covenants may cause us to lose any competitive advantage resulting from advantages provided to us by such confidential information.
We may become subject to claims for remuneration or royalties for assigned service invention rights by our employees, which could result in litigation and adversely affect our business.
A significant portion of our intellectual property has been developed by our Israeli employees in the course of their employment for us. Under the Israeli Patent Law, 5727-1967 (the “Israeli Patent Law”), inventions conceived by an employee during the term and as part of the scope of his or her employment with a company are regarded as "service inventions," which belong to the employer, absent a specific agreement between the employee and employer giving the employee service invention rights. The Israeli Patent Law also provides that if there is no such agreement between an employer and an employee, the Israeli Compensation and Royalties Committee (the “C&R Committee”), a body constituted under the Israeli Patent Law, shall determine whether the employee is entitled to remuneration for his inventions. The C&R Committee (decisions of which have been upheld by the Israeli Supreme Court) has held that employees may be entitled to remuneration for their service inventions despite having specifically waived any such rights. Further, the C&R Committee has not yet set specific guidelines regarding the method for calculating this remuneration or the criteria or circumstances under which an employee’s waiver of his right to remuneration will be disregarded. We generally enter into intellectual property assignment agreements with our employees pursuant to which such employees assign to us all rights to any inventions created in the scope of their employment or engagement with us. Although our employees have agreed to assign to us service invention rights and have specifically waived their right to receive any special remuneration for such assignment beyond their regular salary and benefits, we may face claims demanding remuneration in consideration for assigned inventions. As a consequence of such claims, we could be required to pay additional remuneration or royalties to our current or former employees, or be forced to litigate such claims, which could negatively affect our business.
It may be difficult for investors in the U.S. to enforce any judgments obtained against us or some of our directors or officers.
The majority of our assets are located outside the U.S. In addition, certain of our officers are nationals and/or residents of countries other than the U.S., and all or a substantial portion of such persons’ assets are located outside the U.S. As a result, it may be difficult for investors to enforce within the U.S. any judgments obtained against us or any of our non-U.S. officers, including judgments predicated upon the civil liability provisions of the securities laws of the U.S. or any state thereof. Additionally, it may be difficult to assert U.S. securities law claims in actions originally instituted outside of the U.S. Israeli courts may refuse to hear a U.S. securities law claim because Israeli courts may not be the most appropriate forums in which to bring such a claim. Even if an Israeli court agrees to hear a claim, it may determine that the Israeli law, and not U.S. law, is applicable to the claim. Further, if U.S. law is found to be applicable, certain content of applicable U.S. law must be proved as a fact, which can be a time-consuming and costly process, and certain matters of procedure would still be governed by the Israeli law. Consequently, you may be effectively prevented from pursuing remedies under U.S. federal and state securities laws against us or any of our non-U.S. directors or officers.
The tax benefits that are currently available to us under Israeli law require us to satisfy specified conditions. If we fail to satisfy these conditions, we may be required to pay increased taxes and would likely be denied these benefits in the future.
InspireMD Ltd. has been granted a “Beneficiary Enterprise” status by the Investment Center in the Israeli Ministry of Industry Trade and Labor, and we are therefore eligible for tax benefits under the Israeli Law for the Encouragement of Capital Investments, 1959. The main benefit is a two-year exemption from corporate tax, commencing when we begin to generate net income derived from the beneficiary activities in facilities located in Israel, and a reduced corporate tax rate for an additional five years, depending on the level of foreign investment in each year. In addition, under the January 1, 2011 amendment to the Israeli Law for the Encouragement of Capital Investments, 1959, a uniform corporate tax rate of 16% applies to all qualifying income of “Preferred Enterprise,” which we may be able to apply as an alternative tax benefit.
| 34 |
The tax benefits available to a Beneficiary Enterprise or a Preferred Enterprise are dependent upon the fulfillment of conditions stipulated under the Israeli Law for the Encouragement of Capital Investments, 1959 and its regulations, as amended, which include, among other things, maintaining our manufacturing facilities in Israel. If we fail to comply with these conditions, in whole or in part, the tax benefits could be cancelled and we could be required to refund any tax benefits that we received in the past. If we are no longer eligible for these tax benefits, our Israeli taxable income would be subject to regular Israeli corporate tax rates. The standard corporate tax rate for Israeli companies in 2014 is 26.5% of taxable income. The termination or reduction of these tax benefits would increase our tax liability, which would reduce our profits.
In addition to losing eligibility for tax benefits currently available to us under Israeli law, if we do not maintain our manufacturing facilities in Israel, we will not be able to realize certain tax credits and deferred tax assets, if any, including any net operating losses to offset against future profits.
The tax benefits available to Beneficiary Enterprises may be reduced or eliminated in the future. This would likely increase our tax liability.
The Israeli government may reduce or eliminate in the future tax benefits available to Beneficiary enterprises and Preferred Enterprises. Our Beneficiary Enterprise status and the resulting tax benefits may not continue in the future at their current levels or at any level. The 2011 amendment regarding Preferred Enterprise may not be applicable to us or may not fully compensate us for the change. The termination or reduction of these tax benefits would likely increase our tax liability. The amount, if any, by which our tax liability would increase will depend upon the rate of any tax increase, the amount of any tax benefit reduction, and the amount of any taxable income that we may earn in the future.
Risks Related to Our Organization and Our Common Stock
Our stock price has been and may continue to be volatile, which could result in substantial losses for investors.
The market price of our common stock has been and is likely to continue to be highly volatile and could fluctuate widely in response to various factors, many of which are beyond our control, including the following:
| · | technological innovations or new products and services by us or our competitors; |
| · | additions or departures of key personnel; |
| · | sales of our common stock, particularly under any registration statement for the purposes of selling any other securities, including management shares; |
| · | limited availability of freely-tradable “unrestricted” shares of our common stock to satisfy purchase orders and demand; |
| · | our ability to execute our business plan; |
| · | operating results that fall below expectations; |
| · | loss of any strategic relationship; |
| · | industry developments; |
| · | economic, political and other external factors; and |
| · | period-to-period fluctuations in our financial results. |
In addition, the securities markets have from time to time experienced significant price and volume fluctuations that are unrelated to the operating performance of particular companies. These market fluctuations may also significantly affect the market price of our common stock.
Our common stock could be delisted from the NYSE MKT if we fail to regain compliance with the NYSE MKT’s continued listing standards on the schedule required by the NYSE MKT.
On January 20, 2015, we received a notice indicating that we do not meet certain of the NYSE MKT’s continued listing standards as set forth in Part 10 of the NYSE MKT Company Guide (“Company Guide”). Specifically, we are not in compliance with Section 1003(a)(iii) of the Company Guide because we reported stockholders’ equity of less than $6 million as of September 30, 2014 and had net losses in our five most recent fiscal years. In addition, the NYSE MKT indicated that we are not in compliance with Section 1003(a)(iv) of the Company Guide because we have sustained losses that are substantial in relation to our overall operations or our existing financial resources, or our financial condition has become impaired such that it appears questionable, in the opinion of the NYSE MKT, as to whether we will be able to continue operations and/or meet our obligations as they mature. As a result, we have become subject to the procedures and requirements of Section 1009 of the Company Guide.
In order to maintain our listing on the Exchange, we submitted a plan of compliance to the NYSE MKT on February 19, 2015 addressing how we intend to regain compliance with Section 1003(a)(iii) of the Company Guide by July 20, 2016 and Section 1003(a)(iv) of the Company Guide by June 1, 2015. On March 9, 2015, we closed a public offering of our common stock and warrants that resulted in net proceeds of approximately $12.5 million after deducting placement agent fees and other estimated offering expenses. We believe that this will bring us back into compliance with Section 1003(a)(iv) and Section 1003(a)(iii) as of the end of the first quarter of 2015.
| 35 |
If our compliance plan is not accepted, delisting proceedings will commence. Furthermore, if the plan is accepted but we do not maintain compliance with the continued listing standards by June 1, 2015 for Section 1003(a)(iv) of the Company Guide and July 20, 2016 for Section 1003(a)(iii) of the Company Guide, or if we do not maintain our progress consistent with the plan during the applicable plan period, the NYSE MKT will initiate delisting proceedings. The market price and liquidity of our common stock could be adversely affected by the commencement of such proceedings. If those proceedings resulted in delisting of our common stock and resulting cessation of trading of the stock on the NYSE MKT, we believe that the market price and liquidity of our common stock would be adversely affected.
We do not expect to pay dividends in the future. As a result, any return on investment may be limited to the value of our common stock.
We do not anticipate paying cash dividends on our common stock in the foreseeable future. The payment of dividends on our common stock will depend on our earnings, financial condition and other business and economic factors as our board of directors may consider relevant. We are also subject to certain restrictions pursuant to our loan and security agreement with Hercules Technology Growth Capital, Inc., which prohibits us from paying dividends or distributions on our common stock. If we do not pay dividends, our common stock may be less valuable because a return on an investment in our common stock will only occur if our stock price appreciates.
We are subject to financial reporting and other requirements that place significant demands on our resources.
We are subject to reporting and other obligations under the Securities Exchange Act of 1934, as amended, including the requirements of Section 404 of the Sarbanes-Oxley Act of 2002. Section 404 requires us to conduct an annual management assessment of the effectiveness of our internal controls over financial reporting. It also requires an independent registered public accounting firm to test our internal control over financial reporting and report on the effectiveness of such controls. These reporting and other obligations place significant demands on our management, administrative, operational, internal audit and accounting resources. Any failure to maintain effective internal controls could have a material adverse effect on our business, operating results and stock price. Moreover, effective internal control is necessary for us to provide reliable financial reports and prevent fraud. If we cannot provide reliable financial reports or prevent fraud, we may not be able to manage our business as effectively as we would if an effective control environment existed, and our business and reputation with investors may be harmed.
There are inherent limitations in all control systems, and misstatements due to error or fraud may occur and not be detected.
The ongoing internal control provisions of Section 404 of the Sarbanes-Oxley Act of 2002 require us to identify of material weaknesses in internal control over financial reporting, which is a process to provide reasonable assurance regarding the reliability of financial reporting for external purposes in accordance with accounting principles generally accepted in the U.S. Our management, including our chief executive officer and chief financial officer, does not expect that our internal controls and disclosure controls will prevent all errors and all fraud. A control system, no matter how well conceived and operated, can provide only reasonable, not absolute, assurance that the objectives of the control system are met. In addition, the design of a control system must reflect the fact that there are resource constraints and the benefit of controls must be relative to their costs. Because of the inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that all control issues and instances of fraud, if any, in our company have been detected. These inherent limitations include the realities that judgments in decision-making can be faulty and that breakdowns can occur because of simple errors or mistakes. Further, controls can be circumvented by individual acts of some persons, by collusion of two or more persons, or by management override of the controls. The design of any system of controls is also based in part upon certain assumptions about the likelihood of future events, and there can be no assurance that any design will succeed in achieving its stated goals under all potential future conditions. Over time, a control may be inadequate because of changes in conditions, such as growth of the company or increased transaction volume, or the degree of compliance with the policies or procedures may deteriorate. Because of inherent limitations in a cost-effective control system, misstatements due to error or fraud may occur and not be detected.
In addition, discovery and disclosure of a material weakness, by definition, could have a material adverse impact on our financial statements. Such an occurrence could discourage certain customers or suppliers from doing business with us, cause downgrades in our future debt ratings leading to higher borrowing costs and affect how our stock trades. This could in turn negatively affect our ability to access public debt or equity markets for capital.
Delaware law and our corporate charter and bylaws contain anti-takeover provisions that could delay or discourage takeover attempts that stockholders may consider favorable.
Our board of directors is authorized to issue shares of preferred stock in one or more series and to fix the voting powers, preferences and other rights and limitations of the preferred stock. Accordingly, we may issue shares of preferred stock with a preference over our common stock with respect to dividends or distributions on liquidation or dissolution, or that may otherwise adversely affect the voting or other rights of the holders of common stock. Issuances of preferred stock, depending upon the rights, preferences and designations of the preferred stock, may have the effect of delaying, deterring or preventing a change of control, even if that change of control might benefit our stockholders. In addition, we are subject to Section 203 of the Delaware General Corporation Law. Section 203 generally prohibits a public Delaware corporation from engaging in a “business combination” with an “interested stockholder” for a period of three years after the date of the transaction in which the person became an interested stockholder, unless (i) prior to the date of the transaction, the board of directors of the corporation approved either the business combination or the transaction which resulted in the stockholder becoming an interested stockholder; (ii) the interested stockholder owned at least 85% of the voting stock of the corporation outstanding at the time the transaction commenced, excluding for purposes of determining the number of shares outstanding (a) shares owned by persons who are directors and also officers and (b) shares owned by employee stock plans in which employee participants do not have the right to determine confidentially whether shares held subject to the plan will be tendered in a tender or exchange offer; or (iii) on or subsequent to the date of the transaction, the business combination is approved by the board and authorized at an annual or special meeting of stockholders, and not by written consent, by the affirmative vote of at least 66 2/3% of the outstanding voting stock which is not owned by the interested stockholder.
| 36 |
Section 203 could delay or prohibit mergers or other takeover or change in control attempts with respect to us and, accordingly, may discourage attempts to acquire us even though such a transaction may offer our stockholders the opportunity to sell their stock at a price above the prevailing market price.
Offers or availability for sale of a substantial number of shares of our common stock may cause the price of our common stock to decline.
Sales of a significant number of shares of our common stock in the public market could harm the market price of our common stock and make it more difficult for us to raise funds through future offerings of common stock. Our stockholders and the holders of our options and warrants may sell substantial amounts of our common stock in the public market. The availability of these shares of our common stock for resale in the public market has the potential to cause the supply of our common stock to exceed investor demand, thereby decreasing the price of our common stock.
In addition, the fact that our stockholders, option holders and warrant holders can sell substantial amounts of our common stock in the public market, whether or not sales have occurred or are occurring, could make it more difficult for us to raise additional financing through the sale of equity or equity-related securities in the future at a time and price that we deem reasonable or appropriate.
If securities and/or industry analysts fail to continue publishing research about our business, if they change their recommendations adversely or if our results of operations do not meet their expectations, our stock price and trading volume could decline.
The trading market for our common stock will be influenced by the research and reports that industry or securities analysts publish about us or our business. If one or more of these analysts cease coverage of our company or fail to publish reports on us regularly, we could lose visibility in the financial markets, which in turn could cause our stock price or trading volume to decline. In addition, it is likely that in some future period our operating results will be below the expectations of securities analysts or investors. If one or more of the analysts who cover us downgrade our stock, or if our results of operations do not meet their expectations, our stock price could decline.
Risks Related to our Indebtedness
Our obligations under our $10 million principal term loan are secured by substantially all of our assets, so if we default on those obligations, the lender could foreclose on our assets. As a result of these security interests, such assets would only be available to satisfy claims of our general creditors or to holders of our equity securities if we were to become insolvent at a time when the value of such assets exceeded the amount of our indebtedness and other obligations. In addition, the existence of these security interests may adversely affect our financial flexibility.
The lender under our $10 million principal term loan has a security interest in substantially all of our assets and those of InspireMD Ltd., our wholly-owned subsidiary. As a result, if we default under our obligations to the lender, the lender could foreclose on its security interests and liquidate some or all of these assets, which would harm our business, financial condition and results of operations.
In the event of a default in connection with our bankruptcy, insolvency, liquidation, or reorganization, the lender would have a prior right to substantially all of our assets to the exclusion of our general creditors. In that event, our assets would first be used to repay in full all indebtedness and other obligations secured by the lender, resulting in all or a portion of our assets being unavailable to satisfy the claims of any unsecured indebtedness. Only after satisfying the claims of any unsecured creditors would any amount be available for our equity holders.
The pledge of these assets and other restrictions may limit our flexibility in raising capital for other purposes. Because substantially all of our assets are pledged under the $10 million principal term loan, our ability to incur additional secured indebtedness or to sell or dispose of assets to raise capital may be impaired, which could have an adverse effect on our financial flexibility.
Our loan and security agreement contains customary events of default. In addition, an event of default will include the occurrence of a circumstance that would reasonably be expected to have a material adverse effect upon (i) our business, operations, properties, assets, prospects or condition (financial or otherwise), (ii) our ability to perform our obligations under the agreement and any related loan documents or (iii) the collateral, the lender’s liens on the collateral or the priority of such liens.
We have a substantial amount of indebtedness, which may adversely affect our cash flow and our ability to operate our business.
Pursuant to the terms of our loan and security agreement, the lender made a term loan to us and InspireMD Ltd. in aggregate amount of $10 million. We are required to make monthly payments of interest and principal in the amount of approximately $380,000 per month. The final payment of the loan will be February 1, 2017. The current principal amount of the loan as of March 1, 2015 was $7.9 million.
| 37 |
The terms of our term loan could have negative consequences to us, such as:
| · | we may be unable to obtain additional financing to fund working capital, operating losses, capital expenditures or acquisitions on terms acceptable to us, or at all; |
| · | the amount of our interest expense may increase because our term loan has a variable rate of interest at any time that the prime rate, as reported in the Wall Street Journal, is above 5.5%; |
| · | we will need to use a substantial portion of our cash flows to pay principal and interest on our term loan, which will reduce the amount of money we have for operations, working capital, capital expenditures, expansion, acquisitions or general corporate or other business activities; |
| · | we may have a higher level of debt than some of our competitors, which may put us at a competitive disadvantage; |
| · | we may be unable to refinance our indebtedness on terms acceptable to us, or at all; and |
| · | we may be more vulnerable to economic downturns and adverse developments in our industry or the economy in general. |
Our ability to meet our expenses and debt obligations will depend on our future performance, which will be affected by financial, business, economic, regulatory and other factors. We will be unable to control many of these factors, such as economic conditions. We cannot be certain that our earnings will be sufficient to allow us to pay the principal and interest on our debt and meet any other obligations. If we do not have enough money to service our debt, we may be required, but unable to refinance all or part of our existing debt, sell assets, borrow money or raise equity on terms acceptable to us, if at all, and the lender could foreclose on its security interests and liquidate some or all of our assets.
Our loan and security agreement contains covenants that could limit our financing options and liquidity position, which would limit our ability to grow our business.
Covenants in our loan and security agreement impose operating and financial restrictions on us. These restrictions prohibit or limit our ability, and the ability of InspireMD Ltd., to, among other things:
| · | pay cash dividends to our stockholders; |
| · | redeem or repurchase our common stock or other equity; |
| · | incur additional indebtedness; |
| · | permit liens on assets; |
| · | make certain investments (including through the acquisition of stock, shares, partnership or limited liability company interests, any loan, advance or capital contribution) |
| · | sell, lease, license, lend or otherwise convey an interest in a material portion of our assets; and |
| · | cease making public filings under the Securities Exchange Act of 1934, as amended. |
These restrictions may limit our ability to obtain additional financing, withstand downturns in our business or take advantage of business opportunities. Moreover, additional debt financing we may seek, if permitted, may contain terms that include more restrictive covenants, may require repayment on an accelerated schedule or may impose other obligations that limit our ability to grow our business, acquire needed assets, or take other actions we might otherwise consider appropriate or desirable.
| 38 |
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This Annual Report on Form 10-K contains “forward-looking statements,” which include information relating to future events, future financial performance, strategies, expectations, competitive environment and regulation. Words such as “may,” “should,” “could,” “would,” “predicts,” “potential,” “continue,” “expects,” “anticipates,” “future,” “intends,” “plans,” “believes,” “estimates,” and similar expressions, as well as statements in future tense, identify forward-looking statements. Forward-looking statements should not be read as a guarantee of future performance or results and will probably not be accurate indications of when such performance or results will be achieved. Forward-looking statements are based on information we have when those statements are made or our management’s good faith belief as of that time with respect to future events, and are subject to risks and uncertainties that could cause actual performance or results to differ materially from those expressed in or suggested by the forward-looking statements. Important factors that could cause such differences include, but are not limited to:
| · | our history of recurring losses and negative cash flows from operating activities, significant future commitments and the uncertainty regarding the adequacy of our liquidity to pursue our complete business objectives; |
| · | market acceptance of our existing and new products; |
| · | negative clinical trial results or lengthy product delays in key markets; |
| · | an inability to secure and maintain regulatory approvals for the sale of our products; |
| · | our dependence on single suppliers for certain product components and our ability to comply with stringent manufacturing quality standards and to increase production as necessary; |
| · | intense competition in our industry, with competitors having substantially greater financial, technological, research and development, regulatory and clinical, manufacturing, marketing and sales, distribution and personnel resources than we do; |
| · | entry of new competitors and products and potential technological obsolescence of our products; |
| · | our limited manufacturing capabilities and reliance on subcontractors for assistance; |
| · | loss of a key customer or supplier; |
| · | technical problems with our research and products and potential product liability claims; |
| · | product malfunctions; |
| · | adverse economic conditions; |
| · | insufficient or inadequate reimbursement by governmental and other third party payers for our products; |
| · | our efforts to successfully obtain and maintain intellectual property protection covering our products, which may not be successful; |
| · | legislative or regulatory reform of the healthcare system in both the U.S. and foreign jurisdictions; |
| · | the fact that we will need to raise additional capital to meet our business requirements in the future and that such capital raising may be costly, dilutive or difficult to obtain; |
| · | the fact that we conduct business in multiple foreign jurisdictions, exposing us to foreign currency exchange rate fluctuations, logistical and communications challenges, burdens and costs of compliance with foreign laws and political and economic instability in each jurisdiction; |
| · | the escalation of hostilities in Israel, which could impair our ability to manufacture our products; and |
| · | loss or retirement of key executives and research scientists. |
You should review carefully the risks and uncertainties described under the heading “Item 1A. Risk Factors” in this Annual Report on Form 10-K for a discussion of these and other risks that relate to our business and investing in shares of our common stock. The forward-looking statements contained in this Annual Report on Form 10-K are expressly qualified in their entirety by this cautionary statement. We do not undertake any obligation to publicly update any forward-looking statement to reflect events or circumstances after the date on which any such statement is made or to reflect the occurrence of unanticipated events.
| 39 |
Item 1B. Unresolved Staff Comments.
Not applicable.
Our headquarters are located in Boston, Massachusetts, where we lease approximately 4,130 square feet of executive office space. In addition, in Tel Aviv, Israel, we currently have a 1,000 square meter office and manufacturing facility that has the capacity to manufacture and assemble 4,800 stents per month, based upon the production schedule of one shift per day. We believe that our current facility is sufficient to meet anticipated future demand by adding additional shifts to our current production schedule.
From time to time, we may be involved in litigation that arises through the normal course of business. As of the date of this filing, we are not aware of any material legal proceedings to which we or any of our subsidiaries is a party or to which any of our property is subject, nor are we aware of any such threatened or pending litigation.
There are no material proceedings in which any of our directors, officers or affiliates or any registered or beneficial stockholder of more than 5% of our common stock, or any associate of any of the foregoing, is an adverse party or has a material interest adverse to our interest.
Item 4. Mine Safety Disclosures.
Not applicable.
Item 5. Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities.
Our common stock has been quoted on the NYSE MKT since April 11, 2013 under the symbol “NSPR.” Prior to that date, it was traded on the OTC Bulletin Board
The following table sets forth (i) the intra-day high and low sales price per share for our common stock, as reported on the NYSE MKT, for the period of April 11, 2013 to December 31, 2014, and (ii) the high and low bid prices for our common stock, as reported by the OTC Bulletin Board, for the period July 1, 2012 to April 10, 2013. The quotations reflect inter-dealer prices, without retail mark-up, mark-down or commission, and may not represent actual transactions. The OTC Bulletin Board quotations prior to December 21, 2012 are adjusted for the one-for-four reverse stock split of our common stock that occurred on such date.
| Fiscal Year Ended December 31, 2014 | High | Low | ||||||
| First Quarter | $ | 3.80 | $ | 2.48 | ||||
| Second Quarter | $ | 3.25 | $ | 1.79 | ||||
| Third Quarter | $ | 3.02 | $ | 1.81 | ||||
| Fourth Quarter | $ | 2.23 | $ | 0.70 | ||||
| Transition Period Ended December 31, 2013 | High | Low | ||||||
| First Quarter | $ | 2.68 | $ | 1.80 | ||||
| Second Quarter | $ | 3.67 | $ | 2.27 | ||||
| Fiscal Year Ended June 30, 2013 | High | Low | ||||||
| First Quarter | $ | 10.00 | $ | 3.84 | ||||
| Second Quarter | $ | 10.16 | $ | 3.01 | ||||
| Third Quarter | $ | 4.25 | $ | 1.95 | ||||
| Fourth Quarter | $ | 3.15 | $ | 1.88 | ||||
The last reported sales price of our common stock on the NYSE MKT on March 11, 2015, was $0.26 per share. As of March 11, 2015, there were approximately 223 holders of record of our common stock.
| 40 |
Dividend Policy
In the past, we have not declared or paid cash dividends on our common stock. Our loan and security agreement with Hercules Technology Growth Capital, Inc., dated October 23, 2013, prohibits us from paying dividends or distributions on our common stock. Even if we are permitted to pay cash dividends in the future, we do not intend to do so. Rather, we intend to retain future earnings, if any, to fund the operation and expansion of our business and for general corporate purposes.
Item 6. Selected Financial Data.
The following selected consolidated financial data should be read in conjunction with “Part II—Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations” and “Part II—Financial Statements and Supplementary Data.” The balance sheet data at December 31, 2014 and 2013 and the statement of operations data for the year ended December 31, 2014, the six months ended December 31, 2013 and the year ended June 30, 2013 have been derived from the audited Consolidated Financial Statements for such years, included in “Part II—Financial Statements and Supplementary Data.” The balance sheet data at June 30, 2013 and 2012 and December 31, 2011 and 2010 and the statement of operations data for the six months ended June 30, 2012 and the years ended December 31, 2011 and 2010 have been derived from audited consolidated financial statements not included in this Annual Report on Form 10-K.
The share and per share amounts set forth below reflect the one-for-four reverse stock split of our common stock that occurred on December 21, 2012.
| Statement of Operations Data | ||||||||||||||||||||||||
| Year Ended | Six Months Ended | Year Ended | Six Months Ended | Year Ended | Year Ended December 31, | |||||||||||||||||||
| December 31, 2014 | December 31, 2013 | June 30, 2013 | June 30, 2012 | December 31, 2011 | 2010 | |||||||||||||||||||
| Revenues | 2,818 | 3,105 | 4,873 | 2,071 | 6,004 | 4,949 | ||||||||||||||||||
| Cost of Revenues | 2,034 | 1,442 | 2,283 | 1,377 | 3,011 | 2,696 | ||||||||||||||||||
| Gross Profit | 784 | 1,663 | 2,590 | 694 | 2,993 | 2,253 | ||||||||||||||||||
| Gross Margin | 28 | % | 54 | % | 53 | % | 34 | % | 50 | % | 46 | % | ||||||||||||
| Total Operating Expenses | 24,482 | 10,490 | 17,663 | 7,852 | 16,722 | 5,472 | ||||||||||||||||||
| Loss from Operations | (23,698 | ) | (8,827 | ) | (15,073 | ) | (7,158 | ) | (13,729 | ) | (3,219 | ) | ||||||||||||
| Net Loss | (25,095 | ) | (9,336 | ) | (29,258 | ) | (7,081 | ) | (14,665 | ) | (3,420 | ) | ||||||||||||
| Basic and Diluted loss per common share | (0.71 | ) | (0.27 | ) | (1.39 | ) | (0.41 | ) | (0.95 | ) | (0.28 | ) | ||||||||||||
| Basic and Diluted common shares outstanding | 35,393,644 | 33,963,901 | 20,995,887 | 17,044,221 | 15,359,925 | 12,308,632 | ||||||||||||||||||
| Balance Sheet Data | ||||||||||||||||||||||||
| December 31, 2014 | December 31, 2013 | June 30, 2013 | June 30, 2012 | December 31, 2011 | December 31,
2010 | |||||||||||||||||||
| Cash, Cash equivalents and short term deposits | 6,300 | 17,535 | 14,820 | 10,284 | 5,094 | 636 | ||||||||||||||||||
| Restricted Cash | 93 | 93 | 37 | 91 | 250 | |||||||||||||||||||
| Working Capital | 895 | 15,480 | 14,862 | 10,759 | 6,389 | (53 | ) | |||||||||||||||||
| Total Assets | 11,459 | 23,966 | 20,745 | 16,014 | 10,465 | 4,355 | ||||||||||||||||||
| Total Long-Term Liabilities | 5,773 | 9,203 | 600 | 7,078 | 270 | 1,325 | ||||||||||||||||||
| Shareholder's Equity (Capital Deficiency) | (2,787 | ) | 8,639 | 16,102 | 5,386 | 6,754 | (914 | ) | ||||||||||||||||
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations.
The following discussion and analysis of our financial condition and results of operations should be read in conjunction with the accompanying condensed consolidated financial statements and related notes included elsewhere in this Annual Report on Form 10-K.
Overview
We are a medical device company focused on the development and commercialization of our proprietary stent platform technology, MGuard. MGuard provides embolic protection in stenting procedures by placing a micron mesh sleeve over a stent. Our initial products are marketed for use mainly in patients with acute coronary syndromes, notably acute myocardial infarction (heart attack) and saphenous vein graft coronary interventions (bypass surgery).
| 41 |
We effectuated a one-for-four reverse stock split of our common stock on December 21, 2012. Our authorized shares of common stock were not adjusted as a result of this reverse stock split. All share and related option and warrant information presented in the following discussion and analysis of our financial condition and results of operations and the accompanying consolidated interim financial statements have been retroactively adjusted to reflect the reduced number of shares outstanding which resulted from this action.
On June 1, 2012, our board of directors approved a change in our fiscal year-end from December 31 to June 30, effective June 30, 2012 and on September 16, 2013, our board of directors approved a change in our fiscal year-end from June 30 to December 31, effective December 31, 2013.
Recent Events
During the fourth quarter of 2014, we began implementing a focused spending plan. The plan included reducing the focus of clinical and development expenses related to our bare metal stent product and increasing the focus on our drug eluting stent product. Prior to the fourth quarter of 2014, a large portion of our organization was supporting our MASTER II trial, in which we determined not to resume enrollment, and instead allocated resources to drug eluting stents and the CGuard platform.
During the first quarter of 2015, the board of director approved implementing another cost reduction/focused spending plan. The plan has four components: (i) reducing headcount; (ii) limiting the focus of clinical and development expenses to only the drug eluting stent product; (iii) limiting sales and marketing expenses to only those related to the CGuard EPS stent launch; and (iv) reducing across the board all other expenses (conferences, travel, promotional expenses, executive cash salaries, director cash fees, etc.). Prior to the cost reduction plan, a large portion of our organization was supporting clinical trials and promotional activities related to our bare metal stent platform. We decided to discontinue all work and promotion (such as conferences, clinical studies, and some sales activities) related to the bare metal platform. This decision allowed us to eliminate certain positions that related only to these activities. In addition, we dramatically cut all expenses not directly related to the CGuard launch and drug eluting platform development.
In addition, to reduce the usage of cash, on January 5, 2015, we amended our employment agreements with Alan Milinazzo and James Barry, Ph.D. to provide that, for a limited period of time to be mutually agreed to by us and each of Mr. Milinazzo and Dr. Barry, each of Mr. Milinazzo and Dr. Barry shall receive 50% of his base salary in cash payments, with the remaining 50% to be paid in an equivalent amount of shares of restricted common stock, payable and granted in equal installments in accordance with our normal payroll practices. On the same date, our compensation committee amended its compensation policy for directors to provide that effective as of July 1, 2014, each director would forego any cash compensation in exchange for such number of immediately vested 10 year stock options having a Black-Scholes value equal to the cash compensation otherwise due to such director under our current director compensation policies. On February 22, 2015, Dr. Barry’s employment agreement was further amended to provide that the payment arrangement described above would continue until the earlier of (i) September 30, 2015 and (ii) the Company raising an aggregate of $5 million from investors. Our March 9, 2015 public offering raised in excess of $5 million and therefore Dr. Barry’s payment arrangement will, by the terms of this agreement, no longer be 50% paid in restricted stock.
On March 9, 2015, we sold 34,369,675 shares of our common stock and warrants to purchase 34,369,675 shares of our common stock in a public offering. Each purchaser received a warrant to purchase one share of common stock for each share of common stock that it purchased in the offering. The warrants have a term of exercise of 5 years from the date of issuance and an exercise price of $0.55. This offering resulted in net proceeds to us of approximately $12.5 million after deducting placement agent fees and other estimated offering expenses.
Critical Accounting Policies
We prepared our consolidated financial statements for inclusion in this report in accordance with U.S. Generally Accepted Accounting Principles (“U.S. GAAP”). U.S. GAAP represents a comprehensive set of accounting and disclosure rules and requirements, and applying these rules and requirements requires management judgments and estimates including, in certain circumstances, choices between acceptable U.S. GAAP alternatives. The following is a discussion of our most critical accounting policies, judgments and uncertainties that are inherent in our application of U.S. GAAP.
Use of estimates
The preparation of financial statements in conformity with U.S. GAAP requires management to make estimates using assumptions that affect the reported amounts of assets and liabilities, the disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of sales and expenses during the reporting periods. Actual results could differ from those estimates.
As applicable to these consolidated financial statements, the most significant estimates and assumptions relate to inventory valuations, royalty buyout, legal contingencies and estimation of the fair value of warrants.
Functional currency
The currency of the primary economic environment in which our operations and the operations of our subsidiaries are conducted is the U.S. dollar (“$” or “dollar”). Accordingly, our and our subsidiaries’ functional currency is the U.S. dollar.
The dollar figures are determined as follows: transactions and balances originally denominated in dollars are presented in their original amounts. Balances in foreign currencies are translated into dollars using historical and current exchange rates for non-monetary and monetary balances, respectively. The resulting translation gains or losses are recorded as financial income or expense, as appropriate. For transactions reflected in the statements of operations in foreign currencies, the exchange rates at transaction dates are used. Depreciation and changes in inventories and other changes deriving from non-monetary items are based on historical exchange rates.
| 42 |
Concentration of credit risk and allowance for doubtful accounts
Financial instruments that may potentially subject us to a concentration of credit risk consist of cash and cash equivalents, which are deposited in major financially sound institutions in the U.S, Israel, Germany and the United Kingdom, and trade accounts receivable. Our trade accounts receivable are derived from revenues earned from customers from various countries. We perform ongoing credit evaluations of our customers’ financial condition and, generally, require no collateral from customers. We also have a credit insurance policy for some of customers. We maintain an allowance for doubtful accounts receivable based upon the expected ability to collect the accounts receivable. We review our allowance for doubtful accounts quarterly by assessing individual accounts receivable and all other balances based on historical collection experience and an economic risk assessment. If we determine that a specific customer is unable to meet its financial obligations to us, we provide an allowance for credit losses to reduce the receivable to the amount management reasonably believes will be collected, which is netted against “Accounts receivable – Trade”.
Inventory
Inventories include finished goods, work in process, raw materials and inventory on consignment in hospitals. Inventories are stated at the lower of cost (cost is determined on a “first-in, first-out” basis) or market value. Our inventories generally have a limited shelf life and are subject to impairment as they approach their expiration dates. We regularly evaluate the carrying value of our inventories and when, in our opinion, factors indicate that impairment has occurred, we impair the inventories’ carrying value.
| 43 |
Revenue recognition
Revenue is recognized when delivery has occurred, evidence of an arrangement exists, title and risks and rewards for the products are transferred to the customer, collection is reasonably assured and product returns can be reliably estimated. When estimated right of return exists, we estimate a provision, based on historical experience, which is deducted from revenues.
We recognize revenue net of value added tax (VAT).
Research and development costs
Research and development costs are charged to the statement of operations as incurred.
Share-based compensation
Employee option awards are classified as equity awards and accounted for using the grant-date fair value method. The fair value of share-based awards is estimated using the Black-Scholes valuation model and expensed over the requisite service period, net of estimated forfeitures. We estimate forfeitures based on historical experience and anticipated future conditions.
We elected to recognize compensation expenses for awards with only service conditions that have graded vesting schedules using the accelerated multiple option approach.
In addition, certain share-based awards are performance based and dependent upon achieving certain goals. With respect to these awards, we estimate the expected pre-vesting award probability that the performance conditions will be achieved. We only recognize expense for those shares that are expected to vest.
Uncertain tax and value added tax positions
We follow a two-step approach to recognizing and measuring uncertain tax positions. The first step is to evaluate the tax position for recognition by determining if the weight of available evidence indicates that it is more likely than not that the position will be sustained on audit. If under the first step a tax provision is assessed to be more likely than not of being sustained on audit, the second step is performed, under which the tax benefit is measured as the largest amount that is more than 50% likely to be realized upon ultimate settlement. Such liabilities are classified as long-term, unless the liability is expected to be resolved within twelve months from the balance sheet date. Our policy is to include interest related to unrecognized tax benefits within “Financial expenses (income) – net.”
| 44 |
Fair value measurement
We measure fair value and disclose fair value measurements for financial assets and liabilities. Fair value is based on the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date.
The accounting standard establishes a fair value hierarchy that prioritizes observable and unobservable inputs used to measure fair value into three broad levels, which are described below:
Level 1: Quoted prices (unadjusted) in active markets that are accessible at the measurement date for assets or liabilities. The fair value hierarchy gives the highest priority to Level 1 inputs.
Level 2: Observable prices that are based on inputs not quoted on active markets, but corroborated by market data.
Level 3: Unobservable inputs are used when little or no market data is available. The fair value hierarchy gives the lowest priority to Level 3 inputs.
In determining fair value, we utilize valuation techniques that maximize the use of observable inputs and minimize the use of unobservable inputs to the extent possible and consider counterparty credit risk in our assessment of fair value.
Allocation of issuance proceeds
When debt or equity is issued with other components that are subsequently measured at fair value, the proceeds are allocated first to such components (such as warrants and embedded derivatives in the debt that require bifurcation at their fair values), then the residual amount of the proceeds is allocated to the debt or equity. When other components are classified in equity, the proceeds are allocated based on relative fair values.
Results of Operations
Twelve months ended December 31, 2014 compared to the twelve months ended December 31, 2013
Revenues. For the twelve months ended December 31, 2014, revenue decreased by $3.3 million, or 53.9%, to $2.8 million from $6.1 million during the same period in 2013. This decrease was predominately driven by a decrease in sales volume of $3.2 million, or 52.9%, with price decreases to our repeat distributors driving the remaining decrease of $66,000, or 1.1%. The decrease in sales volume was due to our VFA which resulted in a temporary suspension in sales of MGuard Prime EPS, our primary commercial product. On June 18, 2014, we received European regulatory approval to modify, redeploy and resume the manufacturing of our MGuard Prime EPS. All products sent back to us have been modified. In September 2014, we resumed shipments back to direct hospital customers and the majority of our distributor partners, who have begun shipping modified products back into hospital accounts.
With respect to regions, the decrease in revenue was primarily attributable to a decrease of $3.0 million in revenue from our distributors in Europe, $0.4 million in revenue from our distributors in Latin America and $0.2 million in revenue from our distributors in Africa, partially offset by an increase of $0.3 million in revenue from our distributors in the Middle East.
Gross Profit. For the twelve months ended December 31, 2014, gross profit (revenue less cost of revenues) decreased by 75.3%, or $2.4 million, to $0.8 million from $3.2 million during the same period in 2013. This decrease in gross profit was attributable to the impact of the VFA which included a decrease in revenues of $3.3 million (see above for explanation) as well as $0.4 million of costs associated with the VFA including the costs of modifying and shipping the distributor products sent back to us. This increase, however, was partially offset by a decrease in labor and materials of $0.9 million attributable to the decrease in revenues. In addition, we incurred $0.4 million of expense in the twelve months ended December 31, 2013 pertaining to the consolidation of our manufacturing facilities. No such expense occurred during the twelve months ended December 31, 2014. Gross margin (gross profits as a percentage of revenue) decreased from 51.8% in the twelve months ended December 31, 2013 to 27.8% in the same period in 2014.
Research and Development Expenses. For the twelve months ended December 31, 2014, research and development expenses increased by 66.0%, or $3.4 million, to $8.7 million from $5.3 million during the same period in 2013. This increase in research and development expenses resulted primarily from increases of $0.6 million in related salaries, $0.4 million in related share-based compensation expenses, $0.1 million in related travel expenses, $1.1 million in clinical trial expenses associated with our MASTER II trial and $1.0 million in clinical trial and development expenses associated with our CGuard EPS product. In addition, expenditures related to product development increased by $0.4 million, expenditures related to our eMaster post-market registry increased by $0.2 million and expenditures related to our OCT clinical study, which has subsequently been cancelled, increased by $0.2 million. This increase in research and development expenses, however, was partially offset by a decrease of $0.4 million in expenses associated with our MASTER I trial, which concluded in 2013, and a decrease of $0.2 million in miscellaneous expenses. Research and development expenses as a percentage of revenue increased to 310.3% for the twelve months ended December 31, 2014, from 86.1% in the same period in 2013.
| 45 |
Selling and Marketing Expenses. For the twelve months ended December 31, 2014, selling and marketing expenses increased by 42.1%, or $1.9 million, to $6.6 million from $4.7 million during the same period in 2013. This increase in selling and marketing expenses resulted primarily from an increase of $1.6 million in salaries and an increase of $0.3 million in share-based compensation, as we hired additional sales personnel in an effort to expand our sales activities worldwide, an increase of $0.3 million in travel expenses for the increased number of our sales force and an increase of $0.2 million in miscellaneous expenses. Much of these sales initiatives were driven by our increased efforts to support the new sales strategies in key European and Latin American countries. This increase in selling and marketing expenses, however, was partially offset by a decrease of $0.3 million in trade show expenses and $0.2 million in product promotion expenses. Selling and marketing expenses as a percentage of revenue increased to 234.7% in the twelve months ended December 31, 2014 from 76.1% in the same period in 2013.
General and Administrative Expenses. For the twelve months ended December 31, 2014, general and administrative expenses decreased by 3.9%, or $0.4 million, to $9.1 million from $9.5 million during the same period in 2013. This decrease in general and administrative expenses resulted primarily from a decrease of $0.5 million in share-based compensation and a decrease of $0.3 million in salaries, primarily due to lower bonuses in the twelve months ended December 31, 2014 as compared the same period in 2013. This decrease, however, was partially offset by an increase of $0.3 million in miscellaneous expenses and $0.1 million in legal expenses. General and administrative expenses as a percentage of revenue increased to 323.8% in the twelve months ended December 31, 2014 from 155.3% in the same period in 2013.
Financial Expenses. For the twelve months ended December 31, 2014, financial expenses decreased by 89.3%, or $11.5 million, to $1.4 million from $13.0 million during the same period in 2013. The decrease in financial expenses partially resulted from a decrease of $1.0 million of amortization and interest expenses. In the twelve months ended December 31, 2014, we recognized $1.4 million in amortization and interest expense, in contrast to the twelve months ended December 31, 2013, during which we recognized $2.4 million of amortization and interest expense pertaining to our previously outstanding senior convertible debentures and their related issuance costs. In addition, we incurred $1.7 million of expense in the twelve months ended December 31, 2013 pertaining to our obligation to issue shares of common stock without new consideration to the investors in our March 2011 private placement due to certain anti-dilution rights held by such stockholders and the revaluations of our warrants, as well as $9.9 million of expense pertaining to the adjustment of the conversion ratio of our convertible debentures prior to their retirement in April 2013. No such expenses occurred during the twelve months ended December 31, 2014. This decrease in expenses was partially offset by the absence of any revaluations of our warrants during the twelve months ended December 31, 2014. During the twelve months ended December 31, 2013, we recognized $1.1 million of financial income pertaining to the revaluation of certain of our warrants due to our stock price decreasing from $3.90 to $2.21 during such period. No such income was recognized during the twelve months ended December 31, 2014. Financial expense as a percentage of revenue decreased to 49.1% in the twelve months ended December 31, 2014 from 211.6% in the same period in 2013.
Tax Expenses. For the twelve months ended December 31, 2014, tax expenses increased $43,000, to $12,000 from $31,000 of tax income during the same period in 2013.
Net Loss. Our net loss decreased by $4.1 million, or 14.0%, to $25.1 million for the twelve months ended December 31, 2014 from $29.2 million during the same period in 2013. The decrease in net loss resulted primarily from a decrease of $11.5 million in financial expenses (see above for explanation), partially offset by an increase of $5.0 million in operating expenses primarily associated with research and development and sales and marketing expansion (see above for explanation), and a decrease of $2.4 million in gross profit (see above for explanation).
Six month period ended December 31, 2013 compared to the six month period ended December 31, 2012
Revenues. For the six month period ended December 31, 2013, revenue increased by $1.2 million, or 67.0%, to $3.1 million from $1.9 million during the same period in 2012. This increase was predominantly driven by an increase in sales volume of $1.2 million, or 65.4%, with price increases to our repeat distributors driving the remaining increase of $30,000, or 1.6%. The $1.2 million increase in sales volume reflects the positive impact of steps taken to stabilize our global distribution strategy and targeted selling activities in select European countries.
With respect to regions, the increase in revenue was mainly attributable to an increase of $1.1 million in revenue from our distributors in Europe and an increase of $0.1 million in revenue from our distributors in Latin America.
Gross Profit. For the six month period ended December 31, 2013, gross profit (revenue less cost of revenues) increased 53.7%, or $0.6 million, to $1.7 million from $1.1 million during the same period in 2012. The increase in gross profit was attributable to an increase in revenue of $1.2 million, as described above, partially offset by an increase in cost of revenues of $0.6 million, which was composed of an increase in material and labor costs of $0.4 million associated with our increased sales and increase of $0.2 million in expenses related to the consolidation of our manufacturing facilities. Gross margin (gross profits as a percentage of revenue) decreased from 58.2% in the six month period ended December 31, 2012 to 53.6% in same period in 2013.
| 46 |
Royalties’ Buyout Expenses. For the six month period ended December 31, 2012, we incurred $0.9 million in royalties’ buyout expenses relating to the restructuring of our royalty agreement for MGuard Prime. In connection with the restructuring of this agreement, the licensor of the stent design used for this product agreed to reduce the royalty from 7% of net sales outside of the United States, 7% of the first $10,000,000 of net sales in the United States and 10% of net sales in the United States above $10,000,000 to 2.9% of all net sales both inside and outside the United States in exchange for (i) us waiving $85,000 in regulatory fees owed to us, (ii) us making full payment of royalties owed as of September 30, 2012 in the amount of $205,587 and (iii) $1,763,000, payable in 215,000 shares of our common stock that were valued at $8.20 per share. There was no such expense during the six month period ended December 31, 2013. Royalties’ buyout expenses as a percentage of revenue was 49.4% for the six month period ended December 31, 2012.
Research and Development Expenses. For the six month period ended December 31, 2013, research and development expenses increased 50.0%, or $1.1 million, to $3.3 million, from $2.2 million during the same period in 2012. This increase in research and development expenses resulted primarily from increases of $0.3 million in related salaries, $0.1 million in related travel expenses and $1.4 million in clinical trial expenses associated with our MASTER II trial moving from the pre-clinical stage to the set-up and enrollment phases, triggering costs associated with the selection and qualification of trial sites, contract research organization management fees and patient fees, among others. This increase in research and development expenses, however, was partially offset by a decrease of $0.4 million in expenses associated with our MASTER I trial, which has concluded, a decrease of $0.2 million in expenditures related to the development of the MGuard Carotid product and a decrease of $0.1 million in share based compensation expense. Research and development expense as a percentage of revenue decreased to 106.8% for the six month period ended December 31, 2013, from 118.5% in the same period in 2012.
Selling and Marketing Expenses. For the six month period ended December 31, 2013, selling and marketing expenses increased 64.6%, or $1.0 million, to $2.6 million, from $1.6 million during the same period in 2012. The increase in selling and marketing expenses resulted primarily from an increase of $0.8 million in salaries, as we expanded our sales activities worldwide, an increase of $0.1 million in travel expenses for our increased sales force and an increase of $0.1 million in miscellaneous expenses. Much of these sales initiatives were driven by our efforts to capitalize on the publication of the MASTER I trial results, which represented our first randomized data related to our MGuard technology, and efforts to support our new direct sales channels in key European countries. Selling and marketing expenses as a percentage of revenue decreased to 85.2% in the six month period ended December 31, 2013 from 86.5% in the same period in 2012.
General and Administrative Expenses. For the six month period ended December 31, 2013, general and administrative expenses increased 13.2%, or $0.5 million, to $4.5 million from $4.0 million during the same period in 2012. The increase in general and administrative expenses resulted primarily from an increase of $0.6 million in salaries (which predominately relates to the hiring of our new chief executive officer and bonuses), an increase in share based compensation of $0.2 million, an increase in director’s compensation of $0.1 million and an increase in travel expense of $0.1 million. This increase was partially offset by a decrease in legal fees of $0.3 million and a decrease of $0.2 million in bad debt expense. General and administrative expenses as a percentage of revenue decreased to 145.8% in the six month period ended December 31, 2013 from 215.2% in the same period in 2012.
Financial Expenses. For the six month period ended December 31, 2013, financial expenses decreased 71.2%, or $1.2 million, to $0.5 million from $1.7 million during the same period in 2012. The decrease in financial expenses resulted primarily from a decrease of $1.8 million of amortization and interest expenses. In the six month period ended December 31, 2013, we recognized $0.3 million in amortization and interest expense, in contrast to the six month period ended December 31, 2012, during which we recognized $2.1 million of amortization and interest expense pertaining to our previously outstanding senior convertible debentures and their related issuance costs. This decrease in expenses was partially offset by $0.2 million of expense in the six month period ended December 31, 2013 pertaining to our obligation to issue shares of common stock without new consideration to the investors in our March 2011 private placement due to certain anti-dilution rights held by such stockholders and the absence of any non-cash revaluations of our warrants during the six month period ended December 31, 2013. During the six month period ended December 31, 2012, we recognized $0.3 million of financial income pertaining to the revaluation of certain of our warrants due to our stock price decreasing from $4.24 to $3.90 during such period. No such income was recognized during the six month period ended December 31, 2013. Financial expense as a percentage of revenue decreased to 16.1% in the six month period ended December 31, 2013, from 93.1% in the same period in 2012.
Tax Expenses. For the six month period ended December 31, 2013, tax expenses decreased $39,000 to $10,000 for the six month period ended December 31, 2013, from $49,000 during the same period in 2012.
Net Loss. Our net loss decreased by $0.1 million, or 1.0%, to $9.3 million for the six month period ended December 31, 2013 from $9.4 million during the same period in 2012. The decrease in net loss resulted primarily from a decrease of $1.2 million in financial expenses, (see above for explanation), and an increase of $0.6 million in gross profit (see above for explanation), partially offset by an increase of $1.7 million in operating expenses (see above for explanation).
Twelve months ended June 30, 2013 compared to twelve months ended June 30, 2012
Revenues. For the twelve months ended June 30, 2013, revenue decreased by $0.5 million, or 8.9%, to $4.9 million from $5.3 million during the twelve months ended June 30, 2012. This decrease was predominantly driven by a decrease in sales volume of $0.5 million, or 9.6%, partially offset by price increases to our repeat distributors of $36,000, or 0.7%. The $0.5 million decrease in sales volume was due largely to the fact that we were in the process of replacing certain third party distributors with direct sales channels in key countries where end user average selling prices, along with other limiting factors, continued to impair sales. While we believe that this transition to direct selling will ultimately lead to greater sales in these markets, the transition away from certain distributors adversely impacted revenue for the twelve months ended June 30, 2013, as we had fewer parties selling our products.
| 47 |
With respect to regions, the decrease in revenue was mainly attributable to a decrease of $0.6 million in revenue from our distributors in Latin America and a decrease of $0.2 million in revenue from our distributors throughout the rest of the world. These decreases were partially offset by an increase of $0.3 million in revenue from our distributors in Asia.
Gross Profit. For the twelve months ended June 30, 2013, gross profit increased 3.6%, or $0.1 million, to $2.6 million from $2.5 million during the twelve months ended June 30, 2012. The increase in gross profit was attributable to a decrease in cost of revenues of $0.6 million, primarily attributable to a write-off of $0.4 million of slow moving inventory in the twelve months ended June 30, 2012, which did not occur in the twelve months ended June 30, 2013, as well as a decrease of $0.3 million of material and labor costs due to the decrease in sales of $0.5 million, as discussed above, partially offset by $0.2 million of expenses related to the consolidation of our manufacturing facilities. The decrease of $0.6 million in cost of revenues was partially offset by a decrease in revenue of $0.5 million as discussed above. Gross margin increased from 46.7% in the twelve months ended June 30, 2012 to 53.2% in the twelve months ended June 30, 2013.
Royalties’ Buyout Expenses. For the twelve months ended June 30, 2013, we incurred $0.9 million in royalties’ buyout expenses relating to the restructuring of our royalty agreement for the MGuard Prime version of our MGuard Coronary stent, as described above. There was no such expense during the twelve months ended June 30, 2012. Royalties’ buyout expenses as a percentage of revenue was 18.8% for the twelve months ended June 30, 2013.
Research and Development Expenses. For the twelve months ended June 30, 2013, research and development expenses increased 4.2%, or $0.2 million, to $4.2 million, from $4.0 million during the twelve months ended June 30, 2012. The increase in research and development expenses resulted primarily from an increase of $0.1 million in salaries, an increase of $0.1 million in patent expenses, an increase of $0.1 million in expenditures related to the development of the MGuard Carotid product and an increase of $0.2 million in miscellaneous expense. These increases were partially offset by a decrease in clinical trial expenses of $0.3 million, attributable mainly to fewer expenses associated with our MASTER I trial, as we approach the trial’s conclusion (decrease of $0.2 million), and our MASTER II trial (decrease of $0.1 million). Research and development expense as a percentage of revenue increased to 85.3% for the twelve months ended June 30, 2013 from 74.6% in the twelve months ended June 30, 2012.
Selling and Marketing Expenses. For the twelve months ended June 30, 2013, selling and marketing expenses increased 66.3%, or $1.4 million, to $3.6 million, from $2.2 million during the twelve months ended June 30, 2012. The increase in selling and marketing expenses resulted primarily from an increase of $0.7 million in salaries as we expanded our sales activities worldwide, an increase of $0.4 million in expenditures related to promotional activities related to the Transcatheter Cardiovascular Therapeutics (TCT) conference in Miami, Florida, where we announced our MASTER I trial results, an increase of $0.5 million in product promotion expenses and an increase of $0.3 million in travel expenses for our increased sales force. Much of these sales initiatives were driven by our efforts to capitalize on the publication of the initial MASTER I trial results, which represented our first randomized data related to our MGuard technology. These increases in sales and marketing expenses were partially offset by a decrease of $0.3 million in share-based compensation expenses and a decrease of $0.2 million in miscellaneous expenses. With the growth of our sales force, and associated activities, as described above, selling and marketing expenses as a percentage of revenue increased to 74.2% in the twelve months ended June 30, 2013 from 40.6% in the twelve months ended June 30, 2012.
General and Administrative Expenses. For the twelve months ended June 30, 2013, general and administrative expenses decreased 35.4%, or $4.9 million, to $9.0 million from $13.9 million during the twelve months ended June 30, 2012. The decrease in general and administrative expenses resulted primarily from a decrease in share-based compensation of $6.1 million (which predominantly pertained to director’s compensation paid in 2012) and a decrease of $0.3 million in expenses related to consultants. This decrease was partially offset by an increase in salaries of $0.6 million (which predominately relates to the hiring of our new chief executive officer), an increase of $0.5 million in legal expenses largely associated with our previous financing efforts, an increase of $0.1 million in bad debt expense, an increase of $0.1 million in audit fees, and an increase of $0.2 million in miscellaneous expenses. General and administrative expenses as a percentage of revenue decreased to 184.1% in the twelve months ended June 30, 2013 from 259.5% in the twelve months ended June 30, 2012.
Financial Expenses. For the twelve months ended June 30, 2013, financial expenses increased to $14.1 million from $38,000 during the twelve months ended June 30, 2012. The increase in financial expenses resulted primarily from $9.9 million in non-recurring, effects of the debt inducement related to the adjustment of the conversion ratio of our convertible debentures upon their retirement in April 2013, $4.3 million of amortization expense pertaining to our convertible debentures and their related issuance costs (of which $3.5 million represented the amortization of the discount of the convertible debentures and their related issuance costs). In addition to these expenses, we also incurred $1.5 million of expense pertaining to our obligation to issue shares of common stock without new consideration to the investors in our March 2011 private placement due to certain anti-dilution rights held by such stockholders. These expenses were partially offset by $1.4 million of financial income pertaining to the revaluation of certain of our warrants due to our stock price decreasing to $2.21 on June 30, 2013, from $4.24 on June 30, 2012, and $0.1 million for the favorable impact of exchange rate differences for the twelve months ended June 30, 2013. Financial expense as a percentage of revenue increased from 0.7% in the twelve months ended June 30, 2012, to 290.9% in the twelve months ended June 30, 2013.
Tax Expenses. For the twelve months ended June 30, 2013, tax expenses decreased $6,000 to $8,000 for the twelve months ended June 30, 2013, from $14,000 during the same period in 2012.
| 48 |
Net Loss. Our net loss increased by $11.7 million, or 66.3%, to $29.3 million for the twelve months ended June 30, 2013 from $17.6 million during the twelve months ended June 30, 2012. The increase in net loss resulted primarily from an increase of $14.2 million in financial expenses (see above for explanation), partially offset by a decrease of $2.4 million in operating expenses (see above for explanation) and an increase of $0.1 million in gross profit (see above for explanation).
Liquidity and Capital Resources
We have an accumulated deficit as of December 31, 2014, as well as net losses and negative operating cash flows in recent years and the current year. We expect to continue incurring losses and negative cash flows from operations until our MGuard and CGuard products reach commercial profitability. Management presently anticipates that it has sufficient resources to fund operations through the second quarter of 2016.
Our plans include the continued commercialization of the MGuard and CGuard products and raising capital through the sale of additional equity securities, debt or capital inflows from strategic partnerships. There are no assurances, however, that we will be successful in obtaining the level of financing needed for our operations. If we are unsuccessful in commercializing our MGuard or CGuard products and raising capital, we may need to reduce activities, curtail or cease operations.
On October 23, 2013, we entered into a loan and security agreement, pursuant to which we received a loan of $10 million, before deduction of issuance costs. Interest on the loan is determined on a daily basis at a variable rate equal to the greater of either (i) 10.5%, or (ii) the sum of (A) 10.5% plus (B) the prime rate minus 5.5%. Payments under the loan and security agreement are interest only for 9 months, followed by 30 monthly payments of principal and interest through the scheduled maturity date on February 1, 2017. Our obligations under the loan and security agreement are secured by a grant of a security interest in all of our assets (other than our intellectual property). In addition, in connection with the loan and security agreement, we issued the lender a five year warrant to purchase 168,351 shares of our common stock at a per share exercise price of $2.97.
On October 23, 2013, we entered into an at-the-market issuance sales agreement with MLV & Co. LLC (MLV), pursuant to which we may issue and sell shares of our common stock in an aggregate amount up to $40 million from time to time in an “at-the-market” offering as defined in Rule 415 under the Securities Act of 1933, as amended, through MLV as our sales agent. On August 15, 2014, we sold 948,000 shares of our common stock, at $2.40 per share, pursuant to the at-the-market issuance sales agreement with MLV. These sales resulted in net proceeds to us of approximately $2.2 million. We paid MLV compensation at a commission rate of 3% of the gross sales. Prior to these sales, we have not made any sales under this “at-the-market” equity offering program, and, as of September 30, 2014, shares of our common stock having an aggregate value of approximately $37.7 million remained available for sale under this offering program. Such sales were made pursuant to our effective $75 million shelf registration statement filed with the SEC in October 2013 (File No. 333-191875). Our securities purchase agreement with purchasers of shares of our common stock and warrants to purchase our common stock, dated November 4, 2014, entered into in connection with the registered direct offering described below, prohibits us from issuing and selling additional shares of our common stock under this “at-the-market” equity offering program until November 7, 2016.
On November 7, 2014, we sold 6,261,846 shares of our common stock and warrants to purchase 3,130,923 shares of our common stock in a registered direct offering. The common stock was sold at a negotiated purchase price of $1.30 per share, and each purchaser received a warrant to purchase one-half of a share of common stock for each share of common stock that it purchased in the offering. The warrants are non-exercisable for six months after the date of issuance and have a term of exercise of 42 months after the date of issuance and an exercise price of $1.75. This offering resulted in net proceeds to us of approximately $7.4 million after deducting placement agent fees and other estimated offering expenses. Such sales were made pursuant to the $75 million shelf registration statement.
On March 9, 2015, we sold 34,369,675 shares of our common stock and warrants to purchase 34,369,675 shares of our common stock in a public offering. Each purchaser received a warrant to purchase one share of common stock for each share of common stock that it purchased in the offering. The warrants have a term of exercise of 5 years from the date of issuance and an exercise price of $0.55. This offering resulted in net proceeds to us of approximately $12.5 million after deducting placement agent fees and other estimated offering expenses. Such sales were made pursuant to the $75 million shelf registration statement.
Twelve months ended December 31, 2014 compared to the twelve months ended December 31, 2013
General. At December 31, 2014, we had cash and cash equivalents of $6.3 million, as compared to $17.5 million as of December 31, 2013. We have historically met our cash needs through a combination of issuing new shares, borrowing activities and product sales. Our cash requirements are generally for clinical trials, marketing and sales activities, finance and administrative cost, capital expenditures and general working capital.
| 49 |
Cash used in our operating activities was $19.4 million for the twelve months ended December 31, 2014 and $11.3 million for the same period in 2013. The principal reason for the usage of cash in our operating activities for the twelve months ended December 31, 2014 was a net loss of $25.1 million, offset by $4.1 million in non-cash share-based compensation that was largely paid to our directors and chief executive officer, a decrease in working capital of $0.9 million, $0.4 million of non-cash financial expense and $0.3 million of depreciation and amortization expenses. The principal reasons for the usage of cash in our operating activities for the twelve months ended December 31, 2013 included a net loss of $29.2 million offset by $12.5 million in non-cash financial expenses, $4.0 million in non-cash share-based compensation, a decrease in working capital of $1.2 million and $0.2 million in depreciation and amortization expenses.
Cash used in our investing activities was $86,000 during the twelve months ended December 31, 2014, compared to $435,000 during the same period in 2013. The principal reason for the decrease in cash used in investing activities during 2014 was a $93,000 decrease in restricted cash upon the removal of fixed liens in connection with our credit cards, as well as a decrease of $162,000 in purchases of property, plant and equipment.
Cash provided by financing activities for the twelve months ended December 31, 2014 was $8.3 million, compared to $23.8 million during the same period in 2013. The principal source of the cash provided by financing activities during the twelve months ended December 31, 2014 relates to funds received from the issuance of shares in a registered direct offering of $7.4 million and funds received from the issuance of at-the-market (“ATM”) shares of $2.2 million, offset by the repayment of a loan of $1.2 million. The principal source of the cash provided by financing activities during the twelve months ended December 31, 2013 relates to funds received from the issuance of shares in connection with the underwritten public offering of our common stock of $22.9 million and $9.8 million received pursuant to a loan and security agreement entered into in October 2013, partially offset by the partial satisfaction of our convertible debentures for approximately $8.8 million.
As of December 31, 2014, our current assets exceeded our current liabilities by a multiple of 1.1. Current assets decreased by $12.2 million during the period, mainly due to cash used in operations, and current liabilities increased by $2.3 million during the period. As a result, our working capital surplus decreased by $14.5 million to $0.9 million at December 31, 2014.
Six month period ended December 31, 2013 compared to the six month period ended December 31, 2012
General. At December 31, 2013, we had cash and cash equivalents of $17.5 million, as compared to $14.8 million as of June 30, 2013.
Cash used in our operating activities was $6.8 million for the six month period ended December 31, 2013 and $5.8 million for the same period in 2012. The principal reason for the usage of cash in our operating activities for the six month period ended December 31, 2013 was a net loss of $9.3 million, offset by $1.5 million in non-cash share-based compensation that was largely paid to our directors and chief executive officer, a decrease in working capital of $0.5 million, $0.4 million of non-cash financial expense, and $0.1 million of depreciation expense. The principal reasons for the usage of cash in our operating activities for the six months ended December 31, 2012 include a net loss of approximately $9.4 million and an increase in working capital of approximately $0.2 million, offset by approximately $1.4 million in non-cash share-based compensation, approximately $1.2 million in non-cash financial expenses, approximately $0.9 million in a non-cash royalties buyout, approximately $0.1 million in depreciation and amortization expenses and approximately $0.2 million of all other miscellaneous expenditures.
Cash used in our investing activities was $252,000 during the six month period ended December 31, 2013, compared to $193,000 during the same period in 2012. The principal reason for the increase in cash used in investing activities during 2013 was the purchase of property, plant and equipment of $180,000 (primarily new manufacturing equipment and leasehold improvements for our production facilities) and the funding of employee retirement funds of $72,000.
Cash generated by financing activities for the six month period ended December 31, 2013 was $9.8 million, compared to $1.0 million generated during the same period in 2012. The principal source of cash generated from financing activities during the six month period ended December 31, 2013 was $9.8 million received pursuant to the loan and security agreement entered into in October 2013, net of issuance costs.
As of December 31, 2013, our current assets exceeded our current liabilities by a multiple of 3.5. Current assets increased $2.7 million during the six month period, mainly due to cash received from the loan and security agreement, partially offset by cash used in operations, and current liabilities increased by 2.1 million during the period. As a result, our working capital surplus increased by $0.6 million to $15.5 million at December 31, 2013.
Twelve months ended June 30, 2013 compared to twelve months ended June 30, 2012
General. At June 30, 2013, we had cash and cash equivalents of $14.8 million, as compared to $10.3 million as of June 30, 2012.
Cash used in our operating activities was $10.3 million for the twelve months ended June 30, 2013 and $8.6 million for the same period in 2012. The principal reasons for the usage of cash in our operating activities for the twelve months ended June 30, 2013 include a net loss of $29.3 million, offset by $13.5 million in non-cash financial expenses, $3.8 million in non-cash share-based compensation that was largely paid to our directors, $0.9 million in a non-cash royalties buyout related to the restructuring of our royalty agreement for the MGuard Prime version of our MGuard Coronary stent, as discussed above, a decrease in working capital of $0.4 million, $0.2 million in depreciation and amortization expenses and $0.2 million of miscellaneous expenditures.
| 50 |
Cash used in our investing activities was $376,000 during the twelve months ended June 30, 2013, compared to $43,000 during the same period in 2012. The principal reason for the increase in cash used in investing activities during 2013 was the purchase of property, plant and equipment of $202,000 (primarily new manufacturing equipment and leasehold improvements for our production facilities), an increase in restricted cash of $56,000 and the funding of employee retirement funds of $118,000.
Cash generated by financing activities was $15.1 million for the twelve months ended June 30, 2013, compared to $11.1 million generated during the same period in 2012. The principal source of cash from financing activities during the twelve months ended June 30, 2013 was funds received from the issuance of shares in connection with the underwritten public offering of our common stock of $22.9 million, as well as $1.0 million from the exercise of options and warrants, partially offset by the partial satisfaction of our convertible debentures for $8.8 million as described below. In contrast, during the twelve months ended June 30, 2012, we received $9.9 million from the initial issuance of these convertible debentures and associated warrants and $1.5 million from the exercise of options, partially offset by a repayment of a long term loan of $0.3 million.
As of June 30, 2013, our current assets exceeded our current liabilities by a multiple of 4.68. Current assets increased $4.6 million during the twelve months period, mainly due to cash received from financing activities, and current liabilities increased by $0.5 million during the same period. As a result, our working capital surplus increased by $4.1 million to $14.9 million at June 30, 2013.
Convertible Debentures
On April 5, 2012, we issued senior secured convertible debentures due April 5, 2014 in the original aggregate principal amount of $11,702,128 and five-year warrants to purchase an aggregate of 835,866 shares of our common stock at an exercise price of $7.20 per share in exchange for aggregate gross proceeds of $11.0 million, with corresponding net proceeds of $9.9 million. The convertible debentures were issued with a 6% original issuance discount, bore interest at an annual rate of 8% and were convertible at any time into shares of common stock at an initial conversion price of $7.00 per share. Upon conversion of the convertible debentures, investors were entitled to receive a conversion premium equal to 8%, per annum, with a limit of 12% for the term of the convertible debentures, of the principal amount being converted. In addition, the investors had the right to require us to redeem the convertible debentures at any time after October 5, 2013 (18 months after the date of issuance) for 112% of the then outstanding principal amount, plus all accrued interest, and we had the right to prepay the convertible debentures after six months for 112% of the then outstanding principal amount, plus all accrued interest. In connection with this financing, we paid placement agent fees of $848,750 and issued placement agents warrants to purchase 78,078 shares of common stock, with terms identical to the warrants issued to the investors.
On April 9, 2013, we entered into an exchange and amendment agreement with the holders of these convertible debentures, pursuant to which, simultaneously with the closing of our underwritten public offering on April 16, 2013, and in full satisfaction of our obligations under the convertible debentures, we:
| · | repaid $8,787,234 in cash; |
| · | issued 2,159,574 shares of common stock to the holders of the convertible debentures, reflecting a conversion price of $2.00 per share for the remaining unpaid portion of the convertible debentures; |
| · | issued five year warrants to the holders of these convertible debentures to purchase an aggregate of 659,091 shares of common stock for $3.00 per share; |
| · | amended the securities purchase agreement pursuant to which the convertible debentures were originally issued to prohibit us from issuing securities containing anti-dilution protective provisions; and |
| · | amended the warrants issued in connection with the convertible debentures to (i) eliminate the automatic incorporation of the terms of any securities that are superior to those of such warrants, except with respect to exercise price and warrant coverage and (ii) provide that upon a fundamental transaction, the holders of such warrants will have the right to cause us to repurchase the unexercised portion of such warrants at their Black-Scholes value on the date of such fundamental transaction, payable in shares of common stock, rather than in cash as was previously provided. |
Off Balance Sheet Arrangements
We have no off-balance sheet transactions, arrangements, obligations (including contingent obligations), or other relationships with unconsolidated entities or other persons that have, or may have, a material effect on our financial condition, changes in financial condition, revenues or expenses, results of operations, liquidity, capital expenditures or capital resources.
Recent Accounting Pronouncements
In May 2014, the Financial Accounting Standards Board issued Accounting Standards Codification 606, Revenue from Contracts with Customers. The objective of the new revenue standard is to provide a single, comprehensive revenue recognition model for all contracts with customers to improve comparability within industries, across industries, and across capital markets. The revenue standard contains principles that an entity will apply to determine the measurement of revenue and timing of when it is recognized. The underlying principle is that an entity will recognize revenue to depict the transfer of goods or services to customers in an amount that the entity expects to be entitled in exchange for those goods or services, based on a five step model that includes the identification of the contract with the customer and the performance obligations in the contract, determination of the transaction price, allocation of the transaction price to the performance obligations in the contract and recognizing revenue when (or as) the entity satisfies a performance obligation. The revenue standard is effective for annual periods beginning on or after January 1, 2017. Early adoption is permitted.
| 51 |
In August 2014, the Financial Accounting Standards Board issued Accounting Standards Update 2014-15, Presentation of Financial Statements-Going Concern (Subtopic 205-40): Disclosure of Uncertainties about an Entity’s Ability to Continue as a Going Concern. Continuation of a reporting entity as a going concern is presumed as the basis for preparing financial statements unless and until the entity’s liquidation becomes imminent. Preparation of financial statements under this presumption is commonly referred to as the going concern basis of accounting. Currently, there is no guidance under U.S. GAAP about management’s responsibility to evaluate whether there is substantial doubt about an entity’s ability to continue as a going concern or to provide related footnote disclosures. The amendments in Accounting Standards Update 2014-15 provide that guidance. In doing so, the amendments should reduce diversity in the timing and content of footnote disclosures. This new standard requires management to assess the entity’s ability to continue as a going concern by incorporating and expanding upon certain principles that are currently in U.S. auditing standards. Specifically, the amendments (1) provide a definition of the term substantial doubt, (2) require an evaluation every reporting period including interim periods, (3) provide principles for considering the mitigating effect of management’s plans, (4) require certain disclosures when substantial doubt is alleviated as a result of consideration of management’s plans, (5) require an express statement and other disclosures when substantial doubt is not alleviated, and (6) require an assessment for a period of one year after the date that the financial statements are issued (or available to be issued). Accounting Standards Update 2014-15 will be effective prospectively for annual reporting periods ending after the first annual period ending after December 15, 2016 and interim periods therein. Early application of the standard is permitted for any annual reporting period or interim period for which the entity’s financial statements have not yet been issued.
Tabular Disclosure of Contractual Obligations
The following table summarizes our outstanding contractual obligations as of December 31, 2014:
| Payments Due By Period | ||||||||||||||||||||
| (in thousands) | ||||||||||||||||||||
| Contractual Obligations | Total | Less than 1 year | 1 – 3 years | 3 – 5 years | More than 5 years | |||||||||||||||
| Long-term loan | 10,391 | 4,565 | 5,826 | |||||||||||||||||
| Operating Lease Obligations (1) | 1,111 | 395 | 496 | 211 | 9 | |||||||||||||||
| Purchase Obligations | 2,825 | 2,825 | ||||||||||||||||||
| Liability for Employees’ Rights Upon Retirement | 687 | 687 | ||||||||||||||||||
| Total | 15,014 | 7,785 | 6,322 | 211 | 696 | |||||||||||||||
| (1) | Our operating lease obligations consist of the lease for our offices and manufacturing facilities in Tel Aviv, Israel and Boston, Massachusetts, as well as leases for the majority of our company cars. |
Factors That May Affect Future Operations
We believe that our future operating results will continue to be subject to quarterly variations based upon a wide variety of factors, including the cyclical nature of the ordering patterns of our distributors, timing of regulatory approvals, the implementation of various phases of our clinical trials and manufacturing efficiencies due to the learning curve of utilizing new materials and equipment. Our operating results could also be impacted by a weakening of the Euro and strengthening of the New Israeli Shekel, or NIS, both against the U.S. dollar. Lastly, other economic conditions we cannot foresee may affect customer demand, such as individual country reimbursement policies pertaining to our products.
Item 7A. Quantitative and Qualitative Disclosures About Market Risk.
We are exposed to market risk related to fluctuations in interest rates and in foreign currency exchange rates.
Interest Rate Exposure
Our exposure to market risk relates primarily to our loan for which the interest rate is the greater of either 10.5% or 10.5% plus the Prime Rate less 5.5%. As of December 31, 2014, the Prime Rate had not reached a high enough percentage for the interest of our loan to be changed from 10.5%.
| 52 |
Foreign Currency Exchange Rate Exposure
Our foreign currency exchange rate exposure continues to evolve as we grow internationally. Our exposure to foreign currency transaction gains and losses is the result of certain revenues and expenses being denominated in currencies other than the U.S. dollar, primarily the Euro and the New Israeli Shekel. We do not currently engage in hedging or similar transactions to reduce these risks. Fluctuations in currency exchange rates could impact our results of operations, financial position, and cash flows. At December 31, 2014, a 10% change in the U.S. dollar strengthening against foreign currencies to which we have balance sheet transactional exposure would have reduced financial expenses, net by $127,000 and a 10% change in the U.S. dollar weakening against these foreign currencies would have increased financial expense, net by $127,000.
Item 8. Financial Statements and Supplementary Data.
The following financial statements are included as part of this Report (See Item 15):
| · | Report of Kesselman & Kesselman, Independent Registered Public Accounting Firm |
| · | Consolidated Balance Sheets as of December 31, 2014 and 2013 |
| · | Consolidated Statements of Operations for the Year Ended December 31, 2014, Six Months Ended December 31, 2013 and Years Ended June 30, 2013 and 2012 |
| · | Consolidated Statements of Changes in Equity for the Year Ended December 31, 2014, Six Months Ended December 31, 2013 and Years Ended June 30, 2013 and 2012 |
| · | Consolidated Statements of Cash Flows for the Year Ended December 31, 2014, Six Months Ended December 31, 2013 and Years Ended June 30, 2013 and 2012 |
| · | Notes to Consolidated Financial Statements |
| 53 |
Selected Quarterly Consolidated Financial Data
The table below sets forth selected quarterly consolidated financial information. The information is derived from our unaudited consolidated financial statements and includes, in the opinion of management, all normal and recurring adjustments that management considers necessary for a fair statement of results for such periods. The operating results for any quarter are not necessarily indicative of results for any future period. (in thousands, except percentage and per share data)
Twelve Months Ended December 31, 2014
| Three Months Ended | ||||||||||||||||
| March 31, 2014 | June 30, 2014 | September 30, 2014 | December 31, 2014 | |||||||||||||
| Net Sales | 1,482 | 193 | 273 | 870 | ||||||||||||
| Gross Profit (Loss) | 857 | (391 | ) | (76 | ) | 394 | ||||||||||
| Operating Expenses | 6,392 | 6,844 | 6,405 | 4,841 | ||||||||||||
| Loss from Operations | (5,535 | ) | (7,235 | ) | (6,481 | ) | (4,447 | ) | ||||||||
| Net Loss | (5,968 | ) | (7,562 | ) | (6,775 | ) | (4,790 | ) | ||||||||
| Loss Per Share | (0.18 | ) | (0.22 | ) | (0.20 | ) | (0.12 | ) | ||||||||
Six Months Ended December 31, 2013
| September 30, 2013 | December 31, 2013 | |||||||
| Net Sales | 1,552 | 1,553 | ||||||
| Gross Profit (Loss) | 802 | 861 | ||||||
| Operating Expenses | 4,687 | 5,803 | ||||||
| Loss from Operations | (3,885 | ) | (4,942 | ) | ||||
| Net Loss | (3,945 | ) | (5,391 | ) | ||||
| Loss Per Share | (0.12 | ) | (0.16 | ) | ||||
Twelve Months Ended June 30, 2013
| Three Months Ended | ||||||||||||||||
| September 30, 2012 | December 31, 2012 | March 31, 2013 | June 30, 2013 | |||||||||||||
| Net Sales | 509 | 1,350 | 1,514 | 1,500 | ||||||||||||
| Gross Profit (Loss) | 279 | 803 | 840 | 668 | ||||||||||||
| Operating Expenses | 3,560 | 5,169 | 4,051 | 4,883 | ||||||||||||
| Loss from Operations | (3,281 | ) | (4,366 | ) | (3,211 | ) | (4,215 | ) | ||||||||
| Net Loss | (7,506 | ) | (1,920 | ) | (4,885 | ) | (14,947 | ) | ||||||||
| Loss Per Share | (0.11 | ) | (0.11 | ) | (0.27 | ) | (0.48 | ) | ||||||||
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure.
Not applicable.
Item 9A. Controls and Procedures.
Management’s Conclusions Regarding Effectiveness of Disclosure Controls and Procedures
We conducted an evaluation of the effectiveness of our “disclosure controls and procedures”, as defined by Rules 13a-15(e) and 15d-15(e) of the Securities Exchange Act of 1934, as amended, as of December 31, 2014, the end of the period covered by this Annual Report on Form 10-K. The disclosure controls and procedures evaluation was done under the supervision and with the participation of management, including our chief executive officer and chief financial officer. There are inherent limitations to the effectiveness of any system of disclosure controls and procedures. Accordingly, even effective disclosure controls and procedures can only provide reasonable assurance of achieving their control objectives. Based upon this evaluation, our chief executive officer and chief financial officer have concluded that our disclosure controls and procedures were effective at the reasonable assurance level as of December 31, 2014.
| 54 |
Management's Report on Internal Control Over Financial Reporting
Management is responsible for establishing and maintaining adequate internal control over financial reporting, as defined in Rules 13a-15(f) and 15d-15(f) under the Securities Exchange Act of 1934, as amended. Our internal control over financial reporting is designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of the consolidated financial statements for external reporting purposes in accordance with generally accepted accounting principles.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness of internal control over financial reporting to future periods are subject to the risk that controls may become inadequate because of changes in conditions or that the degree of compliance with the policies or procedures may deteriorate over time.
Management, including our chief executive officer and our chief financial officer, assessed the effectiveness of our internal control over financial reporting as of December 31, 2014. In making this assessment, management used the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission in Internal Control—Integrated Framework 2013. Based on its assessment and those criteria, management has concluded that we maintained effective internal control over financial reporting as of December 31, 2014.
Kesselman & Kesselman, Certified Public Accountants, a member of PricewaterhouseCoopers International Limited, the independent registered public accounting firm that audited the Company's consolidated financial statements included in this Annual Report on Form 10-K, has issued an attestation report on the Company's internal control over financial reporting, which is included herein.
Changes in Internal Control over Financial Reporting
There have been no changes in our internal control over financial reporting during the fiscal quarter ended December 31, 2014 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
Not applicable.
Item 10. Directors, Executive Officers and Corporate Governance.
Executive Officers and Directors
The following table sets forth information regarding our executive officers and the members of our board of directors.
| Name | Age | Position |
| Alan Milinazzo | 55 | President, Chief Executive Officer and Director |
| Craig Shore | 53 | Chief Financial Officer, Secretary and Treasurer |
| James Barry, Ph.D. | 55 | Executive Vice President, Chief Operating Officer and Director |
| Eli Bar | 50 | Senior Vice President of Research and Development and Chief Technical Officer of InspireMD Ltd. |
| Sol J. Barer, Ph.D. | 67 | Chairman of the Board of Directors |
| Michael Berman | 57 | Director |
| James J. Loughlin | 72 | Director |
| Campbell Rogers, M.D. | 53 | Director |
| Paul Stuka | 60 | Director |
Our directors hold office until the earlier of their death, resignation or removal by stockholders or until their successors have been qualified. Our directors are divided into three classes. Alan Milinazzo, Sol J. Barer, Ph.D. and Paul Stuka are our Class 1 directors, with their terms of office to expire at our 2015 annual meeting of stockholders. James J. Loughlin and Michael Berman are our Class 2 directors, with their terms of office to expire at our 2016 annual meeting of stockholders. Campbell Rogers, M.D. and James Barry, Ph.D. are our Class 3 directors, with their terms of office to expire at our 2017 annual meeting of stockholders. At each annual meeting of stockholders, directors elected to succeed those directors whose terms expire shall be elected for a term of office to expire at the third succeeding annual meeting of stockholders after their election, with each director to hold office until his or her successor shall have been duly elected and qualified.
Our officers hold office until the earlier of their death, resignation or removal by our board of directors or until their successors have been selected. They serve at the pleasure of our board of directors.
| 55 |
Alan Milinazzo has served as our president, chief executive officer and director since January 3, 2013. Mr. Milinazzo served as president and chief executive officer of Orthofix International N.V., a Nasdaq-listed medical device company, until August 2011, a position he was promoted to in 2006 after being hired a year earlier as chief operating officer. He also served as a director of Orthofix International N.V. from December 2006 until June 2012, and currently serves as a director of Flexion Therapeutics (NSDQ: FLXN) and the Musculoskeletal Transplant Foundation. From 2002 to 2005, Mr. Milinazzo was the general manager of Medtronic, Inc.’s coronary and peripheral vascular businesses. Mr. Milinazzo also spent 12 years as an executive with Boston Scientific Corporation in numerous roles, including vice president of marketing for SCIMED Europe. Mr. Milinazzo has over 20 years of experience in management and marketing, including positions with Aspect Medical Systems and American Hospital Supply. As chief executive officer, Mr. Milinazzo’s position on the board ensures a unity of vision between the broader goals of our company and our day-to-day operations.
Craig Shore has served as our chief financial officer, secretary and treasurer since March 31, 2011 and as our chief administrative officer since May 3, 2013. In addition, from November 10, 2010 through March 31, 2011, Mr. Shore served as InspireMD Ltd.’s vice president of business development. From February 2008 through June 2009, Mr. Shore served as chief financial officer of World Group Capital Ltd. and Nepco Star Ltd., both publicly traded companies on the Tel Aviv Stock Exchange, based in Tel Aviv, Israel. From March 2006 until February 2008, Mr. Shore served as the chief financial officer of Cellnets Solutions Ltd., a provider of advanced cellular public telephony solutions for low to middle income populations of developing countries based in Azur, Israel. Mr. Shore has over 25 years of experience in financial management in the U.S., Europe and Israel. His experience includes raising capital both in the private and public markets. Mr. Shore graduated with honors and received a B.Sc. in Finance from Pennsylvania State University and an M.B.A. from George Washington University.
James Barry, Ph.D. has served as a director since January 30, 2012 and as our executive vice president and chief operating officer since July 14, 2014. Dr. Barry has served as executive vice president and chief operating officer at Arsenal Medical Inc., a medical device company focused on local therapy, since September 2011. Dr. Barry also heads his own consulting firm, Convergent Biomedical Group LLC, advising medtech companies on product development, strategy, regulatory challenges and fund raising. Until June 2010, he was senior vice president, corporate technology development at Boston Scientific Corporation, where he was in charge of the corporate research and development and pre-clinical sciences functions. Dr. Barry joined Boston Scientific in 1992 and oversaw its efforts in the identification and development of drug, device and biological systems for applications with implantable and catheter-based delivery systems. He currently serves on a number of advisory boards including the College of Biomedical Engineering at Yale University, the College of Sciences at University of Massachusetts-Lowell and the Massachusetts Life Science Center and as a director of pSivida Corp (NASDAQ: PSDV). Dr. Barry received his Ph.D. in Biochemistry from the University of Massachusetts-Lowell and holds a B.A. degree in Chemistry from Saint Anselm College. Dr. Barry brings to the board over 20 years of experience in leadership roles in the medical device industry and significant medical technology experience, in particular with respect to interventional cardiology products.
Eli Bar has served as InspireMD Ltd.’s senior vice president of research and development and chief technical officer since February 2011. Prior to that, he served as InspireMD Ltd.’s vice president of research and development since October 2006 and engineering manager since June 2005. Mr. Bar has over 15 years’ experience in medical device product development. Mr. Bar has vast experience building a complete research and development structure, managing teams from the idea stage to an advanced marketable product. He has been involved with many medical device projects over the years and has developed a synthetic vascular graft for femoral and coronary artery replacement, a covered stent and a fully implantable ventricular assist device. Mr. Bar has more than 21 filed device and method patent applications, has initiated two medical device projects and has two medical publications on STEMI. Mr. Bar is also a director of Blue Surgical Ltd., a medical device company based in Israel. Mr. Bar graduated from New Haven University in Connecticut with a B.Sc. in Mechanical Engineering.
Sol J. Barer, Ph.D. has served as a director since July 11, 2011 and has served as our chairman since November 16, 2011. Dr. Barer has over 25 years of experience with publicly traded biotechnology companies. In 1980, when Dr. Barer was with Celanese Research Company, he formed the biotechnology group that was subsequently spun out to form Celgene Corporation. Dr. Barer spent 18 years leading Celgene Corporation as president, chief operating officer and chief executive officer, culminating with his tenure as Celgene Corporation’s executive chairman and chairman beginning in May 2006 until his retirement in June 2011. Dr. Barer is also a director of Cerecor, Inc., Edge Therapeutics, Inc., Medgenics, Inc., Centrexion Corporation, RestorGenex Corporation, ContraFect Corporation, Amicus Therapeutics, Inc. and Aegerion Pharmaceuticals, Inc. and serves as a senior advisor to a number of other biotechnology companies. Dr. Barer received a Ph.D. in organic chemistry from Rutgers University. Dr. Barer brings to the board significant scientific and executive leadership experience in the U.S. biotechnology industry and prior service on the board of directors of other publicly-held biopharmaceutical companies, as well as a unique perspective on the best methods of growth for a biotechnology company.
Michael Berman has served as our director since February 7, 2013. Mr. Berman is a medical device entrepreneur who works with high-potential development and early-stage commercial companies. From 2005 to 2012, when the company was sold to Boston Scientific, Mr. Berman was a co-founder and the chairman of BridgePoint Medical, Inc., which developed technology to treat coronary and peripheral vascular chronic total occlusions. Mr. Berman was also a member of the board of Lutonix, Inc. from 2007 until 2011, when the company was sold to C.R. Bard, Inc. Mr. Berman has served (i) since 2003 as co-founder and a director of Aetherworks II, a medical device incubator, (ii) since 2004 as a co-founder and director of Benechill, Inc., a company developing a therapeutic hypothermia system for the treatment of cardiac arrest, (iii) since 2011 as an advisor to, and since 2012 as a director of, Cardiosonic, Inc., a company developing a system for hypertension reduction via renal denervation, (iv) since 2005 as a director of PharmaCentra, LLC, which creates customizable marketing programs that help pharmaceutical companies communicate with physicians and patients, (v) since 2011 as a co-founder and director of Rebiotix Inc., a company developing an innovative treatment for C Diff colitis, (vi) since 2011 as a director of AngioSlide Ltd., a medical device company that has developed an embolic capture angioplasty device, (vii) since 2011 as a director of InterValve, Inc., a medical device company developing an aortic valvuloplasty balloon for treatment of calcific aortic stenosis, (viii) since 2013 as a Director of ClearCut Inc., a medical device company that has developed an MRI system for tumor margin assessment, (ix) since 2013 as a director of PulmOne Ltd., a medical device company developing an innovative Pulmonary Function Testing system, (x) since 2014 as a director of Mazor Robotics, Inc., a publicly held company that has developed and markets an innovative system for robotic surgery, (xi) since 2014 as a director of SoniVie, a medical device company and (xii) since 2014 as a venture partner at RiverVest Ventures. Mr. Berman was a member of the Data Sciences International, Inc. board from 2001 until 2012. Mr. Berman brings to the board his extensive executive and entrepreneurial experiences in the field of medical devices and interventional cardiology, which should assist in strengthening and advancing our strategic focus.
| 56 |
James J. Loughlin has served as our director since September 19, 2012. Mr. Loughlin served as the national director of the pharmaceuticals practice at KPMG LLP, and a five-year term as member of the board of directors of KPMG LLP. Additionally, Mr. Loughlin served as chairman of the pension and investment committee of the KPMG LLP board from 1995 through 2001. He also served as partner in charge of human resources, chairman of the personnel and professional development committee, secretary and trustee of the Peat Marwick Foundation and a member of the pension, operating and strategic planning committees. In addition, Mr. Loughlin has served as a member of the board of directors of Celgene Corporation, a global biopharmaceutical company focused on novel therapies for the treatment of cancer and inflammatory diseases, since 2006, including as chairman of the audit committee since June 2008 and a member of the compensation committee since June 2008. Mr. Loughlin served as a member of the board of directors of Alfacell Corporation, a biopharmaceutical company primarily focused on therapeutic drugs for the treatment of cancer and other pathological conditions, until 2008 and Datascope Corp., a medical device company engaged in the interventional cardiology and radiology, cardiovascular and vascular surgery, and critical care fields, until January 2009. Mr. Loughlin brings to the board his valuable experiences as national director of the pharmaceuticals practice at KPMG LLP, an extensive background in accounting and financial reporting, qualifying him as an audit committee financial expert, and prior service on the board of directors of other publicly-held biopharmaceutical companies.
Campbell Rogers, M.D. has served as a director since September 3, 2013. Dr. Rogers has served as chief medical officer of HeartFlow, Inc., a cardiovascular diagnostics company, since March 2012. Prior to joining HeartFlow, Inc., he was the chief scientific officer and global head of research and development at Cordis Corporation, Johnson & Johnson, where he was responsible for leading investments and research in cardiovascular devices, from July 2006 to March 2012. Prior to that, he was associate professor of medicine at Harvard Medical School and the Harvard-M.I.T. Division of Health Sciences and Technology and director of the cardiac catheterization and experimental cardiovascular interventional laboratories at Brigham and Women’s Hospital. He served as principal investigator for numerous interventional cardiology device, diagnostic, and pharmacology trials, is the author of numerous journal articles, chapters, and books in the area of coronary artery and other cardiovascular diseases and was the recipient of research grant awards from the National Institute of Health and the American Heart Association. He received his A.B. from Harvard College and his M.D. from Harvard Medical School. Dr. Rogers’ qualifications to serve on the board include his significant experience in cardiovascular devices, as well as his familiarity with the operations of medical device companies.
Paul Stuka has served as a director since August 8, 2011. Mr. Stuka has served as the managing member of Osiris Partners, LLC, an investment fund, since 2000. Prior to forming Osiris Partners, LLC, Mr. Stuka, with 35 years of experience in the investment industry, was a managing director of Longwood Partners, managing small cap institutional accounts. In 1995, Mr. Stuka joined State Street Research and Management as manager of its Market Neutral and Mid Cap Growth Funds. From 1986 to 1994, Mr. Stuka served as the general partner of Stuka Associates, where he managed a U.S.-based investment partnership. Mr. Stuka began his career in 1980 as an analyst at Fidelity Management and Research. As an analyst, Mr. Stuka followed a wide array of industries including healthcare, energy, transportation, and lodging and gaming. Early in his career he became the assistant portfolio manager for three Fidelity Funds, including the Select Healthcare Fund which was recognized as the top performing fund in the U.S. for the five-year period ending December 31, 1985. Mr. Stuka has served as a director of Caliber Imaging & Diagnostics, Inc. (formerly Lucid, Inc.) since June 2013. Mr. Stuka’s qualifications to serve on the board include his significant strategic and business insight from his years of experience investing in the healthcare industry.
Messrs. Milinazzo, Shore and Bar and Dr. Barry are parties to certain agreements related to their service as executive officers and directors described under “Item 11. Executive Compensation – Agreements with Executive Officers.”
Family Relationships
We have no family relationships amongst our directors and executive officers.
Section 16(a) Beneficial Ownership Reporting Compliance
Section 16(a) of the Securities Exchange Act of 1934, as amended, requires our directors and officers, and persons who own more than ten percent of our common stock, to file with the Securities and Exchange Commission initial reports of ownership and reports of changes in ownership of our common stock. Directors, officers and persons who own more than ten percent of our common stock are required by Securities and Exchange Commission regulations to furnish us with copies of all Section 16(a) forms they file.
To our knowledge, based solely on a review of the copies of such reports furnished to us, during the twelve months ended December 31, 2014, each of our directors, officers and greater than ten percent stockholders complied with all Section 16(a) filing requirements applicable to our directors, officers and greater than ten percent stockholders, except for one late report on Form 4 for Mr. Milinazzo with respect to one transaction, two late reports on Form 4 for Rick Olson, our former vice president of global sales and operations, each with respect to one transaction, and one late report on Form 4 for Dr. Barry with respect to one transaction.
| 57 |
Board Committees
Our board of directors has established an audit committee, a nominating and corporate governance committee and a compensation committee, each of which has the composition and responsibilities described below.
Audit Committee. Our audit committee is currently comprised of Messrs. Loughlin and Stuka and Dr. Barer, each of whom our board has determined to be financially literate and qualify as an independent director under Section 803(B)(2) of the NYSE MKT rules. Mr. Loughlin is the chairman of our audit committee and qualifies as a financial expert, as defined in Item 407(d)(5)(ii) of Regulation S-K. The audit committee’s duties are to recommend to our board of directors the engagement of independent auditors to audit our financial statements and to review our accounting and auditing principles. The audit committee will review the scope, timing and fees for the annual audit and the results of audit examinations performed by the internal auditors and independent public accountants, including their recommendations to improve the system of accounting and internal controls.
Nominating and Corporate Governance Committee. Our nominating and corporate governance committee is currently comprised of Messrs. Berman and Stuka and Dr. Barer, each of whom qualify as an independent director under Section 803(A) of the NYSE MKT rules. Mr. Berman is the chairman of our nominating and corporate governance committee. The nominating and corporate governance committee identifies and recommends to our board of directors individuals qualified to be director nominees. In addition, the nominating and corporate governance committee recommends to our board of directors the members and chairman of each board committee who will periodically review and assess our code of business conduct and ethics and our corporate governance guidelines. The nominating and corporate governance committee also makes recommendations for changes to our code of business conduct and ethics and our corporate governance guidelines to our board of directors, reviews any other matters related to our corporate governance and oversees the evaluation of our board of directors and our management.
Compensation Committee. Our compensation committee is currently comprised of Messrs. Stuka and Loughlin and Dr. Barer, each of whom qualify as an independent director under Sections 803(A) and 805(c)(1) of the NYSE MKT rules. Mr. Stuka is the chairman of our compensation committee. The compensation committee reviews and approves our salary and benefits policies, including compensation of executive officers and directors. The compensation committee also administers our stock option plans and recommends and approves grants of stock options under such plans.
Code of Ethics
We have adopted a code of ethics and business conduct that applies to our officers, directors and employees, including our principal executive officer, principal financial officer and principal accounting officer, which is posted on our website at www.inspire-md.com. We intend to disclose future amendments to certain provisions of the code of ethics, or waivers of such provisions granted to executive officers and directors, on this website within four business days following the date of such amendment or waiver.
Item 11. Executive Compensation.
Compensation Discussion and Analysis
The Compensation Discussion and Analysis discusses the principles underlying our executive compensation policies and decisions for our named executive officers. It provides qualitative information regarding the manner in which compensation is earned by our named executive officers and places in context the data presented in the tables that follow. In addition, we address the compensation paid or awarded during 2014 to our named executive officers: Alan Milinazzo, our president and chief executive officer, Craig Shore, our chief financial officer, secretary and treasurer, James Barry, Ph.D., our executive vice president and chief operating officer, Eli Bar, the senior vice president of research and development and chief technical officer of InspireMD Ltd., and Rick Olson, the former vice president of global sales and operations of InspireMD Ltd. Mr. Olson resigned from employment with us effective February 9, 2015.
The compensation committee of our board of directors reviews at least annually and determines the executive compensation packages for Mr. Milinazzo, including approving any equity grants. Mr. Milinazzo is responsible for making recommendations to our compensation committee with respect to the executive compensation packages for Messrs. Shore and Bar and Dr. Barry, and formerly Mr. Olson, including any equity grants.
In considering compensation for our named executive officers, the board of directors has historically relied upon the officer’s performance and contribution to our development and achievements, as well as the use of formal benchmarking of executive compensation at peer companies. We also consider general compensation trends.
During the compensation committee’s review of named executive officer compensation for 2014, the compensation committee retained the services of Radford, a compensation consultant. The consultant provided a report that included formal benchmarking of our named executive officers’ compensation against that at companies selected by the consultant and approved by our compensation committee. The peer group was comprised of 20 U.S.-based public medical devices companies that were determined to have a comparable business and financial profile to us, in terms of revenue, employee size and/or market value:
| 58 |
| AntriCure | AxoGen | Baxano Surgical |
| BIOLASE | Cardica | Cerus |
| Cryolife | Cutera | Cytori Therapeutics |
| Digrad | Hansen Medical | LDR |
| LeMaitre Vascular | STAAR Surgical | Stereotaxis |
| Sunshine Heart | SurModics | Uroplasty |
| Utah Medical Products | Vascular Solutions |
The compensation consultant’s report and recommendations primarily compared the compensation of our named executive officers to the applicable market 50th percentile. In light of our current financial position and our long-term and short-term goals, the compensation committee determined not to increase named executive officer salary compensation for 2014, except as described below, but to align bonus compensation and equity-based grants at the market 75th percentile. The compensation committee determined to take four actions with respect to increases in named executive officer compensation in 2014, in the form of a base salary increase and an equity grant to Mr. Shore on the terms and for the reasons described under “Named Executive Officer Compensation – Compensation of Chief Financial Officer, Chief Administrative Officer Secretary and Treasurer” below, an increase in the target cash bonus award for Messrs. Shore and Bar, on the terms and for the reasons described under “Named Executive Officer Compensation – Compensation of Chief Financial Officer, Chief Administrative Officer, Secretary and Treasurer” and “Named Executive Officer Compensation – Compensation of Senior Vice President of Research and Development and Chief Technical Officer of InspireMD Ltd” below and an annual equity award grant for Messrs. Shore and Bar, on the terms and for the reasons described under “Named Executive Officer Compensation – Compensation of Chief Financial Officer, Secretary and Treasurer” and “Named Executive Officer Compensation – Compensation of Senior Vice President of Research and Development and Chief Technical Officer of InspireMD Ltd” below.
We have entered into agreements with all of our named executive officers. These agreements are summarized under “Agreements with Executive Officers.”
Philosophy of Compensation
The goals of our compensation policy are to ensure that executive compensation rewards management for helping us achieve our financial goals (increased sales, profitability, etc.) and meet our clinical trial milestones and aligns management’s overall goals and objectives with those of our stockholders. To achieve these goals, our board of directors and, going forward, our compensation committee, aims to:
| · | provide a competitive compensation package that enables us to attract and retain superior management personnel; |
| · | provide incentives that reward the achievement of performance goals that directly correlate to the enhancement of stockholder value and facilitate executive retention; |
| · | reward our officers fairly for their role in our achievements; and |
| · | align executives’ interests with those of stockholders through long-term incentives linked to specific performance. |
We have determined that in order to best meet these objectives, our executive compensation program should balance fixed and bonus compensation, as well as cash and equity compensation, as discussed below. Historically, there has been no pre-established policy or target for the allocation between either cash and non-cash or short-term and long-term incentive compensation for our executive officers.
Components of Compensation
The principal components of compensation for our named executive officers are base salary/consulting fees, equity based grants, personal benefits and perquisites and, potentially in the future, cash bonuses.
Base Salary. The primary component of compensation for our named executive officers is base salary. Base salary levels for our named executive officers have historically been determined based upon an evaluation of a number of factors, including the individual officer’s level of responsibility, length and depth of experience and our assessment of the officer’s future potential with our company, performance and, to the extent available, general compensation levels of similarly situated executives and general compensation trends. Although our employment agreements with our named executive officers set forth a fixed base salary, salaries have been reviewed periodically and changed, when deemed appropriate, by oral or written amendment to the applicable officer’s agreement. For the twelve months ended December 31, 2014, the compensation consultant’s report proposed salary adjustments for most named executive officers to move them towards the applicable market 50th percentile. The compensation committee determined, in light of our current financial position and our long-term and short-term goals, not to align base salary at the 50th percentile, and to compensate for this by aligning bonus compensation and equity-based grants at the market 75th percentile.
| 59 |
Cash Bonus. An additional principal component of our compensation policy for named executive officers is a cash bonus. We consider the amount of cash bonus that each of our named executive officers should be entitled to receive at the end of the year in connection with our annual compensation review, taking into account each executive’s total compensation package, the recommendations of our compensation consultant, and any more formal data we obtain regarding the compensation levels of similarly situated executives. For the twelve months ended December 31, 2014, the compensation consultant’s report proposed a cash bonus target at the market 75th percentile, which was accepted by the compensation committee. Certain financial and operation metrics such as revenue, cash management, clinical enrollment and partnership targets were used in determining the final amount of such awards. We anticipate that similar metrics will be used in determining cash bonuses for 2015.
Equity Based Grants. An additional principal component of our compensation policy for named executive officers consists of grants under the InspireMD, Inc. 2011 UMBRELLA Option Plan and the 2013 Long-Term Incentive Plan. Under these plans, among other awards, executive officers may be granted stock options and restricted shares. The compensation committee administers the grants of awards under the plans. To date, all equity incentive awards have been made either (i) in accordance with negotiated terms set forth in our employment agreements, at levels deemed necessary to attract or retain the executive at the time of such negotiations and determined taking into account the recipient’s overall compensation package and the goal of aligning such executive’s interest with that of our stockholders, or (ii) at the discretion of the compensation committee without reference to any formal targets or objectives, when deemed appropriate in connection with extraordinary efforts or results or necessary in order to retain the executive in light of the executive’s overall compensation package. For 2014, the compensation consultant’s report proposed an annual equity based grant at the market 75th percentile, which was accepted by the compensation committee. These awards were made partially in the form of stock options and partially in the form of restricted stock. A stock option becomes valuable only if our common stock price increases above the option exercise price and the holder of the option remains employed during the period required for the option to “vest,” thus providing an incentive for an option holder to remain our employee. In addition, stock options link a portion of an employee’s compensation to stockholders’ interests by providing an incentive to increase the market price of our stock. Restricted stock consists of shares of common stock that may not be sold, transferred, pledged, hypothecated, encumbered or otherwise disposed of, and that may be forfeited in the event of certain terminations of employment or service, prior to the end of a restricted period specified by the compensation committee. Restricted stock awards with significant vesting periods and other conditions help ensure that those individuals remain with the Company and are incentivized over a long-term horizon to maximize stockholder value.
We believe that equity ownership of our company by our named executive officers will further align the interests of our executive officers with those of our stockholders.
Personal Benefits and Perquisites. Certain of our named executive officers are entitled to additional personal benefits in accordance with what we believe to be customary practice and law in the country in which the individual in located. In Israel, this is comprised primarily of contributions towards pension and vocational studies funds, annual recreational allowances, a company car, a daily food allowance and a company phone. In the U.S., this is comprised primarily of reimbursement for employees’ health insurance. In the U.K, this is comprised primarily of contributions towards pension. We believe these benefits are commonly provided to executives in the applicable country and we therefore believe that it is necessary for us to provide these benefits in order to attract and retain superior management personnel.
Compensation of Named Executive Officers
Compensation of Chief Executive Officer. During the twelve months ended December 31, 2014, Mr. Milinazzo’s total compensation was comprised of salary payments under his employment agreement with us, a cash bonus, option and restricted share grants under the 2013 Long-Term Incentive Plan, as more fully discussed below, and benefits related to health insurance. Mr. Milinazzo’s base salary and target cash bonus amounts for the twelve months ended December 31, 2014 remained unchanged from 2013 (which were set forth in his employment agreement), since they were determined in the compensation consultant’s report to be at the market 50th percentile. Mr. Milinazzo’s 2014 cash bonus amount was calculated based on the Company’s achievements of objectives set early in 2014 that related to revenue, cash management, clinical enrollment and partnership targets in 2014. The bonus payout was approximately 25% of his targeted bonus.
On January 31, 2014, Mr. Milinazzo received 96,400 shares of our restricted common stock and an option to purchases 313,350 shares of our common stock at an exercise price of $2.97 per share as an annual equity grant. The options and restricted shares vest on an annual basis over three years and had a fair market value of $858,929 as of January 31, 2014. The compensation consultant’s report proposed the fair market value of the shares and options to be at the market 75th percentile, which was approved by the compensation committee. The split between options and restricted shares was proposed to be an equal split in the compensation consultant’s report, however, the compensation committee deemed it more appropriate to have two-thirds of the fair market value of the equity grant granted in stock options, because of the long-term incentive component of stock options as compared to restricted shares. In additional to Mr. Milinazzo’s annual equity grant, on January 29, 2014, Mr. Milinazzo received 86,235 shares of our restricted common stock and an option to purchases 86,235 shares of our common stock at an exercise price of $3.10 per share. This award was Mr. Milinazzo’s annual achievement grant under his employment agreement pertaining to the 2013 calendar year, for which he was eligible to receive, in aggregate, up to 0.5% of the actual outstanding shares of our common stock on the date of the grant. Our Board of Directors determined the amount of restricted shares and options based on the achievement of certain performance objectives in the 2013 calendar year, as established by the board. The options and restricted shares vest on an annual basis over three years and had a fair market value of $431,469 as of January 29, 2014.
| 60 |
Compensation of Chief Financial Officer, Chief Administrative Officer, Secretary and Treasurer. During the twelve months ended December 31, 2014, Mr. Shore’s total compensation was comprised of salary payments under his employment agreement with us, a cash bonus, option and restricted share grants under the 2013 Long-Term Incentive Plan, as more fully discussed below, and benefits and perquisites, as more fully discussed below. Mr. Shore’s base salary for the twelve months ended December 31, 2014 was increased as proposed in the compensation consultant’s report and approved by the compensation committee, in order to reduce the gap between his base salary and the market 50th percentile base salary. Mr. Shore’s target cash bonus was increased from 30% of his base salary to 45% of his base salary for the twelve months ended December 31, 2014, as proposed in the compensation consultant’s report and approved by the compensation committee in order for his cash bonus target to be at the market 75th percentile. Mr. Shore’s 2014 cash bonus amount was calculated based on the Company’s achievements of objectives set early in 2014 that related to revenue, cash management, clinical enrollment and partnership targets in 2014. The bonus payout was approximately 25% of his targeted bonus.
Mr. Shore also received various benefits, many of which either are required by Israeli law or we believe are customarily provided to Israeli executives, including contributions to his pension and vocational studies funds, an annual recreation payment, a company car, a company cell phone and daily food allowance.
On January 31, 2014, Mr. Shore received 29,735 shares of our restricted common stock and an option to purchases 96,670 shares of our common stock at an exercise price of $2.97 per share as an annual equity grant. The options and restricted shares vest on an annual basis over three years and had a fair market value of $264,969 as of January 31, 2014. The compensation consultant’s report proposed the fair market value of the shares and options to be at the market 75th percentile, which was approved by the compensation committee. The split between options and restricted shares was proposed to be an equal split in the compensation consultant’s report, however, the compensation committee deemed it more appropriate to have two-thirds of the fair market value of the equity grant granted in stock options, because of the long-term incentive component of stock options as compared to restricted shares. In addition to Mr. Shore’s annual equity grant, on January 29, 2014, Mr. Shore received 77,000 shares of our restricted common stock and an option to purchases 77,000 shares of our common stock at an exercise price of $3.10 per share. The options and restricted shares vest on an annual basis over three years and had a fair market value of $384,860 as of January 29, 2014. This award was recommended in the compensation consultant’s report and approved by the compensation committee given Mr. Show’s below-market cash positioning and his low ownership percentage relative to the market given his tenure with us. The amount of the award was determined in order to bring Mr. Shore’s total ownership up to the market 50th percentile of 1.0%, before giving effect to the annual grant.
Compensation of Executive Vice President and Chief Operating Officer. During the twelve months ended December 31, 2014, Dr. Barry’s total compensation was comprised of salary payments under his employment agreement with us, director fees, a cash bonus, option and restricted share grants under the 2013 Long-Term Incentive Plan, as more fully discussed below, and benefits and perquisites, as more fully discussed below. Dr. Barry’s base salary and target bonus amounts and equity grants as an employee are included in his employment agreement and were negotiated with Mr. Milinazzo and approved by the compensation committee. They were not included in the consultant’s compensation report as he was yet to be employed by us at the time of the report. Dr. Barry’s base salary also includes director’s fees paid to him in the first two quarters of 2014. Dr. Barry’s director’s fees were increased from 2013 as proposed in the compensation consultant’s report and approved by the compensation committee, in order to reduce the gap between his director’s fees and the director’s fees at market 50th percentile. When Dr. Barry became our executive vice president and chief operating officer, he ceased to be paid for his services as a director. Dr. Barry’s 2014 cash bonus amount was calculated based on the Company’s achievements of objectives set early in 2014 that related to revenue, cash management, clinical enrollment and partnership targets in 2014. The bonus payout was approximately 25% of his targeted bonus.
In accordance with his employment agreement, on July 14, 2014, we granted Dr. Barry a nonqualified stock option to purchase 335,058 shares of our common stock, made pursuant to a nonqualified stock option agreement, an incentive stock option to purchase 114,942 shares of our common stock, made pursuant to an incentive stock option agreement, and 150,000 shares of restricted stock, which are subject to forfeiture until the vesting of such shares, made pursuant to a restricted stock award agreement. The options have an exercise price of $2.61, which was the fair market value of our common stock on the date of grant. The options are subject to a three-year vesting period subject to Dr. Barry’s continued service with us. The options and restricted stock had a fair market value of $1,099,136 as of July 14, 2014. We also granted Dr. Barry an option to purchase 50,000 shares of our common stock on January 29, 2014 as compensation for serving as our director. The option has an exercise price of $3.10 per share and vests annually, with one-third vesting in 2015, 2016 and 2017 on the anniversary of the date of grant, provided that if Dr. Barry fails to be reelected or nominated for reelection at the 2017 annual meeting of stockholders, the option vests and becomes exercisable as of such date. The option will expire on January 29, 2024 and had a fair market value of $94,909 as of January 29, 2014.
Compensation of Senior Vice President of Research and Development and Chief Technical Officer of InspireMD Ltd. During the twelve months ended December 31, 2014, Mr. Bar’s total compensation was comprised of salary payments under his employment agreement with us, a cash bonus, option and restricted share grants under the 2013 Long-Term Incentive Plan, as more fully discussed below, and benefits and perquisites, as more fully discussed below. Mr. Bar’s base salary for the twelve months ended December 31, 2014 remained unchanged from 2013 as he received an increase in 2013, and the gap between his base salary and the market 50th percentile was not deemed to be significant enough by the compensation committee to warrant an adjustment. Mr. Bar’s target cash bonus was increased from 30% of his base salary to 45% of his base salary for the twelve months ended December 31, 2014, as proposed in the compensation consultant’s report and approved by the compensation committee in order for his cash bonus target to be at the market 75th percentile. Mr. Bar’s 2014 cash bonus amount was calculated based on the Company’s achievements of objectives set early in 2014 that related to revenue, cash management, clinical enrollment and partnership targets in 2014. The bonus payout was approximately 25% of his targeted bonus.
| 61 |
Mr. Bar also received various benefits, many of which either are required by Israeli law or we believe are customarily provided to Israeli executives, including contributions to his pension and vocational studies funds, an annual recreation payment, a company car, a company cell phone and daily food allowance.
On January 31, 2014, Mr. Bar received 29,735 shares of our restricted common stock and an option to purchases 96,670 shares of our common stock at an exercise price of $2.97 per share as an annual equity grant. The options and restricted shares vest on an annual basis over three years and had a fair market value of $264,969 as of January 31, 2014. The compensation consultant’s report proposed the fair market value of the shares and options to be at the market 75th percentile, which was approved by the compensation committee. The split between options and restricted shares was proposed to be an equal split in the compensation consultant’s report, however, the compensation committee deemed it more appropriate to have two-thirds of the fair market value of the equity grant granted in stock options, because of the long-term incentive component of stock options as compared to restricted shares.
Compensation of Vice President of Global Sales and Operations of InspireMD Ltd. During the twelve months ended December 31, 2014, Mr. Olson’s total compensation was comprised of salary payments under his employment agreement with us, a cash bonus and pension contribution benefits. Mr. Olson’s compensation for the twelve months ended December 31, 2014 was not adjusted as proposed by the compensation consultant’s report due to his short tenure with the Company at the time and that his overall compensation was at market 50th percentile. Mr. Olson’s cash bonus for the twelve months ended December 31, 2014 was calculated based on 0.75% of our 2014 sales.
Impact of Tax Laws
Deductibility of Executive Compensation. Generally, under U.S. law, a company may not deduct compensation of more than $1,000,000 that is paid to an individual employed by the company who, on the last day of the taxable year, either is the company’s principal executive officer or an individual who is among the three highest compensated officers for the taxable year (other than the principal executive officer or the principal financial officer). The $1,000,000 limitation on deductions does not apply to certain types of compensation, including qualified performance-based compensation. We believe that compensation paid under our incentive plans is generally fully deductible for federal income tax purposes. However, in the future, the compensation committee would determine to approve compensation that will not meet these requirements in order to ensure competitive levels of total compensation for our executive officers.
Impact of Israeli Tax Law. The awards granted to employees pursuant to Section 102 of the Tax Ordinance under the InspireMD, Inc. 2011 UMBRELLA Option Plan and the InspireMD, Inc. 2013 Long-Term Incentive Plan may be designated by us as approved options under the capital gains alternative, or as approved options under the ordinary income tax alternative, or as non-approved options.
To qualify for the capital gains alternative, certain requirements must be met, including registration of the options in the name of a trustee. Each option, and any shares of common stock acquired upon the exercise of the option, must be held by the trustee for a period commencing on the date of grant and deposit into trust with the trustee and ending 24 months thereafter.
Under the terms of the capital gains alternative, we may not deduct expenses pertaining to the options for tax purposes.
Termination Payments
Our agreements with Messrs. Milinazzo, Shore Bar and Mr. Olson, and Dr. Barry and Israeli law provide for payments and other compensation in the event of termination under certain circumstances, as more fully described under “Potential Payments Upon Termination or Change of Control.” These provisions are comprised of (i) notice periods of varying length prior to a termination without cause, (ii) severance payments as required by Israeli law or contractually, (iii) vesting of options and restricted shares upon termination in connection with a change of control and (iv) vesting of options and restricted shares automatically upon a change of control if such stock options are not assumed or substituted by the surviving company. We believe that having these provisions in our agreements with our officers enables our officers to focus solely on the performance of their jobs by providing them with security in the event of certain terminations of employment. With respect to the notice provisions, we believe that these provide us with a mechanism to ensure a successful transition if we have to replace one of our named executive officers. In addition, we have provided these benefits to our officers because we believe it is necessary for retention purposes, to attract well qualified and talented executives and, in the case of certain severance payments, to comply with Israeli law. In exchange for these protections, our officers have agreed to be bound by certain restrictive covenants, including confidentiality, non-competition and non-solicitation provisions.
| 62 |
Risk Considerations in our Compensation Programs
Our compensation committee believes that risks arising from our policies and practices for compensating employees are not reasonably likely to have a material adverse effect on us and do not encourage risk taking that is reasonably likely to have a material adverse effect on us. Our compensation committee believes that the structure of our executive compensation program mitigates risks by avoiding any named executive officer placing undue emphasis on any particular performance metric at the expense of other aspects of our business.
Say-on-Pay Vote
In December 2012, we held a stockholder advisory vote to approve the compensation of our then-current named executive officers, commonly referred to as a say-on-pay vote. Our stockholders approved the compensation of our then-current named executive officers, with over 98% of stockholder votes cast in favor of our say-on-pay resolution. The compensation committee has taken the say-on-pay votes into consideration in setting future compensation for our named executive officers. We are mindful of the support our stockholders expressed for our compensation philosophy and the compensation committee remains committed to the goals and objectives enumerated above, and we will continue to consider stockholder concerns and feedback in the future. We intend to conduct an advisory vote to approve executive compensation every three years until the next advisory vote to determine the frequency of future advisory votes on such compensation.
Summary Compensation Table
The table below sets forth the compensation earned by our named executive officers for the twelve month period ended December 31, 2014, the sixth month transition period ended December 31, 2013, the twelve month period ended June 30, 2013 and the six month period ended June 30, 2012.
| Name
and Principal Position |
Year | Salary ($)(1) |
Bonus ($)(1) |
Restricted Stock Awards ($)(2) |
Option Awards($)(2) |
All
Other Compensation ($)(1) |
Total ($)(1) |
||||||||||||||||||
| Alan Milinazzo | 2014 | 450,000 | 69,105 | (3) | 553,916 | 736,482 | 20,460 | (4) | 1,829,963 | ||||||||||||||||
| President and | 2013(6) | 225,000 | 275,000 | (7) | 11,775 | (4) | 511,775 | ||||||||||||||||||
| Chief Executive | 2013(8) | 222,500 | - | 1,988,725 | 1,837,440 | 9,813 | (4) | 4,058,478 | |||||||||||||||||
| Officer (5) | |||||||||||||||||||||||||
| Craig Shore | 2014 | 214,525 | 22,331 | (3) | 327,013 | 322,817 | 59,276 | (9) | 945,962 | ||||||||||||||||
| Chief Financial | 2013(6) | 92,150 | 50,137 | (7) | - | - | 36,925 | (9) | 179,212 | ||||||||||||||||
| Officer, | 2013(8) | 165,083 | - | - | 45,059 | 42,881 | (9) | 253,023 | |||||||||||||||||
| Secretary and | 2012(10) | 76,717 | - | - | 139,499 | 18,180 | (9) | 234,396 | |||||||||||||||||
| Treasurer | |||||||||||||||||||||||||
| James Barry, Ph.D. | 2014 | 183,812 | (11) | 25,914 | (3) | 391,500 | 802,545 | (12) | 123,748 | (13) | 1,527,519 | ||||||||||||||
| Executive Vice | 2013(6) | 12,500 | (14) | - | - | - | - | 12,500 | |||||||||||||||||
| President and | 2013(8) | 6,250 | (14) | - | - | 168,036 | (15) | - | 174,286 | ||||||||||||||||
| Chief Operating | 2012(10) | - | - | 129,696 | (15) | - | 129,696 | ||||||||||||||||||
| Officer | |||||||||||||||||||||||||
| Eli Bar | 2014 | 182,480 | 19,191 | (3) | 88,313 | 176,656 | 61,934 | (16) | 528,574 | ||||||||||||||||
| Senior Vice | 2013(6) | 92,150 | 44,184 | (7) | - | - | 36,714 | (16) | 173,048 | ||||||||||||||||
| President of | 2013(8) | 165,083 | - | - | - | 43,575 | (16) | 208,658 | |||||||||||||||||
| Research and | 2012(10) | 77,100 | 12,850 | - | - | 22,482 | (16) | 112,432 | |||||||||||||||||
| Development and Chief Technical Officer of InspireMD Ltd. | |||||||||||||||||||||||||
| Rick Olson | 2014 | 272,882 | 25,213 | (17) | - | - | 52,371 | (18) | 350,466 | ||||||||||||||||
| Vice President | 2013(6) | 22,773 | (19) | 5,280 | (20) | 253,980 | 4,191 | (18) | 285,565 | ||||||||||||||||
| of Global Sales | |||||||||||||||||||||||||
| and Operations of InspireMD Ltd. | |||||||||||||||||||||||||
| 63 |
| (1) | Compensation amounts received in non-U.S. currency have been converted into U.S. dollars using the average exchange rate for the applicable period, except for bonus amounts which have been converted into U.S. dollars using 3.889 NIS per dollar, which was the exchange rate as of December 31, 2014. The average exchange rate for the twelve month period ended December 31, 2014 was 3.58 NIS per dollar, the average exchange rate for the sixth month transition period ended December 31, 2013 was 3.545 NIS per dollar, the average exchange rate for the twelve month period ended June 30, 2013 was 3.7944 NIS per dollar and the average exchange rate for the six month period ended June 30, 2012 was 3.80 NIS per dollar. | ||
| (2) | The amounts in this column reflect the dollar amounts recognized for financial statement reporting purposes with respect to the twelve month period ended December 31, 2014, the six month transition period ended December 31, 2013, the twelve month period ended June 30, 2013 and the six month period ended June 30, 2012 in accordance with FASB ASC Topic 718. Fair value is based on the Black-Scholes option pricing model using the fair value of the underlying shares at the measurement date. For additional discussion of the valuation assumptions used in determining stock-based compensation and the grant date fair value for stock options, see “Management’s Discussion and Analysis of Financial Condition and Results of Operations - Critical Accounting Policies - Share-based compensation” and Note 2-“Significant Accounting Policies” and Note 9-“Equity” of the Notes to the Consolidated Financial Statements for the Twelve Months Ended December 31, 2014 included herein. | ||
| (3) | Bonuses for the 2014 calendar year were approved by the Compensation Committee in January 2015. | ||
| (4) | Mr. Milinazzo’s other compensation consisted solely of benefits related to health insurance. | ||
| (5) | Mr. Milinazzo served as our director during the twelve months ended December 31, 2014, the six months ended December 31, 2013 and the twelve months ended June 30, 2013 but did not receive any additional compensation for his services as director. | ||
| (6) | Refers to our transition period from July 1, 2013 to December 31, 2013. | ||
| (7) | Bonuses for the 2013 calendar year were approved by the Compensation Committee in January 2014. | ||
| (8) | Refers to the twelve month period ended June 30, 2013. | ||
| (9) | Mr. Shore’s other compensation consisted solely of benefits in the twelve months ended December 31, 2014, the six months ended December 31, 2013, the twelve months ended June 30, 2013 and the six months ended June 30, 2012. In each of the periods reported, Mr. Shore’s benefits included our contributions to his severance, pension, vocational studies and disability funds, an annual recreation payment, a company car and cell phone, and a daily food allowance. | ||
| (10) | Refers to the six month period ended June 30, 2012. | ||
| (11) | Dr. Barry’s salary compensation includes $15,000 of fees earned as a director during the twelve months ended December 31, 2014. | ||
| (12) | Includes the fair value of options granted to Dr. Barry as a director of $94,909 during the twelve months ended December 31, 2014. | ||
| (13) | Dr. Barry’s other compensation in the twelve months ended December 31, 2014 consisted of $115,000 of consulting fees and $8,748 of benefits related to health insurance | ||
| (14) | Dr. Barry’s salary compensation consisted solely of director’s fees. | ||
| (15) | Dr. Barry’s option award compensation consisted solely of option awards received as a director. | ||
| (16) | Mr. Bar’s other compensation in each of the periods reported consisted solely of benefits, including our contributions to his severance, pension, vocational studies and disability funds, an annual recreation payment, a company car and cell phone, and a daily food allowance. | ||
| (17) | Mr. Olson’s bonus for the 2014 calendar year was calculated based on 0.75% of our 2014 sales. | ||
| (18) | Mr. Olson’s other compensation consisted solely of benefits, including our contributions to his pension fund, health insurance, death in service insurance and a car allowance. | ||
| (19) | Mr. Olson’s employment began on December 1, 2013. | ||
| (20) | Mr. Olson’s bonus for the six month period ended December 31, 2013 was calculated based on 0.75% of our December 2013 sales. |
| 64 |
2014 Grants of Plan-Based Awards
| Name | Grant Date | All Other Stock Awards: Number of Shares of Stock or Units (#) | All Other Option Awards: Number of Securities Underlying Options (#) | Exercise or Base Price of Option Awards ($/Sh) | Grant Date Fair Value of Stock and Option Awards ($) | |||||||||||||
| Alan Milinazzo | 01/29/2014 | 86,325 | - | 267,608 | ||||||||||||||
| President and Chief Executive Officer | 01/29/2014 | 86,325 | 3.1 | 163,861 | ||||||||||||||
| 01/31/2014 | 96,400 | - | 286,308 | |||||||||||||||
| 01/31/2014 | 313,350 | 2.97 | 572,621 | |||||||||||||||
| Craig Shore | 01/29/2014 | 77,000 | - | 238,700 | ||||||||||||||
| Chief Financial Officer, Secretary and | 01/29/2014 | 77,000 | 3.1 | 146,160 | ||||||||||||||
| Treasurer | 01/31/2014 | 29,735 | - | 88,313 | ||||||||||||||
| 01/31/2014 | 96,670 | 2.97 | 176,656 | |||||||||||||||
| James Barry, Ph.D. | 07/14/2014 | 150,000 | - | 391,500 | ||||||||||||||
| Executive Vice President and Chief | 07/14/2014 | 450,000 | 2.61 | 707,636 | ||||||||||||||
| Operating Officer | 01/29/2014 | 50,000 | 3.10 | 94,909 | ||||||||||||||
| Eli Bar (2) Senior Vice President, Research and Development and Chief Technical Officer of InspireMD Ltd. | 01/31/2014 | 29,735 | - | 88,313 | ||||||||||||||
| 01/31/2014 | 96,670 | 2.97 | 176,656 | |||||||||||||||
Agreements with Executive Officers
Alan Milinazzo
On January 3, 2013, we entered into an employment agreement with Alan Milinazzo to serve as our president, chief executive officer and a director. The employment agreement has an initial term that ends on January 1, 2016 and will automatically renew for additional one-year periods on January 1, 2016 and on each January 1 thereafter unless either party gives the other party written notice of its election not to extend such employment at least six months prior to the next January 1 renewal date. If a change in control occurs when less than two full years remain in the initial term or during any renewal term, the employment agreement will automatically be extended for two years from the change in control date and will terminate on the second anniversary of the change in control date.
Under this employment agreement, Mr. Milinazzo is entitled to an annual base salary of at least $450,000. Such amount may be reduced only as part of an overall cost reduction program that affects all senior executives of the company and does not disproportionately affect Mr. Milinazzo, so long as such reductions do not reduce the base salary to a rate that is less than 90% of the amount set forth above (or 90% of the amount to which it has been increased). The base salary will be reviewed annually by the board for increase as part of its annual compensation review. Mr. Milinazzo is also eligible to receive an annual bonus of at least $275,000 upon the achievement of reasonable target objectives and performance goals, to be determined by the board of directors in consultation with Mr. Milinazzo on or before the end of the first quarter of the fiscal year to which the bonus relates and, in the event actual performance exceeds the goals, the board may, in its sole discretion, pay Mr. Milinazzo bonus compensation of more than $275,000. The annual bonus amount will be less than $275,000 if the target objectives and performance goals are not met. In addition, Mr. Milinazzo is eligible to receive such additional bonus or incentive compensation as the board may establish from time to time in its sole discretion.
On January 5, 2015, we amended Mr. Milinazzo’s employment agreement to provide that, for a limited period of time to be mutually agreed to by us and Mr. Milinazzo, Mr. Milinazzo will receive 50% of his base salary in cash payments, with the remaining 50% to be paid in an equivalent amount of shares of restricted common stock, payable and granted in equal installments in accordance with our normal payroll practices. These shares of restricted stock will vest immediately and be valued as of the closing price of our common stock on the date of grant.
In accordance with this employment agreement, on January 3, 2013, we granted Mr. Milinazzo a nonqualified stock option to purchase 525,927 shares of our common stock, made pursuant to a nonqualified stock option agreement, an incentive stock option to purchase 74,073 shares of our common stock, made pursuant to an incentive stock option agreement, and 400,000 shares of restricted stock, which are subject to forfeiture until the vesting of such shares, made pursuant to a restricted stock award agreement. The options have an exercise price of $4.05, which was the fair market value of our common stock on the date of grant. The options are subject to a three-year vesting period subject to Mr. Milinazzo’s continued service with us, with one-thirty-sixth (1/36th) of such awards vesting each month. The shares of restricted stock initially vested monthly over thirty-six months, with 1/36 vesting on February 3, 2013, March 3, 2013 and April 3, 2013. The grant was then amended to vest annually over three years, with 9/36 vesting on January 3, 2014, and one-third vesting on January 3, 2015 and January 3, 2016. On or before December 31 of each calendar year, Mr. Milinazzo will be eligible to receive an additional grant of equity awards equal, in the aggregate, to up to 0.5% of actual outstanding shares of our common stock on the date of grant, provided that the actual amount of the grant will be based on his achievement of certain performance objectives as established by the board, in its reasonable discretion, for each such calendar year. Each additional grant will, with respect to any awards that are options, have an exercise price equal to the fair market value of our common stock, and will be subject to a three-year vesting period subject to Mr. Milinazzo’s continued service with us, with one-third of each additional grant vesting equally on the first, second, and third anniversary of the date of grant for such awards.
| 65 |
For a discussion of equity awards granted to Mr. Milinazzo in 2014, see “Compensation Discussion and Analysis.”
Mr. Milinazzo’s employment agreement also contains certain noncompetition, no solicitation, confidentiality, and assignment of inventions requirements for Mr. Milinazzo.
For a description of certain severance payments to which Mr. Milinazzo is entitled under his employment agreement, see “Potential Payments Upon Termination or Change of Control.”
Craig Shore
We have been a party to an employment agreement with Craig Shore since November 28, 2010. Pursuant to the employment agreement, Mr. Shore was initially entitled to a monthly gross salary of $8,750, which amount had increased to $10,620 by 2012. In addition, Mr. Shore’s annual base salary was increased to $175,000 on April 22, 2013, retroactive to January 1, 2013. On May 5, 2014, we entered into an amended and restated employment agreement with Mr. Shore. The employment agreement, as amended, has an initial term that ends on April 20, 2017 and will automatically renew for additional one-year periods on April 21, 2017 and on each April 21st thereafter unless either party gives the other party written notice of its election not to extend such employment at least six months prior to the next April 21st renewal date. If a change in control occurs when less than two full years remain in the initial term or during any renewal term, the employment agreement will automatically be extended for two years from the change in control date and will terminate on the second anniversary of the change in control date. Under the terms of the employment agreement, Mr. Shore is entitled to an annual base salary of at least $220,000, retroactive to January 1, 2014. Such amount may be reduced only as part of an overall cost reduction program that affects all of our senior executives and does not disproportionately affect Mr. Shore, so long as such reduction does not reduce the base salary to a rate that is less than 90% of the amount set forth above (or 90% of the amount to which it has been increased). The base salary will be reviewed annually by our chief executive officer for increase as part of our annual compensation review. Mr. Shore is also eligible to receive an annual bonus in an amount equal to 45% of his then-annual salary upon the achievement of reasonable target objectives and performance goals, to be determined by the board of directors in consultation with Mr. Shore and based on the percentages set forth in his employment agreement. In addition, Mr. Shore is eligible to receive such additional bonus or incentive compensation as the board may establish from time to time in its sole discretion. Mr. Shore will also be considered for grants of equity awards each year as part of the board’s annual compensation review, which will be made at the sole discretion of the board of directors. Each grant will, with respect to any awards that are options, have an exercise price equal to the fair market value of our common stock, and will be subject to a three-year vesting period subject to Mr. Shore’s continued service with us, with one-third of each additional grant vesting equally on the first, second, and third anniversary of the date of grant for such awards.
For a discussion of equity awards granted to Mr. Shore in 2014, see “Compensation Discussion and Analysis.”
The employment agreement also contains certain standard noncompetition, no solicitation, confidentiality, and assignment of inventions requirements for Mr. Shore.
Mr. Shore is also entitled to participate in or receive benefits under our social insurance and benefits plans, including but not limited to our manager’s insurance policy and education fund, which are customary benefits provided to executive employees in Israel. A management insurance policy is a combination of severance savings (in accordance with Israeli law), defined contribution tax-qualified pension savings and disability pension payments. An education fund is a savings fund of pre-tax contributions to be used after a specified period of time for advanced educational training and other permitted purposes, as set forth in the by-laws of the education fund. We will make periodic contributions to these insurance and social benefits plans based on certain percentages of Mr. Shore’s base salary, including (i) 7.5% to the education fund and (ii) 15.83% to the manager’s insurance policy, of which 8.33% will be allocated to severance pay, 5% to pension fund payments and 2.5% to disability pension payments. Upon the termination of Mr. Shore’s employment for any reason other than for cause, Mr. Shore will be entitled to receive the total amount contributed to and accumulated in his manager insurance policy fund.
For a description of certain severance payments to which Mr. Shore is entitled under his employment agreement, see “Potential Payments Upon Termination or Change of Control.”
| 66 |
James Barry
On January 14, 2014, we entered into an employment agreement with James Barry to serve as our executive vice president and chief operating officer. Dr. Barry was previously a director and continued his role as a director. The employment agreement has an initial term that ends on July 14, 2017 and will automatically renew for additional one-year periods on July 17, 2017 and on each July 17 thereafter unless either party gives the other party written notice of its election not to extend such employment at least six months prior to the next July 17 renewal date. If a change in control occurs when less than two full years remain in the initial term or during any renewal term, the employment agreement will automatically be extended for two years from the change in control date and will terminate on the second anniversary of the change in control date.
Under this employment agreement, Dr. Barry is entitled to an annual base salary of at least $365,000. Such amount may be reduced only as part of an overall cost reduction program that affects all senior executives of the company and does not disproportionately affect Dr. Barry, so long as such reductions do not reduce the base salary to a rate that is less than 90% of the amount set forth above (or 90% of the amount to which it has been increased). The base salary will be reviewed annually by the board for increase as part of its annual compensation review. Dr. Barry is also eligible to receive an annual bonus of at least $225,000 upon the achievement of reasonable target objectives and performance goals, to be determined by the board of directors in consultation with Dr. Barry on or before the end of the first quarter of the fiscal year to which the bonus relates and, in the event actual performance exceeds the goals, the board may, in its sole discretion, pay Dr. Barry bonus compensation of more than $225,000. In addition, Dr. Barry is eligible to receive such additional bonus or incentive compensation as the board may establish from time to time in its sole discretion.
On January 5, 2015, we amended Dr. Barry’s employment agreement to provide that, for a limited period of time to be mutually agreed to by us and Dr. Barry, Dr. Barry will receive 50% of his base salary in cash payments, with the remaining 50% to be paid in an equivalent amount of shares of restricted common stock, payable and granted in equal installments in accordance with our normal payroll practices. These shares of restricted stock will vest immediately and be valued as of the closing price of our common stock on the date of grant.
For a discussion of equity awards granted to Dr. Barry in 2014, see “Compensation Discussion and Analysis.”
Dr. Barry’s employment agreement also contains certain noncompetition, no solicitation, confidentiality, and assignment of inventions requirements for Dr. Barry.
For a description of certain severance payments to which Dr. Barry is entitled under his employment agreement, see “Potential Payments Upon Termination or Change of Control.”
Eli Bar
InspireMD Ltd. has been a party to an employment agreement with Eli Bar since June 26, 2005. Pursuant to the employment agreement, Mr. Bar was initially entitled to a monthly gross salary of $8,750, which amount had increased to $10,620 by 2012 and further increased to $175,000 on April 22, 2013, retroactive to January 1, 2013. Mr. Bar is also entitled to certain social and fringe benefits as set forth in the employment agreement including a company car. The employment agreement also contains certain confidentiality, non-competition and non-solicitation requirements for Mr. Bar. On April 22, 2013, we modified the compensation package for Mr. Bar to provide for (i) the aforementioned increase in base salary, (ii) Mr. Bar being eligible to receive an annual bonus equal to up to 30% of his base salary, at the sole discretion of our compensation committee, in consultation with our chief executive officer, and (iii) certain termination benefits, described under “Potential Payments Upon Termination or Change of Control.” On January 29, 2014, we further modified the compensation package for Mr. Bar so that he would be eligible to receive an annual bonus of up to 45% of his base salary for 2014. For a discussion of equity awards granted to Mr. Bar in 2014, see “Compensation Discussion and Analysis.”
For a description of certain severance payments to which Mr. Bar is entitled under his employment agreement, see “Potential Payments Upon Termination or Change of Control.”
Rick Olson
On December 1, 2013, we entered into an employment agreement with Rick Olson to serve as InspireMD Ltd.’s vice president of global sales and operations. Under this employment agreement, Mr. Olson was entitled to an annual base salary of 165,000 Great British Pounds ($270,000 based on the exchange rate on the date of the agreement). Mr. Olson was also entitled to certain social and fringe benefits as set forth in the employment agreement including a car allowance. For 2014, Mr. Olson was eligible to receive a quarterly commission equal to 0.75% of the total sales up to 2013’s total sales, 1.75% of incremental sales up to 2014’s target revenue, as approved by the board of directors, and 2.75% of incremental sales above 2014’s target revenue. In accordance with this employment agreement, on December 2, 2013, we granted Mr. Olson a nonqualified stock option to purchase 150,000 shares of our common stock, made pursuant to a nonqualified stock option agreement. The options have an exercise price of $2.75, which was the fair market value of our common stock on the date of grant. The options are subject to a three-year vesting period subject to Mr. Olson’s continued service with us.
Mr. Olson also signed a noncompetition, no solicitation, confidentiality, and assignment of inventions requirements agreement with us.
| 67 |
For a description of certain severance payments to which Mr. Olson was entitled under his employment agreement, see “Potential Payments Upon Termination or Change of Control.”
Mr. Olson resigned from employment with us effective February 9, 2015.
Outstanding Equity Awards at December 31, 2014
The following table shows information concerning unexercised options and unvested restricted shares outstanding as of December 31, 2014 for each of our named executive officers.
| Option Awards | Stock Awards | |||||||||||||||||||||
| Name | Number of securities underlying unexercised options (#) exercisable | Number of securities underlying unexercised options (#) unexercisable | Option exercise price ($) | Option expiration date | Number of shares of stock that have not vested (#) | Market value of shares of stock that have not vested ($) | ||||||||||||||||
| Alan Milinazzo | 383,333 | 216,667 | (1) | 4.05 | 1/3/2023 | - | - | |||||||||||||||
| 266,667 | (2) | 208,000 | ||||||||||||||||||||
| 99,149 | 198,298 | (3) | 2.05 | 4/23/2023 | - | - | ||||||||||||||||
| 119,911 | (4) | 93,531 | ||||||||||||||||||||
| - | 86,325 | (5) | 3.10 | 1/27/2024 | ||||||||||||||||||
| 86,325 | (6) | 67,334 | ||||||||||||||||||||
| - | 313,350 | (7) | 2.97 | 1/29/2024 | ||||||||||||||||||
| 96,400 | (8) | 75,192 | ||||||||||||||||||||
| Craig Shore | 91,306 | - | 4.928 | 2/27/2021 | - | - | ||||||||||||||||
| 50,000 | 25,000 | (9) | 3.20 | 5/24/2022 | - | - | ||||||||||||||||
| 8,333 | 16,667 | (10) | 2.95 | 5/7/2023 | - | - | ||||||||||||||||
| 77,000 | (5) | 3.10 | 1/27/2024 | |||||||||||||||||||
| 77,000 | (6) | 60,060 | ||||||||||||||||||||
| 96,670 | (7) | 2.97 | 1/29/2024 | |||||||||||||||||||
| 29,735 | (8) | 23,193 | ||||||||||||||||||||
| Eli Bar | 38,044 | - | 0.0001 | 7/25/2020 | - | - | ||||||||||||||||
| 20,290 | - | 4.92 | 7/28/2020 | - | - | |||||||||||||||||
| 50,000 | - | 7.72 | 8/31/2016 | - | - | |||||||||||||||||
| 96,670 | (5) | 2.97 | 1/29/2024 | |||||||||||||||||||
| 29,735 | (6) | 23,193 | ||||||||||||||||||||
| James Barry | 16,667 | 8,333 | (11) | 7.80 | 1/30/2022 | |||||||||||||||||
| 8,333 | 4,167 | (12) | 3.16 | 6/17/2022 | ||||||||||||||||||
| 33,333 | 66,667 | (13) | 2.75 | 5/7/2023 | ||||||||||||||||||
| - | 50,000 | (5) | 3.10 | 1/27/2024 | ||||||||||||||||||
| 150,000 | (14) | 117,000 | ||||||||||||||||||||
| - | 450,000 | (15) | 2.61 | 7/11/2024 | ||||||||||||||||||
| Rick Olson | 50,000 | 100,000 | (16) | 2.75 | 11/30/2023 | |||||||||||||||||
| (1) | These options vest in equal amounts on the third of each month through December 3, 2015. | |
| (2) | These restricted shares vest one-half on each of January 3, 2015 and January 3, 2016. | |
| (3) | These options vest annually, with one-half vesting on each of April 25, 2015 and April 25, 2016. | |
| (4) | These restricted shares vest annually, with one-half vesting on each of April 25, 2015 and April 25, 2016. | |
| (5) | These options vest annually, with one-third vesting on each of January 29, 2015, January 29, 2016 and January 29, 2017. | |
| (6) | These restricted shares vest annually, with one-third vesting on each of January 29, 2015, January 29, 2016 and January 29, 2017. | |
| (7) | These options vest annually, with one-third vesting on each of January 31, 2015, January 31, 2016 and January 31, 2017. | |
| (8) | These restricted shares vest annually, with one-third vesting on each of January 31, 2015, January 31, 2016 and January 31, 2017. | |
| (9) | These options will vest on May 25, 2015. | |
| (10) | These options vest annually, with one-half vesting on each of May 9, 2015 and May 9, 2016. | |
| (11) | These options will vest on January 30, 2015. | |
| (12) | These options will vest on June 18, 2015. | |
| (13) | These options vest annually, with one-half vesting on each of May 9, 2015 and May 9, 2016. |
| 68 |
|
|
(14) | These restricted shares vest annually, with one-third vesting on each of July 14, 2015, July 14, 2016 and July 14, 2017. |
| (15) | These options vest annually, with one-third vesting on each of July 14, 2015, July 14, 2016 and July 14, 2017. | |
| (16) | These options vest annually, with one-half vesting on each of December 2, 2015 and December 2, 2016. |
Option Exercises and Stock Vested
There were no stock options exercised by our named executive officers during the twelve months ended December 31, 2014.
2011 UMBRELLA Option Plan
On March 28, 2011, our board of directors and stockholders adopted and approved the InspireMD, Inc. 2011 UMBRELLA Option Plan, which was subsequently amended on October 31, 2011 and December 21, 2012. Under the InspireMD, Inc. 2011 UMBRELLA Option Plan, we have reserved 5,000,000 shares of our common stock (as adjusted for the one-for-four reverse stock split of our common stock that occurred on December 21, 2012) as awards to the employees, consultants, and service providers to InspireMD, Inc. and its subsidiaries and affiliates worldwide.
The InspireMD, Inc. 2011 UMBRELLA Option Plan currently consists of three components, the primary plan document that governs all awards granted under the InspireMD, Inc. 2011 UMBRELLA Option Plan, and two appendices: (i) Appendix A, designated for the purpose of grants of stock options and restricted stock awards to Israeli employees, consultants, officers and other service providers and other non-U.S. employees, consultants, and service providers, and (ii) Appendix B, which is the 2011 U.S. Equity Incentive Plan, designated for the purpose of grants of stock options and restricted stock awards to U.S. employees, consultants, and service providers who are subject to the U.S. income tax. On December 21, 2012, the stockholders approved the awarding of “incentive stock options” pursuant to the U.S. portion of the plan.
The purpose of the InspireMD, Inc. 2011 UMBRELLA Option Plan is to provide an incentive to attract and retain employees, officers, consultants, directors, and service providers whose services are considered valuable, to encourage a sense of proprietorship and to stimulate an active interest of such persons in our development and financial success. The InspireMD, Inc. 2011 UMBRELLA Option Plan is administered by our compensation committee. Unless terminated earlier by the board of directors, the InspireMD, Inc. 2011 UMBRELLA Option Plan will expire on March 27, 2021.
2013 Long-Term Incentive Plan
On December 16, 2013, our stockholders approved the InspireMD, Inc. 2013 Long-Term Incentive Plan, which was adopted by our board of directors on October 25, 2013.
The purpose of the InspireMD, Inc. 2013 Long-Term Incentive Plan is to provide an incentive to attract and retain employees, officers, consultants, directors, and service providers whose services are considered valuable, to encourage a sense of proprietorship and to stimulate an active interest of such persons in our development and financial success. The InspireMD, Inc. 2013 Long-Term Incentive Plan provides for the granting of incentive stock options, nonqualified stock options, stock appreciation rights, restricted stock, restricted stock units, performance awards, dividend equivalent rights, and other awards, which may be granted singly, in combination, or in tandem. The InspireMD, Inc. 2013 Long-Term Incentive Plan is administered by our compensation committee. A total of 5,000,000 shares of common stock are reserved for awards under the InspireMD, Inc. 2013 Long-Term Incentive Plan.
The InspireMD, Inc. 2013 Long-Term Incentive Plan is intended serve as an “umbrella” plan for us and our subsidiaries worldwide. Therefore, if so required, appendices may be added to the InspireMD, Inc. 2013 Long-Term Incentive Plan in order to accommodate local regulations that do not correspond to the scope of the InspireMD, Inc. 2013 Long-Term Incentive Plan. Attached as Appendix A to the InspireMD, Inc. 2013 Long-Term Incentive Plan is the InspireMD, Inc. 2013 Employee Stock Incentive Plan, for the purpose of making grants of stock options, restricted stock, and other stock incentive awards pursuant to Sections 102 and 3(i) of the Israeli Income Tax Ordinance (New Version), 1961 to Israeli employees and officers and any other service providers or control holders of us who are subject to Israeli Income Tax.
Potential Payments Upon Termination or Change of Control
Our agreements with Messrs. Milinazzo, Shore, Bar and Olson and Dr. Barry, as well as Israeli law, provide for payments and other compensation in the event of their termination or a change of control of us under certain circumstances, as described below.
| 69 |
President and Chief Executive Officer Pursuant to Mr. Milinazzo’s employment agreement, if Mr. Milinazzo’s employment is terminated upon his death or disability, by Mr. Milinazzo for good reason (as such term is defined in Mr. Milinazzo’s employment agreement), or by us without cause (as such term is defined in Mr. Milinazzo’s employment agreement), Mr. Milinazzo will be entitled to receive, in addition to other unpaid amounts owed to him (e.g., for base salary and accrued vacation): (i) the pro rata amount of any bonus for the fiscal year of such termination (assuming full achievement of all applicable goals under the bonus plan) that he would have received had his employment not been terminated; (ii) a one-time lump sum severance payment equal to 200% of his base salary, provided that he executes a release relating to employment matters and the circumstances surrounding his termination in favor of the company, our subsidiaries and our officers, directors and related parties and agents, in a form reasonably acceptable to us at the time of such termination; (iii) vesting of 50% of all unvested stock options, restricted stock, stock appreciation rights or similar stock based rights granted to Mr. Milinazzo, and lapse of any forfeiture included in such restricted or other stock grants; (iv) an extension of the term of any outstanding stock options or stock appreciation rights until the earlier of (a) two (2) years from the date of termination, or (b) the latest date that each stock option or stock appreciation right would otherwise expire by its original terms; (v) to the fullest extent permitted by our then-current benefit plans, continuation of health, dental, vision and life insurance coverage for the lesser of 18 months after termination or until Mr. Milinazzo obtains coverage from a new employer; and (vi) a cash payment of $35,000, which Mr. Milinazzo may use for executive outplacement services or an education program. The payments described above will be reduced by any payments received by Mr. Milinazzo pursuant to any of our employee welfare benefit plans providing for payments in the event of death or disability. If Mr. Milinazzo continues to be employed by us after the term of his employment agreement, unless otherwise agreed by the parties in writing, and Mr. Milinazzo’s employment is terminated upon his death or disability, by Mr. Milinazzo for good reason, or by us without cause, Mr. Milinazzo will be entitled to receive, in addition to other unpaid amounts owed to him, the payments set forth in (i), (ii) and (iv) above. If, during the term of his employment agreement, we terminate Mr. Milinazzo’s employment for cause, Mr. Milinazzo will only be entitled to unpaid amounts owed to him and whatever rights, if any, are available to him pursuant to our stock-based compensation plans or any award documents related to any stock-based compensation.
Mr. Milinazzo has no specific right to terminate the employment agreement or right to any severance payments or other benefits solely as a result of a change in control. However, if within 24 months following a change in control, (a) Mr. Milinazzo terminates his employment for good reason, or (b) we terminate his employment without cause, the lump sum severance payment to which he is entitled will be increased from 200% of his base salary to 250% of his base salary and all stock options, restricted stock units, stock appreciation rights or similar stock-based rights granted to him will vest in full and be immediately exercisable and any risk of forfeiture included in restricted or other stock grants previously made to him will immediately lapse.
Chief Financial Officer, Secretary and Treasurer If during the term of the employment agreement, Mr. Shore’s employment is terminated upon his death or disability or by us without cause (as such term is defined in Mr. Shore’s employment agreement), Mr. Shore will be entitled to receive, in addition to any other unpaid amounts owed to him under the manager’s insurance policy: (i) any unpaid base salary and accrued unpaid vacation or earned incentive compensation plus the pro rata amount of any bonus for the fiscal year of such termination (based on the number of business days he was actually employed by us during the fiscal year of such termination and based on the percentage of the goals that he actually achieved under the bonus plan) that he would have received had his employment not been terminated; (ii) a one-time lump sum severance payment equal to 100% of his base salary, provided that he executes a release relating to employment matters and the circumstances surrounding his termination in favor of us, our subsidiaries and our officers, directors and related parties and agents, in a form reasonably acceptable to us at the time of such termination; (iii) vesting of 50% of all unvested stock options granted to Mr. Shore; (iv) an extension of the exercise period of all vested stock options granted to Mr. Shore until the earlier of (a) two years from the date of termination or (b) the latest date that each stock option would otherwise expire by its original terms; (v) to the fullest extent permitted by our then-current benefit plans, continuation of health, dental, vision and life insurance coverage for the lesser of 12 months after termination or until Mr. Shore obtains coverage from a new employer; and (vi) reimbursement of up to $30,000 for executive outplacement services, subject to certain restrictions. The payments described above will be reduced by any payments received by Mr. Shore pursuant to any of our employee welfare benefit plans providing for payments in the event of death or disability. If, during or after the term of his employment agreement, Mr. Shore’s employment is terminated by us for cause or by Mr. Shore voluntarily, Mr. Shore will only be entitled to unpaid amounts owed to him (e.g., for base salary, accrued vacation and incentive vacation earned through the date of such termination) and whatever rights, if any, are available to him pursuant to our stock-based compensation plan or any award documents related to any stock-based compensation.
Mr. Shore has no specific right to terminate the employment agreement or right to any severance payments or other benefits solely as a result of a change in control. However, if within 24 months following a change in control, (a) Mr. Shore terminates his employment for good reason, or (b) we terminate Mr. Shore’s employment without cause, he is entitled to receive the full lump sum severance payment equal to 100% of his base salary and all stock options, stock appreciation rights or similar stock-based rights granted to him will vest in full and be immediately exercisable and any risk of forfeiture included in restricted or other stock grants previously made to him will immediately lapse. Furthermore, pursuant to terms contained in Mr. Shore’s stock option and restricted stock award agreements, in the event of a change of control of our company, the stock options and restricted stock granted to Mr. Shore that were unvested will vest immediately upon such change of control, in the case of stock options, if such stock options are not assumed or substituted by the surviving company. We have also agreed orally that, upon Mr. Shore’s termination of service as a result of death, disability, resignation for “good reason” or termination by us without “cause,” Mr. Shore will also be entitled to receive: (a) 50% vesting of all unvested stock options, restricted stock, restricted stock units, stock appreciation rights or similar stock based rights outstanding at the time of termination of service; and (b) the right to exercise any outstanding stock options or stock appreciation rights for a period equal to the lesser of (x) two years from the date of termination of service, or (y) the period remaining until the original expiration date of any such outstanding stock options or stock appreciation rights.
If we terminate Mr. Shore’s employment without cause, Mr. Shore will be entitled, under Israeli law, to severance payments equal to his last month’s salary multiplied by the number of years Mr. Shore has been employed with us. In order to finance this obligation, we make monthly contributions equal to 8.33% of Mr. Shore’s salary to a severance payment fund. The total amount accumulated in Mr. Shore’s severance payment fund as of December 31, 2014 was $51,615, as adjusted for conversion from New Israeli Shekels to U.S. Dollars. However, if Mr. Shore’s employment is terminated without cause, on account of a disability or upon his death, as of December 31, 2014, Mr. Shore would have been entitled to receive $67,564 in severance under Israeli law, thereby requiring us to pay Mr. Shore $15,949, in addition to releasing the $51,615 in Mr. Shore’s severance payment fund. On the other hand, pursuant to his employment agreement, Mr. Shore is entitled to the total amount contributed to and accumulated in his severance payment fund in the event of the termination of his employment as a result of his voluntary resignation. In addition, Mr. Shore would be entitled to receive his full severance payment under Israeli law, including the total amount contributed to and accumulated in his severance payment fund, if he retires from our company at or after age 67.
| 70 |
We are entitled to terminate Mr. Shore’s employment immediately at any time for “cause” (as such term is defined in the agreement and the Israeli Severance Payment Act 1963), upon which, after meeting certain requirements under the applicable law and recent Israeli Labor court requirements, we believe we will have no further obligation to compensate Mr. Shore.
Also, upon termination of Mr. Shore’s employment for any reason, we will compensate him for all unused vacation days accrued.
Executive Vice President and Chief Operating Officer Pursuant to Dr. Barry’s employment agreement, if Dr. Barry’s employment is terminated upon his death or disability, by Dr. Barry for good reason (as such term is defined in Dr. Barry’s employment agreement), or by us without cause (as such term is defined in Dr. Barry’s employment agreement), Dr. Barry will be entitled to receive, in addition to other unpaid amounts owed to him (e.g., for base salary and accrued vacation): (i) the pro rata amount of any bonus for the fiscal year of such termination (assuming full achievement of all applicable goals under the bonus plan) that he would have received had his employment not been terminated; (ii) a one-time lump sum severance payment equal to 150% of his base salary, provided that he executes a release relating to employment matters and the circumstances surrounding his termination in favor of the company, our subsidiaries and our officers, directors and related parties and agents, in a form reasonably acceptable to us at the time of such termination; (iii) vesting of 50% of all unvested stock options, restricted stock, stock appreciation rights or similar stock based rights granted to Dr. Barry, and lapse of any forfeiture included in such restricted or other stock grants; (iv) an extension of the term of any outstanding stock options or stock appreciation rights until the earlier of (a) two (2) years from the date of termination, or (b) the latest date that each stock option or stock appreciation right would otherwise expire by its original terms; (v) to the fullest extent permitted by our then-current benefit plans, continuation of health, dental, vision and life insurance coverage for the lesser of 18 months after termination or until Dr. Barry obtains coverage from a new employer; and (vi) a cash payment of $25,000, which Dr. Barry may use for executive outplacement services or an education program. The payments described above will be reduced by any payments received by Dr. Barry pursuant to any of our employee welfare benefit plans providing for payments in the event of death or disability. If Dr. Barry continues to be employed by us after the term of his employment agreement, unless otherwise agreed by the parties in writing, and Dr. Barry’s employment is terminated upon his death or disability, by Dr. Barry for good reason, or by us without cause, Dr. Barry will be entitled to receive, in addition to other unpaid amounts owed to him, the payments set forth in (i), (ii) and (iv) above. If, during the term of his employment agreement, we terminate Dr. Barry’s employment for cause, Dr. Barry will only be entitled to unpaid amounts owed to him and whatever rights, if any, are available to him pursuant to our stock-based compensation plans or any award documents related to any stock-based compensation.
Dr. Barry has no specific right to terminate the employment agreement or right to any severance payments or other benefits solely as a result of a change in control. However, if within 24 months following a change in control, (a) Dr. Barry terminates his employment for good reason, or (b) we terminate his employment without cause, the lump sum severance payment to which he is entitled will be increased from 150% of his base salary to 250% of his base salary and all stock options, restricted stock units, stock appreciation rights or similar stock-based rights granted to him will vest in full and be immediately exercisable and any risk of forfeiture included in restricted or other stock grants previously made to him will immediately lapse.
Senior Vice President of Research and Development and Chief Technical Officer of InspireMD Ltd Either party to our employment agreement with Mr. Bar may terminate the employment agreement without “cause” (as such term is defined in Mr. Bar’s employment agreement with us) upon at least 60 days prior written notice to the other party. During such notice period, we will continue to compensate Mr. Bar according to his employment agreement and Mr. Bar will be obligated to continue to discharge and perform all of his duties and obligations under his employment agreement, and to cooperate with us and use his best efforts to assist with the integration of any persons that we have delegated to assume Mr. Bar’s responsibilities. We believe that our severance arrangement with Mr. Bar will assist us in achieving a successful transition upon Mr. Bar’s departure. In addition, upon termination without “cause,” we have the right to pay Mr. Bar a lump payment representing his compensation for the notice period and terminate Mr. Bar’s employment immediately. We have also agreed orally that, upon Mr. Bar’s termination of service as a result of death, disability, resignation for “good reason” or termination by us without “cause,” Mr. Bar will also be entitled to receive: (a) a one-time lump sum severance payment in an amount equal to 100% of Mr. Bar’s annual base salary; (b) 50% vesting of all unvested stock options, restricted stock, restricted stock units, stock appreciation rights or similar stock based rights outstanding at the time of termination of service; and (c) the right to exercise any outstanding stock options or stock appreciation rights for a period equal to the lesser of (x) two years from the date of termination of service, or (y) the period remaining until the original expiration date of any such outstanding stock options or stock appreciation rights.
If Mr. Bar’s employment is terminated without cause, Mr. Bar will also be entitled under Israeli law to severance payments equal to his last month’s salary multiplied by the number of years Mr. Bar has been employed with us. In order to finance this obligation, we make monthly contributions equal to 8.33% of Mr. Bar’s salary each month to a severance payment fund. The total amount accumulated in his severance payment fund as of December 31, 2014 was $101,658, as adjusted for conversion from New Israeli Shekels to U.S. Dollars. However, if Mr. Bar’s employment was terminated without cause, on account of a disability or upon his death, as of December 31, 2014, Mr. Bar would be entitled to receive $133,880 in severance under Israeli law, thereby requiring us to pay Mr. Bar $32,222, in addition to releasing the $101,658 in his severance payment fund. In addition, Mr. Bar would be entitled to receive his full severance payment under Israeli law, including the total amount contributed to and accumulated in his severance payment fund, if he retires from our company at or after age 67.
| 71 |
We are entitled to terminate Mr. Bar’s employment immediately at any time for “cause” (as such term is defined in the agreement and the Israeli Severance Payment Act 1963), upon which, after meeting certain requirements under the applicable law and recent Israeli Labor court requirements, we believe we will have no further obligation to compensate Mr. Bar.
In addition, pursuant to terms contained in Mr. Bar’s stock option award and restricted stock award agreements, in the event of a change of control of our company, the stock options and restricted stock granted to Mr. Bar that were unvested will vest immediately upon such change of control, in the case of stock options, if such stock options are not assumed or substituted by the surviving company. We have also agreed orally that, with respect to Mr. Bar’s outstanding options, restricted stock or similar awards, upon a termination of service as a result of death, disability, resignation by Mr. Bar for “good reason,” or by us without “cause,” (i) 50% of the remaining unvested portion of such outstanding awards shall vest, and (ii) Mr. Bar has a period equal to the lesser of (A) two years from the date of termination of service, or (B) the period remaining until the original expiration date of such outstanding awards, to exercise such outstanding awards.
Also, upon termination of Mr. Bar’s employment for any reason, we will compensate him for all unused vacation days accrued.
Vice President of Global Sales and Operations of InspireMD Ltd. Subject to certain conditions, either party to our employment agreement with Mr. Olson could terminate the employment agreement without “cause” (as such term was defined in Mr. Olson’s employment agreement with us) upon at least 30 days prior written notice to the other party during the first four years of Mr. Olson’s employment. Under the laws of the United Kingdom, once Mr. Olson had more than four years of continuous service with InspireMD Ltd, he would have been entitled to receive one week’s notice for each completed year of continuous service up to a maximum of twelve weeks’ notice after twelve years of continuous service. During such notice period, we were required to continue to compensate Mr. Olson according to his employment agreement and Mr. Olson would have been obligated to continue to discharge and perform all of his duties and obligations under his employment agreement, and to cooperate with us and use his best efforts to assist with the integration of any persons that we delegated to assume Mr. Olson’s responsibilities. We believed that our notice arrangement with Mr. Olson would assist us in achieving a successful transition upon Mr. Olson’s departure.
In addition, pursuant to terms contained in Mr. Olson’s stock option award agreement, in the event of a change of control of our company, the stock options granted to Mr. Olson that were unvested would vest immediately upon such change of control if such stock options were not assumed or substituted by the surviving company. Also, upon termination of Mr. Olson’s employment for any reason, we would compensate him for all unused vacation days accrued.
The following tables show, as of December 31, 2014, potential payments to our named executive officers for various scenarios involving a resignation, termination, change of control, retirement, death or disability, using, where applicable, the closing price of our common stock of $0.78 (as reported on the NYSE MKT as of December 31, 2014). Compensation amounts to be paid in non-U.S. currency have been converted into U.S. dollars using 3.889 NIS per dollar, which was the exchange rate as of December 31, 2014.
| Type of Event | Voluntary Resignation Upon Breach By Us | Voluntary Resignation | Termination for Cause | Termination Not for Cause | Death | Disability | Termination Not for Cause in Connection with a Change of Control | Change of Control (No Termination) | ||||||||||||||||||||||||
| Alan Milinazzo | ||||||||||||||||||||||||||||||||
| Employment agreement payments | $ | 191,639 | (1) | $ | 125,355 | (2) | 87,855 | (3) | $ | 191,639 | (1) | 154,139 | (4) | 191,639 | (1) | $ | 191,639 | (1) | — | |||||||||||||
| Severance payments | $ | 900,000 | (5) | $ | — | — | $ | 900,000 | (5) | $ | 900,000 | (5) | $ | 900,000 | (5) | $ | 1,125,000 | (6) | — | |||||||||||||
| Accrued vacation payments | $ | 52,855 | $ | 52,855 | $ | 52,855 | $ | 52,855 | $ | 52,855 | $ | 52,855 | $ | 52,855 | — | |||||||||||||||||
| Value of accelerated restricted shares | $ | 222,028 | (7) | — | — | $ | 222,028 | (7) | $ | 222,028 | (7) | $ | 222,028 | (7) | $ | 444,056 | (8) | — | ||||||||||||||
| Craig Shore | ||||||||||||||||||||||||||||||||
| Employment agreement payments | $ | 55,245 | (9) | $ | 55,245 | (9) | 38,788 | (10) | $ | 118,158 | (11) | 38,788 | (10) | 85,245 | (12) | $ | 118,158 | (11) | — | |||||||||||||
| Severance payments | $ | 67,564 | (13) | $ | 51,615 | (14) | — | $ | 274,240 | (15) | $ | 274,240 | (15) | $ | 274,240 | (15) | $ | 274,240 | (15) | — | ||||||||||||
| Accrued vacation payments (16) | $ | 38,598 | $ | 38,598 | $ | 38,598 | $ | 38,598 | $ | 38,598 | $ | 38,598 | $ | 38,598 | — | |||||||||||||||||
| Value of accelerated restricted shares | $ | 41,627 | (17) | — | — | $ | 41,627 | (17) | $ | 83,253 | (18) | $ | 83,253 | (18) | $ | 83,253 | (18) | $ | 83,253 | (18) | ||||||||||||
| James Barry, Ph.D. | ||||||||||||||||||||||||||||||||
| Employment agreement payments | $ | 117,293 | (19) | $ | 71,539 | (20) | 41,122 | (21) | $ | 117,293 | (19) | 86,876 | (22) | 117,293 | (19) | $ | 117,293 | (19) | — | |||||||||||||
| Severance payments | $ | 547,500 | (23) | — | — | $ | 547,500 | (23) | $ | 547,500 | (23) | $ | 547,500 | (23) | $ | 912,500 | (6) | — | ||||||||||||||
| Accrued vacation payments | $ | 8,298 | $ | 8,298 | $ | 8,298 | $ | 8,298 | $ | 8,298 | $ | 8,298 | $ | 8,298 | — | |||||||||||||||||
| Value of accelerated restricted shares | $ | 58,500 | (24) | — | — | $ 58,500 | (24) | $ | 58,500 | (24) | $ | 58,500 | (24) | $ | 117,000 | (25) | — | |||||||||||||||
| Eli Bar | ||||||||||||||||||||||||||||||||
| Employment agreement payments | $ | 41,995 | (26) | $ | 41,995 | (26) | 13,998 | (27) | $ | 41,995 | (26) | 13,998 | (27) | 41,995 | (26) | $ | 41,995 | (26) | — | |||||||||||||
| Severance payments | $ | 133,880 | (13) | $ | 101,659 | (14) | — | $ | 300,503 | (28) | $ | 300,503 | (28) | $ | 300,503 | (28) | $ | 300,503 | (28) | — | ||||||||||||
| Accrued vacation payments (16) | $ | 80,809 | $ | 80,809 | $ | 80,809 | $ | 80,809 | $ | 80,809 | $ | 80,809 | $ | 80,809 | — | |||||||||||||||||
| Value of accelerated restricted shares | $ | 11,597 | (29) | — | — | $ | 11,597 | (29) | 23,193 | (30) | 23,193 | (30) | 23,193 | (30) | $ | 23,193 | (30) | |||||||||||||||
| Rick Olson | ||||||||||||||||||||||||||||||||
| Employment agreement payments | $ | 27,964 | (31) | $ | 27,964 | (31) | — | $ | 27,964 | (31) | — | — | $ | 27,964 | (31) | — | ||||||||||||||||
| Severance payments | — | — | — | — | — | — | — | — | ||||||||||||||||||||||||
| Accrued vacation payments(32) | $ | 3,299 | $ | 3,299 | $ | 3,299 | $ | 3,299 | $ | 3,299 | $ | 3,299 | $ | 3,299 | — | |||||||||||||||||
| (1) | Comprised of (i) $37,500 of salary for the required 30 day notice period, during which time we must continue to compensate the officer according to his agreement and the officer will be obligated to continue to discharge and perform all of his duties and obligations under the agreement, (ii) $18,750 of unpaid salary, (iii) $69,105 of unpaid 2014 bonus, (iv) $31,284 for continuation of health, dental, vision and life insurance coverage for 18 months and (v) $35,000 to be used by the officer for expenses of executive outplacement services or education programs, selected by the officer. |
| (2) | Comprised of (i) $37,500 of salary for the required 30 day notice period, during which time we must continue to compensate the officer according to his agreement and the officer will be obligated to continue to discharge and perform all of his duties and obligations under the agreement, (ii) $18,750 of unpaid salary and (iii) $69,105 of unpaid 2014 bonus. |
| (3) | Comprised of (i) $18,750 of unpaid salary and (ii) $69,105 of unpaid 2014 bonus. |
| (4) | Comprised of (i) $18,750 of unpaid salary, (ii) $69,105 of unpaid 2014 bonus (iii) $31,284 for continuation of health, dental, vision and life insurance coverage and (iv) a $35,000 cash payment. |
| (5) | 200% of the officer’s base salary according to his employment agreement. |
| (6) | 250% of the officer’s base salary according to his employment agreement. |
| (7) | Represents the vesting of 284,651 restricted shares, multiplied by the closing price of our common stock of $0.78 (as reported on the NYSE MKT as of December 31, 2014). |
| (8) | Represents the vesting of 569,303 restricted shares, multiplied by the closing price of our common stock of $0.78 (as reported on the NYSE MKT as of December 31, 2014). |
| (9) | Comprised of (i) $16,457 of salary for the required 30 day notice period, during which time we must continue to compensate the officer according to his agreement and the officer will be obligated to continue to discharge and perform all of his duties and obligations under the agreement, (ii) $16,457 of unpaid salary and (iii) $22,331 of unpaid 2014 bonus. |
| 72 |
| (10) | Comprised of (i) $16,457 of unpaid salary and (ii) $22,331 of unpaid 2014 bonus. |
| (11) | Comprised of (i) $49,370 of salary for the required 90 day notice period, during which time we must continue to compensate the officer according to his agreement and the officer will be obligated to continue to discharge and perform all of his duties and obligations under the agreement, (ii) $16,457 of unpaid salary, (iii) $22,331 of unpaid 2014 bonus and (iv) $30,000 reimbursement of reasonable, documented outplacement expenses actually incurred. |
| (12) | Comprised of (i) $16,457 of salary for the required 30 day notice period, (ii) $16,457 of unpaid salary, (iii) $22,331 of unpaid 2014 bonus and (iv) $30,000 reimbursement of reasonable, documented outplacement expenses actually incurred. |
| (13) | Represents required severance pay under Israeli law. |
| (14) | Represents the total amount contributed to and accumulated in his severance payment fund. |
| (15) | Comprised of (i) $67,564 required severance pay under Israeli law, (ii) a one-time lump sum severance payment of $197,480, which equals 100% of his annual base salary and (iii) $9,196, which equals the cost to the Company of providing an automobile to him for the 12 months immediately preceding the date of termination. |
| (16) | Pursuant to Israeli law, the value of a vacation day is equal to gross salary divided by 22 working days per month. |
| (17) | Represents the vesting of 53,368 restricted shares, multiplied by the closing price of our common stock of $0.78 (as reported on the NYSE MKT as of December 31, 2014). |
| (18) | Represents the vesting of 106,735 restricted shares, multiplied by the closing price of our common stock of $0.78 (as reported on the NYSE MKT as of December 31, 2014). |
| (19) | Comprised of (i) $30,417 of salary for the required 30 day notice period, during which time we must continue to compensate the officer according to his agreement and the officer will be obligated to continue to discharge and perform all of his duties and obligations under the agreement, (ii) $15,208 of unpaid salary, (iii) $25,914 of unpaid 2014 bonus (iv) $20,754 for continuation of health, dental, vision and life insurance coverage for 18 months and (v) $25,000 to be used by the officer for expenses of executive outplacement services or education programs, selected by the officer. |
| (20) | Comprised of (i) $30,417 of salary for the required 30 day notice period, during which time we must continue to compensate the officer according to his agreement and the officer will be obligated to continue to discharge and perform all of his duties and obligations under the agreement, (ii) $15,208 of unpaid salary and (iii) $25,914 of unpaid 2014 bonus. |
| (21) | Comprised of (i) $15,208 of unpaid salary and (ii) $25,914 of unpaid 2014 bonus. |
| (22) | Comprised of (i) $15,208 of unpaid salary, (ii) $25,914 of unpaid 2014 bonus (iii) $20,754 for continuation of health, dental, vision and life insurance coverage and (iv) a $25,000 cash payment. |
| (23) | 150% of the officer’s base salary according to his employment agreement. |
| (24) | Represents the vesting of 75,000 restricted shares, multiplied by the closing price of our common stock of $0.78 (as reported on the NYSE MKT as of December 31, 2014). |
| (25) | Represents the vesting of 150,000 restricted shares, multiplied by the closing price of our common stock of $0.78 (as reported on the NYSE MKT as of December 31, 2014). |
| (26) | Comprised of (i) $27,997 of salary for the required 60 day notice period, during which time we must continue to compensate the officer according to his agreement and the officer will be obligated to continue to discharge and perform all of his duties and obligations under the agreement and (ii) $13,998 of unpaid salary. |
| (27) | Comprised of unpaid salary. |
| (28) | Comprised of (i) $133,880 required severance pay under Israeli law and (ii) a one-time lump sum severance payment of $166,623, which equals 100% of his annual base salary. |
| (29) | Represents the vesting of 14,868 restricted shares, multiplied by the closing price of our common stock of $0.78 (as reported on the NYSE MKT as of December 31, 2014). |
| (30) | Represents the vesting of 29,735 restricted shares, multiplied by the closing price of our common stock of $0.78 (as reported on the NYSE MKT as of December 31, 2014). |
| (31) | Comprised of (i) $21,437 of total compensation for 30 days, during which time we will continue to compensate the officer according to his agreement and the officer will be obligated to continue to discharge and perform all of his duties and obligations under the agreement, and (ii) $6,527 of unpaid 2014 commissions. |
| (32) | Pursuant to UK law, the value of a vacation day is equal to gross salary divided by 22 working days per month. |
| 73 |
Director Compensation
The following table shows information concerning our directors, other than Alan Milinazzo and James Barry, Ph.D., during the twelve months ended December 31, 2014.
| Name | Fees Earned or Paid in Cash ($) | Stock Awards ($) | Option Awards (1) ($) | All Other Compensation ($) | Total ($) | |||||||||||||||
| Sol J. Barer, Ph.D. | 35,000 | — | 161,346 | (2) | — | 196,346 | ||||||||||||||
| Paul Stuka | 39,000 | — | 94,909 | (3) | — | 133,909 | ||||||||||||||
| James J. Loughlin | 41,000 | — | 94,909 | (4) | — | 135,909 | ||||||||||||||
| Michael Berman | 32,000 | — | 94,909 | (5) | — | 126,909 | ||||||||||||||
| Campbell Rogers, M.D. | 27,000 | — | 94,909 | (6) | — | 121,909 | ||||||||||||||
| (1) | The amounts in this column reflect the dollar amounts recognized for financial statement reporting purposes with respect to the twelve months ended December 31, 2014, in accordance with FASB ASC Topic 718. Fair value is based on the Black-Scholes option pricing model using the fair value of the underlying shares at the measurement date. For additional discussion of the valuation assumptions used in determining stock-based compensation and the grant date fair value for stock options, see “Management’s Discussion and Analysis of Financial Condition and Results of Operations - Critical Accounting Policies - Share-based compensation” and Note 2-“Significant Accounting Policies” and Note 9-“Equity” of the Notes to the Consolidated Financial Statements for the Year Ended December 31, 2014 included herein. | |
| (2) | Represents the fair market value on the date of grant of an option to purchase 85,000 shares of our common stock granted to Dr. Barer on January 29, 2014. The option has an exercise price of $3.10 per share and vests annually, with one-third vesting in 2015, 2016 and 2017 on the anniversary of the date of grant, provided that if Dr. Barer fails to be reelected or nominated for reelection at the 2015 annual meeting of stockholders, the option vests and becomes exercisable as of such date. The option will expire on January 29, 2024. This grant was made under the InspireMD, Inc. 2013 Long Term Incentive Plan. | |
| (3) | Represents the fair market value on the date of grant of an option to purchase 50,000 shares of our common stock granted to Mr. Stuka on January 29, 2014. The option has an exercise price of $3.10 per share and vests annually, with one-third vesting in 2015, 2016 and 2017 on the anniversary of the date of grant, provided that if Mr. Stuka fails to be reelected or nominated for reelection at the 2015 annual meeting of stockholders, the option vests and becomes exercisable as of such date. The option will expire on January 29, 2024. This grant was made under the InspireMD, Inc. 2013 Long Term Incentive Plan. | |
| (4) | Represents the fair market value on the date of grant of an option to purchase 50,000 shares of our common stock granted to Mr. Loughlin on January 29, 2014. The option has an exercise price of $3.10 per share and vests annually, with one-third vesting in 2015, 2016 and 2017 on the anniversary of the date of grant, provided that if Mr. Loughlin fails to be reelected or nominated for reelection at the 2016 annual meeting of stockholders, the option vests and becomes exercisable as of such date. The option will expire on January 29, 2024. This grant was made under the InspireMD, Inc. 2013 Long Term Incentive Plan. | |
| (5) | Represents the fair market value on the date of grant of an option to purchase 50,000 shares of our common stock granted to Mr. Berman on January 29, 2014. The option has an exercise price of $3.10 per share and vests annually, with one-third vesting in 2015, 2016 and 2017 on the anniversary of the date of grant, provided that if Mr. Berman fails to be reelected or nominated for reelection at the 2016 annual meeting of stockholders, the option vests and becomes exercisable as of such date. The option will expire on January 29, 2024. This grant was made under the InspireMD, Inc. 2013 Long Term Incentive Plan. | |
| (6) | Represents the fair market value on the date of grant of an option to purchase 50,000 shares of our common stock granted to Dr. Rogers on January 29, 2014. The option has an exercise price of $3.10 per share and vests annually, with one-third vesting in 2015, 2016 and 2017 on the anniversary of the date of grant, provided that if Dr. Rogers fails to be reelected or nominated for reelection at the 2017 annual meeting of stockholders, the option vests and becomes exercisable as of such date. The option will expire on January 29, 2024. This grant was made under the InspireMD, Inc. 2013 Long Term Incentive Plan. |
For the 2014 calendar year, our board approved the following compensation for our independent directors: (i) a $25,000 stipend, payable quarterly; (ii) annual committee chair compensation (effective April 1, 2014) of $12,000 for the chairman of the audit committee, $8,000 for the chairman of the compensation committee and $5,000 for the chairmen of the nominating and corporate governance committee and the research and development committee; (iii) annual committee membership compensation (effective April 1, 2014) of $4,000 for members of the audit committee and the compensation committee and $2,000 for members of the nominating and corporate governance committee and the research and development committee; (iv) an option to purchase 50,000 shares of our common stock for each board member; and (v) an option to purchase an additional 35,000 shares of our common stock for the chairman of the board.
On January 5, 2015, our compensation committee amended its compensation policy for directors to provide that effective as of July 1, 2014, each director would forego any cash compensation in exchange for such number of immediately vested 10 year stock options having a Black-Scholes value equal to the cash compensation otherwise due to such director under the Company’s current director compensation policies. As a result of such amendment, on January 5, 2015, the Company granted to each of Sol Barer, Ph.D., Michael Berman, James Loughlin, Campbell Rogers, M.D. and Paul Stuka options to purchase 41,611, 38,045, 48,745, 32,100 and 46,367 shares of common stock, respectively, in lieu of the cash compensation that was owed to them for their services as directors for the third and fourth calendar quarters of 2014 (which was $17,500, $16,000, $20,500, $13,500 and $19,500, respectively). Each of these options has a term of 10 years, an exercise price of $0.78 per share, the closing price of the Company’s common stock on the date of the grant, and vested immediately.
| 74 |
Directors’ and Officers’ Liability Insurance
We currently have directors’ and officers’ liability insurance insuring our directors and officers against liability for acts or omissions in their capacities as directors or officers, subject to certain exclusions. Such insurance also insures us against losses which we may incur in indemnifying our officers and directors. In addition, we have entered into indemnification agreements with key officers and directors and such persons shall also have indemnification rights under applicable laws, and our certificate of incorporation and bylaws.
Compensation Committee Interlocks and Insider Participation
During the fiscal year ended December 31, 2014, Messrs. Stuka and Loughlin and Dr. Barer served on our compensation committee. None of our executive officers currently serves, or in the past year has served, as a member of the board of directors or compensation committee of any entity that has one or more executive officers serving on our board of directors or compensation committee.
Compensation Committee Report
The compensation committee has reviewed and discussed the Compensation Discussion and Analysis with the members of our management and, based on such review and discussions, the compensation committee recommended to the board of directors that the Compensation Discussion and Analysis be included in this Annual Report on Form 10-K.
| COMPENSATION COMMITTEE | |
| Paul Stuka, Chairman | |
| James J. Loughlin | |
| Sol J. Barer, Ph.D. |
| 75 |
| Item 12. | Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters. |
The following table sets forth information with respect to the beneficial ownership of our common stock as of March 11, 2015 by:
| · | each person known by us to beneficially own more than 5.0% of our common stock; |
| · | each of our directors; |
| · | each of the named executive officers; and |
| · | all of our directors and executive officers as a group. |
The percentages of common stock beneficially owned are reported on the basis of regulations of the Securities and Exchange Commission governing the determination of beneficial ownership of securities. Under the rules of the Securities and Exchange Commission, a person is deemed to be a beneficial owner of a security if that person has or shares voting power, which includes the power to vote or to direct the voting of the security, or investment power, which includes the power to dispose of or to direct the disposition of the security. Except as indicated in the footnotes to this table, each beneficial owner named in the table below has sole voting and sole investment power with respect to all shares beneficially owned and each person’s address is c/o InspireMD, Inc., 321 Columbus Avenue, Boston, MA 02116. As of March 11, 2015, we had 78,152,015 shares outstanding.
| Name of Beneficial Owner | Number of Shares Beneficially Owned (1) | Percentage Beneficially Owned (1) | ||||||
| 5% Owners | ||||||||
| Sabby Management LLC. (2) | 8,671,180 | (3) | 10.6 | % | ||||
| Officers and Directors | ||||||||
| Alan W. Milinazzo | 2,356,241 | (4) | 2.98 | % | ||||
| Craig Shore | 370,222 | (5) | * | |||||
| Sol J. Barer, Ph.D. | 8,233,406 | (6) | 10.07 | % | ||||
| James Barry, Ph.D. | 509,055 | (7) | * | |||||
| Michael Berman | 167,655 | (8) | * | |||||
| James J. Loughlin | 147,078 | (9) | * | |||||
| Campbell Rogers, M.D. | 90,433 | (10) | * | |||||
| Paul Stuka | 2,308,238 | (11) | 2.92 | % | ||||
| Rick Olson | 218,132 | * | ||||||
| Eli Bar | 483,477 | (12) | * | |||||
| All directors and executive officers as a group (10 persons) | 14,883,938 | 17.61 | % | |||||
| * | Represents ownership of less than one percent. |
| (1) | Shares of common stock beneficially owned and the respective percentages of beneficial ownership of common stock assumes the exercise of all options, warrants and other securities convertible into common stock beneficially owned by such person or entity currently exercisable or exercisable within 60 days of March 11, 2015. Shares issuable pursuant to the exercise of stock options and warrants exercisable within 60 days are deemed outstanding and held by the holder of such options or warrants for computing the percentage of outstanding common stock beneficially owned by such person, but are not deemed outstanding for computing the percentage of outstanding common stock beneficially owned by any other person. |
| 76 |
| (2) |
Sabby Management LLC's address is 10 Mountainview Road, Suite 205, Upper Saddle River, New Jersey 07458
| |
| (3) |
Based on Amendment No. 1 to Schedule 13G filed with the Securities and Exchange Commission on January 16, 2015 and comprised of (i) 2,766,913 shares of common stock owned directly by Sabby Healthcare Master Fund, Ltd., (ii) 2,250,421 shares of common stock owned directly by Sabby Volatility Warrant Master Fund, Ltd., (iii) warrants to purchase 576,923 shares of common stock that are currently exercisable or exercisable within 60 days of March , 2015 owned directly by Sabby Healthcare Volatility Master Fund, Ltd., (iv) warrants to purchase 1,826,923 shares of common stock that are currently exercisable or exercisable within 60 days of March , 2015 owned directly by Sabby Volatility Warrant Master Fund, Ltd. and (v) warrants to purchase 1,250,000 shares of common stock that are currently exercisable or exercisable within 60 days of March , 2015 owned directly by Sabby Healthcare Master Fund, Ltd. Sabby Management, LLC serves as the investment manager of Sabby Healthcare Master Fund, Ltd. and Sabby Volatility Warrant Master Fund, Ltd. Hal Mintz serves as manager of Sabby Management, LLC. As such, Sabby Management, LLC and Hal Mintz may be deemed to beneficially own these securities. |
| (4) | Includes options to purchase 932,805 shares of common stock that are currently exercisable or exercisable within 60 days of March 11, 2015. |
| (5) | Includes options to purchase 216,612 shares of common stock that are currently exercisable or exercisable within 60 days of March 11, 2015. |
| (6) | Includes options to purchase 3,591,098 shares of common stock that are currently exercisable or exercisable within 60 days of March 11, 2015. |
| (7) | Includes options to purchase 126,282 shares of common stock that are currently exercisable or exercisable within 60 days of March 11, 2015. |
| (8) | Includes options to purchase 137,655 shares of common stock that are currently exercisable or exercisable within 60 days of March 11, 2015.
| |
| (9) | Includes options to purchase 132,078 shares of common stock that are currently exercisable or exercisable within 60 days of March 11, 2015. | |
| (10) | Includes options to purchase 90,433 shares of common stock that are currently exercisable or exercisable within 60 days of March 11, 2015. |
| (11) |
Paul Stuka is the principal and managing member of Osiris Investment Partners, L.P., and, as such, has beneficial ownership of the (i) 1,370,204 shares of common stock and (ii) currently exercisable warrants to purchase 166,667 shares of common stock held by Osiris Investment Partners, L.P., in addition to personally holding options to purchase 146,367 shares of common stock that are currently exercisable or exercisable within 60 days of March 11, 2015 and warrants to purchase 625,000 shares of common stock that are currently exercisable or exercisable within 60 days of March 11, 2015 |
| (12) | Includes options to purchase 140,558 shares of common stock that are currently exercisable or exercisable within 60 days of March 11, 2015. |
| 77 |
Equity Compensation Plan Information
The following table provides certain information as of December 31, 2014 with respect to our equity compensation plans under which our equity securities are authorized for issuance:
| Number of securities | ||||||||||||
| remaining available for | ||||||||||||
| future issuance under | ||||||||||||
| Number of securities to | Weighted-average | equity compensation | ||||||||||
| be issued upon exercise | exercise price of | plans (excluding | ||||||||||
| of outstanding options, | outstanding options, | securities reflected in | ||||||||||
| warrants and rights | warrants and rights | column (a)) | ||||||||||
| Plan Category | (a) | (b) | (c) | |||||||||
| Equity compensation plans approved by security holders | 4,833,426 | 3.34 | 3,040,121 | |||||||||
| Equity compensation plans not approved by security holders | 1,110,847 | (1) | 5.83 | - | ||||||||
| Total | 5,944,273 | 3.8 | ||||||||||
________________
(1) Comprised of awards made to individuals outside the InspireMD, Inc. 2011 UMBRELLA Option Plan and 2013 Long Term Incentive Plan, as described below:
| · | In April 2008, we issued options to purchase 1,461 shares of common stock to a provider of finder services who assisted InspireMD Ltd. in raising funds in 2008. The exercise price of these options is $4.93 per share. These options are fully vested and expire in June 2016. |
| · | Options issued to a consultant: in May 2006, we issued options to purchase 83,636 shares of common stock to a consultant. The exercise price of these options was $0.76 per share. We believe these options have expired, but they are included above because such expiration is currently under legal dispute. |
| · | Options issued to current director: in November 2011, we issued options to purchase an aggregate of 725,000 shares of common stock to Dr. Barer, the chairman of our board of directors. The exercise price of these options is $7.80 per share. An option to purchase 181,250 shares of common stock vested on April 11, 2013, when our common stock was first listed on a national securities exchange. An option to purchase 181,250 shares of common stock vested on May 10, 2013, after we received research coverage from a second investment bank that ranked in the top twenty investment banks in terms of life science underwritings. The option to purchase 362,500 shares of common stock vests in substantially equal monthly installments (with any fractional shares vesting on the last vesting date) on the last business day of each calendar month over a two year period from the date of grant, with the first installment vesting on November 30, 2011, provided that Dr. Barer is still providing services to us in some capacity as of each such vesting date. |
| · | Warrant issued to current officer: in March 2011, for work performed in connection with the share exchange transactions and as bonus compensation, we issued Craig Shore, our chief financial officer, secretary and treasurer, a five-year warrant to purchase up to 750 shares of common stock at an exercise price of $7.20 per share. |
| · | Options issued to current vice president of global marketing and strategy: in September 2013, we issued options to purchase 150,000 shares of common stock to David Blossom. The exercise price of these options was $2.23 per share. The options vest annually with one-third vesting on September 16, 2014, September 16, 2015 and September 16, 2016. The options expire on September 16, 2023. |
| · | Options issued to current vice president of global sales operations: in December 2013, we issued options to purchase 150,000 shares of common stock to Rick Olson. The exercise price of these options was $2.75 per share. The options vest annually with one-third vesting on December 2, 2014, December 2, 2015 and December 2, 2016. The options expire on December 2, 2023. |
| Item 13. | Certain Relationships and Related Transactions, and Director Independence. |
On November 7, 2014, we closed a registered direct offering of approximately 6.2 million shares of common stock and warrants to purchase up to approximately 3.1 million shares of common stock at a price of $1.30 per share, for gross proceeds of $8.1 million, before deducting placement agents' fees and estimated offering expenses. Each purchaser received a warrant to purchase 0.5 of a share of common stock for each share of common stock that it purchased in the offering. The warrants are non-exercisable for six months and have a term of exercise of 42 months from the date of issuance and an exercise price of $1.75. The purchasers in the offering included Sol J. Barer, Ph.D., the chairman of our board of directors, who purchased 192,308 shares of common stock and warrants to purchase 96,154 shares of common stock, for a purchase price of $250,000, Alan Milinazzo, our president and chief executive officer, and James Barry, Ph.D., our executive vice president and chief operating officer, who each purchased 19,231 shares of common stock and warrants to purchase 9,616 shares of common stock, for a purchase price of $25,000, and Rick Olson, our then-current vice president of global sales and operations of InspireMD Ltd, who purchased 115,385 shares of common stock and warrants to purchase 57,693 shares of common stock, for a purchase price of $150,000.
| 78 |
On March 9, 2015, we closed a public offering of approximately 34.4 million shares of common stock and warrants to purchase up to approximately 34.4 million shares of common stock at a price of $0.55 per share, for gross proceeds of $13.7 million, before deducting placement agents' fees and estimated offering expenses. Each purchaser received a warrant to purchase one share of common stock for each share of common stock that it purchased in the offering. The warrants have a term of exercise of five years from the date of issuance and an exercise price of $0.55. The purchasers in the offering included: Sol J. Barer, Ph.D., the chairman of our board of directors, who purchased 2,500,000 shares of common stock and warrants to purchase 2,500,000 shares of common stock, for a purchase price of $1,000,000, Osiris Investment Partners, L.P., of which Paul Stuka, our director, is the principal and managing member, which purchased 625,000 shares of common stock and warrants to purchase 625,000 shares of common stock, for a purchase price of $250,000 and Alan Milinazzo, our president and chief executive officer, who purchased 125,000 shares of common stock and warrants to purchase 125,000 shares of common stock, for a purchase price of $50,000.
In accordance with our audit committee charter, the audit committee is required to approve all related party transactions. In general, the audit committee will review any proposed transaction that has been identified as a related party transaction under Item 404 of Regulation S-K, which means a transaction, arrangement or relationship in which we and any related party are participants in which the amount involved exceeds $120,000. A related party includes (i) a director, director nominee or executive officer of us, (ii) a security holder known to be an owner of more than 5% of our voting securities, (iii) an immediate family member of the foregoing or (iv) a corporation or other entity in which any of the foregoing persons is an executive, principal or similar control person or in which such person has a 5% or greater beneficial ownership interest.
Director Independence
The board of directors has determined that Drs. Barer and Rogers and Messrs. Loughlin, Stuka and Berman satisfy the requirement for independence set out in Section 803 of the NYSE MKT rules and that each of these directors has no material relationship with us (other than being a director and/or a stockholder). In making its independence determinations, the board of directors sought to identify and analyze all of the facts and circumstances relating to any relationship between a director, his immediate family or affiliates and our company and our affiliates and did not rely on categorical standards other than those contained in the NYSE MKT rule referenced above.
| Item 14. | Principal Accountant Fees and Services. |
The fees billed for professional services provided to us by Kesselman & Kesselman, Certified Public Accountants (“Kesselman”), a member of PricewaterhouseCoopers International Limited, for the year ended December 31, 2014, the six month period ended December 31, 2013 and the twelve month period ended June 30, 2013 are described below.
Audit Fees
Kesselman billed us audit fees in the aggregate amount of $170,500 for the year ended December 31, 2014, $110,000 for the six month period ended December 31, 2013 and $290,000 for the twelve month period ended June 30, 2013. These fees relate to the audit of our annual financial statements and the review of our interim quarterly financial statements.
Audit-Related Fees
Kesselman billed us audit-related fees in the aggregate amount of $67,000 for the year ended December 31, 2014, $35,000 for the six month period ended December 31, 2013 and $135,000 for the twelve month period ended June 30, 2013. The fees for the year ended December 31, 2014 related to performance of audit-related services for our registration statement on Form S-8 initially filed with the Securities and Exchange Commission on June 5, 2014 our prospectus supplement initially filed with the Securities and Exchange Commission on November 5, 2014 and in connection with our evaluation of certain abandoned transactions. The fees for the six month period ended December 31, 2013 related to performance of audit-related services for our registration statement on Form S-3 initially filed with the Securities and Exchange Commission on October 24, 2013. The fees for the twelve month period ended June 30, 2013 related to the performance of audit-related services for our registration statement on Form S-1 initially filed with the Securities and Exchange Commission on September 24, 2012 and amendments thereto.
Tax Fees
Kesselman billed us tax fees in the aggregate amount of $42,275 for the year ended December 31, 2014, $35,000 for the six month period ended December 31, 2013 and $55,500 for the twelve month period ended June 30, 2013. These fees relate to professional services rendered for tax compliance, tax advice and tax planning.
All Other Fees
Kesselman did not bill us for any other fees for the year ended December 31, 2014, the six month period ended December 31, 2013 and the twelve month period ended June 30, 2013.
Our audit committee pre-approves all auditing services, internal control-related services and permitted non-audit services (including the fees and terms thereof) to be performed for us by our independent auditor, except for de minimis non-audit services that are approved by the audit committee prior to the completion of the audit. The audit committee may form and delegate authority to subcommittees consisting of one or more members when appropriate, including the authority to grant pre-approvals of audit and permitted non-audit services, provided that decisions of such subcommittee to grant pre-approvals is presented to the full audit committee at its next scheduled meeting. The Audit Committee pre-approved all of the fees set forth above.
| 79 |
Item 15. Exhibits and Financial Statement Schedules.
Documents filed as part of report:
1. Financial Statements
The following financial statements are included herein:
| · | Report of Kesselman & Kesselman, Independent Registered Public Accounting Firm |
| · | Consolidated Balance Sheets as of December 31, 2014 and 2013 |
| · | Consolidated Statements of Operations for the Year Ended December 31, 2014, Six Months Ended December 31, 2013 and Years Ended June 30, 2013 and 2012 |
| · | Consolidated Statements of Changes in Equity for the Year Ended December 31, 2014, Six Months Ended December 31, 2013 and Years Ended June 30, 2013 and 2012 |
| · | Consolidated Statements of Cash Flows for the Year Ended December 31, 2014, Six Months Ended December 31, 2013 and Years Ended June 30, 2013 and 2012 |
| · | Notes to Consolidated Financial Statements |
2. Financial Statement Schedules
None
3. Exhibits
See Index to Exhibits
| 80 |
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| INSPIREMD, INC. | ||
| Date: March 12, 2015 | By: | /s/ Alan Milinazzo |
| Alan Milinazzo | ||
| President and Chief Executive Officer | ||
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
| Signature | Title | Date | ||
| /s/ Alan Milinazzo | President, Chief Executive Officer and Director | March 12, 2015 | ||
| Alan Milinazzo | (principal executive officer) | |||
| /s/ Craig Shore | Chief Financial Officer, Secretary and Treasurer | March 12, 2015 | ||
| Craig Shore | (principal financial and accounting officer) | |||
| /s/ Sol J. Barer, Ph.D. | Chairman of the Board of Directors | March 12, 2015 | ||
| Sol J. Barer, Ph.D. | ||||
| /s/ James Barry, Ph.D. | Director | March 12, 2015 | ||
| James Barry, Ph.D. | ||||
| /s/ Michael Berman | Director | March 12, 2015 | ||
| Michael Berman | ||||
| /s/ James J. Loughlin | Director | March 12, 2015 | ||
| James J. Loughlin | ||||
| /s/ Campbell Rogers, M.D. | Director | March 12, 2015 | ||
| Campbell Rogers, M.D. | ||||
| /s/ Paul Stuka | Director | March 12, 2015 | ||
| Paul Stuka |
| 81 |
Index to Exhibits
| Exhibit No. | Description | |
| 3.1 | Amended and Restated Certificate of Incorporation (incorporated by reference to Exhibit 3.1 to Current Report on Form 8-K filed with the Securities and Exchange Commission on April 1, 2011) | |
| 3.2 | Amended and Restated Bylaws (incorporated by reference to Exhibit 3.2 to Current Report on Form 8-K filed with the Securities and Exchange Commission on April 1, 2011) | |
| 3.3 | Certificate of Amendment to Amended and Restated Certificate of Incorporation (incorporated by reference to Exhibit 3.1 to Current Report on Form 8-K filed with the Securities and Exchange Commission on December 21, 2012) | |
| 3.4 | Certificate of Designation, Preferences and Rights of Series A Preferred Stock (incorporated by reference to Exhibit 3.1 to Current Report on Form 8-K filed with the Securities and Exchange Commission on October 25, 2013) | |
| 4.1 | Form of Common Stock Certificate (incorporated by reference to Exhibit 4.1 to Amendment No. 3 to Registration Statement on Form S-1 filed with the Securities and Exchange Commission on March 5, 2013) | |
| 4.2 | Rights Agreement dated as of October 22, 2013 between InspireMD, Inc. and Action Stock transfer Corporation, as Rights Agent, including exhibits thereto (incorporated by reference to an exhibit to the Registration Statement on Form 8-A filed with Securities and Exchange Commission on October 25, 2013) | |
| 10.1+ | Amended and Restated 2011 Umbrella Option Plan (incorporated by reference to Exhibit 10.1 to Current Report on Form 8-K filed with the Securities and Exchange Commission on November 4, 2011) | |
| 10.2+ | Form of Stock Option Award Agreement (incorporated by reference to Exhibit 10.2 to Current Report on Form 8-K filed with the Securities and Exchange Commission on April 6, 2011) | |
| 10.3 | Securities Purchase Agreement, dated as of March 31, 2011, by and among InspireMD, Inc. and certain purchasers set forth therein (incorporated by reference to Exhibit 10.5 to Amendment No. 1 to Registration Statement on Form S-1 filed with the Securities and Exchange Commission on August 26, 2011) | |
| 10.4 | Form of $7.20 Warrant (incorporated by reference to Exhibit 10.6 to Current Report on Form 8-K filed with the Securities and Exchange Commission on April 6, 2011) | |
| 10.5 | Form of $4.92 Warrant (incorporated by reference to Exhibit 10.7 to Current Report on Form 8-K filed with the Securities and Exchange Commission on April 6, 2011) | |
| 10.6 | Manufacturing Agreement, by and between InspireMD Ltd. and QualiMed Innovative Medizinprodukte GmbH, dated as of September 11, 2007 (incorporated by reference to Exhibit 10.11 to Amendment No. 1 to Registration Statement on Form S-1 filed with the Securities and Exchange Commission on August 26, 2011) | |
| 10.7 | Development Agreement, by and between InspireMD Ltd. and QualiMed Innovative Medizinprodukte GmbH, dated as of January 15, 2007 (incorporated by reference to Exhibit 10.12 to Amendment No. 1 to Registration Statement on Form S-1 filed with the Securities and Exchange Commission on August 26, 2011) | |
| 10.8 | License Agreement, by and between Svelte Medical Systems, Inc. and InspireMD Ltd., dated as of March 19, 2010 (incorporated by reference to Exhibit 10.5 to Amendment No. 1 to Registration Statement on Form S-1 filed with the Securities and Exchange Commission on August 26, 2011) | |
| 10.9+ | Personal Employment Agreement, by and between InspireMD Ltd. and Eli Bar, dated as of June 26, 2005 (incorporated by reference to Exhibit 10.19 to Current Report on Form 8-K filed with the Securities and Exchange Commission on April 6, 2011) |
| 82 |
| 10.10+ | Employment Agreement, by and between InspireMD Ltd. and Craig Shore, dated as of November 28, 2010 (incorporated by reference to Exhibit 10.21 to Current Report on Form 8-K filed with the Securities and Exchange Commission on April 6, 2011) | |
| 10.11+ | Form of Indemnity Agreement between InspireMD, Inc. and each of the directors and executive officers thereof (incorporated by reference to Exhibit 10.22 to Amendment No. 1 to Registration Statement on Form S-1 filed with the Securities and Exchange Commission on August 26, 2011) | |
| 10.12 | Form of Warrant (incorporated by reference to Exhibit 10.2 to Current Report on Form 8-K filed with the Securities and Exchange Commission on April 22, 2011) | |
| 10.13 | Agreement by and between InspireMD Ltd. and MeKo Laser Material Processing, dated as of April 15, 2010 (incorporated by reference to Exhibit 10.26 to Amendment No. 1 to Registration Statement on Form S-1 filed with the Securities and Exchange Commission on August 26, 2011) | |
| 10.14 | Agreement by and between InspireMD Ltd. and Natec Medical Ltd, dated as of September 23, 2009 (incorporated by reference to Exhibit 10.27 to Amendment No. 1 to Registration Statement on Form S-1 filed with the Securities and Exchange Commission on August 26, 2011) | |
| 10.15 | Exclusive Distribution Agreement by and between InspireMD Ltd. and Hand-Prod Sp. Z o.o, dated as of December 10, 2007 (incorporated by reference to Exhibit 10.28 to Amendment No. 3 to Registration Statement on Form S-1 filed with the Securities and Exchange Commission on October 12, 2011) | |
| 10.16+ | $6.00 Nonqualified Stock Option Agreement, dated as of July 11, 2011, by and between InspireMD, Inc. and Sol J. Barer, Ph.D. (Incorporated by reference to Exhibit 10.1 to Current Report on Form 8-K filed with the Securities and Exchange Commission on July 15, 2011) | |
| 10.17 | Exclusive Distribution Agreement by and between InspireMD GmbH. and IZASA Distribuciones Tecnicas SA, dated as of May 20, 2009 (incorporated by reference to Exhibit 10.36 to Amendment No. 3 to Registration Statement on Form S-1 filed with the Securities and Exchange Commission on October 12, 2011) | |
| 10.18 | Amendment to the Distribution Agreement by and between InspireMD GmbH. and IZASA Distribuciones Tecnicas SA, dated as of February 2011 (incorporated by reference to Exhibit 10.37 to Amendment No. 3 to Registration Statement on Form S-1 filed with the Securities and Exchange Commission on October 12, 2011) | |
| 10.19 | Exclusive Distribution Agreement by and between InspireMD Ltd. and Tzamal-Jacobsohn Ltd., dated as of December 24, 2008 (incorporated by reference to Exhibit 10.38 to Amendment No. 3 to Registration Statement on Form S-1 filed with the Securities and Exchange Commission on October 12, 2011) | |
| 10.20 | Exclusive Distribution Agreement by and between InspireMD Ltd. and Kirloskar Technologies (P) Ltd., dated as of May 13, 2010 (incorporated by reference to Exhibit 10.39 to Amendment No. 3 to Registration Statement on Form S-1 filed with the Securities and Exchange Commission on October 12, 2011) | |
| 10.21 | Letter Agreement by and between InspireMD Ltd. and Tzamal-Jacobsohn Ltd., dated as of May 9, 2011 (incorporated by reference to Exhibit 10.43 to Amendment No. 4 to Registration Statement on Form S-1 filed with the Securities and Exchange Commission on December 1, 2011) | |
| 10.22+ | Stock Award Agreement, dated as of November 16, 2011, by and between InspireMD, Inc. and Sol J. Barer, Ph.D. (Incorporated by reference to Exhibit 10.1 to Current Report on Form 8-K filed with the Securities and Exchange Commission on November 18, 2011) | |
| 10.23+ | Nonqualified Stock Option Agreement, dated as of November 16, 2011, by and between InspireMD, Inc. and Sol J. Barer, Ph.D. (Incorporated by reference to Exhibit 10.2 to Current Report on Form 8-K filed with the Securities and Exchange Commission on November 18, 2011) |
| 83 |
| 10.24 | Amendment No. 1 to Securities Purchase Agreement, dated as of June 21, 2011, by and among InspireMD, Inc. and the purchasers that are signatory thereto (incorporated by reference to Exhibit 10.43 to Annual Report on Form 10-K filed with the Securities and Exchange Commission on March 13, 2012) | |
| 10.25 | Amendment No. 2 to Securities Purchase Agreement, dated as of November 14, 2011, by and among InspireMD, Inc. and the purchasers that are signatory thereto (incorporated by reference to Exhibit 10.44 to Annual Report on Form 10-K filed with the Securities and Exchange Commission on March 13, 2012) | |
| 10.26 | Form of April 2012 $1.80 Warrant (incorporated by reference to Exhibit 10.3 to Current Report on Form 8-K filed with the Securities and Exchange Commission on April 6, 2012) | |
| 10.27 | Registration Rights Agreement, dated April 5, 2012, by and between InspireMD, Inc. and the purchasers set forth therein (incorporated by reference to Exhibit 10.4 to Current Report on Form 8-K filed with the Securities and Exchange Commission on April 6, 2012) | |
| 10.28 | Exclusive Distribution Agreement, dated as of August 1, 2007, by and between InspireMD Ltd. and Kardia Srl. (incorporated by reference to Exhibit 10.59 to Transition Report on Form 10-K/T filed with the Securities and Exchange Commission on September 11, 2012) | |
| 10.29 | Addendum to the Distribution Agreement, dated as of January 18, 2011, by and between InspireMD Ltd. and Kardia Srl. (incorporated by reference to Exhibit 10.60 to Transition Report on Form 10-K/T filed with the Securities and Exchange Commission on September 11, 2012) | |
| 10.30 | Exclusive Distribution Agreement, dated as of May 26, 2011, by and between InspireMD Ltd. and Bosti Trading Ltd. (incorporated by reference to Exhibit 10.62 to Transition Report on Form 10-K/T filed with the Securities and Exchange Commission on September 11, 2012) | |
| 10.31 | Addendum to the Distribution Agreement, dated as of August 29, 2011, by and between InspireMD Ltd. and Bosti Trading Ltd. (incorporated by reference to Exhibit 10.63 to Transition Report on Form 10-K/T filed with the Securities and Exchange Commission on September 11, 2012) | |
| 10.32 | Amendment No. 1 to Registration Rights Agreement, dated May 31, 2012, by and between InspireMD, Inc. and the purchasers set forth therein (incorporated by reference to Exhibit 10.65 to Transition Report on Form 10-K/T filed with the Securities and Exchange Commission on September 11, 2012) | |
| 10.33 | First Amendment to License Agreement, dated October 20, 2012, by and among Svelte Medical Systems, Inc., InspireMD, Inc. and InspireMD Ltd. (incorporated by reference to Exhibit 10.1 to Current Report on Form 8-K filed with the Securities and Exchange Commission on October 23, 2012) | |
| 10.34 | Exclusive Distribution Agreement, dated June 7, 2010, by and between InspireMD Ltd. and Tau Medical Supplies (incorporated by reference to Exhibit 10.68 to Amendment No. 2 to Registration Statement on Form S-1 filed with the Securities and Exchange Commission on November 9, 2012) | |
| 10.35+ | Second Amendment to the InspireMD, Inc. Amended and Restated 2011 UMBRELLA Option Plan (incorporated by reference to Exhibit 10.1 to Current Report on Form 8-K filed with the Securities and Exchange Commission on December 26, 2012) | |
| 10.36+ | Employment Agreement, dated January 3, 2013, by and between InspireMD, Inc. and Alan Milinazzo (incorporated by reference to Exhibit 10.1 to Current Report on Form 8-K filed with the Securities and Exchange Commission on January 9, 2013) | |
| 10.37+ | Nonqualified Stock Option Agreement, dated January 3, 2013, by and between InspireMD, Inc. and Alan Milinazzo (incorporated by reference to Exhibit 10.3 to Current Report on Form 8-K filed with the Securities and Exchange Commission on January 9, 2013) |
| 84 |
| 10.38+ | Incentive Stock Option Agreement, dated January 3, 2013, by and between InspireMD, Inc. and Alan Milinazzo (incorporated by reference to Exhibit 10.4 to Current Report on Form 8-K filed with the Securities and Exchange Commission on January 9, 2013) | |
| 10.39+ | Restricted Stock Award Agreement, dated January 3, 2013, by and between InspireMD, Inc. and Alan Milinazzo (incorporated by reference to Exhibit 10.5 to Current Report on Form 8-K filed with the Securities and Exchange Commission on January 9, 2013) | |
| 10.40 | Form of $3.00 Warrant (incorporated by reference to Exhibit 10.76 to Registration Statement on Form S-1 filed with the Securities and Exchange Commission on April 9, 2013) | |
| 10.41 | Letter Agreement, dated as of April 15, 2013, by and among InspireMD, Inc. and each holder of Senior Secured Convertible Debentures Due April 15, 2014 (incorporated by reference to Exhibit 10.3 to Current Report on Form 8-K filed with the Securities and Exchange Commission on April 15, 2013) | |
| 10.42 | Form of Amended $3.00 Warrant (incorporated by reference to Exhibit 10.4 to Current Report on Form 8-K filed with the Securities and Exchange Commission on April 15, 2013) | |
| 10.43+ | First Amendment to Employment Agreement, dated April 24, 2013, by and between InspireMD, Inc. and Alan Milinazzo (incorporated by reference to Exhibit 10.1 to Current Report on Form 8-K filed with the Securities and Exchange Commission on April 26, 2013) | |
| 10.44+ | First Amendment to Restricted Stock Award Agreement, dated April 24, 2013, by and between InspireMD, Inc. and Alan Milinazzo (incorporated by reference to Exhibit 10.2 to Current Report on Form 8-K filed with the Securities and Exchange Commission on April 26, 2013) | |
| 10.45 | Master Service Agreement, dated May 31, 2013, by and between InspireMD Ltd. and Medpace, Inc. (incorporated by reference to Exhibit 10.1 to Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission on November 12, 2013) | |
| 10.46 | Second Amendment to License Agreement, dated August 22, 2013, by and among Svelte Medical Systems, Inc., InspireMD, Inc. and InspireMD Ltd. (incorporated by reference to Exhibit 10.2 to Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission on November 12, 2013) | |
| 10.47 | Loan and Security Agreement, dated October 23, 2013, by and among InspireMD, Inc., InspireM.D Ltd and Hercules Technology Growth Capital, Inc. (incorporated by reference to Exhibit 10.1 to Current Report on Form 8-K filed with the Securities and Exchange Commission on October 25, 2013) | |
| 10.48 | Fixed Charge Debenture, dated October 23, 2013, by and among InspireMD, Inc., Inspire M.D Ltd and Hercules Technology Growth Capital, Inc. (incorporated by reference to Exhibit 10.2 to Current Report on Form 8-K filed with the Securities and Exchange Commission on October 25, 2013) | |
| 10.49 | Floating Charge Debenture, dated October 23, 2013, by and among InspireMD, Inc., Inspire M.D Ltd and Hercules Technology Growth Capital, Inc. (incorporated by reference to Exhibit 10.3 to Current Report on Form 8-K filed with the Securities and Exchange Commission on October 25, 2013) | |
| 10.50 | Warrant Agreement, dated October 23, 2013, by and between InspireMD, Inc. and Hercules Technology Growth Capital, Inc. (incorporated by reference to Exhibit 10.4 to Current Report on Form 8-K filed with the Securities and Exchange Commission on October 25, 2013) | |
| 10.51 | Account Control Agreement, dated October 23, 2013, among InspireMD, Inc., Hercules Technology Growth Capital, Inc. and Bank Leumi USA (incorporated by reference to Exhibit 10.5 to Current Report on Form 8-K filed with the Securities and Exchange Commission on October 25, 2013) |
| 85 |
| 10.52+ | InspireMD, Inc. 2013 Long-Term Incentive Plan (incorporated by reference to Exhibit 10.1 to Current Report on Form 8-K filed with the Securities and Exchange Commission on December 20, 2013) | |
| 10.53+ | Nonqualified Stock Option Agreement, dated December 2, 2013, by and between InspireMD, Inc. and Rick Olson (incorporated by reference to Exhibit 10.54 to Transition Report on Form 10-KT filed with the Securities and Exchange Commission on February 26, 2014) | |
| 10.54+ | Consulting Agreement, dated February 25, 2014, by and between InspireMD, Inc. and James Barry (incorporated by reference to Exhibit 10.55 to Transition Report on Form 10-KT filed with the Securities and Exchange Commission on February 26, 2014) | |
| 10.55+ | Amended and Restated Employment Agreement, dated May 5, 2014, by and between InspireMD, Inc. and Craig Shore (incorporated by reference to Exhibit 10.2 to Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission on May 7, 2014) | |
| 10.56+ | First Amendment to the InspireMD, Inc. Amended and Restated 2011 UMBRELLA Option Plan (incorporated by reference to Exhibit 10.3 to Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission on May 7, 2014) | |
| 10.57+ | Form of Incentive Stock Option Award Agreement under the InspireMD, Inc. 2013 Long-Term Incentive Plan (incorporated by reference to Exhibit 99.2 to Registration Statement on Form S-8 filed with the Securities and Exchange Commission on June 5, 2014) | |
| 10.58+ | Form of Nonqualified Stock Option Award Agreement under the InspireMD, Inc. 2013 Long-Term Incentive Plan (incorporated by reference to Exhibit 99.3 to Registration Statement on Form S-8 filed with the Securities and Exchange Commission on June 5, 2014) | |
| 10.59+ | Form of Restricted Stock Award Agreement under the InspireMD, Inc. 2013 Long-Term Incentive Plan (incorporated by reference to Exhibit 99.4 to Registration Statement on Form S-8 filed with the Securities and Exchange Commission on June 5, 2014) | |
| 10.60+ | Form of Restricted Stock Unit Award Agreement under the InspireMD, Inc. 2013 Long-Term Incentive Plan (incorporated by reference to Exhibit 99.5 to Registration Statement on Form S-8 filed with the Securities and Exchange Commission on June 5, 2014) | |
| 10.61+ | Form of Section 3(i) Stock Option Award Agreement under the InspireMD, Inc. 2013 Long-Term Incentive Plan (Israeli) (incorporated by reference to Exhibit 99.6 to Registration Statement on Form S-8 filed with the Securities and Exchange Commission on June 5, 2014) | |
| 10.62+ | Form of Section 102 Capital Gain Stock Option Award Agreement under the InspireMD, Inc. 2013 Long-Term Incentive Plan (Israeli) (incorporated by reference to Exhibit 99.7 to Registration Statement on Form S-8 filed with the Securities and Exchange Commission on June 5, 2014) | |
| 10.63+ | Form of Section 102 Capital Gain Restricted Stock Award Agreement under the InspireMD, Inc. 2013 Long-Term Incentive Plan (Israeli) (incorporated by reference to Exhibit 99.8 to Registration Statement on Form S-8 filed with the Securities and Exchange Commission on June 5, 2014) | |
| 10.64+ | Form of Stock Option Award Agreement under the InspireMD, Inc. 2013 Long-Term Incentive Plan (European) (incorporated by reference to Exhibit 99.9 to Registration Statement on Form S-8 filed with the Securities and Exchange Commission on June 5, 2014) | |
| 10.65+ | Form of Restricted Stock Award Agreement under the InspireMD, Inc. 2013 Long-Term Incentive Plan (European) (incorporated by reference to Exhibit 99.10 to Registration Statement on Form S-8 filed with the Securities and Exchange Commission on June 5, 2014) | |
| 10.66+ | Form of Stock Option Award Agreement outside the InspireMD, Inc. 2013 Long-Term Incentive Plan (incorporated by reference to Exhibit 99.11 to Registration Statement on Form S-8 filed with the Securities and Exchange Commission on June 5, 2014) | |
| 10.67+ | Employment Agreement, dated July 14, 2014, by and between InspireMD, Inc. and James J. Barry, Ph.D. (incorporated by reference to Exhibit 10.1 to Current Report on Form 8-K filed with the Securities and Exchange Commission on July 18, 2014) |
| 86 |
|
10.68 |
Form of Securities Purchase Agreement in connection with registered direct offering (incorporated by reference to Exhibit 10.1 to Current Report on Form 8-K filed with the Securities and Exchange Commission on November 5, 2014) | |
| 10.69 | Form of $1.75 Warrant (incorporated by reference to Exhibit 10.2 to Current Report on Form 8-K filed with the Securities and Exchange Commission on November 5, 2014) | |
| 10.70+ | Second Amendment to Employment Agreement, dated January 5, 2015, by and between InspireMD, Inc. and Alan Milinazzo (incorporated by reference to Exhibit 10.1 to Current Report on Form 8-K filed with the Securities and Exchange Commission on January 6, 2015) | |
| 10.71+ | Amendment to Employment Agreement, dated January 5, 2015, by and between InspireMD, Inc. and James J. Barry, PhD (incorporated by reference to Exhibit 10.2 to Current Report on Form 8-K filed with the Securities and Exchange Commission on January 6, 2015) | |
| 10.72+ | First Amendment to Amended and Restated Employment Agreement, dated January 5, 2015, by and between InspireMD, Inc. and Craig Shore (incorporated by reference to Exhibit 10.3 to Current Report on Form 8-K filed with the Securities and Exchange Commission on January 6, 2015) | |
| 10.73* | Exclusive Distribution Agreement, dated December 1, 2014, by and between InspireMD Ltd. and Cardio Medical Sales L.P. | |
| 21.1 | List of Subsidiaries (incorporated by reference to Exhibit 21.1 to Current Report on Form 8-K filed with the Securities and Exchange Commission on April 6, 2011) | |
| 23.1* | Consent of Kesselman & Kesselman, Certified Public Accountants | |
| 31.1* | Certification of Chief Executive Officer Pursuant to Section 302 of Sarbanes-Oxley Act of 2002 | |
| 31.2* | Certification of Chief Financial Officer Pursuant to Section 302 of Sarbanes-Oxley Act of 2002 | |
| 32.1* | Certification of Chief Executive Officer Pursuant to Section 18 U.S.C. Section 1350, as Adopted Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 | |
| 32.2* | Certification of Chief Financial Officer Pursuant to Section 18 U.S.C. Section 1350, as Adopted Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 | |
| 101 | The following materials from the Company’s Annual Report on Form 10-K for the twelve months ended December 31, 2014, formatted in XBRL (eXtensible Business Reporting Language), (i) Condensed Consolidated Balance Sheets, (ii) Condensed Consolidated Statements of Income, (iii) Condensed Consolidated Statements of Comprehensive Income, (iv) Consolidated Statements of Cash Flows, (v) Condensed Consolidated Statement of Stockholders’ Equity and (vi) Notes to Consolidated Financial Statements |
* Filed herewith.
+ Management contract or compensatory plan or arrangement.
| 87 |
INSPIREMD, INC.
CONSOLIDATED FINANCIAL STATEMENTS
AS OF AND FOR THE YEAR ENDED DECEMBER 31, 2014
INSPIREMD, INC.
CONSOLIDATED FINANCIAL STATEMENTS
AS OF AND FOR THE YEAR ENDED DECEMBER 31, 2014
TABLE OF CONTENTS
| Page | |
| REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM | F-2 |
| CONSOLIDATED FINANCIAL STATEMENTS: | |
| Consolidated Balance Sheets | F-3 - F-4 |
| Consolidated Statements of Operations | F-5 |
| Consolidated Statements of Changes in Equity (Capital Deficiency) | F-6 |
| Consolidated Statements of Cash Flows | F-7 |
| Notes to the Consolidated Financial Statements | F-8 - F-42 |
The amounts are stated in U.S. dollars in thousands
_______________
_________________________
_______________

Report of Independent Registered Public Accounting Firm
To the shareholders of
InspireMD Inc.
In our opinion, the accompanying consolidated balance sheets and the related consolidated statements of operations, changes in equity (capital deficiency) and cash flows present fairly, in all material respects, the financial position of InspireMD Inc. (the “Company”) and its subsidiaries at December 31, 2014 and 2013, and the results of their operations and their cash flows for the twelve month period ended December31, 2014 and for the six month period ended December 31, 2013 and for each of the two years in the period ended June 30, 2013 in conformity with accounting principles generally accepted in the United States of America. Also in our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of December 31, 2014, based on criteria established in Internal Control - Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). The Company's management is responsible for these financial statements, for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in “Management's Report on Internal Control Over Financial Reporting” appearing under Item 9(A). Our responsibility is to express opinions on these financial statements and on the Company's internal control over financial reporting based on our audits (which was an integrated audit in 2014). We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the financial statements are free of material misstatement and whether effective internal control over financial reporting was maintained in all material respects. Our audits of the financial statements included examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. Our audit of internal control over financial reporting included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audits also included performing such other procedures as we considered necessary in the circumstances. We believe that our audits provide a reasonable basis for our opinions.
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
| Tel Aviv, Israel | Kesselman & Kesselman | |
March 12, 2015
|
Certified Public Accountants (Isr.) A member of PricewaterhouseCoopers International Limited |
| F-2 |
CONSOLIDATED BALANCE SHEETS
(U.S. dollars in thousands)
| December 31, | ||||||||
| 2014 | 2013 | |||||||
| ASSETS | ||||||||
| CURRENT ASSETS: | ||||||||
| Cash and cash equivalents | $ | 6,300 | $ | 17,535 | ||||
| Restricted cash | - | 93 | ||||||
| Accounts receivable: | ||||||||
| Trade | 635 | 1,855 | ||||||
| Other | 359 | 387 | ||||||
| Prepaid expenses | 150 | 141 | ||||||
| Inventory | 1,924 | 1,593 | ||||||
| Total current assets | 9,368 | 21,604 | ||||||
| NON-CURRENT ASSETS: | ||||||||
| PROPERTY, PLANT AND EQUIPMENT, net | 622 | 652 | ||||||
| Deferred issuance costs | 153 | 310 | ||||||
| Fund in respect of employee rights upon retirement | 498 | 434 | ||||||
| Long-term prepaid expenses | 66 | 114 | ||||||
| Royalties buyout | 752 | 852 | ||||||
| Total non-current assets | 1,469 | 1,710 | ||||||
| Total assets | $ | 11,459 | $ | 23,966 | ||||
| F-3 |
INSPIREMD, INC.
CONSOLIDATED BALANCE SHEETS
(U.S. dollars in thousands other than per share data)
| December 31, | ||||||||
| 2014 | 2013 | |||||||
| LIABILITIES AND EQUITY (NET OF CAPITAL DEFICIENY) | ||||||||
| CURRENT LIABILITIES: | ||||||||
| Accounts payable and accruals: | ||||||||
| Trade | $ | 909 | $ | 1,623 | ||||
| Other | 3,576 | 3,141 | ||||||
| Advanced payment from customers | 179 | 179 | ||||||
| Current maturity of loan | 3,809 | 1,181 | ||||||
| Total current liabilities | 8,473 | 6,124 | ||||||
| LONG-TERM LIABILITIES: | ||||||||
| Liability for employees rights upon retirement | 687 | 610 | ||||||
| Long-term loan | 5,086 | 8,593 | ||||||
| Total long-term liabilities | 5,773 | 9,203 | ||||||
| COMMITMENTS AND CONTINGENT LIABILITIES | ||||||||
| (Note 8) | ||||||||
| Total liabilities | 14,246 | 15,327 | ||||||
| EQUITY (CAPITAL DEFICIENCY): | ||||||||
| Common stock, par value $0.0001 per share; 125,000,000 shares authorized; 41,368,889 and 33,983,346, shares issued and outstanding at December 31, 2014 and 2013, respectively | 4 | 3 | ||||||
| Additional paid-in capital | 104,620 | 90,952 | ||||||
| Accumulated deficit | (107,411 | ) | (82,316 | ) | ||||
| Total equity (capital deficiency) | (2,787 | ) | 8,639 | |||||
| Total liabilities and equity (net of capital deficiency) | $ | 11,459 | $ | 23,966 | ||||
The accompanying notes are an integral part of the consolidated financial statements.
| F-4 |
CONSOLIDATED STATEMENTS OF OPERATIONS
(U.S. dollars in thousands, except per share data)
| Year ended December 31, | 6 month period ended December 31, | Year ended June 30, | ||||||||||||||
| 2014 | 2013 | 2013 | 2012 | |||||||||||||
| REVENUES | $ | 2,818 | $ | 3,105 | $ | 4,873 | $ | 5,349 | ||||||||
| COST OF REVENUES | 2,034 | 1,442 | 2,283 | 2,849 | ||||||||||||
| GROSS PROFIT | 784 | 1,663 | 2,590 | 2,500 | ||||||||||||
| OPERATING EXPENSES: | ||||||||||||||||
| Royalties buyout expenses | - | - | 918 | - | ||||||||||||
| Other research and development expenses | 8,744 | 3,315 | 4,156 | 3,988 | ||||||||||||
| Selling and marketing | 6,613 | 2,647 | 3,616 | 2,174 | ||||||||||||
| General and administrative | 9,125 | 4,528 | 8,973 | 13,883 | ||||||||||||
| Total operating expenses | 24,482 | 10,490 | 17,663 | 20,045 | ||||||||||||
| LOSS FROM OPERATIONS | (23,698 | ) | (8,827 | ) | (15,073 | ) | (17,545 | ) | ||||||||
| FINANCIAL EXPENSES, net: | ||||||||||||||||
| Interest expenses | 1,409 | 273 | 4,268 | 1,238 | ||||||||||||
| Other financial expenses (income) | (24 | ) | 226 | 9,909 | (1,200 | ) | ||||||||||
| Total financial expenses | 1,385 | 499 | 14,177 | 38 | ||||||||||||
| LOSS BEFORE TAX EXPENSES | (25,083 | ) | (9,326 | ) | (29,250 | ) | (17,583 | ) | ||||||||
| TAX EXPENSES | 12 | 10 | 8 | 14 | ||||||||||||
| NET LOSS | $ | (25,095 | ) | $ | (9,336 | ) | $ | (29,258 | ) | $ | (17,597 | ) | ||||
| NET LOSS PER SHARE - basic and diluted | $ | (0.71 | ) | $ | (0.27 | ) | $ | (1.39 | ) | $ | (1.04 | ) | ||||
| WEIGHTED AVERAGE NUMBER OF ORDINARY SHARES USED IN COMPUTING NET LOSS PER SHARE - basic and diluted | 35,393,644 | 33,963,901 | 20,995,887 | 16,707,599 | ||||||||||||
The accompanying notes are an integral part of the consolidated financial statements.
| F-5 |
CONSOLIDATED STATEMENTS OF CHANGES IN EQUITY
| Ordinary shares | ||||||||||||||||||||
| Number of shares | Par value | Additional paid-in capital | Accumulated deficit | Total equity | ||||||||||||||||
| U.S. dollars in thousands | ||||||||||||||||||||
| BALANCE AT JULY 1, 2011 | 16,046,290 | $ | 2 | $ | 33,283 | $ | (26,125 | ) | $ | 7,160 | ||||||||||
| Net loss | (17,597 | ) | (17,597 | ) | ||||||||||||||||
| Employee and non-employee share-based compensation expenses | 748,446 | * | 10,554 | 10,554 | ||||||||||||||||
| Acquisition and cancellation of shares | (4,696 | ) | * | (21 | ) | (21 | ) | |||||||||||||
| Exercise of options by employee | 250,000 | * | 1,500 | 1,500 | ||||||||||||||||
| Beneficial conversion feature of convertible loan | 3,790 | 3,790 | ||||||||||||||||||
| BALANCE AT JUNE 30, 2012 | 17,040,040 | 2 | 49,106 | (43,722 | ) | 5,386 | ||||||||||||||
| Net loss | (29,258 | ) | (29,258 | ) | ||||||||||||||||
| Employee and non-employee share-based compensation expenses | 3,839 | 3,839 | ||||||||||||||||||
| Issuance of shares - public offering, net of $2,121 issuance costs | 12,500,000 | 1 | 22,879 | 22,880 | ||||||||||||||||
| Issuance of shares | 1,168,515 | * | 3,238 | 3,238 | ||||||||||||||||
| Exercise of Warrants | 195,652 | * | 962 | 962 | ||||||||||||||||
| Exercise of options by employees and non-employees | 834,570 | * | 95 | 95 | ||||||||||||||||
| Taxes withheld in respect of share issuance | (9,506 | ) | * | (27 | ) | (27 | ) | |||||||||||||
| 2013 Exchange agreement: | ||||||||||||||||||||
| Induced conversion of convertible debt | 2,159,574 | * | 8,105 | 8,105 | ||||||||||||||||
| Reclassification of 2012 warrants | 314 | 314 | ||||||||||||||||||
| Issuance of warrants | 568 | 568 | ||||||||||||||||||
| BALANCE AT JUNE 30, 2013 | 33,888,845 | 3 | 89,079 | (72,980 | ) | 16,102 | ||||||||||||||
| Net loss | (9,336 | ) | (9,336 | ) | ||||||||||||||||
| Issuance of shares | 17,398 | * | 44 | 44 | ||||||||||||||||
| Issuance of warrants | 280 | 280 | ||||||||||||||||||
| Employee and non-employee share-based compensation expenses | 1,549 | 1,549 | ||||||||||||||||||
| Exercise of options by employees and non-employees | 77,103 | * | * | |||||||||||||||||
| BALANCE AT DECEMBER 31, 2013 | 33,983,346 | 3 | 90,952 | (82,316 | ) | 8,639 | ||||||||||||||
| Net loss | (25,095 | ) | (25,095 | ) | ||||||||||||||||
| Issuance of shares and warrants, including at the market offering, net of $881 issuance costs | 7,259,272 | 1 | 9,645 | 9,646 | ||||||||||||||||
| Employee and non-employee share-based compensation expenses | 4,138 | 4,138 | ||||||||||||||||||
| Vesting of restricted stock | 171,955 | |||||||||||||||||||
| Taxes withheld in respect of share issuance | (45,684 | ) | (115 | ) | (115 | ) | ||||||||||||||
| BALANCE AT DECEMBER 31, 2014 | 41,368,889 | $ | 4 | $ | 104,620 | $ | (107,411 | ) | $ | (2,787 | ) | |||||||||
* Represents an amount less than $1
The accompanying notes are an integral part of the consolidated financial statements.
| F-6 |
CONSOLIDATED STATEMENTS OF CASH FLOWS
(U.S. dollars in thousands)
| Year ended December 31, | 6 month period ended December 31, | Year ended June 30, | ||||||||||||||
| 2014 | 2013 | 2013 | 2012 | |||||||||||||
| CASH FLOWS FROM OPERATING ACTIVITIES: | ||||||||||||||||
| Net loss | $ | (25,095 | ) | $ | (9,336 | ) | $ | (29,258 | ) | $ | (17,597 | ) | ||||
| Adjustments required to reconcile net loss to net cash used in operating activities: | ||||||||||||||||
| Depreciation and amortization | 263 | 110 | 208 | 120 | ||||||||||||
| Change in liability for employees rights upon retirement | 77 | 69 | 246 | 72 | ||||||||||||
| Financial expenses (income) | 350 | 358 | 13,397 | (65 | ) | |||||||||||
| Share-based compensation expenses | 4,138 | 1,549 | 3,839 | 10,554 | ||||||||||||
| Gains on amounts funded in respect of employee rights upon retirement, net | (18 | ) | (15 | ) | (6 | ) | (1 | ) | ||||||||
| Royalties buyout expenses | 918 | |||||||||||||||
| Changes in operating asset and liability items: | ||||||||||||||||
| Decrease (increase) in prepaid expenses | 39 | 17 | (179 | ) | (22 | ) | ||||||||||
| Decrease (increase) in trade receivables | 1,220 | (116 | ) | 85 | (1,210 | ) | ||||||||||
| Decrease (increase) in other receivables | 28 | 1 | (124 | ) | (93 | ) | ||||||||||
| Decrease in inventory on consignment | - | - | 63 | 19 | ||||||||||||
| Decrease (increase) in inventory on hand | (331 | ) | 151 | (273 | ) | |||||||||||
| Increase (decrease) in trade payables | (659 | ) | 737 | 390 | (322 | ) | ||||||||||
| Increase (decrease) in deferred revenues | (10 | ) | 10 | |||||||||||||
| Increase (decrease) in other payable and advanced payment from customers | 626 | (163 | ) | (30 | ) | 228 | ||||||||||
| Net cash used in operating activities | (19,362 | ) | (6,799 | ) | (10,300 | ) | (8,580 | ) | ||||||||
| CASH FLOWS FROM INVESTING ACTIVITIES: | ||||||||||||||||
| Decrease (increase) in restricted cash | 93 | (56 | ) | 306 | ||||||||||||
| Purchase of property, plant and equipment | (133 | ) | (180 | ) | (202 | ) | (290 | ) | ||||||||
| Proceeds from sale of property, plant and equipment | - | - | - | 12 | ||||||||||||
| Amounts funded in respect of employee rights upon retirement, net | (46 | ) | (72 | ) | (118 | ) | (71 | ) | ||||||||
| Net cash used in investing activities | (86 | ) | (252 | ) | (376 | ) | (43 | ) | ||||||||
| CASH FLOWS FROM FINANCING ACTIVITIES: | ||||||||||||||||
| Proceeds from issuance of shares and warrants, net of $881 and $2,121 issuance costs for the years ended December 31, 2014 and June 30, 2013, respectively | 9,535 | 22,880 | ||||||||||||||
| Proceeds from issuance of convertible loan and warrants, net of $1,132 issuance costs | 9,868 | |||||||||||||||
| Exercise of options and warrants | - | 1,057 | 1,500 | |||||||||||||
| Repayment of long-term loan | (1,148 | ) | - | (281 | ) | |||||||||||
| Proceeds from long-term loan and warrants, net of $237 issuance costs | - | 9,763 | ||||||||||||||
| Acquisition and cancellation of shares | (21 | ) | ||||||||||||||
| Taxes withheld in respect of share issuance | (115 | ) | (27 | ) | ||||||||||||
| Induced conversion of convertible debt | (8,787 | ) | ||||||||||||||
| Net cash provided by financing activities | 8,272 | 9,763 | 15,123 | 11,066 | ||||||||||||
| EFFECT OF EXCHANGE RATE CHANGES ON CASH AND CASH EQUIVALENTS | (59 | ) | 3 | 89 | (229 | ) | ||||||||||
| INCREASE (DECREASE) IN CASH AND CASH EQUIVALENTS | (11,235 | ) | 2,715 | 4,536 | 2,214 | |||||||||||
| BALANCE OF CASH AND CASH EQUIVALENTS AT BEGINNING OF PERIOD | 17,535 | 14,820 | 10,284 | 8,070 | ||||||||||||
| BALANCE OF CASH AND CASH EQUIVALENTS AT END OF PERIOD | $ | 6,300 | $ | 17,535 | $ | 14,820 | $ | 10,284 | ||||||||
| SUPPLEMENTAL DISCLOSURES OF CASH FLOW INFORMATION: | ||||||||||||||||
| Taxes on income paid | $ | 14 | $ | 8 | $ | 78 | $ | 17 | ||||||||
| Interest paid | $ | 1,082 | $ | 111 | $ | 745 | $ | 225 | ||||||||
| SUPPLEMENTAL DISCLOSURES OF NON-CASH FINANCING ACTIVITIES: | ||||||||||||||||
| Classification of contingently redeemable warrants from long-term liability to equity | $ | 314 | ||||||||||||||
| Royalties buyout in consideration of shares and waiver | $ | 930 | ||||||||||||||
| Decrease in fund and liabilities in respect of employee rights upon retirement | $ | 59 | ||||||||||||||
| Deferred issuance costs | $ | 90 | ||||||||||||||
| Anti-dilution rights | $ | 110 | ||||||||||||||
In the year ended June 30, 2013, in connection with the Exchange and Amendment Agreement, the Company issued the debentures holders 2,159,574 shares of common stock and five year warrants to purchase 659,091 shares of common stock of the Company. See Note 6.
The accompanying notes are an integral part of the consolidated financial statements.
| F-7 |
NOTE 1 - DESCRIPTION OF BUSINESS
| a. | General |
InspireMD, Inc., a Delaware corporation (the “Company”), together with its subsidiaries, is a medical device company focusing on the development and commercialization of its proprietary MicroNet™ stent platform technology for the treatment of complex coronary and vascular disease. MicroNet, a micron mesh sleeve, is wrapped over a stent to provide embolic protection in stenting procedures. The Company’s coronary products combining MicroNet and a bare-metal stent (MGuard Prime™ EPS) are marketed for use in patients with acute coronary syndromes, notably acute myocardial infarction (heart attack) and saphenous vein graft coronary interventions (bypass surgery). In October 2014, the Company launched a limited market release of its carotid embolic prevention system (CGuard™ EPS), which combines MicroNet and a self-expandable nitinol stent in a single device to treat carotid artery disease. A coronary stent product incorporating a drug-eluting (drug-coated) stent with MicroNet is currently in development. The Company markets its products through distributors in international markets, mainly in Europe, Southeast Asia, India, Latin America and Israel, and through direct sales to hospitals in Europe.
| b. | Liquidity |
The Company has an accumulated deficit as of December 31, 2014, as well as net losses and negative
operating cash flows in recent years and the current year. The Company expects to continue incurring losses and negative cash flows
from operations until its MGuard™ and CGuard™ products reach commercial profitability. Management of the Company presently
anticipates that it has sufficient resources to fund operations through the second quarter of 2016.
Management’s plans
include (i) the continued commercialization of the MGuard®
and CGuard products, (ii) raising capital through the sale
of additional equity securities or debt and (iii) obtaining financing through strategic partnerships. There
are no assurances, however, that the Company will be successful in obtaining
the level of financing needed for its operations. If the Company is unsuccessful
in commercializing its MGuard or CGuard products and raising capital,
it may need to reduce activities, curtail or cease operations.
During the fourth quarter of 2014, the Company began implementing a focused spending plan. The plan included reducing the focus of clinical and development expenses related to the Company’s bare metal stent product and increasing the focus on the Company’s drug eluting stent product. Prior to the fourth quarter of 2014, a large portion of the Company’s organization was supporting the MASTER II trial, in which the Company determined not to resume enrollment, and instead allocated resources to drug eluting stents and the CGuard platform.
During the first quarter of 2015, the board of directors approved implementing another cost reduction/focused spending plan. The plan has four components: (i) reducing headcount; (ii) limiting the focus of clinical and development expenses to only the drug eluting stent product; (iii) limiting sales and marketing expenses to only those related to the CGuard EPS stent launch; and (iv) reducing across the board all other expenses (conferences, travel, promotional expenses, executive cash salaries, director cash fees, etc.). Prior to the cost reduction plan, a large portion of the Company’s organization was supporting clinical trials and promotional activities related to the Company’s bare metal stent platform. The Company decided to discontinue all work and promotion (such as conferences, clinical studies, and some sales activities) related to the bare metal platform. This decision allowed the Company to eliminate certain positions that related only to these activities. In addition, the Company dramatically cut all expenses not directly related to the CGuard launch and drug eluting platform development.
| F-8 |
In addition, to reduce the usage of cash, on January 5, 2015, the Company amended the employment agreements with the Company’s CEO and COO, to provide that, for a limited period of time to be mutually agreed upon by the Company and the CEO and COO, each of the CEO and the COO shall receive 50% of his base salary in cash payments, with the remaining 50% to be paid in an equivalent amount of shares of restricted common stock, payable and granted in equal installments in accordance with the Company’s normal payroll practices. On February 22, 2015, the COO’s employment agreement was further amended to provide that the payment arrangement described above would continue until the earlier of (i) September 30, 2015 and (ii) the Company raising an aggregate of $5 million from investors. The Company’s March 9, 2015 public offering raised in excess of $5 million and therefore the COO’s payment arrangement will, by the terms of this agreement, no longer be 50% paid in restricted stock. On January 5, 2015, the Company’s compensation committee amended its compensation policy for directors to provide that effective as of July 1, 2014, each director would forego any cash compensation in exchange for such number of immediately vested 10 year stock options having a Black-Scholes value equal to the cash compensation otherwise due to such director under the Company’s current director compensation policies.
| c. | Voluntary Field Corrective Action |
On April 30, 2014, the Company initiated a voluntary field corrective action (“VFA”) of its MGuard Prime EPS to address the issue of stent retention following reports of MGuard Prime stent dislodgements. On June 18, 2014, the Company received approval from the European regulatory agency to resume the manufacturing of the MGuard Prime stent with a modified stent securement process. The Company also received approval to modify and re-deploy existing MGuard Prime stents that were sent to the Company by clinical and commercial sites worldwide. These products have been modified and shipped to direct hospital customers and the majority of its distributor partners, who have begun shipping modified product back into hospital accounts. The Company began shipping products to new customers in the Company’s direct markets in Western Europe in October 2014. The VFA had an adverse impact on both the commercial and clinical activities relating to the MGuard Prime EPS from the date of initiation through December 31, 2014.
The expenses associated with the modifications that were performed as a result of the VFA are approximately $326,000 with an additional approximate $55,000 accrued for as of December 31, 2014 which is estimated to be incurred. These expenses were recorded in “Cost of revenues” in the year ended December 31, 2014.
In addition, as a result of the VFA, the Company suspended enrollment in the MASTER II trial, which had been previously launched to support its investigational device exemption application for MGuard Prime EPS with the U.S. Food and Drug Administration (“FDA”), pending a review by the FDA of the manufacturing improvements to the MGuard Prime EPS. The FDA approved the re-commencement of the MASTER II trial in October 2014.
Notwithstanding FDA approval to re-commence enrollment of the Master II trial, in light of current market conditions moving toward the use of drug-eluting stents (DES) over bare-metal stents, the Company elected not to resume enrollment in the MASTER II trial. As a result of this change, the MASTER II trial will no longer be an FDA registration trial.
| F-9 |
| d. | Fundraising |
On November 7, 2014, the Company sold 6,261,846 shares of its common stock and warrants to purchase 3,130,923 shares of common stock in a registered direct offering. See also Note 9a.
On March 9, 2015, the Company sold 34,369,675 shares of its common stock and warrants to purchase 34,369,675 shares of common stock in a registered direct offering. Each purchaser received a warrant to purchase one share of common stock for each share of common stock that it purchased in the offering. The warrants have a term of exercise of 5 years from the date of issuance and an exercise price of $0.55. This offering resulted in net proceeds to the Company of approximately $12.5 million after deducting placement agent fees and other estimated offering expenses.
NOTE 2 - SIGNIFICANT ACCOUNTING POLICIES
| a. | Use of estimates |
The preparation of financial statements in conformity with U.S. GAAP requires management to make estimates using assumptions that affect the reported amounts of assets and liabilities, the disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of sales and expenses during the reporting periods. Actual results could differ from those estimates.
As applicable to these consolidated financial statements, the most significant estimates and assumptions relate to inventory valuations, royalty buyout, legal contingencies and estimation of the fair value of warrants.
| b. | Functional currency |
The currency of the primary economic environment in which the operations of the Company and its subsidiaries are conducted is the U.S. dollar (“$” or “dollar”). Accordingly, the functional currency of the Company and its subsidiaries is the U.S. dollar.
The dollar figures are determined as follows: transactions and balances originally denominated in dollars are presented in their original amounts. Balances in foreign currencies are translated into dollars using historical and current exchange rates for non-monetary and monetary balances, respectively. The resulting translation gains or losses are recorded as financial income or expense, as appropriate. For transactions reflected in the statements of operations in foreign currencies, the exchange rates at transaction dates are used. Depreciation and changes in inventories and other changes deriving from non-monetary items are based on historical exchange rates.
| F-10 |
| c. | Principles of consolidation |
The consolidated financial statements include the accounts of the Company and of its subsidiaries. Intercompany transactions and balances have been eliminated upon consolidation.
| d. | Cash and cash equivalents |
The Company considers all highly liquid investments, which include short-term bank deposits (up to three months from date of deposit), that are not restricted as to withdrawal or use, to be cash equivalents.
| e. | Restricted cash |
Cash amounts restricted as to withdrawal or use, related to credit cards are included in restricted cash.
| f. | Concentration of credit risk and allowance for doubtful accounts |
Financial instruments that may potentially subject the Company to a concentration of credit risk consist of cash and cash equivalents, which are deposited in major financially sound institutions in the U.S, Israel, Germany and the United Kingdom, and trade accounts receivable. The Company’s trade accounts receivable are derived from revenues earned from customers from various countries. The Company performs ongoing credit evaluations of its customers’ financial condition and, generally, requires no collateral from its customers. The Company also has a credit insurance policy for some of its customers. The Company maintains an allowance for doubtful accounts receivable based upon the expected ability to collect the accounts receivable. The Company reviews its allowance for doubtful accounts quarterly by assessing individual accounts receivable and all other balances based on historical collection experience and an economic risk assessment. If the Company determines that a specific customer is unable to meet its financial obligations to the Company, the Company provides an allowance for credit losses to reduce the receivable to the amount management reasonably believes will be collected, which is netted against "Accounts receievable- Trade".
| g. | Inventory |
Inventories include finished goods, work in process, raw materials and inventory on consignment in hospitals. Inventories are stated at the lower of cost (cost is determined on a “first-in, first-out” basis) or market value. The Company’s inventories generally have a limited shelf life and are subject to impairment as they approach their expiration dates. The Company regularly evaluates the carrying value of the Company’s inventories and when, in the Company’s opinion, factors indicate that impairment has occurred, the Company impairs the inventories’ carrying value.
| h. | Property, plant and equipment |
Property, plant and equipment are stated at cost, net of accumulated depreciation and amortization. Depreciation is calculated using the straight-line method over the estimated useful lives of the related assets: over three years for computers and other electronic equipment, and seven to fifteen years for office furniture and equipment and machinery and equipment (mainly seven years). Leasehold improvements are amortized on a straight-line basis over the term of the lease, which is shorter than the estimated life of the improvements.
| F-11 |
| i. | Impairment in value of long-lived assets |
The Company tests long-lived intangible and tangible assets for impairment whenever events or circumstances present an indication of impairment. If the sum of expected future cash flows (undiscounted and without interest charges) of the long-lived assets is less than the carrying amount of such assets, an impairment would be recognized and the assets would be written down to their estimated fair values, based on expected future discounted cash flows.
To date, the Company has not recorded any impairment charges relating to its long-lived assets.
| j. | Revenue recognition |
Revenue is recognized when delivery has occurred, evidence of an arrangement exists, title and risks and rewards for the products are transferred to the customer, collection is reasonably assured and product returns can be reliably estimated. When a right of return exists the Company estimates a provision based on historical experience which is deducted from revenues.
The Company recognizes revenue net of value added tax (VAT).
| k. | Research and development costs |
Research and development costs are charged to the statement of operations as incurred.
| l. | Share-based compensation |
Employee option awards are classified as equity awards and accounted for using the grant-date fair value method. The fair value of share-based awards is estimated using the Black-Scholes valuation model and expensed over the requisite service period, net of estimated forfeitures. The Company estimates forfeitures based on historical experience and anticipated future conditions.
The Company elected to recognize compensation expenses for awards with only service conditions that have graded vesting schedules using the accelerated multiple option approach.
In addition, certain share-based awards of the Company are performance based and dependent upon achieving certain goals. With respect to these awards, the Company estimates the expected pre-vesting award probability that the performance conditions will be achieved. The Company only recognizes expense for those shares that are expected to vest.
| F-12 |
| m. | Uncertain tax positions |
The Company follows a two-step approach to recognizing and measuring uncertain tax positions. The first step is to evaluate the tax position for recognition by determining if the weight of available evidence indicates that it is more likely than not that the position will be sustained on audit. If under the first step a tax provision is assessed to be more likely than not of being sustained on audit, the second step is performed, under which the tax benefit is measured as the largest amount that is more than 50% likely to be realized upon ultimate settlement. Such liabilities are classified as long-term, unless the liability is expected to be resolved within twelve months from the balance sheet date. The Company’s policy is to include interest related to unrecognized tax benefits within “Financial expenses -net”.
| n. | Deferred income taxes |
Deferred taxes are determined utilizing the “asset and liability” method based on the estimated future tax effects of differences between the financial accounting and tax bases of assets and liabilities under the applicable tax laws, and on tax rates anticipated to be in effect when the deferred taxes are expected to be paid or realized. The Company assesses realization of deferred income tax assets and, based on all available evidence, concludes whether it is more likely than not that the net deferred income tax assets will be realized. A valuation allowance is provided for the amount of deferred income tax assets not considered to be realizable.
The Company may incur additional tax liability in the event of intercompany dividend distributions by its subsidiaries. Such additional tax liability in respect of these foreign subsidiaries has not been provided for in these financial statements as it is the Company’s policy to permanently reinvest the subsidiaries’ earnings and to consider distributing dividends only in connection with a specific tax opportunity that may arise.
Taxes that would apply in the event of disposal of investments in a foreign subsidiary have not been taken into account in computing the deferred taxes, as it is the Company’s intention to hold, and not to realize, these investments.
| o. | Advertising |
Costs related to advertising and promotion of products are charged to sales and marketing expense as incurred. Advertising expenses were approximately $0.8 million for the year ended December 31, 2014, $0.8 million for the six month period ended December 31, 2013 and $1.1 million and $0.6 million for the years ended June 30, 2013 and 2012, respectively.
| p. | Net loss per share |
Basic and diluted net loss per share is computed by dividing the net loss for the year by the weighted average number of shares of common stock outstanding during the year. The calculation of diluted net loss per share excludes potential share issuances of common stock upon the exercise of share options, warrants and convertible loans, as the effect is anti-dilutive.
For the year ended December 31, 2014, the six month period ended December 31, 2013, and the years ended June 30, 2013 and 2012, all shares of common stock underlying outstanding options, warrants, convertible loans and restricted stock have been excluded from the calculation of the diluted loss per share since their effect was anti-dilutive. The total number of shares of common stock related to outstanding options and warrants and restricted stock excluded from the calculations of diluted loss per share were 13,523,260 for the year ended December 31, 2014, 8,431,252 for the six month period ended December 31, 2013, and 8,006,837 and 8,117,577 for the years ended June 30, 2013 and 2012, respectively.
| F-13 |
| q. | Segment reporting |
The Company has one operating and reportable segment.
| r. | Fair value measurement: |
The Company measures fair value and discloses fair value measurements for financial assets and liabilities. Fair value is based on the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date.
The accounting standard establishes a fair value hierarchy that prioritizes observable and unobservable inputs used to measure fair value into three broad levels, which are described below:
Level 1: Quoted prices (unadjusted) in active markets that are accessible at the measurement date for assets or liabilities. The fair value hierarchy gives the highest priority to Level 1 inputs.
Level 2: Observable prices that are based on inputs not quoted on active markets, but corroborated by market data.
Level 3: Unobservable inputs are used when little or no market data is available. The fair value hierarchy gives the lowest priority to Level 3 inputs.
In determining fair value, the Company utilizes valuation techniques that maximize the use of observable inputs and minimize the use of unobservable inputs to the extent possible and considers counterparty credit risk in its assessment of fair value.
| s. | Put warrants |
Put warrants that embody an obligation to repurchase the Company’s equity shares, or are indexed to such an obligation, and that require or may require the Company to settle the obligation by transferring assets are within the scope of Accounting Standards Codification (“ASC”) 480-10-25-8, and are recognized as a liability and measured at fair value at each reporting date, with changes in fair value recorded in earnings. See Note 6.
| t. | Beneficial conversion feature (“BCF”) |
When the Company issues convertible debt, if the stock price is greater than the effective conversion price (after allocation of the total proceeds) on the measurement date, the conversion feature is considered "beneficial" to the holder. If there is no contingency, this difference is treated as issued equity and reduces the carrying value of the host debt; the discount is accreted as deemed interest on the debt. See Note 6.
| F-14 |
| u. | Embedded derivatives |
Embedded derivatives in debt contracts that are not clearly and closely related to the host debt are bifurcated and accounted for separately. Those embedded derivatives are measured at fair value each reporting date, with changes in fair value recorded in earnings. See Note 6.
| v. | Allocation of issuance proceeds |
When debt or equity is issued with other components that are subsequently measured at fair value, the proceeds are allocated first to such components (such as warrants and embedded derivatives in the debt that require bifurcation at their fair values) then the residual amount of the proceeds to the debt or equity. When the other components are classified in equity, the proceeds are allocated based on relative fair values. See Note 6.
w. Recently issued accounting pronouncements
| 1. | In May 2014, the Financial Accounting Standards Board (the “FASB”) issued ASC 606, Revenue from contracts with customers. |
The objective of the new revenue standard is to provide a single, comprehensive revenue recognition model for all contracts with customers to improve comparability within industries, across industries, and across capital markets. The revenue standard contains principles that an entity will apply to determine the measurement of revenue and timing of when it is recognized. The underlying principle is that an entity will recognize revenue to depict the transfer of goods or services to customers at an amount that the entity expects to be entitled to in exchange for those goods or services, based on a five step model that includes the identification of the contract with the customer and the performance obligations in the contract, determination of the transaction price, allocation of the transaction price to the performance obligations in the contract and recognizing revenue when (or as) the entity satisfies a performance obligation. The revenue standard is effective for annual periods beginning on or after December 15, 2016. The Company is currently evaluating the impact, if any, the adoption of this guidance will have on its consolidated financial statements.
| 2. | In August 2014, the FASB issued Accounting Standards Update (“ASU”) 2014-15, Presentation of Financial Statements-Going Concern (Subtopic 205-40): Disclosure of Uncertainties about an Entity’s Ability to Continue as a Going Concern. Continuation of a reporting entity as a going concern is presumed as the basis for preparing financial statements unless and until the entity’s liquidation becomes imminent. Preparation of financial statements under this presumption is commonly referred to as the going concern basis of accounting. Currently, there is no guidance under U.S. GAAP about management’s responsibility to evaluate whether there is substantial doubt about an entity’s ability to continue as a going concern or to provide related footnote disclosures. The amendments in ASU 2014-15 provide that guidance. In doing so, the amendments should reduce diversity in the timing and content of footnote disclosures. This new standard requires management to assess the entity’s ability to continue as a going concern by incorporating and expanding upon certain principles that are currently in U.S. auditing standards. Specifically, the amendments (1) provide a definition of the term substantial doubt, (2) require an evaluation every reporting period including interim periods, (3) provide principles for considering the mitigating effect of management’s plans, (4) require certain disclosures when substantial doubt is alleviated as a result of consideration of management’s plans, (5) require an express statement and other disclosures when substantial doubt is not alleviated, and (6) require an assessment for a period of one year after the date that the financial statements are issued (or available to be issued). ASU 2014-15 will be effective prospectively for annual reporting periods ending after the first annual period ending after December 15, 2016 and interim periods therein. Early application of the standard is permitted for any annual reporting period or interim period for which the entity’s financial statements have not yet been issued. |
| F-15 |
NOTE 3 - FAIR VALUE MEASURMENT
Items Measured at Fair Value on a Recurring Basis
The following tables summarize the activity for those financial liabilities where fair value measurements are estimated utilizing Level 3 inputs:
| Anti-Dilution Rights | Embedded Derivative | |||||||
| ($ in thousands) | ||||||||
| Balance as of July 1, 2011 | $ | - | $ | - | ||||
| Issuances | 8 | |||||||
| Total losses (gains) (realized and unrealized) - included in earnings - Financial expenses (income), net | 41 | |||||||
| Balance as of June 30, 2012 | $ | - | $ | 49 | ||||
| Total losses (gains) (realized and unrealized) - included in earnings - Financial expenses (income), net | 1,475 | (19 | ) | |||||
| Settlement by issuance shares | 1,475 | ) | - | |||||
| Conversion of convertible debt | (30 | ) | ||||||
| Balance as of June 30, 2013 | ||||||||
| Total losses (gains) (realized and unrealized) - included in earnings - Financial expenses (income), net | 200 | |||||||
| Settlement by issuance shares | (44 | ) | ||||||
| Balance as of December 31, 2013 | $ | 156 | $ | - | ||||
| Total losses (gains) (realized and unrealized) - included in earnings - Financial expenses (income), net | (46 | ) | ||||||
| Settlement by issuance shares | (110 | ) | ||||||
| Balance as of December 31, 2014 | $ | - | $ | - | ||||
The Company values Level 3 Anti- Dilution Rights using an internally developed valuation model, whose inputs include potential equity transactions (such as fund raising and share based awards), probability of completing successful fund raising during the relevant period and stock prices.
Level 3 liabilities also include an embedded derivative related to the Company’s 2012 Convertible Debentures (as defined in Note 6a). The Company values the Level 3 embedded derivative using an internally developed valuation model, whose inputs include recovery rates, credit spreads, stock prices, and volatilities, as described below.
| F-16 |
The fair value of the warrants classified as Level 2 is estimated using the Black & Scholes model.
For a discussion regarding the calculation of the fair value of the 2012 Warrants as of the transaction date, as of June 30, 2012 and as of the Closing Day (as defined in Note 6), see Note 6.
As of the Closing Day, the Company recalculated the fair value of the embedded derivative of the 2012 Warrants using the following assumptions: the Company's credit spread of 28.5%, the Company's recovery rate of 49.8%, and a 10% probability of non-financial event of default.
The carrying amounts of financial instruments included in working capital approximate their fair value either because these amounts are presented at fair value or due to the relatively short-term maturities of such instruments. The fair value of the Loan (as defined in Note 6) approximated its carrying amount since it bears interest at rates that approximate current market rates.
NOTE 4 - PROPERTY, PLANT AND EQUIPMENT
| a. | Composition of assets, grouped by major classifications, is as follows: |
| December 31, | ||||||||
| 2014 | 2013 | |||||||
| ($ in thousands) | ||||||||
| Cost: | ||||||||
| Computer equipment | $ | 242 | $ | 199 | ||||
| Office furniture and equipment | 110 | 90 | ||||||
| Machinery and equipment | 951 | 891 | ||||||
| Leasehold improvements | 194 | 184 | ||||||
| 1,497 | 1,364 | |||||||
| Less - accumulated depreciation and amortization | (875 | ) | (712 | ) | ||||
| Net carrying amount | $ | 622 | $ | 652 | ||||
| b. | Depreciation and amortization expenses totaled approximately $163,000 for the year ended December 31, 2014, $78,000 for the six month period ended December 31, 2013 and approximately $162,000 and $120,000 for the years ended June 30, 2013 and 2012, respectively. |
NOTE 5 - LIABILITY FOR EMPLOYEES RIGHT UPON RETIREMENT
Israeli labor law generally requires payment of severance pay upon dismissal of an employee or upon termination of employment in certain other circumstances.
Pursuant to section 14 of the Israeli Severance Compensation Act, 1963, some of the Company’s employees are entitled to have monthly deposits, at a rate of 8.33% of their monthly salary, made in their name with insurance companies. Payments in accordance with section 14 relieve the Company from any future severance payments to these employees.
| F-17 |
The severance pay liability of the Company for the rest of its Israeli employees, which reflects the undiscounted amount of the liability, is based upon the number of years of service and the latest monthly salary. The severance pay liability is partly covered by insurance policies and by regular deposits with recognized severance payment funds. The Company may only withdraw funds previously deposited for savings in connection with the payment of severance. The severance pay expenses were approximately $233,000 for the year ended December 31, 2014, $139,000 for the six month period ended December 31, 2013 and $311,000 and $177,000 for the years ended June 30, 2013 and 2012, respectively.
Defined contribution plan expenses were approximately $285,000 for the year ended December 31, 2014, $168,000 for the six month period ended December 31, 2013 and $208,000 and $206,000 for the years ended June 30, 2013 and 2012, respectively.
The Company expects contribution plan expenses in 2015 to be approximately $206,000.
NOTE 6 – FINANCING TRANSACTIONS
| I. | 2012 convertible loan and warrants |
On April 5, 2012, the Company issued senior secured convertible debentures (the “2012 Convertible Debentures”) due April 5, 2014 in the original aggregate principal amount of $11,702,128 and five-year warrants (the “2012 Warrants”) to purchase an aggregate of 835,866 shares of its common stock at an exercise price of $7.20 per share in a private placement transaction in exchange for aggregate gross proceeds of approximately $11 million.
The 2012 Convertible Debentures bore interest at an annual rate of 8% (payable quarterly beginning on July 1, 2012) and were convertible at any time into shares of common stock at an initial conversion price of $7.00 per share, subject to a conversion adjustment mechanism based on a formula stipulated in the agreement.
The terms of the 2012 Convertible Debentures contained, certain conversion and contingent conversion features, as well as a contingent redemption option (by the holder) and a non-contingent redemption option (by the holder and by the Company). For accounting purposes, A BCF charge to equity was recorded at the date of issuance in the amount of $3,790,000, and an embedded derivative related to the holder's contingent redemption option was bifurcated from the debt host and marked to market against the income statement until the date of the April 2013 transaction discussed below.
The terms of the 2012 Warrants contained, a contingent redemption option by the holder upon certain change in control events, as defined in the agreement, for a cash consideration based on the Black-Scholes value of the redeemed warrants. For accounting purposes, the 2012 Warrants were treated as a liability in light of the cash redemption option upon an event deemed outside the Company's control, and were marked to market against the income statement until the date of their amendment, as discussed below.
On April 16, 2013 (“the Closing Day”), simultaneously with the closing of the 2013 Offering (as defined in Note 9a), the Company entered into an exchange and amendment agreement with the holders of the Company’s 2012 Convertible Debentures due April 5, 2014 (the “Exchange Agreement”). In accordance with the Exchange Agreement , the Company:
| F-18 |
| · | repaid $8,787,234 in cash; |
| · | issued 2,159,574 shares of common stock to the holders of the 2012 Convertible Debentures, reflecting a conversion price of $2.00 per share for the remaining unpaid portion of the 2012 Convertible Debentures; and |
| · | issued five year warrants to the holders of the 2012 Convertible Debentures to purchase an aggregate of 659,091 shares of common stock for $3.00 per share (“$3.00 Warrants”); |
In accordance with the provisions of ASC 470-20, the terms of the Exchange Agreement were considered to be an induced conversion and the retirement of the 2012 Convertible Debentures was accounted for as if the 2012 Convertible Debentures had been converted according to their original conversion price of $7 and valued at $3,538,723.
As a result of the Exchange Agreement the Company amortized the deferred debt issuance costs in the amount of $641,000 and incurred approximately $9.9 million of expenses which were recorded in "Financial expenses, net" within the consolidated statements of operations.
In calculating the fair value of the $3.00 Warrants, the Company used the following assumptions: dividend yield of 0% and expected term of 5 years; expected volatility of 68%; and risk-free interest rate of 0.71%. The fair value of the Warrants, using the Black-Scholes option-pricing model was approximately $568,000.
In addition, pursuant to the Exchange Agreement, the Company:
| · | amended the securities purchase agreement pursuant to which the 2012 Convertible Debentures were originally issued to prohibit the Company from issuing securities containing anti-dilution protective provisions; and |
| · | amended the 2012 Warrants to (i) eliminate the Most Favored Nation Adjustment clause which stated that in the event that the Company issued or was deemed to have issued certain securities with terms that were superior to those of the 2012 Warrants, except with respect to exercise price and warrant coverage, the superior terms would have automatically been incorporated into the 2012 Warrants and (ii) provide that upon a fundamental transaction as defined in the agreement, the holders of such warrants will now have the right to cause the Company to repurchase the unexercised portion of such warrants at their Black-Scholes value on the date of such fundamental transaction, payable in shares of common stock, rather than in cash as was previously provided. |
The Company determined, based on the provisions of ASC 480-10-25-8, that following the amendment to the 2012 Warrants described above, equity classification is no longer precluded and accordingly, the 2012 Warrants valued at approximately $314,000 as of the Closing Day of the Exchange Agreement were classified from a liability to equity in the consolidated balance sheets.
| F-19 |
| II. | 2013 Security and Loan Agreement |
| a. | Loan and Security Agreement |
On October 23, 2013, the Company and InspireMD Ltd. entered into a Loan and Security Agreement (the “Loan and Security Agreement”), pursuant to which a lender made a term loan to the Company and InspireMD Ltd. in the aggregate amount of $10 million (the “Loan”). The annual interest rate on the Loan is prime plus 4%, but shall not be reduced below 10.5%. Payments under the Loan and Security Agreement are for the interest portion only for 9 months, followed by 30 monthly payments of principal and interest through the scheduled maturity date on February 1, 2017.
The Company is permitted to prepay all or a portion of the Loan. However, any prepayments of the Loan will be subject to a penalty of (i) 2%, if the prepayment occurs within 12 months of the Loan being requested by the Company and InspireMD Ltd. (the “Advance Date”), (ii) 1%, if the prepayment occurs between 12 and 24 months after the Advance Date, and (iii) 0.5%, if the prepayment occurs more than 24 months after the Advance Date. The Company and InspireMD Ltd. will also pay the lender an aggregate end of term charge (the “End of Term Charge”) of $500,000 when the Loan is paid in full or matures. In addition, upon the occurrence of a change in control, the Company shall prepay the outstanding amount of all principal and accrued interest through the prepayment date and all unpaid lender’s fees and expenses accrued to the date of the repayment (including the End of Term Charge) together with the penalties stated above.
| b. | Warrant Agreement |
On October 23, 2013, in connection with the Loan and Security Agreement, the Company issued the lender a warrant to purchase 168,351 shares of common stock at a per share exercise price of $2.97 (the “ 2013 Warrant”). The Warrant is immediately exercisable and has a five year term. The 2013 Warrant may also be exercised on a cashless basis. The exercise price of the 2013 Warrant and the number of shares issuable upon exercise of the Warrant are subject to adjustments for stock splits, combinations or similar events.
Upon the occurrence of a transaction involving a change of control of the Company in which the consideration is either all cash or securities that are either registered for sale on an exchange or quotation system or otherwise unrestricted, the Warrant, to the extent not previously exercised, may be exchanged, at the holder’s request, for the consideration, to be paid by the acquirer, that the holder would have received, less the exercise price, had the holder exercised the Warrant immediately prior to the change of control. For all other changes of control of the Company, the 2013 Warrant will be assumed by the successor or surviving entity with similar rights to the 2013 Warrant as if it had been exercised immediately prior to the change of control.
| F-20 |
| c. | Security Documents |
On October 23, 2013, InspireMD Ltd. issued the lender a Fixed Charge Debenture and a Floating Charge Debenture (collectively, the “Israeli Security Agreements”) in order to create a security interest in all the assets and property of InspireMD Ltd., securing the Company’s and InspireMD Ltd.’s obligations under the Loan and Security Agreement. In addition, on October 23, 2013 and November 8, 2013, the Company entered into Deposit Account Control Agreements with the lender and two banking instituions in the US (the “Deposit Account Control Agreements”) in order to perfect the lender’s security interest in the Company’s bank account. Pursuant to the Loan and Security Agreement, the Israeli Security Agreements and the Deposit Account Control Agreement, the Company’s obligations to the lender are secured by a first priority perfected security interest in all of the assets and properties of the Company and InspireMD Ltd., other than the intellectual property of the Company and InspireMD Ltd.
The Company is required under the Loan and Security Agreement to maintain at all times in the bank accounts under the Deposit Account Control Agreements, cash and cash equivalents which may include cash collected from Accounts Receivable by Inspire M.D Ltd. and InspireM.D GmbH within the previous 7 days, and cash transferred to Inspire M.D Ltd. for the settlement of Permitted Indebtedness within the following 7 days, in each unrestricted and unencumbered, in an aggregate amount of at least the lesser of (a) an amount equal to one hundred percent of the then outstanding principal amount of the Term Loan Advance and (b) an amount equal to seventy-five percent of the aggregate amount of all of Borrower’s worldwide cash and cash equivalents.
| d. | Accounting treatment |
The Company evaluated whether the 2013 Warrants can be classified as equity and determined that equity classification is appropriate. Accordingly, proceeds from the Loan and 2013 Warrant were allocated to the two elements based on the relative fair value. The portion of the proceeds so allocated to the 2013 Warrant, of approximately $280,000 was recorded in additional paid-in capital. The portion of the proceeds so allocated to the Loan, of approximately $9.7 million (before deduction of approximately $237,000 of issuance costs) was recorded in long term liabilities.
The Loan was subsequently measured at amortized cost on the basis of the effective interest method over the loan period until the maturity date.
Direct transaction costs of approximately $237,000, were allocated to the Loan and the Warrants pro-rata to the amount such instruments were recorded as of the transaction date. The amounts that were allocated to the Warrants were deducted from the Warrants and the amount related to the Loan was recorded as "Deferred issuance costs" in the consolidated balance sheets and is amortized over the loan period until the maturity date.
The Company has evaulted and concluded that the Prepayment options and the prepayment under an event of Change in control are clearly and closely related to the debt host contract and thus should not be bifurcated from the debt host.
In addition the Company evaluated whether the default interest mechanism, as defined in the Loan and Security Agreement, results in an interest rate derivative that should be bifurcated and determined that based on the provisions of ASC 815-15-25-1, an interest rate derivative should not be bifurcated.
| F-21 |
As of December 31, 2014, the future principal payments obligation for the Loan were as follows:
| ($ in thousands) | ||||
| Year Ended December 31: | ||||
| 2015 | 3,809 | |||
| 2016 | 4,234 | |||
| 2017 | 776 | |||
| $ | 8,819 | |||
NOTE 7 - RELATED PARTIES TRANSACTIONS
| a. | Appointment of CEO |
In connection with the appointment of the CEO on January 3, 2013, the Company entered into an Employment Agreement (the “Employment Agreement”) with the CEO. Under the Employment Agreement, the CEO is entitled to an annual base salary of at least $450,000. The CEO is also eligible to receive an annual bonus of at least $275,000 upon the achievement of reasonable target objectives and performance goals, to be determined by the board of directors. The annual bonus amount will be less than $275,000 if the target objectives and performance goals are not met.
During the year ended June 30, 2013, the Company granted the CEO stock options to purchase 897,447 shares of the Company's common stock with an exercise price of $2.05-$4.05 per share, and 579,866 shares of restricted stock, which are subject to forfeiture until vested and vests in three equal annual installments. The fair value of the above options and restricted shares, using the Black-Scholes option-pricing model, was approximately $1,837,000 and $1,989,000, respectively.
During the year ended December 31, 2014, the Company granted the CEO stock options to purchase 399,675 shares of the Company's common stock with an exercise price of $2.97-$3.1 per share, and 182,725 shares of restricted stock, which are subject to forfeiture until vested and vests in three equal annual installments. The fair value of the above options and restricted shares, using the Black-Scholes option-pricing model, was approximately $736,000 and $554,000, respectively.
The CEO has an option to deliver a number of shares with an aggregate fair market value that equals or exceeds (to avoid the issuance of fractional shares) the required tax withholding payment resulted from the vesting of the restricted stock or from the exercise of the options. As of December 31, 2014 and 2013, 45,684 and 9,506 shares were withheld by the Company to satisfy tax withholding obligations. The payment, amounting to $115,000 and $28,000, was deducted from equity.
On or before December 31 of each calendar year, the CEO will be eligible to receive an additional grant of equity awards equal, in the aggregate, to up to 0.5% of the Company’s actual outstanding shares of common stock on the date of grant, provided that the actual amount of the grant will be based on his achievement of certain performance objectives as established by the board, in its reasonable discretion, for each such calendar year. In connection with the equity compensation related to 2013 acheivements, on January 29, 2014 the CEO was granted stock options to purchase 86,325 shares of the Company's common stock and 86,325 restricted shares, (included in total options and restricted shares granted to the CEO during 2014). In connection with the equity compensation related to 2014 acheivements, on January 26, 2015 the CEO was granted stock options to purchase 52,999 shares of the Company's common stock and 52,999 restricted shares.
| F-22 |
If, during the term of the Employment Agreement, the CEO's employment is terminated upon certain conditions as stipulated in the agreement, the CEO will be entitled to receive certain benefits as stipulated in the agreement.
On January 29, 2014, the Company’s board of directors approved an annual bonus for 2013 to the CEO in the amount of $275,000.
On January 28, 2015, the Company’s board of directors approved an annual bonus for 2014 to the CEO in the amount of $69,105.
| b. | During the year ended December 31, 2014, the six month period ended December 31, 2013, the year ended June 30, 2013 and the year ended June 30, 2012 the Company granted stock options to directors to purchase a total of 335,000, 125,000, 499,415 and 937,500 shares of the Company’s common stock, respectively. The options have exercise prices of $3.10, $2.12, $2.75-$9 and $3.16-$10 per share, respectively, which were the fair market value of the Company’s common stock on the date of each respective grant. The options are subject to a three-year vesting period with one-third of such awards vesting each year. The fair value of the above options and restricted shares, using the Black-Scholes option-pricing model, was approximately $636,000, $164,000, $982,399 and $4,072,026, respectively. |
| c. | Balances with related parties: |
| December 31, | ||||||||
| 2014 | 2013 | |||||||
| ($ in thousands) | ||||||||
| Current liabilities: | ||||||||
| Other accounts payable | $ | 241 | $ | 355 | ||||
| d. | Transactions with related parties: |
| Year ended December 31, | 6 month period ended December 31, | Year ended June 30, | ||||||||||||||
| 2014 | 2013 | 2013 | 2012 | |||||||||||||
| ($ in thousands) | ||||||||||||||||
| Expenses: | ||||||||||||||||
| Share-based compensation | $ | 2,502 | $ | 1,293 | $ | 2,973 | $ | 9,517 | ||||||||
| Salaries and related expenses | 578 | 557 | 531 | 305 | ||||||||||||
| Consulting fees | 189 | 82 | 400 | 393 | ||||||||||||
| Rent income | (21 | ) | ||||||||||||||
| F-23 |
NOTE 8 - COMMITMENTS AND CONTINGENT LIABILITIES
| a. | Lease commitments: | |
| 1) | On December 13, 2011, the Company entered into a lease agreement for a facility in Israel, which expired in December 2014. The Company had the option, under the agreement, to extend the agreement for two additional two year periods, for a total of four years. The Company extended the agreement for two additional years. |
In December 2013, the Company entered into a lease agreement for its facilities in the U.S which expires in February 2018. The Company has the right to terminate the lease agreement at the end of the third lease year upon 9 months prior written notice, as stipulated in the agreement.
In August 2014, the Company entered into an amendment (the “First Lease Amendment”) to the lease agreement for its facilities in the U.S. Pursuant to the First Lease Amendment, amongst other things, the Company agreed to lease additional space and extend the expiration of the agreement to February 2019.
Rent expense included in the consolidated statements of operations totaled approximately $388,000 for the year ended December 31, 2014, $180,000 for the six month period ended December 31, 2013 and approximately $383,000 and $220,000 for the years ended June 30, 2013 and 2012, respectively.
As of December 31, 2014, the aggregate future minimum lease obligations for office rent under non-cancelable operating lease agreements were as follows:
| ($ in thousands) | ||||
| Year Ended December 31: | ||||
| 2015 | $ | 382 | ||
| 2016 | 389 | |||
| 2017 | 106 | |||
| 2018 | 104 | |||
| 2019 | 106 | |||
| 2020 | 9 | |||
| $ | 1,096 | |||
| 2) | The Company leases its motor vehicles under operating lease agreements. As of December 31, 2014, the aggregate non-cancelable future minimum lease obligations for motor vehicles were approximately $14,000. |
| b. | License Agreement: |
In March 2010, the Company entered into a license agreement to use a stent design (“MGuard PrimeTM”). Pursuant to the agreement, the licensor was entitled to receive royalty payments of 7% of net sales outside the United States and, for sales within the United States, royalty payments as follows: 7% of net sales for the first $10,000,000 of net sales and 10% of net sales for net sales exceeding $10,000,000.
| F-24 |
On October 20, 2012, the Company, InspireMD Ltd. and the licensor entered into an amendment (the "First Amendment") to the license agreement described above. Pursuant to the First Amendment, amongst other things, the licensor agreed to reduce the royalty owed with respect to sales of MGuard Prime to 2.9% of all net sales both inside and outside the U.S. in exchange for (i) InspireMD Ltd. waiving $85,000 in regulatory fees for the CE Mark that were owed by the licensor to InspireMD Ltd., (ii) InspireMD Ltd. making full payment of royalties in the amount of $205,587 due to the licensor as of September 30, 2012 and (iii) 215,000 shares of the Company’s common stock, that were valued at the closing price of the common stock on October 19, 2012 at $8.20 per share. The total amount paid to the licensor was valued at $1,848,000, inclusive of the shares issued as well as the $85,000 waiver, and was allocated as follows: approximately $930,000 was allocated to royalties’ buyout and approximatly $918,000 was allocated to “research and development” expenses based on the MGuard Prime registration status in the various territories. The royalties’ buyout amortization is calculated using the economic pattern of the Company’s estimated future revenues over the estimated useful life of the royalties’ buyout. The amortization is recorded in “Cost of Revenues” in the consolidated statements of operations.
On August 22, 2013, the Company, InspireMD Ltd. and the Licensor entered into an amendment to the License Agreement (the “Second Amendment”), pursuant to which the Company and the Licensor agreed to amend the royalty fee from 2.9% of all net sales during the term of the agreement to (i) 2% of the first $10.56 million of net sales from July 1, 2013 through June 30, 2015, provided that the Company makes an advance royalty payment of $192,000 on the date of the amendment, (ii) 2.5% of net sales in excess of $10.56 million from July 1, 2013 through June 30, 2015, payable within 45 days of June 30, 2015, and (iii) 2.9% of all net sales beginning on July 1, 2015. The above referenced advance royalty payment has been included in long term prepaid expenses for the six month period ended December 31, 2013.
Royalties accrued for these sales are included in “Accounts payable and accruals –Other” as of June 30, 2013 and 2012, respectively. Royalties expenses for the year ended December 31, 2014 amounted to approximately $148,000. Royalties expenses for the six month period ended December 31, 2013 amounted to approximately $38,000, and for the years ended June 30, 2013 and 2012 amounted to approximately $132,000 and $201,000, respectively.
| c. | Liens and pledges |
| 1) | The Company’s obligations under the Loan and Security Agreement (as defined in Note 6) were secured by the Israeli Security Agreements (as defined in Note 6) and the Deposit Account Control Agreements (as defined in Note 6) on all of the assets and properties of the Company and InspireMD Ltd., other than the intellectual property of the Company and InspireMD Ltd. |
| 2) | As of December 31, 2013, the Company had fixed liens amounting to $93,000 to Bank Mizrahi in connection with the Company’s credit cards. The Company removed the fixed liens as of December 31, 2014. |
| F-25 |
| d. | Litigation: |
In July 2012, a purported assignee of options in InspireMD Ltd. submitted a statement of claim against the Company, InspireMD Ltd., and the Company’s former CEO and President for a declaratory and enforcement order that it is entitled to options to purchase 83,637 shares of the Company’s common stock at an exercise price of $0.76 per share. In December 2014 the court accepted a motion to dismiss the former CEO and president from the lawsuit. After considering the views of its legal counsel as well as other factors, the Company’s management believes that a loss to the Company is neither probable nor in an amount or range of loss that is estimable.
In December 2012, a former service provider of InspireMD GmbH filed a claim with the Labor Court in Buenos Aires, Argentina in the amount of $193,378 plus interest (6% in dollars or 18.5% in pesos), social benefits, legal expenses and fees (25% of the award) against InspireMD Ltd. and InspireMD GmbH. The Company's management, after considering the views of its legal counsel as well as other factors, recorded a provision of $250,000 in the financial statements for the quarter ended December 31, 2012. The related expense has been recorded to "General and administrative" within the consolidated statements of operations. The Company's management estimates that the ultimate resolution of this matter could reasonably result in a loss of up to $80,000 in excess of the amount accrued.
NOTE 9 – EQUITY
| a. | Share capital |
As of December 31, 2014, the Company has authorized 130,000,000 shares of capital stock, par value $0.0001 per share, of which 125,000,000 are shares of common stock and 5,000,000 are shares of “blank check” preferred stock.
On December 19, 2012, the Company filed with the Secretary of State of the State of Delaware a Certificate of Amendment of the Company’s Amended and Restated Certificate of Incorporation to effect a one-for-four reverse stock split of its common stock (the “Reverse Stock Split”).
On January 8, 2013, due to the failure of the Company’s common stock to be listed on a national securities exchange on or before December 31, 2012, the Company issued 178,029 shares of common stock to the purchasers, or their assignees, under the securities purchase agreement that the Company entered into on March 31, 2011 (the "2011 SPA").
On April 16, 2013, the Company consummated an underwritten public offering, pursuant to which it sold a total of 12,500,000 shares of common stock (the “2013 Offering”). The price to the public in the Offering was $2.00 per share, and the aggregate net proceeds of the 2013 Offering to the Company were approximately $22.6 million, after the underwriters’ commissions and offering expenses. On April 11, 2013, following the pricing of the Offering, the Company’s common stock began trading on the NYSE MKT.
Pursuant to the terms of 2011 SPA that provided these investors with certain anti-dilution protections until March 31, 2014. The Company issued the purchasers or their assigns an aggregate of 792,884 and 49,426 shares of common stock during 2013 and 2014, respectively. The related expense has been recorded to “Financial expenses (income), net” within the consolidated statements of operations in the relevant periods.
| F-26 |
On August 15, 2014, the Company sold 948,000 shares of its common stock pursuant to its at-the-market (ATM) issuance sales agreement with MLV & Co. LLC. These sales resulted in net proceeds to the Company of approximately $2.2 million.
On November 7, 2014, the Company sold 6,261,846 shares of its common stock and warrants to purchase 3,130,923 shares of common stock in a registered direct offering (the “2014 Offering”). The common stock was sold at a negotiated purchase price of $1.30 per share, and each purchaser received a warrant to purchase 0.5 of a share of common stock for each share of common stock that it purchased in the offering. The warrants, which are classified in equity, are non-exercisable for six months and have a term of exercise of 42 months from the date of issuance and an exercise price of $1.75. This offering resulted in net proceeds to the Company of approximately $7.4 million after deducting placement agent fees and other estimated offering expenses.
| b. | Share-Based Compensation |
| 1. | On March 28, 2011, the board of directors and stockholders of the Company adopted and approved the InspireMD, Inc. 2011 UMBRELLA Option Plan (the “Umbrella Plan”) which expires on March 27, 2021. Under the Umbrella Plan, as subsequently amended, the Company reserved 5,000,000 shares of common stock as awards to employees, consultants, and service providers. As of December 31, 2014, the Company had reserved 256,049 shares of common stock for issuance under the plans as described above. |
| 2. | On December 16, 2013, the board of directors and stockholders of the Company adopted and approved the InspireMD, Inc. 2013 Long-Term Incentive Plan (the “2013 Plan”). Under the 2013 Plan, the Company reserved 5,000,000 shares of common stock for awards to employees, officers, directors, consultants, and service providers. As of December 31, 2014, the Company had reserved 2,784,072 shares of common stock for issuance under the plans as described above. |
The 2013 Plan provides for the granting of incentive stock options, nonqualified stock options, stock appreciation rights, restricted stock, restricted stock units, performance awards, dividend equivalent rights, and other awards, which may be granted singly, in combination, or in tandem. The 2013 Plan is administered by the Company’s compensation committee.
U.S. federal income tax consequences relating to the transactions described under the Umbrella Plan are set forth in Section 409A of the Internal Revenue Code of 1986, as amended (the “Code”) and treasury regulations in 2004 to regulate all types of deferred compensation.
Pursuant to the current Section 102 of the Israeli Tax Ordinance, which came into effect on January 1, 2003, options may be granted through a trustee (i.e., Approved 102 Options) or not through a trustee (i.e., Unapproved 102 Options).
| F-27 |
| 3. | During the year ended December 31, 2014, the six month period ended December 31, 2013, the year ended June 30, 2013 and the year ended June 30, 2012 the Company granted stock options to the CEO, employees and directors to purchase a total of 1,846,515, 430,000, 1,706,112 and 1,579,250 shares of the Company’s common stock, respectively. The options have exercise prices ranging from $1.14-$3.23, $2.12-$2.75, $1.97-$9.6 and $2.92-$10 per share, respectively, which were the fair market value of the Company’s common stock on the date of each respective grant. The fair value of these options, using the Black-Scholes option-pricing model, was approximately $3,279,000, $633,000, $3,304,000 and $5,425,000, respectively. The options are subject to a three-year vesting period with one-third of such awards vesting each year. See also Note 7a for stock options grants to the CEO and directors. |
Out of the 1,846,515 stock options mentioned above, 450,000 stock options were granted to the COO of the Company, who also serves as one of the Company's directors.
| 4. | During the year ended December 31, 2014 and the year ended June 30, 2013 the Company granted to the CEO, employees and directors 650,757 and 579,866 restricted shares of the Company’s common stock, respectively. The fair value of these restricted shares was approximately $1,890,000 and $1,989,000. The restricted shares are subject to a three-year vesting period with one-third of such awards vesting each year. |
Out of the 650,757 restricted shares mentioned above, 150,000 stock options were granted to the COO of the Company, who also serves as one of the Company's directors.
| F-28 |
| 5. | The following table summarizes information about warrants and share options to employees: |
| Year ended December 31, | 6 month period ended | Year ended June 30, | ||||||||||||||||||||||||||||||
| 2014 | December 31, 2013 | 2013 | 2012 | |||||||||||||||||||||||||||||
| Number
of warrants and options | Weighted
average exercise price | Number
of warrants and options | Weighted
average exercise price | Number
of warrants and options | Weighted
average exercise price | Number
of warrants and options | Weighted
average exercise price | |||||||||||||||||||||||||
| Outstanding - beginning of period | 3,688,175 | $ | 4.55 | 3,407,054 | $ | 4.71 | 2,321,083 | $ | 5.28 | 1,098,631 | $ | 3.60 | ||||||||||||||||||||
| Granted | 1,846,515 | $ | 2.91 | 430,000 | 2.38 | 1,706,112 | 3.19 | 1,579,250 | 6.80 | |||||||||||||||||||||||
| Forfeited | (164,516 | ) | $ | 3.9 | (77,863 | ) | 3.50 | (194,729 | ) | 4.11 | (106,799 | ) | 8.44 | |||||||||||||||||||
| Exercised | (71,016 | ) | 0.001 | (425,412 | ) | 0.001 | (250,000 | ) | 6.00 | |||||||||||||||||||||||
| Outstanding -end of period | 5,370,174 | $ | 4.01 | 3,688,175 | $ | 4.55 | 3,407,054 | $ | 4.71 | 2,321,082 | $ | 5.28 | ||||||||||||||||||||
| Exercisable at the end of the period | 2,292,003 | $ | 5.48 | 1,526,024 | $ | 6.55 | 1,316,979 | $ | 6.23 | 904,108 | $ | 3.04 | ||||||||||||||||||||
| F-29 |
The following table summarizes information about warrants and share options to non-employees:
| Year ended December 31, | 6 month period ended | Year ended June 30, | ||||||||||||||||||||||||||||||
| 2014 | December 31, 2013 | 2013 | 2012 | |||||||||||||||||||||||||||||
| Number
of warrants and options | Weighted
average exercise price | Number
of warrants and options | Weighted
average exercise price | Number
of warrants and options | Weighted
average exercise price | Number
of warrants and options | Weighted average exercise price | |||||||||||||||||||||||||
| Outstanding - beginning of period | 1,543,141 | $ | 4.57 | 1,634,702 | $ | 4.57 | 2,123,943 | $ | 3.80 | 1,999,103 | $ | 3.60 | ||||||||||||||||||||
| Granted* | 115,723 | 5.09 | 239,086 | 5.04 | ||||||||||||||||||||||||||||
| Forfeited | (144,126 | ) | $ | 5.44 | (85,474 | ) | 4.86 | (33,486 | ) | 5.79 | (114,246 | ) | 2.44 | |||||||||||||||||||
| Exercised | (6,087 | ) | 0.00 | (571,478 | ) | 1.86 | - | - | ||||||||||||||||||||||||
| Outstanding - end of period | 1,399,015 | $ | 4.48 | 1,543,141 | $ | 4.57 | 1,634,702 | $ | 4.57 | 2,123,943 | $ | 3.80 | ||||||||||||||||||||
| Exercisable at the end of the period | 1,391,398 | $ | 4.47 | 1,500,713 | $ | 4.53 | 1,546,693 | $ | 4.51 | 2,056,710 | $ | 3.76 | ||||||||||||||||||||
| * | Including 50,000 and 19,479 options with performance conditions in the years ended June 30, 2013 and 2012, respectively. |
| F-30 |
| 6. | The following table summarizes information about restricted shares to employees: |
| Year ended June 30, | ||||||||||||||||
| Year
ended December 31, 2014 | 6
month period ended December 31, 2013 | 2013 | 2012 | |||||||||||||
| Number
of restricted shares | Number
of restricted shares | Number
of restricted shares | Number
of restricted shares | |||||||||||||
| Outstanding - beginning of period | 546,533 | 546,533 | - | - | ||||||||||||
| Granted | 650,757 | 579,866 | ||||||||||||||
| Forfeited | (7,175 | ) | ||||||||||||||
| Vested | (159,955 | ) | (33,333 | ) | ||||||||||||
| Outstanding - end of period | 1,030,160 | 546,533 | 546,533 | - | ||||||||||||
| 7. | On May 7, 2014, the Company the Company granted to a former consultant 12,000 restricted shares of the Company’s common stock. The fair value of these restricted shares was approximately $25,000. The restricted shares vested immediately but are subject to a one-year lock-up period. |
| F-31 |
The following table provides additional information about all warrants and options outstanding and exercisable:
| Outstanding as of December 31, 2014 | ||||||||||||
| Exercise price | Warrants and options outstanding | Weighted average remaining contractual life (years) | Warrants and options exercisable | |||||||||
| 0-1.97 | 572,106 | 4.37 | 462,106 | |||||||||
| 2.05-3.4 | 3,293,377 | 8.81 | 570,204 | |||||||||
| 4.05-4.928 | 998,381 | 6.45 | 775,628 | |||||||||
| 6-7.8 | 1,740,325 | 3.94 | 1,729,631 | |||||||||
| 8-10.4 | 165,000 | 6.44 | 145,833 | |||||||||
| 6,769,189 | 6.77 | 3,683,402 | ||||||||||
The weighted average of the remaining contractual life of total vested and exercisable warrants and options as of December 31, 2014 was 6.48 years.
The aggregate intrinsic value of the total exercisable warrants and options as of December 31, 2014 was approximately $230,817.
The total intrinsic value of options exercised was approximately $0, $177,000, $4,600,000 and $800,000 for the year ended December 31, 2014, the six month period ended December 31, 2013 and for the years ended June 30, 2013 and 2012, respectively.
The weighted average fair value of warrants and options granted was approximately $1.78 , $1.47, $1.98 and $4.24 for the year ended December 31, 2014, the six month period ended December 31, 2013, and for the years ended June 30, 2013 and 2012, respectively. The weighted average fair value of warrants and options granted was estimated using the Black-Scholes option-pricing model.
| 1. | The following table sets forth the assumptions that were used in determining the fair value of options granted to employees for the year ended December 31, 2014 and the six month period ended December 31, 2013, as well as the years ended June 30, 2013 and 2012: |
| F-32 |
| Year ended | 6 month period ended | Year ended June 30, | ||||||||||||||
| December 31, 2014 | December 31, 2013 | 2013 | 2012 | |||||||||||||
| Expected life | 5.5-6.5 years | 5.5-6.5 years | 5.04-6.5 years | 0.17-6.5 years | ||||||||||||
| Risk-free interest rates | 1.64%-2.18% | 1.55%-2.23% | 0.72%-1.28% | 0.03%-2.79% | ||||||||||||
| Volatility | 62.89%-68% | 68%-69% | 67%-70% | 55%-71% | ||||||||||||
| Dividend yield | 0% | 0% | 0% | 0% | ||||||||||||
The following table sets forth the assumptions that were used in determining the fair value of warrants and options granted to non-employees for the years ended June 30, 2013 and 2012. No such options were granted during the year ended December 31, 2014 and during six month period ended December 31, 2013:
| Year ended June 30, | ||||||||
| 2013 | 2012 | |||||||
| Expected life | 2-10 years | 2-10 years | ||||||
| Risk-free interest rates | 0.28%-1.79% | 0.3%-1.97% | ||||||
| Volatility | 60%-73% | 47%-65% | ||||||
| Dividend yield | 0% | 0% | ||||||
The Company does not have sufficient historical exercise data to provide a reasonable basis upon which to estimate expected term. Accordingly, as to ordinary course options granted, the expected term was determined using the simplified method, which takes into consideration the option’s contractual life and the vesting periods (for non-employees, the expected term is equal to the option’s contractual life).
The Company estimates its forfeiture rate based on its employment termination history, and will continue to evaluate the adequacy of the forfeiture rate based on analysis of employee turnover behavior and other factors (for non-employees the forfeiture rate is nil). The annual risk-free rates are based on the yield rates of zero coupon non-index linked U.S. Federal Reserve treasury bonds as both the exercise price and the share price are in dollar terms. The Company’s expected volatility is derived from a blended volatility, based on its historical data and that of a peer group of public companies.
| F-33 |
| 2. | As of December 31, 2014, the total unrecognized compensation cost on employee and non-employee stock options and restricted shares, related to unvested stock-based compensation, amounted to approximately $3.9 million. This cost is expected to be recognized over a weighted-average period of approximately 1.01 years. This expected cost does not include the impact of any future stock-based compensation awards. |
The following table summarizes the allocation of total share-based compensation expense in the consolidated statements of operations:
| Year ended | 6 month period ended | Year ended June 30, | ||||||||||||||
| December 31, 2014 | December 31, 2013 | 2013 | 2012 | |||||||||||||
| ($ in thousands) | ||||||||||||||||
| Deduction from revenue | $ | - | $ | - | $ | - | $ | 68 | ||||||||
| Cost of revenues | 13 | 4 | 44 | 192 | ||||||||||||
| Research and development | 534 | 41 | 284 | 370 | ||||||||||||
| Sales and marketing | 446 | 87 | 78 | 375 | ||||||||||||
| General and administrative | 3,145 | 1,417 | 3,433 | 9,549 | ||||||||||||
| $ | 4,138 | $ | 1,549 | $ | 3,839 | $ | 10,554 | |||||||||
| c. | On April 5, 2012, the Company issued the 2012 Convertible Debenture and 2012 Warrants to purchase an aggregate of 835,866 shares of its common stock at an exercise price of $7.20 per share in a private placement transaction. See Note 6. |
| d. | On October 23, 2013, in connection with the Loan and Security Agreement, the Company issued the 2013 Warrant to purchase 168,351 shares of Common Stock at an exercise price of $2.97 per share. See Note 6. |
| e. | Rights Agreement |
On October 22, 2013, the Board adopted a stockholder rights plan (the “Rights Plan”). The rights, as stipulated in the Rights Plan, were generally exercisable only if a person or group acquired beneficial ownership of 15% or more of the Company's common stock in a transaction not approved by the Company’s board. The plan expired on October 22, 2014.
| f. | At-the-Market Agreement |
On October 23, 2013, The Company entered into an at-the-market issuance sales agreement, or the Sales Agreement, with MLV pursuant to which The Company may issue and sell shares of The Company common stock having an aggregate offering price of up to $40 million directly on The NYSE MKT or sales made to or through a market maker other than on an exchange. With the Company prior written consent, sales may also be made in negotiated transactions and/or any other method permitted by law. MLV will receive a 3% commission from the gross proceeds of any sales. Subject to the terms and conditions of the Sales Agreement, MLV will use its commercially reasonable efforts to sell the shares of the Company common stock from time to time, based upon the Company instructions (including any price, time or size limits or other parameters or conditions that the Company may impose). The Company is not obligated to make any sales of common stock under the Sales Agreement and no assurance can be given that the Company will sell any shares under the Sales Agreement, or, if the Company does, as to the price or amount of shares that the Company will sell, or the dates on which any such sales will take place. The Sales Agreement may be terminated by either party at any time upon 10 days’ notice to the other party, or by MLV at any time in certain circumstances, including the occurrence of a material adverse effect to the Company. In addition, the Sales Agreement will automatically terminate upon the sale of all common stock subject to the Sales Agreement. During the year ended December 31, 2014, the Company sold 948,000 shares of its common stock pursuant to its at-the-market issuance sales agreement with MLV & Co. LLC. These sales resulted in net proceeds to the Company of approximately $2.2 million. Following the 2014 Offering, the Company is prohibited from entering into any variable rate transactions which may impair its ability to make sales under our at-the-market issuance sales agreement absent the consent of the investors in the 2014 Offering until November 7, 2016.
| F-34 |
NOTE 10 - TAXES ON INCOME
| a. | Tax laws applicable to the Company and its subsidiaries |
Taxation in the United States
InspireMD, Inc. is taxed under U.S. tax laws.
Taxation in Israel
InspireMD Ltd. is taxed under the Israeli Income Tax Ordinance.
On December 6, 2011, the "Tax Burden Distribution Law" Legislation Amendment (2011) was published in the Official Gazette. Under this law, the previously approved gradual decrease in the corporate tax rate was cancelled. The Corporate tax rate was increased from 24% in 2011, to 25% beginning 2012.
On August 5, 2013, the Law for Change of National Priorities (Legislative Amendments for Achieving the Budgetary Goals for 2013-2014), 2013 (hereinafter, the "Law") was published in Reshumot (the Israeli government official gazette), and enacts, among other things, the following amendments:
| 1. | Raising the corporate tax rate beginning in 2014 and thereafter to 26.5% (instead of 25%). |
| 2. | Increasing the tax rate on the income of preferred enterprises from the 2014 tax year and thereafter, as stated in the Encouragement of Capital Investment Law, 1959 (hereinafter - the Encouragement Law) of a qualifying company in Development Zone A to 9% (instead of 7% in 2014 and 6% in 2015 and thereafter) and companies located in zones other than Zone A to 16% (instead of 12.5% in 2014 and 12% in 2015 and thereafter). In addition, the tax rate on dividends distributed on January 1, 2014 and thereafter originating from preferred income under the Encouragement Law will be raised to 20% (instead of 15%). |
| 3. | When a company distributes revaluation gains to its shareholders, the asset for which revaluation gains are recognized in the financial statements of the company is deemed as an asset that was sold on distribution day (notional sale) and therefore such revaluation gains are liable to tax. Revaluation gains are defined by the Law as retained earnings not subject to corporate tax, of the kind indicated by the Minister of Finance with approval of the Finance Committee of the Knesset, at over NIS 1 million to be calculated accumulatively from the date of acquiring the asset. |
| F-35 |
Taxation in Germany
InspireMD GmbH is taxed according to the tax laws in Germany. Accordingly, the applicable tax rates are corporate tax rate of 15.825% and trade tax rate of 17.15%.
| b. | Tax benefits under the Law for the Encouragement of Capital Investments, 1959 (the “Law”): |
| 1. | InspireMD Ltd. has been granted a “Beneficiary Enterprises” status under the Investment Law including Amendment No. 60 thereof, which became effective in April 2005. |
The tax benefits derived from any such Beneficiary Enterprise relate only to taxable profits attributable to the specific program of investment to which the status was granted.
The main benefit to which InspireMD Ltd. is entitled, conditional upon the fulfilling of certain conditions stipulated by the above law, is a two-year exemption and five years of a reduced tax rate of 25% from tax on income derived from beneficiary activities in facilities in Israel. The two-year exemption starts only when the Company starts to pay taxes after using all the carryforward tax losses. The tax benefit period is twelve years from the year of election, which means that after a year of election, the two-year exemption and five years of reduced tax rate can only be used within the next twelve years. The Company elected the year 2007 as a year of election and 2011 as an additional year of election.
In the event of a distribution of tax-exempt income attributable to "Beneficiary Enterprises" as a cash dividend, the Company will be required to pay tax at a rate of 25% on the amount distributed. In addition, dividends originating from income attributable to the "Beneficiary Enterprises" will be subject to a 15% withholding tax.
Should InspireMD Ltd. derive income from sources other than the “Beneficiary Enterprises” during the period of benefits, such income shall be taxable at the regular corporate tax rate.
| 2. | Conditions for entitlement to the benefits |
The entitlement to the above benefits is conditional upon InspireMD Ltd. fulfilling the conditions stipulated by the law, regulations published thereunder and the instruments of approval for the specific investments in approved assets. In the event of failure to comply with these conditions, the benefits may be cancelled and InspireMD Ltd. may be required to refund the amount of the benefits, in whole or in part, with the addition of interest.
The Israeli Law for Encouragement of Capital Investments, 1959 was amended as part of the Economic Policy Law for the years 2011-2012, which was passed in the Knesset (the Israeli parliament) on December 29, 2010. The amendment became effective as of January 1, 2011.
| F-36 |
The amendment set alternative benefit tracks to the ones then in place, as follows: (i) an investment grants track designed for enterprises located in national development zone A and (ii) two new tax benefits tracks (for preferred enterprises and for special preferred enterprises), which provide for application of a unified tax rate to all preferred income of the company, as defined in the amendment.
The Company opted not to apply for Preferred Enterprise status.
| c. | Carry forward tax losses |
As of December 31, 2014, InspireMD Ltd. had a net carry forward tax loss of approximately $36 million. Under Israeli tax laws, the carry forward tax losses can be utilized indefinitely. The Company had a net carry forward tax loss of approximately $29 million. Under U.S. tax laws, the Company’s tax losses can be utilized two years back and twenty years forward. As such the Company's carry forward tax losses will begin to expire on December 31, 2031.
| d. | Loss before income taxes |
The components of loss before income taxes are as follows:
| Year ended | 6 month period ended | Year ended June 30, | ||||||||||||||
| December 31, 2014 | December 31, 2013 | 2013 | 2012 | |||||||||||||
| ($ in thousands) | ||||||||||||||||
| Profit (loss) before taxes on income: | ||||||||||||||||
| InspireMD, Inc. | $ | (11,671 | ) | $ | (2,632 | ) | $ | (19,613 | ) | $ | (11,078 | ) | ||||
| Subsidiaries | (13,412 | ) | (6,694 | ) | (9,637 | ) | (6,515 | ) | ||||||||
| $ | (25,083 | ) | $ | (9,326 | ) | $ | (29,250 | ) | $ | (17,583 | ) | |||||
Current taxes on income
The following is a reconciliation of the theoretical tax expense, assuming all income was taxed at the regular tax rates applicable to the Company in the U.S. and the actual tax expense:
| Year ended | 6 month period ended | Year ended June 30, | ||||||||||||||
| December 31, 2014 | December 31, 2013 | 2013 | 2012 | |||||||||||||
| ($ in thousands) | ||||||||||||||||
| Loss before taxes on income, as reported in the statements of operations | $ | 25,083 | $ | 9,326 | $ | 29,250 | $ | 17,583 | ||||||||
| Theoretical tax benefit | (8,529 | ) | (3,171 | ) | (9,945 | ) | (5,984 | ) | ||||||||
| Increase in tax benefit resulting from permanent differences | 390 | 164 | 1,613 | 1,448 | ||||||||||||
| Increase (decrease) in taxes on income resulting from the computation of deferred taxes at a rate which is different from the theoretical rate, and other | 2,038 | (171 | ) | 205 | (75 | ) | ||||||||||
| Increase (decrease) in uncertain tax positions - net | - | - | - | (71 | ) | |||||||||||
| Difference between income reported for tax purposes and income for financial reporting purposes — net | 1,100 | |||||||||||||||
| Decrease in theoretical tax benefit resulting from subsidiaries different tax rate | (73 | ) | (14 | ) | (61 | ) | 1,408 | |||||||||
| Change in corporate tax rates | - | (121 | ) | - | (245 | ) | ||||||||||
| Change in valuation allowance | 5,086 | 3,323 | 8,196 | 3,533 | ||||||||||||
| $ | 12 | $ | 10 | $ | 8 | $ | 14 | |||||||||
| F-37 |
As of December 31, 2014 and 2013, the Company determined that it was more likely than not that the benefit of the operating losses would not be realized and consequently, management concluded that full valuation allowances should be established regarding the Company’s deferred tax assets.
The changes in the valuation allowance for the year ended December 31, 2014, the six month period ended December 31, 2013 and the years ended June 30, 2013 and 2012 were as follows:
| Year ended December 31, | 6 month period ended December 31, | Year ended June 30, | ||||||||||||||
| 2014 | 2013 | 2013 | 2012 | |||||||||||||
| ($ in thousands) | ||||||||||||||||
| Balance at the beginning of the year | $ | 19,569 | $ | 16,246 | $ | 8,050 | $ | 4,517 | ||||||||
| Changes during the year | $ | 5,086 | 3,323 | 8,196 | 3,533 | |||||||||||
| Balance at the end of the year | $ | 24,655 | $ | 19,569 | $ | 16,246 | $ | 8,050 | ||||||||
| e. | Accounting for Uncertain Tax position |
The following is a reconciliation of the total amounts of the Company’s unrecognized tax benefits during the year ended June 30, 2012. During the year ended December 31, 2014, the six month period ended December 31, 2013 and the year ended June 30, 2013 there were no changes in the uncertain tax position.:
| Year ended June 30, 2012 | ||||
| ($ in thousands) | ||||
| Balance at beginning of period | $ | 71 | ||
| Decrease in unrecognized tax benefits as a result of tax positions taken during a prior year | (71 | ) | ||
| Balance at end of period | $ | - | ||
| F-38 |
All of the above amounts of unrecognized tax benefits would affect the effective tax rate if recognized.
A summary of open tax years by major jurisdiction is presented below:
|
Jurisdiction |
Years | |
| U.S. | 2009-2014 | |
| Israel | 2011-2014 | |
| Germany | 2009-2014 | |
| United Kingdom | 2014 |
| f. | Deferred income tax: |
| December 31, | ||||||||
| 2014 | 2013 | |||||||
| ($ in thousands) | ||||||||
| Short-term: | ||||||||
| Allowance for doubtful accounts | $ | 89 | $ | 107 | ||||
| Provision for vacation and recreation pay | 59 | 85 | ||||||
| 148 | 192 | |||||||
| Long-term: | ||||||||
| R&D expenses | 1,738 | 1,085 | ||||||
| Share-based compensation | 2,990 | 2,137 | ||||||
| Carry forward tax losses | 19,729 | 16,111 | ||||||
| Accrued severance pay, net | 50 | 44 | ||||||
| 24,507 | 19,377 | |||||||
| Less-valuation allowance | (24,655 | ) | 19,569 | ) | ||||
| $ | - | $ | - | |||||
NOTE 11 - SUPPLEMENTARY FINANCIAL STATEMENT INFORMATION
Balance sheets:
| a. | Accounts receivable: |
| December 31, | ||||||||
| 2014 | 2013 | |||||||
| ($ in thousands) | ||||||||
| 1) Trade: | ||||||||
| Open accounts | $ | 972 | $ | 2,260 | ||||
| Allowance for doubtful accounts | (337 | ) | (405 | ) | ||||
| $ | 635 | $ | 1,855 | |||||
| 2) Other: | ||||||||
| Due from government institutions | $ | 162 | $ | 221 | ||||
| Advance payments to suppliers | 180 | 152 | ||||||
| Miscellaneous | 17 | 14 | ||||||
| $ | 359 | $ | 387 | |||||
| F-39 |
The changes in “Allowance for doubtful accounts” during the year ended December 31, 2014, the six month period ended December 31, 2013, as well as the years ended June 30, 2013 and 2012 are as follows:
| Year ended | 6 month period ended | Year ended | ||||||||||||||
| December 31, | December 31, | June 30, | ||||||||||||||
| 2014 | 2013 | 2013 | 2012 | |||||||||||||
| ($ in thousands) | ||||||||||||||||
| Balance at beginning of period | $ | 405 | $ | 329 | $ | 215 | 155 | |||||||||
| Additions during the period | 35 | 58 | 245 | 78 | ||||||||||||
| Deductions during the period | (57 | ) | (142 | ) | ||||||||||||
| Exchange rate differences | (46 | ) | 18 | 11 | (18 | ) | ||||||||||
| Balance at end of period | $ | 337 | $ | 405 | $ | 329 | 215 | |||||||||
| b. | Inventories: |
| December 31, | ||||||||
| 2014 | 2013 | |||||||
| ($ in thousands) | ||||||||
| Finished goods | $ | 1,273 | $ | 1,097 | ||||
| Work in process | 326 | 341 | ||||||
| Raw materials and supplies | 325 | 155 | ||||||
| $ | 1,924 | $ | 1,593 | |||||
For the year ended December 31, 2014, as well as December 31, 2013 and June 30, 2013 and 2012 the Company recorded expenses for slow moving inventory in the amounts of $129,000, $40,000, $0 and $443,000, respectively.
| c. | Accounts payable and accruals-other: |
| December 31, | ||||||||
| 2014 | 2013 | |||||||
| ($ in thousands) | ||||||||
| Employees and employee institutions | $ | 1,022 | $ | 1,133 | ||||
| Accrued vacation and recreation pay | 410 | 325 | ||||||
| Accrued clinical trials expenses | 1,016 | 622 | ||||||
| Provision for sales commissions | 120 | 139 | ||||||
| Accrued expenses | 993 | 886 | ||||||
| Taxes payable | 15 | 36 | ||||||
| $ | 3,576 | $ | 3,141 | |||||
| F-40 |
Statements of Operation:
| d. | Financial expenses, net: |
| Year ended December 31, | 6 month period ended December 31, | Year ended June 30, | ||||||||||||||
| 2014 | 2013 | 2013 | 2012 | |||||||||||||
| ($ in thousands) | ||||||||||||||||
| Bank commissions | $ | 43 | $ | 27 | $ | 38 | $ | 50 | ||||||||
| Interest income | (2 | ) | (2 | ) | (28 | ) | (40 | ) | ||||||||
| Exchange rate differences | (18 | ) | 1 | (63 | ) | 112 | ||||||||||
| Induced conversion of convertible debt | 9,330 | |||||||||||||||
| Issuance of warrants | 568 | |||||||||||||||
| Interest expense (including amortization of debt issuance costs) | 1,409 | 273 | 4,268 | 1,238 | ||||||||||||
| Change in fair value of warrants, embedded derivatives and anti-dilution rights | (47 | ) | 200 | 64 | (1,322 | ) | ||||||||||
| $ | 1,385 | $ | 499 | $ | 14,177 | $ | 38 | |||||||||
NOTE 12 - ENTITY WIDE DISCLOSURES
The Company operates in one operating segment.
Disaggregated financial data is provided below as follows:
(1) Revenues by geographic area and
(2) Revenues from principal customers.
Revenues are attributed to geographic areas based on the location of the customers. The following is a summary of revenues by geographic areas:
| Year ended December 31, | 6 month period ended December 31, | Year ended June 30, | ||||||||||||||
| 2014 | 2013 | 2013 | 2012 | |||||||||||||
| ($ in thousands) | ||||||||||||||||
| Middle East | 792 | 337 | 293 | 444 | ||||||||||||
| Germany | 286 | 67 | 246 | 466 | ||||||||||||
| Belarus | 285 | 100 | 321 | 129 | ||||||||||||
| Spain | 242 | 367 | 701 | 422 | ||||||||||||
| Russia | 3 | 759 | 837 | 812 | ||||||||||||
| Other | 1,210 | 1,475 | 2,475 | 3,076 | ||||||||||||
| $ | 2,818 | $ | 3,105 | $ | 4,873 | $ | 5,349 | |||||||||
| F-41 |
By principal customers:
| Year ended December 31, | 6 month period ended December 31, | Year ended June 30, | ||||||||||||||
| 2014 | 2013 | 2013 | 2012 | |||||||||||||
| Customer A | 21 | % | 7 | % | ||||||||||||
| Customer B | 10 | % | 3 | % | 7 | % | 2 | % | ||||||||
| Customer C | 9 | % | 12 | % | 14 | % | 8 | % | ||||||||
| Customer D | 24 | % | 17 | % | 15 | % | ||||||||||
All tangible long lived assets are located in Israel.
NOTE 13 – SUBSEQUENT EVENTS
| During the first quarter of 2015, the board of directors approved implementing a cost reduction/focused spending plan. See Note 1b. |
| On January 5, 2015, the Company amended the employment agreements with the Company’s CEO and COO. See Note 1b. |
| On March 9, 2015, the Company sold 34,369,675 shares of its common stock and warrants to purchase 34,369,675 shares of common stock in a public offering. See Note 1d. |
| F-42 |
