Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Celator Pharmaceuticals Inc | v395905_8-k.htm |
EXHIBIT 99.1

® Corporate Update December 6, 2014 ®

® SAFE HARBOR To the extent that statements contained in this press release are not descriptions of historical facts regarding Celator, they are forward - looking statements reflecting the current beliefs and expectations of management made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. Words such as "may," "will," "expect," "anticipate," "estimate," "intend," and similar expressions (as well as other words or expressions referencing future events, conditions or circumstances) are intended to identify forward - looking statements. Forward - looking statements in this release involve substantial risks and uncertainties that could cause our pre - clinical and clinical research and development programs, future results, working capital requirements, performance or achievements to differ significantly from those expressed or implied by the forward - looking statements. Such risks and uncertainties include, among others, the uncertainties inherent in the initiation and conduct of clinical studies and other research programs, enrollment in clinical studies, availability of data from ongoing clinical studies, the outcome of other research programs, the potential efficacy and therapeutic potential of our drug candidates, our prospects for long - term growth, our ability to raise capital, and other matters that could affect the availability or commercial potential of our drug candidates. Celator undertakes no obligation to update or revise any forward - looking statements. For a further description of the risks and uncertainties that could cause actual results to differ from those expressed in these forward - looking statements, as well as risks relating to the business of the company in general, see Celator's Form 10 - K for the year ended December 31, 2013 and other filings by the company with the U.S. Securities and Exchange Commission. 2

® AGENDA 8:00 – 8:10 pm: Welcome & Introduction Scott Jackson Chief Executive Officer 8:10 – 8:20 pm: CombiPlex Update & Review of CPX - 351 Preclinical Data Selected for Presentation at the American Society of Hematology meeting Dr. Lawrence Mayer, Ph.D. Founder, President & Chief Scientific Officer 8:20 – 8:50 pm: CPX - 351 Update Dr. Jeffrey Lancet Section Chief of Leukemia, Department of Malignant Hematology, Moffitt Cancer Center 8:50 – 9:00 pm: Question & Answer Session 9:00 pm: Closing Remarks Scott Jackson Chief Executive Officer 3

® 4 Welcome & Introduction Scott Jackson Chief Executive Officer

® OUR MISSION: TO IMPROVE AND EXTEND THE LIVES OF PATIENTS WITH CANCER BY TRANSFORMING THE SCIENCE OF COMBINATION THERAPY 5

® DEVELOPING CUTTING EDGE TECHNOLOG IES FOR CANCER TREATMENT • CombiPlex ® is a combination - optimizing technology platform • Elevated, prolonged, selective exposure of synergistic drug ratios to tumors • Broadening applications for CombiPlex • Evaluating 3 novel targeted combination products, that also include single - agent delivery capabilities – data packages in 3Q2015 • CPX - 351, in Phase 3, is the most advanced CombiPlex - based product • Phase 3 study achieved target enrollment ahead of schedule • Induction response rate (secondary endpoint) – 2Q2015 • Overall Survival (primary endpoint) – 1Q2016 • Broad intellectual property and worldwide rights to all products • IP protection to 2029 in U.S., could be extended longer • Orphan Drug Designation in U.S. and Europe • Expect current cash to take the company into second half of 2016 6

® 7 CELATOR’S PROPRIETARY ONCOLOGY PIPELINE CPX - 1 Irinotecan:Floxuridine CPX - 351 Cytarabine:Daunorubicin CPX - 8 (HDPN) Taxane Nanoparticle Research Preclinical Phase 1 Phase 2 Proprietary Technology Platforms Phase 3 Colorectal Cancer Secondary Acute Myeloid Leukemia (Ongoing) Solid Tumors Novel combinations Including targeted therapies Unfit AML due to co - morbidities (Ongoing) Unfit AML due to poor risk (Planned) Pre - conditioning prior to HSCT (Ongoing) COG - Pediatric Pilot (Ongoing) HR - MDS and AML after HMA therapy (Ongoing)

® BUILDING A BROAD PIPELINE FROM THE COMBIPLEX ® PLATFORM • Advancing CombiPlex to widen its application to molecularly targeted therapies • Combinations targeting signaling pathways (i.e. inhibitors of PI3K/AKT/ mTOR pathway and inhibitors of Ras / Raf /MEK/ERK pathway) • Combinations of existing chemotherapeutics with molecularly targeted agents • Combinations of epigenetic modulators • Generate additional data for novel single - agent formulations • Based on formulation work being done in support of CombiPlex • Establish proof of principle to support value of R&D collaborations • Generate product candidates for internal development 8

® 9 CPX - 351 PHASE 3 STUDY DESIGN IN S AML STUDY ACHIEVED TARGET ENROLLMENT AHEAD OF SCHEDULE Eligibility • Confirmation of sAML according to the World Health Organization (WHO) criteria with certain exclusions • Age 60 - 75 years • Able to tolerate intensive chemo • PS 0 - 2 Randomization CPX - 351 7+3 1:1 Randomization Follow - up 150 patients 150 patients Patients were stratified based on age and cytogenetics Primary Endpoint: Overall survival >90% power for HR= 0.635 or better Secondary Endpoints: Leukemia clearance rate, response rate (CR+CRi), response/remission duration, Event - Free Survival (EFS), 60 - day mortality Established with FDA and EMA an acceptable pivotal study design Based on results from randomized, controlled, Phase 2 studies

® ADVANTAGES OF THE CPX - 351 PHASE 3 STUDY 10 • AML is heterogeneous • Single arm studies used to design Phase 3 studies may not be predictive • Two randomized, controlled clinical studies were conducted to identify patient populations to pursue in Phase 3; initial registration study is in sAML patients • Patient eligibility in Phase 3 study is based on population with greatest observed OS benefit in Phase 2 • CPX - 351 demonstrated a statistically significant overall survival benefit • The control arm are the same two drugs used in the Phase 2 study and the control arm median survival (~6 months) was consistent with historical data • Phase 3 study is being conducted in Canada and the U.S., where the Phase 2 study was conducted • Although the study is being conducted in North America, Celator has received scientific advice from EMA and believes this Phase 3 study can be used for regulatory submission in Europe

® • Benefit seen with CPX - 351 vs 7+3 is consistent across all metrics – higher response rate, reduced early mortality and improved overall survival • A positive Phase 3 result can be achieved with half the overall survival observed in the Phase 2 (the population that matches the eligibility being used in the Phase 3 study) • Numerous meetings with FDA, to discuss various components of the NDA, which Celator believes lowers the regulatory risk 11 ADVANTAGES OF THE CPX - 351 PHASE 3 STUDY

® SELECTED PHASE 3 AML PRODUCT CANDIDATES Celator (CPXX) Sunesis (SNSS) Cyclacel (CYCC) Ambit (AMBI) Product CPX - 351 vosaroxin (Qinprezo) sapacitabine quizartinib Patient Population secondary AML >60 and <75 years of age Relapsed/refractory AML > 18 years of age First - line >70 years of age FLT3 - ITD+ relapsed/refractory AML, >18 years of age Status Enrollment complete Did not meet primary endpoint Expect to complete enrollment by Y/E 2014 Estimate enrollment completed in 2H2015 Phase 3 Study Design CPX - 351 vs 7+3 vosaroxin+cytarabine vs cytarabine + placebo sapacitabine alternating cycles with decitabine vs decitabine alone quizartinib (20mg dose titrate, looking to get to 30mg) vs salvage Primary Endpoint Overall survival Overall survival Overall survival Overall survival Phase 2 Geography US and Canada US US US and Western Europe Phase 2 Study Design Control arm No control arm No control arm No control arm Phase 3 Geography US and Canada US, Canada, Europe, Korea, Australia and New Zealand US and Europe US, Canada, Western Europe and Australia 12

® 13 CombiPlex ® Update & CPX - 351 Preclinical Data Being Presented at ASH Lawrence Mayer, Ph.D. Founder, President & Chief Scientific Officer

® COMBIPLEX®: RATIONALE FOR ROLE OF DRUG DELIVERY IN DEVELOPMENT OF TARGETED AGENT COMBINATIONS 14 Conventional Combinations CombiPlex ® Many require simultaneous target inhibition Harmonized PK and tumor cell exposure to both agents Toxicity complications observed with many targeted agent combinations Toxicities a meliorated by shifting drug exposure favoring tumor tissue using delivery complex Growing evidence that drug ratios are important for combinations of targeted agents; difficult to identify best combination regimen in clinical setting Optimized drug ratios established preclinically ; benefits translate from in vitro to in vivo to human

® COMBINATIONS IDENTIFIED FOR COMBIPLEX ® APPLICATION TO MOLECULARLY TARGETED AGENTS Histone deacetylase inhibitors plus Hypomethylating agents • Promising early clinical evidence of favorable interaction • Optimal efficacy with simultaneous exposure • Dose limiting toxicity (encephalopathy) of combination at relatively low doses 15 Inhibitors of PI3K/AKT/ mTOR pathway plus Inhibitors of Ras / Raf /MEK/ERK pathway • Active in many major tumor types • Pathway cross - talk means concurrent inhibition needed for optimal efficacy Docetaxel ) plus Modulators of apoptosis (e.g. HSP90 inhibitors) • Promising preclinical and early clinical results, but several late stage clinical failures • Concurrent and prolonged exposure required for optimal efficacy Data packages projected to be available 3Q2015

® COMBIPLEX ® MAY ADDRESS A VARIETY OF SHORTCOMINGS FOR MOLECULARLY TARGETED COMBINATIONS UNDER INVESTIGATION 16 ISSUE CombiPlex ® PI3K/AKT/ mTOR plus Ras / Raf /MEK/ERK Docetaxel ) plus HSP90 inhibitors HDAC inhibitors plus Hypomethylating agents Pathway cross - talk requires concurrent inhibition for optimal efficacy Concurrent and prolonged exposure required for optimal efficacy Hypomethylators exhibit rapid degradation and elimination Toxicities of the combination limit dose of current formulations HSP90i associated with GI and/or ocular toxicities Inter - patient PK variability plus encephalopathy with combination I ncrease therapeutic index: • Shift exposure away from healthy tissues to tumor • Ensure simultaneous target inhibition at tumor site Enhance PK and Toxicity Profile: • Extend plasma elimination time • Minimize normal tissue exposure • Reduce GI and/or ocular toxicity Enhance Toxicity and Tumor Exposure Profile: • Potential to r educe CNS toxicity • Elevate and prolong exposure of optimal drug ratio

® COMBIPLEX ® MAY ADDRESS A VARIETY OF SHORTCOMINGS FOR MOLECULARLY TARGETED COMBINATIONS UNDER INVESTIGATION 17 ISSUE CombiPlex ® PI3K/AKT/ mTOR plus Ras / Raf /MEK/ERK Docetaxel ) plus HSP90 inhibitors HDAC inhibitors plus Hypomethylating agents Pathway cross - talk requires concurrent inhibition for optimal efficacy Concurrent and prolonged exposure required for optimal efficacy Hypomethylators exhibit rapid degradation and elimination Toxicities of the combination limit dose of current formulations HSP90i associated with GI and/or ocular toxicities Inter - patient PK variability plus encephalopathy with combination I ncrease therapeutic index: • Shift exposure away from healthy tissues to tumor • Ensure simultaneous target inhibition at tumor site Enhance PK and Toxicity Profile: • Extend plasma elimination time • Minimize normal tissue exposure • Reduce GI and/or ocular toxicity Enhance Toxicity and Tumor Exposure Profile: • Potential to r educe CNS toxicity • Elevate and prolong exposure of optimal drug ratio

® COMBIPLEX ® MAY ADDRESS A VARIETY OF SHORTCOMINGS FOR MOLECULARLY TARGETED COMBINATIONS UNDER INVESTIGATION 18 PI3K/AKT/ mTOR plus Ras / Raf /MEK/ERK Docetaxel ) plus HSP90 inhibitors HDAC inhibitors plus Hypomethylating agents Pathway cross - talk requires concurrent inhibition for optimal efficacy Concurrent and prolonged exposure required for optimal efficacy Hypomethylators exhibit rapid degradation and elimination Toxicities of the combination limit dose of current formulations HSP90i associated with GI and/or ocular toxicities Inter - patient PK variability plus encephalopathy with combination I ncrease therapeutic index: • Shift exposure away from healthy tissues to tumor • Ensure simultaneous target inhibition at tumor site Enhance PK and Toxicity Profile: • Extend plasma elimination time • Minimize normal tissue exposure • Reduce GI and/or ocular toxicity Enhance Toxicity and Tumor Exposure Profile: • Potential to r educe CNS toxicity • Elevate and prolong exposure of optimal drug ratio ISSUE CombiPlex ®

® Hours After Administration 0 5 10 15 20 25 Percent Injected Dose 0.0001 0.001 0.01 0.1 1 10 100 1000 Docetaxel prodrug AUY922 prodrug Free AUY922 Free Docetaxel 19 PRODRUG NANOPARTICLE FORMULATION OF DOCETAXEL AND AUY922 DRAMATICALLY INCREASE PLASMA DRUG CONCENTRATION Plasma concentrations of AUY922 and docetaxel in nanoparticle formulation are more than 2 - 4 orders of magnitude greater than conventional IV formulation

® American Society of Hematology Poster Presentations 20

® CPX - 351: ADME (ABSORPTION, DISTRIBUTION, METABOLISM AND EXCRETION) • CPX - 351 Markedly Reduces Renal and Hepatic Clearance Rates for Cytarabine ( Cyt ) and Daunorubicin ( Daun ) in Rats with an Associated Decrease in Excretory and Metabolic Burden Despite Providing Dramatic Increases in Systemic Drug Exposure Compared to Conventional Cyt+Daun Number 2305; Date: Sunday, December 7; Presentation Time: 6:00 pm - 8:00 pm; Location: Moscone Center, North Building, Hall E • Quantitative Whole Body Autoradiography (QWBA) Analysis Reveals that CPX - 351 Shifts the Exposure of Cytarabine ( Cyt ) and Daunorubicin ( Daun ) Away from Many Tissues While Providing Prolonged Exposure to Cytotoxic Drug Concentrations in the Bone Marrow Compared to Conventional Free Drug Administration Number 3740; Date: Monday, December 8; Presentation Time: 6:00 pm - 8:00 pm Location: Moscone Center, North Building, Hall E 21

® SLOWER DRUG ELIMINATION FOR CPX - 351 COMPARED TO FREE DRUGS REDUCES BURDEN ON EXCRETORY / METABOLIC SYSTEMS 22 DAUNORUBICIN CYTARABINE

® QWBA REVEALS DECREASED DRUG DISTRIBUTION TO MOST TISSUES AND INCREASED ACCUMULATION IN BONE MARROW CYTARABINE Treatment Hours after Administration [ 14 C]Cytarabine 1 24 Non-Liposomal CPX-351 23

® QWBA REVEALS DECREASED DRUG DISTRIBUTION TO MOST TISSUES AND INCREASED ACCUMULATION IN BONE MARROW 24 DAUNORUBICIN Treatment Hours after Administration [ 14 C]Daunorubicin 1 48 Non-Liposomal CPX-351

® CONCLUSIONS FROM PRECLINICAL ADME • The slower drug excretion of Cyt and Daun for CPX - 351 suggests that the burden on excretory and metabolic systems may be reduced for CPX - 351 • CPX - 351 limits early tissue distribution of Cyt and Daun compared to the drugs administered as the non - liposomal formulation • CPX - 351 accumulates and persists in the bone marrow for over 4 days at concentrations known to have anti - leukemic activity against AML blasts • Results warrant additional clinical studies to examine whether CPX - 351 provides an improved safety profile for patients with hepatic or renal insufficiencies • Together these results provide additional biologic rationale that support the clinical improvements in both efficacy and safety seen for CPX - 351 compared to conventional Cyt + Daun treatment 25

® CPX - 351 UPDATE DR. JEFFREY LANCET SECTION CHIEF OF LEUKEMIA, DEPARTMENT OF MALIGNANT HEMATOLOGY, MOFFITT CANCER CENTER 26

® Timeline of “progress” in AML 1950s - 1960s 1970s 1980s 1990s 2000s 2010s Daunorubicin Cytarabine Daunorubicin + Cytarabine Idarubicin + Cytarabine High - dose Cytarabine Gemtuzumab Cladribine Vosaroxin CPX - 351 Sapacitabine Midostaurin HMAs HDAC inhibitors 27

® AML survival: patients ≥ 60 years Burnett et al. Semin Hematol 2006: 43(2):96 - 106 0 1 2 3 4 5 6 7 8 0.0 0.2 0.4 0.6 0.8 1.0 Years Survival Probability 28

® Issues delaying the development of effective new therapy in AML • Discovery of molecular anomalies have not yet translated into clear therapeutic targets • Lack of molecular data applied retrospectively to predict response • Trial Design • Empiric testing in a heterogeneous disease • Especially with targeted agents • Inappropriate endpoints in early phase trials? • Lack of randomization in early phase trials 29
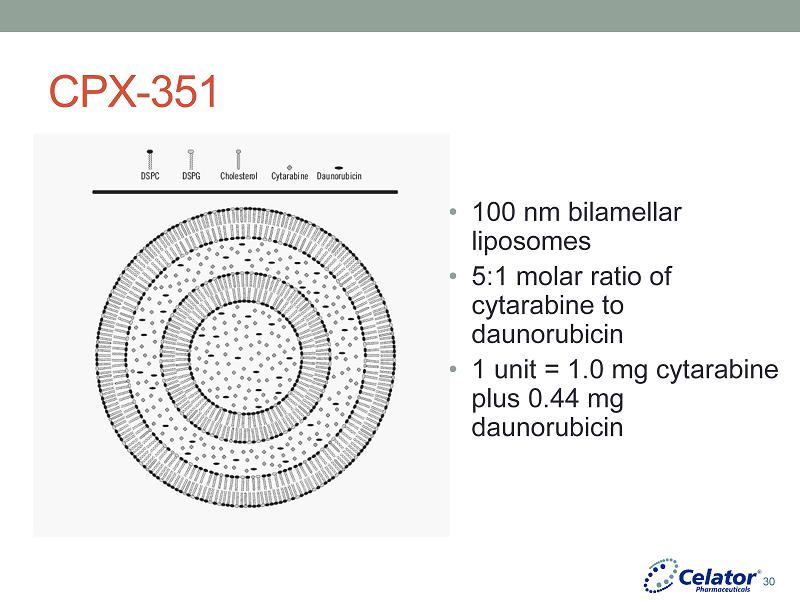
® CPX - 351 • 100 nm bilamellar liposomes • 5:1 molar ratio of cytarabine to daunorubicin • 1 unit = 1.0 mg cytarabine plus 0.44 mg daunorubicin 30

® Pharmacological Basis of CPX - 351 CPX - 351 liposome delivers 5:1 molar ratio for >24 hours Hours After Administration 0 20 40 60 80 100 120 140 160 180 Bone Marrow Cytarabine ( g/ml) 0 1 2 3 CPX-351 Free drug cocktail Cytarabine Accumulates and persists in the bone marrow Preferential uptake and intracellular release in leukemic blasts Plasma Molar Ratio Time (hours) 0 5 10 15 20 25 0 5 10 15 20 30 min 24 hours 31

® Phase 1 study of CPX - 351 in AML • First in human study • Relapsed, refractory AML • Dose escalation – planned up to 134 units/m 2 • Primary endpoints – safety, maximum tolerated dose 32

® Phase 1 study of CPX - 351 in AML: Key Findings • Safety • Dose limiting toxicity in 3 of 6 patients at 134 units/m 2 • Congestive heart failure, hypertension, prolonged cytopenias • Other most common grade 3 - 4 toxicities • Rash, liver function abnormalities, • Clinical congestive heart failure in 2 of 23 pts (both with heavy prior anthracycline exposure) • Maximum tolerated dose: 101 units/m 2 • Efficacy • 10 out of 43 pts (23%) had CR or CR p • CR at dose level as low as 32 units/m 2 • One patient with CR after primary induction failure with 7+3 33

® Pharmacokinetics 0 2,000 4,000 6,000 8,000 10,000 12,000 14,000 16,000 0 24 48 72 96 120 144 168 TIME (hr) Cytarabine Daunorubicin Mean plasma concentration after the day 5 infusion among patients receiving 24 units/m^2 CPX-351 (n=3) D & A detectable 7 days at completion of infusion Fixed molar ratio maintained For 24 hours after final dose 1 2 3 4 5 6 7 8 9 10 0 4 8 12 16 20 24 TIME (hr) 1001 2002 3004 Plasma molar ratio of cytarabine to daunorubicin after the day 5 infusion among patients receiving 24 units/m^2 Feldman, et al. J Clin Oncol 2011; 29:979 34

® Randomized Phase 2 Trial of CPX - 351 vs “7+3” in Older Untreated AML Eligibility • Newly diagnosed • Ages 60 - 75 • Able to tolerate intensive therapy • PS 0 - 2 CPX - 351 (n=85) 100 units/m 2 IV days 1, 3, 5 Up to 2 inductions and 2 consolidations 7+3 (n=41) Daunorubicin 60 mg/m 2 Cytarabine 100 mg/m 2 Primary Endpoint: CR+CR i rate 35
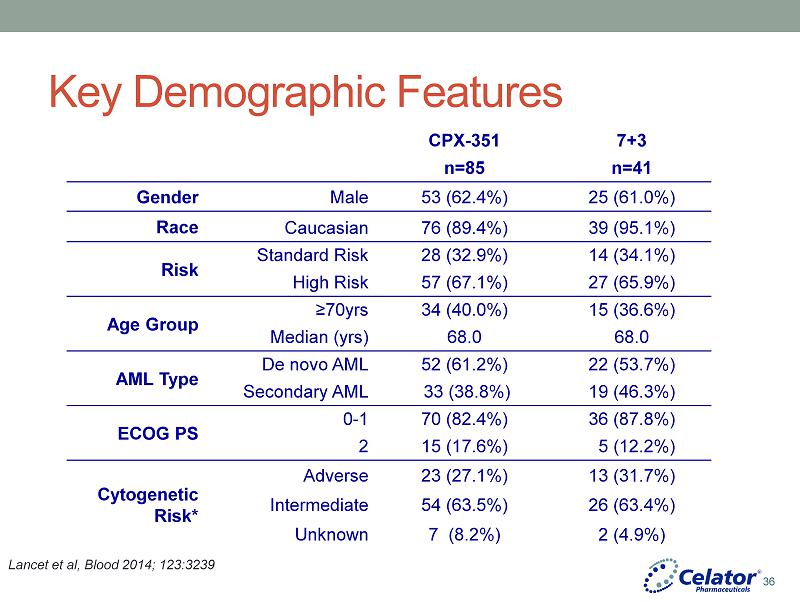
® Key Demographic Features CPX - 351 7+3 n=85 n=41 Gender Male 53 (62.4%) 25 (61.0%) Race Caucasian 76 (89.4%) 39 (95.1%) Risk Standard Risk 28 (32.9%) 14 (34.1%) High Risk 57 (67.1%) 27 (65.9%) Age Group ≥70yrs 34 (40.0%) 15 (36.6%) Median (yrs) 68.0 68.0 AML Type De novo AML 52 (61.2%) 22 (53.7%) Secondary AML 33 (38.8%) 19 (46.3%) ECOG PS 0 - 1 70 (82.4%) 36 (87.8%) 2 15 (17.6%) 5 (12.2%) Cytogenetic Risk* Adverse 23 (27.1%) 13 (31.7%) Intermediate 54 (63.5%) 26 (63.4%) Unknown 7 (8.2%) 2 (4.9%) Lancet et al, Blood 2014; 123:3239 36

® Randomized Phase 2 Trial of CPX - 351 vs “7+3” in Older Untreated AML Parameter CPX - 351 (n=84) “7+3” Regimen (n=41) CR (%) 41 (48.8) 20 (48.8) CRi (%) 15 (17.9) 1 (2.4) CR + CRi (%) 56 (66.6) 21 (51.2) 60 - d mortality (%) 4 (4.7) 6 (14.6) CR + CRi (%) in s - AML 19 (57.5) 6 (31.6) s - AML median EFS (mos) 4.5 1.3 s - AML median OS (mos) 12.1 6.1 Lancet et al, Blood 2014; 123:3239 37

® Randomized Phase 2 Trial of CPX - 351 vs “7+3” in Older Untreated AML Lancet et al, Blood 2014; 123:3239 sAML All Patients Overall Survival Overall Survival by treatment arm 0% 20% 40% 60% 80% 100% 0 12 24 36 48 Months from randomization Arm A: CPX - 351 Arm B: 7+3 Deaths / N 58 / 85 27 / 41 Median in Mon s . 14.7 ( 10.6 , 18.1 ) 12.9 ( 6.7 , 22.4 ) Logrank p - value = 0 .61 , HR=0.88 0% 20% 40% 60% 80% 100% 0 12 24 36 48 Months from randomization 7+3: Secondary CPX - 351: Secondary Deaths / N 18 / 19 23 / 33 Median in Mon s 6.1 ( 1.4 , 7.5 ) 12.1 ( 9.1 , 17.6 ) Logrank p - value = p=0.01, HR=0 . 46 Overall Survival P rotocol - defined sAML 10 patients on the 7+3 arm crossed over; 4 achieved CR; all 4 survived >12 months 6 patients on the 7+3 arm crossed over; 2 achieved CR; both survived >12 months 38

® Safety CPX - 351 (n=85) 7+3 (n=41) n (%) n (%) Grade 3/4 Grade 5 Grade 3/4 Grade 5 Febrile Neutropenia 54 (63.5) 0 (0.0) 21 (51.2) 0 (0.0) Infections 60 (70.6) 3 (3.5) 18 (43.9) 3 (7.3) Bacteraemia 30 (35.3) 0 (0.0) 8 (19.5) 0 (0.0) Epistaxis 6 (7.1) 0 (0.0) 0 (0.0) 0 (0.0) Petechiae 1 (1.2) 0 (0.0) 0 (0.0) 0 (0.0) Early mortality (Day 60): CPX - 351 (4.7%) vs. 7+3 (14.6%) Neutrophils to > 1000/uL 36 days 32 days Platelets to > 100K/uL 37 days 28 days 39

® Outcomes in patients with prior hypomethylating agent exposure Prior Hypomethylating Agent Treatment CPX - 351 n=33 (%) 7+3 n=19 (%) +HMA 13 (39%) - HMA 20 (61%) +HMA 7 (37%) - HMA 12 (63%) Responses 7 (54) 12 (60) 2 (29) 4 (33) CR 3 (23) 9 (45) 2 (29) 4 (33) CRi 4 (31) 3 (15) 0 0 40

® Randomized Phase 2 Trial of CPX - 351 vs Salvage in 1 st Relapse AML Eligibility • 1st Relapse • Initial CR ≥1 month • Ages 18 - 65 • Stratified by European Prognostic Index • PS 0 - 2 CPX - 351 (n=81) 100 units/m 2 IV days 1, 3, 5 Up to 2 inductions and 2 consolidations Salvage (as per investigator choice) : ( n=44) • MEC (mitoxantrone+etoposide+cytarabine) (23pts) • 7+3 (7pts) • Other (14pts) Primary Endpoint: Survival at 1 year 41

® Randomized Phase 2 Trial of CPX - 351 vs Salvage in 1 st Relapse AML Overall (n=125) EPI Poor - Risk ( n=85) CPX - 351 (n=81) Salvage (n=44) CPX - 351 (n=56) Salvage (n=29) Response Rate 49.4% 40.9% 39.3% 27.6% 60 - Day Mortality 14.8% 15.9% 16.1% 24.1% EFS (median, mos.) 4.0 1.4 1.9 1.2 OS (median, mos.) 8.5 6.3 6.6 4.2 42

® Potential reasons why CPX - 351 has a greater benefit in High - Risk AML Patients • Altered signaling/cell cycle control checkpoints • 5:1 molar ratio: constant cytarabine excess → daunorubicin DNA damage without blocking cell cycle progression to S - phase where cytarabine action occurs preventing antagonism • Markedly prolonged cytarabine and daunorubicin half - life • 5 - 8 fold prolonged half - life maintains cytotoxic drug levels, enhances killing of slowly replicating leukemia cells and cells with impaired apoptosis after p53 - function is blocked • Drug transport effects • Direct internalization of CPX - 351 liposomes may circumvent drug efflux resistance mechanisms (MDR1, MRP) • Direct internalization of CPX - 351 liposomes may also reduce cytidine deaminase inactivation of cytarabine 43

® Randomized Phase 3 Trial of CPX - 351 vs “7+3” in Older Patients with sAML Eligibility • Previously untreated sAML or tAML • Ages 60 - 75 • Able to tolerate intensive therapy • PS 0 - 2 CPX - 351 100 units/m 2 IV days 1, 3, 5 Up to 2 inductions and 2 consolidations 7+3 Daunorubicin 60 mg/m 2 Cytarabine 100 mg/m 2 Primary Endpoint: Overall Survival 44

® CPX - 351 vs 7+3 Phase II study: sAML Definition Phase II vs. Phase III *Patient 12 - 004, originally identified as a de novo patient, was diagnosed with Philadelphia chromosome and was not assessed for response Phase III study will use WHO criteria for confirmation of secondary AML 16 patients added by karyotype 10 patients with MPN or MDS / MPN removed 58 patients by Phase III criteria sAML n = 52 45

® What makes AML - 301 unique from other phase 3 AML studies? • Based upon a prior randomized study • Based upon a prior study that identified a distinct group of patients found to benefit from CPX - 351 (secondary AML) • Primary endpoint (overall survival) was modeled upon the primary endpoint from study 204. • Large enough study to assure adequate power for a less robust survival difference than was seen in the 204 study. (Hazard ratio of 0.635 rather than 0.40). • AML - 301 also adequately powered to ensure ability to detect a 20% improvement in response rate of CPX - 351 (less than the difference seen in the 204 study). 46

® Endpoints • Primary • Overall survival with >90% power to detect HR = 0.63 • Detect a 37% decrease in the rate of death with CPX - 351 over the course of the study. • Secondary • Response rate (CR+CRi) • Event - free survival • 60 day mortality • Leukemia clearance rate 47

® Power Calculations Power Hazard Ratio 70 0.726 75 0.712 80 0.697 85 0.679 90 0.657 93.7 0.635 Arm B Rate 31% 42% Power Arm A Rate (%) Difference (%) Arm A Rate (%) Difference (%) 70 46.5 15.5 57.9 15.9 75 47.4 16.4 58.8 16.8 80 48.4 17.4 59.8 17.8 85 49.6 18.6 60.9 18.9 90 51.0 20.0 62.3 20.3 95 53.2 22.2 64.4 22.4 97 54.6 23.6 65.8 23.8 Survival Response 48

® Final Induction Response Rate Analysis • When: • 2Q 2015 • What: • CPX - 351 CR+Cri /n (%) vs. Control CR+CRi /n (%) • No p - value will be calculated • Comments: • Final induction response rate analysis was prospectively defined in the Phase 3 protocol and statistical analytical plan • The analysis occurs after all patients have been randomized and completed all treatment • The analysis has been discussed with FDA 49
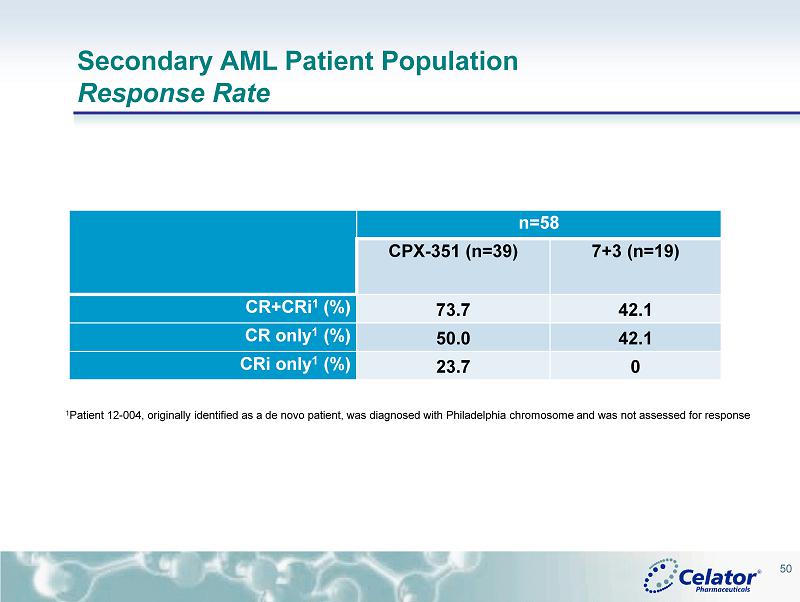
® Secondary AML Patient Population Response Rate n=58 CPX - 351 (n=39) 7+3 (n=19) CR+CRi 1 (%) 73.7 42.1 CR only 1 (%) 50.0 42.1 CRi only 1 (%) 23.7 0 1 Patient 12 - 004, originally identified as a de novo patient, was diagnosed with Philadelphia chromosome and was not assessed for response 50

® Secondary AML Patient Population Early Mortality n=58 CPX - 351 (n=39) 7+3 (n=19) Early mortality (d=60) (%) 2.6 26.3 51

® Secondary AML Patient Population Median Overall Survival n = 58 CPX - 351 ( n=39 1 ) 7+3 (n=19 2 ) All Patients 12.1 months 6.3 months Responders 15.4 months (n=28) 9.4 months (n=8) Non Responders 9.2 months 3.2 months 1 Patient 12 - 004, originally identified as a de novo patient, was diagnosed with Philadelphia chromosome and was not assessed for response 2 Includes patients who clinical investigators deemed non - responders with 7+3 and crossed over to receive CPX - 351 52

® Secondary AML Patient Population Summary of Important Endpoints n = 58 CPX - 351 ( n=39 1 ) 7+3 (n=19 2 ) Morphologic Leukemia Free State 3 35/39 (89.7%) 12/17 (70.6%) Response Rate ( CR+CRi ) (%) 73.7 42.1 Duration of Remission (Months) 6.2 4.3 60 Day Mortality (%) 2.6 26.3 Median Event Free Survival (Months) 8.9 1.4 1 Year Survival (%) 51.3 24.3 2 Median Overall Survival 12.1 6.3 2 1 Patient 12 - 004, originally identified as a de novo patient, was diagnosed with Philadelphia chromosome and was not assessed for response 2 Includes patients who clinical investigators deemed non - responders with 7+3 and crossed over to receive CPX - 351 3 Morphologic leukemia - free state: <5% blasts with no Auer rods at any time during the study; patients with no post - baseline asses sment are excluded from the analysis 53

® American Society of Hematology Poster Presentations 54

® CPX - 351: HR - MDS OR AML PATIENTS AT HIGH - RISK OF TREATMENT - RELATED MORTALITY • Randomized Study of Liposomal Cytarabine and Daunorubicin (CPX - 351) for Adults with Untreated High - Risk Myelodysplastic Syndrome (MDS) and Acute Myeloid Leukemia (AML) at High Risk of Treatment - Related Mortality Number 994; Date: Saturday, December 6; Presentation Time: 5:30 pm – 7:30 pm Location: Moscone Center, North Building, Hall E 55

® • Patients with AML/MDS at high risk of early mortality following treatment are often given palliative care with no serious attempt to induce remissions. • These patients are heterogeneous and causes of early mortality include progressive AML/MDS and treatment - induced toxicities. • The TRM scoring system focuses only on the first 30 - days and a longer time frame for evaluation may be more appropriate. • A TRM score of > 13.1 selected patients with > 41% risk of 30 - day mortality following treatment. CPX - 351: HR - MDS OR AML PATIENTS AT HIGH - RISK OF TREATMENT - RELATED MORTALITY 56

® • Patients were randomized to receive CPX - 351 at 32 or 64 units/m2 by 90 - minute infusion on days 1, 3, and 5. • The 64 unit/m2 dose level observed 3 early deaths and 2 CR among 9 patients. • Based on a comparison with historic controls who principally received 7+3 or high - dose cytarabine - based induction, the number of expected deaths was 3, the same as the number observed. • The 32 unit/m2 dose level continues to enroll and now has 15 patients. • This study is the first of a series of studies that will attempt to define how best to use CPX - 351 in difficult to treat populations of AML/MDS patients. 57 CPX - 351: HR - MDS OR AML PATIENTS AT HIGH - RISK OF TREATMENT - RELATED MORTALITY

® Question & Answer Session 58

® Closing Remarks SCOTT JACKSON CHIEF EXECUTIVE OFFICER 59

® CREATING SHAREHOLDER VALUE • Execution of the Phase 3 study and preparation for regulatory submission and commercialization • Celator to commercialize CPX - 351 in the US • Seek ex - US partner • Continue to evaluate the potential of CPX - 351 in other patient populations (e.g. AML and other hematological malignancies) • Investigator - initiated studies (IIS) • Oncology cooperative groups • Demonstrate the potential of CombiPlex with molecularly targeted therapies • Establish proof of principle to support value of R&D collaborations • Generate product candidates for internal development • Leverage the work with combinations to generate novel single - agent formulations 60
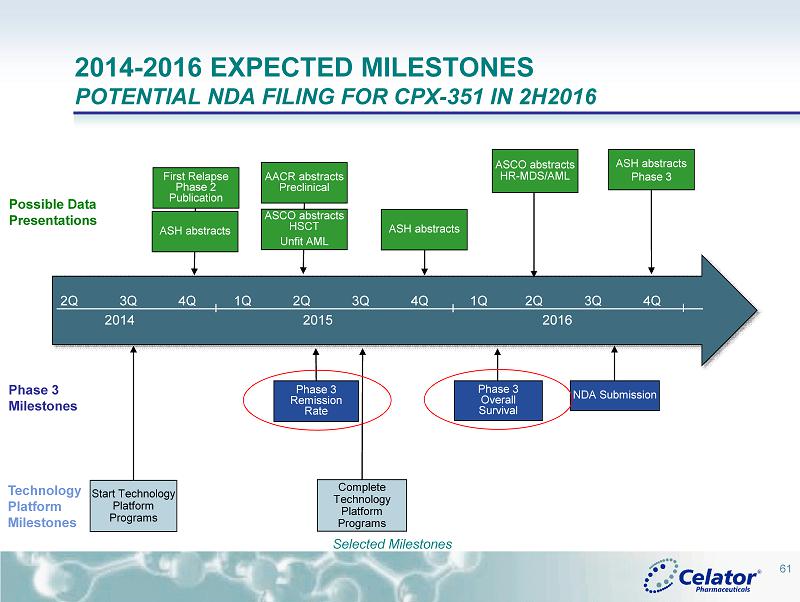
® 2014 - 2016 EXPECTED MILESTONES POTENTIAL NDA FILING FOR CPX - 351 IN 2H2016 Phase 3 Remission Rate Start Technology Platform Programs Phase 3 Overall Survival Complete Technology Platform Programs NDA Submission 2Q 3Q 4Q 1Q 2Q 3Q 4Q 1Q 2Q 3Q 4Q 2014 2015 2016 First Relapse Phase 2 Publication ASH abstracts AACR abstracts Preclinical ASCO abstracts HSCT Unfit AML ASCO abstracts HR - MDS/AML ASH abstracts Phase 3 ASH abstracts 61 Selected Milestones Phase 3 Milestones Possible Data Presentations Technology Platform Milestones

® 62 THANK YOU!!!
