Attached files
| file | filename |
|---|---|
| EX-21.1 - EX-21.1 - GENOMIC HEALTH INC | ghdx-20141231ex211ba4951.htm |
| EXCEL - IDEA: XBRL DOCUMENT - GENOMIC HEALTH INC | Financial_Report.xls |
| EX-31.2 - EX-31.2 - GENOMIC HEALTH INC | ghdx-20141231ex3124fd989.htm |
| EX-32.2 - EX-32.2 - GENOMIC HEALTH INC | ghdx-20141231ex322789f57.htm |
| EX-32.1 - EX-32.1 - GENOMIC HEALTH INC | ghdx-20141231ex3218b220b.htm |
| EX-31.1 - EX-31.1 - GENOMIC HEALTH INC | ghdx-20141231ex311cff683.htm |
| EX-23.1 - EX-23.1 - GENOMIC HEALTH INC | ghdx-20141231ex231fdcc12.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form 10‑K
|
(Mark One) |
|
|
☒ |
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
|
For the fiscal year ended: December 31, 2014 |
|
|
or |
|
|
☐ |
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
|
For the transition period from to . |
|
Commission File Number: 000‑51541
GENOMIC HEALTH, INC.
(Exact name of Registrant as specified in its charter)
|
Delaware |
77‑0552594 |
|
301 Penobscot Drive |
94063 |
(650) 556‑9300
(Registrant’s telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act:
|
Title of Each Class |
Name of Each Exchange on Which Registered: |
|
Common Stock, par value $0.0001 per share |
The NASDAQ Stock Market LLC |
Securities registered pursuant to Section 12(g) of the Act and Title of Class: None
Indicate by check mark if the registrant is a well‑known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☐ No ☒
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ☐ No ☒
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Website, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S‑T during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes ☒ No ☐
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S‑K is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10‑K or any amendment to this Form 10‑K. ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non‑accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b‑2 of the Exchange Act. (Check one):
|
Large accelerated filer ☐ |
Accelerated filer ☒ |
Non‑accelerated filer ☐ |
Smaller reporting company ☐ |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b‑2 of the Act). Yes ☐ No ☒
As of June 30, 2014, the aggregate market value of voting and non‑voting common stock held by non‑affiliates of the registrant was approximately $449.9 million, based on the closing price of the common stock as reported on The NASDAQ Global Select Market for that date.
There were 32,172,849 shares of the registrant’s Common Stock outstanding on February 28, 2015.
DOCUMENTS INCORPORATED BY REFERENCE
Items 10 (as to directors and Section 16(a) Beneficial Ownership Reporting Compliance), 11, 12, 13 and 14 of Part III incorporate by reference information from the registrant’s proxy statement to be filed with the Securities and Exchange Commission in connection with the solicitation of proxies for the registrant’s 2015 Annual Meeting of Stockholders to be held on June 11, 2015.
2
This report contains forward‑looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. When used in this report, the words “expects,” “anticipates,” “intends,” “estimates,” “plans,” “believes,” and similar expressions are intended to identify forward‑looking statements. These are statements that relate to future periods and include statements about our expectation that, for the foreseeable future, a significant amount of our revenues will be derived from Oncotype DX for invasive breast cancer; the factors that may impact our financial results; our ability to achieve sustained profitability; our business strategy and our ability to achieve our strategic goals; our expectations regarding product revenues and the sources of those revenues; the amount of future revenues that we may derive from Medicare patients or categories of patients; our belief that we may become more dependent on Medicare reimbursement in the future; our plans to pursue reimbursement on a case‑by‑case basis; our ability, and expectations as to the amount of time it will take, to achieve reimbursement from third‑party payors and government insurance programs for new indications of tests, new tests or in new markets; the potential impact of changes in reimbursement levels for our tests; our expectations regarding our international expansion and opportunities; our expectations for reimbursement in international markets; our intent to enter into additional foreign distribution arrangements; our beliefs with respect to the benefits and attributes of our tests or tests we may seek to develop in the future; the factors we believe drive demand for our tests and our ability to sustain or increase such demand; our success in increasing patient and physician demand as a result of our direct sales approach and our sales forces’ capacity to sell our tests; plans for, and the timeframe for the development or commercial launch of, future tests, or test enhancements or new technologies; the factors that we believe will drive reimbursement and the establishment of coverage policies; the capacity of our clinical reference laboratory to process tests and our expectations regarding capacity; our expectations regarding expansion of our clinical reference laboratory; our dependence on collaborative relationships to develop tests and the success of those relationships; whether any tests will result from our collaborations or license agreements; the applicability of clinical results to actual outcomes; our estimates and assumptions with respect to disease incidence and potential market opportunities; the occurrence, timing, outcome or success of clinical trials or studies; our plans with respect to additional studies; our expectations regarding timing of the announcement or publication of research results; the benefits of our technology platform; the economic benefits of our tests to the healthcare system; the ability of our tests to impact treatment decisions; our beliefs regarding our competitive position; our expectations regarding new and future technologies, including next generation sequencing and non‑invasive test technology, and their potential benefits; our belief that multi‑gene analysis provides better analytical information; our beliefs regarding the benefits of genomic analysis in various patient populations; our expectations regarding clinical development processes future tests may follow; our beliefs regarding the benefits of individual gene reporting; our expectation that our research and development, general and administrative and sales and marketing expenses will increase and our anticipated uses of those funds; our expectations regarding capital expenditures; our ability to comply with the requirements of being a public company; our expectations regarding future levels of bad debt expense and billing and collections fees; our ability to attract and retain experienced personnel; the adequacy of our product liability insurance; our anticipated cash needs and our estimates regarding our capital requirements; our need for additional financing; our expected future sources of cash; our compliance with federal, state and foreign regulatory requirements; the potential impact resulting from the regulation of our tests by the U.S. Food and Drug Administration, or FDA, and other similar non‑U.S. regulators; our belief that our tests are properly regulated under the Clinical Laboratory Improvement Amendments of 1988, or CLIA; the impact of new or changing policies, regulation or legislation, or of judicial decisions, on our business and reimbursement for our tests; the impact of seasonal fluctuations and economic conditions on our business; our belief that we have taken reasonable steps to protect our intellectual property; the impact of changing interest rates; our beliefs regarding our unrecognized tax benefits or our valuation allowance; the impact of accounting pronouncements and our critical accounting policies, judgments, estimates, models and assumptions on our financial results; the impact of the economy on our business, patients and payors; and anticipated trends and challenges in our business and the markets in which we operate.
Forward‑looking statements are subject to risks and uncertainties that could cause actual results to differ materially from those expected. These risks and uncertainties include, but are not limited to, those risks discussed in Item 1A of this report, as well as our ability to develop and commercialize new products and product enhancements; the risk of unanticipated delays in research and development efforts; the risk that we may not obtain or maintain reimbursement for our existing tests or any future tests we may develop; the risk that reimbursement pricing or coverage may change; the risks and uncertainties associated with the regulation of our tests by the FDA or regulatory agencies outside of the U.S.; the success of our new technology; the results of clinical studies; the applicability of clinical results to actual outcomes; the impact of new legislation or regulations, or of judicial decisions, on our business; our ability to compete against third parties; our ability to obtain capital when needed; the economic environment; and our history of operating losses. These forward‑looking statements speak only as of the date hereof. We expressly disclaim any obligation or undertaking to update any forward‑looking statements
3
contained herein to reflect any change in our expectations with regard thereto or any change in events, conditions or circumstances on which any such statement is based.
This report contains statistical data attributable to both the Kantar Health, Inc.’s CancerMPact database (February 2013) and the Globocan 2008 Data published by the International Agency for Research on Cancer (IARC) 2008, or data that we derived from these sources. These sources generally indicate that they believe their information is reliable but do not guarantee the accuracy and completeness of their information. Although we believe that the sources are reliable, we have not independently verified their data.
In this report, all references to “Genomic Health,” “we,” “us,” or “our” mean Genomic Health, Inc.
Genomic Health, the Genomic Health logo, Oncotype, Oncotype DX, Recurrence Score and DCIS Score are trademarks or registered trademarks of Genomic Health, Inc. We also refer to trademarks of other corporations and organizations in this report.
Company Overview
Genomic Health is a global provider of genomic-based diagnostic tests that address both the overtreatment and optimal treatment of early stage cancer, one of the greatest issues in healthcare today. We are applying our world-class scientific and commercial expertise and infrastructure to lead the translation of massive amounts of genomic data into clinically-actionable results for treatment planning throughout the cancer patient's journey, from screening and surveillance, through diagnosis, treatment selection and monitoring.
We offer our Oncotype DX tests as a clinical laboratory service, where we analyze the expression levels of genes in tumor tissue samples and provide physicians with a quantitative gene expression profile expressed as a single quantitative score, which we call a Recurrence Score for invasive breast cancer and colon cancer, a DCIS Score for ductal carcinoma in situ, or DCIS and a Genomic Prostate Score, or GPS, for prostate cancer. Our Oncotype DX platform utilizes quantitative genomic analysis known as reverse transcription polymerase chain reaction, or RT‑PCR, in standard tumor pathology specimens to provide tumor‑specific information, or the “oncotype” of a tumor. Our Oncotype DX cancer tests analyze the expression levels of multiple genes across multiple biological pathways to predict cancer aggressiveness.
The Oncotype DX breast, colon and prostate cancer tests are commercially available at list prices of $4,510, $4,330 and $4,180, respectively. All of our testing services are made available through our clinical reference laboratory located in Redwood City, California, which is accredited under the Clinical Laboratory Improvement Amendments of 1988, or CLIA, and certified by the College of American Pathologists, or CAP.
As of December 31, 2014, more than 19,000 physicians in over 70 countries had ordered more than 500,000 Oncotype DX tests. We have a direct commercial presence with employees and consultants in the United States and certain other countries, and our tests are also available outside of the United States through a network of distributors.
In January 2004, we launched our first test for early stage invasive breast cancer patients. The Oncotype DX breast cancer test has extensive clinical evidence validating its ability to predict the likelihood of breast cancer recurrence and the likelihood of chemotherapy benefit. Results from a large validation study in node negative, or N−, patients were published in The New England Journal of Medicine and in the Journal of Clinical Oncology. Oncotype DX is the only test incorporated in published American Society of Clinical Oncologists, or ASCO, and National Comprehensive Cancer Network, or NCCN, breast cancer treatment guidelines for patients with N− breast cancer that is estrogen receptor positive, or ER+, and/or progesterone receptor positive, or PR+. The test is also recognized in international guidelines issued by the St. Gallen International Breast Cancer Expert Panel and European Society for Medical Oncology, or ESMO. In addition, the National Institute for Health and Care Excellence (NICE) in the United Kingdom recommends Oncotype DX as the only multi‑gene breast cancer test for use in clinical practice to guide chemotherapy treatment decisions for patients with early‑stage, hormone receptor‑positive, invasive breast cancer.
In December 2007, we presented a study establishing the clinical utility of our Oncotype DX breast cancer test for node positive or, N+, patients. This study, published in Lancet Oncology in December 2009, established that chemotherapy does not appear to benefit patients with either 1‑3 or with 4 or more positive nodes for disease‑free survival over 10 years, if their tumors had a low Recurrence Score result. Based upon these study results we began offering Oncotype DX for invasive breast cancer in N+ patients in early 2008.
4
As of February 2015, our Oncotype DX breast cancer test has been extensively evaluated in invasive breast cancer in 17 clinical studies involving more than 6,500 breast cancer patients worldwide.
In December 2011, we made our Oncotype DX breast cancer test available for patients with DCIS, a pre‑invasive form of breast cancer. The launch of Oncotype DX for DCIS patients was based on positive results presented from a clinical validation study of the Oncotype DX breast cancer test in patients with DCIS. The study met its primary endpoint by demonstrating that a pre‑specified Oncotype DX DCIS Score derived from the Oncotype DX breast cancer test outperforms traditional clinical and pathologic measures to predict the risk of local recurrence, defined as either the development of a new invasive breast cancer or the recurrence of DCIS in the same breast. In May 2013, our Oncotype DX DCIS clinical validation study was published online in the Journal of the National Cancer Institute.
In June 2014, we announced positive top line results of an additional clinical validation study to confirm and extend the observations of the published DCIS clinical validation study, conducted in collaboration with the Ontario DCIS Study Group. The results were presented at the San Antonio Breast Cancer Symposium, or SABCS, in December 2014.
In January 2010, we launched our Oncotype DX colon cancer test, the first multigene expression test developed to assess the risk of recurrence in patients with stage II disease. We collaborated with the National Surgical Adjuvant Breast and Bowel Project, or NSABP, and the Cleveland Clinic on a total of four development studies in more than 1,800 patient samples to analyze patients with stage II colon cancer. The test was then independently evaluated in more than 1,400 stage II colon cancer patients in the QUASAR validation study which demonstrated that the Oncotype DX colon cancer test can independently predict individual recurrence risk in stage II colon cancer patients following surgery. The QUASAR study results were published in the Journal of Clinical Oncology in November 2011.
In June 2012, based on the positive results of our NSABP C‑07 validation study, we began offering the Oncotype DX colon cancer test for use in patients with stage III disease treated with oxaliplatin‑containing adjuvant therapy.
In September 2012, we announced positive topline results from a large clinical validation study of our biopsy‑based prostate cancer test. We launched our Oncotype DX prostate cancer test in May 2013 and made this test available worldwide. The study, performed in collaboration with leading prostate cancer researchers at the University of California, San Francisco, demonstrated that the multi‑gene Oncotype DX GPS, assessed in prostate needle biopsy tumor tissue, is a predictor of adverse pathology for patients with early‑stage prostate cancer. The study supported the results of six earlier feasibility and development studies performed in collaboration with the Cleveland Clinic.
Our research and development activities are focused on developing a pipeline of tests to optimize the treatment of urologic cancers including bladder and kidney cancers, as well as additional treatment decisions in breast, colon and prostate cancers. Additionally, as discussed below, we are incorporating new technologies, such as high‑throughput “next generation” sequencing, or NGS, in our research and development laboratory in order to develop molecular tests that can be performed on tissue, blood or urine. Non‑invasive tests on blood and urine may be used to quantify the presence and burden of cancer as well as predict the sensitivity or resistance to specific drugs, with real time patient monitoring at potentially lower costs.
Scientific Background
Limits of Existing Approaches for Determining Cancer Treatments
Common types of cancer include breast, prostate, lung and colon. Cancer treatment decisions may include whether or not to perform surgery, whether or not to administer chemotherapy or radiation therapy, and whether or not to utilize other targeted therapies. In 2014, approximately 1.7 million people in the United States and 14.1 million people worldwide were diagnosed with cancer.
To treat cancer effectively, physicians diagnose and gauge the stage of a patient’s disease to determine the best course of therapy. For many cancer patients, surgery, radiation therapy, and chemotherapy are commonly used as treatment options, with varying degrees of benefits and side effects that may not always justify the cost of the therapy or the physical and mental burden patients endure.
Historically, physicians have used tumor pathology grade and stage when predicting whether a cancer will recur, as the key determinant in treatment decisions. Because tumor pathology grade and staging are heavily dependent on visual assessment and human interpretation, physicians and patients often make treatment decisions using subjective and qualitative
5
information that may not reflect the molecular nature of the patient’s cancer. As a result, many patients are misclassified as high risk when they are low risk for recurrence or low risk when they are high risk for recurrence, resulting in over‑treatment for some and under‑treatment for others.
Use of Genomics to Understand Cancer
While genomics and genetics may sound similar and are related, each focuses on different information. Genetics involve the study of individual genes and how genes pass on hereditary traits from one generation to the next and how new traits may develop from genetic mutations or changes. Examples of traits include physical traits or predisposition to certain conditions or drug metabolism. Certain genes, which normally help control healthy cell growth, can pass on predispositions to certain types of diseases, including cancer. There are cancer genetic tests that provide information about a person’s inherited genetic make‑up.
Genomics is the study of complex sets of genes, such as the entire set of genes of an organism, their expression and their function in a particular organism. Genomics can be used to understand diseases at the molecular level. Diseases can occur when mutated or defective genes inappropriately activate or block molecular pathways that are important for normal biological function.
Disease can result from inheriting mutated genes or from developing mutations in otherwise normal cells. Such mutations can be the cause of cancer. For most solid tumors, there is great heterogeneity between patients in the tumor mutations that are observed. The ability to detect mutations and their functional results and to understand whether the mutation contributes to disease can be crucial to better diagnosis and ultimately more rational and effective treatment.
The key to utilizing genomics in cancer is identifying specific sets of genes and gene interactions that are important for diagnosing different subsets of cancers. Using our RT‑PCR platform, we have performed studies which link the likelihood of recurrence or response to therapy to the pattern of gene expression in tumors. These results were used by us to develop tests that quantify gene expression of an individual’s tumor, allowing physicians to better understand what treatments are most likely to work for an individual patient or how likely a cancer is to recur.
Next Generation Technologies
Our commercially available tests utilize RT‑PCR technology to quantify gene expression in patient tumor samples. We are also incorporating new technologies, such as high‑throughput NGS in our research and development laboratory. With NGS technology, we can sequence millions of ribonucleic acids, or RNAs, map them back to their respective genes based on their sequence and then count the number of copies and compare the relative expression between different genes.
We have selected NGS to be our primary technology for future biomarker discovery and have begun using NGS for future clinical development in tandem with our existing RT‑PCR based approach. NGS technologies parallelize the sequencing process, producing thousands or millions of sequences at once, and are intended to provide nucleic acid sequence information at lower cost than standard methods. We have created proprietary methods for NGS of fixed paraffin embedded, or FPE, tissue nucleic acids, created bioinformatics programs, and infrastructure for data storage and analysis. In December 2011, we announced positive results of our first clinical outcomes study for biomarker discovery using NGS for whole transcriptome expression profiling. We have also explored the combination and superimposition of certain whole transcriptome derived RNA information (standardized expression; univariate biomarker direction of association) on genomic information to reveal the genomic landscapes of cancers. Employing NGS methods, we have also demonstrated feasibility for fusion transcript and mutation detection in RNA from FPE tissue samples and copy number aberration and structural variation mutations in DNA from FPE samples.
We have begun to further advance our research and development pipeline with proprietary platforms that incorporate emerging molecular technologies in order to develop non‑invasive liquid biopsy tests that can be performed on blood or urine. The positive results from our first two feasibility studies were presented in December 2014, demonstrating our ability to detect the presence of bladder cancer DNA in urine and breast cancer DNA in blood. Based on these positive initial results, we are working to develop non-invasive tests for real-time patient monitoring at potentially lower costs than our current tests. While early‑stage cancer continues to represent a significant opportunity with near‑term potential, we now have the opportunity to expand our business further along the patient’s cancer journey. When the presence of tumor-derived DNA in blood or urine is high and persists or increases over time, the cancer is likely growing and a new course of treatment may be appropriate. Expanding our focus beyond early‑stage treatment decision support toward later‑stage disease includes opportunities to monitor
6
progression and response to therapeutics for patients who are diagnosed with later stage or recurrent disease who can also benefit from precision medicine.
Oncotype DX Platform
Our current Oncotype DX platform uses our RT‑PCR approach to improve cancer treatment decisions. Our diagnostic approach correlates gene expression to clinical outcomes and provides an individualized analysis of each patient’s tumor. We have built a diagnostic infrastructure that allows us to move from research into development through to processing actual patient samples in our clinical reference laboratory. We have optimized this technology for quantitative gene expression on FPE tissue by developing methods and processes for screening hundreds of genes at a time using minimal amounts of tissue.
We believe that our multi‑gene analysis, as opposed to single‑gene analysis, provides a more powerful approach to distinguish tumors as being more or less likely to recur or progress. This information ultimately allows the physician and patient to choose a course of treatment that is individualized for each patient.
We offer Oncotype DX tests as clinical laboratory services, utilizing existing technologies such as RT‑PCR, and information technologies and optimize and integrate them into new processes. We expect to continue to extend the capabilities of the various components of our process to develop effective products. Our technologies allow us to analyze tumor tissue samples in our clinical reference laboratory and provide physicians with genomic information specific to the patient’s tumor. We analyze tissues that are handled, processed and stored under routine clinical pathology laboratory practices.
Once we receive a tumor sample, it is logged in and processed by our pathology department. Suitable samples then undergo a process by which RNA is extracted and purified. We then analyze the resulting material and produce a test result report that shows a single quantitative score on a continuum between 0‑100. Test results are reported as a Recurrence Score for invasive breast cancer and colon cancer, a DCIS Score for DCIS or a Genomic Prostate Score for prostate cancer, and are delivered to the treating physician typically within 10 to 14 days of our receipt of the tissue sample. This is within the crucial decision window after the tumor has been surgically removed or biopsied and before the patient and the treating physician discuss additional treatment options. The continuous range of scores differentiates Oncotype DX tests from other tests that predict only high or low risk by providing an individualized level of risk. The higher the score, the more aggressive the tumor and the more likely it is to recur. The test result report, along with other data and tests that physicians obtain, forms the basis for the treatment decision.
We believe our tests provide information that has the following benefits:
|
· |
Improved Quality of Treatment Decisions. We believe our approach to genomic‑based cancer analysis improves the quality of cancer treatment decisions by providing an individualized analysis of each patient’s tumor that is correlated to clinical outcome, rather than solely using subjective, anatomic and qualitative factors to determine treatments. Oncotype DX has been shown, consistently in more than 30 breast cancer, colon cancer and prostate cancer clinical studies, to classify many patients into recurrence risk categories different from classifications based primarily on tumor pathology grade and stage and to generally change treatment decisions in more than 30% of patients. Thus, our tests enable patients and physicians to make more informed decisions about treatment risk‑benefit considerations and, consequently, design an individualized treatment plan. |
|
· |
Improved Economics of Cancer Care. We believe that improving the quality of treatment decisions can result in significant economic benefits. For example, in early stage invasive breast cancer, our data shows that many patients are misclassified as high or low risk using traditional pathological and other measures. As a result, many low risk patients misclassified as high risk receive toxic and expensive chemotherapy or radiation treatment regimens, which may exceed $20,000, as compared to the cost of an Oncotype DX test. On the other hand, some high risk breast cancer patients misclassified as low risk are not provided chemotherapy or radiation treatment, possibly necessitating future treatment costing up to $50,000 or more if the cancer recurs. |
Oncotype DX Breast Cancer Test
In 2014, approximately 300,000 people in the United States and 1.7 million people worldwide were diagnosed with breast cancer, including both invasive and the pre‑invasive form, DCIS. Breast cancer tumors are classified as stage 0, I, II, III or IV. Stage 0, which includes DCIS, generally refers to a pre‑invasive tumor with reduced risk of recurrence. DCIS is typically not treated with chemotherapy but may be treated with lumpectomy or mastectomy, followed by radiation therapy and hormonal
7
therapy. Stage 0, I and II are generally referred to as early stage breast cancer, and stage III and IV are generally referred to as late stage breast cancer.
Following diagnosis, a physician determines the stage of the breast cancer by examining the following:
|
· |
the pathology of the tumor, |
|
· |
the size of the tumor, |
|
· |
nodal status, referred to as node positive, or N+, where the tumor has spread to the lymph nodes, and node negative, or N−, where the tumor has not spread to the lymph nodes, and |
|
· |
the extent to which the cancer has spread to other parts of the body. |
Prior to the inclusion of our Oncotype DX invasive breast cancer test in clinical guidelines, standard treatment guidelines weighed the stage of the cancer and additional factors to predict cancer recurrence and determine treatment protocol such as:
|
· |
the presence or absence of estrogen receptors, referred to as estrogen receptor positive, or ER+, where estrogen receptors are present, and estrogen receptor negative, or ER−, where estrogen receptors are not present, |
|
· |
the abundance of human epidermal growth factor receptor‑type 2, or HER2, genes or protein in the tumor, |
|
· |
the age of the patient, and |
|
· |
the histological type and grading of the tumor as reported by the pathologist. |
Because these diagnostic factors have limited capability to predict future recurrence and treatment benefit, and some are subjective, a large percentage of breast cancer patients received aggressive treatment while others were undertreated. Most early stage breast cancer patients have N−, ER+ tumors. These patients have been demonstrated to respond well to hormonal therapy, such as tamoxifen or an aromatase inhibitor. Identifying which of these patients to treat with radiation therapy or chemotherapy was a difficult decision.
Node Negative, Estrogen Receptor Positive (N−, ER+)
A National Surgical Adjuvant Breast and Bowel Project study, or NSABP B14, published by The New England Journal of Medicine in December 2004 demonstrated that the incremental survival benefit of chemotherapy in N−, ER+ patients also treated with tamoxifen is only 4%. Our test for invasive breast cancer is designed to help identify those patients with higher risk disease who are most likely to benefit from chemotherapy and to identify those patients with lower risk disease who may receive minimal clinical benefit from chemotherapy.
To develop our Oncotype DX breast cancer test, we evaluated 250 genes in three independent clinical studies which identified a 21‑gene panel whose composite gene expression profile can be represented by a breast cancer Recurrence Score. Our clinical validation study with the NSABP B‑14 population, published by The New England Journal of Medicine in December 2004, demonstrated that the Recurrence Score correlated with an individual’s likelihood of distant recurrence within 10 years of invasive breast cancer diagnosis. Moreover, our study with the NSABP B‑20 population, published in the Journal of Oncology in May 2006, demonstrated that the Recurrence Score also correlates with the likelihood of chemotherapy benefit for invasive breast cancer patients.
Node Positive, Estrogen Receptor Positive (N+, ER+)
We expanded the utility of our Oncotype DX breast cancer test to patients diagnosed with N+ breast cancer that may not benefit from chemotherapy or may have other health issues that increase the risk of chemotherapy treatment. Results from studies of our Oncotype DX breast cancer test in N+ patients utilizing tumor samples from chemotherapy treated patients (anthracycline plus Cytoxan or anthracycline plus Taxotere), completed in collaboration with the Eastern Cooperative Oncology Group, or ECOG, and Aventis, Inc., a member of the sanofi‑aventis group, or Aventis, were published in the Journal of Clinical Oncology in 2008. The results of this study suggest that the Recurrence Score result provides accurate recurrence risk information for patients with ER+ breast cancer, regardless of whether they are N+ or N−. In December 2007, we presented results from a second study conducted in conjunction with SWOG, that reinforced the conclusion that chemotherapy does not
8
appear to benefit patients with either 1‑3 or 4 or more positive nodes for disease‑free survival over 10 years, if their tumors had a low Recurrence Score result. The results were published in The Lancet Oncology in December 2009.
Aromatase Inhibitors
We conducted studies of our Oncotype DX breast cancer test with clinical samples from postmenopausal women with invasive breast cancer who were treated with aromatase inhibitors. Aromatase inhibitors and tamoxifen are both used as standard treatment for early stage ER+ breast cancer patients. In March 2010, the Journal of Clinical Oncology published results from a European study using our test to analyze tumor samples from over 1,200 patients in the ATAC (Arimedix, Tamoxifen, Alone or in Combination) trial, which established the wide use of aromatase inhibitors for adjuvant treatment of postmenopausal women with hormone receptor‑positive breast cancer. The study demonstrated that, along with other standard measures such as tumor size, our Oncotype DX breast cancer test contributes independently to provide a more complete picture of prognosis for N− and N+ patients treated with aromatase inhibitors.
Ductal Carcinoma in Situ (DCIS)
We further expanded the utility of our Oncotype DX breast cancer test to include DCIS patients, which we made available in late December 2011. The test provides an individualized prediction of the 10‑year risk of local recurrence (DCIS or invasive carcinoma), represented by a DCIS Score result, to help guide treatment decision‑making in women with DCIS treated by local excision, with or without tamoxifen. In the United States alone, one out of every five new breast cancer patients each year is diagnosed with DCIS. After breast‑conserving surgery, local recurrences of DCIS or a new invasive breast cancer occur in 20‑25% of patients at 10 years, on average, with surgery alone. The addition of radiation therapy and its attendant costs has been shown in clinical trials to reduce local recurrence risk, but has not been shown to prolong survival.
Development of our Oncotype DX DCIS Score was based on published results for the Oncotype DX breast cancer test showing similarity in the expression profiles of the Recurrence Score genes between DCIS and invasive breast cancer when both are present within the same patient tumor. The DCIS Score algorithm was developed based on published data obtained from the Kaiser Permanente and NSABP B‑14 studies in which the proliferation gene group was found to predict distant recurrence regardless of whether adjuvant tamoxifen therapy was given.
In December 2011, we presented positive results from the ECOG E5194 DCIS clinical validation study at SABCS. The study met its primary endpoint by demonstrating that a pre‑specified Oncotype DX DCIS Score can predict the risk of local recurrence, defined as either the development of a new invasive breast cancer or the recurrence of DCIS in the same breast. The study demonstrated that 75% of patients have a low DCIS Score and may be able to forego radiation therapy. Conversely, the study demonstrated that patients with a high DCIS Score had a 27% likelihood of local recurrence, of which approximately half were likely to develop a new invasive breast cancer. The DCIS Score also demonstrated consistent association with local recurrence across subgroups regardless of lesion size, grade, surgical margins, or menopausal status. This information can assist physicians and patients in deciding on the appropriate course of treatment based on a more complete understanding of the recurrence risk involved.
In May 2013, our Oncotype DX DCIS ECOG E5194 clinical validation study was published online in the Journal of the National Cancer Institute.
In June 2014, we announced positive top line results of a clinical validation study to confirm and extend the observations of the first DCIS clinical validation study, conducted in collaboration with the Ontario DCIS Study Group. Representing the largest genomic study in DCIS to date, the results confirmed and extended the conclusions of the previously published validation study. Additionally, for the first time, the Oncotype DX DCIS score predicted the risk of local recurrence in a group of patients treated with radiation therapy in clinical practice. In December 2014, we presented the study results at the SABCS.
Clinical Decision Studies and Health Economic Benefits of Oncotype DX Breast Cancer Test
We have conducted numerous clinical decision studies intended to support the adoption and reimbursement of our Oncotype DX invasive breast cancer test, both in the United States and in numerous countries outside of the United States. Among these studies is a meta‑analysis of seven studies with a total of 912 patients that demonstrated a consistent and large impact of the Recurrence Score on invasive breast cancer adjuvant treatment decisions. In these studies, physicians who use
9
Oncotype DX in clinical practice changed their treatment decisions in over a third of patients, leading to an overall reduction in chemotherapy use of approximately 28% with the use of the Recurrence Score. The Recurrence Score also led to the addition of chemotherapy to hormonal treatment in approximately 4% of patients who, prior to the Recurrence Score, were considered low risk but were subsequently identified by their Recurrence Score as having high risk disease. The results of this meta‑analysis indicate that the Recurrence Score provides key information for treatment decision‑making that cannot be ascertained from traditional measures.
In addition to clinical decision studies, we sponsor third‑party studies conducted by researchers affiliated with academic institutions to examine the health economic implications of our Oncotype DX breast cancer test. One such study, which was conducted in the United States and published in The American Journal of Managed Care in May 2005, demonstrated that our test provided a more accurate classification of risk than the NCCN guidelines in place at that time as measured by 10 year distant recurrence‑free survival. Based on these results, a model was designed to forecast quality‑adjusted survival and expected costs, or the net present value of all costs of treatment until death, if our Oncotype DX breast cancer test was used in patients classified as low risk or high risk by NCCN guidelines. The model, when applied to a hypothetical population of 100 patients with the demographic and disease characteristics of the patients entered in the NSABP Study B‑14, demonstrated an increase to quality‑adjusted survival in this population of 8.6 years and a reduction in projected aggregate costs of approximately $200,000. Furthermore, the model showed that as the expected costs and anticipated toxicity of chemotherapy regimens increase, the use of the Recurrence Score result to identify which patients would benefit from chemotherapy should lead to larger reductions in projected overall costs. According to this model, if all early stage invasive breast cancer patients and their physicians used our test and acted on the information provided by the breast cancer Recurrence Score result, there would be significant economic benefit to the healthcare system.
These studies reinforce the impact of the Oncotype DX breast cancer test on changing treatment decisions for invasive breast cancer patients and demonstrate its cost effectiveness across multiple healthcare systems. We plan to conduct or support additional clinical decision studies and health economic studies of our breast cancer test with clinical researchers domestically and abroad as we expand distribution of our test.
Oncotype DX Colon Cancer Test
In 2014, approximately 100,000 people in the United States and 955,000 people worldwide were diagnosed with colon cancer. Colon cancer tumors are classified as stage 0, I, II, III or IV. Stage 0 generally refers to a pre‑invasive tumor with reduced risk of recurrence that is typically not treated with chemotherapy but may be treated with surgery.
Following diagnosis, a physician determines the stage of the colon cancer by examining the following:
|
· |
the pathology of the tumor, |
|
· |
the size of the tumor, |
|
· |
nodal status, referred to as node positive, or N+, where the tumor has spread to the lymph nodes, and node negative, or N−, where the tumor has not spread to the lymph nodes, and |
|
· |
the extent to which the cancer has spread to other parts of the body. |
Standard treatment guidelines weigh the stage of the cancer and additional factors to predict cancer recurrence and determine treatment protocol including:
|
· |
the age of the patient, |
|
· |
the histological type and grading of the tumor as reported by the pathologist, |
|
· |
the level of mismatch repair, or MMR, also known as microsatellite instability, or MSI, and |
|
· |
T‑stage, an index of tumor penetration through the bowel. |
In 2014, stage II and stage III colon cancer affected approximately 25,000 and 24,000 people, respectively, in the United States, and the current treatment paradigm is unclear. The decision to treat patients with chemotherapy following surgery
10
is based on an assessment of how likely their disease is to recur. However, accurately identifying those patients with high recurrence risk is a critical issue for physicians because the available markers to determine likelihood of disease recurrence are limited, resulting in both over‑treatment and under‑treatment of patients following surgery. Research indicates that the survival benefit of chemotherapy treatment is only 5% in stage II disease and 10% in stage III disease, however all chemotherapy‑treated colon cancer patients are at risk of significant drug‑related toxicity. While there are existing clinical markers associated generally with higher risk in colon cancer patients, there was no clinically validated genomic test available that predicted the likelihood of recurrence for individual patients prior to the availability of our test.
In developing our colon cancer product, we used the same rigorous clinical development strategy and standardized quantitative technology designed for our Oncotype DX breast cancer test. We developed our gene panel by identifying 761 cancer‑related genes through review of existing research literature and computer analysis of genomic databases. The NSABP conducted three development studies and the Cleveland Clinic Foundation conducted one development study, which we funded, analyzing the 761 candidate genes in over 1,800 patients with stage II colon cancer. Detailed analysis of gene expression and colon cancer recurrence was performed to identify specific genes with the potential to predict the likelihood of cancer recurrence and response to chemotherapy. The 761 candidate genes were also examined to determine whether they would be useful beyond other key variables including tumor stage, tumor grade, lymph nodes examined and MMR/MSI.
We selected a final set of 12 genes which were then independently evaluated in a validation study of over 1,400 stage II colon cancer patients from the Quick and Simple and Reliable, or QUASAR, randomized study of adjuvant chemotherapy in the United Kingdom. This international, multi‑center randomized trial examined the recurrence risk and the benefit associated with 5‑ fluorouracil/leucovorin, or 5FU/LV, adjuvant chemotherapy. Gene expression was quantified by RT‑PCR from manually microdissected FPE primary colon cancer tissue, and recurrence‑free interval, disease‑free survival and overall survival were analyzed.
In May 2009, we presented positive results from this clinical validation study. The study met its primary endpoint to predict the likelihood of recurrence for stage II colon cancer patients following surgery and showed that the colon cancer Recurrence Score provided additional independent clinical value beyond standard measures of risk. The study showed that the colon cancer Recurrence Score result maintained significance, independent of MMR/MSI, T‑stage, nodes examined, grade and lymphovascular invasion. We believe our test addresses an unmet need in the treatment of colon cancer which can significantly improve risk assessment in the treatment planning for stage II colon cancer patients. T4 stage, which indicates growth of the tumor through the wall of the bowel and is associated with higher risk of recurrence, and MMR deficiency were also independently beneficial in predicting recurrence, and together comprise approximately 25% of patients. Patients with tumors identified as MSI high, or MMR deficient, are considered to be at low risk of recurrence. We believe the Oncotype DX colon cancer test result will provide the greatest clinical utility for treatment selection in the more than 70% of patients for whom MMR/MSI and T‑stage are uninformative. In November 2011, the results of the study were published in the Journal of Clinical Oncology.
In January 2010, we presented additional results from a study demonstrating that the Oncotype DX colon cancer test result and number of nodes examined are independent predictors of recurrence in stage II colon cancer and both should be considered when assessing individual recurrence risk in this patient population. In June 2011, a second large study confirming that the Oncotype DX colon cancer test independently predicts individualized recurrence risk for stage II colon cancer was presented.
In June 2012, based on the positive results of the landmark randomized NSABP C‑07 validation study, we began offering the Oncotype DX colon cancer test for use in patients with stage III disease treated with oxaliplatin‑containing adjuvant therapy. In September 2012, at the European Society for Medical Oncology Congress, we presented these positive results from the NSABP C‑07 study, including prediction of risk of recurrence, disease‑free survival and overall survival in stage II and stage III colon cancer patients. In November 2013, the Journal of Clinical Oncology published positive results of the third successful validation of the Oncotype DX colon cancer test in patients with stage II disease and the first validation study in patients with stage III disease. In an exploratory component of the NSABP C‑07 clinical trial, researchers analyzed 735 genes and identified 16 genes as being predictive of oxaliplatin benefit when added to adjuvant therapy. In September 2013, we delayed our plan to utilize these results and initiate a validation study in 2013. The decision to delay was based on analytical performance during the pre‑validation phase that did not meet our standards for a subset of the candidate predictive genes.
We believe these studies and publications will help to support adoption of and further reimbursement for our Oncotype DX colon cancer test. Moreover, current or future studies of our colon cancer test may lead to inclusion of the test in clinical guidelines and as standard of care for indicated patients.
11
Clinical Decision Studies and Health Economic Benefits of Oncotype DX Colon Cancer Test
In January 2012, we presented positive results of the first clinical decision making study of the Oncotype DX colon cancer test that show that Recurrence Score result has a significant impact on treatment recommendations for stage II colon cancer patients. The data demonstrated that knowledge of a patient’s Recurrence Score changes medical oncologists’ treatment recommendations in 29% of cases, with two‑thirds of the changes being decreases in treatment intensity, further confirming the clinical utility of using the Oncotype DX test as an independent predictor of recurrence in stage II colon cancer.
As with our breast cancer test, we sponsor third‑party studies conducted by researchers affiliated with academic institutions to examine the health economic implications of our Oncotype DX colon cancer test. The results of one such study, announced in January 2013, demonstrated after receiving the Recurrence Score for their stage II colon cancer patients, physician recommendations for adjuvant chemotherapy in patients with low risk of recurrence decreased by 22%, which resulted in direct medical care cost savings of $4,200 per patient.
In November 2013, the Current Medical Research & Opinion published positive results from the Partnership for Health Analytic Research clinical utility analysis of the Oncotype DX colon cancer test, demonstrating that use of the assay changed treatment recommendations in 29% of stage II colon cancer patients.
These studies reinforce the impact of the Oncotype DX colon cancer test on changing treatment decisions for stage II and stage III colon cancer patients and demonstrate its cost effectiveness. We plan to conduct or support additional clinical decision studies and health economic studies of our colon cancer test with clinical researchers domestically and abroad as we expand distribution of our test.
Mismatch Repair Testing for Colon Cancer
The QUASAR clinical validation study demonstrated that patients with MMR deficient, or MMR‑D, colon tumors, an alteration observed in approximately 15% of stage II colon cancers, have significantly lower stage II colon cancer recurrence risk, and thus MMR testing can be complementary to the information provided by the Oncotype DX colon cancer test. MMR/MSI testing, although not routinely performed, is currently provided by many pathology laboratories.
In order to advance the incorporation of MMR/MSI testing in colon cancer treatment decisions, we began offering MMR testing in December 2011. The MMR subtyping performed by us is done by standard, non‑proprietary immunohistochemistry processes and as such the testing is billed and reimbursed using reimbursement codes which are subject to coding changes issued by the Centers for Medicare and Medicaid Services, or CMS, and changes in reimbursement applied to the Medicare Physician Fee Schedule. However, we may not be eligible to bill for this service in some instances due to insurance contracts which require in‑network status for billing on non‑proprietary services.
Oncotype DX Prostate Cancer Test
Approximately 1.1 million men worldwide were diagnosed with prostate cancer in 2014. Based upon the results of prostate‑specific antigen, or PSA, testing, biopsies were performed on over one million men in the United States in 2014, and more than 230,000 of these patients were diagnosed with prostate cancer. The vast majority of these patients receive aggressive treatment, including surgery and radiation therapy, and more than half of these patients suffer incontinence and/or impotence after surgery. Less than 10% of patients choose active surveillance even though, for most prostate cancer patients, their disease will not cause clinical symptoms or death.
In February 2011, we presented positive full results from our prostate cancer gene identification study. The study, which applied the same RT‑PCR technology used in our Oncotype DX breast and colon cancer tests, identified 295 genes strongly associated with clinical recurrence of prostate cancer following radical prostatectomy. In June 2012, we presented results of our first development study in prostate tissue obtained from needle biopsies. The study, an analysis of biopsy samples from men with conventionally defined low/intermediate risk prostate cancer, showed that genes and biological pathways associated with clinically‑aggressive prostate cancer in radical prostatectomy specimens can be reliably measured by quantitative RT‑ PCR from fixed prostate needle biopsies.
In September 2012, we announced positive top line results from a clinical validation study of our biopsy‑based prostate cancer test. As a result of this clinical validation study meeting its primary end point, we launched our Oncotype DX prostate cancer test in May 2013, and made the test available worldwide. The test provides a GPS that predicts disease aggressiveness in men with low risk disease. This test may be used to improve treatment decisions for prostate cancer patients, in conjunction with the Gleason score, or tumor grading. In May 2014, European Urology published the positive results from our two development studies, as well as our clinical validation study of diagnostic biopsies from 395 men who were candidates for
12
active surveillance, demonstrating that the use of GPS can potentially increase the number of men who could confidently choose active surveillance by 20% to 30%.
We use our proprietary RT‑PCR process for analyzing very small amounts of fixed prostate tissue obtained by needle biopsy to determine, based on the biopsy, whether a patient has high grade disease or disease that has extended beyond the prostate—verses low grade disease or disease confined to the prostate. Our test is intended to address the well‑known limitation of biopsy sampling, which leads to overtreatment based on the fear of a patient’s tumor being upgraded or upstaged following radical prostatectomy. Our test allows more patients to appropriately select active surveillance, avoiding radical surgery and its lifelong complications.
In August 2014, we announced positive top line results of a second Oncotype DX prostate cancer clinical validation study, demonstrating the ability of our test’s GPS to predict multiple clinical endpoints related to disease aggressiveness among low/intermediate risk patients, as a predictor of biochemical recurrence. The study also confirmed the earlier validation study presented in 2013 and published in May 2014. The results from the clinical validation study were presented at the European Society for Medical Oncology in September 2014, and at the Society of Urologic Oncology meeting in December 2014.
Clinical Decision Studies and Health Economic Benefits of Oncotype DX Prostate Cancer Test
In December 2014, we announced results of the first Oncotype DX prostate cancer test decision impact study, which showed that the use of the test significantly changed urologists’ treatment recommendations across patient risk categories, leading to an overall decrease in treatment intensity and a substantial increase in the number of men for whom active surveillance would be recommended. Additionally, use of the test increased physician confidence in their treatment planning. The complete results have been accepted for publication in Urology Practice, an official journal of the American Urological Association. We also announced results from two studies of the Oncotype DX prostate cancer test demonstrating its value in low- and intermediate-risk prostate cancer to enable physicians and patients to avoid over- and under-treatment of the disease.
Product Development
We developed our Oncotype DX tests generally using the following multi‑phased clinical development program that we are also using to develop future products for breast, colon, prostate and other cancers:
|
· |
Research phase. We conduct studies that are designed to associate genes, pathways or biology with important clinical challenges or endpoints in order to discover biomarkers that will ultimately prove to have clinical utility in oncology. These studies establish technological feasibility so as to determine potential clinical and commercial opportunities. |
|
· |
Development phase. In this phase, we establish a product definition and development plan and perform gene identification either by selecting candidate genes from the approximately 25,000 genes in the human genome or by applying NGS technology to explore both coding and non‑coding regions that could influence tumor biology. Typically, we secure access to archival tumor biopsy samples correlated with clinical data in order to identify genes that correlate with specific clinical outcomes. If early clinical development studies successfully identify genes, we may conduct additional clinical studies to refine the gene set in the specific patient population of interest. We select the final gene panel through statistical modeling of the gene expression and outcome data and considerations of analytical performance. Following establishment of a gene panel, we finalize the remaining assay parameters. |
|
· |
Validation phase. Once the genomic panel, assay chemistry and processes, automation and analysis specifications are finalized, tested and analytically validated, we begin clinical validation. In this phase, we conduct one or more validation studies with prospectively designed endpoints to test our candidate gene panel and the corresponding quantitative expression score. We are often able to conduct large validation studies using archived samples with years of clinical outcomes, thus saving clinical development time. |
|
· |
Clinical utility and product expansion phase. Once a test is commercially available, we may perform additional studies designed to support the test’s clinical utility and to broaden its use in additional patient populations or for additional indications. Clinical utility studies may include a spectrum of studies from retrospective surveys to prospective studies to verify that our test is changing physician behavior so as to determine the impact on patient care and health economics. In addition, further studies may be performed to test a commercial product in new
13 |
patient populations. Finally, through our investigator sponsored trial program, we provide physicians with our tests for use in specific patient populations to be used in treatment decisions. |
Product Development Opportunities
In addition to developing products to address new cancer areas, we continually look to expand the clinical utility and addressable patient populations for our existing tests. These developments efforts may lead to a variety of possible new products covering various treatment decisions, including:
|
· |
Risk assessment; |
|
· |
Screening and prevention; |
|
· |
Early disease diagnosis; |
|
· |
Adjuvant and/or neoadjuvant disease treatment; |
|
· |
Metatastic disease treatment selection; and |
|
· |
Treatment monitoring. |
Potential new products may address a specific clinical need or guide a targeted therapy decision and may also leverage our NGS capabilities to expand our product opportunities. Additionally, potential new products may use non‑invasive tests that can be performed on blood and urine to quantify the presence and burden of cancer, as well as the sensitivity or resistance to specific drug therapies.
Breast Cancer
In breast cancer we have conducted a variety of development studies that could support certain of the opportunities highlighted above. For example, at the May 2009 ASCO meeting, we presented results from a clinical study that summarized the gene signatures of male patients for whom the Oncotype DX breast cancer test was used to guide chemotherapy treatment, indicating that breast cancer in men displays similar gene signatures to female breast cancer. We also presented a separate study at the ASCO meeting demonstrating that there were significant differences in gene expression between hormone receptor negative, or triple negative, breast cancer compared with hormone receptor positive disease.
At the December 2011 SABCS, we presented results of our clinical outcomes study for biomarker discovery using NGS. In addition to re‑confirming the original 21 Oncotype DX breast cancer test genes originally identified by RT‑PCR, this study also revealed more than 1,800 new biological relationships associated with breast cancer recurrence.
At the December 2012 SABCS, we presented results of a large study of early‑stage, node‑positive breast cancer patients treated with anthracycline‑containing chemotherapy as part of the NSABP B‑28 trial supporting the Oncotype DX Recurrence Score as a robust predictor of distant recurrence, disease‑free survival and overall survival in this patient population.
We entered into collaborative agreements with Aventis and ECOG to investigate the ability of gene expression in FPE tissues to predict the likelihood of response to adjuvant chemotherapy, including the taxane Taxotere, in patients with early stage invasive breast cancer and zero to three involved lymph nodes. The agreements provide us with commercial rights to diagnostic tests that may result from the collaboration. Initial study results indicated that a number of candidate genes strongly predicted benefit from treatment with Taxotere in patients with hormone receptor positive disease who had a breast cancer Recurrence Score result indicating intermediate risk of recurrence or above. A genomic classifier predicting differential benefit was identified and, if validated through additional studies, could lead to the development of a test to predict the likelihood of benefit from taxane treatment.
At the December 2013 SABCS, we presented results of a study examining our Oncotype DX breast cancer test and two other commercially available genomic tests, in which we evaluated whether the information those tests provide is equivalent to the Oncotype DX Recurrence Score. Specifically, results indicated more than 44% discordance with the other assays studied when they were compared to Oncotype DX, highlighting the potential of these other tests to misclassify and mistreat patients if they are used to make a decision regarding chemotherapy treatment. Oncotype DX is the only test included
14
in treatment guidelines both for prognosis and for the prediction of chemotherapy benefit and is widely reimbursed by public and private payors for treatment decision making. Two additional studies were presented at the December 2013 SABCS, including one that demonstrated the importance of accurate assessment of ER status to ensure appropriate hormonal treatment, and one that presented the results of a pilot clinical study that demonstrated the feasibility of the large ongoing clinical trial of early endocrine sensitivity prediction by Recurrence Score and conventional parameters in clinical practice.
In November 2013, we entered into an agreement with Almac Diagnostics Limited, or Almac, pursuant to which we obtained an exclusive license to technology and intellectual property to further develop, validate and subsequently commercialize a multi‑gene test to predict benefit from DNA damage‑based chemotherapy drugs, such as the commonly used anthracycline‑based regimens, in breast cancer. We believe such a test would be useful for high‑risk breast cancer patients who are eligible for chemotherapy based on their Oncotype DX Recurrence Score.
Colorectal Cancer
In colon cancer, we have conducted a variety of development studies that could support certain of the opportunities highlighted above. For example, in the NSABP C‑07 clinical trial, which validated the Oncotype DX colon cancer test as a predictor of recurrence in stage III disease, we also performed a gene identification study which analyzed over 700 new genes, and identified 16 genes as being predictive of oxaliplatin benefit for use in patients with stage III disease. In September 2013, we delayed our plan to initiate a validation study in 2013. The decision to delay was based on analytical performance during the pre‑validation phase that did not meet our standards for a subset of the candidate predictive genes.
In 2013, we conducted a clinical validation study to identify the potential use of our Oncotype DX colon cancer test in patients diagnosed with rectal cancer, a cancer that has pathologic features similar to colon cancer, and was diagnosed in approximately 40,000 patients in the United States in 2014. The study, conducted by the Department of Surgery at the Leiden University Medical Centre, evaluated the Oncotype DX colon cancer score and recurrence risk in rectal cancer patients. All 297 patients analyzed in the trial had stage II or III rectal cancer and were treated with surgery alone. The results suggest that the Oncotype DX colon cancer test may help identify high‑risk rectal cancer patients who could benefit from, and low‑risk patients who may forego aggressive therapies.
Prostate Cancer
In August 2014, we announced positive top line results of a second clinical study, demonstrating the ability of our test’s GPS to predict multiple clinical endpoints related to disease aggressiveness among low/intermediate risk patients. The study also confirmed the earlier validation study published in May 2014. The results from the clinical validation study were presented at the European Society for Medical Oncology in September 2014, and at the Society of Urologic Oncology meeting in December 2014.
We plan to continue conducting development studies to provide information to support the relationship of our Oncotype DX prostate cancer test and its benefit with regard to predicting prostate cancer clinical recurrence and biochemical recurrence, as well as its ability to add value for following patients on active surveillance. Also, as with breast and colon cancer, we expect there to be an opportunity in prostate cancer to expand the use of genomic testing to address additional populations. These additional populations may include patients with high risk, based on clinical and pathologic features at the time of diagnosis, the large number of patients with negative biopsies, and patients who receive treatment with radical prostatectomy or radiation who may be considering additional adjuvant therapy with some of the new treatment modalities that are available for advanced disease.
Renal and Other Cancer
In 2014, approximately 50,000 people in the United States and 340,000 people worldwide were diagnosed with renal cancer. In June 2010, we presented results from our first renal gene identification study under our collaboration agreement with Pfizer Inc. for the development of a genomic test to estimate the risk of recurrence following surgery for patients with stage I‑III renal carcinoma, clear cell type that has not spread to other parts of the body. Clear cell renal cell carcinoma, or ccRCC, is the most common type of kidney cancer in adults. The study demonstrated a strong correlation between gene expression and recurrence risk in this patient population. In June 2014, we presented the results of a clinical validation study to predict clinical outcomes in patients with stage I, II and III ccRCC and provide significant information beyond conventional clinical and pathologic characteristics. This panel includes genes from four pathways and may be useful in guiding treatment for specific therapies in the adjuvant setting.
15
Anti‑cancer drugs recently approved by the U.S. Food and Drug Administration, or FDA, and new anti‑cancer drugs in clinical development are designed to provide more targeted treatment, which should improve efficacy and reduce side effects. A need exists to identify those patients who, based on the genomic profile of their tumors, are most likely to benefit from these therapies. We believe genomic analysis has the potential to improve patient selection for these therapies.
Technology
In our Oncotype DX platform we utilize existing technologies, such as RT‑PCR, and information technologies and optimize and integrate them into new processes. We are also incorporating new technologies, such as high‑throughput NGS, in our research and development laboratory. We expect to continue to extend the capabilities of various technologies into proprietary platforms to create new products. Our technology allows us to:
Extract RNA from FPE‑Tumor Biopsies
Our product development process requires that we be able to quantify the relative amounts of RNA in FPE tissue. We have developed proprietary technology, intellectual property and know‑how and are developing new and improved technologies for optimized and automated methods for extraction and analysis of RNA from FPE tissue.
Amplify and Detect Diminished Amounts of RNA Consistently
We currently use RT‑PCR as the basis for our quantitative molecular pathology assays performed in our clinical reference laboratory. This technology uses reverse transcription, RT, coupled to a polymerase chain reaction, or PCR, along with fluorescent detection methods to quantify the relative amount of RNA in a biological specimen. We believe our technology platform has the following advantages:
|
· |
Sensitivity. We have developed protocols for extracting and quantifying RNA utilizing RT‑PCR. Our method for amplifying small fragmented RNA is designed to allow us in the future to conduct studies with hundreds to thousands of genes from 10 micron sections of FPE tissue for our breast and colon cancer tests and significantly smaller tissue samples from the needle biopsy for our prostate cancer test. The ability to amplify RNA allows us to maintain a repository of RNA from limited tissue samples that can be used for later studies. |
|
· |
Specificity. Our RT‑PCR platform is highly specific because it works only when three different test reagents, called DNA primers and probes, independently match each target RNA sequence to be measured. In addition, we have designed and implemented proprietary software for selecting optimal probe and primer sequences in an automated, high‑throughput process. The ability to utilize these sequences allows us to design highly specific assays for closely related sequences. |
|
· |
Precision and Reproducibility. The reagents, materials, instruments and controls in our processes are used by trained personnel following validated standard operating procedures. Validation studies have shown that these standard operating procedures precisely quantify tested RNA with minimal variability in the assay system across days, instruments and operators. This enables our clinical reference laboratory to produce consistently precise and accurate gene expression results. Our quality control methods for our reagents and processes, along with our software for automation, sample tracking, data quality control and statistical analysis, add to the reproducibility and precision of our test. |
|
· |
Dynamic Range. Because our RT‑PCR platform can amplify small amounts of RNA in proportion to the amount present in the sample, we are able to measure RNA levels across as much as a hundred thousand fold range of differing RNA expression. Having a broad range of high resolution testing capability increases the quality of our correlations with clinical outcomes and therefore the predictive power of our tests. |
Analyze Thousands of Biomarkers from Small Amounts of Tissue
The methods and know‑how we have developed allow us to expand RT‑PCR technology to a scale that enables screening of hundreds of genes at a time while using minimal amounts of tissue. With continued investment in miniaturization and automation, we believe that our technology will be capable of continued increases in throughput.
We have developed technologies for assaying low liquid volumes and amplifying trace amounts of RNA in order to develop products that can evaluate minimal amounts of tissue, including breast core biopsies and prostate needle biopsies.
16
We have selected NGS to be our primary technology for future biomarker discovery and begun using NGS for future clinical development in tandem with our existing RT‑PCR based approach. NGS technologies parallelize the sequencing process, producing thousands or millions of sequences at once. These technologies are intended to provide nucleic acid sequence information at lower cost than standard methods. We have created proprietary methods for NGS of FPE tissue nucleic acids, created bioinformatics programs and infrastructure for data storage and analysis, and plan to rely on NGS as the basic source of new biomarker discovery in the future. In December 2011, we announced positive results of our first clinical outcomes study for biomarker discovery using NGS for whole transcriptome expression profiling. The technology allows us to assay the entire transcriptome simultaneously to discover regions of the genome that are turned on or off in disease. From these changes, our researchers are focused on predicting disease outcomes using these comprehensive genomic data sets. The results were successfully generated for all patients using RNA inputs of just 100 nanograms. Additionally, whole transcriptome expression analysis revealed more than 1,800 RNAs associated with breast cancer recurrence risk, many of which belong to gene networks previously unrecognized in their impact on tumor biology.
Our proprietary methods also include the extraction of DNA from FPE tissue and subsequent complete and targeted genome analyses by NGS. We have explored the combination and superimposition of certain whole transcriptome derived RNA information (standardized expression; univariate biomarker direction of association) on genomic information to reveal the genomic landscapes of cancers. This study was reported at the February 2014 Advances in Genome Biology and Technology meeting. We have developed proprietary methods to detect breakpoints in whole transcriptome NGS and in genomic NGS data.
Employing NGS methods we have also demonstrated feasibility for fusion transcript and mutation detection in RNA from FPE tissue samples and copy number aberration and structural variation mutations in DNA from FPE samples.
Employ Advanced Information Technology
We have developed computer programs to automate our RT‑PCR and NGS assay processes. We have also developed and optimized laboratory information management systems to track our gene‑specific reagents, instruments, assay processes and the data generated. Similarly, we have automated data analysis, storage and process quality control. We use statistical methods to optimize and monitor assay performance and to analyze data from our development studies. We are investigating methods to further automate our workflow. In addition, informatics infrastructure investments incorporating a high performance computer cluster, both locally and cloud‑based, to analyze and store large NGS genomic data sets are underway.
Commercial Operations
United States
Our commercial infrastructure, including our sales force, managed care group, and patient support network, is critical to our future success. We are continuing to build a strong domestic sales, marketing and reimbursement effort by interacting directly with medical, radiation, and surgical oncologists, urologists, pathologists and payors. Because oncology and urology are distinct concentrated specialties, we believe that a focused marketing organization and specialized sales force with regional and local experience in the U.S. for each of oncology and urology is necessary in order to effectively serve both specialties. We believe our direct sales approach, targeting oncologists, cancer surgeons and urologists, and our medical education and scientific liaisons, targeting key opinion leaders, coupled with our plans to continue to conduct multiple clinical studies with the objective of having results published in peer‑reviewed journals, is the best approach to increase patient and physician demand and the number of favorable reimbursement coverage decisions by third‑party payors. Due to significant overlap between breast and colon oncologists and surgeons, we believe our current oncology sales force has sufficient capacity to market our Oncotype DX breast and colon cancer tests. In 2014, in connection with the launch of our test for prostate cancer, we expanded our urology sales team in the United States to approximately 30 people to market our prostate cancer test to urologists.
We have a managed care department that works with our contract and reimbursement teams to ensure our tests are being used effectively and appropriately reimbursed. Our call center and patient support network handle benefits investigation, preauthorization, and precertification for patients who use our tests. We have the infrastructure, if needed, to appeal every claim for our tests that is denied by a third‑party payor in order to support the use and encourage adoption of our tests. In addition, we provide patient education through our website, material provided to local advocacy groups, local, national and social media campaigns and materials provided to oncologists, urologists and surgeons.
All Oncotype DX tests are processed in our clinical reference laboratory facility in Redwood City, California. Our current clinical reference laboratory processing capacity is approximately 115,000 tests annually, and has significant expansion
17
capacity with incremental increases in laboratory personnel and equipment. As test processing for our Oncotype DX breast, colon and prostate cancer tests is essentially the same, except that the tests utilize different RNA extraction methods and analyze different genes, we believe that we currently have sufficient capacity to process all of our tests. We may require additional facilities in the future as we expand our business and believe that additional space, when needed, will be available on commercially reasonable terms.
International
We believe our future success is also dependent on our ability to continue to expand our international commercial presence and achieve adequate reimbursement for our tests. We plan to continue to use essentially the same business model internationally as we use in the United States, however, there are significant differences between countries that need to be considered. For example, different countries may have a public healthcare system, a combination of public and private healthcare system or a cash‑based payment system. Treatment costs outside of the United States may be lower, which may impact the cost savings of our tests, and therefore impact the reimbursement amount we can achieve. We have a direct commercial presence with employees and consultants in a number of countries, including Canada, France, Germany, Ireland, Italy, Japan, the Netherlands, Switzerland and the United Kingdom. Additionally, we have exclusive distribution agreements for one or more of our Oncotype DX tests with distributors covering more than 90 countries.
We expect that international sales of our Oncotype DX tests will be heavily dependent on the availability of reimbursement and sample access. In many countries, governments are primarily responsible for reimbursing diagnostic tests. Governments often have significant discretion in determining whether a test will be reimbursed at all, and if so, how much will be paid. In addition, certain countries such as China have prohibitions against exporting tissue samples which will limit our ability to offer our tests in those countries without establishing local laboratory facilities or a method of test delivery which does not require samples to be transported to our U.S. facility.
Coverage, Coding and Reimbursement
Coverage
Medicare coverage for our tests is currently subject to the discretion of the local Medicare Administrative Contractors, or MACs. Palmetto GBA, the MAC that establishes our coverage, coding and reimbursement policies, developed the Molecular Diagnostic Services Program (MolDx) to identify and establish Medicare coverage for molecular diagnostic tests that fall within the scope of its Molecular Diagnostic Test local coverage determination, or LCD. To obtain coverage under the MolDx program, developers of molecular diagnostic tests must submit a detailed dossier of clinical data to substantiate that a test meets Medicare’s requirements for coverage. To date, Palmetto GBA has determined that our breast and colon cancer tests will be covered. Palmetto GBA makes coverage determinations for our tests under the MolDx program; these coverage determinations are adopted by Noridian Healthcare Solutions, the MAC that processes claims submitted by us.
The Protecting Access to Medicare Act of 2014, or PAMA, codified coverage rules for laboratory tests by requiring any local coverage determination to be made following the LCD process. We do not anticipate that the new requirements will meaningfully impact current Medicare coverage policies for our tests.
PAMA also authorizes CMS to consolidate coverage policies for clinical laboratory tests among one to four laboratory-specific MACs. These same contractors may also be designated to process claims if CMS determines that such a model is appropriate. If the MolDx Program is eliminated, or the administrator of the program is changed, it could impact Medicare coverage for our current tests and our ability to obtain Medicare coverage for products for which we do not currently have coverage or any products that we may launch in the future.
State Medicaid programs, which pay for services furnished to the eligible medically indigent, typically make their own decisions with respect to coverage for our tests. Similarly, private payers make their own decisions whether to cover our tests.
Coding
We have received a specific Current Procedural Terminology, or CPT, code for our Oncotype DX breast cancer test. Our other tests, however, are currently billed with an unlisted procedure code. Providers use an unlisted procedure code to bill for a service when no existing specific code accurately describes the service.
Reimbursement
Reimbursement for clinical laboratory tests may come from several sources, including commercial third‑party payors, such as insurance companies and health maintenance organizations, government payors, such as Medicare and Medicaid in the United States, patient self‑pay and, in some cases, from hospitals or referring laboratories who, in turn, may bill third‑party payors for testing.
18
Reimbursement of our tests by third‑party payors is essential to our commercial success. Where there is a payor policy, contract or agreement in place, we bill the third‑party payor, the hospital or referring laboratory as well as the patient (for deductibles and coinsurance or copayments, where applicable) in accordance with established policy, contract or agreement terms. Where there is no payor policy in place, we pursue third-party reimbursement on behalf of each patient on a case‑by‑case basis. Our efforts on behalf of these patients take a substantial amount of time and expense, and bills may not be paid for many months, if at all. Furthermore, if a third‑party payor denies coverage after final appeal, it may take a substantial amount of time to collect from the patient, if we are able to collect at all.
A new CPT code was created for our Oncotype DX breast cancer test effective January 1, 2015. CMS is in the process of establishing a national limitation amount for this new code under the “gapfill” process. Under the gapfill process, the reimbursement rate for the test will be established first by the local MACs in 2015 and then a national limitation amount would be established for 2016. We do not expect the gapfill process to impact our current payment rate for this test.
With regard to Medicare’s current reimbursement of our Oncotype DX colon cancer test, claims are paid at a rate established by the local MAC. Because there is no specific code or national fee schedule rate for our colon cancer test, the payment rate established by our local MAC may be subject to review and adjustment at any time. The MMR subtyping performed by us in connection with our colon cancer test is done according to standard, non‑proprietary immunohistochemistry processes and as such the testing is intended to be billed and reimbursed using established reimbursement codes. These codes changed in 2013, and the associated payment rates were reduced. The codes and payment rates may change again in the future. Additionally, we may not be eligible to bill for this service in some instances due to insurance contracts which require in-network status for billing.
CMS must continue to use the methods for pricing of advanced diagnostic laboratory tests that were in effect prior to enactment of PAMA through December 31, 2016. Under PAMA, laboratories that receive the majority of their Medicare revenues from payments made under the Clinical Laboratory Fee Schedule, or CLFS, or the Physician Fee Schedule will report, beginning January 1, 2016, private payor payment rates and volumes for their tests. CMS will use the rates and volumes reported by laboratories to develop Medicare payment rates for the tests equal to the volume-weighted median of the private payor payment rates for the tests. Laboratories that fail to report the required payment information may be subject to substantial civil money penalties. Rates for “advanced diagnostic laboratory tests” will be reported annually; rates for other diagnostic tests will be reported every three years.
The payment rates calculated under PAMA are expected to apply to our tests beginning January 1, 2017. Any reductions to payment rates (compared to rates established under the CLFS) resulting from the new methodology are limited to 10% per test per year in each of the years 2017 through 2019 and to 15% per test per year in each of 2020 through 2022. Although CMS has not yet issued regulations to implement PAMA, we believe our Oncotype DX tests each would be considered advanced diagnostic laboratory tests. The initial payment rate (for a period not to exceed nine months) for an advanced diagnostic laboratory test will be set at the “actual list charge” for the test as reported by the laboratory. Insofar as the actual list charge substantially exceeds private payor rates (by more than 30%), CMS will have the ability to recoup excess payments made during the initial nine-month payment period.
While we do not believe the new payment rate system under PAMA will have a negative effect on the current payment rates of our Medicare-covered tests beginning in 2017, regulations implementing PAMA have not yet been promulgated. As a result, there can be no assurance that adequate payment rates will continue to be assigned to our tests.
Other, non-PAMA-related policies may affect Medicare reimbursement for our tests. For example, under current Medicare billing rules, payment for Oncotype DX tests performed on Medicare beneficiaries who were hospital inpatients at the time the tumor tissue samples were obtained and whose tests were ordered less than 14 days from discharge is bundled into the payment that the hospital receives for the inpatient services provided. Medicare billing rules also require hospitals to bill for our tests when ordered for hospital outpatients less than 14 days following the date of the hospital procedure where the tumor tissue samples were obtained. Accordingly, we are required to bill individual hospitals for tests performed on Medicare beneficiaries during these time frames. Because we generally do not have a written agreement in place with these hospitals to purchase these tests, we may not be paid for our tests and may have to pursue payment from the hospital on a case‑by‑case basis. We cannot ensure that hospitals will agree to arrangements to pay us for Oncotype DX tests performed on patients falling under these rules. We believe less than 1% of our total claims for the Oncotype DX breast cancer test are subject to these Medicare payment bundling rules.
In 2014, CMS began to bundle payment for clinical laboratory tests together with other services performed during hospital outpatient visits under the Hospital Outpatient Prospective Payment System. While CMS exempted molecular
19
diagnostic tests from this packaging provision (to the extent those tests are not already bundled pursuant to the policy described in the previous paragraph), it is possible that CMS could propose to bundle payment for such tests in the future. Our tests are generally not paid in the hospital outpatient setting, and insofar as they are paid in that setting they likely would be considered molecular tests if billed under specific procedure codes, but it is possible that payment for our tests could be bundled if furnished in a hospital outpatient setting in the future.
On several occasions Congress has considered various cost reduction alternatives, including imposing a 20% co‑insurance amount on clinical laboratory services (which would require beneficiaries to pay a portion of the cost of their clinical laboratory testing). Although these changes have not been enacted at this time, Congress could decide to impose these or other fee reductions or taxes at some point in the future. If so, these additional coinsurance payments for our Oncotype DX tests could be difficult to collect and any new fee reductions or taxes would impact our revenues.
State Medicaid agencies will assign a reimbursement rate equal to or less than the prevailing Medicare rate, often times determined by state law (e.g., a percentage of the Medicare reimbursement rate).
The majority of our international Oncotype DX breast and colon cancer test revenues come from direct payor reimbursement, payments from our distributors, patient self‑pay, and clinical collaborations in various countries. We have obtained coverage for our breast cancer test outside of the United States, including coverage for certain patients in Argentina, Canada, the Czech Republic, Germany, Greece, Ireland, Israel, Saudi Arabia, Spain and the United Kingdom. We expect that it will take several years to establish broad coverage and reimbursement for our Oncotype DX breast, colon and prostate cancer tests with payors in countries outside of the United States.
Oncotype DX Breast Cancer Test
We have focused substantial resources on obtaining reimbursement coverage for our Oncotype DX breast cancer test for invasive breast cancer. We believe the key factors driving adoption of our Oncotype DX breast cancer test include our ongoing commercial efforts, continued publication of peer‑reviewed articles on studies we sponsored, conducted or collaborated on that support the use and reimbursement of our Oncotype DX breast cancer test, clinical presentations at major symposia, and the inclusion of our Oncotype DX breast cancer test in clinical practice guidelines.
Most national and regional third‑party payors in the United States, along with the local MAC for California with jurisdiction for claims submitted by us for Medicare patients, have issued positive coverage determinations for our Oncotype DX breast cancer test for patients with node negative, or N−, estrogen receptor positive, or ER+, invasive disease through contracts, agreements or policy decisions. In addition, the local MAC provides coverage for our breast cancer test for ER+ patients with node positive, or N+, invasive disease (up to three positive lymph nodes). Additionally, some payors provide policy coverage for the use of our test in ER+ patients with N+ disease, including lymph node micro‑metastasis (greater than 0.2 mm, but not greater than 2.0 mm in size). However, we may not be able to obtain reimbursement coverage from other payors for our test for breast cancer patients with N+, ER+ disease.
We have established reimbursement coverage for the use of our Oncotype DX test in DCIS for Medicare patients, as well as limited reimbursement coverage from some private third‑party payors. In many instances our test is covered under existing breast cancer coverage policies with the addition of the indicated diagnosis code for DCIS. We intend to continue to devote resources to gaining expanded private reimbursement for our test in this patient population. We believe it may take several years to achieve reimbursement with a majority of third‑party payors for the use of our test for DCIS patients. However, we cannot predict whether, or under what circumstances, payors will reimburse for this test.
We have established coverage for our Oncotype DX breast cancer test for invasive breast cancer in 28 state Medicaid programs for N− disease. In addition, the Veterans Administration and the Department of Defense hospitals have processes in place that provide coverage for our Oncotype DX test for invasive breast cancer.
Oncotype DX Colon Cancer Test
We expect to continue to pursue global adoption of and reimbursement for our Oncotype DX colon cancer test. We believe the key factors that will drive adoption of this test include publication of peer‑reviewed articles on the QUASAR clinical validation study, published online by the Journal of Clinical Oncology in November 2011, and other studies we sponsored, conducted or collaborated on that support the use of and reimbursement for the test, clinical presentations at major symposia and our ongoing commercial efforts.
20
We are also working with public and private payors and health plans to secure coverage for our Oncotype DX colon cancer test based upon our published and presented results in clinical validation studies and the completed and ongoing studies designed to demonstrate the treatment decision impact of the test in clinical practice. In September 2011, the local carrier with jurisdiction for claims submitted by us for Medicare patients established coverage for our colon cancer test for patients with stage II colon cancer. Additionally, the Veterans Administration, Department of Defense hospitals and a few additional private payors provide coverage and reimbursement. We are beginning to speak with state Medicaid providers regarding coverage and reimbursement for our Oncotype DX colon cancer test. We intend to pursue reimbursement while seeking to obtain formal coverage policies with a substantial number of payors and expect that this test will continue to be reviewed on a case‑by‑case basis until policy decisions have been established. We may need to hire additional commercial, scientific, technical and other personnel to support this process. We believe it may take several years to achieve reimbursement with a majority of third‑party payors for our colon cancer test. However, we cannot predict whether, or under what circumstances, payors will reimburse for this test.
Oncotype DX Prostate Cancer Test
We expect to continue to focus substantial resources on pursuing global adoption of and reimbursement for our Oncotype DX prostate cancer test, which we launched in May 2013. We believe the key factors that will drive adoption of this test include publication of the clinical validation study conducted in collaboration with University of California, San Francisco and other studies we sponsored, conducted or collaborated on that support the use of and reimbursement for the test, clinical presentations at major symposia and our ongoing commercial efforts.
We have not yet obtained reimbursement coverage from third‑party payors for our Oncotype DX prostate cancer test. As a new test, our prostate cancer test may be considered investigational by payors and therefore may not be covered under their reimbursement policies. Consequently, we intend to pursue case‑by‑case reimbursement and expect that this test will continue to be reviewed on this basis until policy decisions have been made by individual payors. We plan to work with public and private payors and health plans to secure coverage for our Oncotype DX prostate cancer test based upon clinical evidence demonstrating the utility of the test. We believe it may take several years to achieve reimbursement with a majority of third‑party payors for our prostate cancer test. However, we cannot predict whether, or under what circumstances, payors will reimburse for this test. We plan to hire additional commercial, scientific, technical and other personnel to support this process.
Competition
We believe that we compete primarily on the basis of:
|
· |
the value of the quantitative information our Oncotype DX platform provides; |
|
· |
the clinical validation of our Oncotype DX breast cancer and DCIS tests’ ability to predict recurrence and demonstrate the test’s ability to predict the likelihood of chemotherapy benefit for invasive and early‑stage breast cancer patients, respectively; |
|
· |
the level of adoption and reimbursement coverage for our tests; |
|
· |
the inclusion of our Oncotype DX invasive breast cancer test in clinical practice guidelines; |
|
· |
the clinical validation of our Oncotype DX colon cancer test’s ability to predict recurrence and survival; |
|
· |
the clinical validation of our Oncotype DX prostate cancer test’s ability to predict the underlying pathology of early stage prostate cancer and reduce over‑treatement; |
|
· |
our ability to perform clinical studies using archival tissue as it is currently processed, handled and stored; |
|
· |
our ability to screen the human genome and cancer genome biomarkers; |
|
· |
our ability to commercialize products through our clinical development platform; |
|
· |
our ability to expand our sales efforts into new areas of medical practice, such as urology, as we launch new products; |
|
· |
our clinical collaborations with clinical study groups; |
21
|
· |
our ability to provide clinical content with sufficient value such that physicians order and payors reimburse for our tests; |
|
· |
the quality of our clinical reference laboratory, which enables consistent, reproducible results; |
|
· |
the level of customer service we provide, both to patients and healthcare professionals; and |
|
· |
our ability to obtain appropriate regulatory approvals in a timely fashion. |
We believe that we compete favorably with respect to these factors, although we cannot assure you that we will be able to continue to do so in the future or that new products that perform better than our Oncotype DX tests will not be introduced. We believe that our continued success depends on our ability to:
|
· |
continue to innovate and maintain scientifically advanced technology; |
|
· |
successfully market and sell our Oncotype DX tests; |
|
· |
enhance our Oncotype DX tests to provide information in response to additional indications; |
|
· |
successfully obtain peer‑reviewed publications of our clinical studies in a timely manner; |
|
· |
continue to validate our tests, especially with respect to treatment benefit; |
|
· |
continue to obtain positive reimbursement decisions from payors; |
|
· |
expand our Oncotype DX platform for use in types of cancer other than breast, colon and prostate; |
|
· |
continue to expand in countries outside of the United States; |
|
· |
attract and retain skilled personnel; |
|
· |
obtain patents or other protection for our products and technology; and |
|
· |
obtain and maintain our clinical reference laboratory accreditations and licenses. |
Historically, our principal competition comes from existing diagnostic methods used by pathologists and oncologists for existing products, however, increasingly inexpensive sequencing platforms, including next generation sequencing and biostatistics tools may change the competitive landscape. Traditional diagnostic methods have been used for many years and are therefore difficult to change or supplement. In addition, companies offering capital equipment and kits or reagents to local pathology laboratories represent another source of potential competition. These kits are used directly by the pathologist, which facilitates adoption more readily than tests like ours that are performed outside the pathology laboratory. In addition, few diagnostic tests are as expensive as our Oncotype DX tests.
We also face competition from companies that offer products or have conducted research to profile genes, gene expression or protein expression in breast, colon or prostate cancer, including public companies such as, GE Healthcare, a business unit of General Electric Company, Hologic, Inc., Myriad Genetics, Inc., NanoString Technologies, Inc., Novartis AG, Qiagen N.V. and Response Genetics, Inc., and many private companies. We face competition from commercial laboratories with strong distribution networks for diagnostic tests, such as Laboratory Corporation of America Holdings and Quest Diagnostics Incorporated. We may also face competition from Illumina, Inc. and Thermo Fisher Scientific Inc., both of which have announced their intention to enter the clinical diagnostics market. Other potential competitors include companies that develop diagnostic tests such as Roche Diagnostics, a division of Roche Holding, Ltd, Siemens AG and Veridex LLC, a Johnson & Johnson company, as well as other companies and academic and research institutions.
In the prostate cancer market, we face comparatively greater competition than in our breast cancer market, including competition from products which were on the market prior to our product launch in May 2013 and which are supported by clinical studies and published data. This existing direct and indirect competition for tests and procedures may make it difficult to gain market share, impact our ability to obtain reimbursement or result in a substantial increase in resources necessary for us to successfully commercialize our Oncotype DX prostate cancer test.
Others may invent and commercialize technology platforms such as next generation sequencing approaches that will compete with our test. Projects related to cancer genomics have received government funding, both in the United States and
22
internationally. As more information regarding cancer genomics becomes available to the public, we anticipate that more products aimed at identifying targeted treatment options will be developed and that these products may compete with ours. In addition, competitors may develop their own versions of our tests in countries where we did not apply for patents or where our patents have not issued and compete with us in those countries, including encouraging the use of their test by physicians or patients in other countries.
Our Oncotype DX tests are considered relatively expensive for diagnostic tests. We have raised the list price of our tests in the past and we may change prices for our tests in the future. Any pricing increases could impact reimbursement of and demand for our tests. Many of our present and potential competitors have widespread brand recognition and substantially greater financial and technical resources and development, production and marketing capabilities than we do. Others may develop lower‑priced, less complex tests that could be viewed by physicians and payors as functionally equivalent to our tests, which could force us to lower the list price of our tests and impact our operating margins and our ability to achieve sustained profitability. Some competitors have developed tests cleared for marketing by the FDA. There may be a marketing differentiation or perception that an FDA‑cleared test is more desirable than Oncotype DX tests, and that may discourage adoption and reimbursement of our tests. If we are unable to compete successfully against current or future competitors, we may be unable to increase market acceptance for and sales of our tests, which could prevent us from increasing or sustaining our revenues or achieving sustained profitability and could cause the market price of our common stock to decline.
Regulation
United States
Clinical Laboratory Improvement Amendments of 1988 (CLIA)
As a clinical reference laboratory, we are required to hold certain federal, state and local licenses, certificates and permits to conduct our business. Under CLIA, we are required to hold a certificate applicable to the types of tests we perform and to comply with standards covering personnel qualifications, facilities administration, quality systems, inspections and proficiency testing.
We have a current Certificate of Accreditation under CLIA to perform high complexity testing and are accredited by the College of American Pathologists, or CAP. To renew our CLIA certificate, we are subject to survey and inspection every two years to assess compliance with program standards and may be subject to additional inspections without prior notice. The standards applicable to the tests we perform may change over time. We cannot assure that we will operate profitably should regulatory compliance requirements become substantially more costly in the future.
If our clinical reference laboratory is out of compliance with CLIA requirements, we may be subject to sanctions such as suspension, limitation or revocation of our CLIA certificate, as well as directed plan of correction, state on‑site monitoring, civil money penalties, civil injunctive suit or criminal penalties. CMS may also cancel our laboratory’s approval to receive Medicare payments if we are found to be out of compliance with CLIA requirements. If we are to be found out of compliance with CLIA program requirements and sanctions are imposed, our business could be harmed.
U.S. Food and Drug Administration (FDA)
Diagnostic test kits that are sold and distributed through interstate commerce are regulated as medical devices by the FDA. Devices subject to FDA regulation must undergo pre‑market review prior to commercialization unless the device is exempt from such review. In addition, manufacturers of medical devices must comply with various regulatory requirements under the Federal Food, Drug and Cosmetic Act and regulations promulgated under that Act, including quality system regulations, unless exempt. Entities that fail to comply with FDA requirements can be liable for criminal or civil penalties, such as recalls, detentions, orders to cease manufacturing and restrictions on labeling and promotion, among other potential sanctions.
Clinical laboratory tests like ours are regulated under CLIA, as administered by CMS, as well as by applicable state laws. Clinical laboratory tests that are developed and validated by a laboratory for its own use, which are referred to as laboratory developed tests, or LDTs, are not currently subject to FDA regulation, although reagents or software provided by third parties and used to perform LDTs may be subject to regulation. We believe that our Oncotype DX tests are not diagnostic kits and also believe that they are LDTs. As a result, we believe our tests should not be subject to regulation at this time under established FDA policies. The container we provide for collection and transport of tumor samples from a pathology laboratory
23
to our clinical reference laboratory may be considered a medical device subject to regulation but is currently exempt from pre‑market review by the FDA.
At various times since 2006, the FDA has issued guidance documents or announced draft guidance regarding initiatives that may require varying levels of FDA oversight of our tests. In October 2014, the FDA issued draft guidance that sets forth a proposed risk-based regulatory framework that would apply varying levels of FDA oversight to LDTs. The FDA has indicated that it does not intend to implement its proposed framework until the draft guidance documents are finalized. It is unclear at this time if or when the draft guidance will be finalized, and even then, the new regulatory requirements are proposed to be phased-in consistent with the schedule set forth in the guidance.
Legislative proposals addressing oversight of genetic testing and LDTs have been introduced in previous Congresses and we expect that new legislative proposals will be introduced from time to time in the future. We cannot provide any assurance that FDA regulation, including pre-market review, will not be required in the future for our tests, whether through finalization of guidance issued by the FDA, new enforcement policies adopted by the FDA or new legislation enacted by Congress. It is possible that legislation will be enacted into law or guidance could be issued by the FDA which may result in increased regulatory burdens for us to continue to offer our tests or to develop and introduce new tests.
We cannot predict the ultimate form of any statutes, regulations or guidance and the potential impact on our existing tests, our tests in development or materials used to perform our tests. If pre‑market review is required, our business could be negatively impacted until such review is completed and clearance or approval is obtained, and the FDA could require that we stop selling our tests pending pre‑market clearance or approval. If our tests are allowed to remain on the market but there is uncertainty about the regulatory status of our tests, if they are labeled investigational by the FDA, or if labeling claims the FDA allows us to make are more limited than the claims we currently make, orders or reimbursement may decline. The regulatory approval process may involve, among other things, successfully completing additional clinical trials and submitting a pre‑market clearance notice or filing a pre-market approval application with the FDA. If pre‑market review is required by the FDA, there can be no assurance that our tests will be cleared or approved on a timely basis, if at all, nor can there be assurance that the labeling claims cleared or approved by the FDA will be consistent with our current claims or adequate to support continued adoption of and reimbursement for our tests. Ongoing compliance with FDA regulations would increase the cost of conducting our business, and subject us to inspection by the FDA and to the regulatory requirements of the FDA, and potentially subject us to penalties for failure to comply with these requirements. We may also decide voluntarily to pursue FDA pre‑market review of our tests if we determine that doing so would be appropriate.
While we qualify all materials used in our tests according to CLIA regulations, we cannot be certain that the FDA will not enact rules or guidance documents which could impact our ability to purchase certain materials necessary for the performance of our tests, such as products labeled for research use only. Should any of the reagents obtained by us from vendors and used in conducting our tests be affected by future regulatory actions, our business could be adversely affected by those actions, including increasing the cost of testing or delaying, limiting or prohibiting the purchase of reagents necessary to perform testing.
Health Insurance Portability and Accountability Act
The federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, required the Department of Health and Human Services, or HHS, to issue regulations to protect the privacy and security of protected health information. HIPAA’s privacy and security requirements are broad in scope and apply to “covered entities,” which include healthcare providers like us who transmit health information in connection with electronic healthcare transactions. In 2009, HIPAA was amended by the Health Information Technology for Economic and Clinical Health Act, or HITECH. The implementing regulations of HIPAA, as amended by HITECH, were last modified in 2013 and resulted in significant changes to the privacy, security, breach notification, and enforcement requirements with which we must comply. Among these changes, covered entities are now vicariously liable for violations of HIPAA that result from acts or omissions of their business associates where the business associate is an agent of the covered entity and was acting within the scope of its agency. Penalties for violations of HIPAA include civil money and criminal penalties.
As a covered entity, we are required to develop and maintain extensive policies and procedures to comply with the HIPAA privacy, security and breach notification requirements. We may not use or disclose protected health information in any form, including electronic, written, or oral, in a way that is not permitted under HIPAA, and we are required to implement security measures to ensure the confidentiality, integrity, and availability of the electronic protected health information that we create, receive, maintain, or transmit. While we have some flexibility in determining which security safeguards are reasonable and appropriate to implement for our operations, it nonetheless requires significant effort and expense to ensure continuing
24
compliance with the HIPAA security rule. Moreover, the requirements under HIPAA’s privacy, security, and breach notification regulations may change periodically and could have an effect on our business operations if compliance becomes substantially more costly than under current requirements. We are also required to comply with the administrative simplification standards under HIPAA when we conduct the electronic transactions regulated by HIPAA, including by using standard code sets and formats and standardized identifiers for health plans and providers.
In addition to HIPPA, a number of state and international laws impose requirements regarding the protection of health or other personal information that are applicable to our operations. Many state laws are not preempted by HIPAA because they are more stringent or are broader in scope than HIPAA. In addition, the United States Department of Commerce, the European Commission and the Federal Data Protection and Information Commissioner of Switzerland have agreed on a set of data protection principles and frequently asked questions, known as the “Safe Harbor Principles,” to enable U.S. companies to satisfy the data protection requirements of the European Union and Switzerland that may apply when the U.S. companies collect personal information from the European Union or Switzerland. The European Commission and Switzerland have also recognized the Safe Harbor Principles as providing adequate data protection.
New laws governing data privacy and security may be adopted in the future as well. We have taken steps to comply with the health information privacy and security requirements to which we are subject. However, we can provide no assurance that we are or will remain in compliance with diverse privacy and security requirements in all of the jurisdictions in which we do business. Failure to comply with privacy and security requirements could result in civil or criminal penalties, which could have a material adverse effect on our business.
Federal and State Physician Self‑Referral Prohibitions
We are subject to the federal physician self‑referral prohibitions, commonly known as the Stark Law. We are also subject to similar restrictions under the self-referral prohibitions of certain states in which we operate. Such state laws are generally interpreted by regulators and the courts in a manner similar to the Stark Law. Together these restrictions generally prohibit us from billing a patient or any governmental or private payor for any test when the physician ordering the test, or any member of such physician’s immediate family, has a financial interest in or compensation arrangement with us, unless the arrangement meets an exception.
For example, under the personal services exception of both the Stark Law and California’s Physician Ownership and Referral Act, or PORA, billing for tests is permitted when the orders for such tests came from physicians whose compensation arrangement with us is for personal services and meets certain written contractual requirements. We have compensation arrangements with a number of physicians for personal services, such as speaking engagements and consulting services. We have structured these arrangements with terms intended to comply with the requirements of the personal services exception of Stark Law and PORA. However, we cannot be certain that regulators would find these arrangements to be in compliance with the safe harbor exceptions of Stark, PORA or similar laws in other states. We would be required to refund any payments we receive pursuant to a referral prohibited by these laws to the patient, the payor or the Medicare program, as applicable.
Sanctions for a violation of the Stark Law include the following:
|
· |
denial of payment for the services provided in violation of the prohibition; |
|
· |
refunds of amounts collected by an entity in violation of the Stark Law; |
|
· |
a civil penalty of up to $15,000 for each service that a person knows or should know was furnished pursuant to a prohibited referral, or for which a timely refund has not been made; |
|
· |
possible exclusion from federal healthcare programs, including Medicare and Medicaid; and |
|
· |
a civil penalty of up to $100,000 against parties that enter into a scheme to circumvent the Stark Law’s prohibition. |
These prohibitions apply regardless of the reasons for the financial relationship and the referral. No finding of intent to violate the Stark Law is required for a violation. In addition, knowing violations of the Stark Law may also serve as the basis for liability under the Federal False Claims Act, which prohibits the knowing presentation of a false, fictitious or fraudulent claim for payment to the U.S. Government or knowingly retaining an overpayment from the U.S. Government.
25
Further, a violation of the self-referral prohibitions of states in which we operate could lead to additional liability. For example, a violation of PORA is a misdemeanor and could result in civil penalties and criminal fines. While we have attempted to comply with the Stark Law, PORA and similar laws of other states, it is possible that our claims for tests ordered by physicians with whom we have a financial relationship could be subject to regulatory scrutiny at some point in the future, and we cannot provide assurance that we will be found to be in compliance following any such regulatory review.
Federal and State Anti‑Kickback Laws
The Federal Anti‑kickback Law makes it a felony for a provider or supplier, including a laboratory, to knowingly and willfully offer, pay, solicit or receive remuneration, directly or indirectly, in order to induce business that is reimbursable under any federal health care program. A violation of the Anti‑kickback Law may result in penalties including imprisonment for up to five years and fines of up to $250,000 in the case of individuals and $500,000 in the case of organizations. Convictions under the Anti‑kickback Law result in mandatory exclusion from federal health care programs for a minimum of five years. In addition, HHS has the authority to impose civil assessments and fines and to exclude health care providers and others engaged in prohibited activities from Medicare, Medicaid and other federal health care programs. Actions that violate the Anti‑kickback Law or similar laws may also involve liability under the Federal False Claims Act.
Although the Anti‑kickback Law applies only to federal health care programs, a number of states in which we operate have passed statutes substantially similar to the Anti‑kickback Law pursuant to which similar types of prohibitions are made applicable to all other health plans and third‑party payors. For example, both California’s fee‑splitting statute, Business and Professions Code Section 650, and its Medi‑Cal anti‑kickback statute, Welfare and Institutions Code Section 14107.2, have been interpreted by the California Attorney General and California courts in substantially the same way that HHS and the courts have interpreted the Anti‑kickback Law. A violation of Section 650 is punishable by imprisonment and fines of up to $50,000. A violation of Section 14107.2 is punishable by imprisonment and fines of up to $10,000.
Federal and state law enforcement authorities scrutinize arrangements between health care providers and potential referral sources to ensure that the arrangements are not designed as a mechanism to induce patient care referrals and opportunities. Law enforcement authorities, the courts and Congress have also demonstrated a willingness to look behind the formalities of a transaction to determine the underlying purpose of payments between health care providers and actual or potential referral sources. Generally, courts have taken a broad interpretation of the scope of the Anti‑kickback Law, holding that the statute may be violated if merely one purpose of a payment arrangement is to induce future referrals.
In addition to statutory exceptions to the Anti‑kickback Law, regulations provide for a number of safe harbors to the law. If an arrangement meets the provisions of a safe harbor, it is deemed not to violate the Anti‑kickback Law. An arrangement must fully comply with each element of an applicable safe harbor in order to qualify for protection. However, failure to meet the terms of the safe harbor does not render an arrangement illegal. Rather, the arrangement must be evaluated under the language of the statute, taking into account all facts and circumstances.
Among the Anti‑kickback Law safe harbors that may be relevant to us is the discount safe harbor. The discount safe harbor potentially applies to discounts provided by providers and suppliers, including laboratories, to physicians or institutions where the physician or institution bills the payor for the test, not when the laboratory bills the payor directly. If the terms of the discount safe harbor are met, the discounts will not be considered prohibited remuneration under the Anti‑kickback Law. This safe harbor may therefore be potentially applicable to our agreements to sell tests to hospitals where the hospital submits a claim to the payor.
Another safe harbor to the Anti-kickback Law that may be relevant to us is the personal services safe harbor. This safe harbor provides that remuneration paid to a referral source for personal services will be deemed not to violate the Anti‑kickback Law provided all of the elements of that safe harbor are met. One element is that, if the agreement is intended to provide for the services of the physician on a periodic, sporadic or part‑time basis, rather than on a full‑time basis for the term of the agreement, the agreement specifies exactly the schedule of such intervals, their precise length, and the exact charge for such intervals. Our personal services arrangements with some physicians do not meet the specific requirement of this safe harbor that the agreement specify exactly the schedule of the intervals of time to be spent on the services because the nature of the services, such as speaking engagements, does not lend itself to exact scheduling and therefore meeting this element of the personal services safe harbor is impractical. However, as noted above, failure to meet the terms of the safe harbor does not render an arrangement illegal, as such arrangements are evaluated under the language of the statute, taking into account all facts and circumstances.
26
Many state anti-kickback statutes have analogous exceptions or safe harbors to those of the Anti-kickback Law. As noted above, these state anti-kickback statutes have generally been interpreted consistently with the Anti‑kickback Law.
While we believe that we are in compliance with the Anti‑kickback Law and similar anti-kickback statutes in the states in which we operate, there can be no assurance that our relationships with physicians, hospitals and other customers will not be subject to investigation or a successful challenge under such laws. If imposed for any reason, sanctions under these laws could have a negative effect on our business.
Many other countries in which we offer our tests also have anti‑kickback regulations, which are discussed below.
Other Federal and State Fraud and Abuse Laws
In addition to the requirements that are discussed above, there are several other health care fraud and abuse laws that could have an impact on our business. For example, provisions of the Social Security Act permit Medicare and Medicaid to exclude an entity that charges the federal health care programs substantially in excess of its usual charges for its services. The terms “usual charge” and “substantially in excess” are ambiguous and subject to varying interpretations.
Further, as stated above, the Federal False Claims Act prohibits a person from knowingly submitting a claim, making a false record or statement in order to secure payment or retaining an overpayment by the federal government. In addition to actions initiated by the government itself, the statute authorizes actions to be brought on behalf of the federal government by a private party having knowledge of the alleged fraud. These lawsuits are known as qui tam or whistle blower lawsuits. Because complaints related to such actions are initially filed under seal, the action may be pending for some time before the defendant is even aware of the action. If the government is ultimately successful in obtaining redress in the matter or if the plaintiff succeeds in obtaining redress without the government’s involvement, then the whistle blower plaintiff will receive a percentage of the recovery.
Finally, the Social Security Act includes its own provisions that prohibit the filing of false claims or submitting false statements in order to obtain payment. Violation of these provisions may result in fines, imprisonment or both, and possible exclusion from Medicare or Medicaid programs. California has an analogous state false claims act applicable to all payors, as do many other states.
California Laboratory Licensing
In addition to federal certification requirements for laboratories under CLIA, California law requires us to maintain a license to conduct testing in the state. Such laws establish standards for the day‑to‑day operation of our clinical reference laboratory, including the training and skills required of personnel and quality control. In addition, California laws require us to participate in a state-approved proficiency testing program, which involves testing of known specimens to verify the accuracy and reliability of our laboratory’s tests.
If our clinical reference laboratory is out of compliance with California standards, the California Department of Public Health, or DPH, may suspend, restrict or revoke our license to operate our clinical reference laboratory, assess substantial civil money penalties, or impose specific corrective action plans, among other potential penalties. If imposed, any such penalties could materially affect our business. We maintain a current license in good standing with DPH. However, we cannot provide assurance that DPH will at all times in the future find us to be in compliance with all applicable laws.
New York Laboratory Licensing
Because we accept specimens from New York State, our clinical reference laboratory is required to be licensed by New York and otherwise comply with New York laws and regulations. New York laws and regulations establish standards for:
|
· |
day‑to‑day operation of a clinical laboratory, including training and skill levels required of laboratory personnel; |
|
· |
physical requirements of the laboratory facility; |
|
· |
equipment; and |
|
· |
quality control. |
27
We maintain New York licensure for our clinical reference laboratory and test-specific licensure for our Oncotype DX tests. New York also mandates proficiency testing for laboratories licensed by the state. If a laboratory is out of compliance with New York statutory or regulatory standards, the New York State Department of Health, or DOH, may suspend, limit, revoke or annul the laboratory’s New York license, censure the holder of the license or assess civil money penalties, among other potential penalties. Should we be found out of compliance with New York laboratory requirements, we could be subject to such sanctions, which could harm our business. We maintain a current license in good standing with DOH. However, we cannot provide assurance that DOH will at all times find us to be in compliance with all such laws.
Other States’ Laboratory Licensing
Maryland, Pennsylvania and Rhode Island require out‑of‑state laboratories that accept specimens from those states to obtain a license. Florida prohibits a clinical laboratory from sending a specimen taken in the state to an out-of-state laboratory unless that laboratory obtains a Florida license. We have obtained licenses in these four states and believe we are in compliance with applicable laws.
From time to time, we may become aware of other states that require out‑of‑state laboratories to obtain a license in order to accept specimens from the state, and it is possible that other states already have such requirements or will have such requirements in the future. If we identify any other state with such requirements or if we are contacted by any other state advising us of such requirements, we intend to follow instructions from the state regulators as to how we should comply with such requirements.
Environmental Laws
We are subject to regulation under federal, state and local laws and regulations governing environmental protection and the use, storage, handling and disposal of hazardous substances. The cost of complying with these laws and regulations may be significant. Our activities currently require the controlled use of potentially harmful biological materials, hazardous materials and chemicals. We cannot eliminate the risk of accidental contamination or injury to employees or third parties from the use, storage, handling or disposal of these materials. In the event of contamination or injury, we could be held liable for any resulting damages, and any liability could exceed our resources or any applicable insurance coverage we may have.
International
Many countries in which we offer our tests in have anti‑kickback regulations prohibiting providers from offering, paying, soliciting or receiving remuneration, directly or indirectly, in order to induce business that is reimbursable under any national health care program. In situations involving physicians employed by state‑funded institutions or national health care agencies, violation of the local anti‑kickback law may also constitute a violation of the U.S. Foreign Corrupt Practices Act, or FCPA.
The FCPA prohibits any U.S. individual, business entity or employee of a U.S. business entity to offer or provide, directly or through a third party, including the distributors we rely on in certain markets, anything of value to a foreign government official with corrupt intent to influence an award or continuation of business or to gain an unfair advantage, whether or not such conduct violate local laws. In addition, it is illegal for a company that reports to the SEC to have false or inaccurate books or records or to fail to maintain a system of internal accounting controls. We are also required to maintain accurate information and control over sales and distributors’ activities that may fall within the purview of the FCPA, its books and records provisions and its anti‑bribery provisions.
The standard of intent and knowledge in the Anti‑Bribery cases is minimal—intent and knowledge are usually inferred from that fact that bribery took place. The accounting provisions do not require intent. Violations of the FCPA’s anti‑bribery provisions for corporations and other business entities are subject to a fine of up to $2 million and officers, directors, stockholders, employees, and agents are subject to a fine of up to $100,000 and imprisonment for up to five years. Other countries, including the United Kingdom and other OECD Anti‑Bribery Convention members, have similar anti‑corruption regulations, such as the United Kingdom Bribery Act.
When marketing our tests outside of the United States, we are subject to foreign regulatory requirements governing human clinical testing, export of tissue and marketing approval for our products. These requirements vary by jurisdiction, differ from those in the United States and may require us to perform additional pre‑clinical or clinical testing. In many countries outside of the United States, coverage, pricing and reimbursement approvals are also required.
28
Patents and Proprietary Technology
In order to remain competitive, we must develop and maintain protection on the proprietary aspects of our technologies. To that end, we rely on a combination of patents, patent applications, copyrights and trademarks, as well as contracts, such as confidentiality, material data transfer, license and invention assignment agreements. We also rely upon trade secret laws to protect unpatented know‑how and continuing technological innovation. In addition, we have what we consider to be reasonable security measures in place to maintain confidentiality. Our intellectual property strategy is intended to develop and maintain our competitive position.
As of December 31, 2014, we had 39 issued patents in the United States and 85 issued patents outside of the United States covering genes and methods that are components of the Oncotype DX breast, colon and prostate cancer tests or research methods and platform technologies. For patents issued by the European Patent Office, we have validated each patent in key European Union countries. In addition, we have a number of pending patent applications in the United States and in other countries, including provisional and non‑provisional filings. Our issued U.S. patents expire at various times between 2023 and 2032. Some of these U.S. patent applications also have corresponding pending or granted applications under the Patent Cooperation Treaty in Canada, Europe, Japan, Australia and other jurisdictions. In these patent applications, we have either sole or joint ownership positions. In certain cases where joint ownership positions were created, we have negotiated contractual provisions providing us with the opportunity to acquire exclusive rights under the patent applications. Under some patent applications, we have elected to allow exclusive options to lapse without exercising the option. The joint ownership agreements generally are in the form of material data transfer agreements that were executed at the onset of our collaborations with third parties.
Our patent applications relate to two main areas: gene expression and sequencing technology methods, and gene biomarkers and methods for predicting cancer recurrence and drug response in certain forms of cancer. We intend to file additional patent applications to strengthen our intellectual property rights. Our pending and future patent applications may not result in issued patents, and we cannot assure you that our issued patents or any patents that might ultimately be issued by the U.S. Patent and Trademark Office, or USPTO, will protect our technology. Any patents issued to United States might be challenged by third parties as being invalid or unenforceable, or third parties may independently develop similar or competing technology that avoids our patents. We cannot be certain that the steps we have taken will prevent the misappropriation of our intellectual property, particularly in foreign countries where the laws may not protect our proprietary rights as fully as in the United States.
We have in the past, and may in the future, receive notices of claims of infringement and misappropriation or misuse of other parties’ proprietary rights and may from time to time receive additional notices. Some of these claims may lead to litigation. We cannot assure you that we will prevail in these actions, or that other actions alleging misappropriation or misuse by us of third‑party trade secrets, alleging infringement by us of third‑party patents and trademarks or challenging the validity of patents issued to us in the future, will not be asserted or prosecuted against us. We may also initiate claims to defend our intellectual property. Assertions of misappropriation, infringement or misuse, or actions seeking to establish the validity of our patents could materially or adversely affect our business, financial condition and results of operations.
An adverse determination in litigation or interference proceedings to which we may become a party relating to any patents issued to us in the future, or any patents owned by third parties, could subject us to significant liabilities to third parties or require us to seek licenses from third parties. Furthermore, if we are found to willfully infringe these patents, we could, in addition to other penalties, be required to pay treble damages. Although patent and intellectual property disputes in this area have often been settled through licensing or similar arrangements, costs associated with such arrangements may be substantial and could include ongoing royalties. We may be unable to obtain necessary licenses on satisfactory or commercially feasible terms, if at all. If we do not obtain necessary licenses, we may not be able to redesign our Oncotype DX tests or other tests to avoid infringement, or such redesign may take considerable time, and force us to reassess our business plans. Adverse determinations in a judicial or administrative proceeding or failure to obtain necessary licenses could prevent us from manufacturing and selling our tests, which could have a significant adverse impact on our business.
All employees and technical consultants working for us are required to execute confidentiality agreements in connection with their employment and consulting relationships with us. Confidentiality agreements provide that all confidential information developed or made known to others during the course of the employment, consulting or business relationship shall be kept confidential except in specified circumstances. In addition, agreements with employees provide that all inventions conceived by the individual while employed by us are our exclusive property. We cannot provide any assurance that employees and consultants will abide by the confidentiality or assignment terms of these agreements. Despite measures taken to protect our intellectual property, unauthorized parties might copy aspects of our technology or obtain and use information that we regard as proprietary.
29
Roche License Agreement
We have non-exclusively licensed a number of U.S. patents claiming nucleic acid amplification processes known as PCR, homogeneous polymerase chain reaction, and RT‑PCR from Roche Molecular Systems, Inc. We use these processes in our research and development activities and in the processing of our Oncotype DX tests. The Roche license is limited to clinical laboratory services performed within the United States and Puerto Rico, and does not include the right to make or sell products using the patented processes. The license continues as long as the underlying patent rights are in effect, but is subject to early termination by Roche under the following circumstances:
|
· |
a change in our ownership; |
|
· |
a declaration of bankruptcy or insolvency, the making of an assignment for the benefit of our creditors, having a receiver appointed, or losing the federal or state licenses necessary for our operation; |
|
· |
a change in our status to a non‑profit entity or government institution; or |
|
· |
our breach of or default under a material term of the license. |
If the Roche license is terminated, we will be unable to use the licensed processes to conduct research and development activities or to perform our tests. As payment for the licenses granted to us, we make royalty payments to Roche consisting of a specified percentage of our net product revenues.
Research and Development Expenses
Research and development expenses were $56.1 million, $66.3 million and $49.1 million for the years ended December 31, 2014, 2013, and 2012, respectively. During 2013, we made a $9.0 million up‑front payment under an exclusive licensing agreement to technology and intellectual property to further develop, validate and subsequently commercialize a multi‑gene test to predict benefit from DNA damage‑based chemotherapy drugs, such as the commonly used anthracycline‑based regimens, in breast cancer. We also continued to conduct research and development studies in breast, colon, prostate and other cancers, including proprietary platforms that incorporate emerging molecular technologies to develop non‑invasive tests that can be performed on blood or urine.
Employees
As of December 31, 2014, we had 752 employees, including 144 in clinical reference laboratory operations, 140 in research and development, including bioinformatics, 294 in sales and marketing, 92 in information technology and systems and 82 in general and administrative functions. None of our employees are covered by collective bargaining arrangements, and our management considers its relationships with employees to be good.
Available Information
We were incorporated in Delaware in August 2000, and our website is located at www.genomichealth.com. We make available free of charge on our website our annual reports on Form 10‑K, quarterly reports on Form 10‑Q, current reports on Form 8‑K and amendments to those reports, as soon as reasonably practicable after we electronically file or furnish such materials to the Securities and Exchange Commission. Our website and the information contained therein or connected thereto are not intended to be incorporated into this Annual Report on Form 10‑K.
We have a history of net losses, we may incur net losses in the future, and we expect to continue to incur significant expenses to develop and market our tests, which may make it difficult for us to achieve sustained profitability.
We have historically incurred substantial net losses. From our inception in August 2000 through December 31, 2014, we had an accumulated deficit of $194.9 million. We expect to continue to invest in our product pipeline, including our current Oncotype DX tests and future products, and in our global commercial infrastructure, our laboratory operations and NGS and other technology. For the year ended December 31, 2014, our research and development expenses were $56.1 million and our selling and marketing expenses were $134.9 million. We expect our expense levels to continue to increase for the foreseeable future as we seek to globally expand the clinical utility of our Oncotype DX breast cancer test, drive adoption of and reimbursement for our Oncotype DX colon cancer and prostate cancer tests and develop and commercialize new tests, including tests based on blood or urine specimens. As a result, we will need to generate significant growth in revenues in order to achieve
30
sustained profitability. Our failure to achieve sustained profitability in the future could cause the market price of our common stock to decline.
Continued weak general economic or business conditions could have a negative impact on our business.
Continuing concerns over prolonged high unemployment levels, entitlement and healthcare reform efforts, regulatory changes and taxation issues, and geopolitical issues have contributed to continued volatility and uncertain expectations for both the U.S. and global economies. These factors, combined with uncertainties in business and consumer confidence, continued concerns regarding the stability of some European Union member countries, and slowing economic growth in China, have contributed to the expectations of slower domestic and global economic growth in the near term. These economic conditions continued to impact product payment cycles, growth in tests delivered and product revenues generated during the year ended December 31, 2014. If the economic environment does not improve or deteriorates, our business, including our patient population, our suppliers and our third‑party payors, could be negatively affected, resulting in a negative impact on our product revenues.
Healthcare policy changes, including recently enacted legislation reforming the U.S. healthcare system, may have a material adverse effect on our financial condition and results of operations.
The Patient Protection and Affordable Care Act, as amended by the Health Care and Education Affordability Reconciliation Act, collectively, the Affordable Care Act, or ACA, enacted in March 2010, makes changes that are expected to significantly impact the pharmaceutical and medical device industries and clinical laboratories. For example, beginning in 2013, each medical device manufacturer must pay sales tax in an amount equal to 2.3% of the price for which such manufacturer sells its medical devices that are listed with the FDA. Although the FDA has issued draft guidance that, if finalized, would regulate certain LDTs as medical devices, none of our LDTs, such as our Oncotype DX breast, colon and prostate cancer tests, are currently listed with the FDA. We cannot assure you that the tax will not apply to services such as ours in the future.
Other significant measures contained in the ACA include, for example, coordination and promotion of research on comparative clinical effectiveness of different technologies and procedures, initiatives to revise Medicare payment methodologies, such as bundling of payments across the continuum of care by providers and physicians, and initiatives to promote quality indicators in payment methodologies. The ACA also includes significant new fraud and abuse measures, including required disclosures of financial arrangements with physician customers, lower thresholds for violations and increasing potential penalties for such violations. In addition, the ACA establishes an Independent Payment Advisory Board, or IPAB, to reduce the per capita rate of growth in Medicare spending if expenditures exceed certain targets. At this point, the triggers for IPAB proposals have not been met; it is unclear when such triggers may be made met in the future and when any IPAB-proposed reductions to payments could take effect. In addition to the ACA, various healthcare reform proposals have also emerged from federal and state governments. We are monitoring the impact of the ACA and these healthcare reform proposals in order to enable us to determine the trends and changes that may potentially impact our business over time.
Under the Budget Control Act of 2011, which went into effect for dates of service on or after April 1, 2013, Medicare payments, including payments to clinical laboratories, are subject to a 2% reduction due to implementation of the automatic expense reductions (sequester). Reductions made by the Congressional sequester are applied to total claims payment made. The sequester reductions do not result in a rebasing of the negotiated or established Medicare or Medicaid reimbursement rates.
State legislation on reimbursement applies to Medicaid reimbursement and Managed Medicaid reimbursement rates within that state. Some states have passed or proposed legislation that would revise reimbursement methodology for clinical laboratory payment rates under those Medicaid programs. In October 2011, CMS approved California’s plan to reduce certain Medi‑Cal payments by 10% retroactive to June 1, 2011. In February 2012, Medi‑Cal began the recoupment process by sporadically adjusting payments on new claims. According to the California Department of Health Care Services, the cut applies to various healthcare providers and outpatient services including laboratory services with certain exceptions. State legislation requires the Department of Health Care Services to develop a new rate-setting methodology for clinical laboratories and laboratory services that is based on the average of the lowest prices other third-party payers are paying for similar services, and to implement an additional 10% reduction to payments for clinical laboratory or laboratory services retroactive to July 1, 2012 with the legislation mandating that these reductions continue until the new rate methodology has been approved by CMS. The Department of Health Care Services has developed the new rate methodology, which involves the use of the range of rates that fell between zero and 80% of the calculated California Medicare rate and the calculation of a weighted average (based on units billed) of such rates, and is targeting a July 1, 2015 effective date for such methodology.
31
Although recent changes to reimbursement methodology in states outside of California have not materially changed the payment rate for our tests, we cannot be certain that these or future changes will not affect payment rates in the future. We also cannot predict whether future healthcare initiatives will be implemented at the federal or state level or in countries outside of the United States in which we may do business, or the effect any future legislation or regulation will have on us. The taxes imposed by new legislation, cost reduction measures and the expansion in government’s role in the U.S. healthcare industry may result in decreased profits to us, lower reimbursements by payors for our products or reduced medical procedure volumes, all of which may adversely affect our business, financial condition and results of operations. In addition, sales of our tests outside the United States make us subject to foreign regulatory requirements and cost‑reduction measures, which may also change over time.
If the FDA were to begin regulating our tests, we could incur substantial costs and time delays associated with meeting requirements for pre‑market clearance or approval or we could experience decreased demand for or reimbursement of our tests.
Clinical laboratory tests like ours are regulated under the Clinical Laboratory Improvement Amendments of 1988, or CLIA, as well as by applicable state laws. Diagnostic kits that are sold and distributed through interstate commerce are regulated as medical devices by the FDA. Most LDTs are not currently subject to FDA regulation, although reagents or software provided by third parties and used to perform LDTs may be subject to regulation. We believe that our Oncotype DX tests are not diagnostic kits and also believe that they are LDTs. As a result, we believe our tests should not be subject to regulation at this time under established FDA policies. The container we provide for collection and transport of tumor samples from a pathology laboratory to our clinical reference laboratory may be a medical device subject to FDA regulation but is currently exempt from pre‑market review by the FDA.
At various times since 2006, the FDA has issued guidance documents or announced draft guidance regarding initiatives that may require varying levels of FDA oversight of our tests. In October 2014, the FDA issued draft guidance that sets forth a proposed risk-based regulatory framework that would apply varying levels of FDA oversight to LDTs. The FDA has indicated that it does not intend to implement its proposed framework until the draft guidance documents are finalized. It is unclear at this time if or when the draft guidance will be finalized, and even then, the new regulatory requirements are proposed to be phased-in consistent with the schedule set forth in the guidance. If this draft guidance is finalized as presently written, it includes an oversight framework that would require pre-market review for high and moderate risk LDTs.
Legislative proposals addressing oversight of genetic testing and LDTs have been introduced in previous Congresses and we expect that new legislative proposals will be introduced from time to time in the future. We cannot provide any assurance that FDA regulation, including pre-market review, will not be required in the future for our tests, whether through finalization of guidance issued by the FDA, new enforcement policies adopted by the FDA or new legislation enacted by Congress. It is possible that legislation will be enacted into law or guidance could be issued by the FDA which may result in increased regulatory burdens for us to continue to offer our tests or to develop and introduce new tests.
If pre‑market review is required, our business could be negatively impacted until such review is completed and clearance or approval is obtained, and the FDA could require that we stop selling our tests pending pre‑market clearance or approval. If our tests are allowed to remain on the market but there is uncertainty about the regulatory status of our tests, if they are labeled investigational by the FDA, or if labeling claims the FDA allows us to make are more limited than the claims we currently make, orders or reimbursement may decline. The regulatory approval process may involve, among other things, successfully completing additional clinical trials and submitting a pre‑market clearance notice or filing a pre‑market approval application with the FDA. If pre‑market review is required by the FDA, there can be no assurance that our tests will be cleared or approved on a timely basis, if at all, nor can there be assurance that the labeling claims cleared or approved by the FDA will be consistent with our current claims or adequate to support continued adoption of and reimbursement for our tests. Ongoing compliance with FDA regulations would increase the cost of conducting our business, and subject us to inspection by and the regulatory requirements of the FDA, for example registration and listing and medical device reporting, and penalties for failure to comply with these requirements. We may also decide voluntarily to pursue FDA pre‑market review of our tests if we determine that doing so would be appropriate.
We cannot predict the ultimate timing or form of final FDA guidance or regulations addressing LDTs and the potential impact on our existing tests, our tests in development or the materials used to perform our tests. While we qualify all materials used in our tests according to CLIA regulations, we cannot be certain that the FDA will not enact rules or guidance documents which could impact our ability to purchase certain materials necessary for the performance of our tests, such as products labeled for research use only. Should any of the reagents obtained by us from suppliers and used in conducting our tests be affected by future regulatory actions, our business could be adversely affected by those actions, including increasing the cost of testing or delaying, limiting or prohibiting the purchase of reagents necessary to perform testing.
32
If we were required to conduct additional clinical trials prior to continuing to sell our breast, colon and prostate cancer tests or launching any other tests we may develop, those trials could result in delays or failure to obtain necessary regulatory approvals, which could harm our business.
If the FDA decides to regulate our tests, it may require additional pre- market clinical testing before clearing or approving such tests for commercial sales. Such pre-market clinical testing could delay the commencement or completion of clinical testing, significantly increase our test development costs, delay commercialization of any future tests, and interrupt sales of our current tests. Many of the factors that may cause or lead to a delay in the commencement or completion of clinical trials may also ultimately lead to delay or denial of regulatory clearance or approval. The commencement of clinical trials may be delayed due to insufficient patient enrollment, which is a function of many factors, including the size of the patient population, the nature of the protocol, the proximity of patients to clinical sites and the eligibility criteria for the clinical trial.
We may find it necessary to engage contract research organizations to perform data collection and analysis and other aspects of our clinical trials, which might increase the cost and complexity of our trials. We may also depend on clinical investigators, medical institutions and contract research organizations to perform the trials. If these parties do not successfully carry out their contractual duties or obligations or meet expected deadlines, or if the quality, completeness or accuracy of the clinical data they obtain is compromised due to the failure to adhere to our clinical protocols or for other reasons, our clinical trials may have to be extended, delayed or terminated. Many of these factors would be beyond our control. We may not be able to enter into replacement arrangements without undue delays or considerable expenditures. If there are delays in testing or approvals as a result of the failure to perform by third parties, our research and development costs would increase, and we may not be able to obtain regulatory clearance or approval for our tests. In addition, we may not be able to establish or maintain relationships with these parties on favorable terms, if at all. Each of these outcomes would harm our ability to market our tests, or to achieve sustained profitability.
If third‑party payors, including managed care organizations and Medicare, do not provide reimbursement, breach, rescind or modify their contracts or reimbursement policies or delay payments for our Oncotype DX tests, or we are unable to successfully renegotiate reimbursement contracts, our commercial success could be compromised.
Physicians and patients may not order our Oncotype DX tests unless third‑party payors, such as managed care organizations as well as government payors such as Medicare and Medicaid and governmental payors outside of the United States, pay a substantial portion of the test price. Reimbursement by a payor may depend on a number of factors, including a payor’s determination that tests using our technologies are:
|
· |
not experimental or investigational, |
|
· |
medically necessary, |
|
· |
appropriate for the specific patient, |
|
· |
cost‑effective, |
|
· |
supported by peer‑reviewed publications, and |
|
· |
included in clinical practice guidelines. |
There is uncertainty concerning third‑party payor reimbursement of any test incorporating new technology, including tests developed using our Oncotype DX platform. Several entities conduct technology assessments of new medical tests and devices and provide the results of their assessments for informational purposes to other parties. These assessments may be used by third‑party payors and health care providers as grounds to deny coverage for a test or procedure. Although there are a number of favorable assessments of our Oncotype DX breast cancer test, the test has received negative assessments in the past and our tests may receive negative assessments in the future. For example, in November 2010, the Medical Advisory Panel of the Blue Cross and Blue Shield Association’s Technology Evaluation Center, a technology assessment group, published its conclusion that the existing clinical data in support of our Oncotype DX breast cancer test did not meet the panel’s technology criteria for clinical effectiveness and appropriateness for usage in patients with N+ disease.
Since each payor makes its own decision as to whether to establish a policy to reimburse our test, seeking these approvals is a time‑consuming and costly process. To date, we have positive coverage determinations for our Oncotype DX
33
breast cancer test for N‑, ER+ patients from most third‑party payors in the United States through contracts, agreements or policy decisions. We cannot be certain that coverage for this test will be provided in the future by additional third‑party payors or that existing contracts, agreements or policy decisions or reimbursement levels, including tests processed as out of network, will remain in place or be fulfilled within existing terms and provisions. From time to time payors change processes that may affect timely payment. These changes may result in uneven cash flow or impact the timing of revenue recognized with these payors.
We have obtained limited reimbursement from private third‑party payors in the United States for our Oncotype DX colon cancer test and for our Oncotype DX breast cancer test for N+ and DCIS patients. Until further clinical data is presented, our N+ and DCIS indication for our breast cancer test and our colon cancer test may be considered investigational by payors and therefore may not be covered under their reimbursement policies.
We believe it may take several years to achieve reimbursement with a majority of third‑party payors for these tests. In addition, the launch of our test for prostate cancer in May 2013 requires that we expend substantial time and resources in order to drive adoption of and reimbursement for this test.
We may not be able to obtain Medicare reimbursement coverage for our prostate cancer test, or obtain third‑party payor reimbursement for patients with colon or prostate cancer or with N+ and DCIS breast cancer patients that is similar to the coverage we have obtained for our invasive breast cancer test for N-, ER+ patients. If we fail to establish broad adoption of and reimbursement for these tests and any future tests we may develop, our reputation could be harmed and our future prospects and our business could suffer.
If we are unable to obtain or maintain reimbursement from private payors, such as the Blue Cross/Blue Shield family, and public payors, such as Medicare and Medicaid programs, for our existing tests or new tests or test enhancements we may develop in the future, our ability to generate revenues could be limited. We have in the past, and will likely in the future, experience delays and temporary interruptions in the receipt of payments from third‑party payors due to modifications in existing contracts or arrangements, contract implementation matters, documentation requirements and other issues, which could cause our revenues to fluctuate from period to period.
If we are unable to obtain or maintain adequate reimbursement for our tests outside of the United States, our ability to expand internationally will be compromised.
The majority of our international Oncotype DX breast and colon cancer test revenues come from direct payor reimbursement, payments from our distributors, patient self‑pay, and clinical collaborations in various countries. In many countries outside of the United States, various coverage, pricing and reimbursement approvals are required. We expect that it will take several years to establish broad coverage and reimbursement for our tests with payors in countries outside of the United States, and our efforts may not be successful. Once established, reimbursement levels outside of the United States may vary considerably from the domestic reimbursement amounts we receive. In addition, because we rely on distributors to obtain reimbursement for our tests, to the extent we do not have direct reimbursement arrangements with payors, we may not be able to retain reimbursement coverage in certain countries with a particular payor if our agreement with a distributor is terminated or expires or a distributor fails to pay us for other reasons. Distributors of our tests may also be negatively affected by the financial instability of, and austerity measures implemented by, several countries in the European Union and elsewhere.
The prices at which our tests are reimbursed may be reduced by Medicare and private and other payors, and any such changes could have a negative impact on our revenues.
Even if we are being reimbursed for our tests, Medicare, Medicaid and private and other payors may withdraw their coverage policies, cancel their contracts with us at any time, review and adjust the rate of reimbursement, require co‑payments from patients or stop paying for our tests, which would reduce our revenues. In addition, insurers, including managed care organizations as well as government payors such as Medicare and Medicaid, have increased their efforts to control the cost, utilization and delivery of healthcare services. These measures have resulted in reduced payment rates for and decreased utilization of clinical laboratory services. Noridian Healthcare Solutions and Palmetto GBA (the Medicare Administrative Contractors, or MACs, that process Medicare claims and set Medicare coverage and payment policies, respectively, for most tests billed by our laboratory) and other MACs review coverage and reimbursement rates annually.
The recently enacted Protecting Access to Medicare Act of 2014, or PAMA, includes a substantial new payment system for clinical laboratory tests under the Clinical Laboratory Fee Schedule, or CLFS. Under PAMA, Medicare payment rates for tests will be equal to the volume-weighted median of the private payor payment rates for the test. The payment rates
34
calculated under PAMA are expected to apply to our tests starting January 1, 2017, and will be reviewed annually for “advanced diagnostic laboratory tests” (and every three years for other tests), based on private payor payment rates and volumes for their tests. Laboratories that fail to report the required payment information may be subject to substantial civil money penalties. Although CMS has not yet issued regulations to implement PAMA, we believe our Oncotype DX tests each would be considered an advanced diagnostic laboratory test. While we do not believe the new payment rate system under PAMA will have a negative effect on the current payment rates of our Medicare-covered tests beginning in 2017, regulations implementing PAMA have not yet been promulgated. As a result, there can be no assurance that adequate payment rates will continue to be assigned to our tests.
We have received a specific Current Procedural Terminology, or CPT, code for our Oncotype DX breast cancer test effective January 1, 2015. Medicare is currently establishing a national limitation amount for this code under the gapfill process through the regional MACs We do not yet know whether the gapfill process for our new test-specific CPT code for Oncotype DX breast cancer test will impact our current payment rate for this test.
Additionally, on a five year competitively bid basis, Medicare requests bids for its regional MAC services. In September 2013, the claims processing function for our jurisdiction transitioned from Palmetto GBA to Noridian Healthcare Solutions, however coverage and payment rate determinations for our tests remain with Palmetto GBA at this time through the MolDx Program. The change in the MAC processing the Medicare claims for our tests delayed reimbursement for a brief period of time. Future changes in the MAC may affect our ability to obtain Medicare coverage and reimbursement for products for which we have coverage, for which we do not yet have coverage or any products we may launch in the future or delay payments, including payments for our Oncotype DX prostate cancer test.
Because of Medicare billing rules, we may not receive reimbursement for all tests provided to Medicare patients.
Under current Medicare billing rules, payment for our Oncotype DX tests performed on Medicare beneficiaries who were hospital inpatients at the time the tumor tissue samples were obtained and whose tests were ordered less than 14 days from discharge must be bundled into the payment that the hospital receives for the inpatient services provided. Medicare billing rules also require hospitals to bill for our tests when ordered for hospital outpatients less than 14 days following the date of the hospital procedure where the tumor tissue samples were obtained. Accordingly, we are required to bill individual hospitals for tests performed on Medicare beneficiaries during these time frames. Because we generally do not have written agreements in place with these hospitals to pay for these tests, we may not be paid or may have to pursue payment from the hospital on a case‑by‑case basis. We cannot ensure that hospitals will pay us for Oncotype DX tests performed on patients falling under these rules.
Although we believe patients coming under these rules represent less than 1% of our total claims, these billing rules may lead to confusion regarding whether Medicare provides adequate reimbursement for our tests, and could discourage providers from ordering our tests for Medicare patients. In addition, compared to our breast cancer tests, a greater proportion of eligible patients for our colon and prostate tests are covered by Medicare. We cannot assure you that Medicare will reverse these billing rules or that Medicare will not extend this limitation in the future.
We depend on Medicare for a significant portion of our product revenues and if Medicare or other significant payors stop providing reimbursement or decrease the amount of reimbursement for our tests, our revenues could decline.
Reimbursement on behalf of patients covered by Medicare accounted for 20%, 21% and 22% of our product revenues for the years ended December 31, 2014, 2013, and 2012, respectively. Accounts receivable on behalf of patients covered by Medicare represented 27%, 28% and 21% of our net accounts receivable at December 31, 2014, 2013, and 2012, respectively. While there were no other third‑party payors representing 10% or more of our product revenues for these periods, there have been in the past, and may be in the future, other payors accounting for 10% or more of our product revenues. Because the majority of stage II and stage III colon cancer patients and prostate cancer patients in the United States are age 65 and over, and thus insured by Medicare, we may become more dependent on Medicare reimbursement in the future. It is possible that Medicare or other third‑party payors that provide reimbursement for our tests may suspend, revoke or discontinue coverage at any time, may require co‑payments from patients, or may reduce the reimbursement rates payable to us. Any such action could have a negative impact on our revenues.
Our financial results depend largely on the sales of one test, our Oncotype DX breast cancer test, and we will need to generate sufficient revenues from this and other tests to run our business and achieve profitability.
For the near future, we expect to continue to derive a substantial majority of our revenues from sales of one test, our Oncotype DX test for invasive breast cancer. While we launched our test for colon cancer in January 2010, we do not expect
35
to recognize significant revenues from this test until significant levels of adoption and reimbursement for this test have been established. We have similar expectations for revenue related to our DCIS breast cancer test, which was launched in December 2011, and our prostate cancer test, which was launched in May 2013. We are in various stages of research and development for other tests that we may offer as well as for enhancements to our existing tests, including those using our liquid biopsy platform. We may not be able to successfully commercialize tests for other cancers or diseases. If we are unable to increase sales of our test for invasive breast cancer, establish adoption of and reimbursement for our colon, or prostate cancer or DCIS tests, or successfully develop and commercialize other tests or enhancements, our revenues and our ability to achieve sustained profitability would be impaired.
Complying with numerous regulations pertaining to our business is an expensive and time‑consuming process, and any failure to comply could result in substantial penalties.
We are subject to CLIA, a federal law that regulates clinical laboratories that perform testing on specimens derived from humans for the purpose of providing information for the diagnosis, prevention or treatment of disease. CLIA regulations mandate specific standards in the areas of personnel qualifications, administration, and participation in proficiency testing, patient test management, quality control, quality assurance and inspections. We have a current certificate of accreditation under CLIA to perform testing through our accreditation by the College of American Pathologists, or CAP. To renew this certificate, we are subject to survey and inspection every two years. Moreover, CLIA inspectors may make random inspections of our clinical reference laboratory.
Although we are required to hold a certificate of accreditation or compliance under CLIA that allows us to perform high complexity testing, we are not required to hold a certificate of accreditation through CAP. We could alternatively maintain a certificate of accreditation from another accrediting organization or a certificate of compliance through inspection by surveyors acting on behalf of the CLIA program. If our accreditation under CAP were to terminate, either voluntarily or involuntarily, we would need to convert our certification under CLIA to a certificate of compliance (or to a certificate of accreditation with another accreditation organization) in order to maintain our ability to perform clinical testing and to continue commercial operations. Whether we would be able to successfully maintain operations through either of these alternatives would depend upon the facts and circumstances surrounding termination of our CAP accreditation, such as whether any deficiencies were identified by CAP as the basis for termination and, if so, whether these were addressed to the satisfaction of the surveyors for the CLIA program (or another accrediting organization).
We are also required to maintain a license to conduct testing in California. California laws establish standards for day‑to‑day operation of our clinical reference laboratory, including the training and skills required of personnel and quality control. In addition, our clinical reference laboratory is required to be licensed on a product‑specific basis by New York State. New York law also mandates proficiency testing for laboratories licensed under New York state law, regardless of whether or not such laboratories are located in New York. Moreover, several other states require that we hold licenses to test specimens from patients in those states. Other states may have similar requirements or may adopt similar requirements in the future. Finally, we may be subject to regulation in foreign jurisdictions as we seek to expand international distribution of our tests, which may require review of our tests in order to offer our services or may have other limitations such as prohibitions on the export of tissue necessary for us to perform our tests that may limit our ability to distribute outside of the United States.
If we were to lose our CLIA accreditation or California license, whether as a result of a revocation, suspension or limitation, we would no longer be able to sell our tests, which would limit our revenues and harm our business. If we were to lose our license in New York or in other states where we are required to hold licenses, we would not be able to test specimens from those states.
We are subject to other regulation in the United States by both the federal government and the states in which we conduct our business, as well as in other jurisdictions outside of the United States, including:
|
· |
Medicare billing and payment regulations applicable to clinical laboratories; |
|
· |
the Federal Anti‑kickback Law and state anti‑kickback prohibitions; |
|
· |
the Federal physician self‑referral prohibition, commonly known as the Stark Law, and the state equivalents; |
|
· |
the Federal Health Insurance Portability and Accountability Act of 1996; |
36
|
· |
the Medicare civil money penalty and exclusion requirements; |
|
· |
the Federal False Claims Act civil and criminal penalties and state equivalents; and |
|
· |
the Foreign Corrupt Practices Act, the United Kingdom Anti‑bribery Act and the European Data Protection Directive, all of which apply to our international activities. |
We have adopted policies and procedures designed to comply with these laws. In the ordinary course of our business, we conduct internal reviews of our compliance with these laws. Our compliance is also subject to governmental review. The growth of our business and sales organization and our expansion outside of the United States may increase the potential of violating these laws or our internal policies and procedures. The risk of our being found in violation of these or other laws and regulations is further increased by the fact that many of them have not been fully interpreted by the regulatory authorities or the courts, and their provisions are open to a variety of interpretations. Any action brought against us for violation of these or other laws or regulations, even if we successfully defend against it, could cause us to incur significant legal expenses and divert our management’s attention from the operation of our business. If our operations are found to be in violation of any of these laws and regulations, we may be subject to any applicable penalty associated with the violation, including civil and criminal penalties, damages and fines, we could be required to refund payments received by us, and we could be required to curtail or cease our operations. Any of the foregoing consequences could seriously harm our business and our financial results.
New test development involves a lengthy and complex process, and we may be unable to commercialize on a timely basis, or at all, any new tests we may develop.
We have multiple tests in development and devote considerable resources to research and development. There can be no assurance that our technologies will be capable of reliably predicting the recurrence of cancers other than breast, colon and prostate cancer with the sensitivity and specificity necessary to be clinically and commercially useful, or that our colon or prostate cancer tests will result in commercially successful products. In addition, before we can develop diagnostic tests for new cancers or other diseases and commercialize any new products, we will need to:
|
· |
conduct substantial research and development; |
|
· |
conduct validation studies; |
|
· |
expend significant funds; |
|
· |
develop and scale our laboratory processes to accommodate different tests; and |
|
· |
develop and scale our infrastructure to be able to analyze increasingly large amounts of data. |
Our product development process involves a high degree of risk and may take several years. Our product development efforts may fail for many reasons, including:
|
· |
failure of the product at the research or development stage; |
|
· |
difficulty in accessing archival tissue samples, especially tissue samples with known clinical results; or |
|
· |
lack of clinical validation data to support the effectiveness of the product. |
Few research and development projects result in commercial products, and success in early clinical trials often is not replicated in later studies. At any point, we may abandon development of a product candidate or we may be required to expend considerable resources repeating clinical trials, which would adversely impact the timing for generating potential revenues from those product candidates. In addition, as we develop products, we will have to make significant investments in product development, marketing and selling resources. If a clinical validation study fails to demonstrate the prospectively defined endpoints of the study, we might choose to abandon the development of the product or product feature that was the subject of the clinical trial, which could harm our business. For example, in September 2013 we delayed our plan to initiate a validation study in 2013 utilizing results from our NSABP C‑07 clinical trial. The decision to delay was based on analytical performance, during the pre‑validation phase, that did not meet our standards for a subset of the candidate predictive genes. In addition, competitors may develop and commercialize competing products faster than we are able to do so.
37
If we are unable to support demand for our tests, including successfully managing the evolution of our technology and manufacturing platforms, our business could suffer.
As our test volume grows, we will need to continue to ramp up our testing capacity, implement increases in scale and related processing, customer service, billing and systems process improvements, and expand our internal quality assurance program, technology and manufacturing platforms to support testing on a larger scale. We will also need additional certified laboratory scientists and other scientific and technical personnel to process higher volumes of our tests. We cannot assure you that any increases in scale, related improvements and quality assurance will be successfully implemented or that appropriate personnel will be available. As additional products are commercialized, such as our prostate cancer test, we will need to bring new equipment on‑line, implement new systems, technology, controls and procedures and hire personnel with different qualifications. We cannot assure you that any such efforts will not result in delays. Failure to implement necessary procedures, transition to new equipment or processes or to hire the necessary personnel could result in higher cost of processing or an inability to meet market demand. There can be no assurance that we will be able to perform tests on a timely basis at a level consistent with demand, that our efforts to scale our commercial operations will not negatively affect the quality of test results, or that we will be successful in responding to the growing complexity of our testing operations. If we encounter difficulty meeting market demand or quality standards for our tests, our reputation could be harmed and our future prospects and our business could suffer.
We may experience limits on our revenues if physicians decide not to order our tests.
If medical practitioners do not order our Oncotype DX tests or any future tests developed or offered by us, we will likely not be able to create or maintain demand for our products in sufficient volume for us to achieve sustained profitability. To generate demand, we will need to continue to make oncologists, urologists, surgeons and pathologists aware of the benefits of each type of test through published papers, presentations at scientific conferences and one‑on‑one education by our sales force. In addition, we will need to demonstrate our ability to obtain and maintain adequate reimbursement coverage from third‑party payors.
Prior to the inclusion of our Oncotype DX breast cancer test in clinical guidelines for treatment of N−, ER+ breast cancer, guidelines and practices regarding the treatment of breast cancer recommended that chemotherapy be considered in most cases, including many cases in which our test might indicate that, based on our clinical trial results, chemotherapy would be of little or no benefit. Accordingly, physicians may be reluctant to order a test that may suggest recommending against chemotherapy in treating breast cancer. Moreover, our test provides quantitative information not currently provided by pathologists and it is performed at our facility rather than by the pathologist in a local laboratory, so pathologists may be reluctant to support our test. These facts may make it difficult for us to convince medical practitioners to order our test for their patients, which could limit our ability to generate revenues and achieve sustained profitability.
Our Oncotype DX colon cancer test predicts recurrence but, unlike our test for invasive breast cancer, does not predict chemotherapy benefit. Our new Oncotype DX prostate cancer test provides physicians and patients with a new way to assess the aggressiveness of a patient’s prostate cancer. We will need to educate physicians, patients and payors about the benefits and cost‑effectiveness of these tests and to establish reimbursement arrangements for these tests with payors. We have and expect to continue to hire additional commercial, sales, scientific, technical and other personnel to support this process. If our marketing and educational efforts do not result in sufficient physician or patient demand, we may not be able to obtain adequate reimbursement for these tests. If we fail to successfully establish adoption of and additional reimbursement beyond Medicare for our colon cancer test, our reputation could be harmed and our business could suffer. If we fail to successfully establish adoption of and reimbursement for our prostate cancer test, our reputation could be harmed and our business could suffer.
We may experience limits on our revenues if patients decide not to use our tests.
Some patients may decide not to use our Oncotype DX tests due to their price, all or part of which may be payable directly by the patient if the applicable payor denies reimbursement in full or in part. Even if medical practitioners recommend that their patients use our tests, patients may still decide not to use our tests, either because they do not want to be made aware of the likelihood of recurrence or they wish to pursue a particular course of therapy regardless of test results. Additionally, the current economic environment in the United States and abroad could continue to negatively impact patients, resulting in higher co‑payments and insurance premiums or the loss of healthcare coverage, which may result in delayed medical checkups or an inability to pay for our tests. If only a small portion of the patient population decides to use our tests, we will experience limits on our revenues and our ability to achieve sustained profitability.
38
Our rights to use technologies licensed from third parties are not within our control, and we may not be able to sell our products if we lose our existing rights or cannot obtain new rights on reasonable terms.
We license from third parties technology necessary to develop our products. For example, we license technology from Roche Molecular Systems, Inc. that we use to analyze genes in our clinical reference laboratory to conduct our tests. In return for the use of a third party’s technology, we may agree to pay the licensor royalties based on sales of our products. Royalties are a component of cost of product revenues and impact the margins on our tests. We may need to license other technologies to commercialize future products. We may also need to negotiate licenses to patents and patent applications after launching any of our commercial products. Our business may suffer if these licenses terminate, if the licensors fail to abide by the terms of the license or fail to prevent infringement by third parties, if the licensed patents or other rights are found to be invalid, if the patents or patent applications are unavailable for license or if we are unable to enter into necessary licenses on acceptable terms. Companies that attempt to replicate our tests could be set up in countries that do not recognize our intellectual property, or in countries in which we do not have intellectual property protection. Such companies could send test results into the United States and therefore reduce sales of our tests.
If we are unable to develop products to keep pace with rapid technological, medical and scientific change, our operating results and competitive position could be harmed.
In recent years, there have been numerous advances in technologies relating to the diagnosis and treatment of cancer. For example, technologies in addition to ours now permit measurement of gene expression in fixed paraffin‑embedded tissue specimens or blood or urine. New chemotherapeutic or biologic strategies are being developed that may increase survival time and reduce toxic side effects. There have also been advances in methods used to analyze very large amounts of genomic information, specifically next generation sequencing, or NGS. These advances require us to continuously develop our technology, develop new products and enhance existing products to keep pace with evolving standards of care. Our tests could become obsolete unless we continually innovate and expand our products to demonstrate recurrence and treatment benefit in patients treated with new therapies. New treatment therapies typically have only a few years of clinical data associated with them, which limits our ability to perform clinical studies and correlate sets of genes to a new treatment’s effectiveness. If we are unable to demonstrate the applicability of our tests to new treatments, sales of our test could decline, which would harm our revenues.
If we are unable to maintain intellectual property protection, our competitive position could be harmed.
Our ability to compete and to achieve sustained profitability is impacted by our ability to protect our proprietary discoveries and technologies. We currently rely on a combination of issued patents, patent applications, copyrights, trademarks, and confidentiality, material data transfer, license and invention assignment agreements to protect our intellectual property rights. We also rely upon trade secret laws to protect unpatented know‑how and continuing technological innovation. Our intellectual property strategy is intended to develop and maintain our competitive position. Patents may be granted to us jointly with other organizations, and while we may have a right of first refusal, we cannot guarantee that a joint owner will not license rights to another party, and we cannot guarantee that a joint owner will cooperate with us in the enforcement of patent rights.
Our pending patent applications may not result in issued patents, and we cannot assure you that our issued patents or any patents that might ultimately be issued by the U.S. Patent and Trademark Office, or USPTO, will protect our technology. In addition, we do not file patent applications in every country nor is patent protection available in every country. We may face competition internationally in jurisdictions where we do not have intellectual property protection. Any patents that may be issued to us might be challenged by third parties as being invalid or unenforceable, or third parties may independently develop similar or competing technology that avoids our patents.
We cannot be certain that the steps we have taken will prevent the misappropriation and use of our intellectual property, particularly in foreign countries where the laws may not protect our proprietary rights as fully as in the United States.
If patent regulations or standards are modified, such changes could have a negative impact on our business.
From time to time, the U.S. Supreme Court, other federal courts, the U.S. Congress or the USPTO may change the standards of patentability and validity and any such changes could have a negative impact on our business. In addition, competitors may develop their own versions of our test in countries where we did not apply for patents or where our patents have not issued and compete with us in those countries, including encouraging the use of their test by physicians or patients in other countries.
39
There have been several cases involving “gene patents” and diagnostic claims that have been considered by the U.S. Supreme Court. In March 2012, the Supreme Court in Mayo Collaborative v. Prometheus Laboratories, or Prometheus, found a patented diagnostic method claim unpatentable because the relationship between a metabolite concentration and optimized dosage was a patent‑ineligible “law of nature.” In June 2013, the Supreme Court ruled in ACLU v. Myriad Genetics, or Myriad, that an isolated genomic DNA sequence is not patent eligible while cDNA is eligible. Both the Prometheus and Myriad decisions affect the legal concept of subject matter eligibility by seemingly narrowing the scope of the statute defining patentable inventions.
In December 2014, the USPTO published revised guidelines for patent examiners to apply when examining process claims for patent eligibility in view of several recent Supreme Court decisions, including Mayo Collaborative Services v. Prometheus Laboratories, Inc., Association for Molecular Pathology v. Myriad Genetics, Inc., and Alice Corporation Pty. Ltd. V. CLS Bank International , et al. The guidance indicates that claims directed to a law of nature, a natural phenomenon, or an abstract idea that do not meet the eligibility requirements should be rejected as non‑statutory, patent ineligible subject matter. We cannot assure you that our patent portfolio will not be negatively impacted by the decisions described above, rulings in other cases or changes in guidance or procedures issued by the USPTO.
Additional substantive changes to patent law, whether new or associated with the American Invents Act, may affect our ability to obtain, enforce or defend our patents. Accordingly, it is not clear what, if any, impact the new law will ultimately have on the cost of prosecuting our patent applications, our ability to obtain patents based on our discoveries and our ability to enforce or defend our issued patents, all of which could have a material adverse effect on our business.
We may face intellectual property infringement claims that could be time‑consuming and costly to defend, and could result in our loss of significant rights and the assessment of treble damages.
We have in the past, and may in the future, receive notices of claims of infringement and misappropriation or misuse of other parties’ proprietary rights and may from time to time receive additional notices. Some of these claims may lead to litigation. We cannot assure you that we will prevail in such actions, or that other actions alleging misappropriation or misuse by us of third‑party trade secrets, alleging infringement by us of third‑party patents and trademarks or challenging the validity of our patents, will not be asserted or prosecuted against us.
We may also initiate claims to defend our intellectual property or to seek relief on allegations that we use, sell, or offer to sell technology that incorporates third party intellectual property. Intellectual property litigation, regardless of outcome, is expensive and time‑consuming, could divert management’s attention from our business and have a material negative effect on our business, operating results or financial condition. If there is a successful claim of infringement against us, we may be required to pay substantial damages (including treble damages if that infringement were found to be willful) to the party claiming infringement, develop non‑infringing technology, stop selling our tests or using technology that contains the allegedly infringing intellectual property or enter into royalty or license agreements that may not be available on acceptable or commercially practical terms, if at all. Our failure to develop non‑ infringing technologies or license the proprietary rights on a timely basis could harm our business. In addition, revising our tests to include the non‑ infringing technologies would require us to re‑validate our tests, which would be costly and time consuming. Also, we may be unaware of pending third-party patent applications that relate to our tests. Parties making infringement claims on future issued patents may be able to obtain an injunction that could prevent us from selling our tests or using technology that contains the allegedly infringing intellectual property, which could harm our business.
It is possible that a third party or patent office might take the position that one or more patents or patent applications constitute prior art in the field of genomic‑based diagnostics. In such a case, we might be required to pay royalties, damages and costs to firms who own the rights to these patents, or we might be restricted from using any of the inventions claimed in those patents.
If we are unable to compete successfully, we may be unable to increase or sustain our revenues or achieve sustained profitability.
Our principal competition for our breast, colon and prostate cancer tests comes from existing diagnostic methods used by pathologists and oncologists. These methods have been used for many years and are therefore difficult to change or supplement. In addition, companies offering capital equipment and kits or reagents to local pathology laboratories represent another source of potential competition. These kits are used directly by the pathologist, which facilitates adoption more readily than tests like ours that are performed outside the pathology laboratory.
40
We also face competition from companies that offer products or have conducted research to profile genes, gene expression or protein expression in breast, colon or prostate cancer, including public companies such as GE Healthcare, a business unit of General Electric Company, Hologic, Inc., Myriad Genetics, Inc., NanoString Technologies, Inc., Novartis AG, Qiagen N.V. and Response Genetics, Inc., and many private companies. We also face competition from commercial laboratories with strong distribution networks for diagnostic tests, such as Laboratory Corporation of America Holdings and Quest Diagnostics Incorporated. We may also face competition from Illumina, Inc. and Thermo Fisher Scientific Inc,, both of which have announced their intention to enter the clinical diagnostics market. Other potential competitors include companies that develop diagnostic tests such as Roche Diagnostics, a division of Roche Holding, Ltd, Siemens AG and Veridex LLC, a Johnson & Johnson company, as well as other companies and academic and research institutions.
In our newly established prostate cancer market, we face comparatively greater competition than in our breast cancer market, including competition from products which were on the market prior to our product launch and which are supported by clinical studies and published data. This existing direct and indirect competition for tests and procedures may make it difficult to gain market share, impact our ability to obtain reimbursement or result in a substantial increase in resources necessary for us to successfully commercialize our Oncotype DX prostate cancer test.
Others may invent and commercialize technology platforms such as next generation sequencing approaches that will compete with our test. Projects related to cancer genomics have received government funding, both in the United States and internationally. As more information regarding cancer genomics becomes available to the public, we anticipate that more products aimed at identifying targeted treatment options will be developed and that these products may compete with ours. In addition, competitors may develop their own versions of our tests in countries where we did not apply for patents, where our patents have not been issued or where our intellectual property rights are not recognized and compete with us in those countries, including encouraging the use of their test by physicians or patients in other countries.
We have changed the list price of our tests in the past and we expect to change prices for our tests in the future. Any increase or decrease in pricing could impact reimbursement of and demand for our tests. Many of our present and potential competitors have widespread brand recognition and substantially greater financial and technical resources and development, production and marketing capabilities than we do. Others may develop lower‑priced tests that could be viewed by physicians and payors as functionally equivalent to our tests, or offer tests at prices designed to promote market penetration, which could force us to lower the list prices of our tests and impact our operating margins and our ability to achieve sustained profitability. Some competitors have developed tests cleared for marketing by the FDA. There may be a marketing differentiation or perception that an FDA‑cleared test is more desirable than Oncotype DX tests, and that may discourage adoption of and reimbursement for our tests. If we are unable to compete successfully against current or future competitors, we may be unable to increase market acceptance for and sales of our tests, which could prevent us from increasing or sustaining our revenues or achieving sustained profitability and could cause the market price of our common stock to decline.
Our research and development efforts will be hindered if we are not able to contract with third parties for access to clinical samples.
Under standard clinical practice, tumor biopsies removed from patients are typically chemically preserved and embedded in paraffin wax and stored. Our clinical development relies on our ability to secure access to these archived tumor biopsy samples, as well as information pertaining to their associated clinical outcomes. Generally, the agreements under which we gain access to archival samples are nonexclusive. Other companies study archival samples and often compete with us for access. Additionally, the process of negotiating access to archived samples is lengthy since it typically involves numerous parties and approval levels to resolve complex issues such as usage rights, institutional review board approval, privacy rights, publication rights, intellectual property ownership and research parameters. If we are not able to negotiate access to clinical samples with hospitals, clinical partners, pharmaceutical companies, or companies developing therapeutics on a timely basis, or at all, or if other laboratories or our competitors secure access to these samples before us, our ability to research, develop and commercialize future products will be limited or delayed.
If we cannot maintain our current clinical collaborations and enter into new collaborations, our product development could be delayed.
We rely on and expect to continue to rely on clinical collaborators to perform a substantial portion of our clinical trial functions. If any of our collaborators were to breach or terminate its agreement with us or otherwise fail to conduct the contracted activities successfully and in a timely manner, the research, development or commercialization of the products contemplated by the collaboration could be delayed or terminated. If any of our collaboration agreements are terminated, or if
41
we are unable to renew those agreements on acceptable terms, we would be required to seek alternatives. We may not be able to negotiate additional collaborations on acceptable terms, if at all, and these collaborations may not be successful.
In the past, we have entered into clinical trial collaborations with highly regarded organizations in the cancer field.. Our success in the future depends in part on our ability to enter into agreements with other leading cancer organizations. This can be difficult due to internal and external constraints placed on these organizations. Some organizations may limit the number of collaborations they have with any one company so as to not be perceived as biased or conflicted. Organizations may also have insufficient administrative and related infrastructure to enable collaborations with many companies at once, which can prolong the time it takes to develop, negotiate and implement collaboration. Additionally, organizations often insist on retaining the rights to publish the clinical data resulting from the collaboration. The publication of clinical data in peer‑reviewed journals is a crucial step in commercializing and obtaining reimbursement for tests such as ours, and our inability to control when, if ever, results are published may delay or limit our ability to derive sufficient revenues from any product that may result from a collaboration.
From time to time we expect to engage in discussions with potential clinical collaborators which may or may not lead to collaborations. However, we cannot guarantee that any discussions will result in clinical collaborations or that any clinical studies which may result will be enrolled or completed in a reasonable time frame or with successful outcomes. Once news of discussions regarding possible collaborations are known in the medical community, regardless of whether the news is accurate, failure to announce a collaboration agreement or the entity’s announcement of a collaboration with an entity other than us could result in adverse speculation about us, our product or our technology, resulting in harm to our reputation and our business.
The loss of key members of our senior management team or our inability to attract and retain highly skilled scientists, clinicians and salespeople could adversely affect our business.
Our success depends largely on the skills, experience and performance of key members of our executive management team and others in key management positions. The efforts of each of these persons together will be critical to us as we continue to develop our technologies and testing processes, continue our international expansion and transition to a company with multiple commercialized products. If we were to lose one or more of these key employees, we may experience difficulties in competing effectively, developing our technologies and implementing our business strategies.
Our research and development programs and commercial laboratory operations depend on our ability to attract and retain highly skilled scientists and technicians, including licensed laboratory technicians, chemists, biostatisticians and engineers. We may not be able to attract or retain qualified scientists and technicians in the future due to the competition for qualified personnel among life science businesses, particularly in the San Francisco Bay Area. In addition, it is expected that there will be a shortage of clinical laboratory scientists in coming years, which would make it more difficult to hire sufficient numbers of qualified personnel. We also face competition from universities and public and private research institutions in recruiting and retaining highly qualified scientific personnel. In addition, our success depends on our ability to attract and retain salespeople with extensive experience in oncology and urology and close relationships with medical oncologists, urologists, surgeons, pathologists and other hospital personnel. We may have difficulties locating, recruiting or retaining qualified salespeople, which could cause a delay or decline in the rate of adoption of our tests. If we are not able to attract and retain the necessary personnel to accomplish our business objectives, we may experience constraints that could adversely affect our ability to support our research and development and sales programs. All of our employees are at‑will, which means that either we or the employee may terminate their employment at any time.
If our sole laboratory facility becomes inoperable, we will be unable to perform our tests and our business will be harmed.
We do not have redundant clinical reference laboratory facilities outside of Redwood City, California. Redwood City is situated near active earthquake fault lines. Our facility and the equipment we use to perform our tests would be costly to replace and could require substantial lead time to repair or replace. The facility may be harmed or rendered inoperable by natural or man‑made disasters, including earthquakes, flooding and power outages, which may render it difficult or impossible for us to perform our tests for some period of time. The inability to perform our tests or the backlog of tests that could develop if our facility is inoperable for even a short period of time may result in the loss of customers or harm our reputation, and we may be unable to regain those customers in the future. Although we possess insurance for damage to our property and the disruption of our business, this insurance may not be sufficient to cover all of our potential losses and may not continue to be available to us on acceptable terms, if at all.
In order to rely on a third party to perform our tests, we could only use another facility with established state licensure and CLIA accreditation under the scope of which Oncotype DX tests could be performed following validation and other
42
required procedures. We cannot assure you that we would be able to find another CLIA‑certified facility willing to comply with the required procedures, that this laboratory would be willing to perform the tests for us on commercially reasonable terms, or that it would be able to meet our quality standards. In order to establish a redundant clinical reference laboratory facility, we would have to spend considerable time and money securing adequate space, constructing the facility, recruiting and training employees, and establishing the additional operational and administrative infrastructure necessary to support a second facility. We may not be able, or it may take considerable time, to replicate our testing processes or results in a new facility. Additionally, any new clinical reference laboratory facility opened by us would be subject to certification under CLIA and licensing by several states, including California and New York, which could take a significant amount of time and result in delays in our ability to begin operations.
International expansion of our business exposes us to business, regulatory, political, operational, financial and economic risks associated with doing business outside of the United States.
Our business strategy incorporates international expansion, including increasing the size of and maintaining direct sales and physician outreach and education capabilities outside of the United States and expanding our relationships with international payors and distributors. Doing business internationally involves a number of risks, including:
|
· |
multiple, conflicting and changing laws and regulations such as tax laws, export and import restrictions, employment laws, regulatory requirements and other governmental approvals, permits and licenses; |
|
· |
competition from local and regional product offerings; |
|
· |
failure by us or our distributors to obtain regulatory approvals for the use of our tests in various countries; |
|
· |
difficulties in staffing and managing foreign operations; |
|
· |
complexities associated with managing multiple payor reimbursement regimes, government payors or patient self‑pay systems; |
|
· |
logistics and regulations associated with shipping tissue samples, including infrastructure conditions and transportation delays; |
|
· |
limits in our ability to penetrate international markets if we are not able to process tests locally; |
|
· |
lack of intellectual property protection in certain markets; |
|
· |
financial risks, such as longer payment cycles, difficulty collecting accounts receivable, the impact of local and regional financial crises on demand and payment for our tests and exposure to foreign currency exchange rate fluctuations; |
|
· |
natural disasters, political and economic instability, including wars, terrorism, and political unrest, outbreak of disease, boycotts, curtailment of trade and other business restrictions; and |
|
· |
regulatory and compliance risks that relate to maintaining accurate information and control over the activities of our sales force and distributors that may fall within the purview of the FCPA, its books and records provisions or its anti‑bribery provisions. |
Any of these factors could significantly harm our future international expansion and operations and, consequently, our revenues and results of operations.
Our dependence on distributors for sales of our Oncotype DX tests outside of the U.S. could limit or prevent us from selling our test in foreign markets and impact our revenue.
As of December 31, 2014, we have entered into exclusive distribution agreements for the sale of our tests with distributors covering more than 90 countries. We may enter into other similar arrangements to distribute our tests in other countries in the future. We intend to continue to grow our business internationally, and to do so we may need to attract additional distributors to expand the territories in which we sell our tests. Distributors may not commit the necessary resources to market and sell our tests to the level of our expectations. If current or future distributors do not perform adequately, or we are unable
43
to enter into arrangements with distributors to market our tests in particular geographic areas, we may not realize long‑term international revenue growth. In addition, our revenue from distributors could be negatively impacted as a result of changes in business cycles, business or economic conditions or other factors that could affect their ability to pay us for tests on a timely basis or at all. Regulatory requirements, costs of doing business outside of the United States and the reimbursement process in foreign markets may also impact our revenues from international sales or impact our ability to increase international sales in the future.
We may acquire other businesses, form joint ventures or make investments in other companies or technologies that could harm our operating results, dilute our stockholders’ ownership, increase our debt or cause us to incur significant expense.
As part of our business strategy, we may pursue acquisitions of complementary businesses and assets, as well as technology licensing arrangements. We also may pursue strategic alliances that leverage our core technology and industry experience to expand our product offerings or distribution, or make investments in other companies. We have recently experienced and may in the future experience losses related to the recognition of our portion of the net losses of equity method investees, and we may in the future experience impairment losses related to our investments in companies if we determine that the value of an investment is impaired. Losses related to our investments in other companies could have a material negative effect on our results of operations. We have no experience with respect to acquiring other companies and limited experience with respect to the formation of strategic alliances and joint ventures. If we make any acquisitions, we may not be able to integrate these acquisitions successfully into our existing business, and we could assume unknown or contingent liabilities. Any future acquisitions by us also could result in significant write‑offs or the incurrence of debt and contingent liabilities, any of which could harm our operating results. Integration of an acquired company also may require management resources that otherwise would be available for ongoing development of our existing business. We may not identify or complete these transactions in a timely manner, on a cost‑effective basis, or at all, and we may not realize the anticipated benefits of any acquisition, technology license, strategic alliance, joint venture or investment.
To finance any acquisitions or investments, we may choose to issue shares of our common stock as consideration, which would dilute the ownership of our stockholders. Periods of upheaval in the capital markets and world economy have in the past, and may in the future, cause volatility in the market price of our common stock. If the price of our common stock is low or volatile, we may not be able to acquire other companies for stock. Alternatively, it may be necessary for us to raise additional funds for acquisitions through public or private financings. Additional funds may not be available on terms that are favorable to us, or at all.
Our marketable securities are subject to risks that could adversely affect our overall financial position.
We invest our cash in accordance with an established internal policy in instruments which historically have been highly liquid and carried relatively low risk. However, similar types of investments have in the past and may in the future experience losses in value or liquidity issues which differ from historical patterns. Should a portion of our marketable securities lose value or have their liquidity impaired, it could adversely affect our overall financial position by imperiling our ability to fund our operations and forcing us to seek additional financing sooner than we would otherwise. Such financing, if available, may not be available on commercially attractive terms.
If it became necessary and we were unable to raise additional capital on acceptable terms in the future, it may limit our ability to develop and commercialize new tests and technologies and expand our operations.
We expect capital outlays and operating expenditures to increase over the next several years as we expand our infrastructure, commercial operations and research and development activities. Specifically, we may need to raise capital to, among other things:
|
· |
sustain commercialization of our Oncotype DX tests and enhancements to those tests; |
|
· |
fund commercialization of any future tests we may develop; |
|
· |
increase our selling and marketing efforts to drive market adoption and address competitive developments; |
|
· |
further expand our clinical laboratory operations; |
|
· |
expand our technologies into other areas of cancer or other diseases; |
44
|
· |
expand our research and development activities; |
|
· |
acquire, license or invest in technologies, including next generation sequencing and liquid biopsy; |
|
· |
acquire or invest in complementary businesses or assets; and |
|
· |
finance capital expenditures and general and administrative expenses. |
Our present and future funding requirements will depend on many factors, including:
|
· |
the rate of progress in establishing and maintaining reimbursement arrangements with domestic and international third‑party payors; |
|
· |
the cost of expanding our commercial and laboratory operations, including our selling and marketing efforts; |
|
· |
the rate of progress and cost of research and development activities associated with expansion of our Oncotype DX breast, colon and prostate cancer tests; |
|
· |
the rate of progress and cost of selling and marketing activities associated with establishing adoption of and reimbursement for our Oncotype DX colon and prostate cancer and DCIS tests; |
|
· |
costs related to future product launches; |
|
· |
the rate of progress and cost of research and development activities associated with products in research and development focused on cancers other than breast, colon and prostate cancer; |
|
· |
the rate of progress and cost of research and development activities associated with next generation sequencing; |
|
· |
the costs of acquiring, licensing or investing in technologies, including next generation sequencing and liquid biopsy; |
|
· |
the cost of acquiring or investing in complementary businesses or assets; |
|
· |
the cost of acquiring or achieving access to tissue samples and technologies; |
|
· |
the cost of filing, prosecuting, defending and enforcing any patent claims and other intellectual property rights; |
|
· |
the effect of competing technological and market developments; |
|
· |
costs related to international expansion; |
|
· |
costs and delays in product development as a result of any changes in regulatory oversight applicable to our products or operations; |
|
· |
the impact of changes in Federal, state and international taxation; and |
|
· |
the economic and other terms and timing of any collaborations, licensing or other arrangements into which we may enter or investments or acquisitions we may seek to effect. |
If we raise funds by issuing equity securities, dilution to our stockholders could result. Any equity securities issued also may provide for rights, preferences or privileges senior to those of holders of our common stock. If we raise funds by issuing debt securities, these debt securities would have rights, preferences and privileges senior to those of holders of our common stock. The terms of debt securities issued or borrowings could impose significant restrictions on our operations. If we raise funds through collaborations and licensing arrangements, we might be required to relinquish significant rights to our technologies or products, or grant licenses on terms that are not favorable to us. Accordingly, additional equity or debt financing might not be available on reasonable terms, if at all. If we are not able to secure additional funding when needed, we may have
45
to delay, reduce the scope of or eliminate one or more research and development programs or selling and marketing initiatives. In addition, we may have to work with a partner on one or more of our product or market development programs, which could lower the economic value of those programs to us.
We are dependent on our information technology and telecommunications systems, and any failure of these systems could harm our business.
We depend on information technology, or IT, and telecommunications systems for significant aspects of our operations. In addition, our third‑party billing and collections provider is dependent upon telecommunications and data systems provided by outside vendors and information it receives from us on a regular basis. These IT and telecommunications systems support a variety of functions, including test processing, sample tracking, quality control, customer service and support, billing and reimbursement, research and development activities, and our general and administrative activities. Failures or significant downtime of our IT or telecommunications systems or those used by our third‑party service providers could prevent us from processing tests, providing test results to physicians, billing payors, processing reimbursement appeals, handling patient or physician inquiries, conducting research and development activities, and managing the administrative aspects of our business. Any disruption or loss of IT or telecommunications systems on which critical aspects of our operations depend could have an adverse effect on our business and our product revenues.
Security breaches, loss of data and other disruptions could compromise sensitive information related to our business or prevent us from accessing critical information and expose us to liability, which could adversely affect our business and our reputation.
In the ordinary course of our business, we and our third party billing and collections provider collect and store sensitive data, including legally protected health information, credit card information, personally identifiable information about our employees, customers and patients, intellectual property, and our proprietary business information and that of our customers, payors and collaboration partners. We manage and maintain our applications and data utilizing a combination of on‑site systems, managed data center systems and cloud‑based data center systems. These applications and data encompass a wide variety of business critical information including research and development information, commercial information and business and financial information. We face four primary risks relative to protecting this critical information, including loss of access risk, inappropriate disclosure risk and inappropriate modification risk combined with the risk of our being able to identify and audit our controls over the first three risks.
The secure processing, storage, maintenance and transmission of this critical information is vital to our operations and business strategy, and we devote significant resources to protecting such information. Although we take measures to protect sensitive information from unauthorized access or disclosure, our information technology and infrastructure, and that of our third party billing and collections provider, may be vulnerable to attacks by hackers or viruses or breached due to employee error, malfeasance or other disruptions. Any such breach or interruption could compromise our networks and the information stored there could be accessed by unauthorized parties, publicly disclosed, lost or stolen. Any such access, disclosure or other loss of information could result in legal claims or proceedings, liability under laws that protect the privacy of personal information, such as the Health Insurance Portability and Accountability Act of 1996, and regulatory penalties. Unauthorized access, loss or dissemination could also disrupt our operations, including our ability to process tests, provide test results, bill payors or patients, process claims and appeals, provide customer assistance services, conduct research and development activities, collect, process and prepare company financial information, provide information about our tests and other patient and physician education and outreach efforts through our website, manage the administrative aspects of our business and damage our reputation, any of which could adversely affect our business.
In addition, the interpretation and application of consumer, health‑related and data protection laws in the U.S., Europe and elsewhere are often uncertain, contradictory and in flux. It is possible that these laws may be interpreted and applied in a manner that is inconsistent with our practices. If so, this could result in government imposed fines or orders requiring that we change our practices, which could adversely affect our business. Complying with these various laws could cause us to incur substantial costs or require us to change our business practices and compliance procedures in a manner adverse to our business.
We rely on a limited number of suppliers or, in some cases, a sole supplier, for some of our laboratory instruments and materials and may not be able to find replacement suppliers or immediately transition to alternative suppliers.
We rely on certain sole suppliers to supply and service some of the laboratory equipment on which we perform our tests. We believe that there are relatively few equipment manufacturers that are currently capable of supplying and servicing the equipment necessary for our tests. Although we have identified alternative suppliers, transition to a new supplier will be
46
time consuming and expensive, and there can be no assurance that we will be able to secure alternative equipment and bring that equipment on line without experiencing interruptions in testing. If we should encounter delays or difficulties in securing the quality and quantity of equipment we require for our tests, we may need to reconfigure our test processes, which could result in an interruption in sales. If any of these events occur, our business and operating results could be harmed.
We also rely on several sole suppliers for certain laboratory reagents and materials which we use to perform our tests. While we have developed alternate sourcing strategies for these materials, we cannot be certain that these strategies will be effective. If we should encounter delays or difficulties in securing these laboratory materials, if the materials do not meet our quality specifications, or if we cannot obtain acceptable substitute materials, an interruption in test processing could occur. Any such interruption may significantly affect future product revenues.
We may be unable to manage our future growth effectively, which could make it difficult to execute our business strategy.
Future growth will impose significant added responsibilities on management, including the need to identify, recruit, train and integrate additional employees. In addition, rapid and significant growth may place strain on our administrative and operational infrastructure, including customer service and our clinical reference laboratory. Our ability to manage our operations and growth will require us to continue to improve our operational, financial and management controls, reporting systems and procedures. We plan to implement new enterprise software affecting a broad range of business processes and functional areas including order fulfillment, sample processing, customer service, supply chain management, and others. The time and resources required to implement these new systems is uncertain, and failure to complete this in a timely and efficient manner could adversely affect our operations. If we are unable to manage our growth effectively, it may be difficult for us to execute our business strategy.
If we were sued for product liability or professional liability, we could face substantial liabilities that exceed our resources.
The marketing, sale and use of our tests could lead to the filing of product liability claims if someone were to allege that our tests failed to perform as it was designed. We may also be subject to liability for errors in the test results we provide to physicians or for a misunderstanding of, or inappropriate reliance upon, the information we provide. For example, physicians sometimes order our Oncotype DX breast cancer test for patients who do not have the same specific clinical attributes indicated on the report form as those for which the test provides clinical experience information from validation studies. It is our practice to offer medical consultation to physicians ordering our test for such patients, including patients with ER‑ breast cancers. A product liability or professional liability claim could result in substantial damages and be costly and time consuming for us to defend. Although we maintain product and professional liability insurance, we cannot assure you that our insurance would fully protect us from the financial impact of defending against product liability or professional liability claims or any judgments, fines or settlement costs arising out of any such claims. Any product liability or professional liability claim brought against us, with or without merit, could increase our insurance rates or prevent us from securing insurance coverage in the future. Additionally, any product liability lawsuit could cause injury to our reputation, result in the recall of our products, or cause current clinical partners to terminate existing agreements and potential clinical partners to seek other partners, any of which could impact our results of operations.
If we use hazardous materials in a manner that causes injury, we could be liable for damages.
Our activities currently require the use of hazardous chemicals. We cannot eliminate the risk of accidental contamination or injury to employees or third parties from the use, storage, handling or disposal of these materials. In the event of contamination or injury, we could be held liable for any resulting damages, and any liability could exceed our resources or any applicable insurance coverage we may have. Additionally, we are subject on an ongoing basis to federal, state and local laws and regulations governing the use, storage, handling and disposal of these materials and specified waste products. The cost of compliance with these laws and regulations may become significant and could negatively affect our operating results.
We must implement additional and expensive business systems, procedures and controls as we grow our business and organization and to satisfy public company reporting requirements, which will increase our costs and require additional management resources.
As a public reporting company, we are required to comply with the Sarbanes‑Oxley Act of 2002 and the related rules and regulations of the Securities and Exchange Commission. Compliance with Section 404 of the Sarbanes‑Oxley Act and other requirements has increased our costs and required additional management resources. We will need to continue to implement additional finance, accounting, and business operating systems, procedures and controls as we grow our business and organization and to satisfy existing reporting requirements. If we fail to maintain or implement adequate controls, if we are unable to complete the required Section 404 assessment as to the adequacy of our internal control over financial reporting in future Form 10‑K filings, or if our independent registered public accounting firm is unable to provide us with an unqualified
47
report as to the effectiveness of our internal control over financial reporting in future Form 10‑K filings, our ability to obtain additional financing could be impaired. In addition, investors could lose confidence in the reliability of our internal control over financial reporting and in the accuracy of our periodic reports filed under the Exchange Act. A lack of investor confidence in the reliability and accuracy of our public reporting could cause our stock price to decline.
We are subject to increasingly complex taxation rules and practices, which may affect how we conduct our business and our results of operations.
As our business grows, we are required to comply with increasingly complex taxation rules and practices. We are subject to tax in multiple U.S. tax jurisdictions and in foreign tax jurisdictions as we expand internationally. The development of our tax strategies requires additional expertise and may impact how we conduct our business. Our future effective tax rates could be unfavorably affected by changes in, or interpretations of, tax rules and regulations in the jurisdictions in which we do business, by lapses of the availability of the U.S. research and development tax credit or by changes in the valuation of our deferred tax assets and liabilities. Furthermore, we provide for certain tax liabilities that involve significant judgment. We are subject to the examination of our tax returns by federal, state and foreign tax authorities, which could focus on our intercompany transfer pricing methodology as well as other matters. If our tax strategies are ineffective or we are not in compliance with domestic and international tax laws, our financial position, operating results and cash flows could be adversely affected.
ITEM 1B. Unresolved Staff Comments.
None.
At December 31, 2014, we leased approximately 144,900 square feet of laboratory and office space in Redwood City, California under operating leases that expire between March 2018 and March 2019, with options for us to extend the term of each lease for an additional five years. We also leased approximately 7,500 square feet of office space in Geneva, Switzerland under an operating lease that expires in May 2016. Additionally, we have offices in France, Germany, Ireland, Italy, Japan and the United Kingdom with short‑term rental agreements. We may need additional facilities in the future as we expand our business and believe that additional space, when needed, will be available on commercially reasonable terms.
We were not a party to any material legal proceedings at December 31, 2014, or at the date of this report. We may from time to time become involved in various legal proceedings arising in the ordinary course of business.
ITEM 4. Mine Safety Disclosures.
Not applicable.
Executive Officers of the Registrant
The names of our executive officers and their ages as of March 1, 2015, are as follows:
|
Name |
|
Age |
Position |
|
Kimberly J. Popovits |
56 |
President and Chief Executive Officer |
|
|
G. Bradley Cole |
59 |
Chief Operating Officer and Chief Financial Officer |
|
|
Steven Shak, M.D. |
64 |
Chief Scientific Officer |
|
|
|
|
|
|
|
|
|
|
|
Kimberly J. Popovits has served as our President and Chief Executive Officer since January 2009, and as Chairman of the Board since March 2012. Prior to that, Ms. Popovits served as our President and Chief Operating Officer since February 2002 and as a director since March 2002. From November 1987 to February 2002, Ms. Popovits served in various roles at Genentech, Inc., a biotechnology company, most recently serving as Senior Vice President, Marketing and Sales from February 2001 to February 2002, and as Vice President, Sales from October 1994 to February 2001. Prior to joining Genentech, she served as Division Manager, Southeast Region, for American Critical Care, a division of American Hospital Supply, a supplier of health care products to hospitals. Ms. Popovits holds a B.A. in Business from Michigan State University.
G. Bradley Cole has served as our Chief Operating Officer since January 2009 and has also served as our Chief Financial Officer since June 2014, and from July 2004 until January 2011. Prior to that, Mr. Cole served as Executive Vice
48
President, Operations from January 2008 and as Executive Vice President and Chief Financial Officer from July 2004 until January 2009. Mr. Cole also served as our Secretary from February 2005 until July 2012. From December 1997 to May 2004, he served in various roles at Guidant Corporation, a medical device company, most recently serving as Vice President, Finance and Business Development for the Endovascular Solutions Group from January 2001 until May 2004. From July 1994 to December 1997, Mr. Cole was Vice President, Finance and Chief Financial Officer of Endovascular Technologies, Inc., a medical device company that was acquired by Guidant Corporation. From December 1988 to February 1994, he served as Vice President, Finance and Chief Financial Officer of Applied Biosystems Incorporated, a life sciences systems company. Mr. Cole holds a B.S. in Business from Biola University and an M.B.A. from San Jose State University.
Steven Shak, M.D., has served as our Chief Scientific Officer since January 2015 and has also served as our Executive Vice President of Research and Development from July 2012 to December 2014 and as our Chief Medical Officer from December 2000 to August 2013. From July 1996 to October 2000, Dr. Shak served in various roles in Medical Affairs at Genentech, most recently as Senior Director and Staff Clinical Scientist. From November 1989 to July 1996, Dr. Shak served as a Director of Discovery Research at Genentech, where he was responsible for Pulmonary Research, Immunology, and Pathology. Prior to joining Genentech, Dr. Shak was an Assistant Professor of Medicine and Pharmacology at the New York University School of Medicine. Dr. Shak holds a B.A. in Chemistry from Amherst College and an M.D. from the New York University School of Medicine, and completed his post‑doctoral training at the University of California, San Francisco.
ITEM 5. Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities.
Our common stock, par value $0.0001 per share, is traded on The NASDAQ Global Select Market under the symbol “GHDX.” The following table sets forth the range of high and low sales prices for our common stock for the periods indicated:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2014 |
|||||||||||||
|
|
|
First |
|
Second |
|
Third |
|
Fourth |
|
||||
|
Quarter |
Quarter |
Quarter |
Quarter |
||||||||||
|
Stock price—high |
|
$ |
33.00 |
|
$ |
28.55 |
|
$ |
30.21 |
|
$ |
36.40 |
|
|
Stock price—low |
$ |
25.95 |
$ |
24.68 |
$ |
25.26 |
$ |
28.54 | |||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2013 |
|
||||||||||
|
|
|
First |
|
Second |
|
Third |
|
Fourth |
|
||||
|
|
|
Quarter |
|
Quarter |
|
Quarter |
|
Quarter |
|
||||
|
Stock price—high |
|
$ |
29.65 |
|
$ |
37.49 |
|
$ |
36.07 |
|
$ |
35.89 |
|
|
Stock price—low |
|
$ |
26.72 |
|
$ |
26.99 |
|
$ |
30.58 |
|
$ |
28.99 |
|
According to the records of our transfer agent, we had 55 stockholders of record as of February 28, 2015.
Dividends
We have never declared or paid any cash dividends on our capital stock, and we do not currently intend to pay any cash dividends on our common stock in the foreseeable future. We expect to retain any future earnings to fund the development and growth of our business. Our board of directors will determine future cash dividends, if any. There are currently no contractual restrictions on our ability to pay dividends.
Stock Performance Graph
The following information is not deemed to be “soliciting material” or to be “filed” with the Securities and Exchange Commission or subject to Regulation 14A or 14C under the Securities Exchange Act of 1934 or to the liabilities of Section 18 of the Securities Exchange Act of 1934, and will not be deemed to be incorporated by reference into any filing under the Securities Act of 1933 or the Securities Exchange Act of 1934, except to the extent we specifically incorporate it by reference into such a filing.
49
Set forth below is a line graph showing the cumulative total stockholder return (change in stock price plus reinvested dividends) assuming the investment of $100 on December 31, 2008 in each of our common stock, the NASDAQ Market Index and the NASDAQ Biotechnology Index for the period commencing on December 31, 2009 and ending on December 31, 2014. The comparisons in the table are required by the Securities and Exchange Commission and are not intended to forecast or be indicative of future performance of our common stock.
COMPARISON OF CUMULATIVE TOTAL RETURN
AMONG GENOMIC HEALTH, INC.,
NASDAQ MARKET INDEX AND NASDAQ BIOTECHNOLOGY INDEX
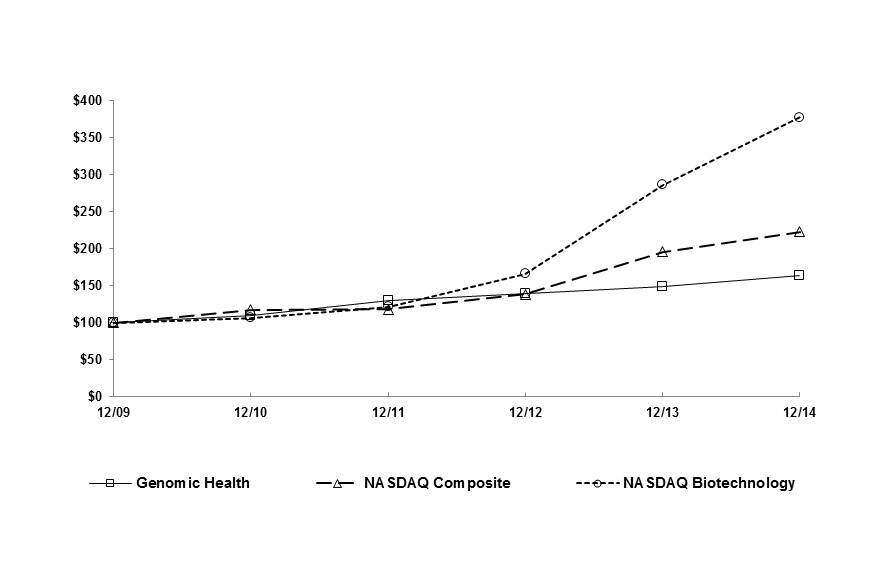
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
December 31, |
|
December 31, |
|
December 31, |
|
December 31, |
|
December 31, |
|
December 31, |
|
||||||
|
|
|
2009 |
|
2010 |
|
2011 |
|
2012 |
|
2013 |
|
2014 |
|
||||||
|
Genomic Health, Inc. |
|
$ |
100.00 |
|
$ |
109.36 |
|
$ |
129.81 |
|
$ |
139.26 |
|
$ |
149.64 |
|
$ |
163.45 |
|
|
NASDAQ Market Index |
|
$ |
100.00 |
|
$ |
117.43 |
|
$ |
118.27 |
|
$ |
138.47 |
|
$ |
196.27 |
|
$ |
223.17 |
|
|
NASDAQ Biotechnology Index |
|
$ |
100.00 |
|
$ |
106.62 |
|
$ |
122.01 |
|
$ |
166.55 |
|
$ |
286.43 |
|
$ |
378.29 |
|
ITEM 6. Selected Financial Data.
The following selected consolidated financial data should be read together with “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our consolidated financial statements and related notes included elsewhere in this report. The selected consolidated balance sheet data at December 31, 2014 and 2013 and the selected consolidated statements of operations data for each year ended December 31, 2014, 2013 and 2012 have been derived from our audited consolidated financial statements that are included elsewhere in this report. The selected consolidated balance sheet data at December 31, 2012, 2011 and 2010 and the selected consolidated statements of operations data for the years ended
50
December 31, 2011 and 2010 have been derived from our audited consolidated financial statements not included in this report. Historical results are not necessarily indicative of the results to be expected in the future.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
Year Ended December 31, |
|
||||||||||||||||
|
|
|
2014 |
|
2013 |
|
2012 |
|
2011 |
|
2010 |
|
||||||||
|
|
|
(In thousands, except per share data) |
|
||||||||||||||||
|
Consolidated Statements of Operations Data: |
|||||||||||||||||||
|
Revenues: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
Product revenues |
$ |
275,706 |
$ |
259,192 |
$ |
233,457 |
$ |
204,766 |
$ |
174,870 | |||||||||
|
Contract revenues |
|
|
— |
|
|
2,403 |
|
|
1,716 |
|
|
1,345 |
|
|
3,231 |
|
|||
|
Total revenues |
|
|
275,706 |
|
|
261,595 |
|
|
235,173 |
|
|
206,111 |
|
|
178,101 |
|
|||
|
Operating expenses(1): |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
Cost of product revenues |
|
|
48,742 |
|
|
42,100 |
|
|
37,018 |
|
|
33,832 |
|
|
34,634 |
|
|||
|
Research and development |
|
|
56,064 |
|
|
66,333 |
|
|
49,104 |
|
|
39,864 |
|
|
33,225 |
|
|||
|
Selling and marketing |
134,858 | 110,602 | 93,553 | 83,613 | 71,405 | ||||||||||||||
|
General and administrative |
|
|
59,669 |
|
|
54,392 |
|
|
47,064 |
|
|
40,543 |
|
|
34,913 |
|
|||
|
Total operating expenses |
|
|
299,333 |
|
|
273,427 |
|
|
226,739 |
|
|
197,852 |
|
|
174,177 |
|
|||
|
Income (loss) from operations |
|
|
(23,627) |
|
|
(11,832) |
|
|
8,434 |
|
|
8,259 |
|
|
3,924 |
|
|||
|
Impairment on investments |
|
|
— |
|
|
(643) |
|
|
— |
|
|
— |
|
|
— |
|
|||
|
Interest income, net |
|
|
192 |
|
|
222 |
|
|
295 |
|
|
221 |
|
|
232 |
|
|||
|
Other income (expense), net |
|
|
(764) |
|
|
(158) |
|
|
(58) |
|
|
(205) |
|
|
(4) |
|
|||
|
Income (loss) before income taxes |
|
|
(24,199) |
|
|
(12,411) |
|
|
8,671 |
|
|
8,275 |
|
|
4,152 |
|
|||
|
Income tax expense (benefit) |
|
|
393 |
|
|
346 |
|
|
422 |
|
|
429 |
|
|
(136) |
|
|||
|
Net income (loss) |
|
$ |
(24,592) |
|
$ |
(12,757) |
|
$ |
8,249 |
|
$ |
7,846 |
|
$ |
4,288 |
|
|||
|
Basic net income (loss) per share |
|
$ |
(0.78) |
|
$ |
(0.42) |
|
$ |
0.27 |
|
$ |
0.27 |
|
$ |
0.15 |
|
|||
|
Diluted net income (loss) per share |
|
$ |
(0.78) |
|
$ |
(0.42) |
|
$ |
0.26 |
|
$ |
0.26 |
|
$ |
0.14 |
|
|||
|
Weighted-average shares used in computing basic net income (loss) per share |
|
|
31,453 |
|
|
30,512 |
|
|
30,326 |
|
|
29,395 |
|
|
28,815 |
|
|||
|
Weighted-average shares used in computing diluted net income (loss) per share |
|
|
31,453 |
|
|
30,512 |
|
|
32,152 |
|
|
30,754 |
|
|
29,653 |
|
|||
|
(1) |
Includes non‑cash charges for employee stock‑based compensation expense as follows: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended December 31, |
|
|||||||||||||
|
|
|
2014 |
|
2013 |
|
2012 |
|
2011 |
|
2010 |
|
|||||
|
|
|
(In thousands) |
|
|||||||||||||
|
Cost of product revenues |
|
$ |
497 |
|
$ |
483 |
|
$ |
441 |
|
$ |
335 |
|
$ |
342 |
|
|
Research and development |
|
|
4,569 |
|
|
4,873 |
|
|
3,992 |
|
|
3,017 |
|
|
2,881 |
|
|
Selling and marketing |
|
|
4,396 |
|
|
4,369 |
|
|
4,191 |
|
|
3,194 |
|
|
3,086 |
|
|
General and administrative |
|
|
7,076 |
|
|
7,732 |
|
|
6,480 |
|
|
5,189 |
|
|
4,035 |
|
|
Total |
|
$ |
16,538 |
|
$ |
17,457 |
|
$ |
15,104 |
|
$ |
11,735 |
|
$ |
10,344 |
|
|
At December 31, |
||||||||||||||||
|
2014 |
2013 |
2012 |
2011 |
2010 |
||||||||||||
|
(In thousands) |
||||||||||||||||
|
Consolidated Balance Sheet Data: |
||||||||||||||||
|
Cash, cash equivalents and marketable securities |
$ |
103,660 |
$ |
105,350 |
$ |
99,065 |
$ |
100,474 |
$ |
76,818 | ||||||
|
Working capital |
110,182 | 115,160 | 104,869 | 102,856 | 76,097 | |||||||||||
|
Total assets |
185,921 | 177,034 | 153,734 | 142,998 | 110,861 | |||||||||||
|
Accumulated deficit |
(194,861) | (170,269) | (157,512) | (165,761) | (173,607) | |||||||||||
|
Total stockholders’ equity |
145,513 | 144,981 | 126,326 | 115,359 | 86,110 | |||||||||||
51
ITEM 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations.
The following discussion of our financial condition and results of operations should be read in conjunction with our consolidated financial statements and the related notes included in Item 8 of this report. Historical results are not necessarily indicative of future results.
Business Overview
We are a global provider of genomic-based diagnostic tests that address both the overtreatment and optimal treatment of early stage cancer, one of the greatest issues in healthcare today. We are applying our world-class scientific and commercial expertise and infrastructure to lead the translation of massive amounts of genomic data into clinically-actionable results for treatment planning throughout the cancer patient's journey, from screening and surveillance, through diagnosis, treatment selection and monitoring. We offer our Oncotype DX tests as a clinical laboratory service, where we analyze the expression levels of genes in tumor tissue samples and provide physicians with a quantitative gene expression profile expressed as a single quantitative score, which we call a Recurrence Score for invasive breast cancer and colon cancer, a DCIS Score for ductal carcinoma in situ, or DCIS and a Genomic Prostate Score, or GPS, for prostate cancer.
In January 2004, we launched our first Oncotype DX test, which is used to predict the likelihood of cancer recurrence and the likelihood of chemotherapy benefit in early stage invasive breast cancer patients. In January 2010, we launched our second Oncotype DX test, the first multigene expression test developed to assess risk of recurrence in stage II colon cancer patients. In late December 2011, we made Oncotype DX available for patients with DCIS, a pre‑invasive form of breast cancer. In June 2012, we extended our offering of the Oncotype DX colon cancer test to patients with stage III disease treated with oxaliplatin‑containing adjuvant therapy. In May 2013, we launched our Oncotype DX prostate cancer test, which is used to predict disease aggressiveness in men with low risk disease. Effective July 1, 2014, the list price of our Oncotype DX breast cancer tests increased from $4,380 to $4,510, the list price of our Oncotype DX colon cancer test increased from $4,030 to $4,330 and the list price of our Oncotype DX prostate cancer test increased from $3,820 to $4,180. The substantial majority of our historical revenues have been derived from the sale of Oncotype DX breast cancer tests ordered by physicians in the United States.
For the year ended December 31, 2014, more than 95,630 Oncotype DX test reports were delivered for use in treatment planning, compared to more than 85,510 and 74,520 test reports delivered for the years ended December 31, 2013 and 2012, respectively. All of our tests are conducted at our clinical reference laboratory in Redwood City, California. Our clinical reference laboratory processing capacity is currently approximately 115,000 tests annually, and has significant expansion capacity with incremental increases in laboratory personnel and equipment. The Oncotype DX breast, colon, and prostate cancer tests analyze different genes. However, all of the tests are based on a similar Oncotype DX reverse transcription polymerase chain reaction, or RT-PCR platform. We believe that we currently have sufficient capacity to process current demand for our tests.
In connection with the May 2013 launch of our prostate cancer test, we have expanded our clinical laboratory processing capacity. We expect our continued commercialization efforts of our prostate cancer test will result in increased costs for laboratory testing, including staffing-related costs, incremental sales and marketing staffing to introduce our product to a new group of physicians and patients, costs for clinical utility studies and costs associated with obtaining reimbursement coverage.
We depend upon third-party payors, both public and private, to provide reimbursement for our tests. Accordingly, we have and expect to continue to focus substantial resources on obtaining reimbursement coverage from third-party payors.
We have continued to expand our business, both in the United States and internationally. We plan to continue to use essentially the same business model internationally as we use in the United States, however, there are significant differences between countries that need to be considered. For example, different countries may have a public healthcare system, a combination of public and private healthcare system or a cash-based payment system. We have a direct commercial presence with employees in Canada and certain European counties. Additionally, we have exclusive distribution agreements for the sale of our breast and colon cancer tests with distributors covering more than 90 countries outside of the United States.
We expect that international sales of our Oncotype DX tests will be heavily dependent on the availability of reimbursement and sample access. In many countries, governments are primarily responsible for reimbursing diagnostic tests. Governments often have significant discretion in determining whether a test will be reimbursed at all, and if so, how much will be paid. In addition, certain countries, such as China, have prohibitions against exporting tissue samples which will limit our
52
ability to offer our tests in those countries without local laboratories or a method of test delivery which does not require samples to be transported to our U.S. laboratory.
The majority of our international Oncotype DX breast and colon cancer test revenues come from direct payor reimbursement, payments from our distributors, patient self-pay, and clinical collaborations in various countries. We have obtained some coverage for our breast cancer test outside of the United States, including in Argentina, Canada, the Czech Republic, Germany, Greece, Ireland, Israel, Saudi Arabia, Spain, Switzerland and the United Kingdom. In September 2013, we announced that the National Institute for Health and Care Excellence (NICE) in the United Kingdom issued its final guidance recommending Oncotype DX as the only multi-gene breast cancer test for use in clinical practice to guide chemotherapy treatment decisions for patients with early-stage, hormone receptor-positive, lymph node negative, human epidermal growth factor receptor 2 negative, invasive breast cancer. We continue to work with NHS England to establish the appropriate reimbursement path following NICE’s exclusive recommendation for our breast cancer test, similar to our contracting process with U.S. insurers. In April 2014, we announced that the Gynecologic Oncology Working Group (AGO) in Germany also updated their guidelines to recommend Oncotype DX as the only breast cancer gene expression test to predict chemotherapy benefit in early-stage, hormone receptor-positive invasive breast cancer. We expect that it will take several years to establish broad coverage and reimbursement for our Oncotype DX breast, colon and prostate cancer tests with payors in countries outside of the United States and there can be no assurance that our efforts will be successful.
Oncotype DX Breast Cancer Test
We expect to continue to focus substantial resources on pursuing global adoption of and reimbursement for our Oncotype DX breast cancer test. We believe increased demand for our Oncotype DX breast cancer test resulted from our ongoing commercial efforts, expanded utility for new breast cancer patient groups, continued publication of peer-reviewed articles on studies we sponsored, conducted or collaborated on that support the use of and reimbursement for the test, clinical presentations at major symposia, and the inclusion of our breast cancer test in clinical practice guidelines for node negative, or N−, estrogen receptor positive, or ER+, invasive disease. However, this increased demand is not necessarily indicative of future growth rates, and we cannot provide assurance that this level of increased demand can be sustained or that publication of articles, future appearances or presentations at medical conferences, increased commercial efforts or expansion of utility to new breast cancer patient groups will have a similar impact on demand for our breast cancer test in the future. Sequential quarterly demand for our breast cancer test may also be impacted by other factors, including the economic environment and continued high unemployment levels, seasonal variations that have historically impacted physician office visits, our shift in commercial focus to our Oncotype DX colon and prostate cancer tests or any future products we may develop, patient enrollment in Oncotype DX clinical studies and the number of clinical trials in process by cooperative groups or makers of other tests conducting experience studies.
Most national and regional third-party payors in the United States, along with the designated regional Medicare contractor for our tests, have issued positive coverage determinations for our Oncotype DX breast cancer test for patients with N−, ER+, invasive disease through contracts, agreements or policy decisions. The local carrier with jurisdiction for claims submitted by us for Medicare patients also provides coverage for our breast cancer test for ER+ patients with node positive, or N+, disease (up to three positive lymph nodes) and invasive breast cancer patients where a lymph node status is unknown or not accessible due to a prior surgical procedure, or when the test is used to guide a neoadjuvant treatment decision. Additionally, some payors provide policy coverage for the use of our test in ER+ patients with N+ disease, including lymph node micro-metastasis (greater than 0.2 mm, but not greater than 2.0 mm in size). In July 2011, the American Journal of Managed Care published results of an economic assessment suggesting use of Oncotype DX in breast cancer patients with 1-3 positive nodes may improve health outcomes without adding incremental cost. However, we may not be able to obtain reimbursement coverage from other payors for our test for breast cancer patients with N+, ER+ disease.
In December 2011, we made the Oncotype DX breast cancer test available for patients with DCIS, a pre-invasive form of breast cancer. The launch of Oncotype DX for DCIS patients was based upon presented positive results from a clinical validation study of the Oncotype DX breast cancer test in patients with DCIS, conducted by the Eastern Cooperative Oncology Group, or ECOG, a clinical trials cooperative group supported by the National Cancer Institute. The study met its primary endpoint by demonstrating that a pre-specified Oncotype DX DCIS Score derived from the Oncotype DX breast cancer test outperforms traditional clinical and pathologic measures to predict the risk of local recurrence, defined as either the development of a new invasive breast cancer or the recurrence of DCIS in the same breast. In May 2013, our Oncotype DX DCIS clinical validation study was published online in the Journal of the National Cancer Institute. Following the publication of the results of this study, the Medicare contractor for our Oncotype DX breast cancer test expanded coverage to include patients with DCIS. Additionally, the Veterans Administration, Department of Defense hospital facilities and some private payors provide coverage for the Oncotype DX DCIS test. We expect that it may take several years to establish coverage with a majority of public and private payors for use of our test in DCIS patients and we may not be able to obtain such coverage.
53
In June 2014, we announced positive top line results of another clinical validation study to confirm and extend the observations of the published DCIS clinical validation study, conducted in collaboration with the Ontario DCIS Study Group. The results were presented at the San Antonio Breast Cancer Symposium in December 2014.
Oncotype DX Colon Cancer Test
We expect to continue to focus resources on pursuing global adoption of and reimbursement for our Oncotype DX colon cancer test. We believe the key factors that will drive further adoption of this test include results from additional studies we sponsor, conduct or collaborate on that support the use of and increased coverage and reimbursement for the test, clinical presentations at major symposia, publications, inclusion of the test in clinical guidelines and our ongoing commercial efforts. In June 2011, a second large study confirming that the Oncotype DX colon cancer test independently predicts individualized recurrence risk for stage II colon cancer was presented. In November 2011, positive results from the QUASAR clinical validation study were published online by the Journal of Clinical Oncology. Current or future studies of our colon cancer test may lead to inclusion of the test in clinical guidelines and as standard of care for indicated patients.
Effective September 18, 2011, the designated regional Medicare contractor for our tests established a formal coverage policy for our Oncotype DX colon cancer test for patients with stage II colon cancer. Additionally, the Veterans Administration, Department of Defense hospital facilities and some private payors provide coverage for the Oncotype DX colon cancer test.
In June 2012, based on the positive results of the landmark randomized NSABP C-07 validation study, we began offering the Oncotype DX colon cancer test for use in patients with stage III disease treated with oxaliplatin-containing adjuvant therapy. In September 2012, we presented these positive results from the NSABP C-07 study, including prediction of risk of recurrence, disease-free survival and overall survival in stage II and stage III colon cancer patients. In November 2013, the Journal of Clinical Oncology published positive results of the third successful validation of the Oncotype DX colon cancer test in patients with stage II disease and the first validation study in patients with stage III disease.
In November 2013, the Current Medical Research & Opinion published positive results from the Partnership for Health Analytic Research clinical utility analysis of the Oncotype DX colon cancer test, demonstrating that use of the test changes treatment recommendations in 29% of stage II colon cancer patients.
We are working with additional public and private payors and health plans to secure coverage for our colon cancer test based upon clinical evidence showing the utility of the test. However, we cannot predict whether, at what rate, or under what circumstances, payors will reimburse for this test.
Oncotype DX Prostate Cancer Test
In June 2012, we presented results of our first development study in prostate tissue obtained from needle biopsies. The study, an analysis of biopsy samples from men with conventionally defined low/intermediate risk prostate cancer, showed that genes and biological pathways associated with clinically-aggressive prostate cancer in radical prostatectomy specimens can be reliably measured by quantitative RT-PCR from fixed prostate needle biopsies.
In September 2012, we announced positive top line results from the clinical validation study of our biopsy-based prostate cancer test. As a result of this clinical validation study meeting its primary end point, we launched our Oncotype DX prostate cancer test in May 2013 and made the test commercially available worldwide. The test provides a Genomic Prostate Score, or GPS, that predicts disease aggressiveness in men with low risk disease. This test may be used to improve treatment decisions for prostate cancer patients, in conjunction with the Gleason score, or tumor grading. In May 2014, European Urology published the positive results from our two development studies, as well as our clinical validation study of diagnostic biopsies from 395 men who were candidates for active surveillance, demonstrating that the use of GPS can potentially increase the number of men who could confidently choose active surveillance by 20 to 30%.
In August 2014, we announced positive top line results of a second clinical study, demonstrating the ability of our test’s GPS to predict multiple clinical endpoints related to disease aggressiveness among low/ intermediate risk patients. The study also confirmed the earlier validation study published in May 2014. The results from the clinical validation study were presented at the European Society for Medical Oncology in September 2014, and at the Society of Urologic Oncology meeting in December 2014.
We expect to continue to invest substantial resources related to continued clinical studies and the global adoption of and reimbursement for our prostate cancer test. We expect our commercialization efforts for our prostate cancer test will result
54
in further increased costs for laboratory testing, including staffing-related costs, incremental sales and marketing staffing to introduce this product to a new group of physicians and patients, costs for clinical utility studies and costs associated with obtaining reimbursement coverage.
Product Development Opportunities
In addition to developing products to address new cancer areas, we continually look to expand the clinical utility and addressable patient populations for our existing cancer tests. These developments efforts may lead to a wide variety of possible new products covering various treatment decisions, including:
|
· |
Risk assessment; |
|
· |
Screening and prevention; |
|
· |
Early disease diagnosis; |
|
· |
Adjuvant and/or neoadjuvant disease treatment; |
|
· |
Metatastic disease treatment selection; and |
|
· |
Treatment monitoring. |
Potential new products may address a specific clinical need or guide a targeted therapy decision and may also leverage our next generation sequencing, or NGS, capabilities to expand our product opportunities. Additionally, potential new products may use non-invasive tests that can be performed on blood and urine to quantify the presence and burden of cancer as well as the sensitivity or resistance to specific drug therapies.
Technology
Our commercially available tests utilize RT-PCR technology to quantify gene expression in patient tumor samples. We are also incorporating new technologies, such as high-throughput NGS, in our research and development laboratory. NGS is typically used to sequence the deoxyribonucleic acids, or DNAs, in the cellular genome of the host tumor. With this technology, we can also sequence millions of ribonucleic acid chains, or RNAs, map them back to their respective genes based on their sequence and then count the number of copies and compare the relative expression between different genes.
We have selected NGS to be our primary technology for future biomarker discovery and have begun using NGS for future clinical development in tandem with our existing RT-PCR based approach. NGS technologies parallelize the sequencing process, producing thousands or millions of sequences at once, and are intended to provide nucleic acid sequence information at lower cost than standard methods. We have created proprietary methods for NGS analysis of fixed paraffin embedded, or FPE, tissue nucleic acids, created bioinformatics programs, and infrastructure for data storage and analysis and plan to rely on NGS as the technology source of new biomarkers in the future.
We have begun to further advance our research and development pipeline with proprietary platforms that incorporate emerging molecular technologies in order to develop non-invasive liquid biopsy tests that can be performed on blood or urine. While early-stage cancer continues to represent a significant opportunity with near-term potential, we now have the opportunity to expand our business further along the patient’s cancer journey. Expanding our focus beyond early-stage treatment decision support toward later-stage disease includes opportunities to monitor progression and response to therapeutics for patients who are diagnosed with later stage or recurrent disease who can also benefit from precision medicine.
Economic Environment
Continuing concerns over prolonged high unemployment levels, entitlement and health care reform efforts, regulatory changes and taxation issues, and geopolitical issues have contributed to uncertain expectations both for the U.S. and global economies. These factors, combined with uncertainties in business and consumer confidence, continued concerns regarding the stability of some European Union member countries and slowing growth in China, have contributed to the expectations of slower domestic and global economic growth in the near term. We periodically evaluate the impact of the economic environment on our cash management, cash collection activities and volume of tests delivered.
55
As of the date of this report, we have not experienced a loss of principal on any of our short-term marketable securities, and we expect that we will continue to be able to access or liquidate these investments as needed to support our business activities. We periodically monitor the financial position of our significant third-party payors, which include Medicare and managed care companies. As of the date of this report, we do not expect the current economic environment to have a material negative impact on our ability to collect payments from third-party payors in the foreseeable future. We believe the economic environment and changes in the healthcare system continued to impact product payment cycles, growth in tests delivered and product revenue generated during the year ended December 31, 2014. We intend to continue to assess the impact of the economic environment on our business activities. If the economic environment does not improve or deteriorates, our business including our patient population, government and third-party payors and our distributors and suppliers could be negatively affected, resulting in a negative impact on our product revenues.
U.S. Healthcare Environment
Healthcare reform proposals and medical cost containment measures are being adopted in the U.S. and in many foreign countries. These reforms and measures, including those envisioned by the adoption in 2010 of the Affordable Care Act, could among other things limit the use of our tests and reduce reimbursement. We also expect that pricing of medical products and services will remain under pressure as alternative payment models such as bundling, value-based purchasing and accountable care organizations develop in the United States.
Sales of our tests in the United States and other countries is dependent upon the coverage decisions and reimbursement policies established by government healthcare programs and private health insurers. Market acceptance of our tests has and will continue to depend upon the ability to obtain an appropriate level of coverage for, and reimbursement from third-party payors for, our tests.
The healthcare industry has undergone significant change driven by various efforts to reduce costs. The effect of the implementation of the Affordable Care Act on our business is uncertain. Among other things, the law requires the medical device makers to pay a 2.3% excise tax on U.S. sales of certain medical devices. Beginning in 2013, each medical device manufacturer must pay sales tax on its medical devices that are listed with the FDA. Although the FDA has issued draft guidance that, if finalized, would regulate certain clinical laboratory tests that are developed and validated by a laboratory for its own use, referred to as LDTs, as medical devices, none of our LDTs, such as our Oncotype DX breast, colon and prostate cancer tests, are currently listed with the FDA. We cannot assure you that the tax will not apply to services such as ours in the future.
In addition, the recently enacted PAMA includes a substantial new payment system for certain clinical laboratory tests that will be effective starting January 1, 2017. Under PAMA, laboratories that receive the majority of their Medicare revenues from payments made under the CLFS or the Physician Fee Schedule would report, beginning January 1, 2016, and then every three years thereafter (or annually for “advanced diagnostic laboratory tests”), private payor payment rates and volumes for their tests. The Centers for Medicare and Medicaid Services, or CMS, will use the rates and volumes reported by laboratories to develop Medicare payment rates for the tests equal to the volume-weighted median of the private payor payment rates for the tests.
Any reductions to payment rates resulting from the new methodology are limited to 10% per test per year in each of the years 2017 through 2019 and to 15% per test per year in each of 2020 through 2022. Although CMS has not yet issued regulations to implement PAMA, we believe our Oncotype DX tests each would be considered an advanced diagnostic laboratory test. The initial payment rate (for a period not to exceed nine months) for an advanced diagnostic laboratory test will be set at the “actual list charge” for the test as reported by the laboratory. Insofar as the actual list charge substantially exceeds private payor rates (by more than 30%), CMS will have the ability to recoup excess payments made during the initial nine-month payment period.
Changes in Medicare Administrative Contractor (MAC) services
On a five year rotational basis, Medicare requests bids for its regional MAC services. In September 2013, the claims processing function transitioned from Palmetto GBA, to our current MAC, Noridian Healthcare Solutions. Palmetto GBA under their MolDx Program is continuing to establish coverage, coding and reimbursement policies for molecular diagnostics located within the jurisdiction applicable to our tests. An elimination of the MolDx Program or a change in the administrator of that program could impact the coverage or payment rates for our current tests and our ability to obtain Medicare coverage for products for which we do not yet have coverage or any products we may launch in the future, or delay payments for our tests.
56
Critical Accounting Policies and Significant Judgments and Estimates
This discussion and analysis of our financial condition and results of operations is based on our consolidated financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States. The preparation of these financial statements requires management to make estimates and judgments that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the financial statements, as well as revenues and expenses during the reporting periods. We evaluate our estimates and judgments on an ongoing basis. We base our estimates on historical experience and on various other factors we believe are reasonable under the circumstances, the results of which form the basis for making judgments about the carrying value of assets and liabilities that are not readily apparent from other sources. Actual results could therefore differ materially from those estimates under different assumptions or conditions.
We believe the following critical accounting policies reflect our more significant estimates and assumptions used in the preparation of our financial statements.
Revenue Recognition
We determine whether revenue is recognized on an accrual basis when test results are delivered or on a cash basis when cash is received from the payor. Our revenues for tests performed are recognized on an accrual basis when the following criteria are met: (1) persuasive evidence that an arrangement exists; (2) delivery has occurred or services have been rendered; (3) the fee is fixed or determinable; and (4) collectability is reasonably assured. When evaluating whether the fee is fixed or determinable and collectible, we consider whether we have sufficient history to reliably estimate the total fee that will be received from a payor and a payor’s individual payment patterns. Based upon at least several months of payment history, we review the number of tests paid against the number of tests billed and the payor’s outstanding balance for unpaid tests to determine whether payments are being made at a consistently high percentage of tests billed and at appropriate amounts given the contracted payment amount. The estimated accrual amounts per test, recorded upon delivery of a patient report, are calculated for each accrual payor and are based on the contracted price adjusted for individual payment patterns resulting from co-payment amounts and excluded services in healthcare plans.
To the extent all criteria set forth above are not met, including where there is no evidence of payment history at the time test results are delivered, product revenues are recognized on a cash basis when cash is received from the payor.
We enter into exclusive distribution agreements for the sale of one or more of our Oncotype DX tests with distributors outside of the United States. In these countries, the distributor generally provides us with certain marketing and administrative services within its territory. As a condition of these agreements, the distributor generally pays us an agreed upon fee per test and we process the tests. The same revenue recognition criteria described above generally apply to tests received through distributors. To the extent all criteria set forth above are not met when test results are delivered, product revenues are generally recognized when cash is received from the distributor.
Test revenue recognized on an accrual basis is recorded upon delivery of each test performed, net of any contractual discount at the amount that we expect to collect. We determine the amount we expect to collect on a per payor, per contract or arrangement basis, based on our analysis of historical average payments. This average amount is typically lower than the agreed upon amount due to several factors, such as the amount of patient co-payments, the existence of secondary payors and claim denials. We typically review our analysis annually, or at the time a contractual price change is implemented or when information comes to our attention that leads us to believe an adjustment may be warranted.
As of December 31, 2014, amounts outstanding for tests delivered, net of write‑downs and adjustments, which were not recognized as revenue upon delivery because our accrual revenue recognition criteria were not met and which had not been collected, totaled approximately $58.1 million. We cannot provide any assurance as to when, if ever, and to what extent these amounts will be collected.
From time to time, we receive requests for refunds of payments, generally due to overpayments made by third‑party payors. Upon becoming aware of a refund request, we establish an accrued liability for tests covered by the refund request until such time as we determine whether or not a refund is due. If we determine that a refund is due, we credit cash and reduce the accrued liability. Accrued refunds were $944,000 and $770,000 at December 31, 2014 and 2013, respectively.
Contract revenues are generally derived from studies conducted with biopharmaceutical and pharmaceutical companies and are recognized on a contract-specific basis. Under certain contracts, revenues are recognized as costs are incurred or assays are processed. We may exercise judgment when estimating full-time equivalent level of effort, costs incurred and time to project completion. For certain contracts, we utilize the performance-based method of revenue recognition, which
57
requires that we estimate the total amount of costs to be expended for a project and recognize revenue equal to the portion of costs expended to date. The estimated total costs to be expended are necessarily subject to revision from time-to-time as the underlying facts and circumstances change.
Accounts Receivable
We accrue an allowance for doubtful accounts against our accounts receivable based on estimates consistent with historical payment experience. Our allowance for doubtful accounts is evaluated quarterly and adjusted when trends or significant events indicate that a change in estimate is appropriate. Historically, the amounts of uncollectible accounts receivable that have been written off have been consistent with management’s expectations. We cannot assure you that we will not experience higher than expected write‑offs in the future. As of December 31, 2014 and 2013, our allowance for doubtful accounts was $3.6 million and $1.9 million, respectively. See “Liquidity and Capital Resources” for additional information, including a summary of accounts receivable aging by payor mix.
Research and Development Expenses
We enter into collaboration and clinical trial agreements with clinical collaborators and record these costs as research and development expenses. We record accruals for estimated study costs comprised of work performed by our collaborators under contract terms. The financial terms of these agreements are subject to negotiation, may vary from contract to contract, and may result in uneven payment flows. We determine our estimates through discussion with internal clinical development personnel and outside service providers as to the progress or stage of completion of services provided and the agreed upon fee to be paid for such services. Advance payments for goods or services that will be used or rendered for future research and development activities are deferred and capitalized and recognized as an expense as the goods are delivered or the related services are performed.
All potential future product programs outside of breast, colon and prostate cancer are in the research or development phase. Although we have estimated the time frame in which some of these products may be brought to market, the timing is uncertain given the technical challenges and clinical variables that exist between different types of cancers. We maintain information regarding costs incurred for activities performed under certain contracts with biopharmaceutical and pharmaceutical companies. However, we do not generally record or maintain information regarding costs incurred in research and development on a program-specific basis. Our research and development staff and associated infrastructure resources are deployed across several programs. Many of our costs are thus not attributable to individual programs. As a result, we are unable to determine the duration and completion costs of our research and development programs or when, if ever, and to what extent we will receive cash inflows from the commercialization and sale of a product.
Stock‑based Compensation Expense
We measure all stock-based payments to employees and directors, including grants of stock options, based on their relative fair values. Fair values of awards granted under our stock option plans and Employee Stock Purchase Plan, or ESPP, were estimated at grant or purchase rights offering dates using a Black Scholes option valuation model. Stock-based compensation expense related to stock option grants is estimated at the date of grant and stock-based compensation expense related to ESPP purchases is estimated at the beginning of each offering period based on these fair value calculations. The expense is recognized ratably over the requisite service period. The application of option valuation models requires significant judgment and the use of estimates, particularly surrounding assumptions used in determining fair value. The Black Scholes option valuation model requires the use of estimates such as stock price volatility and expected option lives to value stock-based compensation. Our assumptions regarding expected volatility are based on the historical volatility of our common stock. The expected life of options is estimated based on historical option exercise data and assumptions related to unsettled options. The expected life of stock issuable pursuant to the ESPP is six months, or the duration of the purchase period. Expected forfeiture rates for stock option grants are based on historical data, and compensation expense is adjusted for actual results. We do not include expected forfeiture rates when calculating stock-based compensation expense for stock issuable pursuant to the ESPP due to the short duration of the purchase period; however, we do adjust the expense for actual results.
Stock-based compensation expense related to restricted stock unit, or RSU, awards is based on the market value of our common stock at the date of grant and is recognized as expense ratably over the requisite service period. Expected forfeiture rates for RSUs are based on historical data, and compensation expense is adjusted for actual results.
58
We review our valuation assumptions on an ongoing basis, and, as a result, our assumptions used to value stock awards granted in future periods may change. See Note 9, “Stock‑based Compensation,” in the Notes to Consolidated Financial Statements in Part II, Item 8 of this Annual Report on Form 10‑K for more information.
Deferred Tax Assets
We are required to reduce our deferred tax assets by a valuation allowance if it is more likely than not that some or all of our deferred tax assets will not be realized. We must use judgment in assessing the potential need for a valuation allowance, which requires an evaluation of both negative and positive evidence. The weight given to the potential effect of negative and positive evidence should be commensurate with the extent to which it can be objectively verified. In determining the need for and amount of our valuation allowance, if any, we assess the likelihood that we will be able to recover our deferred tax assets using historical levels of income, estimates of future income and tax planning strategies. As a result of historical cumulative losses and, based on all available evidence, we believe it is more likely than not that our recorded net deferred tax assets will not be realized. Accordingly, we recorded a valuation allowance against all of our net deferred tax assets at both December 31, 2014 and 2013. We will continue to maintain a full valuation allowance on our deferred tax assets until there is sufficient evidence to support the reversal of all or some portion of this allowance
Results of Operations
Comparison of Years Ended December 31, 2014, 2013 and 2012
We recorded a net loss of $24.6 million for the year ended December 31, 2014, compared to a net loss of $12.8 million and net income of $8.2 million for the years ended December 31, 2013 and 2012, respectively. On a basic per share basis, net income (loss) was $(0.78), $(0.42) and $0.27 for the years ended December 31, 2014, 2013 and 2012, respectively. On a diluted per share basis, net income (loss) was $(0.78), $(0.42) and $0.26 for the years ended December 31, 2014, 2013 and 2012, respectively. We may incur net losses in future periods due to future spending and fluctuations in our business, and we may not achieve or maintain sustained profitability in the future.
Revenues
We derive our revenues primarily from product sales and, to a lesser extent, from contract research arrangements. We operate in one industry segment. As of December 31, 2014, substantially all of our product revenues have been derived from the sale of our Oncotype DX breast cancer test. Payors are billed upon generation and delivery of test results to the physician. Product revenues are recorded on a cash basis unless a contract or arrangement to pay is in place with the payor at the time of billing and collectability is reasonably assured. Contract revenues are derived from studies conducted with biopharmaceutical and pharmaceutical companies and are recorded as contractual obligations are completed.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended December 31, |
||||||||
|
|
|
2014 |
|
2013 |
|
2012 |
|
|||
|
|
|
(In thousands) |
||||||||
|
Product revenues |
|
$ |
275,706 |
|
$ |
259,192 |
|
$ |
233,457 |
|
|
Contract revenues |
|
|
— |
|
|
2,403 |
|
|
1,716 |
|
|
Total revenues |
|
$ |
275,706 |
|
$ |
261,595 |
|
$ |
235,173 |
|
|
Period over period dollar increase in product revenues |
|
$ |
16,514 |
|
$ |
25,735 |
|
|
|
|
|
Period over period percentage increase in product revenues |
|
|
6 |
% |
|
11 |
% |
|
|
|
The year over year increases in product revenues resulted, in part, from increased adoption, as evidenced by a 12% increase in test volume for the year ended December 31, 2014 and a 15% increase in test volume for the year ended December 31, 2013. Test volume increases exceeded revenue increases primarily due to our Oncotype DX prostate cancer test, which does not yet have established reimbursement, and tests from certain international markets where we have not yet established reimbursement. We also experienced 19% and 37% increases in international revenue for the years ended December 31, 2014 and 2013, respectively. Approximately $199.9 million, or 73%, of product revenues for the year ended December 31, 2014, was recorded on an accrual basis and recognized at the time the test results were delivered, compared to $185.7 million, or 72%, and $154.3 million, or 66%, of product revenues for the years ended December 31, 2013 and 2012, respectively. For all periods, the balance of product revenues was recognized upon cash collection as payments were received.
59
Product revenues related to Medicare patients for the year ended December 31, 2014 were $55.9 million, or 20%, of product revenues, compared to $54.4 million, or 21%, and $52.5 million, or 22%, of product revenues for the years ended December 31, 2013 and 2012, respectively. No other third‑party payors comprised product revenues of 10% or more for those years. International product revenues were $45.0 million, or 16% of product revenues, for the year ended December 31, 2014, compared to $37.9 million, or 15%, and $27.7 million, or 12%, of product revenues, for the years ended December 31, 2013 and 2012, respectively.
There were no contract revenues for the year ended December 31, 2014. Contract revenues were $2.4 million and $1.7 million for the years ended December 31, 2013 and 2012, respectively. Contract revenues represented studies assessing our gene expression technology or collaborative work in gene selection and protocol design with our pharmaceutical partners. The decrease in contract revenues for 2014 compared to 2013 was due to a decrease in activities with collaboration partners. The increase in contract revenues for 2013 compared to 2012 was primarily due to $1.5 million of contract revenue recognized in the fourth quarter upon completion of clinical validation work for Pfizer Inc. We expect that our contract revenues will continue to fluctuate based on the number and timing of studies being conducted.
Cost of Product Revenues
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended December 31, |
||||||||
|
|
|
2014 |
|
2013 |
|
2012 |
|
|||
|
|
|
(In thousands) |
||||||||
|
Tissue sample processing costs |
|
$ |
38,712 |
|
$ |
32,679 |
|
$ |
28,540 |
|
|
Stock-based compensation |
|
|
497 |
|
|
483 |
|
|
441 |
|
|
Total tissue sample processing costs |
|
|
39,209 |
|
|
33,162 |
|
|
28,981 |
|
|
License fees |
|
|
9,533 |
|
|
8,938 |
|
|
8,037 |
|
|
Total cost of product revenues |
|
$ |
48,742 |
|
$ |
42,100 |
|
$ |
37,018 |
|
|
Period over period dollar increase |
|
$ |
6,642 |
|
$ |
5,082 |
|
|
|
|
|
Period over period percentage increase |
|
|
16 |
% |
|
14 |
% |
|
|
|
Cost of product revenues represents the cost of materials, direct labor, equipment and infrastructure expenses associated with processing tissue samples (including sample accessioning, histopathology, anatomical pathology, paraffin extraction, RT‑PCR, quality control analyses and shipping charges to transport tissue samples) and license fees. Infrastructure expenses include allocated facility occupancy and information technology costs. Costs associated with performing our tests are recorded as tests are processed. Costs recorded for tissue sample processing represent the cost of all the tests processed during the period regardless of whether revenue was recognized with respect to that test. Royalties for licensed technology calculated as a percentage of product revenues and fixed annual payments relating to the launch and commercialization of Oncotype DX tests are recorded as license fees in cost of product revenues at the time product revenues are recognized or in accordance with other contractual obligations. While license fees are generally calculated as a percentage of product revenues, the percentage increase in license fees does not correlate exactly to the percentage increase in product revenues because certain agreements contain provisions for fixed annual payments and other agreements have tiered rates and payments that may be capped at annual minimum or maximum amounts. License fees represent a significant component of our cost of product revenues and are expected to remain so for the foreseeable future.
Tissue sample processing costs increased $6.0 million, or 18%, in 2014 compared to 2013, and $4.1 million, or 15%, in 2013 compared to 2012, driven primarily by increases in test volume of 12% and 15% in 2014 and 2013, respectively. The increase in 2014 was also due to higher costs related to our prostate cancer test. License fees increased $595,000, or 7%, in 2014 compared to 2013 and increased $901,000, or 11%, in 2013 compared to 2012. The increases in 2014 and 2013 primarily resulted from year over year increases in product revenues of 6 % and 11%, respectively. Also, for the year ended December 31, 2013, the increase in license fees includes a $150,000 milestone license fee paid in connection with the launch of our prostate cancer test in May 2013.
60
Research and Development Expenses
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended December 31, |
||||||||
|
|
|
2014 |
|
2013 |
|
2012 |
||||
|
|
|
(In thousands) |
||||||||
|
Personnel-related expenses |
|
$ |
33,722 |
|
$ |
29,380 |
|
$ |
24,724 |
|
|
Stock-based compensation |
|
|
4,569 |
|
|
4,873 |
|
|
3,992 |
|
|
Collaboration and licensing expenses |
|
|
6,426 |
|
|
12,637 |
|
|
1,907 |
|
|
Reagents and laboratory supplies |
|
|
2,430 |
|
|
3,113 |
|
|
3,152 |
|
|
Allocated information technology, facilities and other costs |
|
|
414 |
|
|
10,933 |
|
|
11,159 |
|
|
Other costs |
|
|
8,503 |
|
|
5,397 |
|
|
4,170 |
|
|
Total research and development expenses |
|
$ |
56,064 |
|
$ |
66,333 |
|
$ |
49,104 |
|
|
Period over period dollar (decrease) increase |
|
$ |
(10,269) |
|
$ |
17,229 |
|
|
|
|
|
Period over period percentage (decrease) increase |
|
|
(15) |
% |
|
35 |
% |
|
|
|
Research and development expenses represent costs incurred to develop our technology, such as NGS, our proprietary liquid platform and continuous process improvement, and carry out clinical studies, primarily related to our ongoing work in breast, colon and prostate cancer. Research and development expenses include personnel‑related expenses, reagents and supplies used in research and development laboratory work, infrastructure expenses, including allocated overhead and facility occupancy costs, contract services and other outside costs. Research and development expenses also include costs related to activities performed under contracts with biopharmaceutical and pharmaceutical companies.
The $10.3 million, or 15%, decrease in research and development expenses for 2014 compared to 2013 included a $10.5 million decrease in allocated information technology, facilities and other costs, a $6.2 million decrease in collaboration and licensing expenses and a $683,000 decrease in reagents and laboratory supplies partially offset by a $4.3 million increase in personnel‑related expenses. The $6.2 million decrease in collaboration and licensing expenses was primarily due to a $9.0 million up-front payment in 2013, under an exclusive licensing agreement for technology and intellectual property to further develop, validate and subsequently commercialize a multi‑gene test to predict benefit from DNA damage‑based chemotherapy drugs in breast cancer. Of the $4.3 million increase in personnel‑related expenses, $2.8 million was attributable to increases in salaries, benefits and related expenses, $1.1 million was attributable to higher contract labor and consulting expenses, and $479,000 was related to higher bonus payments.
The $17.2 million, or 35%, increase in research and development expenses for 2013 compared to 2012 included a $10.7 million increase in collaboration and licensing expenses, a $4.7 million increase in personnel‑related expenses, a $881,000 increase in stock‑based compensation, a $673,000 increase in travel, meetings and seminars expenses and a $555,000 increase in infrastructure expense. Of the $4.7 million increase in personnel‑related expenses, $3.5 million was attributable to increases in salaries, benefits and related expenses, and $767,000 was related to higher bonus payments.
The increase in personnel-related expenses for 2014 and 2013 was primarily attributable to increases in salaries and benefits due to increased headcount to support the launch of our prostate cancer test, as well as projects related to our product pipeline and ongoing work in NGS and our proprietary platform for liquid biopsy tests. The decrease in allocated information technology, facilities and other costs is primarily due to preparing for our prostate cancer product launch in 2013, which included project work from our various information technology groups, allocated based on specific development projects, as well as an increase in research and development support allocated to other functional areas for the year ended December 31, 2014.
The increase in collaboration and licensing expenses for 2013 was primarily attributable to a $9.0 million up‑front payment under the exclusive licensing agreement for technology and intellectual property discussed above. We expect our research and development expenses, exclusive of the up‑front license payment, to increase in future periods due to increased investment in our new product pipeline for breast, colon, prostate, renal and other cancers, along with increased investment in NGS and our proprietary platform for liquid biopsy tests.
We expect our research and development expenses to increase in future periods due to increased investment in our new product pipeline for breast, colon, prostate, renal and other cancers, along with increased investment in NGS and our proprietary liquid platform.
61
Selling and Marketing Expenses
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended December 31, |
|
||||||||
|
|
|
2014 |
|
2013 |
|
2012 |
|
|
|||
|
|
|
(In thousands) |
|
||||||||
|
Personnel-related expenses |
|
$ |
67,952 |
|
$ |
56,077 |
|
$ |
48,189 |
|
|
|
Stock-based compensation |
|
|
4,396 |
|
|
4,369 |
|
|
4,191 |
|
|
|
Promotional and marketing materials |
|
|
18,852 |
|
|
17,604 |
|
|
14,647 |
|
|
|
Travel, meetings and seminars |
|
|
13,749 |
|
|
13,085 |
|
|
11,070 |
|
|
|
Allocated information technology, facilities and other costs |
|
|
26,294 |
|
|
16,273 |
|
|
12,231 |
|
|
|
Other costs |
|
|
3,615 |
|
|
3,194 |
|
|
3,225 |
|
|
|
Total selling and marketing expenses |
|
$ |
134,858 |
|
$ |
110,602 |
|
$ |
93,553 |
|
|
|
Period over period dollar increase |
|
$ |
24,256 |
|
$ |
17,049 |
|
|
|
|
|
|
Period over period percentage increase |
|
|
22 |
% |
|
18 |
% |
|
|
|
|
Our selling and marketing expenses consist primarily of personnel‑related expenses, education and promotional expenses, market analysis and development expenses and infrastructure expenses, including allocated facility occupancy and information technology costs. These expenses include the costs of educating physicians, laboratory personnel and other healthcare professionals regarding our genomic technologies, how our Oncotype DX tests are developed and validated and the value of the quantitative information that our tests provide. Selling and marketing expenses also include the costs of sponsoring continuing medical education, medical meeting participation and dissemination of scientific and economic publications related to our Oncotype DX tests. Our sales force compensation includes annual salaries and eligibility for quarterly commissions based on the achievement of predetermined sales goals and other management objectives.
The $24.3 million, or 22%, increase in selling and marketing expenses for 2014 compared to 2013 was primarily due to U.S. and international sales and operations support and included an $11.9 million increase in personnel‑related expenses, a $10.0 million increase in allocated information technology, facilities and other costs, a $1.2 million increase in promotional and marketing materials and a $664,000 increase in travel, meetings and seminars expenses. Of the $11.9 million increase in personnel‑related expenses, $10.1 million was attributable to increases in salaries, benefits and related expenses due primarily to increased headcount, including new hires related to our launch of our prostate cancer test in May 2013 and annual salary increases, $975,000 was attributable to higher commission and bonus payments and $846,000 was attributable to increased consulting expenses. The increase in allocated information technology, facilities and other costs is primarily due to increased selling activities, related to our newly established prostate sales and marketing programs and information technology allocations for various projects related to scaling our commercial systems worldwide, as well as an increase in research and development support allocated from other functional areas for the year ended December 31, 2014.
The $17.0 million, or 18%, increase in selling and marketing expenses for 2013 compared to 2012 was primarily due to U.S. and international sales and operations support and included a $7.9 million increase in personnel‑related expenses, a $4.0 million increase in allocated information technology, facilities and other costs, a $3.0 million increase in promotional and marketing materials and a $2.0 million increase in travel, meetings and seminars expenses. Of the $7.9 million increase in personnel‑related expenses, $5.4 million was attributable to increases in salaries, benefits and related expenses due primarily to increased headcount, including new hires related to the launch of our prostate cancer test in May 2013 and annual salary increases, $1.9 million was attributable to higher commission and bonus payments and $565,000 was attributable to increased consulting expenses. The increase in allocated information technology, facilities and other costs is primarily due to increased work performed by our various information technology groups, allocated based on specific departmental projects. The increases in promotional and marketing materials expense and travel, meeting and seminars expense are primarily related to the expansion of our international operations and preparing for and executing our prostate cancer product launch.
We expect selling and marketing expenses will continue to increase in future periods due to our efforts to establish adoption of and reimbursement for our new products, continued investment in our global commercial infrastructure and increases in our sales force.
62
General and Administrative Expenses
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended December 31, |
||||||||
|
|
|
2014 |
|
2013 |
|
2012 |
|
|||
|
|
|
(In thousands) |
||||||||
|
Personnel-related expenses |
|
$ |
41,328 |
|
$ |
37,697 |
|
$ |
33,811 |
|
|
Stock-based compensation |
|
|
7,076 |
|
|
7,732 |
|
|
6,480 |
|
|
Occupancy and equipment expenses |
|
|
20,671 |
|
|
18,305 |
|
|
15,793 |
|
|
Billing and collection fees |
|
|
9,348 |
|
|
8,898 |
|
|
8,598 |
|
|
Bad debt expense |
|
|
6,697 |
|
|
6,169 |
|
|
3,408 |
|
|
Professional fees and other expenses |
|
|
8,456 |
|
|
8,016 |
|
|
7,347 |
|
|
Information technology, facilities and other cost allocations |
|
|
(33,907) |
|
|
(32,425) |
|
|
(28,373) |
|
|
Total general and administrative expenses |
|
$ |
59,669 |
|
$ |
54,392 |
|
$ |
47,064 |
|
|
Period over period dollar increase |
|
$ |
5,277 |
|
$ |
7,328 |
|
|
|
|
|
Period over period percentage increase |
|
|
10 |
% |
|
16 |
% |
|
|
|
Our general and administrative expenses consist primarily of personnel‑related expenses, occupancy and equipment expenses, including rent and depreciation expenses, billing and collection fees, bad debt expense, professional fees and other expenses, including intellectual property defense and prosecution costs, and other administrative costs, partially offset by cost allocations to our commercial laboratory operations, research and development, and sales and marketing functions, including allocated information technology and facility occupancy costs.
The $5.3 million, or 10%, increase in general and administrative expenses for 2014 compared to 2013 included a $3.6 million increase in personnel‑related expenses, a $2.4 million increase in occupancy and equipment expenses and a $528,000 increase in bad debt expense partially offset by a $1.5 million decrease in information technology, facilities and other costs allocated to other functional areas and a $656,000 decrease in stock-based compensation. Of the $3.6 million increase in personnel‑related expenses, $2.2 million was attributable to increases in salaries and benefits expenses, primarily resulting from increased headcount, $1.1 million was attributable to higher contract labor and consulting expenses to support growth of our business and $286,000 was related to higher bonus payments.
The $7.3 million, or 16%, increase in general and administrative expenses for 2013 compared to 2012 included a $3.9 million increase in personnel‑related expenses, a $2.8 million increase in bad debt expense, a $2.5 million increase in occupancy and equipment expenses, a $1.3 million increase in stock‑based compensation, a $562,000 increase in investor and public relations expense, partially offset by a $4.1 million increase in information technology, facilities and other costs allocated to other functional areas. Of the $3.9 million increase in personnel‑related expenses, $2.5 million was attributable to increases in salaries and benefits expenses, primarily resulting from increased headcount, $749,000 was attributable to higher bonus payments and $597,000 was attributable to higher contract labor and consulting expenses to support growth of our business.
We expect general and administrative expenses to increase in future periods as we hire additional staff and incur other expenses to support the growth of our business, and to the extent we spend more on both billing and collections fees and bad debt expense.
Impairment of Investments in Privately Held Companies
Each of our equity investments is reviewed at least annually for impairment or whenever events or changes in circumstances indicate that the carrying value of the investment might not be recoverable. At December 31, 2013, we concluded that the indicators of impairment of our investment in a privately held company were other than temporary, and therefore wrote off the remaining asset balance of $643,000. There was no additional impairment recognized for the years ended December 31, 2014, 2013 and 2012.
Interest Income
Interest income was $192,000 for the year ended December 31, 2014, compared to $222,000 and $295,000 for years ended December 31, 2013 and 2012, respectively. We expect our interest income will remain nominal if the current low interest rate environment continues.
63
Other Income (Expense), Net
Other expense, net was $764,000 for the year ended December 31, 2014, compared to other expense, net of $158,000 and $58,000 for the years ended December 30, 2013 and 2012, respectively. Other expense, net for the years ended December 31, 2014 and 2013 was primarily related to $790,000 and $158,000 of net foreign currency transaction losses, respectively, resulting from valuation adjustments to our international accounts receivable balance. Other expense, net for the year ended December 31, 2012 included a $98,000 loss on an investment in a private company accounted for using the equity method and $46,000 of net foreign currency transaction losses, offset by a gain on disposal of assets of $86,000. We expect other income (expense), net to continue to fluctuate based on fluctuations in exchange rates that impact our foreign exchange transaction gains and losses.
Income Tax Expense (Benefit)
For the years ended December 31, 2014, 2013, and 2012, we recorded income tax expense of $393,000, $346,000 and $422,000, respectively. The 2014 and 2013 tax expense is principally comprised of foreign income tax and miscellaneous state income tax. The 2012 tax expense is principally comprised of federal alternative minimum tax, state income taxes and foreign income taxes.
As a result of historical losses and based on all current available evidence, we believe that it is more likely than not that our recorded net deferred tax assets will not be realized. Accordingly, we recorded a full valuation allowance on our net deferred tax assets for the years ended December 31, 2014, 2013 and 2012, respectively. We will continue to maintain a full valuation allowance on our deferred tax assets until there is sufficient evidence to support the reversal of all or some portion of this allowance.
Liquidity and Capital Resources
As of December 31, 2014, we had an accumulated deficit of $194.9 million. We may incur net losses in the future, and we cannot provide assurance as to when, if ever, we will achieve sustained profitability. We expect that our research and development expenses, selling and marketing and general and administrative expenses will increase in future periods and, as a result, we will need to continue to generate significant product revenues to achieve sustained profitability.
Sources (Uses) of Liquidity
At December 31, 2014, we had cash, cash equivalents and short‑term investments of $103.7 million compared to $105.4 million at December 31, 2013. The $1.7 million decrease was attributable to investments in the growth of our business, including research and development, global expansion, and activities related to reimbursement coverage of our tests. In accordance with our investment policy, available cash is invested in short-term and long-term, low-risk, investment-grade debt instruments. Our cash and marketable securities are held in a variety of interest-bearing instruments including money market accounts and high-grade commercial paper and corporate bonds.
Historically we have financed our operations primarily through sales of our equity securities and cash received in payment for our tests. Certain purchases of equipment and leasehold improvements have been partially financed through capital equipment financing arrangements.
In December 2012, we entered into a collared accelerated share repurchase agreement with a financial institution for the purpose of repurchasing up to $30.0 million of our outstanding shares of common stock. Under the terms of this agreement, in December 2012, we paid $30.0 million to a financial institution and received 984,074 shares of our common stock, representing the minimum number of shares deliverable under the agreement. In February 2013, upon termination of the agreement and in accordance with the share delivery provisions of the agreement, we received an additional 77,257 shares of our common stock based on the average of the daily volume weighted-average prices of our common stock during a specified period less a predetermined discount per share. As a result, the average purchase price of our common stock under the accelerated share repurchase program was $28.27 per share.
64
Accounts Receivable
At December 31, 2014 and 2013, $34.9 million, or 19%, and $29.4 million, or 17%, respectively, of our total assets consisted of accounts receivable. The $5.5 million year over year increase in accounts receivable was primarily attributable to increased revenues and additional payors moving from cash basis to accrual basis during 2014. Days sales outstanding, or DSOs, is a measure of the average number of days it takes for us to collect our accounts receivable, calculated from the date that tests are billed. At December 31, 2014 and 2013, our weighted average DSOs were 78 days and 72 days, respectively. The increase in the weighted average DSOs is primarily a result of the overall increase in accounts receivable, an increase in Medicare accounts receivable due to a change in the MAC processing Medicare claims for our tests, and the increase in international payors, who typically have longer payment cycles. The timing of our billing and cash collections also causes fluctuations in our monthly DSOs and accounts receivable.
The following tables summarize accounts receivable by payor mix at December 31, 2014 and 2013:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
December 31, 2014 |
|
|||||||||||||||||||||
|
|
|
|
|
|
% of |
|
|
|
|
31 - 60 |
|
61 - 90 |
|
91 - 120 |
|
121 to 180 |
|
Over 180 |
|
|||||
|
|
|
Total |
|
Total |
|
Current |
|
Days |
|
Days |
|
Days |
|
Days |
|
Days |
|
|||||||
|
|
|
(In thousands) |
|
|||||||||||||||||||||
|
Managed care and other |
|
$ |
28,303 |
|
73 |
% |
$ |
11,469 |
|
$ |
3,637 |
|
$ |
2,649 |
|
$ |
2,085 |
|
$ |
2,485 |
|
$ |
5,978 |
|
|
Medicare |
|
|
10,241 |
|
27 |
|
|
5,187 |
|
|
3,621 |
|
|
469 |
|
|
190 |
|
|
231 |
|
|
543 |
|
|
Total |
|
|
38,544 |
|
100 |
% |
$ |
16,656 |
|
$ |
7,258 |
|
$ |
3,118 |
|
$ |
2,275 |
|
$ |
2,716 |
|
$ |
6,521 |
|
|
Allowance for doubtful accounts |
|
|
(3,628) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net accounts receivable |
|
$ |
34,916 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
December 31, 2013 |
|
|||||||||||||||||||||
|
|
|
|
|
|
% of |
|
|
|
|
31 - 60 |
|
61 - 90 |
|
91 - 120 |
|
121 to 180 |
|
Over 180 |
|
|||||
|
|
|
Total |
|
Total |
|
Current |
|
Days |
|
Days |
|
Days |
|
Days |
|
Days |
|
|||||||
|
|
|
(In thousands) |
|
|||||||||||||||||||||
|
Managed care and other |
|
$ |
22,535 |
|
72 |
% |
$ |
10,083 |
|
$ |
3,513 |
|
$ |
2,186 |
|
$ |
1,641 |
|
$ |
1,988 |
|
$ |
3,124 |
|
|
Medicare |
|
|
8,818 |
|
28 |
|
|
5,453 |
|
|
1,950 |
|
|
344 |
|
|
372 |
|
|
176 |
|
|
523 |
|
|
Total |
|
|
31,353 |
|
100 |
% |
$ |
15,536 |
|
$ |
5,463 |
|
$ |
2,530 |
|
$ |
2,013 |
|
$ |
2,164 |
|
$ |
3,647 |
|
|
Allowance for doubtful accounts |
|
|
(1,907) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net accounts receivable |
|
$ |
29,446 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash Flows
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2014 |
|
2013 |
|
2012 |
|
|||
|
|
|
(In thousands) |
|
|||||||
|
As of December 31: |
|
|
|
|
|
|
|
|
|
|
|
Cash, cash equivalents and short-term investments |
|
$ |
103,660 |
|
$ |
105,350 |
|
$ |
99,065 |
|
|
Working capital |
|
|
110,182 |
|
|
115,160 |
|
|
104,869 |
|
|
For the year ended December 31: |
|
|
|
|
|
|
|
|
|
|
|
Cash provided by (used in): |
|
|
|
|
|
|
|
|
|
|
|
Operating activities |
|
|
2,287 |
|
|
8,552 |
|
|
25,962 |
|
|
Investing activities |
|
|
(14,223) |
|
|
(7,006) |
|
|
(28,223) |
|
|
Financing activities |
|
|
8,383 |
|
|
13,728 |
|
|
(12,603) |
|
|
Capital expenditures (included in investing activities above) |
|
|
(10,455) |
|
|
(11,008) |
|
|
(10,056) |
|
Net cash provided by operating activities for the year ended December 31, 2014 was $2.3 million, compared to net cash provided by operating activities of $8.6 million and $26.0 million for the years ended December 31, 2013 and 2012, respectively. Net cash provided by operating activities includes net income (loss) adjusted for certain non‑cash items and changes in assets and liabilities. Net cash provided by operating activities of $2.3 million for the year ended December 31, 2014 reflected a net loss of $24.6 million, adjusted for $23.4 million of depreciation and stock‑based compensation expense, a $3.8 million increase in accrued compensation expense and employee benefits, a $3.1 million increase in accrued expenses and
65
other liabilities, a $985,000 increase in accounts payable and a $741,000 decrease in prepaid expenses and other assets, partially offset by a $5.5 million increase in accounts receivable.
Net cash provided by operating activities of $8.6 million for the year ended December 31, 2013 reflected net loss of $12.8 million, adjusted for $23.8 million of depreciation and stock‑based compensation expense, a $1.9 million increase in accrued compensation expense and employee benefits, a $1.3 million increase in accrued expenses and other liabilities, a $1.2 million increase in accounts payable and a $643,000 increase in impairment on investments, partially offset by a $7.2 million increase in accounts receivable. Net cash provided by operating activities of $26.0 million for the year ended December 31, 2012 reflected net income of $8.2 million, adjusted for $20.6 million of depreciation and stock‑based compensation expense, a $2.0 million increase in accrued compensation expense and related benefits and a $1.6 million increase in accrued expenses and other liabilities, partially offset by a $2.1 million decrease in accounts payable, a $1.7 million decrease in deferred revenues, a $1.6 million increase in prepaid expenses and other assets and a $1.2 million increase in accounts receivable.
Net cash used in investing activities was $14.2 million for the year ended December 31, 2014, compared to $7.0 million and $28.2 million for the years ended December 31, 2013 and 2012, respectively. Our investing activities have consisted predominantly of purchases and maturities of marketable securities and capital expenditures. Net cash used in investing activities of $14.2 million for the year ended December 31, 2014 included $10.5 million of capital expenditures, a $2.0 million investment in privately held companies and $1.9 million in net purchase of marketable securities. Net cash used in investing activities of $7.0 million for the year ended December 31, 2013 included $11.0 million in capital expenditures and $5.0 million investment in privately held companies, partially offset by $9.0 million in net maturities of marketable securities.
Net cash provided by financing activities was $8.4 million for the year ended December 31, 2014, compared to net cash provided by financing activities of $13.7 million and net cash used in financing activities of $12.6 million for the years ended December 31, 2013 and 2012, respectively. Our financing activities included sales of our equity securities and repurchases of our common stock. Net cash provided by financing activities for the year ended December 31, 2014 comprises $12.0 million in proceeds from the issuance of our common stock upon the exercise of employee stock options and stock purchased pursuant to our ESPP, partially offset by cash paid for tax withholdings in the amount of $3.6 million related to net share settlements of restricted stock units and awards. Net cash provided by financing activities for the year ended December 31, 2013 included $16.6 million in proceeds from the issuance of our common stock upon the exercise of employee stock options and stock purchased pursuant to our ESPP, partially offset by cash paid for tax withholdings in the amount of $2.8 million related to net share settlements of restricted stock units and awards. Net cash used in financing activities for the year ended December 31, 2012 included $30.1 million used to repurchase our common stock under our accelerated share repurchase program, partially offset by $18.8 million in proceeds from the issuance of our common stock upon the exercise of employee stock options and stock purchased pursuant to our ESPP, partially offset by cash paid for tax withholdings in the amount of $1.3 million related to net share settlements of restricted stock units and awards.
Contractual Obligations
The following table summarizes our significant contractual obligations as of December 31, 2014 and the effect those obligations are expected to have on our liquidity and cash flows in future periods:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Payments Due by Period |
|
|||||||||||||
|
|
|
|
|
|
Less Than |
|
|
|
|
|
|
|
More Than |
|
||
|
|
|
Total |
|
1 Year |
|
1 - 3 Years |
|
3 - 5 Years |
|
5 Years |
|
|||||
|
|
|
(In thousands) |
|
|||||||||||||
|
Non-cancelable operating lease obligations |
|
$ |
14,647 |
|
$ |
3,928 |
|
$ |
7,739 |
|
$ |
2,980 |
|
$ |
— |
|
Our non‑cancelable operating lease obligations are for laboratory and office space. We lease various facilities in Redwood City, California, totaling 144,900 square feet. The lease terms expire between March 2018 and March 2019, each with an option for us to extend the terms of the lease for an additional five years. We also lease 7,500 square feet of space in Geneva, Switzerland. This lease expires in May 2016.
We are required to make a series of fixed annual payments under a collaboration agreement beginning with the January 2010 launch of our Oncotype DX colon cancer test. We made payments under this agreement of $450,000, $450,000 and $300,000 in 2014, 2013 and 2012, respectively. We are also required to make a series of fixed annual payments under a collaboration agreement beginning with the May 2013 commercial launch of our Oncotype DX prostate cancer test. We made
66
payments under this agreement of $200,000 and $150,000 in 2014 and 2013, respectively. As of December 31, 2014, future annual payments under these agreements totaled $550,000 and are due in 2015. Further, we are required to make a series of fixed annual payments under a collaboration agreement beginning with a one year anniversary of achieving a key milestone for our DCIS clinical study in June 2014. As of December 31, 2014, future annual payments under this agreement totaled $1.7 million, including payments of $604,000, $604,000, and $504,000 due in 2015, 2016, and 2017, respectively. However, because these agreements may be terminated by either party upon 30 days’ prior written notice, these payments are not included in the table above.
We have also committed to make potential future payments to third parties as part of our collaboration and licensing agreements. Payments under these agreements generally become due and payable only upon achievement of specific project milestones. Because the achievement of these milestones is generally neither probable nor reasonably estimable, such commitments have not been included in the table above.
Off‑Balance Sheet Activities
As of December 31, 2014, we had no material off‑balance sheet arrangements.
Operating Capital and Capital Expenditure Requirements
We achieved positive operating cash flow for the years ended December 31, 2014, 2013 and 2012. We currently anticipate that our cash, cash equivalents and short-term marketable securities, together with payments for our Oncotype DX tests, will be sufficient to fund our operations and facilities expansion plans for at least the next 12 months, including the expansion of our research and development programs, our NGS and proprietary liquid platform development efforts, our efforts to expand adoption of and reimbursement for our Oncotype DX colon and prostate cancer and DCIS tests and our international expansion efforts. We expect to spend approximately $11 million over the next 12 months for planned laboratory equipment, information technology and facilities expansion. We may also use cash to acquire or invest in complementary businesses, technologies, services or products. We expect that our cash, cash equivalents and short term marketable securities will also be used to fund working capital and for other general corporate purposes, such as licensing technology rights, distribution arrangements for our tests both within and outside of the United States or expanding our direct sales capabilities worldwide.
The amount and timing of actual expenditures may vary significantly depending upon a number of factors, such as the amount of cash provided by our operations, the progress of our commercialization efforts, product development, regulatory requirements, progress in reimbursement for our tests and available strategic opportunities for acquisition of or investment in complementary businesses, technologies, services or products.
We cannot be certain that our international expansion plans, efforts to expand adoption of and reimbursement for our Oncotype DX colon and prostate cancer and DCIS tests or the development of future products will be successful or that we will be able to raise sufficient additional funds to see these activities through to a successful result. It may take years to move any one of a number of product candidates in research through development and validation to commercialization.
Our future funding requirements will depend on many factors, including the following:
|
· |
the rate of progress in establishing and maintaining reimbursement arrangements with domestic and international third-party payors; |
|
· |
the cost of expanding our commercial and laboratory operations, including our selling and marketing efforts; |
|
· |
the rate of progress and cost of research and development activities associated with expansion of our current tests and the development of new tests; |
|
· |
the rate of progress and cost of selling and marketing activities associated with expanding adoption of our tests; |
|
· |
the rate of progress and cost of research and development activities associated with NGS and our proprietary liquid platform; |
|
· |
the cost of acquiring, licensing or investing in technologies, including NGS and our proprietary liquid platform; |
67
|
· |
the cost of acquiring or investing in complementary businesses or assets; |
|
· |
costs related to future product launches; |
|
· |
the cost of acquiring or achieving access to tissue samples and technologies; |
|
· |
the cost of filing, prosecuting, defending and enforcing any patent claims and other intellectual property rights; |
|
· |
the effect of competing technological and market developments; |
|
· |
costs related to international expansion; |
|
· |
costs and delays in product development as a result of any changes in regulatory oversight applicable to our products or operations; |
|
· |
the impact of changes in Federal, state and international taxation; and |
|
· |
the economic and other terms and timing of any collaborations, licensing or other arrangements into which we may enter or investments or acquisitions we might seek to effect. |
If we are not able to generate and maintain sustained product revenues to finance our cash requirements, we will need to finance future cash needs primarily through public or private equity offerings, debt financings, borrowings or strategic collaborations or licensing arrangements. If we raise funds by issuing equity securities, dilution to stockholders may result. Any equity securities issued may also provide for rights, preferences or privileges senior to those of holders of our common stock. If we raise funds by issuing debt securities, these debt securities would have rights, preferences and privileges senior to those of holders of our common stock. The terms of debt securities or borrowings could impose significant restrictions on our operations. If we raise funds through collaborations and licensing arrangements, we might be required to relinquish significant rights to our technologies or products, or grant licenses on terms that are not favorable to us. The credit market and financial services industry have in the past, and may in the future, experience periods of upheaval that could impact the availability and cost of equity and debt financing. If we are not able to secure additional funding when needed, on acceptable terms, we may have to delay, reduce the scope of or eliminate one or more research and development programs or selling and marketing initiatives. In addition, we may have to work with a partner on one or more of our product or market development programs, which could lower the economic value of those programs to us.
Recently Issued Accounting Pronouncements
In May 2014, the Financial Accounting Standards Board issued Accounting Standards Update No. 2014-09, Revenue from Contracts with Customers (“ASU 2014-09”) to provide guidance on revenue recognition. ASU 2014-09 requires a company to recognize revenue when it transfers promised goods or services to customers in an amount that reflects the consideration to which the company expects to be entitled in exchange for those goods or services. In doing so, companies will need to use more judgment and make more estimates than under current guidance. These may include identifying performance obligations in the contract, estimating the amount of variable consideration to include in the transaction price and allocating the transaction price to each separate performance obligation. ASU 2014-09 is effective in the first quarter of fiscal 2017. Early adoption is not permitted. Upon adoption, ASU 2014-09 can be applied retrospectively to all periods presented or only to the most current period presented with the cumulative effect of changes reflected in the opening balance of retained earnings in the most current period presented. We are currently evaluating the impact of adopting ASU 2014-09 on our consolidated financial statements
ITEM 7A. Quantitative and Qualitative Disclosures About Market Risk.
Interest Rate Risk
Our exposure to market risk for changes in interest rates relates primarily to interest earned on our cash equivalents and marketable securities. The primary objective of our investment activities is to preserve our capital to fund operations. We also seek to maximize income from our investments without assuming significant risk. Our investment policy provides for investments in short‑term, low‑risk, investment‑grade debt instruments. Our investments in marketable securities, which are comprised primarily of money market funds, obligations of U.S. Government agencies and government‑sponsored entities,
68
commercial paper and corporate bonds, are subject to default, changes in credit rating and changes in market value. These investments are subject to interest rate risk and will decrease in value if market interest rates increase.
Our cash, cash equivalents and marketable securities, totaling $103.7 million at December 31, 2014, did not include any auction preferred stock, auction rate securities or mortgage‑backed investments. We currently do not hedge interest rate exposure, and we do not have any foreign currency or other derivative financial instruments. The securities in our investment portfolio are classified as available for sale and are, due to their short‑ term nature, subject to minimal interest rate risk. To date, we have not experienced a loss of principal on any of our investments. Although we currently expect that our ability to access or liquidate these investments as needed to support our business activities will continue, we cannot ensure that this will not change. We believe that, if market interest rates were to change immediately and uniformly by 10% from levels at December 31, 2014, the impact on the fair value of these securities or our cash flows or income would not be material.
Foreign Currency Exchange Risk
Substantially all of our revenues are recognized in U.S. dollars. Certain expenses related to our international activities are payable in foreign currencies. As a result, factors such as changes in foreign currency exchange rates or weak economic conditions in foreign markets will affect our financial results. We recognized net foreign exchange transaction losses of $790,000, $158,000 and $46,000 for the years ended December 31, 2014, 2013 and 2012, respectively. The functional currency of our wholly‑owned subsidiaries is the U.S. dollar, so we are not currently subject to gains and losses from foreign currency translation of the subsidiary financial statements. We currently do not hedge foreign currency exchange rate exposure. Although the impact of currency fluctuations on our financial results has been immaterial in the past, there can be no guarantee the impact of currency fluctuations related to our international activities will not be material in the future.
69
ITEM 8. Financial Statements and Supplementary Data.
Genomic Health, Inc.
Index to Consolidated Financial Statements
70
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
The Board of Directors and Stockholders of Genomic Health, Inc.
We have audited the accompanying consolidated balance sheets of Genomic Health, Inc. as of December 31, 2014 and 2013, and the related consolidated statements of operations, comprehensive income (loss), stockholders’ equity, and cash flows for each of the three years in the period ended December 31, 2014. Our audits also included the financial statement schedule listed in the Index at Item 15(a). These financial statements and schedule are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements and schedule based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the consolidated financial position of Genomic Health, Inc. at December 31, 2014 and 2013, and the consolidated results of its operations and its cash flows for each of the three years in the period ended December 31, 2014, in conformity with U.S. generally accepted accounting principles. Also, in our opinion, the related financial statement schedule, when considered in relation to the basic financial statements taken as a whole, presents fairly in all material respects the information set forth therein.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), Genomic Health, Inc.’s internal control over financial reporting as of December 31, 2014, based on criteria established in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 framework) and our report dated March 12, 2015 expressed an unqualified opinion thereon.
/s/ Ernst & Young LLP
Redwood City, California
March 12, 2015
71
GENOMIC HEALTH, INC.
(In thousands, except share and per share amounts)
|
|
|
|
|
|
|
|
|
|
|
December 31, |
||||
|
|
|
2014 |
|
2013 |
||
|
ASSETS |
|
|
|
|
|
|
|
Current assets: |
|
|
|
|
|
|
|
Cash and cash equivalents |
|
$ |
29,726 |
|
$ |
33,279 |
|
Short-term marketable securities |
|
|
73,934 |
|
|
72,071 |
|
Accounts receivable (net of allowance for doubtful accounts; 2014—$3,628, 2013—$1,907) |
|
|
34,916 |
|
|
29,446 |
|
Prepaid expenses and other current assets |
|
|
9,944 |
|
|
10,196 |
|
Total current assets |
|
|
148,520 |
|
|
144,992 |
|
Property and equipment, net |
|
|
21,860 |
|
|
18,290 |
|
Other assets |
|
|
15,541 |
|
|
13,752 |
|
Total assets |
|
$ |
185,921 |
|
$ |
177,034 |
|
LIABILITIES AND STOCKHOLDERS’ EQUITY |
|
|
|
|
|
|
|
Current liabilities: |
|
|
|
|
|
|
|
Accounts payable |
|
$ |
6,987 |
|
$ |
5,160 |
|
Accrued compensation and employee benefits |
|
|
17,708 |
|
|
13,884 |
|
Accrued license fees |
|
|
2,656 |
|
|
2,554 |
|
Accrued expenses and other current liabilities |
|
|
10,444 |
|
|
7,356 |
|
Deferred revenues |
|
|
335 |
|
|
586 |
|
Other current liabilities |
|
|
208 |
|
|
292 |
|
Total current liabilities |
|
|
38,338 |
|
|
29,832 |
|
|
|
|
|
|
|
|
|
Other liabilities |
|
|
2,070 |
|
|
2,221 |
|
Commitments |
|
|
|
|
|
|
|
Stockholders’ equity: |
|
|
|
|
|
|
|
Preferred stock, $0.0001 par value, 5,000,000 shares authorized, no shares issued and outstanding |
|
|
— |
|
|
— |
|
Common stock, $0.0001 par value; 100,000,000 shares authorized, 32,969,232 and 32,024,887 shares issued and 31,911,901 and 30,964,086 shares outstanding at December 31, 2014 and 2013, respectively |
|
|
3 |
|
|
3 |
|
Additional paid-in capital |
|
|
370,496 |
|
|
345,345 |
|
Accumulated other comprehensive (loss) income, net |
|
|
(15) |
|
|
12 |
|
Accumulated deficit |
|
|
(194,861) |
|
|
(170,269) |
|
Treasury stock, at cost, 1,061,331 shares at December 31, 2014 and 2013 |
|
|
(30,110) |
|
|
(30,110) |
|
Total stockholders’ equity |
|
|
145,513 |
|
|
144,981 |
|
Total liabilities and stockholders’ equity |
|
$ |
185,921 |
|
$ |
177,034 |
See accompanying notes.
72
GENOMIC HEALTH, INC.
Consolidated Statements of Operations
(In thousands, except per share data)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended December 31, |
|||||||
|
|
|
2014 |
|
2013 |
|
2012 |
|||
|
Revenues: |
|
|
|
|
|
|
|
|
|
|
Product revenues |
|
$ |
275,706 |
|
$ |
259,192 |
|
$ |
233,457 |
|
Contract revenues |
|
|
— |
|
|
2,403 |
|
|
1,716 |
|
Total revenues |
|
|
275,706 |
|
|
261,595 |
|
|
235,173 |
|
Operating expenses: |
|
|
|
|
|
|
|
|
|
|
Cost of product revenues |
|
|
48,742 |
|
|
42,100 |
|
|
37,018 |
|
Research and development |
|
|
56,064 |
|
|
66,333 |
|
|
49,104 |
|
Selling and marketing |
|
|
134,858 |
|
|
110,602 |
|
|
93,553 |
|
General and administrative |
|
|
59,669 |
|
|
54,392 |
|
|
47,064 |
|
Total operating expenses |
|
|
299,333 |
|
|
273,427 |
|
|
226,739 |
|
Income (loss) from operations |
|
|
(23,627) |
|
|
(11,832) |
|
|
8,434 |
|
Impairment of investments |
|
|
— |
|
|
(643) |
|
|
— |
|
Interest income |
|
|
192 |
|
|
222 |
|
|
295 |
|
Other income (expense), net |
|
|
(764) |
|
|
(158) |
|
|
(58) |
|
Income (loss) before income taxes |
|
|
(24,199) |
|
|
(12,411) |
|
|
8,671 |
|
Income tax expense |
|
|
393 |
|
|
346 |
|
|
422 |
|
Net income (loss) |
|
$ |
(24,592) |
|
$ |
(12,757) |
|
$ |
8,249 |
|
Basic net income (loss) per share |
|
$ |
(0.78) |
|
$ |
(0.42) |
|
$ |
0.27 |
|
Diluted net income (loss) per share |
|
$ |
(0.78) |
|
$ |
(0.42) |
|
$ |
0.26 |
|
Shares used in computing basic net income (loss) per share |
|
|
31,453 |
|
|
30,512 |
|
|
30,326 |
|
Shares used in computing diluted net income (loss) per share. |
|
|
31,453 |
|
|
30,512 |
|
|
32,152 |
See accompanying notes.
73
GENOMIC HEALTH, INC.
Consolidated Statements of Comprehensive Income (Loss)
(In thousands)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended December 31, |
|||||||
|
|
|
2014 |
|
2013 |
|
2012 |
|||
|
Net income (loss) |
|
$ |
(24,592) |
|
$ |
(12,757) |
|
$ |
8,249 |
|
Other comprehensive income: |
|
|
|
|
|
|
|
|
|
|
Unrealized gain (loss) on available-for-sale marketable securities, net of tax |
|
|
(27) |
|
|
(3) |
|
|
45 |
|
Comprehensive income (loss) |
|
$ |
(24,619) |
|
$ |
(12,760) |
|
$ |
8,294 |
See accompanying notes.
74
GENOMIC HEALTH, INC.
Consolidated Statements of Stockholders’ Equity
(In thousands)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accumulated |
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
Additional |
|
Other |
|
|
|
Treasury |
|
Total |
|||||
|
|
|
Common Stock |
|
Paid-In |
|
Comprehensive |
|
Accumulated |
|
Stock |
|
Stockholders’ |
||||||||
|
|
|
Shares |
|
Amount |
|
Capital |
|
Income (Loss) |
|
Deficit |
|
at Cost |
|
Equity |
||||||
|
Balance at December 31, 2011 |
|
29,761 |
|
$ |
3 |
|
$ |
281,147 |
|
$ |
(30) |
|
$ |
(165,761) |
|
$ |
— |
|
$ |
115,359 |
|
Issuance of common stock upon exercise of stock options for cash and vesting of restricted stock units |
|
1,025 |
|
|
— |
|
|
14,086 |
|
|
— |
|
|
— |
|
|
— |
|
|
14,086 |
|
Issuance of common stock upon settlement of employee stock purchase plan |
|
146 |
|
|
— |
|
|
3,406 |
|
|
— |
|
|
— |
|
|
— |
|
|
3,406 |
|
Issuance of restricted stock to directors in lieu of fees |
|
6 |
|
|
— |
|
|
172 |
|
|
— |
|
|
— |
|
|
— |
|
|
172 |
|
Stock-based compensation expense related to employee stock options, restricted stock units and employee stock purchase plan |
|
— |
|
|
— |
|
|
15,104 |
|
|
— |
|
|
— |
|
|
— |
|
|
15,104 |
|
Repurchase of common stock |
|
(984) |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
(30,095) |
|
|
(30,095) |
|
Net income |
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
8,249 |
|
|
— |
|
|
8,249 |
|
Unrealized gain on investments, net of tax |
|
— |
|
|
— |
|
|
— |
|
|
45 |
|
|
— |
|
|
— |
|
|
45 |
|
Balance at December 31, 2012 |
|
29,954 |
|
|
3 |
|
|
313,915 |
|
|
15 |
|
|
(157,512) |
|
|
(30,095) |
|
|
126,326 |
|
Issuance of common stock upon exercise of stock options for cash and vesting of restricted stock units |
|
926 |
|
|
— |
|
|
9,725 |
|
|
— |
|
|
— |
|
|
— |
|
|
9,725 |
|
Issuance of common stock upon settlement of employee stock purchase plan |
|
153 |
|
|
— |
|
|
4,018 |
|
|
— |
|
|
— |
|
|
— |
|
|
4,018 |
|
Issuance of restricted stock to directors in lieu of fees |
|
8 |
|
|
— |
|
|
230 |
|
|
— |
|
|
— |
|
|
— |
|
|
230 |
|
Stock-based compensation expense related to employee stock options, restricted stock units and employee stock purchase plan |
|
— |
|
|
— |
|
|
17,457 |
|
|
— |
|
|
— |
|
|
— |
|
|
17,457 |
|
Repurchase of common stock |
|
(77) |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
(15) |
|
|
(15) |
|
Net loss |
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
(12,757) |
|
|
— |
|
|
(12,757) |
|
Unrealized loss on investments, net of tax |
|
— |
|
|
— |
|
|
— |
|
|
(3) |
|
|
— |
|
|
— |
|
|
(3) |
|
Balance at December 31, 2013 |
|
30,964 |
|
|
3 |
|
|
345,345 |
|
|
12 |
|
|
(170,269) |
|
|
(30,110) |
|
|
144,981 |
|
Issuance of common stock upon exercise of stock options for cash and vesting of restricted stock units |
|
748 |
|
|
— |
|
|
4,156 |
|
|
— |
|
|
— |
|
|
— |
|
|
4,156 |
|
Issuance of common stock upon settlement of employee stock purchase plan |
|
191 |
|
|
— |
|
|
4,227 |
|
|
— |
|
|
— |
|
|
— |
|
|
4,227 |
|
Issuance of restricted stock to directors in lieu of fees |
|
8 |
|
|
— |
|
|
230 |
|
|
— |
|
|
— |
|
|
— |
|
|
230 |
|
Stock-based compensation expense related to employee stock options, restricted stock units and employee stock purchase plan |
|
— |
|
|
— |
|
|
16,410 |
|
|
— |
|
|
— |
|
|
— |
|
|
16,410 |
|
Stock-based compensation expense related to consultant restricted stock units |
|
— |
|
|
— |
|
|
128 |
|
|
— |
|
|
— |
|
|
— |
|
|
128 |
|
Net loss |
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
(24,592) |
|
|
— |
|
|
(24,592) |
|
Unrealized loss on investments, net of tax |
|
— |
|
|
— |
|
|
— |
|
|
(27) |
|
|
— |
|
|
— |
|
|
(27) |
|
Balance at December 31, 2014 |
|
31,911 |
|
$ |
3 |
|
$ |
370,496 |
|
$ |
(15) |
|
$ |
(194,861) |
|
$ |
(30,110) |
|
$ |
145,513 |
See accompanying notes.
75
GENOMIC HEALTH, INC.
Consolidated Statements of Cash Flows
(In thousands)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
December 31, |
|||||||
|
|
|
2014 |
|
2013 |
|
2012 |
|||
|
Operating activities |
|
|
|
|
|
|
|
|
|
|
Net income (loss) |
|
$ |
(24,592) |
|
$ |
(12,757) |
|
$ |
8,249 |
|
Adjustments to reconcile net income (loss) to net cash provided by operating activities: |
|
|
|
|
|
|
|
|
|
|
Depreciation and amortization |
|
|
6,870 |
|
|
6,324 |
|
|
5,471 |
|
Employee stock-based compensation |
|
|
16,538 |
|
|
17,457 |
|
|
15,104 |
|
Write-off of previously capitalized software costs |
|
|
— |
|
|
663 |
|
|
— |
|
Impairment of assets held for sale and long-lived assets |
|
|
375 |
|
|
— |
|
|
— |
|
Outside director restricted stock awarded in lieu of fees |
|
|
230 |
|
|
230 |
|
|
172 |
|
Gain on disposal of property and equipment |
|
|
(51) |
|
|
— |
|
|
(86) |
|
Share of loss of equity method investee |
|
|
— |
|
|
— |
|
|
98 |
|
Impairment of investments |
|
|
— |
|
|
643 |
|
|
— |
|
Changes in assets and liabilities: |
|
|
|
|
|
|
|
|
|
|
Accounts receivable |
|
|
(5,470) |
|
|
(7,193) |
|
|
(1,176) |
|
Prepaid expenses and other assets |
|
|
741 |
|
|
(1,448) |
|
|
(1,599) |
|
Accounts payable |
|
|
985 |
|
|
1,238 |
|
|
(2,144) |
|
Accrued compensation and employee benefits |
|
|
3,824 |
|
|
1,886 |
|
|
1,951 |
|
Accrued expenses and other liabilities |
|
|
3,088 |
|
|
1,297 |
|
|
1,608 |
|
Deferred revenues |
|
|
(251) |
|
|
212 |
|
|
(1,686) |
|
Net cash provided by operating activities |
|
|
2,287 |
|
|
8,552 |
|
|
25,962 |
|
Investing activities |
|
|
|
|
|
|
|
|
|
|
Purchases of property and equipment |
|
|
(10,455) |
|
|
(11,008) |
|
|
(10,056) |
|
Proceeds from sale of property and equipment |
|
|
122 |
|
|
16 |
|
|
206 |
|
Purchases of marketable securities |
|
|
(96,800) |
|
|
(107,183) |
|
|
(103,570) |
|
Maturities of marketable securities |
|
|
94,910 |
|
|
116,169 |
|
|
90,160 |
|
Purchase of other investments |
|
|
(2,000) |
|
|
(5,000) |
|
|
(4,963) |
|
Net cash used in investing activities |
|
|
(14,223) |
|
|
(7,006) |
|
|
(28,223) |
|
Financing activities |
|
|
|
|
|
|
|
|
|
|
Net proceeds from issuance of common stock under stock plans |
|
|
12,030 |
|
|
16,588 |
|
|
18,764 |
|
Withholding taxes related to restricted stock units net share settlement |
|
|
(3,647) |
|
|
(2,845) |
|
|
(1,272) |
|
Repurchase of common stock |
|
|
— |
|
|
(15) |
|
|
(30,095) |
|
Net cash provided by (used in) financing activities |
|
|
8,383 |
|
|
13,728 |
|
|
(12,603) |
|
Net (decrease) increase in cash and cash equivalents |
|
|
(3,553) |
|
|
15,274 |
|
|
(14,864) |
|
Cash and cash equivalents at the beginning of period |
|
|
33,279 |
|
|
18,005 |
|
|
32,869 |
|
Cash and cash equivalents at the end of period |
|
$ |
29,726 |
|
$ |
33,279 |
|
$ |
18,005 |
|
Supplemental disclosure of cash flow information |
|
|
|
|
|
|
|
|
|
|
Cash paid for income taxes |
|
$ |
432 |
|
$ |
336 |
|
$ |
173 |
|
Non-cash investing and financing activities |
|
|
|
|
|
|
|
|
|
|
Accrued purchase of property and equipment |
|
$ |
1,809 |
|
$ |
1,138 |
|
$ |
1,168 |
See accompanying notes.
76
Note 1. Organization and Summary of Significant Accounting Policies
The Company
Genomic Health, Inc. (the “Company”) is a global healthcare company that provides actionable genomic information to personalize cancer treatment decisions. The Company develops and globally commercializes genomic‑based clinical laboratory services that analyze the underlying biology of cancer, allowing physicians and patients to make individualized treatment decisions. The Company was incorporated in Delaware in August 2000. The Company’s first product, the Oncotype DX invasive breast cancer test, was launched in 2004 and is used for early stage invasive breast cancer patients to predict the likelihood of breast cancer recurrence and the likelihood of chemotherapy benefit. In January 2010, the Company launched its second product, the Oncotype DX colon cancer test, which is used to predict the likelihood of colon cancer recurrence in patients with stage II disease. In December 2011, the Company made Oncotype DX available for patients with ductal carcinoma in situ (“DCIS”), a pre‑invasive form of breast cancer. This test provides a DCIS score that is used to predict the likelihood of local recurrence. In June 2012, the Company began offering the Oncotype DX colon cancer test for use in patients with stage III disease treated with oxaliplatin‑containing adjuvant therapy. In May 2013, the Company launched the Oncotype DX prostate cancer test. The test provides a Genomic Prostate Score, or GPS, to predict disease aggressiveness in men with low risk disease. This test is used to improve treatment decisions for prostate cancer patients, in conjunction with the Gleason score, or tumor grading.
Principles of Consolidation
These consolidated financial statements include all the accounts of the Company and its wholly‑owned subsidiaries. The Company had two wholly-owned subsidiaries at December 31, 2014: Genomic Health International Holdings, LLC, which was established in Delaware in 2010 and supports the Company’s international sales and marketing efforts; and Oncotype Laboratories, Inc., which was established in 2012, and is inactive. Genomic Health International Holdings, LLC has 10 wholly-owned subsidiaries. The functional currency for the Company’s wholly-owned subsidiaries incorporated outside the United States is the U.S. dollar. All significant intercompany balances and transactions have been eliminated.
Basis of Presentation and Use of Estimates
The accompanying consolidated financial statements have been prepared in accordance with accounting principles generally accepted in the United States (“GAAP”). The preparation of financial statements in conformity with GAAP requires management to make judgments, assumptions and estimates that affect the reported amounts of assets, liabilities, revenues and expenses, and related disclosures in the Company’s consolidated financial statements and accompanying notes. Actual results could differ materially from those estimates.
Certain reclassifications have been made to prior period amounts to conform to the current year presentation. For the year ended December 31, 2013, a reclassification from accrued expenses and other current liabilities was made to accrued compensation and employee benefits in the consolidated balance sheets and the accompanying consolidated statements of cash flows to conform to the current-year presentation.
Cash Equivalents
The Company considers all highly liquid investments with maturities of three months or less when purchased to be cash equivalents.
Marketable Securities
The Company invests in marketable securities, primarily money market funds, obligations of U.S. Government agencies and government‑sponsored entities, corporate bonds and commercial paper. The Company considers all investments with a maturity date of less than one year as of the balance sheet date to be short‑term investments. Those investments with a
77
GENOMIC HEALTH, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
December 31, 2014
maturity date greater than one year as of the balance sheet date are considered to be long‑term investments. As of December 31, 2014 and 2013, respectively, all investments in marketable securities were classified as available for sale. The Company does not intend to sell these securities and management believes it is not more likely than not that the Company will be required to sell these securities prior to the recovery of their amortized cost basis. These securities are carried at estimated fair value with unrealized gains and losses included in stockholders’ equity.
Realized gains and losses and declines in value, if any, judged to be other than temporary on available‑for‑sale securities are reported in other income or expense. When securities are sold, any associated unrealized gain or loss initially recorded as a separate component of stockholders’ equity is reclassified out of stockholders’ equity on a specific‑identification basis and recorded in earnings for the period. The cost of securities sold is determined using specific identification.
Fair Value of Financial Instruments
The Company’s financial instruments consist principally of cash and cash equivalents, marketable securities, trade receivables and accounts payable. The carrying amounts of certain of these financial instruments, including cash and cash equivalents, trade receivables and accounts payable, approximate fair value due to their short maturities.
See Note 3, “Fair Value Measurements” for further information on the fair value of the Company’s financial instruments.
Concentration of Risk
Cash equivalents, marketable securities and trade accounts receivable are financial instruments which potentially subject the Company to concentrations of credit risk. Through December 31, 2014, no material losses had been incurred.
The Company is subject to credit risk from its portfolio of cash equivalents and marketable securities. The Company invests in money market funds through a major U.S. bank and is exposed to credit risk in the event of default by the financial institution to the extent of amounts recorded on the consolidated balance sheets. The Company invests in short‑term, investment‑grade debt instruments and by policy limits the amount in any one type of investment, except for securities issued or guaranteed by the U.S. government. Under its investment policy, the Company limits amounts invested in such securities by credit rating, maturity, industry group, investment type and issuer, except for securities issued by the U.S. government. The Company is not exposed to any significant concentrations of credit risk from these financial instruments. The goals of the Company’s investment policy, in order of priority, are as follows: safety and preservation of principal and diversification of risk; liquidity of investments sufficient to meet cash flow requirements; and a competitive after‑tax rate of return.
The Company is also subject to credit risk from its accounts receivable related to its product sales. The Company performs evaluations of customers’ financial condition and generally does not require collateral. The majority of the Company’s accounts receivable arises from product sales in the United States. As of December 31, 2014, substantially all of the Company’s product revenues have been derived from sales of one product, the Oncotype DX breast cancer test. The majority of the Company’s tests to date have been delivered to physicians in the United States. All Oncotype DX tests are processed in the Company’s clinical reference laboratory facility in Redwood City, California. Medicare accounted for 20%, 21% and 22% of the Company’s product revenues for the years ended December 31, 2014, 2013 and 2012, respectively, and represented 27% and 28% of the Company’s net accounts receivable balance as of December 31, 2014 and 2013, respectively. No other third‑party payor represented more than 10% of the Company’s product revenues or accounts receivable balances for these periods.
Allowance for Doubtful Accounts
The Company accrues an allowance for doubtful accounts against its accounts receivable based on estimates consistent with historical payment experience. Bad debt expense is included in general and administrative expense on the Company’s consolidated statements of operations. Accounts receivable are written off against the allowance when the appeals process is exhausted, when an unfavorable coverage decision is received or when there is other substantive evidence that the account will not be paid. The Company’s allowance for doubtful accounts as of December 31, 2014 and 2013 was $3.6 million and $1.9 million, respectively. Write‑offs for doubtful accounts of $5.4 million were recorded against the allowance during each of
78
GENOMIC HEALTH, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
December 31, 2014
the years ended December 31, 2014 and 2013. Bad debt expense was $6.7 million, $6.2 million, and $3.4 million for the years ended December 31, 2014, 2013 and 2012, respectively.
Property and Equipment
Property and equipment, including purchased software, are stated at cost. Depreciation is calculated using the straight‑line method over the estimated useful lives of the assets, which generally range from three to seven years. Leasehold improvements are amortized using the straight‑line method over the estimated useful lives of the assets or the remaining term of the lease, whichever is shorter.
Intangible Assets
Intangible assets with finite useful lives are recorded at cost, less accumulated amortization. Amortization is recognized over the estimated useful lives of the assets. The Company’s intangible assets with finite lives, which are related to patent licenses, are not material and are included in non‑current other assets on the Company’s consolidated balance sheets.
Investments in Privately Held Companies
The Company determines whether its investments in privately held companies are debt or equity based on their characteristics, in accordance with accounting guidance for investments. The Company also evaluates the investee to determine if the entity is a variable interest entity (“VIE”) and, if so, whether the Company is the primary beneficiary of the VIE, in order to determine whether consolidation of the VIE is required in accordance with accounting guidance for consolidations. If consolidation is not required and the Company owns less than 50.1% of the voting interest of the entity, the investment is evaluated to determine if the equity method of accounting should be applied. The equity method applies to investments in common stock or in‑substance common stock where the Company exercises significant influence over the investee, typically represented by ownership of 20% or more of the voting interests of an entity. If the equity method does not apply, investments in privately held companies determined to be equity securities are accounted for using the cost method. Investments in privately held companies determined to be debt securities are accounted for as available‑for‑sale or held‑to‑maturity securities, in accordance with accounting guidance for investments.
In December 2010, the Company invested $500,000 in the preferred stock of a private company representing 21% of the entity’s outstanding voting shares. The Company determined that is was not the primary beneficiary of this VIE and, accordingly, applied the equity method of accounting. In June 2012, the Company invested an additional $400,000 in the preferred stock of this company as part of a new equity financing, reducing the Company’s holdings to approximately 16%. As of June 30, 2012, as a result of the Company’s ownership falling below 20% and not having the ability to exercise influence over the investee entity, the Company changed its method of accounting for this investment to the cost method. Each of the Company’s equity investments is reviewed at least annually for impairment or whenever events or changes in circumstances indicate that the carrying value of the investment might not be recoverable. At December 31, 2013, the Company concluded that the indicators of impairment of its investment in this privately held company were other than temporary and wrote off the remaining asset balance of $643,000. Therefore, the net carrying value of this investment was $0 at December 31, 2014 and 2013.
In March 2011, the Company invested $2.3 million in the redeemable preferred stock of a private company representing 21% of the entity’s outstanding voting shares. The Company determined that the investment was a held‑to‑maturity debt security and that the investee was not subject to consolidation. In August 2012, the Company participated in the first tranche of a second preferred stock financing of this private company and purchased $1.0 million of preferred stock with no redemption privileges. In connection with this financing, the terms of the Company’s initial redeemable preferred stock investment were modified to become preferred stock with no redemption privileges. As a result of this transaction, the Company’s ownership interest was reduced to approximately 19% and the investment held by the Company is considered to be an investment in non‑marketable equity securities. In October 2012, November 2013 and August 2014, the Company participated in additional rounds of financing and purchased additional preferred stock totaling $10.6 million. At December 31, 2014 and 2013, the Company’s ownership in this entity was approximately 8% and 12%, respectively. The investee is not
79
GENOMIC HEALTH, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
December 31, 2014
consolidated because the Company owns less than 20% of the investee, and the Company does not have the ability to exercise significant influence over the investee. As a result, the Company will continue to use the cost method of accounting for this investment. The carrying value of this investment was $13.9 million at December 31, 2014 and $11.9 million at December 31, 2013, and no impairment was recognized for this investment through December 31, 2014.
The Company’s investments in privately held companies were $13.9 million and $11.9 million at December 31, 2014 and 2013, respectively, and were included in other assets on the Company’s consolidated balance sheets.
Impairment of Long‑lived Assets
The Company reviews long‑lived assets, which include property and equipment, intangible assets and investments in privately held companies, for impairment whenever events or changes in business circumstances indicate that the carrying amounts of the assets may not be fully recoverable. For property and equipment and intangible assets, an impairment loss would be recognized when estimated undiscounted future cash flows expected to result from the use of the asset and its eventual disposition are less than its carrying amount. Impairment, if any, is assessed using undiscounted cash flows. For investments in non‑marketable equity securities, evidence of impairment might include the absence of an ability to recover the carrying amount of the investment or the inability of the investee to sustain an earnings capacity which would justify the carrying amount of the investment. The Company’s assessment as to whether any impairment is other than temporary is based on its ability and intent to hold the investment and whether evidence indicating the carrying value of the investment is recoverable within a reasonable period of time outweighs evidence to the contrary. If the fair value of the investment is determined to be less than the carrying value and the decline in value is considered to be other than temporary, the asset is written down to its fair value. The Company recorded impairment losses of $265,000 for equipment classified as held for sale and $110,000 for equipment disposed prior to placing it in service for the year ended December 31, 2014. There were no impairment losses for the years ended December 31, 2013 and 2012, other than the Company’s $663,000 write off of previously capitalized software costs and the $643,000 write off of an investment in a privately held company for the year ended December 31, 2013, as discussed above.
Income Taxes
The Company uses the liability method for income taxes, whereby deferred income taxes are provided on items recognized for financial reporting purposes over different periods than for income tax purposes. Valuation allowances are provided when the expected realization of tax assets does not meet a more‑likely‑than‑not criterion.
The Company accounts for uncertain income tax positions using a benefit recognition model with a two‑step approach, a more‑likely‑than‑not recognition criterion and a measurement attribute that measures the position as the largest amount of tax benefit that is greater than 50% likely of being realized upon ultimate settlement, in accordance with the accounting guidance for uncertain tax positions. If it is not more likely than not that the benefit will be sustained on its technical merits, no benefit is recorded. Uncertain tax positions that relate only to timing of when an item is included on a tax return are considered to have met the recognition threshold. The Company recognizes accrued interest and penalties related to unrecognized tax benefits in income tax expense when and if incurred. See Note 11, “Income Taxes” for additional information regarding unrecognized tax benefits.
Revenue Recognition
The Company derives its revenues from product sales and contract research arrangements. The majority of the Company’s historical product revenues have been derived from the sale of the Oncotype DX breast cancer test. The Company generally bills third‑party payors upon generation and delivery of a patient report to the physician. As such, the Company takes assignment of benefits and the risk of collection with the third‑party payor. The Company usually bills the patient directly for amounts owed after multiple requests for payment have been denied or only partially paid by the insurance carrier. The Company pursues case‑by‑case reimbursement where policies are not in place or payment history has not been established.
The Company’s product revenues for tests performed are recognized when the following revenue recognition criteria are met: (1) persuasive evidence that an arrangement exists; (2) delivery has occurred or services have been rendered; (3) the fee is fixed or determinable; and (4) collectability is reasonably assured. Criterion (1) is satisfied when the Company has an
80
GENOMIC HEALTH, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
December 31, 2014
arrangement to pay or a contract with the payor in place addressing reimbursement for the Oncotype DX test. In the absence of such arrangements, the Company considers that criterion (1) is satisfied when a third‑party payor pays the Company for the test performed. Criterion (2) is satisfied when the Company performs the test and generates and delivers to the physician, or makes available on its web portal, a patient report. When evaluating whether the fee is fixed or determinable and collectibility, we consider whether we have sufficient history to reliably estimate the total fee that will be received from a payor and a payor’s individual payment patterns. Determination of criteria (3) and (4) are based on management’s judgments regarding whether the fee charged for products or services delivered is fixed or determinable, and the collectability of those fees under any contract or arrangement. Based upon at least several months of payment history, the Company reviews the number of tests paid against the number of tests billed and the payor’s outstanding balance for unpaid tests to determine whether payments are being made at a consistently high percentage of tests billed and at appropriate amounts given the contracted payment amount. The estimated accrual amounts per test, recorded upon delivery of a patient report, are calculated for each accrual payor and are based on the contracted price adjusted for individual payment patterns resulting from co-payment amounts and excluded services in healthcare plans. When a payment received for an individual test is either higher or lower than the estimated accrual amount, we recognize the difference as either cash revenue, in the case of higher payments, or a write off to bad debt expense, in the case of lower payments.
To the extent all criteria set forth above are not met when test results are delivered, product revenues are recognized when cash is received from the payor.
The Company has exclusive distribution agreements for one or more of its Oncotype DX tests with distributors covering more than 90 countries. The distributor generally provides certain marketing and administrative services to the Company within its territory. As a condition of these agreements, the distributor generally pays the Company an agreed upon fee per test and the Company processes the tests. The same revenue recognition criteria described above generally apply to tests received through distributors. To the extent all criteria set forth above are not met when test results are delivered, product revenues are generally recognized when cash is received from the distributor.
From time to time, the Company receives requests for refunds of payments, generally due to overpayments made by third party‑payors. Upon becoming aware of a refund request, the Company establishes an accrued liability for tests covered by the refund request until such time as the Company determines whether or not a refund is due. Accrued refunds were $944,000 and $770,000 at December 31, 2014 and 2013, respectively.
Contract revenues are generally derived from studies conducted with biopharmaceutical and pharmaceutical companies. The specific methodology for revenue recognition is determined on a case‑by‑case basis according to the facts and circumstances applicable to a given contract. Under certain contracts, the Company’s input, measured in terms of full‑time equivalent level of effort or running a set of assays through its clinical reference laboratory under a contractual protocol, triggers payment obligations, and revenues are recognized as costs are incurred or assays are processed. Certain contracts have payments that are triggered as milestones are completed, such as completion of a successful set of experiments. Milestones are assessed on an individual basis and revenue is recognized when these milestones are achieved, as evidenced by acknowledgment from collaborators, provided that (1) the milestone event is substantive and its achievability was not reasonably assured at the inception of the agreement and (2) the milestone payment is non‑refundable. Where separate milestones do not meet these criteria, the Company typically defaults to a performance‑based model, such as revenue recognition following delivery of effort as compared to an estimate of total expected effort.
Advance payments received in excess of revenues recognized are classified as deferred revenue until such time as the revenue recognition criteria have been met.
Cost of Product Revenues
Cost of product revenues includes the cost of materials, direct labor, equipment and infrastructure expenses associated with processing tissue samples (including sample accessioning, histopathology, anatomical pathology, paraffin extraction, reverse transcription polymerase chain reaction (“RT‑PCR”), quality control analyses and shipping charges to transport tissue samples) and license fees. Infrastructure expenses include allocated facility occupancy and information technology costs. Costs associated with performing the Company’s tests are recorded as tests are processed. Costs recorded for tissue sample processing and shipping charges represent the cost of all the tests processed during the period regardless of whether revenue was recognized with respect to that test. Royalties for licensed technology calculated as a percentage of product revenues and fixed annual
81
GENOMIC HEALTH, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
December 31, 2014
payments relating to the launch and commercialization of the Company’s tests are recorded as license fees in cost of product revenues at the time product revenues are recognized or in accordance with other contractual obligations.
Research and Development Expenses
Research and development expenses are comprised of costs incurred to develop technology and carry out clinical studies and include salaries and benefits, reagents and supplies used in research and development laboratory work, infrastructure expenses, including allocated facility occupancy and information technology costs, contract services, and other outside costs. Research and development expenses also include costs related to activities performed under contracts with biopharmaceutical and pharmaceutical companies. Research and development costs are expensed as incurred.
The Company enters into collaboration and clinical trial agreements with clinical collaborators and records these costs as research and development expenses. The Company records accruals for estimated study costs comprised of work performed by its collaborators under contract terms. Advance payments for goods or services that will be used or rendered for future research and development activities are deferred and capitalized and recognized as expense as the goods are delivered or the related services are performed.
Stock‑based Compensation
The Company uses the Black‑Scholes option valuation model, single‑option approach, which requires the use of estimates such as stock price volatility and expected option lives, as well as expected option forfeiture rates, to value employee stock‑based compensation at the date of grant, and recognizes stock‑ based compensation expense ratably over the requisite service period.
Equity instruments granted to non‑employees are also valued using the Black‑Scholes option valuation model and are subject to periodic revaluation over their vesting terms. The Company did not grant any stock options to non‑employee consultants during any of the years presented.
401(k) Plan
Substantially all of the Company’s employees are eligible to participate in its defined contribution plan qualified under Section 401(k) of the Internal Revenue Code. The Company contributed dollar for dollar matching of employee contributions up to a maximum of $2,000, $1,000, and $1,000 for the years ended December 31, 2014, 2013 and 2012, respectively, for each employee per year based on a full calendar year of service. The match is funded concurrently with a participant’s semi‑monthly contributions to the 401(k) Plan. The Company recorded expense for its contributions under the 401(k) Plan of $1.9 million, $718,000 and $610,000 for the years ended December 31, 2014, 2013 and 2012, respectively.
Foreign Currency Transactions
Net foreign currency transaction gains or losses are included in other income (expense), net on the Company’s consolidated statement of operations. Net foreign currency transaction losses totaled $790,000, $158,000 and $46,000 for the years ended December 31, 2014, 2013 and 2012, respectively.
Comprehensive Gain or Loss
Other comprehensive gain or loss consists of unrealized gains and losses on available‑for‑sale securities.
Leases
The Company enters into lease agreements for its laboratory and office facilities. These leases are classified as operating leases. Rent expense is recognized on a straight‑line basis over the term of the lease. Incentives granted under the Company’s facilities leases, including allowances to fund leasehold improvements and rent holidays, are capitalized and are recognized as reductions to rental expense on a straight‑line basis over the term of the lease.
82
GENOMIC HEALTH, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
December 31, 2014
Guarantees and Indemnifications
The Company, as permitted under Delaware law and in accordance with its bylaws, indemnifies its officers and directors for certain events or occurrences, subject to certain limits, while the officer or director is or was serving at the Company’s request in such capacity. The term of the indemnification period is for the officer’s or director’s lifetime. The maximum amount of potential future indemnification is unlimited; however, the Company has a director and officer insurance policy that limits its exposure and may enable it to recover a portion of any future amounts paid. The Company believes the fair value of these indemnification agreements is minimal. Accordingly, the Company has not recorded any liabilities for these agreements as of December 31, 2014 and 2013.
Recently Issued Accounting Pronouncements
In May 2014, the Financial Accounting Standards Board issued Accounting Standards Update No. 2014-09, Revenue from Contracts with Customers (“ASU 2014-09”) to provide guidance on revenue recognition. ASU 2014-09 requires a company to recognize revenue when it transfers promised goods or services to customers in an amount that reflects the consideration to which the company expects to be entitled in exchange for those goods or services. In doing so, companies will need to use more judgment and make more estimates than under current guidance. These may include identifying performance obligations in the contract, estimating the amount of variable consideration to include in the transaction price and allocating the transaction price to each separate performance obligation. ASU 2014-09 is effective in the first quarter of fiscal 2017. Early adoption is not permitted. Upon adoption, ASU 2014-09 can be applied retrospectively to all periods presented or only to the most current period presented with the cumulative effect of changes reflected in the opening balance of retained earnings in the most current period presented. The Company is currently evaluating the impact of adopting ASU 2014-09 on its consolidated financial statements.
Note 2. Net Income (Loss) Per Share
Basic net income (loss) per share is calculated by dividing net income (loss) for the period by the weighted‑average number of common shares outstanding for the period without consideration of potential common shares. Diluted net income (loss) per share is calculated by dividing net income (loss) by the weighted‑ average number of common shares outstanding for the period and dilutive potential common shares for the period determined using the treasury‑stock method. The following table is a reconciliation of the numerator and denominator used in the calculation of basic and diluted net income (loss) per share:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended December 31, |
|||||||
|
|
|
2014 |
|
2013 |
|
2012 |
|||
|
|
|
(In thousands except |
|||||||
|
|
|
per share data) |
|||||||
|
Numerator: |
|
|
|
|
|
|
|
|
|
|
Net income (loss) |
|
$ |
(24,592) |
|
$ |
(12,757) |
|
$ |
8,249 |
|
Denominator: |
|
|
|
|
|
|
|
|
|
|
Weighted-average shares of common stock outstanding used in the calculation of basic net income (loss) per share |
|
|
31,453 |
|
|
30,512 |
|
|
30,326 |
|
Effect of dilutive securities: |
|
|
|
|
|
|
|
|
|
|
Options to purchase common stock |
|
|
— |
|
|
— |
|
|
1,704 |
|
Restricted stock units |
|
|
— |
|
|
— |
|
|
122 |
|
Total |
|
|
— |
|
|
— |
|
|
1,826 |
|
Weighted-average shares of common stock outstanding used in the calculation of diluted net income (loss) per share |
|
|
31,453 |
|
|
30,512 |
|
|
32,152 |
|
Basic net income (loss) per share |
|
$ |
(0.78) |
|
$ |
(0.42) |
|
$ |
0.27 |
|
Diluted net income (loss) per share |
|
$ |
(0.78) |
|
$ |
(0.42) |
|
$ |
0.26 |
Options to purchase 1.1 million shares of the Company’s common stock and 150,000 restricted stock units were outstanding at December 31, 2014, but were not included in the computation of diluted net loss per share because their effect
83
GENOMIC HEALTH, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
December 31, 2014
is anti‑dilutive. Options to purchase 1.5 million shares of the Company’s common stock and 156,000 restricted stock units were outstanding at December 31, 2013, but were not included in the computation of diluted net loss per share because their effect is anti‑dilutive. Options to purchase approximately 119,000 weighted‑average shares of the Company’s common stock were outstanding during the years ended December 31, 2012 but were not included in the computation of diluted net income per share because the options’ exercise prices were greater than the average market price of the Company’s common stock during this period; therefore, their effect is anti‑dilutive.
Note 3. Fair Value Measurements
The Company measures certain financial assets, including cash equivalents and marketable securities, at their fair value on a recurring basis. The fair value of these financial assets was determined based on a hierarchy of three levels of inputs, of which the first two are considered observable and the last unobservable, as follows:
Level 1: Quoted prices in active markets for identical assets or liabilities.
Level 2: Observable inputs other than Level 1 inputs, such as quoted prices for similar assets or liabilities, quoted prices in markets that are not active, or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities.
Level 3: Unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities.
Assets and liabilities measured at fair value are classified in their entirety based on the lowest level of input that is significant to the fair value measurement. The Company’s assessment of the significance of a particular input to the fair value measurement in its entirety requires management to make judgments and consider factors specific to the asset or liability. The Company did not have any non‑financial assets or liabilities that were measured or disclosed at fair value on a recurring basis at December 31, 2014 and 2013, respectively. The following tables set forth the Company’s financial instruments that were measured at fair value on a recurring basis at December 31, 2014 and 2013 by level within the fair value hierarchy:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Actively Quoted |
|
Significant |
|
|
|
|
|
|
||
|
|
|
Markets for |
|
Other |
|
Significant |
|
|
|
|||
|
|
|
Identical |
|
Observable |
|
Unobservable |
|
Balance at |
||||
|
|
|
Assets |
|
Inputs |
|
Inputs |
|
December 31, |
||||
|
|
|
Level 1 |
|
Level 2 |
|
Level 3 |
|
2014 |
||||
|
|
|
(In thousands) |
||||||||||
|
As of December 31, 2014: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Assets |
|
|
|
|
|
|
|
|
|
|
|
|
|
Money market deposits |
|
$ |
12,397 |
|
$ |
— |
|
$ |
— |
|
$ |
12,397 |
|
Commercial paper |
|
|
— |
|
|
29,749 |
|
|
— |
|
|
29,749 |
|
Corporate debt securities |
|
|
— |
|
|
46,435 |
|
|
— |
|
|
46,435 |
|
Total |
|
$ |
12,397 |
|
$ |
76,184 |
|
$ |
— |
|
$ |
88,581 |
84
GENOMIC HEALTH, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
December 31, 2014
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Actively Quoted |
|
Significant |
|
|
|
|
|
|
||
|
|
|
Markets for |
|
Other |
|
Significant |
|
|
|
|||
|
|
|
Identical |
|
Observable |
|
Unobservable |
|
Balance at |
||||
|
|
|
Assets |
|
Inputs |
|
Inputs |
|
December 31, |
||||
|
|
|
Level 1 |
|
Level 2 |
|
Level 3 |
|
2013 |
||||
|
|
|
(In thousands) |
||||||||||
|
As of December 31, 2013: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Assets |
|
|
|
|
|
|
|
|
|
|
|
|
|
Money market deposits |
|
$ |
15,690 |
|
$ |
— |
|
$ |
— |
|
$ |
15,690 |
|
Commercial paper |
|
|
— |
|
|
37,643 |
|
|
— |
|
|
37,643 |
|
Corporate debt securities |
|
|
— |
|
|
35,428 |
|
|
— |
|
|
35,428 |
|
Total |
|
$ |
15,690 |
|
$ |
73,071 |
|
$ |
— |
|
$ |
88,761 |
The Company’s debt securities of U.S. government‑sponsored entities, commercial paper and corporate bonds are classified as Level 2 as they are valued using multi‑dimensional relational pricing models that use observable market inputs, including benchmark yields, reported trades, broker‑dealer quotes, issuer spreads, benchmark securities, bids, offers and reference data. Not all inputs listed are available for use in the evaluation process on any given day for each security evaluation. In addition, market indicators and industry and economic events are monitored and may serve as a trigger to acquire further corroborating market data. There were no transfers between Level 1 and Level 2 categories during the years ended December 31, 2014 and 2013, respectively.
All of the Company’s marketable securities are classified as available for sale. The following tables summarize the Company’s available‑for‑sale marketable securities as of the dates indicated:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
December 31, 2014 |
||||||||||
|
|
|
Amortized |
|
Unrealized |
|
Unrealized |
|
Estimated |
||||
|
|
|
Cost |
|
Gains |
|
Losses |
|
Fair Value |
||||
|
|
|
(In thousands) |
||||||||||
|
Commercial paper |
|
$ |
29,730 |
|
$ |
19 |
|
$ |
— |
|
$ |
29,749 |
|
Corporate debt securities |
|
|
44,219 |
|
|
— |
|
|
(34) |
|
|
44,185 |
|
Total |
|
$ |
73,949 |
|
$ |
19 |
|
$ |
(34) |
|
$ |
73,934 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
December 31, 2013 |
||||||||||
|
|
|
Amortized |
|
Unrealized |
|
Unrealized |
|
Estimated |
||||
|
|
|
Cost |
|
Gains |
|
Losses |
|
Fair Value |
||||
|
|
|
(In thousands) |
||||||||||
|
Commercial paper |
|
$ |
36,625 |
|
$ |
18 |
|
$ |
— |
|
$ |
36,643 |
|
Corporate debt securities |
|
|
35,434 |
|
|
3 |
|
|
(9) |
|
|
35,428 |
|
Total |
|
$ |
72,059 |
|
$ |
21 |
|
$ |
(9) |
|
$ |
72,071 |
The Company had no realized gains or losses on its available‑for‑sale marketable securities for the years ended December 31, 2014, 2013 and 2012, respectively.
85
GENOMIC HEALTH, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
December 31, 2014
The following table provides the breakdown of the available‑for‑sale marketable securities with unrealized losses as of the date indicated:
|
|
|
|
|
|
|
|
|
|
|
In a Loss Position for |
||||
|
|
|
Less Than 12 Months |
||||
|
|
|
Gross |
|
|
|
|
|
|
|
Unrealized |
|
Estimated |
||
|
As of December 31, 2014: |
|
Losses |
|
Fair Value |
||
|
|
|
(In thousands) |
||||
|
Corporate debt securities |
|
$ |
(34) |
|
$ |
42,282 |
|
Total |
|
$ |
(34) |
|
$ |
42,282 |
All of the Company’s available‑for‑sale marketable securities had contractual maturities of one year or less as of December 31, 2014 and 2013, respectively.
Note 4. Property and Equipment
The following table summarizes the Company’s property and equipment as of the dates indicated:
|
|
|
|
|
|
|
|
|
|
|
December 31, |
||||
|
|
|
2014 |
|
2013 |
||
|
|
|
(In thousands) |
||||
|
Laboratory equipment |
|
$ |
24,685 |
|
$ |
23,402 |
|
Computer equipment |
|
|
9,198 |
|
|
8,856 |
|
Computer software—internal use |
|
|
5,075 |
|
|
4,182 |
|
Furniture and fixtures |
|
|
3,607 |
|
|
3,336 |
|
Leasehold improvements |
|
|
17,902 |
|
|
14,886 |
|
Work in progress |
|
|
7,307 |
|
|
7,149 |
|
|
|
|
67,774 |
|
|
61,811 |
|
Less accumulated depreciation and amortization |
|
|
(45,914) |
|
|
(43,521) |
|
Total |
|
$ |
21,860 |
|
$ |
18,290 |
For the years ended December 31, 2014, 2013 and 2012, the Company recognized property and equipment depreciation and amortization expense of $6.7 million, $6.1 million and $5.3 million, respectively.
Note 5. Accrued Expenses and Other Current Liabilities
The following table summarizes the Company’s accrued expenses and other current liabilities as of the dates indicated:
|
|
|
|
|
|
|
|
|
|
|
December 31, |
||||
|
|
|
2014 |
|
2013 |
||
|
|
|
(In thousands) |
||||
|
Accrued expenses |
|
$ |
4,003 |
|
$ |
3,610 |
|
Accrued professional and other service fees |
|
|
1,273 |
|
|
1,401 |
|
Accrued refunds |
|
|
944 |
|
|
770 |
|
Accrued collaboration expense |
|
|
2,862 |
|
|
908 |
|
Accrued taxes payable |
|
|
194 |
|
|
278 |
|
Other current liabilities |
|
|
1,168 |
|
|
389 |
|
Total |
|
$ |
10,444 |
|
$ |
7,356 |
Accrued professional and other service fees include third‑party billing and collections costs, legal expenses,
86
GENOMIC HEALTH, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
December 31, 2014
accounting and audit fees and investor relations expenses. Accrued refunds include overpayments due to third‑party payors.
Note 6. Collaboration and Commercial Technology Licensing Agreements
The Company has entered into a variety of collaboration and specimen transfer agreements relating to its development efforts. The Company recorded collaboration expenses of $6.4 million, $12.6 million and $1.9 million for the years ended December 31, 2014, 2013 and 2012, respectively, relating to services provided by the collaborators in connection with these agreements. In addition to these expenses, some of these agreements contain provisions for royalties from inventions resulting from these collaborations. The Company has specified options and rights relating to joint inventions arising out of these collaborations.
In August 2013, the Company entered into a collaboration agreement to conduct a clinical study to validate the relationship between the Oncotype DX DCIS score and the likelihood of local recurrence in patients with DCIS. The agreement includes a study fee and milestone payments dependent on the completion of certain key milestones. As a result of the primary objective of the study being met, the Company is required to make a series of fixed future annual payments under the collaboration agreement of $604,000, $604,000 and $504,000 in 2015, 2016 and 2017, respectively.
In January 2014, the Company entered into a collaboration agreement to conduct a prostate study with a goal to determine the association between the Genomic Prostate Score, or GPS, provided by the assay and the likelihood of experiencing disease progression while on active surveillance. In July 2014, the Company entered into a collaboration agreement to conduct a prostate observational study in men who choose active surveillance at one and two years after receiving the Oncotype DX prostate cancer GPS. In August 2014, the Company entered into an agreement to provide support to conduct the main phase of a prospective study dealing with individualization of adjuvant decision-making in early-stage breast cancer. As of December 31, 2014, the estimated total remaining obligations for these agreements, including certain milestone payments, is approximately $5.2 million. All future milestone payments are contingent on certain accomplishments, and therefore the timing for any related payments cannot be estimated.
In November 2013, the Company entered into an exclusive license agreement to develop and commercialize a test to predict benefit from DNA damage‑based chemotherapy drugs in high risk breast cancer. The Company made an up‑front payment of $9.0 million, which was recognized in research and development expense in the fourth quarter of 2013, and will make milestone payments as certain clinical and commercial endpoints are achieved in the future. All future milestone payments are contingent on certain milestone accomplishments, and therefore the timing for future milestone payments cannot be estimated. With successful commercialization of a test, the Company will be obligated to pay royalties. In the event that the Company terminates the agreement, a break-up fee of up to $3 million will be payable and the Company will not be obligated to pay future royalty payments or the reminder of the milestone payments.
The Company is a party to various agreements under which it licenses technology on a non‑exclusive basis in the field of human diagnostics. Access to these licenses enables the Company to process its Oncotype DX tests. While certain agreements contain provisions for fixed annual payments, license fees are generally calculated as a percentage of product revenues, with rates that vary by agreement and may be tiered, and payments that may be capped at annual minimum or maximum amounts. The Company recognized costs recorded under these agreements totaling $9.5 million, $8.9 million and $8.0 million for the years ended December 31, 2014, 2013 and 2012, respectively, which were included in cost of product revenues.
At December 31, 2014, fixed future annual payments, exclusive of royalty payments, relating to the launch and commercialization of the Oncotype DX colon cancer test and the Oncotype DX prostate cancer test totaled $550,000 and are fully payable in 2015. These payments are recorded in cost of product revenues as license fees. If at any time the Company discontinues the sale of the products covered by the agreement, no future annual payments will be payable and the Company will have no further obligation under the applicable agreement.
Contract Research Arrangements
In November 2007, the Company entered into a Collaborative Diagnostic Development Agreement with Pfizer Inc.
87
GENOMIC HEALTH, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
December 31, 2014
to provide research and development services for the development of a diagnostic product for renal cell cancer. The Company received an initial payment of $1.5 million and was initially eligible to receive a payment of $2.2 million upon joint agreement on a gene identification plan, $5.0 million in additional payments upon the earlier of Pfizer’s election to initiate the next phase of development or a specified number of months from the date the Company received the sample set and related clinical data necessary to conduct the first phase of development, and a final payment of $1.5 million upon completion of clinical validation. Completion of clinical validation represents a substantive milestone and the Company recognized the $1.5 million payment upon completion in December 2013. All other payments were not considered substantive milestones as they are not based solely on the Company’s past performance. Such payments were recognized using a performance‑based model and revenue is recognized following delivery of effort as compared to an estimate of total expected effort. The Company did not recognize any revenue related to substantive milestones under this arrangement during the years ended December 31, 2014 and 2013.
Note 7. Commitments
Lease Obligations
In September 2005, the Company entered into a non‑cancelable lease for 48,000 square feet of laboratory and office space that the Company currently occupies in Redwood City, California. In November 2010, the Company executed an amendment to extend the term of the lease through March 2019, with an option for the Company to extend the term of the lease for an additional five years. The agreement included lease incentive obligations of $834,000 that are being amortized on a straight‑line basis over the life of the lease.
In January 2007, the Company entered into a non‑cancelable lease for an additional 48,000 square feet of laboratory and office space in a nearby location. In November 2010, the Company executed an amendment to extend the term of the lease through March 2018, with an option for the Company to extend the lease for an additional five years. The agreement included lease incentive obligations totaling $283,000 that are being amortized on a straight‑line basis over the life of the lease.
In October 2009, the Company entered into a non‑cancelable agreement to lease an additional 30,500 square feet of office space near the locations the Company occupied. The lease expires in March 2018, with an option for the Company to extend the term of the lease for an additional five years. The agreement includes lease incentive obligations of $307,000 that are being amortized on a straight‑line basis over the life of the lease.
In August 2013, the Company entered into a non‑cancelable agreement to lease an additional 18,400 square feet of laboratory and office space near the locations the Company currently occupies. The lease expires in March 2019, with an option for the Company to extend the term of the lease for an additional five years. In July 2014, the Company leased an additional 5,500 square feet in the same location on the same terms. The agreements include lease incentive obligations of $358,000 which are being amortized on a straight line basis over the life of the lease.
In May 2010, the Company’s European subsidiary entered into a non‑cancelable lease for approximately 2,500 square feet of office space in Geneva, Switzerland. In May 2014, the Company executed an amendment to extend the terms of the lease and executed a new lease for approximately 5,000 square feet of additional space in the same location. Both lease agreements expire in May 2016. Additionally, the Company has offices in France, Germany, Ireland, Italy, Japan and the United Kingdom with short term rental agreements.
88
GENOMIC HEALTH, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
December 31, 2014
Rent expense under all operating leases amounted to $3.7 million, $3.0 million and $3.7 million for the years ended December 31, 2014, 2013 and 2012, respectively. Future non‑cancelable commitments under these operating leases at December 31, 2014 were as follows:
|
|
|
|
|
|
|
|
Annual |
|
|
|
|
Payments |
|
|
|
|
(In thousands) |
|
|
Years Ending December 31, |
|
|
|
|
2015 |
|
$ |
3,928 |
|
2016 |
|
|
3,872 |
|
2017 |
|
|
3,867 |
|
2018 |
|
|
2,476 |
|
2019 |
|
|
504 |
|
Total minimum payments |
|
$ |
14,647 |
Note 8. Capital Stock
Common Stock
As of December 31, 2014, the Company had 31,911,901 shares of common stock outstanding. Shares of common stock reserved for future issuance as of December 31, 2014 were as follows:
|
|
|
|
|
|
|
Number of |
|
|
|
Shares |
|
|
|
(In thousands) |
|
Shares to be issued upon exercise of outstanding stock options and vesting of restricted stock units (RSUs) |
|
4,420 |
|
Shares available for future stock option and RSU grants, settlement of employee stock purchase plan (ESPP) and restricted stock to be issued to outside directors in lieu of director fees |
|
1,746 |
|
Shares of common stock reserved for future issuance |
|
6,166 |
Treasury Stock
In December 2012, the Company entered into an accelerated share repurchase agreement with a financial institution to repurchase $30.0 million of its common stock on an accelerated basis. Under the terms of this accelerated share repurchase agreement, the Company paid $30.0 million to the financial institution to settle the initial purchase transaction and received 984,074 shares of its common stock, representing the minimum number of shares deliverable under the agreement. In February 2013, upon termination of the agreement and in accordance with the share delivery provisions of the agreement, the Company received an additional 77,257 shares of its common stock based on the average of the daily volume weighted‑average prices of its common stock during a specified period less a predetermined discount per share. As a result, the average purchase price of the Company’s common stock from the accelerated share repurchase program was $28.27 per share.
The Company accounted for the accelerated share repurchase as two separate transactions: (a) as shares of common stock acquired in a treasury stock transaction recorded on the transaction date and (b) as a forward contract indexed to the Company’s common stock. As such, the 984,074 shares repurchased were accounted for as a repurchase of common stock. The 77,257 additional shares that the Company received upon termination of the contract in February 2013 were also recorded in stockholders’ equity. The Company determined that the forward contract indexed to the Company’s common stock met all of the applicable criteria for equity classification, and therefore, the contract was not accounted for as a derivative.
89
GENOMIC HEALTH, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
December 31, 2014
Note 9. Stock‑based Compensation
2005 Stock Incentive Plan
On September 8, 2005, the Board of Directors approved the 2005 Stock Incentive Plan (the “2005 Plan”), which was later approved by the Company’s stockholders. Pursuant to the 2005 Plan, stock options, restricted shares, stock units, including RSUs, and stock appreciation rights may be granted to employees, consultants, and outside directors of the Company. Options granted may be either incentive stock options or nonstatutory stock options. The Company initially reserved 5,000,000 shares of the Company’s common stock for issuance under the 2005 Plan, effective upon the closing of the Company’s initial public offering on October 4, 2005. On June 8, 2009, the Company’s stockholders approved an amendment to the 2005 Plan to increase the shares reserved for issuance under the 2005 Plan by 3,980,000 shares. The amended and restated plan also extends the term under which awards may be granted under the 2005 Plan until January 27, 2019. As of December 31, 2014, options to purchase 985,722 shares of common stock were available for future grant under the 2005 Plan.
Stock options are governed by stock option agreements between the Company and recipients of stock options. Incentive stock options may be granted under the 2005 Plan at an exercise price of not less than 100% of the fair market value of the common stock on the date of grant, determined by the Compensation Committee of the Board of Directors. Nonstatutory stock options may be granted under the 2005 Plan at an exercise price of not less than 80% of the fair market value of the common stock on the date of grant, determined by the Compensation Committee of the Board of Directors. Options become exercisable and expire as determined by the Compensation Committee, provided that the term of incentive stock options may not exceed 10 years from the date of grant. Stock option agreements may provide for accelerated exercisability in the event of an optionee’s death, disability, or retirement or other events.
Under the 2005 Plan, each outside director who joins the board after the effective date of the 2005 Plan will receive an automatic nonstatutory stock option grant that vests at a rate of 25% at the end of the first year, with the remaining balance vesting monthly over the next three years. On the first business day following the annual meeting of the Company’s stockholders, each outside director who is continuing board service and who was not initially elected to the board at the annual meeting will receive an additional nonstatutory stock option grant, which will vest in full on the first anniversary of the date of grant or, if earlier, immediately prior to the next annual meeting of the Company’s stockholders. Nonstatutory stock options granted to outside directors must have an exercise price equal to 100% of the fair market value of the common stock on the date of grant. Nonstatutory stock options terminate on the earlier of the day before the tenth anniversary of the date of grant or the date twelve months after termination of the outside director’s service as a member of the Board of Directors.
In 2011, the Compensation Committee of the Board of Directors revised the Company’s equity incentive guidelines. Under the revised guidelines, most employees receive grants of RSUs in lieu of stock options. Employees with titles of vice president and above are eligible to receive stock options and RSUs. The target percentages of equity grant value for employees with titles of vice president and above other than our executive officers are 50% stock options and 50% RSUs, and the target percentages for executive officers are 75% stock options and 25% RSUs. The RSUs generally vest in three equal annual installments. As of April 2011, outside directors were given the option to elect to receive some or all of their retainers (other than retainers for serving as committee chair) in the form of fully‑vested restricted stock. Restricted shares, stock units and stock appreciation rights granted under the 2005 Plan are governed by agreements between the Company and recipients of the awards. Terms of the agreements are determined by the Compensation Committee.
In March 2014, the Company approved awards of performance-based restricted stock units (“PVRSUs”) for certain senior officers under the 2005 Plan, as amended and restated by the Board of Directors on March 24, 2014. The awards were subject to approval of the amended and restated plan by the Company’s stockholders, which was approved at the June 2014 Annual Meeting. In order for the senior officers to be eligible to earn any of the PVRSUs, the Company must achieve certain corporate-level objectives. The amount potentially available under a PVRSU is subject to the attainment of pre-established, objective performance goals over a specified period. The PVRSUs vest based on achievement of three performance milestones, not to exceed 100% in the aggregate: a revenue milestone, weighted from 0% to 100%; a tests delivered milestone, weighted from 0% to 100%; and a reimbursement-related milestone, weighted from 0% to 33 1/3%. In addition, the awards also have a
90
GENOMIC HEALTH, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
December 31, 2014
service vesting criteria following the achievement of performance criteria through February 2016. As of December 31, 2014, there were 13,533 PVRSUs outstanding with a grant date fair value of $368,000.
The Company recognizes the fair value of these awards to the extent the achievement of the related performance criteria is estimated to be probable. If a performance criteria is subsequently determined to not be probable of achievement, any related expense is reversed in the period such determination is made. Conversely, if a performance criteria is not currently expected to be achieved but is later determined to be probable of achievement, a “catch-up” entry is recorded in the period such determination is made for the expense that would have been recognized had the performance criteria been probable of achievement since the grant of the award.
Employee Stock Purchase Plan
In June 2011, the Company’s stockholders approved the Company’s Employee Stock Purchase Plan (“ESPP”). The ESPP provides eligible employees with an opportunity to purchase common stock from the Company and to pay for their purchases through payroll deductions. The ESPP is implemented through a series of offerings of purchase rights to eligible employees beginning December 1, 2011. Under the ESPP, the Compensation Committee of the Company’s Board of Directors may specify offerings with a duration of not more than 27 months, and may specify shorter purchase periods within each offering. During each purchase period, payroll deductions accumulate without interest. On the last day of the purchase period, accumulated payroll deductions are used to purchase common stock for employees participating in the offering. The purchase price is specified pursuant to the offering, but cannot, under the terms of the ESPP, be less than 85% of the fair market value per share of the Company’s common stock on either the last trading day preceding the offering date or on the purchase date, whichever is less.
The Company’s Board of Directors has determined that the purchase periods initially shall have a duration of six months and that the purchase price will be 85% of the fair market value per share of the Company’s common stock on either the last trading day preceding the offering date or the purchase date, whichever is less. The length of the purchase period applicable to U.S. employees and the purchase price may not be changed without the approval of the independent members of the Company’s Board of Directors.
During 2014, 191,318 shares were issued under the ESPP. A total of 1,250,000 shares of common stock have been reserved for issuance under the ESPP, of which 759,738 shares were available for issuance as of December 31, 2014. During 2013 and 2012, 152,881 and 146,063 shares were issued under the ESPP, respectively.
As of December 31, 2014, there was $592,000 of unrecognized compensation expense related to the ESPP, which is expected to be recognized over a period of five months.
91
GENOMIC HEALTH, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
December 31, 2014
Stock Option Activity
The following table summarizes option activity for the year ended December 31, 2014:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted-Average |
|
|
|
|
|
|
Outstanding Options |
|
Remaining |
|
Aggregate |
||||
|
|
|
Number of |
|
Weighted-Average |
|
Contractual |
|
Intrinsic |
||
|
|
|
Shares |
|
Exercise Price |
|
Life |
|
Value |
||
|
|
|
(In thousands) |
|
(In years) |
|
|
|
(In thousands) |
||
|
Balance at December 31, 2013 |
|
4,109 |
|
$ |
20.57 |
|
|
|
|
|
|
Options granted |
|
442 |
|
$ |
30.35 |
|
|
|
|
|
|
Options exercised |
|
(553) |
|
$ |
14.11 |
|
|
|
|
|
|
Options forfeited |
|
(221) |
|
$ |
30.18 |
|
|
|
|
|
|
Balance at December 31, 2014 |
|
3,777 |
|
$ |
22.10 |
|
5.1 |
|
$ |
37,768 |
|
Exercisable at December 31, 2014 |
|
3,119 |
|
$ |
20.45 |
|
4.3 |
|
$ |
36,327 |
|
Vested and expected to vest at December 31, 2014 |
|
3,729 |
|
$ |
21.99 |
|
5.0 |
|
$ |
37,681 |
The total intrinsic value of stock options exercised during the years ended December 31, 2014, 2013 and 2012 was $8.2 million, $13.5 million and $15.3 million, respectively. The total fair value of stock options vested during the years ended December 31, 2014, 2013 and 2012 was $6.0 million, $7.3 million and $7.9 million, respectively.
Restricted Stock Unit Activity
A following table summarizes RSU activity for the year ended December 31, 2014:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted-Average |
|
|
|
|
Number of |
|
Grant Date Fair |
|
|
|
|
Shares |
|
Value |
|
|
|
|
(In thousands) |
|
|
|
|
Balance at December 31, 2013 |
|
681 |
|
$ |
29.46 |
|
RSUs granted |
|
383 |
|
$ |
29.05 |
|
RSUs vested |
|
(327) |
|
$ |
28.25 |
|
RSUs cancelled |
|
(107) |
|
$ |
30.54 |
|
Balance at December 31, 2014 |
|
630 |
|
$ |
29.65 |
The weighted-average per share grant date fair values of RSUs granted were $29.05, $30.56 and $29.81 during the years ended December 31, 2014, 2013 and 2012, respectively. The fair value of RSUs as of their respective vesting dates were $9.1 million and $6.9 million for the year ended December 31, 2014 and 2013, respectively. There were no RSUs vested prior to 2012.
92
GENOMIC HEALTH, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
December 31, 2014
Performance-Based Restricted Stock Unit Activity
A following table summarizes PVRSU activity for the year ended December 31, 2014:
|
Weighted-Average |
|||||
|
Number of |
Grant Date Fair |
||||
|
Shares |
Value |
||||
|
(In thousands) |
|||||
|
Balance at December 31, 2013 |
— |
$ |
— |
||
|
PVRSUs granted |
14 |
$ |
27.21 | ||
|
PVRSUs vested |
— |
$ |
— |
||
|
PVRSUs cancelled |
— |
$ |
— |
||
|
Balance at December 31, 2014 |
14 |
$ |
27.21 | ||
There were no PVRSUs vested during 2014 and no PVRSUs granted prior to 2014.
Restricted Stock in Lieu of Directors’ Fees
Outside members of the Company’s Board of Directors may elect to receive fully‑vested restricted stock in lieu of cash compensation for services as a director. During the years ended December 31, 2014, 2013 and 2012, the Company issued 8,209, 7,769, and 5,512 shares of restricted stock, respectively, to outside directors, with vesting date fair values of $230,000, $230,000, and $172,000, respectively, and a weighted‑average grant date fair value of $27.97, $29.54, and $30.79 per share, respectively.
Employee Stock‑Based Compensation Expense
The stock-based compensation is recognized as expense over the requisite service periods in our Consolidated Statement of Operations using the straight-line expense attribution approach for stock options and RSUs, and using a graded vesting expense attribution approach for PVRSUs. The Company recognized employee stock‑based compensation expense of $16.5 million, $17.5 million and $15.1 million for the years ended December 31, 2014, 2013 and 2012, respectively. Employee stock‑based compensation expense was calculated based on awards ultimately expected to vest and has been reduced for estimated forfeitures. Forfeitures are estimated at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differ from those estimates. Employee stock‑based compensation expense includes expense related to stock options granted to outside directors of the Company as well as stock purchased under the ESPP. The following table presents the impact of employee stock‑based compensation expense on selected statement of operations line items for the periods indicated:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended December 31, |
|||||||
|
|
|
2014 |
|
2013 |
|
2012 |
|||
|
|
|
(In thousands) |
|||||||
|
Cost of product revenues |
|
$ |
497 |
|
$ |
483 |
|
$ |
441 |
|
Research and development |
|
|
4,569 |
|
|
4,873 |
|
|
3,992 |
|
Selling and marketing |
|
|
4,396 |
|
|
4,369 |
|
|
4,191 |
|
General and administrative |
|
|
7,076 |
|
|
7,732 |
|
|
6,480 |
|
Total |
|
$ |
16,538 |
|
$ |
17,457 |
|
$ |
15,104 |
As of December 31, 2014, unrecognized compensation expense related to unvested stock options, RSUs and PVRSUs, net of estimated forfeitures, were $6.4 million, $10.7 million and $146,000, respectively. The Company expects to recognize these expenses over a weighted‑average period of 2.4 years, 1.8 years, and 0.9 year, respectively.
93
GENOMIC HEALTH, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
December 31, 2014
Valuation Assumptions
Fair values of awards granted under the 2005 Plan and ESPP were estimated at grant or purchase dates using a Black‑Scholes option valuation model. Option valuation models require the input of highly subjective assumptions that can vary over time. The Company’s assumptions regarding expected volatility are based on the historical volatility of the Company’s common stock. The expected life of options granted is estimated based on historical option exercise data and assumptions related to unsettled options. The risk‑free interest rate is estimated using published rates for U.S. Treasury securities with a remaining term approximating the expected life of the options granted. The Company uses a dividend yield of zero as it has never paid cash dividends and does not anticipate paying cash dividends in the foreseeable future. The weighted‑average fair values and assumptions used in calculating such values during each fiscal year are as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended December 31, |
|
|||||||
|
|
|
2014 |
|
2013 |
|
2012 |
|
|||
|
Expected volatility: |
|
|
|
|
|
|
|
|
|
|
|
Stock options |
|
|
44 |
% |
|
46 |
% |
|
46 |
% |
|
ESPP |
|
|
37 |
% |
|
39 |
% |
|
43 |
% |
|
Risk-free interest rate: |
|
|
|
|
|
|
|
|
|
|
|
Stock options |
|
|
1.97 |
% |
|
1.40 |
% |
|
1.23 |
% |
|
ESPP |
|
|
0.08 |
% |
|
0.11 |
% |
|
0.09 |
% |
|
Expected life in years: |
|
|
|
|
|
|
|
|
|
|
|
Stock options |
|
|
6.61 |
|
|
6.64 |
|
|
6.98 |
|
|
ESPP |
|
|
0.50 |
|
|
0.50 |
|
|
0.50 |
|
|
Weighted-average fair value: |
|
|
|
|
|
|
|
|
|
|
|
Stock options |
|
$ |
14.13 |
|
$ |
14.11 |
|
$ |
14.51 |
|
|
ESPP |
|
$ |
7.24 |
|
$ |
7.82 |
|
|
7.67 |
|
Note 10. Segment Information
The Company operates in one business segment, which primarily focuses on the development and global commercialization of genomic‑based clinical laboratory services that analyze the underlying biology of cancer, allowing physicians and patients to make individualized treatment decisions. The Company’s Oncotype DX breast and colon tests have similar economic and other characteristics, including the nature of the products and production processes, type of customers, distribution methods and regulatory environment. As of December 31, 2014, the majority of the Company’s product revenues have been derived from sales of one product, the Oncotype DX breast cancer test.
As of December 31, 2014, the majority of the Company’s tests have been delivered to physicians in the United States. All Oncotype DX tests are processed in the Company’s clinical reference laboratory facility in Redwood City, California. The following table summarizes total revenues from customers, payors and collaboration partners by geographic region (in thousands). Product revenues are attributed to countries based on ship‑to location. Contract revenues are attributed to countries based on the location of the collaboration partner.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended December 31, |
|||||||
|
|
|
2014 |
|
2013 |
|
2012 |
|||
|
|
|
(In thousands) |
|||||||
|
United States |
|
$ |
230,657 |
|
$ |
223,662 |
|
$ |
207,508 |
|
Outside of the United States |
|
|
45,049 |
|
|
37,933 |
|
|
27,665 |
|
Total revenues |
|
$ |
275,706 |
|
$ |
261,595 |
|
$ |
235,173 |
94
GENOMIC HEALTH, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
December 31, 2014
Note 11. Income Taxes
The components of the Company’s income (loss) before income taxes were as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended December 31, |
|||||||
|
|
|
2014 |
|
2013 |
|
2012 |
|||
|
|
|
(In thousands) |
|||||||
|
Domestic |
|
$ |
(25,337) |
|
$ |
(13,294) |
|
$ |
8,018 |
|
Foreign |
|
|
1,138 |
|
|
883 |
|
|
653 |
|
Total income (loss) before income taxes |
|
$ |
(24,199) |
|
$ |
(12,411) |
|
$ |
8,671 |
The components of the Company’s income tax expense (benefit) were as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended |
|||||||
|
|
|
December 31, |
|||||||
|
|
|
2014 |
|
2013 |
|
2012 |
|||
|
|
|
(In thousands) |
|||||||
|
Current expense (benefit): |
|
|
|
|
|
|
|
|
|
|
Federal |
|
$ |
— |
|
$ |
(22) |
|
$ |
111 |
|
State |
|
|
40 |
|
|
57 |
|
|
99 |
|
Foreign |
|
|
353 |
|
|
311 |
|
|
212 |
|
Total income tax expense |
|
$ |
393 |
|
$ |
346 |
|
$ |
422 |
The income tax expense (benefit) differs from the amount computed by applying the statutory federal income tax rate as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended December 31, |
|||||||
|
|
|
2014 |
|
2013 |
|
2012 |
|||
|
|
|
(In thousands) |
|||||||
|
Federal tax at statutory rate |
|
$ |
(8,470) |
|
$ |
(4,344) |
|
$ |
3,035 |
|
Non-deductible officer compensation |
|
|
79 |
|
|
— |
|
|
— |
|
Stock-based compensation |
|
|
789 |
|
|
1,149 |
|
|
1,659 |
|
Non-deductible meals and entertainment |
|
|
531 |
|
|
507 |
|
|
492 |
|
Net operating losses not used (used) |
|
|
7,478 |
|
|
2,999 |
|
|
(4,973) |
|
Federal alternative minimum tax |
|
|
— |
|
|
— |
|
|
120 |
|
State tax, net of federal benefit |
|
|
26 |
|
|
37 |
|
|
64 |
|
Other |
|
|
(40) |
|
|
(2) |
|
|
25 |
|
Total income tax expense |
|
$ |
393 |
|
$ |
346 |
|
$ |
422 |
95
GENOMIC HEALTH, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
December 31, 2014
Deferred income taxes reflect the net tax effects of temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes. Significant components of deferred tax assets and liabilities are as follows:
|
|
|
|
|
|
|
|
|
|
|
December 31, |
||||
|
|
|
2014 |
|
2013 |
||
|
|
|
(In thousands) |
||||
|
Deferred tax assets: |
|
|
|
|
|
|
|
Net operating loss carryforwards |
|
$ |
35,060 |
|
$ |
30,970 |
|
Stock-based compensation |
|
|
10,210 |
|
|
9,150 |
|
Research tax credits |
|
|
12,500 |
|
|
9,580 |
|
Fixed assets |
|
|
3,520 |
|
|
3,200 |
|
Capitalized costs |
|
|
3,590 |
|
|
3,860 |
|
Other |
|
|
6,890 |
|
|
5,080 |
|
Total deferred tax assets |
|
|
71,770 |
|
|
61,840 |
|
Valuation allowance |
|
|
(71,770) |
|
|
(61,840) |
|
Net deferred tax assets |
|
$ |
— |
|
$ |
— |
Based on all available objective evidence, the Company believes that it is more likely than not that the net deferred tax assets will not be fully realizable. Accordingly, the Company recorded a valuation allowance against all of its net deferred tax assets for the years ended December 31, 2014 and 2013, respectively. The Company will continue to maintain a full valuation allowance on its deferred tax assets until there is sufficient evidence to support the reversal of all or some portion of this allowance. The net valuation allowance increased by $9.9 million and $7.2 million, and decreased by $3.8 million during the years ended December 31, 2014, 2013 and 2012, respectively.
As of December 31, 2014, the Company had federal and state net operating loss carryforwards of approximately $115.0 million and $86.0 million, respectively, and federal and state research and development tax credit carryforwards of approximately $9.3 million and $6.7 million, respectively. The federal net operating loss and federal tax credit carryforwards will expire at various dates beginning in 2021. The state net operating loss carryforwards begin to expire in 2015 if not utilized. The state tax credit carryforwards have no expiration date. None of the net operating loss and tax credit carryforwards are subject to the limitations imposed by Sections 382 and 383 of the Internal Revenue Code.
The Company tracks a portion of its deferred tax assets attributable to stock option benefits in a separate memorandum account. Therefore, these amounts are not included in the Company’s gross or net deferred tax assets. The benefit of these stock options will not be recorded in equity unless it reduces taxes payable. As of December 31, 2014, the portion of the federal and state net operating losses related to stock option benefits was approximately $27.9 million.
96
GENOMIC HEALTH, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
December 31, 2014
The Company had $1.6 million, $2.2 million and $875,000 of unrecognized tax benefits as of December 31, 2014, 2013 and 2012, respectively. The unrecognized tax benefits are primarily research tax credits for all years. The following table summarizes the activity related to unrecognized tax benefits:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended |
|||||||
|
|
|
December 31, |
|||||||
|
|
|
2014 |
|
2013 |
|
2012 |
|||
|
|
|
(In thousands) |
|||||||
|
Balance at January 1 |
|
$ |
2,160 |
|
$ |
875 |
|
$ |
839 |
|
Increase (decrease) related to prior year tax positions |
|
|
(907) |
|
|
74 |
|
|
(3) |
|
Increase related to current year tax positions |
|
|
347 |
|
|
1,211 |
|
|
39 |
|
Balance at December 31 |
|
$ |
1,600 |
|
$ |
2,160 |
|
$ |
875 |
Unrecognized tax benefits may change during the next twelve months for items that arise in the ordinary course of business. The Company does not anticipate a material change to its unrecognized tax benefits over the next twelve months that would affect the Company’s effective tax rate.
Accrued interest and penalties related to unrecognized tax benefits are recognized as part of the Company’s income tax provision in its consolidated statement of operations. For the year ended December 31, 2014, 2013 and 2012, the Company recognized $6,400, $5,700 and $6,800 in interest and penalties, respectively, related to unrecognized tax benefits.
The Company files federal, state and foreign income tax returns in many jurisdictions in the United States and abroad. The statute of limitations remain open for fiscal 2000 through 2014 in U.S. federal and state jurisdictions, and for fiscal 2010 through 2014 in foreign jurisdictions. Fiscal years outside the normal statute of limitations remain open to audit by tax authorities due to tax attributes generated in early years which have been carried forward and may be audited in subsequent years when utilized.
Note 12. Selected Quarterly Financial Data (Unaudited)
The following table contains selected unaudited consolidated statement of operations information for each of the fiscal quarters in 2014 and 2013. The Company believes that the following information reflects all adjustments, consisting of only normal recurring adjustments, necessary for a fair presentation of the information for the periods presented. The operating results for any quarter are not necessarily indicative of results for any future period.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Quarter Ended |
|
March 31, |
|
June 30, |
|
September 30, |
|
December 31, |
|
||||
|
|
|
(In thousands, except per share data) |
|
||||||||||
|
2014: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total revenues |
|
$ |
67,002 |
|
$ |
70,477 |
|
$ |
69,101 |
|
$ |
69,126 |
|
|
Product revenues |
|
|
67,002 |
|
|
70,477 |
|
|
69,101 |
|
|
69,126 |
|
|
Cost of product revenues |
|
|
12,055 |
|
|
12,207 |
|
|
11,979 |
|
|
12,501 |
|
|
Net loss |
|
|
(7,445) |
|
|
(4,618) |
|
|
(6,262) |
|
|
(6,267) |
|
|
Basic and diluted net loss per common share |
|
$ |
(0.24) |
|
$ |
(0.15) |
|
$ |
(0.20) |
|
$ |
(0.20) |
|
|
2013: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total revenues |
|
$ |
63,094 |
|
$ |
63,691 |
|
$ |
65,990 |
|
$ |
68,819 |
|
|
Product revenues |
|
|
62,709 |
|
|
63,691 |
|
|
65,732 |
|
|
67,060 |
|
|
Cost of product revenues |
|
|
9,746 |
|
|
10,757 |
|
|
10,781 |
|
|
10,815 |
|
|
Net income (loss) |
|
|
(883) |
|
|
(2,994) |
|
|
488 |
|
|
(9,369) |
|
|
Basic net income (loss) per common share |
|
$ |
(0.03) |
|
$ |
(0.10) |
|
$ |
0.02 |
|
$ |
(0.30) |
|
|
Diluted net income (loss) per common share |
|
$ |
(0.03) |
|
$ |
(0.10) |
|
$ |
0.02 |
|
$ |
(0.30) |
|
The quarterly increases in product revenues during 2014 and 2013 were primarily attributable to increased adoption of the Oncotype DX breast and colon cancer tests by physicians, international expansion, increased revenues recorded on an
97
GENOMIC HEALTH, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
December 31, 2014
accrual basis, and increased reimbursement for these tests by third‑party payors. The increase in cost of product revenues during 2014 and 2013 was primarily due to incremental costs related to test processing associated with the launch of the Oncotype DX prostate cancer test and enhancements to the Company’s laboratory information management system, as well as a milestone license fee paid in connection with the launch of the Company’s prostate cancer test in May 2013. The net loss for the quarter ended December 31, 2013 was primarily due to an up‑front payment of $9.0 million under a license agreement. Per share amounts for the quarters and full year have been calculated separately. Accordingly, quarterly amounts may not add up to the annual amount because of differences in the weighted‑average common shares outstanding during each period, due primarily to the effect of the Company’s issuing shares of its common stock during the year.
For all of the quarters in 2014 and the quarters ended March 31, June 30 and December 31, 2013, basic and diluted net loss per common share were identical as potential common shares were excluded from the calculation because their effects were anti‑dilutive.
98
ITEM 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosures.
Not applicable.
ITEM 9A. Controls and Procedures.
|
(a) Evaluation of disclosure controls and procedures. We maintain “disclosure controls and procedures,” as such term is defined in Rule 13a‑15(e) under the Securities Exchange Act of 1934, or the Exchange Act, that are designed to ensure that information required to be disclosed by us in reports that we file or submit under the Exchange Act is recorded, processed, summarized, and reported within the time periods specified in Securities and Exchange Commission rules and forms, and that such information is accumulated and communicated to our management, including our Chief Executive Officer and Chief Financial Officer, as appropriate, to allow timely decisions regarding required disclosure. In designing and evaluating our disclosure controls and procedures, management recognized that disclosure controls and procedures, no matter how well conceived and operated, can provide only reasonable, not absolute, assurance that the objectives of the disclosure controls and procedures are met. Our disclosure controls and procedures have been designed to meet reasonable assurance standards. Additionally, in designing disclosure controls and procedures, our management necessarily was required to apply its judgment in evaluating the cost‑benefit relationship of possible disclosure controls and procedures. The design of any disclosure controls and procedures also is based in part upon certain assumptions about the likelihood of future events, and there can be no assurance that any design will succeed in achieving its stated goals under all potential future conditions. |
Based on their evaluation as of the end of the period covered by this Annual Report on Form 10‑K, our Chief Executive Officer and Chief Financial Officer have concluded that, as of such date, our disclosure controls and procedures were effective at the reasonable assurance level.
|
(b) Management’s Annual Report on Internal Control over Financial Reporting. Our management is responsible for establishing and maintaining internal control over our financial reporting. Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Projections of any evaluation of the effectiveness of internal control to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with policies or procedures may deteriorate. Our management, with the participation of our Chief Executive Officer and Chief Financial Officer, assessed the effectiveness of our internal control over financial reporting as of December 31, 2014. In making this assessment, our management used the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission, or COSO, in Internal Control—Integrated Framework (2013 Framework). Based on the assessment using those criteria, our management concluded that, as of December 31, 2014, our internal control over financial reporting was effective. Our independent registered public accounting firm, Ernst & Young LLP, audited the effectiveness of our internal control over financial reporting. Their report appears below. |
|
(c) Changes in internal controls. There was no change in our internal control over financial reporting (as defined in Rule 13a‑15(f) under the Exchange Act) identified in connection with the evaluation described in Item 9A(a) above that occurred during our last fiscal quarter that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting. |
99
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
The Board of Directors and Stockholders of Genomic Health, Inc.
We have audited Genomic Health, Inc.’s internal control over financial reporting as of December 31, 2014, based on criteria established in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 framework) (the COSO criteria). Genomic Health, Inc.’s management is responsible for maintaining effective internal control over financial reporting, and for its assessment of the effectiveness of internal control over financial reporting included in the accompanying Management’s Annual Report on Internal Control Over Financial Reporting. Our responsibility is to express an opinion on the company’s internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, Genomic Health, Inc. maintained, in all material respects, effective internal control over financial reporting as of December 31, 2014, based on the COSO criteria.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the consolidated balance sheets of Genomic Health, Inc. as of December 31, 2014 and 2013, and the related consolidated statements of operations, comprehensive income (loss), stockholders’ equity, and cash flows for each of the three years in the period ended December 31, 2014 and our report dated March 12, 2015 expressed an unqualified opinion thereon.
/s/ Ernst & Young LLP
Redwood City, California
March 12, 2015
100
None.
ITEM 10. Directors, Executive Officers and Corporate Governance.
The information required by this item with respect to directors is incorporated by reference from the information under the caption “Election of Directors” contained in our Proxy Statement to be filed with the Securities and Exchange Commission in connection with the solicitation of proxies for our 2015 Annual Meeting of Stockholders to be held on June 11, 2015, or Proxy Statement. Certain information required by this item concerning executive officers is set forth in Part I of this Report under the caption “Executive Officers of the Registrant” and is incorporated herein by reference.
Item 405 of Regulation S‑K calls for disclosure of any known late filing or failure by an insider to file a report required by Section 16(a) of the Exchange Act. This disclosure is contained in the section entitled “Section 16(a) Beneficial Ownership Reporting Compliance” in the Proxy Statement and is incorporated herein by reference.
We have adopted a Code of Business Conduct and Ethics that applies to all of our officers and employees, including our President and Chief Executive Officer, our Chief Financial Officer and other employees who perform financial or accounting functions. The Code of Business Conduct and Ethics sets forth the basic principles that guide the business conduct of our employees. We have also adopted a Senior Financial Officers’ Code of Ethics that specifically applies to our President and Chief Executive Officer, our Chief Financial Officer, and key management employees. Stockholders may request a free copy of our Code of Business Conduct and Ethics and our Senior Financial Officers’ Code of Ethics by contacting Genomic Health, Inc., Attention: Chief Financial Officer, 301 Penobscot Drive, Redwood City, California 94063.
To date, there have been no waivers under our Code of Business Conduct and Ethics or Senior Financial Officers’ Code of Ethics. We intend to disclose future amendments to certain provisions of our Code of Business Conduct and Ethics or Senior Financial Officers’ Code of Ethics or waivers of such Codes granted to executive officers and directors on our website at http://www.genomichealth.com within four business days following the date of such amendment or waiver.
Our Board of Directors has appointed an Audit Committee, comprised of Mr. Randall S. Livingston, as Chairman, Dr. Fred E. Cohen and Ms. Ginger L. Graham. The Board of Directors has determined that Mr. Livingston qualifies as an Audit Committee Financial Expert under the definition outlined by the Securities and Exchange Commission. In addition, each of the members of the Audit Committee qualifies as an “independent director” under the current rules of The NASDAQ Stock Market and Securities and Exchange Commission rules and regulations.
ITEM 11. Executive Compensation.
The information required by this item is incorporated by reference from the information under the captions “Election of Directors—Director Compensation” and “Executive Compensation” contained in the Proxy Statement.
ITEM 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters.
The information required by this item is incorporated by reference from the information under the captions “Security Ownership of Certain Beneficial Owners and Management” and “Executive Compensation—Equity Compensation Plan Information” contained in the Proxy Statement.
ITEM 13. Certain Relationships and Related Transactions, and Director Independence.
The information required by this item is incorporated by reference from the information under the caption “Election of Directors—Certain Relationships and Related Transactions” and “—Director Independence” contained in the Proxy Statement.
ITEM 14. Principal Accounting Fees and Services.
The information required by this item is incorporated by reference from the information under the caption “Ratification of the Appointment of Independent Registered Public Accounting Firm—Principal Accountant Fees and Services” contained in the Proxy Statement.
101
ITEM 15. Exhibits and Financial Statement Schedules.
|
(a) |
Documents filed as part of this report: |
|
(1) |
Financial Statements |
Reference is made to the Index to Consolidated Financial Statements of Genomic Health under Item 8 of Part II hereof.
|
(2) |
Financial Statement Schedules |
The following schedule is filed as part of this Form 10‑K:
Schedule II—Valuation and Qualifying Accounts for the years ended December 31, 2014, 2013, and 2012.
All other financial statement schedules have been omitted because they are not applicable or not required or because the information is included elsewhere in the Consolidated Financial Statements or the Notes thereto.
|
(3) |
Exhibits |
See Item 15(b) below. Each management contract or compensatory plan or arrangement required to be filed has been identified.
|
(b) |
Exhibits |
|
Exhibit No. |
|
Description of Document |
|
|
3(i) |
|
Restated Certificate of Incorporation of the Company (incorporated by reference to exhibit 3.3 filed with Registration Statement on Form S‑1 (File No. 333‑126626), as amended, declared effective on September 28, 2005). |
|
|
3(ii) |
|
Amended and Restated Bylaws of the Company, as amended and restated January 8, 2009 (incorporated by reference to exhibit 3.1 to the Company’s Current Report on Form 8‑K filed on January 9, 2009). |
|
| 4.1 |
|
Specimen Common Stock Certificate (incorporated by reference to the exhibit of the same number filed with Registration Statement on Form S‑1 (File No. 333‑126626), as amended, declared effective on September 28, 2005). |
|
|
10.1# |
|
Form of Indemnification Agreement between the Company and its officers and directors (incorporated by reference to the exhibit of the same number filed with Registration Statement on Form S‑1 (File No. 333‑126626), as amended, declared effective on September 28, 2005). |
|
|
10.2# |
|
2001 Stock Incentive Plan and forms of agreements thereunder (incorporated by reference to the exhibit of the same number filed with Registration Statement on Form S‑1 (File No. 333‑126626), as amended, declared effective on September 28, 2005). |
|
|
10.3.1# |
|
Amended and Restated Genomic Health, Inc. 2005 Stock Incentive Plan (incorporated by reference to exhibit 10.1 filed with the Company’s Quarterly Report on Form 10‑Q for the quarterly period ended June 30, 2014). |
|
|
10.3.2# |
|
Form of Stock Option Agreement under the Company’s Amended and Restated 2005 Stock Incentive Plan (incorporated by reference to exhibit 10.2 filed with the Company’s Quarterly Report on Form 10‑Q for the quarterly period ended June 30, 2009). |
|
|
10.3.3# |
|
Form of Global Restricted Stock Unit Agreement under the Company’s Amended and Restated 2005 Stock Incentive Plan (incorporated by reference to exhibit 10.15 filed with the Company’s Annual Report on Form 10‑K for the year ended December 31, 2010). |
|
102
|
10.3.4# |
|
Form of Non U.S. Employee/Consultant Stock Option Agreement under the Company’s 2005 Stock Incentive Plan (incorporated by reference to exhibit 10.1 filed with the Company’s Quarterly Report on Form 10‑Q for the quarterly period ended September 30, 2008). |
|
|
10.3.5# |
|
Genomic Health, Inc. Employee Stock Purchase Plan (incorporated by reference to exhibit 10.1 filed with the Company’s Quarterly Report on Form 10‑Q for the quarterly period ended June 30, 2011). |
|
|
10.3.6# |
|
Genomic Health, Inc. Executive Cash Bonus Plan (incorporated by reference to exhibit 10.2 filed with the Company’s Quarterly Report on Form 10-Q for the quarterly period ended June 30, 2014). |
|
|
10.4.1† |
|
PCR Patent License Agreement dated February 21, 2005 between the Company and Roche Molecular Systems, Inc. (incorporated by reference to exhibit 10.8 filed with Registration Statement on Form S‑1 (File No. 333‑126626), as amended, declared effective on September 28, 2005). |
|
|
10.4.2† |
|
Amendment to PCR Patent License Agreement dated October 21, 2011 between the Company and Roche Molecular Systems, Inc. (incorporated by reference to exhibit 10.17 filed with the Company’s Annual Report on Form 10‑K for the year ended December 31, 2012). |
|
| 10.5 |
|
Lease dated September 23, 2005 between the Company and Metropolitan Life Insurance Company (incorporated by reference to exhibit 10.10 filed with Registration Statement on Form S‑1 (File No. 333‑126626), as amended, declared effective on September 28, 2005). |
|
| 10.6 |
|
Lease dated January 4, 2007 between the Company and Metropolitan Life Insurance Company (incorporated by reference to exhibit 10.8 filed with the Company’s Annual Report on Form 10‑K for the year ended December 31, 2006). |
|
| 10.7 |
|
Lease dated October 1, 2009 between the Company and Metropolitan Life Insurance Company (incorporated by reference to exhibit 10.1 filed with the Company’s Quarterly Report on Form 10‑Q for the quarterly period ended September 30, 2009). |
|
| 10.8 |
|
First Amendment to Lease dated January 4, 2007 between the Company and Metropolitan Life Insurance Company (incorporated by reference to exhibit 10.13 filed with the Company’s Annual Report on Form 10‑K for the year ended December 31, 2010). |
|
| 10.9 |
|
Second Amendment to Lease dated September 23, 2005 between the Company and Metropolitan Life Insurance Company (incorporated by reference to exhibit 10.14 filed with the Company’s Annual Report on Form 10‑K for the year ended December 31, 2010). |
|
| 10.10 |
|
Lease dated August 30, 2013 between the Company and Metropolitan Life Insurance Company (incorporated by reference to exhibit 10.1 filed with the Company’s Quarterly Report on Form 10‑Q for the quarterly period ended September 30, 2013). |
|
| 10.11 |
|
First Amendment to Lease dated August 30, 2013 between the Company and Metropolitan Life Insurance Company (incorporated by reference to exhibit 10.1 filed with the Company’s Quarterly Report on Form 10‑Q for the quarterly period ended September 30, 2014). |
|
|
21.1* |
|
List of Subsidiaries. |
|
|
23.1* |
|
Consent of Independent Registered Public Accounting firm. |
|
|
24.1* |
|
Power of Attorney (see page 106 of this Form 10‑K). |
|
|
31.1* |
|
Rule 13a‑14(a) Certification of Chief Executive Officer. |
|
|
31.2* |
|
Rule 13a‑14(a) Certification of the Chief Financial Officer. |
|
|
32.1** |
|
Statement of the Chief Executive Officer under Section 906 of the Sarbanes‑Oxley Act of 2002 (18 U.S.C. Section 1350). |
|
|
32.2** |
|
Statement of the Chief Financial Officer under Section 906 of the Sarbanes‑Oxley Act of 2002 (18 U.S.C. Section 1350). |
|
103
| 101 |
|
The following materials from Registrant’s Annual Report on Form 10‑K for the year ended December 31, 2014, formatted in Extensible Business Reporting Language (XBRL), includes: (i) Consolidated Balance Sheets at December 31, 2014 and 2013, (ii) Consolidated Statements of Income for the three years ended December 31, 2014, 2013 and 2012, (iii) Consolidated Statements of Comprehensive Income for the three years ended December 31, 2014, 2013 and 2012, (iv) Consolidated Statements of Stockholders’ Equity for the three years ended December 31, 2014, 2013 and 2012, (v) Consolidated Statements of Cash Flows for the three years ended December 31, 2014, 2013, and 2012, and (vi) Notes to Consolidated Financial Statements. |
|
*Filed herewith.
**In accordance with Item 601(b)(32)(ii) of Regulation S‑K and SEC Release No. 34‑47986, the certifications furnished in Exhibits 32.1 and 32.2 hereto are deemed to accompany this Form 10‑K and will not be deemed “filed” for purposes of Section 18 of the Exchange Act.
†Confidential treatment has been granted with respect to certain portions of this exhibit.
#Indicates management contract or compensatory plan or arrangement.
|
(c) |
Financial Statements and Schedules |
Reference is made to Item 15(a)(2) above.
104
SCHEDULE II
GENOMIC HEALTH, INC.
VALUATION AND QUALIFYING ACCOUNTS
Years Ended December 31, 2014, 2013 and 2012
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance at |
|
|
|
|
|
|
|
Balance at |
||
|
|
|
Beginning of |
|
|
|
|
|
|
|
End of |
||
|
|
|
Period |
|
Expenses |
|
Deductions |
|
Period |
||||
|
|
|
(In thousands) |
||||||||||
|
Allowance for Doubtful Accounts: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Year ended December 31, 2014 |
|
$ |
1,907 |
|
$ |
7,104 |
|
$ |
5,383 |
|
$ |
3,628 |
|
Year ended December 31, 2013 |
|
$ |
1,133 |
|
$ |
6,169 |
|
$ |
5,395 |
|
$ |
1,907 |
|
Year ended December 31, 2012 |
|
$ |
1,206 |
|
$ |
3,408 |
|
$ |
3,481 |
|
$ |
1,133 |
105
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
|
|
GENOMIC HEALTH, INC. |
|
|
|
|
|
|
|
|
|
|
|
By: |
/s/ Kimberly J. Popovits
Kimberly J. Popovits |
Date: March 12, 2015
POWER OF ATTORNEY
KNOW ALL PERSONS BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints Kimberly J. Popovits and G. Bradley Cole, and each of them, his true and lawful attorneys‑in‑fact, each with full power of substitution, for him or her in any and all capacities, to sign any amendments to this report on Form 10-K and to file the same, with exhibits thereto and other documents in connection therewith, with the Securities and Exchange Commission, hereby ratifying and confirming all that each of said attorneys‑in‑fact or their substitute or substitutes may do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
|
Signature |
Title |
Date |
||||
|
|
|
|
||||
|
/s/ Kimberly J. Popovits Kimberly J. Popovits |
President, Chief Executive Officer and Chairman of the Board (Principal Executive Officer) |
March 12, 2015 |
||||
|
|
|
|
||||
|
/s/ G. Bradley Cole G. Bradley Cole |
Chief Operating Officer and Chief Financial Officer (Principal Financial Officer and Principal Accounting Officer) |
March 12, 2015 |
||||
|
|
|
|
||||
|
/s/ Felix J. Baker Felix J. Baker |
Director |
March 12, 2015 |
||||
|
|
|
|
||||
|
/s/ Julian C. Baker Julian C. Baker |
Director |
March 12, 2015 |
||||
|
|
|
|
||||
|
/s/ Fred E. Cohen, M.D., D. Phil. Fred E. Cohen, M.D., D. Phil. |
Director |
March 12, 2015 |
||||
|
|
|
|
||||
|
/s/ Henry J. Fuchs, M.D. Henry J. Fuchs, M.D. |
Director |
March 12, 2015 |
||||
|
|
|
|
||||
|
/s/ Ginger L. Graham Ginger L. Graham |
Director |
March 12, 2015 |
||||
|
|
|
|
||||
|
/s/ Randall S. Livingston Randall S. Livingston |
Director |
March 12, 2015 |
||||
|
|
|
|
||||
|
|
|
|
||||
106
EXHIBIT INDEX
|
Exhibit No. |
|
Description of Document |
|
|
3(i) |
|
Restated Certificate of Incorporation of the Company (incorporated by reference to exhibit 3.3 filed with Registration Statement on Form S‑1 (File No. 333‑126626), as amended, declared effective on September 28, 2005). |
|
|
3(ii) |
|
Amended and Restated Bylaws of the Company, as amended and restated January 8, 2009 (incorporated by reference to exhibit 3.1 to the Company’s Current Report on Form 8‑K filed on January 9, 2009). |
|
| 4.1 |
|
Specimen Common Stock Certificate (incorporated by reference to the exhibit of the same number filed with Registration Statement on Form S‑1 (File No. 333‑126626), as amended, declared effective on September 28, 2005). |
|
|
10.1# |
|
Form of Indemnification Agreement between the Company and its officers and directors (incorporated by reference to the exhibit of the same number filed with Registration Statement on Form S‑1 (File No. 333‑126626), as amended, declared effective on September 28, 2005). |
|
|
10.2# |
|
2001 Stock Incentive Plan and forms of agreements thereunder (incorporated by reference to the exhibit of the same number filed with Registration Statement on Form S‑1 (File No. 333‑126626), as amended, declared effective on September 28, 2005). |
|
|
10.3.1# |
|
Amended and Restated Genomic Health, Inc. 2005 Stock Incentive Plan (incorporated by reference to exhibit 10.1 filed with the Company’s Quarterly Report on Form 10‑Q for the quarterly period ended June 30, 2014). |
|
|
10.3.2# |
|
Form of Stock Option Agreement under the Company’s Amended and Restated 2005 Stock Incentive Plan (incorporated by reference to exhibit 10.2 filed with the Company’s Quarterly Report on Form 10‑Q for the quarterly period ended June 30, 2009). |
|
|
10.3.3# |
|
Form of Global Restricted Stock Unit Agreement under the Company’s Amended and Restated 2005 Stock Incentive Plan (incorporated by reference to exhibit 10.15 filed with the Company’s Annual Report on Form 10‑K for the year ended December 31, 2010). |
|
|
10.3.4# |
|
Form of Non U.S. Employee/Consultant Stock Option Agreement under the Company’s 2005 Stock Incentive Plan (incorporated by reference to exhibit 10.1 filed with the Company’s Quarterly Report on Form 10‑Q for the quarterly period ended September 30, 2008). |
|
|
10.3.5# |
|
Genomic Health, Inc. Employee Stock Purchase Plan (incorporated by reference to exhibit 10.1 filed with the Company’s Quarterly Report on Form 10‑Q for the quarterly period ended June 30, 2011). |
|
|
10.3.6# |
|
Genomic Health, Inc. Executive Cash Bonus Plan (incorporated by reference to exhibit 10.2 filed with the Company’s Quarterly Report on Form 10-Q for the quarterly period ended June 30, 2014). |
|
|
10.4.1† |
|
PCR Patent License Agreement dated February 21, 2005 between the Company and Roche Molecular Systems, Inc. (incorporated by reference to exhibit 10.8 filed with Registration Statement on Form S‑1 (File No. 333‑126626), as amended, declared effective on September 28, 2005). |
|
|
10.4.2† |
|
Amendment to PCR Patent License Agreement dated October 21, 2011 between the Company and Roche Molecular Systems, Inc. (incorporated by reference to exhibit 10.17 filed with the Company’s Annual Report on Form 10‑K for the year ended December 31, 2012). |
|
| 10.5 |
|
Lease dated September 23, 2005 between the Company and Metropolitan Life Insurance Company (incorporated by reference to exhibit 10.10 filed with Registration Statement on Form S‑1 (File No. 333‑126626), as amended, declared effective on September 28, 2005). |
|
| 10.6 |
|
Lease dated January 4, 2007 between the Company and Metropolitan Life Insurance Company (incorporated by reference to exhibit 10.8 filed with the Company’s Annual Report on Form 10‑K for the year ended December 31, 2006). |
|
| 10.7 |
|
Lease dated October 1, 2009 between the Company and Metropolitan Life Insurance Company (incorporated by reference to exhibit 10.1 filed with the Company’s Quarterly Report on Form 10‑Q for the quarterly period ended September 30, 2009). |
|
107
| 10.8 |
|
First Amendment to Lease dated January 4, 2007 between the Company and Metropolitan Life Insurance Company (incorporated by reference to exhibit 10.13 filed with the Company’s Annual Report on Form 10‑K for the year ended December 31, 2010). |
|
| 10.9 |
|
Second Amendment to Lease dated September 23, 2005 between the Company and Metropolitan Life Insurance Company (incorporated by reference to exhibit 10.14 filed with the Company’s Annual Report on Form 10‑K for the year ended December 31, 2010). |
|
| 10.10 |
|
Lease dated August 30, 2013 between the Company and Metropolitan Life Insurance Company (incorporated by reference to exhibit 10.1 filed with the Company’s Quarterly Report on Form 10‑Q for the quarterly period ended September 30, 2013). |
|
| 10.11 |
|
First Amendment to Lease dated August 30, 2013 between the Company and Metropolitan Life Insurance Company (incorporated by reference to exhibit 10.1 filed with the Company’s Quarterly Report on Form 10‑Q for the quarterly period ended September 30, 2014). |
|
|
21.1* |
|
List of Subsidiaries. |
|
|
23.1* |
|
Consent of Independent Registered Public Accounting firm. |
|
|
24.1* |
|
Power of Attorney (see page 106 of this Form 10‑K). |
|
|
31.1* |
|
Rule 13a‑14(a) Certification of Chief Executive Officer. |
|
|
31.2* |
|
Rule 13a‑14(a) Certification of the Chief Financial Officer. |
|
|
32.1** |
|
Statement of the Chief Executive Officer under Section 906 of the Sarbanes‑Oxley Act of 2002 (18 U.S.C. Section 1350). |
|
|
32.2** |
|
Statement of the Chief Financial Officer under Section 906 of the Sarbanes‑Oxley Act of 2002 (18 U.S.C. Section 1350). |
|
| 101 |
|
The following materials from Registrant’s Annual Report on Form 10‑K for the year ended December 31, 2014, formatted in Extensible Business Reporting Language (XBRL), includes: (i) Consolidated Balance Sheets at December 31, 2014 and 2013, (ii) Consolidated Statements of Income for the three years ended December 31, 2014, 2013 and 2012, (iii) Consolidated Statements of Comprehensive Income for the three years ended December 31, 2014, 2013 and 2012, (iv) Consolidated Statements of Stockholders’ Equity for the three years ended December 31, 2014, 2013 and 2012, (v) Consolidated Statements of Cash Flows for the three years ended December 31, 2014, 2013, and 2012, and (vi) Notes to Consolidated Financial Statements. |
|
*Filed herewith.
**In accordance with Item 601(b)(32)(ii) of Regulation S-K and SEC Release No. 34-47986, the certifications furnished in Exhibits 32.1 and 32.2 hereto are deemed to accompany this Form 10-K and will not be deemed “filed” for purposes of Section 18 of the Exchange Act.
†Confidential treatment has been granted with respect to certain portions of this exhibit.
#Indicates management contract or compensatory plan or arrangement.
Copies of above exhibits not contained herein are available to any stockholder, upon payment of a reasonable per page fee, upon written request to: Chief Financial Officer, Genomic Health, Inc., 301 Penobscot Drive, Redwood City, California 94063.
108
