Attached files
| file | filename |
|---|---|
| EX-32.2 - EXHIBIT 32.2 - Edesa Biotech, Inc. | v392175_ex32-2.htm |
| EX-10.17 - EXHIBIT 10.17 - Edesa Biotech, Inc. | v392175_ex10-17.htm |
| EX-10.12 - EXHIBIT 10.12 - Edesa Biotech, Inc. | v392175_ex10-12.htm |
| EX-31.2 - EXHIBIT 31.2 - Edesa Biotech, Inc. | v392175_ex31-2.htm |
| EX-21 - EXHIBIT 21 - Edesa Biotech, Inc. | v392175_ex21.htm |
| EX-31.1 - EXHIBIT 31.1 - Edesa Biotech, Inc. | v392175_ex31-1.htm |
| EX-10.14 - EXHIBIT 10.14 - Edesa Biotech, Inc. | v392175_ex10-14.htm |
| EX-10.11 - EXHIBIT 10.11 - Edesa Biotech, Inc. | v392175_ex10-11.htm |
| EX-32.1 - EXHIBIT 32.1 - Edesa Biotech, Inc. | v392175_ex32-1.htm |
| EX-10.15 - EXHIBIT 10.15 - Edesa Biotech, Inc. | v392175_ex10-15.htm |
| EX-10.16 - EXHIBIT 10.16 - Edesa Biotech, Inc. | v392175_ex10-16.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
| R | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended August 31, 2014
OR
| £ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to
Commission file number: 000-54598
Stellar Biotechnologies, Inc.
(Exact name of registrant as specified in its charter)
| British Columbia, Canada | N/A | |
|
(State or other jurisdiction of incorporation or organization) |
(I.R.S. Employer Identification No.) | |
|
332 E. Scott Street Port Hueneme, California |
93041 | |
| (Address of principal executive offices) | (Zip Code) |
Registrant’s telephone number, including area code: (805) 488-2800
Securities registered pursuant to Section 12(b) of the Act: None
Securities registered pursuant to Section 12(g) of the Act:
Common Shares, without par value
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ¨ No x
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ¨ No x
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes x No ¨
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes ¨ No ¨
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (§229.405 of this chapter) is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. x
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act.
| Large Accelerated Filer ¨ | Accelerated Filer x |
| Non-Accelerated Filer ¨ | Smaller Reporting Company ¨ |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes ¨ No x
The aggregate market value of the voting and non-voting common shares held by non-affiliates of the registrant, computed by reference to the closing price of the registrant’s common shares on the OTCQB marketplace as of February 28, 2014, the last business day of the registrant’s most recently completed second fiscal quarter, was approximately $85,585,181, based on 57,827,825 shares at $1.48 per share.
As of November 1, 2014, the registrant had 79,121,650 common shares issued and outstanding.
Note: As of the last day of the registrant’s most recently completed second fiscal quarter, the registrant determined that it no longer qualified as a “foreign private issuer” under Rule 3b-4 under the Securities Exchange Act of 1934, as amended (the “Exchange Act”). As of September 1, 2014, the beginning of the registrant’s fiscal year, the registrant is now complying with Exchange Act reporting requirements applicable to a U.S. domestic issuer.
Documents incorporated by reference: NONE
| i |
Stellar Biotechnologies, Inc.
Annual Report ON FORM 10-K
Fiscal Year Ended August 31, 2014
Table of Contents
| ii |
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This Annual Report on Form 10-K contains certain forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended (the “Securities Act”) and Section 21E of the Securities Exchange Act of 1934, as amended (the “Exchange Act”) and, as such, may involve known and unknown risks, uncertainties and assumptions. Forward-looking statements are based upon our current expectations, speak only as of the date hereof, and are subject to change. Forward-looking statements are those that predict or describe future events or trends and that do not relate solely to historical matters. You can generally identify forward-looking statements as those statements containing the words “anticipate,” “believe,” “plan,” “estimate,” “expect,” “intend,” “may,” “will,” “would,” “could,” “should,” “might,” “potential,” “continue” or other similar expressions. You should not rely on our forward-looking statements as they are not a guarantee of future performance. There can be no assurance that forward-looking statements will prove to be accurate because the matters they describe are subject to assumptions, known and unknown risks, uncertainties and other unpredictable factors, many of which are beyond our control. Our actual results could differ materially and adversely from those expressed in any forward-looking statements as a result of various factors, some of which are listed under the “Risk Factors” section of this Annual Report. Risks and uncertainties include, among others, the availability of funds and resources to pursue our research and development projects, the successful and timely completion of preclinical or clinical studies by third parties in which our products are utilized, the degree of market acceptance for our products or for other companies’ products in which our products are components, our ability to take advantage of business opportunities in the pharmaceutical industry, changes in our strategy or development plans, our ability to protect our intellectual property, uncertainties related to governmental regulations and regulatory processes, the volatility of our common share price, the effect of competition, the effect of technological changes, reliance on key personnel, and general changes in economic or business conditions. Except as required by law, we undertake no obligation to update forward-looking statements.
As used in this Annual Report on Form 10-K, “Stellar,” “the Company,” “we,” “us,” and “our” and refer to Stellar Biotechnologies, Inc. and our consolidated subsidiaries, except where the context otherwise requires.
EMERGING GROWTH COMPANY STATUS
We are an “emerging growth company,” as defined in the Jumpstart Our Business Startups Act of 2012 (“JOBS Act”), and as a result, we may take advantage of certain exemptions from various reporting requirements that are applicable to other public companies that are not “emerging growth companies.” We will remain an “emerging growth company” for up to five years, or until the earliest of the earliest to occur of: (i) the last day of the fiscal year in which we have more than $1.0 billion in annual revenues; (ii) the date we are deemed a “large accelerated filer” as defined in the Exchange Act, with at least $700 million of equity securities held by non-affiliates; or (iii) the issuance, in any three-year period, by us of more than $1.0 billion in non-convertible debt securities. We may choose to take advantage of some but not all of these reduced reporting burdens. For so long as we remain an emerging growth company, we will not be required to:
| · | have an auditor report on our internal control over financial reporting pursuant to Section 404(b) of Sarbanes-Oxley Act of 2002; |
| · | comply with any requirement that may be adopted by the Public Company Accounting Oversight Board (“PCAOB”), regarding mandatory audit firm rotation or a supplement to the auditor’s report providing additional information about the audit and the financial statements (auditor discussion and analysis); |
| · | submit certain executive compensation matters to shareholders advisory votes pursuant to the “say on frequency” and “say on pay” provisions (requiring a non-binding shareholder vote to approve compensation of certain executive officers) and the “say on golden parachute” provisions (requiring a non-binding shareholder vote to approve golden parachute arrangements for certain executive officers in connection with mergers and certain other business combinations) of the Dodd-Frank Wall Street Reform and Consumer Protection Act of 2010; and |
| · | include detailed compensation discussion and analysis in our filings under the Exchange Act, and instead may provide a reduced level of disclosure concerning executive compensation. |
In addition, Section 107 of the JOBS Act also provides that an “emerging growth company” can take advantage of the extended transition period provided in Section 7(a)(2)(B) of the Securities Act for complying with new or revised accounting standards. In other words, an “emerging growth company” can delay the adoption of certain accounting standards until those standards would otherwise apply to private companies. We have elected not to take advantage of the extended transition period for complying with new or revised accounting standards.
| iii |
| Item 1. | BUSINESS. |
Business Overview
Stellar Biotechnologies, Inc. (“Stellar,” the “Company,” “we,” “our” and “us”) is a biotechnology company engaged in the aquaculture, research and development, manufacture and commercialization of Keyhole Limpet Hemocyanin (“KLH”) protein. KLH is a high molecular weight, immune-stimulating protein with an extensive history (over 40 years) of safe and effective use in immunological applications.
KLH can be used as an active pharmaceutical ingredient (“API”) and combined with a disease-targeting agent to create immunotherapies targeting cancer, immune disorders, Alzheimer’s disease, and inflammatory diseases, or it can be used as a finished, injectable product in the immunodiagnostic market for measuring immune response in patients and research settings. Our mission is to become the world leader in the sustainable manufacture of KLH and use our unique, proprietary methods and intellectual property to serve the growing demand for KLH in immunotherapeutic and immunodiagnostic markets.
Immunotherapies (also known as therapeutic vaccines) involve using the body’s own immune system to target and treat disease. Immunodiagnostics involve assessing the body’s immune status in relation to the effects of a new drug, a disease, or the environment. Our KLH products can be used to stimulate the immune system in both applications.
KLH is refined from the hemolymph of a relatively scarce ocean mollusk, the Giant Keyhole Limpet (Megathura crenulata), which is native only to the rocky Pacific Ocean waters off Southern California and Baja California, Mexico. Based upon our specialized knowledge of aquaculture science and KLH, we have built unique aquaculture, laboratory, and production facilities in Port Hueneme, California, and developed sustainable and commercially viable manufacturing processes to produce KLH using Current Good Manufacturing Practices (“cGMP” or “GMP”). We contract with contract manufacturing organizations (“CMOs”) and contract testing organizations (“CTOs”) for certain steps of the cGMP processing and quality control testing.
Using our proprietary intellectual property and methods related to KLH manufacture, including a patented non-lethal protein extraction process, we are able to raise and sustain commercial-scale colonies of Giant Keyhole Limpets, and extract and purify high quality KLH protein, without relying solely on ocean-harvest techniques. We believe we are positioning our Company to meet the anticipated long-term demand within the pharmaceutical industry for GMP grade KLH by providing a sustainable source for its scalable, controlled, and traceable production.
Our core business is the manufacture and supply of KLH protein under the brand “Stellar KLH™.” We raise Giant Keyhole Limpets in our own land-based aquaculture facilities, extract KLH protein using non-lethal methods, and manufacture and sell GMP and research grade Stellar KLH™ products to third parties. Our products include Stellar KLH™ protein in various grades, formulations and configurations for both preclinical and clinical applications, and certain KLH-based in vitro diagnostic kits for preclinical use. Stellar KLH™ protein can be used for therapeutic vaccine conjugation, as a carrier molecule (API) in immunotherapies under development, and as an immune stimulant in immunotoxicology applications. Our customers and partners include multinational biotechnology and pharmaceutical companies, academic institutions, clinical research organizations, and research centers.
We believe we are the leader in the sustainable manufacture of GMP grade KLH because of our expanding intellectual property portfolio, our achievements in aquaculture science, our KLH production capacity, and our proprietary KLH sustainable manufacturing know-how. The complexity and versatility of the KLH molecule and the growing need for commercial-scale GMP grade KLH provide numerous commercial opportunities for us.
| Page 1 |
Our strategic objectives are to:
| · | Expand our Stellar KLH™ technology portfolio through ongoing research and development and selective acquisitions, while maintaining a strong balance sheet with careful resource management; |
| · | Pursue opportunities for commercial growth that build on our strengths and core competencies in KLH development and manufacture; and |
| · | Identify strategic pathways that leverage our Stellar KLH™ products and expertise into immunotherapy and immunodiagnostics solutions. |
We operate through our wholly-owned subsidiary, Stellar Biotechnologies, Inc., a California corporation which was organized September 9, 1999. We acquired the subsidiary on April 12, 2010 through a reverse merger and began trading on the TSX Venture Exchange under the symbol “KLH” on April 19, 2010. We were originally incorporated in Canada on June 12, 2007 under the name China Growth Capital, Inc. and subsequently changed our name to CAG Capital, Inc. on April 15, 2008. We began trading on the TSX Venture Exchange as a Canadian “capital pool company” on August 29, 2008, and became a British Columbia corporation on November 25, 2009. Our reverse merger in April 2010 constituted our “qualifying transaction” under Canadian law, at which time we changed our name to Stellar Biotechnologies, Inc. As of January 15, 2013, we are quoted on the U.S. OTCQB Marketplace Exchange under the symbol, “SBOTF.” Our executive offices are located at 332 East Scott Street, Port Hueneme, California 93041. Our phone number is (805) 488-2800. Our website address is http://www.stellarbiotechnologies.com. The contents of our website are not incorporated by reference into this report and you should not consider information provided on our website to be part of this report.
Keyhole Limpet Hemocyanin (KLH)
KLH is a safe, potent, immune-stimulating protein. As an API, KLH is an effective and safe carrier molecule for conjugation to vaccine antigens that are used to promote the generation of antibody and cell-mediated immune responses against targeted disease indications such as cancer, immune disorders, Alzheimer’s, and inflammatory diseases. However, the small haptens (partial antigens) and vaccine antigens used to target these diseases are not usually immunogenic enough to awaken the immune system and therefore, require a carrier molecule or adjuvant in order to be effective. The combination of an antigen against specific pathogenic targets, such as tumors, and over-expressed proteins, conjugated to the immunogenic KLH molecule, is the basis for a promising new class of drugs in development known as active immunotherapies or therapeutic vaccines. Unlike preventative vaccines, active immunotherapies are designed to stimulate the body’s own immune system to generate an immune response to target and attack an existing disease or condition. We believe immunotherapies are, and will continue to be, one of the fastest-growing sectors of pharmaceutical research and development.
Biotechnology and pharmaceutical companies currently have KLH-based active immunotherapies and therapeutic vaccines in clinical development for rheumatoid arthritis, Crohn’s disease, systemic lupus erythematous, Alzheimer’s disease, lymphoma, metastatic breast cancer, and various other cancers and diseases.
As a finished injectable product, KLH has been used extensively by pharmaceutical companies and researchers as a safe, immune-stimulating antigen in drug-screening, drug immunotoxicology, and assessment of immune status. KLH is a standard immunogen in T-Cell Dependent Antibody Response (TDAR), a functional assay which is widely recognized as a standard test for monitoring the effects of drugs on the immune system.
KLH is a very large, high molecular weight, oxygen-carrying glycoprotein made of millions of atoms. There are two KLH subunit forms, KLH1 and KLH2, each composed of seven or eight functional units, with each functional unit having an oxygen binding site of two copper atoms. KLH has a distinctive opalescent blue color which is the result of its copper-containing properties. The KLH molecular structure offers numerous sites for conjugation, and can generate multiple product configurations. KLH is a highly effective T-cell dependent carrier protein that induces immune responses via antigen presenting cells. Both the high molecular weight native molecule and subunit forms of KLH are excellent immune stimulants. While KLH is potently immunogenic, it does not cause an adverse immune response in humans. Because of its large size, immune-stimulating properties, numerous sites for conjugation, and safety profile, KLH is sought after by researchers and product developers as a vaccine carrier protein. However, due to its exceptional size and complexity, KLH has not been reproduced synthetically and is more efficiently prepared by purification from the natural hemolymph of the Giant Keyhole Limpet.
| Page 2 |
KLH protein is derived only from the hemolymph of the Giant Keyhole Limpet (Megathura crenulata), which is native only to a limited stretch of the Pacific Ocean coastline along Southern California and Baja California, Mexico. Its natural habitat is the rocky, shallow waters below the low tide line. Historically, suppliers other than us have obtained KLH protein directly from wild and sensitive populations of Giant Keyhole Limpet, or have utilized lethal production processes. We believe that, based on publicly available information and reports, commercial supplies of KLH differ widely in their source, traceability, purity, form, and preparation, as well as in immunogenicity.
However, we believe highly-specialized aquaculture manufacturing methods, like the methods we practice, protect the KLH molecule’s source species and preserve sustainable, scalable supplies of quality KLH protein. The concept of sustainability involves sound, responsible management of environmental resources and, especially where biological systems are concerned, includes protecting native species so that the species thrive and remain diverse and productive over time. Further, we believe that environmentally sound methods associated with professional and specialized aquaculture can minimize variability in KLH products and assure full traceability to their biological source.
Our Stellar KLH™ Technology
We have committed the past 15 years to the advancement of aquaculture science and KLH production methods, specifically focused on protection of the Giant Keyhole Limpet and the non-harmful extraction of KLH protein. We believe our methods will preserve a sustainable supply of GMP grade KLH and meet pharmaceutical industry standards for immune response, consistency, purity, and traceability while protecting the natural source species.
We have developed considerable intellectual property related to KLH manufacture and the environmental protection of the Giant Keyhole Limpet including, but not limited to, patents, patent applications and trade secrets related to specialized aquaculture systems and technologies; spawning, selection and maintenance of the species; non-lethal KLH protein extraction methods; and the processing, purification and production of KLH formulations. This core technology is the basis for our belief that we lead the industry in sustainable manufacture of KLH.
Our Aquaculture Technology & Manufacturing
Our aquaculture technology involves methods we developed and optimized to control the reproduction and growth of the Giant Keyhole Limpet including, but not limited to, culture systems, nutritional requirements, and the recirculation of seawater. We achieved a significant milestone in aquaculture science by developing the capability to sustain the complete life cycle of the Giant Keyhole Limpet. Using our proprietary methods, we can support the marine mollusk from embryo to protein-producing adult. Other KLH suppliers are reliant on scarce, wild populations of limpets. We believe we have the only demonstrated aquaculture system where multiple generations of the Giant Keyhole Limpet are spawned, grown and sustained within a land-based facility, for the purpose of commercial KLH production.
The aquaculture cycle to raise Giant Keyhole Limpets from fertilized eggs to maturity for KLH production is five years, with multiple complex larval and juvenile stages. KLH can be extracted from mature limpets several times per year and, if properly maintained, the average extracted quantity of KLH per year per limpet is predictable and useful in estimating targets for production planning and optimizing the use of the hemolymph. The hemolymph is extracted in a non-harmful manner utilizing our patented methods. Once extracted, the hemolymph is processed through our proprietary methods, which are protected as trade secrets.
We contract with CMOs and CTOs for certain steps of cGMP processing and quality control testing. The services currently performed by these contract vendors include, but may not be limited to, sterile fill/finish and release testing.
The hold-time assigned to KLH pharmaceutical intermediate produced by us is 90 days. Stability studies on our purified subunit KLH (KLH 20MV) support a shelf life of 36 months and stability studies are currently ongoing for our HMW KLH (KLH 01NV). KLH pharmaceutical intermediate is normally produced “just in time” to fill customer orders or to meet our requirements for production of fully purified KLH formulations.
| Page 3 |
We currently maintain a production inventory of limpets sufficient for an annual capacity of a minimum of 1,500 grams/year of KLH pharmaceutical intermediate, with a projected maximum of 2,000 grams/year. Given sufficient funding to continue scale-up, our projected KLH production capacity is 4-5 kilograms per year within the next four years, and up to 20 kilograms in five to seven years. We have developed a five-year plan to incrementally increase hatchery production of limpets, which will thereby increase our KLH production, in order to meet our customers’ forecasts for their anticipated multi-kilogram KLH requirements during product commercialization and future forecasted KLH requirements.
As a result of these operational capabilities, we believe we will be able to supply GMP grade KLH in commercial quantities to meet the anticipated long-term demand within the pharmaceutical industry, while protecting the natural source species. We base these beliefs on our intellectual property, achievements in aquaculture science, KLH production capacity, KLH sustainable manufacturing know-how, and survey data used to estimate population of Giant Keyhole Limpets in the wild.
Our Facilities
We maintain research and manufacturing facilities directly along the Pacific Ocean with dedicated, land-based aquaculture operations in Port Hueneme, California. We have approximately 37,000 square feet of leased aquaculture, manufacturing, and laboratory space. In 2011, we completed a major expansion of our facilities, incorporating significant advances in technology developed by us with support from monetary grants from the National Science Foundation. These advancements included systems for the intensive propagation of the complex larval stages. We believe our waterfront location is a proprietary asset that allows our marine scientists to work in close proximity to naturally resident Giant Keyhole Limpet colonies, and to be at the forefront in developing protective measures and environmentally sound practices for KLH production.
We have developed the capability to support the complete life cycle of the Giant Keyhole Limpet and support multiple generations of limpets grown entirely within our land-based facility. Our aquaculture facility includes, among other specialized infrastructure, systems for spawning, larval development, and maturation of limpets, a fully permitted seawater supply system, recirculating seawater supply systems, environmental controls and regulated seawater return to the ocean. Our facility currently includes 18 production tanks plus 400 individual limpet production modules in two independent closed recirculating aquaculture production systems. Each closed recirculating system is equipped with temperature controlled seawater distribution, filtration and treatment equipment. The facility also contains a fabrication shop for production of equipment and culture apparatus.
Our aquaculture operations were specially developed in the late 1990s for production and research on gastropod mollusks, have been in near continuous operation since that time, and have since been expanded significantly by us for the specialized purpose of conducting the intensive steps required to support the complete life cycle of the Giant Keyhole Limpet and for the commercial production of KLH protein.
Our Stellar KLH™ Products
Our products include Stellar KLH™ protein in various grades, formulations and configurations for both preclinical and clinical applications, and certain KLH-based in vitro diagnostic kits for preclinical use. Stellar KLH™ protein can be used as an API (for therapeutic vaccine conjugation or as a carrier molecule) in immunotherapies under development such as for cancer, immune disorders, Alzheimer’s disease, and inflammatory diseases. Stellar KLH™ protein can also be used as a finished, injectable immune stimulant in immunotoxicology applications. Our customers and partners include multinational biotechnology and pharmaceutical companies, academic institutions, clinical research organizations, and research centers.
We believe our Stellar KLH™ products have advantages over other sources of KLH because:
| · | We are able to produce a product that is fully traceable and controlled from native source to finished product, which are important considerations for our pharmaceutical partners. |
| Page 4 |
| · | Due to the known origin of material and continuity of data, we believe we are able to create a more consistent, high quality, immunogenic product than other proteins in the market. |
| · | Our product is supplied in a stabilized, liquid formulation, rather than freeze-dried, and has low endotoxin and bioburden levels. |
| · | Our viral removal technology in the KLH manufacturing process provides additional assurance of viral clearance. |
| · | Our KLH protein is produced using environmentally sound, sustainable practices. |
| · | Using our proprietary methods, we are able to offer a long-term scalable supply of GMP grade KLH for commercial use. |
Our Stellar KLH™ products include high molecular weight (HMW) and subunit KLH protein in various grades, formulations and configurations, as well as certain in vitro diagnostic kits for preclinical use. Our product offerings and target applications include:
| · | Stellar KLH™ Protein for Vaccine Conjugation and as Carrier Molecule in Immunotherapies: The small haptens (partial antigens) and vaccine antigens used to develop immunotherapies are not usually immunogenic enough and require the aid of a carrier protein to stimulate an immune response. Our offerings include GMP grade subunit and GMP grade HMW KLH for use as a carrier protein in research and therapeutic vaccine applications. |
| · | Stellar KLH™ Protein and Test Kits for Immune Function Testing: KLH plays a vital role in research and clinical studies as an antigen for assessing immune function and in immunotoxicology studies for monitoring the immunosuppressive effects of drug candidates. Our Stellar KLH™ Protein can be used as an immune stimulant for T-Cell Dependent Antibody Response (TDAR) testing. We also offer Stellar KLH™ ELISA assay test kits for the detection of KLH antibodies in preclinical research settings. We launched a line of six Stellar KLH™ ELISA test kits in April 2012. |
| · | Custom KLH formulations, multivalent adjuvants, conjugations and fill finishes for preclinical research and drug development applications. |
We currently have limited revenue from sales of our Stellar KLH™ products. Selling prices for Stellar KLH™ protein vary depending on the purity, grade, preparation, and packaging configuration. Product sales are highly dependent upon the rate of development and clinical trials of the active immunotherapies and other technologies being developed by third party customers, which utilize our products. The advancement and commercial success of these third party products is dependent upon many factors, including available capital, trial recruitment, and regulatory review. Revenue from these customers is highly variable, but historically is not subject to seasonal fluctuations.
Revenues from the sale of Stellar KLH™ products were $143,553 in fiscal 2014, $76,055 in fiscal 2013, and $131,825 in fiscal 2012. Contract services revenues related to Stellar KLH™ products were $192,000 in fiscal 2014, $60,000 in fiscal 2013, and $60,000 in fiscal 2012. The geographic breakdown of revenues in fiscal 2014 was 41% Europe, 40% Asia, 14% U.S., and 6% Canada; fiscal 2013 was 84% Europe, 12% U.S., 3% South America and 1% Canada; and fiscal 2012 was 42% Europe and 58% U.S.
Customers
We believe we are one of only three companies known to manufacture starting material (raw hemolymph) for GMP grade KLH products. Of these three companies, we believe we are the only company that offers GMP grade KLH supported by fully traceable manufacturing methods. We primarily market and distribute our products directly to biotechnology and pharmaceutical companies, academic institutions, clinical research organizations, and research centers. Products are shipped to our customers from our facilities in Port Hueneme, California using a common carrier chosen by the customer. The geographic markets of our potential customers are principally Europe, the United States and Asia.
| Page 5 |
The customers that represent 10% or more of our total consolidated revenue in fiscal 2014, 2013 and 2012 are as follows:
| Customer | 2014 | 2013 | 2012 | |||||||||
| Amaran Biotechnology, Inc. | 35 | % | - | - | ||||||||
| Neovacs SA | 30 | % | 12 | % | 25 | % | ||||||
| SAFC, a division of Sigma Aldrich | - | - | 35 | % | ||||||||
Contracts, Supply Agreements, Collaboration Agreements, and Licensing
We have, and intend to continue to enter into, agreements with third parties that will allow us to supply Stellar KLH™ in exchange for fees, revenues, or royalties. Supply agreements generally involve a customer’s commitment to purchase our Stellar KLH™ for use as an API in the customer’s own immunotherapy products or as a finished product in their development programs. To date, our Stellar KLH™ protein has been used in research and development, preclinical, and clinical phases but has not yet been used in any commercialized and marketed products.
Supply Agreements with Neovacs SA
We entered into two supply agreements with Neovacs SA of Paris, France in 2008 for the use of Stellar KLH™ in development and manufacture of its active immunotherapies. Neovacs has three Kinoid therapeutic vaccine drugs in clinical trials which use Stellar KLH™ as the carrier molecule to stimulate an immune response for the treatment of Lupus, Crohn’s disease, and rheumatoid arthritis. The supply agreements also provide for Neovacs to pay for expenses related to a dedicated colony of limpets.
Collaboration Agreement and Exclusive Licensing Rights with Bayer Innovation GmbH
We entered into a research collaboration agreement with Bayer Innovation GmbH (“Bayer”) in December 2010, where we agreed to allow Bayer access to our information on Stellar KLH™, including manufacturing methods and analytical data, in order to demonstrate the feasibility of improving our process yields. When the research collaboration agreement terminated according to its terms in August 2011, we acquired an exclusive, worldwide sub-licensable and royalty-free license to the technology we developed under collaboration with Bayer for the improved production method. The license included a carve-out by Bayer to use the technology in certain non-Hodgkin Lymphoma active immunotherapies, but we may exclusively commercialize the technology in other fields. We paid Bayer $200,000 in 2011 for the licensing rights, which are jointly owned by Bayer and us. We assessed the licensing rights for impairment and wrote off the unamortized balance in fiscal 2014.
Manufacturing and Supply Agreement with Life Diagnostics
In October 2011, we entered into an exclusive manufacturing and supply agreement with Life Diagnostics, Inc., where it agreed to utilize Stellar KLH™ to develop and manufacture Stellar KLH™ brand ELISA test kits for the detection of anti-KLH antibodies in uses by the preclinical immunotoxicity and immunology markets. The agreement also required Life Diagnostics to supply us with Stellar KLH™ brand ELISA test kits at agreed upon prices.
License Agreement with University of Guelph
In July 2013, we acquired the exclusive, worldwide license to certain patented technology for the development of human immunotherapies against Clostridium difficile infection (“C. diff”), a highly contagious bacteria spread by human contact, from the University of Guelph, Ontario, Canada (the “Guelph License”). Under the terms of the Guelph License, we have the exclusive rights to develop, manufacture, and sell active immunotherapies to treat C. diff infection that derive from the technology covered by certain of the University’s international patents and patent applications.
| Page 6 |
The Guelph License agreement required an initial, non-refundable license fee of $25,000, which was paid in fiscal 2013, payment of an aggregate of $200,000 in delayed license fees, which were paid in fiscal 2014, and a license fee of $20,000 to be paid annually thereafter, creditable against royalties due, if any. Royalties are payable for a percentage of related net sales, if any. License fees are also payable for a percentage of related non-royalty sublicensing revenue, if any. No royalties have been paid to date.
As additional consideration, we also issued 371,200 common shares and warrants to purchase up to 278,400 of our common shares to the University. The warrants expire on January 23, 2015 and have an exercise price of C$1.25 per share. We reimbursed patent filing costs of approximately $34,000 and $50,000 in fiscal 2014 and 2013, respectively, and will reimburse certain future patent filing, prosecution, and maintenance costs.
We are also required to pay up to an aggregate of $6,020,000 in milestone payments to the University upon achievement of various financing and development targets up to the first regulatory approval. Remaining milestone payments totaling $57,025,000 are related to achievement of sales targets. We are required to provide regular reports to the University regarding product development efforts, and progress toward meeting certain milestones. A financing milestone was met during fiscal 2014 and, accordingly, we made a milestone payment of $100,000 to the University. No milestones were met during fiscal 2013, and there can be no assurance that any of the remaining milestones will be met in the future.
The Guelph License agreement expires when the last valid patent claim licensed under the agreement expires. Prior to that time, the agreement can be terminated by the University upon certain conditions including: (i) our failure to make any payments or submit any reports when due; (ii) our failure to diligently pursue development or commercialization of the product based upon our reports; (iii) our material breach of any provision of the agreement; or (iv) providing a false report. We will have 30 days after written notice from the University to cure the problem prior to termination of the agreement. We can terminate the agreement with three months’ prior written notice to the University.
Collaboration Agreement with Amaran Biotechnology
In December 2013, we entered into a collaboration agreement with Amaran Biotechnology, Inc., a privately-held Taiwanese biopharmaceuticals manufacturer and a beneficial owner of over 5% of our common shares. Amaran designs, develops, and manufactures active immunotherapies, potentially such as OBI-822, the lead immunotherapy product of OBI Pharma, Inc. An active immunotherapy uses a patient’s own immune system to recognize and mount an attack against the targeted tumor cells. The primary purpose of our collaboration is to develop and evaluate methods for Amaran’s potential manufacture of the OBI-822 active immunotherapy using our GMP grade Stellar KLH™.
Under the terms of the agreement, which were negotiated at arms’ length, we are responsible for the production and delivery of GMP grade KLH for evaluation as a potential carrier molecule in the OBI-822 active immunotherapy. We are also responsible for method development, product formulation, and process qualification for certain KLH reference standards. Amaran is responsible for development objectives and product specifications.
The agreement also provides for Amaran to pay us fees for certain expenses and costs associated with the collaboration. Subject to certain conditions and timing, the terms of the collaboration also provide for the possible negotiation of a commercial supply agreement for Stellar KLH™ in the future. However, there can be no assurance that any such negotiations will lead to successful execution of any further agreements related to this collaboration.
| Page 7 |
Research and Development
We are committed to applying our Stellar KLH™ technology to improve immunotherapy and immunodiagnostics, and to protecting the natural resource for KLH. To that end, we are actively engaged in research and development focused primarily on the aquaculture of the Giant Keyhole Limpet, improvements in KLH protein analysis and manufacturing, and new uses for KLH in immunotherapy and immunodiagnostic applications. These activities involve both internal programs and external collaborations with other biopharmaceutical companies or research organizations.
Our internal research includes, among other activities, continual improvement of methods for the culture and growth of Giant Keyhole Limpet, innovations in aquaculture systems and infrastructure, biophysical and biochemical characterization of the KLH molecule, analytical processes to enhance performance of our products, KLH manufacturing process improvements, new KLH formulations, and early development of potential new KLH-based immunotherapy.
Our external collaborations involve both development and evaluation projects, with a number of biopharmaceutical companies and research institutions, for the use of Stellar KLH™ in their programs. We believe that these collaborations provide for strategic, revenue and clinical opportunities for our future business by extending the commercial use of Stellar KLH™ and furthering our understanding of the KLH molecule.
For the years ended August 31, 2014, 2013 and 2012, research and development expense amounted to $2,458,934, $2,018,554 and $2,634,119, respectively. Of these amounts, approximately 64%, 20% and 6% in fiscal 2014, 2013 and 2012, respectively, related to our preclinical internal research on new uses for KLH; specifically, the preclinical testing of a potential KLH-based immunotherapy approach against C. diff infection. The remaining amounts related primarily to research and development in aquaculture, improvements in analytical, manufacturing, and purification processes, stability testing, and formulation development. None of these expenses were borne by our customers.
Grants
We have historically financed a portion of our operations through the receipt of monetary grants made available through programs funded and administered by various U.S. government entities. These grants offer non-dilutive funding and are intended to foster and promote research and innovation in important scientific and technological projects.
In the most recent three fiscal years, we recognized, through our California subsidiary, an aggregate of $540,222 in grant funding from the National Science Foundation (“NSF”) Small Business Innovation Research (“SBIR”) through the Technology Enhancement for Commercial Partnerships program under Phase II and Phase IIB grants. Our project was entitled “Megathura Crenulata Post Larval Culture - Bottleneck for a Valuable Medical Resource,” and the purpose of the project was to allow for the full implementation of the commercial scale aquaculture systems for KLH production and development of a validated KLH-based immunogenicity assay. Grant revenues were recorded as we fulfilled the grant requirements.
In addition to NSF grants, we also receive grants from time to time for the development of new technology from the National Institutes of Health, National Cancer Institute (“NIH”), the California Technology Investments Program (CalTIP), and Internal Revenue Service (“IRS”) qualifying therapeutic discovery project grants.
| Page 8 |
Competition
We believe we are one of the world leaders in the manufacture of GMP and research grade KLH. We believe we are one of only three companies known to manufacture starting material (raw hemolymph) for GMP grade KLH products and of these three companies, we believe we are the only company that offers GMP grade KLH supported by fully traceable manufacturing methods. We compete directly with Biosyn Corporation, a pharmaceutical and biotechnology company which manufactures KLH starting material and offers clinical and research grade KLH products. We also compete directly with SAFC, a division of Sigma-Aldrich, which offers clinical and research grade KLH products manufactured from its own starting material from ocean harvested limpets and from aquaculture starting material purchased from us. We compete on the basis of: the advantages and disadvantages of Stellar KLHTM as compared to other KLH proteins manufactured by our competitors; our ability to educate the industry about the high quality, sustainable and traceable qualities of Stellar KLHTM; product efficacy; customer service; and the price and demonstrated cost-effectiveness of Stellar KLHTM as compared to our competitors. However, we believe that our proprietary methods and our unique achievement of an aquaculture production system that now supports multiple generations of the Giant Keyhole Limpet will enable us to compete successfully and meet anticipated future demand for KLH in the pharmaceutical industry.
Intellectual Property and License Agreements
We hold important intellectual property related to KLH development and manufacture and to the environmental protection of the Giant Keyhole Limpet including, but not limited to, patents, and trade secrets related to specialized aquaculture systems and technologies; spawning, selection and maintenance of the Giant Keyhole Limpet; non-lethal KLH protein extraction methods; and the processing, purification and production of KLH formulations. Our proprietary methods also include methods for the control of larval development, metamorphosis and maturation of the Giant Keyhole Limpets, which we protect as trade secrets.
Our success depends in part on our ability to obtain and maintain proprietary protection for our product technology and know-how, to operate without infringing proprietary rights of others, and to prevent others from infringing our proprietary rights. We seek to protect our proprietary position by, among other methods, filing, when possible, U.S. and foreign patent applications relating to our technology, inventions and improvements that are important to our business. We also rely on trade secrets, know-how, continuing technological innovation, and in-licensing opportunities to develop and maintain our proprietary position.
As of November 2014, we have obtained patent protection for our non-lethal extraction methods of hemocyanin in the United States and other countries. We hold one issued patent in the United States, U.S. Patent No. 6,852,338, which currently expires in 2023, and covers a two-step method for obtaining hemolymph from a live gastropod mollusk. This U.S. patent was originally granted to our Chief Executive Officer, Frank Oakes, who assigned the patent to the Company in August 2002. Foreign patent counterparts were granted in Canada, France and Germany.
We have a worldwide exclusive license with the University of Guelph to one issued patent in the United States, U.S. Patent No. 8,597,663, which currently expires in 2030, for certain novel cell surface polysaccharides and their chemical structures and vaccine compositions for the treatment, prevention and diagnosis of C. difficile infection. We also have foreign patent counterparts and foreign patent applications and patents claiming priority therefrom in certain jurisdictions outside the United States, including Europe, Australia, Canada, China, Japan and New Zealand.
The scope of any patent protection may not exclude competitors or provide competitive advantages to us, and any of our patents may not be held valid if subsequently challenged, and others may claim rights in or ownership of our patents and proprietary rights. Furthermore, others may develop products similar to our products and may duplicate any of our products or design around our patents.
| Page 9 |
Our trademarks include, but may not be limited to, “Powering and Improving Immunotherapy™”, “Stellar KLH™” and “KLH Site™”. In addition to patents and trademarks, we rely on trade secrets and other intellectual property laws, nondisclosure agreements and other measures to protect our intellectual property rights. We believe that in order to have a competitive advantage, we must develop and maintain the proprietary aspects of our technologies. We require our employees, consultants and advisors to execute confidentiality agreements in connection with their employment, consulting or advisory relationship with us. We also require our employees, and to the extent practicable, our consultants and advisors with whom we expect to work on our products to agree to disclose and assign to us all inventions made in the course of our working relationship with them, while using our intellectual property or which relate to our business. Despite any measures taken to protect our intellectual property, unauthorized parties may attempt to copy aspects of our products or to wrongfully obtain or use information that we regard as proprietary.
Government Regulation
Our operations, including our aquaculture and harvesting activities, as well as production operations, site development, and drug research, development and sales, are subject to regulation at the local, state and federal levels by a number of regulatory agencies including, but not limited to, the U.S. Food and Drug Administration, the U.S. Environmental Protection Agency, the U.S. Fish and Wildlife Service, the U.S. Secretary of the Navy, the Regional Water Quality Control Board Los Angeles Region, the California Department of Fish and Wildlife, the California Coastal Commission, the California Air Pollution Control Board, the County of Ventura, and the City of Port Hueneme.
We are subject to laws and regulations covering clean water and waste discharge, and are required to hold licenses for the aquaculture production and wild harvesting of the Giant Keyhole Limpet. Our aquaculture facility is subject to regulation by the California Department of Fish and Wildlife and the Regional Water Quality Control Board, Los Angeles Region (“Regional Board”). These agencies impose regulations that restrict any activity that could pose a potential risk to the California marine environment including, but not limited to, seawater waste discharge limitations specified in our National Pollution Discharge Elimination Systems (NPDES) permit. In April 2014, we received notification from the Regional Board of its acceptance of our settlement of a claim related to violations of waste discharge requirements for a de minimis amount, as discussed in further detail under Item 3, “Legal Proceedings.” Apart from this incident, we have operated in compliance with all environmental regulations imposed by these agencies since the formation of our California subsidiary in 1999.
New Drug Development
Currently, none of our products are subject to approval as a drug by any regulatory authority. However, many of our strategic partners are utilizing Stellar KLH™ in the development of pharmaceuticals that are subject to the regulatory approval process in various jurisdictions.
We have submitted Type II Drug Substance Master Files, for both our subunit KLH and HMW KLH, to the U.S. Food and Drug Administration (“FDA”) Center for Biologics Evaluation and Research (CBER) and the U.S. FDA Center for Drug Evaluation and Research (CDER). A Master File is a confidential, detailed dossier kept on file at the FDA that contains the proprietary information on the manufacture and safety of a drug component. These files can be used to support the regulatory approval process for customers’ immunotherapy products that use our Stellar KLH™, while allowing us to control access to our manufacturing data.
The regulatory approval process for new drugs under development by our customers is typically long and expensive. Clinical trials that they conduct may not be successful and such products may not receive regulatory approval. Delays by our customers in obtaining or the inability to obtain regulatory approvals for their products which use Stellar Stellar KLH™ will have a direct effect on the demand for our products.
Good Manufacturing Practices
The FDA and other regulatory agencies regulate and inspect equipment, facilities and processes used in the manufacture of pharmaceutical and biologic products prior to approving a product. If, after receiving approval from regulatory agencies, a company makes a material change in manufacturing equipment, location or process, additional regulatory review and approval may be required. All facilities and manufacturing techniques used for the manufacture of our products must comply with applicable regulations governing the production of pharmaceutical products known as Current Good Manufacturing Practices.
| Page 10 |
The FDA and other regulatory agencies also conduct regular, periodic visits to re-inspect equipment, facilities and processes following initial approval of a product. If, as a result of these inspections, it is determined that our equipment, facilities or processes do not comply with applicable regulations and conditions of product approval, regulatory agencies may issue warning or similar letters or may seek civil, criminal, or administrative sanctions against us. To date, we have not been subject to inspection by the FDA or other drug regulatory agency because none of our customers or partners has filed an application in any country for marketing approval of a product encompassing our Stellar KLH™ protein.
Backlog and Renegotiation of Profits
Orders for our products are generally filled on a current basis, and order backlog is not material to our business. In addition, our business is not subject to renegotiation of profits or termination of contracts at the election of a government.
Employees
We currently have 23 employees. All employees, including our executive officers, are based out of our facilities in Port Hueneme, California.
Available Information
Our website is located at www.stellarbiotechnologies.com. We make available on our website, free of charge, copies of our annual reports on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Securities Exchange of 1934, as amended, as soon as reasonably practicable after we electronically file or furnish such materials to the U.S. Securities and Exchange Commission. Our website and the information contained thereon or connected thereto are not intended to be incorporated into this Annual Report on Form 10-K.
| Item 1A. | RISK FACTORS. |
Investing in our common shares involves a high degree of risk. You should carefully consider the risks and uncertainties described below together with all of the other information contained in this Annual Report, including our financial statements and the related notes, before deciding to invest in our common shares. If any of the following risks actually occur, our business, prospects, operating results and financial condition could suffer materially, the trading price of our common shares could decline and you could lose all or part of your investment. The risks and uncertainties described below are not the only ones we face. Additional risks and uncertainties not presently known to us or that we currently believe to be immaterial may also adversely affect our business.
The following discussion of risk factors contains “forward-looking statements,” which may be important to understanding any statement in this Annual Report on Form 10-K or in our other filings and public disclosures. In particular, the following information should be read in conjunction with Item 7 – Management’s Discussion and Analysis of Financial Condition and Results of Operations and Item 8 – Financial Statements and Supplementary Data of this Annual Report on Form 10-K.
Risks Related to Our Business
We have a history of net losses and limited cash flow to sustain our operations.
We currently have limited revenue from product sales of Stellar KLH™, and anticipate our planned research and development expenditures, as well as our general and administrative expenses, will be greater than our revenues for the foreseeable future. We have incurred net losses of ($8,439,523) in fiscal 2014, ($14,495,779) in fiscal 2013 and ($5,529,278) in fiscal 2012, and as of August 31, 2014, we have an accumulated deficit of ($33,620,190) since inception. To date, we have not paid dividends on our common shares and do not anticipate doing so in the foreseeable future. We have historically relied upon the sale of common shares to help fund our operations and meet our obligations. Any future additional equity financing would cause dilution to current shareholders. If we do not have sufficient capital for our operations, management would be forced to reduce or discontinue our activities, which would have a negative effect on our operations and financial condition.
| Page 11 |
We depend heavily on the success and market acceptance of Stellar KLHTM and we may never recoup our investment into its research and development.
We have invested a significant portion of our time and financial resources into the development of Stellar KLHTM. We anticipate that in the near term our ability to generate revenues will depend solely on the commercial success of Stellar KLHTM, which depends upon its market acceptance by purchasers in the pharmaceutical market and the future market demand and medical need for products and research utilizing KLH. The degree of market acceptance of Stellar KLHTM depends on a number of a factors including: the advantages and disadvantages of Stellar KLHTM as compared to other KLH proteins; our ability to educate the industry about the high quality, sustainable and traceable qualities of Stellar KLHTM; product efficacy; customer service; and the price and demonstrated cost-effectiveness of Stellar KLHTM as compared to our competitors.
We may not be able to meet demand for KLH from either ocean harvest or internally raised sources.
We are dependent upon a supply of Giant Keyhole Limpets (Megathura crenulata) for KLH production. The range of the Giant Keyhole Limpet in the wild is limited, and due to the lack of a regulated harvest, the wild stocks of Giant Keyhole Limpets are believed to be declining. If the wild stocks are depleted, and our hatchery and aquaculture operations are unable to produce sufficient supplies of captive Giant Keyhole Limpets to meet demand, it would have a negative effect on our operations and financial condition.
We compete with other companies in KLH production and manufacturing that may have greater resources than we do.
We believe we are one of only three companies of who are manufacturing KLH starting material for GMP grade KLH products; however, there are other companies offering clinical and research grade KLH products. Of these three companies, we believe we are the only company that offers GMP grade KLH supported by fully traceable manufacturing methods. We compete directly with Biosyn Corporation, a pharmaceutical and biotechnology company which manufactures KLH starting material and offers clinical and research grade KLH products. We also compete directly with SAFC, a division of Sigma-Aldrich, which offers clinical and research grade KLH products manufactured from its own starting material from ocean harvested limpets and from aquaculture starting material purchased from us. Our Stellar KLHTM products use KLH as a main component, which is not unique as a component for immunotherapy application, and is similar to KLH-based products produced by other companies. Some of these other companies, both public and private, have greater financial and personnel resources than us, and have greater sales and marketing experience in the industry than us. If they are able to produce and sell KLH products for less than us, it will have a negative effect on our operations and financial position.
We may not be able to manufacture our products in commercial quantities and currently depend on third parties for certain steps in our manufacturing operations, which could prevent us from marketing our products.
We contract with third party vendors (CMOs and CTOs) for certain steps in the manufacture and testing of our products, and may be unable to establish and maintain relationships with qualified manufacturers in order to produce sufficient supplies of our finished products.
We are currently dependent upon a small number of contractors and locations for certain portions of our manufacturing capacity, namely fill/finish of vialed products and release testing. We do not currently have backup manufacturing capacity for some of our key products. If we are unable to retain our current contractors, or are unable to obtain new contractors to provide manufacturing services in a timely manner and on similar terms, it will have a negative effect on our operations. Further, these contract manufacturers and testing organizations provide services to many biotechnology and research companies, and such third party contractors may not provide acceptable quality, quantity or costs required by us. In addition, they may not be able to provide the services required on a schedule acceptable to us. These issues may result in us being unable to manufacture our products in the required quantities or at an acceptable cost, which would have a negative effect on our operations and financial condition.
| Page 12 |
We have been, and expect to continue to be in the future, significantly dependent on collaboration and supply agreements for the development and sales of Stellar KLHTM.
In conducting our research and development and commercialization activities, we currently rely, and expect to continue to rely, on collaboration and supply agreements with third parties, such as contract research organizations, commercial partners, universities, governmental agencies and not-for-profit organizations, for both strategic and financial resources. The inability to secure agreements on acceptable terms, the termination of these relationships, or failure to perform by us or either of these partners, who are subject to regulatory, competitive and other risks, under their respective agreements or arrangements with us, would substantially disrupt or delay our research and development and commercialization activities, including anticipated commercial sales. Any such loss would likely increase our expenses and materially harm our business, financial condition and results of operation.
Our sales in international markets subject us to foreign currency exchange and other risks and costs which could harm our business.
A substantial portion of our revenues are derived from outside the United States; primarily from Europe and Asia. We anticipate that revenues from international customers will continue to represent a substantial portion of our revenues for the foreseeable future. All our revenues are generated in U.S. dollars. However, if the effective price of our products were to increase as a result of fluctuations in foreign currency exchange rates, demand for our products could decline and adversely affect our results of operations and financial condition.
Our customers face uncertainties related to regulatory approval, which could reduce the market for our products.
A primary market for our Stellar KLHTM products is the commercial manufacture and sale of active immunotherapies. The therapeutic drug industry is subject to significant government regulation, and many of the products developed by our customers that utilize our Stellar KLHTM are not yet approved for commercial sale. Before regulatory approvals for the commercial sale of any products is granted, a drug must be demonstrated through preclinical testing and clinical trials to be safe and effective for their intended use in humans. The process to determine safety and efficacy, including clinical trials, is expensive and prolonged. The time necessary to complete these processes and clinical trials, and to submit applications for the regulatory approvals is difficult to predict and is subject to numerous factors, and such clinical trials may not be successful. Larger or later stage clinical trials may not produce the same results as earlier trials. Successful results in clinical trials may not result in regulatory approval, due to certain factors including unacceptable side effects or safety issues. None of our customers or partners has filed an application in any country for marketing approval of a product encompassing our Stellar KLH™ protein. However, even if regulatory approval is granted for any drug or product that utilizes Stellar KLHTM, it will be subject to ongoing regulatory requirements, which include registration, manufacturing, labeling, advertising and promotion, packaging, distribution, record keeping and reporting, and storage. Manufacturing facilities, both those operated by us and by our contractors, would be subject to continual review and inspection, and failure to meet these regulatory requirements can interrupt delay, or shut down these facilities. Previously unknown problems may result in regulatory restrictions on such products, including withdrawal from the marketplace. Delays in obtaining regulatory approvals for products developed by our customers which use Stellar KLHTM, or failure to obtain or maintain regulatory approvals altogether, would have a negative effect on market demand for our Stellar KLHTM products, and have a negative effect on our operations and financial condition.
The inability to protect our intellectual property rights could result in competitive harm to our Company.
Our success and ability to maintain our competitive position depends on our ability to protect our intellectual property, including by obtaining patent protection in the United States and other countries, or through protection of our trade secrets, including unpatented know-how, technology and other proprietary information. When appropriate, we seek to protect our proprietary position by filing patent applications in the United States and abroad. If we are unable to protect our intellectual property, whether by obtaining patents or through trade secret protection, our competitors could develop and commercialize products similar or identical to ours.
| Page 13 |
We may not have adequate remedies for any infringement or funds to take action against those infringing any of our intellectual property rights, or if our trade secrets otherwise become known or independently developed by competitors. There can be no assurance that any current or future patents held, licensed by or applied for by us will be upheld, if challenged, or that the protections afforded will not be circumvented by others. The patent positions of biotechnology and pharmaceutical companies, which often involve licensing agreements, are frequently uncertain and involve complex legal and factual questions. In addition, the coverage claimed in a patent application can be significantly reduced before the patent is issued. Consequently, our patents, patent applications and licensed rights may not provide protection against competitive technologies or may be held invalid if challenged or could be circumvented. If we enter litigation in regards to our business or to protect or enforce our patents, it may involve substantial expenditures and require significant management attention, even if we ultimately prevail.
The patent position of biotechnology companies is generally highly uncertain. The degree of patent protection we require may be unavailable or severely limited in some cases and may not adequately protect our rights, provide sufficient exclusivity, or preserve our competitive advantage. For example:
| · | we might not have been the first to invent or the first to file patent applications on the inventions covered by each of our pending patent applications; |
| · | others may independently develop similar or alternative technologies or duplicate any of our technologies; |
| · | the patents of others may have an adverse effect on our business; |
| · | any patents we obtain or license from others in the future may not encompass commercially viable products, may not provide us with any competitive advantages or may be challenged by third parties; |
| · | any patents we have obtained, will obtain or license from others in the future may not be valid or enforceable; and |
| · | we may not develop additional proprietary technologies that are patentable. |
Patents have a limited lifespan. In the United States, the natural expiration of a utility patent typically is generally 20 years after it is filed. Various extensions may be available; however, the life of a patent, and the protection it affords, is limited.
In addition, some of our technologies are not covered by any patent application and we rely instead on confidentiality agreements and trade secret law to protect such intellectual property rights. We require all of our employees and consultants to sign confidentiality agreements. The agreements also oblige our employees, and to the extent practicable, our consultants, and advisors, to assign to us ideas, developments, discoveries and inventions made by such persons in connection with their work with us. We cannot be sure that these agreements will maintain confidentiality, will prevent disclosure, or will protect our proprietary information or intellectual property, or that others will not independently develop substantially equivalent proprietary information or intellectual property.
The failure of our patents, patent applications, applicable intellectual property law or our confidentiality agreements to protect our intellectual property and other proprietary information, including our trade secrets, could have a material adverse effect on our competitive advantages and on our operations and financial position.
| Page 14 |
Changes in patent law could diminish the value of patents in general, thereby impairing our ability to protect our products and our technologies.
There are numerous recent changes to the U.S. patent laws and proposed changes to the rules of the United States Patent and Trademark Office (“USPTO”) that may have a significant impact on our ability to obtain and enforce intellectual property rights. In particular, the Leahy-Smith America Invents Act (the “Leahy-Smith Act”) was adopted in September 2011. The Leahy-Smith Act includes a number of significant changes to U.S. patent law, including provisions that affect the way patent applications will be prosecuted and may also affect patent litigation. Under the Leahy-Smith Act, the United States transitioned from a “first-to-invent” system to a “first-inventor-to-file” system for patent applications filed on or after March 16, 2013. With respect to patent applications filed on or after March 16, 2013, if we are the first to invent but not the first to file a patent application, we may not be able to fully protect our intellectual property rights and may be found to have violated the intellectual property rights of others if we continue to operate in the absence of a patent issued to us. Many of the substantive changes to patent law associated with the Leahy-Smith Act have recently become effective. Accordingly, it is not clear what, if any, impact the Leahy-Smith Act will have on the operation of our business. However, the Leahy-Smith Act and its implementation could increase the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of any patents that issue, all of which could have a material adverse effect on our business and financial condition.
In addition, patent reform legislation may pass in the future that could lead to additional uncertainties and increased costs surrounding the prosecution, enforcement, and defense of patent applications and any patents we may obtain. Furthermore, the U.S. Supreme Court and the U.S. Court of Appeals for the Federal Circuit have made, and will likely continue to make, changes in how the patent laws of the United States are interpreted. Similarly, foreign courts have made, and will likely continue to make, changes in how the patent laws in their respective jurisdictions are interpreted. We cannot predict future changes in the interpretation of patent laws or changes to patent laws that might be enacted into law by United States and foreign legislative bodies. Those changes may materially affect our patents and patent applications or any patents we may obtain and our ability to obtain and enforce or defend additional patent protection in the future.
We may not be able to adequately protect our intellectual property rights throughout the world.
Filing, prosecuting and defending patents on our products and technologies in all countries throughout the world would be prohibitively expensive. The requirements for patentability may differ in certain countries, particularly developing countries, and the breadth of patent claims allowed can be inconsistent. In addition, the laws of some foreign countries may not protect our intellectual property rights to the same extent as laws in the United States. Consequently, we may not be able to prevent third parties from practicing our inventions in all countries outside the United States. Competitors may use our technologies in jurisdictions where we have not obtained patent protection to develop their own products and, further, may export otherwise infringing products to territories where we have patent protection, but enforcement on infringing activities is inadequate.
We seek to protect our proprietary position by, among other methods, filing, when possible, U.S. and foreign patent applications relating to our technology, inventions and improvements that are important to our business. We have obtained patent protection for our non-lethal extraction methods of hemocyanin in the United States and other countries. We also rely on trade secrets, know-how, continuing technological innovation, and in-licensing opportunities to develop and maintain our proprietary position.
We plan to file other international patent applications directed to patentable features of our products and technologies from time to time. If patent rights are obtained in foreign jurisdictions, proceedings to enforce such rights could result in substantial costs and divert our efforts and attention from other aspects of our business, could put our patents at risk of being invalidated or interpreted narrowly, could put our pending patent applications at risk of not issuing, and could provoke third parties to assert claims against us. We may not prevail in any lawsuits that we initiate and the damages or other remedies awarded, if any, may not be commercially meaningful. Thus, we may not be able to stop a competitor from marketing and selling in foreign countries products that are the same as or similar to our product.
| Page 15 |
We may become involved in lawsuits to protect or enforce our patents and patent applications, any patents that may be issued to us or other intellectual property, which could be expensive, time consuming and unsuccessful.
Competitors may infringe our patents or patent applications, or other of our intellectual property. To counter infringement or unauthorized use, we may be required to file infringement or misappropriation claims, which can be expensive and time consuming. Any claims we assert against perceived infringers could provoke these parties to assert counterclaims against us alleging that we infringe their patents or claiming that our patents are invalid or unenforceable. Grounds for a validity challenge could be an alleged failure to meet any of several statutory requirements, including lack of novelty, obviousness, non-enablement or lack of statutory subject matter. Grounds for an unenforceability assertion could be an allegation that someone connected with prosecution of the patent withheld relevant material information from the USPTO, or made a materially misleading statement, during prosecution. Third parties may also raise similar validity claims before the USPTO in post-grant proceedings such as ex parte reexaminations, inter partes review, or post-grant review, or oppositions or similar proceedings outside the United States, in parallel with litigation or even outside the context of litigation. The outcome following legal assertions of invalidity and unenforceability is unpredictable. We cannot be certain that there is no invalidating prior art, of which we and the patent examiner were unaware during prosecution. For any patents and patent applications we may license, we may have limited or no right to participate in the defense of any such patents against challenge by a third party. If a defendant were to prevail on a legal assertion of invalidity or unenforceability, we would lose at least part, and perhaps all, of any future patent protection on our products. Such a loss of patent protection could harm our business. In addition, in a patent infringement proceeding, a court may decide that our patent applications or patents, if issued, are invalid or unenforceable, in whole or in part, construe the patent’s claims narrowly, or refuse to stop the other party from using the technology at issue on the grounds that our patent applications do not cover the technology. An adverse result in any litigation proceeding could put one or more of our patents at risk of being invalidated or interpreted narrowly.
Our trade secrets are difficult to protect and misappropriation could reduce the market for our products.
We may not be able to obtain adequate remedies for the unauthorized use or disclosure of our proprietary information, including our trade secrets. Enforcing a claim that a party illegally disclosed or misappropriated a trade secret is difficult, expensive and time consuming, and the outcome is unpredictable. If any of our trade secrets were to be lawfully obtained or independently developed by a competitor, we would have no right to prevent them, or those to whom they communicate it, from using that technology or information to compete with us. If any of our trade secrets were to be disclosed to or independently developed by a competitor, our competitive position could be harmed.
Third parties may initiate legal proceedings alleging that we are infringing their intellectual property rights, the outcome of which would be uncertain and could have a material adverse effect on the success of our business.
Our success will depend, in part, on our ability to operate without infringing the patents and other proprietary intellectual property rights of third parties. This is generally referred to as having the “freedom to operate.” The biotechnology and pharmaceutical industries are characterized by extensive litigation regarding patents and other intellectual property rights. The defense and prosecution of intellectual property claims, interference proceedings and related legal and administrative proceedings, both in the United States and internationally, involve complex legal and factual questions. As a result, such proceedings are lengthy, costly and time-consuming, and their outcome is highly uncertain. We may become involved in protracted and expensive litigation in order to determine the enforceability, scope and validity of the proprietary rights of others, or to determine whether we have the freedom to operate with respect to the intellectual property rights of others.
Patent applications in the United States are, in most cases, maintained in secrecy until approximately 18 months after the patent application is filed. The publication of discoveries in the scientific or patent literature frequently occurs substantially later than the date on which the underlying discoveries were made. Therefore, patent applications relating to a product or method similar to ours may have already been filed by others without our knowledge. In the event that a third party has also filed a patent application covering our products, methods or other claims, we may have to participate in an adversarial proceeding, such as an interference or derivation proceeding in the USPTO or similar proceedings in other countries, to determine the priority of invention. In the event an infringement claim is brought against us, we may be required to pay substantial legal fees and other expenses to defend such a claim and, if we are unsuccessful in defending the claim, we may be subject to injunctions or damage awards.
| Page 16 |
In the future, the USPTO or a foreign patent office may grant patent rights to our claims to third parties. Subject to the issuance of these future patents, the claims of which will be unknown until issued, we may need to obtain a license or sublicense to these rights in order to have the appropriate freedom to further use, develop or commercialize such products or methods. Any required licenses may not be available to us on acceptable terms, if at all. If it is determined that we have infringed an issued patent and do not have the freedom to operate, we could be subject to injunctions, and compelled to pay significant damages, including punitive damages. In any cases where we in-license intellectual property, our failure to comply with the terms and conditions of such licensing agreements could harm our business.
If we become involved in any patent litigation or other legal proceedings, we could incur substantial expense, and the efforts of our technical and management personnel could be significantly diverted. A negative outcome of such litigation or proceedings may expose us to the loss of our proprietary position or to significant liabilities, or require us to seek licenses that may not be available from third parties on commercially acceptable terms, if at all. We may be restricted or prevented from using or developing methods, or manufacturing and selling our products in the event of an adverse determination in a judicial or an administrative proceeding, or if we fail to obtain necessary licenses. Further, even if we are successful in defending against claims of infringement, such litigation could be burdensome and costly, and divert management’s attention away from executing our business plan.
We may be subject to claims that our employees, consultants or independent contractors have wrongfully used or disclosed confidential information of third parties.
Certain of our employees were previously employed at other biotechnology or pharmaceutical companies. We may be subject to claims that we or our employees, consultants or independent contractors have inadvertently or otherwise improperly used or disclosed confidential information of these third parties or our employees’ former employers. We may also be subject to claims that former employees, consultants, independent contractors or other third parties have an ownership interest in our patents or other intellectual property. Litigation may be necessary to defend against these and other claims challenging our right to and use of confidential and proprietary information. If we fail in defending any such claims, we may lose our rights to such information, in addition to paying monetary damages. Such an outcome could have a material adverse effect on our business. Even if we are successful in defending against these claims, litigation could result in substantial cost and be a distraction to our management and employees.
We have limited marketing, sales and distribution experience and capabilities. We will need to establish sales and marketing capabilities or enter into agreements with third parties to market and sell our products.
We currently have limited experience in the marketing, sales and distribution of KLH-based therapeutic or diagnostic products. Depending on market acceptance of our Stellar KLHTM products, we may need to expand our capabilities. We may not be able to establish such additional capabilities in-house, and then will need to enter into agreements with third parties to successfully perform these tasks. If we contract or make arrangements with third parties for the sales and marketing of our products, our revenues will be dependent on the efforts of these third parties, whose efforts may not be successful. If we market any of our products directly, we must either internally develop or acquire a marketing and sales force, which would require substantial resources and management attention.
We rely on the significant experience and specialized expertise of our Chief Executive Officer and other members of our senior management team, and we will need to hire and retain other highly skilled personnel to maintain and grow our business.
Our ability to be successful in the highly competitive biotechnology and pharmaceutical industries depends in large part upon our ability to attract and retain highly qualified managerial, scientific, medical, sales and other personnel. Our performance is substantially dependent on the research and development and business development expertise of Frank Oakes, our President and Chief Executive Officer, and Catherine Brisson, our Chief Operating Officer. We do not have employment agreements currently in effect with Mr. Oakes and Dr. Brisson, and they are free to leave their employment with us at any time.
There is little possibility that this dependence will decrease in the near term. The loss of the services of Mr. Oakes or Dr. Brisson, or the increased demands placed on our key executives and personnel by our continued growth, could adversely affect our financial performance and our ability to execute our strategies. Our continued success also depends on our ability to attract and retain qualified team members to meet our future growth needs. We may not be able to attract and retain necessary team members to operate our business.
| Page 17 |
In addition, our future success depends on our ability to identify, attract, hire, train, retain and motivate highly skilled technical, managerial and research personnel in all areas within our organization. We plan to continue to grow our business and will need to hire additional personnel to support this growth. We believe that there are only a limited number of individuals with the requisite skills to serve in many of our key positions, and we compete for key personnel with other biotechnology companies, as well as universities and research institutions. It is often difficult to hire and retain these persons, and we may be unable to timely replace key persons if they leave or be unable to fill new positions requiring key persons with appropriate experience. If we fail to attract, integrate and retain the necessary personnel, our ability to maintain and grow our business could suffer significantly.
We are subject to the risk of product liability claims, for which we may not have, or be able to obtain, adequate insurance coverage.
The pharmaceutical industry is subject to product liability claims in the event of adverse effects, even in respect to products that have received regulatory approval for commercial sale. Such claims might be made directly by consumers, healthcare providers or by pharmaceutical companies, or others selling or utilizing our Stellar KLHTM products. Although we currently maintain liability insurance of up to $2 million in the aggregate for our products, we may not be able to obtain or maintain sufficient and affordable insurance coverage for all claims that may occur. The cost of any product liability litigation or other proceeding, even if resolved in our favor, could be substantial. In addition, our inability to obtain or maintain sufficient insurance coverage at an acceptable cost or to otherwise protect against potential product liability claims could prevent or inhibit the development and commercial production and sale of our products, which could adversely affect our business, financial condition and results of operations.
We may face environmental risks related to handling regulated substances and hazardous materials.
Our research and clinical development activities, as well as the manufacture of materials and products, are subject to federal, state and local laws, rules, regulations and policies governing the use, generation, manufacture, storage, air emission, effluent discharge, handling and disposal of certain materials, biological specimens and wastes. We may be required to incur significant costs to comply with environmental and health and safety regulations in the future. Our research and clinical development, both now and in the future, may involve the controlled use of hazardous materials, including but not limited to certain hazardous chemicals. We cannot completely eliminate the risk of accidental contamination or injury from these materials. In the event of such an occurrence, we could be held liable for any damages that result and any such liability could exceed our resources.
We deal with hazardous materials and must comply with environmental, health and safety laws and regulations, which can be expensive and restrict how we do business and/or give rise to significant liabilities.
As we operate a manufacturing facility, we are subject to various environmental, health and safety laws and regulations, including those governing air emissions, water and wastewater discharges, noise emissions, the use, management and disposal of hazardous materials and wastes, and the cleanup of contaminated sites. The cost of compliance with these laws and regulations could be significant. In the event of a violation of these requirements, including from accidental contamination or injury, we could be held liable for damages exceeding our available financial resources. We could be subject to monetary fines, penalties or third party damage claims as a result of violations of such laws and regulations or noncompliance with environmental permits required at our facility. As an operator of real property and a generator of hazardous materials and wastes, we also could be subject to environmental cleanup liability, in some cases without regard to fault or whether we were aware of the conditions giving rise to such liability. In addition, we may be subject to liability and may be required to comply with new or existing environmental laws regulating pharmaceuticals in the environment. Environmental laws or regulations (or their interpretation) may become more stringent in the future. If any such future revisions require significant changes in our operations, or if we engage in the development and manufacturing of new products or otherwise expand our operations requiring new or different environmental controls, we will have to dedicate additional management resources and incur additional expenses to comply with such laws and regulations.
| Page 18 |
In the event of an accident, applicable authorities may curtail our use of hazardous materials and interrupt our business operations. In addition, with respect to our manufacturing facility, we may incur substantial costs to comply with environmental regulations and may become subject to the risk of accidental contamination or injury from the use of hazardous materials in our manufacturing process.
Our business is geographically concentrated and if a catastrophic event, such as a hurricane, an earthquake or coastal flooding, were to impact our facilities, our business may be disrupted which could result in serious harm to our business, results of operations and financial condition.
Our aquaculture operations, research and manufacturing facilities, laboratory space, and executive offices are all located in Port Hueneme, California, a coastal city located along the Pacific Ocean. We conduct all of our aquaculture operations, research and manufacturing at these facilities and there are no backup facilities for any of these operations. If a hurricane, an earthquake or other natural disaster, including coastal flooding, or a virus affecting our limpet colony, were to impact our facilities, we may be unable to manufacture our KLH products, which would have a serious disruptive impact on our business and a material adverse effect on our results of operations and financial condition. While we carry personal property insurance, such insurance may not be adequate to compensate us for losses from any damage or interruption of our business operations resulting from a hurricane, an earthquake, coastal flooding or other catastrophic event.
Risks Related to an Investment in Our Securities
Trading in our common shares is limited and the price of our common shares may be subject to substantial volatility.
Our common shares trade over-the-counter in the United States on the OTCQB Marketplace under the symbol “SBOTF” and on the TSX Venture Exchange in Canada under the stock symbol “KLH.” Our common shares have been quoted on the OTCQB Marketplace since January 15, 2013.
Our common shares can be expected to be subject to volatility in both price and volume arising from market expectations, announcements and press releases regarding our business, and changes in estimates and evaluations by securities analysts or other events or factors. Further, despite the existence of a market for trading our common shares, our shareholders may be unable to sell significant quantities of common shares in the public trading markets without a significant reduction in the price of the stock. Broker-dealers may also decline to trade in OTCQB stocks given the market for such securities is often limited, the stocks are more volatile and the risk to investors is greater. These factors may reduce the potential market for our common shares by reducing the number of potential investors, which may make it more difficult for investors in our common shares to sell shares to third parties or to otherwise dispose of their shares and therefore, reduce our stock price.
We will likely require additional financing or financings, which would result in substantial dilution to existing shareholders.
We will likely require additional funds to meet our future obligations, which may include the sale of additional common shares or debt securities in order to raise sufficient capital to meet our budgeted expenditures and obligations. Management currently estimates that our operations, including research and development, capital expenditures and general and administrative expenses, will require approximately $7.5 million for the next 18 months. We believe our cash and cash equivalents at August 31, 2014 are sufficient to meet estimated working capital requirements and fund planned operations for at least the next 18 months. Notwithstanding the above, we may decide to expand operations, undertake strategic acquisitions or determine some other business need. In such case, we would then seek financing for such events. Our ongoing research and development activities may be dependent upon our ability to obtain funds, which is expected to include the sale of common shares, as well as possible debt financings, joint ventures, or other means. Such sources of financing may not be available on acceptable terms, if at all. Failure to obtain such financing may result in delay or indefinite postponement of research and development of our Stellar KLHTM. Any transaction involving the issuance of previously authorized but unissued shares of common shares, or securities convertible into common shares, could result in dilution, possibly substantial, to present and prospective holders of common shares and may be on terms less favorable to us, if at all.
| Page 19 |
If an active, liquid trading market for our common shares does not develop, you may not be able to sell your shares quickly or at or above the price you paid for it.
An active and liquid trading market for our common shares has not yet and may not ever develop or be sustained. You may not be able to sell your shares quickly or at or above the price you paid for our stock if trading in our stock is not active.
“Penny stock” rules may make buying or selling our securities difficult and limit the market for our securities.
Trading in our securities is subject to the SEC’s “penny stock” rules and it is anticipated that trading in our securities will continue to be subject to the penny stock rules for the foreseeable future. The SEC has adopted regulations that generally define a penny stock to be any equity security that has a market price of less than $5.00 per share, subject to certain exceptions. These rules require that any broker-dealer who recommends our securities to persons other than prior customers and accredited investors must, prior to the sale, make a special written suitability determination for the purchaser and receive the purchaser’s written agreement to execute the transaction. Unless an exception is available, the regulations require the delivery, prior to any transaction involving a penny stock, of a disclosure schedule explaining the penny stock market and the risks associated with trading in the penny stock market. In addition, broker-dealers must disclose commissions payable to both the broker-dealer and the registered representative and current quotations for the securities they offer. The additional burdens imposed upon broker-dealers by these requirements may discourage broker-dealers from recommending transactions in our securities, which could severely limit the liquidity of our securities and consequently adversely affect the market price for our securities.
We could be deemed a passive foreign investment company, which could have negative consequences for U.S. investors.
We could be classified as a “passive foreign investment company” (“PFIC”) under the United States tax code. If we are declared a PFIC, then owners of our common shares who are U.S. taxpayers generally will be required to treat any so-called “excess distribution” received on our common shares, or any gain realized upon a disposition of common shares, as ordinary income and to pay an interest charge on a portion of such distribution or gain, unless the taxpayer makes a qualified electing fund (“QEF”) election or a mark-to-market election with respect to our shares. A U.S. taxpayer who makes a QEF election generally must report on a current basis its share of our net capital gain and ordinary earnings for any year in which we are classified as a PFIC, whether or not we distribute any amounts to our shareholders.
Risks Related to an Emerging Growth Company
We are an “emerging growth company” under the JOBS Act of 2012 and we cannot be certain if the reduced disclosure requirements applicable to emerging growth companies will make our common shares less attractive to investors.
We are an “emerging growth company,” as defined in the Jumpstart Our Business Startups Act of 2012 (“JOBS Act”), and as a result, we may take advantage of certain exemptions from various reporting requirements that are applicable to other public companies that are not “emerging growth companies.” We will remain an “emerging growth company” for up to five years, or until the earliest of the earliest to occur of: (i) the last day of the fiscal year in which we have more than $1.0 billion in annual revenues; (ii) the date we are deemed a “large accelerated filer” as defined in the Exchange Act, with at least $700 million of equity securities held by non-affiliates; or (iii) the issuance, in any three-year period, by us of more than $1.0 billion in non-convertible debt securities. We may choose to take advantage of some but not all of these reduced reporting burdens.
| Page 20 |
For so long as we remain an emerging growth company, we will not be required to:
| · | have an auditor report on our internal control over financial reporting pursuant to Section 404(b) of Sarbanes-Oxley; |
| · | comply with any requirement that may be adopted by the Public Company Accounting Oversight Board (“PCAOB”), regarding mandatory audit firm rotation or a supplement to the auditor’s report providing additional information about the audit and the financial statements (auditor discussion and analysis); |
| · | submit certain executive compensation matters to shareholders advisory votes pursuant to the “say on frequency” and “say on pay” provisions (requiring a non-binding shareholder vote to approve compensation of certain executive officers) and the “say on golden parachute” provisions (requiring a non-binding shareholder vote to approve golden parachute arrangements for certain executive officers in connection with mergers and certain other business combinations) of the Dodd-Frank Wall Street Reform and Consumer Protection Act of 2010; and |
| · | include detailed compensation discussion and analysis in our filings under the Exchange Act, and instead may provide a reduced level of disclosure concerning executive compensation. |
In addition, Section 107 of the JOBS Act also provides that an “emerging growth company” can take advantage of the extended transition period provided in Section 7(a)(2)(B) of the Securities Act for complying with new or revised accounting standards. In other words, an “emerging growth company” can delay the adoption of certain accounting standards until those standards would otherwise apply to private companies. We have elected not to take advantage of the extended transition period for complying with new or revised accounting standards.
If we take advantage of any of these reduced reporting burdens in future filings, the information that we provide our security holders may be different than you might get from other public companies in which you hold equity interests. We cannot predict if investors will find our common shares less attractive because we may rely on these exemptions. If some investors find our common shares less attractive as a result, there may be a less active trading market for our common shares and our stock price may be more volatile.
| Page 21 |
| Item 1B. | UNRESOLVED STAFF COMMENTS. |
None.
| Item 2. | PROPERTIES. |
We currently lease 4,300 square feet of executive office and laboratory space in Port Hueneme, California under a lease which was renewed in July 2014 for a two-year term.
Our aquaculture operations are land-based, and encompass three buildings and a 37,000 square foot oceanfront leasehold facility in the Port Hueneme Aquaculture Business Park, located along the Pacific Coast. These facilities include our aquaculture, manufacturing, and laboratory operations, and are leased from the Port Hueneme Surplus Property Authority under sublease agreements that expire in September 2015 with options to extend the leases for an additional five-year term.
| Item 3. | LEGAL PROCEEDINGS. |
In August 2008, we received a Notice of Violations from the Regional Water Quality Control Board, Los Angeles Region (“Regional Board”) for violations of waste discharge requirements in the amount of $69,000 related to our National Pollution Discharge Elimination Systems (NPDES) permit. In March 2014, we settled the matter for a payment of $3,000 in full payment of any civil liability, and waived our right to a hearing to dispute the alleged violations or the amount of civil liability. We received notification of the acceptance of the payment from the Regional Board in April 2014.
From time to time, we may be involved in legal proceedings, claims and litigation arising in the ordinary course of business, including contract disputes, employment matters and intellectual property disputes. We are not currently a party to any material legal proceedings or claims outside the ordinary course of business. Regardless of outcome, litigation can have an adverse impact on us because of defense and settlement costs, diversion of management resources and other factors.
| Item 4. | MINE SAFETY DISCLOSURES. |
Not applicable.
| Page 22 |
| Item 5. | MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES. |
Market Information
Our common shares trade over-the-counter in the United States on the U.S. OTCQB Marketplace Exchange under the symbol “SBOTF” and on the TSX Venture Exchange in Canada under the stock symbol is “KLH.” Our CUSIP number is 85855A 10 4.
Our common shares began trading on the OTCQB on January 15, 2013. The table below lists the high and low bid prices for our common shares on the OTCQB since initiation of trading. The quotations on the OTCQB reflect inter-dealer prices, without retail mark-up, mark-down or commission, and may not necessarily represent actual transactions.
OTCQB Marketplace
Common Shares Trading Activity
| - Bids- | ||||||||
| US Dollars | ||||||||
| Period | High | Low | ||||||
| Fiscal Year 2014 | ||||||||
| Three Months Ended 8/31/14 | $ | 2.36 | $ | 0.60 | ||||
| Three Months Ended 5/31/14 | $ | 1.82 | $ | 0.93 | ||||
| Three Months Ended 2/28/14 | $ | 1.95 | $ | 1.00 | ||||
| Three Months Ended 11/30/13 | $ | 2.30 | $ | 1.17 | ||||
| Fiscal Year 2013 | ||||||||
| Three Months Ended 8/31/13 | $ | 1.66 | $ | 0.62 | ||||
| Three Months Ended 5/31/13 | $ | 0.78 | $ | 0.43 | ||||
| Three Months Ended 2/28/13 (1) | $ | 0.70 | $ | 0.32 | ||||
| (1) | Includes the period from January 15, 2013, the date of initiation of trading of our common shares on the OTCQB, through the three months ended February 28, 2013. |
On August 29, 2008, we commenced trading on the TSX Venture Exchange as a Canadian “capital pool company” under the name “CAG Capital Inc.” On April 7, 2010, we changed our name to Stellar Biotechnologies, Inc. and, on April 12, 2010, we completed our qualifying transaction through a reverse merger with Stellar Biotechnologies, Inc., a California corporation. On April 19, 2010, we began trading on the TSX Venture Exchange under the symbol “KLH.” The table below lists the high and low sale prices as reported by the TSX Venture Exchange for our common shares for the periods indicated.
| Page 23 |
TSX Venture Exchange
Common Shares Trading Activity
| - Sales- | ||||||||
| Canadian Dollars | ||||||||
| Period | High | Low | ||||||
| Fiscal Year 2014 | ||||||||
| Three Months Ended 8/31/14 | $ | 2.55 | $ | 0.65 | ||||
| Three Months Ended 5/31/14 | $ | 1.99 | $ | 0.90 | ||||
| Three Months Ended 2/28/14 | $ | 2.05 | $ | 1.36 | ||||
| Three Months Ended 11/30/13 | $ | 2.15 | $ | 1.23 | ||||
| Fiscal Year 2013 | ||||||||
| Three Months Ended 8/31/13 | $ | 1.74 | $ | 0.63 | ||||
| Three Months Ended 5/31/13 | $ | 0.80 | $ | 0.44 | ||||
| Three Months Ended 2/28/13 | $ | 0.72 | $ | 0.19 | ||||
| Three Months Ended 11/30/12 | $ | 0.38 | $ | 0.20 | ||||
Holders
As of November 1, 2014, we had 26 registered shareholders of record. This figure does not reflect persons or entities that hold their shares in nominee or “street” name through various brokerage firms.
Dividends
We have not declared any dividends on our common shares since our incorporation and do not anticipate that we will do so in the foreseeable future. Our present policy is to retain future earnings, if any, for use in our operations and the expansion of our business.
| Page 24 |
Performance Graph
The following performance graph and related information shall not be deemed to be “soliciting material” or to be “filed” with the SEC, nor shall such information be incorporated by reference into any future filing under the Securities Act, each as amended, except to the extent that we specifically incorporate it by reference into such filing.
The following performance graph compares the cumulative total return of our common shares to the NASDAQ Composite Index and the NASDAQ Biotechnology Index from April 20, 2010, the day after we first officially traded on the TSX Venture Exchange under the symbol “KLH,” through August 31, 2014. The comparison assumes $100 was invested on April 20, 2010 in our common shares and in each of the comparative indices and assumes reinvestment of dividends, if any. The points on the graph are as of August 31st of the year indicated.
Our historic share price performance is not necessarily indicative of future share price performance.
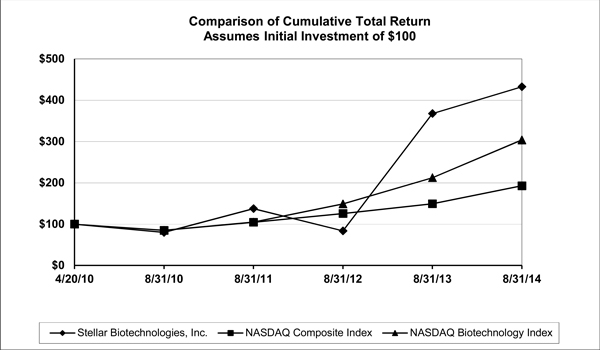
| 8/31/10 | 8/31/11 | 8/31/12 | 8/31/13 | 8/31/14 | ||||||||||||||||
| Stellar Biotechnologies, Inc. | $ | 80.00 | $ | 137.50 | $ | 83.75 | $ | 367.50 | $ | 432.50 | ||||||||||
| NASDAQ Composite Index | $ | 84.86 | $ | 104.53 | $ | 125.71 | $ | 149.28 | $ | 192.80 | ||||||||||
| NASDAQ Biotechnology Index | $ | 85.27 | $ | 105.01 | $ | 148.82 | $ | 212.38 | $ | 303.71 | ||||||||||
Recent Sales of Unregistered Securities; Use of Proceeds from Registered Securities
None.
Purchase of Equity Securities by the Issuer and Affiliated Purchasers
None.
| Page 25 |
| Item 6. | SELECTED FINANCIAL DATA. |
Our selected financial data in the table below is derived from our audited financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States (US GAAP) for the fiscal years ended August 31, 2014, 2013, and 2012; International Financial Reporting Standards (IFRS) for the fiscal year ended August 31, 2011; and Canadian generally accepted accounting principles (CDN GAAP) for the fiscal year ended August 31, 2010. We adopted IFRS effective September 1, 2010. Our auditors for the fiscal year ended August 31, 2014, Moss Adams LLP, conducted the audit in accordance with United States generally accepted auditing standards, and the standards of the Public Company Accounting Oversight Board. Our auditors for the fiscal years ended August 31, 2013 and 2012, D&H Group LLP, conducted the audits in accordance with Canadian generally accepted auditing standards, and the standards of the Public Company Accounting Oversight Board. You should read these selected financial data together with “Management’s Discussion and Analysis of Financial Condition and Results of Operations” in Item 7 and our audited financial statements and notes thereto that are included in this Annual Report on Form 10-K.
Selected Financial Data
Expressed in U.S. dollars
| Year Ended August 31, 2014 | Year Ended August 31, 2013 | Year Ended August 31, 2012 | Year Ended August 31, 2011 | Year Ended August 31, 2010 | ||||||||||||||||
| US GAAP | US GAAP | US GAAP | IFRS | CDN GAAP | ||||||||||||||||
| Net revenues | $ | 372,132 | $ | 545,469 | $ | 286,054 | $ | 697,187 | $ | 854,837 | ||||||||||
| Net loss | (8,439,523 | ) | (14,495,779 | ) | (5,529,278 | ) | (3,597,279 | ) | (589,971 | ) | ||||||||||
| Net loss per share | (0.11 | ) | (0.28 | ) | (0.13 | ) | (0.09 | ) | (0.04 | ) | ||||||||||
| Total assets | 14,473,962 | 8,513,358 | 1,543,878 | 4,750,651 | 2,893,074 | |||||||||||||||
| Long-term obligations | 5,352,663 | 6,835,199 | 124,141 | 1,527,374 | - | |||||||||||||||
| Dividends per share | - | - | - | - | - | |||||||||||||||
Our financial statements have been prepared in accordance with IFRS for the fiscal year ended August 31, 2011 and CDN GAAP for the fiscal year ended August 31, 2010. Therefore, the information in the table above is not comparable with the information for fiscal years ended August 31, 2014, 2013 and 2012. The table below is derived from reconciliations from IFRS and CDN GAAP to US GAAP for the fiscal years ended August 31, 2011 and 2010.
Selected Financial Data
Expressed in U.S. dollars
| Year Ended August 31, 2011 | Year Ended August 31, 2010 | |||||||
| US GAAP | US GAAP | |||||||
| Net revenues | $ | 697,187 | $ | 854,837 | ||||
| Net loss | (3,727,773 | ) | (1,067,205 | ) | ||||
| Net loss per share | (0.10 | ) | (0.07 | ) | ||||
| Total assets | 4,750,651 | 2,893,074 | ||||||
| Long-term obligations | 1,212,115 | 628,005 | ||||||
| Dividends per share | - | - | ||||||
| Page 26 |
Supplementary Financial Information
Selected Quarterly Financial Data
U.S. dollars are shown in thousands, except per share data
| For the Years Ended August 31, | ||||||||||||||||||||||||||||||||
| 2014 | 2013 | |||||||||||||||||||||||||||||||
| Q4 | Q3 | Q2 | Q1 | Q4 | Q3 | Q2 | Q1 | |||||||||||||||||||||||||
| Revenues | $ | 119 | $ | 103 | $ | 91 | $ | 59 | $ | 295 | $ | 73 | $ | 61 | $ | 116 | ||||||||||||||||
| Net income (loss) for period | (4,635 | ) | 1,813 | (238 | ) | (5,380 | ) | (8,915 | ) | (1,171 | ) | (3,644 | ) | (766 | ) | |||||||||||||||||
| Income (loss) per share | (0.06 | ) | 0.02 | (0.00 | ) | (0.08 | ) | (0.17 | ) | (0.02 | ) | (0.07 | ) | (0.02 | ) | |||||||||||||||||
The definition of an employee for purposes of accounting for share based payments differs between US GAAP and IFRS. Therefore, non-employee share based payments were accounted for differently under US GAAP than IFRS. The impact of these differences totaling $146 thousand and $390 thousand decrease in net loss for the years ended August 31, 2014 and 2013, respectively, were recorded in the applicable fourth quarter.
Fluctuations in net income (loss) between quarters can be mainly attributed to changes in fair value of warrant liability shown in thousands as follows:
| For the Years Ended August 31, | ||||||||||||||||||||||||||||||||
| 2014 | 2013 | |||||||||||||||||||||||||||||||
| Q4 | Q3 | Q2 | Q1 | Q4 | Q3 | Q2 | Q1 | |||||||||||||||||||||||||
| Change in fair value of | ||||||||||||||||||||||||||||||||
| warrant liability - gain (loss) | $ | (3,355 | ) | $ | 3,023 | $ | 1,469 | $ | (3,670 | ) | $ | (7,575 | ) | $ | (353 | ) | $ | (2,767 | ) | $ | 139 | |||||||||||
| Item 7. | MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS. |
This discussion contains forward-looking statements that involve risks and uncertainties that could cause actual results or events to differ materially from those expressed or implied by such forward-looking statements as a result of many important factors, including those set forth in Part I of this Annual Report on Form 10-K under the caption “Risk Factors.” Please see “Special Note Regarding Forward-Looking Statements” in Part I above. We do not undertake any obligation to update forward-looking statements to reflect events or circumstances occurring after the date of this Annual Report.
Operating and Financial Review and Prospects
Overview
Our financial statements have been prepared in accordance with accounting principles generally accepted in the United States of America (“GAAP”) and include the accounts of the Company and our wholly-owned subsidiary, Stellar Biotechnologies, Inc.
Since inception, we have primarily financed our activities through the issuance of common shares, exercise of warrants, grant revenues, contract services revenue, and product sales. In September 2013, we closed a private placement with total gross proceeds of $12,000,000. Management believes these financial resources are adequate to support our initiatives at the current level for the next 12 months. Management is also continuing the ongoing effort toward expanding the customer base for our currently marketed products, and we may seek additional financing alternatives, including additional equity financing or nondilutive financing alternatives including applying for grants, entering into collaboration and/or licensing arrangements.
| Page 27 |
Results of Operations
The greatest impact on the comparison of our consolidated statements of operations is from fluctuations in the change in fair value of our warrant liability. As a result of having exercise prices denominated in a currency other than our functional currency, our warrants with Canadian dollar exercise prices meet the definition of derivatives and are therefore classified as derivative liabilities measured at fair value with noncash adjustments to fair value recognized through the consolidated statements of operations. Fair values are based on the Black-Scholes option valuation model. The losses and gains in each year are a reflection of our share price fluctuations with increases in share prices causing greater warrant liability and a resulting loss on fair value of warrant liability, while decreases in share prices cause a resulting gain on fair value of warrant liability. Changes in fair value of warrant liability have no impact on cash flow. If the warrants are exercised, the warrant liability is reclassified to common shares. If the warrants expire, the decrease in warrant liability offsets the changes in fair value.
Fiscal Year Ended August 31, 2014
In September 2013, we closed a private placement with total gross proceeds of $12,000,000.
Our net loss for fiscal 2014 was $8,439,523, or ($0.11) per share, as compared to a net loss of $14,495,779, or ($0.28) per share, for the fiscal 2013. The decrease in net loss of approximately $6 million for fiscal 2014 was primarily due to a large change in the fair value of warrant liability.
Revenue for fiscal 2014 totaled $372,132, as compared to revenue of $545,469 in fiscal 2013. As expected during this early stage of our development, our revenues have high volatility as we establish a market for our products and services. Revenue for fiscal 2014 included product sales of $143,553, as compared to $76,055 in the prior year. The increase in revenue for fiscal 2014 was due to greater product sales volume. Grant revenue for fiscal 2014 decreased to $36,579, as compared to $409,414 in the prior year due to completion of our work associated with the NSF Phase II/IIB grant in fiscal 2013. Contract services revenue was $192,000 for fiscal 2014, as compared to $60,000 in the prior year. The increase for fiscal 2014 was due to new contract services under a collaboration agreement.
Expenses for fiscal 2014 increased to $6,090,648, as compared to $4,393,388 incurred in fiscal 2013. Costs of production and aquaculture increased to $662,946 for fiscal 2014, as compared to $183,276 for the prior year, due to the operations department resuming manufacturing activities during fiscal 2014 and greatly reducing the efforts spent on the NSF grant and on other internal research. It should be noted that we do not capitalize the cost of inventory at this early stage of our development, so manufacturing costs of production are expensed, although related product sales normally occur in a later period. Grant costs decreased to $36,579 from $409,414 in line with the decrease in grant revenue due to completion of NSF Phase II/IIB in fiscal 2013 with the close out period ended November 2013. Research and development was $2,458,934 for fiscal 2014, as compared to $2,018,554 for the prior year, due to preclinical research on C. diff immunotherapy, coupled with a decrease in our other internal research and development activities caused by operations shifting time from process development to manufacturing. General and administration expenses increased to $2,871,455 for fiscal 2014, as compared to $1,770,619 in the prior year, as management executed on strategic initiatives for fiscal 2014, particularly related to corporate development and business development. Share-based compensation is allocated to all expense types but the greatest portion is recorded as general and administration expenses. Share-based compensation was $956,634 for fiscal 2014, which was an increase from $786,585 recorded in fiscal 2013. The increase for fiscal 2014 was related to the timing of granting stock options, increases in our share price that affect the valuation model and the vesting of options granted in prior years.
| Page 28 |
Other income (loss) was an overall loss of $2,693,807 in fiscal 2014, as compared to a loss of $10,647,060 in fiscal 2013. The largest change for fiscal 2014 as compared to the prior year occurred due to a change in fair value of warrant liability, which decreased to a loss of $2,533,305 for fiscal 2014 from a loss of $10,566,208 in the prior year. The loss in fiscal 2014 is a reflection of the increase in our share price from August 31, 2013 to August 31, 2014, but not as much as in the prior year. Our foreign exchange loss in fiscal 2014 was $222,437, as compared to $95,842 in fiscal 2013. The change was due to unfavorable exchange rates for Canadian funds in the fiscal 2014.
Fiscal Year Ended August 31, 2013
Our net loss for fiscal 2013 was $14,495,779, or ($0.28) per share, as compared to a net loss of $5,529,278, or ($0.13) per share, for fiscal 2012. The higher loss reported in fiscal 2013 was primarily due to a large change in the fair value of warrant liability.
Revenue for fiscal 2013 totaled $545,469, as compared to revenue of $286,054 in the prior year. Revenue for fiscal 2013 included product sales of $76,055, as compared to $131,825 in the prior year. A significant portion of the revenue for fiscal 2012 was attributed to a large sale of KLH which occurred in the prior year under a marketing and supply agreement and did not occur in fiscal 2013. Grant revenue rose to $409,414 for fiscal 2013, as compared to $94,229 in the prior year due to timing of grant activities and completion of NSF Phase II/IIB grant in fiscal 2013. Contract services revenue of $60,000 was the same in both periods.
Expenses for the year ended August 31, 2013 declined to $4,393,388 as compared to$6,832,053 incurred in fiscal 2012. During fiscal 2013, management made efforts to temporarily reduce operating costs until additional financing was received, including voluntary salary reductions and reductions in the number of personnel, which resulted in the significant decline in overall operating expenses. Costs of production and aquaculture decreased for fiscal 2013 to $183,276, as compared to $426,310 in the prior year, as the operations department was largely focused on grant and research activities. Grant costs increased to $409,414 for fiscal 2013, as compared to $94,043 in the prior year, consistent with fluctuations in grant revenue due to the timing of grant activities and the completion of a NSF Phase II/IIB grant in fiscal 2013. Research and development was $2,018,554 in fiscal 2013, as compared to $2,634,119 in the prior year, due to the timing of research and development activity, particularly a reduction in outside contracts. General and administration expenses declined to $1,770,619 in fiscal 2013, as compared to $3,662,009 in the prior year, as a result of management’s efforts to reduce operating costs, curtailment of outside contracted services, the voluntary salary reductions and reductions in the number of personnel, and the impact of lower share-based compensation. Share-based compensation was $786,585 in fiscal 2013, which was a decline from $1,923,200 recorded in the prior year. The decline for fiscal 2013 was related to the timing of granting stock options, and share-based compensation under the performance share program fully vesting during the prior year.
Other income (loss) was an overall loss of $10,647,060 in fiscal 2013, as compared to gain of $1,017,521 in fiscal 2012. The largest change occurred due to the change in fair value of warrant liability, which increased to a loss of $10,566,208 in fiscal 2013 from a gain of $897,549 in the prior year. The loss in fiscal 2013 is a reflection of the increase in our share price from August 31, 2012 to August 31, 2013. Foreign exchange in fiscal 2013 was a loss of $95,842 compared to a gain of $10,091 in fiscal 2012. The change was due to unfavorable exchange rates for Canadian funds in fiscal 2013. Loss recovery was $105,000 in fiscal 2012 as we received a settlement for the value of KLH which had been damaged by a vendor. This one-time loss recovery did not recur in fiscal 2013.
| Page 29 |
Capital Expenditures
Our capital expenditures, which primarily consist of scientific, manufacturing, and aquaculture equipment, for the previous three fiscal years are as follows:
| Fiscal Year | Capital Expenditures | Assets Acquired | ||||
| 2014 | $ | 279,065 | Property, plant and equipment | |||
| 2013 | 9,541 | Property, plant and equipment | ||||
| 2012 | 78,338 | Property, plant and equipment | ||||
Liquidity and Capital Resources
Our working capital position at August 31, 2014 was $12,650,004, including cash and cash equivalents of $13,427,404 and net of $879,040 in the noncash current portion of our warrant liability. Management believes the current working capital is sufficient to meet our present requirements, including all contractual obligations and anticipated research and development expenditures for the next 12 months. We expect to finance our future expenditures and obligations through revenues from product sales, contract services income, grant revenues, and sales of common shares. We expect to continue incurring losses for the foreseeable future and may need to raise additional capital to pursue our business plan and continue as a going concern. We cannot provide any assurances that we will be able to raise additional capital. Our management believes that we have access to capital resources through possible public or private equity offerings, debt financings, corporate collaborations or other means, if needed; however, we have not secured any commitment for new financing at this time, nor can we provide any assurance that new financing will be available on commercially acceptable terms, if needed.
Fiscal Year Ended August 31, 2014
As of August 31, 2014, our working capital position was $12,650,004 compared to working capital of $4,260,364 as of August 31, 2013. Working capital is reduced by the noncash current portion of our warrant liability in the amount of $879,040 and $3,454,745 at August 31, 2014 and 2013, respectively.
Our cash and cash equivalents totaled $13,427,404 at August 31, 2014, as compared to cash and cash equivalents of $7,859,889 at August 31, 2013, which represented an increase of $5,567,515.
During fiscal 2014 operating activities used cash of $4,266,707. Items not affecting cash included: depreciation and amortization of $158,313; share-based compensation related to the issuance of stock options of $956,634; unrealized foreign exchange loss of $222,437; change in fair value of warrant liability of $2,533,305 due to adjustment to fair value of warrants previously issued; impairment loss of $90,476 and loss on disposal of property, plant and equipment of $3,670. Changes in non-cash working capital items include a decrease in accounts receivable of $121,075 related mostly to grants; decrease in deferred financing costs related to the private placement of units completed in September 2013 of $60,656; increase in prepaid expenses of $94,974; increase in accounts payable and accrued liabilities of $106,224; and increase in deferred revenue of $15,000 related to contract services billed in advance.
Investing activities used cash of $741,415. The acquisition of property, plant and equipment used cash of $279,065. Proceeds on sale of property, plant and equipment totaled $2,150. Purchase of short-term investments used cash of $464,500. The effect of exchange rate changes on cash and cash equivalents was a reduction of $212,338.
Financing activities provided cash of $10,787,975. The proceeds from exercise of warrants and options provided cash of $4,308,878; share subscription proceeds provided cash of $7,000,000; and share issuance costs used cash of $520,903.
During the year 2014, a total of 20,322,690 common shares were issued:
| · | 11,428,570 common shares were issued pursuant to private placements for gross proceeds of $12,000,000 (with $5,000,000 of these proceeds received as subscriptions in the prior fiscal year). |
| · | 1,515,152 common shares were issued for performance shares under the reverse merger transaction. The vested value had been recorded in prior years and there were no proceeds received in fiscal 2014. |
| · | 5,937,300 common shares were issued pursuant to the exercise of warrants for gross proceeds of $3,920,134 (with $155,674 of these proceeds received as subscriptions in the prior fiscal year). |
| · | 1,441,668 common shares were issued pursuant to the exercise of options for proceeds of $544,418. |
| Page 30 |
Fiscal Year Ended August 31, 2013
As of August 31, 2013, our working capital position was $4,260,364, as compared to working capital of $530,569 as of August 31, 2012. Working capital is reduced by the noncash current portion of our warrant liability of $3,454,745 and $0 at August 31, 2013 and 2012, respectively.
Our cash and cash equivalents totaled $7,859,889 at August 31, 2013, as compared to cash and cash equivalents of $998,998 at August 31, 2012, which represented an increase of $6,860,891.
During fiscal 2013, operating activities used cash of $2,795,823. Items not affecting cash included: depreciation and amortization of $124,833; share-based compensation related to the issuance of stock options and broker units totaling $786,585; unrealized foreign exchange loss of $95,842; change in fair value of warrant liability of $10,556,208 due to adjustment to fair value of warrants previously issued; and fair value of shares and warrants issued for an exclusive license in the amount of $491,408. Changes in non-cash working capital items included: an increase in amounts receivable of $192,067 related mostly to grants; increase in deferred financing costs related to the private placement of units completed after the fiscal year of $62,027 increase in prepaid expenses of $2,658; increase in accounts payable and accrued liabilities of $29,309; and decrease in deferred revenue of $127,477 related to the timing of work performed under grants for which cash had been received in the prior year but earned in the current year.
Investing activities used cash of $9,541, with the entire amount used for the acquisition of property, plant and equipment. The effect of exchange rate changes on cash and cash equivalents was a reduction of $64,571.
Financing activities provided cash of $9,730,826. The proceeds from exercise of warrants and options provided cash of $1,582,739; share subscription proceeds provided cash of $8,271,549, including $5,000,000 received in advance of the closing of our September 2013 private placement; share issuance costs used cash of $125,062; and refund of deposit provided cash of $1,600.
During the year 2013, a total of 12,532,599 common shares were issued:
| · | 9,258,400 common shares were issued pursuant to private placements for gross proceeds of $3,115,875. |
| · | 2,738,000 common shares were issued pursuant to the exercise of warrants for proceeds of $1,510,336. |
| · | 164,999 common shares were issued pursuant to the exercise of options for proceeds of $72,403. |
| · | 371,200 common shares were issued to acquire a license at a deemed value of $491,408 less $195,014 allocated to fair value of warrants for 278,400 non-transferrable share purchase warrants issued to acquire license. |
Research and Development
Our core business is developing and commercializing Keyhole Limpet Hemocyanin for use in immunotherapy and immunodiagnostic applications. Our internal research includes, among other activities, continual improvement of methods for the culture and growth of Giant Keyhole Limpet, innovations in aquaculture systems and infrastructure, biophysical and biochemical characterization of the KLH molecule, analytical processes to enhance performance of our products, KLH manufacturing process improvements, new KLH formulations, and early development of potential new KLH-based immunotherapies.
Research and development costs, including materials and salaries of employees directly involved in research and development efforts, are expensed as incurred.
| Page 31 |
The following table includes our research and development costs for each of the most recent three fiscal years:
| Fiscal Year | Research and Development Expense | |||
| 2014 | $ | 2,458,934 | ||
| 2013 | 2,018,554 | |||
| 2012 | 2,634,119 | |||
Disclosure of Contractual Obligations
We lease three buildings and facilities used in operations under sublease agreements with the Port Hueneme Surplus Property Authority. In September 2010, we exercised our option to extend these sublease agreements for an additional five-year term. We have an option to extend the lease for an additional five-year term to August 2020.
We also lease facilities used for executive offices and laboratories, and we must pay a portion of the common area maintenance. In July 2014, we exercised our option to extend this lease for a two-year term.
We have purchase commitments for contract research organizations and consultants.
The approximate amounts of our contractual obligations are as follows:
Contractual Obligations
As of August 31, 2014
| Less than 1 | 1-3 | 3-5 | More than | |||||||||||||||||
| Total | year | years | years | 5 years | ||||||||||||||||
| Operating lease obligations | $ | 228,000 | $ | 157,000 | $ | 71,000 | $ | - | $ | - | ||||||||||
| Purchase obligations | 78,000 | 78,000 | - | - | - | |||||||||||||||
| Total | $ | 306,000 | $ | 235,000 | $ | 71,000 | $ | - | $ | - | ||||||||||
Basis of Presentation, Significant Accounting Policies and Estimates
Basis of Presentation
The financial statements of the Company have been prepared in accordance with accounting principles generally accepted in the United States of America (“GAAP”) and include the accounts of the Company and its wholly-owned subsidiary Stellar Biotechnologies, Inc. Intercompany balances and transactions are eliminated on consolidation.
Use of Estimates
The preparation of financial statements in conformity with GAAP requires management to make certain estimates, judgments and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the dates of the consolidated financial statements and the reported amounts of revenues and expenses during the reported periods. These estimates include warrant liability, share-based compensation, intangible assets, valuation of accounts receivable, and income taxes. Actual outcomes could differ from these estimates. These consolidated financial statements include estimates, which by their nature are uncertain. The impacts of such estimates are pervasive throughout the consolidated financial statements, and may require accounting adjustments based on future periods if the revision affects both current and future periods. These estimates are based on historical experience, current and future economic conditions and other factors, including expectations of future events that are believed to be reasonable under the circumstances.
| Page 32 |
Cash and Cash Equivalents
Cash and cash equivalents consist of demand deposits with financial institutions and highly liquid investments which are readily convertible into cash with maturities of three months or less when purchased.
Investments
Investments include Canadian enhanced yield deposits with original maturity of 180 days. Investments are classified as held-to-maturity and are reported at amortized cost, which approximates fair value. We regularly review our investments to determine whether a decline in fair value below the cost basis is other than temporary. If the decline in fair value is determined to be other than temporary, the cost basis of the investment is written down to fair value.
Allowance for Doubtful Accounts Receivable
We assess the collectability of our accounts receivable through a review of our current aging, as well as an analysis of our historical collection rate and credit status of our customers. As of August 31, 2014 and 2013, all outstanding accounts receivables were deemed to be fully collectible, and therefore, no allowance for doubtful accounts was recorded.
Property, Plant and Equipment
Property, plant and equipment are recorded at cost less accumulated depreciation and accumulated impairment losses, if any. Depreciation is recorded on the straight-line method over useful lives ranging from 5 to 10 years. Leasehold improvements are depreciated over the shorter of the useful life of the improvement or remaining term of lease. Maintenance and repairs are charged to operations as incurred.
Impairment of Long-Lived Assets
If indicators of impairment exist, we assess the recoverability of the affected long-lived assets by determining whether the carrying value of such assets can be recovered through undiscounted future operating cash flows. If impairment is indicated, the amount of such impairment is measured by comparing the carrying value of the asset to the fair value of the asset and we record the impairment as a reduction in the carrying value of the related asset and a charge to operating results. Estimating the undiscounted future cash flows associated with long-lived assets requires judgment, and assumptions could differ materially from actual results. We incurred impairment loss for licensing rights of $90,476 for the year ended August 31, 2014.
Fair Value of Financial Instruments
We use the fair value measurement framework for valuing financial assets and liabilities measured on a recurring basis in situations where other accounting pronouncements either permit or require fair value measurements.
Fair value of a financial instrument is the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. As of August 31, 2014 and 2013, the carrying value of certain financial instruments such as accounts receivable, accounts payable, accrued liabilities, and deferred revenue approximates fair value due to the short-term nature of such instruments.
We follow the fair value hierarchy which requires an entity to maximize the use of observable inputs and minimize the use of unobservable inputs when measuring fair value. There are three levels of inputs that may be used to measure fair value:
| Level 1: | Quoted prices in active markets for identical or similar assets and liabilities. |
| Page 33 |
| Level 2: | Quoted prices for identical or similar assets and liabilities in markets that are not active or observable inputs other than quoted prices in active markets for identical or similar assets and liabilities. | |
| Level 3: | Unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities. |
We record our cash and cash equivalents at fair value using Level 1 inputs in the fair value hierarchy. We record our warrant liability at fair value using Level 2 input using the Black-Scholes option valuation model.
Revenue Recognition
Contract Services Revenue
We recognize contract services revenue when contract services have been performed and reasonable assurance exists regarding measurement and collectability. An appropriate amount will be recognized as revenue in the period that we are assured of fulfilling the contract requirements. Amounts received in advance of performance of contract services are recorded as deferred revenue.
Contract services include services performed under collaboration agreements and monthly maintenance of limpet colonies designated to meet the needs of the customer. We also have the right to use raw material produced from designated limpet colonies at no cost to us with prior written consent from the customer.
Product Sales
We recognize product sales when KLH product is shipped (for which the risk is typically transferred upon delivery to the shipping carrier) and there is persuasive evidence of an arrangement, the fee is fixed or determinable, and collectability is reasonably assured. We document arrangements with customers with purchase orders and sales agreements.
Product sales include sales made under supply agreements with a customer for a fixed price per gram of KLH products based on quantities ordered, including those produced from the customer’s designated limpet colonies. The supply agreements are on a non-exclusive basis except within that customer’s field of use.
Grants
We have taken the income approach to recognizing grant revenue. We recognize grant revenue when there is reasonable assurance that we will comply with the conditions attached, the benefits have been earned and we are reasonably assured of collection. An appropriate amount of earned revenue will be recognized as revenue in the period that we are assured of fulfilling the grant requirements.
Research and Development
Research and development costs are expensed as incurred.
Equity Financing
We engage in equity financing transactions to obtain the funds necessary to continue operations and perform research and development activities. These equity financing transactions may involve issuance of common shares or units. Units typically comprise a certain number of common shares and share purchase warrants. Depending on the terms and conditions of each equity financing transaction, the warrants are exercisable into additional common shares at a price prior to expiry as stipulated by the terms of the transaction.
| Page 34 |
Share-Based Compensation
We grant options to buy common shares of the Company to our directors, officers, employees and consultants, and grant other equity-based instruments to non-employees.
The fair value of share-based compensation is measured on the date of grant, using the Black-Scholes option valuation model and is recognized over the vesting period net of estimated forfeitures for employees or the service period for non-employees. The Black-Scholes option valuation model requires the input of subjective assumptions, including price volatility of the underlying stock, risk-free interest rate, dividend yield, and expected life of the option.
Foreign Exchange
Items included in the financial statements of our subsidiary are measured using the currency of the primary economic environment in which the entity operates (the “functional currency”). Our functional currency and the functional currency of our subsidiary is the U.S. dollar.
Transactions in currencies other than the U.S. dollar are recorded at exchange rates prevailing on the dates of the transactions.
Income Taxes
Income tax expense comprises current and deferred tax. Income tax is recognized in profit or loss except to the extent that it relates to items recognized directly in equity. Current tax expense is the expected tax payable on taxable income for the year, using tax rates enacted or substantively enacted at year-end, adjusted for amendments to tax payable with regards to previous years.
Deferred tax is recorded using the liability method, providing for temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for taxation purposes. Temporary differences are not provided for relating to goodwill not deductible for tax purposes. The amount of deferred tax provided is based on the expected manner of realization or settlement of the carrying amount of assets and liabilities, using tax rates enacted or substantively enacted at the balance sheet date.
A deferred tax asset is recognized only to the extent that it is more likely than not that future taxable profits will be available against which the asset can be utilized. To the extent that we do not consider it more likely than not that a deferred tax asset will be recovered, we provide a valuation allowance against that excess.
We periodically evaluate our tax positions to determine whether it is more likely than not that a tax position will be sustained upon examination by the appropriate taxing authorities. We have not incurred any interest or penalties as of August 31, 2014 with respect to uncertain income tax matters. We do not expect that there will be unrecognized tax benefits of a significant nature that will increase or decrease within 12 months of the reporting date.
We file income tax returns in the U.S. federal, Canadian and state jurisdictions. Management believes that there are no material uncertain tax positions that would impact the consolidated financial statements. Our policy is to recognize interest and penalties related to unrecognized tax benefits in income tax expense. We may be subject to examination by the Internal Revenue Service for tax years 2010 through 2013 and by the Canada Revenue Agency for tax years 2010 through 2014. We may also be subject to examination on certain state and local jurisdictions for the tax years 2009 through 2013.
Loss Per Share
Basic earnings (loss) per share is calculated by dividing income available to our shareholders by the weighted average number of common shares outstanding during the period.
| Page 35 |
The computation of diluted loss per share assumes the conversion, exercise or contingent issuance of securities only when such conversion, exercise or issuance would have a dilutive effect on loss per share. The dilutive effect of convertible securities is reflected in diluted earnings per share by application of the “if converted” method. The dilutive effect of outstanding options and warrants and their equivalents is reflected in diluted earnings per share by application of the treasury stock method. Conversion of outstanding warrants, broker units and options would have an antidilutive effect on loss per share for the years ended August 31, 2014, 2013 and 2012, and are therefore excluded from the computation of diluted loss per share.
Segments
We operate in one reportable segment and, accordingly, no segment disclosures have been presented. All equipment, leasehold improvements and other fixed assets owned by us are physically located within the United States, and all supply, collaboration and licensing agreements are denominated in U.S. dollars. The geographic markets of our potential customers are principally Europe, the United States and Asia. The geographic breakdown of revenues in fiscal 2014 was 41% Europe, 40% Asia, 14% U.S., and 6% Canada; fiscal 2013 was 84% Europe, 12% U.S., 3% South America and 1% Canada; and fiscal 2012 was 42% Europe and 58% U.S.
CERTAIN TAX CONSIDERATIONS
United States Federal Income Taxation
As used below, a “U.S. holder” is a beneficial owner of a common share that is, for U.S. federal income tax purposes, (i) a citizen or resident alien individual of the United States, (ii) a corporation (or an entity treated as a corporation) created or organized under the law of the United States, any State thereof or the District of Columbia, (iii) an estate the income of which is subject to U.S. federal income tax without regard to its source or (iv) a trust if (1) a court within the United States is able to exercise primary supervision over the administration of the trust, and one or more United States persons have the authority to control all substantial decisions of the trust, or (2) the trust has a valid election in effect under applicable U.S. Treasury Regulations to be treated as a United States person. For purposes of this discussion, a “non-U.S. holder” is a beneficial owner of a common share that is (i) a nonresident alien individual, (ii) a corporation (or an entity treated as a corporation) created or organized in or under the law of a country other than the United States or a political subdivision thereof or (iii) an estate or trust that is not a U.S. Holder. If a partnership (including for this purpose any entity treated as a partnership for U.S. federal tax purposes) is a beneficial owner of a common share, the U.S. federal tax treatment of a partner in the partnership generally will depend on the status of the partner and the activities of the partnership. A holder of a common share that is a partnership and partners in that partnership should consult their own tax advisers regarding the U.S. federal income tax consequences of holding and disposing of common shares. We have not sought a ruling from the Internal Revenue Service (“IRS”) or an opinion of counsel as to any U.S. federal income tax consequence described herein. The IRS may disagree with the description herein, and its determination may be upheld by a court. This discussion does not address U.S. federal tax laws other than those pertaining to U.S. federal income taxation (such as estate or gift tax laws or the recently enacted Medicare tax on investment income), nor does it address any aspects of U.S. state or local or non-U.S. taxation.
GIVEN THE COMPLEXITY OF THE TAX LAWS AND BECAUSE THE TAX CONSEQUENCES TO ANY PARTICULAR SHAREHOLDER MAY BE AFFECTED BY MATTERS NOT DISCUSSED HEREIN, SHAREHOLDERS ARE URGED TO CONSULT THEIR OWN TAX ADVISORS WITH RESPECT TO THE SPECIFIC TAX CONSEQUENCES OF THE ACQUISITION, OWNERSHIP AND DISPOSITION OF COMMON SHARES, INCLUDING THE APPLICABILITY AND EFFECT OF STATE, LOCAL AND NON-U.S. TAX LAWS, AS WELL AS U.S. FEDERAL TAX LAWS.
Taxation of Dividends
U.S. Holders. In general, subject to the passive foreign investment company rules discussed below, a distribution on a common share will constitute a dividend for U.S. federal income tax purposes to the extent that it is made from our current or accumulated earnings and profits as determined under U.S. federal income tax principles. If a distribution exceeds our current and accumulated earnings and profits, it will be treated as a non-taxable reduction of basis to the extent of the U.S. holder’s tax basis in the common share on which it is paid, and to the extent it exceeds that basis it will be treated as capital gain. For purposes of this discussion, the term “dividend” means a distribution that constitutes a dividend for U.S. federal income tax purposes.
| Page 36 |
The gross amount of any dividend on a common share (which will include the amount of any Canadian taxes withheld) generally will be subject to U.S. federal income tax as foreign source dividend income, and will not be eligible for the corporate dividends received deduction. The amount of a dividend paid in Canadian dollars will be its value in U.S. dollars based on the prevailing spot market exchange rate in effect on the day the U.S. holder receives the dividend. A U.S. holder will have a tax basis in any distributed Canadian dollars equal to its U.S. dollar amount on the date of receipt, and any gain or loss realized on a subsequent conversion or other disposition of Canadian dollars generally will be treated as U.S. source ordinary income or loss. If dividends paid in Canadian dollars are converted into U.S. dollars on the date they are received by a U.S. holder, the U.S. holder generally should not be required to recognize foreign currency gain or loss in respect of the dividend income.
Subject to certain exceptions for short-term and hedged positions, as well as the passive foreign investment rules, a dividend that a non-corporate holder receives on a common share will generally be subject to a maximum federal income tax rate of 20% if the dividend is a “qualified dividend.” A dividend on a common share will be a qualified dividend if (i) either (a) the common shares are readily tradable on an established market in the United States or (b) we are eligible for the benefits of a comprehensive income tax treaty with the United States that the Secretary of the Treasury determines is satisfactory for purposes of these rules and that includes an exchange of information program, and (ii) we were not, in the year prior to the year the dividend was paid, and are not, in the year the dividend is paid, a passive foreign investment company (“PFIC”). The common shares are listed on the OTCQB, which may be treated as an established securities market in the United States. In any event, the U.S. Canada Income Convention (the “Treaty”) satisfies the requirements of clause (i)(b), the Company is incorporated in and tax resident of Canada and should be entitled to the benefits of the Treaty. However, based on our audited financial statements and relevant market and shareholder data, we believe that we would likely be classified as a PFIC in the current taxable year and may be classified as a PFIC in future taxable years. Accordingly, no assurance can be made that a dividend paid, if any, would be a “qualified dividend.” In addition, as described in the section below entitled “Passive Foreign Investment Company Rules,” if we were a PFIC in a year while a U.S. holder held a common share, and if the U.S. holder has not made a qualified electing fund election effective for the first year the U.S. holder held the common share, the ordinary share underlying the common share remains an interest in a PFIC for all future years or until such an election is made. The IRS takes the position that such rule will apply for purposes of determining whether a common share is an interest in a PFIC in the year a dividend is paid or in the prior year, even if we do not satisfy the tests to be a PFIC in either of those years. Even if dividends on the common shares would otherwise be eligible for qualified dividend treatment, in order to qualify for the reduced qualified dividend tax rates, a non-corporate holder must hold the ordinary share on which a dividend is paid for more than 60 days during the 120-day period beginning 60 days before the ex-dividend date, disregarding for this purpose any period during which the non-corporate holder has an option to sell, is under a contractual obligation to sell or has made (and not closed) a short sale of substantially identical stock or securities, is the grantor of an option to buy substantially identical stock or securities or, pursuant to Treasury regulations, has diminished their risk of loss by holding one or more other positions with respect to substantially similar or related property. In addition, to qualify for the reduced qualified dividend tax rates, the non-corporate holder must not be obligated to make related payments with respect to positions in substantially similar or related property. Payments in lieu of dividends from short sales or other similar transactions will not qualify for the reduced qualified dividend tax rates.
A non-corporate holder that receives an extraordinary dividend eligible for the reduced qualified dividend rates must treat any loss on the sale of the stock as a long-term capital loss to the extent of the dividend. For purposes of determining the amount of a non-corporate holder’s deductible investment interest expense, a dividend is treated as investment income only if the non-corporate holder elects to treat the dividend as not eligible for the reduced qualified dividend tax rates. Special limitations on foreign tax credits with respect to dividends subject to the reduced qualified dividend tax rates apply to reflect the reduced rates of tax.
The U.S. Treasury has announced its intention to promulgate rules pursuant to which non-corporate holders of stock of non-U.S. corporations, and intermediaries through whom the stock is held, will be permitted to rely on certifications from issuers to establish that dividends are treated as qualified dividends. Because those procedures have not yet been issued, it is not clear whether we will be able to comply with them.
| Page 37 |
Non-corporate holders of ordinary shares are urged to consult their own tax advisers regarding the availability of the reduced qualified dividend tax rates with respect to dividends, if any, received on the common shares in the light of their own particular circumstances.
Any Canadian withholding tax imposed on dividends received with respect to the common shares will be treated as a foreign income tax eligible for credit against a U.S. holder’s U.S. federal income tax liability, subject to generally applicable limitations under U.S. federal income tax law. For purposes of computing those limitations separately under current law for specific categories of income, a dividend generally will constitute foreign source “passive category income” or, in the case of certain holders, “general category income.” A U.S. holder will be denied a foreign tax credit with respect to Canadian income tax withheld from dividends received with respect to the common shares to the extent the U.S. holder has not held the common shares for at least 16 days of the 30-day period beginning on the date which is 15 days before the ex-dividend date or to the extent the U.S. holder is under an obligation to make related payments with respect to substantially similar or related property. Any days during which a U.S. holder has substantially diminished its risk of loss on the common shares are not counted toward meeting the 16-day holding period required by the statute. The rules relating to the determination of the foreign tax credit are complex, and U.S. holders are urged to consult with their own tax advisers to determine whether and to what extent they will be entitled to foreign tax credits as well as with respect to the determination of the foreign tax credit limitation. Alternatively, any Canadian withholding tax may be taken as a deduction against taxable income, provided the U.S. holder takes a deduction and not a credit for all foreign income taxes paid or accrued in the same taxable year. In general, special rules will apply to the calculation of foreign tax credits in respect of dividend income that is subject to preferential rates of U.S. federal income tax.
Non-U.S. Holders. A dividend paid to a non-U.S. holder of a common share will generally not be subject to U.S. federal income tax unless the dividend is effectively connected with the conduct of trade or business by the non-U.S. holder within the United States (and is attributable to a permanent establishment or fixed base the non-U.S. holder maintains in the United States if an applicable income tax treaty so requires as a condition for the non-U.S. holder to be subject to U.S. taxation on a net income basis on income from the common share). A non-U.S. holder generally will be subject to tax on an effectively connected dividend in the same manner as a U.S. holder. A corporate non-U.S. holder under certain circumstances may also be subject to an additional “branch profits tax,” the rate of which may be reduced pursuant to an applicable income tax treaty.
Taxation of Capital Gains
U.S. Holders. Subject to the passive foreign investment company rules discussed below, on a sale or other taxable disposition of a common share, a U.S. holder will recognize capital gain or loss in an amount equal to the difference between the U.S. holder’s adjusted basis in the common share and the amount realized on the sale or other disposition, each determined in U.S. dollars. Such capital gain or loss will be long-term capital gain or loss if at the time of the sale or other taxable disposition the common share has been held for more than one year. In general, any adjusted net capital gain of an individual is subject to a maximum federal income tax rate of 20%. Capital gains recognized by corporate U.S. holders generally are subject to U.S. federal income tax at the same rate as ordinary income. The deductibility of capital losses is subject to limitations.
Any gain a U.S. holder recognizes generally will be U.S. source income for U.S. foreign tax credit purposes, and, subject to certain exceptions, any loss will generally be a U.S. source loss. If a Canadian tax is paid on a sale or other disposition of a common share, the amount realized will include the gross amount of the proceeds of that sale or disposition before deduction of the Canadian tax. The generally applicable limitations under U.S. federal income tax law on crediting foreign income taxes may preclude a U.S. holder from obtaining a foreign tax credit for any Canadian tax paid on a sale or other disposition of a common share. The rules relating to the determination of the foreign tax credit are complex, and U.S. holders are urged to consult with their own tax advisers regarding the application of such rules. Alternatively, any Canadian tax paid on the sale or other disposition of a common share may be taken as a deduction against taxable income, provided the U.S. holder takes a deduction and not a credit for all foreign income taxes paid or accrued in the same taxable year.
| Page 38 |
Non-U.S. Holders. A non-U.S. holder will not be subject to U.S. federal income tax on gain recognized on a sale or other disposition of a common share unless (i) the gain is effectively connected with the conduct of trade or business by the non-U.S. holder within the United States (and is attributable to a permanent establishment or fixed base the non-U.S. holder maintains in the United States if an applicable income tax treaty so requires as a condition for the non-U.S. holder to be subject to U.S. taxation on a net income basis on income from the common share), or (ii) in the case of a non-U.S. holder who is an individual, the holder is present in the United States for 183 or more days in the taxable year of the sale or other disposition and certain other conditions apply. Any effectively connected gain of a corporate non-U.S. holder may also be subject under certain circumstances to an additional “branch profits tax,” the rate of which may be reduced pursuant to an applicable income tax treaty.
Passive Foreign Investment Company Rules
A special set of U.S. federal income tax rules applies to a foreign corporation that is a PFIC for U.S. federal income tax purposes. As noted above, based on our audited financial statements and relevant market and shareholder data, we believe may be classified as a PFIC in the current taxable year. In addition, given that the determination of PFIC status involves the application of complex tax rules, and that it is based on the nature of our income and assets from time to time, no assurances can be provided that we will not be considered a PFIC for any past or future taxable years.
In general, a non-US corporation is a PFIC if in any taxable year either (i) at least 75% of its gross income is “passive income” or (ii) at least 50% of the quarterly average value of its assets is attributable to assets that produce or are held to produce “passive income.” In applying these tests, the Company generally is treated as holding its proportionate share of the assets and receiving its proportionate share of the income of any other corporation in which the Company owns at least 25% by value of the shares. Passive income for this purpose generally includes dividends, interest, royalties, rent and capital gains, but generally does not include certain royalties derived in an active business. Passive assets are those assets that are held for production of passive income or do not produce income at all. Thus cash will be a passive asset. Interest, including interest on working capital, is treated as passive income for purposes of the income test. Without taking into account the value of its goodwill, more than 50% of the Company’s assets by value would be passive so that the Company would be a PFIC under the asset test. Based upon its current operations, its goodwill (the value of which should be based upon the Company’s market capitalization) may be attributable to its activities that will generate active income and accordingly, may be treated as an active asset. Even if the Company is not a PFIC under the asset test, however, the Company may qualify as a PFIC in its current taxable year under the income test, since the Company to date has generally had no gross income from active operations (after deducting cost of sales from gross receipts), but generally has some interest income. The determination of whether a foreign corporation is a PFIC is a factual determination made annually and is therefore subject to change. Subject to exceptions pursuant to certain elections that generally require the payment of tax, once stock in a foreign corporation is stock in a PFIC in the hands of a particular shareholder that is a United States person, it remains stock in a PFIC in the hands of that shareholder.
If we are treated as a PFIC, contrary to the tax consequences described in “Taxation of Dividends” and “Taxation of Capital Gains” above, a U.S. holder that does not make an election described in the succeeding two paragraphs would be subject to special rules with respect to (i) any gain realized on a sale or other disposition of a common share (for purposes of these rules, a disposition of a common share includes many transactions on which gain or loss is not realized under general U.S. federal income tax rules) and (ii) any “excess distribution” by the Company to the U.S. holder (generally, any distribution during a taxable year in which distributions to the U.S. holder on the common share exceed 125% of the average annual taxable distributions (whether actual or constructive and whether or not out of earnings and profits) the U.S. holder received on the common share during the preceding three taxable years or, if shorter, the U.S. holder’s holding period for the common share). Under those rules, (i) the gain or excess distribution would be allocated ratably over the U.S. holder’s holding period for the common share, (ii) the amount allocated to the taxable year in which the gain or excess distribution is realized would be taxable as ordinary income in its entirety and not as capital gain, would be ineligible for the reduced qualified dividend rates, and could not be offset by any deductions or losses, and (iii) the amount allocated to each prior year, with certain exceptions, would be subject to tax at the highest tax rate in effect for that year, and the interest charge generally applicable to underpayments of tax would be imposed in respect of the tax attributable to each of those years. A U.S. holder who owns a common share during any year we are a PFIC will generally have to file IRS Form 8621.
| Page 39 |
The special PFIC rules described above will not apply to a U.S. holder if the U.S. holder makes a timely election, which remains in effect, to treat the Company as a “qualified electing fund” (“QEF”) in the first taxable year in which the U.S. holder owns a common share and the Company is a PFIC and if the Company complies with certain reporting requirements. Instead, a shareholder of a QEF generally is currently taxable on a pro rata share of the Company’s ordinary earnings and net capital gain as ordinary income and long-term capital gain, respectively. Neither that ordinary income nor any actual dividend from the Company would qualify for the 20% maximum federal income tax rate on dividends described above if the Company is a PFIC in the taxable year the ordinary income is realized or the dividend is paid or in the preceding taxable year. A QEF election cannot be made unless the Company provides U.S. Holders the information and computations needed to report income and gains pursuant to a QEF election. The Company expects that will not provide this information. It is, therefore, likely that U.S. holders would not be able to make a QEF election in any year we are a PFIC.
In lieu of a QEF election, a U.S. holder of stock in a PFIC that is considered marketable stock could elect to mark the stock to market annually, recognizing as ordinary income or loss each year an amount equal to the difference as of the close of the taxable year between the fair market value of the stock and the U.S. holder’s adjusted basis in the stock. Losses would be allowed only to the extent of net mark-to-market gain previously included in income by the U.S. holder under the election for prior taxable years. A U.S. holder’s adjusted basis in the common shares will be adjusted to reflect the amounts included or deducted with respect to the mark-to-market election. If the mark-to-market election were made, the rules set forth in the second preceding paragraph would not apply for periods covered by the election. A mark-to-market election will not apply during any later taxable year in which the Company does not satisfy the tests to be a PFIC. In general, the common shares will be marketable stock if the common shares are traded, other than in de minimis quantities, on at least 15 days during each calendar quarter on a national securities exchange that is registered with the SEC or on a designated national market system or on any exchange or market that the Treasury Department determines to have rules sufficient to ensure that the market price accurately represents the fair market value of the stock. Under current law, the mark-to-market election may be available to U.S. holders of common shares because the common shares are listed on the OTCQB and the TSX Venture Exchange (at least one of which should constitute a qualified exchange for this purpose), although there can be no assurance that the common shares will be “regularly traded” for purposes of the mark-to-market election.
Given the complexities of the PFIC rules and their potentially adverse tax consequences, U.S. holders of common shares are urged to consult their tax advisers about the PFIC rules.
Medicare Surtax on Net Investment Income
Non-corporate U.S. Holders whose income exceeds certain thresholds generally will be subject to 3.8% surtax on their “net investment income” (which generally includes, among other things, dividends on, and capital gain from the sale or other taxable disposition of, the common shares). Absent an election to the contrary, if a QEF election is available and made, QEF inclusions will not be included in net investment income at the time a U.S. Holder includes such amounts in income, but rather will be included at the time distributions are received or gains are recognized. Non-corporate U.S. Holders should consult their own tax advisors regarding the possible effect of such tax on their ownership and disposition of the common shares, in particular the applicability of this surtax with respect to a non-corporate U.S. Holder that makes a QEF or mark-to-market election in respect of their common shares.
Information Reporting and Backup Withholding
Dividends paid on, and proceeds from the sale or other disposition of, a common share to a U.S. holder generally may be subject to information reporting requirements and may be subject to backup withholding unless the U.S. holder provides an accurate taxpayer identification number or otherwise establishes an exemption. The amount of any backup withholding collected from a payment to a U.S. holder will be allowed as a credit against the U.S. holder’s U.S. federal income tax liability and may entitle the U.S. holder to a refund, provided certain required information is furnished to the Internal Revenue Service. A non-U.S. holder generally will be exempt from these information reporting requirements and backup withholding tax but may be required to comply with certain certification and identification procedures in order to establish its eligibility for exemption.
| Page 40 |
Under U.S. federal income tax law and U.S. Treasury Regulations, certain categories of U.S. holders must file information returns with respect to their investment in, or involvement in, a foreign corporation. U.S. holders are urged to consult with their own tax advisors concerning such reporting requirements.
Reporting Obligations of Individual Owners of Foreign Financial Assets
Section 6038D of the Code generally requires U.S. individuals (and possibly certain entities that have U.S. individual owners) to file IRS Form 8938 if they hold certain “specified foreign financial assets,” the aggregate value of which exceeds $50,000. The definition of specified foreign financial assets includes not only financial accounts maintained in foreign financial institutions, but also, unless held in accounts maintained by a financial institution, any stock or security issued by a non-US. person, any financial instrument or contract held for investment that has an issuer or counterparty other than a U.S. person and any interest in a foreign entity.
Canadian Federal Income Tax Consequences
The following summary of the material Canadian federal income tax consequences is stated in general terms and is not intended to be legal or tax advice to any particular shareholder. Each shareholder or prospective shareholder is urged to consult his or her own tax advisor regarding the tax consequences of his or her purchase, ownership and disposition of common shares. The tax consequences to any particular holder of common shares will vary according to the status of that holder as an individual, trust, corporation or member of a partnership, the jurisdiction in which that holder is subject to taxation, the place where that holder is resident and, generally, according to that holder’s particular circumstances.
This summary is applicable only to holders who are resident in the United States for income tax purposes, have never been resident in Canada for income tax purposes, deal at arm’s length with the Company, hold their common shares as capital property and who will not use or hold the common shares in carrying on business in Canada. Special rules, which are not discussed in this summary, may apply to a United States holder that is an issuer that carries on business in Canada and elsewhere.
This summary is based upon the provisions of the Income Tax Act (Canada) and the regulations thereunder (collectively, the “Tax Act” or “ITA”) and the Canada-United States Tax Convention (the “Tax Convention”) at the date of this Annual Report and the current administrative practices of the Canada Revenue Agency. This summary does not take into account provincial income tax consequences. The comments in this summary that are based on the Tax Convention are applicable to U.S. holders only if they qualify for benefits under the Tax Convention. Management urges each holder to consult his own tax advisor with respect to the income tax consequences applicable to him in his own particular circumstances.
Non-Resident Holders
The summary below is restricted to the case of a holder (a “Holder”) of one or more common shares who for the purposes of the Tax Act is a non-resident of Canada, holds his common shares as capital property and deals at arm’s length with the Company.
Dividends
A Holder will be subject to Canadian withholding tax (“Part XIII Tax”) equal to 25%, or such lower rates as may be available under an applicable tax treaty, of the gross amount of any dividend paid or deemed to be paid on his common shares. The Company will be required to withhold the applicable amount of Part XIII Tax from each dividend so paid and remit the withheld amount directly to the Receiver General for Canada for the account of the Holder.
| Page 41 |
Disposition of Common Shares
A Holder who disposes of common shares, including by deemed disposition on death, will not be subject to Canadian tax on any capital gain thereby realized unless the common share constituted “taxable Canadian property” as defined by the Tax Act. Generally, a common share of a public corporation will not constitute taxable Canadian property of a Holder unless he held the common share as capital property used by him carrying on a business in Canada, or he, persons with whom he did not deal at arm’s length or (under currently proposed rules) partnerships in which he or persons with whom he did not deal at arm’s length held an interest, alone or together held or held options to acquire, at any time within the 60 months preceding the disposition, 25% or more of the issued shares of any class of the capital stock of the Company and at any time during the 60 months preceding the disposition more than 50% of the value of the common shares is derived from, or from an interest in, Canadian real estate, including Canadian resource or timber resource properties.
Holders Resident in the United States
A Holder who is a resident of the United States and realizes a capital gain on disposition of common shares that was taxable Canadian property will, if qualified for benefits under the Tax Convention, generally be exempt from Canadian tax thereon unless (a) more than 50% of the value of the common shares is derived from, or from an interest in, Canadian real estate, including Canadian mineral resources properties, (b) the common shares formed part of the business property of a permanent establishment that the Holder has or had in Canada within the 12 months preceding disposition, or (c) the Holder (i) was a resident of Canada at any time within the ten years immediately preceding the disposition, and for a total of 120 months during any period of 20 consecutive years, preceding the disposition, (ii) owned the common shares when he ceased to be resident in Canada, and (iii) the common shares were not subject to a deemed disposition on the Holder’s departure from Canada.
Inclusion in Taxable Income
A Holder who is subject to Canadian tax in respect of a capital gain realized on disposition of common shares must include one half of the capital gain (“taxable capital gain”) in computing his taxable income earned in Canada. The Holder may, subject to certain limitations, deduct one half of any capital loss (“allowable capital loss”) arising on disposition of taxable Canadian property from taxable capital gains realized in the year of disposition in respect to taxable Canadian property and, to the extent not so deductible, from such taxable capital gains of any of the three preceding years or any subsequent year.
Subject to certain exceptions, a non-resident person who disposes of taxable Canadian property must notify the Canada Revenue Agency either before or after the disposition (within ten days of the disposition).
Item 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK.
We are exposed to financial market risks associated with foreign exchange rates, concentration of credit, and liquidity. In accordance with our policies, we manage our exposure to various market-based risks and where material, these risks are reviewed and monitored by our Board of Directors.
Foreign Exchange Risk
Our exposure to foreign exchange risk is primarily related to fluctuations between the Canadian dollar and the U.S. dollar. We incur operating expenses and capital expenditures mostly in U.S. dollars, with some operating expenses incurred in Canadian dollars which are subject to foreign currency fluctuations. The fluctuation of the U.S. dollar in relation to the Canadian dollar will have an impact upon our profitability and may also affect the value of our assets and the amount of shareholders’ equity. We have not entered into any agreements or purchased any instruments to hedge possible currency risks. At August 31, 2014, we held approximately $3,946,000 in cash and cash equivalents and short-term investments in Canadian dollars and the U.S. dollar was equal to 1.087 Canadian dollars. Based on the exposure at August 31, 2014, a 5% change in the Canadian/U.S. exchange rate would impact our net loss by approximately $197,000.
| Page 42 |
Concentration of Credit Risk
We are potentially subject to financial instrument concentration of credit risk through our cash equivalents and accounts receivables. We place our cash and cash equivalents in financial institutions believed to be credit worthy and perform periodic evaluations of their relative credit standing. Accounts receivables can be potentially exposed to a concentration of credit risk with our major customers.
Approximately 73%, 73% and 90% of our product sales and contract services revenue during the years ended August 31, 2014, 2013 and 2012, respectively, were from two customers. All of the grant revenue during the years ended August 31, 2014, 2013 and 2012 was received from the National Science Foundation.
Approximately 76% of our accounts receivable at August 31, 2014 were from one customer. Approximately 88% of our accounts receivable at August 31, 2013 was from a grant from the National Science Foundation.
We assess the collectability of our accounts receivable through a review of our current aging, as well as an analysis of our historical collection rate, general economic conditions and credit status of our customers. As of August 31, 2014 and 2013, all outstanding accounts receivable were deemed to be fully collectible, and therefore, no allowance for doubtful accounts was recorded. We determine terms and conditions for our customers primarily based on the volume purchased by the customer, customer creditworthiness and past transaction history.
Management works to mitigate our concentration of credit risk with respect to accounts receivable through our credit evaluation policies, reasonably short payment terms and geographical dispersion of sales. Revenue includes export sales to foreign companies located principally in Europe and Asia.
Liquidity Risk
Liquidity risk is the risk we will not be able to meet our financial obligations as they fall due. We attempt to manage liquidity risk by maintaining sufficient cash and cash equivalent balances. Liquidity requirements are managed based on expected cash flows to ensure that there is sufficient capital in order to meet our short term obligations. At August 31, 2014 and 2013, we had a cash and cash equivalents balance of $13,427,404 and $7,859,889, respectively, to settle current liabilities of $1,420,666 and $3,874,158, respectively. Current liabilities include the current portion of our warrant liability in the amount of $879,040 and $3,454,745, respectively, which will not be settled in cash.
| Item 8. | FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA. |
The financial statements and related financial information required to be filed hereunder are indexed under Item 15 of this Annual Report on Form 10-K and are incorporated herein by reference.
| Page 43 |
| Item 9. | CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE. |
Effective June 3, 2014, we terminated our relationship with D&H Group LLP, Chartered Accountants as our principal independent registered public accounting firm. The report of D&H Group LLP on the financial statements for our fiscal years ended August 31, 2012 through August 31, 2013 previously issued contained no adverse opinion or any disclaimer of opinion, and such reports were not qualified or modified as to uncertainty, audit scope or accounting principles, other than to state that there was substantial doubt as to our ability to continue as a going concern in fiscal 2012. Effective June 3, 2014, we engaged Moss Adams LLP, Certified Public Accountants as our principal independent registered public accounting firm. Our Board of Directors recommended the decision to change our principal independent registered public accounting firm. The termination of D&H Group LLP was not the result of any disagreement or dispute.
During the years ended August 31, 2014 and 2013, and through the interim period preceding such termination, there were no disagreements between us and D&H Group LLP on any matter of accounting principles or practices, financial statement disclosure or auditing scope or procedure, which disagreements, if not resolved to the satisfaction of D&H Group LLP, would have caused D&H Group LLP to make reference to the subject matter of the disagreements in connection with its reports. Further, there were no “reportable events,” as described in Item 304(a)(1)(iv) of Regulation S-K of the SEC promulgated under the Exchange Act.
| Item 9A. | CONTROLS AND PROCEDURES. |
Disclosure Controls and Procedures
Our management is responsible for establishing and maintaining disclosure controls and procedures to provide reasonable assurance that material information related to our Company, including our consolidated subsidiaries, is made known to senior management, including our Chief Executive Officer and the Chief Financial Officer, by others within those entities on a timely basis so that appropriate decisions can be made regarding public disclosure.
We carried out an evaluation, under the supervision and with the participation of our management, including our Principal Executive Officer and our Principal Financial Officer, of the effectiveness of the design and operation of our disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e)) under the Securities and Exchange Act of 1934, as amended) as of August 31, 2014. Our Chief Executive Officer and Chief Financial Officer concluded that the disclosure controls and procedures as of August 31, 2014, were effective to give reasonable assurance that the information required to be disclosed by us in reports that we file or submit under the Exchange Act is (i) recorded, processed, summarized and reported, within the time periods specified in the SEC’s rules and forms, and (ii) accumulated and communicated to management, including the Chief Executive Office and Chief Financial Officer, as appropriate to allow timely decisions regarding required disclosure.
Management’s Annual Report on Internal Control over Financial Reporting
Our management is responsible for designing, establishing and maintaining a system of internal controls over financial reporting (as defined in Exchange Act Rule 13a-15(f)) to provide reasonable assurance that the financial information prepared by us for external purposes is reliable and has been recorded, processed and reported in an accurate and timely manner in accordance with accounting principles generally accepted in the United States. The Board of Directors is responsible for ensuring that management fulfills its responsibilities. The Audit Committee fulfills its role of ensuring the integrity of the reported information through its review of the interim and annual financial statements. Management reviewed the results of their assessment with our Audit Committee.
Because of its inherent limitations, our internal control over financial reporting may not prevent or detect all possible misstatements or frauds. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with policies or procedures may deteriorate.
Management has used the criteria issued by the Committee of Sponsoring Organizations of the Treadway Commission (“COSO”) in “Internal Control — Integrated Framework of 1992” to evaluate the effectiveness of our internal control over financial reporting. Management has assessed the effectiveness of our internal control over financial reporting and concluded that such internal control over financial reporting was effective as of August 31, 2014.
| Page 44 |
Attestation Report of Our Registered Public Accounting Firm
This Annual Report does not include an attestation report from our independent registered public accounting firm. We are an “emerging growth company,” as defined under the JOBS Act, and are subject to reduced public company reporting requirements. The JOBS Act provides that an emerging growth company is not required to have the effectiveness of such company’s internal control over financial reporting audited by its external auditors for as long as such company is deemed to be an emerging growth company.
Limitations on the Effectiveness of Controls
Our management, including the Chief Executive Officer and Chief Financial Officer, does not expect that our disclosure controls or our internal controls will prevent all error and all fraud. A control system, no matter how well designed and operated, can provide only reasonable, not absolute, assurance that the objectives of the control system are met. Further, the design of a control system must reflect the fact that there are resource constraints, and the benefits of controls must be considered relative to their costs. Because of the inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that all control issues and instances of fraud, if any, within our Company have been detected. These inherent limitations include the realities that judgments in decision-making can be faulty, and that breakdowns can occur because of simple error or mistake. Additionally, controls can be circumvented by the individual acts of some persons, by collusion of two or more people, or by management override of the control. The design of any system of controls is also based, in part, upon certain assumptions about the likelihood of future events, and there can be no assurance that any design will succeed achieving its stated goals under all potential future conditions; over time, control may become inadequate because of changes in conditions, or the degree of compliance with the policies or procedures may deteriorate. Because of the inherent limitations in a cost-effective control system, misstatements due to error or fraud may occur and not be detected.
Changes in Internal Control over Financial Reporting
There were no changes in our internal control over financial reporting that occurred during the fourth quarter ended August 31, 2014 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
| Item 9B. | OTHER INFORMATION. |
On June 3, 2014, our Board of Directors approved a change to our fiscal year-end from August 31 to September 30, with effect from September 1, 2014.
| Page 45 |
| Item 10. | DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE. |
Directors
Set forth below is certain information with respect to our directors, including each director’s name, age as of November 1, 2014, and Committee membership. Each director is elected annually to serve until the annual general and special meeting of shareholders, or until his or her successor is duly elected.
| Name | Age | Position(s) Held | Director Since | |||
| Gregory T. Baxter, Ph.D. (1)(2)(3) | 53 | Director | August 15, 2012 | |||
| Tessie M. Che, Ph.D. | 64 | Director | September 25, 2013 | |||
| David L. Hill, Ph.D. (1)(2)(3) | 61 | Director | May 17, 2011 | |||
| Daniel E. Morse, Ph.D. | 71 | Director | April 9, 2010 | |||
| Frank R. Oakes | 63 | President, Chief Executive Officer and Chairman of our Board of Directors | April 9, 2010 | |||
| Mayank D. Sampat (1)(2)(3) | 57 | Director | August 15, 2012 |
(1) Member of Audit Committee.
(2) Member of Compensation Committee.
(3) Member of the Nominating and Corporate Governance Committee.
Biographies and Qualifications. The biographies of our directors and certain information regarding each director’s experience, attributes, skills and/or qualifications that led to the conclusion that the director should be serving as a director of our Company are as follows:
Gregory T. Baxter, Ph.D. has been a director of our Company since August 2012, and serves as chairman of the Nominating and Corporate Governance Committee. Dr. Baxter has served as an executive and scientist for several biotechnology corporations and foundations. Since 2001, he has held the position of Senior Scientist within the Department of Clinical Drug Development for CCS Associates Inc. Dr. Baxter previously served as a Program Director with the National Science Foundation and was also the founder and Chief Science Officer of Hurel Corporation. Prior to his time at Hurel, he was a Senior Scientist at the Cornell Nanoscale Science and Technology Facility and the Biotechnology Liaison for the National Nanofabrication Users Network. He also serves as Adjunct Associate Professor in College of Chemical Engineering at Cornell University and as a member of the Founders Board of Advisors at StartX Stanford Student Startup Accelerator. Dr. Baxter received his B.A. and Ph.D. in Biochemistry/Molecular Biology from University of California, Santa Barbara. Dr. Baxter has extensive scientific, clinical drug development and senior management experience in the pharmaceutical and biotechnology industries.
Tessie M. Che, Ph.D. was appointed a director of our Company as a result of our September 2013 private placement. Dr. Che currently serves as General Manager and Chair of the Board of Directors of Amaran Biotechnology Inc., a privately-held biopharmaceuticals manufacturer based in Taiwan, a position she has held since 2012. She co-founded Optimer Pharmaceuticals Inc. in 1998, and served as Optimer’s Chief Operating Officer and Senior Vice-President of Corporate Affairs from1998 to 2011. During the process development years of Optimer’s flagship drug, DificidTM, Dr. Che built and led the company’s chemistry, manufacturing and quality control (CMC) teams through the successful and cost-effective registration and commercialization of Dificid in the United States, Canada and Europe in 2011. Prior to her founding of Optimer, Dr. Che’s past experience included 20 years in research, management and operations at large companies, including Exxon Mobil Corp., Aventis Pharmaceuticals Inc. and EniChem SpA. She also served as vice president, operations, of M and D Precision Science Group Inc. in 1988, and co-founded Cinogen Pharmaceuticals Inc. (China) serving as vice-president from 1994 to 1996. Cinogen later became a wholly owned subsidiary of Pharmanex Inc., where Dr. Che served as senior director of quality assurance and sourcing. Dr. Che holds bachelor degrees in chemistry from Illinois State University and Fu-Jen Catholic University (Taiwan), a PhD in physical-inorganic chemistry from Brandeis University, and did postdoctoral work at Columbia University. She has authored numerous scientific publications and holds over 20 U.S. patents in material synthesis and applications. Dr. Che currently serves as a director of OBI Pharma USA, a wholly-owned subsidiary of OBI Pharma, Inc., a publicly traded biotechnology corporation in Taiwan. Dr. Che has extensive scientific, operational, manufacturing, quality assurance, product development and senior management experience in the pharmaceutical and biotechnology industries, as well as experience serving on a board of directors within our industry.
| Page 46 |
David L. Hill, Ph.D. has been a director of our Company since May 2011, and serves as chairman of the Compensation Committee. He currently serves as Scientific Director for the ART Reproductive Center, Beverly Hills, California, a position he has held since December 1999. He is also an Assistant Clinical Professor in the Dept. of Obstetrics and Gynecology at the David Geffen School of Medicine, University of California, Los Angeles, and a Research Assistant IV at Cedars-Sinai Medical Center, Los Angeles, California. Dr. Hill received his Ph.D. in Biological Sciences from the Department of Pathology, School of Life Sciences, University of Connecticut and completed a Postdoctoral Fellowship at the Dana Farber Cancer Institute through an appointment by the Department of Physiology and Biophysics, Harvard Medical School, Boston, Massachusetts. Dr. Hill has extensive scientific and clinical research experience in our industry.
Daniel E. Morse, Ph.D. has been a director of our Company since April 2010. Dr. Morse is the Wilcox Professor Emeritus of Molecular Genetics and Biochemistry Biotechnology, Biomolecular Science and Engineering, a position he has held since 2008, and Director of the Marine Biotechnology Center, at the University of California, Santa Barbara, a position he has held since 1986. Previously, he served as Director of the UCSB-MIT-Caltech Institute of Collaborative Biotechnologies from 2003 to 2010, and also served as our Executive Vice-President, Science & Technology from 2010 until December 2011. Dr. Morse is an expert in the structure and function of the KLH molecule and internationally recognized expert in protein chemistry, molecular biology, molluscan reproductive biology, and aquaculture, and has an intimate understanding of our technology.
Frank R. Oakes was appointed our President and Chief Executive Officer and Chairman of our Board of Directors in April 2010. Prior to that time, he served as founder and Chief Executive Officer of our California subsidiary since 1999. He has more than 30 years of management experience in aquaculture including a decade as Chief Executive Officer of The Abalone Farm, Inc., during which he led the company through the R&D, capitalization, and commercialization phases of development to become the largest abalone producer in the United States. Mr. Oakes is the inventor of our patented method for non-lethal extraction of hemolymph from a live gastropod mollusk. He was the principal investigator on our Small Business Innovation Research (“SBIR”) grant from the National Science Foundation and was principal investigator on our Phase I and II SBIR grants from the NIH’s Center for Research Resources, and a California Technology Investment Partnership (“CalTIP”) grant from the Department of Commerce. Mr. Oakes has consulted and lectured for the aquaculture industry around the world. He received his Bachelor of Science degree from California State Polytechnic University, San Luis Obispo and is a graduate of the Los Angeles Regional Technology Alliance University’s management-training program. Mr. Oakes is a valuable member of our Board due to his depth of operating, strategic, and senior management experience in our industry, specifically as related to aquaculture. Additionally, Mr. Oakes holds an intimate knowledge of our Company due to his longevity in the industry and with us.
Mayank (Mike) D. Sampat has been a director of our Company since August 2012, and serves as chairman of the Audit Committee. Mr. Sampat is currently an independent consultant, a position he has held since September 2014. He previously held the position of controller for Zpower, LLC, an emerging manufacturer in the microbattery industry, from June 2012 to September 2014. Prior to that time, he held the position of controller for Imaging Advantage LLC from September 2010 to June 2012, and the position of Chief Financial Officer for Gamma Medica-Ideas, a supplier of imaging equipment to the medical industry, from September 2007 to June 2010. Mr. Sampat received a BBA in accounting from Bombay University and his MBA in Finance at Mercer University. Mr. Sampat is a seasoned finance and accounting executive, having worked with multiple companies ranging from startups to large Fortune 100 companies.
Executive Officers
Set forth below is certain information with respect to the names, ages, and positions of our executive officers as of November 1, 2014. Biographical information pertaining to Mr. Oakes, who is a director and an executive officer, may be found in the above section entitled “Directors.” The executive officers serve at the pleasure of our Board of Directors.
| Page 47 |
| Name | Age | Position(s) Held | Date of Appointment | |||
| Frank R. Oakes | 63 | President, Chief Executive Officer and Chairman of our Board of Directors | April 9, 2010 | |||
| Catherine Brisson, Ph.D. | 40 | Chief Operating Officer | November 1, 2013 | |||
| Kathi Niffenegger | 56 | Chief Financial Officer and Corporate Secretary | November 1, 2013 | |||
| Herbert S. Chow, Ph.D. | 59 | Chief Technology Officer | August 9, 2012 | |||
| Mark A. McPartland | 47 | Vice President of Corporate Development and Communications | November 15, 2013 |
Catherine Brisson, Ph.D. was appointed our Chief Operating Officer in November 2013. She initially joined us in November 2010 and has held positions of increasing responsibility with our Company since that time, including serving as our Executive Director of Quality Assurance and Regulatory Affairs and our Chief Pharmaceutical Officer. Prior to 2010, Dr. Brisson held the position of the Executive Director of Quality Systems at MacuSight, Inc. from 2005 until 2010. Dr. Brisson has more than 20 years of experience in the biotechnology, pharmaceutical, and medical device industries with strong expertise, and broad scientific and operational understanding, in the areas of quality systems, regulatory affairs, manufacturing, and product development. She has extensive background in process development and a strong working knowledge of global regulatory requirements. Dr. Brisson holds a B.S. degree in Chemistry from North Carolina State University and a Ph.D. in Organic Chemistry from the University of North Carolina.
Kathi Niffenegger was appointed our Chief Financial Officer in November 2013 and our Corporate Secretary in June 2013. She initially joined us in May 2012 as Controller, after previously holding the position of our outside Certified Public Accountant since the founding of our California subsidiary in 1999. Ms. Niffenegger has more than 30 years of experience in accounting and finance in a range of industries. She held positions of increasing responsibility in the audit division of Glenn Burdette CPAs from 1988 to 2012 and served most recently as technical partner, obtained CFO experience at Martin Aviation, and began her career at Peat, Marwick, Mitchell & Co. (now KPMG LLP). She has held leadership roles for audits of manufacturing, aquaculture, pharmaceutical, and governmental grant clients, and developed specific expertise in cost accounting systems and internal controls. Ms. Niffenegger holds a B.S. degree in Business Administration, Accounting from California State University, Long Beach and is a member of the American Institute of Certified Public Accountants (AICPA).
Herbert S. Chow, Ph.D. was appointed our Chief Technology Officer in August 2012 and initially joined our Company in May 2010. Dr. Chow has more than 25 years of experience in business management and product development positions in new biologic devices, clinical diagnostic and consumer diagnostic markets. He has operational expertise in developing and commercializing innovative technologies, and building successful strategic partnership in the fields of medical diagnostic and therapeutic devices. Prior to joining us in 2010, Dr. Chow served as a director at MagnaBioScience from 2002-2007 and held the position of a General Manager at Rubicon Consultant from 2000-2010. He has also held key senior management positions with start-up biotechnology companies, as well as international pharmaceutical companies Abbott Labs and Johnson & Johnson. Dr. Chow earned a B.S. in Microbiology and Immunochemistry at Ohio State University and a Ph.D. in Immunopathology at the University of Illinois. Dr. Chow has been granted nine patents in chemical processing, microfluidic devices, liquid sensing devices and medical devices for point-of-care diagnosis.
Mark A. McPartland was appointed our Vice President of Corporate Development and Communications in November 2013. Mr. McPartland has more than 16 years of experience in business development, capital markets advisory, corporate communications and C-suite consulting. Prior to joining us, he served as Senior Vice President at MZ Group, a subsidiary of @titude Global, the world’s largest independent global investor relations consulting firm, from September 2011 to November 2013. Mr. McPartland’s background includes guiding the development and execution of corporate strategy for private and public companies at all stages of commercial evolution, including early- and mid-stage biopharmaceutical entities. His previous positions include Vice President and Partner at Alliance Advisors, LLC from January 2005 until January 2011, and Regional Vice President of Hayden Communications, Inc. from September 1999 until January 2005. Mr. McPartland holds a B.S. in Business Administration and Marketing from Coastal Carolina University.
| Page 48 |
Section 16(a) Beneficial Ownership Reporting Compliance
Section 16(a) did not apply to our Company or executive officers, directors and persons who own more than 10% of our common shares during our fiscal year ended August 31, 2014 because we were a “foreign private issuer” and exempt from such requirements under Rule 3a12-3 under the Securities Exchange Act of 1934, as amended.
Code of Ethics
We have adopted a Code of Ethics and Business Conduct that applies to all of our directors, officers, and employees. A copy of our Code of Ethics and Business Conduct is available on the Investor Relations section of our website at http://ir.stellarbiotechnologies.com. We intend to satisfy the SEC’s disclosure requirements regarding amendments to, or waivers of, our Code of Ethics and Business Conduct by posting such information on our website. Copies of our Code of Ethics and Business Conduct may be obtained, free of charge, by writing to our Corporate Secretary, Stellar Biotechnologies, Inc., 332 East Scott Street, Port Hueneme, California 93041.
Nominations for Board of Directors
The Board of Directors has approved an advance notice policy, which was subsequently approved by our shareholders, that requires advance notice be given to us in certain circumstances where nominations of persons for election to the Board are made by our shareholders.
In the case of an annual meeting of shareholders, notice to the Company must be made not less than 30 days nor more than 65 days prior to the date of the annual meeting. However, in the event that the annual meeting is to be held on a date that is less than 40 days after the date on which the first public announcement of the date of the annual meeting was made, notice may be made not later than the close of business on the tenth (10th) day following such public announcement.
In the case of a special meeting of shareholders (which is not also an annual meeting), notice to the Company must be made not later than the close of business on the fifteenth (15th) day following the day on which the first public announcement of the date of the special meeting was made.
Information about our Board Committees
Our Board of Directors has appointed an Audit Committee, a Compensation Committee, and a Nominating and Corporate Governance Committee. The Board of Directors has determined that each director who serves on these committees is “independent,” as that term is defined by the Nasdaq Listing Rules and rules of the Securities and Exchange Commission. The Board of Directors has adopted written charters for its Audit Committee, its Compensation Committee, and its Nominating and Corporate Governance Committee. Copies of these charters are available on our website at http://ir.stellarbiotechnologies.com.
Audit Committee
Our Audit Committee is composed of Gregory Baxter, David Hill, and Mayank Sampat (chairman). The purpose of the Audit Committee is to oversee our accounting and financial reporting processes and the audits of our financial statements. In that regard, the Audit Committee assists the Board in monitoring: (a) the integrity of our financial statements; (b) our independent auditor’s qualifications, independence, and performance; (c) the performance of our internal audit function, including our system of internal controls, financial reporting, and disclosure controls; and (d) our compliance with legal and regulatory requirements. To fulfill this obligation and perform its duties, the Audit Committee maintains effective working relationships with the Board, management, our internal auditor, and our independent auditor.
| Page 49 |
The Board has identified Mayank Sampat as its audit committee financial expert. Mr. Sampat is the Chairman of our Audit Committee and has extensive financial experience. He received an MBA in Finance from Mercer University, and has served in several financial positions with other companies, including several years as Chief Financial Officer for a medical equipment manufacturer. Mr. Sampat is considered to be “independent” as defined pursuant to the rules of the Nasdaq Listing Rules.
Compensation Committee
Our Compensation Committee is composed of Gregory Baxter, David Hill (chairman), and Mayank Sampat. The purpose of the Compensation Committee is to oversee the Board’s responsibilities relating to compensation of our Company’s Chief Executive Officer and our other executive officers. It has overall responsibility for approving and evaluating all of our compensation plans, policies and programs as such plans, policies and programs affect executive officers.
Nominating and Corporate Governance Committee
Our Nominating and Corporate Governance Committee is composed of Gregory Baxter (chairman), David Hill, and Mayank Sampat. The purpose of the Nominating and Corporate Governance Committee is to identify individuals qualified to become Board members; recommend to the Board individuals to serve as directors; advise the Board with respect to Board composition, procedures and committees; develop, recommend to the Board and annually review a set of corporate governance principles applicable to the Company; and oversee any related matters required by the federal securities laws.
| Item 11. | EXECUTIVE COMPENSATION. |
Executive Compensation
Set forth below is information regarding the all compensation paid or earned by (i) our principal executive officer and (ii) our two most highly compensated executive officers other than our principal executive officer who were serving as executive officers at the end of fiscal 2014. Such officers are collectively referred to as our “named executive officers.”
Summary Compensation Table
| Name and Principal Position | Year | Salary ($) | Bonus ($) | Option Awards ($) (1) | All Other Compensation ($) (2) | Total ($) | ||||||||||||||||||
| Frank R. Oakes, | 2014 | $ | 222,273 | $ | 180,000 | $ | 21,435 | $ | 423,708 | |||||||||||||||
| President, Chief Executive Officer and Chairman of our Board of Directors | 2013 | 33,280 | (3) | 19,602 | 52,882 | |||||||||||||||||||
| Kathi Niffenegger, | 2014 | 175,000 | 162,891 | 15,080 | 352,971 | |||||||||||||||||||
| Chief Financial Officer and | 2013 | 137,500 | 55,201 | 10,957 | 203,658 | |||||||||||||||||||
| Corporate Secretary (4) | ||||||||||||||||||||||||
| Herbert S. Chow, Ph.D., | 2014 | 178,333 | 162,891 | 18,089 | 359,313 | |||||||||||||||||||
| Chief Technology Officer | 2013 | 155,833 | 74,864 | 13,232 | 243,929 | |||||||||||||||||||
| (1) | Represents the aggregate grant date fair value of the stock option award granted in the covered fiscal year as computed in accordance with FASB ASC Topic 718, Compensation — Stock Compensation. The fair value of each stock option award is estimated for the covered fiscal year on the date of grant using the Black-Scholes option valuation model. A discussion of the assumptions used in calculating the amounts in this column may be found in Note 8 to our audited consolidated financial statements for the year ended August 31, 2014 included in this Annual Report. |
| Page 50 |
| (2) | Includes contributions made by us on the executive’s behalf to our Company’s 401(k) plan. Under the plan, we contribute a flat non-elective contribution of 3% of eligible compensation for each plan participant at the end of each fiscal year. |
| (3) | Frank Oakes voluntarily reduced his annual base salary to $33,280 for fiscal 2013. |
| (4) | Ms. Niffenegger was appointed our Chief Financial Officer effective November 1, 2013. Prior to then, she served as our Controller. Ms. Niffenegger was appointed Corporate Secretary in July 2013. |
Employment Agreements
We do not have employment agreements currently in effect with any of our named executive officers. Like our other employees, our executives are eligible for annual salary increases and discretionary equity grants.
Performance Share Plan
Under the merger agreement between our Company and our California subsidiary, we allotted 10,000,000 common shares (the “Performance Shares”) under a performance share plan (the “Plan”). The purpose of the Plan was to encourage the development of our products and business by distributing shares to key management, employees, and consultants upon the meeting of certain milestones. These milestones were set as follows:
| 1. | Completion of method development for commercial-scale manufacture of IMG KLH with applicable good GMP as a pharmaceutical intermediate, evidenced by completion of three GMP lots meeting all quality and product release specifications required for stability studies and process validation; |
| 2. | Compilation and regulatory submittal of all required CMC data compiled in CTD format and evidenced by filing as a DMF with the USFDA; and |
| 3. | Completion of preclinical toxicity and immunogenicity testing of IMG KLH and Subunit KLH in rodent and non-rodent species as evidenced by acceptance by study protocols and completion reports available to support customer United States FDA and EMEA filings. |
As each milestone was met as determined by our Board of Directors, one-third of the Performance Shares were available to be released to the Plan members. In January 2011, it was determined that Milestone No. 3 was successfully completed and the Board authorized the issuance of an aggregate of 3,333,335 Performance Shares to all participants in the Plan. In August 2012, the Board of Directors determined that Milestones No. 1 and 2 had been met and authorized the issuance of an aggregate of 1,313,130 Performance Shares to the non-director participants in the Plan. The Board did not take any action at that time on the issuance of shares to the participants of the Plan who were also directors of the Company. No action was taken regarding the Plan in fiscal 2013. In December 2013, we issued 1,515,152 common shares to a former director of the Company named as an eligible participant in the Plan.
Mr. Oakes, our Chief Executive Officer and Chairman of the Board, and Dr. Morse, our director, are eligible participants in the Plan. 2,356,902 and 1,346,801 shares, respectively, are reserved for future issuance to Mr. Oakes and Dr. Morse under the Plan. No other named executive officer is eligible to participate in the Plan.
| Page 51 |
Outstanding Equity Awards at 2014 Fiscal Year-End
The following table summarizes the equity awards made to our named executive officers that were outstanding at August 31, 2014.
| Option Awards | ||||||||||||||
| Name | Number of securities underlying unexercised options (#) exercisable | Number of securities underlying unexercised options (#) unexercisable (1) | Option exercise price ($) | Option expiration date | ||||||||||
Frank R. Oakes | 1,035,000 | - | CDN$ 0.28 | 04/09/17 | ||||||||||
| 425,600 | - | CDN$ 0.65 | 08/08/18 | |||||||||||
| 375,600 | - | CDN$ 0.42 | 04/13/19 | |||||||||||
| Kathi Niffenegger | 90,000 | - | CDN$ 0.29 | 06/18/19 | ||||||||||
| 50,000 | - | CDN$ 0.25 | 12/19/19 | |||||||||||
| 60,000 | 30,000 | CDN$ 0.58 | 05/14/20 | |||||||||||
| 33,333 | 66,667 | US$ 1.83 | 11/01/20 | |||||||||||
| Herbert S. Chow, Ph.D. | 55,000 | - | CDN$ 0.25 | 05/17/17 | ||||||||||
| 75,000 | - | CDN$ 0.65 | 08/08/18 | |||||||||||
| 75,000 | - | CDN$ 0.42 | 04/13/19 | |||||||||||
| 75,000 | - | CDN$ 0.37 | 08/09/19 | |||||||||||
| 57,500 | - | CDN$ 0.25 | 12/19/19 | |||||||||||
| 66,667 | 33,333 | CDN$ 0.58 | 05/23/20 | |||||||||||
| 33,333 | 66,667 | US$ 1.83 | 11/01/20 | |||||||||||
| (1) | Options granted to the named executive officers are subject to the following vesting schedule: (a) one-third of the option shall vest on the date of grant; (b) one-third of the option shall vest 12 months from the date of grant; and (c) the remaining one-third of the option shall vest 18 months from the date of grant. |
Retirement Benefits
We have established a 401(k) plan to provide retirement benefits to eligible executive officers and employees. Employees may enter the plan after they have been employed by us for at least three consecutive months. Under the plan, we contribute a flat non-elective contribution of 3% of eligible compensation for each plan participant at the end of the fiscal year. Any Company contributions we made to the plan for our named executive officers are reflected in the “All Other Compensation” column of the Summary Compensation Table above.
Other than the funds contributed under our 401(k) plan, no other funds were set aside or accrued by us during fiscal 2014 to provide pension, retirement or similar benefits for our named executive officers.
Director Compensation
The following table sets forth information regarding the compensation of our non-employee directors for the fiscal year ended August 31, 2014.
| Name | Fees earned or paid in cash ($) | Option awards ($) (1) (2) (3) | Total ($) | ||||||
| Gregory T. Baxter, Ph.D. | 4,700 | - | 4,700 | ||||||
Tessie M. Che, Ph.D. | 2,350 | 114,024 (4) | 116,374 | ||||||
David L. Hill, Ph.D. | 4,750 | 40,723 (5) | 45,473 | ||||||
| Daniel E. Morse, Ph.D. | 3,350 | - | 3,350 | ||||||
| Mayank D. Sampat | 5,750 | - | 5,750 | ||||||
| Page 52 |
| (1) | Represents the aggregate grant date fair value of the stock option award granted in the covered fiscal year as computed in accordance with FASB ASC Topic 718, Compensation — Stock Compensation. The fair value of each stock option award is estimated for the covered fiscal year on the date of grant using the Black-Scholes option valuation model. A discussion of the assumptions used in calculating the amounts in this column may be found in Note 8 to our audited consolidated financial statements for the year ended August 31, 2014 included in this Annual Report. |
| (2) | The aggregate number of option awards outstanding at August 31, 2014 held by each non-employee director is as follows: |
| Name | Outstanding Options (#) |
| Gregory T. Baxter, Ph.D. | 70,000 |
Tessie M. Che, Ph.D. |
70,000 |
| David L. Hill, Ph.D. | 75,000 |
| Daniel E. Morse, Ph.D. | 521,000 |
| Mayank D. Sampat | 70,000 |
| (3) | Options granted to directors are subject to the following vesting schedule: (a) one-third of the option shall vest on the date of grant; (b) one-third of the option shall vest 12 months from the date of grant; and (c) the remaining one-third of the option shall vest 18 months from the date of grant. |
| (4) | On November 1, 2013, Dr. Che was awarded an option to purchase up to 70,000 of our common shares. The option expires on November 1, 2020 and has an exercise price of $1.83. |
| (5) | On November 1, 2013, Dr. Hill was awarded an option to purchase up to 25,000 of our common shares. The option expires on November 1, 2020 and has an exercise price of $1.83. |
`
Narrative to Director Compensation Table
Non-Employee Director Compensation Policy
Pursuant to our non-employee director compensation policy, non-employee directors receive $1,000 for each Board meeting attended in person and $350 for each Board meeting attended by telephone. Members of Board committees also receive $350 for each committee meeting attended. Non-executive directors may also receive stock option awards at the discretion of the Board of Directors.
Non-Employee Directors on our Scientific Advisory Board
Dr. Morse and Dr. Baxter are members of our Scientific Advisory Board. As compensation for their services, the members of our Scientific Advisory Board receive certain advisory fees and expense reimbursements. During fiscal 2014, we paid an aggregate of $600 to Dr. Baxter for his services as a member of our Scientific Advisory Board and such amount is not reflected in the Director Compensation table above.
Consulting with Dr. Baxter
During fiscal 2014, we paid Dr. Baxter an aggregate of $600 for his services for our Scientific Advisory Board.
Compensation Committee Interlocks and Insider Participation
The members of our Compensation Committee during the fiscal year ended August 31, 2013 were Gregory Baxter, David Hill, Daniel Morse and Mayank Sampat. The Compensation Committee was reconstituted in June 2014 and is currently composed of Gregory Baxter, David Hill (chairman), and Mayank Sampat.
None of the individuals who served as a member of the Compensation Committee during fiscal 2014 was at any time during fiscal 2014 an officer or employee of our Company. Dr. Morse served as our Executive Vice-President, Science & Technology from 2010 until December 2011.
None of our executive officers currently serves, or in fiscal 2014 served, as a member of the compensation committee or director of any entity that has one or more executive officers serving as a member of our Board of Directors or on our Compensation Committee.
| Page 53 |
| Item 12. | SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS. |
Equity Compensation Plan Information
The following table provides certain information as of August 31, 2014 about our common shares that may be issued under our equity compensation plans:
| Plan category | Number of securities to be issued upon exercise of outstanding options, warrants and rights | Weighted- average exercise price of outstanding options, warrants and rights | Number of securities remaining available for future issuance under equity compensation plans (excluding securities reflected in column (a)) | |||||||||
| (a) | (b) | (c) | ||||||||||
| Equity compensation plans approved by security holders | 5,935,533 | CDN$ | 0.61 | 3,471,966 | ||||||||
| Equity compensation plans not approved by security holders | N/A | N/A | N/A | |||||||||
| Total | 5,935,533 | 3,471,966 | ||||||||||
Security Ownership of Certain Beneficial Owners and Management
The following tables sets forth certain information as of November 1, 2014, with respect to the beneficial ownership of our common shares by: (1) all of our directors; (2) our named executive officers listed in the Summary Compensation Table; (3) all of directors and executive officers as a group; and (4) each person known by us to beneficially own more than 5% of our outstanding common shares.
We have determined beneficial ownership in accordance with the rules of the SEC. Except as indicated by the footnotes below, we believe, based on the information furnished to us, that the persons and entities named in the table below have sole voting and investment power with respect to all common shares that they beneficially own, subject to applicable community property laws.
Common shares subject to options or warrants currently exercisable or exercisable within 60 days of November 1, 2014 are deemed outstanding for computing the share ownership and percentage of the person holding such options and warrants, but are not deemed outstanding for computing the percentage of any other person. The percentage ownership of our common shares of each person or entity named in the following table is based on 79,121,650 common shares outstanding as of November 1, 2014.
Directors and Officers
| Name and Address of Beneficial Owner (1) | Amount and Nature of Beneficial Ownership | Percentage of Shares Beneficially Owned | |||
| Frank R. Oakes | 5,343,846 (2) | 6.6 | % | ||
| Kathi Niffenegger | 296,667 (3) | * | |||
| Herbert S. Chow, Ph.D. | 1,104,334 (4) | 1.4 | % | ||
| Gregory T. Baxter, Ph.D. | 70,000 (5) | * | |||
| Tessie M. Che, Ph.D. | 46,667 (6) | * | |||
| David L. Hill, Ph.D. | 86,667 (7) | * | |||
| Daniel E. Morse, Ph.D. | 1,486,094 (8) | 1.9 | % | ||
| Mayank D. Sampat | 70,000 (9) | * | |||
| All directors and executive officers as a group (10 persons) | 9,134,789 (10) | 11.0 | % |
| Page 54 |
* Percentage of shares beneficially owned does not exceed one percent.
| (1) | Unless otherwise indicated, the address of each beneficial owner is c/o Stellar Biotechnologies, Inc., 332 E. Scott Street, Port Hueneme, California 93041. |
| (2) | This amount includes (i) 1,836,200 shares issuable upon the exercise of options and (ii) 40,000 shares issuable upon the exercise of warrants, each as currently exercisable or exercisable within 60 days of November 1, 2014; and excludes (iii) 464,273 common shares and 50,000 common shares issuable upon the exercise of outstanding options which are held by Mr. Oakes’ spouse who has sole voting and dispositive power over the securities, and as to which Mr. Oakes disclaims beneficial ownership. Mr. Oakes does not have the power to vote or dispose of, or to direct the voting or disposition of, the shares held by his spouse, or with respect to any shares acquired under her outstanding options. |
| (3) | Represents 296,667 shares issuable upon the exercise of options currently exercisable or exercisable within 60 days of November 1, 2014. |
| (4) | This amount includes (i) 504,167 shares issuable upon the exercise of options and (ii) 38,400 shares issuable upon the exercise warrants, each as currently exercisable or exercisable within 60 days of November 1, 2014. |
| (5) | Represents 70,000 shares issuable upon the exercise of options currently exercisable or exercisable within 60 days of November 1, 2014. |
| (6) | Represents 46,667 shares issuable upon the exercise of options currently exercisable or exercisable within 60 days of November 1, 2014. |
| (7) | This amount includes 66,667 shares issuable upon the exercise of options currently exercisable or exercisable within 60 days of November 1, 2014. |
| (8) | This amount includes 521,000 shares issuable upon the exercise of options currently exercisable or exercisable within 60 days of November 1, 2014. |
| (9) | Represents 70,000 shares issuable upon the exercise of options currently exercisable or exercisable within 60 days of November 1, 2014. |
| (10) | This amount includes (i) 3,887,202 shares issuable upon the exercise of options and (ii) 78,400 shares issuable upon the exercise of warrants, currently exercisable or exercisable within 60 days of November 1, 2014. |
Shareholders Known by Us to Own 5% or More of Our Common Shares
| Name and Address of Beneficial Owner | Amount and Nature of Beneficial Ownership | Percent of Shares Beneficially Owned | ||||||
| Ernesto Echavarria (1) | 15,220,266 | (2) | 18.26 | % | ||||
| Amaran Biotechnology Inc. (3) | 7,142,858 | (4) | 8.76 | % | ||||
| Frank R. Oakes | 5,343,846 | (5) | 6.60 | % | ||||
| (1) | The address of Mr. Echavarria is Blvd. Anaya, 1225 Culiacan Sinaloa, Mexico 80040. |
| (2) | Of the amount reported, 4,253,333 common shares represent common shares to be acquired upon exercise of warrants. |
| (3) | The address of Amaran Biotechnology Inc. is 11F-1, No. 308, Sec. 2, BaDe Road, ZhongShan District, Taipei City, 10492, Taiwan (R.O.C.). |
| Page 55 |
| (4) | This amount includes 2,380,953 common shares underlying warrants. |
| (5) | This amount includes (i) 1,836,200 shares issuable upon the exercise of options and (ii) 40,000 shares issuable upon the exercise of warrants, each as currently exercisable or exercisable within 60 days of November 1, 2014; and excludes (iii) 464,273 common shares and 50,000 common shares issuable upon the exercise of outstanding options which are held by Mr. Oakes’ spouse who has sole voting and dispositive power over the securities, and as to which Mr. Oakes disclaims beneficial ownership. Mr. Oakes does not have the power to vote or dispose of, or to direct the voting or disposition of, the shares held by his spouse, or with respect to any shares acquired under her outstanding options. |
| Item 13. | CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE. |
Related Party Transactions
Patent Royalty Agreement
On August 14, 2002, through our California subsidiary, we entered into an agreement with Frank Oakes, our Chief Executive Officer, where he would receive royalty payments in exchange for the assignment of his rights to U.S. Patent No. 6,852,338 to us. The royalty is 5% of gross receipts from products using this invention in excess of $500,000 annually. Our current operations utilize this invention. There were no royalties paid to Mr. Oakes for the year ended August 31, 2014.
Amaran Biotechnology, Inc.
In September 2013, we completed a private placement financing, raising total gross proceeds of $12,000,000 (the “Private Placement”). In connection with the Private Placement, we issued a total of 11,428,570 units. Each unit consisted of one common share and one half of a share purchase warrant, with each whole warrant exercisable into one additional common share at a price of $1.35 for a period of three years from the issuance date of the warrants. The Private Placement included a $5,000,000 investment by Amaran Biotechnology, Inc., a privately-held Taiwan biopharmaceuticals manufacturer. As a result of the Private Placement, we appointed Tessie Che, General Manager and Chair of the Board of Directors of Amaran Biotechnology, to our Board. Following the Private Placement, Amaran Biotechnology holds 8.8% of our common shares.
In December 2013, we entered into a collaboration agreement with Amaran Biotechnology. Under the terms of the agreement, we are responsible for the production and delivery of GMP grade KLH for evaluation as a potential carrier molecule in the OBI-822 active immunotherapy. We are also responsible for method development, product formulation, and process qualification for certain KLH reference standards. Amaran is responsible for development objectives and product specifications. The agreement also provides for Amaran to pay us fees for certain expenses and costs associated with the collaboration. Subject to certain conditions and timing, the terms of the collaboration also provide for the possible negotiation of a commercial supply agreement for Stellar KLH™ in the future. However, there can be no assurance that any such negotiations will lead to successful execution of any further agreements related to this collaboration.
Policies and Procedures for Review of Related Party Transactions
The Audit Committee reviews, approves and oversees any transaction between us and any “related person” (as defined in Item 404 of Regulation S-K) and any other potential conflict of interest situations, on an ongoing basis. Under these policies and procedures, the Audit Committee is to be informed of transactions subject to review before their implementation. The procedures establish our practices for obtaining and reporting information to the Audit Committee regarding such transactions on a periodic and an as-needed basis. The policy provides that such transactions are to be submitted for approval before they are initiated but also provides for ratification of such transactions. No director who is interested in a transaction may participate in the Audit Committee’s determinations as to the appropriateness of such transaction.
| Page 56 |
Director Independence
We are not currently listed on any national securities exchange or quoted on an inter-dealer quotation system that has a requirement that our Board of Directors be independent. However, in evaluating the independence of our Board members and the composition of the committees of our Board of Directors, the Board of Directors utilizes the definition of “independence” as that term is defined by the Securities Exchange Act of 1934, the Nasdaq Listing Rules, and the rules and regulations of the TSX Venture Exchange. Using this standard, the Board of Directors has determined that Gregory Baxter, David Hill, and Mayank Sampat are “independent directors.”
| Item 14. | PRINCIPAL ACCOUNTING FEES AND SERVICES. |
The following table shows the aggregate fees paid or accrued for audit and other services provided for the years ended August 31, 2014 and 2013 rendered by Moss Adams LLP and D&H Group LLP, respectively. Effective June 3, 2014, we dismissed D&H Group LLP, Chartered Accountants as our principal independent registered public accounting firm. That same date, we engaged Moss Adams LLP, Certified Public Accountants as our principal independent registered public accounting firm.
Principal Accountant Fees and Services
| Type of Service | Fiscal Year 2014 | Fiscal Year 2013 | ||||||
| Audit Fees | $ | 88,000 | CDN$ 44,952 | |||||
| Audit-Related Fees | - | - | ||||||
| Tax Fees | - | 4,342 | ||||||
| All Other Fees | - | 2,893 | ||||||
| Total | $ | 88,000 | CDN$ 52,187 | |||||
Audit Fees consisted of fees incurred for professional services rendered for audits of the years ended August 31, 2014 and 2013.
Tax Fees for fiscal year 2013 were related to preparation of Canadian corporate tax returns.
All Other Fees for fiscal year 2013 were related to consents and comments provided for our Annual Report on Form 20-F.
Pre-Approval Policies and Procedures
The Audit Committee is directly responsible for the appointment, compensation and oversight of our auditors. It has established procedures for the receipt, retention, and treatment of complaints received by us regarding accounting, internal accounting controls, or auditing matters, and the confidential, anonymous submission by our employees of concerns regarding questionable accounting or auditing matters. The Audit Committee also has the authority and the funding to engage independent counsel and other outside advisors.
In accordance with the requirements of the Sarbanes-Oxley Act of 2002 and rules issued by the Securities and Exchange Commission, our Audit Committee Charter includes a procedure for the review and pre-approval of all audit and permitted non-audit and tax services, subject to the de minimis exception for non-audit services described in Section 10A(i)(1)(B) of the Exchange Act, the Commission rules promulgated thereunder, and under the rules of the TSX-V, that may be provided by our independent auditor or other registered public accounting firms. The procedure requires that all proposed engagements of the auditor for audit and permitted non-audit services are submitted to the Audit Committee for approval prior to the beginning of any such services. The Audit Committee pre-approved 100% of the audit, audit-related and tax services performed by our independent registered public accounting firm for the fiscal year ended August 31, 2014.
| Page 57 |
| Item 15. | EXHIBITS, FINANCIAL STATEMENT SCHEDULES. |
| (a) | The following documents are filed as a part of this Annual Report: |
(1) Financial Statements
The list of consolidated financial statements and notes required by this Item 15 (a) (1) is set forth in the “Index to Financial Statements” on page F-1 of this Annual Report.
(2) Financial Statement Schedules
All schedules have been omitted because the required information is included in the financial statements or notes thereto.
| (b) | Exhibits |
The exhibits listed on the accompanying Exhibit Index are filed as part of this Annual Report.
| Page 58 |
EXHIBIT INDEX
| Exhibit No. | Description | |
| 3.1 | Certificate of Incorporation of the Company, dated June 12, 2007 (included as Exhibit 1(a) to the Company’s Registration Statement on Form 20-F filed on February 3, 2012, and incorporated herein by reference). | |
| 3.2 | Certificate of Amendment, dated April 15, 2008 (included as Exhibit 1(b) to the Company’s Registration Statement on Form 20-F filed on February 3, 2012, and incorporated herein by reference). | |
| 3.3 | Certificate of Continuation of the Company, dated November 25, 2009 (included as Exhibit 1(c) to the Company’s Registration Statement on Form 20-F filed on February 3, 2012, and incorporated herein by reference). | |
| 3.4 | Certificate of Name Change of the Company, dated April 7, 2010 (included as Exhibit 1(f) to the Company’s Registration Statement on Form 20-F filed on February 3, 2012, and incorporated herein by reference). | |
| 3.5 | Notice of Articles of the Company, dated April 7, 2010 (included as Exhibit 1(g) to the Company’s Registration Statement on Form 20-F filed on February 3, 2012, and incorporated herein by reference). | |
| 3.6 | Articles of the Company, effective November 20, 2009 (included as Exhibit 1(h) to the Company’s Registration Statement on Form 20-F filed on February 3, 2012, and incorporated herein by reference). | |
| 10.1 | Patent Assignment Agreement between the Company and Frank Oakes, dated August 14, 2002 (included as Exhibit 4(a) to the Company’s Registration Statement on Form 20-F filed on February 3, 2012, and incorporated herein by reference). | |
| 10.2 | Sublease Agreement between the Company and the Port Hueneme Surplus Property Authority, dated October 2, 2000 (included as Exhibit 4(j) to the Company’s Registration Statement on Form 20-F filed on February 3, 2012, and incorporated herein by reference). | |
| 10.3 | Sublease Agreement between the Company and the Port Hueneme Surplus Property Authority, dated March 21, 2005 (included as Exhibit 4(k) to the Company’s Registration Statement on Form 20-F filed on February 3, 2012, and incorporated herein by reference). | |
| 10.4 | Lease Agreement between the Company and Beachport Center, dated March 29, 2011(included as Exhibit 4(l) to the Company’s Registration Statement on Form 20-F filed on February 3, 2012, and incorporated herein by reference). | |
| 10.5 | Supply Agreement between the Company and Neovacs S.A. for subunit KLH, effective January 1, 2008 (included as Exhibit 4(14) to the Company’s Amendment No. 2 to its Registration Statement on Form 20-F filed on July 5, 2012, and incorporated herein by reference). | |
| 10.6 | Supply Agreement between the Company and Neovacs S.A. for KLH raw material, effective January 1, 2008 (included as Exhibit 4(15) to the Company’s Amendment No. 2 to its Registration Statement on Form 20-F filed on July 5, 2012, and incorporated herein by reference). | |
| 10.7 | Research Collaboration Agreement between the Company and Bayer Innovation GmbH, dated August 27, 2009 (included as Exhibit 4(16) to the Company’s Amendment No. 2 to its Registration Statement on Form 20-F filed on July 5, 2012, and incorporated herein by reference). | |
| 10.8 | Agreement between the Company and Life Diagnostics, effective October 18, 2011 (included as Exhibit 4(18) to the Company’s Amendment No. 2 to its Registration Statement on Form 20-F filed on July 5, 2012, and incorporated herein by reference). |
| Page 59 |
| 10.9 # | License Agreement between the Company and University of Guelph, dated July 24, 2013 (included as Exhibit 99.1 to the Company’s Report on Form 6-K filed on August 30, 2013, and incorporated herein by reference). | |
| 10.10 | Share Option Plan, as Amended, dated December 13, 2011 (included as Exhibit 10(b) to the Company’s Registration Statement on Form 20-F filed on February 3, 2012, and incorporated herein by reference). | |
| 10.11 | Fixed Share Option Plan dated December 18, 2013 (filed herewith). | |
| 10.12 | Shareholder’s Rights Plan, As Amended, dated January 9, 2014 (filed herewith). | |
| 10.13@ | Performance Share Plan dated April 9, 2010 (included as Exhibit 10(d) to the Company’s Registration Statement on Form 20-F filed on February 3, 2012, and incorporated herein by reference). | |
| 10.14 | Advance Notice Policy, adopted October 31, 2013 (filed herewith). | |
| 10.15 | Amendment One to Lease Agreement between the Company and Beachport Center, dated June 24, 2014 (filed herewith). | |
| 10.16 | Sublease Amendment No. 2 to Sublease Agreement between the Company and the Port Hueneme Surplus Property Authority, dated October 2, 2000 (filed herewith). | |
| 10.17 | Sublease Amendment No. 1 to Sublease Agreement between the Company and the Port Hueneme Surplus Property Authority, dated March 21, 2005 (filed herewith). | |
| 14.1 | Code of Ethics and Business Conduct (included as Exhibit 99.4 to the Company’s Report on Form 6-K filed on August 14, 2014, and incorporated herein by reference). | |
| 21 | Subsidiaries of Stellar Biotechnologies, Inc. (filed herewith). | |
| 31.1^ | Certification of the Chief Executive Officer pursuant to Rule 13a-14(a) under the Securities and Exchange Act of 1934, as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 (filed herewith). | |
| 31.2^ | Certification of the Chief Financial Officer pursuant to Rule 13a-14(a) under the Securities and Exchange Act of 1934, as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 (filed herewith). | |
| 32.1^ | Certification of the Chief Executive Officer pursuant to 18 U.S.C. 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 (filed herewith). | |
| 32.2^ | Certification of the Chief Financial Officer pursuant to 18 U.S.C. 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 (filed herewith). | |
| 101.INS+ | XBRL Instance Document. | |
| 101.SCH+ | XBRL Taxonomy Extension Schema Document. | |
| 101.CAL+ | XBRL Taxonomy Calculation Linkbase Document. | |
| 101.DEF+ | XBRL Taxonomy Extension Definition Linkbase Document. | |
| 101.LAB+ | XBRL Taxonomy Label Linkbase Document. | |
| 101.PRE+ | XBRL Taxonomy Presentation Linkbase Document. |
| @ | Management contract or compensatory plan or arrangement. |
| # | Confidential treatment has been granted for certain portions of this exhibit. Original copies have been filed separately with the Securities and Exchange Commission pursuant to Rule 24B-2 of the Securities Exchange Act of 1934, as amended. |
| ^ | A signed original of this written statement required by Section 906 has been provided and will be retained by the Company and furnished to the Securities and Exchange Commission or its staff upon request. |
| + | IN ACCORDANCE WITH THE TEMPORARY HARDSHIP EXEMPTION PROVIDED BY RULE 201 OF REGULATION S-T, THE DATE BY WHICH THE INTERACTIVE DATA FILE IS REQUIRED TO BE SUBMITTED HAS BEEN EXTENTED BY SIX BUSINESS DAYS. |
| Page 60 |
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| Date: November 14, 2014 | STELLAR BIOTECHNOLOGIES, INC. |
| /s/ Frank R. Oakes | |
|
Frank R. Oakes President and Chief Executive Officer (Principal Executive Officer) |
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
| Signature | Title | Date | ||
| /s/ Frank R. Oakes | President, Chief Executive Officer, | November 14, 2014 | ||
| Frank R. Oakes | and Chairman of the Board of Directors | |||
| (Principal Executive Officer) | ||||
| /s/ Kathi Niffenegger | Chief Financial Officer | November 14, 2014 | ||
| Kathi Niffenegger | (Principal Financial Officer) | |||
| /s/ Gregory T. Baxter | Director | November 14, 2014 | ||
| Gregory T. Baxter | ||||
| /s/ Tessie M. Che | Director | November 14, 2014 | ||
| Tessie M. Che | ||||
| /s/ David l. Hill | Director | November 14, 2014 | ||
| David L. Hill | ||||
| /s/ Daniel e. Morse | Director | November 14, 2014 | ||
| Daniel E. Morse | ||||
| /s/ Mayank D. Sampat | Director | November 14, 2014 | ||
| Mayank D. Sampat |
| Page 61 |
STELLAR BIOTECHNOLOGIES, INC.
INDEX TO FINANCIAL STATEMENTS
| F-1 |

Consolidated Financial Statements
For the Years Ended August 31, 2014, 2013 and 2012
(In U.S. Dollars)
| F-2 |
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
The Board of Directors
Stellar Biotechnologies, Inc.
We have audited the accompanying consolidated balance sheet of Stellar Biotechnologies, Inc. as of August 31, 2014, and the related consolidated statements of operations, changes in equity, and cash flows for the year then ended. These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on these financial statements based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audit included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the consolidated financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall consolidated financial statement presentation. We believe that our audit provides a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the consolidated financial position of Stellar Biotechnologies, Inc. as of August 31, 2014, and the consolidated results of its operations and its cash flows for the year then ended in conformity with accounting principles generally accepted in the United States of America.
/s/ Moss Adams LLP
Los Angeles, California
November 14, 2014
| F-3 |

Independent Auditor’s Report
To the Shareholders of Stellar Biotechnologies, Inc.
We have audited the accompanying consolidated financial statements of Stellar Biotechnologies, Inc., which comprise the consolidated balance sheet as at August 31, 2013, and the consolidated statements of operations, consolidated statements of cash flows and consolidated statements of changes in equity for the years ended August 31, 2013 and August 31, 2012 and a summary of significant accounting policies and other explanatory information.
Management’s Responsibility for the Consolidated Financial Statements
Management is responsible for the preparation and fair presentation of these consolidated financial statements in accordance with generally accepted accounting principles accepted in the United States of America and for such internal control as management determines is necessary to enable the preparation of consolidated financial statements that are free from material misstatement, whether due to fraud or error.
Auditor’s Responsibility
Our responsibility is to express an opinion on these consolidated financial statements based on our audits. We conducted our audits in accordance with Canadian generally accepted auditing standards and the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we comply with ethical requirements and plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free from material misstatement.
An audit involves performing procedures to obtain audit evidence about the amounts and disclosures in the consolidated financial statements. The procedures selected depend on the auditor’s judgment, including the assessment of the risks of material misstatement of the consolidated financial statements, whether due to fraud or error. In making those risk assessments, the auditor considers internal control relevant to the entity’s preparation and fair presentation of the consolidated financial statements in order to design audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the entity’s internal control. An audit also includes evaluating the appropriateness of accounting policies used and the reasonableness of accounting estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements.
We believe that the audit evidence we have obtained in our audits is sufficient and appropriate to provide a basis for our audit opinion.
Opinion
In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of Stellar Biotechnologies, Inc. as at August 31, 2013, and its financial performance and its cash flows for the years ended August 31, 2013 and August 31, 2012 in accordance with generally accepted accounting principles accepted in the United States of America.
| Vancouver, B.C. | “D&H Group LLP” | |
| November 14, 2014 | Chartered Accountants |

| F-4 |
Consolidated Balance Sheets
(Expressed in U.S. Dollars)
| August 31, | August 31, | |||||||
| 2014 | 2013 | |||||||
| Assets: | ||||||||
| Current assets: | ||||||||
| Cash and cash equivalents | $ | 13,427,404 | $ | 7,859,889 | ||||
| Accounts receivable | 56,575 | 177,720 | ||||||
| Short-term investments | 458,098 | - | ||||||
| Deferred financing costs | - | 62,027 | ||||||
| Prepaid expenses | 128,593 | 34,886 | ||||||
| Total current assets | 14,070,670 | 8,134,522 | ||||||
| Noncurrent assets: | ||||||||
| Property, plant and equipment, net | 387,392 | 246,269 | ||||||
| Licensing rights | - | 116,667 | ||||||
| Deposits | 15,900 | 15,900 | ||||||
| Total noncurrent assets | 403,292 | 378,836 | ||||||
| Total Assets | $ | 14,473,962 | $ | 8,513,358 | ||||
| Liabilities and Shareholders' Equity (Deficit): | ||||||||
| Current liabilities: | ||||||||
| Accounts payable and accrued liabilities | $ | 526,626 | $ | 419,413 | ||||
| Deferred revenue | 15,000 | - | ||||||
| Warrant liability, current portion | 879,040 | 3,454,745 | ||||||
| Total current liabilities | 1,420,666 | 3,874,158 | ||||||
| Long-term liabilities: | ||||||||
| Warrant liability, less current portion | 5,352,663 | 6,835,199 | ||||||
| Total Liabilities | 6,773,329 | 10,709,357 | ||||||
| Commitments (Note 7) | ||||||||
| Shareholders' equity (deficit): | ||||||||
| Common shares, unlimited common shares authorized, no par value, 78,268,850 and 57,946,160 issued and outstanding at August 31, 2014 and 2013, respectively | 36,240,838 | 13,180,677 | ||||||
| Shares subscribed | - | 5,155,674 | ||||||
| Accumulated share-based compensation | 5,079,985 | 4,648,317 | ||||||
| Accumulated deficit | (33,620,190 | ) | (25,180,667 | ) | ||||
| Total shareholders' equity (deficit) | 7,700,633 | (2,195,999 | ) | |||||
| Total Liabilities and Shareholders' Equity (Deficit) | $ | 14,473,962 | $ | 8,513,358 | ||||
The accompanying notes are an integral part of these consolidated financial statements.
| F-5 |
Consolidated Statements of Operations
(Expressed in U.S. Dollars)
| Year Ended | ||||||||||||
| August 31, | August 31, | August 31, | ||||||||||
| 2014 | 2013 | 2012 | ||||||||||
| Revenues: | ||||||||||||
| Contract services revenue | $ | 192,000 | $ | 60,000 | $ | 60,000 | ||||||
| Product sales | 143,553 | 76,055 | 131,825 | |||||||||
| Grant revenue | 36,579 | 409,414 | 94,229 | |||||||||
| 372,132 | 545,469 | 286,054 | ||||||||||
| Expenses: | ||||||||||||
| Costs of contract services | 60,734 | 11,525 | 15,572 | |||||||||
| Costs of production and aquaculture | 662,946 | 183,276 | 426,310 | |||||||||
| Grant costs | 36,579 | 409,414 | 94,043 | |||||||||
| Research and development | 2,458,934 | 2,018,554 | 2,634,119 | |||||||||
| General and administration | 2,871,455 | 1,770,619 | 3,662,009 | |||||||||
| 6,090,648 | 4,393,388 | 6,832,053 | ||||||||||
| Other Income (Loss) | ||||||||||||
| Loss recovery | - | - | 105,000 | |||||||||
| Foreign exchange gain (loss) | (222,437 | ) | (95,842 | ) | 10,091 | |||||||
| Change in fair value of warrant liability | (2,533,305 | ) | (10,556,208 | ) | 897,549 | |||||||
| Investment income | 61,935 | 4,990 | 4,881 | |||||||||
| (2,693,807 | ) | (10,647,060 | ) | 1,017,521 | ||||||||
| Loss Before Income Tax | (8,412,323 | ) | (14,494,979 | ) | (5,528,478 | ) | ||||||
| Income tax expense | 27,200 | 800 | 800 | |||||||||
| Net Loss | $ | (8,439,523 | ) | $ | (14,495,779 | ) | $ | (5,529,278 | ) | |||
| Loss per common share - basic and diluted | $ | (0.11 | ) | $ | (0.28 | ) | $ | (0.13 | ) | |||
| Weighted average number of common shares outstanding | 75,826,642 | 51,611,944 | 43,775,766 | |||||||||
The accompanying notes are an integral part of these consolidated financial statements.
| F-6 |
Consolidated Statements of Cash Flows
(Expressed in U.S. Dollars)
| Year Ended | ||||||||||||
| August 31, | August 31, | August 31, | ||||||||||
| 2014 | 2013 | 2012 | ||||||||||
| Cash Flows Used In Operating Activities: | ||||||||||||
| Net loss | $ | (8,439,523 | ) | $ | (14,495,779 | ) | $ | (5,529,278 | ) | |||
| Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||||||
| Depreciation and amortization | 158,313 | 124,833 | 112,144 | |||||||||
| Share-based compensation | 956,634 | 786,585 | 1,923,200 | |||||||||
| Foreign exchange (gain) loss | 222,437 | 95,842 | (10,091 | ) | ||||||||
| Change in fair value of warrant liability | 2,533,305 | 10,556,208 | (897,549 | ) | ||||||||
| Loss on disposal of property, plant and equipment | 3,670 | - | - | |||||||||
| Impairment loss | 90,476 | - | - | |||||||||
| Fair value of shares issued for research license | - | 491,408 | - | |||||||||
| - | ||||||||||||
| Changes in working capital items: | ||||||||||||
| Accounts receivable | 121,075 | (192,067 | ) | 29,740 | ||||||||
| Deferred financing costs | 60,656 | (62,027 | ) | - | ||||||||
| Prepaid expenses | (94,974 | ) | (2,658 | ) | 4,376 | |||||||
| Accounts payable and accrued liabilities | 106,224 | 29,309 | 292,167 | |||||||||
| Deferred revenue | 15,000 | (127,477 | ) | 127,477 | ||||||||
| Net cash used in operating activities | (4,266,707 | ) | (2,795,823 | ) | (3,947,814 | ) | ||||||
| Cash Flows Used In Investing Activities: | ||||||||||||
| Acquisition of property, plant and equipment | (279,065 | ) | (9,541 | ) | (78,338 | ) | ||||||
| Proceeds on sale of property, plant and equipment | 2,150 | - | - | |||||||||
| Purchase of short-term investments | (464,500 | ) | - | - | ||||||||
| Net cash used in investing activities | (741,415 | ) | (9,541 | ) | (78,338 | ) | ||||||
| Cash Flows From Financing Activities: | ||||||||||||
| Proceeds from exercise of warrants and options | 4,308,878 | 1,582,739 | 877,210 | |||||||||
| Proceeds from issuance of common stock, net | 6,479,097 | 8,146,487 | - | |||||||||
| Refund of deposit | - | 1,600 | - | |||||||||
| - | ||||||||||||
| Net cash provided by financing activities | 10,787,975 | 9,730,826 | 877,210 | |||||||||
| Effect of exchange rate changes on cash and cash equivalents | (212,338 | ) | (64,571 | ) | 2,448 | |||||||
| Net change in cash and cash equivalents | 5,567,515 | 6,860,891 | (3,146,494 | ) | ||||||||
| Cash and cash equivalents - beginning of year | 7,859,889 | 998,998 | 4,145,492 | |||||||||
| Cash and cash equivalents - end of year | $ | 13,427,404 | $ | 7,859,889 | $ | 998,998 | ||||||
The accompanying notes are an integral part of these consolidated financial statements.
| F-7 |
Consolidated Statements of Changes in Equity
(Expressed in U.S. Dollars)
| Accumulated | Total | |||||||||||||||||||||||
| Common | Shares | Share-Based | Accumulated | Shareholders' | ||||||||||||||||||||
| Shares | Shares | Subscribed | Compensation | Deficit | Equity | |||||||||||||||||||
| Balance - August 31, 2011 | 41,611,831 | $ | 6,541,810 | $ | - | $ | 2,054,399 | $ | (5,155,610 | ) | $ | 3,440,599 | ||||||||||||
| Performance shares to be issued | - | - | - | 1,209,000 | - | 1,209,000 | ||||||||||||||||||
| Issuance of performance shares | 1,313,130 | 366,363 | - | (366,363 | ) | - | - | |||||||||||||||||
| Proceeds from exercise of warrants | 2,318,600 | 830,716 | - | - | - | 830,716 | ||||||||||||||||||
| Transfer to common stock on exercise of warrants | - | 190,425 | - | - | - | 190,425 | ||||||||||||||||||
| Proceeds from exercise of options | 170,000 | 46,494 | - | - | - | 46,494 | ||||||||||||||||||
| Transfer to common stock on exercise of options | - | 41,087 | - | (41,087 | ) | - | - | |||||||||||||||||
| Share-based compensation | - | - | - | 714,200 | - | 714,200 | ||||||||||||||||||
| Net loss | - | - | - | - | (5,529,278 | ) | (5,529,278 | ) | ||||||||||||||||
| Balance - August 31, 2012 | 45,413,561 | $ | 8,016,895 | $ | - | $ | 3,570,149 | $ | (10,684,888 | ) | $ | 902,156 | ||||||||||||
| Proceeds of private placements | 9,258,400 | 3,115,875 | - | - | - | 3,115,875 | ||||||||||||||||||
| Issuance costs of private placements including fair value of broker units | - | (275,956 | ) | - | 150,894 | - | (125,062 | ) | ||||||||||||||||
| Fair value of warrants issued in private placements | - | (1,749,004 | ) | - | - | - | (1,749,004 | ) | ||||||||||||||||
| Proceeds from exercise of warrants | 2,738,000 | 1,510,336 | - | - | - | 1,510,336 | ||||||||||||||||||
| Transfer to common stock on exercise of warrants | - | 2,139,409 | - | - | - | 2,139,409 | ||||||||||||||||||
| Proceeds from exercise of options | 164,999 | 72,403 | - | - | - | 72,403 | ||||||||||||||||||
| Transfer to common stock on exercise of options | - | 54,325 | - | (54,325 | ) | - | - | |||||||||||||||||
| Share-based compensation | - | - | - | 786,585 | - | 786,585 | ||||||||||||||||||
| Shares issued to acquire license | 371,200 | 491,408 | - | - | - | 491,408 | ||||||||||||||||||
| Fair value of warrants issued to acquire license | - | (195,014 | ) | - | 195,014 | - | - | |||||||||||||||||
| Subscriptions received for private placement and warrants | - | - | 5,155,674 | - | - | 5,155,674 | ||||||||||||||||||
| Net loss | - | - | - | - | (14,495,779 | ) | (14,495,779 | ) | ||||||||||||||||
| Balance - August 31, 2013 | 57,946,160 | $ | 13,180,677 | $ | 5,155,674 | $ | 4,648,317 | $ | (25,180,667 | ) | $ | (2,195,999 | ) | |||||||||||
| Proceeds of private placements | 11,428,570 | 12,000,000 | (5,000,000 | ) | - | - | 7,000,000 | |||||||||||||||||
| Issuance costs of private placements including fair value of broker warrants | - | (907,801 | ) | - | 386,898 | - | (520,903 | ) | ||||||||||||||||
| Issuance of performance shares | 1,515,152 | 422,728 | - | (422,728 | ) | - | - | |||||||||||||||||
| Proceeds from exercise of warrants | 5,937,300 | 3,920,134 | (155,674 | ) | - | - | 3,764,460 | |||||||||||||||||
| Transfer to common stock on exercise of warrants | - | 6,591,546 | - | - | - | 6,591,546 | ||||||||||||||||||
| Proceeds from exercise of options | 1,441,668 | 544,418 | - | - | - | 544,418 | ||||||||||||||||||
| Transfer to common stock on exercise of options | - | 489,136 | - | (489,136 | ) | - | - | |||||||||||||||||
| Share-based compensation | - | - | - | 956,634 | - | 956,634 | ||||||||||||||||||
| Net loss | - | - | - | - | (8,439,523 | ) | (8,439,523 | ) | ||||||||||||||||
| Balance - August 31, 2014 | 78,268,850 | $ | 36,240,838 | $ | - | $ | 5,079,985 | $ | (33,620,190 | ) | $ | 7,700,633 | ||||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
| F-8 |
Notes to Consolidated Financial Statements
For the Years Ended August 31, 2014, 2013 and 2012
(Expressed in U.S. Dollars)
| 1. | Nature of Operations and Going Concern |
Stellar Biotechnologies, Inc. (“the Company”) is listed on the U.S. OTCQB Marketplace Exchange under the trading symbol, “SBOTF” and on the TSX Venture Exchange as a Tier 2 issuer under the trading symbol, “KLH.”
On April 7, 2010, the Company changed its name from CAG Capital, Inc. to Stellar Biotechnologies, Inc. On April 12, 2010, the Company completed a reverse merger transaction with Stellar Biotechnologies, Inc. a California corporation, which was founded in September 1999, and remains the Company’s wholly-owned subsidiary and principal operating entity. The Company’s executive offices are located at 332 E. Scott Street, Port Hueneme, California, 93041, USA, and the registered and records office is Royal Centre, 1055 West Georgia Street, Suite 1500, Vancouver, BC, V6E 4N7, Canada.
The Company’s business is the aquaculture, research and development, manufacture and commercialization of Keyhole Limpet Hemocyanin (“KLH”). The Company markets and distributes its KLH products to biotechnology and pharmaceutical companies, academic institutions, and clinical research organizations in Europe, United States, and Asia.
For the years ended August 31, 2014, 2013 and 2012, the Company reported net losses of approximately $8.4 million, $14.5 million, and $5.5 million, respectively. As of August 31, 2014, the Company had an accumulated deficit of approximately $33.6 million and working capital of approximately $12.7 million.
In the past, operations of the Company have primarily been funded by the issuance of common shares, exercise of warrants, grant revenues, contract services revenue, and product sales. In September 2013, the Company closed a private placement with gross proceeds of $12,000,000. Management believes these financial resources are adequate to support the Company’s initiatives at the current level for the foreseeable future. Management is also continuing the ongoing effort toward expanding the customer base for existing marketed products, and the Company may seek additional financing alternatives, including nondilutive financing through grants, collaboration and licensing arrangements, and additional equity financing.
The accompanying financial statements have been prepared on the going concern basis, which assumes that the Company will continue in operation for the foreseeable future and be able to realize its assets and discharge its liabilities and commitments in the normal course of business.
The consolidated financial statements of the Company are presented in U.S. dollars, unless otherwise stated, which is the functional currency.
| 2. | Basis of Presentation |
The accompanying financial statements of the Company have been prepared in accordance with accounting principles generally accepted in the United States of America (“GAAP”) and include the accounts of the Company and its wholly-owned subsidiary, Stellar Biotechnologies, Inc. Intercompany balances and transactions are eliminated on consolidation.
| F-9 |
Stellar Biotechnologies, Inc.
Notes to Consolidated Financial Statements
For the Years Ended August 31, 2014, 2013 and 2012
(Expressed in U.S. Dollars)
| 3. | Significant Accounting Policies |
| a) | Use of Estimates |
The preparation of financial statements in conformity with GAAP requires management to make certain estimates, judgments and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the dates of the consolidated financial statements and the reported amounts of revenues and expenses during the reported periods. These estimates include warrant liability, share-based compensation, intangible assets, valuation of accounts receivable, and income taxes. Actual outcomes could differ from these estimates. These consolidated financial statements include estimates, which by their nature are uncertain. The impacts of such estimates are pervasive throughout the consolidated financial statements, and may require accounting adjustments based on future periods if the revision affects both current and future periods. These estimates are based on historical experience, current and future economic conditions and other factors, including expectations of future events that are believed to be reasonable under the circumstances.
| b) | Cash and Cash Equivalents |
Cash and cash equivalents consist of demand deposits with financial institutions and highly liquid investments which are readily convertible into cash with maturities of three months or less when purchased.
| c) | Investments |
Investments include Canadian enhanced yield deposits with original maturity of 180 days. Investments are classified as held-to-maturity and are reported at amortized cost, which approximates fair value. The Company regularly reviews its investments to determine whether a decline in fair value below the cost basis is other than temporary. If the decline in fair value is determined to be other than temporary, the cost basis of the investment is written down to fair value.
| d) | Allowance for Doubtful Accounts Receivable |
The Company assesses the collectability of its accounts receivable through a review of its current aging, as well as an analysis of its historical collection rate, general economic conditions and credit status of its customers. As of August 31, 2014 and 2013, all outstanding accounts receivable were deemed to be fully collectible, and therefore, no allowance for doubtful accounts was recorded.
| e) | Property, Plant and Equipment |
Property, plant and equipment are recorded at cost less accumulated depreciation and accumulated impairment losses, if any. Depreciation is recorded on the straight-line method over useful lives ranging from 5 to 10 years. Leasehold improvements are depreciated over the shorter of the useful life of the improvement or remaining term of lease. Maintenance and repairs are charged to operations as incurred.
| f) | Impairment of Long-Lived Assets |
If indicators of impairment exist, the Company assesses the recoverability of the affected long-lived assets by determining whether the carrying value of such assets can be recovered through undiscounted future operating cash flows. If impairment is indicated, the amount of such impairment is measured by comparing the carrying value of the asset to the fair value of the asset and the Company records the impairment as a reduction in the carrying value of the related asset and a charge to operating results. Estimating the undiscounted future cash flows associated with long-lived assets requires judgment, and assumptions could differ materially from actual results. See Note 6 for impairment of licensing rights.
| F-10 |
Stellar Biotechnologies, Inc.
Notes to Consolidated Financial Statements
For the Years Ended August 31, 2014, 2013 and 2012
(Expressed in U.S. Dollars)
| g) | Fair Value of Financial Instruments |
The Company uses the fair value measurement framework for valuing financial assets and liabilities measured on a recurring basis in situations where other accounting pronouncements either permit or require fair value measurements.
Fair value of a financial instrument is the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. As of August 31, 2014 and 2013, the carrying value of certain financial instruments such as accounts receivable, accounts payable, accrued liabilities, and deferred revenue approximates fair value due to the short-term nature of such instruments.
The Company follows the fair value hierarchy which requires an entity to maximize the use of observable inputs and minimize the use of unobservable inputs when measuring fair value. There are three levels of inputs that may be used to measure fair value:
| Level 1: | Quoted prices in active markets for identical or similar assets and liabilities. |
| Level 2: | Quoted prices for identical or similar assets and liabilities in markets that are not active or observable inputs other than quoted prices in active markets for identical or similar assets and liabilities. |
| Level 3: | Unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities. |
The Company records its cash and cash equivalents at fair value using Level 1 inputs in the fair value hierarchy. The Company records its warrant liability at fair value using Level 2 input using the assumptions disclosed in Note 8.
| h) | Revenue Recognition |
Contract services revenue
The Company recognizes contract services revenue when contract services have been performed and reasonable assurance exists regarding measurement and collectability. An appropriate amount will be recognized as revenue in the period that the Company is assured of fulfilling the contract requirements. Amounts received in advance of performance of contract services are recorded as deferred revenue.
Contract services include services performed under collaboration agreements and monthly maintenance of limpet colonies designated to meet the needs of the customer. The Company also has the right to use raw material produced from designated limpet colonies at no cost to the Company with prior written consent from the customer.
Product Sales
The Company recognizes product sales when KLH product is shipped (for which the risk is typically transferred upon delivery to the shipping carrier) and there is persuasive evidence of an arrangement, the fee is fixed or determinable, and collectability is reasonably assured. The Company documents arrangements with customers with purchase orders and sales agreements.
Product sales include sales made under supply agreements with a customer for a fixed price per gram of KLH products based on quantities ordered, including those produced from the customer’s designated limpet colonies. The supply agreements are on a non-exclusive basis except within that customer’s field of use.
| F-11 |
Stellar Biotechnologies, Inc.
Notes to Consolidated Financial Statements
For the Years Ended August 31, 2014, 2013 and 2012
(Expressed in U.S. Dollars)
Grants
The Company has taken the income approach to recognizing grant revenue. The Company recognizes grant revenue when there is reasonable assurance that the Company will comply with the conditions attached, the benefits have been earned and it is reasonably assured of collection. An appropriate amount of earned revenue will be recognized as revenue in the period that the Company is assured of fulfilling the grant requirements.
| i) | Research and Development |
Research and development costs are expensed as incurred.
| j) | Equity Financing |
The Company engages in equity financing transactions to obtain the funds necessary to continue operations and perform research and development activities. These equity financing transactions may involve issuance of common shares or units. Units typically comprise a certain number of common shares and share purchase warrants. Depending on the terms and conditions of each equity financing transaction, the warrants are exercisable into additional common shares at a price prior to expiry as stipulated by the terms of the transaction.
| k) | Share-Based Compensation |
The Company grants options to buy common shares of the Company to its directors, officers, employees and consultants, and grants other equity-based instruments to non-employees.
The fair value of share-based compensation is measured on the date of grant, using the Black-Scholes option valuation model and is recognized over the vesting period net of estimated forfeitures for employees or the service period for non-employees. The Black-Scholes option valuation model requires the input of subjective assumptions, including price volatility of the underlying stock, risk-free interest rate, dividend yield, and expected life of the option.
| l) | Foreign Exchange |
Items included in the financial statements of the Company’s subsidiary are measured using the currency of the primary economic environment in which the entity operates (the “functional currency”). The functional currency of the parent and its subsidiary is the U.S. dollar.
Transactions in currencies other than the U.S. dollar are recorded at exchange rates prevailing on the dates of the transactions.
| F-12 |
Stellar Biotechnologies, Inc.
Notes to Consolidated Financial Statements
For the Years Ended August 31, 2014, 2013 and 2012
(Expressed in U.S. Dollars)
| m) | Income Taxes |
Income tax expense comprises current and deferred tax. Income tax is recognized in profit or loss except to the extent that it relates to items recognized directly in equity. Current tax expense is the expected tax payable on taxable income for the year, using tax rates enacted or substantively enacted at year-end, adjusted for amendments to tax payable with regards to previous years.
Deferred tax is recorded using the liability method, providing for temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for taxation purposes. Temporary differences are not provided for relating to goodwill not deductible for tax purposes. The amount of deferred tax provided is based on the expected manner of realization or settlement of the carrying amount of assets and liabilities, using tax rates enacted or substantively enacted at the balance sheet date.
A deferred tax asset is recognized only to the extent that it is more likely than not that future taxable profits will be available against which the asset can be utilized. To the extent that the Company does not consider it more likely than not that a deferred tax asset will be recovered, it provides a valuation allowance against that excess.
The Company periodically evaluates its tax positions to determine whether it is more likely than not that a tax position will be sustained upon examination by the appropriate taxing authorities. The Company has not incurred any interest or penalties as of August 31, 2014 with respect to uncertain income tax matters. The Company does not expect that there will be unrecognized tax benefits of a significant nature that will increase or decrease within 12 months of the reporting date.
The Company files income tax returns in the U.S. federal, Canadian and state jurisdictions. Management believes that there are no material uncertain tax positions that would impact the accompanying consolidated financial statements. The Company's policy is to recognize interest and penalties related to unrecognized tax benefits in income tax expense. The Company may be subject to examination by the Internal Revenue Service for tax years 2010 through 2013 and by the Canada Revenue Agency for tax years 2010 through 2014. The Company may also be subject to examination on certain state and local jurisdictions for the tax years 2009 through 2013.
| n) | Loss Per Share |
Basic earnings (loss) per share is calculated by dividing income available to common shareholders by the weighted average number of common shares outstanding during the period.
The computation of diluted loss per share assumes the conversion, exercise or contingent issuance of securities only when such conversion, exercise or issuance would have a dilutive effect on loss per share. The dilutive effect of convertible securities is reflected in diluted earnings per share by application of the “if converted” method. The dilutive effect of outstanding options and warrants and their equivalents is reflected in diluted earnings per share by application of the treasury stock method. Conversion of outstanding warrants, broker units and options would have an antidilutive effect on loss per share for the years ended August 31, 2014, 2013 and 2012, and are therefore excluded from the computation of diluted loss per share.
| o) | Segments |
The Company operates in one reportable segment and, accordingly, no segment disclosures have been presented. All equipment, leasehold improvements and other fixed assets owned by the Company are physically located within the United States, and all supply, collaboration and licensing agreements are denominated in U.S. dollars.
| F-13 |
Stellar Biotechnologies, Inc.
Notes to Consolidated Financial Statements
For the Years Ended August 31, 2014, 2013 and 2012
(Expressed in U.S. Dollars)
| p) | Recent Accounting Pronouncements |
In May 2014, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update (“ASU”) 2014-09, Revenue from Contracts with Customers (Topic 606). ASU 2014-09 creates a new topic in the ASC Topic 606 and establishes a new control-based revenue recognition model, changes the basis for deciding when revenue is recognized over time or at a point in time, provides new and more detailed guidance on specific topics, and expands and improves disclosures about revenue. In addition, ASU 2014-09 adds a new Subtopic to the Codification, ASC 340-40, Other Assets and Deferred Costs: Contracts with Customers, to provide guidance on costs related to obtaining a contract with a customer and costs incurred in fulfilling a contract with a customer that are not in the scope of another ASC Topic. The guidance in ASU 2014-09 is effective for public entities for annual reporting periods beginning after December 15, 2016, including interim periods therein. Early application is not permitted. Management is in the process of assessing the impact of ASU 2014-09 on the Company’s financial statements.
In August 2014, the FASB issued ASU 2014-15, Presentation of Financial Statements - Going Concern (Subtopic 205-40): Disclosure of Uncertainties about an Entity's Ability to Continue as a Going Concern. ASU 2014-15 defines management's responsibility to evaluate whether there is substantial doubt about an organization's ability to continue as a going concern and to provide related footnote disclosures. The guidance in ASU 2014-15 is effective for annual reporting periods beginning after December 15, 2016, with early application permitted. Management is in the process of assessing the impact of ASU 2014-15 on the Company’s financial statements.
| 4. | Accounts Receivable |
Accounts receivable consisted of the following:
| August 31, | August 31, | |||||||
| 2014 | 2013 | |||||||
| Accounts receivable | $ | 55,713 | $ | 17,648 | ||||
| Grants receivable | - | 157,297 | ||||||
| Other receivable | 862 | 2,775 | ||||||
| $ | 56,575 | $ | 177,720 | |||||
| F-14 |
Stellar Biotechnologies, Inc.
Notes to Consolidated Financial Statements
For the Years Ended August 31, 2014, 2013 and 2012
(Expressed in U.S. Dollars)
| 5. | Property, Plant and Equipment, net |
Property, plant and equipment, net consisted of the following:
| August 31, | August 31, | |||||||
| 2014 | 2013 | |||||||
| Aquaculture system | $ | 58,923 | $ | 58,923 | ||||
| Laboratory facilities | 62,033 | 62,033 | ||||||
| Computer and office equipment | 77,697 | 56,710 | ||||||
| Tools and equipment | 622,289 | 393,497 | ||||||
| Vehicles | 10,997 | 10,997 | ||||||
| Leasehold improvements | 61,187 | 59,107 | ||||||
| 893,126 | 641,267 | |||||||
| Less: accumulated depreciation | (505,734 | ) | (394,998 | ) | ||||
| $ | 387,392 | $ | 246,269 | |||||
Depreciation expense amounted to $132,122, $96,262 and $83,572 for the years ended August 31, 2014, 2013 and 2012, respectively.
| F-15 |
Stellar Biotechnologies, Inc.
Notes to Consolidated Financial Statements
For the Years Ended August 31, 2014, 2013 and 2012
(Expressed in U.S. Dollars)
| 6. | Intangible Assets - Licensing Rights |
In December 2010, the Company entered into a research collaboration agreement with a customer. When the agreement terminated according to its terms in August 2011, the Company acquired an exclusive, worldwide sub-licensable and royalty-free license for certain technology developed under collaboration with the customer. The Company paid a $200,000 license fee for the licensing rights, which are jointly owned by the Company and the customer. The licensing rights do not have a fixed term or termination provisions. The licensing rights are amortized over the estimated useful life of seven years and are shown net of accumulated amortization and impairment losses. During the year ended August 31, 2014, the Company discontinued its use of these licensing rights and recorded impairment loss for the remaining value of licensing rights. At August 31, 2013, the value of these licensing rights and related accumulated amortization were $200,000 and $83,333, respectively.
Amortization expense amounted to $26,191, $28,571 and $28,572 for the years ended August 31, 2014, 2013 and 2012, respectively. Impairment loss for the year ended August 31, 2014 totaled $90,476 and is included in general and administrative expenses in the accompanying financial statements.
| 7. | Commitments |
Operating leases
The Company leases three buildings and facilities used in its operations under sublease agreements with the Port Hueneme Surplus Property Authority. In September 2010, the Company exercised its option to extend these sublease agreements for an additional five-year term. The Company has an option to extend the lease for an additional five-year term.
The Company also leases facilities used for executive offices and laboratories. The Company must pay a portion of the common area maintenance. In July 2014, the Company exercised its option to extend this lease for a two-year term.
Future minimum lease payments are as follows:
| August 31, | ||||
| For The Year Ending August 31, | 2014 | |||
| 2015 | $ | 157,000 | ||
| 2016 | 71,000 | |||
| $ | 228,000 | |||
Rent expense on these lease agreements amounted to approximately $181,000, $178,000, and $171,000 for the years ended August 31, 2014, 2013 and 2012, respectively.
Purchase obligations
The Company has commitments totaling approximately $78,000 at August 31, 2014, for signed agreements with contract research organizations and consultants, which are scheduled to be paid in accordance with contract terms during the 2015 fiscal year.
| F-16 |
Stellar Biotechnologies, Inc.
Notes to Consolidated Financial Statements
For the Years Ended August 31, 2014, 2013 and 2012
(Expressed in U.S. Dollars)
Supply agreements
The Company has two commitments under certain supply agreements with customers for fixed prices per gram on a non-exclusive basis except within that customer’s field of use. The agreements automatically renew each January unless terminated in writing by either party.
Licensing fees
In July 2013, the Company acquired the exclusive, worldwide license to certain patented technology for the development of human immunotherapies against Clostridium difficile infection (“C. diff”). The license agreement required an initial, non-refundable license fee of $25,000, which was paid in fiscal 2013, and payment of an aggregate of $200,000 in delayed license fees, which were paid in fiscal 2014. Beginning September 2014, the terms also require a license fee of $20,000 to be paid annually, creditable against royalties due, if any. Royalties are payable for a percentage of related net sales, if any. License fees are also payable for a percentage of related non-royalty sublicensing revenue, if any. No royalties have been paid to date. The Company also reimbursed patent filing costs of approximately $34,000 and $50,000 in fiscal 2014 and 2013, respectively, and will reimburse certain future patent filing, prosecution, and maintenance costs. License fees and patent cost reimbursements paid during the years ended August 31, 2014 and 2013, have been accounted for as research and development expense in the accompanying statements of operations.
The license agreement expires when the last valid patent claim licensed under the license agreement expires. Prior to that time, the license agreement can be terminated by the licensor upon certain conditions. The Company will have 30 days after written notice from the licensor to cure the problem prior to termination of the license agreement. The Company can terminate the agreement with three months’ prior written notice.
Upon execution of the license agreement, the Company issued 371,200 common shares and warrants to purchase up to 278,400 of the Company’s common shares to the licensor, as further described in Note 8. The warrants expire on January 23, 2015 and have an exercise price of C$1.25 per share.
The license agreement provides for the Company up to an aggregate of $6,020,000 in milestone payments to the licensor upon achievement of various financing and development targets up to the first regulatory approval. Remaining contingent milestone payments to the licensor totaling $57,025,000 are related to achievement of sales targets A financing milestone was met during the year ended August 31, 2014, and accordingly, the Company made a milestone payment of $100,000. No milestones were met during the year ended August 31, 2013, and there can be no assurance that any of the remaining milestones will be met in the future.
Retirement savings plan 401(k) contributions
The Company sponsors a 401(k) retirement savings plan that requires an annual non-elective safe harbor employer contribution of 3% of eligible employee wages. All employees over 21 years of age are eligible beginning the first payroll after 3 consecutive months of employment. Employees are 100% vested in employer contributions and in any voluntary employee contributions. Contributions to the 401(k) plan were approximately $52,000, $71,000 and $23,000 for the years ended August 31, 2014, 2013 and 2012, respectively.
Related party commitments
See Note 9.
| F-17 |
Stellar Biotechnologies, Inc.
Notes to Consolidated Financial Statements
For the Years Ended August 31, 2014, 2013 and 2012
(Expressed in U.S. Dollars)
| 8. | Share Capital |
Authorized: unlimited common shares without par value.
Private Placements During the Year Ended August 31, 2014:
The Company closed a private placement and issued 11,428,570 units for total gross proceeds of $12,000,000, completed in two closings. The private placement included a brokered portion sold to institutional and accredited investors totaling $5,000,000 (4,761,903 units) (the “brokered offering”) and a non-brokered portion totaling $7,000,000 (6,666,667 units) (the “non-brokered offering”). The non-brokered offering included a $5,000,000 investment by a privately-held Taiwan biopharmaceuticals manufacturer. Each unit, sold for $1.05, consisted of (i) one share of the Company’s common shares and (ii) one half of a share purchase warrant (each whole warrant, a “Warrant”). Each Warrant entitles the holder to purchase one additional share of the Company’s common shares at a purchase price of $1.35 for a period of three years from the issuance date of the Warrants. Subject to additional requirements imposed by the United States Securities Act of 1933, as amended requiring longer hold-periods on certain of the securities for resale by U.S. subscribers in the U.S. market and a lock-up agreement with certain holders of the securities, the securities issued in the initial closing (2,857,143 brokered offering units, 6,666,667 non-brokered offering units, and 200,000 broker warrants) were subject to a hold period that expired January 10, 2014 and the securities issued in the final closing (1,904,760 brokered offering units and 133,333 broker warrants) were subject to a hold period that expired January 21, 2014. The Company issued 333,333 broker warrants valued at $387,000 using the Black Scholes model and paid $521,000 cash share issuance costs in relation to the private placement.
Private Placements During the Year Ended August 31, 2013:
| a) | In October 2012, the Company issued 4,000,000 units at a price of CDN$0.25 per unit for total gross proceeds of $1,007,900 (CDN$1,000,000). Each unit consisted of one common share of the Company and one transferable share purchase warrant. Each warrant entitles the holder to purchase one common share of the Company at a price of CDN$0.40 and is exercisable on or before October 25, 2015. The warrants were valued at $831,000. The Company issued 400,000 broker units valued at $91,000 and paid $50,000 of cash share issuance costs in relation to the private placement. |
| b) | In January 2013, the Company issued 1,998,400 units at a price of CDN$0.25 per unit for total gross proceeds of $502,098 (CDN$499,600). Each unit consisted of one common share of the Company and one transferable share purchase warrant. Each warrant entitles the holder to purchase one common share of the Company at a price of CDN$0.40 and is exercisable on or before January 4, 2016. The warrants were valued at $448,000. The Company issued 97,200 broker units valued at $24,000 and paid $24,000 of cash share issuance costs in relation to the private placement. |
| c) | In April 2013, the Company issued 3,260,000 units at a price of CDN$0.50 per unit for total gross proceeds of $1,605,877 (CDN$1,630,000). Each unit consisted of one common share of the Company and one half of a transferable share purchase warrant. Each whole warrant entitles the holder to purchase one common share of the Company at a price of CDN$0.75 and is exercisable on or before October 2, 2014. The warrants were valued at $470,000. The Company issued 102,000 broker units valued at $36,000 and paid $50,000 of cash share issuance costs in relation to the private placement. |
Performance Shares
There were 10,000,000 common shares were allotted for officers, directors and employees of the Company based on meeting milestones related to completion of method development for commercial-scale manufacture of KLH, compilation and regulatory submittal of all required chemistry, manufacturing and control data and completion of preclinical toxicity and immunogenicity testing of products under a performance share plan (the “Performance Share Plan”). Share-based compensation was recorded over the estimated vesting period.
| F-18 |
Stellar Biotechnologies, Inc.
Notes to Consolidated Financial Statements
For the Years Ended August 31, 2014, 2013 and 2012
(Expressed in U.S. Dollars)
No amounts were recorded as share-based compensation during the years ended August 31, 2014 or 2013 since the performance shares had fully vested during the prior years. Share-based compensation expense for the year ended August 31, 2012 was $1,209,000.
During the year ended August 31, 2011, the Company determined that one of the three milestones set forth in the Performance Share Plan was successfully met and the Board authorized the issuance of an aggregate of 3,333,335 common shares of the Company to all participants in the Performance Share Plan. Accordingly, $930,000 was transferred from accumulated share-based compensation to common shares.
During the year ended August 31, 2012, the Company determined that it had successfully met the final two milestones set forth in the Performance Share Plan and issued an aggregate of 1,313,130 common shares of the Company to non-director participants in the Performance Share Plan. Accordingly, $366,363 was transferred from accumulated share-based compensation to common shares.
During the year ended August 31, 2014, the Company issued 1,515,152 common shares of the Company to a former director named in the Performance Share Plan. Accordingly, $422,728 was transferred from accumulated share-based compensation to common shares.
At August 31, 2014, there are 3,838,383 performance shares outstanding to be issued.
License Agreement
During the year ended August 31, 2013, the Company entered into a license agreement and issued 371,200 common shares and warrants to purchase up to 278,400 of the Company’s common shares to the licensor. Each warrant entitles the holder to purchase one common share of the Company at a price of CDN$1.25 per share on or before January 23, 2015. The common shares were subject to a hold period that ended on November 25, 2013. The value of the shares and warrants were recorded as research and development expense.
Black-Scholes option valuation model
The Company uses the Black-Scholes option valuation model to determine the fair value of warrants, broker units and stock options. Option valuation models require the input of highly subjective assumptions including the expected price volatility. The Company has used historical volatility to estimate the volatility of the share price. Changes in the subjective input assumptions can materially affect the fair value estimates, and therefore the existing models do not necessarily provide a reliable single measure of the fair value of the Company’s warrants, broker units and stock options.
| F-19 |
Stellar Biotechnologies, Inc.
Notes to Consolidated Financial Statements
For the Years Ended August 31, 2014, 2013 and 2012
(Expressed in U.S. Dollars)
Warrants
A summary of the Company’s warrants activity is as follows:
| Number of Warrants | Weighted Average Exercise Price | |||||||||||
| Balance - August 31, 2012 | 7,713,000 | $ | 1.02 | CDN $ | ||||||||
| Granted | 7,908,300 | 0.50 | CDN $ | |||||||||
| Exercised | (2,735,000 | ) | 0.57 | CDN $ | ||||||||
| Expired | (1,560,000 | ) | 0.53 | CDN $ | ||||||||
| Balance - August 31, 2013 | 11,326,300 | $ | 0.57 | CDN $ | ||||||||
| Granted | 38,100 | 0.46 | CDN $ | |||||||||
| Granted | 6,047,612 | 1.33 | ||||||||||
| Exercised | (5,832,300 | ) | 0.68 | CDN $ | ||||||||
| Exercised | (60,000 | ) | 1.35 | |||||||||
| Balance - August 31, 2014 | 11,519,712 | $ | 0.97 | CDN $ | ||||||||
The weighted average contractual life remaining on the outstanding warrants at August 31, 2014 is 1.57 years.
The following table summarizes information about the warrants outstanding at August 31, 2014:
| Exercise Price | Number of Warrants | Expiry Date | ||||||
| CDN$0.75 | 975,300 | October 2, 2014 | ||||||
| CDN$1.25 | 278,400 | January 23, 2015 | ||||||
| CDN$0.40 | 4,000,000 | October 25, 2015 | ||||||
| CDN$0.40 | 278,400 | January 4, 2016 | ||||||
| $1.35 | 4,701,902 | September 9, 2016 | ||||||
| $1.05 | 200,000 | September 9, 2016 | Broker warrants | |||||
| $1.35 | 952,377 | September 20, 2016 | ||||||
| $1.05 | 133,333 | September 20, 2016 | Broker warrants | |||||
| 11,519,712 | ||||||||
| F-20 |
Stellar Biotechnologies, Inc.
Notes to Consolidated Financial Statements
For the Years Ended August 31, 2014, 2013 and 2012
(Expressed in U.S. Dollars)
Warrant Liability
Equity offerings conducted by the Company in prior years included the issuance of warrants with exercise prices denominated in Canadian dollars. The Company’s functional currency is in U.S. dollars. As a result of having exercise prices denominated in other than the Company’s functional currency, these warrants meet the definition of derivatives and are therefore classified as derivative liabilities measured at fair value with adjustments to fair value recognized through the consolidated statements of operations. As these warrants are exercised, the fair value of the recorded warrant liability on date of exercise is included in common shares along with the proceeds from the exercise. If these warrants expire, the related decrease in warrant liability is recognized in profit or loss, as part of the change in fair value of warrant liability. There is no cash flow impact as a result of this accounting treatment.
The fair value of the warrants is determined using the Black-Scholes option valuation model at the end of each reporting period. Upon exercise of the warrants, the fair value of warrants included in derivative liabilities is reclassified to equity.
The fair value of warrants exercised during the years ended August 31, 2014, 2013 and 2012 was determined using the Black-Scholes option valuation model, using the following weighted average assumptions:
| 2014 | 2013 | 2012 | ||||||||||
| Risk free interest rate | 1.07 | % | 1.23 | % | 2.49 | % | ||||||
| Expected life (years) | 0.27 | 1.17 | 0.11 | |||||||||
| Expected share price volitility | 106 | % | 111 | % | 110 | % | ||||||
There were no warrants granted during the year ended August 31, 2012. The fair value of warrants granted during the years ended August 31, 2014 and 2013 was determined using the Black-Scholes option valuation model, using the following weighted average assumptions at the date of the grant:
| 2014 | 2013 | |||||||
| Risk free interest rate | 1.48 | % | 1.18 | % | ||||
| Expected life (years) | 3.00 | 2.76 | ||||||
| Expected share price volitility | 112 | % | 123 | % | ||||
| Expected dividend yield | 0 | % | 0 | % | ||||
| F-21 |
Stellar Biotechnologies, Inc.
Notes to Consolidated Financial Statements
For the Years Ended August 31, 2014, 2013 and 2012
(Expressed in U.S. Dollars)
Broker units
The Company granted broker units as finders’ fees in conjunction with equity offerings in prior years. Broker units are fully vested when granted and allow the holders to purchase equity units. A unit consists of one common share and either one whole warrant or one half warrant.
A summary of broker units activity is as follows:
| Number of Units | Weighted Average Exercise Price | |||||||||||
| Balance - August 31, 2012 | 345,600 | $ | 0.60 | CDN $ | ||||||||
| Granted | 599,200 | 0.29 | CDN $ | |||||||||
| Exercised | (3,000 | ) | 0.50 | CDN $ | ||||||||
| Expired | (345,600 | ) | 0.60 | CDN $ | ||||||||
| Balance - August 31, 2013 | 596,200 | $ | 0.29 | CDN $ | ||||||||
| Exercised | (45,000 | ) | 0.33 | CDN $ | ||||||||
| Balance - August 31, 2014 | 551,200 | $ | 0.29 | CDN $ | ||||||||
The weighted average contractual life remaining on the outstanding broker units is 1.01 years.
The following table summarizes information about the broker units outstanding at August 31, 2014:
| Exercise Price | Number of Units | Expiry Date | ||||||
| CDN$0.50 | 85,200 | October 2, 2014 | ||||||
| CDN$0.25 | 400,000 | October 25, 2015 | ||||||
| CDN$0.25 | 66,000 | January 4, 2016 | ||||||
| 551,200 | ||||||||
The broker units expiring October 2, 2014 include one-half warrant. The broker units expiring October 25, 2015 and January 4, 2016 include one warrant.
There were no broker units granted during the years ended August 31, 2014 or 2012. The estimated fair value of the broker units granted during the year ended August 31, 2013 was determined using a Black-Scholes option valuation model with the following weighted average assumptions:
| 2013 | ||||
| Risk free interest rate | 1.17 | % | ||
| Expected life (years) | 2.83 | |||
| Expected share price volitility | 123 | % | ||
| Expected dividend yield | 0 | % | ||
| F-22 |
Stellar Biotechnologies, Inc.
Notes to Consolidated Financial Statements
For the Years Ended August 31, 2014, 2013 and 2012
(Expressed in U.S. Dollars)
The weighted average fair value of broker units awarded during the year ended August 31, 2013 was CDN$0.25.
Options
The Company has a 2013 fixed stock option plan (“the Plan”) to be administered by the Board of Directors, which has the discretion to grant up to an aggregate of 10,000,000 options. The exercise price of an option is set at the closing price of the Company’s common shares on the date of grant. Stock options granted to directors, officers, employees and consultants are subject to the following vesting schedule:
| (a) | One-third shall vest immediately; |
| (b) | One-third shall vest 12 months from the date of grant; and |
| (c) | One-third shall vest 18 months from the date of grant. |
Stock options granted to investor relations consultants vest over a period of not less than 12 months as to 25% on the date that is three months from the date of grant, and a further 25% on each successive date that is three months from the date of the prior vesting.
Options have been issued under the Plan allowing the holders to purchase common shares of the Company as follows:
| Number of Options | Weighted Average Exercise Price | |||||||||||
| Balance - August 31, 2012 | 5,789,200 | $ | 0.42 | CDN $ | ||||||||
| Granted | 1,200,000 | 0.43 | CDN $ | |||||||||
| Exercised | (164,999 | ) | 0.45 | CDN $ | ||||||||
| Expired | (40,000 | ) | 0.70 | CDN $ | ||||||||
| Forfeited | (195,333 | ) | 0.45 | CDN $ | ||||||||
| Balance - August 31, 2013 | 6,588,868 | $ | 0.42 | CDN $ | ||||||||
| Granted | 195,000 | 1.42 | CDN $ | |||||||||
| Granted | 595,000 | 1.83 | ||||||||||
| Exercised | (1,441,668 | ) | 0.41 | CDN $ | ||||||||
| Expired | (1,667 | ) | 0.42 | CDN $ | ||||||||
| Balance - August 31, 2014 | 5,935,533 | $ | 0.61 | CDN $ | ||||||||
The weighted average contractual life remaining on the outstanding options is 4.17 years.
| F-23 |
Stellar Biotechnologies, Inc.
Notes to Consolidated Financial Statements
For the Years Ended August 31, 2014, 2013 and 2012
(Expressed in U.S. Dollars)
The following table summarizes information about the options under the Plan outstanding and exercisable at August 31, 2014:
| Exercise Price | Number of Options | Exercisable at August 31, 2014 | Expiry Date | |||||||
| CDN$0.25 | 125,000 | 125,000 | October 23, 2015 | |||||||
| CDN$0.28 | 1,645,000 | 1,645,000 | April 9, 2017 | |||||||
| CDN$0.25 | 55,000 | 55,000 | May 17, 2017 | |||||||
| CDN$0.28 | 20,000 | 20,000 | June 28, 2017 | |||||||
| CDN$0.28 | 70,000 | 70,000 | July 13, 2017 | |||||||
| CDN$0.64 | 70,000 | 70,000 | October 25, 2017 | |||||||
| CDN$1.00 | 60,000 | 60,000 | February 10, 2018 | |||||||
| CDN$0.65 | 848,600 | 848,600 | August 8, 2018 | |||||||
| CDN$0.50 | 5,000 | 5,000 | September 26, 2018 | |||||||
| CDN$1.87 | 100,000 | 33,333 | November 7, 2018 | |||||||
| CDN$0.40 | 70,000 | 70,000 | December 22, 2018 | |||||||
| CDN$0.42 | 853,600 | 853,600 | April 13, 2019 | |||||||
| CDN$0.29 | 90,000 | 90,000 | June 18, 2019 | |||||||
| CDN$0.37 | 150,000 | 150,000 | August 9, 2019 | |||||||
| CDN$0.37 | 150,000 | 150,000 | August 16, 2019 | |||||||
| CDN$0.25 | 58,333 | 58,333 | October 23, 2019 | |||||||
| CDN$0.25 | 215,000 | 215,000 | December 19, 2019 | |||||||
| CDN$0.58 | 560,000 | 373,333 | May 14, 2020 | |||||||
| CDN$0.58 | 100,000 | 66,667 | May 23, 2020 | |||||||
| $1.83 | 495,000 | 165,000 | November 1, 2020 | |||||||
| $1.84 | 100,000 | 33,333 | November 15, 2020 | |||||||
| CDN$0.94 | 95,000 | 31,667 | June 27, 2021 | |||||||
| 5,935,533 | 5,188,866 | |||||||||
The estimated fair value of the stock options granted during the years ended August 31, 2014, 2013 and 2012 was determined using a Black-Scholes option valuation model with the following weighted average assumptions:
| 2014 | 2013 | 2012 | ||||||||||
| Risk free interest rate | 2.01 | % | 1.55 | % | 1.63 | % | ||||||
| Expected life (years) | 6.75 | 6.17 | 7.00 | |||||||||
| Expected share price volitility | 120 | % | 123 | % | 147 | % | ||||||
| Expected dividend yield | 0 | % | 0 | % | 0 | % | ||||||
The weighted average fair value of stock options awarded during the years ended August 31, 2014, 2013 and 2012 was CDN$1.58, CDN$0.38 and CDN$0.40, respectively.
As of August 31, 2014, the Company had approximately $327,000 of unrecognized share-based compensation expense, which is expected to be recognized over a period of 1.32 years.
The intrinsic value of the options exercised during the years ended August 31, 2014, 2013 and 2012 was $1.38, $0.67, and $0.05 respectively. The intrinsic value of the vested options at August 31, 2014 was $1.25.
| F-24 |
Stellar Biotechnologies, Inc.
Notes to Consolidated Financial Statements
For the Years Ended August 31, 2014, 2013 and 2012
(Expressed in U.S. Dollars)
| 9. | Related Party Disclosures |
Royalty agreement
On August 14, 2002, through its California subsidiary, the Company entered into an agreement with a director and officer of the Company, where he would receive royalty payments in exchange for assignment of his patent rights to the Company. The royalty is 5% of gross receipts from products using this invention in excess of $500,000 annually. The Company’s current operations utilize this invention. There was no royalty expense incurred during the years ended August 31, 2014, 2013 and 2012.
Collaboration agreement
In December 2013, the Company entered into a collaboration agreement with a privately-held Taiwan biopharmaceuticals manufacturer and a beneficial owner of over 5% of the Company’s common shares. Under the terms of the agreement, the Company will be responsible for the production and delivery of GMP grade KLH for evaluation as a carrier molecule in the collaboration partner’s potential manufacture of OBI-822 active immunotherapy. The Company is also responsible for method development, product formulation, and process qualification for certain KLH reference standards. The collaboration partner will be responsible for development objectives and product specifications. The agreement provides for the collaboration partner to pay fees for certain expenses and costs associated with the collaboration. Subject to certain conditions and timing, the collaboration also provides for the parties to negotiate a commercial supply agreement for Stellar KLH™ in the future. However, there can be no assurance that any such negotiations will lead to successful execution of any further agreements related to this collaboration.
| 10. | Loss Recovery |
A shipment of KLH was damaged by a vendor. The vendor agreed to reimburse the Company for the value of the KLH. The loss recovery of $105,000 was recorded during the year ended August 31, 2012 when the realization of income was virtually certain.
| 11. | Income Taxes |
The breakdown of loss before income tax by jurisdiction is as follows:
| August 31, 2014 | August 31, 2013 | August 31, 2012 | ||||||||||
| U.S. | $ | (4,183,392 | ) | $ | (2,082,441 | ) | $ | (3,773,748 | ) | |||
| Canadian | (4,096,931 | ) | (12,412,538 | ) | (1,754,730 | ) | ||||||
| Other foreign | (132,000 | ) | - | - | ||||||||
| Total Loss Before Income Tax | $ | (8,412,323 | ) | $ | (14,494,979 | ) | $ | (5,528,478 | ) | |||
Deferred income tax assets and liabilities of the Company at August 31, 2014, 2013 and 2012 are as follows:
| August 31, 2014 | August 31, 2013 | August 31, 2012 | ||||||||||
| Deferred income tax assets: | ||||||||||||
| Non-capital loss carry-forwards | $ | 6,418,300 | $ | 4,426,800 | $ | 3,409,600 | ||||||
| Research and development tax credits | 616,600 | 450,400 | 267,900 | |||||||||
| Deferred expenses | 90,000 | 65,100 | 39,400 | |||||||||
| Share issuance costs | 131,800 | 63,300 | 73,400 | |||||||||
| Deferred income tax liabilities: | ||||||||||||
| US federal benefit net of state taxes | (509,000 | ) | (350,600 | ) | (265,200 | ) | ||||||
| Property, plant and equipment | (14,500 | ) | (33,400 | ) | (32,700 | ) | ||||||
| Valuation allowance | (6,733,200 | ) | (4,621,600 | ) | (3,492,400 | ) | ||||||
| Net deferred income tax asset (liability) | $ | - | $ | - | $ | - | ||||||
Realization of the deferred tax assets is dependent upon the generation of future taxable income, the amount and timing of which are uncertain. Accordingly, the net deferred tax assets have been fully offset by a valuation allowance.
| F-25 |
Stellar Biotechnologies, Inc.
Notes to Consolidated Financial Statements
For the Years Ended August 31, 2014, 2013 and 2012
(Expressed in U.S. Dollars)
As of August 31, 2014, the Company had federal and California net operating loss (“NOL”) carryforwards of approximately $13,137,000 and $13,068,000, respectively, that will expire beginning in 2030 and 2015, respectively, and both continue expiring through 2034. Portions of these NOL carryforwards may be used to offset future taxable income, if any.
As of August 31, 2014, the Company also has federal and California research and development tax credit carryforwards of approximately $290,000 and $326,000, respectively, available to offset future taxes. The federal credits begin expiring in 2024 and continue expiring through 2034. The state tax credits do not expire.
Under the provisions of Section 382 of the Internal Revenue Code, substantial changes in the Company's ownership limit the amount of net operating loss carryforwards and tax credit carryforwards that can be utilized annually in the future to offset taxable income. A valuation allowance has been established to reserve the potential benefits of these carryforwards in the Company's consolidated financial statements to reflect the uncertainty of future taxable income required to utilize available tax loss carryforwards and other deferred tax assets.
The recovery of income taxes shown in the consolidated statements of operations differs from the amounts obtained by applying statutory rates to the loss before provision for income taxes due to the following:
| August 31, 2014 | August 31, 2013 | August 31, 2012 | ||||||||||
| Combined Canadian federal and provincial tax rates | 26.0 | % | 25.0 | % | 28.5 | % | ||||||
| Expected income tax (recovery)/expense | $ | (2,187,200 | ) | $ | (3,623,700 | ) | $ | (1,575,600 | ) | |||
| Nondeductible share-based payments | 248,700 | 327,000 | 549,900 | |||||||||
| Nondeductible change in fair value of warrant liability | 659,300 | 2,807,300 | (246,100 | ) | ||||||||
| Effect of higher income tax rate in US | (602,100 | ) | (308,400 | ) | (426,700 | ) | ||||||
| Foreign currency differences | (50,900 | ) | (26,300 | ) | 9,000 | |||||||
| Other | (219,800 | ) | (322,500 | ) | (137,300 | ) | ||||||
| Change in valuation allowance on deferred tax assets | 2,179,200 | 1,147,400 | 1,827,600 | |||||||||
| Income tax expense | $ | 27,200 | $ | 800 | $ | 800 | ||||||
| F-26 |
Stellar Biotechnologies, Inc.
Notes to Consolidated Financial Statements
For the Years Ended August 31, 2014, 2013 and 2012
(Expressed in U.S. Dollars)
The components of income tax provision (benefits) are as follows:
| August 31, 2014 | August 31, 2013 | August 31, 2012 | ||||||||||
| Current tax provision | ||||||||||||
| U.S. federal | $ | - | $ | - | $ | - | ||||||
| Canadian | - | - | - | |||||||||
| Other foreign | 26,400 | - | - | |||||||||
| State | 800 | 800 | 800 | |||||||||
| Deferred tax provision | ||||||||||||
| U.S. federal | (1,431,400 | ) | (738,000 | ) | (1,165,700 | ) | ||||||
| Canadian | (289,800 | ) | (157,500 | ) | (249,200 | ) | ||||||
| Other foreign | - | - | - | |||||||||
| State | (458,000 | ) | (251,900 | ) | (412,700 | ) | ||||||
| Change in valuation allowance on deferred tax assets | 2,179,200 | 1,147,400 | 1,827,600 | |||||||||
| Total | $ | 27,200 | $ | 800 | $ | 800 | ||||||
12. Supplemental Disclosure of Cash Flow and Non-Cash Transactions
Supplemental disclosure of cash flow information follows:
| August 31, | August 31, | August 31, | ||||||||||
| 2014 | 2013 | 2012 | ||||||||||
| Cash paid during the period for taxes | 30,200 | 800 | 800 | |||||||||
| Cash paid during the period for interest | - | - | - | |||||||||
| F-27 |
Stellar Biotechnologies, Inc.
Notes to Consolidated Financial Statements
For the Years Ended August 31, 2014, 2013 and 2012
(Expressed in U.S. Dollars)
Supplemental disclosure of non-cash financing and investing activities follows:
| August 31, | August 31, | August 31, | ||||||||||
| 2014 | 2013 | 2012 | ||||||||||
| Share issuance costs - broker units and warrants | $ | 386,898 | $ | 150,894 | $ | - | ||||||
| Transfer to common stock on exercise of warrants | 6,591,546 | 2,139,409 | 190,425 | |||||||||
| Transfer to common stock on exercise of options | 489,136 | 54,325 | 41,087 | |||||||||
| Transfer to common stock on issuance of performance shares | 422,728 | - | 366,363 | |||||||||
| Shares subscribed transferred to common stock | 5,155,674 | - | - | |||||||||
| Warrant valuations on private placements | - | 1,749,004 | - | |||||||||
| Fair value of shares issued for acquisition of license | - | 491,408 | - | |||||||||
| Warrant valuation on acquisition of license | - | 195,014 | - | |||||||||
| 13. | Concentrations of Credit Risk |
Credit risk is the risk of an unexpected loss if a customer or third party to a financial instrument fails to meet its contractual obligations. Financial instruments that potentially subject the Company to a concentration of credit risk consist primarily of cash, cash equivalents and accounts receivable. Management’s assessment of the Company’s credit risk for cash and cash equivalents is low as cash and cash equivalents are held in financial institutions believed to be credit worthy. The Company limits its exposure to credit loss by placing its cash with major financial institutions and invests only in short-term obligations.
Approximately 73%, 73% and 90% of the Company’s product sales and contract services revenue during the years ended August 31, 2014, 2013 and 2012, respectively, were from two customers. All of the grant revenue during the years ended August 31, 2014, 2013 and 2012 was received from the National Science Foundation.
Approximately 76% of the Company’s accounts receivable at August 31, 2014, was from one customer. Approximately 88% of the Company’s accounts receivable at August 31, 2013 was from a grant from National Science Foundation.
While the Company is exposed to credit losses due to the non-performance of its counterparties, the Company considers the risk of this remote. The Company estimates its maximum credit risk for accounts receivable at the amount recorded on the balance sheet.
| 14. | Subsequent Events |
Subsequent to August 31, 2014, the Company issued 1,152,800 common shares upon the exercise of 1,102,800 warrants for gross proceeds of CDN$805,800 and the exercise of 50,000 stock options for gross proceeds of CDN$12,500 through November 1, 2014.
| F-28 |
