Attached files
| file | filename |
|---|---|
| EXCEL - IDEA: XBRL DOCUMENT - Cascadian Therapeutics, Inc. | Financial_Report.xls |
| EX-31.2 - EX-31.2 - Cascadian Therapeutics, Inc. | d884239dex312.htm |
| EX-31.1 - EX-31.1 - Cascadian Therapeutics, Inc. | d884239dex311.htm |
| EX-21.1 - EX-21.1 - Cascadian Therapeutics, Inc. | d884239dex211.htm |
| EX-32.1 - EX-32.1 - Cascadian Therapeutics, Inc. | d884239dex321.htm |
| EX-32.2 - EX-32.2 - Cascadian Therapeutics, Inc. | d884239dex322.htm |
| EX-23.1 - EX-23.1 - Cascadian Therapeutics, Inc. | d884239dex231.htm |
| EX-10.1 - EX-10.1 - Cascadian Therapeutics, Inc. | d884239dex101.htm |
| EX-10.26 - EX-10.26 - Cascadian Therapeutics, Inc. | d884239dex1026.htm |
| EX-10.2 - EX-10.2 - Cascadian Therapeutics, Inc. | d884239dex102.htm |
Table of Contents
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form 10-K
(Mark One)
| x | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
FOR THE FISCAL YEAR ENDED DECEMBER 31, 2014
OR
| ¨ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
FOR THE TRANSITION PERIOD FROM TO
Commission file number: 001-33882
ONCOTHYREON INC.
(Exact name of registrant as specified in its charter)
| Delaware | 26-0868560 | |
| (State or other jurisdiction of incorporation or organization) |
(I.R.S. Employer Identification Number) | |
| 2601 Fourth Ave, Suite 500 Seattle, Washington |
98121 | |
| (Address of principal executive offices) | (Zip Code) | |
Registrant’s telephone number, including area code:
(206) 801-2100
Securities registered pursuant to Section 12(b) of the Act:
| Title of Each Class |
Name of Exchange on Which Registered | |
| Common Stock, $0.0001 par value | The NASDAQ Stock Market LLC (The NASDAQ Global Market) |
Securities registered pursuant to Section 12(g) of the Act:
None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ¨ No x
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ¨ No x
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes x No ¨
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes x No ¨
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of the Form 10-K or any amendment to this Form 10-K. ¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check one):
| Large accelerated filer | ¨ | Accelerated filer | x | |||
| Non-accelerated filer | ¨ (Do not check if a smaller reporting company) | Smaller reporting company | ¨ | |||
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ¨ No x
The aggregate market value of the voting and non-voting stock held by non-affiliates of the Registrant, based on the closing sale price of the Registrant’s common stock on the last day of its most recently completed second fiscal quarter, as reported on the NASDAQ Global Market, was approximately $197million. Shares of common stock held by each executive officer and director and by each person who owns 10% or more of the outstanding common stock, based on filings with the Securities and Exchange Commission, have been excluded from this computation since such persons may be deemed affiliates of the Registrant. The determination of affiliate status for this purpose is not necessarily a conclusive determination for other purposes.
There were 102,301,012 shares of the Registrant’s common stock, $0.0001 par value, outstanding on March 10, 2015.
DOCUMENTS INCORPORATED BY REFERENCE
None.
Table of Contents
ONCOTHYREON INC.
ANNUAL REPORT ON FORM 10-K
FOR THE FISCAL YEAR ENDED DECEMBER 31, 2014
-i-
Table of Contents
PART I
| ITEM 1. | Business |
This annual report on Form 10-K, including Part I, Item 1, “Business,” Part II, Item 7, “Management’s Discussion and Analysis of Financial Condition and Results of Operation” and other sections in this annual report on Form 10-K, contain forward-looking statements or incorporate by reference forward-looking statements. The words “believe,” “may,” “will,” “estimate,” “continue,” “anticipate,” “intend,” “expect,” and similar expressions are intended to identify forward-looking statements. You should read these statements carefully because they discuss future expectations, contain projections of future results of operations or financial condition, or state other “forward-looking” information. These statements relate to our, or in some cases our partners’ future plans, objectives, expectations, intentions and financial performance and the assumptions that underlie these statements.
All forward-looking statements are based on information available to us on the date of this annual report and we will not update any of the forward-looking statements after the date of this annual report, except as required by law. Our actual results could differ materially from those discussed in this annual report. The forward-looking statements contained in this annual report, and other written and oral forward-looking statements made by us from time to time, are subject to certain risks and uncertainties that could cause actual results to differ materially from those anticipated in the forward-looking statements. Factors that might cause such a difference include, but are not limited to, those discussed in the following discussion and within Part I, Item 1A,“Risk Factors” of this annual report.
Throughout this discussion, unless the context specifies or implies otherwise, the terms “Company,” “Oncothyreon,” “Biomira,” “we,” “us,” and “our” refer to Oncothyreon Inc., its predecessor, Biomira Inc., and its subsidiaries.
Overview
We are a clinical-stage biopharmaceutical company focused primarily on the development of therapeutic products for the treatment of cancer. Our goal is to discover, develop and commercialize novel compounds that have the potential to improve the lives and outcomes of cancer patients. Our current clinical-stage product candidates include ONT-380, an orally active and selective small-molecule HER2 inhibitor, and ONT-10, a therapeutic vaccine targeting the Mucin 1 peptide antigen (MUC1). We are developing preclinical product candidates in oncology, and potentially certain rare diseases, using our recently acquired protocell technology. We also collaborate with partners to discover and develop additional product candidates.
We are developing ONT-380 for the treatment of HER2-positive metastatic breast cancer. ONT-380 is a small molecule inhibitor of HER2, also known as ErbB2, a receptor tyrosine kinase that is over-expressed in breast cancer and other cancers, such as gastric and ovarian cancer. Over-expression of HER2 in breast cancer is associated with increased mortality in early stage disease, decreased time to relapse and increased incidence of metastases. We have an exclusive license agreement with Array BioPharma Inc., (Array) to develop, manufacture and commercialize ONT-380.
We are currently conducting two Phase 1b trials of ONT-380, one in combination with Kadcyla® (ado-trastuzumab emtansine or TDM-1) and another in combination with Xeloda® (capecitabine) and/or Herceptin® (trastuzumab). In December 2014, we announced that interim data from these Phase 1b trials indicated preliminary clinical activity and tolerability in a heavily pretreated patient population. Each of these ongoing trials is also enrolling a cohort of patients with HER2-positive breast cancer metastatic to the central nervous system (CNS). ONT-380 has demonstrated superior activity, based on overall survival, compared to Tykerb® (lapatinib) and to the investigational drug, neratinib, in an intracranial HER2+ breast cancer xenograft model. This provides a rationale to explore whether ONT-380 can provide benefit to patients with brain metastases, which occur in approximately one-third of women with metastatic HER2+ breast cancer.
We are conducting a Phase 1 trial for ONT-10, a cancer vaccine directed against the MUC1. Results from this trial have demonstrated that ONT-10 activates the humoral arm of the immune system and elicits antibodies specific for MUC1. Natural antibodies against MUC1 have been shown to correlate with improved survival in patients with tumors expressing MUC1. This trial is also the first-in-man trial for our novel vaccine adjuvant PET-Lipid A, a fully-synthetic toll-like receptor 4 agonist. We are also conducting a Phase 1b trial of ONT-10 in combination with the T-cell agonist antibody varlilumab in collaboration with Celldex Therapeutics, Inc. (Celldex).
1
Table of Contents
We are increasingly focused on expanding our pipeline of product candidates through both internal research and collaborative efforts. To support our internal efforts, in August 2014 we acquired Alpine Biosciences, Inc., of Seattle, Washington (Alpine), a privately held biotechnology company developing protocells, a nanoparticle platform technology designed to enable the targeted delivery of multiple therapeutic agents, including nucleic acids, proteins, peptides and small molecules. We intend to utilize the protocell technology to develop new product candidates for the treatment of cancer and rare diseases, either on our own or with partners. We are also collaborating with Sentinel Oncology Ltd., of Cambridge, United Kingdom (Sentinel), for the development of novel small molecule Chk1 kinase inhibitors. We have identified a lead product candidate molecule, for which we currently expect to file an Investigational New Drug application (IND) in late 2015. In addition, we recently initiated a collaboration with Adimab LLC of Lebanon, New Hampshire (Adimab), for the discovery of novel antibodies against undisclosed immunotherapy targets in oncology.
We have not developed a therapeutic product to the commercial stage. As a result, our revenue has been limited to date, and we do not expect to recognize any material revenue for the foreseeable future. In particular, our ability to generate revenue in future periods will depend substantially on the progress of ongoing and/or future clinical trials for ONT-380 and ONT-10, our success in obtaining regulatory approval for ONT-380 and ONT-10, and our ability to establish commercial markets for these drugs. As ONT-380 and ONT-10 are in early clinical development, we do not expect to realize any revenues associated with the commercialization of these product candidates for the foreseeable future.
The continued research and development of our product candidates will require significant additional expenditures, including preclinical studies, clinical trials, manufacturing costs and the expenses of seeking regulatory approval. We rely on third parties to conduct a portion of our preclinical studies, all of our clinical trials and all of the manufacturing of current good manufacturing practice (cGMP) material. We expect expenditures associated with these activities to increase in future years as we continue the development of ONT-380 and ONT-10, and as we advance the development of our preclinical product candidates.
We were incorporated in 1985 in Canada under the name Biomira Inc. (Biomira). On December 10, 2007, Oncothyreon became the successor corporation to Biomira by way of a plan of arrangement effected pursuant to Canadian law. The plan of arrangement represents a transaction among entities under common control. The assets and liabilities of our predecessor Biomira have been reflected at their historical cost in the accounts of Oncothyreon.
Our executive office is located at 2601 Fourth Avenue, Suite 500, Seattle, Washington 98121 and our telephone number is (206) 801-2100. Our common stock trades on the NASDAQ Global Market under the symbol “ONTY”.
Available Information
We make available free of charge through the investor relations section of our website, www.oncothyreon.com, our annual reports, quarterly reports, current reports, proxy statements and all amendments to those reports as soon as reasonably practicable after such material is electronically filed or furnished with the Securities and Exchange Commission (SEC). These reports may also be obtained without charge by contacting Investor Relations, Oncothyreon Inc., 2601 Fourth Avenue, Suite 500, Seattle, Washington 98121, e-mail: IR@oncothyreon.com. Our Internet website and the information contained therein or incorporated therein are not intended to be incorporated into this Annual Report on Form 10-K. In addition, the public may read and copy any materials we file or furnish with the SEC at the SEC’s Public Reference Room at 100 F Street, N.E., Washington, D.C. 20549 or may obtain information on the operation of the Public Reference Room by calling the SEC at 1-800-SEC-0330. Moreover, the SEC maintains an Internet site that contains periodic reports, proxy statements, and other information that we file or furnish electronically with them at www.sec.gov.
Our Strategy
Our strategy is primarily focused on the development of therapeutic products for the treatment of cancer. Our pipeline includes the clinical-stage product candidates, ONT-380 and ONT-10, and a pre-clinical inhibitor of checkpoint kinase 1. We intend to supplement our product pipeline through internal discovery research and selective in-licensing. We believe the development of multiple products increases our opportunity for success, diversifies risk, creates development synergies and allows us to establish strategic partnerships. Our pipeline is the foundation on which we intend to build a valuable oncology franchise. Key elements of our strategy are to:
Advance our clinical stage product candidates. Our primary focus is advancing our pipeline of clinical-stage product candidates: ONT-380 and ONT-10. To that end, we maintain internal expertise in our research and development, regulatory and clinical groups. We also have relationships with key scientific advisors, research organizations and contract manufacturers to supplement our internal efforts.
2
Table of Contents
Increase our product pipeline through discovery research and preclinical development. We seek to develop new product candidates through internal discovery research. We intend to use our recently acquired protocell technology to develop product candidates in oncology, and potentially, certain rare diseases. To support these efforts we have expanded our internal research capability and maintain expertise in pre-clinical and technical development. We also supplement our internal efforts with collaborations to expand the range of potential product opportunities. For example, in 2013 we initiated a collaboration with Sentinel to perform chemistry and analytical services for the development of novel Chk1 kinase inhibitors. In addition, we recently initiated a collaboration with Adimab for the discovery of novel antibodies against undisclosed immunotherapy targets in oncology.
Acquire or in-license attractive product candidates and technologies. In addition to our internal research and development initiatives, we have ongoing efforts to identify products and technologies to acquire or in-license from biotechnology and pharmaceutical companies and academic institutions. For example, in late 2014 we completed an exclusive license agreement with Array for the development, manufacture and commercialization of ONT-380, replacing a prior collaboration agreement. In addition, in August 2014 we acquired Alpine for its protocell technology. We plan to continue supplementing our internal development programs through strategic acquisition or in-licensing transactions.
Support our internal activities with strategic collaborations and out-licensing. We believe that our protocell technology has the potential to create more product opportunities than we will be able to develop ourselves. We intend to license these opportunities to collaborators, potentially providing resources to support our internal activities. We may also enter into collaborations, acquisitions or license arrangements at appropriate stages in our research and development process to advance the development or potential commercialization of our product candidates. Such relationships can supplement our own internal expertise in areas such as discovery research, clinical trials and manufacturing, as well as provide us with access to licensees’ marketing, sales and distribution capabilities.
Product Candidates Overview
| Product Candidate |
Technology |
Most Advanced Indication |
Development Stage | |||
| ONT-380 |
Small Molecule | Breast cancer | Phase 1b | |||
| ONT-10 |
Immunotherapy | To be determined | Phase 1b |
In the table above, under the heading “Development Stage,” “Phase 1b” indicates initial clinical testing of safety, dosage tolerance, pharmacokinetics and pharmacodynamics. “Pre-clinical” indicates undergoing toxicology and pharmacology studies intended to support subsequent clinical development.
Clinical-Stage Product Candidates
ONT-380
ONT-380 (previously known as ARRY-380) is an orally active, reversible and selective small-molecule HER2 inhibitor. HER2, also known as ErbB2, is a receptor tyrosine kinase that is over-expressed in breast cancer and other cancers such as gastric and ovarian cancer. We are developing ONT-380 under an exclusive license arrangement with Array. We intend to develop ONT-380 primarily for the treatment of metastatic breast cancer, including patients with brain metastases.
Breast cancer. Breast cancer is the most common form of cancer in women worldwide, and the second leading cause of cancer-related death in women in North America. The American Cancer Society estimates that in 2015 more than 230,000 women in the U.S. will develop breast cancer and more than 40,000 will die from the disease. Approximately 15-20% of breast cancers overexpress HER2.
The treatment of breast cancer differs by stage and includes surgery and radiation for most earlier stage patients. The addition of HER2 targeted agents, including antibody-based therapies and a small molecule, has led to significant improvements in progression-free and overall survival, both in the adjuvant setting and for patients with metastatic disease. There are currently four approved agents for the treatment of HER2-positive breast cancer, Herceptin (trastuzumab), Perjeta (pertuzumab), Kadcyla (ado-trastuzumab emantasine, TDM1) and Tykerb (lapatinib). The size of the worldwide market for these agents in 2014 exceeded $8 billion.
The prevention and treatment of metastatic disease in the central nervous system (CNS) remains a significant unmet medical need for patients with HER2-positive breast cancer. The incidence of first relapse occurring in the CNS is increasing in patients who have received Herceptin as adjuvant therapy, and approximately 30% of patients with HER2-positive metastatic disease will develop CNS metastases.
3
Table of Contents
Prior results and status. In multiple preclinical tumor models, ONT-380 was well tolerated and demonstrated significant dose-related tumor growth inhibition that was superior to Herceptin and Tykerb® (lapatinib). Additionally, in these models, ONT-380 demonstrated synergistic or additive tumor growth inhibition when dosed in combination with the standard-of-care therapeutics Herceptin or Taxotere® (docetaxel). ONT-380 has also demonstrated superior activity, based on overall survival, compared to Tykerb® and to the investigational drug, neratinib, in an intracranial HER2+ breast cancer xenograft model.
A Phase 1 trial of ONT-380, with both dose-escalation and expansion components, was completed by Array in 50 patients, 43 of whom had HER2+ metastatic breast cancer. All HER2+ breast cancer patients had progressed on a Herceptin-containing regimen. In addition, over 80% had been treated with Tykerb, with many having progressed on therapy. In this study, ONT-380 demonstrated an acceptable safety profile; treatment-related adverse events were primarily Grade 1. Because ONT-380 is selective for HER2 and does not inhibit the epidermal growth factor receptor (EGFR), there was a low incidence and severity of treatment-related diarrhea, rash and fatigue. Additionally, there were no treatment-related cardiac events or Grade 4 treatment-related adverse events reported. Twenty-two HER2+ breast cancer patients with measurable disease were treated with ONT-380 at doses greater than or equal to 600 mg BID. In this heavily pretreated patient population, there was a clinical benefit rate (partial response [n = 3] plus stable disease for at least 6 months [n = 3]) of 27%.
In February 2014 we initiated two Phase 1b trials of ONT-380. The first trial is studying ONT-380 in combination with Xeloda® (capecitabine) and/or Herceptin® (trastuzumab) in patients who have been previously treated with Herceptin and Kadcyla® (ado-trastuzumab emtansine or TDM-1) for metastatic breast cancer. The primary objective is to determine the maximum-tolerated and/or recommended Phase 2 dose (MTD/RP2D) of ONT-380 in combination with the approved dose of either Xeloda or Herceptin or both. Secondary objectives include an evaluation of the safety and preliminary anti-tumor activity of the combinations. The study includes an expansion arm at the MTD/RP2D of ONT-380 in combination with both Xeloda and Herceptin, with the option to include expansion arms in combination with either agent alone. Patients with treated stable central nervous system (CNS) metastases are eligible for the dose escalation portions of the trial, while patients with CNS metastases which are either asymptomatic and untreated or progressive following local therapy may be included in the expansion cohorts.
The second Phase 1b trial is studying ONT-380 in combination with Kadcyla in patients with metastatic HER2+ breast cancer. The trial is a dose-escalation study in up to 48 patients who have been previously treated with Herceptin and a taxane for metastatic breast cancer. The primary objective is to determine the MTD/RP2D of ONT-380 in combination with the approved dose of Kadcyla. Secondary objectives include an evaluation of the safety and preliminary anti-tumor activity of the combination. Following determination of the MTD/RP2D, the study includes an expansion arm at the MTD/RP2D as well as an optional second expansion arm in patients with central nervous system metastases.
In December 2014, we announced that interim data from these two ongoing Phase 1b trials indicated preliminary clinical activity and tolerability in a heavily pretreated patient population. In the trial of ONT-380 in combination with Herceptin and/or Xeloda, data were available for 21 patients, including seven in the ONT-380 plus Xeloda cohort, eight in the ONT-380 plus Herceptin cohort, four in the ONT-380 plus Xeloda and Herceptin cohort, and two in an ongoing expansion cohort in patients with untreated or progressive central nervous system (CNS) metastases, both treated with ONT-380 plus Herceptin.
Seventeen of the patients were evaluable for best overall response using RECIST 1.1 criteria. In the ONT-380 plus Xeloda cohort, four patients had a partial response (PR) and three patients had stable disease (SD), for an overall clinical benefit rate of 100 percent (defined as either PR/CR or stable disease for > 6 months). In the ONT-380 plus Herceptin cohort, best response was a complete response (CR) in one patient, PR in two patients, SD in four patients, and progressive disease (PD) in one patient. Two patients in the ONT-380 plus Xeloda and Herceptin cohort were evaluable for response, one of whom had a PR and one PD. One patient in the CNS expansion cohort had a PR and the other SD.
Fourteen of the 21 patients in this trial had a history of CNS metastases, of whom six had evaluable CNS target lesions per modified RECIST 1.1 at the time of entry into the trial. Of these, best initial CNS response was SD, with decreases in CNS target lesions in four patients.
In the trial of ONT-380 in combination with Kadcyla, data were available for 17 patients, of whom 16 were evaluable for response. Patients in this trial were heavily pre-treated, having received a median of two prior systemic treatments for metastatic disease, including prior pertuzumab in six, and prior lapatinib in five. Best overall response was PR in five patients, SD in seven patients, and PD in four patients. Nine patients in this trial had a history of CNS metastases, of whom four had evaluable CNS target lesions per modified RECIST 1.1 at the time of entry into the trial. Three of these four patients had SD in the CNS, including two with decreases in measurable target lesions.
4
Table of Contents
ONT-380 was well-tolerated in both studies and in all combinations tested. The most common adverse events included nausea and vomiting, diarrhea, fatigue and elevated liver function tests. Most adverse events were grade 1 or 2 in severity. Elevated liver function tests were more common in patients also receiving Kadcyla. No grade 3 diarrhea was seen in either trial; anti-diarrheal prophylaxis was not a study requirement.
The Dana-Farber Cancer Institute, Boston, Massachusetts, is also currently conducting an investigator-sponsored trial of ONT-380 in combination with Herceptin in patients with brain metastases from HER2+ breast cancer.
ONT-10
We have developed a completely synthetic MUC1-based liposomal glycolipopeptide cancer vaccine, ONT-10, for potential use in several cancer indications, including breast, thyroid, colon, stomach, pancreas, ovarian and prostate, as well as certain types of lung cancer. The ONT-10 glycolipopeptide combines carbohydrate and peptide determinates in a multi-epitopic vaccine that evokes both cellular and humoral immune responses against major cancer-associated epitopes expressed on adenocarcinomas. ONT-10 includes our synthetic adjuvant PET Lipid A and is manufactured using our proprietary liposomal delivery technology. We currently own all rights to ONT-10.
We are conducting a two-part Phase 1 clinical trial of ONT-10. Part 1 is complete and studied a dose escalation schedule in 49 patients to determine the maximally tolerated and/or recommended dose of ONT-10 administered either once every other week or once every week over an 8 week period. Part 2 is investigating the safety of ONT-10 at the maximally tolerated or recommended dose in up to 15 additional patients in each of breast or ovarian cancer. Enrollment in Part 2 is complete. The ability of ONT-10 to induce both a humoral and a cellular immune response is being investigated in both parts of the study. Patients with stable disease for at least twelve weeks after starting treatment with ONT-10 are eligible for a separate maintenance protocol in which the same dose of the vaccine is administered every six weeks until tumor progression. Patients from both parts of the Phase 1 trial are currently enrolled in this maintenance protocol.
Data from the dose escalation portion of this trial were reported in June 2014. As of that date, the trial had enrolled 49 patients with malignancies of types associated with the expression of the tumor-associated antigen MUC1, including ovarian cancer (14), breast cancer (10), colorectal cancer (7), pancreatic cancer (5), endometrial cancer (4), and lung cancer (4). The patients were extensively pretreated, having received multiple lines of prior therapy (median 3, range 1-11). ONT-10 was administered at doses from 250 µg up to 2000 µg weekly, and from 250 µg up to 2000 µg every other week.
ONT-10 was well-tolerated in this trial, with no treatment-related serious adverse events identified. The most common treatment-related adverse events were fatigue and injection site reactions, all of which were mild to moderate in severity. A dose-limiting toxicity was not identified, and 2000 µg weekly was selected as the dose for future studies.
Patients without disease progression by Immune-Related Response Criteria (irRC) for at least twelve weeks after starting treatment with ONT-10 were eligible for the maintenance protocol. As of the time of the June 2014 update, 31 of 43 patients (72%) evaluable for response had entered the maintenance protocol. Eleven patients were without disease progression for at least six months (range, 6.4-26 months). A decrease in the size of nodal disease was seen in two patients with ovarian cancer.
A significant endpoint of the trial was to determine if ONT-10 stimulated the production of MUC1-specific antibodies as seen in preclinical animal models. IgM and IgG anti-MUC1 antibodies were observed in the majority of patients with many titers exceeding 1:50,000. The data support a dose and schedule response, with the greatest IgG response occurring at 1000-2000 µg weekly.
We recently initiated a Phase 1b trial of ONT-10 in combination with the T-cell agonist antibody varlilumab in collaboration with Celldex. Varlilumab is a fully human monoclonal antibody that targets CD27, a critical molecule in the activation pathway of lymphocytes. The trial is an open-label Phase 1b study of ONT-10 administered at the recommended single agent dose in combination with varlilumab at two dose levels in up to 42 patients with advanced breast or ovarian cancer. The primary objective of the trial is to determine the safety and tolerability of the combined therapy. Additional objectives include evaluations of the impact of combination treatment on MUC1-specific humoral and cellular immune responses, T-cell activation markers and levels of regulatory T-cells, and anti-tumor effects.
5
Table of Contents
Pre-clinical Product Candidates
Checkpoint kinase 1 inhibitors
Checkpoint kinase 1 (Chk1) is a protein kinase that is activated in response to DNA damage and DNA replication stress. Together with other cellular factors, Chk1 provides a coordinated “checkpoint” to arrest the cell division cycle in response to damaged DNA. The induction of this cell cycle checkpoint enables cells to repair DNA lesions and ensures the fidelity of the cell division process. Cancer cells commonly have mutations that reduce or eliminate the activity of DNA damage response factors that function in parallel with Chk1. These mutations make tumor cells more reliant on the activity of Chk1 to provide cell cycle checkpoint control, which may make them more sensitive to Chk1 inhibitors and produce a synergistic tumor killing effect when combined with DNA targeted chemotherapy drugs.
In pre-clinical studies, Chk1 inhibitors have been shown to inhibit tumor growth as single agents and can substantially increase the effectiveness of anti-cancer drugs that induce DNA damage or target DNA replication. There are currently no marketed drugs which specifically target Chk1. Genentech is conducting a Phase 1 trial of an oral Chk1 inhibitor in patients with refractory solid tumors or lymphoma. Eli Lilly and Company is developing an intravenous Chk1 inhibitor in patients with cancer, currently in Phase 2.
We are developing a series of highly potent, selective, and orally active Chk1 inhibitors in collaboration with Sentinel . We have identified lead molecules, and currently expect to initiate IND-enabling studies this year. Under terms of our agreement with Sentinel, we are responsible for clinical development, manufacture and commercialization.
Discovery Research
Protocells
Protocells are nanoparticles designed to enable the targeted delivery of multiple therapeutic agents, including nucleic acids, proteins, peptides and small molecules. We intend to use the protocell technology to develop product candidates for the treatment of cancer and rare diseases, either alone or with partners.
Each protocell consists of a very small silica sphere surrounded by a lipid bilayer. The silica sphere is synthesized with many small pores and tunnels, creating a large surface area to which the cargo to be delivered is adsorbed. This large surface area enables the delivery of significantly greater quantities of payload than comparably sized liposomes with the payload in solution. The silica also supports the lipid bilayer, which stabilizes the layer and allows the use of fluid lipids. Targeting ligands incorporated in the lipid bilayer can rapidly diffuse, allowing the ligands to interact with multiple receptors to increase the strength of binding to the target. The ability of the targeting ligands to diffuse limits the overall number required, which may decrease any potential immune response to the protocell.
We are currently focusing our activities with protocells on the delivery of mini-circle DNA or mRNA to produce proteins intracellularly in specifically targeted organs or cells. For example, protocells may allow in vivo delivery of nucleic acids encoding for a chimeric antigen receptor to T cells, thereby creating an “off-the-shelf” CAR-T program. As another example, protocells may allow for the delivery of nucleic acids coding for a specific enzyme and thus be applicable to the treatment of certain protein or enzyme deficiency diseases. We are also investigating the targeting of protocells specifically to tumors. In oncology, protocells may be used for the delivery of small molecules, including cytotoxic drugs, targeted agents or protein toxins.
We believe that the potential of the protocell technology to deliver a wide variety of targets may allow us to create multiple product candidates, not all of which we will be able to develop ourselves. We intend to seek partners to develop these additional product candidates, while retaining the rights to the technology platform for ourselves.
Immunotherapy
We are collaborating with Adimab for the discovery of novel antibodies against undisclosed immunotherapy targets in oncology. We are utilizing our internal expertise in immunotherapy to screen antibodies identified by Adimab against one or more targets which we have selected. The program is at an early discovery phase.
License Agreements
Array BioPharma Inc. In December 2014 we entered into a license agreement with Array. Pursuant to the license agreement, Array has granted us an exclusive license to develop, manufacture and commercialize ONT-380. The license agreement replaced a development and commercialization agreement under which we and Array were previously jointly developing ONT-380. As part of the agreement, we paid Array $20 million as an upfront fee. In addition, we will pay
6
Table of Contents
Array a portion of any payments received from sublicensing ONT-380 rights. If we are acquired within three years of the effective date of the license agreement, Array will be eligible for up to $280 million in commercial milestone payments. Array is also entitled to receive up to a double-digit royalty based on net sales of ONT-380
STC.UNM. In August 2014, we acquired Alpine and by way of assignment became a party to an exclusive license agreement with STC.UNM, a New Mexico nonprofit corporation affiliated with the University of New Mexico with respect to technology relating to protocells, a mesoporous silica nanoparticle delivery platform. Under the terms of the license, we have the right to conduct research, clinical development and commercialize all inventions and products that are developed from the platform technology in certain fields of use as described in the license. Under the license, STC.UNM is eligible to receive success-based milestone payments up to $5 million, a double-digit royalty on commercial sublicensing income and a low single-digit royalty on net sales based on events as outlined in the license agreement.
Merck KGaA. In May 2001, we and Merck KGaA entered into a collaborative arrangement to pursue joint global product research, clinical development and commercialization for two product candidates, including tecemotide (formerly known as L-BLP25 or Stimuvax), a MUC1-based liposomal cancer vaccine. This collaboration agreement was subsequently revised and ultimately replaced in 2008 with a license agreement. Under the 2008 license agreement, (1) we licensed to Merck KGaA the exclusive right to develop, commercialize and manufacture tecemotide and the right to sublicense to other persons all rights licensed to Merck KGaA by us, (2) we transferred certain manufacturing know-how, (3) we agreed not to develop any product, other than ONT-10, that is competitive with tecemotide and (4) if we intend to license the development or commercialization rights to ONT-10, Merck KGaA will have a right of first negotiation with respect to such rights. In 2014 Merck KGaA announced that it does not intend to continue the clinical development of tecemotide. Merck KGaA is continuing to support certain investigator-sponsored studies of tecemotide.
Collaborations
Sentinel Oncology Ltd. In 2014 we signed a research collaboration agreement with Sentinel for the discovery of novel Chk1 inhibitors. Under the agreement we make payments to Sentinel to support their chemistry research. We are responsible for pre-clinical and clinical development, manufacture and commercialization of any resulting compounds. Sentinel is eligible to receive success-based development and commercial milestone payments up to $90 million GBP based on development and commercialization events, including the initiation of cGMP toxicology studies, the initiation of certain clinical trials, regulatory approval and first commercial sale. Sentinel is also entitled to a single-digit royalty based on net sales.
Celldex Therapeutics, Inc. We are collaborating with Celldex on a combined clinical trial of ONT-10 and varlilumab, a fully human monoclonal antibody that targets CD27. The trial is being conducted by Oncothyreon at our expense. We and Celldex will jointly own the data from the trial and will make any plans for potential future development of the combination therapy together. Neither Oncothyreon nor Celldex has granted the other a license, or any other rights, to its product candidate. There are no payments due under this agreement.
Adimab LLC. In late 2014 we initiated a collaboration with Adimab for the discovery of novel antibodies against undisclosed immunotherapy targets in oncology. Oncothyreon will have sole responsibility for the manufacture, development and commercialization of any antibody products which result. The collaboration is currently at an early discovery stage. Adimab is entitled to research funding, success-based development milestone payments up to $17 million and a low single digit royalty based on net sales.
Acquisitions
On August 8, 2014, we entered into an Agreement and Plan of Reorganization (Merger Agreement) with Alpine, a privately held biotechnology company developing protocells, a nanoparticle capable of delivery of nucleic acids, proteins, peptides and small molecules. Pursuant to the terms and conditions set forth in the Merger Agreement, on August 8, 2014, through a merger sub, we consummated the acquisition of Alpine. In connection with the closing of the acquisition, we issued 9,245,344 shares of our common stock in exchange for all of the outstanding capital stock of Alpine (Merger Consideration). The issued shares represented ten percent of our capital stock on a fully-diluted basis immediately following the acquisition and reflected adjustments made pursuant to the Merger Agreement. The shares are subject to certain resale restrictions. An amount of stock equal to 12.5% of the Merger Consideration was placed in escrow as security for the indemnification obligations of Alpine’s stockholders. We intend to utilize the protocell technology to develop new product candidates for the treatment of cancer and rare diseases, either on our own or with partners.
7
Table of Contents
Patents and Proprietary Information
We seek appropriate patent protection for our proprietary technologies by filing patent applications in the United States and other countries. As of December 31, 2014, we owned approximately 15 U.S. patents and patent applications, as well as the corresponding foreign patents and patent applications and held exclusive or partially exclusive licenses to 21 U.S. patents and patent applications, as well as the corresponding foreign patents and patent applications.
Our patents and patent applications are directed to our product candidates as well as to our protocell technology and liposomal formulation technology. Although we believe our patents and patent applications provide us with a competitive advantage, the patent positions of biotechnology and pharmaceutical companies can be uncertain and involve complex legal and factual questions. We and our corporate collaborators may not be able to develop patentable products or processes or obtain patents from pending patent applications. Even if patent claims are allowed, the claims may not issue, or in the event of issuance, may not be sufficient to protect the technology owned by or licensed to us or our corporate collaborators.
Our clinical product candidates are protected by composition and use patents and patent applications. Patent protection afforded by the patents and patent applications covering our product candidates will expire over the following time frames:
| Product Candidate |
Expiration of U.S. Patent Protection | |
| ONT-10 |
2023 (patent) – 2032 (patent, application) | |
| ONT-380 |
2031 (patent) – 2033 (patent application) |
In addition, our composition of matter patents for ONT-10 will expire in 2032. We also rely on trade secrets and proprietary know-how, especially when we do not believe that patent protection is appropriate or can be obtained. Our policy is to require each of our employees, consultants and advisors to execute a confidentiality and inventions assignment agreement before beginning their employment, consulting or advisory relationship with us. These agreements provide that the individual must keep confidential and not disclose to other parties any confidential information developed or learned by the individual during the course of their relationship with us except in limited circumstances. These agreements also provide that we shall own all inventions conceived by the individual in the course of rendering services to us.
Manufacturing
We currently outsource the manufacturing of drug substances and drug products for all of our products in clinical development. This arrangement allows us to use contract manufacturers that already have extensive cGMP manufacturing experience. We have a staff with experience in the management of contract manufacturing and the development of efficient commercial manufacturing processes for our products. We currently intend to outsource the supply of all our commercial products.
We manufacture the active pharmaceutical ingredients (API’s) used in ONT-10 at contract manufacturing organizations. The MUC-1 peptide API has been manufactured at two different sites and we believe we could successfully manufacture at other sites if necessary. PET Lipid A is currently manufactured at a single site, and a second manufacturing site has been selected and can be used if needed. If our current contract manufacturer failed to manufacture PET Lipid A, clinical trials could be delayed while we transfer manufacturing to the secondary site. Because there are specific requirements for manufacturing ONT-10, there are a limited number of contract manufacturers available to us. If our contract manufacturer would not have capacity to manufacture ONT-10, future clinical trials could be delayed.
For our small molecule programs, we rely on third parties to manufacture both the API and drug product. Under our prior collaboration agreement with Array for the development of ONT-380, Array was responsible for the manufacture of ONT-380, which they outsourced to third parties. In December 2014, we entered into an exclusive license agreement with Array to develop, manufacture and commercialize ONTY-380. Under the exclusive license agreement, which superseded the collaboration agreement, we are responsible for the manufacture of ONT-380, which we plan to outsource to third parties.
We believe that our existing supplies of drug product and our contract manufacturing relationships with our existing and other potential contract manufacturers with whom we are in discussions, will be sufficient to accommodate our planned clinical trials. However, we may need to obtain additional manufacturing arrangements, if available on commercially reasonable terms, or increase our own manufacturing capability to meet our future needs, both of which would require significant capital investment. We may also enter into collaborations with pharmaceutical or larger biotechnology companies to enhance the manufacturing capabilities for our product candidates.
8
Table of Contents
Competition
The pharmaceutical and biotechnology industries are intensely competitive, and any product candidate developed by us would compete with existing drugs and therapies. There are many pharmaceutical companies, biotechnology companies, public and private universities, government agencies and research organizations that compete with us in developing various approaches to cancer therapy. Many of these organizations have substantially greater financial, technical, manufacturing and marketing resources than we have. Several of them have developed or are developing therapies that could be used for treatment of the same diseases that we are targeting. In addition, many of these competitors have significantly greater commercial infrastructures than we have. Our ability to compete successfully will depend largely on our ability to:
| • | design and develop products that are superior to other products in the market and under development; |
| • | attract and retain qualified scientific, product development and commercial personnel; |
| • | obtain patent and/or other proprietary protection for our product candidates and technologies; |
| • | obtain required regulatory approvals; |
| • | successfully collaborate with pharmaceutical companies in the design, development and commercialization of new products; |
| • | compete on, among other things, product efficacy and safety, time to market, price, extent of adverse side effects and the basis of and convenience of treatment procedures; and |
| • | identify, secure the rights to and develop products and exploit these products commercially before others are able to develop competitive products. |
In addition, our ability to compete may be affected if insurers and other third party payors seek to encourage the use of generic products, making branded products less attractive to buyers from a cost perspective.
ONT-380. ONT-380 is an inhibitor of the receptor tyrosine kinase HER2, also known as ErbB2. There are multiple marketed products which target HER2, including the antibodies trastuzumab (Herceptin®) and pertuzumab (Perjeta®) and the antibody toxin conjugate ado-trastuzumab emtansine (Kadcyla®), all from Roche/Genentech. In addition, GlaxoSmithKline markets the dual HER1/HER2 oral kinase inhibitor lapatinib (Tykerb®) for the treatment of metastatic breast cancer, and Puma Biotechnology is developing the HER1/HER2/HER4 inhibitor neratinib in Phase 3.
ONT-10. ONT-10 is a MUC1-based liposomal glycolipopeptide cancer vaccine. It is currently in the early stages of development for many indications, for which there are likely to be other competitors.
Government Regulation
Government authorities in the United States, at the federal, state and local level, and other countries extensively regulate, among other things, the research, development, testing, manufacture, labeling, promotion, advertising, distribution, marketing and export and import of biopharmaceutical products such as those we are developing.
U.S. Government Regulation
In the United States, the information that must be submitted to the Food and Drug Administration (FDA) in order to obtain approval to market a new drug varies depending on whether the drug is a new product whose safety and effectiveness has not previously been demonstrated in humans or a drug whose active ingredient(s) and certain other properties are the same as those of a previously approved drug. A new drug will follow the New Drug Application (NDA) route for approval, a new biologic will follow the biologics license application (BLA) route for approval, and a drug that claims to be the same as an already approved drug may be able to follow the Abbreviated New Drug Application (ANDA) route for approval.
NDA and BLA Approval Process
In the United States, the FDA regulates drugs and biologics under the Federal Food, Drug and Cosmetic Act, and, in the case of biologics, also under the Public Health Service Act, and implementing regulations. If we fail to comply with the applicable U.S. requirements at any time during the product development process, approval process or after approval, we may become subject to administrative or judicial sanctions. These sanctions could include the FDA’s refusal to approve pending applications, license suspension or revocation, withdrawal of an approval, a clinical hold, warning letters, product recalls, product seizures, total or partial suspension of production or distribution, injunctions, fines, civil penalties or criminal prosecution. Any agency or judicial enforcement action could have a material adverse effect on us.
9
Table of Contents
The steps required before a drug or biologic may be marketed in the United States include:
| • | completion of pre-clinical laboratory tests, animal studies and formulation studies under the FDA’s good laboratory practices regulations; |
| • | submission to the FDA of an IND for human clinical testing, which must become effective before human clinical trials may begin; |
| • | performance of adequate and well-controlled clinical trials to establish the safety and efficacy of the product for each indication; |
| • | submission to the FDA of an NDA or BLA; |
| • | satisfactory completion of an FDA inspection of the manufacturing facility or facilities at which the product is produced to assess compliance with cGMP; and |
| • | FDA review and approval of the NDA or BLA. |
Preclinical tests include laboratory evaluations of product chemistry, toxicity and formulation, as well as animal studies. An IND sponsor must submit the results of the pre-clinical tests, together with manufacturing information and analytical data, to the FDA as part of the IND. The IND must become effective before human clinical trials may begin. An IND will automatically become effective 30 days after receipt by the FDA, unless before that time the FDA raises concerns or questions about issues such as the conduct of the trials as outlined in the IND. In that case, the IND sponsor and the FDA must resolve any outstanding FDA concerns or questions before clinical trials can proceed. Submission of an IND may not result in the FDA allowing clinical trials to commence.
Clinical trials involve the administration of the investigational product to human subjects under the supervision of qualified investigators. Clinical trials are conducted under protocols detailing, among other things, the objectives of the study, the parameters to be used in monitoring safety and the effectiveness criteria to be evaluated. Each clinical protocol must be submitted to the FDA as part of the IND.
Clinical trials typically are conducted in three sequential phases, but the phases may overlap or be combined. Each trial must be reviewed and approved by an independent institutional review board at each site where the trial will be conducted before it can begin at that site. Phase 1 clinical trials usually involve the initial introduction of the investigational drug into humans to evaluate the product’s safety, dosage tolerance, pharmacokinetics and pharmacodynamics and, if possible, to gain an early indication of its effectiveness. Phase 2 clinical trials usually involve controlled trials in a limited patient population to evaluate dosage tolerance and appropriate dosage, identify possible adverse effects and safety risks, and evaluate preliminarily the efficacy of the drug for specific indications. Phase 3 clinical trials usually further evaluate clinical efficacy and further test for safety in an expanded patient population. Phase 1, Phase 2 and Phase 3 testing may not be completed successfully within any specified period, if at all. The FDA or we may suspend or terminate clinical trials at any time on various grounds, including a finding that the subjects or patients are being exposed to an unacceptable health risk.
Assuming successful completion of the required clinical testing, the results of the pre-clinical studies and of the clinical studies, together with other detailed information, including information on the chemistry, manufacture and control criteria of the product, are submitted to the FDA in the form of an NDA or BLA requesting approval to market the product for one or more indications. The FDA reviews an NDA to determine, among other things, whether a product is safe and effective for its intended use. The FDA reviews a BLA to determine, among other things, whether the product is safe, pure and potent and whether the facility in which it is manufactured, processed, packed or held meets standards designed to assure the product’s continued safety, purity and potency. In connection with the submission of an NDA or BLA, an applicant may seek a special protocol assessment (SPA), which is an agreement between an applicant and the FDA on the design and size of clinical trials that is intended to form the basis of an NDA or BLA.
Before approving an application, the FDA will inspect the facility or the facilities at which the product is manufactured. The FDA will not approve the product unless cGMP compliance is satisfactory. If the FDA determines the application, manufacturing process or manufacturing facilities are not acceptable, it will outline the deficiencies in the submission and often will request additional testing or information. Notwithstanding the submission of any requested additional information, the FDA ultimately may decide that the application does not satisfy the regulatory criteria for approval.
The testing and approval process requires substantial time, effort and financial resources, and each may take several years to complete. The FDA may not grant approval on a timely basis, or at all. We may encounter difficulties or unanticipated costs in our efforts to secure necessary governmental approvals, which could delay or preclude us from marketing our product candidates. The FDA may limit the indications for use or place other conditions on any approvals
10
Table of Contents
that could restrict the commercial application of our product candidates. After approval, some types of changes to the approved product, such as adding new indications, manufacturing changes and additional labeling claims, are subject to further FDA review and approval.
Fast Track Designation / Priority Review
A Fast Track product is defined as a new drug or biologic intended for the treatment of a serious or life-threatening condition that demonstrates the potential to address unmet medical needs for this condition. Under the Fast Track program, the sponsor of a new drug or biologic may request that the FDA designate the drug or biologic as a Fast Track product at any time during the development of the product, prior to marketing.
The FDA can base approval of a marketing application for a Fast Track product on an effect on a surrogate endpoint, or on another endpoint that is reasonably likely to predict clinical benefit. The FDA may condition approval of an application for a Fast Track product on a commitment to do post-approval studies to validate the surrogate endpoint or confirm the effect on the clinical endpoint and require prior review of all promotional materials. In addition, the FDA may withdraw approval of a Fast Track product in an expedited manner on a number of grounds, including the sponsor’s failure to conduct any required post-approval study in a timely manner.
The FDA also has established priority and standard review classifications for original NDAs and efficacy supplements. Priority review applies to the time frame for FDA review of completed marketing applications and is separate from and independent of the Fast Track and accelerated approval mechanisms. The classification system, which does not preclude the FDA from doing work on other projects, provides a way of prioritizing NDAs upon receipt and throughout the application review process.
Priority designation applies to new drugs that have the potential for providing significant improvement compared to marketed products in the treatment or prevention of a disease. Hence, even if an NDA is initially classified as a priority application, this status can change during the FDA review process, such as in the situation where another product is approved for the same disease for which previously there was no available therapy. In addition, priority review does not guarantee that a product candidate will receive regulatory approval. To date, none of our product candidates have obtained priority designation from the FDA.
Post-Approval Requirements
After regulatory approval of a product is obtained, we are required to comply with a number of post-approval requirements. Holders of an approved NDA or BLA are required to report certain adverse reactions and production problems to the FDA, to provide updated safety and efficacy information and to comply with requirements concerning advertising and promotional labeling for their products. Also, quality control and manufacturing procedures must continue to conform to cGMP after approval. The FDA periodically inspects manufacturing facilities to assess compliance with cGMP, which imposes certain procedural, substantive and recordkeeping requirements. Accordingly, manufacturers must continue to expend time, money and effort in the area of production and quality control to maintain compliance with cGMP and other aspects of regulatory compliance.
We use, and in at least the near-term will continue to use, third party manufacturers to produce our product candidates in clinical and commercial quantities. Future FDA inspections may identify compliance issues at our facilities or at the facilities of our contract manufacturers that may disrupt production or distribution, or require substantial resources to correct. In addition, discovery of problems with a product or the failure to comply with applicable requirements may result in restrictions on a product, manufacturer or holder of an approved NDA or BLA, including withdrawal or recall of the product from the market or other voluntary, FDA-initiated or judicial action that could delay or prohibit further marketing. Also, new government requirements may be established that could delay or prevent regulatory approval of our product candidates under development.
In addition, as a condition of approval of an NDA or BLA, the FDA may require post-marketing testing and surveillance to monitor the product’s safety or efficacy.
Canadian and Foreign Regulation
In addition to regulations in the United States, we will be subject to a variety of foreign regulations governing clinical trials and commercial sales and distribution of our product candidates. Whether or not we obtain FDA approval for a product candidate, we must obtain approval of a product candidate by the comparable regulatory authorities of foreign countries before we can commence clinical trials or marketing of the product candidate in those countries. The approval process
11
Table of Contents
varies from country to country, and the time may be longer or shorter than that required for FDA approval. The requirements governing the conduct of clinical trials, product licensing, pricing and reimbursement vary greatly from country to country.
Under the Canadian regulatory system, Health Canada is the regulatory body that governs the sale of drugs for the purposes of use in clinical trials. Accordingly, any company that wishes to conduct a clinical trial in Canada must submit a clinical trial application to Health Canada. Health Canada reviews the application and notifies the company within 30 days if the application is found to be deficient. If the application is deemed acceptable, Health Canada will issue a letter to the company within the 30-day review period which means the company may proceed with its clinical trial(s).
Under European Union regulatory systems, we may submit marketing authorizations either under a centralized or decentralized procedure. The centralized procedure provides for the grant of a single marketing authorization that is valid for all European Union member states. The decentralized procedure provides for mutual recognition of national approval decisions. Under this procedure, the holder of a national marketing authorization from one member state may submit an application to the remaining member states. Within 90 days of receiving the application and assessment report, each member state must decide whether to recognize approval.
Reimbursement
Sales of biopharmaceutical products depend in significant part on the availability of third party reimbursement, including Medicare. Each third party payer may have its own policy regarding what products it will cover, the conditions under which it will cover such products, and how much it will pay for such products. It is time consuming and expensive for us to seek reimbursement from third party payers. Reimbursement may not be available or sufficient to allow us to sell our products on a competitive and profitable basis.
In addition, in some foreign countries, the proposed pricing for a drug must be approved before it may be lawfully marketed. The requirements governing drug pricing vary widely from country to country. For example, the European Union provides options for its member states to restrict the range of medicinal products for which their national health insurance systems provide reimbursement and to control the prices of medicinal products for human use. A member state may approve a specific price for the medicinal product or it may instead adopt a system of direct or indirect controls on the profitability of the company placing the medicinal product on the market.
Political, economic and regulatory influences are subjecting the healthcare industry in the United States to fundamental changes. There have been, and we expect there will continue to be, legislative and regulatory proposals to change the healthcare system in ways that could significantly affect our business, including the Affordable Care Act of 2010. We anticipate that the U.S. Congress, state legislatures and the private sector will continue to consider and may adopt healthcare policies intended to curb rising healthcare costs. These cost containment measures include:
| • | controls on government funded reimbursement for drugs; |
| • | controls on healthcare providers; |
| • | challenges to the pricing of drugs or limits or prohibitions on reimbursement for specific products through other means; |
| • | reform of drug importation laws; and |
| • | expansion of use of managed care systems in which healthcare providers contract to provide comprehensive healthcare for a fixed cost per person. |
We are unable to predict what legislation, regulations or policies, if any, relating to the healthcare industry or third party coverage and reimbursement may be enacted in the future or what the magnitude of the effect such legislation, regulations or policies would have on our business. Any cost containment measures, including those listed above, or other healthcare system reforms that are adopted could have a material adverse effect on our business, financial condition and profitability.
Research and Development
We devote a substantial portion of our resources to developing new product candidates. During the years ended December 31, 2014, 2013 and 2012, we expended approximately $41.9 million, $33.2 million and $22.0 million, respectively, on research and development activities. Our research and development expenses included a $10.0 million upfront license payment to Array in 2013 upon initiation of our ONT-380 collaboration, and a $20.0 million upfront license payment to Array in 2014 in connection with our exclusive license of ONT-380.
12
Table of Contents
Employees
As of December 31, 2014, we had 49 employees, 36 of whom are engaged in development activities, 13 in finance and administration, and 12 of whom hold Ph.D. and/or M.D. degrees. A number of our management and professional employees have had prior experience with other pharmaceutical or medical products companies.
Our ability to develop marketable products and to establish and maintain our competitive position in light of technological developments will depend, in part, on our ability to attract and retain qualified personnel. Competition for such personnel is intense. We have also chosen to outsource activities where skills are in short supply or where it is economically prudent to do so.
None of our employees are covered by collective bargaining agreements and we believe that our relations with our employees are good.
Financial Information
We manage our operations and allocate resources as a single reporting segment. Financial information regarding our operations, including net loss, for the years ended December 31, 2014, 2013 and 2012, our total assets, liabilities and stockholders’ equity as of December 31, 2014 and 2013, is included in our audited financial statements located elsewhere in this Annual Report on Form 10-K.
13
Table of Contents
| Item 1A. | Risk Factors |
Set forth below and elsewhere in this report, and in other documents we file with the SEC are descriptions of risks and uncertainties that could cause actual results to differ materially from the results contemplated by the forward-looking statements contained in this report. Because of the following factors, as well as other variables affecting our operating results, past financial performance should not be considered a reliable indicator of future performance and investors should not use historical trends to anticipate results or trends in future periods. The risks and uncertainties described below are not the only ones facing us. Other events that we do not currently anticipate or that we currently deem immaterial may also affect our results of operations and financial condition.
Risks Relating to our Business
Products that appear promising in research and development may be delayed or may fail to reach later stages of clinical development.
The successful development of pharmaceutical products is highly uncertain. Products that appear promising in research and development may be delayed or fail to reach later stages of development. For example, in September 2014 Merck KGaA announced that its biopharmaceutical division Merck Serono decided to discontinue the clinical development program of tecemotide as a monotherapy in Stage III NSCLC, including the Phase III START2 and INSPIRE studies. In addition, the ongoing Phase 1 trials for ONT-380 and ONT-10 may fail to demonstrate that either product candidate is sufficiently safe and effective to warrant further development.
Furthermore, decisions regarding the further development of product candidates must be made with limited and incomplete data, which makes it difficult to accurately predict whether the allocation of limited resources and the expenditure of additional capital on specific product candidates will result in desired outcomes. Preclinical and clinical data can be interpreted in different ways, and negative or inconclusive results or adverse medical events during a clinical trial could delay, limit or prevent the development of a product candidate, which could harm our business, financial condition or the trading price of our securities. There can be no assurance as to whether or when we will receive regulatory approvals for any of our product candidates, including ONT-380 or ONT-10.
There is no assurance that ONT-380 will be safe, effective or receive regulatory approval.
ONT-380 is an early stage clinical development candidate and the risks associated with its development are significant. Promising pre-clinical data in animal models and early clinical data may not be predictive of later clinical trial results. Additional clinical data may fail to establish that ONT-380 is effective in treating breast cancer or central nervous system disease or may indicate safety profile concerns not indicated by early clinical data. In December 2014, we announced that interim data from these ongoing Phase 1b trials indicated preliminary clinical activity and tolerability in a heavily pretreated patient population. However, these trials are not yet complete, and even if final Phase 1 data are encouraging, further trials will be necessary to establish safety and efficacy.
If the results of the current Phase 1 ONT-380 trials, or of future ONT-380 trials, do not indicate a favorable safety and efficacy profile for ONT-380, or otherwise fail to support the continued development of ONT-380, a substantial decline in the price of our common stock could result. There can be no assurance as to whether we will be able to successfully develop and commercialize ONT-380.
Our pipeline as a whole is subject to the inherent risks of early stage pharmaceutical development.
As a function of their development stage, preclinical programs and product candidates in early clinical development are inherently subject to a high degree of risk. Research programs to identify new product candidates require substantial technical, financial and human resources. Because our current product pipeline is comprised of product candidates in pre-clinical development and Phase 1 trials, our business is heavily subject to the risks of early stage pharmaceutical development.
If we are not able to advance our preclinical programs, including our investigation of the utility of checkpoint kinase 1 inhibitors, and our Phase 1 product candidates fail, our pipeline of products in development could be reduced or eliminated. This would cause our stock price to decline and could have a material adverse effect on our business, including but not limited to our ability to raise capital to rebuild our pipeline and develop future product candidates.
14
Table of Contents
We may not be successful in our efforts to use our protocell platform to develop a pipeline of product candidates or create partnership opportunities.
We intend to use our protocell platform to discover and develop our own product candidates. Our protocell platform is at an early stage of development and has not yet, and may never, lead to the development of product candidates. Even if we are successful in developing new product candidates, such product candidates may not be suitable for clinical development, including as a result of their harmful side-effects, limited efficacy or other characteristics that make it unlikely such product candidates will receive regulatory approval or achieve commercial success.
We also intend to enter into strategic partnerships with respect to our protocell platform, including business development transactions that license certain rights to our protocell platform to third parties and research collaborations. We may not be successful in entering into any capital-generating transactions with respect to this technology. Establishing strategic partnerships is difficult and time-consuming. Potential partners may reject partnerships based upon their assessment of our technology or product offerings or our financial, regulatory or intellectual property position. If we fail to establish a sufficient number of partners on acceptable terms, we may not be able to commercialize our products or generate sufficient revenue to fund further research and development efforts. Even if we establish new partnerships, these relationships may never result in the successful development or commercialization of any product candidates.
We have a history of net losses, we anticipate additional losses and we may never become profitable.
Other than the year ended December 31, 2008, we have incurred net losses in each fiscal year since we commenced our research activities. The net income we realized in 2008 was due entirely to our December 2008 transactions with Merck KGaA, and we do not anticipate realizing net income again for the foreseeable future. As of December 31, 2014, our accumulated deficit was approximately $482.0 million. Our losses have resulted primarily from expenses incurred in research and development of our product candidates. We may make significant capital commitments to fund the development of our product candidates. If these development efforts are unsuccessful, the development costs would be incurred without any future revenue, which could have a material adverse effect on our financial condition. We do not know when or if we will complete our product development efforts, receive regulatory approval for any of our product candidates, or successfully commercialize any approved products. As a result, it is difficult to predict the extent of any future losses or the time required to achieve profitability, if at all. Any failure of our products to complete successful clinical trials and obtain regulatory approval and any failure to become and remain profitable could adversely affect the price of our common stock and our ability to raise capital and continue operations.
If we fail to acquire and develop products or product candidates at all or on commercially reasonable terms, we may be unable to grow our business.
The success of our product pipeline strategy depends, in part, on our ability to identify, select and acquire product candidates. Proposing, negotiating and implementing an economically viable product acquisition or license is a lengthy and complex process. We compete for partnering arrangements and license agreements with pharmaceutical and biotechnology companies and academic research institutions. Our competitors may have stronger relationships with third parties with whom we are interested in collaborating or may have more established histories of developing and commercializing products. As a result, our competitors may have a competitive advantage in entering into partnering arrangements with such third parties. In addition, even if we find promising product candidates, and generate interest in a partnering or strategic arrangement to acquire such product candidates, we may not be able to acquire rights to additional product candidates or approved products on terms that we find acceptable, if at all. If we fail to acquire and develop product candidates from others, we may be unable to grow our business.
We expect that any product candidate to which we acquire rights will require additional development efforts prior to commercial sale, including extensive clinical evaluation and approval by the U.S. Food and Drug Administration (FDA) and non-U.S. regulatory authorities. All product candidates are subject to the risks of failure inherent in pharmaceutical product development, including the possibility that the product candidate will not be shown to be sufficiently safe and effective for approval by regulatory authorities. Even if the product candidates are approved, we can make no assurance that we would be capable of economically producing the product or that the product would be commercially successful.
There is no assurance that we will be granted regulatory approval for any of our product candidates.
We are currently conducting two Phase 1b trials for ONT-380, one Phase 1 trial for ONT-10 and collaborating with Celldex to conduct a combination Phase 1b trial of ONT-10 and varlilumab. There can be no assurance that these and future trials will demonstrate sufficient safety and efficacy to obtain the requisite regulatory approvals. A number of companies in the
15
Table of Contents
biotechnology and pharmaceutical industries, including our company, have suffered significant setbacks in advanced clinical trials, even after promising results in earlier trials. For example, in September 2014, we and Merck KGaA announced that Merck KGaA decided to discontinue the clinical development program of tecemotide in NSCLC, including the Phase III INSPIRE and START2 studies.
Further, we may be unable to submit applications to regulatory agencies within the time frame we currently expect. Once submitted, applications must be approved by various regulatory agencies before we can commercialize the product described in the application. Additionally, even if applications are submitted, regulatory approval may not be obtained for any of our product candidates, and regulatory agencies could require additional studies to verify safety or efficacy, which could make further development of our product candidates impracticable. If our product candidates are not shown to be safe and effective in clinical trials, we may not receive regulatory approval, which would have a material adverse effect on our business, financial condition and results of operations.
We currently rely on third-party manufacturers to supply our product candidates. Any disruption in production, inability of these third-party manufacturers to produce adequate quantities to meet our needs or other impediments with respect to development or manufacturing could adversely affect our ability to continue our research and development activities or successfully complete pre-clinical studies and clinical trials, delay submissions of our regulatory applications or adversely affect our ability to commercialize our product candidates in a timely manner, or at all.
Under our prior collaboration agreement with Array for the development of ONT-380, Array was responsible for the manufacture of ONT-380, which they outsourced to third parties. In December 2014, we entered into an exclusive license agreement with Array to develop, manufacture and commercialize ONT-380. Under the exclusive license agreement, which superseded the collaboration agreement, we are responsible for the manufacture of ONT-380, which we plan to outsource to third parties. Celldex is responsible for the manufacture of varlilumab, and we are responsible for the manufacture of ONT-10, which we outsource to third parties for the planned combination trial of ONT-10 and varlilumab. If our or Celldex’s third-party manufacturers cease or interrupt production or if our or Celldex’s third-party manufacturers and other service providers fail to supply materials, products or services to them for any reason, or there are challenges in transferring the ONT-380 manufacturing process from Array to us, such interruption could delay progress on our programs, with the potential for additional costs. Our product candidates have not yet been manufactured on a commercial scale. In order to commercialize a product candidate, the third-party manufacturer may need to increase its manufacturing capacity, which may require the manufacturer to fund capital improvements to support the scale up of manufacturing and related activities. With respect to certain of our product candidates, we may be required to provide all or a portion of these funds. The third-party manufacturer may not be able to successfully increase its manufacturing capacity for our product candidate for which we obtain marketing approval in a timely or economic manner, or at all. If any manufacturer is unable to provide commercial quantities of a product candidate, we will need to successfully transfer manufacturing technology to a new manufacturer. Engaging a new manufacturer for a particular product candidate could require us to conduct comparative studies or use other means to determine equivalence between product candidates manufactured by a new manufacturer and those previously manufactured by the existing manufacturer, which could delay or prevent commercialization of our product candidates. If any of these manufacturers is unable or unwilling to increase its manufacturing capacity or if alternative arrangements are not established on a timely basis or on acceptable terms, the development and commercialization of our product candidates may be delayed or there may be a shortage in supply.
Any manufacturer of our products must comply with cGMP requirements enforced by the FDA through its facilities inspection program or by foreign regulatory agencies. These requirements include quality control, quality assurance and the maintenance of records and documentation. Manufacturers of our products may be unable to comply with these cGMP requirements and with other FDA, state and foreign regulatory requirements. We have little control over our manufacturers’ compliance with these regulations and standards. A failure to comply with these requirements may result in fines and civil penalties, suspension of production, suspension or delay in product approval, product seizure or recall, or withdrawal of product approval. If the safety of any quantities supplied is compromised due to our manufacturers’ failure to adhere to applicable laws or for other reasons, we may not be able to obtain regulatory approval for or successfully commercialize our products.
16
Table of Contents
Pre-clinical and clinical trials are expensive and time consuming, and any failure or delay in commencing or completing clinical trials for our product candidates could severely harm our business.
We are currently conducting Phase 1 clinical trials for ONT-380 and ONT-10. Each of our product candidates must undergo extensive pre-clinical studies and clinical trials as a condition to regulatory approval. Pre-clinical studies and clinical trials are expensive and take many years to complete. The commencement and completion of clinical trials for our product candidates may be delayed by many factors, including:
| • | safety issues or side effects; |
| • | delays in patient enrollment and variability in the number and types of patients available for clinical trials; |
| • | poor effectiveness of product candidates during clinical trials; |
| • | governmental or regulatory delays and changes in regulatory requirements, policy and guidelines; |
| • | our ability to obtain regulatory approval to commence a clinical trial and conduct a trial in accordance with good clinical practices; |
| • | our ability to manufacture or obtain from third parties materials sufficient for use in pre-clinical studies and clinical trials; and |
| • | varying interpretation of data by the FDA and similar foreign regulatory agencies. |
It is possible that none of our product candidates will complete clinical trials in any of the markets in which we intend to sell those product candidates. Accordingly, we may not receive the regulatory approvals necessary to market our product candidates. Any failure or delay in commencing or completing clinical trials or obtaining regulatory approvals for product candidates would prevent or delay their commercialization and severely harm our business and financial condition.
The failure to enroll patients for clinical trials may cause delays in developing our product candidates.
We may encounter delays if we are unable to enroll enough patients to timely initiate or complete clinical trials. Patient enrollment depends on many factors, including, the size of the patient population, the nature of the protocol, the proximity of patients to clinical sites and the eligibility criteria for the trial. Moreover, when one product candidate is evaluated in multiple clinical trials simultaneously, patient enrollment in ongoing trials can be adversely affected by negative results from completed trials. Our product candidates are focused in oncology, which can be a difficult patient population to recruit. If we fail to enroll patients for clinical trials, our clinical trials may be delayed or suspended, which could delay our ability to generate revenues.
We rely on third parties to conduct our clinical trials. If these third parties do not perform as contractually required or otherwise expected, we may not be able to obtain regulatory approval for or be able to commercialize our product candidates.
We rely on third parties, such as contract research organizations, medical institutions, clinical investigators and contract laboratories, to assist in conducting our clinical trials. We have, in the ordinary course of business, entered into agreements with these third parties. Nonetheless, we are responsible for confirming that each of our clinical trials is conducted in accordance with its general investigational plan and protocol. Moreover, the FDA and foreign regulatory agencies require us to comply with regulations and standards, commonly referred to as good clinical practices, for conducting, recording and reporting the results of clinical trials to assure that data and reported results are credible and accurate and that the trial participants are adequately protected. Our reliance on third parties does not relieve us of these responsibilities and requirements. If these third parties do not successfully carry out their contractual duties or regulatory obligations or meet expected deadlines, if the third parties need to be replaced or if the quality or accuracy of the data they obtain is compromised due to the failure to adhere to our clinical protocols or regulatory requirements or for other reasons, our pre-clinical development activities or clinical trials may be extended, delayed, suspended or terminated, and we may not be able to obtain regulatory approval for our product candidates.
17
Table of Contents
Our product candidates may never achieve market acceptance even if we obtain regulatory approvals.
Even if we receive regulatory approvals for the commercial sale of our product candidates, the commercial success of these product candidates will depend on, among other things, their acceptance by physicians, patients, third-party payers such as health insurance companies and other members of the medical community as a therapeutic and cost-effective alternative to competing products and treatments. New patterns of care, alternative new treatments or different reimbursement and payor paradigms, possibly due to economic conditions or governmental policies, could negatively impact the commercial viability of our product candidates. If our product candidates fail to gain market acceptance, we may be unable to earn sufficient revenue to continue our business. Market acceptance of, and demand for, any product that we may develop and commercialize will depend on many factors, including:
| • | our ability to provide acceptable evidence of safety and efficacy; |
| • | the prevalence and severity of adverse side effects; |
| • | availability, relative cost and relative efficacy of alternative and competing treatments; |
| • | the effectiveness of our marketing and distribution strategy; |
| • | publicity concerning our products or competing products and treatments; and |
| • | our ability to obtain sufficient third-party insurance coverage or reimbursement. |
If our product candidates do not become widely accepted by physicians, patients, third-party payers and other members of the medical community, our business, financial condition and results of operations would be materially and adversely affected.
The termination of Merck’s 2008 license agreement with us could harm our business and negatively affect the development prospects for ONT-10.
Pursuant to our 2008 license agreement with Merck KGaA, Merck KGaA has the exclusive right to develop, manufacture and commercialize tecemotide in return for our right to receive cash payments upon the occurrence of certain events and royalties based on net sales. Merck KGaA has the right to terminate the license agreement upon thirty days’ prior written notice if, in its reasonable judgment, it determines there are issues concerning the safety or efficacy of tecemotide that would materially and adversely affect tecemotide’s medical, economic or competitive viability. In September 2014, Merck KGaA announced that its biopharmaceutical division Merck Serono decided to discontinue the clinical development program of tecemotide as a monotherapy in Stage III NSCLC. Merck KGaA may ultimately decide not to continue development of tecemotide as a combination therapy or in any manner and may terminate the 2008 license agreement. Any future payments under the license agreement, including royalties to us, will depend on whether Merck KGaA decides to advance tecemotide through development and commercialization.
Merck KGaA’s decisions regarding the development of tecemotide and the license agreement may also negatively impact the development of ONT-10, as both ONT-10 and tecemotide are targeted at the MUC1 antigen. Merck KGaA’s recent announcement of Merck Serono’s decision to discontinue the Phase III START2 and INSPIRE studies of tecemotide substantially decreases the likelihood that Merck KGaA will exercise its right of first negotiation with respect to ONT-10. These developments may also make it more difficult to find other co-development partners for ONT-10. In addition, if Merck KGaA were to terminate the license agreement, we would have to assume certain patent prosecution expenses with respect to ONT-10 that are currently paid for by Merck KGaA.
ONT-10 is based on novel technology, which may raise new regulatory issues that could delay or make FDA or foreign regulatory approval more difficult.
The process of obtaining required FDA, and other regulatory approvals, including foreign approvals, is expensive, often takes many years and can vary substantially based upon the type, complexity and novelty of the products involved. ONT-10 is novel; therefore, regulatory agencies may lack experience with similar product candidates, which may lengthen the regulatory review process, increase our development costs and delay or prevent commercialization of ONT-10.
To date, the FDA has approved for commercial sale in the United States only one active vaccine designed to stimulate an immune response against cancer. Consequently, there is limited precedent for the successful development or commercialization of products based on technologies in this area. This may lengthen the regulatory review process, increase our development costs and delay or prevent commercialization of ONT-10.
18
Table of Contents
Even if regulatory approval is received for our product candidates, the later discovery of previously unknown problems with a product, manufacturer or facility may result in restrictions, including withdrawal of the product from the market.
Approval of a product candidate may be conditioned upon certain limitations and restrictions as to the drug’s use, or upon the conduct of further studies, and may be subject to continuous review. After approval of a product, if any, there will be significant ongoing regulatory compliance obligations, and if we fail to comply with these requirements, we could be subject to penalties, including:
| • | warning letters; |
| • | fines; |
| • | product recalls; |
| • | withdrawal of regulatory approval; |
| • | operating restrictions; |
| • | disgorgement of profits; |
| • | injunctions; and |
| • | criminal prosecution. |
Regulatory agencies may require us to delay, restrict or discontinue clinical trials on various grounds, including a finding that the subjects or patients are being exposed to an unacceptable health risk. In addition, all statutes and regulations governing the conduct of clinical trials are subject to change in the future, which could affect the cost of such clinical trials. Any unanticipated delays in clinical studies could delay our ability to generate revenues and harm our financial condition and results of operations.
Failure to obtain regulatory approval in foreign jurisdictions would prevent us from marketing our products internationally.
We intend to have our product candidates marketed outside the United States. In order to market our products in the European Union and many other non-U.S. jurisdictions, we must obtain separate regulatory approvals and comply with numerous and varying regulatory requirements. To date, we have not filed for marketing approval for any of our product candidates and may not receive the approvals necessary to commercialize our product candidates in any market.
The approval procedure varies among countries and may include all of the risks associated with obtaining FDA approval. The time required to obtain foreign regulatory approval may differ from that required to obtain FDA approval, and additional testing and data review may be required. We may not obtain foreign regulatory approvals on a timely basis, if at all. Additionally, approval by the FDA does not ensure approval by regulatory agencies in other countries, and approval by one foreign regulatory authority does not ensure approval by regulatory agencies in other foreign countries or by the FDA. However, a failure or delay in obtaining regulatory approval in one jurisdiction may have a negative effect on the regulatory approval process in other jurisdictions, including approval by the FDA. The failure to obtain regulatory approval in foreign jurisdictions could limit commercialization of our products, reduce our ability to generate profits and harm our business.
Our ability to continue with our planned operations is dependent on our success at raising additional capital sufficient to meet our obligations on a timely basis. If we fail to obtain additional financing when needed, we may be unable to complete the development, regulatory approval and commercialization of our product candidates.
We have expended and continue to expend substantial funds in connection with our product development activities and clinical trials and regulatory approvals. The very limited funds generated currently from our operations will be insufficient to enable us to bring all of our products currently under development to commercialization. Accordingly, we need to raise additional funds from the sale of our securities, partnering arrangements or other financing transactions in order to finance the commercialization of our product candidates. We cannot be certain that additional financing will be available when and as needed or, if available, that it will be available on acceptable terms. If financing is available, it may be on terms that adversely affect the interests of our existing stockholders or restrict our ability to conduct our operations. To the extent that we raise additional funds through collaboration and licensing arrangements, we may be required to relinquish some rights to our technologies or product candidates, or grant licenses on terms that are not favorable to us. If adequate financing is not available, we may need to continue to reduce or eliminate our expenditures for research and development, testing, production and marketing for some of our product candidates. Our actual capital requirements will depend on numerous factors, including:
| • | activities and arrangements related to the commercialization of our product candidates; |
| • | the progress of our research and development programs; |
19
Table of Contents
| • | the progress of pre-clinical and clinical testing of our product candidates; |
| • | the time and cost involved in obtaining regulatory approvals for our product candidates; |
| • | the cost of filing, prosecuting, defending and enforcing any patent claims and other intellectual property rights with respect to our intellectual property; |
| • | our capacity to enter into collaborative or licensing agreements with respect to our protocell technology; |
| • | the effect of competing technological and market developments; |
| • | the effect of changes and developments in our existing licensing and other relationships; and |
| • | the terms of any new collaborative, licensing and other arrangements that we may establish. |
If we require additional financing and cannot secure sufficient financing on acceptable terms, we may need to delay, reduce or eliminate some or all of our research and development programs, any of which would be expected to have a material adverse effect on our business, operating results, and financial condition.
We may expand our business through the acquisition of companies or businesses or by entering into collaborations or in-licensing product candidates that could disrupt our business and harm our financial condition.
We have in the past and may in the future seek to expand our pipeline and capabilities by acquiring one or more companies or businesses, entering into collaborations or in-licensing one or more product candidates. For example, in May 2013, we began collaborating with Array to develop ONT-380 and in December 2014, we entered into a license agreement with Array for exclusive rights to develop and commercialize ONT-380. In August 2014, we acquired Alpine Biosciences, Inc., a biotechnology company developing protocells. Acquisitions, collaborations and in-licenses, including our ONT-380 license agreement and Alpine acquisition, involve numerous risks, including:
| • | substantial cash expenditures; |
| • | potentially dilutive issuance of equity securities; |
| • | incurrence of debt and contingent liabilities, some of which may be difficult or impossible to identify at the time of acquisition; |
| • | difficulties in assimilating the operations and technology of the acquired companies; |
| • | potential disputes regarding contingent consideration; |
| • | the assumption of unknown liabilities of the acquired businesses; |
| • | diverting our management’s attention away from other business concerns; |
| • | entering markets in which we have limited or no direct experience; and |
| • | potential loss of our key employees or key employees of the acquired companies or businesses. |
Our experience in making acquisitions, entering collaborations and in-licensing product candidates is limited. We cannot assure you that any acquisition, collaboration or in-license will result in short-term or long-term benefits to us. We may incorrectly judge the value or worth of an acquired company or business or in-licensed product candidate. In addition, our future success would depend in part on our ability to manage the rapid growth associated with some of these acquisitions, collaborations and in-licenses. We cannot assure you that we would be able to successfully combine our business with that of acquired businesses, manage collaboration or integrate in-licensed product candidates or that such efforts would be successful. Furthermore, the development or expansion of our business or any acquired business or company or any collaboration or in-licensed product candidate may require a substantial capital investment by us. We may also seek to raise funds by selling shares of our capital stock, which could dilute our current stockholders’ ownership interest, or securities convertible into our capital stock, which could dilute current stockholders’ ownership interest upon conversion.
20
Table of Contents
If we are unable to maintain and enforce our proprietary rights, we may not be able to compete effectively or operate profitably.
Our success is dependent in part on maintaining and enforcing our patents and other proprietary rights and will depend in large part on our ability to:
| • | defend patents once issued; |
| • | preserve trade secrets; and |
| • | operate without infringing the patents and proprietary rights of third parties. |
The degree of future protection for our proprietary rights is uncertain. For example:
| • | we might not have been the first to make the inventions covered by any of our patents, if issued, or our pending patent applications; |
| • | we might not have been the first to file patent applications for these inventions; |
| • | under our license agreement with Array, Array is responsible for the prosecution of patents related to ONT-380, and they may not effectively prosecute and protect those patents; |
| • | others may independently develop similar or alternative technologies or products and/or duplicate any of our technologies and/or products; |
| • | it is possible that none of our pending patent applications will result in issued patents or, if issued, these patents may not be sufficient to protect our technology or provide us with a basis for commercially-viable products and may not provide us with any competitive advantages; |
| • | if our pending applications issue as patents, they may be challenged by third parties as infringed, invalid or unenforceable under U.S. or foreign laws; |
| • | if issued, the patents under which we hold rights may not be valid or enforceable; or |
| • | we may develop additional proprietary technologies that are not patentable and which may not be adequately protected through trade secrets, if for example a competitor were to independently develop duplicative, similar or alternative technologies. |
The patent position of biotechnology and pharmaceutical firms is highly uncertain and involves many complex legal and technical issues. There is no clear policy involving the breadth of claims allowed in patents or the degree of protection afforded under patents. Although we believe our potential rights under patent applications provide a competitive advantage, it is possible that patent applications owned by or licensed to us will not result in patents being issued, or that, if issued, the patents will not give us an advantage over competitors with similar products or technology, nor can we assure you that we can obtain, maintain and enforce all ownership and other proprietary rights necessary to develop and commercialize our product candidates.
In addition to the intellectual property and other rights described above, we also rely on unpatented technology, trade secrets, trademarks and confidential information, particularly when we do not believe that patent protection is appropriate or available. However, trade secrets are difficult to protect and it is possible that others will independently develop substantially equivalent information and techniques or otherwise gain access to or disclose our unpatented technology, trade secrets and confidential information. We require each of our employees, consultants and advisors to execute a confidentiality and invention assignment agreement at the commencement of an employment or consulting relationship with us. However, it is possible that these agreements will not provide effective protection of our confidential information or, in the event of unauthorized use of our intellectual property or the intellectual property of third parties, provide adequate or effective remedies or protection.
If we are unable to obtain intellectual property rights to develop or market our products or we infringe on a third-party patent or other intellectual property rights, we may need to alter or terminate a product development program.
If our vaccine technology, protocell platform or our product candidates infringe or conflict with the rights of others, we may not be able to manufacture or market our product candidates, which could have a material and adverse effect on us.
Issued patents held by others may limit our ability to develop commercial products. All issued patents are entitled to a presumption of validity under the laws of the United States. If we need licenses to such patents to permit us to develop or
21
Table of Contents
market our product candidates, we may be required to pay significant fees or royalties, and we cannot be certain that we would be able to obtain such licenses on commercially reasonable terms, if at all. Competitors or third parties may obtain patents that may cover subject matter we use in developing the technology required to bring our products to market, that we use in producing our products, or that we use in treating patients with our products.
We know that others have filed patent applications in various jurisdictions that relate to several areas in which we are developing products. Some of these patent applications have already resulted in the issuance of patents and some are still pending. We may be required to alter our processes or product candidates, pay licensing fees or cease activities. Certain parts of our vaccine technology, including the MUC1 antigen, originated from third-party sources.
These third-party sources include academic, government and other research laboratories, as well as the public domain. If use of technology incorporated into or used to produce our product candidates is challenged, or if our processes or product candidates conflict with patent rights of others, third parties could bring legal actions against us, in Europe, the United States and elsewhere, claiming damages and seeking to enjoin manufacturing and marketing of the affected products. Additionally, it is not possible to predict with certainty what patent claims may issue from pending applications. In the United States, for example, patent prosecution can proceed in secret prior to issuance of a patent. As a result, third parties may be able to obtain patents with claims relating to our product candidates or technology, which they could attempt to assert against us. Further, as we develop our products, third parties may assert that we infringe the patents currently held or licensed by them and it is difficult to provide the outcome of any such action. Ultimately, we could be prevented from commercializing a product, or forced to cease some aspect of our business operations, as a result of claims of patent infringement or violation of other intellectual property rights, which could have a material and adverse effect on our business, financial condition and results of operations.
We may incur substantial costs as a result of litigation or other proceedings relating to patent and other intellectual property rights, and we may be unable to protect our rights in, or to use, our technology.
There has been significant litigation in the biotechnology industry over patents and other proprietary rights and if we become involved in any litigation, it could consume a substantial portion of our resources, regardless of the outcome of the litigation. Others may challenge the validity, inventorship, ownership, enforceability or scope of our patents or other technology used in or otherwise necessary for the development and commercialization of our product candidates. We may not be successful in defending against any such challenges. If these legal actions are successful, in addition to any potential liability for damages, we could be required to obtain a license, grant cross-licenses and pay substantial royalties in order to continue to manufacture or market the affected products.
Moreover, the cost of litigation to uphold the validity of patents to prevent infringement or to otherwise protect our proprietary rights can be substantial. If the outcome of litigation is adverse to us, third parties may be able to use the challenged technologies without payment to us. There is also the risk that, even if the validity of a patent were upheld, a court would refuse to stop the other party from using the inventions, including on the ground that its activities do not infringe that patent. There is no assurance that we would prevail in any legal action or that any license required under a third-party patent would be made available on acceptable terms or at all. If any of these events were to occur, our business, financial condition and results of operations would be materially and adversely effected.
If any products we develop become subject to unfavorable pricing regulations, third-party reimbursement practices or healthcare reform initiatives, our ability to successfully commercialize our products will be impaired.
Our future revenues, profitability and access to capital will be affected by the continuing efforts of governmental and private third-party payers to contain or reduce the costs of health care through various means. We expect a number of federal, state and foreign proposals to control the cost of drugs through government regulation. We are unsure of the impact recent health care reform legislation may have on our business or what actions federal, state, foreign and private payers may take in response to the recent reforms. Therefore, it is difficult to predict the effect of any implemented reform on our business. Our ability to commercialize our products successfully will depend, in part, on the extent to which reimbursement for the cost of such products and related treatments will be available from government health administration authorities, such as Medicare and Medicaid in the United States, private health insurers and other organizations. Significant uncertainty exists as to the reimbursement status of newly approved health care products, particularly for indications for which there is no current effective treatment or for which medical care typically is not sought. Adequate third-party coverage may not be available to enable us to maintain price levels sufficient to realize an appropriate return on our investment in product research and development. If adequate coverage and reimbursement levels are not provided by government and third-party payers for use of our products, our products may fail to achieve market acceptance and our results of operations will be harmed.
22
Table of Contents
Governments often impose strict price controls, which may adversely affect our future profitability.
We intend to seek approval to market our future products in both the United States and foreign jurisdictions. If we obtain approval in one or more foreign jurisdictions, we will be subject to rules and regulations in those jurisdictions relating to our product. In some foreign countries, particularly in the European Union, prescription drug pricing is subject to government control. In these countries, pricing negotiations with governmental authorities can take considerable time after the receipt of marketing approval for a drug candidate. To obtain reimbursement or pricing approval in some countries, we may be required to conduct a clinical trial that compares the cost-effectiveness of our future product to other available therapies.
Domestic and foreign governments continue to propose and pass legislation designed to reduce the cost of healthcare, including drugs. In the United States, there have been, and we expect that there will continue to be, federal and state proposals to implement similar governmental control. In addition, increasing emphasis on managed care in the United States will continue to put pressure on the pricing of pharmaceutical products. For example, in March 2010, the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Affordability Reconciliation Act, or collectively, PPACA, became law in the United States. PPACA substantially changed the way healthcare is financed by both governmental and private insurers and significantly affected the pharmaceutical industry.
We anticipate that the PPACA, as well as other healthcare reform measures that may be adopted in the future, may result in more rigorous coverage criteria and downward pressure on the price for any approved product, and could seriously harm our prospects. Any reduction in reimbursement from Medicare or other government programs may result in a similar reduction in payments from private payers. If reimbursement of our future products is unavailable or limited in scope or amount, or if pricing is set at unsatisfactory levels, we may be unable to achieve or sustain profitability.
We face potential product liability exposure, and if successful claims are brought against us, we may incur substantial liability for a product candidate and may have to limit its commercialization.
The use of our product candidates in clinical trials and the sale of any products for which we obtain marketing approval expose us to the risk of product liability claims. Product liability claims might be brought against us by consumers, health care providers, pharmaceutical companies or others selling our products. If we cannot successfully defend ourselves against these claims, we will incur substantial liabilities. Regardless of merit or eventual outcome, liability claims may result in:
| • | decreased demand for our product candidates; |
| • | impairment of our business reputation; |
| • | withdrawal of clinical trial participants; |
| • | costs of related litigation; |
| • | substantial monetary awards to patients or other claimants; |
| • | loss of revenues; and |
| • | the inability to commercialize our product candidates. |
Although we currently have product liability insurance coverage for our clinical trials for expenses or losses up to a $10 million aggregate annual limit, our insurance coverage may not reimburse us or may not be sufficient to reimburse us for any or all expenses or losses we may suffer. Moreover, insurance coverage is becoming increasingly expensive and, in the future, we may not be able to maintain insurance coverage at a reasonable cost or in sufficient amounts to protect us against losses due to liability. We intend to expand our insurance coverage to include the sale of commercial products if we obtain marketing approval for our product candidates in development, but we may be unable to obtain commercially reasonable product liability insurance for any products approved for marketing. On occasion, large judgments have been awarded in class action lawsuits based on products that had unanticipated side effects. A successful product liability claim or series of claims brought against us could cause our stock price to fall and, if judgments exceed our insurance coverage, could decrease our cash and adversely affect our business.
23
Table of Contents
We face substantial competition, which may result in others discovering, developing or commercializing products before, or more successfully, than we do.
The life sciences industry is highly competitive, and we face significant competition from many pharmaceutical, biopharmaceutical and biotechnology companies that are researching and marketing products designed to address cancer indications for which we are currently developing products or for which we may develop products in the future. Our future success depends on our ability to demonstrate and maintain a competitive advantage with respect to the design, development and commercialization of our product candidates. We expect any product candidate that we commercialize with our collaborative partners or on our own will compete with existing, market-leading products and products in development.
ONT-380. ONT-380 is an inhibitor of the receptor tyrosine kinase HER2, also known as ErbB2. There are multiple marketed products which target HER2, including the antibodies trastuzumab (Herceptin ®) and pertuzumab (Perjeta ®) and the antibody toxin conjugate ado-trastuzumab emtansine (Kadcyla®), all from Roche/Genentech. In addition, GlaxoSmithKline markets the dual HER1/HER2 oral kinase inhibitor lapatinib (Tykerb ®) for the treatment of metastatic breast cancer, and Puma Biotechnology is developing the HER1/HER2/HER4 inhibitor neratinib in Phase 3.
ONT-10. ONT-10 is a MUC1-based liposomal glycolipopeptide cancer vaccine. It is currently in the early stages of development for many indications, for which there are likely to be other competitors.
Many of our potential competitors have substantially greater financial, technical and personnel resources than we have. In addition, many of these competitors have significantly greater commercial infrastructures than we have. Our ability to compete successfully will depend largely on our ability to:
| • | design and develop products that are superior to other products in the market; |
| • | attract qualified scientific, medical, sales and marketing and commercial personnel; |
| • | obtain patent and/or other proprietary protection for our processes and product candidates; |
| • | obtain required regulatory approvals; and |
| • | successfully collaborate with others in the design, development and commercialization of new products. |
Established competitors may invest heavily to quickly discover and develop novel compounds that could make our product candidates obsolete. In addition, any new product that competes with a generic market-leading product must demonstrate compelling advantages in efficacy, convenience, tolerability and safety in order to overcome severe price competition and to be commercially successful. If we are not able to compete effectively against our current and future competitors, our business will not grow and our financial condition and operations will suffer.
If we are unable to enter into agreements with partners to perform sales and marketing functions, or build these functions ourselves, we will not be able to commercialize our product candidates.
We currently do not have any internal sales, marketing or distribution capabilities. In order to commercialize any of our product candidates, we must either acquire or internally develop a sales, marketing and distribution infrastructure or enter into agreements with partners to perform these services for us. We may not be able to enter into such arrangements on commercially acceptable terms, if at all. Factors that may inhibit our efforts to commercialize our product candidates without entering into arrangements with third parties include:
| • | our inability to recruit and retain adequate numbers of effective sales and marketing personnel; |
| • | the inability of sales personnel to obtain access to or persuade adequate numbers of physicians to prescribe our products; |
| • | the lack of complementary products to be offered by sales personnel, which may put us at a competitive disadvantage relative to companies with more extensive product lines; and |
| • | unforeseen costs and expenses associated with creating a sales and marketing organization. |
If we are not able to partner with a third party and are not successful in recruiting sales and marketing personnel or in building a sales and marketing and distribution infrastructure, we will have difficulty commercializing our product candidates, which would adversely affect our business and financial condition.
24
Table of Contents
If we lose key personnel, or we are unable to attract and retain highly-qualified personnel on a cost-effective basis, it would be more difficult for us to manage our existing business operations and to identify and pursue new growth opportunities.
Our success depends in large part upon our ability to attract and retain highly qualified scientific, clinical, manufacturing, and management personnel. In addition, future growth will require us to continue to implement and improve our managerial, operational and financial systems, and continue to retain, recruit and train additional qualified personnel, which may impose a strain on our administrative and operational infrastructure. Any difficulties in hiring or retaining key personnel or managing this growth could disrupt our operations. The competition for qualified personnel in the biopharmaceutical field is intense. We are highly dependent on our continued ability to attract, retain and motivate highly-qualified management, clinical and scientific personnel. Due to our limited resources, we may not be able to effectively recruit, train and retain additional qualified personnel. If we are unable to retain key personnel or manage our growth effectively, we may not be able to implement our business plan.
Furthermore, we have not entered into non-competition agreements with all of our key employees. In addition, we do not maintain “key person” life insurance on any of our officers, employees or consultants. The loss of the services of existing personnel, the failure to recruit additional key scientific, technical and managerial personnel in a timely manner, and the loss of our employees to our competitors would harm our research and development programs and our business.
Our business is subject to increasingly complex environmental legislation that has increased both our costs and the risk of noncompliance.
Our business may involve the use of hazardous material, which will require us to comply with environmental regulations. We face increasing complexity in our product development as we adjust to new and upcoming requirements relating to the materials composition of many of our product candidates. If we use biological and hazardous materials in a manner that causes contamination or injury or violates laws, we may be liable for damages. Environmental regulations could have a material adverse effect on the results of our operations and our financial position. We maintain insurance under our general liability policy for any liability associated with our hazardous materials activities, and it is possible in the future that our coverage would be insufficient if we incurred a material environmental liability.
If we fail to establish and maintain proper and effective internal controls, our ability to produce accurate financial statements on a timely basis could be impaired, which would adversely affect our consolidated operating results, our ability to operate our business, and our stock price, and could result in litigation or similar actions.
Ensuring that we have adequate internal financial and accounting controls and procedures in place to produce accurate financial statements on a timely basis is a costly and time-consuming effort that needs to be re-evaluated frequently. Failure on our part to have effective internal financial and accounting controls would cause our financial reporting to be unreliable, could have a material adverse effect on our business, operating results, and financial condition, and could cause the trading price of our common stock to fall dramatically. Our management is responsible for establishing and maintaining adequate internal control over financial reporting to provide reasonable assurance regarding the reliability of our financial reporting and the preparation of financial statements for external purposes in accordance with U.S. GAAP. Our management does not expect that our internal control over financial reporting will prevent or detect all errors and all fraud. A control system, no matter how well designed and operated, can provide only reasonable, not absolute, assurance that the control system’s objectives will be met. Because of the inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that misstatements due to error or fraud will not occur or that all control issues and instances of fraud, if any, within the company will have been detected.
We cannot be certain that the actions we have taken to ensure we have adequate internal controls over financial reporting will be sufficient. In future periods, if the process required by Section 404 of the Sarbanes-Oxley Act reveals any material weaknesses or significant deficiencies, the correction of any such material weaknesses or significant deficiencies could require remedial measures which could be costly and time-consuming. In addition, in such a case, we may be unable to produce accurate financial statements on a timely basis. Any associated accounting restatement could create a significant strain on our internal resources and cause delays in our release of quarterly or annual financial results and the filing of related reports, increase our cost and cause management distraction. Any of the foregoing could cause investors to lose confidence in the reliability of our consolidated financial statements, which could cause the market price of our common stock to decline and make it more difficult for us to finance our operations and growth.
25
Table of Contents
We may face risks related to securities litigation that could result in significant legal expenses and settlement or damage awards.
We have in the past been, and may in the future become, subject to claims and litigation alleging violations of the securities laws or other related claims, which could harm our business and require us to incur significant costs. For example, in April 2013, a putative shareholder derivative action was filed in the United States District Court for the Western District of Washington, purportedly on behalf of Oncothyreon and naming certain executive officers and the members of our board of directors as defendants. The complaint asserted claims for breaches of fiduciary duty, unjust enrichment, abuse of control, and mismanagement based on allegedly false statements made by us in public filings and press releases in 2011 and 2012. In September 2013, the court entered an order granting our motion to dismiss the lawsuit with prejudice, which means that the plaintiff was not permitted to further amend his complaint to bolster his claims. The period to appeal the dismissal order has now expired, with no appeal being filed, so the lawsuit is concluded. We are generally obliged, to the extent permitted by law, to indemnify our current and former directors and officers who are named as defendants in these types of lawsuits. Any future litigation may require significant attention from management and could result in significant legal expenses, settlement costs or damage awards that could have a material impact on our financial position, results of operations, and cash flows.
Risks Related to the Ownership of Our Common Stock
The trading price of our common stock may be volatile.
The market prices for and trading volumes of securities of biotechnology companies, including our securities, have been historically volatile. In particular, we experienced significant volatility after we and Merck KGaA announced in December 2012 that tecemotide failed to meet its primary endpoint in a Phase 3 trial. We experienced additional volatility in May 2013 following an additional release regarding the Merck KGaA study of tecemotide and the release of the results of our trials of PX-866. The market has from time to time experienced significant price and volume fluctuations unrelated to the operating performance of particular companies. The market price of our common shares may fluctuate significantly due to a variety of factors, including:
| • | the results of pre-clinical testing and clinical trials by us, our competitors and/or companies that are developing products that are similar to ours (regardless of whether such products are potentially competitive with ours); |
| • | public concern as to the safety of products developed by us or others; |
| • | technological innovations or new therapeutic products; |
| • | governmental regulations; |
| • | developments in patent or other proprietary rights; |
| • | litigation; |
| • | comments by securities analysts; |
| • | the issuance of additional shares of common stock, or securities convertible into, or exercisable or exchangeable for, shares of our common stock in connection with financings, acquisitions or otherwise; |
| • | the incurrence of debt; |
| • | general market conditions in our industry or in the economy as a whole; and |
| • | political instability, natural disasters, war and/or events of terrorism. |
We may seek to raise additional capital in the future; however, such capital may not be available to us on reasonable terms, if at all, when or as we require additional funding. If we issue additional shares of our common stock or other securities that may be convertible into, or exercisable or exchangeable for, our common stock, our existing stockholders would experience further dilution.
We expect that we will seek to raise additional capital from time to time in the future. For example, in connection with our February 2015 public offering, we sold an aggregate of 14,699,660 shares of our common stock and 1,333 shares of our Series B convertible preferred stock.
Future financings may involve the issuance of debt, equity and/or securities convertible into or exercisable or exchangeable for our equity securities. These financings may not be available to us on reasonable terms or at all when and as we require funding. Additionally, if we are unable to increase our authorized capital stock, we may not have sufficient authorized but unissued capital stock to issue or sell additional capital stock in potential financings. If we are
26
Table of Contents
able to consummate financings, the trading price of our common stock could be adversely affected and/or the terms of such financings may adversely affect the interests of our existing stockholders. Any failure to obtain additional working capital when required would have a material adverse effect on our business and financial condition and would be expected to result in a decline in our stock price. Any issuances of our common stock, preferred stock, or securities such as warrants or notes that are convertible into, exercisable or exchangeable for, our capital stock, would have a dilutive effect on the voting and economic interest of our existing stockholders.
Because we do not expect to pay dividends on our common stock, stockholders will benefit from an investment in our common stock only if it appreciates in value.
We have never paid cash dividends on our common shares and have no present intention to pay any dividends in the future. We are not profitable and do not expect to earn any material revenues for at least several years, if at all. As a result, we intend to use all available cash and liquid assets in the development of our business. Any future determination about the payment of dividends will be made at the discretion of our board of directors and will depend upon our earnings, if any, capital requirements, operating and financial conditions and on such other factors as our board of directors deems relevant. As a result, the success of an investment in our common stock will depend upon any future appreciation in its value. There is no guarantee that our common stock will appreciate in value or even maintain the price at which stockholders have purchased their shares.
We can issue shares of preferred stock that may adversely affect the rights of a stockholder of our common stock.
Our certificate of incorporation authorizes us to issue up to 10,000,000 shares of preferred stock with designations, rights, and preferences determined from time-to-time by our board of directors. Accordingly, our board of directors is empowered, without stockholder approval, to issue preferred stock with dividend, liquidation, conversion, voting or other rights superior to those of holders of our common stock. For example, an issuance of shares of preferred stock could:
| • | adversely affect the voting power of the holders of our common stock; |
| • | make it more difficult for a third party to gain control of us; |
| • | discourage bids for our common stock at a premium; |
| • | limit or eliminate any payments that the holders of our common stock could expect to receive upon our liquidation; or |
| • | otherwise adversely affect the market price or our common stock. |
We have in the past issued, and we may at any time in the future issue, additional shares of authorized preferred stock. For example, in connection with our September 2014 and February 2015 public offerings, we issued 10,000 shares of Series A convertible preferred stock and 1,333 shares of Series B convertible preferred stock, respectively, each share of which is convertible into 1,000 shares of the Company’s common stock, subject to certain ownership restrictions. Concurrent but separate from these offerings, we entered into an exchange agreement with certain affiliates of Biotechnology Value Fund (BVF) to exchange 4,000,000 shares of common stock previously purchased by BVF for 4,000 shares of Series B Convertible Preferred Stock.
We expect our quarterly operating results to fluctuate in future periods, which may cause our stock price to fluctuate or decline.
Our quarterly operating results have fluctuated in the past, and we believe they will continue to do so in the future. Some of these fluctuations may be more pronounced than they were in the past as a result of the issuance by us in September 2010 of warrants to purchase shares of our common stock in connection with equity financings. As of December 31, 2014, there were outstanding warrants from the September 2010 financing exercisable for up to 3,182,147 shares of our common stock. These warrants are classified as a liability. Accordingly, the fair value of the warrants is recorded on our consolidated balance sheet as a liability, and such fair value is adjusted at each financial reporting date with the adjustment to fair value reflected in our consolidated statement of operations. The fair value of the warrants is determined using the Black-Scholes option-pricing model. Fluctuations in the assumptions and factors used in the Black-Scholes model can result in adjustments to the fair value of the warrants reflected on our balance sheet and, therefore, our statement of operations. Due to the classification of such warrants and other factors, quarterly results of operations are difficult to forecast, and period-to-period comparisons of our operating results may not be predictive of future performance. In one or more future quarters, our results of operations may fall below the expectations of securities analysts and investors. In that event, the market price of our common stock could decline. In addition, the market price of our common stock may fluctuate or decline regardless of our operating performance.
27
Table of Contents
Our management will have broad discretion over the use of proceeds from the sale of shares of our common stock and may not use such proceeds in ways that increase the value of our stock price.
In July 2013, we commenced selling our common stock through the “at the market” equity offering program under our Sales Agreement with Cowen. As of December 31, 2014, we had sold an aggregate of 8,364,379 shares under this equity offering program for net proceeds of approximately $16.1 million. In connection with our September 2014 public offering, we terminated the Sales Agreement as of September 17, 2014. In our September 2014 public offering, we sold 11,500,000 shares of our common stock and 10,000 shares of our Series A convertible preferred stock for net proceeds of approximately $40.2 million. In our February 2015 public offering, we sold 14,699,660 shares of common stock and 1,333 shares of Series B convertible preferred stock for net proceeds of approximately $22.4 million. We will have broad discretion over the use of proceeds from the sale of those shares, and we could spend the proceeds in ways that do not improve our results of operations or enhance the value of our common stock. Our failure to apply these funds effectively could have a material adverse effect on our business, delay the development of our product candidates and cause the price of our common stock to decline.
| ITEM 1B. | Unresolved Staff Comments |
None.
| ITEM 2. | Properties |
Description of Property
In May 2008, we entered into a lease for a facility in Seattle, Washington totaling approximately 17,000 square feet, which includes laboratory space, to house our research and development and administrative activities. The lease expires in December 2018. We believe that our Seattle facility is in good condition, adequately maintained and suitable for the conduct of our business.
| ITEM 3. | Legal Proceedings |
From time to time, we may be involved in litigation relating to claims arising out of our operations in the normal course of business. We are not currently a party to any legal proceedings, the adverse outcome of which, in management’s opinion, individually or in the aggregate, would have a material adverse effect on the results of our operations or financial position. There are no material proceedings to which any director, officer or any of our affiliates, any owner of record or beneficially of more than five percent of any class of our voting securities, or any associate of any such director, officer, our affiliates, or security holder, is a party adverse to us or our consolidated subsidiary or has a material interest adverse thereto.
| ITEM 4. | Mine Safety Disclosures |
Not applicable.
28
Table of Contents
PART II
| ITEM 5. | Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities |
Market Information for Common Stock
Our common stock is quoted on the NASDAQ Global Market under the symbol “ONTY”. The following table sets forth for the indicated periods the high and low sales prices of our common stock as reported by the NASDAQ Global Market.
| High | Low | |||||||
| Fiscal year ended December 31, 2014: |
||||||||
| First Quarter |
$ | 4.08 | $ | 1.73 | ||||
| Second Quarter |
3.56 | 2.16 | ||||||
| Third Quarter |
3.60 | 1.92 | ||||||
| Fourth Quarter |
2.35 | 1.52 | ||||||
| Fiscal year ended December 31, 2013: |
||||||||
| First Quarter |
$ | 2.42 | $ | 1.80 | ||||
| Second Quarter |
2.81 | 1.55 | ||||||
| Third Quarter |
2.43 | 1.57 | ||||||
| Fourth Quarter |
2.15 | 1.67 | ||||||
Dividends
We have never declared nor paid cash dividends on our common stock. We currently expect to retain future earnings, if any, to finance the operation and expansion of our business, and we do not anticipate paying any cash dividends in the foreseeable future. Any future determination related to our dividend policy will be made at the discretion of our board of directors.
Stockholders
As of February 28, 2015, there were 106,301,012 shares of our common stock outstanding held by approximately 658 stockholders of record and approximately 22,165 stockholders in nominee name.
Securities Authorized for Issuance under Equity Compensation Plans
For information concerning our equity compensation plans see the section of this Annual Report on Form 10-K captioned “Part III — Item 12 — Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters.”
29
Table of Contents
Stock Performance Graph
The following information relating to the price performance of our common stock shall not be deemed to be “filed” with the SEC or to be “soliciting material” under the Securities Exchange Act of 1934, as amended (the Exchange Act) and it shall not be deemed to be incorporated by reference into any of our filings under the Exchange Act or the Securities Act of 1933, as amended, except to the extent we specifically incorporate it by reference into such filing.
The graph below compares the cumulative total stockholder return of our common stock with that of the NASDAQ Composite Index, NASDAQ Pharmaceutical Index, NASDAQ Biotechnology Index, RDG MicroCap Biotechnology Index and a composite S&P/TSX index from December 31, 2009 through December 31, 2014. The comparisons in this graph below are based on historical data and are not intended to forecast or be indicative of future performance of our common stock. The graph assumes that $100 was invested and that all dividends were reinvested.
COMPARISON OF 5 YEAR CUMULATIVE TOTAL RETURN*
Among Oncothyreon Inc, the S&P/TSX Composite Index,
the NASDAQ Composite Index, the NASDAQ Pharmaceutical Index, the NASDAQ
Biotechnology Index and the RDG MicroCap Biotechnology Index
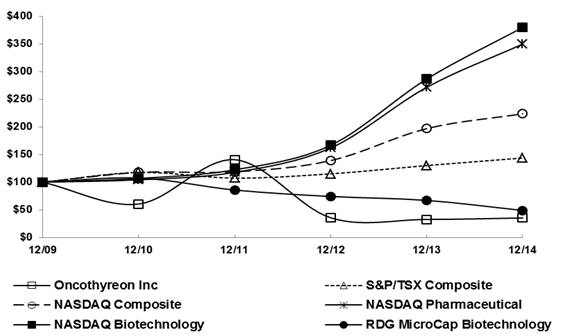
| * | $100 invested on 12/31/09 in stock or index, including reinvestment of dividends. |
Fiscal year ending December 31.
Copyright© 2014 S&P, a division of The McGraw-Hill Companies Inc. All rights reserved.
Unregistered Sale of Equity Securities
During the three months ended December 31, 2014, we did not issue or sell any shares of our common stock or other equity securities pursuant to unregistered transactions in reliance upon exemption from the registration requirements of the Securities Act of 1933, as amended.
Issuer Purchases of Equity Securities
We did not make any purchases of our outstanding common stock during the three months ended December 31, 2014.
30
Table of Contents
| ITEM 6. | Selected Financial Data |
The data set forth below is not necessarily indicative of the results of future operations and should be read in conjunction with the consolidated financial statements and notes thereto included elsewhere in this annual report on Form 10-K and also with Part II, Item 7, “Management’s Discussion and Analysis of Financial Condition and Results of Operations.”
| Year Ended December 31, | ||||||||||||||||||||
| 2014 | 2013 | 2012 | 2011 | 2010 | ||||||||||||||||
| (Amounts in thousands, except share and per share data.) | ||||||||||||||||||||
| Consolidated Statements of Operations Data: |
||||||||||||||||||||
| Total revenues |
$ | — | $ | — | $ | — | $ | 145 | $ | 18 | ||||||||||
| Total operating expenses |
50,835 | 41,223 | 28,499 | 24,844 | 19,502 | |||||||||||||||
| Loss from operations |
(50,835 | ) | (41,223 | ) | (28,499 | ) | (24,699 | ) | (19,484 | ) | ||||||||||
| Net loss (1) |
$ | (49,963 | ) | $ | (38,759 | ) | $ | (3,415 | ) | $ | (42,656 | ) | $ | (15,618 | ) | |||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Loss per share — basic |
$ | (0.64 | ) | $ | (0.62 | ) | $ | (0.06 | ) | $ | (1.12 | ) | $ | (0.58 | ) | |||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Loss per share — diluted |
$ | (0.64 | ) | $ | (0.62 | ) | $ | (0.53 | ) | $ | (1.12 | ) | $ | (0.72 | ) | |||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Weighted average number of common shares outstanding — basic |
77,619,807 | 62,387,616 | 53,728,672 | 38,197,666 | 26,888,588 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Weighted average number of common shares outstanding — diluted |
77,619,807 | 62,387,616 | 54,899,955 | 38,197,666 | 26,972,969 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| As of December 31, | ||||||||||||||||||||
| 2014 | 2013 | 2012 | 2011 | 2010 | ||||||||||||||||
| (Amounts in thousands, except share data.) | ||||||||||||||||||||
| Consolidated Balance Sheets Data: |
||||||||||||||||||||
| Cash, cash equivalents and short-term investments |
$ | 57,671 | $ | 60,027 | $ | 81,254 | $ | 63,876 | $ | 28,877 | ||||||||||
| Total assets |
$ | 103,401 | $ | 77,746 | $ | 89,435 | $ | 71,539 | $ | 34,445 | ||||||||||
| Total long-term liabilities |
$ | 7,430 | $ | 1,536 | $ | 4,041 | $ | 33,236 | $ | 13,727 | ||||||||||
| Stockholders’ equity |
$ | 91,266 | $ | 71,550 | $ | 82,323 | $ | 33,433 | $ | 18,857 | ||||||||||
| Common shares outstanding |
91,601,352 | 70,673,143 | 57,216,237 | 43,613,107 | 30,088,628 | |||||||||||||||
| (1) | Net loss includes income (expense) from the change in fair market value of warrant liability of $0.8, $2.3, $25.5, ($17.6) and 3.0 million for the years ended December 31, 2014, 2013, 2012, 2011 and 2010, respectively. Please refer to the audited financial statements included elsewhere in this Annual Report on Form 10-K for details on net loss for the years ended December 31, 2014, 2013 and 2012. For additional information on net loss for the year ended December 31, 2011 and 2010, please refer to our Annual Reports on Form 10-K for such years. |
31
Table of Contents
| ITEM 7. | Management’s Discussion and Analysis of Financial Condition and Results of Operations |
The following discussion and analysis of our financial condition and results of operations should be read in conjunction with our consolidated financial statements and related notes included elsewhere in this report. This discussion contains forward-looking statements that involve risks and uncertainties. Our actual results could differ materially from those discussed below. Factors that could cause or contribute to such differences include, but are not limited to, those identified below, and those discussed in the section titled “Risk Factors” included elsewhere in this report. All dollar amounts included in this discussion and analysis of our financial condition and results of operations represent U.S. dollars unless otherwise specified. Throughout this discussion, unless the context specifies or implies otherwise, the terms “Company,” “Oncothyreon,” “Biomira,” “we,” “us,” and “our” refer to Oncothyreon Inc., its predecessor, Biomira Inc., and its subsidiaries.
Overview
We are a clinical-stage biopharmaceutical company focused primarily on the development of therapeutic products for the treatment of cancer. Our goal is to discover, develop and commercialize novel compounds that have the potential to improve the lives and outcomes of cancer patients. Our current clinical-stage product candidates include ONT-380, an orally active and selective small-molecule HER2 inhibitor, and ONT-10, a therapeutic vaccine targeting MUC1. We are developing preclinical product candidates in oncology, and potentially certain rare diseases, using our recently acquired protocell technology. We also collaborate with partners to discover and develop additional product candidates.
We are developing ONT-380 for the treatment of HER2-positive metastatic breast cancer. ONT-380 is a small molecule inhibitor of HER2, also known as ErbB2, a receptor tyrosine kinase that is over-expressed in breast cancer and other cancers, such as gastric and ovarian cancer. Over-expression of HER2 in breast cancer is associated with increased mortality in early stage disease, decreased time to relapse, and increased incidence of metastases. We have an exclusive license agreement with Array to develop, manufacture and commercialize ONT-380.
We are currently conducting two Phase 1b trials of ONT-380, one in combination with Kadcyla® (ado-trastuzumab emtansine or TDM-1) and another in combination with Xeloda® (capecitabine) and/or Herceptin® (trastuzumab). In December 2014, we announced that interim data from these Phase 1b trials indicated preliminary clinical activity and tolerability in a heavily pretreated patient population. Each of these ongoing trials is also enrolling a cohort of patients with HER2-positive breast cancer metastatic to the central nervous system (CNS). ONT-380 has demonstrated superior activity, based on overall survival, compared to Tykerb® (lapatinib) and to the investigational drug, neratinib, in an intracranial HER2+ breast cancer xenograft model. This provides a rationale to explore whether ONT-380 can provide benefit to patients with brain metastases, which occur in approximately one-third of women with metastatic HER2+ breast cancer.
We are conducting a Phase 1 trial for ONT-10, a cancer vaccine directed against the Mucin 1 peptide antigen (MUC1). Results from this trial have demonstrated that ONT-10 activates the humoral arm of the immune system and elicits antibodies specific for MUC1. Natural antibodies against MUC1 have been shown to correlate with improved survival in patients with tumors expressing MUC1. This trial is also the first-in-man trial for our novel vaccine adjuvant PET-Lipid A, a fully-synthetic toll-like receptor 4 agonist. We are also conducting a Phase 1b trial of ONT-10 in combination with the T-cell agonist antibody varlilumab in collaboration with Celldex.
We are increasingly focused on expanding our pipeline of product candidates through both internal research and collaborative efforts. To support our internal efforts, in August 2014 we acquired Alpine Biosciences, Inc., of Seattle, Washington (Alpine), a privately held biotechnology company developing protocells, a nanoparticle platform technology designed to enable the targeted delivery of multiple therapeutic agents, including nucleic acids, proteins, peptides and small molecules. We intend to utilize the protocell technology to develop new product candidates for the treatment of cancer and rare diseases, either on our own or with partners. We are also collaborating with Sentinel for the development of novel small molecule Chk1 kinase inhibitors. We have identified a lead product candidate molecule, for which we currently expect to file an Investigational New Drug application (IND) in late 2015. In addition, we recently initiated a collaboration with Adimab for the discovery of novel antibodies against undisclosed immunotherapy targets in oncology.
We have not developed a therapeutic product to the commercial stage. As a result, our revenue has been limited to date, and we do not expect to recognize any material revenue for the foreseeable future. In particular, our ability to generate revenue in future periods will depend substantially on the progress of ongoing and/or future clinical trials for ONT-380 and ONT-10, our success in obtaining regulatory approval for ONT-380 and ONT-10, and our ability to establish commercial markets for these drugs. As ONT-380 and ONT-10 are in early clinical development, we do not expect to realize any revenues associated with the commercialization of these product candidates for the foreseeable future.
32
Table of Contents
The continued research and development of our product candidates will require significant additional expenditures, including preclinical studies, clinical trials, manufacturing costs and the expenses of seeking regulatory approval. We rely on third parties to conduct a portion of our preclinical studies, all of our clinical trials and all of the manufacturing of current good manufacturing practice (cGMP) material. We expect expenditures associated with these activities to increase in future years as we continue the development of ONT-380 and ONT-10, and as we advance the development of our preclinical product candidates.
We have incurred substantial losses since our inception. As of December 31, 2014, our accumulated deficit totaled $482.0 million. We incurred a net loss of $50.0 million for the year ended December 31, 2014 compared to a net loss of $38.8 million for the same period in 2013. The increase in loss for the year ended December 31, 2014 was primarily due to higher research and development expenses, primarily as a result of an increase of $10.0 million in license fees paid to Array. In December 2014 we paid Array $20.0 million upon entering into an exclusive license agreement. The exclusive license agreement superseded the collaboration agreement with Array under which we paid Array $10.0 million in 2013. See the section captioned “Note 8 - Collaborative and License Agreements” of the audited financial statements included elsewhere in this Annual Report on Form 10-K for additional information. The increase in loss was also due to increases in general and administrative expenses and lower non-cash income from the change in the fair value of our warrant liability, which was $0.8 million for the year ended December 31, 2014 compared to $2.3 million for the year ended December 31, 2013. The change in the fair value of our warrant liability is attributable to changes in our stock price, volatility and expected life of our warrants that were classified as liabilities. In addition, the change in fair value was also due to the expiration of our May 2009 warrants. In future periods, we expect to continue to incur substantial net losses as we expand our research and development activities with respect to our product candidates. To date we have funded our operations principally through the sale of our equity securities, cash received through our strategic alliance with Merck KGaA, government grants, debt financings and equipment financings.
Key Financial Metrics
Expenses
Research and Development. Research and development expense consists of costs associated with research activities as well as costs associated with our product development efforts, conducting preclinical studies and clinical trial and manufacturing costs. These expenses include external research and development expenses incurred pursuant to collaboration agreements; agreements with third-party manufacturing and contract research organizations; technology access and licensing fees related to the use of proprietary third-party technologies; employee related expenses, including salaries, share-based compensation expense, benefits and related costs; allocated facility overhead which includes depreciation and amortization; and third-party consulting and supplier expenses. We recognize research and development expenses, including those paid to third parties, as they have been incurred.
General and Administrative. General and administrative expense consists principally of salaries, benefits, share-based compensation expense and related costs for personnel in our executive, finance, accounting, legal, human resource functions and information technology services. Other general and administrative expenses include professional fees for legal, consulting, accounting services and allocation of our facility costs, which includes depreciation and amortization.
Investment and Other Income (Expense), Net. Net investment and other income (expense) consisted of interest and other income on our cash and short-term and long-term investments, debt, foreign exchange gains and losses and other non-operating income (expense). Our investments consist of debt securities of U.S government agencies and corporate bonds. In 2012 we incurred a loss on extinguishment of debt which consists of a prepayment penalty of 3% on the outstanding principal, the write-off of unamortized deferred financing costs and unamortized debt discount and legal expenses.
Interest Expense. Interest expense consists of interest paid and accrued and includes non-cash amortization of the debt discount and capitalized loan fees. For more information, see Note 9 to our consolidated financial statements included elsewhere in this Annual Report on Form 10-K.
Change in Fair Value of Warrants. Warrants issued in connection with our securities offerings in May 2009 and September 2010 are classified as a liability due to their potential settlement in cash and other terms, and as such, were recorded at their estimated fair value on the date of the closing of the respective transactions. The warrants issued in
33
Table of Contents
connection with our May 2009 securities offering expired in May 2014. The warrants are marked to market for each financial reporting period, with changes in estimated fair value recorded as a gain or loss in our consolidated statements of operations. The fair value of the warrants is determined using the Black-Scholes option-pricing model, which requires the use of significant judgment and estimates for the inputs used in the model. For more information, see “Note 3 — Fair Value Measurements” and “Note 6 — Share Capital” of the audited financial statements included elsewhere in this Annual Report on Form 10-K.
Income Tax Benefit (Provision) for Income Tax. Due to our history of significant losses, we do not recognize the benefit of net operating losses and have established a full valuation allowance against our net deferred tax assets since the realization of these benefits is not reasonably assured.
Critical Accounting Policies and Significant Judgments and Estimates
We have prepared this management’s discussion and analysis of financial condition and results of operations based on our audited consolidated financial statements, which have been included in this report beginning on page F-1 and which have been prepared in accordance with U.S. generally accepted accounting principles. These accounting principles require us to make significant estimates and judgments that can affect the reported amounts of assets and liabilities as of the dates of our consolidated financial statements as well as the reported amounts of revenue and expense during the periods presented. We believe that the estimates and judgments upon which we rely are reasonable based upon historical experience and information available to us at the time that we make these estimates and judgments. To the extent there are material differences between these estimates and actual results, our consolidated financial statements will be affected.
The SEC considers an accounting policy to be critical if it is important to a company’s financial condition and results of operations and if it requires the exercise of significant judgment and the use of estimates on the part of management in its application. We have discussed the selection and development of our critical accounting policies with the audit committee of our board of directors, and our audit committee has reviewed our related disclosures in this report. Although we believe that our judgments and estimates are appropriate, actual results may differ from these estimates.
We believe the following to be our critical accounting policies because they are important to the portrayal of our financial condition and results of operations and because they require critical management judgment and estimates about matters that are uncertain:
| • | goodwill impairment; |
| • | indefinite-lived intangible assets — in-process research and development (IPR&D); |
| • | share-based compensation; |
| • | warrant liability; and |
| • | business combinations. |
Goodwill Impairment
Goodwill is not amortized, but is reviewed annually for impairment on October 1 of each year, or more frequently when events or changes in circumstances indicate that the asset may be impaired. As of December 31, 2014, we had one reporting unit and there was an excess of fair value compared to the carrying value. There were no impairment charges recorded for any of the periods presented.
Indefinite-lived Intangible Assets — IPR&D
Intangible assets with indefinite lives represent the value assigned to IPR&D that, as of the acquisition date, the Company determined that technological feasibility had not been established, and the IPR&D had no alternative future use. The IPR&D will be subject to annual impairment testing until completion or abandonment of the projects. Upon completion of the project, the Company will make a separate determination of useful life of the IPR&D and the related amortization will be recorded as an expense over the estimated useful life. If the IPR&D is abandoned, the carrying value of the asset will be expensed. All research and development costs incurred subsequent to the acquisition of Alpine are expensed as incurred. The Company performs an annual impairment assessment on October 1 of each year for the IPR&D assets, or more frequently if indicators of potential impairment exist, to determine whether it is more likely than not that the carrying value of the assets may not be recoverable. Recoverability of IPR&D is measured by comparing the carrying amount of the asset to the fair value. If the Company determines that an individual asset is impaired, the amount of any impairment is measured as the difference between the carrying value and the fair value of the impaired asset. As of December 31, 2014, no impairment charges were recorded for any of the periods presented.
34
Table of Contents
Share-based Compensation
We maintain a share option plan under which an aggregate of 5,217,535 shares of common stock underlie outstanding options and, as of December 31, 2014, an aggregate of 1,825,858 shares of common stock were available for future issuance. We maintain an Employee Stock Purchase Plan (ESPP) under which a total of 900,000 shares of common stock were reserved for sale to employees of the Company. As of December 31, 2014, there were 600,533 shares reserved for future purchases under the ESPP. We maintain a restricted share unit plan. On June 6, 2014, our stockholders approved an increase of 500,000 shares in the number of shares of our common stock reserved for issuance under the RSU Plan. As of December 31, 2014, an aggregate of 163,204 shares of common stock underlie restricted stock units (RSUs) were outstanding and an aggregate of 530,910 shares of common stock were available for future issuance. We have generally granted options to our employees and directors under the share option plan, and we have granted RSUs to non-employee directors under the RSU plan. Pursuant to an October 2011 amendment to the RSU plan, approximately 25% of each RSU represents a contingent right to receive cash upon vesting, and we are required to deliver an amount in cash equal to the fair market value of approximately 25% of the vesting shares on the vesting date to facilitate the satisfaction of the non-employee directors’ U.S. federal income tax obligation with respect to the vested RSUs. The outstanding RSU awards are required to be re-measured at each reporting date until settlement of the award, and changes in valuation are recorded as compensation expense for the period. To the extent that the liability recorded in the balance sheet is less than the original award value, the difference is recognized in equity.
We use the closing share price of our shares in The NASDAQ Global Market at the reporting or settlement date to determine the fair value of RSUs. We use the Black-Scholes option pricing model for determining the estimated fair value for our share option plan and employee stock purchase plan awards, which requires the use of highly subjective and complex assumptions to determine the fair value of stock-based awards, including the option’s expected term and the price volatility of the underlying stock. We recognize the value of the portion of the awards that is ultimately expected to vest as non-cash expense over the requisite vesting periods on a straight-line basis for the entire award in our consolidated statements of operations. Forfeitures are estimated at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differ from those estimates. We base our risk free interest rate for the expected term of the option on the yield available on a U.S. Treasury security with an equivalent expected term. The expected life of options in years represents the period of time stock-based awards are expected to be outstanding and was determined based on the simplified method. The expected volatility is based on the historical volatility of our common stock for a period equal to the stock option’s expected life. For more information, see “Note 7 — Share-based Compensation” of the audited financial statements included elsewhere in this Annual Report on Form 10-K.
Warrant Liability
In September 2010, we issued warrants to purchase 3,182,147 shares of our common stock respectively in connection with a registered direct offering of our common stock and warrants. These warrants are classified as liabilities due to potential cash settlement upon the occurrence of certain transactions specified in the warrant agreement. Accordingly, the estimated fair value of the outstanding warrants is recorded on our consolidated balance sheet as a liability, and such fair value is adjusted at each financial reporting period with the adjustment to fair value reflected in our consolidated statement of operations. The fair value of the warrants is determined using the Black-Scholes option pricing model. Fluctuations in the assumptions and factors used in the Black-Scholes model can result in adjustments to the fair value of the warrants reflected on our balance sheet and, therefore, our statement of operations. Warrants to purchase 2,691,242 shares of our common stock from a May 2009 financing expired in May 2014. As of December 31, 2014, warrants to purchase 3,182,147 shares of our common stock from the September 2010 financings were outstanding.
Business Combination
In a business combination, we determine if the acquired property and activities meet the definition of a business under current accounting guidance. If the combination meets the definition of a business, we measure the significance of the combination to determine the required reporting and disclosure requirements for the transaction. Business combinations are required to be accounted for under the acquisition method which requires that identifiable assets acquired, liabilities assumed and any noncontrolling interest in the acquiree be recognized and measured as of the acquisition date at fair value. In addition, all consideration transferred must be measured at its acquisition-date fair value.
When necessary, we use a third party valuation expert to determine the fair value of the identifiable assets and liabilities acquired. The estimated fair values of in-process research and development (IPR&D) acquired in a business combination which have not been fully developed are capitalized as indefinite-lived intangible assets and impairment testing is conducted periodically.
35
Table of Contents
Results of Operations for the years ended December 31, 2014, 2013 and 2012
The following table sets forth selected consolidated statements of operations data for each of the periods indicated.
Overview
| Years Ended December 31, | ||||||||||||
| 2014 | 2013 | 2012 | ||||||||||
| (In millions, except per share amounts) | ||||||||||||
| Operating expenses |
$ | (50.8 | ) | $ | (41.2 | ) | $ | (28.5 | ) | |||
| Interest expense |
$ | — | $ | — | $ | (0.3 | ) | |||||
| Change in fair value of warrant liability |
$ | 0.8 | $ | 2.3 | $ | 25.5 | ||||||
| Net loss |
$ | (50.0 | ) | $ | (38.8 | ) | $ | (3.4 | ) | |||
Operating expenses were higher for the year ended December 31, 2014 compared to the year ended December 31, 2013 primarily as a result of an increase of $10.0 million in license fees paid to Array. In December 2014 we paid Array $20.0 million upon entering into an exclusive license agreement. The exclusive license agreement superseded the collaboration agreement with Array under which we paid Array $10.0 million in 2013. See “Note 8 — Collaborative and License Agreements” of the audited financial statements included elsewhere in this Annual Report on Form 10-K for additional information.
We incurred a net loss of $50.0 million for the year ended December 31, 2014 compared to a net loss of $38.8 million for the year ended December 31, 2013. The increase in our net loss was primarily due to increases in operating expenses and lower non-cash income from the change in the fair value of our warrant liability, which was $0.8 million for the year ended December 31, 2014 compared to $2.3 million for the year ended December 31, 2013.
Income or expense associated with the change in fair value of the warrant liability is the result of the re-measurement of the fair value of the warrant liability at each reporting date. Changes in the fair value of the warrant liability are attributable to increases or decreases in our stock price, volatility and expected life of our liability-classified warrants. In addition, the change in fair value was also due to the expiration of our May 2009 warrants. For more information, see “Note 3 — Fair Value Measurements” of the audited financial statements included elsewhere in this Annual Report on Form 10-K.
We incurred a net loss of $38.8 million for the year ended December 31, 2013 compared to a net loss of $3.4 million for the year ended December 31, 2012. The increase in our net loss was primarily due to $2.3 million in non-cash income from the change in the fair value of our warrant liability during the year ended December 31, 2013 compared to a $25.5 million non-cash income during the year ended December 31, 2012, as well as increases in research and development expenses, which included an upfront fee of $10.0 million paid to Array and increases in general and administrative expenses.
Based on our development plans for our product candidates, we will continue to incur operating losses for the foreseeable future.
Research and Development
| Years Ended December 31, | ||||||||||||
| 2014 | 2013 | 2012 | ||||||||||
| (In millions) | ||||||||||||
| Research and development |
$ | 41.9 | $ | 33.2 | $ | 22.0 | ||||||
The $8.7 million, or 26.2%, increase in research and development expenses for the year ended December 31, 2014 compared to the year ended December 31, 2013 was principally due to an increase of $10.0 million in license fees paid to Array. In December 2014 we paid Array $20.0 million upon entering into an exclusive license agreement. The exclusive license agreement superseded the collaboration agreement with Array under which we paid Array $10.0 million in 2013. See “Note 8 — Collaborative and License Agreements” of the audited financial statements included elsewhere in this Annual Report on Form 10-K for additional information. In addition, the increase in research and development expense was also due to increased salaries and benefits of $0.7 million attributable to increased headcount, which was partly offset by a decrease in clinical trials expense of $2.5 million attributable to less activity related to the development of prior programs.
36
Table of Contents
The $11.2 million, or 50.9%, increase in research and development expenses for the year ended December 31, 2013 compared to the year ended December 31, 2012 was principally due to an upfront payment of $10.0 million to Array. For more information, see “Note 8 — Collaborative and License Agreements” of the audited financial statements included elsewhere in this Annual Report on Form 10-K. In addition, the increase in research and development expense was also due to higher manufacturing development and preclinical expense of $2.7 million attributable to greater activity related to the development of our product candidates and increased salaries and benefits of $1.3 million attributable to increased headcount. The increase was partly offset by decrease in clinical trials expense of $2.8 million attributable to less activity related to the development of prior programs.
General and Administrative
| Years Ended December 31, | ||||||||||||
| 2014 | 2013 | 2012 | ||||||||||
| (In millions) | ||||||||||||
| General and administrative |
$ | 9.0 | $ | 8.0 | $ | 6.5 | ||||||
The $1.0 million, or 12.5%, increase in general and administrative expense for the year ended December 31, 2014 relative to the year ended December 31, 2013 was principally due to a $0.5 million increase in professional fees primarily related to our August 2014 acquisition of Alpine. For more information, see “Note 5 — Acquisition” of the audited financial statements included elsewhere in this Annual Report on Form 10-K. In addition, the increase in general and administrative expenses was also due to a $0.3 million increase in salaries and benefits expense attributable to increased headcount and a $0.2 million increase in director compensation expense primarily related to grants and the change in fair value of RSUs on conversion. For more information related to the liability-classified RSUs, see “Note 7 — Share-based Compensation” of the audited financial statements included elsewhere in this Annual Report on Form 10-K.
We expect general and administrative expenses to be slightly lower in 2015 compared to 2014; however, these expenses will be subject to fluctuations related to the changes in the fair value of the RSU liability.
The $1.5 million, or 23.1%, increase in general and administrative expense for the year ended December 31, 2013 relative to the year ended December 31, 2012 was principally due to a $0.8 million increase in salaries and benefits expense attributable to increased headcount and a $0.7 million increase in director compensation expense primarily related to the change in fair value of the outstanding liability-classified RSUs, which was attributable to the change in the price of our common stock.
Interest Expense
| Years Ended December 31, | ||||||||||||
| 2014 | 2013 | 2012 | ||||||||||
| (In millions) | ||||||||||||
| Interest expense |
$ | — | $ | — | $ | 0.3 | ||||||
There was no interest expense during the year ended December 31, 2014 and 2013. The $0.3 million interest expense for year ended December 31, 2012 included cash interest payments and non-cash amortization of debt issuance costs and debt discount associated with our term loan with General Electric Capital Corporation (GECC). We paid off the outstanding balance of the term loan in June 2012. For additional information, see Note 9 to our consolidated financial statements included elsewhere in this Annual Report on Form 10-K.
Change in Fair Value of Warrant Liability
| Years Ended December 31, | ||||||||||||
| 2014 | 2013 | 2012 | ||||||||||
| (In millions) | ||||||||||||
| Change in fair value of warrant liability |
$ | 0.8 | $ | 2.3 | $ | 25.5 | ||||||
The $0.8 million, $2.3 million and $25.5 million non-cash income recorded during the year ended December 31, 2014, 2013 and 2012, respectively, was due to the change in the estimated fair value of warrant liability during that period. Such change was attributable to the change in the price of our common stock, volatility and expected life of our liability-
37
Table of Contents
classified warrants. In addition, the change in fair value was also due to the expiration of our May 2009 warrants in May 2014. We determined the fair value of the warrants using the Black-Scholes model. For more information, see “Note 3 — Fair Value Measurements” of the audited financial statements included elsewhere in this Annual Report on Form 10-K.
Liquidity and Capital Resources
Cash, Cash Equivalents, Short-Term Investments, Long-Term Investments and Working Capital
As of December 31, 2014, our principal sources of liquidity consisted of cash and cash equivalents of $10.5 million, short-term investments of $47.2 million and long-term investments of $6.0 million. Our cash and cash equivalents consist of cash, money market funds and securities with an initial maturity of less than 90 days. Our short-term investments are invested in debt securities of U.S government agencies and corporate bonds with maturities not exceeding 12 months from the reporting date. Our long-term investments are invested in debt securities of U.S government agencies with maturities exceeding 12 months from the reporting date. Our primary source of cash has historically been proceeds from the issuance of equity securities, exercise of warrants, debt, and payments to us under grants, licensing and collaboration agreements. These proceeds have been used to fund our operations.
Our cash and cash equivalents were $10.5 million as of December 31, 2014 compared to $9.3 million as of December 31, 2013, an increase of $1.2 million, or 12.9%. The increase was primarily attributable to net proceeds of $40.2 million from our September 2014 concurrent but separate underwritten offerings of our common stock and Series A convertible preferred stock, which resulted in net proceeds of $21.6 million and $18.6 million, respectively. In addition, the increase was also the result of net investment redemption of $9.5 million, partly offset by cash used to fund our operations of $48.4 million and capital equipment purchases of $0.4 million.
As of December 31, 2014, our working capital (defined as current assets less current liabilities) was $54.1 million compared to $56.3 million as of December 31, 2013, a decrease of $2.2 million, or 3.9%. The decrease in working capital was primarily attributable to a decrease in short-term investments of $3.5 million, partly offset by an increase in cash and cash equivalents of $1.2 million and an increase in prepaid and other current assets of $0.2 million.
On February 11, 2015, we closed concurrent but separate underwritten offerings of 13,500,000 shares of our common stock at a price to the public of $1.50 per share, for estimated gross proceeds of approximately $20 million and 1,333 shares of our Series B Convertible Preferred Stock at a price to the public of $1,500 per share, for estimated gross proceeds of approximately $2 million. Each share of Series B Convertible Preferred Stock is non-voting and convertible into 1,000 shares of the Company’s common stock, provided that conversion will be prohibited if, as a result, the holder and its affiliates would beneficially own more than 4.99% of the common stock then outstanding. As part of the common stock offering, we also granted the underwriters a 30-day option to purchase 2,025,000 additional shares of our common stock. On February 18, 2015, we closed a partial exercise of the underwriter’s option to purchase 1,199,660 additional shares of our common stock, at a price to the public of $1.50 per share, less underwriting discounts and commissions, which resulted in net proceeds to us of approximately $1.7 million. Aggregate gross proceeds from the offerings were approximately $24.0 million. Aggregate net proceeds from the offerings, after underwriting discounts and commissions and estimated expenses of $1.6 million, were approximately $22.4 million.
On September 23, 2014, we closed concurrent but separate underwritten offerings of 10,000,000 shares of our common stock at a price of $2.00 per share and 10,000 shares of our Series A convertible preferred stock at a price of $2,000 per share. Each share of Series A convertible preferred stock is non-voting and convertible into 1,000 shares of our common stock at the option of the holder, provided that conversion will be prohibited if, as a result, the holder and its affiliates would beneficially own more than 4.99% of our common stock then outstanding. As part of the common stock offering, the underwriters exercised a 30-day option to purchase 1,500,000 additional shares of our common stock. Aggregate gross proceeds from the offerings were approximately $43.0 million. Aggregate net proceeds from the offerings, after commissions and estimated expenses of $2.8 million, were approximately $40.2 million.
On July 1, 2013, we commenced selling our common stock through the “at the market” equity offering program under a Sales Agreement with Cowen and Company, LLC. During the year ended December 31, 2013, we sold an aggregate of 8,346,901 shares of our common stocks for gross proceeds of $16.5 million. The net proceeds from the sale of the shares, after deducting commission of $0.5 million, were approximately $16.0 million. In connection with our September 2014 equity offerings, we terminated the Sales Agreement, effective September 17, 2014. For more information, see “Note 6 — Share Capital” of the audited financial statements included elsewhere in this Annual Report on Form 10-K.
On June 4, 2013, we closed a registered direct offering of 5,000,000 units, with each unit consisting of one share of our common stock and a warrant to purchase one share of our common stock, at $2.00 per unit for gross proceeds of $10.0 million. After deducting offering expenses, net proceeds were approximately $9.9 million.
38
Table of Contents
We believe that our currently available cash and cash equivalents and investments will be sufficient to finance our operations for at least the next 12 months. Nevertheless, we expect that we will require additional capital from time to time in the future in order to continue the development of products in our pipeline and to expand our product portfolio. We would expect to seek additional financing from the sale and issuance of equity or debt securities, but we cannot predict whether financing will be available when and as we need financing or that, if available, the financing terms will be commercially reasonable. If we are unable to raise additional capital when and if we require, it would have a material adverse effect on our business and results of operations. To the extent we issue additional equity securities, our existing shareholders could experience substantial dilution.
Cash Flows from Operating Activities
Cash used in operating activities is primarily driven by our net loss. However, operating cash flows differ from net loss as a result of non-cash charges or differences in the timing of cash flows and changes in warrant liabilities.
Cash used by operating activities totaled $48.4 million for the year ended December 31, 2014, compared to $36.3 million for the year ended December 31, 2013. The increase was attributable primarily to an increase in general and administrative expense of $1.2 million and an increase of $10.0 million in license fees paid to Array. In December 2014, we paid Array $20.0 million upon entering into an exclusive license agreement. The exclusive license agreement superseded the collaboration agreement with Array under which we paid Array $10.0 million in 2013. See “Note 8 — Collaborative and License Agreements” of the audited financial statements included elsewhere in this Annual Report on Form 10-K for additional information.
Net cash used in operating activities totaled $36.3 million for the year ended December 31, 2013, compared to $26.5 million for the year ended December 31, 2012. The increase was primarily due to an upfront payment of $10.0 million to Array in connection with our collaborative agreement in May 2013.
Cash Flows from Investing Activities
Cash provided by investing activities was $9.2 million for the year ended December 31, 2014, compared to cash used in investing activities of $2.7 million for the year ended December 31, 2013. This change was attributable primarily to redemption of investments, net of purchases, of $9.5 million for the year ended December 31, 2014 as compared to purchases, net of redemptions, of investments of $2.5 million for the year ended December 31, 2013.
Cash used in investing activities was $2.7 million for the year ended December 31, 2013, compared to cash used in investing activities of $8.3 million for the year ended December 31, 2012. This change was attributable primarily to a decrease in net investments purchases of $5.1 million for the year ended December 31, 2013 compared to the year ended December 31, 2012. In addition, purchases of property and equipment decreased by $0.5 million during the year ended December 31, 2013 compared to the year ended December 31, 2012.
Cash Flows from Financing Activities
Cash provided by financing activities was $40.4 million during the year ended December 31, 2014, which consisted of net proceeds of approximately $40.2 million from our September 2014 concurrent but separate underwritten common stock and Series A convertible preferred stock offerings. Net proceeds from our common stock offering were $21.6 million and net proceeds from our Series A convertible preferred stock offering were $18.6 million.
Cash provided by financing activities was $26.1 million during the year ended December 31, 2013, which consisted of net proceeds of $16.0 million received from the sale of our common stock through our “at the market” equity offering program under the Sales Agreement with Cowen, net proceeds of $9.9 million received from a registered direct offering completed in June 2013 and cash received of $0.1 million from ESPP purchases.
Cash provided by financing activities during 2012 was $45.4 million, which consisted primarily $50.3 million of proceeds from our April 2012 common stock offering. The proceeds from this offering were partially offset by principal payments made on the GECC term loan of $0.9 million for the first half year of 2012 and the repayment of the GECC term loan of $4.1 million in June 2012.
39
Table of Contents
Contractual Obligations and Contingencies
In our continuing operations, we have entered into long-term contractual arrangements from time to time for our facilities, the provision of goods and services, and the acquisition of technology access rights, among others. The following table presents contractual obligations arising from these arrangements as of December 31, 2014:
| Payments Due by Period | ||||||||||||||||||||
| Total | Less than 1 Year |
1 — 3 Years |
3 — 5 Years |
After 5 Years |
||||||||||||||||
| (In thousands) | ||||||||||||||||||||
| Operating leases |
$ | 2,453 | $ | 609 | $ | 1,240 | $ | 604 | $ | — | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
In May 2008, we entered into a lease for an office and laboratory facility in Seattle, Washington totaling approximately 17,000 square feet. The lease provides for a base monthly rent of $47,715, increasing to $52,259 in 2018. We also have entered into operating lease obligations through July 2017 for certain office equipment.
Under certain licensing arrangements for technologies incorporated into our product candidates, we are contractually committed to payments for ongoing licensing fees and royalties, as well as contingent payments when certain milestones (as defined in the agreements) have been achieved.
Guarantees and Indemnification
In the ordinary course of our business, we have entered into agreements with our licensors, vendors, and other persons and entities that include guarantees or indemnity provisions. For example, our agreements with licensors, clinical trial sites, manufacturers and other contract partners contain indemnification provisions and we have entered into indemnification agreements with our officers and directors. Based on information known to us as of December 31, 2014, we believe that our exposure related to these guarantees and indemnification obligations is not material.
Off-Balance Sheet Arrangements
During the period presented, we did not have any relationships with unconsolidated entities or financial partnerships, such as entities often referred to as structured finance or special purpose entities, which would have been established for the purpose of facilitating off-balance sheet arrangements or for another contractually narrow or limited purpose.
Recent Accounting Pronouncements
In November 2014, the Financial Accounting Standards Board (FASB) issued Accounting Standards Update 2014-16, Derivatives and Hedging (Topic 815), Determining Whether the Host Contract in a Hybrid Financial Instrument Issued in the Form of a Share is More Akin to Debt or Equity, a consensus of the FASB Emerging Issues Task Force. The standard eliminates diversity in the practice of determining whether the nature of a host contract with a hybrid financial instrument issued in the form of a share is more akin to debt or equity and applies to all reporting entities that are issuers of hybrid financial instruments issued in the form of a share. This standard provides that the determination would be based on a consideration of all economic characteristics and the risk of the entire hybrid financial instrument, including the embedded derivative function. Upon adoption, each issued hybrid share instrument must be evaluated to determine whether it contains embedded features that require bifurcation or no longer require bifurcation under the new standard. Retrospective application and early adoption would both be permitted. The standard is effective for public business entities for fiscal years, and interim periods within those years, beginning after 15 December 2015. We are currently evaluating the impact this standard will have on our consolidated financial position or results of operations.
In August 2014, FASB issued Accounting Standard Update 2014-15, Presentation of Financial Statements—Going Concern (Subtopic 205-40): Disclosure of Uncertainties about an Entity’s Ability to Continue as a Going Concern, which provides guidance on determining when and how to disclose going-concern uncertainties in the financial statements. The new standard requires management to perform interim and annual assessments of an entity’s ability to continue as a going concern within one year of the date the financial statements are issued. An entity must provide certain disclosures if conditions or events raise substantial doubt about the entity’s ability to continue as a going concern. This standard applies to all entities and is effective for annual periods ending after December 15, 2016, and interim periods thereafter, with early adoption permitted. We are currently evaluating the impact this standard will have on our consolidated financial position or results of operations.
In May 2014, FASB issued Accounting Standard Update 2014-09, Revenue from Contracts with Customers (Topic 606) that will supersede most revenue recognition standards. Under the new standard, an entity will recognize revenue to
40
Table of Contents
depict the transfer of goods or services to customers in amounts that reflect the payment to which the entity expects to be entitled in exchange for those goods or services. An entity would recognize revenue through a five-step process: (1) identify the contract with a customer; (2) identify the performance obligations in the contract; (3) determine the transaction price; (4) allocate the transaction price to the performance obligations in the contract; and (5) recognize revenue when (or as) the entity satisfies a performance obligation. This standard also requires enhanced disclosures and provides more comprehensive guidance for transactions such as service revenue and contract modifications. Guidance for multiple-element arrangements also has been enhanced. The standard will take effect for public entities for annual reporting periods beginning after December 15, 2016, including interim reporting periods. Early application is not permitted. We are currently evaluating the impact this standard will have on our consolidated financial position or results of operations.
In July 2013, FASB issued guidance on presentation of an unrecognized tax benefit in financial statements when a NOL carryforward, a similar tax loss, or a tax credit carryforward exists. This guidance requires an entity to present an unrecognized tax benefit as a reduction of a deferred tax asset for an NOL carryforward, or similar tax loss or tax credit carryforward, rather than as a liability when (1) the uncertain tax position would reduce the NOL or other carryforward under the tax law of the applicable jurisdiction and (2) the entity intends to use the deferred tax asset for that purpose. The guidance does not require new recurring disclosures. The guidance is effective prospectively for fiscal years, and interim periods within those years, beginning after December 15, 2013 for public entities. Early adoption and retrospective application are permitted. We adopted this standard on January 1, 2014. The adoption of this standard had no impact on the presentation of our unrecognized tax benefits or on our consolidated financial position or results of operations.
| ITEM 7A. | Quantitative and Qualitative Disclosure About Market Risk |
Interest Rate Sensitivity
We had cash, cash equivalents, short-term investments and long-term investment totaling $63.7 million and $72.6 million as of December 31, 2014 and 2013, respectively. We do not enter into investments for trading or speculative purposes. We believe that we do not have any material exposure to changes in the fair value of these assets as a result of changes in interest rates since a majority of these assets are of a short term nature. Declines in interest rates, however, would reduce future investment income. A 10 basis point decline in interest rates, occurring January 1, 2014 and sustained throughout the period ended December 31, 2014, would have resulted in a decline in investment income of approximately $68,000 for that same period.
| ITEM 8. | Financial Statements and Supplementary Data |
See Financial Statements beginning on page F-1.
| ITEM 9. | Changes in and Disagreements with Accountants on Accounting and Financial Disclosure |
None.
| ITEM 9A. | Controls and Procedures |
Evaluation of Disclosure Controls and Procedures
Under the supervision and with the participation of our management, including our chief executive officer and chief financial officer, we conducted an evaluation of the effectiveness, as of the end of the period covered by this report, of our disclosure controls and procedures, as defined in Rules 13a-15(e) and 15d-15(e) under the Securities Exchange Act of 1934, as amended, or the Exchange Act. The purpose of this evaluation was to determine whether as of the evaluation date our disclosure controls and procedures were effective to provide reasonable assurance that the information we are required to disclose in our filings with the SEC, under the Exchange Act (1) is recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms and (2) accumulated and communicated to our management, including our chief executive officer and chief financial officer, as appropriate to allow timely decisions regarding required disclosure. Based on this evaluation, our chief executive officer and chief financial officer have concluded that, as of December 31, 2014, our disclosure controls and procedures were effective.
41
Table of Contents
Management’s Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting as defined in Rules 13a-15(f) and 15d-15(f) of the Exchange Act. We have designed our internal controls to provide reasonable assurance that our financial statements are prepared in accordance with generally accepted accounting principles in the United States (U.S. GAAP), and include those policies and procedures that:
| • | pertain to the maintenance of records that in reasonable detail accurately and fairly reflect the transactions and disposition of our assets; |
| • | provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures are being made only in accordance with authorization of our management and directors; and |
| • | provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of our assets that could have a material effect on the financial statements. |
Our management conducted an evaluation of the effectiveness of our internal controls based on the COSO criteria (2013 framework) as of December 31, 2014.
Based on our evaluation, our management concluded that our internal control over financial reporting was effective as of December 31, 2014. The effectiveness of our internal control over financial reporting as of December 31, 2014 has been audited by Ernst & Young LLP, an independent registered public accounting firm, as stated in their report thereto, appearing below.
Inherent Limitation on the Effectiveness of Internal Controls
The effectiveness of any system of internal control over financial reporting, including ours, is subject to inherent limitations, including the exercise of judgment in designing, implementing, operating, and evaluating the controls and procedures, and the inability to eliminate misconduct completely. Accordingly, any system of internal control over financial reporting, including ours, no matter how well designed and operated, can only provide reasonable, not absolute assurances. In addition, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate. We intend to continue to monitor and upgrade our internal controls as necessary or appropriate for our business, but cannot assure you that such improvements will be sufficient to provide us with effective internal control over financial reporting.
Changes in Internal Control over Financial Reporting
There have been no changes in our internal control over financial reporting during the quarter ended December 31, 2014 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
Report of Independent Registered Public Accounting Firm
The Board of Directors and Stockholders of Oncothyreon Inc.
We have audited Oncothyreon Inc.’s internal control over financial reporting as of December 31, 2014, based on criteria established in Internal Control — Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 framework) (the COSO criteria). Oncothyreon Inc.’s management is responsible for maintaining effective internal control over financial reporting, and for its assessment of the effectiveness of internal control over financial reporting included in the accompanying Management’s Report on Internal Control Over Financial Reporting. Our responsibility is to express an opinion on the company’s internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
42
Table of Contents
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, Oncothyreon Inc. maintained, in all material respects, effective internal control over financial reporting as of December 31, 2014, based on the COSO criteria.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the 2014 consolidated financial statements of Oncothyreon Inc. and our report dated March 10, 2015 expressed an unqualified opinion thereon.
| /s/ Ernst & Young LLP |
| Seattle, Washington |
| March 10, 2015 |
| ITEM 9B. | Other Information |
None.
43
Table of Contents
PART III
| ITEM 10. | Directors, Executive Officers and Corporate Governance |
Executive Officers
The names , ages and positions of each of our executive officers as of March 10, 2015 are set forth below.
| Name |
Age | Office | ||||
| Executive Officers |
||||||
| ROBERT KIRKMAN, M.D. |
66 | President, Chief Executive Officer and Director | ||||
| JAY VENKATESAN M.D. |
43 | Executive Vice President and General Manager | ||||
| JULIA M. EASTLAND |
50 | Chief Financial Officer, Secretary and Vice President, Corporate Development | ||||
| GARY CHRISTIANSON |
60 | Chief Operating Officer | ||||
| DIANA HAUSMAN, M.D. |
51 | Chief Medical Officer | ||||
| SCOTT PETERSON, Ph.D. |
53 | Chief Scientific Officer | ||||
Robert Kirkman, M.D. See “Directors, Executive Officers and Corporate Governance — Our Directors” included elsewhere in this Annual Report on Form 10-K for Dr. Kirkman’s biographical information.
Jay Venkatesan, M.D. has served as our executive vice president & general manager since August 2014 when we acquired Alpine Biosciences, Inc. From January 2014 until August 2014, Dr. Venkatesan served as chief executive officer of Alpine Biosciences, Inc following Alpine’s merger with Andaman Therapeutics, Inc. Dr Venkatasen was the chairman of Andaman Therapeutics, Inc. which he founded in October 2011. From May 2008 to August 2014, Dr. Venkatesan was the founder, portfolio manager and managing director of Ayer Capital Management, LP, a global healthcare long-short equity fund. Prior to founding Ayer Capital, he was a director at Brookside Capital Partners, the hedge fund group affiliated with Bain Capital, where he participated in overseeing the portfolio’s healthcare investments and was involved in evaluating public and private investments in all healthcare subsectors. Dr. Venkatesan also founded and served as the chief executive officer of Varro Technologies, a knowledge management software company focused on life sciences and was involved in healthcare venture investing at Patricof & Co. Ventures and consulting at McKinsey & Company. Dr. Venkatesan serves on the board of directors of Lion Biotechnologies, Inc. and AuraSense Therapeutics, both biopharmaceutical companies. Dr. Venkatesan received an M.D. from the University of Pennsylvania, School of Medicine, an M.B.A. from the Wharton School of the University of Pennsylvania and a B.A., magna cum laude, from Williams College.
Julia M. Eastland has served as our chief financial officer and vice president, corporate development since August 2010 and as our secretary since October 2010. From February 2006 to 2010, Ms. Eastland served as chief financial officer and vice president Finance and Operations of VLST Corporation, a privately held biotechnology company. From 2000 to 2005, Ms. Eastland held various finance positions at Dendreon Corporation, a publicly-traded biotechnology company, most recently as the vice president of strategic planning. Prior to Dendreon, Ms. Eastland worked for Amgen, Inc. as area finance manager and assistant controller for its Colorado operations. Ms. Eastland has also worked as director of finance and planning for Encore Media Group, international finance and business manager and senior financial analyst for SCIENCE Magazine and financial manager for the Discovery Channel. Ms. Eastland received an M.B.A. from Edinburgh University Management School and a B.S. in finance from Colorado State University.
Gary Christianson has served as our chief operating officer since July 2007. From 2005 to 2007, Mr. Christianson was site director for the Biologics Unit of GlaxoSmithKline plc, a global healthcare company. From 1999 to 2003, Mr. Christianson was vice president, technical operations at Corixa Corp., a biopharmaceutical and biotechnology company, and from 2003 to 2005, he was general manager of the Hamilton, Montana site in addition to his duties as vice president. From 1987 to 1999, Mr. Christianson held various positions at RIBI ImmunoChem Research, Inc., a biopharmaceuticals company. Mr. Christianson received a B.S. in mechanical engineering technology from Montana State University.
Diana Hausman, M.D. has served as our chief medical officer since January 2012. Prior to that, from August 2009 until January 2012, she served as our vice president, clinical development. From 2005 to 2009, Dr. Hausman served in a variety of positions at Zymogenetics, Inc., a biopharmaceutical company, most recently as senior director, clinical research. From 2002 until 2009, Dr. Hausman served as senior associate medical director at Berlex Inc., a biopharmaceutical company. From 2001 to 2002, Dr. Hausman worked in drug safety at Immunex Corporation, a biopharmaceutical company. Dr. Hausman received her A.B. in Biology from Princeton University, and her M.D. from the University of Pennsylvania School of Medicine. She was trained in internal medicine and hematology/oncology at the University of Washington and is board certified in medical oncology.
44
Table of Contents
Scott Peterson, Ph.D. was appointed as our chief scientific officer in June 2012. From June 2009 until June 2012, Dr. Peterson served as our vice president, research and development. From 2007 until 2009 Dr. Peterson served as director and department head, oncology research at Zymogenetics, Inc., a biopharmaceutical company. From 1999 to 2007, Dr. Peterson held a variety of positions at ICOS Corporation, a biopharmaceutical company. Dr. Peterson received his Ph.D. in chemistry (biochemistry) from the University of Colorado, Boulder and holds a B.S. in biology from Washington State University.
Our Directors
The name, age, position(s), term, board committee membership and biographical information for each member of our Board of Directors is set forth below as of March 10, 2015:
Directors Continuing in Office Until the 2015 Annual Meeting of Stockholders
Christopher Henney, Ph.D., age 74, has served as the chairman of our board of directors since September 2006 and as a member of our board of directors since March 2005. Dr. Henney is a member of our compensation and corporate governance and nominating committees. From 1995 to 2003, Dr. Henney was chairman and chief executive officer of Dendreon Corporation, a publicly-traded biotechnology company that he co-founded and from 2003 to 2005 continued as executive chairman. Dr. Henney was also a co-founder of Immunex Corporation and ICOS Corporation, both publicly-traded biotechnology companies before being sold. Our corporate governance and nominating committee believes that Dr. Henney’s qualifications for membership on the board of directors include his roles as co-founder of Dendreon, Immunex and ICOS, as well as his membership on the boards of directors of several development-stage biotechnology companies. Through his experience in working with biotechnology companies from founding until commercialization of their product candidates, Dr. Henney provides our board of directors with significant insights into the strategic, operational and clinical development aspects of the company. Dr. Henney currently serves as vice-chairman of the board of directors of Cyclacel Pharmaceuticals, Inc., a development-stage biopharmaceuticals company, chairman of the board of directors of Anthera Pharmaceuticals, Inc., a biopharmaceutical company and as a member of the board of directors of Prothena Corporation plc, a biotechnology company. Dr. Henney was the chairman of SGX Pharmaceuticals, Inc., a biotechnology company acquired by Eli Lilly in 2008, and a member of the board of directors of AVI BioPharma, Inc., a biopharmaceuticals company, until June 2010 and Mymetics Corp. during 2011. Dr. Henney received a Ph.D. in experimental pathology from the University of Birmingham, England, where he also obtained his D.Sc. for contributions in the field of immunology. In 2011, he received the honorary degree of Doctor of the University from his alma mater for contributions to the biotechnology industry and in 2012 was elected to the Hall of Fame of the Association of International Biotechnology CEOs. Dr. Henney is a former professor of immunology and microbiology and has held faculty positions at Johns Hopkins University, the University of Washington and the Fred Hutchinson Cancer Research Center.
Steven P. James, age 56, was appointed as a member of our board of directors in February 2015. Mr. James is a member of our audit committee. Mr. James served as President and Chief Executive Officer of Labrys Biologics, Inc., from December 2012 until its acquisition by Teva Pharmaceuticals in July 2014. He was President and Chief Executive Officer of KAI Pharmaceuticals, Inc., from October 2004 until its acquisition by Amgen in July 2012. He was Senior Vice President, Commercial Operations, at Exelixis, Inc., from 2003 until 2004. Previously he held senior business roles at Sunesis Pharmaceuticals, Inc., and Isis Pharmaceuticals, Inc. He began his career in new product planning at Eli Lilly and Company. Mr. James is also a member of the board of directors of Ocera Therapeutics, Inc., and Chrono Therapeutics, both biotechnology companies. Our corporate governance and nominating committee believes that Mr. James’ qualifications for membership on the board of directors include his extensive experience in the leadership of development stage biotechnology companies and in business development. Mr. James earned a Bachelor of Arts degree in biology from Brown University and a Masters in Management degree from the Kellogg Graduate School of Management at Northwestern University.
W. Vickery Stoughton, age 69, has been a member of our board of directors since June 1997. Mr. Stoughton is a member of our audit and compensation committees. Since September 2011, Mr. Stoughton has been the president and chief executive officer of Radia Genetics, a private gene therapy company. From August 2006 until September 2007, Mr. Stoughton served as president and chief executive officer of MagneVu Corporation, a medical devices company, which filed for bankruptcy in September 2007. From 1996 to 2002, Mr. Stoughton was chairman and chief executive officer of Careside Inc., a research and development medical devices company. From October 1995 to July 1996, Mr. Stoughton was president of SmithKline Beecham Diagnostics Systems Co., a diagnostic services and product company, and prior to October 1995 he served as president of SmithKline Beecham Clinical Laboratories, Inc., a clinical laboratory company. From 1988 until May 2008, Mr. Stoughton was a member of the board of directors of Sun Life Financial Inc., a financial services company. Our corporate governance and nominating committee believes that Mr. Stoughton’s qualifications for membership on the board of directors include his involvement in several medical device
45
Table of Contents
companies, his role as president of SmithKline Beecham Clinical Laboratories, and his broader business background. Through this experience, Mr. Stoughton provides our board of directors with significant insights into the operational aspects of the company. Mr. Stoughton received his B.S. in chemistry from St. Louis University and his M.B.A. from the University of Chicago.
Directors Continuing in Office Until the 2016 Annual Meeting of Stockholders
Richard Jackson, Ph.D., age 75, has been a member of our board of directors since May 2003. Dr. Jackson is the chairman of our compensation committee and a member of our corporate governance and nominating committee. Dr. Jackson is president of Jackson Associates, LLC, a biotechnology and pharmaceutical consulting company. From September 2006 to August 2014, Dr. Jackson was president and chief executive officer of Ausio Pharmaceuticals, LLC, a drug development company. From May 2002 to May 2003, Dr. Jackson was president, chief executive officer and chairman of the board of directors of EmerGen, Inc., a biotechnology company. From November 1998 to January 2002, Dr. Jackson served as senior vice president, research and development for Atrix Laboratories, Inc., a biotechnology company. From January 1993 to July 1998, Dr. Jackson served as senior vice president, discovery research, at Wyeth Ayerst Laboratories, the pharmaceuticals division of American Home Products Corporation. Our corporate governance and nominating committee believes that Dr. Jackson’s qualifications for membership on the board of directors include over 20 years of experience in academic medicine and over 25 years of experience at several pharmaceutical and biotechnology companies, with positions in both research and development and senior management. This experience allows Dr. Jackson to provide our board of directors with significant insights into the clinical development of our product candidates. Dr. Jackson served as a director of Inflazyme Pharmaceuticals Ltd. until 2007. Dr. Jackson received his Ph.D. in microbiology and his B.S. in chemistry from the University of Illinois.
Robert Kirkman, M.D., age 66, has served as a member of our board of directors and as our president and chief executive officer since September 2006. From 2005 to 2006, Dr. Kirkman was acting president and chief executive officer of Xcyte Therapies, Inc., which concluded a merger with Cyclacel Pharmaceuticals, Inc., both development stage biopharmaceuticals companies, in March of 2006. From 2004 to 2005, Dr. Kirkman was chief business officer and vice president of Xcyte. From 1998 to 2003, Dr. Kirkman was vice president, business development and corporate communications of Protein Design Labs, Inc., a biopharmaceuticals company. Dr. Kirkman also serves as a member of the board of directors of Trillium Therapeutics Inc., a public biopharmaceutical company. Our corporate governance and nominating committee believes that Dr. Kirkman’s qualifications for membership on the board of directors include his previous experience at development stage biotechnology companies and his position as our president and chief executive officer. Dr. Kirkman’s scientific understanding along with his corporate vision and operational knowledge provide strategic guidance to our management team and our board of directors. Dr. Kirkman holds an M.D. degree from Harvard Medical School and a B.A. in economics from Yale University.
Ted W. Love, M.D., age 56, has been a member of our board of directors since September 2013. Since June 2014, Dr. Love has served as chief executive officer of Global Blood Therapeutics, Inc. From February 2010 until August 2012, Dr. Love served as executive vice president and head of research and development and technical operations at Onyx Pharmaceuticals, Inc.. From 2001 to January 2009, Dr. Love served as chairman and chief executive officer of Nuvelo, Inc. Dr. Love joined Nuvelo from Theravance, Inc., where he served as senior vice president of development from 1998 to 2001. Previously, he spent six years at Genentech, Inc., where he held a number of senior management positions in medical affairs and product development and served as chairman of Genentech’s product development committee. Dr. Love also serves as a member of the boards of directors of biopharmaceutical companies Amicus Therapeutics, Inc. and KaloBios Pharmaceuticals, Inc. Our corporate governance and nominating committee believes that Dr. Love’s qualifications for membership on the board of directors include over 15 years of experience in the biotechnology industry. This experience provides our board of directors with significant insights into the strategic and operational issues facing our company. Until April 2012, he served on the California Independent Citizens’ Oversight Committee. Dr. Love earned his Bachelor of Science in molecular biology from Haverford College and his M.D. from Yale Medical School.
Directors Continuing in Office Until the 2017 Annual Meeting of Stockholders
Daniel Spiegelman, M.B.A., age 56, has been a member of our board of directors since June 2008. Mr. Spiegelman is the chairman of our audit committee and a member of our corporate governance and nominating committee. Since May 2012, Mr. Spiegelman has been the executive vice president and chief financial officer of Biomarin Pharmaceuticals Inc., a biopharmaceutical company focused on the development and commercialization of therapies for rare diseases. From October 2009 to May 2012, Mr. Spiegelman served as a consultant to provide strategic financial support to a portfolio of public and private life sciences companies. From 1998 to 2009, Mr. Spiegelman was employed at CV Therapeutics, Inc., a biopharmaceutical company acquired in 2009 by Gilead, most recently as senior vice president and chief financial officer. From 1992 to 1998, Mr. Spiegelman was an employee at Genentech, Inc., a biotechnology company, serving most
46
Table of Contents
recently as its treasurer. Mr. Spiegelman also serves as a member of the board of directors of Relypsa, Inc., a biopharmaceutical company. Our corporate governance and nominating committee believes that Mr. Spiegelman’s qualifications for membership on the board of directors include his extensive background in the financial and commercial issues facing growing biotechnology companies. Additionally, as chief financial officer of CV Therapeutics prior to its sale to Gilead Sciences, Mr. Spiegelman was involved in transitioning the company from a research and development focus to a commercial entity with two approved products. This experience allows Mr. Spiegelman to provide our board of directors with significant insights into financial strategy and organizational development. Mr. Spiegelman received his B.A. and M.B.A. from Stanford University.
Section 16(a) Beneficial Ownership Reporting Compliance
Section 16(a) of the Exchange Act requires our directors and executive officers, and persons who own more than ten percent of a registered class of our equity securities, to file reports of ownership of, and transactions in, our securities with the Securities and Exchange Commission and NASDAQ. Such directors, executive officers, and ten percent stockholders are also required to furnish us with copies of all Section 16(a) forms that they file.
Based solely on a review of the copies of such forms received by us, or written representations from certain reporting persons, we believe that during 2014, our directors, executive officers, and ten percent stockholders complied with all Section 16(a) filing requirements applicable to them. Two Form 4 reports regarding three dispositions of common stock by Mr. Spiegelman in 2013 were not timely filed.
Code of Conduct
Our board of directors adopted a Code of Business Conduct and Ethics (the Code of Conduct) for all our officers, directors, and employees in March 2008, which was last amended in September 2010, and a Code of Ethics for the President and Chief Executive Officer, the Chief Financial Officer and Corporate Controller on March 25, 2003, which was subsequently amended on March 13, 2008, (the Code of Ethics). The Code of Conduct details the responsibilities of all our officers, directors, and employees to conduct our affairs in an honest and ethical manner and to comply with all applicable laws, rules, and regulations. The Code of Conduct addresses issues such as general standards of conduct, avoiding conflicts of interest, communications, financial reporting, safeguarding our assets, responsibilities to our customers, suppliers, and competitors, and dealing with governments. The Code of Ethics imposes additional requirements on our senior executive, financial and accounting officers with respect to conflicts of interest, accuracy of accounting records and periodic reports and compliance with laws. Each of the Code of Conduct and Code of Ethics is available on our website at www.oncothyreon.com.
Stockholder Nominations and Recommendations for Director Candidates
We have not made any material changes to the procedures by which our stockholders may recommend nominees to our board of directors since we last disclosed the procedures by which stockholders may nominate director candidates under the caption “Corporate Governance and Board Matters — Committees of the Board of Directors — Corporate Governance and Nominating Committee” in our proxy statement for the 2014 annual meeting of Oncothyreon filed with the SEC on April 24, 2014.
Audit Committee
We have a standing audit committee, which reviews with our independent registered public accounting firm the scope, results, and costs of the annual audit and our accounting policies and financial reporting. Our audit committee (1) has direct responsibility for the appointment, compensation, retention, and oversight of our independent registered public accounting firm, (2) establishes procedures for handling complaints regarding our accounting practices, (3) has authority to engage any independent advisors it deems necessary to carry out its duties, and (4) has appropriate funding to engage any necessary outside advisors. The current members of the audit committee are Daniel Spiegelman (Chairman), Steven P. James, Dr. Ted W. Love and W. Vickery Stoughton. The board of directors has determined that Mr. Spiegelman, the chairman of the audit committee, is an “audit committee financial expert” as that term is defined in Item 407(d)(5) of Regulation S-K promulgated by the SEC and is an “independent director” as that term is defined under the applicable rules and regulations of The NASDAQ Stock Market. The audit committee reviews and reassesses the adequacy of its charter on an annual basis.
47
Table of Contents
| ITEM 11. | Executive Compensation |
Compensation Discussion and Analysis
This compensation discussion and analysis describes our executive compensation policies for our named executive officers, Dr. Kirkman, Ms. Eastland, Mr. Christianson, Dr. Hausman and Dr. Peterson.
Compensation Philosophy and Objectives
The principal objectives of our compensation policies and programs have been to attract and retain senior executive management, to motivate their performance toward clearly defined corporate goals, and to align their long term interests with those of our stockholders. In addition, our compensation committee believes that maintaining and improving the quality and skills of our management and appropriately incentivizing their performance are critical factors affecting our stockholders’ realization of long-term value.
Our compensation programs have reflected, and we expect that they will continue to reflect, the fact that we are a biopharmaceutical company whose principal compounds are in pre-clinical and clinical development and subject to regulatory approval. As a result, our revenues have been and will continue to be limited, and we expect to continue to incur net losses for at least the next several years. In an effort to preserve cash resources, our historical compensation programs have focused on long-term equity incentives relative to cash compensation. This approach seeks to place a substantial portion of executive compensation at risk by rewarding our executive officers, in a manner comparable to our stockholders, for achieving our business and financial objectives.
In addition to long-term equity incentives, we have also implemented a performance-based cash bonus program for our executive officers. Payments under this performance-based cash bonus program are based on achievement of pre-established corporate performance goals. All of the performance goals of our executive officers are tied to corporate objectives to reflect the fact that our executive officers make key strategic decisions influencing our company as a whole.
We design and implement compensation programs that combine both cash incentive elements based on annual performance objectives and long-term equity elements. Our compensation committee has not, however, adopted any formal or informal policies or guidelines for allocating compensation between cash and equity compensation or among different forms of non-cash compensation. The compensation committee’s philosophy is that a substantial portion of an executive officer’s compensation should be performance-based. In that regard, we expect to continue to use options or other equity incentives as a significant component of compensation because we believe that they align individual compensation with the creation of stockholder value, and we expect any payments under cash incentive plans to be tied to annual performance targets.
Our executive compensation programs have remained substantially the same for several years. We believe our programs are effectively designed and work well in aligning the interests of our executive officers and stockholders and are instrumental to achieving our company objectives. In determining executive compensation for 2014, our compensation committee considered the stockholder support that the “Say-on-Pay” proposal received at our 2011 and 2014 annual meetings of stockholders. As a result, the compensation committee continued to apply the same effective principles and philosophy it has used in previous years in determining executive compensation and will continue to consider stockholder concerns and feedback in the future. With respect to the frequency of future “Say-on-Pay” advisory votes, consistent with the recommendation of our board of directors and the outcome of the stockholder vote regarding the proposal at our 2011 annual meeting of stockholders, we determined to hold an advisory “Say-on-Pay” vote on the compensation of our executive officers every three years. Our next advisory “Say-on-Pay” vote will occur at our 2017 annual meeting of stockholders.
Role of Our Compensation Committee
Our compensation committee is comprised of three non-employee members of our board of directors, Dr. Jackson, Dr. Henney and Mr. Stoughton, each of whom is an independent director under the applicable rules and regulations of The NASDAQ Stock Market and a “non-employee director” for purposes of Rule 16b-3 under the Exchange Act.
48
Table of Contents
Our compensation committee approves and oversees our executive compensation and benefit policies. Our compensation committee acts as the administrator of our equity incentive plans and approves all grants to our executive officers. Our compensation committee operates pursuant to a written charter under which our board of directors has delegated specific authority with respect to compensation determinations. Among the responsibilities of our compensation committee are the following:
| • | evaluating our compensation practices and assisting in developing and implementing our executive compensation program and philosophy; |
| • | establishing a practice, in accordance with the applicable rules and regulations of The NASDAQ Stock Market, of determining the compensation earned, paid, or awarded to our chief executive officer independent of input from him; and |
| • | establishing a policy, in accordance with the applicable rules and regulations of The NASDAQ Stock Market, of reviewing on an annual basis the performance of our other executive officers with assistance from our chief executive officer and determining what we believe to be appropriate compensation levels for such officers. |
The compensation committee’s charter allows the committee to form subcommittees for any purpose that the committee deems appropriate and may delegate to such subcommittees such power and authority as the committee deems appropriate. For example, the compensation committee has delegated certain powers and authority to the new employee option committee as set forth in “— Share Option Plan” included elsewhere in this Annual Report on Form 10-K.
Our chief executive officer actively supports the compensation committee’s work by providing information relating to our financial plans, performance assessments of our executive officers, and other personnel related data. In particular, our chief executive officer, as the person to whom our other executive officers report, is responsible for evaluating individual officers’ contributions to corporate objectives. Our chief executive officer, on an annual basis at or shortly after the end of each year, makes recommendations to the compensation committee with respect to merit salary increases, cash bonuses, and stock option grants or other equity incentives for our other executive officers. Our compensation committee meets to evaluate, discuss, modify or approve these recommendations. Without the participation of the chief executive officer, the compensation committee as part of the annual review process conducts a similar evaluation of the chief executive officer’s contribution and performance and makes determinations, at or shortly after the end of each year, with respect to merit salary increases, bonus payments, stock option grants, or other forms of compensation for our chief executive officer.
Our compensation committee has the authority under its charter to engage the services of outside advisors, experts, and others for assistance. The compensation committee did not rely on any outside advisors for purposes of structuring our 2014 compensation plan but did consider the survey data described below.
Competitive Market Review for 2014
The market for experienced management is highly competitive in the life sciences and biopharmaceutical industries. We seek to attract and retain the most highly qualified executives to manage each of our business functions, and we face substantial competition in recruiting and retaining management from companies ranging from large and established pharmaceutical companies to entrepreneurial early stage companies. We expect competition for appropriate technical, commercial, and management skills to remain strong for the foreseeable future.
In making our executive compensation determinations for 2014, we benchmarked our compensation levels using the Radford Global Life Sciences Salary Survey. This survey includes life sciences companies based predominantly in biotechnology markets in the U.S. with which we compete for executive talent.
In evaluating the survey data, we compared our compensation practices and levels with the survey data. This information was used to determine appropriate levels of compensation based on market benchmarks for similarly situated officers.
Principal Elements of Executive Compensation
Our executive compensation program consists of five components:
| • | base salary; |
| • | annual performance-based cash bonuses; |
| • | equity-based incentives; |
49
Table of Contents
| • | benefits; and |
| • | severance/termination protection. |
We believe that each of these components, combining both short and long-term incentives, offers a useful element in achieving our compensation objectives and that collectively these components have been effective in achieving our corporate goals.
Annual Review Process
Our compensation committee reviews data and makes executive compensation decisions on an annual basis, typically during the last quarter of the year and the first quarter of the new year. From time to time, the compensation committee may make mid-year changes to executive compensation based on new developments in our business or industry.
In connection with the annual goal setting process, executive officers are responsible for establishing and submitting for review to our chief executive officer (and in the case of our chief executive officer, directly to the compensation committee) their departmental goals and financial objectives. Our chief executive officer then compiles the information submitted and provides it, along with information relating to his own personal goals and objectives, to our compensation committee and board for review in the form of draft corporate objectives. Subsequently, our compensation committee, including our chief executive officer with respect to all officers other than himself and excluding our chief executive officer with respect to discussions of his own compensation, reviews, considers, and may amend the draft objectives prior to the compensation committee’s final approval of the objectives.
Weighting of Compensation Elements
Our compensation committee’s determination of the appropriate use and weight of each element of executive compensation is subjective, based on its view of the relative importance of each element in meeting our overall objectives and factors relevant to the individual executive. Like many biopharmaceutical companies with pre-clinical and clinical-stage products, we seek to place a significant amount of each executive’s total potential compensation “at risk” based on performance.
Base Salary
As part of the annual review process, our compensation committee makes its determinations of changes in annual base salary for executive officers. Base salary for our executive officers reflects the scope of their respective responsibilities, their relative seniority and experience, and competitive market factors including our compensation committee’s review of market compensation for executive officers of U.S. biopharmaceutical companies. Salary adjustments are typically based on competitive conditions, individual performance, changes in job duties, and our budget requirements. The compensation committee determined based on the factors above that it was appropriate to increase base salaries for each of our executive officers by 3% for 2014 in order to keep salaries for these officers at approximately the 50th percentile of the Radford Global Life Sciences Salary Survey. Effective January 2014, Dr. Kirkman’s base salary was increased to $435,000, Ms. Eastland’s base salary to $276,000, Mr. Christianson’s base salary to $310,000, Dr. Hausman’s base salary to $355,500 and Dr. Peterson’s base salary to $270,500. With respect to each executive officer, these base salaries were approximately consistent with the 50th percentile of the salaries reported in the Radford Global Life Sciences Salary Survey.
Variable Cash Compensation — Incentive Bonuses
We pay performance-based bonuses to our executive officers pursuant to our performance review policy, which we believe enhances each executive’s incentive to contribute to corporate objectives and aligns their interests with our stockholders. Under the performance review policy, our executive officers are eligible to receive bonuses based on achievement of pre-established corporate performance goals. The weighting among the goals is individualized based on the nature of the executive’s role within the company. As further described in the paragraphs below, each goal is assigned a percentage for each executive based on the importance to us that the goal be achieved by that executive and the extent to which the goal falls within the executive’s area of operational control. Generally, achievement of a particular goal will result in the payment of the expected level of incentive compensation associated with such goal. Partial achievement can result in the payment of reduced or no incentive compensation and superior achievement of any performance goal may result in the payment in excess of the target level of incentive compensation; however, there is not a fixed formula for determining the amount of incentive compensation for partial or above target achievement. Rather, the compensation committee retains discretion to increase or decrease variable cash incentive compensation to our officers as it determines appropriate, based on actual achievement against the goals.
50
Table of Contents
Typically, the maximum incentive compensation to which an executive officer is entitled is based on a percentage of such executive’s base salary. For example, if (1) an executive’s base salary is $100,000, (2) he or she is eligible to receive a bonus up to 50% of his base salary, or $50,000, (3) the compensation committee has established four performance goals, each weighted at 25% and (4) the compensation committee determines that the executive has achieved two of the four performance goals, then, the executive would be eligible to receive, subject to the discretion of the compensation committee, a bonus of $25,000.
Performance goals may be both qualitative and quantitative and are designed to be specific, measurable and completed within a fixed period of time. Although performance goals are intended to be achievable with significant effort, we do not expect that every goal will be actually attained in any given year. Our compensation committee is responsible for setting performance goals, assessing whether such goals have been achieved and determining the amount of bonuses (if any) to be paid with respect to our executive officers. Performance goals for the upcoming year are typically established at or shortly after the end of the prior year. Assuming that a determination is made that a bonus has been earned, we typically pay bonuses to executive officers shortly after the first scheduled meeting of the compensation committee each year. An individual must remain actively employed by the company through the actual date of payment to receive a bonus.
The 2014 performance goals approved by the compensation committee in January 2014 for each executive officer are set forth in the table below. With input from the board, the compensation committee selected these particular corporate objectives based on its judgment that they represented areas in which each of the executive officers have significant operational control and on which the board and compensation committee believed each of the executive officers should focus to move our strategic plan forward and enhance stockholder value. As is reflected in the table below, the weighting of specific performance goals varies among executive officers based on each executive officer’s role and position within the company. For example, because Dr. Hausman holds a position as our chief medical officer, the compensation committee felt it was appropriate to more heavily weight her bonus on achievement of certain clinical development milestones. Mr. Christianson and Dr. Peterson’s goals are more heavily weighted to the achievement of technical operations and pre-clinical assessment goals, respectively, to align their goals with their respective roles as our chief operating officer and chief scientific officer. Dr. Kirkman and Ms. Eastland’s goals are more heavily weighted toward the achievement of goals relating to cash position, investor perception and business development. Since the compensation committee believes that our performance is also determined by the performance of executive management acting collaboratively as a team, no corporate goal was assigned a weight of less than 5% for any of our executive officers.
| Named Executive Officer |
Cash Position (1) |
Investor Perception (2) |
Clinical Assessment (3) |
Pre-Clinical Assessment (4) |
Technical Operations (5) |
Business Development (6) |
||||||||||||||||||
| Robert Kirkman |
25 | % | 20 | % | 10 | % | 10 | % | 10 | % | 25 | % | ||||||||||||
| Julia Eastland |
30 | 25 | 10 | 10 | 10 | 15 | ||||||||||||||||||
| Gary Christianson |
10 | 5 | 10 | 10 | 50 | 15 | ||||||||||||||||||
| Diana Hausman |
10 | 5 | 50 | 10 | 10 | 15 | ||||||||||||||||||
| Scott Peterson |
10 | 5 | 10 | 50 | 10 | 15 | ||||||||||||||||||
| (1) | Have cash and investments as of December 31, 2014 sufficient to fund ONT-380 through Phase 2 data release. |
| (2) | Improve investor perception of the company. |
| (3) | Timely complete dose escalation in ONT-380 trials; if supported by clinical data, timely initiate a Phase 2 trial of ONT-380; timely complete enrollment in expansion cohorts of ONT-10 Phase 1 trial and timely initiate an additional trial of ONT-10. |
| (4) | Timely complete evaluations of in-licensing candidates and complete studies in connection with ONT-380, ONT-10 and in-licensed molecule. |
| (5) | Timely complete supply, formulation and manufacturing goals with respect to ONT-10, PET Lipid A, and in-licensed molecule. |
| (6) | In-license or acquire a preclinical drug development candidate, complete partnership for ONT-10. |
51
Table of Contents
In September 2014, the compensation committee revised the 2014 performance goals to reflect goals and objectives related to our acquisition of protocell technology from Alpine in August 2014 and our entry into a collaboration with Adimab for the discovery of novel antibodies in October 2014. Accordingly, the pre-clinical assessment measurement was revised to include, in addition to the goals described above, the timely development of a strategy for immune-oncology antibody discovery and the timely initiation of two protocell toxicology studies. Additionally, the technical operations measurement was revised to include, in additional to the goals described above, the timely evaluation of manufacturing organizations for protocells and timely manufacturing goals with respect to ONT-380.
The target and actual bonus amounts for 2014 for our named executive officers are set forth below, based on achievement against the corporate performance goals. Specifically, the compensation committee determined the following: (1) the cash position goal was fully achieved; (2) the investor perception goal was fully achieved; (3) clinical goals were fully achieved; (4) preclinical goals were fully achieved; (5) technical operations goals were fully achieved; and (6) business development goals were fully achieved.
| Named Executive Officer |
Base Salary ($) |
2014 Annual Target as Percentage of Base Salary |
Target Bonus ($) |
Target Goals Achieved |
2014 Incentive Bonus Actually Paid ($) |
|||||||||||||||
| Robert Kirkman |
$ | 435,000 | 50 | % | $ | 217,500 | 100 | % | $ | 217,500 | ||||||||||
| Julia Eastland |
276,000 | 30 | 82,800 | 100 | 82,800 | |||||||||||||||
| Gary Christianson |
310,000 | 35 | 108,500 | 100 | 108,500 | |||||||||||||||
| Diana Hausman |
355,500 | 35 | 124,425 | 100 | 124,425 | |||||||||||||||
| Scott Peterson |
270,500 | 30 | 81,150 | 100 | 81,150 | |||||||||||||||
In January 2015, the compensation committee approved performance goals for 2015. Dr. Kirkman, Ms. Eastland, Mr. Christianson, Dr. Hausman and Dr. Peterson are eligible to receive in 2015 incentive bonuses under our performance review policy of up to 50%, 35%, 35%, 35% and 35%, respectively, of their base salary. The compensation committee increased Ms. Eastland’s and Dr. Peterson’s bonus targets from 30% to 35% to reflect the relative importance of their contributions to the company and increase parity in the executive officer bonus targets. The 2015 performance goals for our executive officers relate to various corporate objectives, including objectives related to our financial condition, development of our product candidates and proprietary technologies, technical operations and certain business development activities (although the weighting for such performance goals will differ between such executive officers as described above).
Equity-based Incentives
We grant equity-based incentives to employees, including our named executive officers, in order to create a corporate culture that aligns employee interests with stockholder interests. We have not adopted any specific stock ownership guidelines, and our equity incentive plans have provided the principal method for named executive officers to acquire an equity position in our company.
Historically, we have granted options to our executive officers under our share option plan. Our share option plan permits the grant of stock options for shares of common stock. All equity incentive programs are administered by our compensation committee (other than grants of restricted share units to non-employee directors, which are overseen by the corporate governance and nominating committee and grants of stock options to certain new employees, which are overseen by the new employee option committee). To date, our equity incentive grants to executive officers have consisted of options under the share option plan. We use stock options as a long-term incentive vehicle because stock options align the interests of executives with those of our stockholders, support a pay-for-performance culture, foster employee stock ownership and focus the management team on increasing value for our stockholders. In addition, stock options help to provide a balance to the overall executive compensation program as base salary and our bonus program focus on nearer-term achievements, while the grant and vesting of stock options is intended to focus executive efforts toward increasing stockholder value over the longer term.
The practice of our compensation committee has been to consider the annual grant of options to our executive officers in connection with the annual compensation review process. In making its determination of the size of annual option grants for our executive officers, the compensation committee considers the individual performance of the executive officer in the prior year, the industry experience and background of the executive officer, and the value of the executive officer’s outstanding equity grants in the then-current competitive environment, including the value of such outstanding equity grants as a retention tool. Adjustments may be made as the compensation committee deems reasonable to attract and retain executive officers in the competitive environment for highly qualified employees in which we operate.
52
Table of Contents
In determining the annual option grants to executive officers, the compensation committee considered the roles and performance of each executive officer in the context of the companies goals and priorities, and the long-term retention value of the overall outstanding option grants held by each of the executive officers. Accordingly, the compensation committee awarded stock option grants to the executive officers as set forth in the table below. The compensation committee decided that the somewhat larger size of the grants relative to historical grants was appropriate in light of our long-term retention objectives. The compensation committee also decided to grant Dr. Kirkman a comparatively larger award because of the importance of Dr. Kirkman’s continued leadership to the business operations and strategy of our company. The compensation committee also decided to grant Dr. Hausman and Dr. Peterson comparatively larger grants in light of the strategic importance of the achievement of our current clinical and pre-clinical objectives.
| Named Executive Officer |
Options (#) | |||
| Dr. Robert Kirkman |
300,000 | |||
| Julia Eastland |
100,000 | |||
| Gary Christianson |
100,000 | |||
| Dr. Diana Hausman |
150,000 | |||
| Dr. Scott Peterson |
150,000 | |||
Our compensation committee believes that the size and terms of these stock option grants were reasonable given the need to ensure that equity incentive grants held by our executive officers effectively serve as a retention instrument.
These options vest as follows: 25% of the shares underlying the option will vest and become exercisable on the first anniversary of the grant date and thereafter 1/48th of the shares underlying the option will vest and become exercisable on each monthly anniversary of the grant date, subject to the executive officer’s continued service, such that the option will be fully exercisable on the fourth anniversary of the grant date.
The exercise price of these options was the closing sales price of our common stock on the date of grant, December 16, 2014, or $1.76.
Our practice has been to provide equity incentives principally in the form of stock option grants that vest over time. The stock option vesting period encourages executive retention over the term of the option. Our compensation committee may also consider alternative forms of equity in the future, such as performance shares, restricted share units or restricted stock awards with alternative vesting strategies based on the achievement of performance milestones or financial metrics.
Benefits
We provide the following benefits to our executive officers, generally on the same basis provided to all of our employees:
| • | health, dental insurance and vision (for the employee and eligible dependents); |
| • | flexible spending accounts for medical and dependent care; |
| • | life insurance; |
| • | employee assistance plan (for the employee and eligible dependents); |
| • | short- and long-term disability, accidental death and dismemberment; and |
| • | a 401(k) plan with an employer match into the plan. |
Severance/Termination Protection
We are a party to agreements with our executive officers that provide for benefits payable in connection with the termination of employment or a change in control. The compensation committee considers such benefits in order to be competitive in the hiring and retention of employees, including executive officers. When establishing the termination and change of control provisions in our agreements with our executive officers, the compensation committee considered industry practice and an analysis of current market trends.
53
Table of Contents
In addition, these benefits are intended to incentivize and retain our officers during the pendency of a proposed change in control transaction and align the interests of our officers with our stockholders in the event of a change in control. The compensation committee believes that proposed or actual change in control transactions can adversely impact the morale of officers and create uncertainty regarding their continued employment. Without these benefits, officers may be tempted to leave the company prior to the closing of the change in control, especially if they do not wish to remain with the entity after the transaction closes. Such departures could jeopardize the consummation of the transaction or our interests if the transaction does not close and we remain independent.
All arrangements with the named executive officers and the potential payments that each of the named executive officers would have received if a change in control or termination of employment would have occurred on December 31, 2014, are described in “— Termination and Change of Control Provisions under Offer Letters” and “— Potential Payments Upon Termination or Change in Control” included elsewhere in this Annual Report on Form 10-K.
Accounting and Tax Considerations
Section 162(m) of the United States Internal Revenue Code of 1986, as amended (Section 162(m)), limits the amount that we may deduct for compensation paid to our chief executive officer and to each of our four most highly compensated officers to $1,000,000 per person, unless certain exemption requirements are met. Exemptions to this deductibility limit may be made for various forms of “performance-based” compensation. In addition to salary and bonus compensation, upon the exercise of stock options that are not treated as incentive stock options, the excess of the current market price over the option price, or option spread, is treated as compensation and accordingly, in any year, such exercise may cause an officer’s total compensation to exceed $1,000,000. Under certain regulations, option spread compensation from options that meet certain requirements will not be subject to the $1,000,000 cap on deductibility. Our options do not meet the requirements for exemption towards the $1,000,000 cap. While the compensation committee cannot determine with certainty how the deductibility limit may impact our compensation program in future years, the compensation committee intends to maintain an approach to executive compensation that strongly links pay to performance. While the compensation committee has not adopted a formal policy regarding tax deductibility of compensation paid to our chief executive officer and our four most highly compensated officers, the compensation committee intends to consider tax deductibility under Section 162(m) as a factor in compensation decisions.
Compensation Committee Interlocks and Insider Participation
During 2014, Richard Jackson, Christopher Henney and W. Vickery Stoughton served on our compensation committee. During 2014, no member of our compensation committee was an officer or employee or formerly an officer of our company, and no member had any relationship that would require disclosure under Item 404 of Regulation S-K of the Exchange Act. None of our executive officers has served on the board of directors or the compensation committee (or other board committee performing equivalent functions) of any other entity, one of whose executive officers served on our board of directors or on our compensation committee.
Risk Analysis of Compensation Plans
The mix and design of the elements of executive compensation do not encourage management to assume excessive risks. Any risks arising from our compensation policies and practices for our employees are not reasonably likely to have a material adverse effect on the company.
The compensation committee extensively reviewed the elements of executive compensation to determine whether any portion of executive compensation encouraged excessive risk taking and concluded:
| • | significant weighting towards long-term incentive compensation discourages short-term risk taking; and |
| • | several categories of goals generally apply, so that if any particular goal is not achieved, then a disproportionate amount of total compensation is not forfeited. |
Compensation Committee Report
The information contained in this report will not be deemed to be “soliciting material” or to be “filed” with the SEC, nor will such information be incorporated by reference into any future filing under the Securities Act or the Exchange Act, except to the extent that we specifically incorporate it by reference in such filing.
54
Table of Contents
In reliance on the reviews and discussions referred to above and the review and discussion of the section captioned “Compensation Discussion and Analysis” with our management, the compensation committee has recommended to the board of directors and the board of directors has approved, that the section captioned “Compensation Discussion and Analysis” be included in this Annual Report on Form 10-K and the proxy statement for our annual meeting of stockholders.
| COMPENSATION COMMITTEE |
| Dr. Richard Jackson, Chairman |
| Dr. Christopher Henney |
| W. Vickery Stoughton |
55
Table of Contents
Summary Compensation Table — 2014, 2013 and 2012
The following table sets forth the compensation earned by or awarded to, as applicable, our named executive officers during each of 2014, 2013 and 2012.
| Name and Principal Position |
Year | Salary ($) |
Option Awards ($)(1) |
Non-Equity Incentive Plan Compensation ($)(2) |
All Other Compensation ($)(3) |
Total ($) |
||||||||||||||||||
| Robert Kirkman |
2014 | $ | 435,000 | $ | 360,000 | $ | 217,500 | $ | 12,455 | $ | 1,024,955 | |||||||||||||
| President, Chief Executive Officer and Director |
|
2013 2012 |
|
|
422,300 410,000 |
|
|
756,000 336,000 |
|
|
202,704 159,900 |
|
|
13,161 12,792 |
|
|
1,394,165 918 ,692 |
| ||||||
| Julia Eastland |
2014 | 276,000 | 120,000 | 82,800 | 8,292 | 487,092 | ||||||||||||||||||
| Chief Financial Officer, |
2013 | 267,800 | 189,000 | 76,725 | 8,526 | 542,051 | ||||||||||||||||||
| Secretary and Vice President, Corporate Development |
2012 | 260,000 | 168,000 | 60,840 | 8,292 | 497,132 | ||||||||||||||||||
| Gary Christianson |
2014 | 310,000 | 120,000 | 108,500 | 9,017 | 547,517 | ||||||||||||||||||
| Chief Operating Officer |
2013 | 300,760 | 189,000 | 100,529 | 9,515 | 599,804 | ||||||||||||||||||
| 2012 | 292,000 | 168,000 | 87,892 | 9,252 | 557,144 | |||||||||||||||||||
| Diana Hausman |
2014 | 355,500 | 180,000 | 124,425 | 10,268 | 670,193 | ||||||||||||||||||
| Chief Medical Officer |
2013 | 345,050 | 189,000 | 96,787 | 10,843 | 641,680 | ||||||||||||||||||
| 2012 | 335,000 | 168,000 | 82,410 | 10,542 | 595,952 | |||||||||||||||||||
| Scott Peterson |
2014 | 270,500 | 180,000 | 81,150 | 8,292 | 539,942 | ||||||||||||||||||
| Chief Scientific Officer |
2013 | 262,650 | 189,000 | 76,825 | 8,371 | 536,847 | ||||||||||||||||||
| 2012 | 239,091 | 168,000 | 62,730 | 7,665 | 477,486 | |||||||||||||||||||
| (1) | These amounts represent the aggregate grant date fair value of option awards for fiscal years 2014, 2013 and 2012. These amounts do not represent the actual amounts paid to or realized by the named executive officer for these awards during fiscal years 2014, 2013 and 2012. The value as of the grant date for stock options is recognized over the number of days of service required for the grant to become vested. For a more detailed description of the assumptions used for purposes of determining grant date fair value, see “Note 7 — Share-based Compensation” of the audited financial statements included elsewhere in this Annual Report on Form 10-K. |
| (2) | The amounts in this column represent total performance-based bonuses earned for services rendered during the year under our performance review policy by our executive officers. Under the applicable bonus plan for each year, each executive was eligible to receive a cash bonus based on achievement of a combination of corporate objectives. See “— Compensation Discussion and Analysis — Variable Cash Compensation — Incentive Bonuses” included elsewhere in this Annual Report on Form 10-K for additional information regarding our variable cash compensation policies for executive officers. |
| (3) | The amounts in this column consist of contributions made by us pursuant to our 401(k) plan and life insurance premiums. |
56
Table of Contents
Grants of Plan-Based Awards
The following table sets forth each grant of an award made to an executive officer during 2014 under any of our incentive plans or equity plans.
| Name |
Grant Date (1) |
Estimated Possible Payouts Under Non- Equity Incentive Plan Awards($)(2) (3) |
All Other Option Awards: Number of Securities Underlying Options (#) |
Exercise or Base Price of Option Awards ($/Sh)(1) |
Grant Date Fair Value of Stock and Option Awards ($)(4) |
|||||||||||||||
| Robert L. Kirkman |
December 16, 2014 | $ | 217,500 | 300,000 | $ | 1.76 | $ | 360,000 | ||||||||||||
| Julia Eastland |
December 16, 2014 | 82,800 | 100,000 | 1.76 | 120,000 | |||||||||||||||
| Gary Christianson |
December 16, 2014 | 108,500 | 100,000 | 1.76 | 120,000 | |||||||||||||||
| Diana Hausman |
December 16, 2014 | 124,425 | 150,000 | 1.76 | 180,000 | |||||||||||||||
| Scott Peterson |
December 16, 2014 | 81,150 | 150,000 | 1.76 | 180,000 | |||||||||||||||
| (1) | Except as otherwise noted below and consistent with the provisions of our share option plan in effect at the date of grant, all options reflected in the table had an exercise price equal to the closing sales price of our common stock as reported by The NASDAQ Global Market on the grant date. All options were granted under our share option plan. |
| (2) | In January 2015, the compensation committee determined that 100% of the target performance bonuses were earned in 2014. |
| (3) | Amounts represent the “Target” amount of each award. There was no set “Threshold” or “Maximum” performance bonus amounts established with respect to our 2014 non-equity incentive plan awards. For more information regarding the 2014 non-equity incentive plan awards, see “— Compensation Discussion and Analysis — Variable Cash Compensation — Incentive Bonuses” included elsewhere in this Annual Report on Form 10-K. |
| (4) | These amounts represent the grant date fair value of option awards granted in 2014. These amounts do not represent the actual amounts paid to or realized by the named executive officer for these awards during fiscal year 2014. The value as of the grant date for stock options is recognized over the number of days of service required for the grant to become vested. For a more detailed description of the assumptions used for purposes of determining grant date fair value, see “Part II — Item 7 — Management’s Discussion and Analysis of Financial Condition and Results of Operations — Critical Accounting Policies and Significant Judgments and Estimates — Share-based Compensation” and “Note 7 — Share-based Compensation” of the audited financial statements included elsewhere in this Annual Report on Form 10-K. |
57
Table of Contents
Outstanding Equity Awards at 2014 Fiscal Year-End
The following table sets forth the equity awards outstanding at December 31, 2014 for each of the named executive officers. Except as set forth in the footnotes to the following table, each stock option is fully vested.
| Option Awards | ||||||||||||||||
| Name |
Number of Securities Underlying Unexercised Options (#) Exercisable |
Number of Securities Underlying Unexercised Options (#) Unexercisable |
Option Exercise Price ($Cdn. or $U.S.)(1) |
Option Expiration Date |
||||||||||||
| Robert Kirkman |
137,537 | — | (2) | Cdn. $ | 8.04 | May 3, 2015 | ||||||||||
| 45,000 | — | (3) | $ | 3.43 | June 4, 2016 | |||||||||||
| 100,000 | — | (4) | $ | 1.10 | March 11, 2017 | |||||||||||
| 200,000 | — | (5) | $ | 4.71 | December 3, 2017 | |||||||||||
| 100,000 | — | (6) | $ | 3.32 | December 1, 2018 | |||||||||||
| 75,000 | 25,000 | (11) | $ | 6.92 | December 1, 2019 | |||||||||||
| 50,000 | 50,000 | (12) | $ | 4.74 | December 12, 2020 | |||||||||||
| 150,000 | 450,000 | (13) | $ | 1.74 | December 12, 2021 | |||||||||||
| — | 300,000 | (14) | $ | 1.76 | December 16, 2022 | |||||||||||
| Julia Eastland |
40,000 | — | (7) | $ | 3.31 | November 10, 2018 | ||||||||||
| 50,000 | — | (6) | $ | 3.32 | December 1, 2018 | |||||||||||
| 37,500 | 12,500 | (11) | $ | 6.92 | December 1, 2019 | |||||||||||
| 25,000 | 25,000 | (12) | $ | 4.74 | December 12, 2020 | |||||||||||
| 37,500 | 112,500 | (13) | $ | 1.74 | December 12, 2021 | |||||||||||
| — | 100,000 | (14) | $ | 1.76 | December 16, 2022 | |||||||||||
| Gary Christianson |
16,666 | — | (8) | Cdn. $ | 6.72 | June 29, 2015 | ||||||||||
| 15,000 | — | (3) | $ | 3.43 | June 4, 2016 | |||||||||||
| 30,000 | — | (4) | $ | 1.10 | March 11, 2017 | |||||||||||
| 100,000 | — | (5) | $ | 4.71 | December 3, 2017 | |||||||||||
| 50,000 | — | (6) | $ | 3.32 | December 1, 2018 | |||||||||||
| 37,500 | 12,500 | (11) | $ | 6.92 | December 1, 2019 | |||||||||||
| 25,000 | 25,000 | (12) | $ | 4.74 | December 12, 2020 | |||||||||||
| 37,500 | 112,500 | (13) | $ | 1.74 | December 12, 2021 | |||||||||||
| — | 100,000 | (14) | $ | 1.76 | December 16, 2022 | |||||||||||
| Diana Hausman |
30,000 | — | (9) | $ | 4.96 | October 1, 2017 | ||||||||||
| 50,000 | — | (5) | $ | 4.71 | December 3, 2017 | |||||||||||
| 50,000 | — | (6) | $ | 3.32 | December 1, 2018 | |||||||||||
| 37,500 | 12,500 | (11) | $ | 6.92 | December 1, 2019 | |||||||||||
| 25,000 | 25,000 | (12) | $ | 4.74 | December 12, 2020 | |||||||||||
| 37,500 | 112,500 | (13) | $ | 1.74 | December 12, 2021 | |||||||||||
| — | 150,000 | (14) | $ | 1.76 | December 16, 2022 | |||||||||||
| Scott Peterson |
25,000 | — | (10) | $ | 6.56 | August 1, 2017 | ||||||||||
| 50,000 | — | (5) | $ | 4.71 | December 3, 2017 | |||||||||||
| 50,000 | — | (6) | $ | 3.32 | December 1, 2018 | |||||||||||
| 37,500 | 12,500 | (11) | $ | 6.92 | December 1, 2019 | |||||||||||
| 25,000 | 25,000 | (12) | $ | 4.74 | December 12, 2020 | |||||||||||
| 37,500 | 112,500 | (13) | $ | 1.74 | December 12, 2021 | |||||||||||
| — | 150,000 | (14) | $ | 1.76 | December 16, 2022 | |||||||||||
| (1) | In April 2008, the board of directors approved an amendment to our share option plan, which provided that the exercise price of any future grants would equal the closing price of our common stock traded on The NASDAQ Global Market on the date of grant. Unless otherwise indicated, all exercise prices are denominated in U.S. dollars. |
| (2) | This stock option fully vested on May 3, 2011. |
| (3) | This stock option fully vested on June 4, 2012. |
| (4) | This stock option fully vested on March 11, 2013. |
| (5) | This stock option fully vested on December 3, 2013. |
| (6) | This stock option fully vested on December 1, 2014. |
58
Table of Contents
| (7) | This stock option fully vested on September 7, 2014. |
| (8) | This stock option fully vested on June 29, 2011. |
| (9) | This stock option fully vested on September 1, 2013. |
| (10) | This stock option fully vested on August 1, 2013. |
| (11) | This stock option fully vests on December 1, 2015, and 1/4 vests on the first anniversary of grant, with the balance vesting in monthly increments for 36 months following the first anniversary of grant. |
| (12) | This stock option fully vests on December 12, 2016, and 1/4 vests on the first anniversary of grant, with the balance vesting in monthly increments for 36 months following the first anniversary of grant. |
| (13) | This stock option fully vests on December 12, 2017, and 1/4 vests on the first anniversary of grant, with the balance vesting in monthly increments for 36 months following the first anniversary of grant. |
| (14) | This stock option fully vests on December 16, 2018, and 1/4 vests on the first anniversary of grant, with the balance vesting in monthly increments for 36 months following the first anniversary of grant. |
Option Exercises and Stock Vested
None of our executive officers exercised stock options during 2014. We have not granted any stock awards to date to any of our executive officers.
Employee Benefit Plans
Our share option plan, in which our employees and officers participate, provides for the acceleration of vesting of awards in connection with or following a change in control of the company. A “change in control” shall be deemed to have occurred if (i) our board of directors passes a resolution to the effect that, for purposes of the share option plan, a change in control has occurred or (ii) any person or any group of two or more persons acting jointly or in concert becomes the beneficial owner, directly or indirectly, or acquires the right to control or direct, 25% or more of our outstanding voting securities or any successor entity in any manner, including without limitation as a result of a takeover bid or an amalgamation with any other corporation or any other business combination or reorganization. See “— Share Option Plan” included elsewhere in this Annual Report on Form 10-K.
Termination and Change of Control Provisions under Offer Letters
Dr. Robert Kirkman
We are a party to an offer letter with Dr. Kirkman, our president and chief executive officer, dated August 29, 2006 as amended in December 2008 and December 2009. Pursuant to the terms of the offer letter as amended, Dr. Kirkman will receive the following benefits if we undergo a change of control transaction (as defined in the share option plan), in addition to the stock option vesting acceleration described above:
| • | lump sum payment of two year’s base salary, less required withholding; and |
| • | lump sum payment of two year’s equivalent of performance review bonus at target, less required withholding. |
Additionally, if Dr. Kirkman is terminated for reasons other than cause (as defined in the December 2009 amendment), he will receive the following benefits:
| • | lump sum payment of one year’s base salary, less required withholding; and |
| • | lump sum payment of one year’s equivalent of performance review bonus at target, less required withholding. |
Julia Eastland
We are a party to an offer letter dated August 17, 2010 with Julia Eastland, our chief financial officer, secretary and vice president, corporate development.
Pursuant to the terms of the offer letter, Ms. Eastland will receive the following benefits if we undergo a change of control transaction (as defined in the share option plan), in addition to the stock option vesting acceleration described above:
| • | lump sum payment of one year’s base salary, less required withholding; and |
| • | lump sum payment of one year’s equivalent of performance review bonus at target, less required withholding. |
59
Table of Contents
Additionally, if Ms. Eastland is terminated for reasons other than cause (as defined in the offer letter), she will receive the following benefits:
| • | lump sum payment of nine month’s base salary, less required withholding; and |
| • | lump sum payment of nine month’s equivalent of performance review bonus at target, less required withholding. |
Gary Christianson
We are a party to an offer letter dated June 29, 2007 as amended in December 2008 and December 2009, with Gary Christianson, our chief operating officer. Pursuant to the terms of the offer letter as amended, Mr. Christianson will receive the following benefits if we undergo a change of control transaction (as defined in the share option plan), in addition to the stock option vesting acceleration described above:
| • | lump sum payment of one year’s base salary, less required withholding; and |
| • | lump sum payment of one year’s equivalent of performance review bonus at target, less required withholding. |
Additionally, if Mr. Christianson is terminated for reasons other than cause (as defined in the June 2007 offer letter), he will receive the following benefits:
| • | lump sum payment of nine month’s base salary, less required withholding; |
| • | lump sum payment of nine month’s equivalent of performance review bonus at target, less required withholding; and |
| • | health insurance coverage for a period of nine months. |
Dr. Diana Hausman
We are a party to an offer letter dated July 6, 2009, as amended December 2009, with Diana Hausman, M.D., our chief medical officer. Pursuant to the terms of the offer letter as amended, Dr. Hausman will receive the following benefits if we undergo a change of control transaction (as defined in the share option plan), in addition to the stock option vesting acceleration described above:
| • | lump sum payment of one year’s base salary, less required withholding; and |
| • | lump sum payment of one year’s equivalent of performance review bonus at target, less required withholding. |
Additionally, if Dr. Hausman is terminated for reasons other than cause (as defined in the July 2009 offer letter), she will receive the following benefits:
| • | lump sum payment of six month’s base salary, less required withholding; and |
| • | lump sum payment of six month’s equivalent of performance review bonus at target, less required withholding. |
Dr. Scott Peterson
We are a party to an offer letter dated June 4, 2009, as amended in December 2009 and January 2015, with Scott Peterson, Ph.D., our chief scientific officer. Pursuant to the terms of his offer letter as amended, Dr. Peterson will receive the following benefits if we undergo a change of control transaction (as defined in the share option plan), in addition to the stock option vesting acceleration described above:
| • | lump sum payment of one year’s base salary, less required withholding; and |
| • | lump sum payment of one year’s equivalent of performance review bonus at target, less required withholding. |
Additionally, if Dr. Peterson is terminated for reasons other than cause (as defined in the offer letter), he will receive the following benefits:
| • | lump sum payment of nine month’s base salary, less required withholding; and |
| • | lump sum payment of nine month’s equivalent of performance review bonus at target, less required withholding. |
60
Table of Contents
Potential Payments Upon Termination or Change in Control
The tables below describe the payments and benefits our executive officers would be entitled to receive assuming the occurrence on December 31, 2014 of either a change of control transaction or termination of their employment without “cause” (as defined below). For additional details regarding the payments and benefits our named executive officers are entitled to, please see “— Termination and Change of Control Provisions under Offer Letters” included elsewhere in this Annual Report on Form 10-K.
Dr. Robert Kirkman
| Change of Control | Termination Other Than for Cause(3) | |||||||||||||||||||||||
| Name |
Equity Acceleration (1) |
Salary (2) |
Insurance Benefits |
Equity Acceleration (4) |
Salary (5) |
Insurance Benefits |
||||||||||||||||||
| Robert Kirkman |
$ | 114,000 | $ | 1,305,000 | $ | — | $ | — | $ | 652,500 | $ | — | ||||||||||||
| (1) | The amount shown in this column is calculated as the spread value of all unvested stock options held by Dr. Kirkman on December 31, 2014, assuming a stock price of $1.90 per share, the last reported sale price of our common stock on The NASDAQ Global Market on December 31, 2014. |
| (2) | The amount shown in this column is a lump sum payment equal to two times Dr. Kirkman’s base salary for 2014 plus two year’s equivalent of his performance review bonus at target. Such payments will be made within 60 days following a change of control, provided that, within 45 days of a change of control, Dr. Kirkman signs a separation agreement in a form reasonably satisfactory to us, which shall include a general release of all claims against us. |
| (3) | For purposes of Dr. Kirkman’s offer letter, “cause” includes, among other things (a) willful engaging in illegal conduct or gross misconduct which is injurious to us, (b) being convicted of, or entering a plea of nolo contendere or guilty to, a felony or a crime of moral turpitude, (c) engaging in fraud, misappropriation, embezzlement or any other act or acts of dishonesty resulting or intended to result directly or indirectly in a gain or personal enrichment of him at our expense, (d) material breach of any of our written policies, or (e) willful and continual failure substantially to perform his duties, which failure has continued for a period of at least 30 days after written notice by us. |
| (4) | Pursuant to the terms of our share option plan, there is no acceleration of vesting if Dr. Kirkman is terminated without cause. |
| (5) | The amount shown in this column is a lump sum payment equal to Dr. Kirkman’s base salary for 2014 plus one year’s equivalent of his performance review bonus at target. Such payments will be made within 60 days following termination other than for cause, subject to any payment delay in order to comply with Section 409A of the Internal Revenue Code. |
Julia Eastland
| Change of Control | Termination Other Than for Cause(3) | |||||||||||||||||||||||
| Name |
Equity Acceleration (1) |
Salary (2) |
Insurance Benefits |
Equity Acceleration (4) |
Salary (5) |
Insurance Benefits |
||||||||||||||||||
| Julia Eastland |
$ | 32,000 | $ | 358,800 | $ | — | $ | — | $ | 269,100 | $ | — | ||||||||||||
| (1) | The amount shown in this column is calculated as the spread value of all unvested stock options held by Ms. Eastland on December 31, 2014, assuming a stock price of $1.90 per share, the last reported sale price of our common stock on The NASDAQ Global Market on December 31, 2014. |
| (2) | The amount shown in this column is a lump sum payment equal to Ms. Eastland’s base salary for 2014 plus one year’s equivalent of her performance review bonus at target. Such payments will be made within 60 days following a change of control, provided that, within 45 days of a change of control, Ms. Eastland signs a separation agreement in a form reasonably satisfactory to us, which shall include a general release of all claims against us. |
| (3) | For purposes of Ms. Eastland’s offer letter, “cause” includes, among other things (a) willful engaging in illegal conduct or gross misconduct which is injurious to us, (b) being convicted of, or entering a plea of nolo contendere or guilty to, a felony or a crime of moral turpitude, (c) engaging in fraud, misappropriation, embezzlement or any other act or acts of dishonesty resulting or intended to result directly or indirectly in a gain or personal enrichment of her at our expense, (d) material breach of any of our written policies, or (e) willful and continual failure substantially to perform her duties, which failure has continued for a period of at least 30 days after written notice by us. |
| (4) | Pursuant to the terms of our share option plan, there is no acceleration of vesting if Ms. Eastland is terminated without cause. |
61
Table of Contents
| (5) | The amount shown in this column is a lump sum payment equal to nine months of Ms. Eastland’s base salary for 2014 plus nine month’s equivalent of her performance review bonus at target. |
Gary Christianson
| Change of Control | Termination Other Than for Cause(3) | |||||||||||||||||||||||
| Name |
Equity Acceleration (1) |
Salary (2) |
Insurance Benefits |
Equity Acceleration (4) |
Salary (5) |
Insurance Benefits |
||||||||||||||||||
| Gary Christianson |
$ | 32,000 | $ | 418,500 | $ | — | $ | — | $ | 313,875 | $ | 18,033 | ||||||||||||
| (1) | The amount shown in this column is calculated as the spread value of all unvested stock options held by Mr. Christianson on December 31, 2014, assuming a stock price of $1.90 per share, the last reported sale price of our common stock on The NASDAQ Global Market on December 31, 2014. |
| (2) | The amount shown in this column is a lump sum payment equal to Mr. Christianson’s base salary for 2014 plus one year’s equivalent of his performance review bonus at target. Such payments will be made within 60 days following a change of control, provided that, within 45 days of a change of control, Mr. Christianson signs a separation agreement in a form reasonably satisfactory to us, which shall include a general release of all claims against us. |
| (3) | For purposes of Mr. Christianson’s offer letter, “cause” includes, among other things (a) willful engaging in illegal conduct or gross misconduct which is injurious to us, (b) being convicted of, or entering a plea of nolo contendere or guilty to, a felony or a crime of moral turpitude, (c) engaging in fraud, misappropriation, embezzlement or any other act or acts of dishonesty resulting or intended to result directly or indirectly in a gain or personal enrichment of him at our expense, (d) material breach of any of our written policies, or (e) willful and continual failure substantially to perform his duties, which failure has continued for a period of at least 30 days after written notice by us. |
| (4) | Pursuant to the terms of our share option plan, there is no acceleration of vesting if Mr. Christianson is terminated without cause. |
| (5) | The amount shown in this column is a lump sum payment equal to nine months of Mr. Christianson’s base salary for 2014 plus nine month’s equivalent of his performance review bonus at target. If Mr. Christianson is a “specified employee” within the meaning of Section 409A of the Internal Revenue Code and any final regulations and official guidance promulgated thereunder, at the time of his separation from service, then, if required, the amounts shown in this column, which are otherwise due on or within the six-month period following the separation from service will accrue, to the extent required, during such six-month period and will become payable in a lump sum payment six months and one day following the date of separation from service. |
Dr. Diana Hausman
| Change of Control | Termination Other Than for Cause(3) | |||||||||||||||||||||||
| Name |
Equity Acceleration (1) |
Salary (2) |
Insurance Benefits |
Equity Acceleration (4) |
Salary (5) |
Insurance Benefits |
||||||||||||||||||
| Diana Hausman |
$ | 39,000 | $ | 479,925 | $ | — | $ | — | $ | 239,963 | $ | — | ||||||||||||
| (1) | The amount shown in this column is calculated as the spread value of all unvested stock options held by Dr. Hausman on December 31, 2014, assuming a stock price of $1.90 per share, the last reported sale price of our common stock on The NASDAQ Global Market on December 31, 2014. |
| (2) | The amount shown in this column is a lump sum payment equal to Dr. Hausman’s base salary for 2014 plus one year’s equivalent of her performance review bonus at target. Such payments will be made within 60 days following a change of control, provided that, within 45 days of a change of control, Dr. Hausman signs a separation agreement in a form reasonably satisfactory to us, which shall include a general release of all claims against us. |
| (3) | For purposes of Dr. Hausman’s offer letter, “cause” includes, among other things (a) willful engaging in illegal conduct or gross misconduct which is injurious to us, (b) being convicted of, or entering a plea of nolo contendere or guilty to, a felony or a crime of moral turpitude, (c) engaging in fraud, misappropriation, embezzlement or any other act or acts of dishonesty resulting or intended to result directly or indirectly in a gain or personal enrichment of her at our expense, d) material breach of any of our written policies, or (e) willful and continual failure substantially to perform her duties, which failure has continued for a period of at least 30 days after written notice by us. |
| (4) | Pursuant to the terms of our share option plan, there is no acceleration of vesting if Dr. Hausman is terminated without cause. |
| (5) | The amount shown in this column is a lump sum payment equal to six months of Dr. Hausman’s base salary for 2014 plus six month’s equivalent of her performance review bonus at target. |
62
Table of Contents
Dr. Scott Peterson
| Change of Control | Termination Other Than for Cause(3) | |||||||||||||||||||||||
| Name |
Equity Acceleration (1) |
Salary (2) |
Insurance Benefits |
Equity Acceleration (4) |
Salary (5) |
Insurance Benefits |
||||||||||||||||||
| Scott Peterson |
$ | 39,000 | $ | 351,650 | $ | — | $ | — | $ | 263,738 | $ | — | ||||||||||||
| (1) | The amount shown in this column is calculated as the spread value of all unvested stock options held by Dr. Peterson on December 31, 2014, assuming a stock price of $1.90 per share, the last reported sale price of our common stock on The NASDAQ Global Market on December 31, 2014. |
| (2) | The amount shown in this column is a lump sum payment equal to Dr. Peterson’s base salary for 2014 plus one year’s equivalent of his performance review bonus at target. Such payments will be made within 60 days following a change of control, provided that, within 45 days of a change of control, Dr. Peterson signs a separation agreement in a form reasonably satisfactory to us, which shall include a general release of all claims against us. |
| (3) | For purposes of Dr. Peterson’s offer letter, “cause” includes, among other things (a) willful engaging in illegal conduct or gross misconduct which is injurious to us, (b) being convicted of, or entering a plea of nolo contendere or guilty to, a felony or a crime of moral turpitude, (c) engaging in fraud, misappropriation, embezzlement or any other act or acts of dishonesty resulting or intended to result directly or indirectly in a gain or personal enrichment of his at our expense, (d) material breach of any of our written policies or (e) willful and continual failure substantially to perform his duties, which failure has continued for a period of at least 30 days after written notice by us. |
| (4) | Pursuant to the terms of our share option plan, there is no acceleration of vesting if Dr. Peterson is terminated without cause. |
| (5) | The amount shown in this column is a lump sum payment equal to nine months of Dr. Peterson’s base salary for 2014 plus nine month’s equivalent of her performance review bonus at target. |
Share Option Plan
Our board of directors adopted our share option plan on December 9, 1992 and our stockholders approved it on May 26, 1993. Our share option plan was amended and restated as of May 3, 2007, April 3, 2008, October 22, 2009, March 14, 2011, December 1, 2011 and December 4, 2014. Unless further amended by our stockholders, our share option plan will terminate on May 3, 2017. Our share option plan provides for the grant of nonstatutory stock options to selected employees, directors and persons or companies engaged to provide ongoing management or consulting services for us, or any entity controlled by us. The employees, directors and consultants who have been selected to participate in our share option plan are referred to below as “participants.”
Share Reserve
The total number of shares of common stock issuable pursuant to options granted under our share option plan shall, at any time, be 10% of our issued and outstanding shares of common stock. We had reserved a total of 7,067,314 shares of our common stock for issuance pursuant to our share option plan as of December 31, 2014. As of December 31, 2014, options to purchase 5,217,535 shares of our common stock were outstanding and 1,825,858 shares of our common stock were available for future grant under our share option plan.
Administration
Our compensation committee administers our share option plan. Under our share option plan, the plan administrator has the power, subject to certain enumerated restrictions in our share option plan, to determine the terms of the awards, including the employees, directors and consultants who will receive awards, the exercise price of the award, the number of shares subject to each award, the vesting schedule and exercisability of each award and the form of consideration payable upon exercise.
In addition, the compensation committee has delegated to the new employee option committee the authority to approve grants of stock options to newly hired employees who are not our chief executive officer, president, chief financial officer (or principal financial officer, if no person holds the office of chief financial officer), vice president or a Section 16 officer (as determined pursuant to the rules promulgated under the Securities Exchange Act of 1934). The new employee option committee is composed of our chief executive officer, our principal financial officer and our head of human resources. The new employee option committee meets during the last full week of each month and may only grant stock option awards. The stock options granted by the new employee option committee must have an exercise price equal to the closing sales price of our common stock as reported by The NASDAQ Global Market on the last trading day of the month in which such grants were approved. These grants must fall within a predetermined range approved by the compensation committee
63
Table of Contents
and may not deviate from the standard vesting terms (i.e., awards vest over a four year period, with 25% of the shares subject to an award vesting on the first anniversary of the optionee’s commencement of employment and the balance vesting in equal monthly increments for 36 months following the first anniversary of the commencement of employment).
Share Options
The exercise price of the shares subject to options granted under our share option plan shall be determined by our compensation committee or board of directors, but shall not be less than the fair market value of the shares. Generally, the exercise price will be the closing price of our common stock on the day of the option grant.
Termination of Service Provider Relationship
Upon the termination without cause of a participant’s employment or service with us (or any of our subsidiaries), other than a termination due to death or retirement (as such terms are defined in our share option plan), the participant’s option will continue to vest and may be exercised at any time up to and including, but not after, the date which is 180 days after the date of the termination or the date prior to the close of the business on the expiry date of the option, whichever is the earlier. If termination is for cause, the option will immediately terminate in its entirety. An option may never be exercised after the expiration of its term.
For our president or any of our vice presidents, in the event of a termination of the participant’s service or employment with us (or any of our subsidiaries) without cause, any option granted to the participant will continue to vest and may be exercised at any time up to and including, but not after, the date which is the second anniversary of the date of his or her termination or the date before the close of business on the expiry date of his or her option, whichever is the earlier.
In the event of the retirement, as such term is defined in our share option plan, of the participant while in the employment of us (or any of our subsidiaries), any option granted to the participant will continue to vest and may be exercised by the participant in accordance with the terms of the option at any time up to and including, but not after, the expiry date of the option.
In the event of the death of the participant (other than Dr. Kirkman) while in the employment or service of us (or any of our subsidiaries), the option will continue to vest and may be exercised by a legal representative of the participant at any time up to and including, but not after, the date which is 180 days after the date of the death of the optionee or before the close of business on the expiry date of the option, whichever is earlier.
In the event of the termination of service on account of disability of the participant (other than Dr. Kirkman) while in the employment or service of us (or any of our subsidiaries), the option will continue to vest and may be exercised by participant at any time up to and including, but not after, the date which is 180 days after the date of the disability of the participant or before the close of business on the expiry date of the option, whichever is earlier. In the event of Dr. Kirkman’s death or disability, options would continue to vest for 180 days, but would be exercisable at any time prior to the close of business on the expiry date of the option.
Effect of a Change in Control
Our share option plan provides that, if a change in control occurs, as such term is defined in our share option plan, including our merger with or into another corporation or the sale of all or substantially all of our assets, or if there is an offer to purchase, a solicitation of an offer to sell, or an acceptance of an offer to sell our shares of common stock made to all or substantially all of the holders of shares of common stock, a participant, who at the time of the change of control is an employee, director or service provider, shall have the right to immediately exercise his or her option as to all shares of common stock subject to such option, including as to those shares of common stock with respect to which such option cannot be exercised immediately prior to the occurrence of the change of control, and the participant shall have 90 days from the date of the change of control to exercise his or her option (unless the option expires prior to such date).
Transferability
Unless otherwise determined by the plan administrator, our share option plan generally does not allow for the sale or transfer of awards under our share option plan other than by will or the laws of descent and distribution, and awards may be exercised only during the lifetime of the participant and only by that participant or by the participant’s legal representative for up to 180 days following the participant’s death.
64
Table of Contents
Additional Provisions
Our board of directors has the authority to amend (subject to stockholder approval in some circumstances) or discontinue our share option plan, so long as that action does not materially and adversely affect any option rights granted to a participant without the written consent of that participant.
During the period from January 1 to December 31, 2014, options to purchase 1,444,500 shares of common stock were granted under our share option plan at a weighted average exercise price of $1.82 per share.
Restricted Share Unit Plan
Our board of directors adopted our RSU plan on May 18, 2005 and our stockholders approved it on May 18, 2005. Our RSU plan was amended and restated as of June 12, 2009 to add additional shares to the plan and again as of October 22, 2009 to remove references to the Toronto Stock Exchange and make certain other housekeeping changes necessitated by our voluntary delisting from the TSX. Our RSU plan provides for the grant of RSUs to non-employee members of our board of directors. Pursuant to an October 2011 amendment to the RSU plan, we withhold 25% of the shares of our common stock otherwise deliverable in connection with the vesting of any RSU and instead deliver to each non-employee director an amount in cash equal to the fair market value of the withheld shares on the vesting date. The amendment is designed to facilitate satisfaction of the non-employee directors’ income tax obligation with respect to the vested RSUs. On June 6, 2014, our stockholders approved an increase of 500,000 shares in the number of shares of our common stock reserved for issuance under the RSU Plan and our board approved an administrative amendment to our RSU plan. The directors who receive RSUs under our restricted share unit plan are referred to below as participants.
Share Reserve
We have reserved a total of 966,666 of our shares of common stock for issuance pursuant to our restricted share unit plan. As of December 31, 2014, grants covering 163,204 shares of our common stock were outstanding, 530,910 shares of our common stock were available for future grant under our restricted share unit plan and 272,552 shares had been issued upon conversion of RSUs.
Administration
The corporate governance and nominating committee of our board of directors administers our restricted share unit plan. Under our restricted share unit plan, the plan administrator has the power, subject to certain enumerated restrictions in our restricted share unit plan, to determine the terms of the grants, including the directors who will receive grants, the grant period (as such term is defined in our restricted share unit plan) of any awards, and any applicable vesting terms in order for the restricted share units to be issued, and such other terms and conditions as the board of directors deems appropriate.
Each grant of restricted share units will be evidenced by a written notice, which we call the notice of grant, with such notice, along with our restricted share unit plan, governing the terms and conditions of the grant. Each notice of grant will state the number of restricted share units granted to the participant and state that each restricted share unit, subject to and in accordance with the terms of our restricted share unit plan, will entitle the participant to receive one share of our common stock in settlement of a restricted share unit granted pursuant to our restricted share unit plan.
Right to Restricted Share Units in the event of Death, Disability, Retirement, or Resignation
In the event of the death or disability of a participant while a director of us, and with respect to each grant of restricted share units for which the grant period has not ended and for which the restricted share units have not been otherwise issued prior to the date of death, all unvested restricted share units will immediately vest and the shares of our common stock subject to such restricted share units will be issued by the later of the end of the calendar year of the date of death, or by the 15th day of the third calendar month following the participant’s date of death.
In the event the participant’s service as a director terminates for any reason other than death or disability, and provided such participant is not a specified employee (as such term is defined in our restricted share unit plan) on the date of his or termination, with respect to the restricted share units as to which the release date (as such term is defined in our restricted share unit plan) has not occurred, and for which shares of our common stock have not been issued, the participant will receive such shares as if the grant period had ended and such shares will be issued by the later of the end of the calendar year of the date of termination or by the 15th day of the third calendar month following the date of the termination. If the participant is a specified employee on the date of his or her termination, and if such termination is for any reason other than death, with respect to the restricted share units as to which the release date has not occurred, and for which shares of our common stock have not been issued, the participant will receive such shares as if the grant period had ended and such shares will be delivered by the 30th day of the date following the date which is six months following the participant’s date of termination.
65
Table of Contents
Effect of a Change in Control
In the event of a change in control (as such term is defined in our restricted share unit plan), with respect to all grants of restricted share units that are outstanding as of the date of such change in control, all unvested restricted share units will immediately vest and each participant who has received any such grants will be entitled to receive, on the date that is ten business days following the change in control date, an amount in full settlement of each restricted share unit covered by the grant. Such amount will be either one share of our common stock for each restricted share unit, or if so specified in a written election by the participant, a cash payment equal to the special value (as such term is defined in our restricted share unit plan) for each covered restricted share unit.
Transferability
The rights or interests of a participant under our restricted share unit plan will not be assignable or transferable, other than by will or the laws governing the devolution of property in the event of death and such rights or interests will not be encumbered.
Additional Provisions
Our board of directors has the authority to amend (subject to stockholder approval in some circumstances), suspend or terminate our restricted share unit plan in whole or in part from time to time.
Compensation of Directors
We pay our non-employee directors an annual cash fee of $50,000 for their service on our board of directors and its committees. We also pay the chairman of our board an additional annual fee of $50,000, the Chairman of our audit committee an additional annual fee of $25,000, and the chairmen of our other standing committees of the board of directors an additional annual fee of $5,000 each. In addition, each non-employee director is entitled to annual restricted share grant equal to the greater of (1) 7,500 and (2) $50,000 divided by the closing price of our common stock on the NASDAQ Global Market on the date of grant. Board members receive cash compensation in U.S. dollars. We also reimburse our directors for travel and other necessary business expenses incurred in the performance of their services for us.
Fiscal Year 2014 Director Compensation
The following table sets forth compensation information for our non-employee directors for the year ended December 31, 2014. The table excludes Dr. Kirkman who did not receive any compensation from us in his role as director in the year ended December 31, 2014. All compensation numbers are expressed in U.S. dollars.
| Name |
Fees Earned or Paid in Cash ($) |
Stock Awards ($) (1)(2)(3) |
Total ($) | |||||||||
| Christopher Henney |
$ | 105,000 | $ | 50,000 | $ | 155,000 | ||||||
| Daniel Spiegelman |
75,000 | 50,000 | 125,000 | |||||||||
| Richard Jackson |
55,000 | 50,000 | 105,000 | |||||||||
| Ted W. Love |
50,000 | 50,000 | 100,000 | |||||||||
| W. Vickery Stoughton |
50,000 | 50,000 | 100,000 | |||||||||
| (1) | These amounts represent the aggregate grant date fair value of RSUs granted in 2014. |
| (2) | As of December 31, 2014, our non-employee directors held RSUs and outstanding options to purchase the number of shares of common stock as follows: Dr. Henney (zero options, 32,381 RSUs); Dr. Jackson (zero options, 32,381 RSUs); Mr. Stoughton (zero options, 32,381 RSUs); Mr. Spiegelman (zero options, 32,381 RSUs); Dr. Love (zero options, 33,680 RSUs). |
| (3) | Each RSU may be converted into one share of our common stock at the end of the grant period, which is two years for each of the RSUs granted. |
66
Table of Contents
| ITEM 12. | Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters |
Equity Compensation Plan Information as of December 31, 2014
The following table sets forth the securities authorized for issuance under Oncothyreon’s equity compensation plans.
| Plan Category |
(A) Number of Securities to be Issued Upon Exercise of Outstanding Options, Warrants and Rights |
(B) Weighted Average Exercise Price of Outstanding Options, Warrants and Rights |
(C) Number of Securities Remaining Available for Future Issuance Under Equity Compensation Plans (Excluding Securities Reflected in Column (A))(1) |
|||||||||
| Equity compensation plans approved by security holders: |
||||||||||||
| Share option plan ($Cdn.)(2) |
172,535 | $ | 7.80 | — | ||||||||
| Share option plan ($U.S.)(2) |
5,045,000 | $ | 2.97 | 1,825,858 | ||||||||
| RSU plan |
163,204 | N.A. | 530,910 | |||||||||
| Equity compensation plans not approved by security holders |
— | N.A. | — | |||||||||
| Total |
5,380,739 | N.A. | 2,356,768 | |||||||||
| (1) | All of these are available for issuance under the respective plans pursuant to the grant of restricted stock, restricted share units and other equity awards, as well as for grants of stock options and stock appreciation rights. |
| (2) | Under the terms of the share option plan, the total number of shares issuable pursuant to options under the plan is 10% of the issued and outstanding shares. Shares issued upon the exercise of options do not reduce the percentage of shares which may be issuable pursuant to options under the share option plan. |
For more information regarding our share option plan and amended and restated restricted share unit plan, see Note 7 to our consolidated financial statements included elsewhere in this Annual Report on Form 10-K.
Security Ownership of Certain Beneficial Owners and Management
The following table sets forth certain information regarding beneficial ownership of our capital stock as of February 28, 2015 by (i) each person known by us to be the beneficial owner of more than 5% of any class of our voting securities, (ii) each of our directors, (iii) each of our executive officers and (iv) our directors and executive officers as a group, including shares they had the right to acquire within 60 days after February 28, 2015. Beneficial ownership excludes stockholders of Series A convertible preferred stock which is convertible into 1,000 shares of the Company’s common stock at any time at the holder’s option.
| Common Stock Beneficially Owned | ||||||||
| Name of Beneficial Owner(1) |
Number of Shares(2) | Percent of Class(3) | ||||||
| 5% Stockholders: |
||||||||
| BVF, Inc.(4) |
18,762,260 | 16.9 | % | |||||
| BlackRock, Inc(5) |
5,665,289 | 5.3 | % | |||||
| Directors and Named Executive Officers: |
||||||||
| Christopher Henney(6) |
123,776 | * | ||||||
| Richard Jackson(7) |
47,526 | * | ||||||
| Steven P. James |
— | — | ||||||
| Ted W. Love(8) |
— | — | ||||||
| W. Vickery Stoughton(9) |
46,692 | * | ||||||
| Daniel Spiegelman(10) |
20,644 | * | ||||||
| Robert Kirkman(11) |
932,536 | * | ||||||
| Gary Christianson(12) |
352,962 | * | ||||||
| Julia Eastland(13) |
230,850 | * | ||||||
67
Table of Contents
| Common Stock Beneficially Owned | ||||||||
| Name of Beneficial Owner(1) |
Number of Shares(2) | Percent of Class(3) | ||||||
| Diana Hausman(14) |
250,834 | * | ||||||
| Scott Peterson(15) |
256,258 | * | ||||||
| All directors and executive officers as a group (12 persons)(16) |
7,559,345 | 6.9 | % | |||||
| * | Represents less than 1% of class or combined classes. |
| (1) | Except as otherwise indicated, the address of each stockholder identified is c/o Oncothyreon Inc., 2601 Fourth Avenue, Suite 500, Seattle, Washington 98121. Except as indicated in the other footnotes to this table, each person named in this table has sole voting and investment power with respect to all shares of stock beneficially owned by that person. |
| (2) | Options, RSUs and warrants exercisable within 60 days after February 28, 2015 are deemed outstanding for the purposes of computing the percentage of shares owned by that person, but are not deemed outstanding for purposes of computing the percentage of shares owned by any other person. |
| (3) | Based on 106,301,012 shares of common stock issued and outstanding as of February 28, 2015. |
| (4) | Based on information of beneficial ownership as of December 31, 2014, included in a Schedule13G/A filed with the SEC on February 17, 2015. Includes 13,762,260 shares of common stock and 5,000,000 warrants exercisable within 60 days after February 28, 2015 and are beneficially owned by BVF Inc. and various affiliated entities and one individual. The address of BVF Inc. is 900 North Michigan Avenue, Suite 1100, Chicago, Ill 60611. |
| (5) | Based on information of beneficial ownership as of December 31, 2014, included in a Schedule 13G/A filed with the SEC on February 2, 2015. Includes shares of common stock beneficially owned by BlackRock, Inc. and various affiliated entities. The address of BlackRock, Inc. is 55 East 52nd Street, New York, New York 10022. |
| (6) | Shares attributable to restricted stock units owned by Dr. Henney are not reflected as none of the shares underlying the RSUs have vested or will vest within 60 days after February 28, 2015. |
| (7) | Shares attributable to restricted stock units owned by Dr. Jackson are not reflected as none of the shares underlying the RSUs have vested or will vest within 60 days after February 28, 2015. |
| (8) | Shares attributable to restricted stock units owned Dr. Love are not reflected as none of the shares underlying the RSUs have vested or will vest within 60 days after February 28, 2015. |
| (9) | Shares attributable to restricted stock units owned by Mr. Stoughton are not reflected as none of the shares underlying the RSUs have vested or will vest within 60 days after February 28, 2015. |
| (10) | Shares attributable to restricted stock units owned by Mr. Spiegelman are not reflected as none of the shares underlying the RSUs have vested or will vest within 60 days after February 28, 2015. |
| (11) | Includes 924,203 shares of common stock that Dr. Kirkman has the right to acquire under outstanding options exercisable within 60 days after February 28, 2015. |
| (12) | Includes 332,500 shares of common stock that Mr. Christianson has the right to acquire under outstanding options exercisable within 60 days after February 28, 2015. |
| (13) | Includes 210,834 shares of common stock that Ms. Eastland has the right to acquire under outstanding options exercisable within 60 days after February 28, 2015. |
| (14) | Includes 250,834 shares of common stock that Dr. Hausman has the right to acquire under outstanding options exercisable within 60 days after February 28, 2015. |
| (15) | Includes 245,834 shares of common stock that Dr. Peterson has the right to acquire under outstanding options exercisable within 60 days after February 28, 2015. |
| (16) | Includes 2,921,256 shares of common stock issuable upon exercise of options and warrants exercisable within 60 days after February 28, 2015 and zero shares of common stock that would be fully vested and issuable upon the vesting of RSUs within 60 days after February 28, 2015. |
| ITEM 13. | Certain Relationships and Related Transactions and Director Independence |
Certain Relationships and Related Transactions
We have entered into the arrangements which are described where required under the heading titled “Part III — Item 11 — Executive Compensation — “Termination and Change of Control Provisions under Offer Letters” and “Part III — Item 11 — Executive Compensation — Potential Payments Upon Termination or Change in Control” included elsewhere in this Annual Report on Form 10-K.
One of our executive officers, Dr. Jay Venkatesan, was a stockholder in Alpine Biosciences, Inc., which we acquired through a merger in August 2014. Pursuant to the acquisition, Dr. Venkatesan received 3,304,633 shares of our common stock and Andaman Orphan Therapies, LLC, an entity controlled by Dr. Venkatesan, received 230,557 shares of our common stock. An additional 472,090 common shares and 32,936 common shares are held in escrow in the accounts of
68
Table of Contents
Dr. Venkatesan and of Andaman Orphan Therapies, LLC, respectively, and are subject to future release subject to the merger agreement. In connection with the Alpine acquisition, we also entered into a piggy back registration rights agreement with Dr. Venkatesan in which we agreed to provide certain registration rights to Dr. Venkatesan with respect to the Company’s common shares he acquired in the acquisition in the event we file a registration statement after February 4, 2015.
Approval of Related Party Transactions
We have adopted a formal, written policy that our executive officers, directors (including director nominees), holders of more than 5% of any class of our voting securities, or any member of the immediate family of or any entities affiliated with any of the foregoing persons, are not permitted to enter into a related party transaction with us without the prior approval or, in the case of pending or ongoing related party transactions, ratification of our audit committee. For purposes of our policy, a related party transaction is a transaction, arrangement or relationship where the company was, is or will be involved and in which a related party had, has or will have a direct or indirect material interest. Certain transactions with related parties, however, are excluded from the definition of a related party transaction including, but not limited to (1) transactions involving the purchase or sale of products or services in the ordinary course of business, not exceeding $120,000, (2) transactions where a related party’s interest derives solely from his or her service as a director of another entity that is a party to the transaction, (3) transactions where a related party’s interest derives solely from his or her ownership of less than 10% of the equity interest in another entity that is a party to the transaction, and (4) transactions where a related party’s interest derives solely from his or her ownership of a class of our equity securities and all holders of that class received the same benefit on a pro rata basis. No member of the audit committee may participate in any review, consideration or approval of any related party transaction where such member or any of his or her immediate family members is the related party. In approving or rejecting the proposed agreement, our audit committee shall consider the relevant facts and circumstances available and deemed relevant to the audit committee, including, but not limited to (1) the benefits and perceived benefits to the company, (2) the materiality and character of the related party’s direct and indirect interest, (3) the availability of other sources for comparable products or services, (4) the terms of the transaction, and (5) the terms available to unrelated third parties under the same or similar circumstances. In reviewing proposed related party transactions, the audit committee will only approve or ratify related party transactions that are in, or not inconsistent with, the best interests of the company and our stockholders.
Determinations Regarding Director Independence
The board of directors has determined that each of our current directors, except Dr. Kirkman, is an “independent director” as that term is defined by the applicable rules and regulations of The NASDAQ Stock Market. The independent directors generally meet in executive session at each quarterly board of directors meeting.
The board of directors has also determined that each member of the audit committee, the compensation committee, and the corporate governance and nominating committee meets the independence standards applicable to those committees prescribed by the applicable rules and regulations of The NASDAQ Stock Market, the SEC, and the Internal Revenue Service.
Finally, the board of directors has determined that Mr. Spiegelman, the chairman of the audit committee, is an “audit committee financial expert” as that term is defined in Item 407(d)(5) of Regulation S-K promulgated by the SEC.
| ITEM 14. | Principal Accountant Fees and Services |
Audit Fees
Fees and related expenses for the 2014 audit by Ernst & Young LLP of our annual financial statements, its review of the financial statements included in our 2014 quarterly reports and other services, which include comfort letters, consents and accounting consultations that are provided in connection with statutory and regulatory filings totaled $594,935. Fees and related expenses for 2013 totaled $464,000.
Audit-Related Fees
None.
Tax Fees
For the years 2014 and 2013, Ernst & Young LLP billed us $21,000 and $99,939, respectively, for professional services related to preparation of our tax return and tax consultations on tax related matters.
69
Table of Contents
All Other Fees
Ernst & Young LLP billed us $1,995 for each of the years 2014 and 2013 for a subscription to their technical accounting database.
Policy on Audit Committee Pre Approval of Fees
In its pre-approval policy, the audit committee has authorized our chief executive officer or our chief financial officer to engage the services of Ernst & Young LLP with respect to the following:
| • | audit related services that are outside the scope of our annual audit and generally are (1) required on a project, recurring, or on a one-time basis, (2) requested by one of our business partners (for example, a review or audit of royalty payments), or (3) needed by us to assess the impact of a proposed accounting standard; |
| • | audits of the annual statutory financial statements required by the non-U.S. governmental agencies for our overseas subsidiaries; |
| • | accounting services related to potential or actual acquisitions or investment transactions that if consummated would be reflected in our financial results or tax returns (this does not include any due diligence engagements, which must be pre-approved by the audit committee separately); and |
| • | other accounting and tax services, such as routine consultations on accounting and/or tax treatments for contemplated transactions. |
Notwithstanding this delegation of authorization, the audit committee pre-approves all audit and non-audit related services performed by Ernst & Young LLP. On an annual basis prior to the completion of the audit, the audit committee will review a listing prepared by management of all proposed non-audit services to be performed by the external auditor for the upcoming fiscal year, such listing to include scope of activity and estimated budget amount. The audit committee, if satisfied with the appropriateness of the services, will provide pre-approval of such services. If non-audit services are required subsequent to the annual pre-approval of services, management will seek approval of such services at the next regularly scheduled audit committee meeting. If such services are required prior to the next audit committee meeting, management will confer with the audit committee chairman regarding either conditional approval subject to full audit committee ratification or the necessity to reconvene a meeting. The audit committee has considered the non-audit services provided to us by our independent registered public accountants and has determined that the provision of such services is compatible with their independence.
All audit-related, tax and other fees were approved by the audit committee.
PART IV
| ITEM 15. | Exhibits and Financial Statement Schedules |
| (a) | The following documents are filed as part of this Annual Report on Form 10-K: |
1. Financial Statements:
Our consolidated financial statements are contained in Item 8 of this annual report on Form 10-K.
2. Financial Statement Schedules:
All financial statement schedules have been omitted because the required information is either included in the financial statements or notes thereto, or is not applicable.
3. Exhibits:
The exhibits required by Item 601 of Regulation S-K are listed in paragraph (b) below.
70
Table of Contents
| (b) | Exhibits: |
The following exhibits are filed herewith or are incorporated by reference to exhibits previously filed with the SEC:
| Incorporated by Reference | ||||||||||
| Exhibit |
Exhibit Description |
Form |
Exhibit |
Filing Date |
Filed/ Furnished | |||||
| 2.1(a) | Agreement and Plan of Reorganization among ProlX Pharmaceuticals Corporation, D. Lynn Kirkpatrick, Garth Powis and Biomira Inc., dated October 30, 2006. | S-4/A | 2.1 | October 29, 2007 | ||||||
| 2.1(b) | Amendment No. 1 to Agreement and Plan of Reorganization dated November 7, 2007. | 10-K | 2.1(b) | May 6, 2010 | ||||||
| 2.1(c) | Agreement and Plan of Reorganization, dated August 8, 2014, among Oncothyreon Inc., AB Acquisition (DE) Corp., Alpine Biosciences, Inc. and Mitchell H. Gold, M.D., as Stockholders’ Agent | 8-K | 2.1 | August 11, 2014 | ||||||
| 3.1 | Amended and Restated Certificate of Incorporation of Oncothyreon Inc. | S-4/A | 3.1 | September 27, 2007 | ||||||
| 3.2 | Certificate of Amendment to the Amended and Restated Certificate of Incorporation of Oncothyreon Inc. | 8-K | 3.1 | June 10, 2014 | ||||||
| 3.3 | Certificate of Designation of Preferences, Rights and Limitations of Series A Convertible Preferred Stock | 8-K | 3.1 | September 23, 2014 | ||||||
| 3.4 | Certificate of Designation of Preferences, Rights and Limitations of Series B Convertible Preferred Stock | 8-K | 3.1 | February 11, 2014 | ||||||
| 3.5 | Bylaws of Oncothyreon Inc. | 10-Q | 3.1 | August 14, 2009 | ||||||
| 4.1 | Form of registrant’s common stock certificate. | S-4/A | 4.1 | September 27, 2007 | ||||||
| 4.2 | Form of Series A Convertible Preferred Stock Certificate. | 8-K | 4.1 | September 23, 2014 | ||||||
| 4.3 | Form of Series B Convertible Preferred Stock Certificate. | 8-K | 4.1 | February 1, 2014 | ||||||
| 4.4 | Form of Warrant issued pursuant to the terms of the Securities Purchase Agreement, dated September 23, 2010, by and among Oncothyreon Inc. and the signatories thereto, as amended. | S-1 | 10.49 | October 4, 2010 | ||||||
| 4.5 | Form of Warrant to Purchase Common Stock issued by Oncothyreon Inc. to the Lenders pursuant to the terms of the Loan and Security Agreement. | 8-K | 10.3 | February 9, 2011 | ||||||
| 4.6 | Form of Warrant issued by Oncothyreon Inc. to BVF Partners L.P. and certain of its affiliates. | 8-K | 4.1 | May 30, 2013 | ||||||
| 4.7 | Piggyback Registration Rights Agreement, dated August 8, 2014, by and between Oncothyreon and each of Jay Venkatesan and Mitchell H. Gold | 10-Q | 4.2 | November 6, 2014 | ||||||
| 10.1* | Amended and Restated Share Option Plan. | X | ||||||||
| 10.2* | Form of Stock Option Agreement under the Amended and Restated Share Option Plan. | X | ||||||||
| 10.3* | Amended and Restated Restricted Share Unit Plan. | S-8 | 99.2 | June 6, 2014 | ||||||
| 10.4* | Form of Restricted Share Unit Agreement under the Amended and Restated Restricted Share Unit Plan. | 10-K | 10.4 | March 9, 2012 | ||||||
71
Table of Contents
| Incorporated by Reference | ||||||||||
| Exhibit |
Exhibit Description |
Form |
Exhibit |
Filing Date |
Filed/ Furnished | |||||
| 10.5* | 2010 Employee Stock Purchase Plan. | 8-K | 10.1 | June 8, 2010 | ||||||
| 10.6* | Form of Subscription Agreement and Notice of Withdrawal under the 2010 Employee Stock Purchase Plan. | 8-K | 10.2 | June 8, 2010 | ||||||
| 10.7* | Form of Indemnification Agreement. | S-4/A | 10.1 | September 27, 2007 | ||||||
| 10.8* | Offer letter with Robert Kirkman, dated August 29, 2006. | S-4 | 10.27 | September 12, 2007 | ||||||
| 10.8(a)* | Amendment to Robert Kirkman Offer Letter dated December 31, 2008. | 10-K | 10.18(a) | March 30, 2009 | ||||||
| 10.8(b)* | Amendment to Robert Kirkman Offer Letter dated December 3, 2009. | 8-K | 10.1 | December 7, 2009 | ||||||
| 10.9* | Offer Letter with Gary Christianson, dated June 29, 2007. | 10-Q | 10.1 | November 10, 2008 | ||||||
| 10.9(a)* | Amendment to Gary Christianson Offer Letter dated December 31, 2008. | 10-K | 10.40(a) | March 30, 2009 | ||||||
| 10.9(b)* | Amendment to Gary Christianson Offer Letter dated December 3, 2009. | 8-K | 10.2 | December 7, 2009 | ||||||
| 10.10* | Offer Letter dated June 9, 2009 between Oncothyreon Inc. and Scott Peterson, Ph.D. | 8-K | 10.2 | June 15, 2009 | ||||||
| 10.10(a)* | Amendment to Scott Peterson Offer Letter dated December 3, 2009. | 8-K | 10.4 | December 7, 2009 | ||||||
| 10.10(b)* | Amendment to Scott Peterson Offer Letter dated January 8, 2015. | 8-K | 10.1 | January 8, 2015 | ||||||
| 10.11* | Offer Letter dated July 6, 2009 between Oncothyreon Inc. and Diana Hausman, M.D. | 8-K | 10.1 | August 4, 2009 | ||||||
| 10.11(a)* | Amendment to Diana Hausman Offer Letter dated December 3, 2009. | 8-K | 10.3 | December 7, 2009 | ||||||
| 10.12* | Offer letter with dated August 17, 2010 between Oncothyreon Inc. and Julia M. Eastland | 8-K | 10.1 | August 31, 2010 | ||||||
| 10.13* | Offer letter effective August 8, 2014 between Oncothyreon Inc. and Jay Venkatesan | 10-Q | 10.2 | November 6, 2014 | ||||||
| 10.14 | Lease Agreement between Selig Holdings Company and Oncothyreon Inc., dated May 9, 2008. | 10-Q | 10.3 | November 10, 2008 | ||||||
| 10.15† | License Agreement between Biomira Inc. and the Dana-Farber Cancer Institute, Inc., dated November 22, 1996. | S-4 | 10.6 | September 12, 2007 | ||||||
| 10.16† | Amended and Restated License Agreement between Imperial Cancer Research Technology Limited and Biomira Inc., dated November 14, 2000. | S-4/A | 10.11 | September 27, 2007 | ||||||
| 10.17 | Consent and Acknowledgement among Biomira Inc., Biomira International Inc., Biomira Europe B.V., Imperial Cancer Research Technology Limited and Merck KGaA, dated February 5, 2002. | S-4 | 10.13 | September 12, 2007 | ||||||
| 10.18† | License Agreement between the Governors of the University of Alberta and Biomira Inc., dated December 1, 2001. | S-4/A | 10.14 | September 27, 2007 | ||||||
72
Table of Contents
| Incorporated by Reference | ||||||||||
| Exhibit |
Exhibit Description |
Form |
Exhibit |
Filing Date |
Filed/ Furnished | |||||
| 10.19† | Amended and Restated License Agreement between Biomira Management, Inc. and Merck KGaA, dated December 18, 2008. | 10-Q | 10.1 | May 15, 2009 | ||||||
| 10.20 | Common Stock Purchase Agreement by and among Biomira Inc., Biomira International Inc. and Merck KGaA dated May 2, 2001. | 10-K | 10.41 | May 6, 2010 | ||||||
| 10.21 | Tax Indemnity Agreement by and between Biomira International Inc. and Merck KGaA dated May 3, 2001. | 10-K | 10.42 | May 6, 2010 | ||||||
| 10.22 | Letter Amendment, dated April 30, 2013, to Amended and Restated License Agreement between Merck KGaA and Oncothyreon Inc. | 10-Q | 10.2 | August 6, 2013 | ||||||
| 10.23 | Form of Subscription Agreement between BVF Partners L.P. and certain of its affiliates and Oncothyreon Inc. | 8-K | 10-1 | May 30, 2013 | ||||||
| 10.24† | Phase 1 Co-Development Agreement, dated May 28, 2014, between Celldex Therapeutics, Inc. and Oncothyreon | 10-Q | 10.1 | August 15, 2014 | ||||||
| 10.25† | Patent License Agreement, effective June 30, 2014, between STC.UNM and Alpine Biosciences, Inc. | 10-Q | 10.1 | November 6, 2014 | ||||||
| 10.26†† | License Agreement, dated December 11, 2014, between Oncothyreon and Array BioPharma Inc. | X | ||||||||
| 21.1 | Subsidiaries of Oncothyreon Inc. | X | ||||||||
| 23.1 | Consent of Independent Registered Public Accounting Firm. | X | ||||||||
| 24.1 | Power of Attorney (included on signature page). | X | ||||||||
| 31.1 | Certification of Robert L. Kirkman, M.D., President and Chief Executive Officer, pursuant to Exchange Act Rules 13a-14(a) and 15d-14(a), as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. | X | ||||||||
| 31.2 | Certification of Julia Eastland, Chief Financial Officer, pursuant to Exchange Act Rules 13a-14(a) and 15d-14(a), as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. | X | ||||||||
| 32.1 | Certification of Robert L. Kirkman, M.D., President and Chief Executive Officer, pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002(1). | X | ||||||||
| 32.2 | Certification of Julia Eastland, Chief Financial Officer, pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002(1). | X | ||||||||
| 101.INS | XBRL Instance Document. | X | ||||||||
| 101.SCH | XBRL Taxonomy Extension Schema Document. | X | ||||||||
| 101.CAL | XBRL Taxonomy Extension Calculation Linkbase Document. | X | ||||||||
| 101.DEF | XBRL Taxonomy Extension Definition Linkbase Document. | X | ||||||||
73
Table of Contents
| Incorporated by Reference | ||||||||||
| Exhibit |
Exhibit Description |
Form |
Exhibit |
Filing Date |
Filed/ Furnished | |||||
| 101.LAB | XBRL Taxonomy Extension Labels Linkbase Document. | X | ||||||||
| 101.PRE | XBRL Taxonomy Extension Presentation Linkbase Document. | X | ||||||||
| (1) | This certification is deemed not filed for purposes of section 18 of the Securities Exchange Act of 1934, as amended, or Exchange Act, or otherwise subject to the liability of that section, nor shall it be deemed incorporated by reference into any filing under the Securities Act of 1933, as amended, or Securities Act or the Exchange Act. |
| * | Executive Compensation Plan or Agreement. |
| † | Confidential treatment has been granted for portions of this exhibit. |
| †† | Confidential treatment has been requested for portions of this exhibit pursuant to Rule 24b-2 under the Exchange Act. The omitted portions of this exhibit have been filed separately with the SEC. |
74
Table of Contents
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this Report to be signed on its behalf by the undersigned, thereunto duly authorized in the City of Seattle, County of King, State of Washington on March 10, 2015.
| ONCOTHYREON INC. | ||
| By: | /s/ Robert L. Kirkman, M.D. | |
| Robert L. Kirkman, M.D. | ||
| President, CEO and Director (Principal Executive Officer) | ||
POWER OF ATTORNEY
Each person whose signature appears below hereby constitutes and appoints Robert L. Kirkman and Julia M. Eastland and each of them, his or her true and lawful attorneys-in-fact and agents, with full power to act without the other and with full power of substitution and resubstitution, to execute in his or her name and on his or her behalf, individually and in each capacity stated below, any and all amendments and supplements to this Report, and any and all other instruments necessary or incidental in connection herewith, and to file the same with the Commission.
Pursuant to the requirements of the Securities Exchange Act of 1934, this Report has been signed below by the following persons on behalf of the Registrant and in the capacities and on the dates indicated.
| Signature |
Title |
Date | ||
| /s/ Robert L. Kirkman, M.D. |
President, Chief Executive Officer and Director (Principal Executive Officer) | March 10, 2015 | ||
| Robert L. Kirkman, M.D. | ||||
| /s/ Julia M. Eastland Julia M. Eastland |
Chief Financial Officer, Secretary and Vice President of Corporate Development (Principal Financial and Accounting Officer) |
March 10, 2015 | ||
| /s/ Christopher S. Henney, Ph.D. Christopher S. Henney, Ph.D. |
Chairman and Director | March 10, 2015 | ||
| /s/ Richard L. Jackson, Ph.D. Richard L. Jackson, Ph.D. |
Director | March 10, 2015 | ||
| /s/ Steven P. James Steven P. James |
Director | March 10, 2015 | ||
| /s/ Ted W. Love, M.D. Ted W. Love, M.D. |
Director | March 10, 2015 | ||
| /s/ Daniel K. Spiegelman Daniel K. Spiegelman |
Director | March 10, 2015 | ||
| /s/ W. Vickery Stoughton W. Vickery Stoughton |
Director | March 10, 2015 | ||
75
Table of Contents
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
F-1
Table of Contents
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders of
Oncothyreon Inc.
We have audited the accompanying consolidated balance sheets of Oncothyreon Inc. as of December 31, 2014 and 2013, and the related consolidated statements of operations, comprehensive loss, stockholders’ equity, and cash flows for each of the three years in the period ended December 31, 2014. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the consolidated financial position of Oncothyreon Inc. at December 31, 2014 and 2013, and the consolidated results of its operations and its cash flows for each of the three years in the period ended December 31, 2014, in conformity with U.S. generally accepted accounting principles.
We have also audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), Oncothyreon Inc.’s internal control over financial reporting as of December 31, 2014, based on criteria established in Internal Control — Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 framework) and our report dated March 10, 2015 expressed an unqualified opinion thereon.
/s/ Ernst & Young LLP
Seattle, Washington
March 10, 2015
F-2
Table of Contents
Consolidated Balance Sheets
(In thousands, except share and per share amounts)
| As of December 31, | ||||||||
| 2014 | 2013 | |||||||
| ASSETS | ||||||||
| Current: |
||||||||
| Cash and cash equivalents |
$ | 10,454 | $ | 9,279 | ||||
| Short-term investments |
47,217 | 50,748 | ||||||
| Accounts and other receivables |
298 | 197 | ||||||
| Prepaid and other current assets |
888 | 720 | ||||||
|
|
|
|
|
|||||
| Total current assets |
58,857 | 60,944 | ||||||
| Long-term investments |
6,043 | 12,535 | ||||||
| Property and equipment, net |
1,576 | 1,695 | ||||||
| Indefinite-lived intangible assets |
19,738 | — | ||||||
| Goodwill |
16,659 | 2,117 | ||||||
| Other assets |
538 | 455 | ||||||
|
|
|
|
|
|||||
| Total assets |
$ | 103,411 | $ | 77,746 | ||||
|
|
|
|
|
|||||
| LIABILITIES AND STOCKHOLDERS’ EQUITY | ||||||||
| Current |
||||||||
| Accounts payable |
$ | 689 | $ | 533 | ||||
| Accrued and other liabilities |
2,129 | 2,622 | ||||||
| Accrued compensation and related liabilities |
1,614 | 1,311 | ||||||
| Current portion of restricted share unit liability |
155 | 194 | ||||||
| Current portion of warrant liability |
128 | — | ||||||
|
|
|
|
|
|||||
| Total current liabilities |
4,715 | 4,660 | ||||||
| Deferred rent |
337 | 439 | ||||||
| Restricted share unit liability |
155 | 143 | ||||||
| Warrant liability |
— | 924 | ||||||
| Deferred tax liability |
6,908 | — | ||||||
| Class UA preferred stock, 12,500 shares authorized, 12,500 shares issued and outstanding |
30 | 30 | ||||||
| Commitments and contingencies |
||||||||
| Stockholders’ equity: |
||||||||
| Preferred stock, $0.0001 par value; 10,000,000 shares authorized as of December 31, 2014 and 2013; Series A Convertible Preferred Stock – 10,000 shares and zero shares issued and outstanding as of December 31, 2014 and 2013, respectively |
— | — | ||||||
| Common stock, $0.0001 par value; 200,000,000 shares and 100,000,000 shares authorized as of December 31, 2014 and 2013, respectively; 91,601,352 shares and 70,673,143 shares issued and outstanding as of December 31, 2014 and 2013, respectively |
353,856 | 353,854 | ||||||
| Additional paid-in capital |
224,549 | 154,832 | ||||||
| Accumulated deficit |
(482,048 | ) | (432,085 | ) | ||||
| Accumulated other comprehensive loss |
(5,091 | ) | (5,051 | ) | ||||
|
|
|
|
|
|||||
| Total stockholders’ equity |
91,266 | 71,550 | ||||||
|
|
|
|
|
|||||
| Total liabilities and stockholders’ equity |
$ | 103,411 | $ | 77,746 | ||||
|
|
|
|
|
|||||
See accompanying notes to the consolidated financial statements
F-3
Table of Contents
Consolidated Statements of Operations
(In thousands, except share and per share amounts)
| Years Ended December 31, | ||||||||||||
| 2014 | 2013 | 2012 | ||||||||||
| Operating expenses |
||||||||||||
| Research and development |
$ | 41,884 | $ | 33,221 | $ | 22,001 | ||||||
| General and administrative |
8,951 | 8,002 | 6,498 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total operating expenses |
50,835 | 41,223 | 28,499 | |||||||||
|
|
|
|
|
|
|
|||||||
| Loss from operations |
(50,835 | ) | (41,223 | ) | (28,499 | ) | ||||||
| Other income (expense) |
||||||||||||
| Investment and other income (expense), net |
76 | 137 | (127 | ) | ||||||||
| Interest expense |
— | — | (309 | ) | ||||||||
| Change in fair value of warrant liability |
796 | 2,327 | 25,520 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total other income (expense), net |
872 | 2,464 | 25,084 | |||||||||
| Loss before income taxes |
(49,963 | ) | (38,759 | ) | (3,415 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Net loss |
$ | (49,963 | ) | $ | (38,759 | ) | $ | (3,415 | ) | |||
|
|
|
|
|
|
|
|||||||
| Loss per share — basic |
$ | (0.64 | ) | $ | (0.62 | ) | $ | (0.06 | ) | |||
|
|
|
|
|
|
|
|||||||
| Loss per share — diluted (Note 6) |
$ | (0.64 | ) | $ | (0.62 | ) | $ | (0.53 | ) | |||
|
|
|
|
|
|
|
|||||||
| Shares used to compute basic loss per share |
77,619,807 | 62,387,616 | 53,728,672 | |||||||||
|
|
|
|
|
|
|
|||||||
| Shares used to compute diluted loss per share |
77,619,807 | 62,387,616 | 54,899,955 | |||||||||
|
|
|
|
|
|
|
|||||||
See accompanying notes to the consolidated financial statements
F-4
Table of Contents
Consolidated Statements of Comprehensive Loss
(In thousands)
| Years Ended December 31, | ||||||||||||
| 2014 | 2013 | 2012 | ||||||||||
| Net loss |
$ | (49,963 | ) | $ | (38,759 | ) | $ | (3,415 | ) | |||
| Other comprehensive income (loss): |
||||||||||||
| Available-for-sale securities: |
||||||||||||
| Unrealized gains (loss) during the period, net |
(34 | ) | (15 | ) | 9 | |||||||
| Reclassification adjustment |
(6 | ) | — | (1 | ) | |||||||
|
|
|
|
|
|
|
|||||||
| Other comprehensive income (loss) |
(40 | ) | (15 | ) | 8 | |||||||
|
|
|
|
|
|
|
|||||||
| Comprehensive loss |
$ | (50,003 | ) | $ | (38,774 | ) | $ | (3,407 | ) | |||
|
|
|
|
|
|
|
|||||||
See accompanying notes to the consolidated financial statements
F-5
Table of Contents
Consolidated Statements of Stockholders’ Equity
(In thousands, except share amounts)
| Common Stock | Preferred Stock | Additional Paid-in Capital |
Accumulated Deficit |
Accumulated Other Comprehensive Loss |
Stockholders’ Equity |
|||||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | |||||||||||||||||||||||||||||
| Balance at December 31, 2011 |
43,613,107 | $ | 353,851 | — | $ | — | $ | 74,537 | $ | (389,911 | ) | $ | (5,044 | ) | $ | 33,433 | ||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Net loss |
— | — | — | — | — | (3,415 | ) | — | (3,415 | ) | ||||||||||||||||||||||
| Unrealized gains on available-for-sale securities |
— | — | — | — | — | — | 8 | 8 | ||||||||||||||||||||||||
| Common stock issued, net of offering costs of $3.8 million |
13,512,500 | 2 | — | — | 50,283 | — | — | 50,285 | ||||||||||||||||||||||||
| Issuances under employee stock purchase plan |
55,424 | — | — | — | 182 | — | — | 182 | ||||||||||||||||||||||||
| Restricted stock units converted |
32,551 | — | — | — | 231 | — | — | 231 | ||||||||||||||||||||||||
| Share-based compensation expense |
— | — | — | — | 1,590 | — | — | 1,590 | ||||||||||||||||||||||||
| Stock options exercised |
2,655 | — | — | — | 9 | — | — | 9 | ||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Balance at December 31, 2012 |
57,216,237 | 353,853 | — | — | 126,832 | (393,326 | ) | (5,036 | ) | 82,323 | ||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Net loss |
— | — | — | — | — | (38,759 | ) | — | (38,759 | ) | ||||||||||||||||||||||
| Unrealized losses on available-for-sale securities |
— | — | — | — | — | — | (15 | ) | (15 | ) | ||||||||||||||||||||||
| Common stock issued, net of offering costs of $0.6 million |
13,346,901 | 1 | — | — | 25,955 | — | — | 25,956 | ||||||||||||||||||||||||
| Issuances under employee stock purchase plan |
74,829 | — | — | — | 113 | — | — | 113 | ||||||||||||||||||||||||
| Restricted stock units converted |
35,176 | — | — | — | 66 | — | — | 66 | ||||||||||||||||||||||||
| Share-based compensation expense |
— | — | — | — | 1,866 | — | — | 1,866 | ||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Balance at December 31, 2013 |
70,673,143 | 353,854 | — | — | 154,832 | (432,085 | ) | (5,051 | ) | 71,550 | ||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Net loss |
— | — | — | — | — | (49,963 | ) | — | (49,963 | ) | ||||||||||||||||||||||
| Unrealized losses on available-for-sale securities |
— | — | — | — | — | — | (40 | ) | (40 | ) | ||||||||||||||||||||||
| Common stock issued, net of offering costs of $1.4 million |
11,517,478 | 1 | — | — | 21,552 | — | — | 21,553 | ||||||||||||||||||||||||
| Series A Convertible Preferred Stock issued, net of offering costs of $1.4 million |
— | — | 10,000 | — | 18,693 | — | — | 18,693 | ||||||||||||||||||||||||
| Acquisition of Alpine Biosciences, Inc. (Alpine) |
9,245,344 | 1 | — | — | 27,232 | 27,233 | ||||||||||||||||||||||||||
| Issuances under employee stock purchase plan |
76,811 | — | — | — | 114 | — | — | 114 | ||||||||||||||||||||||||
| Restricted stock units converted |
82,576 | — | — | — | 287 | — | — | 287 | ||||||||||||||||||||||||
| Share-based compensation expense |
— | — | — | — | 1,832 | — | — | 1,832 | ||||||||||||||||||||||||
| Stock options exercised |
6,000 | — | — | 7 | 7 | |||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Balance at December 31, 2014 |
91,601,352 | $ | 353,856 | 10,000 | $ | — | $ | 224,549 | $ | (482,048 | ) | $ | (5,091 | ) | $ | 91,266 | ||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
See accompanying notes to the consolidated financial statements
F-6
Table of Contents
Consolidated Statements of Cash Flows
(In thousands)
| Years Ended December 31, | ||||||||||||
| 2014 | 2013 | 2012 | ||||||||||
| Cash flows from operating activities |
||||||||||||
| Net loss |
$ | (49,963 | ) | $ | (38,759 | ) | $ | (3,415 | ) | |||
| Adjustments to reconcile net loss to net cash used in operating activities: |
||||||||||||
| Depreciation and amortization |
512 | 489 | 520 | |||||||||
| Amortization of discount and deferred financing costs on notes payable |
— | — | 78 | |||||||||
| Amortization of premiums and accretion of discounts on securities |
533 | 651 | 835 | |||||||||
| Share-based compensation expense |
2,187 | 2,021 | 1,041 | |||||||||
| Change in fair value of warrant liability |
(796 | ) | (2,327 | ) | (25,520 | ) | ||||||
| Cash settled on conversion of restricted share units |
(96 | ) | (22 | ) | (39 | ) | ||||||
| Other |
(1 | ) | (45 | ) | 255 | |||||||
| Net changes in assets and liabilities: |
||||||||||||
| Accounts and other receivables |
(101 | ) | 126 | 11 | ||||||||
| Prepaid and other current assets |
(168 | ) | 120 | (302 | ) | |||||||
| Other long-term assets |
(83 | ) | 57 | (307 | ) | |||||||
| Accounts payable |
144 | (600 | ) | 674 | ||||||||
| Accrued and other liabilities |
(736 | ) | 1,770 | (435 | ) | |||||||
| Accrued compensation and related liabilities |
303 | 269 | 184 | |||||||||
| Deferred rent |
(102 | ) | (94 | ) | (83 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Net cash used in operating activities |
(48,367 | ) | (36,344 | ) | (26,503 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Cash flows from investing activities |
||||||||||||
| Purchases of investments |
(62,411 | ) | (70,775 | ) | (72,037 | ) | ||||||
| Redemption of investments |
71,861 | 68,315 | 64,518 | |||||||||
| Purchases of property and equipment |
(380 | ) | (252 | ) | (752 | ) | ||||||
| Cash assumed in connection with the acquisition of Alpine |
104 | — | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Net cash provided by (used in) investing activities |
9,174 | (2,712 | ) | (8,271 | ) | |||||||
|
|
|
|
|
|
|
|||||||
| Cash flows from financing activities |
||||||||||||
| Proceeds from issuance of common stock and warrants, net of issuance costs |
21,668 | 26,069 | 50,466 | |||||||||
| Proceeds from issuance of Series A convertible preferred stock, net of issuance cost |
18,693 | — | — | |||||||||
| Proceeds from stock options exercised |
7 | — | 9 | |||||||||
| Principal payment on notes payable |
— | — | (909 | ) | ||||||||
| Repayment on notes payable |
— | — | (4,135 | ) | ||||||||
|
|
|
|
|
|
|
|||||||
| Net cash provided by financing activities |
40,368 | 26,069 | 45,431 | |||||||||
|
|
|
|
|
|
|
|||||||
| Increase (decrease) in cash and cash equivalents |
1,175 | (12,987 | ) | 10,657 | ||||||||
| Cash and cash equivalents, beginning of year |
9,279 | 22,266 | 11,609 | |||||||||
|
|
|
|
|
|
|
|||||||
| Cash and cash equivalents, end of year |
$ | 10,454 | $ | 9,279 | $ | 22,266 | ||||||
|
|
|
|
|
|
|
|||||||
| Supplemental disclosure of cash flow information |
||||||||||||
| Interest paid |
$ | — | $ | — | $ | 276 | ||||||
|
|
|
|
|
|
|
|||||||
| Non-cash activities |
||||||||||||
| Issuance of common stock in connection with the acquisition of Alpine |
$ | 27,233 | $ | — | $ | — | ||||||
|
|
|
|
|
|
|
|||||||
See accompanying notes to the consolidated financial statements
F-7
Table of Contents
Notes to the Consolidated Financial Statements
| 1. | DESCRIPTION OF BUSINESS |
Oncothyreon Inc. (the “Company”) is a clinical-stage biopharmaceutical company incorporated in the State of Delaware on September 7, 2007. The Company is focused primarily on the development of therapeutic products for the treatment of cancer. The Company’s goal is to discover, develop and commercialize novel compounds that have the potential to improve the lives and outcomes of cancer patients. The Company’s operations are not subject to any seasonality or cyclicality factors.
| 2. | SIGNIFICANT ACCOUNTING POLICIES |
Basis of presentation
These consolidated financial statements have been prepared using accounting principles generally accepted in the United States of America (“U.S. GAAP”) and reflect the following significant accounting policies.
Basis of consolidation
The Company’s consolidated financial statements include the accounts of the company and its wholly-owned subsidiaries, including Protocell Therapeutics Inc., Oncothyreon Canada Inc., Biomira Management Inc., ProlX Pharmaceuticals Corporation, Biomira BV and Oncothyreon Luxembourg. All intercompany balances and transactions have been eliminated upon consolidation.
Accounting estimates
The preparation of financial statements in accordance with U.S. GAAP requires management to make complex and subjective judgments and estimates that affect the reported amounts of assets and liabilities, the disclosure of contingent assets and liabilities at the date of the financial statements, and the reported amounts of revenues and expenses during the reporting period. By their nature, these judgments are subject to an inherent degree of uncertainty and as a consequence actual results may differ from those estimates.
Cash and cash equivalents
Cash equivalents include short-term, highly liquid investments that are readily convertible to known amounts of cash with original maturities of 90 days or less at the time of purchase. At December 31, 2014, cash and cash equivalents was comprised of $6.4 million in cash, and $4.1 million in money market funds. As of December 31, 2013, cash and cash equivalents was comprised of $3.2 million in cash and $6.1 million in money market funds. The carrying value of cash equivalents approximates their fair value.
Investments
Investments are classified as available-for-sale securities and are carried at fair value with unrealized temporary holding gains and losses, where applicable, excluded from net income or loss and reported in other comprehensive income or loss and also as a net amount in accumulated other comprehensive income or loss until realized. Available-for-sale securities are written down to fair value through income whenever it is necessary to reflect an other-than-temporary impairment. The Company determined that the unrealized losses on its marketable securities as of December 31, 2014 were temporary in nature, and the Company currently does not intend to sell these securities before recovery of their amortized cost basis. All short-term investments are limited to a final maturity of less than one year from the reporting date. The Company’s long-term investments are investments with maturities exceeding 12 months but less than five years from the reporting date. The Company is exposed to credit risk on its cash equivalents, short-term investments and long-term investments in the event of non-performance by counterparties, but does not anticipate such non-performance and mitigates exposure to concentration of credit risk through the nature of its portfolio holdings. If a security falls out of compliance with the Company’s investment policy, it may be necessary to sell the security before its maturity date in order to bring the investment portfolio back into compliance. The cost basis of any securities sold is determined by specific identification. The fair value of available-for-sale securities is based on prices obtained from a third-party pricing service. The Company utilizes third-party pricing services for all of its marketable debt security valuations. The Company reviews the pricing methodology used by the third-party pricing services including the manner employed to collect market information. On a periodic basis, the Company also performs review and validation procedures on the pricing information
F-8
Table of Contents
received from the third-party pricing services. These procedures help ensure that the fair value information used by the Company is determined in accordance with applicable accounting guidance. Proceeds from sales of available-for-sale securities were $12.5 million for the year ended December 31, 2014. The amortized cost, unrealized gain or losses and fair value of the Company’s cash, cash equivalents and investments for the periods presented are summarized below:
| Amortized Cost |
Gross Unrealized Gains |
Gross Unrealized Losses |
Fair Value | |||||||||||||
| (In thousands) | ||||||||||||||||
| As of December 31, 2014: |
||||||||||||||||
| Cash |
$ | 6,351 | $ | — | $ | — | $ | 6,351 | ||||||||
| Money market funds |
4,103 | — | — | 4,103 | ||||||||||||
| Debt securities of U.S. government agencies |
43,862 | 1 | (19 | ) | 43,844 | |||||||||||
| Corporate bonds |
9,423 | 2 | (9 | ) | 9,416 | |||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total |
$ | 63,739 | $ | 3 | $ | (28 | ) | $ | 63,714 | |||||||
|
|
|
|
|
|
|
|
|
|||||||||
| As of December 31, 2013: |
||||||||||||||||
| Cash |
$ | 3,221 | $ | — | $ | — | $ | 3,221 | ||||||||
| Money market funds |
6,058 | — | — | 6,058 | ||||||||||||
| Debt securities of U.S. government agencies |
49,878 | 18 | (6 | ) | 49,890 | |||||||||||
| Corporate bonds |
13,390 | 4 | (1 | ) | 13,393 | |||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total |
$ | 72,547 | $ | 22 | $ | (7 | ) | $ | 72,562 | |||||||
|
|
|
|
|
|
|
|
|
|||||||||
The following table summarizes the Company’s available for sale securities by contractual maturity:
| As of December 31, 2014 | As of December 31, 2013 | |||||||||||||||
| Amortized Cost | Fair Value | Amortized Cost | Fair Value | |||||||||||||
| (In thousands) | ||||||||||||||||
| Less than one year |
$ | 51,338 | $ | 51,319 | $ | 56,789 | $ | 56,806 | ||||||||
| Greater than one year but less than five years |
6,050 | 6,044 | 12,537 | 12,535 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total |
$ | 57,388 | $ | 57,363 | $ | 69,326 | $ | 69,341 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
Warrants
Warrants issued in connection with the Company’s May 2009 and September 2010 financings are recorded as liabilities as both have the potential for cash settlement upon the occurrence of a fundamental transaction (as defined in the warrant; see “Note 6 — Share Capital”). Changes in the fair value of the warrants are recognized as other income (expense) in the consolidated statements of operations. Warrants issued in connection with the Company’s May 2009 financing expired on May 26, 2014.
Accounts and other receivables
Accounts and other receivables are reviewed whenever circumstances indicate that the carrying amount of the receivable may not be recoverable. At this time, the Company does not deem an allowance to be necessary.
Property and equipment, depreciation and amortization
Property and equipment are recorded at cost and depreciated over their estimated useful lives on a straight-line basis, as follows:
| Scientific and office equipment |
5 years | |||
| Computer software and equipment |
3 years | |||
| Leasehold improvements and leased equipment |
|
Shorter of useful life or the term of the lease |
|
F-9
Table of Contents
Long-lived assets
Long-lived assets, such as property and equipment, are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. If circumstances require a long-lived asset be tested for impairment, the Company first compares the undiscounted cash flows expected to be generated by the asset to the carrying value of the asset. If the carrying value of the long-lived asset is not recoverable on an undiscounted cash flow basis, impairment is recognized to the extent that the carrying value exceeds its estimated fair value. Fair value is determined by management through various valuation techniques, including discounted cash flow models, quoted market values and third-party independent appraisals, as considered necessary. No impairment charges were recorded for any of the periods presented.
Indefinite-lived intangible assets — IPR&D
Intangible assets related to In Process Research & Development (IPR&D) are considered to be indefinite-lived until the completion or abandonment of the associated research and development efforts. Upon completion of the project, the Company will make a separate determination of useful life of the IPR&D and the related amortization will be recorded as an expense over the estimated useful life. If the IPR&D is abandoned, the carrying value of the asset will be expensed. During the period the assets are considered indefinite-lived, they will not be amortized but will be tested for impairment on October 1 of each year or more frequently when events or changes in circumstances indicate that the asset may be impaired. In the event that the carrying value of IPR&D exceeds its fair value, an impairment loss would be recognized. Subsequent research and development costs associated with the initial recognition of IPR&D assets are expensed as incurred. No impairment charges were recorded for any of the periods presented.
Goodwill
Goodwill is not amortized, but is reviewed annually for impairment on October 1 of each year or more frequently when events or changes in circumstances indicate that the asset may be impaired. In the event that the carrying value of goodwill exceeds its fair value, an impairment loss would be recognized. No impairment charges were recorded for any of the periods presented.
Deferred rent
Rent expense is recognized on a straight-line basis over the term of the lease. Lease incentives, including rent holidays provided by lessors, and rent escalation provisions are accounted for as deferred rent.
Revenue recognition
The Company recognizes revenue when there is persuasive evidence that an arrangement exists, delivery has occurred, the price is fixed and determinable, and collection is reasonably assured.
Research and development costs
Research and development expenses include personnel and facility related expenses, which includes depreciation and amortization, outside contract services including clinical trial costs, manufacturing and process development costs, research costs and other consulting services. Research and development costs are expensed as incurred. In instances where the Company enters into agreements with third parties for clinical trials, manufacturing and process development, research, licensing arrangements and other consulting activities, costs are expensed as services are performed. Amounts due under such arrangements may be either fixed fee or fee for service, and may include upfront payments, monthly payments, and payments upon the completion of milestones or receipt of deliverables.
The Company’s accruals for clinical trials are based on estimates of the services received and pursuant to contracts with numerous clinical trial centers and clinical research organizations. In the normal course of business, the Company contracts with third parties to perform various clinical trial activities in the ongoing development of potential products. The financial terms of these agreements are subject to negotiation and variation from contract to contract and may result in uneven payment flows. Payments under the contracts depend on factors such as the achievement of certain events, the successful accrual of patients, and the completion of portions of the clinical trial or similar conditions. The objective of the Company’s accrual policy is to match the recording of expenses in its consolidated financial statements to the actual services received. As such, expense accruals related to clinical trials are recognized based on its estimate of the degree of completion of the event or events specified in the specific clinical study or trial contract.
F-10
Table of Contents
Income or loss per share
Basic net loss per share is calculated by dividing net loss by the weighted average number of shares outstanding for the period. Diluted net loss per share is calculated by adjusting the numerator and denominator of the basic net loss per share calculation for the effects of all potentially dilutive common shares. Potential dilutive shares of the Company’s common stock include stock options, restricted share units, warrants, Series A convertible preferred stock and shares granted under the 2010 ESPP. The calculation of diluted loss per share requires that, to the extent the average market price of the underlying shares for the reporting period exceeds the exercise price of the warrants and the presumed exercise of such securities are dilutive to loss per share for the period, adjustments to net loss used in the calculation are required to remove the change in fair value of the warrants for the period. Furthermore, adjustments to the denominator are required to reflect the addition of the related dilutive shares. Basic net loss per share equaled the diluted loss per share for the year ended December 31, 2014 and 2013, since the effect of the shares potentially issuable upon the exercise or conversion was anti-dilutive. For additional information regarding the income or loss per share, see “Note 6 — Share Capital.”
Income taxes
The Company follows the asset and liability method of accounting for income taxes. Under this method,deferred tax assets and liabilities are recognized for the future income tax consequences attributable to differences between the carrying amounts and tax bases of assets and liabilities and losses carried forward and tax credits. Deferred tax assets and liabilities are measured using enacted tax rates and laws applicable to the years in which the differences are expected to reverse. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period that includes the enactment date. A valuation allowance is provided to the extent that it is more likely than not that deferred tax assets will not be realized.
The Company recognizes the financial statement effects of a tax position when it is more likely than not,based on the technical merits, that the position will be sustained upon examination. The Company does not believe any uncertain tax positions currently pending will have a material adverse effect on its consolidated financial statements nor expects any material change in its position in the next twelve months. Penalties and interest, of which there are none, would be reflected in income tax expense. Tax years are open to the extent the Company has net operating loss carryforwards available to be utilized currently.
Accumulated other comprehensive income (loss)
Comprehensive income or loss is comprised of net income or loss and other comprehensive income or loss. Other comprehensive income or loss includes unrealized gains and losses on the Company’s available-for-sale investments. In addition to unrealized gains and losses on investments, accumulated other comprehensive income or loss consists of foreign currency translation adjustments which arose from the conversion of the Canadian dollar functional currency consolidated financial statements to the U.S. dollar reporting currency consolidated financial statements prior to January 1, 2008. Should the Company liquidate or substantially liquidate its investments in its foreign subsidiaries, the Company would be required to recognize the related cumulative translation adjustments pertaining to the liquidated or substantially liquidated subsidiaries, as a charge to earnings in the Company’s consolidated statements of operations and comprehensive loss.
Realized gains of approximately $6,000 on sales of available-for-sale securities were reclassified out of accumulated other comprehensive loss and recorded as part of net other income (expense) on the Company’s consolidated statements of operations for the year ended December 31, 2014. The table below shows the changes in accumulated balances of each component of accumulated other comprehensive loss for the twelve months ended December 31, 2014, 2013 and 2012:
| Net unrealized gains/(losses) on Available-for-sale Securities |
Foreign Currency Translation Adjustment |
Accumulated Other Comprehensive Loss |
||||||||||
| (In thousands) | ||||||||||||
| Balance at December 31, 2011 |
$ | 22 | $ | (5,066 | ) | $ | (5,044 | ) | ||||
| Other comprehensive income |
8 | — | 8 | |||||||||
|
|
|
|
|
|
|
|||||||
| Balance at December 31, 2012 |
30 | (5,066 | ) | (5,036 | ) | |||||||
| Other comprehensive loss |
(15 | ) | — | (15 | ) | |||||||
|
|
|
|
|
|
|
|||||||
| Balance at December 31, 2013 |
15 | (5,066 | ) | (5,051 | ) | |||||||
F-11
Table of Contents
| Net unrealized gains/(losses) on Available-for-sale Securities |
Foreign Currency Translation Adjustment |
Accumulated Other Comprehensive Loss |
||||||||||
| (In thousands) | ||||||||||||
| Other comprehensive loss |
(40 | ) | — | (40 | ) | |||||||
|
|
|
|
|
|
|
|||||||
| Balance at December 31, 2014 |
$ | (25 | ) | $ | (5,066 | ) | $ | (5,091 | ) | |||
|
|
|
|
|
|
|
|||||||
Share-based compensation
The Company recognizes in the statements of operations the estimated grant date fair value of share-based compensation awards granted to employees over the requisite service period. Share-based compensation expense in the consolidated statements of operations is recorded on a straight-line basis over the requisite service period for the entire award, which is generally the vesting period, with the offset to additional paid-in capital. The Company uses the Black-Scholes option pricing model to estimate the fair value of stock options granted to employees. Forfeitures are estimated at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differ from those estimates.
For non-employee directors, the Company sponsors a RSU Plan that was established in 2005. According to an amendment to the RSU Plan in October 2011, approximately 25% of each RSU represents a contingent right to receive cash upon vesting, and the Company is required to deliver an amount in cash equal to the fair market value of the shares on the vesting date to facilitate the satisfaction of the non-employee directors’ U.S. federal income tax obligation with respect to the vested RSUs. This amendment resulted in the RSUs being classified as a liability. The outstanding RSU awards are required to be re-measured at each reporting date until settlement of the award, and changes in valuation are recorded as compensation expense for the period. To the extent that the liability recorded in the balance sheet is less than the original award value, the difference is recognized in equity. The Company uses the closing share price of its shares on the NASDAQ Global Market at the reporting or settlement date to determine the fair value of RSUs. In June 2014, the Company’s stockholders approved an increase of 500,000 shares in the number of shares of the Company’s common stock reserved for issuance under the RSU Plan.
The Company maintains an ESPP under which a total of 900,000 shares of common stock were reserved for sale to employees of the Company. The Company recognizes the estimated fair value of the ESPP which determined by the Black-Scholes option pricing model in the statement of operations.
For additional information regarding share-based compensation, see “Note 7 — Share-based Compensation.”
Business Combinations
In a business combination, the Company determines if the acquired property and activities meet the definition of a business under current accounting guidance. If the combination meets the definition of a business, the Company measures the significance of the combination to determine the required reporting and disclosure requirements for the transaction. Business combinations are required to be accounted for under the acquisition method which requires that identifiable assets acquired, liabilities assumed and any noncontrolling interest in the acquiree be recognized and measured as of the acquisition date at fair value. In addition, all consideration transferred must be measured at its acquisition-date fair value.
When necessary, the Company uses a third party valuation expert to determine the fair value of the identifiable assets and liabilities acquired. The estimated fair values of in-process research and development acquired in a business combination which have not been fully developed are capitalized as indefinite-lived intangible assets and impairment testing is conducted periodically.
Segment information
The Company operates in a single business segment — research and development of therapeutic products for the treatment of cancer.
Recent accounting pronouncements
In November 2014, FASB issued Accounting Standards Update 2014-16, Derivatives and Hedging (Topic 815), Determining Whether the Host Contract in a Hybrid Financial Instrument Issued in the Form of a Share is More Akin to Debt or Equity, a consensus of the FASB Emerging Issues Task Force. The standard eliminates diversity in the practice of
F-12
Table of Contents
determining whether the nature of a host contract with a hybrid financial instrument issued in the form of a share is more akin to debt or equity and applies to all reporting entities that are issuers of hybrid financial instruments issued in the form of a share. This standard provides that the determination would be based on a consideration of all economic characteristics and the risk of the entire hybrid financial instrument, including the embedded derivative function. Upon adoption, each issued hybrid share instrument must be evaluated to determine whether it contains embedded features that require bifurcation or no longer require bifurcation under the new standard. Retrospective application and early adoption would both be permitted. The standard is effective for public business entities for fiscal years, and interim periods within those years, beginning after 15 December 2015. The Company is currently evaluating the impact this standard will have on the consolidated financial position or results of operations.
In August 2014, FASB issued Accounting Standard Update 2014-15, Presentation of Financial Statements—Going Concern (Subtopic 205-40): Disclosure of Uncertainties about an Entity’s Ability to Continue as a Going Concern, which provides guidance on determining when and how to disclose going-concern uncertainties in the financial statements. The standard requires management to perform interim and annual assessments of an entity’s ability to continue as a going concern within one year of the date the financial statements are issued. An entity must provide certain disclosures if conditions or events raise substantial doubt about the entity’s ability to continue as a going concern. This standard applies to all entities and is effective for annual periods ending after December 15, 2016, and interim periods thereafter, with early adoption permitted. The Company is currently evaluating the impact this standard will have on the consolidated financial position or results of operations.
In May 2014, FASB issued Accounting Standard Update 2014-09, Revenue from Contracts with Customers (Topic 606) that will supersede most revenue recognition standards. Under the new standard, an entity will recognize revenue to depict the transfer of goods or services to customers in amounts that reflect the payment to which the entity expects to be entitled in exchange for those goods or services. An entity would recognize revenue through a five-step process: (1) identify the contract with a customer; (2) identify the performance obligations in the contract; (3) determine the transaction price; (4) allocate the transaction price to the performance obligations in the contract; and (5) recognize revenue when (or as) the entity satisfies a performance obligation. This standard also requires enhanced disclosures and provides more comprehensive guidance for transactions such as service revenue and contract modifications. Guidance for multiple-element arrangements also has been enhanced. The standard will take effect for public entities for annual reporting periods beginning after December 15, 2016, including interim reporting periods. Early application is not permitted. The Company is currently evaluating the impact this standard will have on the consolidated financial position or results of operations.
In July 2013, FASB issued guidance on presentation of an unrecognized tax benefit in financial statements when a net operating loss (NOL) carryforward, a similar tax loss, or a tax credit carryforward exists. This guidance requires an entity to present an unrecognized tax benefit as a reduction of a deferred tax asset for an NOL carryforward, or similar tax loss or tax credit carryforward, rather than as a liability when (1) the uncertain tax position would reduce the NOL or other carryforward under the tax law of the applicable jurisdiction and (2) the entity intends to use the deferred tax asset for that purpose. The guidance does not require new recurring disclosures. The guidance is effective prospectively for fiscal years, and interim periods within those years, beginning after December 15, 2013 for public entities. Early adoption and retrospective application are permitted. The Company adopted this standard on January 1, 2014. The adoption of this standard had no impact on the presentation of the Company’s unrecognized tax benefits or on the consolidated financial position or results of operations.
F-13
Table of Contents
| 3. | FAIR VALUE MEASUREMENTS |
The Company measures certain financial assets and liabilities at fair value in accordance with a hierarchy which requires an entity to maximize the use of observable inputs which reflect market data obtained from independent sources and minimize the use of unobservable inputs. There are three levels of inputs that may be used to measure fair value:
| • | Level 1 — quoted prices in active markets for identical assets or liabilities; |
| • | Level 2 — observable inputs other than Level 1 prices such as quoted prices for similar assets or liabilities, quoted prices in markets that are not active, or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities; and |
| • | Level 3 — unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities. |
The Company’s financial assets and liabilities measured at fair value on a recurring basis consisted of the following as of December 31, 2014 and 2013:
| December 31, 2014 | December 31, 2013 | |||||||||||||||||||||||||||||||
| Level 1 | Level 2 | Level 3 | Total | Level 1 | Level 2 | Level 3 | Total | |||||||||||||||||||||||||
| (In thousands) | ||||||||||||||||||||||||||||||||
| Financial Assets: |
||||||||||||||||||||||||||||||||
| Money market funds |
$ | 4,103 | $ | — | $ | — | $ | 4,103 | $ | 6,058 | $ | — | $ | — | $ | 6,058 | ||||||||||||||||
| Debt securities of U.S. government agencies |
— | 43,844 | — | 43,844 | — | 49,890 | — | 49,890 | ||||||||||||||||||||||||
| Corporate bonds |
— | 9,416 | — | 9,416 | — | 13,393 | — | 13,393 | ||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| $ | 4,103 | $ | 53,260 | $ | — | $ | 57,363 | $ | 6,058 | $ | 63,283 | $ | — | $ | 69,341 | |||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Financial Liability: |
||||||||||||||||||||||||||||||||
| Restricted Share Units |
$ | 310 | $ | — | $ | — | $ | 310 | $ | 337 | $ | — | $ | — | $ | 337 | ||||||||||||||||
| Warrants |
— | — | 128 | 128 | — | — | 924 | 924 | ||||||||||||||||||||||||
If quoted market prices in active markets for identical assets are not available to determine fair value, then the Company uses quoted prices of similar instruments and other significant inputs derived from observable market data obtained from third-party data providers. These investments are included in Level 2 and consist of debt securities of U.S government agencies and corporate bonds.
There were no transfers between Level 1 and Level 2 during 2014.
The Company classifies its warrant liability within Level 3 because the warrant liability is valued using valuation models with significant unobservable inputs. The estimated fair value of warrants accounted for as liabilities was determined on the issuance date and are subsequently remeasured to fair value at each reporting date. The change in fair value of the warrants is recorded in the statement of operations as other income or other expense by using the Black-Scholes option-pricing model with the following inputs:
| As of December 31, 2014 | ||||
| September 2010 Warrants |
||||
| Exercise price |
$ | 4.24 | ||
| Market value of stock at end of period |
$ | 1.90 | ||
| Expected dividend rate |
0.0 | % | ||
| Expected volatility |
60.3 | % | ||
| Risk-free interest rate |
0.2 | % | ||
| Expected life (in years) |
0.78 | |||
F-14
Table of Contents
| As of December 31, 2013 | ||||||||
| May 2009 Warrants |
September 2010 Warrants |
|||||||
| Exercise price |
$ | 3.74 | $ | 4.24 | ||||
| Market value of stock at end of period |
$ | 1.76 | $ | 1.76 | ||||
| Expected dividend rate |
0.0 | % | 0.0 | % | ||||
| Expected volatility |
45.3 | % | 77.4 | % | ||||
| Risk-free interest rate |
0.1 | % | 0.3 | % | ||||
| Expected life (in years) |
0.40 | 1.78 | ||||||
The table below shows the reconciliation of warrant liability measured and recorded at fair value on a recurring basis, using significant unobservable inputs (Level 3):
| Years Ended December 31, | ||||||||
| 2014 | 2013 | |||||||
| (In thousands) | ||||||||
| Balance at beginning of period |
$ | 924 | $ | 3,251 | ||||
| Change in fair value of warrant liability included in Other expense (income) |
(796 | ) | (2,327 | ) | ||||
|
|
|
|
|
|||||
| Balance at the end of period |
$ | 128 | $ | 924 | ||||
|
|
|
|
|
|||||
Expected volatility is an unobservable input that is inter-related with the market value or price of the Company’s stock, since the calculation of volatility is based on the Company’s historical closing prices. If volatility were to increase by 10%, the value of the warrant liability would increase by approximately $64,000 or if volatility were to decrease by 10%, the value of the warrant liability would decrease by approximately $32,000.
| 4. | PROPERTY AND EQUIPMENT |
The table below outlines the cost, accumulated depreciation and amortization and net carrying value of the Company’s property and equipment for the years ended December 31, 2014 and 2013:
| 2014 | ||||||||||||
| Cost | Accumulated Depreciation and Amortization |
Net Carrying Value |
||||||||||
| (In thousands) | ||||||||||||
| Scientific equipment |
$ | 2,273 | $ | (1,429 | ) | $ | 844 | |||||
| Leasehold improvements |
1,590 | (948 | ) | 642 | ||||||||
| Computer software and equipment |
414 | (327 | ) | 87 | ||||||||
| Office equipment |
34 | (31 | ) | 3 | ||||||||
|
|
|
|
|
|
|
|||||||
| $ | 4,311 | $ | (2,735 | ) | $ | 1,576 | ||||||
|
|
|
|
|
|
|
|||||||
| 2013 | ||||||||||||
| Cost | Accumulated Depreciation and Amortization |
Net Carrying Value |
||||||||||
| (In thousands) | ||||||||||||
| Scientific equipment |
$ | 1,985 | $ | (1,096 | ) | $ | 889 | |||||
| Leasehold improvements |
1,579 | (787 | ) | 792 | ||||||||
| Office equipment |
34 | (26 | ) | 8 | ||||||||
| Computer software and equipment |
325 | (319 | ) | 6 | ||||||||
|
|
|
|
|
|
|
|||||||
| $ | 3,923 | $ | (2,228 | ) | $ | 1,695 | ||||||
|
|
|
|
|
|
|
|||||||
Depreciation and leasehold improvement amortization expense was $0.5 million for each of the years ended December 31, 2014, 2013 and 2012, respectively.
F-15
Table of Contents
| 5. | ACQUISITION |
On August 8, 2014, the Company entered into an Agreement and Plan of Reorganization (Merger Agreement) with Alpine Biosciences, Inc. (Alpine), a privately held biotechnology company developing protocells, a nanoparticle capable of delivery of nucleic acids, proteins, peptides and small molecules. Pursuant to the terms and conditions set forth in the Merger Agreement, on August 8, 2014, the Company, through a reverse-triangular merger with Alpine into a fully-owned subsidiary of the Company, known as Protocell Therapeutics Inc, consummated the acquisition of Alpine. The merger consideration received by Alpine stockholders was 10% of the Company’s total capital stock determined on a fully-diluted basis immediately following the closing of the merger (Merger Consideration). The total value of the acquisition was approximately $27.2 million based on the closing price of Oncothyreon’s common stock on the day of the merger, which was $2.93 per share. An amount of stock equal to 12.5% of the Merger Consideration was placed in escrow as security for the indemnification obligations of Alpine’s stockholders. The Company intends to utilize the protocell technology to develop new product candidates for the treatment of cancer and rare diseases, either on its own or with partners.
The transaction has been accounted for using the acquisition method of accounting. This method requires, among other things, that assets acquired and liabilities assumed in a business combination be recognized at their estimated fair values as of the acquisition date and that intangible assets with indefinite lives be recorded at fair value on the balance sheet for IPR&D activities, regardless of the likelihood of success of the related product or technology. The excess of the aggregate fair value of consideration exchanged for an acquired business over the fair value of assets acquired including tangible assets and indefinite-lived intangible assets and liabilities assumed is recorded as Goodwill. Goodwill represents the anticipated synergies from combining the acquired assets with the Company.
Recognition and Measurement of Assets Acquired and Liabilities Assumed at Estimated Fair Value
The total purchase consideration has been allocated to the assets acquired and liabilities assumed, including identifiable intangible assets, based on their respective fair values at the acquisition date. Goodwill was derived from the excess of the aggregate fair value of consideration exchanged for the acquisition of Alpine over the fair value of assets acquired and liabilities assumed. Based upon the fair values determined by the Company, in which the Company considered or relied in part upon a valuation report of a third-party expert. These fair value measurements were based on Level 3 measurements under the fair value hierarchy. The following table summarizes the allocation of the purchase price for the acquisition (in thousands):
| Indefinite-lived intangible assets |
$ | 19,738 | ||
| Goodwill |
14,542 | |||
| Net tangible assets |
(139 | ) | ||
| Deferred tax liabilities |
(6,908 | ) | ||
|
|
|
|||
| Total purchase price allocation |
$ | 27,233 | ||
|
|
|
Goodwill
The changes in the carrying amount of goodwill for the year ended December 31, 2014 were as follows (in thousands):
| Balance as of December 31, 2013 |
$ | 2,117 | ||
| Goodwill recorded in connection with the acquisition of Alpine |
14,542 | |||
|
|
|
|||
| Balance as of December 31, 2014 |
$ | 16,659 | ||
|
|
|
The goodwill recognized from the acquisition of Alpine is not deductible for tax purposes.
Indefinite-lived Intangible Assets — IPR&D
Intangible assets with indefinite lives represent the value assigned to IPR&D that, as of the acquisition date, the Company determined that technological feasibility had not been established, and the IPR&D had no alternative future use. IPR&D represents a series of awarded patents and filed patent applications that are the basis of the platform which forms a major part of the planned future products. The indefinite-lived intangible assets will be subject to annual impairment testing until completion or abandonment of the projects. Upon completion of the project, the Company will make a separate determination of useful life of the indefinite-lived intangible assets and the related amortization will be recorded as an expense over the estimated useful life.
F-16
Table of Contents
The fair value of the indefinite-lived intangible assets of $19.7 million was determined by the Company, which relied upon a valuation report from an independent third party valuation expert using the income approach and estimates and assumptions provided by the Company’s management. The income approach is a valuation technique that provides an estimate of the fair value of an asset based on market participant expectations of the cash flows an asset would generate over its remaining useful life. The rates utilized to discount net cash flows to their present values were based on a range of discount rates of 40% to 60% applied to the intangible assets to reflect the risk of the asset revenues derived from the respective intangible asset. Subsequent to the closing of the merger, research and development cost incurred on the IPR&D and general and administrative expenses associated with salaries and legal costs are expensed as incurred. From August 8, 2014 to December 31, 2014, the Company incurred nominal expenses from Alpine.
Deferred Tax Liabilities
Deferred tax liabilities of $6.9 million were the result of book versus tax difference attributable to the identifiable intangible asset multiplied by the statutory tax rate for the relevant jurisdiction.
Acquisition-Related Expenses
Acquisition-related expenses of $0.5 million, including legal and regulatory costs, were expensed as incurred and recorded in general and administrative expense in the Company’s condensed consolidated statements of operations for the twelve months ended December 31, 2014.
Unaudited Pro Forma Financial Information
The following pro forma condensed combined financial information gives effect to the acquisition of Alpine as if it were consummated on January 1, 2013 (the beginning of the comparable prior reporting period), and includes pro forma adjustments related to share-based compensation expense and direct and incremental transaction costs reflected in the historical financial statements. The pro forma condensed combined financial information is presented for informational purposes only. The pro forma condensed combined financial information is not intended to represent or be indicative of the results of operations that would have been reported had the acquisition occurred on January 1, 2013 and should not be taken as representative of future results of operations of the combined company.
The following table presents the unaudited pro forma condensed combined financial information (in thousands, except per share amounts):
| Year Ended December 31, | ||||||||
| 2014 | 2013 | |||||||
| Net loss |
$ | (51,297 | ) | $ | (39,287 | ) | ||
| Net loss per share – basic and diluted |
$ | (0.66 | ) | $ | (0.63 | ) | ||
| 6. | SHARE CAPITAL |
Class UA preferred stock
As of December 31, 2014 and 2013, the Company had 12,500 shares of Class UA preferred stock authorized, issued and outstanding. The Class UA preferred stock has the following rights, privileges, and limitations:
Voting. Each share of Class UA preferred stock will not be entitled to receive notice of, or to attend and vote at, any Stockholder meeting unless the meeting is called to consider any matter in respect of which the holders of the shares of Class UA preferred stock would be entitled to vote separately as a class, in which case the holders of the shares of Class UA preferred stock shall be entitled to receive notice of and to attend and vote at such meeting. Amendments to the certificate of incorporation of Oncothyreon that would increase or decrease the par value of the Class UA preferred stock or alter or change the powers, preferences or special rights of the Class UA preferred stock so as to affect them adversely would require the approval of the holders of the Class UA preferred stock.
Conversion. The Class UA preferred stock is not convertible into shares of any other class of Oncothyreon capital stock.
F-17
Table of Contents
Dividends. The holders of the shares of Class UA preferred stock will not be entitled to receive dividends.
Liquidation preference. In the event of any liquidation, dissolution or winding up of the Company, the holders of the Class UA preferred stock will be entitled to receive, in preference to the holders of the Company’s common stock, an amount equal to the lesser of (1) 20% of the after tax profits (“net profits”), determined in accordance with Canadian generally accepted accounting principles, where relevant, consistently applied, for the period commencing at the end of the last completed financial year of the Company and ending on the date of the distribution of assets of the Company to its stockholders together with 20% of the net profits of the Company for the last completed financial year and (2) CDN $100 per share.
Holders of Class UA preferred stock are entitled to mandatory redemption of their shares if the Company realizes “net profits” in any year. For this purpose, “net profits … means the after tax profits determined in accordance with generally accepted accounting principles, where relevant, consistently applied.” The Company has taken the position that this applies to Canadian GAAP and, accordingly, there have been no redemptions to date.
Redemption. The Company may, at its option and subject to the requirements of applicable law, redeem at any time the whole or from time to time any part of the then-outstanding shares of Class UA preferred stock for CDN $100 per share. The Company is required each year to redeem at CDN $100 per share that number of shares of Class UA preferred stock as is determined by dividing 20% of the net profits by CDN $100.
The difference between the redemption value and the book value of the Class UA preferred stock will be recorded at the time that the fair value of the shares increases to redemption value based on the Company becoming profitable as measured using Canadian GAAP.
Preferred stock
As of December 31, 2014 and 2013, the Company had authorized 10,000,000 shares of undesignated preferred stock, $0.0001 par value per share. As of December 31, 2014 and 2013, the Company had 10,000 shares and zero shares of Series A convertible preferred stock issued and outstanding, respectively. Shares of preferred stock may be issued in one or more series from time to time by the board of directors of the Company, and the board of directors is expressly authorized to fix by resolution or resolutions the designations and the powers, preferences and rights, and the qualifications, limitations and restrictions thereof, of the shares of each series of preferred stock. Subject to the determination of the board of directors of the Company, the preferred stock would generally have preferences over common stock with respect to the payment of dividends and the distribution of assets in the event of the liquidation, dissolution or winding up of the Company.
On September 22, 2014, in connection with the public offering of 10,000 shares of the Company’s Series A convertible preferred stock, the Company designated 10,000 shares of its authorized and unissued preferred stock as Series A convertible preferred stock and filed a Certificate of Designation of Preferences, Rights and Limitations of Series A Convertible Preferred Stock with the Delaware Secretary of State. Each share of Series A convertible preferred stock is convertible into 1,000 shares of the Company’s common stock at any time at the holder’s option. The holder, however, will be prohibited from converting Series A convertible preferred stock into shares of common stock if, as a result of such conversion, the holder, together with its affiliates, would own more than 4.99% of the shares of the Company’s common stock then issued and outstanding. In the event of the Company’s liquidation, dissolution, or winding up, holders of Series A convertible preferred stock will receive a payment equal to $0.0001 per share of Series A convertible preferred stock before any proceeds are distributed to the holders of common stock, but after any proceeds are distributed to the holder of the Company’s Class UA preferred stock. Shares of Series A convertible preferred stock will generally have no voting rights, except as required by law and except that the consent of holders of a majority of the outstanding Series A convertible preferred stock will be required to amend the terms of the Series A convertible preferred stock. Shares of Series A convertible preferred stock will not be entitled to receive any dividends, unless and until specifically declared by the Company’s board of directors, and will rank:
| • | senior to all common stock; |
| • | senior to any class or series of capital stock hereafter created specifically ranking by its terms junior to the Series A convertible preferred stock; |
| • | on parity with any class or series of capital stock hereafter created specifically ranking by its terms on parity with the Series A convertible preferred stock; and |
| • | junior to the Company’s Class UA preferred stock and any class or series of capital stock hereafter created specifically ranking by its terms senior to the Series A convertible preferred stock; in each case, as to distribution of assets upon the Company’s liquidation, dissolution or winding up whether voluntarily or involuntarily. |
F-18
Table of Contents
On February 11, 2015, the Company closed concurrent but separate underwritten offerings of 13,500,000 shares of its common stock at a price to the public of $1.50 per share, for estimated gross proceeds of , approximately $20 million and 1,333 shares of its Series B Convertible Preferred Stock at a price to the public of $1,500 per share, for estimated gross proceeds of approximately $2 million. Each share of Series B Convertible Preferred Stock is non-voting and convertible into 1,000 shares of the Company’s common stock, provided that conversion will be prohibited if, as a result, the holder and its affiliates would beneficially own more than 4.99% of the common stock then outstanding. As part of the common stock offering, the Company also granted the underwriters a 30-day option to purchase 2,025,000 additional shares of the Company’s common stock. On February 18, 2015, the Company closed a partial exercise of the underwriter’s option to purchase 1,199,660 additional shares of the Company’s common stock, at a price to the public of $1.50 per share, less underwriting discounts and commissions, which resulted in net proceeds to the Company of approximately $1.7 million. Aggregate gross proceeds from the offerings were approximately $24.0 million. Aggregate net proceeds from the offerings, after underwriting discounts, commissions and estimated expenses of $1.6 million, were approximately $22.4 million.
Concurrent but separate from these offerings, the Company entered into an exchange agreement with certain affiliates of Biotechnology Value Fund (BVF) to exchange 4,000,000 shares of common stock previously purchased by BVF for 4,000 shares of Series B Convertible Preferred Stock.
Common stock
On June 6, 2014, the stockholders approved an amendment to the Company’s Amended and Restated Certificate of Incorporation to increase the number of the Company’s authorized common shares from 100,000,000 to 200,000,000. On June 6, 2014, the Company filed a Certificate of Amendment with the Delaware Secretary of State to effect such amendment.
As of December 31, 2014 and 2013, the Company had 200,000,000 shares and 100,000,000 shares of common stock, $0.0001 par value per share, authorized, respectively. The holders of common stock are entitled to receive such dividends or distributions as are lawfully declared on the Company’s common stock, to have notice of any authorized meeting of stockholders, and to exercise one vote for each share of common stock on all matters which are properly submitted to a vote of the Company’s stockholders. As a Delaware corporation, the Company is subject to statutory limitations on the declaration and payment of dividends. In the event of a liquidation, dissolution or winding up of the Company, holders of common stock have the right to a ratable portion of assets remaining after satisfaction in full of the prior rights of creditors, including holders of the Company’s indebtedness, all liabilities and the aggregate liquidation preferences of any outstanding shares of preferred stock. The holders of common stock have no conversion, redemption, preemptive or cumulative voting rights.
Amounts pertaining to issuances of common stock are classified as common stock on the consolidated balance sheet, approximately $9,160 and $7,067 of which represents par value of common stock as of December 31, 2014 and 2013 respectively. Additional paid-in capital primarily relates to amounts for share-based compensation (see “Note 7 — Share-based Compensation”).
Warrants
In connection with certain equity and debt financings, the Company issued warrants to purchase shares of its common stock.
In September 2010, the Company issued warrants to purchase 3,182,147 shares of its common stock in connection with a registered direct offering of its common stock and warrants. These warrants are classified as liabilities, as opposed to equity, due to the potential cash settlement upon the occurrence of certain transactions specified in the warrant agreement. Warrants to purchase 2,691,242 shares of the Company’s common stock from a May 2009 financing expired on May 26, 2014.
In February 2011, the Company issued 48,701 warrants, which were classified as equity, to purchase shares of common stock in connection with a Loan and Security Agreement entered into with General Electric Capital Corporation.
In June 2013, the Company issued warrants to purchase 5,000,000 shares of common stock, which were classified as equity, in connection with a registered direct offering to Biotechnology Value Fund, L.P. and other affiliates of BVF Partners L.P. (collectively, “BVF”).
F-19
Table of Contents
A summary of outstanding warrants as of December 31, 2014 and 2013 and changes during the years is presented below.
| 2014 | 2013 | |||||||
| Shares Underlying Warrants |
Shares Underlying Warrants |
|||||||
| Balance, beginning of year |
10,922,090 | 5,922,090 | ||||||
| Warrants issued |
— | 5,000,000 | ||||||
| Warrants expired |
(2,691,242 | ) | — | |||||
|
|
|
|
|
|||||
| Balance, end of year |
8,230,848 | 10,922,090 | ||||||
|
|
|
|
|
|||||
The following table summarizes information regarding warrants outstanding at December 31, 2014:
| Exercise Prices |
Shares Underlying Outstanding Warrants |
Expiry Date | ||||
| $3.08 |
48,701 | February 8, 2018 | ||||
| $4.24 |
3,182,147 | October 12, 2015 | ||||
| $5.00 |
5,000,000 | December 5, 2018 | ||||
|
|
|
|||||
| 8,230,848 | ||||||
|
|
|
|||||
| Years Ended December 31, | ||||||||
| 2014 | 2013 | |||||||
| Shares underlying warrants outstanding classified as liabilities |
3,182,147 | 5,873,389 | ||||||
| Shares underlying warrants outstanding classified as equity |
5,048,701 | 5,048,701 | ||||||
Equity Financings
On September 18, 2014, the Company entered into two underwriting agreements (each, an Underwriting Agreement) with Cowen and Company, LLC (Cowen) as representative of the underwriters named therein (Underwriters) for concurrent but separate offerings of the Company’s securities. On September 23, 2014, the Company closed concurrent but separate underwritten offerings of 10,000,000 shares of its common stock at a price of $2.00 per share, for gross proceeds of $20 million, and 10,000 shares of its Series A convertible preferred stock at a price of $2,000 per share, for gross proceeds of $20 million. Each share of Series A convertible preferred stock is non-voting and convertible into 1,000 shares of the Company’s common stock at any time at the option of the holder, provided that conversion will be prohibited if, as a result, the holder and its affiliates would beneficially own more than 4.99% of the common stock then outstanding. As part of the common stock offering, the Company also granted the underwriters, and the underwriters exercised, a 30-day option to purchase 1,500,000 additional shares of the Company’s common stock. Aggregate gross proceeds from the offerings were approximately $43.0 million. Aggregate net proceeds from the offerings, after commissions and estimated expenses of $2.8 million, was approximately $40.2 million which included $21.6 million from the Company’s common stock offering and $18.6 million from the Company’s Series A convertible preferred stock offering.
On June 4, 2013, the Company closed a registered direct offering of 5,000,000 units, with each unit consisting of one share of the Company’s common stock and a warrant to purchase one share of the Company’s common stock, at $2.00 per unit for gross proceeds of $10 million. The warrants are exercisable at an exercise price of $5.00 per share any time on or after December 5, 2013 and expire December 5, 2018. The shares and warrants were sold to Biotechnology Value Fund, L.P. and other affiliates of BVF Partners L.P. in a registered direct offering conducted without an underwriter or placement agent. The net proceeds from the offering, after deducting estimated offering expenses, were approximately $9.9 million.
“At-the-Market” Program
On February 3, 2012, the Company entered into a Sales Agreement (the Sales Agreement) with Cowen to sell shares of the Company’s common stock, having aggregate gross sales proceeds up to $50,000,000, from time to time, through an “at-the-market” equity offering program under which Cowen acted as sales agent. Under the Sales Agreement, the
F-20
Table of Contents
Company set the parameters for the sale of shares, including the number of shares to be issued, the time period during which sales are requested to be made, limitation on the number of shares that may be sold in any one trading day and any minimum price below which sales may not be made. The Sales Agreement provided that Cowen would be entitled to compensation for its services that would not exceed, but could be lower than, 3.0% of the gross sales price per share of all shares sold through Cowen under the Sales Agreement. On July 1, 2013, the Company commenced selling its common stock through the “at the market” equity offering program under the Sales Agreement. On September 17, 2014, the Company terminated the Sales Agreement in connection with the Company’s September 2014 equity offerings. The Company was not subject to any termination penalties related to termination of the Sales Agreement. As of September 17, 2014, the Company had sold an aggregate of 8,364,379 shares of its common stock under the Sales Agreement for gross proceeds of $16.6 million. The net proceeds from the sale of the shares, after deducting commission of approximately $0.5 million, were approximately $16.1 million.
Net loss per share
Basic net loss per share is calculated by dividing net loss by the weighted average number of shares outstanding for the period. Diluted net loss per share is calculated by adjusting the numerator and denominator of the basic net loss per share calculation for the effects of all potentially dilutive common shares. Potential dilutive shares of the Company’s common stock include stock options, restricted share units, warrants, Series A convertible preferred stock and shares granted under the 2010 ESPP. The calculation of diluted loss per share requires that, to the extent the average market price of the underlying shares for the reporting period exceeds the exercise price of the warrants and the presumed exercise of such securities are dilutive to loss per share for the period, adjustments to net loss used in the calculation are required to remove the change in fair value of the warrants for the period. Furthermore, adjustments to the denominator are required to reflect the addition of the related dilutive shares.
The following table is a reconciliation of the numerators and denominators used in the calculation of basic and diluted net loss per share computations for the years ended December 31, 2014, 2013 and 2012:
| Years Ended December 31, | ||||||||||||
| 2014 | 2013 | 2012 | ||||||||||
| (in thousands, except share and per share amounts) | ||||||||||||
| Numerator: |
||||||||||||
| Net loss used to compute net loss per share |
||||||||||||
| Basic |
$ | (49,963 | ) | $ | (38,759 | ) | $ | (3,415 | ) | |||
| Adjustments for change in fair value of warrant liability |
— | — | (25,520 | ) | ||||||||
|
|
|
|
|
|
|
|||||||
| Diluted |
$ | (49,963 | ) | $ | (38,759 | ) | $ | (28,935 | ) | |||
|
|
|
|
|
|
|
|||||||
| Denominator: |
||||||||||||
| Weighted average shares outstanding used to compute net loss per share: |
||||||||||||
| Basic |
77,619,807 | 62,387,616 | 53,728,672 | |||||||||
| Dilutive effect of warrants |
— | — | 1,171,283 | |||||||||
|
|
|
|
|
|
|
|||||||
| Diluted |
77,619,807 | 62,387,616 | 54,899,955 | |||||||||
|
|
|
|
|
|
|
|||||||
| Net loss per share—basic |
$ | (0.64 | ) | $ | (0.62 | ) | $ | (0.06 | ) | |||
|
|
|
|
|
|
|
|||||||
| Net loss per share—diluted |
$ | (0.64 | ) | $ | (0.62 | ) | $ | (0.53 | ) | |||
|
|
|
|
|
|
|
|||||||
The following table presents the number of shares that were excluded from the number of shares used to calculate diluted net loss per share:
| Years Ended December 31, | ||||||||||||
| 2014 | 2013 | 2012 | ||||||||||
| Director and employee stock options |
5,217,535 | 4,415,033 | 2,934,453 | |||||||||
| Warrants |
8,230,848 | 10,922,090 | 48,701 | |||||||||
| Series A convertible preferred stock (as converted to common stock) |
10,000,000 | — | — | |||||||||
| Non-employee director restricted share units |
163,204 | 191,613 | 140,968 | |||||||||
| Employee stock purchase plan |
3,997 | 2,765 | 4,758 | |||||||||
F-21
Table of Contents
| 7. | SHARE-BASED COMPENSATION |
Share option plan
The Company sponsors a Share Option Plan (“Option Plan”) under which a maximum fixed reloading percentage of 10% of the issued and outstanding common shares of the Company may be granted to employees, directors, and service providers. Prior to April 1, 2008, options were granted with a per share exercise price, in Canadian dollars, equal to the closing market price of the Company’s shares of common stock on the Toronto Stock Exchange on the date immediately preceding the date of the grant. After April 1, 2008, options were granted with a per share exercise price, in U.S. dollars, equal to the closing price of the Company’s shares of common stock on The NASDAQ Global Market on the date of grant. Canadian dollar amounts reflected in the tables below, which approximates their U.S. dollar equivalents as differences between the U.S. dollar and Canadian dollar exchange rates for the periods reflected below are not material. Prior to January 2010, options granted under the Option Plan begin to vest after one year from the date of the grant, are exercisable in equal amounts over four years on the anniversary date of the grant, and expire eight years following the date of grant. After January 2010, options granted under the Option Plan begin to vest 25% on the first anniversary of the hiring date, with the balance vesting in monthly increments for 36 months following the first anniversary of hiring, and expire eight years following the date of grant. The current maximum number of shares of common stock reserved for issuance under the Option Plan is 9,160,135. As of December 31, 2014, 1,825,858 shares of common stock remain available for future grant under the Option Plan. A summary of option activity under the Option Plan as of December 31, 2014, and changes during such year is presented below. As described above, prior to April 1, 2008, exercise prices were denominated in Canadian dollars and in U.S. dollars thereafter. The weighted average exercise prices listed below are in their respective dollar denominations.
| Options |
Stock Options | Weighted Average Exercise Price |
Weighted Average Remaining Contractual Term |
Aggregate Intrinsic Value |
||||||||||||
| In Canadian dollars ($CDN): |
||||||||||||||||
| Outstanding at January 1, 2014 |
687,533 | $ | 7.53 | |||||||||||||
| Granted |
— | — | ||||||||||||||
| Exercised |
— | — | ||||||||||||||
| Forfeited |
(6,666 | ) | 8.64 | |||||||||||||
| Expired |
(508,332 | ) | 7.42 | |||||||||||||
|
|
|
|||||||||||||||
| Outstanding at December 31, 2014 |
172,535 | $ | 7.80 | 0.37 | $ | — | ||||||||||
|
|
|
|||||||||||||||
| Vested or expected to vest at December 31, 2014 |
172,535 | $ | 7.80 | 0.37 | $ | — | ||||||||||
|
|
|
|||||||||||||||
| Vested and exercisable at December 31, 2014 |
172,535 | $ | 7.80 | 0.37 | $ | — | ||||||||||
|
|
|
|||||||||||||||
| In US dollars ($US): |
||||||||||||||||
| Outstanding at January 1, 2014 |
3,727,500 | $ | 3.41 | |||||||||||||
| Granted |
1,444,500 | 1.82 | ||||||||||||||
| Exercised |
(6,000 | ) | 1.10 | |||||||||||||
| Forfeited |
(71,000 | ) | 3.76 | |||||||||||||
| Expired |
(50,000 | ) | 1.83 | |||||||||||||
|
|
|
|||||||||||||||
| Outstanding at December 31, 2014 |
5,045,000 | $ | 2.97 | 6.01 | $ | 543,010 | ||||||||||
|
|
|
|||||||||||||||
| Vested or expected to vest at December 31, 2014 |
4,938,695 | $ | 2.99 | 5.97 | $ | 529,330 | ||||||||||
|
|
|
|||||||||||||||
| Vested and exercisable at December 31, 2014 |
2,172,303 | $ | 3.96 | 4.35 | $ | 186,549 | ||||||||||
|
|
|
|||||||||||||||
The aggregate intrinsic value is calculated as the difference between the exercise price of the underlying awards and the quoted price of the Company’s common stock for all options that were in-the-money at December 31, 2014. The total fair value of stock options vested during the years ended December 31, 2014, 2013 and 2012 was $6.29 million, $4.73 million and $2.93 million, respectively. There were 6,000, zero and 2,655 stock options exercised for the year ended December 31, 2014, 2013 and 2012, respectively. Cash received from stock option exercises and the total intrinsic value of stock option exercises for the years ended December 31, 2014 and 2012 were immaterial and for the year ended December 31, 2013 were zero. As of December 31, 2014, there were 535,188 exercisable, in-the-money stock options based on the Company’s closing share price of $1.90 on The NASDAQ Global Market.
F-22
Table of Contents
Share-based compensation expense related to the stock option plan of $1.7 million, $1.7 million and $1.4 million was recognized for the years ended December 31, 2014, 2013 and 2012, respectively. Total compensation cost related to non-vested stock options not yet recognized was $3.3 million as of December 31, 2014, which is expected to be recognized over the next 35 months on a weighted-average basis. The Company uses the Black-Scholes option pricing model to value options upon grant date, under the following weighted average assumptions:
| 2014 | 2013 | 2012 | ||||||||||
| Weighted average grant-date fair value per stock option $US |
$ | 1.24 | $ | 1.26 | $ | 3.41 | ||||||
| Expected dividend rate |
— | — | — | |||||||||
| Expected volatility |
78.67 | % | 86.53 | % | 84.68 | % | ||||||
| Risk-free interest rate |
1.65 | % | 1.90 | % | 0.89 | % | ||||||
| Expected life of options in years |
5.82 | 6.0 | 6.0 | |||||||||
The expected term represents the period that the Company’s stock options are expected to be outstanding and was determined based on the simplified method, which calculates the expected life as the average of the vesting term and the contractual term of the option. The Company’s historical stock option exercise data was impacted by a restructuring of its business in 2008. Because the Company does not have sufficient historical stock option exercise data to accurately estimate the expected term used for its valuation of stock options, the Company continues to use the simplified method to calculate the expected term of new stock option grants. As the Company accumulates more data and history related to the exercises of stock option awards, the Company will reassess its use of the simplified method to determine the expected term. The expected volatility is based on the historical volatility of the Company’s common stock for a period equal to the stock option’s expected life. The risk-free interest rate is based on the yield at the time of grant of a U.S. Treasury security with an equivalent expected term of the option. The Company does not expect to pay dividends on its common stock. The amounts estimated according to the Black-Scholes option pricing model may not be indicative of the actual values realized upon the exercise of these options by the holders.
Share-based compensation guidance requires forfeitures to be estimated at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differ from estimates. The Company estimates forfeitures based on its historical experience.
Restricted share unit plan
The Company also sponsors a RSU Plan for non-employee directors that was established in 2005. The RSU Plan provides for grants to be made from time to time by the Board of Directors or a committee thereof. Each restricted stock unit granted will be made in accordance with the RSU Plan and terms specific to that grant and will be converted into one share of common stock less the cash payment provisions described below at the end of the grant period (not to exceed five years) without any further consideration payable to the Company in respect thereof. On June 6, 2014, the Company’s stockholders approved an increase of 500,000 shares in the number of shares of the Company’s common stock reserved for issuance under the RSU Plan. The current maximum number of common shares of the Company reserved for issuance pursuant to the RSU Plan is 966,666. As of December 31, 2014, 530,910 shares of common stock remain available for future grant under the RSU Plan. The fair value of the restricted share units has been determined to be the equivalent of the Company’s common share closing trading price on the date of grant as quoted on the NASDAQ Global Market.
Approximately 25% of each RSU represents a contingent right to receive cash upon vesting, and the Company is required to deliver an amount in cash equal to the fair market value of such shares on the vesting date to facilitate the satisfaction of the non-employee directors’ U.S. federal income tax obligation with respect to the vested RSUs. The outstanding RSU awards are required to be re-measured at each reporting date until settlement of the award, and changes in valuation are recorded as compensation expense for the period. To the extent that the liability recorded in the balance sheet is less than the original award value, the difference is recognized in equity. The fair value of the outstanding RSUs on the reporting date is determined to be the closing trading price of the Company’s common shares on that date.
Upon vesting, RSUs of 110,104, 46,906 and 43,397 with a weighted average fair value of $3.47, $1.84 and $3.55 were converted into 110,104, 46,906 and 43,397 shares of common stock for the years ended December 31, 2014, 2013 and 2012, respectively. Pursuant to an October 2011 amendment to the Company’s RSU Plan, the Company withheld 27,528 shares of the 110,104 RSUs for the year ended December 31, 2014, 11,730 shares of the 46,906 RSUs for the year ended December 31, 2013 and 10,846 shares of the 43,397 RSUs for the year ended December 31, 2012. The Company
F-23
Table of Contents
delivered to non-employee directors cash totaling $95,653, $21,636 and $38,544, which was equal to the fair value of the shares withheld on the vesting date in order to facilitate satisfaction of the non-employee directors’ income tax obligation with respect to the vested RSUs for the years ended December 31, 2014, 2013 and 2012, respectively.
A summary of the RSU activity under the Company’s RSU Plan as of December 31, 2014, and changes during such year is presented below:
| Restricted Share Units |
Restricted Share Units |
Weighted Average Fair Value per Unit |
||||||
| Outstanding at January 1, 2014 |
191,613 | $ | 1.76 | |||||
| Granted |
81,695 | 3.06 | ||||||
| Converted |
(110,104 | ) | 3.47 | |||||
|
|
|
|||||||
| Outstanding at December 31, 2014 |
163,204 | $ | 1.90 | |||||
|
|
|
|||||||
| Expected to vest at December 31, 2014 |
163,204 | $ | 1.90 | |||||
|
|
|
|||||||
As of December 31, 2014, there was no unrecognized compensation cost related to unvested RSUs. The re-measurement of the outstanding RSUs together with the grant and conversion of the RSUs resulted in an additional $0.4 million, $0.2 million and a reduction of $0.5 million in share-based compensation expense recorded in general and administrative expenses in the consolidated statement of operations for the years ended December 31, 2014, 2013 and 2012, respectively.
Employee Stock Purchase Plan
The Company adopted an ESPP on June 3, 2010, pursuant to which a total of 900,000 shares of common stock were reserved for sale to employees of the Company. The ESPP is administered by the compensation committee of the board of directors and is open to all eligible employees of the Company. Under the terms of the ESPP, eligible employees may purchase shares of the Company’s common stock at six month intervals during 18-month offering periods through their periodic payroll deductions, which may not exceed 15% of any employee’s compensation and may not exceed a value of $25,000 in any calendar year, at a price not less than the lesser of an amount equal to 85% of the fair market value of the Company’s common stock at the beginning of the offering period or an amount equal to 85% of the fair market value of the Company’s common stock on each purchase date. The maximum aggregate number of shares that may be purchased by each eligible employee during each offering period is 15,000 shares of the Company’s common stock.
Fair value of shares purchases under the Company’s ESPP was estimated at subscription dates using a Black-Scholes valuation model, which requires the input of highly subjective assumptions including expected stock price volatility and expected term. The expected volatility is based on the historical volatility of the Company’s common stock for a period equal to the ESPP’s expected life, which is determined by length of time between the subscription date and the purchase date. The risk-free interest rate is based on the yield at the time of grant of a U.S. Treasury security with an equivalent expected term of the ESPP. The Company does not expect to pay dividends on its common stock.
For the year ended December 31, 2014, 2013 and 2012, expense related to this plan was $101,796, $149,674 and $184,960, respectively. Under the ESPP, the Company issued 76,811 shares to employees at a purchase price of $1.49 per share during the year ended December 31, 2014. The Company issued 38,934 and 35,895 shares to employees at a purchase price of $1.46 and $1.57 per share respectively for the year ended December 31, 2013. The Company issued 49,086 and 6,338 shares to employees at a purchase price of $3.33 and $2.82 per share respectively for the year ended December 31, 2012. As of December 31, 2014, there are 600,533 shares reserved for future purchases and there was $25,559 of unrecognized compensation cost related to the ESPP, which is expected to be recognized over an estimated weighted-average period of 0.7 year.
| 8. | COLLABORATIVE AND LICENSE AGREEMENTS |
Array BioPharma Agreements
On December 11, 2014, the Company entered into a License Agreement (the “License Agreement”) with Array BioPharma Inc. (“Array”). Pursuant to the License Agreement, Array has granted the Company an exclusive license to develop, manufacture and commercialize ONT-380, an orally active, reversible and selective small-molecule HER2 inhibitor. The License Agreement replaces and terminates the prior Development and Commercialization Agreement under which Oncothyreon and Array were jointly developing ONT-380, and going forward, the Company will be solely responsible for all pre-clinical and clinical development, regulatory and commercialization activities relating to ONT-380.
F-24
Table of Contents
Under the terms of the License Agreement, the Company paid Array an upfront fee of $20 million, which was recorded as part of research and development expense upon initiation of the exclusive license agreement. In addition, if the Company sublicenses rights to ONT-380 to a third party, the Company will pay Array a percentage of any sublicense payments it receives, with the percentage varying according to the stage of development of ONT-380 at the time of the sublicense. If the Company is acquired within three years of the effective date of the License Agreement, and ONT-380 has not been sublicensed to another entity prior to such acquisition, then the acquirer will be required to make certain milestone payments of up to $280 million to Array, which are primarily based on potential ONT-380 sales. Array is also entitled to receive up to a double-digit royalty based on net sales of ONT-380.
The License Agreement will expire on a county-by-country basis ten years following the first commercial sale of the product in each respective country, but may be terminated earlier by either party upon material breach of the License Agreement by the other party or the other party’s insolvency, or by the Company on 180 days’ notice to Array. The Company and Array have also agreed to indemnify the other party for certain of their respective warranties and obligations under the License Agreement.
Pursuant to the terms of the License Agreement, the Company and Array agreed to terminate the Development and Commercialization Agreement, dated May 29, 2013 by and between the Company and Array (the “Collaboration Agreement”), pursuant to which the companies collaborated on the development and commercialization of ONT-380 for the treatment of cancer, including breast cancer. The Company paid Array an upfront fee of $10 million in 2013, which was recorded as part of research and development expense upon initiation of the collaboration.
The License Agreement replaces the Collaboration Agreement, and the termination of the Collaboration Agreement was effective on the date the parties entered into the License Agreement. The Company did not incur any early termination penalties as a result of termination of the Collaboration Agreement.
Celldex Therapeutics, Inc.
On May 28, 2014, the Company entered into a Co-Development Agreement with Celldex Therapeutics, Inc. (Celldex) to collaborate on a combined Phase 1b clinical trial of ONT-10 and varlilumab. The primary objective of the trial is to determine the safety and tolerability of the combined therapy. Additional objectives include evaluations of the impact of combination treatment on MUC1-specific humoral and cellular immune responses and anti-tumor effects.
The agreement provides that the Company will supply ONT-10 and Celldex will supply varlilumab. The Phase 1b trial will be conducted and funded by the Company. The Company and Celldex will jointly own the data from the trial and will make any plans for potential future development of the combination therapy together. There are no payments due under this agreement.
STC.UNM
Effective June 30, 2014, Alpine entered into an exclusive license agreement with STC.UNM, by assignment from The Regents of the University of New Mexico, to license the rights to use certain technology relating to protocells, a mesoporous silica nanoparticle delivery platform. Under the terms of the license agreement, the Company, as successor to Alpine, has the right to conduct research, clinical development and commercialize all inventions and products that are developed from the platform technology in certain fields of use as described in the license agreement. In exchange for the exclusive license, the Company is obligated to make a series of payments including on-going annual license payments, reimbursement of patent costs, success based milestones up to $5 million, a double-digit royalty on commercial sublicensing income and a low single-digit royalty based on net sales, if any. In addition, Alpine issued STC.UNM a number of shares of common stock such that STC.UNM owned 5% of the outstanding equity of Alpine prior to the merger between the Company and Alpine. Please refer to “Note 5 - Acquisition” of the audited financial statements included in this report for additional information regarding the Company’s acquisition of Alpine.
Merck KGaA
In May 2001, the Company and Merck KGaA entered into a collaborative arrangement to pursue joint global product research, clinical development and commercialization for two product candidates, including tecemotide (formerly known as L-BLP25 or Stimuvax), a MUC1-based liposomal cancer vaccine. This collaboration agreement was subsequently revised and ultimately replaced in 2008 with a license agreement. Under the 2008 license agreement, (1) the Company licensed to Merck KGaA the exclusive right to develop, commercialize and manufacture tecemotide and the right to
F-25
Table of Contents
sublicense to other persons all rights licensed to Merck KGaA by us, (2) the Company transferred certain manufacturing know-how, (3) the Company agreed not to develop any product, other than ONT-10, that is competitive with tecemotide and (4) if the Company intends to license the development or commercialization rights to ONT-10, Merck KGaA will have a right of first negotiation with respect to such rights. In 2014, Merck KGaA announced that it does not intend to continue the clinical development of tecemotide. Merck KGaA is continuing to support certain investigator-sponsored studies of tecemotide.
| 9. | NET INVESTMENT AND OTHER INCOME (EXPENSE) AND INTEREST EXPENSE |
Net investment and other income (expense) include the following components for the periods indicated:
| Years Ended December 31, | ||||||||||||
| 2014 | 2013 | 2012 | ||||||||||
| (In thousands) | ||||||||||||
| Investment income, net |
$ | 73 | $ | 95 | $ | 128 | ||||||
| Loss on extinguishment of debt |
— | — | (279 | ) | ||||||||
| Net foreign exchange gain (loss) |
(4 | ) | (3 | ) | 1 | |||||||
| Gain (loss) on sale of equipment |
1 | 45 | 24 | |||||||||
| Gain (loss) on sale of investment |
6 | — | (1 | ) | ||||||||
| Other income |
— | — | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Total investment and other income (expense), net |
$ | 76 | $ | 137 | $ | (127 | ) | |||||
|
|
|
|
|
|
|
|||||||
During part of 2012, the Company had a $5 million term loan outstanding which carried a fixed rate of 10.64% per annum and was payable over a 42-month period.
On June 29, 2012, the Company paid approximately $4.1 million to extinguish the outstanding balance of its term loan prior to its scheduled maturity. During the year ended December 31, 2012, the Company incurred a $0.3 million loss on early extinguishment of debt, which consisted of a prepayment penalty of 3% on the outstanding principal, the write-off of unamortized deferred financing costs and unamortized debt discount and legal expenses related to extinguishment of debt.
Interest expense for the years ended December 31, 2012 was $308,745. Interest expense is calculated using the effective interest method and includes non-cash amortization of debt discount and capitalized loan fees in the amount of $77,504 for the years ended December 31, 2012.
There were no interest expenses for the year ended December 31, 2014 and 2013.
| 10. | INCOME TAX |
There was no income tax provision or benefit for the years ended December 31, 2014, 2013 and 2012.
The provision for income taxes was different from the expected statutory federal income tax rate as follows:
| 2014 | 2013 | 2012 | ||||||||||
| Tax benefit at statutory rate |
35.0 | % | 35.0 | % | 35.0 | % | ||||||
| Change in fair value of warrant liability |
0.6 | 2.1 | 261.5 | |||||||||
| Stock based compensation |
(2.1 | ) | (0.1 | ) | (1.9 | ) | ||||||
| Other |
0.5 | (0.0 | ) | (1.9 | ) | |||||||
| Change in valuation allowance |
(30.1 | ) | (37.0 | ) | (292.7 | ) | ||||||
| Expiration of loss carryforwards and credits |
(2.9 | ) | (0.0 | ) | (0.0 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Income tax benefit (provision) |
0.0 | % | 0.0 | % | 0.0 | % | ||||||
|
|
|
|
|
|
|
|||||||
F-26
Table of Contents
The Company’s net deferred tax assets and deferred tax liabilities were recorded in other assets and accrued and other liabilities, respectively on the Consolidated Balance Sheets and consist of the following as of December 31, 2014 and 2013:
| 2014 | 2013 | |||||||
| (In thousands) | ||||||||
| Deferred tax assets |
||||||||
| Current |
||||||||
| Accrued expenses and other |
$ | 676 | $ | 569 | ||||
| Valuation allowance |
(673 | ) | (567 | ) | ||||
|
|
|
|
|
|||||
| Net current deferred tax assets |
3 | 2 | ||||||
| Non-current deferred tax assets |
||||||||
| Tax benefits from losses carried forward and tax credits |
154,559 | 156,938 | ||||||
| Stock based compensation |
2,088 | 2,506 | ||||||
| Intangible assets |
11,449 | 1,030 | ||||||
| Other |
107 | 114 | ||||||
|
|
|
|
|
|||||
| 168,203 | 160,588 | |||||||
| Valuation allowance |
(167,895 | ) | (160,354 | ) | ||||
|
|
|
|
|
|||||
| Net non-current deferred tax assets |
308 | 234 | ||||||
|
|
|
|
|
|||||
| Deferred tax liabilities |
||||||||
| Current |
||||||||
| Prepaid expenses |
311 | 236 | ||||||
|
|
|
|
|
|||||
| Total current deferred tax liabilities |
311 | 236 | ||||||
| Noncurrent |
||||||||
| Intangible asset |
6,908 | — | ||||||
|
|
|
|
|
|||||
| Total noncurrent deferred tax liabilities |
6,908 | — | ||||||
| Net deferred tax liability |
$ | 6,908 | $ | — | ||||
|
|
|
|
|
|||||
Based on the available evidence, the Company has recorded a full valuation allowance against its net deferred income tax assets as it is more likely than not that the benefit of these deferred tax assets will not be realized. The valuation allowance increased by $7.6 million and $7.6 million during the years ended December 31, 2014 and December 31, 2013, respectively.
On August 8, 2014 Alpine Biosciences Inc., merged into and with Protocell Therapeutics Inc., a wholly owned subsidiary of Oncothyreon Inc. For tax purposes this transaction is treated as a stock acquisition and therefore the tax attributes of Alpine were recorded through purchase accounting. There was no release of the valuation allowance due to the acquisition.
The Company has recorded the following reserve for uncertain tax positions as of December 31, 2014, 2013 and 2012:
| 2014 | 2013 | 2012 | ||||||||||
| (In thousands) | ||||||||||||
| Balance at January 1 |
$ | 662 | $ | 662 | $ | 729 | ||||||
| Increase related to prior year tax positions |
— | — | 12 | |||||||||
| Decrease related to current year tax positions |
(117 | ) | — | — | ||||||||
| Lapses of statute of limitations |
— | — | (79 | ) | ||||||||
|
|
|
|
|
|
|
|||||||
| Balance at December 31 |
$ | 545 | $ | 662 | $ | 662 | ||||||
|
|
|
|
|
|
|
|||||||
None of the unrecognized tax benefits that, if recognized, would affect the effective tax rate due to valuation allowance. We are currently not under audit by the federal, state and foreign tax authorities. We do not believe that it is reasonably possible that the total amounts of unrecognized tax benefit will materially increase or decrease within the next 12 months.
United States
The Company has accumulated net operating losses in the United States of $183.9 million and $168.4 million for United States federal tax purposes at December 31, 2014 and 2013, respectively, some of which are restricted pursuant to Section 382 of the Internal Revenue Code, and which may not be available entirely for use in future years. These losses expire in fiscal years 2018 through 2034. The Company has federal research and development tax credit carry forwards of $0.5 million that will expire in fiscal years 2018 through 2023, if not utilized.
F-27
Table of Contents
Canada
The Company has unclaimed Canada federal investment tax credits of $17.6 million and $19.1 million at December 31, 2014 and 2013, respectively, that expire in fiscal years 2018 through 2029. The Company has scientific research & experimental development expenditures of $118.0 million and $128.3 million for Canada federal purposes and $51.7 million and $56.2 million for provincial purposes at December 31, 2014 and 2013, respectively. These expenditures may be utilized in any period and may be carried forward indefinitely. The Company also has Canada federal capital losses of $160.3 million and $174.3 million and provincial capital losses of $160.4 million and $174.4 million at December 31, 2014 and 2013, respectively, that can be carried forward indefinitely to offset future capital gains. The Company has accumulated net operating losses of $5.5 million and $5.7 million at December 31, 2014 and 2013 for Canada federal tax purposes and $3.5 million and $3.6 million at December 31, 2014 and 2013 for provincial purposes which expire between 2027 and 2033. The Company is subject to examination by the Canada Revenue Agency for years after 2008. However carryforward attributes that were generated prior to 2008 may still be adjusted by a taxing authority upon examination if the attributes have been or will be used in a future period.
Other
The Company files federal and foreign income tax returns in the United States and abroad. For U.S. federal income tax purposes, the statute of limitations is open for 1998 and onward for the United States and Canada due to net operating loss carried forwards.
| 11. | CONTINGENCIES, COMMITMENTS, AND GUARANTEES |
Royalties
Pursuant to various license agreements, the Company is obligated to make payments based both on the achievement of certain milestones and a percentage of revenues derived from the licensed technology and royalties on net sales.
Employee benefit plan
Under a defined contribution plan available to permanent employees, the Company is committed to matching employee contributions up to limits set by the terms of the plan, as well as limits set by U.S. tax authorities. The Company’s matching contributions to the plan totaled $0.2 million for each of the three year ended December 31, 2014, 2013 and 2012. There were no changes to the plan during the year ended December 31, 2014.
Lease obligations — operating leases
The Company is committed to annual minimum payments under operating lease agreements for its office and laboratory space and equipment) as follows (in thousands):
| Year Ending December 31, |
||||
| 2015 |
$ | 609 | ||
| 2016 |
618 | |||
| 2017 |
622 | |||
| 2018 |
604 | |||
| Thereafter |
— | |||
|
|
|
|||
| $ | 2,453 | |||
|
|
|
|||
Rental expense for operating leases in the amount of $0.5 million has been recorded in the consolidated statements of operations for each of the years ended December 31, 2014, 2013 and 2012, respectively. In May 2008, the Company entered into a lease agreement to lease office and laboratory space for its headquarters in Seattle, Washington totaling approximately 17,000 square feet. The lease, which expires in December 2018, provides for a monthly base rent of $47,715 increasing to $52,259 in 2018. The Company has also entered into operating lease obligations through July 2017 for certain office equipment, which are included in the table above.
F-28
Table of Contents
Guarantees
In the normal course of operations, the Company indemnifies counterparties in transactions such as purchase and sale contracts for assets or shares, service agreements, director/officer contracts and leasing transactions. These indemnification agreements may require the Company to compensate the counterparties for costs incurred as a result of various events, including environmental liabilities, changes in (or in the interpretation of) laws and regulations, or as a result of litigation claims or statutory sanctions that may be suffered by the counterparties as a consequence of the transaction. The terms of these indemnification agreements vary based upon the contract, the nature of which prevents the Company from making a reasonable estimate of the maximum potential amount that could be required to pay to counterparties. Historically, the Company has not made any significant payments under such indemnification agreements and no amounts have been accrued in the accompanying condensed consolidated financial statements with respect to these indemnification guarantees.
| 12. | SUBSEQUENT EVENTS |
On February 11, 2015, the Company closed concurrent but separate underwritten offerings of 13,500,000 shares of its common stock at a price to the public of $1.50 per share, for estimated gross proceeds of , approximately $20 million and 1,333 shares of its Series B Convertible Preferred Stock at a price to the public of $1,500 per share, for estimated gross proceeds of approximately $2 million. Each share of Series B Convertible Preferred Stock is non-voting and convertible into 1,000 shares of the Company’s common stock, provided that conversion will be prohibited if, as a result, the holder and its affiliates would beneficially own more than 4.99% of the common stock then outstanding. As part of the common stock offering, the Company also granted the underwriters a 30-day option to purchase 2,025,000 additional shares of the Company’s common stock. On February 18, 2015, the Company closed a partial exercise of the underwriter’s option to purchase 1,199,660 additional shares of the Company’s common stock, at a price to the public of $1.50 per share, less underwriting discounts and commissions, which resulted in net proceeds to the Company of approximately $1.7 million. Aggregate gross proceeds from the offerings were approximately $24.0 million. Aggregate net proceeds from the offerings, after underwriting discounts, commissions and estimated expenses of $1.6 million, were approximately $22.4 million. Concurrent but separate from these offerings, the Company entered into an exchange agreement with certain affiliates of Biotechnology Value Fund (BVF) to exchange 4,000,000 shares of common stock previously purchased by BVF for 4,000 shares of Series B Convertible Preferred Stock.
| 13. | CONDENSED QUARTERLY FINANCIAL DATA (unaudited) |
The following table contains selected unaudited statement of operations information for each quarter of 2014 and 2013. The unaudited information should be read in conjunction with the Company’s audited financial statements and related notes included elsewhere in this report. The Company believes that the following unaudited information reflects all normal recurring adjustments necessary for a fair presentation of the information for the periods presented. The operating results for any quarter are not necessarily indicative of results for any future period.
Quarterly Financial Data:
| Three Months Ended, | ||||||||||||||||
| March 31 | June 30 | September 30 | December 31 | |||||||||||||
| (In thousands, except per share data) | ||||||||||||||||
| 2014 |
||||||||||||||||
| Operating expenses(1) |
$ | 7,160 | $ | 7,797 | $ | 8,020 | $ | 27,858 | ||||||||
| Net loss(1)(2) |
(9,616 | ) | (6,032 | ) | (6,736 | ) | (27,578 | ) | ||||||||
| Net loss per share — basic and diluted |
(0.14 | ) | (0.09 | ) | (0.09 | ) | (0.30 | ) | ||||||||
| 2013 |
||||||||||||||||
| Operating expenses(3) |
$ | 7,991 | $ | 18,047 | $ | 7,602 | $ | 7,583 | ||||||||
| Net loss(3)(4) |
(8,288 | ) | (16,398 | ) | (7,710 | ) | (6,363 | ) | ||||||||
| Net loss per share — basic and diluted |
(0.14 | ) | (0.28 | ) | (0.12 | ) | (0.09 | ) | ||||||||
| (1) | Operating expenses and net loss for the three months ended December 31, 2014 includes an upfront fee of $20.0 million paid to Array in connection with our license agreement in December 2014 (see Note 8). |
| (2) | Net loss for the three months ended March 31, June 30, September 30 and December 31, 2014 includes change in fair value of warrants income (expense) of approximately $(2.5) million, $1.7 million, $1.3 million and $0.3 million respectively (see Note 3). |
| (3) | Operating expenses and net loss for the three months ended June 30, 2013 includes an upfront fee of $10.0 million paid to Array upon initiation of a collaboration agreement that the Company entered into in May 2013 (see Note 8). |
F-29
Table of Contents
| (4) | Net loss for the three months ended March 31, June 30, September 30 and December 31, 2013 includes change in fair value of warrants income (expense) of approximately $(0.3) million, $1.6 million, $(0.2) million and $1.2 million respectively (see Note 3). |
F-30
