Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Ignyta, Inc. | d804210d8k.htm |
 ®
Exhibit 99.1
Catalyzing Precision Medicine with Integrated Rx/Dx in Oncology
Investor Day
Palace
Hotel
–
NYC
October 14, 2014
8am
–
9:30am
ET |
 ____ __ ____
_____ ____ ______ _____ _____
____ _____
_____ _____
____ _____
Click to edit Master text styles
Second level
Third level
Fourth level
Fifth level
2
This document contains forward-looking statements, as that term is defined in Section 27A of the
Securities Act of 1933 and Section
21E
of
the
Securities
Exchange
Act
of
1934,
about
Ignyta,
Inc.
(“us”
or
the
“Company”).
Statements
that
are
not
purely historical are forward-looking statements. These include statements regarding, among other
things: the clinical and/or non-clinical data or plans underlying RXDX-101,
RXDX-103, RXDX-104 or any of our other development programs; our ability to design and
conduct development activities for RXDX-101, RXDX-103, RXDX-104 and our other development
programs;
our
ability
to
develop
or
access
companion
diagnostics
for
our
product
candidates;
our
ability
to
obtain
and
maintain intellectual property protection for our product candidates; our ability to adequately fund
our development programs; our ability to obtain regulatory approvals in order to market any of
our product candidates; and our ability to successfully commercialize any approved
products. Forward-looking statements involve known and unknown risks that relate to future
events or the Company’s future financial performance, some of which may be beyond our
control, and the actual results could differ materially from those discussed in this
document. Accordingly, the Company cautions investors not to place undue reliance on the forward-looking
statements contained in, or made in connection with, this document.
Important factors that could cause actual results to differ materially from those indicated by such
forward-looking statements, include, among others, the potential for results of past or
ongoing clinical or non-clinical studies to differ from expectations or previous results;
the interpretation of data from our clinical and non-clinical studies; our ability to initiate and complete clinical
trials and non-clinical studies; the potential advantages of our product candidates; the markets
any approved products are intended to serve; and our capital needs; as well as those set forth
under the headings “Special Note Regarding Forward- Looking Statements,”
“Risk Factors”
and “Management’s Discussion and Analysis of Financial Condition and Results of
Operations”
contained in the Company’s Form 10-K filed with the Securities and Exchange Commission
(“SEC”) on February 28, 2014, and similar disclosures made in the Company’s Form
10-Q filings and other SEC filings and press releases. The forward-looking
statements contained in this document represent our estimates and assumptions only as of the date of
this document, and we undertake no duty or obligation to update or revise publicly any
forward-looking statements contained in this document as a result of new information,
future events or changes in our expectations. Third-party
information
included
herein
has
been
obtained
from
sources
believed
to
be
reliable,
but
the
accuracy
or
completeness of such information is not guaranteed by, and should not be construed as a representation
by, the Company. Safe Harbor Statement |
 Time
Speaker
8:00am
–
8:15am
8:15am –
8:35am
Garrett Brodeur, MD –
Children’s Hospital of
Philadelphia
8:35am –
8:55am
Alexander Drilon, MD –
Memorial Sloan-Kettering
Cancer Center
8:55am –
9:15am
Question & Answer
3
Jonathan Lim, MD –
Ignyta Chairman and CEO
Agenda |
 Despite
progress
in
drug
development,
likelihood
of
approval
for
early
phase
cancer
drugs is only 6.7%*
4
*Nature Biotechnology, Jan. 2014; LOA 10.4% for NME’s, all indications
Barriers to precision medicine: challenges with patient selection, market size, sample
procurement, sample prep, tumor heterogeneity, resistance to single agent
therapy….
On the bright side, there are a significant number of new initiatives and technologies
in precision (and personalized) medicine
-
NCI’s
n-of-1;
Medicine’s
“Manhattan
Project”
&
other
Big
Data
initiatives;
personalized genomic analyses; “Universal Diagnostics”….
-
Targeting the right patient with the right drug at the right time can boost success
rates for new patient treatments
Precision Medicine |
 5
Harnessing the power of molecularly targeted Rx and Dx
for the benefit of cancer patients everywhere
Ignyta’s Goal Is to Catalyze Precision Medicine |
 Migrating from 1:1
targeting of single markers for single targets with single drugs to
multi-/combi-Rx/Dx
-
Multiple alterations: gene rearrangements, SNPs, copy number variants,
overexpression
-
Multiomic: genomic, epigenomic, proteomic biomarker analyses
-
Multiplex: signatures or patterns of biomarkers
-
Combination therapies to combat resistance to single agent treatment
6
Integrating Rx/Dx solutions to optimize the ability to help patients with low frequency molecular
alterations -
Building in-house central diagnostics laboratory
-
Seamless communication and workflows between Rx drug developers and CDx
developers
Seeking
biomarkers
for
patient
selection
and
for
disease
monitoring
-
Strong
foundation
of
biological
understanding
of
the
targets
and
indications
we
are pursuing, to underpin both our Rx and Dx efforts
-
Baseline and longitudinal assessments, including non-invasive methods
Our Approach |
 Developing and commercializing highly targeted drugs for patients with
low frequency molecular alterations that we assay with our own
companion diagnostics (e.g., RXDX-101)
Repositioning drugs by targeting selected patient populations, instead
of all-comers, using insights from our proprietary database
(Oncolome™) and biomarker development capabilities (e.g., RXDX-
103)
Leveraging Oncolome and our discovery capabilities to identify novel
targets and/or biomarker hypotheses to develop potential first-in-class
cancer therapies (e.g., Spark programs)
7
Precision Oncology Programs |
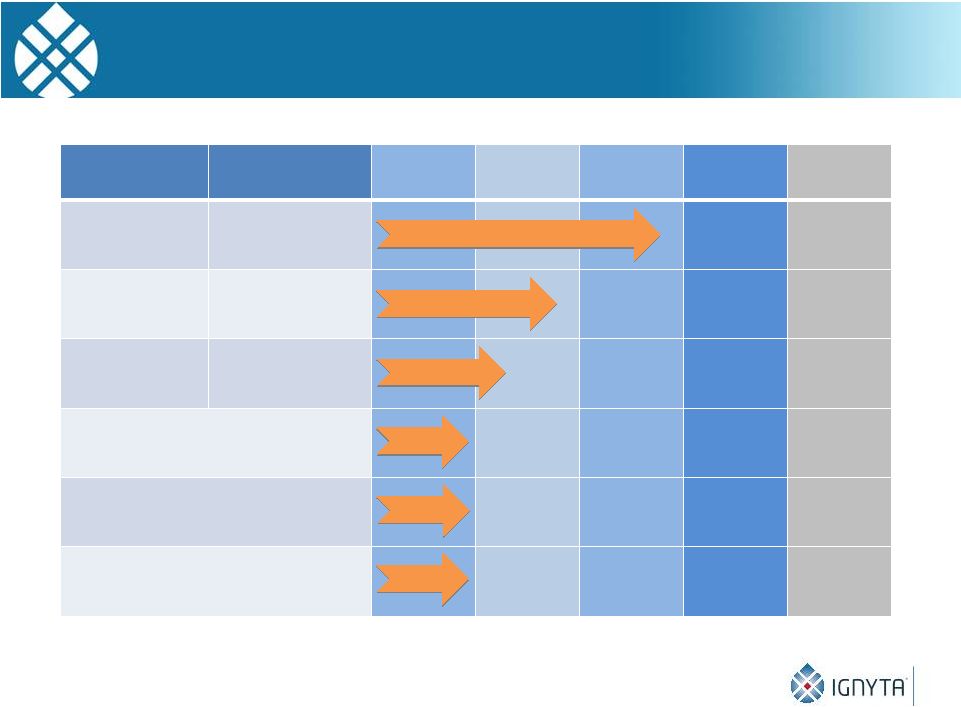 Ignyta Rx/Dx
Pipeline 8
1
In-licensed from Nerviano Medical Sciences (NMS); RXDX-102 Pan-Trk inhibitor is a
back-up to RXDX-101 Compound
Target
Discovery
Preclinical
Phase 1
Phase 2
Phase 3
RXDX-101
1
Pan-Trk, ROS1,
ALK
RXDX-103
1
Cdc7
RXDX-104
1
RET
Spark-1 Rx/Dx Program
Spark-2 Rx/Dx Program
Spark-3 Rx/Dx Program |
 9
Central
Lab
Clinical Sites
Specimens
Platforms
Output
FFPE
Fresh Tissue
FISH
IHC
PCR
NGS
Oncolome®
Trial Enrollment
CDx
Ignyta’s Dx Capabilities Enable Leadership
in Precision Medicine
•
GXP
•
CLIA
•
CNVs
•
Splice variants
•
Mutations
•
Overexpression
•
Rearrangements |
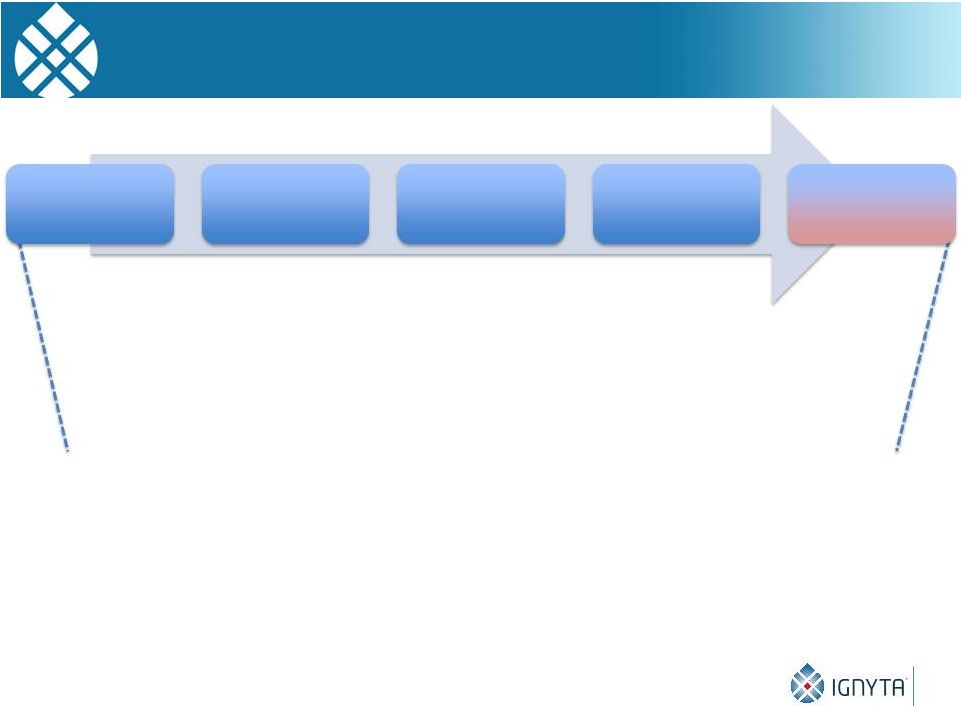 Ignyta’s
Goal Is to Catalyze Precision Medicine 10
Harnessing the power of molecularly targeted Rx and Dx
for the benefit of cancer patients everywhere
2030 Vision
2021 –
2025
Scale pipeline revenue
2026 –
2030
Drive sustainable
profitability
Leading precision
medicine
company
2011 –
2015
Advance clinical
pipeline
2016 –
2020
Commercialize RXDX
lead |
 Speaker
Biographies 11
Garrett Brodeur, M.D.
-
Associate Chair for Research in the Department of Pediatrics and
Associate Director of the Abramson
Cancer Center in the University of Pennsylvania School of Medicine
-
Professor of Pediatrics and the Audrey E. Evans Chair in Molecular Oncology at the
Children’s Hospital of Philadelphia. Served as Oncology Division Chief from
1998-2008, during which time he initiated programs in Cancer Predisposition,
Experimental Therapeutics, Palliative Care and Proton Therapy, while expanding
programs in Neuro-Oncology, Cancer Survivorship, and Stem Cell Transplants
-
Research focuses on identifying the genes, proteins and pathways
responsible for the pathogenesis of
neuroblastoma (NB), including extensive work in examining the role that Trk receptors play in
these tumors
Alexander Drilon, M.D.
-
Medical Oncologist in the Developmental Therapeutics Clinic and Thoracic Oncology Service at
Memorial Sloan Kettering Cancer Center
-
Recipient of multiple awards, including an American Society of Clinical Oncology (ASCO) Young
Investigator Award, an ASCO Career Development Award, and an International Association
for the Study of Lung Cancer (IASLC) Fellowship Award
-
Research focuses on early-phase clinical trials of targeted therapeutics for
molecularly-enriched cohorts of advanced solid tumors. Spearheads research into
lung cancers with recurrent gene rearrangements, including those involving the genes
ROS1, NTRK1, and RET |
 TRK
Receptors as Oncogenic Drivers in Pediatric And Adult Cancers
Garrett M. Brodeur, MD
Children’s Hospital of Philadelphia
University of Pennsylvania
12
® |
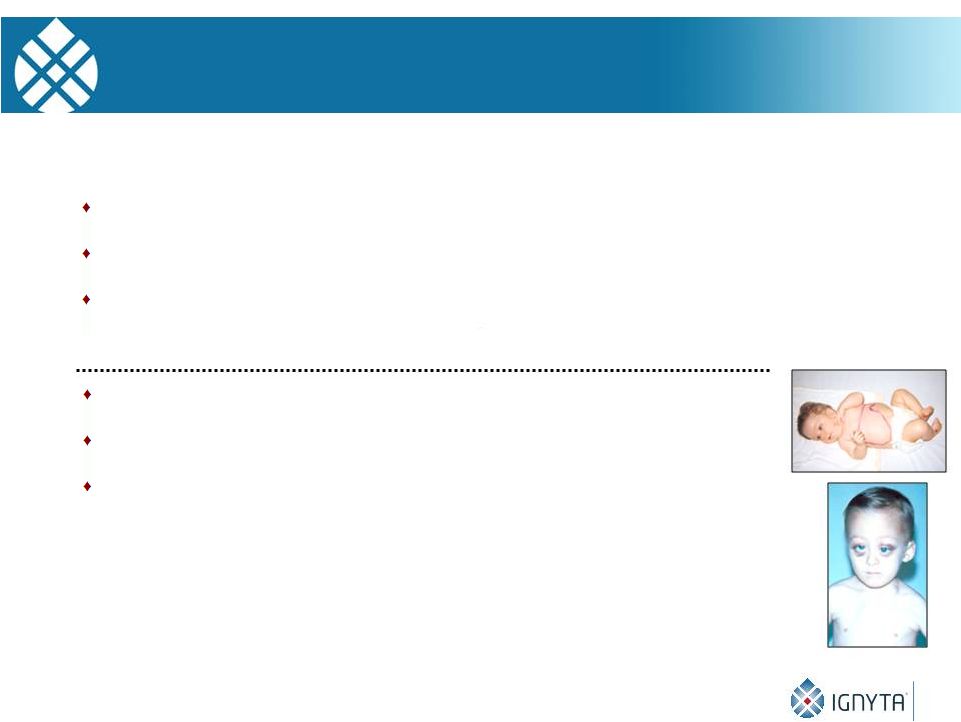 13
Tumor of the peripheral nervous system
Most common extracranial solid tumor
Accounts for 8% of childhood cancers, and 12%
of deaths from cancer in children
Spontaneous regression in some infants
Maturation to ganglioneuroma in older pts.
Most patients are over 1 year of age with
metastatic disease and a poor prognosis
Neuroblastoma—An Enigmatic Pediatric Cancer |
 14
Low-
and intermediate-risk NB patients: cure rate 90%
High-risk NB patients: cure rate 40-50%
Improvements in cure of HR-NB patients has plateaued
Intense, multimodality therapy has many acute toxicities
Multimodality therapy has many long-term toxicities:
-
Growth, sterility, hearing, endocrine, dental, cardiac,
second malignancies
Thus, more effective, less toxic therapy is needed
Therapy targeted to specific genes, proteins and
pathways is likely to provide a selective advantage
Unmet Need #1: More Effective, Less Toxic Therapy |
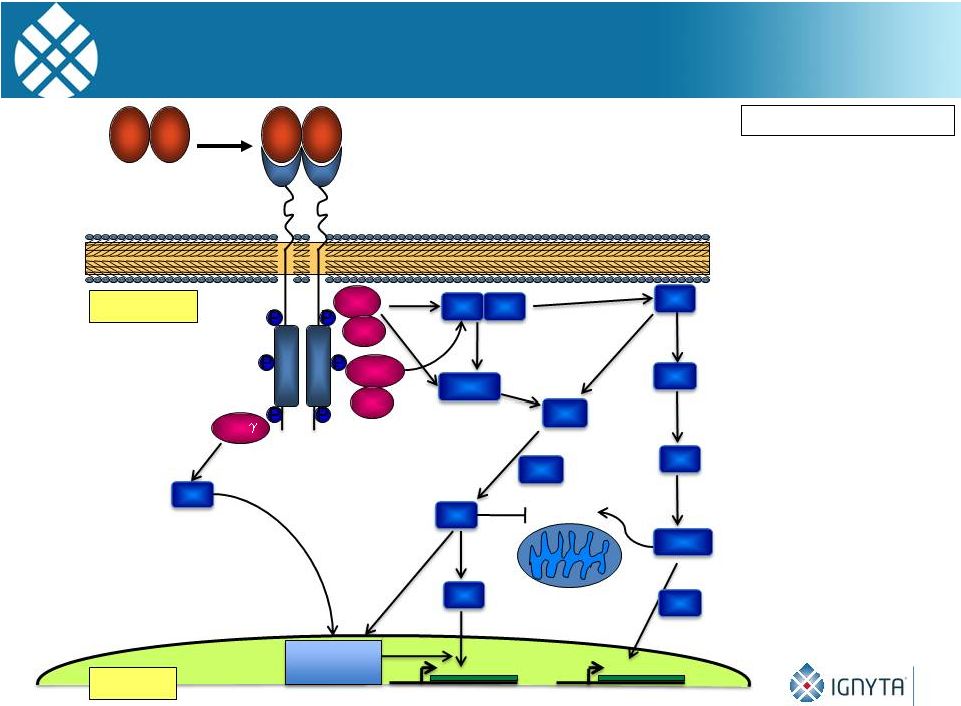 TrkA
Signaling 15
Survival
Brodeur GM: CCRes, 2009
Survival
Differentiation
Gab1/2
SOS
Grb2
Ras
Raf
MEK
MAPK
PDK1
PI3K
Bad Bcl2
Akt
APS
SH2B
Frs2
Shc
PLC
1
PKC
mTor
RSK
Cytoplasm
NGF
NGF
NGF
NGF
Transcription
Factors
Nucleus |
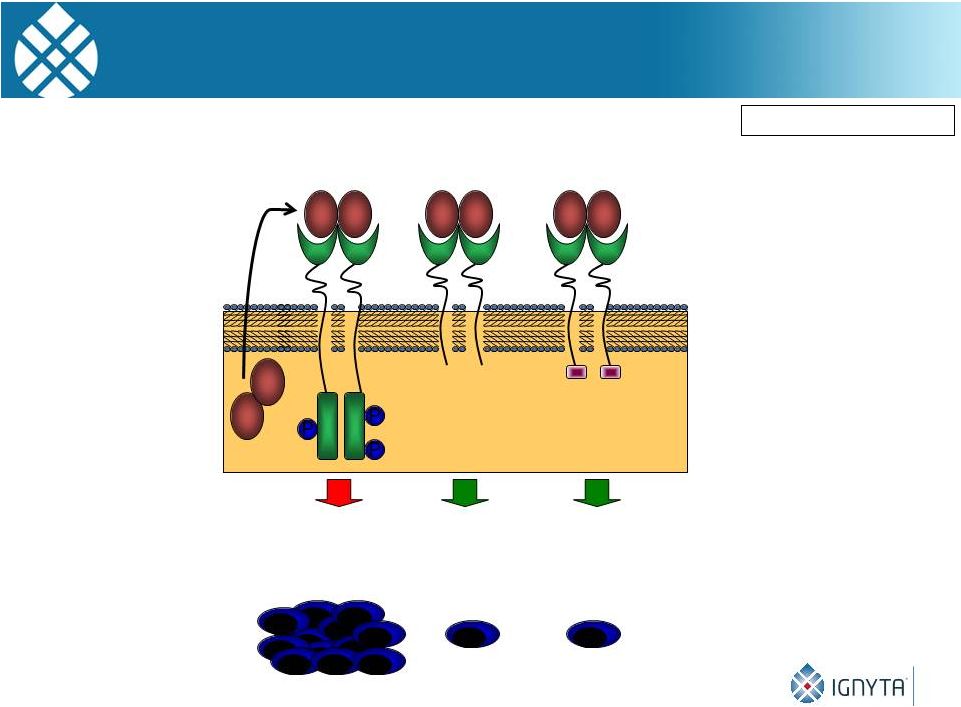 TrkB Autocrine
Pathway 16
Brodeur GM: CCRes, 2009
BDNF
BDNF
BDNF
BDNF
BDNF
BDNF
BDNF
BDNF
•
Inactive
•
Decoy receptor
TrkB-Shc
BDNF+
•
PI3K+
•
Metastasis
•
Chemoresistance
•
Angiogenesis
TrkB-full
BDNF+
TrkB-T1
BDNF+
•
Inactive
•
Decoy receptor |
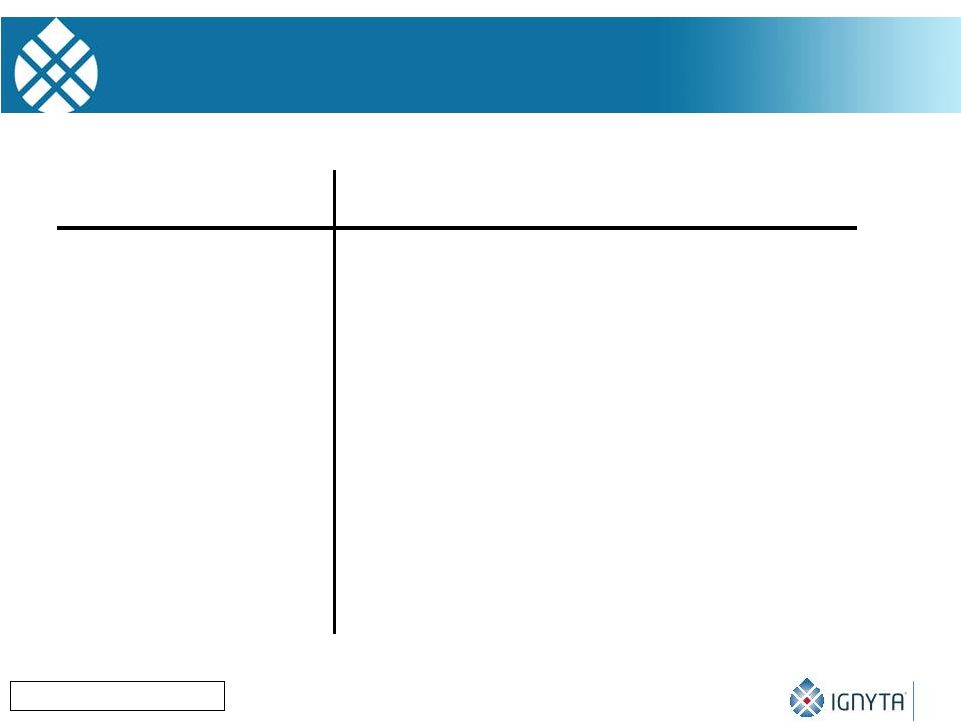 Role of TRK Receptors
in Human Neuroblastomas 17
Brodeur GM: CCRes, 2009
Features
TrkA (NTRK1) TrkB (NTRK2)
Clinical group
Favorable
Unfavorable
Ligand (expr.)
NGF (No)
BDNF (Yes)
Cell Survival
Yes
Yes
Differentiation
Yes
No
Invasion
Inhibits
Promotes
Metastasis
Inhibits
Promotes
Angiogenesis
Inhibits
Promotes
Drug resistance
Inhibits
Promotes |
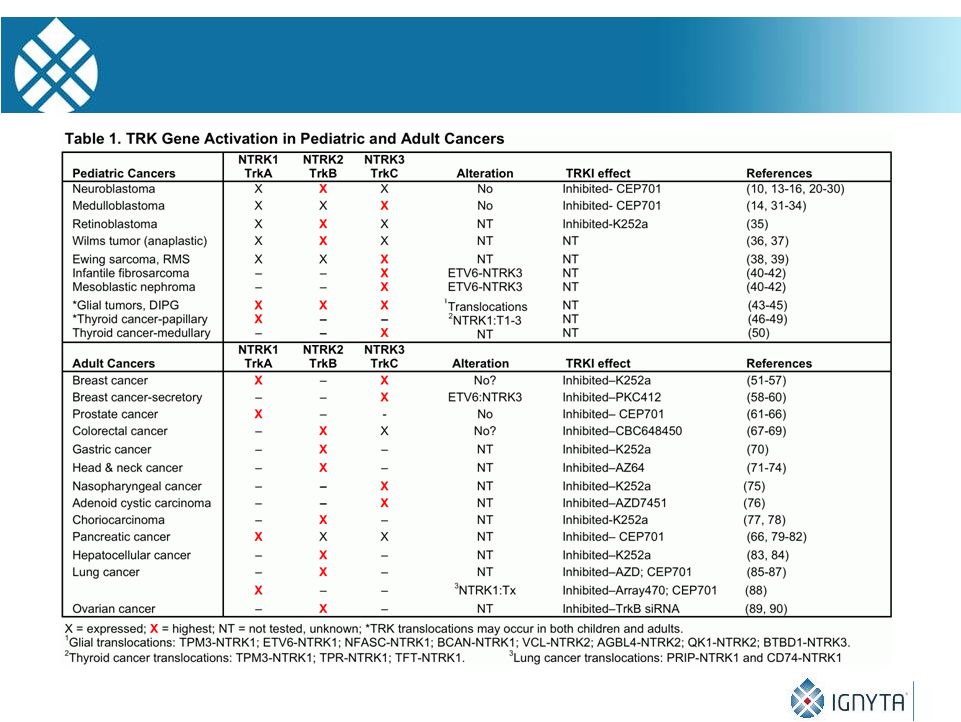 18
NTRK
Gene Activation in Pediatric and Adult Cancers |
 Unmet Need #2: Potent
TRK Receptor Inhibitor There
are
relatively
few
actionable
targets
to
oncogenic
drivers
in
NB
MYCN is amplified in 22%, but it is difficult to target
ALK is activated by mutation, duplication, amplification in 8-10%
TrkB/BDNF autocrine activation occurs in 50-60% of HR-NB
Phase 1 trial with Lestaurtinib was promising, but it was discontinued
19 |
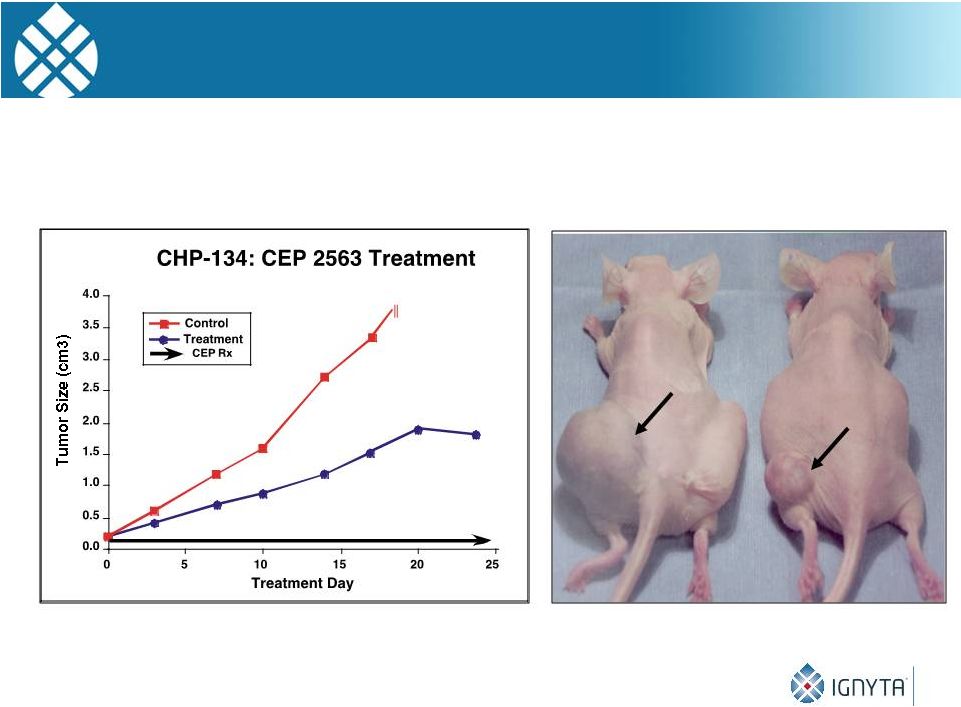 Efficacy of CEP-2563* in NB Xenografts
P=0.0025
7
7
Control
Treatment
*CEP-2563 is an alanyl-lysinyl prodrug of CEP-701 (Lestaurtinib)
20 |
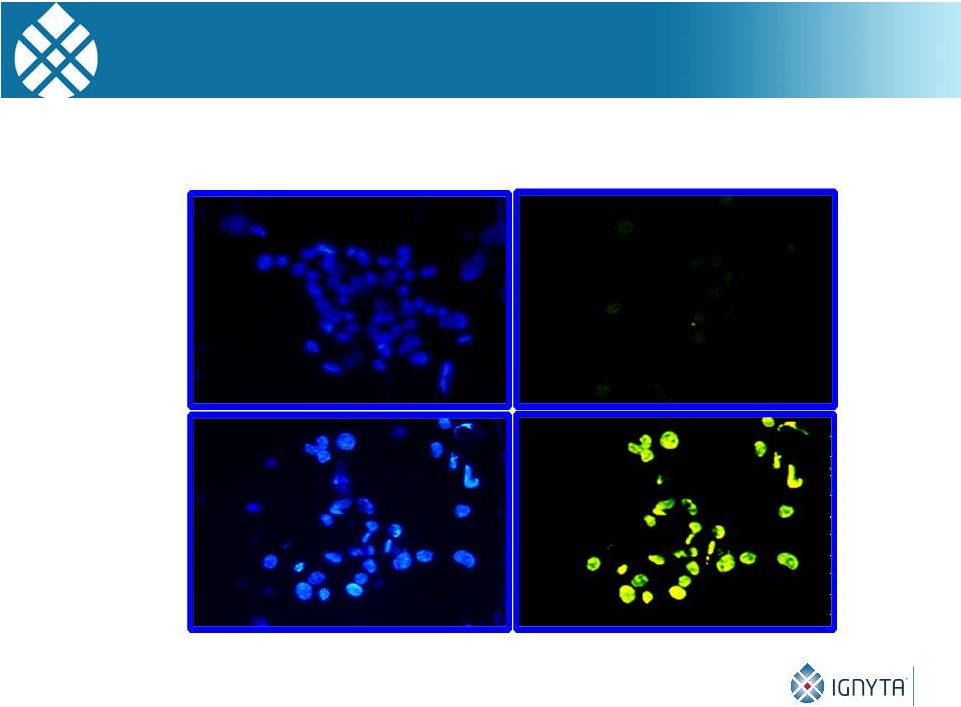 Apoptosis in NB Tumors After CEP-2563 Treatment
DAPI
Staining TUNEL Staining
Control
Tumor
CEP-2563
Treated
21 |
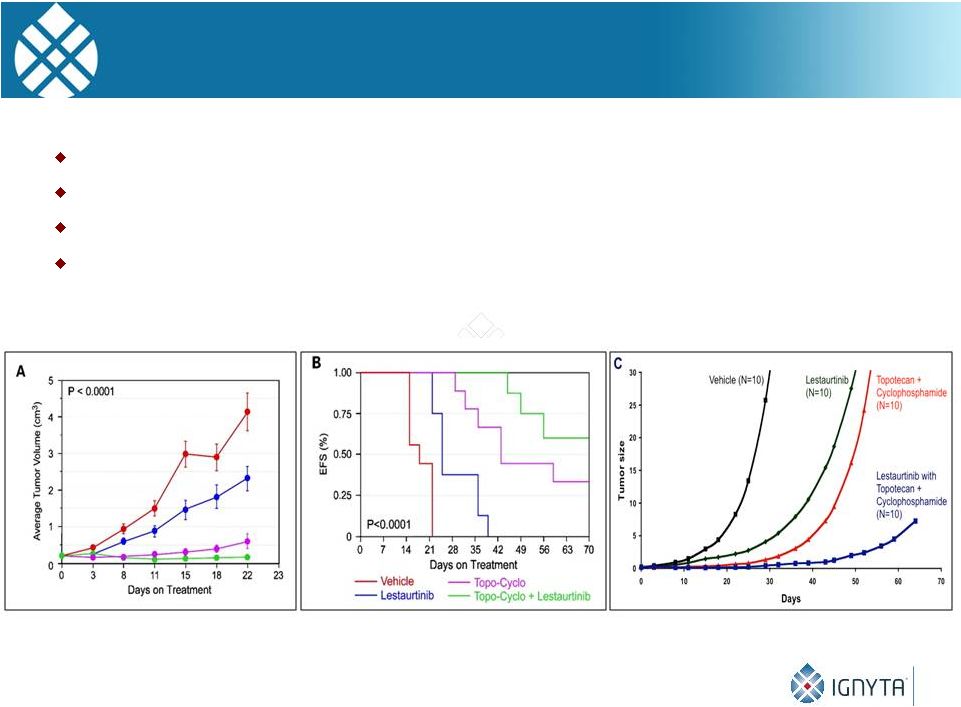 22
Effect of Lestaurtinib
± Topo-Cyclo on NB
xenografts. Lestaurtinib enhances the efficacy of chemotherapy
Tested with Topo-Cyclo (below) and Irino-TMZ (not shown)
TRK inhibition also enhances effect of radiation therapy
Effects are at least additive, if not synergistic
Tumor
Growth Life-table
Analysis Linear Mixed Effects Model |
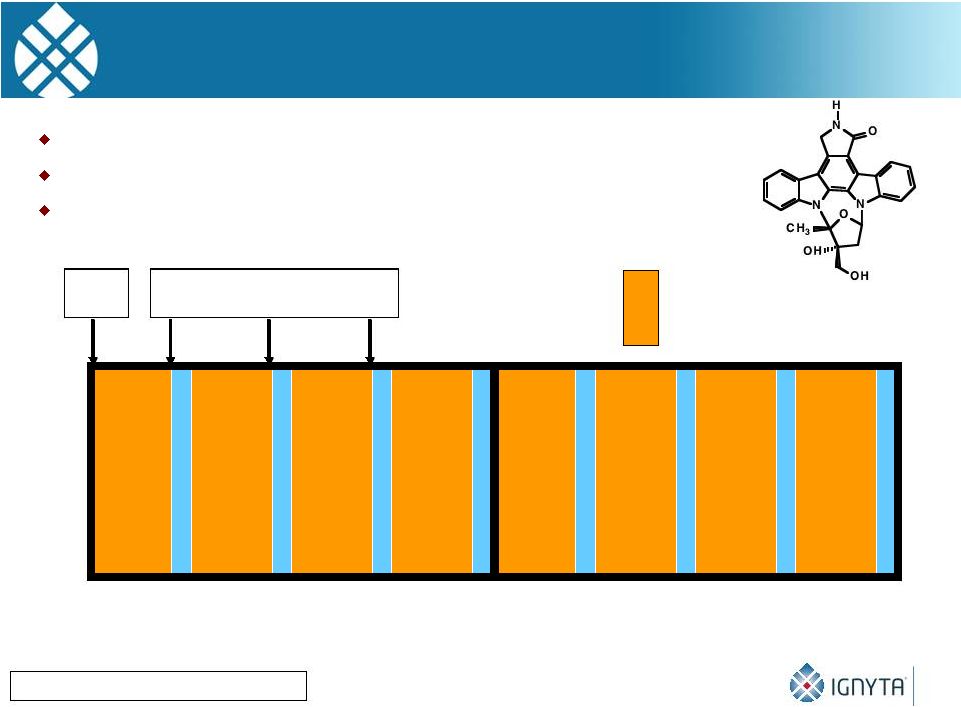 0
28
56
Day
pK
Trough Levels
= CEP-701 BID
Lestaurtinib Phase I Trial: Clinical Trial Design
Minturn JE, Can. Chemo. Pharm, 2011
Conventional dose-
and toxicity/MTD finding study
Nine dose levels from 25-195 mg/m2/dose BID
Bioassay performed to assess therapeutic efficacy
23 |
 Lestaurtinib Phase I
Trial: Target Inhibition Adult Phase 2 Dose
Minturn JE, Can. Chemo. Pharm, 2011
24
Dose Levels
Dose of C
EP –
701
(mg/M
2
/dos
e
B.I.D.)
Dose Level 1
25
Dose Level 2
35
Dose Level 3
45
Dose Level 4
55
Dose Level 5
70
Dose Level 6*
90
Dose Level 7#
115
Dose Level 8#
150
Dose Level 9#
195 |
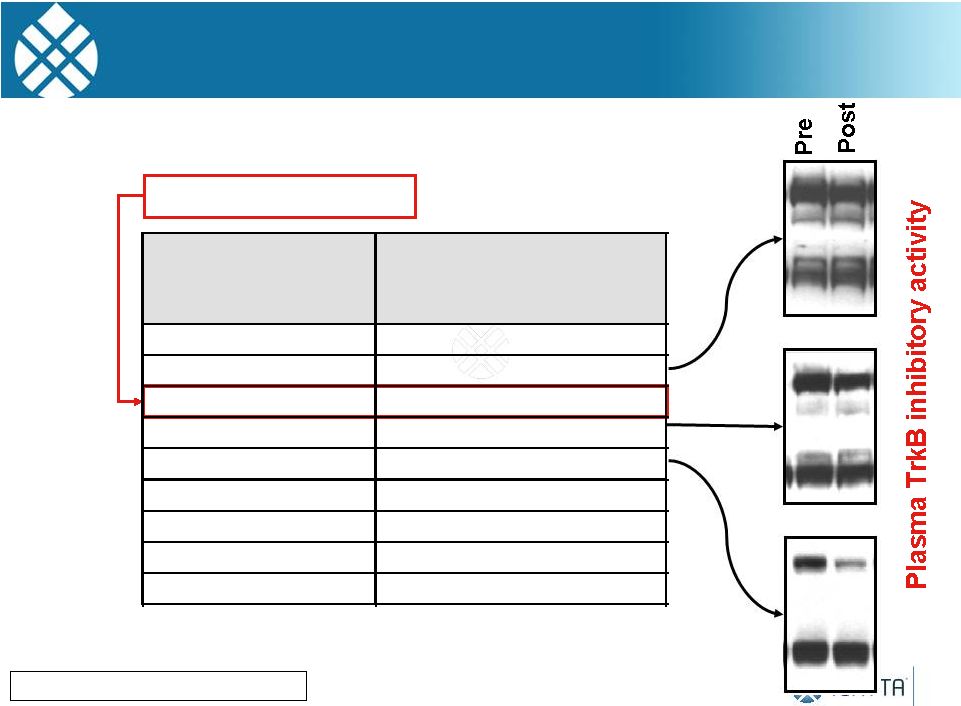
 CEP-701 Clinical Trial: Conclusions
Lestaurtinib is tolerated in pediatric patients at doses above those
used in adults. Mild hepatotoxicity encountered, but MTD not reached.
Doses
of
70
mg/M
twice
daily
was
required
for
significant
target
inhibition
8 of 16 NB patients treated at effective dose respond: 2 PR’s, 2
MR’s and 4 SD (median 10 mo, 4-28+ mo) 50% ORR
Preclinical data support a follow-up phase 1/2 study combining a TRK
inhibitor with other agents
However, Cephalon was taken over by TEVA and the R&D for
Lestaurtinib ended
Minturn JE, Can. Chemo. Pharm, 2011
25
2 |
 26
RXDX-101 is a Potent, TRK-Selective Multikinase Inhibitor
RXDX-101 inhibits TrkA, TrkB, TrkC, ALK and ROS1 at
low nanomolar concentrations
RXDX-101 has shown promise in a Phase 1 dose
escalation study in Italy
The TrkB/BDNF autocrine pathway is important in 50-
60% of high-risk NB patients
We tested the antitumor efficacy of RXDX-101 in vitro
and in our SY5Y-TrkB xenograft model |
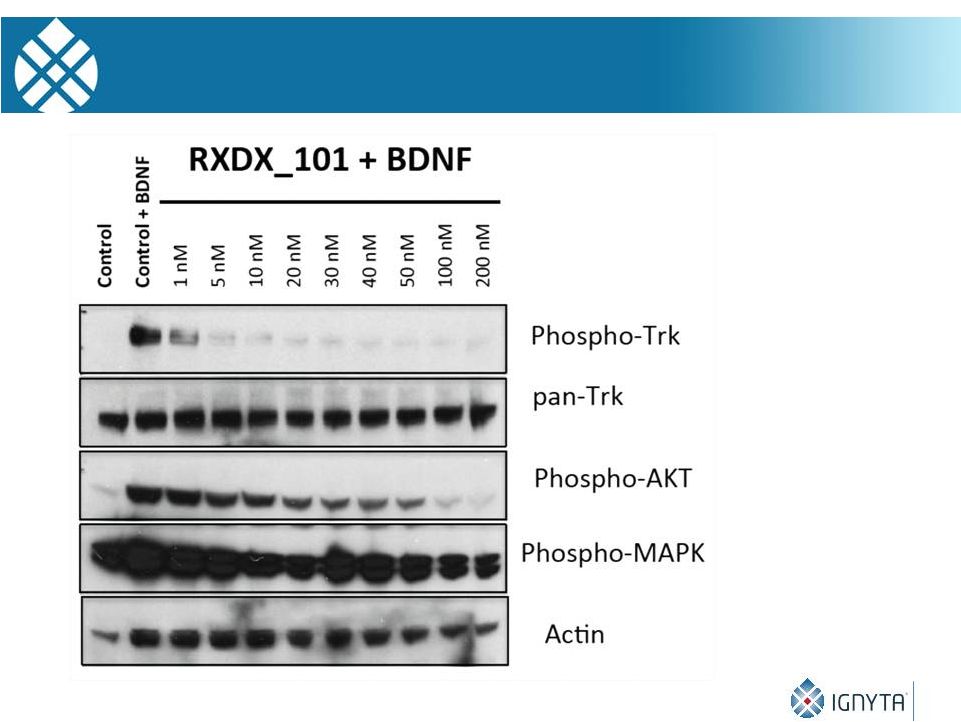 27
RXDX-101 Is a Potent Inhibitor of TRK Phosphorylation |
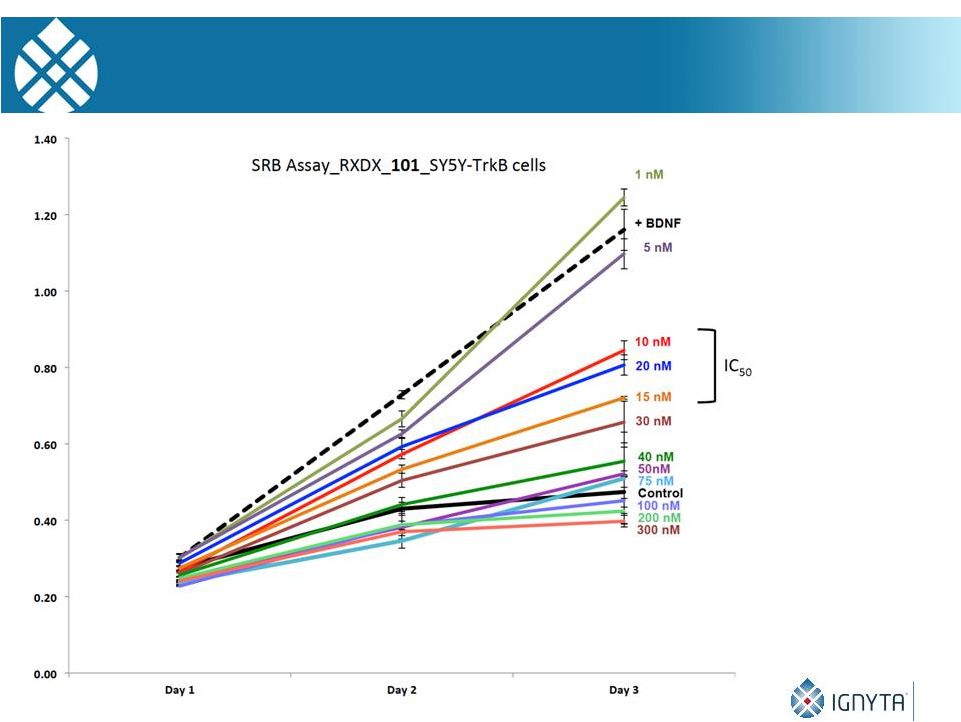 28
RXDX-101 and SY5Y-TrkB Growth Inhibition in Vitro |
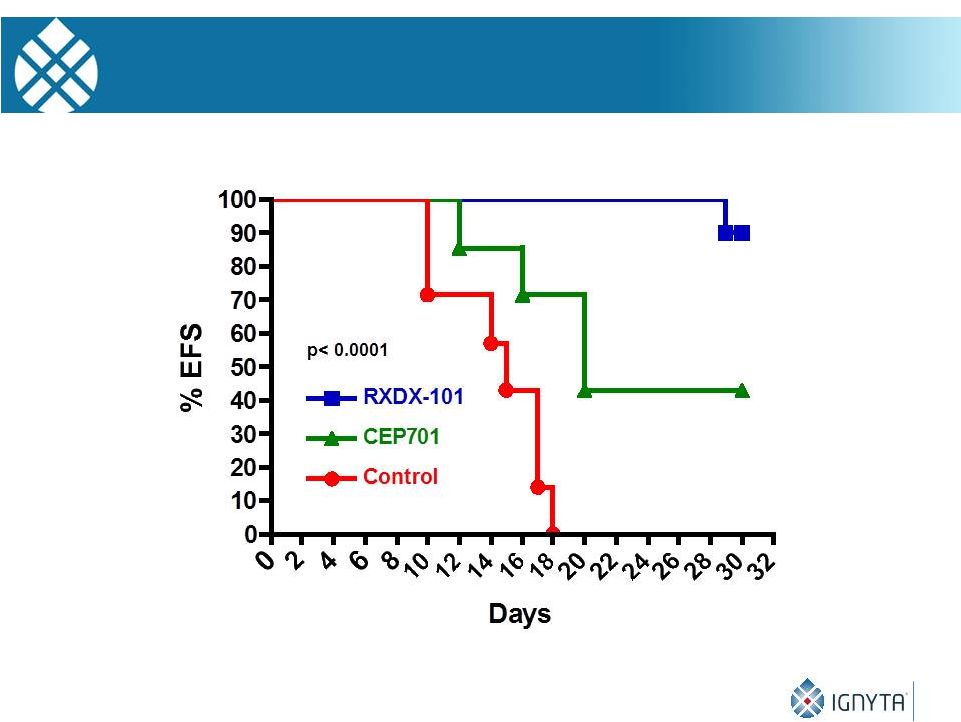 29
RXDX-101 and SY5Y-TrkB Growth Inhibition in Vivo |
 We have tested ~12
different TRK inhibitors from 8 companies over the past 20 years
RXDX-101 is the most potent TRK receptor inhibitor we have tested
We are planning a Phase 1/2 industry-sponsored, study of RXDX-101
in recurrent/refractory tumors at CHOP, pending an adult Phase 2
dose, a pediatric formulation, and regulatory agency concurrence
We are planning phase 2 expansion cohorts for NB and brain tumors
We are performing studies to identify mechanisms of RXDX-101
resistance
We are also performing combination studies with RXDX-101 and 1)
conventional chemotherapy, 2) other RTK inhibitors, 3) signaling
inhibitors, and 4) other targeted agents in the pediatric pipeline
30
RXDX-101: Conclusions |
 General Mechanisms of Resistance to RTK Inhibitors
Target-dependent mechanisms:
-
Mutations/rearrangement/
alternate
splicing
that
block
inhibitor
binding
-
Amplification, overexpression of target RTK
Target-independent mechanisms: bypass pathways
-
Activation of another RTK
-
Activation of downstream signaling: Ras/Raf/Mek/Erk, PI3K/Akt, mTOR
Target-independent
mechanisms:
EMT,
differentiation
Target-independent mechanisms: resistance to apoptosis
31 |
 32
Targeted Therapy for Patients with Genomic Alterations
Involving NTRK 1/2/3, ROS1, ALK, and RET
Alexander Drilon, MD
Attending
Physician,
Thoracic
Oncology
Service
and
Developmental
Therapeutics
Memorial Sloan-Kettering Cancer Center, New York, NY |
 33
Targeted Therapy In Oncology
A Rapidly Expanding Field |
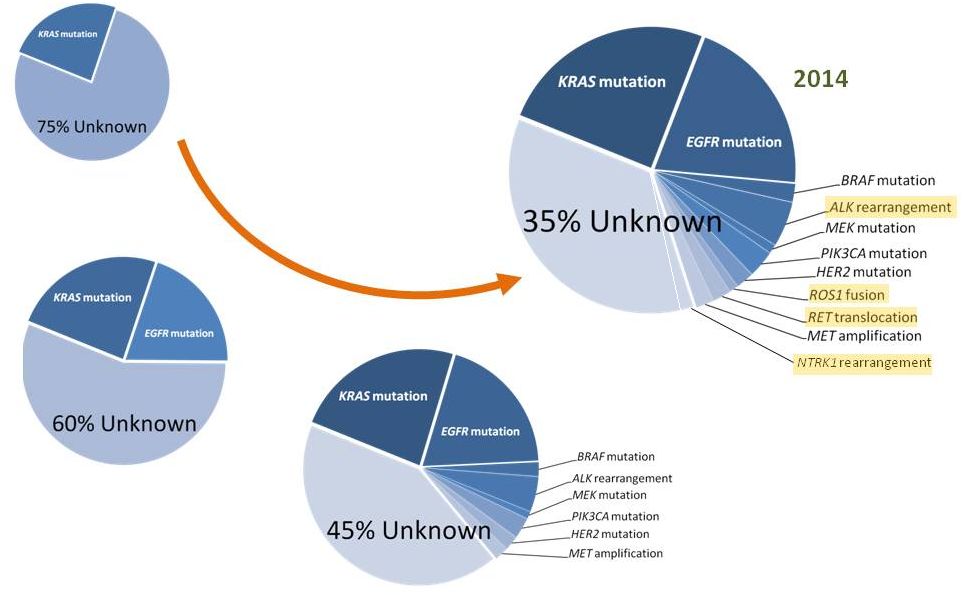 1999
2004
2009
Timeline of Driver Discovery in Lung Cancers
34
Drilon Am J Hematol Oncol 2014 |
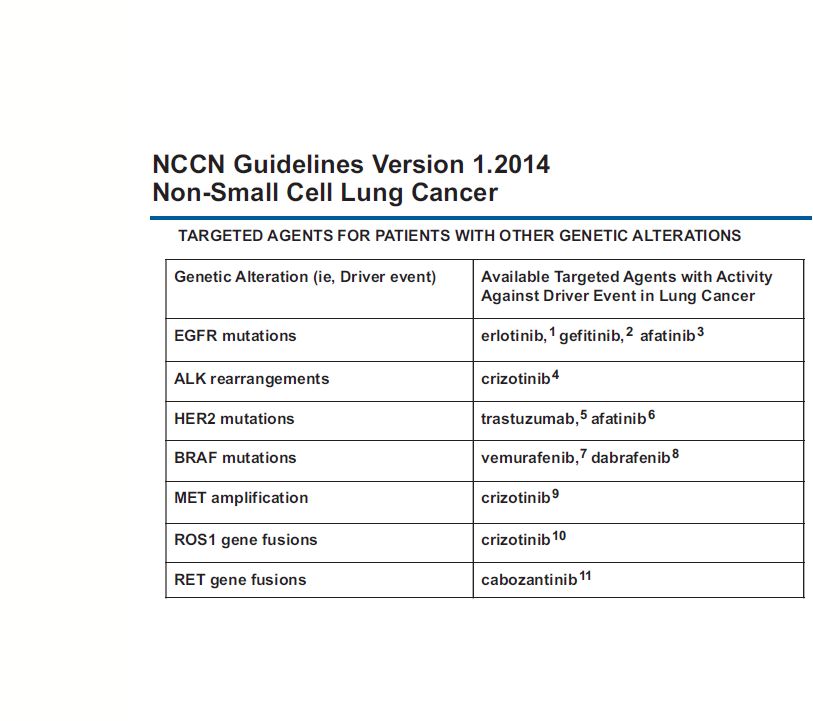 Targeted
Therapy in Lung Cancers 35
NCCN Guidelines NSCLC 2014 |
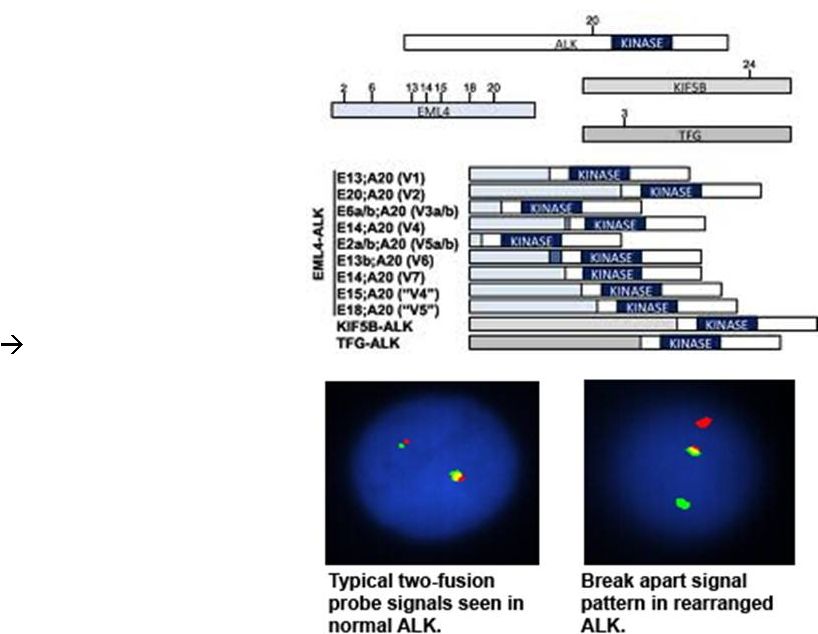 ALK-
•
recurrent gene rearrangements
•
varying upstream partners
•
intact ALK
TK domain
•
oncogenic in vivo and in vitro,
expression in transgenic mouse
model
multiple lung
adenocarcinomas
•
3-5% of NSCLCs, commonly in
adenocarcinomas from never or
former light smokers
•
efficacy of ALK inhibition is well-
established
36
Shaw Oncogene 2014
Rearranged
Lung Cancers |
 ALK
•
ALK-
rearranged
cancers
–
5% NSCLCs (EML4-ALK)
–
50% adult ALCL (NPM-ALK)
–
50% inflammatory myofibroblastic
tumor (TPM3-ALK)
–
10% spitzoid neoplasms (TPM3-ALK)
–
breast CA, esophageal SCC, renal cell
CA, colorectal CA, DLBCL
•
ALK-mutated cancers
–
10% anaplastic thyroid
–
10% sporadic NBL
–
100% hereditary NBL
–
missense, kinase domain
•
ALK inhibition
–
ORR 60-80%, PFS 8 months in ALK-
rearranged NSCLCs
–
phase 3: superior to chemo
–
responses in ALK-rearranged ALCL
–
CR in ALK-mutated neuroblastoma
Mosse Lancet Oncol 2013, Butrynski NEJM 2010, Rikova J Clin Oncol 2007, Shaw NEJM 2013
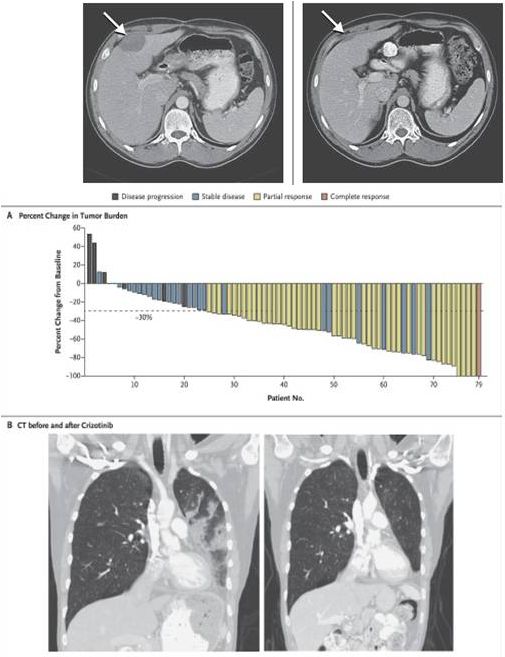
44/M IMT
containing
RANBP2-
ALK
with
PR to
crizotinib
37
ALK inhibition with crizotinib in ALK-fused NSCLC
Alterations |
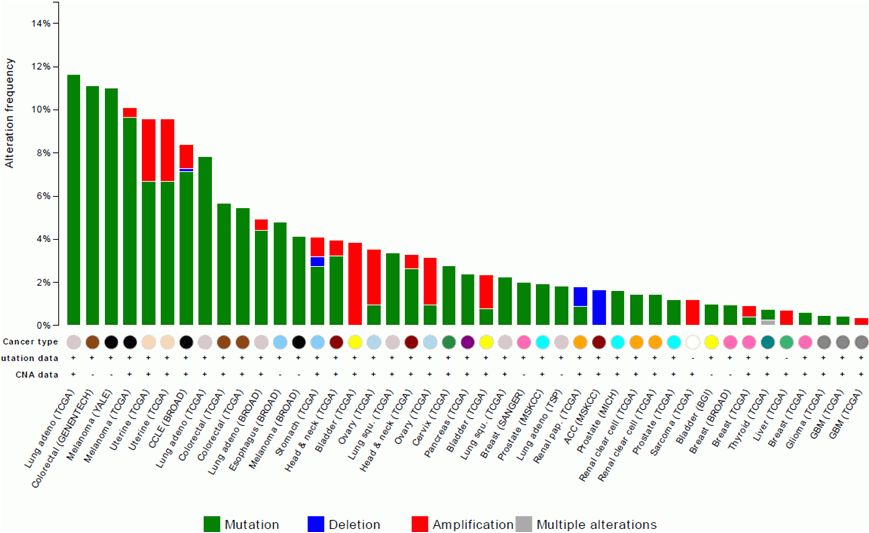 ALK
Alterations
38
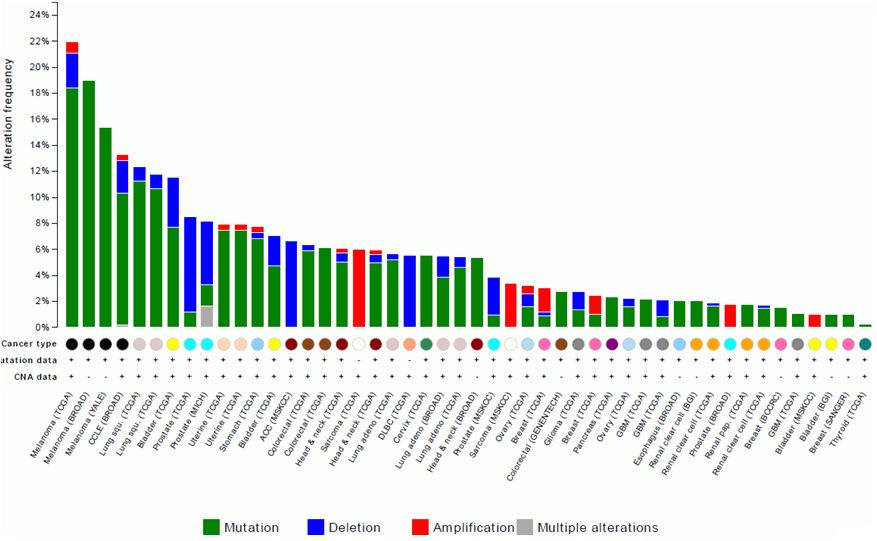
cBio Portal, accessed 9/2014 |
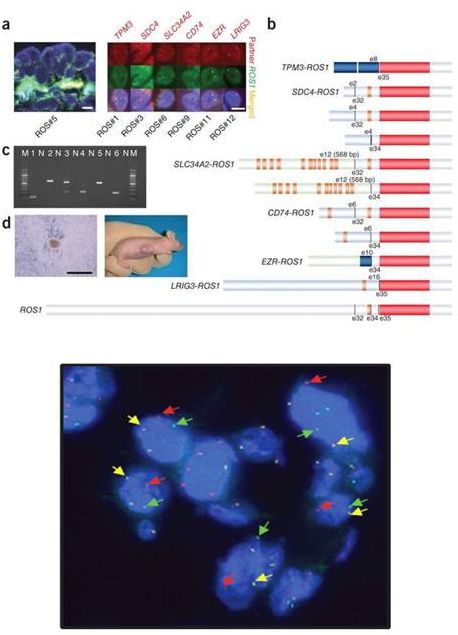 ROS1-
•
fusion biology similar to ALK
•
ROS1
TK domain intact, partners
w/ transmembrane & coiled-coil
domains
•
oncogenic in vivo and in vitro
•
1-2% of NSCLCs: adenoCA, never
or former light smokers
•
strong efficacy data for ROS1
inhibition
39
Shaw Oncogene 2014, Gu PLoS One 2011, Bergethon JCO 2012
Lung Cancers
Rearranged |
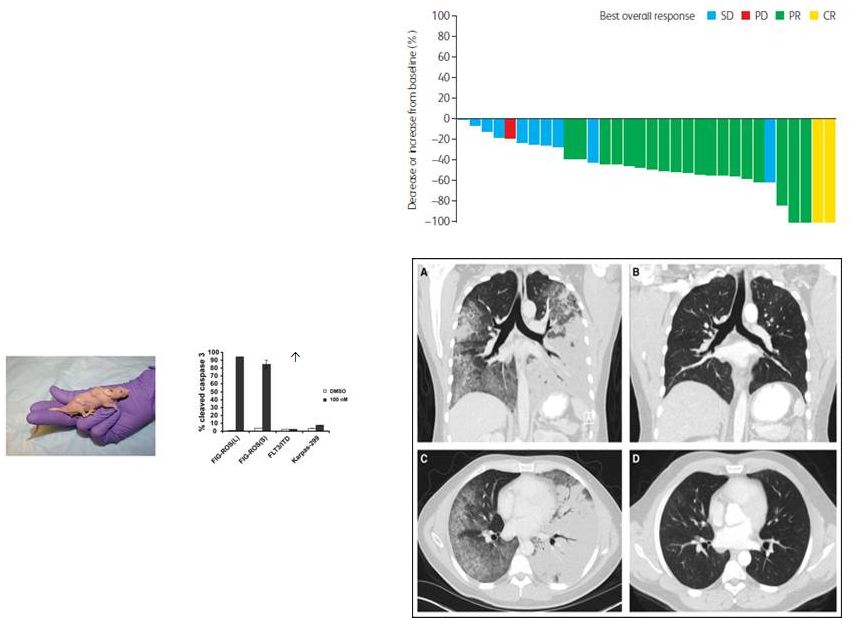 ROS1
Alterations Charest Genes Chrom Cancer 2003, Gu PLoS One 2011, Bergethon JCO
2012, Shaw ASCO 2012 •
ROS1-rearranged cancers
–
1-2% NSCLCs (CD74-ROS1)
–
9% cholangiocarcinoma (FIG-ROS1)
–
17% spitzoid neoplasms (TPM3-ROS1)
–
1-2% glioblastoma (FIG-ROS1)
–
ovarian CA, gastric CA, colorectal CA,
IMT, angiosarcoma, epithelioid
hemangioendothelioma
•
ROS1 inhibition
–
response rate ~60%
–
comparable to efficacy in ALK
fusion-
positive lung cancers
ROS1-rearranged cholangioCA
TAE64
apoptosis
40
ROS1 inhibition with crizotinib in ROS1-fused NSCLC |
 ROS1
Alterations
41
cBio Portal, accessed 9/2014 |
 For Cancers
With NTRK, ROS1, and ALK Alterations
RXDX-101
42 |
 RXDX-101
(NMS-E628) •
oral multikinase
inhibitor
•
activity against TRKA,
TRKB, TRKC, ROS1, and
ALK
•
ongoing Trials
–
Phase I First-In-Human Trial
(Italy)
–
Phase I/II Trial of
Continuous Dosing (US)
Kinase
IC50 nM
TRKA
1.7
TRKB
0.1
TRKC
0.1
ROS1
0.2
ALK
1.6
RET, VEGFR, IGF1R,
FGFR1, FLT3
>10 fold
selectivity
43 |
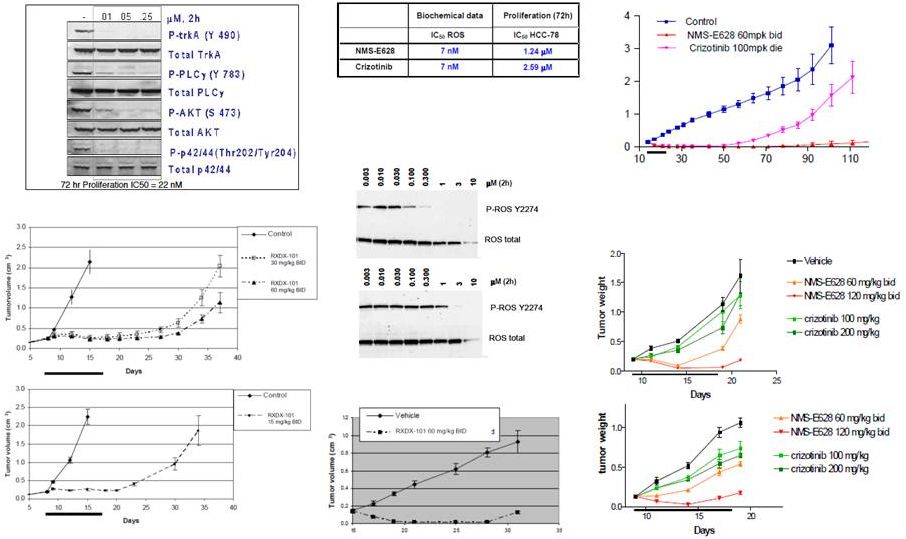 RXDX-101
(NMS-E628) Preclinical Activity EML4-ALK
in vivo (NCI-H228 lung cancer xenografts)
ALK C1156Y
and L1196M
in vivo (Ba/F3 in SCID mice)
crizotinib
in vitro (Ba/F3 expr TEL-ROS1)
TEL-ROS1
SLC34A2-ROS1
in vivo (Ba/F3 in SCID mice)
in vitro (HCC78 lung cancer cell line)
44
TPM-TRKA
in vivo (KM12 colorectal xenograft)
in vitro (KM12 colorectal cell line)
RXDX-101 |
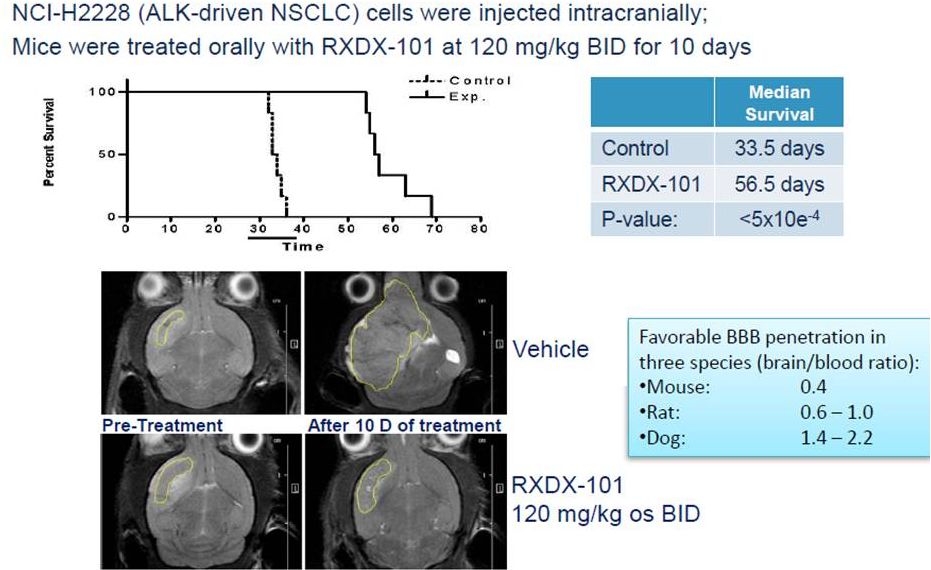 RXDX-101
and Blood Brain Penetration 45 |
 ALKA-372-001:
First-in-Human (FIH) Study of RXDX-101
Break
1
2
3
4
Week
Figure 1a. Schedule A
Dose
Break
1
2
3
4
Week
Figure 1c. Schedule C
1
2
3
4
Week
Figure 1b. Schedule B
-
phase 1, open-label
study of RXDX-101
-evaluating the safety,
PK, antitumor activity
-
advanced/metastatic
solid tumors that harbor
a ROS1, ALK, or NTRK
molecular alteration
46
Dose
Dose |
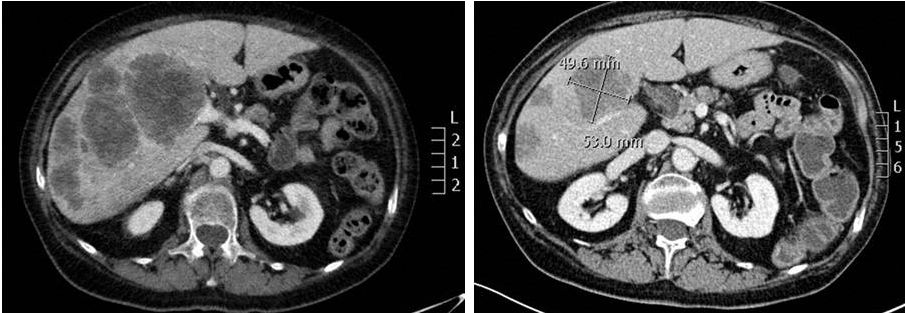 ALKA
First-in Human Study -
75/F, NTRK1-rearranged colorectal cancer, s/p chemotherapy and cetuximab
-
RXDX-101 1600 mg/m
, PR via RECIST, 4 month duration
RXDX-101: Clinical Activity
as a TRK Inhibitor
47
Baseline, 3/2014
Week 4, 4/2014
Data as of ESMO September 2014
2 |
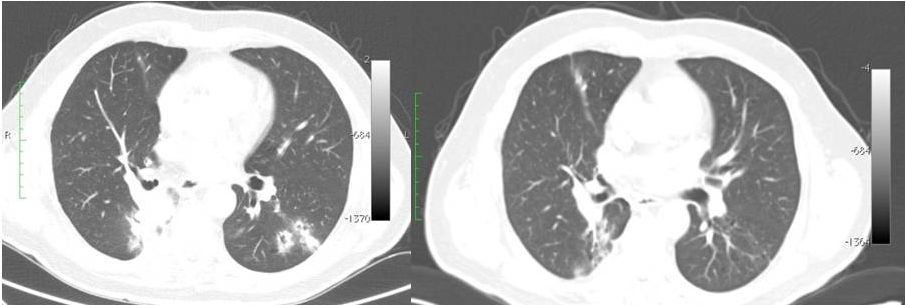 Baseline,
7/2014 Week 4, 8/2014
48
RXDX-101: Clinical Activity
as a ROS1 Inhibitor
ALKA First-in Human Study
-
ROS1-
-
partial response via RECIST, ongoing benefit at 3+ months
Data as of ESMO September 2014
2
rearranged NSCLC: RXDX-101 400 mg/m
|
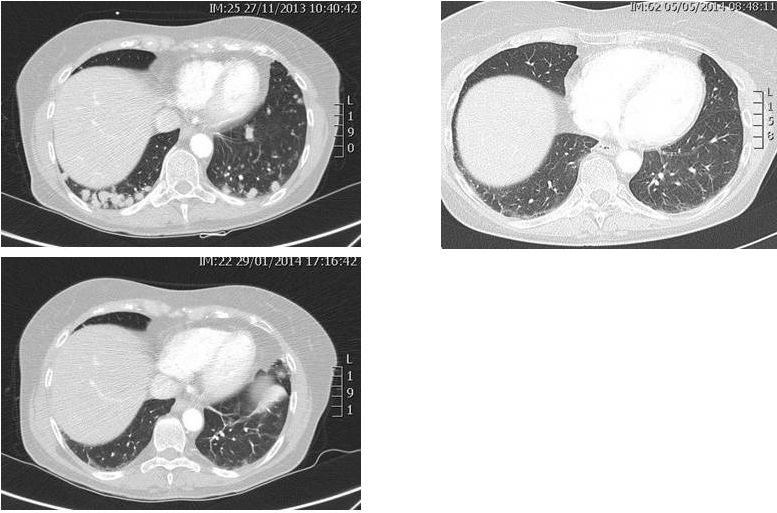 ALKA
First-in Human Study -
63/F ALK-rearranged NSCLC
(s/p 4 cycles chemo, crizotinib)
-
RXDX-101 1200 mg/m
-
PR via RECIST
-
11 months on therapy with
continued disease control
Baseline, 11/2013
Week 8, 1/2014
Week 20, 5/2014
RXDX-101: Clinical Activity
as an ALK Inhibitor
49
Data as of ESMO September 2014
2 |
 Clinical Antitumor
Activity in Each of TrkA, ROS1 and ALK Patients
PD at C11
PD at C4
50
* Unconfirmed
** All
PRs
were
in
Schedule
A
except
1
patient
with
ROS1+
NSCLC
in
Schedule
C
*** CR was in Schedule C
Timing of PR
Timing of CR
Data as of ESMO September 2014
Dose
(mg/m²)
Treatment Cycles / Months
200
400
800 1200
PR
200
400
800
SD
400
800
SD
1200
800
PR
1200
PR
1600
PR
400**
PR
400***
CR*
2
4
6
8
10
12
14
16
18
20
22
24
Neuroblastoma
(ALK)
NSCLC
(ALK)
Pancreatic
(ROS1)
NSCLC
(ALK)
NSCLC
(ROS1)
CRC
(TrkA)
NSCLC
(ROS1)
NSCLC
(ROS1)
Tumor type
(Alteration)
Best
Response |
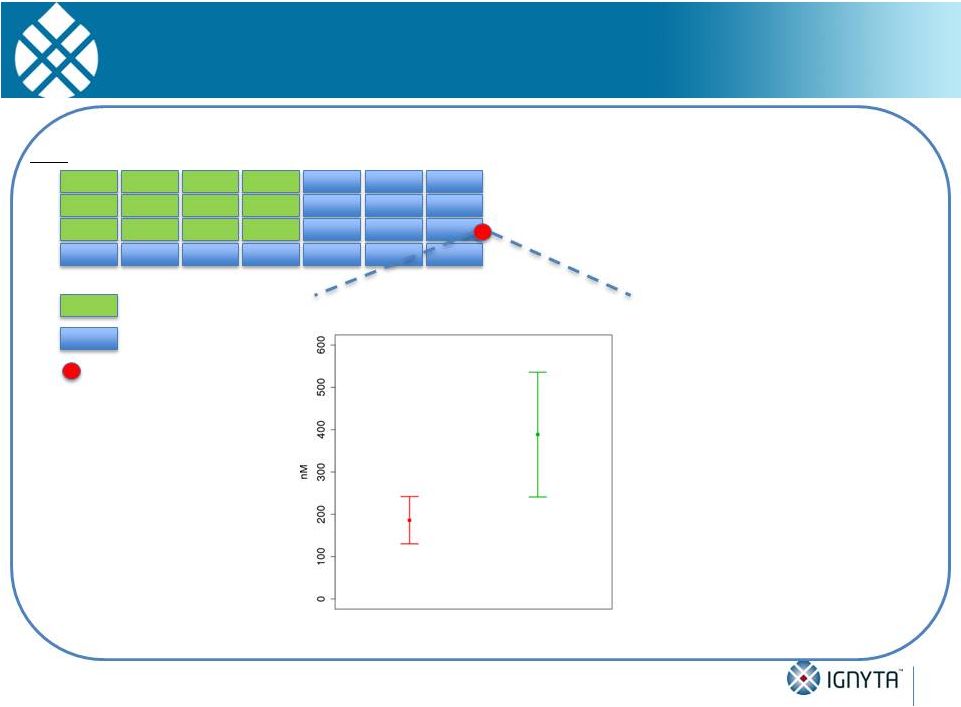 Responders Tend to Have Higher Exposure than
Non-Responders Throughout the Entire Dosing Cycle
Note:
Analysis
includes
patients
in
the
800,
1200
and
1600
mg/m
cohorts;
mean
is
represented
by
square,
and
standard error of the mean is represented by error bars
Dose
1
2
3
4
Week
Non-responders
(n = 6)
Responders
(n = 3)
Day 18, T
96h
RXDX-101 Exposure Levels of
Responders and Non-Responders
Intermittent Dosing Schedule (Schedule A, fasted)
(4 days on, 3 days off, 1 week break)
The following may further increase
RXDX-101 exposure:
•
•
•
Additional PK Optimization
51
Break
Fed (vs. fasted) state
New formulation
Continuous dosing schedules
without breaks
2 |
 STARTRK-1
Dose Escalation
•
n = 20-30 patients
•
Advanced solid tumors
•Genetic alterations in
NTRK1/2/3, ROS1, ALK
Expansion
•
Patients treated at
RP2D
•
n = 20 patients per
expansion cohort
ALK+ pre-treated
ROS1+ cohort
TRKA+ cohort
TRKB/C+ cohort
Endpoints
Primary:
I -
MTD/RP2D, DLTs
Secondary: Safety, PKs, PFS, OS, RR
3+3 Design
ALK+ TKI naïve
Dose
1
2
3
4
Week
Continuous Daily
Schedule
52
Dose
Escalation
Study Targeting ALK, ROS1, TRKA/B/C
|
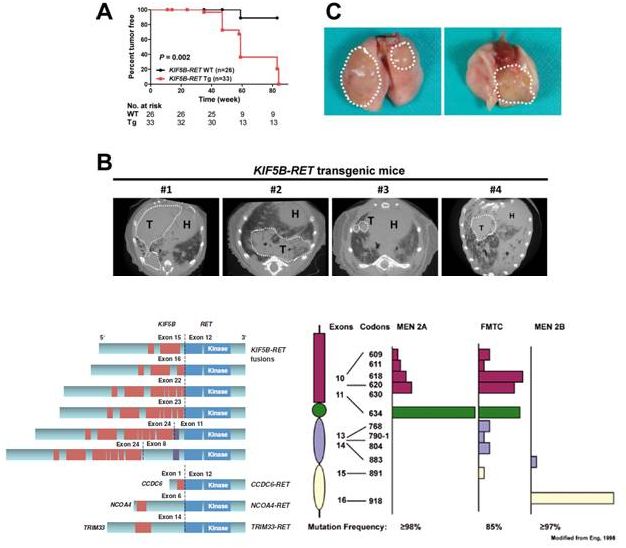 RET
Alterations in Cancer
Drilon et al
Cancer Disc 2013;3:630, Saito et al Carcinogenesis 2014
•
Ranged
–
20-40% papillary thyroid
cancers
–
60-80% radiation-induced
papillary thryoid cancers
–
1-2% of NSCLCs
–
chronic myelomonocytic
leukemia
–
Oncogenic in vitro
and in
vivo:
multiple
lung
cancers
in
transgenic mice expressing
KIF5B-RET
•
RET-mutant
cancers
–
medullary thyroid CAs:
–
MEN2A 100%
–
MEN2B 100%
–
FMTC 100%
53
•
RET-
•
RET-
rearranged cancers
mutant cancers |
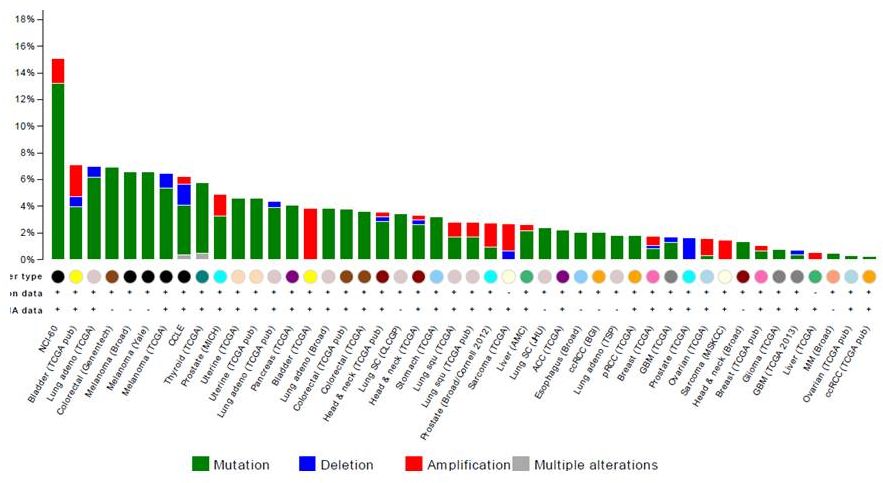 RET
Alterations
54
cBio Portal, accessed 9/2014 |
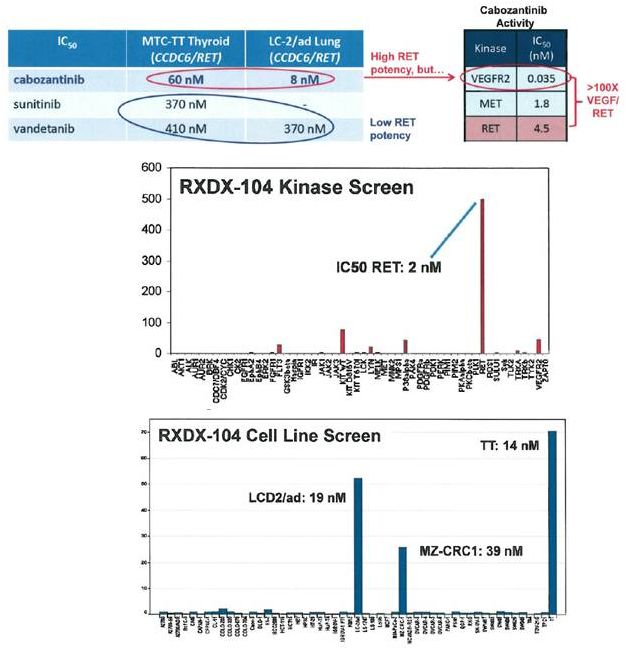 RXDX-104: A
Potent RET
Inhibitor
•
RET inhibition
–
pre-clinical activity of various RET
inhibitors, documented clinical
responses to RET inhibition
–
issues with tolerance secondary
to VEGFR activity of RET TKIs and
associated adverse events
•
RXDX-104
–
potent, highly-selective RET
inhibitor (2 nM)
–
Good selectivity in panel of 54
tyrosine/serine-threonine kinases
–
active against RET-mutated and
RET-rearranged cell lines
•
LC-2/ad (CCDC6-RET lung)
•
MZ-CRC1 (M918T CRC)
•
TT (C634W thyroid)
55 |
 56 |
