Attached files
| file | filename |
|---|---|
| EX-31.1 - EX-31.1 - TETRALOGIC PHARMACEUTICALS Corp | tlog-20141231ex311c788b2.htm |
| EX-32.1 - EX-32.1 - TETRALOGIC PHARMACEUTICALS Corp | tlog-20141231ex321d36e65.htm |
| EX-31.2 - EX-31.2 - TETRALOGIC PHARMACEUTICALS Corp | tlog-20141231ex312f78d8d.htm |
| EX-32.2 - EX-32.2 - TETRALOGIC PHARMACEUTICALS Corp | tlog-20141231ex322c7bcad.htm |
| EX-21 - EX-21 - TETRALOGIC PHARMACEUTICALS Corp | tlog-20141231ex21d920052.htm |
| EX-23.1 - EX-23.1 - TETRALOGIC PHARMACEUTICALS Corp | tlog-20141231ex2314c58f0.htm |
| EXCEL - IDEA: XBRL DOCUMENT - TETRALOGIC PHARMACEUTICALS Corp | Financial_Report.xls |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10‑K
|
(Mark One) |
|
|
☒ |
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
|
For the fiscal year ended December 31, 2014 |
|
|
or |
|
|
☐ |
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
|
For the transition period from to |
|
Commission File Number 001‑36208
TetraLogic Pharmaceuticals Corporation
(Exact Name of Registrant as Specified in Its Charter)
|
Delaware |
42‑1604756 |
|
343 Phoenixville Pike |
19355 |
Registrant’s telephone number, including area code: (610) 889‑9900
Securities registered pursuant to Section 12(b) of the Act:
|
Title of each class |
Name of each exchange on which registered |
|
Common Stock, par value $0.0001 per share |
NASDAQ Global Market |
Securities registered pursuant to Section 12(g) of the Act: None
|
|
(Title of Class) |
|
Indicate by check mark if the registrant is a well‑known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☐ No ☒
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ☐ No ☒
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S‑T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes ☒ No ☐
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S‑K is not contained herein, and will not be contained, to the best of the registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10‑K or any amendment to this Form 10‑K. ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non‑accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer,” and “smaller reporting company” in Rule 12b‑2 of the Exchange Act.:
|
Large accelerated filer ☐ |
Accelerated filer ☐ |
Non‑accelerated filer ☐ |
Smaller reporting company ☒ |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b‑2 of the Exchange Act). Yes ☐ No ☒
The aggregate market value of the voting and non-voting common equity held by non‑affiliates of the registrant, as of June 30, 2014, the last business day of the registrant’s most recently completed second quarter, was approximately $68.5 million. Such aggregate market value was computed by reference to the closing price of the common stock as reported on the NASDAQ Global Market on June 30, 2014. For purposes of making this calculation only, the registrant has defined affiliates as including only directors and executive officers and stockholders holding greater than 10% of the voting common stock of the registrant as of June 30, 2014.
The number of shares of the registrant’s common stock outstanding as of February 20, 2015 was 22,790,782.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the registrant’s definitive proxy statement for its 2015 annual meeting of stockholders are incorporated by reference into Items 10, 11, 12, 13, and 14 of Part III of this Form 10‑K.
TABLE OF CONTENTS
i
Cautionary Note Regarding Forward‑Looking Statements and Industry Data
In addition to historical facts or statements of current condition, this report and the documents into which this report is and will be incorporated contain forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, or the Securities Act, and Section 21E of the Securities Exchange Act of 1934, as amended, or the Exchange Act. We may, in some cases, use terms such as “believes,” “estimates,” “anticipates,” “expects,” “plans,” “projects,” “intends,” “potential,” “may,” “could,” “might,” “will,” “should,” “approximately” or other words that convey uncertainty of future events or outcomes to identify these forward-looking statements. Forward-looking statements appear in a number of places throughout this report and include statements regarding our intentions, beliefs, projections, outlook, analyses or current expectations concerning, among other things, our ability to develop and commercialize our product candidates, birinapant and suberohydroxamic acid phenyl-ester, or SHAPE; status, timing and results of pre-clinical studies and clinical trials; the potential benefits of our product candidates; the timing of seeking regulatory approval of our product candidates; our ability to obtain and maintain regulatory approval for our product candidates; our estimates of expenses and future revenues and profitability; our estimates regarding our capital requirements and our needs for additional financing; difficulties with increasing the size and complexity of our organization to assist with the expansion of our operations; our plans to develop and market our product candidates and the timing of our development programs; our estimates of the size of the potential markets for our product candidates; our selection and licensing of our product candidates; our ability to attract collaborators with acceptable development, regulatory and commercial expertise; the benefits to be derived from corporate collaborations, license agreements, and other collaborative or acquisition efforts, including those relating to the development and commercialization of our product candidates; sources of revenues and anticipated revenues, including contributions from corporate collaborations, license agreements, and other collaborative efforts for the development and commercialization of products; our ability to create an effective sales and marketing infrastructure if we elect to market and sell our product candidates directly; the rate and degree of market acceptance of our product candidates; the timing and amount or reimbursement for our product candidates; the success of other competing therapies that may become available; the manufacturing capacity for our product candidates; our ability to manage the growth and size of our organization as a result of our acquisition of Shape Pharmaceuticals, Inc., or Shape Pharmaceuticals; our intellectual property position; our ability to maintain and protect our intellectual property rights; our results of operations; financial condition, liquidity, prospects, and growth and strategies; the industry in which we operate; the trends that may affect the industry or us; the market price of our stock; our potential obligation to repurchase certain shares of common stock; our ability to pay existing indebtedness; the effect of our indebtedness on our business and liquidity; the decrease in the market price of our common stock from the issuance of additional shares of or instruments convertible into common stock; the effect on our financial results from the conversion of our convertible notes; and the dilution of existing stockholders from the conversion of our convertible notes.
By their nature, forward-looking statements involve risks and uncertainties because they relate to events, competitive dynamics and industry change, and depend on the economic circumstances that may or may not occur in the future or may occur on longer or shorter timelines than anticipated. Although we believe that we have a reasonable basis for each forward-looking statement contained in this report, we caution you that forward-looking statements are not guarantees of future performance and that our actual results of operations, financial condition and liquidity, and the development of the industry in which we operate may differ materially from the forward-looking statements contained in this report. In addition, even if our results of operations, financial condition and liquidity, and events in the industry in which we operate are consistent with the forward-looking statements contained in this report, they may not be predictive of results or developments in future periods.
Actual results could differ materially from our forward-looking statements due to a number of factors, including risks related to:
|
· |
our estimates regarding expenses, future revenues, capital requirements and needs for additional financing; |
|
· |
the success and timing of our pre-clinical studies and clinical trials; |
|
· |
the difficulties with increasing the size and complexity of our organization to assist with the expansion of our operations; |
1
|
· |
the potential that results of pre-clinical studies and clinical trials indicate birinapant or SHAPE is unsafe or ineffective; |
|
· |
our exposure to business disruptions; |
|
· |
our dependence on third parties in the conduct of our pre-clinical studies and clinical trials; |
|
· |
the difficulties and expenses associated with obtaining and maintaining regulatory approval of our product candidates, and the labeling under any approval we may obtain; |
|
· |
our plans and ability to develop and commercialize our product candidates; |
|
· |
our ability to acquire or license additional product candidates; |
|
· |
our failure to recruit or retain key scientific or management personnel or to retain our executive officers; |
|
· |
the size and growth of the potential markets for our product candidates, market acceptance of our product candidates and our ability to serve those markets; |
|
· |
legal and regulatory developments in the U.S. and foreign countries; |
|
· |
our ability to limit our exposure to product liability lawsuits; |
|
· |
our exposure to scrutiny and increased expenses as a result of being a public company; |
|
· |
the rate and degree of market acceptance of our product candidates; |
|
· |
obtaining and maintaining intellectual property protection for our product candidates and our proprietary technology; |
|
· |
the successful development of our commercialization capabilities, including sales and marketing capabilities; |
|
· |
recently enacted and future legislation regarding the healthcare system; |
|
· |
the success of competing therapies and products that are or become available; |
|
· |
our ability to acquire products or product candidates with acceptable economics; |
|
· |
our ability to raise additional capital; |
|
· |
our ability to pay existing indebtedness; |
|
· |
the decrease in the market price of our common stock from the issuance of additional shares of or instruments convertible into common stock; |
|
· |
the effect on our financial results from the conversion of our convertible notes; and |
|
· |
the dilution of existing stockholders from the conversion of our convertible notes. |
2
Birinapant and SHAPE are investigational drugs undergoing clinical development and have not been approved by the U.S. Food and Drug Administration, or FDA, or submitted to the FDA for approval. Birinapant and SHAPE have not been, nor may ever be, approved by any regulatory agency or marketed anywhere in the world. Statements contained in this report should not be deemed to be promotional.
Any forward-looking statement that we make in this report speaks only as of the date of such statement, and, except as required by law, we undertake no obligation to update such statements to reflect events or circumstances after the date of this report or to reflect the occurrence of unanticipated events. Comparisons of results for current and any prior periods are not intended to express any future trends or indications of future performance, unless expressed as such, and should only be viewed as historical data.
You should also read carefully the factors described in the “Risk Factors” section of this annual report and elsewhere to better understand the risks and uncertainties inherent in our business and underlying any forward‑looking statements. As a result of these factors, we cannot assure you that the forward‑ looking statements in this annual report will prove to be accurate. Furthermore, if our forward‑looking statements prove to be inaccurate, the inaccuracy may be material. In light of the significant uncertainties in these forward‑looking statements, you should not regard these statements as a representation or warranty by us or any other person that we will achieve our objectives and plans in any specified timeframe, or at all.
We obtained the industry, market and competitive position data in this annual report from our own internal estimates and research as well as from industry and general publications and research surveys and studies conducted by third parties. Industry publications and surveys generally state that the information contained therein has been obtained from sources believed to be reliable. We believe this data is accurate in all material respects as of the date of this annual report.
3
Overview
We are a clinical-stage biopharmaceutical company focused on discovering and developing novel small molecule therapeutics in oncology and infectious diseases. We currently have two clinical-stage product candidates in development: birinapant and suberohydroxamic acid 4-methoxycarbonyl phenyl ester, or SHAPE.
Birinapant is a novel small molecule therapeutic that mimics Second Mitochondrial Activator of Caspases, or SMAC-mimetic, which leads to apoptosis, or cell-death, in damaged cells. We have treated over 300 oncology subjects with birinapant, and in non-randomized clinical trials to date, we have seen activity in subjects with (i) higher risk myelodysplastic syndromes, or MDS, where we have observed complete bone marrow responses with birinapant administered with azacitidine (Vidaza®); (ii) end-stage acute myeloid leukemia, where birinapant was administered as a single-agent and subjects who have previously relapsed or were refractory to standard therapy experienced declines in blast counts; (iii) ovarian cancer, where birinapant was administered with conatumumab (AMG 655), we have observed disease stabilization and a partial response, or PR, in women who previously relapsed or were refractory to standard therapy; and (iv) colorectal cancer, or CRC, where birinapant was administered with irinotecan, we have observed evidence of anti-tumor activity or prolonged disease stabilization in subjects who have progressed after multiple prior therapies, including irinotecan.
Most recently, we have generated pre-clinical data indicating that birinapant induces apoptosis in-vivo in mouse hepatocytes infected with human hepatitis B virus, or HBV. In a mouse model, we have seen clearance of HBV surface antigen, or HBsAg, and the appearance of antibodies directed against HBsAg, a clinical finding considered equivalent to a functional cure. We have also seen activity of birinapant in other infectious disease models, including human mononuclear cells infected with human immunodeficiency virus, or HIV, in-vitro, and in-vivo in mouse models of Mycobacterium tuberculosis and legionella pneumophila.
We are currently conducting the following three clinical programs with birinapant:
|
· |
In June 2014, we commenced a randomized, double-blind placebo-controlled Phase 2 clinical trial of birinapant administered with azacitidine in subjects with previously untreated, higher risk MDS. Interim data is expected in 2015. This clinical trial follows our Phase 1b/2a open-label clinical trial of birinapant administered with azacitidine. |
|
· |
We are continuing enrollment in a Phase 1/2 open-label, non-randomized clinical trial of birinapant administered with conatumumab in third-line ovarian cancer. In December 2014, this clinical trial proceeded into a Phase 2a expansion based upon data in the Phase 1 portion of the clinical trial. Phase 2a clinical trial data is expected in 2015. |
|
· |
We recently initiated a randomized, placebo-controlled, multiple ascending dose Phase 1 clinical trial in subjects with chronic HBV currently taking entecavir or tenofovir. Data is expected in 2015. |
We discovered birinapant, and its composition of matter patent in the U.S. extends until at least 2030. We have retained worldwide development and commercialization rights for all indications.
SHAPE, our second clinical-stage product candidate, is a histone deacetylase, or HDAC, inhibitor that we are developing for topical use for the treatment of early-stage cutaneous T-cell lymphoma, or CTCL. HDAC is a validated cancer target, and HDAC inhibitors, or HDACi, are a proven class of anti-cancer drugs for CTCL. SHAPE is a novel therapeutic, designed to maximize HDAC inhibition locally in the skin with limited systemic exposure, and it has characteristics that could allow its topical use over large body surface areas with minimal systemic absorption. By potentially avoiding toxicities typical of systemically-administered HDACi’s, SHAPE may provide a more favorable safety profile than current HDACi’s delivered orally or intravenously. SHAPE has been evaluated in a randomized,
4
placebo-controlled dose escalation Phase 1 clinical trial in early-stage CTCL. SHAPE was well-tolerated, and it demonstrated evidence of clinical activity with PRs observed in certain subjects after 28 days of application. We commenced a randomized Phase 2 clinical trial of SHAPE in subjects with early-stage CTCL in December 2014, which will assess clinical activity after six months of application. Data for this clinical trial is expected in 2015.
SHAPE’s composition of matter patent in the U.S. extends until at least 2028. In addition, SHAPE has been granted U.S. orphan drug designation for CTCL. We have acquired worldwide development and commercialization rights to SHAPE for all indications.
We incurred research and development expenses of $9.5 million for the year ended December 31, 2013 and $20.3 million for the year ended December 31, 2014.
Introduction to Apoptosis
The mechanism of controlling programmed cell death in both normal and abnormal cells is fundamentally important to maintaining human health. The process of cellular self‑destruction is known as apoptosis. There are multiple checks and balances within a cell to ensure that healthy cells do not undergo apoptosis, and that abnormal cells, do undergo apoptosis and are cleared from the body. In certain cancers and intra-cellular infections, abnormal cells that should be naturally cleared from the body manage to escape apoptosis. As a result, cells that should self‑destruct actually survive and even proliferate or propagate infection, leading to multiple disease complications. .
Tumor Necrosis Factor, or TNF, is an extracellular signaling molecule that should induce apoptosis in abnormal or damaged cells. Within the TNF receptor key molecules that block this signal and protect cells from apoptosis are called Inhibitor of Apoptosis proteins, or IAPs. A key molecule that promotes apoptosis is Second Mitochondrial Activator of Caspases, or SMAC, a naturally occurring IAP inhibitor.
Cancer cells and certain virally infected cells can use IAPs to block the TNF‑induced self‑destruction signal and convert it into a pro‑survival signal through a protein complex called NF‑κB (a nuclear factor kappa-light-chain-enhancer of activated B cells). Thus, while a number of cancer therapies induce TNF, the resulting TNF self‑destruction signal may be blocked by IAPs. Normally, IAPs can be disabled by their natural inhibitor SMAC, but this natural blocking mechanism is rendered ineffective in many cancers and certain intra-cellular infections due to the overexpression of IAPs.
We believe that novel small molecule therapies that mimic SMAC, or SMAC‑mimetics, have the potential to inhibit IAPs and re‑establish the TNF self‑destruction signal. Our therapeutic focus is centered on the development of SMAC‑mimetics . This is a novel approach and there are no SMAC mimetics currently on the market.
IAPs have multiple and distinct regions that are responsible for different functions. As shown in Figure 1 below, the principal target of birinapant is the IAP, cIAP1.
5
Figure 1. Birinapant is designed to mimic SMAC and enable TNF‑activated apoptosis.
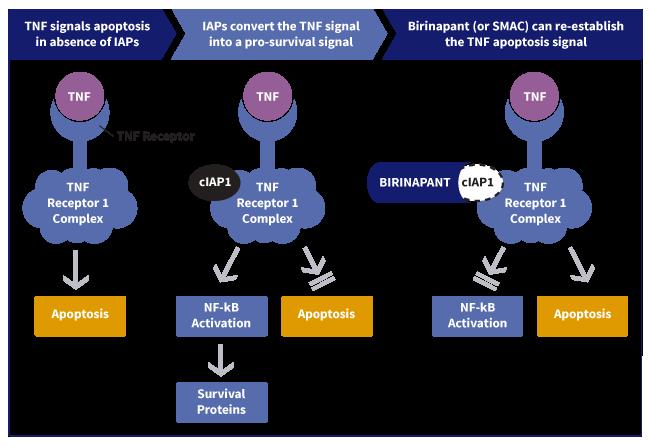
The interaction between the IAPs and SMAC is regulated via a critical functional region of the IAPs called the E3 ubiquitin ligase domain, or E3 domain. Two IAP E3 domains must interact with each other to be functional. Accordingly, when two IAPs come together to form a “homodimer” allowing the two E3 domains to interact, this results in the self‑degradation of IAPs. This self‑degradation of two IAP molecules as a result of coming together and interacting with each other through their E3 domains is enabled by SMAC. Product candidates under development that interact with IAPs fall into two classes: a “monovalent” compound that interacts with a single IAP molecule or a “bivalent” compound that, like the endogenous protein, interacts with two IAP molecules. Based upon our pre‑clinical studies, we believe that bivalent SMAC‑mimetics more closely mimic the activity of endogenous SMAC and are more potent inhibitors of TNF induced NF‑κB activation than monovalent IAP inhibitors. Birinapant is a bivalent, small molecule IAP-inhibitor that acts within the cell to mimic the activity of endogenous SMAC.
6
Figure 2. Birinapant interacts with two IAP molecules.
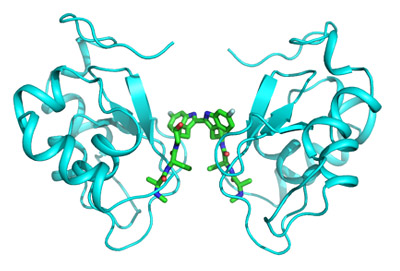
Birinapant
Birinapant is a bivalent SMAC-mimetic in clinical development for the treatment of certain cancers and infectious diseases. It was selected from our chemical library of over 3,000 IAP-inhibitor compounds designed to bind to a greater or lesser extent with multiple IAPs. IAPs, including cIAP1, cIAP2, XIAP and ML‑IAP, are a group of structurally‑related proteins that can suppress apoptosis. Based on our pre‑clinical studies and clinical trials, we believe that birinapant’s potential ability to inhibit IAPs will block this suppression across multiple cancers and viral infections.
In pre‑clinical cancer studies, birinapant was synergistic (or super‑additive) with agents that induce TNF, including established anti‑cancer chemotherapies (such as azacitidine, gemcitabine and irinotecan), other anti‑cancer therapies (such as radiotherapy), biological agents (such as granulocyte-macrophage colony-stimulating factor (GM‑CSF) and interferon, or IFN, and with TNF and other members of the TNF superfamily, including TNF‑related apoptosis‑inducing ligand, or TRAIL and TRAIL‑Receptor 2 (also known as Death‑Receptor 5, or DR5) agonists. Our clinical strategy is to administer birinapant with therapies (such as azacitidine) that induce the production of TNF or related molecules.
In pre‑clinical studies, a significant number of tumor types resistant to single agent treatment with either TNF or TRAIL became sensitive to that agent in the presence of low concentrations of birinapant. The requirement for TNF underpins our clinical program. While birinapant may have some activity when administered as the sole therapy, we believe its maximum anti‑cancer activity will occur when administered with chemotherapies or other agents that further induce TNF.
We believe that birinapant has the potential to be superior to other IAP inhibitors for two reasons. First, birinapant is a bivalent molecule similar to endogenous SMAC and allows for direct engagement of two IAP molecules. Our pre‑clinical studies suggest that bivalent SMAC‑mimetics are more potent inhibitors of TNF induced NF‑κB activation than monovalent IAP inhibitors. To our knowledge, birinapant is the only bivalent SMAC‑mimetic in clinical development in the U.S. Second, based on our pre‑clinical studies, birinapant inhibits cIAP1 more than cIAP2. Complete degradation of cIAP2 is believed to be associated with increased toxicities and, therefore, we believe that birinapant will be better tolerated than SMAC‑mimetics that are less selective and result in increased degradation of cIAP2. Consistent with these pre‑clinical studies, birinapant has been generally well tolerated in our clinical trials.
Activity in Clinical Trials
Approximately 350 subjects have been treated with birinapant. In clinical trials, birinapant was generally well tolerated, meaning that treatment‑related side effects were mild or moderate in severity in the majority of treated subjects. Birinapant has also shown favorable pharmacokinetic, or PK, properties, meaning how the body metabolizes birinapant, including the length of time birinapant remains in a subject’s blood or tumor, with similar behavior among
7
treated subjects. In addition, our clinical trials show evidence that birinapant is interacting with its intended target, with inhibition of cIAP1, inhibition of NF‑κB, and activation of apoptosis in subjects’ tumor cells. Most importantly, birinapant has shown early clinical activity in subjects with both hematological malignancies and solid tumors, including MDS, acute myelogenous leukemia, or AML, relapsed/refractory ovarian cancer and CRC; thus providing a rationale for further clinical development of birinapant in later phase clinical trials.
Most recently, we have generated pre-clinical data indicating that birinapant induces apoptosis in-vivo in mouse hepatocytes infected with HBV. In a mouse model, we have seen clearance of HBsAg and the appearance of antibodies directed against HBsAg, a clinical finding considered equivalent to a functional cure; thus providing a strong rationale for our ongoing randomized, placebo-controlled, multiple ascending dose Phase 1 clinical trial in subjects with chronic HBV currently taking entecavir or tenofovir.
Safety Data
Birinapant has been generally well tolerated in Phase 1 and Phase 2 clinical trials, which have collectively enrolled approximately 350subjects. In these trials, side effects were predominantly dose‑related, transient and mild or moderate in severity.
In single-agent studies of birinapant, the most frequent treatment‑related adverse events, or AEs, occurring in at least 20% of subjects were abdominal pain, back pain, constipation, decreased appetite, dehydration, diarrhea, fatigue, headache, hypotension, lymphopenia, nausea and vomiting. In clinical trials of birinapant administered with standard chemotherapies, the most frequent treatment‑related AEs, occurring in at least 20% of subjects, were fatigue and nausea. The majority of these treatment‑related events were Grade 1 (mild) or 2 (moderate) in severity, and reversible without clinical complications.
In the single agent dose‑escalation Phase 1 clinical trial that sought to define the dose‑limiting toxicities of birinapant, the birinapant‑related AEs that were Grade 3 or greater (severe to life‑threatening) occurred in 9 of 50 (18%) subjects and included fatigue, headache, hypophosphatemia, increased serum amylase, increased serum lipase, lymphocytopenia, nausea, rash, thrombocytopenia and vomiting. In the clinical trials of birinapant administered with standard chemotherapies, the treatment‑related AEs that were Grade 3 or greater in severity occurred in 34 of 176 (19%) subjects and those that occurred in more than 5% of subjects included anemia, diarrhea, fatigue, neutropenia, thrombocytopenia and vomiting. In the clinical trial of birinapant administered with azacitidine, the treatment-related AEs that were Grade 3 or higher to date were abdominal cellulitis at the site of subcutaneous injection of azacitidine, anemia, asymptomatic increases in serum lipase, decrease in neutrophils/neutropenia, decrease in platelets/thrombocytopenia and decrease in white blood cell count/leukopenia.
Cranial nerve palsies
In clinical trials, birinapant has been associated with the onset of cranial nerve palsies, meaning a weakness or paralysis of the areas served by the affected cranial nerve. Across the entirety of the clinical program to date, there have been 18 cases of cranial nerve palsy, of which all but two have been mild to moderate in severity. These events have been transient and responsive to therapy. Some subjects who reported a cranial nerve palsy elected to continue birinapant treatment, and none had a recurrent event of cranial nerve palsy.
The majority of events have affected the VII cranial nerve and caused a Bell’s palsy, i.e., a weakness affecting one side of the face. All but one case of Bell’s palsy has occurred in subjects receiving a weekly cumulative dose of birinapant of 22mg/m2 or higher. However, one event was reported in a subject receiving a cumulative dose of 4.2mg/m2. The onset of Bell’s palsy typically occurs after the third dose of birinapant and is preceded by a prodromal syndrome of headache, ear or eye pain. Thus, we believe it may be possible to mitigate the onset of the cranial nerve palsy by intervening with either non-steroidal anti-inflammatory drugs ( NSAIDs) or corticosteroids at the time of the prodromal syndrome and deferring the next dose of birinapant. This procedure is being implemented in all of the clinical programs.
8
Clinical Trials
Prior to evaluating birinapant in any particular indication, we conducted a single agent Phase 1 clinical trial with birinapant in 50 subjects with multiple solid tumor cancer types to determine the maximum tolerated dose, or MTD, and gather PK and safety data. An Investigational New Drug Application, or IND, for birinapant was submitted to the U.S. Food and Drug Administration, or the FDA, in September 2009. The clinical trial began in December 2009 and was conducted at Fox Chase Cancer Center in Philadelphia, PA, Roswell Park Cancer Institute in Buffalo, NY and the University of Pennsylvania in Philadelphia, PA, and included subjects who had received a median of four prior therapies for their cancers. Birinapant was generally well tolerated. There was evidence of anti‑tumor activity or prolonged disease stabilization in two subjects with CRC, one subject with non‑small cell lung cancer, or NSCLC, and one subject with liposarcoma. Methods for showing activity were a blood test measuring declines in carcinoembryonic antigen and computed tomography (CT) scanning. The liposarcoma subject had disease stabilization for nine months despite progression on three prior therapies. This clinical trial was completed in March 2012.
Our second clinical trial was a Phase 1 clinical trial of birinapant administered with one of five different standard chemotherapy regimens: carboplatin, docetaxel, gemcitabine, irinotecan, or liposomal doxorubicin and paclitaxel. This clinical trial included 124 subjects with multiple solid tumor cancer types to determine MTD, and gather PK and safety data. Secondary objectives were to assess anti‑tumor activity, pharmacodynamic, or PD, data and potential translational biomarkers. This clinical trial began in October 2010 and was conducted at the three sites listed above as well as Barbara Ann Karmanos Cancer Institute in Detroit, MI, the Holy Cross Hospital Cancer Center in Fort Lauderdale, FL, the Mary Crowley Cancer Research Centers in Dallas, TX and START (South Texas Accelerated Research Therapeutics) in San Antonio, TX. Subjects treated had a variety of tumor types, the most common being CRC, lung cancer, melanoma and ovarian cancer. The subjects in this clinical trial had failed a median of three prior chemotherapies. Birinapant did not appear to substantially exacerbate the toxicities commonly associated with any of these regimens. Fourteen subjects showed anti‑tumor activity (as defined by Response Evaluation Criteria in Solid Tumors, or RECIST). One NSCLC subject had a complete response, or CR (defined as the disappearance of all lesions), and 13 subjects had PRs (defined as at least a 30% decrease in the sum of all lesions), including responses in anal cancer, CRC, gallbladder cancer, melanoma, NSCLC, ovarian cancer and small‑cell lung cancer.
Overview and Rationale for Our Birinapant Clinical Programs
Myelodysplastic Syndromes (MDS)
Background
MDS is a form of cancer of bone‑marrow stem cells resulting in fewer than normal mature blood cells in the circulation. In MDS, bone marrow cells are dysplastic, or abnormal, and as such do not function correctly. The blood cells produced do not develop normally, such that too few healthy blood cells are released into the blood stream, which leads to cytopenias. In advanced stages of the disease, the abnormal blood cells or “blasts” leave the bone marrow and enter the blood stream and may lead to AML, which occurs in approximately one‑third of patients with MDS. We believe that there is a medical need for a treatment option that improves outcomes of standard of care regimens for patients with MDS.
According to the American Cancer Society, MDS is diagnosed in approximately 12,000 people in the U.S. annually, for an age‑adjusted incidence rate of approximately 4.4 to 4.6 cases per 100,000 people. MDS occurs predominantly in older patients (usually those older than 60 years). The median age at diagnosis is approximately 70 years. MDS may arise de novo or secondarily after treatment with chemotherapy and/or radiation therapy for other cancers or, rarely, after environmental exposures.
MDS has two main classification systems, the French-American-British (FAB) and the World Health Organization, or the WHO. Revised in 2008, the WHO classification system is widely accepted because it incorporates morphologic and cytogenetic factors and correlates with prognosis. The categories are distinguished by specific characteristics of peripheral blood and bone marrow. Unlike many other cancers, MDS is not “staged.” Rather, prognostic systems have been devised to predict the risk of transformation to AML and to predict overall survival. Most
9
recently in 2012, a revised International Prognostic Scoring System (IPSS-R) for MDS was published, in which risk of death or progression to AML is assessed by the quantity of bone marrow blasts, the presence of specific cytogenetic abnormalities in the bone marrow and the degree of bone marrow suppression evident in the peripheral blood, through the assessment of anemia, neutropenia and thrombocytopenia. Age also likely plays a role in assessing risk.
Azacitidine was initially approved in the U.S. in 2004 for the treatment of patients with MDS. Azacitidine is a pyrimidine nucleoside analog of cytidine and is believed to exert antineoplastic effect by causing hypomethylation of deoxyribonucleic acid, or DNA, and direct cytotoxicity on abnormal hematopoietic cells in the bone marrow. While azacitidine has become a standard of care for first‑line therapy for higher‑risk MDS, the overall response rate is only 30% and responders typically demonstrate progressive disease within 2 years, often progressing to AML. Thus, there continues to be a significant unmet need for a more effective initial therapy that produces an increase in response rates and prolongs the response duration.
Rationale for treatment with birinapant
Dysregulation of IAPs may be critical for the development and progression of hematological malignancies, including AML and MDS. Although classified as distinct disease entities, MDS and AML are closely related diseases. In a number of third‑party studies, the over‑expression of IAPs has been associated with “escape‑from‑apoptosis” and poor prognosis in AML. Consistent with these third‑party findings suggesting that IAPs exert adverse effects on the outcome of AML, in other third‑party studies, SMAC (the endogenous inhibitor of IAPs) over‑expression has been associated with improved prognosis in AML. The natural evolution of MDS involves molecular changes that make those cells increasingly dependent on anti‑apoptotic pathways, the same pathways that birinapant is designed to inhibit.
In in vitro studies of both established AML cell lines and freshly‑derived AML blast cells, single agent birinapant demonstrated activity at clinically achievable study drug exposures. This was evident in assays of unfractionated AML cells, and in studies of AML “stem/progenitor” cells (CD34+, CD38 negative cells). In these studies, we observed increased apoptosis after 48 hours of in vitro culture. An independent study by a separate investigator observed clonal suppression of AML‑derived colonies in 14 day cultures by birinapant, with sparing of normal progenitor cells. Studies of human acute lymphoblastic leukemia, or ALL, cells in tumor models have demonstrated cytotoxic activity in in vitro and in vivo studies.
We believe our pre‑clinical studies of birinapant administered with azacitidine support a distinct mechanism of anti‑tumor synergy that may provide clinical benefit compared to individual agents alone. Birinapant administered with azacitidine demonstrated anti‑tumor activity compared to single agent azacitidine in primary AML blast cells. In our in vivo studies of AML cells, we also observed activity for birinapant as either a single agent or administered with azacitidine. We have observed similar pre‑clinical activity when birinapant is administered with two other AML/MDS therapies, cytosine arabinoside and decitabine.
In our in vitro studies, we observed that azacitidine leads to TNF induction, which we believe supports the rationale that the administration of birinapant with azacitidine may have additive activity over azacitidine treatment alone for MDS subjects. Additionally, we believe these studies support the rationale that administration of birinapant with azacitidine offers possible therapeutic benefit to subjects who relapse following, or are refractory to, azacitidine therapy. Our belief is further supported by clinical evidence of a potential for retreatment efficacy, which has been demonstrated for CRC subjects who obtained a clinical benefit from the administration of birinapant with irinotecan and likewise MDS subjects obtained a clinical benefit from the administration of birinapant with azacitidine after previously progressing on these same chemotherapy agents.
Clinical trials
A Phase 1/2 investigator-initiated clinical trial in AML, MDS and ALL is complete. Subjects were treated with birinapant as the sole agent or administered with hydroxyurea. The majority of subjects enrolled were elderly (over 70 years) with AML secondary to MDS and had received multiple prior treatments. The following were observed:
|
· |
some subjects experienced a decrease in the leukemic blast cells; |
10
|
· |
some subjects showed an increase in neutrophils with the first birinapant dose; |
|
· |
PK measurements indicated that birinapant had comparable plasma and tumor drug exposure in subjects with AML compared to subjects with solid tumors; |
|
· |
PD measurements of several subjects showed cIAP1 and NF‑κB target suppression in peripheral blood AML blast cells; |
|
· |
one subject with AML who had progressed from MDS and had progressive disease after prior cytotoxic regimens, had stable disease and no cumulative toxicities with 10 months of therapy; and |
|
· |
one other subject experienced a decrease from 60% to 10% in bone marrow blast count. |
Safety data is available from the first 16 AML subjects. Treatment-related AEs were neutropenia (low blood levels of neutrophils), leukopenia (low blood levels of leukocytes), oral pain (mouth sores), fatigue, fever, increased serum amylase (protein in multiple organs, including pancreas), increased serum lipase (protein in multiple organs, including pancreas), increased aspartate aminotransferase (an enzyme in multiple organs, including the liver), increased alkaline phosphatase (an enzyme in multiple organs, including the liver), dysesthesia (abnormal sensation), dysgeusia (abnormal taste), headaches and sweating. Eight serious AEs (AEs that may result in a hospitalization, are life-threatening or cause death), that were considered related to birinapant treatment, as determined by the clinical investigator, included febrile neutropenia (fever with low blood levels of neutrophils), fever, increased serum amylase and increased serum lipase.
The following table sets forth the AEs occurring during treatment of AML, MDS and ALL subjects who received birinapant and are considered to be related to such treatment as determined by the principal investigator as of October 26, 2012:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
No. of |
|
No. of |
|
No. of |
|
No. of |
|
|
|
|
|
|
Grade 1 |
|
Grade 2 |
|
Grade 3 |
|
Grade 4 |
|
Total No of |
|
|
|
|
Adverse |
|
Adverse |
|
Adverse |
|
Adverse |
|
Adverse |
|
|
Adverse Event |
|
Events |
|
Events |
|
Events |
|
Events |
|
Events(1) |
|
|
Dysethesia |
|
1 |
|
1 |
|
— |
|
— |
|
1 |
|
|
Dysgeusia |
|
— |
|
1 |
|
— |
|
— |
|
1 |
|
|
Fatigue |
|
1 |
|
— |
|
— |
|
— |
|
2 |
|
|
Fever |
|
2 |
|
1 |
|
— |
|
— |
|
2 |
|
|
Headache |
|
2 |
|
1 |
|
— |
|
— |
|
3 |
|
|
Increased alkaline phosphatase |
|
— |
|
— |
|
— |
|
— |
|
1 |
|
|
Increased aspartate aminotransferase (AST) |
|
1 |
|
— |
|
— |
|
— |
|
1 |
|
|
Increased serum amylase |
|
2 |
|
— |
|
2 |
|
— |
|
4 |
|
|
Increased serum lipase |
|
1 |
|
— |
|
1 |
|
1 |
|
3 |
|
|
Leukopenia |
|
1 |
|
— |
|
— |
|
— |
|
1 |
|
|
Neutropenia |
|
— |
|
— |
|
— |
|
1 |
|
2 |
|
|
Oral pain (mouth sores) |
|
1 |
|
— |
|
— |
|
— |
|
1 |
|
|
Sweating |
|
1 |
|
— |
|
— |
|
— |
|
1 |
|
|
(1) |
There were no Grade 5 (death) adverse events. |
Based on the synergy we observed in pre-clinical studies between birinapant and azacitidine, the current standard of care for MDS, and the observed clinical activity of birinapant as a single agent in subjects with AML secondary to MDS, we are conducting a Phase 1b/2a open-label clinical trial of birinapant administered with azacitidine in higher-risk MDS subjects who have relapsed or are refractory to azacitidine. We expanded this clinical trial to include subjects who have not been previously treated with, or are naïve to, azacitidine. The primary objective of this Phase 1b/2a clinical trial is to characterize the safety and tolerability and determine the recommended Phase 2 dose of birinapant when administered with azacitidine. Additional objectives of the clinical trial are to assess any preliminary indications of efficacy and PD of the combination.
11
In December of 2014, we published data at the American Society of Hematology Annual Meeting that demonstrated that birinapant, administered with azacitidine, can induce a complete bone marrow remission in some subjects. To date, no dose limiting hematological toxicities of the combination have been reported. A number of subjects who received subcutaneous azacitidine experienced local injection site skin reactions or cellulitis. Although cellulitis is a known effect of azacitidine, several of these were considered to be of increased severity with the combination, possibly reflecting a localized synergistic PD effect in the skin. We believe this toxicity should be mitigated by the use of intravenous, or IV, azacitidine, as no subject whose route of administration has been changed to IV azacitidine experienced further injection site cellulitis. Also, based on data from this clinical trial, the recommended dose of birinapant administered with IV azacitidine for further clinical trials is 13mg/m2 twice weekly for three out of four weeks. Birinapant demonstrated inhibition of NF-κB in circulating blast cells at this dose. In June 2014, we commenced a randomized, double-blind placebo-controlled Phase 2 clinical trial of birinapant administered with azacitidine in subjects with previously untreated, higher risk MDS. Interim data is expected in 2015.
Ovarian Cancer
Background
Ovarian cancer is among the five most common cancers in women and ranks fifth as the cause of cancer death in the U.S. It is the leading cause of gynecologic mortality in the U.S. According to the SEER Cancer Statistics Review, it is estimated that 21,980 women will be diagnosed with, and 14,270 women will die of, ovarian cancer in 2014. Ovarian cancer accounts for 5% of cancer deaths among women and causes more deaths than any other cancer of the female reproductive system.
Although over 70% of women with advanced disease respond to optimal debulking surgery followed by platinum‑taxane based chemotherapy, duration of response is short and relapse is common. Subsequent responses to salvage therapy regimens tend to be brief (less than six months) due to the tumors’ progressive resistance to chemotherapy. Relapsed ovarian cancers represent a significant challenge. Objective response rates to second‑line therapies (such as doxorubicin, gemcitabine, and topotecan) are in the range of 20% and median overall survival is less than 1 year. In a third‑party Phase 2 clinical trial of docetaxel, given every 21 days in paclitaxel‑resistant ovarian and peritoneal carcinoma, the response rate (combined CR and PR) was 22.4%. A similar third‑party study showed a response rate of 23%.
Rationale for treatment with birinapant administered with conatumumab
Cancer cells use IAPs to convert the TNF self‑destruction signal into a pro‑survival signal through a protein complex called NF‑κB. Published data have shown that the NF‑κB pathway may be over‑activated in aggressive ovarian cancers as evidenced by intrinsic NF‑κB activation in serous ovarian cancer. The addition of a SMAC‑mimetic such as birinapant has shown potential in pre‑clinical studies to inhibit NF‑κB activity, down‑regulate cell survival pathways and overcome blocks to the apoptotic pathway resulting in increased tumor cell destruction.
TRAIL is a member of the TNF super‑family, and can induce apoptosis by binding to two cell surface receptors called death receptor 4, or DR4 (also known as TRAIL Receptor‑1) and DR5 (also known as TRAIL Receptor‑2), respectively. TRAIL binding to DR4 and DR5 initiates an intracellular cascade inducing apoptosis in many transformed cell lines but not non‑cancerous cells.
Conatumumab, an investigational product candidate, owned by Amgen, Inc., or Amgen, is a fully‑human monoclonal agonist antibody designed to partially mimic endogenous TRAIL by binding DR5, thereby inducing apoptosis in sensitive cells. Such a property of conatumumab, being a TRAIL agonist, may induce apoptosis selectively in cancer cells and enhance the activity of standard cancer therapy, molecularly targeted agents or both. Approximately 985 subjects have been enrolled in 10 ongoing or completed clinical trials of conatumumab; 55 have received monotherapy and 930 have received conatumumab or placebo administered with chemotherapy and/or other biologic agents.
12
We believe that birinapant may have the potential to remove blockades imposed by IAPs in the TRAIL‑induced apoptotic pathway. Pre‑clinical studies have evaluated birinapant’s ability to convert TRAIL‑resistant cells into TRAIL‑responsive cells and demonstrated that administering birinapant with conatumumab may allow enhanced activation of DR5‑induced apoptosis by removing IAP‑mediated inhibition. There continues to be an unmet medical need for subjects with many kinds of solid tumors, including ovarian cancer. Ovarian cancer provides an opportunity for testing this novel combination. We believe that pre‑clinical studies suggest that the clinical administration of birinapant with conatumumab in solid tumor malignancies may potentially result in greater clinical activity than either agent alone.
Clinical trial
In collaboration with Amgen, we are exploring the combination of birinapant administered with Amgen’s TRAIL receptor agonist antibody, conatumumab. A Phase 1/2 open‑label, non‑randomized clinical trial of birinapant administered with conatumumab in subjects with relapsed epithelial ovarian cancer, fallopian tube cancer or primary peritoneal cancer is ongoing. The primary objectives of the Phase 1/2 clinical trial are to determine the recommended Phase 2 dose of birinapant when administered with conatumumab in subjects who have relapsed after two prior standard therapies. The secondary objectives are to determine the clinical activity of birinapant administered with conatumumab, to determine the PK characteristics of birinapant and conatumumab in plasma and tumor and to assess PD and predictive biomarkers.
Results from the Phase 1 portion of the clinical trial suggest a PD interaction between birinapant and conatumumab, manifested as the appearance of AEs at lower than anticipated doses. In addition, one subject showed a PR and four subjects have maintained stable disease, one through four cycles of treatment, and three through two cycles of treatment.
Based upon the data from the Phase 1 portion of the clinical trial, this clinical trial proceeded into a Phase 2a expansion in December 2014, with a reduction in the dose of birinapant and a limit of dosing to three weeks out of four. Phase 2a data is expected in 2015.
Hepatitis B Virus (HBV)
Background
Hepatitis B is a liver disease that results from infection with HBV. Hepatitis B can range in severity from a mild illness lasting a few weeks to a serious, lifelong illness. In the U.S., 804,000 to 1.4 million persons are estimated to be chronically infected with HBV.
HBV infections can be either acute or chronic. Acute HBV infection is a self-limiting illness that occurs within the first six months after someone is exposed to HBV. Chronic HBV infection is a long-term illness that occurs when HBV remains in a person’s body. Chronic HBV is a serious disease that can result in long-term health problems, such as cirrhosis of the liver and liver (hepatocellular) cancer. The risk for chronic HBV infection decreases with increasing age at infection. Approximately 5% of adults with acute HBV infections progress to chronic infection.
For an acute infection, there is generally no treatment other than rest and supportive measures to manage any symptoms. There are several approved drugs in the U.S. for chronic HBV, including Intron A (interferon alpha-2b), Pegasys (peg-INF alpha-2b), Epivir-HBV (lamivudine), Hepsera (adefovir), Baraclude (entecavir), Tyzeka (telbivudine) and Viread (tenofivir). These drugs slow down viral replication by suppressing the production and release of viral particles, but they do not suppress the production and release of viral proteins. In rare cases, they may even get rid of the virus completely.
Rationale for treatment with birinapant
We have conducted pre‑clinical studies to evaluate the potential of birinapant as a therapeutic for chronic HBV. There are no drugs currently on the market that specifically target IAPs to induce apoptosis in HBV infected cells as a strategy for therapy. Using a mouse model of HBV, birinapant was well tolerated and showed activity in the clearance of
13
cells infected with HBV. The clearance was additive when administered with entecavir, the standard of care therapy for HBV. Birinapant caused eradication of HBsAg and the formation of antibodies to HBsAb whereas entecavir when used alone did not. These pre-clinical studies are ongoing to understand the action of birinapant in greater detail in HBV, and to determine the spectrum of potential therapeutic activity of birinapant in other infectious diseases. Consistent with these results, birinapant demonstrated activity at clinically achievable study drug exposures in studies of HIV in human blood cells in vitro. It was also active in a mouse model of tuberculosis and in a mouse model of legionella. We have entered into a research collaboration with the Walter and Eliza Hall Institute of Medical Research, or WEHI, based in Melbourne, Australia, to examine SMAC-mimetics, including birinapant, in the treatment of infectious disease.
Clinical trial
A randomized, placebo-controlled, multiple ascending dose Phase 1 clinical trial of birinapant in subjects with chronic HBV was initiated in the fourth quarter of 2014. The clinical trial is being conducted in subjects over the age of 18 with HBV who are receiving treatment with either tenofavir or entecavir and who are HBsAg positive. The clinical trial is expected to enroll approximately six cohorts of eight subjects each, who will receive four weekly treatments with either birinapant or placebo in a 3:1 ratio. The clinical trial is being conducted at multiple clinical sites in Australia and New Zealand. Although predominantly a clinical trial to determine safety and tolerability, subjects will also be monitored for reductions in HBsAg and the development of HBsAb as an indication of therapeutic activity. Data is expected in 2015.
Colorectal Cancer (CRC)
The American Cancer Society estimates that in the U.S. there will have been approximately 136,000 new cases and approximately 50,000 deaths from CRC in 2014, accounting for 9% of all cancer deaths. Twenty relapsed and/or refractory CRC subjects were treated with birinapant plus irinotecan during the previously mentioned 124 subject Phase 1 trial. An additional 50 CRC subjects were treated in a Phase 2 expansion, for a total of 71 subjects CRC subjects. Subjects received a median of four prior therapies before birinapant with irinotecan. There were signs of activity in subjects treated with birinapant and irinotecan as determined by objective responses and progression free survival, or PFS. Six subjects showed PRs. Five of these six had previously failed prior irinotecan‑based therapies, including three with KRAS mutant tumors. The median PFS for birinapant and irinotecan was 2.2 months, and 34% of subjects treated with birinapant and irinotecan were alive without disease progression at four months and 21% were alive without disease progression at six months.
Of the 71 subjects in this Phase 1/2 clinical trial of birinapant administered with irinotecan, 23 CRC subjects had failed irinotecan therapy immediately prior to starting treatment with birinapant and re‑treatment with irinotecan. It is generally not expected that subjects who fail chemotherapy will respond to immediate re‑treatment with the same therapy. Of these 23 subjects who previously failed irinotecan as their immediate prior therapy, there were three (13%) partial responders to treatment, seven subjects (32%) were alive without disease progression at four months and four subjects (18%) were alive without disease progression at six months. In addition, of the 71 subjects in this Phase 1/2 clinical trial, 37 CRC subjects with KRAS mutant tumors who had previously failed an irinotecan‑based therapy were treated with birinapant and irinotecan. Of these 37 subjects, three subjects (8%) demonstrated PRs, 38% of subjects were alive without progression of their disease at four months, and 24% of subjects were alive without progression of disease at six months.
The combination of birinapant administered with irinotecan was generally well tolerated. Compared to treatment with irinotecan alone, birinapant administered with irinotecan led to a modest increase in anemia (or a decrease in red blood cells) and a modest increase in thrombocytopenia (or a decrease in platelets). Irinotecan is one of the chemotherapies that induce TNF. As the majority of subjects had disease progression on prior irinotecan treatment (65 of 71, or 92%), we believe that this data supports the view that the activity seen in this study is being driven by the synergistic effect of birinapant and irinotecan. Based on the clinical data that has emerged from the study of birinapant administered with irinotecan, we continue to critically evaluate the potential for birinapant therapy in CRC.
14
Introduction to Epigenetics
Epigenetics refers to the regulation of gene expression by post-translational modification of protein complexes associated with DNA without altering the DNA sequence. Maintenance of normal cell growth and differentiation is highly dependent on coordinated and tight transcriptional regulation of genes. In cancer, genes encoding growth regulators are abnormally expressed. Conversely, tumor suppressor genes are silenced. These changes can occur as a result of chromatin modifications that cause this altered gene expression. Thus, mechanisms that regulate chromatin structure and gene expression have become attractive targets for anti-cancer therapy. The basic structure of chromatin consists of the nucleosome, which comprises a sequence of DNA wrapped around core histones. Histone proteins compact massive amounts of genomic DNA into a size and structure that can be easily housed in the nucleus of a cell. These proteins are post-translationally modified, which impacts their interactions with chromatin-associated proteins and levels of gene activity.
One such post-translational modification is acetylation, which is regulated by HDACs. HDACs catalyze the removal of acetyl groups from specific lysine side chains in histones, resulting in modification of chromatin structure and modulation of gene transcription. HDACs also deacetylate non-histone proteins, such as transcription factors. Histone acetylation and deacetylation, and imbalances between the two, can result in gene overexpression on the one hand and gene silencing on the other.
HDACs play a broad role in numerous signaling pathways critical to cancer cell survival. Inhibition of HDAC activity results in an open chromatin structure and alteration of transcriptional activity of specific genes. Restoring the balance between acetylation and deacetylation with HDACi’s has been extensively studied in both pre-clinical and clinical models.
SHAPE
SHAPE is an HDACi being developed for topical use for the treatment of CTCL. HDAC is a validated cancer target and HDACi’s are a proven class of anti-cancer drugs. SHAPE is a novel therapeutic designed to maximize HDAC inhibition locally in the skin with limited systemic exposure. As a result, SHAPE has characteristics that could allow it to be used topically over large body surface areas with minimal systemic absorption. By potentially avoiding toxicities typical of systemically-administered HDACi’s, SHAPE may provide a more favorable safety profile than an HDACi delivered systemically.
SHAPE has been evaluated in a randomized, dose escalation placebo-controlled Phase 1 clinical trial in early-stage CTCL subjects that met safety endpoints and demonstrated clinical activity. Pre-clinical studies and clinical trials have demonstrated that topical application of SHAPE produces substantial HDAC inhibition in the skin and no detectable systemic exposure of SHAPE or systemic toxicity. We commenced a randomized Phase 2 clinical trial of SHAPE in subjects with early-stage CTCL in December 2014, which will assess clinical activity after six months of application. Data for this clinical trial is expected in 2015. SHAPE has been granted U.S. orphan drug designation for CTCL.
SHAPE’s design to minimize systemic impact creates an opportunity for the treatment of other diseases of the skin. Potential for topical HDAC mediation exists for a wide range of inflammatory and proliferative skin conditions, including acne, dermatitis and psoriasis, as well as actinic keratosis and superficial basal cell carcinoma.
Overview of SHAPE Clinical and Pre‑clinical Programs
Our most advanced clinical program with SHAPE is in CTCL.
15
Cutaneous T-Cell Lymphoma (CTCL)
Background
CTCL is comprised of indolent non-Hodgkin T-cell lymphomas, which have their primary manifestations in the skin. According to the Cutaneous Lymphoma Foundation, the incidence of CTCL is increasing in the U.S. with approximately 3,000 new cases being diagnosed annually. Since patients have a very long survival, it is estimated that there may be as many as 30,000 patients living with CTCL in the U.S. and Canada. CTCL is twice as common in men as in women, occurs most often in people older than 55 and affects twice as many African-Americans as Caucasians.
The prognosis of patients with CTCL is based on the extent of disease at presentation, or disease stage. CTCL patients with early-stage disease typically present with skin symptoms and lesions. These lesions may remain as patches or plaques confined to the skin for many years prior to the development of cutaneous tumors or visceral disease. Stages IA-IIA, the stages of the disease restricted to the skin and includes the most common CTCL subtype mycosis fungoides, or MF, comprise approximately 75% of the CTCL patient population. Stages IIB-IV, which are later stages of the disease that have spread to the blood or lymph nodes, comprise approximately 25% of the CTCL patient population. The median survival following diagnosis varies according to stage. Patients with Stage IA disease have a median survival of 20 or more years. The majority of deaths for this group are not caused by CTCL. In contrast, more than 50% of patients with Stage III through Stage IV disease die of CTCL, with a median survival of less than 5 years.
Generic topical corticosteroids are the primary first line treatment for Stage IA-IIA CTCL. This includes both Class I and Class II corticosteroids. Because of side-effects, the duration of corticosteroid use is rather limited, and patients generally progress to other therapies that include topical retinoids, topical nitrogen mustards, phototherapy or local radiation. After patients relapse or fail first- and second-line therapies, they may then transition to an IV form of mechlorethamine or nitrogen mustard. Bexarotene (Targretin®, Valeant) is a retinoid available as a topical gel and an oral capsule. Targretin® is approved and used in all stages of CTCL. Mechlorethamine (Valchlor™, Actelion) is a topical gel approved for Stage IA-IIA MF patients who have received prior skin directed therapy.
When CTCL advances beyond the skin, patients are then treated with systemic therapies, which include Ontak® (denileukin diftitox, Eisai Co., Ltd., or Eisai) and two systemic HDACi: vorinostat (Zolinza®, Merck & Co., Inc., or Merck) and romidepsin (Istodax®, Celgene Corporation, or Celgene). These therapies have demonstrated significant durable anti-cancer activity with rapid onset of action. They have also demonstrated substantial improvement in pruritus (itching), which is one of the hallmarks of CTCL. However, use of these agents has been limited to patients who have progressive or persistent disease following prior systemic therapies.. Consequently, the majority of CTCL patients, those with early stage disease, do not receive these medications.
Rationale for treatment with SHAPE
SHAPE, our second clinical-stage product candidate, is an HDACi being developed for topical use for the treatment of CTCL. SHAPE is a novel therapeutic intentionally designed to maximize HDAC inhibition locally in the skin when applied topically (or locally) with limited systemic exposure. SHAPE has characteristics that could allow it to be used topically over large body surface areas with minimal systemic absorption. By potentially avoiding toxicities typical of a systemically-administered HDACi, SHAPE may provide a more favorable safety profile.
Pre-clinical studies
SHAPE was evaluated in vitro for its ability to inhibit histone deacetylase and for their cytotoxic activity in CTCL cell lines. Studies have demonstrated that SHAPE inhibits the activity of HDAC1, HDAC2, HDAC3 and HDAC6 enzymes in vitro and is cytotoxic to CTCL cell lines. SHAPE inhibited HDAC activity and decreased proliferation of malignant CTCL cells in vitro.
SHAPE was also tested in vivo for its ability to cause hyperacetylation of skin cells when applied topically to human skin xenografts in mice or to the skin of minipigs. Hyperacetylation is a known PD effect of HDAC inhibition.
16
SHAPE produced immunohistochemical staining consistent with hyperacetylation in skin cells when administered topically to minipigs or to human skin xenografts in mice.
Studies were undertaken to determine exposure of SHAPE locally in the skin and systemically in blood. In vitro and in vivo studies of the uptake of SHAPE through skin showed that dermal application of SHAPE largely obviates exposure to the HDACi in the blood. Administering SHAPE intravenously to minipigs and rats resulted in higher blood concentrations of SHAPE than those achieved in the in vivo dermal study, and, in both IV and dermal studies, SHAPE was metabolized rapidly in the blood to the two primary metabolites: SHP‑100 and methylparaben. Systemic levels of SHAPE were negligible in all studies, even at maximum feasible dose strengths. These findings suggest that dermal application of SHAPE largely obviates systemic exposure to HDACi.
SHAPE has been tested in safety pharmacology studies. When administered topically to minipigs, SHAPE was well tolerated. Slight irritation of the skin was observed in some animal studies and was likely related to the ethanol excipient in the formulation.
Clinical trial
SHAPE was evaluated in a randomized, double-blind, placebo-controlled Phase 1 clinical trial of escalating doses of SHAPE in subjects with Stage IA-IIA CTCL. SHAPE doses of 0.1%, 0.5%, 1.0% or placebo were applied to skin areas defined as index lesions in up to 5% of body surface area twice daily over a 28-day treatment period. A total of 18 subjects, ages 32 to 84 years, were randomized at 5 study sites in the U.S. Five subjects were in each of the SHAPE treatment groups and a total of three subjects received placebo.
The primary objective of this clinical trial was to investigate the safety and tolerability of topical SHAPE administered directly to affected skin lesions in subjects with Stage IA, IB or IIA CTCL. The secondary objectives of this clinical trial were to evaluate the histological and clinical effect of SHAPE on treated skin lesions, to investigate systemic PK of SHAPE and metabolites following topical SHAPE administration and to investigate the local PD effect, including chromatin hyperacetylation of the skin, of topical SHAPE administration.
Local pharmacokinetic (PK) effect of SHAPE on treated skin lesions
The clinical effect of SHAPE on treated skin lesions was evaluated using the Composite Assessment of Index Lesion Severity, or CAILS, scoring system. The CAILS scoring system is a composite scale to evaluate the clinical signs for each index lesion. Subjects had 1–5 index lesions for assessment. Individual index lesions were graded at each visit. Assessments of each lesion included scaling, erythema, plaque elevation, and index lesion area. A CAILS score was generated by a summation of the grades for each index lesion for each of the assessments. A CR is defined as a 100% decrease in the CAILS score and a PR is defined as a 50% to 99% decrease. Stable disease is defined as less than a 25% increase to less than a 50% increase and progressive disease is defined as a greater than 25% increase.
Based upon the above criteria, four of 15 subjects treated with SHAPE (27%) had evidence of a PR. No clear dose response was evident. No placebo subjects approached the magnitude of reduction necessary to meet accepted response criteria.
Systemic pharmacokinetics (PK) of topical SHAPE administration
Topical administration of SHAPE gel at 0.1%, 0.5% and 1.0% concentrations twice daily for 28 days was well-tolerated. For all SHAPE doses, plasma concentrations of SHAPE were at or below the lower limit of quantitation of the assay, indicating negligible/lack of SHAPE systemic exposure. The lack of measurable levels of SHAPE in the blood is consistent with rendering the drug to an inactive form by endogeneous esterases. Low systemic drug availability is also consistent with the lack of systemic side effects observed in this clinical trial that are typically reported with oral or parenterally administered HDACi drugs.
17
Pharmacodynamic (PD) effect of topical SHAPE administration
Punch biopsies of skin within index lesions were taken on Day 1 (Visit 2) and after 14 and 28 days of treatment with study medication (Visits 4 and 6). The biopsy on Day 1 was used for central pathology use in characterizing CTCL and was taken prior to dosing while the other biopsies were taken after dosing. Immunohistochemical analysis of skin biopsy specimens confirmed evidence of a dose dependent augmented HDACi activity using a specific acetyl-lysine antibody. This was most prominent in the 1.0% SHAPE dose group where subjects in this group most clearly demonstrated augmented nuclear staining of the epidermis after dosing. This increase in staining intensity was not observed in any placebo subject.
Randomized Phase 2 clinical trial
Even with the short dosing interval of 28 days in the Phase 1 clinical trial, we believe that the PD and safety data provide evidence of clinical benefit and safety profile sufficient to pursue further clinical development. In addition, we believe the data provides a rationale that SHAPE may address an important unmet medical need in early stage CTCL by feasibly offering clinical benefits of an HDACi without the attendant safety concerns associated with systemic administration of HDACi.
Safety data of SHAPE is available from 15 subjects in subjects with Stage IA-IIA CTCL. In the Phase 1 clinical trial, no deaths, serious AEs, discontinuations due to AEs or dose-limiting toxicities occurred during the study. The majority of AE’s were Grade 1 and not related to administration of study medication. Two subjects in the 1.0% SHAPE treatment group experienced AEs considered by the reporting investigators to be greater than or equal to Grade 2. Of these AEs, only one event of contact dermatitis was considered treatment related. The other AEs that were greater than or equal to Grade 2 (joint dislocation, radius fracture and tendonitis) were related to other etiologies and not treatment related.
Eight subjects experienced AEs that were classified by the reporting investigators as having a possible or probable relationship to treatment. All treatment-related AEs were confined to those associated with topical administration of study medication to treatment sites/index lesions. Treatment-related cutaneous events were reported in one of three (33%) of placebo subjects and four of 15 (27%) of SHAPE subjects. Skin burning and irritation were the most frequently reported treatment-related AEs. None of these AEs required remedial therapy during the treatment period and none resulted in discontinuing dosing of study drug. Review of other safety-related assessments (such as clinical laboratories, concomitant medication usage, Eastern Cooperative Oncology Group Performance Status, electrocardiograms and vital signs) did not reveal any significant clinical findings.
Based upon the data from the Phase 1 clinical trial, we commenced in the fourth quarter of 2014 a randomized Phase 2 clinical trial of SHAPE in approximately 60 subjects with Stage IA-IIA CTCL. The objectives of the Phase 2 clinical trial are to evaluate the dose, clinical effect at six months, time to response and tolerability of treatment of greater than 5% body surface area. The treatment effect identified in the randomized Phase 2 clinical trial will determine the design of a Phase 3 registration trial. Data for this clinical trial is expected in 2015.
Our Strategy
Our goal is to maximize the potential value of both birinapant as a first‑ in‑class and best‑in‑class SMAC‑mimetic and SHAPE as a novel skin‑targeted HDACi. The key elements of our strategy to achieve these goals include:
|
· |
pursuing regulatory approval for birinapant administered with azacitidine for the treatment of first‑line higher‑risk MDS. The data from our randomized Phase 2 clinical trial will determine the size of the treatment effect of birinapant administered with azacitidine versus azacitidine alone and will form the basis of a Phase 3 clinical trial in first‑line higher‑risk MDS; |
18
|
· |
continuing a Phase 1/2 clinical trial that started in December 2013 for birinapant administered with conatumumab in third-line ovarian cancer. In December 2014, this clinical trial proceeded into a Phase 2a expansion based upon data in the Phase 1 portion of the clinical trial. Phase 2a clinical trial data is expected in 2015; |
|
· |
assessing the clinical effect of birinapant in reducing HBsAg and developing HBsAb in the ongoing randomized, placebo-controlled, multiple ascending dose Phase 1 clinical trial of birinapant in subjects with chronic HBV. The data from this clinical trial will determine the extent of further studies to be undertaken in this patient population. In addition, pre-clinical studies of birinapant in other infectious disease models will be evaluated in order to determine other potential clinical programs; |
|
· |
continuing to evaluate the possibility of undertaking further clinical trials of birinapant in CRC and other solid tumors; |
|
· |
pursuing regulatory approval of SHAPE in early stage CTCL. The data from our randomized Phase 2 clinical trial will determine the size of the treatment effect of SHAPE after 6 months of treatment and will form the basis of a Phase 3 clinical trial in CTCL; |
|
· |
considering collaborations to accelerate development of our clinical programs outside of the U.S.; and |
|
· |
in‑licensing or acquisitions of assets and companies to expand our existing technologies and operations. |
Other elements of our business strategy include exploiting our understanding of the role of SMAC‑mimetics more broadly in infectious disease, leveraging our library of SMAC‑mimetic compounds to develop novel molecules to expand the utility of this developing class and pursuing potential collaborations.
License Agreement with Princeton University
In November 2003, we entered into an exclusive license agreement with Princeton University, subsequently amended in June 2004, August 2006, and October 2006, which grants us the rights to certain U.S. patents controlled by the university relating to SMAC‑mimetic compounds, including birinapant, and a non‑exclusive right to certain know‑how and technology relating thereto. The agreement contains a right by us to sublicense. We have paid an aggregate of $100,000 in license fees to Princeton University. As part of the consideration paid, we issued to Princeton University 9,734 shares of our common stock and agreed to pay Princeton University certain royalties. In particular, we are obligated to pay royalties as a percentage of net product sales of 2.0% for direct licensed products, such as birinapant, and 0.5% of derived licensed products, if such products are covered by the applicable Princeton University patent rights. We have the right to reduce the amount of royalties owed to Princeton University by the amount of any royalties paid to a third‑party in a pro rata manner, provided that the royalty rate may not be less than 1.0% of net sales for direct licensed products and 0.25% for derived licensed products. The obligation to pay royalties outside the U.S. expires, on a country by country basis, on the later of 10 years from the first commercial sale of a licensed product in each country and expiration, lapse or abandonment of the last of the licensed patent rights that covers the manufacture, use or sale of the licensed product, if it had been made, used or sold in the U.S. The licensed patent rights were developed using federal funds from the National Institutes of Health and are subject to certain overriding rights of and obligations to the federal government as provided in the Bayh‑Dole Act. This agreement expires upon expiration of the last of the licensed patent rights in 2023 (absent extensions).
The agreement also requires that we pay to Princeton University 2.5% of the non‑royalty consideration that we receive from a sublicensee.
Unless otherwise assigned, we are responsible for the patent prosecution and maintenance activities pertaining to the licensed patents, while Princeton University is afforded reasonable opportunities to review and comment on such activities. In the event that we do not wish to continue to maintain any patent within the licensed patents, Princeton University may assume responsibility and control over the necessary maintenance for such patent or patent application
19
subject to our review and comment. We have the sole right to enforce (or defend against any declaratory judgment action) the licensed patents against third parties for suspected infringement, provided however that Princeton University must consent to any proposed settlement, which shall not be unreasonably withheld. We also have the sole right to defend against any third‑party challenge to Princeton University’s exclusive right, title and interest in the licensed technology, including the licensed patents, provided however that Princeton University must consent to any proposed settlement, which shall not be unreasonably withheld.
Under the license agreement, we are obligated to use reasonable efforts to develop, test, obtain regulatory approval, manufacture, market, and sell licensed products in all countries worldwide.
If we materially breach or fail to perform our obligations under this agreement (including failure to make payments to Princeton University when due for royalties and other sub‑license revenues, failure to use reasonable efforts to develop, test, obtain regulatory approval, manufacture, market and sell licensed products, failure to file annual progress reports, commencement of bankruptcy or insolvency proceedings against us or failure to prosecute and maintain the licensed patents), Princeton University has the right to terminate our license, and upon the effective date of such termination, our right to practice the licensed Princeton University patent rights and related technology.
License Agreement with Walter and Eliza Hall Institute
In January 2014, we entered into a license agreement with WEHI in Melbourne, Australia for worldwide exclusive rights to a patent application and any patents issuing therefrom relating to a method of treating intracellular infections involving the administration of an IAP antagonist, which is being used to further our HBV program. WEHI will perform research and development services for which we will be making payments over the first four years of the agreement in the amount of $1 million, of which we have paid $500,000 to date. We are obligated to pay royalties as a percentage of net sales of 2% for products that are based either on the licensed patents or any patents arising from research performed by WEHI. We are also obligated to pay royalties as a percentage of net income of 15% received from sublicensing the licensed patents or any patents arising from research performed by WEHI to a third party. We may also be required to make milestone payments to WEHI of up to $3,750,000 for the first indication and up to $1,875,000 for each of the next two indications based on the commencement of certain clinical trials and the filing and approval of new drug applications.
Under the license agreement, we are required to use reasonable efforts to commercialize the licensed patents and to promote and develop the sale of products that are based either on the licensed patents or any patents arising from research performed by WEHI. We are responsible for obtaining and maintaining the licensed patents, including patent filing, patent application prosecution, and any patent application costs or ongoing maintenance fees.
If we materially breach or fail to perform our obligations under this agreement (including failure to make payments when due for royalties, failure to use reasonable efforts to develop, test, obtain regulatory approval, manufacture, market and sell licensed products, failure to file annual progress reports, commencement of bankruptcy or insolvency proceedings against us or failure to prosecute and maintain the licensed patents), this license agreement will terminate, and upon the effective date of such termination, all further rights and obligations under the agreement, including the right to use the licensed patents, will cease.
License Agreement with Harvard University and Dana-Farber Cancer Institute
In October 2008, Shape Pharmaceuticals entered into a license agreement with Harvard University and Dana-Farber Cancer Institute, Inc., or the Licensors, to grant a license under its interest in certain patent rights as defined in the license agreement, which include claims covering the composition of the SHAPE molecule. The agreement contains a right by us to sublicense. The Licensors received 400,000 shares of common stock of Shape Pharmaceuticals, in consideration for the grant of the license, for which they received a payment of $213,317 when we acquired Shape Pharmaceuticals in April 2014. We also paid the Licensors an annual maintenance fee of $100,000 in 2012 and will pay $50,000 on the fifth anniversary of the effective date of the agreement and on each subsequent anniversary date thereafter as long as the license agreement remains in full force and effect. The annual maintenance fee of $50,000 was paid in 2013 and 2014. As defined in the license agreement, we may be required to pay milestones on an indication-by-
20
indication basis of up to $4,450,000 in the aggregate and/or royalties of net sales of developed products, if and when achieved. Annual maintenance fee payments can be used to offset milestone obligations. We paid a milestone payment of $100,000 during the year ended December 31, 2011. We have the right to terminate the agreement upon 60 days’ written notice.
We have the first right, but not the obligation, to take action in the patent prosecution and maintenance activities pertaining to the licensed patents, while Harvard University is afforded reasonable opportunities to review and comment on such activities. In the event that we do not wish to continue to maintain any patent within the licensed patents, Harvard University may assume responsibility and control over the necessary maintenance for such patent or patent application subject to our review and comment. We have the first right, but not the obligation, to enforce (or defend against any declaratory judgment action) the licensed patents against third parties for suspected infringement, provided however that Harvard University must consent to any proposed settlement, which shall not be unreasonably withheld. We also have the first right, but not the obligation, to defend against any third‑party challenge to Harvard University’s exclusive right, title and interest in the licensed technology, including the licensed patents, provided however that Harvard University must consent to any proposed settlement, which shall not be unreasonably withheld. If we exercise our right to take action, then we shall first reimburse ourselves out of any settlement for all costs and expenses, including reasonable attorney’s fees, incurred in the prosecution of the action, with any remaining amounts distributed 10% to Harvard University and 90% to us.
If we choose not to take action in the patent prosecution and maintenance activities pertaining to the licensed patents, or not to enforce (or defend against any declaratory judgment action) the licensed patents against third parties for suspected infringement, then Harvard University may elect to do so, and we must consent to any proposed settlement, which shall not be unreasonably withheld. If Harvard University exercises its right to take action, then they shall first reimburse themselves out of any settlement for all costs and expenses, including reasonable attorney’s fees, incurred in the prosecution of the action, with any remaining amounts distributed 10% to us and 90% to Harvard University.
Under the license agreement, we are obligated to use reasonable efforts to develop, test, obtain regulatory approval, manufacture, market, and sell licensed products in all countries worldwide.
If we materially breach or fail to perform our obligations under this agreement (including failure to make payments to the Licensors when due for royalties and other sub‑license revenues, failure to use reasonable efforts to develop, test, obtain regulatory approval, manufacture, market and sell licensed products, failure to file annual progress reports, commencement of bankruptcy or insolvency proceedings against us or failure to prosecute and maintain the licensed patents), the Licensors have the right to terminate our license, and upon the effective date of such termination, our right to practice the licensed patent rights and related technology.
CTCL Trial with The Leukemia and Lymphoma Society
In June 2010, we entered into a funding agreement with The Leukemia and Lymphoma Society, or LLS, to fund the development of SHAPE. Under the LLS funding agreement, we are obligated to use the funding received exclusively for the payment or reimbursement of the costs and expenses for clinical development activities for SHAPE. Under this agreement, we retain ownership and control of all intellectual property pertaining to works of authorship, inventions, know-how, information, data and proprietary material.
Under the LLS funding agreement, as amended, we received funding of $2.695 million from LLS through 2014. We terminated the funding agreement effective as of February 2014. We are required to make specified payments to LLS, including payments payable upon execution of the first out-license; first filing of approval for marketing by a regulatory body; first approval for marketing by a regulatory body; and completion of the first commercial sale of SHAPE. The extent of these payments and our obligations will depend on whether we out-license rights to develop or commercialize SHAPE to a third party, we commercialize SHAPE on our own or we combine with or are sold to another company. In addition, we will pay to LLS a single-digit percentage royalty of our net sales of SHAPE, if any. The sum of our payments to LLS is capped at three times the total funding received from LLS, or $8.085 million.
21
In addition, some of our obligations under the funding agreement will remain in effect until the completion of specified milestones and payments to LLS. Assuming the successful outcome of the development activities covered by the LLS funding agreement and our receipt of necessary regulatory approvals, we will be required to take commercially reasonable steps through 2019 to advance the development of SHAPE in clinical trials and to bring SHAPE to practical application for CTCL in a major market country, provided that we reasonably believe the product is safe and effective. We believe that we can satisfy our obligation by out-licensing SHAPE to, or partnering SHAPE with, a third party. We are required to report to LLS on our efforts and results with respect to continuing development of SHAPE. Our failure to perform these diligence obligations, even if we successfully achieve the specified development milestones, would require us to pay back to LLS the total amount of the funding we received from them, unless an exception applies. If LLS were to claim that such failure occurred and we disagreed with such claim, the dispute would be settled through binding arbitration. In connection with the accounting for the acquisition of Shape Pharmaceuticals, we estimated the fair value of this potential obligation to be $200,000 and have accrued this amount in our December 31, 2014 balance sheet.
Ovarian Cancer Trial with Amgen TRAIL Antibody
We are currently collaborating with Amgen on the conduct of our Phase 1/2 clinical trial in relapsed epithelial ovarian cancer, fallopian tube cancer or primary peritoneal cancer for birinapant administered with conatumumab, an investigational fully‑human monoclonal agonist antibody designed to partially mimic endogenous TRAIL, which is owned by Amgen. In support of that trial, we have entered into an agreement with Amgen for the purchase of clinical supplies of conatumumab sufficient to conduct the study. Neither party has acquired any developmental or commercial rights to the other party’s product candidate.
Intellectual Property
Birinapant
In addition to our license of the Princeton University patents, we own more than 120 patents and patent applications worldwide, all relating to SMAC‑mimetics and uses thereof, including U.S. patents that we own which have been granted with claims that cover birinapant as a new chemical entity. In particular, we have issued patents in the U.S. that specifically claim birinapant as a composition of matter. The patents relating to composition of matter expire in June 2030, without accounting for possible patent term extensions under the Drug Price Competition and Patent Term Restoration Act of 1984, or the Hatch‑Waxman Act, or for possible pediatric exclusivity. We have not licensed any rights to practice any of these patents or patent applications to any third party.
Patents that we own with claims that cover birinapant as a new chemical entity have also been granted or allowed in several foreign jurisdictions, including the European Patent Office, Japan, India and China, among others. These patents are generally set to expire in 2026, without accounting for possible extensions, such as by issuance of supplementary protection certificates or grant of pediatric exclusivity. In addition to the U.S., we have also filed patent applications with claims that specifically cover birinapant as a new chemical entity in the following countries or regions, which, if granted, would expire in 2030, without considering any possible extensions: Argentina, African Regional Intellectual Property Organization (ARIPO), Australia, Brazil, Canada, Chile, China, Colombia, Ecuador, Egypt, Eurasian Patent Organization (EAPO), Europe, Patent Office of the Cooperation Council for the Arab States of the Gulf (GCC), Hong Kong, Israel, India, Japan, Malaysia, Mexico, New Zealand, African Intellectual Property Organization (OAPI), Peru, Philippines, Singapore, South Africa, South Korea, Taiwan, Thailand, Ukraine and Venezuela. In addition to the new chemical entity patents claiming birinapant, we hold an exclusive license from Princeton University under a U.S. patent that broadly claims SMAC‑mimetics as a class of compounds and that expires in 2023 unless extended. Other patents and patent applications owned by us are directed to SMAC‑mimetics other than birinapant and various methods of treatment and biomarkers relevant to birinapant and other SMAC‑mimetics. We do not believe that birinapant would infringe any valid third‑party patent to which we do not have a license. We continue to monitor patent filings in major commercial jurisdictions.
We expect to continue to file additional patent applications with claims that cover uses of birinapant and other SMAC‑mimetics, methods of treatment, and biomarkers and other diagnostic tools. In addition, pursuant to material
22
transfer agreements and other agreements in place with third‑party researchers, including our CRADA with NCI, we have the opportunity to license additional technologies that may complement our current programs.
SHAPE
Our license agreement with Harvard University and Dana-Farber Cancer Institute, Inc. grants us the worldwide rights to certain patent applications and patents issuing therefrom as defined in the license agreement, which include claims covering the composition of the SHAPE molecule. SHAPE’s composition of matter patent in the U.S. extends until at least 2028. In addition, SHAPE has been granted U.S. orphan drug designation for CTCL. We have acquired worldwide development and commercialization rights to SHAPE for all indications.
General Considerations
As with other biotechnology and pharmaceutical companies, our ability to maintain and solidify a proprietary position for our product candidates will depend upon our success in obtaining effective patent claims and enforcing those claims once granted.
Our commercial success will depend in part upon not infringing upon the proprietary rights of third parties. It is uncertain whether the issuance of any third‑party patent would require us to alter our development or commercial strategies, obtain licenses, or cease certain activities. The biotechnology and pharmaceutical industries are characterized by extensive litigation regarding patents and other intellectual property rights.
The term of a patent that covers an FDA‑approved drug may be eligible for patent term extension, which provides patent term restoration as compensation for the patent term lost during the FDA regulatory review process. The Hatch‑ Waxman Act permits a patent term extension of up to five years beyond the expiration of the patent. The length of the patent term extension is related to the length of time the drug is under regulatory review. Patent extension cannot extend the remaining term of a patent beyond a total of 14 years from the date of product approval and only one patent applicable to an approved drug may be extended. Similar provisions are available in Europe and other foreign jurisdictions to extend the term of a patent that covers an approved drug. In the future, if and when our pharmaceutical products receive FDA approval, we expect to apply for patent term extensions on patents covering those products.
Many pharmaceutical companies, biotechnology companies and academic institutions are competing with us in the fields of oncology and infectious disease and filing patent applications potentially relevant to our business. Even when a third‑party patent is identified, we may conclude upon a thorough analysis, that we do not infringe upon the patent or that the patent is invalid. If the third‑party patent owner disagrees with our conclusion and we continue with the business activity in question, we may be subject to patent litigation. Alternatively, we might decide to initiate litigation in an attempt to have a court declare the third‑party patent invalid or non‑infringed by our activity. In either scenario, patent litigation typically is costly and time‑consuming, and the outcome can be favorable or unfavorable.
In addition to patents, we rely upon unpatented trade secrets, know‑how and continuing technological innovation to develop and maintain a competitive position. We seek to protect our proprietary information, in part, through confidentiality agreements with our employees, collaborators, contractors and consultants, and invention assignment agreements with our employees and some of our collaborators. The confidentiality agreements are designed to protect our proprietary information and, in the case of agreements or clauses requiring invention assignment, to grant us ownership of technologies that are developed through a relationship with a third party.
Competition
The pharmaceutical industry is highly competitive and subject to rapid and significant technological change. While we believe that our development experience and scientific knowledge provide us with competitive advantages, we face competition from both large and small pharmaceutical and biotechnology companies; specifically with companies that are actively researching and developing products that target apoptosis and HDACi’s as mechanisms to treat various cancers and infectious diseases.
23
Our product candidates are presently being developed primarily as cancer and infectious disease therapeutics. There are a variety of available therapies and supportive care products marketed for cancer and infectious disease patients. In many cases, these products are administered in combination to enhance efficacy or to reduce side effects. Some of these drugs are branded and subject to patent protection, some are in clinical development and not yet approved, and others are available on a generic basis. Many of these approved drugs are well established therapies or products and are widely accepted by physicians, patients and third‑party payors. Insurers and other third‑party payors may also encourage the use of generic products. In addition, birinapant is delivered intravenously, which will require a visit to an oncologist office or a hospital. Some of our competitors are seeking to develop drugs that can be administered by oral delivery, and thus would not require a visit to a doctor for each administration. These factors may make it difficult for us to achieve market acceptance at desired levels in a timely manner to ensure viability of our business.
More established companies have a competitive advantage over us due to their greater size, cash flows and institutional experience. Compared to us, many of our competitors have significantly greater financial, technical and human resources.
As a result of these factors, our competitors may obtain regulatory approval of their products before we are able to obtain patent protection or other intellectual property rights, which will limit our ability to develop or commercialize our product candidates. Our competitors may also develop drugs that are safer, more effective, more widely used and less costly than ours, and may also be more successful than us in manufacturing and marketing their products. These appreciable advantages could render our product candidates obsolete or non‑competitive before we can recover the expenses of their development and commercialization.
Mergers and acquisitions in the pharmaceutical and biotechnology industries may result in even more resources being concentrated among a smaller number of our competitors. Smaller and other early‑stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies. These third parties compete with us in recruiting and retaining qualified scientific, management and commercial personnel, establishing clinical trial sites and subject registration for clinical trials, as well as in acquiring technologies complementary to, or necessary for, our programs.
SMAC‑Mimetic Competitive Landscape
It is not possible to know with certainty what competitors are or may be doing in the field of SMAC‑mimetics. It appears, however, that, in the field of SMAC‑mimetics, our principal competitors in clinical development include Curis Inc. (Phase 1), Debiopharma S.A. (Phase 1) and Novartis AG, or Novartis, (Phase 2). Companies with earlier stage (discovery or pre‑clinical studies) SMAC‑ mimetic programs include Astex Pharmaceuticals, or Astex, Bristol‑Myers Squibb Company, or BMS, and Ensemble Therapeutics.
Myelodysplastic Syndromes (MDS)
The hypomethylating agents/DNA methyltransferase inhibitors, azacitidine and decitabine are the standard of care treatment for higher‑risk MDS patients. The companies that are marketing these drugs include Celgene and Eisai. Allogeneic stem cell transplant is the only treatment that is potentially curative for those patients who are candidates for the procedure which is generally considered only for the small proportion of younger MDS patients. Currently there are no FDA approved drugs for MDS patients who fail hypomethylating agents.
Due to the unmet medical need, there are several companies developing new agents for higher‑risk MDS. The majority of the competitive therapies in development for higher‑risk MDS consists of histone deacetylase inhibitors, multi‑kinase inhibitors, new hypomethylating agents and nucleoside analogues; many of which are also in development for AML. Companies with investigational product candidates in Phase 2 or 3 clinical trials for MDS include Astex, Cyclacel Pharmaceuticals Inc., Merck, Onconova Therapeutics, Inc. and others.
24
Ovarian Cancer
Platinum‑taxane based chemotherapy is the first‑line standard of care therapy for advanced ovarian cancer, however, most patients will eventually experience recurrence or progression of their cancer. Bevacizumab is approved in Europe as a first‑line treatment for women with advanced ovarian cancer, and administered with standard chemotherapy (carboplatin and gemcitabine) as a treatment for women with first recurrence of platinum‑sensitive ovarian cancer. Patients with platinum‑resistant ovarian cancer are much more resistant to standard chemotherapy and will typically receive a non‑platinum chemotherapy (such as gemcitabine, liposomal doxorubicin, or topotecan) or participate in a clinical trial. The companies that are marketing these drugs include Eli Lilly and Company, or Eli Lilly, GlaxoSmithKline plc, or GlaxoSmithKline, Janssen Pharmaceuticals, Inc., or Janssen, and others.
There are several competitive therapies in clinical development for ovarian cancer including antiangiogenic inhibitors, cytotoxic agents, immunotherapies, kinase inhibitors, and PARP inhibitors. Companies with investigational drugs in Phase 2 or 3 clinical trials for advanced ovarian cancer include AstraZeneca plc, Clovis Oncology, Endocyte, Inc., Sanofi S.A. and others.
Hepatitis B Virus (HBV)
There are currently no FDA approved products available to cure HBV. FDA approved treatments for patients with chronic HBV include INFs such as Intron A (INF alpha-2b) and Pegasys (peg-INF alpha-2A), and nucleoside analogues such as Epivir-HBV (lamivudine), Hepsera (adefovir dipivoxil), Baraclude (entecavir), Tyzeka (telbivudine) and Viread (tenofovir). The companies that are marketing these drugs include Merck, Genentech Inc., GlaxoSmithKline, Gilead Sciences, BMS and Novartis. These treatments are designed to decrease the risk of liver damage from HBV by slowing down or stopping the virus from reproducing. In addition, several pharmaceutical and biotechnology companies are developing additional therapies to address HBV, including non-nucleotide antivirals and non-INF immune enhancers.
There are also FDA approved vaccinations available for children and high-risk adults that protect against HBV. Three doses are generally required to complete the HBV vaccine series. These vaccines are manufactured by Merck and GlaxoSmithKline and are widely available in the U.S. (and less available in the rest of the world), and have limited side effects. Although the vaccines are effective against HBV in non-infected individuals, they will not reverse or cure the disease in people who have already contracted the virus, such as children of women who are already HBV positive.
HDAC Inhibitors (HDACi) Competitive Landscape
The most successful clinical applications of HDACi against refractory CTCL have been the use of vorinostat (Zolinza®, Merck) and romidepsin (Istodax®, Celgene). In addition to these two FDA-approved agents, HDACi that are in clinical development against various cancers include valproic acid (Abbott Laboratories) and newer compounds such as panobinostat (Novartis, Phase 3), quisinostat (Janssen, Phase 2), givinostat (Italfarmaco S.p.A., Phase 2), mocetinostat (MethylGene Inc., Phase 2), belinostat (Spectrum Pharmaceuticals, Inc., Phase 2), pracinostat (MEI Pharma Inc., Phase 2) and entinostat (Merck, Phase 2). Of these, only panobinostat and quisinostat are being actively studied in CTCL, according to the FDA web-site.
Cutaneous T-Cell Lymphoma (CTCL)
Due to the unmet medical need described above, there are several companies investigating other therapies for CTCL. The HDACi panobinostat and quisinostat are currently in Phase 1/2 and Phase 2 clinical trials for CTCL, respectively. Other agents in clinical development for CTCL include Mogamulizumab (Kyowa Hakko Kirin Co., Ltd., Phase 3), IL-12 plasmid (OncoSec Medical, Phase 2) and CD5789 cream (Galderma S.A., Phase 1). FDA approved agents that are currently being evaluated for CTCL are bortezomib (Velcade®, Phase 2), lenalidomide (Revlimid®, Phase 2) and everolimus (Afinitor®, Phase 2).
25
Manufacturing
Manufacturing of drugs and product candidates, including birinapant and SHAPE, must comply with FDA current good manufacturing practices, or cGMP regulations. Both of our investigational product candidates are synthetic small molecules made through a series of organic chemistry steps starting with commercially available organic chemical raw materials. We conduct manufacturing activities under individual purchase orders with independent contract manufacturing organizations, or CMOs to supply our clinical trials. We have an internal quality program; and accordingly, we have qualified and signed quality agreements with our CMOs, and we conduct periodic quality audits of their facilities. We believe that our existing suppliers of our active pharmaceutical ingredients and finished products will be capable of providing sufficient quantities of each to meet our clinical trial supply needs. Other CMOs may be used in the future for clinical supplies and, subject to approval, commercial manufacturing.
Commercial Operations
We do not currently have an organization for the sales, marketing and distribution of pharmaceutical products. We may rely on licensing and co‑promotion agreements with strategic collaborators for the commercialization of our products in the U.S. and other territories. If we choose to build a commercial infrastructure to support marketing in the U.S., such commercial infrastructure could be expected to include a sales force supported by sales management, internal sales support, an internal marketing group and distribution support. To develop the appropriate commercial infrastructure internally, we would have to invest financial and management resources, some of which would have to be deployed prior to any confirmation that our product candidates will be approved.
Government Regulation
As a clinical‑stage biopharmaceutical company that operates in the U.S., we are subject to extensive regulation by the FDA, and other federal, state, and local regulatory agencies. The Federal Food, Drug, and Cosmetic Act, or the FDC Act, and its implementing regulations set forth, among other things, requirements for the research, testing, development, manufacture, quality control, safety, effectiveness, approval, labeling, storage, record keeping, reporting, distribution, import, export, advertising and promotion of our products. Although the discussion below focuses on regulation in the U.S., we anticipate seeking approval for, and marketing of, our products in other countries. Generally, our activities in other countries will be subject to regulation that is similar in nature and scope as that imposed in the U.S., although there can be important differences. Additionally, some significant aspects of regulation in Europe are addressed in a centralized way through the EMA, but country‑ specific regulation remains essential in many respects. The process of obtaining regulatory marketing approvals and the subsequent compliance with appropriate federal, state, local and foreign statutes and regulations require the expenditure of substantial time and financial resources and may not be successful.
U.S. Government Regulation
The FDA is the main regulatory body that controls pharmaceuticals in the U.S., and its regulatory authority is based in the FDC Act. Pharmaceutical products are also subject to other federal, state and local statutes. A failure to comply with any requirements during the product development, approval, or post‑approval periods, may lead to administrative or judicial sanctions. These sanctions could include the imposition of a hold on clinical trials, refusal to approve pending marketing applications or supplements, withdrawal of approval, warning letters, product recalls, product seizures, total or partial suspension of production or distribution, injunctions, fines, civil penalties or criminal prosecution.
The steps required before a new drug may be marketed in the U.S. generally include:
|
· |
completion of pre‑clinical studies, animal studies and formulation studies in compliance with the FDA’s Good Laboratory Practices, or GLP regulations; |
|
· |
submission to the FDA of an IND to support human clinical testing; |
26
|
· |
approval by an IRB at each clinical site before each trial may be initiated; |
|
· |
performance of adequate and well‑controlled clinical trials in accordance with federal regulations and with current Good Clinical Practices, or GCPs to establish the safety and efficacy of the investigational product candidate for each targeted indication; |
|
· |
submission of new drug applications, or NDA to the FDA; |
|
· |
satisfactory completion of an FDA Advisory Committee review, if applicable; |
|
· |
satisfactory completion of an FDA inspection of the manufacturing facilities at which the investigational product candidate is produced to assess compliance with cGMP, and to assure that the facilities, methods and controls are adequate; and |
|
· |
FDA review and approval of the NDA. |
Clinical Trials
An IND is a request for authorization from the FDA to administer an investigational product candidate to humans. This authorization is required before interstate shipping and administration of any new drug product to humans that is not the subject of an approved NDA. A 30‑day waiting period after the submission of each IND is required prior to the commencement of clinical testing in humans. If the FDA has neither commented on nor questioned the IND within this 30‑day period, the clinical trial proposed in the IND may begin. Clinical trials involve the administration of the investigational product candidate to subjects under the supervision of qualified investigators following GCPs, an international standard meant to protect the rights and health of subjects and to define the roles of clinical trial sponsors, administrators and monitors. Clinical trials are conducted under protocols that detail the parameters to be used in monitoring safety, and the efficacy criteria to be evaluated. Each protocol involving testing on U.S. subjects and subsequent protocol amendments must be submitted to the FDA as part of the IND. The informed written consent of each participating subject is required. The clinical investigation of an investigational product candidate is generally divided into three phases. Although the phases are usually conducted sequentially, they may overlap or be combined. The three phases of an investigation are as follows:
|
· |
Phase 1. Phase 1 includes the initial introduction of an investigation product candidate into humans. Phase 1 clinical trials may be conducted in subjects with the target disease or condition or healthy volunteers. These studies are designed to evaluate the safety, metabolism, PKs and pharmacologic actions of the investigational product candidate in humans, the side effects associated with increasing doses, and if possible, to gain early evidence on effectiveness. During Phase 1 clinical trials, sufficient information about the investigational product candidate’s PKs and pharmacological effects may be obtained to permit the design of Phase 2 clinical trials. The total number of participants included in Phase 1 clinical trials varies, but is generally in the range of 20 to 80. |
|
· |
Phase 2. Phase 2 includes the controlled clinical trials conducted to evaluate the effectiveness of the investigational product candidate for a particular indication(s) in subjects with the disease or condition under study, to determine dosage tolerance and optimal dosage, and to identify possible adverse side effects and safety risks associated with the product candidate. Phase 2 clinical trials are typically well‑controlled, closely monitored, and conducted in a limited subject population, usually involving no more than several hundred participants. |
|
· |
Phase 3. Phase 3 clinical trials are controlled clinical trials conducted in an expanded subject population at geographically dispersed clinical trial sites. They are performed after preliminary evidence suggesting effectiveness of the investigational product candidate has been obtained, and are intended to further evaluate dosage, clinical effectiveness and safety, to establish the overall benefit‑risk relationship of the product candidate, and to provide an adequate basis for drug approval.
27 |
Phase 3 clinical trials usually involve several hundred to several thousand participants. In most cases, the FDA requires two adequate and well controlled Phase 3 clinical trials to demonstrate the efficacy of the drug. A single Phase 3 trial with other confirmatory evidence may be sufficient in rare instances where the study is a large multicenter trial demonstrating internal consistency and a statistically very persuasive finding of a clinically meaningful effect on mortality, irreversible morbidity or prevention of a disease with a potentially serious outcome and confirmation of the result in a second trial would be practically or ethically impossible. |
The decision to terminate development of an investigational product candidate may be made by either a health authority body, such as the FDA or IRB/ethics committees, or by a company for various reasons. The FDA may order the temporary, or permanent, discontinuation of a clinical trial at any time, or impose other sanctions, if it believes that the clinical trial either is not being conducted in accordance with FDA requirements or presents an unacceptable risk to the clinical trial subjects. In some cases, clinical trials are overseen by an independent group of qualified experts organized by the trial sponsor, or the clinical monitoring board. This group provides authorization for whether or not a trial may move forward at designated check points. These decisions are based on the limited access to data from the ongoing trial. The suspension or termination of development can occur during any phase of clinical trials if it is determined that the participants or subjects are being exposed to an unacceptable health risk. In addition, there are requirements for the registration of ongoing clinical trials of product candidates on public registries and the disclosure of certain information pertaining to the trials as well as clinical trial results after completion.
A sponsor may be able to request a special protocol assessment, or SPA, the purpose of which is to reach agreement with the FDA on the Phase 3 clinical trial protocol design and analysis that will form the primary basis of an efficacy claim. A sponsor meeting the regulatory criteria may make a specific request for an SPA and provide information regarding the design and size of the proposed clinical trial. An SPA request must be made before the proposed trial begins, and all open issues must be resolved before the trial begins. If a written agreement is reached, it will be documented and made part of the record. The agreement will be binding on the FDA and may not be changed by the sponsor or the FDA after the trial begins except with the written agreement of the sponsor and the FDA or if the FDA determines that a substantial scientific issue essential to determining the safety or efficacy of the product candidate was identified after the testing began. An SPA is not binding if new circumstances arise, and there is no guarantee that a study will ultimately be adequate to support an approval even if the study is subject to an SPA. We expect to test birinapant in several advanced stage clinical trials, including a Phase 3 clinical trial for which we may request an SPA. Having an SPA does not guarantee that a product will receive FDA approval.
Assuming successful completion of all required testing in accordance with all applicable regulatory requirements, detailed investigational product candidate information is submitted to the FDA in the form of an NDA to request market approval for the product in specified indications.
New Drug Applications
In order to obtain approval to market a drug in the U.S., a marketing application must be submitted to the FDA that provides data establishing the safety and effectiveness of the product candidate for the proposed indication. The application includes all relevant data available from pertinent pre‑ clinical studies and clinical trials, including negative or ambiguous results as well as positive findings, together with detailed information relating to the product’s chemistry, manufacturing, controls and proposed labeling, among other things. Data can come from company‑sponsored clinical trials intended to test the safety and effectiveness of a product, or from a number of alternative sources, including studies initiated by investigators. To support marketing approval, the data submitted must be sufficient in quality and quantity to establish the safety and effectiveness of the investigational product candidate to the satisfaction of the FDA.
In most cases, the NDA must be accompanied by a substantial user fee; there may be some instances in which the user fee is waived. The FDA will initially review the NDA for completeness before it accepts the NDA for filing. The FDA has 60 days from its receipt of an NDA to determine whether the application will be accepted for filing based on the agency’s threshold determination that it is sufficiently complete to permit substantive review. After the NDA submission is accepted for filing, the FDA begins an in‑depth review. The FDA has agreed to certain performance goals in the review of NDAs. Most such applications for standard review product candidates are reviewed within ten to twelve
28
months. The FDA can extend this review by three months to consider certain late‑submitted information or information intended to clarify information already provided in the submission. The FDA reviews the NDA to determine, among other things, whether the proposed product is safe and effective for its intended use, and whether the product is being manufactured in accordance with cGMP. The FDA may refer applications for novel product candidates which present difficult questions of safety or efficacy to an advisory committee, typically a panel that includes clinicians and other experts, for review, evaluation and a recommendation as to whether the application should be approved and under what conditions. The FDA is not bound by the recommendations of an advisory committee, but it considers such recommendations carefully when making decisions.
Before approving an NDA, the FDA will inspect the facilities at which the product is manufactured. The FDA will not approve the product unless it determines that the manufacturing processes and facilities are in compliance with cGMP requirements and adequate to assure consistent production of the product within required specifications. Additionally, before approving an NDA, the FDA will typically inspect one or more clinical sites to assure compliance with GCP. After the FDA evaluates the NDA and the manufacturing facilities, it issues either an approval letter or a complete response letter. A complete response letter generally outlines the deficiencies in the submission and may require substantial additional testing or information in order for the FDA to reconsider the application. If, or when, those deficiencies have been addressed to the FDA’s satisfaction in a resubmission of the NDA, the FDA will issue an approval letter. The FDA has committed to reviewing such resubmissions in two or six months depending on the type of information included. Notwithstanding the submission of any requested additional information, the FDA ultimately may decide that the application does not satisfy the regulatory criteria for approval.
An approval letter authorizes commercial marketing of the drug with specific prescribing information for specific indications. As a condition of NDA approval, the FDA may require a Risk Evaluation and Mitigation Strategy, or REMS to help ensure that the benefits of the drug outweigh the potential risks. REMS can include medication guides, communication plans for healthcare professionals, and elements to assure safe use, or ETASU. ETASU can include, but are not limited to, special training or certification for prescribing or dispensing, dispensing only under certain circumstances, special monitoring, and the use of patient registries. The requirement for a REMS can materially affect the potential market and profitability of the drug. Moreover, product approval may require substantial post‑approval testing and surveillance to monitor the drug’s safety or efficacy. Once granted, product approvals may be withdrawn if compliance with regulatory standards is not maintained or problems are identified following initial marketing.
Changes to some of the conditions established in an approved application, including changes in indications, labeling, or manufacturing processes or facilities, require submission and FDA approval of a new NDA or NDA supplement before the change can be implemented. An NDA supplement for a new indication typically requires clinical data similar to that in the original application, and the FDA uses the same procedures and actions in reviewing NDA supplements as it does in reviewing NDAs.
Advertising and Promotion
The FDA and other federal regulatory agencies closely regulate the marketing and promotion of drugs through, among other things, standards and regulations for direct‑to‑consumer advertising, communications regarding unapproved uses, industry‑sponsored scientific and educational activities, and promotional activities involving the Internet. A product cannot be commercially promoted before it is approved. After approval, product promotion can include only those claims relating to safety and effectiveness that are consistent with the labeling approved by the FDA. Healthcare providers are permitted to prescribe drugs for “off‑label” uses—that is, uses not approved by the FDA and therefore not described in the drug’s labeling—because the FDA does not regulate the practice of medicine. However, FDA regulations impose stringent restrictions on manufacturers’ communications regarding off‑label uses. Broadly speaking, a manufacturer may not promote a drug for off‑label use, but may engage in non‑promotional, balanced communication regarding off‑ label use under specified conditions. Failure to comply with applicable FDA requirements and restrictions in this area may subject a company to adverse publicity and enforcement action by the FDA, the Department of Justice, or the DOJ, or the Office of the Inspector General of the Department of Health and Human Services, or HHS, as well as state authorities. This could subject a company to a range of penalties that could have a significant commercial impact, including civil and criminal fines and agreements that materially restrict the manner in which a company promotes or distributes drug products.
29
Post‑Approval Regulations
After regulatory approval of a drug is obtained, a company is required to comply with a number of post‑approval requirements. For example, as a condition of approval of an NDA, the FDA may require post‑marketing testing, including Phase 4 clinical trials, and surveillance to further assess and monitor the product’s safety and effectiveness after commercialization. Regulatory approval of oncology products often requires that subjects in clinical trials be followed for long periods to determine the overall survival benefit of the drug. In addition, as a holder of an approved NDA, a company would be required to report adverse reactions and production problems to the FDA, to provide updated safety and efficacy information, and to comply with requirements concerning advertising and promotional labeling for any of its products. Also, quality control and manufacturing procedures must continue to conform to cGMP after approval to assure and preserve the long term stability of the drug or biological product. The FDA periodically inspects manufacturing facilities to assess compliance with cGMP, which imposes extensive procedural and substantive record keeping requirements. In addition, changes to the manufacturing process are strictly regulated, and, depending on the significance of the change, may require prior FDA approval before being implemented. FDA regulations also require investigation and correction of any deviations from cGMP and impose reporting and documentation requirements upon a company and any third‑party manufacturers that a company may decide to use. Accordingly, manufacturers must continue to expend time, money and effort in the area of production and quality control to maintain compliance with cGMP and other aspects of regulatory compliance.
We rely, and expect to continue to rely, on third parties for the production of clinical and commercial quantities of our products. Future FDA and state inspections may identify compliance issues at our facilities or at the facilities of our contract manufacturers that may disrupt production or distribution, or require substantial resources to correct. In addition, discovery of previously unknown problems with a product or the failure to comply with applicable requirements may result in restrictions on a product, manufacturer or holder of an approved NDA, including withdrawal or recall of the product from the market or other voluntary, FDA‑initiated or judicial action that could delay or prohibit further marketing.
Newly discovered or developed safety or effectiveness data may require changes to a product’s approved labeling, including the addition of new warnings and contraindications, and also may require the implementation of other risk management measures. Also, new government requirements, including those resulting from new legislation, may be established, or the FDA’s policies may change, which could delay or prevent regulatory approval of our products under development.
The Hatch‑Waxman Amendments to the FDC Act
Orange Book Listing
In seeking approval for a drug through an NDA, applicants are required to list with the FDA each patent whose claims cover the applicant’s product or a method of using the product. Upon approval of a drug, each of the patents listed in the application for the drug is then published in the FDA’s Approved Drug Products with Therapeutic Equivalence Evaluations, commonly known as the Orange Book. Drugs listed in the Orange Book can, in turn, be cited by potential generic competitors in support of approval of an abbreviated new drug application, or ANDA or 505(b)(2) application. An ANDA provides for marketing of a drug product that has the same active ingredients, generally in the same strengths and dosage form, as the listed drug and has been shown through PK testing to be bioequivalent to the listed drug. Other than the requirement for bioequivalence testing, ANDA applicants are generally not required to conduct, or submit results of, pre‑clinical studies or clinical tests to prove the safety or effectiveness of their drug product. 505(b)(2) applications provide for marketing of a drug product that may have the same active ingredients as the listed drug and contains full safety and effectiveness data as an NDA, but at least some of this information comes from studies not conducted by or for the applicant. Drugs approved in this way are commonly referred to as “generic equivalents” to the listed drug, and can often be substituted by pharmacists under prescriptions written for the original listed drug.
The ANDA or 505(b)(2) applicant is required to certify to the FDA concerning any patents listed for the approved product in the FDA’s Orange Book. Specifically, the applicant must certify that: (i) the required patent information has not been filed; (ii) the listed patent has expired; (iii) the listed patent has not expired, but will expire on a
30
particular date and approval is sought after patent expiration; or (iv) the listed patent is invalid or will not be infringed by the new product. The ANDA or 505(b)(2) applicant may also elect to submit a statement certifying that its proposed ANDA label does not contain (or carves out) any language regarding a patented method of use rather than certify to such listed method of use patent. If the applicant does not challenge the listed patents by filing a certification that the listed patent is invalid or will not be infringed by the new product, the ANDA or 505(b)(2) application will not be approved until all the listed patents claiming the referenced product have expired.
A certification that the new product will not infringe the already approved product’s listed patents, or that such patents are invalid, is called a Paragraph IV certification. If the ANDA or 505(b)(2) applicant has provided a Paragraph IV certification to the FDA, the applicant must also send notice of the Paragraph IV certification to the NDA and patent holders once the ANDA or 505(b)(2) application has been accepted for filing by the FDA. The NDA and patent holders may then initiate a patent infringement lawsuit in response to the notice of the Paragraph IV certification. The filing of a patent infringement lawsuit within 45 days of the receipt of a Paragraph IV certification automatically prevents the FDA from approving the ANDA or 505(b)(2) application until the earliest of 30 months, expiration of the patent, settlement of the lawsuit, and a decision in the infringement case that is favorable to the ANDA or 505(b)(2) applicant.
The ANDA or 505(b)(2) application also will not be approved until any applicable non‑patent exclusivity listed in the Orange Book for the referenced product has expired.
Marketing Exclusivity
Upon NDA approval of a new chemical entity, which is a drug that contains no active moiety that has been approved by the FDA in any other NDA, that drug receives five years of marketing exclusivity during which the FDA cannot approve any ANDA seeking approval of a generic version of that drug. Certain changes to a drug, such as the addition of a new indication to the package insert, are associated with a three‑year period of exclusivity during which the FDA cannot approve an ANDA for a generic drug that includes the change.
An ANDA may be submitted one year before marketing exclusivity expires if a Paragraph IV certification is filed. In this case, the 30 months stay, if applicable, runs from the end of the five years marketing exclusivity period. If there is no listed patent in the Orange Book, there may not be a Paragraph IV certification, and, thus, no ANDA may be filed before the expiration of the exclusivity period.
Patent Term Extension
After NDA approval, owners of relevant drug patents may apply for up to a five year patent extension. The allowable patent term extension is calculated as half of the drug’s testing phase—the time between IND application and NDA submission—and all of the review phase—the time between NDA submission and approval up to a maximum of five years. The time can be shortened if the FDA determines that the applicant did not pursue approval with due diligence. The total patent term after the extension may not exceed 14 years.
Many other countries also provide for patent term extensions or similar extensions of patent protection for pharmaceutical products. For example, in Japan, it may be possible to extend the patent term for up to five years and in Europe, it may be possible to obtain a supplementary patent certificate that would effectively extend patent protection for up to five years.
The Foreign Corrupt Practices Act
The Foreign Corrupt Practices Act, or FCPA prohibits any U.S. individual or business from paying, offering, or authorizing payment or offering of anything of value, directly or indirectly, to any foreign official, political party or candidate for the purpose of influencing any act or decision of the foreign entity in order to assist the individual or business in obtaining or retaining business. The FCPA also obligates companies whose securities are listed in the U.S. to comply with accounting provisions requiring such companies to maintain books and records that accurately and fairly
31
reflect all transactions of the corporation, including international subsidiaries, and to devise and maintain an adequate system of internal accounting controls for international operations.
European and Other International Government Regulation
In addition to regulations in the U.S., we will be subject to a variety of regulations in other jurisdictions governing, among other things, clinical trials and any commercial sales and distribution of our products. Whether or not we obtain FDA approval for a product, we must obtain the requisite approvals from regulatory authorities in foreign countries prior to the commencement of clinical trials or marketing of the product in those countries. Some countries outside of the U.S. have a similar process that requires the submission of a clinical trial application, or CTA, much like the IND prior to the commencement of human clinical trials. In Europe, for example, a CTA its actually an IMPD must be submitted to each country’s national health authority and an independent ethics committee, much like the FDA and IRB, respectively. Once the CTA is approved in accordance with a country’s requirements, clinical trial development may proceed.
To obtain regulatory approval to commercialize a new drug under European Union regulatory systems, we must submit a marketing authorization application, or MAA. The MAA is similar to the NDA, with the exception of, among other things, country‑specific document requirements.
For other countries outside of the European Union, such as countries in Eastern Europe, Latin America,Asia, or Australia the requirements governing the conduct of clinical trials, product licensing, pricing and reimbursement vary from country to country. Internationally, clinical trials are generally required to be conducted in accordance with GCPs, applicable regulatory requirements of each jurisdiction and the medical ethics principles that have their origin in the Declaration of Helsinki.
Compliance
During all phases of development (pre‑ and post‑marketing), failure to comply with applicable regulatory requirements may result in administrative or judicial sanctions. These sanctions could include the FDA’s imposition of a clinical hold on trials, refusal to approve pending applications, withdrawal of an approval, warning letters, product recalls, product seizures, total or partial suspension of production or distribution, product detention or refusal to permit the import or export of products, injunctions, fines, civil penalties or criminal prosecution. Any agency or judicial enforcement action could have a material adverse effect on us.
Other Special Regulatory Procedures
Orphan Drug Designation
The FDA may grant Orphan Drug Designation to drugs intended to treat a rare disease or condition that affects fewer than 200,000 individuals in the U.S., or, if the disease or condition affects more than 200,000 individuals in the U.S., there is no reasonable expectation that the cost of developing and making the drug would be recovered from sales in the U.S. In the European Union, the European Medicines Agency, or EMA’s Committee for Orphan Medicinal Products grants Orphan Drug Designation to promote the development of products that are intended for the diagnosis, prevention or treatment of life‑threatening or chronically debilitating conditions affecting not more than five in 10,000 persons in the European Union community. Additionally, designation is granted for products intended for the diagnosis, prevention or treatment of a life‑threatening, seriously debilitating or serious and chronic condition and when, without incentives, it is unlikely that sales of the drug in the European Union would be sufficient to justify the necessary investment in developing the drug.
In the U.S., Orphan Drug Designation entitles a party to financial incentives, such as opportunities for grant funding towards clinical trial costs, tax credits for certain research and user fee waivers under certain circumstances. In addition, if a product receives the first FDA approval for the indication for which it has orphan designation, the product is entitled to seven years of market exclusivity, which means the FDA may not approve any other application for the same drug for the same indication for a period of seven years, except in limited circumstances, such as a showing of
32
clinical superiority over the product with orphan exclusivity. Orphan drug exclusivity does not prevent the FDA from approving a different drug for the same disease or condition, or the same drug for a different disease or condition.
In the European Union, Orphan Drug Designation also entitles a party to financial incentives such as reduction of fees or fee waivers and ten years of market exclusivity is granted following drug approval. This period may be reduced to six years if the Orphan Drug Designation criteria are no longer met, including where it is shown that the product is sufficiently profitable not to justify maintenance of market exclusivity.
Orphan drug designation must be requested before submission of an application for marketing approval. Orphan drug designation does not convey any advantage in, or shorten the duration of the regulatory review and approval process.
Priority Review (U.S.) and Accelerated Review (European Union)
Based on results of the Phase 3 clinical trial(s) submitted in an NDA, upon the request of an applicant, a priority review designation may be granted to a product by the FDA, which sets the target date for FDA action on the application at six months from FDA filing, or eight months from the sponsor’s submission. Priority review is given where preliminary estimates indicate that a product, if approved, has the potential to provide a safe and effective therapy where no satisfactory alternative therapy exists, or a significant improvement compared to marketed products is possible. If criteria are not met for priority review, the standard FDA review period is ten months from FDA filing, or 12 months from sponsor submission. Priority review designation does not change the scientific/medical standard for approval or the quality of evidence necessary to support approval.
Under the Centralized Procedure in the European Union, the maximum timeframe for the evaluation of a MAA is 210 days (excluding “clock stops,” when additional written or oral information is to be provided by the applicant in response to questions asked by the Committee for Medicinal Products for Human Use, or CHMP). Accelerated evaluation might be granted by the CHMP in exceptional cases, when a medicinal product is expected to be of a major public health interest, defined by three cumulative criteria: the seriousness of the disease (e.g., heavy disabling or life‑threatening diseases) to be treated; the absence or insufficiency of an appropriate alternative therapeutic approach; and anticipation of high therapeutic benefit. In this circumstance, EMA ensures that the opinion of the CHMP is given within 150 days.
Healthcare Reform
In March 2010, President Obama signed one of the most significant healthcare reform measures in decades. The Affordable Care Act substantially changes the way healthcare will be financed by both governmental and private insurers, and significantly impacts the pharmaceutical industry. The Affordable Care Act will impact existing government healthcare programs and will result in the development of new programs. For example, the Affordable Care Act provides for Medicare payment for performance initiatives and improvements to the physician quality reporting system and feedback program.
Among the Affordable Care Act’s provisions of importance to the pharmaceutical industry are the following:
|
· |
an annual, nondeductible fee on any covered entity engaged in manufacturing or importing certain branded prescription drugs and biological products, apportioned among such entities in accordance with their respective market share in certain government healthcare programs; |
|
· |
an increase in the statutory minimum rebates a manufacturer must pay under the Medicaid Drug Rebate Program, retroactive to January 1, 2010, to 23.0% and 13.0% of the AMP for most branded and generic drugs, respectively; |
|
· |
expansion of healthcare fraud and abuse laws, including the False Claims Act and the Anti‑Kickback Statute, new government investigative powers, and enhanced penalties for noncompliance; |
33
|
· |
a new partial prescription drug benefit for Medicare recipients, or Medicare Part D, coverage gap discount program, in which manufacturers must agree to offer 50.0% point‑of‑sale discounts off negotiated prices of applicable brand drugs to eligible beneficiaries during their coverage gap period, as a condition for the manufacturers’ outpatient drugs to be covered under Medicare Part D; |
|
· |
extension of manufacturers’ Medicaid rebate liability to covered drugs dispensed to individuals who are enrolled in Medicaid managed care organizations; |
|
· |
expansion of eligibility criteria for Medicaid programs by, among other things, allowing states to offer Medicaid coverage to additional individuals beginning in 2014 and by adding new mandatory eligibility categories for individuals with income at or below 133.0% of the Federal Poverty Level, thereby potentially increasing manufacturers’ Medicaid rebate liability; |
|
· |
expansion of the entities eligible for discounts under the Public Health Service pharmaceutical pricing program; |
|
· |
new requirements to report annually specified financial arrangements with physicians and teaching hospitals, as defined in the Affordable Care Act and its implementing regulations, including reporting any “payments or transfers of value” made or distributed to prescribers, teaching hospitals, and other healthcare providers and reporting any ownership and investment interests held by physicians and other healthcare providers and their immediate family members and applicable group purchasing organizations during the preceding calendar year, with data collection to be required beginning August 1, 2013 and reporting to the Centers for Medicare and Medicaid Services to be required by March 31, 2014 and by the 90th day of each subsequent calendar year; |
|
· |
a new requirement to annually report drug samples that manufacturers and distributors provide to physicians; |
|
· |
a new Patient‑Centered Outcomes Research Institute to oversee, identify priorities in, and conduct comparative clinical effectiveness research, along with funding for such research; and |
|
· |
a mandatory nondeductible payment for employers with 50 or more full‑time employees (or equivalents) who fail to provide certain minimum health insurance coverage for such employees and their dependents, beginning in 2015 (pursuant to relief enacted by the Treasury Department). |
The Affordable Care Act also establishes an Independent Payment Advisory Board, or IPAB, to reduce the per capita rate of growth in Medicare spending. Beginning in 2014, IPAB is mandated to propose changes in Medicare payments if it determines that the rate of growth of Medicare expenditures exceeds target growth rates. The IPAB has broad discretion to propose policies to reduce expenditures, which may have a negative impact on payment rates for pharmaceutical products. A proposal made by the IPAB is required to be implemented by the U.S. federal government’s Centers for Medicare & Medicaid Services unless Congress adopts a proposal with savings greater than those proposed by the IPAB. IPAB proposals may impact payments for physician and free‑standing services beginning in 2015 and for hospital services beginning in 2020.
We anticipate that the Affordable Care Act will result in additional downward pressure on coverage and the price that we receive for any approved product, and could seriously harm our business. Any reduction in reimbursement from Medicare and other government programs may result in a similar reduction in payments from private payors. The implementation of cost containment measures or other healthcare reforms may prevent us from being able to generate revenue, attain profitability, or commercialize our products. In addition, it is possible that there will be further legislation or regulation that could harm our business, financial condition and results of operations.
34
Coverage and Reimbursement
Significant uncertainty exists as to the coverage and reimbursement status of any drug products for which we obtain regulatory approval. In the U.S. and markets in other countries, sales of any products for which we receive regulatory approval for commercial sale will depend in part on the availability of reimbursement from third‑party payors. Third‑party payors include government health administrative authorities, managed care providers, private health insurers and other organizations. The process for determining whether a payor will provide coverage for a drug product may be separate from the process for setting the price or reimbursement rate that the payor will pay for the drug product. Third‑party payors may limit coverage to specific drug products on an approved list, or formulary, which might not include all of the FDA‑approved drugs for a particular indication. Third‑party payors are increasingly challenging the price and examining the medical necessity and cost‑ effectiveness of medical products and services, in addition to their safety and efficacy. We may need to conduct expensive pharmacoeconomic studies in order to demonstrate the medical necessity and cost‑effectiveness of our products, in addition to the costs required to obtain FDA approvals. Our products may not be considered medically necessary or cost‑effective. A payor’s decision to provide coverage for a drug product does not imply that an adequate reimbursement rate will be approved. Adequate third‑party reimbursement may not be available to enable us to maintain price levels sufficient to realize an appropriate return on our investment in product development.
In 2003, the U.S. Congress enacted legislation providing Medicare Part D, which became effective at the beginning of 2006. Government payment for some of the costs of prescription drugs may increase demand for any products for which we receive marketing approval. However, to obtain payments under this program, we would be required to sell products to Medicare recipients through prescription drug plans operating pursuant to this legislation. These plans will likely negotiate discounted prices for our products. Federal, state and local governments in the U.S. continue to consider legislation to limit the growth of healthcare costs, including the cost of prescription drugs. Future legislation could limit payments for pharmaceuticals such as the drug candidates that we are developing.
Different pricing and reimbursement schemes exist in other countries. In the European Union, governments influence the price of pharmaceutical products through their pricing and reimbursement rules and control of national healthcare systems that fund a large part of the cost of those products to consumers. Some jurisdictions operate positive and negative list systems under which products may only be marketed once a reimbursement price has been agreed upon. To obtain reimbursement or pricing approval, some of these countries may require the completion of clinical trials that compare the cost‑effectiveness of a particular product candidate to currently available therapies. Other member states allow companies to fix their own prices for medicines, but monitor and control company profits. The downward pressure on healthcare costs in general, particularly prescription drugs, has become more intense. As a result, increasingly high barriers are being erected to the entry of new products. The European Union provides options for its member states to restrict the range of medicinal products for which their national health insurance systems provide reimbursement and to control the prices of medicinal products for human use. A member state may approve a specific price for the medicinal product or it may instead adopt a system of direct or indirect controls on the profitability of the company placing the medicinal product on the market. We may face competition for our products from lower‑priced products in foreign countries that have placed price controls on pharmaceutical products. In addition, in some countries, cross‑border imports from low‑priced markets exert a commercial pressure on pricing within a country.
The marketability of any products for which we receive regulatory approval for commercial sale may suffer if the government and third‑party payors fail to provide adequate coverage and reimbursement. In addition, an increasing emphasis on managed care in the U.S. has increased and will continue to increase the pressure on pharmaceutical pricing. Coverage policies and third‑party reimbursement rates may change at any time.
Even if favorable coverage and reimbursement status is attained for one or more products for which we receive regulatory approval, less favorable coverage policies and reimbursement rates may be implemented in the future.
Other Healthcare Laws and Compliance Requirements
The federal Anti‑Kickback Statute prohibits, among other things, knowingly and willfully offering, paying, soliciting or receiving remuneration to induce or in return for purchasing, leasing, ordering or arranging for the purchase,
35
lease or order of any healthcare item or service reimbursable under Medicare, Medicaid or other federally financed healthcare programs. This statute has been interpreted to apply to arrangements between pharmaceutical manufacturers on one hand and prescribers, purchasers, and formulary managers on the other. Although there are a number of statutory exemptions and regulatory safe harbors protecting some business arrangements from prosecution, the exemptions and safe harbors are drawn narrowly and practices that involve remuneration intended to induce prescribing, purchasing or recommending may be subject to scrutiny if they do not qualify for an exemption or safe harbor. Our practices may not in all cases meet all of the criteria for safe harbor protection from federal Anti‑Kickback Statute liability. The reach of the Anti‑Kickback Statute was broadened by the Affordable Care Act, which, among other things, amends the intent requirement of the federal Anti‑Kickback Statute. Pursuant to the statutory amendment, a person or entity no longer needs to have actual knowledge of this statute or specific intent to violate it in order to have committed a violation. In addition, the Affordable Care Act provides that the government may assert that a claim including items or services resulting from a violation of the federal Anti‑Kickback Statute constitutes a false or fraudulent claim for purposes of the civil False Claims Act (discussed below) or the civil monetary penalties statute, which imposes penalties against any person who is determined to have presented or caused to be presented a claim to a federal health program that the person knows or should know is for an item or service that was not provided as claimed or is false or fraudulent.
The federal False Claims Act prohibits any person from knowingly presenting, or causing to be presented, a false claim for payment to the federal government or knowingly making, using, or causing to be made or used a false record or statement material to a false or fraudulent claim to the federal government. As a result of a modification made by the Fraud Enforcement and Recovery Act of 2009, a claim includes “any request or demand” for money or property presented to the U.S. government. Recently, several pharmaceutical and other healthcare companies have been prosecuted under these laws for allegedly providing free product to customers with the expectation that the customers would bill federal programs for the product. Other companies have been prosecuted for causing false claims to be submitted because of the companies’ marketing of the product for unapproved, and thus non‑reimbursable, uses. The Federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, created new federal criminal statutes that prohibit knowingly and willfully executing a scheme to defraud any healthcare benefit program, including private third‑party payors and knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false, fictitious or fraudulent statement in connection with the delivery of or payment for healthcare benefits, items or services. Also, many states have similar fraud and abuse statutes or regulations that apply to items and services reimbursed under Medicaid and other state programs, or, in several states, apply regardless of the payor.
In addition, we may be subject to data privacy and security regulation by both the federal government and the states in which we conduct our business. HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act of 2009, or HITECH, and its implementing regulations, imposes requirements relating to the privacy, security and transmission of individually identifiable health information. Among other things, HITECH makes HIPAA’s privacy and security standards directly applicable to “business associates”—independent contractors or agents of covered entities that receive or obtain protected health information in connection with providing a service on behalf of a covered entity. HITECH also increased the civil and criminal penalties that may be imposed against covered entities, business associates and possibly other persons, and gave state attorneys general new authority to file civil actions for damages or injunctions in federal courts to enforce the federal HIPAA laws and seek attorney’s fees and costs associated with pursuing federal civil actions. In addition, state laws govern the privacy and security of health information in specified circumstances, many of which differ from each other in significant ways and may not have the same effect, thus complicating compliance efforts.
In the U.S., our activities are potentially subject to additional regulation by various federal, state and local authorities in addition to the FDA, including the Centers for Medicare and Medicaid Services, other divisions of HHS (for example, the Office of Inspector General), the DOJ and individual U.S. Attorney offices within the DOJ, and state and local governments. If a drug product is reimbursed by Medicare or Medicaid, pricing and rebate programs must comply with, as applicable, the Medicare Modernization Act as well as the Medicaid rebate requirements of the Omnibus Budget Reconciliation Act of 1990, or the OBRA, and the Veterans Health Care Act of 1992, or the VHCA, each as amended. Among other things, the OBRA requires drug manufacturers to pay rebates on prescription drugs to state Medicaid programs and empowers states to negotiate rebates on pharmaceutical prices, which may result in prices for our future products that will likely be lower than the prices we might otherwise obtain. If products are made available to authorized users of the Federal Supply Schedule of the General Services Administration, additional laws and
36
requirements apply. Under the VHCA, drug companies are required to offer some drugs at a reduced price to a number of federal agencies including the U.S. Department of Veterans Affairs and the U.S. Department of Defense, or DoD, the Public Health Service and some private Public Health Service designated entities in order to participate in other federal funding programs including Medicaid. Recent legislative changes require that discounted prices be offered for specified DoD purchases for its TRICARE program via a rebate system. Participation under the VHCA requires submission of pricing data and calculation of discounts and rebates pursuant to complex statutory formulas, as well as the entry into government procurement contracts governed by the Federal Acquisition Regulation.
Because of the breadth of these laws and the narrowness of available statutory and regulatory exemptions, it is possible that some of our business activities could be subject to challenge under one or more of such laws. If our operations are found to be in violation of any of the federal and state laws described above or any other governmental regulations that apply to us, we may be subject to penalties, including criminal and significant civil monetary penalties, damages, fines, imprisonment, exclusion from participation in government programs, injunctions, recall or seizure of products, total or partial suspension of production, denial or withdrawal of pre‑marketing product approvals, private “qui tam” actions brought by individual whistleblowers in the name of the government or refusal to allow us to enter into supply contracts, including government contracts, and the curtailment or restructuring of our operations, any of which could adversely affect our ability to operate our business and our results of operations. To the extent that any of our products are sold in a foreign country, we may be subject to similar foreign laws and regulations, which may include, for instance, applicable post‑ marketing requirements, including safety surveillance, anti‑fraud and abuse laws, and implementation of corporate compliance programs and reporting of payments or transfers of value to healthcare professionals.
In order to distribute products commercially, we must comply with state laws that require the registration of manufacturers and wholesale distributors of pharmaceutical products in a state, including, in some states, manufacturers and distributors who ship products into the state even if such manufacturers or distributors have no place of business within the state. Some states also impose requirements on manufacturers and distributors to establish the pedigree of product in the chain of distribution, including some states that require manufacturers and others to adopt new technology capable of tracking and tracing product as it moves through the distribution chain. Several states have enacted legislation requiring pharmaceutical companies to, among other things, establish marketing compliance programs, file periodic reports with the state, make periodic public disclosures on sales, marketing, pricing, clinical trials and other activities, and/or register their sales representatives, as well as to prohibit pharmacies and other healthcare entities from providing specified physician prescribing data to pharmaceutical companies for use in sales and marketing, and to prohibit other specified sales and marketing practices. All of our activities are potentially subject to federal and state consumer protection and unfair competition laws.
CORPORATE INFORMATION
We were originally incorporated in July 2001 as Apop Corp., a New Jersey corporation. Pursuant to a merger effective as of October 2003 with and into our wholly owned subsidiary, we became a Delaware corporation and commenced operations in 2003. Our name was changed to Apop Corporation in March 2004, to Gentara Corporation in June 2004 and to TetraLogic Pharmaceuticals Corporation in January 2006.
Our executive offices are located at 343 Phoenixville Pike, Malvern, PA 19355 and our telephone number is (610) 889‑9900.
We have registered TetraLogic Pharmaceuticals as a U.S. trademark. All other trademarks, trade names or service marks referred to in this annual report are the property of their respective owners.
AVAILABLE INFORMATION
All periodic and current reports, registration statements, code of conduct and other material that we are required to file with the Securities and Exchange Commission, or the SEC, including our annual report on Form 10‑K, quarterly reports on Form10‑Q, current reports on Form 8‑K, and amendments to those reports filed or furnished pursuant to Section 13(a) of the Exchange Act, are or will be available free of charge through our investor relations page at
37
www.tlog.com. Such documents are available as soon as reasonably practicable after electronic filing of the material with the SEC. The inclusion of our website address above and elsewhere in this annual report is, in each case, intended to be an inactive textual reference only and not an active hyperlink to our website. The information contained in, or that can be accessed through, our website is not part of this annual report.
The public may also read and copy any materials filed by the Company with the SEC at the SEC’s Public Reference Room at 100 F Street, NE, Washington, DC 20549. The public may obtain information on the operation of the Public Reference Room by calling the SEC at 1‑800‑SEC‑0330. The SEC maintains an Internet site, www.sec.gov, which contains reports, proxy and information statements, and other information regarding issuers that file such information electronically with the SEC.
From time to time we will post updated versions of our corporate presentation slides on our website. Such updates may include information regarding our pipeline, including adjustments of expected timing of certain events, and other information about the progress of our business.
EMPLOYEES
As of December 31, 2014, we had 30 full‑time employees and one part‑time employee, of whom 10 hold Ph.D. degrees and four hold M.D. (or international M.D.‑equivalent) degrees. We have no collective bargaining agreements with our employees and none are represented by labor unions. We have not experienced any work stoppages. We believe our relationship with our employees is satisfactory.
You should carefully consider the risks described below, together with the other information contained in this report, including our financial statements and the related notes appearing in this report. We cannot assure you that any of the events discussed in the risk factors below will not occur. These risks could have a material and adverse impact on our business, results of operations, financial condition and cash flows and if so our future prospects would likely be materially and adversely affected. If any of such events were to happen, the trading price of our common stock could decline, and you could lose all or part of your investment. You should understand that it is not possible to predict or identify all such risks. Consequently, you should not consider the following to be a complete discussion of all potential risks or uncertainties.
Risks Related to Our Business and Industry
Our future success is dependent on the successful clinical development, regulatory approval and commercialization of our product candidates, birinapant and SHAPE, which are currently undergoing clinical trials and will require significant capital resources and years of additional clinical development effort.
We do not have any products that have gained regulatory approval. Currently, our only clinical‑stage product candidates are birinapant and SHAPE. As a result, our business is dependent on our ability to successfully complete clinical development of, obtain regulatory approval for, and, if approved, to successfully commercialize our product candidates in a timely manner. We cannot commercialize our product candidates in the U.S. without first obtaining regulatory approval from the FDA; similarly, we cannot commercialize our product candidates outside of the U.S. without obtaining regulatory approval from comparable foreign regulatory authorities. Before obtaining regulatory approvals for the commercial sale of our product candidates for any target indication, we must demonstrate with substantial evidence gathered in pre‑clinical studies and well‑controlled clinical trials, and, with respect to approval in the U.S., to the satisfaction of the FDA, that our product candidates are safe and effective for use for that target indication and that the manufacturing facilities, processes and controls are adequate. Even if our product candidates were to successfully obtain approval from the FDA and comparable foreign regulatory authorities, any approval might contain significant limitations related to use restrictions for specified age groups, warnings, precautions or contraindications, or may be subject to burdensome post‑approval study or risk management requirements. If we are unable to obtain regulatory approval for our product candidates in one or more jurisdictions, or any approval contains significant limitations, we may not be able to obtain sufficient funding or generate sufficient revenue to continue the development of
38
any other product candidate that we may discover, in‑license, develop or acquire in the future. Furthermore, even if we obtain regulatory approval for our product candidates, we will still need to develop a commercial organization, establish commercially viable pricing and obtain approval for adequate reimbursement from third‑party and government payors. If we are unable to successfully commercialize our product candidates, we may not be able to earn sufficient revenues to continue our business.
Because the results of pre‑clinical studies or earlier clinical trials are not necessarily predictive of future results, our product candidates may not have favorable results in later clinical trials or receive regulatory approval.
Success in pre‑clinical studies and early clinical trials does not ensure that later clinical trials will generate adequate data to demonstrate the efficacy and safety of our product candidates. A number of companies in the pharmaceutical and biotechnology industries, including those with greater resources and experience, have suffered significant setbacks in clinical trials, even after seeing promising results in earlier clinical trials. Despite the results reported in earlier clinical trials for our product candidates, we do not know whether the clinical trials we may conduct will demonstrate adequate efficacy and safety to result in regulatory approval to market our product candidates in any particular jurisdiction. If later‑stage clinical trials do not produce favorable results, our ability to achieve regulatory approval for our product candidates may be adversely impacted.
The therapeutic efficacy of our product candidates is unproven in humans, and we may not be able to successfully develop and commercialize our product candidates pursuant to these programs.
Birinapant and SHAPE are novel compounds and their potential benefit as a therapeutic cancer or antiviral drug, as the case may be, is unproven. Our ability to generate revenues from our product candidates, which we do not expect will occur in the short term, if ever, will depend heavily on their successful development and commercialization after approval, if achieved, which is subject to many potential risks. For example, birinapant or SHAPE may not prove to be an effective inhibitor of the cancer or viral targets, as the case may be, that each is being designed to act against and may not demonstrate in study subjects any or all of the pharmacological data points that may have been demonstrated in pre‑clinical studies or earlier clinical trials. Birinapant or SHAPE may interact with human biological systems in unforeseen, ineffective or harmful ways. If birinapant or SHAPE is associated with undesirable side effects or has characteristics that are unexpected, we may need to abandon its development or limit development to certain uses or subpopulations in which the undesirable side effects or other characteristics are less prevalent, less severe or more acceptable from a risk‑benefit perspective. Many compounds that initially showed promise in early stage testing for treating cancer or infectious diseases have later been found to cause side effects that prevented further development of such compounds. As a result of these and other risks described herein that are inherent in the development of novel therapeutic agents, we may never successfully develop, enter into or maintain third‑party licensing or collaboration transactions with respect to, or successfully commercialize our product candidates, in which case we will not achieve profitability and the value of our stock may decline.
Clinical development of product candidates involves a lengthy and expensive process with an uncertain outcome.
Clinical testing is expensive, can take many years to complete, and its outcome is inherently uncertain. Failure can occur at any time during the clinical trial process. Product candidates in later stages of clinical trials may fail to show the desired safety and efficacy traits despite having progressed through pre‑clinical studies and early clinical trials.
|
· |
We may experience delays in our ongoing or future clinical trials and we do not know whether planned clinical trials will begin or enroll subjects on time, need to be redesigned or be completed on schedule, if at all. There can be no assurance that the FDA or other foreign regulator authority will not put clinical trials of birinapant, SHAPE or any other product candidates on clinical hold now or in the future. Clinical trials may be delayed, suspended or prematurely terminated for a variety of reasons, such as: |
|
· |
delay or failure in reaching agreement with the FDA or a comparable foreign regulatory authority on a trial design that we are able to execute; |
39
|
· |
delay or failure in obtaining authorization to commence a trial or inability to comply with conditions imposed by a regulatory authority regarding the scope or design of a clinical trial; |
|
· |
delay or failure in reaching agreement on acceptable terms with prospective contract research organizations, or CROs, and clinical trial sites, the terms of which can be subject to extensive negotiation and may vary significantly among different CROs and trial sites; |
|
· |
delay or failure in obtaining institutional review board, or IRB, approval or the approval of other reviewing entities, including comparable foreign regulatory authorities, to conduct a clinical trial at each site; |
|
· |
withdrawal of clinical trial sites from our clinical trials as a result of changing standards of care or the ineligibility of a site to participate in our clinical trials; |
|
· |
delay or failure in recruiting and enrolling suitable study subjects to participate in a trial; |
|
· |
delay or failure in study subjects completing a trial or returning for post‑treatment follow‑up; |
|
· |
clinical sites and investigators deviating from trial protocol, failing to conduct the trial in accordance with regulatory requirements, or dropping out of a trial; |
|
· |
inability to identify and maintain a sufficient number of trial sites, many of which may already be engaged in other clinical trial programs, including some that may be for competing product candidates with the same indication; |
|
· |
failure of our third‑party clinical trial managers to satisfy their contractual duties or meet expected deadlines; |
|
· |
delay or failure in adding new clinical trial sites; |
|
· |
ambiguous or negative interim results or results that are inconsistent with earlier results; |
|
· |
feedback from the FDA, the IRB, data safety monitoring boards, or a comparable foreign regulatory authority, or results from earlier stage or concurrent pre‑clinical studies and clinical trials, that might require modification to the protocol for the trial; |
|
· |
decision by the FDA, the IRB, a comparable foreign regulatory authority, or us, or recommendation by a data safety monitoring board or comparable foreign regulatory authority, to suspend or terminate clinical trials at any time for safety issues or for any other reason; |
|
· |
unacceptable risk‑benefit profile, unforeseen safety issues or adverse side effects or adverse events; |
|
· |
failure of a product candidate to demonstrate any benefit; |
|
· |
difficulties in manufacturing or obtaining from third parties sufficient quantities of a product candidate for use in clinical trials; |
|
· |
lack of adequate funding to continue the clinical trial, including the incurrence of unforeseen costs due to enrollment delays, requirements to conduct additional clinical studies or increased expenses associated with the services of our CROs and other third parties; or |
|
· |
changes in governmental regulations or administrative actions or lack of adequate funding to continue the clinical trial. |
40
Study subject enrollment, a significant factor in the timing of clinical trials, is affected by many factors including the size and nature of the subject population, the proximity of subjects to clinical sites, the eligibility criteria for the trial, the design of the clinical trial, ability to obtain and maintain subject consents, risk that enrolled subjects will drop out before completion, competing clinical trials and clinicians’ and subjects’ perceptions as to the potential advantages of the product candidate being studied in relation to other available therapies, including any new drugs that may be approved or product candidates that may be studied in competing clinical trials for the indications we are investigating. We rely on CROs and clinical trial sites to ensure the proper and timely conduct of our clinical trials, and while we have agreements governing their committed activities, we have limited influence over their actual performance.
If we experience delays in the completion of any clinical trial of our product candidates, their commercial prospects might be harmed, and our ability to generate product revenues from our product candidates, if approved, will be delayed. In addition, any delays in completing our clinical trials will increase our costs, slow down our development and approval process for our product candidates and jeopardize our ability to commence product sales and generate revenues. In addition, many of the factors that could cause a delay in the commencement or completion of clinical trials may also ultimately lead to the denial of regulatory approval of our product candidates.
Our commercial success depends upon attaining significant market acceptance of our product candidates, if approved, among physicians, patients, healthcare payors and the major operators of cancer or infectious disease clinics.
Even if we obtain regulatory approval for our product candidates, they may not gain market acceptance among physicians, healthcare payors, patients or the medical community. Market acceptance of our product candidates, if we receive approval, depends on a number of factors, including the:
|
· |
efficacy and safety of such product candidate or such product candidate administered with other drugs, each as demonstrated in clinical trials and post‑marketing experience; |
|
· |
clinical indications for which such product candidate is approved; |
|
· |
acceptance by physicians, major operators of cancer or infectious disease clinics and patients of the product candidate as a safe and effective treatment; |
|
· |
potential and perceived advantages of such product candidate over alternative treatments; |
|
· |
safety of such product candidate seen in a broader patient group, including its use outside the approved indications should physicians choose to prescribe for such uses; |
|
· |
prevalence and severity of any side effects; |
|
· |
product labeling or product insert requirements of the FDA or other regulatory authorities; |
|
· |
timing of market introduction of such product candidate as well as competitive products; |
|
· |
cost of treatment in relation to alternative treatments; |
|
· |
availability of coverage and adequate reimbursement and pricing by third‑party payors and government authorities; |
|
· |
relative convenience and ease of administration; and |
|
· |
effectiveness of our sales and marketing efforts. |
41
Moreover, if either birinapant or SHAPE is approved but fails to achieve market acceptance among physicians, patients, or healthcare payors or the products or product candidates that are being administered with birinapant are restricted, withdrawn or recalled or fail to be approved, as the case may be, we may not be able to generate significant revenues, which would compromise our ability to become profitable.
Our commercial success could depend upon the continued marketing of an approved product, or the approval of a product candidate, to be administered with birinapant.
Some of our clinical trials involve products marketed, or product candidates being developed, by other pharmaceutical companies and some of the indications for which we are developing birinapant involve its use in combination with these other products and product candidates. These products or product candidates may be administered in a clinical trial in combination with birinapant. In the event that any of these pharmaceutical companies have unforeseen issues that negatively impact their clinical development or marketing approval for these products and product candidates or otherwise negatively affect their ability to continue to clinically develop or market these products and product candidates, our ability to complete our applicable clinical trials and/or evaluate clinical results and, ultimately, our ability to receive regulatory approval for birinapant for the indications we are pursuing may also be negatively impacted. As a result, this could adversely affect our ability to file for, gain or maintain regulatory approvals for birinapant on a timely basis, if at all.
Our product candidates may cause undesirable side effects or have other properties that could delay or prevent their regulatory approval, limit the commercial profile of an approved label, or result in significant negative consequences following any marketing approval.
Undesirable side effects caused by our product candidates could cause us or regulatory authorities to interrupt, delay or halt clinical trials and could result in a restrictive label or the delay or denial of regulatory approval by the FDA or other comparable foreign regulatory authorities. For example, even though birinapant has generally been well tolerated by subjects in our earlier‑stage clinical trials, in some cases there were side effects, some of which were severe. In clinical trials where birinapant was administered as monotherapy, treatment‑related side effects that were Grade 3 or Grade 4, meaning they were more than mild or moderate in severity, include increase of serum levels of amylase or lipase protein concentrations, fatigue, headache, hypophosphatemia, lymphopenia, nausea, rash, thrombocytopenia and vomiting. Treatment‑related Grade 3 or Grade 4 side effects observed in trials where birinapant was administered with other cancer therapies included abdominal cellulitis, abdominal pain, alanine aminotransferase increase, amylase increase, anemia, aspartate aminotransferase increase, caecitis, dehydration, diarrhea, dyspnea, fatigue, febrile neutropenia, granulocytopenia, headache, hyponatraemia, hypotension, leukopenia, lipase increase, lymphocytopenia, mucositis, nausea, neutropenia, pancytopenia, sepsis, stomatitis, stress cardiomyopathy, thrombocytopenia, vomiting, and weight decrease. In dose escalation studies of birinapant combined with chemotherapies that deliberately sought to define the dose‑limiting toxicities, and thus the maximum tolerated dose, the most common dose‑limiting side effect was Grade 2 Bell’s Palsy, or weakness or inability to control facial muscles on one side of the face. Minor side effects have been reported in some cases in clinical trials of SHAPE, including blisters, erythema, itching and burning sensations.
As a result of these side effects or further safety or toxicity issues that we may experience in our clinical trials in the future, we may not receive approval to market our product candidates, which could prevent us from ever generating revenues or achieving profitability. Results of our trials could reveal an unacceptably high severity and prevalence of side effects. In such an event, our trials could be suspended or terminated and the FDA or comparable foreign regulatory authorities could order us to cease further development of or deny approval of our product candidates for any or all targeted indications. The study drug‑related side effects could affect study subject recruitment or the ability of enrolled subjects to complete the trial or result in potential product liability claims.
Additionally, if our product candidates receive marketing approval, and we or others later identify undesirable side effects caused by our product candidates, a number of potentially significant negative consequences could result, including:
|
· |
we may be forced to suspend marketing of the product candidate; |
42
|
· |
regulatory authorities may withdraw their approvals of the product candidate; |
|
· |
regulatory authorities may require additional warnings on the label that could diminish the usage or otherwise limit the commercial success of the product candidate; |
|
· |
we may be required to conduct post‑market studies; |
|
· |
we could be sued and held liable for harm caused to subjects or patients; and |
|
· |
our reputation may suffer. |
Any of these events could prevent us from achieving or maintaining market acceptance of our product candidates, if approved.
Even if our product candidates receive regulatory approval, we may still face future development and regulatory difficulties.
Even if we obtain regulatory approval for our product candidates, they would be subject to ongoing requirements by the FDA and comparable foreign regulatory authorities governing the manufacture, quality control, further development, labeling, packaging, storage, distribution, safety surveillance, import, export, advertising, promotion, recordkeeping and reporting of safety and other post‑market information. The safety profile of our product candidates will continue to be closely monitored by the FDA and comparable foreign regulatory authorities after approval. If new safety information becomes available after approval of our product candidates, the FDA or comparable foreign regulatory authorities may require labeling changes or establishment of a Risk Evaluation and Mitigation Strategy, or similar strategy, impose significant restrictions on indicated uses or marketing, or impose ongoing requirements for potentially costly post‑approval studies or post‑market surveillance. For example, the label ultimately approved for our product candidates, if they achieve marketing approval, may include restrictions on use.
In addition, manufacturers of drug products and their facilities are subject to continual review and periodic inspections by the FDA and other regulatory authorities for compliance with current good manufacturing practices, or cGMP, and other regulations. If we or a regulatory agency discover previously unknown problems with a product, such as adverse events of unanticipated severity or frequency, or problems with the facility where the product is manufactured, a regulatory agency may impose restrictions on that product, the manufacturing facility or us, including requiring recall or withdrawal of the product from the market or suspension of manufacturing. If we, birinapant, SHAPE or the manufacturing facilities for our product candidates fail to comply with applicable regulatory requirements, a regulatory agency may:
|
· |
issue warning letters or untitled letters; |
|
· |
mandate modifications to promotional materials or require us to provide corrective information to healthcare practitioners; |
|
· |
require us to enter into a consent decree, which can include imposition of various fines, reimbursements for inspection costs, required due dates for specific actions and penalties for noncompliance; |
|
· |
seek an injunction or impose civil or criminal penalties or monetary fines; |
|
· |
suspend or withdraw regulatory approval; |
|
· |
suspend any ongoing clinical trials; |
|
· |
refuse to approve pending applications or supplements to applications filed by us; |
43
|
· |
suspend or impose restrictions on operations, including costly new manufacturing requirements; or |
|
· |
seize or detain products, refuse to permit the import or export of products, or require us to initiate a product recall. |
The occurrence of any event or penalty described above may inhibit or preclude our ability to commercialize our product candidates and generate revenue.
Advertising and promotion of any product candidate that obtains approval in the U.S. will be heavily scrutinized by, among others, the FDA, the Department of Justice, or DOJ, the Office of the Inspector General of the Department of Health and Human Services, or HHS, state attorneys general, members of Congress and the public. Violations, including promotion of our products for unapproved or off‑label uses, are subject to enforcement letters, inquiries and investigations, and civil and criminal sanctions by the FDA or other government agencies. Additionally, advertising and promotion of any product candidate that obtains approval outside of the U.S. will be heavily scrutinized by comparable foreign regulatory authorities.
In the U.S., engaging in impermissible promotion of our product candidates for off‑label uses can also subject us to false claims litigation under federal and state statutes, and other litigation and/or investigation, which can lead to civil and criminal penalties and fines and agreements that materially restrict the manner in which we promote or distribute our drug products. These false claims statutes include the federal False Claims Act, which allows any individual to bring a lawsuit against a pharmaceutical company on behalf of the federal government alleging submission of false or fraudulent claims, or causing to present such false or fraudulent claims, for payment by a federal program such as Medicare or Medicaid. If the government prevails in the lawsuit, the individual will share in any fines or settlement funds. Since 2004, these False Claims Act lawsuits against pharmaceutical companies have increased significantly in volume and breadth, leading to several substantial civil and criminal settlements based on certain sales practices promoting off‑label drug uses. This increasing focus and scrutiny has increased the risk that a pharmaceutical company will have to defend a false claim action, pay settlement fines or restitution, agree to comply with burdensome reporting and compliance obligations, and be excluded from the Medicare, Medicaid and other federal and state healthcare programs. If we do not lawfully promote our approved products, we may become subject to such litigation and/or investigation and, if we are not successful in defending against such actions, those actions could compromise our ability to become profitable.
Failure to obtain regulatory approval in international jurisdictions would prevent our product candidates from being marketed abroad.
In order to market and sell our products in the European Union and many other jurisdictions, including Japan and South Korea, we must obtain separate marketing approvals and comply with numerous and varying regulatory requirements. The approval procedure varies among countries and can involve additional testing. The time required to obtain approval may differ substantially from that required to obtain FDA approval. The regulatory approval process outside the U.S. generally includes all of the risks associated with obtaining FDA approval. In addition, in many countries outside the U.S., it is required that the product be approved for reimbursement before the product can be approved for sale in that country. We may not obtain approvals from regulatory authorities outside the U.S. on a timely basis, if at all. Approval by the FDA does not ensure approval by regulatory authorities in other countries or jurisdictions, and approval by one regulatory authority outside the U.S. does not ensure approval by regulatory authorities in other countries or jurisdictions or by the FDA. We may not be able to file for marketing approvals and may not receive necessary approvals to commercialize our products in any market. If we are unable to obtain approval of our product candidates by regulatory authorities in the European Union, Japan, South Korea or another country or jurisdiction, the commercial prospects of our product candidates may be significantly diminished and our business prospects could decline.
44
Current and future legislation, including potentially unfavorable pricing regulations or other healthcare reform initiatives, may increase the difficulty and cost for us to obtain marketing approval of and commercialize our product candidates and affect the prices we may obtain.
The regulations that govern, among other things, marketing approvals, coverage, pricing and reimbursement for new drug products vary widely from country to country. In the U.S. and some foreign jurisdictions, there have been a number of legislative and regulatory changes and proposed changes regarding the healthcare system that could prevent or delay marketing approval of our product candidates, restrict or regulate post‑approval activities and affect our ability to successfully sell our product candidates if we may obtain marketing approval.
In the U.S., the Medicare Prescription Drug, Improvement, and Modernization Act of 2003, or Medicare Modernization Act, changed the way Medicare covers and pays for pharmaceutical products. The legislation expanded Medicare coverage for drug purchases by the elderly and introduced a new reimbursement methodology based on average sales prices for physician administered drugs. In recent years, Congress has considered further reductions in Medicare reimbursement for drugs administered by physicians. The Centers for Medicare and Medicaid Services, the agency that runs the Medicare program, also has the authority to revise reimbursement rates and to implement coverage restrictions for some drugs. Cost reduction initiatives and changes in coverage implemented through legislation or regulation could decrease utilization of and reimbursement for any approved products, which in turn would affect the price we can receive for those products. While the Medicare Modernization Act and Medicare regulations apply only to drug benefits for Medicare beneficiaries, private payors often follow Medicare coverage policy and payment limitations in setting their own reimbursement rates. Therefore, any reduction in reimbursement that results from federal legislation or regulation may result in a similar reduction in payments from private payors.
In March 2010, President Obama signed into law the Patient Protection and Affordable Care Act and the Health Care and Education Affordability Reconciliation Act of 2010, or the Affordable Care Act, a sweeping law intended to broaden access to health insurance, reduce or constrain the growth of healthcare spending, enhance remedies against fraud and abuse, add new transparency requirements for healthcare and health insurance industries, impose new taxes and fees on pharmaceutical and medical device manufacturers and impose additional health policy reforms. The Affordable Care Act expanded manufacturers’ rebate liability to include covered drugs dispensed to individuals who are enrolled in Medicaid managed care organizations, increased the minimum rebate due for innovator drugs from 15.1% of average manufacturer price, or AMP, to 23.1% of AMP and capped the total rebate amount for innovator drugs at 100.0% of AMP. The Affordable Care Act and subsequent legislation also changed the definition of AMP. Furthermore, the Affordable Care Act imposes a significant annual, nondeductible fee on companies that manufacture or import certain branded prescription drug products. Substantial new provisions affecting compliance have also been enacted, which may affect our business practices with healthcare practitioners, and a significant number of provisions are not yet, or have only recently become, effective. Although it is too early to determine the effect of the Affordable Care Act, it appears likely to continue the pressure on pharmaceutical pricing, especially under the Medicare program, and may also increase our regulatory burdens and operating costs.
In addition, other legislative changes have been proposed and adopted since the Affordable Care Act was enacted. In August 2011, President Obama signed into law the Budget Control Act of 2011, which, among other things, creates the Joint Select Committee on Deficit Reduction to recommend to Congress proposals in spending reductions. The Joint Select Committee did not achieve a targeted deficit reduction of an amount greater than $1.2 trillion for the years 2013 through 2021, triggering the legislation’s automatic reduction to several government programs. This included aggregate reductions to Medicare payments to healthcare providers of up to 2.0% per fiscal year, starting in 2013. In January 2013, President Obama signed into law the American Taxpayer Relief Act of 2012, which, among other things, reduced Medicare payments to several categories of healthcare providers and increased the statute of limitations period for the government to recover overpayments to providers from three to five years. If we ever obtain regulatory approval and commercialization of our product candidates, these new laws may result in additional reductions in Medicare and other healthcare funding, which could have a material adverse effect on our customers and accordingly, our financial operations. Legislative and regulatory proposals have been made to expand post‑approval requirements and restrict sales and promotional activities for pharmaceutical products. We cannot be sure whether additional legislative changes will be enacted, or whether the FDA regulations, guidance or interpretations will be changed, or what the impact of such changes on the marketing approvals of our product candidates may be.
45
In the U.S., the European Union and other potentially significant markets for our product candidates, government authorities and third‑party payors are increasingly attempting to limit or regulate the price of medical products and services, particularly for new and innovative products and therapies, which has resulted in lower average selling prices. Furthermore, the increased emphasis on managed healthcare in the U.S. and on country and regional pricing and reimbursement controls in the European Union will put additional pressure on product pricing, reimbursement and usage, which may adversely affect our future product sales and results of operations. These pressures can arise from rules and practices of managed care groups, judicial decisions and governmental laws and regulations related to Medicare, Medicaid and healthcare reform, pharmaceutical reimbursement policies and pricing in general.
Some countries require approval of the sale price of a drug before it can be marketed. In many countries, the pricing review period begins after marketing or product licensing approval is granted. In some foreign markets, prescription pharmaceutical pricing remains subject to continuing governmental control even after initial approval is granted. As a result, we might obtain marketing approval for one of our product candidates in a particular country, but then be subject to price regulations that delay our commercial launch of such product, possibly for lengthy time periods, which could negatively impact the revenues we are able to generate from the sale of such product in that particular country. Adverse pricing limitations may hinder our ability to recoup our investment in our product candidates even if they obtain marketing approval.
Laws and regulations governing international operations may preclude us from developing, manufacturing and selling product candidates outside of the U.S. and require us to develop and implement costly compliance programs.
As we seek to expand our operations outside of the U.S., we must comply with numerous laws and regulations in each jurisdiction in which we plan to operate. The creation and implementation of international business practices compliance programs is costly and such programs are difficult to enforce, particularly where reliance on third parties is required.
The Foreign Corrupt Practices Act of 1977, or FCPA, prohibits any U.S. individual or business from paying, offering, authorizing payment or offering of anything of value, directly or indirectly, to any foreign official, political party or candidate for the purpose of influencing any act or decision of the foreign entity in order to assist the individual or business in obtaining or retaining business. The FCPA also obligates companies whose securities are listed in the U.S. to comply with certain accounting provisions requiring such companies to maintain books and records that accurately and fairly reflect all transactions of the corporation, including international subsidiaries, and to devise and maintain an adequate system of internal accounting controls for international operations. The anti‑bribery provisions of the FCPA are enforced primarily by the DOJ. The SEC is involved with enforcement of the books and records provisions of the FCPA.
Compliance with the FCPA is expensive and difficult, particularly in countries in which corruption is a recognized problem. In addition, the FCPA presents particular challenges in the pharmaceutical industry, because, in many countries, hospitals are operated by the government, and doctors and other hospital employees are considered foreign officials. Certain payments to hospitals in connection with clinical trials and other work have been deemed to be improper payments to government officials and have led to FCPA enforcement actions.
Various laws, regulations and executive orders also restrict the use and dissemination outside of the U.S., or the sharing with certain non‑U.S. nationals, of information classified for national security purposes, as well as certain products and technical data relating to those products. Our expanding presence outside of the U.S. will require us to dedicate additional resources to comply with these laws, and these laws may preclude us from developing, manufacturing, or selling our product candidates outside of the U.S., which could limit our growth potential and increase our development costs.
The failure to comply with laws governing international business practices may result in substantial penalties, including suspension or debarment from government contracting. Violation of the FCPA can result in significant civil and criminal penalties. Indictment alone under the FCPA can lead to suspension of the right to do business with the U.S. government until the pending claims are resolved. Conviction of a violation of the FCPA can result in long‑term disqualification as a government contractor. The termination of a government contract or relationship as a result of our failure to satisfy any of our obligations under laws governing international business practices would have a negative
46
impact on our operations and harm our reputation and ability to procure government contracts. The SEC also may suspend or bar issuers from trading securities on U.S. exchanges for violations of the FCPA’s accounting provisions.
Even if we are able to commercialize our product candidates, these products may not receive coverage and adequate reimbursement from third‑party payors, which could harm our business.
Our ability to commercialize our product candidates successfully will depend, in part, on the extent to which coverage and adequate reimbursement for our product candidates and related treatments will be available from government health administration authorities, private health insurers and other organizations. Government authorities and third‑party payors, such as private health insurers and health maintenance organizations, determine which medications they will cover and establish reimbursement levels. A primary trend in the U.S. healthcare industry and elsewhere is cost containment. Government authorities and third‑party payors have attempted to control costs by limiting coverage and the amount of reimbursement for particular medications. Increasingly, third‑party payors are requiring that drug companies provide them with predetermined discounts from list prices and are challenging the prices charged for medical drugs. Third‑party payors may also seek additional clinical evidence, beyond the data required to obtain marketing approval, demonstrating clinical benefits and value in specific patient populations before covering product candidates for those patients. We cannot be sure that coverage and adequate reimbursement will be available for our product candidates and, if reimbursement is available, what the level of reimbursement will be. Coverage and reimbursement may impact the demand for, or the price of, our product candidates, if we obtain marketing approval. If reimbursement is not available or is available only at limited levels, we may not be able to successfully commercialize our product candidates, if we obtain marketing approval.
There may be significant delays in obtaining coverage and reimbursement for newly approved drugs, and coverage may be more limited than the purposes for which the drug is approved by the FDA or comparable foreign regulatory authorities. Moreover, eligibility for coverage and reimbursement does not imply that any drug will be paid for in all cases or at a rate that covers our costs, including research, development, manufacture, sale and distribution. Interim reimbursement levels for new drugs, if applicable, may also not be sufficient to cover our costs and may only be temporary. Reimbursement rates may vary according to the use of the drug and the clinical setting in which it is used, may be based on reimbursement levels already set for lower cost drugs and may be incorporated into existing payments for other services. Net prices for drugs may be reduced by mandatory discounts or rebates required by government healthcare programs or private payors and by any future relaxation of laws that presently restrict imports of drugs from countries where they may be sold at lower prices than in the U.S. Third‑party payors often rely upon Medicare coverage policy and payment limitations in setting their own reimbursement policies. Our inability to obtain coverage and profitable reimbursement rates from both government‑funded and private payors for any approved products that we develop could have a material adverse effect on our operating results, our ability to raise capital needed to commercialize products and our overall financial condition.
If we are unable to establish sales and marketing capabilities or enter into agreements with third parties to market and sell our product candidates, we may be unable to generate any revenue.
We do not currently have an organization for the sale, marketing and distribution of pharmaceutical products and the cost of establishing and maintaining such an organization may exceed the cost‑effectiveness of doing so. In order to market any products approved by the FDA or comparable foreign regulatory authorities, we must build our sales, marketing, managerial and other non‑technical capabilities or make arrangements with third parties to perform these services. If we are unable to establish adequate sales, marketing and distribution capabilities, whether independently or with third parties, we may not be able to generate product revenue and may not become profitable. We will be competing with many companies that currently have extensive and well‑funded sales and marketing operations. Without an internal commercial organization or the support of a third party to perform sales and marketing functions, we may be unable to compete successfully against these more established companies.
47
Our relationships with customers and third‑party payors will be subject to applicable anti‑kickback, fraud and abuse and other healthcare laws and regulations, which could expose us to criminal sanctions, civil penalties, contractual damages, reputational harm and diminished profits and future earnings.
Healthcare providers, physicians and third‑party payors will play a primary role in the recommendation and prescription of any product candidates for which we obtain marketing approval. Our future arrangements with third‑party payors and customers may expose us to broadly applicable fraud and abuse and other healthcare laws and regulations that may affect the business or financial arrangements and relationships through which we would market, sell and distribute our products. Even though we do not and will not control referrals of healthcare services or bill directly to Medicare, Medicaid or other third‑party payors, federal and state healthcare laws and regulations pertaining to fraud and abuse and patients’ rights are and will be applicable to our business. Restrictions under applicable federal and state healthcare laws and regulations that may affect our operations (including our marketing, promotion, educational programs, pricing, and relationships with healthcare providers or other entities, among other things) and expose us to areas of risk include the following:
|
· |
the federal healthcare Anti‑Kickback Statute prohibits, among other things, persons from knowingly and willfully soliciting, offering, receiving or providing remuneration, directly or indirectly, in cash or in kind, to induce or reward, or in return for, either the referral of an individual for, or the purchase, order or recommendation of, any good or service, for which payment may be made under a federal healthcare program such as Medicare and Medicaid; |
|
· |
federal civil and criminal false claims laws and civil monetary penalty laws impose criminal and civil penalties, including through civil whistleblower or qui tam actions, against individuals or entities for knowingly presenting, or causing to be presented, to the federal government, including the Medicare and Medicaid programs, claims for payment that are false or fraudulent or making a false statement to avoid, decrease or conceal an obligation to pay money to the federal government; |
|
· |
the Health Insurance Portability and Accountability Act of 1996, or HIPAA, and the rules and regulations promulgated thereunder, establish federal standards for maintaining the privacy and security of certain patient health information known as Protected Health Information. As amended by the Health Information Technology for Economic and Clinical Health Act, HIPAA also establishes federal standards for administrative, technical and physical safeguards relevant to the electronic transmission of Protected Health Information and imposes certain notification obligations in the event of a breach of the privacy or security of Protected Health Information. In addition to adhering to the requirements of HIPAA, entities considered “covered entities” under HIPAA (such as health plans, health care clearinghouses, and certain health care providers) are also required to obtain assurances in the form of a written contract from certain business associates to which they transmit Protected Health Information to ensure that the privacy and security of such information is maintained in accordance with HIPAA requirements; |
|
· |
HIPAA also criminalizes health care fraud and makes it a felony to knowingly and willfully execute or attempt to execute a scheme or artifice to defraud any health care benefit program or to obtain money or other property owned or controlled by a health care benefit program by means of false or fraudulent pretenses, representations, or promises; |
|
· |
failure to comply with HIPAA can result in civil and criminal liability, including civil money penalties, fines and imprisonment; |
|
· |
the federal physician sunshine requirements under the Affordable Care Act requires manufacturers of drugs, devices, biologics and medical supplies to report annually to HHS information related to payments and other transfers of value to physicians, other healthcare providers, and teaching hospitals, and ownership and investment interests held by physicians and other healthcare providers and their immediate family members and applicable group purchasing organizations; and |
48
|
· |
analogous state and foreign laws and regulations, such as state anti‑kickback and false claims laws, may apply to sales or marketing arrangements and claims involving healthcare items or services reimbursed by non‑governmental third‑party payors, including private insurers; some state laws require pharmaceutical companies to comply with the pharmaceutical industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government and may require drug manufacturers to report information related to payments and other transfers of value to physicians and other healthcare providers or marketing expenditures; and state and foreign laws govern the privacy and security of health information in specified circumstances, many of which differ from each other in significant ways and often are not preempted by HIPAA, thus complicating compliance efforts. |
Efforts to ensure that our business arrangements with third parties are compliant with applicable healthcare laws and regulations will involve the expenditure of appropriate, and possibly significant, resources. Nonetheless, it is possible that governmental authorities will conclude that our business practices may not comply with current or future statutes, regulations or case law involving applicable fraud and abuse or other healthcare laws and regulations. If our operations are found to be in violation of any of these laws or any other governmental regulations that may apply to us, we may be subject to significant civil, criminal and administrative penalties, damages, fines, imprisonment, exclusion from government funded healthcare programs, such as Medicare and Medicaid, and the curtailment or restructuring of our operations. If any physicians or other healthcare providers or entities with whom we expect to do business are found to not be in compliance with applicable laws, they may be subject to criminal, civil or administrative sanctions, including exclusions from government funded healthcare programs.
Our employees may engage in misconduct or other improper activities, including noncompliance with regulatory standards and requirements, which could cause significant liability for us and harm our reputation.
We are exposed to the risk of employee fraud or other misconduct, including intentional failures to comply with FDA regulations or similar regulations of comparable foreign regulatory authorities, provide accurate information to the FDA or comparable foreign regulatory authorities, comply with manufacturing standards we have established, comply with federal and state healthcare fraud and abuse laws and regulations and similar laws and regulations established and enforced by comparable foreign regulatory authorities, report financial information or data accurately or disclose unauthorized activities to us. Employee misconduct could also involve the improper use of information obtained in the course of clinical trials, which could result in regulatory sanctions and serious harm to our reputation. We have adopted a code of conduct for our directors, officers and employees, or the Code of Conduct, but it is not always possible to identify and deter employee misconduct, and the precautions we take to detect and prevent this activity may not be effective in controlling unknown or unmanaged risks or losses or in protecting us from governmental investigations or other actions or lawsuits stemming from a failure to be in compliance with such laws or regulations. If any such actions are instituted against us, and we are not successful in defending ourselves or asserting our rights, those actions could have a significant impact on our business and results of operations, including the imposition of significant fines or other sanctions.
We face substantial competition, which may result in others discovering, developing or commercializing products before or more successfully than we do.
The development and commercialization of new drug products is highly competitive. We face competition with respect to our product candidates and will face competition with respect to any other product candidates that we may seek to develop or commercialize in the future, from major pharmaceutical companies, specialty pharmaceutical companies and biotechnology companies worldwide. There are a number of large pharmaceutical and biotechnology companies that currently market and sell products or are pursuing the development of products for the treatment of the disease indications for which we are developing birinapant, as well as for the treatment of early‑stage CTCL for which we are developing SHAPE. Some of these competitive products and therapies are based on scientific approaches that are the same as or similar to our approach, and others are based on entirely different approaches. For example, there are several companies developing product candidates that target the same cancer pathways that we are targeting or that are testing product candidates in the same cancer indications that we are testing. For example, Curis Inc., or Curis (Phase 1), Debiopharma SA, or Debiopharma (Phase 1), and Novartis AG, or Novartis (Phase 2), are all developing IAP inhibitors. Similarly, Merck (Zolinza) and Celgene (Istodax) have each received approval for HDAC inhibitors as therapeutic
49
options for patients with advanced CTCL, and Eisai (Targretin) and Actelion (Valchlor) have been approved as topical treatments for early‑stage CTCL. Potential competitors also include academic institutions, government agencies and other public and private research organizations that conduct research, seek patent protection and establish collaborative arrangements for research, development, manufacturing and commercialization.
Birinapant and SHAPE are presently being developed primarily as cancer therapeutics. There are a variety of available therapies and supportive care products marketed for cancer patients. Some of these other drugs are branded and subject to patent protection, some are in clinical development and not yet approved, and others are available on a generic basis. Many of the approved drugs are well established therapies or products and are widely accepted by physicians, patients and third‑party payors. Insurers and other third‑party payors may also encourage the use of generic products. In addition, birinapant is delivered intravenously, which will require a visit to an oncologist office or a hospital. Some of our competitors are seeking to develop drugs that can be administered by oral delivery, and thus would not require a visit to a doctor for each administration. These factors may make it difficult for us to achieve market acceptance at desired levels and/or in a timely manner to ensure viability of our business.
More established companies may have a competitive advantage over us due to their greater size, cash flows and institutional experience. Compared to us, many of our competitors may have significantly greater financial, technical and human resources.
As a result of these factors, our competitors may obtain regulatory approval of their products before we are able to, which may limit our ability to develop or commercialize our product candidates. Our competitors may also develop drugs that are safer, more effective, more widely used and cheaper than ours, and may also be more successful than us in manufacturing and marketing their products. These appreciable advantages could render our product candidates obsolete or non‑competitive before we can recover the expenses relating to the development and commercialization of our product candidates.
Mergers and acquisitions in the pharmaceutical and biotechnology industries may result in even more resources being concentrated among a smaller number of our competitors. Smaller and other early‑stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies. These third parties compete with us in recruiting and retaining qualified scientific, management and commercial personnel, establishing clinical trial sites and subject registration for clinical trials, as well as in acquiring technologies complementary to, or necessary for, our programs.
Product liability lawsuits against us could cause us to incur substantial liabilities and to limit commercialization of the product candidates that we may develop.
We face an inherent risk of product liability exposure related to the testing of our product candidates by us or our investigators in human clinical trials and will face an even greater risk if we commercially sell our product candidates after obtaining regulatory approval. Product liability claims may be brought against us by study subjects enrolled in our clinical trials, patients, healthcare providers or others using, administering or selling such product candidate. If we cannot successfully defend ourselves against claims that such product candidate caused injuries, we could incur substantial liabilities. Regardless of merit or eventual outcome, liability claims may result in, for example:
|
· |
decreased demand for such product candidate; |
|
· |
termination of clinical trial sites or entire trial programs; |
|
· |
injury to our reputation and significant negative media attention; |
|
· |
withdrawal of clinical trial subjects; |
|
· |
significant costs to defend the related litigation; |
50
|
· |
substantial monetary awards to clinical trial subjects or patients; |
|
· |
loss of revenue; |
|
· |
diversion of management and scientific resources from our business operations; |
|
· |
the inability to commercialize such product candidate; and |
|
· |
increased scrutiny and potential investigation by, among others, the FDA, DOJ, the Office of the Inspector General of the HHS, state attorneys general, members of Congress and the public. |
We currently have $10.0 million in product liability insurance coverage in the aggregate, which may not be adequate to cover all liabilities that we may incur. Insurance coverage is increasingly expensive. We may not be able to maintain insurance coverage at a reasonable cost or in an amount adequate to satisfy any liability that may arise. We intend to expand our product liability insurance coverage to include the sale of commercial products if we obtain marketing approval for our product candidates, but we may be unable to obtain commercially reasonable product liability insurance for either product candidate, if approved for marketing. Large judgments have been awarded in class action lawsuits based on drugs that had unanticipated side effects. A successful product liability claim or series of claims brought against us, particularly if judgments exceed our insurance coverage, could decrease our cash and adversely affect our business.
We will need to grow the size of our organization, and we may experience difficulties in managing this growth.
As our development and commercialization plans and strategies develop, or as a result of any future acquisitions, we will need additional managerial, operational, sales, marketing, financial and other resources. Our management, personnel and systems currently in place may not be adequate to support this future growth. Future growth would impose significant added responsibilities on members of management, including:
|
· |
managing our clinical trials effectively; |
|
· |
identifying, recruiting, maintaining, motivating and integrating additional employees; |
|
· |
managing our internal development efforts effectively while complying with our contractual obligations to licensors, licensees, contractors and other third parties; |
|
· |
improving our managerial, development, operational and finance systems; and |
|
· |
expanding our facilities. |
As our operations expand, we will need to manage additional relationships with various strategic partners, suppliers and other third parties. Our future financial performance and our ability to commercialize our product candidates, if either product candidate is approved, and to compete effectively will depend, in part, on our ability to manage any future growth effectively. To that end, we must be able to manage our development efforts and clinical trials effectively and hire, train and integrate additional management, administrative and sales and marketing personnel. Our failure to accomplish any of these tasks could prevent us from successfully growing our company.
Our future success depends on our ability to retain our executive officers and to attract, retain and motivate qualified personnel.
We are highly dependent upon J. Kevin Buchi, our President and Chief Executive Officer, Lesley Russell, M.B.Ch.B., M.R.C.P., our Chief Operating Officer and Chief Medical Officer, Pete A. Meyers, our Chief Financial Officer and Treasurer, C. Glenn Begley, M.B.B.S., Ph.D., F.R.A.C.P., F.R.C.P.A., F.R.C.Path., our Chief Scientific Officer and Senior Vice President, Research & Development, and Richard L. Sherman, J.D., our Senior Vice President,
51
Strategic Transactions, General Counsel and Secretary. The employment agreements we have with the persons named above do not prevent such persons from terminating their employment with us at any time. We do not maintain “key person” insurance for any of our executives or other employees. The loss of the services of any of these persons could impede the achievement of our research, development and commercialization objectives.
If we are unable to attract and retain highly qualified employees, we may not be able to grow effectively.
Our future growth and success depend on our ability to recruit, retain, manage and motivate our employees. The loss of any member of our senior management team or the inability to hire or retain experienced management personnel could compromise our ability to execute our business plan and harm our operating results.
Because of the specialized scientific and managerial nature of our business, we rely heavily on our ability to attract and retain qualified scientific, technical and managerial personnel. The competition for qualified personnel in the pharmaceutical field is intense and as a result, we may be unable to continue to attract and retain qualified personnel necessary for the development of our business.
Our business and operations would suffer in the event of computer system failures.
Despite the implementation of security measures, our internal computer systems, and those of our CROs and other third parties on which we rely, are vulnerable to damage from computer viruses, unauthorized access, natural disasters, fire, terrorism, war and telecommunication and electrical failures. In addition, our systems safeguard important confidential personal data regarding our subjects. If a disruption event were to occur and cause interruptions in our operations, it could result in a material disruption of our drug development programs. For example, the loss of clinical trial data from completed, ongoing or planned clinical trials could result in delays in our regulatory approval efforts and significantly increase our costs to recover or reproduce the data. To the extent that any disruption or security breach results in a loss of or damage to our data or applications, or inappropriate disclosure of confidential or proprietary information, we could incur liability and the further development of our product candidates could be delayed.
If we fail to comply with environmental, health and safety laws and regulations, we could become subject to fines or penalties or incur costs that could have a material adverse effect on the success of our business.
We are subject to numerous environmental, health and safety laws and regulations, including those governing laboratory procedures and the handling, use, storage, treatment and disposal of hazardous materials and wastes. Our operations involve the use of hazardous and flammable materials, including chemicals and biological materials. Our operations also produce hazardous waste products. We generally contract with third parties for the disposal of these materials and wastes. We cannot eliminate the risk of contamination or injury from these materials. In the event of contamination or injury resulting from our use of hazardous materials, we could be held liable for any resulting damages, and any liability could exceed our resources. We also could incur significant costs associated with civil or criminal fines and penalties.
Although we maintain workers’ compensation insurance to cover us for costs and expenses we may incur due to injuries to our employees resulting from the use of hazardous materials, this insurance may not provide adequate coverage against potential liabilities. We do not maintain insurance for environmental liability or toxic tort claims that may be asserted against us in connection with our storage or disposal of biological or hazardous materials.
In addition, we may incur substantial costs in order to comply with current or future environmental, health and safety laws and regulations. These current or future laws and regulations may impair our research, development or production efforts. Failure to comply with these laws and regulations also may result in substantial fines, penalties or other sanctions.
52
Business disruptions could seriously harm our future revenues and financial condition and increase our costs and expenses.
Our operations could be subject to earthquakes, power shortages, telecommunications failures, water shortages, floods, hurricanes, typhoons, fires, extreme weather conditions, medical epidemics and other natural or man‑made disasters or business interruptions, for which we are predominantly self‑insured. The occurrence of any of these business disruptions could seriously harm our operations and financial condition and increase our costs and expenses. We rely on third‑party manufacturers to produce our product candidates. Our ability to obtain clinical supplies of our product candidates could be disrupted if the operations of these suppliers are affected by a man‑made or natural disaster or other business interruption. The ultimate impact on us, our significant suppliers and our general infrastructure of being in certain geographical areas is unknown, but our operations and financial condition could suffer in the event of a major earthquake, fire or other natural disaster.
Risks Related to Our Dependence on Third Parties
We rely on third parties to conduct our pre‑clinical studies and clinical trials. If these third parties do not successfully carry out their contractual duties or meet expected deadlines, we may not be able to obtain regulatory approval for or commercialize our product candidates.
We rely on third‑party CROs to monitor and manage data for our ongoing pre‑clinical and clinical programs. We rely on these parties for execution of our pre‑clinical studies and clinical trials, and we control only some aspects of their activities. Nevertheless, we are responsible for ensuring that each of our pre‑clinical studies and clinical trials are conducted in accordance with the applicable protocol and legal, regulatory and scientific standards, and our reliance on the CROs does not relieve us of our regulatory responsibilities. We also rely on third parties to assist in conducting our pre‑clinical studies in accordance with GLP regulations, and the Animal Welfare Act requirements. We and our CROs are required to comply with federal regulations and current GCPs which are international standards meant to protect the rights and health of subjects that are enforced by the FDA, the Competent Authorities of the Member States of the European Economic Area and comparable foreign regulatory authorities for our product candidates and any future product candidates in clinical development. Regulatory authorities enforce GCP through periodic inspections of trial sponsors, principal investigators and trial sites. If we or any of our CROs fail to comply with applicable GCP, the clinical data generated in our clinical trials may be deemed unreliable and the FDA or comparable foreign regulatory authorities may require us to perform additional clinical trials before approving our marketing applications. We cannot assure you that upon inspection by a given regulatory authority, such regulatory authority will determine that any of our clinical trials comply with GCP requirements. In addition, our clinical trials must be conducted with product produced under cGMP requirements. Failure to comply with these regulations may require us to repeat pre‑clinical studies and clinical trials, which would delay the regulatory approval process.
Our CROs are not our employees, and except for remedies available to us under our agreements with such CROs, we cannot control whether or not they devote sufficient time and resources to our ongoing clinical, nonclinical and pre‑clinical programs. If CROs do not successfully carry out their contractual duties or obligations or meet expected deadlines or if the quality or accuracy of the data they obtain is compromised due to the failure to adhere to our protocols, regulatory requirements or for other reasons, our pre‑clinical studies and clinical trials may be extended, delayed or terminated and we may not be able to obtain regulatory approval for or successfully commercialize our product candidates. As a result, our results of operations and the commercial prospects for our product candidates would be harmed, our costs could increase and our ability to generate revenues could be delayed.
Because we have relied on third parties, our internal capacity to perform these functions is limited. Outsourcing these functions involves risk that third parties may not perform to our standards, may not produce results in a timely manner or may fail to perform at all. In addition, the use of third‑party service providers requires us to disclose our proprietary information to these parties, which could increase the risk that this information will be misappropriated. We currently have a small number of employees, which limits the internal resources we have available to identify and monitor our third‑party providers. To the extent we are unable to identify and successfully manage the performance of third‑party service providers in the future, our business may be adversely affected. Though we carefully manage our
53
relationships with our CROs, there can be no assurance that we will not encounter challenges or delays in the future or that these delays or challenges will not have a material adverse impact on our business, financial condition and prospects.
If we lose our relationships with CROs, our drug development efforts could be delayed.
We rely on third‑party vendors and CROs for pre‑clinical studies and clinical trials related to our drug development efforts. Switching or adding additional CROs would involve additional cost and requires management time and focus. Our CROs generally have the right to terminate their agreements with us in the event of an uncured material breach. In addition, some of our CROs have an ability to terminate their respective agreements with us and/or research projects pursuant to such agreements if the safety of the subjects participating in our clinical trials warrants such termination in accordance with the reasonable opinion of the relevant CRO, if we make a general assignment for the benefit of our creditors or if we are liquidated. Identifying, qualifying and managing performance of third‑party service providers can be difficult, time consuming and cause delays in our development programs. In addition, there is a natural transition period when a new CRO commences work and the new CRO may not provide the same type or level of services as the original provider. If any of our relationships with our third‑party CROs terminate, we may not be able to enter into arrangements with alternative CROs or to do so on commercially reasonable terms.
Our experience manufacturing our product candidates is limited to the needs of our pre‑clinical studies and clinical trials. We have no experience manufacturing on a commercial scale and have no manufacturing facility. We are dependent on third‑party manufacturers for the manufacture of our product candidates as well as on third parties for our supply chain, and if we experience problems with any such third parties, the manufacturing of our product candidates could be delayed.
We do not own or operate facilities for the manufacture of our product candidates. We currently have no plans to build our own clinical or commercial scale manufacturing capabilities. We currently rely on contract manufacturing organizations, or CMOs, for the chemical manufacture of the active pharmaceutical ingredient for our product candidates and another CMO for the production of the birinapant intravenous formulation. To meet our projected needs for pre‑clinical and clinical supplies to support our activities through regulatory approval and commercial manufacturing, the CMOs with whom we currently work will need to increase the scale of production. We may need to identify additional CMOs for continued production of supply for our product candidates. Although alternative third‑party suppliers with the necessary manufacturing and regulatory expertise and facilities exist, it could be expensive and take a significant amount of time to arrange for alternative suppliers. If we are unable to arrange for alternative third‑party manufacturing sources, or to do so on commercially reasonable terms or in a timely manner, we may not be able to complete development of our product candidates, or market or distribute our product candidates.
Reliance on third‑party manufacturers entails risks to which we would not be subject if we manufactured our product candidates ourselves, including reliance on the third party for regulatory compliance and quality assurance, the possibility of breach of the manufacturing agreement by the third party because of factors beyond our control, including a failure to synthesize and manufacture our product candidates or any products we may eventually commercialize in accordance with our specifications, and the possibility of termination or nonrenewal of the agreement by the third party, based on its own business priorities, at a time that is costly or damaging to us. In addition, the FDA and other regulatory authorities would require that our product candidates and any products that we may eventually commercialize be manufactured according to cGMP and similar foreign standards. Any failure by our third‑party manufacturers to comply with cGMP or failure to scale up manufacturing processes, including any failure to deliver sufficient quantities of our product candidates in a timely manner, could lead to a delay in, or failure to obtain, regulatory approval of our product candidates. In addition, such failure could be the basis for the FDA to issue a warning letter, withdraw approvals for such product candidate previously granted to us, or take other regulatory or legal action, including recall or seizure of outside supplies of our product candidates, total or partial suspension of production, suspension of ongoing clinical trials, refusal to approve pending applications or supplemental applications, detention or product, refusal to permit the import or export of products, injunction, or imposing civil and criminal penalties.
Any significant disruption in our supplier relationships could harm our business. Any significant delay in the supply of our product candidates or their respective key materials for an ongoing pre‑clinical study or clinical trial could considerably delay completion of such pre‑clinical study or clinical trial, product testing and potential regulatory
54
approval of our product candidates. If our manufacturers or we are unable to purchase these key materials after regulatory approval has been obtained for one of our product candidates, the commercial launch of such product candidate would be delayed or there would be a shortage in supply, which would impair our ability to generate revenues from the sale of that product candidate.
We may elect to enter into licensing or collaboration agreements with third parties to develop, obtain regulatory approvals for and commercialize our product candidates. Our dependence on such relationships may adversely affect our business.
Because we have limited resources, we may seek to enter into collaboration agreements with other pharmaceutical or biotechnology companies. Any failure by our partners to perform their obligations or any decision by our partners to terminate these agreements could negatively impact our ability to successfully develop, obtain regulatory approvals for and commercialize our product candidates. In the event we grant exclusive rights to such partners, we would be precluded from potential commercialization of our product candidates within the territories in which we have a partner. In addition, any termination of our collaboration agreements will terminate the funding we may receive under the relevant collaboration agreement and may impair our ability to fund further development efforts and our progress in our development programs.
Our commercialization strategy for our product candidates may depend on our ability to enter into agreements with collaborators to obtain assistance and funding for the development and potential commercialization of the relevant product candidate in the territories in which we seek to partner. Despite our efforts, we may be unable to secure additional collaborative licensing or other arrangements that are necessary for us to further develop and commercialize our product candidates. Supporting diligence activities conducted by potential collaborators and negotiating the financial and other terms of a collaboration agreement are long and complex processes with uncertain results. Even if we are successful in entering into one or more collaboration agreements, collaborations may involve greater uncertainty for us, as we have less control over certain aspects of our collaborative programs than we do over our proprietary development and commercialization programs. We may determine that continuing a collaboration under the terms provided is not in our best interest, and we may terminate the collaboration. Our potential future collaborators could delay or terminate their agreements, and as a result our product candidates may never be successfully commercialized.
Further, our potential future collaborators may develop alternative products or pursue alternative technologies either on their own or in collaboration with others, including our competitors, and the priorities or focus of our collaborators may shift such that our product candidates receive less attention or resources than we would like, or they may be terminated altogether. We may also enter into agreements with collaborators to share in the burden of conducting clinical trials, manufacturing and marketing our product candidates. Any such actions by our potential future collaborators may adversely affect our business prospects and ability to earn revenues. In addition, we could have disputes with our potential future collaborators, such as the interpretation of terms in our agreements. Any such disagreements could lead to delays in the development or commercialization of our product candidates, or could result in time‑consuming and expensive litigation or arbitration, which may not be resolved in our favor.
Risks Related to Our Intellectual Property
If we are unable to protect our intellectual property rights or if our intellectual property rights are inadequate for our technology and product candidates, our competitive position could be harmed.
Our commercial success will depend in large part on our ability to obtain and maintain patent and other intellectual property protection in the U.S. and other countries with respect to our proprietary technology and products. We rely on trade secret, patent, copyright and trademark laws, and confidentiality, licensing and other agreements with employees and third parties, all of which offer only limited protection. We seek to protect our proprietary position by filing and prosecuting patent applications in the U.S. and abroad related to our novel technologies and products that are important to our business.
The patent positions of biotechnology and pharmaceutical companies generally are highly uncertain, involve complex legal and factual questions and have in recent years been the subject of much litigation. As a result, the
55
issuance, scope, validity, enforceability and commercial value of our patents, including those patent rights licensed to us by third parties, are highly uncertain. The steps we or our licensors have taken to protect our proprietary rights may not be adequate to preclude misappropriation of our proprietary information or infringement of our intellectual property rights, both inside and outside the U.S. Further, the examination process may require us or our licensors to narrow the claims for our pending patent applications, which may limit the scope of patent protection that may be obtained if these applications issue. The rights already granted under any of our currently issued patents or those licensed to us and those that may be granted under future issued patents may not provide us with the proprietary protection or competitive advantages we are seeking. If we or our licensors are unable to obtain and maintain patent protection for our technology and products, or if the scope of the patent protection obtained is not sufficient, our competitors could develop and commercialize technology and products similar or superior to ours, and our ability to successfully commercialize our technology and products may be adversely affected. It is also possible that we or our licensors will fail to identify patentable aspects of inventions made in the course of our development and commercialization activities before it is too late to obtain patent protection on them.
With respect to patent rights, we do not know whether any of the pending patent applications for any of our compounds will result in the issuance of patents that protect our technology or products, or if any of our or our licensors’ issued patents will effectively prevent others from commercializing competitive technologies and products. Publications of discoveries in the scientific literature often lag behind the actual discoveries, and patent applications in the U.S. and other jurisdictions are typically not published until 18 months after filing or in some cases not at all, until they are issued as a patent. Therefore we cannot be certain that we or our licensors were the first to make the inventions claimed in our owned or licensed patents or pending patent applications, or that we or our licensors were the first to file for patent protection of such inventions.
Our pending applications cannot be enforced against third parties practicing the technology claimed in such applications unless and until a patent issues from such applications. Because the issuance of a patent is not conclusive as to its inventorship, scope, validity or enforceability, issued patents that we own or have licensed from third parties may be challenged in the courts or patent offices in the U.S. and abroad. Such challenges may result in the loss of patent protection, the narrowing of claims in such patents or the invalidity or unenforceability of such patents, which could limit our ability to stop others from using or commercializing similar or identical technology and products, or limit the duration of the patent protection for our technology and products. Protecting against the unauthorized use of our or our licensors’ patented technology, trademarks and other intellectual property rights is expensive, difficult and may in some cases not be possible. In some cases, it may be difficult or impossible to detect third‑party infringement or misappropriation of our intellectual property rights, even in relation to issued patent claims, and proving any such infringement may be even more difficult.
Third parties may initiate legal proceedings alleging that we are infringing their intellectual property rights, the outcome of which would be uncertain and could harm our business.
Our commercial success depends upon our ability to develop, manufacture, market and sell our product candidates, and to use our related proprietary technologies. We may become party to, or threatened with, future adversarial proceedings or litigation regarding intellectual property rights with respect to our product candidates, including interference or derivation proceedings before the U.S. Patent and Trademark Office, or USPTO. Third parties may assert infringement claims against us based on existing patents or patents that may be granted in the future. If we are found to infringe a third party’s intellectual property rights, we could be required to obtain a license from such third party to continue commercializing our product candidates. However, we may not be able to obtain any required license on commercially reasonable terms or at all. Under certain circumstances, we could be forced, including by court order, to cease commercializing our product candidates. In addition, in any such proceeding or litigation, we could be found liable for monetary damages. A finding of infringement could prevent us from commercializing our product candidates or force us to cease some of our business operations, which could materially harm our business. Any claims by third parties that we have misappropriated their confidential information or trade secrets could have a similar negative impact on our business.
While our product candidates are in pre‑clinical studies and clinical trials, we believe that the use of our product candidates in these pre‑clinical studies and clinical trials falls within the scope of the exemptions provided by 35 U.S.C.
56
Section 271(e) in the U.S., which exempts from patent infringement liability activities reasonably related to the development and submission of information to the FDA. As our product candidates progress toward commercialization, the possibility of a patent infringement claim against us increases. We attempt to ensure that birinapant, SHAPE and the methods we employ to manufacture them, as well as the methods for their use we intend to promote, do not infringe other parties’ patents and other proprietary rights. There can be no assurance they do not, however, and competitors or other parties may assert that we infringe their proprietary rights in any event.
In addition, we are testing birinapant administered with other product candidates and regulatory approved products that are covered by patents held by other companies or institutions. In the event that a labeling instruction is required in product packaging recommending that combination, we could be accused of, or held liable for, infringement of the third‑party patents covering the product candidate or regulatory approved products recommended for administration with birinapant. In such a case, we could be required to obtain a license from the other company or institution to use the required or desired package labeling, which may not be available on commercially reasonable terms, or at all.
We are aware of certain U.S. and foreign patents owned by a certain third party with claims that are broadly directed to pro‑apoptotic SMAC peptide mimetic monomer and dimer compounds, as well as to their use in treating cancer. These patents could be construed to cover birinapant. Generally, conducting clinical trials and other development activities in the U.S. is not considered an act of infringement. If and when birinapant is approved by the FDA, that certain third party may then seek to enforce its patents by filing a patent infringement lawsuit against us. In such lawsuit, we may incur substantial expenses defending our rights to commercialize birinapant, and in connection with such lawsuit and under certain circumstances, it is possible that we could be required to cease or delay the commercialization of birinapant and/or be required to pay monetary damages or other amounts, including royalties on the sales of birinapant. Moreover, such lawsuit may also consume substantial time and resources of our management team and board of directors. The threat or consequences of such a lawsuit may also result in royalty and other monetary obligations, which may adversely affect our results of operations and financial condition.
If we breach any of our license agreements with the respective exclusive licensors of our product candidates, or related licenses, this could have a material adverse effect on our commercialization efforts for birinapant, SHAPE or other related compounds which may be covered by the claims of our in‑ licensed patents.
In November 2003, we entered into an exclusive license agreement with Princeton University, subsequently amended in June 2004, August 2006 and October 2006, which grants us the rights to certain U.S. patents controlled by the university relating to SMAC‑mimetic compounds, including birinapant, and a non‑exclusive right to certain know‑how and technology relating thereto. In October 2008, Shape Pharmaceuticals entered into an exclusive license agreement with Harvard University and Dana‑Farber Cancer Institute, Inc., which grants us the worldwide rights to certain patent applications and patents issuing therefrom as defined in the license agreement, which include claims covering the composition of the SHAPE molecule. Additionally, in January 2014, we entered into a license agreement with the Walter and Eliza Hall Institute of Medical Research in Melbourne, Australia for worldwide exclusive rights to a patent application and any patents issuing therefrom relating to a method of treating intracellular infections involving the administration of an IAP antagonist. Each license agreement includes the right for us to sublicense.
If we materially breach or fail to perform any provision under any of our license agreements (including, generally, failure to make payments when due for royalties and other sub‑license revenues, failure to use reasonable efforts to develop, test, obtain regulatory approval, manufacture, market and sell licensed products, failure to file annual progress reports, commencement of bankruptcy or insolvency proceedings against us or failure to prosecute and maintain the licensed patents), the licensor under each license may have the right to terminate the applicable license, and upon the effective date of such termination, our right to practice the applicable licensed patent rights (or our rights in the relevant patent application) would end. To the extent the licensed technology, methodology or patent rights relate to our product candidates or uses thereof, we would expect to exercise all rights and remedies available to us, including attempting to cure any breach by us, and otherwise seek to preserve our rights under the patent rights or other technology or methodologies licensed to us, but we may not be able to do so in a timely manner, at an acceptable cost to us or at all. Any uncured, material breach under such licenses could result in our loss of rights to practice the patent rights, technology or methodology licensed to us under the applicable license agreement, and to the extent such patent rights or
57
other technology or methodology relate to birinapant, SHAPE or other of our compounds, it could have a material adverse effect on our commercialization efforts for birinapant, SHAPE or such other compounds, as the case may be.
We may not be able to protect our intellectual property rights throughout the world.
Filing, prosecuting and defending patents on birinapant, SHAPE and any future product candidates throughout the world would be prohibitively expensive, and our or our licensors’ intellectual property rights in some countries outside the U.S. can be less extensive than those in the U.S. In addition, the laws and practices of some foreign countries do not protect intellectual property rights to the same extent as federal and state laws in the U.S. Consequently, we and our licensors may not be able to prevent third parties from practicing our and our licensors’ inventions in all countries outside the U.S., or from selling or importing products made using our and our licensors’ inventions in and into the U.S. or other jurisdictions. Competitors may use our technologies in jurisdictions where we have not obtained patent protection to develop their own products, and may export otherwise infringing products to territories where we or our licensors have patent protection, but where enforcement is not as strong as that in the U.S. These products may compete with our products in jurisdictions where we do not have any issued patents and our patent claims or other intellectual property rights may not be effective or sufficient to prevent them from so competing.
Many companies have encountered significant problems in protecting and defending intellectual property rights in certain foreign jurisdictions. The legal systems of certain countries, particularly certain developing countries, do not favor the enforcement of patents and other intellectual property protection, particularly those relating to biopharmaceuticals, which could make it difficult for us to stop the infringement of our or our licensor’s patents or marketing of competing products in violation of our proprietary rights generally in those countries. Proceedings to enforce our patent rights in foreign jurisdictions could result in substantial cost and divert our efforts and attention from other aspects of our business, could put our and our licensors’ patents at risk of being invalidated or interpreted narrowly and our and our licensors’ patent applications at risk of not issuing and could provoke third parties to assert claims against us or our licensors. We or our licensors may not prevail in any lawsuits that we or our licensors initiate and the damages or other remedies awarded, if any, may not be commercially meaningful.
The laws of certain foreign countries may not protect our rights to the same extent as the laws of the U.S., and these foreign laws may also be subject to change. For example, methods of treatment and manufacturing processes may not be patentable in certain jurisdictions, and the requirements for patentability may differ in certain countries, particularly developing countries. Furthermore, generic drug manufacturers or other competitors may challenge the scope, validity or enforceability of our or our licensors’ patents, requiring us or our licensors to engage in complex, lengthy and costly litigation or other proceedings. Generic drug manufacturers may develop, seek approval for, and launch generic versions of our products. Many countries, including European Union countries, India, Japan and China, have compulsory licensing laws under which a patent owner may be compelled under certain circumstances to grant licenses to third parties. In those countries, we and our licensors may have limited remedies if patents are infringed or if we or our licensors are compelled to grant a license to a third party, which could materially diminish the value of those patents. This could limit our potential revenue opportunities. Accordingly, our and our licensors’ efforts to enforce intellectual property rights around the world may be inadequate to obtain a significant commercial advantage from the intellectual property that we own or license.
Patent term may be inadequate to protect our competitive position on our products for an adequate amount of time.
Given the amount of time required for the development, testing and regulatory review of new product candidates, such as birinapant and SHAPE, patents protecting such candidates might expire before or shortly after such candidates are commercialized. We expect to seek extensions of patent terms in the U.S. and, if available, in other countries where we are prosecuting patents. In the U.S., the Drug Price Competition and Patent Term Restoration Act of 1984 permits a patent term extension of up to five years beyond the normal expiration of the patent, which is limited to the approved indication (or any additional indications approved during the period of extension). However, the applicable authorities, including the FDA and the USPTO in the U.S., and any equivalent regulatory authority in other countries, may not agree with our assessment of whether such extensions are available, and may refuse to grant extensions to our patents, or may grant more limited extensions than we request. If this occurs, our competitors may be able to take
58
advantage of our investment in development and clinical trials by referencing our clinical and pre‑clinical data and launch their product earlier than might otherwise be the case.
Changes in patent law, including recent patent reform legislation, could increase the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of our issued patents.
As is the case with other pharmaceutical companies, our success is heavily dependent on intellectual property, particularly patents. Obtaining and enforcing patents in the pharmaceutical industry involve technological and legal complexity, and obtaining and enforcing pharmaceutical patents is costly, time‑consuming, and inherently uncertain. Changes in either the patent laws or interpretation of the patent laws in the U.S. and other countries may diminish the value of our patents or narrow the scope of our patent protection. For example, the U.S. Supreme Court has ruled on several patent cases in recent years, either narrowing the scope of patent protection available in certain circumstances or weakening the rights of patent owners in certain situations. In addition to increasing uncertainty with regard to our and our licensors’ ability to obtain patents in the future, this combination of events has created uncertainty with respect to the value of patents, once obtained. Depending on decisions by Congress, the federal courts, and the USPTO, the laws and regulations governing patents could change in unpredictable ways that would weaken our and our licensors’ ability to obtain new patents or to enforce existing patents and patents we and our licensors may obtain in the future. Recent patent reform legislation could increase the uncertainties and costs surrounding the prosecution of our and our licensors’ patent applications and the enforcement or defense of our or our licensors’ issued patents and those licensed to us.
In September 2011, the Leahy‑Smith America Invents Act, or the Leahy‑Smith Act, was signed into law. The Leahy‑Smith Act includes a number of significant changes to U.S. patent law. These include provisions that affect the way patent applications will be prosecuted and may also affect patent litigation. In particular, under the Leahy‑Smith Act, the U.S. transitioned in March 2013 to a “first to file” system in which the first inventor to file a patent application will be entitled to the patent. Third parties are allowed to submit prior art before the issuance of a patent by the USPTO and may become involved in opposition, derivation, reexamination, inter‑partes review or interference proceedings challenging our patent rights or the patent rights of our licensors. An adverse determination in any such submission, proceeding or litigation could reduce the scope of, or invalidate, our or our licensors’ patent rights, which could adversely affect our competitive position.
The USPTO is currently developing regulations and procedures to govern administration of the Leahy‑Smith Act, and many of the substantive changes to patent law associated with the Leahy‑Smith Act, and in particular, the first to file provisions, did not become effective until March 16, 2013. Accordingly, it is not clear what, if any, impact the Leahy‑Smith Act will have on the operation of our business. However, the Leahy‑Smith Act and its implementation could increase the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of our issued patents.
Obtaining and maintaining our patent protection depends on compliance with various procedural, document submissions, fee payment and other requirements imposed by governmental patent agencies, and our patent protection could be reduced or eliminated for non‑compliance with these requirements.
Periodic maintenance fees on any issued patent are due to be paid to the USPTO and foreign patent agencies in several stages over the lifetime of the patent. The USPTO and various foreign governmental patent agencies require compliance with a number of procedural, documentary, fee payment and other similar provisions during the patent application process. While an inadvertent lapse can in many cases be cured by payment of a late fee or by other means in accordance with the applicable rules, there are situations in which noncompliance can result in abandonment or lapse of the patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction. Non‑ compliance events that could result in abandonment or lapse of a patent or patent application include, but are not limited to, failure to respond to official actions within prescribed time limits, non‑payment of fees and failure to properly legalize and submit formal documents. If we or our licensors fail to maintain the patents and patent applications covering our product candidates, our competitive position would be adversely affected.
59
We may become involved in lawsuits to protect or enforce our intellectual property, which could be expensive, time consuming and unsuccessful and have a material adverse effect on the success of our business.
Competitors may infringe our patents or misappropriate or otherwise violate our intellectual property rights. To counter infringement or unauthorized use, litigation may be necessary in the future to enforce or defend our intellectual property rights, to protect our trade secrets or to determine the validity and scope of our own intellectual property rights or the proprietary rights of others. Also, third parties may initiate legal proceedings against us or our licensors to challenge the validity or scope of intellectual property rights we own or control. These proceedings can be expensive and time consuming. Many of our current and potential competitors have the ability to dedicate substantially greater resources to defend their intellectual property rights than we can. Accordingly, despite our efforts, we may not be able to prevent third parties from infringing upon or misappropriating our intellectual property. Litigation could result in substantial costs and diversion of management resources, which could harm our business and financial results. In addition, in an infringement proceeding, a court may decide that a patent owned by or licensed to us is invalid or unenforceable, or may refuse to stop the other party from using the technology at issue on the grounds that our patents do not cover the technology in question. An adverse result in any litigation proceeding could put one or more of our patents at risk of being invalidated, held unenforceable or interpreted narrowly. Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of our confidential information could be compromised by disclosure during this type of litigation. There could also be public announcements of the results of hearings, motions or other interim proceedings or developments. If securities analysts or investors perceive these results to be negative, it could have a material adverse effect on the price of shares of our common stock.
We may be subject to claims by third parties asserting that our licensors, employees or we have misappropriated their intellectual property, or claiming ownership of what we regard as our own intellectual property.
Many of our employees and our licensors’ employees, including our senior management, were previously employed at universities or at other biotechnology or pharmaceutical companies, including our competitors or potential competitors. Some of these employees, including each member of our senior management, executed proprietary rights, non‑disclosure and non‑competition agreements, or similar agreements, in connection with such previous employment. Although we try to ensure that our employees do not use the proprietary information or know‑how of others in their work for us, we may be subject to claims that we or these employees have used or disclosed intellectual property, including trade secrets or other proprietary information, of any such third party. Litigation may be necessary to defend against such claims. If we fail in defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights or personnel or sustain damages. Such intellectual property rights could be awarded to a third party, and we could be required to obtain a license from such third party to commercialize our technology or products. Such a license may not be available on commercially reasonable terms or at all. Even if we are successful in defending against such claims, litigation could result in substantial costs and be a distraction to management.
Intellectual property rights do not necessarily address all potential threats to our competitive advantage.
The degree of future protection afforded by our intellectual property rights is uncertain because intellectual property rights have limitations, and may not adequately protect our business, or permit us to maintain our competitive advantage. The following examples are illustrative:
|
· |
others may be able to make compounds that are the same as or similar to birinapant or SHAPE, as the case may be, but that are not covered by the claims of the patents that we own or have exclusively licensed; |
|
· |
we or our licensors or any strategic partners might not have been the first to make the inventions covered by the issued patents or pending patent applications that we own or have exclusively licensed; |
|
· |
we or our licensors might not have been the first to file patent applications covering certain of our inventions; |
60
|
· |
others may independently develop similar or alternative technologies or duplicate any of our technologies without infringing our intellectual property rights; |
|
· |
it is possible that our pending patent applications will not lead to issued patents; |
|
· |
issued patents that we own or have exclusively licensed may not provide us with any competitive advantages, or may be held invalid or unenforceable as a result of legal challenges; |
|
· |
our competitors might conduct research and development activities in the U.S. and other countries that provide a safe harbor from patent infringement claims for certain research and development activities, as well as in countries where we do not have patent rights and then use the information learned from such activities to develop competitive products for sale in our major commercial markets; |
|
· |
we may not develop additional proprietary technologies that are patentable; and |
|
· |
the patents of others may have an adverse effect on our business. |
Risks Related to Our Financial Position and Capital Needs
We have incurred significant losses since our inception and anticipate that we will continue to incur losses in the future.
We are a clinical‑stage biopharmaceutical company. Investment in biopharmaceutical product development is highly speculative because it entails substantial upfront capital expenditures and significant risk of failure to gain regulatory approval or become commercially viable. We have two product candidates, birinapant and SHAPE, at the early stages of clinical development and all of our other compounds are pre‑clinical. We do not have any products approved by regulatory authorities for marketing and have not generated any revenue from product sales, and we continue to incur significant research, development and other expenses related to our ongoing operations. As a result, we are not profitable and have incurred losses in every reporting period since our inception in 2003.
We expect to continue to incur significant expenses and operating losses for the foreseeable future. We anticipate these losses to increase as we continue the research and development of, and seek regulatory approvals for, our product candidates, and potentially begin to commercialize our product candidates, if they receive regulatory approval. We may encounter unforeseen expenses, difficulties, complications, delays and other unknown factors that may adversely affect our business. The size of our future net losses will depend, in part, on the rate of future growth of our expenses and our ability to generate revenues. If our product candidates fail in clinical trials or do not gain regulatory approval, or if approved, fail to achieve market acceptance, we may never become profitable. Even if we achieve profitability in the future, we may not be able to sustain profitability in subsequent periods.
We currently have no source of product revenue and may never become profitable.
We have not generated any revenues from commercial product sales (and we have no commercial products). Our ability to generate revenue from product sales and achieve profitability will depend upon our ability to successfully gain regulatory approval and commercialize birinapant, SHAPE or other product candidates that we may develop, in‑license or acquire in the future. Even if we are able to successfully achieve regulatory approval for our product candidates, we do not know when it will generate revenue from product sales for us, if at all. Our ability to generate revenue from product sales from birinapant, SHAPE or any other future product candidates also depends on a number of additional factors, including our ability to:
|
· |
successfully complete development activities, including enrollment of study participants and completion of the necessary clinical trials; |
|
· |
complete and submit NDAs to the FDA and obtain regulatory approval for indications for which there is a commercial market; |
|
· |
complete and submit applications to, and obtain regulatory approval from, foreign regulatory authorities; |
61
|
· |
successfully commercialize any products, if approved; |
|
· |
make or have made commercial quantities of our products at acceptable cost levels; |
|
· |
develop a commercial organization capable of manufacturing, sales, marketing and distribution for any products we intend to sell ourselves in the markets in which we choose to commercialize on our own; |
|
· |
find suitable partners to help us market, sell and distribute our approved products in other markets; and |
|
· |
obtain adequate pricing, coverage and reimbursement from third parties, including government and private payors. |
In addition, because of the numerous risks and uncertainties associated with product development, including that birinapant or SHAPE may not advance through development or achieve the endpoints of applicable clinical trials, we are unable to predict the timing or amount of increased expenses, or when or if we will be able to achieve or maintain profitability. Even if we are able to complete the development and regulatory process for our product candidates, we anticipate incurring significant costs associated with commercializing these products.
Even if we are able to generate revenues from the sale of birinapant, SHAPE or any future commercial products, we may not become profitable and will need to obtain additional funding to continue operations. If we fail to become profitable or are unable to sustain profitability on a continuing basis, and we are not successful in obtaining additional funding, then we may be unable to continue our operations at planned levels.
We intend to expend our limited resources to pursue birinapant and SHAPE, and may fail to capitalize on other technologies, product candidates or other indications that may be more profitable or for which there is a greater likelihood of success.
Because we have limited financial and managerial resources, we are focusing on research programs relating to birinapant and SHAPE, which concentrates the risk of product failure in the event birinapant or SHAPE proves to be unsafe or ineffective or the SMAC‑mimetic class of product candidates is considered to be inadequate for clinical development or commercialization. As a result, we may forego or delay pursuit of opportunities with other technologies, product candidates or for other indications that later prove to have greater commercial potential. Our resource allocation decisions may cause us to fail to capitalize on viable commercial products or profitable market opportunities. Our spending on proprietary research and development programs relating to birinapant and SHAPE may not yield any commercially viable products. If we do not accurately evaluate the commercial potential or target market for birinapant or SHAPE, we may relinquish valuable rights to birinapant or SHAPE through collaboration, licensing or other royalty arrangements in cases in which it would have been more advantageous for us to retain sole development and commercialization rights to birinapant or SHAPE.
We will require additional capital to fund our operations and if we fail to obtain necessary financing, we may be unable to complete the development and potential commercialization of birinapant or SHAPE.
Our operations have consumed substantial amounts of cash since inception. We expect to continue to spend substantial amounts to advance the clinical development of birinapant and SHAPE and launch and commercialize birinapant and SHAPE, if we receive regulatory approval. We will require additional capital for the further development and potential commercialization of birinapant and SHAPE, and may also need to raise additional funds sooner to pursue a more accelerated development of birinapant and SHAPE. If we are unable to raise capital when needed or on attractive terms, we could be forced to delay, reduce or eliminate our research and development programs or any future commercialization efforts.
As of the date of this report, we anticipate that our cash on hand will enable us to fund our operating expenses and capital expenditures, including the payment of interest on our convertible notes, through the first quarter of 2016. We have based this estimate on assumptions that may prove to be wrong, and we could deploy our available capital
62
resources sooner than we currently expect. Our future funding requirements, both near and long‑term, will depend on many factors, including, but not limited to the:
|
· |
initiation, progress, timing, costs and results of pre‑clinical studies and clinical trials for birinapant, SHAPE or any other future product candidates; |
|
· |
clinical development plans we establish for birinapant, SHAPE and any other future product candidates; |
|
· |
our obligation to make milestone payments, royalty and non‑royalty sublicense receipt payments to third‑party licensors, if any, under our licensing agreements; |
|
· |
number and characteristics of product candidates that we discover or in‑license and develop; |
|
· |
outcome, timing and cost of regulatory review by the FDA and comparable foreign regulatory authorities, including the potential for the FDA or comparable foreign regulatory authorities to require that we perform more studies than those that we currently expect; |
|
· |
costs of filing, prosecuting, defending and enforcing any patent claims and maintaining and enforcing other intellectual property rights; |
|
· |
effect of competing technological and market developments; |
|
· |
costs and timing of the implementation of commercial‑scale manufacturing activities; and |
|
· |
costs and timing of establishing sales, marketing and distribution capabilities for any product candidates for which we may receive regulatory approval. |
If we are unable to obtain necessary financing, our ability to complete the development and potential commercialization of birinapant and SHAPE will be compromised. We cannot assure you that we will be able to continue to obtain financing on terms we deem to be commercially reasonable, if at all or that the third-party financing that we do obtain will be sufficient to cover our operating expenses, debt expenses and other payment obligations. If we fail to obtain the third-party financing we require, we may be unable to continue our operations at the planned levels or at all.
Raising additional capital may cause dilution to our existing stockholders, restrict our operations or require us to relinquish rights to our technologies, birinapant or SHAPE.
Until we can generate substantial revenue from product sales, if ever, we expect to seek additional capital through a combination of private and public equity offerings, debt financings, strategic collaborations and alliances and licensing arrangements. To the extent that we raise additional capital through the sale of equity or convertible debt securities, the ownership interests of existing stockholders will be diluted, and the terms may include liquidation or other preferences that adversely affect the rights of existing stockholders. The indenture for the notes contains certain covenants limiting our and our subsidiaries’ ability to incur certain additional debt and liens, subject to certain exceptions. Future additional debt financings, if available, may involve agreements that include liens or other restrictive covenants limiting our ability to take important actions, such as incurring additional debt, making capital expenditures or declaring dividends. These covenants may restrict our operations and ability to raise additional financing. If we raise additional funds through strategic collaborations and alliances or licensing arrangements with third parties, we may have to relinquish valuable rights to our technologies in particular countries, or grant licenses on terms that are not favorable to us. If we are unable to raise additional funds through equity or debt financing when needed, we may be required to delay, limit, reduce or terminate our product development or commercialization efforts or grant rights to develop and market our technologies that we would otherwise prefer to develop and market ourselves.
63
We may acquire other assets or businesses, or form collaborations or make investments in other companies or technologies that could harm our operating results, dilute our stockholders’ ownership, increase our debt or cause us to incur significant expense.
As part of our business strategy, we may pursue acquisitions of assets, including pre‑clinical, clinical or commercial stage products or product candidates, or businesses, or strategic alliances and collaborations, to expand our existing technologies and operations. We may not identify or complete these transactions in a timely manner, on a cost‑effective basis, or at all, and we may not realize the anticipated benefits of any such transaction, any of which could have a detrimental effect on our financial condition, results of operations and cash flows. We have limited experience with acquiring other companies, products or product candidates, and limited experience with forming strategic alliances and collaborations. We may not be able to find suitable acquisition candidates, and if we make any acquisitions, we may not be able to integrate these acquisitions successfully into our existing business and we may incur additional debt or assume unknown or contingent liabilities in connection therewith. Integration of an acquired company or assets may also disrupt ongoing operations, require the hiring of additional personnel and the implementation of additional internal systems and infrastructure, especially the acquisition of commercial assets, and require management resources that would otherwise focus on developing our existing business. We may not be able to find suitable strategic alliance or collaboration partners or identify other investment opportunities, and we may experience losses related to any such investments.
To finance any acquisitions or collaborations, we may choose to issue debt or shares of our common stock as consideration. Any such issuance of shares would dilute the ownership of our stockholders. If the price of our common stock is low or volatile, we may not be able to acquire other assets or companies or fund a transaction using our stock as consideration. Alternatively, it may be necessary for us to raise additional funds for acquisitions through public or private financings. Additional funds may not be available on terms that are favorable to us, or at all. In addition, the covenants under the indenture governing the notes limit our ability to raise additional funds for acquisitions and our ability to assume debt of acquired businesses.
Risks Relating to Securities Markets and Investment in Our Common Stock
The market price of our common stock may be volatile.
The trading price of our common stock is likely to be highly volatile and could be subject to wide fluctuations in response to various factors, some of which are beyond our control. In addition to the factors discussed in this “Risk Factors” section, these factors include:
|
· |
trading volatility of low‑priced stock; |
|
· |
the success of competitive products or technologies; |
|
· |
regulatory actions with respect to our products or our competitors’ products; |
|
· |
actual or anticipated changes in our growth rate relative to our competitors; |
|
· |
announcements by us or our competitors of significant acquisitions, strategic partnerships, joint ventures, collaborations or capital commitments; |
|
· |
results of clinical trials of birinapant, SHAPE or product candidates of our competitors; |
|
· |
regulatory or legal developments in the U.S. and other countries; |
|
· |
developments or disputes concerning patent applications, issued patents or other proprietary rights; |
|
· |
the recruitment or departure of key personnel; |
64
|
· |
the level of expenses related to our clinical development programs; |
|
· |
the results of our efforts to in‑license or acquire additional product candidates or products; |
|
· |
actual or anticipated changes in estimates as to financial results, development timelines or recommendations by securities analysts; |
|
· |
variations in our financial results or those of companies that are perceived to be similar to us; |
|
· |
fluctuations in the valuation of companies perceived by investors to be comparable to us; |
|
· |
share price and volume fluctuations attributable to inconsistent trading volume levels of our shares; |
|
· |
announcement or expectation of additional financing efforts; |
|
· |
sales of our common stock by us, our insiders or our other stockholders; |
|
· |
changes in the structure of healthcare payment systems; |
|
· |
market conditions in the pharmaceutical and biotechnology sectors; and |
|
· |
general economic, industry and market conditions. |
In addition, the stock market in general, and pharmaceutical and biotechnology companies in particular, have experienced extreme price and volume fluctuations that have often been unrelated or disproportionate to the operating performance of these companies. Broad market and industry factors may negatively affect the market price of our common stock, regardless of our actual operating performance. Moreover, some institutional investors and mutual funds cannot invest in stocks priced below $5.00 per share. The realization of any of these risks or any of a broad range of other risks, including those described in these “Risk Factors,” could have a dramatic and material adverse impact on the market price of our common stock.
Future sales and issuances of our common stock or rights to purchase common stock, including pursuant to our equity incentive plans, could result in additional dilution of the percentage ownership of our stockholders and could cause our stock price to fall.
We expect that significant additional capital will be needed in the future to continue our planned operations. To raise capital, we may sell substantial amounts of common stock or securities convertible into or exchangeable for common stock. These future issuances of common stock or common stock‑related securities, together with the exercise of stock options, warrants outstanding or granted in the future, any additional shares issued in connection with acquisitions and any shares issued upon the conversion of the notes offered hereby, if any, may result in material dilution to our investors. Such sales may also result in material dilution to our existing stockholders, and new investors could gain rights, preferences and privileges senior to those of holders of our common stock.
In addition, persons who were our stockholders prior to the sale of shares in our initial public offering continue to hold a substantial number of shares of our common stock that they may be able to sell in the public market, subject to the limitations of Rule 144 of the Securities Act. Significant portions of these shares are held by a small number of stockholders. Sales by our current stockholders of a substantial number of shares, or the expectation that such sales may occur, could significantly reduce the market price of our common stock. Moreover, the holders of a substantial number of shares of common stock may have rights, subject to certain conditions, to require us to file registration statements to permit the resale of their shares in the public market or to include their shares in registration statements that we may file for ourselves or other stockholders.
65
Pursuant to our equity incentive plans, our compensation committee is authorized to grant equity‑based incentive awards to our directors, executive officers and other employees and service providers. Future equity incentive grants and issuances of common stock under our existing equity incentive plans may have an adverse effect on the market price of our common stock.
We have also registered all common stock that we may issue under our employee benefits plans. As a result, these shares can be freely sold in the public market upon issuance, subject to restrictions under the securities laws. In addition, our directors and executive officers may in the future establish programmed selling plans under Rule 10b5‑1 of the Exchange Act for the purpose of effecting sales of our common stock. If any of these events cause a large number of our shares to be sold in the public market, the sales could reduce the trading price of our common stock and impede our ability to raise future capital.
We may be subject to securities litigation, which is expensive and could divert our management’s attention.
The market price of our common stock may be volatile, and in the past companies that have experienced volatility in the market price of their stock have been subject to securities class action litigation. We may be the target of this type of litigation in the future. Securities litigation against us could result in substantial costs and divert our management’s attention from other business concerns, which could seriously harm our business.
Insiders have substantial influence over us and could delay or prevent a change in corporate control.
As of December 31, 2014, our executive officers, directors and beneficial owners of over 5% of our common stock held a majority of our common stock. This concentration of ownership could harm the market price of our common stock by:
|
· |
delaying, deferring or preventing a change in control of our company; |
|
· |
impeding a merger, consolidation, takeover or other business combination involving our company; or |
|
· |
discouraging a potential acquirer from making a tender offer or otherwise attempting to obtain control of our company. |
The interests of this group of stockholders may not always coincide with your interests or the interests of other stockholders and they may act in a manner that advances their best interests and not necessarily those of other stockholders, including seeking a premium value for their common stock, and might negatively affect the prevailing market price for our common stock.
We have never paid dividends on our capital stock, and because we do not anticipate paying any cash dividends in the foreseeable future, capital appreciation, if any, of our common stock will be your sole source of gain on an investment in our stock if converted.
We have never paid any cash dividends on our common stock, and we currently intend to retain any future earnings to support our operations and finance the growth and development of our business. We do not intend to pay cash dividends on our common stock for the foreseeable future. As a result, capital appreciation, if any, of our common stock will be your sole source of gain for the foreseeable future after you have converted your notes.
We are an “emerging growth company” and we take advantage of reduced disclosure and governance requirements applicable to emerging growth companies, which could result in our common stock being less attractive to investors.
We are an “emerging growth company,” as defined in the Jumpstart Our Business Startups, or JOBS, Act, and we take advantage of certain exemptions from various reporting requirements that are applicable to other public companies that are not emerging growth companies including, but not limited to, not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes‑Oxley Act of 2002, or the Sarbanes‑Oxley Act, reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements, and exemptions
66
from the requirements of holding a non‑binding advisory vote on executive compensation and stockholder approval of any golden parachute payments not previously approved. We cannot predict if investors will find our common stock less attractive because we will rely on these exemptions. If some investors find our common stock less attractive as a result, there may be a less active trading market for our common stock and our stock price may be more volatile. We may take advantage of these reporting exemptions until we are no longer an emerging growth company, which in certain circumstances could be for up to five years from the end of the fiscal year in which we completed our initial public offering, which was December 31, 2013.
Our status as an “emerging growth company” under the JOBS Act may make it more difficult to raise capital as and when we need it.
Because of the exemptions from various reporting requirements provided to us as an “emerging growth company” we may be less attractive to investors and it may be difficult for us to raise additional capital as and when we need it. Investors may be unable to compare our business with other companies in our industry if they believe that our financial accounting is not as transparent as other companies in our industry. If we are unable to raise additional capital as and when we need it, our financial condition and results of operations may be materially and adversely affected.
We will remain an “emerging growth company” until the earliest of (a) the last day of the first fiscal year in which our annual gross revenues exceed $1.0 billion, (b) the date that we become a “large accelerated filer” as defined in Rule 12b‑2 under the Exchange Act, which would occur if the market value of our shares that are held by non‑affiliates exceeds $700 million as of the last business day of our most recently completed second fiscal quarter, (c) the date on which we have issued more than $1.0 billion in nonconvertible debt during the preceding three‑year period and (d) the last day of our fiscal year containing the fifth anniversary of the date on which shares of our common stock become publicly traded in the U.S., which was December 31, 2013.
If we fail to maintain an effective system of internal control over financial reporting in the future, we may not be able to accurately report our financial condition, results of operations or cash flows, which may adversely affect investor confidence in us and, as a result, the value of our common stock.
The Sarbanes‑Oxley Act requires, among other things, that we maintain effective internal controls for financial reporting and disclosure controls and procedures. We are required, under Section 404 of the Sarbanes‑Oxley Act, to furnish a report by management on, among other things, the effectiveness of our internal control over financial reporting with each of our Annual Reports on Form 10-K.. This assessment will include disclosure of any material weaknesses identified by our management in our internal control over financial reporting. A material weakness is a deficiency, or combination of deficiencies, in internal control over financial reporting that results in more than a reasonable possibility that a material misstatement of annual or interim financial statements will not be prevented or detected on a timely basis. Section 404 of the Sarbanes‑Oxley Act also generally requires an attestation from our independent registered public accounting firm on the effectiveness of our internal control over financial reporting. For as long as we remain an emerging growth company as defined in the JOBS Act, we intend to take advantage of the exemption permitting us not to comply with the independent registered public accounting firm attestation requirement.
Our compliance with Section 404 will require that we incur substantial accounting expense and expend significant management efforts. We currently do not have an internal audit group, and we will need to hire additional accounting and financial staff with appropriate public company experience and technical accounting knowledge, and compile the system and process documentation necessary to perform the evaluation needed to comply with Section 404. We may not be able to complete our evaluation, testing and any required remediation in a timely fashion. During the evaluation and testing process, if we identify one or more material weaknesses in our internal control over financial reporting, we will be unable to assert that our internal control over financial reporting is effective. We cannot assure you that there will not be material weaknesses or significant deficiencies in our internal control over financial reporting in the future. Any failure to maintain internal control over financial reporting could severely inhibit our ability to accurately report our financial condition, results of operations or cash flows. If we are unable to conclude that our internal control over financial reporting is effective, or if our independent registered public accounting firm determines we have a material weakness or significant deficiency in our internal control over financial reporting once that firm begin its Section 404 reviews, we could lose investor confidence in the accuracy and completeness of our financial reports, the
67
market price of our common stock could decline, and we could be subject to sanctions or investigations by the NASDAQ Stock Market, the SEC or other regulatory authorities. Failure to remedy any material weakness in our internal control over financial reporting, or to implement or maintain other effective control systems required of public companies, could also restrict our future access to the capital markets.
Our disclosure controls and procedures may not prevent or detect all errors or acts of fraud.
We are subject to the periodic reporting requirements of the Exchange Act. Our disclosure controls and procedures are designed to reasonably assure that information required to be disclosed by us in reports we file or submit under the Exchange Act is accumulated and communicated to management, recorded, processed, summarized and reported within the time periods specified in the rules and forms of the SEC. We believe that any disclosure controls and procedures or internal controls and procedures, no matter how well conceived and operated, can provide only reasonable, not absolute, assurance that the objectives of the control system are met.
These inherent limitations include the realities that judgments in decision‑ making can be faulty, and that breakdowns can occur because of simple error or mistake. Additionally, controls can be circumvented by the individual acts of some persons, by collusion of two or more people or by an unauthorized override of the controls. Accordingly, because of the inherent limitations in our control system, misstatements or insufficient disclosures due to error or fraud may occur and not be detected.
We incur significant expenses as a result of being a public company, which negatively impacts our financial performance and could cause our results of operations and financial condition to suffer.
We are required to incur significant legal, accounting, insurance, compliance and other expenses as a result of being a public company. These expenses may increase even more after we are no longer an “emerging growth company.” We are obligated to file annual and quarterly information and other reports with the SEC. In addition, we also became subject to other reporting and corporate governance requirements which impose significant compliance obligations upon us. The Sarbanes‑Oxley Act of 2002, together with related rules implemented by the SEC and by the NASDAQ Stock Market, have imposed increased regulation and disclosure and have required enhanced corporate governance practices of public companies. If we fail to maintain an effective system of internal control over financial reporting in the future, we may not be able to accurately report our financial condition, results of operations or cash flows, which may adversely affect investor confidence in us and, as a result, the value of our common stock” above, substantially increase our expenses, including our legal and accounting costs, and make some activities more time‑consuming and costly.
We also expect these laws, rules and regulations to make it more expensive for us to obtain director and officer liability insurance, and we may be required to accept reduced policy limits and coverage or incur substantially higher costs to obtain the same or similar coverage. As a result, it may be more difficult for us to attract and retain qualified persons to serve on our board of directors or as officers. As a result of the foregoing, we have begun to incur substantial increases in legal, accounting and insurance compliance and we expect to incur certain other expenses in the future, which will negatively impact our financial performance and could cause our results of operations and financial condition to suffer.
Some provisions of our charter documents and Delaware law may have anti‑takeover effects that could discourage an acquisition of us by others, even if an acquisition would be beneficial to our stockholders and may prevent attempts by our stockholders to replace or remove our current management.
Provisions in our certificate of incorporation and bylaws, as well as provisions of Delaware law, could make it more difficult for a third party to acquire us or increase the cost of acquiring us, even if doing so would benefit our stockholders, or remove our current management. These include provisions that:
|
· |
permit our board of directors to issue up to 25 million shares of preferred stock, with any rights, preferences and privileges as it may designate; |
68
|
· |
provide that all vacancies on our board of directors, including as a result of newly created directorships, may, except as otherwise required by law, be filled by the affirmative vote of a majority of directors then in office, even if less than a quorum; |
|
· |
establish a classified board of directors such that only one of three classes of directors is elected each year; |
|
· |
provide that directors can only be removed for cause; |
|
· |
require that any action to be taken by our stockholders must be effected at a duly called annual or special meeting of stockholders and not be taken by written consent; |
|
· |
provide that stockholders seeking to present proposals before a meeting of stockholders or to nominate candidates for election as directors at a meeting of stockholders must provide advance notice in writing, and also specify requirements as to the form and content of a stockholder’s notice; |
|
· |
require that the amendment of certain provisions of our certificate of incorporation and bylaws relating to anti‑takeover measures may only be approved by a vote of 75% of our outstanding capital stock; |
|
· |
not provide for cumulative voting rights, thereby allowing the holders of a majority of the shares of common stock entitled to vote in any election of directors to elect all of the directors standing for election; and |
|
· |
provide that special meetings of our stockholders may be called only by the board of directors or by such person or persons designated by a majority of the board of directors to call such meetings. |
These provisions may frustrate or prevent any attempts by our stockholders to replace or remove our current management by making it more difficult for stockholders to replace members of our board of directors, who are responsible for appointing the members of our management.
Because we are incorporated in Delaware, we are governed by the provisions of Section 203 of the Delaware General Corporation Law, which may discourage, delay or prevent someone from acquiring us or merging with us whether or not it is desired by or beneficial to our stockholders. Under Delaware law, a corporation may not, in general, engage in a business combination with any holder of 15.0% or more of its capital stock unless the holder has held the stock for three years or, among other things, the board of directors has approved the transaction. Any provision of our certificate of incorporation or bylaws or Delaware law that has the effect of delaying or deterring a change in control could limit the opportunity for our stockholders to receive a premium for their shares of our common stock, and could also affect the price that some investors are willing to pay for our common stock.
If securities or industry analysts publish inaccurate or unfavorable research about our business, our stock price and trading volume could decline.
The trading market for our common stock will depend in part on the research and reports that securities or industry analysts publish about us or our business. If one or more of the analysts who cover us downgrade our stock or publish inaccurate or unfavorable research about our business, our stock price would likely decline. If one or more of these analysts cease coverage of our company or fail to publish reports on us regularly, demand for our stock could decrease, which might cause our stock price and trading volume to decline.
69
Risks Related to our Convertible Notes
We may issue additional shares of our common stock or instruments convertible into shares of our common stock, including in connection with the conversion of our 8% Notes, and thereby materially and adversely affect the market price of our common stock.
We may conduct future offerings of our common stock or, subject to certain conditions and limitations, preferred stock or other securities convertible into our common stock to fund acquisitions, finance operations or for other purposes. The market price of shares of our common stock or the trading price of the notes could decrease significantly if we conduct such future offerings, if any of our existing stockholders sells a substantial amount of our common stock, or if the market perceives that such offerings or sales may occur. Moreover, any issuance of additional common stock will dilute the ownership interest of our existing common stockholders.
Servicing our debt requires a significant amount of cash, and we may not have sufficient cash flow from our business to pay our substantial debt.
We have $47.0 million face value of total long‑term indebtedness as of December 31, 2014 pursuant to our 8% Notes. We may not have the ability to repay our 8% Notes when they become due. Since our inception, we have not generated any revenues or cash flows from operations and our operations have been financed primarily through the issuance of equity securities and convertible notes. We expect to continue to incur losses for the next several years. Our ability to make scheduled payments of the principal of, to pay interest on or to refinance our indebtedness, including the 8% Notes, depends on our future performance, which is subject to regulatory, economic, financial, competitive and other factors beyond our control, and our ability to raise equity capital. Our business may not generate cash flow from operations in the future sufficient to service our debt and make necessary capital expenditures. If we are unable to generate such cash flow, we may be required to adopt one or more alternatives, such as selling assets, restructuring debt or obtaining additional equity capital on terms that may be onerous or highly dilutive. Our ability to refinance our indebtedness will depend on the capital markets and our financial condition at such time. We may not be able to engage in any of these activities or engage in these activities on desirable terms, which could result in a default on our debt obligations.
Our indebtedness and other obligations are substantial and could adversely affect our business and liquidity.
Our substantial indebtedness, including our 8% Notes, could have important consequences. For example, they:
|
· |
require us to dedicate a substantial portion of our available cash flows to payments on our indebtedness and other obligations, thereby reducing the funds available for other purposes; |
|
· |
contain restrictive covenants that limit our ability to incur additional indebtedness or liens on our assets, which could interfere with our ability to obtain additional equity or debt financing for general corporate purposes, acquisitions, investments, capital expenditures or other strategic purposes; and |
|
· |
make us more vulnerable to economic downturns and catastrophic external events. |
The above factors could limit our financial and operational flexibility, and as a result could have a material adverse effect on our business, financial condition and results of operations.
The conversion of our 8% Notes will dilute the ownership interests of our existing stockholders .
The issuance of shares of our common stock to satisfy all or a portion of our conversion obligation will dilute the ownership interests of our existing stockholders.
70
ITEM 1B. UNRESOLVED STAFF COMMENTS
None.
Our corporate headquarters and research facilities are located in Malvern, Pennsylvania, where we lease approximately 16,190 square feet of office and laboratory space, pursuant to a lease agreement which expires in July 2016. When our lease expires, we may exercise renewal options or look for additional or alternate space for our operations. We believe that suitable additional or alternative space will be available if required in July 2016 and thereafter on commercially reasonable terms.
We are not currently a party to any legal proceedings.
ITEM 4. MINE SAFETY DISCLOSURES
Not applicable.
ITEM 5. MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
Market Information and Holders
Our common stock is traded on the NASDAQ Global Market under the symbol “TLOG.” Trading of our common stock commenced on December 12, 2013, in connection with our initial public offering. Before then, there was no public market for our common stock. The following table sets forth, for the periods indicated, the high and low sales prices for our common stock as reported on the NASDAQ Global Market.
|
|
|
|
|
|
|
|
|
|
Year Ended December 31, 2014 |
|
HIGH |
|
LOW |
|
||
|
First quarter |
|
$ |
14.75 |
|
$ |
5.89 |
|
|
Second quarter |
|
$ |
8.26 |
|
$ |
3.84 |
|
|
Third quarter |
|
$ |
6.22 |
|
$ |
3.55 |
|
|
Fourth quarter |
|
$ |
6.30 |
|
$ |
3.51 |
|
|
Year Ended December 31, 2013 |
|
|
|
|
|
|
|
|
Fourth quarter (beginning December 12, 2013) |
|
$ |
10.31 |
|
$ |
6.91 |
|
On February 20, 2015, there were approximately 1,900 beneficial holders of our common stock. On February 20, 2015, the closing price of our common stock was $4.94.
Dividends
We have never declared or paid any cash dividends on our capital stock. We currently intend to retain all available funds and any future earnings to support our operations and finance the growth and development of our business. We do not intend to pay cash dividends on our common stock for the foreseeable future.
Securities Authorized for Issuance Under Equity Compensation Plans
See Item 12. “Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters” for information regarding securities authorized for issuance under equity compensation plans.
71
Recent Sales of Unregistered Securities
Set forth below is information regarding certain shares of common stock, preferred stock and stock options issued by us during 2013 and 2014 that were not registered under the Securities Act. Also included is the consideration, if any, received by us for such shares and information relating to the section of the Securities Act, or rule of the SEC, under which exemption from registration was claimed. The information set forth below gives effect to our 1‑for‑17 reverse stock split that was effected on November 18, 2013.
(a)Issuances of Capital Stock:
|
(1) |
In September 2013, we issued 900,085 shares of common stock as a result of a conversion of all of the shares of preferred stock held by Quaker BioVentures, L.P., Quaker BioVentures Tobacco Fund, L.P. and Bio Advance Ventures, L.P., each an existing stockholder, pursuant to a mandatory conversion provision in a note and warrant purchase agreement and our certificate of incorporation. |
|
(2) |
On December 17, 2013, we converted all of our Series A, Series B, Series C and Series C‑1 Convertible Preferred stock into an aggregate of 8,692,906 shares of our common stock. |
|
(3) |
On December 17, 2013, we issued an aggregate of 2,876,419 shares of our common stock upon the mandatory conversion of $19.2 million in principal amount and accrued interest of our outstanding convertible notes. The shares were issued upon the closing of our initial public offering, |
|
(4) |
On December 17, 2013, we issued 64,970 shares of our common stock upon the mandatory exercise of our Series B warrants upon the closing of our initial public offering. |
(b)Issuance of Convertible Notes and Warrants:
|
(1) |
In June 2014, we issued convertible notes in the aggregate principal amount of $47.0 million to Nomura Securities International, Inc. as representative for the initial purchasers named therein. We may not redeem our 8% Notes at our option prior to maturity. Our 8% Notes are convertible prior to maturity, subject to certain conditions, into shares of our common stock at an initial conversion rate of 148.3019 shares per $1,000 principal amount of our 8% Notes (equivalent to an initial conversion price of approximately $6.74 per share of common stock). This conversion rate is subject to adjustment upon the occurrence of certain specified events but will not be adjusted for accrued and unpaid interest. |
|
(2) |
In May 2013, we issued a convertible note in the aggregate principal amount of $3.0 million to Amgen, one of our existing stockholders. On December 17, 2013, these convertible notes were converted into an aggregate 448,767 shares of our common stock, as described in paragraph (a)(3) above. |
|
(3) |
In April 2013 and October 2013, we issued convertible notes to certain of our existing stockholders. The original aggregate principal amount of the notes was $5.0 million and $6.2 million, respectively, for a combined total of aggregate principal amount of $11.2 million. We also issued warrants to certain stockholders in the combined aggregate amount of approximately $4.1 million in connection with the convertible promissory notes. On December 17, 2013, these convertible notes were converted into an aggregate 1,653,413 shares of our common stock, as described in paragraph (a)(3) above, and these warrants were net exercised into an aggregate 54,966 shares of our common stock, as described in paragraph (a)(4) above. |
(c)Grants of Stock Options:
|
(1) |
From January 1, 2013 through December 15, 2013, we granted stock options to purchase an aggregate of 620,967 shares of our common stock with an exercise prices ranging from $1.53 to $6.12 per share, to certain of our employees and consultants in connection with services provided by such parties to us. |
72
|
(2) |
On October 2, 2013, we granted stock options to purchase a total of 1,455,723 shares of common stock at an exercise price of $6.12 per share to three employees pursuant to our 2004 Equity Incentive Plan. |
We deemed the offers, sales and issuances of the securities described in paragraph (b)(1) above to be exempt from registration under Section 5 of the Securities Act provided by Rule 144A of the Securities Act.
We deemed the offers, sales and issuances of the securities described in paragraphs (b)(2), (b)(3) and (c)(2) above to be exempt from registration under the Securities Act, in reliance on Section 4(2) of the Securities Act, including Regulation D and Rule 506 promulgated thereunder, relative to transactions by an issuer not involving a public offering.
The issuances of the securities described in paragraphs (a)(1), (a)(2), (a)(3) and (a)(4) above were exempt from registration under Section 3(a)(9) of the Securities Act.
We deemed the grant of stock options described in paragraph (c)(1) to be exempt from registration under the Securities Act in reliance on Rule 701 pursuant to the Securities Act as offers and sales of securities under compensatory benefit plans and contracts relating to compensation in compliance with Rule 701.
All purchasers of securities in transactions exempt from registration pursuant to Regulation D represented to us that they were accredited investors and were acquiring the shares for investment purposes only and not with a view to, or for sale in connection with, any distribution thereof and that they could bear the risks of the investment and could hold the securities for an indefinite period of time. The purchasers received written disclosures that the securities had not been registered under the Securities Act and that any resale must be made pursuant to a registration statement or an available exemption from the registration requirements of the Securities Act.
All of the foregoing securities are deemed restricted securities for purposes of the Securities Act. The book entries representing the issued shares of common stock described above include appropriate notations setting forth that the applicable securities have not been registered and the applicable restrictions on transfer. There were no underwriters employed in connection with any of the transactions set forth above.
Issuer Purchases of Equity Securities
We did not purchase any shares of our common stock for the three months ended December 31, 2014.
ITEM 6. SELECTED FINANCIAL DATA
The following selected financial data should be read together with “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our financial statements and accompanying notes included as Items 7 and 8, respectively, in this annual report.
We have derived the following statement of operations data for the years ended December 31, 2013 and 2014 and balance sheet data as of December 31, 2013 and 2014 from our audited financial statements included elsewhere in this annual report. Our historical results are not necessarily indicative of the results that may be expected in the future.
73
Selected Financial Data
|
|
|
|
|
|
|
|
|
|
|
|
Year ended December 31, |
|
||||
|
|
|
2013 |
|
2014 |
|
||
|
Revenue |
|
$ |
— |
|
$ |
— |
|
|
Expenses: |
|
|
|
|
|
|
|
|
General and administrative |
|
|
8,467,467 |
|
|
10,926,777 |
|
|
Research and development |
|
|
9,523,427 |
|
|
20,286,826 |
|
|
Change in fair value of contingent consideration |
|
|
— |
|
|
2,572,000 |
|
|
Total expenses |
|
|
17,990,894 |
|
|
33,785,603 |
|
|
Loss from operations |
|
|
(17,990,894) |
|
|
(33,785,603) |
|
|
Change in fair value of derivative liabilities |
|
|
807,291 |
|
|
1,073,198 |
|
|
Interest and other income |
|
|
629 |
|
|
112,815 |
|
|
Interest expense |
|
|
(2,767,422) |
|
|
(3,359,389) |
|
|
Net loss |
|
$ |
(19,950,396) |
|
$ |
(35,958,979) |
|
|
Cumulative preferred stock dividends |
|
|
(3,249,948) |
|
|
— |
|
|
Net loss attributable to common shareholders |
|
$ |
(23,200,344) |
|
$ |
(35,958,979) |
|
|
Per share information: |
|
|
|
|
|
|
|
|
Basic net loss per share of common stock |
|
$ |
(10.11) |
|
$ |
(1.61) |
|
|
Basic weighted average shares outstanding |
|
|
2,295,636 |
|
|
22,290,069 |
|
|
Diluted net loss per share of common stock |
|
$ |
(10.11) |
|
$ |
(1.63) |
|
|
Diluted weighted average shares outstanding |
|
|
2,295,636 |
|
|
22,351,689 |
|
|
|
|
|
|
|
|
|
|
|
|
|
As of December 31, |
|
||||
|
|
|
2013 |
|
2014 |
|
||
|
Balance Sheet Data: |
|
|
|
|
|
|
|
|
Cash, cash equivalents and short-term investments |
|
$ |
55,135,508 |
|
$ |
53,697,486 |
|
|
Total assets |
|
|
55,502,421 |
|
|
118,291,045 |
|
|
Convertible notes payable |
|
|
— |
|
|
28,979,342 |
|
|
Total liabilities |
|
|
4,953,368 |
|
|
84,888,870 |
|
|
Accumulated deficit |
|
|
(89,872,238) |
|
|
(125,831,217) |
|
|
Total stockholders’ equity (deficit) |
|
|
50,549,053 |
|
|
33,402,176 |
|
74
ITEM 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
You should read the following discussion and analysis of our financial condition and results of operations together with our financial statements and related notes appearing in this annual report. Some of the information contained in this discussion and analysis or set forth elsewhere in this annual report, including information with respect to our plans and strategy for our business and related financing, includes forward‑looking statements that involve risks and uncertainties. As a result of many factors, including those factors set forth in the “Risk Factors” section of this annual report, our actual results could differ materially from the results described in or implied by the forward‑ looking statements contained in the following discussion and analysis.
Overview
We are a clinical-stage biopharmaceutical company focused on discovering and developing novel small molecule therapeutics in oncology and infectious diseases. We currently have two clinical-stage product candidates in development: birinapant and suberohydroxamic acid 4-methoxycarbonyl phenyl ester, or SHAPE.
Birinapant is a novel small molecule therapeutic that mimics Second Mitochondrial Activator of Caspases, or SMAC-mimetic, which leads to apoptosis, or cell-death, in damaged cells. We have treated over 300 oncology subjects with birinapant, and in non-randomized clinical trials to date, we have seen activity in subjects with (i) higher risk myelodysplastic syndromes, or MDS, where we have observed complete bone marrow responses with birinapant administered with azacitidine (Vidaza®); (ii) end-stage acute myeloid leukemia, where birinapant was administered as a single-agent and subjects who have previously relapsed or were refractory to standard therapy experienced declines in blast counts; (iii) ovarian cancer, where birinapant was administered with conatumumab (AMG 655), we have observed disease stabilization and a partial response, or PR, in women who previously relapsed or were refractory to standard therapy; and (iv) colorectal cancer, or CRC, where birinapant was administered with irinotecan, we have observed evidence of anti-tumor activity or prolonged disease stabilization in subjects who have progressed after multiple prior therapies, including irinotecan.
Most recently, we have generated pre-clinical data indicating that birinapant induces apoptosis in-vivo in mouse hepatocytes infected with human hepatitis B virus, or HBV. In a mouse model, we have seen clearance of HBV surface antigen, or HBsAg, and the appearance of antibodies directed against HBsAg, a clinical finding considered equivalent to a functional cure. We have also seen activity of birinapant in other infectious disease models, including human mononuclear cells infected with human immunodeficiency virus, or HIV, in-vitro, and in-vivo in mouse models of Mycobacterium tuberculosis and legionella pneumophila.
We are currently conducting the following three clinical programs with birinapant:
|
· |
In June 2014, we commenced a randomized, double-blind placebo-controlled Phase 2 clinical trial of birinapant administered with azacitidine in subjects with previously untreated, higher risk MDS. Interim data is expected in 2015. This clinical trial follows our Phase 1b/2a open-label clinical trial of birinapant administered with azacitidine. |
|
· |
We are continuing enrollment in a Phase 1/2 open-label, non-randomized clinical trial of birinapant administered with conatumumab in third-line ovarian cancer. In December 2014, this clinical trial proceeded into a Phase 2a expansion based upon data in the Phase 1 portion of the clinical trial. Phase 2a clinical trial data is expected in 2015. |
|
· |
We recently initiated a randomized, placebo-controlled, multiple ascending dose Phase 1 clinical trial in subjects with chronic HBV currently taking entecavir or tenofovir. Data is expected in 2015. |
We discovered birinapant, and its composition of matter patent in the U.S. extends until at least 2030. We have retained worldwide development and commercialization rights for all indications.
75
SHAPE, our second clinical-stage product candidate, is a histone deacetylase, or HDAC, inhibitor that we are developing for topical use for the treatment of early-stage cutaneous T-cell lymphoma, or CTCL. HDAC is a validated cancer target, and HDAC inhibitors, or HDACi, are a proven class of anti-cancer drugs for CTCL. SHAPE is a novel therapeutic, designed to maximize HDAC inhibition locally in the skin with limited systemic exposure, and it has characteristics that could allow its topical use over large body surface areas with minimal systemic absorption. By potentially avoiding toxicities typical of systemically-administered HDACi’s, SHAPE may provide a more favorable safety profile than current HDACi’s delivered orally or intravenously. SHAPE has been evaluated in a randomized, placebo-controlled dose escalation Phase 1 clinical trial in early-stage CTCL. SHAPE was well-tolerated, and it demonstrated evidence of clinical activity with PRs observed in certain subjects after 28 days of application. We commenced a randomized Phase 2 clinical trial of SHAPE in subjects with early-stage CTCL in December 2014, which will assess clinical activity after six months of application. Data for this clinical trial is expected in 2015.
SHAPE’s composition of matter patent in the U.S. extends until at least 2028. In addition, SHAPE has been granted U.S. orphan drug designation for CTCL. We have acquired worldwide development and commercialization rights to SHAPE for all indications.
We have incurred operating losses since inception, have not generated any product sales revenues and have not achieved profitable operations. Our accumulated deficit through December 31, 2014 was $125.8 million, and we expect to continue to incur substantial losses in future periods.
We incurred research and development expenses of $9.5 million and $20.3 million during the years ended December 31, 2013 and 2014, respectively. We anticipate that a significant portion of our operating expenses will continue to be related to research and development as we continue to advance our clinical-stage product candidates, birinapant and SHAPE. We funded our operations as a private company primarily through the sale of preferred stock and the issuance of convertible notes for gross proceeds totaling $85.4 million, which have been converted into shares of our common stock in connection with our initial public offering. We also received amounts under collaboration and grant arrangements totaling $13.7 million. In addition, in December 2013 we sold 8,222,115 shares of common stock in our initial public offering for net proceeds of $49.1 million after payment of underwriting fees and other expenses, and in June 2014 we issued convertible notes for proceeds of $44.1 million, net of underwriting fees and other expenses. As of December 31, 2013 and December 31, 2014, we had $55.1 million and $53.7 million in cash, cash equivalents and short-term investments, respectively.
We are highly dependent on the success of our research, development and licensing efforts and, ultimately, upon regulatory approval and market acceptance of birinapant and SHAPE. Our short- and long-term capital requirements depend upon a variety of factors, including our clinical development plan and various other factors discussed below. We believe that our existing cash, cash equivalents and short-term investments as of December 31, 2014 will enable us to fund our operating expenses and capital expenditure requirements through early 2016. However, we will need to secure additional funding in the future, from one or more equity or debt financings, collaborations, or other sources, in order to carry out all of our planned research and development activities with respect to our product candidates.
Our future funding requirements, both near- and long-term, will depend on many factors, including, but not limited to the:
|
· |
initiation, progress, timing, costs and results of pre-clinical studies and clinical trials for birinapant, SHAPE or any other future product candidates; |
|
· |
clinical development plans we establish for birinapant, SHAPE and any other future product candidates; |
|
· |
our obligation to make milestone payments and royalty and non-royalty sublicense receipt payments to third-party licensors, if any, under our licensing agreements; |
|
· |
number and characteristics of product candidates that we discover or in-license and develop; |
76
|
· |
outcome, timing and cost of regulatory review by the FDA and comparable foreign regulatory authorities, including the potential for the FDA or comparable foreign regulatory authorities to require that we perform more studies than those that we currently expect; |
|
· |
costs of filing, prosecuting, defending and enforcing any patent claims and maintaining and enforcing or defending other intellectual property rights; |
|
· |
effect of competing technological and market developments; |
|
· |
costs and timing of the implementation of commercial-scale manufacturing activities; and |
|
· |
costs and timing of establishing sales, marketing and distribution capabilities for birinapant, SHAPE and any other future product candidates for which we may receive regulatory approval. |
The following discussion and analysis provides information that we believe is relevant to an assessment and understanding of our results of operations for the years ended December 31, 2013 and 2014, and our financial condition as of December 31, 2013 and 2014.
Critical Accounting Policies and Significant Judgments and Estimates
This management’s discussion and analysis of our financial condition and results of operations is based on our financial statements, which have been prepared in accordance with GAAP. The preparation of our financial statements requires us to make estimates and judgments that affect the reported amounts of assets, liabilities, revenues and expenses and the disclosure of contingent assets and liabilities in our financial statements. We evaluate our estimates and judgments, including those related to derivative liabilities, stock‑based compensation and accrued expenses on an ongoing basis. We base our estimates on historical experience, known trends and events and various other factors that we believe to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions. You should consider your evaluation of our financial condition and results of operations with these policies, judgments and estimates in mind.
While our significant accounting policies are described in the notes to our financial statements appearing elsewhere in this annual report, we believe the following accounting policies to be most critical to the judgments and estimates used in the preparation of our financial statements.
Stock‑Based Compensation
We account for stock‑based compensation in accordance with the provisions of Accounting Standards Codification, or ASC, Topic 718, Compensation—Stock Compensation, or ASC 718, which requires the recognition of expense related to the fair value of stock‑based compensation awards in the statements of operations and comprehensive loss. For stock options issued to employees and members of our board of directors for their services on our board of directors, we estimate the grant date fair value of each option using the Black‑Scholes option pricing model. The use of the Black‑Scholes option pricing model requires management to make assumptions with respect to the expected term of the option, the expected volatility of the common stock consistent with the expected life of the option, risk‑free interest rates, the value of the common stock and expected dividend yields of the common stock. For awards subject to service‑based vesting conditions, we recognize stock‑based compensation expense, net of estimated forfeitures, equal to the grant date fair value of stock options on a straight‑line basis over the requisite service period, which is generally the vesting term. For awards subject to performance‑based vesting conditions, we recognize stock‑based compensation expense using the accelerated attribution method when it is probable that the performance condition will be achieved. Forfeitures are required to be estimated at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differ from those estimates. Stock‑based payments issued to non‑employees are recorded at their fair values, and are periodically revalued as the equity instruments vest and are recognized as expense over the related service period in accordance with the provisions of ASC 718 and ASC Topic 505, Equity.
77
Stock‑based compensation expense recognized by award type is as follows:
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended December 31, |
|
||||
|
|
|
2013 |
|
2014 |
|
||
|
Option awards |
|
$ |
3,160,067 |
|
$ |
3,299,870 |
|
|
Restricted stock awards |
|
|
548,325 |
|
|
84,930 |
|
|
Total stock-based compensation expense |
|
$ |
3,708,392 |
|
$ |
3,384,800 |
|
Total compensation cost recognized for all stock‑based compensation awards in the statements of operations is as follows:
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended December 31, |
|
||||
|
|
|
2013 |
|
2014 |
|
||
|
Research and development |
|
$ |
886,808 |
|
$ |
933,814 |
|
|
General and administrative |
|
|
2,821,584 |
|
|
2,450,986 |
|
|
Total stock-based compensation expense |
|
$ |
3,708,392 |
|
$ |
3,384,800 |
|
Prior to our initial public offering, we were required to estimate the fair value of the common stock underlying our stock‑based awards when performing the fair value calculations at each grant date. We engaged an independent third‑party valuation firm to assist our board of directors in determining the fair value of the common stock underlying our stock‑based awards. All options to purchase shares of our common stock have been granted with an exercise price per share no less than the fair value per share of our common stock underlying those options on the date of grant, based on the information known to us on the date of grant.
Clinical Trial Expense Accruals
As part of the process of preparing our financial statements, we are required to estimate our accrued expenses. Our clinical trial accrual process seeks to account for expenses resulting from our obligations under contracts with vendors, consultants and CROs and clinical site agreements in connection with conducting clinical trials. The financial terms of these contracts are subject to negotiations, which vary from contract to contract and may result in payment flows that do not match the periods over which materials or services are provided to us under such contracts. Our objective is to reflect the appropriate clinical trial expenses in our financial statements by matching the appropriate expenses with the period in which services and efforts are expended. We account for these expenses according to the progress of the trial as measured by subject progression and the timing of various aspects of the trial. We determine accrual estimates through financial models that take into account discussion with applicable personnel and outside service providers as to the progress or state of completion of trials. During the course of a clinical trial, we adjust our clinical expense recognition if actual results differ from our estimates. We make estimates of our accrued expenses as of each balance sheet date in our financial statements based on the facts and circumstances known to us at that time. Our clinical trial accrual and prepaid assets are dependent, in part, upon the receipt of timely and accurate reporting from CROs and other third‑party vendors. Although we do not expect our estimates to be materially different from amounts actually incurred, our understanding of the status and timing of services performed relative to the actual status and timing of services performed may vary and may result in us reporting amounts that are too high or too low for any particular period.
Contingent Consideration
In April 2014, we acquired by merger 100% of Shape Pharmaceuticals, a development stage pharmaceutical company developing SHAPE. The acquisition of Shape Pharmaceuticals includes a contingent consideration arrangement that may require us to pay additional consideration in the form of milestone payments and tiered royalty payments upon commercialization. We account for contingent consideration in accordance with applicable guidance provided within ASC 805, Business Combinations. It is currently estimated that the Shape Pharmaceuticals milestone payments will occur between 2016 and 2020. The range of undiscounted milestones we could be required to pay under
78
our agreement is between zero and $64.5 million. We determined the fair value of the liability for the contingent consideration based on a probability-weighted discounted cash flow analysis. This fair value measurement is based on significant inputs not observable in the market and thus represents a Level 3 measurement within the fair value hierarchy. The fair value of the contingent consideration liability associated with future milestone payments was based on several factors including:
|
· |
estimated cash flows projected from the success of unapproved product candidates in the U.S. and Rest of World, or ROW; |
|
· |
the probability of success for product candidates including risks associated with uncertainty, achievement and payment of milestone events; |
|
· |
the time and resources needed to complete the development and approval of product candidates; |
|
· |
the life of the potential commercialized products and associated risks of obtaining regulatory approvals in the U.S. and ROW; and |
|
· |
the risk adjusted discount rate for fair value measurement. |
The contingent consideration payments have been recorded as a liability and the fair value is evaluated quarterly or more frequently if circumstances dictate. Changes in the fair value of contingent consideration are recorded in earnings.
Interest Make-whole Derivative
Our 8% Notes include an interest make-whole feature whereby if a noteholder converts any of the Notes after December 31, 2014, they are entitled, in addition to the other consideration payable or deliverable in connection with such conversion, to an interest make-whole payment through the earlier of (i) the date that is three years after the conversion date and (ii) the maturity date if the notes had not been so converted. We have determined that this feature is an embedded derivative and have recognized the fair value of this derivative as a liability on our balance sheet, with subsequent changes to fair value recorded through earnings at each reporting period on our statements of operations and comprehensive loss as change in fair value of derivative liabilities. The fair value of this embedded derivative was determined based on a binomial lattice model.
Financial Overview
Revenue
We have not generated any revenue from commercial product sales since we commenced operations. In the future, if our product candidates are approved for commercial sale, we may generate revenue from product sales, or alternatively, we may choose to select a collaborator or licensee to commercialize our product candidates.
General and Administrative Expenses
General and administrative expenses consist principally of salaries and related costs for executive and other administrative personnel, including stock‑based compensation and travel expenses. Other general and administrative expenses include professional fees for legal, patent review, consulting and accounting services. General and Administrative Expenses are expensed when incurred.
For the years ended December 31, 2013 and 2014, our general and administrative expenses totaled approximately $8.5 million, and $10.9 million, respectively. The increase in general and administrative expenses is due primarily to the increase in ongoing costs associated with becoming a public company of $2.4 million, including increases in headcount, recruiting, Board and other fees, insurance, and investor relations. This increase is also due to an
79
increase in legal expenses of $0.8 million, including $0.4 million of transaction costs related to the acquisition of Shape Pharmaceuticals in April 2014. This increase was offset by the recognition of $1.0 million of bonus and severance expenses for a former executive in 2013.
Research and Development Expenses
Our research and development expenses consist primarily of costs incurred for the development of our product candidates, which include:
|
· |
employee‑related expenses, including salaries, benefits, travel and stock‑based compensation expense; |
|
· |
expenses incurred under agreements with CROs and investigative sites that conduct our clinical trials and pre‑clinical studies; |
|
· |
the cost of acquiring, developing and manufacturing clinical trial materials; |
|
· |
facilities, depreciation and other expenses, which include direct and allocated expenses for rent and maintenance of facilities, insurance and other supplies; and |
|
· |
costs associated with pre‑clinical activities and regulatory operations. |
Research and development costs are expensed when incurred. We record costs for some development activities, such as clinical trials, based on an evaluation of the progress to completion of specific tasks using data such as subject enrollment, clinical site activations or information provided to us by our vendors.
During the years ended December 31, 2013 and 2014, we incurred approximately $9.5 million, and $20.3 million, respectively, in research and development expenses. Research and development expenses increased during the year ended December 31, 2014 primarily due to increased manufacturing and formulation activities related to birinapant and SHAPE and increased expenses relating to our MDS, ovarian, HBV and SHAPE clinical trials, offset by a decrease in expenses relating to our CRC clinical trial.
Research and development activities are central to our business model. Product candidates in later stages of clinical development generally have higher development costs than those in earlier stages of clinical development, primarily due to the increased size and duration of later‑stage clinical trials. We do not currently utilize a formal time allocation system to capture expenses on a project‑by‑project basis as the majority of our past and planned expenses have been and will be in support of birinapant. However, we do allocate some portion of our research and development expenses by functional area, as shown below.
The following table summarizes our research and development expenses for the years ended December 31, 2013 and 2014:
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended |
|
||||
|
|
|
December 31, |
|
||||
|
|
|
2013 |
|
2014 |
|
||
|
Clinical development |
|
$ |
2,605,788 |
|
$ |
8,969,010 |
|
|
Manufacturing and formulation |
|
|
577,026 |
|
|
3,100,528 |
|
|
Personnel related |
|
|
4,340,532 |
|
|
5,424,838 |
|
|
Stock-based compensation |
|
|
886,808 |
|
|
933,814 |
|
|
Consulting |
|
|
893,332 |
|
|
496,929 |
|
|
Other research and non-clinical development |
|
|
219,941 |
|
|
1,361,707 |
|
|
|
|
$ |
9,523,427 |
|
$ |
20,286,826 |
|
80
The following table summarizes our research and development expenses by targeted indication for the years ended December 31, 2013 and 2014:
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended |
|
||||
|
|
|
December 31, |
|
||||
|
|
|
2013 |
|
2014 |
|
||
|
Blood cancers (MDS) |
|
$ |
3,574,730 |
|
$ |
10,188,957 |
|
|
Solid tumors (CRC and ovarian) |
|
|
4,371,717 |
|
|
1,858,584 |
|
|
Infectious diseases (HBV) |
|
|
— |
|
|
578,837 |
|
|
Shape (CTCL) |
|
|
— |
|
|
2,158,114 |
|
|
Other pre-clinical and non-indication specific |
|
|
1,576,980 |
|
|
5,502,334 |
|
|
|
|
$ |
9,523,427 |
|
$ |
20,286,826 |
|
We will need to secure additional funding in the future in order to carry out all of our planned research and development activities with respect to our product candidates. We will incur substantial costs beyond our present and planned clinical trials in order to file NDAs in target indications for both birinapant and SHAPE, and in each case, the nature, design, size and cost of further studies and trials will depend in large part on the outcome of preceding studies and trials and discussions with regulators.
It is difficult to determine with certainty the costs and duration of our current or future clinical trials and pre-clinical studies, or if, when or to what extent we will generate revenues from the commercialization and sale of our product candidates if we obtain regulatory approval. We may never succeed in achieving regulatory approval for our product candidates. The duration, costs and timing of clinical trials and development of our product candidates will depend on a variety of factors, including the uncertainties of future clinical trials and pre-clinical studies, uncertainties in clinical trial enrollment rate and significant and changing government regulation.
In addition, the probability of success for our product candidates will depend on numerous factors, including competition, manufacturing capability and commercial viability. See Risk Factors in Item 1A of this Annual Report.
Market acceptance of our product candidates, if we receive approval, depends on a number of factors, including the:
|
· |
efficacy and safety of our product candidates administered alone or with other drugs, and post-marketing experience; |
|
· |
clinical indications for which our product candidates are approved; |
|
· |
acceptance by physicians, major operators of cancer or infectious disease clinics and patients of our product candidates as safe and effective treatments; |
|
· |
potential and perceived advantages of our product candidates over alternative treatments; |
|
· |
prevalence and severity of any side effects; |
|
· |
product labeling or package insert requirements of the FDA or other regulatory authorities; |
|
· |
timing of market introduction of our product candidates as well as competitive products; |
|
· |
cost of treatment in relation to alternative treatments; |
|
· |
availability of coverage and adequate reimbursement and pricing by third-party payors and government authorities; |
|
· |
relative convenience and ease of administration; and |
81
|
· |
effectiveness of our sales and marketing efforts. |
We will determine which programs to pursue and how much to fund each program in response to the scientific and clinical success of our product candidates, as well as an assessment of their commercial potential.
Change in fair value of contingent consideration
The acquisition of Shape Pharmaceuticals includes a contingent consideration arrangement that may require us to pay additional consideration in the form of milestone payments and tiered royalties. We recorded the liability for the contingent consideration based on its fair value on the date of the acquisition, and we record any change in fair value of the contingent consideration in our statements of operations and comprehensive loss as a change in fair value of contingent consideration included in loss from operations. The change in fair value of the contingent consideration of $2.6 million recorded for the year ended December 31, 2014 was due to accretion related to the passage of time
Change in fair value of derivative liability
Certain of our warrants to purchase our common stock are classified as derivative liabilities and recorded at fair value. These derivative liabilities are subject to re-measurement at each balance sheet date and we recognize any change in fair value in our statements of operations and comprehensive loss as a change in fair value of the derivative liability. The change in the fair value of these derivative liabilities of $(0.4) million for the year ended December 31, 2014 is due primarily to the decrease in the value of our common stock from December 2013 to December 2014.
We have classified the interest make-whole provision of our 8% Notes due 2019 issued in June 2014 as a derivative liability that is recorded at fair value. This derivative liability is subject to re-measurement at each balance sheet date and we recognize any change in fair value in our statements of operations and comprehensive loss as a change in fair value of the derivative liability. The change in the fair value of this derivative liability of $(0.7) million for the year ended December 31, 2014 is due primarily to the change in the value of our common stock from the date of issuance of our 8% Notes to December 31, 2014.
Interest and Other Income
Interest and other income consists principally of interest income earned on cash and cash equivalent balances.
Interest Expense
Interest expense is mostly attributable to coupon interest on our convertible notes and to non-cash interest expense resulting from the accretion of the debt discount and beneficial conversion feature associated with our convertible notes. Interest expense recognized for the year ended December 31, 2013 represented interest expense and the accretion of debt discount in connection with our convertible notes and warrants issued in November 2012, April 2013 and October 2013, plus interest expense on our collaboration loan received in May 2013. All of our then outstanding convertible notes and warrants and the collaboration loan were converted into our common stock in December 2013. Interest expense recognized for the year ended December 31, 2014 is related to our 8% Notes issued in June 2014.
Cash Flows
Operating Activities. Cash used in operating activities during the year ended December 31, 2014 increased to $32.4 million as compared to $12.8 million used in the year ended December 31, 2013. This increase was driven primarily by increased costs for both research and development and administration.
Investing Activities. Cash used in investing activities was $0.1 million and $54.0 million for the years ended December 31, 2013 and 2014, respectively. The increase in cash used in investing activities for the year ended December 31, 2014 is attributable primarily to the purchase of Shape Pharmaceuticals in April 2014 for $12.8 million,
82
net of cash received, and to the purchase of short-term investments in corporate bonds and commercial paper during 2014.
Financing Activities. Cash provided by financing activities was $63.5 million and $44.3 million for the years ended December 31, 2013 and 2014, respectively. Cash provided by financing activities is attributable to proceeds from the issuance of our 8% Notes in 2014. In 2013, cash provided from financing activities is mostly attributable to $49.1 million from our initial public offering of common stock and $14.2 million from the sale of convertible notes.
JOBS Act
We are an “emerging growth company” under Section 107 of the JOBS Act. As an emerging growth company, we can take advantage of the extended transition period provided in Section 7(a)(2)(B) of the Securities Act for complying with new or revised accounting standards. In other words, an “emerging growth company” can delay the adoption of new or revised accounting standards until those standards would otherwise apply to private companies. We have irrevocably elected not to avail ourselves of this exemption from new or revised accounting standards and, therefore, we will be subject to the same new or revised accounting standards as other public companies that are not emerging growth companies.
Liquidity and Capital Resources
Since our inception, we have incurred net losses and negative cash flows from our operations. We incurred net losses of $20.0 million and $36.0 million for the years ended December 31, 2013 and 2014, respectively. Our operating activities used $12.8 million and $32.4 million of cash flows during the years ended December 31, 2013 and 2014, respectively. At December 31, 2014, we had an accumulated deficit of $125.8 million, working capital of $50.7 million and cash, cash equivalents and short-term investments of $53.7 million. We funded our operations as a private company primarily through the sale of preferred stock and the issuance of convertible notes for gross proceeds totaling $85.4 million, which were converted into shares of our common stock in connection with our initial public offering. We also received amounts under collaboration and grant arrangements totaling $13.7 million. In addition, in December 2013 we sold 8,222,115 shares of common stock in our initial public offering for net proceeds of $49.1 million after payment of underwriting fees and other expenses, and in June 2014 we issued our 8% Notes for proceeds of $44.1 million, net of underwriting fees and other expenses, as further described below.
On June 23, 2014, we issued through a private placement $47.0 million in aggregate principal amount of our 8% Notes, all of which remain outstanding as of December 31, 2014. Interest on our 8% Notes is payable semi-annually in arrears on June 15 and December 15 of each year, which commenced December 15, 2014. Our 8% Notes are general unsecured and unsubordinated obligations and rank senior in right of payment to all of our indebtedness that is expressly subordinated in right of payment to the notes, rank equal in right of payment to our existing and future indebtedness and other liabilities that are not so subordinated, are effectively subordinated to any of our future secured indebtedness to the extent of the value of the assets securing such indebtedness, and rank structurally junior to all indebtedness and other liabilities incurred by our subsidiaries, including trade payables. We may not redeem our 8% Notes at our option prior to maturity. Our 8% Notes are convertible prior to maturity, subject to certain conditions described below, into shares of our common stock at an initial conversion rate of 148.3019 shares per $1,000 principal amount of our 8% Notes (equivalent to an initial conversion price of approximately $6.74 per share of common stock). This conversion rate is subject to adjustment upon the occurrence of certain specified events but will not be adjusted for accrued and unpaid interest. If we receive stockholder approval, we may satisfy our conversion obligation by paying or delivering, as the case may be, cash, shares of our common stock or a combination thereof, at our election. See Note 8 of Notes to Consolidated Financial Statements for additional information. The indenture for our 8% Notes contains certain covenants which limit our and our subsidiaries’ ability to incur certain additional indebtedness except for certain permitted debt, and to incur liens except for certain permitted liens. We will also be restricted from taking certain actions that could result in an adjustment to the conversion rate of our 8% Notes until we receive stockholder approval. We are in compliance with all the covenants set forth in the indenture governing our 8% Notes.
In April 2014, we acquired by merger Shape Pharmaceuticals, a development stage pharmaceutical company developing SHAPE. We acquired this company to add a second clinical-stage oncology compound to the TetraLogic
83
portfolio. We paid $13.0 million in cash at closing and entered into a contingent consideration arrangement that may require us to pay additional consideration, including milestone payments and tiered royalty payments upon commercialization. We currently estimate that the milestone payments will occur between 2016 and 2020. The range of undiscounted amounts that we could be required to pay under our agreement for the milestone payments is between zero and $64.5 million.
Similar to other clinical-stage biopharmaceutical companies, our access to traditional bank credit is limited. Although we have had a revolving line of credit in the past, we do not currently have an open revolving line of credit or access to bank finance. We have limited assets which can be used as collateral to secure potential indebtedness. Moreover, as noted above, we have not received any material revenues since inception. Therefore, our ability to fund our operations and sustain our clinical development programs is dependent on our ability to raise additional funding, including through issuances of debt or equity securities. None of our current investors is required to invest any additional capital in us. Thus, there can be no assurances that we will be able to raise sufficient capital in the future from these or other similar sources or the public markets to fund our operations, and failure to do so could have a material adverse effect on our operations. In addition, the need to raise capital is expected to consume management resources, time and attention and, to a lesser extent, the time and attention of our scientific staff.
Operating and Capital Expenditure Requirements
We have not achieved profitability since our inception and we expect to continue to incur net losses for the foreseeable future. We expect our cash expenditures to increase in the near term as we fund our planned clinical trials for our product candidates. Following our initial public offering, we are now a publicly traded company and have incurred significant additional legal, accounting and other expenses that we were not required to incur as a private company. In addition, the Sarbanes‑Oxley Act, as well as rules adopted by the SEC and the NASDAQ Stock Market, requires public companies to implement specified corporate governance practices that were inapplicable to us as a private company. These rules and regulations have increased our legal and financial compliance costs and will make some activities more time‑consuming and costly.
We believe that our existing cash and cash equivalents as of December 31, 2014 will enable us to fund our operating expenses and capital expenditure requirements through early 2016. However, we will need to secure additional funding in the future, from one or more equity or debt financings, collaborations, or other sources, in order to carry out all of our planned research and development activities with respect to our product candidates. In order to meet these additional cash requirements, we may seek to sell additional equity or convertible debt securities that may result in dilution to our stockholders. If we raise additional funds through the issuance of convertible debt securities, these securities could have rights senior to those of our common stock and could contain covenants that restrict our operations. There can be no assurance that we will be able to obtain additional equity or debt financing on terms acceptable to us, if at all. Our failure to obtain sufficient funds on acceptable terms when needed could have a negative impact on our business, results of operations, and financial condition. Our future capital requirements will depend on many factors, including:
|
· |
the results of our pre-clinical studies and clinical trials; |
|
· |
the development and commercialization of birinapant and SHAPE; |
|
· |
the scope, progress, results and costs of researching and developing birinapant, SHAPE or any other future product candidates, and conducting pre-clinical studies and clinical trials; |
|
· |
the timing of, and the costs involved in, obtaining regulatory approvals for birinapant, SHAPE or any other future product candidates; |
|
· |
the cost of commercialization activities if birinapant, SHAPE or any other future product candidates are approved for sale, including marketing, sales and distribution costs; |
84
|
· |
the cost of manufacturing birinapant, SHAPE or any other future product candidates in pre-clinical studies, clinical trials and, if approved, in commercial sale; |
|
· |
our ability to establish and maintain strategic collaborations, licensing or other arrangements and the financial terms of such agreements; |
|
· |
any product liability infringement or other lawsuits related to our products; |
|
· |
the expenses needed to attract and retain skilled personnel; |
|
· |
the costs associated with being a public company; |
|
· |
the costs involved in preparing, filing, prosecuting, maintaining, defending and enforcing patent claims, including litigation costs and the outcome of such litigation; |
|
· |
the timing, receipt and amount of sales of, or royalties on, future approved products, if any; and |
|
· |
the costs associated with any future acquisitions or in-licensing of additional assets or companies to further expand our technology base. |
In April 2014, we acquired by merger Shape Pharmaceuticals, a development stage pharmaceutical company developing SHAPE. We acquired this company to add a second clinical-stage oncology compound to the TetraLogic portfolio. We paid $13 million in cash at closing and entered into a contingent consideration arrangement that may require us to pay additional consideration, including milestone payments and tiered royalty payments upon commercialization. We may in-license or acquire additional assets or companies in the future to further expand our technology base. However, we may not have sufficient cash reserves or other liquid assets to consummate such acquisitions and it may be necessary for us to raise additional funds to complete future transactions.
Contractual Obligations and Commitments
The following is a summary of our long‑term contractual cash obligations as of December 31, 2014:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Less Than |
|
|
|
|
|
|
|
More Than |
|
||
|
|
|
Total |
|
One Year |
|
1 ‑ 3 Years |
|
3 ‑ 5 Years |
|
5 Years |
|
|||||
|
Operating lease obligations |
|
$ |
748,287 |
|
$ |
438,939 |
|
$ |
309,348 |
|
$ |
— |
|
$ |
— |
|
|
Convertible notes |
|
|
47,000,000 |
|
|
— |
|
|
— |
|
|
47,000,000 |
|
|
— |
|
|
Total contractual obligations |
|
$ |
47,748,287 |
|
$ |
438,939 |
|
$ |
309,348 |
|
$ |
47,000,000 |
|
$ |
— |
|
Royalty‑Based and Other Commitments
License Agreement with Princeton University
In November 2003, we entered into an exclusive license agreement with Princeton University, subsequently amended in June 2004, August 2006 and October 2006, which grants us the rights to certain U.S. patents controlled by the university relating to SMAC‑mimetic compounds, including birinapant, and a non‑exclusive right to certain know‑how and technology relating thereto. The agreement contains a right by us to sublicense. To date, we have paid an aggregate of $100,000 in license fees under the license agreement. As part of the consideration paid, we issued to Princeton University 9,734 shares of our common stock and agreed to pay Princeton University certain royalties. In particular, we are obligated to pay royalties as a percentage of net product sales of 2.0% for direct licensed products, such as birinapant, and 0.5% of derived licensed products, if such products are covered by the applicable Princeton University patent rights. We have the right to reduce the amount of royalties owed to Princeton University by the amount of any royalties paid to a third‑party in a pro rata manner, provided that the royalty rate may not be less than 1.0% of net sales for direct licensed products and 0.25% for derived licensed products. The obligation to pay royalties in the U.S.
85
expires upon the expiration, lapse or abandonment of the last of the licensed patent rights that covers the manufacture, use or sale of the direct licensed products. The obligation to pay royalties outside the U.S. expires, on a country by country basis, 10 years from the first commercial sale of a licensed product in each country. The licensed patent rights were developed using federal funds from the National Institutes of Health and are subject to certain overriding rights of and obligations to the federal government as provided in the Bayh—Dole Act. This agreement expires upon expiration of the last of the licensed patent rights in 2023 (absent extensions).
The agreement also requires that we pay to Princeton University 5.0% of the non‑royalty consideration that we receive from a sublicensee until October 5, 2014 and 2.5% thereafter. Under the license agreement, we are obligated to use reasonable efforts to develop, test, obtain regulatory approval, manufacture, market and sell licensed products in all countries worldwide.
License Agreement with Walter and Eliza Hall Institute
In January 2014, we entered into a license agreement with the Walter and Eliza Hall Institute of Medical Research, or WEHI, in Melbourne, Australia for worldwide exclusive rights to a patent application and any patents issuing therefrom relating to a method of treating intracellular infections involving the administration of an IAP antagonist. WEHI will perform research and development services for which we will be making payments over the first 4 years of the agreement in the amount of $1 million, of which we have paid $500,000 to date. We are obligated to pay royalties as a percentage of net sales of 2% for products that are based either on the licensed patents or any patents arising from research performed by WEHI. We are also obligated to pay royalties as a percentage of net income of 15% received from sublicensing the licensed patents or any patents arising from research performed by WEHI to a third party. We may also be required to make milestone payments to WEHI of up to $3,750,000 for the first indication and up to $1,875,000 for each of the next two indications based on the commencement of certain clinical trials and the filing and approval of new drug applications.
License Agreement with Harvard University and Dana-Farber Cancer Institute
In October 2008, Shape Pharmaceuticals entered into a license agreement with Harvard University and Dana-Farber Cancer Institute, Inc. (the “Licensors”) to grant a license under its interest in certain patent rights as defined in the license agreement, which include claims covering the composition of the SHAPE molecule. The agreement contains a right by us to sublicense. The Licensors received 400,000 shares of common stock of Shape Pharmaceuticals in consideration for the grant of the license, for which they received a payment of $213,317 when we acquired Shape Pharmaceuticals in April 2014. We also paid the Licensors an annual maintenance fee of $100,000 in 2012 and will pay $50,000 on the fifth anniversary of the effective date of the agreement and on each subsequent anniversary date thereafter as long as the license agreement remains in full force and effect. The annual maintenance fee of $50,000 was paid in 2013 and 2014. As defined in the license agreement, we may be required to pay milestones on an indication-by-indication basis of up to $4,450,000 in the aggregate and/or royalties of net sales of developed products, if and when achieved. Annual maintenance fee payments can be used to offset milestone obligations. We paid a milestone payment of $100,000 during the year ended December 31, 2011. We have the right to terminate the agreement upon 60 days’ written notice.
CTCL Trial with The Leukemia and Lymphoma Society
In June 2010, we entered into a funding agreement with The Leukemia and Lymphoma Society, or LLS, to fund the development of SHAPE. Under the LLS funding agreement, we are obligated to use the funding received exclusively for the payment or reimbursement of the costs and expenses for clinical development activities for SHAPE. Under this agreement, we retain ownership and control of all intellectual property pertaining to works of authorship, inventions, know-how, information, data and proprietary material.
Under the LLS funding agreement, as amended, we received funding of $2.695 million from LLS through 2014. We terminated the funding agreement effective as of February 2014. We are required to make specified payments to LLS, including payments payable upon execution of the first out-license; first filing of approval for marketing by a regulatory body; first approval for marketing by a regulatory body; and completion of the first commercial sale of
86
SHAPE. The extent of these payments and our obligations will depend on whether we out-license rights to develop or commercialize SHAPE to a third party, we commercialize SHAPE on our own or we combine with or are sold to another company. In addition, we will pay to LLS a single-digit percentage royalty of our net sales of SHAPE, if any. The sum of our payments to LLS is capped at three times the total funding received from LLS, or $8.085 million.
In addition, some of our obligations under the funding agreement will remain in effect until the completion of specified milestones and payments to LLS. Assuming the successful outcome of the development activities covered by the LLS funding agreement and our receipt of necessary regulatory approvals, we will be required to take commercially reasonable steps through 2019 to advance the development of SHAPE in clinical trials and to bring SHAPE to practical application for CTCL in a major market country, provided that we reasonably believe the product is safe and effective. We believe that we can satisfy our obligation by out-licensing SHAPE to, or partnering SHAPE with, a third party. We are required to report to LLS on our efforts and results with respect to continuing development of SHAPE. Our failure to perform these diligence obligations, even if we successfully achieve the specified development milestones, would require us to pay back to LLS the total amount of the funding we received from them, unless an exception applies. If LLS were to claim that such failure occurred and we disagreed with such claim, the dispute would be settled through binding arbitration. In connection with the accounting for the acquisition of Shape Pharmaceuticals, we estimated the fair value of this potential obligation to be $200,000 and have accrued this amount in our December 31, 2014 balance sheet.
Because the achievement and timing of our net sales is dependent on successful completion of our clinical programs and is therefore not fixed and determinable, our commitments under these agreements have not been included on our balance sheets or in the Contractual Obligations and Commitments table above.
Off‑Balance Sheet Arrangements
We do not have any off‑balance sheet arrangements, as defined by applicable SEC regulations.
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
We are exposed to market risks in the ordinary course of our business. These market risks are principally limited to interest rate fluctuations.
We had cash, cash equivalents and short-term investments of $55.1 million and $53.7 million at December 31, 2013 and 2014, respectively, consisting primarily of funds in cash, money market accounts, corporate bonds, and commercial paper. The primary objective of our investment activities is to preserve principal and liquidity while maximizing income without significantly increasing risk. We do not enter into investments for trading or speculative purposes. Due to the short‑term nature of our investment portfolio, we do not believe an immediate 10.0% increase or decrease in interest rates would have a material effect on the fair market value of our portfolio, and accordingly we do not expect our operating results or cash flows to be materially affected by a sudden change in market interest rates.
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
The financial statements required by this Item are set forth on pages 92 to 122 hereto.
REPORT OF MANAGEMENT
Management’s Report on Financial Statements
Our management is responsible for the preparation, integrity and fair presentation of information in our consolidated financial statements, including estimates and judgments. The consolidated financial statements presented in this Annual Report on Form 10-K have been prepared in accordance with accounting principles generally accepted in the United States of America. Our management believes the consolidated financial statements and other financial information included in this Annual Report on Form 10-K fairly present, in all material respects, our financial condition, results of operations and cash flows as of and for the periods presented in this Annual Report on Form 10-K. The
87
consolidated financial statements have been audited by Ernst & Young LLP, an independent registered public accounting firm, as stated in their report, which is included herein.
Management's Report on Internal Control Over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting. Our internal control over financial reporting is designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with accounting principles generally accepted in the United States of America.
Our internal control over financial reporting includes those policies and procedures that:
|
· |
pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect our transactions and dispositions of our assets; |
|
· |
provide reasonable assurance that our transactions are recorded as necessary to permit preparation of our financial statements in accordance with accounting principles generally accepted in the United States of America, and that our receipts and expenditures are being made only in accordance with authorization of our management and our directors; and |
|
· |
provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of our assets that could have a material effect on the consolidated financial statements. |
Because of its inherent limitations, internal control over financial reporting can provide only reasonable assurance and may not prevent or detect misstatements. Also, projections of any evaluation of the effectiveness of such controls in future periods are subject to the risk that the controls may become inadequate because of changes in conditions or that the degree of compliance with the policies and procedures may deteriorate.
Our management conducted an assessment of the effectiveness of internal control over financial reporting based on the framework in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission. Based on this assessment, our management concluded that, as of December 31, 2014, our internal control over financial reporting was effective to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with accounting principles generally accepted in the United States of America.
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
None.
ITEM 9A. CONTROLS AND PROCEDURES
Evaluation of Disclosure Controls and Procedures
We maintain disclosure controls and procedures that are designed to ensure that information required to be disclosed in our reports under the Securities Exchange Act of 1934, as amended, or the Exchange Act, and the rules and regulations thereunder, is recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms and that such information is accumulated and communicated to our management, including our principal executive officer and principal financial officer, as appropriate, to allow for timely decisions regarding required disclosure. In designing and evaluating the disclosure controls and procedures, management recognizes that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving the desired control objectives, and management is required to apply its judgment in evaluating the cost‑benefit relationship of possible controls and procedures.
88
As required by Rule 13a‑15(b) under the Exchange Act, our management, under the supervision and with the participation of our principal executive officer and principal financial officer, has evaluated the effectiveness of the design and operation of our disclosure controls and procedures (as such term is defined in Rules 13a‑15(e) and 15d‑15(e) under the Exchange Act) as of December 31, 2014. Based on such evaluation, our principal executive officer and principal financial officer have concluded that, as of December 31, 2014, our disclosure controls and procedures were effective at the reasonable assurance level.
Management's Report on Internal Control Over Financial Reporting
The information required by this section which includes the “Management’s Report on Consolidated Financial Statements and Internal Control over Financial Reporting” incorporated by reference from “Item 8. Financial Statements and Supplementary Data.”
Attestation Report on Internal Control Over Financial Reporting
This annual report does not include an attestation report of our registered public accounting firm regarding internal control over financial reporting due to the rules of the SEC for emerging growth companies.
Changes in Internal Control over Financial Reporting
There have been no changes in our internal control over financial reporting during our most recent fiscal quarter that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
None.
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
Directors
The information required by Item 10 is incorporated herein by reference to the information contained under the caption “Proposal 1—Election of Directors” in our definitive proxy statement related to the 2015 annual meeting of stockholders.
Executive Officers
The information required by Item 10 is incorporated herein by reference to the information contained under the caption “Executive Officers” in our definitive proxy statement related to the 2015 annual meeting of stockholders.
Section 16(a) Beneficial Ownership Reporting Compliance
The information concerning Section 16(a) Beneficial Ownership Reporting Compliance by our directors and executive officers is incorporated by reference to the information contained under the caption “Section 16(a) Beneficial Ownership Reporting Compliance” in our definitive proxy statement related to the 2015 annual meeting of stockholders.
Code of Business Conduct and Ethics
The information concerning our Code of Business Conduct and Ethics is incorporated by reference to the information contained under the caption “Board of Directors” in our definitive proxy statement related to the 2015 annual meeting of stockholders.
89
ITEM 11. EXECUTIVE COMPENSATION
The information required by this Item 11 is incorporated by reference to the information contained under the captions “Executive and Director Compensation” in our definitive proxy statement related to the 2015 annual meeting of stockholders.
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
The information required by Item 12 is incorporated by reference to the information contained under the captions “Security Ownership of Certain Beneficial Owners and Management” and “Securities Authorized For Issuance Under Equity Compensation Plans December 31, 2014” in our definitive proxy statement related to the 2015 annual meeting of stockholders.
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
The information required by Item 13 is incorporated by reference to the information contained under the captions “– Director Independence” and “Certain Relationships and Related Party Transactions” in our definitive proxy statement related to the 2015 annual meeting of stockholders..
ITEM 14. PRINCIPAL ACCOUNTANT FEES AND SERVICES
The information required by Item 14 is incorporated by reference to the information contained under the caption “Proposal No. 2: Ratification of Independent Registered Public Accounting Firm” in our definitive proxy statement related to the 2015 annual meeting of stockholders.
ITEM 15. EXHIBITS AND FINANCIAL STATEMENT SCHEDULES
(a)1.Financial Statements
See Index to our financial statements on page 91 of this annual report on Form 10‑K.
2.Financial Statement Schedules
None, as all information required in these schedules is included in the notes to our financial statements.
3.Exhibits
Reference is made to the Exhibit Index on pages 123 to 125 of this annual report on Form 10‑K for a list of exhibits required by Item 601 of Regulation S‑K to be filed as part of this annual report on Form 10‑K.
90
TETRALOGIC PHARMACEUTICALS CORPORATION
INDEX TO FINANCIAL STATEMENTS
|
92 |
|
|
93 |
|
|
94 |
|
|
95 |
|
|
Consolidated Statements of Cash Flows for the years ended December 31, 2013 and 2014 |
96 |
|
97 |
91
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
The Board of Directors and Stockholders of TetraLogic Pharmaceuticals Corporation
We have audited the accompanying consolidated balance sheets of TetraLogic Pharmaceuticals Corporation (the Company) as of December 31, 2013 and 2014, and the related consolidated statements of operations and comprehensive loss, convertible preferred stock and stockholders’ equity (deficit), and cash flows for the years then ended. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. We were not engaged to perform an audit of the Company’s internal control over financial reporting. Our audits included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the consolidated financial position of TetraLogic Pharmaceuticals Corporation at December 31, 2013 and 2014, and the consolidated results of its operations and its cash flows for the years then ended, in conformity with U.S. generally accepted accounting principles.
|
|
/s/ Ernst & Young LLP |
Philadelphia, Pennsylvania
February 26, 2015
92
TETRALOGIC PHARMACEUTICALS CORPORATION
|
|
|
|
|
|
|
|
|
|
|
|
December 31 |
|
||||
|
|
|
2013 |
|
2014 |
|
||
|
Assets |
|
|
|
|
|
|
|
|
Current assets: |
|
|
|
|
|
|
|
|
Cash and cash equivalents |
|
$ |
55,135,508 |
|
$ |
13,073,137 |
|
|
Short-term investments |
|
|
— |
|
|
40,624,349 |
|
|
Deferred financing costs, short-term |
|
|
— |
|
|
375,808 |
|
|
Prepaid expenses and other current assets |
|
|
153,210 |
|
|
1,784,069 |
|
|
Restricted cash |
|
|
20,000 |
|
|
— |
|
|
Total current assets |
|
|
55,308,718 |
|
|
55,857,363 |
|
|
Property and equipment, net |
|
|
139,577 |
|
|
528,476 |
|
|
Intangible assets |
|
|
— |
|
|
41,575,516 |
|
|
Goodwill |
|
|
— |
|
|
16,902,466 |
|
|
Deferred financing costs, long-term |
|
|
— |
|
|
1,299,674 |
|
|
Other assets |
|
|
54,126 |
|
|
2,127,551 |
|
|
Total assets |
|
$ |
55,502,421 |
|
$ |
118,291,046 |
|
|
|
|
|
|
|
|
|
|
|
Liabilities and stockholders’ equity |
|
|
|
|
|
|
|
|
Current liabilities: |
|
|
|
|
|
|
|
|
Accounts payable |
|
$ |
1,215,198 |
|
$ |
1,138,470 |
|
|
Accrued expenses |
|
|
2,921,458 |
|
|
3,727,784 |
|
|
Derivative liabilities |
|
|
793,744 |
|
|
256,027 |
|
|
Total current liabilities |
|
|
4,930,400 |
|
|
5,122,281 |
|
|
Convertible notes payable, net of discount of $18,020,658 |
|
|
— |
|
|
28,979,342 |
|
|
Derivative liabilities |
|
|
— |
|
|
2,400,000 |
|
|
Deferred taxes |
|
|
— |
|
|
16,879,659 |
|
|
Contingent consideration and other liabilities |
|
|
22,968 |
|
|
31,507,588 |
|
|
Total liabilities |
|
|
4,953,368 |
|
|
84,888,870 |
|
|
|
|
|
|
|
|
|
|
|
Commitments and contingencies (Note 9) |
|
|
|
|
|
|
|
|
Stockholders’ equity (deficit): |
|
|
|
|
|
|
|
|
Preferred stock, $0.0001 par value; 25,000,000 shares authorized, none issued and outstanding |
|
|
— |
|
|
— |
|
|
Common stock, $0.0001 par value; 100,000,000 shares authorized, 22,199,256 and 22,334,901 shares issued and outstanding at December 31, 2013 and December 31, 2014, respectively |
|
|
2,220 |
|
|
2,234 |
|
|
Additional paid‑in capital |
|
|
140,419,071 |
|
|
159,308,307 |
|
|
Cumulative translation adjustment |
|
|
— |
|
|
(69,031) |
|
|
Cumulative unrealized loss on investments |
|
|
— |
|
|
(8,117) |
|
|
Accumulated deficit |
|
|
(89,872,238) |
|
|
(125,831,217) |
|
|
Total stockholders’ equity |
|
|
50,549,053 |
|
|
33,402,176 |
|
|
Total liabilities and stockholders’ equity |
|
$ |
55,502,421 |
|
$ |
118,291,046 |
|
See accompanying notes to financial statements.
93
TETRALOGIC PHARMACEUTICALS CORPORATION
CONSOLIDATED STATEMENTS OF OPERATIONS AND COMPREHENSIVE LOSS
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended December 31, |
|
||||
|
|
|
2013 |
|
2014 |
|
||
|
|
|
|
|
|
|
|
|
|
Revenue |
|
$ |
— |
|
$ |
— |
|
|
|
|
|
|
|
|
|
|
|
Expenses: |
|
|
|
|
|
|
|
|
General and administrative |
|
|
8,467,467 |
|
|
10,926,777 |
|
|
Research and development |
|
|
9,523,427 |
|
|
20,286,826 |
|
|
Change in fair value of contingent consideration |
|
|
— |
|
|
2,572,000 |
|
|
Total expenses |
|
|
17,990,894 |
|
|
33,785,603 |
|
|
|
|
|
|
|
|
|
|
|
Loss from operations |
|
|
(17,990,894) |
|
|
(33,785,603) |
|
|
|
|
|
|
|
|
|
|
|
Change in fair value of derivative liabilities |
|
|
807,291 |
|
|
1,073,198 |
|
|
Interest and other income |
|
|
629 |
|
|
112,815 |
|
|
Interest expense |
|
|
(2,767,422) |
|
|
(3,359,389) |
|
|
Net loss |
|
$ |
(19,950,396) |
|
$ |
(35,958,979) |
|
|
Cumulative preferred stock dividends |
|
|
(3,249,948) |
|
|
— |
|
|
Net loss attributable to common stockholders |
|
$ |
(23,200,344) |
|
$ |
(35,958,979) |
|
|
|
|
|
|
|
|
|
|
|
Per share information: |
|
|
|
|
|
|
|
|
Net loss per common share: |
|
|
|
|
|
|
|
|
Basic |
|
$ |
(10.11) |
|
$ |
(1.61) |
|
|
Diluted |
|
$ |
(10.11) |
|
$ |
(1.63) |
|
|
|
|
|
|
|
|
|
|
|
Weighted average shares outstanding: |
|
|
|
|
|
|
|
|
Basic |
|
|
2,295,636 |
|
|
22,290,069 |
|
|
Diluted |
|
|
2,295,636 |
|
|
22,351,689 |
|
|
|
|
|
|
|
|
|
|
|
Net loss |
|
$ |
(19,950,396) |
|
$ |
(35,958,979) |
|
|
Foreign currency losses |
|
|
— |
|
|
(69,031) |
|
|
Unrealized gains/(losses) on available-for-sale securities |
|
|
— |
|
|
(8,117) |
|
|
Comprehensive loss |
|
$ |
(19,950,396) |
|
$ |
(36,036,127) |
|
See accompanying notes to financial statements.
94
TETRALOGIC PHARMACEUTICALS CORPORATION
CONSOLIDATED STATEMENTS OF CONVERTIBLE PREFERRED STOCK AND STOCKHOLDERS’ EQUITY (DEFICIT)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Convertible Preferred Stock |
|
Stockholders’ Equity (Deficit) |
|
||||||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total |
|
|||||
|
|
|
Series A |
|
Series B |
|
Series C |
|
Series C‑1 |
|
|
|
|
|
|
Additional |
|
Cumulative |
|
Cumulative |
|
|
|
Stockholders’ |
|
|||||||||||||||||
|
|
|
Preferred Stock |
|
Preferred Stock |
|
Preferred Stock |
|
Preferred Stock |
|
Common Stock |
|
Paid‑in |
|
Unrealized Loss |
|
Translation |
|
Accumulated |
|
Equity |
|
||||||||||||||||||||
|
|
|
Shares |
|
Amount |
|
Shares |
|
Amount |
|
Shares |
|
Amount |
|
Shares |
|
Amount |
|
Shares |
|
Amount |
|
Capital |
|
on Investments |
|
Adjustment |
|
Deficit |
|
(Deficit) |
|
||||||||||
|
Balance, December 31, 2012 |
|
8,000,000 |
|
$ |
7,847,860 |
|
33,333,334 |
|
$ |
14,788,379 |
|
98,693,337 |
|
$ |
36,856,348 |
|
13,276,686 |
|
$ |
5,919,616 |
|
1,352,635 |
|
$ |
135 |
|
$ |
1,486,309 |
|
$ |
— |
|
$ |
— |
|
$ |
(69,921,842) |
|
$ |
(68,435,398) |
|
|
Stock‑based compensation expense |
|
— |
|
|
— |
|
— |
|
|
— |
|
— |
|
|
— |
|
— |
|
|
— |
|
— |
|
|
— |
|
|
3,708,392 |
|
|
— |
|
|
— |
|
|
— |
|
|
3,708,392 |
|
|
Cancellation of unvested restricted stock |
|
— |
|
|
— |
|
— |
|
|
— |
|
— |
|
|
— |
|
— |
|
|
— |
|
(10,395) |
|
|
(1) |
|
|
1 |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
Exercise of stock options |
|
— |
|
|
— |
|
— |
|
|
— |
|
— |
|
|
— |
|
— |
|
|
— |
|
100,521 |
|
|
10 |
|
|
109,985 |
|
|
— |
|
|
— |
|
|
— |
|
|
109,995 |
|
|
Conversion of preferred stock to common stock |
|
— |
|
|
— |
|
(8,333,334) |
|
|
(3,749,999) |
|
(6,968,137) |
|
|
(2,624,201) |
|
|
|
|
|
|
900,085 |
|
|
90 |
|
|
6,374,110 |
|
|
— |
|
|
— |
|
|
— |
|
|
6,374,200 |
|
|
Conversion of Series A convertible preferred stock to common |
|
(8,000,000) |
|
|
(7,847,860) |
|
— |
|
|
— |
|
— |
|
|
— |
|
— |
|
|
— |
|
1,045,749 |
|
|
105 |
|
|
7,847,755 |
|
|
— |
|
|
— |
|
|
— |
|
|
7,847,860 |
|
|
common stock on a 2.22‑for‑one basis at IPO—December 2013 |
|
— |
|
|
— |
|
— |
|
|
— |
|
— |
|
|
— |
|
— |
|
|
— |
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
Conversion of Series B convertible preferred stock to common |
|
— |
|
|
— |
|
(25,000,000) |
|
|
(11,038,380) |
|
— |
|
|
— |
|
— |
|
|
— |
|
1,470,584 |
|
|
147 |
|
|
11,038,233 |
|
|
— |
|
|
— |
|
|
— |
|
|
11,038,380 |
|
|
common stock on a one‑for‑one basis at IPO—December 2013 |
|
— |
|
|
— |
|
— |
|
|
— |
|
— |
|
|
— |
|
— |
|
|
— |
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
Conversion of Series C convertible preferred stock to common |
|
— |
|
|
— |
|
— |
|
|
— |
|
(91,725,200) |
|
|
(34,232,147) |
|
— |
|
|
— |
|
5,395,592 |
|
|
540 |
|
|
34,231,607 |
|
|
— |
|
|
— |
|
|
— |
|
|
34,232,147 |
|
|
common stock on a one‑for‑one basis at IPO—December 2013 |
|
— |
|
|
— |
|
— |
|
|
— |
|
— |
|
|
— |
|
— |
|
|
— |
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
Conversion of Series C‑1 convertible preferred stock to common |
|
— |
|
|
— |
|
— |
|
|
— |
|
— |
|
|
— |
|
(13,276,686) |
|
|
(5,919,616) |
|
780,981 |
|
|
78 |
|
|
5,919,538 |
|
|
— |
|
|
— |
|
|
— |
|
|
5,919,616 |
|
|
common stock on a one‑for‑one basis at IPO—December 2013 |
|
— |
|
|
— |
|
— |
|
|
— |
|
— |
|
|
— |
|
— |
|
|
— |
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
Conversion of convertible notes and accrued interest to Common Stock |
|
— |
|
|
— |
|
— |
|
|
— |
|
— |
|
|
— |
|
— |
|
|
— |
|
2,876,419 |
|
|
288 |
|
|
20,134,701 |
|
|
— |
|
|
— |
|
|
— |
|
|
20,134,989 |
|
|
at IPO—December 2013 |
|
— |
|
|
— |
|
— |
|
|
— |
|
— |
|
|
— |
|
— |
|
|
— |
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
Conversion of warrants at IPO—December 2013 |
|
— |
|
|
— |
|
— |
|
|
— |
|
— |
|
|
— |
|
— |
|
|
— |
|
64,970 |
|
|
6 |
|
|
454,784 |
|
|
— |
|
|
— |
|
|
— |
|
|
454,790 |
|
|
Sale of common stock, net of expenses |
|
— |
|
|
— |
|
— |
|
|
— |
|
— |
|
|
— |
|
— |
|
|
— |
|
8,222,115 |
|
|
822 |
|
|
49,113,656 |
|
|
— |
|
|
— |
|
|
— |
|
|
49,114,478 |
|
|
Net loss |
|
— |
|
|
— |
|
— |
|
|
— |
|
— |
|
|
— |
|
— |
|
|
— |
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
(19,950,396) |
|
|
(19,950,396) |
|
|
Balance, December 31, 2013 |
|
— |
|
$ |
— |
|
— |
|
$ |
— |
|
— |
|
$ |
— |
|
— |
|
$ |
— |
|
22,199,256 |
|
$ |
2,220 |
|
$ |
140,419,071 |
|
$ |
— |
|
$ |
— |
|
$ |
(89,872,238) |
|
$ |
50,549,053 |
|
|
Stock‑based compensation expense |
|
— |
|
|
— |
|
— |
|
|
— |
|
— |
|
|
— |
|
— |
|
|
— |
|
— |
|
|
— |
|
|
3,384,800 |
|
|
— |
|
|
— |
|
|
— |
|
|
3,384,800 |
|
|
Cancellation of unvested restricted stock |
|
— |
|
|
— |
|
— |
|
|
— |
|
— |
|
|
— |
|
— |
|
|
— |
|
(9,193) |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
Exercise of stock options |
|
— |
|
|
— |
|
— |
|
|
— |
|
— |
|
|
— |
|
— |
|
|
— |
|
121,816 |
|
|
12 |
|
|
181,547 |
|
|
— |
|
|
— |
|
|
— |
|
|
181,559 |
|
|
Conversion of warrants |
|
— |
|
|
— |
|
— |
|
|
— |
|
— |
|
|
— |
|
— |
|
|
— |
|
23,022 |
|
|
2 |
|
|
139,086 |
|
|
— |
|
|
— |
|
|
— |
|
|
139,088 |
|
|
Issuance of convertible notes |
|
— |
|
|
— |
|
— |
|
|
— |
|
— |
|
|
— |
|
— |
|
|
— |
|
— |
|
|
— |
|
|
15,183,803 |
|
|
— |
|
|
— |
|
|
— |
|
|
15,183,803 |
|
|
Unrealized loss on investments |
|
— |
|
|
— |
|
— |
|
|
— |
|
— |
|
|
— |
|
— |
|
|
— |
|
— |
|
|
— |
|
|
— |
|
|
(8,117) |
|
|
— |
|
|
— |
|
|
(8,117) |
|
|
Cumulative translation adjustment |
|
— |
|
|
— |
|
— |
|
|
— |
|
— |
|
|
— |
|
— |
|
|
— |
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
(69,031) |
|
|
— |
|
|
(69,031) |
|
|
Net loss |
|
— |
|
|
— |
|
— |
|
|
— |
|
— |
|
|
— |
|
— |
|
|
— |
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
(35,958,979) |
|
|
(35,958,979) |
|
|
Balance, December 31, 2014 |
|
— |
|
$ |
— |
|
— |
|
$ |
— |
|
— |
|
$ |
— |
|
— |
|
$ |
— |
|
22,334,901 |
|
$ |
2,234 |
|
$ |
159,308,307 |
|
$ |
(8,117) |
|
$ |
(69,031) |
|
$ |
(125,831,217) |
|
$ |
33,402,176 |
|
See accompanying notes to financial statement.
95
TETRALOGIC PHARMACEUTICALS CORPORATION
CONSOLIDATED STATEMENTS OF CASH FLOWS
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended December 31, |
|
||||
|
|
|
2013 |
|
2014 |
|
||
|
Cash flows from operating activities |
|
|
|
|
|
|
|
|
Net loss |
|
$ |
(19,950,396) |
|
$ |
(35,958,979) |
|
|
Adjustments to reconcile net loss to net cash used in operating activities: |
|
|
|
|
|
|
|
|
Depreciation and amortization |
|
|
99,591 |
|
|
125,956 |
|
|
Stock‑based compensation expense |
|
|
3,708,392 |
|
|
3,384,800 |
|
|
Change in fair value of derivative liabilities |
|
|
(807,291) |
|
|
(1,073,198) |
|
|
Change in fair value of contingent consideration |
|
|
— |
|
|
2,572,000 |
|
|
Non‑cash interest expense |
|
|
2,767,422 |
|
|
1,395,833 |
|
|
Changes in operating assets and liabilities: |
|
|
|
|
|
|
|
|
Prepaid expenses and other assets |
|
|
538,890 |
|
|
(3,383,820) |
|
|
Accounts payable, accrued expenses and other liabilities |
|
|
874,441 |
|
|
571,234 |
|
|
Net cash used in operating activities |
|
|
(12,768,951) |
|
|
(32,366,174) |
|
|
|
|
|
|
|
|
|
|
|
Cash flows from investing activities |
|
|
|
|
|
|
|
|
Purchase of property and equipment |
|
|
(67,319) |
|
|
(514,855) |
|
|
Restricted cash |
|
|
— |
|
|
20,000 |
|
|
Acquisition of Shape Pharmaceuticals, net of cash acquired |
|
|
— |
|
|
(12,847,803) |
|
|
Purchase of short‑term investments |
|
|
— |
|
|
(66,295,197) |
|
|
Sales and maturities of short‑term investments |
|
|
— |
|
|
25,662,731 |
|
|
Net cash used in investing activities |
|
|
(67,319) |
|
|
(53,975,124) |
|
|
|
|
|
|
|
|
|
|
|
Cash flows from financing activities |
|
|
|
|
|
|
|
|
Proceeds from exercise of stock options and warrants |
|
|
109,995 |
|
|
201,128 |
|
|
Proceeds from convertible notes payable, net |
|
|
14,235,416 |
|
|
44,146,830 |
|
|
Proceeds from issuance of common stock, net |
|
|
49,114,478 |
|
|
— |
|
|
Net cash provided by financing activities |
|
|
63,459,889 |
|
|
44,347,958 |
|
|
Effect of exchange rate changes on cash and cash equivalents |
|
|
— |
|
|
(69,031) |
|
|
Net increase (decrease) in cash and cash equivalents |
|
|
50,623,619 |
|
|
(42,062,371) |
|
|
Cash and cash equivalents—beginning of period |
|
|
4,511,889 |
|
|
55,135,508 |
|
|
Cash and cash equivalents—end of period |
|
$ |
55,135,508 |
|
$ |
13,073,137 |
|
|
|
|
|
|
|
|
|
|
|
Supplemental disclosure of cash flow information |
|
|
|
|
|
|
|
|
Noncash investing and financing activities: |
|
|
|
|
|
|
|
|
Conversion of convertible notes principal and accrued interest to common stock |
|
$ |
20,134,701 |
|
$ |
— |
|
|
Conversion of preferred stock to common stock |
|
$ |
65,412,203 |
|
$ |
— |
|
|
Cash paid for interest |
|
$ |
— |
|
$ |
1,796,444 |
|
See accompanying notes to financial statements.
96
TETRALOGIC PHARMACEUTICALS CORPORATION
NOTES TO FINANCIAL STATEMENTS
December 31, 2014
1. Organization and Description of the Business
We are a clinical-stage biopharmaceutical company focused on discovering and developing novel small molecule therapeutics in oncology and infectious diseases. We have two clinical-stage product candidates in development: birinapant and suberohydroxamic acid phenyl ester (SHAPE). We were originally incorporated in July 2001 as Apop Corp., a New Jersey corporation. Pursuant to a merger effective as of October 2003 with and into our wholly owned subsidiary, we became a Delaware corporation and commenced operations in 2003. Our name was changed to Apop Corporation in March 2004, to Gentara Corporation in June 2004 and to TetraLogic Pharmaceuticals Corporation in January 2006. Our operations to date have been directed primarily toward developing business strategies, raising capital, research and development activities, conducting pre‑clinical testing and human clinical trials of our product candidates and recruiting personnel.
Initial Public Offering
On December 17, 2013, 7,150,000 shares of common stock were sold on our behalf at an initial public offering price of $7.00 per share, for aggregate gross proceeds of $50.0 million, managed by Oppenheimer & Co. Inc., Guggenheim Securities, LLC and Needham & Company, LLC. On December 31, 2013, in connection with the exercise by the underwriters of our initial public offering of a portion of the over‑allotment option granted to them in connection with the initial public offering, 1,072,115 additional shares of common stock were sold on our behalf at the initial public offering price of $7.00 per share, for aggregate gross proceeds of approximately $7.5 million. In addition, as part of the initial public offering, we converted all of our existing convertible preferred stock and convertible notes, and a portion of our existing warrants, into 12,534,380 shares of common stock.
The underwriters received underwriting discounts of approximately $4.0 million in connection with our initial public offering. In addition, we incurred expenses of approximately $4.4 million in connection with our initial public offering, which when added to the underwriting discounts, amounts to total expenses of approximately $8.4 million. Thus, the net offering proceeds to us, after deducting underwriting discounts and offering expenses, were approximately $49.1 million.
Liquidity
At December 31, 2014, we had working capital of $50.7 million and cash, cash equivalents and short-term investments of $53.7 million. We have not generated any product revenues and have not yet achieved profitable operations. There is no assurance that profitable operations will ever be achieved, and if achieved, could be sustained on a continuing basis. In addition, development activities, clinical and pre‑clinical testing, and commercialization of our products will require significant additional financing.
We believe that our existing cash and cash equivalents as of December 31, 2014 will enable us to fund our operating expenses and capital expenditure requirements through the first quarter of 2016. However, we will need to secure additional funding in the future, from one or more equity or debt financings, collaborations, or other sources, in order to carry out all of our planned research and development activities. We plan to finance our future operations with a combination of proceeds from the issuance of equity securities, the issuance of additional debt, potential collaborations and revenues from potential future product sales, if any.
Reverse stock split
Our board of directors and our stockholders approved a 1‑for‑17 reverse stock split of our common stock. The reverse split became effective on November 18, 2013. All common share and per common share amounts in the financial
97
TETRALOGIC PHARMACEUTICALS CORPORATION
NOTES TO FINANCIAL STATEMENTS (Continued)
December 31, 2014
statements and notes have been retroactively adjusted to give effect to the reverse stock split, including reclassifying an amount equal to the reduction in par value of common stock to additional paid‑in capital.
2. Summary of Significant Accounting Policies
Use of Estimates
The preparation of financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from such estimates.
Principles of Consolidation
The consolidated financial statements include the results of our operations and our wholly-owned subsidiaries. All intercompany accounts and transactions have been eliminated.
Fair Value of Financial Instruments and Credit Risk
At December 31, 2013 and 2014, our financial instruments included cash and cash equivalents, short-term investments, restricted cash, accounts payable, accrued expenses and derivative liabilities. The carrying amount of cash and cash equivalents, restricted cash, accounts payable and accrued expenses approximates fair value, given their short‑term nature. Short-term investments are carried at fair market value. The carrying value of the derivative liabilities are the estimated fair value of the liability as more fully described below.
Cash and cash equivalents and short-term investments subject us to concentrations of credit risk. However, we invest our cash and short-term investments in accordance with a policy objective that seeks to ensure both liquidity and safety of principal. We had restricted cash of $20,000 at December 31, 2013 as a deposit securing our corporate credit card.
Cash and Cash Equivalents and Short-Term Investments
We consider all highly liquid investments that have maturities of three months or less when acquired to be cash equivalents. We consider our short-term investments to be “available-for-sale” and carry them at fair market value. Unrealized gains and losses have been recorded as a separate component of accumulated other comprehensive income included in stockholders’ equity. All realized gains and losses on our available-for-sale securities are recognized in results of operations.
Property and Equipment
Property and equipment consist of computer and laboratory equipment, furniture, and leasehold improvements and are recorded at cost. Property and equipment are depreciated on a straight‑line basis over their estimated useful lives. We estimate a life of three years for computer equipment, including software, and five years for laboratory equipment, office equipment, and furniture. Leasehold improvements are amortized over the shorter of the lease term or the estimated useful life of the asset. When property and equipment are sold or otherwise disposed of, the cost and related accumulated depreciation are removed from the accounts and the resulting gain or loss is included in operating expenses.
98
TETRALOGIC PHARMACEUTICALS CORPORATION
NOTES TO FINANCIAL STATEMENTS (Continued)
December 31, 2014
Intangible Assets, Goodwill and Other Long‑Lived Assets
Goodwill represents the excess of consideration transferred over the fair value of net assets acquired. Goodwill and indefinite lived intangible assets are not amortized; rather, they are subject to a periodic assessment for impairment by applying a fair-value-based test. We perform an annual test of impairment of goodwill in the fourth quarter of each year or more frequently if events or changes in circumstances indicate that the asset might be impaired. We utilize a two-step method for determining goodwill impairment. In the first step, we compare the fair value of our single reporting unit with its carrying value based on the market value of our common stock. If the carrying value exceeds the fair value, then we would perform the second step of the impairment test and allocate the fair value to all assets and liabilities using the authoritative guidance for business combinations, with any residual fair value amount assigned to goodwill. An impairment charge would be recognized if the implied fair value of our goodwill is less than its carrying value.
We review indefinite lived intangible assets for impairment on an annual basis and review all intangible assets for impairment whenever changes in circumstances indicate the carrying value of the asset may not be recoverable. If impairment is indicated, we measure the amount of such impairment by comparing the carrying value to the fair value of the assets, which is usually based on the present value of the expected future cash flows associated with the use of the asset. We review other long‑lived assets, including property and equipment, for impairment whenever events or changes in business circumstances indicate that the carrying amount of an asset may not be fully recoverable. If the estimated undiscounted future cash flows expected to result from the use of the asset and its eventual disposition is less than its carrying amount an impairment loss would be recognized if the carrying value of the asset exceeded its fair value. Fair value is generally determined using discounted cash flows. Through December 31, 2014, no impairment has occurred.
Research and Development
Research and development costs are expensed as incurred. Costs for certain development activities, such as clinical trials, are recognized based on an evaluation of the progress to completion of specific tasks using data such as subject enrollment, clinical site activations, or information provided to us by our vendors with respect to their actual costs incurred. Payments for these activities are based on the terms of the individual arrangements, which may differ from the pattern of costs incurred, and are reflected in the financial statements as prepaid or accrued research and development expense, as the case may be.
Income Taxes
We recognize current tax liabilities or receivables for the amount of taxes we estimate are payable or refundable for the current year. We recognize deferred tax assets and liabilities for temporary differences between the financial reporting basis and the tax basis of our assets and liabilities and the expected benefits of net operating loss and credit carryforwards. The impact of changes in tax rates and laws on deferred taxes, if any, applied during the years in which temporary differences are expected to be settled, is reflected in the financial statements in the period of enactment. The measurement of deferred tax assets is reduced, if necessary, if based on weight of the evidence, it is more likely than not that some, or all, of the deferred tax assets will not be realized. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in the period that such tax rate changes are enacted. At December 31, 2013 and 2014, we have concluded that a full valuation allowance is necessary for our net deferred tax assets. We have no amounts recorded for uncertain tax positions, interest or penalties in the accompanying financial statements.
99
TETRALOGIC PHARMACEUTICALS CORPORATION
NOTES TO FINANCIAL STATEMENTS (Continued)
December 31, 2014
Loss Per Share of Common Stock
Basic net loss per common share is computed by dividing net loss applicable to common stockholders by the weighted average number of shares of common stock outstanding during each period. Diluted net loss per common share is computed by giving effect to all potentially dilutive securities outstanding for the period.
In accordance with ASC Topic 260, Earnings per Share, when calculating diluted net loss per common share, the gain associated with the decrease in the fair value of certain warrants classified as derivative liabilities results in an adjustment to the net loss; and the dilutive impact of the assumed exercise of the warrants results in an adjustment to the weighted average common shares outstanding. We utilize the treasury stock method to calculate the dilutive impact of the assumed exercise of the warrants. For the year ended December 31, 2014, the effect of the adjustments for our 2006 Warrants and our 2009/2010 Warrants were dilutive. For the year ended December 31, 2013, the effect of the adjustments for all warrants classified as derivative liabilities was non-dilutive.
The following table sets forth the computation of diluted earnings per share for the years ended December 31, 2013 and 2014:
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended December 31, |
|
||||
|
|
|
2013 |
|
2014 |
|
||
|
Numerator: |
|
|
|
|
|
|
|
|
Net loss |
|
$ |
(19,950,396) |
|
$ |
(35,958,979) |
|
|
Less: income from change in fair value of warrant liability |
|
|
— |
|
|
(418,198) |
|
|
Dividends on series C and C-1 convertible preferred stock |
|
|
(3,249,948) |
|
|
— |
|
|
Numerator for diluted net loss per common share |
|
$ |
(23,200,344) |
|
$ |
(36,377,177) |
|
|
Denominator: |
|
|
|
|
|
|
|
|
Basic weighted average common shares outstanding |
|
|
2,295,636 |
|
|
22,290,069 |
|
|
Dilutive common shares from assumed warrant exercises |
|
|
— |
|
|
61,620 |
|
|
Diluted weighted average shares of common stock outstanding |
|
|
2,295,636 |
|
|
22,351,689 |
|
|
Diluted net loss per share of common stock |
|
$ |
(10.11) |
|
$ |
(1.63) |
|
|
|
|
|
|
|
|
|
|
The following potentially dilutive securities outstanding at December 31, 2013 and 2014 have been excluded from the computation of diluted weighted average shares outstanding, as they would be antidilutive:
|
|
|
|
|
|
|
|
|
|
December 31, |
|
||
|
|
|
2013 |
|
2014 |
|
|
Convertible preferred stock |
|
— |
|
— |
|
|
Warrants |
|
100,319 |
|
23,747 |
|
|
Stock options |
|
3,263,417 |
|
3,270,363 |
|
|
Unvested restricted stock |
|
80,237 |
|
11,765 |
|
|
|
|
3,443,973 |
|
3,305,875 |
|
100
TETRALOGIC PHARMACEUTICALS CORPORATION
NOTES TO FINANCIAL STATEMENTS (Continued)
December 31, 2014
In addition, the common shares issuable upon conversion of the 8% notes have been excluded from the computation of diluted weighted average shares outstanding.
Comprehensive Loss
Comprehensive loss is defined as the change in equity of a business enterprise during a period from transactions and other events and circumstances from non‑owner sources. Comprehensive loss was equal to net loss for the year ended December 31, 2013. Comprehensive loss for the year ended December 31, 2014 comprised net loss, foreign currency losses, and net unrealized gains and losses on available-for-sale securities.
Segment Information
Operating segments are defined as components of an enterprise about which separate discrete information is available for evaluation by the chief operating decision maker, or decision‑making group, in deciding how to allocate resources and in assessing performance. We view our operations and manage our business in one segment, which is the identification and development of novel small molecule therapies in oncology and infectious diseases.
Stock‑Based Compensation
We account for stock‑based compensation in accordance with the provisions of ASC Topic 718, Compensation—Stock Compensation, or ASC 718, which requires the recognition of expense related to the fair value of stock‑based compensation awards in the Statements of Operations and Comprehensive Loss. For stock options issued to employees and members of our board of directors for their services on our board of directors, we estimate the grant date fair value of each option using the Black‑Scholes option pricing model. The use of the Black‑Scholes option pricing model requires management to make assumptions with respect to the expected term of the option, the expected volatility of the common stock consistent with the expected life of the option, risk‑free interest rates, the value of the common stock and expected dividend yields of the common stock. For awards subject to service‑based vesting conditions, we recognize stock‑based compensation expense, net of estimated forfeitures, equal to the grant date fair value of stock options on a straight‑line basis over the requisite service period, which is generally the vesting term. For awards subject to performance‑ based vesting conditions, we recognize stock‑based compensation expense using the accelerated attribution method when it is probable that the performance condition will be achieved. Forfeitures are required to be estimated at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differ from those estimates. Stock‑ based payments issued to non‑employees are recorded at their fair values, and are periodically revalued as the equity instruments vest and are recognized as expense over the related service period in accordance with the provisions of ASC 718 and ASC Topic 505, Equity.
Clinical Trial Expense Accruals
As part of the process of preparing our financial statements, we are required to estimate our expenses resulting from our obligations under contracts with vendors, clinical research organizations and consultants and under clinical site agreements in connection with conducting clinical trials. The financial terms of these contracts are subject to negotiations, which vary from contract to contract and may result in payment flows that do not match the periods over which materials or services are provided under such contracts. Our objective is to reflect the appropriate trial expenses in our financial statements by matching those expenses with the period in which services are performed and efforts are expended. We account for these expenses according to the progress of the trial as measured by patient progression and the timing of various aspects of the trial. We determine accrual estimates through financial models taking into account discussion with applicable personnel and outside service providers as to the progress or state of consummation of trials. During the course of a clinical trial, we adjust our clinical expense recognition if actual results differ from its estimates. We make estimates of our accrued expenses as of each balance sheet date based on the facts and circumstances known at
101
TETRALOGIC PHARMACEUTICALS CORPORATION
NOTES TO FINANCIAL STATEMENTS (Continued)
December 31, 2014
that time. Our clinical trial accruals are dependent upon the timely and accurate reporting of contract research organizations and other third‑party vendors. Although we do not expect our estimates to be materially different from amounts actually incurred, our understanding of the status and timing of services performed relative to the actual status and timing of services performed may vary and may result in reporting amounts that are too high or too low for any particular period. For the years ended December 31, 2013 and 2014, there were no material adjustments to our prior period estimates of accrued expenses for clinical trials.
Recent Accounting Pronouncements
In June 2014, the Financial Accounting Standards Board (FASB) issued Accounting Standards Update (ASU) 2014-10, Development Stage Entities. This Update eliminates the distinction between development stage entities and other reporting entities under GAAP and also eliminates the requirement to present inception-to-date information in financial statements. The Update is effective for reporting periods beginning after December 31, 2014, but early adoption is permitted. We have elected to adopt this Update beginning with the quarterly period ended June 30, 2014.
3. Acquisitions
Shape Pharmaceuticals
On April 14, 2014 (“the acquisition date”), we acquired by merger 100% of Shape Pharmaceuticals. Shape Pharmaceuticals was a development stage pharmaceutical company developing SHAPE, a novel, tissue-targeted HDAC inhibitor in a topical gel formulation to treat stage IA-IIA Cutaneous T-Cell Lymphoma (CTCL). The acquisition added a second clinical-stage oncology compound to the TetraLogic portfolio. A member of our Board of Directors also served as Chief Executive Officer and a member of the Board of Directors of Shape Pharmaceuticals.
The acquisition date fair value of consideration transferred totaled $13.0 million, all of which was in cash.
The following summarizes the estimated fair values of the assets acquired and liabilities assumed at the acquisition date:
|
|
|
|
|
|
|
Allocation of purchase price |
|
|
|
|
|
Cash and cash equivalents |
|
$ |
71,792 |
|
|
Other current assets |
|
|
320,464 |
|
|
Accounts payable |
|
|
(138,645) |
|
|
Deferred taxes |
|
|
(16,879,659) |
|
|
Contingent consideration |
|
|
(28,932,339) |
|
|
Legal expenses |
|
|
121,873 |
|
|
Indefinite-lived intangible assets |
|
|
41,575,516 |
|
|
Goodwill |
|
|
16,902,466 |
|
|
Total purchase price |
|
$ |
13,041,468 |
|
The purchase price allocation has been finalized based on fair values of the acquired assets and liabilities. The intangible assets acquired represent indefinite-lived intangible assets relating to the technology acquired from Shape Pharmaceuticals. These intangibles will not be amortized until FDA approval has been obtained for a product that uses this technology. Goodwill results primarily from the recognition of deferred tax liabilities in connection with the acquisition. There is no goodwill recognized or deductible for tax purposes.
The acquisition of Shape Pharmaceuticals includes a contingent consideration arrangement that may require us to pay additional consideration in the form of milestone payments and tiered royalty payments upon commercialization. We currently estimate that the milestone payments will occur between 2016 and 2020. The range of undiscounted
102
TETRALOGIC PHARMACEUTICALS CORPORATION
NOTES TO FINANCIAL STATEMENTS (Continued)
December 31, 2014
amounts that we could be required to pay under our agreement for the milestone payments is between zero and $64.5 million. We determined the fair value of the liability for the contingent consideration based on a probability-weighted discounted cash flow analysis. This fair value measurement is based on significant inputs not observable in the market and thus represents a Level 3 measurement within the fair value hierarchy. The fair value of the contingent consideration liability associated with future milestones and royalties was based on several factors including:
|
· |
estimated cash flows projected from the success of unapproved product candidates in the U.S. and Rest of World, or ROW; |
|
· |
the probability of success for product candidates including risks associated with uncertainty, achievement, and payment of milestone events; |
|
· |
the time and resources needed to complete the development and approval of product candidates; |
|
· |
the life of the potential commercialized products and associated risks of obtaining regulatory approvals in the U.S. and ROW; and |
|
· |
the risk adjusted discount rate for fair value measurements. |
The fair value of the liability for the contingent consideration recognized on the acquisition date was $22.6 million. In connection with the finalization of the purchase accounting, the contingent consideration was increased by approximately $6.3 million primarily as a result of an increase in estimated revenue resulting in a higher level of milestones and royalties expected to be paid. The contingent consideration payments have been recorded as a liability and the fair value is evaluated quarterly or more frequently if circumstances dictate. Changes in the fair value of contingent consideration are recorded in earnings. The change in fair value that was recognized as an operating expense for the period between April 14, 2014 and December 31, 2014 was $2.6 million. At December 31, 2014, the fair value of the liability was $31.5 million.
We recognized $0.4 million of acquisition related costs that were expensed in 2014. These costs are included in operating expenses in our consolidated statement of operations. In 2014, we have included $5.6 million of net losses attributable to Shape in our consolidated financial statements.
The following unaudited pro forma information presents results as if the acquisition occurred at the beginning of each annual reporting period presented:
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended December 31, |
|
||||
|
|
|
2013 |
|
2014 |
|
||
|
|
|
|
|
|
|
|
|
|
Revenue |
|
$ |
— |
|
$ |
— |
|
|
Net loss attributable to TetraLogic Pharmaceuticals Corporation (after cumulative preferred stock dividends) |
|
|
(25,509,981) |
|
|
(36,469,877) |
|
|
Basic loss per common share attributable to TetraLogic Pharmaceuticals Corporation |
|
$ |
(11.11) |
|
$ |
(1.64) |
|
|
Diluted loss per common share attributable to TetraLogic Pharmaceuticals Corporation |
|
$ |
(11.11) |
|
$ |
(1.65) |
|
4. Fair Value Measurements
FASB accounting guidance defines fair value as the price that would be received to sell an asset or paid to transfer a liability (the exit price) in an orderly transaction between market participants at the measurement date. The accounting guidance outlines a valuation framework and creates a fair value hierarchy in order to increase the
103
TETRALOGIC PHARMACEUTICALS CORPORATION
NOTES TO FINANCIAL STATEMENTS (Continued)
December 31, 2014
consistency and comparability of fair value measurements and the related disclosures. In determining fair value, we use quoted prices and observable inputs. Observable inputs are inputs that market participants would use in pricing the asset or liability based on market data obtained from independent sources.
The fair value hierarchy is broken down into three levels based on the source of inputs as follows:
|
· |
Level 1—Valuations based on unadjusted quoted prices in active markets for identical assets or liabilities. |
|
· |
Level 2—Valuations based on observable inputs and quoted prices in active markets for similar assets and liabilities. |
|
· |
Level 3—Valuations based on inputs that are unobservable and models that are significant to the overall fair value measurement. |
The following fair value hierarchy table presents information about each major category of our financial assets and liabilities measured at fair value on a recurring basis:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Level 1 |
|
Level 2 |
|
Level 3 |
|
Total |
|
||||
|
December 31, 2013 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Assets |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Money market funds (cash equivalents) |
|
$ |
42,000,000 |
|
$ |
— |
|
$ |
— |
|
$ |
42,000,000 |
|
|
Certificate of deposit (restricted cash) |
|
|
20,000 |
|
|
— |
|
|
— |
|
|
20,000 |
|
|
Total assets |
|
$ |
42,020,000 |
|
$ |
— |
|
$ |
— |
|
$ |
42,020,000 |
|
|
Liabilities |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common stock warrant liability |
|
|
— |
|
|
— |
|
$ |
793,744 |
|
$ |
793,744 |
|
|
Total liabilities |
|
$ |
— |
|
$ |
— |
|
$ |
793,744 |
|
$ |
793,744 |
|
|
December 31, 2014 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Assets |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Money market funds (cash equivalents) |
|
$ |
13,073,137 |
|
$ |
— |
|
$ |
— |
|
$ |
13,073,137 |
|
|
Short-term investments |
|
|
— |
|
|
40,624,349 |
|
|
— |
|
|
40,624,349 |
|
|
Total assets |
|
$ |
13,073,137 |
|
$ |
40,624,349 |
|
$ |
— |
|
$ |
53,697,486 |
|
|
Liabilities |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Interest make-whole derivative |
|
|
— |
|
|
— |
|
$ |
2,400,000 |
|
$ |
2,400,000 |
|
|
Shape contingent consideration |
|
|
— |
|
|
— |
|
|
31,491,686 |
|
|
31,491,686 |
|
|
Common stock warrant liability |
|
|
— |
|
|
— |
|
|
256,027 |
|
|
256,027 |
|
|
Total liabilities |
|
$ |
— |
|
$ |
— |
|
$ |
34,147,713 |
|
$ |
34,147,713 |
|
We had outstanding warrants to purchase our series B convertible preferred stock, or the 2006 Warrants (as defined below), that converted into warrants to purchase our common stock at the time of our initial public offering. The 2006 Warrants were redeemable for cash upon an event that was not within our control. In accordance with the guidance for accounting for certain financial instruments with characteristics of both liabilities and equity, the 2006 Warrants are recorded as a derivative liability on our balance sheets with subsequent changes to fair value recorded through earnings at each reporting period on our statement of operations and comprehensive loss as change in fair value of derivative liabilities. The fair value of the warrant liability is estimated using an option‑pricing model, which requires inputs such as the expected volatility based on comparable public companies (90%), the fair value of our common stock, and the remaining contractual term of the warrant (1.3 years). For this liability, we developed our own assumptions that do not have observable inputs or available market data to support the fair value.
In 2009 and 2010, we issued warrants to purchase our common stock, or the 2009/2010 Warrants, which contain a provision whereby the exercise price may be reduced upon the occurrence of certain events within our control, such as the future issuance of equity securities or rights to purchase equity securities at a price below the current exercise price of the 2009/2010 Warrants. Accordingly, the 2009/2010 Warrants are recorded as a derivative liability on our
104
TETRALOGIC PHARMACEUTICALS CORPORATION
NOTES TO FINANCIAL STATEMENTS (Continued)
December 31, 2014
balance sheets, with subsequent changes to fair value recorded through earnings at each reporting period on our statement of operations and comprehensive loss as change in fair value of derivative liabilities. The fair value of each 2009/2010 Warrant is estimated using an option‑pricing model, which requires inputs such as the expected volatility based on comparable public companies (90%), the fair value of the common stock, and the contractual term of the warrant (4.9 to 5.2 years). For this liability, we developed our own assumptions that do not have observable inputs or available market data to support the fair value.
In 2012 and 2013, we issued detachable warrants, or the 2012/2013 Warrants, in conjunction with the 2012/2013 Notes (as defined below). The 2012/2013 Warrants are freestanding financial instruments that are legally detachable and separately exercisable from the 2012/2013 Notes. The 2012/2013 Warrants contain one or more underlying securities, one or more notional amounts or payment provisions or both, minimal to no initial net investment and net settlement provisions. As such, the 2012/2013 Warrants are recorded as a derivative liability on our balance sheets, with subsequent changes to fair value recorded through earnings at each reporting period on our statement of operations and comprehensive loss as change in fair value of derivative liabilities. The fair value of each 2012/2013 Warrant is estimated by applying probability‑weighted expected return methods to a multiple scenario option‑pricing model, which requires inputs such as the expected volatility based on comparable public companies (90%), the estimated fair value of certain equity securities ($0.47 to $6.12 per share), and the expected term to various liquidity events (1‑ 2 years). For this liability, we developed our own assumptions that do not have observable inputs or available market data to support the fair value. All of the 2012/2013 Warrants were converted to common stock at the time of our initial public offering in December 2013.
Significant decreases in our stock price volatility will significantly decrease the overall valuation of our derivative liabilities, while significant increases in our stock price volatility will significantly increase the overall valuation. As discussed above, the strike price of our 2009/2010 Warrants may be decreased. Accordingly, a significant decrease in the strike price of the 2009/2010 Warrants will substantially increase the overall valuation.
In April 2014, we acquired by merger 100% of Shape Pharmaceuticals. The acquisition of Shape Pharmaceuticals includes a contingent consideration arrangement that may require us to pay additional consideration in the form of milestone payments and tiered royalty payments upon commercialization. We account for contingent consideration in accordance with applicable guidance provided within Accounting Standards Codification (ASC) 805, Business Combinations. It is currently estimated that the Shape Pharmaceuticals milestone payments will occur between 2016 and 2020. The range of undiscounted milestones we could be required to pay under our agreement is between zero and $64.5 million. We determined the fair value of the liability for the contingent consideration based on a probability-weighted discounted cash flow analysis. This fair value measurement is based on significant inputs not observable in the market and thus represents a Level 3 measurement within the fair value hierarchy. The fair value of the contingent consideration liability associated with future milestone and royalty payments was based on several factors including:
|
· |
estimated cash flows projected from the success of unapproved product candidates in the U.S. and ROW; |
|
· |
the probability of success for product candidates including risks associated with uncertainty, achievement and payment of milestone events; |
|
· |
the time and resources needed to complete the development and approval of product candidates; |
|
· |
the life of the potential commercialized products and associated risks of obtaining regulatory approvals in the U.S. and ROW; and |
|
· |
the risk adjusted discount rate for fair value measurement. |
105
TETRALOGIC PHARMACEUTICALS CORPORATION
NOTES TO FINANCIAL STATEMENTS (Continued)
December 31, 2014
In June 2014, we issued $47.0 million in aggregate principal amount of 8.00% convertible senior notes due June 15, 2019 (the “8% Notes”). The 8% Notes include an interest make-whole feature whereby if a noteholder converts any of the Notes after December 31, 2014, they are entitled, in addition to the other consideration payable or deliverable in connection with such conversion, to an interest make-whole payment through the earlier of (i) the date that is three years after the conversion date and (ii) the maturity date if the notes had not been so converted. We have determined that this feature is an embedded derivative and have recognized the fair value of this derivative as a liability in our balance sheet, with subsequent changes to fair value recorded through earnings at each reporting period on our statements of operations and comprehensive loss as change in fair value of derivative liabilities. The fair value of this embedded derivative was determined based on a binomial lattice model.
The following tables set forth a summary of changes in the fair value of Level 3 liabilities for the years ended December 31, 2013 and 2014:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
December 31, |
|
|
|
|
|
|
|
|
|
|
Change in |
|
December 31, |
|
|||
|
|
|
2012 |
|
Issuances |
|
Exercises |
|
Conversions |
|
Fair Value |
|
2013 |
|
||||||
|
Preferred stock warrant liability |
|
$ |
272,079 |
|
$ |
1,686,652 |
|
$ |
— |
|
$ |
(569,604) |
|
$ |
(1,389,127) |
|
$ |
— |
|
|
Common stock warrant liability |
|
|
97,094 |
|
|
— |
|
|
— |
|
|
114,814 |
|
|
581,836 |
|
|
793,744 |
|
|
|
|
$ |
369,173 |
|
$ |
1,686,652 |
|
$ |
— |
|
$ |
(454,790) |
|
$ |
(807,291) |
|
$ |
793,744 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
December 31, |
|
|
|
|
|
|
|
|
|
Change in |
|
December 31, |
|
||||
|
|
|
2013 |
|
Additions |
|
Deductions |
|
Conversions |
|
Fair Value |
|
2014 |
|
||||||
|
Interest make-whole derivative |
|
|
— |
|
$ |
3,055,000 |
|
|
— |
|
|
— |
|
$ |
(655,000) |
|
$ |
2,400,000 |
|
|
Shape contingent consideration |
|
|
— |
|
|
28,932,339 |
|
$ |
(12,653) |
|
|
— |
|
|
2,572,000 |
|
|
31,491,686 |
|
|
Common stock warrant liability |
|
$ |
793,744 |
|
|
— |
|
|
— |
|
$ |
(119,519) |
|
|
(418,198) |
|
|
256,027 |
|
|
Total liabilities |
|
$ |
793,744 |
|
$ |
32,298,339 |
|
$ |
(12,653) |
|
$ |
(119,519) |
|
$ |
1,498,802 |
|
$ |
34,147,713 |
|
As of December 31, 2014, the fair value and carrying value of our convertible debt, based on quoted market prices was:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Fair Value^ |
|
Carrying Value |
|
Face Value |
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
8.00% convertible senior notes due June 15, 2019 |
|
$ |
43,500,000 |
|
$ |
28,979,342 |
|
$ |
47,000,000 |
|
^The fair value shown above represents the fair value of the total debt instrument, inclusive of both the liability and equity components, while the carrying value represents the carrying value of the liability.
5. Investments
The Company’s investments are classified as available-for-sale pursuant to ASC 320, Investments—Debt and Equity Securities. The Company classifies investments available to fund current operations as current assets on its balance sheets. We consider all investments that have maturities of three months or less when acquired to be cash equivalents. Investments are classified as long-term assets on the balance sheets if (i) the Company has the intent and ability to hold the investments for a period of at least one year and (ii) the contractual maturity date of the investments is greater than one year.
Investments are carried at fair value with unrealized gains and losses included as a component of accumulated other comprehensive loss, until such gains and losses are realized. If a decline in the fair value is considered other-than-temporary, based on available evidence, the unrealized loss is transferred from other comprehensive loss to the statements of operations. There were no charges taken for other-than-temporary declines in fair value of short-term or
106
TETRALOGIC PHARMACEUTICALS CORPORATION
NOTES TO FINANCIAL STATEMENTS (Continued)
December 31, 2014
long-term investments in 2013 and 2014. The Company recorded $0 and $8,117 of unrealized (gains)/losses in 2013 and 2014, respectively. Realized gains and losses are included in interest income in the statements of operations. In 2013 and 2014, the Company sold investments for gross proceeds of $0 and $2,496,731. The Company recorded $0 and $148 of realized gains in 2013 and 2014, respectively. The Company utilizes the specific identification method as a basis to determine the cost of securities sold.
The Company reviews investments for other-than-temporary impairment whenever the fair value of an investment is less than the amortized cost and evidence indicates that an investment’s carrying amount is not recoverable within a reasonable period of time. To determine whether an impairment is other-than-temporary, the Company considers the intent to sell, or whether it is more likely than not that the Company will be required to sell, the investment before recovery of the investment’s amortized cost basis. Evidence considered in this assessment includes reasons for the impairment, compliance with the Company’s investment policy, the severity and the duration of the impairment and changes in value subsequent to year end. As of December 31, 2014, there were no investments with a fair value that was significantly lower than the amortized cost basis or any investments that had been in an unrealized loss position for a significant period.
At December 31, 2013, cash and cash equivalents consisted of $13.1 million in cash accounts and $42.0 million in money market accounts. We did not hold any investments at December 31, 2013.
Cash, cash equivalents and investments at December 31, 2014 consist of the following:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Gross |
|
Gross |
|
|
|
|
||
|
|
|
Amortized |
|
Unrealized |
|
Unrealized |
|
Fair |
|
||||
|
|
|
Cost |
|
Gains |
|
Losses |
|
Value |
|
||||
|
December 31, 2014 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash and money market accounts |
|
$ |
13,073,137 |
|
|
— |
|
|
— |
|
$ |
13,073,137 |
|
|
Total cash and cash equivalents |
|
$ |
13,073,137 |
|
$ |
— |
|
$ |
— |
|
$ |
13,073,137 |
|
|
Short-term investments: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Corporate bonds |
|
$ |
37,136,494 |
|
|
— |
|
$ |
(11,908) |
|
$ |
37,124,586 |
|
|
Commercial paper |
|
|
3,495,972 |
|
|
3,791 |
|
|
|
|
|
3,499,763 |
|
|
Total short-term investments |
|
$ |
40,632,466 |
|
$ |
3,791 |
|
$ |
(11,908) |
|
$ |
40,624,349 |
|
|
Total cash, cash equivalents, and investments |
|
$ |
53,705,603 |
|
$ |
3,791 |
|
$ |
(11,908) |
|
$ |
53,697,486 |
|
6. Property and Equipment
Property and equipment consisted of the following:
|
|
|
|
|
|
|
|
|
|
|
|
December 31 |
|
||||
|
|
|
2013 |
|
2014 |
|
||
|
Laboratory equipment |
|
$ |
1,086,825 |
|
$ |
1,536,432 |
|
|
Computers and software |
|
|
258,340 |
|
|
315,640 |
|
|
Furniture, office equipment and leasehold improvements |
|
|
237,811 |
|
|
237,810 |
|
|
Office equipment under capital leases |
|
|
125,014 |
|
|
125,014 |
|
|
|
|
|
1,707,990 |
|
|
2,214,896 |
|
|
Less: accumulated depreciation and amortization |
|
|
(1,568,413) |
|
|
(1,686,420) |
|
|
|
|
$ |
139,577 |
|
$ |
528,476 |
|
107
TETRALOGIC PHARMACEUTICALS CORPORATION
NOTES TO FINANCIAL STATEMENTS (Continued)
December 31, 2014
Depreciation and amortization expense was $99,591 and $125,956 for the years ended December 31, 2013 and 2014, respectively.
7. Accrued Expenses
At December 31, 2013 and 2014, accrued expenses consisted of the following:
|
|
|
|
|
|
|
|
|
|
|
|
December 31 |
|
||||
|
|
|
2013 |
|
2014 |
|
||
|
Payroll and related costs |
|
$ |
1,674,869 |
|
$ |
1,399,264 |
|
|
Research and development related costs |
|
|
730,161 |
|
|
1,445,369 |
|
|
Professional fees |
|
|
368,945 |
|
|
411,096 |
|
|
Interest |
|
|
— |
|
|
167,111 |
|
|
Other |
|
|
147,483 |
|
|
304,944 |
|
|
|
|
$ |
2,921,458 |
|
$ |
3,727,784 |
|
8. Notes Payable
In November 2012, we entered into an agreement with certain of our preferred stockholders, authorizing us to borrow up to $10,000,000 in convertible debt, or the 2012/2013 Notes. In both November 2012 and April 2013, we issued convertible promissory notes to such preferred stockholders in the amount of $5,000,000 for an aggregate of $10,000,000. The 2012/2013 Notes bear interest at the rate of 8% per annum, and were originally due, with interest, on the earlier of July 1, 2013, or upon a liquidation, as defined in our certificate of incorporation. Subsequent to issuance, the 2012/2013 Notes were amended by the noteholders and us to extend their maturity to April 2014. Upon the closing of a qualified financing, as defined in the agreement, the 2012/2013 Notes, including all outstanding principal and accrued interest, shall automatically convert into the type of equity securities issued in the qualified financing at the share price paid by the participating investors in the financing. Upon the occurrence of a non‑qualified financing, the outstanding amount of principal and accrued interest is convertible at the option of the noteholder at the share price paid by the participating investors in the non‑qualified financing. If the 2012/2013 Notes have not been converted or repaid prior to twelve months following issuance, the notes, including all outstanding principal and accrued interest, may be converted, at the option of the holder, into series C convertible preferred stock at the original purchase price for the series C convertible preferred stock. For the years ended December 31, 2013 and 2014, we incurred interest expense of $656,463 and $0, respectively, in connection with these outstanding convertible notes. In December 2013, all of the 2012/2013 Notes and related accrued interest were converted into 1,527,507 shares of common stock at the time of our initial public offering.
In connection with the 2012/2013 Notes, in November 2012 and April 2013 certain of the noteholders received 10‑year warrants to purchase $750,000 and $2,250,000, respectively, of our preferred stock. The number of shares the holder may purchase by exercising these warrants is equal to the warrant dollar value divided by the per share price paid by investors in the qualified or non‑qualified equity financing, as defined in the applicable agreement. If a qualified or non‑qualified financing has not occurred within twelve months of issuance, or the Trigger Date, then, at the election of the holder, the warrants may be exercisable into series C convertible preferred stock or the preferred stock issued in the first preferred stock financing completed after the Trigger Date. In the event of an initial public offering (such as our recent initial public offering), the number of warrants and the exercise price will be set based on the series C conversion ratio (6.4022) and net settle into a number of shares of common stock equal to the in‑the‑money value at the initial public offering price. The fair value of the 2012/2013 Warrants was calculated to be $847,441, applying probability‑weighted expected return methods to multiple scenario option pricing models and was recorded as debt discount with a corresponding credit to the preferred stock warrant liability. A total of $811,218 and $0 of debt discount was amortized
108
TETRALOGIC PHARMACEUTICALS CORPORATION
NOTES TO FINANCIAL STATEMENTS (Continued)
December 31, 2014
to interest expense during the years ended December 31, 2013 and 2014, respectively. In December 2013, the 2012/2013 Warrants were converted into 40,017 shares of common stock at the time of our initial public offering.
In connection with a clinical study being conducted in collaboration with a pharmaceutical company, in May 2013, we received a $3,000,000 loan from such company and in return we issued a convertible note to such company, or the Collaboration Note, in the amount of $3,000,000. The Collaboration Note bears interest at the rate of 8% per annum, and is due, with interest, on the earlier of May 16, 2015 or in the event of a liquidation (as more fully described in our certificate of incorporation). Upon the closing of a qualified financing, as defined in the agreement, the Collaboration Note, including all outstanding principal and accrued interest, shall automatically convert into the type of equity securities issued in the qualified financing at the share price paid by the participating investors in the financing. Upon the occurrence of a non‑qualified financing, the outstanding amount of principal and accrued interest is convertible at the option of the noteholder at the share price paid by the participating investors in the non‑qualified financing. If the Collaboration Note has not been converted or repaid prior to twenty-four months following issuance, such note, including all outstanding principal and accrued interest, may be converted, at the option of the holder, into series C‑1 convertible preferred stock at the original purchase price for the series C‑1 convertible preferred stock. For the year ended December 31, 2013 and 2014, interest expense of $141,370 and $0, respectively, was recorded in connection with the outstanding Collaboration Note. In December 2013, the Collaboration Note and related accrued interest was converted into 448,767 shares of common stock at the time of our initial public offering.
In October 2013, we issued additional convertible notes in the aggregate amount of $6.2 million, or the October 2013 Notes. The October 2013 Notes bear interest at the rate of 8% per annum, and mature, with interest, on the earlier of October 30, 2014 or in the event of a liquidation (as more fully described in our certificate of incorporation). Upon the closing of a qualified financing, as defined in the agreement, the October 2013 Notes, including all outstanding principal and accrued interest, shall automatically convert into the type of equity securities issued in the qualified financing at the share price paid by the participating investors in the financing. Upon the occurrence of a non‑qualified financing, the outstanding amount of principal and accrued interest is convertible at the option of the noteholder at the share price paid by the participating investors in the non‑qualified financing. If the October 2013 Notes have not been converted or repaid prior to twelve months following issuance, the notes, including all outstanding principal and accrued interest, may be converted, at the option of the holder, into series C convertible preferred stock at the original purchase price for the series C convertible preferred stock. For the years ended December 31, 2013 and 2014, we incurred interest expense of $65,600 and $0, respectively, in connection with these outstanding convertible notes. In December 2013, the October 2013 Notes and related accrued interest were converted into 900,145 shares of common stock at the time of our initial public offering.
In connection with this additional issuance of the October 2013 Notes, the noteholders received additional warrants to purchase $1.9 million of our preferred stock. The number of shares the holder may purchase by exercising these warrants is equal to the warrant dollar value divided by the per share price paid by investors in the qualified or non‑qualified equity financing, as defined in the applicable agreement. If a qualified or non‑qualified financing has not occurred within twelve months of issuance, or the Trigger Date, then, at the election of the holder, the warrants may be exercisable into series C convertible preferred stock or the preferred stock issued in the first preferred stock financing completed after the Trigger Date. In the event of an initial public offering, the number of warrants and the exercise price will be set based on the series C conversion ratio (6.4022) and net settle into a number of shares of common stock equal to the in‑the‑money value at the initial public offering price. The fair value of the October 2013 Warrants was calculated to be $1,092,771, applying probability‑weighted expected return methods to multiple scenario option pricing models and was recorded as debt discount with a corresponding credit to the preferred stock warrant liability. A total of $1,092,771 and $0 of debt discount was amortized to interest expense during the year ended December 31, 2013 and 2014, respectively. In December 2013, the October 2013 Warrants were converted into 24,953 shares of common stock at the time of our initial public offering.
109
TETRALOGIC PHARMACEUTICALS CORPORATION
NOTES TO FINANCIAL STATEMENTS (Continued)
December 31, 2014
8% Convertible Senior Notes Due 2019
On June 23, 2014, we issued through a private placement $47.0 million in aggregate principal amount of 8% convertible senior notes due June 15, 2019 (the “8% Notes”), all of which remain outstanding as of December 31, 2014. Interest on the 8% Notes is payable semi-annually in arrears on June 15 and December 15 of each year, commencing December 15, 2014.
The 8% Notes are general unsecured and unsubordinated obligations and will rank senior in right of payment to all of our indebtedness that is expressly subordinated in right of payment to the notes, rank equal in right of payment to our existing and future indebtedness and other liabilities that are not so subordinated, are effectively subordinated to any of our future secured indebtedness to the extent of the value of the assets securing such indebtedness, and rank structurally junior to all indebtedness and other liabilities incurred by our subsidiaries, including trade payables. We may not redeem the 8% Notes at our option prior to maturity. The 8% Notes are convertible prior to maturity, subject to certain conditions described below, into shares of our common stock at an initial conversion rate of 148.3019 shares per $1,000 principal amount of the 8% Notes (equivalent to an initial conversion price of approximately $6.74 per share of common stock). This conversion rate is subject to adjustment upon the occurrence of certain specified events but will not be adjusted for accrued and unpaid interest. If we receive stockholder approval, we will satisfy our conversion obligation by paying or delivering, as the case may be, cash, shares of our common stock or a combination thereof, at our election.
The Holders of the 8% Notes may surrender their notes for conversion any time prior to the close of business immediately preceding February 15, 2019 only if any of the following conditions is satisfied:
|
· |
during any fiscal quarter (and only during such fiscal quarter) commencing after December 31, 2014, if, for at least 20 trading days (whether or not consecutive) during the 30 consecutive trading day period ending on the last trading day of the immediately preceding fiscal quarter, the last reported sale price of our common stock for each such trading day is greater than or equal to 100% of the applicable conversion price on such trading day; |
|
· |
during the five consecutive business day period immediately following any 10 consecutive trading day period, or the “measurement period”, in which, for each trading day of such measurement period, the “trading price” per $1,000 principal amount of notes for such trading day was less than 98% of the product of the last reported sale price of our common stock for such trading day and the applicable conversion rate on such trading day; or |
|
· |
upon the occurrence of specified corporate events. |
Holders also may surrender their 8% Notes for conversion at any time on or after February 15, 2019 and on or prior to the close of business on the business day immediately prior to the stated maturity date regardless if any of the foregoing conditions have been satisfied.
Each $1,000 principal amount of 8% Notes is convertible into shares of our common stock equal to the conversion rate in effect on the conversion date, together with cash in lieu of fractional shares issuable upon conversion. If we receive stockholder approval prior to the relevant conversion date, we may deliver upon conversion cash, shares, or a combination thereof, at our election. With respect to any conversions after obtaining stockholder approval, settlement amounts will be computed as follows:
|
· |
if we elect (or are deemed to have elected) physical settlement, we will deliver to the converting holder in respect of each $1,000 principal amount of notes being converted a number of shares of common
110 |
TETRALOGIC PHARMACEUTICALS CORPORATION
NOTES TO FINANCIAL STATEMENTS (Continued)
December 31, 2014
stock equal to the conversion rate in effect on the conversion date together with cash in lieu of fractional shares issuable upon conversion and the interest make-whole payment, if applicable; |
|
· |
if we elect cash settlement, we will pay to the converting holder in respect of each $1,000 principal amount of notes being converted cash in an amount equal to the sum of the daily conversion values for each of the 60 consecutive trading days during the related observation period and the interest make-whole payment, if applicable; and |
|
· |
if we elect combination settlement, we will pay or deliver, as the case may be, to the converting holder in respect of each $1,000 principal amount of notes being converted a “settlement amount” equal to the sum of the daily settlement amounts for each of the 60 consecutive trading days during the relevant observation period (plus cash in lieu of any fractional share of our common stock issuable upon conversion) and the interest make-whole payment, if applicable. |
“Daily settlement amount” means, for each of the 60 consecutive trading days in the relevant observation period:
|
· |
cash equal to the lesser of (i) the maximum cash amount per $1,000 principal amount of notes to be received upon conversion as specified in the notice specifying our chosen settlement method (the “specified dollar amount”), if any, divided by 60 (such quotient, the “daily measurement value”) and (ii) the daily conversion value; and |
|
· |
if the daily conversion value exceeds the daily measurement value, a number of shares equal to (i) the difference between the daily conversion value and the daily measurement value, divided by (ii) the daily volume-weighted average price for such trading day. |
“Daily conversion value” means, for each of the 60 consecutive trading days in the observation period for a note, one sixtieth (1/60th) of the product of (i) the applicable conversion rate on such trading day and (ii) the daily volume-weighted average price on such trading day.
If the 8% Notes are converted in connection with certain fundamental changes that occur prior to maturity of the 8% Notes, we may also be obligated to pay an additional (or “make whole”) premium with respect to the 8% Notes so converted. In addition, if certain fundamental changes occur with respect to TetraLogic, holders of the 8% Notes will have the option to require us to purchase for cash all or a portion of the 8% Notes at a purchase price equal to 100% of the principal amount of the 8% Notes plus accrued and unpaid interest.
On or after December 31, 2014, if, for at least 20 trading days (whether or not consecutive) during the 30 consecutive trading day period ending within five trading days prior to a conversion date the last reported sale price of our common stock exceeds the applicable conversion price on each such trading day, we will, in addition to the other consideration payable or deliverable in connection with such conversion, make an interest make-whole payment to the converting holder equal to the present value of all scheduled payments of interest (using a discount rate equal to 2%) through the earlier of (i) the date that is three years after the conversion date and (ii) the maturity date if the notes had not been so converted. We will satisfy our obligation to pay any interest make-whole payment in shares of our common stock, without a cash payment in lieu of any fractional shares and without further obligation to deliver any shares of our common stock or pay any cash in excess of the threshold described in the succeeding paragraph. However, if we receive stockholder approval, we may pay any interest make-whole payment either in cash or in shares of our common stock, at our election. Notwithstanding the foregoing, until we receive stockholder approval, the number of shares we may deliver in connection with a conversion of notes, including those delivered in connection with an interest make-whole payment, will not exceed 163.1321 shares per $1,000 principal amount of notes, subject to adjustment at the same time and in the
111
TETRALOGIC PHARMACEUTICALS CORPORATION
NOTES TO FINANCIAL STATEMENTS (Continued)
December 31, 2014
same manner as the conversion rate adjustments on the 8% Notes, including those adjustments made in connection with certain fundamental changes. If we pay an interest make-whole payment in shares of our common stock, then the number of shares of common stock a holder will receive will be that number of shares that have a value equal to the amount of the interest make-whole payment to be paid to such holder in shares, divided by the product of the simple average of the daily value weighted average price of our common stock for the 10 trading days immediately preceding the conversion date multiplied by 92.5%. Notwithstanding the foregoing, if in connection with any conversion the conversion rate is adjusted for a make-whole fundamental change, then such holder will not receive the interest make-whole payment with respect to such note.
The indenture for the notes contains certain covenants which limit our and our subsidiaries’ ability to incur certain additional indebtedness except for certain permitted debt, and to incur liens except for certain permitted liens.
We account for convertible debt instruments that may be settled in cash upon conversion (including partial cash settlement) by recording the liability and equity components of the convertible debt separately. The liability component is computed based on the fair value of a similar liability that does not include the conversion option. The liability component includes both the value of the embedded interest make-whole derivative and the carrying value of the 8% Notes. The equity component is computed based on the total debt proceeds less the fair value of the liability component. The equity component is also recorded as debt discount and amortized as interest expense over the expected term of the 8% Notes.
The liability component of the 8% Notes on the date of issuance was computed as $30.8 million, consisting of the value of the embedded interest make-whole derivative of $3.1 million and the carrying value of the 8% Notes of $27.7 million. Accordingly, the equity component on the date of issuance was $16.2 million. The discount on the 8% Notes is being amortized to interest expense over the term of the Notes, using the effective interest method. The carrying value of the 8% Notes was $29.0 million at December 31, 2014.
If our debt is considered current at the balance sheet date, the liability component of our convertible notes will be classified as current liabilities and presented in current portion of long-term debt and the equity component of our convertible debt will be considered a redeemable security and presented as redeemable equity on our consolidated balance sheet.
Transaction costs of $2.9 million related to the issuance of the 8% Notes are allocated to the liability and equity components in proportion to the allocation of the proceeds and accounted for as deferred financing costs and equity issuance costs, respectively. Approximately $1.0 million of this amount was allocated to equity and the remaining $1.9 million have been capitalized as deferred financing costs and are being amortized over the term of the 8% Notes.
The following table summarizes how the issuance of the 8% Notes is reflected in our balance sheet at December 31, 2014:
|
|
|
|
|
|
|
|
|
December 31, 2014 |
|
|
|
Gross proceeds |
|
$ |
47,000,000 |
|
|
Conversion option reported in equity |
|
|
(16,165,238) |
|
|
Interest make-whole derivative |
|
|
(3,055,000) |
|
|
Amortization of debt discount |
|
|
1,199,580 |
|
|
Carrying value |
|
$ |
28,979,342 |
|
112
TETRALOGIC PHARMACEUTICALS CORPORATION
NOTES TO FINANCIAL STATEMENTS (Continued)
December 31, 2014
The following table sets forth our interest expense incurred for the years ended December 31, 2013 and 2014:
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended December 31, |
|
||||
|
|
|
2013 |
|
2014 |
|
||
|
|
|
|
|
|
|
|
|
|
2012/2013 Notes |
|
$ |
656,463 |
|
$ |
— |
|
|
Debt discount - 2012/2013 Warrants |
|
|
811,218 |
|
|
— |
|
|
Collaboration Note |
|
|
141,370 |
|
|
— |
|
|
October 2013 Notes |
|
|
65,600 |
|
|
— |
|
|
Debt discount - October 2013 Notes |
|
|
1,092,771 |
|
|
— |
|
|
8% Convertible Senior Notes due 2019 - coupon |
|
|
— |
|
|
1,963,556 |
|
|
8% Convertible Senior Notes due 2019 - amortization of debt discount |
|
|
— |
|
|
1,199,580 |
|
|
Amortization of deferred financing costs |
|
|
— |
|
|
196,253 |
|
|
|
|
$ |
2,767,422 |
|
$ |
3,359,389 |
|
9. Convertible Preferred Stock and Stockholders’ Equity (Deficit)
Convertible Preferred Stock
In March 2004, we issued 8,000,000 shares of series A convertible preferred stock at $1.00 per share generating gross proceeds of $8.0 million. Costs associated with the offering were $152,140. The series A convertible preferred stock originally had a redemption feature that was revoked as part of the Series B convertible preferred stock issuance in June 2006. The carrying value of the series A convertible preferred stock was being accreted to its redemption value on the redemption date, based upon the effective interest method. With the redemption feature being revoked in June 2006, all previous accretion was reversed. In December 2013, all 8,000,000 shares of series A convertible preferred stock were converted into 1,045,749 shares of common stock at the time of our initial public offering.
In June 2006, we issued 31,835,223 shares of series B convertible preferred stock at $0.45 per share generating gross proceeds of $14,325,850. Costs associated with the offering were $211,621. An additional 1,498,111 shares of series B convertible preferred stock were issued in connection with the conversion of $666,666 of 2006 Notes and $7,484 of accrued interest associated with the 2006 Notes. We also authorized the issuance of an additional 47,037,035 shares of series B convertible preferred stock under our certificate of incorporation; however, in connection with the issuance of the series C convertible preferred stock in July 2010, the number of authorized shares of series B convertible preferred stock was reduced to 33,703,699. On September 1, 2013, 8,333,334 shares of series B convertible preferred stock were converted to 490,197 shares of common stock. In December 2013, the remaining 25,000,000 shares of series B convertible preferred stock were converted into 1,470,584 shares of common stock at the time of our initial public offering.
In July 2010, we authorized the sale of series C convertible preferred stock. In 2010 we issued 95,143,072 shares of series C convertible preferred stock at $0.3766 per share generating gross proceeds of $35,830,884, including the conversion of $4,388,000 of 2009/2010 Notes and $167,914 of accrued interest associated with the 2009/2010 Notes. Costs associated with the offering were $303,880. An additional 3,550,265 shares of series C convertible preferred stock were issued in January 2011 at $0.3766 per share generating gross proceeds of $1,337,030. On September 1, 2013, 6,968,137 shares of series C convertible preferred stock were converted to 409,888 shares of common stock. In December 2013, the remaining 91,725,200 shares of series C convertible preferred stock were converted into 5,395,592 shares of common stock at the time of our initial public offering.
In May 2011, we authorized and issued 13,276,686 shares of series C‑1 convertible preferred stock at $0.4519 per share, generating gross proceeds of $5,999,734. Costs associated with the offering were $80,118. In December 2013,
113
TETRALOGIC PHARMACEUTICALS CORPORATION
NOTES TO FINANCIAL STATEMENTS (Continued)
December 31, 2014
all 13,276,686 shares of series C‑1 convertible preferred stock were converted into 780,981 shares of common stock at the time of our initial public offering.
Preferred Stock
We are authorized to issue 25,000,000 shares of preferred stock, with a par value of $0.0001, of which none were issued and outstanding at December 31, 2013 and 2014.
Common Stock
We are authorized to issue 100,000,000 shares of common stock, with a par value of $0.0001, of which 22,199,256 and 23,334,901 were issued and outstanding at December 31, 2013 and 2014, respectively.
Equity Compensation Plans
In March 2004, we adopted the 2004 Equity Compensation Plan, or the 2004 Plan, that authorizes us to grant option and restricted stock awards. In October 2013, our board of directors approved the reservation of an additional 1,847,738 shares of common stock for issuance under the 2004 Plan, to accommodate grants of non‑qualified stock options to new members of our executive management, our former President and Chief Executive Officer and other employees and consultants. The amount, terms of grants, and exercisability provisions are determined and set by our board of directors.
In December 2013, we adopted the 2013 Equity Compensation Plan, or the 2013 Plan, that authorizes us to grant option and restricted stock awards. Our board of directors approved the reservation of 3,659,158 shares of common stock for issuance under the 2013 Plan.
As of December 31, 2014, the number of shares of common stock reserved for issuance under the 2004 and 2013 Equity Compensation Plans was 2,942,629.
Stock‑based compensation expense
Stock‑based payments to employees, including grants of employee stock options, are recognized in the statement of operations and comprehensive loss based on fair value. Stock‑based payments to non‑employees are recognized at fair value on the date of grant and re‑measured at each subsequent reporting date through the settlement of the instrument. The fair value of stock options is calculated using the Black‑ Scholes valuation model, and is recognized over the vesting period.
Stock‑based compensation expense recognized by award type is as follows:
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
Year Ended December 31 |
||||
|
|
|
2013 |
|
2014 |
||
|
Options awards |
|
$ |
3,160,067 |
|
$ |
3,299,870 |
|
Restricted stock awards |
|
|
548,325 |
|
|
84,930 |
|
Total stock-based compensation expense |
|
$ |
3,708,392 |
|
$ |
3,384,800 |
114
TETRALOGIC PHARMACEUTICALS CORPORATION
NOTES TO FINANCIAL STATEMENTS (Continued)
December 31, 2014
Total compensation cost recognized for all stock‑based compensation awards in the statements of operations is as follows:
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
Year Ended December 31 |
||||
|
|
|
2013 |
|
2014 |
||
|
Research and development |
|
$ |
886,808 |
|
$ |
933,814 |
|
General and administrative |
|
|
2,821,584 |
|
|
2,450,986 |
|
Total stock-based compensation expense |
|
$ |
3,708,392 |
|
$ |
3,384,800 |
Stock Options
A summary of activity for all options is presented below:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Fair Value |
|
|
|
|
|
|
|
|
|
|
Weighted Average |
|
Weighted Average |
|
of |
|
Aggregate |
|
|||
|
|
|
|
|
Exercise Price |
|
Contractual |
|
Options |
|
Intrinsic |
|
|||
|
|
|
Shares |
|
Per Share |
|
Life (In Years) |
|
Granted |
|
Value |
|
|||
|
Outstanding—December 31, 2012 |
|
716,780 |
|
$ |
1.36 |
|
6.9 |
|
|
|
|
$ |
121,853 |
|
|
Granted |
|
2,825,746 |
|
|
5.11 |
|
|
|
$ |
13,139,719 |
|
|
|
|
|
Exercised |
|
(100,521) |
|
|
1.09 |
|
|
|
|
|
|
|
|
|
|
Forfeited |
|
(178,588) |
|
|
5.22 |
|
|
|
|
|
|
|
|
|
|
Outstanding—December 31, 2013 |
|
3,263,417 |
|
$ |
5.36 |
|
9.0 |
|
|
|
|
$ |
13,575,815 |
|
|
Granted |
|
253,750 |
|
|
5.77 |
|
|
|
$ |
1,145,545 |
|
|
|
|
|
Exercised |
|
(121,816) |
|
|
1.49 |
|
|
|
|
|
|
|
|
|
|
Forfeited |
|
(118,043) |
|
|
5.56 |
|
|
|
|
|
|
|
|
|
|
Outstanding—December 31, 2014 |
|
3,277,308 |
|
$ |
5.53 |
|
8.5 |
|
|
|
|
$ |
— |
|
|
Vested and expected to vest—December 31, 2014 |
|
3,270,963 |
|
$ |
5.53 |
|
8.5 |
|
|
|
|
$ |
— |
|
|
Exercisable at December 31, 2014 |
|
1,690,999 |
|
$ |
5.11 |
|
8.1 |
|
|
|
|
$ |
— |
|
Options issued under the 2013 Plan may have a contractual life of up to 10 years and may be exercisable in cash or as otherwise determined by the board of directors. Vesting generally occurs over a period of not greater than four years.
The per‑share weighted‑average fair value of the options granted to employees and non‑employees was estimated at the date of grant using the Black‑Scholes option‑pricing model with the following weighted‑average assumptions:
|
|
|
|
|
|
|
|
|
|
|
|
|
2013 |
|
|
2014 |
|
||
|
Expected stock price volatility |
|
|
90 |
% |
|
|
90 |
% |
|
Expected term of options |
|
|
6.2 Years |
|
|
|
6.9 years |
|
|
Risk‑free interest rate |
|
|
1.89 |
% |
|
|
2.19 |
% |
|
Expected annual dividend yield |
|
|
— |
% |
|
|
— |
% |
|
Weighted average grant date fair value |
|
$ |
4.65 |
|
|
$ |
4.51 |
|
The weighted‑average valuation assumptions were determined as follows:
|
· |
Expected stock price volatility: The expected volatility is based on historical volatilities of similar entities within our industry which were commensurate with our expected term assumption as described in the SEC’s Staff Accounting Bulletin, or SAB, No. 107. |
115
TETRALOGIC PHARMACEUTICALS CORPORATION
NOTES TO FINANCIAL STATEMENTS (Continued)
December 31, 2014
|
· |
Expected term of options: We estimated the expected term of our stock options with service‑based vesting using the “simplified” method, as prescribed in SAB No. 107, whereby the expected life equals the arithmetic average of the vesting term and the original contractual term of the option due to our lack of sufficient historical data. |
|
· |
Risk‑free interest rate: We base the risk‑free interest rate on the interest rate payable on U.S. Treasury securities in effect at the time of grant for a period that is commensurate with the assumed expected option term. |
|
· |
Expected annual dividend yield: The estimated annual dividend yield is 0% because we have not historically paid, and do not expect for the foreseeable future to pay, a dividend on our common stock. |
As of December 31, 2014, there was $7,331,186 of total unrecognized compensation expense related to unvested options granted under the 2004 and 2013 Plans. That expense is expected to be recognized in the years ended as follows:
|
|
|
|
|
|
|
December 31, 2015 |
|
$ |
3,401,176 |
|
|
December 31, 2016 |
|
|
3,305,382 |
|
|
December 31, 2017 |
|
|
541,701 |
|
|
December 31, 2018 |
|
|
82,927 |
|
|
|
|
$ |
7,331,186 |
|
During the years ended December 31, 2013 and 2014, we granted options to purchase 248,745 and 0 shares of common stock, respectively, to employees and consultants that contain performance‑based vesting criteria. Milestone events are specific to our corporate goals, including but not limited to certain clinical and corporate development milestones. Stock‑based compensation expense associated with these performance‑based stock options is recognized if the performance condition is considered probable of achievement using management’s best estimates. We have concluded that certain performance‑based milestones were probable of achievement, and compensation expense of $384,850 and $8,329 was recognized for the years ended December 31, 2013 and 2014, respectively. At December 31, 2014, there are no remaining shares of common stock issuable upon vesting of stock options subject to performance‑based vesting criteria.
The intrinsic value of stock options exercised for the years ended December 31, 2013 and 2014 were $162,636 and $948,417, respectively. The estimated fair value of shares that vested for the years ended December 31, 2013 and 2014 were $3,331,419 and $3,235,533, respectively.
116
TETRALOGIC PHARMACEUTICALS CORPORATION
NOTES TO FINANCIAL STATEMENTS (Continued)
December 31, 2014
Restricted Stock
A summary of our restricted stock activity and related information is as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted |
|
|
|
|
|
|
|
Average |
|
|
|
|
|
|
|
Grant Date |
|
|
|
|
|
|
|
Fair Value |
|
|
|
|
|
Shares |
|
per Share |
|
|
|
Unvested Balance—December 31, 2012 |
|
285,167 |
|
|
|
|
|
Granted |
|
— |
|
|
— |
|
|
Vested |
|
(194,536) |
|
|
|
|
|
Forfeited |
|
(10,394) |
|
|
|
|
|
Unvested Balance—December 31, 2013 |
|
80,237 |
|
|
|
|
|
Granted |
|
— |
|
|
— |
|
|
Vested |
|
(59,279) |
|
|
|
|
|
Forfeited |
|
(9,193) |
|
|
|
|
|
Unvested Balance—December 31, 2014 |
|
11,765 |
|
|
|
|
As of December 31, 2014, there was $18,000 of total unrecognized compensation expense related to unvested shares of restricted stock granted under the Plan.
We record restricted stock based on its estimated fair value on the date of issuance. The expense related to restricted stock is recognized over the vesting period, which is generally over a three to four year period.
Warrants
We presently have the following warrants outstanding to purchase shares of our common stock:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Number of |
|
|
|
|
|
|
|
|
|
|
|
Shares |
|
Exercise |
|
|
|
|
|
Warrant Series |
|
Underlying Equity Security |
|
Issuable |
|
Price |
|
Expiration Date |
|
|
|
2007 Warrant |
|
common stock |
|
1,961 |
|
$ |
7.65 |
|
May 2017 |
|
|
2006 Warrants |
|
common stock |
|
21,786 |
|
$ |
7.65 |
|
March 2016-May 2016 |
|
|
2009/2010 Warrants |
|
common stock |
|
52,815 |
|
$ |
0.85 |
|
November 2019-March 2020 |
|
Our 2006 Warrants and 2009/2010 Warrants are classified as derivative liabilities on our balance sheets with subsequent changes to fair value recorded through earnings at each reporting period on our statement of operations and comprehensive loss as change in fair value of derivative liabilities. See Note 8 for additional information and Note 3 with respect to fair value.
10. Commitments and Contingencies
Leases
We lease office space and office equipment under operating leases. Rent expense under these operating leases was $344,389 and $390,126 in 2013 and 2014, respectively.
117
TETRALOGIC PHARMACEUTICALS CORPORATION
NOTES TO FINANCIAL STATEMENTS (Continued)
December 31, 2014
As of December 31, 2014, we have commitments for $748,287 of future minimum lease payments to be made in 2015 and 2016.
Employee Benefit Plan
We maintain a Section 401(k) retirement plan for all employees who are 21 years of age or older. Employees can contribute up to 90.0% of their eligible pay, subject to maximum amounts allowed under law. We may make discretionary profit sharing contributions, which vest over a period of four years from each employee’s commencement of employment with us. We have not made any discretionary contributions.
License Agreement with Princeton University
In November 2003, we entered into an exclusive license agreement with Princeton University, subsequently amended in June 2004, August 2006, and October 2006, which grants us the rights to certain U.S. patents controlled by the university relating to SMAC‑mimetic compounds, including birinapant, and a non‑exclusive right to certain know‑how and technology relating thereto. The agreement contains a right by us to sublicense. To date, we have paid an aggregate of $100,000 in license fees under the license agreement. As part of the consideration paid, we issued to Princeton University 9,734 shares of our common stock and agreed to pay Princeton University certain royalties. In particular, we are obligated to pay royalties as a percentage of net product sales of 2.0% for direct licensed products, such as birinapant, and 0.5% of derived licensed products, if such products are covered by the applicable Princeton University patent rights. We have the right to reduce the amount of royalties owed to Princeton University by the amount of any royalties paid to a third‑party in a pro rata manner, provided that the royalty rate may not be less than 1.0% of net sales for direct licensed products and 0.25% for derived licensed products. The obligation to pay royalties in the U.S. expires upon the expiration, lapse or abandonment of the last of the licensed patent rights that covers the manufacture, use or sale of the direct licensed products. The obligation to pay royalties outside the U.S. expires, on a country by country basis, 10 years from the first commercial sale of a licensed product in each country. The licensed patent rights were developed using federal funds from the National Institutes of Health and are subject to certain overriding rights and obligations of the federal government. This agreement expires upon expiration of the last of the licensed patent rights in 2023 (absent extensions).
The agreement also requires that we pay to Princeton University 5.0% of the non‑royalty consideration that we receive from a sublicensee until October 5, 2014 and 2.5% thereafter. Under the license agreement, we are obligated to use reasonable efforts to develop, test, obtain regulatory approval, manufacture, market and sell licensed products in all countries worldwide.
License Agreement with Walter and Eliza Hall Institute
In January 2014, we entered into a license agreement with the Walter and Eliza Hall Institute of Medical Research, or WEHI, in Melbourne, Australia for worldwide exclusive rights to a patent application and any patents issuing therefrom relating to a method of treating intracellular infections involving the administration of an IAP antagonist. WEHI will perform research and development services for which we will be making payments over the first 4 years of the agreement in the amount of $1 million, of which we have paid $500,000 to date. We are obligated to pay royalties as a percentage of net sales of 2% for products that are based either on the licensed patents or any patents arising from research performed by WEHI. We are also obligated to pay royalties as a percentage of net income of 15% received from sublicensing the licensed patents or any patents arising from research performed by WEHI to a third party. We may also be required to make milestone payments to WEHI of up to $3,750,000 for the first indication and up to $1,875,000 for each of the next two indications based on the commencement of certain clinical trials and the filing and approval of new drug applications.
118
TETRALOGIC PHARMACEUTICALS CORPORATION
NOTES TO FINANCIAL STATEMENTS (Continued)
December 31, 2014
License Agreement with Harvard University and Dana-Farber Cancer Institute
In October 2008, Shape Pharmaceuticals entered into a license agreement with Harvard University and Dana-Farber Cancer Institute, Inc. (the “Licensors”) to grant a license under its interest in certain patent rights as defined in the license agreement, which include claims covering the composition of the SHAPE molecule. The agreement contains a right by us to sublicense. The Licensors received 400,000 shares of common stock of Shape Pharmaceuticals in consideration for the grant of the license, for which they received a payment of $213,317 when we acquired Shape Pharmaceuticals in April 2014. We also paid the Licensors an annual maintenance fee of $100,000 in 2012 and will pay $50,000 on the fifth anniversary of the effective date of the agreement and on each subsequent anniversary date thereafter as long as the license agreement remains in full force and effect. The annual maintenance fee of $50,000 was paid in 2013 and 2014. As defined in the license agreement, we may be required to pay milestones on an indication-by-indication basis of up to $4,450,000 in the aggregate and/or royalties of net sales of developed products, if and when achieved. Annual maintenance fee payments can be used to offset milestone obligations. We paid a milestone payment of $100,000 during the year ended December 31, 2011. We have the right to terminate the agreement upon 60 days’ written notice.
CTCL Trial with The Leukemia and Lymphoma Society
In June 2010, we entered into a funding agreement with The Leukemia and Lymphoma Society, or LLS, to fund the development of SHAPE. Under the LLS funding agreement, we are obligated to use the funding received exclusively for the payment or reimbursement of the costs and expenses for clinical development activities for SHAPE. Under this agreement, we retain ownership and control of all intellectual property pertaining to works of authorship, inventions, know-how, information, data and proprietary material.
Under the LLS funding agreement, as amended, we received funding of $2.695 million from LLS through 2014. We terminated the funding agreement effective as of February 2014. We are required to make specified payments to LLS, including payments payable upon execution of the first out-license; first filing of approval for marketing by a regulatory body; first approval for marketing by a regulatory body; and completion of the first commercial sale of SHAPE. The extent of these payments and our obligations will depend on whether we out-license rights to develop or commercialize SHAPE to a third party, we commercialize SHAPE on our own or we combine with or are sold to another company. In addition, we will pay to LLS a single-digit percentage royalty of our net sales of SHAPE, if any. The sum of our payments to LLS is capped at three times the total funding received from LLS, or $8.085 million.
In addition, some of our obligations under the funding agreement will remain in effect until the completion of specified milestones and payments to LLS. Assuming the successful outcome of the development activities covered by the LLS funding agreement and our receipt of necessary regulatory approvals, we will be required to take commercially reasonable steps through 2019 to advance the development of SHAPE in clinical trials and to bring SHAPE to practical application for CTCL in a major market country, provided that we reasonably believe the product is safe and effective. We believe that we can satisfy our obligation by out-licensing SHAPE to, or partnering SHAPE with, a third party. We are required to report to LLS on our efforts and results with respect to continuing development of SHAPE. Our failure to perform these diligence obligations, even if we successfully achieve the specified development milestones, would require us to pay back to LLS the total amount of the funding we received from them, unless an exception applies. If LLS were to claim that such failure occurred and we disagreed with such claim, the dispute would be settled through binding arbitration. In connection with the accounting for the acquisition of Shape Pharmaceuticals, we estimated the fair value of this potential obligation to be $200,000 and have accrued this amount in our December 31, 2014 balance sheet.
119
TETRALOGIC PHARMACEUTICALS CORPORATION
NOTES TO FINANCIAL STATEMENTS (Continued)
December 31, 2014
Management Transition Agreement
In connection with the Management Transition Agreement with John M. Gill, our former President and Chief Executive Officer, we recorded a charge of $1,726,599 included in general and administrative expenses for the year ended December 31, 2013. Pursuant to the Transition Agreement, Mr. Gill will receive:
|
· |
continuation of his base salary (at the rate of $34,386.50 per month) for 15 months from August 12, 2013; |
|
· |
a lump sum payment of $50,000 plus 1.0% of the amounts committed to be paid to us over three years pursuant to (i) partnership or collaboration agreements executed on or before December 31, 2013 with any entity with which Mr. Gill had substantive discussions over the 18 months immediately prior to the Resignation Date, that contractually commit payment to us over the first three years of such agreement(s) of no less than $15,000,000 of non‑dilutive funding, and/or (ii) the first closing(s) of financings that contractually commit, on or prior to December 31, 2013, payment to us of no less than $20,000,000 from new investors over three years after such first closing(s) (not including the conversion of any notes held by investors as of August 12, 2013), including as commitments future closings of a tranched financing that are subject to the satisfaction of a funding milestones, in each case provided that the total amount committed to us pursuant to clauses (i) and (ii) during such three‑year periods shall not total less than $35,000,000; |
|
· |
accelerated vesting of all of his unvested restricted stock; and |
|
· |
certain additional stock option grants which vested in 2013 based on the achievement of certain defined milestones. |
11. Income Taxes
We provide for income taxes under FASB ASC 740. Deferred income tax assets and liabilities are determined based upon differences between financial reporting and tax bases of assets and liabilities, which are measured using the enacted tax rates and laws that will be in effect when the differences are expected to reverse.
As of December 31, 2013 and 2014, we had approximately $82.1 million and $106.7 million of federal net operating loss(“NOL”) carryforwards and $81.2 million and $101.5 million of state NOL carryforwards, respectively, available to offset future federal and state taxable income that will expire beginning in the year 2023 and through the year 2034. As of December 31, 2013 and 2014, we had approximately $4.6 million and $3.6 million, respectively, of federal research and development (“R&D”) tax credit carryforwards to offset future federal tax liabilities that will expire beginning in the year 2028 and through the year 2034.
In assessing the realizability of deferred tax assets, we consider whether it is more‑likely‑than‑not that some portion or all of the deferred tax assets will not be realized. The ultimate realization of deferred tax assets is dependent upon the generation of future taxable income during the periods in which the temporary differences representing net future deductible amounts become deductible. Our deferred tax liability related to the indefinite-lived intangible asset may not be considered a source of future taxable income available to offset our deferred tax assets. After consideration of all the evidence, both positive and negative, we have recorded a full valuation allowance against our net deferred tax assets at December 31, 2013 and 2014, respectively, because our management has determined that is it more likely than not that these assets will not be fully realized. The valuation allowance increased by approximately $8.0 million and $4.5 million during the years ended December 31, 2013 and December 31, 2014, respectively, due primarily to the generation of NOLs during those periods. However, for the year ended December 31, 2014, approximately $2.0 million of the increase was related to purchase accounting for the acquisition of Shape Pharmaceuticals and an approximately $6.6
120
TETRALOGIC PHARMACEUTICALS CORPORATION
NOTES TO FINANCIAL STATEMENTS (Continued)
December 31, 2014
million tax benefit from reversing the valuation allowance was recorded as an adjustment to APIC attributable to recording the deferred tax liability associated with the issuance of the 8% notes.
The NOL carryforwards, as well as the R&D tax credit carryforwards, are subject to review and possible adjustment by the Internal Revenue Service and state tax authorities. NOL and tax credit carryforwards may become subject to an annual limitation in the event of certain cumulative changes in the ownership interest of significant stockholders over a three‑year period in excess of 50%, as defined under Sections 382 and 383 of the Internal Revenue Code of 1986, as amended, as well as similar state tax provisions. This could limit the amount of NOLs that we can utilize annually to offset future taxable income or tax liabilities. The amount of the annual limitation, if any, will be determined based on the value of our company immediately prior to an ownership change. Subsequent ownership changes may further affect the limitation in future years. Additionally, U.S. tax laws limit the time during which these carry forwards may be applied against future taxes, therefore, we may not be able to take full advantage of these carry forwards for federal income tax purposes. The company performed an analysis during the year ended December 31, 2014 to determine an estimate of the Section 382 limitation on the net operating losses and R&D tax credit carryforwards. Accordingly, we recorded a reduction as of December 31, 2014 of approximately $9.2 million to the federal and state NOL carryforwards and approximately $2.1 million to the R&D credit carryforwards available for future utilization.
For all years through December 31, 2014, we generated research credits but have not conducted a study to document the qualified activities. This study may result in an adjustment to our R&D credit carryforwards; however, until a study is completed and any adjustment is known, no amounts are being presented as an uncertain tax position for these years. A full valuation allowance has been provided against our research and development credits and, if an adjustment is required, this adjustment to the deferred tax asset established for the research and development credit carryforwards would be offset by an adjustment to the valuation allowance.
The components of the net deferred tax asset are as follows:
|
|
|
|
|
|
|
|
|
|
|
|
December 31, |
|
||||
|
|
|
2013 |
|
2014 |
|
||
|
Gross deferred tax assets: |
|
|
|
|
|
|
|
|
Net operating loss carryovers |
|
$ |
33,352,581 |
|
$ |
43,399,282 |
|
|
Accrued expenses |
|
|
112,584 |
|
|
— |
|
|
Vacation pay/compensation |
|
|
311,835 |
|
|
550,165 |
|
|
Contributions |
|
|
7,049 |
|
|
9,196 |
|
|
Depreciation |
|
|
62,620 |
|
|
— |
|
|
Stock compensation |
|
|
885,304 |
|
|
2,222,537 |
|
|
Contingent liability |
|
|
— |
|
|
81,187 |
|
|
R&D credit |
|
|
4,482,639 |
|
|
3,598,910 |
|
|
Total gross deferred tax assets |
|
|
39,214,612 |
|
|
49,861,277 |
|
|
Gross deferred tax liabilities: |
|
|
|
|
|
|
|
|
Depreciation |
|
|
— |
|
|
(28,069) |
|
|
Conversion option discount |
|
|
— |
|
|
(6,079,535) |
|
|
Indefinite-lived intangible asset |
|
|
— |
|
|
(16,879,659) |
|
|
Total gross deferred tax liabilities |
|
|
— |
|
|
(22,987,263) |
|
|
Net deferred tax assets |
|
|
39,214,612 |
|
|
26,874,014 |
|
|
Less: valuation allowance |
|
|
(39,214,612) |
|
|
(43,753,673) |
|
|
Net deferred tax liabilities after valuation allowance |
|
$ |
— |
|
$ |
(16,879,659) |
|
We did not have unrecognized tax benefits as of December 31, 2013 and December 31, 2014, respectively, and do not expect this to change significantly over the next twelve months. We recognize interest and penalties, if any,
121
TETRALOGIC PHARMACEUTICALS CORPORATION
NOTES TO FINANCIAL STATEMENTS (Continued)
December 31, 2014
accrued on any unrecognized tax benefits as a component of income tax expense. As of December 31, 2013 and December 31, 2014, we have not accrued interest or penalties related to any uncertain tax positions.
Loss from continuing operations before income taxes of $36.0 million is considered a domestic loss, because the results of foreign operations are included in the company’s domestic tax returns as realized. Income tax expense from continuing operations is zero.
A reconciliation of income tax expense (benefit) at the statutory federal income tax rate and income taxes as reflected in the financial statements is as follows:
|
|
|
|
|
|
|
|
|
|
Year Ended |
|
||
|
|
|
December 31, |
|
||
|
|
|
2013 |
|
2014 |
|
|
Federal income tax expense at statutory rate |
|
34.0 |
% |
34.0 |
% |
|
Permanent items |
|
(3.0) |
|
(1.9) |
|
|
State income tax, net of federal benefit |
|
6.6 |
|
6.4 |
|
|
Adjustment to NOL and credit carryforwards |
|
— |
|
(16.3) |
|
|
Tax credits generated |
|
2.8 |
|
2.0 |
|
|
Change in valuation allowance |
|
(40.4) |
|
(24.2) |
|
|
Effective income tax rate |
|
— |
% |
— |
% |
We file U.S. federal, state and foreign income tax returns, which are generally subject to tax examinations for the tax years ended December 31, 2011 through December 31, 2014. To the extent we utilize our NOL or tax credit attribute carryforwards, the tax years in which the attribute was generated may still be adjusted upon examination by the Internal Revenue Service or state or foreign tax authorities of the tax return in which the attribute was utilized.
12. Related Party Transactions
Consulting Agreements
We had consulting agreements with two founding scientists and stockholders, under which $95,000 and $0 were paid in 2013 and 2014, respectively. The consulting agreement with one of our founding scientists, signed in November 2003, required payment of consulting fees totaling $200,000 over four-years in return for specified services. A second consulting agreement with this founding scientist was signed in March 2011 providing for consulting fees of $30,000 per year. This agreement automatically renews for one-year terms unless terminated by either party. This scientist also received shares of our common stock. Payments to this founding scientist ceased as of January 1, 2013. A four-year consulting agreement with the second founding scientist was signed in 2006, requiring payment of $65,000 per annum for specified services. This agreement automatically renews for one-year terms unless terminated by either party. This agreement with the second founding scientist was terminated in 2014 and payments ceased effective December 31, 2013.
Acquisition of Shape Pharmaceuticals
A member of our Board of Directors also served as Chief Executive Officer and a member of the Board of Directors of Shape Pharmaceuticals. See Note 3.
122
(b) INDEX TO EXHIBITS
|
Exhibit |
Exhibit Description |
|
3.1* |
Sixth Amended and Restated Certificate of Incorporation of TetraLogic Pharmaceuticals Corporation, incorporated by reference to the designated exhibit of the Company’s Current Report on Form 8‑K filed on December 18, 2013. |
|
|
|
|
3.2* |
Amended and Restated Bylaws of TetraLogic Pharmaceuticals Corporation, incorporated by reference to the designated exhibit of the Company’s Amendment No. 4 to Registration Statement on Form S‑1 (File No. 333‑191811) filed on November 6, 2013. |
|
|
|
|
4.1* |
Form of Certificate of Common Stock, incorporated by reference to the designated exhibit of the Company’s Amendment No. 4 to Registration Statement on Form S‑1 (File No. 333‑191811) filed on November 6, 2013. |
|
|
|
|
4.2* |
Warrant to Purchase Common Stock, dated May 2, 2007, by and between TetraLogic Pharmaceuticals Corporation and Silicon Valley Bank, incorporated by reference to the designated exhibit of the Company’s Registration Statement on Form S‑1 (File No. 333‑191811) filed on October 18, 2013. |
|
|
|
|
4.3* |
Form of Warrant to Purchase Convertible Preferred Stock of TetraLogic Pharmaceuticals Corporation, dated March 30, 2006, issued to HealthCare Ventures VII, L.P., Novitas Capital III, L.P. and Kammerer & Associates, L.P., and schedule of omitted material details thereto, incorporated by reference to the designated exhibit of the Company’s Registration Statement on Form S‑1 (File No. 333‑191811) filed on October 18, 2013. |
|
|
|
|
4.4* |
Form of Warrant to Purchase Convertible Preferred Stock of TetraLogic Pharmaceuticals Corporation, dated May 5, 2006, issued to HealthCare Ventures VII, L.P., Novitas Capital III, L.P. and Kammerer & Associates, L.P., and schedule of omitted material details thereto, incorporated by reference to the designated exhibit of the Company’s Registration Statement on Form S‑1 (File No. 333‑191811) filed on October 18, 2013. |
|
|
|
|
4.5* |
Form of Warrant to Purchase Common Stock of TetraLogic Pharmaceuticals Corporation, dated November 25, 2009, issued to HealthCare Ventures VII, L.P., Novitas Capital III, L.P., Latterell Venture Partners III, L.P., LVP III Associates, L.P., LVP III Partners, L.P., Vertical Fund I, L.P., Vertical Fund II, L.P., Quaker BioVentures, L.P., Quaker BioVentures Tobacco Fund, L.P., BioAdvance Ventures, L.P., Amgen Ventures LLC, George McLendon and Pecora & Co., LLC, and schedule of omitted material details thereto, incorporated by reference to the designated exhibit of the Company’s Registration Statement on Form S‑1 (File No. 333‑ 191811) filed on October 18, 2013. |
|
|
|
|
4.6* |
Form of Warrant to Purchase Common Stock of TetraLogic Pharmaceuticals Corporation, dated March 11, 2010, issued to HealthCare Ventures VII, L.P., Novitas Capital III, L.P., Latterell Venture Partners III, L.P., LVP III Associates, L.P., LVP III Partners, L.P., Vertical Fund I, L.P., Vertical Fund II, L.P., Quaker BioVentures, L.P., Quaker BioVentures Tobacco Fund, L.P., BioAdvance Ventures, L.P., Amgen Ventures LLC, George McLendon and Pecora & Co., LLC, and schedule of omitted material details thereto, incorporated by reference to the designated exhibit of the Company’s Registration Statement on Form S‑1 (File No. 333‑ 191811) filed on October 18, 2013. |
|
|
|
|
4.7* |
Indenture, dated June 23, 2014, by and between TetraLogic Pharmaceuticals Corporation and U.S. Bank National Association, incorporated by reference to the designated exhibit of the Company’s Current Report on Form 8-K filed on June 23, 2014. |
|
|
|
123
|
Exhibit |
Exhibit Description |
|
4.8* |
Form of 8% Convertible Senior Notes due 2019, incorporated by reference to the designated exhibit of the Company’s Current Report on Form 8-K filed on June 23, 2014. |
|
|
|
|
10.1* |
Amended and Restated License Agreement, effective as of October 6, 2006, by and between Princeton University and TetraLogic Pharmaceuticals Corporation, incorporated by reference to the designated exhibit of the Company’s Registration Statement on Form S‑1 (File No. 333‑191811) filed on October 18, 2013. |
|
10.2*+ |
TetraLogic Pharmaceuticals Corporation 2004 Equity Incentive Plan, and Amendment 2007‑1 thereto, and forms of agreement thereunder, incorporated by reference to the designated exhibit of the Company’s Registration Statement on Form S‑8 (File No. 333‑192875) filed on December 16, 2013. |
|
|
|
|
10.3*+ |
TetraLogic Pharmaceuticals Corporation Amended and Restated 2013 Equity Incentive Plan, and forms of agreement thereunder, incorporated by reference to the designated exhibit of the Company’s Registration Statement on Form S‑ 8 (File No. 333‑192875) filed on December 16, 2013. |
|
|
|
|
10.4*+ |
TetraLogic Pharmaceuticals Corporation Performance Bonus Plan, incorporated by reference to the designated exhibit of the Company’s Amendment No. 4 to Registration Statement on Form S‑1 (File No. 333‑191811) filed on November 6, 2013. |
|
|
|
|
10.5*+ |
Executive Employment Agreement, dated August 12, 2013, by and between TetraLogic Pharmaceuticals Corporation and J. Kevin Buchi, incorporated by reference to the designated exhibit of the Company’s Registration Statement on Form S‑1 (File No. 333‑191811) filed on October 18, 2013. |
|
|
|
|
10.6*+ |
Executive Employment Agreement, dated August 12, 2013, by and between TetraLogic Pharmaceuticals Corporation and Pete Meyers, incorporated by reference to the designated exhibit of the Company’s Registration Statement on Form S‑1 (File No. 333‑191811) filed on October 18, 2013. |
|
|
|
|
10.7*+ |
Executive Employment Agreement, dated August 12, 2013, by and between TetraLogic Pharmaceuticals Corporation and Lesley Russell, M.B.Ch.B., M.R.C.P., incorporated by reference to the designated exhibit of the Company’s Registration Statement on Form S‑1 (File No. 333‑191811) filed on October 18, 2013. |
|
|
|
|
10.8*+ |
Executive Employment Agreement, dated August 14, 2012, by and between C. Glenn Begley, M.B.B.S., Ph.D., F.R.A.C.P., F.R.C.P.A., and TetraLogic Pharmaceuticals Corporation, incorporated by reference to the designated exhibit of the Company’s Registration Statement on Form S‑1 (File No. 333‑191811) filed on October 18, 2013. |
|
|
|
|
10.9*+ |
Executive Employment Agreement, dated April 22, 2014, by and between Richard Sherman and TetraLogic Pharmaceuticals Corporation., incorporated by reference to the designated exhibit of the Company’s Current Report on Form 8-K filed on June 5, 2014. |
|
|
|
|
10.10*+ |
Advisory Services Agreement, dated March 8, 2013, by and between Andrew Pecora, M.D. and TetraLogic Pharmaceuticals Corporation, incorporated by reference to the designated exhibit of the Company’s Registration Statement on Form S‑1 (File No. 333‑191811) filed on October 18, 2013. |
|
|
|
|
10.11* |
Office/Laboratory Lease Agreement, dated April 30, 2004, by and between APOP Corporation and 335‑95 Phoenixville Pike Associates, as amended, incorporated by reference to the designated exhibit of the Company’s Registration Statement on Form S‑1 (File No. 333‑191811) filed on October 18, 2013. |
|
|
|
|
10.12* |
Third Amended and Restated Investor Rights Agreement, dated November 18, 2013, by and among TetraLogic Pharmaceuticals Corporation and certain stockholders named therein, incorporated by reference to the designated exhibit of the Company’s Amendment No. 5 to Registration Statement on Form S‑1 (File No. 333‑191811) filed on November 18, 2013. |
124
|
Exhibit |
Exhibit Description |
|
|
|
|
10.13* |
Merger Agreement, dated April 7, 2014, by and among Shape Pharmaceuticals, Inc., TetraLogic Pharmaceuticals Corporation, TLOG Acquisition Sub, Inc. and Augustine Lawlor as Holder Representative, incorporated by reference to the designated exhibit of the Company’s Quarterly Report on Form 10-Q filed on August 5, 2014. |
|
|
|
|
10.14* |
License Agreement, dated October 7, 2008, by and among Shape Pharmaceuticals, Inc., President and Fellows of Harvard College and Dana-Farber Cancer Institute, Inc., incorporated by reference to the designated exhibit of the Company’s Quarterly Report on Form 10-Q filed on August 5, 2014. |
|
|
|
|
10.15* |
Licence Agreement, undated, by and between TetraLogic Pharmaceuticals Corporation and The Walter and Eliza Hall Institute of Medical Research., incorporated by reference to the designated exhibit of the Company’s Quarterly Report on Form 10-Q filed on August 5, 2014. |
|
|
|
|
10.16* |
Form of Indemnification Agreement (for directors and officers), and a schedule of parties thereto, incorporated by reference to the designated exhibit of the Company’s Current Report on Form 8-K filed on November 10, 2014. |
|
|
|
| 21.1 |
List of Subsidiaries |
|
|
|
| 23.1 |
Consent of Ernst & Young LLP. |
|
|
|
| 31.1 |
Certifying Statement of the Chief Executive Officer pursuant to Section 302 of the Sarbanes‑Oxley Act of 2002. |
|
|
|
| 31.2 |
Certifying Statement of the Chief Financial Officer pursuant to Section 302 of the Sarbanes‑Oxley Act of 2002. |
|
|
|
| 32.1 |
Certifying Statement of the Chief Executive Officer pursuant to Section 1350 of Title 18 of the United States Code. |
|
|
|
| 32.2 |
Certifying Statement of the Chief Financial Officer pursuant to Section 1350 of Title 18 of the United States Code. |
|
|
|
| 101 |
The following financial information from this Annual Report on Form 10-K for the year ended December 31, 2014, formatted in XBRL: (i) Consolidated Balance Sheets as of December 31, 2013 and 2014, (ii) Consolidated Statements of Operations and Comprehensive Loss for the years ended December 31, 2013 and 2014, (iii) Consolidated Statements of Cash Flows for the years ended December 31, 2013 and 2014, and (iv) Notes to Consolidated Financial Statements, tagged as blocks of text. |
+Indicates management contract or compensatory plan.
*Previously filed.
125
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, as amended, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
Date: February 26, 2015
|
|
TETRALOGIC PHARMACEUTICALS CORPORATION |
|
|
|
|
|
|
|
By: |
/s/ J. Kevin Buchi
J. Kevin Buchi |
Pursuant to the requirements of the Securities Exchange Act of 1934, as amended, this report has been signed by the following persons in the capacities and on the dates indicated.
|
Signature |
|
Title |
|
Date |
|
|
|
|
|
|
|
/s/ J. Kevin Buchi |
President and Chief Executive Officer (Principal Executive Officer) and Director |
February 26, 2015 |
||
|
J. Kevin Buchi |
||||
|
|
|
|
|
|
|
/s/ Pete A. Meyers |
Chief Financial Officer and Treasurer (Principal Financial and Accounting Officer) |
February 26, 2015 |
||
|
Pete A. Meyers |
||||
|
|
|
|
|
|
|
/s/ Andrew Pecora |
Chairman, Board of Directors |
February 26, 2015 |
||
|
Andrew Pecora, M.D. |
||||
|
|
|
|
|
|
|
/s/ Mary Ann Gray |
Director |
February 26, 2015 |
||
|
Mary Ann Gray |
||||
|
|
|
|
|
|
|
/s/ Michael D. Kishbauch |
Director |
February 26, 2015 |
||
|
Michael D. Kishbauch |
||||
|
|
|
|
|
|
|
/s/ Douglas E. Onsi |
Director |
February 26, 2015 |
||
|
Douglas E. Onsi |
||||
|
|
|
|
|
|
|
/s/ Douglas Reed |
Director |
February 26, 2015 |
||
|
Douglas Reed, M.D. |
||||
|
|
|
|
|
|
|
/s/ Paul J. Schmitt |
Director |
February 26, 2015 |
||
|
Paul J. Schmitt |
||||
|
|
|
|
|
|
|
/s/ Michael Steinmetz |
Director |
February 26, 2015 |
||
|
Michael Steinmetz, Ph.D. |
||||
|
|
|
|
|
|
|
/s/ James N. Woody |
Director |
February 26, 2015 |
||
|
James N. Woody, M.D., Ph.D. |
126
