Attached files
| file | filename |
|---|---|
| EXCEL - IDEA: XBRL DOCUMENT - ACURA PHARMACEUTICALS, INC | Financial_Report.xls |
| EX-32 - EXHIBIT 32 - ACURA PHARMACEUTICALS, INC | v400005_ex32.htm |
| EX-10.9 - EXHIBIT 10.9 - ACURA PHARMACEUTICALS, INC | v400005_ex10-9.htm |
| EX-31.1 - EXHIBIT 31.1 - ACURA PHARMACEUTICALS, INC | v400005_ex31-1.htm |
| EX-31.2 - EXHIBIT 31.2 - ACURA PHARMACEUTICALS, INC | v400005_ex31-2.htm |
| EX-10.8 - EXHIBIT 10.8 - ACURA PHARMACEUTICALS, INC | v400005_ex10-8.htm |
| EX-23.1 - EXHIBIT 23.1 - ACURA PHARMACEUTICALS, INC | v400005_ex23-1.htm |
| EX-10.10 - EXHIBIT 10.10 - ACURA PHARMACEUTICALS, INC | v400005_ex10-10.htm |
| EX-10.13 - EXHIBIT 10.13 - ACURA PHARMACEUTICALS, INC | v400005_ex10-13.htm |
| EX-10.11 - EXHIBIT 10.11 - ACURA PHARMACEUTICALS, INC | v400005_ex10-11.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
| (Mark One) | |
| x |
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 FOR THE FISCAL YEAR ENDED DECEMBER 31, 2014 |
| Or | |
| ¨ |
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 For the transition period from _____ to _____ |
Commission file number 1-10113
ACURA PHARMACEUTICALS, INC.
(Exact name of registrant as specified in its charter)
| New York | 11-0853640 |
| (State or other jurisdiction of Incorporation or organization) | (I.R.S. Employer Identification No.) |
| 616 N. North Court, Suite 120, Palatine, Illinois | 60067 |
| (Address of principal administrative office) | (Zip code) |
Registrant's telephone number, including area code: 847 705 7709
|
Securities registered pursuant to section 12(b) of the Act: Common Stock, par value $0.01 per share |
Name of each exchange on which registered: NASDAQ Capital Market |
Securities registered pursuant to section 12(g) of the Act:
(Title of Class)
None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act.
Yes ¨ No x
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act.
Yes ¨ No x
Indicate by check mark whether the registrant: (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes x No ¨
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes x No ¨
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (§229.405 of this chapter is not contained herein, and will not be contained, to the best of registrant's knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. x
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company.
¨ Large Accelerated Filer ¨ Accelerated Filer ¨ Non-Accelerated Filer x Smaller Reporting Company.
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes o No x
Based on the last sale price on the NASDAQ Capital Market of the Common Stock of $1.09 on June 30, 2014 (the last business day of the registrant's most recently completed second fiscal quarter), the aggregate market value of the voting stock held by non-affiliates of the registrant was approximately $29.0 million.
As of February 27, 2015, the registrant had 48,947,247 shares of Common Stock, par value $0.01, outstanding.
DOCUMENTS INCORPORATED BY REFERENCE: Portions of the Proxy Statement for the registrant’s Annual Meeting of Shareholders to be held on or about April 30, 2015 are incorporated by reference into Part III of this Annual Report on Form 10-K.
Acura Pharmaceuticals, Inc.
Form 10-K
For the Fiscal Year Ended December 31, 2014
Tablet of Contents
| (i) |
Forward-Looking Statements
Certain statements in this Report constitute “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995. Such forward-looking statements involve known and unknown risks, uncertainties and other factors which may cause our actual results, performance or achievements to be materially different from any future results, performance, or achievements expressed or implied by such forward-looking statements. Forward-looking statements may include, but are not limited to:
| · | our and our licensee’s ability to successfully launch and commercialize our products and technologies, including Oxaydo Tablets and our Nexafed products; |
| · | the pricing and price discounting that may be offered by Egalet for Oxaydo; |
| · | the results of our development of our Limitx™ technology; |
| · | our ability to fund, or obtain funding for, products developed utilizing our Aversion®, Impede® and Limitx™ technologies; |
| · | the results of our meetings or discussions with the U.S. Food and Drug Administration (“FDA”), or any appeals of prior FDA determinations, relating to our Aversion hydrocodone/acetaminophen product; |
| · | whether the results of studies AP-ADF-302, AP-ADF-303, and AP-ADF-304 relating to our Aversion hydrocodone/acetaminophen product will be acceptable to the FDA; |
| · | whether we will conduct an additional intranasal abuse liability study on our Aversion hydrocodone/acetaminophen product and, if conducted, whether the results of such study will support the filing of a New Drug Application and/or a claim of intranasal abuse deterrence; |
| · | our and our licensee’s ability to obtain necessary regulatory approvals and commercialize
products utilizing our technologies; |
| · | the market acceptance of and competitive environment for any of our products; |
| · | the willingness of wholesalers and pharmacies to stock our Nexafed products; |
| · | expectations regarding potential market share for our products and the timing of first sales; |
| · | our ability to enter into additional license agreements for our Aversion Technology product candidates; |
| · | our exposure to product liability and other lawsuits in connection with the commercialization of our products; |
| · | the increasing cost of insurance and the availability of product liability insurance coverage; |
| · | the ability to avoid infringement of patents, trademarks and other proprietary rights of third parties; |
| · | the ability of our patents to protect our products from generic competition and our ability to protect and enforce our patent rights in any paragraph IV patent infringement litigation; |
| · | the ability to fulfill the FDA requirements for approving our product candidates for commercial manufacturing and distribution in the United States, including, without limitation, the adequacy of the results of the laboratory and clinical studies completed to date, the results of laboratory and clinical studies we may complete in the future to support FDA approval of our product candidates and the sufficiency of our development process to meet over-the-counter (“OTC”) Monograph standards as applicable; |
| 1 |
| · | the adequacy of the development program for our product candidates, including whether additional clinical studies will be required to support FDA approval of our product candidates; |
| · | changes in regulatory requirements; |
| · | adverse safety findings relating to our commercialized products or product candidates in development; |
| · | whether the FDA will agree with our analysis of our clinical and laboratory studies; |
| · | whether further studies of our product candidates will be required to support FDA approval; |
| · | whether or when we are able to obtain FDA approval of labeling for our product candidates for the proposed indications and will be able to promote the features of our abuse discouraging technologies; and |
| · | whether Oxaydo or our Aversion and Limitx™ product candidates will ultimately deter abuse in commercial settings and whether our Nexafed products and Impede technology product candidates will disrupt the processing of pseudoephedrine into methamphetamine. |
In some cases, you can identify forward-looking statements by terms such as “may,” “will,” “should,” “could,” “would,” “expects,” “plans,” “anticipates,” “believes,” “indicates,” “estimates,” “projects,” “predicts,” “potential” and similar expressions intended to identify forward-looking statements. These statements reflect our current views with respect to future events and are based on assumptions and subject to risks and uncertainties. Given these uncertainties, you should not place undue reliance on these forward-looking statements. We discuss many of these risks in greater detail in Item 1A of this Report. In light of these risks, uncertainties and assumptions, the forward-looking events and circumstances discussed in this Report may not occur and actual results could differ materially and adversely from those anticipated or implied in the forward-looking statements.
Unless required by law, we undertake no obligation to update or revise any forward-looking statements to reflect new information or future events or developments. Accordingly, you should not assume that our silence over time means that actual events are bearing out as expressed or implied in such forward-looking statements.
| 2 |
Overview
We are a specialty pharmaceutical company engaged in the research, development and commercialization of technologies and products intended to address medication abuse and misuse. We have discovered and developed three proprietary platform technologies which can be used to develop multiple products. Our Aversion® Technology is a mixture of inactive ingredients incorporated into pharmaceutical tablets and capsules intended to address some common methods of product tampering associated with opioid abuse. Oxaydo™ Tablets (formerly known as Oxecta®)(oxycodone HCl, CII), is the first approved product utilizing Aversion in the United States and we have 7 additional opioid products utilizing Aversion in various stages of development. On January 7, 2015, we entered into a Collaboration and License Agreement with Egalet US, Inc. and Egalet Ltd., each a subsidiary of Egalet Corporation (collectively, “Egalet”) pursuant to which we exclusively licensed to Egalet worldwide rights to manufacture and commercialize Oxaydo. Oxaydo is currently approved by the FDA for marketing in the United States in 5mg and 7.5 mg strengths. We are advised that Egalet plans to launch Oxaydo in the United States in the third quarter of 2015. We have also developed Impede® Technology which is a combination of inactive ingredients that prevent the extraction of pseudoephedrine from tablets and disrupt the direct conversion of pseudoephedrine from tablets into methamphetamine. We launched our first Impede Technology product, Nexafed®, into the United States market in December 2012 and launch our Nexafed Sinus Pressure + Pain product in the United States in February 2015. We have multiple pseudoephedrine products in development utilizing our Impede Technology. In August 2014, we received a grant from the National Institute on Drug Abuse to advance early stage development of our third abuse deterrent technology, Limitx™. Limitx is designed to retard the release of active drug ingredients when too many tablets are accidently or purposefully ingested.
Opioid analgesics are one of the largest prescription drug markets in the United States with 250 million prescriptions dispensed in 2014. Prescription opioids are also the most widely abused drugs with 12 million people abusing or misusing these products annually. We expect our Aversion Technology opioid products to compete primarily in the immediate-release opioid product segment. Because immediate-release opioid products are used for both acute and chronic pain, a prescription, on average, contains 65 tablets or capsules. According to IMS Health, in 2014, sales in the immediate-release opioid product segment were approximately 235 million prescriptions and $3.0 billion, of which ~98% was attributable to generic products. Immediate-release oxycodone tablets represent 14.8 million of these prescriptions or almost 1.6 billion tablets. The FDA approved label for our Oxaydo product describes the unique, and we believe promotable, abuse deterrent features of our product which we believe makes prescribing our product attractive to some healthcare providers.
Hydrocodone bitartrate with acetaminophen, or hydrocodone/acetaminophen, is the most widely prescribed and often abused opioid product in the United States. Our Aversion hydrocodone/acetaminophen product is our most advanced opioid product in development and the primary focus of our opioid development efforts. On December 5, 2013, we met with the FDA to discuss the results of Study AP-ADF-301, or Study 301, a key abuse liability study for our Aversion hydrocodone/acetaminophen product and whether the results from Study 301 are acceptable for submission in a New Drug Application, or NDA. On May 27, 2014, we announced that the FDA advised us that the data from our Study 301 was insufficient to support an intranasal abuse deterrence description in our product labeling. The FDA indicated that a product will have to have an impact on “drug liking” to support a description of abuse-deterrence through a relevant route of abuse. The FDA’s advice also questioned whether the intranasal route is a relevant route of abuse for hydrocodone/ acetaminophen products. We met with the FDA on August 14, 2014 to discuss the development pathway for our Aversion hydrocodone/acetaminophen tablet development candidate, which is intended to provide abuse-deterrent features to address abuse by nasal snorting and injection. The FDA continues to question the relevance of abuse of hydrocodone with acetaminophen products by the intranasal route of administration.
| 3 |
On September 11, 2014, we submitted a formal dispute resolution request with the FDA. The dispute pertains to the FDA's determination that nasal snorting abuse of hydrocodone with acetaminophen products lacks relevance. On October 16, 2014, we announced that the FDA denied on procedural grounds our appeal of the position taken by the Division of Anesthesia, Analgesia and Addiction Products (“DAAAP”) that abuse by snorting hydrocodone with acetaminophen products lacks relevance. In a letter decision from the Office of Drug Evaluation II, the FDA indicated that DAAAP’s comments and correspondence with us to date, as well as the FDA’s Draft Guidance on abuse deterrent opioids, should be viewed only as recommendations and opinions, and do not preclude us from completing clinical development of our hydrocodone with acetaminophen product and submitting an NDA for consideration by the FDA. We are currently assessing our development strategy for our Aversion hydrocodone with acetaminophen product, including the merits of appealing the FDA’s decision. Even if we were to file an appeal and succeed in such proceeding, in order to continue the development of our hydrocodone/acetaminophen product we will be required to conduct an additional abuse liability study that will need to demonstrate a statistically significant reduction in Drug Liking, of which no assurance can be provided. We are currently in the process of designing this next study and will make a decision on commencing this study in 2015.
We expect that the development program for all our Aversion opioid products in development will be consistent with that of Oxaydo and our hydrocodone/acetaminophen product candidate.
In 2009, the United States market for over-the-counter market, or OTC, cold and allergy products containing an oral nasal decongestant was approximately $1 billion. In 2012, the DEA reported 11,210 laboratory incidents involving the illegal use of OTC pseudoephedrine products to manufacture the highly addictive drug methamphetamine, or meth. According to the Substance Abuse and Mental Health Services Administration, users of methamphetamine surged in 2013 to 595,000 people up from 440,000 in 2012. Nexafed, our 30mg pseudoephedrine hydrochloride immediate-release tablet, is stocked in approximately 19% of the estimated 65,000 U.S. pharmacies. Many of these pharmacies are either actively recommending Nexafed to their patients or carry Nexafed as their only 30mg pseudoephedrine product. We launched our first line extension, Nexafed Sinus Pressure + Pain, a 30/325mg pseudoephedrine HCl and acetaminophen tablet using our Impede technology in February 2015. The category for meth-resistant pseudoephedrine products has also been the focus of some, as yet unsuccessful, state legislation seeking to incentivize consumers and pharmacists to utilize these meth-resistant technologies.
We have an active development program to develop an extended-release version of our Impede technology to capitalize on higher sales products in the category. We also are investigating new technologies that would improve on our meth-resistant capabilities.
We also have discovered an early-stage technology, Limitx™, which, in proof of concept laboratory tests, demonstrates the ability to limit the release of the active ingredient from tablets when multiple tablets are consumed simultaneously. We are currently undertaking formulation optimization work for a hydromorphone HCl product using our Limitx technology.
Our Strategy
Our goal is to become a leading specialty pharmaceutical company focused on addressing the growing societal problem of pharmaceutical drug abuse by developing a broad portfolio of products with abuse deterrent features and benefits. Specifically, we intend to:
| · | Capitalize on our experience and expertise in the research and development of technologies that address medication abuse and misuse. We have one FDA approved product containing our Aversion Technology that is expected to be launched in the United States by our licensee in the third quarter of 2015, and two products commercially launched containing our Impede Technology. We continue to invest in improvements in these technologies and innovate new technologies to address medication abuse and misuse. |
| · | Leverage our technologies by developing a full line of pharmaceutical products which utilize our proprietary technologies. Medication abuse and misuse is not limited to single drugs but often pervades entire drug categories. We intend to develop or collaborate with strategically focused pharmaceutical companies to develop multiple products in the prescription opioid and OTC cold/allergy markets with our technologies. |
| 4 |
| · | Commercialize our products with our internal resources or license to strategically focused companies in the United States and other geographic territories. We have developed a small infrastructure to commercialize our OTC products that utilize the Impede Technology. We have licensed our Oxaydo product to Egalet for commercialization and we are seeking licensing partners for our products in development utilizing our Aversion and Impede technologies. |
| · | Maintain an efficient internal cost structure. Our internal cost structure is focused on discovering new technologies and developing product formulations using those technologies. We also have a small, focused OTC marketing and sales team. We outsource many high cost elements of development and commercialization, such as clinical trials and commercial manufacturing that minimize required fixed overhead and capital investment and thereby reduces our business risk. |
| · | In-license or acquire technologies and/or products to expand our portfolio of technologies and products. We intend to pursue the in-license or acquisition of product candidates and technologies that will allow us to expand our portfolio of products. Such in-licensing or acquisition transactions, if successfully completed, of which no assurance can be given, may include product candidates or technologies for pain relief, addiction, and other drugs. |
Aversion Technology Overview
Aversion Technology is a unique composition of inactive pharmaceutical ingredients utilized with an opioid or other drug susceptible of abuse to provide abuse deterrent functionality. All of our Aversion Technology opioid products are covered by claims in five issued U.S. patents, which expire between 2023 and 2025. Our Aversion Technology products are intended to provide the same therapeutic benefits of the active drug ingredient as currently marketed products containing the same active pharmaceutical ingredient, while simultaneously discouraging the following common methods of pharmaceutical product misuse and abuse:
· Drug abusers may dissolve pharmaceutical tablets or capsules in water, alcohol, or other common solvents, filter the dissolved solution into a syringe, and inject the resulting fluid intravenously to obtain euphoric effects. Aversion Technology tablets dissolved in generally available solvents, including water or alcohol, into a volume and form suitable for intravenous injection, converts the tablet into a viscous gel mixture. We believe this gel will limit or impede drug abusers from extracting and injecting the active ingredients from our tablets.
· Drug abusers may crush pharmaceutical tablets or capsules and intranasally snort the resulting powder to absorb active ingredient through the nasal passages to obtain euphoric effects. The combination of Aversion Technology inactive ingredients is intended to induce nasal passage discomfort if the tablets are snorted. We believe products which utilize Aversion Technology may be disliked and will discourage prospective nasal drug abusers from snorting crushed tablets or capsules.
The extent and manner in which any of the features described above may be described in the FDA approved label for our pipeline products will be dependent on the results of and the acceptance by the FDA of our and our licensees’ studies for each product.
Oxaydo Tablets
Oxaydo (oxycodone HCI tablets) is a Schedule II narcotic indicated for the management of acute and chronic moderate to severe pain where the use of an opioid analgesic is appropriate. Oxaydo was FDA approved on June 17, 2011 and introduced, by our former licensee, Pfizer Inc., into the U.S. market in February 2012. Pfizer strategically deprioritized their efforts in immediate-release opioids commencing in summer of 2012 and we reacquired the rights to Oxaydo in April 2014, shortly after Pfizer initiated minimal, non-sales representative promotion in late 2013. On January 7, 2015, we entered into a Collaboration and License Agreement with Egalet pursuant to which we exclusively licensed to Egalet worldwide rights to manufacture and commercialize Oxaydo. Oxaydo is approved in 5mg and 7.5mg strengths. We are advised that Egalet plans to launch Oxaydo in the United States in the third quarter of 2015.
| 5 |
The 2014 market for immediate-release oxycodone products was 14.8 million dispensed prescription or 1.6 billion tablets. The current market is predominately serviced by generic formulations that contain no abuse deterrent features and sell for approximately $0.10 to $0.40 per tablet, depending on strength. Immediate-release opioids are prescribed by a broad cross-section of healthcare providers including primary care physicians, surgeons and pain specialists. We believe Oxaydo, given its differentiated label compared to generic products, can offer an alternative for opioid prescribing physicians concerned with the abuse or diversion for abuse of their prescriptions even at premium pricing to generics. Because the target audience for promoting our opioid products is so large, we will seek marketing partners to best capitalize on our opioid opportunities.
The safety and efficacy of Oxaydo 5mg and 7.5mg tablets was established by demonstrating bioequivalence to commercially available oxycodone immediate-release tablets in the fasted state. Oxaydo differs from oxycodone tablets when taken with a high fat meal though these differences are not considered clinically relevant, and Oxaydo can be taken without regard to food. The FDA-approved label for Oxaydo describes elements unique to our Aversion Technology, which differs from current commercially available oxycodone immediate-release tablets. The label for Oxaydo includes the results from a clinical study that evaluated the effects of nasally snorting crushed Oxaydo and commercially available oxycodone tablets, and limitations on exposing Oxaydo tablets to water and other solvents and administration through feeding tubes. The clinical study evaluated 40 non-dependent recreational opioid users, who self-administered the equivalent of 15mg of oxycodone. After accounting for a first sequence effect, the study demonstrated:
| · | 30% of subjects exposed to Oxaydo responded that they would not take the drug again compared to 5% of subjects exposed to immediate-release oxycodone; |
| · | subjects taking Oxaydo reported a higher incidence of nasopharyngeal and facial adverse events compared to immediate-release oxycodone; |
| · | a decreased ability to completely insufflate two crushed Oxaydo tablets within a fixed time period (21 of 40 subjects), while all subjects were able to completely insufflate the entire dose of immediate-release oxycodone; and |
| · | small numeric differences in the median and mean drug liking scores, which were lower in response to Oxaydo than immediate-release oxycodone. |
Although we believe these abuse deterrent characteristics differentiate Oxaydo from immediate-release oxycodone products currently on the market, consistent with FDA guidance which requires epidemiology studies to support a claim of abuse deterrence, the clinical significance of the difference in drug liking and difference in response to taking the drug again in this study has not been established. There is no evidence that Oxaydo has a reduced abuse liability compared to immediate release oxycodone. We have a post-approval commitment with the FDA to perform an epidemiology study to assess the actual impact on abuse of Oxaydo tablets.
Further, the Oxaydo product label guides patients not to crush and dissolve the tablets or pre-soak, lick or otherwise wet the tablets prior to administration. Similarly, caregivers are advised not to crush and dissolve the tablets or otherwise use Oxaydo for administration via nasogastric, gastric or other feeding tubes as it may cause an obstruction. Our laboratory studies demonstrated that the Oxaydo tablet may gel when Oxaydo is exposed to certain solvents, including water.
Aversion Technology Opioid Products in Development
We have the following opioid products utilizing our Aversion Technology in various stages of development:
| 6 |
| Aversion Technology Tablets | Comparable Brand Name1 |
Status | ||
| Hydrocodone bitartrate/acetaminophen |
Vicodin®, Lortab®, Norco® |
All clinical work is complete except a repeat nasal snorting abuse liability study will be required. We are assessing FDA’s view that abuse by nasal snorting lacks relevance before continuing the development program. | ||
| Hydromorphone HCl | Dilaudid® | Proof of Concept2 | ||
| Methadone HCl | Methadose | Proof of Concept2 | ||
| Morphine Sulfate | MSIR® | Proof of Concept2 | ||
| Oxycodone HCl/acetaminophen | Percocet® | Proof of Concept2 | ||
| Oxymorphone HCl | Opana® | Proof of Concept2 | ||
| Tramadol HCl | Ultram® | Proof of Concept2 |
1 Comparable Brand Name refers to currently marketed prescription products in the United States containing the same active analgesic ingredient(s) as in the corresponding Aversion Technology product.
2 Proof of Concept is attained upon demonstration of product stability and bioavailability parameters. All proof of concept formulations contain niacin (derived from the initial Aversion formulation) and will require reformulation.
We anticipate the development program for each of our Aversion opioid products will be consistent with that of Oxaydo. Because our products use known active ingredients in approved dosage strengths, the safety and efficacy of the Aversion opioid will be established by a series of pharmacokinetic studies demonstrating: (a) bioequivalence to an approved reference drug, (b) food effect of our formulations, and (c) dose proportionality of our formulation.
The abuse deterrent studies of the Aversion products will be consistent with FDA’s draft guidance for the development of abuse deterrent opioids with the objective to obtain a description of our studies and/or abuse deterrent features in the product’s label. These studies may include in vitro laboratory studies to determine, among other things, syringeability of the formulation, extractability of the opioid, and particle size of the crushed product. We also may conduct human abuse liability studies comparing the abuse liability of Aversion products to currently marketed products.
We may have to perform additional safety and/or efficacy assessment as may be identified by the FDA for each specific formulation during the IND or NDA phase of development. In accordance with the FDA draft guidance, we will likely have a post-approval requirement for each of our products, if approved, to perform an epidemiology study to assess the in-market impact on abuse of our formulation.
We believe that the time to develop each Aversion opioid product from IND to NDA submission can be as short as 18 months to 24 months, provided all studies meet their primary study objectives. There can be no assurance, however, that such development timeline will be achieved.
Aversion Hydrocodone/Acetaminophen Development
Our most advanced opioid development product is Aversion hydrocodone/acetaminophen. Our clinical development program for our hydrocodone/acetaminophen product is expected to consist of:
| · | A nasal abuse liability liking study in about 40 recreational drug users against a reference drug, or Study AP-ADF-301 (complete); |
| · | A pharmacokinetic study (Study AP-ADF-302) in about 36 fasted subjects to establish bioequivalence to the FDA’s reference listed drug and determine the food effect on our drug (complete); |
| · | A pharmacokinetic study (Study AP-ADF-303) in about 24 subjects demonstrating dose proportionality of our formulation (complete); |
| · | A pharmacokinetic study (Study AP-ADF-304) in about 24 subjects to establish safety compared to the reference listed drugs tramadol/acetaminophen (for acetaminophen) and hydrocodone bitartrate/ibuprofen (for hydrocodone) (complete); |
| · | Laboratory studies demonstrating extraction, syringing, swelling and particle size characteristics of our product (in progress); |
| · | An assessment of the routes of abuse of hydrocodone products (complete); and |
| · | An additional nasal abuse liability study in recreational drug users against a reference drug (under strategic review). |
| 7 |
On August 26, 2013, we announced top-line results from Study AP-ADF-301 (Study 301), a phase II clinical study in 40 recreational drug abusers assessing the abuse liability of snorting of our crushed hydrocodone/acetaminophen product. Study 301’s primary endpoint indicated Aversion hydrocodone/acetaminophen had slightly lower numeric mean maximum drug liking (Emax: 72.1) compared to an equivalent dose of a generic hydrocodone/acetaminophen tablet (Emax: 75.6) currently on the market, however these results were not statistically significant (p=0.22). The secondary endpoints demonstrated the effects of the Aversion ingredients on drug snorting. Aversion hydrocodone/acetaminophen’s mean minimum liking (Emin: 40.2) was less than the comparator (Emin: 50.4) (the difference being statistically significant at p=0.0003). The mean minimum drug liking for Aversion hydrocodone/acetaminophen and the placebo control were 40.2 and 48.8, respectively (the difference being statistically significant at p=0.0042). A score below 50 indicates a subject disliked the drug they were taking at some point during the treatment (a score of 50 means neither like or dislike), and a score greater than 50 indicates they liked the drug they were taking.
The mean minimum liking results correlated closely the Overall Drug Liking score (ODL) and Take Drug Again assessment (TDA). ODL assessed the subject like or dislike for the drug experience 12 hours after taking the dose. The ODL for Aversion hydrocodone/acetaminophen (52.7) was lower than the generic comparator (71.0) (the difference being statistically significant at p=0.0001) with a score of 50 indicating neither a like nor dislike. TDA assessed a subject’s willingness to take the drug again assessed 12 hours after taking the dose. The TDA for Aversion hydrocodone/acetaminophen (45.1) was lower than the generic comparator (71.0) (the difference being statistically significant at p=0.0001) with the Aversion hydrocodone/acetaminophen score below 50 indicating an unwillingness to take the drug again.
There were no serious adverse events reported for Aversion hydrocodone/acetaminophen. There was no sequence effect identified in the study but a carryover effect between the 5 study crossover periods was identified for the Emax measure but not the Emin measure. Due to this observed carryover effect, the FDA may review the results of our study differently than we have and/or limit the amount of data we collected in the label for our product if approved by the FDA. As such, we are strategically considering the need to complete an additional nasal abuse liability study.
On December 5, 2013, we met with FDA to discuss if the FDA will consider whether the results of Study 301 are acceptable for submission in a NDA. On May 27, 2014, we announced that the FDA advised us that the data from our Study 301 was insufficient to support an intranasal abuse deterrence claim. The FDA indicated that a product will have to have an impact on “drug liking” to support a claim of abuse-deterrence through a relevant route of abuse. The FDA’s advice also questioned whether the intranasal route is a relevant route of abuse for hydrocodone/ acetaminophen products and recommended that we identify variables that could have impacted the findings from Study 301 before considering or conducting an additional intranasal abuse liability study on our Aversion hydrocodone/ acetaminophen product. We have previously submitted a report to the FDA on the prevalence of abusing hydrocodone products by intranasal administration. We met with the FDA on August 14, 2014 to discuss the development pathway for our Aversion hydrocodone/acetaminophen tablet development candidate, which is intended to provide abuse-deterrent features to address abuse by nasal snorting and injection. The FDA continues to question the relevance of abuse of hydrocodone with acetaminophen products by the intranasal route of administration. The FDA indicated that we may conduct an additional nasal abuse liability study for our Aversion hydrocodone/acetaminophen product candidate.
On September 11, 2014, we submitted a formal dispute resolution request with the FDA. The dispute pertains to the FDA's determination that nasal snorting abuse of hydrocodone with acetaminophen products lacks relevance. We believe the available data, as contained in the multiple sources provided to the FDA, strongly supports the conclusion that hydrocodone containing products are known to be abused through snorting, a standard explicitly identified in FDA's January 2013 "Guidance for Industry Abuse-Deterrent Opioids — Evaluation and Labeling". On October 16, 2014, we announced that the FDA denied on procedural grounds our appeal of the position taken by the Division of Anesthesia, Analgesia and Addiction Products (“DAAAP”) that abuse by snorting hydrocodone with acetaminophen products lacks relevance. In a letter decision from the Office of Drug Evaluation II, the FDA indicated that DAAAP’s comments and correspondence with us to date, as well as the FDA’s Draft Guidance on abuse deterrent opioids, should be viewed only as recommendations and opinions, and do not preclude us from completing clinical development of our hydrocodone with acetaminophen product and submitting an NDA for consideration by the FDA. The FDA noted that an Advisory Committee meeting may greatly inform their considerations. The FDA letter ruling also advised us that we may appeal the decision of the Office of Drug Evaluation II to the next level within the FDA. We are currently assessing our development strategy for our Aversion hydrocodone with acetaminophen product, including the merits of appealing the FDA’s decision. Even if we were to file an appeal and succeed in such proceeding, in order to continue the development of our hydrocodone/acetaminophen product we will be required to conduct an additional abuse liability study that will need to demonstrate a statistically significant reduction in Drug Liking, of which no assurance can be provided. We are currently in the process of designing this next study and will make a decision on commencing this study in 2015.
| 8 |
We have completed scale-up activities for our Aversion hydrocodone/acetaminophen product at the proposed commercial manufacturer and have manufactured our registration batches. We have also completed the pharmacokinetic studies (302, 303 and 304) for Aversion hydrocodone/acetaminophen, the results of which have demonstrated conformance with the FDA’s standard for bioequivalence when compared to the reference drug, and demonstrated dose proportionality, or relatively consistent blood exposure, across all three dosage strengths. Such studies also evaluated blood levels of each of hydrocodone and acetaminophen compared to their respective comparator drugs, and demonstrated that our Aversion hydrocodone/acetaminophen blood levels of hydrocodone were consistent with the comparator product, while acetaminophen peak blood levels were 23% higher than the comparator product based on the geometric mean. A large variability in acetaminophen results was observed in the study. We believe the results of Studies 302, 303 and 304 satisfy the requirement for a NDA to establish the safety and pain efficacy of our Aversion hydrocodone/acetaminophen product, however, the interpretation of these results will be subject to FDA’s review and acceptance of our conclusions. Before submitting and NDA, we will need to complete an additional nasal abuse liability study which is currently undergoing an internal strategic review.
U.S. Market Opportunity for Opioid Analgesic Products Utilizing Aversion Technology
The misuse and abuse of controlled prescription drugs (CPDs) in general, and opioid analgesics in particular, continues to constitute a dynamic and challenging threat to the United States and is the nation’s fastest growing drug problem. Results from the 2013 National Drug Threat Assessment conducted by the DEA report that CPD rates of abuse remain high, with individuals abusing CPDs at a higher prevalence rate than any illicit drug except marijuana. Opioid analgesics are the most common type of CPDs taken illicitly and are the CPDs most commonly involved in overdose incidents. According to the Drug Abuse Warning Network (DAWN), the estimated number of emergency department visits involving nonmedical use of prescription opiates/ opioids increased 112 percent—84,671 to 179,787— between 2006 and 2010. Immediate release, or IR, opioid products comprise the vast majority of this abuse compared with extended release, or ER, opioid products.
It is estimated that more than 75 million people in the United States suffer from pain and the FDA estimates more than 45 million people receive a prescription for the opioid hydrocodone annually. For many pain sufferers, opioid analgesics provide their only pain relief. As a result, opioid analgesics are among the largest prescription drug classes in the United States with over 250 million tablet and capsule prescriptions dispensed in 2014 of which approximately 235 million were for IR opioid products and 15 million were for ER opioid products. However, physicians and other health care providers at times are reluctant to prescribe opioid analgesics for fear of misuse, abuse, and diversion of legitimate prescriptions for illicit use.
We expect our Aversion Technology opioid products, to compete primarily in the IR opioid product segment of the United States opioid analgesic market. Because IR opioid products are used for both acute and chronic pain, a prescription, on average, contains 65 tablets or capsules. According to IMS Health, in 2014, sales in the IR opioid product segment were approximately $3.0 billion, of which ~98% was attributable to generic products. Due to fewer identified competitors and the significantly larger market for dispensed prescriptions for IR opioid products compared to ER opioid products, we have initially focused on developing IR opioid products utilizing our Aversion Technology. Aversion oxycodone and our Aversion Technology products in development include the active opioid ingredients representing approximately 76% of the U.S. IR Opioid Product segment. A summary of the IR opioid product prescription data for 2014 is provided below:
| 9 |
IR Opioid Products(1) | 2014
US Prescriptions (Millions)(2) | % of Total | ||||||
| Hydrocodone | 119 | 50 | % | |||||
| Oxycodone | 54 | 23 | ||||||
| Tramadol | 45 | 19 | ||||||
| Codeine | 12 | 5 | ||||||
| 3 Others | 5 | 3 | ||||||
| Total | 235 | 100 | % | |||||
1 Includes all salts and
esters of the opioid and opioids in combination
with other active ingredients such as acetaminophen.
2 IMS Health, 2014
Despite considerable publicity regarding the abuse of OxyContin® extended-release tablets and other ER opioid products, U.S. government statistics suggest that far more people have used IR opioid products non-medically than ER opioid products. These statistics estimate that nearly four times as many people have misused the IR opioid products Vicodin®, Lortab® and Lorcet® (hydrocodone bitartrate/acetaminophen brands and generics) than OxyContin®. We estimate 60-95% of the 37 million lifetime U.S. opioid abusers have engaged in the non-medical use of the active ingredients in our IR opioid product candidates. As indicated in the following chart, the top five abused opioid products are available only as IR opioid products.
Lifetime Non-Medical Use of Selected Pain Relievers, Age 12 or Older: 2013
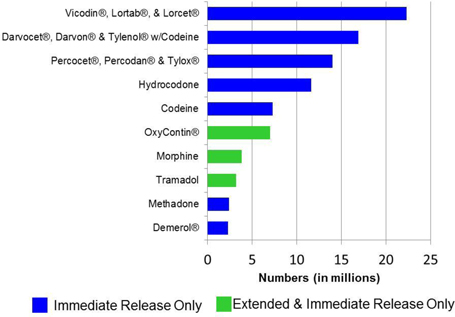
Source: SAMHSA, Office of Applied Studies, 2013 National Survey on Drug Use and Health.
In a 2011 survey of 400 opioid prescribing physicians conducted for us by an independent research firm, 39% of physicians indicated they were highly concerned with the diversion of their opioid prescriptions for non-medical purposes and 42% were highly concerned about opioid misuse by their patients. However, less than 17% of these same physicians indicated they were confident they could adequately identify patients who are diverting or misusing their opioid prescriptions. Further, 77% and 66% of the physicians indicated that abuse of their opioid prescription by injection and snorting, respectively, would likely lead to serious adverse health consequences for the abuser as compared to only 38% for abuse by oral administration.
| 10 |
A majority of pharmaceutical products in the United States are paid for by third-party payers such as insurers, pharmacy benefit managers, self-insured companies and the federal and state governments through Medicare, Medicaid and other health care programs. We believe our product candidates must demonstrate a clinical benefit to the patient and/or an economic benefit to third-party payers and/or a benefit to health care providers to receive favorable reimbursement status by the third-party payers, of which no assurance can be given.
Several independent organizations have estimated the potential cost impact of prescription opioid abuse to insurers. An analysis of health and pharmacy insurance claims between 1998 and 2002 for almost two million Americans conducted by Analysis Group, Inc. and others indicated that enrollees with a diagnosis of opioid abuse had average claims of approximately $14,000 per year higher than an age-gender matched non-opioid abuse sample. A 2007 report by the Coalition Against Insurance Fraud, after adjusting for inflation, estimated this excess cost per patient at more than $16,000 for 2007. By applying the U.S. government’s estimated 4.4 million annual opioid abusers, this organization concluded that abuse of IR and ER opioid products could cost health insurers up to $72.5 billion a year.
Product Labeling for Aversion Technology Products
In January 2013, the FDA published draft guidance for industry on the evaluation and labeling of abuse-deterrent opioids. While this guidance is non-binding on the FDA, it outlines FDA’s current thinking on the labeling of abuse-deterrent products. FDA encourages sponsors to seek approval of proposed product labeling that sets forth the results of physiochemical, physiologic, pharmacodynamic, pharmacokinetic, and/or formal post-marketing studies that appropriately characterizes the abuse-deterrent properties of a product. To date, FDA has limited data correlating the potentially abuse-deterrent properties of certain opioid drug products with actual reduction in abuse or adverse events associated with abuse. When the data predict or show that a product’s potentially abuse-deterrent properties can be expected to, or actually do, result in a significant reduction in that product’s abuse potential, these data, together with an accurate characterization of what the data mean, should be included in product labeling.
We or our licensee may seek to include descriptions of studies that characterize the abuse-deterrent properties in the label for our Aversion Technology products in development. Although the FDA approved label for Oxaydo contains limitations on exposing Oxaydo tablets to water and other solvents and administration through feeding tubes, the FDA approved Oxaydo label does not contain a description of the I.V. injection studies we performed to characterize the abuse deterrent properties of Oxaydo. We have committed to the FDA to undertake epidemiological studies to assess the actual consequences of abuse of Oxaydo in the market. The extent to which a description of the abuse-deterrent properties or results of epidemiological or other studies will be added to or included in the FDA approved product label for our products in development will be the subject of our discussions with the FDA as part of the NDA review process, even after having obtained approval of Oxaydo. Further, because the FDA closely regulates promotional materials, even if FDA initially approves labeling that includes a description of the abuse deterrent properties of the product, the FDA’s Office of Prescription Drug Promotion, or OPDP, will continue to review the acceptability of promotional labeling claims and product advertising campaigns for our marketed products.
Egalet Agreement Covering Oxaydo
On January 7, 2015, we and Egalet US, Inc. and Egalet Ltd., each a subsidiary of Egalet Corporation, (collectively, “Egalet”) entered into a Collaboration and License Agreement (the “Egalet Agreement”) to commercialize Oxaydo™ tablets containing our Aversion® Technology. Oxaydo is approved by the FDA for marketing in the United States in 5 mg and 7.5 mg strengths. Under the terms of the Egalet Agreement, we are transferring the approved NDA for Oxaydo to Egalet and Egalet is granted an exclusive license under our intellectual property rights for development and commercialization of Oxaydo worldwide (the “Territory”) in all strengths, subject to our right to co-promote Oxaydo in the United States.
| 11 |
In accordance with the Egalet Agreement, we and Egalet have formed a joint steering committee to coordinate commercialization strategies and the development of product line extensions. Egalet will pay a significant portion of the expenses relating to (i) annual NDA PDUFA product fees, (ii) expenses of the FDA required post-marketing study for Oxaydo and (iii) expenses of clinical studies for product line extensions (additional strengths) of Oxaydo for the United States and will bear all of the expenses of development and regulatory approval of Oxaydo for sale outside the United States. Egalet is responsible for all manufacturing and commercialization activities in the Territory for Oxaydo. Subject to certain exceptions, Egalet will have final decision making authority with respect to all development and commercialization activities for Oxaydo, including pricing, subject to our co-promotion right. Egalet may develop Oxaydo for other countries and in additional strengths, in its discretion.
Egalet paid us an upfront payment of $5 million upon signing of the Egalet Agreement and will pay us a $2.5 million milestone on the earlier to occur of (A) the launch of Oxaydo and (B) January 1, 2016, but in no event earlier than June 30, 2015. In addition, we will be entitled to a one-time $12.5 million milestone payment when worldwide Oxaydo net sales reach $150 million in a calendar year. In addition, we will receive from Egalet a stepped royalty at percentage rates ranging from mid-single digits to double-digits on net sales during a calendar year based on Oxaydo net sales during such year (excluding net sales resulting from our co-promotion efforts). In any calendar year in which net sales exceed a specified threshold, we will receive a double digit royalty on all Oxaydo net sales in that year (excluding net sales resulting from our co-promotion efforts). If we exercise our co-promotion rights, we will receive a share of the gross margin attributable to incremental Oxaydo net sales from our co-promotion activities. Egalet’s royalty payment obligations commence on the first commercial sale of Oxaydo and expire, on a country-by-country basis, upon the expiration of the last to expire valid patent claim covering Oxaydo in such country (or if there are no patent claims in such country, then upon the expiration of the last valid claim in the United States or the date when no valid and enforceable listable patent in the FDA’s Orange Book remains with respect to the Product). Royalties will be reduced upon the entry of generic equivalents, as well for payments required to be made by Egalet to acquire intellectual property rights to commercialize Oxaydo, with an aggregate minimum floor.
The Egalet Agreement expires upon the expiration of Egalet’s royalty payment obligations in all countries. Either party may terminate the Egalet Agreement in its entirety if the other party breaches a payment obligation, or otherwise materially breaches the Egalet Agreement, subject to applicable cure periods, or in the event the other party makes an assignment for the benefit of creditors, files a petition in bankruptcy or otherwise seeks relief under applicable bankruptcy laws. We also may terminate the Egalet Agreement with respect to the U.S. and other countries if Egalet materially breaches its commercialization obligations. Egalet may terminate the Egalet Agreement for convenience on 120 days prior written notice, which termination may not occur prior to the second anniversary of Egalet’s launch of Oxaydo. Egalet also may terminate the Agreement prior to the launch of Oxaydo on 30 days prior written notice upon the occurrence of serious safety issues, regulatory restrictions and intellectual property issues, in each case involving Oxaydo. Termination does not affect a party’s rights accrued prior thereto, but there are no stated payments in connection with termination other than payments of obligations previously accrued. For all terminations (but not expiration), the Egalet Agreement provides for the transition of development and marketing of Oxaydo from Egalet to us, including the conveyance by Egalet to us of the trademarks and all regulatory filings and approvals relating to Oxaydo, and for Egalet’s supply of Oxaydo for a transition period.
Impede 1.0 Technology
Our Impede 1.0 Technology, a proprietary mixture of inactive ingredients, prevents the extraction of pseudoephedrine, or PSE, from tablets using known extraction methods and disrupts the direct conversion of PSE from tablets into methamphetamine. The chemical structure of PSE is very similar to methamphetamine, facilitating a straight-forward chemical conversion to methamphetamine. OTC PSE products are sometimes purchased and used for this conversion. There are multiple known processes to convert PSE to methamphetamine, all of which are not complex and do not require specialized equipment; however, many do require readily available but uncommon ingredients. Two of the three most popular processes follow two general processing steps: (1) dissolving the PSE tablets in a solvent to isolate, by filtration, purified PSE and (2) a chemical reduction of the PSE into methamphetamine for drying into crystals. The third method, or the “one-pot” method, involves the direct chemical reduction of the PSE to methamphetamine in the presence of the tablet’s inactive ingredients. All the solvents used are ultimately dried off or otherwise removed so a vast range of solvents are amenable to the process.
| 12 |
Studies sponsored by us at an international, independent laboratory demonstrated our Impede 1.0 Technology prevents the extraction of PSE from tablets for conversion into methamphetamine using what we believe are the two most common extraction methods, each requiring extraction of PSE as an initial step. Laboratory tests conducted on our behalf by an independent Clinical Research Organization, or CRO, using the “one-pot” method demonstrated that our Impede Technology disrupted the direct conversion of PSE from the tablets into methamphetamine. The study compared the amount of pure methamphetamine hydrochloride produced from Nexafed and Johnson & Johnson’s Sudafed® tablets. Using one hundred 30 mg tablets of both products, multiple one-pot tests and a variety of commonly used solvents, the study demonstrated an average of 38% of the maximum 2.7 grams of pure methamphetamine hydrochloride was recovered from Nexafed. Comparatively, approximately twice as much pure methamphetamine hydrochloride was recovered from Sudafed tablets. Both products yielded a substantial amount of additional solids such that the purity of the total powder provided contained approximately 65% methamphetamine hydrochloride.
Impede 2.0 Technology
We have developed several next generation, or Impede 2.0, prototypes of our Impede Technology to improve the meth-resistance of our technology. We have completed one-pot, direct conversion meth testing performed by our CRO with results as follows:
| Product/Formulation | Meth Resistant Technology | Meth Recovery1 | Purity2 | |||||||
| Sudafed® 30mg Tablets | none | 67 | % | 62 | % | |||||
| Nexafed 30mg Technology | Impede® 1.0 | 38 | % | 65 | % | |||||
| Zephrex-D® 30mg Pills | Tarex® | 28 | % | 51 | % | |||||
| Nexafed 120mg Extended-release tablets | Impede® 2.0 | 17 | % | 34 | % | |||||
1 Total methamphetamine HCl recovered from 100 PSE 30mg tablets divided by the maximum theoretical yield of 2.7 grams.
2 Total methamphetamine HCl recovered from 100 PSE 30mg tablets divided by the total weight of powder recovered.
We are assessing two experimental formulations of Nexafed extended-release tablets in a pilot pharmacokinetic study compared to an FDA-approved 120mg PSE extended-release product. We expect this study to inform possible formulation changes before undertaking a formal bioequivalence study. We also are assessing the one-pot results of immediate-release Impede 2.0 formulations, along with manufacturability and other pertinent information to determine our strategy for introducing Impede 2.0 into our Nexafed product line.
Nexafed Products
Our Nexafed product line consists of immediate release tablets which utilize our patented Impede 1.0 Technology. Nexafed is a 30mg pseudoephedrine tablet and Nexafed Sinus Pressure + Pain is a 30/325 mg pseudoephedrine and acetaminophen tablet. PSE is a widely-used nasal decongestant available in many non-prescription and prescription cold, sinus and allergy products. While the 30mg PSE tablet is not the largest selling PSE product on the market, we believe it is the most often used product to make meth due to: (a) its relatively low selling price and (b) its simpler formulation provides better meth. However, as meth-resistant products become pervasive, we believe meth cooks will migrate to other, larger selling, PSE containing products.
We have demonstrated that our Nexafed 30mg tablets is bioequivalent to Johnson & Johnson’s Sudafed 30mg Tablets when a single 2 tablets dose is administered. Commencing in 2006, the CMEA, required all non-prescription PSE products to be held securely behind the pharmacy counter, has set monthly consumer purchase volume limits, and has necessitated consumer interaction with pharmacy personnel to purchase PSE-containing products. We are capitalizing on this consumer-pharmacist interaction at the point of sale by soliciting distribution to pharmacies and educating and encouraging pharmacists to recommend Nexafed to their customers. We are using telemarketing, direct mail, and online and journal advertising to educate pharmacists about Nexafed and encourage pharmacists to recommend Nexafed to their customers.
| 13 |
We launched Nexafed commercially in mid-December 2012 into the United States OTC market for cold and allergy products. We have built a distribution system of several regional and national drug wholesalers for redistribution to pharmacies which includes the three largest U.S. drug wholesalers: McKesson, Cardinal Health and AmerisourceBergen. We also ship directly to the warehouses of certain pharmacy chains. Nexafed is currently stocked in approximately 12,600 U.S. pharmacies or about 19% of the estimated 65,000 U.S. pharmacy outlets. Initial adoption was primarily in independent pharmacies in predominately rural communities with high meth awareness. Chain pharmacies, with more centralized control of the pharmacy operations, began adopting in mid-2013, including Kroger, Publix, Fruth and Bartells. Some pharmacists are actively recommending Nexafed to their customers while some have replaced all 30mg PSE products, brand and generic, with Nexafed. Rite Aid, the nation’s fourth largest pharmacy operator, began purchasing Nexafed in late 2013. In late 2014, Kmart and Kroger initiated chain-wide stocking of Nexafed.
We estimate that approximately 50% of Nexafed stocking pharmacies are repeat customers, excluding Rite Aid and Kroger which purchase directly from us and we therefore do not have individual store data.
In February 2015, we began initial shipments of Nexafed Sinus Pressure + Pain. We are marketing this product consistent with our Nexafed marketing efforts to pharmacists concerned with meth abuse of their products. We are not aware of any branded non-prescription product that contains PSE and acetaminophen believing that brands containing these ingredients have either been discontinued or reformulated with phenylephrine. We expect Nexafed Sinus Pressure + Pain to compete primarily against Advil® Cold and Sinus (PSE/ibuprofen) and to a lesser extent Aleve®-D and Sudafed® Pressure + Pain which are extended-release products.
We shipped approximately $161 thousand in Nexafed product during the quarter ended December 31, 2014 and $327 thousand during the year ended December 31, 2014. We are marketing our Nexafed product and our Nexafed Sinus Pressure + Pain product under FDA’s regulations applicable to OTC Monograph products. Nexafed and Nexafed Sinus Pressure + Pain tablets are offered in 24-count blister packaged cartons.
Impede Technology Products in Development
Given the fragmented nature of the PSE market with products containing multiple active ingredients, we are developing additional products for our Nexafed franchise:
| Impede Technology Product | Status | |
| Immediate-release pseudoephedrine HCl in combination with other cold and allergy active ingredients | Nexafed Sinus Pressure + Pain launched Other formulations being considered | |
| Extended-release formulation | Initial test formulations using Impede 2.0 undergoing pharmacokinetic testing |
We are undertaking pharmacokinetic testing of two different test formulations of an extended-release PSE product that have exhibit suitable in vitro release profiles against a comparator product. These test formulations contain Impede 2.0 technology. We currently expect to initiate a pre-IND meeting with the FDA to discuss the results from our pharmacokinetic and meth testing studies to determine the development path for our extended-release development product, which, we believe, will require and NDA or ANDA submission to the FDA.
Our objective is to establish our own Nexafed franchise in the United States with multiple product offerings, including both immediate and extended release products utilizing both single and combinations of active ingredients. We aim to make meth-resistant PSE product the standard of care in all U.S. pharmacies. We will evaluate possible licensing of our Impede Technology with commercial partners. Within the United States, we may consider licenses with appropriate partners that can: (a) help advance our distribution network with the goal of making meth-resistant products the standard of care in all U.S. pharmacies, and/or (b) extend our internal development resources to develop difficult to formulate products, such as extended-release.
| 14 |
U.S. Methamphetamine Problem and the Role Meth Resistant Technologies
Methamphetamine is a highly addictive illicit drug used non-medically by an estimated 12 million people at some point in their lifetime and 1.2 million in 2013. In 2006, the Combat Methamphetamine Epidemic Act, or CMEA, was enacted in response to an alarming increase in and widespread conversion of PSE containing products into methamphetamine. Among other things, CMEA, requires retail stores to maintain their inventory of PSE containing products in a secured location and restricts the amount of PSE products a store can sell to an individual customer. Implementation of CMEA initially reduced the number of illegal methamphetamine laboratory seizures reported by the Drug Enforcement Administration, or DEA, as the then most commonly used process for conversion of PSE to methamphetamine required substantial quantities of PSE. However, a newer process for converting PSE to methamphetamine requires less PSE. Possibly as a result of this new conversion process, the DEA reported 2010 clandestine methamphetamine laboratory seizures increased 84% over the low reported in 2007. Laboratory seizures were down 12% and 5.5% in 2011 and 2012, respectively, although certain states continue to see increases. In response to the ongoing methamphetamine problem, several local jurisdictions (state, counties and/or local municipalities) have enacted or propose to enact legislation to require a physician’s prescription to obtain a PSE-containing product or have tightened consumer purchase limits beyond that established by CMEA. Further, federal funding for federal meth lab clean-up has changed which may be impacting law enforcement’s policing and accounting of meth labs.
In January 2014, local media in Scott County Tennessee reported that substantially all pharmacies located in such county removed single ingredient PSE products from their shelves in favor of Nexafed. We believe similar changes took place in neighboring Campbell County. Based on local media reports, authorities in these counties subsequently reported a 90% and 88%, respectively, reduction in meth labs seizures.
In late 2013, West Virginia considered legislation requiring all PSE products to have a prescription with an exemption for meth-resistant products like Nexafed. Although this bill failed to pass, by the end of 2013, many West Virginia retailers, including Fruth’s and Rite Aid had voluntarily removed single-ingredient PSE products from their shelves, some in favor of using only Nexafed. In the first half of 2014, West Virginia seized 207 meth labs or a reported 25% reduction from 2013 year-to-date (there were 530 seizures in 2013). In July 2014, CVS pharmacies announced the removal of older single-ingredient PSE products from their West Virginia stores. We believe the vast majority of West Virginia pharmacies now stock either no single-ingredient PSE products or exclusively meth-resistant products. The West Virginia Gazette reported in December 2014 that authorities seized only 83 meth labs between July and November 2014, compared to 207 meth labs in the first half of 2014 and 530 for all of 2013.
The DEA may grant exemptions from the purchase requirements of PSE under the CMEA. We believe a more robust formulation along with in-market data demonstrating a reduction in meth lab incidents may qualify for this exemption, although there can be no assurance this will be the case.
U.S. Market Opportunity for Impede PSE Products
PSE is a widely-used nasal decongestant available in many non-prescription and prescription cold, sinus and allergy products. PSE is sold in products as the only active ingredient in both immediate and extended-release products. In addition, PSE is combined with other cold, sinus and allergy ingredients such as pain relievers, cough suppressants and antihistamines. PSE also competes against phenylephrine, an alternate nasal decongestant available in non-prescription products. In 2009, AC Nielsen reported approximately $1.0 billion in retail sales of non-prescription products containing either PSE or phenylephrine as a nasal decongestant, of which approximately 47% contained PSE. The top selling brands of OTC cold/allergy products in 2009 were:
| Brand1 | Company | Active Ingredient(s) | 2009 Retail Sales ($ Millions) | |||||
| Claritin-D | Merck | PSE & Loraditine2 | $ | 113.0 | ||||
| Mucinex-D | Rickett Benckiser | PSE & Guaifenesin2 | $ | 72.2 | ||||
| Zyrtec-D | Pfizer | PSE & Ceterizine2 | $ | 52.2 | ||||
| Advil Sinus | Pfizer | PSE & Ibuprofen | $ | 30.9 | ||||
| Sudafed 12 Hour | J&J | PSE2 | $ | 24.9 | ||||
| Sudafed 30mg | J&J | PSE | $ | 20.8 | ||||
1 Branded product only. Does not include store brand sales.
2 Extended release PSE formulations
| 15 |
The 2009 market for 30mg PSE tablets, including store brands was approximately 372 million tablets or 15.5 million boxes of 24 tablets. Nexafed is currently priced at $3.99 for a box of 24 tablets and Nexafed Sinus Pressure + Pain is currently priced at $7.50 for a box of 24 tablets.
The market for cold, sinus and allergy products is highly competitive and many products have strong consumer brand recognition and, in some cases, prescription drug heritage. Category leading brands are often supported by national mass marketing and promotional efforts. Consumers often have a choice to purchase a less expensive store brand. Store brands contain the same active ingredients as the more popular national brands but are not supported by large marketing campaigns and are offered at a lower price. Non-prescription products are typically distributed through retail outlets including drug store chains, food store chains, independent pharmacies and mass merchandisers. The distribution outlets for PSE products are highly consolidated. According to Chain Drug Review, the top 50 drug, food and mass merchandising chains operate approximately 40,000 pharmacies in the U.S., of which 58% are operated by the four largest chains. Stocking decisions and pharmacists recommendations for these chain pharmacies are often centralized at the corporate headquarters.
Our 2010 market research study showed that 93% of the 204 pharmacists surveyed believe that PSE has superior efficacy as a nasal decongestant compared to phenylephrine. In our 2012 survey of 215 chain and independent pharmacists, 164 indicated they had influence over the pharmacies’ product offerings. Of such pharmacists, 70% indicated they were likely to stock or recommend stocking Nexafed in their pharmacies. The 215 surveyed pharmacists also indicated a willingness to recommend Nexafed to over 50% of their customers who seek a pharmacist’s advice for a single ingredient nasal decongestant.
Product Labeling for Impede Technology Products
We are marketing our Nexafed and Nexafed Sinus Pressure + Pain products pursuant to the FDA’s OTC Monograph regulations, which require that our product have labeling as specified in the regulations. We are advertising the extraction characteristics and methamphetamine-resistant benefits of our Nexafed products which is supported by our published research studies.
We expect that any of our other Impede Technology products that are marketed pursuant to an NDA or ANDA will be subject to a label approved by the FDA. We expect that such a label will require submission of our scientifically derived abuse liability data and we intend to seek descriptions of our abuse liability studies in the FDA approved product label, although there can be no assurance that this will be the case.
Limitx™ Technology
Limitx™ technology is a novel, early stage technology intended to address abuse by excess oral consumption of multiple tablets and provide a margin of safety during accidental over-ingestion of tablets. In proof of concept laboratory tests, Limitx™ tablets demonstrated the ability to limit the release of the active ingredient from tablets when multiple tablets are simultaneously introduced into simulated gastric fluid. Using .055N HCl dissolution bath, a single Limitx tablet released most of its active ingredient within 15 minutes while eight Limitx tablets in the same bath released the equivalent of one tablet’s active ingredient in 15 minutes. Eight immediate-release tablets of a marketed product comparator released the most of its active ingredient in 15 minutes compared with over 2 hours for the eight Limitx tablets.
While the initial Limitx™ formulation utilizes hydromorphone as its sole active ingredient, if such development proves successful, of which no assurance can be given, it is expected that the technology could incorporate other opioids as well. The need for abuse deterrent formulations which address excess oral consumption was stressed in the January 2013 FDA draft guidance for abuse deterrent opioids. We have patent applications pending with the USPTO covering our Limitx™ technology.
Limitx™ is being developed pursuant to a $300,000 grant (the “Grant”) by the National Institute On Drug Abuse (“NIDA”) of the National Institutes of Health. Phase I of development is to create an optimized formulation that can be commercially manufactured and is suitable for human testing. In Phase I, we will be developing a formulation and manufacturing process that mimics, at research scale batches, commercial manufacturing scale equipment and test and evaluate the tablets in our proof of concept dissolution apparatus. We have successfully manufactured small scale batches of the key micro-particle at our Culver facility but believe the manufacturing process used will not be scalable for commercial batches. We have tested and are in the process of installing new equipment for use in this process.
| 16 |
In Phase II, we will perform human pharmacokinetic testing to characterize the release of drug in vivo. NIDA funding of Phase II development, for which an application has already been submitted, will be contingent upon (1) assessment by NIDA of the Phase I progress report and its determination that the Phase I milestones were achieved, (2) review and approval of other documents necessary for continuation, and (3) availability of funds. No assurance can be given that Phase II development funding will be provided by NIDA.
Phase I research on the Company’s hydromorphone tablet utilizing Limitx™ technology is supported by the National Institute On Drug Abuse of the National Institutes of Health under Award Number R44DA037921. The results and content of any such research is solely the responsibility of Acura and does not necessarily represent the official views of the National Institutes of Health.
Patents and Patent Applications
We have the following issued patents covering, among other things, Oxaydo and our Aversion technology:
| Patent No. (Jurisdiction) | Subject Matter | Issued | Expires | |||
| 7,201,902 (US) | Pharmaceutical compositions including a mixture of functional inactive ingredients and specific opioid analgesics | Apr. 2007 | Mar. 2025 | |||
| 7,510,726 (US) | A wider range of compositions than those described in the 7,201,920 Patent | Mar. 2009 | Nov. 2023 | |||
| 7,981,439 (US) | Pharmaceutical compositions including any water soluble drug susceptible to abuse | Jul. 2011 | Aug. 2024 | |||
| 8,409,616 (US) | Pharmaceutical compositions of immediate-release abuse deterrent dosage forms | Apr. 2013 | Nov. 2023 | |||
| 8,637,540 (US) | Pharmaceutical compositions of immediate-release abuse deterrent opioid products | Jan. 2014 | Nov. 2023 |
We have the following issued patents related to our Aversion technology:
| Patent No. (Jurisdiction) | Subject Matter | Issued | Expires | |||
| 7,476,402 (US) | Pharmaceutical compositions of certain combinations of kappa and mu opioid receptor agonists and other ingredients intended to deter opioid analgesic product misuse and abuse | Jan. 2009 | Nov. 2023 | |||
| 8,822,489 (US) | Pharmaceutical compositions of certain abuse deterrent products that contain polymers, surfactant and polysorb 80 | Jul. 2014 | Nov. 2023 | |||
| 2004294953 (AUS) | Abuse deterrent pharmaceuticals | Apr. 2010 | Nov. 2024 | |||
| 2010200979 (AUS) | Abuse deterrent pharmaceuticals | Aug. 2010 | Nov. 2024 | |||
| 2,547,334 (CAN) | Abuse deterrent pharmaceuticals | Aug. 2010 | Nov. 2024 | |||
| 2,647,360 (CAN) | Abuse deterrent pharmaceuticals | May 2012 | Apr. 2027 | |||
| 175863 (ISR) | Abuse deterrent pharmaceuticals | Nov. 2004 | Nov. 2024 | |||
| 221018 (ISR) | Abuse deterrent pharmaceuticals | Nov. 2004 | Nov. 2024 |
We have the following issued patent covering, among other things, our Nexafed product line and Impede 1.0 and 2.0 technologies:
| Patent No. (Jurisdiction) | Subject Matter | Issued | Expires | |||
| 8,901,113 (US) | Pharmaceutical compositions suitable for reducing the chemical conversion of precursor compounds | Dec. 2014 | Feb. 2032 |
| 17 |
In January 2012, the USPTO issued to us U.S. Patent No. 8,101,630, or the 630 Patent with a single claim that encompasses an extended release abuse deterrent dosage form of oxycodone or a pharmaceutically acceptable salt thereof. The 630 Patent expires in August 2024. In July 2014, we ceded priority of the ‘630 patent to a patent application filed by Purdue Pharma and expect this patent to be rescinded.
In addition to our issued U.S. patents, we have filed multiple U.S. patent applications and international patent applications relating to compositions containing abusable active pharmaceutical ingredients as well as applications covering our Impede 1.0 and 2.0 Technologies and filed U.S. patent applications for our Limitx Technology. Except for the rights granted in the Egalet Agreement, we have retained all intellectual property rights to our Aversion Technology, Impede Technology, Limitx Technology and related product candidates.
In 2012 and 2013, we received Paragraph IV Certification Notices from five generic sponsors of an ANDA for a generic drug listing our Oxaydo product as the reference listed drug. The Paragraph IV Notices referred to our 920, 726 and 439 Patents, which cover our Aversion® Technology and our Oxaydo product. We filed suit against each of such generic sponsors, Watson Laboratories, Inc., Par Pharmaceutical, Inc., Impax Laboratories, Inc., Sandoz Inc. and Ranbaxy Inc., in the United States District Court for the District of Delaware alleging infringement of our 726 Patent listed in the FDA’s Orange Book. Our litigation against Watson Laboratories was dismissed by us following Watson Laboratories’ change of its Paragraph IV Certification to a Paragraph III Certification, indicating it would not launch its generic product until the expiry of our applicable Patents. Our litigation against the remaining generic sponsors was settled during the period October 2013 through May 2014 on an individual basis, upon mutual agreement between us and such generic sponsors. None of such settlements impacted the validity or enforceability of our Patents. See “Item 3 – Legal Proceedings – Paragraph IV ANDA Litigation” for a discussion of the settlements relating to such patent litigation.
Reference is made to the Risk Factors contained in Item 1A of this Report for a discussion, among other things, of patent applications and patents owned by third parties, including claims that may encompass our Aversion Technology and Oxaydo tablets.
Research and Manufacturing
We conduct research, development, manufacture of laboratory clinical trial supplies, and warehousing activities at our operations facility in Culver, Indiana and lease an administrative office in Palatine, Illinois. The 25,000 square foot Culver facility is registered with the DEA to perform research, development and manufacture of certain DEA-scheduled active pharmaceutical ingredients and finished dosage form products. We have obtained quotas for supply of DEA-scheduled active pharmaceutical ingredients from the DEA and develop finished dosage forms in our Culver facility. We manufacture clinical trial supplies of drug products in our Culver facility. In addition to internal capabilities and activities, we engage numerous clinical research organizations, or CROs, with expertise in regulatory affairs, clinical trial design and monitoring, clinical data management, biostatistics, medical writing, laboratory testing and related services. Egalet is responsible for commercial manufacture of Oxaydo under the Egalet Agreement. We expect that future opioid product candidates developed and licensed by us will be commercially manufactured by our licensees or other qualified third-party contract manufacturers.
We rely on a contract manufacturer to manufacture, package and supply our commercial quantities of Nexafed and Nexafed Sinus Pressure + Pain products. Initially, we will source our commercial requirements of our Nexafed products from a single manufacturer and will not have a second source. Although we believe there are alternate sources of supply that can satisfy our commercial requirements, replacing or adding a contract manufacture will result in additional costs and may delay or interrupt the supply of these products.
Competition
Our products and technologies will, if marketed, compete to varying degrees against both brand and generic products offering similar therapeutic benefits and being developed and marketed by small and large pharmaceutical (for prescription products) and consumer packaged goods (for OTC products) companies. Many of our competitors have substantially greater financial and other resources and are able to expend more funds and effort than us in research, development and commercialization of their competitive technologies and products. Prescription generic products and OTC store brand products will offer cost savings to third-party payers and/or consumers that will create pricing pressure on our products. Also, these competitors may have a substantial sales volume advantage over our products, which may result in our costs of manufacturing being higher than our competitors’ costs.
| 18 |
We believe potential competitors may be developing opioid abuse deterrent technologies and products. Such potential competitors include, but may not be limited to, Pain Therapeutics, Pfizer Inc., Purdue Pharma, Atlantic Pharmaceuticals, Egalet Corporation, KemPharm, Shionogi, Nektar Therapeutics, Signature Therapeutics, QRx Pharma, Tris Pharma, Pisgah Labs, and Collegium Pharmaceuticals, Inc. Egalet, our partner for Oxaydo, is also developing other analgesic products, all of which will compete for development and commercialization resources for Oxaydo, which may adversely impact the sales of Oxaydo. In August 2014, Purdue Pharma announced the submission of an NDA for an immediate-release oxycodone HCl product with reported abuse deterrent properties.
Our Impede Technology products containing PSE will compete in the highly competitive market for cold, sinus and allergy products generally available to the consumer without a prescription. Some of our competitors will have multiple consumer product offerings both within and outside the cold, allergy and sinus category providing them with substantial leverage in dealing with a highly consolidated pharmacy distribution network. The competing products may have well established brand names and may be supported by national or regional advertising. Nexafed will compete primarily with Johnson & Johnson’s Sudafed® brand and Nexafed Sinus Pressure + Pain with Pfizer’s Advil® Cold and Sinus, as well as generic/store brand formulations of such products manufactured by Perrigo Company and others. A competing product from Westport Pharmaceuticals is being marketed with claims of methamphetamine-resistance.
We are also aware that some large pharmaceutical companies in the past have sought to develop PSE technologies or products that resist conversion into methamphetamine.
We may consider licensing our Impede Technology or products utilizing such technology for commercialization.
Government Regulation
All pharmaceutical firms, including us, are subject to extensive regulation by the federal government, principally by the FDA under the Federal Food, Drug and Cosmetic Act, or the FD&C Act, and, to a lesser extent, by state and local governments. Before our prescription products and some OTC products may be marketed in the U.S., they must be approved by the FDA for commercial distribution. Other OTC products must comply with applicable FDA regulations, known as OTC Monographs, in order to be marketed, but do not require FDA review and approval before marketing. Additionally, we are subject to extensive regulation by the DEA under the Controlled Substances Act, the Combat Methamphetamine Act of 2005, and related laws and regulations for research, development, manufacturing, marketing and distribution of controlled substances and certain other pharmaceutical active ingredients that are regulated as Listed Chemicals. Extensive FDA, DEA, and state regulation of our products and commercial operations continues after drug product approvals, and the requirements for our continued marketing of our products may change even after initial approval. We are also subject to regulation under federal, state and local laws, including requirements regarding occupational safety, laboratory practices, environmental protection and hazardous substance control, and may be subject to other present and future local, state, federal and foreign regulations, including possible future regulations of the pharmaceutical industry. We cannot predict the extent to which we may be affected by legislative and other regulatory developments concerning our products and the healthcare industry in general.
The FD&C Act, the Controlled Substances Act and other federal statutes and regulations govern the testing, manufacture, quality control, export and import, labeling, storage, record keeping, approval, pricing, advertising, promotion, sale and distribution of pharmaceutical products. Noncompliance with applicable requirements both before and after approval, can subject us, our third-party manufacturers and other collaborative partners to administrative and judicial sanctions, such as, among other things, warning letters, fines and other monetary payments, recall or seizure of products, criminal proceedings, suspension or withdrawal of regulatory approvals, interruption or cessation of clinical trials, total or partial suspension of production or distribution, injunctions, limitations on or the limitation of claims we can make for our products, and refusal of the government to enter into supply contracts for distribution directly by governmental agencies, or delay in approving or refusal to approve new drug applications. The FDA also has the authority to revoke or withhold approvals of new drug applications.
| 19 |
FDA approval is required before any "new drug," can be marketed. A "new drug" is one not generally recognized, by experts qualified by scientific training and experience, as safe and effective for its intended use. Our products not subject to and in compliance with an OTC Monograph are new drugs and require prior FDA approval. Such approval must be based on extensive information and data submitted in a NDA, including but not limited to adequate and well controlled laboratory and clinical investigations to demonstrate the safety and effectiveness of the drug product for its intended use(s). In addition to providing required safety and effectiveness data for FDA approval, a drug manufacturer's practices and procedures must comply current Good Manufacturing Practices, or with cGMPs, which apply to manufacturing, receiving, holding and shipping. Accordingly, manufacturers must continue to expend time, money and effort in all applicable areas relating to quality assurance and regulatory compliance, including production and quality control to comply with cGMPs. Failure to so comply risks delays in approval of drug products and possible FDA enforcement actions, such as an injunction against shipment of products, the seizure of non-complying products, criminal prosecution and/or any of the other possible consequences described above. We are subject to periodic inspection by the FDA and DEA, which inspections may or may not be announced in advance.
The FDA Drug Approval Process
The process of drug development is complex and lengthy. The activities undertaken before a new pharmaceutical product may be marketed in the U.S. generally include, but are not limited to, preclinical studies; submission to the FDA of an Investigational New Drug application, or IND, which must become active before human clinical trials may commence; adequate and well-controlled human clinical trials to establish the safety and efficacy of the product; submission to the FDA of an NDA; acceptance for filing of the NDA by FDA; satisfactory completion of an FDA pre-approval inspection of the clinical trial sites and manufacturing facility or facilities at which both the active ingredients and finished drug product are produced to assess compliance with, among other things, patient informed consent requirements, the clinical trial protocols, current Good Clinical Practices, or GCP, and cGMPs; and FDA review and approval of the NDA prior to any commercial sale and distribution of the product in the U.S.
Preclinical studies include laboratory evaluation of product chemistry and formulation, and in some cases, animal studies and other studies to preliminarily assess the potential safety and efficacy of the product candidate. The results of preclinical studies together with manufacturing information, analytical data, and detailed information including protocols for proposed human clinical trials are then submitted to the FDA as a part of an IND. An IND must become effective, and approval must be obtained from an Institutional Review Board, or IRB, must be obtained prior to the commencement of human clinical trials. The IND becomes effective 30 days following its receipt by the FDA unless the FDA objects to, or otherwise raises concerns or questions and imposes a clinical hold. We, the FDA or the IRB may suspend or terminate a clinical trial at any time after it has commenced due to safety or efficacy concerns or for commercial reasons. In the event that FDA objects to the IND and imposes a clinical hold, the IND sponsor must address any outstanding FDA concerns or questions to the satisfaction of the FDA before clinical trials can proceed or resume. There can be no assurance that submission of an IND will result in FDA authorization to commence clinical trials.
Human clinical trials are typically conducted in three phases that may sometimes overlap or be combined:
Phase 1: This phase is typically the first involving human participants, and involves the smallest number of human participants (typically, 20-50). The investigational drug is initially introduced into healthy human subjects or patients and tested for safety, dosage tolerance, absorption, metabolism, distribution and excretion. In addition, it is sometimes possible to gain a preliminary indication of efficacy.
Phase 2: Once the preliminary safety and tolerability of the drug in humans is confirmed during phase 1, phase 2 involves studies in a somewhat larger group of study subjects. Unlike phase 1 studies, which typically involve healthy subjects, participants in phase 2 studies may be affected by the disease or condition for which the product candidate is being developed. Phase 2 studies are intended to identify possible adverse effects and safety risks, to evaluate the efficacy of the product for specific targeted diseases, and to determine appropriate dosage and tolerance.
| 20 |
Phase 3: Phase 3 trials typically involve a large numbers of patients affected by the disease or condition for which the product candidate is being developed. Phase 3 clinical trials are undertaken to evaluate clinical efficacy and safety under conditions resembling those for which the product will be used in actual clinical practice after FDA approval of the NDA. Phase 3 trials are typically the most costly and time-consuming of the clinical phases.
Phase 4 or Post-Marketing Requirements: Phase 4 trials may be required by FDA after the approval of the NDA for the product, as a condition of the approval, or may be undertaken voluntarily by the sponsor of the trial. The purpose of phase 4 trials is to continue to evaluate the safety and efficacy of the drug on a long-term basis and in a much larger and more diverse patient population than was included in the prior phases of clinical investigation.
After clinical trials have been completed, and if they were considered successful, the sponsor may submit a NDA or Abbreviated New Drug Application, or ANDA, to the FDA including the results of the preclinical and clinical testing, together with, among other things, detailed information on the chemistry, manufacturing, quality controls, and proposed product labeling. There are two types of NDAs; a 505(b)(1) NDA and a 505(b)(2) NDA. A 505(b)(1) NDA is also known as a "full NDA" and is described by section 505(b)(1) of the FD&C Act as an application containing full reports of investigations of safety and effectiveness, in addition to other information. The data in a full NDA is either owned by the applicant or are data for which the applicant has obtained a right of reference. A 505(b)(2) application is one described under section 505(b)(2) of the FD&C Act as an application for which information, or one or more of the investigations relied upon by the applicant for approval "were not conducted by or for the applicant and for which the applicant has not obtained a right of reference or use from the person by or for whom the investigations were conducted". This provision permits the FDA to rely for approval of an NDA on data not developed by the applicant, such as published literature or the FDA's finding of safety and effectiveness of a previously approved drug. 505(b)(2) applications are submitted under section 505(b)(1) of the FD&C Act and are therefore subject to the same statutory provisions that govern 505(b)(1) applications that require among other things, "full reports" of safety and effectiveness.
505(b)(2) NDAs must include one of several different types of patent certifications to each patent that is listed in the FDA publication known as the Orange Book in connection with any previously approved drug, the approval of which is relied upon for approval of the 505(b)(2) NDA. Depending on the type of certification made, the approval of the 505(b)(2) NDA may be delayed until the relevant patent(s) expire, or in the case of a Paragraph IV Certification may lead to patent litigation against the applicant and a potential automatic approval delay of 30 months or more.
Each NDA requires payment of a user fee, pursuant to the requirements of the Prescription Drug User Fee Act, or PDUFA, as periodically amended. According to FDA’s fee schedule, effective on October 1, 2014, for the 2015 fiscal year, the user fee for an application fee requiring clinical data, such as an NDA is $2,335,200. The FDA adjusts PDUFA user fees on an annual basis. PDUFA also imposes annual product and facility fees. A written request can be submitted for a waiver of the application fee for the first human drug application that is filed by a small business, but no waivers for product or establishment fees are available. Where we are subject to these fees, they are significant expenditures that may be incurred in the future and must be paid at the time of submission of each application to FDA.
After an NDA is submitted by an applicant, and if it is accepted for filing by the FDA, the FDA will then review the NDA and, if and when it determines that the data submitted are adequate to show that the product is safe and effective for its intended use, the FDA will approve the product for commercial distribution in the U.S. There can be no assurance that any of our products in development will receive FDA approval or that even if approved, they will be approved with labeling that includes descriptions of its abuse deterrent features. Moreover, even if our products in development are approved with labeling that includes descriptions of the abuse deterrent features of our products, advertising and promotion for the products will be limited to the specific claims and descriptions in the FDA approved product labeling.
| 21 |
The FDA requires drug manufacturers to establish and maintain quality control procedures for manufacturing, processing and holding drugs and investigational products, and products must be manufactured in accordance with defined specifications. Before approving an NDA, the FDA usually will inspect the facility(ies) at which the active pharmaceutical ingredients and finished drug product is manufactured, and will not approve the product unless it finds that cGMP compliance at those facility(ies) are satisfactory. If the FDA determines the NDA is not acceptable, the FDA may outline the deficiencies in the NDA and often will request additional information, thus delaying the approval of a product. Notwithstanding the submission of any requested additional testing or information, the FDA ultimately may decide that the application does not satisfy the criteria for approval. After a product is approved, changes to the approved product, such as adding new indications, manufacturing changes, or changes in or additions to the approved labeling for the product, may require submission of a new NDA or, in some instances, an NDA amendment, for further FDA review. Post-approval marketing of products in larger or different patient populations than those that were studied during development can lead to new findings about the safety or efficacy of the products. This information can lead to a product sponsor’s requesting approval for and/or the FDA requiring changes in the labeling of the product or even the withdrawal of the product from the market.
The Best Pharmaceuticals for Children Act, or BPCA, became law in 2002 and was subsequently reauthorized and amended by FDAAA. The reauthorization of BPCA provides an additional six months of market exclusivity beyond the expiration date of existing market exclusivities or eligible patents to NDA applicants that conduct acceptable pediatric studies of new and currently-marketed drug products for which pediatric information would be beneficial, as identified by FDA in a Pediatric Written Request. The FD&C Act, as amended by the Pediatric Research Equity Act, or PREA, requires that most applications for drugs and biologics include a pediatric assessment (unless waived or deferred) to ensure the drugs' and biologics' safety and effectiveness in children. Such pediatric assessment must contain data, gathered using appropriate formulations for each age group for which the assessment is required, that are adequate to assess the safety and effectiveness of the drug or the biological product for the claimed indications in all relevant pediatric subpopulations, and to support dosing and administration for each pediatric subpopulation for which the drug or the biological product is safe and effective. The pediatric assessments can only be deferred provided there is a timeline for the completion of such studies. FDA may waive (partially or fully) the pediatric assessment requirement for several reasons, including if the applicant can demonstrate that reasonable attempts to produce a pediatric formulation necessary for that age group have failed. The FDA has indicated our Oxaydo product is exempt from the pediatric studies requirement of the PREA.
The terms of approval of any NDA for our product candidates, including the indication and product labeling (and, consequently permissible advertising and promotional claims we can make) may be more restrictive than what is sought in the NDA or what is desired by us. Additionally, the FDA conditioned approval of our Oxaydo product on our commitment to conduct Phase 4 epidemiological studies to assess the actual abuse levels of Oxaydo in the market. The testing and FDA approval process for our product candidates requires substantial time, effort, and financial resources, and we cannot be sure that any approval will be granted on a timely basis, if at all.
Further, drug products approved by FDA may be subject to continuing obligations intended to assure safe use of the products. Specifically, under the FD&C Act, as amended by the Food and Drug Administration Amendments Act of 2007, or FDAAA, FDA may require Risk Evaluation and Mitigation Strategies, or REMS, to manage known or potential serious risks associated with drugs or biological products. If FDA finds, at the time of approval or afterward, that a REMS is necessary to ensure that the benefits of our products outweigh the risks associated with the products, FDA will require a REMS and, consequently, that we take additional measures to ensure safe use of the product. Components of a REMS may include, but are not limited to, a Medication Guide and/or Patient Package Insert, a marketing and sales communication plan for patients or healthcare providers concerning the drug, Elements To Assure Safe Use, or ETASUs such as, but not limited to, patient, prescriber, and pharmacy registries, and restrictions on the extent or methods of distribution, a REMS implementation system, and a timetable for assessment of the effectiveness of the REMS.
In addition, we, our suppliers and our licensees are required to comply with extensive FDA requirements both before and after approval. For example, we or our licensees are required to report certain adverse reactions and production problems, if any, to the FDA, and to comply with certain requirements concerning the advertising and promotion of our products, which, as discussed above, may significantly affect the extent to which we can include statements or claims referencing our abuse deterrent technology in product labeling and advertising. Also, quality control and manufacturing procedures must continue to conform to cGMP after approval to avoid the product being rendered misbranded and/or adulterated under the FD&C Act as a result of manufacturing problems. In addition, discovery of any material safety issues may result in changes to product labeling or restrictions on a product manufacturer, potentially including removal of the product from the market.
| 22 |
Whether or not FDA NDA approval in the U.S. has been obtained, approvals from comparable governmental regulatory authorities in foreign countries must be obtained prior to the commencement of commercialization of our drug products in those countries. The approval procedure varies in complexity from country to country, and the time required may be longer or shorter than that required for FDA approval.
FDA’s OTC Monograph Process
The FDA regulates certain non-prescription drugs using an OTC Monograph which, when final, is published in the Code of Federal Regulations at 21 C.F.R. Parts 330-358. For example, 21 C.F.R. Part 341 sets forth the products, such as pseudoephedrine hydrochloride, that may be marketed as an OTC cold, cough, allergy, bronchodilator, or antiasthmatic drug product in a form suitable for oral, inhalant, or topical administration and is generally recognized as safe and effective and is not misbranded. Such products that meet each of the conditions established in the OTC Monograph regulations and the other applicable regulations may be marketed without prior approval by the FDA.
The general conditions set forth for OTC Monograph products include, among other things:
| · | the product is manufactured at FDA registered establishments and in accordance with cGMPs; |
| · | the product label meets applicable format and content requirements including permissible “Indications” and all required dosing instructions and limitations, warnings, precautions and contraindications that have been established in an applicable OTC Monograph; |
| · | the product contains only permissible active ingredients in permissible strengths and dosage forms; |
| · | the product contains only suitable inactive ingredients which are safe in the amounts administered and do not interfere with the effectiveness of the preparation; and |
| · | the product container and container components meet FDA’s requirements. |
The advertising for OTC drug products is regulated by the Federal Trade Commission, or FTC, which generally requires that advertising claims be truthful, not misleading, and substantiated by adequate and reliable scientific evidence. False, misleading, or unsubstantiated OTC drug advertising may be subject to FTC enforcement action and may also be challenged in court by competitors or others under the federal Lanham Act or similar state laws. Penalties for false or misleading advertising may include monetary fines or judgments as well as injunctions against further dissemination of such advertising claims.
A product marketed pursuant to an OTC Monograph must be registered with the FDA and have a National Drug Code listing which is required for all marketed drug products. After marketing, the FDA may test the product or otherwise investigate the manufacturing and development of the product to ensure compliance with the OTC Monograph. Should the FDA determine that a product is not marketed in compliance with the OTC Monograph or is advertised outside of its regulations, the FDA may require corrective action up to and including market withdrawal and recall.
In March 2014, the FDA held a workshop to discuss potential changes to the OTC Monograph regulations, including the requirement for sponsor companies to determine that their innovative formulations of inactive ingredients do not interfere with the effectiveness of the product.
| 23 |
DEA Regulation
Our Oxaydo product and several of our products in development, if approved and marketed, will be regulated as “controlled substances” as defined in the CSA, which establishes registration, security, recordkeeping, reporting, storage, distribution and other requirements administered by the DEA. The DEA is concerned with the loss and diversion of potentially abused drugs into illicit channels of commerce and closely monitors and regulates handlers of controlled substances, and the equipment and raw materials used in their manufacture and packaging.
The DEA designates controlled substances as Schedule I, II, III, IV or V or as List I Chemicals. Schedule I substances by definition have no established medicinal use, and may not be marketed or sold in the United States. A pharmaceutical product may be listed as Schedule II, III, IV or V, with Schedule II substances considered to present the highest risk of abuse and Schedule V substances the lowest relative risk of abuse among such substances. List I Chemicals are used to regulate potentially abused raw materials, such as pseudoephedrine HCl. We believe all of our products will receive DEA Scheduling consistent with current DEA Scheduling standards. For example, Oxaydo Tablets are listed as a Schedule II controlled substances under the CSA, the same as all other oxycodone HCl products. Consequently, their manufacture, shipment, storage, sale and use will be subject to a high degree of regulation. For example, generally, all Schedule II drug prescriptions must be signed by a physician, physically presented to a pharmacist and may not be refilled without a new prescription.
Annual DEA registration is required for any facility that manufactures, tests, distributes, dispenses, imports or exports any controlled substance or List I Chemical. Except for certain DEA defined co-incidental activities, each registration is specific to a particular location and activity. For example, separate registrations are needed for import and manufacturing, and each registration must specify which schedules of controlled substances are authorized.
The DEA typically inspects a facility to review its security measures prior to issuing a registration and, thereafter, on a periodic basis. Security requirements vary by controlled substance schedule, with the most stringent requirements applying to Schedule I and Schedule II substances. Required security measures include, among other things, background checks on employees and physical control of inventory through measures such as vaults, cages, surveillance cameras and inventory reconciliations. Records must be maintained for the handling of all controlled substances and List I Chemicals, and periodic reports made to the DEA, for example distribution reports for Schedule I and II controlled substances, Schedule III substances that are narcotics, and other designated substances. Reports must also be made for thefts or significant losses of any controlled substance and List I Chemicals, and to obtain authorization to destroy any controlled substance and List I Chemicals. In addition, special authorization, notification and permit requirements apply to imports and exports.
In addition, a DEA quota system controls and limits the availability and production of controlled substances in Schedule I or II and List I Chemicals. Distributions of any Schedule I or II controlled substance must also be accomplished using special order forms, with copies provided to the DEA. Because Oxaydo Tablets are Schedule II they are subject to the DEA’s production and procurement quota scheme. The DEA establishes annually an aggregate quota for how much oxycodone active ingredient may be produced in total in the United States based on the DEA’s estimate of the quantity needed to meet legitimate scientific and medicinal needs. This limited aggregate amount of oxycodone that the DEA allows to be produced in the United States each year is allocated among individual companies, who must submit applications annually to the DEA for individual production and procurement quotas. We or our licensees must receive an annual quota from the DEA in order to produce or procure any Schedule I or Schedule II substance and List I Chemicals. The DEA may adjust aggregate production quotas and individual production and procurement quotas from time to time during the year, although the DEA has substantial discretion in whether or not to make such adjustments. Our or our licensees’ quota of an active ingredient may not be sufficient to meet commercial demand or complete the manufacture or purchase of material required for clinical trials. Any delay or refusal by the DEA in establishing our or our licensees’ quota for controlled substances or List I Chemicals could delay or stop our clinical trials or product launches, or interrupt commercial sales of our products which could have a material adverse effect on our business, financial position and results of operations.
| 24 |
The DEA also regulates Listed Chemicals, which are chemicals that may be susceptible to abuse, diversion, and use in the illicit manufacture of controlled substances. Some Listed Chemicals, including pseudoephedrine, are used in various prescription and OTC drug products. DEA and state laws and regulations impose extensive recordkeeping, security, distribution, and reporting requirements for companies that handle, manufacture, or distribute Listed Chemicals, including lawful drug products containing Listed Chemicals. In particular, OTC drug products containing certain Listed Chemicals, including pseudoephedrine, are required to be secured behind the pharmacy counter and dispensed to customers directly by a pharmacist only in limited quantities. Pharmacists must obtain proof of identity from customers, and must keep detailed records and make reports to the DEA regarding sales of such products. Individual states may, and in some cases have, imposed stricter requirements on the sale of drug products containing Listed Chemicals, including requiring a doctor’s prescription prior to dispensing such products to a customer.
The DEA conducts periodic inspections of registered establishments that handle controlled substances and Listed Chemicals. Failure to maintain compliance with applicable requirements, particularly as manifested in loss or diversion, can result in enforcement action that could have a material adverse effect on our business, results of operations and financial condition. The DEA may seek civil penalties, refuse to renew necessary registrations, or initiate proceedings to revoke those registrations. In certain circumstances, violations could lead to criminal prosecution.
Individual states also regulate controlled substances and List I Chemicals, and we or our licensees are subject to such regulation by several states with respect to the manufacture and future distribution of these products.
Pharmaceutical Coverage, Pricing and Reimbursement
In the United States, the commercial success of our product candidates will depend, in part, upon the availability of coverage and reimbursement from third-party payers at the federal, state and private levels. Government payer programs, including Medicare and Medicaid, private health care insurance companies and managed care plans may deny coverage or reimbursement for a product or therapy in whole or in part if they determine that the product or therapy is not medically appropriate or necessary. Also, third-party payers have attempted to control costs by limiting coverage and the amount of reimbursement for particular procedures or drug treatments. The United States Congress and state legislatures from time to time propose and adopt initiatives aimed at cost containment, which could impact our ability to sell our products profitably.
For example, in March 2010, President Obama signed into law the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act, which we refer to collectively as the Health Care Reform Law, a sweeping law intended to broaden access to health insurance, reduce or constrain the growth of healthcare spending, enhance remedies against fraud and abuse, add new transparency requirements for healthcare and health insurance industries, impose new taxes and fees on the health industry and impose additional health policy reforms. Among other cost containment measures, the Healthcare Reform Law establishes:
| · | An annual, nondeductible fee on any entity that manufactures or imports certain branded prescription drugs and biologic agents; |
| · | A new Medicare Part D coverage gap discount program, in which pharmaceutical manufacturers who wish to have their drugs covered under Part D must offer discounts to eligible beneficiaries during their coverage gap period (the “donut hole”); and |
| · | A new formula that increases the rebates a manufacturer must pay under the Medicaid Drug Rebate Program. |
Many of the Healthcare Reform Law’s most significant reforms were implemented in 2014, with others thereafter, and their details will be shaped significantly by implementing regulations, some of which have yet to be finalized. If such reforms result in an increase in the proportion of uninsured patients who are prescribed products resulting from our proprietary or partnered programs, this could adversely impact future sales of our products and our business and results of operations. Where patients receive insurance coverage under any of the new options made available through the Healthcare Reform Law, the possibility exists that manufacturers may be required to pay Medicaid rebates on that resulting drug utilization, a decision that could impact manufacturer revenues. In addition, the Administration has also announced delays in the implementation of key provisions of the Healthcare Reform Law. The implications of these delays for our sales, business and financial condition, if any, are not yet clear.
| 25 |
Although it is too early to determine the effect of the Health Care Reform Law, the new law appears likely to continue the pressure on pharmaceutical pricing, especially under government programs, and may also increase our or our licensees’ regulatory burdens and operating costs. Moreover, in the coming years, additional changes could be made to governmental healthcare programs that could significantly impact the success of our products.
The cost of pharmaceuticals continues to generate substantial governmental and third-party payer interest. We expect that the pharmaceutical industry will experience pricing pressures due to the trend toward managed healthcare, the increasing influence of managed care organizations and additional legislative proposals. In addition to the Healthcare Reform Law, there will continue to be proposals by legislators at both the federal and state levels, regulators and third-party payers to keep healthcare costs down while expanding individual healthcare benefits. Economic pressure on state budgets may result in states increasingly seeking to achieve budget savings through mechanisms that limit coverage or payment for drugs. State Medicaid programs are increasingly requesting manufacturers to pay supplemental rebates and requiring prior authorization by the state program for use of any drug for which supplemental rebates are not being paid. Managed care organizations continue to seek price discounts and, in some cases, to impose restrictions on the coverage of particular drugs. Government efforts to reduce Medicaid expenses may lead to increased use of managed care organizations by Medicaid programs. This may result in managed care organizations influencing prescription decisions for a larger segment of the population and a corresponding constraint on prices and reimbursement for our products. Certain of these changes could limit the prices that can be charged for drugs we develop or the amounts of reimbursement available for these products from governmental agencies or third-party payers, or may increase the tax obligations on pharmaceutical companies, or may facilitate the introduction of generic competition with respect to products we are able to commercialize. In short, our or our licensees’ results of operations could be adversely affected by current and future healthcare reforms.
In international markets, reimbursement and healthcare payment systems vary significantly by country, and many countries have instituted price ceilings on specific products and therapies. There can be no assurance that our products will be considered medically reasonable and necessary for a specific indication, that our products will be considered cost-effective by third-party payers, that an adequate level of coverage or payment will be available so that the third-party payers’ reimbursement policies will not adversely affect our ability to sell our products profitably.
Other Healthcare Laws and Compliance Requirements
We and our licensees that commercialize our products are subject to various federal and state laws targeting fraud and abuse in the healthcare industry. For example, the federal Anti-Kickback Statute prohibits persons from knowingly and willfully soliciting, receiving, offering or paying remuneration, directly or indirectly, to induce either the referral of an individual, or the furnishing, recommending, or arranging for a good or service, for which payment may be made under a federal healthcare program, such as the Medicare and Medicaid programs. The reach of the Anti-Kickback Statute was broadened by the Health Care Reform Law, which, among other things, amends the intent requirement of the statute so that a person or entity no longer needs to have actual knowledge of this statute or specific intent to violate it in order to have committed a violation. The Healthcare Reform Law also provides that the government may assert that a claim including items or services resulting from a violation of the federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the civil False Claims Act or the civil monetary penalties statute. The civil False Claims Act imposes liability on any person who, among other things, knowingly presents, or causes to be presented, a false or fraudulent claim for payment by a federal healthcare program. The “qui tam” provisions of the False Claims Act allow a private individual to bring civil actions on behalf of the federal government alleging that the defendant has submitted a false claim to the federal government, and to share in any monetary recovery. Violations of these laws or any other federal or state fraud and abuse laws may subject our licensees to civil and criminal penalties, including fines, imprisonment and exclusion from participation in federal healthcare programs, which could harm the commercial success of our products and materially affect our business, financial condition and results of operations.
Segment Reporting
We operate in one business segment; the research, development and manufacture of innovative abuse deterrent, orally administered pharmaceutical products.
| 26 |
Environmental Compliance
We are subject to regulation under federal, state and local environmental laws and believe we are in material compliance with such laws. We incur the usual waste disposal cost associated with a pharmaceutical research, development and manufacturing operation.
Employees
We have 15 full-time employees, 9 of whom are engaged in the research, development and manufacture of product candidates utilizing our proprietary Aversion, Impede, and Limitx Technologies. The remaining employees are engaged in administrative legal, accounting, finance, marketing, market research, and business development activities. All of our senior management and most of our other employees have had prior experience in pharmaceutical or biotechnology companies. None of our employees are covered by collective bargaining agreements. We believe that our relations with our employees are good.
Our future operating results may vary substantially from anticipated results due to a number of factors, many of which are beyond our control. The following discussion highlights some of these factors and the possible impact of these factors on future results of operations. If any of the following factors actually occur, our business, financial condition or results of operations could be materially harmed. In that case, the value of our common stock could decline substantially and you may lose all or part of your investment.
Risks Related to Our Business and Industry
We are largely dependent on the commercial success of OXAYDO
We anticipate that, for at least fiscal 2015 and 2016, our ability to generate revenues and become profitable will depend in large part on the commercial success of our only FDA approved product, OXAYDO, which in turn will depend on several factors, including our and our licensee Egalet’s ability to:
| · | obtain and increase market demand for, and sales of, OXAYDO; |
| · | obtain acceptance of OXAYDO by physicians and patients; |
| · | obtain and maintain adequate levels of coverage and reimbursement for OXAYDO from commercial health plans and government health programs, which we refer to collectively as third-party payors, particularly in light of the availability of other branded and generic competitive products; |
| · | maintain compliance with regulatory requirements; |
| · | price OXAYDO competitively and enter into price discounting contracts with third-party payors; |
| · | establish and maintain agreements with wholesalers and distributors on commercially reasonable terms; |
| · | manufacture and supply OXAYDO to meet commercial demand, including obtaining sufficient quota from the DEA; and |
| · | maintain intellectual property protection for OXAYDO and obtain favorable drug listing treatment by the FDA to minimize generic competition. |
There can be no assurance that Egalet will devote sufficient resources to the marketing and commercialization of OXAYDO. Egalet’s marketing of OXAYDO may result in low market acceptance and insufficient demand for, and sales of, the product. If Egalet fails to successfully commercialize OXAYDO and generate and increase sales, we may be unable to generate sufficient revenues to sustain or grow our business and we may never become profitable, and our business, financial condition and results of operations will be materially adversely affected.
| 27 |
If we are not successful in commercializing our NEXAFED Products and other IMPEDE Technology products, our revenues and business will suffer.
We commenced the launch and commercial distribution of NEXAFED in mid-December 2012 and launched our NEXAFED Sinus Pressure + Pain product in February 2015. Our NEXAFED products compete in the highly competitive market for cold, sinus and allergy products generally available to the consumer without a prescription. Many of our competitors have substantially greater financial and other resources and are able to expend more funds and effort than us in marketing their competing products. Category leading brands are often supported by regional and national advertising and promotional efforts. Our NEXAFED products will compete with national brands as well as pharmacy store brands that are offered at a lower price. There can be no assurance that we will succeed in commercializing our NEXAFED products, or that the pricing of our NEXAFED products will allow us to generate significant revenues or profit. Regulations have been enacted in several state or local jurisdictions requiring a doctor’s prescription to obtain pseudoephedrine products. An expansion of such restrictions to other jurisdictions or even nationally will adversely impact our ability to market our NEXAFED products as OTC products and generate revenue from NEXAFED products sales. Our failure to successfully commercialize our NEXAFED® products and to develop and commercialize other IMPEDE Technology products will have a material adverse effect on our business and financial condition.
If Egalet is not successful in commercializing OXAYDO, our revenues and our business will suffer.
Pursuant to our Collaboration and License Agreement with Egalet, or the Egalet Agreement, Egalet is responsible for manufacturing, marketing, pricing, promotion, selling and distribution of OXAYDO. If the Egalet Agreement is terminated in accordance with its terms, including due to a party’s failure to perform its obligations or responsibilities under the Agreement, then we would need to commercialize OXAYDO ourselves, for which we currently have no infrastructure, or alternatively enter into a new agreement with another pharmaceutical company, of which no assurance can be given. If we are unable to build the necessary infrastructure to commercialize OXAYDO ourselves, which would substantially increase our expenses and capital requirements, which we are currently unable to fund, or are unable to find a suitable replacement commercialization partner, we would be unable to generate any revenue from OXAYDO. Even if we are successful at replacing the commercialization capabilities of Egalet, our revenues and/or royalties from OXAYDO could be adversely impacted.
Egalet’s third-party manufacturing facility will be the sole commercial source of supply of OXAYDO. If Egalet’s manufacturing facility fails to obtain sufficient DEA quotas for oxycodone, fails to source adequate quantities of active and inactive ingredients, fails to comply with regulatory requirements, or otherwise experiences disruptions in commercial supply of OXAYDO, product revenue and our royalties could be adversely impacted.
Egalet has various products in development for which OXAYDO will vie for such licensee’s development, promotional, marketing, and selling resources. If Egalet fails to commit sufficient promotional, marketing and selling resources to OXAYDO, our expected royalties could be adversely impacted. Additionally, there can be no assurance that Egalet will commit the resources required for the successful commercialization of OXAYDO.
The market for our opioid product candidates is highly competitive with many marketed non-abuse deterrent brand and generic products and other abuse deterrent product candidates in development. If Egalet prices OXAYDO inappropriately, fails to position OXAYDO properly, targets inappropriate physician specialties, or otherwise does not provide sufficient promotional support, product revenue and our royalties could be materially adversely impacted.
Egalet’s promotional, marketing and sales activities in connection with OXAYDO are subject to various federal and state fraud and abuse laws, including, without limitation, the federal Anti-Kickback Statute and the federal False Claims Act. The federal Anti-Kickback Statute prohibits persons from knowingly and willfully soliciting, receiving, offering or paying remuneration, directly or indirectly, to induce, or in return for, the purchase or recommendation of an item or service reimbursable under a federal healthcare program. The federal False Claims Act imposes liability on any person who, among other things, knowingly presents, or causes to be presented, a false or fraudulent claim for payment by a federal healthcare program. If Egalet’s activities are found to be in violation of these laws or any other federal and state fraud and abuse laws, Egalet may be subject to penalties, including civil and criminal penalties, damages, fines and the curtailment or restructuring of its activities with regard to the commercialization of OXAYDO, which could harm the commercial success of Oxaydo and have a material adverse effect on our business, financial condition and results of operations.
| 28 |
Our failure to continue the development of our AVERSION opioid products, including hydrocodone/acetaminophen, , or to successfully establish a license agreement with a pharmaceutical company for the development and commercialization of such products, will adversely impact our ability to develop, market and sell such products and our revenues and business will suffer.
We have developed to various stages additional AVERSION opioid products. Our plan for developing, manufacturing and commercializing the AVERSION opioid products includes entering into an agreement similar to the Egalet Agreement with a strategically focused pharmaceutical company. However, there can be no assurance that we will be successful in entering into such an agreement. Pending any such agreement, we expect to continue the development of our AVERSION hydrocodone bitartrate with acetaminophen product on our own. The continued development of our hydrocodone bitartrate with acetaminophen product and the other opioid products will likely require additional financing, which may not be available on acceptable terms, or at all. In the absence of available financing, or our failure to successfully enter into a license agreement with a pharmaceutical company to develop and commercialize the returned products, we may have to limit the size or scope of, or delay or abandon, the development of some or all of the returned products, which would adversely impact our financial condition and results of operations.
We have a history of operating losses and may not achieve profitability sufficient to generate a positive return on shareholders’ investment.
We had a net loss of $13.2, $13.9 million and $9.7 million for the years ended December 31, 2014, 2013 and 2012, respectively. Our future profitability will depend on several factors, including:
| · | our receipt of royalties relating to Egalet’s sale of OXAYDO; |
| · | our successful marketing and sale of our NEXAFED® products and other products utilizing our IMPEDE Technology, and market acceptance, increased demand for and sales of our NEXAFED products; |
| · | our receipt of milestone payments and royalties relating to our AVERSION Technology products in development from future licensees, of which no assurance can be given; and |
| · | the receipt of FDA approval and the successful commercialization by future licensees (if any) of products utilizing our AVERSION Technology and our ability to commercialize our IMPEDE Technology without infringing the patents and other intellectual property rights of third parties. |
We cannot assure you that OXAYDO or our NEXAFED products will be successfully commercialized or our AVERSION Technology or IMPEDE Technology products in development will be successfully developed or be approved for commercialization by the FDA.
Even if Egalet succeeds in commercializing OXAYDO, or if we or a licensee succeed in developing and commercializing one or more of our pipeline AVERSION Technology products, or if we are successful in commercializing our NEXAFED products or other IMPEDE Technology products, we expect to continue using cash reserves for the foreseeable future. Our expenses may increase in the foreseeable future as a result of continued research and development of our product candidates, maintaining and expanding the scope of our intellectual property, commercializing our NEXAFED products, and hiring of additional research and development staff.
We will need to generate revenues from direct product sales or indirectly from royalties on sales to achieve and maintain profitability. If we cannot successfully commercialize our NEXAFED products, if Egalet does not successfully commercialize OXAYDO, or if we or our licensee (if any) cannot successfully develop, obtain regulatory approval and commercialize our products in development, we will not be able to generate such royalty revenues or achieve future profitability. Our failure to achieve or maintain profitability would have a material adverse impact on our operations, financial condition and on the market price of our common stock.
| 29 |
We must rely on current cash reserves, milestones payable by Egalet under the Egalet Agreement, royalties from Egalet on Egalet’s sale of OXAYDO, and revenues from our NEXAFED product sales to fund operations.
Pending the receipt of the milestone payments and royalties under the Egalet Agreement relating to OXAYDO, and milestone payments and royalties under license agreements similar to the Egalet Agreement that we may enter into with other pharmaceutical companies relating to our products in development, in each case of which no assurance can be given, we must rely on our current cash reserves and revenues from our sales of our NEXAFED products to fund operations and product development activities. No assurance can be given that current cash reserves and revenues from our NEXAFED product sales will be sufficient to fund continued operations and the development of our product candidates until such time as we generate revenues from Egalet’s commercialization of OXAYDO or from any of our products in development. Moreover, no assurance can be given that we will be successful in raising additional financing or, if funding is obtained, that such funding will be sufficient to fund operations until we generate sufficient revenues from OXAYDO, or until product candidates utilizing our AVERSION or IMPEDE Technologies may be commercialized. In the event our cash reserves are insufficient to fund continued operations, we may need to suspend some or all of our product development efforts or possibly discontinue operations.
Our and our licensees’ ability to market and promote OXAYDO and other AVERSION Technology products by describing the abuse deterrent features of such products will be determined by the FDA approved label for such products.
The commercial success of OXAYDO and our AVERSION Technology products in development will depend upon our and our licensees’ ability to obtain FDA approved labeling describing such products’ abuse deterrent features or benefits. Our or our licensees’ failure to achieve FDA approval of product labeling containing such information will prevent or substantiality limit our and our licensees’ advertising and promotion of such abuse deterrent features in order to differentiate AVERSION Technology products from other immediate release opioid products containing the same active ingredients, and would have a material adverse impact on our business and results of operations. The FDA’s January 2013 draft guidance, while not binding on the FDA, outlines the FDA’s current views on the labeling of abuse deterrent products. The FDA encourages sponsors to seek approval of proposed product labeling that sets forth the results of physiochemical, physiologic, pharmacodynamic, pharmacokinetic, and/or formal post-marketing studies that appropriately characterizes the abuse-deterrent properties of a product. To date, the FDA has limited data correlating the potentially abuse-deterrent properties of certain opioid drug products with actual reduction in abuse or adverse events associated with abuse. When the data predict or show a product’s potential abuse-deterrent properties can be expected to, or actually do, result in a significant reduction in that product’s abuse potential, those data, together with an accurate characterization of what the data mean, should be included in product labeling. We intend to utilize certain clinical and laboratory studies for our opioid products in development to support a label describing the abuse-deterrent features of such products. However, the extent to which such information is included in the FDA approved product label is the subject of our and our licensees’ discussions with, and agreement by, the FDA as part of the NDA review process for each of our product candidates. The outcome of those discussions with the FDA will determine whether we or our licensees will be able to market our products with labeling that sufficiently differentiates them from other products that have comparable therapeutic profiles. While the FDA approved label for OXAYDO includes the results from a clinical study which evaluated the effects of nasally snorting crushed Oxaydo and commercially available oxycodone tablets and limitations on wetting or dissolving OXAYDO, it does not, however, include the results of our laboratory studies intended to evaluate OXAYDO’s potential to limit extraction of oxycodone HCl from dissolved OXAYDO Tablets and resist conversion into an injectable, or IV solution. The absence of the results of these extraction and syringe studies in the FDA approved label for OXAYDO may substantially limit our licensee’s ability to differentiate OXAYDO from other immediate release oxycodone products, which would have a material adverse effect on market acceptance of OXAYDO and on our business and results of operations.
Notwithstanding the FDA approved labeling for OXAYDO, there can be no assurance that our AVERSION Technology products in development will receive FDA approved labeling that describes the abuse deterrent features of such products. If the FDA does not approve labeling containing such information, we or our licensees will not be able to promote such products based on their abuse deterrent features, may not be able to differentiate such products from other immediate release opioid products containing the same active ingredients, and may not be able to charge a premium above the price of such other products which could materially adversely affect our business and results of operations.
| 30 |
Because the FDA closely regulates promotional materials and other promotional activities, even if the FDA initially approves product labeling that includes a description of the abuse deterrent characteristics of our product, as in the case of OXAYDO, the FDA may object to our or our licensee’s marketing claims and product advertising campaigns. This could lead to the issuance of warning letters or untitled letters, suspension or withdrawal of OXAYDO from the market, recalls, fines, disgorgement of money, operating restrictions, injunctions or criminal prosecution, which could harm the commercial success of our product and materially affect our business, financial condition and results of operations.
Our product candidates are unproven and may not be approved by the FDA.
We are committing a majority of our resources to the development of product candidates utilizing our AVERSION and IMPEDE Technologies. Notwithstanding the receipt of FDA approval of OXAYDO and our marketing of our NEXAFED products, there can be no assurance that any other product candidate utilizing our AVERSION, IMPEDE or LIMITX Technologies will meet FDA’s standards for commercial distribution. Further, there can be no assurance that other product candidates that may be developed using AVERSION, IMPEDE or LIMITX Technologies will achieve the targeted end points in the required clinical studies or perform as intended in other pre-clinical and clinical studies or lead to an NDA submission or filing acceptance. Our failure to successfully develop and achieve final FDA approval of our product candidates in development will have a material adverse effect on our financial condition.
If the FDA disagrees with our determination that certain of our products meet the over-the-counter, or OTC, Monograph requirements, once those products are commercialized, they may be removed from the market; the FDA or the U.S. Federal Trade Commission, or FTC, may object to our advertisement and promotion of the extraction characteristics and benefits of our NEXAFED products.
Drugs that have been deemed safe and effective by the FDA for use by the general public without a prescription are classified as OTC drug products. Certain OTC drug products may be commercialized without premarket review by the FDA if the standards set forth in the applicable regulatory monograph are met. An OTC monograph provides the marketing conditions for the applicable OTC drug product, including active ingredients, labeling, and other general requirements, such as compliance with cGMP and establishment registration. Any product which fails to conform to each of the general conditions and a monograph is subject to regulatory action. Further, although the FDA regulates OTC drug product labeling, the FTC regulates the advertising and marketing of OTC drug products. We believe that our NEXAFED products are classified for OTC sale under an FDA OTC monograph, which will allow us to commercialize them without submitting an NDA or ANDA to the FDA. We have also determined that, provided we adhere to the FDA’s requirements for OTC monograph products, including product labeling, we can advertise and promote the extraction characteristics and benefits of our NEXAFED products which are supported by our research studies. No assurance can be given, however, that the FDA will agree that our NEXAFED products may be sold under the FDA’s OTC monograph product regulations or that the FDA or FTC will not object to our advertisement and promotion of our NEXAFED products’ extraction characteristics and benefits. If the FDA determines that our NEXAFED products do not conform to the OTC monograph or if we fail to meet the general conditions, once commercialized, the products may be removed from the market and we may face various actions including, but not limited to, restrictions on the marketing or distribution of such products, warning letters, fines, product seizure, or injunctions or the imposition of civil or criminal penalties. Any of these actions may materially and adversely affect our financial condition and operations. Additionally, the FDA has recently announced that it is considering material changes to how it regulates OTC drug products and held hearing in late March 2014 for public comment. Changes to the existing OTC regulations could result in a requirement that Acura file an NDA or ANDA for our NEXAFED products or other IMPEDE Technology products in order to commercialize such products. If the FDA requires that we submit a NDA or ANDA to obtain marketing approval for our NEXAFED® products or other IMPEDE Technology products, this would result in substantial additional costs, suspend the commercialization of our NEXAFED products and require FDA approval prior to sale, of which no assurance can be provided. In such case, the label for our NEXAFED products or other IMPEDE Technology products would be subject to FDA review and approval and there can be no assurance that we will be able to market NEXAFED or other IMPEDE Technology products with labeling sufficient to differentiate it from products that have comparable therapeutic profiles. If we are unable to advertise and promote the extraction characteristics of NEXAFED or other IMPEDE Technology products, we may be unable to compete with national brands and pharmacy chain store brands.
| 31 |
Our AVERSION, IMPEDE, and LIMITX Technology products may not be successful in limiting or impeding abuse or misuse upon commercialization.
We are committing a majority of our resources to the development of products utilizing our AVERSION and IMPEDE Technologies, as well as LIMITX. Notwithstanding the receipt of FDA approval of OXAYDO and the results of our numerous clinical and laboratory studies for OXAYDO, our NEXAFED products, and our AVERSION, IMPEDE, and LIMITX Technology products in development, there can be no assurance that OXAYDO, our NEXAFED products or any other product utilizing our AVERSION, IMPEDE, or LIMITX Technologies will perform as tested and limit or impede the actual abuse or misuse of such products in commercial settings. Moreover, there can be no assurance that the post-approval epidemiological study required by the FDA as a condition of approval of OXAYDO will show a reduction in the consequences of abuse and misuse by patients for whom OXAYDO is prescribed. The failure of OXAYDO, our NEXAFED products or other products utilizing our AVERSION, IMPEDE, and LIMITX Technologies to limit or impede actual abuse or misuse in practice will have a material adverse impact on market acceptance for such products and on our financial condition and results of operations.
Relying on third-party CROs may result in delays in our pre-clinical, clinical or laboratory testing. If pre-clinical, clinical or laboratory testing for our product candidates are unsuccessful or delayed, we will be unable to meet our anticipated development and commercialization timelines.
To obtain FDA approval to commercially sell and distribute in the United States any of our prescription product candidates, we or our licensees must submit to the FDA a NDA demonstrating, among other things, that the product candidate is safe and effective for its intended use. As we do not possess the resources or employ all the personnel necessary to conduct such testing, we rely on CROs for the majority of this testing with our product candidates. As a result, we have less control over our development program than if we performed the testing entirely on our own. Third parties may not perform their responsibilities on our anticipated schedule. Delays in our development programs could significantly increase our product development costs and delay product commercialization.
The commencement of clinical trials with our product candidates may be delayed for several reasons, including, but not limited to, delays in demonstrating sufficient pre-clinical safety required to obtain regulatory approval to commence a clinical trial, reaching agreements on acceptable terms with prospective CROs, clinical trial sites and licensees, manufacturing and quality assurance release of a sufficient supply of a product candidate for use in our clinical trials and/or obtaining institutional review board approval to conduct a clinical trial at a prospective clinical site. Once a clinical trial has begun, it may be delayed, suspended or terminated by us or regulatory authorities due to several factors, including ongoing discussions with regulatory authorities regarding the scope or design of our clinical trials, a determination by us or regulatory authorities that continuing a trial presents an unreasonable health risk to participants, failure to conduct clinical trials in accordance with regulatory requirements, lower than anticipated recruitment or retention rate of patients in clinical trials, inspection of the clinical trial operations or trial sites by regulatory authorities, the imposition of a clinical hold by FDA, lack of adequate funding to continue clinical trials, and/or negative or unanticipated results of clinical trials.
Clinical trials required by the FDA for commercial approval may not demonstrate safety or efficacy of our product candidates. Success in pre-clinical testing and early clinical trials does not assure that later clinical trials will be successful. Results of later clinical trials may not replicate the results of prior clinical trials and pre-clinical testing. Even if the results of our pivotal phase III clinical trials are positive, we and our licensees may have to commit substantial time and additional resources to conduct further pre-clinical and clinical studies before we or our licensees can submit NDAs or obtain regulatory approval for our product candidates.
Clinical trials are expensive and at times, difficult to design and implement, in part because they are subject to rigorous regulatory requirements. Further, if participating subjects or patients in clinical studies suffer drug-related adverse reactions during the course of such trials, or if we, our licensees or the FDA believes that participating patients are being exposed to unacceptable health risks, we or our licensees may suspend the clinical trials. Failure can occur at any stage of the trials, and we or our licensees could encounter problems causing the abandonment of clinical trials or the need to conduct additional clinical studies, relating to a product candidate.
| 32 |
Even if our clinical trials and laboratory testing are completed as planned, their results may not support commercially viable product label claims. The clinical trial process may fail to demonstrate that our product candidates are safe and effective for their intended use. Such failure may cause us or our licensees to abandon a product candidate and may delay the development of other product candidates.
We have no commercial manufacturing capacity and rely on third-party contract manufacturers to produce commercial quantities of our products.
We do not have the facilities, equipment or personnel to manufacture commercial quantities of our products and therefore must rely on our licensees or other qualified third-party contract manufactures with appropriate facilities and equipment to contract manufacture commercial quantities of products utilizing our AVERSION and IMPEDE Technologies. These licensees and third-party contract manufacturers are also subject to cGMP regulations, which impose extensive procedural and documentation requirements. Any performance failure on the part of our licensees or contract manufacturers could delay commercialization of any approved products, depriving us of potential product revenue.
Our drug products, including our NEXAFED products, require precise, high quality manufacturing. Failure by our contract manufacturers to achieve and maintain high manufacturing standards could result in patient injury or death, product recalls or withdrawals, delays or failures in testing or delivery, cost overruns, or other problems that could materially adversely affect our business. Contract manufacturers may encounter difficulties involving production yields, quality control, and quality assurance. These manufacturers are subject to ongoing periodic unannounced inspection by the FDA and corresponding state and foreign agencies to ensure strict compliance with cGMP and other applicable government regulations; however, beyond contractual remedies that may be available to us, we do not have control over third-party manufacturers’ compliance with these regulations and standards.
If for some reason our contract manufacturers cannot perform as agreed, we may be required to replace them. Although we believe there are a number of potential replacements, we will incur added costs and delays in identifying and qualifying any such replacements. In addition, a new manufacturer would have to be educated in, or develop substantially equivalent processes for, production of our products or drug candidates.
We or our licensees may not obtain required FDA approval; the FDA approval process is time-consuming and expensive.
The development, testing, manufacturing, marketing and sale of pharmaceutical products are subject to extensive federal, state and local regulation in the United States and other countries. Satisfaction of all regulatory requirements typically takes years, is dependent upon the type, complexity and novelty of the product candidate, and requires the expenditure of substantial resources for research, development and testing. Substantially all of our operations are subject to compliance with FDA regulations. Failure to adhere to applicable FDA regulations by us or our licensees would have a material adverse effect on our operations and financial condition. In addition, in the event we are successful in developing product candidates for distribution and sale in other countries, we would become subject to regulation in such countries. Such foreign regulations and product approval requirements are expected to be time consuming and expensive.
We or our licensees may encounter delays or rejections during any stage of the regulatory review and approval process based upon the failure of clinical or laboratory data to demonstrate compliance with, or upon the failure of the product candidates to meet, the FDA’s requirements for safety, efficacy and quality; and those requirements may become more stringent due to changes in regulatory agency policy or the adoption of new regulations. After submission of a NDA, the FDA may refuse to file the application, deny approval of the application, require additional testing or data and/or require post-marketing testing and surveillance to monitor the safety or efficacy of a product. For instance, the FDA’s approval of OXAYDO is conditioned on us or Egalet conducting a post-approval epidemiological study to assess the actual abuse levels and consequences of OXAYDO in the market. The Prescription Drug User Fee Act, or PDUFA, sets time standards for the FDA’s review of NDA’s. The FDA's timelines described in the PDUFA guidance are flexible and subject to change based on workload and other potential review issues and may delay the FDA’s review of an NDA. Further, the terms of approval of any NDA, including the product labeling, may be more restrictive than we or our licensees desire and could affect the marketability of our products.
| 33 |
Even if we comply with all the FDA regulatory requirements, we or our licensees may not obtain regulatory approval for any of our product candidates in development. For example, we previously submitted a NDA to the FDA for an AVERSION Technology product containing niacin, intended to provide impediments to over-ingesting the product. Such niacin containing product was not approved by the FDA. If we or our licensees fail to obtain regulatory approval for any of our product candidates in development, we will have fewer commercialized products and correspondingly lower revenues. Even if regulatory approval of our products in development is received, such approval may involve limitations on the indicated uses or promotional claims we or our licensees may make for our products, or otherwise not permit labeling that sufficiently differentiates our product candidates from competitive products with comparable therapeutic profiles but without abuse deterrent features (see risk factor above entitled “Our and our licensees ability to market and promote OXAYDO and other AVERSION Technology products by describing the abuse deterrent features of such products will be determined by the FDA approved label for such products”). Such events would have a material adverse effect on our operations and financial condition. We may market certain of our products without the prior application to and approval by the FDA. The FDA may subsequently require us to withdraw such products and submit NDA’s for approval prior to re-marketing.
The FDA also has the authority to revoke or suspend approvals of previously approved products for cause, to debar companies and individuals from participating in the drug-approval process, to request recalls of allegedly violative products, to seize allegedly violative products, to obtain injunctions to close manufacturing plants allegedly not operating in conformity with current cGMP and to stop shipments of allegedly violative products. In the event the FDA takes any such action relating to our products, such actions would have a material adverse effect on our operations and financial condition.
We must maintain FDA approval to manufacture clinical supplies of our product candidates at our facility; failure to maintain compliance with FDA requirements may prevent or delay the manufacture of our product candidates and costs of manufacture may be higher than expected.
We have installed the equipment necessary to manufacture clinical trial supplies of our AVERSION and IMPEDE Technology product candidates in tablet formulations at our Culver, Indiana facility. To be used in clinical trials, all of our product candidates must be manufactured in conformity with cGMP regulations. All such product candidates must be manufactured, packaged, and labeled and stored in accordance with cGMPs. Modifications, enhancements or changes in manufacturing sites of marketed products are, in many circumstances, subject to FDA approval, which may be subject to a lengthy application process or which we may be unable to obtain. Our Culver, Indiana facility, and those of any third-party manufacturers that we or our licensees may use, are periodically subject to inspection by the FDA and other governmental agencies, and operations at these facilities could be interrupted or halted if the FDA deems such inspections are unsatisfactory. Failure to comply with FDA or other governmental regulations can result in fines, unanticipated compliance expenditures, recall or seizure of products, total or partial suspension of production or distribution, suspension of FDA review of our product candidates, termination of ongoing research, disqualification of data for submission to regulatory authorities, enforcement actions, injunctions and criminal prosecution.
We develop our products, and manufacture clinical supplies, at a single location. Any disruption at this facility could adversely affect our business and results of operations.
We rely on our Culver, Indiana facility for developing our product candidates and the manufacture of clinical supplies of our product candidates. If the Culver, Indiana facility were damaged or destroyed, or otherwise subject to disruption, it would require substantial lead-time to repair or replace. If our Culver facility were affected by a disaster, we would be forced to rely entirely on CROs and third-party contract manufacturers for an indefinite period of time. Although we believe we possess adequate insurance for damage to our property and for the disruption of our business from casualties, such insurance may not be sufficient to cover all of our potential losses and may not continue to be available to us on acceptable terms, or at all. Moreover, any disruptions or delays at our Culver, Indiana facility could impair our ability to develop our product candidates utilizing the AVERSION, IMPEDE, or LIMITX Technologies, which could adversely affect our business and results of operations.
| 34 |
Our operations are subject to environmental, health and safety, and other laws and regulations, with which compliance is costly and which exposes us to penalties for non-compliance.
Our business, properties and product candidates are subject to federal, state and local laws and regulations relating to the protection of the environment, natural resources and worker health and safety and the use, management, storage and disposal of hazardous substances, waste and other regulated materials. Because we own and operate real property, various environmental laws also may impose liability on us for the costs of cleaning up and responding to hazardous substances that may have been released on our property, including releases unknown to us. These environmental laws and regulations also could require us to pay for environmental remediation and response costs at third-party locations where we dispose of or recycle hazardous substances. The costs of complying with these various environmental requirements, as they now exist or may be altered in the future, could adversely affect our financial condition and results of operations.
Our failure to successfully establish new license agreements with pharmaceutical companies for the development and commercialization of our other products in development may adversely impair our ability to develop, market and sell such products.
The Egalet Agreement grants Egalet an exclusive worldwide license to develop and commercialize OXAYDO. We believe that opportunities exist to enter into license agreements similar to the Egalet Agreement with other pharmaceutical company partners for the development and commercialization of our AVERSION product candidates in development in the United States and worldwide, and for the development and commercialization of additional AVERSION Technology and IMPEDE Technology product candidates for other abused and misused drugs, such as tranquilizers, stimulants, sedatives and nasal decongestants in the United States and worldwide. However, there can be no assurance that we will be successful in entering into such license agreements in the future. If we are unable to enter into such agreements, our ability to develop and commercialize our product candidates, and our financial condition and results of operations, would be materially adversely affected.
If our licensees do not satisfy their obligations, we will be unable to develop our licensed product candidates.
As part of our Egalet Agreement or any license agreement we may enter into relating to any of our AVERSION or IMPEDE Technology products in development, we will not have day-to-day control over the activities of our licensees with respect to any product candidate. If a licensee fails to fulfill its obligations under an agreement with us, we may be unable to assume the development and/or commercialization of the product covered by that agreement or to enter into alternative arrangements with another third party. In addition, we may encounter delays in the commercialization of the products that are the subject of a license agreement. Accordingly, our ability to receive any revenue from the products covered by such agreements will be dependent on the efforts of our licensee. We could be involved in disputes with a licensee, which could lead to delays in or termination of, our development and/or commercialization programs and result in time consuming and expensive litigation or arbitration. In addition, any such dispute could diminish our licensee’s commitment to us and reduce the resources they devote to developing and/or commercializing our products. If any licensee terminates or breaches its agreement, or otherwise fails to complete its obligations in a timely manner, our chances of successfully developing and/or commercializing our product candidates would be materially adversely effected. Additionally, due to the nature of the market for OXAYDO and our AVERSION product candidates, it may be necessary for us to license a significant portion of our product candidates to a single company, thereby eliminating our opportunity to commercialize other product candidates with other licensees.
| 35 |
If we fail to maintain our license agreement with Eaglet, we may have to commercialize OXAYDO on our own.
Our plan for manufacturing and commercializing OXAYDO currently requires us to maintain our license agreement with Egalet. In addition to other customary termination provisions, the Egalet Agreement provides that Egalet may terminate the Egalet Agreement upon certain conditions prior to the launch OXAYDO, or following launch, upon certain notice periods. If Egalet elects to terminate the Egalet Agreement, or if we are otherwise unable to maintain our existing relationship with Egalet, we would have to commercialize OXAYDO ourselves for which we currently have no infrastructure, or alternatively enter into a new agreement with another pharmaceutical company, of which no assurance can be given. Our ability to commercialize OXAYDO on our own may require additional financing, which may not be available on acceptable terms, or at all.
The market may not be receptive to products incorporating our AVERSION or IMPEDE Technologies.
The commercial success of our products will depend on acceptance by health care providers and others that such products are clinically useful, cost-effective and safe. There can be no assurance given that our products utilizing the AVERSION or IMPEDE Technologies would be accepted by health care providers and others. Factors that may materially affect market acceptance of our product candidates include but are not limited to:
| · | the relative advantages and disadvantages of our products compared to competitive products; |
| · | the relative timing to commercial launch of our products compared to competitive products; |
| · | the relative safety and efficacy of our products compared to competitive products; |
| · | the product labeling approved by the FDA for our products; |
| · | the perception of health care providers of their role in helping to prevent abuse and their willingness to prescribe abuse-deterrent products to do so; |
| · | the willingness of third-party payers to reimburse for our prescription products; |
| · | the willingness of pharmacy chains to stock our NEXAFED products; |
| · | the willingness of pharmacists to recommend our NEXAFED products to their customers; and |
| · | the willingness of consumers to pay for our products. |
OXAYDO and our product candidates, if successfully developed and commercially launched, will compete with both currently marketed and new products launched in the future by other companies. Health care providers may not accept or utilize any of our products. Physicians and other prescribers may not be inclined to prescribe our prescription products unless our products demonstrate commercially viable advantages over other products currently marketed for the same indications. Pharmacy chains may not be willing to stock our NEXAFED products and pharmacists may not recommend such products to consumers. Further, consumers may not be willing to purchase our products. If our products do not achieve market acceptance, we may not be able to generate significant revenues or become profitable.
If we, our licensees or others identify serious adverse events or deaths relating to any of our products once on the market, we may be required to withdraw our products from the market, which would hinder or preclude our ability to generate revenues.
We or our licensees are required to report to relevant regulatory authorities all serious adverse events or deaths involving our product candidates or approved products. If we, our licensees, or others identify such events, regulatory authorities may withdraw their approvals of such products; we or our licensees may be required to reformulate our products; we or our licensees may have to recall the affected products from the market and may not be able to reintroduce them onto the market; our reputation in the marketplace may suffer; and we may become the target of lawsuits, including class actions suits. Any of these events could harm or prevent sales of the affected products and could materially adversely affect our business and financial condition.
| 36 |
Our revenues may be adversely affected if we fail to obtain insurance coverage or adequate reimbursement for our products from third-party payers.
The ability of our licensees to successfully commercialize our products may depend in part on the availability of reimbursement for our prescription products from government health administration authorities, private health insurers, and other third-party payers and administrators, including Medicaid and Medicare. We cannot predict the availability of reimbursement for newly-approved products utilizing our AVERSION Technology. Third-party payers and administrators, including state Medicaid programs and Medicare, are challenging the prices charged for pharmaceutical products. Government and other third-party payers increasingly are limiting both coverage and the level of reimbursement for new drugs. Third-party insurance coverage may not be available to patients for any of our products candidates. The continuing efforts of government and third-party payers to contain or reduce the costs of health care may limit our commercial opportunity. If government and other third-party payers do not provide adequate coverage and reimbursement for any product utilizing our AVERSION Technology, health care providers may not prescribe them or patients may ask their health care providers to prescribe competing products with more favorable reimbursement. In some foreign markets, pricing and profitability of pharmaceutical products are subject to government control. In the United States, we expect there may be federal and state proposals for similar controls. In addition, we expect that increasing emphasis on managed care in the United States will continue to put pressure on the pricing of pharmaceutical products. Cost control initiatives could decrease the price that we or our licensees charge for any of our products in the future. Further, cost control initiatives could impair our ability or the ability of our licensees to commercialize our products and our ability to earn revenues from commercialization.
In both the United States and certain foreign jurisdictions, there have been and we expect there will continue to be a number of legislative and regulatory changes to the health care system that could impact our or our licensees’ ability to sell our products profitably. In particular, in 2010, the Patient Protection Affordable Care Act, as amended by the Health Care and Education Reconciliation Act, collectively, the Healthcare Reform Law, was enacted. The Healthcare Reform Law substantially changes the way healthcare is financed by both governmental and private insurers and significantly affects the pharmaceutical industry. Among the provisions of the Healthcare Reform Law of greatest importance to the pharmaceutical industry are the following:
| · | An annual, nondeductible fee on any entity that manufactures or imports certain branded prescription drugs and biologic agents; |
| · | An increase in the minimum rebates a manufacturer must pay under the Medicaid Drug Rebate Program; |
| · | A new Medicare Part D coverage gap discount program, under which manufacturers must agree to offer 50 percent point-of-sale discounts off negotiated prices of applicable brand drugs to eligible beneficiaries during their coverage gap period, as a condition for the manufacturer’s outpatient drugs to be covered under Medicare Part D; |
| · | Extension of manufacturer’s Medicaid rebate liability to covered drugs dispensed to individuals who are enrolled in Medicaid managed care organizations; |
| · | A new Patient-Centered Outcomes Research Institute to oversee, identify priorities in, and conduct comparative clinical effectiveness research; |
| · | A revision to the definition of “average manufacturer price” for reporting purposes; and |
| · | Encouragement for the development of comparative effectiveness research, which may reduce the extent of reimbursement for our products if such research results in any adverse findings. |
At this time, it remains uncertain what the full impact of these provisions will be on the pharmaceutical industry generally or our business in particular. The full effects of these provisions will become apparent as these laws are implemented and the Centers for Medicare & Medicaid Services and other agencies issue applicable regulations or guidance as required by the Healthcare Reform Law. Moreover, in the coming years, additional changes could be made to governmental healthcare programs that could significantly impact the success of our products.
If we are unable to establish sales and marketing capabilities for our products that are not licensed to third parties, our revenues and our business will suffer.
We do not currently have an extensive organization for the sales, marketing and distribution of pharmaceutical products and the cost of establishing and maintaining such an organization may exceed the cost-effectiveness of doing so. If we do not license the commercialization of a product, we may have to build our sales, marketing, managerial and other non-technical capabilities or make arrangements with third parties to perform these services. If we are unable to establish or fund adequate sales, marketing and distribution capabilities, whether independently or with third parties, it will impair our ability to sell products and have a material adverse effect on our operations.
| 37 |
Consolidation in the healthcare industry could lead to demands for price concessions or for the exclusion of some suppliers from certain of our markets, which could have an adverse effect on our business, financial condition or results of operations.
Because healthcare costs have risen significantly, numerous initiatives and reforms by legislatures, regulators and third-party payers to curb these cost increases have resulted in a trend in the healthcare industry to consolidate product suppliers and purchasers. As the healthcare industry consolidates, competition among suppliers to provide products to purchasers has become more intense. This in turn has resulted, and will likely continue to result, in greater pricing pressures and the exclusion of certain suppliers from important market segments as group purchasing organizations, and large single accounts continue to use their market power to influence product pricing and purchasing decisions. We expect that market demand, government regulation, third-party reimbursement policies and societal pressures will continue to influence the worldwide healthcare industry, resulting in further business consolidations, which may exert further downward pressure on the prices of our anticipated products. This downward pricing pressure may adversely impact our business, financial condition or results of operations. Under the Egalet Agreement, Egalet controls the price of OXAYDO, and we expect that our licensees, if any, of our products in development, will control the price of such products and may provide price discounts and price reductions in its discretion. Such price discounts and reductions will reduce the net sales of our licensed products and, correspondingly, our royalty payments under such license agreements.
Our success depends on our ability to protect our intellectual property.
Our success depends on our ability to obtain and maintain patent protection for products developed utilizing our technologies, in the United States and in other countries, and to enforce these patents. The patent positions of pharmaceutical firms, including us, are generally uncertain and involve complex legal and factual questions. Notwithstanding our receipt of U.S. patents covering our AVERSION Technology and IMPEDE Technology, there is no assurance that any of our patent claims in our other pending non-provisional and provisional patent applications relating to our technologies will issue or if issued, that any of our existing and future patent claims will be held valid and enforceable against third-party infringement or that our products will not infringe any third-party patent or intellectual property. Moreover, any patent claims relating to our technologies may not be sufficiently broad to protect our products. In addition, issued patent claims may be challenged, potentially invalidated or potentially circumvented. Our patent claims may not afford us protection against competitors with similar technology or permit the commercialization of our products without infringing third-party patents or other intellectual property rights.
Our success also depends on our not infringing patents issued to others. We may become aware of patents belonging to competitors and others that could require us to obtain licenses to such patents or alter our technologies. Obtaining such licenses or altering our technology could be time consuming and costly. We may not be able to obtain a license to any technology owned by or licensed to a third party that we or our licensees require to manufacture or market one or more of our products. Even if we can obtain a license, the financial and other terms may be disadvantageous.
Our success also depends on maintaining the confidentiality of our trade secrets and know-how. We seek to protect such information by entering into confidentiality agreements with employees, potential licensees, raw material suppliers, contract research organizations, contract manufacturers, consultants and other parties. These agreements may be breached by such parties. We may not be able to obtain an adequate, or perhaps any, remedy to such a breach. In addition, our trade secrets may otherwise become known or be independently developed by our competitors. Our inability to protect our intellectual property or to commercialize our products without infringing third-party patents or other intellectual property rights would have a material adverse effect on our operations and financial condition.
| 38 |
We also rely on or intend to rely on our or our licensees’ trademarks, trade names and brand names to distinguish our products from the products of our competitors, and have registered or applied to register many of these trademarks. However, our trademark applications may not be approved. Third parties may also oppose our or our licensees’ trademark applications or otherwise challenge our use of the trademarks. In the event that our or our licensees’ trademarks are successfully challenged, we or our licensees could be forced to rebrand our product, which could result in loss of brand recognition and could require us or our licensees to devote resources to advertising and marketing these new brands. Further, our competitors may infringe our trademarks, or we may not have adequate resources to enforce our trademarks.
We may become involved in patent litigation or other intellectual property proceedings relating to our AVERSION or IMPEDE Technologies or product candidates, which could result in liability for damages or delay or stop our development and commercialization efforts.
The pharmaceutical industry has been characterized by significant litigation and other proceedings regarding patents, patent applications and other intellectual property rights. The situations in which we may become parties to such litigation or proceedings may include:
| · | litigation or other proceedings we or our licensee(s) may initiate against third parties to enforce our patent rights or other intellectual property rights, including the Paragraph IV Proceedings described below; |
| · | litigation or other proceedings we or our licensee(s) may initiate against third parties seeking to invalidate the patents held by such third parties or to obtain a judgment that our products do not infringe such third parties’ patents; |
| · | litigation or other proceedings third parties may initiate against us or our licensee(s) to seek to invalidate our patents or to obtain a judgment that third-party products do not infringe our patents; |
| · | if our competitors file patent applications that claim technology also claimed by us, we may be forced to participate in interference or opposition proceedings to determine the priority of invention and whether we are entitled to patent rights on such invention; and |
| · | if third parties initiate litigation claiming that our products infringe their patent or other intellectual property rights, we will need to defend against such proceedings. |
The costs of resolving any patent litigation, including the Paragraph IV Proceedings, or other intellectual property proceeding, even if resolved in our favor, could be substantial. Many of our potential competitors will be able to sustain the cost of such litigation and proceedings more effectively than we can because of their substantially greater resources. Uncertainties resulting from the initiation and continuation of patent litigation or other intellectual property proceedings could have a material adverse effect on our ability to compete in the marketplace. Patent litigation, including the Paragraph IV Proceedings, and other intellectual property proceedings may also consume significant management time.
In the event that a competitor infringes upon our patent or other intellectual property rights, enforcing those rights may be costly, difficult and time consuming. Even if successful, litigation to enforce our intellectual property rights or to defend our patents against challenge could be expensive and time-consuming and could divert our management’s attention. We may not have sufficient resources to enforce our intellectual property rights or to defend our patent or other intellectual property rights against a challenge. If we are unsuccessful in enforcing and protecting our intellectual property rights and protecting our products, it could harm our business. In certain circumstances, we expect that our licensees will have first right to control the enforcement of certain of our patents against third-party infringers. Our licensees may not put adequate resources or effort into such enforcement actions or otherwise fail to restrain infringing products. In addition, in an infringement proceeding, including the Paragraph IV Proceedings, a court may decide that a patent of ours is invalid or is unenforceable, or may refuse to stop the other party from using the technology at issue on the grounds that our patents do not cover the technology in question. An adverse result in any litigation, including the Paragraph IV Proceedings, or defense proceedings could put one or more of our patents at risk of being invalidated or interpreted narrowly and could put our patent applications at risk of not issuing.
| 39 |
Our technologies or products may be found to infringe claims of patents owned by others. If we determine that we are, or if we are found to be infringing a patent held by another party, we, our suppliers or our licensees might have to seek a license to make, use, and sell the patented technologies and products. In that case, we, our suppliers or our licensees might not be able to obtain such license on acceptable terms, or at all. The failure to obtain a license to any third-party technology that may be required would materially harm our business, financial condition and results of operations. If a legal action is brought against us or our licensee(s), we could incur substantial defense costs, and any such action might not be resolved in our favor. If such a dispute is resolved against us, we may have to pay the other party large sums of money and use of our technology and the testing, manufacturing, marketing or sale of one or more of our products could be restricted or prohibited. Even prior to resolution of such a dispute, use of our technology and the testing, manufacturing, marketing or sale of one or more of our products could be restricted or prohibited.
We are aware of certain United States and international pending patent applications owned by third parties with claims potentially encompassing OXAYDO and our AVERSION products in development. While we do not expect the claims contained in such pending patent applications will issue in their present form, there can be no assurance that such patent applications will not issue as patents with claims encompassing one or more of our product candidates. If such patent applications result in valid and enforceable issued patents, containing claims in their current form or otherwise encompassing our products we or our licensees may be required to obtain a license to such patents, should one be available, or alternatively, alter our products so as to avoid infringing such third-party patents. If we or our licensees are unable to obtain a license on commercially reasonable terms, or at all, we or our licensees could be restricted or prevented from commercializing our products. Additionally, any alterations to our products or our technologies could be time consuming and costly and may not result in technologies or products that are non-infringing or commercially viable.
We are aware of an issued United States patent owned by a third party having claims encompassing the use of one of our AVERSION inactive ingredients in a controlled release pharmaceutical preparation. We are also aware of an issued United States patent owned by a third party having claims encompassing a pharmaceutical preparation containing viscosity producing ingredients that can be drawn into a syringe when dissolved in 10mL’s or less of aqueous solution. While we believe that our AVERSION products do not infringe these patents, or that such patents are otherwise invalid, there can be no assurance that we or our licensees will not be sued for infringing these patents, and if sued, there can be no assurance that we or our licensees will prevail in any such litigation. If we or our licensees are found to infringe either or both of these patents, we or our licensees may seek a license to use the patented technology. If we are unable to obtain such a license, of which no assurance can be given, we or our licensees may be restricted or prevented from commercializing our AVERSION products.
We are aware of certain issued United States patents owned by a third party having claims encompassing a process used to manufacture oxycodone HCl of high purity and pharmaceutical products resulting therefrom. As required by the FDA, OXAYDO contains a similar high purity oxycodone HCl manufactured by a supplier that is not the owner or licensee of such patents. The owner of these patents has filed patent infringement actions relating to these patents against companies that have filed abbreviated new drug applications with the FDA for extended-release versions of oxycodone HCl. To our knowledge, the patent owner has not initiated any patent infringement actions against the sellers of immediate-release oxycodone HCl products or their suppliers of oxycodone HCl, however, we cannot be certain that these immediate-release products actually utilize a high purity oxycodone. We cannot provide assurance that our licensee or its oxycodone HCl supplier will not be sued for infringing these patents. In the event of an infringement action, our licensee and their oxycodone HCl supplier would have to either: (a) demonstrate that the manufacture of the oxycodone HCl used in OXAYDO does not infringe the patent claims, (b) demonstrate the patents are invalid or unenforceable, or (c) enter into a license with the patent owner. If our licensee or their oxycodone HCl supplier is unable to demonstrate the foregoing, or obtain a license to these patients, our licensee may be required or choose to withdraw OXAYDO from the market.
We are aware of a certain issued United States patent owned by a third party having claims similar to our second generation IMPEDE Technology directed to ingredient amounts that are generally more than the amounts used in our technology. While we believe our technology does not infringe this patent, we cannot provide assurance that we will not be sued under such patent or if sued, that we will prevail in any such suit.
We cannot assure you that our technologies, products and/or actions in developing our products will not infringe third-party patents. Our failure to avoid infringing third-party patents and intellectual property rights in the development and commercialization of our products would have a material adverse effect on our operations and financial condition.
| 40 |
Generic manufacturers are using litigation and regulatory means to seek approval for generic versions of OXAYDO, which could cause Egalet’s sales to suffer.
Under the Hatch-Waxman Act, the FDA can approve an ANDA for a generic version of a branded drug and what is referred to as a Section 505(b)(2) NDA, for a branded variation of an existing branded drug, without requiring such applicant to undertake the full clinical testing necessary to obtain approval to market a new drug. An ANDA applicant usually needs to only submit data demonstrating that its product has the same active ingredient(s) and is bioequivalent to the branded product, in addition to any data necessary to establish that any difference in strength, dosage form, inactive ingredients, or delivery mechanism does not result in different safety or efficacy profiles, as compared to the reference drug.
The Hatch-Waxman Act requires an applicant for a drug that references one of our branded drugs to notify us of their application if they assert in their application that the patents we have listed in the Orange Book will not be infringed or otherwise are invalid or unenforceable (a Paragraph IV Certification). Upon receipt of this notice, we or our licensee will have 45 days to bring a patent infringement suit in federal district court against such applicant. If such a suit is commenced, the FDA is generally prohibited from granting approval of the ANDA or Section 505(b)(2) NDA until the earliest of 30 months from the date the FDA accepted the application for filing, the conclusion of litigation in the generic’s favor or expiration of the patent(s). If the litigation is resolved in favor of the applicant or the challenged patent expires during the 30-month stay period, the stay is lifted and the FDA may thereafter approve the application based on the standards for approval of ANDAs and Section 505(b)(2) NDAs. Frequently, the unpredictable nature and significant costs of patent litigation leads the parties to settle to remove this uncertainty. Settlement agreements between branded companies and generic applicants may allow, among other things, a generic product to enter the market prior to the expiration of any or all of the applicable patents covering the branded product, either through the introduction of an authorized generic or by providing a license to the applicant for the patents subject to the litigation.
On September 20, 2012, we announced that we had received a Paragraph IV Certification Notice under 21 U.S.C. 355(j) (a Paragraph IV Notice) from a generic sponsor of an ANDA for a generic drug listing OXAYDO (formerly known as OXECTA) as the reference listed drug. Since such date, we have received similar Paragraph IV Notices from three other generic pharmaceutical companies that have filed ANDAs listing OXAYDO as the reference drug. The Paragraph IV Notices refer to our U.S. Patent Numbers 7,201,920, 7,510,726 and 7,981,439, which cover our AVERSION Technology and OXAYDO. The Paragraph IV Notices state that each generic sponsor believes that such patents are invalid, unenforceable or not infringed. On October 31, 2012, we initiated suit against each of Watson Laboratories, Inc. – Florida (Watson), Par Pharmaceutical, Inc., Impax Laboratories, Inc. and Sandoz Inc., and on April 29, 2013, we initiated suit against Ranbaxy, Inc., each in the United States District Court for the District of Delaware alleging infringement of our U.S. Patent No. 7,510,726 listed in the FDA’s Orange Book. The commencement of such litigation prohibits the FDA from granting approval of the filed ANDAs until the earliest of 30 months from the date the FDA accepted the application for filing, or the conclusion of litigation. In January 2013, we dismissed our suit against Watson on the grounds that Watson had amended its ANDA from a Paragraph IV Certification to a Paragraph III Certification, which indicated its intent not to market its generic OXAYDO product in advance of our patent expiring.
On October 9, 2013, we announced that we had entered into distinct Settlement Agreements with each of Par and Impax, to settle our patent infringement action pending against them in the United States District Court for the District of Delaware. In the suit, we alleged that a generic OXAYDO product for which each of Par and Impax is separately seeking approval to market in the United States pursuant to an ANDA filing with the FDA infringes a U.S. patent owned by us. Par is the first filer of an ANDA for a generic OXAYDO product and is entitled to the 180-day first filer exclusivity under applicable law and FDA regulations.
Under the terms of the Settlement Agreement with Par, Par may launch its generic OXAYDO product in the U.S., through the grant of a non-exclusive, royalty-bearing license from us that would trigger on January 1, 2022. We currently have Orange Book patents that are due to expire between November 2023 and March 2025. In certain limited circumstances, our license to Par would become effective prior to January 1, 2022. Par is required to pay us royalties in the range of 10% to 15% of Par’s net profits from the sale of its generic OXAYDO product.
| 41 |
Under the Settlement Agreement with Impax, Impax may launch its generic OXAYDO product in the U.S., through the grant of a non-exclusive, royalty-free license from us that would trigger 180 days following the first sale of a generic OXAYDO product in the U.S. by an entity that is entitled to the 180 day first-filer exclusivity under applicable law and FDA regulations (or if no entity is entitled to such 180 day exclusivity period, the date on which a generic OXAYDO product is first sold in the U.S. or November 27, 2021, whichever date occurs first). In certain circumstances, our license to Impax would become effective prior to such time.
On May 8, 2014, we announced that we had entered into a Settlement Agreement with Ranbaxy Inc. to settle our patent infringement action pending in the United States District Court for the District of Delaware. In the suit, we alleged that a generic of our OXAYDO product for which Ranbaxy is seeking approval to market in the United States pursuant to an ANDA filed with the FDA infringes U.S. patents owned by us. The Settlement Agreement provides that Ranbaxy’s current generic of our OXAYDO product that is the subject of its ANDA filing does not infringe our Orange Book listed patents with the FDA. We have not provided Ranbaxy a license to our patents and we may re-commence patent infringement litigation against Ranbaxy if Ranbaxy changes the formulation of its current generic OXAYDO product.
On May 21, 2014, we announced that we had entered into a Settlement Agreement with Sandoz Inc. to settle our patent infringement action pending against Sandoz in the United States District Court for the District of Delaware. In the suit, we alleged that a generic of our OXAYDO product for which Sandoz is seeking approval to market in the United States pursuant to an ANDA filed with the FDA infringes a U.S. patent owned by us. Under the Settlement Agreement, Sandoz may launch its generic to the OXAYDO product in the U.S., through the grant of a non-exclusive license from us that would trigger 180 days following the first sale of a generic to the OXAYDO product in the U.S. by an entity that is entitled to the 180 day first-filer exclusivity under applicable law and FDA regulations (or if no entity is entitled to such 180 day exclusivity period, the date on which a generic to the OXAYDO product is first sold in the U.S). In certain circumstances, our license to Sandoz would become effective prior to such time. Sandoz is not obligated to pay us a royalty if its current formulation of its generic to the OXAYDO product is approved by the FDA. In the event Sandoz changes or modifies the structure of its generic OXAYDO product, or materially changes or modifies the amounts or type of any excipient used in the Sandoz formulation disclosed in its ANDA filing with the FDA as of July 30, 2013, Sandoz is required to pay us a royalty based upon the Net Profits (as defined in the Settlement Agreement) derived from the net sales of such changed or modified Sandoz generic OXAYDO product in the United States.
It is possible that other generic manufacturers may also seek to launch a generic version of OXAYDO and challenge our patents. Any determination in any such infringement actions that our patents covering our Aversion Technology and OXAYDO are invalid or unenforceable, in whole or in part, or that the products covered by generic sponsors’ ANDAs do not infringe our patents could have a material adverse effect on our operations and financial condition.
We may be exposed to product liability claims and may not be able to obtain or maintain adequate product liability insurance.
Our business exposes us to potential product liability risks, which are inherent in the testing, manufacturing, marketing and sale of pharmaceutical products. Product liability claims might be made by patients, health care providers or others that sell or consume our products. These claims may be made even with respect to those products that possess regulatory approval for commercial sale. We are currently covered by clinical trial product liability insurance on a claims-made basis and for product liability insurance covering our sale and distribution of our NEXAFED products. This coverage may not be adequate to cover any product liability claims. Product liability coverage is expensive. In the future, we may not be able to maintain such product liability insurance at a reasonable cost or in sufficient amounts to protect us against losses due to product liability claims. Any claims that are not covered by product liability insurance could have a material adverse effect on our business, financial condition and results of operations.
The pharmaceutical industry is characterized by frequent litigation. Those companies with significant financial resources will be better able to bring and defend any such litigation. No assurance can be given that we would not become involved in future litigation, in addition to the ongoing Reglan/Metoclopramide mass tort litigation discussed in “Item 3. Legal Proceedings – Reglan/Metoclopramide Litigation” of this Report. Such litigation may have material adverse consequences to our financial condition and results of operations.
| 42 |
We face significant competition, which may result in others developing or commercializing products before or more successfully than we do.
Our products and technologies compete to varying degrees against both brand and generic products offering similar therapeutic benefits and being developed and marketed by small and large pharmaceutical (for prescription products) and consumer packaged goods (for OTC products) companies. Many of our competitors have substantially greater financial and other resources and are able to expend more funds and effort than us in research, development and commercialization of their competitive technologies and products. Prescription generic products and OTC store brand products will offer cost savings to third-party payers and/or consumers that will create pricing pressure on our products. Also, these competitors may have a substantial sales volume advantage over our products, which may result in our costs of manufacturing being higher than our competitors’ costs. If our products are unable to capture and maintain market share, we or our licensees may not achieve significant product revenues and our financial condition and results of operations will be materially adversely affected.
We believe potential competitors may be developing opioid abuse deterrent technologies and products. Such potential competitors include, but may not be limited to, Pain Therapeutics, Pfizer Inc., Purdue Pharma, Atlantic Pharmaceuticals, Egalet Corporation, KemPharm, Shionogi, Nektar Therapeutics, Signature Therapeutics, QRx Pharma, Tris Pharma, Pisgah Labs, and Collegium Pharmaceuticals, Inc. These companies appear to be focusing their development efforts on ER Opioid Products, except for Atlantic Pharmaceuticals, while the majority of our AVERSION Technology opioid analgesic product candidates under development are IR Opioid Products.
Our IMPEDE Technology products containing PSE, including our NEXAFED products, will compete in the highly competitive market for cold, sinus and allergy products generally available to the consumer without a prescription. Some of our competitors will have multiple consumer product offerings both within and outside the cold, allergy and sinus category providing them with substantial leverage in dealing with a highly consolidated pharmacy distribution network. The competing products may have well established brand names and may be supported by national or regional advertising. Our NEXAFED products compete directly with Johnson & Johnson’s Sudafed® brand as well as generic formulations manufactured by Perrigo Company and others. In addition, Highland Pharmaceuticals is commercializing a PSE product that is stated to resist PSE extraction in aqueous solutions.
We are concentrating a substantial majority of our efforts and resources on developing product candidates utilizing our AVERSION and IMPEDE Technologies. The commercial success of products utilizing such technologies will depend, in large part, on the intensity of competition, FDA approved product labeling for our products compared to competitive products, and the relative timing and sequence for commercial launch of new products by other companies developing, marketing, selling and distributing products that compete with the products utilizing our AVERSION and IMPEDE Technologies. Alternative technologies and non-opioid products are being developed to improve or replace the use of opioid analgesics. In the event that such alternatives to opioid analgesics are widely adopted, then the market for products utilizing our AVERSION and IMPEDE Technologies may be substantially decreased, thus reducing our ability to generate future revenues and adversely affecting our ability to generate a profit.
If we fail to comply with the covenants and other obligations under our term loan, the lender may be able to accelerate amounts owed under the facility and may foreclose upon the assets securing our obligations.
In December 2013, we (including our wholly-owned subsidiary Acura Pharmaceutical Technologies, Inc. (“APT”)) entered into a loan and security agreement with Oxford Finance LLC (“Oxford”) pursuant to which we borrowed $10 million from Oxford. Our loan and security agreement with Oxford was amended on January 7, 2015 in connection with our collaboration and license agreement with Egalet. Under the Oxford loan agreement, as amended, we are subject to a variety of affirmative and negative covenants, including required financial reporting, limitations on certain dispositions and licensing of assets, limitations on the incurrence of additional debt, the requirement to maintain at least $2.5 million in cash reserves until the principal amount of the Oxford loan is reduced below $5.0 million, and other requirements. To secure our performance of our obligations under this loan and security agreement, we granted Oxford a security interest in substantially all of our assets, other than intellectual property assets, and pledged to Oxford the stock of APT. Our failure to comply with the terms of the loan and security agreement, the occurrence of a material adverse change in our business, operations or condition (financial or otherwise) or prospects, the material impairment in our prospect of repayment, a material impairment in the perfection or priority of the Oxford’s lien on our assets or the value of Oxford’s collateral, or the occurrence of certain other specified events could result in an event of default that, if not cured or waived, could result in the acceleration of all or a substantial portion of our loan, coupled with prepayment penalties, an additional interest payment of $795,000, potential foreclosure on our assets, and other adverse results.
| 43 |
Key personnel are critical to our business and our success depends on our ability to retain them.
We are dependent on our management and scientific team, including Robert Jones, our President and Chief Executive Officer, Peter A. Clemens, our Chief Financial Officer, and Albert W. Brzeczko, Ph.D., our Vice President of Technical Affairs. We may not be able to attract and retain personnel on acceptable terms given the competition for such personnel among biotechnology, pharmaceutical and healthcare companies, universities and non-profit research institutions. While we have employment agreements with our CEO and CFO, all of our employees are at-will employees who may terminate their employment at any time. We do not have key personnel insurance on any of our officers or employees. The loss of any of our key personnel, or the inability to attract and retain such personnel, may significantly delay or prevent the achievement of our product and technology development and business objectives and could materially adversely affect our business, financial condition and results of operations.
Our products are subject to regulation by the U.S. Drug Enforcement Administration, or DEA, and such regulation may affect the development and sale of our products.
The DEA regulates certain finished drug products and active pharmaceutical ingredients, including certain opioid active pharmaceutical ingredients and pseudoephedrine HCl that are contained in our products. Consequently, their manufacture, research, shipment, storage, sale and use are subject to a high degree of regulation. Furthermore, the amount of active ingredients we can obtain for our clinical trials is limited by the DEA and our quota may not be sufficient to complete clinical trials. There is a risk that DEA regulations may interfere with the supply of the products used in our clinical trials.
In addition, we and our contract manufacturers are subject to ongoing DEA regulatory obligations, including, among other things, annual registration renewal, security, recordkeeping, theft and loss reporting, periodic inspection and annual quota allotments for the raw material for commercial production of our products. The DEA, and some states, conduct periodic inspections of registered establishments that handle controlled substances. Facilities that conduct research, manufacture, store, distribute, import or export controlled substances must be registered to perform these activities and have the security, control and inventory mechanisms required by the DEA to prevent drug loss and diversion. Failure to maintain compliance, particularly non-compliance resulting in loss or diversion, can result in regulatory action that could have a material adverse effect on our business, results of operations, financial condition and prospects. The DEA may seek civil penalties, refuse to renew necessary registrations, or initiate proceedings to revoke those registrations. In certain circumstances, violations could lead to criminal proceedings.
Individual states also have controlled substances laws. Though state controlled substances laws often mirror federal law, because the states are separate jurisdictions, they may separately schedule drugs, as well. While some states automatically schedule a drug when the DEA does so, in other states there has to be a rulemaking or a legislative action. State scheduling may delay commercial sale of any controlled substance drug product for which we obtain FDA approval and adverse scheduling could have a material adverse effect on the attractiveness of such product. We or our licensees must also obtain separate state registrations in order to be able to obtain, handle, and distribute controlled substances for clinical trials or commercial sale, and failure to meet applicable regulatory requirements could lead to enforcement and sanctions from the states in addition to those from the DEA or otherwise arising under federal law.
| 44 |
We are increasingly dependent on information technology and our systems and infrastructure face certain risks, including cybersecurity and data storage risks.
Significant disruptions to our information technology systems or breaches of information security could adversely affect our business. In the ordinary course of business, we collect, store and transmit confidential information, and it is critical that we do so in a secure manner in order to maintain the confidentiality and integrity of such confidential information. Our information technology systems are potentially vulnerable to service interruptions and security breaches from inadvertent or intentional actions by our employees, partners, vendors, or from attacks by malicious third parties. Maintaining the secrecy of this confidential, proprietary, and/or trade secret information is important to our competitive business position. While we have taken steps to protect such information and invested in information technology, there can be no assurance that our efforts will prevent service interruptions or security breaches in our systems or the unauthorized or inadvertent wrongful access or disclosure of confidential information that could adversely affect our business operations or result in the loss, dissemination, or misuse of critical or sensitive information. A breach of our security measures or the accidental loss, inadvertent disclosure, unapproved dissemination or misappropriation or misuse of trade secrets, proprietary information, or other confidential information, whether as a result of theft, hacking, or other forms of deception, or for any other cause, could enable others to produce competing products, use our proprietary technology and/or adversely affect our business position. Further, any such interruption, security breach, loss or disclosure of confidential information could result in financial, legal, business, and reputational harm to us and could have a material effect on our business, financial position, results of operations and/or cash flow.
Prior ownership changes limit our ability to use our tax net operating loss carryforwards.
Significant equity restructuring often results in an Internal Revenue Section 382 ownership change that limits the future use of Net Operating Loss, or NOL, carryforwards and other tax attributes. We have determined that an ownership change (as defined by Section 382 of the Internal Revenue Code) did occur as a result of restructuring that occurred in 2004. Neither the amount of our NOL carryforwards nor the amount of limitation of such carryforwards claimed by us have been audited or otherwise validated by the Internal Revenue Service, which could challenge the amount we have calculated. The recognition and measurement of our tax benefit includes estimates and judgment by our management, which includes subjectivity. Changes in estimates may create volatility in our tax rate in future periods based on new information about particular tax positions that may cause management to change its estimates.
Risks Relating to our Common Stock
Our quarterly results of operations will fluctuate, and these fluctuations could cause our stock price to decline.
Our quarterly and annual operating results are likely to fluctuate in the future. These fluctuations could cause our stock price to decline. The nature of our business involves variable factors, such as the timing of any license agreement, the timing of launch and market acceptance of our products, and the timing of the research, development and regulatory submissions of our products in development that could cause our operating results to fluctuate. The forecasting of the timing and amount of sales of our products is difficult due to market uncertainty and the uncertainty inherent in seeking FDA and other necessary approvals for our product candidates. As a result, in some future quarters or years, our clinical, financial or operating results may not meet the expectations of securities analysts and investors, which could result in a decline in the price of our stock.
Our stock price has been and may continue to be volatile, and the value of an investment in our common stock may decline.
During the year ended December 31, 2014, our stock traded as high as $2.12 per share and as low as $0.41 per share. The trading price of our common stock is likely to continue to be highly volatile and could be subject to wide fluctuations in response to various factors, some of which are beyond our control. These factors could include:
| · | results from our clinical development programs, including the data from our ongoing Phase 3 clinical trial evaluating our AVERSION® hydrocodone/acetaminophen product; |
| · | FDA actions related to our products in development; |
| · | FDA actions related to any of our potential products; |
| 45 |
| · | announcements regarding the launch and sales of OXAYDO; |
| · | announcements regarding the progress of sales of OXAYDO; |
| · | announcements regarding the progress of our preclinical programs; |
| · | our success in the commercialization of our NEXAFED products; |
| · | announcements regarding the sales of our NEXAFED products; |
| · | failure of any of our products in development, if approved, to achieve commercial success; |
| · | quarterly variations in our results of operations or those of our competitors; |
| · | our ability to develop and mark new and enhanced products on a timely basis; |
| · | announcements by us or our competitors of acquisitions, regulatory approvals, clinical milestones, new products, significant contracts, commercial relationships or capital commitments; |
| · | third-party coverage and reimbursement policies; |
| · | additions or departures of key personnel; |
| · | commencement of, or our involvement in, litigation; |
| · | the inability of our contract manufacturers to provide us with adequate commercial supplies of our products; |
| · | changes in governmental regulations or in the status of our regulatory approvals; |
| · | changes in earnings estimates or recommendations by securities analysts; |
| · | any major change in our board or management; |
| · | general economic conditions and slow or negative growth of our market; and |
| · | political instability, natural disasters, war and/or events of terrorism. |
From time to time, we estimate the timing of the accomplishment of various scientific, clinical, regulatory and other product development goals or milestones. These milestones may include the commencement or completion of scientific studies and clinical trials and the submission of regulatory filings. Also, from time to time, we expect that we will publicly announce the anticipated timing of some of these milestones. All of these milestones are based on a variety of assumptions. The actual timing of these milestones can vary dramatically compared to our estimates, in some cases for reasons beyond our control. If we do not meet these milestones as publicly announced, our stock price may decline and the commercialization of our products and potential products may be delayed.
In addition, the stock market has experienced extreme price and volume fluctuations that have often been unrelated or disproportionate to the operating performance of publicly traded companies. Broad market and industry factors may seriously affect the market price of companies’ stock, including ours, regardless of actual operating performance. These fluctuations may be even more pronounced in the trading market for our stock. In addition, in the past, following periods of volatility in the overall market and the market price of a particular company’s securities, securities class action litigation has often been instituted against these companies. This litigation, if instituted against us, could result in substantial costs and a diversion of our management’s attention and resources.
We do not have a history of paying dividends on our common stock.
Historically, we have not declared and paid any cash dividends on our common stock. We intend to retain all of our earnings for the foreseeable future to finance the operation and expansion of our business. As a result, you may only receive a return on your investment in our common stock if the market price of our common stock increases.
Any future sale of a substantial number of shares included in our current registration statement could depress the trading price of our stock, lower our value and make it more difficult for us to raise capital.
In order to raise additional capital, we may in the future offer additional shares of our common stock or other securities convertible into or exchangeable for our common stock at prices that may not be the same as the then current trading price of our common stock. The price per share at which we sell additional shares of our common stock, or securities convertible or exchangeable into common stock, in future transactions may be higher or lower than the then current trading price of our common stock.
| 46 |
In accordance with the terms of the Securities Purchase Agreement dated August 20, 2007 between us and the investors named therein, we filed a registration statement with and declared effective by the SEC, to register the shares included in our Units issued pursuant to the Securities Purchase Agreement, including shares underlying warrants included in the Units. In addition, pursuant to the exercise of previously granted piggyback registration rights, each of Galen Partners III, L.P., Galen Partners International III, L.P., Galen Employee Fund III, L.P., Care Capital Investments II, LP, Care Capital Offshore Investments II, LP and Essex Woodlands Health Ventures V, L.P. have exercised their piggyback registration rights to include an aggregate of 26,584,016 shares in such registration statement. As a result, approximately 26,278,000 shares (representing approximately 48% of our shares outstanding on a fully-diluted basis, including all derivative securities, whether or not currently exercisable) are available for resale by selling stockholders under the registration statement. If some or all of the shares included in such registration statement are sold by our affiliates and others it may have the effect of depressing the trading price of our common stock. In addition, such sales could make it more difficult for us to raise capital if needed in the future.
In April 2013, we entered into an at-the-market equity facility, or ATM, with MLV & Co. LLC, or MLV, as sales agent under which we may sell up to approximately $13.0 million of our common stock under our prospectus supplement by any method deemed to be an “at-the-market” offering under SEC rules. As of December 31, 2014 we sold cumulatively approximately $3.3 million of common stock and issued 1,339,275 shares under the ATM. If we continue to sell shares under the ATM, such sales will dilute our existing shareholders and could cause the market price of our common stock to decline significantly. The availability of the ATM to us, as well as any sales of our common stock under the ATM, should we elect to continue to use it, could encourage short sales by third parties, which could contribute to the further decline of our stock price.
If we do not meet the continued listing standards of the NASDAQ Capital Market, our common stock could be delisted from trading, which could limit investors’ ability to make transactions in our common stock and subject us to additional trading restrictions.
Our common stock is listed on the NASDAQ Capital Market, a national securities exchange, which imposes continued listing requirements with respect to listed shares. If we fail to satisfy its continued listing standards, such as, for example, NASDAQ Listing Rule 5450(a)(1), which requires that the closing bid price of our common stock shall not fall below $1.00 for thirty consecutive business days. Failure to comply with NASDAQ’s continued listing standards will result in the issuance of a non-compliance letter and/or initiation of delisting proceedings by NASDAQ.
On September 18, 2014, we received a letter from the Listing Qualifications Staff of the NASDAQ Stock Market notifying us that because the closing bid price of our common stock has been below $1.00 for 30 consecutive business days, it no longer complies with the requirements for continued listing on the NASDAQ Capital Market. The NASDAQ notice does not impact our current listing on the NASDAQ Capital Market at this time and our common stock will continue to trade under the symbol “ACUR”. In accordance with NASDAQ rules, we have been provided a period of 180 calendar days, or until March 17, 2015, in which to regain compliance. In order to regain compliance with the minimum bid price requirement, the closing bid price of our common stock must be at least $1.00 per share for a minimum of 10 consecutive business days during this 180 day period. If we do not satisfy this requirement by March 17, 2015, NASDAQ will determine whether the Company meets the applicable market value of publicly held shares requirement for continued listing and all other applicable standards for initial listing on the NASDAQ Capital Market (except the bid price requirement). If we meet such criteria, of which no assurance can be given, we may be eligible for an additional 180 day compliance period. As part of NASDAQ’s determination of whether to grant us an additional 180 day compliance period, we will likely be required to commit to undertake a reverse stock split (including seeking shareholder approval for the reverse split) during such compliance period in order to meet NASDAQ’s minimum bid price requirement. If we do not regain compliance, our common stock will be subject to delisting.
We intend to monitor the bid price of our common stock between now and March 17, 2015, and will consider available options to regain compliance with the listing requirements, including seeking an additional 180 day compliance period, if needed. There can be no assurance that we will be able to regain compliance with the minimum bid price requirement or maintain compliance with other listing requirements.
If our securities are delisted from trading on the NASDAQ Capital Market and we are not able to list our securities on another exchange, such as the NYSE, our securities could then be quoted on the OTC Bulletin Board or on the “pink sheets.” As a result, we could face significant adverse consequences including:
| 47 |
| · | a limited availability of market quotations for our securities; |
| · | a determination that our common stock is a “penny stock,” which will require brokers trading in our common stock to adhere to more stringent rules and possibly result in a reduced level of trading activity in the secondary trading market for our securities; |
| · | a limited amount of news and analyst coverage for us; and |
| · | a decreased ability to issue additional securities (including pursuant to short-form registration statements on Form S-3 or obtain additional financing in the future). |
ITEM 1B. UNRESOLVED STAFF COMMENTS
The Company has received no written comments regarding periodic or current reports from the staff of the SEC that were issued 180 days or more preceding the end of its 2014 fiscal year that remain unresolved.
We lease from an unaffiliated Lessor, approximately 1,600 square feet of administrative office space at 616 N. North Court, Suite 120, Palatine, Illinois 60067. The lease agreement has a term expiring March 31, 2016. The lease agreement provides for rent, property taxes, common area maintenance, and janitorial services on an annualized basis of approximately $25,000 per year. We utilize this lease space for our administrative, marketing and business development functions.
We conduct research, development, laboratory, development scale and NDA submission batch scale manufacturing and other activities relating to developing product candidates using Aversion and Impede Technologies at the facility we own located at 16235 State Road 17, Culver, Indiana. At this location, our wholly-owned subsidiary Acura Pharmaceutical Technologies, Inc., is a 25,000 square foot facility with 7,000 square feet of warehouse, 8,000 square feet of manufacturing space, 4,000 square feet of research and development labs and 6,000 square feet of administrative and storage space. The facility is located on 28 acres of land.
Paragraph IV ANDA Litigation
On or about September 17, 2012, we believe the FDA internally changed the status of OXAYDO (then known as OXECTA) to be considered a Reference Listed Drug, or RLD. An RLD is the standard to which all generic versions must be shown to be bioequivalent and a drug company seeking approval to market a generic equivalent must refer to the RLD. By designating Oxaydo as an RLD, the FDA was allowed to accept ANDAs referencing OXAYDO.
On September 20, 2012, we announced that we had received a Paragraph IV Certification Notice under 21 U.S.C. 355(j) (a Paragraph IV Notice) from a generic sponsor of an ANDA for a generic drug listing OXAYDO as the reference listed drug. Since such date, we have received similar Paragraph IV Notices from three other generic pharmaceutical companies that have filed ANDAs listing OXAYDO as the reference drug. The Paragraph IV Notices refer to our U.S. Patent Numbers 7,201,920, 7,510,726 and 7,981,439, which cover our Aversion Technology and OXAYDO. The Paragraph IV Notices state that each generic sponsor believes that such patents are invalid, unenforceable or not infringed. On October 31, 2012, we initiated suit against each of Watson Laboratories, Inc. – Florida (Watson), Par Pharmaceutical, Inc., Impax Laboratories, Inc. and Sandoz Inc., and on April 29, 2013, we initiated suit against Ranbaxy, Inc., each in the United States District Court for the District of Delaware alleging infringement of our U.S. Patent No. 7,510,726 listed in the FDA’s Orange Book. The commencement of such litigation prohibits the FDA from granting approval of the filed ANDAs until the earliest of 30 months from the date the FDA accepted the application for filing, or the conclusion of litigation. In January 2013, we dismissed our suit against Watson on the grounds that Watson had amended its ANDA from a Paragraph IV Certification to a Paragraph III Certification, which indicated its intent not to market its generic OXAYDO product in advance of our patent expiring.
| 48 |
On October 9, 2013, we announced that we had entered into distinct Settlement Agreements with each of Par and Impax, to settle our patent infringement action pending against them in the United States District Court for the District of Delaware. In the suit, we alleged that a generic OXAYDO product for which each of Par and Impax is separately seeking approval to market in the United States pursuant to an ANDA filing with the FDA infringes a U.S. patent owned by us. Par is the first filer of an ANDA for a generic OXAYDO product and is entitled to the 180-day first filer exclusivity under applicable law and FDA regulations.
Under the terms of the Settlement Agreement with Par, Par may launch its generic OXAYDO product in the U.S., through the grant of a non-exclusive, royalty-bearing license from us that would trigger on January 1, 2022. We currently have Orange Book patents that are due to expire between November 2023 and March 2025. In certain limited circumstances, our license to Par would become effective prior to January 1, 2022. Par is required to pay us royalties in the range of 10% to 15% of Par’s net profits from the sale of its generic OXAYDO product.
Under the Settlement Agreement with Impax, Impax may launch its generic OXAYDO product in the U.S., through the grant of a non-exclusive, royalty-free license from us that would trigger 180 days following the first sale of a generic OXAYDO product in the U.S. by an entity that is entitled to the 180 day first-filer exclusivity under applicable law and FDA regulations (or if no entity is entitled to such 180 day exclusivity period, the date on which a generic OXAYDO product is first sold in the U.S. or November 27, 2021, whichever date occurs first). In certain circumstances, our license to Impax would become effective prior to such time.
On May 8, 2014, we announced that we had entered into a Settlement Agreement with Ranbaxy Inc. to settle our patent infringement action pending in the United States District Court for the District of Delaware. In the suit, we alleged that a generic of our OXAYDO product for which Ranbaxy is seeking approval to market in the United States pursuant to an ANDA filed with the FDA infringes U.S. patents owned by us. The Settlement Agreement provides that Ranbaxy’s current generic of our OXAYDO product that is the subject of its ANDA filing does not infringe our Orange Book listed patents with the FDA. We have not provided Ranbaxy a license to our patents and we may re-commence patent infringement litigation against Ranbaxy if Ranbaxy changes the formulation of its current generic OXAYDO product.
On May 21, 2014, we announced that we had entered into a Settlement Agreement with Sandoz Inc. to settle our patent infringement action pending against Sandoz in the United States District Court for the District of Delaware. In the suit, we alleged that a generic of our OXAYDO product for which Sandoz is seeking approval to market in the United States pursuant to an ANDA filed with the FDA infringes a U.S. patent owned by us. Under the Settlement Agreement, Sandoz may launch its generic to the OXAYDO product in the U.S., through the grant of a non-exclusive license from us that would trigger 180 days following the first sale of a generic to the Oxaydo product in the U.S. by an entity that is entitled to the 180 day first-filer exclusivity under applicable law and FDA regulations (or if no entity is entitled to such 180 day exclusivity period, the date on which a generic to the OXAYDO product is first sold in the U.S). In certain circumstances, our license to Sandoz would become effective prior to such time. Sandoz is not obligated to pay us a royalty if its current formulation of its generic to the OXAYDO product is approved by the FDA. In the event Sandoz changes or modifies the structure of its generic OXAYDO product, or materially changes or modifies the amounts or type of any excipient used in the Sandoz formulation disclosed in its ANDA filing with the FDA as of July 30, 2013, Sandoz is required to pay us a royalty based upon the Net Profits (as defined in the Settlement Agreement) derived from the net sales of such changed or modified Sandoz generic OXAYDO product in the United States.
Notwithstanding the settlement of these prior infringement actions, it is possible that other generic manufacturers may also seek to launch a generic version of OXAYDO and challenge our patents. Any determination in such infringement actions that our patents covering our Aversion Technology and OXAYDO are invalid or unenforceable, in whole or in part, or that the products covered by generic sponsors’ ANDAs do not infringe our patents could have a material adverse effect on our operations and financial condition.
| 49 |
By designating OXAYDO as an RLD, we believe the FDA has acknowledged that OXAYDO contains unique properties and/or a unique label that is different from other FDA approved immediate-release oxycodone HCl tablets that do not contain abuse resistant characteristics. As required by the Food and Drug Administration Safety and Innovation Act of July 2012, the FDA published for comment draft guidance on the development of abuse-deterrent drug products in January 2013. We believe the ANDA applicants that refer to OXAYDO as an RLD will have to have substantially equivalent, if not identical, abuse deterrent characteristics to be considered by the FDA as therapeutically equivalent to OXAYDO. There can be no assurance, however, that FDA will rely on such guidance for ANDA applicants.
Reglan®/Metoclopramide Litigation
Halsey Drug Company, as predecessor to us, has been named along with numerous other companies as a defendant in cases filed in three separate state coordinated litigations pending in Pennsylvania, New Jersey and California, respectively captioned In re: Reglan®/Metoclopramide Mass Tort Litigation, Philadelphia County Court of Common Pleas, January Term, 2010, No. 01997; In re: Reglan Litigation, Superior Court of New Jersey, Law Division, Atlantic County, Case No. 289, Master Docket No. ATL-L-3865-10; and Reglan/Metoclopramide Cases, Superior Court of California, San Francisco County, Judicial Council Coordination Proceeding No. 4631, Superior Court No.: CJC-10-004631. In addition, Acura was served with a similar complaint by two individual plaintiffs in Nebraska federal court, which plaintiffs voluntarily dismissed in December 2014. In this product liability litigation against numerous pharmaceutical product manufacturers and distributors, including us, plaintiffs claim injuries from their use of the Reglan brand of metoclopramide and generic metoclopramide.
In the Pennsylvania action, over 200 lawsuits have been filed against us and Halsey Drug Company alleging that plaintiffs developed neurological disorders as a result of their use of the Reglan brand and/or generic metoclopramide. In the New Jersey action, plaintiffs filed approximately 150 lawsuits against us, but served less than 50 individual lawsuits upon us. In the California action, there are 89 pending cases against us, with more than 445 individual plaintiffs.
In the lawsuits filed to date, plaintiffs have not confirmed they ingested any of the generic metoclopramide manufactured by us. We discontinued manufacture and distribution of generic metoclopramide more than 18 years ago. In addition, we believe the June 23, 2011 decision by the U.S. Supreme Court in PLIVA v. Mensing (“Mensing decision”) holding that state tort law failure to warn claims against generic drug companies are pre-empted by the 1984 Hatch-Waxman Act Amendments and federal drug regulations will assist us in favorably resolving these cases. We have consistently maintained the position that these claims are without merit and intend to vigorously defend these actions.
In New Jersey, Generic Defendants, including Acura, filed dispositive motions based on the Mensing decision, which the Court granted with a limited exception. As of September 2012, the New Jersey trial court dismissed Acura with prejudice. In Pennsylvania, and California, Generic Defendants, including Acura, also filed dispositive motions based on the Mensing decision.
In Pennsylvania, on November 18, 2011, the trial court denied Generic Defendants’ dispositive preemption motions. On appeal, the Pennsylvania Superior Court held in a July 29, 2013 decision that federal preemption applied, but that Mensing did not completely bar all claims and refused to dismiss these cases. On September 17, 2014, the Pennsylvania Supreme Court declined to hear a further appeal. On December 16, 2014, Generic Defendants filed a Petition for a Writ of Certiorari requesting that the United States Supreme Court agree to hear a further appeal on the grounds that federal preemption under Mensing should completely bar all of these claims. All trial court proceedings have been stayed pending resolution of this lengthy appeal process. To the extent that plaintiffs intend to pursue their claims in the future, if the appeal is denied, Acura nonetheless remains optimistic that most, if not all, of these Philadelphia cases will eventually be dismissed against us based upon the favorable aspects of the Superior Court’s narrow preemption ruling and lack of product identification, although there can be no assurance in this regard. Legal fees related to this matter are currently covered by our insurance carrier.
| 50 |
In California, the trial court entered a May 25, 2012 Order denying Generic Defendants’ dispositive preemption motions. The Generic Defendants’ appeals from this order were denied by the California appellate courts. Therefore, subject to further developments, plaintiffs may be permitted to proceed with these lawsuits including state law claims based on (1) failing to communicate warnings to physicians through “Dear Doctor” letters; and (2) failure to update labeling to adopt brand labeling changes. The California trial court also has acknowledged the preemptive effect of Mensing so that any claim “that would render the generic defendants in violation of federal law if they are found responsible under a state law cause of action, would not be permissible.” To date, however, none of these plaintiffs have confirmed they ingested any of the generic metoclopramide manufactured by us. Therefore, we expect the number of plaintiffs with possible claims to be reduced voluntarily or by motion practice. Action will be taken in an effort to dismiss Acura from these cases, although there can be no assurance in this regard. Legal fees related to this matter are currently covered by our insurance carrier.
As any potential loss is neither probable nor estimable, we have not accrued for any potential loss related to these matters as of December 31, 2014 and we are presently unable to determine if any potential loss would be covered by our insurance carrier.
ITEM 4. MINE SAFETY DISLCOSURES
Not Applicable.
| 51 |
ITEM 5. MARKET FOR REGISTRANT'S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
Market and Market Prices of Common Stock
Set forth below for the periods indicated are the high and low sales prices for trading in our common stock on the NASDAQ Capital Market as reported by the NASDAQ Capital Market.
| Period | Sale Prices | |||||||
| High | Low | |||||||
| 2013 Fiscal Year | ||||||||
| First Quarter | $ | 3.62 | $ | 1.81 | ||||
| Second Quarter | 3.78 | 1.85 | ||||||
| Third Quarter | 2.59 | 1.36 | ||||||
| Fourth Quarter | 2.23 | 1.50 | ||||||
| 2014 Fiscal Year | ||||||||
| First Quarter | 2.12 | 1.44 | ||||||
| Second Quarter | 1.55 | 0.98 | ||||||
| Third Quarter | 1.13 | 0.68 | ||||||
| Fourth Quarter | 0.78 | 0.41 | ||||||
| 2015 Fiscal Year | ||||||||
| First Quarter (through January 31, 2015) | $ | 0.70 | $ | 0.45 | ||||
On September 18, 2014, we received a letter from the Listing Qualifications Staff of the NASDAQ Stock Market notifying us that because the closing bid price of our common stock has been below $1.00 for 30 consecutive business days, it no longer complies with the requirements for continued listing on the NASDAQ Capital Market. The NASDAQ notice does not impact our current listing on the NASDAQ Capital Market at this time and our common stock will continue to trade under the symbol “ACUR”. In accordance with NASDAQ rules, we have been provided a period of 180 calendar days, or until March 17, 2015, in which to regain compliance. In order to regain compliance with the minimum bid price requirement, the closing bid price of our common stock must be at least $1.00 per share for a minimum of 10 consecutive business days during this 180 day period. If we do not satisfy this requirement by March 17, 2015, NASDAQ will determine whether the Company meets the applicable market value of publicly held shares requirement for continued listing and all other applicable standards for initial listing on the NASDAQ Capital Market (except the bid price requirement). If we meet such criteria, of which no assurance can be given, we may be eligible for an additional 180 day compliance period. As part of NASDAQ’s determination of whether to grant us an additional 180 day compliance period, we will likely be required to commit to undertake a reverse stock split (including seeking shareholder approval for the reverse split) during such compliance period in order to meet NASDAQ’s minimum bid price requirement. If we do not regain compliance, our common stock will be subject to delisting.
We intend to monitor the bid price of our common stock between now and March 17, 2015, and will consider available options to regain compliance with the listing requirements, including seeking an additional 180 day compliance period, if needed. There can be no assurance that we will be able to regain compliance with the minimum bid price requirement or maintain compliance with other listing requirements.
Holders
There were approximately 750 holders of record of our common stock on February 27, 2015. This number, however, does not reflect the ultimate number of beneficial holders of our common stock.
| 52 |
Dividend Policy
The payment of cash dividends is subject to the discretion of our Board of Directors and is dependent upon many factors, including our earnings, our capital needs and our general financial condition. Historically, we have not paid any cash dividends.
Securities Authorized for Issuance under Equity Compensation Plans
Reference is made to the Company’s Proxy Statement for its 2014 Annual Meeting of Shareholders under the caption “Compensation of Executive Officers and Directors - Restricted Stock Unit Award Plan; and Securities Authorized for Issuance under Equity Compensation Plans”.
ITEM 6. SELECTED FINANCIAL DATA
The selected consolidated financial data presented below for the years ended December 31, 2014, 2013, 2012, 2011 and 2010 are derived from our audited Consolidated Financial Statements. The Consolidated Financial Statements as of December 31, 2014 and 2013 and for each of the years in the three-year period ended December 31, 2014, and the reports thereon, are included elsewhere in this Report. The selected financial information presented for our 2011 and 2010 operations and for our 2012, 2011 and 2010 balance sheets are derived from our audited Consolidated Financial Statements not presented in this Report.
The information set forth below is qualified by reference to, and should be read in conjunction with, the Consolidated Financial Statements and related notes thereto included elsewhere in this Report and "Item 7. Management's Discussion and Analysis of Financial Condition and Results of Operations".
| OPERATING DATA (in thousands) except per share data | 2014 | 2013 | 2012 | 2011 | 2010 | |||||||||||||||
| Revenues, net | $ | 751 | $ | 123 | $ | — | $ | 20,466 | $ | 3,311 | ||||||||||
| Operating expenses: | ||||||||||||||||||||
| Cost of sales | 428 | 364 | — | — | — | |||||||||||||||
| Research and development(1) | 4,582 | 4,923 | 3,726 | 4,037 | 7,177 | |||||||||||||||
| Selling, marketing, general and administrative(2) | 7,940 | 8,926 | 6,013 | 5,895 | 8,858 | |||||||||||||||
| Interest expense | (1,212 | ) | (9 | ) | — | — | — | |||||||||||||
| Investment income | 198 | 194 | 79 | 32 | 42 | |||||||||||||||
| Other (expense) income | 4 | 4 | (8 | ) | (34 | ) | (14 | ) | ||||||||||||
| (Loss) income before income tax | (13,209 | ) | (13,901 | ) | (9,668 | ) | 10,532 | (12,696 | ) | |||||||||||
| Provision for income taxes | - | — | — | 147 | 11 | |||||||||||||||
| Net (loss) income applicable to common stockholders | $ | (13,209 | ) | $ | (13,901 | ) | (9,668 | ) | $ | 10,385 | $ | (12,707 | ) | |||||||
| (Loss) earnings per share: Basic | $ | (0.27 | ) | $ | (0.29 | ) | $ | (0.20 | ) | $ | 0.22 | $ | (0.27 | ) | ||||||
| (Loss) earnings per share: Diluted | $ | (0.27 | ) | $ | (0.29 | ) | $ | (0.20 | ) | $ | 0.22 | $ | (0.27 | ) | ||||||
| Weighted average shares used in computing net earnings (loss) per share: Basic | 48,893 | 47,764 | 47,521 | 47,496 | 47,029 | |||||||||||||||
| Weighted average shares used in computing net earnings (loss) per share: Diluted | 48,893 | 47,764 | 47,521 | 48,007 | 47,029 | |||||||||||||||
| (1) Includes stock-based compensation expense of approximately $220, $300, $375, $500 and $1,700 for years 2014, 2013, 2012, 2011, and 2010, respectively. |
| (2) Includes stock-based compensation expense of approximately $700, $900, $1,360, $1,900, and $5,100 for years 2014, 2013, 2012, 2011 and 2010, respectively. |
| 53 |
BALANCE SHEET DATA (in thousands) | 2014 | 2013 | 2012 | 2011 | 2010 | |||||||||||||||
| Working capital | $ | 10,239 | $ | 26,346 | $ | 26,572 | $ | 35,599 | $ | 23,289 | ||||||||||
| Total assets | 16,195 | 28,630 | 29,054 | 37,173 | 25,493 | |||||||||||||||
| Total liabilities | 11,143 | 10,707 | 1,424 | 530 | 1,152 | |||||||||||||||
| Accumulated deficit | (362,321 | ) | (349,112 | ) | (335,211 | ) | (325,543 | ) | (335,928 | ) | ||||||||||
| Stockholders' equity | $ | 5,052 | $ | 17,923 | $ | 27,630 | $ | 36,643 | $ | 24,341 | ||||||||||
ITEM 7. MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
This discussion and analysis should be read in conjunction with our financial statements and accompanying notes included elsewhere in this Report. Operating results are not necessarily indicative of results that may occur in the future periods. Certain statements in this Report under this Item 7, Item 1, "Business", Item 1A, “Risk Factors” and elsewhere in this Report constitute "forward-looking statements" within the meaning of the Private Securities Litigation Reform Act of 1995. Such forward-looking statements involve known and unknown risks, uncertainties and other factors which may cause our actual results, performance or achievements or industry results, to be materially different from any future results, performance, or achievements expressed or implied by such forward-looking statements. See page 1 of this Report under the caption “Forward-Looking Statements” for a description of the most significant of such factors.
Company Overview
We are a specialty pharmaceutical company engaged in the research, development and commercialization of technologies and products intended to address medication abuse and misuse. We have discovered and developed three proprietary platform technologies which can be used to develop multiple products. Our Aversion® Technology is a mixture of inactive ingredients incorporated into pharmaceutical tablets and capsules intended to address some common methods of product tampering associated with opioid abuse. Oxaydo Tablets (formerly known as Oxecta)(oxycodone HCl CII), is the first FDA approved product utilizing Aversion in the United States and is exclusively licensed to Egalet for commercialization. We have 7 additional opioid products utilizing Aversion in various stages of development. The FDA approved label for our Oxaydo product describes the unique, and we believe promotable, abuse deterrent features of our product which we believe makes prescribing our product attractive to some healthcare providers. We have also developed Impede® Technology which is a combination of inactive ingredients that prevent the extraction of pseudoephedrine from tablets and disrupt the direct conversion of pseudoephedrine from tablets into methamphetamine. We launched our first Impede Technology product, Nexafed®, into the United States market in December 2012 and launched our Nexafed Sinus Pressure + Pain product in February 2015. We have multiple pseudoephedrine products in development utilizing Impede. In August 2014, we received a grant from the National Institute on Drug Abuse to advance early stage development of our third abuse deterrent technology, Limitx™. Limitx is designed to retard the release of active drug ingredients when too many tablets are accidently or purposefully ingested.
Hydrocodone bitartrate with acetaminophen, or hydrocodone/acetaminophen, is the most widely prescribed and often abused opioid product in the United States. Our Aversion hydrocodone/acetaminophen product is the most advanced opioid product in development and the primary focus of our opioid development efforts. On December 5, 2013, we met with the FDA to discuss the results of Study AP-ADF-301, or Study 301, a key abuse liability study for our Aversion hydrocodone/acetaminophen product and whether the results from Study 301 are acceptable for submission in a New Drug Application, or NDA. On May 27, 2014, we announced that the FDA advised us that the data from our Study 301 was insufficient to support an intranasal abuse deterrence description in our product labeling. The FDA indicated that a product will have to have an impact on “drug liking” to support a description of abuse-deterrence through a relevant route of abuse. The FDA’s advice also questioned whether the intranasal route is a relevant route of abuse for hydrocodone/ acetaminophen products.
| 54 |
On September 11, 2014, we submitted a formal dispute resolution request with the FDA. The dispute pertains to the FDA's determination that nasal snorting abuse of hydrocodone with acetaminophen products lacks relevance. On October 16, 2014, we announced that the FDA denied on procedural grounds our appeal of the position taken by the Division of Anesthesia, Analgesia and Addiction Products (“DAAAP”) that abuse by snorting hydrocodone with acetaminophen products lacks relevance. We are currently assessing our development strategy for our Aversion hydrocodone with acetaminophen product, including the merits of appealing the FDA’s decision. Even if we were to file an appeal and succeed in such proceeding, in order to continue the development of our hydrocodone/acetaminophen product we will be required to conduct an additional abuse liability study that will need to demonstrate a statistically significant reduction in Drug Liking, of which no assurance can be provided. We are currently in the process of designing this next study and will make a decision on commencing this study in 2015.
We expect that the development program for all our Aversion opioid products in development will be consistent with that of Aversion Oxycodone and our hydrocodone/acetaminophen product candidate.
In 2009, the United States market for over-the-counter market, or OTC, cold and allergy products containing an oral nasal decongestant was approximately $1 billion. In 2012, the DEA reported 11,210 laboratory incidents involving the illegal use of OTC pseudoephedrine products to manufacture the highly addictive drug methamphetamine, or meth. Nexafed, our 30mg pseudoephedrine hydrochloride immediate-release tablet is stocked in approximately 19% of the estimated 65,000 U.S. pharmacies. Nexafed Sinus Pressure + Pain, our 30/325 mg pseudoephedrine and acetaminophen immediate–release tablet was launched in February 2015. Both Nexafed products utilizing our Impede 1.0 Technology. Many of these pharmacies are either actively recommending Nexafed to their patients or carry Nexafed as their only 30mg pseudoephedrine product. The category for meth-resistant pseudoephedrine products has also been the focus of some, as yet unsuccessful, state legislation seeking to incentivize consumers and pharmacists to utilize these meth-resistant technologies.
We have an active development program to develop an extended-release version of our technology to capitalize on higher sales products in the category. We also are investigating new technologies that would improve on our meth-resistant capabilities.
We also have discovered an early-stage technology, Limitx™, which, in proof of concept laboratory tests, demonstrates the ability to limit the release of the active ingredient from tablets when multiple tablets are consumed simultaneously.
Company’s Present Financial Condition
At December 31, 2014, we had cash, cash equivalents and marketable securities of $12.1 million compared to $26.1 million of cash, cash equivalents and marketable securities at December 31, 2013. We had working capital of $10.2 million at December 31, 2014, compared to working capital of $26.3 million at December 31, 2013. We had an accumulated deficit of approximately $362.3 million and $349.0 million at December 31, 2014 and December 31, 2013, respectively. We had a loss from operations of $12.2 million and a net loss of $13.2 million for the year ended December 31, 2014, compared to a loss from operation of $14.1 million and a net loss of $13.9 million for the year ended December 31, 2013. As of January 31, 2015, we had cash, cash equivalents and marketable securities of approximately $15.9 million.
During the year ended December 31, 2014, we recognized $247 thousand of product sales on gross shipments of Nexafed which totaled $327 thousand. Certain of our customers have accepted a pricing allowance in exchange for forfeiting the right to return Nexafed and therefore we are recognizing revenue upon product shipment to them. Additionally, we are recognizing revenue from certain of our other customers based on a pharmacy’s Nexafed reorder activity with their own drug wholesaler supplier. For all other customers, given the limited sales history of Nexafed, the Company currently cannot reliably estimate expected returns of the product from these customers at the time of shipment. Accordingly, the Company is deferring recognition of revenue on these product shipments of Nexafed until the right of return no longer exists or adequate history and information is available to estimate product returns.
| 55 |
To fund our continued operations, we expect to rely on our current cash resources, net proceeds, if any, from our “at-the-market” offering of our common stock pursuant to our Sales Agreement with MLV & Co., milestone and royalty payments, if any, that may be made under Egalet Agreement, milestones and royalty payments that may be made under future license agreements with other pharmaceutical company partners, of which there can be no assurance of obtaining, and revenues from our commercialization of our Nexafed products. Our cash requirements for operating activities may increase in the future as we continue to conduct pre-clinical studies and clinical trials for our product candidates, maintain, defend, and expand the scope of our intellectual property, hire additional personnel, commercialize our Nexafed products, or invest in other areas.
Our losses have resulted principally from costs incurred in connection with research and development activities, salaries and other personnel-related costs and general corporate expenses. Research and development activities include costs of pre-clinical studies, clinical trials, and clinical trial product supplies associated with our product candidates. Salaries and other personnel-related costs include the non-cash stock-based compensation associated with stock options and restricted stock units granted to employees and non-employee directors.
Results of Operations for the Years Ended December 31, 2014 and 2013.
| December 31 | ||||||||||||||||
| 2014 | 2013 | Change | ||||||||||||||
| $000’s | $000’s | Percent | ||||||||||||||
| Revenues: | ||||||||||||||||
| Royalty revenue | $ | 4 | $ | 10 | $ | (6 | ) | 60 | ||||||||
| Product sales, net | 247 | 113 | 134 | 118 | ||||||||||||
| License fee | 500 | — | 500 | 100 | ||||||||||||
| Total revenues, net | 751 | 123 | 628 | 510 | ||||||||||||
| Operating expenses: | ||||||||||||||||
| Cost of sales | 428 | 364 | 64 | 17 | ||||||||||||
| Research and development | 4,582 | 4,923 | (341 | ) | (7 | ) | ||||||||||
| Selling, marketing, general and administrative | 7,940 | 8,926 | (986 | ) | (11 | ) | ||||||||||
| Total operating expenses | 12,950 | 14,213 | (1,263 | ) | (9 | ) | ||||||||||
| Operating loss | (12,199 | ) | (14,090 | ) | (1,895 | ) | (13 | ) | ||||||||
| Non-Operating income (expense): | ||||||||||||||||
| Investment income | 198 | 194 | 4 | 2 | ||||||||||||
| Other expense, net | (1,208 | ) | (5 | ) | (1,203 | ) | 24,060 | |||||||||
| Total other income (expense), net | (1,010 | ) | 189 | (1,199 | ) | (634 | ) | |||||||||
| Loss before income taxes | (13,209 | ) | (13,901 | ) | 692 | 5 | ||||||||||
| Provision for income taxes | — | — | — | — | ||||||||||||
| Net loss | $ | (13,209 | ) | $ | (13,901 | ) | $ | 692 | 5 | |||||||
Revenue
Product Sales
Nexafed® was launched in mid-December 2012. The Company sells Nexafed® in the United States to wholesale pharmaceutical distributors and as well as directly to chain drug stores. Nexafed® is sold subject to the right of return for a period of up to twelve months after the product expiration. Nexafed® currently has a shelf life of twenty-four months from the date of manufacture. Given the limited sales history of Nexafed®, the Company currently cannot reliably estimate expected returns of the product at the time of shipment to certain customers. Accordingly, the Company has deferred the recognition of revenue on Nexafed® shipments to these customers until the right of return no longer exists or adequate history and information becomes available to estimate product returns. We have recognized revenue of $247 thousand in 2014 for shipments to customers where the right of return no longer existed either because a pricing allowance was accepted in exchange for forfeiting the right of return or because information became available on pharmacy’s reorder activity with their drug wholesaler. We will continue to analyze information to assess the recognition of our revenue. As of December 31, 2014, we had $353 thousand of deferred revenue on our balance sheet related to Nexafed shipments which occurred since its commercial launch.
| 56 |
Royalty Revenue
In connection with our License, Development, and Commercialization Agreement dated October 30, 2007 with King Pharmaceuticals Research and Development, Inc., now a subsidiary of Pfizer Inc., (which agreement has since been terminated effective April 2014) we began to earn royalties on Oxecta net sales starting in February 2013. We recorded royalties of approximately $4 thousand for 2014 (January 1 to April 2014) on Pfizer’s net sales of Oxecta of approximately $80 thousand.
License Fee
The Company entered into an agreement with Purdue Pharma to settle a patent interference action regarding certain intellectual property held by Acura (U.S. Patent No. 8,101,630). The dispute centered upon the issue of which company has priority in developing the invention. The parties agreed to forgo protracted litigation and the uncertainties arising therefrom by entering an agreement whereby Acura conceded Purdue’s claim of priority in exchange for certain financial consideration including an immediate non-refundable payment of $500,000.
Cost of Sales
Cost of sales includes third-party acquisition costs, third-party warehousing and product distribution charges and inventory reserve charges for the Nexafed product line.
Operating Expenses
Research and development expense (R&D) during 2014 and 2013 was primarily for our Aversion or our Impede Technologies development including costs of preclinical, clinical trials, clinical supplies and related formulation and design costs, salaries and other personnel related expenses, and facility costs. Included in each of the 2014 and 2013 results are non-cash share-based compensation expenses of $0.2 million and $0.3 million, respectively. Excluding the share-based compensation expense, our R&D expenses decreased approximately $0.2 million between reporting periods primarily from a reduction in Aversion development expenses on our hydrocodone/acetaminophen product candidate as we awaited response from the FDA to clinical results before continuing with additional development.
Selling and marketing expenses during 2014 and 2013 primarily consisted of advertising and marketing activities on Nexafed which was launched in December 2012. Our Nexafed advertising and marketing activities will continue in 2015. Our general and administrative expenses primarily consisted of legal, audit and other professional services, corporate insurance, and payroll. Included in the 2014 and 2013 results are non-cash share-based compensation expenses of $0.7 million and $0.9 million, respectively. Excluding the share-based compensation expense our selling, marketing, general and administrative expenses decreased approximately $0.8 million between reporting periods, resulting primarily from a reduction in legal services on our paragraph IV litigation, the majority of which was settled in 2013.
Non-Operating Income (Expense)
During 2014 non-operating expense consisted principally of interest expense on the $10.0 million promissory note entered into in December 2013 less investment income derived from our investments. During 2013 non-operating income consisted principally of investment income derived from our investments.
| 57 |
Income Taxes
The net loss for 2014 and 2013 includes no federal or state income tax benefit provisions due to uncertainty of their future utilization. The Company did record $4 thousand of state tax expense in 2014.
Results of Operations for the Years Ended December 31, 2013 and 2012
| December 31 | ||||||||||||||||
| 2013 | 2012 | Change | ||||||||||||||
| $000’s | $000’s | Percent | ||||||||||||||
| Revenues: | ||||||||||||||||
| Royalty revenue | $ | 10 | $ | - | $ | 10 | 100 | |||||||||
| Product sales, net | 113 | - | 113 | 100 | ||||||||||||
| Total revenues, net | 123 | — | 123 | 100 | ||||||||||||
| Operating expenses: | ||||||||||||||||
| Cost of sales | 364 | — | 364 | 100 | ||||||||||||
| Research and development | 4,923 | 3,726 | 1,197 | 32 | ||||||||||||
| Selling, marketing, general and administrative | 8,926 | 6,013 | 2,913 | 48 | ||||||||||||
| Total operating expenses | 14,213 | 9,739 | 4,474 | 46 | ||||||||||||
| Operating loss | (14,090 | ) | (9,739 | ) | 4,351 | 45 | ||||||||||
| Non-Operating income (expense): | ||||||||||||||||
| Investment income | 194 | 79 | 115 | 146 | ||||||||||||
| Other expense, net | (5 | ) | (8 | ) | (3 | ) | (3 | ) | ||||||||
| Total other income, net | 189 | 71 | 118 | 166 | ||||||||||||
| Loss before income taxes | (13,901 | ) | (9,668 | ) | 4,233 | 44 | ||||||||||
| Provision for income taxes | — | — | — | — | ||||||||||||
| Net loss | $ | (13,901 | ) | $ | (9,668 | ) | $ | 4,233 | 44 | |||||||
Revenue
Product Sales
Nexafed® was launched in mid-December 2012. The Company sells Nexafed® in the United States to wholesale pharmaceutical distributors and as well as directly to chain drug stores. Nexafed® is sold subject to the right of return for a period of up to twelve months after the product expiration. Nexafed® currently has a shelf life of twenty-four months from the date of manufacture. Given the limited sales history of Nexafed®, the Company currently cannot reliably estimate expected returns of the product at the time of shipment to certain customers. Accordingly, the Company has deferred the recognition of revenue on $0.3 million of Nexafed® shipments to these customers until the right of return no longer exists or adequate history and information becomes available to estimate product returns. We have recognized revenue of $113 thousand in 2013 for shipments to customers where the right of return no longer existed either because a pricing allowance was accepted in exchange for forfeiting the right of return or because information became available on pharmacy’s reorder activity with their drug wholesaler. We will continue to analyze information to assess the recognition of our revenue but also expect to continue the deferral of some revenue in the foreseeable future. As of December 31, 2013, we had $0.3 million in deferred revenue on our balance sheet related to Nexafed shipments which occurred since its commercial launch. We had no net product sales during 2012.
Royalty Revenue
In connection with our License, Development, and Commercialization Agreement dated October 30, 2007 with King Pharmaceuticals Research and Development, Inc., now a subsidiary of Pfizer Inc., (which agreement has since been terminated effective April 2014) we began to earn royalties on Oxecta net sales starting in February 2013. We recorded royalties of approximately $10 thousand for 2013 on Pfizer’s net sales of Oxecta of approximately $0.2 million.
| 58 |
Cost of Sales
Cost of sales includes third-party acquisition costs, third-party warehousing and product distribution charges and inventory reserve charges for the Nexafed product line. During 2013, we established an inventory reserve of $0.25 million.
Operating Expenses
Research and development expense (R&D) during 2013 were primarily for our Aversion development expenses and during 2012 were for product candidates utilizing either our Aversion or our Impede® Technologies, including costs of preclinical, clinical trials, clinical supplies and related formulation and design costs, salaries and other personnel related expenses, and facility costs. Included in each of the 2013 and 2012 results are non-cash share-based compensation expenses of $0.3 million and $0.4 million, respectively. Excluding the share-based compensation expense, our R&D expenses increased approximately $1.3 million between reporting periods primarily from our Aversion development expenses on our hydrocodone/acetaminophen product candidate.
Selling and marketing expenses during 2013 primarily consisted of advertising and marketing activities on Nexafed which was launched in December 2012. Selling and marketing expenses during 2012 primarily consisted of market research studies on our Aversion and Impede® Technologies. Our Nexafed advertising and marketing activities will continue in 2014. Our general and administrative expenses primarily consisted of legal, audit and other professional services, corporate insurance, and payroll. Included in the 2013 and 2012 results are non-cash share-based compensation expenses of $0.9 million and $1.3 million, respectively. Excluding the share-based compensation expense our selling, marketing, general and administrative expenses increased approximately $3.4 million between reporting periods, primarily for the marketing, advertising and promotional programs on Nexafed of $1.1 million, compensation costs of $0.2 million, legal services on our paragraph IV litigation of $1.7 million, patent and trademark services of $0.3 million and general corporate legal matters of $0.1 million.
Non-Operating Income
During 2013 and 2012, non-operating income consisted principally of investment income derived from our cash reserves being invested in accordance with a Board of Director approved investment policy.
Income Taxes
The net loss for 2013 and 2012 includes no federal or state income tax benefit provisions due to uncertainty of their future utilization.
Liquidity and Capital Resources
At December 31, 2014, we had cash, cash equivalents and marketable securities of $12.1 million compared to $26.1 million in cash and cash equivalents at December 31, 2013. We had working capital of $10.2 million at December 31, 2014 compared to $26.3 million at December 31, 2013. Our investing activities for capital expenditures were $135 thousand in 2014 and $23 thousand in 2013.
Pending the receipt of milestone and royalty payments under the Egalet Agreement and similar agreements for our products in development anticipated to be negotiated and executed with other pharmaceutical company partners, of which no assurance can be given, we must rely on revenues from our Nexafed products sales, the net proceeds, if any, from our “at-the-market” offering of our common stock pursuant to our Sales Agreement with MLV & Co., and our current investments, including interest income from investments, to fund the development of our Aversion Technology, Impede Technology, Limitx Technology and related administrative and operating expenses. Our future sources of revenue, if any, will be derived from milestone payments and royalties under the Egalet Agreement and similar agreements for our products in development with other pharmaceutical company partners, for which there can be no assurance, and from the commercialization of our Nexafed products and other Impede Technology products that we expect to develop.
| 59 |
At January 31, 2015, we had cash, cash equivalents and marketable securities of approximately $15.9 million. We estimate that such cash reserves will be sufficient to fund the development of Aversion Technology and Impede Technology product candidates, and related operating expenses at least through the next 12 months.
The amount and timing of our future cash requirements will depend on regulatory and market acceptance of our product candidates and the resources we devote to the development and commercialization of our product candidates.
The following table presents our expected cash payments on contractual obligations outstanding as of December 31, 2014:
| Payments due by period (in thousands) | ||||||||||||||||||||
| Total | Less than 1 year | 1-3 years | 3-5 years | More than 5 years | ||||||||||||||||
| Operating leases | $ | 33 | $ | 26 | $ | 7 | $ | — | $ | — | ||||||||||
| Contract manufacturing | 492 | 492 | — | — | — | |||||||||||||||
| Clinical studies | 104 | 104 | — | — | — | |||||||||||||||
| Marketing and advertising | — | — | — | — | — | |||||||||||||||
| Employment agreements | 677 | 677 | — | — | — | |||||||||||||||
| Debt principal | 10,000 | 1,758 | 5,263 | 2,979 | — | |||||||||||||||
| Debt interest | 2,686 | 787 | 967 | 932 | — | |||||||||||||||
| Total | $ | 14,497 | $ | 4,349 | $ | 6,237 | $ | 3,911 | $ | — | ||||||||||
Term Loan with Oxford Finance
On December 27, 2013, we and our subsidiary, Acura Pharmaceutical Technologies, Inc. entered into a Loan and Security Agreement (the “Loan Agreement”) with Oxford Finance LLC (“Oxford”), as collateral agent and as a lender, pursuant to which the Oxford made a term loan to us in the principal amount of $10.0 million (the “Term Loan”), subject to the terms and conditions set forth in the Loan Agreement. We are using the proceeds of the Loan Agreement for general working capital and to fund our business requirements.
The full principal amount of the Term Loan was funded on December 27, 2013. The Term Loan accrues interest at a fixed rate of 8.35% per annum (with a default rate of 13.35% per annum). We are required to make monthly interest−only payments until April 1, 2015 and starting on April 1, 2015, we are required to make payments of principal and accrued interest in equal monthly installments sufficient to amortize the Term Loan through the maturity date of December 1, 2018. All unpaid principal and accrued and unpaid interest with respect to the Term Loan is due and payable in full on December 1, 2018. As security for our obligations under the Loan Agreement, we granted Oxford a security interest in substantially all of our existing and after−acquired assets, exclusive of intellectual property assets. Pursuant to the Loan Agreement, we are not allowed to pledge our intellectual property assets to others.
On January 7, 2015, we and Oxford entered into an amendment (the “Amendment”) to the Loan Agreement. Pursuant to the Amendment, (i) the exercise price of the warrant previously issued to the Lender to purchase 297,805 shares of our Common Stock was lowered from $1.59 to $0.504 per share (the average closing price of our common stock on Nasdaq for the 10 trading days preceding the date of the Amendment), (ii) we agreed to maintain a $2.5 million cash balance until such time as we have repaid $5 million in principal of the Term Loan, and (iii) the Lender consented to the terms of our Collaboration and License Agreement with Egalet relating to our Oxaydo product.
| 60 |
We may voluntarily prepay the Term Loan in full, but not in part, and any prepayment is subject to a prepayment premium equal to 2% of the principal prepaid, if prepaid prior to December 27, 2015, and 1% of the principal prepaid if prepaid after December 27, 2015. In addition, at the maturity, termination or upon voluntary or mandatory prepayment of the Term Loan, we must pay Oxford an additional one-time interest payment of $795,000.
The Loan Agreement contains customary representations and warranties and customary affirmative and negative covenants, including, among others, limits or restrictions on our ability to incur liens, incur indebtedness, pay dividends, redeem stock, and merge or consolidate and dispose of assets. In addition, it contains customary events of default that entitles Oxford to cause any or all of our indebtedness under the Loan Agreement to become immediately due and payable. The events of default (some of which are subject to applicable grace or cure periods), include, among other things, non−payment defaults, covenant defaults, a material adverse change affecting us or our operations, bankruptcy and insolvency defaults and material judgment defaults.
The warrants to purchase 297,805 shares of our common stock we issued to Oxford in connection with the Term Loan, having an exercise price of $0.504 per share (as adjusted pursuant to the Amendment) are immediately exercisable for cash or by net exercise and will expire December 27, 2020.
Off-Balance Sheet Arrangements
We do not engage in transactions or arrangements with unconsolidated or other special purpose entities.
Critical Accounting Policies
The preparation of our financial statements in accordance with United States generally accepted accounting principles requires us to make estimates and assumptions that affect the reported amounts of assets, liabilities, revenues and expenses in our financial statements and accompanying notes. We evaluate our estimates on an ongoing basis, including those estimates related to contract agreements, research collaborations and investments. We base our estimates on historical experience and various other assumptions that we believe to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions. The following items in our financial statements require significant estimates and judgments:
Revenue Recognition
Revenue is generally realized or realizable and earned when there is persuasive evidence an arrangement exists, delivery has occurred or services rendered, the price is fixed and determinable, and collection is reasonably assured. The Company records revenue from its Nexafed product sales when the price is fixed and determinable at the date of sale, title and risk of ownership have been transferred to the customer, and returns can be reasonably estimated.
Nexafed was launched in mid-December 2012. The Company sells Nexafed in the United States to wholesale pharmaceutical distributors and as well as directly to chain drug stores. Nexafed is sold subject to the right of return for a period of up to twelve months after the product expiration. Nexafed currently has a shelf life of twenty-four months from the date of manufacture. Given the limited sales history of Nexafed, the Company currently cannot reliably estimate expected returns of the product at the time of shipment to certain customers. Accordingly, the Company has deferred revenue recognition on Nexafed shipments of $353 thousand since the product’s launch to these customers until the right of return no longer exists or adequate history and information is available to estimate product returns.
| 61 |
Research and Development
Research and Development, or R&D, expenses include internal R&D activities, external CRO services and their clinical research sites, and other activities. Internal R&D activity expenses include facility overhead, equipment and facility maintenance and repairs, laboratory supplies, pre-clinical laboratory experiments, depreciation, salaries, benefits, and share-based compensation expenses. CRO activity expenses include preclinical laboratory experiments and clinical trial studies. Other activity expenses include regulatory consulting, and regulatory legal counsel. Internal R&D activities and other activity expenses are charged to operations as incurred. We make payments to the CRO's based on agreed upon terms and may include payments in advance of the study starting date. We review and accrue CRO expenses and clinical trial study expenses based on services performed and rely upon estimates of those costs applicable to the stage of completion of a study as provided by the CRO. Accrued CRO costs are subject to revisions as such studies progress to completion. Revisions are charged to expense in the period in which the facts that give rise to the revision become known. We have entered into several cancelable CRO arrangements and our obligations under these arrangements were approximately $0.1 million and $0.7 million at December 31, 2014 and 2013, respectively, for services to be incurred as subjects are enrolled and progress through the studies.
Income Taxes
We account for income taxes under the liability method. Under this method, deferred income tax assets and liabilities are determined based on differences between financial reporting and income tax basis of assets and liabilities and are measured using the enacted income tax rates and laws that will be in effect when the differences are expected to reverse. Additionally, net operating loss and tax credit carryforwards are reported as deferred income tax assets. The realization of deferred income tax assets is dependent upon future earnings. A valuation allowance against deferred income tax assets is required if, based on the weight of available evidence, it is more likely than not that some or all of the deferred income tax assets may not be realized. Because we realized taxable income in 2011 we were able to utilize a portion of our net operating loss carryforwards. At December 31, 2014, 100% of the remaining deferred income tax assets are offset by a valuation allowance due to uncertainties with respect to future utilization of net operating loss carryforwards. If in the future it is determined that amounts of our deferred income tax assets would likely be realized, the valuation allowance would be reduced in the period in which such determination is made and a benefit from income taxes in such period would be recognized.
Stock Compensation
Compensation cost related to stock-based payment transactions is measured based on fair value of the equity or liability instrument issued. For purposes of estimating the fair value of each stock option unit on the date of grant, we utilized the Black-Scholes option-pricing model. The Black-Scholes option valuation model was developed for use in estimating the fair value of traded options, which have no vesting restrictions and are fully transferable. In addition, option valuation models require the input of highly subjective assumptions including the expected volatility factor of the market price of our common stock (as determined by reviewing its historical public market closing prices). Our accounting for stock-based compensation for restricted stock units, or RSUs, is based on the fair-value method. The fair value of the RSUs is the market price of our common stock on the date of grant, less its exercise cost.
Capital Expenditures
Our capital expenditures during 2014, 2013 and 2012 were $135,000, $23,000 and $147,000, respectively. Capital expenditures in each such year were primarily attributable to the purchase of scientific equipment and improvements to the Culver, Indiana facility.
Impact of Inflation
We believe that inflation did not have a material impact on our operations for the periods reported.
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
Some of the securities that we invest in may be subject to market risk. Our primary objective in our cash management activities is to preserve principal while at the same time maximizing income we receive from our investments. A change in the prevailing interest rates may cause the principal amount of our investments to fluctuate. We have no holdings of derivative financial and commodity instruments. As of December 31, 2014, our investments consisted of corporate bonds and exchange-traded funds.
| 62 |
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
The consolidated financial statements of Acura Pharmaceuticals, Inc. and Subsidiary and the Report of the Independent Registered Public Accounting Firm thereon, to be filed pursuant to Item 8 are included in Item 15.
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
Not applicable.
ITEM 9A. CONTROLS AND PROCEDURES
Evaluation of Disclosure Controls and Procedures. We have conducted an evaluation, under the supervision and with the participation of our management, including our Chief Executive Officer and Chief Financial Officer of the effectiveness of the design and operation of our disclosure controls and procedures pursuant to Exchange Act Rule 13a-15(e). Based upon that evaluation, our Chief Executive Officer and Chief Financial Officer concluded that our disclosure controls and procedures are effective in timely alerting them to material information relating to the Company (including our subsidiary) required to be included in our periodic Securities and Exchange Commission filings.
Management’s Report on Internal Control Over Financial Reporting. Our management is responsible for establishing and maintaining adequate internal control over financial reporting. Internal control over financial reporting is defined in Rule 13a-15(f) and 15d-15(f) promulgated under the Exchange Act as a process designated by, or under the supervision of, our principal executive and principal financial officers and effected by our board of directors, management and other personnel, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles and includes those policies and procedures that:
| · | Pertain to the maintenance of records that in reasonable detail accurately and fairly reflect the transactions and disposition of our assets; |
| · | Provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that our receipts and expenditures are being made only in accordance with authorizations of our management and directors; and |
| · | Provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of our assets that could have a material effect on the financial statements. |
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Therefore, even those systems determined to be effective can provide only reasonable assurance with respect to financial statement preparation and presentation. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Our management assessed the effectiveness of our internal control over financial reporting as of December 31, 2014. In making this assessment, management used the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission (COSO) in Internal Control – Integrated Framework (1992). Based on our assessment, our Chief Executive Officer and our Chief Financial Officer both believe that, as of December 31, 2014, our internal control over financial reporting is effective based on those criteria.
The Company’s independent registered public accounting firm was not required to and did not express an opinion on the effectiveness of the Company’s internal control over financial reporting.
| 63 |
Changes in Internal Control Over Financial Reporting. There was no change in our internal control over financial reporting that occurred during the Fourth Quarter 2014 that materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
Not Applicable.
| 64 |
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
Reference is made to 2015 Proxy Statement to be filed with the SEC on or about March 17, 2015 with respect to Directors, Executive Officers and Corporate Governance, which is incorporated herein by reference and made a part hereof in response to the information required by Item 10.
ITEM 11. EXECUTIVE COMPENSATION
Reference is made to our 2015 Proxy Statement to be filed with the SEC on or about March 17, 2015 with respect to Executive Compensation, which is incorporated herein by reference and made a part hereof in response to the information required by Item 11.
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
Reference is made to our 2015 Proxy Statement to be filed with the SEC on or about March 17, 2015 with respect to the to the security ownership of certain beneficial owners and management and related stockholder matters, which is incorporated herein by reference and made a part hereof in response to the information required by Item 12.
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
Reference is made to our 2015 Proxy Statement to be filed with the SEC on or about March 17, 2015 with respect to certain relationships and related transactions and direct independence, which is incorporated herein by reference and made a part hereof in response to the information required by Item 13.
ITEM 14. PRINCIPAL ACCOUNTANT FEES AND SERVICES
Reference is made to our 2015 Proxy Statement to be filed with the SEC on or about March 17, 2015 with respect to auditor fees, which is incorporated herein by reference and made a part in response to the information required by Item 14.
ITEM 15. EXHIBITS AND FINANCIAL STATEMENT SCHEDULES
(a) The following documents are filed as part of this report:
1. All Financial Statements: See Index to Financial Statements
2. Financial Statement Schedules: None
3. Exhibits: See Index to Exhibits
| 65 |
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| Date: February 27, 2015 | ACURA PHARMACEUTICALS, INC. | |
| By: | /s/ ROBERT B. JONES | |
| Robert B. Jones | ||
| President and Chief Executive Officer | ||
| (Principal Executive Officer) | ||
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
| Signature | Title(s) | Date | ||
| /s/Robert B. Jones | President, Chief Executive Officer and Director | |||
| Robert B. Jones | (Principal Executive Officer) | February 27, 2015 | ||
| /s/Peter A. Clemens | Senior Vice President and Chief Financial Officer | |||
| Peter A. Clemens | (Principal Financial and Accounting Officer) | February 27, 2015 | ||
| /s/William G. Skelly | ||||
| William G. Skelly | Director | February 27, 2015 | ||
| /s/Bruce F Wesson | ||||
| Bruce F. Wesson | Director | February 27, 2015 | ||
| /s/Immanuel Thangaraj | ||||
| Immanuel Thangaraj | Director | February 27, 2015 | ||
| /s/George K. Ross | ||||
| George K. Ross | Director | February 27, 2015 |
| 66 |
ACURA PHARMACEUTICALS, INC
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
| F-1 |
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
Board of Directors and Shareholders
Acura Pharmaceuticals, Inc.
Palatine, Illinois
We have audited the accompanying consolidated balance sheets of Acura Pharmaceuticals Inc. as of December 31, 2014 and 2013 and the related consolidated statements of operations and comprehensive loss, stockholders’ equity, and cash flows for each of the three years in the period ended December 31, 2014. These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these consolidated financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audits included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the consolidated financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall consolidated financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of Acura Pharmaceuticals Inc. at December 31, 2014 and 2013, and the results of its operations and its cash flows for each of the three years in the period ended December 31, 2014, in conformity with accounting principles generally accepted in the United States of America.
/s/ BDO USA, LLP
Chicago, Illinois
March 2, 2015
| F-2 |
ACURA PHARMACEUTICALS, INC.
DECEMBER 31, 2014 and 2013
(in thousands, except par value)
| 2014 | 2013 | |||||||
| ASSETS | ||||||||
| Current assets: | ||||||||
| Cash and cash equivalents | $ | 774 | $ | 12,340 | ||||
| Marketable securities (Note 4) | 11,322 | 13,733 | ||||||
| Accounts receivable, net of allowances of $5 and $28 | 76 | 194 | ||||||
| Accrued investment income | 66 | 120 | ||||||
| Inventories, net (Note 5) | 304 | 251 | ||||||
| Prepaid expenses and other current assets | 471 | 629 | ||||||
| Other current deferred assets | 218 | 186 | ||||||
| Total current assets | 13,231 | 27,453 | ||||||
| Property, plant and equipment, net (Note 6) | 957 | 941 | ||||||
| Deferred debt issuance costs, net of accumulated amortization of $69 and $- (Note 8) | 162 | 231 | ||||||
| Other assets | - | 5 | ||||||
| Intangible asset, net of accumulated amortization of $155 | 1,845 | - | ||||||
| Total assets | $ | 16,195 | $ | 28,630 | ||||
| LIABILITIES AND STOCKHOLDERS’ EQUITY | ||||||||
| Current liabilities: | ||||||||
| Accounts payable | $ | 217 | $ | 274 | ||||
| Accrued expenses (Note 7) | 568 | 541 | ||||||
| Accrued interest | 70 | - | ||||||
| Other current liabilities | 26 | 5 | ||||||
| Deferred revenue | 353 | 287 | ||||||
| Current maturities of long-term debt (Note 8) | 1,758 | - | ||||||
| Total current liabilities | 2,992 | 1,107 | ||||||
| Long-term debt, net of discount of $281 and $400 (Note 8) | 7,961 | 9,600 | ||||||
| Long-term portion of accrued interest | 190 | - | ||||||
| Total liabilities | 11,143 | 10,707 | ||||||
| Commitments and contingencies (Note 13) | ||||||||
| Stockholders’ equity: | ||||||||
| Common stock: $.01 par value per share; 100,000 shares authorized, 48,848 and 48,325 shares issued and outstanding at 2014 and 2013, respectively | 488 | 483 | ||||||
| Additional paid-in capital | 366,898 | 366,533 | ||||||
| Accumulated deficit | (362,321 | ) | (349,112 | ) | ||||
| Accumulated other comprehensive income (loss) | (13 | ) | 19 | |||||
| Total stockholders’ equity | 5,052 | 17,923 | ||||||
| Total liabilities and stockholders’ equity | $ | 16,195 | $ | 28,630 | ||||
See accompanying Notes to Consolidated Financial Statements.
| F-3 |
ACURA PHARMACEUTICALS, INC.
CONSOLIDATED STATEMENTS OF OPERATIONS AND COMPREHENSIVE LOSS
YEARS ENDED DECEMBER 31, 2014, 2013 and 2012
(in thousands, except per share amounts)
| 2014 | 2013 | 2012 | ||||||||||
| Revenues: | ||||||||||||
| Royalty revenue | $ | 4 | $ | 10 | $ | - | ||||||
| Product sales, net | 247 | 113 | - | |||||||||
| License fee revenue | 500 | - | - | |||||||||
| Total revenues, net | 751 | 123 | - | |||||||||
| Operating expenses: | ||||||||||||
| Cost of sales (excluding inventory write-down) | 227 | 114 | - | |||||||||
| Inventory write-down (Note 5) | 201 | 250 | - | |||||||||
| Research and development | 4,582 | 4,923 | 3,726 | |||||||||
| Selling, marketing, general and administrative | 7,940 | 8,926 | 6,013 | |||||||||
| Total operating expenses | 12,950 | 14,213 | 9,739 | |||||||||
| Operating loss | (12,199 | ) | (14,090 | ) | (9,739 | ) | ||||||
| Non-Operating income (expense): | ||||||||||||
| Investment income | 198 | 194 | 79 | |||||||||
| Gain on sales of marketable securities | 4 | 4 | - | |||||||||
| Interest expense (Note 8) | (1,212 | ) | (9 | ) | - | |||||||
| Other expense, net | - | - | (8 | ) | ||||||||
| Total other income (expense), net | (1,010 | ) | 189 | 71 | ||||||||
| Loss before income taxes | (13,209 | ) | (13,901 | ) | (9,668 | ) | ||||||
| Provision for income taxes | - | - | - | |||||||||
| Net loss | $ | (13,209 | ) | $ | (13,901 | ) | $ | (9,668 | ) | |||
| Other comprehensive income (loss): | ||||||||||||
| Unrealized gains (losses) on securities | (32 | ) | 59 | (40 | ) | |||||||
| Total other comprehensive income (loss) | (32 | ) | 59 | (40 | ) | |||||||
| Comprehensive loss | $ | (13,241 | ) | $ | (13,842 | ) | $ | (9,708 | ) | |||
| Loss per share: | ||||||||||||
| Basic | $ | (0.27 | ) | $ | (0.29 | ) | $ | (0.20 | ) | |||
| Diluted | $ | (0.27 | ) | $ | (0.29 | ) | $ | (0.20 | ) | |||
| Weighted average shares outstanding: | ||||||||||||
| Basic | 48,893 | 47,764 | 47,521 | |||||||||
| Diluted | 48,893 | 47,764 | 47,521 | |||||||||
See accompanying Notes to Consolidated Financial Statements.
| F-4 |
ACURA PHARMACEUTICALS, INC.
CONSOLIDATED STATEMENTS OF STOCKHOLDERS' EQUITY
YEARS ENDED DECEMBER 31, 2014, 2013 and 2012
(in thousands)
| Common Stock | Additional | Accumulated Other | ||||||||||||||||||||||
| Shares | $ Amount | Paid-in Capital | Accumulated Deficit | Comprehensive Income (Loss) | Total | |||||||||||||||||||
| Balance at Dec. 31, 2011 | 45,320 | $ | 453 | $ | 361,733 | $ | (325,543 | ) | $ | - | $ | 36,643 | ||||||||||||
| Net loss | - | - | - | (9,668 | ) | - | (9,668 | ) | ||||||||||||||||
| Other comprehensive loss | - | - | - | - | (40 | ) | (40 | ) | ||||||||||||||||
| Share-based compensation | - | - | 1,733 | - | - | 1,733 | ||||||||||||||||||
| Net distribution of common stock pursuant to restricted stock unit award plan | 827 | 8 | (7 | ) | - | - | 1 | |||||||||||||||||
| Common shares withheld for withholding taxes on distribution of restricted stock units | (296 | ) | (2 | ) | (1,031 | ) | - | - | (1,033 | ) | ||||||||||||||
| Net issuance of common stock pursuant to cashless exercise of stock options | 14 | - | - | - | - | - | ||||||||||||||||||
| Common shares withheld for withholding taxes on cashless exercise of stock options | (5 | ) | - | (15 | ) | - | - | (15 | ) | |||||||||||||||
| Issuance of common stock for exercise of stock options | 7 | - | 9 | - | - | 9 | ||||||||||||||||||
| Balance at Dec. 31, 2012 | 45,867 | $ | 459 | $ | 362,422 | $ | (335,211 | ) | $ | (40 | ) | $ | 27,630 | |||||||||||
| Net loss | - | - | - | (13,901 | ) | - | (13,901 | ) | ||||||||||||||||
| Other comprehensive income | - | - | - | - | 59 | 59 | ||||||||||||||||||
| Share-based compensation | - | - | 1,215 | - | - | 1,215 | ||||||||||||||||||
| Warrants issued with promissory notes | - | - | 400 | - | - | 400 | ||||||||||||||||||
| Net distribution of common stock pursuant to restricted stock unit award plan | 826 | 8 | (7 | ) | - | - | 1 | |||||||||||||||||
| Common shares withheld for withholding taxes on distribution of restricted stock units | (321 | ) | (3 | ) | (709 | ) | - | - | (712 | ) | ||||||||||||||
| Net issuance of common stock pursuant to cashless exercise of stock options | 7 | - | - | - | - | - | ||||||||||||||||||
| Common shares withheld for withholding taxes on cashless exercise of stock options | (1 | ) | - | (4 | ) | - | - | (4 | ) | |||||||||||||||
| Issuance of common stock under “at the market” offerings, net of offering costs of $102 | 1,940 | 19 | 3,207 | - | - | 3,226 | ||||||||||||||||||
| Issuance of common stock for exercise of stock options | 7 | - | 9 | - | - | 9 | ||||||||||||||||||
| Balance at Dec. 31, 2013 | 48,325 | $ | 483 | $ | 366,533 | $ | (349,112 | ) | $ | 19 | $ | 17,923 | ||||||||||||
| Net loss | - | - | - | (13,209 | ) | - | (13,209 | ) | ||||||||||||||||
| Other comprehensive loss | - | - | - | - | (32 | ) | (32 | ) | ||||||||||||||||
| Share-based compensation | - | - | 890 | - | - | 890 | ||||||||||||||||||
| Net distribution of common stock pursuant to restricted stock unit award plan | 825 | 8 | (7 | ) | - | - | 1 | |||||||||||||||||
| Common shares withheld for withholding taxes on distribution of restricted stock units | (315 | ) | (3 | ) | (522 | ) | - | - | (525 | ) | ||||||||||||||
| Net issuance of common stock pursuant to cashless exercise of stock options | 8 | - | - | - | - | - | ||||||||||||||||||
| Common shares withheld for withholding taxes on cashless exercise of stock options | (2 | ) | - | (4 | ) | - | - | (4 | ) | |||||||||||||||
| Issuance of common stock for exercise of stock options | 7 | - | 8 | - | - | 8 | ||||||||||||||||||
| Balance at Dec. 31, 2014 | 48,848 | $ | 488 | $ | 366,898 | $ | (362,321 | ) | $ | (13 | ) | $ | 5,052 | |||||||||||
See accompanying Notes to Consolidated Financial Statements.
| F-5 |
ACURA PHARMACEUTICALS, INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS
YEARS ENDED DECEMBER 31, 2014, 2013, and 2012
(in thousands)
| 2014 | 2013 | 2012 | ||||||||||
| Cash Flows from Operating Activities: | ||||||||||||
| Net loss | $ | (13,209 | ) | $ | (13,901 | ) | $ | (9,668 | ) | |||
| Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||||||
| Depreciation | 119 | 134 | 131 | |||||||||
| Provision to reduce inventory to net realizable value | 201 | 250 | - | |||||||||
| Share-based compensation | 890 | 1,215 | 1,733 | |||||||||
| Amortization of debt discount and deferred debt issue costs | 188 | - | - | |||||||||
| Amortization of bond premium in marketable securities | 250 | - | - | |||||||||
| Amortization of intangible asset | 155 | - | - | |||||||||
| Gain on sales of marketable securities | (4 | ) | (4 | ) | - | |||||||
| Loss on disposal of property, plant and equipment | - | - | 8 | |||||||||
| Changes in assets and liabilities: | ||||||||||||
| Accounts receivable | 118 | (194 | ) | - | ||||||||
| Accrued investment income | 54 | (84 | ) | - | ||||||||
| Inventories | (254 | ) | (282 | ) | (219 | ) | ||||||
| Income taxes refundable | - | 43 | 110 | |||||||||
| Prepaid expenses and other current assets | 158 | (358 | ) | (16 | ) | |||||||
| Other current deferred assets | (32 | ) | (186 | ) | - | |||||||
| Other assets | 5 | 6 | - | |||||||||
| Accounts payable | (57 | ) | (720 | ) | 941 | |||||||
| Accrued expenses | 27 | 128 | (64 | ) | ||||||||
| Deferred revenue | 66 | 287 | - | |||||||||
| Accrued interest – current and long term | 260 | - | - | |||||||||
| Other current and non-current liabilities | 21 | (12 | ) | 6 | ||||||||
| Net cash used in operating activities | (11,044 | ) | (13,678 | ) | (7,038 | ) | ||||||
| Cash Flows from Investing Activities: | ||||||||||||
| Purchases of marketable securities | (2,203 | ) | (7,611 | ) | (20,306 | ) | ||||||
| Proceeds from sale and maturities of marketable securities | 4,336 | 13,887 | 320 | |||||||||
| Acquisition of product rights | (2,000 | ) | - | - | ||||||||
| Additions to property, plant and equipment | (135 | ) | (23 | ) | (147 | ) | ||||||
| Net cash (used in) provided by investing activities | (2 | ) | 6,253 | (20,133 | ) | |||||||
| Cash Flows from Financing Activities: | ||||||||||||
| Proceeds from exercise of stock options | 8 | 9 | 9 | |||||||||
| Proceeds from distribution of restricted stock units | 1 | 1 | 1 | |||||||||
| Statutory minimum withholding taxes paid on the distribution of common stock pursuant to restricted stock unit plan and exercise of stock options | (529 | ) | (716 | ) | (1,048 | ) | ||||||
| Long-term debt borrowings | - | 10,000 | - | |||||||||
| Capitalized debt issuance costs | - | (231 | ) | - | ||||||||
| Proceeds from “at the market offering” | - | 3,328 | - | |||||||||
| “At the market offering” transaction costs | - | (102 | ) | - | ||||||||
| Net cash (used in) provided by financing activities | (520 | ) | 12,289 | (1,038 | ) | |||||||
| Net (decrease) increase in cash and cash equivalents | (11,566 | ) | 4,864 | (28,209 | ) | |||||||
| Cash and cash equivalents at beginning of year | 12,340 | 7,476 | 35,685 | |||||||||
| Cash and cash equivalents at end of year | $ | 774 | $ | 12,340 | $ | 7,476 | ||||||
| Supplemental Disclosures of Cash Flow Information: | ||||||||||||
| Cash paid (refunded) during the year for: | ||||||||||||
| Interest | $ | 765 | $ | 9 | $ | - | ||||||
| Income taxes, net of refunds | $ | 14 | $ | (42 | ) | $ | (108 | ) | ||||
See accompanying Notes to Consolidated Financial Statements.
| F-6 |
ACURA PHARMACEUTICALS, INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS (CONTINUED)
YEAR ENDED DECEMBER 31, 2014, 2013, and 2012
Supplemental disclosures of noncash investing and financing activities (amounts presented are rounded to the nearest thousand):
Year ended December 31, 2014
| 1. | 829 thousand shares of common stock were eligible for distribution pursuant to our 2005 Restricted Stock Unit (RSU) Plan utilizing various cashless exercise features of the plan. After withholding 4 thousand shares for $7 in exercise costs and withholding 315 thousand shares for $525 in statutory minimum payroll taxes, we issued 510 thousand shares of common stock. | |
| 2. | Options to purchase 24 thousand shares of common stock were exercised utilizing various cashless exercise features of our stock option plan. After withholding 16 thousand shares for $32 in exercise costs and withholding 2 thousand shares for $4 in statutory minimum payroll taxes, we issued 6 thousand shares of common stock. |
Year ended December 31, 2013
| 1. | 829 thousand shares of common stock were eligible for distribution pursuant to our RSU Plan utilizing various cashless exercise features of the plan. After withholding 3 thousand shares for $7 in exercise costs and withholding 321 thousand shares for $712 in statutory minimum payroll taxes, we issued 505 thousand shares of common stock. | |
| 2. | Options to purchase 24 thousand shares of common stock were exercised utilizing various cashless exercise features of the stock option plan. After withholding 17 thousand shares for $32 in exercise costs and withholding 1 thousand shares for $4 in statutory minimum payroll taxes, we issued 6 thousand shares of common stock. | |
| 3. | In connection with a debt issuance of $10 million, we issued the lender 298 thousand warrants with an exercise price of $1.595. The fair value of these warrants of $400 was recorded as a debt discount and is being amortized as interest expense over the term of this debt. |
Year ended December 31, 2012
| 1. | 829 thousand shares of common stock were eligible for distribution pursuant to our RSU Plan utilizing various cashless exercise features of the plan. After withholding 2 thousand shares for $7 in exercise costs and withholding 296 thousand shares for $1.0 million in statutory minimum payroll taxes, we issued 531 thousand shares of common stock. | |
| 2. | Options to purchase 24 thousand shares of common stock were exercised utilizing various cashless exercise features of the stock option plan. After withholding 10 thousand shares for $31 in exercise costs and withholding 5 thousand shares for $15 in statutory minimum payroll taxes, we issued 9 thousand shares of common stock. |
See accompanying Notes to Consolidated Financial Statements.
| F-7 |
ACURA PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2014, 2013 and 2012
NOTE 1 - DESCRIPTION OF BUSINESS
Acura Pharmaceuticals, Inc., a New York corporation, and its subsidiary (the “Company”, “We”, or “Our”) is a specialty pharmaceutical company engaged in the research, development and commercialization of technologies and products intended to address medication abuse and misuse. We have discovered and developed three proprietary platform technologies which can be used to develop multiple products. Our Aversion® Technology is a mixture of inactive ingredients incorporated into pharmaceutical tablets and capsules intended to address some common methods of product tampering associated with opioid abuse. Oxaydo™ Tablets (formerly known as Oxecta®) (oxycodone HCl, CII), is the first approved product utilizing Aversion in the United States and we have 7 additional opioid products utilizing Aversion in various stages of development. On January 7, 2015, we entered into a Collaboration and License Agreement with Egalet US, Inc. and Egalet Ltd., each a subsidiary of Egalet Corporation (collectively, “Egalet”) pursuant to which we exclusively licensed to Egalet worldwide rights to manufacture and commercialize Oxaydo. Oxaydo is currently approved by the FDA for marketing in the United States in 5mg and 7.5 mg strengths. We are advised that Egalet plans to launch Oxaydo in the United States in the third quarter of 2015. We have also developed Impede® Technology which is a combination of inactive ingredients that prevent the extraction of pseudoephedrine from tablets and disrupt the direct conversion of pseudoephedrine from tablets into methamphetamine. We launched our first Impede Technology product, Nexafed®, into the United States market in December 2012 and our Nexafed Sinus Pressure + Pain product in the United States in February 2015. We have multiple pseudoephedrine products in development utilizing our Impede Technology. In August 2014, we received a grant from the National Institute on Drug Abuse to advance early stage development of our third abuse deterrent technology, Limitx™. Limitx is designed to retard the release of active drug ingredients when too many tablets are accidently or purposefully ingested.
NOTE 2 - SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Basis of Presentation
The Company’s consolidated financial statements are prepared in accordance with accounting principles generally accepted in the United States of America (“GAAP”). The consolidated financial statements include the accounts of our wholly-owned subsidiary, Acura Pharmaceutical Technologies Inc., after elimination of intercompany accounts and transactions.
Reclassifications
Certain reclassifications have been made to the prior years' amounts to conform to the current year's presentation.
Management Estimates
Management is required to make certain estimates and assumptions in order to prepare consolidated financial statements in conformity with GAAP. Such estimates and assumptions affect the reported amounts of assets, liabilities, revenues and expenses and disclosure of contingent assets and liabilities in the consolidated financial statements and accompanying notes. Actual results could differ from those estimates. Management periodically evaluates estimates used in the preparation of the consolidated financial statements for continued reasonableness. Appropriate adjustments, if any, to the estimates used are made prospectively based on such periodic evaluations.
Cash and Cash Equivalents
The Company considers cash and cash equivalents to include cash in financial institutions and money market funds. The Company considers all highly liquid investments with an original maturity of three months or less to be cash equivalents. Our cash and cash equivalents are governed by our investment policy as approved by our Board of Directors. The carrying amount of cash and cash equivalents approximates its fair value due to its short-term nature.
| F-8 |
Marketable Securities
The Company’s marketable securities at December 31, 2014 and 2013 primarily consist of corporate debt securities and exchange-traded funds. Our marketable securities are governed by our investment policy as approved by our Board of Directors. The Company’s marketable securities are classified as available-for-sale and are recorded at fair value, based upon quoted market prices or net asset value. Unrealized temporary adjustments to fair value are included in a separate component of stockholders’ equity as unrealized gains and losses and reported as a component of accumulated other comprehensive income (loss). No gains or losses on marketable securities are realized until shares are sold or a decline in fair value is determined to be other-than-temporary. If a decline in fair value is determined to be other-than-temporary, an impairment charge is recorded and a new cost basis in the investment is established.
Fair Value of Other Financial Instruments
The Company’s financial instruments consist primarily of cash and cash equivalents, marketable securities, accounts and other receivables, trade accounts payable, accrued expenses and our long-term debt. The carrying amounts of these financial instruments, other than marketable securities and our long-term debt, are representative of their respective fair values due to their relatively short maturities. The Company believes the fair value of long-term debt approximates its carrying value based upon the borrowing rates currently available to the Company for loans with similar terms. As discussed below, marketable securities are recorded at fair value.
Concentration of Credit Risk
Credit Risk: Financial instruments that potentially subject the Company to a significant concentration of credit risk consist primarily of cash and cash equivalents, marketable securities and accounts receivable. The Company maintains deposits in federally insured financial institutions in excess of federally insured limits. However, management believes the Company is not exposed to significant credit risk due to the financial position of the depository institutions in which those deposits are held. Additionally, the Company’s excess cash is invested in accordance with the investment policy approved by our Board of Directors that seeks a combination of both liquidity and safety of principal using diversification of investments, through investments such as investment grade corporate debt securities with varying maturities. To date, the Company has not experienced any material realized losses on its cash, cash equivalents, and marketable securities.
Customers: We launched our first Impede Technology product, Nexafed®, into the United States market in December 2012. Our accounts receivable arise from our product sales of Nexafed and represents amounts due from wholesalers in the health care and pharmaceuticals industries and from chain drug stores. The Company has performed a credit evaluation of its customers and may maintain an allowance for potentially uncollectable accounts. We have not experienced any losses on uncollectable accounts from launch through December 31, 2014.
Sales to certain of our customers during 2014 and 2013 accounting for 10% or more of our annual product sales, whether the product shipment was recognized or deferred, are presented below:
| Customer | 2014 | |||
| Rite Aid Corporation | 28 | % | ||
| Cardinal Health, Inc. | 24 | % | ||
| AmerisourceBergen Corporation | 13 | % | ||
| McKesson Corporation | 13 | % | ||
| Kroger Foods | 11 | % | ||
| 2013 | ||||
| Rite Aid Corporation | 52 | % | ||
| Cardinal Health, Inc. | 15 | % | ||
| F-9 |
Accounts receivable from certain of our customers at December 31, 2014 and 2013 accounting for 10% or more of our gross accounts receivable are presented below:
| Customer | 2014 | |||
| Kroger Foods | 13 | % | ||
| Rite Aid Corporation | 83 | % | ||
| 2013 | ||||
| Rite Aid Corporation | 95 | % | ||
Inventories
Inventories consist of both raw and packaging materials on our Oxaydo product and finished goods held for distribution and sale on our Nexafed® product. During 2014 we purchased raw and packaging material inventories for $260 thousand from Pfizer on the Oxaydo product we reacquired from them. Inventories are stated at the lower of cost (first-in, first-out method) or market (net realizable value). The Company writes down inventories to net realizable value based on forecasted demand and market conditions, which may differ from actual results.
Our purchases of active pharmaceutical ingredients and raw materials required for our development and clinical trial manufacture of product candidates utilizing our Aversion®, Impede® or Limitx Technologies are expensed as incurred.
Property, Plant and Equipment
Property, plant and equipment are stated at cost, less accumulated depreciation. We have no leasehold improvements. Betterments are capitalized and maintenance and repairs are charged to operations as incurred. When a depreciable asset is retired from service, the cost and accumulated depreciation is removed from the respective accounts.
Depreciation expense is recorded on a straight-line basis over the estimated useful lives of the related assets. The estimated useful lives of the major classification of depreciable assets are:
| Building and improvements | 10 - 40 years | |
| Land and improvements | 20 - 40 years | |
| Machinery and equipment | 7 - 10 years | |
| Scientific equipment | 5 - 10 years | |
| Computer hardware and software | 3 - 10 years |
Debt Issuance Costs and Debt Discount
Deferred debt issuance costs include costs of debt financing undertaken by the Company, including legal fees, placement fees and other direct costs of the financing. Debt discount is the value attributable to warrants issued in conjunction with the financing. Debt issuance costs and debt discount are amortized into interest expense over the term of the related debt using the effective interest method.
Revenue Recognition
Revenue is generally realized or realizable and earned when there is persuasive evidence an arrangement exists, delivery has occurred or services rendered, the price is fixed and determinable, and collection is reasonably assured. The Company records revenue from its Nexafed® product sales when the price is fixed and determinable at the date of sale, title and risk of ownership have been transferred to the customer, and returns can be reasonably estimated.
Royalty Revenue
In connection with our License, Development, and Commercialization Agreement dated October 30, 2007 with King Pharmaceuticals Research and Development, Inc., now a subsidiary of Pfizer Inc. (the “Pfizer Agreement”), we began earning royalties on Oxecta starting in February 2013. We recorded royalties of approximately $4 thousand and $10 thousand for the years ended December 31, 2014 and 2013, respectively. Effective April 9, 2014, the Pfizer Agreement was terminated and the rights to Oxecta were returned to us after making a one-time payment of $2.0 million to Pfizer.
On January 7, 2015, we, Egalet US, Inc., Egalet Ltd., and Egalet Corporation (collectively, “Egalet”) entered into a Collaboration and License Agreement to commercialize Oxaydo™ tablets (formerly known as Oxecta) containing our Aversion® Technology. We will receive a stepped royalty at percentage rates ranging from mid-single digits to double-digits on net sales during a calendar year based on Oxaydo net sales during such year (excluding net sales resulting from our co-promotion efforts) (see Note 3).
| F-10 |
Product Sales
Nexafed® was launched in December 2012. The Company sells Nexafed® in the United States to wholesale pharmaceutical distributors as well as directly to chain drug stores. Nexafed® is generally sold subject to the right of return beginning six months prior to and ending twelve months following the product expiration. Nexafed® currently has a shelf life of twenty-four months from the date of manufacture. Given the limited sales history of Nexafed®, the Company currently cannot reliably estimate expected returns of the product at the time of shipment to certain customers. Accordingly, the Company has deferred the recognition of revenue on $0.4 million of Nexafed® shipments to these customers until the right of return no longer exists or adequate history and information becomes available to estimate product returns.
License Fees
The Company entered into an agreement with Purdue Pharma to settle a patent interference action regarding certain intellectual property held by Acura (U.S. Patent No. 8,101,630). The dispute centered upon the issue of which company has priority in developing the invention. The parties agreed to forgo protracted litigation and the uncertainties arising therefrom by entering an agreement whereby Acura conceded Purdue’s claim of priority in exchange for certain financial consideration to us including an immediate non-refundable payment of $500 thousand.
On January 7, 2015, we and Egalet US, Inc. and Egalet Ltd., each a subsidiary of Egalet Corporation (collectively, “Egalet”) entered into a Collaboration and License Agreement to commercialize Oxaydo™ tablets (formerly known as Oxecta) containing our Aversion® Technology. Egalet paid us an upfront payment of $5 million upon signing of the Egalet Agreement (see Note 3).
Advertising Costs
The Company records the cost of its advertising efforts when services are performed or goods are delivered.
Shipping and Handling Costs
The Company records shipping and handling costs in selling expenses. The amounts recorded from the sales of Nexafed® were not material.
Impairment of Long-Lived Assets
Long-lived assets such as the intangible asset and property, plant and equipment are reviewed for impairment whenever events or changes in circumstances indicate that the carrying value of an asset may not be recoverable. Recoverability of the assets to be held and used is measured by a comparison of the carrying amount of the asset to estimated undiscounted future cash flows expected to be generated by the asset. If the carrying value of an asset exceeds its estimated future cash flows, an impairment charge is recognized by the amount by which the carrying amount of the asset exceeds the fair value of the asset. We had no impairment charges for years ended 2014, 2013 and 2012.
Research and Development Activities
Research and Development (“R&D”) expenses include internal R&D activities, external Contract Research Organization (“CRO”) services and their clinical research sites, and other activities. Internal R&D activity expenses include facility overhead, equipment and facility maintenance and repairs, laboratory supplies, pre-clinical laboratory experiments, depreciation, salaries, benefits, and share-based compensation expenses. CRO activity expenses include preclinical laboratory experiments and clinical trial studies. Other activity expenses include regulatory consulting, and regulatory legal counsel. Internal R&D activities and other activity expenses are charged to operations as incurred. We make payments to the CRO's based on agreed upon terms and may include payments in advance of a study starting date. We review and accrue CRO expenses and clinical trial study expenses based on services performed and rely on estimates of those costs applicable to the stage of completion of a study as provided by the CRO. Accrued CRO costs are subject to revisions as such studies progress to completion. Revisions are charged to expense in the period in which the facts that give rise to the revision become known. We did not have any accrued CRO costs and clinical trial study expenses at either December 31, 2014 or 2013. We had no prepaid CRO costs and clinical trial study expenses at either December 31, 2014 or 2013.
| F-11 |
Share-based Compensation
We have several share-based compensation plans covering stock options and RSUs for our employees and directors, which are described more fully in Note 11.
We measure our compensation cost related to share-based payment transactions based on fair value of the equity or liability instrument issued. For purposes of estimating the fair value of each stock option unit on the date of grant, we utilize the Black-Scholes option-pricing model. The Black-Scholes option valuation model was developed for use in estimating the fair value of traded options, which have no vesting restrictions and are fully transferable. In addition, option valuation models require the input of highly subjective assumptions including the expected volatility factor of the market price of our common stock (as determined by reviewing our historical public market closing prices). Our accounting for share-based compensation for RSUs is based on the fair-value method. The fair value of the RSUs is the market price of our common stock on the date of grant, less its exercise cost.
Our share-based compensation expense recognized in the Company’s results of operations comprised the following:
| Year ended December 31, | ||||||||||||
| 2014 | 2013 | 2012 | ||||||||||
| (in thousands) | ||||||||||||
| Research and development: | ||||||||||||
| Stock option awards | $ | 220 | $ | 315 | $ | 375 | ||||||
| RSU awards | - | - | - | |||||||||
| $ | 220 | $ | 315 | $ | 375 | |||||||
| Selling, general and administrative: | ||||||||||||
| Stock option awards | 550 | 900 | 1,358 | |||||||||
| RSU awards | 146 | - | - | |||||||||
| $ | 696 | $ | 900 | $ | 1,358 | |||||||
| Total share-based compensation expense | $ | 916 | $ | 1,215 | $ | 1,733 | ||||||
Comprehensive Income (Loss)
Comprehensive income (loss) includes all changes in equity during a period except those that resulted from investments by or distributions to the Company’s stockholders. Other comprehensive income (loss) refers to revenues, expenses, gains and losses that, under GAAP, are included in comprehensive income (loss), but excluded from net income (loss) as these amounts are recorded directly as an adjustment to stockholders’ equity. Acura’s other comprehensive income (loss) is composed of unrealized gains (losses) on certain holdings of marketable securities, net of any realized gains (losses) included in net income (loss).
Income Taxes
We account for income taxes under the liability method. Under this method, deferred income tax assets and liabilities are determined based on differences between the financial reporting and the income tax basis of assets and liabilities and are measured using the enacted income tax rates and laws that will be in effect when the differences are expected to reverse. Additionally, net operating loss and tax credit carryforwards are reported as deferred income tax assets. The realization of deferred income tax assets is dependent upon future earnings. A valuation allowance is required against deferred income tax assets if, based on the weight of available evidence, it is more likely than not that some or all of the deferred income tax assets may not be realized. At both December 31, 2014 and 2013, 100% of all remaining net deferred income tax assets were offset by a valuation allowance due to uncertainties with respect to future utilization of net operating loss carryforwards. If in the future it is determined that additional amounts of our deferred income tax assets would likely be realized, the valuation allowance would be reduced in the period in which such determination is made and an additional benefit from income taxes in such period would be recognized.
| F-12 |
Earnings Per Share (“EPS”)
Basic EPS is computed by dividing net income or loss by the weighted average common shares outstanding during a period, including shares weighted related to vested Restricted Stock Units (“RSUs”) (see Note 11). Diluted EPS is based on the treasury stock method and computed based on the same number of shares used in the basic share calculation and includes the effect from potential issuance of common stock, such as shares issuable pursuant to the exercise of stock options and stock warrants, assuming the exercise of all in-the-money stock options and warrants. Common stock equivalents are excluded from the computation where their inclusion would be anti-dilutive. No such adjustments were made for 2014, 2013 or 2012 as the Company reported a net loss for the years and including the effects of common stock equivalents in the diluted EPS calculation would have been antidilutive.
A reconciliation of the numerators and denominators of basic and diluted EPS consisted of the following:
| Years ended December 31, | ||||||||||||
| 2014 | 2013 | 2012 | ||||||||||
| (in thousands except per share data) | ||||||||||||
| EPS - basic | ||||||||||||
| Numerator: net loss | $ | (13,209 | ) | $ | (13,901 | ) | $ | (9,668 | ) | |||
| Denominator: | ||||||||||||
| Common shares | 48,847 | 46,935 | 45,863 | |||||||||
| Vested RSUs | 46 | 829 | 1,658 | |||||||||
| Basic weighted average shares outstanding | 48,893 | 47,764 | 47,521 | |||||||||
| EPS - basic | $ | (0.27 | ) | $ | (0.29 | ) | $ | (0.20 | ) | |||
| EPS – assuming dilution | ||||||||||||
| Numerator: net loss | $ | (13,209 | ) | $ | (13,901 | ) | $ | (9,668 | ) | |||
| Denominator: | ||||||||||||
| Common shares | 48,847 | 46,935 | 45,863 | |||||||||
| Vested RSUs | 46 | 829 | 1,658 | |||||||||
| Stock options | - | - | - | |||||||||
| Common stock warrants | - | - | - | |||||||||
| Diluted weighted average shares outstanding | 48,893 | 47,764 | 47,521 | |||||||||
| EPS - diluted | $ | (0.27 | ) | $ | (0.29 | ) | $ | (0.20 | ) | |||
| Excluded dilutive securities: | ||||||||||||
| Common stock issuable: | ||||||||||||
| Stock options | 4,556 | 3,738 | 3,296 | |||||||||
| Common stock warrants | 298 | 2,154 | 1,856 | |||||||||
| Total excluded potentially dilutive shares | 4,854 | 5,892 | 5,152 | |||||||||
Recent Accounting Pronouncements
In May 2014, the FASB issued Accounting Standards Update No. 2014-09, Revenue from Contracts with Customers (ASU 2014-09), which supersedes nearly all existing revenue recognition guidance under U.S. GAAP. The core principle of ASU 2014-09 is to recognize revenues when promised goods or services are transferred to customers in an amount that reflects the consideration to which an entity expects to be entitled for those goods or services. ASU 2014-09 defines a five step process to achieve this core principle and, in doing so, more judgment and estimates may be required within the revenue recognition process than are required under existing U.S. GAAP.
The standard is effective for annual periods beginning after December 15, 2016, and interim periods therein, using either of the following transition methods: (i) a full retrospective approach reflecting the application of the standard in each prior reporting period with the option to elect certain practical expedients, or (ii) a retrospective approach with the cumulative effect of initially adopting ASU 2014-09 recognized at the date of adoption (which includes additional footnote disclosures). We are currently evaluating the impact of our pending adoption of ASU 2014-09 on our consolidated financial statements and have not yet determined the method by which we will adopt the standard in 2017.
| F-13 |
NOTE 3 – LICENSE, DEVELOPMENT, AND COMMERCIALIZATION AGREEMENTS
Pfizer Agreement
In October 2007, we entered into a License, Development and Commercialization Agreement, or the Pfizer Agreement, with King Pharmaceuticals Research and Development, Inc., now a subsidiary of Pfizer, to develop and commercialize in the United States, Canada and Mexico certain opioid analgesic products utilizing our Aversion Technology. Aversion Oxycodone was approved by the U.S. Food and Drug Administration, or FDA, on June 17, 2011 and sales of Aversion Oxycodone, under Pfizer’s brand name Oxecta, commenced in February 2012. For sales of Aversion Oxycodone occurring on and following February 2, 2013 (the one year anniversary of first commercial sale), Pfizer paid us a royalty of 5% of net sales of Aversion Oxycodone.
On September 24, 2012,
we entered into a letter agreement with Pfizer which provided for the termination of Pfizer’s license to our Aversion Technology
used in three development-stage products licensed to Pfizer and for the return of these products to us. On April 9, 2014, we entered
into a second letter agreement with Pfizer providing for the termination of the Pfizer Agreement and the return of Aversion Oxycodone
to us effective April 9, 2014. The letter agreement further provides that (i) Pfizer will cease the development, marketing and
sale of any product using our technologies effective April 9, 2014, (ii) Pfizer will retain its Oxecta® trademark, (iii) Pfizer
transferred to us all studies, data, regulatory filings (including the NDA) and all other information relating to Aversion
Oxycodone pursuant to a transition process described in the letter agreement, (iv) we will remit to Pfizer a one-time termination
payment of $2.0 million, and (v) each party waives all claims against the other relating to the Pfizer Agreement. Pfizer’s
royalty payment obligations relating to Aversion Oxycodone ceased effective April 9, 2014 and all royalty payments due to us have
been received. Our termination payment of $2.0 million has been recorded on our financial statements as an intangible asset and
will be amortized over the remaining useful life of the patent for Aversion Oxycodone. The recorded value of the intangible asset
will be periodically assessed for impairment. We also purchased raw and packaging material inventories for $260 thousand from Pfizer
on the Aversion Oxycodone product.
Egalet Agreement
On January 7, 2015, we and Egalet entered into a Collaboration and License Agreement (the “Egalet Agreement”) to commercialize Aversion Oxycodone under the tradename Oxaydo™. Oxaydo is approved by the FDA for marketing in the United States in 5 mg and 7.5 mg strengths. Under the terms of the Egalet Agreement, we are transferring the approved NDA for Oxaydo to Egalet and Egalet is granted an exclusive license under our intellectual property rights for development and commercialization of Oxaydo worldwide (the “Territory”) in all strengths, subject to our right to co-promote Oxaydo in the United States.
In accordance with the Egalet Agreement, we and Egalet have formed a joint steering committee to coordinate commercialization strategies and the development of product line extensions. Egalet will pay a significant portion of the expenses relating to (i) annual NDA PDUFA product fees, (ii) expenses of the FDA required post-marketing study for Oxaydo and (iii) expenses of clinical studies for product line extensions (additional strengths) of Oxaydo for the United States and will bear all of the expenses of development and regulatory approval of Oxaydo for sale outside the United States. Egalet is responsible for all manufacturing and commercialization activities in the Territory for Oxaydo. Subject to certain exceptions, Egalet will have final decision making authority with respect to all development and commercialization activities for Oxaydo, including pricing, subject to our co-promotion right. Egalet may develop Oxaydo for other countries and in additional strengths, in its discretion.
Egalet paid us an upfront payment of $5 million upon signing of the Egalet Agreement and will pay us a $2.5 million milestone on the earlier to occur of (A) the launch of Oxaydo and (B) January 1, 2016, but in no event earlier than June 30, 2015. In addition, we will be entitled to a one-time $12.5 million milestone payment when worldwide Oxaydo net sales reach $150 million in a calendar year. We will receive from Egalet a stepped royalty at percentage rates ranging from mid-single digits to double-digits on net sales during a calendar year based on Oxaydo net sales during such year (excluding net sales resulting from our co-promotion efforts). In any calendar year in which net sales exceed a specified threshold, we will receive a double digit royalty on all Oxaydo net sales in that year (excluding net sales resulting from our co-promotion efforts). If we exercise our co-promotion rights, we will receive a share of the gross margin attributable to incremental Oxaydo net sales from our co-promotion activities. Egalet’s royalty payment obligations commence on the first commercial sale of Oxaydo and expire, on a country-by-country basis, upon the expiration of the last to expire valid patent claim covering Oxaydo in such country (or if there are no patent claims in such country, then upon the expiration of the last valid claim in the United States or the date when no valid and enforceable listable patent in the FDA’s Orange Book remains with respect to Oxaydo. Royalties will be reduced upon the entry of generic equivalents, as well for payments required to be made by Egalet to acquire intellectual property rights to commercialize Oxaydo, with an aggregate minimum floor.
| F-14 |
The Egalet Agreement expires upon the expiration of Egalet’s royalty payment obligations in all countries. Either party may terminate the Egalet Agreement in its entirety if the other party breaches a payment obligation, or otherwise materially breaches the Egalet Agreement, subject to applicable cure periods, or in the event the other party makes an assignment for the benefit of creditors, files a petition in bankruptcy or otherwise seeks relief under applicable bankruptcy laws. We also may terminate the Egalet Agreement with respect to the U.S. and other countries if Egalet materially breaches its commercialization obligations. Egalet may terminate the Egalet Agreement for convenience on 120 days prior written notice, which termination may not occur prior to the second anniversary of Egalet’s launch of Oxaydo. Egalet also may terminate the Agreement prior to the launch of Oxaydo on 30 days prior written notice upon the occurrence of serious safety issues, regulatory restrictions and intellectual property issues, in each case involving Oxaydo. Termination does not affect a party’s rights accrued prior thereto, but there are no stated payments in connection with termination other than payments of obligations previously accrued. For all terminations (but not expiration), the Egalet Agreement provides for the transition of development and marketing of Oxaydo from Egalet to us, including the conveyance by Egalet to us of the trademarks and all regulatory filings and approvals relating to Oxaydo, and for Egalet’s supply of Oxaydo for a transition period.
Paragraph IV ANDA Litigation
On or about September 17, 2012, we believe the FDA internally changed the status of Oxecta® to be considered a Reference Listed Drug, or RLD. An RLD is the standard to which all generic versions must be shown to be bioequivalent and a drug company seeking approval to market a generic equivalent must refer to the RLD. By designating Oxecta® as an RLD, the FDA was allowed to accept ANDAs referencing Oxecta®.
On September 20, 2012, we announced that we had received a Paragraph IV Certification Notice under 21 U.S.C. 355(j) (a Paragraph IV Notice) from a generic sponsor of an ANDA for a generic drug listing Oxecta® as the reference listed drug. Since such date, we have received similar Paragraph IV Notices from four other generic pharmaceutical companies that have filed ANDAs listing Oxecta® as the reference drug. The Paragraph IV Notices refer to our U.S. Patent Numbers 7,201,920, 7,510,726 and 7,981,439, which cover our Aversion® Technology and Oxecta®. The Paragraph IV Notices state that each generic sponsor believes that such patents are invalid, unenforceable or not infringed. On October 31, 2012, we initiated suit against each of Watson Laboratories, Inc. – Florida (Watson), Par Pharmaceutical, Inc., Impax Laboratories, Inc. and Sandoz Inc., and on April 29, 2013 we initiated suit against Ranbaxy, Inc., each in the United States District Court for the District of Delaware alleging infringement of our U.S. Patent No. 7,510,726 listed in the FDA’s Orange Book. The commencement of such litigation prohibits the FDA from granting approval of the filed ANDAs until the earliest of 30 months from the date the FDA accepted the application for filing, or the conclusion of litigation. In January 2013, we dismissed our suit against Watson on the grounds that Watson had amended its ANDA from a Paragraph IV Certification to a Paragraph Certification III, which indicated its intent not to market its generic Oxaydo product in advance of our patent expiry.
On October 9, 2013, we announced that we had entered into distinct Settlement Agreements with each of Par and Impax, to settle our patent infringement action pending against them in the United States District Court for the District of Delaware. In the suit, we alleged that a generic Oxecta® product for which each of Par and Impax is separately seeking approval to market in the United States pursuant to an ANDA filing with the FDA infringes a U.S. patent owned by us. Par is the first filer of an ANDA for a generic Oxecta® product and is entitled to the 180-day first filer exclusivity under applicable law and FDA regulations.
| F-15 |
Under the terms of the Settlement Agreement with Par, Par may launch its generic Oxecta® product in the U.S., through the grant of a non-exclusive, royalty-bearing license from us that would trigger on January 1, 2022. We currently have Orange Book patents that are due to expire between November 2023 and March 2025. In certain limited circumstances, our license to Par would become effective prior to January 1, 2022. Par is required to pay us royalties in the range of 10% to 15% of Par’s net profits from the sale of its generic Oxecta® product.
Under the Settlement Agreement with Impax, Impax may launch its generic Oxecta® product in the U.S., through the grant of a non-exclusive, royalty-free license from us that would trigger 180 days following the first sale of a generic Oxecta® product in the U.S. by an entity that is entitled to the 180 day first-filer exclusivity under applicable law and FDA regulations (or if no entity is entitled to such 180 day exclusivity period, the date on which a generic Oxecta® product is first sold in the U.S. or November 27, 2021, whichever date occurs first). In certain circumstances, our license to Impax would become effective prior to such time.
On May 8, 2014, we announced that we had entered into a Settlement Agreement with Ranbaxy Inc. to settle our patent infringement action pending in the United States District Court for the District of Delaware. In the suit, we alleged that a generic of our Oxaydo product for which Ranbaxy is seeking approval to market in the United States pursuant to an ANDA filed with the FDA infringes U.S. patents owned by us. The Settlement Agreement provides that Ranbaxy’s current generic of our Oxaydo product that is the subject of its ANDA filing does not infringe our Orange Book listed patents with the FDA. We have not provided Ranbaxy a license to our patents and we may re-commence patent infringement litigation against Ranbaxy if Ranbaxy changes the formulation of its current generic Oxaydo product.
On May 21, 2014, we announced that we had entered into a Settlement Agreement with Sandoz Inc. to settle our patent infringement action pending against Sandoz in the United States District Court for the District of Delaware. In the suit, we alleged that a generic of our Oxaydo product for which Sandoz is seeking approval to market in the United States pursuant to an ANDA filed with the FDA infringes a U.S. patent owned by us. Under the Settlement Agreement, Sandoz may launch its generic to the Oxaydo product in the U.S., through the grant of a non-exclusive license from us that would trigger 180 days following the first sale of a generic to the Oxaydo product in the U.S. by an entity that is entitled to the 180 day first-filer exclusivity under applicable law and FDA regulations (or if no entity is entitled to such 180 day exclusivity period, the date on which a generic to the Oxaydo product is first sold in the U.S). In certain circumstances, our license to Sandoz would become effective prior to such time. Sandoz is not obligated to pay us a royalty if its current formulation of its generic to the Oxaydo product is approved by the FDA. In the event Sandoz changes or modifies the structure of its generic Oxaydo product, or materially changes or modifies the amounts or type of any excipient used in the Sandoz formulation disclosed in its ANDA filing with the FDA as of July 30, 2013, Sandoz is required to pay us a royalty based upon the Net Profits (as defined in the Settlement Agreement) derived from the net sales of such changed or modified Sandoz generic Oxaydo product in the United States.
Notwithstanding the settlement of these prior infringement actions, it is possible that other generic manufacturers may also seek to launch a generic version of Oxaydo and challenge our patents. Any determination in such infringement actions that our patents covering our Aversion Technology and Oxaydo are invalid or unenforceable, in whole or in part, or that the products covered by generic sponsors’ ANDAs do not infringe our patents could have a material adverse effect on our operations and financial condition.
NOTE 4 – INVESTMENTS IN MARKETABLE SECURITIES
Investments in marketable securities consisted of the following:
| December 31, 2014 | December 31, 2013 | |||||||
| (in millions) | (in millions) | |||||||
| Marketable securities: | ||||||||
| Corporate bonds — maturing within 1 year | $ | 3.5 | $ | 3.1 | ||||
| Corporate bonds — maturing after 1 year and through March 2017 | 2.8 | 6.8 | ||||||
| Exchange-traded funds | 5.0 | 3.8 | ||||||
| Total marketable securities | $ | 11.3 | $ | 13.7 | ||||
| F-16 |
The Company’s marketable securities are classified as available-for-sale and are recorded at fair value based on quoted market prices or net asset value using the specific identification method. The purchase cost of corporate bonds may include a purchase price premium or discount which will be amortized or accreted against earned interest income to the maturity date of the bond. Our investments are classified as current in the Company’s Consolidated Balance Sheets as they may be sold within one year in response to changes in market prices or interest rates, to realign our investment concentrations or to meet our working capital needs.
The following tables provide a summary of the fair value and unrealized gains (losses) related to the Company’s available-for-sale securities (in millions):
| December 31, 2014 | ||||||||||||||||
| (in millions) | ||||||||||||||||
| Cost | Gross Unrealized Gains | Gross Unrealized Losses | Fair Value | |||||||||||||
| Available-for-sale: | ||||||||||||||||
| Corporate bonds | $ | 6.3 | $ | - | $ | - | $ | 6.3 | ||||||||
| Exchange-traded funds | 5.0 | - | - | 5.0 | ||||||||||||
| Total - Current | $ | 11.3 | $ | - | $ | - | $ | 11.3 | ||||||||
| December 31, 2013 | ||||||||||||||||
| (in millions) | ||||||||||||||||
| Cost | Gross Unrealized Gains | Gross Unrealized Losses | Fair Value | |||||||||||||
| Available-for-sale: | ||||||||||||||||
| Corporate bonds | $ | 9.9 | $ | - | $ | - | $ | 9.9 | ||||||||
| Exchange-traded funds | 3.8 | - | - | 3.8 | ||||||||||||
| Total - Current | $ | 13.7 | $ | - | $ | - | $ | 13.7 | ||||||||
Fair Value Measurement
Fair value is the price that would be received to sell an asset or paid to transfer a liability (an exit price) in an orderly transaction between market participants. Fair values determined based on Level 1 inputs utilize quoted prices (unadjusted) in active markets for identical assets or liabilities. Fair values determined based on Level 2 inputs utilize observable quoted prices for similar assets and liabilities in active markets and observable quoted prices for identical or similar assets in markets that are not very active. Fair values determined based on Level 3 inputs utilize unobservable inputs and include valuations of assets or liabilities for which there is little, if any, market activity. A financial asset or liability’s classification within the above hierarchy is determined based on the lowest level input that is significant to the fair value measurement.
Our assets measured at fair value or disclosed at fair value on a recurring basis as at December 31, 2014 and 2013 consisted of the following (in millions):
| December 31, 2014 | ||||||||||||||||
| (in millions) | ||||||||||||||||
| Total | Level 1 | Level 2 | Level 3 | |||||||||||||
| Assets: | ||||||||||||||||
| Corporate bonds | 6.3 | 6.3 | - | |||||||||||||
| Exchange-traded funds | 5.0 | 5.0 | - | - | ||||||||||||
| Total | $ | 11.3 | $ | 5.0 | $ | 6.3 | $ | - | ||||||||
| December 31, 2013 | ||||||||||||||||
| (in millions) | ||||||||||||||||
| Total | Level 1 | Level 2 | Level 3 | |||||||||||||
| Assets: | ||||||||||||||||
| Corporate bonds | 9.9 | - | 9.9 | - | ||||||||||||
| Exchange-traded funds | 3.8 | $ | 3.8 | $ | - | $ | - | |||||||||
| Total | $ | 13.7 | 3.8 | 9.9 | - | |||||||||||
| F-17 |
Accumulated Other Comprehensive Income (Loss)
Unrealized gains or losses on marketable securities are recorded in accumulated other comprehensive income (loss). Accumulated other comprehensive income (loss) at December 31, 2014 consisted of unrealized losses on securities of $13 thousand. Accumulated other comprehensive income (loss) at December 31, 2013 consisted of unrealized gains on securities of $19 thousand.
NOTE 5 – INVENTORIES
Inventories consist of both raw and packaging materials on our Oxaydo product and finished goods held for distribution and sale on our Nexafed product. During 2014 we purchased raw and packaging material inventories for $260 thousand from Pfizer on the Oxaydo product we reacquired from them. Inventories are stated at the lower of cost (first-in, first-out method) or market (net realizable value). We write down inventories to net realizable value based on forecasted demand and market conditions, which may differ from actual results.
We have recorded Nexafed deferred revenue of $0.35 million and $0.29 million at December 31, 2014 and 2013, respectively. The related cost of sales of $0.22 million and $0.19 million at December 31, 2014 and 2013, respectively, is reported in our balance sheet in the other current deferred assets account and excluded from the reported year end inventories. We will recognize both the revenue and cost of sales on these Nexafed shipments once the right of return no longer exists or adequate history and information becomes available to estimate product returns.
Our purchases of ingredients and other materials required in our development and clinical trial activities are expensed as incurred.
Inventories are summarized as follows:
| December 31, | ||||||||
| 2014 | 2013 | |||||||
| (in thousands) | ||||||||
| Raw and packaging materials | $ | 260 | $ | - | ||||
| Finished goods | 44 | 501 | ||||||
| Total inventory | 304 | 501 | ||||||
| Less: inventory reserve for finished goods | ( -) | (250 | ) | |||||
| Net inventory | $ | 304 | $ | 251 | ||||
Our inventory reserve activity during the year ended December 31, 2014 and 2013 was as follows:
| Inventory reserve | ||||||||
| 2014 | 2013 | |||||||
| (in thousands) | ||||||||
| Beginning of year | $ | 250 | $ | - | ||||
| Reserve expense for finished goods | 201 | 250 | ||||||
| 451 | 250 | |||||||
| Inventory write-offs | (451 | ) | (-) | |||||
| End of year | $ | - | $ | 250 | ||||
NOTE 6 – PROPERTY, PLANT AND EQUIPMENT
Property, plant and equipment are summarized as follows:
| December 31, | ||||||||
| 2014 | 2013 | |||||||
| (in thousands) | ||||||||
| Building and improvements | $ | 1,259 | $ | 1,259 | ||||
| Scientific equipment | 595 | 595 | ||||||
| Computer hardware and software | 252 | 252 | ||||||
| Machinery and equipment | 386 | 252 | ||||||
| Land and improvements | 162 | 162 | ||||||
| Other personal property | 70 | 70 | ||||||
| Office equipment | 27 | 27 | ||||||
| 2,751 | 2,617 | |||||||
| Less: accumulated depreciation | (1,794 | ) | (1,676 | ) | ||||
| Total property, plant and equipment, net | $ | 957 | $ | 941 | ||||
| F-18 |
Depreciation and amortization expense was approximately $0.1 million for each of the years ended December 31, 2014, 2013, and 2012.
NOTE 7 – ACCRUED EXPENSES
Accrued expenses are summarized as follows:
| December 31, | ||||||||
| 2014 | 2013 | |||||||
| (in thousands) | ||||||||
| Professional services | $ | 253 | $ | 293 | ||||
| Other fees and services | 110 | 140 | ||||||
| Payroll, payroll taxes and benefits | 94 | 78 | ||||||
| Clinical and regulatory services | 83 | - | ||||||
| Contract manufacture services | - | 14 | ||||||
| Property taxes | 15 | 15 | ||||||
| Franchise taxes | 13 | 1 | ||||||
| $ | 568 | $ | 541 | |||||
NOTE 8 – DEBT
On December 27, 2013, we entered into a Loan and Security Agreement (the “Loan Agreement”) with Oxford Finance LLC (“Oxford” or the “Lender”), for a term loan to the Company in the principal amount of $10.0 million (the “Term Loan”). The full principal amount of the Term Loan was funded on December 27, 2013. We are using the proceeds of the Loan Agreement for general working capital and to fund our business requirements. The Term Loan accrues interest at a fixed rate of 8.35% per annum (with a default rate of 13.35% per annum). The Company is required to make monthly interest−only payments until the April 1, 2015 (“Amortization Date”) and starting on the Amortization Date, the Company is required to make payments of principal and accrued interest in equal monthly installments sufficient to amortize the Term Loan through the maturity date of December 1, 2018. All unpaid principal and accrued and unpaid interest with respect to the Term Loan is due and payable in full on December 1, 2018. As security for its obligations under the Loan Agreement, the Company granted Lender a security interest in substantially all of its existing and after−acquired assets, exclusive of its intellectual property assets. Pursuant to the Loan Agreement, the Company is not allowed to pledge its intellectual property assets to others.
The Company may voluntarily prepay the Term Loan in full, but not in part, and any prepayment is subject to a prepayment premium equal to 2% of the principal prepaid, if prepaid prior to December 27, 2015, and 1% of the principal prepaid if prepaid after December 27, 2015. In addition, at the maturity, termination or upon voluntary or mandatory prepayment of the Term Loan the Company must pay the Lender an additional one-time interest payment of $795 thousand. We will incur and accrue additional monthly interest expense over the term of the loan for this additional one-time interest payment using the loan’s effective cash interest rate.
The Company was obligated to pay customary lender fees and expenses, including a one-time facility fee of $50 thousand and the Lender’s expenses in connection with the Loan Agreement. Combined with the Company’s own expenses and a $100 thousand consulting placement fee, the Company incurred $231 thousand in deferred debt issue costs. We are amortizing these costs into interest expense over the term of the loan using the loan’s effective interest rate.
The Loan Agreement contains customary representations and warranties and customary affirmative and negative covenants, including, among others, limits or restrictions on the Company’s ability to incur liens, incur indebtedness, pay dividends, redeem stock, and merge or consolidate and dispose of assets. In addition, it contains customary events of default that entitles the Lender to cause any or all of the Company’s indebtedness under the Loan Agreement to become immediately due and payable. The events of default (some of which are subject to applicable grace or cure periods), include, among other things, non−payment defaults, covenant defaults, a material adverse change in the Company, bankruptcy and insolvency defaults and material judgment defaults.
| F-19 |
Our debt is summarized below:
| 2014 | ||||||||||||
| (in thousands) | ||||||||||||
| Debt | Current | Long-term | Total | |||||||||
| Beginning of year | $ | - | $ | 10,000 | $ | 10,000 | ||||||
| Principal payments | - | - | - | |||||||||
| Classification | 1,758 | (1,758 | ) | - | ||||||||
| End of year | $ | 1,758 | $ | 8,242 | $ | 10,000 | ||||||
| Debt Discount | Current | Long-term | Total | |||||||||
| Beginning of year | $ | - | $ | (400 | ) | $ | (400 | ) | ||||
| Amortization expense | - | 119 | 119 | |||||||||
| End of year | $ | - | $ | (281 | ) | $ | (281 | ) | ||||
| Debt, net | $ | 1,758 | $ | 7,961 | $ | 9,719 | ||||||
Upon the execution of the Loan Agreement on December 27, 2013, we issued to the Lender warrants to purchase an aggregate of up to 298 thousand shares of our common stock at an exercise price equal to $1.595 per share (the “Warrants”). We recorded $400 thousand as debt discount associated with the fair value of the Warrants and are amortizing it to interest expense over the term of the loan using the loan’s effective interest rate. The Warrants are immediately exercisable for cash or by net exercise and will expire December 27, 2020. The fair value of the warrants was determined using the Black-Scholes option-pricing model. Significant assumptions used in the Black-Scholes model were:
| Expected dividend yield | 0.0 | % | ||
| Risk-free interest rate | 2.4 | % | ||
| Expected volatility | 92 | % | ||
| Expected term (years) | 7 |
On January 7, 2015, we and Oxford entered into an amendment (the “Amendment”) to the Loan Agreement. Pursuant to the Amendment, (i) the exercise price of the warrant previously issued to the Lender to purchase 297,805 shares of our Common Stock was lowered from $1.595 to $0.504 per share (the average closing price of our common stock on Nasdaq for the 10 trading days preceding the date of the Amendment), (ii) we agreed to maintain a $2.5 million cash balance until such time as we have repaid $5 million in principal of the Term Loan, and (iii) the Lender consented to the terms of our Collaboration and License Agreement with Egalet relating to our Oxaydo product.
Our interest expense consisted of the following:
| 2014 | 2013 | |||||||
| (in thousands) | ||||||||
| Interest expense: | ||||||||
| Secured Promissory notes | $ | 1,024 | $ | 9 | ||||
| Debt discount | 119 | - | ||||||
| Debt issue costs | 69 | - | ||||||
| Total interest expense | $ | 1,212 | $ | 9 | ||||
The annual principal payments of the debt at December 31, 2014 are as follows:
| AnnualPrincipal Payments | ||||
| (in thousands) | ||||
| 2015 | $ | 1,758 | ||
| 2016 | 2,522 | |||
| 2017 | 2,741 | |||
| 2018 | 2,979 | |||
| Total | $ | 10,000 | ||
| F-20 |
NOTE 9 – EQUITY FINANCING
Our universal shelf registration statement on Form S-3 was declared effective by the Securities and Exchange Commission (“SEC”) on March 15, 2013. On April 18, 2013, we filed a prospectus supplement with the SEC pursuant to which we may sell shares of our common stock from time to time in “at the market” offerings and certain other transactions, having sales proceeds of up to $13 million. During the year ended December 31, 2014, we did not sell any shares of our common stock pursuant to our prospectus supplement. During the year ended December 31, 2013, we sold approximately 1.94 million shares of our common stock under a Sales Agreement with MLV & Co., our sales agent, through an “at the market” offering, for gross proceeds of approximately $3.3 million. Transaction costs were approximately $0.1 million. The net proceeds of these transactions for year ended ending December 31, 2013 were approximately $3.2 million and were used for general corporate purposes, including working capital, capital expenditures, research, development and marketing expenditures and clinical trial expenditures.
NOTE 10 – COMMON STOCK WARRANTS
In connection with the issuance of the $10.0 million secured promissory notes in December 2013, we issued common stock purchase warrants (“warrants”) to acquire approximately 298 thousand shares of our common stock having an exercise price of $1.595 per share with an expiration date in December 2020. In August 2014, warrants exercisable for 1.9 million shares of our common stock with an exercise price of $3.40 per share expired unexercised. At December 31, 2014, we have total outstanding warrants exercisable for 298 thousand shares of our common stock having a weighted average exercise price of $1.595 per share with an expiration date in December 2020. In January 2015, the exercise price of these warrants was reduced to $0.504 per share. The warrants contain a cashless exercise feature.
Our warrant activity during the years ended December 31, 2014 and 2013 is shown below:
| December 31, | ||||||||||||||||
| 2014 | 2013 | |||||||||||||||
| Number (000’s) | Weighted Average Exercise Price | Number (000’s) | Weighted Average Exercise Price | |||||||||||||
| Outstanding, beginning | 2,154 | $ | 3.15 | 1,856 | $ | 3.40 | ||||||||||
| Issued | - | - | 298 | 1.60 | ||||||||||||
| Exercised | - | - | - | - | ||||||||||||
| Expired | (1,856 | ) | 3.40 | - | - | |||||||||||
| Outstanding, ending | 298 | $ | 1.60 | 2,154 | $ | 3.15 | ||||||||||
NOTE 11 – EMPLOYEE BENEFIT PLANS
401(k) and Profit-Sharing Plan
We have a 401(k) and Profit-Sharing Plan (the “Plan”) for our employees. Employees may elect to make a basic contribution of up to 80% of their annual earnings subject to certain regulatory restrictions on their total contribution. The Plan provides that the Company can make discretionary matching contributions along with a discretionary profit-sharing contribution. We did not contribute matching or profit sharing contributions for the Plan in years 2014, 2013, and 2012.
| F-21 |
Stock Option Plans
We maintain various stock option plans. A summary of our stock option plans as of December 31, 2014, 2013, and 2012 and for the years then ended consisted of the following:
| Years Ended December 31, | ||||||||||||||||||||||||
| 2014 | 2013 | 2012 | ||||||||||||||||||||||
| (in thousands except price data) | ||||||||||||||||||||||||
| Number of Options | Weighted Average Exercise Price | Number of Options | Weighted Average Exercise Price | Number
of Options) | Weighted Average Exercise Price | |||||||||||||||||||
| Outstanding, beginning | 3,738 | $ | 4.99 | 3,296 | $ | 5.50 | 3,556 | $ | 6.41 | |||||||||||||||
| Granted | 900 | 0.52 | 548 | 1.66 | 475 | 2.80 | ||||||||||||||||||
| Exercised | (31 | ) | 1.30 | (31 | ) | 1.30 | (31 | ) | 1.30 | |||||||||||||||
| Forfeited or expired | (51 | ) | 3.50 | (75 | ) | 5.02 | (704 | ) | 8.43 | |||||||||||||||
| Outstanding, ending | 4,556 | $ | 4.14 | 3,738 | $ | 4.99 | 3,296 | $ | 5.50 | |||||||||||||||
| Options exercisable | 3,476 | $ | 5.21 | 3,115 | $ | 5.61 | 2,763 | $ | 5.99 | |||||||||||||||
The following table summarizes information about nonvested stock options outstanding at December 31, 2014:
| Number of Options Not Exercisable | Weighted Average Fair Value | |||||||
| (in thousands except per price data) | ||||||||
| Outstanding at December 31, 2013 | 623 | $ | 1.71 | |||||
| Granted | 900 | 0.46 | ||||||
| Vested | (443 | ) | 1.73 | |||||
| Forfeited | - | - | ||||||
| Outstanding at December 31, 2014 | 1,080 | $ | 0.66 | |||||
We estimate the option’s fair value on the date of grant using the Black-Scholes option-pricing model. Black-Scholes utilizes assumptions related to volatility, the risk-free interest rate, the dividend yield (which is assumed to be zero, as we have not paid any cash dividends) and employee exercise behavior. Expected volatilities utilized in the Black-Scholes model are based on the historical volatility of our common stock price. The risk-free interest rate is derived from the U.S. Treasury yield curve in effect at the time of grant. The expected life of the grants is derived from historical exercise activity. Historically, the majority of our stock options have been held until their expiration date.
The assumptions used in the Black-Scholes model to determine fair value for the 2014, 2013 and 2012 stock option grants were:
| 2014 | 2013 | 2012 | ||||||||||
| Expected dividend yield | 0.0 | % | 0.0 | % | 0.0 | % | ||||||
| Risk-free interest rates | 2.2 | % | 1.9% to 2.9 | % | 1.7% to 2.0 | % | ||||||
| Average expected volatility | 97 | % | 111 | % | 114 | % | ||||||
| Expected term (years) | 10 | 10 | 10 | |||||||||
| Weighted average grant date fair value | $ | 0.46 | $ | 1.54 | $ | 2.60 | ||||||
As of December 31, 2014, there was no intrinsic value of the option awards which were vested and outstanding. As of December 31, 2013 and 2012 the aggregate intrinsic value of the option awards which were vested and outstanding was $13 thousand and $0.1 million, respectively. In addition, the aggregate intrinsic value of option awards exercised during the year ended December 31, 2014, 2013 and 2012 was $0.00, $28 thousand and $0.1 million, respectively. The total remaining unrecognized compensation cost on unvested option awards outstanding at December 31, 2014 was $0.8 million and is expected to be recognized in operating expense in varying amounts over the twenty-three months remaining in the requisite service period.
| F-22 |
Information about the cashless stock option exercises during the last three years is as follows:
| · | During 2014, options to purchase 24 thousand shares of common stock were exercised utilizing various cashless exercise features of our stock option plan and after withholding 16 thousand shares for $32 in exercise costs and withholding 2 thousand shares for $4 in statutory minimum withholding payroll taxes, we issued 6 thousand shares of common stock. | |
| · | During 2013, options to purchase 24 thousand shares of common stock were exercised utilizing various cashless exercise features of our stock option plan and after withholding 17 thousand shares for $32 in exercise costs and withholding 1 thousand shares for $4 in statutory minimum withholding payroll taxes, we issued 6 thousand shares of common stock. | |
| · | During 2012, options to purchase 24 thousand shares of common stock were exercised utilizing various cashless exercise features of the stock option plan and after withholding 10 thousand shares for $31 in exercise costs and withholding 5 thousand shares for $15 in statutory minimum payroll taxes, we issued 9 thousand shares of common stock. |
Restricted Stock Unit Award Plan
We have two Restricted Stock Unit Award Plans for our employees and non-employee directors, a 2005 Restricted Stock Unit Award Plan (the “2005 RSU Plan”) and a 2014 Restricted Stock Unit Award Plan (the “2014 RSU Plan”). Vesting of an RSU entitles the holder to receive a share of our common stock on a distribution date. The share-based compensation cost to be incurred on a granted RSU is the RSU’s fair value, which is the market price of our common stock on the date of grant, less its exercise cost. The compensation cost is amortized to expense over the vesting period of the RSU award.
A summary of the grants under the RSU Plans as of December 31, 2014, 2013, and 2012, and for the years then ended consisted of the following (in thousands):
| Years Ended December 31, | ||||||||||||||||||||||||
| 2014 | 2013 | 2012 | ||||||||||||||||||||||
| (in thousands) | ||||||||||||||||||||||||
| Number of RSUs | Number of Vested RSUs | Number of RSUs | Number of Vested RSUs | Number
of RSUs | Number of Vested RSUs | |||||||||||||||||||
| Outstanding, beginning | 829 | 829 | 1,658 | 1,658 | 2,487 | 2,487 | ||||||||||||||||||
| Granted | 147 | 147 | - | - | - | - | ||||||||||||||||||
| Distributed | (829 | ) | (829 | ) | (829 | ) | (829 | ) | (829 | ) | (829 | ) | ||||||||||||
| Vested | - | - | - | - | - | - | ||||||||||||||||||
| Forfeited or expired | - | - | - | - | - | - | ||||||||||||||||||
| Outstanding, ending | 147 | 147 | 829 | 829 | 1,658 | 1,658 | ||||||||||||||||||
2005 Restricted Stock Unit Award Plan
Under our 2005 RSU Plan, one-fourth of vested shares of common stock underlying RSU awards of 3.3 million shares were distributed (after payment of exercise costs of $0.01 par value per share) on January 1 of each of years 2011 thru 2014. All RSUs granted under the 2005 RSU Plan had been distributed effective January 1, 2014.
Information about the distribution of 0.83 million shares under the 2005 RSU Plan during each of the last three years are as follows:
| · | On January 1, 2014, 0.51 million shares were distributed to the holders while 0.32 million shares were withheld by the Company upon elections made to exchange RSUs in satisfaction of $0.5 million withholding tax obligations; and | |
| · | On January 1, 2013, 0.51 million shares were distributed to the holders while 0.32 million shares were withheld by the Company upon elections made to exchange RSUs in satisfaction of $0.7 million withholding tax obligations. | |
| · | On January 1, 2012, 0.53 million shares were distributed to the holders while 0.30 million shares were withheld by the Company upon elections made to exchange RSUs in satisfaction of $1.0 million withholding tax obligations. |
| F-23 |
2014 Restricted Stock Unit Award Plan
Our 2014 RSU Plan was approved by shareholders on May 1, 2014 and permits the grant of up to 2.0 million shares of our common stock pursuant to awards under the 2014 RSU Plan.
Information about the RSU grants under the 2014 RSU Plan is as follows:
| · | On May 1, 2014, we awarded 37 thousand RSUs to each of our 4 non-employee directors. Such RSU awards vested 50% on June 30, 2014 and 25% on each of September 30 and December 31, 2014. Such non-employee director awards allow for non-employee directors to receive payment in cash, instead of stock, for up to 40% of each RSU award. The RSU awards subject to cash settlement are recorded as a liability in the Company’s balance sheet. The liability was $26 thousand at December 31, 2014. Accordingly the vested portion of the awards containing the cash settlement feature are being marked-to-market each reporting period until they are distributed. Marked-to-market accounting can create fluctuations in our compensation expense including the need to record additional expense. RSU awards are generally distributed on the first business day of the year after vesting, but such distribution can be deferred until a later date at the election of the non-employee director. | |
| · | On January 2, 2015, we awarded 52 thousand RSUs to each of our 4 non-employee directors which also allow for them to receive payment in cash, instead of stock, for up to 40% of each RSU award. Such awards vest 25% at the end of each calendar quarter in 2015. The RSU awards subject to cash settlement are subject to marked-to market accounting. Distributions of stock under the January 2, 2015 award cannot be deferred until a later date and the stock under such awards will be distributed on January 4, 2016. |
Information about the distribution of shares under the 2014 RSU Plan is as follows:
| · | On January 2, 2015, 129 thousand RSUs from the May 1, 2014 award were distributed and 18 thousand RSUs were deferred until a future distribution date. Of the 129 thousand RSUs distributed, 99 thousand RSUs were distributed in common stock and 30 thousand RSUs were settled in cash. |
NOTE 12 – INCOME TAXES
Provision for Income Taxes
The reconciliation between our provision for income taxes and the amounts computed by multiplying our loss before taxes by the U.S. statutory tax rate is as follows:
| December 31, | ||||||||||||
| 2014 | 2013 | 2012 | ||||||||||
| (in thousands) | ||||||||||||
| Benefit at U.S. statutory 34% tax rate | $ | (4,491 | ) | $ | (4,690 | ) | $ | (3,287 | ) | |||
| State taxes (benefit), net of federal effect | 65 | (238 | ) | - | ||||||||
| Research and development tax credits | (30 | ) | (185 | ) | - | |||||||
| Share-based compensation | 262 | 369 | 473 | |||||||||
| Other | 2 | 2 | 2 | |||||||||
| Change in valuation allowance | 4,192 | 4,742 | 2,812 | |||||||||
| Provision for income taxes | $ | - | $ | - | $ | - | ||||||
Deferred Tax Assets and Valuation Allowance
Deferred tax assets reflect the tax effects of net operating losses (“NOLs”), tax credit carryovers, and temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes. The most significant item of our deferred tax assets is derived from our Federal NOLs. We have approximately $51.5 million federal income tax benefits at December 31, 2014 derived from $151.4 million Federal NOLs at the U.S. statutory tax rate of 34% and $2.9 million state NOLs, available to offset future taxable income, some of which have limitations for use as prescribed under IRC Section 382. Our Federal and state NOLs will expire in varying amounts between 2016 and 2034 if not used, and those expirations will cause fluctuations in our valuation allowances. In 2013 we adjusted the estimated future value of NOLs under IRC Section 382 resulting in increasing those NOLs by $15.5 million with an equally offsetting valuation allowance. The net change in the valuation allowance in 2014, 2013, and 2012 was approximately $2.4 million, $18.0 million, and $2.8 million, respectively.
| F-24 |
As of December 31, 2014 we had federal research and development tax credits of approximately $1.1 million, which expire in the years 2024 through 2034. We also had approximately $0.3 million of Indiana state research and development tax credits, which expire in the years 2015 through 2017. The components of our deferred tax assets are as follows:
| December 31, | ||||||||
| 2014 | 2013 | |||||||
| (in thousands) | ||||||||
| Deferred tax assets: | ||||||||
| Estimated future value of NOLs | ||||||||
| - Federal | $ | 51,503 | $ | 46,830 | ||||
| - State | 2,898 | 2,843 | ||||||
| Research and development tax credits | 1,398 | 1,433 | ||||||
| Share-based compensation | 45 | 2,261 | ||||||
| Other, net | 151 | 119 | ||||||
| Total deferred taxes | 55,995 | 53,486 | ||||||
| Valuation allowance | (55,995 | ) | (53,486 | ) | ||||
| Net deferred tax assets | $ | - | $ | - | ||||
Realization of deferred tax assets is dependent upon future earnings, if any, and the timing and amount of which may be uncertain. Valuation allowances are placed on deferred tax assets when uncertainty exists on their near term utilization. We make periodic reviews of our valuation allowances and fluctuations can occur. Those fluctuations may be reflected as income tax expenses or benefits in the period they occur. We continue to maintain full valuation allowance against all of our deferred tax assets at December 31, 2014 due to uncertainties with respect to future utilization of net operating loss carryforwards. If in the future it is determined that amounts of our deferred tax assets would likely be realized, the valuation allowance would be reduced in the period in which such determination is made and a benefit from income taxes in such period would be recognized.
Uncertainty in Income Taxes
We adopted FASB’s statement regarding accounting for uncertainty in income taxes which defined the threshold for recognizing the benefits of tax-return positions in the financial statements as "more-likely-than-not" to be sustained by the taxing authorities. Our adoption of the standard did not result in establishing a contingent tax liability reserve or a corresponding charge to retained earnings. At each of December 31, 2014, 2013 and 2012 we had no liability for income tax associated with uncertain tax positions. If in the future we establish a contingent tax liability reserve related to uncertain tax positions, our practice will be to recognize the interest in interest expense and the penalties in other non-operating expense.
The Company files federal and state income tax returns and in the normal course of business the Company is subject to examination by these taxing authorities. As of December 31, 2014, the Company’s tax years 2011, 2012 and 2013 are subject to examination by the taxing authorities. With few exceptions, we believe the Company is no longer subject to U.S. federal, state and local examinations by taxing authorities for years before 2011. As of December 31, 2013 the Company’s tax year of 2010 was included in the tax years subject to examination.
NOTE 13 – COMMITMENTS AND CONTINGENCIES
Facility Lease
The Company leases administrative office space in Palatine, Illinois under a lease expiring March 31, 2016 for approximately $25 thousand annually.
Reglan®/Metoclopramide Litigation
Halsey Drug Company, as predecessor to us, has been named along with numerous other companies as a defendant in cases filed in three separate state coordinated litigations pending in Pennsylvania, New Jersey and California, respectively captioned In re: Reglan®/Metoclopramide Mass Tort Litigation, Philadelphia County Court of Common Pleas, January Term, 2010, No. 01997; In re: Reglan Litigation, Superior Court of New Jersey, Law Division, Atlantic County, Case No. 289, Master Docket No. ATL-L-3865-10; and Reglan/Metoclopramide Cases, Superior Court of California, San Francisco County, Judicial Council Coordination Proceeding No. 4631, Superior Court No.: CJC-10-004631. In addition, Acura was served with a similar complaint by two individual plaintiffs in Nebraska federal court, which plaintiffs voluntarily dismissed in December 2014. In this product liability litigation against numerous pharmaceutical product manufacturers and distributors, including us, plaintiffs claim injuries from their use of the Reglan brand of metoclopramide and generic metoclopramide.
| F-25 |
In the Pennsylvania action, over 200 lawsuits have been filed against us and Halsey Drug Company alleging that plaintiffs developed neurological disorders as a result of their use of the Reglan brand and/or generic metoclopramide. In the New Jersey action, plaintiffs filed approximately 150 lawsuits against us, but served less than 50 individual lawsuits upon us. In the California action, there are 89 pending cases against us, with more than 445 individual plaintiffs.
In the lawsuits filed to date, plaintiffs have not confirmed they ingested any of the generic metoclopramide manufactured by us. We discontinued manufacture and distribution of generic metoclopramide more than 19 years ago. In addition, we believe the June 23, 2011 decision by the U.S. Supreme Court in PLIVA v. Mensing (“Mensing decision”) holding that state tort law failure to warn claims against generic drug companies are pre-empted by the 1984 Hatch-Waxman Act Amendments and federal drug regulations will assist us in favorably resolving these cases. We have consistently maintained the position that these claims are without merit and intend to vigorously defend these actions.
In New Jersey, Generic Defendants, including Acura, filed dispositive motions based on the Mensing decision, which the Court granted with a limited exception. As of September 2012, the New Jersey trial court dismissed Acura with prejudice. In Pennsylvania, and California, Generic Defendants, including Acura, also filed dispositive motions based on the Mensing decision.
In Pennsylvania, on November 18, 2011, the trial court denied Generic Defendants’ dispositive preemption motions. On appeal, the Pennsylvania Superior Court held in a July 29, 2013 decision that federal preemption applied, but that Mensing did not completely bar all claims and refused to dismiss these cases. On September 17, 2014, the Pennsylvania Supreme Court declined to hear a further appeal. On December 16, 2014, Generic Defendants filed a Petition for a Writ of Certiorari requesting that the United States Supreme Court agree to hear a further appeal on the grounds that federal preemption under Mensing should completely bar all of these claims. All trial court proceedings have been stayed pending resolution of this lengthy appeal process. To the extent that plaintiffs intend to pursue their claims in the future, if the appeal is denied, Acura nonetheless remains optimistic that most, if not all, of these Philadelphia cases will eventually be dismissed against us based upon the favorable aspects of the Superior Court’s narrow preemption ruling and lack of product identification, although there can be no assurance in this regard. Legal fees related to this matter are currently covered by our insurance carrier.
In California, the trial court entered a May 25, 2012 Order denying Generic Defendants’ dispositive preemption motions. The Generics Defendants’ appeals from this order were denied by the California appellate courts. Therefore, subject to further developments, plaintiffs may be permitted to proceed with these lawsuits including state law claims based on (1) failing to communicate warnings to physicians through “Dear Doctor” letters; and (2) failure to update labeling to adopt brand labeling changes. California trial court also has acknowledged the preemptive effect of Mensing so that any claim “that would render the generic defendants in violation of federal law if they are found responsible under a state law cause of action, would not be permissible.” To date, however, none of these plaintiffs have confirmed they ingested any of the generic metoclopramide manufactured by us. Therefore, we expect the number of plaintiffs with possible claims to be reduced voluntarily or by motion practice. Action will be taken in an effort to dismiss Acura from these cases, although there can be no assurance in this regard. Legal fees related to this matter are currently covered by our insurance carrier.
As any potential loss is neither probable nor estimable, we have not accrued for any potential loss related to these matters as of December 31, 2014 and we are presently unable to determine if any potential loss would be covered by our insurance carrier.
| F-26 |
SUPPLEMENTARY DATA (UNAUDITED)
Selected unaudited quarterly consolidated financial data is shown below (in thousands except per share amounts):
| For Three Month Periods Ended | ||||||||||||||||
| Mar. 31, 2014 | June 30, 2014 | Sept. 30, 2014 | Dec. 31, 2014 | |||||||||||||
| Net revenues (i) | $ | 42 | $ | 35 | $ | 145 | $ | 529 | ||||||||
| Operating expenses | 3,868 | 3,307 | 2,791 | 2,984 | ||||||||||||
| Operating loss | (3,826 | ) | (3,272 | ) | (2,646 | ) | (2,455 | ) | ||||||||
| Net loss | $ | (4,088 | ) | $ | (3,521 | ) | $ | (2,904 | ) | $ | (2,696 | ) | ||||
| Basic loss per share | $ | (0.08 | ) | $ | (0.07 | ) | $ | (0.06 | ) | $ | (0.06 | ) | ||||
| Diluted loss per share | $ | (0.08 | ) | $ | (0.07 | ) | $ | (0.06 | ) | $ | (0.06 | ) | ||||
| For Three Month Periods Ended | ||||||||||||||||
| Mar. 31, 2013 | June 30, 2013 | Sept. 30, 2013 | Dec. 31, 2013 | |||||||||||||
| Net revenues (i) | $ | 4 | $ | 1 | $ | 83 | $ | 35 | ||||||||
| Operating expenses | 4,248 | 3,141 | 3,308 | 3,516 | ||||||||||||
| Operating loss | (4,244 | ) | (3,140 | ) | (3,225 | ) | (3,481 | ) | ||||||||
| Net loss | $ | (4,218 | ) | $ | (3,076 | ) | $ | (3,190 | ) | $ | (3,417 | ) | ||||
| Basic loss per share | $ | (0.09 | ) | $ | (0.07 | ) | $ | (0.07 | ) | $ | (0.07 | ) | ||||
| Diluted loss per share | $ | (0.09 | ) | $ | (0.07 | ) | $ | (0.07 | ) | $ | (0.07 | ) | ||||
(i) See Note 2 for revenue recognition.
| F-27 |
ACURA PHARMACEUTICALS, INC.
EXHIBIT INDEX
The following exhibits are included as a part of this Annual Report on Form 10-K or incorporated herein by reference.
|
Exhibit Number |
Exhibit Description | |
| 1.1 | At Market Issuance Sales Agreement dated April 18, 2013 between Acura Pharmaceuticals, Inc. and MLV & Co. LLC (incorporated by reference to Exhibit 1.1 to the Registrant’s Form 8-K filed on April 18, 2013) | |
|
3.1
|
Restated Certificate of Incorporation of the Registrant (incorporated by reference to Exhibit 3.1 to the Registrant’s Form 8-K filed on June 25, 2009). | |
| 3.2 | Certificate of Amendment Reverse Splitting Common Stock and restating but not changing text of part of Article III of Restated Certificate of Incorporation (incorporated by Reference to Exhibit 3.1 to the Form 8-K filed December 4, 2007). | |
| 3.3 | Restated Bylaws of the Registrant (incorporated by reference to Exhibit 3.1 to the Form 8-K filed on March 3, 2009). | |
| 10.1 | License, Development and Commercialization Agreement dated October 30, 2007 by and between the Registrant and King Pharmaceuticals Research and Development, Inc. (incorporated by reference to Exhibit 10.1 of the Form 8-K filed on November 2, 2007) | |
| 10.2 | Letter Agreement dated as of September 24, 2012 by and between the Registrant and King Pharmaceuticals Research and Development, Inc. (incorporated by reference to Exhibit 10.1 of the Form 8-K filed on September 26, 2012) (confidential treatment has been granted for portions of this Exhibit). | |
| 10.3 | Letter Agreement dated April 9, 2014 between King Pharmaceuticals Research and Development Inc. and Registrant terminating License, Development and Commercialization Agreement dated October 30, 2007 (Incorporated by Reference to Exhibit 10.1 to the Registrant’s Form 10-Q for the quarter ending June 30, 2014 filed on August 4, 2014) (confidential treatment has been granted for portions of this Exhibit). | |
| 10.4 | Manufacturing Services Agreement dated as of July 19, 2011 between the Registrant and Patheon Pharmaceuticals Inc. (incorporated by reference to Exhibit 10.1 to our Form 8-K filed July 27, 2011) (confidential treatment has been granted for portions of this Exhibit) | |
| 10.5 |
Securities Purchase Agreement dated as of August 20, 2007 (“PIPE SPA”) among the Registrant, Vivo Ventures Fund VI, L.P., Vivo Ventures VI Affiliates Fund, L.P., GCE Holdings LLC, and certain other signatories thereto (incorporated by reference to Exhibit 10.1 to the Form 8-K filed on August 21, 2007).
| |
| 10.6 | Form of Warrant dated as of August 20, 2007 issued pursuant to the PIPE SPA (incorporated by reference to Exhibit 4.1 to the Form 8-K filed on August 21, 2007)(These warrants expired in 2014). | |
| 10.7 | Loan and Security Agreement dated as of December 27, 2013 between Acura Pharmaceuticals, Inc. Acura Pharmaceutical Technologies, Inc. and Oxford Finance LLC (incorporated by reference to Exhibit 10.6 to the Form 10-K filed March 3, 2014) |
| 1 |
|
Exhibit Number |
Exhibit Description | |
| *10.8 | First Amendment to Loan and Security Agreement entered into as of January 7, 2015 between Oxford Finance LLC , the Registrant and APT | |
| *10.9 | Amended and Restated Warrant A-1 issued to Oxford Finance LLC on January 7, 2015 | |
| *10.10 | Amended and Restated Warrant A-2 issued to Oxford Finance LLC on January 7, 2015 | |
| *10.11 | Amended and Restated Warrant A-3 issued to Oxford Finance LLC on January 7, 2015 | |
| 10.12 | Form of Mortgage dated December 27, 2013(incorporated by reference to Exhibit 10.8 to the Form 10-K filed March 3, 2014) | |
| *10.13 | Collaboration and License Agreement between the Registrant, Egalet US, Inc., Egalet Limited and with respect to Section 17.21, Egalet Corporation (certain information has been omitted and filed separately with the Securities and Exchange Commission and confidential treatment has been requested with respect to the omitted portion). | |
| 10.14 | Amended and Restated Voting Agreement dated as of February 6, 2004 among the Registrant, Care Capital Investments II, LP, Essex Woodlands Health Ventures V, L.P., Galen Partners III, L.P., and others (incorporated by reference to Exhibit 10.5 of the Form 8-K filed on February 10, 2004 (the “February 2004 Form 8-K”)). | |
| 10.15 | Joinder and Amendment to Amended and Restated Voting Agreement dated November 9, 2005 between the Registrant, GCE Holdings, Essex Woodlands Health Ventures V, L.P., Care Capital Investments II, LP, Galen Partners III, L.P. and others (incorporated by reference to Exhibit 10.1 to the Form 8-K filed November 10, 2005). | |
| 10.16 | Second Amendment to Amended and Restated Voting Agreement dated as of January 24, 2008 between the Registrant and GCE Holdings, LLC (incorporated by reference to Exhibit 10.1 to the Form 8-K filed January 28, 2008). | |
| 10.17 | Third Amendment to Amended and Restated Voting Agreement dated as of October 1, 2012 between the Registrant, Care Capital Investments II, LP, Essex Woodlands Health Ventures V, L.P., Galen Partners III, L.P., and others (incorporated by reference to Exhibit 10.1 of the Form 8-K filed on October 3, 2012). | |
| †10.18 | Registrant’s 1995 Stock Option and Restricted Stock Purchase Plan (incorporated by reference to Exhibit 4.1 to the Registrant's Registration Statement on Form S-8, File No. 33-98396). | |
| †10.19 | Registrant’s 1998 Stock Option Plan, as amended (incorporated by reference to Appendix C to the Registrant’s Proxy Statement filed on May 12, 2009). | |
| †10.20 | Registrant’s 2005 Restricted Stock Unit Award Plan, as amended (incorporated by reference to Appendix B to the Registrant’s Proxy Statement filed on April 2, 2008). | |
| †10.21 | Registrant’s 2014 Restricted Stock Unit Award Plan, (incorporated by reference to Appendix A to the Registrant’s Proxy Statement filed on March 12, 2014). |
| 2 |
|
Exhibit Number |
Exhibit Description | |
| †10.22 | Registrant’s 2008 Stock Option Plan, as amended on June 25, 2009 (incorporated by reference to Appendix B to our Proxy Statement filed on May 12, 2009). | |
| †10.23 | Employment Agreement dated as of March 10, 1998 between the Registrant and Peter Clemens (“Clemens”) (incorporated by reference to Exhibit 10.44 to the Form 10-K for the period ending December 31, 2007, filed on April 15, 1998). | |
| †10.24 | First Amendment to Employment Agreement made as of June 28, 2000 between the Registrant and Clemens (incorporated by reference to Exhibit 10.44A to the Registrant’s 2005 Form 10-K). | |
| †10.25 | Second Amendment to Executive Employment Agreement between Registrant and Clemens, dated as of January 5, 2005 (incorporated by reference to Exhibit 99.1 to the Registrant's Form 8-K filed January 31, 2005). | |
| †10.26 | Third Amendment to Executive Employment Agreement dated December 22, 2005 between Registrant and Clemens (incorporated by reference to Exhibit 10.3 to the December 2005 Form 8-K). | |
| †10.27 | Fourth Amendment to Executive Employment Agreement dated December 16, 2007 between Registrant and Clemens (incorporated by reference to Exhibit 10.28 to the Form 10-K for the year ending December 31, 2007, filed on March 5, 2008). | |
| †10.28 | Fifth Amendment to Executive Employment Agreement executed July 9, 2008 between Registrant and Clemens (incorporated by reference to Exhibit 10.4 to our Form 8-K filed on July 10, 2008). | |
| †10.29 |
Sixth Amendment to Executive Employment Agreement executed December 14, 2012 between the Registrant and Clemens (incorporated by reference to Exhibit 10.2 to our Form 8-K filed on December 17, 2012). | |
| †10.30 |
Seventh Amendment to Executive Employment Agreement executed December 12, 2013 between the Registrant and Clemens. (incorporated by reference to Exhibit 10.24 to the Form 10-K for the year ending December 31, 2013 filed on March 3, 2014) | |
| †10.31 | Employment Agreement dated as of March 18, 2008 between the Registrant and Robert B. Jones (incorporated by reference to Exhibit 10.1 to our Form 8-K filed on March 24, 2008). | |
| †10.32 | Amendment to Executive Employment Agreement dated as of April 28, 2011 between the Registrant and Robert B. Jones (incorporated by reference to Exhibit 10.1 to our Form 10-Q filed July 28, 2011) | |
| †10.33 | Second Amendment to Executive Employment Agreement between Registrant and Robert B. Jones executed December 14, 2012 (incorporated by reference to Exhibit 10.1 to our Form 8-K filed December 17, 2012). | |
| †10.34 | Strategic Transaction Bonus Grant Agreement dated February 28, 2013 between the Registrant and Robert B. Jones (incorporated by reference to Exhibit 10.1 of our Form 10-Q for the quarter ending March 31, 2013, filed May 2, 2013) |
| 3 |
|
Exhibit Number |
Exhibit Description | |
| †10.35 | Strategic Transaction Bonus Grant Agreement dated February 28, 2013 between the Registrant and Peter A. Clemens (incorporated by reference to Exhibit 10.2 of our Form 10-Q filed for the quarter ending March 31, 2013, filed May 2, 2013). | |
| 10.36 | Stipulation of Settlement dated October 31, 2011 re: Class Action Litigation (incorporated by reference to Exhibit 10.1 to our Form 8-K filed November 4, 2011) | |
| 14.1 | Code of Ethics (incorporated by reference to Exhibit 14.1 of the Form 8-K filed on December 10, 2007). | |
| 21 | Subsidiaries of the Registrant (incorporated by reference to Exhibit 21 to the Form 10-K for the fiscal year ended December 31, 2006 filed on March 15, 2007). | |
| *23.1 | Consent of BDO USA, LLP, Independent Registered Public Accounting Firm. | |
| *31.1 | Certification of Periodic Report by Chief Executive Officer pursuant to Rule 13a-14 and 15d-14 of the Securities Exchange Act of 1934. | |
| *31.2 | Certification of Periodic Report by Chief Financial Officer pursuant to Rule 13a-14 and 15d-14 of the Securities Exchange Act of 1934. | |
|
*32 |
Certification of Chief Executive Officer and Chief Financial Officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002.
| |
| *101.INS | XBRL Instance Document | |
| *101.SCH | XBRL Taxonomy Extension Schema Document | |
| *101.CAL | XBRL Extension Calculation Linkbase | |
| *101.LAB | XBRL Extension Label Linkbase | |
| *101.PRE | XBRL Extension Presentation Linkbase | |
| *101.DEF | XBRL Taxonomy Extension Definition Linkbase |
*Filed or furnished herewith.
† Management contract or compensatory plan or arrangement
| 4 |
