Attached files
| file | filename |
|---|---|
| EX-23.1 - EX-23.1 - ARIAD PHARMACEUTICALS INC | d862791dex231.htm |
| EX-31.2 - EX-31.2 - ARIAD PHARMACEUTICALS INC | d862791dex312.htm |
| EX-32.1 - EX-32.1 - ARIAD PHARMACEUTICALS INC | d862791dex321.htm |
| EX-31.1 - EX-31.1 - ARIAD PHARMACEUTICALS INC | d862791dex311.htm |
| EX-21.1 - EX-21.1 - ARIAD PHARMACEUTICALS INC | d862791dex211.htm |
| EX-10.16 - EX-10.16 - ARIAD PHARMACEUTICALS INC | d862791dex1016.htm |
| EX-10.9.1 - EX-10.9.1 - ARIAD PHARMACEUTICALS INC | d862791dex1091.htm |
| EX-10.9.2 - EX-10.9.2 - ARIAD PHARMACEUTICALS INC | d862791dex1092.htm |
| EXCEL - IDEA: XBRL DOCUMENT - ARIAD PHARMACEUTICALS INC | Financial_Report.xls |
| EX-10.8 - EX-10.8 - ARIAD PHARMACEUTICALS INC | d862791dex108.htm |
Table of Contents
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 10-K
(Mark One)
| x | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2014
OR
| ¨ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to
Commission file number 001-36172
ARIAD Pharmaceuticals, Inc.
(Exact name of registrant as specified in its charter)
| Delaware | 22-3106987 | |
| (State or other jurisdiction of incorporation or organization) |
(I.R.S. Employer Identification No.) | |
| 26 Landsdowne Street, Cambridge, Massachusetts | 02139-4234 | |
| (Address of principal executive offices) | (Zip Code) | |
Registrant’s telephone number, including area code: (617) 494-0400
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class |
Name of each exchange on which registered | |
| Common Stock, $.001 par value Preferred Stock Purchase Rights |
The NASDAQ Global Select Market |
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes x No ¨
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Exchange Act. Yes ¨ No x
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes x No ¨
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes x No ¨
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. x
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer or a smaller reporting company. See definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check one):
| Large accelerated filer | x | Accelerated filer | ¨ | |||
| Non-accelerated filer | ¨ (Do not check if a smaller reporting company) | Smaller reporting company | ¨ | |||
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ¨ No x
The aggregate market value of the registrant’s common stock held by nonaffiliates of the registrant (without admitting that any person whose shares are not included in such calculation is an affiliate), computed by reference to the price at which the common stock was last sold, as of the last business day of the registrant’s most recently completed second fiscal quarter was approximately $1.1 billion.
As of January 31, 2015, the registrant had 187,531,908 shares of common stock outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
The following documents (or parts thereof) are incorporated by reference into the following parts of this Form 10-K: Certain information required in Part III of this Annual Report on Form 10-K is incorporated from the Registrant’s Definitive Proxy Statement for the 2015 Annual Meeting of Stockholders.
Table of Contents
Table of Contents
PART I
The following Business Section contains forward-looking statements. Our actual results could differ materially from those anticipated in these forward-looking statements as a result of certain risks, uncertainties and other factors including the risk factors set forth in Part I, Item 1A of this annual report. Unless the content requires otherwise, references to “ARIAD,” “company,” “we,” “our,” and “us,” in this annual report refer to ARIAD Pharmaceuticals, Inc. and our subsidiaries.
Overview
ARIAD is a global oncology company focused on transforming the lives of cancer patients with breakthrough medicines. Our mission is to discover, develop and commercialize small-molecule drugs to treat cancer in patients with the greatest unmet medical need – aggressive cancers where current therapies are inadequate. We are focused on value-driving investments in commercialization, research and development, and new business development initiatives that we expect will lead to sustained profitability beginning in 2018 and increased shareholder value.
As described in further detail below, we are currently commercializing or developing the following three products and product candidates:
| • | Iclusig® (ponatinib) is our first approved cancer medicine, which we are commercializing in the United States, Europe and other territories for the treatment of certain patients with rare forms of leukemia. In 2014, we generated $55.7 million in net product revenue from sales of Iclusig, and we intend to continue to focus our commercial efforts in the United States and in 16 major European countries, while we seek to extend our geographic reach outside these territories through regional distributorships and collaborations. In late 2014, we secured an exclusive agreement for the co-development and commercialization of Iclusig in Japan and nine other Asian countries with Otsuka Pharmaceutical Co., Ltd., or Otsuka, for which we received an upfront payment of $77.5 million and are eligible to receive additional milestone payments. We plan to initiate three new randomized clinical trials beginning in 2015 to evaluate Iclusig in earlier lines of treatment and potentially to expand its addressable market. |
| • | Brigatinib (previously known as AP26113) is our next most advanced drug candidate, which we are developing for the treatment of certain patients with a form of non-small cell lung cancer, or NSCLC. In 2014, brigatinib received a Breakthrough Therapy designation from the FDA for the treatment of patients with ALK+ NSCLC who are resistant to crizotinib, and we initiated a pivotal Phase 2 trial in these patients. We plan to complete enrollment of this pivotal clinical trial in the third quarter of 2015, and, assuming favorable results, expect to file for regulatory approval in the United States in 2016. We are focused on securing a co-development and co-commercialization partnership for brigatinib that we expect will allow us to continue to leverage our existing infrastructure and capabilities, as well as support a randomized trial of brigatinib as a first line therapy. |
| • | AP32788 is our most recent, internally discovered drug candidate. In December 2014, we nominated AP32788 for clinical development. We are conducting studies necessary to support the filing of an investigational new drug application, or IND, which we expect to submit in 2015. We expect to commence clinical trials of AP32788 in 2016. |
These products and product candidates complement our earlier discovery of ridaforolimus, which we have out-licensed for development in drug-eluting stents and other medical devices for cardiovascular indications, and rimiducid (AP1903), which we have out-licensed for development in novel cellular immunotherapies. All of our product candidates were discovered internally by our scientists based on our expertise in computational chemistry and structure-based drug design.
Iclusig (ponatinib)
Iclusig is a tyrosine kinase inhibitor, or TKI, which is approved in the United States, Europe and Australia for the treatment of adult patients with chronic myeloid leukemia, or CML, and Philadelphia chromosome-positive acute lymphoblastic leukemia, or Ph+ ALL.
Background of CML and Ph+ ALL
CML is a rare form of leukemia that is characterized by an excessive and unregulated production of white blood cells by the bone marrow due to a genetic abnormality that produces the BCR-ABL protein. After a chronic phase of production of too many white blood cells, CML typically evolves to the more aggressive phases referred to as accelerated phase and blast phase. Ph+ ALL is a subtype of acute lymphoblastic leukemia that carries the Ph+ chromosome that produces BCR-ABL. It has a more aggressive course than CML and is often treated with a combination of chemotherapy and TKIs. The BCR-ABL protein is expressed in both of these diseases.
1
Table of Contents
We estimate that currently there are approximately 14,600 new cases of CML and Ph+ ALL diagnosed each year in the United States, Europe and Japan who are eligible for TKI therapy. CML and Ph+ ALL patients treated with TKIs can develop resistance or intolerance over time to these therapies. Iclusig was designed by ARIAD scientists to inhibit the BCR-ABL protein, including drug-resistant mutants that arise during treatment. Iclusig is the only approved TKI that is currently known to demonstrate activity against all resistant mutations, including the T315I gatekeeper mutation of BCR-ABL, the most common mutation occurring in approximately 10 percent of patients with drug resistance. Other TKIs currently approved to treat patients with CML and Ph+ ALL are imatinib, dasatinib, nilotinib and bosutinib. Based on the currently approved label in the United States and Europe, we estimate that there are approximately 4,000 patients with CML and Ph+ ALL in the United States and Europe who annually develop resistance or intolerance to other TKIs and are eligible for treatment with Iclusig.
United States
Iclusig is approved in the United States for the treatment of adult patients with:
| • | T315I-positive CML (chronic phase, accelerated phase, or blast phase) or T315I-positive Ph+ ALL, and |
| • | Chronic phase, accelerated phase, or blast phase CML or Ph+ ALL for whom no other tyrosine-kinase inhibitor therapy is indicated. |
The full prescribing information for Iclusig includes a boxed warning alerting patients and healthcare professionals to the risk of vascular occlusive events, heart failure and hepatotoxicity as well as warnings about hypertension, neuropathy, ocular toxicity, hemorrhage, fluid retention, cardiac arrhythmias, myelosuppression, pancreatitis and tumor lysis syndrome, compromised wound healing and gastrointestinal perforation and embryo-fetal toxicity. The prescribing information also includes recommendations regarding dose reductions based on disease response or for toxicity. The recommended dose of Iclusig is a 45mg tablet taken once daily, with or without food.
We employ an experienced and trained sales force and other professional staff, including account specialists, regional business directors, corporate account directors and medical science liaisons, who target the approximate 5,000 physicians who generate the majority of TKI prescriptions. We are currently distributing Iclusig in the United States through a single specialty pharmacy.
We initially obtained approval, on an accelerated basis, from the U.S. Food and Drug Administration, or FDA, to sell Iclusig in the United States in December 2012.
On October 31, 2013, we temporarily suspended marketing and commercial distribution of Iclusig in the United States due to safety concerns raised by the FDA, while we negotiated updates to the United States prescribing information for Iclusig and implementation of a risk mitigation strategy with the FDA. In December 2013, the FDA approved revised U.S. Prescribing Information, or USPI, and a Risk Evaluation and Mitigation Strategy, or REMS, for Iclusig that allowed for resumption of the marketing and commercial distribution of Iclusig, which we commenced in January 2014.
Europe
We obtained marketing authorization for Iclusig in July 2013 from the European Commission, or EC, as an orphan medicinal product for the treatment of adult patients with:
| • | Chronic phase, accelerated phase or blast phase CML who are resistant to dasatinib or nilotinib; who are intolerant to dasatinib or nilotinib and for whom subsequent treatment with imatinib is not clinically appropriate; or who have the T315I mutation, and |
2
Table of Contents
| • | Ph+ ALL who are resistant to dasatinib; who are intolerant to dasatinib and for whom subsequent treatment with imatinib is not clinically appropriate; or who have the T315I mutation. |
In Europe, we are now selling Iclusig in Germany, the United Kingdom, Austria, the Netherlands, Norway, Sweden, and Italy. In addition, we are distributing Iclusig to patients in France prior to pricing and reimbursement approval as permitted under French regulations. Full pricing and reimbursement in France and other European countries is expected by mid-2015. In total, we anticipate that Iclusig will be available for sale in 16 European countries by the third quarter of 2015.
Following the temporary suspension of marketing and commercial distribution of Iclusig in the United States in October 2013, we engaged in discussions with the European Medicines Agency, or EMA, regarding potentially revised prescribing information for Iclusig. In November 2013, the EMA announced that Iclusig’s product information should be updated to include strengthened warnings for cardiovascular risk and guidance on optimizing patients’ cardiovascular therapy before starting treatment. In addition, the EMA commenced an in-depth review, known as an Article 20 referral, of the benefits and risks of Iclusig to better understand the nature, frequency and severity of events obstructing the arteries or veins, the potential mechanism that leads to these side effects and whether there needs to be a revision in the European prescribing information for Iclusig.
In October 2014, the Pharmacovigilance Risk Assessment Committee, or PRAC, of the EMA concluded its review of Iclusig under the Article 20 referral procedure and recommended that Iclusig continue to be used in Europe in accordance with its already approved indications. The PRAC also recommended that the Summary of Medicinal Product Characteristics, or SmPC, for Iclusig include (1) patient monitoring for response according to standard clinical guidelines, (2) consideration of Iclusig dose-reduction following achievement of major cytogenetic response with subsequent monitoring of response, and (3) consideration of Iclusig discontinuation if a complete haematologic response has not been achieved by three months. The PRAC also recommended that further information be provided in the Iclusig SmPC indicating that the risk of vascular occlusive events is likely dose-related, and recommend an update of the Warning and Precautions and Undesirable Effects sections. The PRAC’s recommendation was considered and adopted by the Committee for Medicinal Products for Human Use, or CHMP, of the EMA in October 2014. In January 2015, the EC endorsed the final opinion adopted by the CHMP, which is binding throughout the European Union.
We have established headquarters for our European operations in Switzerland where we manage all aspects of our business in Europe, including sales and marketing, distribution and supply chain, regulatory, medical affairs and supporting functions. We also employ personnel in key countries in Europe to build company and brand awareness, manage the local country pricing and reimbursement process and sell Iclusig upon obtaining all necessary approvals. In order to provide for access to Iclusig in countries where we do not employ personnel, we enter into relationships with distributors in such countries. These distributorship arrangements provide for the exclusive right to sell and distribute Iclusig in a specific territory for a specified period of time in exchange for fees and payments related to purchase of Iclusig from us and/or sales of Iclusig in the territory. In December 2014, we entered into an agreement with Angelini Pharma, through its Austrian subsidiary, CSC Pharmaceuticals, to distribute Iclusig in countries in central and eastern Europe.
Other Markets
In territories outside the United States and Europe, we provide for the sale and distribution of Iclusig through partnership or distributorship arrangements. In December 2014, we entered into a co-development and commercialization agreement with Otsuka Pharmaceutical Co., Ltd., or Otsuka, under which Otsuka has exclusive rights to commercialize Iclusig in Japan and nine other Asian countries and
3
Table of Contents
will fund agreed-upon future clinical development in those countries. We expect to file for marketing approval in Japan in collaboration with Otsuka in the second half of 2015. In Australia, we obtained marketing approval for Iclusig in November 2014 and will distribute Iclusig through Specialised Therapeutics Australia Pty Ltd. under a distribution agreement we entered into in January 2014 that covers Australia and New Zealand. In Israel, we entered into a distribution agreement with Medison Pharma Ltd. in August 2014 and filed for marketing approval, which is expected in 2015. We have also filed a marketing authorization application for Iclusig in Canada, which is expected to be approved in 2015.
Ongoing Development of Iclusig
Initial approvals of Iclusig in the United States and Europe were based on results from the pivotal Phase 2 PACE (Ponatinib Ph+ ALL and CML Evaluation) trial in patients with CML or Ph+ ALL who were resistant or intolerant to prior TKI therapy, or who had the T315I mutation of BCR-ABL. In that trial, Iclusig demonstrated robust anti-leukemic activity, with 54 percent of chronic phase CML patients, including 70 percent of patients with the T315I mutation, achieving a major cytogenetic response, or MCyR, which was the primary endpoint of the PACE trial for chronic phase patients. A MCyR means that 35 percent or less of the cells in a patient’s bone marrow test positive for the Philadelphia chromosome. In patients with advanced disease, 52 percent of accelerated phase CML patients, 31 percent of blast phase CML patients and 41 percent of Ph+ ALL patients achieved a major hematologic response, or MaHR, to Iclusig. MaHR was the primary endpoint in the trial for patients with advanced disease. A MaHR, as measured through the counting of white blood cells in blood and bone marrow, means that either a complete hematologic response has occurred or there is no evidence of leukemia. The most common non-hematologic adverse reactions reported (greater than or equal to 20 percent) were hypertension, rash, abdominal pain, fatigue, headache, dry skin, constipation, arthralgia, nausea, and pyrexia. Hematologic adverse reactions included thrombocytopenia, anemia, neutropenia, lymphopenia, and leukopenia.
We continue to follow patients who remain on treatment in the PACE trial and, in December 2014, in connection with a presentation at the 56th Annual Meeting of the American Society of Hematology, or ASH, we announced long-term follow-up data from the PACE trial. The study now shows that with a median follow-up of approximately three years for chronic phase CML patients in the trial, Iclusig continues to demonstrate anti-leukemic activity in patients with limited treatment options. Deep and durable responses have been maintained in chronic phase CML patients with 83 percent of such patients who achieved a response estimated to remain in major cytogenetic response at three years. Additionally, the rate of maintenance of response in chronic phase CML patients was high (greater than 90 percent) in patients who underwent Iclusig dose reductions. Long-term safety data confirm that careful benefit-risk evaluations should guide the decision to use and then maintain Iclusig therapy, particularly in patients who may be at increased risk for arterial thrombotic events.
Also at the ASH Annual Meeting, we announced long-term follow-up data from our Phase 1 clinical trial of Iclusig in heavily pretreated patients with resistant or intolerant CML or Ph+ ALL. The study now shows that with a median follow-up of four years in chronic phase CML patients, Iclusig continues to demonstrate anti-leukemic activity in patients with limited treatment options and that responses have been maintained in chronic phase CML patients with 72 percent having an MCyR, 65 percent having a complete cytogenetic response, or CCyR, and 56 percent having a major molecular response, or MMR.
In addition, we announced at the ASH Annual Meeting safety and efficacy follow-up data on Iclusig in patients with a baseline T315I mutation from our Phase 1 and PACE trials in heavily pretreated patients with resistant or intolerant CML or Ph+ ALL. With a median follow-up of three years for chronic phase CML patients and nine months overall, Iclusig continues to exhibit responses in patients with the T315I mutation, for whom there is no other approved TKI therapy. Among patients with the T315I mutation, MCyR was achieved by 92 percent of chronic phase CML patients in the Phase 1 trial and 72 percent of chronic phase CML patients in the PACE trial; taken together, the combined MCyR rate for all T315I chronic phase CML patients was 75 percent.
4
Table of Contents
In 2015, we plan to invest in studies designed to better understand the safety profile of Iclusig in patients with resistant and intolerant CML or Ph+ ALL, with the objective of improving the balance of benefit and risk for these patients, as well as studies designed to evaluate its use in earlier lines of therapy. These studies include:
| • | a dose-ranging, trial of Iclusig in patients with chronic phase CML, who have become resistant to at least two prior TKIs. This global, randomized trial will evaluate three starting doses of Iclusig in patients with refractory chronic phase CML. The trial is expected to inform the optimal use of Iclusig in these patients and is expected to begin by mid-2015. We expect that approximately 450 patients will be enrolled in this trial. |
| • | a randomized, Phase 3 trial in patients with chronic phase CML who have experienced treatment failure after imatinib therapy. This second-line, global trial will evaluate two doses of Iclusig vs. the standard dose of nilotinib. The primary endpoint of the trial will be major molecular response (MMR) by 12 months. The trial is expected to open to patient enrollment in the second half of 2015 and will be integral to potentially expanding Iclusig into earlier lines of treatment. We expect that approximately 500 patients will be enrolled in this trial. |
| • | an early-switch trial of Iclusig in second-line chronic phase CML patients that will be conducted in the United Kingdom. This investigator-sponsored trial, named SPIRIT3, will be coordinated by the Newcastle University, U.K., on behalf of the U.K. National Cancer Research Institute (NCRI) CML Working Group. It will enroll newly diagnosed patients with chronic phase CML, who will be randomized to either imatinib or nilotinib. Patients failing to reach an early molecular response at three months will then be switched to Iclusig in the second line. We expect the trial to inform the use of Iclusig as part of the emerging paradigm in CML for early switching of TKIs in patients with suboptimal responses. We anticipate that the trial will begin in the first half of 2015 and will enroll approximately 1,000 patients. |
Additionally, 10 investigator-sponsored trials, or ISTs, in the ponatinib program are open to patient enrollment, and three additional ISTs are pending regulatory or institutional review board approval. This includes two trials in RET-driven non-small cell lung cancer (NSCLC) and one in medullary thyroid cancer, five trials in CML or Ph+ALL, two trials in acute myeloid leukemia (AML) and three in various other cancers with genetic aberrations in FGFR. We expect that additional investigator-sponsored clinical trials will be initiated in 2015 in various indications.
Pipeline
In addition to Iclusig, our product pipeline currently consists of three product candidates – brigatinib (previously known as AP26113), AP32788 and ridaforolimus.
Brigatinib (AP26113)
Brigatinib (AP26113) is an investigational inhibitor of anaplastic lymphoma kinase, or ALK. ALK was first identified as a chromosomal rearrangement in anaplastic large-cell lymphoma, or ALCL. Genetic studies indicate that abnormal expression of ALK is a key driver of certain types of non-small cell lung cancer, or NSCLC, and neuroblastomas, as well as ALCL. Since ALK is generally not expressed in normal adult tissues, it represents a highly promising molecular target for cancer therapy.
Brigatinib has received a Breakthrough Therapy designation from the FDA for the treatment of patients with ALK+ metastatic NSCLC whose tumors are resistant to crizotinib. This designation is based on results from the ongoing Phase 1/2 trial, described below, that show anti-tumor activity of brigatinib in patients with ALK+ NSCLC, including patients with active brain metastases. A Breakthrough Therapy designation is intended to expedite the development and FDA review of drugs for serious or life-threatening conditions.
5
Table of Contents
We estimate that currently there are approximately 18,000 ALK-positive patients with advanced and metastatic NSCLC in the United States, Europe and Japan, based on healthcare information providers, who would be potentially eligible for treatment with brigatinib, if it were approved.
We initiated patient enrollment in a Phase 1/2 clinical trial of brigatinib in the third quarter of 2011. In September 2014, we announced updated clinical results from the ongoing Phase 1/2 clinical trial in which we had enrolled a total of 137 patients. The study results show robust anti-tumor activity in patients with TKI-naïve and crizotinib-resistant ALK-positive NSCLC, including in patients with brain metastases after crizotinib treatment. Crizotinib is the currently available first-generation ALK inhibitor.
Objective responses were observed in ALK+ NSCLC patients at the lowest dose tested in these patients (60 mg), and responses were observed in patients who were either TKI-naïve or resistant to crizotinib. Of the 72 ALK+ NSCLC patients evaluable for response, 52 (72 percent) demonstrated an objective response. The data demonstrated tumor shrinkage in nearly all ALK+ NSCLC patients, with 16 patients experiencing 100 percent shrinkage of the target lesion. The median duration of response was 49 weeks, and the median progression-free survival was 56 weeks.
Of the seven evaluable TKI-naïve ALK+ NSCLC patients treated with brigatinib, all demonstrated an objective response, including two complete responses. Of the 65 evaluable ALK+ NSCLC patients with prior crizotinib therapy treated with brigatinib, 45 (69 percent) demonstrated an objective response. Median progression-free survival was 47.3 weeks. In a subgroup analysis, 10 of 14 (71 percent) ALK+ NSCLC patients with active, untreated or progressing brain metastases had evidence of radiographic improvement in those metastases. Four of these patients demonstrated a complete response by independent review. Of the 10 patients who responded, six remained on study, with treatment durations up to 105 weeks. In addition, improvement in a patient with leptomeningeal metastasis was observed.
The most common adverse events, regardless of treatment relationship and including all grades, were nausea (45 percent), diarrhea (37 percent), and fatigue (37 percent). Adverse events, grade 3 or higher, occurring in three or more patients were dyspnea (4 percent), increased lipase (4 percent), hypoxia (4 percent), fatigue (3 percent), alanine aminotransferase (ALT) increased (2 percent) and amylase increased (2 percent). Serious adverse events occurring in three or more patients were dyspnea (7 percent), pneumonia (5 percent), hypoxia (4 percent), neoplasm progression (4 percent), pyrexia (2 percent) and pulmonary embolism (2 percent). Early onset pulmonary symptoms were observed in 13 of 137 (10 percent) patients enrolled in the study and occurred more frequently with starting doses of 180 mg per day and higher compared to the lower starting doses. These symptoms occurred within seven days of treatment initiation, and in some cases, included dyspnea, hypoxia, and new pulmonary opacities on chest imaging. To minimize the early-onset pulmonary symptoms observed, two additional dosing regimens were examined in the Phase 2 portion of the trial: 90 mg per day for one week followed by 180 mg per day (n=32) and 90 mg per day continuously (n=18). The incidence of early-onset pulmonary symptoms in these two dosing regimens was 0 of 32 and 2 of 18 patients, respectively. Overall, 2 out of 50 patients (4 percent) who began dosing at 90 mg per day had early-onset pulmonary symptoms and both continued on treatment.
We commenced a pivotal trial of brigatinib in the first quarter of 2014 in patients with locally advanced or metastatic NSCLC who were previously treated with crizotinib. The ALTA (ALK in Lung Cancer Trial of AP26113) trial is designed to determine the safety and efficacy of brigatinib in refractory ALK+ NSCLC patients. The trial will enroll approximately 220 patients including those with brain metastasis. Patients are being randomized 1:1 to receive either 90 mg of brigatinib once per day continuously or a lead-in dose of 90 mg per day for 7 days followed by 180 mg once per day continuously. We anticipate that the results of the ALTA trial will form the basis for our application for initial regulatory approval. We expect to achieve full patient enrollment in the ALTA trial in the third quarter of 2015 and to file for approval of brigatinib in the United States in 2016.
We are currently pursuing a corporate partnership to co-develop and co-commercialize brigatinib. We expect that such a partnership will allow us to continue to leverage our existing infrastructure and capabilities, as well as support a randomized, first-line trial of brigatinib vs. crizotinib in ALK+ NSCLC. A partnership may also support the exploration of new combination therapies in lung cancer that include brigatinib and other approved and unapproved medicines.
6
Table of Contents
AP32788
At the end of 2014, we nominated our next internally discovered development candidate, AP32788. This orally active TKI has a unique profile against a validated class of mutated targets in non-small cell lung cancer and certain other solid tumors and may address an unmet medical need. We expect to file an investigational new drug (IND) application for AP32788 in 2015 and to begin a Phase 1/2 proof-of-concept trial in 2016.
Ridaforolimus
Ridaforolimus is an investigational inhibitor of the mammalian target of rapamycin, or mTOR, that we discovered and developed internally and later licensed in 2010 to Merck & Co., Inc., or Merck, for oncology applications. Under the license agreement, Merck was responsible for all activities and funding of all of the costs related to the development, manufacturing and commercialization of ridaforolimus in oncology. In February 2014, we received notice from Merck that it was terminating the license agreement. Per the terms of the license agreement, this termination became effective in November 2014 at which time all rights to ridaforolimus in oncology licensed to Merck were returned to us. At the current time, we have no plans to develop ridaforolimus internally for oncology indications.
As an mTOR inhibitor, ridaforolimus has also been shown in preclinical studies to block the proliferation and migration of vascular smooth muscle cells, the primary cause of narrowing and blockage of injured arteries, and is an analog of sirolimus, another mTOR inhibitor that has been approved for use in drug-eluting stents. Clinical studies have found lower reblockage rates in patients treated with stents that deliver small-molecule drugs, such as sirolimus, everolimus or paclitaxel, a cytotoxic agent, locally to the site of vascular injury. Such stents have become the standard of care for many patients undergoing interventional procedures to open narrowed coronary arteries.
We have entered into non-exclusive license agreements with Medinol Ltd., or Medinol, a leading innovator in stent technology, and ICON Medical Corp., or ICON, an emerging medical device company, to develop and commercialize stents and other medical devices to deliver ridaforolimus to prevent restenosis, or reblockage, of injured vessels following interventions in which stents are used in conjunction with balloon angioplasty, as further described below under the caption “Our Licenses and Collaborations with Third Parties”.
Our Discovery Programs
Our research and development programs are focused on discovering and developing small-molecule drugs that regulate cell signaling for the treatment of cancer. Many of the critical functions of cells, such as cell growth, differentiation, gene transcription, metabolism, motility and survival, are dependent on signals carried back and forth from the cell surface to the nucleus and within the cell through a system of molecular pathways. When disrupted or over-stimulated, such pathways may trigger diseases such as cancer. Our research focuses on exploring cell-signaling pathways, identifying their role in specific cancers and cancer subtypes, and discovering drug candidates to treat those cancers by interfering with the aberrant signaling pathways of cells. The specific cellular proteins blocked by our product candidates have been well characterized as cancer targets. Iclusig and our product candidates, brigatinib, AP32788 and ridaforolimus, have been developed by us through the integrated use of structure-based drug design and computational chemistry, and their targets have been validated with techniques such as functional genomics, proteomics, and chemical genetics.
7
Table of Contents
Our Intellectual Property
Patents and other intellectual property rights are essential to our business. In general, we file patent applications to protect our technology, inventions and improvements to our inventions that we consider to be patentable and important to our business.
We solely own the following patents and patent applications for our product candidates:
| • | Iclusig, our pan BCR-ABL inhibitor, is protected by composition of matter claims of U.S. Patent No. 8,114,874, which is expected to expire on December 22, 2026, subject to a patent term adjustment, and corresponding international counterparts; |
| • | Brigatinib (AP26113), our investigational ALK inhibitor, is covered by composition of matter claims of a pending U.S. patent application, which, if granted, is expected to expire on or after 2029, depending on possible patent term adjustment and / or extension, and corresponding international counterparts; |
| • | Ridaforolimus, our mTOR inhibitor, is protected by composition of matter claims of U.S. Patent No. 7,091,213, which expires on February 3, 2023, and corresponding international counterparts; and |
| • | AP32788, our most recent oncology development candidate, is covered by composition of matter claims of a pending U.S. patent application, which, if granted, is expected to expire in or after 2035, depending on possible patent term adjustment and / or extension. |
In addition to the composition of matter patents and patent applications mentioned above, we also own other patents and patent applications covering manufacturing processes, polymorphs, formulations and uses that may provide additional protection of the respective product or product candidate.
The remainder of our patent portfolio is focused primarily on inventions involving additional classes of chemical compounds, the mTOR gene, and the components, configurations and use of our ARGENT regulation technologies, which we out-licensed in 2011 and are no longer pursuing internally.
We also rely on unpatented trade secrets and proprietary know-how, some of which is not believed to be adequately protectable through patents. In order to protect our trade secrets, we enter into confidentiality agreements with our employees, consultants, investigators, clinical trial sites, contractors, collaborators and other third parties to whom we disclose confidential information, although protection of trade secrets is generally recognized as challenging.
Our Licenses and Collaboration Agreements with Third Parties
Our Iclusig Commercialization and Co-Development Agreement with Otsuka
In December 2014, we entered into an agreement with Otsuka Pharmaceutical Co., Ltd., or Otsuka, for the commercialization and co-development of Iclusig in Japan and nine other Asian countries. Under the terms of the agreement, we will lead the completion of the Japanese New Drug Application, or JNDA, for Iclusig, and, based on current plans, Otsuka will file in 2015 the JNDA on behalf of both companies for regulatory approval in resistant and intolerant CML and Ph+ALL. The countries that are included in the agreement are Japan, China, South Korea, Indonesia, Malaysia, the Philippines, Singapore, Taiwan, Thailand and Vietnam.
Under the terms of the agreement, Otsuka was granted an exclusive license to market Iclusig in the specified territory and will perform certain development activities, at its expense. Otsuka will promote Iclusig as its sole tyrosine kinase inhibitor in the territory and, in connection therewith, has agreed to end its co-promotion arrangements for dasatinib in Japan. In addition, Otsuka will purchase Iclusig from us in bulk form, or potentially in final packaged form for certain countries, in dosage strengths approved in the territory.
In consideration for the exclusive license and other rights contained in the agreement, Otsuka paid us an upfront payment of $77.5 million, less a refundable Japan withholding tax of $15.8 million, and has agreed to pay us a milestone payment upon regulatory approval in Japan for patients with resistant and intolerant Philadelphia-positive leukemias, and additional milestone payments for approval in other indications. We will also be entitled to receive royalties and other payments representing a substantial share of net product sales. Following approvals in each country in the territory, Otsuka will conduct sales activities and record sales.
We have agreed to continue to fund the completion of our ongoing pivotal trial of Iclusig that will form the basis of the filing for regulatory approval in Japan, while Otsuka has agreed to fund additional agreed-upon clinical studies in the territory. For ARIAD-sponsored global studies that include sites in Japan, Otsuka has the option to contribute to the funding and gain access to the data for use in its territory.
8
Table of Contents
Unless terminated earlier, the agreement has a term that continues until the later of (x) the expiration of all royalty obligations in the territory, or (y) the last sale by Otsuka or its sub-licensees of a product in the territory under approvals obtained under the agreement. The agreement may be terminated (i) by either party based on insolvency or uncured breach by the other party or an unresolved, extended force majeure event affecting the other party, (ii) by Otsuka at its discretion on 180 days prior written notice, or (iii) by us upon the failure of Otsuka to submit an application for marketing authorization or launch Iclusig in Japan within specified dates, assuming such failure is not due to our failure to meet certain obligations, or other reasons beyond Otsuka’s control.
We and Otsuka have established a joint development and commercialization committee to oversee clinical development and commercialization of Iclusig in the territory, including approval of any development or commercialization plans. Each party has ultimate decision making authority with respect to a specified limited set of issues, and for all other issues, the matter must be resolved by consensus or by an expedited arbitration process.
For additional information on our agreement with Otsuka, please see Note 2 to our consolidated financial statements included in this report.
Our Ridaforolimus Collaboration and License Agreements with Merck
In July 2007, we entered into a collaboration agreement with Merck for the joint global development, manufacture and commercialization of ridaforolimus for use in cancer, referred to as the Collaboration Agreement. Under the Collaboration Agreement, Merck paid us an initial upfront fee of $75 million in 2007 and milestone payments of $53.5 million through the second quarter of 2010.
In May 2010, we entered into an amended and restated agreement with Merck, referred to as the License Agreement, which replaced the Collaboration Agreement, and a related supply agreement. Under the terms of the License Agreement, we granted Merck an exclusive license to develop, manufacture and commercialize ridaforolimus in oncology, and Merck assumed responsibility for all activities related to the development, manufacture and commercialization of ridaforolimus and agreed to fund 100 percent of all ridaforolimus costs incurred after January 1, 2010. Under the License Agreement, Merck paid us an initial upfront fee of $50 million in the second quarter of 2010 and a $25 million milestone payment in the third quarter of 2011.
In February 2014, we received notice from Merck that it was terminating the License Agreement, effective in November 2014. Upon termination of this agreement, all rights to ridaforolimus that we had licensed to Merck were returned to us.
Our Ridaforolimus Stent Collaborations
In January 2005, we entered into a license agreement with Medinol and in October 2007 we entered into a license agreement with ICON, to develop and commercialize ridaforolimus-eluting stents and other medical devices to prevent restenosis, or reblockage, of injured vessels following interventions in which stents are used in conjunction with balloon angioplasty. Under these agreements, we granted to each of Medinol and ICON a non-exclusive, world-wide, royalty-bearing license, under our patents and
9
Table of Contents
technology relating to ridaforolimus, to develop, manufacture and sell the stents and certain other medical devices that deliver ridaforolimus. We are responsible for supplying Medinol and ICON with, and they have agreed to purchase from us, certain quantities of ridaforolimus for use in its development, manufacture and sale of the stents and other medical devices.
The agreement with Medinol provides for the payment by Medinol to us of up to $39.3 million, which includes an upfront license fee and payments based upon achievement of development, regulatory and commercial milestones, if two products are developed. Through December 31, 2014, we had received an upfront fee of $750,000 and milestone payments of $3.8 million following the initiation of two registration trials and the filing of an IDE with the FDA in January 2014. In addition, we are eligible to receive tiered single-digit royalties based on various minimum levels of stents or other medical devices sold under the agreement. As of December 31, 2014, no products have been approved by regulatory authorities for sale under this agreement.
The agreement with ICON provides for the payment by ICON to us of up to $27.4 million based upon achievement of certain clinical, regulatory and commercial milestones, if two products are developed. Through December 31, 2014, we have received no such payments under the agreement. In addition, we are eligible to receive single-digit royalties based on net sales of stents or other medical devices sold under the agreement. As of December 31, 2014, no products have been approved by regulatory authorities for sale under this agreement. Concurrent with the execution of the license agreement with ICON, we received shares of ICON common stock equal to an ownership interest in ICON of less than 10 percent and certain other rights, including maintenance, anti-dilution and registration rights.
The terms of both the Medinol and ICON agreements extend to the later to occur of the expiration of our patents relating to the rights licensed to Medinol or ICON under the agreement or 15 years after the first commercial sale of a product. The agreements may be terminated by either party for breach following the failure to cure after a 90-day cure period. In addition, either Medinol or ICON may terminate its agreement upon 30 days’ notice to us upon certain events, including if it determines, in its reasonable business judgment, that it is no longer in its business interest to continue the development of a medical device to deliver ridaforolimus. We may terminate the agreements upon 30 days’ notice to Medinol or ICON, if we determine that it is no longer in our business interest to continue our development and regulatory approval efforts with respect to ridaforolimus.
Licenses Related to ARGENT Technology
In 2011, we executed three exclusive out-license agreements for separate aspects of our ARGENT cell-signaling regulation technology, which we are no longer pursuing internally. The licenses to these non-core assets provide us with a combination of equity ownership in the licensees, upfront payments, ongoing fees for supply of certain research reagents, and potential milestone and royalty payments linked to clinical, regulatory and sales achievements of the licensees.
Under one of these license agreements, Bellicum Pharmaceuticals, Inc., or Bellicum, is developing cell therapies to treat cancers and other chronic and life-threatening diseases. Bellicum’s proprietary cell-based therapies utilize our ARGENT technology and our small-molecule drug rimiducid (AP1903), under the terms of the license agreement. In October 2014, we and Bellicum entered into an Omnibus Amendment Agreement, or the Omnibus Agreement, that restructures the previous license agreement. Under the terms of the Omnibus Agreement, we received $15 million upon execution of the agreement and were entitled to receive two payments of $20 million and $15 million by specified dates in the future in exchange for granting Bellicum a fully paid-up license to this technology and return of shares of Bellicum common stock owned by us. The second and third installment payments could be accelerated under certain circumstances and prepaid at any time. On December 23, 2014, Bellicum completed its initial public offering, and on December 29, 2014, Bellicum paid the $20 million and $15 million payments owed to us under the agreement and we returned the shares of Bellicum common stock owned by us.
10
Table of Contents
The Omnibus Agreement gives Bellicum a worldwide exclusive license, with the right to sublicense, to our cell-signaling technology for broad use in human cell therapies for all diseases on a royalty- and milestone-free basis. The Omnibus Agreement can be terminated by either party upon a specified uncured material breach of the license agreement or the Omnibus Agreement.
The ARGENT technology platform combines chemistry and genetics to allow specific cell-signaling and gene-expression events to be chemically activated in whole animals and cultured cells. The technology platform includes a portfolio of distinct small-molecule “dimerizer” compounds optimized for specific applications. Dimerizers bring specific proteins together in cells. The technology allows intracellular processes to be controlled with small molecules, which may be useful in the development of therapeutic vaccines and gene and cell therapy products, and which provide versatile tools for applications in cell biology, functional genomics and drug-discovery research. The technology is being developed to treat human disease through cancer vaccines, cell therapy and gene therapy, in each case featuring small-molecule regulation of cellular activation.
Manufacturing
Iclusig and our drug candidates and preclinical compounds are small molecules that can be readily synthesized by processes that we have developed. We are able to manufacture in-house the quantities of our product candidates necessary for certain preclinical studies. We do not own or operate manufacturing facilities for the production of clinical or commercial quantities of Iclusig or our product candidates. We contract with third-party manufacturers to assist in the development and optimization of our manufacturing processes and methods and to supply our product candidates in sufficient bulk quantities and in suitable dosage forms for use in our clinical trials. We also expect to continue to depend on third-party manufacturers for the supply of our products for commercialization in the United States, Europe and other territories in which we may sell Iclusig.
Iclusig and brigatinib are produced by established manufacturing processes using conventional organic chemical synthesis. The production of Iclusig and brigatinib is based on technology that we believe is proprietary to us. We have established relationships with third parties for the manufacture of Iclusig clinical and commercial supply and have existing agreements for our supply of drug substance, drug product and distribution.
Ridaforolimus is produced by an established manufacturing process using conventional synthetic and natural-product fermentation techniques. The production of ridaforolimus is based in part on technology that we believe is proprietary to us. Upon termination of the license agreement with Merck in November 2014, all rights to ridaforolimus were returned to us and we will be responsible for all manufacturing required for any continued development of the drug candidate. We have established relationships with third parties for the manufacture of ridaforolimus drug substance for our use, including any use by our medical device collaborators.
Contract manufacturers are subject to extensive governmental regulation and we depend on them to manufacture Iclusig and our product candidates in accordance with the FDA’s current good manufacturing practice regulations, or cGMPs. We have an established quality assurance program designed to ensure that our contract manufacturers produce our compounds in accordance with cGMPs, and other applicable domestic and foreign regulations. We believe that our current contractors comply with such regulations.
Competition
The pharmaceutical and biotechnology industries are intensely competitive. We compete directly and indirectly with other pharmaceutical companies, biotechnology companies and academic and research organizations, many of whom have greater resources than we do. We compete with companies who have products on the market or in development in the same class or for the same indications as our product candidates. We may also compete with organizations that are developing similar technology platforms.
11
Table of Contents
In the area of oncology, pharmaceutical and biotechnology companies such as Amgen Inc., AstraZeneca PLC, Bristol-Myers Squibb Company, Celgene Corporation, Eli Lilly and Company, the Roche Group, GlaxoSmithKline plc, Johnson & Johnson, Merck, Merck KGaA, Novartis AG, Pfizer, Inc., Sanofi-Aventis, Takeda Pharmaceutical Co., Ltd., and Teva Pharmaceutical Industries Ltd. are developing and marketing drugs to treat cancer.
Bristol-Myers Squibb, Novartis and Pfizer are currently marketing TKIs for the treatment of patients with CML that compete with Iclusig. Novartis’ imatinib is marketed in the first-line setting, Bristol-Myers Squibb’s dasatinib and Novartis’ nilotinib are marketed for patients in the first-line setting, as well as in those patients who have failed imatinib therapy; and Pfizer’s bosutinib is marketed for patients for whom therapy with other TKIs has failed. These drugs generated approximately $6 billion in revenues during 2014, according to reported results from Bristol-Myers Squibb, Novartis and Pfizer. In the resistance/intolerance market, Teva’s omacetaxine mepesuccinate, a non-TKI, is also marketed in the United States for patients for whom therapy with other TKIs has failed. Other than imatinib, all of these products compete with Iclusig in the resistance/intolerance market. In January 2012, Il-Yang Pharmaceutical gained approval in South Korea for radotinib, a locally developed TKI for the treatment of CML patients.
Pfizer, Novartis and Roche/Chugai have launched ALK inhibitors that will compete with brigatinib should it gain marketing approval. In addition, there are several other ALK inhibitors in various stages of development that could also compete with brigatinib. In August 2011, Pfizer obtained US approval for and is currently marketing crizotinib for patients with ALK-positive non-small cell lung cancer. In 2012, crizotinib was approved for marketing in Japan and the European Union. In April 2014, Novartis obtained US approval for ceritinib and filed for approval in the European Union. In September 2014, Chugai Pharmaceutical Co. obtained approval for alectinib in Japan. Tesaro, Ignyta and Xcovery also have ALK inhibitors in development and Pfizer is developing a follow-on compound to crizotinib.
We cannot be certain that Iclusig or brigatinib, if approved, will be commercially successful. In addition to the other challenges related to a company launching its first commercial drug, we face competition from TKIs and ALK inhibitors that are currently approved for the treatment of patients with CML and NSCLC, respectively, and any new products that may be approved. While we believe that Iclusig and brigatinib have competitive commercial profiles compared to the existing therapies on the market and products in late-stage development, our current estimates of the potential competitiveness of Iclusig and brigatinib compared to existing therapies and the revenues that Iclusig and brigatinib, if approved, could generate in future periods are subject to various risks and uncertainties, including those set forth in “Risk Factors” in Part I, Item 1A of this Annual Report under the caption “Risks relating to the development and commercialization of our products and product candidates.”
We may also experience competition from companies that have acquired or may acquire technology from companies, universities, and other research institutions. As these companies develop their technologies, they may develop proprietary positions that may materially and adversely affect us.
Government Regulation and Product Approval
Governmental authorities in the United States, at the federal, state and local level, and in other countries extensively regulate, among other things, the research, development, testing, manufacture, quality control, approval, labeling, packaging, storage, record-keeping, promotion, advertising, distribution, marketing and export and import of products such as Iclusig and others we are developing. Our product candidates must be approved by the FDA through the new drug application, or NDA, process before they may be legally marketed in the United States and by the EMA through the marketing authorization application, or MAA, process before they may be legally marketed in Europe. Our product candidates will be subject to
12
Table of Contents
similar requirements in other countries prior to marketing in those countries. The process of obtaining regulatory approvals in the United States and in foreign countries, along with subsequent compliance with applicable statutes and regulations, requires the expenditure of substantial time and financial resources.
United States Drug Development Process
In the United States, the FDA regulates drugs under the Federal Food, Drug, and Cosmetic Act, or FDCA, and implementing regulations. Failure to comply with the applicable U.S. requirements at any time during the product development process or approval process, or after approval, may subject an applicant to administrative or judicial sanctions, any of which could have a material adverse effect on the applicant. These sanctions could include:
| • | refusal to approve pending applications; |
| • | withdrawal of an approval; |
| • | imposition of a clinical hold; |
| • | warning letters; |
| • | product seizures; |
| • | total or partial suspension of production or distribution; or |
| • | injunctions, fines, disgorgement, or civil or criminal penalties. |
The process required by the FDA before a drug may be marketed in the United States generally involves the following:
| • | completion of preclinical laboratory tests, animal studies and formulation studies according to Good Laboratory Practices, or GLPs, or other applicable regulations; |
| • | submission to the FDA of an Investigational New Drug Application, or IND, which must become effective before human clinical trials may begin; |
| • | performance of adequate and well-controlled human clinical trials according to Good Clinical Practices, or GCPs, and other applicable requirements to establish the safety and efficacy of the proposed drug for its intended use; |
| • | submission to the FDA of an NDA; |
| • | satisfactory completion of an FDA inspection of the manufacturing facility or facilities at which the drug is produced to assess compliance with current Good Manufacturing Practices, or cGMP, to assure that the facilities, methods and controls are adequate to preserve the drug’s identity, strength, quality and purity; and |
| • | FDA review and approval of the NDA. |
As part of the IND, an IND sponsor must submit to the FDA the results of preclinical tests, which may include laboratory evaluations and animal studies, together with manufacturing information and analytical data, and the proposed clinical protocol for the first phase of the clinical trial of the drug. The IND automatically becomes effective 30 days after receipt by the FDA, unless the FDA, within the 30-day time period, places the clinical trial on a “clinical hold” because of safety concerns or perceived procedural deficiencies. In such a case, the IND sponsor and the FDA must resolve any outstanding concerns before clinical trials may begin. A clinical hold may be imposed by the FDA at any time during the life of an IND, and may affect one or more specific studies or all studies conducted under the IND.
All clinical trials must be conducted under the supervision of one or more qualified investigators in accordance with GCP’s. They must be conducted under protocols detailing the objectives of the trial, dosing procedures, research subject selection and exclusion criteria and the safety and effectiveness criteria to be evaluated. Each protocol must be submitted to the FDA as part of the IND, and progress reports detailing the status of the clinical trials must be submitted to the FDA annually. Sponsors also must timely report to FDA serious and unexpected adverse reactions, any clinically important increase in the rate of serious suspected adverse reactions over those listed in the protocol or investigators’ brochure, or any findings from other studies or animal or in vitro testing that suggest a significant risk to humans exposed to the drug. An institutional review board, or IRB, must also review and approve each new clinical protocol and patient informed consent form prior to commencement of the corresponding clinical trial at each institution where a trial is to be performed.
13
Table of Contents
Human clinical trials are typically conducted in three sequential phases that may overlap or be combined:
| • | Phase 1: The drug is initially introduced into healthy human subjects and tested for safety, dosage tolerance, absorption, metabolism, distribution and excretion. In the case of some products for severe or life-threatening diseases, such as cancer, especially when the product may be inherently too toxic to ethically administer to healthy volunteers, the initial human testing is often conducted in patients. |
| • | Phase 2: Clinical trials are performed on a limited patient population intended to identify possible adverse effects and safety risks, to preliminarily evaluate the efficacy of the product for specific targeted diseases and to determine dosage tolerance and optimal dosage. |
| • | Phase 3: Clinical trials are undertaken to further evaluate dosage, clinical efficacy and safety in an expanded patient population at geographically dispersed clinical study sites. These studies are intended to establish the overall risk-benefit ratio of the product and provide, if appropriate, an adequate basis for product labeling. |
Human clinical trials are inherently uncertain and Phase 1, Phase 2 and Phase 3 testing may not be successfully completed. The FDA or the sponsor may suspend a clinical trial at any time for a variety of reasons, including a finding that the research subjects or patients are being exposed to an unacceptable health risk. Similarly, an IRB can suspend or terminate approval of a clinical trial at its institution if the clinical trial is not being conducted in accordance with the IRB’s requirements or if the drug has been associated with unexpected serious harm to patients.
During the development of a new drug, sponsors are given an opportunity to meet with the FDA at certain points. These points may be prior to submission of an IND, at the end of Phase 2, and before an NDA is submitted. These meetings can provide an opportunity for the sponsor to share information about the data gathered to date and for the FDA to provide advice on the next phase of development. Sponsors typically use the end-of-Phase 2 meeting to discuss their Phase 2 clinical results and present their plans for the pivotal Phase 3 clinical trial that they believe will support approval of the new drug.
Concurrent with clinical trials, sponsors usually complete additional animal safety studies and also develop additional information about the chemistry and physical characteristics of the drug and finalize a process for manufacturing commercial quantities of the product in accordance with cGMP requirements. The manufacturing process must be capable of consistently producing quality batches of the drug and the manufacturer must develop methods for testing the quality, purity and potency of the drug. Additionally, appropriate packaging must be selected and tested and stability studies must be conducted to demonstrate that the drug candidate does not undergo unacceptable deterioration over its proposed shelf-life.
United States Drug Review and Approval Processes
The results of product development, preclinical studies and clinical trials, along with descriptions of the manufacturing process, analytical tests conducted on the chemistry of the drug, proposed labeling, and other relevant information are submitted to the FDA as part of an NDA requesting approval to market the product.
The FDA reviews all NDAs submitted to ensure that they are sufficiently complete for substantive review before it accepts them for filing. It may request additional information rather than accept an NDA for filing. In this event, the NDA must be resubmitted with the additional information. The resubmitted application also is subject to review before the FDA accepts it for filing.
14
Table of Contents
Once the submission is accepted for filing, the FDA begins an in-depth and substantive review. The FDA reviews an NDA to determine, among other things, whether a product is safe and effective for its intended use and whether its manufacturing is cGMP-compliant. The FDA may seek advice and a recommendation from an external advisory committee as to whether the application should be approved and under what conditions. The FDA is not bound by the recommendation of an advisory committee, but it generally follows such recommendations. Before approving an NDA, the FDA will inspect the facility or facilities where the product is manufactured and tested. The FDA may refuse to approve an NDA if the applicable regulatory criteria are not satisfied or may require submission of additional clinical or other data and information which, upon agency review and interpretation, may or may not be deemed by the FDA to satisfy the criteria for approval. The FDA may also issue a “complete response” letter, which may require additional clinical or other data or impose other conditions that must be met in order to secure final approval of the NDA.
NDAs receive either standard or priority review. A drug representing a significant improvement in treatment, prevention or diagnosis of disease may receive priority review. In addition, products studied for their safety and effectiveness in treating serious or life-threatening illnesses and that provide meaningful therapeutic benefit over existing treatments may receive accelerated approval and may be approved on the basis of adequate and well-controlled clinical trials establishing that the drug product has an effect on a surrogate endpoint that is reasonably likely to predict clinical benefit or on the basis of an effect on a clinical endpoint other than survival or irreversible morbidity. In such a situation, a drug may be approved based on a Phase 2 pivotal trial, as was the case with Iclusig. As a condition of approval, the FDA may require that a sponsor of a drug receiving accelerated approval perform adequate and well-controlled post-marketing clinical trials. Priority review and accelerated approval do not change the standards for approval, but may expedite the approval process.
Expedited Review and Approval
The FDA has various programs, including Fast Track, Breakthrough Therapy designation, priority review, and accelerated approval, which are intended to expedite or simplify the process for reviewing drugs, and/or provide for the approval of a drug on the basis of a surrogate endpoint. Even if a drug qualifies for one or more of these programs, the FDA may later decide that the drug no longer meets the conditions for qualification or that the time period for FDA review or approval will be shortened. Generally, drugs that are eligible for these programs are those for serious or life-threatening conditions, those with the potential to address unmet medical needs and those that offer meaningful benefits over existing treatments. For example, Fast Track is a process designed to facilitate the development and expedite the review of drugs to treat serious or life-threatening diseases or conditions and fill unmet medical needs. Priority review is designed to give drugs that offer major advances in treatment or provide a treatment where no adequate therapy exists an initial review within six months as compared to a standard review time of ten months.
Although Fast Track and priority review do not affect the standards for approval, the FDA will attempt to facilitate early and frequent meetings with a sponsor of a Fast Track designated drug and expedite review of the application for a drug designated for priority review. Accelerated approval, which is described in Subpart H of 21 Code of Federal Regulations, or 21 CFR Part 314, provides for an earlier approval for a new drug that is intended to treat a serious or life-threatening disease or condition and that fills an unmet medical need based on a surrogate endpoint. A surrogate endpoint is a clinical measurement or other biomarker used as an indirect or substitute measurement to predict a clinically meaningful outcome. As a condition of approval, the FDA may require that a sponsor of a product candidate receiving accelerated approval perform post-marketing clinical trials. We received accelerated approval for Iclusig and are required to perform post-marketing clinical trials.
In the Food and Drug Administration Safety and Innovation Act, or FDASIA, which was signed into law in July 2012, Congress encouraged the FDA to utilize innovative and flexible approaches to the assessment of products under accelerated approval. The law required the FDA to issue related draft guidance within a year after the law’s enactment and also promulgate confirming regulatory changes. In June 2013, the FDA
15
Table of Contents
published a draft Guidance for Industry entitled, “Expedited Programs for Serious Conditions––Drugs and Biologics” which provides guidance on FDA programs that are intended to facilitate and expedite development and review of new drugs as well as threshold criteria generally applicable to concluding that a drug is a candidate for these expedited development and review programs. In addition to the Fast Track, accelerated approval and priority review programs discussed above, the FDA also provided guidance on a new program for Breakthrough Therapy designation. A Breakthrough Therapy designation is intended to expedite the development and FDA review of drugs for serious or life-threatening conditions. A request for Breakthrough Therapy designation should be submitted concurrently with, or as an amendment to an IND. In 2014, brigatinib received a Breakthrough Therapy designation from the FDA for the treatment of patients with ALK-positive NSCLC whose tumors are resistant to crizotinib.
Risk Evaluation and Mitigation Strategy
The Food and Drug Administration Amendments Act of 2007, or FDAAA, created a new section of the FDCA which authorizes the FDA to require a risk evaluation and mitigation strategy, or REMS, when necessary to ensure that the benefits of a drug outweigh the risks. The FDA may require, among other things, that an applicant develop a Medication Guide for distribution to each patient when the drug is dispensed, a communication plan to health care providers, and other elements to insure safe use, or ETASUs. Medication Guides may be safety-related, addressing serious risk(s) (relative to benefits) of which patients should be made aware, and/or efficacy-related, when patient adherence to directions for use is crucial to the drug’s effectiveness. Since the enactment of FDAAA, the FDA has considered any new Medication Guide (or safety-related changes to an existing Medication Guide) to be part of a REMS. However, the FDA has the authority to determine, based on the risks of a drug and public health concern, whether a Medication Guide should be required as part of a REMS and may decide the Medication Guide should be required as labeling but not part of a REMS if the FDA determines that a REMS is not necessary to ensure the benefits of the drug outweigh its risks. Depending on the known or anticipated risks, the ETASU may require prescribers to have specific experience, or require pharmacies, practitioners or healthcare settings that dispense the drug to be specially certified and patients receiving the drug to be regularly monitored or enrolled in a registry. In addition, the drug’s sponsor may be required to take reasonable steps to monitor and evaluate those in the healthcare system responsible for implementing ETASU measures. All REMS include a timetable for assessments and may be modified as necessary.
As part of our discussions with the FDA in December 2013 to allow us to resume marketing and commercial distribution of Iclusig in the United States, we implemented a REMS for Iclusig.
Orphan Drug Designation and Approval
Under the Orphan Drug Act, the FDA may grant orphan drug designation to a drug intended to treat a rare disease or condition which is defined as one affecting fewer than 200,000 individuals in the United States or more than 200,000 individuals where there is no reasonable expectation that the product development cost will be recovered from product sales in the United States. Orphan drug designation must be requested before submitting an NDA and does not convey any advantage in, or shorten the duration of, the regulatory review and approval process.
If an orphan drug-designated product subsequently receives the first FDA approval for the disease for which it was designed, the product will be entitled to seven years of product exclusivity, which means that the FDA may not approve any other applications to market the same drug for the same indication, except in very limited circumstances, for seven years. In 2013, we received orphan drug exclusivity approval of Iclusig for the treatment of adult patients with chronic, accelerated or blast phase CML, who are resistant or intolerant to prior TKI therapy, and the treatment of adult patients with Ph+ ALL who are resistant or intolerant to prior TKI therapy. This seven year exclusivity period began on December 14, 2012, the date of approval of our NDA.
16
Table of Contents
Patent Term Restoration and Marketing Exclusivity
Some of our U.S. patents may be eligible for limited patent term extension under the Drug Price Competition and Patent Term Restoration Act of 1984, referred to as the Hatch-Waxman Act. The Hatch-Waxman Act permits a patent restoration term of up to five years as compensation for patent term lost during product development and the FDA regulatory review process. However, patent term restoration cannot extend the remaining term of a patent beyond a total of 14 years from the product’s approval date. The patent term restoration period is generally one-half the time between the effective date of an IND, and the submission date of an NDA, plus the time between the submission date of an NDA and the approval of that application. Only one patent applicable to an approved drug is eligible for the extension and the application for extension must be made prior to expiration of the patent. The United States Patent and Trademark Office, in consultation with the FDA, reviews and approves the application for any patent term extension or restoration.
We have applied for restorations of patent term for some of our patents to add patent life beyond their current expiration date, as described above under the caption “Our Intellectual Property”. We may apply for restorations of patent terms for our other patents depending on the expected length of clinical trials and other factors involved in the submission of the relevant NDA.
Market exclusivity provisions under the FDCA also can delay the submission or the approval of certain applications. The FDCA provides a five-year period of non-patent marketing exclusivity within the United States to the first applicant to gain approval of an NDA for a new chemical entity. A drug is a new chemical entity if the FDA has not previously approved any other new drug containing the same active moiety, which is the molecule or ion responsible for the action of the drug substance. During the exclusivity period, the FDA may not accept for review an abbreviated new drug application, or ANDA, or a 505(b)(2) NDA submitted by another company for another version of such drug where the applicant does not own or have a legal right of reference to all the data required for approval. However, an application may be submitted after four years if it contains a certification of patent invalidity or non-infringement. The FDCA also provides three years of marketing exclusivity for an NDA, 505(b)(2) NDA or supplement to an approved NDA if new clinical investigations, other than bioavailability studies, that were conducted or sponsored by the applicant are deemed by the FDA to be essential to the approval of the application, for example, for new indications, dosages or strengths of an existing drug. This three-year exclusivity covers only the conditions associated with the new clinical investigations and does not prohibit the FDA from approving ANDAs for drugs containing the original active ingredient. Five-year and three-year exclusivity will not delay the submission or approval of a full NDA; however, an applicant submitting a full NDA would be required to conduct or obtain a right of reference to all of the preclinical studies and adequate and well-controlled clinical trials necessary to demonstrate safety and effectiveness or perform that work itself.
Pediatric Exclusivity and Pediatric Use
Under the Best Pharmaceuticals for Children Act, or BPCA, certain drugs may obtain an additional six months of exclusivity, if the sponsor submits information requested in writing by the FDA, or a Written Request, relating to the use of the active moiety of the drug in children. The FDA may not issue a Written Request for studies on unapproved or approved indications or where it determines that information relating to the use of a drug in a pediatric population, or part of the pediatric population, may not produce health benefits in that population.
To receive the six-month pediatric market exclusivity, we would have to receive a Written Request from the FDA, conduct the requested studies in accordance with a written agreement with the FDA or, if there is no written agreement, in accordance with commonly accepted scientific principles, and submit reports of the studies. A Written Request may include studies for indications that are not currently in the labeling if the FDA determines that such information will benefit the public health. The FDA will accept the
17
Table of Contents
reports upon its determination that the studies were conducted in accordance with and are responsive to the original Written Request or commonly accepted scientific principles, as appropriate, and that the reports comply with the FDA’s filing requirements. We have not received a Written Request for such pediatric studies, although we may ask the FDA to issue a Written Request for such studies in the future.
In addition, the Pediatric Research Equity Act, or PREA, requires a sponsor to conduct pediatric studies for most drugs and biologicals, for a new active ingredient, new indication, new dosage form, new dosing regimen or new route of administration. Under PREA, original NDAs, biologics license applications and supplements thereto, must contain a pediatric assessment unless the sponsor has received a deferral or waiver. The required assessment must address the safety and effectiveness of the product for the claimed indications in all relevant pediatric subpopulations and support dosing and administration for each pediatric subpopulation for which the product is safe and effective. The sponsor or FDA may request a deferral of pediatric studies for some or all of the pediatric subpopulations. A deferral may be granted for several reasons, including a finding that the drug or biologic is ready for approval for use in adults before pediatric studies are complete or that additional safety or effectiveness data needs to be collected before the pediatric studies begin. After April 2013, the FDA must send a non-compliance letter to any sponsor that fails to submit the required assessment, to keep a deferral current or to submit a request for approval of a pediatric formulation.
As part of the FDASIA, Congress made a few revisions to BPCA and PREA, which were slated to expire on September 30, 2012, and made both laws permanent.
Post-Approval Requirements
Once an approval is granted, the FDA may withdraw the approval if compliance with regulatory requirements is not maintained or if problems occur after the product reaches the market. Later discovery of previously unknown problems with a product may result in restrictions on the product or even complete withdrawal of the product from the market. After approval, some types of changes to the approved product, such as adding new indications, manufacturing changes and additional labeling claims, are subject to further FDA review and approval. In addition, the FDA may require testing and surveillance programs to monitor the effect of approved products that have been commercialized, and the FDA has the power to prevent or limit further marketing of a product based on the results of these post-marketing programs. Since October 2013, Iclusig has been subject to these types of post-approval restrictions and requirements, as described above under the caption “Iclusig (ponatinib)”
Any drug products manufactured or distributed by us pursuant to FDA approvals are subject to continuing regulation by the FDA, including, among other things:
| • | record-keeping requirements; |
| • | reporting of adverse experiences with the drug; |
| • | providing the FDA with updated safety and efficacy information; |
| • | drug sampling and distribution requirements; |
| • | notifying the FDA and gaining its approval of specified manufacturing or labeling changes; and |
| • | complying with FDA promotion and advertising requirements. |
Drug manufacturers and other entities involved in the manufacture and distribution of approved drugs are required to register their establishments with the FDA and certain state agencies, and are subject to periodic unannounced inspections by the FDA and some state agencies for compliance with cGMP and other laws.
We rely, and expect to continue to rely, on third parties for the production of clinical and commercial supplies of our products. Future FDA and state inspections may identify compliance issues at the facilities of our contract manufacturers that may disrupt production or distribution, or require substantial resources to correct.
18
Table of Contents
From time to time, legislation is drafted, introduced and passed in Congress that could significantly change the statutory provisions governing the approval, manufacturing and marketing of products regulated by the FDA. In addition, FDA regulations and guidance are often revised or reinterpreted by the agency in ways that may significantly affect our business and our products. It is impossible to predict whether legislative changes will be enacted, or FDA regulations, guidance or interpretations changed or what the impact of such changes, if any, may be.
Foreign Regulation
In addition to regulations in the United States, we are subject to a variety of foreign regulations governing clinical trials and commercial sales and distribution of our products. Whether or not we obtain FDA approval for a product, we must obtain approval by the comparable regulatory authorities of foreign countries before we can commence clinical trials or marketing of the product in those countries. The approval process varies from country to country and the time may be longer or shorter than that required for FDA approval. The requirements governing the conduct of clinical trials, product licensing, pricing and reimbursement vary greatly from country to country.
Under European Union regulatory systems, a company may submit marketing authorization applications either under a centralized or decentralized procedure. The centralized procedure, which is compulsory for medicines produced by biotechnology or those medicines intended to treat AIDS, cancer, neurodegenerative disorders or diabetes and optional for those medicines which are highly innovative, provides for the grant of a single marketing authorization that is valid for all European Union member states. The decentralized procedure provides for mutual recognition of national approval decisions. Under this procedure, the holder of a national marketing authorization may submit an application to the remaining member states. Within 90 days of receiving the applications and assessments report, each member state must decide whether to recognize approval. If a member state does not recognize the marketing authorization, the disputed points are eventually referred to the European Commission, whose decision is binding on all member states.
As in the United States, the EMA may grant orphan drug status for specific indications if the request is made before an MAA is made. The EMA considers an orphan medicinal product to be one that affects less than five of every 10,000 people in the European Union. A company whose application for orphan drug designation in the European Union is approved is eligible to receive, among other benefits, regulatory assistance in preparing the marketing application, protocol assistance and reduced application fees. Orphan drugs in the European Union also enjoy economic and marketing benefits, including up to ten years of market exclusivity for the approved indication, unless another applicant can show that its product is safer, more effective or otherwise clinically superior to the orphan designated product.
Reimbursement
Sales of Iclusig and any of our product candidates, if approved, will depend, in part, on the extent to which the costs of the products will be covered by third-party payors, including government health programs such as Medicare and Medicaid, commercial health insurers and managed care organizations. These third-party payors are increasingly challenging the prices charged for health care products and services. The U.S. government, state legislatures and foreign governments have shown significant interest in implementing cost-containment programs, including price controls, restrictions on reimbursement and requirements for substitution of generic products. Adoption of price controls and cost-containment measures, and adoption of more restrictive policies in jurisdictions with existing controls and measures, could further limit our net revenue. If these third-party payors do not consider our products to be cost-effective compared to other available therapies, they may not cover our products after approval as a benefit under their plans or, if they do, the level of payment may not be sufficient to allow us to sell our products on a profitable basis.
19
Table of Contents
The Medicare Prescription Drug, Improvement, and Modernization Act of 2003, or the “MMA,” imposes requirements for the distribution and pricing of prescription drugs for Medicare beneficiaries. Under Part D (the Medicare prescription drug benefit), Medicare beneficiaries may enroll in prescription drug plans offered by private entities that provide coverage of outpatient prescription drugs not covered under Medicare Part B (fee-for-service medical insurance). Part D prescription drug plan sponsors are not required to pay for all covered Part D drugs. Each drug plan can develop its own drug formulary that identifies which drugs it will cover and at what tier or level. Federal regulations require Part D prescription drug formularies to include drugs within each therapeutic category and class of covered Part D drugs, although not necessarily all the drugs in each category or class.
In general, government payment for some of the costs of prescription drugs may increase demand for products for which we receive marketing approval. However, any negotiated prices for our products covered by a Part D prescription drug plan will likely be lower than the prices we might otherwise obtain. Moreover, while the MMA applies only to drug benefits for Medicare beneficiaries, private payors often follow Medicare coverage policy and payment limitations in setting their own payment rates. Any reduction in payment that results from the MMA or other Medicare regulations may result in a similar reduction in payments from non-governmental payors.
The Patient Protection and Affordable Care Act and the Health Care and Education Affordability Reconciliation Act of 2010 (collectively, the Affordable Care Act or “ACA,”) was enacted in March 2010 and has had a significant impact on the health care industry. The ACA mandated prescription drug coverage as one of ten essential health benefits that most health plans must offer, requiring coverage of at least one drug in every category and class. The ACA also expanded coverage for the uninsured through the new health insurance exchanges and a significant increase in the number of individuals eligible for Medicaid coverage. The ACA also prevents health insurers from charging more, denying, or limiting coverage for individuals with pre-existing conditions (i.e. individuals whose health care costs are typically higher).
Because of the significant increase in the number of individuals covered and the expansion of the coverage that must be provided to them, commercial insurers and government programs have increased their emphasis on cost controls to reduce overall spending. For example, the ACA expanded and increased mandatory industry rebates for drugs covered under Medicaid. Pharmaceutical manufacturers are required to provide drug rebates to the federal government and most state governments in order to have the product eligible for Medicaid coverage. In addition, commercial insurers offering Medicaid managed care products seek to negotiate additional rebates. The ACA also made changes to the drug coverage requirements under the Medicare Part D program. Although the changes have not yet been enacted, the Center for Medicine Services, or CMS, has proposed decreasing the number of categories and classes of drugs Part D plan sponsors must cover. Because of these cost controls, it is hard to determine what impact the ACA’s expansion in coverage might have on pharmaceutical companies.
We expect that there will continue to be a number of federal and state proposals to implement governmental pricing controls and limit the growth of health care costs, including the cost of prescription drugs, while at the same time increasing access to health care services. At the present time, Medicare is prohibited from negotiating directly with pharmaceutical companies for drugs. There have been legislative proposals seeking to allow such direct negotiation. It is possible that the adoption of other legislative or regulatory proposals could have a material adverse effect on our business, financial condition and results of operations.
In addition, in some foreign countries, the proposed pricing for a drug must be approved before it may be lawfully marketed. The requirements governing drug pricing vary widely from country to country. For example, the European Union provides options for its member states to restrict the range of medicinal products for which their national health insurance systems provide reimbursement and to control the prices of medicinal products for human use. A member state may approve a specific price for the
20
Table of Contents
medicinal product or it may instead adopt a system of direct or indirect controls on the profitability of the company placing the medicinal product on the market. There can be no assurance that any country that has price controls or reimbursement limitations for pharmaceutical products will allow favorable reimbursement and pricing arrangements for any of our products. Historically, products launched in the European Union do not follow price structures of the United States and generally tend to be significantly lower.
Other United States Regulations
Pharmaceutical companies also are subject to various federal and state laws pertaining to health care “fraud and abuse,” including anti-kickback laws and false claims laws, and the reporting of payments to physicians and teaching hospitals.
Anti-Kickback Laws
U.S. federal laws prohibit fraud and abuse involving state and federal health care programs, such as Medicare and Medicaid. The anti-kickback law prohibits, among other things, knowingly and willfully offering, paying, soliciting, receiving or providing remuneration, directly or indirectly, in exchange for or to induce either the referral of an individual, or the furnishing, arranging for or recommending of an item or service that is reimbursable, in whole or in part, by a federal health care program. Remuneration is broadly defined to include anything of value, such as cash payments, gifts or gift certificates, discounts, or the furnishing of services, supplies or equipment. Many states have implemented their own health care anti-kickback laws, regulating similar conduct as the federal anti-kickback law. State anti-kickback laws can extend more broadly to services covered by private insurers or third party payors, as well as government payors. Anti-kickback laws are broad and prohibit many arrangements and practices that are lawful in businesses outside of the health care industry.
The penalties for violating the anti-kickback law can be severe. The sanctions include criminal and civil penalties, and possible exclusion from the federal health care programs.
State and Federal Prohibitions on False Claims
The federal False Claims Act imposes liability on any person or entity that, among other things, knowingly presents, or causes to be presented, a false or fraudulent claim for payment to the federal government. Under the False Claims Act, a person acts knowingly if he has actual knowledge of the information or acts in deliberate ignorance or in reckless disregard of the truth or falsity of the information. Specific intent to defraud is not required. Provisions of the False Claims Act allow a private individual to bring an action on behalf of the federal government and to share in any amounts paid by the defendant to the government in connection with the action. The number of filings under these provisions has increased significantly in recent years. When an entity is determined to have violated the False Claims Act, it may be required to pay up to three times the actual damages sustained by the government, plus civil penalties for each false claim. Conduct that violates the False Claims Act may also lead to exclusion from the federal health care programs. Given the number of claims likely to be at issue, potential damages under the False Claims Act for even a single inappropriate arrangement could be significant. In addition, various states have enacted similar laws modeled after the False Claims Act that apply to items and services reimbursed under Medicaid and other state health care programs, and, in several states, such laws apply to claims submitted to all payors.
HIPAA
Under the administrative simplification provisions of the Health Insurance Portability and Accountability Act of 1996, or HIPAA, and state laws, there are numerous regulations for protecting the privacy and security of protected health information. Additional administrative simplification provisions created the following new federal crimes: health care fraud, false statements relating to health care matters, theft or embezzlement in connection with a health benefit program and obstruction of criminal investigation of
21
Table of Contents
health care offenses. The health care fraud statute prohibits knowingly and willfully executing a scheme to defraud any health care benefit program, including a private insurer. The false statements statute prohibits knowingly and willfully falsifying, concealing, or covering up a material fact or making any materially false, fictitious, or fraudulent statement in connection with the delivery of or payment for health care benefits, items, or services. The theft or embezzlement statute prohibits knowingly and willfully embezzling, stealing or otherwise converting or misapplying the money or property of a health care benefit program. The obstruction of criminal investigations of health care offenses statute prohibits willfully preventing, obstructing, misleading or delaying the communication of information and records relating to a violation of a federal health care offense to a criminal investigator. A violation of any of these laws is a felony and may result in fines, or exclusion from the federal health care programs.
HIPAA’s privacy and security regulations apply to “covered entities” or health care providers, health plans and health care clearinghouses and limit the permissible uses and disclosures of individually identifiable health information, also called “protected health information” or “PHI.” Amendments to HIPAA under the Health Information Technology for Economic and Clinical Health Act, or “HITECH,” require notification to the government and affected individuals of certain breaches of PHI that has not been secured in accordance with federal standards, such as encrypted. HIPAA does not directly regulate us, but it does limit the types of communications and data sharing that health care providers and plans may engage in with us. HITECH created liability for third parties in possession of PHI in violation of HIPAA, so we must ensure that our own uses or disclosures and requests for PHI are compliant. Penalties for HIPAA violations include fines, civil monetary penalties, criminal prosecution and imprisonment as well as significant reputational damage for reported breaches.
State Privacy and Data Security Laws
State laws governing the privacy and security of health and other personal information apply along with HIPAA and impose additional compliance obligations as well as breach notification requirements. Unlike HIPAA, state privacy and data security laws apply more broadly. In particular, state data security laws apply to any organization maintaining personal information, such as employee information. There is significant variability among state privacy and data security laws, and these laws are rapidly evolving. They typically apply based on the residence of the subject of the information, which means that multiple state laws and compliance requirements may be implicated at any given time. Failure to comply with these laws and an ensuing breach may lead to litigation, including class action lawsuits, fines, civil penalties and reputational damage.
The Physician Payment Sunshine Act
The Physician Payment Sunshine Act, or Sunshine Act, which was enacted as part of ACA, requires applicable manufacturers of drugs, devices, biologicals, or medical supplies covered under Medicare and other programs to report annually payments or other transfers of value made by that entity, or by a third party as directed by that entity, to physicians and teaching hospitals, or to third parties on behalf of physicians or teaching hospitals, during the course of the preceding calendar year. Failure to comply with the reporting requirements can result in significant civil monetary penalties. We are required to collect data on these payments and report such payments.
The Foreign Corrupt Practices Act
The Foreign Corrupt Practices Act prohibits U.S. companies and their representatives from offering, promising, authorizing or making payments to foreign officials for the purpose of obtaining or retaining business abroad. In many countries, the health care professionals we regularly interact with may meet the definition of a foreign government official for purposes of the Foreign Corrupt Practices Act.
22
Table of Contents
Financial Information and Significant Customers
Financial information about (i) our net product revenues and other revenues generated in the principal geographic regions in which we operate and our significant customers is set forth in Note 1, “Segment Reporting and Geographical Information” and “Concentration of Credit Risk,” to our consolidated financial statements included in this Annual Report on Form 10-K, (ii) net loss per share attributable to ARIAD common shareholders and our total assets are provided in our consolidated financial statements included in this Annual Report on Form 10-K and (iii) our research and development expenses in each of the last three fiscal years is provided in Item 7, “Management’s Discussion and Analysis of Financial Condition and Results of Operations.” A discussion of the risks attendant to our international operations is set forth in the “Risk Factors” section of this Annual Report on Form 10-K.
Our Employees
As of December 31, 2014, we had 379 employees, of whom more than half held post-graduate professional, medical or science degrees. Of these employees, 264 were based in the United States and 115 were based in Europe. We have entered into confidentiality, assignment of inventions and non-disclosure agreements with all of our employees and non-competition agreements with all of our senior level employees. None of our employees are covered by a collective bargaining agreement, and we consider relations with our employees to be good.
Our Company
ARIAD was organized as a Delaware corporation in April 1991. Our principal executive offices are located at 26 Landsdowne Street, Cambridge, Massachusetts 02139-4234, and our telephone number is (617) 494-0400.
Information Available on the Internet
We maintain an internet website at http://www.ariad.com, the contents of which are not incorporated herein. Our Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q and Current Reports on Form 8-K and all amendments to such reports are made available free of charge through www.sec.gov and the Investor Relations section of our website as soon as reasonably practicable after they have been electronically filed with or furnished to the United States Securities and Exchange Commission, or SEC.
Trademarks
ARIAD, the ARIAD logo and Iclusig are our registered trademarks. ARGENT is our trademark. Other service marks, trademarks and trade names appearing in this report are the property of their respective owners.
23
Table of Contents
THE RISKS AND UNCERTAINTIES DESCRIBED BELOW ARE THOSE THAT WE CURRENTLY BELIEVE MAY MATERIALLY AFFECT OUR COMPANY. IF ANY OF THE FOLLOWING RISKS ACTUALLY OCCUR, THEY MAY MATERIALLY HARM OUR BUSINESS, OUR FINANCIAL CONDITION AND OUR RESULTS OF OPERATIONS.
Risks relating to the development and commercialization of our products and product candidates
We depend heavily on the commercial success of our only approved cancer medicine, Iclusig® (ponatinib). If we do not achieve commercial success with Iclusig, our business, results of operations and financial condition will suffer, and we will be dependent on the success of our other product candidates.
We obtained accelerated approval from the U.S. Food and Drug Administration, or FDA, in December 2012, to sell our first new cancer medicine, Iclusig, for the treatment of adult patients with chronic, accelerated or blast phase chronic myeloid leukemia (CML) that is resistant or intolerant to prior tyrosine kinase inhibitor (TKI) therapy or Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ ALL) that is resistant or intolerant to prior TKI therapy. When Iclusig was approved by the FDA, its United States prescribing information, or USPI, included a boxed warning concerning arterial thrombosis, hepatotoxicity and other precautions. We commenced sales and marketing of Iclusig in the United States in January 2013.
In October 2013, we suspended marketing and commercial distribution of Iclusig in the United States based on the FDA’s concerns about updated safety data from the pivotal PACE trial of Iclusig. In addition, our clinical trials for Iclusig were placed on partial clinical hold and we discontinued our Phase 3 EPIC trial of Iclusig in adult patients with newly diagnosed CML in the chronic phase. In December 2013, the FDA approved revised USPI, a risk evaluation and mitigation strategy, and a revised box warning, allowing us to resume marketing and commercial distribution, which we commenced in January 2014. Iclusig is now indicated for adult patients with T315I-positive CML (chronic phase, accelerated phase, or blast phase) or T315I-positive Ph+ ALL, and chronic phase, accelerated phase, or blast phase CML or Ph+ ALL for whom no other TKI therapy is indicated.
Iclusig has also been approved for marketing and commercial distribution in the European Union, and from late 2013 through 2014, was subject to review by the European Medicines Agency, or EMA, of the benefits and risks of Iclusig to better understand the nature, frequency and severity of events obstructing the arteries or veins, the potential mechanism that leads to these side effects and whether there needed to be a revision in the dosing recommendation, patient monitoring and a risk management plan for Iclusig. This review was completed in January 2015, with additional warnings in the product information but without any change in the approved indications.
Prior to the approval of Iclusig, we had not marketed a therapeutic product. We had no revenues from product sales in 2012, $45.2 million in revenues from product sales in 2013 and $55.7 million in revenues from product sales in 2014. We expect that a majority of our total revenues in the next several years will be attributable to sales of Iclusig. We cannot be certain that Iclusig will be commercially successful. In addition to the other challenges related to a company launching its first commercial drug, we have faced the challenges of having Iclusig removed from the market in the United States in the fourth quarter of 2013 and re-launching the drug in the first quarter of 2014 with revised prescribing information that reduces the addressable patient population and an updated box warning. Moreover, the FDA, EMA or other regulatory authorities could take additional actions in the future that could further reduce the commercial potential of Iclusig. If we do not achieve commercial success with Iclusig, our business, results of operations and financial condition will suffer, and we will be dependent on the success of our other product candidates.
24
Table of Contents
The commercial success of Iclusig depends on numerous factors, some of which are outside our control. If one or more of these factors negatively affects sales of Iclusig, our business, results of operations and financial condition will be materially harmed.
Our future sales of Iclusig depend on numerous factors, including:
| • | the impact of the changes required by the FDA in the USPI that reduced the addressable patient population and changes required by the EMA in the Iclusig SmPC following its review of the risks and benefits of Iclusig, along with any additional changes that may be required in the future; |
| • | the safety profile of Iclusig, including whether previously unknown side-effects or the increased incidence or severity of known side-effects are identified with the increased use of Iclusig, such as we announced in the fourth quarter of 2013; |
| • | competition from other TKIs, which compete with Iclusig on the basis of, among other things, efficacy, cost, breadth of approved use and the safety and side-effect profile; |
| • | competition from any additional products for the treatment of CML that are approved by the FDA, the EMA and other regulatory authorities in the future; |
| • | the effectiveness of our commercial strategy for marketing Iclusig and our execution of that strategy, including our pricing strategy and the effectiveness of our efforts to obtain adequate third-party reimbursements; |
| • | receipt of regulatory approvals for Iclusig, and any applicable pricing and reimbursement approvals, in Europe, Japan and other countries or territories outside of the United States and the European Union; |
| • | the acceptance of Iclusig by patients, the medical community and third-party payors, particularly following the temporary suspension of commercial distribution in the fourth quarter of 2013 and the updated prescribing information, including a revised boxed warning; |
| • | results from clinical trials and the receipt of regulatory approvals in any other indications that we may decide to pursue in blood cancers and solid tumors; and |
| • | our ability to meet the demand for commercial supplies of Iclusig and to maintain and successfully monitor commercial manufacturing arrangements for Iclusig with third-party manufacturers to ensure they meet our standards and those of regulatory authorities. |
While we believe that Iclusig is an important medicine for patients with resistant or intolerant Philadelphia-positive leukemias, the net revenues that Iclusig may generate in future periods may change based upon the above factors, and could be lower than expected. If our revenues, market share and/or other indicators of market acceptance for Iclusig do not meet the expectations of investors or public market analysts, the market price of our common stock would likely decline. In addition, if one or more of the factors above negatively affects sales of Iclusig, our business, results of operations and financial condition will be materially harmed.
We have never marketed a drug prior to Iclusig, and if we are unable to maintain an effective and specialized sales force and marketing infrastructure, we will not be able to commercialize Iclusig successfully.
In order to successfully commercialize Iclusig, we have built a marketing organization and a specialized sales force for Iclusig in the United States and Europe. Factors that may hinder our ability to successfully market and commercially distribute Iclusig include:
| • | inability to maintain the relationship with the single specialty pharmacy with whom we have contracted for distribution of Iclusig in the United States and our other distributors, suppliers and manufacturers; |
25
Table of Contents
| • | inability of sales personnel to obtain access to or convince adequate numbers of physicians to prescribe our products; |
| • | inability to recruit, retain and effectively manage adequate numbers of effective sales and marketing personnel; |
| • | lack of complementary products to be offered by sales personnel, which may put us at a competitive disadvantage relative to companies that have more extensive product lines; and |
| • | unforeseen delays, costs and expenses associated with maintaining our international capabilities, including our international sales and marketing organization and international supply chain and pricing and reimbursement capabilities. |
If we are unable to maintain our sales force and marketing capability for Iclusig in the United States and in the European Union, we may not be able to generate sufficient product revenue, may generate increased expenses and may never become profitable.
We will need to continue to expend significant time and resources to train our Iclusig sales forces in the United States and the European Union to be credible, persuasive and compliant in discussing Iclusig with the specialists treating the patients indicated under the product’s label. In addition, if we are unable to effectively train our sales force and equip them with effective materials, including medical and sales literature to help them inform and educate potential customers about the benefits and risks of Iclusig and its proper administration, our ability to successfully commercialize Iclusig could be diminished, which would have a material adverse effect on our business, results of operations and financial condition.
If Iclusig and any of our product candidates are not accepted by patients, physicians and third-party payors, we will not be successful.
Our success is dependent on the commercial acceptance of Iclusig and any of our other product candidates that may be approved. Iclusig and any other approved product candidates may not achieve market acceptance among patients, physicians or third-party payors, even if we have obtained necessary regulatory and any applicable pricing and reimbursement approvals. Physicians and health care payors may conclude that Iclusig or our product candidates are not as safe and/or effective as competing therapies or are not as attractive based on a cost and risk/benefit analysis as alternative treatments. For example, physicians may elect not to prescribe our drugs, and patients may elect not to request or take them for a variety of reasons, including lower demonstrated or perceived clinical safety and efficacy compared to other drugs; prevalence and severity of adverse events or other side effects; lack of cost-effectiveness; lack of reimbursement availability from third-party payors; a decision to wait for the approval of other therapies that are believed to have significant advantages over our drugs and drug candidates; convenience and ease of administration; other potential advantages of alternative treatment methods; or ineffective marketing and distribution support.
We believe that recommendations by physicians and acceptance by health care payors will be essential for market acceptance of Iclusig and our product candidates. If Iclusig fails to achieve market acceptance, or our product candidates are approved and fail to achieve market acceptance, we will not be able to generate revenues sufficient to be successful.
26
Table of Contents
Competing drugs or technologies may render some or all of our products or future products noncompetitive or obsolete.
Many well-known pharmaceutical and biotechnology companies, which have substantially greater capital, research and development capabilities and experience than us, are presently engaged in developing drug candidates focused on the same biological targets or the treatment of the same disease indications on which we are focused. Some of these entities already have competitive products on the market or product candidates in clinical trials or in more advanced preclinical studies than we do.
For example, Iclusig currently competes with existing therapies that are approved for the treatment of patients with CML who are resistant or intolerant to prior TKI therapies, such as nilotinib marketed by Novartis, dasatinib marketed by Bristol-Myers Squibb, bosutinib marketed by Pfizer and omacetaxine mepesuccinate marketed by Teva Pharmaceutical Industries. At this time, based on the revised USPI for Iclusig approved by the FDA in December 2013, Iclusig may only be promoted for treatment of adult patients with T315I-positive CML and Ph+ ALL and patients for whom no other TKI therapy is indicated, which limits the addressable patient population and therefore revenues that can be generated from sales of the drug.
In addition, Pfizer, Novartis and Roche/Chugai have launched ALK inhibitors that will compete with brigatinib should it gain marketing approval. Tesaro, Ignyta and Xcovery also have ALK inhibitors in development and Pfizer is developing a follow-on compound to its currently marketed product, crizotinib.
Competing drugs or technologies may render some or all of our products or future products noncompetitive or obsolete, and we may not be able to make the enhancements to our products necessary to compete successfully with newly emerging drug products. Competing products on the market or in development may also lead us and our collaborators to revise or cease development of our product candidates in one or more indications for commercial reasons, even where clinical data may be promising. If we are unable to successfully compete in our chosen markets, we will not become profitable.
In order to execute our business plan and achieve the full commercial potential of Iclusig, we will need to obtain regulatory approval to commercialize Iclusig in additional markets and in additional indications and lines of therapy. If we are not successful in these efforts, our business, results of operations and financial condition could be materially harmed.
Based on sales of existing TKIs for the treatment of CML, we believe that there are commercial opportunities for the use of Iclusig globally in additional therapeutic indications and in additional lines of therapy, and we are pursuing these opportunities. In December 2014, we entered into a co-development and commercialization agreement with Otsuka Pharmaceutical Co., Ltd., or Otsuka, under which Otsuka has exclusive rights to commercialize Iclusig in Japan and nine other Asian countries and to fund future clinical trials in those countries. We expect to file for marketing approval in Japan in 2015. In Australia, we obtained marketing approval for Iclusig in November 2014 and will distribute Iclusig through a third party distributor that covers Australia and New Zealand. In addition, we have filed for marketing approval of Iclusig in Canada and Israel.
In addition, Iclusig is being studied in various investigator-sponsored trials in settings including first line and second line CML, acute myeloid leukemia, or AML, non-small cell lung cancer, or NSCLC, and medullary thyroid cancer, or MTC. We expect that additional investigator-sponsored clinical trials will be initiated in 2015 in various indications. If we are not successful in obtaining regulatory approval of Iclusig in additional foreign countries and in additional indications and lines of therapy, our business, results of operations and financial condition could be materially harmed.
We may not succeed in developing, obtaining regulatory approval and generating product revenues from our product candidate brigatinib (AP26113) or any other product candidate, which would materially harm our business, results of operations and financial condition.
As with all scientific endeavors, we face much trial and error, and we and our collaborators may fail at numerous stages along the way, which could prevent us and our collaborators from successfully developing, obtaining approval for and marketing our product candidate brigatinib (AP26113) and any
27
Table of Contents
other product candidate. Factors that could affect the timing and the ability to obtain additional regulatory approvals and to achieve market acceptance and gain market share for brigatinib and any other product candidate include, among others:
| • | product formulation; |
| • | dose and dosage regimen; |
| • | the ability to obtain timely and sufficient patient enrollment in clinical trials; |
| • | the risk of occurrence of adverse events and other side effects in patients participating in clinical trials; |
| • | the attainment of clinical data that is sufficient to support regulatory approval; |
| • | the ability to manufacture sufficient quantities of product candidates at commercially reasonable costs; |
| • | the ability to fund commercial development and to build or access a sales force in the marketplace for that product candidate; |
| • | the ability to successfully differentiate product candidates from competitive products; |
| • | the ability to educate physicians and build awareness about our product candidates; and |
| • | the ability to sell, market and distribute such product candidates. |
We may not receive regulatory approvals within the timeframes we anticipate, or at all, and ultimately we may not succeed in developing or commercializing additional products which will generate revenues for our company. If we are not successful in developing our product candidates, obtaining regulatory approvals and marketing any approved products, our business, results of operations and financial condition will be materially harmed.
Positive results from earlier stage clinical trials may not be replicated in later-stage clinical trials, increased adverse events or safety issues could arise, or regulatory authorities may conclude that clinical data from later-stage clinical trials are not sufficient to support approval or that data from post-approval clinical trials are not sufficient to support broader use of drugs after approval. Regulatory authorities may require changes in the permitted usage of any approved products.
A number of companies in the pharmaceutical and biotechnology industries have suffered significant setbacks in late-stage clinical trials or following regulatory approval even after achieving promising results in earlier-stage development. Accordingly, the results to date from preclinical studies and clinical trials for Iclusig and brigatinib may not be predictive of the results to be obtained from ongoing or future clinical trials. In addition, regulatory authorities may conclude that data generated from later-stage clinical trials are not sufficient to support approval, or that data obtained following approval may require changes to be made in the permitted usage of any approved products. For example, although we were able to obtain accelerated approval for Iclusig on the basis of data from our pivotal Phase 2 PACE trial without conducting a Phase 3 trial, and we believe that similar prospects for regulatory approval exist for brigatinib, we may be required to conduct more clinical trials for brigatinib than we currently anticipate. Moreover, we announced in October 2013 updated data from the PACE trial, in which it was observed that with a median follow up of 24 months, serious arterial thrombosis occurred in 11.8 percent of Iclusig-treated patients, compared to 8 percent after 11 months of follow-up reflected in the then-current USPI. Based on this and other follow-up data, we suspended marketing and commercial distribution of Iclusig in October 2013 and, following discussions with the FDA, we were permitted to resume in December 2013 with revised prescribing information and a revised boxed warning that reduces the addressable patient population for whom Iclusig is indicated.
If positive results from earlier stage trials are not replicated in later-stage trials, if increased adverse events or safety issues arise, if we or our collaborators are required to conduct additional clinical trials or other testing of our product candidates beyond those currently contemplated, or if the permitted usage of Iclusig or any of our other product candidates that are approved is constrained or withdrawn by the FDA or other regulatory authorities, we or our collaborators may be delayed in obtaining, or may not be able to obtain or maintain, marketing approval for these products, and we may lose the opportunity to generate
28
Table of Contents
product revenues or to earn additional development or regulatory milestones or royalties. Furthermore, potential competitive commercial factors and our resources may influence future decisions and directions by us or our collaborators on which clinical indications to pursue and when.
Risks relating to our financial position and capital requirements
We have incurred significant losses to date and may never be profitable.
We have incurred significant losses since our formation in 1991, and had an accumulated deficit of $1.2 billion at December 31, 2014. Our losses have resulted principally from costs incurred in research and development of Iclusig and our product candidates, from our discovery research activities and from general and administrative costs, including costs incurred to prosecute and protect our intellectual property. In addition, we have incurred significant expenses in building a commercial organization to market, sell and distribute Iclusig and our other products upon potential regulatory approval in the United States, Europe and other select markets, worldwide. It is likely that we will incur significant operating losses for the foreseeable future, as we continue our research, development and commercialization activities. If our losses continue and we and our existing collaborators or potential future collaborators are unable to successfully develop, commercialize, manufacture and market Iclusig, brigatinib, ridaforolimus and any other product candidates and/or we are unable to enter into additional collaboration agreements or licenses for our intellectual property, we may never generate sufficient revenues to achieve profitability. Even if we and our collaborators are able to commercialize products and we are able to enter into collaboration agreements or licenses in the future, we may never generate sufficient revenues to be profitable.
Insufficient funding may jeopardize our research and development programs and may require us to reduce our operations or prevent commercialization of our products and technologies.
We have financed our operations and investments to date primarily through sales of our common stock and convertible notes in public and private offerings, through the receipt of upfront and milestone payments from collaborations and licenses with pharmaceutical and biotechnology companies and, to a lesser extent, through issuances of our common stock pursuant to our equity incentive and employee stock purchase plans, supplemented by the borrowing of long-term debt from commercial lenders. We sell securities and incur debt when the terms of such transactions are deemed favorable to us and as necessary to fund our current and projected cash needs. We seek to balance the level of cash, cash equivalents and marketable securities on hand with our projected needs and to allow us to withstand periods of uncertainty relative to the availability of funding on favorable terms.
With sales of Iclusig in the United States from January through October 2013 and commencing again in January 2014, as well as sales in Europe since the second half of 2013, we have generated product revenues that have contributed to our cash flows. However, our cash flows generated from sales of Iclusig are not currently sufficient to fund operations and we will need to seek additional sources to fund our operations.
As of December 31, 2014, we had cash and cash equivalents totaling $352.7 million. We expect that our cash and cash equivalents as of December 31, 2014, together with estimated sales of Iclusig and funding we expect to receive from new collaborative agreements or strategic alliances, will be sufficient to fund our operations for the foreseeable future. The adequacy of our available funds to meet our future operating and capital requirements will depend on many factors, including the amount of future revenue generated by Iclusig, our ability to enter into a partnership to co-develop and co-commercialize brigatinib and the amount of funding generated from such partnership, the potential introduction of one or more of our other drug candidates to the market, and the number, breadth, cost and prospects of our research and development programs. However, changes to our operating plan or assumptions, economic factors or strategic opportunities may require us to raise additional funds through one or more financings of equity or debt securities or other transactions.
We have from time to time accessed funding by issuing common stock or other securities in private placements or public offerings. We are currently a “well-known seasoned issuer,” or WKSI, pursuant to rules of the U.S. Securities and Exchange Commission, or SEC, which permits us to file registration statements for offerings of shares of our common stock and other securities that are effective upon filing. We may also from time to time seek additional funding from technology licensing, or the issuance of debt or other structured funding alternatives. However, such additional funding may not be available at all, or on terms acceptable to us.
29
Table of Contents
If we are not able to secure additional funding if or when required to fund our operations including our research and development programs and commercialization activities at their current levels or at levels that may be required in the future, we may be required to reduce our operations or to delay, scale back, eliminate or terminate commercialization or further clinical development of Iclusig or clinical trials for one or more of our product candidates.
Our ability to use net operating loss and tax credit carryforwards to offset future taxable income may be limited in the future if we do not have sufficient taxable income or due to ownership changes that have occurred or may occur in the future.
As of December 31, 2014, we had tax assets, including net operating loss carryforwards, or NOLs, of $562.1 million in the United States and $194.9 million in foreign territories and U.S. federal research tax credits of $28.9 million, which could be used in certain circumstances to offset our future taxable income or otherwise payable taxes and therefore reduce our federal and state income tax liabilities. Based on current income tax rates, if fully utilized, our NOLs and other carryforwards could provide a benefit to us of significant future tax savings. However, our ability to use these tax benefits in future years will depend upon our ability to comply with the rules relating to the preservation and use of NOLs and the amount of our otherwise taxable income. If we do not have sufficient taxable income in future years to use the tax benefits before they expire, we will lose the benefit of these NOLs permanently. Consequently, our ability to use the tax benefits associated with our NOLs will depend significantly on our success in generating income from Iclusig and any other product candidates that may be approved.
Additionally, if we undergo an ownership change, the NOLs and tax credit carryforwards would be subject to an annual limit on the amount of the taxable income that may be offset by our NOLs generated prior to the ownership change, and we may be unable to use a significant portion or all of our NOLs to offset taxable income. Our common stock is very actively traded on a daily basis. As a result of high trading volume, there may be a shift of ownership among certain of our stockholders that could result in an ownership change, which could limit our ability to utilize our NOL and tax credit carryforwards under applicable section of the Internal Revenue Code. Limitations imposed on the ability to use NOLs and tax credits to offset future taxable income could require us to pay U.S. federal income taxes earlier than would otherwise be required if such limitations were not in effect and could cause such NOLs and tax credits to expire unused, in each case reducing or eliminating the benefit of such NOLs and tax credits. Similar rules and limitations may apply for state income tax purposes. We have adopted a shareholder rights plan in the form of a Section 382 Rights Plan with the intention of reducing the likelihood of an ownership change. However, we have granted exemptions under the plan in limited circumstances and cannot be sure that the plan will be effective in deterring or preventing such an ownership change. The plan was approved by our stockholders in June 2014, expires in October 2016 and may not be amended to extend the expiration date.
Raising additional capital by issuing securities or through collaboration and licensing arrangements may cause dilution to existing stockholders, restrict our operations or require us to relinquish proprietary rights.
We may seek to raise the additional capital necessary to fund our operations through public or private equity offerings, debt financings, and collaboration and licensing arrangements. To the extent that we raise additional capital through the sale of equity or convertible debt securities, our stockholders’ ownership interest will be diluted, and the terms of such securities may include liquidation or other preferences that adversely affect our stockholders’ rights or, in the case of debt securities, require us to pay interest that would reduce our cash flows from operations or comply with certain covenants that could restrict our operations. If we raise additional funds through collaboration and licensing arrangements with third parties, we may have to relinquish valuable rights to our technologies or product candidates, or grant licenses on terms that are not favorable to us.
30
Table of Contents
Forecasting sales of Iclusig may be difficult and revenue recognition may be deferred. If our revenue projections are inaccurate or revenue is deferred, our business may be harmed and our future prospects may be adversely affected.
Our sales of Iclusig can be difficult to forecast. Factors that can increase the difficulty of forecasting sales of Iclusig include the following:
| • | cautionary prescribing behavior, confusion or other concerns regarding the safety and risk-benefit of Iclusig; |
| • | decisions that may be made by physicians regarding dosing of patients with Iclusig in response to safety concerns or adverse events; |
| • | difficulty in identifying appropriate patients for treatment with Iclusig; |
| • | the cost and availability of reimbursement for the product; |
| • | treatment guidelines issued by government and non-government agencies; |
| • | types of cancer for which the product is approved; |
| • | timing of market entry relative to competitive products; |
| • | availability of alternative therapies; |
| • | price of our product relative to alternative therapies, including generic versions of our product, or generic versions of innovative products that compete with our product; |
| • | patients’ reliance on patient assistance programs, under which we provide free drug; |
| • | rates of returns and rebates; |
| • | uncertainty of launch trajectory; |
| • | the ability of our third-party manufacturers to manufacture and deliver Iclusig in commercially sufficient quantities; |
| • | the ability of our single specialty pharmacy distributor in the United States and our distributors in other markets to process orders in a timely manner and satisfy their other obligations to us; |
| • | the extent of marketing efforts by us and any third-party agents retained by us; and |
| • | side effects or unfavorable publicity concerning our products or similar products. |
The extent to which any of these or other factors individually or in the aggregate may impact future sales of Iclusig is uncertain and difficult to predict. Our management must make forecasting decisions regarding future revenue in the course of business planning despite this uncertainty, and actual results of operations may deviate materially from projected results. This may lead to inefficiencies and increased difficulties in operational planning. If our revenues from Iclusig sales are lower than we anticipate or revenue is deferred, we will incur costs in the short term that will result in losses that are unavoidable. A shortfall in our revenue would have a direct impact on our cash flow and on our business generally. In addition, fluctuations in our quarterly results can adversely and significantly affect the market price of our common stock.
31
Table of Contents
Our financial results are impacted by management’s selection of accounting methods and certain assumptions and estimates.
Our accounting policies and methods are fundamental to how we record and report our financial condition and results of operations. Our management must exercise judgment in selecting and applying many of these accounting policies and methods so they comply with generally accepted accounting principles and reflect management’s judgment of the most appropriate manner to report our financial condition and results. In some cases, management must select the accounting policy or method to apply from two or more alternatives, any of which may be reasonable under the circumstances, yet may result in our reporting materially different results than would have been reported under a different alternative.
Certain accounting policies are critical to the presentation of our financial condition and results of operations. The preparation of our financial statements requires us to make significant estimates, assumptions and judgments that affect the amounts of assets, liabilities, revenues and expenses and related disclosures. Significant estimates that may be made by us include assumptions used in the determination of revenue recognition, accrued product development expenses, inventory, leased buildings under construction and stock-based compensation. Although we base our estimates and judgments on historical experience, our interpretation of existing accounting literature and on various other assumptions that we believe to be reasonable under the circumstances, if our assumptions prove to be materially incorrect, actual results may differ materially from these estimates.
Risks relating to our reliance on third parties
We depend on third-party manufacturers, including sole source suppliers, to manufacture Iclusig and our product candidates and the materials we require for our clinical trials. We may not be able to maintain these relationships and could experience supply disruptions outside of our control.
We rely on a network of third-party manufacturers to manufacture and supply Iclusig for commercial sale and post-approval clinical trials, and our drug candidates for clinical trials and any commercial sales if they are approved. As a result of our reliance on these third-party manufacturers and suppliers, including sole source suppliers of certain components of Iclusig and our product candidates, we could be subject to significant supply disruptions. Our supply chain for sourcing raw materials and manufacturing drug product ready for distribution is a multi-step endeavor. Third-party contract manufacturers supply us with raw materials, and contract manufacturers in the United States convert these raw materials into drug substance and convert the drug substance into final dosage form. Establishing and managing this supply chain requires a significant financial commitment and the creation and maintenance of numerous third-party contractual relationships. Although we attempt to effectively manage the business relationships with companies in our supply chain, we do not have control over their operations.
We require a supply of Iclusig for sale in the United States, Europe and Australia and we will require a supply of Iclusig for sale in other markets if we obtain additional marketing approvals. We currently rely, and expect to continue to rely, on sole source third-party manufacturers to produce starting materials, drug substance, and final drug product, and to package and label Iclusig and our product candidates, until we enter into arrangements with additional or alternative suppliers. While we have identified and expect to qualify and engage back-up third-party manufacturers as additional or alternative suppliers for the commercial supply of Iclusig, we currently do not have such arrangements in place. Moreover, some of these alternative manufacturers will have to be approved by the FDA before we can use them for manufacturing Iclusig. It is also possible that supplies of materials that cannot be second-sourced can be managed with inventory planning. There can be no assurance, however, that failure of any of our original sole source third party manufacturers to meet our commercial demands for Iclusig in a timely manner, or our failure to engage qualified additional or back-up suppliers for the commercial supply of Iclusig, would not have a material adverse effect on commercialization of Iclusig and our business.
32
Table of Contents
Supply disruptions may result from a number of factors, including shortages in product raw materials, labor or technical difficulties, regulatory inspections or restrictions, shipping or customs delays or any other performance failure by any third-party manufacturer on which we rely. Any supply disruptions could disrupt sales of Iclusig and/or the timing of our clinical trials, which could have a material adverse impact on our business. Furthermore, we may be required to modify our production methods to permit us to economically manufacture our drugs for sale and our drug candidates for clinical trials. These modifications may require us to re-evaluate our resources and the resources of our third-party manufacturers, which could result in abrupt changes in our production methods and supplies.
In the course of providing its services, a contract manufacturer may develop process technology related to the manufacture of our products or drug candidates that the manufacturer owns, either independently or jointly with us. This would increase our reliance on that manufacturer or require us to obtain a license from that manufacturer in order to have Iclusig or our drug candidates manufactured by other suppliers utilizing the same process.
The failure of our third party manufacturers to meet our commercial demands for Iclusig in a timely manner, or our failure to engage qualified additional or back-up suppliers for the commercial supply of Iclusig, would have a material adverse effect on our business, results of operations and financial position.
We rely on a single specialty pharmacy for distribution of Iclusig in the United States, and the loss of that specialty pharmacy or its failure to distribute Iclusig effectively would adversely affect sales of Iclusig.
We rely on a single specialty pharmacy for distribution of Iclusig in the United States. A specialty pharmacy is a pharmacy that specializes in the dispensing of medications for complex or chronic conditions, which often require a high level of patient education and ongoing management. The use of specialty pharmacies involves certain risks, including, but not limited to, risks that these specialty pharmacies will:
| • | not provide us accurate or timely information regarding their inventories, the number of patients who are using our products or complaints about our products; |
| • | reduce or discontinue their efforts to sell or support or otherwise not effectively sell or support our products; |
| • | not devote the resources necessary to sell our products in the volumes and within the time frames that we expect; |
| • | be unable to satisfy financial obligations to us or others; or |
| • | cease operations. |
In addition, our agreement with the specialty pharmacy may be terminated by either party on 30 days’ notice. If our single specialty pharmacy does not fulfill its contractual obligations to us, or refuses or fails to adequately serve patients, or the agreement is terminated without adequate notice, shipments of Iclusig, and associated revenues, would be adversely affected. In addition, we expect that it may take a significant amount of time if we were required to change our specialty pharmacy.
We have limited experience in conducting clinical trials and are dependent upon the ability of third parties, including contract research organizations, collaborative academic groups, clinical trial sites and investigators, to conduct or to assist us in conducting clinical trials for our product candidates.
Notwithstanding our successful development of Iclusig to date, we have limited experience compared to many other biopharmaceutical companies in designing, initiating, conducting and monitoring the clinical trials necessary to obtain regulatory approval of our product candidates. We are currently conducting clinical trials of Iclusig and brigatinib. We are dependent upon our ability and/or the ability of our collaborators, licensees, contract research organizations, clinical trial sites and investigators to successfully design, initiate, conduct and monitor clinical trials. Failure by us or any of these parties to timely and
33
Table of Contents
effectively initiate, conduct and monitor our clinical trials could significantly delay or materially impair our ability to complete clinical trials and/or obtain regulatory approval of our product candidates and, consequently, could delay or materially impair our ability to generate revenues from them.
If any collaborator or licensee terminates its agreement with us or fails to perform its obligations under its agreement with us, or fails to comply with applicable law, the development and commercialization of our product candidates could be delayed or terminated.
Our current or future collaborations and licenses may not result in product candidates that are scientifically or commercially successful or result in the development or commercialization of any product candidates. In addition, disputes may arise in the future with respect to the ownership of rights to technology or product candidates developed with collaborators and licensees, which could have an adverse effect on our ability to develop and commercialize any affected product candidate.
Our current collaboration and license agreements allow, and we expect that any future collaborations and licenses will allow, either party to terminate the agreement for specified material breaches by the other party. If a collaborator or licensee terminates its agreement with us, for breach or otherwise, it may be difficult for us to attract new collaborators or licensees and could adversely affect how we are perceived in the business and financial communities. In addition, a collaborator or licensee could determine that it is in its financial interest to:
| • | pursue alternative technologies or develop alternative products, either on its own or jointly with others, that may be competitive with the products on which it is collaborating with us or has licensed from us, which could affect its commitment to us; |
| • | pursue higher-priority programs or change the focus of its development programs, which could affect the collaborator’s or licensee’s commitment to us; or |
| • | choose to devote fewer resources to the marketing of our product candidates, if any are approved for marketing, than it does for product candidates of its own development. |
If any of these events occur, the development and commercialization of one or more of our product candidates could be delayed, curtailed or terminated because we may not have sufficient financial resources or capabilities to continue such development and commercialization on our own.
Risks relating to our intellectual property
If our patents do not protect Iclusig or our product candidates, our exclusive commercial rights in the product or product candidate could be compromised, and if any of our approved drugs or product candidates infringe third-party patents, we could be subject to litigation and substantial liabilities.
We have numerous issued patents and patent applications pending in the United States, as well as counterparts in other countries. Our success will depend, in significant part, on our ability to obtain and maintain United States and foreign patent protection for Iclusig and our product candidates, their manufacture and uses; to preserve our trade secrets; and to operate without infringing the proprietary rights of third parties. In particular, we believe that composition-of-matter claims are the most significant patent claims for companies in our segment of the pharmaceutical industry that focus on small molecule drug candidates that are new chemical compounds. While we have patents or patent applications with composition-of-matter claims for Iclusig and each of our product candidates, only a portion of these patents have been granted to date. We cannot be certain that any patents will issue from our patent applications or, even if patents issue or have issued, that the issued claims will provide us with any significant protection against competitive products or otherwise be valuable commercially.
34
Table of Contents
Due to evolving legal standards relating to the patentability, validity and enforceability of patents covering pharmaceutical inventions and the scope of claims made under these patents, our ability to maintain, obtain and enforce patents is uncertain and involves complex legal and factual questions. U.S. and foreign patent applications typically are maintained in confidence for a period of time after they initially are filed with the applicable patent office. Similarly, publication of discoveries in the scientific literature often lag behind actual discoveries. Consequently, we cannot be certain that we or our licensors were the first to invent, or the first to file patent applications covering Iclusig, our product candidates or their manufacture or use.
Third parties, including a number of our competitors, have developed competing and/or complementary technologies upon which patent applications have been filed and patents have been granted. These third-party technologies concern in part compounds, compositions, methods of use and production of such compounds and compositions, targets, genes and gene mutations, and the use of such targets, genes and gene mutations to identify drug candidates and develop companion diagnostic methods and corresponding kits. Third-party intellectual property protecting such technologies that are related to our business may cover or conflict with our patent applications, technologies or product candidates as well as those of complementary businesses which our business relies upon. Such conflicts could limit the scope of the patents that we may be able to obtain or may result in the denial of our patent applications. If a third party were to obtain intellectual property protection for any of the foregoing, we may be required to challenge such protection, terminate or modify our programs impacted by such protection or obtain licenses from such third parties, which might not be available on acceptable terms. Also, if a third party were to introduce a product into the market which we believe infringes our patents, we may be required to enforce our patent rights or seek to obtain an injunction or other relief, which could be time-consuming and expensive.
Our patents may be challenged by third parties, in connection with a third party’s Abbreviated New Drug Application, or ANDA, or otherwise, resulting in the patent being deemed invalid, unenforceable or narrowed in scope, which could compromise the scope or duration of our exclusive rights in the relevant product. An ANDA can be filed as early as four years after FDA approval of a drug. Other challenges to a patent may be mounted without regard to the date of an FDA approval. Also, our pending patent applications may not issue, and we may not receive any additional patents. Our patents as issued or as subsequently limited by any litigation might not contain claims that are sufficiently broad to prevent others from circumventing our patent protection and utilizing our technologies. For instance, the issued patents relating to Iclusig and our product candidates may be limited to a particular molecule or molecules and may not cover similar molecules that have similar clinical properties. Consequently, our competitors may independently develop competing products that do not infringe our patents or other intellectual property.
The laws of some foreign jurisdictions do not protect intellectual property rights to the same extent as in the United States and other companies have encountered significant difficulties in protecting and defending such rights in foreign jurisdictions. If we encounter such difficulties or are otherwise precluded from effectively protecting our intellectual property rights in foreign jurisdictions, our business could be substantially harmed.
Because of the extensive time required for development, testing and regulatory review of a drug candidate, it is possible that, before any of our drug candidates can be commercialized, any related patent may expire or remain in force for only a short period following commercialization of our drug candidates, thereby reducing any advantages of the patent. To the extent our approved drugs or drug candidates are not commercialized significantly ahead of the expiration date of any applicable patent, or to the extent we have no other patent protection on such approved drugs or drug candidates, those drugs and drug candidates would not be protected by patents, and we would then need to rely solely on other types of exclusivity, such as orphan drug exclusivity and other types of regulatory exclusivity available under the Food, Drug and Cosmetic Act.
35
Table of Contents
If we are unable to protect the confidentiality of our trade secrets, our business and competitive position could be harmed.
In addition to seeking patents for some of our technology and products, we also rely on trade secrets, including unpatented know-how, technology, and other proprietary information, to maintain our competitive position. We seek to protect these trade secrets, in part, by entering into non-disclosure and confidentiality agreements with parties that have access to them, such as our employees, collaborators, outside scientific collaborators, sponsored researchers, contract manufacturers, consultants, advisors and other third parties. We also have entered into confidentiality and invention or patent assignment agreements with our employees and our consultants. Any of these parties may breach the agreements and disclose our proprietary information, and we may not have adequate remedies for any such breach. Enforcing a claim that a party illegally disclosed or misappropriated a trade secret is difficult, expensive and time-consuming, and the outcome is unpredictable. In addition, some courts inside and outside the United States are less willing or unwilling to protect trade secrets. If any of our trade secrets were to be lawfully obtained or independently developed by a competitor, we would have no right to prevent them from using that technology or information to compete with us. If any our trade secrets were to be disclosed to or independently developed by a competitor, our competitive position could be harmed.
Risks relating to our operations
If we fail to manage the size of our workforce and other resources effectively, our business may suffer.
The number of our employees more than doubled prior to our initial launch of Iclusig in January 2013 as we built out the commercial organization that was responsible for the commercialization of Iclusig in the United States and Europe, but was then reduced by approximately 40 percent of our employees in the United States in the fourth quarter of 2013 in connection with our announcements concerning the updated safety, marketing and commercial distribution and further clinical development of Iclusig. With the re-launch of Iclusig in the United States in January 2014, we have hired necessary personnel to manage all the requirements of the business in this re-launch period.
We have also entered into a new lease to move our corporate headquarters and laboratory facilities to two buildings being constructed by the landlord at 75 Binney Street and 125 Binney Street in Cambridge, Massachusetts. We had planned to move into the new buildings in early 2015. However, in light of our announcements in the fourth quarter of 2013 concerning Iclusig, we re-assessed our current and future need for space in Cambridge and expect to require less space than previously planned. We could not terminate the new lease without substantial cost, and we would have been required to make substantial expenditures to make necessary improvements to our existing facilities. We are, therefore, planning to sub-lease approximately 170,000 square feet of the 386,000 square feet of the space comprising the new buildings. We are currently planning the fit-out of the space and expect the space to be ready for occupancy in 2016. Due to delays in the timing of the fit-out and occupancy of the buildings, we expect to be required to commence lease payments for the space prior to occupancy. If we are unable to sub-lease the space in the buildings that we will not occupy, our cost of occupancy of the space we will use will increase. We are currently in discussion with the landlord regarding our revised plans and timing and other matters in the lease.
In addition, because our drug discovery and development activities are highly technical in nature, we require the services of highly qualified and trained scientists who have the skills necessary to conduct these activities. We need to attract and retain employees with experience in these fields. We face intense competition for our personnel from our competitors, our collaborators and other companies throughout our industry. The growth of local biotechnology companies and the expansion of major pharmaceutical companies into the Cambridge, Massachusetts area have increased competition for the available pool of skilled employees, especially in technical fields, and the high cost of living in the Cambridge area makes it difficult to attract employees from other parts of the country to these areas.
We must respond effectively to these demands and manage our internal organization to accommodate our anticipated needs. If we are unable to manage the size of our workforce and our other resources effectively, there could be a material adverse effect on our business, results of operations and financial condition.
36
Table of Contents
We are currently subject to securities class action litigation and derivative litigation and may be subject to similar or other litigation such as products liability litigation in the future.
The market price of our common stock declined significantly following our October 2013 announcements concerning the safety, marketing and commercial distribution and further clinical development of Iclusig in the United States. Class action lawsuits have been filed alleging, among other things, that we and certain of our officers violated federal securities laws by making allegedly material misrepresentations and/or omissions of material fact regarding clinical and safety data for Iclusig in our public disclosures. The plaintiffs seek unspecified monetary damages on behalf of the putative class and an award of costs and expenses, including attorney’s fees.
In addition, in November and December 2013, purported derivative lawsuits were filed alleging that our directors and certain of our officers breached their fiduciary duties related to the clinical development and commercialization of Iclusig and by making misrepresentations regarding the safety and commercial marketability of Iclusig. The lawsuits also assert claims for unjust enrichment and corporate waste, and for misappropriation of information and insider trading by the officers named as defendants. The plaintiffs seek unspecified monetary damages, changes in our corporate governance policies and internal procedures, restitution and disgorgement from the individually named defendants, and an award of costs and expenses, including attorney’s fees.
While we believe we have meritorious defenses, we cannot predict the outcome of these lawsuits. Monitoring and defending against legal actions, whether or not meritorious, is time-consuming for our management and detracts from our ability to fully focus our internal resources on our business activities, and we cannot predict how long it may take to resolve these matters. In addition, we may incur substantial legal fees and costs in connection with litigation. Although we have insurance, coverage could be denied or prove to be insufficient. We are not currently able to estimate the possible cost to us from these lawsuits, as they are currently at an early stage and we cannot be certain how long it may take to resolve these matters or the possible amount of any damages that we may be required to pay. We have not established any reserves for any potential liability relating to these lawsuits. It is possible that we could, in the future, incur judgments or enter into settlements of claims for monetary damages. A decision adverse to our interests on these actions could result in the payment of substantial damages and could have a material adverse effect on our business, results of operations and financial condition. In addition, the uncertainty of the currently pending lawsuits could lead to more volatility in our stock price.
Our business has a substantial risk of product liability claims. If we are unable to obtain appropriate levels of insurance, product liability claims could adversely affect our business.
Our business exposes us to potential product liability risks inherent in the testing, manufacturing, marketing and sale of pharmaceutical products. Prior to obtaining regulatory approval to market our products, we or our collaborators are required to test such products in human clinical trials at health care institutions pursuant to agreements which indemnify such institutions in case of harm caused to patients by our products. We may not be able to avoid significant product liability exposure resulting from use of our products.
If product liability lawsuits are brought against us for injuries or deaths allegedly due to patients’ adverse reactions to Iclusig, we may be subject to additional liability. In any event, a potential product liability lawsuit would require significant financial and management resources. Regardless of the outcome, product liability claims may result in injury to our reputation, withdrawal of clinical trial participants, significant costs, diversion of management’s attention and resources, substantial monetary awards, loss of revenue, and additional distractions from our efforts to resume marketing and commercial distribution of Iclusig in the United States. Although we have product liability insurance, coverage could be denied or prove to be insufficient. Also, we may not be able to renew or retain sufficient product liability insurance at an acceptable cost to protect against potential product liability claims, which could also impact the resumption of marketing and commercial distribution of Iclusig in the United States.
37
Table of Contents
Risks associated with operating in foreign countries could materially adversely affect our business.
We have operations in Europe in order to market Iclusig internationally. In addition, we have distribution, manufacturing, collaborative and clinical trial relationships outside the United States. Consequently, we are, and will continue to be, subject to risks related to operating in foreign countries. Risks associated with conducting operations in foreign countries include:
| • | differing regulatory requirements for drug approvals and regulation of approved drugs in foreign countries; |
| • | changes in tariffs, trade barriers and regulatory requirements; |
| • | economic weakness, including inflation, or political instability in particular foreign economies and markets; |
| • | compliance with tax, employment, immigration and labor laws for employees living or traveling abroad; |
| • | compliance with country-specific regulations limiting the use of individuals’ personal information and restricting the transfer of this information to the United States; |
| • | foreign taxes, including withholding of payroll taxes; |
| • | foreign currency fluctuations, which could result in increased operating expenses or reduced revenues, and other obligations incident to doing business or operating in another country; |
| • | workforce uncertainty in countries where labor unrest is more common than in the United States; |
| • | production shortages resulting from any events affecting raw material supply or manufacturing capabilities abroad; and |
| • | business interruptions resulting from geo-political actions, including war and terrorism. |
These and other risks associated with our international operations could materially adversely affect our business.
In addition, our international operations are subject to regulation under United States law. For example, the Foreign Corrupt Practices Act prohibits United States companies and their representatives from offering, promising, authorizing or making payments to foreign officials for the purpose of obtaining or retaining business abroad. In many countries, the health care professionals we regularly interact with may meet the definition of a foreign government official for purposes of the Foreign Corrupt Practices Act. We are also subject to import and export control laws. Failure to comply with domestic or foreign laws could result in various adverse consequences, including the possible delay in approval or refusal to approve a product, recalls, seizures, withdrawal of an approved product from the market, the imposition of civil or criminal sanctions, the prosecution of executives overseeing our international operations and corresponding adverse publicity and negative perception of our company in foreign countries.
The loss of key members of our scientific and management staff could delay and may prevent the achievement of our research, development and business objectives.
We are substantially dependent on our senior management team and other employees responsible for areas such as drug development, clinical trials, regulatory affairs, drug discovery, manufacturing, commercial operations, business development and intellectual property protection and licensing. A loss of key personnel or a failure to properly integrate new personnel could be disruptive. While we have entered into employment agreements with all of our executive officers, these officers may terminate their
38
Table of Contents
employment with us at any time. The value to employees of stock-related benefits that vest over time, such as options and restricted stock units, will be significantly affected by movements in our stock price that we cannot control, and may at any point in time be insufficient to counteract more lucrative offers from other companies. The loss of, and failure to promptly replace, any member of our senior management team could significantly delay and may prevent the achievement of our research, development and business objectives.
If we do not comply with laws regulating the protection of the environment and health and human safety, our business could be adversely affected.
Our research and development efforts involve the controlled use of hazardous materials, chemicals and various radioactive compounds. Although we believe that our safety procedures for handling and disposing of these materials comply with the standards prescribed by state, federal and foreign regulations, the risk of accidental contamination or injury from these materials cannot be eliminated. If an accident occurs, we could be held liable for resulting damages, which could be substantial. We are also subject to numerous environmental, health and workplace safety laws and regulations, including those governing laboratory procedures, exposure to blood-borne pathogens and the handling of biohazardous materials. Although we maintain workers’ compensation insurance to cover us for costs we may incur due to injuries to our employees resulting from the use of these materials, this insurance may not provide adequate coverage against potential liabilities. We maintain insurance to cover pollution conditions or other extraordinary or unanticipated events relating to our use and disposal of hazardous materials that we believe is appropriate based on the small amount of hazardous materials we generate. Additional federal, state and local laws and regulations affecting our operations may be adopted in the future. We may incur substantial costs to comply with, and substantial fines or penalties if we violate any of these laws or regulations.
Security breaches, loss of data and other disruptions could compromise sensitive information related to our business, prevent us from accessing critical information or expose us to liability, which could adversely affect our business and our reputation.
In the ordinary course of our business, we collect and store sensitive data, including legally protected patient health information, credit card information, personally identifiable information about our employees, intellectual property, and proprietary business information. We manage and maintain our applications and data utilizing on-site systems. These applications and data encompass a wide variety of business critical information including research and development information, commercial information and business and financial information.
The secure processing, storage, maintenance and transmission of this critical information is vital to our operations and business strategy, and we devote significant resources to protecting such information. Although we take measures to protect sensitive information from unauthorized access or disclosure, our information technology and infrastructure may be vulnerable to attacks by hackers, or viruses, breaches or interruptions due to employee error, malfeasance or other disruptions, or lapses in compliance with privacy and security mandates. Any such virus, breach or interruption could compromise our networks and the information stored there could be accessed by unauthorized parties, publicly disclosed, lost or stolen. We have measures in place that are designed to detect and respond to such security incidents and breaches of privacy and security mandates. Any such access, disclosure or other loss of information could result in legal claims or proceedings, liability under laws that protect the privacy of personal information, such as the Health Insurance Portability and Accountability Act of 1996, government enforcement actions and regulatory penalties. Unauthorized access, loss or dissemination could also disrupt our operations, including our ability to bill payors or patients, provide customer support services, conduct research and development activities, process and prepare company financial information, manage various general and administrative aspects of our business and damage our reputation, any of which could adversely affect our business.
39
Table of Contents
Risks relating to regulatory approvals, pricing and reimbursement
The manufacture, distribution and sale of Iclusig and our product candidates are subject to significant regulatory oversight and restrictions. Regulatory authorities may require the use of a risk mitigation strategy such as the Risk Evaluation and Mitigation Strategy (REMS) required by the FDA for Iclusig. These restrictions and requirements subject us to increased risks and uncertainties, any of which could negatively impact sales of Iclusig.
In connection with the approval to resume marketing and commercial distribution of Iclusig in the United States in December 2013, the FDA required us to implement a Risk Evaluation and Mitigation Strategy, or REMS. The objective of the REMS program is to inform prescribers of the risk of vascular occlusion associated with Iclusig and of the revised USPI. The REMS program includes letters to physicians and professional societies, a fact sheet and information that will be communicated through professional journals and displayed at scientific meetings. In addition, we are fulfilling a series of post-marketing requirements to better understand the risks of vascular occlusion and to further explore various doses of Iclusig, including enhanced assessment and prospective observation of patients with vascular occlusive events, continued follow-up monitoring of patients from our Iclusig trials, and a randomized multi-arm trial to characterize a range of Iclusig doses and safe use.
Any REMS required by the FDA or other regulatory agencies may lead to increased costs to assure compliance with new post-approval regulatory requirements and potential requirements or restrictions on the sale of Iclusig, all of which could lead to lower sales volume and revenue. As required by the FDA and other regulatory agencies, the information that we collect while implementing our REMS could result in further required changes to the Iclusig label and prescribing information or require us to take other actions that could have an adverse effect on Iclusig’s commercial success, which would have a material adverse effect on our business, financial condition and results of operations.
Clinical trials for our product candidates are expensive and time consuming, may take longer than we expect or may not be completed at all, and their outcome is uncertain.
We and our collaborators are currently conducting multiple clinical trials for our clinical product candidates, and we and our collaborators expect to commence additional trials of Iclusig and our product candidates in the future. Each of our clinical trials requires the investment of substantial expense and time and the timing of the commencement, continuation and completion of these clinical trials may be subject to significant delays attributable to various causes, including scheduling conflicts with participating clinicians and clinical institutions, difficulties in identifying and enrolling patients who meet trial eligibility criteria, failure of patients to complete the clinical trial, delay in or failure to obtain IRB approval to conduct a clinical trial at a prospective site, shortages of available drug supply, or the imposition of a clinical hold by regulatory agencies, such as the partial clinical hold the FDA imposed on additional patient enrollment in all clinical trials of Iclusig in October 2013. Patient enrollment is a function of many factors, including the size of the target patient population, the proximity of patients to clinical sites, the eligibility criteria for the trial, the existence of competing clinical trials and the availability of alternative established or investigational treatments.
We depend on medical institutions and clinical research organizations, or CROs, to conduct our clinical trials in compliance with FDA and other applicable requirements and guidelines, often referred to as Good Clinical Practices, and to the extent they fail to enroll patients for our clinical trials, are delayed for a significant time in achieving full enrollment, or fail to follow proper procedures, we may be affected by increased costs, program delays or both, which may harm our business. In addition, we conduct clinical trials in foreign countries which may subject us to further delays and expenses as a result of increased drug shipment costs, additional regulatory requirements and the need to engage foreign CROs, as well as expose us to risks associated with less experienced clinical investigators who are unknown to the FDA, different standards of medical care, and fluctuating foreign currency exchange rates.
40
Table of Contents
Clinical trials must be conducted in accordance with Good Clinical Practices and are subject to oversight by the FDA, other foreign governmental agencies and IRBs at the medical institutions where the clinical trials are conducted. In addition, clinical trials must be conducted with product candidates produced under current Good Manufacturing Practices, or cGMP, conditions. We, the FDA, the EMA or other foreign governmental agencies could delay, suspend or halt our clinical trials of a product candidate for numerous reasons, including:
| • | deficiencies in the conduct of the clinical trial, including failure to conduct the clinical trial in accordance with regulatory requirements or clinical protocols; |
| • | the product candidate may have unforeseen adverse side effects, including fatalities, or a determination may be made that a clinical trial presents unacceptable health risks, such as was reflected in our announcement in October 2013 that the FDA had imposed a partial clinical hold on all clinical trials for Iclusig following our review of additional clinical data from the PACE trial showing that a higher percentage of patients taking Iclusig had experienced serious arterial thrombosis and serious venous occlusion after a median of 24 months of follow-up; |
| • | the time required to determine whether the product candidate is effective may be longer than expected; |
| • | the product candidate may not be more effective than current therapies; |
| • | the quality or stability of the product candidate may fall below acceptable standards; |
| • | our inability to produce or obtain sufficient quantities of the product candidate to complete the trials; |
| • | our inability to obtain IRB approval to conduct a clinical trial at a prospective site; |
| • | lack of adequate funding to continue the clinical trial, including the incurrence of unforeseen costs due to enrollment delays, requirements to conduct additional trials and studies and increased expenses associated with the services of our CROs and other third parties; |
| • | our inability to recruit and enroll patients to participate in clinical trials for reasons including competition from other clinical trial programs for the same or similar indications; or |
| • | our inability to retain patients who have initiated a clinical trial but may be prone to withdraw due to side effects from the therapy, lack of efficacy or personal issues, or who are lost to further follow-up. |
In addition, clinical trial results are frequently susceptible to varying interpretations that may delay, limit or prevent regulatory approvals. Negative or inconclusive results or adverse medical events, including patient fatalities that may be attributable to our product candidates, during a clinical trial could cause it to be redone or terminated. Further, some of our clinical trials may be overseen by an independent data monitoring committee, or DMC, and a DMC may recommend a delay or suspension in one or more of these trials due to safety or futility findings based on events occurring during a clinical trial.
If clinical trials of any of our product candidates fail, we or our collaborators may not be able to obtain marketing approval for the product candidate that is the subject of the failed clinical trials. The FDA and foreign regulatory agencies could also require additional clinical trials before or after granting of marketing approval for any products, which could result in increased costs and significant delays in the development and commercialization of such products and could result in the withdrawal of such products from the market after initially obtaining marketing approval. Our failure, or the failure of our collaborators, to adequately demonstrate the safety and efficacy of a product candidate in clinical
41
Table of Contents
development could delay or prevent obtaining marketing approval of the product candidate and, after obtaining marketing approval, data from post-approval studies could result in the product being withdrawn from the market, either of which would likely have a material adverse effect on our business.
Iclusig and each of our product candidates will remain subject to ongoing regulatory review even if they receive marketing approval. If we or our collaborators or contractors fail to comply with applicable regulations, we or they may be subject to enforcement action that could adversely affect us.
We and our collaborators and contractors will continue to be subject to extensive regulation by the FDA and other regulatory authorities even after our product candidates are approved. We and our collaborators and contractors will continue to be subject to FDA requirements governing, among other things, the manufacture, packaging, sale, promotion, adverse event reporting, storage and recordkeeping of our approved products. For example, in October 2013, the FDA placed a partial clinical hold on all clinical trials of Iclusig and, at the request of the FDA, we suspended marketing and commercial distribution of Iclusig in the United States following our review of additional clinical data from the pivotal PACE trial of Iclusig. The FDA has also issued Drug Safety Communications concerning Iclusig and revised the approved indications and prescribing information for Iclusig and the EMA has required additional safety warnings.
If we or any of our collaborators fails to comply with the requirements of the FDA and other U.S. or foreign governmental or regulatory authorities with jurisdiction over our products or operations or previously unknown problems with our products, manufacturers or manufacturing processes are discovered, we or our collaborator could be subject to administrative or judicially imposed sanctions, including warning letters; civil or criminal penalties; fines; injunctions; product seizures or detentions; import bans; voluntary or mandatory product recalls; suspension or withdrawal of regulatory approvals; total or partial suspension of production; and refusal to approve pending applications for marketing approval of new drugs or supplements to approved applications.
We rely on third-party contractors for numerous services, and if those contractors fail to fulfill their contractual obligations to us, which affects our regulatory compliance, we could be adversely affected. For example, during 2014 we conducted a routine quality control audit of a third-party vendor and learned that this vendor did not meet its obligations to report to us all of the adverse events which its employees had become aware of while it performed patient support services for Iclusig during 2013. As a result, these adverse events in patients receiving Iclusig obtained pursuant to health care providers’ prescriptions were not reported to the FDA and other regulatory authorities as required. In response, we initiated a corrective action plan with the vendor to identify and report all adverse events from these patients, and we informed the FDA, the EMA and other regulatory authorities of these findings. We submitted a full report summarizing our review and findings to the regulatory authorities during the fourth quarter of 2014. We also accelerated quality control audits of all third-party contractors providing patient support services for us in the United States and Europe.
Based on our assessment to date of previously unreported adverse events, we do not expect any changes to the benefit/risk profile of Iclusig. Moreover, these adverse events were identified during commercial use of Iclusig, which are assessed separately from adverse events observed in clinical trials. However, it is too early to determine whether additional information may arise that could alter our assessment. In addition, the FDA or other regulatory authorities may conduct their own evaluation of the cases, may require additional changes to Iclusig’s prescribing information, or may take or require us to take other actions that could be costly or time-consuming and/or adversely affect our commercialization of Iclusig.
We may not be able to obtain government regulatory approval to market our product candidates.
Other than Iclusig, none of our product candidates has been approved for commercialization in any country. Prior to commercialization, each product candidate will be subject to an extensive and lengthy review process in the United States and in other countries. We or our collaborators may not be able to obtain regulatory approval for any product candidates, or even if approval is obtained, the labeling for
42
Table of Contents
such products may place restrictions on their use that could materially impact the marketability and profitability of those products. Satisfaction of regulatory requirements, which includes satisfying the FDA and foreign regulatory authorities that the product is both safe and effective for its intended uses, typically takes several years or more depending upon the type, complexity, novelty and safety profile of the product and requires the expenditure of substantial resources. Uncertainty with respect to meeting the regulatory requirements governing our product candidates may result in excessive costs or extensive delays in the regulatory review process.
We will not be able to sell our product candidates if we or our third-party manufacturers fail to comply with current good manufacturing practice requirements.
Before approving any of our product candidates, the FDA will inspect the facility or facilities at which the drug product is manufactured and will not approve the drug candidate unless it is satisfied with our or our third-party manufacturer’s compliance with cGMPs. The manufacturing of our product candidates must comply with cGMP requirements of the FDA and similar requirements of regulatory agencies in other countries. These requirements govern, among other things, manufacturing, quality control and documentation procedures. We, or any third-party manufacturer of our product candidates, may not be able to comply with these requirements, which would prevent us from obtaining approval for commercialization of our products. Material changes to the manufacturing processes or a change in manufacturer of products after approvals have been granted are also subject to review and approval by the FDA or other regulatory agencies. Following approval, such facilities are subject to continuing FDA and foreign regulatory requirements including inspections and failure to comply with cGMPs or similar regulations could result in regulatory action including market withdrawals and recalls.
Government and other third-party payors seek to contain costs of health care through legislative and other means. If they fail to provide coverage and adequate reimbursement rates for our products, our revenues will be harmed.
In both domestic and foreign markets, sales of products depend in part upon the availability of reimbursement from third-party payors. Third-party payors include government health programs such as Medicare and Medicaid, managed care providers, and private health insurers. Governments and other third-party payors continually try to contain or reduce the costs of health care through various means. For example, in certain foreign markets, pricing of pharmaceutical products is subject to governmental control. In the United States, there have been, and we expect that there will continue to be, a number of federal and state proposals to implement similar governmental control. The Patient Protection and Affordable Care Act and the Health Care and Education Affordability Reconciliation Act of 2010, collectively, the Affordable Care Act or ACA, will require discounts under the Medicare drug benefit program and increases the rebates paid by pharmaceutical companies on drugs covered by Medicaid. In addition, the ACA imposes an annual fee, which will increase annually, on sales by branded pharmaceutical manufacturers. The financial impact of these discounts, increased rebates and fees and the other provisions of the ACA on our business is unclear, and there can be no assurance that our business will not be materially harmed by future implementation of the ACA.
In addition, third-party payors are increasingly attempting to contain health care costs by demanding price discounts or rebates and limiting both the types and variety of drugs that they will cover and the amounts that they will pay for drugs. As a result, they may not cover or provide adequate payment for our products. We may have to conduct post-marketing or competitive effectiveness studies in order to demonstrate the cost-effectiveness of Iclusig or any other of our future drugs to such payors’ satisfaction.
Such studies might require us to commit a significant amount of management’s time and financial and other resources. Our products might not ultimately be proven to be cost-effective. Adequate third-party reimbursement might not be available to enable us to maintain price levels sufficient to realize an appropriate return on our investment in product development.
43
Table of Contents
Reimbursement rates may vary according to the use of the drug and the clinical setting in which it is used, may be based on payments allowed for lower-cost products that already are reimbursed, may be incorporated into existing payments for other products or services, and may reflect budgetary constraints and/or imperfections in Medicare or Medicaid data used to calculate these rates. Net prices for products are often reduced by mandatory discounts or rebates required by government health care programs or by privately-negotiated discounts. Moreover, the United States federal government, state governments and private payors frequently pursue actions against pharmaceutical companies alleging that the companies have overstated prices in order to inflate reimbursement rates. Any such action could adversely affect the pricing of and the commercial success of our products.
Any legislation or regulatory changes or relaxation of laws that restrict imports of drugs from other countries also could reduce the net price we receive for our products.
If we market any of our products in a manner that violates federal or state health care laws, including fraud and abuse laws, laws prohibiting off-label promotion, disclosure laws or other similar laws, we may be subject to civil or criminal penalties.
We are subject to health care “fraud and abuse” laws, such as the federal False Claims Act and the anti-kickback provisions of the federal Social Security Act, laws prohibiting off-label product promotion and other similar state and federal laws and regulations. While we have a corporate compliance program designed to actively identify, prevent and mitigate risk through the implementation of compliance policies and systems and the promotion of a culture of compliance, if we are found not to be in full compliance with these laws our business could be materially harmed.
The federal anti-kickback law prohibits knowingly and willfully offering, paying, soliciting, receiving or providing remuneration, directly or indirectly, in exchange for or to induce either the referral of an individual, or the ordering, furnishing, arranging for or recommending of an item or service that is reimbursable, in whole or in part, by a federal health care program, such as Medicare or Medicaid. The federal statute has been interpreted to apply to arrangements between pharmaceutical manufacturers on the one hand and prescribers, patients, purchasers and formulary managers on the other hand, and therefore constrains our marketing practices and our various service arrangements with physicians, including physicians who make clinical decisions to use our products. Although there are a number of statutory exemptions and regulatory safe harbors protecting certain common activities from prosecution, the exemptions and safe harbors are drawn narrowly. Practices that involve remuneration and that can be construed as inducements for prescribing, purchasing, or recommending our products may be subject to scrutiny or penalty if they do not qualify for an exemption or safe harbor.
Federal false claims laws prohibit any person from knowingly presenting, or causing to be presented, a false claim for payment to the federal government, or knowingly making, or causing to be made, a false statement to get a false claim paid. Pharmaceutical companies have been prosecuted under these laws for a variety of alleged promotional and marketing activities, such as providing free product to customers with the expectation that the customers would bill federal programs for the product; reporting to pricing services inflated average wholesale prices that were then used by federal programs to set reimbursement rates; engaging in promotion for uses that the FDA has not approved, known as “off-label” uses, that caused claims to be submitted to Medicaid for non-covered off-label uses; and submitting inflated “best price” information to the Medicaid Rebate Program.
Although physicians are permitted to, based on their medical judgment, prescribe products for indications other than those cleared or approved by the FDA, manufacturers are prohibited from promoting their products for such off-label uses. We market Iclusig for the treatment of adult patients with T315I-positive CML (chronic phase, accelerated phase, or blast phase) or T315I-positive Ph+ ALL and chronic phase, accelerated phase, or blast phase CML or Ph+ ALL for whom no other TKI therapy is indicated, and provide promotional materials to physicians regarding the use of Iclusig in these patient populations. If the FDA determines that our promotional materials or other activities constitute off-label promotion, it could request that we modify our promotional materials or other activities or subject us to regulatory
44
Table of Contents
enforcement actions, including the issuance of a warning letter, injunction, seizure, civil fine and criminal penalties. It also is possible that other federal, state or foreign enforcement authorities might take action if they believe that the alleged improper promotion led to the submission and payment of claims for an off-label use, which could result in significant fines or penalties under other statutory authorities, such as laws prohibiting false claims for reimbursement. Even if it is later determined we were not in violation of these laws, we may be faced with negative publicity, incur significant expenses defending our actions and have to divert significant management resources from other matters.
Also applicable to some of our practices is the Health Insurance Portability and Accountability Act of 1996, or HIPAA, and its implementing regulations, which created federal criminal laws that prohibit executing a scheme to defraud any health care benefit program or making false statements relating to health care matters and which also imposes certain regulatory and contractual requirements regarding the privacy, security and transmission of individually identifiable health information.
The majority of states also have statutes or regulations similar to the federal anti-kickback law and false claims laws, which apply to items and services reimbursed under Medicaid and other state programs, or, in several states, apply regardless of the payor. In addition, certain states have laws governing the privacy and security of certain health information and personal data, which may differ from each other in significant ways and often are not preempted by HIPAA and are rapidly evolving, complicating compliance efforts. Sanctions under these federal and state laws may include civil monetary penalties, exclusion of a pharmaceutical manufacturer’s products from reimbursement under government programs and criminal fines. Even if we are not determined to have violated these laws, government investigations into these issues typically require the expenditure of significant resources and generate negative publicity, which could harm our business.
In recent years, several states and localities, including California, the District of Columbia, Maine, Massachusetts, Minnesota, Nevada, New Mexico, Vermont and West Virginia, have enacted legislation requiring pharmaceutical companies to establish marketing compliance programs, file periodic reports with the state or make periodic public disclosures on sales, marketing, pricing, clinical trials, health care provider, or HCP payments and other activities. Similar legislation is being considered in other states. Additionally, as part of the ACA, the federal government has enacted the Physician Payment Sunshine provisions, which require pharmaceutical manufacturers to publicly report gifts and payments made to physicians and teaching hospitals beginning in March 2014. Many of these requirements are new and the penalties for failure to comply with these requirements will be significant. If we are found not to be in full compliance with these laws, we could face enforcement action, fines and other penalties, and could receive adverse publicity.
The ACA also includes various provisions designed to strengthen significantly fraud and abuse enforcement, such as increased funding for enforcement efforts and the lowering of the intent requirement of the federal anti-kickback statute and criminal health care fraud statute such that a person or entity no longer needs to have actual knowledge of this statute or specific intent to violate it.
If our past or present operations are found to be in violation of any such laws or any other governmental regulations that may apply to us, we may be subject to penalties, including civil and criminal penalties, damages, fines, exclusion from federal health care programs and/or the curtailment or restructuring of our operations. The risk of our being found in violation of these laws is increased by the fact that many of them have not been fully interpreted by the regulatory authorities or the courts, and their provisions are subject to a variety of interpretations. Any action against us for violation of these laws, even if we successfully defend against them, could cause us to incur significant legal expenses and divert our management’s attention from the operation of our business.
The sales and marketing practices of our industry have been the subject of increased scrutiny from federal and state government agencies, and we believe that this trend will continue. Any action against us for violation of these laws, even if we successfully defend against them, could cause us to incur significant legal expenses and divert our management’s attention from the operation of our business.
45
Table of Contents
Future health care reform measures could hinder or prevent commercial success of our drugs and drug candidates.
The U.S. federal government and other governments have shown significant interest in pursuing health care reform. Any government-adopted reform measures could adversely affect the pricing of health care products, including Iclusig and/or any future product candidates approved for sale. The continuing efforts of governments, insurance companies, managed care organizations and other payors for health care products to contain or reduce health care costs may adversely affect our ability to set prices we believe are fair for Iclusig or any drugs we may develop and commercialize.
New laws, regulations and judicial decisions, or new interpretations of existing laws, regulations and decisions, relating to health care availability, methods of delivery or payment for drugs, or sales, marketing or pricing, may limit our potential revenues, and we may need to revise our research and development or commercialization programs. The pricing and reimbursement environment may change in the future and become more challenging for any of several reasons, including policies advanced by the U.S. government, new health care legislation or fiscal challenges faced by government health administration authorities. Specifically, in the United States and in some foreign jurisdictions there have been a number of legislative and regulatory proposals and initiatives to change the health care system in ways that could affect our ability to sell products. Some of these proposed and implemented reforms could result in reduced reimbursement rates for our current or future products, which would adversely affect our business, operations and financial results. As discussed above, the ACA may have far reaching consequences for companies like us. As a result of this new legislation, substantial changes could be made to the current system for paying for health care in the United States, including changes made in order to extend medical benefits to those who currently lack health insurance coverage. Extending coverage to a large population could substantially change the structure of the health insurance system and the methodology for reimbursement. If reimbursement for our products is substantially less than we expect in the future, or rebate obligations associated with them are substantially increased, our business could be materially and adversely affected.
Further federal and state proposals and health care reforms in and outside of the United States could limit the prices that can be charged for our products and may further limit our commercial opportunity. Our results of operations could be materially adversely affected by the ACA, by the Medicare prescription drug coverage legislation, by the possible effect of such current or future legislation on amounts that private insurers will pay and by other health care reforms that may be enacted or adopted in the future.
Risks relating to our convertible senior notes and related hedge transactions
We have indebtedness in the form of convertible senior notes. The indebtedness created by the sale of the notes and any future indebtedness we incur expose us to risks that could adversely affect our business, results of operations and financial condition.
In June 2014, we completed an offering of $200 million of 3.625 percent convertible senior notes due 2019, or the Notes. As a result of the issuance of the Notes, we incurred $200 million principal amount of indebtedness, the principal amount of which we may be required to pay at maturity on June 15, 2019, or upon the occurrence of a fundamental change (as defined in the indenture). There can be no assurance that we will be able to repay this indebtedness when due, or that we will be able to refinance this indebtedness on acceptable terms or at all. In addition, this indebtedness could have significant negative consequences for our business, results of operations and financial condition, including:
| • | making it difficult for us to pay other obligations; |
46
Table of Contents
| • | making it difficult to obtain favorable terms for any necessary future financing for working capital, capital expenditures, debt service requirements or other purposes; |
| • | requiring us to dedicate a portion of our cash flow from operations to service the indebtedness, reducing the amount of cash flow available for other purposes; and |
| • | limiting our flexibility in planning for and reacting to changes in our business. |
Conversion of the Notes may affect the price of our common stock.
The conversion of some or all of the Notes may dilute the ownership interest of existing stockholders to the extent we deliver shares of common stock upon conversion. Holders of the outstanding Notes will be able to convert them only upon the satisfaction of certain conditions prior to the close of business on the business day immediately preceding December 15, 2018. Upon conversion, holders of the Notes will receive cash, shares of common stock or a combination of cash and shares of common stock, at our election. Any sales in the public market of shares of common stock issued upon conversion of such notes could adversely affect the trading price of our common stock. We cannot predict the size of future issuances or the effect, if any, that they may have on the market price for our common stock. The issuance and sale of substantial amounts of common stock, or the perception that such issuances and sales may occur, could adversely affect the market price of our common stock and impair our ability to raise capital through the sale of additional equity or convertible debt securities.
We may incur substantially more debt or take other actions which would intensify the risks discussed above; and we may not generate cash flow from operations in the future sufficient to satisfy our obligations under the Notes and any future indebtedness we may incur.
We and our subsidiaries may incur substantial additional debt in the future, subject to the restrictions contained in any debt instruments that we enter into in the future, some of which may be secured debt. We are not restricted under the terms of the indenture governing the Notes from incurring additional debt, securing existing or future debt, recapitalizing our debt or taking a number of other actions that are not limited by the terms of the indenture governing the Notes that could have the effect of diminishing our ability to make payments on the Notes when due.
Our ability to make scheduled payments of the principal of, to pay interest on or to refinance our indebtedness, including the Notes, depends on our future performance, which is subject to economic, financial, regulatory, competitive and other factors beyond our control. Our business may not generate cash flow from operations in the future sufficient to satisfy our obligations under the Notes and any future indebtedness we may incur, as well as to make necessary capital expenditures. If we are unable to generate such cash flow, we may be required to adopt one or more alternatives, such as reducing or delaying our research, development or commercialization efforts, investments or capital expenditures, selling or licensing assets, refinancing or obtaining additional debt or equity financing on terms that may be onerous or highly dilutive. Our ability to refinance the Notes or future indebtedness will depend on the capital markets and our financial condition at such time.
In addition, agreements that govern any future indebtedness that we may incur may contain financial and other restrictive covenants that will limit our ability to engage in activities that may be in our long-term best interests. Our failure to comply with those covenants could result in an event of default that, if not cured or waived, could result in the acceleration of some or all of our debt.
We may not be able to raise the funds necessary to settle conversions of the Notes or to repurchase the Notes upon a fundamental change, and our future debt may contain limitations on our ability to pay cash upon conversion or repurchase of the Notes.
Holders of the Notes will have the right to require us to repurchase their Notes upon the occurrence of a fundamental change at a fundamental change repurchase price equal to 100 percent of the principal amount of the Notes to be repurchased, plus accrued and unpaid interest, if any. In addition, upon conversion of the Notes, unless we elect to deliver solely shares of our common stock to settle such conversion (other than
47
Table of Contents
paying cash in lieu of delivering any fractional share), we will be required to make cash payments in respect of the Notes being converted. However, we may not have enough available cash or be able to obtain financing at the time we are required to make repurchases of Notes surrendered therefor or Notes being converted.
In addition, our ability to repurchase the Notes or to pay cash upon conversions of the Notes may be limited by law, by regulatory authority or by agreements governing our future indebtedness. Our failure to repurchase Notes at a time when the repurchase is required by the indenture governing the Notes or to pay any cash payable on future conversions of the Notes as required by the indenture governing the Notes would constitute a default under the indenture governing the Notes. A default under the indenture governing the Notes or the fundamental change itself could also lead to a default under agreements governing our future indebtedness. If the repayment of the related indebtedness were to be accelerated after any applicable notice or grace periods, we may not have sufficient funds to repay the indebtedness and repurchase the Notes or make cash payments upon conversions of the Notes.
The Notes contain a conditional conversion feature, which, if triggered, may adversely affect our financial condition and operating results.
The Notes contain a conditional conversion feature. If such feature is triggered, holders of Notes will be entitled to convert the Notes at any time during specified periods at their option. If one or more holders elect to convert their Notes, unless we elect to satisfy our conversion obligation by delivering solely shares of our common stock (other than paying cash in lieu of delivering any fractional share), we would be required to settle a portion or all of our conversion obligation through the payment of cash, which could adversely affect our liquidity. In addition, even if holders do not elect to convert their Notes, we could be required under applicable accounting rules to reclassify all or a portion of the outstanding principal of the Notes as a current rather than long-term liability, which would result in a material reduction of our working capital.
The convertible note hedge and warrant transactions we entered into in connection with our issuance of the Notes may affect the value of the Notes and our common stock.
In connection with our offering of the Notes, we entered into a convertible note hedge transaction with a financial institution, or the hedge counterparty. We entered into this convertible note hedge transaction with the expectation that it will reduce the potential dilution to our common stock and/or offset potential cash payments in excess of the principal amount of the Notes, as the case may be, upon conversion of the Notes. In the event that the hedge counterparty fails to deliver shares to us or potential cash payments, as the case may be, as required under the convertible note hedge document, we would not receive the benefit of such transaction. Separately, we also entered into a warrant transaction with the hedge counterparty. The warrant transaction could separately have a dilutive effect to the extent that the market price per share of our common stock as measured over the valuation period at the maturity of the warrants exceeds the strike price of the warrants.
In connection with hedging this transaction, the hedge counterparty and/or its affiliates may enter into various derivative transactions with respect to our common stock, and may enter into, or may unwind, various derivative transactions and/or purchase or sell our common stock or other securities of ours in secondary market transactions prior to maturity of Notes (and are likely to do so during any conversion period related to any conversion of the Notes). These activities could have the effect of increasing or preventing a decline in, or could have a negative effect on, the value of our common stock.
In addition, we intend to exercise options under the convertible note hedge transaction whenever the Notes are converted. Depending upon the method we elect to exercise such options, in order to unwind its hedge position with respect to the options we exercise, the hedge counterparty and/or its affiliates may sell shares of our common stock or other securities in secondary market transactions or unwind various derivative transactions with respect to our common stock. The effect, if any, of any of these transactions
48
Table of Contents
and activities on the trading price of our common stock or the Notes will depend in part on market conditions and cannot be ascertained at this time, but any of these activities could adversely affect the value of our common stock and the value of the Notes. The derivative transactions that the hedge counterparty and/or its affiliates expect to enter into to hedge this transaction may include cash-settled equity swaps referenced to our common stock. In certain circumstances, the hedge counterparty and/or its affiliates may have derivative positions that, when combined with the hedge counterparty’s and its affiliates’ ownership of our common stock, if any, would give them economic exposure to the return on a significant number of shares of our common stock.
The accounting method for convertible debt securities that may be settled in cash, such as the Notes, could have a material effect on our reported financial results.
In May 2008, the Financial Accounting Standards Board (“FASB”) issued FASB Staff Position No. APB 14-1, Accounting for Convertible Debt Instruments That May Be Settled in Cash Upon Conversion (Including Partial Cash Settlement), which has subsequently been codified as Accounting Standards Codification 470-20, Debt with Conversion and Other Options (“ASC 470-20”). Under ASC 470-20, an entity must separately account for the liability and equity components of the convertible debt instruments (such as the Notes) that may be settled entirely or partially in cash upon conversion in a manner that reflects the issuer’s economic interest cost. The effect of ASC 470-20 on the accounting for the Notes is that the equity component is required to be included in the additional paid-in capital section of stockholders’ equity on our consolidated balance sheet, and the value of the equity component would be treated as original issue discount for purposes of accounting for the debt component of the Notes. As a result, we will be required to record a greater amount of non-cash interest expense in current periods presented as a result of the amortization of the discounted carrying value of the Notes to their face amount over the term of the Notes. We will report lower net income or higher net loss in our financial results because ASC 470-20 will require interest to include both the current period’s amortization of the debt discount and the instrument’s coupon interest, which will adversely affect our reported or future financial results. The trading price of our common stock and the trading price of the Notes may also be adversely affected.
In addition, convertible debt instruments (such as the Notes) that may be settled entirely or partly in cash are, where the issuer has the intent to settle such instruments partly in a cash amount equal to the principal amount, currently accounted for utilizing the treasury stock method, the effect of which is that the shares issuable upon conversion of the Notes are not included in the calculation of diluted earnings per share except to the extent that the conversion value of the Notes exceeds their principal amount. Under the treasury stock method, for diluted earnings per share purposes, the transaction is accounted for as if the number of shares of common stock that would be necessary to settle such excess, if we elected to settle such excess in shares, are issued. We cannot be sure that the accounting standards in the future will continue to permit the use of the treasury stock method. If we are unable to use the treasury stock method in accounting for the shares issuable upon conversion of the Notes, then our diluted earnings per share would be adversely affected. We expect to use the “if converted” method to compute earnings per share, which could be more dilutive than using the “treasury stock” method.
Certain provisions in the Notes and the indenture could delay or prevent an otherwise beneficial takeover or takeover attempt of us and, therefore, the ability of holders to exercise their rights associated with a fundamental change.
Certain provisions in the Notes and the indenture could make it more difficult or more expensive for a third party to acquire us. For example, if an acquisition event constitutes a fundamental change, holders of the Notes will have the right to require us to purchase their Notes in cash. In addition, if an acquisition event constitutes a make-whole fundamental change, we may be required to increase the conversion rate for holders who convert their Notes in connection with such make-whole fundamental change. Accordingly, our obligations under the Notes and the indenture as well as provisions of our organizational documents and other agreements could increase the cost of acquiring us or otherwise discourage a third party from acquiring us or removing incumbent management.
49
Table of Contents
Risks relating to our common stock
Results of our operations, new clinical and safety data, FDA and other regulatory actions, general market conditions for biotechnology stocks and other factors could result in a sudden change in the value of our stock.
As a biopharmaceutical company, we continue to experience significant volatility in the price of our common stock. In the twelve months ended December 31, 2014, our stock price ranged from a high of $9.83 per share to a low of $4.90 per share. In connection with our October 2013 announcements concerning the safety, marketing and commercial distribution and further clinical development of Iclusig in the United States, our stock price declined by 87 percent, from a closing price of $17.14 per share before on the day the first announcements to a low of $2.15 per share following the announcements. Some of the many factors that could contribute to such volatility include:
| • | actual or anticipated results of our commercialization and continued development of Iclusig, including potential approvals of Iclusig in additional markets; |
| • | our development and the expected timing of any regulatory approvals of brigatinib and our product candidates; |
| • | additional actual or anticipated actions taken by regulatory agencies that may impact Iclusig and our product candidates; |
| • | evidence of the safety or efficacy of Iclusig and our product candidates, including additional data on serious arterial thrombosis and serious venous occlusion of CML patients being treated with Iclusig or the occurrence of other serious adverse events; |
| • | litigation, including the securities class action and derivative lawsuits pending against us and certain of our officers; |
| • | announcements regarding results and timing of preclinical studies and clinical trials for our product candidates; |
| • | announcements of financial results and other operating performance measures, including product revenues; |
| • | our funding resources and requirements, including announcements of new equity or debt financings; |
| • | the timing of our receipt of, or our failure to receive, future milestones under our license agreements with our collaborators; |
| • | announcements regarding existing collaborations or new collaborations or our failure to enter into collaborations; |
| • | announcements regarding product developments or regulatory approvals obtained by companies developing competing products; |
| • | announcements of technological innovations or new therapeutic product candidates; |
50
Table of Contents
| • | developments relating to intellectual property rights, including licensing, litigation and governmental regulation; |
| • | healthcare or cost-containment legislation and public policy pronouncements; |
| • | sales of our common stock by us, our insiders or our other stockholders; |
| • | market conditions for biopharmaceutical stocks in general; |
| • | announcements regarding the potential proxy contest for our 2015 annual meeting or the outcome thereof, as well as any potential related litigation; and |
| • | general economic and market conditions. |
The stock markets, and the markets for biotechnology stocks in particular, have experienced volatility that has often been unrelated to the operating performance of particular companies. These broad market fluctuations may adversely affect the trading price of our common stock. Investors may not be able to sell when they desire due to insufficient buyer demand and may realize less than, or lose all of, their investment.
A potential proxy contest for the election of directors at our 2015 annual meeting could distract our management, divert our resources and adversely impact our business and financial condition
Sarissa Capital Domestic Fund LP and its affiliates (collectively, “Sarissa”) submitted notices of its intention to nominate three individuals for election to our Board of Directors at our 2015 annual meeting of stockholders. Sarissa has also stated that it is seeking the imminent retirement of Harvey J. Berger, M.D., the Chairman of our Board of Directors, as the company’s Chief Executive Officer.
If Sarissa does not withdraw its nominations and we are unable to reach an agreement with Sarissa relating to our 2015 annual meeting, a proxy contest is likely to occur. A proxy contest could adversely affect our business and financial condition because:
| • | a proxy contest can be disruptive, costly and time-consuming, and divert the attention of our Board of Directors, management and employees; |
| • | perceived uncertainties as to our future direction, including, but not limited to, uncertainties related to our CEO and Board of Directors, may result in the loss of potential business opportunities, and may make it more difficult to attract and retain qualified personnel, customers or other business partners; |
| • | if individuals are elected to our Board of Directors with a specific agenda, it may adversely affect our ability to effectively implement our business strategy and create additional value for our stockholders; |
| • | the expenses for legal and advisory fees and the administrative and associated costs incurred in connection with a potential proxy contest may be substantial; and |
| • | we may choose to initiate, or may become subject to, litigation as a result of the potential proxy contest or matters resulting from the potential proxy contest, which could serve as a further distraction to our Board of Directors, management and employees and could require us to incur significant additional costs. |
The market price of our common stock could be subject to significant fluctuation or otherwise be adversely affected by the events, risks and uncertainties described above.
Anti-takeover provisions of Delaware law, provisions in our charter and bylaws and our shareholder rights plan could delay, discourage or make more difficult a third-party acquisition of control of us.
Because we are a Delaware corporation, certain provisions of Delaware law could delay, discourage or make more difficult a third-party acquisition of control of us, even if the change in control would be beneficial to stockholders or the stockholders regard it as such. We are subject to the provisions of Section 203 of the Delaware General Corporation Law, or DGCL, which prohibits certain “business combination” transactions (as defined in Section 203) with an “interested stockholder” (defined in Section 203 as a 15 percent or greater stockholder) for a period of three years after a stockholder becomes an “interested stockholder”, unless the attaining of “interested stockholder” status or the transaction is pre-approved by our board of directors, the transaction results in the attainment of at least an 85 percent ownership level by an acquirer or the transaction is later approved by our board of directors and by our stockholders by at least a 66 2/3 percent vote of our stockholders other than the “interested stockholder”, each as specifically provided in Section 203.
In addition, because our board of directors is a classified board, as described below, Section 141(k)(1) of the DGCL provides that directors may only be removed by the stockholders and then only for “cause”. Further, Section 242(b)(1) of the DGCL provides that amendment of our certificate of incorporation requires that the amendment be determined by the board of directors to be advisable and be submitted by our board of directors to our stockholders for action by them and then approved by our stockholders holding a majority of the outstanding shares of our common stock.
Our certificate of incorporation and our bylaws, each as currently in effect, also contain certain provisions that may delay, discourage or make more difficult a third-party acquisition of control of us:
| • | a classified board of directors, with three classes of directors, each serving for a staggered three-year term, such that not all members of the board of directors may be elected at one time; |
| • | the authorized number of directors may be changed only by resolution of the board of directors; |
| • | any vacancies on the board of directors may only be filled by a majority of the directors then serving, although not a quorum, and not by the stockholders; |
| • | the ability of the board of directors to issue preferred stock that could dilute the stock ownership of a potential unsolicited acquirer and so possibly hinder an acquisition of control of us that is not approved by our board of directors, including through the use of preferred stock in connection with a shareholder rights plan which we could adopt by action of the board of directors; |
51
Table of Contents
| • | record date-setting provisions for annual and special meetings of stockholders and actions by written consent, provisions regulating the conduct of meetings of stockholders and action by written consent, and “advance notice” timing and informational requirements for stockholder nominations to our board of directors at stockholder meetings or for stockholder proposals that can be acted on at stockholder meetings or by written consent; and |
| • | the inability of our stockholders to call a special meeting of stockholders, the limitation of matters to be acted upon at an annual meeting of stockholders to those matters proposed by us or properly brought before the meeting and the limitation of matters to be acted upon at a special meeting of stockholders to matters which we place on the agenda for the meeting. |
We have adopted a shareholder rights plan in the form of a Section 382 Rights Plan, designed to help protect and preserve our substantial tax attributes primarily associated with our NOLs and research tax credits under Sections 382 and 383 of the Internal Revenue Code and related U.S. Treasury regulations. The plan was approved by our stockholders in June 2014, expires in October 2016 and may not be amended to extend the expiration date. Although this is not the purpose of the Section 382 Rights Plan, it could have the effect of making it uneconomical for a third party to acquire us on a hostile basis.
These provisions of the DGCL, our certificate of incorporation and bylaws, and our Section 382 Rights Plan may delay, discourage or make more difficult certain types of transactions in which our stockholders might otherwise receive a premium for their shares over the current market price, and might limit the ability of our stockholders to approve transactions that they think may be in their best interest.
ITEM 1B: UNRESOLVED STAFF COMMENTS
None.
We have leased approximately 100,000 square feet of laboratory and office space at 26 Landsdowne Street, Cambridge, Massachusetts through July 2019, with two five-year renewal options. In May 2012, we entered into a lease agreement for an additional 26,000 square feet of office space in Cambridge, Massachusetts which now extends to July 2017. We have also leased approximately 22,000 square feet of office space in a building in Lausanne, Switzerland which we occupied in 2014 and which serves as our European headquarters. We believe that any additional space that we may require will be available on commercially reasonable terms.
We have entered into a long-term lease for approximately 386,000 square feet of laboratory and office space in two adjacent, connected buildings under construction in Cambridge, Massachusetts. The lease has a term of 15 years with options to renew for three terms of five years each. We had planned to move into the new buildings in early 2015. However, in light of our announcements in the fourth quarter of 2013 concerning Iclusig, we re-assessed our current and future need for space in Cambridge and expect to require less space than previously planned. We could not terminate the new lease without substantial cost, and we would have been required to make substantial expenditures to make necessary improvements to our existing facilities. We are, therefore, planning to sub-lease approximately 170,000 square feet of the 386,000 square feet of the space comprising the new buildings.
Construction of the core and shell of the buildings is expected to be completed in the first half of 2015, at which time construction of tenant improvements in the building will commence. Construction of the tenant improvements is expected to be completed in the first half of 2016, at which time we expect to occupy the buildings. In connection with this lease, the landlord is providing a tenant improvement allowance for the costs associated with the design, engineering, and construction of tenant improvements for the leased facility. The tenant improvements will be in accordance with our plans and include fit-out of the buildings to construct appropriate laboratory and office space, subject to approval by the landlord. Due to delays in fit-out and occupancy of the facility, we expect to be required to commence rent payments for the facility prior to occupancy. We are currently in discussions with the landlord regarding revisions to our plans, the timing related to such plans and the completion of tenant improvements and other matters in the lease.
52
Table of Contents
On October 10, 2013, October 17, 2013, December 3, 2013 and December 6, 2013, purported shareholder class actions, styled Jimmy Wang v. ARIAD Pharmaceuticals, Inc., et al., James L. Burch v. ARIAD Pharmaceuticals, Inc., et al., Greater Pennsylvania Carpenters’ Pension Fund v. ARIAD Pharmaceuticals, Inc., et al, and Nabil Elmachtoub v. ARIAD Pharmaceuticals, Inc., et al, respectively, were filed in the United States District Court for the District of Massachusetts (the District Court), naming us and certain of our officers as defendants. The lawsuits allege that we made material misrepresentations and/or omissions of material fact regarding clinical and safety data for Iclusig in our public disclosures during the period from December 12, 2011 through October 8, 2013 or October 17, 2013, in violation of Sections 10(b) and 20(a) of the Securities Exchange Act of 1934, as amended, and Rule 10b-5 promulgated thereunder. On January 9, 2014, the District Court consolidated the actions and appointed lead plaintiffs. On February 18, 2014, the lead plaintiffs filed an amended complaint as contemplated by the order of the District Court. The amended complaint extends the class period for the Securities Exchange Act claims through October 30, 2013. In addition, plaintiffs allege that certain of our officers, present and former directors and certain underwriters made material misrepresentations and/or omissions of material fact regarding clinical and safety data for Iclusig in connection with our January 24, 2013 follow-on public offering of common stock in violation of Sections 11 and 15 of the Securities Act of 1933, as amended. The plaintiffs seek unspecified monetary damages on behalf of the putative class and an award of costs and expenses, including attorney’s fees. On April 14, 2014, the defendants and the underwriters filed separate motions to dismiss the amended complaint. On June 10, 2014, the District Court heard oral argument on the motion to dismiss. The court has not yet ruled on the motion.
On November 6, 2013, a purported derivative lawsuit, styled Yu Liang v. ARIAD Pharmaceuticals, Inc., et al., was filed in the United States District Court for the District of Massachusetts (the District Court), on behalf of us naming our directors and certain of our officers as defendants. On December 6, 2013, an additional purported derivative lawsuit, styled Arkady Livitz v. Harvey J. Berger, et al, was filed in the District Court. The lawsuits allege that our directors and certain of our officers breached their fiduciary duties related to the clinical development and commercialization of Iclusig and by making misrepresentations regarding the safety and commercial marketability of Iclusig. The lawsuits also assert claims for unjust enrichment and corporate waste, and for misappropriation of information and insider trading by the officers named as defendants. On January 23, 2014, the District Court consolidated the actions. On February 3, 2014, plaintiffs designated the Yu Liang complaint as the operative complaint as contemplated by the order of the District Court. The plaintiffs seek unspecified monetary damages, changes in our corporate governance policies and internal procedures, restitution and disgorgement from the individually named defendants, and an award of costs and expenses, including attorney fees. On March 5, 2014, the defendants filed a motion to dismiss the complaint. In response, on March 26, 2014, the plaintiffs filed an amended complaint. On April 23, 2014, the defendants filed a motion to dismiss the amended complaint. On July 23, 2014, the District Court heard oral argument on the motion to dismiss. The court recently scheduled supplemental oral argument for March 2, 2015.
We believe that these actions are without merit. At this time, we have not recorded a liability related to damages in connection with these matters because we believe that any potential loss is not currently probable or reasonably estimable under U.S. generally accepted accounting principles. In addition, due to the early stages of the matters described above, we cannot reasonably estimate the possible loss or range of loss, if any, that may result from these matters.
From time to time, we may be subject to various claims and legal proceedings. If the potential loss from any claim, asserted or unasserted, or legal proceedings is considered probable and the amount is reasonably estimated, we will accrue a liability for the estimated loss.
53
Table of Contents
ITEM 4: MINE SAFETY DISCLOSURES
None.
PART II
| ITEM 5: | MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES |
Market Information
Our common stock is traded on the NASDAQ Global Select Market under the symbol “ARIA”. The following table sets forth the high and low sales prices of our common stock as quoted on this market for the periods indicated.
| High | Low | |||||||
| 2014: |
||||||||
| First Quarter |
$ | 9.83 | $ | 6.32 | ||||
| Second Quarter |
8.72 | 6.23 | ||||||
| Third Quarter |
6.65 | 4.97 | ||||||
| Fourth Quarter |
7.65 | 4.90 | ||||||
| 2013: |
||||||||
| First Quarter |
$ | 22.49 | $ | 17.15 | ||||
| Second Quarter |
20.32 | 15.35 | ||||||
| Third Quarter |
23.00 | 16.95 | ||||||
| Fourth Quarter |
19.18 | 2.15 | ||||||
On February 17, 2015, the last reported sale price of our common stock was $7.36 per share.
Stock Performance Graph
The following graph compares the yearly percentage change in the cumulative total stockholder return on our common stock since December 31, 2009, with the total cumulative return of the NASDAQ Biotechnology Index and the Russell 3000® Index, in each of which ARIAD is listed. The Russell 3000 Index measures the stock performance of the largest 3,000 U.S. companies representing approximately 98 percent of the investable U.S. equity market. Since the Russell 3000 Index is specifically designed to provide a comprehensive, unbiased and stable barometer of the broad stock market, we believe it is a meaningful index against which to compare our stock price performance.
The price of a share of common stock is based upon the closing price per share as quoted on NASDAQ on the last trading day of the year shown. The graph lines merely connect year-end values and do not reflect fluctuations between those dates. The comparison assumes $100 was invested on December 31, 2009 in our common stock and in each of the foregoing indices. We did not declare or pay any dividends during the comparison period. The stock price performance as shown in the graph below is not necessarily indicative of future stock price performance.
54
Table of Contents
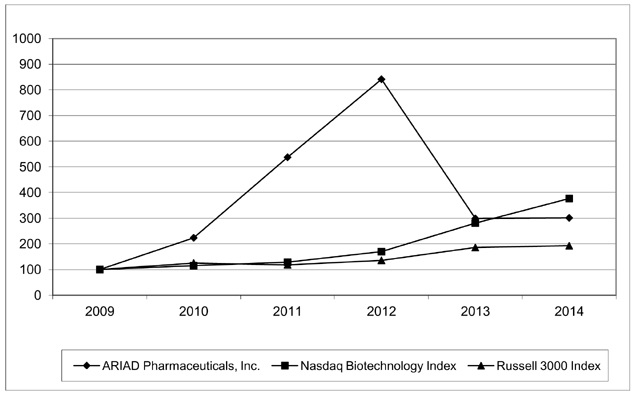
| 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | |||||||||||||||||||
| ARIAD Pharmaceuticals, Inc. |
100.00 | 223.68 | 537.28 | 841.23 | 299.12 | 301.32 | ||||||||||||||||||
| Nasdaq Biotechnology Index |
100.00 | 115.01 | 128.59 | 169.61 | 280.89 | 376.68 | ||||||||||||||||||
| Russell 3000 Index |
100.00 | 125.31 | 118.47 | 135.81 | 186.07 | 192.63 | ||||||||||||||||||
The performance graph shall not be deemed to be incorporated by reference by means of any general statement incorporating by reference this Form 10-K into any filing under the Securities Act of 1933, as amended or the Securities Exchange Act of 1934, as amended, except to the extent that we specifically incorporate such information by reference, and shall not otherwise be deemed filed under such acts.
Stockholders
As of February 17, 2015, the approximate number of holders of record of our common stock was 323.
Dividends
We have not declared or paid dividends on our common stock in the past and do not intend to declare or pay such dividends in the foreseeable future.
Unregistered Sales of Securities
Not applicable.
Issuer Purchases of Equity Securities
Not applicable.
55
Table of Contents
| ITEM 6: | SELECTED FINANCIAL DATA |
The selected financial data set forth below as of December 31, 2014, 2013, 2012, 2011 and 2010 and for each of the years then ended have been derived from our audited consolidated financial statements, of which the financial statements as of December 31, 2014 and 2013 and for the years ended December 31, 2014, 2013 and 2012 are included elsewhere in this Annual Report on Form 10-K. The information set forth below should be read in conjunction with “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and the audited consolidated financial statements, and the notes thereto, and other financial information included herein.
| Years Ended December 31, | ||||||||||||||||||||
| In thousands, except per share data | 2014 | 2013 | 2012 | 2011 | 2010 | |||||||||||||||
| Consolidated Statements of Operations Data: |
||||||||||||||||||||
| Revenue: |
||||||||||||||||||||
| Product revenue, net |
$ | 55,720 | $ | 45,238 | $ | — | $ | — | $ | — | ||||||||||
| License and collaboration revenue (1) |
49,688 | 296 | 514 | 25,189 | 174,460 | |||||||||||||||
| Service revenue (1) |
4 | 27 | 44 | 111 | 4,520 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total revenue |
105,412 | 45,561 | 558 | 25,300 | 178,980 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Operating expenses: |
||||||||||||||||||||
| Cost of product revenue |
5,224 | 9,612 | — | — | — | |||||||||||||||
| Research and development expense |
120,593 | 162,900 | 144,709 | 77,743 | 57,985 | |||||||||||||||
| Selling, general and administrative expense |
139,790 | 146,615 | 60,909 | 24,380 | 16,095 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total operating expenses |
265,607 | 319,127 | 205,618 | 102,123 | 74,080 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Income (loss) from operations |
(160,195 | ) | (273,566 | ) | (205,060 | ) | (76,823 | ) | 104,900 | |||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Other income (expense): |
||||||||||||||||||||
| Interest income (expense), net |
(7,990 | ) | (23 | ) | 41 | (65 | ) | (120 | ) | |||||||||||
| Gain on disposition of stock |
4,768 | — | — | — | — | |||||||||||||||
| Revaluation of warrant liability (2) |
— | — | (15,924 | ) | (46,715 | ) | (19,532 | ) | ||||||||||||
| Foreign exchange gain (loss) |
1,445 | (130 | ) | 71 | — | — | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Other income (expense), net |
(1,777 | ) | (153 | ) | (15,812 | ) | (46,780 | ) | (19,652 | ) | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Provision for income taxes |
630 | 439 | — | — | — | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Net income (loss) |
$ | (162,602 | ) | $ | (274,158 | ) | $ | (220,872 | ) | $ | (123,603 | ) | $ | 85,248 | ||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Net income (loss) per share – basic |
$ | (0.87 | ) | $ | (1.49 | ) | $ | (1.34 | ) | $ | (0.93 | ) | $ | 0.75 | ||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
|
– diluted |
$ | (0.87 | ) | $ | (1.49 | ) | $ | (1.34 | ) | $ | (0.93 | ) | $ | 0.74 | ||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Weighted average number of shares of common stock |
186,835 | 183,575 | 164,964 | 132,375 | 113,020 | |||||||||||||||
|
– diluted |
186,835 | 183,575 | 164,964 | 132,375 | 114,734 | |||||||||||||||
| As of December 31, | ||||||||||||||||||||
| In thousands | 2014 | 2013 | 2012 | 2011 | 2010 | |||||||||||||||
| Consolidated Balance Sheet Data: |
||||||||||||||||||||
| Cash, cash equivalents and marketable securities |
$ | 352,688 | $ | 237,179 | $ | 164,414 | $ | 306,256 | $ | 103,630 | ||||||||||
| Working capital |
295,644 | 172,769 | 119,484 | 282,195 | 88,775 | |||||||||||||||
| Total assets |
603,870 | 370,894 | 180,193 | 320,712 | 120,030 | |||||||||||||||
| Long-term debt, capital lease and facility lease obligations |
352,935 | 104,312 | 9,100 | 11,215 | 8,294 | |||||||||||||||
| Warrant liability (2) |
— | — | — | 58,639 | 28,815 | |||||||||||||||
| Accumulated deficit |
(1,214,595 | ) | (1,051,993 | ) | (777,835 | ) | (556,963 | ) | (433,360 | ) | ||||||||||
| Stockholders’ equity |
80,801 | 185,517 | 112,851 | 220,141 | 64,076 | |||||||||||||||
| (1) | During 2010, we modified our collaboration agreement with Merck and entered into a license agreement, as described in Note 2 to the consolidated financial statements. As a result of this modification, additional payments were received and deferred revenue was recognized. Pursuant to the license agreement, we provided services to Merck, recognized as service revenue. |
56
Table of Contents
| (2) | In 2009, we issued warrants that were accounted for as a derivative liability. The change in fair value of outstanding warrants was recorded in our consolidated statements of operations. Upon exercise of all remaining warrants in January and February 2012, the balance of the warrant liability was credited to stockholders’ equity and the liability was eliminated. See Note 11 to the consolidated financial statements. |
57
Table of Contents
| ITEM 7. | MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS |
The information set forth below should be read in conjunction with the audited consolidated financial statements, and the notes thereto, and other financial information included herein.
Overview
ARIAD is a global oncology company focused on transforming the lives of cancer patients with breakthrough medicines. Our mission is to discover, develop and commercialize small-molecule drugs to treat cancer in patients with the greatest and most urgent unmet medical need – aggressive cancers where current therapies are inadequate. We are focused on value-driving investments in commercialization, research and development, and new business development initiatives that we expect will lead to sustained profitability beginning in 2018 and increased shareholder value.
We are currently commercializing or developing the following three products and product candidates:
| • | Iclusig® (ponatinib) is our first approved cancer medicine, which we are commercializing in the United States, Europe and other territories for the treatment of certain patients with rare forms of leukemia. In 2014, we generated $55.7 million in net product revenue from sales of Iclusig, and we intend to continue to focus our commercial efforts in the United States and in 16 major European countries, while we seek to extend our geographic reach outside these territories through regional distributorships and collaborations. In late 2014, we secured an exclusive agreement for the co-development and commercialization of Iclusig in Japan and nine other Asian countries with Otsuka Pharmaceutical Co., Ltd., or Otsuka, for which we received an upfront payment of $77.5 million and are eligible to receive additional milestone payments. We plan to initiate three new randomized clinical trials beginning in 2015 to evaluate Iclusig in earlier lines of treatment and potentially to expand its addressable market. |
| • | Brigatinib (previously known as AP26113) is our next most advanced drug candidate, which we are developing for the treatment of certain patients with a form of non-small cell lung cancer, or NSCLC. In 2014, brigatinib received a Breakthrough Therapy designation from the FDA for the treatment of patients with ALK+ NSCLC who are resistant to crizotinib, and we initiated a pivotal Phase 2 trial in these patients. We plan to complete enrollment of this pivotal clinical trial in the third quarter of 2015, and, assuming favorable results, expect to file for regulatory approval in the United States in 2016. We are focused on securing a co-development and co-commercialization partnership for brigatinib that we expect will allow us to continue to leverage our existing infrastructure and capabilities, as well as support a randomized trial of brigatinib as a first line therapy. |
| • | AP32788 is our most recent, internally discovered drug candidate. In December 2014, we nominated AP32788 for clinical development. We are conducting studies necessary to support the filing of an investigational new drug application, or IND, which we expect to submit in 2015. We expect to commence clinical trials of AP32788 in 2016. |
These products and product candidates complement our earlier discovery of ridaforolimus, which we have out-licensed for development in drug-eluting stents and other medical devices for cardiovascular indications, and rimiducid (AP1903), which we have out-licensed for development in novel cellular immunotherapies. All of our product candidates were discovered internally by our scientists based on our expertise in computational chemistry and structure-based drug design.
Iclusig (ponatinib)
Commercialization
As noted above, Iclusig is approved in the United States, Europe and other territories for the treatment of certain patients with chronic myeloid leukemia, or CML, and Philadelphia chromosome-positive acute lymphoblastic leukemia, or Ph+ ALL.
In the United States, we are distributing Iclusig through a single specialty pharmacy. We employ an experienced and trained sales force and other professional staff, including account specialists, regional business directors, corporate account directors and medical science liaisons, who target the approximate 5,000 physicians who generate the majority of TKI prescriptions in the United States.
In Europe, we are now selling Iclusig in Germany, the United Kingdom, Austria, the Netherlands, Norway, Sweden, and Italy. In addition, we are distributing Iclusig to patients in France prior to pricing and reimbursement approval as permitted under French regulations. Full pricing and reimbursement in France and other European countries is expected by mid-2015. In total, we anticipate that Iclusig will be available for sale in 16 European countries by the third quarter of 2015.
We have established headquarters for our European operations in Switzerland where we manage all aspects of our business in Europe, including sales and marketing, distribution and supply chain, regulatory, medical affairs and supporting functions. We employ personnel in key countries in Europe to build company and brand awareness, manage the local country pricing and reimbursement process and sell Iclusig upon obtaining all necessary approvals.
58
Table of Contents
In order to provide for access to Iclusig in countries where we do not employ personnel, we enter into relationships with distributors in such countries. These distributorship arrangements provide for the exclusive right to sell and distribute Iclusig in a specific territory for a specified period of time in exchange for fees and payments related to purchase of Iclusig from us and/or sales of Iclusig in the territory. In December 2014, we entered into an agreement with Angelini Pharma, through its Austrian subsidiary, CSC Pharmaceuticals, whereby it will distribute Iclusig in countries in central and eastern Europe.
In territories outside the United States and Europe, we provide for the sale and distribution of Iclusig through partnership or distributorship arrangements. In December 2014, we entered into a co-development and commercialization agreement with Otsuka Pharmaceutical Co., Ltd., or Otsuka, under which Otsuka has exclusive rights to commercialize Iclusig in Japan and nine other Asian countries and to fund future clinical development in those countries. We expect to file for marketing approval in Japan in collaboration with Otsuka in the second half of 2015. In Australia, we obtained marketing approval for Iclusig in November 2014 and will distribute Iclusig through Specialised Therapeutics Australia Pty Ltd, under a distribution agreement we entered into in January 2014 that covers Australia and New Zealand. In Israel, we entered into a distribution agreement with Medison Pharma Ltd. in August 2014 and filed for marketing approval which is expected in 2015. We have also filed a marketing authorization application for Iclusig in Canada which is expected to be approved in 2015.
Ongoing Development of Iclusig
Initial approval of Iclusig in the United States and Europe was based on results from the pivotal Phase 2 PACE (Ponatinib Ph+ ALL and CML Evaluation) trial in patients with CML or Ph+ ALL who were resistant or intolerant to prior TKI therapy, or who had the T315I mutation of BCR-ABL. The PACE trial is fully enrolled and we continue to treat and follow patients in this trial who continue to respond to treatment. We also continue to treat and follow patients in our first, Phase 1 clinical trial of Iclusig in heavily pre-treated patients with resistant or intolerant CML or Ph+ALL, which was initiated in 2008; our Phase 2 clinical trial in CML patients in Japan; and our Phase 2 clinical trial in patients with gastrointestinal stromal tumors. In addition, Iclusig is being studied in ongoing investigator-sponsored trials in settings including first line and second line CML, acute myeloid leukemia, or AML, non-small cell lung cancer, or NSCLC, and medullary thyroid cancer, or MTC. Our development of Iclusig also includes ongoing activities in manufacturing, quality, safety and regulatory functions.
In 2015, we plan to initiate studies designed to better understand the safety profile of Iclusig in resistant and intolerant CML and Ph+ ALL patients, with the objective of improving the balance of benefit and risk for these patients as post-marketing commitments, as well as studies designed to evaluate its use in earlier lines of therapy. These studies include:
| • | a dose-ranging trial of Iclusig in approximately 450 patients with chronic phase CML, who have become resistant to at least two prior TKIs, designed to inform the optimal use of Iclusig in these patients. |
| • | a randomized, Phase 3 trial in approximately 500 patients with chronic phase CML who have experienced treatment failure after imatinib therapy, designed to evaluate two doses of Iclusig compared to the standard dose of nilotinib; and |
| • | an investigator-sponsored, early-switch trial of Iclusig in approximately 1,000 second-line chronic phase CML patients that will be conducted in the United Kingdom, designed to inform the use of Iclusig as part of the emerging paradigm in CML for early switching of TKIs in patients with suboptimal responses. |
In addition, we expect that additional investigator-sponsored clinical trials will be initiated in 2015 in various indications.
59
Table of Contents
Brigatinib (AP26113)
Brigatinib (AP26113) is an investigational inhibitor of anaplastic lymphoma kinase, or ALK. ALK was first identified as a chromosomal rearrangement in anaplastic large-cell lymphoma, or ALCL. Genetic studies indicate that abnormal expression of ALK is a key driver of certain types of non-small cell lung cancer, or NSCLC, and neuroblastomas, as well as ALCL. Since ALK is generally not expressed in normal adult tissues, it represents a highly promising molecular target for cancer therapy.
We are currently conducting a Phase 1/2 clinical trial of brigatinib, which we initiated in 2011. The primary objectives of the Phase 1 portion of the trial were to determine the maximum tolerated dose and the recommended dose for further study and to characterize its safety and preliminary anti-tumor activity. The primary purpose of the Phase 2 portion of the trial is to evaluate the efficacy of brigatinib in patients with TKI-naïve and crizotinib-resistant ALK-positive NSCLC, including in patients with brain metastases after crizotinib treatment. Results of this trial to date show robust anti-tumor activity in patients with TKI-naïve and crizotinib-resistant ALK-positive NSCLC, including in patients with brain metastases after crizotinib treatment. Crizotinib is the currently available first-generation ALK inhibitor.
We commenced a pivotal trial of brigatinib in the first quarter of 2014 in patients with locally advanced or metastatic NSCLC who were previously treated with crizotinib. The ALTA (ALK in Lung Cancer Trial of AP26113) trial is designed to determine the safety and efficacy of brigatinib in refractory ALK+ NSCLC patients. We anticipate that the results of the ALTA trial will form the basis for our application for initial regulatory approval. We expect to achieve full patient enrollment in the ALTA trial in the third quarter of 2015 and to file for approval of brigatinib in the United States in 2016.
Our development of brigatinib also includes key product and process development activities to support our clinical trials as well as the anticipated filing of an NDA and potential commercial launch of the product, quality and stability studies and pharmacovigilance and regulatory activities.
We are currently pursuing and a partnership to co-develop and co-commercialize brigatinib. We expect that such a partnership will allow us to continue to leverage our existing infrastructure and capabilities, as well as support a randomized, first-line trial of brigatinib vs. crizotinib in ALK+ NSCLC. A partnership may also support the exploration of new combination therapies in lung cancer that include brigatinib and other approved and unapproved medicines.
AP32788
At the end of 2014, we nominated our next internally discovered development candidate, AP32788. This orally active TKI has a unique profile against a validated class of mutated targets in non-small cell lung cancer and certain other solid tumors and may address an unmet medical need. We are currently performing pharmacology, toxicology and other studies necessary to support the filing of an investigational new drug (IND) application for AP32788 in 2015. We expect to begin a Phase 1/2 proof-of-concept trial in 2016.
Ridaforolimus
We have entered into license agreements with Medinol and ICON pursuant to which they are developing drug-eluting stents and other medical devices to deliver ridaforolimus to prevent restenosis, or reblockage, of injured vessels following interventions in which stents are used in conjunction with balloon angioplasty. In 2014, Medinol initiated two registration trials in the United States and other countries of its NIRsupremeTM Ridaforolimus-Eluting Coronary Stent System incorporating ridaforolimus and submitted an investigational device exemption, or IDE, to the FDA. These actions triggered milestone payments to us of $3.8 million in 2014.
In 2010, we had licensed ridaforolimus to Merck & Co., Inc., or Merck, for oncology applications. In February 2014, we received notice from Merck that it was terminating the license agreement. Per the terms of the license agreement, this termination became effective in November 2014 at which time all rights to ridaforolimus in oncology licensed to Merck were returned to us.
60
Table of Contents
Our Discovery Programs
Our research and development programs are focused on discovering and developing small-molecule drugs that regulate cell signaling for the treatment of cancer. Many of the critical functions of cells, such as cell growth, differentiation, gene transcription, metabolism, motility and survival, are dependent on signals carried back and forth from the cell surface to the nucleus and within the cell through a system of molecular pathways. When disrupted or over-stimulated, such pathways may trigger diseases such as cancer. Our research focuses on exploring cell-signaling pathways, identifying their role in specific cancers and cancer subtypes, and discovering drug candidates to treat those cancers by interfering with the aberrant signaling pathways of cells. The specific cellular proteins blocked by our product candidates have been well characterized as cancer targets. Iclusig and our product candidates brigatinib, AP32788 and ridaforolimus, have been developed in-house through the integrated use of structure-based drug design and computational chemistry, and their targets have been validated with techniques such as functional genomics, proteomics, and chemical genetics.
Critical Accounting Policies and Estimates
Our financial position and results of operations are affected by subjective and complex judgments, particularly in the areas of revenue recognition, accrued product development expenses, inventory, leased buildings under construction and stock-based compensation.
Revenue Recognition
Revenue is recognized when the four basic criteria of revenue recognition are met: (1) persuasive evidence that an arrangement exists; (2) delivery has occurred or services have been rendered; (3) the fee is fixed or determinable; and (4) collectability is reasonably assured. When the revenue recognition criteria are not met, we defer the recognition of revenue by recording deferred revenue until such time that all criteria are met.
Product Revenue, net
We commercially launched Iclusig in the United States on January 3, 2013 and in certain European countries in the second half of 2013. As noted above, in the fourth quarter of 2013, we temporarily suspended the marketing and commercial distribution of Iclusig in the United States. In January 2014, we resumed marketing and commercial distribution of Iclusig in the United States under the revised USPI and the REMS program.
Through October 31, 2013, we sold Iclusig in the United States through a limited number of specialty distributors and specialty pharmacies. Title and risk of loss transferred upon receipt of Iclusig by these customers. Specialty pharmacies dispensed Iclusig directly to patients in fulfillment of prescriptions. Specialty distributors sold Iclusig to hospital pharmacies and community practice pharmacies, referred to as healthcare providers, for treatment of patients. We provided the right of return to customers in the United States for unopened product for a limited time before and after its expiration date. Iclusig’s shelf-life in the United States is eighteen months for 15mg tablets and twelve months for 45mg tablets, measured from date of manufacture. In the United States, we deferred the recognition of revenue until Iclusig was sold to the healthcare providers or dispensed to the patients. Revenue recognition was deferred due to the inherent uncertainties in estimating future returns of Iclusig from our United States customers, due to factors such as estimated product demand and the limited shelf-life of Iclusig. Upon the temporary suspension of marketing and commercial distribution in the United States in the fourth quarter of 2013, we terminated our existing contracts with these specialty distributors and specialty pharmacies and we accepted product returns of Iclusig from these customers. The majority of these product returns related to product held by specialty distributors and specialty pharmacies that had not yet been sold to healthcare
61
Table of Contents
providers or dispensed to patients and thus not yet recognized as revenue. As a result, these returns were recorded as a reduction of accounts receivable and deferred revenue. For customers that had already paid for delivered product, a liability was recorded in other current liabilities. Upon receiving approval to resume marketing and commercial distribution in the United States, we revised our distribution strategy and sell Iclusig through a single specialty pharmacy which dispenses Iclusig to patients. Title and risk of loss transfers to the specialty pharmacy upon its receipt of Iclusig.
In Europe, we sell Iclusig to retail pharmacies and hospital pharmacies that dispense product directly to patients. These customers are provided with a limited right of return, such as instances of damaged product, and revenue is recognized at the time of shipment to these customers provided all other revenue recognition criteria are met.
Product revenues are recorded net of estimated gross to net deductions, including government-mandated rebates and chargebacks, distribution fees and other deductions. Reserves are established for these deductions and actual amounts incurred are offset against applicable reserves. We reflect these reserves as either a reduction in the related account receivable from the customer or as an accrued liability, depending on the nature of the deduction. These reserves are based on management’s estimates that consider payor mix in target markets, industry benchmarks and experience to date. These estimates involve a high degree of judgment and are periodically reviewed and adjusted as necessary.
We have individual contracts or standard terms of sale with our customers and delivery occurs when a customer receives Iclusig. We evaluate the creditworthiness of each of our customers to determine whether collection is reasonably assured and have determined that all of our customers are creditworthy. In order to conclude that the price is fixed or determinable, we must be able to calculate our gross product revenues from our customers and reasonably estimate our net product revenues. Our gross product revenues are based on the fixed wholesale acquisition cost for Iclusig that we charge our customers. We estimate our net product revenues by deducting from our gross product revenues (i) trade allowances, such as invoice discounts for prompt payment and customer fees, (ii) estimated government and private payor rebates, chargebacks and discounts, such as Medicare and Medicaid reimbursements in the United States, and (iii) estimated costs of incentives offered to certain indirect customers including patients. These gross to net deductions, and in particular the estimates for rebates, chargebacks and discounts, require us to make significant judgments that materially affect our recognition of net product revenues related to Iclusig.
Trade Allowances: We provide invoice discounts on Iclusig sales to certain of our customers for prompt payment and pay fees for certain services, such as fees for certain data that customers provide to us. We expect our customers to earn these discounts and fees, and therefore deduct these discounts and fees from our gross product revenues when revenue is recognized.
Rebates, Chargebacks and Discounts: In the United States, we contract with Medicare, Medicaid, other government agencies and various private organizations (collectively, payors) to make Iclusig, when commercially available, eligible for purchase by, or for partial or full reimbursement from, such payors. We estimate the rebates, chargebacks and discounts we will provide to payors and deduct these estimated amounts from our gross product revenue at the time the revenue is recognized. We estimate rebates, chargebacks and discounts based on (1) the contractual terms of agreements in place with payors, (2) the government-mandated discounts applicable to government-funded programs, and (3) the estimated payor mix. Government rebates that are invoiced directly to us are recorded in accrued liabilities on our consolidated balance sheet. For qualified programs that can purchase our product at a lower contractual government or commercial price, the customers charge back to us the difference between their acquisition cost and the lower contractual government or commercial price, which we record as an allowance against accounts receivable on our consolidated balance sheet. In Europe, we are subject to mandatory rebates and discounts in markets where government-sponsored healthcare systems are the primary payers for healthcare. These rebates and discounts are recorded when revenue is recognized and included in accrued liabilities on our consolidated balance sheet.
62
Table of Contents
The value of the rebates, chargebacks and discounts provided to third-party payors per course of treatment vary significantly and are based on government-mandated discounts and our arrangements with other third-party payors. In the United States, typically, government-mandated discounts are significantly larger than discounts provided to other third-party payors. In order to estimate our total rebates, chargebacks and discounts, we estimate the percentage of prescriptions that will be covered by each third-party payor, which is referred to as the payor mix. In order to estimate the payor mix for Iclusig, we used both (i) information obtained from our customers and third parties regarding the payor mix for Iclusig, (ii) historical industry information regarding the payor mix for competitive products, and (iii) our actual history since our commercial launch of Iclusig. We track available information regarding changes, if any, to the payor mix for Iclusig, to our contractual terms with third-party payors and to applicable governmental programs and regulations. If necessary, we adjust our estimated rebates, chargebacks and discounts based on new information, including information regarding actual rebates, chargebacks and discounts for Iclusig, as it becomes available. If we increased our estimate of the percentage of patients receiving Iclusig covered by third-party payors entitled to government-mandated discounts by five percentage points, our United States net product revenues would have decreased by approximately 1 percent in 2014. In the European countries where we currently sell Iclusig, rebates, as applicable, are fixed percentages of product sales.
Other Incentives: Other incentives that we offer include co-pay assistance for our patients and distribution fees offered to our customers. Our co-pay assistance programs assist commercially insured patients who have coverage for Iclusig and who reside in states that permit co-pay assistance programs. Our co-pay assistance program is intended to reduce each participating patient’s portion of the financial responsibility for Iclusig’s purchase price to a specified dollar amount. In each period, we record the amount of co-pay assistance provided to eligible patients based on the terms of the program.
In 2012, prior to obtaining marketing authorization for Iclusig in Europe, the French regulatory authority granted an Autorisation Temporaire d’Utilisation (ATU), or Temporary Authorization for Use, for Iclusig for the treatment of patients with CML and Ph+ ALL under a nominative program on a patient-by-patient basis. We began shipping Iclusig under this program during the year ended December 31, 2012. This program concluded on September 30, 2013 and all outstanding amounts due from customer under this program ($7.7 million) have been received. Upon completion of this program, we became eligible to ship Iclusig directly to customers in France as of October 1, 2013. These shipments totaled $10.6 million for the period from October 1, 2013 to December 31, 2014, of which $17.1 million was received as of December 31, 2014. Shipments under these programs have not met the criteria for revenue recognition as the price for these shipments is not yet fixed or determinable. The price of Iclusig in France will become fixed or determinable upon completion of pricing and reimbursement negotiations, which is expected in the first half of 2015. At that time, we will record revenue related to cumulative shipments as of that date in France, net of amounts that will be refunded to the health authorities based on the results of the pricing and reimbursements negotiations.
License Revenue
We generate revenue from license and collaboration agreements with third parties related to use of our technology and/or development and commercialization of products. Such agreements typically include payment to us of non-refundable upfront license fees, regulatory, clinical and commercial milestone payments, payment for services or supply of product and royalty payments on net sales. Revenue arrangements with multiple elements are divided into separate units of accounting if certain criteria are met, including whether the delivered element has stand-alone value to the customer. When deliverables are separable, consideration received is allocated to the separate units of accounting based on the relative selling price of each deliverable and the appropriate revenue recognition principles are applied to each unit. For arrangements with multiple elements, where we determine there is one unit of accounting, revenue associated will be recognized over the period beginning with the commencement of the final deliverable in the arrangement and over a period reflective of our longest obligation period within the arrangement on a straight-line basis.
At the inception of each agreement that includes milestone payments, we evaluate whether each milestone is substantive on the basis of the contingent nature of the milestone. This evaluation includes an assessment of whether:
| • | the consideration is commensurate with either (1) our performance to achieve the milestone, or (2) the enhancement of the value of the delivered item(s) as a result of a specific outcome resulting from our performance to achieve the milestone, |
| • | the consideration relates solely to past performance, and |
| • | the consideration is reasonable relative to all of the deliverables and payment terms within the arrangement. |
In making this assessment, we evaluate factors such as the clinical, regulatory, commercial and other risks that must be overcome to achieve the respective milestone, the level of effort and investment required, and whether the milestone consideration is reasonable relative to all deliverables and payment terms in the arrangement. We recognizes revenues related to substantive milestones in full in the period in which the substantive milestone is achieved. If a milestone payment is not considered substantive, we recognize the applicable milestone over the remaining period of performance.
We will recognize royalty revenue, if any, based upon actual and estimated net sales by the licensee of licensed products in licensed territories, and in the period the sales occur.
63
Table of Contents
Accrued Product Development Expenses
We accrue expenses for our product development activities based on our estimates of services performed or progress achieved pursuant to contracts and agreements with multiple vendors including research laboratories, contract manufacturers, contract research organizations and clinical sites. These estimates are recorded in research and development expenses in our consolidated statements of operations and are reflected in accrued product development expenses on our consolidated balance sheet. At December 31, 2014, we reported accrued product development expenses of $14.0 million on our consolidated balance sheet.
Our estimates of services performed or progress achieved are based on all available information we have from reports, correspondence and discussions with our vendors. Our estimates of accrued expenses based on such information require judgment. Actual costs may vary from such estimates. When such variances become known, we adjust our expenses accordingly.
Inventory
Inventory costs consist of costs related to the manufacturing of Iclusig, which are primarily the costs of contract manufacturing. We value our inventory at the lower of cost or market. We determine the cost of our inventory on a specific identification basis. We evaluate our inventory on a quarterly basis and, if we identify excess, obsolete or unsalable inventory, we adjust the carrying value of such inventory to net realizable value in the period in which it is identified. Estimates of excess inventory consider inventory levels, our projected sales of the product for the foreseeable future and the estimated shelf-lives of our inventory components. At the time of launch in the United States, Iclusig 15mg tablets had a shelf-life of twelve months, which has subsequently been extended to eighteen months, and Iclusig 45mg tablets have a shelf life of twelve months. In Europe, the shelf-life of both 15mg and 45mg tablets is twenty-four months. Inventory that is not expected to be used within one year is recorded as a non-current asset.
Prior to receiving approval from the FDA in December 2012 to sell Iclusig, we expensed all costs incurred related to the manufacture of Iclusig as research and development costs because of the inherent risks associated with the development of a drug candidate, the uncertainty about the regulatory approval process and the lack of history for our Company of regulatory approval of drug candidates. Accordingly, the manufacturing costs for Iclusig included in our cost of product revenue for 2013 and 2014 were significantly lower than full manufacturing cost, as most of these costs had been recorded as research and development expenses in prior periods.
Following the temporary suspension of Iclusig in the United States and the revised USPI, which reduced the addressable patient population for whom Iclusig is currently indicated, we revised our forecasts of demand for Iclusig and have incorporated such revisions in our evaluations of the carrying value of our inventory. Consequently, in the fourth quarter of 2013, we recorded charges to cost of product revenue of $8.9 million for excess inventory and finished goods inventory that was estimated to reach the end of its shelf life prior to sale. In 2014, we recoded an additional $4.2 million in charges for excess inventory. Based on our current inventory levels, projected production schedules and levels and updated forecasts of demand for Iclusig, we do not anticipate any material charges for excess inventory in the foreseeable future. Total inventory at December 31, 2014 and 2013 was $1.4 million and $3.4 million, respectively.
Due to the inventory costs that were recorded as research and development expenses as well as the write-downs of inventory, we expect that our cost of inventory sold in the future will be significantly less than full manufacturing cost. The time period over which this reduced-cost inventory is consumed will depend on a number of factors, including the amount of future sales of Iclusig in the United States and in Europe, the ultimate use of this inventory in either commercial sales, clinical development or other research activities and the ability to utilize inventory prior to its expiration date. We expect that as this reduced-cost inventory is used in the future, the cost of product revenue, before consideration of any future required inventory write-downs or reserves, will be in the low single digits as a percentage of net product revenue.
64
Table of Contents
Leased Buildings Under Construction
In January 2013, we entered into a lease agreement for approximately 244,000 square feet of laboratory and office space in two adjacent, connected buildings which are under construction in Cambridge, Massachusetts. Under the terms of the original lease, we leased all of the rentable space in one of the two buildings and a portion of the available space in the second building. In September 2013, we entered into a lease amendment to lease all of the remaining space, approximately 142,000 square feet, in the second building, for an aggregate of 386,000 square feet in both buildings. The terms of the lease amendment were consistent with the terms of the original lease. Construction of the core and shell of the buildings is expected to be completed in the first half of 2015 at which time construction of tenant improvements in the buildings will commence. Construction of the tenant improvements is expected to be completed in the first half of 2016.
In connection with this lease, the landlord is providing a tenant improvement allowance for the costs associated with the design, engineering, and construction of tenant improvements for the leased facility. The tenant improvements will be in accordance with our plans and include fit-out of the buildings to construct appropriate laboratory and office space, subject to approval by the landlord. To the extent the stipulated tenant allowance provided by the landlord is exceeded, we are obligated to fund all costs incurred in excess of the tenant allowance. The scope of the planned tenant improvements do not qualify as “normal tenant improvements” under the lease accounting guidance. Accordingly, for accounting purposes, we are the deemed owner of the buildings during the construction period.
As construction progresses, we record the project construction costs incurred as an asset, which reflects estimated replacement cost, along with a corresponding facility lease obligation, on the consolidated balance sheet for the total amount of project costs incurred whether funded by us or the landlord. Upon completion of the buildings, we will determine if the asset and corresponding financing obligation should continue to be carried on our consolidated balance sheet under the accounting guidance. Based on the current terms of the lease, we expect to continue to be the deemed owner of the buildings upon completion of the construction period.
Following our announcements in the fourth quarter of 2013 concerning Iclusig, we re-assessed our current and future need for space in Cambridge and expect to require less space than previously planned. We could not terminate the new lease without substantial cost, and we would have been required to make substantial expenditures to make necessary improvements to our existing facilities. We are, therefore, planning to sub-lease approximately 170,000 square feet of the 386,000 square feet of the space comprising the new buildings. We are now planning the fit-out of the buildings and expect the space to be ready for occupancy in the first half of 2016. Due to delays in completion of the construction and occupancy of the buildings, we expect to be required to commence lease payments for the facility prior to occupancy. We are currently in discussions with the landlord regarding revisions to our plans, the timing related to construction of tenant improvements and occupancy of the buildings and other matters in the lease.
Stock-Based Compensation Expense
Stock-based compensation cost is estimated at the grant date based on the fair value of the award and is recognized as expense over the requisite service period of the award if performance and service conditions are expected to be achieved. We use the Black-Scholes option-pricing model to estimate the fair value of stock options. Option valuation models require the input of assumptions, including the expected life of the stock-based awards, the estimated stock price volatility, the risk-free interest rate, and the expected dividend yield. Estimated volatility is based on a combination of historical and implied volatility. Expected life is based on our historical experience. The risk-free interest rate is based on U.S. Treasury interest rates with terms consistent with the expected life of the stock-based award. Expected dividend yield was not considered in the option pricing formula since we do not pay dividends and have no current plans to do so in the future. The forfeiture rate is based upon historical experience. We adjust the estimated forfeiture rate based upon our actual experience. In addition, we have performance based awards that are valued at the fair value of shares as of the grant date and compensation expense is recognized based on the number of shares expected to vest under the terms of the award under which
65
Table of Contents
they are granted, if performance conditions are expected to be met. The determination of whether the performance condition will be met requires significant judgment, including estimating the probability and timing of future events. Compensation cost for certain awards may increase by up to 60 percent of the cost estimated at target award levels based upon the eventual outcome of the performance conditions. We recognized stock compensation expense of $5.0 million as of December 31, 2014 for performance awards that are expected to vest in the future.
Results of Operations
For the Years Ended December 31, 2014 and 2013
Revenue
Our revenues for 2014, as compared to 2013, were as follows:
| Year Ended December 31, | Increase/ | |||||||||||
| In thousands | 2014 | 2013 | (decrease) | |||||||||
| Product revenue, net |
$ | 55,720 | $ | 45,238 | $ | 10,482 | ||||||
| License revenue |
49,688 | 296 | 49,392 | |||||||||
| Service revenue |
4 | 27 | (23 | ) | ||||||||
|
|
|
|
|
|
|
|||||||
| $ | 105,412 | $ | 45,561 | $ | 59,851 | |||||||
|
|
|
|
|
|
|
|||||||
Product revenue is stated net of adjustments for trade allowances, rebates, chargebacks and discounts and other incentives, summarized as follows:
| Year Ended December 31, | Increase/ | |||||||||||
| In thousands | 2014 | 2013 | (decrease) | |||||||||
| Trade allowances |
$ | 723 | $ | 1,158 | $ | (435 | ) | |||||
| Rebates, chargebacks and discounts |
3,798 | 2,721 | 1,077 | |||||||||
| Other incentives |
1,253 | 180 | 1,073 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total adjustments |
$ | 5,774 | $ | 4,059 | $ | 1,715 | ||||||
|
|
|
|
|
|
|
|||||||
| Gross product revenue |
$ | 61,494 | $ | 49,297 | $ | 12,197 | ||||||
|
|
|
|
|
|
|
|||||||
| Percentage of gross product revenue |
9.4 | % | 8.2 | % | ||||||||
|
|
|
|
|
|||||||||
Product revenue, net reflects the commercial launch of Iclusig in the United States in January 2013 and in Europe in mid-2013. In the United States, we recognize revenue on a sell-through basis. In Europe, we generally recognize revenue upon shipment to our customers. The increase in product revenue in 2014 when compared to 2013 primarily reflects a full year of sales in Europe and, in general, increasing demand for Iclusig over time. Product revenue is reduced by certain gross to net deductions. In 2014, these gross to net deductions, as a percentage of gross revenue, were approximately 9.4 percent as compared to 8.2 percent in 2013 and related to increased government-mandated discounts as well as returns of Iclusig in the United States.
We expect that our product revenue will increase in 2015 compared to 2014 due primarily to increasing acceptance of and demand for Iclusig, as well as pricing adjustments, in the United States, and increasing sales of Iclusig in Europe as we obtain pricing and reimbursement approval in various countries in Europe during the year. With the re-launch of Iclusig in the United States in January 2014, we have re-established marketing and distribution activities that have driven increasing product revenue that we expect will continue in 2015 and beyond. In Europe, our growth in revenues is dependent on the successful completion of pricing and reimbursement negotiations in countries throughout Europe. We currently have pricing and reimbursement approval in eight countries in Europe and expect to receive approval and formally launch commercial distribution in an additional eight to twelve countries in Europe in 2015, which we expect will drive additional increases in Iclusig product revenue. In addition, we have obtained marketing approval for Iclusig in Australia, have filed for approval in Israel and Canada and expect to file for approval in Japan in the second half of 2015. In these and other territories, we plan to rely on partnerships and distribution arrangements to make Iclusig available in these markets. Actual revenues will be subject to the outcome of pricing and reimbursement negotiations in Europe and the ultimate resolution of pricing negotiations and refunds in France.
License revenue increased in 2014 when compared to 2013 due to the receipt of $3.8 million in milestone payments from Medinol triggered by the commencement of patient enrollment in two clinical trials of Medinol’s ridaforolimus – eluting stent system as well as the receipt of $50 million in payments from Bellicum Pharmaceuticals, Inc., or Bellicum, pursuant to the Omnibus Amendment Agreement signed in October 2014 related to our previous license agreement for our ARGENT technology. Of the $50 million received from Bellicum, $4.8 million was recorded as other income related to the value of Bellicum common stock we surrendered to Bellicum and the remainder of $45.2 million was recorded as license revenue.
We expect our license revenue will significantly decrease in 2015 compared to 2014 due primarily to the non-recurring license revenue in 2014 related to the Bellicum agreement. There are no continuing services or payments being provided by us or Bellicum going forward. In 2015, our license revenue will primarily relate to the Otsuka agreement which became effective in December 2014.
66
Table of Contents
Operating Expenses
Cost of Product Revenue
Our cost of product revenue relates to sales of Iclusig upon commercial launch in the United States and in Europe. Our cost of product revenue for 2014 as compared to 2013, was as follows:
| Year Ended December 31, | Increase/ | |||||||||||
| In thousands | 2014 | 2013 | (decrease) | |||||||||
| Inventory cost of Iclusig sold |
$ | 457 | $ | 183 | $ | 274 | ||||||
| Shipping and handling costs |
545 | 572 | (27 | ) | ||||||||
| Inventory reserves/write-downs |
4,222 | 8,857 | (4,635 | ) | ||||||||
|
|
|
|
|
|
|
|||||||
| $ | 5,224 | $ | 9,612 | $ | (4,388 | ) | ||||||
|
|
|
|
|
|
|
|||||||
Prior to receiving regulatory approval for Iclusig from the FDA in December 2012, we expensed as research and development costs all costs incurred in the manufacturing of Iclusig to be sold upon commercialization. In addition, we recorded charges to cost of product revenue of $4.2 million and $8.9 million in 2014 and 2013, respectively, for excess inventory as described below. As a result, for Iclusig sold in 2014 and 2013, the majority of manufacturing costs incurred had previously been expensed. Therefore, the cost of inventory sold included limited manufacturing costs and the cost of packaging and labeling for commercial sales. If product-related costs had not previously been expensed as research and development prior to receiving FDA approval, or written down as excess inventory, the cost to produce the Iclusig sold would have been approximately $581,000 and $594,000, in 2014 and 2013, respectively.
Following the temporary suspension of U.S. marketing and commercial distribution and the revised USPI, which reduced the addressable patient population for whom Iclusig is currently indicated, we revised our forecasts of demand for Iclusig and have incorporated such revisions in our evaluations of the carrying value of our inventory. Consequently, in the fourth quarter of 2013, we recorded charges to cost of product revenue of $8.9 million for excess inventory and finished goods inventory that was estimated to reach the end of its shelf life prior to sale. In 2014, we recorded an additional $4.2 million in charges for excess inventory. Based on our current inventory levels, projected production schedules and levels and updated forecasts of demand for Iclusig, we do not anticipate any material charges for excess inventory in the foreseeable future.
Research and Development Expenses
Research and development expenses decreased by $42.3 million, or 26 percent, to $120.6 million in 2014, compared to $162.9 million in 2013, for the reasons set forth below.
The research and development process necessary to develop a pharmaceutical product for commercialization is subject to extensive regulation by numerous governmental authorities in the United States and other countries. This process typically takes years to complete and requires the expenditure of substantial resources. Current requirements include:
| • | preclinical toxicology, pharmacology and metabolism studies, as well as in vivo efficacy studies in relevant animal models of disease; |
| • | manufacturing of drug product for preclinical studies and clinical trials and ultimately for commercial supply; |
67
Table of Contents
| • | submission of the results of preclinical studies and information regarding manufacturing and control and proposed clinical protocol to the FDA in an Investigational New Drug application, or IND (or similar filings with regulatory agencies outside the United States); |
| • | conduct of clinical trials designed to provide data and information regarding the safety and efficacy of the product candidate in humans; and |
| • | submission of all the results of testing to the FDA in a New Drug Application, or NDA (or similar filings with regulatory agencies outside the United States). |
Upon approval by the appropriate regulatory authorities, including in some countries approval of product pricing, we may commence commercial marketing and distribution of the product. As a condition of approval, the FDA may require that a sponsor of a product candidate receiving accelerated approval perform post-marketing studies.
We group our research and development, or R&D, expenses into two major categories: direct external expenses and all other R&D expenses. Direct external expenses consist of costs of outside parties to conduct and manage clinical trials, to develop manufacturing processes and manufacture product candidates, to conduct laboratory studies and similar costs related to our clinical programs. These costs are accumulated and tracked by product candidate. All other R&D expenses consist of costs to compensate personnel, to purchase lab supplies and services, to lease, operate and maintain our facility, equipment and overhead and similar costs of our research and development efforts. These costs apply to our clinical programs as well as our preclinical studies and discovery research efforts. Product candidates are designated as clinical programs once we have filed an IND with the FDA, or a similar filing with regulatory agencies outside the United States, for the purpose of commencing clinical trials in humans.
Our R&D expenses for 2014, as compared to 2013, were as follows:
| Year Ended December 31, | Increase/ | |||||||||||
| In thousands | 2014 | 2013 | (decrease) | |||||||||
| Direct external expenses: |
||||||||||||
| Iclusig |
$ | 25,143 | $ | 59,593 | $ | (34,450 | ) | |||||
| Brigatinib |
17,061 | 17,439 | (378 | ) | ||||||||
| All other R&D expenses |
78,389 | 85,868 | (7,479 | ) | ||||||||
|
|
|
|
|
|
|
|||||||
| $ | 120,593 | $ | 162,900 | $ | (42,307 | ) | ||||||
|
|
|
|
|
|
|
|||||||
In 2014 and 2013, our clinical programs consisted of (i) Iclusig, our pan BCR-ABL inhibitor, and (ii) brigatinib (AP26113), our ALK inhibitor.
Direct external expenses for Iclusig were $25.1 million in 2014, a decrease of $34.5 million, or 59 percent, as compared to the corresponding period in 2013. The decrease is primarily due to decreases in clinical trial costs of $28.4 million, contract manufacturing costs of $3.9 million and other costs of $2.2 million. The decrease in clinical trial costs relates primarily to the discontinuation of the Phase 3 EPIC trial and pediatric trial in October 2013, the impact of clinical holds placed on certain trials, including investigator sponsored trials, and decreasing activities in the Phase 2 PACE trial and other trials. We also reduced our expenses in 2014 through the suspension of new enrollment in certain clinical trials of Iclusig in the fourth quarter of 2013, as well as by focusing near term development activities for Iclusig on key activities to address the safety concerns raised by the FDA in 2013. The decrease in manufacturing costs relates to the completion of large scale development activities for Iclusig at a contract manufacturer. Other costs decreased due to reduction in toxicology, stability and other studies related to use of new manufacturers for Iclusig and refunds of certain regulatory fees.
68
Table of Contents
Direct external expenses for brigatinib were $17.1 million in 2014, a decrease of $378,000, or 2 percent, as compared to the corresponding period in 2013. The decrease in expenses for brigatinib was due primarily to decreases of $4.4 million in contract manufacturing costs and $312,000 in other supporting costs; this decrease was offset in part by an increase in clinical trial costs of $4.3 million. Contract manufacturing costs decreased due to decreased process and formulation development and validation activities in the corresponding period for this program. Clinical trial costs increased due to costs related to initiation and on-going enrollment in the ALTA pivotal Phase 2 trial for brigatinib, which we initiated in March 2014.
We expect that our direct external R&D expenses will increase in 2015 compared to 2014 as we continue to invest in Iclusig and our product candidates brigatinib and AP32788. We continue to treat and follow patients in on-going clinical trials of Iclusig and plan to commence additional trials in 2015 designed to better understand the safety profile of Iclusig and to evaluate its use in earlier lines of therapy, as well as conduct additional studies to support continued development of Iclusig. For brigatinib, we continue to treat and follow patients in our Phase 1/2 clinical trial and continue to enroll patients in the ALTA pivotal trial, and plan to conduct additional studies to support continued development and potential regulatory approval of brigatinib. For AP32788, which we recently nominated as our next development candidate, we plan to conduct various studies that are necessary to support the filing of an investigational new drug application with the FDA.
All other R&D expenses decreased by $7.5 million or 9 percent, in 2014 as compared to 2013. This decrease was due to a decrease in personnel costs of $6.6 million as well as decreases in general expenses of $650,000 and lab expenses of $1.0 million, due to the reduction in workforce in the United States in November 2013. These decreases were offset in part by an increase in overhead expenses of $1.1 million due primarily to increased facility and related expenses. We expect that all other R&D expenses will increase in 2015 compared to 2014 due to increased personnel expenses in support of additional clinical trials and increased discovery research initiatives.
The successful development of our product candidates is uncertain and subject to a number of risks. We cannot be certain that any of our products or product candidates will prove to be safe and effective or will meet all of the applicable regulatory requirements needed to receive and maintain marketing approval. Data from preclinical studies and clinical trials are susceptible to varying interpretations that could delay, limit or prevent regulatory approval or could result in label warnings related to or recalls of approved products. Our ability to obtain sources of product revenue from the successful development our product candidates will depend on, among other things, our efforts to develop Iclusig in other patient populations and cancers, as well as the success of brigatinib, AP32788 and other product candidates, if any. Other risks associated with our products and product candidates are described in the section entitled “Risk Factors” in Part I of this report.
Selling, General and Administrative Expenses
Selling, general and administrative expenses decreased by $6.8 million, or 5 percent, to $139.8 million in 2014, compared to $146.6 million in 2013. Following the suspension of marketing and commercial distribution of Iclusig in the United States in the fourth quarter of 2013 and the subsequent approval by the FDA of revised U.S. prescribing information and Risk Evaluations and Mitigation Strategy, we re-launched Iclusig in the United States in January 2014, with a revised distribution model utilizing a single specialty pharmacy and a smaller sales and marketing workforce, resulting in lower costs to market and sell Iclusig in the United States. We also reduced our expenses in 2014 through a reduction in workforce in the United States, implemented in the fourth quarter of 2013.
Personnel expenses decreased by $2.0 million in 2014 as compared to 2013 primarily due to the impact of the reduction in our work force in the United States announced in the fourth quarter of 2013, offset in part by an increase in personnel expenses in Europe as we added personnel in connection with the launch of Iclusig in various European countries starting in the second half of 2013. Expenses for outside professional services decreased by $6.5 million in 2014 as compared to 2013 primarily due to a reduction in sales and marketing initiatives and other consulting services that supported initial commercial launch of Iclusig in the United States in 2013, offset in part by an increase in legal expenses related to litigation matters. Overhead expenses increased by $1.9 million in 2014 as compared to 2013 primarily due to increases in facility-related costs in Europe. We expect that selling, general and administrative costs in 2015 will remain at approximately the same level as in 2014.
We expect that operating expenses in total will increase in 2015 compared to 2014 as described in the sections above. The actual amount of operating expenses will depend on, among other things, costs related to the progress of our product development programs including the initiation of an increase in enrollment in our clinical trials of Iclusig and brigatinib, the status of regulatory reviews and timing of pricing and reimbursement approvals of Iclusig in various countries in Europe, and the status of pre-clinical studies of AP32788.
69
Table of Contents
Other Income (Expense)
Interest Income/Expense
Interest income decreased to $85,000 in 2014 from $130,000 in 2013, as a result of a lower average balance of funds invested in 2014.
Interest expense increased to $8.1 million in 2014 from $153,000 in 2013 as a result of the issuance of $200 million in convertible notes in June 2014, including coupon interest of $3.9 million and amortization of debt discount of $4.1 million in 2014.
Gain on Disposition of Stock
We recorded a gain on the disposition of stock of $4.8 million in 2014 related to the surrender of Bellicum common stock we owned upon Bellicum’s payment of the amounts owed to us under in the Omnibus Amendment Agreement signed in October 2014, as described in the section “Revenue,” above.
Foreign Exchange Gain (Loss)
We recognized net foreign exchange transaction gains of $1.4 million in 2014 compared to net foreign exchange losses of $130,000 in 2013. The gains and losses are a result of our expansion into Europe as we carry accounts denominated in foreign currencies and are caused by changes in exchange rates during these periods.
Provision for Income Taxes
Our provision for income taxes for 2014 was $630,000 compared to $439,000 in 2013 and reflects estimated expenses for state taxes and taxes associated with certain foreign subsidiaries.
Operating Results
We reported a loss from operations of $160.2 million in 2014 compared to a loss from operations of $273.6 million in 2013, a decrease of $113.4 million, or 41 percent. We also reported a net loss of $162.6 million in 2014, compared to a net loss of $274.2 million in 2013, a decrease in net loss of $111.6 million or 41 percent, and a net loss per share of $0.87 and $1.49, respectively. The decrease in net loss for 2014 is largely due to an increase in Iclusig product revenue of $10.5 million, an increase in license revenue of $49.4 million and a decrease in our operating expenses, all as described above. Our results of operations for 2015 will vary from those of 2014 and actual results will depend on a number of factors, including our ability to successfully grow Iclusig product revenue in the United States, Europe and other territories, the status of pricing and reimbursement approvals in Europe, the progress of our product development programs, ongoing employee and related personnel costs, the progress of our discovery research programs, our ability to secure a partnership to co-develop and co-commercialize brigatinib and the impact of any commercial and business development activities, the impact of costs and potential sub-leasing activity for our building currently under construction in Cambridge, Massachusetts and other factors. The extent of changes in our results of operations will also depend on the sufficiency of funds on hand or available from time to time, which will influence the amount we will spend on operations and capital expenditures and the development timelines for our product candidates.
70
Table of Contents
Results of Operations
For the Years Ended December 31, 2013 and 2012
Revenue
Our revenues for 2013, as compared to 2012, were as follows:
| Year Ended December 31, | Increase/ | |||||||||||
| In thousands | 2013 | 2012 | (decrease) | |||||||||
| Product revenue, net |
$ | 45,238 | $ | — | $ | 45,238 | ||||||
| License revenue |
296 | 514 | (218 | ) | ||||||||
| Service revenue |
27 | 44 | (17 | ) | ||||||||
|
|
|
|
|
|
|
|||||||
| $ | 45,561 | $ | 558 | $ | 45,003 | |||||||
|
|
|
|
|
|
|
|||||||
The increase in product revenue, net reflects the commercial launch of Iclusig, our first approved cancer medicine, in the United States and in Europe in 2013. Prior to the temporary suspension of marketing and commercial distribution of Iclusig in the United States in the fourth quarter of 2013, we recognized revenue on a sell-through basis. In Europe, we generally recognize revenue upon shipment to our customers. Product revenue is reduced by certain gross to net deductions. In 2013, these gross to net deductions, as a percentage of gross revenue, were approximately 8.7 percent and related to reductions in the United States including government-related discounts, as well as government-mandated rebates in Europe.
We recognized $296,000 of license revenue in 2013, pursuant to a license agreement related to our ARGENT technology. Revenue in 2012 also consisted of $514,000 of license revenue related to our ARGENT technology.
Operating Expenses
Cost of Product Revenue
For 2013, our cost of product revenue totaled $9.6 million and included the following:
| In thousands | Year Ended December 31, 2013 |
|||
| Inventory cost of Iclusig sold |
$ | 183 | ||
| Shipping and handling costs |
572 | |||
| Inventory reserves/write-downs |
8,857 | |||
|
|
|
|||
| $ | 9,612 | |||
|
|
|
|||
Prior to receiving regulatory approval for Iclusig from the FDA in December 2012, we expensed as research and development costs all costs incurred in the manufacturing of Iclusig to be sold upon commercialization. For Iclusig sold in 2013, the majority of manufacturing costs incurred had previously been expensed. Therefore, the cost of inventory sold included limited manufacturing costs and the cost of packaging and labeling for commercial sales. If product-related costs had not previously been expensed as research and development prior to receiving FDA approval, the cost to produce the Iclusig sold would have been approximately $594,000 and total cost of product revenue would have been approximately $10.0 million in 2013.
Due to the temporary suspension of U.S. marketing and commercial distribution and the expected decrease in demand for Iclusig, we assessed the need for an inventory write-down for excess inventory for our inventory on hand at December 31, 2013. This review included all components of inventory, including raw materials, work-in-process and finished goods inventory. Inventory balances on hand were compared to the expected uses of inventory in the foreseeable future, taking into account the estimated future global demand for Iclusig as well as the estimated shelf-life of our inventory components. From this analysis, we determined that approximately $7.1 million of our inventory was excess at December 31, 2013 and we recorded a charge to cost of product revenue to write down inventory in the fourth quarter of 2013. In connection with this review, we also charged $1.3 million of vendor advances to cost of product revenue as the advances relate to future inventory production that will result in excess inventory. In addition, during 2013 we also wrote down finished goods that will expire and not be sold. Our total charges for 2013 for excess inventory, as well as finished goods inventory that will expire before it is sold, was approximately $8.9 million.
71
Table of Contents
Research and Development Expenses
Research and development expenses increased by $18.2 million, or 13 percent, to $162.9 million in 2013, compared to $144.7 million in 2012, were as follows:
| Year Ended December 31, | Increase/ | |||||||||||
| In thousands | 2013 | 2012 | (decrease) | |||||||||
| Direct external expenses: |
||||||||||||
| Iclusig |
$ | 59,593 | $ | 54,588 | $ | 5,005 | ||||||
| Brigatinib |
17,439 | 6,034 | 11,405 | |||||||||
| All other R&D expenses |
85,868 | 84,087 | 1,781 | |||||||||
|
|
|
|
|
|
|
|||||||
| $ | 162,900 | $ | 144,709 | $ | 18,191 | |||||||
|
|
|
|
|
|
|
|||||||
In 2013 and 2012, our clinical programs consisted of (i) Iclusig, our pan BCR-ABL inhibitor, and (ii) brigatinib (AP26113), our ALK inhibitor.
Direct external expenses for Iclusig were $59.6 million in 2013, an increase of $5.0 million, or 9 percent, as compared to 2012. The increase is due primarily to an increase in clinical trial costs of $13.6 million which was due to increases related to ongoing treatment of patients in existing and new trials, including the EPIC trial and the related comparator drug purchases, our Phase 2 GIST trial, our Phase 1/2 clinical trial of Iclusig in Japan, our pediatric trial of Iclusig, and increased costs associated with investigator-sponsored trials, offset by a decrease in costs related to our Phase 2 PACE clinical trial as treatment of patients and other related activities decreased in 2013 compared to 2012. As noted above, we announced the discontinuation of the EPIC trial in October 2013 and a pause on additional patient enrollment, as well as a partial FDA clinical hold, for all other Iclusig trials. The increase in the clinical trial costs was offset, in part, by a decrease in contract manufacturing costs of $5.9 million, and a decrease in other non-clinical support costs of $2.2 million. Contract manufacturing costs decreased as costs to manufacture Iclusig in 2013 are now being capitalized in inventory while such costs in 2012, prior to the FDA approval of Iclusig, were included in research and development expense. Non-clinical support costs decreased due primarily to the completion of the regulatory filings in support of the Iclusig NDA and in support of the initiation of the Phase 1/2 clinical trial in Japan.
Direct external expenses for brigatinib were $17.4 million in 2013, an increase of $11.4 million, or 189 percent, as compared to 2012. The increase in expenses for brigatinib was due primarily to an increase in clinical trial costs related to the initiation of additional trials and an increase in contract manufacturing costs due to the manufacture of additional material to supply the Phase 1/2 clinical trial and readiness to support additional trials.
All other R&D expenses increased by $1.8 million, or 2 percent, in 2013, as compared to 2012. This increase was primarily due to an increase in personnel costs of $7.4 million, due to higher compensation and related costs from an increase in the number of employees to support expanding R&D activities and costs associated with the employee workforce reduction in the fourth quarter of 2013, an increase in lab expenses of $1.2 million, related to increased discovery efforts, and an increase in general and other expenses of $861,000 related to travel expenses and training costs. These increases were offset primarily by a decrease in professional fees of $3.9 million and a decrease in intellectual property costs of $3.6 million due primarily to an impairment charge recorded for ridaforolimus technology in 2012.
Selling, General and Administrative Expenses
Selling, general and administrative expenses increased by $85.7 million, or 141 percent, to $146.6 million in 2013, compared to $60.9 million in 2012. This increase was due primarily to an increase in personnel costs of $43.4 million related primarily to compensation and related costs associated with an increase in the
72
Table of Contents
number of employees, including sales and marketing related personnel to support the commercial launch of Iclusig, expanding business activities in the United States and in Europe during 2013 and costs associated with the employee workforce reduction that occurred in the fourth quarter of 2013; an increase in professional services of $23.5 million due to corporate and commercial development initiatives supporting the launch of Iclusig; an increase in general expenses of $10.4 million primarily related to increases in technology costs and travel costs in support of the launch of Iclusig; and an increase in overhead and other expenses of $6.1 million primarily related to increased office, overhead and administrative expenses incurred to support the commercial operations associated with the launch of Iclusig.
Other Income (Expense)
Interest Income/Expense
Interest income decreased to $130,000 in 2013 from $240,000 in 2012, as a result of a lower average balance of funds invested in 2013.
Interest expense decreased to $153,000 in 2013 from $199,000 in 2012 as a result of lower average borrowings in 2013.
Foreign Exchange Gain (Loss)
We recognized net foreign exchange transaction losses of $130,000 in 2013 compared to net foreign exchange gains of $71,000 in 2012. The gains and losses are a result of our expansion into Europe as we carry accounts denominated in foreign currencies.
Provision for Income Taxes
Our provision for income taxes for 2013 was $439,000 and reflects estimated taxes for state taxes and taxable income associated with certain foreign subsidiaries. There was no provision for income taxes in 2012.
Operating Results
We reported a loss from operations of $273.6 million in 2013 compared to a loss from operations of $205.1 million in 2012, an increase of $68.5 million, or 33 percent. We also reported a net loss of $274.2 million in 2013, compared to a net loss of $220.9 million in 2012, an increase in net loss of $53.3 million or 24 percent, and a net loss per share of $1.49 and $1.34, respectively. The increase in net loss is largely due to the increase in our operating expenses described above offset in part by product revenue related to the commercial launch of Iclusig in January 2013.
73
Table of Contents
Selected Quarterly Financial Data
Summarized unaudited quarterly financial data are as follows:
| 2014 | ||||||||||||||||
| In thousands, except per share amounts | First | Second | Third | Fourth(a) | ||||||||||||
| Total revenue |
$ | 11,782 | $ | 12,114 | $ | 14,682 | $ | 66,835 | ||||||||
| Gross profit (loss) |
10,494 | 9,719 | 14,088 | 65,887 | ||||||||||||
| Net loss |
(49,822 | ) | (56,921 | ) | (50,108 | ) | (5,751 | ) | ||||||||
| Net loss per share – basic and diluted |
(0.27 | ) | (0.30 | ) | (0.27 | ) | (0.03 | ) | ||||||||
| 2013 | ||||||||||||||||
| In thousands, except per share amounts | First | Second | Third | Fourth(b) | ||||||||||||
| Total revenue |
$ | 6,464 | $ | 14,011 | $ | 16,732 | $ | 8,354 | ||||||||
| Gross profit (loss) |
6,195 | 13,783 | 16,317 | (346 | ) | |||||||||||
| Net loss |
(64,670 | ) | (68,985 | ) | (66,339 | ) | (74,164 | ) | ||||||||
| Net loss per share – basic and diluted |
(0.36 | ) | (0.37 | ) | (0.36 | ) | (0.40 | ) | ||||||||
| (a) | Our results for the fourth quarter of 2014 include $50 million in payments received from Bellicum Pharmaceuticals, Inc. of which $45.2 million is included in revenues and $4.8 million is included in other income. |
| (b) | Our results for the fourth quarter of 2013 include expenses related to the write-down of excess inventory and vendor advances of $8.5 million and restructuring charges of $4.8 million. |
Liquidity and Capital Resources
At December 31, 2014, we had cash and cash equivalents totaling $352.7 million and working capital of $295.6 million, compared to cash, cash equivalents and marketable securities totaling $237.2 million and working capital of $172.8 million at December 31, 2013. This increase was due primarily to the receipt of net proceeds of $177.3 million from our convertible note offering in June 2014, $111.7 million in license payments from Otsuka and Bellicum and net product revenue of $55.7 million, offset by our operating expenses. Of the $352.7 million of cash and cash equivalents at December 31, 2014, $11.9 million was in accounts held by our international subsidiaries. For 2014, we reported a net loss of $162.6 million and cash used in operating activities of $57.8 million. We expect to continue to incur losses on a quarterly basis until we can substantially increase revenues as a result of increased sales of Iclusig and potential future regulatory approvals of our product candidates, the timing of which are uncertain. However, pursuant to our current operating plan, we are focused on investments in commercialization, research and development, and new business development initiatives that we expect will lead to sustained profitability in 2018 and increased shareholder value.
Our balance sheet at December 31, 2014 includes property and equipment of $203.0 million, which represents an increase of $ 94.3 million from December 31, 2013. The increase is primarily due to the accounting, as described below, for our lease of new laboratory and office space in two adjacent, connected buildings currently under construction in Cambridge, Massachusetts. Construction of the core and shell of the buildings is expected to be completed in the first half of 2015, at which time construction of tenant improvements will commence. Construction of tenant improvements is expected to be completed in the first half of 2016, at which time we expect to occupy the buildings. Under the relevant accounting guidance, we are the deemed owner for the project during the construction period and accordingly, we record the project construction costs as an asset ($196.0 million at December 31, 2014) and a corresponding facility lease obligation. As construction continues on the facility, the asset and corresponding facility lease obligation will continue to increase.
Sources of Funds
We have financed our operations and investments to date primarily through sales of our common stock and convertible notes in public and private offerings, through the receipt of upfront and milestone payments from collaborations and licenses with pharmaceutical and biotechnology companies and, to a lesser extent, through issuances of our common stock pursuant to our equity incentive and employee stock purchase plans, supplemented by the borrowing of long-term debt from commercial lenders.
With the sales of Iclusig in the United States from January through October 2013 and commencing again in January 2014 as well as sales in Europe since the second half of 2013, we have generated product revenues that have contributed to our cash flows. However, our cash flows generated from sales of Iclusig are not currently sufficient to fund operations and we rely on funding from other sources to fund our operations.
74
Table of Contents
During the years ended December 31, 2014, 2013 and 2012, our sources of cash were as follows:
| In thousands | 2014 | 2013 | 2012 | |||||||||
| Issuance of convertible debt and related transactions, net |
$ | 177,281 | $ | — | $ | — | ||||||
| Upfront payment from Otsuka, net |
61,675 | — | — | |||||||||
| License payments from Bellicum |
50,000 | — | — | |||||||||
| Sales/issuances of common stock: |
||||||||||||
| In common stock offerings |
— | 310,037 | — | |||||||||
| Pursuant to warrant exercises |
— | — | 12,483 | |||||||||
| Pursuant to stock option and purchase plans |
3,695 | 6,284 | 10,511 | |||||||||
| Reimbursement of amounts related to facility lease obligation |
— | 2,741 | — | |||||||||
|
|
|
|
|
|
|
|||||||
| $ | 292,651 | $ | 319,062 | $ | 22,994 | |||||||
|
|
|
|
|
|
|
|||||||
In December 2014, we received from Otsuka Pharmaceutical Co., Ltd. an upfront payment of $61.7 million, which is net of a refundable withholding tax of $15.8 million that we expect to receive in 2015, in connection with the execution of an exclusive collaboration and license agreement to commercialize and further develop Iclusig in Japan and nine other Asian countries. Also, in the fourth quarter of 2014, we received a total of $50 million in payments from Bellicum Pharmaceuticals, Inc. in connection with an amendment to our license agreement with Bellicum. The amounts received from Otsuka and $45.2 million of the payments received from Bellicum are included in cash used in operating activities in our consolidated statement of cash flows but are presented separately in this analysis due to the non-recurring nature of these payments.
In June 2014, we sold $200 million aggregate principal amount of convertible notes to investors through JPMorgan Securities, LLC and other initial purchasers in a private placement to qualified institutional buyers pursuant to Rule 144A under the Securities Act of 1933, as amended. Net proceeds of this offering were approximately $177.3 million after deducting fees and expenses of approximately $7.1 million and the cost of convertible bond hedges of $15.6 million (after such cost was partially offset by the proceeds to us from the sale of warrants). Details of the convertible notes, the bond hedges and the warrants are included in Note 8, “Long term debt”, to our consolidated financial statements included in this Annual Report on Form 10-K.
In January 2013, we sold 16,489,893 shares of our common stock in an underwritten public offering at a purchase price of $19.60 per share. Net proceeds of this offering, after underwriting discounts and commissions and expenses, were approximately $310.0 million.
75
Table of Contents
We intend to rely on our existing cash and cash equivalents, cash flows from sales of Iclusig and funding from new collaborative agreements or strategic alliances, as our primary sources of liquidity. In the near-term, we expect cash flows from sales of Iclusig to increase as we continue to increase the number of patients that are treated with Iclusig and launch the product in new markets. In addition, we are currently pursuing a partnership arrangement to co-develop and co-commercialize brigatinib. We expect that such an agreement will further improve our liquidity, allow us to continue to leverage our existing infrastructure and capabilities, and support key development activities. We believe that these and similar non-dilutive funding transactions will provide necessary resources until such time that we generate revenues from sales of Iclusig and our product candidates sufficient to fund operations.
Uses of Funds
The primary uses of our cash are to fund our operations and working capital requirements and, to a lesser degree, to repay our long-term debt and to invest in our property and equipment as needed for our business. Our uses of cash during the years ended December 31, 2014, 2013 and 2012 were as follows:
| In thousands | 2014 | 2013 | 2012 | |||||||||
| Net cash used in operating activities |
$ | 57,794 | $ | 221,882 | $ | 153,681 | ||||||
| Upfront payment from Otsuka, net |
61,675 | — | — | |||||||||
| License payments from Bellicum |
45,232 | — | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Adjusted net cash used in operating activities |
164,701 | 221,882 | 153,681 | |||||||||
| Repayment of long-term borrowings and capital leases |
9,100 | 2,115 | 1,454 | |||||||||
| Change in restricted cash |
— | 10,319 | 289 | |||||||||
| Investment in intangible assets |
— | — | 633 | |||||||||
| Investment in property and equipment |
2,787 | 8,543 | 4,424 | |||||||||
| Payment of tax withholding obligations related to stock compensation |
816 | 3,363 | 4,336 | |||||||||
|
|
|
|
|
|
|
|||||||
| $ | 177,404 | $ | 246,222 | $ | 164,817 | |||||||
|
|
|
|
|
|
|
|||||||
The net cash used in operating activities is comprised of our net losses, adjusted for non-cash expenses, deferred revenue and working capital requirements. As noted previously, our net loss in 2014 decreased by $111.6 million as compared to 2013 while our net cash used in operating activities decreased by $164.1 million, the difference being due substantially to the receipt of an upfront payment from Otsuka Pharmaceutical Co. Ltd. of $61.7 million, which is net of a refundable withholding tax amount of $15.8 million, in December 2014 related to our Iclusig commercialization and co-development agreement for Japan and other Asian territories. After adjusting for the non-recurring payments from Otsuka and Bellicum, our adjusted net cash used in operating activities decreased by $57.2 million in 2014 as compared to 2013, reflecting primarily the decrease in our operating expenses discussed above. Another significant use of cash in 2014 compared to 2013 was the pay-off of our term loan with a bank resulting in debt principal payments for 2014 of $9.1 million. Also as noted previously, our net loss for 2013 increased by $53.3 million as compared to 2012, while our net cash used in operating activities increased by $68.2 million in 2013 as compared to 2012, reflecting decreases in non-cash adjustments and a decrease in working capital items. Another significant use of cash in 2013 compared to 2012 was an increase in restricted cash held as collateral for a letter of credit associated with the lease we executed in January 2013, and amended in September 2013, for new laboratory and office space in Cambridge, Massachusetts.
We currently occupy facilities in Cambridge, Massachusetts and Switzerland from which we conduct and manage our business. We also plan to occupy space in Cambridge, Massachusetts currently under construction. We had planned to move into the new buildings in early 2015. However, in light of our announcements in the fourth quarter of 2013 concerning Iclusig, we re-assessed our current and future need for space in Cambridge and expect to require less space than previously planned. We could not terminate the new lease without substantial cost, and we would have been required to make substantial expenditures to make necessary improvements to our existing facilities. We are, therefore, planning to sub-lease approximately 170,000 square feet of the 386,000 square feet of the space comprising the new buildings. If we are not successful in subleasing this space, our cost of the space we occupy will increase. Tenant improvements and the fit-out of the facility are expected to commence in the first half of 2015. The landlord has provided a tenant improvement allowance for such costs. To the extent such costs exceed the allowance, we will be responsible for funding such excess. We are currently planning the fit-out of the buildings and expect the space to be ready for occupancy in the first half of 2016. Due to delays in the timing for fit-out and occupancy of the buildings, we expect to be required to commence rent payments for the facility prior to occupancy. We are currently in discussions with the landlord regarding revisions to our plans, the timing related to construction of tenant improvements and occupancy of the buildings and other matters in the lease.
Liquidity
We incur substantial operating expenses to conduct research and development and commercialization activities and operate our business. In addition, we must pay interest on the $200.0 million principal amount of convertible notes we issued in June 2014 and will be required to repay the principal amount of the notes in June 2019, or earlier in specified circumstances, if the notes are not converted into shares of our common stock. We also have a substantial facility lease obligation, as noted above. We expect that cash flows from Iclusig together with our current cash and cash equivalents and funding we expect to receive from new collaborative agreements or strategic alliances will be sufficient to fund our operations for the foreseeable future.
The adequacy of our available funds to meet our future operating and capital requirements will depend on many factors, including the amounts of future revenue generated by Iclusig, our ability to enter into a partnership to co-develop and co-commercialize brigatinib and the amount of funding generated from such partnership, the potential introduction of one or more of our other drug candidates to the market, and the number, breadth, cost and prospects of our research and development programs.
To the extent that product revenues or non-dilutive funding transactions are not sufficient to fund our operations, we may seek to fund our operations by issuing common stock, debt or other securities in one or more public or private offerings, as market conditions permit, or through the incurrence of additional debt from commercial lenders or other financing transactions. Under SEC rules, we currently qualify as a “well-known seasoned issuer,” which allows us to file shelf registration statements to register an unspecified amount of securities that are effective upon filing, giving us the opportunity to raise funding when needed or otherwise considered appropriate. To the extent that we raise additional capital through the sale of equity or convertible debt securities, the ownership interest of our existing stockholders will be diluted, and the terms may include liquidation or other preferences that adversely affect the rights of our stockholders. Debt financing, if available, may involve agreements that include covenants limiting or restricting our ability to take specific actions, such as incurring debt, making capital expenditures or declaring dividends. In addition, we may raise additional capital through securing new collaborative agreements or other methods of financing. We will continue to manage our capital structure and to consider all financing opportunities, whenever they may occur, that could strengthen our long-term liquidity profile.
There can be no assurance that additional funds will be available when we need them on terms that are acceptable to us, or at all. If adequate funds are not available to us on a timely basis, we may be required to: (1) delay, limit, reduce or terminate our commercialization of Iclusig; (2) delay, limit, reduce or terminate preclinical studies, clinical trials or other clinical development activities for one or more of our approved products or product candidates; (3) delay, limit, reduce or terminate our discovery research or preclinical development activities; or (4) enter into licenses or other arrangements with third parties on terms that may be unfavorable to us or sell, license or relinquish rights to develop or commercialize our product candidates, approved products, technologies or intellectual property.
76
Table of Contents
Off-Balance Sheet Arrangements
As part of our ongoing business, we do not participate in transactions that generate relationships with unconsolidated entities for financial partnerships, such as entities often referred to as structured finance or special purpose entities which would have been established for the purpose of facilitating off-balance sheet arrangements or other contractually narrow or limited purposes. As of December 31, 2014, we maintained outstanding letters of credit of $11.3 million in accordance with the terms of our existing leases for our office and laboratory space, our new lease for office and laboratory space under construction, and for other purposes.
Contractual Obligations
We have substantial fixed contractual obligations under our long-term debt agreement, lease agreements, employment agreements, purchase commitments and benefit plans. These non-cancellable contractual obligations were comprised of the following as of December 31, 2014:
| Payments Due By Period | ||||||||||||||||||||
| In thousands | Total | In 2015 |
2016 through 2018 |
2019 through 2020 |
After 2020 |
|||||||||||||||
| Long-term debt |
$ | 232,927 | $ | 7,552 | $ | 21,750 | $ | 203,625 | $ | — | ||||||||||
| Lease agreements |
487,582 | 13,860 | 86,725 | 68,100 | 318,897 | |||||||||||||||
| Employment agreements |
15,468 | 7,734 | 7,734 | — | — | |||||||||||||||
| Purchase commitments |
38,489 | 2,767 | 24,290 | 3,918 | 7,514 | |||||||||||||||
| Other long-term obligations |
3,063 | 485 | 2,343 | 135 | 100 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total fixed contractual obligations |
$ | 777,529 | $ | 32,398 | $ | 142,842 | $ | 275,778 | $ | 326,511 | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
Long-term debt reflects the payment at maturity of our $200 million of convertible notes issued in June 2014 and due on June 15, 2019. Interest on this debt accrues at a rate of 3.625 percent of the principal, or $7.25 million, annually and is payable in arrears in December and June of each year. We may not redeem the convertible notes prior to the maturity date and no “sinking fund” is provided for the convertible notes, which means that we are not required to periodically redeem or retire the convertible notes. Upon the occurrence of certain fundamental changes involving our company, holders of the convertible notes may require us to repurchase for cash all or part of their convertible notes at a repurchase price equal to 100 percent of the principal amount of the convertible notes to be repurchased, plus accrued and unpaid interest.
Leases consist of payments to be made on our building leases in Cambridge, Massachusetts and Lausanne, Switzerland, including future lease commitments related to leases executed for office and laboratory space in two buildings currently under construction in Cambridge and office space in a building in Lausanne that completed construction in early 2014. The minimum non-cancelable payments for the facility being constructed in Cambridge are included in the table above and include amounts related to the original lease and the lease amendment. We are the deemed owner for accounting purposes and have recognized a financing obligation associated with the cost of the buildings incurred to date for the buildings under construction in Cambridge, Massachusetts. In addition to minimum lease payments, the leases require us to pay additional amounts for our share of taxes, insurance, maintenance and operating expenses, which are not included in the above table. Employment agreements represent base salary payments under agreements with officers. Purchase commitments represent non-cancelable contractual commitments associated with certain clinical trial activities. Other long-term obligations are comprised primarily of our liability for unrecognized tax positions, which is expected to be determined by 2016.
77
Table of Contents
Recently Adopted or Issued Accounting Pronouncements
In August 2014, FASB issued ASU 2014-15, Disclosure of Uncertainties about an Entity’s Ability to Continue as a Going Concern. The amendments in this update will explicitly require a company’s management to assess an entity’s ability to continue as a going concern, and to provide related footnote disclosures in certain circumstances. The new standard will be effective in the first annual period ending after December 15, 2016. Early application is permitted. The Company is currently evaluating the potential impact of the adoption of this standard, but believes its adoption will have no impact on its financial position, results of operations or cash flows.
In May 2014, the Financial Accounting Standards Board (FASB) and the International Accounting Standards Board (IASB) jointly issued Accounting Standards Update (ASU) No. 2014-9, Revenue from Contracts with Customers (Topic 606), which clarifies the principles for recognizing revenue and develops a common revenue standard for GAAP and International Financial Reporting Standards (IFRS). The core principle of the guidance is that an entity should recognize revenue to depict the transfer of promised goods or services to customers in an amount that reflects the consideration to which the entity expects to
78
Table of Contents
be entitled in exchange for those goods and services. The ASU is effective for public entities for annual and interim periods beginning after December 15, 2016. Early adoption is not permitted under GAAP and retrospective application is permitted, but not required. We are currently evaluating the impact of adopting this guidance on its financial position and results of operations.
In July 2013, the FASB issued ASU 2013-11, Presentation of an Unrecognized Tax Benefit When a Net Operating Loss Carryforward, a Similar Tax Loss, or a Tax Credit Carryforward Exists. ASU 2013-11 requires, unless certain conditions exists, an unrecognized tax benefit or a portion of an unrecognized tax benefit to be presented in the financial statements as a reduction to a deferred tax asset for a net operating loss carryforward, similar to a tax loss or a tax credit carryforward. We will apply this standard beginning January 1, 2014. The adoption of the standard is not expected to have a material impact on our consolidated financial statements.
ITEM 7A: QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
We invest our available funds in accordance with our investment policy to preserve principal, maintain proper liquidity to meet operating needs and maximize yields. Our investment policy specifies credit quality standards for our investments and limits the amount of credit exposure to any single issue, issuer or type of investment.
We invest cash balances in excess of operating requirements first in short-term, highly liquid securities, and money market accounts. Depending on our level of available funds and our expected cash requirements, we may invest a portion of our funds in marketable securities, consisting generally of corporate debt and U.S. government and agency securities. Maturities of our marketable securities are generally limited to periods necessary to fund our liquidity needs and may not in any case exceed three years. These securities are classified as available-for-sale.
At December 31, 2014, our available funds are invested solely in cash and cash equivalents and we do not have significant market risk related to interest rate movements.
As a result of our foreign operations, we face exposure to movements in foreign currency exchange rates, primarily the Euro, Swiss Franc and British Pound against the U.S. dollar. The current exposures arise primarily from cash, accounts receivable, intercompany receivables, payables and inventories. Both positive and negative affects to our net revenues from international product sales from movements in foreign currency exchange rates are partially mitigated by the natural, opposite affect that foreign currency exchange rates have on our international operating costs and expenses.
Certain Factors That May Affect Future Results of Operations
The SEC encourages companies to disclose forward-looking information so that investors can better understand a company’s future prospects and make informed investment decisions. This Annual Report on Form 10-K contains such “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995. These statements may be made directly in this Annual Report, and they may also be made a part of this Annual Report by reference to other documents filed with the SEC, which is known as “incorporation by reference.” Such statements in connection with any discussion of future operating or financial performance are identified by use of words such as “may,” “anticipate,” “estimate,” “expect,” “project,” “intend,” “plan,” “believe,” and other words and terms of similar meaning. Such statements are based on management’s expectations and are subject to certain factors, risks and uncertainties that may cause actual results, outcome of events, timing and performance to differ materially from those expressed or implied by such forward-looking statements. These risks and uncertainties include, but are not limited to, our ability to meet anticipated clinical trial commencement, enrollment and completion dates for our products and product candidates and to move new development candidates into the clinic; our ability to secure a partnership for brigatinib (AP26113); difficulties or delays in obtaining regulatory and pricing and reimbursement approvals to market our products; our ability to successfully commercialize and generate profits from sales of Iclusig; competition from alternative therapies, our
79
Table of Contents
reliance on the performance of third-party manufacturers and specialty pharmacies for the distribution of Iclusig; the occurrence of adverse safety events with our products and product candidates; preclinical data and early-stage clinical data that may not be replicated in later-stage clinical studies; the costs associated with our research, development, manufacturing and other activities; the conduct and results of preclinical and clinical studies of our product candidates; the adequacy of our capital resources and the availability of additional funding; patent protection and third-party intellectual property claims; the potential proxy contest for the election of directors at our 2015 annual meeting; litigation, including our pending securities class action and derivative lawsuits and any potential litigation related to the potential proxy contest; our operations in foreign countries; risks related to key employees, markets, economic conditions, health care reform, prices and reimbursement rates; and other factors. Please also see the discussion under “Risk Factors” in Part I, Item 1A appearing elsewhere in this Annual Report on Form 10-K for more details regarding these and other risks.
In light of these assumptions, risks and uncertainties, the results and events discussed in the forward-looking statements contained in this Annual Report or in any document incorporated by reference might not occur. Stockholders are cautioned not to place undue reliance on the forward-looking statements, which speak only as of the date of this report or the date of the document incorporated by reference in this Annual Report. We are not under any obligation, and we expressly disclaim any obligation, to update or alter any forward-looking statements, whether as a result of new information, future events or otherwise. All subsequent forward-looking statements attributable to us or to any person acting on our behalf are expressly qualified in their entirety by the cautionary statements contained or referred to in this section.
80
Table of Contents
ITEM 8: FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
Report of Independent Registered Public Accounting Firm
To the Board of Directors and Stockholders of
ARIAD Pharmaceuticals, Inc.
Cambridge, Massachusetts
We have audited the accompanying consolidated balance sheets of ARIAD Pharmaceuticals, Inc. and subsidiaries (the “Company”) as of December 31, 2014 and 2013, and the related consolidated statements of operations, comprehensive loss, stockholders’ equity, and cash flows for each of the three years in the period ended December 31, 2014. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, such consolidated financial statements present fairly, in all material respects, the financial position of ARIAD Pharmaceuticals, Inc. and subsidiaries as of December 31, 2014 and 2013, and the results of their operations and their cash flows for each of the three years in the period ended December 31, 2014, in conformity with accounting principles generally accepted in the United States of America.
We have also audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the Company’s internal control over financial reporting as of December 31, 2014, based on the criteria established in Internal Control—Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission and our report dated March 2, 2015 expressed an unqualified opinion on the Company’s internal control over financial reporting.
/s/ Deloitte & Touche LLP
Boston, Massachusetts
March 2, 2015
81
Table of Contents
ARIAD PHARMACEUTICALS, INC. AND SUBSIDIARIES
CONSOLIDATED BALANCE SHEETS
| December 31, | ||||||||
| In thousands, except share and per share data | 2014 | 2013 | ||||||
| ASSETS |
||||||||
| Current assets: |
||||||||
| Cash and cash equivalents |
$ | 352,688 | $ | 237,179 | ||||
| Accounts receivable |
8,397 | 1,305 | ||||||
| Inventory, net |
979 | 419 | ||||||
| Other current assets |
23,578 | 6,043 | ||||||
|
|
|
|
|
|||||
| Total current assets |
385,642 | 244,946 | ||||||
| Restricted cash |
11,308 | 11,357 | ||||||
| Property and equipment, net |
203,027 | 108,777 | ||||||
| Intangible and other assets, net |
3,893 | 5,814 | ||||||
|
|
|
|
|
|||||
| Total assets |
$ | 603,870 | $ | 370,894 | ||||
|
|
|
|
|
|||||
| LIABILITIES AND STOCKHOLDERS’ EQUITY |
||||||||
| Current liabilities: |
||||||||
| Accounts payable |
$ | 10,819 | $ | 11,363 | ||||
| Current portion of long-term debt |
— | 4,200 | ||||||
| Current portion of long-term facility lease obligation |
6,707 | — | ||||||
| Accrued compensation and benefits |
21,095 | 12,778 | ||||||
| Accrued product development expenses |
13,958 | 16,740 | ||||||
| Other accrued expenses |
11,514 | 8,977 | ||||||
| Current portion of deferred executive compensation |
— | 2,511 | ||||||
| Current portion of deferred revenue |
8,075 | 472 | ||||||
| Other current liabilities |
17,830 | 15,136 | ||||||
|
|
|
|
|
|||||
| Total current liabilities |
89,998 | 72,177 | ||||||
|
|
|
|
|
|||||
| Long-term debt |
156,908 | 4,900 | ||||||
|
|
|
|
|
|||||
| Long-term facility lease obligation |
189,320 | 99,412 | ||||||
|
|
|
|
|
|||||
| Other long-term liabilities |
11,338 | 8,580 | ||||||
|
|
|
|
|
|||||
| Deferred revenue |
75,505 | 308 | ||||||
|
|
|
|
|
|||||
| Commitments (Note 9) |
||||||||
| Stockholders’ equity: |
||||||||
| Preferred stock, $.01 par value, authorized 10,000,000 shares, none issued and outstanding |
||||||||
| Common stock, $.001 par value, authorized 450,000,000 shares in 2014 and in 2013; shares issued and outstanding 187,294,094 in 2014 and 185,896,080 shares in 2013 |
187 | 186 | ||||||
| Additional paid-in capital |
1,299,394 | 1,238,859 | ||||||
| Accumulated other comprehensive loss |
(4,185 | ) | (1,535 | ) | ||||
| Accumulated deficit |
(1,214,595 | ) | (1,051,993 | ) | ||||
|
|
|
|
|
|||||
| Total stockholders’ equity |
80,801 | 185,517 | ||||||
|
|
|
|
|
|||||
| Total liabilities and stockholders’ equity |
$ | 603,870 | $ | 370,894 | ||||
|
|
|
|
|
|||||
See notes to consolidated financial statements.
82
Table of Contents
ARIAD PHARMACEUTICALS, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF OPERATIONS
| Years Ended December 31, | ||||||||||||
| In thousands, except per share data | 2014 | 2013 | 2012 | |||||||||
| Revenue: |
||||||||||||
| Product revenue, net |
$ | 55,720 | $ | 45,238 | $ | — | ||||||
| License and collaboration revenue |
49,688 | 296 | 514 | |||||||||
| Service revenue |
4 | 27 | 44 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total revenue |
105,412 | 45,561 | 558 | |||||||||
|
|
|
|
|
|
|
|||||||
| Operating expenses: |
||||||||||||
| Cost of product revenue |
5,224 | 9,612 | — | |||||||||
| Research and development expense |
120,593 | 162,900 | 144,709 | |||||||||
| Selling, general and administrative expense |
139,790 | 146,615 | 60,909 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total operating expenses |
265,607 | 319,127 | 205,618 | |||||||||
|
|
|
|
|
|
|
|||||||
| Loss from operations |
(160,195 | ) | (273,566 | ) | (205,060 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Other income (expense): |
||||||||||||
| Interest income |
85 | 130 | 240 | |||||||||
| Interest expense |
(8,075 | ) | (153 | ) | (199 | ) | ||||||
| Gain on disposition of stock |
4,768 | — | — | |||||||||
| Revaluation of warrant liability |
— | — | (15,924 | ) | ||||||||
| Foreign exchange gain (loss) |
1,445 | (130 | ) | 71 | ||||||||
|
|
|
|
|
|
|
|||||||
| Other expense, net |
(1,777 | ) | (153 | ) | (15,812 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Loss before provision for income taxes |
(161,972 | ) | (273,719 | ) | (220,872 | ) | ||||||
| Provision for income taxes |
630 | 439 | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Net loss |
$ | (162,602 | ) | $ | (274,158 | ) | $ | (220,872 | ) | |||
|
|
|
|
|
|
|
|||||||
| Net loss per share – basic and diluted |
$ | (0.87 | ) | $ | (1.49 | ) | $ | (1.34 | ) | |||
|
|
|
|
|
|
|
|||||||
| Weighted-average number of shares of common stock outstanding – basic and diluted |
186,835 | 183,575 | 164,964 | |||||||||
|
|
|
|
|
|
|
|||||||
CONSOLIDATED STATEMENTS OF COMPREHENSIVE LOSS
| Years Ended December 31, | ||||||||||||
| In thousands | 2014 | 2013 | 2012 | |||||||||
| Net loss |
$ | (162,602 | ) | $ | (274,158 | ) | $ | (220,872 | ) | |||
|
|
|
|
|
|
|
|||||||
| Other comprehensive income (loss): |
||||||||||||
| Net unrealized gains (reclassification adjustment) on marketable securities |
— | (20 | ) | 20 | ||||||||
| Cumulative translation adjustment |
214 | (40 | ) | — | ||||||||
| Defined benefit pension obligation |
(2,864 | ) | (1,495 | ) | — | |||||||
| Other comprehensive income (loss) |
(2,650 | ) | (1,555 | ) | 20 | |||||||
|
|
|
|
|
|
|
|||||||
| Comprehensive loss |
$ | (165,252 | ) | $ | (275,713 | ) | $ | (220,852 | ) | |||
|
|
|
|
|
|
|
|||||||
See notes to consolidated financial statements.
83
Table of Contents
ARIAD PHARMACEUTICALS, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY
| Additional | Accumulated Other |
|||||||||||||||||||||||
| Common Stock | Paid-in | Comprehensive | Accumulated | Stockholders’ | ||||||||||||||||||||
| In thousands, except share data | Shares | Amount | Capital | Income (Loss) | Deficit | Equity | ||||||||||||||||||
| Balance, January 1, 2012 |
157,608,702 | $ | 158 | $ | 776,946 | $ | — | $ | (556,963 | ) | $ | 220,141 | ||||||||||||
| Issuance of shares pursuant to ARIAD stock plans |
3,661,213 | 3 | 10,508 | 10,511 | ||||||||||||||||||||
| Issuance of common stock from warrant exercise |
5,805,843 | 6 | 87,040 | 87,046 | ||||||||||||||||||||
| Stock-based compensation |
20,341 | 20,341 | ||||||||||||||||||||||
| Payments of tax withholding obligations related to stock compensation |
(4,336 | ) | (4,336 | ) | ||||||||||||||||||||
| Net loss |
(220,872 | ) | (220,872 | ) | ||||||||||||||||||||
| Other comprehensive income |
20 | 20 | ||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Balance, December 31, 2012 |
167,075,758 | 167 | 890,499 | 20 | (777,835 | ) | 112,851 | |||||||||||||||||
| Issuance of shares pursuant to ARIAD stock plans |
2,330,429 | 2 | 6,282 | 6,284 | ||||||||||||||||||||
| Issuance of common stock, net of issuance costs |
16,489,893 | 17 | 310,020 | 310,037 | ||||||||||||||||||||
| Stock-based compensation |
35,421 | 35,421 | ||||||||||||||||||||||
| Payments of tax withholding obligations related to stock compensation |
(3,363 | ) | (3,363 | ) | ||||||||||||||||||||
| Net loss |
(274,158 | ) | (274,158 | ) | ||||||||||||||||||||
| Other comprehensive loss |
(1,555 | ) | (1,555 | ) | ||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Balance, December 31, 2013 |
185,896,080 | $ | 186 | $ | 1,238,859 | $ | (1,535 | ) | $ | (1,051,993 | ) | $ | 185,517 | |||||||||||
| Issuance of shares pursuant to ARIAD stock plans |
1,398,014 | 1 | 3,693 | 3,694 | ||||||||||||||||||||
| Equity component of convertible debt issuance |
25,255 | 25,255 | ||||||||||||||||||||||
| Stock-based compensation |
32,403 | 32,403 | ||||||||||||||||||||||
| Payments of tax withholding obligations related to stock compensation |
(816 | ) | (816 | ) | ||||||||||||||||||||
| Net loss |
(162,602 | ) | (162,602 | ) | ||||||||||||||||||||
| Other comprehensive loss |
(2,650 | ) | (2,650 | ) | ||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Balance, December 31, 2014 |
187,294,094 | $ | 187 | $ | 1,299,394 | $ | (4,185 | ) | $ | (1,214,595 | ) | $ | 80,801 | |||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
See notes to consolidated financial statements
84
Table of Contents
ARIAD PHARMACEUTICALS, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CASH FLOWS
| Years Ended December 31, | ||||||||||||
| In thousands | 2014 | 2013 | 2012 | |||||||||
| Cash flows from operating activities: |
||||||||||||
| Net loss |
$ | (162,602 | ) | $ | (274,158 | ) | $ | (220,872 | ) | |||
| Adjustments to reconcile net loss to net cash used in operating activities: |
||||||||||||
| Depreciation, amortization and impairment charges |
8,814 | 4,136 | 8,307 | |||||||||
| Stock-based compensation |
32,403 | 35,421 | 20,341 | |||||||||
| Deferred executive compensation expense |
119 | 963 | 2,810 | |||||||||
| Gain on disposition of stock |
(4,768 | ) | — | — | ||||||||
| Revaluation of warrant liability |
— | — | 15,924 | |||||||||
| Increase (decrease) from: |
||||||||||||
| Accounts receivable |
(7,092 | ) | (1,305 | ) | — | |||||||
| Inventory |
(560 | ) | (413 | ) | — | |||||||
| Other current assets |
(17,536 | ) | (2,113 | ) | (4,714 | ) | ||||||
| Other assets |
2,645 | (2,714 | ) | — | ||||||||
| Accounts payable |
(137 | ) | 2,478 | 2,664 | ||||||||
| Accrued compensation and benefits |
8,317 | 913 | 10,656 | |||||||||
| Accrued product development expenses |
(2,782 | ) | 2,679 | 2,113 | ||||||||
| Other accrued expenses |
2,570 | 1,340 | 4,669 | |||||||||
| Other liabilities |
2,644 | 14,834 | 5,714 | |||||||||
| Deferred revenue |
82,801 | 10 | (230 | ) | ||||||||
| Deferred executive compensation paid |
(2,630 | ) | (3,953 | ) | (1,063 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Net cash used in operating activities |
(57,794 | ) | (221,882 | ) | (153,681 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Cash flows from investing activities: |
||||||||||||
| Acquisitions of marketable securities |
— | — | (89,554 | ) | ||||||||
| Proceeds from maturities of marketable securities |
— | 45,000 | 44,500 | |||||||||
| Change in restricted cash |
— | (10,319 | ) | (289 | ) | |||||||
| Investment in property and equipment |
(2,787 | ) | (8,543 | ) | (4,424 | ) | ||||||
| Gain on disposition of stock |
4,768 | — | — | |||||||||
| Investment in intangible assets |
— | — | (633 | ) | ||||||||
|
|
|
|
|
|
|
|||||||
| Net cash provided by (used in) investing activities |
1,981 | 26,138 | (50,400 | ) | ||||||||
|
|
|
|
|
|
|
|||||||
| Cash flows from financing activities: |
||||||||||||
| Proceeds from issuance of convertible debt, including equity component, net of issuance costs, $7,079,000 |
192,921 | — | — | |||||||||
| Proceeds from issuance of warrants |
27,580 | — | — | |||||||||
| Purchase of convertible bond hedges |
(43,220 | ) | — | — | ||||||||
| Repayment of long-term borrowings |
(9,100 | ) | (2,100 | ) | (1,400 | ) | ||||||
| Principal payments under capital lease obligations |
— | (15 | ) | (54 | ) | |||||||
| Proceeds from issuance of common stock, net of issuance costs |
— | 310,037 | — | |||||||||
| Reimbursements of amounts related to facility lease obligation |
— | 2,741 | — | |||||||||
| Proceeds from issuance of common stock pursuant to warrants |
— | — | 12,483 | |||||||||
| Proceeds from issuance of common stock pursuant to stock option and purchase plans |
3,695 | 6,284 | 10,511 | |||||||||
| Payment of tax withholding obligations related to stock compensation |
(816 | ) | (3,363 | ) | (4,336 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Net cash provided by financing activities |
171,060 | 313,584 | 17,204 | |||||||||
|
|
|
|
|
|
|
|||||||
| Effect of exchange rates on cash |
262 | (40 | ) | — | ||||||||
|
|
|
|
|
|
|
|||||||
| Net increase (decrease) in cash and cash equivalents |
115,509 | 117,800 | (186,877 | ) | ||||||||
| Cash and cash equivalents, beginning of year |
237,179 | 119,379 | 306,256 | |||||||||
|
|
|
|
|
|
|
|||||||
| Cash and cash equivalents, end of year |
$ | 352,688 | $ | 237,179 | $ | 119,379 | ||||||
|
|
|
|
|
|
|
|||||||
| Supplemental disclosures: |
||||||||||||
| Interest paid |
$ | 8,077 | $ | 153 | $ | 206 | ||||||
|
|
|
|
|
|
|
|||||||
| Income taxes paid |
$ | 185 | $ | 17 | $ | — | ||||||
|
|
|
|
|
|
|
|||||||
| Capitalization of construction-in-progress related to facility lease obligation |
$ | 96,558 | $ | 96,671 | $ | — | ||||||
|
|
|
|
|
|
|
|||||||
| Non-cash transaction – property and equipment included in accounts payable or accruals |
$ | 298 | $ | 738 | $ | 579 | ||||||
|
|
|
|
|
|
|
|||||||
See notes to consolidated financial statements.
85
Table of Contents
ARIAD PHARMACEUTICALS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
1. Nature of Business and Summary of Significant Accounting Policies
Nature of Business
ARIAD is a global oncology company whose vision is to transform the lives of cancer patients with breakthrough medicines. The Company’s mission is to discover, develop and commercialize small-molecule drugs to treat cancer in patients with the greatest unmet medical need – aggressive cancers where current therapies are inadequate.
In addition to commercializing Iclusig® (ponatinib), the Company is developing Iclusig for approval in additional countries and cancer indications and has three other product candidates in development, brigatinib (AP26113), ridaforolimus and AP32788. Brigatinib is being studied in patients with advanced solid tumors, including non-small cell lung cancer. Ridaforolimus is being developed for cardiovascular indications by Medinol, Ltd. and ICON Medical Corp. AP32788 is being developed for the treatment of non-small cell lung cancer and other solid tumors. In addition to its clinical development programs, the Company has a focused drug discovery program centered on small-molecule therapies that are molecularly targeted to cell-signaling pathways implicated in cancer.
Principles of Consolidation
The consolidated financial statements include the accounts of ARIAD Pharmaceuticals, Inc. and its wholly-owned subsidiaries. Intercompany accounts and transactions have been eliminated in consolidation.
Foreign Currency
A subsidiary’s functional currency is the currency of the primary economic environment in which the subsidiary operates; normally, that is the currency of the environment in which a subsidiary primarily generates and expends cash. In making the determination of the appropriate functional currency for a subsidiary, the Company considers cash flow indicators, local market indicators, financing indicators and the subsidiary’s relationship with both the parent company and other subsidiaries. For subsidiaries that are primarily a direct and integral component or extension of the parent entity’s operations, the U.S. dollar is the functional currency.
For foreign subsidiaries that transact in functional currency other than the U.S. dollar, assets and liabilities are translated at current rates of exchange at the balance sheet date. Income and expense items are translated at the average foreign exchange rate for the period. Adjustments resulting from the translation of the financial statements of the Company’s foreign subsidiaries into U.S. dollars are excluded from the determination of net loss and are recorded in accumulated other comprehensive loss, a separate component of stockholders’ equity. For foreign subsidiaries where the functional currency is the U.S. dollar, monetary assets and liabilities are re-measured into U.S. dollars at the current exchange rate on the balance sheet date. Nonmonetary assets and liabilities are re-measured into U.S. dollars at historical exchange rates. Revenue and expense items are translated at average rates of exchange prevailing during each period.
86
Table of Contents
The net total of realized and unrealized transaction gains and losses was a gain of $1.2 million in 2014 and a loss of $194,000 in 2013. The Company did not have significant subsidiary operations with the functional currency denominated as the local currency in 2012.
Accounting Estimates
The preparation of the consolidated financial statements in conformity with U.S. generally accepted accounting principles (“GAAP”) requires management to make estimates and assumptions that affect the reported amounts and disclosure of assets and liabilities at the date of the consolidated financial statements and the reported amounts and disclosure of revenue and expenses during the reporting period. Significant estimates included in the Company’s financial statements include estimates associated with revenue recognition and the related adjustments, research and development accruals, inventory, leased buildings under construction and stock-based compensation. Actual results could differ from those estimates.
Cash Equivalents
Cash equivalents include short-term, highly liquid investments, with remaining maturities at the date of purchase of 90 days or less, and money market accounts.
Restricted Cash
Restricted cash consists of cash balances held as collateral for outstanding letters of credit related to the lease of the Company’s laboratory and office facilities, including those currently under construction in Cambridge, Massachusetts and for other purposes.
Inventory
The Company outsources the manufacturing of Iclusig and uses contract manufacturers that produce the raw and intermediate materials used in the production of Iclusig as well as the finished product. The Company currently has one supplier qualified for each step in the manufacturing process and is in the process of qualifying additional suppliers for certain steps of the production process of Iclusig. Accordingly, the Company has concentration risk associated with its manufacturing process and relies on its currently approved contract manufacturers for supply of its product.
In connection with production of inventory, the Company may be required to provide payments to vendors in advance of production. These amounts are included in other current assets on the accompanying consolidated balance sheets.
Inventory is composed of raw materials, intermediate materials, which are classified as work-in-process, and finished goods, which are goods that are available for sale. The Company records inventory at the lower of cost or market. The Company determines the cost of its inventory on a specific identification basis. The Company evaluates its inventory balances quarterly and if the Company identifies excess, obsolete or unsalable inventory, it writes down its inventory to its net realizable value in the period it is identified. These adjustments are recorded based upon various factors, including the level of product manufactured by the Company, the level of product in the distribution channel, current and projected demand for the foreseeable future and the expected shelf-life of the inventory components. The Company recorded such adjustments of $4.2 million and $8.9 million in the years ended December 31, 2014 and 2013, respectively, which are recorded as a component of cost of product revenue in the accompanying consolidated statements of operations. Inventory that is not expected to be used within one year is included in other assets, net, on the accompanying consolidated balance sheet.
Prior to receiving approval from the FDA on December 14, 2012 to sell Iclusig, the Company expensed all costs incurred related to the manufacture of Iclusig as research and development expense because of the inherent risks associated with the development of a drug candidate, the uncertainty about the regulatory approval process and the lack of history for the Company of regulatory approval of drug candidates.
87
Table of Contents
Shipping and handling costs for product shipments are recorded as incurred in cost of product revenue along with costs associated with manufacturing the product sold and any inventory reserves or write-downs.
Property and Equipment
Property and equipment are recorded at cost. Depreciation is recorded using the straight-line method over the estimated useful lives of the assets (3 to 10 years). Leasehold improvements and assets under capital leases are amortized over the shorter of their useful lives or lease term using the straight-line method.
In connection with a lease for a facility being constructed in Cambridge, Massachusetts, the landlord is providing the Company with a tenant improvement allowance for the costs associated with the design, engineering, and construction of tenant improvements for the leased facility. The tenant improvements will be constructed in accordance with the Company’s plans and include fit-out of the buildings to construct appropriate laboratory and office space, subject to approval by the landlord. To the extent the stipulated tenant allowance provided by the landlord is exceeded, the Company is obligated to fund all costs incurred in excess of the tenant allowance. The scope of the planned tenant improvements do not qualify as “normal tenant improvements” under the lease accounting guidance. Accordingly, for accounting purposes, the Company is the deemed owner of the buildings during the construction period.
As construction progresses, the Company records the project construction costs incurred as an asset, along with a corresponding facility lease obligation, on the consolidated balance sheet for the total amount of project costs incurred whether funded by the Company or the landlord. Upon completion of the buildings, the Company will determine if the asset and corresponding financing obligation should continue to be carried on its consolidated balance sheet under the appropriate accounting guidance. Based on the current terms of the lease, the Company expects to continue to be the deemed owner of the buildings upon completion of the construction period.
Intangible Assets
Intangible assets consist primarily of purchased technology and capitalized patent and license costs. The cost of purchased technology, patents and patent applications, costs incurred in filing patents and certain license fees are capitalized when recovery of the costs is probable. Capitalized costs related to purchased technology are amortized over the estimated useful life of the technology. Capitalized costs related to issued patents are amortized over a period not to exceed seventeen years or the remaining life of the patent, whichever is shorter, using the straight-line method. Capitalized license fees are amortized over the periods to which they relate. In addition, capitalized costs are expensed when it becomes determinable that the related patents, patent applications or technology will not be pursued.
Impairment of Long-Lived Assets
The Company reviews its long-lived assets, including the above-mentioned intangible assets, for impairment when events or changes in circumstances indicate that the carrying amount of a long-lived asset may not be recoverable. Recoverability of assets to be held and used is measured by a comparison of the carrying amount of an asset to future net cash flows expected to be generated by the asset. If such assets are considered to be impaired, the impairment to be recognized is measured by the amount by which the carrying amount of the assets exceeds the fair value of the assets.
Accrued Rent
The Company recognizes rent expense for leases with increasing annual rents on a straight-line basis over the term of the lease. The amount of rent expense in excess of cash payments is classified as accrued rent. Any lease incentives received are deferred and amortized over the term of the lease. At December 31, 2014 and 2013, the amount of accrued rent was $5.3 million and $5.1 million, respectively. Of these
88
Table of Contents
amounts, at December 31, 2014 and 2013, $4.7 million and $4.6 million, respectively, were included in other long-term liabilities, with the remaining $0.6 million and $0.5 million as of December 31, 2014 and 2013, respectively, included in other current liabilities.
Revenue Recognition
Revenue is recognized when the four basic criteria of revenue recognition are met: (1) persuasive evidence that an arrangement exists; (2) delivery has occurred or services have been rendered; (3) the fee is fixed or determinable; and (4) collectability is reasonably assured. When the revenue recognition criteria are not met, we defer the recognition of recording deferred revenue until such time that all criteria are met.
Product Revenue, Net
From the launch of Iclusig in January 2013 until its temporary suspension in October 2013, the Company sold Iclusig in the United States to a limited number of specialty pharmacies, which dispensed the product directly to patients, and specialty distributors, which in turn sold the product to hospital pharmacies and community practice pharmacies (collectively, healthcare providers) for the treatment of patients. Commencing with the re-launch of Iclusig in January 2014, the Company now sells Iclusig in the United States through a single specialty pharmacy, Biologics, Inc. (“Biologics”). Biologics dispenses the product directly to patients. In Europe, the Company sells Iclusig to retail pharmacies and hospital pharmacies, which dispense product directly to patients. These specialty distributor, specialty pharmacies, retail pharmacies and hospital pharmacies are referred to as the Company’s customers.
The Company provides the right of return to customers in the United States for unopened product for a limited time before and after its expiration date. European customers are provided the right to return product only in limited circumstances, such as damaged product. Given the Company’s limited sales history for Iclusig and the inherent uncertainties in estimating product returns, the Company has determined that the shipments of Iclusig to its United States customers, thus far, do not meet the criteria for revenue recognition at the time of shipment. The Company invoices Biologics upon shipment of Iclusig and records accounts receivable, with a corresponding liability for deferred revenue equal to the gross invoice price. The Company then recognizes revenue, assuming all other revenue recognition criteria have been met, when Iclusig is sold through, which occurs when Biologics dispenses Iclusig directly to the patient. For European customers, who are provided with a limited right of return, the criteria for revenue recognition is met at the time of shipment and revenue is recognized at that time, provided all other revenue recognition criteria are met.
In connection with the temporary suspension of marketing and commercial distribution of Iclusig in October 2013, the Company terminated its then existing contracts with specialty pharmacies and specialty distributors in the United States. In addition, the Company accepted product returns for Iclusig in connection with the temporary suspension. These returns primarily related to Iclusig held by specialty pharmacies and specialty distributors for which revenue had not yet been recognized.
The Company has written contracts or standard terms of sale with each of its customers and delivery occurs when the customer receives Iclusig. The Company evaluates the creditworthiness of each of its customers to determine whether collection is reasonably assured. In order to conclude that the price is fixed and determinable, the Company must be able to (i) calculate its gross product revenues from the sales to its customers and (ii) reasonably estimate its net product revenues. The Company calculates gross product revenues based on the wholesale acquisition cost that the Company charges its customers for Iclusig. The Company estimates its net product revenues by deducting from its gross product revenues (i) trade allowances, such as invoice discounts for prompt payment and customer fees, (ii) estimated government and private payor rebates, chargebacks and discounts, such as Medicare and Medicaid reimbursements in the United States, and (iii) estimated costs of incentives offered to certain indirect customers including patients. These deductions from gross revenue to determine net revenue are also referred to as gross to net deductions.
89
Table of Contents
Trade Allowances: The Company provides invoice discounts on Iclusig sales to certain of its customers for prompt payment and pays fees for certain distribution services, such as fees for certain data that its customers provide to the Company. The Company deducts the full amount of these discounts and fees from its gross product revenues at the time such discounts and fees are earned by such customers.
Rebates, Chargebacks and Discounts: In the United States, the Company contracts with Medicare, Medicaid, and other government agencies (collectively, “payers”) to make Iclusig eligible for purchase by, or for partial or full reimbursement from, such payers. The Company estimates the rebates, chargebacks and discounts it will provide to payers and deducts these estimated amounts from its gross product revenues at the time the revenues are recognized. The Company’s estimates of rebates, chargebacks and discounts are based on (1) the contractual terms of agreements in place with payers, (2) the government-mandated discounts applicable to government funded programs, and (3) the estimated payer mix. Government rebates that are invoiced directly to the Company are recorded in accrued liabilities on the consolidated balance sheet. In Europe, the Company is subject to mandatory rebates and discounts in markets where government-sponsored healthcare systems are the primary payers for healthcare. These rebates and discounts are recorded in accrued expenses on the consolidated balance sheet.
Other Incentives: Other incentives that the Company offers to indirect customers include co-pay assistance rebates provided by the Company to commercially insured patients who have coverage for Iclusig and who reside in states that permit co-pay assistance programs. The Company’s co-pay assistance program is intended to reduce each participating patient’s portion of the financial responsibility for Iclusig’s purchase price to a specified dollar amount. In each period, the Company records the amount of co-pay assistance provided to eligible patients based on the terms of the program. Other incentives in the year ended December 31, 2014 include product returns from customers related to the temporary suspension of marketing and distribution of Iclusig in the United States.
The following table summarizes the activity in each of the above product revenue allowances and reserve categories for 2014 and 2013:
| In thousands | Trade Allowances |
Rebates, Chargebacks and Discounts |
Other Incentives/ Returns |
Total | ||||||||||||
| Balance, January 1, 2013 |
$ | — | $ | — | $ | — | $ | — | ||||||||
| Provision |
1,158 | 2,721 | 180 | 4,059 | ||||||||||||
| Payments or credits |
(1,140 | ) | (2,206 | ) | (103 | ) | (3,449 | ) | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Balance, December 31, 2013 |
18 | 515 | 77 | 610 | ||||||||||||
| Provision |
723 | 3,798 | 1,253 | 5,774 | ||||||||||||
| Payments or credits |
(669 | ) | (2,218 | ) | (970 | ) | (3,857 | ) | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Balance, December 31, 2014 |
$ | 72 | $ | 2,095 | $ | 360 | $ | 2,527 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
The reserves above included in the Company’s consolidated balance sheets as of December 31, 2014 and 2013 are summarized as follows:
| December 31, | ||||||||
| In thousands | 2014 | 2013 | ||||||
| Reductions of accounts receivable |
$ | — | $ | 64 | ||||
| Component of other accrued expenses |
2,527 | 546 | ||||||
|
|
|
|
|
|||||
| Total |
$ | 2,527 | $ | 610 | ||||
|
|
|
|
|
|||||
90
Table of Contents
In 2012, prior to the Company obtaining market authorization for Iclusig in Europe, the French regulatory authority granted an Autorisation Temporaire d’Utilisation (ATU), or Temporary Authorization for Use, for Iclusig for the treatment of patients with CML and Ph+ ALL under a nominative program on a patient-by-patient basis. The Company began shipping Iclusig under this program during the year ended December 31, 2012. This program concluded on September 30, 2013. Upon completion of this program, the Company became eligible to ship Iclusig directly to customers in France as of October 1, 2013. Shipments under these programs have not met the criteria for revenue recognition as the price for these shipments is not yet fixed or determinable. The price of Iclusig in France will become fixed or determinable upon completion of pricing and reimbursement negotiations, which is expected in the first half of 2015. At that time, the Company will record revenue related to cumulative shipments as of that date in France, net of amounts that will be refunded to the health authority based on the results of the pricing and reimbursement negotiations. The aggregate gross selling price of the shipments under these programs amounted to $18.3 million through December 31, 2014, of which $17.1 million was received as of December 31, 2014.
License Revenue
The Company generates revenue from license and collaboration agreements with third parties related to use of the Company’s technology and/or development and commercialization of products. Such agreements typically include payment to the Company’s of non-refundable upfront license fees, regulatory, clinical and commercial milestone payments, payment for services or supply of product and royalty payments on net sales. Revenue arrangements with multiple elements are divided into separate units of accounting if certain criteria are met, including whether the delivered element has stand-alone value to the customer. When deliverables are separable, consideration received is allocated to the separate units of accounting based on the relative selling price of each deliverable and the appropriate revenue recognition principles are applied to each unit. For arrangements with multiple elements, where the Company determines there is one unit of accounting, revenue associated will be recognized over the period beginning with the commencement of the final deliverable in the arrangement and over a period reflective of the Company’s longest obligation period within the arrangement on a straight-line-basis.
At the inception of each agreement that includes milestone payments, the Company evaluates whether each milestone is substantive on the basis of the contingent nature of the milestone. This evaluation includes an assessment of whether:
| • | the consideration is commensurate with either (1) our performance to achieve the milestone, or (2) the enhancement of the value of the delivered item(s) as a result of a specific outcome resulting from our performance to achieve the milestone, |
| • | the consideration relates solely to past performance, and |
| • | the consideration is reasonable relative to all of the deliverables and payment terms within the arrangement. |
In making this assessment, the Company evaluates factors such as the clinical, regulatory, commercial and other risks that must be overcome to achieve the respective milestone, the level of effort and investment required, and whether the milestone consideration is reasonable relative to all deliverables and payment terms in the arrangement. The Company recognizes revenues related to substantive milestones in full in the period in which the substantive milestone is achieved. If a milestone payment is not considered substantive, the Company recognizes the applicable milestone over the remaining period of performance.
The Company will recognize royalty revenue, if any, based upon actual and estimated net sales by the licensee of licensed products in licensed territories, and in the period the sales occur.
Concentration of Credit Risk
For the year-ended December 31, 2014, one individual customer accounted for 72 percent of net product revenue. As of December 31, 2014, one individual customer accounted for 75 percent of accounts receivable. For the year ended December 31, 2013, three individual customers accounted for 24 percent, 15 percent and 13 percent of net product revenue, respectively. As of December 31, 2013, two individual customers accounted for 15 percent, and 13 percent of accounts receivable, respectively. No other customer accounted for more than 10 percent of net product revenue for either 2014 or 2013 or accounts receivable as of either December 31, 2014 or 2013.
Financial instruments which potentially subject the Company to concentrations of credit risk consist of accounts receivable from customers and cash held at financial institutions. The Company believes that such customers and financial institutions are of high credit quality. As of December 31, 2014, a portion of the Company’s cash and cash equivalent accounts were concentrated at a single financial institution, which potentially exposes the Company to credit risks. The Company does not believe that there is significant risk of non-performance by the financial institution and the Company’s cash on deposit at this financial institution is fully liquid.
Revenues in 2012 primarily related to one license agreement discussed in Note 2.
Advertising Costs
In connection with the commercial launch of Iclusig during 2013, the Company began incurring advertising costs. Advertising costs are expensed as incurred. For the years ended December 31, 2014 and 2013, advertising costs totaled $0.6 million and $1.0 million, respectively.
91
Table of Contents
Income Taxes
The Company accounts for income taxes using an asset and liability approach to financial accounting and reporting for income taxes. Deferred income tax assets and liabilities are computed annually for differences between the financial statement basis and the income tax basis of assets and liabilities that will result in taxable or deductible amounts in the future and for loss and other tax carry forwards. Such deferred income tax computations are based on enacted tax laws and rates applicable to the years in which the differences are expected to affect taxable income. A valuation allowance is established when it is necessary to reduce deferred income tax assets to the amount that is considered to be more-likely-than-not realizable.
The Company does not recognize a tax benefit unless it is more likely than not that the tax position will be sustained upon examination, including resolution of any related appeals or litigation processes, based on the technical merits of the position. The tax benefit that is recorded for these positions is measured at the largest amount of benefit that is greater than 50 percent likely of being realized upon ultimate settlement. Any interest and penalties on uncertain tax positions are included within the tax provision.
Stock-Based Compensation
The Company awards stock options and other equity-based instruments to its employees, directors and consultants and provides employees the right to purchase common stock (collectively “share-based payments”), pursuant to stockholder approved plans. Compensation cost related to such awards is measured based on the fair value of the instrument on the grant date and is recognized on a straight-line basis over the requisite service period, which generally equals the vesting period.
Segment Reporting and Geographic Information
The Company organizes itself into one operating segment reporting to the Chief Executive Officer.
For the years ended December 31, 2014 and 2013, product revenue from customers outside the United States totaled 28 percent and 9 percent, respectively with 19 percent and 7 percent, respectively, representing product revenue from customers in Germany. All other product, license and collaboration and service revenues in 2014, 2013, and 2012 were generated within the United States. Long lived assets outside the United States totaled 1.4 million at December 31, 2014 and were not material at December 31, 2013.
Subsequent Events
The Company considers events or transactions that occur after the balance sheet date but before the financial statements are issued to provide additional evidence relative to certain estimates or to identify matters that require additional disclosure.
Recent Accounting Pronouncements
In August 2014, FASB issued ASU 2014-15, Disclosure of Uncertainties about an Entity’s Ability to Continue as a Going Concern. The amendments in this update will explicitly require a company’s management to assess an entity’s ability to continue as a going concern, and to provide related footnote disclosures in certain circumstances. The new standard will be effective in the first annual period ending after December 15, 2016. Early application is permitted. The Company is currently evaluating the potential impact of the adoption of this standard, but believes its adoption will have no impact on its financial position, results of operations or cash flows.
In May 2014, the Financial Accounting Standards Board (FASB) and the International Accounting Standards Board (IASB) jointly issued Accounting Standards Update (ASU) No. 2014-9, Revenue from Contracts with Customers (Topic 606), which clarifies the principles for recognizing revenue and develops a
92
Table of Contents
common revenue standard for GAAP and International Financial Reporting Standards (IFRS). The core principle of the guidance is that an entity should recognize revenue to depict the transfer of promised goods or services to customers in an amount that reflects the consideration to which the entity expects to be entitled in exchange for those goods and services. The ASU is effective for public entities for annual and interim periods beginning after December 15, 2016. Early adoption is not permitted under GAAP and retrospective application is permitted, but not required. The Company is evaluating the impact of adopting this guidance on its financial position and results of operations.
In July 2013, the FASB issued ASU 2013-11, Presentation of an Unrecognized Tax Benefit When a Net Operating Loss Carryforward, a Similar Tax Loss, or a Tax Credit Carryforward Exists. ASU 2013-11 requires, unless certain conditions exists, an unrecognized tax benefit or a portion of an unrecognized tax benefit to be presented in the financial statements as a reduction to a deferred tax asset for a net operating loss carryforward, similar to a tax loss or a tax credit carryforward. The Company adopted the standard beginning January 1, 2014. The adoption of the standard has not had a material impact on our consolidated financial statements.
2. License and Collaboration Agreements
Otsuka Pharmaceutical Co. Ltd
On December 22, 2014, the Company entered into a collaboration agreement (the “Collaboration Agreement”) with Otsuka Pharmaceutical Co., Ltd. (“Otsuka”) pursuant to which Otsuka will commercialize and further develop Iclusig in Japan, China, South Korea, Indonesia, Malaysia, the Philippines, Singapore, Taiwan, Thailand and Vietnam (the “Territory”).
Key provisions of the Collaboration Agreement include the following:
| • | The Company has granted an exclusive, non-assignable (except to affiliates) license to Otsuka to commercialize and distribute Iclusig in the Territory. |
| • | The Company has granted a co-exclusive license to Otsuka to conduct research and development in the Territory. |
| • | The Company will complete its ongoing pivotal trial of Iclusig in Japan and lead the preparation of the Japanese new drug application (the “JNDA”) on behalf of Otsuka and the Company. |
| • | Otsuka is responsible for filing of the JNDA on behalf of Otsuka and the Company, which is expected to occur in 2015. |
| • | The Company and Otsuka will form and participate on a joint development and commercialization committee (the “JDCC”) to oversee activities related to Iclusig in the Territory. |
| • | The Company is responsible for manufacture and supply of Iclusig to Otsuka in either bulk form or in final packaged form, as requested by Otsuka. |
| • | Otsuka is responsible for completion of final manufacturing, consisting of packaging and labeling of Iclusig for distribution in the Territory, as well as pricing and all other commercial activities by Otsuka within the Territory. |
Following approvals in each country, Otsuka will market and sell Iclusig and record sales. Otsuka is not allowed to manufacture bulk product, but must purchase its supply from the Company. Otsuka will be responsible for medical affairs activities, determining pricing and reimbursement and all commercial activities in the Territory. With respect to the JDCC, each party has ultimate decision making authority with respect to a specified limited set of issues, and for all other issues, the matter must be resolved by consensus or by an expedited arbitration process.
In consideration for the licenses and other rights contained in the Collaboration Agreement, Otsuka paid the Company a non-refundable upfront payment of $77.5 million, less a refundable withholding tax in Japan of $15.8 million, and has agreed to pay the Company up to $80 million in future milestone payments upon obtaining further regulatory approvals in the Territory. Otsuka will pay royalties based on a percentage of net sales in each country until the later of (i) the expiry date of the composition patent in each country, (ii) the expiration of any orphan drug exclusivity period or other statutory designation that provides similar exclusivity, or (iii) 10 years after the date of first commercial sale in such country. Otsuka will also pay for the supply of Iclusig purchased from the Company at a price based on a percentage of net sales in each country.
The Collaboration Agreement continues until the later of (x) the expiration of all royalty obligations in the Territory, or (y) the last sale by Otsuka in the Territory, or the last to expire patent in the Territory which is currently expected to be 2029. Under certain conditions, the Collaboration Agreement may be terminated by either party, in which case the Company would receive all rights to the regulatory filings related to Iclusig at our request, and the licenses granted to Otsuka would be terminated.
93
Table of Contents
For accounting purposes, because Otsuka’s ability to access the value of the distribution rights in the license absent the delivery of the other elements of the arrangement, in particular the manufacturing deliverables which remain within the Company’s control, the Company has concluded that the licenses and other deliverables do not have standalone value, and have combined all deliverables into a single unit of accounting. The nonrefundable upfront cash payment has been recorded as deferred revenue on our balance sheet and will be recognized as revenue on a straight-line basis over the estimated term (currently estimated to extend through 2029), beginning at the point at which the Company has provided or is providing all elements included in the Collaboration Agreement.
The upfront payment was subject to a Japan withholding tax of $15.8 million which was remitted by Otsuka to the Japanese tax authorities. The Company has determined that the release of those funds to the Company is probable and therefore has recorded a receivable for such amounts with an offsetting amount included in deferred revenue.
Bellicum Pharmaceuticals, Inc.
On October 3, 2014, the Company entered into an Omnibus Amendment Agreement (the “Agreement”) with Bellicum Pharmaceuticals, Inc. (“Bellicum”) that restructures a previous license agreement dated March 7, 2011 for the Company’s cell-signaling technology. The Agreement gives Bellicum a worldwide exclusive license, with the right to sublicense, to the Company’s cell-signaling technology for broad use in human cell therapies for all diseases on a royalty- and milestone-free basis. Under the terms of the Agreement, the Company would receive $50 million, payable in three installments, in exchange for granting Bellicum a fully paid-up license to this technology and the return of the Company’s equity stake in Bellicum upon receipt of the second installment payment. The Agreement could be terminated by either party upon a specified uncured material breach.
Under the terms of the Agreement, the Company would receive $50 million in three installments: $15 million, which was paid upon signing of the Agreement, $20 million by June 30, 2015, and $15 million by June 30, 2016. The second and third installments, which were subject to a subordinated promissory note issued by Bellicum, could be accelerated under certain circumstances and prepaid at any time. On December 29, 2014, Bellicum paid the $20 million and $15 million payments owed to the Company under the Agreement and the Company returned the shares of Bellicum common stock it owned.
The Company has recorded as other income a gain on the disposition of the Bellicum common stock it owned, which had no book value, of $4.8 million based on the estimated fair value of the Bellicum common stock as of October 3, 2014. The remaining $45.2 million of the total of $50 million paid by Bellicum was recorded as license revenue in the accompanying consolidated statement of operations.
Medinol Ltd.
The Company entered into an agreement with Medinol Ltd. (“Medinol”) in 2005 pursuant to which the Company granted to Medinol a non-exclusive, world-wide, royalty-bearing license, under its patents and technology, to develop, manufacture and sell stents and other medical devices to deliver the Company’s mTOR inhibitor, ridaforolimus, to prevent reblockage of injured vessels following stent-assisted angioplasty. The term of the license agreement extends to the later to occur of the expiration of the Company’s patents relating to the rights granted to Medinol under the license agreement or fifteen years after the first commercial sale of a product developed under the agreement.
Medinol is required under the license agreement to use commercially reasonable efforts to develop products. The Company is required under a related supply agreement to use commercially reasonable efforts to supply agreed-upon quantities of ridaforolimus to Medinol, and Medinol shall purchase such supply of ridaforolimus from the Company, for the development, manufacture and sale of products. The supply agreement is coterminous with the license agreement. These agreements may be terminated by either party for breach after a 90-day cure period. In addition, Medinol may terminate the agreements upon 30-day notice to the Company upon certain events, including if it determines, in its reasonable business judgment, that it is not in its business interest to continue the development of any product, and the Company may terminate the agreements upon 30-day notice to Medinol, if it determines that it is not in its business interest to continue development and regulatory approval efforts with respect to ridaforolimus.
94
Table of Contents
The license agreement provides for the payment by Medinol to the Company of an upfront license fee, payments based on achievement of development, regulatory and commercial milestones and royalties based on commercial sale of products developed under the agreement. In January 2014, Medinol initiated two registration trials of its ridaforolimus-eluting stent system. The commencement of enrollment in these clinical trials along with the submission of an investigational device exemption with the FDA triggered milestone payments to the Company of $3.75 million, which are recorded as license revenue in the accompanying consolidated statement of operations. The Company is eligible to receive additional, regulatory, clinical and commercial milestone payments of up to $34.75 million under the agreement if two products are successfully developed and commercialized.
Merck & Co., Inc.
In July 2007, the Company entered into a collaboration agreement with Merck & Co. Inc., or Merck, for the joint global development, manufacture and commercialization of ridaforolimus, the Company’s investigational oral mTOR inhibitor, for use in cancer (the “Collaboration Agreement”). In May 2010, the Company entered into an amended and restated agreement with Merck for ridaforolimus (the “License Agreement”), which replaced the Collaboration Agreement. These agreements are described below.
The Collaboration Agreement (July 2007 to May 2010)
Under the terms of the Collaboration Agreement, as in effect until May 4, 2010, Merck and the Company were conducting a broad-based development program for the use of ridaforolimus in multiple types of cancer. Each party funded 50 percent of global development costs incurred. Under the terms of the Collaboration Agreement, Merck paid the Company an initial upfront payment of $75 million in July 2007 and milestone payments of $53.5 million through May 4, 2010, based on the achievement of specified clinical and regulatory events.
The License Agreement (May 2010 to termination)
Under the terms of the License Agreement, the Company granted Merck an exclusive license to develop, manufacture and commercialize ridaforolimus in oncology, and Merck assumed full responsibility for all activities related to the development, manufacture and commercialization of ridaforolimus and agreed to fund 100 percent of all ridaforolimus costs incurred after January 1, 2010. The License Agreement provided that Merck would develop ridaforolimus in multiple oncology indications. If ridaforolimus received regulatory approval, Merck would be responsible for selling ridaforolimus worldwide, and would pay the Company tiered double-digit royalties on global net sales.
Under the License Agreement, in 2010, Merck paid the Company an initial upfront fee of $50 million and approximately $12.8 million for its share of costs incurred in the period from January 1, 2010 to May 4, 2010 related to development, manufacture and commercial activities for ridaforolimus in accordance with the cost-sharing provisions of the Collaboration Agreement as in effect during that period. In addition, in 2011, Merck paid the Company a $25 million milestone payment for the acceptance of the marketing authorization application in Europe for the sarcoma indication, which was subsequently withdrawn by Merck in November 2012.
For the years ended December 31, 2014, 2013 and 2012, the Company recorded service revenue of approximately $4,000, $27,000 and $44,000, respectively. The cost of such services is reflected in operating expenses in the year in which they were incurred.
95
Table of Contents
On February 20, 2014, the Company received notice from Merck that it was terminating the license agreement. Per the terms of the license agreement, this termination became effective in November 2014 at which time all rights to ridaforolimus in oncology licensed to Merck were returned to the Company.
3. Inventory
All of the Company’s inventories relate to the manufacturing of Iclusig. The following table sets forth the Company’s inventories as of December 31, 2014 and 2013:
| In thousands | 2014 | 2013 | ||||||
| Raw materials |
$ | — | $ | — | ||||
| Work in process |
460 | 3,170 | ||||||
| Finished goods |
979 | 234 | ||||||
|
|
|
|
|
|||||
| Total |
1,439 | 3,404 | ||||||
| Current portion |
(979 | ) | (419 | ) | ||||
|
|
|
|
|
|||||
| Non-current portion included in intangible and other assets, net |
$ | 460 | $ | 2,985 | ||||
|
|
|
|
|
|||||
Upon approval of Iclusig by the FDA on December 14, 2012, the Company began capitalizing inventory costs for Iclusig manufactured in preparation for the product launch in the United States. In periods prior to December 14, 2012, the Company expensed costs associated with Iclusig, including raw materials, work-in-process and finished goods, as development expenses. The Company has not capitalized inventory costs related to its other drug development programs. Non-current inventory consists of work-in-process inventory that was manufactured in order to provide adequate supply of Iclusig in the United States and Europe and to support continued clinical development.
4. Property and Equipment, Net
Property and equipment, net, was comprised of the following at December 31, 2014 and 2013:
| In thousands | 2014 | 2013 | ||||||
| Leasehold improvements |
$ | 22,315 | $ | 25,714 | ||||
| Construction in progress |
196,027 | 99,908 | ||||||
| Equipment and furniture |
23,511 | 23,466 | ||||||
|
|
|
|
|
|||||
| 241,853 | 149,088 | |||||||
| Less accumulated depreciation and amortization |
(38,826 | ) | (40,311 | ) | ||||
|
|
|
|
|
|||||
| $ | 203,027 | $ | 108,777 | |||||
|
|
|
|
|
|||||
As of December 31, 2014 and 2013, the Company has recorded construction in progress and a facility lease obligation of $196.0 million and $99.4 million, respectively, related to a lease for a new facility under construction in Cambridge, Massachusetts. See Note 9 for further information.
Depreciation and amortization expense for the years ended December 31, 2014, 2013 and 2012 was $4.7 million, $4.1 million and $2.8 million, respectively.
96
Table of Contents
5. Intangible and Other Assets, Net
Intangible and other assets, net, were comprised of the following at December 31, 2014 and 2013:
| In thousands | 2014 | 2013 | ||||||
| Capitalized patent and license costs |
$ | 5,975 | $ | 5,975 | ||||
| Less accumulated amortization |
(5,036 | ) | (5,007 | ) | ||||
|
|
|
|
|
|||||
| 939 | 968 | |||||||
| Inventory, non-current |
460 | 2,985 | ||||||
| Other assets |
2,494 | 1,861 | ||||||
|
|
|
|
|
|||||
| $ | 3,893 | $ | 5,814 | |||||
|
|
|
|
|
|||||
Amortization expense for intangible assets was $29,000, $25,000 and $218,000 in 2014, 2013 and 2012, respectively. The weighted average amortization period for intangible assets was 17.0 years, for each of 2014, 2013 and 2012, respectively. The estimated future amortization expense is $31,000 per year for 2015, 2016, 2017, 2018 and 2019 and $241,000 thereafter.
In 2012, the Company recorded charges to operating expenses of $5.2 million to reflect impairment of the carrying value of certain capitalized patents and licenses or purchased technology. This amount included a charge of $4.8 million to reflect impairment of the carrying value of intangible assets associated with ridaforolimus, following the decision in June 2012 by the FDA not to approve the NDA filed by Merck for the treatment of patients with soft tissue or bone sarcomas. The impairment of the carrying value of intangible assets was based on management’s assessment of the uncertainty related to the timing and amount of future cash flows anticipated from these assets. No impairment charges were recorded in 2014 or 2013.
6. Other Current Liabilities
Other current liabilities consisted of the following at December 31, 2014 and 2013:
| In thousands | 2014 | 2013 | ||||||
| Amounts received in advance of revenue recognition |
$ | 17,186 | $ | 10,434 | ||||
| Amounts due to former customers |
122 | 4,172 | ||||||
| Other |
522 | 530 | ||||||
|
|
|
|
|
|||||
| Total |
$ | 17,830 | $ | 15,136 | ||||
|
|
|
|
|
|||||
Amounts received in advance of revenue recognition consists of payments received from customers in France. Amounts due to former customers consists of amounts due for product returns.
7. Long-term Debt
3.625 percent Convertible Notes due 2019
On June 17, 2014, the Company issued $200.0 million aggregate principal amount of 3.625 percent convertible senior notes due 2019 (the “convertible notes”). The Company received net proceeds of $192.9 million from the sale of the convertible notes, after deducting fees of $6.0 million and expenses of $1.1 million. At the same time, the Company used $43.2 million of the net proceeds from the sale of the convertible notes to pay the cost of the convertible bond hedges, as described below, which cost was partially offset by $27.6 million in proceeds to the Company from the sale of warrants in the warrant transactions also described below.
The convertible notes are governed by the terms of an indenture between the Company, as issuer, and Wells Fargo Bank, National Association, as the trustee. The convertible notes are senior unsecured obligations and bear interest at a rate of 3.625 percent per year, payable semi-annually in arrears on June 15 and December 15 of each year, beginning on December 15, 2014. The convertible notes will mature on June 15, 2019, unless earlier repurchased or converted. The convertible notes are convertible, subject to adjustment
97
Table of Contents
as described below, into cash, shares of the Company’s common stock, or a combination thereof, at the Company’s election, at an initial conversion rate of approximately 107.5095 shares of common stock per $1,000 principal amount of the convertible notes, which corresponds to an initial conversion price of approximately $9.30 per share of the Company’s common stock and represents a conversion premium of approximately 32.5 percent based on the last reported sale price of the Company’s common stock of $7.02 on June 11, 2014, the date the notes offering was priced. The principal amount of the notes exceeded if-converted value as of December 31, 2014.
The conversion rate is subject to adjustment from time to time upon the occurrence of certain events, but will not be adjusted for any accrued and unpaid interest. At any time prior to the close of business on the business day immediately preceding December 15, 2018, holders may convert their convertible notes at their option only under the following circumstances:
| • | during any calendar quarter commencing after the calendar quarter ending on December 31, 2014 (and only during such calendar quarter), if the last reported sale price of our common stock for at least 20 trading days (whether or not consecutive) during a period of 30 consecutive trading days ending on the last trading day of the immediately preceding calendar quarter is greater than or equal to 130 percent of the conversion price, or approximately $12.00 per share, on each applicable trading day; |
| • | during the five business day period after any five consecutive trading day period, or the measurement period, in which the trading price per $1,000 principal amount of the convertible notes for each trading day of the measurement period was less than 98 percent of the product of the last reported sale price of the Company’s common stock and the conversion rate on each such trading day; or |
| • | upon the occurrence of specified corporate events. |
On or after December 15, 2018 until the close of business on the second scheduled trading day immediately preceding the maturity date, holders may convert all or any portion of their convertible notes, in multiples of $1,000 principal amount, at their option regardless of the foregoing circumstances. Upon conversion, the Company will satisfy its conversion obligation by paying or delivering, as the case may be, cash, shares of common stock, or a combination thereof, at its election.
If a make-whole fundamental change, as described in the indenture, occurs and a holder elects to convert its convertible notes in connection with such make-whole fundamental change, such holder may be entitled to an increase in the conversion rate as described in the indenture.
The Company may not redeem the convertible notes prior to the maturity date and no “sinking fund” is provided for the convertible notes, which means that the Company is not required to periodically redeem or retire the convertible notes. Upon the occurrence of certain fundamental changes involving the Company, holders of the convertible notes may require the Company to repurchase for cash all or part of their convertible notes at a repurchase price equal to 100 percent of the principal amount of the convertible notes to be repurchased, plus accrued and unpaid interest.
The indenture does not contain any financial or maintenance covenants or restrictions on the payments of dividends, the incurrence of indebtedness or the issuance or repurchase of securities by the Company or any of its subsidiaries. The indenture contains customary terms and covenants and events of default. If an event of default (other than certain events of bankruptcy, insolvency or reorganization involving the Company or any of its significant subsidiaries) occurs and is continuing, the trustee by notice to the Company, or the holders of at least 25 percent in principal amount of the outstanding convertible notes by written notice to the Company and the trustee, may declare 100 percent of the principal and accrued and unpaid interest, if any, on all of the convertible notes to be due and payable. Upon such a declaration of acceleration, such principal and accrued and unpaid interest, if any, will be due and payable
98
Table of Contents
immediately. Upon the occurrence of certain events of bankruptcy, insolvency or reorganization involving the Company or any of its significant subsidiaries, 100 percent of the principal of and accrued and unpaid interest, if any, on all of the convertible notes will become due and payable automatically. Notwithstanding the foregoing, the indenture provides that, to the extent the Company elects and for up to 180 days, the sole remedy for an event of default relating to certain failures by the Company to comply with certain reporting covenants in the indenture consists exclusively of the right to receive additional interest on the convertible notes.
In accordance with accounting guidance for debt with conversion and other options, the Company separately accounted for the liability and equity components of the convertible notes by allocating the proceeds between the liability component and the embedded conversion option, or equity component, due to the Company’s ability to settle the convertible notes in cash, common stock or a combination of cash and common stock, at the Company’s option. The carrying amount of the liability component was calculated by measuring the fair value of a similar liability that does not have an associated convertible feature. The allocation was performed in a manner that reflected the Company’s non-convertible debt borrowing rate for similar debt. The equity component of the convertible notes was recognized as a debt discount and represents the difference between the proceeds from the issuance of the convertible notes and the fair value of the liability of the convertible notes on their date of issuance. The excess of the principal amount of the liability component over its carrying amount, or debt discount, is amortized to interest expense using the effective interest method over the five year life of the convertible notes. The approximate remaining discount amortization period as of December 31, 2014 was 53.5 months. The equity component will not be remeasured for changes in fair value as long as it continues to meet the conditions for equity classification.
The outstanding convertible note balances as of December 31, 2014 consisted of the following:
| In thousands | ||||
| Liability component: |
||||
| Principal |
$ | 200,000 | ||
| Less: debt discount, net |
(43,092 | ) | ||
|
|
|
|||
| Net carrying amount |
$ | 156,908 | ||
|
|
|
|||
| Equity component |
$ | 40,896 | ||
|
|
|
|||
In connection with the issuance of the convertible notes, the Company incurred approximately $1.1 million of debt issuance costs, which primarily consisted of legal, accounting and other professional fees, and allocated these costs to the liability and equity components based on the allocation of the proceeds. Of the total $1.1 million of debt issuance costs, $254,000 was allocated to the equity component and recorded as a reduction to additional paid-in capital and $825,000 was allocated to the liability component and recorded in other assets on the balance sheet. The portion allocated to the liability component is amortized to interest expense over the expected life of the convertible notes using the effective interest method.
The Company determined the expected life of the debt was equal to the five-year term on the convertible notes. The effective interest rate on the liability component was 9.625 percent for the period from the date of issuance through December 31, 2014. The following table sets forth total interest expense recognized related to the convertible notes during the period from issuance through December 31, 2014:
| In thousands | ||||
| Contractual interest expense |
$ | 3,887 | ||
| Amortization of debt discount |
4,058 | |||
| Amortization of debt issuance costs |
71 | |||
|
|
|
|||
| Total interest expense |
$ | 8,016 | ||
|
|
|
|||
99
Table of Contents
Convertible Bond Hedge and Warrant Transactions
In connection with the pricing of the convertible notes and in order to reduce the potential dilution to the Company’s common stock and/or offset any cash payments in excess of the principal amount due upon conversion of the convertible notes, on June 12, 2014, the Company entered into convertible note hedge transactions covering approximately 21.5 million shares of the Company’s common stock underlying the $200.0 million aggregate principal amount of the convertible notes with JPMorgan Chase Bank, National Association, an affiliate of JPMorgan Securities LLC (the “Counter Party”). The convertible bond hedges have an exercise price of approximately $9.30 per share, subject to adjustment upon certain events, and are exercisable when and if the convertible notes are converted. Upon conversion of the convertible notes, if the price of the Company’s common stock is above the exercise price of the convertible bond hedges, the Counter Party will deliver shares of the Company’s common stock and/or cash with an aggregate value approximately equal to the difference between the price of the Company’s common stock at the conversion date and the exercise price, multiplied by the number of shares of the Company’s common stock related to the convertible bond hedges being exercised. The convertible bond hedges are separate transactions entered into by the Company and are not part of the terms of the convertible notes or the warrants, discussed below. Holders of the convertible notes will not have any rights with respect to the convertible bond hedges. The Company paid $43.2 million for these convertible bond hedges and recorded this amount as a reduction to additional paid-in capital.
At the same time, the Company also entered into separate warrant transactions with the Counter Party relating to, in the aggregate, approximately 21.5 million shares of the Company’s common stock underlying the $200.0 million aggregate principal amount of the convertible notes. The initial exercise price of the warrants is $12.00 per share, subject to adjustment upon certain events, which is approximately 70 percent above the last reported sale price of the Company’s common stock of $7.02 per share on June 11, 2014. Upon exercise, the Company will deliver shares of the Company’s common stock and /or cash with an aggregate value equal to the excess of the price of the Company’s common stock on the exercise date and the exercise price, multiplied by the number of shares, of the Company’s common stock underlying the exercise. The warrants will be exercisable and will expire in equal installments for a period of 100 trading days beginning on September 15, 2019. The warrants were issued to the Counter Party pursuant to the exemption from registration set forth in Section 4(a)(2) of the Securities Act. The Company received $27.6 million for these warrants and recorded this amount as an increase to additional paid-in capital.
Aside from the initial payment of a $43.2 million premium to the Counter Party under the convertible bond hedges, which cost is partially offset by the receipt of a $27.6 million premium under the warrants, the Company is not required to make any cash payments to the Counter Party under the convertible bond hedges and will not receive any proceeds if the warrants are exercised.
Other Long Term Debt
Other long-term debt consisted of the following at December 31, 2014 and 2013:
| In thousands | 2014 | 2013 | ||||||
| Bank term loan |
$ | — | $ | 9,100 | ||||
| Less current portion |
— | (4,200 | ) | |||||
|
|
|
|
|
|||||
| $ | — | $ | 4,900 | |||||
|
|
|
|
|
|||||
The bank term loan provided for quarterly payments of principal and interest with final scheduled maturity on December 31, 2015. The loan bore interest at LIBOR plus 1.25 to 2.25 percent, depending on the percentage of the Company’s liquid assets on deposit with or invested through the bank, or at the prime rate. The loan was secured by a lien on all assets of the Company excluding intellectual property, which the Company agreed not to pledge to any other party. The loan required the Company to
100
Table of Contents
maintain a minimum of $15.0 million in unrestricted cash, cash equivalents and investments. The loan also contained certain covenants that restricted additional indebtedness, additional liens and sales of assets, and dividends, distributions or repurchases of common stock. The term loan was paid in full on June 17, 2014.
8. Executive Compensation Plan
Under the Company’s deferred executive compensation plan, the Company accrued a liability for the value of the awards ratably over the vesting period. The grant date value of awards made in 2012 was $1.1 million. There were no awards in 2014 or 2013. The net expense for this plan was $119,000, $963,000 and $2.8 million in 2014, 2013 and 2012, respectively.
9. Leases, Licensed Technology and Other Commitments
Facility Leases
The Company conducts the majority of its operations in a 100,000 square foot office and laboratory facility under a non-cancelable operating lease that extends to July 2019 with two consecutive five-year renewal options. The Company maintains an outstanding letter of credit of $1.4 million in accordance with the terms of the amended lease. In May 2012, the Company entered into a three-year operating lease agreement for an additional 26,000 square feet of office space. Future non-cancelable minimum annual rental payments through July 2019 under these leases are $6.7 million in 2015, $5.9 million in 2016, $6.0 million in 2017, $6.1 million in 2018 and $3.6 million in 2019.
Binney Street, Cambridge, Massachusetts
In January 2013, the Company entered into a lease agreement for approximately 244,000 square feet of laboratory and office space in two adjacent, connected buildings which are under construction in Cambridge, Massachusetts. Under the terms of the original lease, the Company leased all of the rentable space in one of the two buildings and a portion of the available space in the second building. In September 2013, the Company entered into a lease amendment to lease all of the remaining space, approximately 142,000 square feet, in the second building, for an aggregate of 386,000 square feet in both buildings. The terms of the lease amendment were consistent with the terms of the original lease. Construction of the core and shell of the building is expected to be completed in the first half of 2015 at which time construction of tenant improvements in the building will commence. Construction of the tenant improvements is expected to be completed in the first half of 2016.
In connection with this lease, the landlord is providing a tenant improvement allowance for the costs associated with the design, engineering, and construction of tenant improvements for the leased facility. The tenant improvements will be in accordance with the Company’s plans and include fit-out of the buildings to construct appropriate laboratory and office space, subject to approval by the landlord. To the extent the stipulated tenant allowance provided by the landlord is exceeded, the Company is obligated to fund all costs incurred in excess of the tenant allowance. The scope of the planned tenant improvements do not qualify as “normal tenant improvements” under the lease accounting guidance. Accordingly, for accounting purposes, the Company is the deemed owner of the buildings during the construction period.
As construction progresses, the Company records the project construction costs incurred as an asset, along with a corresponding facility lease obligation, on the consolidated balance sheet for the total amount of project costs incurred whether funded by the Company or the landlord. Upon completion of the buildings, the Company will determine if the asset and corresponding financing obligation should continue to be carried on its consolidated balance sheet under the appropriate accounting guidance. Based on the current terms of the lease, the Company expects to continue to be the deemed owner of the
101
Table of Contents
buildings upon completion of the construction period. As of December 31, 2014, the Company has recorded construction in progress and a facility lease obligation of $196.0 million (including the current portion of the obligation of $6.7 million included in current liabilities).
The initial term of the lease is for 15 years from substantial completion of the buildings with options to renew for three terms of five years each at market-based rates. The base rent is subject to increases over the term of the lease. Based on the original and amended leased space, the non-cancelable minimum annual lease payments for the annual periods beginning upon commencement of the lease are $5.3 million, $8.4 million, $26.6 million, $30.4 million and $30.9 million in the first five years of the lease and $347.1 million in total thereafter, plus the Company’s share of the facility operating expenses and other costs that are reimbursable to the landlord under the lease. The Company has the right to sublease portions of the space and is currently planning to sublease approximately 170,000 square feet of the total of 386,000 square feet available.
The Company maintains a letter of credit as security for the lease of $9.2 million, which is supported by restricted cash.
Lausanne, Switzerland
In January 2013, the Company entered into a lease agreement for approximately 22,000 square feet of office space in a building, which the Company occupied in 2014. The term of the lease is for ten years, with options for extension of the term and an early termination at the Company’s option after five years. Future non-cancelable minimum annual lease payments under their lease are expected to be approximately $1.0 million in 2015, 2016, 2017 and 2018, $1.1 million in 2019 and $4.3 million in total thereafter.
Total rent expense for the leases described above as well as other Company leases for 2014, 2013 and 2012 was $7.6 million, $6.1 million and $5.9 million, respectively. Contingent rent for 2014, 2013 and 2012 was $0.7 million, $0.7 million and $0.6 million, respectively. Total future non-cancelable minimum annual rental payments for the leases described above as well as other Company leases, for the next five years and thereafter are $13.9 million, $15.5 million, $33.7 million, $37.5 million, $35.6 million and $351.4 million, respectively.
Licensed Technology
The Company has entered into agreements with several universities under the terms of which the Company has received exclusive licenses to technology and intellectual property. The agreements, which are generally cancelable by the Company, provide for the payment of license fees and/or minimum payments, which are generally creditable against future royalties. Fees paid by the Company amounted to $470,000 in 2014 and $145,000 in each of 2013 and 2012, and are expected to amount to $485,000 in 2015, $110,000 in 2016, $105,000 in 2017, $100,000 in 2018, and $85,000 in 2019. In addition, the agreements provide for payments upon the achievement of certain milestones in product development. The agreements also require the Company to fund certain costs associated with the filing and prosecution of patent applications.
Other Commitments
The Company has entered into employment agreements with twenty officers of the Company. The agreements for these officers have remaining terms as of December 31, 2014 through the end of 2016, providing for aggregate base salaries of $7.7 million for 2015 and 2016.
102
Table of Contents
10. Stockholders’ Equity and Warrants
Preferred Stock
The Company has authorized 10,000,000 shares of preferred stock which the Board of Directors is authorized to designate and issue in different series. In connection with the Section 382 Rights Plan discussed below, the Company designated 500,000 shares of preferred stock as Series A Junior Participating Preferred Stock (the “Series A Junior Preferred Stock”). The Series A Junior Preferred Stock, when and if issued, has certain rights and privileges including rights to dividends, voting rights and preferential rights in the event of a liquidation of the Company. Each share of Series A Junior Preferred Stock participates in dividends and voting rights on a 1,000 to 1 basis with each share of common stock.
Common Stock and Warrants
On June 20, 2013, following stockholder approval, the Company filed a Certificate of Amendment to its Certificate of Incorporation, as amended, to increase the number of authorized shares of the Company’s common stock from 240,000,000 to 450,000,000 shares.
On February 25, 2009, the Company sold 14,378,698 shares of its common stock in a registered direct offering to institutional investors, at a purchase price of $1.69 per share, resulting in net proceeds after fees and expenses of $22.8 million. The investors also received warrants to purchase an additional 10,784,024 shares of the Company’s common stock exercisable at a price of $2.15 per share in cash or pursuant to the net exercise provisions of the warrants. The warrants became exercisable on August 25, 2009 and were scheduled to expire on February 25, 2012 if not exercised by that date. In 2012, the then remaining 5,805,843 warrants were exercised for proceeds to the Company of approximately $12.5 million. Prior to exercise, the warrant liability was recorded at fair value, with the adjustment to carrying value recognized in earnings. Upon exercise, the sum of the fair value of the exercised warrants and the proceeds received were credited to additional paid-in-capital and totaled $87.0 million in 2012. Upon the exercise of these remaining warrants, the balance of the warrant liability was credited to stockholders’ equity and the liability was eliminated.
On January 29, 2013, the Company sold 16,489,893 shares of its common stock in an underwritten public offering at a purchase price of $19.60 per share. Net proceeds of this offering, after underwriting discounts and commissions and expenses, were approximately $310.0 million.
On December 14, 2011, the Company filed a shelf registration statement with the SEC for the issuance of an unspecified amount of common stock, preferred stock, various series of debt securities and/or warrants to purchase any of such securities, either individually or in units, from time to time at prices and on terms to be determined at the time of any such offering. This registration statement was effective upon filing and expired in December 2014.
Section 382 Rights Plan
On November 1, 2013, the Company announced that the Company’s Board of Directors adopted a shareholder rights plan in the form of a Section 382 Rights Plan designed to preserve the Company’s tax assets. As a part of the plan, on October 31, 2013, the Company’s Board of Directors declared a dividend of one Series A Junior Preferred Stock fractional share purchase right for each share of the Company’s common stock outstanding as of November 11, 2013. Effective on November 1, 2013, if any group or person acquires 4.99 percent or more of the Company’s outstanding shares of common stock, or if a group or person that already owns 4.99 percent or more of the Company’s common stock acquires additional shares representing 0.5 percent or more of the Company’s common stock, then, subject to certain exceptions, there would be a triggering event under the plan. The rights would then separate from the Company’s common stock and would be adjusted to become exercisable to purchase 1/1000 share of the Company’s Series A Junior Preferred Stock having a market value equal to twice the exercise price of $20.00, resulting in significant dilution in the ownership interest of the acquiring person or group. The Company’s Board of Directors has the discretion to exempt any acquisition of the Company’s common stock from the provisions of the plan and has the ability to terminate the plan prior to a triggering event. The plan was approved by the Company’s stockholders in June 2014, expires in October 2016 and may not be amended to extend the expiration date.
103
Table of Contents
11. Fair Value of Financial Instruments
The Company provides disclosure of financial assets and financial liabilities that are carried at fair value based on the price that would be received upon sale of an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. Fair value measurements may be classified based on the amount of subjectivity associated with the inputs to fair valuation of these assets and liabilities using the following three levels:
Level 1—Inputs are unadjusted quoted prices in active markets for identical assets or liabilities that the Company has the ability to access at the measurement date.
Level 2—Inputs include quoted prices for similar assets and liabilities in active markets, quoted prices for identical or similar assets or liabilities in markets that are not active, inputs other than quoted prices that are observable for the asset or liability (i.e., interest rates, yield curves, etc.) and inputs that are derived principally from or corroborated by observable market data by correlation or other means (market corroborated inputs).
Level 3—Unobservable inputs that reflect the Company’s estimates of the assumptions that market participants would use in pricing the asset or liability. The Company develops these inputs based on the best information available, including its own data.
As of December 31, 2014 and 2013, the Company did not hold any assets recorded at fair value.
At December 31, 2014 and 2013, the carrying amounts of cash equivalents, accounts payable and accrued liabilities approximate fair value because of their short-term nature. The carrying amount of the Company’s bank term loan at December 31, 2013 approximated fair value due to its variable interest rate and other terms. All such measurements are Level 2 measurements in the fair value hierarchy. The carrying amount of the Company’s leased buildings under construction in Cambridge, Massachusetts and the related long term facility lease obligation reflect replacement cost, which approximates fair value. This measurement is a Level 3 fair value measurement. The fair value of the convertible notes, which differs from their carrying value, is influenced by interest rates and stock price and stock price volatility and is determined by prices for the convertible notes observed in market trading. The market for trading of the convertible notes is not considered to be an active market and therefore the estimate of fair value is based on Level 2 inputs. The estimated fair value of the convertible notes, face value of $200 million, was $203.4 million at December 31, 2014.
12. Stock Compensation
ARIAD Stock Option and Stock Plans
The Company’s 2001, 2006 and 2014 stock option and stock plans (the “Plans”) provide for the awarding of nonqualified and incentive stock options, stock grants, restricted stock units, performance share units and other equity-based awards to officers, directors, employees and consultants of the Company. Stock options become exercisable as specified in the related option certificate, typically over a three or four-year period, and expire ten years from the date of grant. Stock grants, restricted stock units and performance share units provide the recipient with ownership of common stock subject to terms of vesting, any rights the Company may have to repurchase the shares granted or other restrictions. The 2001 and 2006 Plans have no shares remaining available for grant, although existing stock options granted under these Plans remain outstanding. As of December 31, 2014, there were 13,840,313 shares available for awards under the 2014 Plan. The Company generally issues new shares upon the exercise or vesting of stock plan awards.
104
Table of Contents
Employee Stock Purchase Plan
In 1997, the Company adopted the 1997 Employee Stock Purchase Plan (“ESPP”) and reserved 500,000 shares of common stock for issuance under this plan. The ESPP was amended in June 2008 to reserve an additional 500,000 shares of common stock for issuance and the plan was further amended in 2009 and in June 2014 to reserve an additional 750,000 shares of common stock for issuance pursuant to each of those amendments. Under this plan, substantially all of the Company’s employees may, through payroll withholdings, purchase shares of the Company’s common stock at a price of 85 percent of the lesser of the fair market value at the beginning or end of each three-month withholding period. In 2014, 2013 and 2012, 250,776, 101,300 and 66,531 shares of common stock were issued under the plan, respectively. Compensation cost is equal to the fair value of the discount on the date of grant and is recognized as compensation in the period of purchase.
Stock-Based Compensation
The Company’s statements of operations included total compensation cost from awards under the Plans and purchases under the ESPP for the years ended December 31, as follows:
| In thousands | 2014 | 2013 | 2012 | |||||||||
| Compensation cost from: |
||||||||||||
| Stock options |
$ | 16,608 | $ | 16,364 | $ | 10,626 | ||||||
| Stock and stock units |
15,346 | 18,458 | 9,467 | |||||||||
| Purchases of common stock at a discount |
449 | 599 | 248 | |||||||||
|
|
|
|
|
|
|
|||||||
| $ | 32,403 | $ | 35,421 | $ | 20,341 | |||||||
|
|
|
|
|
|
|
|||||||
| Compensation cost included in: |
||||||||||||
| Research and development expense |
$ | 13,856 | $ | 15,150 | $ | 9,846 | ||||||
| Selling, general and administrative expense |
18,547 | 20,271 | 10,495 | |||||||||
|
|
|
|
|
|
|
|||||||
| $ | 32,403 | $ | 35,421 | $ | 20,341 | |||||||
|
|
|
|
|
|
|
|||||||
Stock Options
Stock options are granted with an exercise price equal to the closing market price of the Company’s common stock on the date of grant. Stock options generally vest ratably over three or four years and have contractual terms of ten years. Stock options are valued using the Black-Scholes option valuation model and compensation cost is recognized based on such fair value over the period of vesting on a straight-line basis.
The following table summarizes information about stock options as of and for the years ended December 31 as follows:
| In thousands, except per share amounts | 2014 | 2013 | 2012 | |||||||||
| Weighted average fair value of options granted, per share |
$ | 5.20 | $ | 8.38 | $ | 13.04 | ||||||
| Total cash received from exercises of stock options |
2,356 | 4,847 | 9,677 | |||||||||
| Total intrinsic value of stock options exercised |
1,619 | 13,829 | 27,572 | |||||||||
The weighted average fair value of options granted in the years ended December 31, 2014, 2013 and 2012, reflect the following weighted-average assumptions:
| 2014 | 2013 | 2012 | ||||||||||
| Expected life of options granted (in years) |
6.8 | 6.9 | 7.1 | |||||||||
| Expected volatility |
84 | % | 76 | % | 76 | % | ||||||
| Risk-free rate |
2.16 | % | 1.74 | % | 1.32 | % | ||||||
| Expected dividends |
0 | % | 0 | % | 0 | % | ||||||
105
Table of Contents
The expected life assumption is based on an analysis of historical behavior of participants related to options awarded over time. The expected volatility assumption is based on an average of the historical volatility and the implied volatility of the Company’s common stock, derived from an analysis of historical traded and quoted options on the Company’s common stock. The risk-free rate is based on the forward U.S. Treasury yield curve. The expected dividends reflect the Company’s current and expected future policy for dividends on its common stock.
Stock option activity under the Company’s stock plans for the year ended December 31, 2014 was as follows:
| Number of Shares |
Weighted Average Exercise Price Per Share |
|||||||
| Options outstanding, January 1, 2014 |
10,379,668 | $ | 10.83 | |||||
| Granted |
1,983,420 | $ | 6.94 | |||||
| Forfeited |
(1,529,855 | ) | $ | 13.54 | ||||
| Exercised |
(685,146 | ) | $ | 4.44 | ||||
|
|
|
|||||||
| Options outstanding, December 31, 2014 |
10,148,087 | $ | 10.09 | |||||
|
|
|
|||||||
The following table summarizes information about stock options outstanding as of December 31, 2014:
| Options Outstanding |
Options Exercisable |
Options Vested and Expected To Vest |
||||||||||
| Number of options |
10,148,087 | 5,091,290 | 9,369,517 | |||||||||
| Weighted average exercise price per share |
$ | 10.09 | $ | 9.37 | $ | 10.16 | ||||||
| Aggregate intrinsic value (in 000’s) |
$ | 9,717 | $ | 5,746 | $ | 8,853 | ||||||
| Weighted average remaining contractual term (years) |
6.97 | 5.27 | 6.82 | |||||||||
Options expected to vest consist of options scheduled to vest in the future less expected forfeitures.
At December 31, 2014, total unrecognized compensation cost related to non-vested stock options outstanding amounted to $21.4 million. That cost is expected to be recognized over a weighted-average period of 2.2 years.
Stock and Stock Unit Grants
Stock and stock unit grants carry restrictions as to resale for periods of time or vesting provisions over time as specified in the grant. Stock and stock unit grants are valued at the closing market price of the Company’s common stock on the date of grant and compensation expense is recognized over the requisite service period, vesting period or period during which restrictions remain on the common stock or stock units granted.
Stock and stock unit activity under the Company’s stock plans for the year ended December 31, 2014 was as follows:
| Number of Shares |
Weighted Average Grant Date Fair Value |
|||||||
| Outstanding, January 1, 2014 |
2,047,600 | $ | 14.18 | |||||
| Granted / awarded |
2,479,500 | $ | 7.35 | |||||
| Forfeited |
(321,718 | ) | $ | 9.30 | ||||
| Vested or restrictions lapsed |
(702,229 | ) | $ | 13.28 | ||||
|
|
|
|||||||
| Outstanding, December 31, 2014 |
3,503,153 | $ | 9.97 | |||||
|
|
|
|||||||
106
Table of Contents
The total fair value of stock and stock unit awards that vested in 2014, 2013 and 2012 was $5.2 million, $25.5 million and $27.8 million, respectively. The total unrecognized compensation expense for restricted shares or units that have been granted or are probable to be awarded was $19.3 million at December 31, 2014 and will be recognized over 1.5 years on a weighted average basis.
Included in stock and stock units outstanding in the above table are 524,800 performance share units awarded in March 2012 of which 274,400 vested upon the achievement of the performance condition, marketing authorization of Iclusig by the European Commission in July 2013, 129,200 that vested in July 2014 and 121,200 that will vest in July 2015.
Stock and stock units outstanding in the table above also include 316,000 performance share units awarded in 2013. The number of shares that may vest, if any, related to the 2013 performance share unit awards is dependent on the achievement, and timing of the achievement, of the performance criteria defined for the award. The compensation cost for such performance-based stock awards will be based on the awards that ultimately vest and the grant date fair value of those awards. The Company begins to recognize compensation expense related to performance share units when achievement of the performance condition is probable. In 2013 and 2014, the Company concluded that it was probable that the performance condition related to the 2013 performance share unit awards would be met. The performance condition is based upon continued success in specific research and development initiatives.
Stock and stock units outstanding in the above table as of December 31, 2014 also include 997,000 and 47,000 performance share units awarded on January 31, 2014 and June 25, 2014, respectively. The vesting of fifty percent of the award is dependent upon the achievement of specific commercial objectives by the end of 2015 and the vesting of the remainder is dependent upon the achievement, and timing of the achievement, of specific research and development objectives. The Company has concluded that it is probable that these performance conditions will be met. The total compensation expense for the portion related to the research and development objectives may be up to 60 percent higher depending on the timing of the achievement of the specific performance objectives.
13. Net Loss Per Share
Basic net loss per share amounts have been computed based on the weighted-average number of common shares outstanding. Diluted net loss per share amounts have been computed based on the weighted-average number of common shares outstanding plus the dilutive effect, if any, of potential common shares. The computation of potential common shares has been performed using the treasury stock method. Because of the net loss reported in each period, diluted and basic net loss per share amounts are the same.
The calculation of net loss and the number of shares used to compute basic and diluted earnings per share for the years ended December 31, 2014, 2013 and 2012 are as follows:
| In thousands, except per share amounts | 2014 | 2013 | 2012 | |||||||||
| Net loss |
$ | (162,602 | ) | $ | (274,158 | ) | $ | (220,872 | ) | |||
|
|
|
|
|
|
|
|||||||
| Weighted average shares outstanding – basic and diluted |
186,835 | 183,575 | 164,964 | |||||||||
|
|
|
|
|
|
|
|||||||
| Net loss per share – basic and diluted |
$ | (0.87 | ) | $ | (1.49 | ) | $ | (1.34 | ) | |||
|
|
|
|
|
|
|
|||||||
For the years ended December 31, 2014, 2013 and 2012, the following potentially dilutive securities were not included in the computation of net loss per share because the effect would be anti-dilutive:
| In thousands | 2014 | 2013 | 2012 | |||||||||
| Stock options |
10,148 | 10,380 | 8,228 | |||||||||
| Restricted stock and restricted stock units |
3,503 | 2,048 | 1,903 | |||||||||
|
|
|
|
|
|
|
|||||||
| 13,651 | 12,428 | 10,131 | ||||||||||
|
|
|
|
|
|
|
|||||||
107
Table of Contents
14. Accumulated Other Comprehensive Income (Loss)
The changes in accumulated other comprehensive income (loss) for the years ended December 31, 2014 and 2013 were as follows:
| In thousands | Net Unrealized Gains (reclassification adjustment) on Marketable Securities |
Cumulative Translation Adjustment |
Defined Benefit Pension Obligation |
Total | ||||||||||||
| Balance, January 1, 2013 |
$ | 20 | $ | — | $ | — | $ | 20 | ||||||||
| Reclassification adjustment |
(20 | ) | — | — | (20 | ) | ||||||||||
| Other comprehensive loss |
— | (40 | ) | (1,495 | ) | (1,535 | ) | |||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Balance, December 31, 2013 |
— | (40 | ) | (1,495 | ) | (1,535 | ) | |||||||||
| Other comprehensive income (loss) |
— | 214 | (2,864 | ) | (2,650 | ) | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Balance, December 31, 2014 |
$ | — | $ | 174 | $ | (4,359 | ) | $ | (4,185 | ) | ||||||
|
|
|
|
|
|
|
|
|
|||||||||
15. Income Taxes
The Company is subject to U.S. federal and various state corporate income taxes as well as taxes in foreign jurisdictions where subsidiaries have been established. Loss before provision for income taxes and the provision for income taxes consist of the following for the years ended December 31, 2014, 2013 and 2012:
| In thousands | 2014 | 2013 | 2012 | |||||||||
| Loss before provision for income taxes |
||||||||||||
| Domestic |
$ | (100,860 | ) | $ | (192,998 | ) | $ | (182,974 | ) | |||
| Foreign |
(61,112 | ) | (80,721 | ) | (37,898 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Total |
$ | (161,972 | ) | $ | (273,719 | ) | $ | (220,872 | ) | |||
|
|
|
|
|
|
|
|||||||
| Provision for income taxes |
||||||||||||
| Current: |
||||||||||||
| Federal |
$ | — | $ | — | $ | — | ||||||
| State |
237 | 216 | — | |||||||||
| Foreign |
393 | 223 | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Total |
$ | 630 | $ | 439 | $ | — | ||||||
|
|
|
|
|
|
|
|||||||
108
Table of Contents
The Company did not incur a deferred income tax provision or benefit in 2014, 2013 or 2012.
A reconciliation of the federal statutory corporate income tax rate to the effective income tax rate for the years ended December 31, 2014, 2013 and 2012 is as follows:
| 2014 | 2013 | 2012 | ||||||||||
| Statutory federal income tax rate |
(35 | )% | (35 | )% | (35 | )% | ||||||
| State income tax rate, net of federal benefit |
(4 | ) | (2 | ) | (4 | ) | ||||||
| Revaluation of warrant liability |
— | — | 3 | |||||||||
| Other permanent differences |
(5 | ) | — | (1 | ) | |||||||
| Foreign rate differential |
9 | 9 | 6 | |||||||||
| Change in valuation allowance |
35 | 28 | 31 | |||||||||
|
|
|
|
|
|
|
|||||||
| Effective tax rate |
— | % | — | % | — | % | ||||||
|
|
|
|
|
|
|
|||||||
The components of deferred income taxes were as follows at December 31:
| In thousands | 2014 | 2013 | ||||||
| Deferred tax liabilities: |
||||||||
| Intangibles |
$ | 377 | $ | 388 | ||||
| Unrealized currency gain |
— | 10,892 | ||||||
|
|
|
|
|
|||||
| Total deferred tax liabilities |
377 | 11,280 | ||||||
|
|
|
|
|
|||||
| Deferred tax assets: |
||||||||
| Net operating loss carryforwards |
205,793 | 166,951 | ||||||
| Federal and state tax credit carryovers |
32,318 | 28,645 | ||||||
| Depreciation |
4,383 | 3,607 | ||||||
| Stock-based compensation |
12,803 | 7,780 | ||||||
| Other |
9,008 | 7,977 | ||||||
| Debt-related deductions |
934 | — | ||||||
|
|
|
|
|
|||||
| Total deferred tax assets |
265,239 | 214,960 | ||||||
|
|
|
|
|
|||||
| Deferred tax assets, net |
264,862 | 203,680 | ||||||
| Valuation allowance |
(264,862 | ) | (203,680 | ) | ||||
|
|
|
|
|
|||||
| Total deferred taxes |
$ | — | $ | — | ||||
|
|
|
|
|
|||||
At December 31, 2014, the Company had available estimated net operating loss carryforwards and research and development credit carryforwards for federal, foreign and state tax reporting purposes as follows:
| Amount | Expiring if not utilized | |||||||
| (in thousands) | ||||||||
| Net operating loss carryforwards: |
||||||||
| Federal |
$ | 562,120 | 2024 through 2035 | |||||
| State |
$ | 226,804 | 2034 through 2035 | |||||
| Foreign |
$ | 194,944 | 2020 through 2022 | |||||
| Research and development credit carryforwards: |
||||||||
| Federal |
$ | 28,930 | 2019 through 2035 | |||||
| State |
$ | 4,251 | 2026 through 2030 | |||||
109
Table of Contents
Included in the federal net operating loss carryforwards above is approximately $35 million related to stock-based compensation tax deductions in excess of book compensation expense which will be credited to additional paid-in-capital when such reductions reduce taxes payable. Although these net operating losses are included in the carryforwards above, they are not reflected in the table of deferred tax assets as the excess tax benefits are not yet realized.
During 2012, the Company transferred certain intellectual property rights related to Iclusig to its wholly-owned subsidiary in Switzerland. Although the transfer of intellectual property rights between consolidated entities did not result in any gain in the consolidated results of operations, the Company generated a taxable gain in the U.S. that was substantially offset by existing tax loss and credit carryforwards. Any taxes incurred related to the intercompany transactions are treated as a prepaid tax in the Company’s consolidated balance sheet and amortized to income tax expense over the life of the intellectual property. The amount of tax amortized to the provision for income taxes for the year ended December 31, 2014 was approximately $153,498.
Since the Company has not yet achieved sustained profitable operations, management believes its deferred tax assets do not satisfy the more likely than not realization criteria and has recorded a valuation allowance for all deferred tax assets as of December 31, 2014 and 2013. The valuation allowance increased by $61.2 million in 2014 and $80.8 million in 2013 and decreased by $70.5 million in 2012.
The Company does not recognize a tax benefit unless it is more likely than not that the tax position will be sustained upon examination by tax authorities, including resolution of any related appeals or litigation processes, based on the technical merits of the position. The tax benefit recognized for these positions is measured at the largest amount of benefit that is greater than 50 percent likelihood of being realized upon ultimate settlement. Deferred tax assets that do not meet these recognition criteria are not recorded and the Company recognizes a liability for uncertain tax positions that may result in tax payments. The Company recognizes interest and penalties as a component of the provision for incomes taxes. In 2014 and 2013 the Company recorded approximately $84,000 and $65,000, respectively, of interest expense as a component of the provision for income taxes.
In 2014, the Company’s uncertain tax positions increased to approximately $25 million. If such unrecognized tax benefits were realized and not subject to valuation allowances, the Company would recognize a tax benefit of $21.2 million. A reconciliation of the reserve for uncertain tax benefits (including state tax matters without federal benefits) is as follows:
| In thousands | 2014 | 2013 | ||||||
| Uncertain tax positions, beginning of the year: |
$ | 24,653 | $ | 24,404 | ||||
| Gross increases – tax positions in current period |
331 | 249 | ||||||
|
|
|
|
|
|||||
| Uncertain tax positions, end of year |
$ | 24,984 | $ | 24,653 | ||||
|
|
|
|
|
|||||
Due to the Company’s historical net operating loss position, the Company’s U.S. federal and Massachusetts tax returns remain open to examination for three years after the Company utilizes that year’s net operating loss carryforward. The Company’s earliest year which generated a net operating loss included in the Company’s current net operating loss carryforward is 2004 for U.S. federal tax purposes. The Company’s Massachusetts state tax returns from 2009 to 2013 remain open to examination. All tax years for foreign subsidiaries are also open to audit in their respective jurisdictions.
110
Table of Contents
In 2015, the Internal Revenue Service (“IRS”) commenced an examination of our U.S. income tax return for 2012 that is anticipated to be completed within the next twelve months. As a result of this audit, it is possible that the amount of the liability for unrecognized tax benefits could change over the next twelve months. The impact to the Company’s unrecognized tax benefits cannot be determined at this time based on the preliminary stage of the audit. As of December 31, 2014, the Company has not been notified of any significant proposed adjustments by the IRS.
16. Restructuring Actions
In the fourth quarter of fiscal 2013, the Company incurred expenses of $4.8 million associated with employee workforce reductions of approximately 155 positions, designed to reduce the Company’s operating expenses and extend its cash runway. The Company recorded $2.2 million of the employee separation costs in research and development expense and $2.6 million in selling, general and administrative expense. A rollforward of the restructuring liability for 2014 and 2013 is as follows:
| In thousands | 2014 | 2013 | ||||||
| Balance, beginning of the year |
$ | 2,220 | $ | — | ||||
| Charges |
— | 4,751 | ||||||
| Amounts paid |
(2,220 | ) | $ | (2,531 | ) | |||
|
|
|
|
|
|||||
| Balance, end of the year |
$ | — | $ | 2,220 | ||||
|
|
|
|
|
|||||
On the accompanying consolidated balance sheet at December 31, 2013, the restructuring liability balance of $2.2 million was classified as a component of accrued compensation and benefits.
17. Defined Benefit Pension Obligation
On March 1, 2013, the Company established a defined benefit pension plan for employees in its Switzerland subsidiary. The plan provides benefits to employees upon retirement, death or disability. The Company uses December 31 as the year-end measurement date for this plan.
Summarized information regarding changes in the plan obligations and plan assets, the funded status and the amounts recorded as of December 31, 2014 is as follows:
| In thousands | 2014 | 2013 | ||||||
| Benefit obligation, beginning of year |
$ | 7,876 | $ | — | ||||
| Benefit obligation at plan establishment |
— | 5,621 | ||||||
| Service cost |
1,081 | 994 | ||||||
| Interest cost |
163 | 105 | ||||||
| Plan participants’ contributions |
486 | 283 | ||||||
| Actuarial loss (gain) |
3,057 | (192 | ) | |||||
| Employee vested funds brought to plan, net |
2,183 | 1,065 | ||||||
| Translation gain |
(636 | ) | ||||||
|
|
|
|
|
|||||
| Benefit obligation, end of year |
14,210 | 7,876 | ||||||
|
|
|
|
|
|||||
| Fair value of plan assets, beginning of year |
5,998 | — | ||||||
| Fair value of plan assets at plan establishment |
— | 3,853 | ||||||
| Actual return on plan assets |
130 | 77 | ||||||
| Employer contributions |
1,285 | 720 | ||||||
| Plan participants’ contributions |
483 | 283 | ||||||
| Employee vested funds brought to plan, net |
2,183 | 1,065 | ||||||
| Translation loss |
(459 | ) | — | |||||
|
|
|
|
|
|||||
| Fair value of plan assets, end of year |
9,620 | 5,998 | ||||||
|
|
|
|
|
|||||
| Unfunded liability as of December 31, 2014 |
$ | 4,590 | $ | 1,878 | ||||
|
|
|
|
|
|||||
111
Table of Contents
This unfunded liability is recognized in other long-term liabilities in the accompanying consolidated balance sheet as of December 31, 2014 and 2013.
The projected benefit obligation, the accumulated benefit obligation and the fair value of the plan assets as of December 31, 2014 and 2013 were as follows:
| In thousands | 2014 | 2013 | ||||||
| Projected benefit obligation |
$ | 14,210 | $ | 7,876 | ||||
| Accumulated benefit obligation |
$ | 13,839 | $ | 7,947 | ||||
| Fair value of plan assets |
$ | 9,619 | $ | 5,998 | ||||
The net periodic benefit cost for the defined benefit pension plan for the year ended December 31, 2014 and 2013 were as follows:
| In thousands | 2014 | 2013 | ||||||
| Service cost |
$ | 1,081 | $ | 994 | ||||
| Interest cost |
163 | 105 | ||||||
| Expected return on plan assets |
(137 | ) | (85 | ) | ||||
| Amortization of prior service cost |
150 | 134 | ||||||
|
|
|
|
|
|||||
| Net periodic benefit cost |
$ | 1,257 | $ | 1,148 | ||||
|
|
|
|
|
|||||
Other changes in the plan assets and the benefit obligation that are recognized in accumulated other comprehensive income (loss) and other comprehensive loss for the years ended December 31, 2014 and 2013 were as follows:
| In thousands | 2014 | 2013 | ||||||
| Pension liability OCI, beginning of year |
$ | (1,495 | ) | |||||
| Establishment of plan |
— | $ | (1,768 | ) | ||||
| Net gain |
(3,014 | ) | 139 | |||||
| Amortization of prior service cost |
150 | 134 | ||||||
|
|
|
|
|
|||||
| Total |
$ | (4,359 | ) | $ | (1,495 | ) | ||
|
|
|
|
|
|||||
The prior service cost for the defined benefit pension plan that will be amortized from accumulated other comprehensive income (loss) into net periodic benefit cost over the next fiscal year is $275.
The assumptions used to determine the benefit obligation at December 31, 2014 and 2013 were as follows:
| 2014 | 2013 | |||||||
| Discount rate |
1.25 | % | 2.25 | % | ||||
| Rate of compensation |
1.50 | % | 1.50 | % | ||||
The assumptions used to determine net periodic benefit costs for 2014 and 2013 were as follows:
| 2014 | 2013 | |||||||
| Discount rate |
1.25 | % | 2.00 | % | ||||
| Expected long-term return on plan |
1.25 | % | 2.00 | % | ||||
| Rate of compensation increase |
1.50 | % | 1.50 | % | ||||
The assets of the plan are held in a collective investment account. All plan investments are classified as level 2 within the fair value hierarchy.
The Company expects to contribute approximately $1.8 million to its defined benefit pension plan in 2015.
112
Table of Contents
The following benefit payments, which reflect expected future service, as appropriate, are expected to be paid:
| In thousands | ||||
| 2015 |
$ | 588 | ||
| 2016 |
639 | |||
| 2017 |
678 | |||
| 2018 |
704 | |||
| 2019 |
730 | |||
| 2020-2024 |
4,126 | |||
|
|
|
|||
| $ | 7,465 | |||
|
|
|
|||
18. Litigation
On October 10, 2013, October 17, 2013, December 3, 2013 and December 6, 2013, purported shareholder class actions, styled Jimmy Wang v. ARIAD Pharmaceuticals, Inc., et al., James L. Burch v. ARIAD Pharmaceuticals, Inc., et al., Greater Pennsylvania Carpenters’ Pension Fund v. ARIAD Pharmaceuticals, Inc., et al, and Nabil Elmachtoub v. ARIAD Pharmaceuticals, Inc., et al, respectively, were filed in the United States District Court for the District of Massachusetts (the “District Court”), naming the Company and certain of its officers as defendants. The lawsuits allege that the defendants made material misrepresentations and/or omissions of material fact regarding clinical and safety data for Iclusig in its public disclosures during the period from December 12, 2011 through October 8, 2013 or October 17, 2013, in violation of Sections 10(b) and 20(a) of the Securities Exchange Act of 1934, as amended, and Rule 10b-5 promulgated thereunder. On January 9, 2014, the District Court consolidated the actions and appointed lead plaintiffs. On February 18, 2014, the lead plaintiffs filed an amended complaint as contemplated by the order of the District Court. The amended complaint extends the class period for the Securities Exchange Act claims through October 30, 2013. In addition, plaintiffs allege that certain of the Company’s officers, directors and certain underwriters made material misrepresentations and/or omissions of material fact regarding clinical and safety data for Iclusig in connection with the Company’s January 24, 2013 follow-on public offering of common stock in violation of Sections 11 and 15 of the Securities Act of 1933, as amended. The plaintiffs seek unspecified monetary damages on behalf of the putative class and an award of costs and expenses, including attorney’s fees. On April 14, 2014, the defendants and the underwriters filed separate motions to dismiss the amended complaint. On June 10, 2014, the District Court heard oral argument on the motion to dismiss. The court has not yet ruled on the motion.
On November 6, 2013, a purported derivative lawsuit, styled Yu Liang v. ARIAD Pharmaceuticals, Inc., et al., was filed in the United States District Court for the District of Massachusetts (the “District Court”), on behalf of the Company naming its directors and certain of its officers as defendants. On December 6, 2013, an additional purported derivative lawsuit, styled Arkady Livitz v. Harvey J. Berger, et al, was filed in the District Court. The lawsuits allege that the Company’s directors and certain of its officers breached their fiduciary duties related to the clinical development and commercialization of Iclusig and by making misrepresentations regarding the safety and commercial marketability of Iclusig. The lawsuits also assert claims for unjust enrichment and corporate waste, and for misappropriation of information and insider trading by the officers named as defendants. On January 23, 2014, the District Court consolidated the actions. On February 3, 2014, the plaintiffs designated the Yu Liang complaint as the operative complaint as contemplated by the order of the District Court. The plaintiffs seek unspecified monetary damages, changes in the Company’s corporate governance policies and internal procedures, restitution and disgorgement from the individually named defendants, and an award of costs and expenses, including attorney fees. On March 5, 2014, the defendants filed a motion to dismiss the complaint. In response, on March 26, 2014, the plaintiffs filed an amended complaint. On April 23, 2014, the defendants filed a motion to dismiss the amended complaint. On July 23, 2014, the District Court heard oral argument on the motion to dismiss. The court recently scheduled supplemental oral argument for March 2, 2015.
113
Table of Contents
The Company believes that these actions are without merit. At this time, the Company has not recorded a liability related to damages in connection with these matters because it believes that any potential loss is not currently probable or reasonably estimable under U.S. GAAP. In addition, due to the early stages of the matters described above, the Company cannot reasonably estimate the possible loss or range of loss, if any, that may result from these matters.
From time to time, the Company may be subject to various claims and legal proceedings. If the potential loss from any claim, asserted or unasserted, or legal proceedings is considered probable and the amount is reasonably estimated, the Company will accrue a liability for the estimated loss.
114
Table of Contents
| ITEM 9: | CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE |
Not applicable.
ITEM 9A: CONTROLS AND PROCEDURES
(a) Evaluation of Disclosure Controls and Procedures. Our principal executive officer and principal financial officer, after evaluating the effectiveness of our disclosure controls and procedures (as defined in paragraph (e) of Rules 13a-15 and 15d-15 under the Securities Exchange Act of 1934) as of the end of the period covered by this Annual Report on Form 10-K, have concluded that, based on such evaluation, our disclosure controls and procedures were effective at the reasonable assurance level to ensure that information required to be disclosed by us in the reports that we file or submit under the Securities Exchange Act of 1934 is recorded, processed, summarized and reported, within the time periods specified in the SEC’s rules and forms and is accumulated and communicated to management, including the Chief Executive Officer and Chief Financial Officer, or persons performing similar functions, as appropriate to allow timely decisions regarding required disclosure, particularly during the period in which this Annual Report on Form 10-K was being prepared.
(b) Changes in Internal Controls. There were no changes in our internal control over financial reporting, identified in connection with the evaluation of such internal control that occurred during our last fiscal quarter that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
Management’s Report on Internal Control over Financial Reporting
The management of the Company is responsible for establishing and maintaining adequate internal control over financial reporting as defined in Rules 13a-15(f) and 15d-15(f) under the Securities Exchange Act of 1934, as amended. The Company’s internal control system was designed to provide reasonable assurance to the Company’s management and board of directors regarding the preparation and fair presentation of published financial statements. Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
The Company’s management assessed the effectiveness of the Company’s internal control over financial reporting as of December 31, 2014. In making this assessment, it used the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission (COSO) in Internal Control-Integrated Framework (2013). Based on our assessment we believe that, as of December 31, 2014, the Company’s internal control over financial reporting is effective based on those criteria.
Deloitte & Touche LLP, the independent registered public accounting firm that audited the Company’s consolidated financial statements, has issued an attestation report on the Company’s internal control over financial reporting as of December 31, 2014, which is included below.
115
Table of Contents
Report of Independent Registered Public Accounting Firm
To the Board of Directors and Stockholders of
ARIAD Pharmaceuticals, Inc.
Cambridge, Massachusetts
We have audited the internal control over financial reporting of ARIAD Pharmaceuticals, Inc. and subsidiaries (the “Company”) as of December 31, 2014, based on criteria established in Internal Control—Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission. The Company’s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying Management’s Report on Internal Control over Financial Reporting. Our responsibility is to express an opinion on the Company’s internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company’s internal control over financial reporting is a process designed by, or under the supervision of, the company’s principal executive and principal financial officers, or persons performing similar functions, and effected by the company’s board of directors, management, and other personnel to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of the inherent limitations of internal control over financial reporting, including the possibility of collusion or improper management override of controls, material misstatements due to error or fraud may not be prevented or detected on a timely basis. Also, projections of any evaluation of the effectiveness of the internal control over financial reporting to future periods are subject to the risk that the controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of December 31, 2014, based on the criteria established in Internal Control—Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission.
We have also audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the consolidated financial statements as of and for the year ended December 31, 2014 of the Company and our report dated March 2, 2015 expressed an unqualified opinion on those financial statements.
/s/ Deloitte & Touche LLP
Boston, Massachusetts
March 2, 2015
116
Table of Contents
PART III
Portions of our definitive Proxy Statement for the 2015 Annual Meeting of Shareholders, or 2015 Proxy Statement, during which we expect to, among other things, (i) elect our Class 3 Directors, (ii) conduct the non-binding advisory vote on our executive compensation program and (iii) ratify the appointment of our independent registered accounting firm, are incorporated by reference into this Part III of our Annual Report on Form 10-K.
| ITEM 10: | DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE |
The information required by this Item 10 will be included in our 2015 Proxy Statement and is incorporated herein by reference. We expect this information to be provided under the captions “Board of Directors,” “Executive Officers,” “Recommendations for Board Nominees; Board Diversity” “Section 16(a) Beneficial Ownership Reporting Compliance,” “Director Independence and Committee Qualifications,” “Board Committees” and “Corporate Code of Conduct and Ethics.”
| ITEM 11: | EXECUTIVE COMPENSATION |
The information required by this Item 11 will be included in our 2015 Proxy Statement and is incorporated herein by reference. We expect this information to be provided under the captions “Executive Compensation,” “Compensation Committee Interlocks and Insider Participation,” “Director Compensation” and “Compensation Practices and Policies Relating to Risk Management.”
| ITEM 12: | SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS |
The information required by this Item 12 will be included in our 2015 Proxy Statement and is incorporated herein by reference. We expect this information to be provided under the captions “Security Ownership of Certain Beneficial Owners and Management” and “Equity Compensation Plan Information.”
| ITEM 13: | CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE |
The information required by this Item 13 will be included in our 2015 Proxy Statement and is incorporated herein by reference. We expect this information to be provided under the captions “Board of Directors,” “Director Independence and Committee Qualifications” and “Certain Relationships and Related Transactions.”
| ITEM 14: | PRINCIPAL ACCOUNTING FEES AND SERVICES |
The information required by this Item 10 will be included in our 2015 Proxy Statement and is incorporated herein by reference. We expect this information to be provided under the caption “Ratification of Selection of Independent Registered Public Accounting Firm.”
117
Table of Contents
PART IV
| ITEM 15: | EXHIBITS, FINANCIAL STATEMENT SCHEDULES |
| (a)(1) | The following Consolidated Financial Statements, Notes thereto and Report of Independent Registered Public Accounting Firm have been presented in Item 8: |
Report of Independent Registered Public Accounting Firm
Consolidated Balance Sheets
Consolidated Statements of Operations
Consolidated Statements of Comprehensive Loss
Consolidated Statements of Stockholders’ Equity
Consolidated Statements of Cash Flows
Notes to Consolidated Financial Statements
| (a)(2) | Financial Statement Schedules: |
Schedules have been omitted because of the absence of conditions under which they are required or because the required information is included in the financial statements or notes thereto.
| (a)(3) | The Exhibits listed in the Exhibit Index are filed herewith in the manner set forth therein. |
| (b) | See (a) (3) above. |
| (c) | See (a) (2) above. |
118
Table of Contents
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Cambridge and Commonwealth of Massachusetts on the 2nd day of March, 2015.
| ARIAD PHARMACEUTICALS, INC. | ||
| By: | /s/ Harvey J. Berger, M.D. | |
| Name: | Harvey J. Berger, M.D. | |
| Title: | Chairman, Chief Executive Officer and President | |
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
| Signature | Title | Date | ||
| /s/ Harvey J. Berger, M.D. Harvey J. Berger, M.D. |
Chairman of the Board of Directors, Chief Executive Officer and President (Principal Executive Officer) |
March 2, 2015 | ||
| /s/ Edward M. Fitzgerald Edward M. Fitzgerald |
Executive Vice President, Chief Financial Officer and Treasurer (Principal Financial Officer and Principal Accounting Officer) |
March 2, 2015 | ||
| Director | ||||
| Alexander J. Denner, Ph.D. | ||||
| /s/ Jay R. LaMarche |
Director | March 2, 2015 | ||
| Jay R. LaMarche | ||||
| /s/ Athanase Lavidas, Ph.D. |
Director | March 2, 2015 | ||
| Athanase Lavidas, Ph.D. | ||||
| /s/ Massimo Radaelli, Ph.D. |
Director | March 2, 2015 | ||
| Massimo Radaelli, Ph.D. | ||||
| /s/ Norbert G. Riedel, Ph.D. |
Director | March 2, 2015 | ||
| Norbert G. Riedel, Ph.D. | ||||
| /s/ Sarah J. Schlesinger, M.D. |
Director | March 2, 2015 | ||
| Sarah J. Schlesinger, M.D. | ||||
| /s/ Wayne Wilson |
Director | March 2, 2015 | ||
| Wayne Wilson | ||||
119
Table of Contents
ARIAD Pharmaceuticals, Inc.
Form 10-K for the Year Ended December 31, 2014
Exhibit List
| Exhibit Number |
Exhibit Description |
Filed with this Report |
Incorporated by |
Filing Date |
SEC File/ Reg. Number | |||||||
| 3.1 | .1 | Certificate of Incorporation of ARIAD Pharmaceuticals, Inc., as amended | 10-Q (Exhibit 3.1) |
05/10/10 | 000-21696 | |||||||
| .2 | Certificate of Amendment of Certificate of Incorporation of ARIAD Pharmaceuticals, Inc., dated June 20, 2013 | 8-K (Exhibit 3.1) |
06/24/13 | 000-21696 | ||||||||
| .3 | Amended Certificate of Designation of Series A Preferred Stock of ARIAD Pharmaceuticals, Inc., dated November 1, 2013 | 8-K/A (Exhibit 3.1) |
11/05/13 | 001-36172 | ||||||||
| 3.2 | ARIAD Pharmaceuticals, Inc. Amended and Restated Bylaws | 8-K (Exhibit 3.1) |
05/01/14 | 001-36172 | ||||||||
| 4.1 | Specimen common stock certificate of ARIAD Pharmaceuticals, Inc. | 10-K (Exhibit 4.1) |
03/03/14 | 001-36172 | ||||||||
| 4.2 | .1 | Section 382 Rights Agreement, dated October 31, 2013, between ARIAD Pharmaceuticals, Inc. and Computershare Trust Company, N.A., as Rights Agent | 8-K (Exhibit 4.1) |
11/01/13 | 000-21696 | |||||||
| .2 | Amendment to Section 382 Rights Agreement, dated June 24, 2014, between ARIAD Pharmaceuticals, Inc. and Computershare Trust Company, N.A., as Rights Agent | 8-K (Exhibit 4.1) |
06/24/14 | 001-36172 | ||||||||
| 4.3 | Indenture, dated June 17, 2014 by and between ARIAD Pharmaceuticals, Inc. and Wells Fargo Bank, National Association, as trustee | 8-K (Exhibit 4.1) |
06/17/14 | 001-36172 | ||||||||
| 4.4 | Form of 3.625 percent Convertible Senior Note due 2019 (included in Exhibit 4.3) | 8-K (Exhibit 4.1) |
06/17/14 | 001-36172 | ||||||||
| 4.5 | Convertible Note Hedge Confirmation, dated June 12, 2014, by and between ARIAD Pharmaceuticals, Inc. and JP Morgan Chase Bank, National Association | 8-K (Exhibit 10.2) |
06/17/14 | 001-36172 | ||||||||
| 4.6 | Warrant Confirmation, dated June 12, 2014, by and between ARIAD Pharmaceuticals, Inc. and JP Morgan Chase Bank, National Association | 8-K (Exhibit 10.3) |
06/17/14 | 001-36172 | ||||||||
| Lease Agreements |
||||||||||||
| 10.1 | .1 | Lease Agreement, dated January 8, 1992, between ARIAD Pharmaceuticals, Inc. and Forest City Cambridge, Inc. | 10-Q (Exhibit 10.1) |
04/30/93 | 000-21696 | |||||||
| .2 | Eighth Amendment to Lease dated October 30, 2006 | 10-K (Exhibit 10.57) |
03/14/07 | 000-21696 | ||||||||
| .3 | Ninth Amendment to Lease dated May 20, 2011, between ARIAD Corporation and UP 26 Landsdowne LLC | 10-Q (Exhibit 10.1) |
08/09/11 | 000-21696 | ||||||||
| .4 | Assignment and Assumption dated December 31, 2011, by and between ARIAD Corporation and ARIAD Pharmaceuticals, Inc. (for lease at 26 Landsdowne Street) | 10-K (Exhibit 10.1.4) |
02/29/12 | 000-21696 | ||||||||
| 10.2 | .1 | Lease Agreement, dated January 4, 2013 between ARIAD Pharmaceuticals, Inc. and ARE-MA REGION NO. 48, LLC (for lease at 75 Binney Street and 25 Binney Street)* | 10-K (Exhibit 10.2) |
03/01/13 | 000-21696 | |||||||
120
Table of Contents
| Exhibit Number |
Exhibit Description |
Filed with this Report |
Incorporated by |
Filing Date |
SEC File/ Reg. Number | |||||||
| .2 | First Amendment to Lease, dated September 16, 2013, between ARIAD Pharmaceuticals, Inc. and ARE-MA REGION NO. 48, LLC (for lease at 75 Binney Street and 25 Binney Street)* | 10-Q (Exhibit 10.1) |
11/12/13 | 001-36172 | ||||||||
| Collaboration, License and Commercial Agreements | ||||||||||||
| 10.3 | Amended and Restated Agreement, dated as of December 12, 1997, between The Board of Trustees of The Leland Stanford Junior University and ARIAD Gene Therapeutics, Inc.* | 10-K (Exhibit 10.14) |
03/10/98 | 000-21696 | ||||||||
| 10.4 | License Agreement, effective January 26, 2005, by and between ARIAD Pharmaceuticals, Inc. and Medinol Ltd.* | 10-Q (Exhibit 10.1) |
05/10/05 | 000-21696 | ||||||||
| 10.5 | Supply Agreement, entered into as of January 26, 2005, by and between ARIAD Pharmaceuticals, Inc. and Medinol Ltd.* | 10-Q (Exhibit 10.2) |
05/10/05 | 000-21696 | ||||||||
| 10.6 | License Agreement, dated October 9, 2007, among ARIAD Pharmaceuticals, Inc., ARIAD Gene Therapeutics, Inc. and ICON Medical Corp.* | 10-K (Exhibit 10.13) |
03/16/10 | 000-21696 | ||||||||
| 10.7 | Master Services Agreement, effective as of January 21, 2014, between ARIAD Pharmaceuticals, Inc. and Biologics, Inc. * | 10-K (Exhibit 10.12) |
03/03/14 | 001-36172 | ||||||||
| 10.8 | Collaboration Agreement, effective December 22, 2014, by and between ARIAD Pharmaceuticals, Inc. and Otsuka Pharmaceutical Co., Ltd. ** | X | ||||||||||
| 10.9 | .1 | Amended and Restated License Agreement, dated March 7, 2011, by and between ARIAD Pharmaceuticals, Inc. and Bellicum Pharmaceuticals, Inc.** | X | |||||||||
| .2 | Omnibus Amendment Agreement, dated October 3, 2014, by and between ARIAD Pharmaceuticals, Inc. and Bellicum Pharmaceuticals, Inc.** | X | ||||||||||
| Agreements with Executive Officers and Directors |
||||||||||||
| 10.10 | Amended and Restated Executive Employment Agreement, dated April 30, 2010, between ARIAD Pharmaceuticals, Inc. and Harvey J. Berger, M.D.+ | 8-K (Exhibit 10.1) |
05/03/10 | 000-21696 | ||||||||
| 10.11 | Amended and Restated Executive Employment Agreement, dated May 15, 2010, between ARIAD Pharmaceuticals, Inc. and David L. Berstein, Esq.+ | 10-Q (Exhibit 10.4) |
08/09/10 | 000-21696 | ||||||||
| 10.12 | Amended and Restated Executive Employment Agreement, dated May 15, 2010, between ARIAD Pharmaceuticals, Inc. and Daniel M. Bollag, Ph.D.+ | 10-Q (Exhibit 10.5) |
08/09/10 | 000-21696 | ||||||||
| 10.13 | .1 | Amended and Restated Executive Employment Agreement, dated May 1, 2010, between ARIAD Pharmaceuticals, Inc. and Maria Cantor+ | 10-Q (Exhibit 10.1) |
05/09/12 | 000-21696 | |||||||
| .2 | First Amendment to Amended and Restated Executive Employment Agreement, dated January 25, 2012, between ARIAD Pharmaceuticals, Inc. and Maria Cantor+ | 10-Q (Exhibit 10.2) |
05/09/12 | 000-21696 | ||||||||
| 10.14 | Amended and Restated Executive Employment Agreement, dated May 15, 2010, between ARIAD Pharmaceuticals, Inc. and Timothy P. Clackson, Ph.D.+ | 10-Q (Exhibit 10.6) |
08/09/10 | 000-21696 | ||||||||
| 10.15 | Executive Employment Agreement, dated February 28, 2014, between ARIAD Pharmaceuticals, Inc., and Hugh M. Cole+ | 10-Q (Exhibit 10.4) |
05/09/14 | 001-36172 | ||||||||
121
Table of Contents
| Exhibit Number |
Exhibit Description |
Filed with this Report |
Incorporated by |
Filing Date |
SEC File/ Reg. Number | |||||||
| 10.16 | Executive Employment Agreement, dated January 20, 2015, between ARIAD Pharmaceuticals, Inc., and Thomas J. DesRosier, Esq.+ | X | ||||||||||
| 10.17 | Executive Employment Agreement, dated September 3, 2011, between ARIAD Pharmaceuticals, Inc. and Martin J. Duvall+ | 10-Q (Exhibit 10.1) |
11/07/11 | 000-21696 | ||||||||
| 10.18 | Amended and Restated Executive Employment Agreement, dated May 15, 2010, between ARIAD Pharmaceuticals, Inc. and Edward M. Fitzgerald+ | 10-Q (Exhibit 10.8) |
08/09/10 | 000-21696 | ||||||||
| 10.19 | .1 | Amended and Restated Executive Employment Agreement, dated May 1, 2010, between ARIAD Pharmaceuticals, Inc. and Frank G. Haluska, M.D., Ph.D.+ | 10-Q (Exhibit 10.9) |
08/09/10 | 000-21696 | |||||||
| .2 | First Amendment to Amended and Restated Executive Employment Agreement, dated January 25, 2012 between ARIAD Pharmaceuticals, Inc. and Frank G. Haluska, M.D., Ph.D.+ | 10-Q (Exhibit 10.3) |
05/09/12 | 000-21696 | ||||||||
| 10.20 | Amendments to Executive Employment Agreements, dated April 28, 2014, for David L. Berstein, Esq., Daniel M. Bollag, Ph.D., Maria E. Cantor, Timothy P. Clackson, Ph.D., Martin J. Duvall, Edward M. Fitzgerald, and Frank G. Haluska, M.D., Ph.D. (solely to extend term)+ | 8-K (Exhibit 10.1) |
05/01/14 | 001-36172 | ||||||||
| 10.21 | Nomination and Standstill Agreement, dated February 20, 2014, by and between ARIAD Pharmaceuticals, Inc., Dr. Alexander J. Denner, Sarissa Capital Management LP, Sarissa Capital Domestic Fund LP, Sarissa Capital Offshore Master Fund LP, Sarissa Capital Fund GP LP and Sarissa Capital Offshore Fund GP LLC+ | 8-K (Exhibit 99.1) |
02/21/14 | 001-36172 | ||||||||
| 10.22 | Confidentiality Agreement, dated February 20, 2014, by and between ARIAD Pharmaceuticals, Inc., Dr. Alexander J. Denner, Sarissa Capital Management LP, Sarissa Capital Domestic Fund LP, Sarissa Capital Offshore Master Fund LP, Sarissa Capital Fund GP LP and Sarissa Capital Offshore Fund GP LLC+ | 8-K (Exhibit 99.2) |
02/21/14 | 001-36172 | ||||||||
| 10.23 | Form of Indemnity Agreement between ARIAD Pharmaceuticals, Inc. and its directors and officers+ | 10-K (Exhibit 10.33) |
03/16/09 | 000-21696 | ||||||||
| Equity and Other Compensation Plans |
||||||||||||
| 10.24 | .1 | ARIAD Pharmaceuticals, Inc. 1997 Executive Compensation Plan+ | 10-K (Exhibit 10.41) |
03/10/98 | 000-21696 | |||||||
| .2 | Amendment to ARIAD Pharmaceuticals, Inc. 1997 Executive Compensation Plan+ | 10-Q (Exhibit 10.2) |
11/09/05 | 000-21696 | ||||||||
| 10.25 | ARIAD Pharmaceuticals, Inc. 2005 Executive Compensation Plan (as amended and restated effective October 1, 2008)+ | 10-K (Exhibit 10.31) |
03/16/09 | 000-21696 | ||||||||
| 10.26 | Director Compensation Arrangements+ | 10-K (Exhibit 10.25) |
03/03/14 | 001-36172 | ||||||||
| 10.27 | .1 | ARIAD Pharmaceuticals, Inc. 1991 Stock Option Plan for Employees and Consultants, as amended+ | 10-K (Exhibit 10.13) |
03/31/95 | 000-21696 | |||||||
| .2 | Amendment to the 1991 Stock Option Plan for Employees and Consultants+ | 10-Q (Exhibit 10.36) |
08/12/97 | 000-21696 | ||||||||
| 10.28 | ARIAD Pharmaceuticals, Inc. 1991 Stock Option Plan for Directors+ | 10-Q (Exhibit 10.15) |
04/30/93 | 000-21696 | ||||||||
| 10.29 | .1 | ARIAD Pharmaceuticals, Inc. 1994 Stock Option Plan for Non-Employee Directors+ | 10-K (Exhibit 10.24) |
03/31/95 | 000-21696 | |||||||
122
Table of Contents
| Exhibit Number |
Exhibit Description |
Filed with this Report |
Incorporated by |
Filing Date |
SEC File/ Reg. Number | |||||||
| .2 | Amendment to the 1994 Stock Option Plan for Non-Employee Directors.+ | 10-Q (Exhibit 10.37) |
08/12/97 | 000-21696 | ||||||||
| 10.30 | Amended and Restated ARIAD Pharmaceuticals, Inc. 1997 Employee Stock Purchase Plan, as amended+ | Def 14A (Appendix C) |
05/09/14 | 000-36172 | ||||||||
| 10.31 | ARIAD Pharmaceuticals, Inc. 2001 Stock Plan, as amended and restated+ | 10-Q (Exhibit 10.3) |
11/09/05 | 000-21696 | ||||||||
| 10.32 | .1 | ARIAD Pharmaceuticals, Inc. 2006 Long-Term Incentive Plan, as amended+ | Def 14A (Appendix A) |
04/30/12 | 000-21696 | |||||||
| .2 | Form of Stock Option Certificate under the ARIAD Pharmaceuticals, Inc. 2006 Long-Term Incentive Plan+ | 10-K (Exhibit 10.30.2) |
02/29/12 | 000-21696 | ||||||||
| .3 | Form of Stock Grant Certificate under the ARIAD Pharmaceuticals, Inc. 2006 Long-Term Incentive Plan+ | 10-K (Exhibit 10.30.3) |
02/29/12 | 000-21696 | ||||||||
| .4 | Form of Restricted Stock Unit Certificate under the ARIAD Pharmaceuticals, Inc. 2006 Long-Term Incentive Plan+ | 10-K (Exhibit 10.30.4) |
02/29/12 | 000-21696 | ||||||||
| .5 | Form of Restricted Stock Grant Certificate under the ARIAD Pharmaceuticals, Inc. 2006 Long-Term Incentive Plan+ | 10-K (Exhibit 10.30.5) |
02/29/12 | 000-21696 | ||||||||
| .6 | Form of 2012 Performance Share Certificate under the ARIAD Pharmaceuticals, Inc. 2006 Long-Term Incentive Plan+ | 10-Q (Exhibit 10.5) |
05/09/12 | 000-21696 | ||||||||
| 10.33 | .1 | ARIAD Pharmaceuticals, Inc. 2014 Long-Term Incentive Plan | Def 14A (Appendix B) |
05/09/14 | 001-36172 | |||||||
| .2 | Form of Option Agreement under the ARIAD Pharmaceuticals, Inc. 2014 Long-Term Incentive Plan+ | 10-Q (Exhibit 10.1) |
11/07/14 | 001-36172 | ||||||||
| .3 | Form of Restricted Stock Unit Agreement under the ARIAD Pharmaceuticals, Inc. 2014 Long-Term Incentive Plan | 10-Q (Exhibit 10.2) |
11/07/14 | 001-36172 | ||||||||
| .4 | Form of Restricted Stock Agreement under the ARIAD Pharmaceuticals, Inc. 2014 Long-Term Incentive Plan | 10-Q (Exhibit 10.3) |
11/07/14 | 001-36172 | ||||||||
| .5 | Form of Performance Share Agreement under the ARIAD Pharmaceuticals, Inc. 2014 Long-Term Incentive Plan | 10-Q (Exhibit 10.4) |
11/07/14 | 001-36172 | ||||||||
| 21.1 | Subsidiaries of ARIAD Pharmaceuticals, Inc. | X | ||||||||||
| 23.1 | Consent of Deloitte & Touche LLP | X | ||||||||||
| 31.1 | Certification of the Chief Executive Officer | X | ||||||||||
| 31.2 | Certification of the Chief Financial Officer | X | ||||||||||
| 32.1 | Certification pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 | X | ||||||||||
| 101 | The following materials from ARIAD Pharmaceuticals, Inc.’s Annual Report on Form 10-K for the year ended December 31, 2014, formatted in XBRL (eXtensible Business Reporting Language): (i) Consolidated Balance Sheets, (ii) Consolidated Statements of Operations and Comprehensive Loss, (iii) Consolidated Statements of Stockholders’ Equity, (iv) Consolidated Statements of Cash Flows, and (v) Notes to Consolidated Financial Statements. | X | ||||||||||
| (+) | Management contract or compensatory plan or arrangement. |
| (*) | Confidential treatment has been granted by the Securities and Exchange Commission as to certain portions. |
| (**) | Confidential treatment has been requested from the Securities and Exchange Commission as to certain portions. |
123
