Attached files
| file | filename |
|---|---|
| 8-K - 8-K - LA JOLLA PHARMACEUTICAL CO | ljpc8-koctober2014.htm |

Corporate Presentation NasdaqCM: LJPC October 2014 Developing Innovative Therapies for Patients Suffering from Life-threatening Diseases

Forward-Looking Statements These slides contain "forward-looking" statements within the meaning of the Private Securities Litigation Reform Act of 1995. These statements may be identified by the use of forward-looking terminology such as "anticipate", "believe", "continue", "could", "estimate", "expect", "intend", "may", "might", "plan", "potential", "predict", "should" or "will" and include statements regarding La Jolla Pharmaceutical’s product candidates and clinical trial progress and results. These forward-looking statements are based on our current expectations, speak only as of the date of this presentation and involve risks and uncertainties, many of which are outside of our control, that can cause actual results to differ materially from those in the forward-looking statements. Potential risks and uncertainties include, but are not limited to, our ability to complete our anticipated clinical trials, the time and expense required to conduct such clinical trials, the ability to manufacture clinical or commercial product, issues arising in the regulatory process, use of proceeds from financings and the results of such clinical trials (including product safety issues and efficacy results). Further information is included in La Jolla Pharmaceutical’s periodic reports filed with the SEC at www.sec.gov. We disclaim any duty to update any forward-looking statements. 2

Overview of LJPC LJPC-501 (Angiotensin II) for CRH GCS-100 (IV Galectin-3 Inhibitor) for CKD LJPC-1010 (Oral Galectin-3 Inhibitor) for NASH LJPC-401 (Hepcidin) for Iron Overload Financial Position and Milestones

Product Pipeline GCS-100 IV Galectin-3 Inhibitor LJPC-1010 Oral Galectin-3 Inhibitor LJPC-501 Angiotensin II LJPC-401 Hepcidin Other R&D Preclinical IND Phase 1 Phase 2 Phase 3 CKD NASH Underway Completed ESRD Planned H2 2016 H1 2016 HRS CRH H1 2016 4 Early 2015 H2 2016 2b Early 2015 2015 Iron Overload 2a Extension Phase 1/2

Overview of LJPC LJPC-501 (Angiotensin II) for CRH GCS-100 (IV Galectin-3 Inhibitor) for CKD LJPC-1010 (Oral Galectin-3 Inhibitor) for NASH LJPC-401 (Hepcidin) for Iron Overload Financial Position and Milestones

LJPC-501 for CRH: Overview 6 • LJPC-501 is a proprietary formulation of angiotensin II, a naturally occurring regulator of blood pressure • Catecholamine-resistant hypotension (CRH) is an acute, life-threatening condition in which blood pressure drops to dangerously low levels and is unresponsive to current treatments • LJPC-501 has been shown to raise blood pressure in a pilot RPC clinical trial in CRH, as well as animal models of hypotension • Initiation of registration Phase 3 planned for early 2015 Meeting held with FDA at which agreement was reached that blood pressure can be the primary endpoint for approval • Orphan Drug Designation application submitted (75,000-100,000 cases/ year in U.S.)

What Is CRH? Hypotension = low blood pressure that is below the normal perfusion blood pressure (MAP < 60-65 mmHg) • Treated with fluids and catecholamines [epinephrine (adrenaline), norepinephrine (noradrenaline) and dopamine] • Some patients require excessive doses and are found to be resistant 7 All patients at risk for acute hypotension ~1.5M (sepsis, cardiogenic shock, trauma, drug reactions) 75,000-100,000 estimated patients per year in U.S. 5-7% catecholamine-resistant
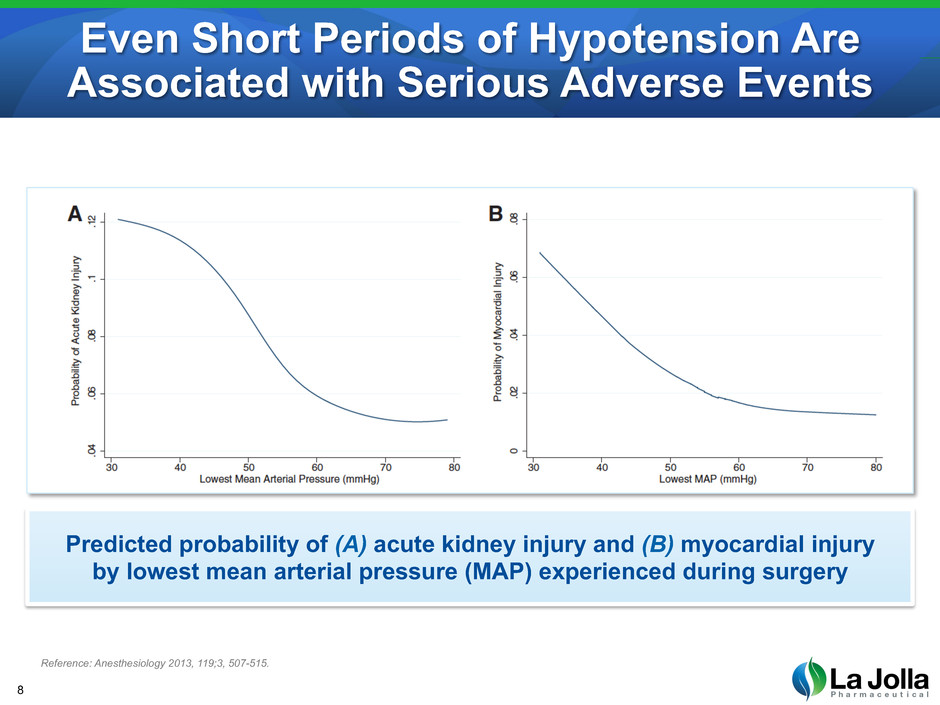
Even Short Periods of Hypotension Are Associated with Serious Adverse Events 8 Reference: Anesthesiology 2013, 119;3, 507-515. Predicted probability of (A) acute kidney injury and (B) myocardial injury by lowest mean arterial pressure (MAP) experienced during surgery

What Is LJPC-501? • Proprietary, stable formulation of synthetic angiotensin II • Endogenous eight-amino acid peptide that is central to the renin-angiotensin system ACE inhibitors lower BP by inhibiting synthesis of angiotensin II • Currently being investigated in a Phase 1/2 trial in Hepatorenal Syndrome (HRS) 9

Angiotensin II Raises Blood Pressure in Hypotensive Pig Model 10 Reference: Correa,TD, et al. 2014 Crit Care Med. Epubication ahead of print 0 20 40 60 80 100 BL EOP RP 12h RP 24h RP 36h RP 48h M A P (m m H g) Time NE E AT-II norepinephrine enalapril angiotensin II
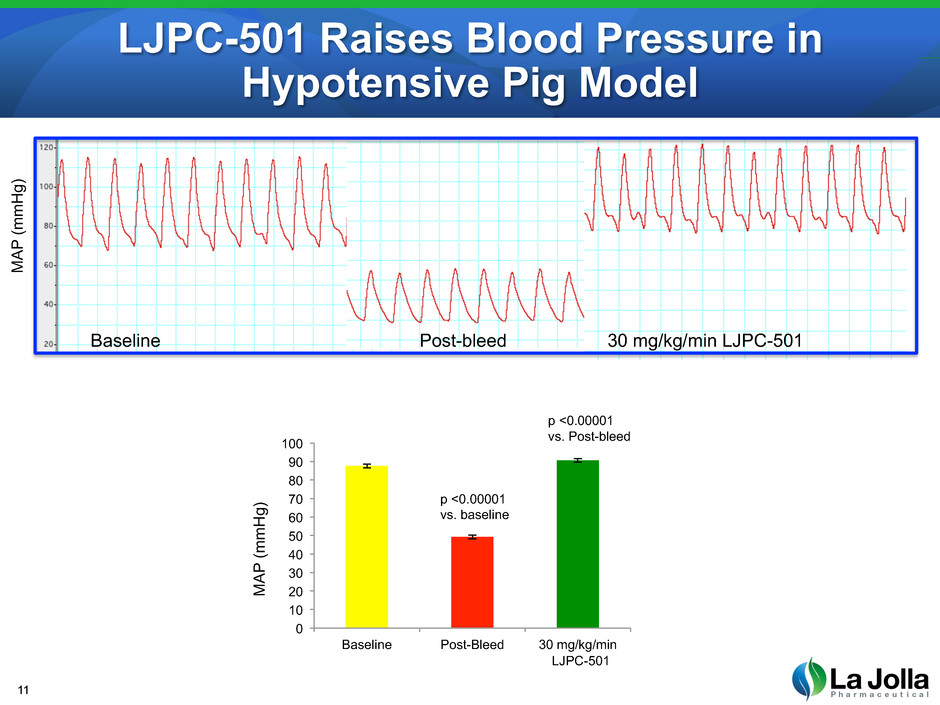
11 LJPC-501 Raises Blood Pressure in Hypotensive Pig Model 0 10 20 30 40 50 60 70 80 90 100 Baseline Post-Bleed 30 mg/kg/min p <0.00001 vs. baseline p <0.00001 vs. Post-bleed Baseline Post-bleed 30 mg/kg/min LJPC-501 LJPC-501 MA P (mm H g) MA P (mm H g)
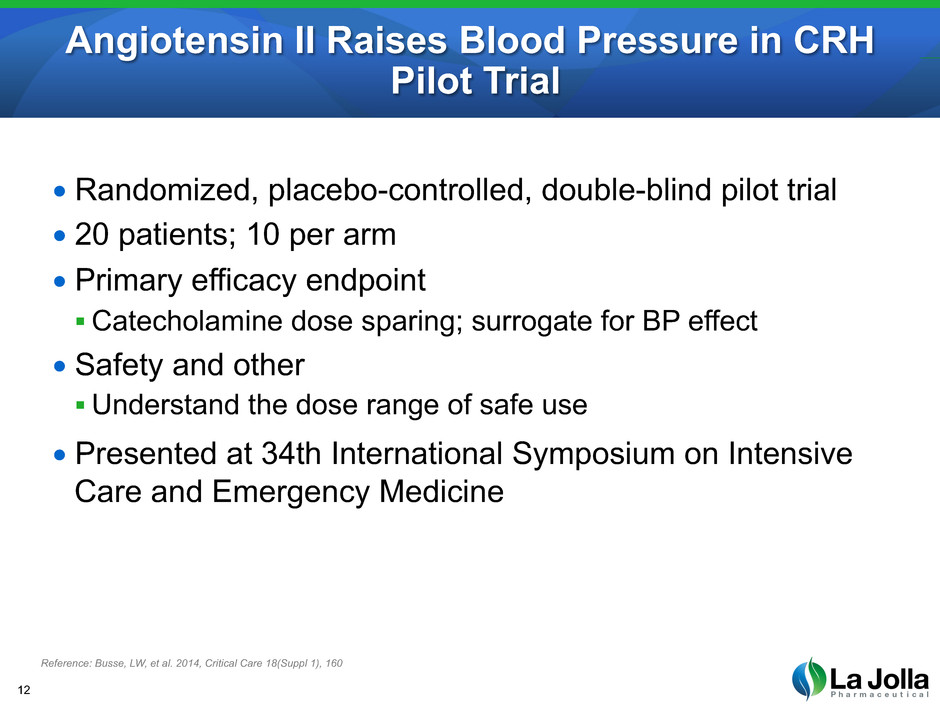
Angiotensin II Raises Blood Pressure in CRH Pilot Trial 12 Reference: Busse, LW, et al. 2014, Critical Care 18(Suppl 1), 160 • Randomized, placebo-controlled, double-blind pilot trial • 20 patients; 10 per arm • Primary efficacy endpoint Catecholamine dose sparing; surrogate for BP effect • Safety and other Understand the dose range of safe use • Presented at 34th International Symposium on Intensive Care and Emergency Medicine

Norepinephrine Dose Decreases with Angiotensin II Surrogate Effect on Blood Pressure 13 0 5 10 15 20 25 30 35 40 45 50 Pre2 Pre1 Hr0 Hr1 Hr2 Hr3 Hr4 Hr5 Hr6 Post1 Post2 N or ep in ep hr in e D os e (m cg /m in ) Placebo AT-II Arm Angio dose % of Patients with Rise in BP: Placebo Group 20% vs. Angiotensin II Group 80% p<0.05 Reference: Busse, LW, et al. 2014, Critical Care 18(Suppl 1), 160 placebo group angiotensin II group angiotensin II dose
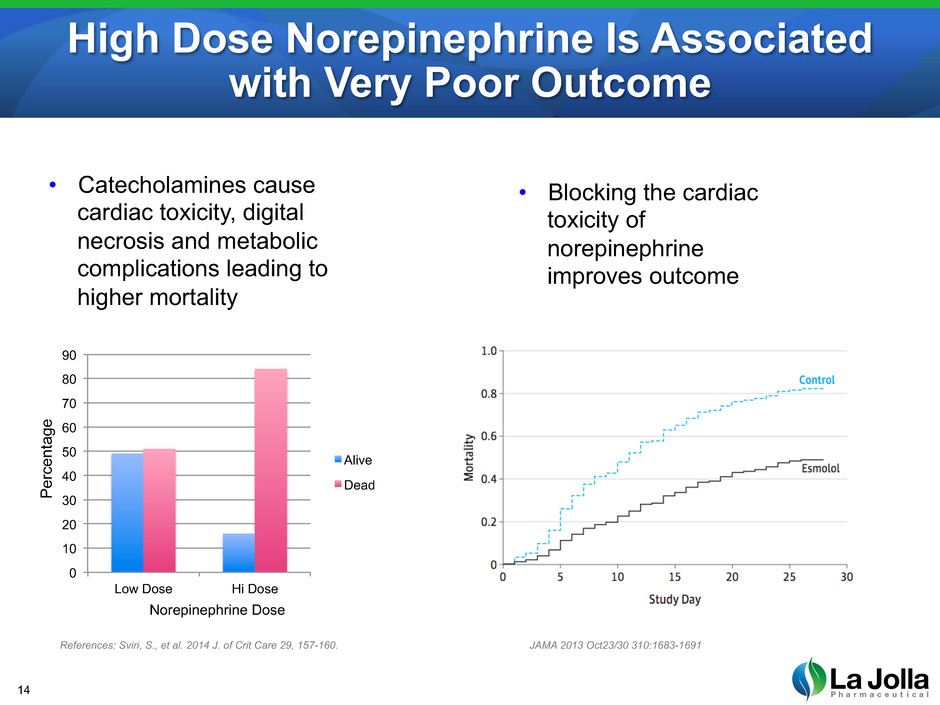
High Dose Norepinephrine Is Associated with Very Poor Outcome 14 References: Sviri, S., et al. 2014 J. of Crit Care 29, 157-160. JAMA 2013 Oct23/30 310:1683-1691 • Catecholamines cause cardiac toxicity, digital necrosis and metabolic complications leading to higher mortality 0 10 20 30 40 50 60 70 80 90 Low Dose Hi Dose Alive Dead P erc en ta ge Norepinephrine Dose • Blocking the cardiac toxicity of norepinephrine improves outcome

Planned Phase 3 Trial of LJPC-501 in CRH 15 • Randomized, placebo-controlled, blinded Phase 3 trial • Patient population: catecholamine-resistant, based on CV SOFA* score • Primary endpoint: blood pressure • Primary endpoint duration: short-term to primary endpoint (hours) • Size: 200-300 patients, 25-35 sites • Start: early 2015 *Cardiovascular Sequential Organ Failure Assessment
Summary of LJPC-501 in CRH 16 • LJPC-501 is a proprietary formulation of angiotensin II, a naturally occurring regulator of blood pressure • Catecholamine-resistant hypotension (CRH) is a high, unmet medical need with no approved therapies • LJPC-501 has been shown to raise blood pressure in a pilot RPC clinical trial in CRH, as well as animal models of hypotension • Initiation of registration Phase 3 planned for early 2015 Meeting held with FDA at which agreement was reached that blood pressure can be the primary endpoint for approval

Overview of LJPC LJPC-501 (Angiotensin II) for CRH GCS-100 (IV Galectin-3 Inhibitor) for CKD LJPC-1010 (Oral Galectin-3 Inhibitor) for NASH LJPC-401 (Hepcidin) for Iron Overload Financial Position and Milestones

Galectins and Galectin-3 18 • Galectin-3 is unique because it has the ability to self-associate (multimerize) up to a pentameric form, allowing it to bind to several receptors at once • Galectin-3 is normally present at low concentration, but is up-regulated in organ failure and cancer; increased levels correlated with reduced survival, disease onset 1 CRD Galectin with Amino Acid Monomer Tail (3) Multimerized Galectin-3 Dimerized 1 CRD Galectin Galectin-3 • Galectins are proteins that can bind to specific sugars on other proteins to modulate cellular function and communication
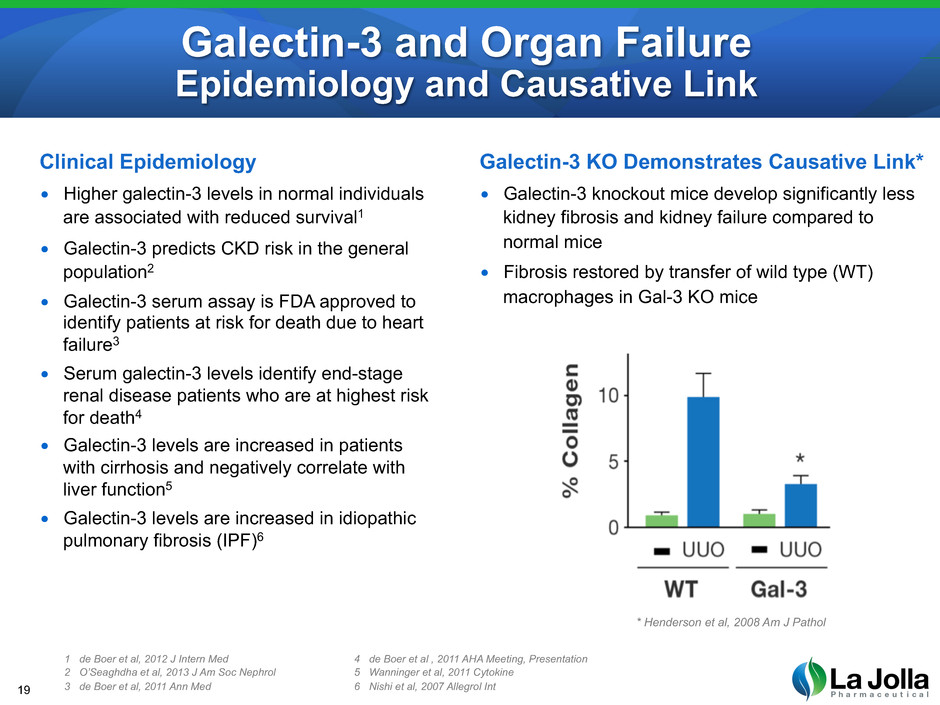
Galectin-3 and Organ Failure Epidemiology and Causative Link Clinical Epidemiology • Higher galectin-3 levels in normal individuals are associated with reduced survival1 • Galectin-3 predicts CKD risk in the general population2 • Galectin-3 serum assay is FDA approved to identify patients at risk for death due to heart failure3 • Serum galectin-3 levels identify end-stage renal disease patients who are at highest risk for death4 • Galectin-3 levels are increased in patients with cirrhosis and negatively correlate with liver function5 • Galectin-3 levels are increased in idiopathic pulmonary fibrosis (IPF)6 19 1 de Boer et al, 2012 J Intern Med 2 O’Seaghdha et al, 2013 J Am Soc Nephrol 3 de Boer et al, 2011 Ann Med 4 de Boer et al , 2011 AHA Meeting, Presentation 5 Wanninger et al, 2011 Cytokine 6 Nishi et al, 2007 Allegrol Int Galectin-3 KO Demonstrates Causative Link* • Galectin-3 knockout mice develop significantly less kidney fibrosis and kidney failure compared to normal mice • Fibrosis restored by transfer of wild type (WT) macrophages in Gal-3 KO mice * Henderson et al, 2008 Am J Pathol

GCS-100: The Leading Galectin-3 Antagonist Mechanism of Action 20 • GCS-100 is a well-characterized, complex sugar derived from pectin • GCS-100 binds to and neutralizes galectin-3; binding activity is localized to the galactose containing side-branches • Patented manufacturing process required for biologic activity; issued patent claims cover all modified pectin compositions with defined size

Phase 1 Data Validates Galectin-3 as a Target in CKD r²=0.36191 p=0.003 0 10 20 30 40 50 60 70 80 0 10 20 30 40 50 60 G al -3 n g/ m L eGFR (mL/min/1.73m2) 21 • Single-dose, Phase 1 dose escalation trial complete • 30 mg/m2 MTD • 1/6 patients at 30 mg/m2 experienced reversible, self-limited muscle cramps • Baseline serum galectin-3 levels strongly correlated with reduced kidney function

Phase 2a CKD Trial Design 22 Day 1 Day 57 Dosing, IV PBO or GCS-100 at 1.5 or 30 mg/m2 D -1 D -8 D -2 2 D -2 9 D -3 6 D -4 3 D -5 0 1o Endpoint: eGFR change vs. placebo 2o Endpoint: safety and tolerability Exploratory Endpoints: circulating galectin-3, markers of inflammation, fibrosis and renal injury Key GCS-100 Dosing D -1 5 Screening Visit Placebo, n=41 1.5 mg/m2, n=41 30 mg/m2, n=39 Randomized, N=121 Study Day: Phase 1 MTD Active in retrospective review Day 50 Day 29 Day 43 Day 36 Day 22 Day 8 Day 15

23 Evaluation of Phase 2a Doses 30 mg/m2 Is Too High Based on PK/PD 0 200 400 600 800 1000 1200 1400 1600 0 50 100 150 200 M ol es G C S- 10 0/ M ol e G al -3 Hours post-infusion GCS-100/Gal-3 Ratio 30 mg/m2 Pharmacologic overdose may lead to adverse off-target effects 0 5 10 15 20 25 30 35 40 45 50 0 50 100 150 200 M ol es G C S- 10 0/ M ol e G al -3 Hours post-infusion GCS-100/Gal-3 Ratio 1.5 mg/m2

-2.5 -2 -1.5 -1 -0.5 0 0.5 0.0 0.5 1.0 1.5 2.0 2.5 3.0 Published CKD Natural Hx Phase 2 Study Placebo Patients Time Course of Activity Consistent with MoA Placebo Group Consistent with Natural Hx of CKD 24 Efficacy Endpoint Also Met at End of Study Time Point (5 Weeks post Last Dose) Placebo Data Consistent with the Natural History of eGFR in CKD -2.00 -1.50 -1.00 -0.50 0.00 0.50 1.00 1.50 0 2 4 6 8 10 12 14 C ha ng e in e G FR (m L/ m in /1 .7 3m 2) Study Week plac 1.5 mg/m2 30 mg/m2 p=0.07 p=0.04 End of Dosing Months eG FR C ha ng e Nephrol Dial Transplant (2012) 27: 2255–2263 Pl cebo Primary Endpoint

Early Terminations • 117 of 121 patients enrolled completed the study Placebo: 40/41 completed; 1 withdrew consent after the first dose 1.5 mg/m2: 41/41 completed 30 mg/m2: 36/39 completed; 1 withdrew consent before the first dose, 1 withdrew consent after the second dose due to scheduling conflict, 1 subject failed to come back after the 6th dose without explanation Overall AE profile comparable between groups • Similar event rate among the groups, although more grade 3 events in the 30 mg/m2 group 4 Serious Adverse Events: none at the 1.5 mg/m2 dose Safety Data Summary 25 Dose (mg/m2) Event Description Relationship to drug Resolution 0 Worsening urinary tract infection None Resolved 0 Cerebrovascular accident None Resolved with sequelae 30 Shortness of breath, secondary to congestive heart failure exacerbation None Resolved 30 Progressive chronic kidney disease and volume overload None Resolved

• Primary endpoint reached for 1.5 mg/m2 dose • eGFR effect correlates with change in galectin-3 • Multiple secondary endpoints improved and consistent with primary endpoint analysis • No deaths on study, and no Serious Adverse Events (SAE) and no Grade 3/4 events at 1.5 mg/m2 • No significant adverse effects on other lab parameters • Data presentation at ASN Nov. 13-16 GCS-100 CKD Phase 2 Results 26

• OBJECTIVE Compare efficacy of 3 doses versus placebo • PRIMARY ENDPOINT Sustained improvement in eGFR versus placebo • SECONDARY ENDPOINTS Rate of CV events Potential Phase 2b Study Design 27 • Stage: 3 and 4 • eGFR: high baseline variability excluded • Other: • Etiology of CKD • Biomarkers GCS-100 0.5 mg/m2 IV weekly for up to 6 months GCS-100 1.5 mg/m2 IV weekly for up to 6 months • N = 280; 70 patients/arm • # of sites: 15 • PK: 20 patients/arm • Duration of treatment: 6 months • Plan to initiate late 2014 Placebo Saline IV weekly for up to 6 months GCS-100 6 mg/m2 IV weekly for up to 6 months

Overview of LJPC LJPC-501 (Angiotensin II) for CRH GCS-100 (IV Galectin-3 Inhibitor) for CKD LJPC-1010 (Oral Galectin-3 Inhibitor) for NASH LJPC-401 (Hepcidin) for Iron Overload Financial Position and Milestones

LJPC-1010: Overview 29 • LJPC-1010 is a more potent and purified derivative of GCS-100 that can be delivered orally • Nonalcoholic steatohepatitis (NASH) is the more serious form of nonalcoholic fatty liver disease (NAFLD), which can lead to liver failure; NASH affects 2 to 5 percent of Americans • In a preclinical study, LJPC-1010 showed a significant reduction in NAFLD activity score, a system of scoring the features of NAFLD • IND activities for LJPC-1010 in NASH are underway; initiation of Phase 1 trial planned for early 2015
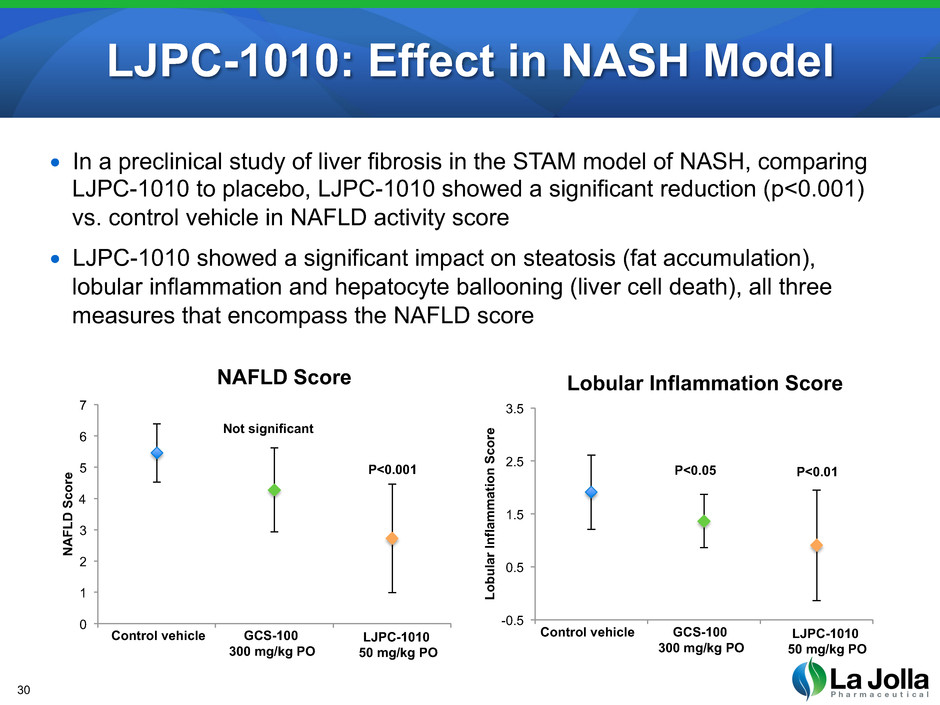
LJPC-1010: Effect in NASH Model 30 • In a preclinical study of liver fibrosis in the STAM model of NASH, comparing LJPC-1010 to placebo, LJPC-1010 showed a significant reduction (p<0.001) vs. control vehicle in NAFLD activity score • LJPC-1010 showed a significant impact on steatosis (fat accumulation), lobular inflammation and hepatocyte ballooning (liver cell death), all three measures that encompass the NAFLD score 0 1 2 3 4 5 6 7 0 1 2 3 N A FL D S co re NAFLD Score Control vehicle GCS-100 300 mg/kg PO LJPC-1010 50 mg/kg PO Not significant P<0.001 -0.5 0.5 1.5 2.5 3.5 0 1 2 3 Lo bu la r In fla m m at io n S co re Lobular Inflammation Score Control vehicle GCS-100 300 mg/kg PO LJPC-1010 50 mg/kg PO P<0.05 P<0.01

LJPC-1010: Improves Histology Phase 1/2 Planned for Early 2015 31 Control Vehicle GCS-100 LJPC-1010 • Next Steps: • Bridging toxicology studies • GMP Manufacturing • Phase 1/2 early 2015

Overview of LJPC LJPC-501 (Angiotensin II) for CRH GCS-100 (IV Galectin-3 Inhibitor) for CKD LJPC-1010 (Oral Galectin-3 Inhibitor) for NASH LJPC-401 (Hepcidin) for Iron Overload Financial Position and Milestones

LJPC-401: Overview • LJPC-401 is a formulation of hepcidin, which is an endogenous peptide hormone that controls and regulates iron metabolism • Several large indications Acquired iron overload – Currently treated with chelators; 50% of patients are intolerant to or fail – Approximately 25,000 patients in the U.S. Hereditary hemochromatosis – Disorder of hepcidin production – 1,000,000 patients in the U.S.; 5% fail or are intolerant to phlebotomy 33
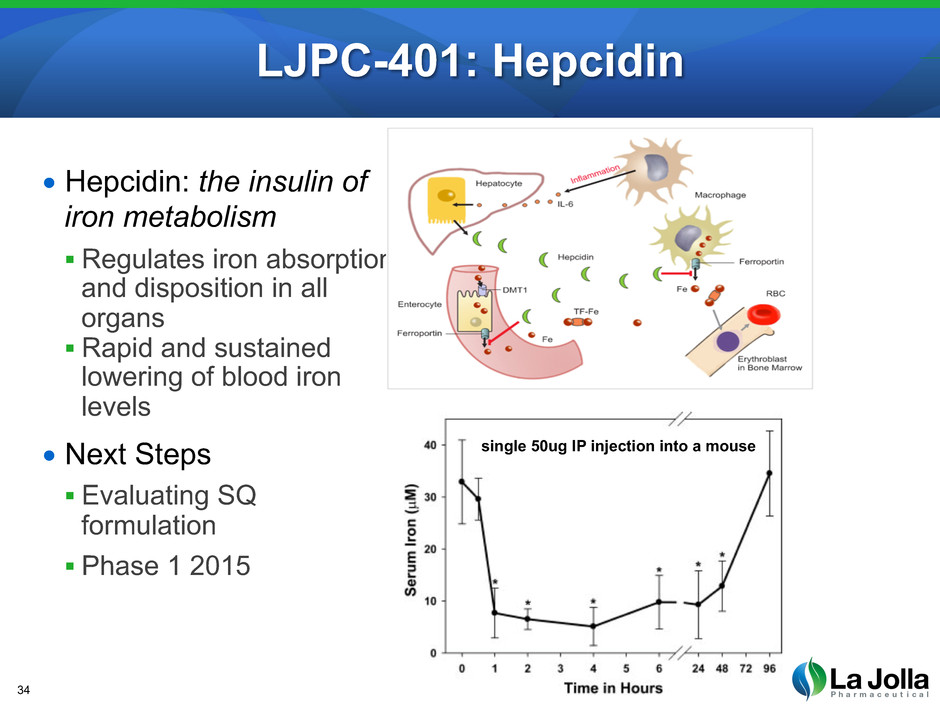
LJPC-401: Hepcidin • Hepcidin: the insulin of iron metabolism Regulates iron absorption and disposition in all organs Rapid and sustained lowering of blood iron levels • Next Steps Evaluating SQ formulation Phase 1 2015 34 single 50ug IP injection into a mouse

Overview of LJPC LJPC-501 (Angiotensin II) for CRH GCS-100 (IV Galectin-3 Inhibitor) for CKD LJPC-1010 (Oral Galectin-3 Inhibitor) for NASH LJPC-401 (Hepcidin) for Iron Overload Financial Position and Milestones

Condensed Statement of Cash Flow Data Twelve Months Ended June 30, 2014 (in millions) Cash used for operating activities $7.9 Financial Position 36 Cash resources expected to fund Company through 2016 Condensed Balance Sheet Data As of June 30, 2014 (in millions)* Cash $57.0 Total liabilities $1.1 Total shareholders’ equity $56.2 Fully Diluted, As-Converted Shares Outstanding** 23,321,185 *Reflects proceeds from July 28, 2014 equity financing **Includes common stock, preferred stock & outstanding equity awards as of October 10, 2014

Development Plan 37 Cash resources through 2016 2017 2015 2016 2014 Today Phase 2b G C S- 10 0 Phase 1 Phase 2a extension Phase 3 LJ P C -5 01 Phase 2/3 Phase 3 Phase 1/2 CRH HRS CKD ESRD IND H2 2014 H2 2015 [DATE] H1 2016 H1 2015 Late 2014 H2 2016 H2 2016 Not Budgeted LJ P C -4 01 Ir on O ve rl oa d Phase 1 Preclinical / IND LJ P C -1 01 0 N A S H Phase 1 Phase 2/3 Preclinical / IND Early 2015 H1 2016 2015 H1 2015 H1 2016 H1 2015 Not Budgeted Not Budgeted Early 2015

Thank You
