Attached files
| file | filename |
|---|---|
| 8-K - 8-K - AMAG PHARMACEUTICALS, INC. | a14-23365_18k.htm |
| EX-99.1 - EX-99.1 - AMAG PHARMACEUTICALS, INC. | a14-23365_1ex99d1.htm |
Exhibit 99.2
|
|
AMAG Pharmaceuticals Third Quarter Financial Results October 30, 2014 |
|
|
Forward Looking Statements This presentation contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 and other federal securities laws. Any statements contained herein which do not describe historical facts, including but not limited to, statements regarding: (i) our plans to build a high-growth, multi-product specialty pharmaceutical company, (ii) expectations regarding our pending acquisition of Lumara Health, including market dynamics, the strategic rationale (including our ability to gain entry into specialty women’s health market and the impact on future product acquisitions), expected combined annual sales and synergies, the impact on adjusted earnings per share and our use of combined net operating loss carryforwards, as well as the expected timing for the closing of the transaction, (iii) beliefs regarding the Makena Care Connection and the lifecycle management program for Makena, (iv) the expected financial profile of the combined company following the closing of the Lumara Health acquisition, including revenues and adjusted EBITDA, (v) integrations plans for the acquisition of Lumara Health, including expectations for the management team, synergies and value-capture, the post-closing organization and integration support, (vi) beliefs about the competitive landscape for the U.S. IV iron market, (vii) Feraheme growth opportunities, including an increase in our current chronic kidney disease (CKD) indication and plans for potential label expansion to cover the broader iron deficiency anemia (IDA) market, including the anticipated call audiences and potential future call points in the women’s health arena, (viii) expectations regarding regulatory developments in the U.S., the EU and Canada, including the belief that we may be able to determine in the fourth quarter of 2014 the potential path forward for possibly pursuing a broader label in the U.S. and the implementation of our proposed label changes in the U.S. and the likelihood and timing of approval for the broader indication in the EU and Canada,, (ix) future expansion opportunities through 2020, (x) our 2014 financial outlook, including revenues, cost of goods sold, operating expenses, net loss and cash and investments (including our expected cash balance assuming the consummation of the Lumara Health acquisition), (xi) our 2014 goals, including plans to maximize Feraheme opportunities, drive MuGard sales, execute on business development transactions (such as the Lumara Health acquisition) and continue to operate the business with financial discipline and (xii) our statement that AMAG is well positioned for success in 2014 and beyond are forward-looking statements which involve risks and uncertainties that could cause actual results to differ materially from those discussed in such forward-looking statements. Statements about AMAG’s and Lumara Health’s past financial results do not, and are not meant to, predict future results. AMAG can provide no assurance that such results and performance will continue. Such risks and uncertainties include, among others: (1) the possibility that the closing conditions set forth in the definitive agreement for the acquisition of Lumara Health will not be met and that the parties will be unable to consummate the proposed transaction, (2) the chance that, despite having a commitment in place for the financing of the Lumara Health acquisition, we will be unable to secure such financing, or financing on satisfactory terms, in amounts sufficient to consummate the acquisition of Lumara Health, (3) the possibility that if the Lumara Health acquisition is consummated, we may not realize the expected benefits, synergies and opportunities anticipated in connection with the transaction, including the anticipated costs synergies of $20 million, annualized sales of more than $180 million, continued revenue growth, annualized EBITDA of $110 million and $1 billion market opportunity, (4) the challenges of integrating the Makena commercial team into AMAG, (5) the impact on sales of Makena from competitive, commercial payor, government (including federal and state Medicaid reimbursement policies), physician, patient or public responses with respect to product pricing, product access and sales and marketing initiatives, (6) the impact of patient compliance on units sales, (7) the uncertainty of achieving sales of Feraheme to OB/GYN specialists for the treatment of women who suffer from IDA, even assuming FDA approval for the broader indication, (8) we may face challenges in leveraging our in-office injectables commercial expertise, which could result in unforeseen expenses and disrupt business operations, (9) liabilities we assume from Lumara Health, including the class action litigation In Re K-V Pharmaceutical Company Securities Litigation, Case No. 4:11CV1816 AGF, may be higher than expected, (10) the possibility that sales of Makena will not meet expectations as a result of current and future competition from compounded products and/or future competition from generic alternatives upon expiration of exclusivity in February 2018, (11) the impact of reimbursement policies for Makena and the resulting coverage decisions and/or impact on pricing, (12) the number of preterm birth risk pregnancies for which Makena may be prescribed, its safety and side effects profile and acceptance of pricing, (13) in connection with the Lumara Health acquisition, we will incur a substantial amount of indebtedness and will have to comply with restrictive and affirmative debt covenants, including a requirement that we reduce our leverage over time, (14) the possibility that we will need to raise additional capital from the sale of our common stock, which will cause significant dilution to our stockholders, in order to satisfy our contractual obligations, including our debt service, milestone payments that may become payable to Lumara Health’s stockholders and/or in order to pursue business development activities, (15) upon consummation of the Lumara Health transaction, we will be highly leveraged and have limited cash and cash equivalent resources which may limit our ability to take advantage of attractive business development opportunities and execute on our strategic plan, (16) the possibility that our stockholders will not approve the amendment to our shareholder rights plan and that our tax benefits, including those acquired upon consummation of the Lumara Health acquisition, will not be available in the future, (17) the likelihood and timing of potential approval of Feraheme in the U.S. in the broader IDA indication in light of the complete response letter we received from the FDA informing us that our supplemental new drug application (sNDA) for the broader indication could not be approved in its present form and stating that we had not provided sufficient information to permit labeling of Feraheme for safe and effective use for the proposed broader indication, (18) the possibility that following FDA review of post-marketing safety data, including reports of serious anaphylaxis, cardiovascular events, and death, and/or in light of the label changes requested by the European Medicines Agency’s (EMA) Pharmacovigilance Risk Assessment Committee (PRAC) and confirmed by the Committee for Medicinal Products for Human Use (CHMP), the FDA (or other regulators) will request additional technical or scientific information, new studies or reanalysis of existing data, on-label warnings, post-marketing requirements/commitments or risk evaluation and mitigation strategies (REMS) in the current indication for Feraheme for IDA in adult patients with CKD and the additional costs and expenses that will or may be incurred in connection with such activities, (19) whether our proposed label changes will be acceptable to the FDA or other regulatory authorities and what impact such changes, or such additional changes as the FDA, CHMP or other regulators may require, will have on sales of Feraheme/ Rienso™ (Rienso is the trade name for ferumoxytol outside of the U.S. and Canada), (20) our and Takeda Pharmaceutical Company Limited’s ability to successfully compete in the IV iron replacement market both in the U.S. and outside the U.S., including the EU, as a result of limitations, restrictions or warnings in Feraheme’s/Rienso’s current or future label, including that Feraheme/Rienso be administered to patients by infusion over at least 15-minutes (replacing injection) and that it be contraindicated for patients with any known history of drug allergy, (21) our ability to execute on our long-term strategic plan or to realize the expected results from our long-term strategic plan, (22) Takeda’s ability to obtain regulatory approval for Feraheme in Canada, and Rienso in the EU, in the broader IDA patient population, (23) the possibility that significant safety or drug interaction problems could arise with respect to Feraheme/Rienso and in turn affect sales, or our ability to market the product both in the U.S. and outside of the U.S., including the EU, (24) the relationship between Takeda and AMAG and the impact on commercialization efforts for Feraheme/Rienso in the EU and Canada, (25) the likelihood and timing of milestone payments, if any, in connection with AMAG’s licensing arrangement with Takeda, (26) the manufacture of Feraheme/Rienso or MuGard (or Makena if the acquisition is consummated), including any significant interruption in the supply of raw materials or finished product, (27) our patents and proprietary rights both in the U.S. and outside the U.S. (including those that we acquire from the acquisition of Lumara), (28) the risk of an Abbreviated New Drug Application (ANDA) filing for generic ferumoxytol (or, following the consummation of the Lumara Health acquisition, hydroxyprogesterone caproate), especially following the FDA’s draft bioequivalence recommendation for ferumoxytol published in December 2012, (29) the possibility that AMAG (or Takeda) will disseminate future Dear Healthcare Provider letters, (30) uncertainties regarding our ability to compete in the oral mucositis market in the U.S. and in the women’s maternal health market and (31) other risks identified in our filings with the U.S. Securities and Exchange Commission (SEC), including our Quarterly Report on Form 10-Q for the quarter ended June 30, 2014 and subsequent filings with the SEC. Any of the above risks and uncertainties could materially and adversely affect our results of operations, our profitability and our cash flows, which would, in turn, have a significant and adverse impact on our stock price. Use of the term “including” in the two paragraphs above shall mean in each case “including, but not limited to.” We caution you not to place undue reliance on any forward-looking statements, which speak only as of the date they are made. We disclaim any obligation to publicly update or revise any such statements to reflect any change in expectations or in events, conditions or circumstances on which any such statements may be based, or that may affect the likelihood that actual results will differ from those set forth in the forward-looking statements. AMAG Pharmaceuticals and Feraheme are registered trademarks of AMAG Pharmaceuticals, Inc. MuGard is a registered trademark of Access Pharmaceuticals, Inc. Rienso is a registered trademark of Takeda Pharmaceutical Company Limited. Lumara Health is a trademark of Lumara Health Inc. Makena is a registered trademark of Lumara Health Inc. |
|
|
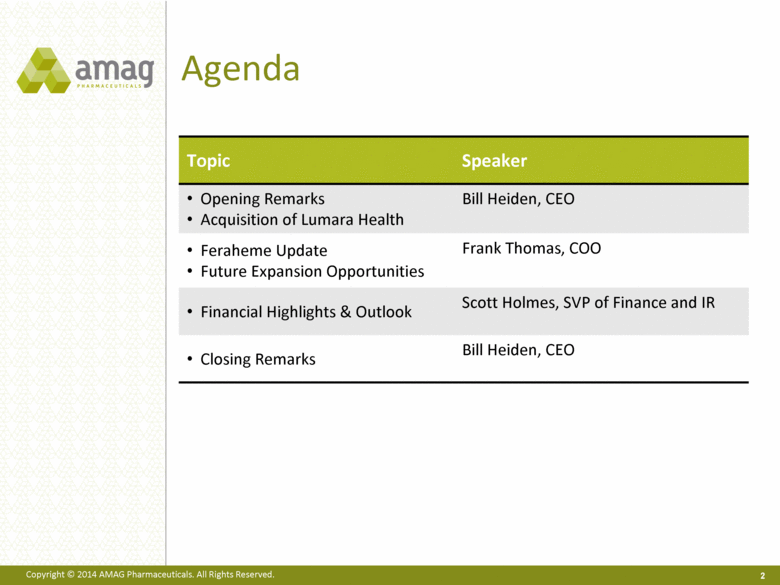
Agenda 2 Topic Speaker Opening Remarks Acquisition of Lumara Health Bill Heiden, CEO Feraheme Update Future Expansion Opportunities Frank Thomas, COO Financial Highlights & Outlook Scott Holmes, SVP of Finance and IR Closing Remarks Bill Heiden, CEO |
|
|
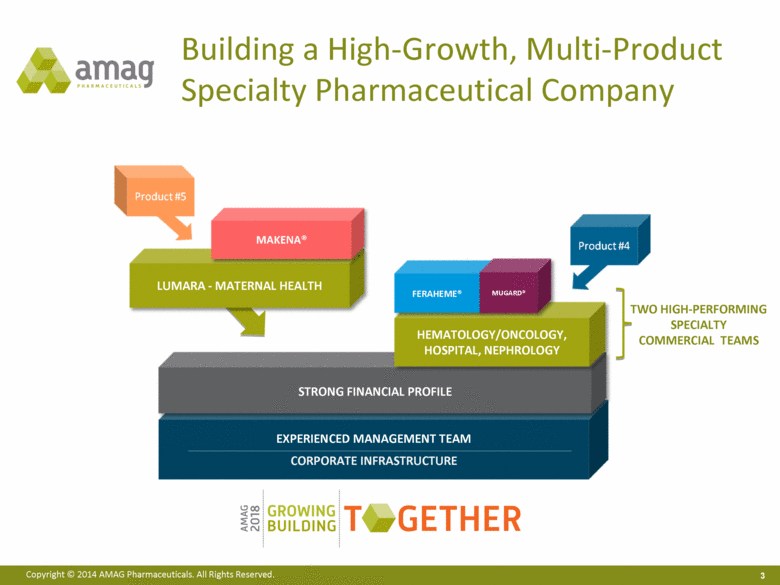
EXPERIENCED MANAGEMENT TEAM CORPORATE INFRASTRUCTURE Building a High-Growth, Multi-Product Specialty Pharmaceutical Company HEMATOLOGY/ONCOLOGY, HOSPITAL, NEPHROLOGY FERAHEME® MUGARD® TWO HIGH-PERFORMING SPECIALTY COMMERCIAL TEAMS LUMARA - MATERNAL HEALTH MAKENA® |
|
|
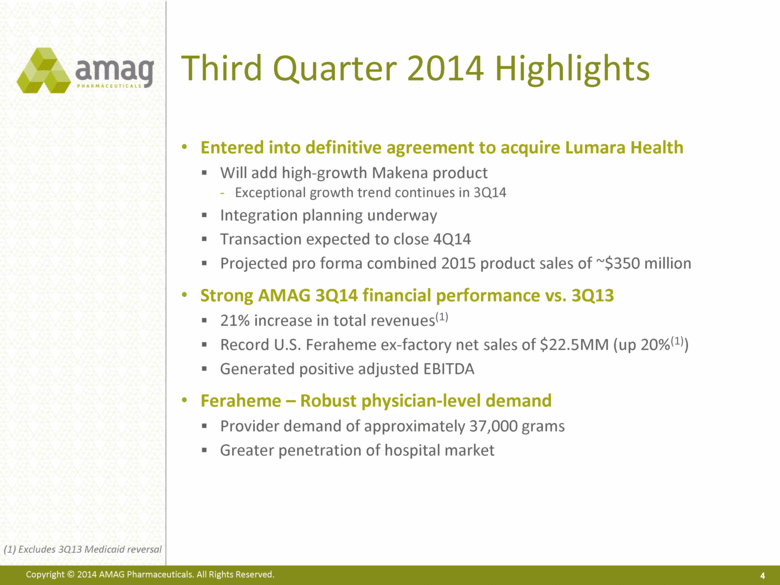
Third Quarter 2014 Highlights Entered into definitive agreement to acquire Lumara Health Will add high-growth Makena product Exceptional growth trend continues in 3Q14 Integration planning underway Transaction expected to close 4Q14 Projected pro forma combined 2015 product sales of ~$350 million Strong AMAG 3Q14 financial performance vs. 3Q13 21% increase in total revenues(1) Record U.S. Feraheme ex-factory net sales of $22.5MM (up 20%(1)) Generated positive adjusted EBITDA Feraheme – Robust physician-level demand Provider demand of approximately 37,000 grams Greater penetration of hospital market 4 (1) Excludes 3Q13 Medicaid reversal |
|
|
ACQUISITION OF LUMARA HEALTH |
|
|
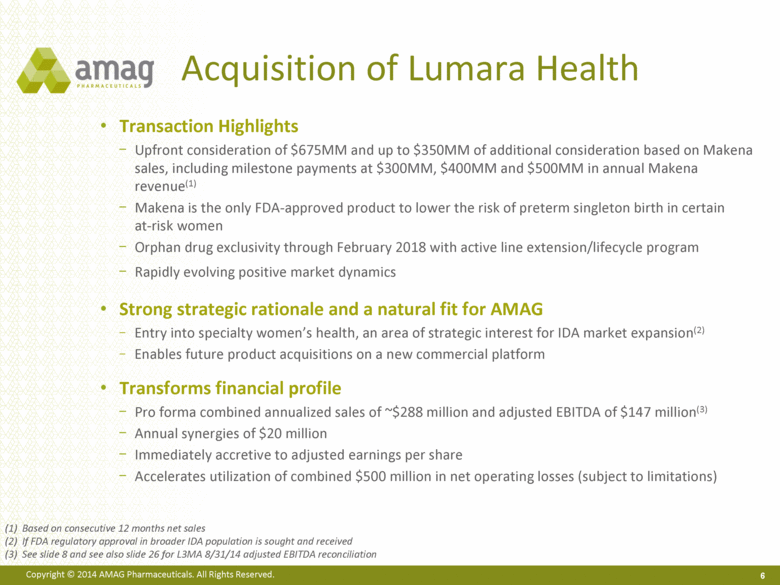
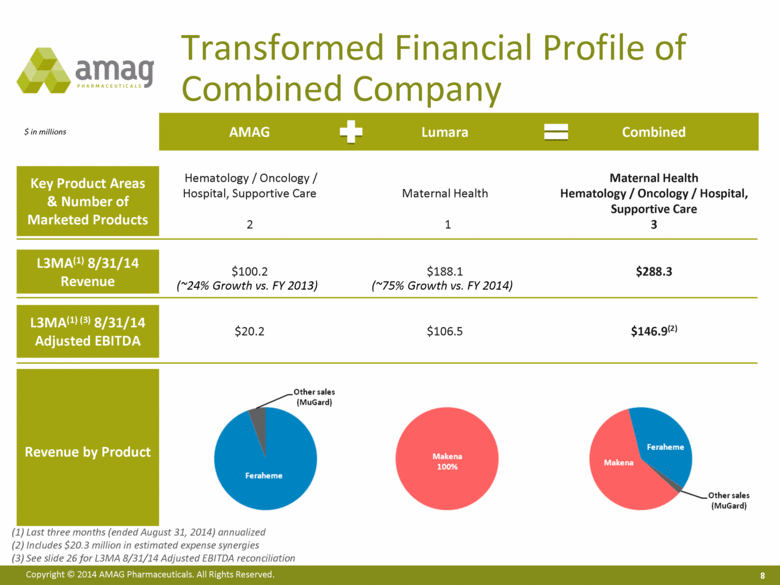
Acquisition of Lumara Health Transaction Highlights Upfront consideration of $675MM and up to $350MM of additional consideration based on Makena sales, including milestone payments at $300MM, $400MM and $500MM in annual Makena revenue(1) Makena is the only FDA-approved product to lower the risk of preterm singleton birth in certain at-risk women Orphan drug exclusivity through February 2018 with active line extension/lifecycle program Rapidly evolving positive market dynamics Strong strategic rationale and a natural fit for AMAG Entry into specialty women’s health, an area of strategic interest for IDA market expansion(2) Enables future product acquisitions on a new commercial platform Transforms financial profile Pro forma combined annualized sales of ~$288 million and adjusted EBITDA of $147 million(3) Annual synergies of $20 million Immediately accretive to adjusted earnings per share Accelerates utilization of combined $500 million in net operating losses (subject to limitations) 6 (1) Based on consecutive 12 months net sales (2) If FDA regulatory approval in broader IDA population is sought and received (3) See slide 8 and see also slide 26 for L3MA 8/31/14 adjusted EBITDA reconciliation |
|
|
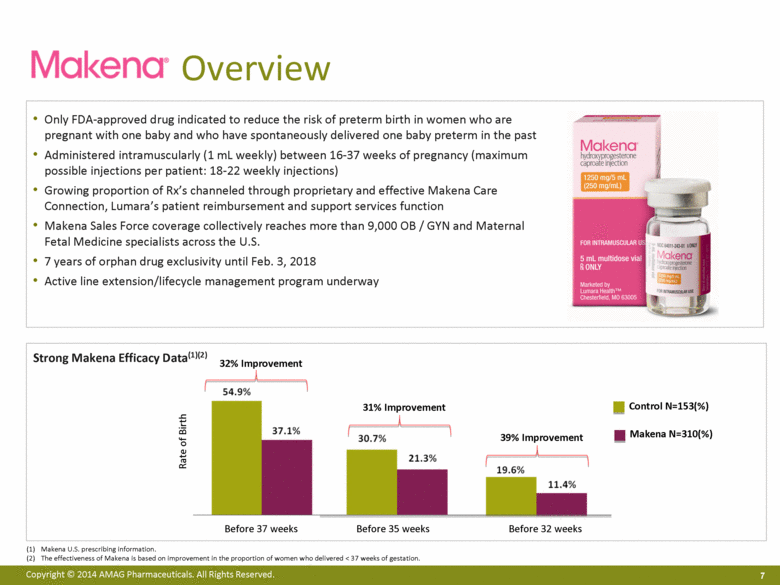
Strong Makena Efficacy Data(1)(2) Before 37 weeks Overview 7 Only FDA-approved drug indicated to reduce the risk of preterm birth in women who are pregnant with one baby and who have spontaneously delivered one baby preterm in the past Administered intramuscularly (1 mL weekly) between 16-37 weeks of pregnancy (maximum possible injections per patient: 18-22 weekly injections) Growing proportion of Rx’s channeled through proprietary and effective Makena Care Connection, Lumara’s patient reimbursement and support services function Makena Sales Force coverage collectively reaches more than 9,000 OB / GYN and Maternal Fetal Medicine specialists across the U.S. 7 years of orphan drug exclusivity until Feb. 3, 2018 Active line extension/lifecycle management program underway Rate of Birth 32% Improvement Control N=153(%) Makena N=310(%) Before 32 weeks 31% Improvement 39% Improvement Before 35 weeks Before 37 weeks Makena U.S. prescribing information. The effectiveness of Makena is based on improvement in the proportion of women who delivered < 37 weeks of gestation. |
|
|
$ in millions AMAG Lumara Combined Key Product Areas & Number of Marketed Products Hematology / Oncology / Hospital, Supportive Care 2 Maternal Health 1 Maternal Health Hematology / Oncology / Hospital, Supportive Care 3 L3MA(1) 8/31/14 Revenue $100.2 $188.1 $288.3 L3MA(1) (3) 8/31/14 Adjusted EBITDA $20.2 $106.5 $146.9(2) Revenue by Product Transformed Financial Profile of Combined Company Last three months (ended August 31, 2014) annualized Includes $20.3 million in estimated expense synergies See slide 26 for L3MA 8/31/14 Adjusted EBITDA reconciliation (~24% Growth vs. FY 2013) (~75% Growth vs. FY 2014) |
|
|
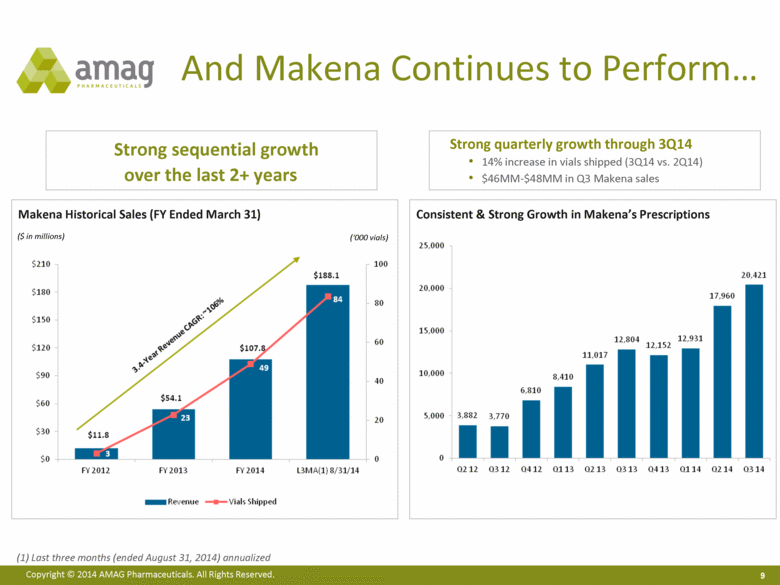
And Makena Continues to Perform Strong sequential growth over the last 2+ years ($ in millions) (‘000 vials) Consistent & Strong Growth in Makena’s Prescriptions Makena Historical Sales (FY Ended March 31) Strong quarterly growth through 3Q14 14% increase in vials shipped (3Q14 vs. 2Q14) $46MM-$48MM in Q3 Makena sales Last three months (ended August 31, 2014) annualized |
|
|
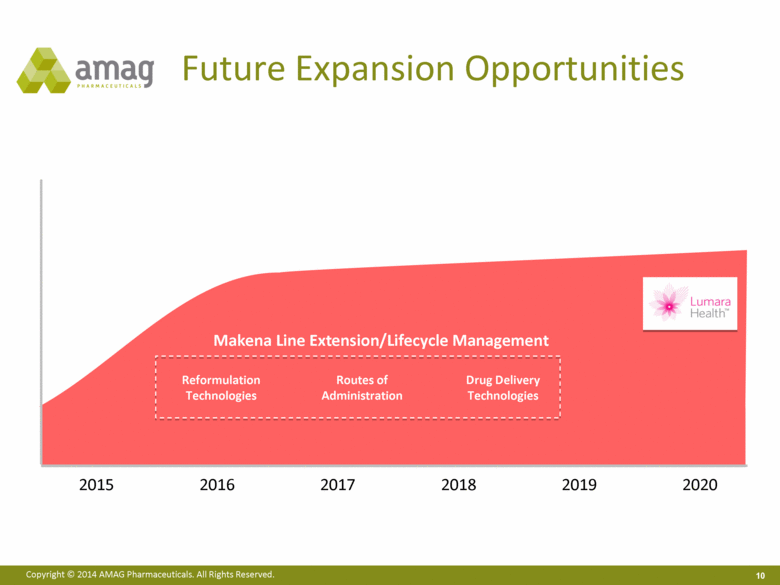
2016 2017 2018 2019 2020 2015 Future Expansion Opportunities Makena Line Extension/Lifecycle Management Routes of Administration Drug Delivery Technologies Reformulation Technologies |
|
|
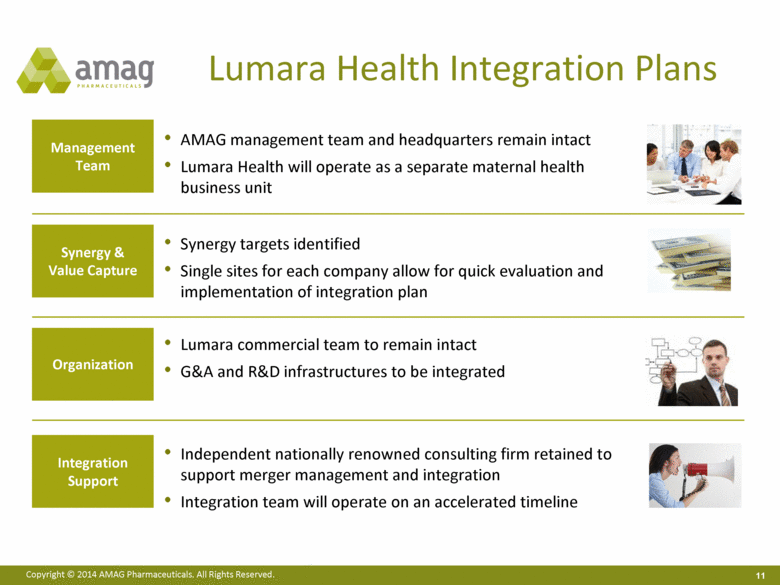
Integration Support Synergy & Value Capture Organization Management Team AMAG management team and headquarters remain intact Lumara Health will operate as a separate maternal health business unit Synergy targets identified Single sites for each company allow for quick evaluation and implementation of integration plan Lumara commercial team to remain intact G&A and R&D infrastructures to be integrated Independent nationally renowned consulting firm retained to support merger management and integration Integration team will operate on an accelerated timeline Lumara Health Integration Plans |
|
|
FERAHEME UPDATE |
|
|
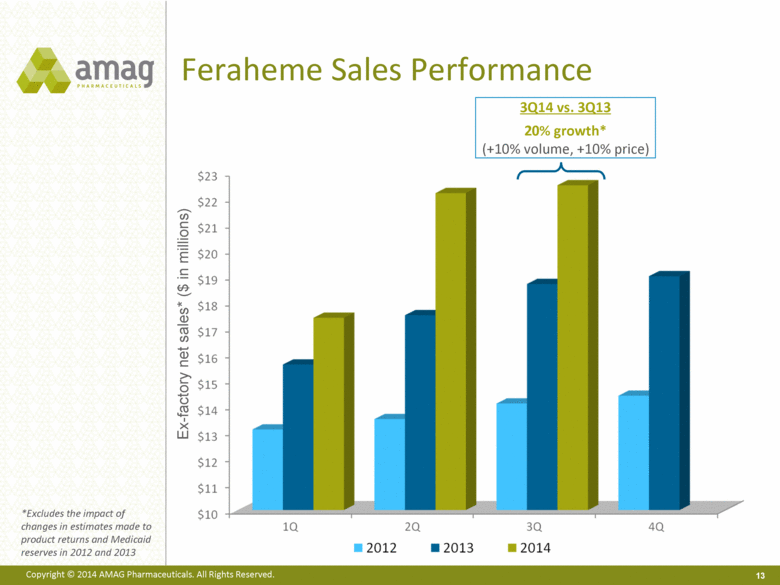
Feraheme Sales Performance Ex-factory net sales* ($ in millions) *Excludes the impact of changes in estimates made to product returns and Medicaid reserves in 2012 and 2013 3Q14 vs. 3Q13 20% growth* (+10% volume, +10% price) |
|
|
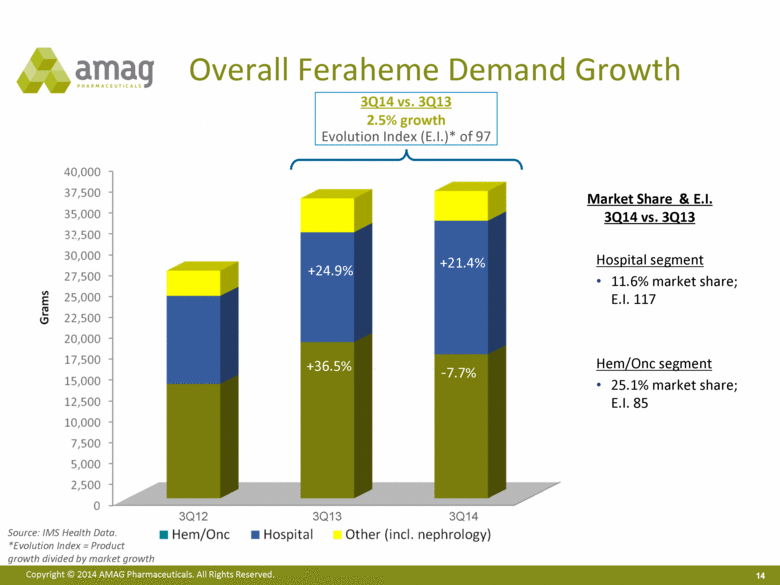
Source: IMS Health Data. *Evolution Index = Product growth divided by market growth Overall Feraheme Demand Growth Market Share & E.I. 3Q14 vs. 3Q13 Hospital segment 11.6% market share; E.I. 117 Hem/Onc segment 25.1% market share; E.I. 85 -7.7% +21.4% 3Q12 3Q14 3Q14 vs. 3Q13 2.5% growth Evolution Index (E.I.)* of 97 +24.9% +36.5% 3Q13 |
|
|
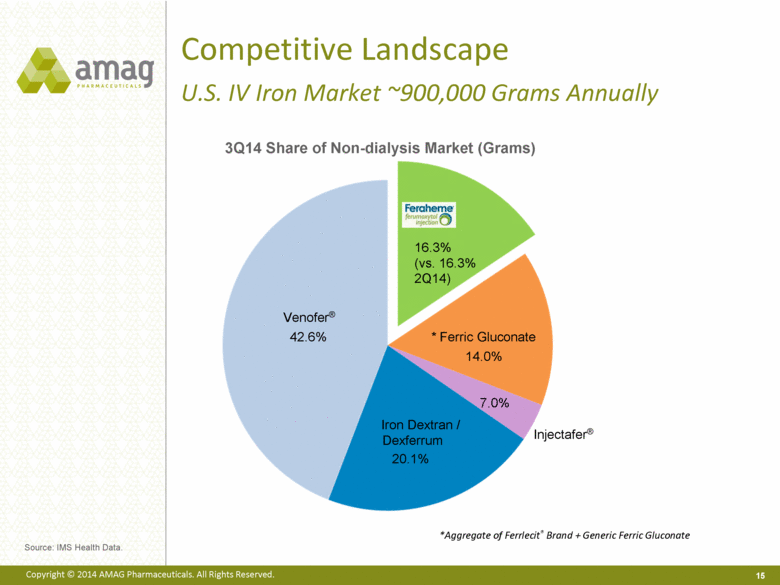
Competitive Landscape U.S. IV Iron Market ~900,000 Grams Annually *Aggregate of Ferrlecit® Brand + Generic Ferric Gluconate 3Q14 Share of Non-dialysis Market (Grams) Venofer® Iron Dextran / Dexferrum Injectafer® * Ferric Gluconate 16.3% (vs. 16.3% 2Q14) Source: IMS Health Data. 14.0% 42.6% 7.0% 20.1% |
|
|
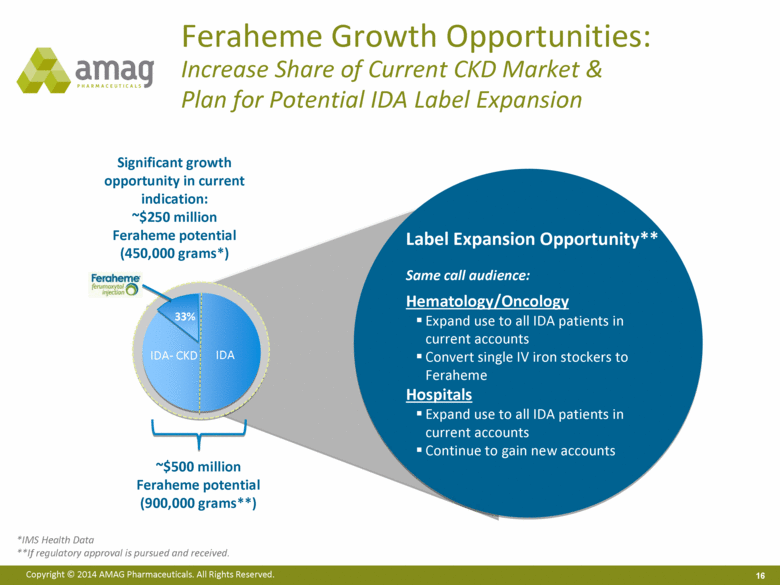
Feraheme Growth Opportunities: Increase Share of Current CKD Market & Plan for Potential IDA Label Expansion ~$500 million Feraheme potential (900,000 grams**) IDA- CKD IDA Label Expansion Opportunity** Same call audience: Hematology/Oncology Expand use to all IDA patients in current accounts Convert single IV iron stockers to Feraheme Hospitals Expand use to all IDA patients in current accounts Continue to gain new accounts *IMS Health Data **If regulatory approval is pursued and received. 33% Significant growth opportunity in current indication: ~$250 million Feraheme potential (450,000 grams*) |
|
|
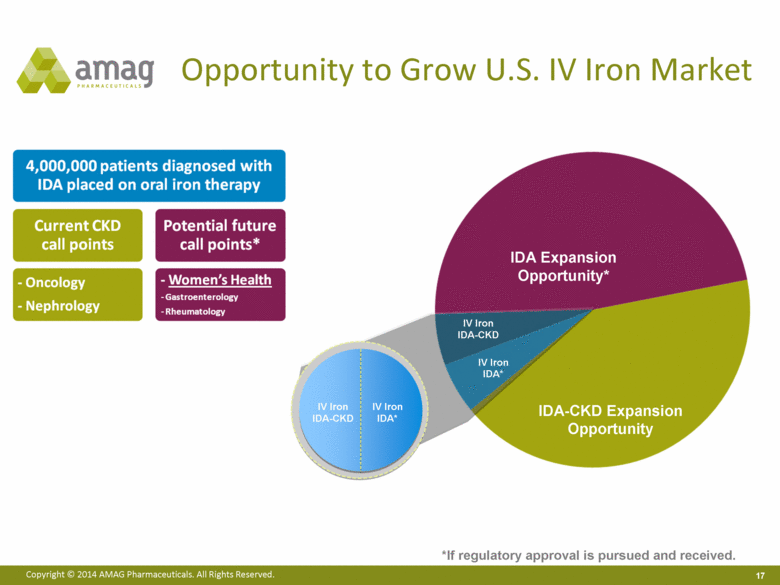
Opportunity to Grow U.S. IV Iron Market IV Iron IDA-CKD IV Iron IDA* IV Iron IDA* IV Iron IDA-CKD IDA Expansion Opportunity* IDA-CKD Expansion Opportunity *If regulatory approval is pursued and received. |
|
|
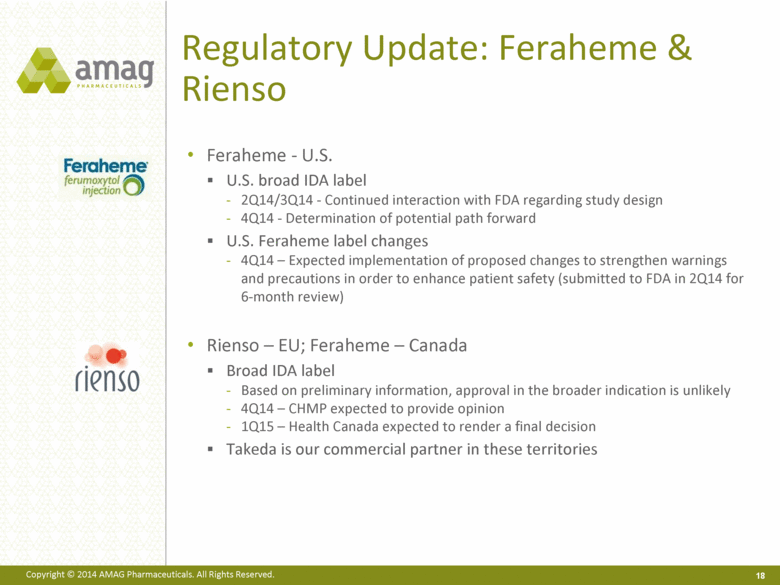
Regulatory Update: Feraheme & Rienso Feraheme - U.S. U.S. broad IDA label 2Q14/3Q14 - Continued interaction with FDA regarding study design 4Q14 - Determination of potential path forward U.S. Feraheme label changes 4Q14 – Expected implementation of proposed changes to strengthen warnings and precautions in order to enhance patient safety (submitted to FDA in 2Q14 for 6-month review) Rienso – EU; Feraheme – Canada Broad IDA label Based on preliminary information, approval in the broader indication is unlikely 4Q14 – CHMP expected to provide opinion 1Q15 – Health Canada expected to render a final decision Takeda is our commercial partner in these territories |
|
|
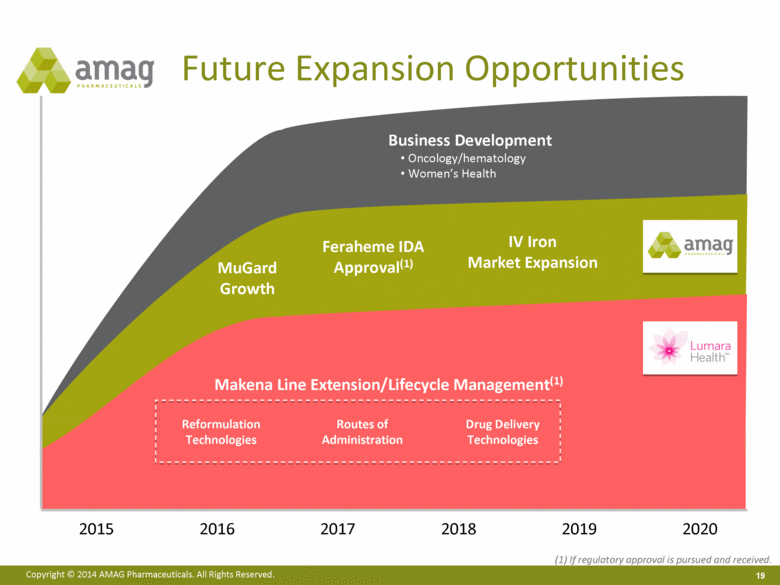
2016 2017 2018 2019 2020 2015 Future Expansion Opportunities Feraheme IDA Approval(1) IV Iron Market Expansion Business Development Oncology/hematology Women’s Health Makena Line Extension/Lifecycle Management(1) Routes of Administration Drug Delivery Technologies Reformulation Technologies MuGard Growth (1) If regulatory approval is pursued and received. |
|
|
FINANCIAL HIGHLIGHTS & OUTLOOK |
|
|
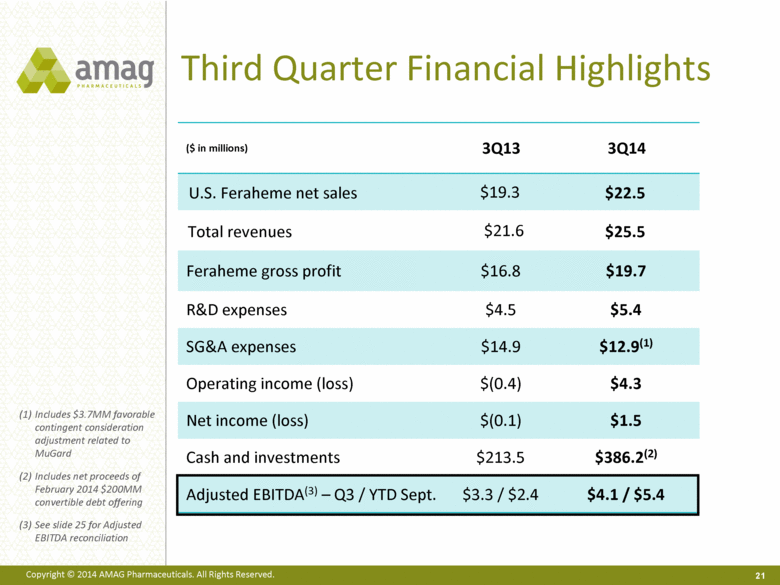
Third Quarter Financial Highlights ($ in millions) 3Q13 3Q14 U.S. Feraheme net sales $19.3 $22.5 Total revenues $21.6 $25.5 Feraheme gross profit $16.8 $19.7 R&D expenses $4.5 $5.4 SG&A expenses $14.9 $12.9(1) Operating income (loss) $(0.4) $4.3 Net income (loss) $(0.1) $1.5 Cash and investments $213.5 $386.2(2) Adjusted EBITDA(3) – Q3 / YTD Sept. $3.3 / $2.4 $4.1 / $5.4 21 Includes $3.7MM favorable contingent consideration adjustment related to MuGard Includes net proceeds of February 2014 $200MM convertible debt offering See slide 25 for Adjusted EBITDA reconciliation |
|
|
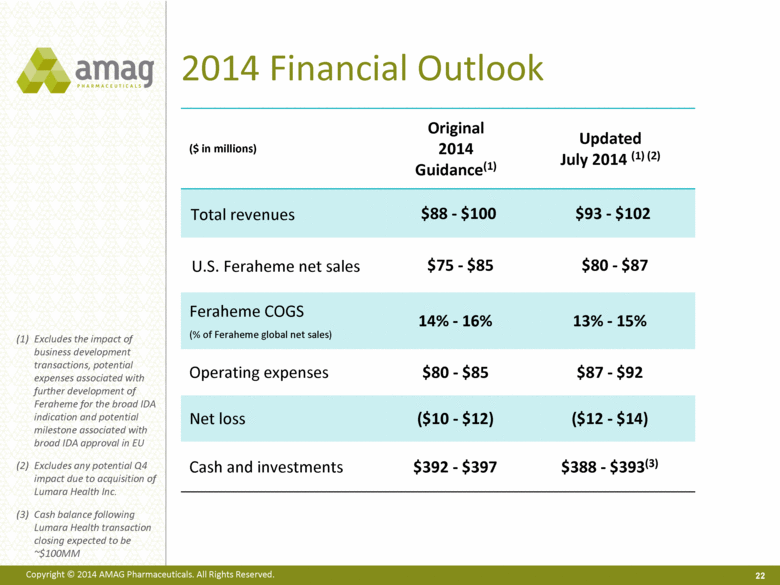
2014 Financial Outlook ($ in millions) Original 2014 Guidance(1) Updated July 2014 (1) (2) Total revenues $88 - $100 $93 - $102 U.S. Feraheme net sales $75 - $85 $80 - $87 Feraheme COGS (% of Feraheme global net sales) 14% - 16% 13% - 15% Operating expenses $80 - $85 $87 - $92 Net loss ($10 - $12) ($12 - $14) Cash and investments $392 - $397 $388 - $393(3) 22 Excludes the impact of business development transactions, potential expenses associated with further development of Feraheme for the broad IDA indication and potential milestone associated with broad IDA approval in EU Excludes any potential Q4 impact due to acquisition of Lumara Health Inc. Cash balance following Lumara Health transaction closing expected to be ~$100MM |
|
|
2014 Goals 23 Maximize Feraheme opportunities Drive market share within current U.S. IDA-CKD indication Optimize net revenue per gram Potential label expansion: broad IDA Determine regulatory path forward in U.S. Expected CHMP opinion – 4Q14 Pursue IV iron market expansion initiatives in IDA-CKD Drive MuGard sales through focused targeting, product differentiation and expanded payer coverage Execute quality business development transactions Complete Lumara Health transaction Execute seamless integration and Makena growth plans Continue to operate the business with financial discipline |
|
|
Well positioned for success in 2014. . . and beyond. |
|
|
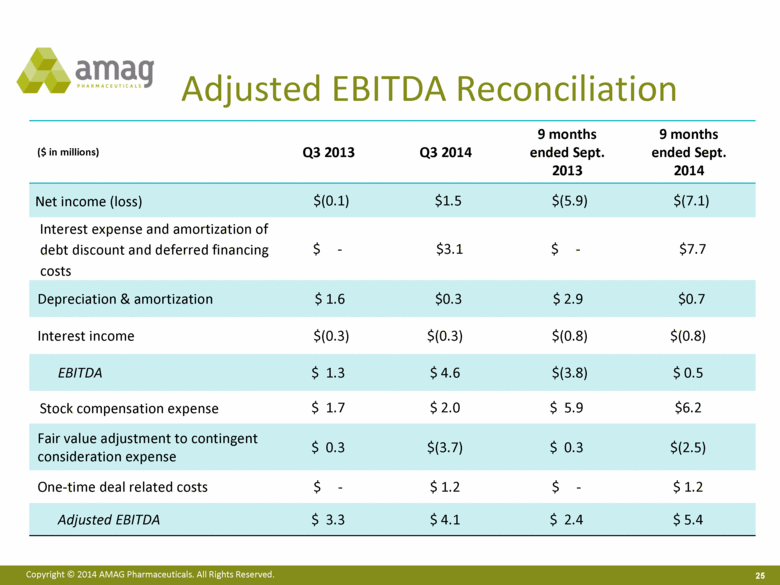
Adjusted EBITDA Reconciliation ($ in millions) Q3 2013 Q3 2014 9 months ended Sept. 2013 9 months ended Sept. 2014 Net income (loss) $(0.1) $1.5 $(5.9) $(7.1) Interest expense and amortization of debt discount and deferred financing costs $ - $3.1 $ - $7.7 Depreciation & amortization $ 1.6 $0.3 $ 2.9 $0.7 Interest income $(0.3) $(0.3) $(0.8) $(0.8) EBITDA $ 1.3 $ 4.6 $(3.8) $ 0.5 Stock compensation expense $ 1.7 $ 2.0 $ 5.9 $6.2 Fair value adjustment to contingent consideration expense $ 0.3 $(3.7) $ 0.3 $(2.5) One-time deal related costs $ - $ 1.2 $ - $ 1.2 Adjusted EBITDA $ 3.3 $ 4.1 $ 2.4 $ 5.4 |
|
|
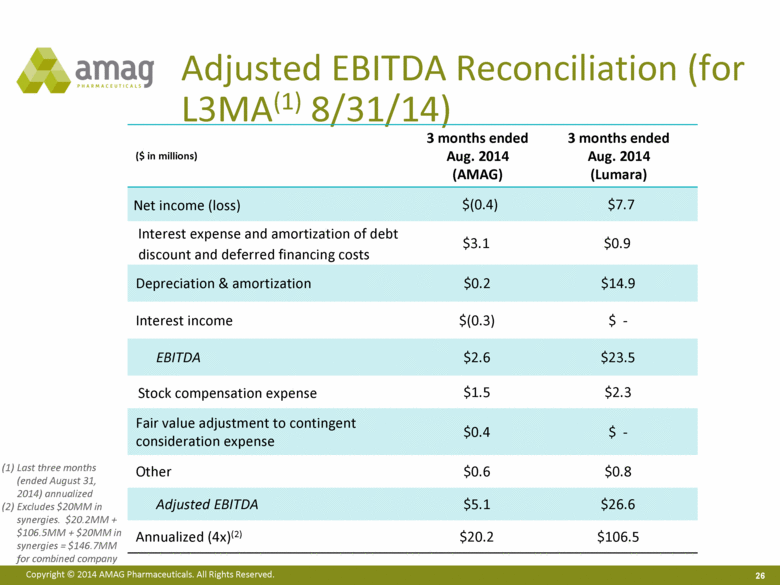
Adjusted EBITDA Reconciliation (for L3MA(1) 8/31/14) ($ in millions) 3 months ended Aug. 2014 (AMAG) 3 months ended Aug. 2014 (Lumara) Net income (loss) $(0.4) $7.7 Interest expense and amortization of debt discount and deferred financing costs $3.1 $0.9 Depreciation & amortization $0.2 $14.9 Interest income $(0.3) $ - EBITDA $2.6 $23.5 Stock compensation expense $1.5 $2.3 Fair value adjustment to contingent consideration expense $0.4 $ - Other $0.6 $0.8 Adjusted EBITDA $5.1 $26.6 Annualized (4x)(2) $20.2 $106.5 Last three months (ended August 31, 2014) annualized Excludes $20MM in synergies. $20.2MM + $106.5MM + $20MM in synergies = $146.7MM for combined company |