Attached files
| file | filename |
|---|---|
| 8-K - 8-K - ATOSSA THERAPEUTICS, INC. | v398781_8k.htm |

1 The Breast Care Company™ NASDAQ: ATOS WWW . ATOSSAGENETICS . COM J ANUARY 13, 2015 ©2015, A TOSSA G ENETICS , I NC . A LL R IGHTS R ESERVED .

2 Some of the information presented herein may contain projections or other forward - looking statements regarding future events or the future financial performance of the Company . For example, our revenue forecast is a forward - looking statement . These statements are based on management’s current expectations and are subject to risks and uncertainties that may cause actual results to differ materially from the anticipated or estimated future results, including the risks and uncertainties associated with planned and ongoing product launches, expected levels of future revenues, expenditures, actions by the FDA, regulatory clearances, responses to regulatory matters, A tossa's ability to continue to manufacture and sell its products, the efficacy of A tossa's products and services, the market demand for and acceptance of A tossa's products and services, performance of distributors and other risks detailed from time to time in A tossa's filings with the S ecurities and Exchange C ommission, including without limitation its most recent annual report on form 10 - K and subsequent quarterly reports on form 10 - Q, each as amended and supplemented from time to time . Forward - Looking Statements
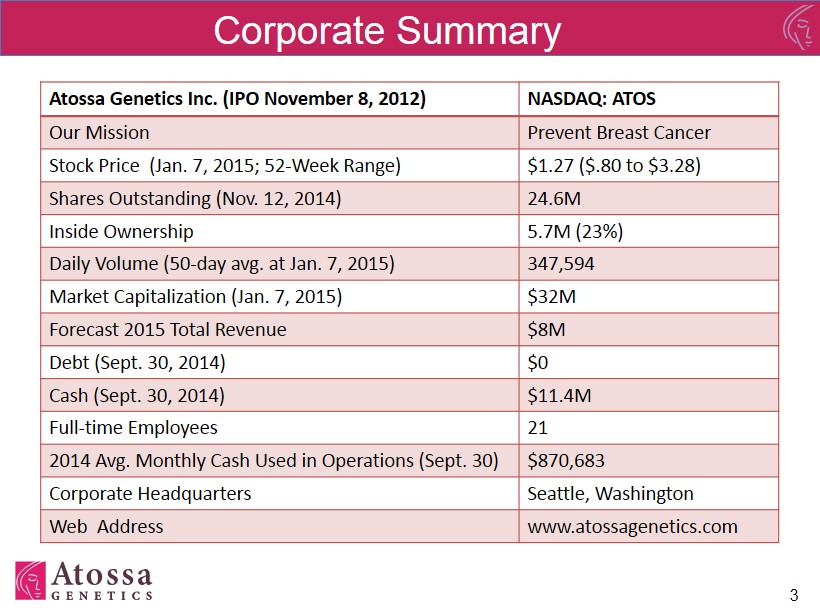
Atossa Genetics Inc. (IPO November 8, 2012) NASDAQ: ATOS Our Mission Prevent Breast Cancer Stock Price (Jan. 7 , 2015; 52 - Week Range) $1.27 ($.80 to $3.28) Shares Outstanding (Nov. 12, 2014) 24.6M Inside Ownership 5.7M (23%) Daily Volume (50 - day avg. at Jan. 7, 2015) 347,594 Market Capitalization (Jan. 7, 2015 ) $32M Forecast 2015 Total Revenue $8M Debt (Sept. 30 , 2014 ) $0 Cash (Sept. 30 , 2014) $11.4M Full - time Employees 21 2014 Avg. Monthly Cash Used in Operations (Sept. 30) $870,683 Corporate Headquarters Seattle, Washington Web Address www.atossagenetics.com Corporate Summary 3
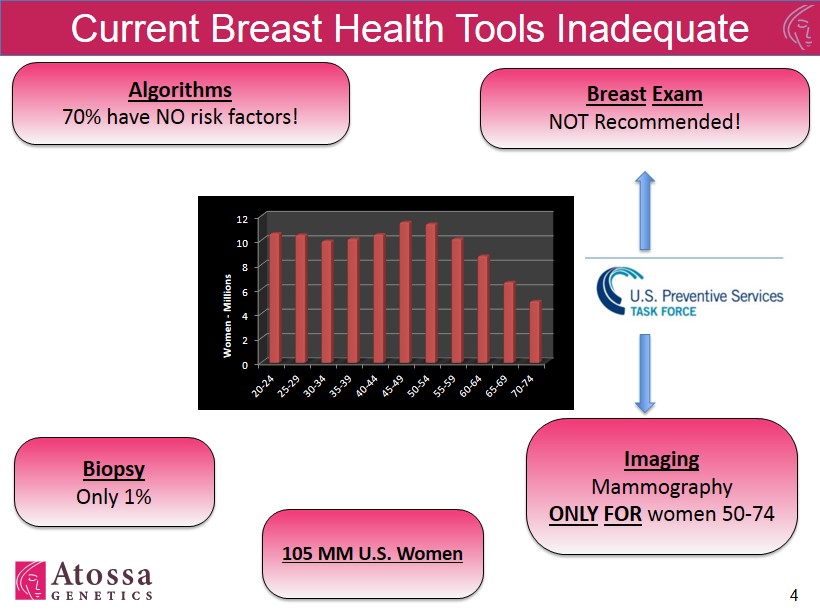
Current Breast Health Tools Inadequate 4 Breast Exam NOT Recommended! Imaging Mammography ONLY FOR women 50 - 74 Biopsy Only 1% Algorithms 70% have NO risk factors! 0 2 4 6 8 10 12 Women - Millions 105 MM U.S. Women
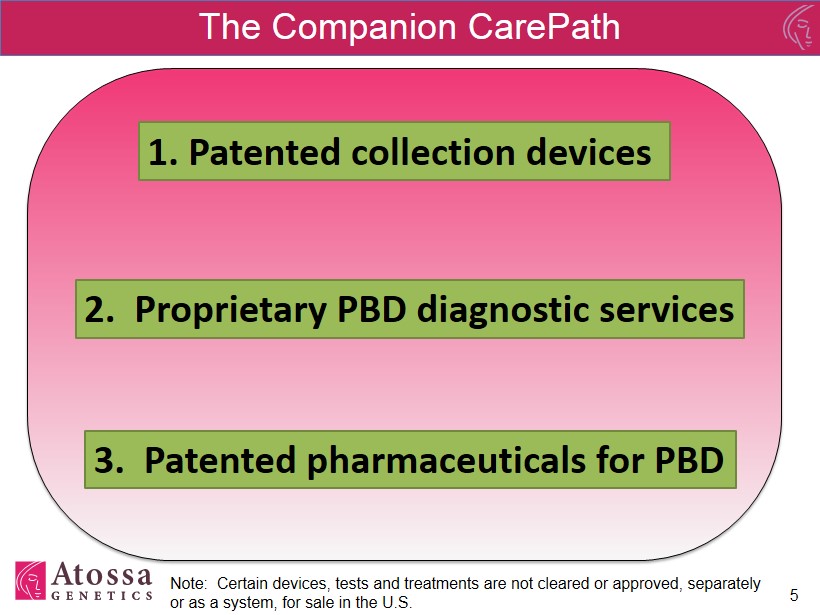
The Companion CarePath 5 3. Patented p harmaceuticals for PBD 1. Patented collection devices 2. Proprietary PBD diagnostic services Note: Certain devices, tests and treatments are not cleared or approved, separately or as a system, for sale in the U.S.

6 Patented Collection Devices Non U.S. Market – ForeCYTE* Intended for the collection of NAF for cytological testing . Reusable, hand - held pump. CE Marked for Q1 European launch U.S. Market – FullCYTE Intended for the collection of ductal fluid for cytological testing. Single use only. Third party literature Q1 US launch *Not cleared or approved for sale in the U.S.

7 Additional Patented Devices FullCYTE Microcatheters* ▪ Intended for ductal l avage – collects NAF from each duct. ▪ Also intended for targeted drug delivery. ▪ 2015 launch expected. *Not cleared or approved for sale in the U.S.
Laboratory Services Breast specimen cytology Pharmacogenomics 8
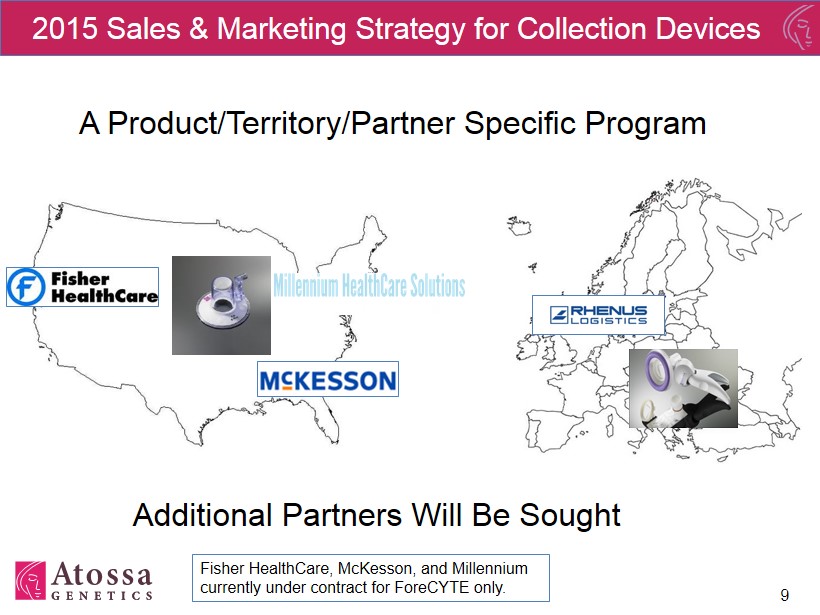
9 2015 Sales & Marketing Strategy for Collection Devices A Product/Territory/Partner Specific Program Additional Partners Will Be Sought Fisher HealthCare, McKesson, and Millennium currently under contract for ForeCYTE only.
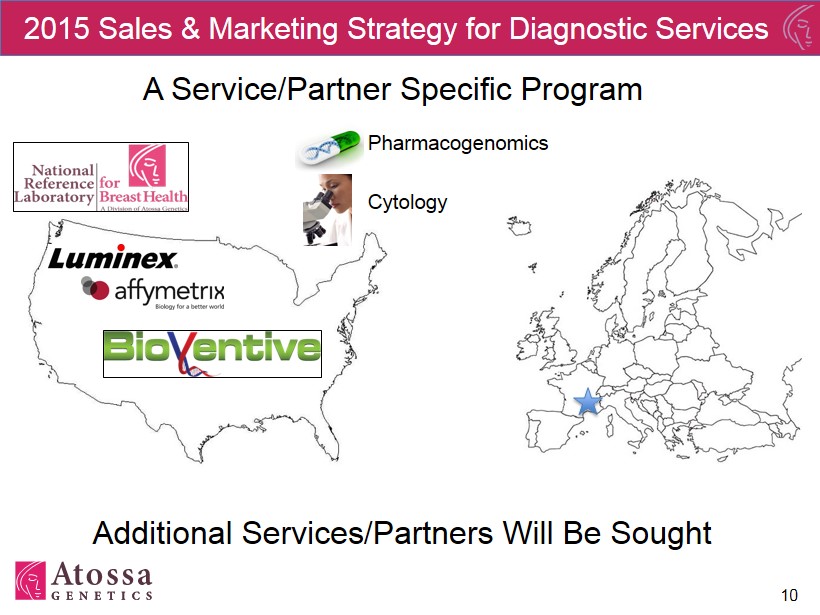
10 2015 Sales & Marketing Strategy for Diagnostic Services A Service/Partner Specific Program Additional Services/Partners Will Be Sought Pharmacogenomics Cytology
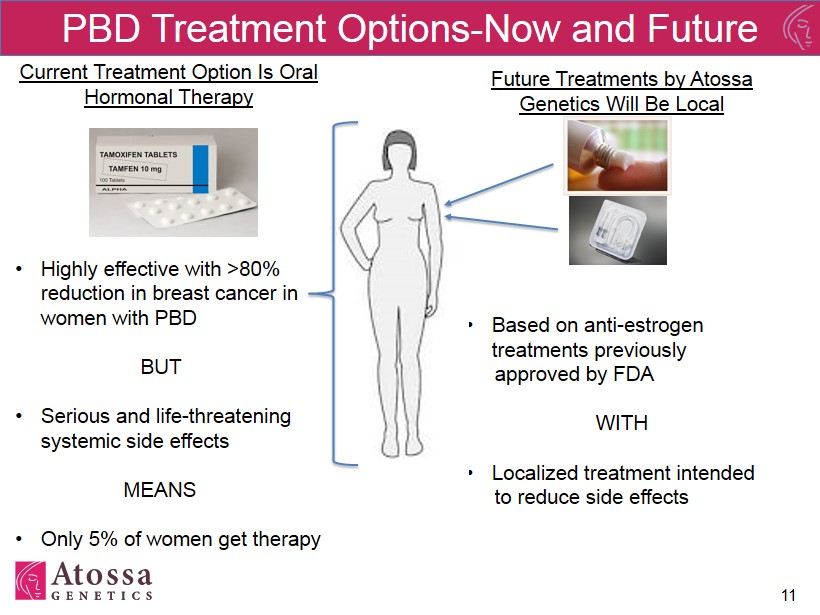
11 Future Treatments by Atossa Genetics Will Be Local • Based on anti - estrogen treatments previously approved by FDA WITH • Localized treatment intended to reduce side effects PBD Treatment Options - Now and Future Current Treatment Option Is Oral Hormonal Therapy • Highly effective with >80% reduction in breast cancer in women with PBD BUT • Serious and life - threatening systemic side effects MEANS • Only 5% of women get therapy
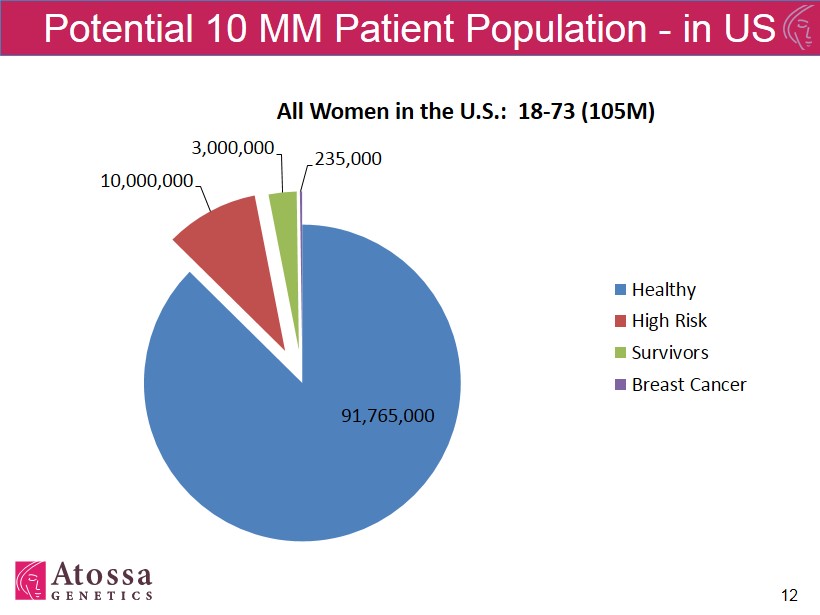
105M Women (Stratify) 12 Potential 10 MM Patient Population - in US 91,765,000 10,000,000 3,000,000 235,000 All Women in the U.S.: 18 - 73 (105M) Healthy High Risk Survivors Breast Cancer
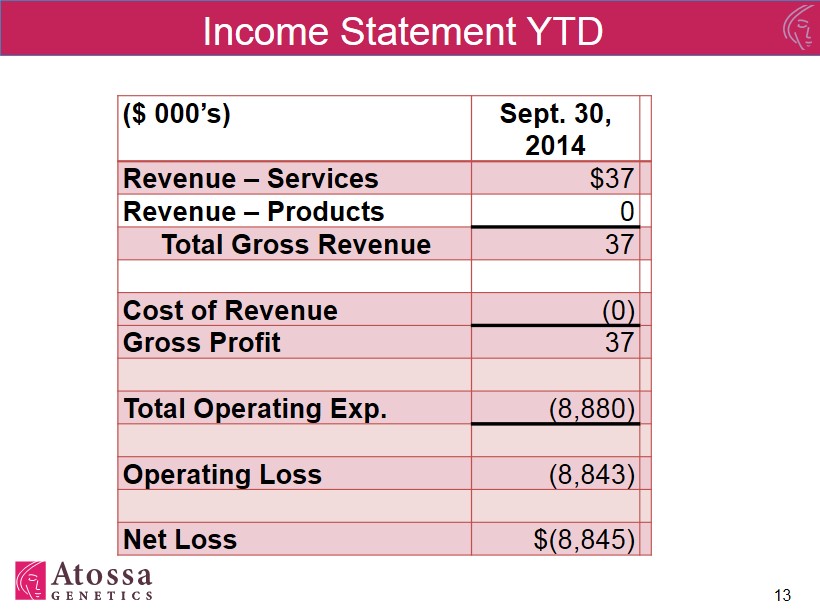
Income Statement YTD ($ 000’s) Sept. 30, 2014 Revenue – Services $37 Revenue – Products 0 Total Gross Revenue 37 Cost of Revenue (0) Gross Profit 37 Total Operating Exp. (8,880) Operating Loss (8,843) Net Loss $(8,845) 13
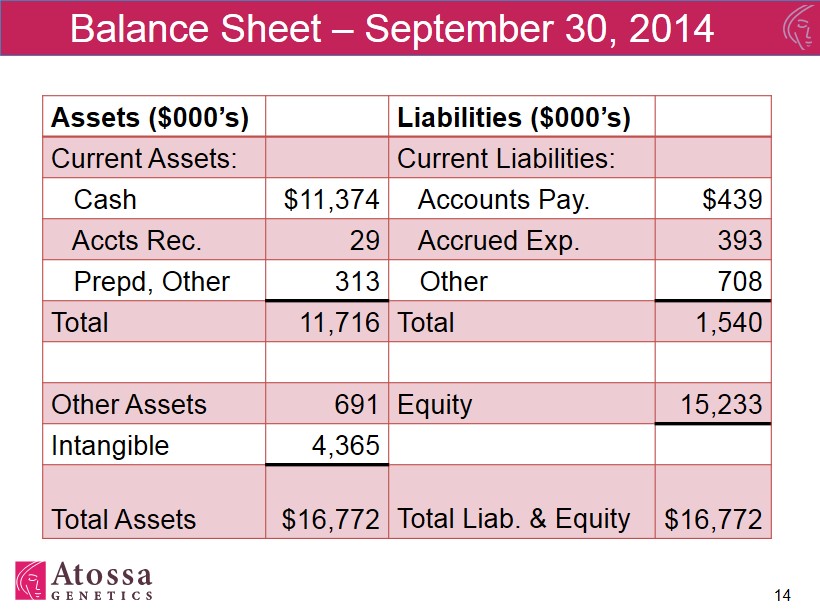
Balance Sheet – September 30, 2014 Assets ($000’s) Liabilities ($000’s) Current Assets: Current Liabilities: Cash $11,374 Accounts Pay. $439 Accts Rec. 29 Accrued Exp. 393 Prepd, Other 313 Other 708 Total 11,716 Total 1,540 Other Assets 691 Equity 15,233 Intangible 4,365 Total Assets $16,772 Total Liab. & Equity $16,772 14
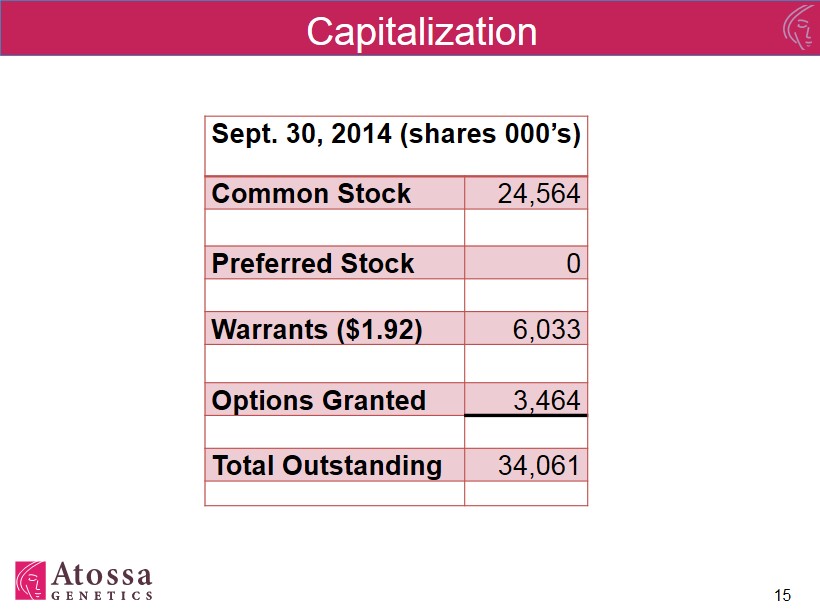
Capitalization Sept. 30 , 2014 (shares 000’s ) Common Stock 24,564 Preferred Stock 0 Warrants ($1.92) 6,033 Options Granted 3,464 Total Outstanding 34,061 15
16 x Forecasted 2015 Total Revenue of $8.0 M x 34.1 M shares outstanding provides leverage x No debt, no p referred s tock x Scalable contract m anufacturing and low - capital laboratory services Financial Highlights

17 » Steven Quay, M.D., Ph.D., Chairman and Chief Executive Officer » Kyle Guse, CPA, Esq., MBA, CFO and General Counsel » Jelle Kylstra, M.D., MBA, VP Clinical R&D » Christopher Destro, Sr. VP Sales and Marketing » Scott Youmans, Sr. VP Operations » John Sawyer, Sr. VP Global Regulatory and QA » Pieter van der Poel, VP European Commercial Operations Management – Comprehensive and Experienced

x 2015 Forecasted Total Revenue of $8.0 M from Two - Pronged Product - Service Financial Model x 2015 Pharmaceutical Clinical T rials D rive A dditional V alue x Multi - Billion Dollar Diagnostic and Therapeutic Markets - Women’s Health Driven x 148 Issued Patents Secure Shareholder Value x Experienced Management Team Supports Execution 18 Investment Highlights

19 The Breast Care Company™ NASDAQ: ATOS WWW . ATOSSAGENETICS . COM ©2015, A TOSSA G ENETICS , I NC . A LL R IGHTS R ESERVED .
