Attached files
| file | filename |
|---|---|
| EX-21.1 - EXHIBIT 21.1 - Option Care Health, Inc. | bios-ex211xx20141231x10k.htm |
| EX-23.1 - EXHIBIT 23.1 - Option Care Health, Inc. | bios-ex231xx20141231x10k.htm |
| EX-31.2 - EXHIBIT 31.2 - Option Care Health, Inc. | bios-ex312xx20141231x10k.htm |
| EX-23.2 - EXHIBIT 23.2 - Option Care Health, Inc. | bios-ex232xx20141231x10k.htm |
| EX-32.1 - EXHIBIT 32.1 - Option Care Health, Inc. | bios-ex321xx20141231x10k.htm |
| EX-31.1 - EXHIBIT 31.1 - Option Care Health, Inc. | bios-ex311xx20141231x10k.htm |
| EX-32.2 - EXHIBIT 32.2 - Option Care Health, Inc. | bios-ex322xx201412x31x10k.htm |
| EX-10.7 - EXHIBIT 10.7 - Option Care Health, Inc. | bios-ex107xx201412x31x10k.htm |
| EX-10.17 - EXHIBIT 10.17 - Option Care Health, Inc. | bios-ex1017xx201412x31x10xk.htm |
| EXCEL - IDEA: XBRL DOCUMENT - Option Care Health, Inc. | Financial_Report.xls |
| EX-12 - EXHIBIT 12 - Option Care Health, Inc. | bios-ex12xx20141231x10k.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form 10-K
(Mark One) | |
þ | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2014 | |
OR | |
o | PERIODIC REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to | |
Commission file number: 0-28740

BioScrip, Inc.
(Exact name of registrant as specified in its charter)
Delaware | 05-0489664 |
(State of incorporation) | (I.R.S. Employer Identification No.) |
100 Clearbrook Road, Elmsford NY | 10523 |
(Address of principal executive offices) | (Zip Code) |
Registrant’s telephone number, including area code:
914-460-1600
Securities registered pursuant to Section 12(b) of the Act:
Title of each class | Name of each exchange on which registered |
Common Stock, $0.0001 par value per share | NASDAQ Global Market |
Securities registered pursuant to section 12(g) of the Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes o No þ
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act Yes o No þ
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes þ No o
Indicate by check mark whether the registrant has submitted electronically and posted to its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes þ No £
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (§ 229.405 of this chapter) is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. o
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, or a non-accelerated filer. See definition of “large accelerated filer”, “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act.
Large accelerated filer þ Accelerated filer o Non-accelerated filer o Smaller reporting company o
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes o No þ
The aggregate market value of the registrant’s Common Stock held by non-affiliates of the registrant as of June 30, 2014, the last business day of the registrant’s most recently completed second fiscal quarter, was approximately $566,219,922 based on the closing price of the Common Stock on the Nasdaq Global Market on such date.
On February 25, 2015, there were 68,636,965 shares of the registrant’s Common Stock outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the registrant’s definitive proxy statement for its 2015 Annual Meeting of Stockholders to be filed with the SEC within 120 days after the close of the registrant’s fiscal year are incorporated by reference into Part III of this Annual Report.
TABLE OF CONTENTS
Page Number | ||
PART I | ||
PART II | ||
PART III | ||
PART IV | ||
2
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
This Annual Report on Form 10-K (“Annual Report”) contains “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995. Forward-looking statements relate to expectations, beliefs, future plans and strategies, anticipated events or trends concerning matters that are not historical facts or that necessarily depend upon future events. In some cases, you can identify forward-looking statements by terms such as “may,” “will,” “should,” “could,” “would,” “expect,” “plan,” “anticipate,” “believe,” “estimate,” “project,” “predict,” “potential” and similar expressions. Specifically, this Annual Report contains, among others, forward-looking statements about:
• | our ability to make principal and interest payments on our debt and unsecured notes and satisfy the other covenants contained in our senior secured credit facility and other debt agreements; |
• | our high level of indebtedness; |
• | our expectations regarding financial condition or results of operations in future periods; |
• | our future sources of, and needs for, liquidity and capital resources; |
• | our expectations regarding economic and business conditions; |
• | our expectations regarding potential legislative and regulatory changes impacting the level of reimbursement received from the Medicare and state Medicaid programs; |
• | our internal control over financial reporting |
• | periodic reviews and billing audits from governmental and private payors; |
• | our expectations regarding the size and growth of the market for our products and services; |
• | our business strategies and our ability to grow our business; |
• | the implementation or interpretation of current or future regulations and legislation, particularly governmental oversight of our business; |
• | our ability to maintain contracts and relationships with our customers; |
• | our ability to avoid delays in payment from our customers; |
• | sales and marketing efforts; |
• | status of material contractual arrangements, including the negotiation or re-negotiation of such arrangements; |
• | our ability to address cybersecurity risks; |
• | our ability to maintain supplies and services, which could be impacted by force majeure events such as war, strike, riot, crime or “acts of God” such as hurricanes, flooding, blizzards or earthquakes; |
• | future capital expenditures; |
• | our ability to hire and retain key employees; |
• | our ability to successfully execute our succession plans; |
• | our ability to execute our acquisition and growth strategy; |
• | our ability to successfully integrate businesses we may acquire; and |
• | other risks and uncertainties described from time to time in our filings with the U.S. Securities and Exchange Commission (the “SEC”). |
The forward-looking statements contained in this Annual Report reflect our current views about future events, are based on assumptions, and are subject to known and unknown risks and uncertainties. Many important factors could cause actual results or achievements to differ materially from any future results or achievements expressed in or implied by our forward-looking statements. Many of the factors that will determine future events or achievements are beyond our ability to control or predict. Certain of these are important factors that could cause actual results or achievements to differ materially from the results or achievements reflected in our forward-looking statements.
The forward-looking statements contained in this Annual Report reflect our views and assumptions only as of the date this Annual Report is signed. The reader should not place undue reliance on forward-looking statements. Except as required by law, we assume no responsibility for updating any forward-looking statements.
We qualify all of our forward-looking statements by these cautionary statements. In addition, with respect to all of our forward-looking statements, we claim the protection of the safe harbor for forward-looking statements contained in the Private Securities Litigation Reform Act of 1995.
3
PART I
Item 1. | Business |
Overview
BioScrip, Inc. (“BioScrip”, “we”, “us”, “our” or the “Company”) is a national provider of infusion and home care management solutions. We partner with physicians, hospital systems, skilled nursing facilities, healthcare payors and pharmaceutical manufacturers to provide patients access to post-acute care services. We operate with a commitment to bring customer-focused pharmacy and related healthcare infusion therapy services into the home or alternate-site setting. By collaborating with the full spectrum of healthcare professionals and the patient, we aim to provide cost-effective care that is driven by clinical excellence, customer service and values that promote positive outcomes and an enhanced quality of life for those whom we serve.
Our platform provides nationwide service capabilities and the ability to deliver clinical management services that offer patients a high-touch, community-based and home-based care environment. Our core services are provided in coordination with, and under the direction of, the patient’s physician. Our multidisciplinary team of clinicians, including pharmacists, nurses, dieticians and respiratory therapists, work with the physician to develop a plan of care suited to our patient’s specific needs. Whether in the home, physician office, ambulatory infusion center, skilled nursing facility or other alternate sites of care, we provide products, services and condition-specific clinical management programs tailored to improve the care of individuals with complex health conditions such as gastrointestinal abnormalities, infectious diseases, cancer, multiple sclerosis, organ and blood cell transplants, bleeding disorders, immune deficiencies and heart failure.
We were incorporated in Delaware in 1996 as MIM Corporation, with our primary business and operations being pharmacy benefit management services. Over the years, we expanded our service offerings to include home infusion services and home health services.
Strategic Assessment and Transactions
In 2010, we commenced a strategic assessment of our business and operations. The assessment examined our market strengths and opportunities and compared our position to that of our competitors. As a result of this assessment and subsequent assessments, we have focused our growth on investments in the Infusion Services segment, which remains the primary driver of our growth strategy. We continue to assess the value of each of our business lines, including those not targeted for growth.
On February 1, 2012, we entered into a Community Pharmacy and Mail Business Purchase Agreement by and among Walgreen Co. and certain of its subsidiaries for the sale of certain assets, rights and properties (the “Pharmacy Services Asset Sale”) related to our traditional and specialty pharmacy mail operations and community retail pharmacy stores. We received a total purchase price of $173.8 million resulting in a pretax gain of $108.2 million net of transaction costs and other one-time charges.
Following the completion of the Pharmacy Services Asset Sale, we continued to execute our strategic plan by deploying the proceeds toward strategic business acquisitions.
On July 31, 2012, we acquired 100% of InfuScience, Inc. (“InfuScience”) for a cash payment of $38.3 million at closing and an additional $3.0 million of contingent consideration that was paid based on the results of operations during the 24 month period following the acquisition. InfuScience historically acquired, developed and operated businesses providing alternate site infusion pharmacy services through five infusion centers located in Eagan, Minnesota; Omaha, Nebraska; Chantilly, Virginia; Charleston, South Carolina; and Savannah, Georgia.
On February 1, 2013, we acquired 100% of the ownership interest in HomeChoice Partners, Inc. (“HomeChoice”) for a cash purchase price of $72.9 million at closing. The purchase agreement provided that the purchase price may also be increased by contingent consideration of up to $10.0 million if HomeChoice reaches certain performance milestones in the first year following the closing and an additional $10.0 million if HomeChoice reaches certain performance milestones in the second year following the closing, for total possible contingent consideration of up to $20.0 million. HomeChoice has not achieved its performance milestones for the period from acquisition through December 31, 2014 and, as a result, the probability of payment of any contingent consideration is negligible. We funded the acquisition with a combination of cash on hand and drawing on our revolving credit facility. HomeChoice was a provider of alternate-site infusion pharmacy services. Prior to our acquisition, HomeChoice serviced approximately 15,000 patients annually and had 14 infusion pharmacy locations in Pennsylvania, the District of Columbia, Maryland, Virginia, North Carolina, South Carolina, Georgia, Missouri and Alabama.
4
On August 23, 2013, we completed the acquisition of substantially all of the assets and assumption of certain liabilities that constituted the home infusion business (the “CarePoint Business”) of CarePoint Partners Holdings LLC for a cash purchase price of $211.1 million at closing. The purchase agreement provided that the purchase price could have increased by contingent consideration of $10.0 million if the CarePoint Business achieved a specified level of product gross profit during the one year measurement period following the closing date. The CarePoint Business did not achieve the specified level of product gross profit during the measurement period and, as a result, it is unlikely that any contingent consideration would be due to the sellers. We funded the cash payment at closing with a combination of cash on hand and $150.0 million in borrowings under the Delayed Draw Term Loan Facility. CarePoint was a provider of home and alternate-site infusion therapy for patients with complex, acute and chronic illnesses. CarePoint serviced approximately 20,500 patients annually and had 28 sites of service in nine states in the East Coast and Gulf Coast regions prior to our acquisition.
Consistent with our continuing strategic evaluation of our non-core businesses and our decision to continue to focus growth initiatives and capital in the Infusion Services segment, we completed the sale of substantially all of our Home Health Services segment to LHC Group, Inc. and certain of its subsidiaries on March 31, 2014. We received total consideration of approximately $60.6 million in cash including adjustments to net working capital. A portion of the net proceeds from the sale was used to pay down a portion of our outstanding debt.
Business Outlook
The Pharmacy Services Asset Sale caused us to perform further strategic assessments of our business and operations in order to align our corporate structure with our remaining business operations. As a result of the reassessment and subsequent realignment, we have focused on expanding revenue opportunities and lowering corporate overhead as well as redeploying our resources strategically. These actions have resulted in employee severance, retention bonus payments, write-downs of certain long-lived assets and accelerated recognition of expense associated with certain of our contractual obligations. The impact of these efforts included a reduction in salaries, benefits, rent and other facility costs. The redeployment of resources following the Pharmacy Services Asset Sale has better positioned us for growth in our strategic areas of operation; however, the impact of these actions on our future consolidated financial statements cannot be estimated.
Our Strengths
Our company has a number of competitive strengths, including:
We Have a Local Competitive Market Position within Our National Platform and Infrastructure
As of December 31, 2014, we had a total of 77 service locations in 29 states, executive offices in New York and corporate offices in Minnesota. Our model combines local presence with comprehensive clinical programs for multiple therapies and specific delivery technologies (infusible and injectible). We also have the capabilities and payor relationships to distribute pharmaceuticals to all 50 states. We have relationships with more than 1,000 payors, including Managed Care Organizations (“MCOs”), government programs such as Medicare and Medicaid and other commercial insurers (“Third Party Payors”). We believe payors generally favor fully integrated vendors that can provide high-touch pharmacy solutions to their patients. We believe we are one of a limited number of pharmacy providers that can offer a truly national, integrated and comprehensive approach to managing a patient’s chronic or acute conditions.
Diversified and Favorable Payor Base
We provide prescription drugs, infusion and clinical management services for a broad range of commercial and governmental payors. Approximately 77% of our payor base is comprised of commercial payors that operate at a national, regional or local level. One national commercial payor, UnitedHealthcare, accounted for 22% of consolidated revenue during the year ended December 31, 2014. No other commercial payor accounted for more than 4% of consolidated revenue during the year ended December 31, 2014. Government payors, including Medicare, state Medicaid and other government payors, accounted for 23% of consolidated revenue during the year ended December 31, 2014. For the year ended December 31, 2014, Medicare accounted for 11.3% of our consolidated revenue with no single state Medicaid program accounting for more than 5% of consolidated revenue.
The costs savings realized by administering infusion therapies in the home versus hospitals, skilled nursing facilities or other post-acute care facilities positions our business to benefit from healthcare reform. Under the current plan, Medicare offers limited reimbursement for home infusion therapy products and services. As healthcare reform continues to focus on cost-reduction initiatives, home infusion and other low-cost in-home therapeutic alternatives are expected to be impacted favorably by revised
5
coverage. Significant health plan cost savings per infusion can be achieved when therapy is provided at an alternative treatment site compared to other patient settings.
Effective Care Management Clinical Programs that are Designed to Produce Positive Clinical Outcomes and Reduce Readmissions
Our diversified and comprehensive clinical programs, which span numerous therapeutic areas, are designed to improve patient outcomes. Our home infusion business provides traditional infusion therapies for acute conditions with accompanying clinical management and home care. Our infusion product offerings and services are also designed to treat patients with chronic infusion needs. In addition to the long-term treatment associated with these chronic conditions, these conditions require ongoing caregiver and patient counseling and education regarding patient treatment and ongoing monitoring and communication with physicians to encourage patients to follow therapies prescribed by the physicians.
Our Centers of Excellence focus on interdisciplinary teams to provide clinical excellence with outstanding personal service. Externally qualified by a panel of leading industry experts, the Centers employ evidence-based standards of care, policies and procedures built on industry-recognized best practices. They are led by specialists with advanced certifications and training who are dedicated to developing, improving and sustaining clinical services to achieve optimal patient outcomes and exceed the expectations of patients and referral sources.
Our clinical management programs in multiple disease-state therapy provide us opportunities to cross-sell services and technologies. We believe we have earned a positive reputation among patients, physicians, payors and pharmaceutical manufacturers by providing quality service and favorable clinical outcomes. We believe our platform provides the necessary programs and services for better and more efficient clinical outcomes for our patients.
Operating and Reporting Segments
Following the sale of our Home Health Business on March 31, 2014, our operating and reportable segments are “Infusion Services” and “PBM Services.” These segments reflect how our chief operating decision maker reviews our results in terms of allocating resources and assessing performance.
The Infusion Services operating and reportable segment provides services consisting of home infusion therapy, respiratory therapy and the provision of durable medical equipment, products and services. Infusion services include the dispensing and administering of infusion-based drugs, which typically require additional nursing and clinical management services, equipment to administer the correct dosage and patient training designed to improve patient outcomes. Home infusion services also include the dispensing of certain self-injectable therapies.
The integrated pharmacy benefit management (“PBM”) Services operating and reportable segment consists of integrated PBM services, which primarily consists of discount card programs. The discount card programs provide a cost effective alternative for individuals who may be uninsured, underinsured or may have restrictive coverage that disallows reimbursement for certain medications. Under these discount programs, individuals who present a discount card at one of our participating network pharmacies receive prescription medications at a discounted price compared to the retail price.
Products and Services
Infusion Services
We are one of the largest providers of home infusion services in the United States. Home infusion involves the preparation, delivery, administration and clinical monitoring of pharmaceutical treatments that are administered to a patient via intravenous (into the vein), subcutaneous (into the fatty layer under the skin), intramuscular (into the muscle), intra-spinal (into the membranes around the spinal cord) and enteral (into the gastrointestinal tract) methods. These methods are employed when a physician determines that the best outcome can be achieved through utilization of one or more of the therapies provided through the routes of administration described above.
Our home infusion services primarily involve the intravenous administration of medications treating a wide range of acute and chronic conditions, such as infections, nutritional deficiencies, various immunologic and neurologic disorders, cancer, pain and palliative care. Our services are usually provided in the patient’s home but may also be provided at outpatient clinics, skilled nursing facilities, the physician’s office or at one of our ambulatory infusion centers. We receive payment for our home health services and medications, pursuant to provider agreements with government sources, such as Medicare and Medicaid programs, MCOs and Third Party Payers.
6
We provide a wide array of home infusion products and services to meet the diverse needs of physicians, patients and payors. Diseases commonly requiring infusion therapy include infections that are unresponsive to oral antibiotics, cancer and cancer-related pain, dehydration and gastrointestinal diseases or disorders that prevent normal functioning of the gastrointestinal tract, which require IV fluids, parenteral or enteral nutrition. Other conditions treated with infusion therapies may include chronic diseases such as heart failure, Crohn’s disease, hemophilia, immune deficiencies, multiple sclerosis, rheumatoid arthritis, growth disorders and genetic enzyme deficiencies, such as Gaucher’s or Pompe’s disease. The therapies most commonly provided are listed below:
Therapy Type | Description |
Parenteral Nutrition (PN) | Provide intravenous nutrition customized to the nutritional needs of the patient. PN is used in patients that cannot meet their nutritional needs via other means due to disease process or as a complication of a disease process, surgical procedure or congenital anomaly. PN may be used short term or chronically. |
Enteral Nutrition (EN) | Provide nutrition directly to the stomach or intestine in patients who cannot chew or swallow nutrients in the usual manner. EN may be delivered via a naso-gastric tube or a tube placed directly into the stomach or intestine. EN may be used short term or chronically. |
Antimicrobial Therapy | Provide intravenous antimicrobial medications used in the treatment of patients with various infectious processes such as: HIV/AIDS, wound infections, pneumonia, osteomyelitis, cystic fibrosis, Lyme disease and cellulitis. May also be used in patients with disease processes or therapies that may lead to infections when oral antimicrobials are not effective. |
Chemotherapy | Provide injectable and/or infused medications in the home or the prescriber’s office for the treatment of cancer. Adjuvant medications may also be provided to minimize the side effects associated with chemotherapy. |
Immune Globulin (IG) Therapy | Provide immune globulins intravenously or subcutaneously on an as-needed basis in patients with immune deficiencies or auto-immune diseases. This therapy may be chronic based on the etiology of the immune deficiency. |
Pain Management | Provide analgesic medications intravenously, subcutaneously or epidurally. This therapy is generally administered as a continuous infusion via an internal or external infusion pump to treat severe pain associated with diseases such as COPD, cancer and severe injury. |
Blood Factor Therapies | Provide medications to patients with one of several inherited bleeding disorders in which a patient does not manufacture the clotting factors necessary or use the clotting factors their liver makes appropriately in order to halt an external or internal bleed in response to a physical injury or trauma. |
Inotropes Therapy | Provide intravenous inotropes in the home for the treatment of heart failure, either in anticipation of cardiac transplant or to provide palliation of heart failure symptoms. Inotropes increase the strength of weak heart muscles to pump blood. The therapy is only started in late phase heart failure when alternative therapies proved inadequate. |
Respiratory Therapy/Home Medical Equipment | Provide oxygen systems, continuous or bi-level positive airway pressure devices, nebulizers, home ventilators, respiratory devices, respiratory medications and other medical equipment. |
Patients generally are referred to us by physicians, hospital discharge planners, MCOs and other referral sources. Our medications are compounded and dispensed under the supervision of a registered pharmacist in a state licensed pharmacy that is accredited by an independent accrediting organization. We only compound pursuant to a patient specific prescription and do so in compliance with USP 797 standards. A national accrediting organization surveys our pharmacies for compliance with the USP 797 standards for sterile drug compounding pharmacies and has confirmed that we are in compliance with such standards. The therapy is typically administered in the patient’s home by a registered nurse or trained caregiver. Depending on the preferences of the patient or the payor, these services may also be provided at one of our ambulatory infusion centers, a physician's office or another alternate site of administration.
7
We currently have relationships with a large number of MCOs and other Third Party Payors to provide home infusion services. These relationships are at a national, regional or local level. A key element of our business strategy is to leverage our relationships, geographic coverage, clinical expertise and reputation in order to gain contracts with payors. Our infusion service contracts typically provide for us to receive a fee for preparing and delivering medications and related equipment to patients in their homes. Pricing is typically negotiated in advance on the basis of Average Wholesale Price (“AWP”) minus some percentage of contractual discount, or Average Sales Price (“ASP”) plus some percentage. In addition, we typically receive a per diem payment for the service and supplies component of care provided to patients in connection with infusion services and a visit rate for the associated skilled nursing provided.
PBM Services
We also provide prescription discount card programs and integrated PBM services. These services are designed to offer employers, MCOs, Third Party Administrators (“TPAs”) and other Third Party Payors (collectively, “Plan Sponsors”) cost-effective delivery of pharmacy benefit plans including the low cost distribution of mail services for plan members who receive traditional maintenance medications through our network pharmacies that deliver traditional and specialty medications through mail facilities and retail stores.
Prescription Discount Card Programs
Our discount card services provide a cost effective alternative for individuals who may be uninsured or underinsured, or who may have restrictive coverage that disallows reimbursement for certain medications. Under these discount programs, individuals who present a discount card at one of our participating network pharmacies receive prescription medications at a discounted price as compared to the retail price. The discount card programs are designed and marketed by consumer marketing organizations to whom we provide administrative and claims processing services under contract. Our marketing organization clients receive a broker fee or commission for the sales generated.
PBM Formulary and Benefit Design
Our funded PBM business involves working with our Plan Sponsors to offer formularies and benefit plan designs that meet their specific program requirements. Formulary design assists in controlling program costs to the extent consistent with accepted medical and pharmacy practices and applicable law, primarily through three principal techniques: (i) tiered co-pay or percentage coinsurance designs, which provide lower co-pays for formulary preferred medications and higher co-pays for non-preferred medications, or charge a percentage of the prescription price to the member at different percentages based on the preferred or non-preferred status of a drug; (ii) generic substitution, which involves the selection of a generic drug as a cost-effective alternative to its bio-equivalent brand name drug; and/or (iii) therapeutic interchange, which involves the selection of a lower cost brand name drug as an alternative to a higher priced brand name drug within a therapeutic class. Formulary rebates on brand name drugs are negotiated with drug manufacturers based on the drug’s preferred status and are typically shared with Plan Sponsors. Our rebates are managed and administered by third party vendors.
PBM Drug Usage Evaluation
Drug usage is evaluated on a concurrent, prospective and/or retrospective basis utilizing the real-time POS system and information systems for multiple drug interactions, duplication of therapy, step therapy protocol enforcement, minimum/maximum dose range edits, compliance with prescribed utilization levels and early refill notification. In addition, we maintain a drug utilization review program through which select medication therapies are reviewed and data is collected, analyzed and reported for management applications.
Sales and Marketing
We have over 225 sales and marketing representatives and over 1,000 payor relationships including MCOs, Medicare Part D pharmacy networks, other government programs such as Medicare and Medicaid and other Third Party Payors. Our sales and marketing efforts are focused on payors, healthcare systems and physician prescribers and are driven by dedicated managed care and physician sales teams as well as home health care consultants. Our sales and marketing strategies include the development of strong relationships with key referral sources, such as physicians, hospital discharge planners, case managers, long-term care facilities and other healthcare professionals, primarily through regular contact with the referral sources and by fulfilling the care and service expectations of our many customers. Contracts with Third Party Payors, including MCOs, are an integral component for sales success.
8
Through our PBM Services, we also have over 100 relationships with PBM clients, including Medicaid MCOs, employers, TPAs, workers compensation providers and discount card marketers. A range of direct sales methods are used to promote the discount card program and add new marketing organizations.
Intellectual Property
We own and use a variety of trademarks, trade names and service marks, including without limitation “BioScrip”, “BioScrip Infusion Services”, “BioScrip Medical Supply Services”, “BioScrip Nursing Services”, “BioScrip PBM Services”, “BioScrip Pharmacy Services”, “Applied Health Care”, “CarePoint Partners”, “Critical Homecare Solutions”, “HomeChoice Partners”, “InfuScience”, “InfusionCare”, “Infusion Partners”, “Infusion Solutions”, “New England Home Therapies”, “Option Health”, “Professional Home Care Services”, “Wilcox Home Infusion” and “Wilcox Medical”, each of which has either been registered at the state or federal level or is being used pursuant to common law rights. We are recognized in local markets by several of these trade names, but we do not consider the marks material to our business.
Competition
Infusion Services
The home infusion services market is highly competitive with a limited number of national providers and numerous local and regional companies. Providers strive to differentiate their services based on their responsiveness to patient needs, quality of care, reputation with referral sources and cost of service. Our Centers of Excellence offer a high touch, high service approach to care on a local basis, which we believe differentiates our service.
Our competitors within the home infusion market include Walgreen Infusion Services, Coram CVS/specialty infusion services (a division of CVS Health), Accredo Health Group, Inc. (a subsidiary of Express Scripts Holding Company) and various regional and local providers of alternate site healthcare services such as hospitals and physician practices.
Pharmacy Benefits Management and Discount Card Services
In the PBM market we compete with large national PBMs and a number of smaller and regional PBMs. The large PBMs have integrated mail service and specialty pharmacy services and are very competitive with all Plan Sponsors. These national PBM companies include Express Scripts, Inc., Catamaran Corp. and CVS/Caremark Corp. In the discount card services market there are numerous competitors of various sizes. Generally, PBMs contract with marketing and sales organizations that market the cards either regionally or nationally via various sources, such as direct mail, internet, email, and sub-brokers/sales representatives.
Existing and potential competitors within the pharmacy discount card market include Catamaran Corp., MedImpact, Argus, Agility, Inc. and local marketers across the country.
Information Technology
In 2014 our Information Technology (“IT”) department had completed several significant consolidation projects including the primary transactional platform, and the datacenters from previous acquisitions. In addition, our IT department completed the divestiture of the home health care division, as well as, completing the majority of the data center migration to a hosted managed data center.
In 2015, in addition to completing the data center migration, our IT investments are expected to include a nurse resource scheduling platform and an upgrade to the Voice-Over-IP (VOIP) / Call Center platform.
Financial Information about Segments
Segment financial information is provided in Note 11 of the Notes to the Consolidated Financial Statements.
Government Regulation
The healthcare industry is subject to extensive regulation by a number of governmental entities at the federal, state and local level. The healthcare regulatory landscape is also subject to frequent change. Laws and regulations in the healthcare industry are extremely complex and, in many instances, the industry does not have the benefit of significant regulatory or judicial interpretation. Moreover, our business is impacted not only by those laws and regulations that are directly applicable to us but also by certain
9
laws and regulations that are applicable to our payors, vendors and referral sources. While our management believes we are in substantial compliance with all of the existing laws and regulations applicable to us, such laws and regulations are subject to rapid change and often are uncertain in their application. Further, to the extent we engage in new business initiatives, we must continue to evaluate whether new laws and regulations are applicable to us. As controversies continue to arise in the healthcare industry, federal and state regulation and enforcement priorities in this area may increase, the impact of which cannot be predicted. There can be no assurance that we will not be subject to scrutiny or challenge under one or more of these laws or that any such challenge would not be successful. Any such challenge, whether or not successful, could have a material adverse effect upon our business and consolidated financial statements. In addition, the Patient Protection and Affordable Care Act, or PPACA, and the Health Care and Education Reconciliation Act of 2010, which amended PPACA (collectively, the “Health Reform Law”), may have a considerable impact on the financing and delivery of health care and conceivably could have a material adverse effect on our business.
Among the various federal and state laws and regulations which may govern or impact our current and planned operations are the following:
Medicare and Medicaid Reimbursement
Many of the products and services that we provide are reimbursed by Medicare and state Medicaid programs and are therefore subject to extensive government regulation. Medicare is a federally funded program that provides health insurance coverage for qualified persons age 65 or older and for some disabled persons with certain specific conditions. The Medicare Program currently consists of four parts: Medicare Part A, which covers, among other things, inpatient hospital, skilled nursing facility, home nursing and certain other types of healthcare services; Medicare Part B, which covers physicians' services, outpatient services, items and services provided by medical suppliers and a limited number of prescription drugs; Medicare Part C, which generally allows beneficiaries to enroll in private healthcare plans (known as Medicare Advantage plans); and Medicare Part D, established by the Medicare Prescription, Drug, Improvement and Modernization Act of 2003 (“Medicare Modernization Act”), which provides for a voluntary prescription drug benefit.
The Medicaid Program provides medical benefits to groups of low-income and disabled individuals, some of whom may have inadequate or no medical insurance. Although the federal government establishes general guidelines for the program, Medicaid is a state administered program and each state sets its own guidelines regarding eligibility and covered services, subject to certain minimum federal requirements.
Congress often enacts legislation that affects, positively or negatively, the reimbursement rates of Medicare providers and also may impact Medicaid providers. Generally, Medicare provider payment modifications occur in the context of budget reconciliation; however, Medicare changes also may occur in the context of broader healthcare policy legislation, including the Health Reform Law. In the last several years, Congress has reduced Medicare reimbursement for various providers, including Medicare Part B suppliers.
Approximately 23% of our revenue for the year ended December 31, 2014 was derived directly from Medicare, Medicaid or other government-sponsored healthcare programs. Also, we indirectly provide services to beneficiaries of Medicare, Medicaid and other government-sponsored healthcare programs through managed care entities. Should there be material changes to federal or state reimbursement methodologies, regulations or policies, our direct reimbursements from government-sponsored healthcare programs, as well as service fees that relate indirectly to such reimbursements, could be adversely affected. In addition, certain state Medicaid programs only allow for reimbursement to pharmacies residing in the state or in a border state. While we believe we can service our current Medicaid patients through our existing infusion pharmacies, there can be no assurance that additional states will not enact in-state dispensing requirements for their Medicaid programs. To the extent such requirements are enacted, certain therapeutic pharmaceutical reimbursements could be adversely affected.
Medicare Parts B and D
We receive reimbursement for infusion therapy under both Medicare Part B and Medicare Part D. In connection with the enactment of the Medicare Modernization Act, the Centers for Medicare and Medicaid Services (“CMS”) promulgated a substantial volume of new regulations implementing the federal government’s Voluntary Prescription Drug Benefit Program, known as Medicare Part D. CMS has attempted to clarify issues regarding coverage of infused drugs under Medicare Part D and the relationship with existing coverage under Medicare Part B. In certain cases, both Medicare Parts B and D will cover identical infused drugs. CMS has stated that coverage is generally determined by the diagnosis and the method of drug delivery.
Under Medicare Part D, the ingredient costs and dispensing fees associated with the administration of home infusion therapies are covered. Under Medicare Part B, no separate dispensing reimbursement is available. For eligible Medicare beneficiaries, the
10
cost of equipment and supplies associated with infused drugs covered under Medicare Part D will continue to be reimbursed on a limited basis under Medicare Part A or Part B, as applicable, and the cost of professional services associated with infused covered Medicare Part D drugs will continue to be reimbursed on a limited basis under Medicare Part A. For beneficiaries who are dually eligible for benefits under Medicare and a state Medicaid program, Medicaid covered infused drugs will be reimbursed under individual state coverage guidelines if coverage is denied by Medicare.
The U.S. Department of Health and Human Services (“HHS”), Office of the Inspector General (“OIG”) and CMS continue to issue guidance with regard to the Medicare Part D program and compliance with related federal laws and regulations by Medicare Part D sponsors and their subcontractors. For example, on May 23, 2014, CMS finalized regulations that made a number of changes to Medicare Part D. The receipt of funds made available through this program may be subject to compliance with these new regulations, the established laws and regulations governing the federal government’s payment for healthcare goods and services, and provisions in contracts with the prescription drug plans. There are many uncertainties about the financial and regulatory risks of participating in the Medicare Part D program, and these risks could negatively impact our business in future periods.
Medicare Part C - Medicare Advantage
Under Medicare Part C, beneficiaries can choose to enroll in a Medicare Advantage plan sponsored by an MCO. Providers who serve these beneficiaries must contract with the applicable MCO plan. Reimbursement and other requirements imposed on the provider are governed by the agreement with the MCO plan rather than by statute or regulation and as such vary from plan to plan. Medicare advantage plans are permitted to cover certain services that fee-for-service Medicare does not cover. We currently have contracts with a number of Medicare advantage plans.
Legislative Changes to Medicare Reimbursement
The Medicare Prescription Drug, Improvement, and Modernization Act of 2003 established requirements for a competitive bidding program for determining Medicare reimbursement rates for certain items of durable medical equipment, prosthetics, orthotics and supplies (“DMEPOS”), including enteral nutrients, supplies and equipment, certain respiratory therapy and home medical equipment products and external infusion pumps and supplies. CMS has the discretion to determine which products will be subject to competitive bidding.
The first round of competitive bidding occurred in nine metropolitan areas around the country, called Competitive Bidding Areas (“CBAs”) and was effective from January 1, 2011 through December 31, 2013. Round 1 did not have a material impact on our business. A Round 1 Recompete was also conducted in the same nine CBAs and included six product categories, including external infusion pumps. The prices for the Round 1 Recompete went into effect January 1, 2014 and will expire December 31, 2016.
The second round of competitive bidding was conducted in 100 additional CBAs for eight product categories. New prices for the Round 2 CBAs went into effect July 1, 2013 and will expire June 30, 2016. Bids for a Round 2 Recompete are expected in the first quarter of 2015. The Round 2 Recompete is for the same geographic areas that were included in the second round of competitive bidding, although due to the Office of Management and Budget’s updates there are 117 CBAs in the Round 2 Recompete. The Round 2 Recompete includes seven product categories. The prices for the Round 2 Recompete will go into effect July 1, 2016.
The Health Reform Law requires that CMS institute competitive bidding or use competitive bidding prices in all areas of the country by January 1, 2016. Final regulations were published November 6, 2014, which define the methodologies that will be used to implement the use of information from the competitive bidding program to adjust the fee schedule amounts for DME in areas where competitive bidding programs are not implemented.
Medicare currently covers home infusion therapy for selected therapies primarily through the durable medical equipment benefit. Congressional legislation was introduced in January 2015 that would establish Medicare coverage of home infusion therapy services. The bill would provide for reimbursement for the professional services, supplies and equipment associated with infusion therapy in the home under Medicare Part B and would provide for coordination between drug coverage under Part D and coverage for home infusion therapy services under Part B. We cannot predict whether this bill will be passed and if it is, what impact it will have on our business. The Health Reform Law did not change Medicare coverage for home infusion therapy or home infusion drugs.
State Legislation and Other Matters Affecting Drug Prices
Many states have adopted legislation that limits the amount a pharmacy participating in the state Medicaid program is paid based on the pharmacy's prices applicable to third party plans, or in some instances, self-pay patients (“most favored nation”
11
legislation). Because of these limitations, we may not receive the full Medicaid fee schedule amounts in some instances. There is wide variation in drafting, interpretation and enforcement of states’ “most favored nation” legislation. Our management carefully considers these laws and believes that each of our respective companies is in material compliance therewith, however, we cannot predict whether the regulators will disagree with our interpretation or change their interpretation of the laws or their enforcement priorities.
Effective September 26, 2009, First DataBank and Medi-Span agreed to reduce the mark-up factor applied to Wholesale Acquisition Cost (“WAC”), on which AWP is based, from 1.25 to 1.20 for the approximately 1,400 drug codes that were the subject of the lawsuits. These AWP publishers also similarly reduced the mark-up factor on all other national drug codes on which they had marked up AWP. This voluntary reduction affected approximately 18,000 national drug codes. First DataBank ceased publication of the AWP pricing benchmarks on September 28, 2011. As of the date of this report, a viable generally accepted alternative to the AWP benchmark has not been developed by the industry, and Medi-Span has announced they will continue to publish AWP until a new benchmark is widely accepted. See “Risk Factors - Risks Related to Our Business - Changes in industry pricing benchmarks could adversely affect our financial performance.”
Medicaid
We are also sensitive to possible changes in state Medicaid programs as we do business with several state Medicaid programs. Budgetary concerns in many states have resulted in, and may continue to result in, reductions to Medicaid reimbursement and Medicaid eligibility as well as delays in payment of outstanding claims. Any reductions to or delays in collecting amounts reimbursable by state Medicaid programs for our products or services, or changes in regulations governing such reimbursements, could cause our revenue and profitability to decline and increase our working capital requirements. For further discussion on state Medicaid reductions, refer to “Management’s Discussion and Analysis of Financial Condition and Results of Operations” in Part II, Item 7.
Healthcare Reform Legislation - The Health Reform Law
In March 2010, the President signed into law the Health Reform Law. The Health Reform Law has resulted in sweeping changes to the existing U.S. system for the delivery and financing of health care. In general, among other things, the reforms increase the number of persons covered under government program and private insurance; furnish economic incentives for measurable improvements in health care quality outcomes; promote a more integrated health care delivery system and the creation of new health care delivery models; revise payment for health care services under the Medicare and Medicaid programs; and increase government enforcement tools and sanctions for combating fraud and abuse by health care providers. In addition, the Health Reform Law reduces cost sharing for Medicare beneficiaries under the Part D prescription drug benefit program and provides funding for medication management services by licensed pharmacists to individuals with chronic conditions.
While many regulations for many requirements have been promulgated, further implementation of certain of the requirements under the Health Reform Law will depend on the promulgation of regulations by a number of federal government agencies, including the HHS. It is impossible to predict the outcome of these changes and the net effect of those requirements on us.
Regulation of the Pharmacy Industry
Every state's laws require each of our pharmacy locations to be licensed as an in-state pharmacy to dispense pharmaceuticals. Pharmacy and controlled substances laws often address the qualifications of an applicant's personnel, the adequacy of its prescription fulfillment and inventory control practices and the adequacy of its facilities. In general, pharmacy licenses are renewed annually. We believe our pharmacy locations materially comply with all state licensing laws applicable to their practice. If our pharmacy locations become subject to additional licensure requirements, are unable to maintain their required licenses or if states place overly burdensome restrictions or limitations on pharmacies, our ability to operate in some states would be limited, which could have an adverse impact on our business. We believe the impact of any such requirements would be mitigated by our ability to shift business among our numerous locations.
Many states, as well as the federal government, are considering imposing, or have already begun to impose, more stringent requirements on compounding pharmacies including the Drug Quality and Security Act (“DQSA”) (see Food, Drug, and Cosmetic Act below). We believe that our compounding is done in safe environments with clinically appropriate policies and procedures in place. Those compounding pharmacies adhere to rigorous safety and quality standards for compounded sterile preparations and only fill prescriptions for individually identified patients pursuant to a valid prescription from a prescriber. All compounding is done in compliance with USP 797 standards.
12
Many of the states into which we deliver pharmaceuticals have laws and regulations that require out-of-state pharmacies to register with, or be licensed by, the boards of pharmacy or similar regulatory bodies in those states. These states generally permit the dispensing pharmacy to follow the laws of the state within which the dispensing pharmacy is located. However, various state pharmacy boards have enacted laws and/or adopted rules or regulations directed at restricting or prohibiting the operation of out-of-state pharmacies by, among other things, requiring compliance with all laws of the states into which the out-of-state pharmacy dispenses medications, whether or not those laws conflict with the laws of the state in which the pharmacy is located, or requiring the pharmacist-in-charge to be licensed in that state. To the extent that such laws or regulations are applicable to our operations, we believe we comply with them. To the extent that the foregoing laws or regulations prohibit or restrict the operation of out-of-state pharmacies and are found to be applicable to us, they could have an adverse effect on our operations.
Laws enforced by the U.S. Drug Enforcement Administration (“DEA”) require each of our pharmacy locations to register with the DEA in order to handle and dispense controlled substances. A separate registration is required at each principal place of business where we dispense controlled substances. Federal and state laws also require us to follow specific labeling, reporting and record-keeping requirements for controlled substances. We maintain federal and state controlled substance registrations for each of our facilities that require such registration and follow procedures intended to comply with all applicable federal and state requirements regarding controlled substances. These laws can change from time to time. We continuously review these changes to laws and believe we are in material compliance with the applicable federal and state controlled substances laws. If any of our pharmacy locations is deemed to be out of compliance, it could have an adverse impact on our business.
Many states in which we operate also require home infusion companies to be licensed as home health agencies. We believe we materially comply with these laws. If our infusion locations become subject to new licensure requirements, are unable to maintain required licenses or if states place burdensome restrictions or limitations on home health agencies or home nursing agencies, our infusion locations’ ability to provide nursing services in some states would be limited, which could have an adverse impact on our business.
Professional Licensure
Nurses, pharmacists and certain other professionals employed by us are required to be individually licensed and/or certified under applicable state law. We perform criminal and other background checks on employees and confirm that our employees possess all licenses and certifications required in order to provide healthcare-related services. We believe our employees comply with applicable licensure laws.
Food, Drug and Cosmetic Act
Pharmacy operations
Certain provisions of the Federal Food, Drug and Cosmetic Act (“FDCA”) govern the preparation, handling, storage, marketing and distribution of pharmaceutical products. This law exempts many pharmaceuticals and medical devices we dispense from certain federal requirements as long as they are not adulterated or misbranded and are dispensed in accordance with, and pursuant to, a valid prescription.
Since the passage of DQSA, the U.S. Food and Drug Administration (“FDA”) directly regulates outsourcing facilities, but does not directly regulate non-outsourcing facilities or pharmacies. Outsourcing facilities are pharmacies that are engaged in sterile compounding of drugs that are not for an individually identifiable patient. As such, these outsourcing facilities are subject to a standard relating to sterilization and the physical facility that are the same as pharmaceutical manufacturers (“cGMP”). Because we only fill prescriptions pursuant to valid prescriptions for individually identifiable patients, we do not qualify as an outsourcing facility, and therefore, should not be required to comply with the cGMP standards. The FDA has been conducting inspections of pharmacies that engage in compounding, including ours, and has been attempting to apply the cGMP standards even though those pharmacies are not outsourcing facilities. While the FDA has issued reports following their surveys, to date, no enforcement action has been taken against us. We cannot predict what further actions the FDA may take. We believe our operations are in compliance with applicable laws and that the requirements for outsourcing facilities are not applicable to our operations. We cannot predict the impact of increased scrutiny on or new regulation of compounding pharmacies.
In addition, the FDCA governs pharmaceutical products’ movement in interstate commerce. The FDA has begun scrutinizing more closely compounding pharmacies’ operations and compounded pharmaceuticals’ movement in interstate commerce. Specifically, the FDA has proposed regulations that could have the effect of limiting our ability to ship prescriptions out of state by pharmacies that hold valid licenses but do not comply with cGMP standards. We do not know if these regulations, as proposed, will be adopted, but if they are, we will likely need to modify our operations to comply. While we cannot predict the new regulatory
13
environment under the DQSA, we believe we comply in all material respects with all applicable requirements of a non-outsourcing-facility pharmacy.
Infusion services
Certain medical devices (e.g., infusion pumps) essential to the company’s infusion services are governed by the FDCA and regulated by FDA. An infusion pump, like any medical device, is subject to failure. Since 2010, due to the relatively large number of adverse events associated with the use of infusion pumps, FDA has begun to change its approach to overseeing infusion pumps. Changes have included introducing higher levels of scrutiny, intensifying manufacturer engagement and bolstering user education and adverse event reporting. The shifting regulatory climate around infusion pumps; the requirement to maintain high levels of proficiency in using and training patients in the safe use of infusion pumps; cybersecurity issues, including modification and misuse of infusion pumps, and unauthorized use of information that is stored on or accessed from infusion pumps; and, finally, the need to stay current in infusion pump design and “best practices,” present elements of risk. Nevertheless, we believe we comply in all material respects with all applicable requirements and that our employees are adequately trained and equipped to use these devices.
Antitrust Laws
Numerous lawsuits have been filed throughout the United States by retail pharmacies against drug manufacturers challenging certain brand drug pricing practices under various state and federal antitrust laws. A settlement or decision in this type of lawsuit could have an impact on pricing and discounts and could reduce or eliminate the availability to us of certain discounts, rebates and fees currently received in connection with our drug purchasing and formulary administration programs. In addition, to the extent that we or an associated business appear to have actual or potential market power in a relevant market, business arrangements and practices may be subject to heightened scrutiny from an anti-competitive perspective and possible challenge by state or federal regulators or private parties.
Regulation of the PBM Industry
Licensure Laws
Many states have licensure or registration laws governing certain types of ancillary healthcare organizations, including preferred provider organizations, PBMs, TPAs, discount card prescription drug programs and companies that provide utilization review services. The scope of these laws differs significantly from state to state, and the application of such laws to the activities of PBMs often is unclear. We have registered or are registering under such laws in those states in which we have concluded that such registration or licensure is required.
Legislation Imposing Plan Design Mandates
Some states have enacted legislation that prohibits Plan Sponsors from implementing certain restrictions on design features, and many states have introduced legislation to regulate various aspects of managed care plans including legislation that prohibits or restricts therapeutic substitution, requires coverage of all drugs approved by the FDA, or prohibits denial of coverage for non-FDA approved uses. For example, some states provide that members may not be required to use network providers, but that they must instead be provided with benefits even if they choose to use non-network providers (“freedom of choice” legislation), or provide that a member may sue his or her health plan if care is denied. Some states have enacted, and other states have introduced, legislation regarding plan design mandates. Some states mandate coverage of certain benefits or conditions. Such legislation does not generally apply to our business, but it may apply to certain of our customers (generally, health maintenance organizations (“HMOs”) and health insurers). We do not believe the widespread enactment of these regulations would have a material adverse effect on our PBM business.
Consumer Protection Laws
Most states have consumer protection laws that have been the basis for investigations and multi-state settlements relating to financial incentives provided by drug manufacturers to pharmacies in connection with drug switching programs. Consumer protection laws have also been the basis for governmental investigations and settlements relating to the improper marketing and advertising of discount medical plans. No assurance can be given that we will not be subject to scrutiny under one or more of these laws.
14
Comprehensive PBM Regulation
Although no state has passed legislation regulating PBM activities in a comprehensive manner, such legislation has been introduced in the past in several states. Since we do not derive significant PBM revenues from business in any particular state,we do not believe such legislation, if currently enacted in a state, would not have a material adverse impact on our operations.
Fraud and Abuse Laws
Anti-Kickback Laws
Subject to certain statutory and regulatory exceptions (including exceptions relating to certain managed care, discount, bona fide employment arrangements, group purchasing and personal services arrangements), the federal “anti-kickback” law prohibits the knowing and willful offer or payment of any remuneration to induce the referral of an individual or the purchase, lease or order (or the arranging for or recommending of the purchase, lease or order) of healthcare items or services paid for in whole or in part by Medicare, Medicaid or other government-funded healthcare programs. Violation of the federal anti-kickback statute could subject us to criminal and/or civil penalties including suspension or exclusion from Medicare and Medicaid programs and other government-funded healthcare programs. A number of states also have enacted anti-kickback laws that sometimes apply not only to state-sponsored healthcare programs but also to items or services that are paid for by private insurance and self-pay patients. State anti-kickback laws can vary considerably in their applicability and scope and sometimes have fewer statutory and regulatory exceptions than does the federal law. Our management carefully considers the importance of such anti-kickback laws when structuring each company’s operations and believes that each of our respective companies is in compliance therewith.
The federal anti-kickback law has been interpreted broadly by courts, the OIG and other administrative bodies. Because of the broad scope of those statutes, federal regulations establish certain safe harbors from liability. Safe harbors exist for certain properly reported discounts received from vendors, certain investment interests held by a person or entity, certain properly disclosed payments made by vendors to group purchasing organizations, payments made for leases of space and equipment and payments for personal services as well as for other transactions or relationships. Nonetheless, a practice that does not fall within a safe harbor is not necessarily unlawful, but may be subject to scrutiny and challenge. In the absence of an applicable exception or safe harbor, a violation of the statute may occur even if only one purpose of a payment arrangement is to induce patient referrals or purchases.
Certain governmental entities have commenced investigations of PBM companies and other companies having dealings with the PBM industry and have identified issues concerning selection of drug formularies, therapeutic substitution programs and discounts or rebates from prescription drug manufacturers and whether best pricing requirements are being complied with. Additionally, at least one state has filed a lawsuit concerning similar issues against a health plan.
Governmental entities have also investigated pharmacies and their dealings with pharmaceutical manufacturers concerning, among other things, retail distribution, sales and marketing practices and product conversion or product switching programs. Governmental entities have also investigated pharmacies with respect to their relationships with physicians and other referral sources. There can be no assurance that we will not receive subpoenas or be requested to produce documents in pending investigations or litigation from time to time. In addition, we may be the target or subject of one or more such investigations or named parties in corresponding actions.
We believe we are in compliance with the legal requirements imposed by the anti-kickback laws and regulations, and we believe there are material and substantial differences between drug switching programs that have been challenged under these laws and the generic substitution and therapeutic interchange practices and formulary management programs offered by us to our Plan Sponsors since no remuneration or other incentives are provided to patients, pharmacists or others. However, there can be no assurance that we will not be subject to scrutiny or challenge under such laws or regulations, or that any such challenge would not have a material adverse effect on us.
On April 18, 2003, the OIG released Compliance Program Guidance for Pharmaceutical Manufacturers (the “Guidance”), which is designed to provide voluntary, nonbinding guidance in devising effective compliance programs to assist companies that develop, manufacture, market and sell pharmaceutical products or biological products. The Guidance provides the OIG's view of the fundamental elements of a pharmaceutical manufacturer’s compliance program and principles that should be considered when creating and implementing an effective compliance program, or as a benchmark for companies with existing compliance programs. While we are not a manufacturer, we believe that many aspects of it are useful to our business and therefore we currently maintain a compliance program that includes the key compliance program elements described in the Guidance. We believe the fundamental elements of our compliance programs are consistent with the principles, policies and intent of the Guidance.
15
The Stark Laws
The federal self-referral law, commonly known as the “Stark Law,” prohibits physicians from referring Medicare patients for “designated health services” (which include, among other things, outpatient prescription drugs, durable medical equipment and supplies and home health services) to an entity with which the physician, or an immediate family member of the physician, has a direct or indirect financial relationship, unless the financial relationship is structured to meet an applicable exception. Possible penalties for violation of the Stark Law include denial of payment, refund of amounts collected in violation of the statute, civil monetary penalties and program exclusion. Our management carefully considers the Stark Law and its accompanying regulations in structuring our financial relationships with physicians and believes we are in compliance therewith.
State Self-Referral Laws
We are subject to state statutes and regulations that prohibit payments for the referral of patients and referrals by physicians to healthcare providers with whom the physicians have a financial relationship. Some state statutes and regulations apply to services reimbursed by governmental as well as private payors. Violation of these laws may result in prohibition of payment for services rendered, loss of pharmacy or health provider licenses, fines and criminal penalties. The laws and exceptions or safe harbors may vary from the federal Stark Law and vary significantly from state to state. Certain of these state statutes mirror the federal Stark Law while others may be more restrictive. The laws are often vague, and in many cases, have not been widely interpreted by courts or regulatory agencies; however, we believe we are in compliance with such laws.
Statutes Prohibiting False Claims and Fraudulent Billing Activities
A range of federal civil and criminal laws target false claims and fraudulent billing activities. One of the most significant is the federal False Claims Act, which we refer to as the False Claims Act, which imposes civil penalties for knowingly making or causing to be made false claims in order to secure a reimbursement from government-sponsored programs, such as Medicare and Medicaid. Investigations or actions commenced under the False Claims Act may be brought either by the government or by private individuals on behalf of the government, through a “whistleblower” or “qui tam” action. The False Claims Act authorizes the payment of a portion of any recovery to the individual bringing suit. Such actions are initially required to be filed under seal pending their review by the Department of Justice. If the government intervenes in the lawsuit and prevails, the whistleblower (or plaintiff filing the initial complaint) may share with the federal government in any settlement or judgment. If the government does not intervene in the lawsuit, the whistleblower plaintiff may pursue the action independently. The False Claims Act generally provides for the imposition of civil penalties and for treble damages, resulting in the possibility of substantial financial penalties for small billing errors that are replicated in a large number of claims, as each individual claim could be deemed to be a separate violation of the False Claims Act. Significantly, the Health Reform Law amended the False Claims Act to require that an overpayment must be reported and returned to the government within 60 days after an overpayment is identified. The failure to comply with this requirement now constitutes a violation of the federal False Claims Act.
Some states also have enacted statutes similar to the False Claims Act which may include criminal penalties, substantial fines, and treble damages. In recent years, federal and state governments have launched several initiatives aimed at uncovering practices that violate false claims or fraudulent billing laws. Under Section 1909 of the Social Security Act, which became effective January 1, 2007, if a state false claim act meets certain requirements as determined by the OIG in consultation with the U.S. Attorney General, the state is entitled to an increase of ten percentage points in the state medical assistance percentage with respect to any amounts recovered under a state action brought under such a law. Some of the larger states in terms of population that have had the OIG review such laws include: California, Connecticut, Florida, Georgia, Illinois, Louisiana, Massachusetts, Michigan, New Jersey, New York, North Carolina, Texas, and Virginia. We operate in all of these states and we submit claims for Medicaid reimbursement to the respective state Medicaid agencies. We expect the list of states that enact qualifying false claims acts to continue to grow. This legislation has led to increased auditing activities by state healthcare regulators. As such, we have been the subject of an increased number of audits. Further, a number of states, including states in which we operate, have adopted their own false claims statutes as well as statutes that allow individuals to bring qui tam actions. We believe we have procedures in place to ensure the accuracy of our claims. While we believe we are in compliance with Medicaid and Medicare billing rules and requirements, there can be no assurance that regulators would agree with the methodology employed by us in billing for our products and services, and a material disagreement between us, on the one hand, and these governmental agencies, on the other hand, on the manner in which we provide products or services could have a material adverse effect on our business and Consolidated Financial Statements.
The False Claims Act also has been used by the federal government and private whistleblowers to bring enforcement actions under so-called “fraud and abuse” laws like the federal anti-kickback statute and the Stark Law. Such actions are not based on a contention that an entity has submitted claims that are factually invalid. Instead, such actions are based on the theory that when an entity submits a claim, it either expressly or impliedly certifies that it has provided the underlying services in compliance with applicable laws, and therefore that services provided and billed for during an anti-kickback statute or Stark Law violation result
16
in false claims, even if such claims are billed accurately for appropriate and medically necessary services. The existence of the False Claims Act, which enforces alleged fraud and abuse violations, has increased the potential for such actions to be brought and has increased the potential financial exposure for such actions. These actions are costly and time-consuming to defend.
Civil Monetary Penalties Act
The Civil Monetary Penalties Act authorizes the U.S. Secretary of HHS to impose civil money penalties, assessments and program supervision or exclusion for various forms of fraud and abuse involving the Medicare and Medicaid programs. Penalties range from $2,000 to $100,000 for each violation, depending on the specific misconduct involved. The Inspector General must only prove liability by a “preponderance of the evidence” rather than the more demanding “beyond a reasonable doubt” standard required in criminal actions. A health care provider may be held liable based on its own negligence and the negligence of its employees. There is no requirement that intent to defraud must be proved. The availability of the Civil Money Penalties Act to enforce alleged fraud and abuse violations has increased the potential for such actions and has increased the potential financial exposure for such actions. These actions are costly and time-consuming to defend.
Confidentiality, Privacy and HIPAA
Most of our activities involve the receipt, use and disclosure of confidential medical, pharmacy or other health-related information concerning individual patients or members, including the disclosure of such confidential information to an individual's health benefit plan. In addition, we may use de-identified and aggregated data for research and analysis purposes.
The Health Insurance Portability and Accountability Act of 1996 and the federal regulations implemented thereunder (collectively, “HIPAA”), as amended by the Health Information for Economic and Clinical Health Act of 2009 (“HITECH”), give people greater control over the privacy of their medical information. The federal privacy regulations (the “Privacy Regulations”) are designed to protect the medical information of a healthcare patient or health plan enrollee that could be used to identify the individual. We refer to this information as protected health information (“PHI”). Among numerous other requirements, the Privacy Regulations, as amended by HITECH: (i) limit certain uses and disclosures of PHI; (ii) limit most disclosures of PHI to the minimum necessary for the intended purpose; (iii) require patient authorization for certain uses and disclosures of PHI; and (iv) guarantee patients the right to access their medical records and to receive an accounting of disclosures. The federal security regulations (the “Security Regulations”) set certain standards regarding the storage, utilization of, access to and transmission of electronic PHI. The federal breach notification regulations (the “Breach Notification Regulations”) require notification to individuals and the federal government in the event of a breach of PHI.
These regulations apply to “covered entities,” which include most healthcare providers and health plans, and some of these regulations apply to “business associates,” which are persons or entities that perform or assist in performing services or activities for or on behalf of a covered entity, if the performance of those services or activities involves the use or disclosure of a patient’s PHI. HIPAA also requires that a covered entity and its business associates enter into written contracts whereby the business associate agrees to certain restrictions regarding its use and disclosure of PHI. We provide a varied line of services to patients and other entities. Depending on the purpose or function of the service line, we may be functioning as a covered entity or a business associate for purposes of complying with HIPAA. For example, in our role as a pharmacy and home infusion therapy service provider, we are a covered entity. In our role as a PBM, we are a business associate.
The requirements imposed by HIPAA are extensive, and it has required substantial cost and effort to assess and implement those requirements. We have taken and intend to continue to take steps that we believe are reasonably necessary to ensure our policies and procedures are in compliance with the Privacy Regulations, the Security Regulations and the Breach Notification Regulations. The requirements imposed by HIPAA have increased our burden and costs of regulatory compliance (including our health improvement programs and other information-based products), altered our reporting and reduced the amount of information we can use or disclose if patients or members do not authorize such uses or disclosures.
In addition, most states have enacted privacy and security laws, including laws that protect particularly sensitive medical information (such as HIV status or mental health records) and the so-called “security breach” notification laws that impose an obligation to notify persons when their personal information has or may have been accessed by an unauthorized person. Which state's laws are implicated is generally based on the state of the patient’s residence. Many of these laws apply to our business and have increased and will continue to increase our burden and costs of privacy and security-related regulatory compliance.
17
Employees
As of December 31, 2014, we had 1,955 full-time, 71 part-time and 464 per diem employees. Per diem employees are defined as those available on an as-needed basis. None of our employees are represented by any union and, in our opinion, relations with our employees are satisfactory.
Available Information
We maintain a website at www.bioscrip.com. The information contained on our website is not incorporated by reference into this Annual Report and should not be considered part of this report. We file annual, quarterly and current reports, proxy statements and other information with the SEC. We make available, free of charge through our website, our reports on Forms 10-K, 10-Q, and 8-K, and amendments to those reports, as soon as reasonably practicable after they are filed with or furnished to the SEC.
We have adopted a Code of Business Conduct and Ethics policy for our Company, including our directors, officers and employees. Our Code of Business Conduct and Ethics policy and the charters of the Audit Committee, Management Development and Compensation Committee, and Governance, Compliance and Nominating Committee of our board of directors are available on our website at www.bioscrip.com.
Item 1A. | Risk Factors |
Risks Related to Our Business
Pressures relating to downturns in the economy could adversely affect our business and consolidated financial statements.
Medicare and other federal and state payors account for a significant portion of our revenues. During economic downturns and periods of stagnant or slow economic growth, federal and state budgets are typically negatively affected, resulting in reduced reimbursements or delayed payments by the federal and state government health care coverage programs in which we participate, including Medicare, Medicaid and other federal or state assistance plans. Government programs could also slow or temporarily suspend payments on Medicaid obligations, negatively impacting our cash flow and increasing our working capital needs and interest payments. We have seen, and believe we will continue to see, Medicare and state Medicaid programs institute measures aimed at controlling spending growth, including reductions in reimbursement rates.
Higher unemployment rates and significant employment layoffs and downsizings may lead to lower numbers of patients enrolled in employer-provided plans. Adverse economic conditions could also cause employers to stop offering, or limit, healthcare coverage, or modify program designs, shifting more costs to the individual and exposing us to greater credit risk from patients or the discontinuance of therapy.
Existing and new government legislative and regulatory action could adversely affect our business and financial results.
Our business is subject to numerous federal, state and local laws and regulations. See “Business - Government Regulation.” Changes in these regulations may require extensive changes to our systems and operations that may be difficult to implement. Untimely compliance or noncompliance with applicable laws and regulations could adversely affect the continued operation of our business, including, but not limited to: imposition of civil or criminal penalties; suspension of payments from government programs; loss of required government certifications or approvals; suspension of authorizations to participate in or exclusion from government reimbursement programs; or loss of licensure. Reduction in reimbursement by Medicare, Medicaid and other governmental payors could adversely affect our business as well. The regulations to which we are subject include, but are not limited to, Anti-Kickback laws; federal and state laws prohibiting self-referrals or “Stark laws”; HIPAA, as amended by HITECH; False Claims Act; Civil Monetary Penalties Act; regulations of the FDA, U.S. Federal Trade Commission, and the DEA, and regulations of individual state regulatory authorities. In that regard, our business and consolidated financial statements could be affected by one or more of the following:
• | federal and state laws and regulations governing the purchase, distribution, management, compounding, dispensing and reimbursement of prescription drugs and related services, including state and federal controlled substances laws and regulations; |
• | FDA and/or state regulation affecting the pharmacy or PBM industries; |
• | rules and regulations issued pursuant to HIPAA and HITECH; and other federal and state laws affecting the use, disclosure and transmission of health information, such as state security breach notification laws and state laws limiting the use and disclosure of prescriber information; |
18
• | administration of Medicare and state Medicaid programs, including legislative changes and/or rulemaking and interpretation; |
• | federal and state laws and regulations that require reporting and public dissemination of payments to and between various health care providers and other industry participants; |
• | government regulation of the development, administration, review and updating of formularies and drug lists; |
• | managed care reform and plan design legislation, including state laws regarding out-of-network charges and participation; |
• | federal or state laws governing our relationships with physicians or others in a position to refer to us; and |
• | interpretation and enforcement of the DQSA. |
The Health Reform Law and its implementation could have a material adverse effect on our business.
The Health Reform Law has resulted and will continue to result in sweeping changes to the existing U.S. system for the delivery and financing of health care. While many regulations have already been promulgated, further implementation of certain of the requirements under the Health Reform Law will depend on the promulgation of regulations by a number of federal government agencies, including the HHS. It is impossible to predict the outcome of these changes and the net effect of those requirements on us. As such, we cannot predict the impact of the Health Reform Law on our business, operations or financial performance.
Federal actions and legislation may reduce reimbursement rates from governmental payors and adversely affect our results of operations.
In August 2011, Congress passed a deficit reduction agreement that created a committee tasked with proposing legislation to reduce the federal deficit by November 23, 2011. Because the committee did not act, automatic Medicare cuts were scheduled to go into effect January 1, 2013. However, Congress passed legislation extending the time for such cuts by two months. Thus, Medicare reimbursement to providers was reduced overall by 2% (as part of sequestration) beginning April 1, 2013. The automatic spending cuts did not and will not have an impact on Medicaid reimbursement. The reductions in Medicare reimbursement have not yet been significant but they could have an adverse impact on our results of operations.
These reductions are in addition to reductions mandated by the Health Reform Law, which provides for material reductions in the growth of Medicare program spending. From time to time, CMS revises the reimbursement systems used to reimburse health care providers, which may result in reduced Medicare payments. Because most states must operate with balanced budgets and because the Medicaid program is often a state’s largest program, some states have enacted or may consider enacting legislation designed to reduce their Medicaid expenditures. Further, many states have also adopted, or are considering, legislation designed to reduce coverage and/or enroll Medicaid recipients in managed care programs. The current economic environment has increased the budgetary pressures on many states, and these budgetary pressures have resulted, and likely will continue to result, in decreased spending, or decreased spending growth, for Medicaid programs and the Children’s Health Insurance Program in many states.
In some cases, Third Party Payors rely on all or portions of Medicare payment systems to determine payment rates. Changes to government health care programs that reduce payments under these programs may negatively impact payments from Third Party Payors. Current or future health care reform and deficit reduction efforts, changes in laws or regulations regarding government health care programs, other changes in the administration of government health care programs and changes to Third Party Payors in response to health care reform and other changes to government health care programs could have a material, adverse effect on our financial position and results of operations.
We face periodic reviews and billing audits from governmental and private payors, and these audits could have adverse findings that may negatively impact our business.
As a result of our participation in the Medicare and Medicaid programs, we are subject to various governmental reviews and audits to verify our compliance with these programs and applicable laws and regulations. We also are subject to audits under various government programs in which third party firms engaged by CMS conduct extensive reviews of claims data and medical and other records to identify potential improper payments under the Medicare program. Private pay sources also reserve the right to conduct audits. If billing errors are identified in the sample of reviewed claims, the billing error can be extrapolated to all claims filed which could result in a larger overpayment than originally identified in the sample of reviewed claims. Our costs to respond to and defend reviews and audits may be significant and could have a material adverse effect on our business and consolidated financial condition, results of operations and cash flows. Moreover, an adverse review or audit could result in:
• | required refunding or retroactive adjustment of amounts we have been paid by governmental or private payors; |
• | state or Federal agencies imposing fines, penalties and other sanctions on us; |
• | loss of our right to participate in the Medicare program, state programs, or one or more private payor networks; or |
19
• | damage to our business and reputation in various markets. |
These results could have a material adverse effect on our business and consolidated financial condition, results of operations and cash flows.
If any of our pharmacies fail to comply with the conditions of participation in the Medicare program, that pharmacy could be terminated from Medicare, which could adversely affect our consolidated financial statements.
Our pharmacies must comply with the extensive conditions of participation in the Medicare program. These conditions vary depending on the type of facility, but, in general, require our facilities to meet specified standards relating to licensure, personnel, patient rights, patient care, patient records, physical site, administrative reporting and legal compliance. If a pharmacy fails to meet any of the Medicare supplier standards, that pharmacy could be terminated from the Medicare program. We respond in the ordinary course to deficiency notices issued by surveyors, and none of our pharmacies has ever been terminated from the Medicare program for failure to comply with the supplier standards. Any termination of one or more of our pharmacies from the Medicare program for failure to satisfy the Medicare supplier standards could adversely affect our consolidated financial statements.
We cannot predict the impact of new requirements on compounding pharmacies.
Compounding pharmacies have come under increasing scrutiny from federal and state governmental agencies. We have been responding to requests for additional information on our practices as we receive them. We believe that our compounding is done in safe environments and we have clinically appropriate policies and procedures in place. We only compound pursuant to a patient specific prescription and do so in compliance with USP 797 standards. In November 2013, Congress passed the DQSA, which creates a new category of compounders called outsourcing facilities, which are newly-regulated by the FDA. We do not believe that our current compounding practices qualify us as an outsourcing facility and therefore we continue to operate in compliance with USP 797 standards. Should state regulators or the FDA disagree, or should our business practices change to qualify us as an outsourcing facility, there is a risk of regulatory action and/or increased resources required to comply with federal requirements imposed by the DQSA on outsourcing facilities that would significantly increase our costs or otherwise affect our results of operations. Furthermore, we cannot predict the implications and overall impact of increased scrutiny on compounding pharmacies.
Competition in the healthcare industry could reduce profit margins.
The healthcare industry is very competitive. Our competitors include large and well-established companies that may have greater financial, marketing and technological resources than we do. Some of our competitors are under common control with, or owned by, pharmaceutical wholesalers and distributors, managed care organizations, pharmacy benefit managers or retail pharmacy chains and may be better positioned with respect to the cost-effective distribution of pharmaceuticals. In addition, some of our competitors may have secured long-term supply or distribution arrangements for prescription pharmaceuticals necessary to treat certain chronic disease states on price terms substantially more favorable than the terms currently available to us. As a result of such advantageous pricing, we may be less price competitive than some of these competitors with respect to certain pharmaceutical products. Our competitive position could also be adversely affected by any inability to obtain access to new biotech pharmaceutical products.
Changes in the case mix of patients, as well as payment methodologies, payor mix or pricing could adversely affect our consolidated financial statements.
The sources and amounts of our patient revenue are determined by a number of factors, including the mix of patients and the rates of reimbursement among payors. Changes in the case mix of the patients, payment methodologies, payor mix or pricing among private pay, Medicare and Medicaid may significantly affect our consolidated financial statements.
Changes in industry pricing benchmarks could adversely affect our financial performance.
Contracts within our business generally use certain published benchmarks to establish pricing for the reimbursement of prescription medications dispensed by us. These benchmarks include AWP, wholesale acquisition cost and average manufacturer price. Many of our contracts utilize the AWP benchmark. As a part of the settlement of class-action lawsuits brought against First DataBank and Medi-Span, effective September 26, 2009, both companies announced they would cease publication of the AWP pricing benchmarks at the end of 2011. First DataBank ceased publication of the AWP pricing benchmarks on September 28, 2011. Without a suitable pricing benchmark in place many of our contracts will have to be modified and could potentially change the economic structure of our agreements. As of the date of this report, a viable generally accepted alternative to the AWP benchmark has not been developed by the industry, and Medi-Span has announced they will continue to publish AWP until a new benchmark is widely accepted.
20
Competitive bidding could reduce our volumes and profitability.
The Medicare Prescription Drug, Improvement, and Modernization Act of 2003 established requirements for a competitive bidding program for determining Medicare reimbursement rates for certain items of durable medical equipment, prosthetics, orthotics and supplies (“DMEPOS”), including enteral nutrients, supplies and equipment, certain respiratory therapy and home medical equipment products and external infusion pumps and supplies. CMS has the discretion to determine which products will be subject to competitive bidding.
Although we are contract suppliers under the Round 1 Recompete and Round 2 of competitive bidding and have entered into strategic relationships in the Competitive Bidding Areas (“CBAs”) in which we were not awarded contracts, the prices paid under the competitive bid contracts are below what Medicare had previously paid. Because of this, even in CBAs where we continue to provide competitively bid items to Medicare beneficiaries, we have seen and may continue to see decreased revenues. Continued expansion of the competitive bidding program could also have a negative impact on our revenue if we are not a successful bidder in many or all of the CBAs for the product categories included that we offer. Further, the Affordable Care Act mandated use of information from the DMEPOS competitive bidding program to adjust the fee schedule amounts by January 1, 2016 for DMEPOS in areas where competitive bidding programs are not implemented. The establishment of new fee schedule pricing for areas where competitive bidding are not implemented, which is based on competitive bid prices, could have a further negative impact on our revenue.
Our inability to effectively and timely transition to the new ICD-10 coding system could disrupt our operations.
CMS has mandated that all providers implement the use of new patient codes for medical coding, referred to as ICD-10 codes, on or before October 1, 2015. This mandate substantially increases the number of medical billing codes by which providers will seek reimbursement, increasing the complexity of submitting claims for reimbursement. Claims submitted after October 1, 2015 must use ICD-10 codes or they will not be paid. Transition to the new ICD-10 system requires changes to our clinical software system as well as the training of staff involved in the coding and billing processes. In addition to these upfront costs of transition to ICD-10, it is possible that we could experience disruption or delays in payment due to implementation issues, including software errors, coding errors or a decrease in the productivity of our staff involved in the coding and billing processes. Any such delays in payment could disrupt our operations and materially and adversely affect our business.
PBM client demands for enhanced service levels or possible loss or unfavorable modification of contracts with clients or providers could adversely affect our consolidated financial statements.
As our PBM clients face long-term, sustained increases in prescription drug costs, they may demand additional services and enhanced service levels to help mitigate the increase in spending. We operate in a very competitive environment, and we may not be able to increase our fees to compensate for these increased services, which could put pressure on our margins.
Our contracts with PBM clients generally do not have terms longer than three years and, in some cases, may be terminated by the client on relatively short notice, typically 90 days. Our PBM clients generally seek bids from other PBM or specialty providers in advance of the expiration of their contracts. If several of these clients elect not to extend their relationship with us, and we are not successful in generating sales to replace the lost business, our future business and operating results could be materially and adversely affected. In addition, we believe the managed care industry is undergoing substantial consolidation, and another party that is not our client could acquire some of our managed care clients. In such case, there is a risk of contract loss and a loss of the associated revenues and profit.
There are approximately 60,000 retail pharmacies in the United States. All major retail chain pharmacies and a vast majority of independent pharmacies participate in our pharmacy network. The top ten retail pharmacy chains represent approximately 50% of the total number of stores and over 90% of prescriptions filled in our network. Our contracts with retail pharmacies, which are non-exclusive, are generally terminable on relatively short notice. If one or more of the top pharmacy chains elects to terminate its relationship with us, our members’ access to retail pharmacies and our business could be materially and adversely affected. In addition, many large pharmacy chains either own PBMs today, or could attempt to acquire a PBM in the future. Increased ownership of PBMs by retail pharmacy chains could materially and adversely affect our relationships with those pharmacy chains and, accordingly, our consolidated financial statements.
21
Contract renewals, or lack thereof, with key revenue sources and key business relationships could result in less favorable pricing, loss of exclusivity and/or reduced distribution and access to customers, which could have an adverse effect on our business, financial condition and results of operations.
We are renegotiating, on a rolling basis, contracts and business relationships with key revenue sources, including Third Party Payors, Plan Sponsors, network pharmacies, and discount card brokers. Our future growth and success depends on our ability to maintain these relationships and renew such contracts on acceptable terms. However, we may not be able to continue to maintain these relationships which grant us access to certain customers and distribution channels. Any break in these key business relationships could result in lost contracts and reduce our access to certain customers and distribution channels. Further, when such contracts near expiration, we may not be able to successfully renegotiate acceptable terms. Any increase in pricing or loss of exclusivity could result in reduced margins. Accordingly, it is possible that our ongoing efforts to renew contracts and business relationships with such key revenue sources as Third Party Payors, Plan Sponsors, network pharmacies and discount card brokers could result in less favorable pricing, loss of exclusivity or even reduced access to customers and distribution channels, any of which could have an adverse effect on our business, financial condition and results of operations. As discussed in the risk factor titled “PBM client demands for enhanced service levels or possible loss or unfavorable modification of contracts with clients or providers could adversely affect our consolidated financial statements,” even when such contracts are renewed, they may be renewed for only a short term or may be terminable on relatively short notice.
We and certain of our directors and executive officers have been named as defendants in a consolidated class action lawsuit that could result in substantial costs and divert management’s attention, and we may be subject to similar lawsuits in the future.
We, and certain of our current and former directors and executive officers, have been named as defendants in two purported class action lawsuits that generally allege that we and certain of our directors and officers violated Sections 11, 12(a)(2) and 15 of the Securities Act of 1933, as amended (the “Securities Act”), Sections 10(b) and 20(a) of the Securities Exchange Act of 1934, as amended (the “Exchange Act”) and Rule 10b-5 promulgated under the Exchange Act by making allegedly false and misleading statements and/or omissions pertaining to (i) the distribution of the Novartis Pharmaceutical Corporation’s product Exjade® (the “Medication”) by our legacy specialty pharmacy division that was divested in May 2012 (the “Legacy Division”) and (ii) our PBM Services segment. On December 19, 2013, the two class action lawsuits were consolidated into a single consolidated class action lawsuit and a lead plaintiff was appointed. The lead plaintiff filed a consolidated complaint on February 19, 2014. The consolidated complaint seeks damages and other relief. All defendants in the case moved to dismiss the consolidated complaint on April 28, 2014. Briefing on the motion to dismiss was complete on July 28, 2014.
We intend to engage in a vigorous defense of the consolidated lawsuit. However, we are unable to predict the outcome of this matter at this time. Moreover, any conclusion of this matter in a manner adverse to us would have an adverse effect on our financial condition and business. Even if we were to be successful in the defense of the litigation, we could incur substantial costs not covered by our directors’ and officers’ liability insurance, suffer a significant adverse impact on our reputation and divert management’s attention and resources from other priorities, including the execution of business plans and strategies that are important to our ability to grow our business, any of which could have an adverse effect on our business. In addition, while we believe based on current information that this matter is covered by applicable insurance and we intend to engage in a vigorous defense of the consolidated lawsuit, nevertheless, this matter could require payments (including payments with respect of legal expenses) that are not covered by, or exceed the limits of, our available directors’ and officers’ liability insurance, which could adversely impact our financial condition, results of operations or cash flows.
Pending and future litigation could subject us to significant monetary damages and/or require us to change our business practices.
We are subject to risks relating to litigation and other proceedings in connection with our operations, including the dispensing of pharmaceutical products. See Item 3-Legal Proceedings for a description of material proceedings pending against us. We believe that these suits are without merit and, to the extent not already concluded, intend to contest them vigorously. However, an adverse outcome in one or more of these suits may have a material adverse effect on our consolidated results of operations, consolidated financial position and/or consolidated cash flow from operations, or may require us to make material changes to our business practices. For instance, effective January 8, 2014 we entered into a Stipulation and Order of Settlement and Dismissal (the “Federal Settlement Agreement”) with the U.S. Department of Justice (the “DOJ”) and qui tam relator David M. Kester (the “Relator”), and effective February 11, 2014, we entered into State Settlement Agreements (collectively, the “State Settlement Agreements”, and together with the Federal Settlement Agreement the “Settlement Agreements”) with the offices of the Attorneys General of 34 states (collectively, the “Settling States”). The Settlement Agreements provide for aggregate payments of $15.0 million plus interest to settle civil claims under the False Claims Act and related statutes and common law claims that could be brought by the DOJ, Relator or Settling States that arise out of the Legacy Division’s distribution of the Medication.
22
We periodically respond to subpoenas and requests for information from governmental agencies, including the civil investigative demand from the United States Attorney’s Office (the “USAO”) for the Southern District of New York (the “SDNY”) and the subpoena from relevant state governments related to certain operations by our Legacy Division, as discussed above. We confirm that, to our knowledge, we are not a target or a potential subject of a criminal investigation. Except to the extent already concluded as discussed above, we cannot predict with certainty what the outcome of any of the foregoing might be or whether we may in the future become a target or potential target of an investigation or the subject of further inquiries or ultimately settlements with respect to the subject matter of these subpoenas. In addition to potential monetary liability arising from these suits and proceedings, from time to time we incur costs in providing documents to government agencies. Current pending claims and associated costs may be covered by our insurance, but certain other costs are not insured. Such costs may increase and/or continue to be material to our performance in the future.
In addition, as we continue our strategic assessment and cost reduction efforts, there is an increased risk of employment and workers compensation-related litigation and/or administrative claims brought against us. We would defend against any and all such litigation and claims, as appropriate. Such claims could have a material adverse effect on our consolidated financial statements in any particular reporting period.
We may face liabilities relating to the sale of the Home Health Business.
We are still subject to potential liabilities relating to the sale of the Home Health Business. Under the terms of the Stock Purchase Agreement, we are obligated to indemnify the Buyer against certain potential liabilities related to operations prior to the sale and for breaches of representations, warranties and covenants under the Stock Purchase Agreement.
Our acquisition strategy exposes us to a variety of operational and financial risks.
A principal element of our business strategy has been to grow by acquiring other companies and assets in the home infusion and complementary businesses. Growth, especially rapid growth, through acquisitions exposes us to a variety of operational and financial risks. We summarize the most significant of these risks below.
Integration risks. We must integrate our acquisitions with our existing operations. This process includes the integration of the various components of our business (including the following) and of the businesses we have acquired or may acquire in the future:
• | health care professionals and employees who are not familiar with our policies and procedures; |
• | clients who may terminate their relationships with us; |
• | key employees who may seek employment elsewhere; |
• | patients who may elect to switch to another health care provider; |
• | regulatory compliance programs; and |
• | disparate operating, information and record keeping systems and technology platforms. |
Integrating an acquisition could be expensive and time consuming and could disrupt our ongoing business, negatively affect cash flow and distract management and other key personnel from day-to-day operations.
We may not be able to combine successfully the operations of recently acquired companies with our operations, and, even if such integration is accomplished, we may never realize the potential benefits of the acquisition. The integration of acquisitions requires significant attention from management, may impose substantial demands on our operations or other projects and may impose challenges on the combined business including, but not limited to, consistencies in business standards, procedures, policies and business cultures. If we fail to complete ongoing integration efforts, we may never fully realize the potential benefits of the related acquisitions.
Benefits may not materialize. When evaluating potential acquisition targets, we identify potential synergies and cost savings that we expect to realize upon the successful completion of the acquisition and the integration of the related operations. We may, however, be unable to achieve or may otherwise never realize the expected benefits. Our ability to realize the expected benefits from improvements to companies we acquire are subject to significant business, economic and competitive uncertainties and contingencies, many of which are beyond our control, such as changes to government regulation governing or otherwise impacting our industry, reductions in reimbursement rates from Third Party Payors, reductions in service levels under our contracts, operating difficulties, client preferences, changes in competition and general economic or industry conditions. If we are unsuccessful in implementing these improvements or if we do not achieve our expected results, it may adversely impact our results of operations.
Assumptions of unknown liabilities. Companies that we acquire may have unknown or contingent liabilities, including, but not limited to, liabilities for failure to comply with healthcare laws and regulations. We may incur material liabilities for the past
23
activities of acquired operations. Such liabilities and related legal or other costs and/or resulting damage to our reputation could negatively impact our business through lower-than-expected operating results, charges for impairment of acquired intangible assets or otherwise.
Competing for acquisitions. We face competition for acquisition candidates primarily from other home infusion and other healthcare companies. Some of our competitors have greater resources than we do. As a result, we may pay more to acquire a target business or may agree to less favorable deal terms than we would have otherwise. Accurately assessing the value of acquisition candidates is often very challenging. Also, suitable acquisitions may not be available due to unfavorable terms.
Further, the cost of an acquisition could result in a dilutive effect on our results of operations, depending on various factors, including the amount paid for in an acquisition, the acquired entity’s results of operations, the fair value of assets acquired and liabilities assumed, effects of subsequent legislation and limits on rate increases.
Improving financial results. Some of the operations we have acquired or may acquire in the future may have had significantly lower operating margins than our current operations. If we fail to improve the operating margins of the companies we acquire, operate such companies profitably or effectively integrate the operations of the acquired companies, our results of operations could be negatively impacted.
Acquisitions, strategic investments and strategic relationships involve certain risks.
We intend to pursue opportunistic strategic acquisitions of, or investments in, businesses and technologies. Acquisitions may entail numerous risks, including difficulties in assessing values for acquired businesses, intangible assets and technologies, difficulties in the assimilation of acquired operations and products, diversion of management’s attention from other business concerns, assumption of unknown material liabilities of acquired companies, amortization of acquired intangible assets which could reduce future reported earnings, and potential loss of clients or key employees of acquired companies. We may not be able to successfully fully integrate the operations, personnel, services or products that we have acquired or may acquire in the future. Strategic investments may also entail some of the risks described above. If these investments are unsuccessful, we may need to incur charges against earnings. We may also pursue a number of strategic relationships. These relationships and others we may enter into in the future may be important to our business and growth prospects. We may not be able to maintain these relationships or develop new strategic alliances.
We may not be able to identify suitable acquisition candidates or business and investment opportunities.
We intend to continue to explore strategic alternatives and identify new business acquisition opportunities. We may not be able to identify such new business acquisition opportunities or strategic alternatives to continue to execute our strategy.
Strategic investments, relationships and alternatives involve certain risks, and we may incur significant costs in connection with our evaluation of new business opportunities and suitable acquisition candidates.
Our management intends to identify, analyze and evaluate potential new business opportunities, including possible acquisition and merger candidates. We may incur significant costs, such as due diligence and legal and other professional fees and expenses, as part of these efforts. Notwithstanding these efforts and expenditures, we may not be able to identify an appropriate new business opportunity, or any acquisition opportunity, in the near term, or at all.
If our remedial measures are insufficient to address material weaknesses and we are unable to maintain an effective system of internal control over financial reporting, we may not be able to accurately report our financial results, timely file our periodic reports, maintain our reporting status or prevent fraud.
In connection with our management’s assessment of the effectiveness of our internal control over financial reporting as of December 31, 2013, we concluded there were two material weaknesses. The first material weakness related to the establishment of accounts receivable related reserves and the timely recognition of bad debt expense, and the second related to certain clerical errors and documentation omissions in the contingent consideration calculations that were provided to our auditors.
In addition, in connection with our management’s assessment of the effectiveness of our internal control over financial reporting as of December 31, 2014, we concluded there were two new material weaknesses. The first new material weakness related to our general information technology controls (“GITCs”) not being complete, and the second related to our internal control over the accounting for significant and unusual transactions not being adequate to detect a material misstatement in our consolidated financial statements.
24
Under standards established by the Public Company Accounting Oversight Board, a material weakness is a deficiency, or combination of deficiencies, in internal control over financial reporting such that there is a reasonable possibility that a material misstatement of our annual or interim financial statements will not be prevented, detected or corrected on a timely basis.
The material weakness related to the contingent consideration calculations was remediated in the first quarter of 2014. In order to remediate the material weakness related to the establishment of accounts receivable related reserves, we (i) standardized processes, procedures and productivity measures for intake, billing, collection and cash application processes; (ii) converted all sites to a single version of our pharmacy and accounts receivable system; (iii) centralized the cash application function and implemented technology upgrades to improve the accuracy and timeliness of cash application and secondary payor billing; and (iv) developed a robust estimation methodology for the allowance for doubtful accounts and contractual adjustments that uses historical collection and write-off data from acquired sites. We implemented and utilized these new measures throughout 2014. However, management evaluated and tested the effectiveness of the design and operation of our internal control over financial reporting and concluded that the material weakness related to the establishment of accounts receivable related reserves and the timely recognition of bad debt expense still existed as of December 31, 2014.
In order to remediate the material weaknesses our management identified as of December 31, 2014, we are (i) reviewing the GITCs to ensure that specific roles and responsibilities are evaluated and duties within our information technology environments are segregated; (ii) reviewing the need for additional corporate accounting and finance personnel and external resources to ensure that we have an effective system of internal controls related to significant and unusual transactions; and (iii) improving our processes and analyses performed by management that support the estimate of the allowance for doubtful accounts and the related bad debt expense.
If our remedial measures are insufficient to address the material weaknesses, or if additional material weaknesses or significant deficiencies in our internal control over financial reporting are discovered or occur in the future, then there exists a risk that our consolidated financial statements may contain material misstatements that are unknown to us at that time, and such misstatements could require us to restate our financial results. Our management or our independent registered public accounting firm may identify other material weaknesses in our internal control over financial reporting in the future. The existence of a material weakness in our internal control over financial reporting may result in current and potential stockholders losing confidence in our financial reporting, which could negatively impact the market price of our common stock.
In addition, the existence of material weaknesses in our internal control over financial reporting may affect our ability to timely file periodic reports under the Exchange Act and may consequently result in the SEC revoking the registration of our common stock, the NASDAQ Global Market delisting our common stock or a default or an event of default under our Senior Credit Facilities and our 2021 Notes (each, as defined below). Any of these events could have a material adverse effect on the market price of our common stock or on our business, financial condition and results of operations.
We may be subject to liability claims for damages and other expenses that are not covered by insurance.
A successful product or professional liability claim in excess of our insurance coverage could harm our consolidated financial statements. Various aspects of our business may subject us to litigation and liability for damages. For example, a prescription drug dispensing error could result in a patient receiving the wrong or incorrect amount of medication, leading to personal injury or death. Our business and consolidated financial statements could suffer if we pay damages or defense costs in connection with a claim that is outside the scope of any applicable contractual indemnity or insurance coverage.
Changes in our relationships with pharmaceutical suppliers, including changes in drug availability or pricing, could adversely affect our business and financial results.
We have contractual relationships with pharmaceutical manufacturers to purchase the drugs that we dispense. Any changes to these relationships, including, but not limited, to loss of a manufacturer relationship, drug shortages or changes in pricing, could have an adverse effect on our business and financial results.
We purchase a majority of our pharmaceutical products from one vendor and a disruption in our purchasing arrangements could adversely impact our business.
We purchase a majority of our prescription products, subject to certain minimum periodic purchase levels and excluding purchases of therapeutic plasma products, from a single wholesaler, AmerisourceBergen Drug Corporation, or ABDC, pursuant to a prime vendor agreement. The term of this agreement extends until December 2018, subject to extension for up to two additional years. Any significant disruption in our relationship with ABDC, or in ABDC’s supply and timely delivery of products to us, would make it difficult and possibly more costly for us to continue to operate our business until we are able to execute a replacement
25
wholesaler agreement. We may not be able to find a replacement wholesaler on a timely basis or that such wholesaler would be able to fulfill our demands on similar financial terms and service levels. If we are unable to identify a replacement on substantially similar financial terms and/or service levels, our consolidated financial statements may be materially and adversely affected.
A disruption in supply could adversely impact our business.
We also source pharmaceuticals, medical supplies and equipment from other manufacturers, distributors and wholesalers. Most of the pharmaceuticals that we purchase are available from multiple sources, and we believe they are available in sufficient quantities to meet our needs and the needs of our patients. We keep safety stock to ensure continuity of service for reasonable, but limited, periods of time. Should a supply disruption result in the inability to obtain especially high margin drugs and compound components, our consolidated financial statements could be negatively impacted.
Prescription volumes may decline, and our net revenues and profitability may be negatively impacted, when products are withdrawn from the market or when increased safety risk profiles of specific drugs result in utilization decreases.
We dispense significant volumes of prescription medications from our pharmacies. Our dispensing volume is the principal driver of revenue and profitability. When products are withdrawn by manufacturers, or when increased safety risk profiles of specific drugs or classes of drugs result in utilization decreases, physicians may cease writing or reduce the numbers of prescriptions written for these higher-risk drugs. Additionally, negative media reports regarding drugs with higher safety risk profiles may result in reduced consumer demand for such drugs. In cases where there are no acceptable prescription drug equivalents or alternatives for these prescription drugs, our prescription volumes, net revenues, profitability and cash flows may decline.
Home infusion joint ventures formed with hospitals could adversely affect our financial results.
The home infusion industry is currently seeing renewed activity in the formation of equity-based infusion joint ventures formed with hospitals. This activity stems, in part, from hospitals seeking to position themselves for new paradigms in the delivery of coordinated healthcare and new methods of payment, including an emerging interdisciplinary care model forming that is being labeled as an accountable care organization, or ACO. These organizations are encouraged by the new Health Reform Law. These entities are being designed in order to save money and improve quality of care by better integrating care, with the healthcare provider possibly sharing in the financial benefits of the new efficiencies.
Participation in equity-based joint ventures offer hospitals and other providers an opportunity to more efficiently transfer patients to less expensive care settings, while keeping the patient within its network. Additionally, it provides many hospitals with a mechanism to invest accumulated profits in a growing sector with attractive margins.
If these home infusion joint ventures continue to expand and we lose referrals as a result, our consolidated financial statements could be adversely affected.
Network lock-outs by health insurers and PBMs could adversely affect our financial results.
Many Plan Sponsors and PBMs continue to create exclusive pharmacy networks which limit a member’s access to a mail service facility or network of preferred pharmacies. To the extent our pharmacies are excluded from these networks, we are unable to dispense medications to those members and bill for prescriptions to those member’s insurance carriers. If these specialty networks continue to expand and we are locked out from dispensing infusion medications to members of exclusive networks, our consolidated financial statements could be adversely affected.
A shortage of qualified registered nursing staff, pharmacists and other professionals could adversely affect our ability to attract, train and retrain qualified personnel and could increase operating costs.
Our business relies significantly on its ability to attract and retain nursing staff, pharmacists and other professionals who possess the skills, experience and licenses necessary to meet the requirements of their job responsibilities. From time to time and particularly in recent years, there have been shortages of nursing staff, pharmacists and other professionals in certain local and regional markets. As such, we are often required to compete for personnel with other healthcare systems and our competitors. Our ability to attract and retain personnel depends on several factors, including our ability to provide them with engaging assignments and competitive salaries and benefits. We may not be successful in any of these areas.
In addition, where labor shortages arise in markets in which we operate, we may face higher costs to attract personnel, and we may have to provide them with more attractive benefit packages than originally anticipated or are being paid in other markets where such shortages don’t exist at the time. In either case, such circumstances could cause our profitability to decline. Finally,
26
if we expand our operations into geographic areas where healthcare providers historically have unionized or unionization occurs in our existing geographic areas, negotiating collective bargaining agreements may have a negative effect on our ability to timely and successfully recruit qualified personnel and on our financial results. If we are unable to attract and retain nursing staff, pharmacists and other professionals, the quality of our services may decline and we could lose patients and referral sources.
Introduction of new drugs or accelerated adoption of existing lower margin drugs could cause us to experience lower revenues and profitability when prescribers prescribe these drugs for their patients or they are mandated by Plan Sponsors.
The pharmaceutical industry pipeline of new drugs includes many drugs that over the long term may replace older, more expensive therapies. As a result of such older drugs going off patent and being replaced by generic substitutes, new and less expensive delivery methods (such as when an infusion or injectable drug is replaced with an oral drug) or additional products are added to a therapeutic class, thereby increasing price competition among competing manufacturer’s products in that therapeutic category. In such cases, manufacturers have the ability to increase drug acquisition costs or lower the selling price of replaced products. This could have the effect of lowering our revenues and/or margins.
Any changes to our relationships with our discount card brokers or changes in their efforts could negatively impact our business and financial results.
We contract with over 95 marketing companies that provide pharmacy discount cards to the uninsured and underinsured. Depending on the amount of revenue generated by any broker agreement, one or more terminations could have a material and adverse effect on our consolidated financial statements. The brokers we use are typically small, privately held marketing companies. Several of the large chain pharmacies are heavily promoting their own store discount cards, which has had a negative impact on volume for the discount card business. Because of the reduced volume, some of our discount card brokers have reduced their efforts because response rates to their marketing campaigns have decreased. These decreases in volume could negatively impact our business and financial results.
Increases in costs to fulfill discount card claims could reduce our profitability.
The discount card portion of our PBM business relies on participating network pharmacies to fulfill drug prescriptions and reimburse us for the utilization of the card. Our fees are based on negotiated rates with the pharmacies. Should these fees decrease, operating profit will be reduced.
Acts of God such as major weather disturbances could disrupt our business.
We operate in a network of prescribers, providers, patients and facilities that can be negatively impacted by local weather disturbances and other force majeure events. For example, in anticipation of major weather events, patients with impaired health may be moved to alternate sites. After a major weather event, availability of electricity, clean water and transportation can impact our ability to provide service in the home. In addition, acts of God and other force majeure events may cause a reduction in our business or increased costs, such as increased costs in our operations as we incur overtime charges or redirect services to other locations, delays in our ability to work with payors, hospitals, physicians and other strategic partners on new business initiatives, and disruption to referral patterns as patients are moved out of facilities affected by such events or are unable to return to sites of service in the home.
Failure to develop new services may adversely affect our business.
We operate in a highly competitive environment. We develop new services from time to time to assist our clients. If we are unsuccessful in developing innovative services, our ability to attract new clients and retain existing clients may suffer.
Technology is also an important component of our business as we continue to utilize new and better channels to communicate and interact with our clients, members and business partners. If our competitors are more successful than us in employing this technology, our ability to attract new clients, retain existing clients and operate efficiently may suffer.
Cybersecurity risks could compromise our information and expose us to liability, which may harm our ability to operate effectively and may cause our business and reputation to suffer.
Cybersecurity refers to the combination of technologies, processes and procedures established to protect information technology systems and data from unauthorized access, attack, or damage. We rely on our information systems to provide security for processing, transmission and storage of confidential information about our patients, customers and personnel, such as names, addresses and other individually identifiable information protected by HIPAA and other privacy laws. Cyber-attacks are
27
increasingly more common, including in the health care industry. The regulatory environment surrounding information security and privacy is increasingly demanding, with the frequent imposition of new and changing requirements. Compliance with changes in privacy and information security laws and with rapidly evolving industry standards may result in our incurring significant expense due to increased investment in technology and the development of new operational processes.
We have not experienced any known attacks on our information technology systems that compromised any confidential information. We maintain our information technology systems with safeguard protection against cyber-attacks including passive intrusion protection, firewalls and virus detection software. However, these safeguards do not ensure that a significant cyber-attack could not occur. Although we have taken steps to protect the security of our information systems and the data maintained in those systems, it is possible that our safety and security measures will not prevent the systems’ improper functioning or damage or the improper access or disclosure of personally identifiable information such as in the event of cyber-attacks.
Security breaches, including physical or electronic break-ins, computer viruses, attacks by hackers and similar breaches can create system disruptions or shutdowns or the unauthorized disclosure of confidential information. If personal information or protected health information is improperly accessed, tampered with or disclosed as a result of a security breach, we may incur significant costs to notify and mitigate potential harm to the affected individuals, and we may be subject to sanctions and civil or criminal penalties if we are found to be in violation of the privacy or security rules under HIPAA or other similar federal or state laws protecting confidential personal information. In addition, a security breach of our information systems could damage our reputation, subject us to liability claims or regulatory penalties for compromised personal information and could have a material adverse effect on our business, financial condition and results of operations.
The success of our business depends on maintaining a well-secured business and technology infrastructure.
We are dependent on our infrastructure, including our information systems, for many aspects of our business operations. A fundamental requirement for our business is the secure storage and transmission of protected health information and other confidential data. Our business and operations may be harmed if we do not maintain our business processes and information systems in a secure manner, and maintain and continually improve the integrity of our confidential information. Although we have developed systems and processes that are designed to protect information against security breaches, failure to protect our confidential information or mitigate harm caused by such breaches may adversely affect our operating results. Malfunctions in our business processes, breaches of our information systems or the failure to maintain effective and up-to-date information systems could disrupt our business operations, result in customer and member disputes, damage our reputation, expose us to risk of loss or litigation, result in regulatory violations and related costs and penalties, increase administrative expenses or lead to other adverse consequences.
Our business is dependent on the services provided by third party information technology vendors.
Our information technology infrastructure includes hosting services provided by third parties. While we believe these third parties are high-performing organizations with secure platforms and customary certifications, they could suffer a security breach or business interruption which in turn could impact our operations negatively. In addition, changes in pricing terms charged by our technology vendors may adversely affect our financial performance.
Our failure to maintain controls and processes over billing and collecting could have a significant negative impact on our consolidated financial statements.
The collection of accounts receivable is a significant challenge, and requires constant focus and involvement by management and ongoing enhancements to information systems and billing center operating procedures. If we are unable to properly bill and collect our accounts receivable, our results could be materially and adversely affected. While management believes that our controls and processes are satisfactory, our accounts receivable collectability may not remain at current levels.
Delays in payment may adversely affect our working capital.
Our business is characterized by delays from the time we provide services to the time we receive payment for these services. If we have difficulty in obtaining documentation, experience information system problems or experience other issues that arise with Medicare or other payors, we may encounter additional delays in our payment cycle.
In addition, timing delays may cause working capital shortages. Working capital management, including prompt and diligent billing and collection, is an important factor in achieving our financial results and maintaining liquidity. It is possible that documentation support, system problems, Medicare or other provider issues or industry trends may extend our collection period,
28
which may materially adversely affect our working capital, and our working capital management procedures may not successfully mitigate this risk.
Our ability to use net operating loss carryforwards to offset future taxable income for U.S. federal tax purposes is subject to limitation, and our issuance of common stock in the merger with Critical Homecare Solutions Holdings, Inc. ("“CHS”") increased the risk that we could experience an “ownership change” in the future that could further limit our ability to utilize our net operating losses.
Under U.S. federal income tax law, a corporation’s ability to utilize its net operating losses (“NOLs”) to offset future taxable income may be significantly limited if it experiences an “ownership change” as defined in Section 382 of the Internal Revenue Code, as amended. In general, an ownership change will occur if there is a cumulative change in a corporation’s ownership by “5-percent shareholders” that exceeds 50 percentage points over a rolling three-year period. A corporation that experiences an ownership change will generally be subject to an annual limitation on the use of its pre-ownership change NOLs equal to the value of the corporation immediately before the ownership change, multiplied by the long-term tax-exempt rate (subject to certain adjustments). The annual limitation for a taxable year generally is increased by the amount of any “recognized built-in gains” for such year and the amount of any unused annual limitation in a prior year. Any limitation to our annual use of NOLs could require us to pay a greater amount of U.S. federal (and in some cases, state) income taxes, which could reduce our after-tax income from operations for future taxable years and adversely impact our financial condition.
Risks Related to Our Indebtedness
We incurred substantial additional indebtedness to refinance our prior indebtedness and to finance our acquisition of the CarePoint Business, which imposes operating and financial restrictions on us that, together with the resulting debt service obligations, may significantly limit our ability to execute our business strategy and may increase the risk of default under our debt obligations.
We have entered into (i) a senior secured first-lien revolving credit facility in an aggregate principal amount of $75.0 million (the “Revolving Credit Facility”), (ii) a senior secured first-lien term loan B in an aggregate principal amount of $250.0 million (the “Term Loan B Facility”) and (iii) a senior secured first-lien delayed draw term loan B in an aggregate principal amount of $150.0 million (the “Delayed Draw Term Loan Facility” and, together with the Revolving Credit Facility and the Term Loan B Facility, the “Senior Credit Facilities”). A portion of the proceeds of the loans advanced to us on the closing date of the Senior Credit Facilities were used to refinance certain existing indebtedness of ours and our subsidiaries, including the repayment in full of all amounts outstanding under the Prior Credit Facility, the payment of the purchase price for our 10 1/4% senior unsecured notes due 2015 (the “2015 Notes”) tendered and accepted for purchase in the Offer and the payment of the redemption price for the 2015 Notes that remained outstanding after completion of the Offer. The Delayed Draw Term Loan Facility was fully funded in connection with the closing of our acquisition of the CarePoint Business, and the proceeds were used to fund a portion of the purchase price for such acquisition. The proceeds of all other loans advanced under the Senior Credit Facilities have been or will be used to fund working capital and other general corporate purposes of BioScrip and its subsidiaries, including acquisitions, investments and capital expenditures. Our indebtedness may significantly limit our ability to execute our business strategy.
In addition, we have issued $200.0 million in aggregate principal amount of 8.875% senior notes due 2021 (the “2021 Notes”). See “Item 7 - Management’s Discussion and Analysis of Financial Condition and Results of Operations - Liquidity and Capital Resources.” The 2021 Notes are our senior unsecured obligations and are fully and unconditionally guaranteed by certain of our subsidiaries. Pursuant to the terms of the Second Amendment to the Senior Credit Facilities, we used approximately $194.5 million of the net proceeds of the 2021 Notes offering to repay $59.3 million of our Revolving Credit Facility and $135.2 million related to the Term Loans Facilities. Interest is payable semi-annually on February 1 and August 1. At our option, we may redeem some or all of the 2021 Notes prior to maturity.
The operating and financial restrictions and covenants of our debt instruments, including the Senior Credit Facilities and the indenture governing the 2021 Notes, may adversely affect our ability to finance our future operations or capital needs or engage in other business activities that may be in our interest. The terms of the Senior Credit Facilities require us to comply with certain financial covenants, including a maximum leverage ratio (which will be tested to the extent that advances under the Revolving Credit Facility exceed 25% of the maximum amount able to be drawn thereunder). In addition, subject to a number of important exceptions, the Senior Credit Facilities contain certain restrictions on our ability to, among other things:
• | incur or guarantee additional indebtedness or issue certain preferred stock; |
• | transfer or sell assets; |
• | make certain investments and loans; |
• | pay dividends or distributions, redeem subordinated indebtedness, or make other restricted payments; |
29
• | create or incur liens; |
• | incur dividend or other payment restrictions affecting certain subsidiaries; |
• | issue capital stock of our subsidiaries; |
• | enter into hedging transactions or sale and leaseback transactions; |
• | consummate a merger, consolidation or sale of all or substantially all of our assets or the assets of any of our subsidiaries; and |
• | enter into transactions with affiliates. |
The indenture governing the 2021 Notes contains similar restrictions. Our ability to comply with these covenants, including the financial covenant, may be affected by events beyond our control. Therefore, in order to engage in some corporate actions, we may need to seek permission from our lenders or the note holders, whose interests may be different from ours and we cannot guarantee that we will be able to obtain such consent when needed. If we do not comply with the restrictions and covenants in our Senior Credit Facilities, we may not be able to finance our future operations, make acquisitions or pursue business opportunities. The restrictions contained in our Senior Credit Facilities may prevent us from taking actions that we believe would be in the best interest of our business and may make it difficult for us to successfully execute our business strategy or effectively compete with companies that are not similarly restricted. Additionally, we cannot assure you that we will be able to satisfy the maximum leverage ratio in the event that such financial covenant is tested or that the lenders under the Senior Credit Facilities will waive any failure to meet that test.
A breach of any of these covenants or the inability to comply with the required financial ratio could result in a default under the Senior Credit Facilities. If any such default occurs, the lenders under the Senior Credit Facilities may elect to declare all of their respective outstanding debt, together with accrued interest and other amounts payable thereunder, to be immediately due and payable. Under such circumstances, we may not have sufficient funds or other resources to satisfy all of our obligations. In addition, the limitations imposed on our ability to incur additional debt and to take other corporate actions might significantly impair our ability to obtain other financing.
Although we entered into a First Amendment, Second Amendment and Third Amendment with respect to the Senior Credit Facilities on December 23, 2013, January 31, 2014 and March 1, 2015, respectively, there can be no assurance that we will be granted future waivers or amendments to the restrictions in the Senior Credit Facilities if for any reason we are unable to comply with such restrictions or that we will be able to refinance our debt on terms acceptable to us, or at all.
The lenders under the Senior Credit Facilities also have the right in these circumstances to terminate any commitments they have to provide further borrowings. If we were unable to pay such amounts, the lenders under the Senior Credit Facilities could recover amounts owed to them by foreclosing against the collateral pledged to them. We have pledged a substantial portion of our assets to the lenders under the Senior Credit Facilities, including the equity of all of the Company’s subsidiaries.
In addition, the degree to which we are leveraged as a result of the indebtedness incurred in connection with the acquisition of the CarePoint Business or otherwise could:
• | make us more vulnerable to general adverse economic, regulatory and industry conditions; |
• | limit our flexibility in planning for, or reacting to, changes and opportunities in the markets in which we compete; |
• | place us at a competitive disadvantage compared to our competitors that have less debt; |
• | require us to dedicate a substantial portion of our cash flow to service our debt, reducing the availability of our cash flow and such proceeds to fund working capital, capital expenditures and other general corporate purposes; or |
• | restrict us from making strategic acquisitions or exploiting other business opportunities. |
We may be unable to obtain a required modification of the Revolving Credit Facility if our Revolving Credit Facility usage exceeds certain thresholds.
If the PBM business continues to decline and if we are unable to offset this decline through the growth of our other business segments or otherwise improve our cash flow from operations and reduce our borrowing needs, the Revolving Credit Facility financial covenant that limits advances under the Revolving Credit Facility will become applicable to us. This covenant becomes applicable when Revolving Credit Facility usage exceeds certain thresholds. In such event, we would be required to seek modification of this financial covenant to better align with our expectations for the PBM business. There can be no assurance that the lenders under the Revolving Credit Facility will grant our request for such modification, nor is there any assurance that the terms and conditions of such modification, if granted by the lenders, would be acceptable to us. If this covenant becomes applicable and we do not obtain the required modification of the covenant, we will not be in compliance with this covenant.
30
Despite our substantial indebtedness, we may still incur significantly more debt. This could exacerbate the risks associated with our substantial leverage.
We may incur substantial additional indebtedness, including additional secured indebtedness, in the future, in connection with future acquisitions, strategic investments and strategic relationships. Although the Senior Credit Facilities and the indenture governing the 2021 Notes contain restrictions on the incurrence of additional debt, these restrictions are subject to a number of qualifications and exceptions and, under certain circumstances, debt incurred in compliance with these restrictions, including secured debt, could be substantial. The Senior Credit Facilities permit, among other things, credit borrowings of up to $475.0 million. Adding additional debt to current debt levels could exacerbate the leverage-related risks described above.
To service our indebtedness and other obligations, we will require a significant amount of cash. Our ability to generate cash depends on many factors beyond our control, and any failure to meet our debt obligations could harm our business, financial condition and results of operations.
Our ability to make payments on and to refinance our indebtedness, including the Senior Credit Facilities and the 2021 Notes, and to fund working capital needs and planned capital expenditures will depend on our ability to generate cash in the future. A significant reduction in our operating cash flows resulting from changes in economic conditions, changes in government reimbursement rates or methods, increased competition or other events beyond our control could increase the need for additional or alternative sources of liquidity and could have a material adverse effect on our business, consolidated financial statements, prospects and our ability to service our debt and other obligations.
We cannot assure you that our business will generate sufficient cash flows from operations or that future borrowings will be available to us under the Senior Credit Facilities or otherwise in an amount sufficient to enable us to pay our indebtedness, including our indebtedness under the Senior Credit Facilities and 2021 Notes, or to fund our other liquidity needs. Our inability to pay our debts would require us to pursue one or more alternative strategies, such as selling assets, refinancing all or a portion of our indebtedness or selling equity capital. However, our alternative strategies may not be feasible at the time or may not provide adequate funds to allow us to pay our debts as they come due and fund our other liquidity needs. In addition, some alternative strategies are likely to require the prior consent of our senior secured lenders, which we may not be able to obtain.
The 2021 Notes are structurally subordinated to the liabilities of our subsidiaries that do not guarantee the 2021 Notes.
The 2021 Notes are guaranteed on a senior unsecured basis by each of our current and future wholly owned domestic subsidiaries that are guarantors under our Senior Credit Facilities. The 2021 Notes are structurally subordinated to indebtedness and other liabilities, including trade payables, of any of our existing and future subsidiaries that are not guarantors of the 2021 Notes.
The indenture governing the 2021 Notes allows non-guarantor subsidiaries to incur certain additional indebtedness in the future. In the event of a bankruptcy, liquidation or reorganization of any of our non-guarantor subsidiaries, these non-guarantor subsidiaries will pay the holders of their debts, holders of their preferred equity interests and their trade creditors before they will be able to distribute any of their assets to us.
A subsidiary guarantee could be voided if it constitutes a fraudulent transfer under U.S. bankruptcy or similar state law, which would prevent the lenders under the Senior Credit Facilities from relying on that subsidiary to satisfy claims.
The indebtedness outstanding under our Senior Credit Facilities is guaranteed by our domestic subsidiaries. The guarantees may be subject to review under U.S. federal bankruptcy law and comparable provisions of state fraudulent conveyance laws if a bankruptcy or another similar case or lawsuit is commenced by or on behalf of our or a guarantor subsidiary’s unpaid creditors or another authorized party. Under these laws, if a court were to find that, at the time any guarantor subsidiary issued a guarantee of the indebtedness under the Senior Credit Facilities, either it issued the guarantee to delay, hinder or defraud present or future creditors or it received less than reasonably equivalent value or fair consideration for issuing the guarantee and at the time:
• | it was insolvent or rendered insolvent by reason of issuing the guarantee; |
• | it was engaged, or about to engage, in a business or transaction for which its remaining unencumbered assets constituted unreasonably small capital to carry on its business; |
• | it intended to incur, or believed that it would incur, debts beyond its ability to pay as they mature; or |
• | it was a defendant in an action for money damages, or had a judgment for money damages docketed against it if, in either case, after final judgment, the judgment is unsatisfied, then the court could void the obligations under the guarantee, or subordinate the guarantee of the indebtedness outstanding under the Senior Credit Facilities to other debt. |
31
We cannot be sure as to the standard that a court would use to determine whether a guarantor subsidiary was solvent at the relevant time, or, regardless of the standard that the court uses, that the issuance of the guarantees would not be voided or that the guarantees would not be subordinated to other debt. If such a case were to occur, the guarantee could also be subject to the claim that, since the guarantee was incurred for our benefit, and only indirectly for the benefit of the guarantor subsidiary, the obligations of the applicable guarantor subsidiary were incurred for less than fair consideration. A court could thus void the obligations under the guarantee, subordinate the guarantee to the applicable guarantor subsidiary’s other debt or take other action detrimental to the lenders under the Senior Credit Facilities. If a court were to void a guarantee, the lenders under the Senior Credit Facilities would no longer have a claim against the guarantor subsidiary. Sufficient funds to repay amounts outstanding under the Senior Credit Facilities may not be available from other sources, including the remaining guarantor subsidiaries, if any. In addition, the court might direct the lenders under the Senior Credit Facilities to repay any amounts already received from or that are attributable to the guarantor subsidiary.
Each subsidiary guarantee contains a provision intended to limit the guarantor subsidiary’s liability to the maximum amount that it could incur without causing the incurrence of obligations under its subsidiary guarantee to be a fraudulent transfer. This provision may not be effective to protect the subsidiary guarantees from being voided under fraudulent transfer law.
Our subsidiary guarantors may be unable to fulfill their obligations under their guarantees.
The ability of our subsidiary guarantors to make any required payments under their guarantees depends on our future operating performance, which will be affected by financial, business, economic and other factors, many of which we cannot control. Such subsidiaries’ businesses may not generate sufficient cash flow from operations in the future and their anticipated growth in revenue and cash flow may not be realized, either or both of which could result in their being unable to honor their guarantees or to fund other liquidity needs. If such subsidiaries do not have enough money, they may be required to refinance all or part of their then-existing debt, sell assets or borrow more money. They may not be able to accomplish any of these alternatives on terms acceptable to them, or at all. In addition, the terms of existing or future debt agreements, including the Senior Credit Facilities, may restrict such subsidiaries from adopting any of these alternatives. The failure of our subsidiaries to generate sufficient cash flow or to achieve any of these alternatives could materially and adversely affect the ability of such subsidiaries to pay the amounts due under their guarantees, if any.
Item 1B. | Unresolved Staff Comments |
None.
32
Item 2. | Properties |
Our executive offices are located in Elmsford, New York and we maintain a corporate office in Eden Prairie, Minnesota. We currently lease all of our properties from third parties under various lease terms expiring over periods extending through 2023, in addition to a number of non-material month-to-month leases. Our properties mainly consist of infusion pharmacies equipped with clean room and compounding capabilities. Some infusion pharmacies are co-located with an ambulatory infusion center where patients receive infusion treatments. As of December 31, 2014 our property locations, all in support of our Infusion Services segment, were as follows:
Birmingham, AL | Savannah, GA | Pearl, MS | Jackson, TN | |||
Burbank, CA | Elmhurst, IL | Durham, NC | Knoxville, TN | |||
Irvine, CA | Silvis, IL | Fayetteville, NC | Memphis, TN | |||
Ontario, CA | Lexington, KY | Omaha, NE | Nashville, TN | |||
Rohnert Park, CA | Alexandria, LA | Bedford, NH | Austin, TX | |||
San Diego, CA | Baton Rouge, LA | Morris Plains, NJ | Dallas, TX | |||
Cromwell, CT (two locations) | Covington, LA | Chestnut Ridge, NY | Houston, TX | |||
Norwalk, CT | Hammond, LA | Lake Success, NY | Richardson, TX | |||
Vernon, CT | Houma, LA | New York, NY | Texarkana, TX | |||
Coral Springs, FL | Lafayette, LA | Canfield, OH | Annandale, VA | |||
Gainesville, FL | Metairie, LA | Cincinnati, OH | Ashland, VA | |||
Jacksonville, FL | Monroe, LA | Columbus, OH | Chantilly, VA | |||
Melbourne, FL | Shreveport, LA | Sylvania, OH | Fredericksburg, VA | |||
Miami Lakes, FL | Southborough, MA | Dunmore, PA | Norfolk, VA | |||
Tampa, FL | Columbia, MD | Sharpsburg, PA | Roanoke, VA | |||
Albany, GA | Auburn, ME | West Chester, PA | Rutland, VT | |||
Augusta, GA | Auburn Hills, MI | Pawtucket, RI | Charleston, WV | |||
Brunswick, GA | Eagan, MN | Duncan, SC | Morgantown, WV | |||
Norcross, GA | Chesterfield, MO | Mount Pleasant, SC | Fairmont, WV | |||
33
Item 3. | Legal Proceedings |
Securities Class Action Litigation in the Southern District of New York
On September 30, 2013, a putative securities class action lawsuit was filed against the Company and certain of its officers on behalf of the putative class of purchasers of our securities between August 8, 2011 and September 20, 2013, inclusive.
On November 15, 2013, a putative securities class action lawsuit was filed against the Company and certain of its directors and officers and certain underwriters in the Company’s April 2013 underwritten public offering of its common stock, on behalf of the putative class of purchasers of our securities between August 8, 2011 and September 23, 2013, inclusive.
On December 19, 2013, the United States District Court for the SDNY entered an order consolidating the two class action lawsuits and appointing a lead plaintiff. The Company denies any allegations of wrongdoing in the consolidated class action lawsuit. The lead plaintiff filed a consolidated complaint on February 19, 2014 against the Company, certain of its directors and officers, certain underwriters in the Company’s April 2013 underwritten public offering of its common stock, and a certain stockholder of the Company. The consolidated complaint is brought on behalf of a putative class of purchasers of the Company’s securities between November 9, 2012 and November 6, 2013, inclusive, and persons and entities who purchased the Company’s securities pursuant or traceable to two underwritten public offerings of the Company’s common stock conducted in April 2013, and August 2013. The consolidated complaint alleges generally that the defendants made material misstatements and/or failed to disclose matters related the Legacy Division’s distribution of the Medication as well as the Company’s PBM Services segment. The consolidated complaint asserts claims under Sections 11, 12(a)(2) and 15 of the Securities Act and Sections 10(b) and 20(a) of the Exchange Act and Rule 10b-5 promulgated thereunder. All defendants in the case moved to dismiss the consolidated complaint on April 28, 2014. Briefing on the motion to dismiss was complete on July 28, 2014. The Company believes all of the claims in these class action lawsuits are without merit and intends to vigorously defend against these claims. However, there is no assurance that the Company will be successful in its defense or that insurance will be available or adequate to fund any settlement or judgment or the litigation costs of these actions. Additional similar lawsuits may be filed. Moreover, the Company is not able to predict the outcome or reasonably estimate a range of possible loss at this time.
Item 4. | Mine Safety Disclosures |
Item not applicable.
34
PART II
Item 5. | Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities |
Our common stock, par value $0.0001 per share (“Common Stock”), is traded on the NASDAQ Global Market under the symbol “BIOS.” The following table represents the range of high and low sale prices for our Common Stock for the last eight quarters. These prices reflect interdealer prices, without retail mark-up, mark-down or commission and may not necessarily represent actual transactions.
High | Low | ||||||||
2014 | First Quarter | $ | 9.05 | $ | 6.63 | ||||
Second Quarter | $ | 8.45 | $ | 5.93 | |||||
Third Quarter | $ | 8.75 | $ | 6.75 | |||||
Fourth Quarter | $ | 7.01 | $ | 5.44 | |||||
2013 | First Quarter | $ | 12.92 | $ | 10.57 | ||||
Second Quarter | $ | 16.93 | $ | 11.62 | |||||
Third Quarter | $ | 17.62 | $ | 8.29 | |||||
Fourth Quarter | $ | 8.93 | $ | 5.61 | |||||
As of February 25, 2015, there were 194 stockholders of record of our Common Stock. On February 25, 2015 the closing sale price of our Common Stock on the NASDAQ Global Market was $5.99 per share.
We have never paid cash dividends on our Common Stock and do not anticipate doing so in the foreseeable future.
Information regarding securities authorized for issuance under our equity compensation plans required by this Item 5 is included in our definitive proxy statement to be filed with the SEC on or before April 30, 2015 in connection with our 2015 Annual Meeting of Stockholders and is hereby incorporated by reference.
35
The graph below compares our total cumulative return to holders of our Common Stock with the total cumulative returns of the NASDAQ Composite Index and the NASDAQ Health Services Index for the five-year period from December 31, 2009 through December 31, 2014. The graph shows the performance of a $100 investment in our Common Stock and in each index as of December 31, 2009.
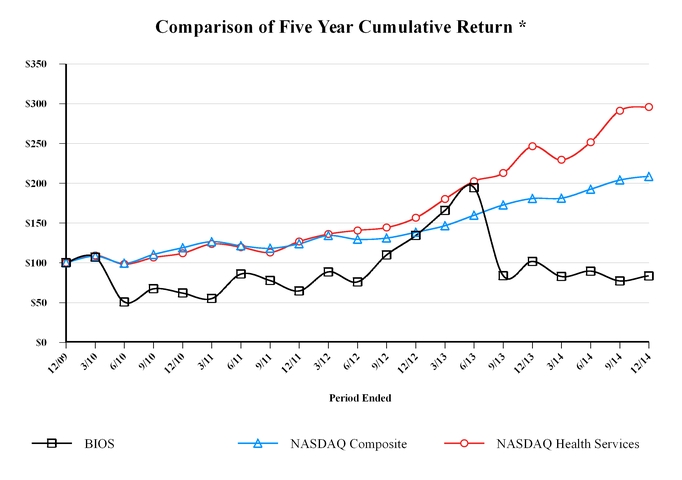
* $100 invested on December 31, 2009 in stock or index including reinvestment of dividends.
Item 6. | Selected Consolidated Financial Data |
The selected consolidated financial data presented below should be read in conjunction with, and is qualified in its entirety by reference to, Management’s Discussion and Analysis and our Consolidated Financial Statements and the Notes thereto appearing elsewhere in this Annual Report. Acquisitions during the periods below include CHS beginning March 2010, DS Pharmacy beginning July 2010, InfuScience beginning August 2012, HomeChoice beginning February 2013 and CarePoint Business beginning August 2013. Divestitures during this period include the Pharmacy Services Asset Sale in February 2012 and the sale of substantially all of the Home Health Business in March 2014. All historical amounts have been restated to reclassify amounts directly associated with these divested operations as discontinued operations. The amounts below are not necessarily indicative of what the actual results would have been if the Pharmacy Services Asset Sale and the sale of the Home Health Business were divested at the beginning of the period.
36
December 31, | |||||||||||||||||||
Balance Sheet Data | 2014 | 2013 | 2012 | 2011 | 2010 | ||||||||||||||
(in thousands) | |||||||||||||||||||
Working capital (1) | $ | 25,902 | $ | 51,891 | $ | 122,352 | $ | 31,603 | $ | (2,224 | ) | ||||||||
Total assets (2) | $ | 824,713 | $ | 871,900 | $ | 579,131 | $ | 557,831 | $ | 530,016 | |||||||||
Total debt | $ | 423,803 | $ | 435,579 | $ | 226,379 | $ | 293,459 | $ | 306,469 | |||||||||
Stockholders’ equity | $ | 216,805 | $ | 354,583 | $ | 293,409 | $ | 215,279 | $ | 200,101 | |||||||||
Total assets of discontinued operations | $ | — | $ | 64,958 | $ | 63,245 | $ | 119,271 | $ | 133,971 | |||||||||
Year Ended December 31, | |||||||||||||||||||
Statement of Operations Data | 2014 | 2013 | 2012 | 2011 | 2010 | ||||||||||||||
(in thousands, except per share amounts) | |||||||||||||||||||
Revenue | $ | 984,055 | $ | 769,458 | $ | 593,447 | $ | 484,871 | $ | 375,920 | |||||||||
Gross profit | 261,066 | 243,613 | 195,902 | 184,150 | 134,384 | ||||||||||||||
Selling, general and administrative expenses | 239,810 | 209,627 | 162,879 | 145,457 | 117,261 | ||||||||||||||
Change in fair value of contingent consideration | (7,364 | ) | (5,786 | ) | — | — | — | ||||||||||||
Bad debt expense | 79,574 | 19,625 | 13,201 | 10,418 | 6,257 | ||||||||||||||
Acquisition and integration expenses (3) | 17,924 | 16,130 | 4,046 | — | 5,924 | ||||||||||||||
Restructuring and other expenses (4) | 15,646 | 7,718 | 5,143 | 7,904 | 3,952 | ||||||||||||||
Amortization of intangibles | 6,555 | 6,671 | 3,957 | 3,376 | 2,522 | ||||||||||||||
Income (loss) from operations | (91,079 | ) | (10,372 | ) | 6,676 | 16,995 | (1,532 | ) | |||||||||||
Interest expense, net | 38,539 | 28,198 | 26,068 | 25,544 | 23,561 | ||||||||||||||
Loss on extinguishment of debt (5) | 2,373 | 15,898 | — | — | 2,954 | ||||||||||||||
Loss from continuing operations, before income taxes | (131,991 | ) | (54,468 | ) | (19,392 | ) | (8,549 | ) | (28,047 | ) | |||||||||
Income tax expense (benefit) (6) | 11,391 | 2,523 | (7,117 | ) | (2,977 | ) | 45,201 | ||||||||||||
Loss from continuing operations, net of income taxes | (143,382 | ) | (56,991 | ) | (12,275 | ) | (5,572 | ) | (73,248 | ) | |||||||||
Income (loss) from discontinued operations, net of income taxes | (4,086 | ) | (12,663 | ) | 76,982 | 13,444 | 4,106 | ||||||||||||
Net income (loss) | $ | (147,468 | ) | $ | (69,654 | ) | $ | 64,707 | $ | 7,872 | $ | (69,142 | ) | ||||||
Income (loss) per common share: | |||||||||||||||||||
Loss from continuing operations, basic and diluted | $ | (2.09 | ) | $ | (0.89 | ) | $ | (0.22 | ) | $ | (0.10 | ) | $ | (1.45 | ) | ||||
Income (loss) from discontinued operations, basic and diluted | (0.06 | ) | (0.19 | ) | 1.37 | 0.24 | 0.08 | ||||||||||||
Net income (loss), basic and diluted (7) | $ | (2.15 | ) | $ | (1.08 | ) | $ | 1.15 | $ | 0.14 | $ | (1.37 | ) | ||||||
Weighted average common shares outstanding, basic and diluted | 68,476 | 64,560 | 56,239 | 54,505 | 50,374 | ||||||||||||||
(1) | Working capital calculation excludes current assets of discontinued operations and current liabilities of discontinued operations as of December 31, 2013, 2012, 2011 and 2010. |
(2) | Total assets excludes total assets of discontinued operations as of December 31, 2013, 2012, 2011 and 2010. |
(3) | Acquisition and integration expenses are related to the acquisitions of the CarePoint Partners Business (acquired August 23, 2013), HomeChoice Partners (February 1, 2013), InfuScience (July 31, 2012), DS Pharmacy (July 29, 2010) and CHS (March 25, 2010) as well as costs associated with the divestiture resulting from the Pharmacy Services Asset Sale (February 1, 2012) and the sale of the Home Health Business (March 31, 2014). |
(4) | Restructuring and other expenses are related to our strategic assessment and related restructuring plans consisting primarily of employee severance and other benefit-related costs, third-party consulting costs and facility-related costs. Other costs include training and transitional costs, redundant salaries and certain fees incurred as a result of restructuring. |
(5) | The total loss on extinguishment of debt in 2010 was $9.6 million of which $6.6 million is included in loss from discontinued operations. |
37
(6) | The income tax expense of $45.2 million in 2010 relates to the recognition of a valuation allowance on deferred tax assets. |
(7) | The net income (loss) per diluted share excludes the effect of all common stock equivalents for all years as their inclusion would be anti-dilutive to loss per share from continuing operations. |
Item 7. | Management’s Discussion and Analysis of Financial Condition and Results of Operations |
The following Management’s Discussion and Analysis of Financial Condition and Results of Operations (MD&A) is designed to assist the reader in understanding our Consolidated Financial Statements, the changes in certain key items in those financial statements from year-to-year and the primary factors that accounted for those changes, as well as how certain accounting principles affect our Consolidated Financial Statements. The discussion also provides information about the financial results of the segments of our business to provide a better understanding of how those segments and their results affect our financial condition and results of operations as a whole.
Except for the historical information contained herein, the following discussion contains forward-looking statements that are subject to known and unknown risks, uncertainties and other factors that may cause our actual results to differ materially from those expressed or implied by such forward-looking statements. We discuss such risks, uncertainties and other factors throughout this Annual Report and specifically under the caption “Cautionary Note Regarding Forward-Looking Statements” and under “Item 1A. Risk Factors” in this Annual Report. In addition, the following discussion of financial condition and results of operations should be read in conjunction with the Consolidated Financial Statements and Notes thereto appearing elsewhere in this Annual Report.
Business Overview
We are a national provider of infusion and home care management solutions. We partner with physicians, hospital systems, skilled nursing facilities, healthcare payors and pharmaceutical manufacturers to provide patients access to post-acute care services. We operate with a commitment to bring customer-focused pharmacy and related healthcare infusion therapy services into the home or alternate site setting. By collaborating with the full spectrum of healthcare professionals and the patient, we aim to provide cost-effective care that is driven by clinical excellence, customer service and values that promote positive outcomes and an enhanced quality of life for those whom we serve. As of December 31, 2014, we had a total of 77 service locations in 29 states.
Our platform provides nationwide service capabilities and the ability to deliver clinical management services that offer patients a high-touch, community-based and home-based care environment. Our core services are provided in coordination with, and under the direction of, the patient’s physician. Our multidisciplinary team of clinicians, including pharmacists, nurses, dieticians and respiratory therapists, work with the physician to develop a plan of care suited to our patient’s specific needs. Whether in the home, physician office, ambulatory infusion center, skilled nursing facility or other alternate sites of care, we provide products, services and condition-specific clinical management programs tailored to improve the care of individuals with complex health conditions such as gastrointestinal abnormalities, infectious diseases, cancer, multiple sclerosis, organ and blood cell transplants, bleeding disorders, immune deficiencies and heart failure.
Segments
Following the sale of our Home Health Business on March 31, 2014, our business is reported under two operating segments: “Infusion Services” and “PBM Services.” These two segments reflect how our chief operating decision maker reviews our results in terms of allocating resources and assessing operating and financial performance.
Our Infusion Services segment provides services consisting of home infusion therapy, respiratory therapy and the provision of durable medical equipment products and services. Infusion services include the dispensing and administering of infusion-based drugs, which typically require nursing support and clinical management services, equipment to administer the correct dosage and patient training designed to improve patient outcomes.
The PBM Services segment consists of integrated pharmacy benefit management services, primarily discount card programs. The discount card programs provide a cost effective alternative for individuals who may be uninsured, underinsured or may have restrictive coverage that disallows reimbursement for certain medications. Under these discount programs, individuals who present a discount card at one of our participating network pharmacies receive prescription medications at a discounted price compared to the retail price. In addition, in our capacity as a pharmacy benefit manager, it has fully funded prescription benefit programs under which we reimburse our network pharmacies and Third Party Payors in turn reimburse us based on Medi-Span reported pricing for those claims fulfilled for their plan participants.
38
Strategic Transactions
In 2010, we commenced a strategic assessment of our business and operations. The assessment examined our market strengths and opportunities and compared our position to that of our competitors. As a result of this assessment and ensuing assessments, we have focused our growth on investments in the Infusion Services segment, which is now the primary driver of our growth strategy. We continue to assess the value of each of our business lines, including those not targeted for growth.
On February 1, 2012, we entered into a Community Pharmacy and Mail Business Purchase Agreement by and among Walgreen Co. and certain of its subsidiaries for the sale of certain assets, rights and properties (the “Pharmacy Services Asset Sale”) related to our traditional and specialty pharmacy mail operations and community retail pharmacy stores. We received a total purchase price of $173.8 million resulting in a pretax gain of $108.2 million net of transaction costs and other one-time charges.
Following the completion of the Pharmacy Services Asset Sale, we continued to execute our strategic plan by deploying the proceeds toward strategic business acquisitions.
On July 31, 2012, we acquired 100% of InfuScience, Inc. (“InfuScience”) for a cash payment of $38.3 million at closing and an additional $3.0 million of contingent consideration that was paid based on the results of operations during the 24 month period following the acquisition. InfuScience historically acquired, developed and operated businesses providing alternate site infusion pharmacy services through five infusion centers located in Eagan, Minnesota; Omaha, Nebraska; Chantilly, Virginia; Charleston, South Carolina; and Savannah, Georgia.
On February 1, 2013, we acquired 100% of the ownership interest in HomeChoice Partners, Inc. (“HomeChoice”) for a cash purchase price of $72.9 million at closing. The purchase agreement provided that the purchase price may also be increased by contingent consideration of up to $10.0 million if HomeChoice reaches certain performance milestones in the first year following the closing and an additional $10.0 million if HomeChoice reaches certain performance milestones in the second year following the closing, for total possible contingent consideration of up to $20.0 million. HomeChoice has not achieved its performance milestones for the period from acquisition through December 31, 2014 and, as a result, the probability of payment of any contingent consideration is negligible. We funded the acquisition with a combination of cash on hand and drawing on our revolving credit facility. HomeChoice was a provider of alternate-site infusion pharmacy services. Prior to our acquisition, HomeChoice serviced approximately 15,000 patients annually and had 14 infusion pharmacy locations in Pennsylvania, the District of Columbia, Maryland, Virginia, North Carolina, South Carolina, Georgia, Missouri and Alabama.
On August 23, 2013, we completed the acquisition of substantially all of the assets and assumption of certain liabilities that constituted the home infusion business (the “CarePoint Business”) of CarePoint Partners Holdings LLC for a cash purchase price of $211.1 million at closing. The purchase agreement provided that the purchase price could have increased by contingent consideration of $10.0 million if the CarePoint Business achieved a specified level of product gross profit during the one year measurement period following the closing date. The CarePoint Business did not achieve the specified level of product gross profit during the measurement period. We funded the cash payment at closing with a combination of cash on hand and $150.0 million in borrowings under the Delayed Draw Term Loan Facility. CarePoint was a provider of home and alternate-site infusion therapy for patients with complex, acute and chronic illnesses. CarePoint serviced approximately 20,500 patients annually and had 28 sites of service in nine states in the East Coast and Gulf Coast regions prior to our acquisition.
Consistent with our continuing strategic evaluation of our non-core businesses and our decision to continue to focus growth initiatives and capital in the Infusion Services segment, we completed the sale of substantially all of our Home Health Services segment to LHC Group, Inc. and certain of its subsidiaries on March 31, 2014. We received total consideration of approximately $60.6 million in cash including adjustments to net working capital. A portion of the net proceeds from the sale was used to pay down a portion of our outstanding debt.
Regulatory Matters Update
Approximately 23% of revenue for the year ended December 31, 2014 was derived directly from Medicare, state Medicaid programs and other government payors. We also provide services to beneficiaries of Medicare, Medicaid and other government-sponsored healthcare programs through managed care entities. Medicare Part D, for example, is administered through managed care entities. In the normal course of business, we and our customers are subject to legislative and regulatory changes impacting the level of reimbursement received from the Medicare and state Medicaid programs.
39
Medicare
In August 2011, Congress passed a deficit reduction agreement that created a committee tasked with proposing legislation to reduce the federal deficit by November 23, 2011. Because the committee did not act, automatic Medicare cuts were scheduled to go into effect January 1, 2013. However, Congress passed legislation extending the time for such cuts by two months. Thus, Medicare reimbursement to providers was reduced overall by 2% (as part of sequestration) beginning April 1, 2013. The automatic spending cuts did not and will not have an impact on Medicaid reimbursement. The reductions in Medicare reimbursement have not yet been significant. There may also be other impacts from the automatic spending reductions that we cannot predict. Also, the staff at CMS and Medicare administrative contractors may be reduced, which could result in delays in claims processing.
These reductions will be in addition to reductions mandated by the Health Reform Law, which provides for material reductions in the growth of Medicare program spending. Further, from time to time, CMS revises the reimbursement systems used to reimburse health care providers, which may result in reduced Medicare payments. The Medicare Prescription Drug, Improvement, and Modernization Act of 2003 established requirements for a competitive bidding program for determining Medicare reimbursement rates for certain items of DMEPOS that we provide to patients, including enteral nutrients, supplies and equipment, certain respiratory therapy and home medical equipment products and external infusion pumps and supplies. The first round of competitive bidding occurred in nine metropolitan areas around the country, called Competitive Bidding Areas (“CBAs”) and was effective from January 1, 2011 through December 31, 2013. Round 1 did not have a material impact on our business. A Round 1 Recompete was also conducted in the same nine CBAs and included six product categories, including external infusion pumps. The prices for the Round 1 Recompete went into effect January 1, 2014 and will expire December 31, 2016. The second round of competitive bidding was conducted in 100 additional CBAs for eight product categories. New prices for the Round 2 CBAs went into effect July 1, 2013 and will expire June 30, 2016. The impact of the Round 1 Recompete and Round 2 on our business was insignificant.
State Medicaid Programs
Because most states must operate with balanced budgets and because the Medicaid program is often a state’s largest program, some states have enacted or may consider enacting legislation designed to reduce their Medicaid expenditures. Further, many states have also adopted, or are considering, legislation designed to reduce coverage and/or enroll Medicaid recipients in managed care programs. The current economic environment has increased the budgetary pressures on many states, and these budgetary pressures have resulted, and likely will continue to result, in decreased spending, or decreased spending growth, for Medicaid programs and the Children’s Health Insurance Program in many states.
No single state Medicaid program represents greater than 5% of our consolidated revenue for the year ended December 31, 2014 and no individual state Medicaid reimbursement reduction to us as a provider is expected to have a material effect on our consolidated financial statements. We are continually assessing the impact of the state Medicaid reimbursement cuts as states propose, finalize and implement various cost-saving measures.
Given the reimbursement pressures, we continue to improve operational efficiencies and reduce costs to mitigate the impact on results of operations where possible. In some cases, reimbursement rate reductions may result in negative operating results, and we would likely exit some or all services where rate reductions result in unacceptable returns to the Company.
Critical Accounting Estimates
Our Consolidated Financial Statements have been prepared in accordance with United States generally accepted accounting principles (“GAAP”). In preparing our financial statements, we are required to make estimates and assumptions that affect the reported amounts of assets and liabilities, the disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenue and expenses during the reporting period. We evaluate our estimates and judgments on an ongoing basis. We base our estimates and judgments on historical experience and on various other factors that we believe to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that may not be readily apparent from other sources. Our actual results may differ from these estimates, and different assumptions or conditions may yield different estimates. The following discussion highlights what we believe to be the critical accounting estimates and judgments made in the preparation of our Consolidated Financial Statements.
The following discussion is not intended to be a comprehensive list of all the accounting estimates or judgments made in the preparation of our financial statements and in many cases the accounting treatment of a particular transaction is specifically dictated by GAAP, with no need for management’s judgment on its application. See our audited Consolidated Financial Statements and notes thereto appearing elsewhere in this Annual Report, which contain a description of our accounting policies and other disclosures required by GAAP.
40
Revenue Recognition
We generate revenue principally through the provision of home infusion and other home healthcare services to provide clinical management services and the delivery of cost effective prescription medications. Prescription drugs are dispensed through pharmacies owned by us. Fee-for-service agreements include: (i) pharmacy agreements, where we dispense prescription medications through our pharmacy facilities and (ii) PBM agreements, where prescription medications are dispensed through pharmacies participating in our retail pharmacy network.
Financial Accounting Standards Board Accounting Standards Codification (“ASC”) Subtopic 605-25, Revenue Recognition: Multiple-Element Arrangements (“ASC 605-25”), addresses situations in which there are multiple deliverables under one revenue arrangement with a customer and provides guidance in determining whether multiple deliverables should be recognized separately or in combination.
For infusion-related therapies, we frequently provide multiple deliverables of drugs and related nursing services. After applying the criteria of ASC 605-25, we concluded that separate units of accounting exist in revenue arrangements with multiple deliverables. If the drug is shipped, the drug revenue is recognized at the time of shipment, and nursing revenue is recognized on the date of service. We allocate revenue consideration based on the relative fair value as determined by our best estimate of selling price to separate the revenue where there are multiple deliverables under one revenue arrangement. We recognize infusion nursing revenue as the estimated net realizable amounts from patients and payors for services rendered and products provided. This revenue is recognized as the treatment plan is administered to the patient and is recorded at amounts estimated to be received under reimbursement or payment arrangements with payors.
Revenue generated under PBM agreements is classified as either gross or net based on whether we are acting as a principal or an agent in the fulfillment of prescriptions through our pharmacy network. When we independently have a contractual obligation to pay a network pharmacy provider for benefits provided to a Plan Sponsors’ members, and therefore are the “primary obligor” as defined in ASC Topic 605, Revenue Recognition, we include payments (which include the drug ingredient cost) from these Plan Sponsors as revenue and payments to the network pharmacy providers as cost of revenue. These transactions require us to pay network pharmacy providers, assume credit risk of Plan Sponsors and act as a principal. If we merely act as an agent, and consequently administer Plan Sponsors’ network pharmacy contracts, we do not have the primary obligation to pay the network pharmacy and assume credit risk and as such record only the administrative fees (and not the drug ingredient cost) as revenue.
Revenue generated under discount card agreements is recognized when the discount card is used to purchase a prescription drug. The revenue is based on contractual rates per transaction. Broker fees associated with the marketing of the discount cards are incurred and recognized at the time the card is used and classified as selling, general and administrative expense in the Consolidated Statements of Operations.
Allowance for Doubtful Accounts
The allowance for doubtful accounts is based on estimates of losses related to receivable balances. The risk of collection varies based upon the service/product, the payor (commercial health insurance and government) and the patient’s ability to pay the amounts not reimbursed by the payor. We estimate the allowance for doubtful accounts based upon several factors including the age of the outstanding receivables, the historical experience of collections, adjusting for current economic conditions and, in some cases, evaluating specific customer accounts for the ability to pay. Collection agencies are employed and legal action is taken when we determine that taking collection actions is reasonable relative to the probability of receiving payment on amounts owed. Management judgment is used to assess the collectability of accounts and the ability of our customers to pay. Judgment is also used to assess trends in collections and the effects of systems and business process changes on our expected collection rates. We review the estimation process quarterly and make changes to the estimates as necessary. When it is determined that a customer account is uncollectible, that balance is written off against the existing allowance.
41
The following table shows the aging of our net accounts receivable (net of allowance for contractual adjustments and prior to allowance for doubtful accounts), aged based on date of service and categorized based on the three primary overall types of accounts receivable characteristics (in thousands):
December 31, 2014 | December 31, 2013 | |||||||||||||||||||||||
0 - 180 days | Over 180 days | Total | 0 - 180 days | Over 180 days | Total | |||||||||||||||||||
Government | $ | 25,812 | $ | 13,036 | $ | 38,848 | $ | 27,622 | $ | 7,864 | $ | 35,486 | ||||||||||||
Commercial | 117,699 | 35,302 | 153,001 | 122,661 | 26,975 | 149,636 | ||||||||||||||||||
Patient | 4,899 | 10,562 | 15,461 | 2,792 | 2,110 | 4,902 | ||||||||||||||||||
Gross accounts receivable | $ | 148,410 | $ | 58,900 | 207,310 | $ | 153,075 | $ | 36,949 | 190,024 | ||||||||||||||
Allowance for doubtful accounts | (66,500 | ) | (17,836 | ) | ||||||||||||||||||||
Net accounts receivable | $ | 140,810 | $ | 172,188 | ||||||||||||||||||||
Change in Estimate of the Collectability of Accounts Receivable
During the year ended December 31, 2014, the Company experienced deterioration in the aging of certain accounts receivable primarily due to delays and disruptions related to the integration of its acquisitions in 2013. The disruption to billing and collection processes was attributable in part to the following:
• | Re-licensure and new managed care credentialing was required in connection with the CarePoint Business; |
• | Medicare claims were not filed until retraining and review of eligibility was performed; |
• | Merged facilities and work teams in seven large markets and related employee turnover; |
• | Conversion to a single version of our dispensing and billing system while still managing accounts receivable run-off on five other legacy versions; and |
• | Cash posting challenges that delayed secondary and patient billings and patient statement issuance. |
The Company outsourced collections to third party agency partners and hired and trained billing and collection personnel to mitigate the effects of the disruption, however, the Company has experienced more difficulty collecting the aged balances than it originally estimated. While the Company has provided incremental allowances in the prior quarters of 2014 to address the developing deterioration, during the third quarter of 2014, the Company materially changed its estimates based on actual collection experience during and after the acquisition disruption period. The Company also recorded adjustments to reserves in the fourth quarter of approximately $31.7 million consisting of $32.3 million to its allowance for bad debts partially offset by a favorable adjustment of $0.6 million to its contractual adjustment reserves due to the deterioration of the Infusion Services segment accounts receivable aging. The reserves as of December 31, 2014 represent a 100% reserve on all amounts over one year and 80% of receivables outstanding in the nine to twelve month age category. For the year ended December 31, 2014, the incremental adjustments are approximately $60.4 million consisting of $55.4 million to the allowance for doubtful accounts and $5.0 million of contractual adjustment reserves.
Allowance for Contractual Discounts
We are reimbursed by payors for products and services we provide. Payments for medications and services covered by payors average less than billed charges. We monitor revenue and receivables from payors for each of our branches and record an estimated contractual allowance for certain revenue and receivable balances as of the revenue recognition date to properly account for anticipated differences between amounts estimated in our billing system and amounts reimbursed. Accordingly, the total revenue and receivables reported in our financial statements are recorded at the amounts expected to be received from these payors. For the significant portion of our Infusion Services revenue, the contractual allowance is estimated based on several criteria, including unbilled claims, historical trends based on actual claims paid, current contract and reimbursement terms and changes in customer base and payor/product mix. Contractual allowance estimates are adjusted to actual amounts as cash is received and claims are settled. We do not believe these changes in estimates are material. The billing functions for the remaining portion of our revenue are largely computerized, which enables on-line adjudication (i.e., submitting charges to third-party payors electronically, with simultaneous feedback of the amount the primary insurance plan expects to pay) at the time of sale to record net revenue, exposure to estimating contractual allowance adjustments is limited on this portion of the business.
42
Amounts Due to Plan Sponsors
Payables to Plan Sponsors primarily represent payments received from Plan Sponsors in excess of the contractually required reimbursement. These amounts are refunded to Plan Sponsors. These payables also include the sharing of manufacturers’ rebates with Plan Sponsors.
Contingent Consideration
Liabilities that may be owed to sellers after the closing of an acquisition transaction are recorded at fair value as of the opening balance sheet established for the acquired target. These contingent consideration provisions are frequently referred to as earnouts and are the subject of negotiation between the seller and the buyer. An earnout provision can compensate the seller with the value they believe the asset will deliver while also providing downside risk protection to the buyer should projections not materialize. As such, the terms of potential earnouts vary with each transaction. Fair value is assigned using multiple payout scenarios which each have a probability assigned based on factors including actual performance, evidence of business plans that have been implemented, and current market conditions that influence the ability to achieve the earnout. The probable payout amount is discounted to the current balance sheet date using the current weighted average cost of capital. Each quarter, the fair value of the contingent consideration is updated to reflect relevant factors such as post-closing operating results and future forecasts for the acquired business or entity. The fair value of contingent consideration may be included in current liabilities or other non-current liabilities depending on the payment date specified in the purchase agreement.
Income Taxes
As part of the process of preparing our Consolidated Financial Statements, management is required to estimate income taxes in each of the jurisdictions in which we operate. We account for income taxes under ASC Topic 740, Income Taxes (“ASC 740”). ASC 740 requires the use of the asset and liability method of accounting for income taxes. Under this method, deferred taxes are determined by calculating the future tax consequences attributable to differences between the financial accounting and tax bases of existing assets and liabilities. A valuation allowance is recorded against deferred tax assets when, in the opinion of management, it is more likely than not that we will not be able to realize the benefit from our deferred tax assets. A valuation allowance is reversed when sufficient evidence exists that we will be able to realize the benefits of our deferred tax assets.
As of December 31, 2014, we have a full valuation allowance of $111.2 million recorded against our deferred tax assets. We will maintain this valuation allowance until an appropriate level of profitability is sustained or we are able to develop tax planning strategies that enable us to conclude that it is more likely than not that our deferred tax assets are realizable. As of December 31, 2014, we have deferred tax liabilities of $21.3 million relating to indefinite-lived goodwill and intangibles. These deferred tax liabilities cannot be used as a future source of taxable income because of the indefinite nature of the assets and therefore cannot be used to offset the deferred tax assets that require a valuation allowance. The deferred tax liability for these indefinite-lived goodwill and intangibles will continue to increase as we continue to amortize the tax deductible amounts of these assets. The tax amortization related to these assets will increase the deferred tax liability as well as create tax expense in future years until the full valuation allowance is reversed or the asset is fully amortized for tax purposes.
We file income tax returns, including returns for our subsidiaries, as required by federal tax laws and the tax laws of the state and local jurisdictions in which we operate. Our uncertain tax positions are related to tax years that remain subject to examination and are recognized in the financial statements when the recognition threshold and measurement attributes of ASC 740 are met. Interest and penalties related to unrecognized tax benefits are recorded as income tax expense.
Goodwill and Intangible Assets
Goodwill and indefinite-lived intangible assets are not subject to amortization and, in accordance with ASC Topic 350, Intangibles – Goodwill and Other, we evaluate goodwill and indefinite lived intangible assets for impairment on an annual basis and whenever events or circumstances exist that indicate that the carrying value of goodwill or indefinite-lived intangible assets may no longer be recoverable.
The goodwill evaluation is based on a two-step process. The first step compares the fair value of a reporting unit with its carrying amount including goodwill. If the first step indicates that the fair value of the reporting unit is less than its carrying amount, the second step must be performed which determines the implied fair value of reporting unit goodwill. The measurement of possible impairment is based on the comparison of the implied fair value of reporting unit to its carrying value. We used a third party valuation specialist to assist in the annual impairment evaluation that was performed as of December 31, 2014.
43
As of December 31, 2014, goodwill of $573.3 million is included in our Consolidated Balance Sheets of which $560.6 million is related to the Infusion Services reporting unit and $12.7 million related to the PBM Services reporting unit. The goodwill of the Infusion Services reporting unit was recorded primarily as a result of the acquisition of CHS in 2010 and the CarePoint Business in 2013.
In performing an annual evaluation of goodwill, a reporting unit’s fair value is determined based on discounted future cash flows and a market-based comparison to industry peers. Significant estimates are used in the determination of fair value that include future forecasted earnings and the working capital requirements of the business to generate estimated cash flows. Our estimates could be materially impacted by factors such as competitive forces, changes in growth trends and specific industry conditions, with the potential for an adverse effect on financial condition and operating results of our reporting units that could potentially result in impairment of the goodwill. Also, the cash flow model used to determine fair value is sensitive to the discount rate used. We performed a sensitivity analysis on the discount rate and determined that an increase in the discount rate used by a factor of 50% would not impair the goodwill of each of our reporting units. Carrying value of each of the reporting units is determined based on the specific assets and liabilities of each reporting unit and allocations of corporate assets, liabilities and expenses. Each of our reporting units have fair values in excess of their carrying value at December 31, 2014.
Impairment of Long Lived Assets
We evaluate whether events and circumstances have occurred that indicate that the remaining estimated useful life of long-lived assets may warrant revision or that the remaining balance of an asset may not be recoverable in accordance with the provisions of ASC Topic 360, Property, Plant and Equipment. The measurement of possible impairment of property, plant and equipment is based on the ability to recover the balance of assets from expected future operating cash flows on an undiscounted basis. Impairment losses, if any, would be determined based on the present value of the cash flows using discount rates that reflect the inherent risk of the underlying business.
Accounting for Stock-Based Compensation
Compensation cost for all share-based payments are based on the grant-date fair value estimated in accordance with the provisions of ASC Topic 718, Compensation – Stock Compensation. The fair value of each option award is estimated on the date of grant using a binomial option-pricing model that uses the following assumptions: (i) expected volatility is based on the historical volatility of our stock, (ii) the risk-free interest rate for periods within the contractual life of the option is based on the U.S. Treasury yield curve in effect at the time of the grant, and (iii) the expected life of options granted is derived from previous history of stock exercises from the grant date and represents the period of time that options granted are expected to be outstanding. We use historical data to estimate option exercise and employee termination assumptions under the valuation model. The fair value of the award is amortized to expense on a straight line basis over the requisite service period. We expense restricted stock awards based on vesting requirements, including time elapsed, market conditions, and/or performance conditions. Because of these requirements, the weighted average period for which the expense is recognized varies. We expense stock appreciation right (“SAR”) awards based on vesting requirements. In addition, because they are settled with cash, the fair value of the SAR awards are revalued on a quarterly basis.
Off-Balance Sheet Arrangements
Variable Interest Entity
We previously had an affiliate equity investment in a variable interest entity that developed a platform that facilitates the flow, management and sharing of vital health and medical information with stakeholders across the healthcare system. On April 19, 2013, we, along with all other minority investors, completed the sale of our affiliate equity investment in this variable interest entity. At closing, we received cash payments from the sale of $8.5 million, with an additional $1.1 million held in escrow pending any working capital adjustments that may be necessary. As of December 31, 2014, we have received all amounts held in escrow. We also expect to receive additional services or cash from an existing guarantee during the two years following close. The terms of the services to be provided or the cash guarantee to be paid will be determined by us and the parties involved in the sale. As of December 31, 2014, a receivable of $2.4 million is included in prepaid expenses and other current assets in the accompanying Consolidated Balance Sheets.
Reclassifications
With the sale of the Home Health Services segment on March 31, 2014, all prior period financial statements have been reclassified to include the Home Health Services segment as discontinued operations. In addition, certain prior period amounts have been reclassified to conform to the current year presentation. Such reclassifications had no material effect on our previously reported Consolidated Financial Statements.
44
Results of Operations
The following discussion is based on our Consolidated Financial Statements. It compares our annual results of operations with the prior year results of operations.
Year ended December 31, 2014 compared to year ended December 31, 2013
Year Ended December 31, (in thousands) | |||||||||||||||
2014 | 2013 | Change | |||||||||||||
Revenue | $ | 984,055 | $ | 769,458 | $ | 214,597 | |||||||||
Gross profit | $ | 261,066 | 26.5 | % | $ | 243,613 | 31.7 | % | $ | 17,453 | |||||
Loss from operations | $ | (91,079 | ) | (9.3 | )% | $ | (10,372 | ) | (1.3 | )% | $ | (80,707 | ) | ||
Interest expense, net | $ | 38,539 | 3.9 | % | $ | 28,198 | 3.7 | % | $ | 10,341 | |||||
Loss from continuing operations, before income taxes | $ | (131,991 | ) | (13.4 | )% | $ | (54,468 | ) | (7.1 | )% | $ | (77,523 | ) | ||
Loss from continuing operations, net of income taxes | $ | (143,382 | ) | (14.6 | )% | $ | (56,991 | ) | (7.4 | )% | $ | (86,391 | ) | ||
Loss from discontinued operations, net of income taxes | $ | (4,086 | ) | (0.4 | )% | $ | (12,663 | ) | (1.6 | )% | $ | 8,577 | |||
Net loss | $ | (147,468 | ) | (15.0 | )% | $ | (69,654 | ) | (9.1 | )% | $ | (77,814 | ) | ||
Revenue. Revenue for the year ended December 31, 2014 was $984.1 million compared to revenue of $769.5 million for the year ended December 31, 2013.
Infusion Services segment revenue for the year ended December 31, 2014 was $922.7 million, compared to revenue of $696.9 million for the year ended December 31, in 2013, an increase of $225.8 million, or 32.4%. Product revenue increased $226.0 million or 33.4% during 2014 as a result of organic growth and due to a full year of revenue related to the operations of HomeChoice and the CarePoint Business that were acquired during 2013. Organic growth was approximately 18.3% during the year ended December 31, 2014.
PBM Services segment revenue for the year ended December 31, 2014 was $61.4 million compared to revenue of $72.6 million for the year ended December 31, in 2013, a decrease of $11.2 million, or 15.4%. This decrease results from a decrease of $28.3 million in discount cash card revenue partially offset by an increase in funded business of $17.0 million. The decline in discount cash card revenue results from the termination of a large contract effective December 31, 2013 and volume declines during 2014.
Cost of Revenue. Cost of revenue for the year ended December 31, 2014 was $723.0 million compared to $525.8 million for the year ended December 31, in 2013 or an increase of $197.1 million or 37.5%. The increase in cost of revenue primarily results from organic growth and the acquisitions in the Infusion Services Segment partially offset by declines in discount card volumes in the PBM Services segment.
Gross Profit. Gross profit for the year ended December 31, 2014 was $261.1 million compared to $243.6 million for the year ended December 31, in 2013, an increase of $17.5 million, or 7.2%. The increase in gross profit dollars was due to growth in Infusion Services segment partially offset by lower PBM Services gross profit. The increase in Infusion Services segment gross profit is due to organic growth and the inclusion of a full year of operations of HomeChoice and CarePoint acquired in 2013 partially offset by a $5.0 million increase in contractual reserve provisions over prior run rates due to the disruption that occurred related to acquisition integration, particularly in merged markets where facilities, work teams and information systems were consolidated. Gross profit as a percentage of revenue declined to 26.5% during the year ended December 31, 2014 as compared to 31.7% in the year ended December 31, 2013. The decline in gross profit as a percentage of revenue is due to the growth in revenue in the lower margin Infusion Services segment as a percentage of total revenue as compared to the higher margin PBM Services segment. In addition, gross profit in Infusion Services declined due the mix of revenue, particularly revenue related to chronic therapies which is lower margin relative to the rest of Infusion Services therapies.
Selling, General and Administrative Expenses. Selling, general and administrative expenses (“SG&A”) for the year ended December 31, 2014 were $239.8 million, or 24.4% of total revenue, compared to $209.6 million, or 27.2% of total revenue, for the year ended December 31, 2013. The increase in SG&A expense is due mainly to acquisitions and also due to higher expenses required to drive and manage organic growth. The decrease in SG&A as a percentage of revenue was due to operating leverage attained on Infusion segment growth and due to a reduction of the PBM Services segment cash card business which incurs high selling costs as a percentage of revenue.
45
Change in Fair Value of Contingent Consideration. The change in fair value of contingent consideration for the year ended December 31, 2014 was $7.4 million compared to $5.8 million for the year ended December 31, 2013. The adjustments recorded in 2014 resulted from decreases of the fair value of contingent consideration of $2.1 million related to the HomeChoice acquisition and $5.2 million related to the CarePoint acquisition that were remeasured in 2014 based on gross profit performance versus targets. The 2013 change in fair value of contingent consideration was primarily due to a $5.9 million adjustment related to the HomeChoice acquisition.
Bad Debt Expense. For the year ended December 31, 2014, bad debt expense was $79.6 million, or 8.1% of revenue, compared to $19.6 million, or 2.6% of revenue, for the year ended December 31, 2013. The increase in bad debt expense during 2014 is the result of approximately $55.4 million of incremental bad debt reserves that we recorded over prior year run rates in Infusion Services. These reserves were necessary due to the disruption that occurred related to acquisition integration, particularly in merged markets where facilities, work teams and information systems were consolidated. While we focused on acquisition integration requirements, billing and collections efforts and accounts receivable aging were negatively impacted. Despite efforts to quickly outsource to collection agency partners and internal hiring and training of billing and collection personnel, we have experienced more difficulty collecting aged balances than we originally estimated. Collection of billed revenues has now returned to historical Infusion Services segment levels experienced prior to the disruption related to acquisition integration.
Acquisition and Integration Expenses. During the years ended December 31, 2014 and 2013 we incurred acquisition and integration expenses of $17.9 million and $16.1 million, respectively. These costs include legal and financial advisory fees associated with the acquisitions; integration costs to convert to common policies, procedures and information systems; and costs related to branch consolidation and severance related to the acquisitions of HomeChoice and the CarePoint Business. In addition, the 2014 amount includes $5.4 million of revenue reserve adjustments to the allowance for doubtful accounts and allowance for contractual discounts related to acquired accounts receivable that are no longer deemed to be collectible.
Restructuring and Other Expenses. We incurred restructuring and other expenses of $15.6 million during the year ended December 31, 2014 compared to $7.7 million in 2013. During 2014 we incurred $11.2 million of costs resulting from the execution of our strategic assessment and related restructuring plans, consisting primarily of employee severance and other benefit-related costs, third-party consulting costs, facility-related costs and certain other costs compared to $3.4 million during 2013. During the year ended December 31, 2014, we also incurred $4.4 million of training and transition costs compared to $3.7 million during the year ended December 31, 2013. Training and transition costs include costs related to training, redundant salaries and wages, and retention bonuses for certain critical personnel. In addition, restructuring and other expenses for the year ended December 31, 2013 includes approximately $0.6 million related to our share in the loss of the unconsolidated affiliate equity investment that was sold in April 2013.
Amortization of Intangibles. During the year ended December 31, 2014, we recorded amortization of intangible assets of $6.6 million compared to $6.7 million for the prior year.
Interest Expense, Net. Net interest expense was $38.5 million for the year ended December 31, 2014, compared to $28.2 million for the year ended December 31, 2013. The increase in interest expense between years is the result of higher debt levels during 2014 as compared to 2013 that were partially offset by more favorable interest rates in 2014. Additionally, the 2014 interest expense includes amortization of $3.7 million of deferred financing costs as compared to amortization of $2.3 million during the prior year period.
Loss on Extinguishment of Debt. During 2014 we incurred a loss on the partial extinguishment of the Senior Credit Facilities of $2.4 million related to the write-off of deferred financing costs. During 2013 we incurred a loss on extinguishment of debt of $15.9 million due to the repurchase and redemption of the 2015 Notes in July 2013. This loss includes $12.2 million of premium paid to repurchase and redeem the 2015 Notes and $3.5 million related to the write-off of deferred financing costs.
Income Tax Expense (Benefit). Income tax expense for the year ended December 31, 2014 was $11.4 million on pre-tax net loss from continuing operations of $132.0 million. The 2014 income tax expense includes a federal tax benefit of $46.2 million offset by state tax benefit of $3.7 million at statutory tax rates offset by a $61.2 million adjustment related to deferred tax asset valuation allowances and other items of $0.1 million. The income tax expense of $2.5 million in 2013 includes a federal tax benefit of $18.1 million, state tax benefit of $2.5 million at statutory rates and other items of $0.7 million offset by a $23.8 million adjustment to deferred tax asset valuation allowances.
Net Loss and Loss Per Share from Continuing Operations. Net loss from continuing operations for the year ended year ended December 31, 2014 was $143.4 million, or $2.09 per basic and diluted share. Net loss from continuing operations was $57.0 million or $0.89 per basic and diluted share for the year ended December 31, 2014.
46
Income (Loss) from Discontinued Operations, Net of Income Taxes. The loss from discontinued operations for the year ended December 31, 2014 was $4.1 million compared to a loss of $12.7 million for the year ended December 31, 2013. The 2014 loss primarily results from legal fees and interest expense related to the settlement of the civil claims regarding the distribution of medication by our legacy specialty pharmacy division (See Note 10 - Commitments and Contingencies). These expenses were partially offset by income, net of income taxes, of $0.7 million related to the operations and sale of our Home Health Services segment. The 2013 loss is due to the accrual of a $15.0 million settlement in connection with the civil investigation offset by the recognition of a pre-tax gain of $6.5 million related to the favorable resolution of contingent consideration from the Pharmacy Services Asset Sale and income of $3.4 million related to the operations of the Home Health Services segment.
Year ended December 31, 2013 compared to year ended December 31, 2012
Year Ended December 31, (in thousands) | |||||||||||||||
2013 | 2012 | Change | |||||||||||||
Revenue | $ | 769,458 | $ | 593,447 | $ | 176,011 | |||||||||
Gross profit | $ | 243,613 | 31.7 | % | $ | 195,902 | 33.0 | % | $ | 47,711 | |||||
Income (loss) from operations | $ | (10,372 | ) | (1.3 | )% | $ | 6,676 | 1.1 | % | $ | (17,048 | ) | |||
Interest expense, net | $ | 28,198 | 3.7 | % | $ | 26,068 | 4.4 | % | $ | 2,130 | |||||
Income (loss) from continuing operations, before income taxes | $ | (54,468 | ) | (7.1 | )% | $ | (19,392 | ) | (3.3 | )% | $ | (35,076 | ) | ||
Loss from continuing operations, net of income taxes | $ | (56,991 | ) | (7.4 | )% | $ | (12,275 | ) | (2.1 | )% | $ | (44,716 | ) | ||
Income (loss) from discontinued operations, net of income taxes | $ | (12,663 | ) | (1.6 | )% | $ | 76,982 | 13.0 | % | $ | (89,645 | ) | |||
Net income (loss) | $ | (69,654 | ) | (9.1 | )% | $ | 64,707 | 10.9 | % | $ | (134,361 | ) | |||
Revenue. Revenue for the year ended December 31, 2013 was $769.5 million compared to revenue of $593.4 million for the year ended December 31, 2012.
Infusion Services segment revenue for the year ended December 31, 2013 was $696.9 million, compared to revenue of $481.6 million for the year ended December 31, 2012, an increase of $215.3 million, or 44.7%. Product revenue increased $204.2 million or 43.3% as a result of additional revenue related to the acquisitions of HomeChoice and the CarePoint Business during 2013 and due to organic growth. Infusion service revenue increased $11.1 million, or 110.1% as a result of increases in the volume of infusion nursing visits.
PBM Services segment revenue for the year ended December 31, 2013 was $72.6 million compared to revenue of $111.9 million for the year ended December 31, 2012, a decrease of $39.3 million, or 35.1%. This decrease results from $27.1 million related to the termination of a large but low margin client during the first quarter of 2013, declines in discount card revenue and pricing pressure related to a large discount pharmacy retailer.
Cost of Revenue. Cost of revenue for the year ended December 31, 2013 was $525.8 million compared to $397.5 million for the year ended December 31, 2012 or an increase of $128.3 million or 32.3%. The increase in cost of revenue primarily results from the acquisitions and organic growth in the Infusion Services Segment partially offset by declines in discount card volumes.
Gross Profit. Gross profit for the year ended December 31, 2013 was $243.6 million compared to $195.9 million for the year ended December 31, 2012, an increase of $47.7 million, or 24.4%. The increase in gross profit is due to the acquisitions of HomeChoice and CarePoint and organic growth. Gross profit as a percentage of revenue declined to 31.7% in the year ended December 31, 2013 as compared to 33.0% in the year ended December 31, 2012. The decline in gross profit as a percentage of revenue is due to the growth in revenue in the lower margin Infusion Services segment as a percentage of total revenue as compared to the higher margin PBM Services segment.
Selling, General and Administrative Expenses. Selling, general and administrative expenses (“SG&A”) for the year ended December 31, 2013 were $209.6 million, or 27.2% of total revenue, compared to $162.9 million, or 27.4% of total revenue, for the year ended December 31, 2012. The increase in SG&A was primarily related to support for the Infusion Segment growth
Change in Fair Value of Contingent Consideration. For the year ended December 31, 2013, the change in the fair value of contingent consideration was $5.8 million or 0.8% of total revenue. There was no change in the fair value of contingent consideration
47
for the year ended December 31, 2012. The amount recorded in 2013 was due to reevaluation of the probability of sellers of HomeChoice earning the contingent consideration based on gross profit performance versus targets. While the acquisition has generated expected revenues, the contingent consideration was an incentive for the sellers to partner with the business which would result in performance significantly over and the transaction valuation model. Based on performance in 2013 and the 2014 business plans for these branches, we reduced the probability of payout and the fair value of this liability.
Bad Debt Expense. For the year ended December 31, 2013, bad debt expense was $19.6 million, or 2.6% of revenue, compared to $13.2 million, or 2.2% of revenue, for the year ended December 31, 2012. Bad debt expense increased 48.7% between periods as a result of higher Infusion Services segment revenue and higher bad debt experience largely related to the aging of insurance claims during our acquisition integration process and due to patients’ ability to pay and continuing trends toward high-deductible plans.
Acquisition and Integration Expenses. During the year ended December 31, 2013 we incurred acquisition and integration expenses of $16.1 million associated with the acquisitions of the HomeChoice and the CarePoint Business. These costs include legal and financial advisory fees associated with the acquisitions; integration costs to convert to common policies, procedures and information systems; and costs related to branch consolidation and severance. In addition, a $2.3 million settlement was paid related to merger and acquisition activities during the year ended December 31, 2013. During the year ended December 31, 2012 we incurred acquisition and integration expenses of $4.0 million associated with the acquisitions of InfuScience and the preliminary stages of the HomeChoice acquisition that closed on February 1, 2013.
Restructuring and Other Expenses. We incurred restructuring and other expenses of $7.7 million during the year ended December 31, 2013 compared to $5.1 million in 2012. During 2013 we incurred $3.4 million of costs resulting from the execution of our strategic assessment and related restructuring plans, consisting primarily of employee severance and other benefit-related costs, third-party consulting costs, facility-related costs, and certain other costs compared to $2.2 million during 2012. During the year ended December 31, 2013, we also incurred $3.7 million of training and transition costs compared to $1.7 million during the year ended December 31, 2012. Training and transition costs include costs related to training, redundant salaries and wages, and retention bonuses for certain critical personnel.
Amortization of Intangibles. During the year ended December 31, 2013, we recorded amortization of intangible assets of $6.7 million compared to $4.0 million for the prior year. The increase in amortization is due to the intangible assets recognized from the acquisitions of the CarePoint Business and HomeChoice Partners.
Interest Expense, Net. Net interest expense was $28.2 million for the year ended December 31, 2013, compared to $26.1 million for the year ended December 31, 2012. Interest expense for the year ended year ended December 31, 2013 included $11.2 million related to the Senior Credit Facilities that were entered into on July 31, 2013 and $14.7 million related to our 2015 Notes that were redeemed during July 2013. Additionally, the 2013 interest expense includes amortization of $1.4 million of deferred financing costs related to the Senior Credit Facilities. Interest expense for the year ended December 31, 2012 included $23.1 million of interest expense related to our 2015 Notes and $2.7 million related to our Prior Credit Facility.
Loss on Extinguishment of Debt. During 2013 we incurred a loss on extinguishment of debt of $15.9 million due to the repurchase and redemption of the 2015 Notes in July 2013. This loss includes $12.2 million of premium paid to repurchase and redeem the 2015 Notes, $3.5 million related to the write-off of deferred financing costs and $0.2 million of other costs.
Income Tax Expense (Benefit). Income tax expense for the year ended December 31, 2013 was $2.5 million on pre-tax net loss from continuing operations of $54.5 million. The 2013 income tax expense includes a federal tax benefit of $18.1 million, a state tax benefit of $2.5 million at statutory tax rates and other items of $0.7 million offset by a $23.8 million adjustment related to deferred tax asset valuation allowances. The income tax benefit of $7.1 million in 2012 includes a federal tax benefit of $6.8 million, a state tax benefit of $0.9 million at statutory rates and other items of $0.5 million partially offset by a $1.1 million adjustment to deferred tax asset valuation allowances.
Net Loss and Loss Per Share from Continuing Operations. Net loss from continuing operations for the year ended year ended December 31, 2013 was $57.0 million, or $0.89 per basic and diluted share. Net loss from continuing operations was $12.3 million or $0.22 per basic and diluted share for the year ended December 31, 2012.
Net Income (Loss) from Discontinued Operations, Net of Income Taxes. The net loss from discontinued operations for the year ended December 31, 2013 was $12.7 million compared to net income of $77.0 million for the year ended December 31, 2012. The 2013 net loss includes the accrual of a $15.0 million legal settlement and other restructuring costs of $7.6 million, partially offset by a $6.5 million increase in the gain on sale primarily as a result of the favorable resolution of a contingent liability and $3.4 million of income, net of income taxes, related to the operations of the Home Health Services segment. Net income from
48
discontinued operations for the year ended December 31, 2012 was $77.0 million. This reflects a gain of $115.0 million before taxes from the Pharmacy Services Asset Sale offset by one-time charges of approximately $13.4 million as a result of the transaction, a net loss from the operations of the traditional and specialty pharmacy mail operations and community retail pharmacy stores for the period ended May 4, 2012 of $1.5 million, and additional costs of $18.7 million, including incremental bad debt expense of $9.6 million, associated with the subsequent resolution of retained receivables and working capital liabilities relating to the operations subject to the sale. The net income from discontinued operations for 2012 also includes $3.9 million related to the operations of our Home Health Segment.
Non-GAAP Measures
The following table reconciles Segment Adjusted EBITDA to Consolidated Adjusted EBITDA to GAAP net loss from continuing operations, net of income taxes. EBITDA is net (loss) income from continuing operations adjusted for net interest expense, loss on extinguishment of debt, income tax benefit (expense), depreciation, amortization and stock-based compensation expense. Adjusted EBITDA also excludes certain acquisition-related charges such as transaction costs and acquisition integration expenses; costs associated with restructuring such as employee severance, third party consulting costs and facility closure costs; training and transitional costs as well as redundant salaries; losses in the short-term investment in the unconsolidated affiliate; and investments in start-up branch locations.
Consolidated Adjusted EBITDA and Segment Adjusted EBITDA are measures of earnings that management monitors as an important indicator of financial performance, particularly future earnings potential and recurring cash flow. Adjusted EBITDA is also a primary objective of the management bonus plan.
Non-GAAP financial measures have limitations as analytical tools and should not be considered in isolation or as a substitute for our financial results prepared in accordance with GAAP. The Company encourages investors to review these reconciliations. We qualify our use of non-GAAP financial measures with cautionary statements as to their limitations.
Year Ended December 31, | |||||||||||
2014 | 2013 | 2012 | |||||||||
Results of Operations: | (in thousands) | ||||||||||
Adjusted EBITDA by Segment before corporate overhead: | |||||||||||
Infusion Services | $ | 6,501 | $ | 60,686 | $ | 35,421 | |||||
PBM Services | 6,731 | 17,094 | 25,644 | ||||||||
Total Segment Adjusted EBITDA | 13,232 | 77,780 | 61,065 | ||||||||
Corporate overhead | (36,264 | ) | (32,042 | ) | (26,755 | ) | |||||
Consolidated Adjusted EBITDA | (23,032 | ) | 45,738 | 34,310 | |||||||
Interest expense, net | (38,539 | ) | (28,198 | ) | (26,068 | ) | |||||
Loss on extinguishment of debt | (2,373 | ) | (15,898 | ) | — | ||||||
Income tax benefit (expense) | (11,391 | ) | (2,523 | ) | 7,117 | ||||||
Depreciation | (16,388 | ) | (13,381 | ) | (8,367 | ) | |||||
Amortization of intangibles | (6,555 | ) | (6,671 | ) | (3,957 | ) | |||||
Stock-based compensation expense | (8,570 | ) | (9,450 | ) | (6,122 | ) | |||||
Acquisition and integration expenses | (17,924 | ) | (16,130 | ) | (4,046 | ) | |||||
Restructuring and other expenses and investments | (18,610 | ) | (10,478 | ) | (5,142 | ) | |||||
Loss from continuing operations, net of taxes | $ | (143,382 | ) | $ | (56,991 | ) | $ | (12,275 | ) | ||
Infusion Services segment Adjusted EBITDA decreased during the year ended December 31, 2014 as a result of the incremental adjustments to the allowance for bad debts and contractual adjustment reserves of $60.4 million partially offset by the full year of operations from the 2013 acquisitions of HomeChoice Partners and CarePoint Business and organic growth.
PBM Services segment Adjusted EBITDA decreased during the year ended December 31, 2014 compared to 2013 compared to the prior year due to decreases in discount cash card revenue partially offset by an increase in funded business. The decline in discount cash card revenue results from the termination of a large contract effective December 31, 2013 and volume declines during 2014.
Non-GAAP Reconciliation -- Adjusted EPS. In an effort to provide better transparency into the operational results of the business and better comparability to other market participants, we have identified non-operating (non-GAAP) categories of earnings per
49
share (Non-GAAP Adjusted EPS) from continuing operations. Non-GAAP Adjusted EPS is a measure that excludes the effects of amortization of intangibles and stock-based compensation expense. Non-GAAP Adjusted EPS also excludes certain acquisition-related charges such as transaction costs and acquisition and integration expenses; costs associated with restructuring such as employee severance, third party consulting and facility closure costs, training and transitional costs as well as redundant salaries; losses in the short-term investment in the unconsolidated affiliate; and investments in start-up branch locations. We consider these costs to be outside the operational performance of the business.
The tables below provide a reconciliation of our net loss from continuing operations, net of income taxes, and basic and diluted loss per common share from continuing operations as reported under GAAP to its Adjusted EPS presentation, which is a non-GAAP measure. Our calculation of Non-GAAP Adjusted EPS, as presented, may differ from similarly titled measures reported by other companies.
Year Ended December 31, | ||||||||||||
2014(1) | 2013(2) | 2012(3) | ||||||||||
(in thousands, except per share amounts) | ||||||||||||
Loss from continuing operations, net of income taxes | $ | (143,382 | ) | $ | (56,991 | ) | $ | (12,275 | ) | |||
Non-GAAP adjustments, net of income taxes: | ||||||||||||
Restructuring and other expenses and investments (4) | 18,610 | 10,478 | 5,142 | |||||||||
Loss on extinguishment of debt | 2,373 | 15,894 | — | |||||||||
Acquisition and integration expenses | 17,924 | 16,126 | 4,046 | |||||||||
Amortization of intangibles | 6,555 | 6,669 | 3,957 | |||||||||
Stock-based compensation expense | 8,570 | 9,448 | 6,122 | |||||||||
Non-GAAP net income (loss) from continuing operations | $ | (89,350 | ) | $ | 1,624 | $ | 6,992 | |||||
Loss per share from continuing operations, basic and diluted | $ | (2.09 | ) | $ | (0.89 | ) | $ | (0.22 | ) | |||
Non-GAAP adjustments, net of income taxes: | ||||||||||||
Restructuring and other expenses and investments (4) | 0.27 | 0.16 | 0.09 | |||||||||
Loss on extinguishment of debt | 0.03 | 0.25 | — | |||||||||
Acquisition and integration expenses | 0.26 | 0.25 | 0.07 | |||||||||
Amortization of intangibles | 0.10 | 0.10 | 0.07 | |||||||||
Stock-based compensation expense | 0.13 | 0.15 | 0.11 | |||||||||
Non-GAAP earnings (loss) per share from continuing operations, basic and diluted | $ | (1.30 | ) | $ | 0.02 | 0.12 | ||||||
Weighted average shares outstanding, basic and diluted | 68,476 | 64,560 | 56,239 | |||||||||
(1) | For the year ended December 31, 2014 non-GAAP net loss from continuing operations adjustments are net of tax, calculated using an annual effective tax rate method. However, the Company has recorded a full valuation allowance on its deferred tax assets and, as a result, no tax benefit is being recognized for the non-GAAP net loss from continuing operations. The tax expense in continuing operations relates to indefinite-lived intangible assets and an insignificant amount of state tax expense which would not be impacted by the non-GAAP adjustments above. Accordingly, no tax expense has been allocated to the non-GAAP adjustments. |
(2) | For the year ended December 31, 2013, non-GAAP net income from continuing operations adjustments are net of tax, calculated using an annual effective tax rate method. The tax expense netted against restructuring and other expenses and investments, loss on extinguishment of debt, acquisition and integration expenses, amortization of intangibles, and stock-based compensation expense was $3, $4, $4, $2 and $2, respectively. The tax effect of these adjustments on a per share basis is not meaningful. |
(3) | For the year ended December 31, 2012, non-GAAP net income from continuing operations adjustments are net of tax, calculated using an annual effective tax rate method. However, the Company has recorded a full valuation allowance on its deferred tax assets and, as a result, no tax benefit is being recognized for the non-GAAP net loss from continuing operations. The tax expense in continuing operations relates to indefinite-lived intangible assets and an insignificant amount of state tax expense which would not be impacted by the non-GAAP adjustments above. Accordingly, no tax expense has been allocated to the non-GAAP adjustments. |
50
(4) | Restructuring and other expenses and investments include costs associated with restructuring such as employee severance, third party consulting costs and facility closure costs; training and transitional costs as well as redundant salaries; losses in the short-term investment in the unconsolidated affiliate; and investments in start-up branch locations. |
Liquidity and Capital Resources
Sources and Uses of Funds
We utilize funds generated from operations for general working capital needs, capital expenditures and acquisitions.
Net cash used by operating activities from continuing operations totaled $24.6 million during the year ended December 31, 2014 compared to net cash used of $46.0 million during the year ended December 31, 2013. This $24.6 million of cash used in operating activities from continuing operations during 2014 results from the loss from continuing operations net of income taxes of $143.4 million reduced by net non-cash expenses of $43.0 million and offset by the decrease in the net accounts receivable balance of $30.7 million and increases in current liability balances, primarily accounts payable of $26.0 million and other accruals of $16.6 million. Net cash used by operating activities during the prior period results from the loss from continuing operations net of income taxes of $57.0 million and increases in accounts receivable of $33.5 million partially offset by increases in accounts payable of $22.3 million.
Net cash used in investing activities from continuing operations during the year ended December 31, 2014 was $13.4 million compared to $302.3 million of cash used during the same period in 2013. The 2013 amount is primarily due to $283.0 million of cash used for the acquisitions of HomeChoice and the CarePoint Business. Capital expenditures were $13.8 million and $25.5 million for the years ended December 31, 2014 and 2013, respectively. In addition, the year ended December 31, 2014 includes $57.7 million of cash provided from investing activities from discontinued operations related to the net proceeds from the sale of our Home Health Business.
Net cash used by financing activities during the year ended December 31, 2014 was $13.1 million compared to cash provided of $295.8 million during the same period in 2013. The cash used during 2014 results from principal payments of $172.2 million related to our Senior Credit Facilities and a $35.0 million reduction of our revolving credit facility partially offset by the net proceeds of our 2021 notes of $194.5 million. The cash provided in 2013 includes net proceeds of $118.4 million related to our public stock offering and $378.1 million related to our Senior Credit Facilities offset by $237.4 million utilized to repurchase and redeem our 2015 Notes.
At December 31, 2014, we had working capital of $25.9 million compared to $51.9 million of net working capital at December 31, 2013. Working capital at December 31, 2013 excludes the current assets and current liabilities related to discontinued operations. The $26.0 million decrease in working capital results from the reduction of current assets of $33.4 million was partially offset by lower current liabilities of $7.4 million at December 31, 2014 compared to December 31, 2013. The reduction of current assets is primarily due to lower accounts receivable, net balance as a result of the increased accounts receivable and contractual allowance reserves recorded during 2014. Current liabilities were lower at December 31, 2014 as compared to 2013 primarily due to the reduction of the current maturities of long-term debt of $54.9 million that was partially offset by increases in accounts payable and other current liabilities. As of December 31, 2014, approximately $70.0 million of our Revolving Credit Facility was available for working capital needs.
Public Stock Offering
On April 24, 2013, we completed an underwritten primary public offering of 10,406,250 shares of our common stock at an offering price to the public of $12.00 per share, less underwriting discounts and commissions and other offering expenses payable by us. In addition, 3,968,750 shares of common stock were offered and sold by certain existing stockholders in an underwritten secondary offering completed on the same date and at the same offering price to the public, less underwriting discounts and commissions and other offering expenses payable by the selling stockholders.
We received net proceeds of $118.4 million after underwriting discounts, commissions and other offering expenses. We did not receive any proceeds from the sale of shares of common stock by the selling stockholders. We used $21.0 million and approximately $61.1 million of the net proceeds, respectively, (i) to repay outstanding borrowings under the Prior Credit Facility and (ii) to fund a portion of the CarePoint acquisition. We used the remaining net proceeds from the offering for general corporate purposes, which included, among other things, capital expenditures, repurchases of outstanding debt or equity securities, debt servicing requirements or redemption of our short-term or long-term borrowings, or for other working capital requirements.
51
Repurchase and Redemption of 2015 Notes
On June 3, 2013, we commenced an Offer to Purchase and Consent Solicitation (the “Offer”) to the holders of our outstanding 2015 Notes to purchase any and all of the 2015 Notes at $1,056.25 cash for each $1,000.00 of principal plus accrued but unpaid interest to the date of purchase. On July 31, 2013, we received and accepted for purchase approximately 56.1% of the aggregate principal amount of our outstanding 2015 Notes that were tendered by the Offer’s expiration date of July 30, 2013. The $133.3 million aggregate repurchase price plus accrued but unpaid interest of $4.3 million of the 2015 Notes tendered in connection with the Offer was paid from proceeds received under the Term Loan B (defined below).
In connection with the Offer, we solicited and received sufficient consents from the holders of the 2015 Notes to amend certain provisions of the indenture governing the 2015 Notes (the “2015 Notes Indenture”) that eliminated substantially all of the restrictive covenants, certain events of default and other provisions included in the 2015 Notes Indenture. On July 31, 2013, we entered into a supplemental indenture with the trustee for the 2015 Notes, giving effect to the proposed amendments to the 2015 Notes Indenture and eliminating substantially all of the restrictive covenants and certain default provisions contained in the 2015 Notes Indenture.
On July 31, 2013, we satisfied and discharged our obligations under the 2015 Notes Indenture by depositing with the trustee approximately $107.8 million (the “Discharge Amount”) from proceeds received under the Term Loan B Facility. From the Discharge Amount, the trustee paid all remaining outstanding 2015 Notes on August 19, 2013 at a redemption price equal to $1,051.25 cash for each $1,000.00 of the principal amount plus accrued and unpaid interest as of such date.
As a result of the above repurchase and redemption, all amounts under the 2015 Notes were fully satisfied and we incurred a loss on extinguishment of debt of $15.9 million during the year ended December 31, 2013.
Senior Credit Facilities
On July 31, 2013, we entered into (i) a senior secured first-lien revolving credit facility in an aggregate principal amount of $75.0 million (the “Revolving Credit Facility”), (ii) a senior secured first-lien term loan B in an aggregate principal amount of $250.0 million (the “Term Loan B Facility”) and (iii) a senior secured first-lien delayed draw term loan B in an aggregate principal amount of $150.0 million (the “Delayed Draw Term Loan Facility” and, together with the Revolving Credit Facility and the Term Loan B Facility, the “Senior Credit Facilities”) with SunTrust Bank, Jefferies Finance LLC and Morgan Stanley Senior Funding, Inc.., as lead arrangers, SunTrust Bank, as administrative agent, and a syndicate of lenders. On July 31, 2013, in connection with our entering into the Senior Credit Facilities, we also terminated the Prior Credit Facility.
Advances under the Senior Credit Facilities bear interest at a floating rate or rates equal to the Eurodollar rate plus 5.25% or the base rate plus 4.25% specified in the Senior Credit Facilities. As of December 31, 2014, the interest rates for the Term Loan B Facility and Delayed Draw Term Loan Facility (collectively, the “Term Loan Facilities”) are approximately 6.5% and the interest rate for the Revolving Credit Facility is approximately 7.50%. The interest rates may vary in the future depending on our consolidated net leverage ratio.
The Revolving Credit Facility matures on July 31, 2018 at which time all principal amounts outstanding are due and payable. The Term Facilities each mature on July 31, 2020 and require equal consecutive quarterly repayments of 1.25% of the original principal amount funded commencing on December 31, 2013. Once repaid, amounts under the Term Loan Facilities may not be re-borrowed. The Senior Credit Facilities are secured by substantially all of the Company’s and its subsidiaries’ assets.
The Senior Credit Facilities contain customary events of default that include, among others, non-payment of principal, interest or fees, violation of covenants, inaccuracy of representations and warranties, bankruptcy and insolvency events, material judgments, cross-defaults to material indebtedness, events constituting a change in control and any other development that results in, or would reasonably be expected to result in, a material adverse effect to the debtor’s ability to perform its obligation under the facility. The occurrence of certain events of default may increase the applicable rate of interest by 2% and could result in the acceleration of our obligations under the Senior Credit Facilities to pay the full amount of the obligations. If we draw down in excess of 25% of the available borrowing capacity under the Revolving Credit Facility, the net leverage covenants under the Revolving Credit Facility will become applicable such that our consolidated net leverage ratio will not be permitted to exceed certain thresholds until maturity of the Revolving Credit Facility. The required maximum consolidated net leverage ratio thresholds for the Revolving Credit Facility are defined for each measurement quarter. The Term Loan Facilities are not subject to any financial covenants.
The proceeds of the Term Loan B Facility were used to refinance certain of our existing indebtedness, including the payment of the purchase price for the 2015 Notes tendered and accepted for purchase in the Offer and the payment of the redemption price for the 2015 Notes that remained outstanding after completion of the Offer. The Delayed Draw Term Loan Facility and the
52
Revolving Credit Facility were used to fund a portion of the CarePoint Purchase Price and may be used for other general corporate purposes, including acquisitions, investments, capital expenditures and working capital needs.
On December 23, 2013, we entered into the First Amendment to the Senior Credit Facilities pursuant to which we obtained the required consent of the lenders to enter into the Settlement Agreements and to begin making payments, in accordance with the payment terms, on the settlement amount of $15.0 million. In exchange for this consent, we paid the lenders a fee of $0.5 million.
On January 31, 2014, we entered into the Second Amendment to the Senior Credit Facilities, which, among other things (i) provides additional flexibility with respect to compliance with the maximum net leverage ratio for the fiscal quarters ending December 31, 2013 through and including December 31, 2014, (ii) provides additional flexibility under the indebtedness covenants to permit up to $150.0 million of second-lien debt and up to $250.0 million of unsecured bonds, provided that all of the net proceeds are applied first to the Revolving Credit Facility, with no corresponding permanent commitment reduction, and then to the Term Loan B Facility, (iii) provides the requisite flexibility to sell non-core assets, subject to the satisfaction of certain conditions, provided that all of the net proceeds are applied first to the Revolving Credit Facility, with no corresponding permanent commitment reduction, and then to the Term Loan B Facility, and (iv) increased the applicable interest rates for the Term Loan Facilities to the Eurodollar rate plus 6.00% or the base rate plus 5.00%, until the occurrence of certain pricing decrease triggering events, as defined in the amendment. Upon the occurrence of a pricing decrease triggering event, the interest rates for the Senior Credit Facilities may revert to the Eurodollar rate plus 5.25% or the base rate plus 4.25%.
On March 1, 2015, we entered into the Third Amendment to the Senior Credit Facilities, which establishes an alternate leverage test for the fiscal quarters ending March 31, 2015 through and including March 31, 2016. The maximum net leverage ratio for these quarters is consistent with that in effect for the prior four fiscal quarters. The amendment eliminated the need to meet progressively lower leverage ratio requirements at each quarter end date for the next four quarters. The amendment also reduces the Revolver Covenant Triggering Event from 25% of the Aggregate Revolving Commitment Amount to 5% of the Aggregate Revolving Commitment Amount beginning with the quarter ended June 30, 2015 and provides for certain additional financial reporting.
As discussed below, the net proceeds of approximately $194.5 million from the issuance on February 11, 2014 of 8.875% senior notes due 2021 (the “2021 Notes”) were used to repay $59.3 million of the Revolving Credit Facility and $135.2 million of the Term Loan Facilities. In addition, approximately $54.2 million of the net proceeds from the sale of the Home Health Business were used to repay $17.2 million of the Revolving Credit Facility and $37.0 million of the Term Loan Facilities. These repayments were used to prepay the required quarterly principal repayments such that no principal repayments will be required for the Term Loan Facilities until their maturity on July 31, 2020. Once repaid, amounts under Term Loan Facilities may not be reborrowed. The Senior Credit Facilities are secured by substantially all of the Company’s and its subsidiaries’ assets.
The partial repayments of the Senior Credit Facilities as a result of the issuance of the 2021 Notes and from the sale of the Home Health Business were pricing decrease triggering events that resulted in the interest rates reverting to the Eurodollar rate plus 5.25% or the base rate plus 4.25%. The interest rates may vary in the future depending on our consolidated net leverage ratio.
At December 31, 2014, the Company had $70.0 million of unused capacity under its Revolving Credit Facility.
Issuance of 2021 Notes
On February 11, 2014 we issued $200.0 million in aggregate principal amount of 8.875% Senior Notes due 2021 (the “2021 Notes”). The 2021 Notes are senior unsecured obligations and are fully and unconditionally guaranteed by certain of our subsidiaries. We used approximately $194.5 million of the net proceeds of the offering to repay $59.3 million of the Revolving Credit Facility and $135.2 million related to the Term Loan Facilities. The 2021 Notes were issued under Rule 144A of the Securities Act and Regulation S.
The 2021 Notes have a fixed annual interest rate of 8.875%. The 2021 Notes rank equally to all of our and the guarantors’ other unsecured and unsubordinated indebtedness, but are effectively junior to all of our and the guarantors’ secured indebtedness, to the extent of the collateral securing such indebtedness. The 2021 Notes rank effectively junior to all liabilities of our future subsidiaries that do not guarantee the 2021 Notes. We may redeem the 2021 Notes in whole or in part on and after February 15, 2017. In addition, we may redeem up to 35% of the 2021 Notes before February 15, 2017 with the proceeds of certain equity offerings. We may also redeem all or part of the 2021 Notes before February 15, 2017, at a redemption price equal to 100% of the principal amount of the 2021 Notes redeemed, plus accrued and unpaid interest, to the date of redemption, plus a make-whole premium. If we sell certain assets or experience specific kinds of changes in control, we must offer to repurchase the 2021 Notes. On February 6, 2015, we and the guarantors filed a registration statement on Form S-4 pursuant to which we will offer to exchange the 2021 Notes for substantially similar notes that are registered under the Securities Act.
53
Income Taxes
At December 31, 2014, we had federal NOL carryforwards of approximately $174.9 million, of which $21.1 million is subject to an annual limitation, which will begin expiring in 2026 and later. Of our federal NOLs, $18.0 million are related to the exercise of non-qualified stock options and restricted stock grants and will be recorded in additional paid-in capital when realized. We have post-apportioned state NOL carryforwards of approximately $245.7 million, the majority of which will begin expiring in 2017 and later.
Future Cash Requirements
As of the filing of this report, we expect that cash generated from operating activities combined with available borrowings under our Revolving Credit Facility will be sufficient to fund our anticipated working capital, information technology systems investments, scheduled interest repayments and other cash needs for at least the next twelve months, based on historical levels.
Cash generated from operations turned positive in the second half of 2014 as we increased cash collections and reduced days sales outstanding (“DSO”). Our plan in 2015 is to continue to reduce DSO and tightly manage operating expenses. Under the terms of our credit facility, a springing covenant becomes applicable if our borrowings on the revolver are over 25% of availability on the last day of the quarter. The covenant is a consolidated first lien net leverage ratio which uses first lien debt net of cash divided by last twelve months Adjusted EBITDA as defined in the credit facility. Should DSO rise, or if we have other unforeseen needs for liquidity, or if we are unable to manage cash to ensure compliance with our debt covenants, we would pursue alternate financing arrangements to meet our working capital requirements. From time to time we may evaluate market conditions and financing options that would improve our current liquidity profile and enhance our financial flexibility. This may include, but is not limited to, opportunities to raise additional funds through the issuance of various forms of equity and/or debt securities or other instruments. However, there is no assurance that, if necessary, we would be able to raise capital to provide required liquidity.
We intend to continue exploring strategic alternatives anticipated to maximize shareholder value going forward. We may pursue joint venture arrangements, additional business acquisitions and other transactions designed to expand our business.
The following table sets forth our contractual obligations affecting future cash flows as of December 31, 2014 (in thousands):
Payments Due in Year Ending December 31, | |||||||||||||||||||||||||||
Contractual Obligations | Total | 2015 | 2016 | 2017 | 2018 | 2019 | 2020 and Beyond | ||||||||||||||||||||
Long-term debt (1) | $ | 618,974 | $ | 32,229 | $ | 32,229 | $ | 32,229 | $ | 32,229 | $ | 32,229 | $ | 457,829 | |||||||||||||
Operating lease obligations | 31,313 | 8,590 | 7,243 | 6,205 | 4,308 | 2,634 | 2,333 | ||||||||||||||||||||
Capital lease obligations (1) | 614 | 418 | 122 | 62 | 12 | — | — | ||||||||||||||||||||
Settlement agreement (2) | 12,557 | 6,181 | 6,376 | — | — | — | — | ||||||||||||||||||||
Purchase commitment (3) | 47,362 | 47,362 | — | — | — | — | — | ||||||||||||||||||||
Total | $ | 710,820 | $ | 94,780 | $ | 45,970 | $ | 38,496 | $ | 36,549 | $ | 34,863 | $ | 460,162 | |||||||||||||
(1) | Includes principal and estimated interest. |
(2) | Includes estimated interest. |
(3) | Commitment to purchase prescription drugs from drug manufacturers. |
Item 7A. | Quantitative and Qualitative Disclosures About Market Risk |
We are exposed to market risk from changes in interest rates related to our outstanding debt. At December 31, 2014, we had total debt of $423.8 million of which $227.8 million is related to the Senior Credit Facilities and is subject to floating interest rates. Advances under the Senior Credit Facilities bear interest at a floating rate or rates equal to the Eurodollar rate plus 5.25% or the base rate plus 4.25% specified in the Senior Credit Facilities. Interest rates for the Term Loan Facilities are subject to a 1.25% minimum to determine our interest rate. As of December 31, 2014, the Eurodollar rate is approximately 0.27% therefore, an increase in the current market rate of 1.00% would not impact our interest expense. Interest rates under the Revolving Credit Facility are not subject to a minimum rate, therefore, an increase in the current market of 1.00% would increase our interest expense by approximately $0.5 million annually based on the amount outstanding under the Revolving Credit Facility at December 31, 2014.
54
On February 11, 2014, we issued $200.0 million in aggregate principal amount of the 2021 Notes. The interest rate on the 2021 Notes, 8.875%, is fixed and not subject to market risk.
We regularly assess the significance of interest rate market risk as part of our treasury operations and as circumstances change and will enter into interest rate swaps as appropriate in accordance with the terms of the Senior Credit Facilities. We do not use financial instruments for trading or other speculative purposes and are not a party to any derivative financial instruments at this time.
At December 31, 2014, the carrying values of accounts receivable, accounts payable, claims payable, payables to plan sponsors and others approximate fair value due to their short-term nature. We believe the carrying value of our long-term debt under our Senior Credit Facilities, which is subject to variable interest rates, also approximates fair market value.
55
Item 8. | Financial Statements and Supplementary Data |
Report of Independent Registered Public Accounting Firm
The Board of Directors and Stockholders
BioScrip, Inc.:
We have audited the accompanying consolidated balance sheet of BioScrip, Inc. and subsidiaries as of December 31, 2014, and the related consolidated statements of operations, stockholders’ equity, and cash flows for the year ended December 31, 2014. Our audit also included the financial statement schedule listed in the Index at Item 15. These consolidated financial statements and schedule are the responsibility of the Company’s management. Our responsibility is to express an opinion on these consolidated financial statements and schedule based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audit provides a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of BioScrip, Inc. and subsidiaries as of December 31, 2014, and the results of their operations and their cash flows for the year ended December 31, 2014, in conformity with U.S. generally accepted accounting principles. Also, in our opinion, the related financial statement schedule, when considered in relation to the basic financial statements taken as a whole, presents fairly in all material respects the information set forth therein.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), BioScrip, Inc.’s internal control over financial reporting as of December 31, 2014, based on criteria established in Internal Control - Integrated Framework (1992) issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO), and our report dated March 2, 2015 expressed an adverse opinion on the effectiveness of the Company’s internal control over financial reporting.
/s/ KPMG LLP
Minneapolis, Minnesota
March 2, 2015
56
Report of Independent Registered Public Accounting Firm
The Board of Directors and Stockholders
BioScrip, Inc.
We have audited the accompanying consolidated balance sheet of BioScrip, Inc. and subsidiaries as of December 31, 2013, and the related consolidated statements of operations, stockholders' equity and cash flows for each of the two years in the period ended December 31, 2013. Our audits also included the financial statement schedule listed in the Index at Item 15. These financial statements and schedule are the responsibility of the Company's management. Our responsibility is to express an opinion on these financial statements and schedule based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the consolidated financial position of BioScrip, Inc. and subsidiaries at December 31, 2013, and the consolidated results of its operations and its cash flows for each of the two years in the period ended December 31, 2013, in conformity with U.S. generally accepted accounting principles. Also, in our opinion, the related financial statement schedule, when considered in relation to the basic financial statements taken as a whole, presents fairly in all material respects the information set forth therein.
/s/ Ernst & Young LLP
Minneapolis, Minnesota
March 3, 2014, except as to Note 5, as to which the date is March 2, 2015
57
BIOSCRIP, INC. AND SUBSIDIARIES
CONSOLIDATED BALANCE SHEETS
(in thousands, except for share amounts)
December 31, 2014 | December 31, 2013 | ||||||
ASSETS | |||||||
Current assets | |||||||
Cash and cash equivalents | $ | 740 | $ | 1,001 | |||
Receivables, less allowance for doubtful accounts of $66,500 and $17,836 at December 31, 2014 and December 31, 2013, respectively | 140,810 | 172,188 | |||||
Inventory | 37,215 | 34,341 | |||||
Prepaid expenses and other current assets | 9,450 | 14,110 | |||||
Current assets of discontinued operations | — | 15,316 | |||||
Total current assets | 188,215 | 236,956 | |||||
Property and equipment, net | 38,171 | 41,182 | |||||
Goodwill | 573,323 | 571,337 | |||||
Intangible assets, net | 10,269 | 16,824 | |||||
Deferred financing costs | 13,463 | 17,184 | |||||
Other non-current assets | 1,272 | 3,733 | |||||
Non-current assets of discontinued operations | — | 49,642 | |||||
Total assets | $ | 824,713 | $ | 936,858 | |||
LIABILITIES AND STOCKHOLDERS’ EQUITY | |||||||
Current liabilities | |||||||
Current portion of long-term debt | $ | 5,395 | $ | 60,257 | |||
Accounts payable | 90,032 | 63,575 | |||||
Claims payable | 8,162 | 2,547 | |||||
Amounts due to plan sponsors | 5,779 | 4,826 | |||||
Accrued interest | 6,853 | 2,173 | |||||
Accrued expenses and other current liabilities | 46,092 | 36,371 | |||||
Current liabilities of discontinued operations | — | 6,576 | |||||
Total current liabilities | 162,313 | 176,325 | |||||
Long-term debt, net of current portion | 418,408 | 375,322 | |||||
Deferred taxes | 19,058 | 6,935 | |||||
Other non-current liabilities | 8,129 | 17,540 | |||||
Non-current liabilities of discontinued operations | — | 6,153 | |||||
Total liabilities | 607,908 | 582,275 | |||||
Stockholders’ equity | |||||||
Preferred stock, $.0001 par value; 5,000,000 shares authorized; no shares issued or outstanding | — | — | |||||
Common stock, $.0001 par value; 125,000,000 shares authorized; 71,274,064 and 70,711,439 shares issued and 68,636,965 and 68,128,919 shares outstanding as of December 31, 2014 and 2013, respectively | 8 | 7 | |||||
Treasury stock, 2,637,099 and 2,582,520 shares, at cost, as of December 31, 2014 and 2013 | (10,679 | ) | (10,311 | ) | |||
Additional paid-in capital | 529,682 | 519,625 | |||||
Accumulated deficit | (302,206 | ) | (154,738 | ) | |||
Total stockholders’ equity | 216,805 | 354,583 | |||||
Total liabilities and stockholders’ equity | $ | 824,713 | $ | 936,858 | |||
See accompanying Notes to the Consolidated Financial Statements.
58
BIOSCRIP, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF OPERATIONS
(in thousands, except per share amounts)
Years Ended December 31, | |||||||||||
2014 | 2013 | 2012 | |||||||||
Product revenue | $ | 901,653 | $ | 675,684 | $ | 471,506 | |||||
Service revenue | 82,402 | 93,774 | 121,941 | ||||||||
Total revenue | 984,055 | 769,458 | 593,447 | ||||||||
Cost of product revenue | 645,419 | 466,155 | 325,271 | ||||||||
Cost of service revenue | 77,570 | 59,690 | 72,274 | ||||||||
Total cost of revenue | 722,989 | 525,845 | 397,545 | ||||||||
Gross profit | 261,066 | 243,613 | 195,902 | ||||||||
Selling, general and administrative expenses | 239,810 | 209,627 | 162,879 | ||||||||
Change in fair value of contingent consideration | (7,364 | ) | (5,786 | ) | — | ||||||
Bad debt expense | 79,574 | 19,625 | 13,201 | ||||||||
Acquisition and integration expenses | 17,924 | 16,130 | 4,046 | ||||||||
Restructuring and other expenses | 15,646 | 7,718 | 5,143 | ||||||||
Amortization of intangibles | 6,555 | 6,671 | 3,957 | ||||||||
Income (loss) from operations | (91,079 | ) | (10,372 | ) | 6,676 | ||||||
Interest expense, net | 38,539 | 28,198 | 26,068 | ||||||||
Loss on extinguishment of debt | 2,373 | 15,898 | — | ||||||||
Loss from continuing operations, before income taxes | (131,991 | ) | (54,468 | ) | (19,392 | ) | |||||
Income tax provision (benefit) | 11,391 | 2,523 | (7,117 | ) | |||||||
Loss from continuing operations, net of income taxes | (143,382 | ) | (56,991 | ) | (12,275 | ) | |||||
Income (loss) from discontinued operations, net of income taxes | (4,086 | ) | (12,663 | ) | 76,982 | ||||||
Net income (loss) | $ | (147,468 | ) | $ | (69,654 | ) | $ | 64,707 | |||
Income (loss) per common share: | |||||||||||
Loss from continuing operations, basic and diluted | $ | (2.09 | ) | $ | (0.89 | ) | $ | (0.22 | ) | ||
Income (loss) from discontinued operations, basic and diluted | (0.06 | ) | (0.19 | ) | 1.37 | ||||||
Income (loss), basic and diluted | $ | (2.15 | ) | $ | (1.08 | ) | $ | 1.15 | |||
Weighted average common shares outstanding, basic and diluted | 68,476 | 64,560 | 56,239 | ||||||||
See accompanying Notes to the Consolidated Financial Statements.
59
BIOSCRIP, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY
(in thousands)
Common Stock | Treasury Stock | Additional Paid-in Capital | Accumulated Deficit | Total Stockholders' Equity | |||||||||||||||
Balance at December 31, 2011 | $ | 6 | $ | (10,461 | ) | $ | 375,525 | $ | (149,791 | ) | $ | 215,279 | |||||||
Exercise of employee stock compensation plans | — | — | 8,611 | — | 8,611 | ||||||||||||||
Surrender of stock to satisfy minimum tax withholding | — | (174 | ) | — | — | (174 | ) | ||||||||||||
Issuance of treasury stock for restricted stock vesting | — | 324 | (324 | ) | — | — | |||||||||||||
Compensation under employee stock compensation plan | — | — | 4,986 | — | 4,986 | ||||||||||||||
Net income | — | — | — | 64,707 | 64,707 | ||||||||||||||
Balance at December 31, 2012 | 6 | (10,311 | ) | 388,798 | (85,084 | ) | 293,409 | ||||||||||||
Net proceeds of public stock offering | 1 | — | 118,381 | — | 118,382 | ||||||||||||||
Exercise of stock options | — | — | 2,549 | — | 2,549 | ||||||||||||||
Compensation under employee stock compensation plan | — | — | 9,498 | — | 9,498 | ||||||||||||||
Exercise of warrants | — | — | 399 | — | 399 | ||||||||||||||
Net loss | — | — | — | (69,654 | ) | (69,654 | ) | ||||||||||||
Balance at December 31, 2013 | 7 | (10,311 | ) | 519,625 | (154,738 | ) | 354,583 | ||||||||||||
Exercise of stock options | 1 | — | 1,467 | — | 1,468 | ||||||||||||||
Surrender of stock to satisfy minimum tax withholding | — | (368 | ) | — | — | (368 | ) | ||||||||||||
Compensation under employee stock compensation plan | — | — | 8,590 | — | 8,590 | ||||||||||||||
Net loss | — | — | — | (147,468 | ) | (147,468 | ) | ||||||||||||
Balance at December 31, 2014 | $ | 8 | $ | (10,679 | ) | $ | 529,682 | $ | (302,206 | ) | $ | 216,805 | |||||||
See accompanying Notes to the Consolidated Financial Statements.
60
BIOSCRIP, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CASH FLOWS
(in thousands)
Years Ended December 31, | |||||||||||
2014 | 2013 | 2012 | |||||||||
Cash flows from operating activities: | |||||||||||
Net income (loss) | $ | (147,468 | ) | $ | (69,654 | ) | $ | 64,707 | |||
Less: Income (loss) from discontinued operations, net of income taxes | (4,086 | ) | (12,663 | ) | 76,982 | ||||||
Loss from continuing operations, net of income taxes | (143,382 | ) | (56,991 | ) | (12,275 | ) | |||||
Adjustments to reconcile net loss from continuing operations to net cash provided by (used in) operating activities: | |||||||||||
Depreciation | 16,388 | 13,381 | 8,367 | ||||||||
Amortization of intangibles | 6,555 | 6,671 | 3,957 | ||||||||
Amortization of deferred financing costs and debt discount | 4,153 | 2,259 | 1,261 | ||||||||
Change in fair value of contingent consideration | (7,364 | ) | (5,786 | ) | — | ||||||
Change in deferred income tax | 12,318 | 4,801 | (32 | ) | |||||||
Compensation under stock-based compensation plans | 8,570 | 9,450 | 6,122 | ||||||||
Loss on disposal of fixed assets | — | — | 156 | ||||||||
Loss on extinguishment of debt | 2,373 | 15,898 | — | ||||||||
Equity in earnings of unconsolidated affiliate | — | 675 | — | ||||||||
Changes in assets and liabilities, net of acquired businesses: | |||||||||||
Receivables, net of bad debt expense | 30,650 | (33,511 | ) | 103,937 | |||||||
Inventory | (2,952 | ) | 4,939 | (15,249 | ) | ||||||
Prepaid expenses and other assets | 5,464 | (456 | ) | 3,805 | |||||||
Accounts payable | 26,021 | 22,260 | (48,369 | ) | |||||||
Claims payable | 5,614 | (4,864 | ) | (4,354 | ) | ||||||
Amounts due to plan sponsors | 953 | (13,105 | ) | (7,025 | ) | ||||||
Accrued interest | 4,681 | (3,627 | ) | (22 | ) | ||||||
Accrued expenses and other liabilities | 5,313 | (8,005 | ) | 9,062 | |||||||
Net cash provided by (used in) operating activities from continuing operations | (24,645 | ) | (46,011 | ) | 49,341 | ||||||
Net cash provided by (used in) operating activities from discontinued operations | (6,771 | ) | (8,542 | ) | (22,457 | ) | |||||
Net cash provided by (used in) operating activities | (31,416 | ) | (54,553 | ) | 26,884 | ||||||
Cash flows from investing activities: | |||||||||||
Purchases of property and equipment, net | (13,829 | ) | (25,525 | ) | (10,658 | ) | |||||
Cash consideration paid for acquisitions, net of cash acquired | (454 | ) | (282,998 | ) | (43,046 | ) | |||||
Net cash proceeds from sale of unconsolidated affiliate | 852 | 8,617 | — | ||||||||
Cash advances to unconsolidated affiliate | — | (2,363 | ) | — | |||||||
Cash consideration paid to DS Pharmacy | — | — | (2,935 | ) | |||||||
Cash consideration paid for unconsolidated affiliate, net of cash acquired | — | — | (10,652 | ) | |||||||
Net cash used in investing activities from continuing operations | (13,431 | ) | (302,269 | ) | (67,291 | ) | |||||
Net cash provided by (used in) investing activities from discontinued operations | 57,688 | (101 | ) | 161,171 | |||||||
Net cash provided by (used in) investing activities | 44,257 | (302,370 | ) | 93,880 | |||||||
Cash flows from financing activities: | |||||||||||
Proceeds from public stock offering | — | 118,382 | — | ||||||||
Proceeds from senior notes due 2021, net of discount, lenders' fees and other expenses | 194,539 | — | — | ||||||||
Proceeds from senior credit facilities, net of fees paid to issuers | — | 378,091 | — | ||||||||
Repayment of 10 1/4% senior unsecured notes | — | (237,397 | ) | — | |||||||
Deferred and other financing costs | (1,135 | ) | — | — | |||||||
Borrowings on revolving credit facility | 244,700 | 449,559 | 1,244,050 | ||||||||
Repayments on revolving credit facility | (279,703 | ) | (409,559 | ) | (1,307,872 | ) | |||||
Principal payments of long-term debt | (172,243 | ) | (5,000 | ) | — | ||||||
Repayments of capital leases | (360 | ) | (802 | ) | (3,278 | ) | |||||
Net proceeds from exercise of employee stock compensation plans | 1,468 | 2,549 | 8,611 | ||||||||
Surrender of stock to satisfy minimum tax withholding | (368 | ) | — | (174 | ) | ||||||
Net cash provided by (used in) financing activities | (13,102 | ) | 295,823 | (58,663 | ) | ||||||
Net change in cash and cash equivalents | (261 | ) | (61,100 | ) | 62,101 | ||||||
Cash and cash equivalents - beginning of period | 1,001 | 62,101 | — | ||||||||
Cash and cash equivalents - end of period | $ | 740 | $ | 1,001 | $ | 62,101 | |||||
DISCLOSURE OF CASH FLOW INFORMATION: | |||||||||||
Cash paid during the period for interest | $ | 34,133 | $ | 25,589 | $ | 27,528 | |||||
Cash paid during the period for income taxes, net of refunds | $ | 1,651 | $ | 3,137 | $ | 1,042 | |||||
DISCLOSURE OF NON-CASH TRANSACTIONS: | |||||||||||
Capital lease obligations incurred to acquire property and equipment | $ | 107 | $ | 20 | $ | 6,631 | |||||
See accompanying Notes to the Consolidated Financial Statements.
61
BIOSCRIP, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 1-- NATURE OF BUSINESS
Corporate Organization and Business
BioScrip, Inc. and subsidiaries (the “Company” or “BioScrip”) is a national provider of infusion and home care management solutions that partners with physicians, hospital systems, skilled nursing facilities, healthcare payors and pharmaceutical manufacturers to provide patients access to post-acute care services. The Company operates with a commitment to bring customer-focused pharmacy and related healthcare infusion therapy services into the home or alternate-site setting. By collaborating with the full spectrum of healthcare professionals and the patient, the Company aims to provide cost-effective care that is driven by clinical excellence, customer service and values that promote positive outcomes and an enhanced quality of life for those whom we serve.
The Company’s platform provides nationwide service capabilities and the ability to deliver clinical management services that offer patients a high-touch, community-based and home-based care environment. The Company’s core services are provided in coordination with, and under the direction of, the patient’s physician. The Company's multidisciplinary team of clinicians, including pharmacists, nurses, dieticians and respiratory therapists, work with the physician to develop a plan of care suited to the patient’s specific needs. Whether in the home, physician office, ambulatory infusion center, skilled nursing facility or other alternate sites of care, the Company provides products, services and condition-specific clinical management programs tailored to improve the care of individuals with complex health conditions such as gastrointestinal abnormalities, infectious diseases, cancer, multiple sclerosis, organ and blood cell transplants, bleeding disorders, immune deficiencies and heart failure.
On March 31, 2014, the Company completed the sale of substantially all of its Home Health Services segment to LHC Group, Inc. (see Note 5 - Discontinued Operations). As a result of the sale of the Home Health Services segment, the Company has two operating and reportable segments, “Infusion Services” and “PBM Services”. These operating and reportable segments reflect how the Company's chief operating decision maker reviews the Company’s results in terms of allocating resources and assessing performance.
Basis of Presentation
The Company’s Consolidated Financial Statements have been prepared in accordance with U.S. generally accepted accounting principles (“GAAP”).
Reclassifications
With the sale of the Home Health Services segment on March 31, 2014, all prior period financial statements have been reclassified to include the Home Health Services segment as discontinued operations. In addition, certain prior period amounts have been reclassified to conform to the current year presentation. Such reclassifications had no material effect on our previously reported Consolidated Financial Statements.
NOTE 2-- SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Consolidation
The Consolidated Financial Statements include the accounts of the Company and its wholly-owned subsidiaries. All significant intercompany accounts and transactions have been eliminated in consolidation.
Use of Estimates
The preparation of financial statements in conformity with GAAP requires management to make certain estimates and assumptions. These estimates and assumptions affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenue and expenses during the reporting period. Actual results could differ from those estimates.
62
Fair Value Measurements
The fair value measurement accounting standard, ASC Topic 820, Fair Value Measurement (“ASC 820”), provides a framework for measuring fair value and defines fair value as the price that would be received to sell an asset or paid to transfer a liability. Fair value is a market-based measurement that should be determined using assumptions that market participants would use in pricing an asset or liability. The standard establishes a valuation hierarchy for inputs used in measuring fair value that maximizes the use of observable inputs and minimizes the use of unobservable inputs by requiring that the most observable inputs be used when available. Observable inputs are inputs market participants would use in valuing the asset or liability developed based on independent market data sources. Unobservable inputs are inputs that reflect the Company’s assumptions about the factors market participants would use in valuing the asset or liability developed based upon the best information available.
The valuation hierarchy is composed of three categories. The categorization within the valuation hierarchy is based on the lowest level of input that is significant to the fair value measurement. The categories within the valuation hierarchy are described as follows:
• | Level 1 - Inputs to the fair value measurement are quoted prices in active markets for identical assets or liabilities. |
• | Level 2 - Inputs to the fair value measurement include quoted prices in active markets for similar assets or liabilities, quoted prices for identical or similar assets or liabilities in markets that are not active, and inputs other than quoted prices that are observable for the asset or liability, either directly or indirectly. |
• | Level 3 - Inputs to the fair value measurement are unobservable inputs or valuation techniques. |
Cash and Cash Equivalents
Highly liquid investments with a maturity of three months or less when purchased are classified as cash equivalents.
Receivables
Receivables include amounts due from government sources, such as Medicare and Medicaid programs, PBMs, Managed Care Organizations and other commercial insurance (“Plan Sponsors”); amounts due from patient co-payments; amounts due from pharmaceutical manufacturers for rebates; and service fees resulting from the distribution of certain drugs through retail pharmacies.
Allowance for Doubtful Accounts
The allowance for doubtful accounts is based on estimates of losses related to receivable balances. The risk of collection varies based upon the product, the payor (commercial health insurance and government) and the patient’s ability to pay the amounts not reimbursed by the payor. We estimate the allowance for doubtful accounts based on several factors including the age of the outstanding receivables, the historical experience of collections, adjusting for current economic conditions and, in some cases, evaluating specific customer accounts for the ability to pay. Collection agencies are employed and legal action is taken when we determine that taking collection actions is reasonable relative to the probability of receiving payment on amounts owed. Management judgment is used to assess the collectability of accounts and the ability of our customers to pay. Judgment is also used to assess trends in collections and the effects of systems and business process changes on our expected collection rates. The Company reviews the estimation process quarterly and makes changes to the estimates as necessary. When it is determined that a customer account is uncollectible, that balance is written off against the existing allowance.
Change in Estimate of the Collectability of Accounts Receivable
During the year ended December 31, 2014, the Company experienced deterioration in the aging of certain accounts receivable primarily due to delays and disruptions related to the integration of its acquisitions in 2013. The disruption to billing and collection processes was attributable in part to the following:
• | Re-licensure and new managed care credentialing was required in connection with the CarePoint Business; |
• | Medicare claims were not filed until retraining and review of eligibility was performed; |
• | Merged facilities and work teams in seven large markets and related employee turnover; |
• | Conversion to a single version of our dispensing and billing system while still managing accounts receivable run-off on five other legacy versions; and |
• | Cash posting challenges that delayed secondary and patient billings and patient statement issuance. |
The Company outsourced collections to third party agency partners and hired and trained billing and collection personnel to mitigate the effects of the disruption; however, the Company has experienced more difficulty collecting the aged balances than it
63
originally estimated. While the Company provided incremental allowances during each of the first two quarters of 2014 to address the developing deterioration, during the third quarter, the Company materially changed its estimates based on actual collection experience during and after the acquisition disruption period. The Company also recorded adjustments to reserves in the fourth quarter of approximately $31.7 million consisting of $32.3 million to its allowance for bad debts partially offset by a favorable adjustment of $0.6 million to its contractual adjustment reserves due to the deterioration of the Infusion Services segment accounts receivable aging. The reserves as of December 31, 2014 represent a 100% reserve on all amounts over one year and 80% of receivables outstanding in the nine to twelve month age category. For the year ended December 31, 2014, the incremental adjustments are approximately $60.4 million consisting of $55.4 million to the allowance for doubtful accounts and $5.0 million of contractual adjustment reserves.
The following table sets forth the aging of our net accounts receivable (net of allowance for contractual adjustments and prior to allowance for doubtful accounts), aged based on date of service and categorized based on the three primary overall types of accounts receivable characteristics (in thousands):
December 31, 2014 | December 31, 2013 | |||||||||||||||||
0 - 180 days | Over 180 days | Total | 0 - 180 days | Over 180 days | Total | |||||||||||||
Government | 25,812 | 13,036 | 38,848 | 27,622 | 7,864 | 35,486 | ||||||||||||
Commercial | 117,699 | 35,302 | 153,001 | 122,661 | 26,975 | 149,636 | ||||||||||||
Patient | 4,899 | 10,562 | 15,461 | 2,792 | 2,110 | 4,902 | ||||||||||||
Gross accounts receivable | 148,410 | 58,900 | 207,310 | 153,075 | 36,949 | 190,024 | ||||||||||||
Allowance for doubtful accounts | (66,500 | ) | (17,836 | ) | ||||||||||||||
Net accounts receivable | 140,810 | 172,188 | ||||||||||||||||
Allowance for Contractual Discounts
The Company is reimbursed by payors for products and services the Company provides. Payments for medications and services covered by payors average less than billed charges. The Company monitors revenue and receivables from payors for each of our branches and records an estimated contractual allowance for certain revenue and receivable balances as of the revenue recognition date to properly account for anticipated differences between amounts estimated in our billing system and amounts reimbursed. Accordingly, the total revenue and receivables reported in our financial statements are recorded at the amounts expected to be received from these payors. For the significant portion of the Company’s revenue, the contractual allowance is estimated based on several criteria, including unbilled claims, historical trends based on actual claims paid, current contract and reimbursement terms and changes in customer base and payor/product mix. Contractual allowance estimates are adjusted to actual amounts as cash is received and claims are settled. We do not believe these changes in estimates are material. The billing functions for the remaining portion of the Company’s revenue are largely computerized, which enables on-line adjudication (i.e., submitting charges to third-party payors electronically with simultaneous feedback of the amount the primary insurance plan expects to pay) at the time of sale to record net revenue, exposure to estimating contractual allowance adjustments is limited on this portion of the business.
Inventory
Inventory is recorded at the lower of cost or market. Cost is determined using the first-in, first-out method. Inventory consists principally of purchased prescription drugs and related supplies. Included in inventory is a reserve for inventory waste and obsolescence.
Property and Equipment
Property and equipment is stated at cost less accumulated depreciation. Depreciation is calculated using the straight-line method over the estimated useful lives of assets as follows:
Asset | Useful Life | |||||
Computer hardware and software | 3 | years | - | 5 | years | |
Office equipment | 5 | years | ||||
Vehicles | 4 | years | - | 5 | years | |
Medical equipment | 13 | months | - | 5 | years | |
Furniture and fixtures | 5 | years | ||||
64
Leasehold improvements and assets leased under capital leases are depreciated using a straight-line basis over the related lease term or estimated useful life of the assets, whichever is less. The cost and related accumulated depreciation of assets sold or retired are removed from the accounts with the gain or loss, if applicable, recorded in the statement of operations. Maintenance and repair costs are expensed as incurred.
Costs relating to the development of software for internal purposes are charged to expense until technological feasibility is established in accordance with FASB ASC Topic 350, Intangibles – Goodwill and Other (“ASC 350”). Thereafter, the remaining software production costs up to the date placed into production are capitalized and included in Property and Equipment. Costs of customization and implementation of computer software purchased for internal use are likewise capitalized. Depreciation of the capitalized amounts commences on the date the asset is ready for its intended use and is calculated using the straight-line method over the estimated useful life of the software.
Goodwill
Goodwill is not subject to amortization and is tested for impairment annually and whenever events or circumstances exist that indicate that the carrying value of goodwill may no longer be recoverable in accordance with ASC 350. Impairment testing is performed for each of our operating and reporting segments. The impairment testing is based on a two-step process. The first step compares the fair value of a reporting segment to its carrying amount including goodwill. If the first step indicates that the fair value of the reporting unit is less than its carrying amount, the second step must be performed to determine the implied fair value of reporting unit goodwill. The measurement of possible impairment is based on the comparison of the implied fair value of reporting unit goodwill to its carrying value.
Intangible Assets
The Company evaluates the useful lives of its intangible assets to determine if they are finite or indefinite-lived. Finite-lived intangible assets, primarily acquired customer relationships, trademarks and non-compete agreements, are amortized on a straight-line basis over their estimated useful lives.
Impairment of Long Lived Assets
The Company evaluates whether events and circumstances have occurred that indicate the remaining estimated useful life of long lived assets, including intangible assets, may warrant revision or that the remaining balance of an asset may not be recoverable. The measurement of possible impairment is based on the ability to recover the balance of assets from expected future operating cash flows on an undiscounted basis. Impairment losses, if any, are determined based on the fair value of the asset, calculated as the present value of related cash flows using discount rates that reflect the inherent risk of the underlying business.
Variable Interest Entity
The Company previously had an affiliate equity investment in a variable interest entity that developed a platform that facilitates the flow, management and sharing of vital health and medical information with stakeholders across the healthcare ecosystem. On April 19, 2013, the Company, along with all other minority investors, completed the sale of its affiliate equity investment in this variable interest entity. At closing, the Company received cash payments from the sale of $8.5 million, with an additional $1.1 million held in escrow pending any working capital adjustments that may be necessary. As of December 31, 2014, all amounts held in escrow have been received by the Company. The Company also expects to receive additional services or cash from an existing guarantee during the two years following close. The terms of the services to be provided or the cash guarantee to be paid will be determined by the Company and the parties involved in the sale. As of December 31, 2014, a receivable of $2.4 million is included in prepaid expenses and other current assets in the accompanying Consolidated Balance Sheets.
Amounts due to Plan Sponsors
Amounts due to Plan Sponsors primarily represent payments received from Plan Sponsors in excess of the contractually required reimbursement. These amounts are refunded to Plan Sponsors. These payables also include the sharing of manufacturers’ rebates with Plan Sponsors.
Contingent Consideration
Liabilities that may be owed to sellers after the closing of an acquisition transaction are recorded at fair value as of the opening balance sheet established for the acquired target. These contingent consideration provisions are frequently referred to as earnouts
65
and are the subject of negotiation between the seller and the buyer. An earnout provision can compensate the seller with the value they believe the asset will deliver while also providing downside risk protection to the buyer should projections not materialize. As such, the terms of potential earnouts vary with each transaction. Fair value is assigned using multiple payout scenarios which each have a probability assigned based on factors including actual performance, evidence of business plans that have been implemented, and current market conditions that influence the ability to achieve the earnout. The probable payout amount is discounted to the current balance sheet date using a risk free rate. Each quarter, the fair value of the contingent consideration is updated to reflect relevant factors such as post-closing operating results and future forecasts for the acquired business or entity. The fair value of contingent consideration may be included in current liabilities or other non-current liabilities depending on the payment date specified in the purchase agreement.
Revenue Recognition
The Company generates revenue principally through the provision of home infusion and other home healthcare services to provide clinical management services and the delivery of cost effective prescription medications. Prescription drugs are dispensed through pharmacies owned by the Company. Prescription drugs are dispensed either through a pharmacy participating in the Company’s pharmacy network or a pharmacy owned by the Company. Fee-for-service agreements include: (i) pharmacy agreements, where we dispense prescription medications through the Company’s pharmacy facilities and (ii) PBM agreements, where prescription medications are dispensed through pharmacies participating in the Company’s retail pharmacy network.
FASB ASC Subtopic 605-25, Revenue Recognition: Multiple-Element Arrangements (“ASC 605-25”), addresses situations in which there are multiple deliverables under one revenue arrangement with a customer and provides guidance in determining whether multiple deliverables should be recognized separately or in combination. The Company provides a variety of therapies to patients. For infusion-related therapies, the Company frequently provides multiple deliverables of drugs and related nursing services. After applying the criteria from ASC 605-25, the Company concluded that separate units of accounting exist in revenue arrangements with multiple deliverables. Drug revenue is recognized at the time the drug is shipped, and nursing revenue is recognized on the date of service. The Company allocates revenue consideration based on the relative fair value as determined by the Company’s best estimate of selling price to separate the revenue where there are multiple deliverables under one revenue arrangement.
Revenue generated under PBM agreements is classified as either gross or net based on whether the Company is acting as a principal or an agent in the fulfillment of prescriptions through our retail pharmacy network. When the Company independently has a contractual obligation to pay a network pharmacy provider for benefits provided to its Plan Sponsors’ members, and therefore is the “primary obligor” as defined in FASB ASC 605, Revenue Recognition (“ASC 605”) the Company includes payments (which include the drug ingredient cost) from these Plan Sponsors as revenue and payments to the network pharmacy providers as cost of revenue. These transactions require the Company to pay network pharmacy providers, assume credit risk of Plan Sponsors and act as a principal. If the Company merely acts as an agent, and consequently administers Plan Sponsors’ network pharmacy contracts, the Company does not have the primary obligation to pay the network pharmacy and assume credit risk, and as such, records only the administrative fees (and not the drug ingredient cost) as revenue.
Revenue generated under discount card agreements is recognized when the discount card is used to purchase a prescription drug. The revenue is based on contractual rates per transaction. Broker fees associated with the marketing of the discount cards are incurred and recognized at the time the card is used and classified as selling, general and administrative expense in the Consolidated Statements of Operations.
In the Company’s Infusion Services segment, the Company also recognizes nursing revenue as the estimated net realizable amounts from patients and Plan Sponsors for services rendered and products provided. This revenue is recognized as the treatment plan is administered to the patient and is recorded at amounts estimated to be received under reimbursement or payment arrangements with payors.
Under the Medicare Prospective Payment System program, net revenue is recorded based on a reimbursement rate which varies based on the severity of the patient’s condition, service needs and certain other factors. Revenue is recognized ratably over a 60-day episode period and is subject to adjustment during this period if there are significant changes in the patient’s condition during the treatment period or if the patient is discharged but readmitted to another agency within the same 60-day episodic period. Medicare cash receipts under the prospective payment system are initially recognized as deferred revenue and are subsequently recognized as revenue over the 60-day episode period. The process for recognizing revenue under the Medicare program is based on certain assumptions and judgments, the appropriateness of the clinical assessment of each patient at the time of certification, and the level of adjustments to the fixed reimbursement rate relating to patients who receive a limited number of visits, have significant changes in condition or are subject to certain other factors during the episode.
66
Cost of Revenue
Cost of revenue includes the costs of prescription medications, pharmacy claims, fees paid to pharmacies, shipping and other direct and indirect costs associated with pharmacy management and administration, claims processing operations, and nursing services, offset by volume and prompt pay discounts received from pharmaceutical manufacturers and distributors and total manufacturer rebates.
Rebates
Manufacturers’ rebates are part of each of the Company’s segments. Rebates are generally volume-based incentives that are earned and recorded upon purchase of the inventory. Rebates are recorded as a reduction of both inventory and cost of goods sold.
PBM rebates are recorded on historical PBM results and trends and are revised on a regular basis depending on the Company’s latest forecasts, as well as information received from rebate sources. Should actual results differ, adjustments will be recorded in future earnings when the adjustment becomes known. In some instances, rebate payments are shared with the Company’s Plan Sponsors. PBM rebates earned by the Company are recorded as a reduction of cost of goods sold. PBM rebates shared with clients are recorded as a reduction of revenue consistent with the sales incentive provisions of ASC 605.
Lease Accounting
The Company accounts for operating leasing transactions by recording rent expense on a straight-line basis over the expected term of the lease starting on the date it gains possession of leased property. The Company includes tenant improvement allowances and rent holidays received from landlords and the effect of any rent escalation clauses, as adjustments to straight-line rent expense over the expected term of the lease.
Capital lease transactions are reflected as a liability at the inception of the lease based on the present value of the minimum lease payments or, if lower, the fair value of the property. Assets recorded under capital leases are depreciated in the same manner as owned property.
Income Taxes
As part of the process of preparing the Company’s Consolidated Financial Statements, management is required to estimate income taxes in each of the jurisdictions in which it operates. The Company accounts for income taxes under ASC Topic 740, Income Taxes (“ASC 740”). ASC 740 requires the use of the asset and liability method of accounting for income taxes. Under this method, deferred taxes are determined by calculating the future tax consequences attributable to differences between the financial accounting and tax bases of existing assets and liabilities. A valuation allowance is recorded against deferred tax assets when, in the opinion of management, it is more likely than not that the Company will not be able to realize the benefit from its deferred tax assets.
The Company files income tax returns, including returns for its subsidiaries, as prescribed by Federal tax laws and the tax laws of the state and local jurisdictions in which it operates. The Company’s uncertain tax positions are related to tax years that remain subject to examination and are recognized in the Consolidated Financial Statements when the recognition threshold and measurement attributes of ASC 740 are met. Interest and penalties related to unrecognized tax benefits are recorded as income tax expense.
Financial Instruments
The Company’s financial instruments consist mainly of cash and cash equivalents, receivables, accounts payable, accrued interest and its line of credit. The carrying amounts of cash and cash equivalents, receivables, accounts payable, accrued interest and its line of credit approximate fair value due to their fully liquid or short-term nature.
Accounting for Stock-Based Compensation
The Company accounts for stock-based compensation expense under the provisions of ASC Topic 718, Compensation – Stock Compensation (“ASC 718”). At December 31, 2014, the Company has two stock-based compensation plans pursuant to which incentive stock options (“ISOs”), non-qualified stock options (“NQSOs”), stock appreciation rights (“SARs”), restricted stock, performance shares and performance units may be granted to employees and non-employee directors. Option and stock awards are typically settled by issuing authorized but unissued shares of the Company.
67
The Company estimates the fair value of each stock option award on the measurement date using a binomial option-pricing model. The fair value of the award is amortized to expense on a straight-line basis over the requisite service period. The Company expenses restricted stock awards based on vesting requirements, including time elapsed, market conditions and/or performance conditions. Because of these requirements, the weighted average period for which the expense is recognized varies. The Company expenses SAR awards based on vesting requirements. In addition, because they are settled with cash, the fair value of the SAR awards are revalued on a quarterly basis.
Income (Loss) Per Share
The following table sets forth the computation of basic and diluted income (loss) per common share (in thousands, except for per share amounts):
Year Ended December 31, | |||||||||||
2014 | 2013 | 2012 | |||||||||
Numerator: | |||||||||||
Loss from continuing operations, net of income taxes | $ | (143,382 | ) | $ | (56,991 | ) | $ | (12,275 | ) | ||
Income from discontinued operations, net of income taxes | (4,086 | ) | (12,663 | ) | 76,982 | ||||||
Net income (loss) | $ | (147,468 | ) | $ | (69,654 | ) | $ | 64,707 | |||
Denominator - Basic and Diluted: | |||||||||||
Weighted average number of common shares outstanding | 68,476 | 64,560 | 56,239 | ||||||||
Earnings Per Common Share: | |||||||||||
Loss from continuing operations, basic and diluted | $ | (2.09 | ) | $ | (0.89 | ) | $ | (0.22 | ) | ||
Income (loss) from discontinued operations, basic and diluted | (0.06 | ) | (0.19 | ) | 1.37 | ||||||
Income (loss) per common share, basic and diluted | $ | (2.15 | ) | $ | (1.08 | ) | $ | 1.15 | |||
The computation of diluted shares for the years ended December 31, 2014, 2013 and 2012 excludes the effect of 3.1 million, 3.1 million and 3.4 million warrants with an exercise price of $10.00 issued in connection with the acquisition of CHS as their inclusion would be anti-dilutive to income (loss) per common share from continuing operations. In addition, the computation of diluted shares for the years ended December 31, 2014, 2013 and 2012 excludes the effect of 7.0 million, 6.1 million and 5.0 million, respectively, of other common stock equivalents as their inclusion would be anti-dilutive to income (loss) per common share from continuing operations. ASC Topic 260, Earnings Per Share, requires that income from continuing operations be used as the basis for determining whether the inclusion of common stock equivalents would be anti-dilutive.
Recent Accounting Pronouncements
In July 2013, the FASB issued ASU 2013-11, Presentation of an Unrecognized Tax Benefit When a Net Operating Loss Carryforward, a Similar Tax Loss, or a Tax Credit Carryforward Exists (“ASU 2013-11”). ASU 2013-11 provides that a liability related to an unrecognized tax benefit would be offset against a deferred tax asset for a net operating loss carryforward, a similar tax loss or a tax credit carryforward if such settlement is required or expected in the event the uncertain tax position is disallowed. In that case, the liability associated with the unrecognized tax benefit is presented in the financial statements as a reduction to the related deferred tax asset for a net operating loss carryforward, a similar tax loss or a tax credit carryforward. In situations in which a net operating loss carryforward, a similar tax loss or a tax credit carryforward is not available at the reporting date under the tax law of the jurisdiction or the tax law of the jurisdiction does not require, and the entity does not intend to use, the deferred tax asset for such purpose, the unrecognized tax benefit will be presented in the financial statements as a liability and will not be combined with deferred tax assets. The Company adopted ASU 2013-11 effective January 1, 2014 with no material impact on its Consolidated Financial Statements.
In April 2014, the FASB issued ASU 2014-08 Reporting Discontinued Operations and Disclosures of Disposals of Components of an Entity (“ASU 2014-08”) which amends the existing GAAP discontinued operations classification criteria to require a significant strategic shift in an entity’s operations. The new guidance also simplifies the classification analysis by eliminating the continuing involvement criteria. ASU 2014-08 also requires additional disclosures for discontinued operations and new disclosures for individually material disposal transactions that do not meet the definition of a discontinued operation. ASU 2014-08 becomes effective for the Company on January 1, 2015. The Company is currently evaluating the impact of adopting ASU 2014-08 and anticipates that the adoption will have no material impact on the Consolidated Financial Statements.
68
In May 2014, the FASB issued guidance codified in Accounting Standards Codification (“ASC”) 606, Revenue Recognition - Revenue from Contracts with Customers, which supersedes the guidance in former ASC 605, Revenue Recognition. ASC 606 becomes effective for the Company on January 1, 2017. The Company is currently evaluating the impact of the provisions of ASC 606.
In June 2014, the FASB issued ASU 2014-12, Compensation - Stock Compensation, Accounting for Share-Based Payments When the Terms of an Award Provide That a Performance Target Could Be Achieved after the Requisite Service Period (“ASU 2014-12”). ASU 2014-12 provides new guidance on accounting for share-based payments requiring a specific performance target to be achieved in order for employees to become eligible to vest in the awards when that performance target may be achieved after the requisite service period for the award. ASU 2014-12 is effective January 1, 2016. Early adoption is permitted. The Company is currently evaluating the impact adoption of this guidance will have on its Consolidated Financial Statements.
In August 2014, the FASB issued ASU 2014-15, “Disclosure of Uncertainties About an Entity’s Ability to Continue as a Going Concern” (“ASU 2014-15”). ASU 2014-15 requires management to evaluate, at each annual and interim reporting period, whether there are conditions or events that raise substantial doubt about the entity’s ability to continue as a going concern and provide related disclosures. ASU 2014-15 is effective for annual and interim reporting periods beginning January 1, 2017 and is not expected to have a material impact on the Company’s Consolidated Financial Statements.
NOTE 3-- STOCKHOLDERS’ EQUITY
Stock Offering
The Company filed a shelf registration statement on Form S-3 on March 18, 2013 and related amendment on April 2, 2013, which was declared effective on April 4, 2013. On April 24, 2013, the Company completed an underwritten primary public offering of 10,406,250 shares of its common stock at an offering price to the public of $12.00 per share. In addition, 3,968,750 shares of common stock were offered and sold by certain existing stockholders in an underwritten secondary offering completed on the same date and at the same offering price to the public.
Net proceeds to the Company were $118.4 million after underwriting discounts, commissions and other offering expenses. The Company did not receive any proceeds from the sale of shares of common stock by the selling stockholders. The Company used $21.0 million and approximately $61.1 million of the net proceeds to (i) repay outstanding borrowings under the Company's prior credit facility with Healthcare Finance Group (the “Prior Credit Facility”) and (ii) fund a portion of the CarePoint acquisition as described in Note 4 - Acquisitions below, respectively. The Company used the remaining net proceeds from the offering for general corporate purposes, which included, among other things, capital expenditures, repurchases of outstanding debt or equity securities, debt servicing requirements or redemption of our short-term or long-term borrowings, or for other working capital requirements.
Treasury Stock
During the years ended December 31, 2014 and 2012, 54,579 and 25,999 shares, respectively, were surrendered to satisfy tax withholding obligations on the vesting of restricted stock awards. The Company holds a total of 2,637,099 shares of treasury stock at December 31, 2014 acquired under current and prior repurchase programs as well as forfeitures to satisfy tax obligations in the vesting of restricted stock awards. During the year ended December 31, 2013, no shares of treasury stock were acquired or issued.
Common Stock Purchase Warrants
In connection with the acquisition of Critical Homecare Solutions Holdings, Inc. (“CHS”) in March 2010, the Company issued 3.4 million warrants exercisable for BioScrip common stock. The warrants have a five year term with an exercise price of $10.00 per share. They are exercisable at any time prior to the expiration date. The warrants also contain provisions whereby the number of shares to be issued upon exercise of the warrants will be increased if the Company were to execute certain dilutive transactions such as stock splits, stock dividends or the issuance of shares below 90% of market value at the time of issuance. The Company has determined that the warrants meet the conditions for equity classification in accordance with GAAP. Therefore, these warrants were classified as equity and included in additional paid-in capital.
During the year ended December 31, 2013, the Company issued 78,567 shares of common stock pursuant to the cashless exercise of 256,175 of the warrants. No warrants were exercised during the year ended December 31, 2014. As of December 31, 2014, 3.1 million of the warrants remain outstanding.
69
The fair value of the warrants of $12.3 million was calculated as of the acquisition date using the Black-Scholes model. The Black-Scholes model used the following assumptions: volatility of 62%, risk free interest rate of 2.63%, dividend yield of 0% and expected term of five years. In addition, there was a discount applied for lack of marketability of 13.5%. This discount was considered appropriate because the warrants were not registered under the Securities Act of 1933, as amended (the "Securities Act") and the shares issued upon exercise of the warrants were unregistered shares and subject to transfer restrictions as of the acquisition date. The shares were subsequently registered on Form S-3 on April 2, 2013 which was declared effective on April 4, 2013.
NOTE 4-- | ACQUISITIONS |
CarePoint Partners Holdings LLC
On August 23, 2013, the Company closed on the acquisition of substantially all of the assets and assumption of certain liabilities that constituted the home infusion business (the “CarePoint Business”) of CarePoint Partners Holdings LLC, a Delaware limited liability company, and its subsidiaries (collectively “CarePoint”). CarePoint was a provider of home and alternate-site infusion therapy for patients with complex, acute and chronic illnesses. CarePoint serviced approximately 20,500 patients annually through 28 sites of service in nine states in the East Coast and Gulf Coast regions.
The cash purchase price paid at closing was $211.1 million. The purchase agreement contemplated a targeted level of net working capital. Subsequent to the closing, the Company and the sellers agreed that additional net working capital adjustments of approximately $1.8 million were due to the Company. These working capital adjustments were primarily related to the value of accounts receivable and prepaid expenses as of the date of acquisition. The Company received payment for these amounts during the year ended December 31, 2014.
In addition, the purchase agreement provided that the purchase price could be increased by contingent consideration of $10.0 million if the CarePoint Business achieved a specified level of product gross profit during the one-year period following the closing date. If the specified level of product gross profit was not achieved, no contingent consideration would be due to the sellers. The Company reported actual product gross profit for the measurement period from September 1, 2013 to August 31, 2014 to the sellers on October 15, 2014. The report indicated that the requisite level of product gross profit was not achieved during the measurement period. Should the sellers disagree with the Company’s report, the purchase agreement provides for the dispute to be settled through arbitration.
At the date of acquisition, the fair value of the $10.0 million contingent consideration was estimated at $9.8 million. The fair value of the contingent consideration was determined using Level 3 inputs based on the present value of various payout scenarios, weighted on the basis of probability. The most important factor in determining the probability of payout at various balance sheet dates has been the business forecasts and actual results for the CarePoint Business standalone and merged market sites.
As of December 31, 2014, the fair value of the contingent consideration was remeasured considering the required product gross profit was not achieved during the measurement period and the uncertainties around any potential arbitration process. As a result, the fair value of the contingent consideration was maintained at $4.6 million as of December 31, 2014. Should an arbitrator rule against the Company, an additional expense of $5.4 million will be recorded over and above the accrual of $4.6 million estimated at December 31, 2014. Should an arbitrator rule in favor of the Company, the liability for contingent consideration will be reversed and, as a result, additional income of $4.6 million will be recorded. The liability for the contingent consideration is included in accrued expenses and other current liabilities in the accompanying Consolidated Balance Sheets.
The $5.2 million of income resulting from the reduction of the fair value of the contingent consideration for the year ended December 31, 2014 is included in the change in fair value of contingent consideration in the accompanying Consolidated Statements of Operations.
The Company funded the cash payment at closing with a combination of cash on hand and $150.0 million in borrowings under the Senior Credit Facilities (see Note 9 - Debt).
70
The table below summarizes the Company’s assessment of the fair values of the assets acquired and liabilities assumed as of the date of closing of the acquisition of the CarePoint Business (in thousands):
Fair Value | |||
Cash | $ | 14 | |
Accounts receivable | 15,917 | ||
Inventories | 3,184 | ||
Other current assets | 215 | ||
Property and equipment | 3,266 | ||
Identifiable intangible assets(1) | 16,700 | ||
Current liabilities | (8,697 | ) | |
Non-current liabilities | (721 | ) | |
Total identifiable net assets | 29,878 | ||
Goodwill | 189,214 | ||
Total cash and fair value of contingent consideration | $ | 219,092 | |
(1) | The following table summarizes the amounts and useful lives assigned to identifiable intangible assets (in thousands): |
Weighted- Average Useful Lives | Amounts Recognized as of the Closing Date | ||||
Customer relationships | 2 - 4 years | $ | 13,600 | ||
Trademarks | 2 years | 2,600 | |||
Non-compete agreements | 5 years | 500 | |||
Total identifiable intangible assets acquired | $ | 16,700 | |||
The excess of the purchase price over the fair value of tangible and identifiable intangible assets acquired and liabilities assumed in the acquisition was allocated to goodwill. The value of goodwill represents the value the Company expects to be created by combining the various operations of the CarePoint Business with the Company’s operations, including the expansion into new infusion markets, the opportunity to consolidate and upgrade certain existing facilities, access to new patients and potential cost savings and synergies. The CarePoint transaction was structured such that the amount allocated to goodwill will be deductible for income tax purposes in accordance with applicable tax rules.
The accompanying Consolidated Statements of Operations for the year ended December 31, 2014 include revenues and income from continuing operations of the CarePoint Business of $157.9 million and $1.8 million. The accompanying Consolidated Statements of Operations include revenues and income from continuing operations of $55.8 million and $2.3 million related to the CarePoint Business for the period from the date of acquisition to December 31, 2013.
HomeChoice Partners, Inc.
On February 1, 2013, the Company acquired 100% of the ownership interest in HomeChoice Partners, Inc., a Delaware corporation (“HomeChoice”). Prior to the Company's acquisition, HomeChoice was a provider of alternate-site infusion pharmacy services that serviced approximately 15,000 patients annually and had 14 infusion pharmacy locations in Pennsylvania, the District of Columbia, Maryland, Virginia, North Carolina, South Carolina, Georgia, Missouri and Alabama.
The cash purchase price of the HomeChoice acquisition was $72.9 million paid at the closing date. In addition, the purchase agreement provides that the purchase price could be increased by contingent consideration of up to $10.0 million if HomeChoice were to attain certain performance milestones in the first year following the closing and an additional $10.0 million if HomeChoice were to attain certain performance milestones in the second year following the closing, for total possible contingent consideration of up to $20.0 million.
At the date of acquisition, the fair value of the potential contingent consideration, using Level 3 inputs, was estimated at $8.0 million. The $20.0 million maximum contingent consideration was established using aggressive growth targets meant to achieve operating results in excess of transaction valuation model assumptions. Given the aggressiveness of the earnout target threshold, the Company assigned less than 50% probability of payout among the various payout scenarios considered.
71
While the acquisition has generated revenues as expected in the transaction valuation model, revenues through December 31, 2014 have not exceeded the aggressive earnout performance pace required. Specifically, revenue generating opportunities through various potential business relationships have not come to fruition and thus the probability of attaining the high level of growth required to achieve the earnout has been diminishing over the past year resulting in a lower probability of a future payout of contingent consideration. As of December 31, 2014, the fair value of the contingent consideration was again remeasured at fair value using actual operating results through December 31, 2014 and forecasted operating results for the remainder of the measurement period. As a result of this remeasurement, the probability of the payment of any contingent consideration appeared negligible and the fair value of the contingent consideration was reduced to $0 as of December 31, 2014. The $2.1 million and $5.9 million of income resulting from the reduction in the fair value of the contingent liability is included in change in fair value of contingent consideration in the accompanying Consolidated Statements of Operations for the years ended December 31, 2014 and 2013, respectively.
The Company funded the acquisition with a combination of cash and the Prior Credit Facility.
The table below summarizes the Company’s assessment of the fair values of the assets acquired and liabilities assumed as of the acquisition date of HomeChoice (in thousands):
Fair Value | |||
Accounts receivable | $ | 9,693 | |
Inventories | 1,984 | ||
Other current assets | 154 | ||
Property and equipment | 2,432 | ||
Identifiable intangible assets(1) | 4,000 | ||
Other non-current assets | 30 | ||
Current liabilities | (4,073 | ) | |
Total identifiable net assets | 14,220 | ||
Goodwill | 66,701 | ||
Total cash and fair value of contingent consideration | $ | 80,921 | |
(1) | The following table summarizes the amounts and useful lives assigned to identifiable intangible assets (in thousands): |
Weighted- Average Useful Lives | Amounts Recognized at the Closing Date | ||||
Customer relationships | 5 mo. - 3 years | $ | 2,000 | ||
Trademarks | 23 months | 1,000 | |||
Non-compete agreements | 1 year | 1,000 | |||
Total identifiable intangible assets acquired | $ | 4,000 | |||
The excess of the purchase price over the fair value of tangible and identifiable intangible assets acquired and liabilities assumed in the acquisition was allocated to goodwill. The value of goodwill represented the value the Company expected to be created by combining the various operations of HomeChoice with the Company’s operations, including the expansion into new infusion markets, the opportunity to consolidate and upgrade certain existing facilities, access to new patients and potential cost savings and synergies. The HomeChoice transaction was structured such that the amount allocated to goodwill will be deductible for income tax purposes in accordance with applicable tax rules.
The accompanying Consolidated Statements of Operations includes revenues and income from operations of $87.9 million and $8.1 million related to HomeChoice for the year ended December 31, 2014. The accompanying Consolidated Statements of Operations includes revenues and income from continuing operations related to HomeChoice for the period from the date of acquisition through December 31, 2013, of $67.7 million and $2.6 million, respectively.
InfuScience, Inc.
On July 31, 2012, the Company acquired 100% of InfuScience, Inc. (“InfuScience”) for a cash payment of $38.3 million. InfuScience historically acquired, developed and operated businesses providing alternate site infusion pharmacy services through
72
five infusion centers located in Eagan, Minnesota; Omaha, Nebraska; Chantilly, Virginia; Charleston, South Carolina; and Savannah, Georgia.
In addition, the purchase agreement provided that the purchase price could increase by contingent consideration of $3.0 million based on the results of operations during the 24 month period through July 31, 2014. At the date of acquisition, the fair value of the potential contingent payments of $3.0 million was estimated at $2.9 million. The fair value of the contingent liability was determined using Level 3 inputs based on the present value of various payout scenarios, weighted on the basis of probability. The $0.1 million of expense resulting from the increase in the fair value of the contingent liability is included in change in fair value of contingent consideration in the accompanying Consolidated Statements of Operations for the year ended December 31, 2013. The Company has made contingent payments of $1.3 million and $1.7 million during the years ended December 31, 2014 and 2013, respectively, based on the achievement of expected operating results. As of December 31, 2014, the contingent consideration has been fully paid.
The table below summarizes the Company’s assessment of the fair values of the assets acquired and liabilities assumed as of the acquisition date of InfuScience (in thousands):
Fair Value | |||
Cash | $ | 23 | |
Accounts receivable | 4,938 | ||
Inventories | 586 | ||
Other current assets | 371 | ||
Property and equipment | 751 | ||
Identifiable intangible assets(1) | 400 | ||
Other non-current assets | 349 | ||
Current liabilities | (4,428 | ) | |
Total identifiable net assets | 2,990 | ||
Goodwill | 38,429 | ||
Total cash and fair value of contingent consideration | $ | 41,419 | |
(1) | The following table summarizes the amounts and useful lives assigned to identifiable intangible assets (in thousands): |
Weighted- Average Useful Lives | Amounts Recognized at the Closing Date | ||||
Customer relationships | 5 months | $ | 400 | ||
Total identifiable intangible assets acquired | $ | 400 | |||
The excess of the purchase price over the fair value of tangible and identifiable intangible assets acquired and liabilities assumed in the acquisition was allocated to goodwill. The value of goodwill represented the value the Company expected to be created by combining the various operations of InfuScience with the Company’s operations, including the ability to cross-sell their respective services on a national basis with an expanded footprint in Infusion Services segment. Of the goodwill recorded in the InfuScience acquisition, $7.7 million is estimated to be deductible for income tax purposes.
The accompanying Consolidated Statements of Operations for the years ended December 31, 2014 and 2013 includes revenues of $52.2 million and $46.7 million and income from operations of $5.0 million and $4.4 million related to the operations of InfuScience, respectively. The accompanying Consolidated Statements of Operations for the year ended December 31, 2012, includes revenues of $16.5 million and loss from operations of $1.7 million related to InfuScience for the period from the date of acquisition through December 31, 2012.
73
Acquisition and Integration Costs
Acquisition and integration expenses in the accompanying Consolidated Statements of Operations for the years ended December 31, 2014, 2013 and 2012 include the following costs related to the CarePoint Business, HomeChoice Partners, and InfuScience acquisitions (in thousands):
Year Ended December 31, | |||||||||||
2014 | 2013 | 2012 | |||||||||
Legal and professional fees | $ | 6,931 | $ | 5,113 | $ | 2,941 | |||||
Financial advisory fees | — | 2,413 | — | ||||||||
Employee costs including redundant salaries and benefits and severance | 2,016 | 3,554 | 806 | ||||||||
Facilities consolidation and discontinuation | 1,401 | 1,621 | 110 | ||||||||
Bad debt expense and contractual adjustments related to acquired accounts receivable | 5,430 | — | — | ||||||||
Legal settlement | 334 | 2,300 | — | ||||||||
Other | 1,812 | 1,129 | 189 | ||||||||
Total | $ | 17,924 | $ | 16,130 | $ | 4,046 | |||||
Pro Forma Impact of Acquisitions
The following table shows summarized unaudited pro forma combined operating results of the Company for the years ended December 31, 2013 and 2012 as if the InfuScience, HomeChoice and CarePoint Business acquisitions had occurred on the same terms as of January 1, 2012. Pro forma adjustments have been made related to amortization of intangibles, interest expense, and income tax expense for the years ended December 31, 2013 and 2012. No proforma adjustments are required for the year ended December 31, 2014. The pro forma financial information does not reflect revenue opportunities and cost savings which the Company expected to realize as a result of the acquisitions or estimates of charges related to the integration activity. Amounts are in thousands, except for earnings per share:
Year Ended December 31, | |||||||
2013 | 2012 | ||||||
Revenues | $ | 876,942 | $ | 824,624 | |||
Net loss from continuing operations | $ | (58,829 | ) | $ | (27,287 | ) | |
Basic loss per common share from continuing operations | $ | (0.91 | ) | $ | (0.49 | ) | |
Diluted loss per common share from continuing operations | $ | (0.91 | ) | $ | (0.49 | ) | |
The unaudited pro forma combined results of operations were prepared using the acquisition method of accounting and are based on the historical financial operating results of the Company, CarePoint Business, HomeChoice and InfuScience. Except to the extent realized in the years ended December 31, 2013 and 2012, the unaudited pro forma information does not reflect any cost savings, operating synergies and other benefits that the Company may achieve as a result of these acquisitions, or the expenses to be incurred to achieve these savings, operating synergies and other benefits. In addition, except to the extent recognized in the years ended December 31, 2013 and 2012, the unaudited pro forma information does not reflect the costs to integrate the operations of the Company with CarePoint Business, HomeChoice and InfuScience.
The unaudited pro forma information is not necessarily indicative of what the Company’s consolidated results of operations actually would have been had the CarePoint Business, and HomeChoice and InfuScience acquisitions been completed on January 1, 2012. In addition, the unaudited pro forma information does not purport to project the future results of operations of the Company. The unaudited pro forma information primarily reflects the following net adjustments to the historical results of the acquired entities prior to acquisition (in thousands):
Year Ended December 31, | |||||||
2013 | 2012 | ||||||
Interest expense | $ | (3,734 | ) | $ | (8,613 | ) | |
Amortization expense | $ | (576 | ) | $ | (4,094 | ) | |
Income tax expense (benefit) | $ | (2,785 | ) | $ | (4,357 | ) | |
74
NOTE 5-- DISCONTINUED OPERATIONS
Sale of Home Health Business
On March 31, 2014, the Company completed the sale of substantially all of the Company’s Home Health Services segment (the “Home Health Business”) pursuant to the Stock Purchase Agreement dated as of February 1, 2014 (the “Stock Purchase Agreement”), as amended, by and among LHC Group, Inc., a Delaware corporation, and certain of its subsidiaries (collectively, the “Buyer”) and the Company and Elk Valley Professional Affiliates, Inc. (“EVPA”), South Mississippi Home Health, Inc. (“SMHH”), and Deaconess Homecare, LLC (collectively the “Seller”). The Buyer agreed to acquire the Home Health Business, consisting of (1) all of the issued shares of capital stock of EVPA owned by the Seller, (2) all of the issued shares of capital stock of SMHH owned by the Seller, and (3) all of the issued membership interests in two limited liability companies (collectively, the “Holding Newcos” and, together with EVPA and SMHH, the “Subject Companies”) that were wholly-owned subsidiaries of the Seller. On the closing date, the Company also entered into an Amendment No. 1 (the “Amendment”) to the Stock Purchase Agreement in connection with the closing. The Amendment modified the Stock Purchase Agreement to (i) exclude from the home health business conducted by the Company at one of its locations, and (ii) reduce by $0.5 million the total consideration to be received by the Company, to approximately $59.5 million.
Pursuant to the terms of the Stock Purchase Agreement, as amended, the Company received total consideration of approximately $59.5 million paid in cash (the “Purchase Price”) at closing. The Company used a portion of the net proceeds from the sale to pay down a portion of the Company’s outstanding debt. Subsequently, the Purchase Price was adjusted for net working capital of the Subject Companies as of the closing date that resulted in an additional payment to the Company of approximately $1.1 million. As a result of this adjustment, the final Purchase Price received by the Company was approximately $60.6 million. The Company has classified the net proceeds received from this sale in cash provided by investing activities from discontinued operations in the accompanying consolidated statements of cash flows.
The sale of the Home Health Business is consistent with the Company’s continuing strategic evaluation of its non-core businesses and its decision to continue to focus growth initiatives and capital in the Infusion Services segment. As a result, the Company decided in the second quarter of 2014 to cease the material portion of its Home Health operations at the one location excluded from the Stock Purchase Agreement, as amended, and reclassified its operations to discontinued operations for all prior periods in the accompanying Consolidated Financial Statements.
As of the March 31, 2014 closing date of the sale of the Home Health Business, the carrying value of the net assets of the Subject Companies was as follows (in thousands):
Carrying Value | ||||
Net accounts receivable | $ | 12,597 | ||
Prepaid expenses and other current assets | 242 | |||
Total current assets | 12,839 | |||
Property and equipment, net | 402 | |||
Goodwill | 33,784 | |||
Intangible assets | 15,400 | |||
Other non-current assets | 28 | |||
Total assets | 62,453 | |||
Accounts payable | 673 | |||
Amounts due to plan sponsors | 229 | |||
Accrued expenses and other current liabilities | 3,008 | |||
Total liabilities | 3,910 | |||
Net assets | $ | 58,543 | ||
The pre-tax gain on sale of the Home Health Business is approximately $2.1 million based on the March 31, 2014 net asset balances above and before financial advisory fees, legal expenses and other one-time transactions costs and including the net working capital adjustment. The net assets of the Subject Companies have been reclassified to discontinued operations for all prior periods in the accompanying Consolidated Financial Statements.
75
The operating results included in discontinued operations of the Home Health Business for the years ended December 31, 2014, 2013 and 2012 are summarized as follows (in thousands):
Year Ended December 31, | |||||||||||
2014 | 2013 | 2012 | |||||||||
Revenue | $ | 18,551 | $ | 72,737 | $ | 69,190 | |||||
Gross profit | $ | 6,918 | $ | 28,201 | $ | 29,058 | |||||
Selling, general and administrative expenses | 8,219 | 23,464 | 21,612 | ||||||||
Bad debt expense | 902 | 1,338 | 834 | ||||||||
Income (loss) from operations | (2,203 | ) | 3,399 | 6,612 | |||||||
Gain on sale before income taxes | 2,067 | — | — | ||||||||
Financial advisor fee and legal expenses | (2,875 | ) | — | — | |||||||
Impairment of assets | (452 | ) | — | — | |||||||
Other costs and expenses | (47 | ) | 1 | 1 | |||||||
Income (loss) before income taxes | (3,510 | ) | 3,400 | 6,613 | |||||||
Income tax expense (benefit) | (4,257 | ) | 15 | 2,678 | |||||||
Income from discontinued operations, net of income taxes | $ | 747 | $ | 3,385 | $ | 3,935 | |||||
Pharmacy Services Asset Sale
On February 1, 2012, the Company and certain of its subsidiaries (collectively, the “Sellers”) entered into a purchase agreement (the “2012 Asset Purchase Agreement”) with Walgreen Co. and certain of its subsidiaries (collectively, the “Pharmacy Services Buyers”) with respect to the sale of certain assets, rights and properties (the “Pharmacy Services Asset Sale”) relating to the Sellers’ traditional and specialty pharmacy mail operations and community retail pharmacy stores. The transaction included the sale of 27 community pharmacy locations, and certain assets of three community pharmacy locations, and three traditional and specialty mail service operations, which constituted all of the Company’s operations in the community pharmacy and mail order lines of business.
Pursuant to the terms of the 2012 Asset Purchase Agreement, the Company received a total purchase price of approximately $173.8 million. As a result of the Pharmacy Services Asset Sale, the Company has recognized a total pretax gain of approximately $108.2 million, net of transaction costs and other one-time charges as a result of the transaction. The Company used a portion of the net proceeds from the sale to pay down the Company’s outstanding debt and a portion was used to invest in the Infusion Services segment.
The purchase price excluded all accounts receivable and working capital liabilities relating to the operations subject to the Pharmacy Services Asset Sale, which were retained by the Company. No amounts related to the net accounts receivable retained by the Company remained at December 31, 2013.
On May 4, 2012, the carrying value of the assets included in the Pharmacy Services Asset Sale was as follows (in thousands):
Carrying Value | |||
Inventory | $ | 30,560 | |
Prepaid expenses and other current assets | 299 | ||
Total current assets | 30,859 | ||
Property and equipment, net | 1,592 | ||
Goodwill | 11,754 | ||
Intangible assets, net | 2,503 | ||
Total assets | $ | 46,708 | |
In addition, the Company and its subsidiaries and certain subsidiaries of the Pharmacy Services Buyers entered into an agreement concurrently with the 2012 Asset Purchase Agreement which provided that the Company cease to be the sole fulfillment pharmacy for customers who utilized the drugstore.com website. The agreement provided for a cash payment of $3.0 million to the Company and the payment of $2.9 million to the Pharmacy Services Buyers related to contingent consideration from the
76
Company’s 2010 acquisition of the prescription pharmacy business of DS Pharmacy, Inc. both of which occurred during the year ended December 31, 2012.
As a result of the divestiture process, the Company assessed its continuing operations in order to align its corporate structure with its remaining operations. As part of these efforts, the Company has incurred and expects to continue to incur additional expenses that may impact the Company’s future consolidated financial statements. These additional costs, including employee severance and other benefit-related costs, facility-related costs, and other one-time charges are included in income (loss) from discontinued operations, net of income taxes in the Consolidated Statements of Operations.
Effective January 8, 2014, the Company entered into a Stipulation and Order of Settlement and Dismissal (the “Federal Settlement Agreement”) with the U.S. Department of Justice (the “DOJ”) and a qui tam relator (the “Relator”). The Federal Settlement Agreement represented the federal and private component of an agreement in principle to settle all civil claims under the False Claims Act and related statutes and all common law claims that could have been brought by the DOJ and Relator that arose out of the distribution of the Novartis Pharmaceutical Corporation’s product Exjade® (the “Medication”) by the Company’s traditional and specialty pharmacy mail operations and community retail pharmacy stores prior to its divestiture in May 2012. Further, effective February 11, 2014, the Company entered into State Settlement Agreements with the offices of the Attorneys General of thirty-four states (the “Settling States”). The State Settlement Agreements represented the state component of the Company’s agreement in principle to settle the claims that could have been brought by the Settling States that arose out of the distribution of the Medication. During the year ended December 31, 2013, the Company accrued $15.0 million related to the Settlement Agreements and included the amount and related legal fees and expenses in income (loss) from discontinued operations, net of income taxes in the Consolidated Statements of Operations (see Note 10 - Commitments and Contingencies).
As of December 31, 2014, there were accruals of $13.0 million related to legal settlement and other costs, of which $7.0 million is included in accrued expenses and other current liabilities and $6.0 million is included in other non-current liabilities on the Consolidated Balance Sheets. The accrual activity consisted of the following (in thousands):
Legal Settlement | Employee Severance and Other Benefits | Other Costs | Total | ||||||||||||
Balance at December 31, 2012 | $ | — | $ | 45 | $ | 89 | $ | 134 | |||||||
Expenses | 15,000 | 186 | 7,410 | 22,596 | |||||||||||
Cash payments | — | (103 | ) | (6,261 | ) | (6,364 | ) | ||||||||
Non-cash charges | — | (36 | ) | (43 | ) | (79 | ) | ||||||||
Balance at December 31, 2013 | 15,000 | 92 | 1,195 | 16,287 | |||||||||||
Expenses | 403 | — | 4,510 | 4,913 | |||||||||||
Cash payments | (3,014 | ) | (92 | ) | (5,015 | ) | (8,121 | ) | |||||||
Non-cash charges | — | — | (81 | ) | (81 | ) | |||||||||
Balance at December 31, 2014 | $ | 12,389 | $ | — | $ | 609 | $ | 12,998 | |||||||
The operating results of the divested traditional and specialty pharmacy mail operations and community pharmacies for the years ended December 31, 2014, 2013 and 2012 are summarized below (in thousands):
Years ended December 31, | |||||||||||
2014 | 2013 | 2012 | |||||||||
Revenue | $ | — | $ | (75 | ) | $ | 466,747 | ||||
Gross profit | (439 | ) | (519 | ) | 29,844 | ||||||
Operating expenses | 3,995 | 7,118 | 38,612 | ||||||||
Legal settlement expense | — | 15,000 | — | ||||||||
Bad debt expense | — | — | 12,931 | ||||||||
Interest (income) expense | 403 | (41 | ) | 761 | |||||||
Gain on sale | 4 | 6,548 | 101,624 | ||||||||
Income tax expense | — | — | 6,117 | ||||||||
Income (loss) from discontinued operations, net of income taxes | $ | (4,833 | ) | $ | (16,048 | ) | $ | 73,047 | |||
77
NOTE 6-- GOODWILL AND INTANGIBLE ASSETS
Goodwill, and the changes in the carrying amount of goodwill by operating and reportable segment for the years ended December 31, 2014 and 2013, are as follows (in thousands):
Infusion Services | PBM Services | Total | |||||||||
Balance at December 31, 2012 | $ | 304,282 | $ | 12,744 | $ | 317,026 | |||||
Acquisitions | 254,304 | — | 254,304 | ||||||||
Other adjustments | 7 | — | 7 | ||||||||
Balance at December 31, 2013 | 558,593 | 12,744 | 571,337 | ||||||||
Other adjustments | 1,986 | — | 1,986 | ||||||||
Balance at December 31, 2014 | $ | 560,579 | $ | 12,744 | $ | 573,323 | |||||
At December 31, 2013, goodwill of $33.8 million related to the Home Health Business that was sold on March 31, 2014 is included in non-current assets of discontinued operations in the accompanying Consolidated Balance Sheets (see Note 5 - Discontinued Operations).
The $254.3 million increase in the Infusion Services segment goodwill primarily results from the acquisitions of the HomeChoice and CarePoint Businesses during the year ended December 31, 2013. Purchase price adjustments of $2.0 million related to the CarePoint Business acquisition net working capital adjustments related to the value of accounts receivable and prepaid expenses were recorded during the year ended December 31, 2014 (see Note 4 - Acquisitions).
In accordance with ASC 350, Intangibles--Goodwill and Other, the Company evaluates goodwill for impairment on an annual basis and whenever events or circumstances exist that indicate that the carrying value of goodwill may no longer be recoverable. The impairment evaluation is based on a two-step process. The first step compares the fair value of a reporting unit with its carrying amount, including goodwill. If the first step indicates that the fair value of the reporting unit is less than its carrying amount, the second step must be performed which determines the implied fair value of reporting unit goodwill. The measurement of possible impairment is based upon the comparison of the implied fair value of reporting unit to its carrying value.
The Company evaluated goodwill for possible impairment in accordance with ASC 350, Intangibles--Goodwill and Other (see Note 2 - Significant Accounting Policies) as of December 31, 2014, and determined that no impairment of goodwill was indicated.
Intangible assets consisted of the following as of December 31, 2014 and 2013 (in thousands):
December 31, 2014 | December 31, 2013 | |||||||||||||||||||||||
Gross Carrying Amount | Accumulated Amortization | Net Carrying Amount | Gross Carrying Amount | Accumulated Amortization | Net Carrying Amount | |||||||||||||||||||
Finite Lived Assets | ||||||||||||||||||||||||
Infusion customer relationships | 25,650 | (16,615 | ) | 9,035 | 25,650 | (12,062 | ) | 13,588 | ||||||||||||||||
Infusion trademarks | 6,200 | (5,333 | ) | 867 | 6,200 | (3,514 | ) | 2,686 | ||||||||||||||||
Non-compete agreements | 1,500 | (1,133 | ) | 367 | 1,500 | (950 | ) | 550 | ||||||||||||||||
$ | 33,350 | $ | (23,081 | ) | $ | 10,269 | $ | 33,350 | $ | (16,526 | ) | $ | 16,824 | |||||||||||
Indefinite lived intangible assets of $15.4 million related to the Home Health Business sold on March 31, 2014 are included in non-current assets of discontinued operations in the accompanying Consolidated Balance Sheets at December 31, 2013 (see Note 5 - Discontinued Operations).
78
Finite lived intangible assets are amortized on a straight-line basis over their estimated useful lives as follows:
Estimated Useful Life | ||||||
Infusion customer relationships | 5 | months | - | 4 | years | |
Infusion trademarks | 23 | months | - | 3 | years | |
Non-compete agreements | 1 | year | - | 5 | years | |
Total amortization expense of intangible assets was $6.6 million, $6.7 million, and $4.0 million for the years ended December 31, 2014, 2013, and 2012, respectively. Amortization expense is expected to be the following (in thousands):
Year ending December 31, | Estimated Amortization | ||
2015 | $ | 5,142 | |
2016 | 3,078 | ||
2017 | 1,983 | ||
2018 | 66 | ||
2019 | — | ||
Thereafter | $ | — | |
Total estimated amortization expense | $ | 10,269 | |
NOTE 7-- RESTRUCTURING AND OTHER EXPENSES
Restructuring and other expenses include expenses resulting from the execution of our strategic assessment and related restructuring plans, consisting primarily of employee severance and other benefit-related costs, third-party consulting costs, facility-related costs, and certain other costs. It also includes other transitional costs such as training, redundant salaries prior to defined separation dates, and retention bonuses for certain critical personnel.
In 2010, the Company commenced a strategic assessment of its business and operations (“Restructuring Phase I”). This assessment focused on expanding revenue opportunities and lowering corporate overhead, including workforce and benefit reductions and facility rationalization. In addition to addressing corporate overhead, the strategic assessment examined the Company’s market strengths and opportunities and compared the Company’s position to that of its competitors. As a result of the assessment, the Company focused its growth on investments in the Infusion and Home Health Services segments and elected to pursue offers for its traditional and specialty pharmacy mail operations and community retail pharmacy stores. Accordingly, the Company consummated the Pharmacy Services Asset Sale relating to its traditional and specialty pharmacy mail operations and community retail pharmacy stores.
In 2012, as a result of the divestiture process, the Company’s management team commenced an assessment of the Company’s continuing operations in order to align its corporate structure with its remaining operations (“Restructuring Phase II”).
The Company anticipates that additional restructuring will occur and thus we may incur significant additional charges such as the write down of certain long-lived assets, employee severance, other restructuring type charges, temporary redundant expenses, potential cash bonus payments and potential accelerated payments or termination costs for certain of its contractual obligations, which impact the Company’s future Consolidated Financial Statements.
Restructuring Phase I
As a result of the execution of the strategic assessment and related restructuring plan, the Company incurred restructuring expenses of approximately $0.2 million, and $(0.1) million, during the years ended December 31, 2014 and 2013, respectively. Restructuring costs for the year ended December 31, 2014 relate primarily to the closing of facilities. The Company did not incur any significant restructuring expenses related to Restructuring Phase I during 2013, though some amounts previously accrued were adjusted in 2013.
Since inception of the strategic assessment and related restructuring plan, the Company has incurred approximately $10.3 million in total expenses, including $4.3 million of third-party consulting costs, $4.1 million of employee severance and other
79
benefit-related costs related to workforce reductions, and $1.9 million of facility-related costs. A large part of the third-party consulting costs and other costs were associated with the analysis of our assets and their long-term strategic value relative to other assets in which we could invest. The assessment process culminated in the Pharmacy Services Asset Sale (see Note 5--Discontinued Operations).
The restructuring costs are included in restructuring and other expenses on the Consolidated Statements of Operations. As of December 31, 2014, there are restructuring accruals of $0.4 million related to Phase I included in accrued expenses and other current liabilities and other non-current liabilities on the Consolidated Balance Sheets. The restructuring accrual activity consisted of the following (in thousands):
Employee Severance and Other Benefits | Consulting Costs | Facility-Related Costs | Total | |||||||||||||
Balance at December 31, 2012 | $ | 163 | $ | 20 | $ | 841 | $ | 1,024 | ||||||||
Expenses | (163 | ) | (20 | ) | 118 | (65 | ) | |||||||||
Cash payments | — | — | (438 | ) | (438 | ) | ||||||||||
Balance at December 31, 2013 | — | — | 521 | 521 | ||||||||||||
Expenses | — | — | 248 | 248 | ||||||||||||
Cash payments | — | — | (351 | ) | (351 | ) | ||||||||||
Balance at December 31, 2014 | $ | — | $ | — | $ | 418 | $ | 418 | ||||||||
Restructuring Phase II
As a result of Restructuring Phase II, the Company incurred restructuring expenses of approximately $10.9 million and $3.4 million during the years ended December 31, 2014 and 2013. Restructuring expense during the year ended December 31, 2014 consisted of approximately $2.9 million of employee severance and other benefit related costs associated with workforce reductions, $6.2 million of third-party consulting and $1.7 million of other costs, primarily incremental bad debt reserves related to branch closures. Restructuring expenses during the year ended December 31, 2013 consisted of approximately $1.5 million of employee severance and other benefit related costs associated with workforce reductions, $1.6 million of third-party consulting costs and $0.4 million in other costs.
The restructuring costs are included in restructuring and other expenses on the Consolidated Statements of Operations. As of December 31, 2014, there are restructuring accruals of $3.3 million related to Phase II included on the Consolidated Balance Sheets. The restructuring accrual activity consisted of the following (in thousands):
Employee Severance and Other Benefits | Consulting Costs | Other Costs | Total | |||||||||||||
Balance at December 31, 2012 | $ | 559 | $ | 145 | $ | — | $ | 704 | ||||||||
Expenses | 1,496 | 1,561 | 378 | 3,435 | ||||||||||||
Cash payments | (1,159 | ) | (155 | ) | (344 | ) | (1,658 | ) | ||||||||
Balance at December 31, 2013 | 896 | 1,551 | 34 | 2,481 | ||||||||||||
Expenses | 2,949 | 6,242 | 1,721 | 10,912 | ||||||||||||
Cash payments | (2,460 | ) | (7,312 | ) | (279 | ) | (10,051 | ) | ||||||||
Balance at December 31, 2014 | $ | 1,385 | $ | 481 | $ | 1,476 | $ | 3,342 | ||||||||
Other transitional costs included in restructuring and other expenses on the Consolidated Statements of Operations totaled $4.4 million, $4.4 million and $3.0 million, in the years ended December 31, 2014, 2013 and 2012, respectively. During the year ended December 31, 2014, $0.7 million of the transitional costs related to the issuance of the 2021 Notes (see Note 9). During the year ended December 31, 2012, transitional costs included $0.8 million for certain state sales taxes associated with prior year sales of acquired companies.
80
NOTE 8-- PROPERTY AND EQUIPMENT
Property and equipment consists of the following (in thousands):
December 31, | |||||||
2014 | 2013 | ||||||
Computer and office equipment, including equipment acquired under capital leases | $ | 22,662 | $ | 19,961 | |||
Software capitalized for internal use | 14,914 | 13,746 | |||||
Vehicles, including equipment acquired under capital leases | 2,106 | 2,056 | |||||
Medical equipment | 27,668 | 22,247 | |||||
Work in progress | 3,287 | 8,815 | |||||
Furniture and fixtures | 4,487 | 4,291 | |||||
Leasehold improvements | 13,690 | 12,082 | |||||
Property and equipment, gross | 88,814 | 83,198 | |||||
Less: Accumulated depreciation | (50,643 | ) | (42,016 | ) | |||
Property and equipment, net | $ | 38,171 | $ | 41,182 | |||
The Company had an insignificant amount of vehicles and medical equipment under capital lease for the years ended December 31, 2014, 2013 and 2012.
Work in progress at December 31, 2014 and 2013 includes $0.6 million and $0.7 million, respectively, of internally developed software costs to be capitalized upon completion.
Depreciation expense, including expense related to assets under capital lease, for the years ended December 31, 2014, 2013 and 2012 was $16.4 million, $13.4 million, and $8.4 million, respectively. Depreciation expense for the years ended December 31, 2014, 2013 and 2012 includes $2.4 million, $1.7 million, and $1.3 million, respectively, related to costs related to software capitalized for internal use.
There were no significant gains or losses on sales of property and equipment for the years ended December 31, 2014 and 2013. Losses of $0.2 million were incurred on disposal of property and equipment for the year ended December 31, 2012.
Impairment
The Company assesses the impairment of its assets whenever events or changes in circumstances indicate that the carrying value may not be recoverable. As a result of the Pharmacy Services Asset Sale (see Note 5 - Discontinued Operations), the Company evaluated certain facilities that were retained by the Company following the divestiture. As a result of the evaluation, the Company determined that a triggering event occurred, giving rise to the need to assess the recoverability of certain of our assets previously used in the specialty pharmacy mail operations and community retail pharmacy operations, which consisted primarily of software capitalized for internal use, leasehold improvements and work in progress. Based on our analysis, we recorded a $5.8 million impairment charge in income (loss) from discontinued operations, net of income taxes during the year ended December 31, 2012. In connection with the sale of substantially all of the Home Health Services segment, the Company recorded an impairment charge of $0.5 million that is included in income (loss) from discontinued operations, net of income taxes during the year ended December 31, 2014. No impairment charges were incurred during the year ended December 31, 2013.
81
NOTE 9-- DEBT
As of December 31, 2014 and 2013 the Company’s debt consisted of the following (in thousands):
December 31, | |||||||
2014 | 2013 | ||||||
Revolving Credit Facility | $ | 5,000 | $ | 40,003 | |||
Term Loan Facilities | 222,757 | 395,000 | |||||
2021 Notes, net of unamortized discount | 195,462 | — | |||||
Capital leases | 584 | 576 | |||||
Total Debt | 423,803 | 435,579 | |||||
Less: Current portion | 5,395 | 60,257 | |||||
Long-term debt, net of current portion | $ | 418,408 | $ | 375,322 | |||
Senior Credit Facilities
On July 31, 2013, the Company entered into (i) a senior secured first-lien revolving credit facility in an aggregate principal amount of $75.0 million (the “Revolving Credit Facility”), (ii) a senior secured first-lien term loan B in an aggregate principal amount of $250.0 million (the “Term Loan B Facility”) and (iii) a senior secured first-lien delayed draw term loan B in an aggregate principal amount of $150.0 million (the “Delayed Draw Term Loan Facility” and, together with the Revolving Credit Facility and the Term Loan B Facility, the “Senior Credit Facilities”) with SunTrust Bank, Jefferies Finance LLC and Morgan Stanley Senior Funding, Inc.
Advances under the Senior Credit Facilities bear interest at a floating rate or rates equal to the Eurodollar rate plus 5.25% or the base rate plus 4.25% specified in the Senior Credit Facilities agreement. The Eurodollar rate used in the interest rate calculation for Term Loan B Facility and the Delayed Draw Term Loan Facility (collectively, the “Term Loan Facilities”) is subject to a floor of 1.25%. There is no floor applied to interest rate calculation for the Revolving Credit Facility. In addition, there is a 0.50% commitment fee on the unused portion of the Revolving Credit Facility. As of December 31, 2014, the interest rate for the Term Loan Facilities is approximately 6.50% and the interest rate for the Revolving Credit Facility is approximately 7.50%. The interest rates may vary in the future depending on the Company’s consolidated net leverage ratio.
The Revolving Credit Facility matures on July 31, 2018, at which time all principal amounts outstanding are due and payable. The Term Loan Facilities each mature on July 31, 2020, and require equal consecutive quarterly repayments of 1.25% of the original principal amount funded commencing on December 31, 2013. Once repaid, amounts under Term Loan Facilities may not be re-borrowed. The Senior Credit Facilities are secured by substantially all of the Company’s and its subsidiaries’ assets.
The Senior Credit Facilities contain customary events of default that include, among others, non-payment of principal, interest or fees, violation of covenants, inaccuracy of representations and warranties, bankruptcy and insolvency events, material judgments, cross-defaults to material indebtedness, events constituting a change of control and any other development that results in, or would reasonably be expected to result in, a material adverse effect to the debtor’s ability to perform its obligation under the facility. The occurrence of certain events of default may increase the applicable rate of interest by 2% and could result in the acceleration of the Company’s obligations under the Senior Credit Facilities to pay the full amount of the obligations. If the Company draws down in excess of 25% of the available borrowing capacity under the Revolving Credit Facility, the net leverage covenants under the Revolving Credit Facility will become applicable such that the Company’s consolidated net leverage ratio will not be permitted to exceed certain thresholds until maturity of the Revolving Credit Facility. The required maximum consolidated net leverage ratio thresholds for the Revolving Credit Facility are defined for each measurement quarter. The Term Loan Facilities are not subject to any financial covenants.
The proceeds of the Term Loan B Facility were used to refinance certain existing indebtedness of the Company, including the payment of the purchase price for the 10.25% senior unsecured notes (the “2015 Notes”) tendered and accepted for purchase in the Offer (defined below) and the payment of the redemption price for the 2015 Notes that remained outstanding after completion of the Offer. The Delayed Draw Term Loan Facility and the Revolving Credit Facility were used to fund a portion of the CarePoint Business acquisition and may be used for other general corporate purposes of the Company, including acquisitions, investments, capital expenditures and working capital needs.
82
On December 23, 2013, the Company entered into the First Amendment to the Senior Credit Facilities pursuant to which the Company obtained the required consent of the lenders to enter into the Settlement Agreements (see Note 10 - Commitments and Contingencies) and to begin making payments, in accordance with the payment terms, on the settlement amount of $15.0 million. In exchange for this consent, the Company paid the lenders a fee of $0.5 million and included this amount in loss from discontinued operations in the Consolidated Statements of Operations.
On January 31, 2014, the Company entered into the Second Amendment to the Senior Credit Facilities, which, among other things (i) provides additional flexibility with respect to compliance with the maximum net leverage ratio for the fiscal quarters ending December 31, 2013 through and including December 31, 2014, (ii) provides additional flexibility under the indebtedness covenants to permit the Company to obtain up to $150.0 million of second-lien debt and issue up to $250.0 million of unsecured bonds, provided that 100% of the net proceeds are applied first to the Revolving Credit Facility, with no corresponding permanent commitment reduction, and then on a pro rata basis to the Term Loan B Facility and the Delayed Draw Term Loan Facility (collectively, the “Term Loan Facilities”), (iii) provides the requisite flexibility to sell non-core assets, subject to the satisfaction of certain conditions, and (iv) increased the applicable interest rates for each of the Term Loan Facilities to the Eurodollar rate plus 6.00% or the base rate plus 5.00%, until the occurrence of certain pricing decrease triggering events, as defined in the amendment. Upon the occurrence of a pricing decrease triggering event, the interest rates for the Senior Credit Facilities may revert to the Eurodollar rate plus 5.25% or the base rate plus 4.25%.
As discussed below, the net proceeds of approximately $194.5 million from the issuance on February 11, 2014 of 8.875% senior notes due 2021 (the “2021 Notes”) were used to repay $59.3 million of the Revolving Credit Facility and $135.2 million of the Term Loan Facilities. In addition, approximately $54.2 million of the net proceeds from the sale of the Home Health Business (see Note 5 - Discontinued Operations) were used to repay $17.2 million of the Revolving Credit Facility and $37.0 million of the Term Loan Facilities. These repayments were used to prepay the required quarterly principal repayments such that no principal repayments will be required for the Term Loan Facilities until their maturity on July 31, 2020. Once repaid, amounts under Term Loan Facilities may not be reborrowed. The Senior Credit Facilities are secured by substantially all of the Company’s and its subsidiaries’ assets.
The partial repayments of the Senior Credit Facilities as a result of the issuance of the 2021 Notes and from the sale of the Home Health Business were pricing decrease triggering events that resulted in the interest rates reverting to the Eurodollar rate plus 5.25% or the base rate plus 4.25%.
At December 31, 2014, the Company had $70.0 million undrawn under its Revolving Credit Facility.
On March 1, 2015, the Company entered into the Third Amendment to the Senior Credit Facilities (see Note 17--Subsequent Events).
2021 Notes
On February 11, 2014, the Company issued $200.0 million aggregate principal amount of the 2021 Notes. The 2021 Notes are senior unsecured obligations of the Company and are fully and unconditionally guaranteed by all existing and future subsidiaries of the Company. The 2021 Notes were offered in the United States to qualified institutional buyers in reliance on Rule 144A under the Securities Act of 1933, as amended (the “Securities Act”), and outside the United States to non-U.S. persons in reliance on Regulation S under the Securities Act pursuant to an Indenture (the “2021 Notes Indenture”), dated February 11, 2014, by and among the Company, the guarantors named therein and U.S. Bank National Association, as trustee.
Interest on the 2021 Notes accrues at a fixed rate of 8.875% per annum and is payable in cash semi-annually, in arrears, on February 15 and August 15 of each year, commencing on August 15, 2014. The debt discount of $5.0 million at issuance is being amortized as interest expense through maturity which will result in the accretion over time of the outstanding debt balance to the principal amount. As of December 31, 2014, there are no quoted prices or active markets for the 2021 Notes. The 2021 Notes are the Company’s senior unsecured obligations and rank equally in right of payment with all of its other existing and future senior unsecured indebtedness and senior in right of payment to all of its existing and future subordinated indebtedness.
The 2021 Notes are guaranteed on a full, joint and several basis by each of the Company’s existing and future domestic restricted subsidiaries that is a borrower under any of the Company’s credit facilities or that guarantees any of the Company’s debt or that of any of its restricted subsidiaries, in each case incurred under the Company’s credit facilities.
The Company may redeem some or all of the 2021 Notes prior to February 15, 2017 by paying a “make-whole” premium. The Company may redeem some or all of the 2021 Notes on or after February 15, 2017 at specified redemption prices. In addition, prior to February 15, 2017, the Company may redeem up to 35% of the 2021 Notes with the net proceeds of certain equity offerings
83
at a price of 108.875% plus accrued and unpaid interest, if any. The Company is obligated to offer to repurchase the 2021 Notes at a price of 101% of their principal amount plus accrued and unpaid interest, if any, as a result of certain change of control events. These restrictions and prohibitions are subject to certain qualifications and exceptions.
The 2021 Notes Indenture contains covenants that, among other things, limit the Company’s ability and the ability of certain of the Company’s subsidiaries to (i) grant liens on its assets, (ii) make dividend payments, other distributions or other restricted payments, (iii) incur restrictions on the ability of the Company’s restricted subsidiaries to pay dividends or make other payments, (iv) enter into sale and leaseback transactions, (v) merge, consolidate, transfer or dispose of substantially all of their assets, (vi) incur additional indebtedness, (vii) make investments, (viii) sell assets, including capital stock of subsidiaries, (ix) use the proceeds from sales of assets, including capital stock of restricted subsidiaries, and (x) enter into transactions with affiliates. In addition, the 2021 Notes Indenture requires, among other things, the Company to provide financial and current reports to holders of the 2021 Notes or file such reports electronically with the U.S. Securities and Exchange Commission (the “SEC”). These covenants are subject to a number of exceptions, limitations and qualifications set forth in the 2021 Notes Indenture.
Pursuant to the terms of the Second Amendment to the Senior Credit Facilities, the Company used the net proceeds of the 2021 Notes of approximately $194.5 million to repay $59.3 million of the Revolving Credit Facility and $135.2 million of the Term Loan Facilities.
In connection with the issuance of the 2021 Notes, the Company entered into a registration rights agreement on February 11, 2014 with certain guarantors of the 2021 Notes named therein and Jefferies LLC, on behalf of itself and the other initial purchasers named therein (the “Registration Rights Agreement”). Pursuant to the Registration Rights Agreement, the Company on February 6, 2015 filed on Form S-4 an exchange offer registration statement to exchange the 2021 Notes for substantially identical notes registered under the Securities Act. The Company has agreed to use commercially reasonable efforts to have the exchange offer registration statement declared effective within 450 days of the issue date and to complete the exchange offer with respect to the 2021 Notes within 30 days of effectiveness. If the Company fails to satisfy its registration obligations under the Registration Rights Agreement, it will be required to pay additional interest to the holders of the 2021 Notes under certain circumstances.
Prior Revolving Credit Facility
On July 3, 2012, the Company entered into a Third Amendment to the Second Amended and Restated Credit Agreement, by and among the Company, as borrower, all of its subsidiaries as guarantors thereto, the lenders, Healthcare Finance Group, LLC, an administrative agent, and the other parties thereto to provide an available line of credit of up to $125.0 million. The Prior Credit Facility bore interest at LIBOR rate plus 3.5%. On July 31, 2013, the Company entered into its Senior Credit Facilities and terminated this agreement. At the date of termination, no amounts were outstanding under this agreement.
2015 Notes
On June 3, 2013, the Company commenced an Offer to Purchase and Consent Solicitation (the “Offer”) to the holders of the Company’s outstanding $225.0 million aggregate principal 2015 Notes to purchase any and all of the 2015 Notes at $1,056.25 cash for each $1,000.00 of principal plus accrued but unpaid interest to the date of purchase.
On July 31, 2013, the Company received and accepted for purchase approximately 56.1% of the aggregate principal amount of its outstanding 2015 Notes that were validly tendered by the Offer’s expiration date of July 30, 2013. The $133.3 million aggregate repurchase price plus accrued but unpaid interest of $4.3 million, of the 2015 Notes tendered in connection with the Offer was paid from proceeds received under the Term Loan B Facility.
In connection with the Offer, the Company solicited and received sufficient consents from the holders of the 2015 Notes to amend certain provisions of the indenture governing the 2015 Notes (the “2015 Notes Indenture”) that would eliminate substantially all of the restrictive covenants, certain events of default and other provisions included in the Indenture. On July 31, 2013, the Company entered into a supplemental indenture with the trustee for the 2015 Notes, giving effect to the proposed amendments to the 2015 Notes Indenture and eliminating substantially all of the restrictive covenants and certain default provisions contained in the 2015 Notes Indenture.
On July 31, 2013, the Company satisfied and discharged its obligations under the 2015 Notes Indenture by depositing with the trustee approximately $107.8 million from proceeds received under the Term Loan B Facility. On August 19, 2013, the trustee paid all remaining outstanding 2015 Notes at a redemption price equal to $1,051.25 cash for each $1,000.00 of the principal amount plus accrued and unpaid interest as of such date.
84
Loss on Extinguishment of Debt
As a result of the $54.2 million repayment of the Senior Credit Facilities from the net proceeds of the sale of the Home Health Business on March 31, 2014, the Company recognized a partial extinguishment of debt and wrote off approximately $2.4 million of deferred financing costs during the three months ended December 31, 2014. During the year ended December 31, 2013, the Company recognized a $15.9 million loss on extinguishment of debt as a result of the repurchase and redemption of all outstanding principal and interest amounts under the 2015 Notes.
The accompanying Consolidated Statements of Operations include losses on extinguishment of debt during the years ended December 31, 2014 and 2013 as follows (in thousands):
Year Ended December 31, | |||||||
2014 | 2013 | ||||||
2015 Note redemption premium | $ | — | $ | 12,162 | |||
Write-off of deferred financing costs | 2,373 | 3,501 | |||||
Legal fees and other expenses | — | 235 | |||||
Loss on extinguishment of debt | $ | 2,373 | $ | 15,898 | |||
Deferred Financing Costs
In connection with Senior Credit Facilities, the Company incurred underwriting fees, agent fees, legal fees and other expenses of $21.9 million that are being amortized over the terms of the Senior Credit Facilities. As discussed above, approximately $2.4 million of these deferred financing costs were written off during the three months ended December 31, 2014.
In connection with the issuance of the 2021 Notes, the Company incurred underwriting fees, agent fees, legal fees and other expenses of $0.5 million that are being amortized over the term of the 2021 Notes.
Future Maturities
The estimated future maturities of the Company’s long-term debt as of December 31, 2014, are as follows (in thousands):
Year Ending December 31, | Amount | |||
2015 | $ | — | ||
2016 | — | |||
2017 | — | |||
2018 | — | |||
2019 | — | |||
Thereafter | 422,757 | |||
Total future maturities | $ | 422,757 | ||
85
Interest Expense, net
Interest expense consisted of the following for each of the three years ended December 31, 2014, 2013 and 2012 (in thousands):
Year ended December 31, | |||||||||||
2014 | 2013 | 2012 | |||||||||
Revolving Credit Facility | $ | 1,829 | $ | 873 | $ | — | |||||
Term Loan Facilities | 16,820 | 10,313 | — | ||||||||
2021 Notes | 15,926 | — | — | ||||||||
Prior Credit Facility | — | 765 | 2,675 | ||||||||
2015 Notes | — | 13,960 | 23,063 | ||||||||
Amortization of deferred financing costs | 3,691 | 2,259 | 1,261 | ||||||||
Amortization of debt discount | 462 | — | — | ||||||||
Expense allocated to discontinued operations | — | 41 | (761 | ) | |||||||
Other, net | (189 | ) | (13 | ) | (170 | ) | |||||
Interest expense, net | $ | 38,539 | $ | 28,198 | $ | 26,068 | |||||
The weighted average interest rate on the Company’s short-term borrowings under its revolving credit facilities during the years ended December 31, 2014 and 2013 was 8.55% and 5.43%, respectively.
Liquidity
The Company expects that cash generated from operating activities combined with available borrowings under the Revolving Credit Facility will be sufficient to fund anticipated working capital, information technology systems investments, scheduled interest repayments and other cash needs for at least the next twelve months, based on historical levels.
Cash generated from operations turned positive in the second half of 2014 as the Company increased cash collections and reduced days sales outstanding (“DSO”). The Company’s plan in 2015 is to continue to reduce DSO and tightly manage operating expenses. Under the terms of its credit facility, a springing covenant becomes applicable if the Company’s borrowings on the revolver are over 25% of availability on the last day of the quarter. The covenant is a consolidated first lien net leverage ratio which uses first lien debt net of cash divided by last twelve months Adjusted EBITDA as defined in the credit facility. Should DSO rise, or if other unforeseen needs for liquidity develop, or if the Company does not manage cash to ensure compliance with debt covenants, the Company would pursue alternate financing arrangements to meet its working capital requirements. From time to time the Company may evaluate market conditions and financing options that would improve its current liquidity profile and enhance its financial flexibility. This may include, but is not limited to, opportunities to raise additional funds through the issuance of various forms of equity and/or debt securities or other instruments. However, there is no assurance that, if necessary, the Company would be able to raise capital to provide required liquidity.
86
NOTE 10-- COMMITMENTS AND CONTINGENCIES
Legal Proceedings
United States Attorney’s Office for the Southern District of New York and New York State Attorney General investigation
Effective January 8, 2014, the Company entered into the Federal Settlement Agreement with the DOJ and David M. Kester (the “Relator”). The Federal Settlement Agreement represented the federal and private component of the Company’s agreement to settle all civil claims under the False Claims Act and related statutes and all common law claims (collectively, the “Claims”) that could have been brought by the DOJ and Relator in the qui tam lawsuit filed in the Southern District of New York (the “SDNY”) by the Relator relating to the distribution of the Medication by the Company’s legacy specialty pharmacy division (the “Legacy Division”) that was divested in May 2012 (the “Civil Action”). Until January 8, 2014, the Company was prohibited from publicly disclosing any information related to the existence of the Civil Action. On January 8, 2014, the Civil Action was unsealed and made public on order of the court. Effective February 11, 2014, the Company entered into the State Settlement Agreements with the Settling States. The State Settlement Agreements represented the state component of the Company’s agreement to settle the Claims that could have been brought by the Settling States that arose out of the Legacy Division’s distribution of the Medication.
With the execution of the Federal Settlement Agreement and the State Settlement Agreements (collectively the “Settlement Agreements”), the Company expects the Civil Action to be fully resolved, and also expects to be fully resolved the federal and state claims that were or could have been raised in the Civil Action. All federal claims and all state claims by the Settling States that have been or could be brought against it in the Civil Action have been dismissed with prejudice. The State Settlement Agreements expressly recognize and affirmatively provide that, by entering into the State Settlement Agreements, the Company has not made any admission of liability and the Company expressly denies the allegations in the Civil Action.
As a part of the State Settlement Agreements, the Company has also resolved any and all claims that the Settling States or their representatives, including the National Association of Medicaid Fraud Control Units (the “NAMFCU”) (which represented the offices of the Attorneys General of the Settling States), could bring for attorney’s fees, investigative fees and/or administrative costs related to the Civil Action. The Company has also separately resolved any and all claims for certain investigative/administrative costs and attorney’s fees related to the Civil Action incurred by the DOJ, Relator and the NAMFCU for approximately $1.1 million in the aggregate. The Company does not anticipate any further claims relating to the matters involved in the Settlement Agreements. The Settlement Agreements do not, however, preclude the U.S. Department of Health and Human Services, the Office of the Inspector General or any state from taking any administrative actions.
Under the Settlement Agreements, the Company will pay an aggregate of $15.0 million, plus interest (at an annual rate of 3.25%) in three approximately annual payments from January 2014 through January 2016. The Settlement Agreements represented a compromise to avoid the costs, distraction and uncertainty of protracted litigation. The Settlement Agreements do not include any admission of wrongdoing, illegal activity, or liability by the Company or its employees, directors, officers or agents.
During the year ended December 31, 2013, the Company included in its results of discontinued operations an accrual of $15.0 million in connection with the government’s investigation regarding certain operations of the Legacy Division. As of December 31, 2014, the Company has paid $3.0 million, including interest, related to the Settlement Agreements and $450,000 of fees to the Relator.
Securities Class Action Litigation in the Southern District of New York
On September 30, 2013, a putative securities class action lawsuit was filed against the Company and certain of its officers on behalf of the putative class of purchasers of our securities between August 8, 2011 and September 20, 2013, inclusive.
On November 15, 2013, a putative securities class action lawsuit was filed against the Company and certain of its directors and officers and certain underwriters in the Company’s April 2013 underwritten public offering of its common stock, on behalf of the putative class of purchasers of our securities between August 8, 2011 and September 23, 2013, inclusive.
On December 19, 2013, the United States District Court for the SDNY entered an order consolidating the two class action lawsuits and appointing a lead plaintiff. The Company denies any allegations of wrongdoing in the consolidated class action lawsuit. The lead plaintiff filed a consolidated complaint on February 19, 2014 against the Company, certain of its directors and officers, certain underwriters in the Company’s April 2013 underwritten public offering of its common stock, and a certain stockholder of the Company. The consolidated complaint is brought on behalf of a putative class of purchasers of the Company’s securities between November 9, 2012 and November 6, 2013, inclusive, and persons and entities who purchased the Company’s securities pursuant or traceable to two underwritten public offerings of the Company’s common stock conducted in April 2013,
87
and August 2013. The consolidated complaint alleges generally that the defendants made material misstatements and/or failed to disclose matters related the Legacy Division’s distribution of the Medication as well as the Company’s PBM Services segment. The consolidated complaint asserts claims under Sections 11, 12(a)(2) and 15 of the Securities Act and Sections 10(b) and 20(a) of the Exchange Act and Rule 10b-5 promulgated thereunder. All defendants in the case moved to dismiss the consolidated complaint on April 28, 2014. Briefing on the motion to dismiss was complete on July 28, 2014. The Company believes all of the claims in these class action lawsuits are without merit and intends to vigorously defend against these claims. However, there is no assurance that the Company will be successful in its defense or that insurance will be available or adequate to fund any settlement or judgment or the litigation costs of these actions. Additional similar lawsuits may be filed. Moreover, the Company is not able to predict the outcome or reasonably estimate a range of possible loss at this time.
Professional Home Care Services Litigation
On March 31, 2009, Professional Home Care Services, Inc. (“PHCS”), a subsidiary of the Company, was sued by Alexander Infusion, LLC, a New York-based home infusion company (“Alexander Infusion”), in the Supreme Court of the State of New York (the “Lawsuit”). The complaint alleged principally breach of contract arising in connection with PHCS’s failure to consummate an acquisition of Alexander Infusion after failing to satisfy the conditions to PHCS’s obligation to close. On April 4, 2014, PHCS and the Company entered into a settlement agreement with Alexander Infusion and its affiliate Avantiscripts, LLC (collectively the “Alexander Parties”) to resolve all outstanding claims arising out of the Lawsuit in exchange for payment by PHCS to the Alexander Parties in the amount of $325,000, and the Lawsuit was dismissed on April 8, 2014. The Company did not pay any cash under the settlement agreement. Rather, the settlement amount of $325,000 was offset against an amount of $325,000 on accounts receivable due to the Company from the Alexander Parties. In addition, under the merger agreement dated as of January 24, 2010, by and among the Company, CHS and the former CHS stockholders, the former CHS stockholders agreed to indemnify the Company, subject to certain limits, in connection with any losses arising from claims made in respect of the acquisition agreement entered into between PHCS and Alexander Infusion.
PBM Services Payment Delay
The Company had historically engaged a third party processor to process PBM Services cash card claims. The third party processor has ceased paying amounts due to the Company. As of December 31, 2014, the total amount owed to the Company is approximately $6.8 million. The Company has initiated arbitration to collect approximately $6.8 million due from the third party processor. As of December 31, 2014, no reserve has been provided for the amounts due to the Company as we believe the amounts owed will be paid in full however there are uncertainties around any arbitration process.
Government Regulation
Various federal and state laws and regulations affecting the healthcare industry do or may impact the Company’s current and planned operations, including, without limitation, federal and state laws prohibiting kickbacks in government health programs, federal and state antitrust and drug distribution laws, and a wide variety of consumer protection, insurance and other state laws and regulations. While management believes the Company is in substantial compliance with all existing laws and regulations material to the operation of its business, such laws and regulations are subject to rapid change and often are uncertain in their application. As controversies continue to arise in the healthcare industry, federal and state regulation and enforcement priorities in this area can be expected to increase, the impact of which cannot be predicted.
From time to time, the Company responds to subpoenas and requests for information from governmental agencies. The Company cannot predict with certainty what the outcome of any of the foregoing might be. While the Company believes it is in substantial compliance with all laws, rules and regulations that affects its business and operations, there can be no assurance that the Company will not be subject to scrutiny or challenge under one or more existing laws or that any such challenge would not be successful. Any such challenge, whether or not successful, could have a material effect upon the Company’s Consolidated Financial Statements. A violation of the Federal anti-kickback statute, for example, may result in substantial criminal penalties, as well as suspension or exclusion from the Medicare and Medicaid programs. Moreover, the costs and expenses associated with defending these actions, even where successful, can be significant. Further, there can be no assurance the Company will be able to obtain or maintain any of the regulatory approvals that may be required to operate its business, and the failure to do so could have a material effect on the Company’s Consolidated Financial Statements.
Leases
The Company leases its facilities and certain equipment under various operating leases with third parties. The majority of these leases contain escalation clauses that increase base rent payments based upon either the Consumer Price Index or an agreed upon schedule.
88
In addition, the Company utilizes capital leases agreements with third parties to obtain certain assets such as telecommunications equipment and vehicles. Interest rates on capital leases are both fixed and variable and range from 3% to 7%.
As of December 31, 2014, future minimum lease payments under operating and capital leases were as follows (in thousands):
Operating Leases | Capital Leases | Total | |||||||||
2015 | $ | 8,590 | $ | 418 | $ | 9,008 | |||||
2016 | 7,243 | 122 | 7,365 | ||||||||
2017 | 6,205 | 62 | 6,267 | ||||||||
2018 | 4,308 | 12 | 4,320 | ||||||||
2019 | 2,634 | — | 2,634 | ||||||||
Thereafter | 2,333 | — | 2,333 | ||||||||
Total Future Minimum Lease Payments | $ | 31,313 | $ | 614 | $ | 31,927 | |||||
Rent expense for leased facilities and equipment was approximately $7.6 million, $7.9 million and $6.2 million for the years ended December 31, 2014, 2013 and 2012, respectively.
Purchase Commitments
As of December 31, 2014, the Company had commitments to purchase prescription drugs from drug manufacturers of approximately $47.4 million in 2015. These purchase commitments are made at levels expected to be used in the normal course of business.
NOTE 11-- OPERATING AND REPORTABLE SEGMENTS
Following the sale of substantially all of the Company’s Home Health Services segment on March 31, 2014, the Company’s operating and reportable segments, “Infusion Services,” and “PBM Services,” reflect how the Company’s chief operating decision maker reviews the Company’s results in terms of allocating resources and assessing performance. All prior period Segment Operating Results and Supplementary Data have been reclassified to exclude the Home Health Services segment.
The Infusion Services operating and reportable segment provides services consisting of home infusion therapy, respiratory therapy and the provision of durable medical equipment, products and services. Infusion services include the dispensing and administering of infusion-based drugs, which typically require nursing support and clinical management services, equipment to administer the correct dosage and patient training designed to improve patient outcomes.
The PBM Services operating and reportable segment consists of PBM services, which primarily consists of discount card programs. The discount card programs provide a cost effective alternative for individuals who may be uninsured, underinsured or may have restrictive coverage that disallows reimbursement for certain medications. Under these discount programs, individuals who present a discount card at one of the Company’s participating network pharmacies receive prescription medications at a discounted price compared to the retail price. In addition, in the Company’s capacity as a pharmacy benefit manager, it has fully funded prescription benefit programs where the Company reimburses its network pharmacies and third party payors in turn reimburse the Company based on Medi-Span reported pricing for those claims fulfilled for their plan participants.
The Company’s chief operating decision maker evaluates segment performance and allocates resources based on Segment Adjusted EBITDA. Segment Adjusted EBITDA is defined as income (loss) from continuing operations, net of income taxes adjusted for net interest expense, income tax expense (benefit), depreciation, amortization of intangibles and stock-based compensation expense and prior to the allocation of certain corporate expenses. Segment Adjusted EBITDA excludes acquisition, integration and transitional expenses; restructuring and other expense; and other expenses related to the Company’s strategic assessment. Segment Adjusted EBITDA also excludes the operating losses of start-up branch locations that the Company has invested in organically rather than through acquisition. Segment Adjusted EBITDA is a measure of earnings that management monitors as an important indicator of operating and financial performance. The accounting policies of the operating and reportable segments are consistent with those described in the Company's summary of significant accounting policies.
89
Segment Reporting Information (in thousands) | |||||||||||
Year Ended December 31, | |||||||||||
2014 | 2013 | 2012 | |||||||||
Results of Operations: | |||||||||||
Revenue: | |||||||||||
Infusion Services - product revenue | $ | 901,653 | $ | 675,684 | $ | 471,506 | |||||
Infusion Services - service revenue | 21,001 | 21,182 | 10,080 | ||||||||
Total Infusion Services revenue | 922,654 | 696,866 | 481,586 | ||||||||
PBM Services - service revenue | 61,401 | 72,592 | 111,861 | ||||||||
Total revenue | $ | 984,055 | $ | 769,458 | $ | 593,447 | |||||
Adjusted EBITDA by Segment before corporate overhead: | |||||||||||
Infusion Services | $ | 6,501 | $ | 60,686 | $ | 35,421 | |||||
PBM Services | 6,731 | 17,094 | 25,644 | ||||||||
Total Segment Adjusted EBITDA | 13,232 | 77,780 | 61,065 | ||||||||
Corporate overhead | (36,264 | ) | (32,042 | ) | (26,755 | ) | |||||
Interest expense, net | (38,539 | ) | (28,198 | ) | (26,068 | ) | |||||
Loss on extinguishment of debt | (2,373 | ) | (15,898 | ) | — | ||||||
Income tax benefit (expense) | (11,391 | ) | (2,523 | ) | 7,117 | ||||||
Depreciation | (16,388 | ) | (13,381 | ) | (8,367 | ) | |||||
Amortization of intangibles | (6,555 | ) | (6,671 | ) | (3,957 | ) | |||||
Stock-based compensation expense | (8,570 | ) | (9,450 | ) | (6,122 | ) | |||||
Acquisition and integration expenses | (17,924 | ) | (16,130 | ) | (4,046 | ) | |||||
Restructuring and other expenses and investments | (18,610 | ) | (10,478 | ) | (5,142 | ) | |||||
Loss from continuing operations, net of income taxes | $ | (143,382 | ) | $ | (56,991 | ) | $ | (12,275 | ) | ||
90
Segment Reporting Information (continued) (in thousands) | |||||||||||
Year Ended December 31, | |||||||||||
2014 | 2013 | 2012 | |||||||||
Supplemental Operating Data: | |||||||||||
Capital Expenditures: | |||||||||||
Infusion Services | $ | 9,754 | $ | 15,972 | $ | 6,685 | |||||
PBM Services | — | — | — | ||||||||
Corporate unallocated | 4,075 | 9,553 | 3,973 | ||||||||
Total Capital Expenditures | $ | 13,829 | $ | 25,525 | $ | 10,658 | |||||
Depreciation Expense: | |||||||||||
Infusion Services | $ | 10,203 | $ | 8,541 | $ | 4,312 | |||||
PBM Services | — | — | — | ||||||||
Corporate unallocated | 6,185 | 4,840 | 4,055 | ||||||||
Total Depreciation Expense | $ | 16,388 | $ | 13,381 | $ | 8,367 | |||||
Total Assets: | |||||||||||
Infusion Services | $ | 755,955 | $ | 793,475 | $ | 438,171 | |||||
PBM Services | 29,147 | 25,239 | 36,354 | ||||||||
Corporate unallocated | 39,611 | 53,169 | 95,423 | ||||||||
Assets from discontinued operations | — | 64,959 | 63,245 | ||||||||
Assets associated with discontinued operations, not sold | — | 16 | 9,183 | ||||||||
Total Assets | $ | 824,713 | $ | 936,858 | $ | 642,376 | |||||
Goodwill: | |||||||||||
Infusion Services | $ | 560,579 | $ | 558,593 | $ | 304,282 | |||||
PBM Services | 12,744 | 12,744 | 12,744 | ||||||||
Total Goodwill | $ | 573,323 | $ | 571,337 | $ | 317,026 | |||||
NOTE 12-- CONCENTRATION OF RISK
Customer and Credit Concentration Risk
The Company provides trade credit to its customers in the normal course of business. One commercial payor, UnitedHealthcare, accounted for approximately 22%, 22% and 21% of revenue during the years ended December 31, 2014, 2013 and 2012, respectively. Medicare accounted for 11%, 11% and 9% of revenue during the years ended December 31, 2014, 2013 and 2012, respectively.The majority of the revenue is related to the Infusion Services segment.
Therapy Revenue Concentration Risk
The Company sells products related to the Immune Globulin (IG) therapy, which represented 17%, 19%, and 22% of revenue during the years ended December 31, 2014, 2013 and 2012, respectively. The revenue is related to the Infusion Services segment.
91
NOTE 13-- INCOME TAXES
The Company’s federal and state income tax provision (benefit) from continuing operations is summarized in the following table (in thousands):
Year Ended December 31, | |||||||||||
2014 | 2013 | 2012 | |||||||||
Current | |||||||||||
Federal | $ | (886 | ) | $ | (866 | ) | $ | (6,095 | ) | ||
State | (41 | ) | (1,412 | ) | (990 | ) | |||||
Total current | (927 | ) | (2,278 | ) | (7,085 | ) | |||||
Deferred | |||||||||||
Federal | 9,951 | 4,424 | 97 | ||||||||
State | 2,367 | 377 | (129 | ) | |||||||
Total deferred | 12,318 | 4,801 | (32 | ) | |||||||
Total tax provision (benefit) | $ | 11,391 | $ | 2,523 | $ | (7,117 | ) | ||||
The effect of temporary differences that give rise to a significant portion of deferred taxes is as follows (in thousands):
December 31, | |||||||
2014 | 2013 | ||||||
Deferred tax assets: | |||||||
Reserves not currently deductible | $ | 28,424 | $ | 9,785 | |||
Net operating loss carryforwards | 65,097 | 39,265 | |||||
Goodwill and intangibles (tax deductible) | 8,499 | 7,556 | |||||
Accrued expenses | 38 | — | |||||
Property basis differences | 301 | — | |||||
Stock based compensation | 8,201 | 6,277 | |||||
Other | 610 | 417 | |||||
Total deferred tax assets | 111,170 | 63,300 | |||||
Deferred tax liabilities: | |||||||
Property basis differences | — | (496 | ) | ||||
Accrued expenses | — | (679 | ) | ||||
Indefinite-lived goodwill and intangibles | (21,272 | ) | (9,969 | ) | |||
Less: valuation allowance | (111,170 | ) | (61,110 | ) | |||
Net deferred tax liability | (21,272 | ) | (8,954 | ) | |||
Less: Amount included in accrued expenses and other current liabilities | (2,214 | ) | (2,019 | ) | |||
Deferred taxes | $ | (19,058 | ) | $ | (6,935 | ) | |
During the fourth quarter of 2010, the Company concluded that it was more likely than not that its deferred tax assets would not be realized. The Company continually assesses the necessity of a valuation allowance. Based on this assessment, the Company concluded that a valuation allowance, in the amount of $111.2 million and $61.1 million, was required as of December 31, 2014 and 2013, respectively. If the Company determines in a future period that it is more likely than not that part or all of the deferred tax assets will be realized, the Company will reverse part or all of the valuation allowance.
At December 31, 2014, the Company had federal net operating loss (“NOL”) carryforwards of approximately $174.9 million, of which $21.1 million is subject to an annual limitation, which will begin expiring in 2026 and later. Of the Company’s $174.9 million federal NOLs, $18.0 million will be recorded in additional paid-in capital when realized as these NOLs are related to the exercise of non-qualified stock options and restricted stock grants. The Company has post-apportioned state NOL carryforwards of approximately $245.7 million, the majority of which will begin expiring in 2017 and later.
92
The Company’s reconciliation of the statutory rate to the effective income tax rate from continuing operations is as follows (in thousands):
Year Ended December 31, | |||||||||||
2014 | 2013 | 2012 | |||||||||
Tax (benefit) provision at statutory rate | $ | (46,197 | ) | $ | (18,107 | ) | $ | (6,788 | ) | ||
State tax (benefit) provision, net of federal taxes | (3,704 | ) | (2,459 | ) | (901 | ) | |||||
Valuation allowance changes affecting income tax expense | 61,227 | 23,762 | 1,077 | ||||||||
Change in tax contingencies | (109 | ) | (1,157 | ) | (633 | ) | |||||
Non-deductible transaction costs | — | 317 | — | ||||||||
Other | 174 | 167 | 128 | ||||||||
Tax provision (benefit) | $ | 11,391 | $ | 2,523 | $ | (7,117 | ) | ||||
As of December 31, 2014, the Company had $1.1 million of gross unrecognized tax benefits, of which $0.1 million, if recognized, would favorably affect the effective income tax rate in future periods. A reconciliation of the beginning and ending amount of gross unrecognized tax benefits is as follows (in thousands):
Year Ended December 31, | |||||||||||
2014 | 2013 | 2012 | |||||||||
Unrecognized tax benefits balance at January 1, | $ | 1,172 | $ | 2,754 | $ | 2,605 | |||||
Gross increases for tax positions taken in current year | — | — | 636 | ||||||||
Lapse of statute of limitations | (76 | ) | (1,582 | ) | (487 | ) | |||||
Unrecognized tax benefits balance at December 31, | $ | 1,096 | $ | 1,172 | $ | 2,754 | |||||
The Company’s policy for recording interest and penalties associated with uncertain tax positions is to record such items as a component of income tax expense in the Consolidated Statements of Operations. As of December 31, 2014 and 2013, the Company had approximately $0.1 million and $0.1 million of accrued interest related to uncertain tax positions, respectively.
The Company files income tax returns, including returns for its subsidiaries, with federal, state and local jurisdictions. The Company’s uncertain tax positions are related to tax years that remain subject to examination. As of December 31, 2014, U.S. tax returns for the years 2011 through 2014 remain subject to examination by federal tax authorities. Tax returns for the years 2010 through 2014 remain subject to examination by state and local tax authorities for a majority of the Company's state and local filings.
NOTE 14-- STOCK-BASED COMPENSATION
BioScrip Equity Incentive Plans
Under the Company’s Amended and Restated 2008 Equity Incentive Plan (the “2008 Plan”), the Company may issue, among other things, incentive stock options (“ISOs”), non-qualified stock options (“NQSOs”), stock appreciation rights (“SARs”), restricted stock, performance shares and performance units to key employees and directors. While SARS are authorized under the 2008 Plan, they may also be issued outside of the plan. The 2008 Plan is administered by the Company's Management Development and Compensation Committee (the “Compensation Committee”), a standing committee of the Board of Directors.
On May 7, 2013, the Company’s stockholders approved an amendment to the 2008 Plan to increase, by 300,000 shares (from 500,000 to 800,000), the aggregate number of shares within the 2008 Plan that may be granted to directors.
On May 8, 2014, the Company’s stockholders (i) approved an amendment to the 2008 Plan to increase the number of authorized shares of common stock available for issuance by 2,500,000 shares (the “2014 Additional Shares”) to 9,355,000 shares and to clarify that cash dividends or dividend equivalents may not be paid to holders of unvested restricted stock units, restricted stock grants and performance units until such awards are vested and non-forfeitable; and (ii) re-approved the material terms of the performance goals that are a part of the 2008 Plan. On September 19, 2014, the Company filed a Registration Statement on Form S-8 to register the issuance of the 2014 Additional Shares that were approved by the Company’s stockholders on May 8, 2014.
As of December 31, 2014, there were 1,874,672 shares that remained available for grant under the 2008 Plan.
93
BioScrip/CHS Equity Plan
Effective upon closing of the acquisition of CHS, the CHS 2006 Equity Incentive Plan was adopted by the Company and renamed the “BioScrip/CHS 2006 Equity Incentive Plan”. In connection with the May 8, 2014 amendment to the 2008 Plan noted above, the Company determined to cease issuance of equity instruments from the BioScrip/CHS 2006 Equity Incentive Plan. As of December 31, 2014, no shares remained available under the BioScrip/CHS Plan.
Annual Equity Grants
During the year ended December 31, 2014, the Compensation Committee approved grants of approximately 1.9 million NQSO awards and 0.1 million restricted stock awards to key employees and members of the board of directors consistent with the Compensation Committee’s historic grant practices.
Stock Options
Options granted under the Equity Compensation Plans: (a) typically vest over a three-year period and, in certain instances, fully vest upon a change in control of the Company, (b) have an exercise price that may not be less than 100% of its fair market value on the date of grant and (c) are generally exercisable for ten years after the date of grant, subject to earlier termination in certain circumstances.
Option expense is amortized on a straight-line basis over the requisite service period. The Company recognized compensation expense related to stock options of $6.9 million, $6.0 million, and $4.6 million, in the years ended December 31, 2014, 2013 and 2012, respectively.
The weighted-average, grant-date fair value of options granted during the years ending December 31, 2014, 2013 and 2012 was $4.32, $6.24, and $4.00, respectively. A binomial lattice-based valuation model is used to estimate the fair value of each option granted. Because of the limitations with closed-form valuation models, such as the Black-Scholes model, we have determined that this more flexible binomial model provides a better estimate of the fair value of our options. The fair value of each stock option award on the date of the grant was calculated using the following weighted-average assumptions:
2014 | 2013 | 2012 | |||||||||
Expected volatility | 61.0 | % | 61.8 | % | 64.8 | % | |||||
Risk-free interest rate | 2.50 | % | 2.13 | % | 1.98 | % | |||||
Expected life of options | 5.7 years | 5.5 years | 5.8 years | ||||||||
Dividend rate | — | — | — | ||||||||
Fair value of options | $ | 4.32 | $ | 6.24 | $ | 4.00 | |||||
Stock option activity for the Equity Compensation Plans through December 31, 2014 was as follows:
Options | Weighted Average Exercise Price | Aggregate Intrinsic Value (thousands) | Weighted Average Remaining Contractual Life | |||||||||
Balance at December 31, 2013 | 5,732,821 | $ | 7.59 | $ | 6,185 | 7.7 years | ||||||
Granted | 1,906,000 | $ | 7.68 | |||||||||
Exercised | (272,622 | ) | $ | 5.38 | ||||||||
Forfeited and expired | (529,193 | ) | $ | 8.33 | ||||||||
Balance at December 31, 2014 | 6,837,006 | $ | 7.65 | $ | 4,150 | 7.4 years | ||||||
Outstanding options less expected forfeitures at December 31, 2014 | 6,415,834 | $ | 7.56 | $ | 4,127 | 7.4 years | ||||||
Exercisable at December 31, 2014 | 3,523,697 | $ | 6.69 | $ | 3,933 | 6.2 years | ||||||
Cash received from option exercises under share-based payment arrangements for the years ended December 31, 2014, 2013, and 2012 was $1.5 million, $2.5 million, and $8.6 million, respectively.
94
The maximum term of stock options under these plans is ten years. Options outstanding as of December 31, 2014 expire on various dates ranging from March 2015 through October 2024. The following table outlines our outstanding and exercisable stock options as of December 31, 2014:
Options Outstanding | Options Exercisable | |||||||||||||||
Range of Option Exercise Price | Outstanding Options | Weighted Average Exercise Price | Weighted Average Remaining Contractual Life | Options Exercisable | Weighted Average Exercise Price | |||||||||||
$1.71 - $4.24 | 409,250 | $ | 2.73 | 4.4 years | 409,250 | $ | 2.73 | |||||||||
$4.42 - $6.61 | 981,256 | $ | 5.16 | 5.7 years | 932,756 | $ | 5.12 | |||||||||
$6.62- $6.65 | 1,681,000 | $ | 6.63 | 6.8 years | 1,226,000 | $ | 6.63 | |||||||||
$6.67 - $9.16 | 2,471,500 | $ | 7.82 | 8.6 years | 524,334 | $ | 8.27 | |||||||||
$11.04 - $16.63 | 1,294,000 | $ | 12.08 | 8.2 years | 431,357 | $ | 12.08 | |||||||||
All options | 6,837,006 | $ | 7.65 | 7.4 years | 3,523,697 | $ | 6.69 | |||||||||
As of December 31, 2013 and 2012 the exercisable portion of outstanding options was approximately 2.5 million shares and 1.9 million shares, respectively.
As of December 31, 2014 there was $9.3 million of unrecognized compensation expense related to unvested option grants that is expected to be recognized over a weighted-average period of 1.9 years. The total intrinsic value of options exercised during the years December 31, 2014, 2013 and 2012 was $0.6 million, $3.8 million, and $4.4 million, respectively.
As compensation expense for options granted is recorded over the requisite service period of options, future stock-based compensation expense may be greater as additional options are granted.
Restricted Stock
Under the Equity Compensation Plans, stock grants subject solely to an employee’s or director’s continued service with the Company will not become fully vested less than (a) three years from the date of grant to employees and, in certain instances, may fully vest upon a change in control of the Company, and (b) one year from the date of grant for directors. Stock grants subject to the achievement of performance conditions will not vest less than one year from the date of grant. Such performance shares may vest after one year from grant. No such time restrictions applied to stock grants made under the Company’s prior equity compensation plans.
The Company recognized compensation expense related to restricted stock awards of $1.6 million, $3.5 million, and $0.4 million for the years ended December 31, 2014, 2013 and 2012, respectively.
Since the Company records compensation expense for restricted stock awards based on the vesting requirements, which generally includes time elapsed, market conditions and/or performance conditions, the weighted average period over which the expense is recognized varies. Also, future equity-based compensation expense may be greater if additional restricted stock awards are made.
Each share of restricted stock issued reduces the number of shares available for grant under the Equity Compensation Plans by 1.53 shares.
Restricted stock award activity through December 31, 2014 was as follows:
Restricted Stock | Weighted Average Award Date Fair Value | Weighted Average Remaining Recognition Period | ||||||
Balance at December 31, 2013 | 370,000 | $ | 12.31 | 0.3 years | ||||
Granted | 100,000 | $ | 6.25 | |||||
Awards Vested | (290,003 | ) | $ | 12.08 | ||||
Canceled | — | $ | — | |||||
Balance at December 31, 2014 | 179,997 | $ | 9.31 | 0.4 years | ||||
95
As of December 31, 2014, there was $0.2 million of unrecognized compensation expense related to unvested restricted stock awards. That expense is expected to be recognized over a weighted average period of 0.4 years. The total grant date fair value of awards vested during the years ended December 31, 2014, 2013 and 2012 was $3.5 million, $0.5 million, and $0.8 million, respectively. The total fair value of restricted stock awards vested during the years December 31, 2014, 2013 and 2012 was $2.0 million, $0.5 million, and $2.3 million, respectively.
Performance Units
Under the 2008 Plan, the Compensation Committee may grant performance units to key employees. The Compensation Committee will establish the terms and conditions of any performance units granted, including the performance goals, the performance period and the value for each performance unit. If the performance goals are satisfied, the Company would pay the key employee an amount in cash equal to the value of each performance unit at the time of payment. In no event may a key employee receive an amount in excess of $1.0 million with respect to performance units for any given year. As of December 31, 2014, no performance units have been granted under the 2008 Plan.
Stock Appreciation Rights
The Company has granted and has outstanding cash-based phantom stock appreciation rights (“SARs”), which are independent of the Company's 2008 Equity Incentive Plan, with respect to 320,000 shares of the Company's common stock. The SARs vest in three equal annual installments and will fully vest in connection with a change of control (as defined in the grantee’s employment agreement). The SARs may be exercised, in whole or in part, to the extent each SAR has been vested and will receive in cash the amount by which the closing stock price on the exercise date exceeds the Grant Price, if any. Upon the exercise of any SARs, as soon as practicable under the applicable federal and state securities laws, the grantee may be required to use the net after-tax proceeds of such exercise to purchase shares of the Common Stock from the Company at the closing stock price of the Common Stock on that date and hold such shares of Common Stock for a period of not less than one year from the date of purchase, except that the grantee will not be required to purchase any shares of Common Stock if the SAR is exercised on or after a change of control of the Company. The grantee’s right to exercise the SAR will expire on the earliest of (1) the tenth anniversary of the grant date, or (2) under certain conditions as a result of termination of the grantee’s employment.
SAR activity through December 31, 2014 was as follows:
Stock Appreciation Rights | Weighted Average Exercise Price | Weighted Average Remaining Recognition Period | ||||||
Balance at December 31, 2013 | 330,000 | $ | 6.73 | 1.7 years | ||||
Granted | — | $ | — | |||||
Exercised | — | $ | — | |||||
Canceled | (10,000 | ) | $ | 9.16 | ||||
Balance at December 31, 2014 | 320,000 | $ | 6.65 | 0.3 years | ||||
The SARs are recorded as a liability in other non-current liabilities in the accompanying Consolidated Balance Sheets. Compensation expense (benefit) related to the SARs for the year ended December 31, 2014, 2013 and 2012 was $(20) thousand, $(48) thousand and $1.1 million As of December 31, 2014 there was $0.1 million of unrecognized compensation expense related to the SARs that is expected to be recognized over a weighted-average period of 0.3 years. In addition, because they are settled with cash, the fair value of the SAR awards is revalued on a quarterly basis. During the years ended December 31, 2014, 2013 and 2012 the Company paid $0, $0 and $0.3 million related to the exercise of SAR awards.
Employee Stock Purchase Plan
On May 7, 2013, the Company’s stockholders approved the BioScrip, Inc. Employee Stock Purchase Plan (the “ESPP”). The ESPP provides all eligible employees, as defined under the ESPP, the opportunity to purchase up to a maximum number of shares of Common Stock of the Company as determined by the Compensation Committee. Participants in the ESPP may acquire the Common Stock at a cost of 85% of the lower of the fair market value on the first or last day of the Plan Year from January 1st through December 31st. The Company has filed a Registration Statement on Form S-8 to register 750,000 shares of Common Stock for issuance under the ESPP. As of December 31, 2014, the ESPP has not yet been implemented and, as a result, no shares have been issued and no expense has been incurred related to the ESPP.
96
NOTE 15-- DEFINED CONTRIBUTION PLAN
The Company maintains a deferred compensation plan under Section 401(k) of the Internal Revenue Code. Under the Plan, employees may elect to defer up to 100% of their salary, subject to Internal Revenue Service limits, and the Company may make a discretionary matching contribution. The Company recorded matching contributions in selling, general and administrative expenses in the Consolidated Statements of Operations of $1.6 million and $0.5 million during the years ended December 31, 2014 and 2013. The Company elected not to a make matching contributions during the year ended December 31, 2012.
NOTE 16-- SELECTED QUARTERLY FINANCIAL DATA (UNAUDITED)
A summary of unaudited quarterly financial information for the years ended December 31, 2014 and 2013 is as follows (in thousands except per share data):
First Quarter | Second Quarter | Third Quarter | Fourth Quarter | ||||||||||||
Year ended December 31, 2014 | |||||||||||||||
Revenue | $ | 239,293 | $ | 247,125 | $ | 243,959 | $ | 253,678 | |||||||
Gross profit | $ | 65,100 | $ | 65,356 | $ | 65,002 | $ | 65,608 | |||||||
Loss from continuing operations, before income taxes | $ | (21,765 | ) | $ | (15,548 | ) | $ | (35,645 | ) | $ | (59,033 | ) | |||
Net loss from discontinued operations, net of income taxes | $ | (58 | ) | $ | (1,207 | ) | $ | (1,135 | ) | $ | (1,686 | ) | |||
Net loss | $ | (25,314 | ) | $ | (19,818 | ) | $ | (38,710 | ) | $ | (63,626 | ) | |||
Loss per share from continuing operations, basic and diluted | $ | (0.37 | ) | $ | (0.27 | ) | $ | (0.55 | ) | $ | (0.90 | ) | |||
Loss per share from discontinued operations, basic and diluted | — | (0.02 | ) | (0.02 | ) | (0.02 | ) | ||||||||
Loss per share, basic and diluted | $ | (0.37 | ) | $ | (0.29 | ) | $ | (0.57 | ) | $ | (0.92 | ) | |||
Year ended December 31, 2013 | |||||||||||||||
Revenue | $ | 181,020 | $ | 172,323 | $ | 190,631 | $ | 225,484 | |||||||
Gross profit | $ | 55,987 | $ | 57,775 | $ | 61,655 | $ | 68,196 | |||||||
Loss from continuing operations, before income taxes | $ | (8,538 | ) | $ | (9,150 | ) | $ | (23,769 | ) | $ | (13,011 | ) | |||
Net income (loss) from discontinued operations, net of income taxes | $ | 235 | $ | 416 | $ | (10,331 | ) | $ | (2,983 | ) | |||||
Net loss | $ | (8,128 | ) | $ | (8,880 | ) | $ | (34,087 | ) | $ | (18,559 | ) | |||
Loss per share from continuing operations, basic and diluted | $ | (0.15 | ) | $ | (0.14 | ) | $ | (0.37 | ) | $ | (0.23 | ) | |||
Income (loss) per share from discontinued operations, basic and diluted | 0.01 | — | (0.16 | ) | (0.04 | ) | |||||||||
Loss per share, basic and diluted | $ | (0.14 | ) | $ | (0.14 | ) | $ | (0.53 | ) | $ | (0.27 | ) | |||
With the sale of the Home Health Services segment on March 31, 2014 (see Note 5 - Discontinued Operations), all prior period financial statements have been reclassified to include the Home Health Services segment as discontinued operations. In addition, the Company decided in the second quarter of 2014 to cease the material portion of its Home Health operations at the one location excluded from the Stock Purchase Agreement, as amended. As a result, the Unaudited Consolidated Statements of Operations previously reported by the Company as of March 31, 2014 have been reclassified to include this Home Health location in discontinued operations. The effect of this reclassification reduced total revenue by $0.4 million to $239.3 million and reduced loss from continuing operations, net of income taxes by $0.2 million to $25.3 million for the three months ended March 31, 2014. The reclassification had no effect on previously reported net loss or net loss per common share for the three months ended March 31, 2014.
Company recognized a partial extinguishment of debt and wrote off approximately $2.4 million f deferred financing costs during the three months ended December 31, 2014. The expense related to early repayment of the Senior Credit Facilities from the net proceeds of the sale of the Home Health Business on March 31, 2014. The Company also recognized $1.1 million of income tax expense during the three months ended December 31, 2014 that related to prior years. The tax adjustment was related to estimated state tax rates applied the years ended December 31, 2010 through 2013. The effect of the adjustments was not material to prior and current periods.
97
NOTE 17-- SUBSEQUENT EVENTS
Third Amendment to Senior Credit Facilities
On March 1, 2015, the Company entered into the Third Amendment to the Senior Credit Facilities, which establishes an alternate leverage test for the fiscal quarters ending March 31, 2015 through and including March 31, 2016. The maximum net leverage ratio for these quarters is consistent with that in effect for the prior four fiscal quarters. The amendment eliminated the need to meet progressively lower leverage ratio requirements at each quarter end date for the next four quarters. The amendment also reduces the Revolver Covenant Triggering Event from 25% of the Aggregate Revolving Commitment Amount to 5% of the Aggregate Revolving Commitment Amount beginning with the quarter ended June 30, 2015 and provides for certain additional financial reporting.
98
Item 9. | Changes in and Disagreements with Accountants on Accounting and Financial Disclosure |
Effective on September 8, 2014, the Audit Committee (the “Audit Committee”) of the Company’s Board of Directors (the “Board”) approved the appointment of KPMG LLP to serve as the Company’s independent registered public accounting firm. Ernst & Young LLP (“EY”), the Company’s former independent registered public accounting firm, notified the Company on September 2, 2014 that it was resigning as the Company’s independent public accounting firm effective after the Company filed its Form 10-Q for its fiscal quarter ended September 30, 2014. Incorporated herein by reference is Item 4.01 from the Current Report on Form 8-K, including the letter of EY filed as Exhibit 16.1 thereto, filed by the Company with the Commission on September 8, 2014. There were no changes in or disagreements with accountants on accounting and financial disclosure requiring disclosure pursuant to Item 304(b) of Regulation S-K.
Item 9A. | Controls and Procedures |
(a) Evaluation of Disclosure Controls and Procedures
As of the end of the period covered by this Annual Report, we evaluated the effectiveness of the design and operation of our disclosure controls and procedures. This evaluation was performed under the supervision and with the participation of management, including our Chief Executive Officer (“CEO”) and Chief Financial Officer (“CFO”). Disclosure controls and procedures (as defined in the Securities Exchange Act Rules 13a-15(e) and 15d-15(e)) are designed to ensure that information required to be disclosed in our reports filed under the Securities Exchange Act, such as this Annual Report, is recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms. Disclosure controls and procedures also include, without limitation, controls and procedures that are designed to ensure that such information is accumulated and communicated to our management, including our CEO and CFO, as appropriate, to allow timely decisions regarding required disclosure.
The evaluation of our disclosure controls and procedures included a review of the controls objectives and design, our implementation of the controls and the effect of the controls on the information generated for use in this Annual Report. In conducting this evaluation, our CEO and CFO concluded there are material weaknesses in the design and operating effectiveness of our internal control over financial reporting, as described below. As a result of such evaluation and this conclusion, our CEO and CFO also have concluded that our disclosure controls and procedures were not effective as of December 31, 2014 in ensuring that (1) information required to be disclosed in our reports filed under the Securities Exchange Act was recorded, processed, summarized and reported within the time periods prescribed by SEC rules and regulations, and (2) such information was accumulated and communicated to our management to allow timely decisions regarding required disclosure.
(b) Management Report on Internal Control over Financial Reporting
Our management is responsible for the preparation and accuracy of the consolidated financial statements and other information included in this Annual Report. Our management is also responsible for establishing and maintaining adequate internal control over financial reporting, as such term is defined in Securities Exchange Act Rules 13a-15(f) and 15d-15(f). Under the supervision and with the participation of management, including our CEO and CFO, we conducted an evaluation of the effectiveness of internal control over financial reporting as of December 31, 2014, based on the criteria set forth in Internal Control - Integrated Framework (1992) (the “Framework”) issued by the Committee of Sponsoring Organizations of the Treadway Commission (“COSO”). Based on this assessment, management identified material weaknesses in our internal control over financial reporting, as described below. As a result of these material weaknesses, management concluded that, as of December 31, 2014, our internal control over financial reporting was not effective based on the Framework.
A material weakness is a deficiency, or combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of the annual or interim financial statements will not be prevented or detected on a timely basis.
The following control deficiencies were identified and were determined to be material weaknesses in our internal control over financial reporting as of December 31, 2014:
• | Our internal control over the accounting for the establishment of accounts receivable related reserves and the timely recognition of bad debt expense was not designed appropriately in that the methodology averaged potential estimated reserve levels using various assumptions rather than selecting an estimate that emphasized the growth in aged balances during the year ended December 31, 2014. |
99
• | Our internal controls over significant and unusual transactions were not designed appropriately to ensure that the related accounting conclusions were sufficiently reviewed for compliance with generally accepted accounting principles. |
• | Our general information technology controls (“GITCs”) intended to ensure that access to certain data is restricted to the appropriate personnel were not operating effectively. This impacted our ability to rely on related internal controls that used this data. |
The material weaknesses resulted in (a) an initial conclusion related to the adequacy of the allowance for doubtful accounts receivable and related bad debt expense that was materially misstated and that was corrected by the Company prior to the issuance of the annual consolidated financial statements, and (b) inaccurate conclusions related to the accounting for deferred debt issuance costs that resulted in a misstatement that also was corrected by the Company prior to the issuance of the annual consolidated financial statements, and for which a reasonable possibility existed that a material misstatement in the Company’s consolidated financial statements would not be prevented or detected on a timely basis.
KPMG LLP, our independent registered public accounting firm, has audited the effectiveness of our internal control over financial reporting as of December 31, 2014, and has issued an adverse report which is included in Item 8 of this Annual Report.
(c) Management Remediation Plan
Due to the three material weaknesses reported as of December 31, 2014, management performed additional analysis and procedures to ensure that our consolidated financial statements and schedules included in this Annual Report were presented fairly in conformity with generally accepted accounting principles and fairly present in all material respects our financial position, results of operations and cash flows for the periods presented.
Management has implemented and continues to implement changes to our internal control over financial reporting to remediate the control deficiencies that gave rise to the material weaknesses. We are undertaking the following remediation plans and actions:
• | Further improvements in our processes and analyses that support the estimate of the allowance for doubtful accounts and the related bad debt expense. |
• | Performing a comprehensive review of the need for additional corporate accounting and finance personnel, supplemented by external resources as appropriate, with the requisite skill and technical expertise to execute an effective system of internal controls related to significant and unusual transactions. |
• | Performing a comprehensive review of our GITCs, including evaluating specific roles and responsibilities to ensure sufficient maintenance of segregation of duties within our information technology environments. |
(d) Prior Material Weaknesses
Based on our assessment of the effectiveness of our internal control over financial reporting as of December 31, 2013, management identified two material weaknesses, including a material weakness in internal control over financial reporting related to the establishment of accounts receivable related reserves and the timely recognition of bad debt expense. This material weakness was the result of the aggregation of control deficiencies in the following categories:
• | Controls to aggregate and analyze historical collection patterns by major payors over the history of legacy and acquired businesses, |
• | Follow-up collection actions and tracking thereof in order to influence establishment of accounts receivable reserves, and |
• | Controls to identify and categorize the nature of claims in a denied status or various states of appeal in order to be able to assess collectability. |
This material weakness affected the establishment of accounts receivable related reserves for both contractual adjustments and bad debt. Each of these reserves could have potentially resulted in a material misstatement of the consolidated financial statements.
Remediation efforts related to this material weakness occurred throughout our business during 2014 as we integrated the acquired companies and implemented common processes, systems and measures. Specifically, the following was implemented:
• | Standardized processes, procedures, and productivity measures for intake, billing, collection and cash application processes, |
100
• | Converted all sites to a single version of our pharmacy and accounts receivable system, |
• | Centralized the cash application function and implemented technology upgrades to improve the accuracy and timeliness of cash application and secondary payor billing, and |
• | Developed a robust estimation methodology for the allowance for doubtful accounts and contractual adjustments that uses historical collection and write-off data from acquired sites. |
While we implemented the action plan noted above, we also engaged a third-party collection firm to apply temporary resources to the backlog of claims that need to be worked through the collection process. We also hired additional internal resources to bill and collect outstanding accounts receivable balances on a timely basis and to manage the revenue and collection cycle.
While progress was made in 2014 regarding the establishment of accounts receivable related reserves and the timely recognition of bad debt expense, management evaluated and tested the appropriateness of the design of our internal controls and concluded that the material weakness reported in the 2013 Form 10-K related to the establishment of accounts receivable related reserves and the timely recognition of bad debt expense still existed as of December 31, 2014.
We remediated the material weakness reported in the 2013 Form 10-K related to certain clerical errors and documentation omissions in contingent consideration calculations.
(e) Inherent Limitations on Control Systems
Because of the inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that all control issues and instances of fraud, if any, will be or have been detected. These inherent limitations include the realities that judgments in decision-making can be faulty, and that breakdowns can occur because of simple error or mistake. Additionally, controls can be circumvented by the individual acts of some persons, by collusion of two or more people, or by management override of the control. The design of any system of controls also is based in part upon certain assumptions about the likelihood of future events, and there can be no assurance that any design will succeed in achieving its stated goals under all potential future conditions; over time, controls may become inadequate because of changes in conditions, or the degree of compliance with the policies or procedures may deteriorate.
(f) Changes in Internal Control over Financial Reporting
During the three months ended December 31, 2014, we continued to improve accounts receivable billing and collections monitoring controls across all acquired locations, as described in section (d) above.
We remediated one of the material weaknesses previously reported as of December 31, 2013, related to our documentation and support of our contingent consideration determination as described in section (d) above. Other than the ongoing remediation plans described above, there have been no changes in our internal control over financial reporting that occurred during the quarter ended December 31, 2014 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
101
Report of Independent Registered Public Accounting Firm
The Board of Directors and Stockholders
BioScrip, Inc.:
We have audited BioScrip, Inc.’s internal control over financial reporting as of December 31, 2014, based on criteria established in Internal Control ‑ Integrated Framework (1992) issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). BioScrip, Inc.’s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying Management Report on Internal Control over Financial Reporting (Item 9A.(b)). Our responsibility is to express an opinion on the Company’s internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audit also included performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of the company’s annual or interim financial statements will not be prevented or detected on a timely basis. Material weaknesses related to the establishment of accounts receivable related reserves and the timely recognition of bad debt expense, significant and unusual transactions, and general information technology controls have been identified and included in management’s assessment. We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the consolidated balance sheet of BioScrip, Inc. and subsidiaries as of December 31, 2014 and the related consolidated statements of operations, stockholders’ equity, and cash flows for the year then ended. These material weaknesses were considered in determining the nature, timing, and extent of audit tests applied in our audit of the 2014 consolidated financial statements, and this report does not affect our report dated March 2, 2015, which expressed an unqualified opinion on those consolidated financial statements.
In our opinion, because of the effect of the aforementioned material weaknesses on the achievement of the objectives of the control criteria, BioScrip, Inc. has not maintained effective internal control over financial reporting as of December 31, 2014, based on criteria established in Internal Control-Integrated Framework (1992) issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO)..
/s/ KPMG LLP
Minneapolis, Minnesota
March 2, 2015
102
Item 9B. | Other Information |
None.
PART III
Item 10. | Directors, Executive Officers and Corporate Governance |
The information required by this item is incorporated by reference from the information contained in our definitive proxy statement to be filed with the SEC on or before April 30, 2015 in connection with our 2015 Annual Meeting of Stockholders.
Item 11. | Executive Compensation |
The information required by this item is incorporated by reference from the information contained in our definitive proxy statement to be filed with the SEC on or before April 30, 2015 in connection with our 2015 Annual Meeting of Stockholders.
Item 12. | Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters |
The information required by this item is incorporated by reference from the information contained in our definitive proxy statement to be filed with the SEC on or before April 30, 2015 in connection with our 2015 Annual Meeting of Stockholders.
Item 13. | Certain Relationships and Related Transactions, and Director Independence |
The information required by this item is incorporated by reference from the information contained in our definitive proxy statement to be filed with the SEC on or before April 30, 2015 in connection with our 2015 Annual Meeting of Stockholders.
Item 14. | Principal Accountant Fees and Services |
The information required by this item is incorporated by reference from the information contained in our definitive proxy statement to be filed with the SEC on or before April 30, 2015 in connection with our 2015 Annual Meeting of Stockholders.
103
PART IV
Item 15. | Exhibits, Financial Statement Schedules and Reports on Form 8-K |
Page | |
1. Financial Statements: | |
Report of Independent Registered Public Accounting Firm | |
Consolidated Balance Sheets as of December 31, 2014 and 2013 | |
Consolidated Statements of Operations for the years ended December 31, 2014, 2013 and 2012 | |
Consolidated Statements of Stockholders’ Equity for the years ended December 31, 2014, 2013 and 2012 | |
Consolidated Statements of Cash Flows for the years ended December 31, 2014, 2013 and 2012 | |
Notes to Consolidated Financial Statements | |
2. Financial Statement Schedule: | |
Valuation and Qualifying Accounts for the years ended December 31, 2014, 2013 and 2012 | |
All other schedules not listed above have been omitted since they are not applicable or are not required.
104
3. Exhibits | |
Exhibit Number | Description | Location | |
2.1 | Agreement and Plan of Merger, dated as of January 24, 2010, by and among BioScrip, Inc. (the “Company”), Camelot Acquisition Corp., Critical Homecare Solutions Holdings, Inc., Kohlberg Investors V, L.P. (“Kohlberg Investors”), Kohlberg Partners V, L.P., Kohlberg Offshore Investors V, L.P., Kohlberg TE Investors V, L.P., KOCO Investors V, L.P. (collectively with Kohlberg Investors, Kohlberg Partners V, L.P., Kohlberg Offshore Investors V, L.P. and Kohlberg TE Investors V, L.P., the “Kohlberg Entities”), Robert Cucuel, Mary Jane Graves, Nitin Patel, Joey Ryan, Blackstone Mezzanine Partners II L.P. (“Blackstone”), Blackstone Mezzanine Holdings II L.P. (together with Blackstone, the “Blackstone Entities”), and S.A.C. Domestic Capital Funding, Ltd. (“S.A.C.”). Pursuant to Item 601(b)(2) of Regulation S-K, certain schedules and exhibits to this agreement are omitted. The Company agrees to furnish supplementally a copy of any omitted schedule or exhibit to the U.S. Securities and Exchange Commission (the “SEC”) upon request. | (1) | |
2.2 | Community Pharmacy and Mail Business Purchase Agreement, dated as of February 1, 2012, by and among Walgreen Co., Walgreens Mail Service, Inc., Walgreens Specialty Pharmacy, LLC, and Walgreen Eastern Co., Inc., the Company and subsidiaries of the Company listed on Annex A thereto (the “Pharmacy Business Purchase Agreement”). Pursuant to Item 601(b)(2) of Regulation S-K, certain schedules and exhibits to this agreement are omitted. The Company agrees to furnish supplementally a copy of any omitted schedule or exhibit to the SEC upon request. | (2) | |
2.3 | Amendment No. 1, dated as of May 4, 2012, to the Pharmacy Business Purchase Agreement. | (3) | |
2.4 | Stock Purchase Agreement, dated as of December 12, 2012, by and among HomeChoice Partners, Inc., DaVita HealthCare Partners Inc. Mary Ann Cope, R.Ph., Kathy F. Puglise, RN, CRNI, Joseph W. Boyd, R.Ph., Barbara J. Exum, PharmD and the Company. Pursuant to Item 601(b)(2) of Regulation S-K, certain schedules and exhibits to this agreement are omitted. The Company agrees to furnish supplementally a copy of any omitted schedule or exhibit to the SEC upon request. | (4) | |
2.5 | Asset Purchase Agreement, dated as of June 16, 2013, among the Company, CarePoint Partners Holdings LLC (“CarePoint”), the direct and indirect subsidiaries of CarePoint, and the members of CarePoint. Pursuant to Item 601(b)(2) of Regulation S-K, certain schedules and exhibits to this agreement are omitted. The Company agrees to furnish supplementally a copy of any omitted schedule or exhibit to the SEC upon request. | (5) | |
2.6 | Stock Purchase Agreement, dated as of February 1, 2014, by and among Elk Valley Professional Affiliates, Inc., South Mississippi Home Health, Inc., Deaconess Homecare, LLC, and the Buyers identified on the signature pages thereto, the Company and LHC Group, Inc. (the “Stock Purchase Agreement”). Pursuant to Item 601(b)(2) of Regulation S-K, certain schedules and exhibits to this agreement are omitted. The Company agrees to furnish supplementally a copy of any omitted schedule or exhibit to the SEC upon request. | (6) | |
2.7 | Amendment No. 1, dated as of March 31, 2014, to the Stock Purchase Agreement. Pursuant to Item 601(b)(2) of Regulation S-K, certain schedules and exhibits to this agreement are omitted. The Company agrees to furnish supplementally a copy of any omitted schedule or exhibit to the SEC upon request. | (7) | |
3.1 | Second Amended and Restated Certificate of Incorporation. | (8) | |
3.2 | Amendment to the Second Amended and Restated Certificate of Incorporation. | (9) | |
3.3 | Amended and Restated By-Laws. | (10) | |
4.1 | Specimen Common Stock Certificate. | (11) | |
4.2 | Warrant Agreement, dated as of March 25, 2010, by and among the Company, the Kohlberg Entities, Robert Cucuel, Mary Jane Graves, Nitin Patel, Joey Ryan, Colleen Lederer, the Blackstone Entities and S.A.C. | (12) | |
4.3 | Form of Cash-only Stock Appreciation Right Agreement. | (13) | |
105
4.4 | Indenture, dated as of February 11, 2014, by and among the Company, the Guarantors party thereto and U.S. Bank National Association, as Trustee. | (14) | |
4.5 | Specimen of 8.875% Notes due 2021 (included in Exhibit 4.8) | (15) | |
4.6 | Registration Rights Agreement, dated February 11, 2014, by and among the Company, the guarantors named therein and Jefferies LLC, on behalf of itself and the other initial purchasers named therein. | (16) | |
10.1† | MIM Corporation Amended and Restated 2001 Incentive Stock Plan. | (17) | |
10.2† | Amendment to BioScrip, Inc. 2001 Incentive Stock Plan. | (18) | |
10.3† | Amended and Restated BioScrip, Inc. 2008 Equity Incentive Plan. | (19) | |
10.4† | BIOSCRIP/CHS 2006 Equity Incentive Plan, as Amended and Restated. | (20) | |
10.5† | Employee Stock Purchase Plan. | (21) | |
10.6† | Form of Restricted Stock Grant Certificate. | (22) | |
10.7†* | Form of Stock Option Agreement. | ||
10.8† | Employment Offer Letter, dated as of June 21, 2007, by and between the Company and Pat Bogusz. | (23) | |
10.9† | Amendment dated May 26, 2011, to the Employment Offer Letter by and between the Company and Pat Bogusz. | (24) | |
10.10† | Engagement Letter, dated April 19, 2012, by and between the Company and Hai Tran. | (25) | |
10.11† | Employment Offer Letter, dated January 30, 2009, by and between the Company and David Evans. | (26) | |
10.12† | Employment Offer Letter, dated January 13, 2010, by and between the Company and Vito Ponzio, Jr. | (27) | |
10.13† | Amended and Restated Employment Agreement, dated as of November 25, 2013, by and between the Company and Richard M. Smith. | (28) | |
10.14† | Employment Offer Letter, dated March 10, 2009, by and between the Company and Brian Stiver. | (29) | |
10.15† | Employment Offer Letter, dated July 30, 2012, by and between the Company and Brian Stiver. | (30) | |
10.16† | Employment Offer Letter, dated July 18, 2014, by and between the Company and Thomas Petit. | (31) | |
10.17†* | Employment Offer Letter, dated December 1, 2013, by and between the Company and Karen Cain. | ||
10.18 | Form of Indemnification Agreement. | (32) | |
10.19 | Credit Agreement, dated July 31, 2013, by and among the Company, the several banks and other financial institutions and lenders from time to time party thereto, and SunTrust Bank, in its capacity as administrative agent (the “Administrative Agent”). | (33) | |
10.20 | First Amendment to Credit Agreement, dated as of December 23, 2013, by and among the Company, each of the Subsidiaries of the Company identified on the signature pages thereto, the Lenders party thereto, and the Administrative Agent. | (34) | |
10.21 | Second Amendment to Credit Agreement, dated as of January 31, 2014, by and among the Company, each of the Subsidiaries of the Company identified on the signature pages thereto, the Lenders party thereto, and the Administrative Agent. | (35) | |
10.22 | Third Amendment to Credit Agreement, dated as of March 1, 2015, by and among the Company, each of the Subsidiaries of the Company identified on the signature pages thereto, the Lenders party thereto, and the Administrative Agent. | (36) | |
10.23 | Guaranty and Security Agreement, dated July 31, 2013, made by the Company and the Guarantors identified on the signature pages thereto, in favor of the Administrative Agent. | (37) | |
10.24 # | Prime Vendor Agreement dated as of July 1, 2009, between AmerisourceBergen Drug Corporation, the Company and the other parties thereto (the “Prime Vendor Agreement”). | (38) | |
10.25 | First Amendment, dated as of March 25, 2010, to the Prime Vendor Agreement. | (39) | |
106
10.26 # | Second Amendment, dated as of June 1, 2010 to the Prime Vendor Agreement. | (40) | |
10.27 # | Third Amendment, dated as of August 1, 2010, to the Prime Vendor Agreement. | (41) | |
10.28 # | Fourth Amendment, dated as of May 1, 2011, to the Prime Vendor Agreement. | (42) | |
10.29 # | Fifth Amendment, dated as of January 1, 2012, to the Prime Vendor Agreement. | (43) | |
10.30 | Stockholders' Agreement, dated as of January 24, 2010, by and among the Company, the Kohlberg Entities, Robert Cucuel, Mary Jane Graves, Nitin Patel, Joey Ryan, Colleen Lederer, the Blackstone Entities and S.A.C. (the “Stockholders’ Agreement”). | (44) | |
10.31 | Amendment No. 1 to the Stockholders’ Agreement, dated as of March 8, 2013, by and between the Company and Kohlberg Investors. | (45) | |
10.32 | Amendment No. 2 to the Stockholders’ Agreement, dated as of March 14, 2013, by and between the Company and Kohlberg Investors. | (46) | |
10.33 | Amendment No. 3 & Waiver to the Stockholders’ Agreement, dated as of August 13, 2013, by and between the Company and Kohlberg Investors. | (47) | |
10.34 | Amendment No. 4 & Waiver to the Stockholders’ Agreement, dated as of March 26, 2014, by and between the Company and Kohlberg Investors. | (48) | |
10.35 | Indemnification Agreement, dated as of April 3, 2013, by and among the Company and the Kohlberg Entities, Robert Cucuel, Mary Jane Graves, Nitin Patel, Joey Ryan, Colleen Lederer, the Blackstone Entities and S.A.C. | (49) | |
10.36 | Stipulation and Order of Settlement and Dismissal, effective January 8, 2014, by and among the Company, the United States of America, acting through the U.S. Department of Justice and on behalf of the Office of Inspector General of the Department of Health and Human Services, and relator David Kester. | (50) | |
10.37 | Investor Agreement, dated as of February 6, 2015, by and among the Company, Cloud Gate Capital LLC and DSC Advisors, LLC. | (51) | |
12 * | Computation of Ratio of Earnings to Fixed Charges | ||
21.1 * | List of Subsidiaries of the Company. | ||
23.1 * | Consent of Independent Registered Public Accounting Firm. | ||
23.2 * | Consent of Independent Registered Public Accounting Firm. | ||
31.1 * | Certification of Chief Executive Officer pursuant to Rule 13a-14(a) promulgated under the Exchange Act. | ||
31.2 * | Certification of Chief Financial Officer pursuant to Rule 13a-14(a) promulgated under the Exchange Act. | ||
32.1 * | Certification of Chief Executive Officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. | ||
32.2 * | Certification of Chief Financial Officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. | ||
101 ** | The following financial information from the Company’s Form 10-K for the fiscal year ended December 31, 2014, formatted in XBRL (Extensible Business Reporting Language): (i) Consolidated Statements of Operations for the fiscal years ended December 31, 2014, 2013 and 2012, (ii) Consolidated Balance Sheets as of December 31, 2014 and 2013, (iii) Consolidated Statements of Stockholders' Equity for the fiscal years ended December 31, 2014, 2013 and 2012, (iv) Consolidated Statements of Cash Flows for the fiscal years ended December 31, 2014, 2013 and 2012, and (v) Notes to Consolidated Financial Statements. | ||
(1) | Incorporated by reference to Exhibit 2.1 to the Company’s Form 8-K filed on January 27, 2010, SEC File Number 000-28740. |
(2) | Incorporated by reference to Exhibit 2.1 to the Company’s Form 8-K filed on February 3, 2012, SEC File Number 000-28740. |
(3) | Incorporated by reference to Exhibit 2.1 to the Company’s Form 8-K filed on May 10, 2012, SEC File Number 000-28740. |
(4) | Incorporated by reference to Exhibit 2.1 to the Company’s Form 8-K filed on February 4, 2013, SEC File Number 000-28740. |
(5) | Incorporated by reference to Exhibit 2.1 to the Company’s Form 8-K filed on June 18, 2013, SEC File Number 000-28740. |
(6) | Incorporated by reference to Exhibit 2.1 to the Company’s Form 8-K filed on February 3, 2014, SEC File Number 000-28740. |
(7) | Incorporated by reference to Exhibit 2.2 to the Company's Form 8-K filed on April 1, 2014, SEC File Number 000-28740. |
107
(8) | Incorporated by reference to Exhibit 4.1 to the Company’s Form 8-K filed on March 17, 2005, SEC File Number 000-28740. |
(9) | Incorporated by reference to Exhibit 3.1 to the Company’s Form 8-K filed on June 10, 2010, SEC File Number 000-28740. |
(10) | Incorporated by reference to Exhibit 3.2 to the Company’s Form 8-K filed on April 28, 2011, SEC File Number 000-28740. |
(11) | Incorporated by reference to Exhibit 4.1 to the Company’s Form 10-K filed on March 31, 2006, SEC File Number 000-28740. |
(12) | Incorporated by reference to Exhibit 4.2 to the Company’s Form 8-K filed on March 31, 2010, SEC File Number 000-28740. |
(13) | Incorporated by reference to Exhibit 10.40 to the Company’s Form 10-K filed on March 16, 2011, SEC File Number 000-28740. |
(14) | Incorporated by reference to Exhibit 4.1 to the Company’s Form 8-K filed on February 11, 2014, SEC File Number 000-28740. |
(15) | Incorporated by reference to Exhibit 4.2 to the Company’s Form 8-K filed on February 11, 2014, SEC File Number 000-28740. |
(16) | Incorporated by reference to Exhibit 4.3 to the Company’s Form 8-K filed on February 11, 2014, SEC File Number 000-28740. |
(17) | Incorporated by reference to the definitive proxy statement filed on April 30, 2003, SEC File Number 000-28740. |
(18) | Incorporated by reference to Exhibit 10.1 to the Company’s Form 8-K filed on August 10, 2011, SEC File Number 000-28740. |
(19) | Incorporated by reference to Exhibit 10.1 to the Company’s Form 8-K filed on May 14, 2014, SEC File Number 000-28740. |
(20) | Incorporated by reference to Exhibit 10.3 to the Company’s Form 8-K filed on May 2, 2011, SEC File Number 000-28740. |
(21) | Incorporated by reference to the definitive proxy statement filed on April 2, 2013, SEC File Number 000-28740. |
(22) | Incorporated by reference to Exhibit 99.3 to the Company's Registration Statement on Form S-8 filed on filed on May 16, 2008. |
(23) | Incorporated by reference to Exhibit 10.14 to the Company’s Form 10-K/A filed on December 16, 2013, SEC File Number 000-28740. |
(24) | Incorporated by reference to Exhibit 10.15 to the Company’s Form 10-K/A filed on December 16, 2013, SEC File Number 000-28740. |
(25) | Incorporated by reference to Exhibit 10.1 to the Company’s Form 8-K filed on April 23, 2012, SEC File Number 000-28740. |
(26) | Incorporated by reference to Exhibit 10.23 to the Company’s Form 10-K/A filed on December 16, 2013, SEC File Number 000-28740. |
(27) | Incorporated by reference to Exhibit 10.24 to the Company’s Form 10-K/A filed on December 16, 2013, SEC File Number 000-28740. |
(28) | Incorporated by reference to Exhibit 10.1 to the Company’s Form 8-K filed on November 27, 2013, SEC File Number 000-28740. |
(29) | Incorporated by reference to Exhibit 10.24 to the Company’s Form 10-K/A filed on June 6, 2014, SEC File Number 000-28740. |
(30) | Incorporated by reference to Exhibit 10.25 to the Company’s Form 10-K/A filed on June 6, 2014, SEC File Number 000-28740. |
(31) | Incorporated by reference to Exhibit 10.1 to the Company’s Form 8-K filed on July 18, 2014, SEC File Number 000-28740. |
(32) | Incorporated by reference to Exhibit 10.1 to the Company’s Form 8-K filed on March 14, 2013, SEC File Number 000-28740. |
(33) | Incorporated by reference to Exhibit 10.1 to the Company’s Form 8-K filed on August 1, 2013, SEC File Number 000-28740. |
(34) | Incorporated by reference to Exhibit 99.1 to the Company’s Form 8-K filed on February 3, 2014, SEC File Number 000-28740. |
(35) | Incorporated by reference to Exhibit 10.1 to the Company’s Form 8-K filed on February 3, 2014, SEC File Number 000-28740. |
(36) | Incorporated by reference to Exhibit 10.1 to the Company’s Form 8-K filed on March 2, 2015, SEC File Number 000-28740. |
(37) | Incorporated by reference to Exhibit 10.2 to the Company’s Form 8-K filed on August 1, 2013, SEC File Number 000-28740. |
(38) | Incorporated by reference to Exhibit 10.1 to the Company’s Form 10-Q/A filed on December 2, 2009, SEC File Number 000-28740. |
(39) | Incorporated by reference to Exhibit 10.3 to the Company’s Form 8-K filed on March 31, 2010, SEC File Number 000-28740. |
(40) | Incorporated by reference to Exhibit 10.1 to the Company’s Form 10-Q filed on August 3, 2010, SEC File Number 000-28740. |
(41) | Incorporated by reference to Exhibit 10.1 to the Company’s Form 8-K filed on May 2, 2011, SEC File Number 000-28740. |
(42) | Incorporated by reference to Exhibit 10.2 to the Company’s Form 8-K filed on May 2, 2011, SEC File Number 000-28740. |
(43) | Incorporated by reference to Exhibit 10.1 to the Company’s Form 8-K filed on January 26, 2012, SEC File Number 000-28740. |
(44) | Incorporated by reference to Exhibit 10.1 to the Company’s Form 8-K filed on January 27, 2010, SEC File Number 000-28740. |
(45) | Incorporated by reference to Exhibit 10.1 to the Company’s Form 10-Q filed on May 9, 2013, SEC File Number 000-28740. |
(46) | Incorporated by reference to Exhibit 10.2 to the Company’s Form 10-Q filed on May 9, 2013, SEC File Number 000-28740. |
(47) | Incorporated by reference to Exhibit 1.2 to the Company’s Form 8-K filed on August 19, 2013, SEC File Number 000-28740. |
(48) | Incorporated by reference to Exhibit 10.1 to the Company’s Form 8-K filed on April 1, 2014, SEC File Number 000-28740. |
(49) | Incorporated by reference to Exhibit 10.1 to the Company’s Form 8-K filed on April 5, 2013, SEC File Number 000-28740. |
(50) | Incorporated by reference to Exhibit 10.1 to the Company’s Form 8-K filed on January 8, 2014, SEC File Number 000-28740. |
(51) | Incorporated by reference to Exhibit 10.1 to the Company’s Form 8-K filed on February 9, 2015, SEC File Number 000-28740. |
* | Filed herewith. |
108
** | Pursuant to Rule 406T of Regulation S-T, these interactive data files are deemed not filed or part of a registration statement or prospectus for purposes of Sections 11 or 12 of the Securities Act of 1933 or Section 18 of the Securities Exchange Act of 1934 and otherwise are not subject to liability under those sections. |
† | Designates the Company’s management contracts or compensatory plan or arrangement. |
# | The SEC has granted confidential treatment of certain provisions of these exhibits. Omitted material for which confidential treatment has been granted has been filed separately with the SEC. |
109
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized, on March 2, 2015.
BIOSCRIP, INC. |
/s/ Hai Tran |
Hai Tran |
Chief Financial Officer and Treasurer |
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
Signature | Title(s) | Date |
/s/ Richard M. Smith Richard M. Smith | Chief Executive Officer, President and Director (Principal Executive Officer) | March 2, 2015 |
/s/ Hai Tran Hai Tran | Chief Financial Officer and Treasurer (Principal Financial Officer) | March 2, 2015 |
/s/ Patricia Bogusz Patricia Bogusz | Vice President of Finance (Principal Accounting Officer) | March 2, 2015 |
/s/ Myron Z. Holubiak Myron Z. Holubiak | Non-Executive Chairman of the Board | March 2, 2015 |
/s/ Charlotte W. Collins Charlotte W. Collins | Director | March 2, 2015 |
/s/ Samuel P. Frieder Samuel P. Frieder | Director | March 2, 2015 |
/s/ David R. Hubers David R. Hubers | Director | March 2, 2015 |
/s/ Tricia Huong Thi Nguyen Tricia Huong Thi Nguyen | Director | March 2, 2015 |
/s/ Yon Y. Jorden Yon Y. Jorden | Director | March 2, 2015 |
/s/ Stuart A. Samuels Stuart A. Samuels | Director | March 2, 2015 |
/s/ Gordon H. Woodward Gordon H. Woodward | Director | March 2, 2015 |
110
Bioscrip, Inc. and Subsidiaries
Schedule II-- Valuation and Qualifying Accounts
(in thousands)
Balance at Beginning of Period | Write-Off of Receivables | Charged to Costs and Expenses | Balance at End of Period | ||||||||||||
Year ended December 31, 2012 | |||||||||||||||
Allowance for doubtful accounts | $ | 21,272 | $ | (13,349 | ) | $ | 13,201 | $ | 21,124 | ||||||
Year ended December 31, 2013 | |||||||||||||||
Allowance for doubtful accounts | $ | 21,124 | $ | (22,913 | ) | $ | 19,625 | $ | 17,836 | ||||||
Year ended December 31, 2014 | |||||||||||||||
Allowance for doubtful accounts | $ | 17,836 | $ | (30,910 | ) | $ | 79,574 | $ | 66,500 | ||||||
111
(Exhibits being filed with this Annual Report on Form 10-K)
10.7 | Form of Stock Option Agreement. |
10.17 | Employment Offer Letter, dated December 1, 2013, by and between the Company and Karen Cain. |
12 | Computation of Ratio of Earnings to Fixed Charges |
21.1 | List of Subsidiaries of the Company. |
23.1 | Consent of Independent Registered Public Accounting Firm. |
23.2 | Consent of Independent Registered Public Accounting Firm. |
31.1 | Certification of Chief Executive Officer pursuant to Rule 13a-14(a) promulgated under the Exchange Act. |
31.2 | Certification of Chief Financial Officer pursuant to Rule 13a-14(a) promulgated under the Exchange Act. |
32.1 | Certification of Chief Executive Officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. |
32.2 | Certification of Chief Financial Officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. |
101 | The following financial information from the Company’s Form 10-K for the fiscal year ended December 31, 2014, formatted in XBRL (Extensible Business Reporting Language): (i) Consolidated Statements of Operations for the fiscal years ended December 31, 2014, 2013 and 2012, (ii) Consolidated Balance Sheets as of December 31, 2014 and 2013, (iii) Consolidated Statements of Stockholders’ Equity for the fiscal years ended December 31, 2014, 2013 and 2012, (iv) Consolidated Statements of Cash Flows for the fiscal years ended December 31, 2014, 2013 and 2012, and (v) Notes to Consolidated Financial Statements. |
112
