Attached files
| file | filename |
|---|---|
| 8-K/A - AMENDMENT NO. 1 - CAPRICOR THERAPEUTICS, INC. | v394138_8ka1.htm |
Exhibit 99.2

QUARTERLY CONFERENCE CALL 3 rd Quarter, 2014 November 12, 2014 4:30p.m. EST / 1:30p.m. PST

Forward Looking Statements This presentation contains forward - looking statements and information that are based on the beliefs of the management of Capricor Therapeutics, Inc. (Capricor) as well as assumptions made by and information currently available to Capricor. Statements in this presentation regarding the efficacy, safety, and intended utilization of Capricor’s product candidates; the conduct, size, timing and results of discovery efforts and clinical trials; scope, duration, validity and enforceability of intellectual property rights; plans regarding regulatory filings, future research and clinical trials; plans regarding current and future collaborative activities and the ownership of commercial rights; future royalty streams, and any other statements about Capricor’s management team’s future expectations, beliefs, goals, plans or prospects constitute forward - looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. Any statements that are not statements of historical fact (including statements containing the words "believes," "plans," "could," "anticipates," "expects," "estimates," "should," "target," "will," "would" and similar expressions) should also be considered to be forward - looking statements. There are a number of important factors that could cause actual results or events to differ materially from those indicated by such forward - looking statements. More information about these and other risks that may impact our business are set forth in our Annual Report on Form 10 - K for the year ended December 31, 2013, as filed with the Securities and Exchange Commission on March 31, 2014, in our Amendment No. 1 to Registration Statement on Form S - 1, as filed with the Securities and Exchange Commission on May 23, 2014, and in our Quarterly Report on Form 10 - Q for the quarter ended June 30, 2014, as filed with the Securities and Exchange Commission on August 14, 2014. All forward - looking statements in this press release are based on information available to us as of the date hereof, and we assume no obligation to update these forward - looking statements. 2

Capricor Therapeutics Overview Clinical - stage biotechnology company with a diversified pipeline and near - term focus on cardiovascular diseases including orphan indications Peptide therapy for heart failure ( Cenderitide ) Cardiac - derived stem cells (CDCs) Micro - RNA Platform (Exosomes) 3

Balance Sheet Highlights (approximate) 9.30.14 12.31.13 Cash (Includes restricted cash and marketable securities) $13.8M $3.5M Outstanding Common Shares 11,703,774 11,687,747 P&L Highlights (approximate) Three months ended 9.30.14 Three months Ended 9.30.13 Income $1.3M $0.1M R&D Expenses $2.0M $1.0M G&A Expenses $0.8M $0.6M Net Loss $(1.5M) $(1.5M) Net Loss per share (basic and diluted) $(0.13) $(0.15) Quarterly Financial Highlights 4

Notable AHA Presentations American Heart Association Scientific Sessions Chicago, IL (November 15 - 19) Mon. 11/17 4:00pm – 6:00pm South Hall - A2 ALLogeneic Heart STem Cells to Achieve Myocardial Regeneration (ALLSTAR): the One Year Phase I Results Mon. 11/17 4:00pm – 6:00pm South Hall - A2 Heart - derived Cell Therapy for Duchenne Cardiomyopathy: Cardiosphere - derived Cells and their Exosomes Improve Function, Restore Mitochondrial Integrity and Reverse Degenerative Changes in the Hearts of Mdx Mice Tues. 11/18 3:00pm - 4:30pm South Hall - A2 Regenerative Effects of Exosomes Secreted by Cardiospheres in a Rat Model of Chronic Myocardial Infarction Are Mimicked by Exosome - primed Fibroblasts Wed. 11/19 9:00am – 10:15am S404bc d Intracoronary Delivery of Exosomes Secreted by Cardiosphere - Derived Cells Confers Cardioprotection with Delayed Administration After Ischemia - Reperfusion Injury in Rats Wed. 11/19 9:00 am - 12:00 South Hall A2 - Core 5 Dose - Escalation Study Using Novel Continuous Flow Intracoronary Delivery of Allogeneic Cardiosphere - Derived Stem Cells: Is There a Threshold for Cell Therapy? 5

Clinical Update

CDCs: Clinical Development Post Myocardial Infarction (30 days – 1 year after MI) ALLSTAR Clinical Trial NYHA Class III or ambulatory Class IV heart failure DYNAMIC Clinical Trial IND granted, Phase II currently enrolling IND granted, Phase I plan to commence in 2014 Phase I – 14 patients Phase II – 300 patients Phase Ia – 14 patients Phase Ib – 28 patients DMD - related cardiomyopathy Duchenne Muscular Dystrophy Potential Orphan Designation by FDA Planned Phase I (~10 patients) 7

ALLSTAR Phase I – 12 month MRI Analysis ▪ ALLSTAR Phase I ▪ Met safety endpoint (1 month) ▪ No control group ▪ Preliminary 12 month MRI analysis on Phase II equivalent population (defined by tissue type compatibility) ▪ Ejection fraction improved by 5.2% ▪ Relative reduction in scar size of 20.7% ▪ Measurements of viable mass and regional function also showed quantifiable improvements ▪ Additional data on Phase I to be presented at AHA (November 17, 2014) 8

ALLSTAR: Phase II Clinical Trial ▪ Ejection Fraction ≤ 45% and Infarct Size ≥ 15 % (MRI ) ▪ Primary Endpoint – Infarct Size by MRI at 1 year ▪ Secondary Endpoints – multiple; EF, volumes, quality of life, etc. ▪ Phase II: Est. 300 patients – currently enrolling ▪ 23 sites activated ALL ogeneic heart ST em cells to A chieve myocardial R egeneration

New Indication: Duchenne Muscular Dystrophy

Duchenne Muscular Dystrophy ▪ Affects 1 in 3,500 male births worldwide ▪ ~20,000 male children affected in the US (~275,000 worldwide) ▪ The disease is usually fatal; a majority of deaths occur due to cardiomyopathy ▪ Compelling pre - clinical data to be presented at AHA in November 2014 ▪ Targeting IND in early 2015 ▪ Phase I planned for 2015 Reference: McNeil et. al, Muscle & Nerve, 2010 11

Upcoming Conference Call ▪ Tuesday – November 18, 2014 ▪ 9:30AM (EST) ▪ Hosted by Dr. Linda and Eduardo Marbán ▪ Topic: Cardiosphere - derived Cells and their Exosomes Improve Function, Restore Mitochondrial Integrity and Reverse Degenerative Changes in the Hearts of Mdx Mice ▪ Data to be presented at AHA on November 17, 2014 ▪ Call - in number: 1 - 866 - 652 - 5200 ▪ Webcast Link: http://services.choruscall.com/links/capr141112.html Reference: McNeil et. al, Muscle & Nerve, 2010 12

Cenderitide Update

Cenderitide: A Unique Protein Drug - S - S - K L L D R I G S M S G L G G F C C K G S L G NH 2 P S L R D P R P N A P S T S A CD - NP ▪ Developed by scientists at the Mayo Clinic and derived from the venom of the green mamba snake ▪ Cenderitide : ▪ Cardiac unloading ▪ Renal function preserved ▪ Aldosterone suppressing ▪ Anti - fibrotic, apoptotic, and hypertrophic ▪ 270 patients with acute decompensated heart failure have been treated 14

Annual U.S. Cenderitide Market Opportunity Reference: Go AS et al., Circulation , 2013, Desai AS et al., Circulation ,2012 ▪ Heart failure - leading cause of hospitalizations among adults older than 65 years of age in the US ▪ Costs $17 billion annually ▪ Responsible for over 1 million hospital admissions annually ▪ No new class of heart f ailure d rug approved for more than a decade ▪ Medicare has implemented financial penalties for re - hospitalizations within 30 days ▪ Complementary with LCZ696 (Novartis ) ▪ Cenderitide’s treatment goal is to prevent readmission to hospital within 90 days due to cardiac events 15

The Post - Acute Hospitalization Period (90 days): When the Rate of Re - hospitalization and Death are Highest 0% 20% 40% 60% - 60 120 180 240 300 360 Percent of Patients Days After Hospital Admission for Heart Failure % of Patients with Death or Re - hospitalization after admission for Acute HF Reference: estimate from analysis of DOSE, PROTECT, ASCEND, OPTIMIZE, & ADHERE ▪ As days in hospital have decreased, patient’s physiology is unstable at discharge ▪ HF patients are frequently non - compliant with their chronic medications Post - Acute Period 16

Continuous Subcutaneous Infusion Using the Insulet Omnipod ® Technology ▪ Entered into a Research Support Agreement with Insulet Corporation (NASDAQ: PODD ) ▪ Insulet will supply Omnipod ® for planned clinical trial: ▪ Engage in product development ▪ Project management ▪ D esign control activities Sample shown: Omnipod® Press Start Apply the Pod Fill the Pod 17

Acquisition of Medtronic IP ▪ Entered into an agreement to acquire patent rights from Medtronic, Inc. (NYSE: MDT ) ▪ Related to the formulation and pump delivery of natriuretic peptides. ▪ Assigned to Capricor all of its right, title and interest in all natriuretic peptide patents and patent applications previously owned by Medtronic or co - owned by each of the companies as part of their collaborative natriuretic peptide delivery program 18
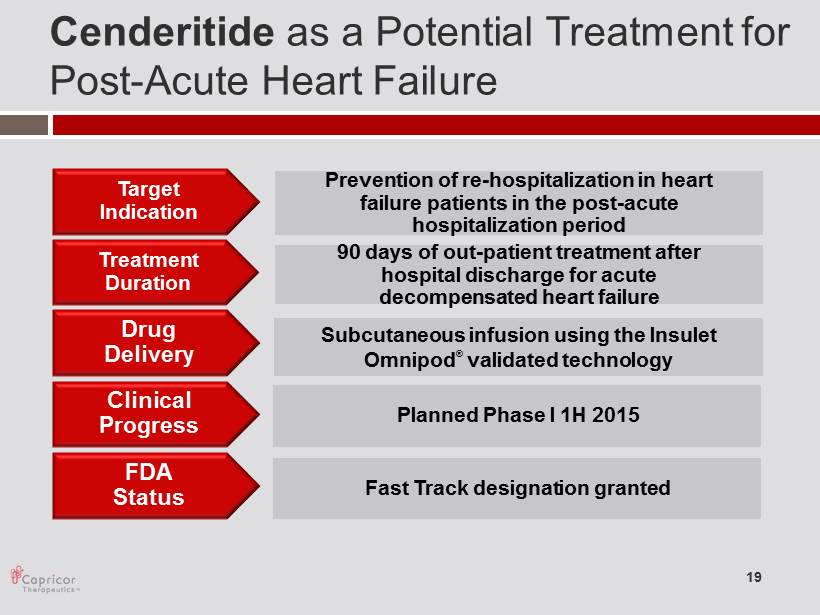
Cenderitide as a Potential T reatment for Post - Acute Heart Failure Target Indicatio n Treatmen t Duration 90 days of out - patient treatment after hospital discharge for acute decompensated heart failure Drug Delivery FDA Status Subcutaneous infusion using the Insulet Omnipod ® validated technology Fast Track designation granted Prevention of re - hospitalization in heart failure patients in the post - acute hospitalization period Clinical Progress Planned Phase I 1H 2015 19

Anticipated Milestones ▪ 2014 ▪ Report additional ALLSTAR Phase I results (AHA) ▪ Initiate DYNAMIC clinical trial ▪ 2015 ▪ Initiate Duchenne Muscular Dystrophy trial ▪ Initiate Cenderitide trial ▪ Report initial Cenderitide results ▪ Report initial DYNAMIC results 20

Thank you
