Attached files
| file | filename |
|---|---|
| 8-K - 8-K - LDR HOLDING CORP | form8k.htm |
| EX-99.1 - EX-99.1 - LDR HOLDING CORP | ex991.htm |

Mobi-C® 5 Year 1 and 2 Level IDE Study Results
2 • Prospective, randomized, multi-center concurrently controlled, FDA IDE clinical trial: – Investigational device: Mobi-C® Cervical Disc. – Control: ACDF. – Non-inferiority study with 2:1 randomization (TDR:ACDF). • One-level and two-level trial arms were conducted at 24 sites across the U.S. • Outcome measures were collected pre-operatively, at 6 weeks, 3, 6, 12, 18, 24, 36, 48 and 60 months post-operatively. Study Design
3 • One-level trial arm enrolled 245 patients randomized in a 2:1 ratio (TDR:ACDF): – 164 TDR patients and 81 ACDF patients. • Two-level trial arm enrolled 330 patients randomized in a 2:1 ratio (TDR:ACDF): – 225 TDR patients and 105 ACDF patients. • One investigational training case was included for each site, resulting in 24 non- randomized cases. • All patients were diagnosed with symptomatic cervical DDD at either one or two contiguous levels of the cervical spine and had no previous cervical fusions. • Follow-up rate at 60 months for TDR patients was 88.5% and for ACDF patients was 83.1%. Patient Sample

4 • FDA predefined protocol – NDI Success • Improvement in NDI of at least 15/50 points in subjects with a baseline NDI score of ≥ 30/50 points, or a 50% improvement in subjects with a baseline NDI score of <30/50 points – No study failures due to secondary surgical interventions at the index level – Neurologic Success – No adverse event determined by CEC – Radiographic Success Overall Success
5 • 245 patients enrolled into a prospective, non-inferiority, unmasked, concurrently controlled, randomized 2:1 ratio (TDR:ACDF) study. – 164 TDR patients and 81 ACDF patients • All patients were treated for intractable radiculopathy with or without neck pain or myelopathy due to a single-level abnormality localized to the level of the disc space (C3-C7), with at least one of the following conditions confirmed by radiographic imaging (CT, MRI, X-rays): – Herniated nucleus pulposus – Spondylosis – Visible loss of disc height compared to adjacent levels in skeletally mature patients • Patients had no previous cervical fusions. • Patients were treated between April 2006 and March 2008. Results from one-level comparative study of Mobi-C to ACDF
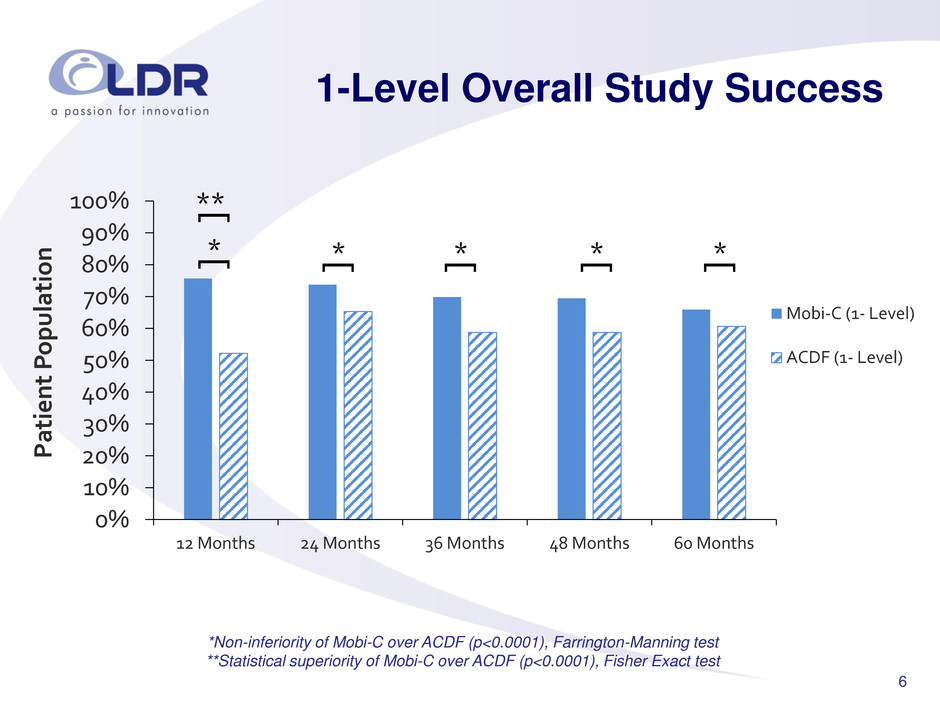
6 1-Level Overall Study Success *Non-inferiority of Mobi-C over ACDF (p<0.0001), Farrington-Manning test **Statistical superiority of Mobi-C over ACDF (p<0.0001), Fisher Exact test 0% 10% 20% 30% 40% 50% 60% 70% 80% 90% 100% 12 Months 24 Months 36 Months 48 Months 60 Months Pa ti e nt P o pulatio n Mobi-C (1- Level) ACDF (1- Level) ** [ * [ * [ * [ * [ * [
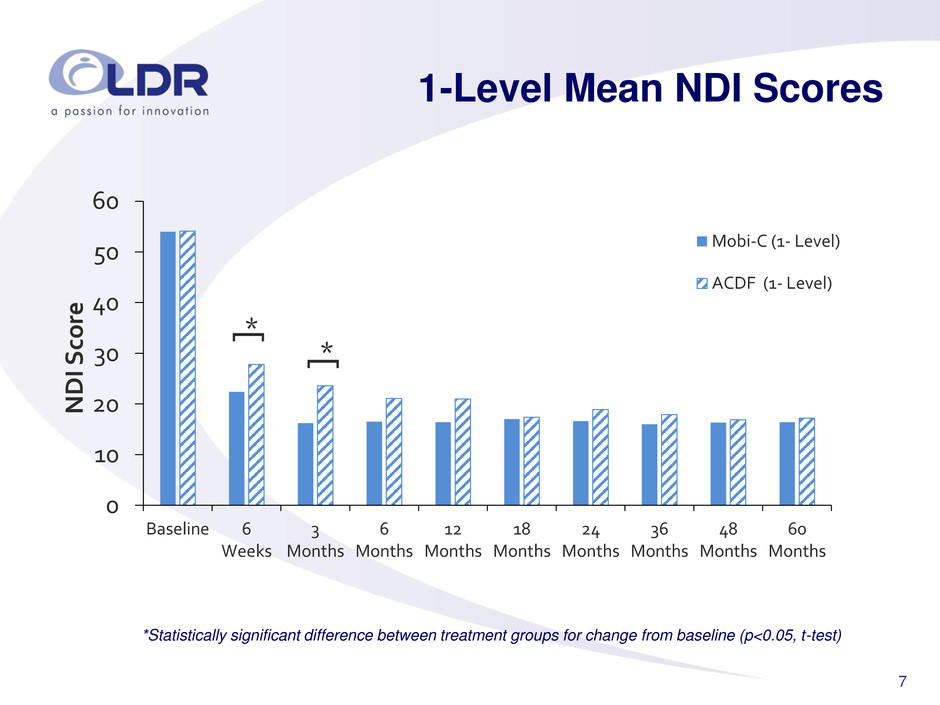
7 1-Level Mean NDI Scores *Statistically significant difference between treatment groups for change from baseline (p<0.05, t-test) 0 10 20 30 40 50 60 Baseline 6 Weeks 3 Months 6 Months 12 Months 18 Months 24 Months 36 Months 48 Months 60 Months NDI Sc o re Mobi-C (1- Level) ACDF (1- Level) * [ * [
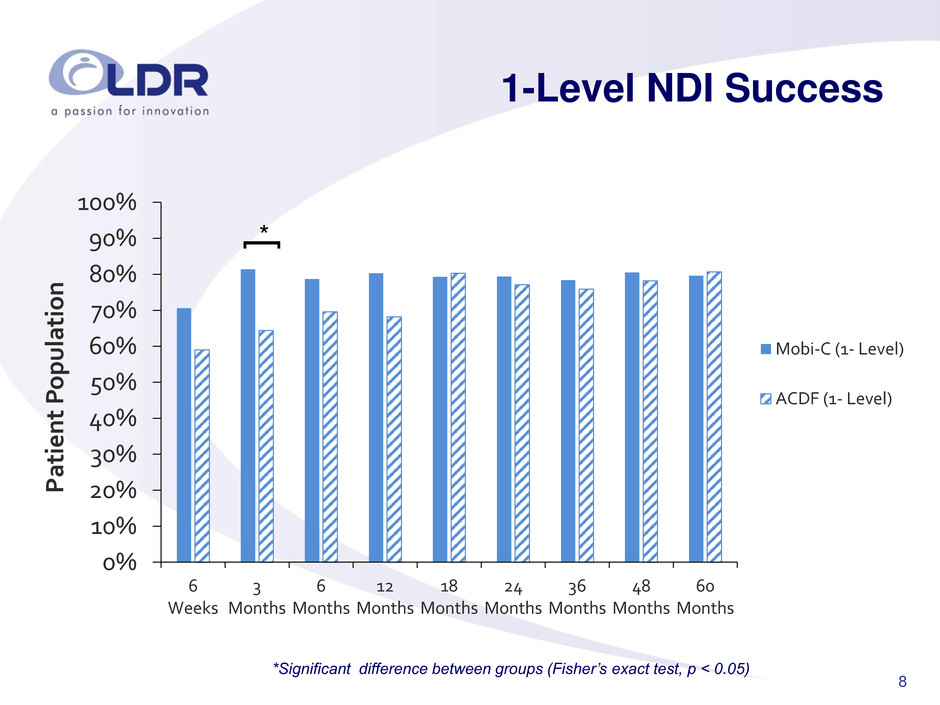
8 1-Level NDI Success 0% 10% 20% 30% 40% 50% 60% 70% 80% 90% 100% 6 Weeks 3 Months 6 Months 12 Months 18 Months 24 Months 36 Months 48 Months 60 Months Pa ti e nt P o pulatio n Mobi-C (1- Level) ACDF (1- Level) * [ *Significant difference between groups (Fisher’s exact test, p < 0.05)
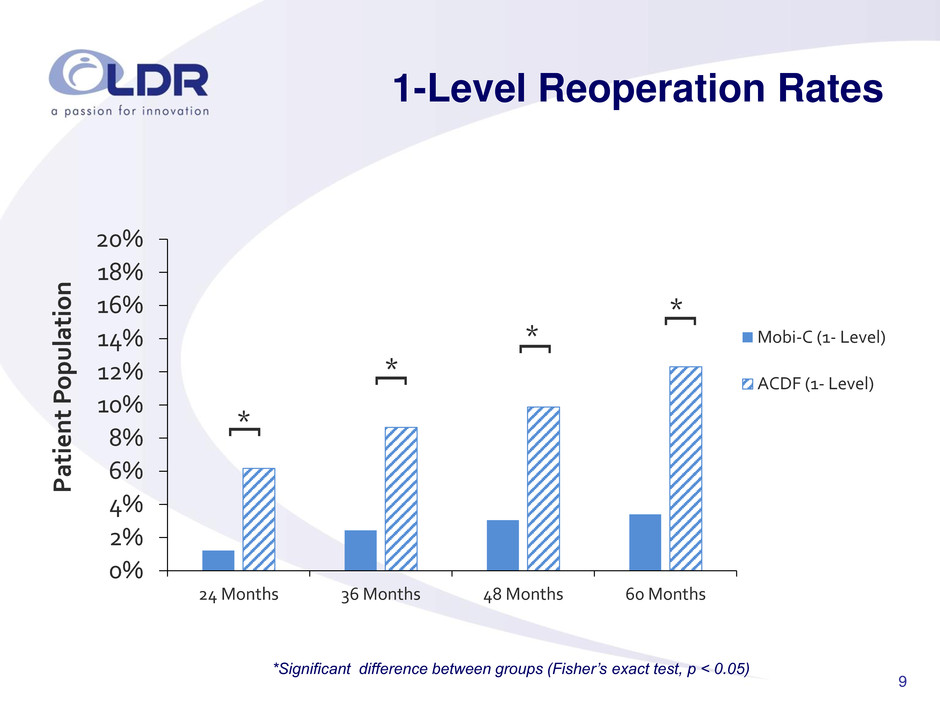
9 1-Level Reoperation Rates 0% 2% 4% 6% 8% 10% 12% 14% 16% 18% 20% 24 Months 36 Months 48 Months 60 Months Pa ti e nt P o pulatio n Mobi-C (1- Level) ACDF (1- Level) * [ * [ * [ * [ *Significant difference between groups (Fisher’s exact test, p < 0.05)
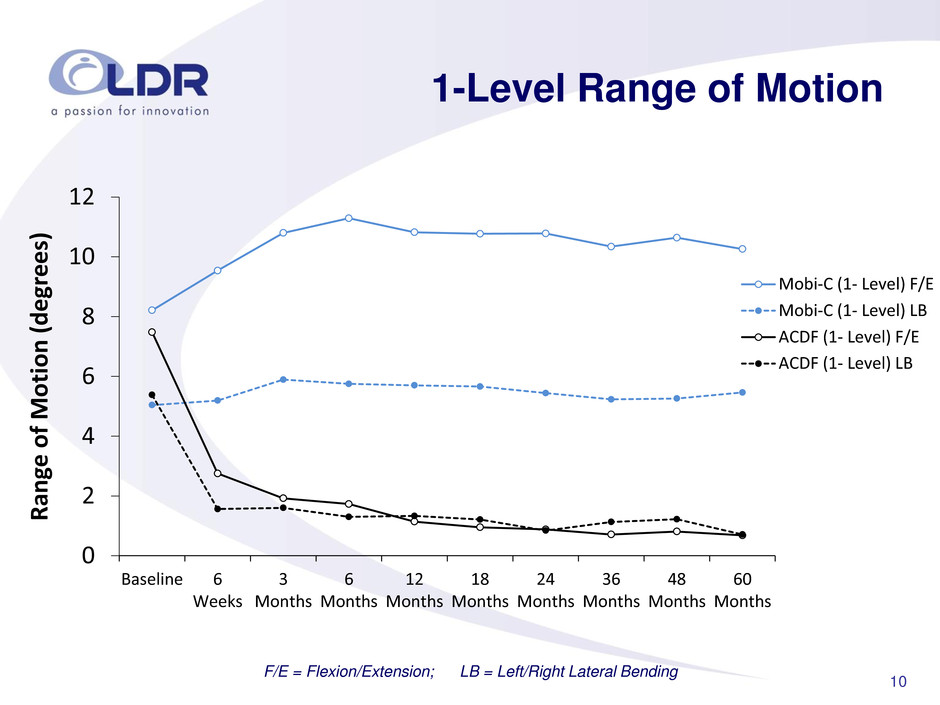
10 1-Level Range of Motion F/E = Flexion/Extension; LB = Left/Right Lateral Bending 0 2 4 6 8 10 12 Baseline 6 Weeks 3 Months 6 Months 12 Months 18 Months 24 Months 36 Months 48 Months 60 Months R an ge of Motion (deg rees ) Mobi-C (1- Level) F/E Mobi-C (1- Level) LB ACDF (1- Level) F/E ACDF (1- Level) LB
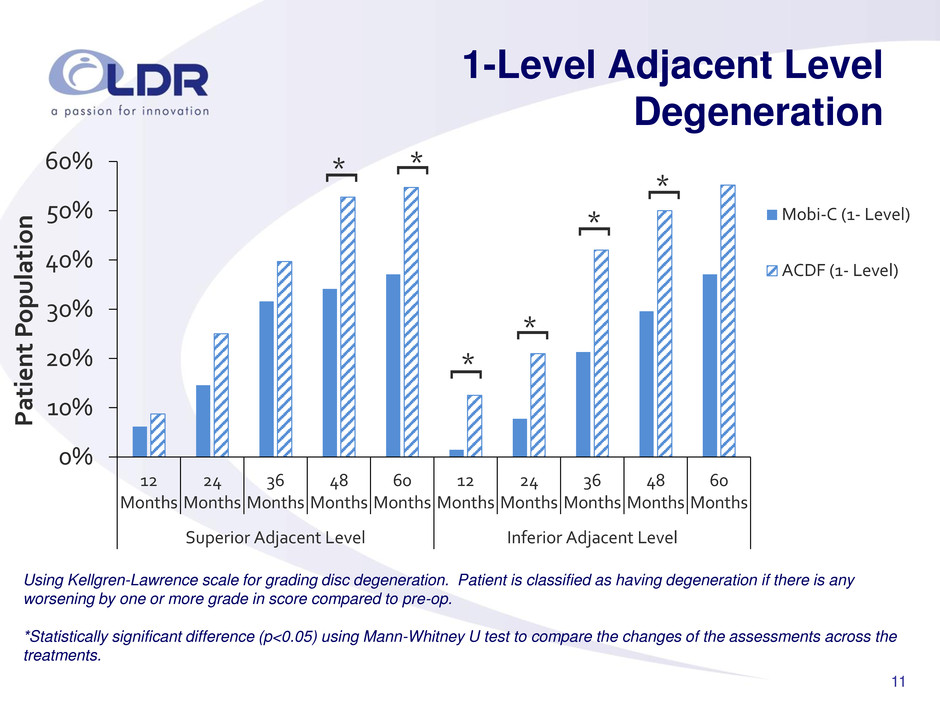
11 1-Level Adjacent Level Degeneration 0% 10% 20% 30% 40% 50% 60% 12 Months 24 Months 36 Months 48 Months 60 Months 12 Months 24 Months 36 Months 48 Months 60 Months Superior Adjacent Level Inferior Adjacent Level Pati e n t P opulatio n Mobi-C (1- Level) ACDF (1- Level) * [ * [ * [ * [ * [ * [ Using Kellgren-Lawrence scale for grading disc degeneration. Patient is classified as having degeneration if there is any worsening by one or more grade in score compared to pre-op. *Statistically significant difference (p<0.05) using Mann-Whitney U test to compare the changes of the assessments across the treatments.
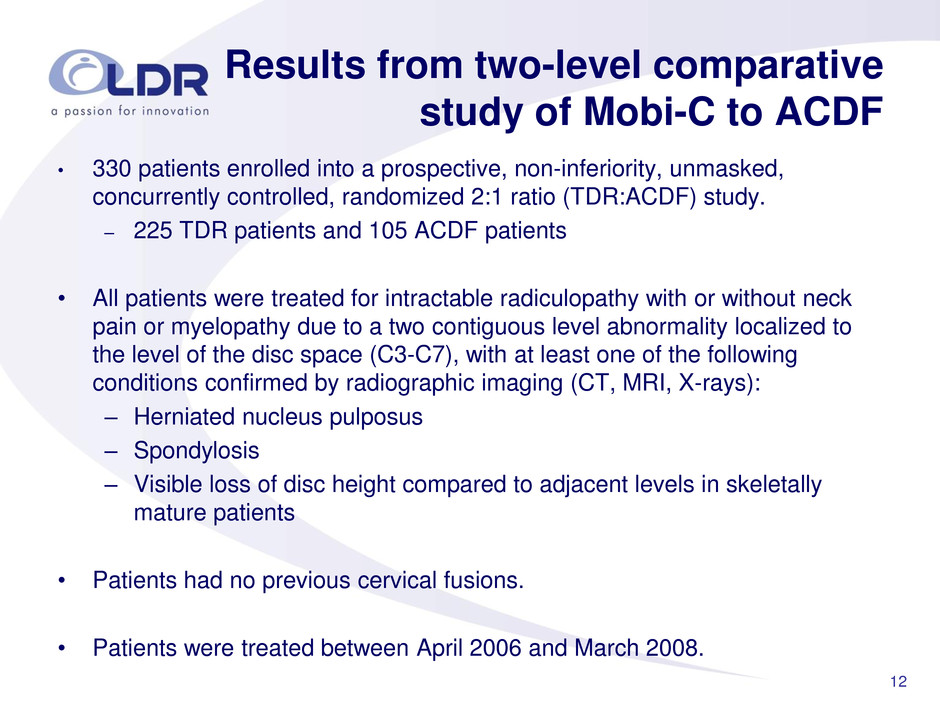
12 • 330 patients enrolled into a prospective, non-inferiority, unmasked, concurrently controlled, randomized 2:1 ratio (TDR:ACDF) study. – 225 TDR patients and 105 ACDF patients • All patients were treated for intractable radiculopathy with or without neck pain or myelopathy due to a two contiguous level abnormality localized to the level of the disc space (C3-C7), with at least one of the following conditions confirmed by radiographic imaging (CT, MRI, X-rays): – Herniated nucleus pulposus – Spondylosis – Visible loss of disc height compared to adjacent levels in skeletally mature patients • Patients had no previous cervical fusions. • Patients were treated between April 2006 and March 2008. Results from two-level comparative study of Mobi-C to ACDF
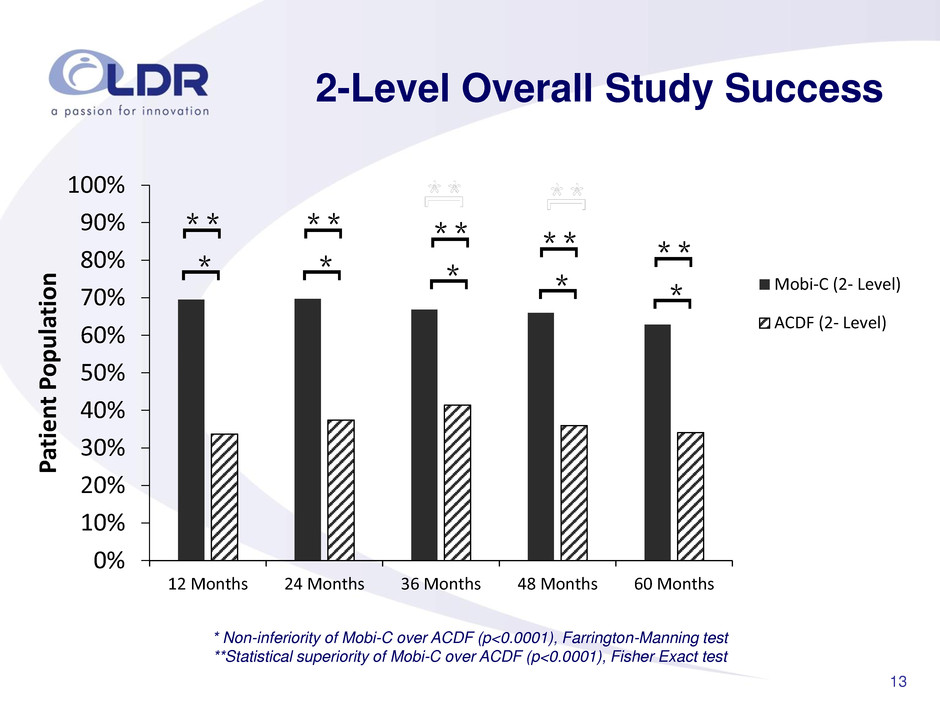
13 0% 10% 20% 30% 40% 50% 60% 70% 80% 90% 100% 12 Months 24 Months 36 Months 48 Months 60 Months P atie n t P op u la tio n Mobi-C (2- Level) ACDF (2- Level) 2-Level Overall Study Success * Non-inferiority of Mobi-C over ACDF (p<0.0001), Farrington-Manning test **Statistical superiority of Mobi-C over ACDF (p<0.0001), Fisher Exact test [ * * * [ [ * * * [ [ * * * [ [ * * * [ [ * * * [
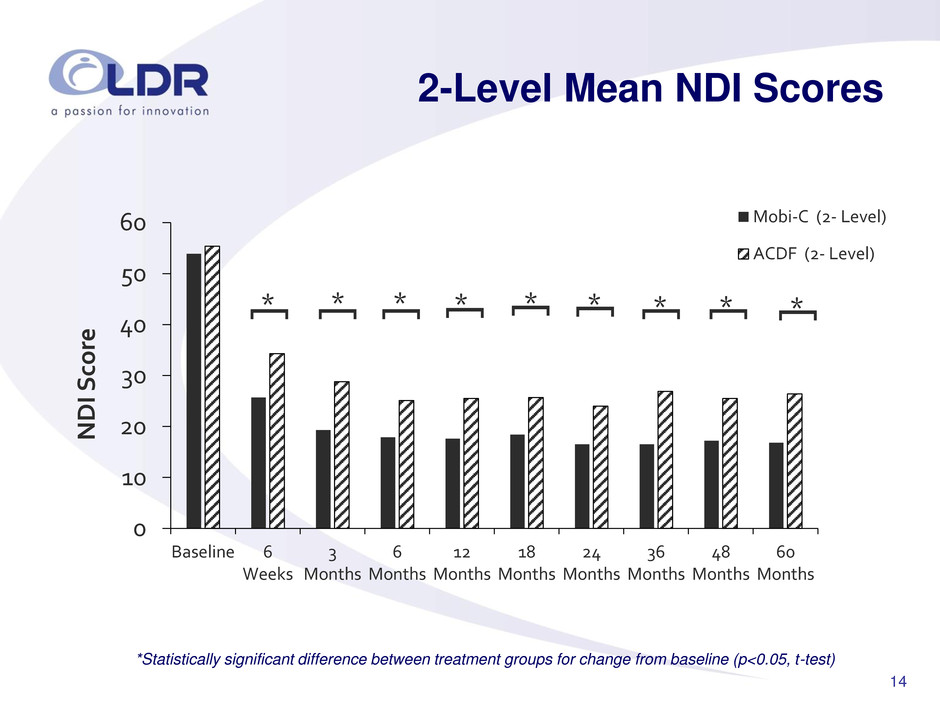
14 2-Level Mean NDI Scores 0 10 20 30 40 50 60 Baseline 6 Weeks 3 Months 6 Months 12 Months 18 Months 24 Months 36 Months 48 Months 60 Months NDI Sc o re Mobi-C (2- Level) ACDF (2- Level) * [ * [ * [ * [ * [ * [ * [ * [ * [ *Statistically significant difference between treatment groups for change from baseline (p<0.05, t-test)
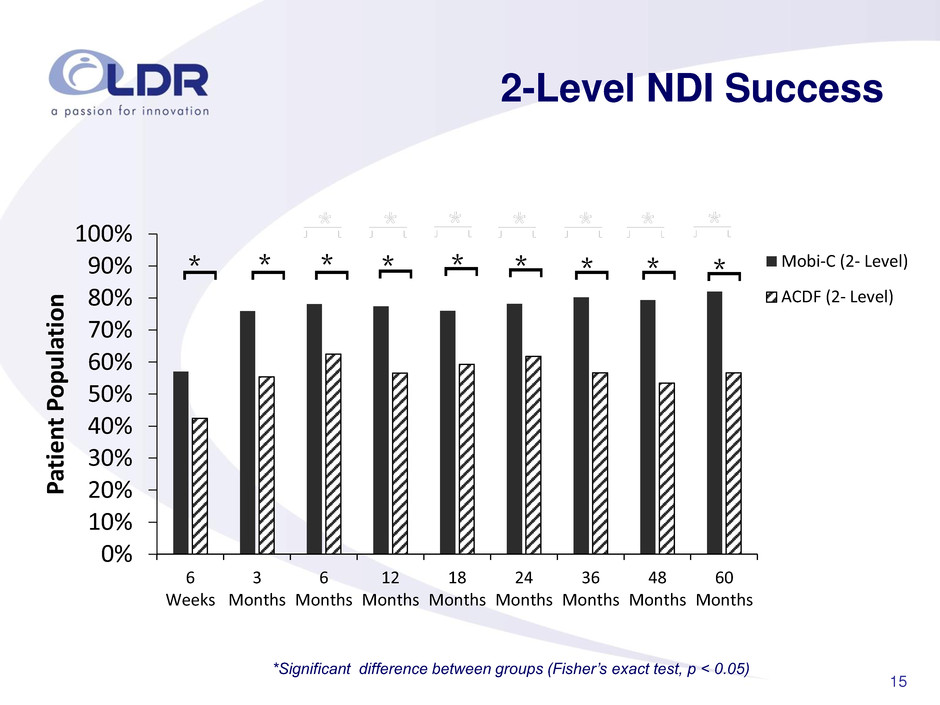
15 2-Level NDI Success 0% 10% 20% 30% 40% 50% 60% 70% 80% 90% 100% 6 Weeks 3 Months 6 Months 12 Months 18 Months 24 Months 36 Months 48 Months 60 Months P atie n t P op u la tio n Mobi-C (2- Level) ACDF (2- Level) * [ * [ * [ * [ * [ * [ * [ * [ * [ *Significant difference between groups (Fisher’s exact test, p < 0.05)
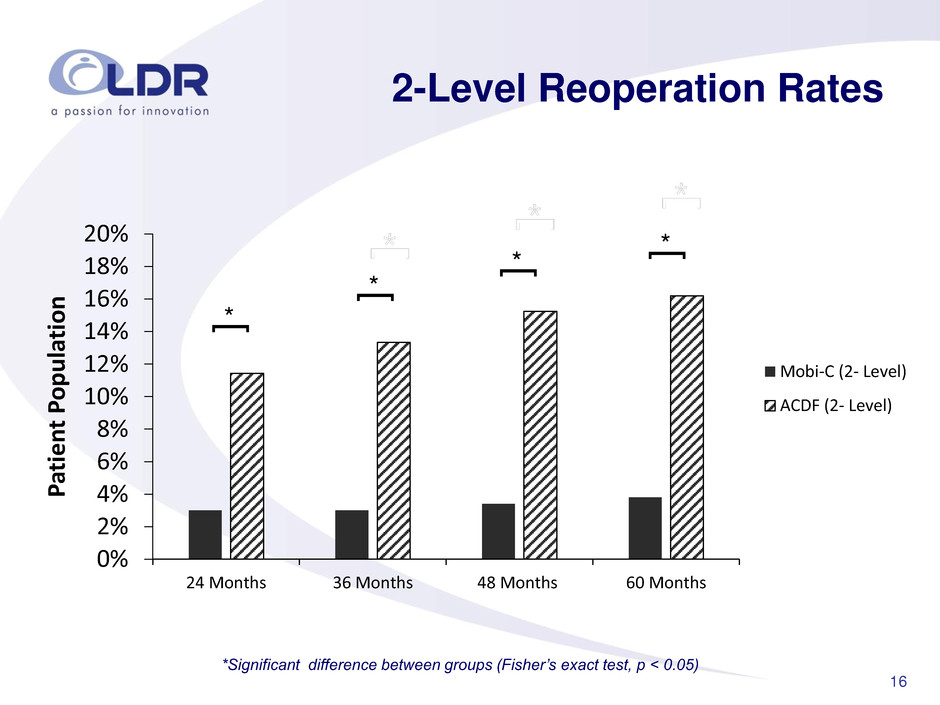
16 2-Level Reoperation Rates *Significant difference between groups (Fisher’s exact test, p < 0.05) * [ 0% 2% 4% 6% 8% 10% 12% 14% 16% 18% 20% 24 Months 36 Months 48 Months 60 Months P atie n t P o p u la tio n Mobi-C (2- Level) ACDF (2- Level) * [ * [ * [ * [
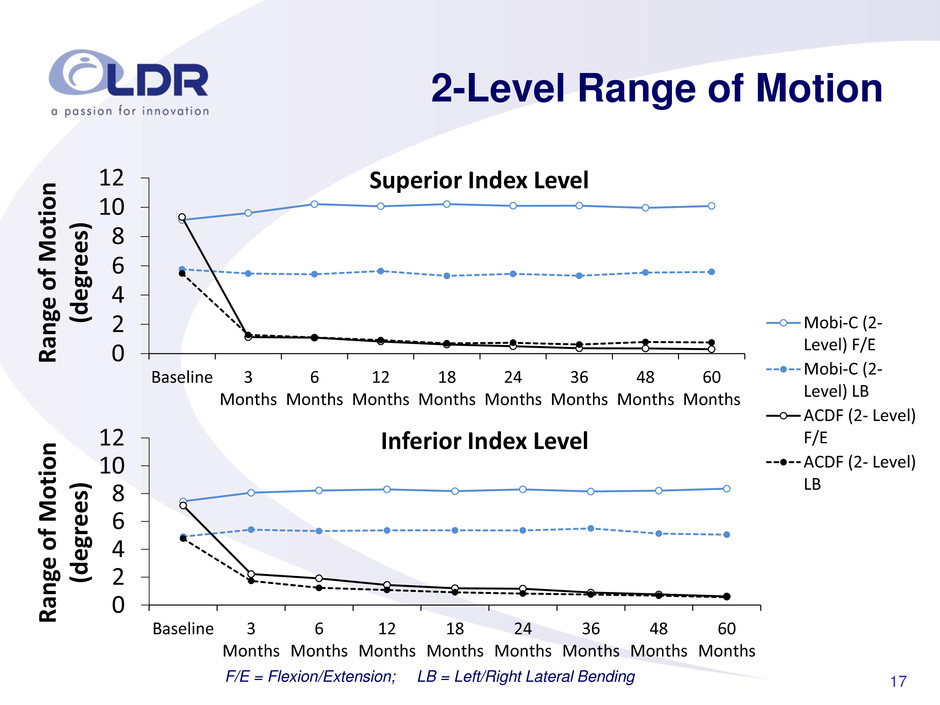
17 2-Level Range of Motion F/E = Flexion/Extension; LB = Left/Right Lateral Bending 0 2 4 6 8 10 12 Baseline 3 Months 6 Months 12 Months 18 Months 24 Months 36 Months 48 Months 60 Months R an ge of Motion (deg rees ) Superior Index Level Mobi-C (2- Level) F/E Mobi-C (2- Level) LB ACDF (2- Level) F/E ACDF (2- Level) LB 0 2 4 6 8 10 12 Baseline 3 Months 6 Months 12 Months 18 Months 24 Months 36 Months 48 Months 60 Months R an ge of Motion (deg rees ) Inferior Index Level
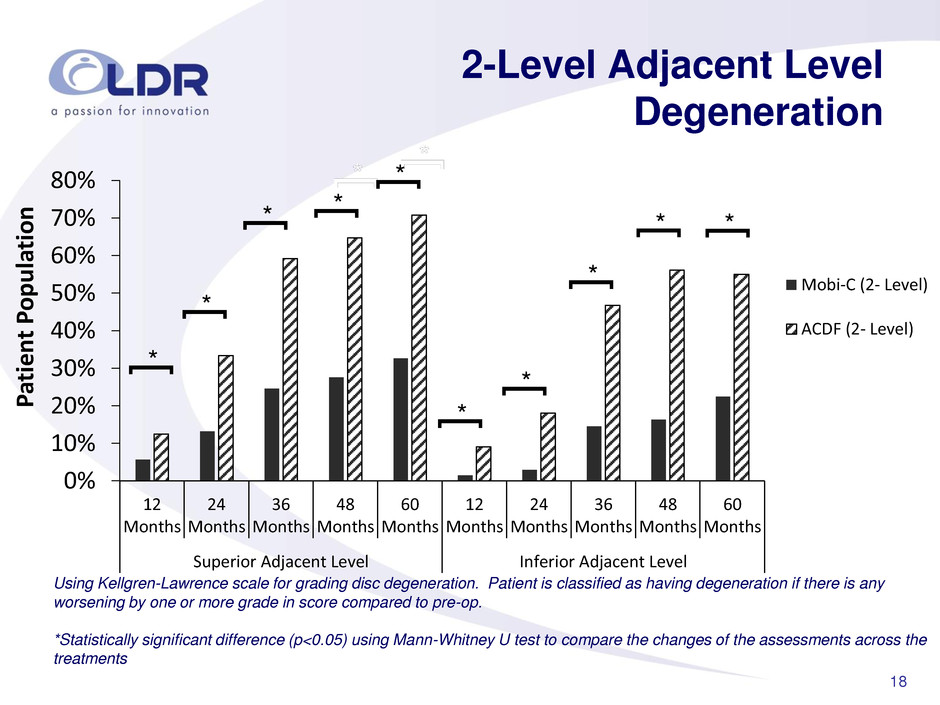
18 0% 10% 20% 30% 40% 50% 60% 70% 80% 12 Months 24 Months 36 Months 48 Months 60 Months 12 Months 24 Months 36 Months 48 Months 60 Months Superior Adjacent Level Inferior Adjacent Level P atie n t P op u la tion Mobi-C (2- Level) ACDF (2- Level) 2-Level Adjacent Level Degeneration Using Kellgren-Lawrence scale for grading disc degeneration. Patient is classified as having degeneration if there is any worsening by one or more grade in score compared to pre-op. *Statistically significant difference (p<0.05) using Mann-Whitney U test to compare the changes of the assessments across the treatments * [ * [ * [ * [ * [ * [ * [ * [ * [ * [
