Attached files
| file | filename |
|---|---|
| 8-K - CURRENT REPORT - ROCK CREEK PHARMACEUTICALS, INC. | v398545_8k.htm |
EXHIBIT 99.1

January 12, 2015 Biotech Showcase 2015

Forward Looking Statements Certain statements that will be made during this presentation constitute forward - looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. Such statements include, but are not limited to statements identified by words such as "believes," "expects," "anticipates," "estimates," "intends," "plans," "targets," "projects" and similar expressions. These statements are based upon the current beliefs and expectations of our company's management and are subject to significant risks and uncertainties. Actual results may differ from those set forth in the forward - looking statements. Numerous factors could cause or contribute to such differences, including, but not limited to, results of clinical trials and/or other studies, the challenges inherent in new product development initiatives, including the continued development, market and regulatory acceptance of our dietary supplement products, the effect of any competitive products, our ability to license and protect our intellectual property, our ability to raise additional capital in the future that is necessary to maintain our business, changes in government policy and/or regulation, potential litigation by or against us, any governmental review of our products or practices and the outcome of the ongoing investigations as well as other risks discussed from time to time in our filings with the Securities and Exchange Commission, including, without limitation, our annual report on Form 10 - K for the fiscal year ended December 31, 2013 filed on March 17, 2014., and our latest 10 - Q Report filed on November 10, 2014. We undertake no duty to update any forward - looking statement or any information contained in this presentation or in other public disclosures at any time. 2

Corporate Overview • Drug development company focused on the discovery, development and commercialization of new drugs to provide therapies for chronic inflammatory diseases, neurological disorders and behavioral health • Advancing into human clinical development in the UK and US • Novel lead compound Anatabine Citrate backed by strong IP and peer reviewed publications • Large human exposure database through pre - clinical and clinical studies as a dietary supplement • Recent corporate reorganization (Strategy, Management, & Board) • Publicly traded (NASDAQ:RCPI) 3

Anatabine: Key Attributes • Small molecule • Exhibits anti - inflammatory pharmacological characteristics via Cholinergic Receptor Binding • Crosses blood brain barrier • Excellent bioavailability • Well tolerated as dietary supplement by several hundred thousand users 4
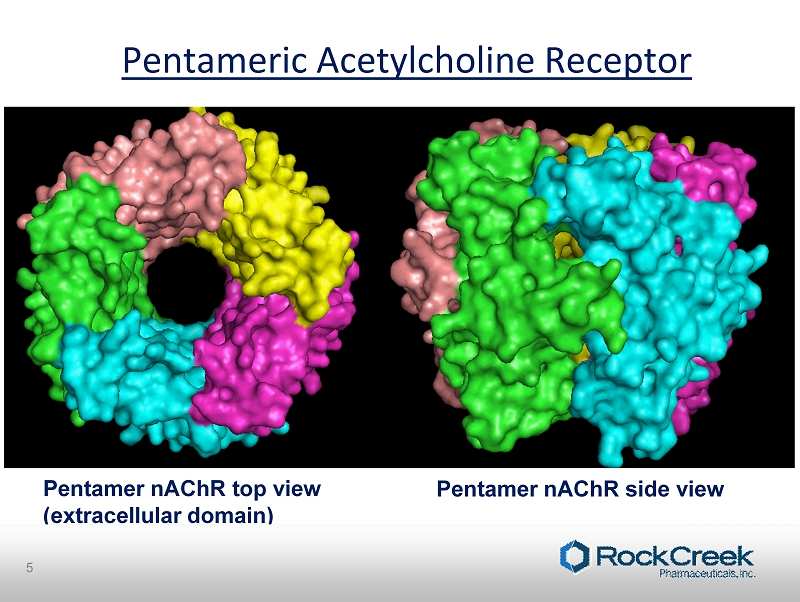
Pentameric Acetylcholine Receptor Pentamer nAChR top view (extracellular domain) Pentamer nAChR side view 5
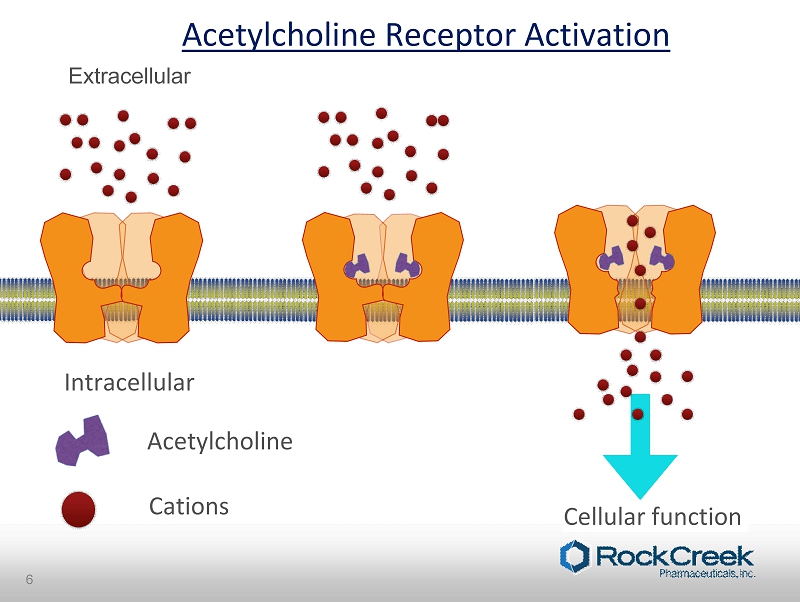
Extracellular Intracellular Cations Acetylcholine Cellular function Acetylcholine Receptor Activation 6

Acetylcholine Binding at the interface of α7 - nAChR Anatabine Binding at the interface of α7 - nAChR ElectroStatic Ribbon Model 7
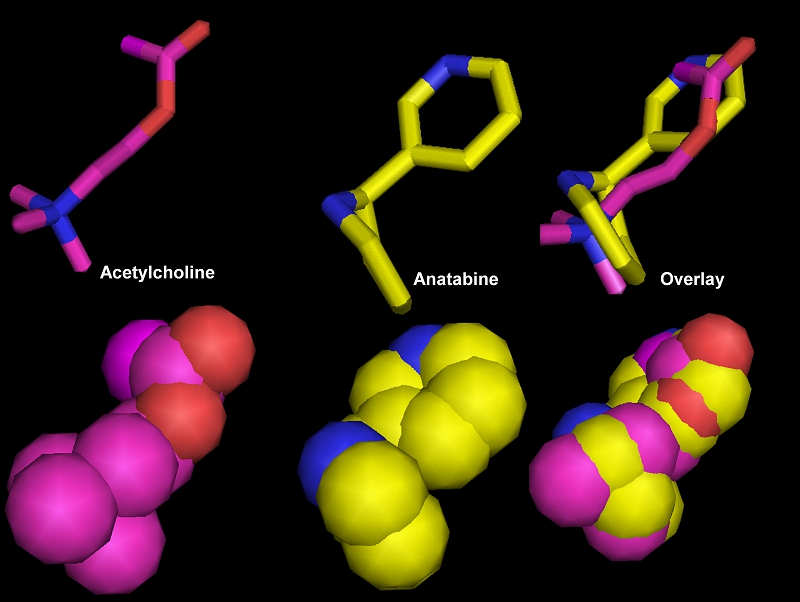
Acetylcholine Anatabine Overlay

Nuclear Factor Kappa B (NF - ƙB ) • NF - κB is a protein complex that controls the transcription of DNA. • Found in almost all animal cell types and is involved in cellular responses to external stimuli. • NF - κB plays a key role in regulating cellular response to inflammation. • Specifically, NF - κB controls inflammatory molecule production. • Chronic NF - κB activation is associated with chronic inflammatory conditions, therefore reducing NF - kB activity is a therapeutic approach in these conditions. 9
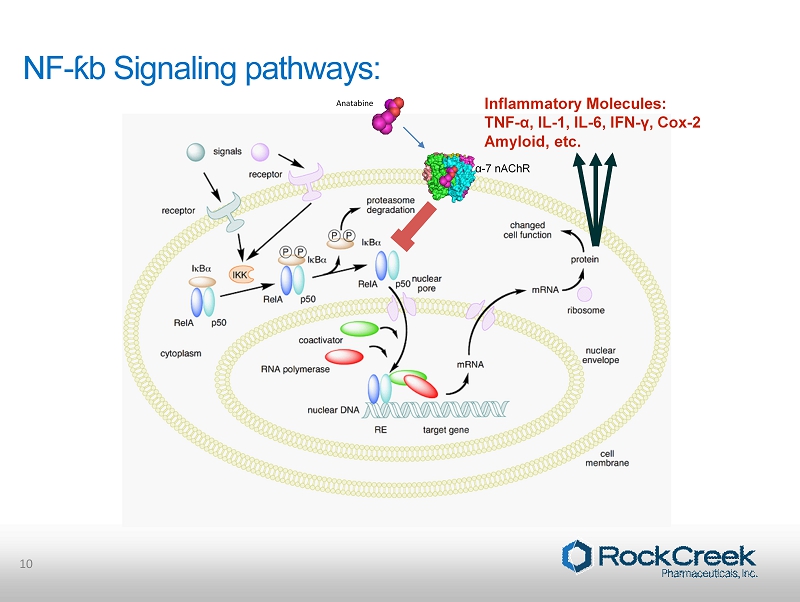
NF - ƙb Signaling pathways: 10 Anatabine Inflammatory Molecules: TNF - α, IL - 1, IL - 6, IFN - γ, Cox - 2 Amyloid, etc. α - 7 nAChR

Proposed Common Mechanism(s) of Action of Anatabine across Different CNS (and non - CNS) Conditions Suppression of inflammatory activity (via ህ NF - kB) Activation of alpha 7 nAchR on microglia (macrophage, monocytes, B - cells, T - cells) Reduced inflammatory cytokine release Reduced tissue damage Reduced organ dysfunction 11

Anatabine Lowers Alzheimer’s Aβ Production in vitro and in vivo Publication: European Journal of Pharmacology, Sept 2011 Anti - inflammatory Activity of Anatabine via Inhibition of STAT 3 Phosphorylation Publication: European Journal of Pharmacology , Nov 2012 Anatabine Ameliorates Experimental Autoimmune Thyroiditis Publication: Endocrinology, July 2012 Amelioration of Experimental Autoimmune Encephalomyelitis by Anatabine Publication: PLOS ONE , Jan 2013 Anatabine Supplementation Decreases Thyroglobulin Antibodies in Patients With Chronic Lymphocytic Autoimmune (Hashimoto’s) Thyroiditis: A Randomized Controlled Clinical Trial Publication: Journal Clinical Endocrinology Metabolism, January 2014 Anatabine Attenuates Tau Phosphorylation and Oligomerization in P 301 S Tau Transgenic Mice Publication: Brain Disorders & Therapy, May 2014 Peer Reviewed Published Studies 12

• Mice injected at 0 hr and 48 hrs with 300µg of Pertussis toxin • Day 1, 10 Week - old C57Bl6/J Female mice immunized with 300 µg of murine MOG35 - 55 (myelin oligodendrocyte glycoprotein) • Mice randomized in placebo group (n=15) receiving regular drinking water and Anatabine group (n=15) receiving 20 mg/Kg of anatabine diluted in drinking water • Mice evaluated for clinical signs daily (0= no clinical sign; 1= tail paresis; 2= paresis of one hind limb; 3= paresis of two hind limbs; 4= moribund; 5= death) * Paris D, Beaulieu - Abdelahad D, Mullan M, Ait - Ghezala G, Mathura V, et al. (2013) Amelioration of Experimental Autoimmune Encephalomyelitis by Anatabine. PLoS ONE 8(1): e55392. doi:10.1371/journal.pone.0055392 13 Pre - Clinical Analysis: Experimental Autoimmune Encephalomyelitis (EAE)
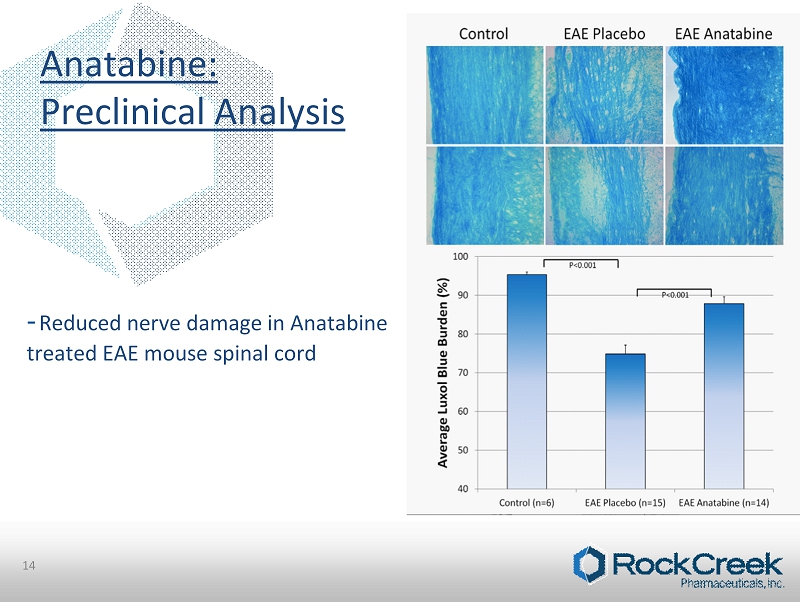
- Reduced nerve damage in Anatabine treated EAE mouse spinal cord Anatabine: Preclinical Analysis 14
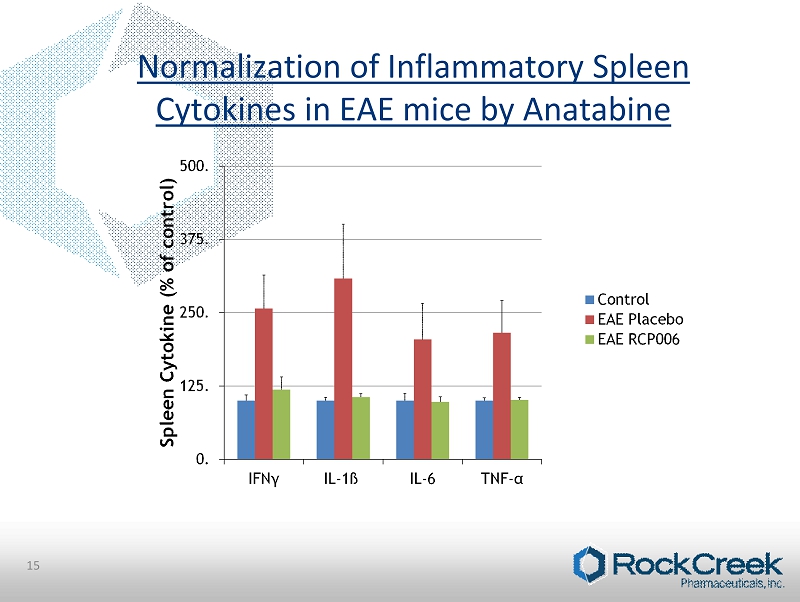
15 Normalization of Inflammatory Spleen Cytokines in EAE mice by Anatabine

Hashimoto's thyroiditis cytology showing lymphocytes and Hurthle cell metaplasia 17 Lowell R. Schmeltz et al, (2014) Anatabine Supplementation Decreases Thyroglobulin Antibodies in Patients With Chronic Lympho cyt ic Autoimmune (Hashimoto’s) Thyroiditis: A Randomized Controlled Clinical Trial. J Clin Endocrinol Metab, January 2014, 99(1):E137 – E142 Auto - Immune Thyroiditis: Human Trial 16 • Evaluating Anatabine in Human Thyroid Health • Primary Outcome: Safety and Tolerability • Secondary Outcome: Anti - thyroid auto - antibodies, thyroid structure, and thyroid function • Enrollment 165 people: 5 - visits, 12 - week, double - blind, randomized, placebo - controlled, parallel - group study

Reduced Anti - thyroglobulin antibodies in Anatabine treated Thyroiditis patients Lowell R. Schmeltz et al, (2014) Anatabine Supplementation Decreases Thyroglobulin Antibodies in Patients With Chronic Lympho cyt ic Autoimmune (Hashimoto’s) Thyroiditis: A Randomized Controlled Clinical Trial. J Clin Endocrinol Metab, January 2014, 99(1):E137 – E142 17
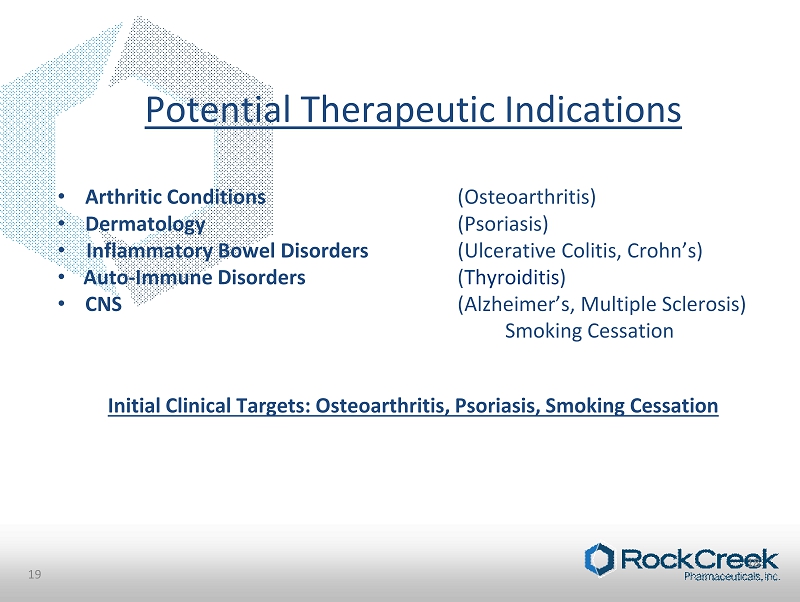
Potential Therapeutic Indications • Arthritic Conditions (Osteoarthritis) • Dermatology (Psoriasis) • Inflammatory Bowel Disorders (Ulcerative Colitis, Crohn’s) • Auto - Immune Disorders ( Thyroiditis ) • CNS (Alzheimer’s, Multiple Sclerosis) Smoking Cessation Initial Clinical Targets: Osteoarthritis, Psoriasis, Smoking Cessation 19 18

Intellectual Property 03/01/14 • Six Issued US/Foreign Anatabine related patents: 1. Anatabine Citrate - Novel Compound - US Patent 8,577,999 2. Method of Anatabine synthesis • Six (+) US and foreign patents pending for use of Anatabine, its isomers and derivatives for inflammatory conditions/smoking cessation 19 20

Anatabine: UK and US Regulatory Strategy • Clinical Trial Application (CTA) filed with the Medicines and Healthcare Products Regulatory Agency (MHRA) in the UK – CTA Response Received, responding to minor comments – Expect CTA approval Q1 – Engaged Quotient Clinical as CRO for Phase I: Q1 start – Formulation development (controlled release formulations) – Phase I complete: Q2 2015 – Phase IIa commences: Q3 2015 • Investigational New Drug Application (IND) filed with US Food and Drug Administration (FDA) – On clinical hold – Responding to FDA’s comments 20
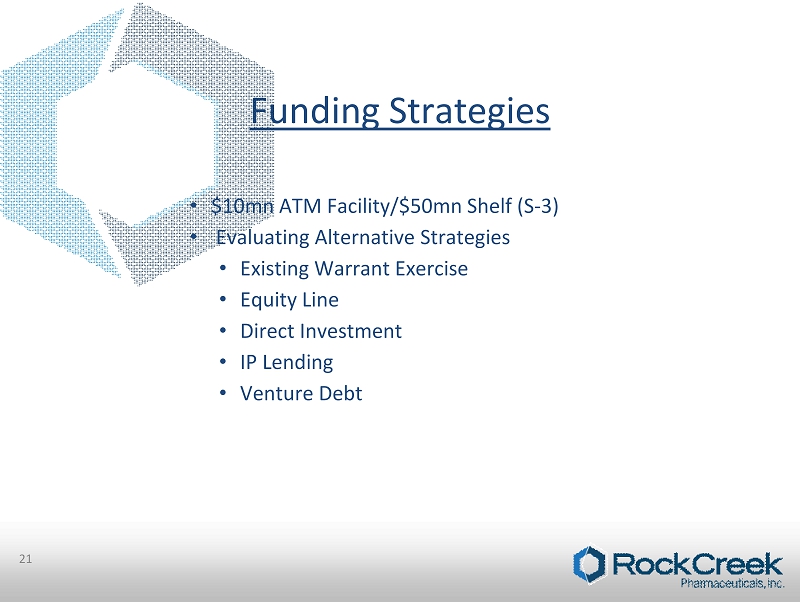
Funding Strategies 21 • $10mn ATM Facility/$50mn Shelf (S - 3) • Evaluating Alternative Strategies • Existing Warrant Exercise • Equity Line • Direct Investment • IP Lending • Venture Debt

Dr. Michael Mullan, MD, PhD., Chairman and Chief Executive Officer Recognized authority on Alzheimer's disease and related disorders. Trained as a physician. Medical degree and PhD in molecular genetics, from London University. Inventor on the patents covering the first genetic mutations linked to familial Alzheimer's disease. Authored and co - authored over 200 papers on Alzheimer's disease and related disorders. Former CEO of The Roskamp Institute, which is a non - profit research organization, funded by multiple agencies, including the NIH and the Department of Defense. "Top 100 Cited Alzheimer Investigators" Dr. Christopher C. Chapman, MD. President Chairman, Chapman Pharmaceutical Consulting, Inc. Senior Director of Medical Affairs with Quintiles/BRI, 1995 - 2000. Oversight responsibility for the support of new drug applications (NDA), clinical studies and device submissions to the FDA for approval. From 1992 - 1994, Medical Director at Regeneron Pharmaceuticals. Dr. Chapman is a graduate of the Georgetown University School of Medicine in Washington, DC. Park A. Dodd, MBA, CPA ; Chief Financial Officer/Treasurer Senior Manager and Director of Financial Planning and Analysis of Philip Morris (20 Year Finance Career). Director in Accounting and Reporting of Capital One Financial Corporation. Senior Accounting Advisor to the United States Olympic Committee. Theodore Jenkins; VP, Corporate Strategy, Development, Investor Relations 18 Years Wall Street, Institutional Capital Markets Professional; Alex Brown & Sons, Salomon Brothers, Credit Suisse, Oppenheimer. 9 Years, US Naval Officer (Lieutenant Commander). MS Business Administration, Carey School, Johns Hopkins University. Management 22

Benjamin M. Dent : CEO of the Dent Consulting Group LLC and an adjunct professor with the St. Louis University graduate program. Mr. Dent previously has served in senior financial management positions with several public and private companies, and also has served as a management consultant to Lehman Brothers Investment Management Portfolio Company; Scott P. Sensenbrenner : President and CEO of Enzymedica, Inc. Mr. Sensenbrenner has executive experience in marketing, supply chain, operations and financial management in the natural products industry. Overseen sales and marketing programs in public and private companies, including in his role as Group Director, Nutrition Division with Perrigo, Inc. (NYSE: PRGO); Lee Musgrove Canaan: Currently the portfolio manager of a private money management firm she founded in 2003. Ms.Canaan brings 25 years of financial and public capital markets experience and 35 years of scientific and technical experti se to our board. Prior to that, she was a fixed income analyst for the high yield debt team at a global mutual fund company aft er making the transition from corporate financial professional in the treasury operations of a Fortune 100 energy company. Ed McDonnell, MPH: A long, distinguished career in both the private, public and government sector.President at Compliance Management Consulting , providing expert advice, regularity assessments and compliance management to FDA regulated industries since 2000. Mr. McDonnell ’ s government experience includes a twenty eight year career with the Federal Drug Administration (FDA). Mr. McDonnell has numerous awards, honors and professional affiliations, Dr. Christopher C. Chapman, MD: President Dr. Michael Mullan, MBBS, PhD: Chairman and Chief Executive Officer Board of Directors 23

FDA/EMA regulatory timeline progression change Clinical studies do not reach desired endpoints. Ongoing operating losses, access to cash, sustainable funding and growth. Inability to attract joint venture/licensing partners Risk Factors 24

Upcoming Milestones 15 Q115 CTA Approval Phase I under CTA Safety, PK, PD data Drug Formulation development Phase IIA POC studies Q 215 Q315 Q415 2016 Phase II studies CTA Phase I Complete 25 US IND Protocol Development

Summary • Drug development company focused on the discovery, development and commercialization of new drugs to provide therapies for chronic inflammatory disease, neurological disorders and behavioral health • Advancing into human clinical development in the UK and US • Novel lead compound Anatabine Citrate backed by strong IP and peer reviewed publications • Large human exposure database through pre - clinical and clinical studies as a dietary supplement • Recent corporate reorganization (Strategy, Management, & Board) • Publicly traded (NASDAQ:RCPI) 26

January 12, 2015 Biotech Showcase 2015
