Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Auspex Pharmaceuticals, Inc. | d838939d8k.htm |
| Exhibit 99.1
|
Treating Movement Disorders
Huntington’s Tardive Tourette Disease Dyskinesia Syndrome
DECEMBER 16, 2014
1
|
|
Forward Looking Statements
Statements made in this presentation regarding matters that are not historical facts are “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995. Because such statements are subject to risks and uncertainties, actual results may differ materially from those expressed or implied by such forward-looking statements. Such statements include, but are not limited to, statements regarding Auspex’s ability to successfully complete its ongoing clinical trials and development programs, Auspex’s ability to obtain regulatory approval for its product candidates, and market penetration and acceptance of its product candidates. Risks that contribute to the uncertain nature of the forward-looking statements include: Auspex’s future preclinical studies and clinical trials may not be successful; changes in regulatory requirements in the United States and foreign countries may prevent or significantly delay regulatory approval of Auspex’s product candidates; Auspex may change its plans to develop and commercialize its product candidates; the U.S. Food and Drug Administration (FDA) may not agree with Auspex’s interpretation of the data from clinical trials of its product candidates; Auspex may decide, or the FDA may require Auspex, to conduct additional clinical trials or to modify Auspex’s ongoing clinical trials; Auspex may experience delays in the commencement, enrollment, completion or analysis of clinical testing for its product candidates, or significant issues regarding the adequacy of its clinical trial designs or the execution of its clinical trials, which could result in increased costs and delays, or limit Auspex’s ability to obtain regulatory approval; the third parties with whom Auspex has partnered with for the development of its product candidates and upon whom Auspex relies to conduct its clinical trials and manufacture its product candidates may not perform as expected; Auspex’s product candidates may not receive regulatory approval or be successfully commercialized; unexpected adverse side effects or inadequate therapeutic efficacy of Auspex’s product candidates could delay or prevent regulatory approval or commercialization; Auspex may be unable to obtain and maintain intellectual property protection for its product candidates; the loss of key scientific or management personnel; and Auspex’s ability to obtain additional financing.
This presentation also contains estimates, projections and other information concerning our industry, our business, and the markets for our drug candidates, as well as data regarding market research, estimates and forecasts prepared by our management. Information that is based on estimates, forecasts, projections, market research or similar methodologies is inherently subject to uncertainties and actual events or circumstances may differ materially from events and circumstances reflected in this information. New risk factors and uncertainties may emerge from time to time, and it is not possible for management to predict all risk factors and uncertainties.
All forward-looking statements contained in this presentation speak only as of the date on which they were made. Other risks and uncertainties affecting Auspex are described more fully in Auspex’s filings with the Securities and Exchange Commission. Auspex undertakes no obligation to update such statements to reflect events that occur or circumstances that exist after the date on which they were made.
December 16, 2014 2
|
|
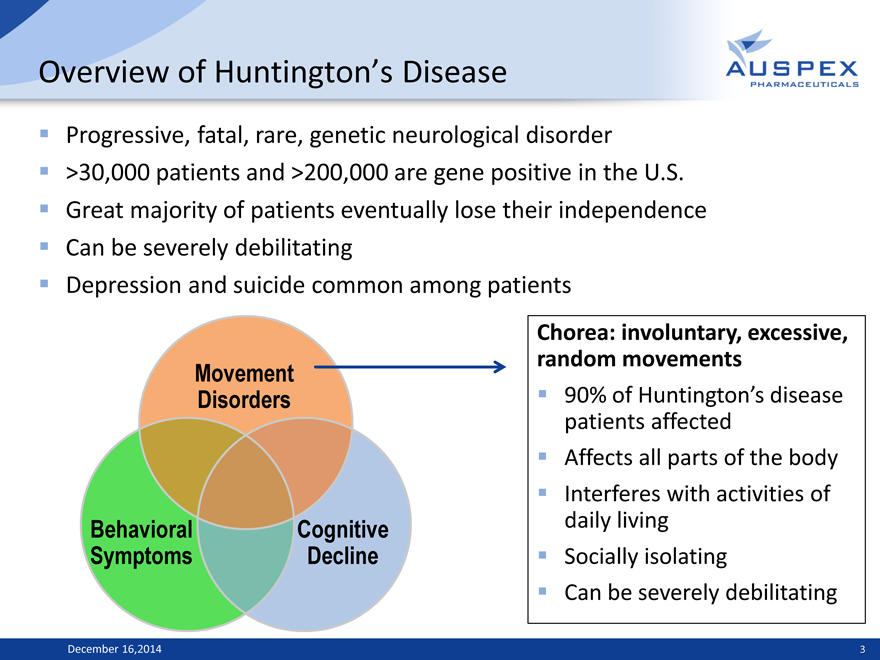
Overview of Huntington’s Disease
Progressive, fatal, rare, genetic neurological disorder >30,000 patients and >200,000 are gene positive in the U.S. Great majority of patients eventually lose their independence Can be severely debilitating Depression and suicide common among patients
Movement Disorders
Behavioral Cognitive Symptoms Decline
Chorea: involuntary, excessive, random movements
90% of Huntington’s disease patients affected Affects all parts of the body Interferes with activities of daily living Socially isolating Can be severely debilitating
December 16, 2014 3
|
|
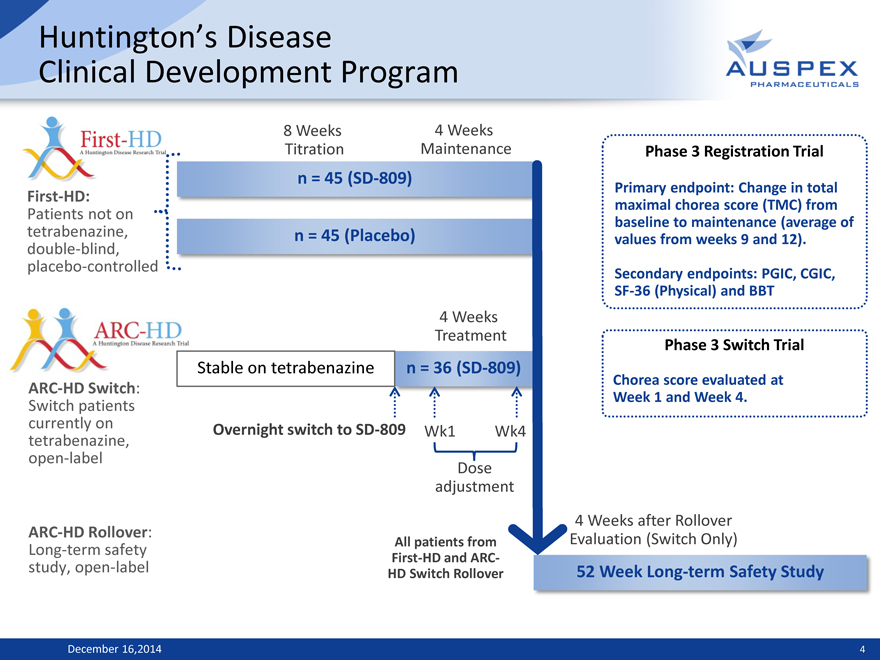
Huntington’s Disease
Clinical Development Program
First-HD:
Patients not on tetrabenazine, double-blind, placebo-controlled
8 Weeks 4 Weeks Titration Maintenance n = 45 (SD-809)
n = 45 (Placebo)
ARC-HD Switch: Switch patients currently on tetrabenazine, open-label
ARC-HD Rollover: Long-term safety study, open-label
4 Weeks Treatment
Stable on tetrabenazine n = 36 (SD-809)
Overnight switch to SD-809 Wk1 Wk4 Dose adjustment
All patients from First-HD and ARC-HD Switch Rollover
Phase 3 Registration Trial
Primary endpoint: Change in total maximal chorea score (TMC) from baseline to maintenance (average of values from weeks 9 and 12). Secondary endpoints: PGIC, CGIC, SF-36 (Physical) and BBT
Phase 3 Switch Trial
Chorea score evaluated at Week 1 and Week 4.
4 Weeks after Rollover Evaluation (Switch Only)
52 Week Long-term Safety Study
December 16, 2014 4
|
|
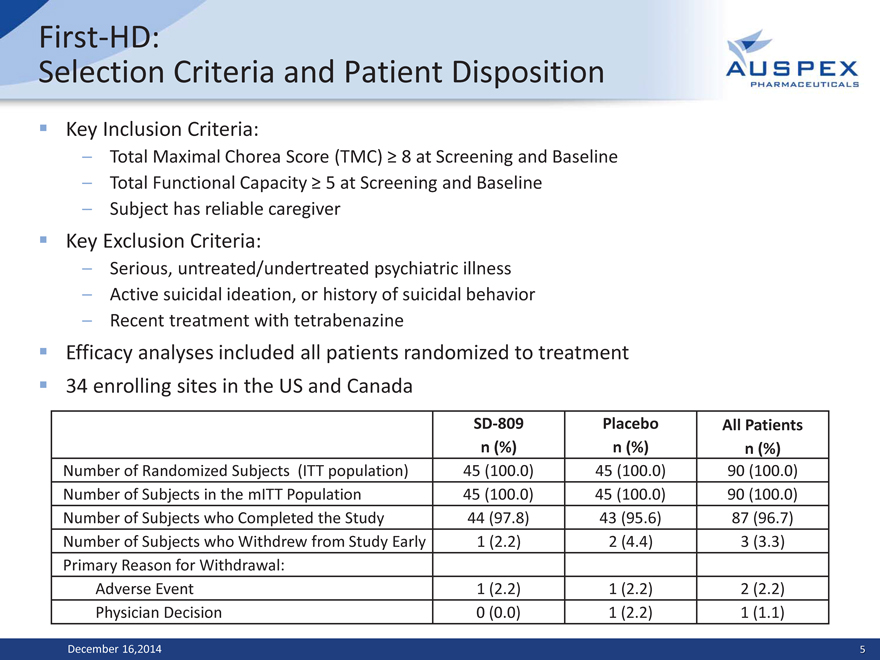
First-HD:
Selection Criteria and Patient Disposition
Key Inclusion Criteria:
– Total Maximal Chorea Score (TMC) ? 8 at Screening and Baseline
– Total Functional Capacity ? 5 at Screening and Baseline
– Subject has reliable caregiver
Key Exclusion Criteria:
– Serious, untreated/undertreated psychiatric illness
– Active suicidal ideation, or history of suicidal behavior
– Recent treatment with tetrabenazine
Efficacy analyses included all patients randomized to treatment 34 enrolling sites in the US and Canada
SD-809 Placebo All Patients
n (%) n (%) n (%)
Number of Randomized Subjects (ITT population) 45 (100.0) 45 (100.0) 90 (100.0)
Number of Subjects in the mITT Population 45 (100.0) 45 (100.0) 90 (100.0)
Number of Subjects who Completed the Study 44 (97.8) 43 (95.6) 87 (96.7)
Number of Subjects who Withdrew from Study Early 1 (2.2) 2 (4.4) 3 (3.3)
Primary Reason for Withdrawal:
Adverse Event 1 (2.2) 1 (2.2) 2 (2.2)
Physician Decision 0 (0.0) 1 (2.2) 1 (1.1)
December 16, 2014 5
|
|
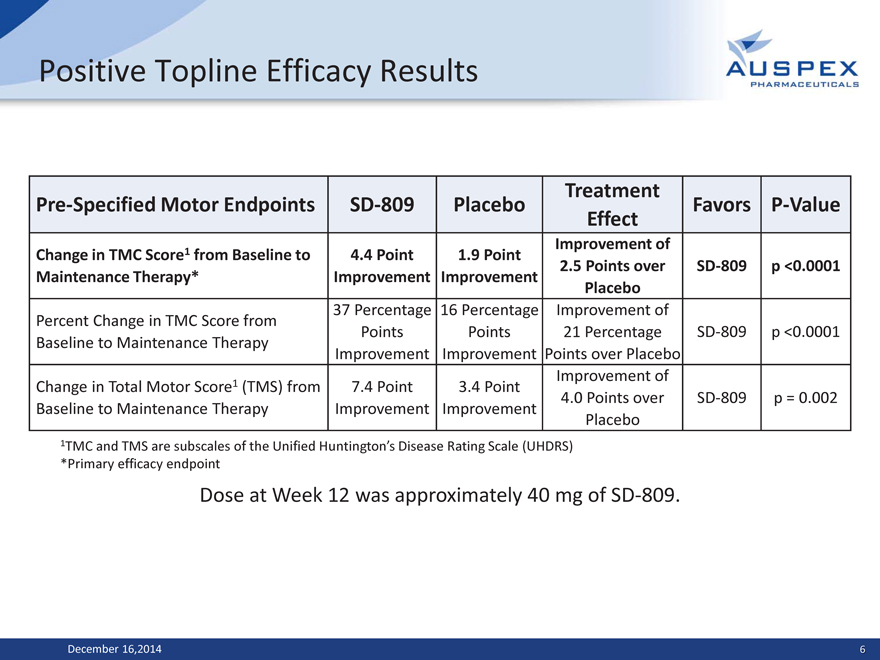
Positive Topline Efficacy Results
Treatment
Pre-Specified Motor Endpoints SD-809 Placebo Favors P-Value
Effect
Improvement of
Change in TMC Score1 from Baseline to 4.4 Point 1.9 Point
2.5 Points over SD-809 p <0.0001
Maintenance Therapy* Improvement Improvement
Placebo
37 Percentage 16 Percentage Improvement of
Percent Change in TMC Score from
Points Points 21 Percentage SD-809 p <0.0001
Baseline to Maintenance Therapy
Improvement Improvement Points over Placebo
Improvement of
Change in Total Motor Score1 (TMS) from 7.4 Point 3.4 Point
4.0 Points over SD-809 p = 0.002
Baseline to Maintenance Therapy Improvement Improvement
Placebo
1TMC and TMS are subscales of the Unified Huntington’s Disease Rating Scale (UHDRS)
*Primary efficacy endpoint
Dose at Week 12 was approximately 40 mg of SD-809.
December 16,2014
6
|
|
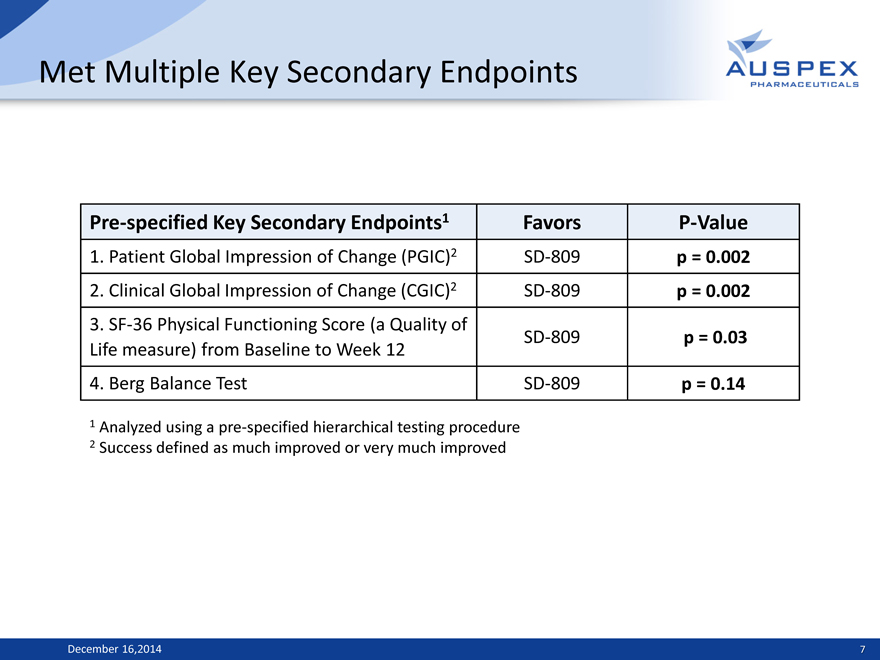
Met Multiple Key Secondary Endpoints
Pre-specified Key Secondary Endpoints1 Favors P-Value
1. Patient Global Impression of Change (PGIC)2 SD-809 p = 0.002
2. Clinical Global Impression of Change (CGIC)2 SD-809 p = 0.002
3. SF-36 Physical Functioning Score (a Quality of
Life measure) from Baseline to Week 12 SD-809 p = 0.03
4. Berg Balance Test SD-809 p = 0.14
1 Analyzed using a pre-specified hierarchical testing procedure
2 Success defined as much improved or very much improved
December 16,2014 7
|
|
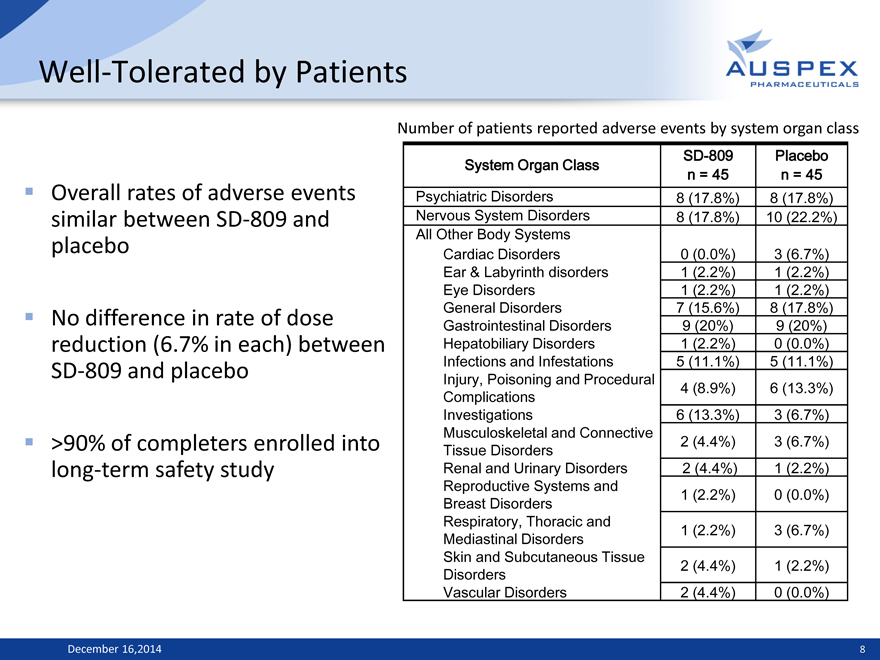
Well-Tolerated by Patients
Overall rates of adverse events
similar between SD-809 and
placebo
No difference in rate of dose
reduction (6.7% in each) between
SD-809 and placebo
>90% of completers enrolled into
long-term safety study
Number of patients reported adverse events by system organ class
System Organ Class SD-809 Placebo
n = 45 n = 45
Psychiatric Disorders 8 (17.8%) 8 (17.8%)
Nervous System Disorders 8 (17.8%) 10 (22.2%)
All Other Body Systems
Cardiac Disorders 0 (0.0%) 3 (6.7%)
Ear & Labyrinth disorders 1 (2.2%) 1 (2.2%)
Eye Disorders 1 (2.2%) 1 (2.2%)
General Disorders 7 (15.6%) 8 (17.8%)
Gastrointestinal Disorders 9 (20%) 9 (20%)
Hepatobiliary Disorders 1 (2.2%) 0 (0.0%)
Infections and Infestations 5 (11.1%) 5 (11.1%)
Injury, Poisoning and Procedural 4 (8.9%) 6 (13.3%)
Complications
Investigations 6 (13.3%) 3 (6.7%)
Musculoskeletal and Connective 2 (4.4%) 3 (6.7%)
Tissue Disorders
Renal and Urinary Disorders 2 (4.4%) 1 (2.2%)
Reproductive Systems and 1 (2.2%) 0 (0.0%)
Breast Disorders
Respiratory, Thoracic and 1 (2.2%) 3 (6.7%)
Mediastinal Disorders
Skin and Subcutaneous Tissue
Disorders 2 (4.4%) 1 (2.2%)
Vascular Disorders 2 (4.4%) 0 (0.0%)
December 16, 2014 8
|
|
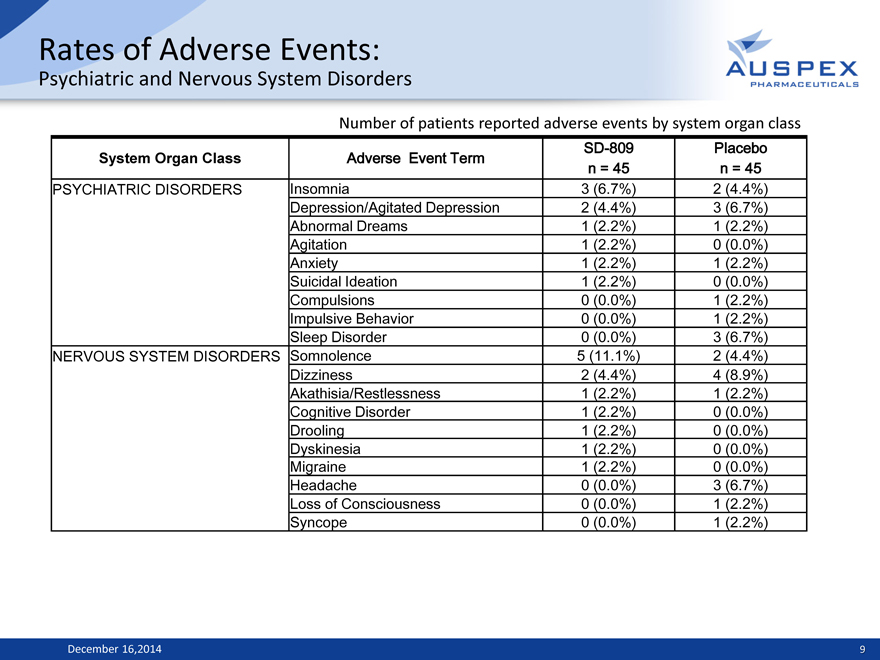
Rates of Adverse Events:
Psychiatric and Nervous System Disorders
Number of patients reported adverse events by system organ class
System Organ Class Adverse Event Term SD-809 Placebo
n = 45 n = 45
PSYCHIATRIC DISORDERS Insomnia 3 (6.7%) 2 (4.4%)
Depression/Agitated Depression 2 (4.4%) 3 (6.7%)
Abnormal Dreams 1 (2.2%) 1 (2.2%)
Agitation 1 (2.2%) 0 (0.0%)
Anxiety 1 (2.2%) 1 (2.2%)
Suicidal Ideation 1 (2.2%) 0 (0.0%)
Compulsions 0 (0.0%) 1 (2.2%)
Impulsive Behavior 0 (0.0%) 1 (2.2%)
Sleep Disorder 0 (0.0%) 3 (6.7%)
NERVOUS SYSTEM DISORDERS Somnolence 5 (11.1%) 2 (4.4%)
Dizziness 2 (4.4%) 4 (8.9%)
Akathisia/Restlessness 1 (2.2%) 1 (2.2%)
Cognitive Disorder 1 (2.2%) 0 (0.0%)
Drooling 1 (2.2%) 0 (0.0%)
Dyskinesia 1 (2.2%) 0 (0.0%)
Migraine 1 (2.2%) 0 (0.0%)
Headache 0 (0.0%) 3 (6.7%)
Loss of Consciousness 0 (0.0%) 1 (2.2%)
Syncope 0 (0.0%) 1 (2.2%)
December 16, 2014 9
|
|
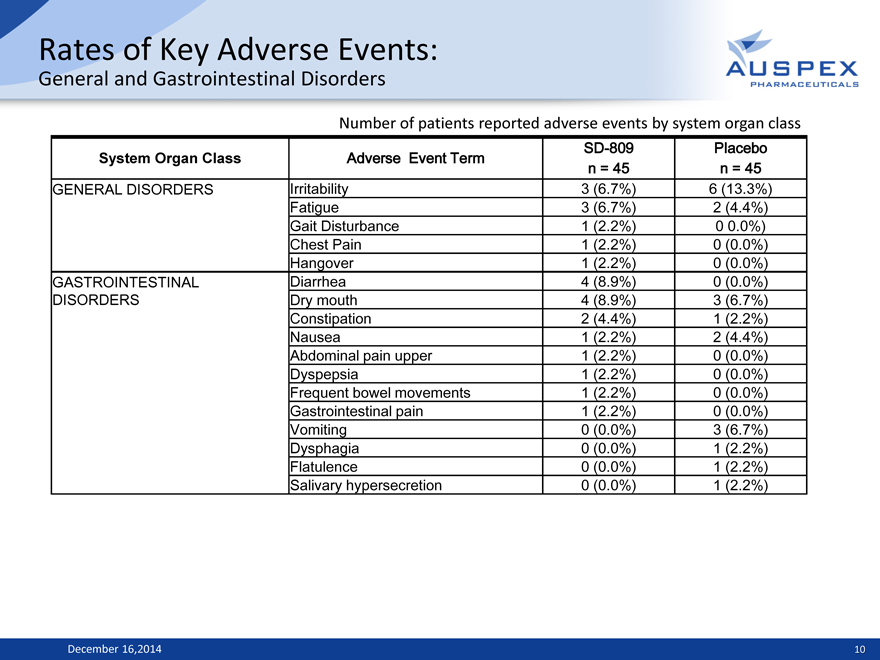
Rates of Key Adverse Events:
General and Gastrointestinal Disorders
Number of patients reported adverse events by system organ class
System Organ Class Adverse Event Term SD-809 Placebo
n = 45 n = 45
GENERAL DISORDERS Irritability 3 (6.7%) 6 (13.3%)
Fatigue 3 (6.7%) 2 (4.4%)
Gait Disturbance 1 (2.2%) 0 0.0%)
Chest Pain 1 (2.2%) 0 (0.0%)
Hangover 1 (2.2%) 0 (0.0%)
GASTROINTESTINAL Diarrhea 4 (8.9%) 0 (0.0%)
DISORDERS Dry mouth 4 (8.9%) 3 (6.7%)
Constipation 2 (4.4%) 1 (2.2%)
Nausea 1 (2.2%) 2 (4.4%)
Abdominal pain upper 1 (2.2%) 0 (0.0%)
Dyspepsia 1 (2.2%) 0 (0.0%)
Frequent bowel movements 1 (2.2%) 0 (0.0%)
Gastrointestinal pain 1 (2.2%) 0 (0.0%)
Vomiting 0 (0.0%) 3 (6.7%)
Dysphagia 0 (0.0%) 1 (2.2%)
Flatulence 0 (0.0%) 1 (2.2%)
Salivary hypersecretion 0 (0.0%) 1 (2.2%)
December 16, 2014 10
|
|
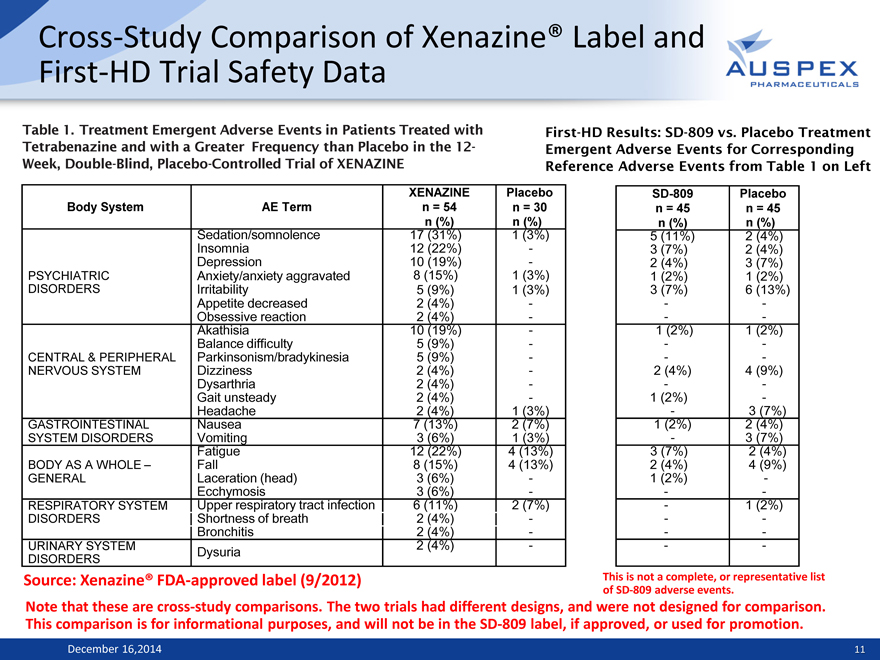
Cross-Study Comparison of Xenazine® Label and First-HD Trial Safety Data
Table 1. Treatment Emergent Adverse Events in Patients Treated with Tetrabenazine and with a Greater Frequency than Placebo in the 12-Week, Double-Blind, Placebo-Controlled Trial of XENAZINE
First-HD Results: SD-809 vs. Placebo Treatment Emergent Adverse Events for Corresponding Reference Adverse Events from Table 1 on Left
XENAZINE Placebo
Body System AE Term n = 54 n = 30
n (%) n (%)
Sedation/somnolence 17 (31%) 1 (3%)
Insomnia 12 (22%) -
Depression 10 (19%) -
PSYCHIATRIC Anxiety/anxiety aggravated 8 (15%) 1 (3%)
DISORDERS Irritability 5 (9%) 1 (3%)
Appetite decreased 2 (4%) -
Obsessive reaction 2 (4%) -
Akathisia 10 (19%) -
Balance difficulty 5 (9%) -
CENTRAL & PERIPHERAL Parkinsonism/bradykinesia 5 (9%) -
NERVOUS SYSTEM Dizziness 2 (4%) -
Dysarthria 2 (4%) -
Gait unsteady 2 (4%) -
Headache 2 (4%) 1 (3%)
GASTROINTESTINAL Nausea 7 (13%) 2 (7%)
SYSTEM DISORDERS Vomiting 3 (6%) 1 (3%)
Fatigue 12 (22%) 4 (13%)
BODY AS A WHOLE – Fall 8 (15%) 4 (13%)
GENERAL Laceration (head) 3 (6%) -
Ecchymosis 3 (6%) -
RESPIRATORY SYSTEM Upper respiratory tract infection 6 (11%) 2 (7%)
DISORDERS Shortness of breath 2 (4%) -
Bronchitis 2 (4%) -
URINARY SYSTEM Dysuria 2 (4%) -
DISORDERS
SD-809 Placebo
n = 45 n = 45
n (%) n (%)
5 (11%) 2 (4%)
3 (7%) 2 (4%)
2 (4%) 3 (7%)
1 (2%) 1 (2%)
3 (7%) 6 (13%)
—
—
1 (2%) 1 (2%)
—
—
2 (4%) 4 (9%)
—
1 (2%) -
- 3 (7%)
1 (2%) 2 (4%)
- 3 (7%)
3 (7%) 2 (4%)
2 (4%) 4 (9%)
1 (2%) -
—
- 1 (2%)
—
—
—
Source: Xenazine® FDA-approved label (9/2012)
This is not a complete, or representative list of SD-809 adverse events.
Note that these are cross-study comparisons. The two trials had different designs, and were not designed for comparison. This comparison is for informational purposes, and will not be in the SD-809 label, if approved, or used for promotion.
December 16, 2014 11
|
|
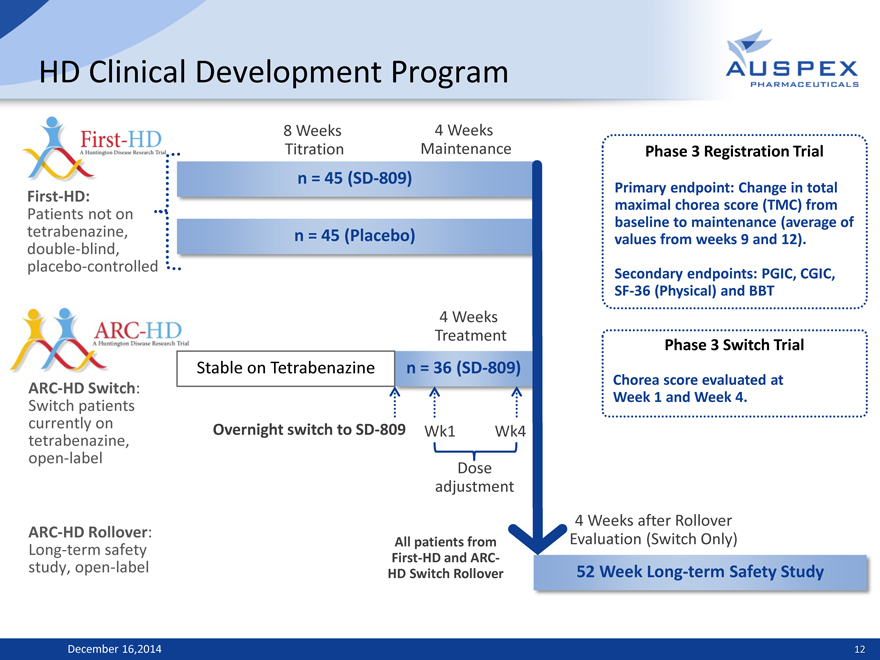
HD Clinical Development Program
First-HD:
Patients not on tetrabenazine, double-blind, placebo-controlled
ARC-HD Switch: Switch patients currently on tetrabenazine, open-label
ARC-HD Rollover: Long-term safety study, open-label
8 Weeks 4 Weeks Titration Maintenance n = 45 (SD-809)
n = 45 (Placebo)
4 Weeks Treatment
Stable on Tetrabenazine n = 36 (SD-809)
Overnight switch to SD-809 Wk1 Wk4 Dose adjustment
All patients from First-HD and ARC-HD Switch Rollover
Phase 3 Registration Trial
Primary endpoint: Change in total maximal chorea score (TMC) from baseline to maintenance (average of values from weeks 9 and 12). Secondary endpoints: PGIC, CGIC, SF-36 (Physical) and BBT
Phase 3 Switch Trial
Chorea score evaluated at Week 1 and Week 4.
4 Weeks after Rollover Evaluation (Switch Only)
52 Week Long-term Safety Study
December 16, 2014 12
|
|
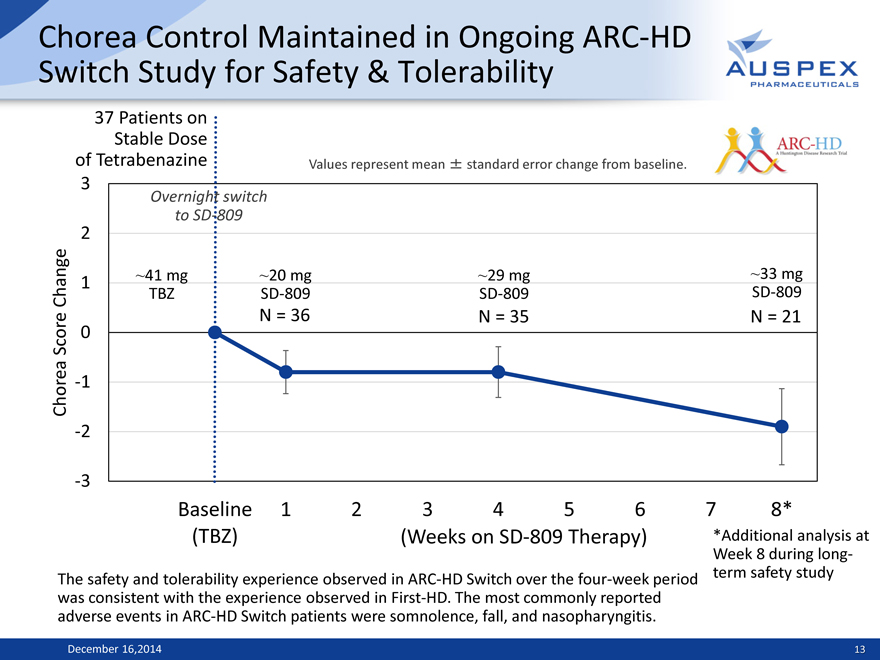
Chorea Control Maintained in Ongoing ARC-HD Switch Study for Safety & Tolerability
37 Patients on Stable Dose
of Tetrabenazine Values represent mean ±standard error change from baseline.
3
Overnight switch 2 to SD-809
1 ~41 mg ~20 mg ~29 mg ~33 mg Change TBZ SD-809 SD-809 SD-809
N = 36 N = 35 N = 21 Score 0 Chorea -1 -2
-3
Baseline 1 2 3 4 5 6 7 8*
(TBZ) (Weeks on SD-809 Therapy) *Additional analysis at
Week 8 during long-The safety and tolerability experience observed in ARC-HD Switch over the four-week period term safety study was consistent with the experience observed in First-HD. The most commonly reported adverse events in ARC-HD Switch patients were somnolence, fall, and nasopharyngitis.
December 16, 2014 13
|
|
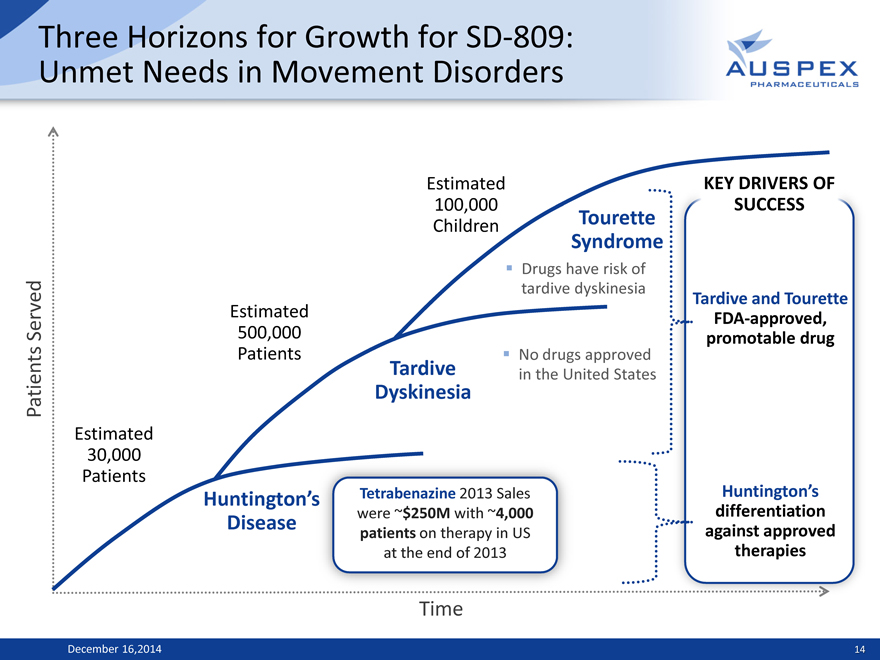
Three Horizons for Growth for SD-809: Unmet Needs in Movement Disorders
Estimated KEY DRIVERS OF
100,000 SUCCESS Children Tourette
Syndrome
Drugs have risk of tardive dyskinesia
Tardive and Tourette Estimated FDA-approved, Served 500,000 promotable drug
Patients
No drugs approved Tardive in the United States
Patients Dyskinesia
Estimated 30,000 Patients
Huntington’ Tetrabenazine 2013 Sales Huntington’s were ~$250M with ~4,000 differentiation Disease against approved patients on therapy in US therapies at the end of 2013
Time
December 16, 2014 14
|
|
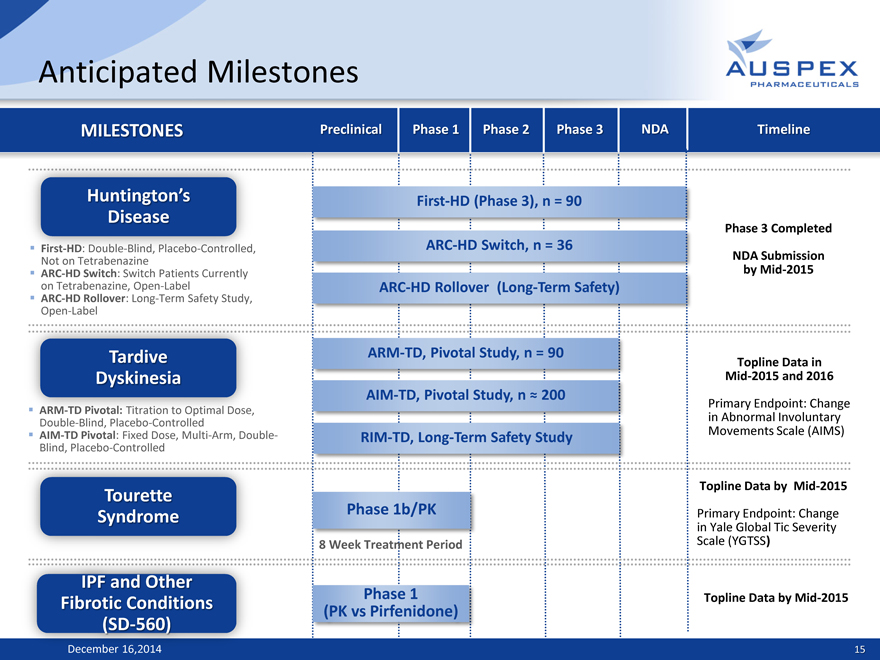
Anticipated Milestones
MILESTONES Preclinical Phase 1 Phase 2 Phase 3 NDA Timeline
Huntington’s First-HD (Phase 3), n = 90
Disease Phase 3 Completed
First-HD: Double-Blind, Placebo-Controlled, ARC-HD Switch, n = 36
Not on Tetrabenazine NDA Submission
ARC-HD Switch: Switch Patients Currently by Mid-2015
on Tetrabenazine, Open-Label ARC-HD Rollover (Long-Term Safety)
ARC-HD Rollover: Long-Term Safety Study,
Open-Label
Tardive ARM-TD, Pivotal Study, n = 90 Topline Data in
Dyskinesia Mid-2015 and 2016
AIM-TD, Pivotal Study, n ? 200
ARM-TD Pivotal: Titration to Optimal Dose, Primary Endpoint: Change
Double-Blind, Placebo-Controlled in Abnormal Involuntary
AIM-TD Pivotal: Fixed Dose, Multi-Arm, Double- RIM-TD, Long-Term Safety Study Movements Scale (AIMS)
Blind, Placebo-Controlled
Tourette Topline Data by Mid-2015
Syndrome Phase 1b/PK Primary Endpoint: Change
in Yale Global Tic Severity
8 Week Treatment Period Scale (YGTSS)
IPF and Other Phase 1
Fibrotic Conditions (PK vs Pirfenidone) Topline Data by Mid-2015
(S 560)
December 16, 2014 15