Attached files
| file | filename |
|---|---|
| EXCEL - IDEA: XBRL DOCUMENT - ThermoGenesis Holdings, Inc. | Financial_Report.xls |
| EX-31.2 - EXHIBIT 31.2 - ThermoGenesis Holdings, Inc. | ex31_2.htm |
| EX-31.1 - EXHIBIT 31.1 - ThermoGenesis Holdings, Inc. | ex31_1.htm |
| EX-23.1 - EXHIBIT 23.1 - ThermoGenesis Holdings, Inc. | ex23_1.htm |
| EX-32 - EXHIBIT 32 - ThermoGenesis Holdings, Inc. | ex32.htm |
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15 (d) OF THE
SECURITIES EXCHANGE ACT OF 1934
For the Fiscal Year Ended: June 30, 2014
Commission File Number: 000-16375
Cesca Therapeutics Inc.
(Formerly known as ThermoGenesis Corp.)
(Exact name of registrant as specified in its charter)
|
Delaware
|
|
94-3018487
|
|
(State of incorporation)
|
(I.R.S. Employer Identification No.)
|
2711 Citrus Road
Rancho Cordova, California 95742
(Address of principal executive offices) (Zip Code)
(916) 858-5100
(Registrant’s telephone number, including area code)
Securities Registered Pursuant to Section 12(b) of the Act:
|
Title of each class
|
Name of each exchange on which registered
|
|
Common Stock, $0.001 par value
|
Nasdaq Stock Market, LLC
|
Securities Registered Pursuant to Section 12(g) of the Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. o Yes x No
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. o Yes x No
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. x Yes o No
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files.) x Yes o No
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K, is not contained herein, and will not be contained, to the best of the registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment of this Form 10-K. o
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer” and “small reporting company” in Rule 12b-2 of the Exchange Act.
|
Large accelerated filer o
|
Accelerated filer o
|
|
Non-accelerated filer o (Do not check if a smaller reporting company)
|
Smaller reporting company x
|
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act)
o Yes x No
The aggregate market value of the common stock held by non-affiliates as of December 31, 2013 (the last business day of the most recently completed second quarter) was $16,790,804 based on the closing sale price on such day.
As of September 26, 2014, 40,268,811 shares of the registrant’s Common Stock were outstanding.
Documents Incorporated By Reference: Portions of the registrant’s proxy statement for its 2014 Annual Meeting of Stockholders are incorporated by reference into Part III hereof.
|
Part I
|
|
|
|
|
|
Page Number
|
|
ITEM 1.
|
2
|
|
|
ITEM 1A.
|
16
|
|
|
ITEM 1B.
|
26
|
|
|
ITEM 2.
|
26
|
|
|
ITEM 3.
|
26
|
|
|
ITEM 4.
|
27
|
|
|
|
|
|
|
Part II
|
|
|
|
|
|
|
|
ITEM 5.
|
28
|
|
|
ITEM 6.
|
29
|
|
|
ITEM 7.
|
30
|
|
|
ITEM 7A.
|
38
|
|
|
ITEM 8.
|
39
|
|
|
ITEM 9.
|
66
|
|
|
ITEM 9A.
|
66
|
|
|
ITEM 9B.
|
67
|
|
|
|
|
|
|
Part III
|
|
|
|
|
|
|
|
ITEM 10.
|
68
|
|
|
ITEM 11.
|
68
|
|
|
ITEM 12.
|
68
|
|
|
ITEM 13.
|
68
|
|
|
ITEM 14.
|
68
|
|
|
|
|
|
|
Part IV
|
|
|
|
|
|
|
|
ITEM 15.
|
69
|
PART I
All dollar amounts are presented in thousands except as otherwise noted.
Business Overview
Cesca Therapeutics Inc. (the “Company”, “we”, “our”, formerly known as ThermoGenesis Corp) is focused on the research, development, and commercialization of autologous cell-based therapeutics for use in regenerative medicine. We are a leader in developing and manufacturing automated blood and bone marrow processing systems that enable the separation, processing and preservation of cell and tissue therapy products. We focus in three target markets to serve patients, physicians and partners:
• Cellular Therapeutics
• Medical/Diagnostic Device Development and Commercialization
• Cell Manufacturing and Banking
On February 18, 2014, TotipotentRX (“TRX”) Corporation merged with and into ThermoGenesis Corp. In connection with the merger, ThermoGenesis changed its name from ThermoGenesis Corp. to Cesca Therapeutics Inc. The Company believes that TotipotentRX has the depth of clinical, scientific and biological engineering experience necessary to commercialize cell therapies with diseases having significant unmet medical needs. As a result of the merger, Cesca is a fully integrated regenerative medicine company with the ability and expertise to research, design, and develop devices and disposables necessary to facilitate clinical protocols and applications directed at cell therapies at the point of care.
Our business strategy includes:
• Practical, Commercializable Cell Therapies. Deliver proprietary, commercially viable, highly effective autologous (patient’s own cells) cell therapies to treat major medical diseases.
• Ability to Rapidly and Cost-Effectively Implement New Clinical Trials. Rapidly initiate early clinical development of new cell therapies at its United States Food and Drug Administration (“FDA”)-registered clinical research organization in India and generate high quality data at a fraction of the cost of clinical trials undertaken in the U.S. or Europe.
• Positioned to Commercialize in Both Developed and Emerging Markets. Utilize our existing U.S. and Asian footprints to uniquely position us to meet the needs of patients, hospitals and physicians across the globe. This footprint allows flexibility to meet the variable market demands in service and price.
• Proprietary and Protected. Possess an unmatched suite of proprietary technological and clinical assets to be deployed in the regenerative medicine markets. Our cell-therapy-related devices and platform technologies, unique cell formulations and treatment protocols are protected via a broad portfolio of patents and intellectual property filings.
Cesca Therapeutics Inc., formerly ThermoGenesis Corp., was founded in 1986, and our principal executive offices are located at 2711 Citrus Road, Rancho Cordova, California 95742. Unless otherwise indicated, information regarding us and our business includes information regarding TotipotentRX Corporation which merged with and into us on February 18, 2014.
Key Events and Accomplishments
In addition to the merger with TotipotentRX discussed above, the following are key events and accomplishments that occurred in fiscal 2014:
|
·
|
Announced Statistically Significant Phase 1b Clinical Trial Results in Critical Limb Ischemia
|
The trial achieved both its primary safety and secondary efficacy endpoints at 12 months, achieving statistical significance in five key areas including, major amputation free survival rates (82.4%), both resting and walking pain reduction, improved walking distance, open wound healing and vasculogenesis (generation of new blood vessels) in the treated leg. Also, there were no serious adverse events determined to be related to the therapy.
|
·
|
Raised $16 million in Net Proceeds From Two Stock Offerings
|
On January 30, 2014, we completed a private placement of 3,336,800 shares of our common stock at $2.00 per share, together with warrants to purchase up to an aggregate of 1,668,400 shares of common stock. The warrants may be exercised at a price of $2.81 per share until January 29, 2019. Net proceeds after expenses from the offering were approximately $5.9 million.
On June 18, 2014, we completed a public offering of 7,530,000 shares of common stock at $1.50 per share, together with warrants to purchase up to an aggregate of 2,259,000 shares of common stock. The warrants may be exercised at a price of $1.55 per share until June 18, 2019. Net proceeds after expenses from the offering were approximately $10.1 million.
|
·
|
Formed Clinical and Scientific Advisory Board
|
In May 2014, we formed a Clinical and Scientific Advisory Board (“CSAB”) and appointed Solomon Hamburg MD, Ph.D. to the Board. The CSAB will serve to help set strategic goals for the advancement of research towards the development and commercialization of autologous cellular therapies to improve patient care in the fields of hematology/oncology, cardio/vascular and orthopedic indications.
|
·
|
Signed Direct Agreement with Cord Blood Registry Systems, Inc. (“CBR”)
|
On December 31, 2013, we entered into a Sale and Purchase Agreement with CBR in which we will supply CBR with the AXP cord blood processing system and disposables. The agreement is for 5 years with automatic two-year renewal options unless CBR provides a 6 month notice of non-renewal.
Market Overview
Regenerative Medicine Market
Regenerative cell therapy relies on replacing diseased, damaged or dysfunctional cells with healthy, functioning ones or repairing damaged or diseased tissue. A great range of cells and cell components can serve in cell therapy, including cells found in peripheral blood, umbilical cord blood and bone marrow.
The regenerative medicine market continues to experience meaningful advances in clinical efficacy using cells and cell components as measured by the number of FDA and European Union (“EU”) therapeutic product approvals and product commercialization of cell based therapies. The vast majority of this progress has been achieved through the broader application of adult stem cells, reflecting a greater awareness and appreciation of their therapeutic potential.
Positive results generated from the application of adult stem cells have resulted in greater government and private sector investment in the research and development of new cell therapies, including the continued advancement of existing treatments.
The regenerative medicine market is comprised of companies developing components that harvest, process, purify, expand, modify, cryopreserve, store or administer cells (i.e. devices and methods) or therapeutic providers commercializing cellular therapeutic agents (i.e. cell therapeutics). These cells and cell constituents can be stem cells, modified autologous cells and cell carrier packages for therapeutic cytokines and growth factors, i.e. platelets, cytokine or growth factor(s) as purified biologicals, and gene or plasmid therapies for in vivo production of protein having a direct impact on regeneration. Key success factors in regenerative medicine include:
|
·
|
Target or purified cell recovery rates
|
|
·
|
Efficiency of cell processing, including time
|
|
·
|
Cost of care
|
|
·
|
Product quality and dose specific efficacy
|
|
·
|
Purity, viability and potency of stem cells
|
|
·
|
Obtaining regulatory approval / FDA clearance
|
Generally, cell therapies include a process whereby, target cells are harvested from a donor or patient, further processed or expanded, manufactured into an effective safe dose, and implanted into a patient through a specific device. Cells are processed in the laboratory as well as in the operating room or point-of-care setting. Point-of-care applications involve the processing of patient cells in conjunction with a surgical procedure in an operating room or in an outpatient clinical setting. Requirements for the point-of-care include sterile field packaging, portability, minimal processing steps, predictable target cell recovery rates, and speed of processing. These market requirements must be considered and translated into product features and benefits for successful market adoption. Laboratory applications require Good Manufacturing Practices (“cGMP”), objective quality assurance and the ability to process multiple samples at one time.
The availability of therapeutic cells, including stem cells, at the point-of-care enables physicians to apply cells across an array of applications. In the United States the regulations governing the use of tissue and cells are defined in the Public Health Services Act under Sections 351 and 361. Cells intended to treat patients which are autologous, minimally manipulated, homologous and not combined with another regulated article are categorized as 361 agents and may be prescribed by physicians without a PreMarket Approval (“PMA”) or Biological License Approval (“BLA”). All other cell products are therefore regulated as 351 tissue or cell treatments and can only be used within an approved clinical trial or as defined in the PMA/BLA license. Therefore, many physicians are now choosing to study patient outcomes to understand the benefits of the therapeutic cells under their own independently-sponsored and regulated studies. Such research efforts are growing and include studies using cells derived from bone marrow, peripheral blood, cord blood, adipose, and placenta sources in diverse areas such as spinal fusion, non-healing fractures, wound healing, radiation injury, breast reconstruction and augmentation, cardiovascular applications, peripheral vascular disease and liver disease among many others.
With respect to large market opportunities, we believe that commercial products will come first in orthopedics, cardiology, skin and wound healing, diabetes and central nervous system disorders.
We believe regenerative medicine will be a critical catalyst in addressing the global increase in health care costs. As healthcare costs rise, there has been an increase in efforts to limit expenses by employers, payers and the government. If regenerative medicine therapies can provide a cost-effective alternative to current standards of care, we believe physicians and hospitals will have an incentive to more readily adopt these therapies. The need for baseline clinical and cost data developed through comprehensive studies is critical for the successful adoption of regenerative medicine therapies.
Cord Blood Market
Since the first cord blood transplant was carried out in 1988, stem cells derived from umbilical cord blood have been used in more than 30,000 transplants worldwide to treat a wide range of blood diseases, genetic and metabolic disorders, immunodeficiency’s and various forms of cancer. Today over 4,000 cord blood transplants are performed annually and that number is expected to grow.
Cord blood banks now exist in nearly every developed country, as well as in a large number of developing nations.
Cord blood banking can be divided into two categories; private banks serving individual families and public banks serving the broader public. In some cases a third model exists which is referred to as a hybrid private/public bank. Various hybrid models are possible; however, all derive a portion of their revenue from individual family sales as well as in part from public funding.
Cord blood use in clinical applications is now widely acceptable as a standard treatment for blood-based cancers and genetic disorders; an important but limited application base. To support this usage the FDA has approved several BLA licenses for certain public cord blood products which is considered a critical step in the maturation of the industry as well as a testament to the improvements in clinical cord blood quality. To address further growth and adoption of cord blood as a valuable therapeutic cell source, additional research and clinical trials are essential and currently underway in the United States and other countries.
Therapeutic Products, Approach and Clinical Delivery
Our clinical program is designed with two models of clinical delivery:
|
·
|
SurgWerks® – Rapid Intra-operative Use
|
|
·
|
CellWerks™ – Rapid Laboratory Use under the direction of a licensed physician
|
Our vision is to provide fully optimized therapeutic “kits“, which are under investigational use, ultimately seeking marketing approval with the FDA (or appropriate equivalent in markets outside the U.S.). We believe SurgWerks® and CellWerks™ kits will revolutionize how autologous cellular therapies are administered to patients. At the core of successful clinical outcomes is the achievement to rapidly harvest, process and deliver an autologous therapeutic dose at the bedside. The SurgWerks® process maintains cell viability and potency throughout the process of source material collection, target cell selection, characterization/dose determination, and final delivery of the therapeutic cells to the patient. We are developing a unique and patent protected system designed to achieve cGMP cell manufacturing process control in a rapid 60-90min process either intra-operatively (SurgWerks®) or in the processing lab (CellWerks™).
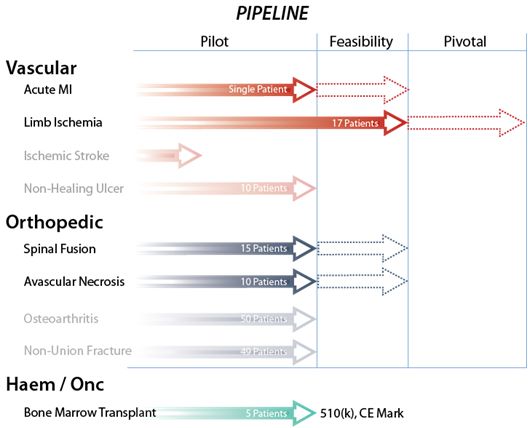
Cesca Clinical Pipeline
The SurgWerks® Platform
We have designed a fully integrated protocol, disposable and equipment product for rapid intra-operative use in 60-90 minutes called SurgWerks.®
SurgWerks consists of the following:
|
·
|
Protocol : A defined standard operating procedure containing step-by-step instructions on the operation of all components necessary to produce a defined cellular dose starting from the autologous collection of source material (i.e. bone marrow) through final delivery to targeted tissue/organ in the same patient.
|
|
·
|
Disposables : A complete sterile “single-use” kit containing all medical disposables for harvesting, processing and delivery of the autologous cells including the testing reagents necessary to ensure the production of a high quality defined cellular dose.
|
|
·
|
Equipment : An easy-to-use equipment “cart” containing all equipment/devices necessary to produce and test the defined cellular dose (i.e. centrifuge for cell processing and purification).
|
We completed the following SurgWerk’s clinical trials in fiscal 2014:
|
·
|
SurgWerks-AMI pilot trial for acute myocardial infarction in patients having low ejection fractions three to ten days after an ST elevated heart attack and having successful reperfusion of the affected heart artery. The goal of this study was to prove proof of principle.
|
|
·
|
SurgWerks-CLI feasibility trial on no-option Rutherford 4 and 5 patients suffering from non-reconstructable critical limb ischemia. This study met the primary endpoints of demonstrating safety, while also demonstrating the salvage of the afflicted limb in 82.4% of the Intent-To-Treat (“ITT”) study patients.
|
We intend to initiate the following SurgWerk’s clinical trials in fiscal 2015:
SurgWerks-AMI feasibility (Phase II) trial on acute myocardial infarction patients having low ejection fractions three to ten days after the heart attack and having successful reperfusion of the affected heart artery.
SurgWerks-CLI pivotal trial on no-option Rutherford 5 patients suffering from non-reconstructable critical limb ischemia.
The company plans to initiate the following SurgWerk’s pre-clinical evaluations in fiscal 2015:
|
·
|
SurgWerks-Stroke pre-clinical development targeting patients with sub-acute ischemic brain injury
|
The CellWerks™ Platform
We offer the CellWerks ™ Platform for the optimal processing of targeted cells used in the treatment of oncological and hematological disorders. The equipment platform includes a “smart vision” control module and a corresponding disposable to process blood and bone marrow sourced tissue.
We plan to complete the following internally sponsored CellWerk’s clinical study in fiscal 2015:
|
·
|
Pilot study in pediatric allogeneic ABO mismatched bone marrow transplant
|
In fiscal 2014 the company completed an optimization of its CellWerks Platform that included several significant upgrades to address the emerging needs of the cell banking, biopharmaceutical and cellular therapeutic manufacturing sectors. Our goal for this Generation II automated platform is that CellWerks will be beneficial to cellular manufacturers in increasing cell yield over currently available commercial cell processing systems without the necessity to add any extraneous chemicals.
Our Product Overview
We design, manufacture and sell advanced devices created specifically for the regenerative medicine bioprocessing market. This market includes biologic collection, transport, processing/washing, characterization/analysis, and cryopreservation. We view the regenerative medicine bioprocessing market as essential to the success of clinical trials through the control of quality of small and large scale cellular manufacturing. Our current product offering includes:
|
·
|
The MarrowXpress® or MXP System, a derivative product of the AXP and its accompanying disposable bag set, isolates and concentrates stem cells from bone marrow. The product is an automated, closed, sterile system that volume-reduces blood from bone marrow to a user-defined volume in 30 minutes, while retaining over 90% of the MNCs, a clinically important cell fraction. Self-powered and microprocessor-controlled, the MXP System contains flow control optical sensors that achieve precise separation. We have received the CE-Mark, enabling commercial sales in Europe, and we received authorization from the FDA to begin marketing the MXP as a Class I device in the U.S. for the preparation of cell concentrate from bone marrow. However, the safety and effectiveness of this device for in vivo use has not been established.
|
|
·
|
The AXP System is a medical device with an accompanying disposable bag set that isolates and retrieves stem cells from umbilical cord blood. The AXP System provides cord blood banks with an automated method to separate and capture adult stem cells which reduce the overall processing and labor costs with a reduced risk of contamination under cGMP conditions. The AXP System retains over 97% of the mononuclear cells (MNCs). High MNC recovery has significant clinical importance to patient transplant survival rates. Self-powered and microprocessor-controlled, the AXP device contains flow control optical sensors that achieve precise separation of the cord blood fractions.
|
|
·
|
The BioArchive System is a robotic cryogenic medical device used to cryopreserve and archive stem cells for future transplant and treatment. Launched in fiscal 1998, our BioArchive Systems have been purchased by over 110 umbilical cord blood banks in over 35 countries to archive, cryopreserve and store stem cell preparations extracted from human placentas and umbilical cords for future use.
|
|
·
|
The Res-Q 60 BMC, is a rapid, reliable, and easy to use product for cell processing. The product is a centrifuge-based disposable device designed for the isolation and extraction of specific stem cell populations from bone marrow. The key advantages of the Res-Q 60 BMC include (a) delivering a high number of target cells from a small sample of bone marrow, and (b) providing a disposable that is highly portable and packaged for the sterile field. These features allow users to process bone marrow to isolate and capture certain cells in 15 minutes. However, the safety and effectiveness of this device for in vivo use has not been established.
|
|
·
|
The Res-Q 60 PRP is designed to be used for the safe and rapid preparation of autologous platelet rich plasma (PRP) from a small sample of blood at the point of care. The product allows PRP to be mixed with autograft and/or allograft bone prior to application to a bony defect in the body. The Res-Q 60 PRP received FDA 510(k) clearance in June of 2011.
|
Cell Manufacturing and Banking Services
At our international subsidiaries, we operate advanced clinical cell manufacturing, processing, testing, and storage facilities compliant with cGMP, Good Tissue Practices (“GTP”), and Good Laboratory Practices (“GLP”). We can support the production of a personalized medicine cell prescription or a large scale batch process. Patient samples, batch samples, and therapeutic aliquots are all labeled in accordance with ISBT 128 and stored in our cryogenics facility. In addition, our clinical research organization (“CRO”) is the only specialized, in-hospital, cell therapy CRO globally. We have the unique expertise in designing, managing, and completing cell based clinical trials including the ability to support various device prototyping and validation typically required in a combination product. These services ensure patient safety under Good Clinical Practices (GCP), quality laboratory documentation under GLP, and quality cell processing and handling under both cGMP and GTP. In partnership with Fortis Healthcare we have assembled the industry’s only fully integrated cell therapy CRO team to execute all elements in our in-house clinical trials, providing complete and seamless cellular drug and device clinical services.
Sales and Distribution Channels
We market and sell our products primarily through independent distributors, except in North America. We utilize integrated distribution arrangements whereby our suite of cord blood products are distributed into specific territories by a single distributor. These arrangements have improved the customer experience by streamlining their product, service and support needs through a single point of contact.
Competition
The regenerative medicine market is characterized by rapidly evolving technology and intense competition from medical device companies, pharmaceutical companies and stem cell companies operating in the fields of cardiac, vascular, orthopedics and neural medicine. The primary competitors for our current product mix include automated cell processing systems from BioSafe SA, MacoPharma, BioE, SynGen and Pall Corporation. Our competitors in the field of cell therapy development are MesoBlast, Ltd., Osiris Therapeutics, Inc., Baxter International, Inc., Athersys, Ltd., Neostem, Inc., Aastrom Biosciences, Inc., Cytori Therapeutics, Inc., Cytomedix, Inc., Pluristem Therapeutics Inc., and Bioheart, Inc.
Research and Development
Our research and development activities in fiscal 2014 focused on AutoXpress AXP and MXP platform improvements, transitioning the platform to Point-of-Care applications, and compliance with new environmental regulations. Also, the activities were aimed to develop or expand contract manufacturing capabilities for low cost disposables and building on our product quality leadership position. Significant investments were made to support product registration in China, Taiwan, India and South Korea. In fiscal 2015, we plan to introduce new features and enhancements to the AXP and MXP platforms to support our clinical trial initiatives and the expansion of the platform applications. Research and development expenses were $3,468, $2,991 and $3,729 for the years ended June 30, 2014, 2013 and 2012, respectively. Research and development activities include expenses related to engineering, regulatory, scientific and clinical affairs.
Manufacturing
Our long-term manufacturing strategy continues to utilize high quality, low cost contract manufacturers for production of high volume, consumable products while maintaining in-house manufacturing capabilities for low volume, high complexity devices. We will continue to evaluate in-house manufacturing versus out-sourcing programs to balance cost, quality, capacity and assurance of supply. As we expand our product offering in the point-of-care area, our third party sourcing of complex, hardware devices will increase. This will be accomplished by signing strategic, long-term supply agreements.
Quality System
Our quality system is compliant with domestic and international standards and is appropriate for the specific devices we manufacture. Our corporate quality policies govern the methods used in, and the facilities and controls used for, the design, manufacture, packaging, labeling, storage, installation, and servicing of all finished devices intended for human use. These requirements are intended to ensure that finished devices will be safe and effective and otherwise in compliance with the FDA Quality System Regulation (“QSR”) (21 CFR 820) administered by the FDA and the applicable rules of other governmental agencies.
We and our contract manufacturers are subject to inspections by the FDA and other regulatory agencies for compliance with applicable regulations, codified in the QSR which include requirements relating to manufacturing processes, testing, documentation control and other quality assurance processes. Our facilities have undergone International Organization of Standards (“ISO”) 13485:2012 and EU Medical Device Directive (“MDD”) (93/42/EEC) inspections and we have obtained approval to CE-Mark our products. Failure to obtain or maintain necessary regulatory approvals to market our products would have a material adverse impact on our business.
Regulatory Scheme and Strategy
The development, clinical trials and marketing of our cell therapy products are subject to the laws and regulations of the FDA, European Medicine Agencies (EMEA) and other countries including India.
Our trials conducted in India are compliant with the applicable Indian Council for Medical Research, and Ministry of Health Order No. V.25011/375/2010-HR rules specific to oversight and rulemaking related to stem cell research and therapy in addition to requisite institutional ethics board and institutional stem cell committee approvals. Both the U.S. and E.U. regulatory agencies are experienced with accepting Indian clinical trial data. The FDA issued a Final Rule in October 2008 revising §21 CFR 312.120(a) and further clarifying their position in a Guidance Document in March 2012, where they will accept as support for an Investigational new Drug (IND) or application for marketing approval a well-designed and well-conducted foreign clinical study not conducted under a U.S. IND if the study is conducted in accordance with the GCP and where the sponsor is able to validate the data from the study through an onsite inspection by FDA if necessary. GCP includes review and approval by an IEC before initiating a study, continuing review of an ongoing study by an IEC, and obtaining and documenting the freely given informed consent of the subject before initiating a study.
Our regulatory activities focus on obtaining PMA from the FDA or the equivalent via the EMEA and national authorities in Europe as well as other national territories. Therefore, we have designed our studies to comply with the guidelines of these regulatory authorities per the Combination Products as defined by the FDA.
We have a quality and regulatory compliance management system that complies with the requirements of the ISO 13485: 2012 standard, the FDA’s QSR, the EU MDD, the Canadian Medical Device Regulations (“SOR 98-282”), and other applicable local, state, national and international regulations.
Our medical devices are subject to regulation by numerous government agencies, including the FDA and comparable state and foreign agencies. To varying degrees, each of these agencies requires us to comply with laws and regulations governing the development, testing, manufacturing, labeling, marketing, distribution, installation and servicing, clinical testing, post-market surveillance and approval of our products, including investigational, and commercially-distributed medical devices. These international, national, state, and local agencies set the legal requirements for ensuring our products are safe and effective, as well as manufactured, packaged and labeled in conformity with cGMP established by the FDA, as well as comparable regulations under the MDD of the EU. Virtually every activity associated with the manufacture and sale of our products and services are scrutinized on a defined basis and failure to implement and maintain a Quality Management System could subject the Company to civil and criminal penalties.
Class III Devices
Before certain medical devices may be marketed in the U.S., they must be approved by the FDA. FDA approval depends on the classification of the device. If the product is a Class III device, such as the SurgWerks-CLI therapy kit, the FDA approval process includes the following:
|
·
|
Extensive pre-clinical laboratory and animal testing,
|
|
·
|
Submission and approval of an Investigational Device Exemption (“IDE”) application,
|
|
·
|
Human clinical trials to establish the safety and efficacy of the medical device for the intended indication, and
|
|
·
|
Submission and approval of a PMA application to the FDA.
|
Pre-clinical trials typically include laboratory evaluation, through in vitro and in vivo animal studies, to obtain safety and if possible dosage information about the product to justify future clinical trials in human subjects. Safety testing is performed to demonstrate the biocompatibility of the device, particularly if the device is intended to come into contact with blood or other body tissues. Pre-clinical studies must be performed by laboratories which comply with the FDA’s Good Laboratory Practices regulations. The results of the pre-clinical studies are submitted to the FDA as part of an IDE application and are reviewed by the FDA before human clinical trials can begin.
Clinical trials involve the application of the medical device or biologic produced by the medical device to patients by a qualified medical investigator, after approval from an Institutional Review Board (“IRB”), and in certain jurisdictions having authorization for the trial under investigational use. Medical device trials which are conducted inside the U.S. are subject to FDA preapproval under an IDE application (21 C.F.R. Part 812), or an Investigational New Drug (“IND”) application (21 C.F.R. Part 312). Clinical trials conducted outside the U.S., and the data collected therefrom are allowed in accordance with the requirements outlined in 21 C.F.R. Part 312.120.
Medical device clinical trials are typically conducted as a Phase III clinical trial. A Phase II or combined Phase I/II safety pilot trial may be performed prior to initiating the Phase III clinical trial to determine the safety of the product for specific targeted indications or dosage optimization studies. The FDA, the clinical trial sponsor, the investigators, the IRB or the Data Safety Monitoring Board may suspend clinical trials at any time if any one of them believes that study participants are being exposed to an unacceptable health risk.
The combined results of product development, pre-clinical studies, and Phase III clinical studies are submitted to the FDA as a PMA application for approval of the marketing and commercialization of the medical device in the U.S. The FDA may deny the approval of a PMA application if applicable regulatory criteria are not satisfied or it may require additional clinical testing. Even if the appropriate data is submitted, the FDA may ultimately decide the PMA application does not satisfy the criteria for approval. Product approvals, once obtained, may be withdrawn if compliance with regulatory standards is not maintained or if safety concerns arise after the product reaches the market. The FDA may require post-marketing testing and surveillance programs to monitor the effect of the medical devices that have been commercialized and has the power to prevent or limit future marketing of the product based on the results of such programs.
Class II Devices
Several of our medical devices, including the BioArchive, Res-Q 60 PRP and AXP are categorized as Class II. These devices have a lower potential safety risk to the patient, user, or caregiver. A PMA submission is not a requirement for these devices. A simpler and shorter process of premarket notification, known as a 510(k) submission, is required to demonstrate substantial equivalence to another legally U.S. marketed device. Substantial equivalence means that the new device is at least as safe and effective as the predicate. Once the FDA has notified us that the product file has been cleared, the medical device may be marketed and distributed in the U.S.
Class I Devices
Some of our products, including the MXP and Res-Q 60 BMC that have minimal risk to the intended user have been deemed by the FDA as being exempt from FDA approval or clearance processes. While submissions to the FDA are not a requirement for Class I devices (low risk), compliance with the QSR is still mandated.
Other U.S. Regulatory Information
Failure to comply with applicable FDA requirements can result in fines, injunctions, civil penalties, recall or seizure of products, total or partial suspension of production or loss of distribution rights. It may also include the refusal of the FDA to grant approval of a PMA or clearance of a 510(k). Actions by the FDA may also include withdrawal of marketing clearances and possibly criminal prosecution. Such actions, if taken by the FDA, could have a material adverse effect on our business, financial condition, and results of operation.
Each manufacturing establishment must register with the FDA and is subject to a biennial inspection for compliance with the Federal Food, Drug, and Cosmetic Act and the QSRs. In addition, each manufacturing establishment in California must be registered with the California State Food and Drug Branch of the California Department of Public Health and be subject to an annual inspection by the State of California for compliance with the applicable state regulations. Companies are also subject to various environmental laws and regulations, both within and outside the U.S. Our operations involve the use of substances regulated under environmental laws, primarily manufacturing. Workplace safety, hazardous material, and controlled substances regulations also govern our activities. We have a California Environmental Protection Agency Identification number for the disposal of bio-hazardous waste from our research and development biological lab. Our cost associated with environmental law compliance is immaterial. The California State Food and Drug Branch of the California Department of Public Health completed a quality system compliance audit resulting with zero observations in fiscal 2011. The FDA audited us in fiscal 2012 resulting in two minor non-conformances that were resolved before the end of the audit.
International Regulatory Requirements
Internationally, we are required to comply with a multitude of other regulatory requirements. These regulations may differ from the FDA regulatory scheme. In the EU, a single regulatory approval process has been created and approval is represented by the CE-Mark. To be able to affix the CE-Mark to our medical devices and distribute them in the EU, we must meet minimum standards for safety and quality (known as the essential requirements) and comply with one or more conformity rules. A notified body assesses our quality management system and compliance to the MDD. Marketing authorization for our products is subject to revocation by the applicable governmental agency or notified body under the EU which are subject to annual audit confirmations with respect to our quality system.
In India, the regulatory body having oversight of medical devices, therapies, and cell banking is the Central Drugs Standard Control Organization (“CDSCO”), and specifically the Drugs Controller General India office. Our marketing and facilities licenses are subject to revocation as allowed by state and national laws by the applicable state Drug Controller in Haryana or DCGI.
Patents and Proprietary Rights
We believe that patent protection is important for our products and our current and proposed business. In the U.S., we currently hold 14 patents, and have 5 patents pending to protect our products. It is our policy to seek foreign patent protection in relevant markets around the world.
Patent positions of regenerative medicine companies, such as ours, are uncertain and involve complex and evolving legal and factual questions. The coverage sought in a patent application can be denied or significantly reduced either before or after the patent is issued. Consequently, there can be no assurance that any of our pending patent applications will result in an issued patent. There is also no assurance that any existing or future patent will provide significant protection or commercial advantage, or whether any existing or future patent will be circumvented by a more basic patent, thus requiring us to obtain a license to produce and sell the product. Generally, patent applications can be maintained in secrecy for at least 18 months after their earliest priority date. In addition, publication of discoveries in the scientific or patent literature often lags behind actual discoveries. Therefore, we cannot be certain that we were the first to invent or the first to file a patent application for the subject matter covered by each of our pending U.S. and foreign patent applications.
If a third party files a patent application relating to an invention claimed in our patent application, we may be required to participate in an interference or derivation proceeding conducted by the U.S. Patent and Trademark Office to determine who owns the patent. Such proceeding could involve substantial uncertainties and cost, even if the eventual outcome is favorable to us. There can be no assurance that our patents, if issued, would be upheld as valid in court.
Certain Agreements
The following are certain agreements involving our business.
Fortis Healthcare Limited (“Fortis”)
On August 1, 2014 we entered into an agreement with Fortis which renews and expands their existing agreement in the areas of cord blood banking services, point-of-care technology sales and support services, bone marrow transplant technology and laboratory services, and clinical/patient management of clinical trials for our internally developed therapeutics and third party marketed clinical research organization services. The term of the agreement is for three years.
Cord Blood Registry Systems, Inc. (“CBR”)
On December 31, 2013, we entered into a Sale and Purchase Agreement with CBR in which we will supply CBR with the AXP cord blood processing system and disposables. The term of the agreement is for 5 years with automatic two-year renewal options unless CBR provides a 6 month notice of non-renewal. Additionally, we entered into the Fourth Amended and Restated Technology License and Escrow Agreement to delete or reduce the financial covenants that we must meet in order to avoid an event of default to one financial covenant, maintain a cash balance and short-term investments net of debt or borrowed funds of not less than $2,000 at any month end.
In June 2010, we entered into a License and Escrow Agreement with CBR as a method to provide assurances to CBR of continuity of product delivery and manufacturing for CBR’s business, and to alleviate concerns about long term supply risk. We are the sole provider to CBR of devices and disposables used in the processing of cord blood samples in CBR’s operations. Under the agreement, we granted CBR a non-exclusive, royalty-free license to certain intellectual property necessary for the potential manufacture and supply of AXP devices and certain AXP disposables. The license is for the sole and limited purpose of manufacturing and supplying the AXP and related disposables for use by CBR. The licensed intellectual property will be maintained in escrow and will be released to and used by CBR if and only if we default under the agreement.
Golden Meditech
In August 2012, we entered into a Product Purchase and International Distributor Agreement with Golden Meditech. Under the terms of the agreement, Golden Meditech obtained the exclusive, subject to existing distributors and customers, rights to develop an installed base for our AXP System in specified countries. This right includes the right to distribute AXP Disposable Blood Processing Sets and use rights to the AXP System, and other accessories used for the processing of stem cells from cord blood. Golden Meditech has rights in the People’s Republic of China (excluding Hong Kong and Taiwan), India, Singapore, Indonesia, and the Philippines and may begin selling once relevant approval has been obtained in each respective country. Additionally, Golden Meditech is subject to certain annual minimum purchase commitments. The term of the agreement is for 5 years with one year renewal options by mutual agreement.
Asahi
Effective June 30, 2012 Asahi exercised its option to purchase certain intellectual property rights from us for the CryoSeal System, including, but not limited to, patents and patent applications, trademarks and any and all commercial and technical know-how. The intellectual property rights were sold for $2,000 which was received in August 2012.
In June 2010, we entered into an amendment to a Distribution and License Agreement with Asahi, originally effective March 28, 2005. Under the terms of the amendment, Asahi obtained exclusive rights to distribute the CryoSeal System in South Korea, North Korea, Taiwan, the People’s Republic of China, the Philippines, Thailand, Singapore, India and Malaysia. These rights included the exclusive right to market, distribute and sell the processing disposables and thrombin reagent for production of thrombin in a stand-alone product. We will provide support to Asahi in the form of maintaining manufacturing capabilities of the CryoSeal System until the earlier of when Asahi receives regulatory approval from the Ministry of Health, Labour and Welfare (“MHLW”) or December 31, 2012, upon which we shall have no further obligation to manufacture. Asahi received regulatory approval on August 31, 2011. Asahi shall continue to have the right to manufacture such products in Japan and shall additionally have a non-exclusive right to manufacture such products outside of Japan and would make royalty payments to us for products it manufactures and sells. The amendment extends the agreement eight years with automatic one year renewals. Asahi paid us a $1,000 license fee, which was fully earned and non-refundable as of June 30, 2012. Concurrent with exercising the purchase option, the terms and conditions of the amendment terminated.
Arthrex
In January 2012, we entered into an agreement with Arthrex. Under the terms of the agreement, Arthrex obtained exclusive rights in certain territories to sell, distribute and service our Res-Q 60 System technology for use in the preparation of autologous PRP and BMC for sports medicine applications and orthopedic procedures. We granted Arthrex a limited license to use our intellectual property as part of enabling Arthrex to sell the products. Arthrex will purchase products from us to distribute and service at certain purchase prices, which may be changed after an initial period. The agreement contains purchase minimums that must be met on a yearly basis for Arthrex to maintain its exclusivity. Arthrex also pays a certain royalty rate based upon volume of products sold. The term of the agreement is for five years, subject to an extension right of an additional three years.
BioParadox LLC (“BioParadox”)
In October 2010, the Company and BioParadox entered into a License and Distribution Agreement. Under the terms of the agreement BioParadox obtained exclusive world-wide rights for the use, research and commercialization of the Res-Q technology in the production of PRP in the diagnosis, treatment and prevention of cardiovascular disease. The term of the agreement will depend on the satisfaction by BioParadox of certain milestones, or the payment of extension fees. If certain delivery or financial metrics are not maintained, the agreement requires the Company to place in escrow the detailed instructions for manufacturing the products. BioParadox will have the right to manufacture the product for the cardiac field for the term of the agreement in the event of a default by the Company or if certain on-time delivery metrics or supply requirements are not met.
GEHC
In January 2010, we signed an amendment with GEHC to extend the Amended and Restated International Distribution Agreement, effective February 1, 2010. Under the terms of the amendment, the contract ran through July 31, 2012, GEHC continued to distribute the AXP product line in the U.S., Canada and approximately 25 countries throughout the world, excluding certain countries in Latin America, Asia, CIS, Eastern Europe and the Middle East. The amendment provided incentives for both parties related to sales success, product quality and delivery. Under the original agreement, signed October 13, 2005, we received fees for the rights granted under the agreement. The amounts received are being recognized as revenue on the straight-line method over the initial five year term of the contract.
In January 2012, we signed an amendment, effective August 1, 2012. Under the terms of the amendment, GEHC will continue to distribute the AXP product line in the United States and Canada. The purchase prices for the products are fixed. The amendment will automatically renew for one year terms unless terminated by either party with 90 day notice. On August 26, 2013, the Company sent GEHC a 90 day notice of termination, which terminated the agreement effective November 24, 2013.
In May 2010, we signed a non-exclusive distribution agreement for the Res-Q 60 BMC System with GEHC. Under the agreement, GEHC had the right to distribute the Res-Q 60 BMC in the U.S., excluding orthopedic indications, Canada and 19 European countries. The agreement has a two and a half year term, with automatic one year renewals, unless terminated by either party with six months advance notice. The agreement provides for a price reduction mechanism should we fail to meet certain product quality and delivery metrics. The parties mutually agreed to terminate effective December 31, 2011.
Celling
In September 2008, we signed a distribution agreement for our MXP and Res-Q 60 BMC product lines with Celling. The distribution rights are for the field of use in orthopedic intraoperative or point-of-care applications. The agreement provides Celling with an initial two-year period of exclusive distribution rights in the U.S. and non-exclusive distribution rights throughout the rest of the world, excluding Central and South America, Russia and certain Eastern European countries. The exclusivity period and field of use may be extended under certain circumstances. The parties amended the agreement in July 2009 to provide shared funding for clinical studies to demonstrate the clinical effectiveness of the products in orthopedic applications. The parties amended the agreement in January 2012. The revised distribution rights are world-wide, non-exclusive within field of use for the MXP and exclusive within field of use in the United States and non-exclusive in Mexico for the Res-Q. The parties have until January 31, 2015 to terminate the agreement otherwise it renews for another five years.
New York Blood Center (“NYBC”)/Pall Medical
In March 1997, we and NYBC, as licensors, entered into a license agreement with Pall Medical, a subsidiary of Pall Corporation, as a licensee through which Pall Medical became the exclusive worldwide manufacturer (excluding Japan) for a system of sterile, disposable containers developed by us and NYBC for the processing of hematopoietic stem cells sourced from placental cord blood (“PCB”). The system is designed to simplify and streamline the harvesting of stem cells from umbilical cord blood and the manual concentration, cryopreservation (freezing) and transfusion of the PCB stem cells while maintaining the highest stem cell population and viability from each PCB donation. In May 1999, we and Pall Medical amended the original agreement, and we regained the rights to distribute the bag sets outside North America and Europe under our name. In fiscal 2012, we and NYBC signed an agreement which provides for the equal sharing of royalties between the two parties effective July 1, 2011, except for calendar 2012, in which NYBC received 75% and we received 25%.
Employees
As of June 30, 2014, we had approximately 95 employees, 60 of whom were employed in the U.S. and 35 in India. We also utilize temporary employees throughout the year to address business needs and significant fluctuations in orders and product manufacturing. None of our employees are represented by a collective bargaining agreement, nor have we experienced any work stoppage.
Foreign Sales and Operations
See footnote 8 of our Notes to Consolidated Financial Statements for information on our sales and operations outside of the U.S.
Where you can Find More Information
We are required to file annual reports on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K and other information, including our proxy statement with the Securities and Exchange Commission (“SEC”). The public can obtain copies of these materials by visiting the SEC’s Public Reference Room at 100 F Street, NE, Room 1580, Washington, DC 20549, by calling the SEC at 1-800-732-0330, or by accessing the SEC’s website at http://www.sec.gov. In addition, as soon as reasonably practicable after these materials are filed with or furnished to the SEC, we will make copies available to the public free of charge through our website, www.cescatherapeutics.com. The information on our website is not incorporated into, and is not part of, this annual report.
An investment in Cesca Therapeutics Inc. common stock is subject to risks inherent to our business. The material risks and uncertainties that management believes affect us are described below. Before making an investment decision, you should carefully consider the risks and uncertainties described below together with all of the other information included or incorporated by reference in this report. The risks and uncertainties described below are not the only ones facing us. Additional risks and uncertainties that we are not aware of or focused on or that we currently deem immaterial may also impair our business operations. This report is qualified in its entirety by these risk factors.
If any of the following risks actually occur, our financial condition and results of operations could be materially and adversely affected. If this were to happen, the value of our common stock could decline significantly, and you could lose all or part of your investment.
Risks Related to Our Business
Lack of Demonstrated Clinical Utility of Cord Blood Derived Stem Cells Beyond Hematopoietic Transplantation May Result in a Decline in Demand for Cord Blood Banking Services, Adversely Affecting Sales of Our Products. Transplants using stem cells derived from cord blood and cord tissue have become a standard procedure for treating blood cell lineage disorders including leukemia, lymphoma and anemia. However, clinical research demonstrating the utility of cord blood stem cells for use in treating other diseases or injuries has been minimal, leaving claims of broad clinical utility of cord blood stem cells by cord blood banks largely unsubstantiated. The low utilization rate of banked cord blood samples coupled with the lack of demonstrated clinical results for multiple treatment indications has led to consumer skepticism regarding the benefits of cord blood banking and in turn, a significant reduction in collection rates in a number of geographies in Europe and the U.S. A continued lack of investment in the research and development of supporting clinical data for additional applications may lead to greater skepticism globally, further adversely affecting demand for cord blood banking services and our revenues.
We have Limited Operating History In the Emerging Regenerative Medicine Industry. Through the merger with TotipotentRX, we are in the business of research, development and commercialization of autologous cell-based therapeutics for use in the emerging regenerative medicine industry, and therefore, we have a limited operating history in such industry on which to base an evaluation of our business and prospects. We will be subject to the risks inherent in the operation of a company in an emerging industry such as regulatory setbacks and delays, fluctuations in expenses, competition, and governmental regulation.
Our Potential Products and Technologies Are In Early Stages Of Development. The development of new cell therapy products is a highly risky undertaking, and there can be no assurance that any future research and development efforts we may undertake will be successful. Our potential products in vascular, orthopedic, hematological/oncological and wound care indications will require extensive additional research and development and regulatory approval before any commercial introduction. There can be no assurance that any future research, development and clinical trial efforts will result in viable products or meet efficacy standards.
We Intend To Rely On Third Parties For Certain Functions In Conducting Clinical Trials Of Our Product Candidates. We intend to rely on third parties for certain clinical trial activities of our products. In this regard, we have renewed and expanded our agreement with Fortis Healthcare Limited, a hospital chain networked throughout India and Asia, for contract clinical trial services programs among other services. The agreement expires in August 2017. Termination of this agreement could jeopardize or delay development of our products.
Delays In The Commencement Or Completion Of Clinical Testing Of Our Products Could Result In Increased Costs To Us And Delay Our Ability To Generate Revenues. Delays in the commencement or completion of clinical testing could significantly impact our product development costs. We do not know whether current or planned clinical trials will begin on time or be completed on schedule, if at all. The commencement of clinical trials can be delayed for a variety of reasons, including delays in:
|
·
|
Obtaining regulatory approval to commence a clinical trial;
|
|
·
|
Reaching agreement on acceptable terms with prospective contract research organizations and clinical trial sites for Phase II and III trials;
|
|
·
|
Obtaining proper devices for any or all of the product candidates;
|
|
·
|
Obtaining institutional review board approval to conduct a clinical trial at a prospective site; and
|
|
·
|
Recruiting participants for a clinical trial.
|
In addition, once a clinical trial has begun, it may be suspended or terminated by us or the FDA or other regulatory authorities due to a number of factors, including:
|
·
|
Failure to conduct the clinical trial in accordance with regulatory requirements;
|
|
·
|
Inspection of the clinical trial operations or clinical trial site by the FDA or other regulatory authorities resulting in the imposition of a clinical hold;
|
|
·
|
Failure to achieve certain efficacy and/or safety standards;
|
|
·
|
Reports of serious adverse events including but not limited to death of trial subjects; or
|
|
·
|
Lack of adequate funding to continue the clinical trial.
|
Our clinical therapy candidates may produce negative or inconclusive results, and we may decide, or regulators may require us, to conduct additional clinical trials or abandon product development programs that we expect to be pursuing.
We May Seek To Enter Into Collaborative Arrangements To Develop and Commercialize Our Products Which May Not Be Successful. We may seek to enter into collaborative arrangements to develop and commercialize some of our potential products both in North America and international markets. There can be no assurance that we will be able to negotiate collaborative arrangements on favorable terms or at all or that our current or future collaborative arrangements will be successful.
A Significant Portion of our Revenue is Derived from Customers Outside the United States. We may Lose Revenues, Market Share, and Profits due to Exchange Rate Fluctuations, Political and Economic Changes Related to our Foreign Business. In the year ended June 30, 2014, sales to customers outside the U.S. comprised approximately 57% of our revenues. This compares to 55% in fiscal 2013. Our foreign business is subject to economic, political and regulatory uncertainties and risks that are unique to each area of the world. Fluctuations in exchange rates may also affect the prices that our foreign customers are willing to pay, and may put us at a price disadvantage compared to other competitors. Potentially volatile shifts in exchange rates may negatively affect our financial position and results.
The Loss of a Significant Distributor or End User Customer may Adversely Affect our Financial Condition and Results of Operations. Revenues from three significant distributors/customers comprised 39% of our revenues for the year ended June 30, 2014. The loss of a large end user customer or distributor may decrease our revenues.
We are Reliant on Highly Specialized Distributors and Regulatory Approval to Market and Sell Our Bone Marrow Processing System. Although we have added distributors in other territories, we may not be able to expand our sales of in vivo applications utilizing bone marrow processing devices until clinical trials are conducted. Since the MXP, Res-Q, and VXP products are projected as a significant portion of our near-term revenue growth, a delay in finding competent distributors in the clinical space and/or a delay or failure to complete clinical trials and each on-label regulatory approval may adversely affect our future revenues and competitive advantage.
We may be Exposed to Liabilities under the Foreign Corrupt Practices Act and any Determination that we Violated these Laws could have a Material Adverse Effect on our Business. We are subject to the Foreign Corrupt Practices Act (“FCPA”), and other laws that prohibit improper payments or offers of payments to foreign governments and their officials and political parties by U.S. persons and issuers as defined by the statute, for the purpose of obtaining or retaining business. It is our policy to implement safeguards to discourage these practices by our employees. However, our existing safeguards and any future improvements may prove to be less than effective and the employees, consultants, sales agents or distributors of our Company may engage in conduct for which we might be held responsible. Violations of the FCPA may result in severe criminal or civil sanctions and we may be subject to other liabilities, which could negatively affect our business, operating results and financial condition.
Adverse Results of Legal Proceedings could have a Material Adverse Effect on Us. We are subject to, and may in the future be subject to, a variety of legal proceedings and claims that arise out of the ordinary conduct of our business. Results of legal proceedings cannot be predicted with certainty. Irrespective of their merits, legal proceedings may be both lengthy and disruptive to our operations and may cause significant expenditure and diversion of management attention. We may be faced with significant monetary damages or injunctive relief against us that could have a material adverse effect on a portion of our business operations or a material adverse effect on our financial condition and results of operations.
Risks Related to Our Operations
We May Not Be Able to Successfully Integrate our Business, or to Realize the Anticipated Synergies of the Combined Businesses. Our completed merger with TotipotentRX represents a significant investment by both companies. The integration of the two companies is requiring significant attention and resources of management which could reduce the likelihood of achievement of other corporate goals. Failure to quickly and adequately integrate operations and personnel could adversely affect the combined company’s business and its ability to achieve its objectives and strategy. The additional financing needs created by the combined company will also require additional management time to address. There is no assurance that we will realize synergies in the scientific, clinical, regulatory, or other areas as we currently contemplate.
We Do Not Have Commercial-Scale Manufacturing Capability And Lack Commercial Manufacturing Experience. We operate GMP manufacturing facilities for both devices and cellular production; however, they are not of sufficient size for medium to large commercial production of product candidates. We will not have large scale experience in cell-drug formulation or manufacturing, and will lack the resources and the capability to manufacture any of our product candidates on a clinical or commercial scale. Accordingly, we expect to depend on third-party contract manufacturers for the foreseeable future. Any performance failure on the part of our contract manufacturers could delay clinical development, regulatory approval or commercialization of our current or future products, depriving us of potential product revenues and resulting in additional losses.
We Have Limited Sales, Marketing and Distribution Experience in Pharmaceutical Products. We have limited experience in the sales, marketing, and distribution of pharmaceutical products. There can be no assurance that we will be able to establish sales, marketing, and distribution capabilities or make arrangements with current collaborators or others to perform such activities or that such effort will be successful. If we decide to market any of our new products directly, we must either partner, acquire or internally develop a marketing and sales force with technical expertise and with supporting distribution capabilities. The acquisition or development of a sales, marketing and distribution infrastructure would require substantial resources, which may not be available to us or, even if available, divert the attention of our management and key personnel, and have a negative impact on further product development efforts.
Our Inability to Protect our Patents, Trademarks, Trade Secrets and other Proprietary Rights could Adversely Impact our Competitive Position. We believe that our patents, trademarks, trade secrets and other proprietary rights are important to our success and our competitive position. Accordingly, we commit substantial resources to the establishment and protection of our patents, trademarks, trade secrets and proprietary rights. We use various methods, including confidentiality agreements with employees, vendors, and customers, to protect our trade secrets and proprietary know-how for our products. We currently hold patents for products, and have patents pending in certain countries for additional products that we market or intend to market. However, our actions to establish and protect our patents, trademarks, and other proprietary rights may be inadequate to prevent imitation of our products by others or to prevent others from claiming violations of their trademarks and proprietary rights by us. If our products are challenged as infringing upon patents of other parties, we may be required to modify the design of the product, obtain a license, or litigate the issues, all of which may have an adverse business effect on us.
We may be Subject to Claims that our Products or Processes Infringe the Intellectual Property Rights of Others, which may Cause us to Pay Unexpected Litigation Costs or Damages, Modify our Products or Processes or Prevent us from Selling our Products. Although it is our intention to avoid infringing or otherwise violating the intellectual property rights of others, third parties may nevertheless claim that our processes and products infringe their intellectual property and other rights. Our strategies of capitalizing on growing international demand as well as developing new innovative products across multiple business lines present similar infringement claim risks both internationally and in the U.S. as we expand the scope of our product offerings and markets. We compete with other companies for contracts in some small or specialized industries, which increases the risk that the other companies will develop overlapping technologies leading to an increased possibility that infringement claims will arise. Whether or not these claims have merit, we may be subject to costly and time-consuming legal proceedings, and this could divert our management’s attention from operating our business. In order to resolve such proceedings, we may need to obtain licenses from these third parties or substantially re-engineer or rename our products in order to avoid infringement. In addition, we might not be able to obtain the necessary licenses on acceptable terms, or at all, or be able to re-engineer or rename our products successfully.
We may not be able to Protect our Intellectual Property in Countries Outside the United States. Intellectual property law outside the United States is uncertain and in many countries is currently undergoing review and revisions. The laws of some countries do not protect our patent and other intellectual property rights to the same extent as United States laws. This is particularly relevant to us as a significant amount of our current and projected future sales are outside of the United States. Third parties may attempt to oppose the issuance of patents to us in foreign countries by initiating opposition proceedings. Opposition proceedings against any of our patent filings in a foreign country could have an adverse effect on our corresponding patents that are issued or pending in the United States. It may be necessary or useful for us to participate in proceedings to determine the validity of our patents or our competitors’ patents that have been issued in countries other than the U.S. This could result in substantial costs, divert our efforts and attention from other aspects of our business, and could have a material adverse effect on our results of operations and financial condition.
Any Failure to Achieve and Maintain the High Design and Manufacturing Standards that our Products Require may Seriously Harm our Business. Our products require precise, high-quality manufacturing. Achieving precision and quality control requires skill and diligence by our personnel as well as our vendors. Our failure to achieve and maintain these high manufacturing standards, including the incidence of manufacturing errors, design defects or component failures could result in patient injury or death, product recalls or withdrawals, delays or failures in product testing or delivery, cost overruns or other problems that could seriously hurt our business. Additionally, the large amount of AXP disposable inventory certain distributors and end-users maintain may delay the identification of a manufacturing error and expand the financial impact. A manufacturing error or defect, or previously undetected design defect, or uncorrected impurity or variation in a raw material component, either unknown or undetected, could affect the product. Despite our very high manufacturing standards, we cannot completely eliminate the risk of errors, defects or failures. If we or our vendors are unable to manufacture our products in accordance with necessary quality standards, our business and results of operations may be negatively affected.
Our Revenues and Operating Results may be Adversely Affected as a Result of our Required Compliance with the Adopted EU Directive on the Restriction of the Use of Hazardous Substances in Electrical and Electronic Equipment, as well as other Standards Around the World. A number of domestic and foreign jurisdictions seek to restrict the use of various substances, a number of which have been or are currently used in our products or processes. For example, the EU Restriction of Hazardous Substances in Electrical and Electronic Equipment (“RoHS”) Directive now requires that certain substances, which may be found in certain products we have manufactured in the past, be removed from all electronics components. Eliminating such substances from our manufacturing processes requires the expenditure of additional research and development funds to seek alternative substances for our products, as well as increased testing by third parties to ensure the quality of our products and compliance with the RoHS Directive. Other countries, such as China, have enacted or may enact laws or regulations similar to RoHS. While we have implemented a compliance program to ensure our product offerings meet these regulations, there may be instances where alternative substances will not be available or commercially feasible, or may only be available from a single source, or may be significantly more expensive than their restricted counterparts. Additionally, if we were found to be non-compliant with any such rule or regulation, we could be subject to fines, penalties and/or restrictions imposed by government agencies that could adversely affect our operating results.
Compliance with Government Regulations Regarding the Use of “Conflict Minerals” may Result in Additional Expense and Affect our Operations. The SEC has adopted a final rule to implement Section 1502 of the Dodd-Frank Wall Street Reform and Consumer Protection Act of 2010, which imposes new disclosure requirements regarding the use of “conflict minerals” mined from the Democratic Republic of Congo and adjoining countries. These minerals include tantalum, tin, gold and tungsten. We may incur significant costs associated with complying with the new disclosure requirements, including but not limited to costs related to determining which of our products may be subject to the rules and identifying the source of any “conflict minerals” used in those products. Additionally, implementing the new requirements could adversely affect the sourcing, supply and pricing of materials used in the manufacture of our products. We may also face reputational challenges if we are unable to verify through our compliance procedures the origins for all metals used in our products.
Our Products may be Subject to Product Recalls which may Harm our Reputation and Divert our Managerial and Financial Resources. The FDA and similar governmental authorities in other countries have the authority to order the mandatory recall of our products or order their removal from the market if the governmental entity finds our products might cause adverse health consequences or death. The FDA may also seize product or prevent further distribution. A government-mandated or voluntary recall by us could occur as a result of component failures, manufacturing errors or design defects (including labeling defects). In the past we have initiated voluntary recalls of some of our products and we could do so in the future. Any recall of our products may harm our reputation with customers, divert managerial and financial resources and negatively impact our profitability.
We are Dependent on our Suppliers and Manufacturers to Meet Existing Regulations. Certain of our suppliers and manufacturers are subject to heavy government regulations, including FDA QSR compliance, in the operation of their facilities, products and manufacturing processes. Any adverse action by the FDA against our suppliers or manufacturers could delay supply or manufacture of component products required to be integrated or sold with our products. Although we attempt to mitigate this risk through inventory held directly or through distributors, and audit our suppliers, there are no assurances we will be successful in identifying issues early enough to allow for corrective action or transition to an alternative supplier, or in locating an alternative supplier or manufacturer to meet product shipment or launch deadlines. As a result, our sales, contractual commitments and financial forecasts may be significantly affected by any such delays.
Dependence on Suppliers for Disposable Products and Custom Components may Impact the Production Schedule. The Company obtains certain disposable products and custom components from a limited number of suppliers. If the supplier raises the price or discontinues production, the Company may have to find another qualified supplier to provide the item or re-engineer the item. In the event that it becomes necessary for us to find another supplier, we would first be required to qualify the quality assurance systems and product quality of that alternative supplier. Any operational issues with re-engineering or the alternative qualified supplier may impact the production schedule, therefore delaying revenues, and this may cause the cost of disposables or key components to increase.
Failure to Meet Certain Financial Covenants could Decrease our AXP Revenues. Under certain license and escrow agreements, if we fail to meet certain financial covenants, other companies may take possession of the escrowed intellectual property and initiate manufacturing of the applicable device and disposables. If this were to occur, our revenues would be negatively impacted.
Failure to Retain or Hire Key Personnel may Adversely Affect our Ability to Sustain or Grow our Business. Our ability to operate successfully and manage our potential future growth depends significantly upon retaining key research, technical, clinical, regulatory, sales, marketing and managerial personnel. Our future success partially depends upon the continued services of key technical and senior management personnel. Our future success also depends on our continuing ability to attract, retain and motivate highly qualified managerial and technical personnel. The inability to retain or attract qualified personnel could have a significant negative effect upon our efforts and thereby materially harm our business and future financial condition.
Most of Our Operations Are Conducted At A Single Location. Any Disruption At Our Facilities Could Delay Revenues Or Increase Our Expenses. Our U.S. device operations are conducted at a single location although we contract the manufacturing of certain devices, disposables and components. Further, through the TotiPotentRX merger, we have research, clinical and manufacturing operations in Emeryville, CA and Gurgaon, India. We take precautions to safeguard our facilities, through insurance, health and safety protocols, and off-site storage of computer data. However, a natural disaster, such as a fire, flood or earthquake, could cause substantial delays in our operations, damage or destroy our manufacturing equipment or inventory, and cause us to incur additional expenses. The insurance we maintain against fires, floods, and other natural disasters may not be adequate to cover our losses in any particular case.
Risks Related to Our Industry
Our Business is Heavily Regulated, Resulting in Increased Costs of Operations and Delays in Product Sales. Many of our products require FDA approval or clearance to sell in the U.S. and will require approvals from comparable agencies to sell in foreign countries. These authorizations may limit the U.S. or foreign markets in which our products may be sold. Further, our products must be manufactured under requirements of our quality system for continued CE-Marking so they can continue to be marketed and sold in Europe. These requirements are similar to the QSR of both the FDA and California Department of Public Health. Failure to comply with or incorrectly interpret these quality system requirements and regulations may subject the Company to delays in production while it corrects deficiencies found by the FDA, the State of California, or the Company’s notifying body as a result of any audit of our quality system. If we are found to be out of compliance, we could receive a Warning Letter or an untitled letter from the FDA or even be temporarily shut down in manufacturing and product sales while the non-conformances are rectified. Also, we may have to recall products and temporarily cease their manufacture and distribution, which would increase our costs and reduce our revenues. The FDA may also invalidate our PMA or 510(k) if appropriate regulations relative to the PMA or 510(k) product are not met. The notified bodies may elect to not renew CE-Mark certification. Any of these events would negatively impact our revenues and costs of operations.
Changes in Governmental Regulations may Reduce Demand for our Products or Increase our Expenses. We compete in many markets in which we and our customers must comply with federal, state, local and international regulations, such as environmental, health and safety and food and drug regulations. We develop, configure and market our products to meet customer needs created by those regulations. Any significant change in regulations could reduce demand for our products or increase our expenses. For example, many of our instruments are marketed to the industry for enabling new regenerative therapies. Changes in the FDA’s regulation of the devices and products directed at regenerative medicine, and development process for new therapeutic applications could have an adverse effect on the demand for these products.
To Sell in International Markets, we will be Subject to Regulation in Foreign Countries. In cooperation with our distribution partners, we intend to market our current and future products both domestically and in many foreign markets. A number of risks are inherent in international transactions. In order for us to market our products in certain non-U.S. jurisdictions, we need to obtain and maintain required regulatory approvals or clearances and must comply with extensive regulations regarding safety, manufacturing processes and quality. These regulations, including the requirements for approvals or clearances to market, may differ from the FDA regulatory scheme. International sales also may be limited or disrupted by political instability, price controls, trade restrictions and changes in tariffs. Additionally, fluctuations in currency exchange rates may adversely affect demand for our products by increasing the price of our products in the currency of the countries in which the products are sold.
There can be no assurance that we will obtain regulatory approvals or clearances in all of the countries where we intend to market our products, or that we will not incur significant costs in obtaining or maintaining foreign regulatory approvals or clearances, or that we will be able to successfully commercialize current or future products in various foreign markets. Delays in receipt of approvals or clearances to market our products in foreign countries, failure to receive such approvals or clearances or the future loss of previously received approvals or clearances could have a substantial negative effect on our results of operations and financial condition.
To Operate In Foreign Jurisdictions, We Are Subject to Regulation by Non-U.S. Authorities. As a result of the merger, we have operations in India, and as such are subject to Indian regulatory agencies. A number of risks are inherent in conducting business and clinical operations overseas. In order for us to operate as a majority owned foreign corporation in India, we are subject to financial regulations imposed by the Reserve Bank of India. This includes the rules specific to the capital funding, repatriation of funds and payment of dividends from and to the foreign subsidiaries and from and to us in the U.S.
In order for us to manufacture and/or market our services and products in India, we need to obtain and maintain required regulatory approvals or clearances and must comply with extensive regulations regarding safety, manufacturing processes and quality. These regulations, including the requirements for approvals or clearances to market, and/or export may differ from the FDA regulatory scheme. Additionally, In order for us to complete clinical trials, clinical trial services and cell banking in India, and other foreign jurisdictions, we need to obtain and maintain approvals and licenses which comply with extensive regulations of the appropriate regulatory body.
International operations also may be limited or disrupted by political, economic or social instability, price controls, trade restrictions and changes in tariffs as ordered by various governmental agencies. Additionally, fluctuations in currency exchange rates may adversely affect the cost of production for our products by increasing the price of materials and other inputs for our products in the currency of the countries in which the products are sold.
If Our Competitors Develop and Market Products That Are More Effective Than Our Product Candidates Or Obtain Regulatory and Market Approval For Similar Products Before We Do, Our Commercial Opportunity May Be Reduced Or Eliminated. The development and commercialization of new pharmaceutical products which target cardiovascular, orthopedic, chronic dermal wounds and other conditions addressed by our current and future products is competitive, and we will face competition from numerous sources, including major biotechnology and pharmaceutical companies worldwide. Many of our competitors have substantially greater financial and technical resources, and development, production and marketing capabilities than we do. In addition, many of these companies have more experience than we do in pre-clinical testing, clinical trials and manufacturing of compounds, as well as in obtaining FDA and foreign regulatory approvals. As a result, there is a risk that one of the competitors will develop a more effective product for the same indications for which we are developing a product or, alternatively, bring a similar product to market before we can. With regards to the BioArchive and AXP Systems, numerous larger and better-financed medical device manufacturers may choose to enter this market as it develops.
Influence by the Government and Insurance Companies may Adversely Impact Sales of our Products. Our business may be materially affected by continuing efforts by government, third party payers such as Medicare, Medicaid, and private health insurance plans, to reduce the costs of healthcare. For example, in certain foreign markets the pricing and profit margins of certain healthcare products are subject to government controls. In addition, increasing emphasis on managed care in the U.S. will continue to place pressure on the pricing of healthcare products. As a result, continuing efforts to contain healthcare costs may result in reduced sales or price reductions for our products. To date, we are not aware of any direct impact on our pricing or product sales due to such efforts by governments to contain healthcare costs, and we do not anticipate any impact in the near future.
Product Liability and Uninsured Risks May Adversely Affect the Continuing Operations. We operate in an industry susceptible to significant product liability claims. We may be liable if any of our products cause injury, illness, or death. These claims may be brought by individuals seeking relief or by groups seeking to represent a class. We also may be required to recall certain of our products should they become damaged or if they are defective. We are not aware of any material product liability claims against us. However, product liability claims may be asserted against us in the future based on events we are not aware of at the present time. We maintain a product liability policy for $3,000 and a general liability policy that includes product liability coverage of $1,000 per occurrence and $2,000 per year in the aggregate. However, a product liability claim against us could have a material adverse effect on our business or future financial condition.
Risks Related to Operating Results and Financial Markets
We Have Incurred Net Losses since our Inception and Losses will Continue. We have not been profitable for a significant period. For the fiscal year ended June 30, 2014, we had a net loss of $8,631 and an accumulated deficit at June 30, 2014, of $122,822. We will continue to incur significant costs as we develop and market our current products and related applications. Although we are executing our business plan to develop, market and launch new products, continuing losses may impair our ability to fully meet our objectives for new product sales.
We Will Need to Raise Additional Capital in Furtherance of Our Business Plan. We will need to raise additional capital in furtherance of our business plan, including progression of the CLI and Acute Myocardial Infarction Rapid Stem Cell Therapy (“AMIRST”) clinical trials and development of other new products. Any proposed financing may include shares of common stock, shares of preferred stock, warrants to purchase shares of common stock or preferred stock, debt securities, units consisting of the forgoing securities, equity investments from strategic development partners or some combination of each. Any additional equity financings may be financially dilutive to, and will be dilutive from an ownership perspective to our stockholders.
The Preparation of our Consolidated Financial Statements in Accordance with U.S. Generally Accepted Accounting Principles (“GAAP”) Requires Us to Make Estimates, Judgments, and Assumptions that may Ultimately Prove to be Incorrect. The accounting estimates and judgments that management must make in the ordinary course of business affect the reported amounts of assets and liabilities at the date of the consolidated financial statements and the reported amounts of revenue and expenses during the periods presented. If the underlying estimates are ultimately proven to be incorrect, subsequent adjustments could have a material adverse effect on our operating results for the period or periods in which the change is identified. Additionally, subsequent adjustments could require us to restate our consolidated financial statements. Restating consolidated financial statements could result in a material decline in the price of our stock.
Our Future Financial Results Could be Adversely Impacted by Asset Impairment Charges. We are required to test both goodwill and intangible assets for impairment on an annual basis based upon a fair value approach. We have chosen to perform our annual impairment reviews of goodwill and other intangible assets during the fourth quarter of each fiscal year. We also are required to test for impairment between annual tests if events occur or circumstances change that would more likely than not reduce our enterprise fair value below its book value. These events or circumstances could include results of our on-going clinical trials, activities and results of our competitor’s clinical trials, a significant change in the regulatory climate, legal factors, operating performance indicators, or other factors. If the fair market value is less than the book value of goodwill, we could be required to record an impairment charge. The valuation requires judgment in estimating future cash flows, discount rates and estimated product life cycles. In making these judgments, we evaluate the financial health of the business, including such factors as industry performance, changes in technology and operating cash flows.
As of June 30, 2014 we have a goodwill balance of $13,254 and a net intangible assets balance of $21,928, out of total assets of $62,888. As a result, the amount of any annual or interim impairment could be significant and could have a material adverse effect on our reported financial results for the period in which the charge is taken.
Risks Related to Our Common Stock
If the Price of our Common Stock does not Meet the Requirements of the NASDAQ Capital Market Stock Exchange (“NASDAQ”), Our Shares may be Delisted. Our Ability to Publicly or Privately Sell Equity Securities and the Liquidity of Our Common Stock Could be Adversely Affected if We Are Delisted. The listing standards of NASDAQ provide, among other things, that a company may be delisted if the bid price of its stock drops below $1.00 for a period of 30 consecutive business days. Delisting from NASDAQ could adversely affect our ability to raise additional financing through the public or private sale of equity securities, would significantly affect the ability of investors to trade our securities and would negatively affect the value and liquidity of our common stock. Delisting could also have other negative results, including the potential loss of confidence by employees, the loss of institutional investor interest and fewer business development opportunities.
Certain Principal Stockholders Have Significant Influence Over Us. As a result of the merger with TotiPotentRX, Messrs. Harris and Sivilotti, our President and a key employee, respectively, own approximately 23% of our current outstanding common stock. As a result, they will be able to exert a significant degree of influence over our management and affairs and over matters requiring stockholder approval, including the election of directors, any merger, consolidation or sale of all or substantially all of the combined company’s assets, and any other significant corporate transaction. Their interests may not always coincide with those of our other stockholders.
We Are Integrating a Private Company That Has Not Been Subject to the Sarbanes-Oxley Act Of 2002 or the Rules and Regulations of the SEC. Prior to the merger, TotipotentRX was a private company and was not subject to the Sarbanes-Oxley Act of 2002, the rules and regulations of the SEC, or other corporate governance requirements to which public reporting companies may be subject. During the audit of TotipotentRX’s financial statements for the year ended December 31, 2012, TotipotentRX’s independent auditors determined that a material weakness existed in its internal control over financial reporting as TotipotentRX did not have adequate personnel and information systems in place to prepare financial statements on a timely basis, including accrual accounting, non-routine data processes and estimation processes. As result of the merger, we are required to implement the appropriate internal control processes and procedures over financial accounting and reporting. However, there is a risk that we may incur significant legal, accounting and other expenses to ensure that TotipotentRX meets these requirements. Such requirements include, but are not limited to, that we will be required to report on the effectiveness of our internal control over financial reporting. Implementing the controls and procedures required to comply with the various applicable laws and regulations may place a significant burden on our management and internal resources.
We do not Pay Cash Dividends. We have never paid any cash dividends on our common stock and may not pay cash dividends in the future. Instead, we intend to apply earnings to the expansion and development of our business. Thus, the liquidity of your investment is dependent upon your ability to sell stock at an acceptable price. The price can go down as well as up and may limit your ability to realize any value from your investment, including the initial purchase price.
None.
The Company leases a facility with approximately 28,000 square feet of space located in Rancho Cordova, California. Approximately 50% of the facility is devoted to warehouse space and manufacturing of products. The other 50% is comprised of office space, a biologics lab, and a research and development lab. The lease expires May 31, 2019.
We also sub-lease approximately 7,819 square feet for an office and research facility located in Emeryville, California. The sub-lease expires April 30, 2017.
In Gurgaon India we lease approximately 5,800 square feet for an office and manufacturing facility. The lease expires March 1, 2015.
Additionally in Gurgaon India, as part of our agreement with Fortis Healthcare, we occupy and manage a 2,800 square foot cord blood banking facility in the Fortis Memorial Research Institute.
In the normal course of operations, we may have disagreements or disputes with distributors, vendors or employees. Such potential disputes are seen by management as a normal part of business.
On September 9, 2014, Cesca Therapeutics Inc. (Cesca) filed a complaint against SynGen Inc., PHC Medical Inc, Philip Coelho and others (the Defendants) in the case captioned as Cesca Therapeutics, Inc. v. SynGen, Inc., et al, United States District Court, Eastern District of California, Case No. 2:14-cv-02085-GEB-KJN. In the complaint, Cesca contends that SynGens’ product the SynGenX-1000 and the patent application entitled “System for Purifying Certain Cell Populations in Blood or Bone Marrow by Depleting Others” were developed using Cesca confidential information and that Cesca is the equitable owner. The complaint is based on misappropriation of trade secrets, breach of contract and other claims.
On December 17, 2013, the Company filed a lawsuit against OriGen Bimedical, Inc. ThermoGenesis Corp. v. Origen Biomedical, Inc., 2:13-cv-02619, U.S. District Court, Eastern District of California (Sacramento) claiming that OriGen’s freezer bag products are infringing on one of our patents and a patent developed from our partnership with New York Blood Center, which although owned by the New York Blood Center, has had all rights thereunder assigned to us. Origen subsequently filed for re-examination of the patents in the U.S. patent and trademark office. The case is proceeding.
On October 24, 2012, Harvest Technologies Corp. filed suit against us in the case Harvest Technologies Corp. v. ThermoGenesis Corp., 12-cv-01354, U.S. District Court, District of Delaware (Wilmington) claiming our Res-Q 60 System infringes certain Harvest patents. We have been served, and on April 11, 2013, we filed an answer and counter-claims in response. The counter-claims are based on anti-trust and other alleged improper conduct by Harvest and further seek declarations that the Res-Q 60 System does not infringe the patents and that the patents are invalid. The Company intends to vigorously defend itself against the Harvest claims, while aggressively pursuing its separate claims against Harvest. Harvest has answered our counter-claims and the litigation is proceeding.
Not applicable.
PART II
| ITEM 5. | MARKET FOR THE REGISTRANT’S COMMON EQUITY AND RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES. |
The Company’s common stock, $0.001 par value, is listed on the Nasdaq Stock Market under the symbol KOOL. The following table sets forth the range of high and low bid prices for the Company’s common stock for the past two fiscal years as reported on the Nasdaq Stock Market.
|
Fiscal 2014
|
High
|
Low
|
Fiscal 2013
|
High
|
Low
|
||||||||||||
|
|
|
||||||||||||||||
|
First Quarter (Sep. 30)
|
$
|
1.52
|
$
|
1.01
|
First Quarter (Sep. 30)
|
$
|
1.29
|
$
|
0.87
|
||||||||
|
Second Quarter (Dec. 31)
|
$
|
1.12
|
$
|
0.72
|
Second Quarter (Dec. 31)
|
$
|
1.01
|
$
|
0.67
|
||||||||
|
Third Quarter (Mar. 31)
|
$
|
2.82
|
$
|
1.05
|
Third Quarter (Mar. 31)
|
$
|
1.00
|
$
|
0.82
|
||||||||
|
Fourth Quarter (June 30)
|
$
|
2.06
|
$
|
1.39
|
Fourth Quarter (June 30)
|
$
|
1.53
|
$
|
0.77
|
||||||||
The Company has not paid cash dividends on its common stock and does not intend to pay a cash dividend in the foreseeable future. There were approximately 265 stockholders of record on June 30, 2014 (not including street name holders).
The following selected financial data should be read in conjunction with our consolidated financial statements and the related notes and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” appearing elsewhere in this report. Unless otherwise indicated in this Item 6, reference to dollar amounts are in thousands except for share and per share amounts.
CESCA THERAPEUTICS INC.
FIVE-YEAR REVIEW OF SELECTED FINANCIAL DATA
|
|
Year Ended June 30,
|
|||||||||||||||||||
|
Summary of Operations
|
2014
|
2013
|
2012
|
2011
|
2010
|
|||||||||||||||
|
Net revenues
|
$
|
15,987
|
$
|
17,963
|
$
|
19,023
|
$
|
23,400
|
$
|
23,088
|
||||||||||
|
|
||||||||||||||||||||
|
Cost of revenues
|
(10,101
|
)
|
(11,598
|
)
|
(12,690
|
)
|
(14,563
|
)
|
(15,643
|
)
|
||||||||||
|
|
||||||||||||||||||||
|
Gross profit
|
5,886
|
6,365
|
6,333
|
8,837
|
7,445
|
|||||||||||||||
|
|
||||||||||||||||||||
|
Sales and marketing
|
(2,968
|
)
|
(2,955
|
)
|
(2,761
|
)
|
(3,195
|
)
|
(2,889
|
)
|
||||||||||
|
Research and development
|
(3,468
|
)
|
(2,991
|
)
|
(3,729
|
)
|
(3,003
|
)
|
(5,013
|
)
|
||||||||||
|
General and administrative
|
(8,490
|
)
|
(5,645
|
)
|
(5,222
|
)
|
(5,474
|
)
|
(4,797
|
)
|
||||||||||
|
Gain on sale of product lines
|
--
|
2,161
|
--
|
--
|
--
|
|||||||||||||||
|
|
||||||||||||||||||||
|
Loss from operations
|
(9,040
|
)
|
(3,065
|
)
|
(5,379
|
)
|
(2,835
|
)
|
(5,254
|
)
|
||||||||||
|
Interest and other income (expense), net
|
6
|
(21
|
)
|
393
|
268
|
61
|
||||||||||||||
| Loss before income tax benefits | (9,034 | ) | (3,086 | ) | (4,986 | ) | (2,567 | ) | (5,193 | ) | ||||||||||
| Deferred income tax benefit | 403 |
--
|
--
|
--
|
--
|
|||||||||||||||
|
Net loss
|
$
|
(8,631
|
)
|
$
|
(3,086
|
)
|
$
|
(4,986
|
)
|
$
|
(2,567
|
)
|
$
|
(5,193
|
)
|
|||||
|
Per share data:
|
||||||||||||||||||||
|
Basic and diluted net loss per common share
|
$
|
(0.36
|
)
|
$
|
(0.19
|
)
|
$
|
(0.30
|
)
|
$
|
(0.17
|
)
|
$
|
(0.37
|
)
|
|||||
|
Balance Sheet Data
|
2014
|
2013
|
2012
|
2011
|
2010
|
|||||||||||||||
|
Cash and cash equivalents
|
$
|
14,811
|
$
|
6,884
|
$
|
7,879
|
$
|
12,309
|
$
|
10,731
|
||||||||||
|
|
||||||||||||||||||||
|
Working capital
|
$
|
18,947
|
$
|
11,125
|
$
|
14,034
|
$
|
18,976
|
$
|
16,587
|
||||||||||
|
|
||||||||||||||||||||
|
Total assets
|
$
|
62,888 |
$
|
18,529
|
$
|
21,080
|
$
|
24,399
|
$
|
24,030
|
||||||||||
|
|
||||||||||||||||||||
|
Total liabilities
|
$
|
14,190
|
$
|
5,211
|
$
|
5,182
|
$
|
4,306
|
$
|
6,251
|
||||||||||
|
|
||||||||||||||||||||
|
Total stockholders’ equity
|
$
|
48,698
|
$
|
13,318
|
$
|
15,898
|
$
|
20,093
|
$
|
17,779
|
||||||||||
|
Other Data
|
2014
|
2013
|
2012
|
2011
|
2010
|
|||||||||||||||
|
Adjusted EBITDA1
|
$
|
(7,368
|
)
|
$
|
(3,961
|
)
|
$
|
(3,984
|
)
|
$
|
(1,409
|
)
|
$
|
(4,244
|
)
|
|||||
1Adjusted EBITDA represents loss from operations excluding amounts for depreciation and amortization, stock-based compensation expense, impairment of intangible asset and gain on sale of product lines. Adjusted EBITDA is a common measure of operating performance and helps us evaluate our performance by removing from our operating results non-cash items and items which do not relate to our core operating performance.
Non-GAAP Measures
In addition to the results reported in accordance with US GAAP, we also use a non-GAAP measure, adjusted EBITDA, to evaluate operating performance and to facilitate the comparison of our historical results and trends. This financial measure is not a measure of financial performance under US GAAP and should not be considered in isolation or as a substitute for loss as a measure of performance. The calculation of this non-GAAP measure may not be comparable to similarly titled measures used by other companies. Reconciliations to the most directly comparable GAAP measure are provided below.
|
|
2014
|
2013
|
2012
|
2011
|
2010
|
|||||||||||||||
|
Loss from operations
|
$
|
(9,040
|
)
|
$
|
(3,065
|
)
|
$
|
(5,379
|
)
|
$
|
(2,835
|
)
|
$
|
(5,254
|
)
|
|||||
|
|
||||||||||||||||||||
|
Add (subtract):
|
||||||||||||||||||||
|
Depreciation and amortization
|
993
|
538
|
604
|
466
|
492
|
|||||||||||||||
|
Stock-based compensation expense
|
679
|
563
|
791
|
960
|
518
|
|||||||||||||||
|
Impairment of intangible asset
|
--
|
164
|
--
|
--
|
--
|
|||||||||||||||
|
Gain on sale of product lines
|
--
|
(2,161
|
)
|
--
|
--
|
--
|
||||||||||||||
|
Adjusted EBITDA
|
$
|
(7,368
|
)
|
$
|
(3,961
|
)
|
$
|
(3,984
|
)
|
$
|
(1,409
|
)
|
$
|
(4,244
|
)
|
|||||
(amounts in thousands, except share and per share amounts)
Certain statements contained in this section and other parts of this report on Form 10-K which are not historical facts are forward looking statements and are subject to certain risks and uncertainties. The Company’s actual results may differ significantly from the projected results discussed in the forward looking statements. Factors that might affect actual results include, but are not limited to, those discussed in ITEM 1A “RISK FACTORS” and other factors identified from time to time in the Company’s reports filed with the U.S. Securities and Exchange Commission. The following discussion should be read in conjunction with the Company’s consolidated financial statements contained in this report.
Overview
Cesca Therapeutics is focused on the research, development, and commercialization of autologous cell-based therapies for use in regenerative medicine. We are a leader in developing and manufacturing automated blood and bone marrow processing systems that enable the separation, processing and preservation of cell and tissue therapy products. The Company was founded in 1986 and is headquartered in Rancho Cordova, California. Our strategy is to expand our offerings in the development of regenerative medicine tools and partner with other pioneers in the stem cell arena to accelerate our clinical therapies and our worldwide penetration in the regenerative medicine market.
On February 18, 2014, TotipotentRX Corporation merged with and into ThermoGenesis Corp. In connection with the merger, ThermoGenesis changed its name from ThermoGenesis Corp. to Cesca Therapeutics Inc. The Company believes that TotipotentRX has the depth of clinical, scientific and biological engineering experience necessary to develop cell-based therapies in the vascular, orthopedic and oncological areas. As a result of the merger, Cesca is a fully integrated regenerative medicine company with the ability and expertise to research, design, and develop cell therapies targeting unmet clinical needs in large patient populations using our cost effective, clinically proven, point-of-care delivery system- SurgWerks. TotipotentRX was a privately held biomedical technology company specializing in human clinical trials in the field of regenerative medicine and the exclusive provider of cell-based therapies to the Fortis Healthcare System. TotipotentRX had two wholly-owned subsidiaries, TotipotentRX Cell Therapy Pvt. Ltd. (TotiRX India) and TotipotentSC Product Pvt. Ltd. (TotiSC India). The two subsidiaries are located in Gurgaon, a suburb of New Delhi, India. The operations of TotipotentRX have been included in our consolidated results as of February 18, 2014.
Our business strategy includes:
• Develop and deliver proprietary, commercially viable, highly effective autologous (patient’s own cells) cell therapies to treat major medical diseases.
• Rapidly and Cost-Effectively Implement New Clinical Trials. Rapidly initiate early clinical development of new cell therapies at our United States Food and Drug Administration (“FDA”)-registered clinical research organization in India and generate high quality data at a fraction of the cost of clinical trials undertaken in the U.S. or Europe.
• Commercialize in Both Developed and Emerging Markets. Utilize our existing U.S. and Asian footprints to uniquely position us to meet the needs of patients, hospitals and physicians across the globe. This footprint allows flexibility to meet the variable market demands in service and price.
• Maintain and expand our unmatched suite of proprietary technological and clinical assets to be deployed in the regenerative medicine markets. Ensure cell-therapy-related devices and platform technologies, unique cell formulations and treatment protocols are protected via a broad portfolio of patents and intellectual property filings.
Stem Cell Therapies
We are currently focusing our clinical therapy efforts in three areas:
|
·
|
Critical Limb Ischemia (CLI) - The CLI Phase 1b trial enrolled 17 patients who were considered “no option” patients. CLI is the last phase of peripheral vascular disease, where the leg is so deprived of blood flow and oxygen, that it has visible signs of gangrenous ulceration. In each of these cases the surgeon had determined that the patient required major amputation (below the knee) of the leg. Alternatively, the patient was asked to participate in the study where their bone marrow stem cells were harvested and processed through a Cesca device, and injected into multiple sites along the afflicted limb. After 12 months 82.4% of the patients had retained their leg and showed measurable improvement in blood flow and pain.
|
|
·
|
Acute Myocardial Infarction (AMI) – This therapy is designed to treat patients who have suffered an acute ST-elevated myocardial infarction (STEMI), a particular and most threatening type of heart attack. The SurgWerks-AMI treatment is designed to minimize remodeling of the heart from dysfunctional blood pumping action by minimizing the dysfunctional enlarging of the heart. The entire 4-step bedside treatment takes less than 90 minutes to complete in a single procedure in the heart catheterization laboratory.
|
|
·
|
Bone Marrow Transplant (BMT) – This therapy automates the processing of bone marrow for transplant which has significant advantages over the current standard of care. Improving cell yield and quality among clinical mismatch and haplod-identical transplants can also yield major advantages. Due to the lack of qualified donors for bone marrow transplants and the lack of a qualified process, patients typically see poor outcomes. Our therapy optimizes harvest yield and empowers BMT specialists to find best-case results in determining the balance between a match and GvHD.
|
Our Products
|
·
|
The SurgWerks Platform, a proprietary stem cell therapy point-of-care kit system for treating vascular, orthopedic and oncological indications that integrate the following indication specific devices and biologic protocols in a seamless delivery under statistical process control:
|
|
-
|
Cell harvesting
|
|
-
|
Cell processing and selection
|
|
-
|
Cell diagnostics
|
|
-
|
Cell delivery
|
|
·
|
The MarrowXpress® or MXP System, a derivative product of the AXP and its accompanying disposable bag set, isolates and concentrates stem cells from bone marrow. The product is an automated, closed, sterile system that volume-reduces blood from bone marrow to a user-defined volume in 30 minutes, while retaining over 90% of the MNCs, a clinically important cell fraction. Self-powered and microprocessor-controlled, the MXP System contains flow control optical sensors that achieve precise separation. We have received the CE-Mark, enabling commercial sales in Europe, and we received authorization from the FDA to begin marketing the MXP as a Class I device in the U.S. for the preparation of cell concentrate from bone marrow. However, the safety and effectiveness of this device for in vivo use has not been established. MXP Platform is an integrated component of The SurgWerks Kit and performs the cell processing and selection.
|
|
·
|
The AXP System is a medical device with an accompanying disposable bag set that isolates and retrieves stem cells from umbilical cord blood. The AXP System provides cord blood banks with an automated method to separate and capture adult stem cells which reduces the overall processing and labor costs with a reduced risk of contamination under cGMP conditions. The AXP System retains over 97% of the mononuclear cells (MNCs). High MNC recovery has significant clinical importance to patient transplant survival rates. Self-powered and microprocessor-controlled, the AXP device contains flow control optical sensors that achieve precise separation of the cord blood fractions.
|
Results of Operations
The following is Management’s discussion and analysis of certain significant factors which have affected the Company’s financial condition and results of operations during the periods included in the accompanying consolidated financial statements.
Results of Operations for the Fiscal Year Ended June 30, 2014 Versus the Fiscal Year Ended June 30, 2013
Net Revenues
Net revenues for the year ended June 30, 2014 were $15,987 compared to $17,963 for the year ended June 30, 2013, a decrease of $1,976, or 11%. The decrease is primarily due to a decrease in AXP disposable revenues that occurred in the first quarter of fiscal 2014 due to the termination of the GE distribution agreement and the related wind-down of their product inventory resulting in a temporary slowdown of orders from customers consuming said inventory and a decrease as our distributors in Asia ordered less product due to delays in the implementation of our Automated AXP Platform. Also, other revenues decreased as we were selling ThermoLine and CryoSeal products during the year ended June 30, 2013.
The following represents the Company’s revenues by product platform for the years ended:
|
|
June 30,
2014 |
June 30,
2013 |
||||||
|
AXP
|
$
|
6,143
|
$
|
7,687
|
||||
|
BioArchive
|
4,776
|
4,258
|
||||||
|
Manual Disposables
|
1,706
|
2,286
|
||||||
|
Bone Marrow
|
2,542
|
2,390
|
||||||
|
Other
|
820
|
1,342
|
||||||
|
|
$
|
15,987
|
$
|
17,963
|
||||
Gross Profit
Gross profit was $5,886 or 37% of revenues for 2014 compared to $6,365 or 35% of revenues for 2013. Our gross profit decreased due to a decline in revenues. The increase in gross profit percentage from 35% to 37% for 2014 is primarily due to lower warranty costs and a favorable mix of products sold.
Sales and Marketing Expenses
Sales and Marketing expenses include costs primarily associated with generating revenues from the sale of cord blood and bone marrow disposables and BioArchive devices.
Sales and Marketing expenses were $2,968 for 2014, compared to $2,955 for 2013, an increase of $13. Sales and marketing expenses increased due to our direct sales representation in Asia and expenses associated with our TRX operations. These increases were largely offset by a reduction in salaries and benefits as a result of a strategic reorganization in October 2013.
Research and Development Expenses
Research and development expenses include costs associated with our engineering, regulatory, scientific and clinical functions.
Research and development expenses for 2014, were $3,468, compared to $2,991 for 2013, an increase of $477 or 16%. The increase is primarily due to costs associated with developing our clinical therapies program. During the latter half of fiscal 2014 we increased personnel in our clinical therapies group and incurred certain legal and advisory fees to support the preparation of our IDE application with FDA for our forthcoming pivotal trial for our Critical Limb Ischemia Rapid Stem Cell Therapy (“CLIRST”) Therapy. We also incurred costs associated with the development of our MXP and VXP Cell Processing device to be used in our forthcoming CLIRST and AMIRST clinical trial.
General and Administrative Expenses
General and administrative expenses include costs associated with our accounting, finance, human resources, information system and executive functions.
General and administrative expenses were $8,490 for 2014, compared to $5,645 for 2013, an increase of $2,845 or 50%. The increase is primarily due to professional and legal fees associated with consummating the merger with TRX, legal costs incurred to defend our Res-Q patents and costs associated with transforming our business from a device oriented company to one focused on developing clinical therapies using autologous stem cells.
Deferred Income Tax Benefit
Our deferred income tax benefit was $403 for 2014, compared to $0 for 2013. The increase was due to certain intangible assets and the related deferred tax liabilities acquired in the merger with TRX. The recognition of a deferred income tax benefit resulted from netting the deferred tax liabilities against previously generated, but fully reserved, deferred tax assets.
Adjusted EBITDA
Our adjusted EBITDA loss of approximately $7,368 was driven by significant investments made to consummate the merger with TRX, develop and advance our clinical program and design and re-engineer our MXP and VXP cell processing devices to be used in our forthcoming CLIRST and AMIRST clinical trials.
Results of Operations for the Fiscal Year Ended June 30, 2013 versus the Fiscal Year Ended June 30, 2012
Net Revenues
Net revenues for the year ended June 30, 2013 were $17,963 compared to $19,023 for the year ended June 30, 2012, a decrease of $1,060, or 6%. The decrease in revenues is primarily due to the sale of the ThermoLine and CryoSeal product lines in 2013. These two product lines represented $2,240 in revenues for the year ended June 30, 2012 compared to $944 for the year ended June 30, 2013. This decrease in revenues was offset by an increase in revenues from Res-Q disposables of $403 primarily due to an increase in the number of bone marrow procedures performed and an increase in new customers.
Sales analysis for the year ended June 30:
|
|
June 30,
2013 |
June 30,
2012 |
||||||
|
|
||||||||
|
AXP
|
$
|
7,687
|
$
|
7,814
|
||||
|
BioArchive
|
4,258
|
4,310
|
||||||
|
Manual Disposables
|
2,286
|
2,200
|
||||||
|
Bone Marrow
|
2,390
|
2,076
|
||||||
|
Other
|
1,342
|
2,623
|
||||||
|
Total Company revenues
|
$
|
17,963
|
$
|
19,023
|
||||
Gross Profit
The Company’s gross profit was $6,365 or 35% of revenues for the year ended June 30, 2013, as compared to $6,333 or 33% of revenues for the year ended June 30, 2012. The increase in gross profit for the year ended June 30, 2013, is primarily due to lower inventory reserves and the mix of products sold in the prior fiscal year. We sold 25 CryoSeal devices to Asahi at cost during the quarter ended March 31, 2012. Inventory reserves recorded in the prior year were higher primarily due to the deceleration in sales of the ThermoLine freezers.
Sales and Marketing Expenses
Sales and marketing expenses were $2,955 for the year ended June 30, 2013, compared to $2,761 for the year ended June 30, 2012, an increase of $194 or 7%. The increase is primarily due to establishing direct representation in Asia.
Research and Development Expenses
Research and development expenses for the year ended June 30, 2013, were $2,991, compared to $3,729 for fiscal 2012, a decrease of $738 or 20%. The decrease is primarily due to lower personnel costs primarily as a result of the January 2012 restructuring and lower costs for clinical studies, offset by an increase in consulting expenses for quality assurance and regulatory projects.
General and Administrative Expenses
General and administrative expenses were $5,645 for the year ended June 30, 2013, compared to $5,222 for the year ended June 30, 2012, an increase of $423 or 8%. The increase is primarily due to legal and professional fees of $835 associated with the proposed merger with TotipotentRX and $670 due to the legal diligence associated with the Res-Q patent litigation and the development of our counterclaim. These increases were offset by a decrease in severance costs of $360 as a result of the January 2012 restructuring.
Gain on Sale of Product Lines
During the year ended June 30, 2013, the Company recognized a gain of $2,000 on the sale of certain intangible assets related to the CryoSeal product line, including all associated patents and engineering files and $161 on the sale of the ThermoLine product line.
Adjusted EBITDA
The adjusted EBITDA loss was $3,961 for the year ended June 30, 2013 compared to $3,984 for the year ended June 30, 2012. The adjusted EBITDA loss was consistent with the prior year as we offset a decrease in revenues from a change in the mix of products sold in our global markets with a decrease in expenses resulting from our cost reduction initiatives.
Liquidity and Capital Resources
At June 30, 2014, the Company had cash and cash equivalents of $14,811 and working capital of $18,947. This compared to cash and cash equivalents of $6,884 and working capital of $11,125 at June 30, 2013. The Company has primarily financed operations through private and public placement of equity securities and the sale of certain non-core assets. On January 30, 2014, we completed a private placement of 3,336,800 shares of common stock, plus 1,668,400 warrants for net proceeds of $5.9 million and on June 18, 2014, we completed a public offering of 7,530,000 shares of common stock, plus 2,259,000 warrants for net proceeds of $10.1 million.
Our net cash used in operating activities for the year ended June 30, 2014 of $7,836, and our net loss of approximately $8,631, were primarily due to costs associated with transforming the company from solely a device oriented company to a fully integrated regenerative medicine company. Significant investments were made to consummate the merger with TRX, develop and advance our clinical program and design and re-engineer our MXP and VXP cell processing devices to be used in our forthcoming CLIRST and AMIRST clinical trials.
Based on our cash balance after the June 18, 2014 public offering, historical trends, and future revenue projections, we believe our current funds are sufficient to provide for our projected needs to maintain operations and working capital requirements for at least the next 12 months. However, in order to maximize the value of our clinical trials and accelerate the planned commercialization of our products in connection with the merger with TotipotentRX, we intend to raise a minimum of $10 million for investing in the planned clinical development strategy over 24 months. Effective December 31, 2013, we amended the Technology License and Escrow Agreement with Cord Blood Registry Systems, Inc. The amendment removed the financial covenants, except the minimum cash and short-term investments balance covenant which it reduced to $2,000 at any month end. Our ability to fund our longer-term cash needs is subject to various risks, many of which are beyond our control. Should we require additional funding, such as additional capital investments, we may need to raise the required additional funds through bank borrowings or public or private sales of debt or equity securities. We cannot assure that such funding will be available in needed quantities or on terms favorable to us, if at all see Part I Item 1A – Risk Factors.
The Company generally does not require extensive capital equipment to produce or sell its current products. In fiscal 2012 and 2013, the Company spent $545 and $391, respectively. These expenditures were primarily for tooling at contract manufacturers. In fiscal 2014, we spent $402 primarily for tooling at a contract manufacturer and equipment to be used in our clinical trials.
At June 30, 2014, we had four distributors that accounted for 16%, 16%, 14% and 10% of accounts receivable. At June 30, 2013, we had four distributors that accounted for 28%, 18%, 10% and 10% of accounts receivable.
Revenues from one distributor totaled $2,288 or 14%, $2,299 or 13% and $1,870 or 10% of net revenues for the years ended June 30, 2014, 2013 and 2012, respectively. Revenues from another distributor totaled $2,102 or 13% and $2,057 or 11% of net revenues for the years ended June 30, 2014 and 2013, respectively. Revenues from a customer totaled $1,849 or 12% for the year ended June 30, 2014.
The Company manages the concentration of credit risk with these customers through a variety of methods including, letters of credit with financial institutions, pre-shipment deposits, credit reference checks and credit limits. Although management believes that these customers are sound and creditworthy, a severe adverse impact on their business operations could have a corresponding material effect on their ability to pay timely and therefore on our net revenues, cash flows and financial condition.
Critical Accounting Policies
The preparation of these consolidated financial statements requires the Company to make estimates and judgments that affect the reported amounts of assets, liabilities, revenues and expenses and related disclosure of contingent assets and liabilities. On an on-going basis, the Company evaluates its estimates, including those related to stock-based compensation, bad debts, inventories, warranties, contingencies and litigation. The Company bases its estimates on historical experience and on various other assumptions that are believed to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions.
The Company believes the following critical accounting policies affect its more significant judgments and estimates used in the preparation of its consolidated financial statements.
Goodwill, Intangible Assets and Impairment Assessments
Goodwill represents the excess of the purchase price in a business combination over the fair value of net tangible and intangible assets acquired. Intangible assets that are not considered to have an indefinite useful life are amortized over their useful lives, which generally range from three to ten years. Clinical protocols are not expected to provide economic benefit until they are introduced to the marketplace or licensed to an independent entity. Each period we evaluate the estimated remaining useful lives of purchased intangible assets and whether events or changes in circumstances warrant a revision to the remaining periods of amortization.
The carrying amounts of these assets are periodically reviewed for impairment (at least annually) and whenever events or changes in circumstances indicate that the carrying value of these assets may not be recoverable. According to ASC 350, Intangibles-Goodwill and Other, we can opt to perform a qualitative assessment, if we determine that the fair value of a reporting unit is more likely than not (i.e., a likelihood of more than 50 percent) to be less than its carrying amount, the two step impairment test will be performed. In the first step, we compare the fair value of our sole reporting unit to its carrying value. If the fair value of the reporting unit exceeds the carrying value, goodwill is not considered impaired and we are not required to perform further testing. If the fair value of the reporting unit does not exceed the carrying value, then we must perform the second step of the impairment test in order to determine the implied fair value of the goodwill. If the carrying value of goodwill exceeds its implied fair value, then we would record an impairment loss equal to the difference. Recoverability of finite lived intangible assets is measured by comparison of the carrying amount of the asset to the future undiscounted cash flows the asset is expected to generate.
Revenue Recognition
Revenues from the sale of the Company’s products are recognized when persuasive evidence of an arrangement exists, delivery has occurred (or services have been rendered), the price is fixed or determinable, and collectability is reasonably assured. We generally ship products F.O.B. shipping point. There is no conditional evaluation on any product sold and recognized as revenue. Amounts billed in excess of revenue recognized are recorded as deferred revenue on the balance sheet.
The Company’s sales are generally through distributors. There is no right of return provided for distributors. For sales of products made to distributors, we consider a number of factors in determining whether revenue is recognized upon transfer of title to the distributor, or when payment is received. These factors include, but are not limited to, whether the payment terms offered to the distributor are considered to be non-standard, the distributor history of adhering to the terms of its contractual arrangements with the Company, the level of inventories maintained by the distributor, whether we have a pattern of granting concessions for the benefit of the distributor, and whether there are other conditions that may indicate that the sale to the distributor is not substantive. We currently recognize revenue primarily on the sell-in method with our distributors.
Revenue arrangements with multiple deliverables are divided into units of accounting if certain criteria are met, including whether the deliverable item(s) has (have) value to the customer on a stand-alone basis. Revenue for each unit of accounting is recognized as the unit of accounting is delivered. Arrangement consideration is allocated to each unit of accounting based upon the relative estimated selling prices of the separate units of accounting contained within an arrangement containing multiple deliverables. Estimated selling prices are determined using VSOE, when available, or an estimate of selling price when VSOE is not available for a given unit of accounting. Significant inputs for the estimates of the selling price of separate units of accounting include market and pricing trends and a customer’s geographic location. We account for training and installation, and service agreements and the collection, processing and testing of the umbilical cord blood and the storage as separate units of accounting.
Service revenue generated from contracts for providing maintenance of equipment is amortized over the life of the agreement. Revenue generated from storage contracts is deferred and recorded ratably over the life of the agreement, up to 21 years. All other service revenue is recognized at the time the service is completed.
For licensing agreements pursuant to which the Company receives up-front licensing fees for products or technologies that will be provided by the Company over the term of the arrangements, the Company defers the up-front fees and recognizes the fees as revenue on a straight-line method over the term of the respective license. For license agreements that require no continuing performance on the Company’s part, license fee revenue is recognized immediately upon grant of the license.
Revenues are net of normal discounts. Shipping and handling fees billed to customers are included in net revenues, while the related costs are included in cost of revenues.
Stock-Based Compensation
The Company uses the Black-Scholes-Merton option-pricing formula in determining the fair value of the Company’s options at the grant date and applies judgment in estimating the key assumptions that are critical to the model such as the expected term, volatility and forfeiture rate of an option. The Company’s estimate of these key assumptions is based on historical information and judgment regarding market factors and trends. If any of the key assumptions change significantly, stock-based compensation expense for new awards may differ materially in the future from that recorded in the current period. The compensation expense is then amortized over the vesting period.
Income Taxes
The Company’s estimates of income taxes and the significant items resulting in the recognition of deferred tax assets and liabilities reflect our assessment of future tax consequences of transactions that have been reflected in the financial statements or tax returns for each taxing jurisdiction in which the Company operates. We base our provision for income taxes on our current period results of operations, changes in deferred income tax assets and liabilities, income tax rates, and changes in estimates of uncertain tax positions in the jurisdictions in which the Company operates. The Company recognizes deferred tax assets and liabilities when there are temporary differences between the financial reporting basis and tax basis of assets and liabilities and for the expected benefits of using net operating loss and tax credit loss carryforwards. The Company establishes valuation allowances when necessary to reduce the carrying amount of deferred income tax assets to the amounts that the Company believes are more likely than not to be realized. The Company evaluates the need to retain all or a portion of the valuation allowance on recorded deferred tax assets. When a change in the tax rate or tax law has an impact on deferred taxes, the Company applies the change based on the years in which the temporary differences are expected to reverse. As the Company operates in more than one state, changes in the state apportionment factors, based on operational results, may affect future effective tax rates and the value of recorded deferred tax assets and liabilities. The Company records a change in tax rates in the consolidated financial statements in the period of enactment.
Income tax consequences that arise in connection with a business combination include identifying the tax basis of assets and liabilities acquired and any contingencies associated with uncertain tax positions assumed or resulting from the business combination. Deferred tax assets and liabilities related to temporary differences of an acquired entity are recorded as of the date of the business combination and are based on the Company’s estimate of the appropriate tax basis that will be accepted by the various taxing authorities and its determination as to whether any of the acquired deferred tax liabilities could be a source of taxable income to realize the Company’s pre-existing deferred tax assets.
Warranty
The Company provides for the estimated cost of product warranties at the time revenue is recognized. While the Company engages in extensive product quality programs and processes, including actively monitoring and evaluating the quality of its component suppliers, the Company’s warranty obligation is affected by product failure rates, material usage and service delivery costs incurred in correcting a product failure. Should actual product failure rates, material usage or service delivery costs differ from the Company’s estimates, revisions to the estimated warranty liability could have a material impact on the Company’s financial position, cash flows or results of operations.
Inventory Valuation
The Company states inventories at lower of cost or market value determined on a first-in, first-out basis. The Company provides inventory allowances when conditions indicate that the selling price could be less than cost due to physical deterioration, obsolescence, changes in price levels, or other causes, which it includes as a component of cost of revenues. Additionally, the Company provides valuation allowances for excess and slow-moving inventory on hand that are not expected to be sold to reduce the carrying amount of slow-moving inventory to its estimated net realizable value. The valuation allowances are based upon estimates about future demand from our customers and distributors and market conditions. Because some of the Company’s products are highly dependent on government and third-party funding, current customer use and validation, and completion of regulatory and field trials, there is a risk that we will forecast incorrectly and purchase or produce excess inventories. As a result, actual demand may differ from forecasts and the Company may be required to record additional inventory valuation allowances that could adversely impact our gross margins. Conversely, favorable changes in demand could result in higher gross margins when those products are sold.
Off Balance Sheet Arrangements
The Company has no off- balance sheet arrangements.
We are a smaller reporting company as defined by Rule 12b-2 of the SEC Act of 1934 and are not required to provide information under this item.
|
|
Page
Number
|
|
|
|
|
Report of Independent Registered Public Accounting Firm
|
40
|
|
|
|
|
Consolidated Balance Sheets at June 30, 2014 and 2013
|
41
|
|
|
|
|
Consolidated Statements of Operations and Comprehensive Loss for the years ended June 30, 2014, 2013 and 2012
|
42
|
|
|
|
|
Consolidated Statements of Stockholders’ Equity for the years ended June 30, 2014, 2013 and 2012
|
43
|
|
|
|
|
Consolidated Statements of Cash Flows for the years ended June 30, 2014, 2013 and 2012
|
44
|
|
|
|
|
Notes to Consolidated Financial Statements
|
45
|
Report of Independent Registered Public Accounting Firm
The Board of Directors and Stockholders of Cesca Therapeutics Inc.
We have audited the accompanying consolidated balance sheets of Cesca Therapeutics Inc. as of June 30, 2014 and 2013, and the related consolidated statements of operations and comprehensive loss, stockholders’ equity, and cash flows for each of the three years in the period ended June 30, 2014. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. We were not engaged to perform an audit of the Company’s internal control over financial reporting. Our audits included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the consolidated financial position of Cesca Therapeutics Inc. at June 30, 2014 and 2013, and the consolidated results of its operations and its cash flows for each of the three years in the period ended June 30, 2014, in conformity with U.S. generally accepted accounting principles.
|
|
/s/ Ernst & Young LLP
|
|
|
|
|
Sacramento, California
|
|
|
September 29, 2014
|
|
CESCA THERAPEUTICS INC.
CONSOLIDATED BALANCE SHEETS
(in thousands, except share and per share amounts)
|
ASSETS
|
June 30, 2014
|
June 30, 2013
|
||||||
|
|
||||||||
|
Current assets:
|
||||||||
|
Cash and cash equivalents
|
$
|
14,811
|
$
|
6,884
|
||||
|
Accounts receivable, net of allowance for doubtful accounts of $47 ($47 at June 30, 2013)
|
4,693
|
4,898
|
||||||
|
Inventories
|
5,606
|
4,259
|
||||||
|
Prepaid expenses and other current assets
|
217
|
232
|
||||||
|
Total current assets
|
25,327
|
16,273
|
||||||
|
|
||||||||
|
Equipment at cost less accumulated depreciation of $4,099 ($3,277 at June 30, 2013)
|
2,298
|
2,208
|
||||||
|
Goodwill
|
13,254 |
--
|
||||||
|
Intangible assets, net
|
21,928
|
--
|
||||||
|
Other assets
|
81
|
48
|
||||||
|
Total assets
|
$
|
62,888 |
$
|
18,529
|
||||
|
LIABILITIES AND STOCKHOLDERS’ EQUITY
|
||||||||
|
|
||||||||
|
Current liabilities:
|
||||||||
|
Accounts payable
|
$
|
3,590
|
$
|
3,106
|
||||
|
Accrued payroll and related expenses
|
599
|
477
|
||||||
|
Deferred revenue
|
638
|
377
|
||||||
|
Other current liabilities
|
1,553
|
1,188
|
||||||
|
Total current liabilities
|
6,380
|
5,148
|
||||||
|
Noncurrent deferred tax liability
|
7,641 |
--
|
||||||
|
Other noncurrent liabilities
|
169
|
63
|
||||||
|
|
||||||||
|
Commitments and contingencies (Footnote 5)
|
||||||||
|
|
||||||||
|
Stockholders’ equity:
|
||||||||
|
Preferred stock, $0.001 par value; 2,000,000 shares authorized, none issued and outstanding at June 30, 2014 and 2013
|
--
|
--
|
||||||
|
|
||||||||
|
Common stock, $0.001 par value; 80,000,000 shares authorized; 40,200,529 issued and outstanding (16,557,627 at June 30, 2013)
|
40
|
16
|
||||||
|
Paid in capital in excess of par
|
171,422
|
127,493
|
||||||
|
Accumulated deficit
|
(122,822
|
)
|
(114,191
|
)
|
||||
|
Accumulated other comprehensive income
|
58
|
--
|
||||||
|
|
||||||||
|
Total stockholders’ equity
|
48,698
|
13,318
|
||||||
|
|
||||||||
|
Total liabilities and stockholders’ equity
|
$
|
62,888 |
$
|
18,529
|
||||
CESCA THERAPEUTICS INC.
CONSOLIDATED STATEMENTS OF OPERATIONS AND COMPREHENSIVE LOSS
(in thousands, except share and per share amounts)
|
|
Years ended June 30
|
|||||||||||
|
|
2014
|
2013
|
2012
|
|||||||||
|
Net revenues
|
$
|
15,987
|
$
|
17,963
|
$
|
19,023
|
||||||
|
Cost of revenues
|
10,101
|
11,598
|
12,690
|
|||||||||
|
Gross profit
|
5,886
|
6,365
|
6,333
|
|||||||||
|
|
||||||||||||
|
Expenses:
|
||||||||||||
|
Sales and marketing
|
2,968
|
2,955
|
2,761
|
|||||||||
|
Research and development
|
3,468
|
2,991
|
3,729
|
|||||||||
|
General and administrative
|
8,490
|
5,645
|
5,222
|
|||||||||
|
Gain on sale of product lines
|
--
|
(2,161
|
)
|
--
|
||||||||
|
Total operating expenses
|
14,926
|
9,430
|
11,712
|
|||||||||
|
Loss from operations
|
(9,040
|
)
|
(3,065
|
)
|
(5,379
|
)
|
||||||
|
|
||||||||||||
|
Interest and other income (expense), net
|
6
|
(21
|
)
|
393
|
||||||||
|
Loss before income tax benefits
|
(9,034 | ) |
(3,086
|
) |
(4,986
|
) | ||||||
| Deferred income tax benefit | 403 |
--
|
--
|
|||||||||
|
Net loss
|
$
|
(8,631
|
)
|
$
|
(3,086
|
)
|
$
|
(4,986
|
)
|
|||
|
|
||||||||||||
|
Net loss
|
$
|
(8,631
|
)
|
$
|
(3,086
|
)
|
$
|
(4,986
|
)
|
|||
|
Other comprehensive income:
|
||||||||||||
|
Foreign currency translation adjustments
|
58
|
--
|
--
|
|||||||||
|
Comprehensive loss
|
$
|
(8,573
|
)
|
$
|
(3,086
|
)
|
$
|
(4,986
|
)
|
|||
|
|
||||||||||||
|
Per share data:
|
||||||||||||
|
Basic and diluted net loss per common share
|
$
|
(0.36
|
)
|
$
|
(0.19
|
)
|
$
|
(0.30
|
)
|
|||
|
|
||||||||||||
|
Shares used in computing per share data
|
24,234,348
|
16,526,578
|
16,389,008
|
|||||||||
See accompanying notes.
CESCA THERAPEUTICS INC.
CONSOLIDATED STATEMENTS OF STOCKHOLDERS' EQUITY
(in thousands, except share and per share amounts)
|
Common Stock
|
||||||||||||||||||||||||
|
|
Shares
|
Amount
|
Paid in
capital in
excess of par
|
Accumulated
deficit
|
Accumulated
other
comprehensive
income
|
Total stockholders’
equity
|
||||||||||||||||||
|
Balance at June 30, 2011
|
16,346,366
|
$
|
16
|
$
|
126,196
|
$
|
(106,119
|
)
|
--
|
$
|
20,093
|
|||||||||||||
|
Issuance of common shares and compensation related to unrestricted common stock awards
|
60,000
|
--
|
88
|
--
|
--
|
88
|
||||||||||||||||||
|
Issuance of common shares and compensation related to restricted common stock awards
|
6,700
|
--
|
326
|
--
|
--
|
326
|
||||||||||||||||||
|
Stock-based compensation expense
|
--
|
--
|
377
|
--
|
--
|
377
|
||||||||||||||||||
|
Net loss
|
--
|
--
|
--
|
(4,986
|
)
|
--
|
(4,986
|
)
|
||||||||||||||||
|
Balance at June 30, 2012
|
16,413,066
|
16
|
126,987
|
(111,105
|
)
|
--
|
15,898
|
|||||||||||||||||
|
Issuance of common shares and compensation related to restricted common stock awards, net of stock surrenders
|
115,944
|
--
|
275
|
--
|
--
|
275
|
||||||||||||||||||
|
Stock-based compensation expense
|
--
|
--
|
198
|
--
|
--
|
198
|
||||||||||||||||||
|
Common stock issued to directors in lieu of cash compensation
|
28,617
|
--
|
33
|
--
|
--
|
33
|
||||||||||||||||||
|
Net loss
|
--
|
--
|
--
|
(3,086
|
)
|
--
|
(3,086
|
)
|
||||||||||||||||
|
Balance at June 30, 2013
|
16,557,627
|
16
|
127,493
|
(114,191
|
)
|
--
|
13,318
|
|||||||||||||||||
|
Issuance of common shares and compensation related to restricted common stock awards, net of stock surrenders
|
140,591
|
1
|
333
|
--
|
--
|
334
|
||||||||||||||||||
|
Stock-based compensation expense
|
--
|
--
|
209
|
--
|
--
|
209
|
||||||||||||||||||
|
Common stock issued to directors in lieu of cash compensation
|
52,582
|
--
|
68
|
--
|
--
|
68
|
||||||||||||||||||
|
Issuance of common shares & warrants in public offering
|
7,530,000
|
8
|
10,053
|
--
|
--
|
10,061
|
||||||||||||||||||
|
Issuance of common shares & warrants in private placement
|
3,336,800
|
3
|
5,940
|
--
|
--
|
5,943
|
||||||||||||||||||
|
Issuance of common shares & warrants pursuant to acquisition
|
12,490,841
|
12
|
27,118
|
--
|
--
|
27,130
|
||||||||||||||||||
|
Issuance of common shares for repayment of related party notes payable
|
82,713
|
--
|
187
|
--
|
--
|
187
|
||||||||||||||||||
|
Issuance of common shares for exercise of options
|
9,375
|
--
|
21
|
--
|
--
|
21 | ||||||||||||||||||
|
Foreign currency translation
|
--
|
--
|
--
|
--
|
$
|
58
|
58
|
|||||||||||||||||
|
Net loss
|
--
|
--
|
--
|
(8,631
|
)
|
--
|
(8,631
|
)
|
||||||||||||||||
|
Balance at June 30, 2014
|
40,200,529
|
$
|
40
|
$
|
171,422
|
$
|
(122,822
|
)
|
$
|
58
|
$
|
48,698
|
||||||||||||
See accompanying notes.
CESCA THERAPEUTICS INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS
(amounts in thousands, except share and per share amounts)
|
|
Years ended June 30,
|
|||||||||||
|
|
2014
|
2013
|
2012
|
|||||||||
|
|
||||||||||||
|
Cash flows from operating activities:
|
||||||||||||
|
Net loss
|
$
|
(8,631
|
)
|
$
|
(3,086
|
)
|
$
|
(4,986
|
)
|
|||
|
Adjustments to reconcile net loss to net cash used in operating activities:
|
||||||||||||
| Deferred income tax benefit | (403 | ) |
--
|
--
|
||||||||
|
Depreciation and amortization
|
993
|
538
|
604
|
|||||||||
|
Stock-based compensation expense
|
679
|
563
|
791
|
|||||||||
|
Loss on retirement of equipment
|
--
|
25
|
17
|
|||||||||
|
Impairment of intangible asset
|
--
|
164
|
--
|
|||||||||
|
Gain on sale of product lines
|
--
|
(2,161
|
)
|
--
|
||||||||
|
Net changes in operating assets and liabilities:
|
||||||||||||
|
Accounts receivable, net
|
515
|
(340
|
)
|
(655
|
)
|
|||||||
|
Inventories
|
(1,279
|
)
|
795
|
120
|
||||||||
|
Prepaid expenses and other current assets
|
64
|
106
|
(38
|
)
|
||||||||
|
Other assets
|
(9
|
)
|
--
|
1
|
||||||||
|
Accounts payable
|
(295
|
)
|
619
|
696
|
||||||||
|
Accrued payroll and related expenses
|
96
|
(130
|
)
|
223
|
||||||||
|
Deferred revenue
|
282
|
(47
|
)
|
2
|
||||||||
|
Other liabilities
|
152
|
(128
|
)
|
(660
|
)
|
|||||||
|
Net cash used in operating activities
|
(7,836
|
)
|
(3,082
|
)
|
(3,885
|
)
|
||||||
|
Cash flows from investing activities:
|
||||||||||||
|
Capital expenditures
|
(402
|
)
|
(391
|
)
|
(545
|
)
|
||||||
|
Cash acquired in acquisition
|
351
|
--
|
--
|
|||||||||
|
Proceeds from sale of product lines
|
--
|
2,535
|
--
|
|||||||||
|
Net cash provided by(used in) investing activities
|
(51
|
)
|
2,144
|
(545
|
)
|
|||||||
|
Cash flows from financing activities:
|
||||||||||||
|
Repayment of related party notes payable
|
(150
|
)
|
--
|
--
|
||||||||
|
Repurchase of common stock
|
(68
|
)
|
(57
|
)
|
--
|
|||||||
|
Exercise of stock options
|
21
|
--
|
--
|
|||||||||
|
Issuance of common stock
|
16,004
|
--
|
--
|
|||||||||
|
Net cash provided by(used in) financing activities
|
15,807
|
(57
|
)
|
--
|
||||||||
|
Effects of foreign currency rate changes on cash and cash equivalents
|
7
|
--
|
--
|
|||||||||
|
Net (decrease)increase in cash and cash equivalents
|
7,927
|
(995
|
)
|
(4,430
|
)
|
|||||||
|
Cash and cash equivalents at beginning of year
|
6,884
|
7,879
|
12,309
|
|||||||||
|
Cash and cash equivalents at end of year
|
$
|
14,811
|
$
|
6,884
|
$
|
7,879
|
||||||
|
|
||||||||||||
|
Supplemental non-cash financing and investing information:
|
||||||||||||
|
Transfer of inventories to equipment
|
$
|
99
|
$
|
834
|
--
|
|||||||
|
Transfer of other current assets to inventories
|
--
|
--
|
$
|
120
|
||||||||
|
Acquisitions of intangible asset in exchange for forgiveness of accounts receivable and assumption of liabilities
|
--
|
--
|
$
|
390
|
||||||||
|
Stock issued for repayment of related party note payable
|
$
|
187
|
--
|
--
|
||||||||
See accompanying notes.
CESCA THERAPEUTICS INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(in thousands except shares and per share amounts)
|
1.
|
Summary of Significant Accounting Policies
|
Organization and Basis of Presentation
Cesca Therapeutics Inc. (the Company, we or our) is focused on the research, development, and commercialization of autologous cell-based therapeutics for use in regenerative medicine. We are a leader in developing and manufacturing automated blood and bone marrow processing systems that enable the separation, processing and preservation of cell and tissue therapy products. During the quarter ended March 31, 2014, Cesca Therapeutics Inc. was formed by the merger of ThermoGenesis Corp. and TotipotentRX. See footnote 2 for details of the transaction.
Principles of Consolidation
The consolidated financial statements include the accounts of Cesca Therapeutics Inc., and our wholly-owned subsidiaries, TotipotentRX Cell Therapy, Pvt. Ltd. and TotipotentSC Scientific Product Pvt. Ltd. All significant intercompany accounts and transactions have been eliminated upon consolidation.
Use of Estimates
Preparation of financial statements in conformity with U.S. generally accepted accounting principles and pursuant to the rules and regulations of the SEC requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities at the date of the consolidated financial statements and the reported amounts of revenues and expenses during the reporting period. Estimates are used for, but not limited to, the allowance for doubtful accounts, allocation of arrangement consideration, slow-moving inventory reserves, depreciation, warranty costs, certain accruals and contingencies. Actual results could materially differ from the estimates and assumptions used in the preparation of our consolidated financial statements. Events subsequent to the balance sheet date have been evaluated for inclusion in the accompanying consolidated financial statements through the date of issuance.
Revenue Recognition
Revenues from the sale of the Company’s products are recognized when persuasive evidence of an arrangement exists, delivery has occurred (or services have been rendered), the price is fixed or determinable, and collectability is reasonably assured. We generally ship products F.O.B. shipping point. There is no conditional evaluation on any product sold and recognized as revenue. Amounts billed in excess of revenue recognized are recorded as deferred revenue on the balance sheet.
The Company’s sales are generally through distributors. There is no right of return provided for distributors. For sales of products made to distributors, we consider a number of factors in determining whether revenue is recognized upon transfer of title to the distributor, or when payment is received. These factors include, but are not limited to, whether the payment terms offered to the distributor are considered to be non-standard, the distributor history of adhering to the terms of its contractual arrangements with the Company, the level of inventories maintained by the distributor, whether we have a pattern of granting concessions for the benefit of the distributor, and whether there are other conditions that may indicate that the sale to the distributor is not substantive. We currently recognize revenue primarily on the sell-in method with our distributors.
CESCA THERAPEUTICS INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (CONTINUED)
(in thousands except shares and per share amounts)
| 1. | Summary of Significant Accounting Policies (Continued) |
Revenue Recognition (Continued)
Revenue arrangements with multiple deliverables are divided into units of accounting if certain criteria are met, including whether the deliverable item(s) has (have) value to the customer on a stand-alone basis. Revenue for each unit of accounting is recognized as the unit of accounting is delivered. Arrangement consideration is allocated to each unit of accounting based upon the relative estimated selling prices of the separate units of accounting contained within an arrangement containing multiple deliverables. Estimated selling prices are determined using VSOE, when available, or an estimate of selling price when VSOE is not available for a given unit of accounting. Significant inputs for the estimates of the selling price of separate units of accounting include market and pricing trends and a customer’s geographic location. We account for training and installation, and service agreements and the collection, processing and testing of the umbilical cord blood and the storage as separate units of accounting.
Service revenue generated from contracts for providing maintenance of equipment is amortized over the life of the agreement. Revenue generated from storage contracts is deferred and recorded ratably over the life of the agreement, up to 21 years. All other service revenue is recognized at the time the service is completed.
For licensing agreements pursuant to which the Company receives up-front licensing fees for products or technologies that will be provided by the Company over the term of the arrangements, the Company defers the up-front fees and recognizes the fees as revenue on a straight-line method over the term of the respective license. For license agreements that require no continuing performance on the Company’s part, license fee revenue is recognized immediately upon grant of the license.
Revenues are net of normal discounts. Shipping and handling fees billed to customers are included in net revenues, while the related costs are included in cost of revenues.
Cash and Cash Equivalents
We consider all highly liquid investments with a maturity of three months or less at the time of purchase to be cash equivalents.
Foreign Currency Translation
Our reporting currency is the US dollar. The functional currency of our subsidiaries in India is the Indian rupee (INR). Assets and liabilities are translated into US dollars at period end exchange rates. Revenue and expenses are translated at average rates of exchange prevailing during the periods presented. Cash flows were also translated at average exchange rates for the period, therefore, amounts reported on the consolidated statement of cash flows did not necessarily agree with changes in the corresponding balances on the consolidated balance sheet. Equity accounts other than retained earnings are translated at the historic exchange rate on the date of investment. A translation adjustment of $58 gain for the year ended June 30, 2014 resulting from this process is recorded as a component of other comprehensive income.
Goodwill, Intangible Assets and Impairment Assessments
Goodwill represents the excess of the purchase price in a business combination over the fair value of net tangible and intangible assets acquired. Intangible assets that are not considered to have an indefinite useful life are amortized over their useful lives, which generally range from three to ten years. Clinical protocols are not expected to provide economic benefit until they are introduced to the marketplace or licensed to an independent entity. Each period we evaluate the estimated remaining useful lives of purchased intangible assets and whether events or changes in circumstances warrant a revision to the remaining periods of amortization.
The carrying amounts of these assets are periodically reviewed for impairment (at least annually) and whenever events or changes in circumstances indicate that the carrying value of these assets may not be recoverable. According to ASC 350, Intangibles-Goodwill and Other, we can opt to perform a qualitative assessment, if we determine that the fair value of a reporting unit is more likely than not (i.e., a likelihood of more than 50 percent) to be less than its carrying amount, the two step impairment test will be performed. In the first step, we compare the fair value of our sole reporting unit to its carrying value. If the fair value of the reporting unit exceeds the carrying value, goodwill is not considered impaired and we are not required to perform further testing. If the fair value of the reporting unit does not exceed the carrying value, then we must perform the second step of the impairment test in order to determine the implied fair value of the goodwill. If the carrying value of goodwill exceeds its implied fair value, then we would record an impairment loss equal to the difference. Recoverability of finite lived intangible assets is measured by comparison of the carrying amount of the asset to the future undiscounted cash flows the asset is expected to generate.
CESCA THERAPEUTICS INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (CONTINUED)
(in thousands except shares and per share amounts)
| 1. | Summary of Significant Accounting Policies (Continued) |
Fair Value of Financial Instruments
The carrying values of cash and cash equivalents, accounts receivable, accounts payable and accrued liabilities, approximate fair value due to their short duration. As of June 30, 2014, we had approximately $354 in cash equivalents classified as Level 1 assets, which are based on quoted market prices in active markets for identical assets. As of June 30, 2013, we had no Level 1 financial instruments. As of June 30, 2014 and 2013, we did not have any Level 2 or 3 financial instruments.
Accounts Receivable and Allowance for Doubtful Accounts
The Company’s receivables are recorded when billed and represent claims against third parties that will be settled in cash. The carrying value of the Company’s receivables, net of the allowance for doubtful accounts, represents their estimated net realizable value. We estimate our allowance for doubtful accounts based on historical collection trends, age of outstanding receivables and existing economic conditions. If events or changes in circumstances indicate that a specific receivable balance may be impaired, further consideration is given to the collectability of those balances and the allowance is adjusted accordingly. A customer’s receivable balance is considered past-due based on its contractual terms. Past-due receivable balances are written-off when the Company’s internal collection efforts have been unsuccessful in collecting the amount due.
Inventories
Inventories are stated at the lower of cost or market and include the cost of material, labor and manufacturing overhead. Cost is determined on the first-in, first-out basis. The Company provides inventory allowances to write down inventory to its estimated net realizable value when conditions indicate that the selling price could be less than cost due to physical deterioration, obsolescence, changes in price levels, or other causes, which it includes as a component of cost of revenues.
Equipment
Equipment is recorded at cost. Repairs and maintenance costs are expensed as incurred. Depreciation for office, computer, machinery and equipment is computed under the straight-line method over the estimated useful lives. Leasehold improvements are depreciated under the straight line method over their estimated useful lives or the remaining lease period, whichever is shorter.
Warranty
We provide for the estimated cost of product warranties at the time revenue is recognized. The Company’s warranty obligation is calculated based on estimated product failure rates, material usage and estimated service delivery costs incurred in correcting a product failure.
CESCA THERAPEUTICS INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (CONTINUED)
(in thousands except shares and per share amounts)
| 1. | Summary of Significant Accounting Policies (Continued) |
Stock-Based Compensation
We have three stock-based compensation plans, which are described more fully in Note 6.
Valuation and Amortization Method – We estimate the fair value of stock options granted using the Black-Scholes-Merton option-pricing formula. This fair value is then amortized on a straight-line basis over the requisite service periods of the awards, which is generally the vesting period.
Expected Term – For options which we have limited available data, the expected term of the option is based on the simplified method. This simplified method averages an award’s vesting term and its contractual term. For all other options, the Company's expected term represents the period that the Company's stock-based awards are expected to be outstanding and was determined based on historical experience of similar awards, giving consideration to the contractual terms of the stock-based awards, vesting schedules and expectations of future employee behavior.
Expected Volatility – Expected volatility is based on historical volatility. Historical volatility is computed using daily pricing observations for recent periods that corresponded to the expected term of the options.
Expected Dividend – We have not declared dividends and we do not anticipate declaring any dividends in the foreseeable future. Therefore, we use a zero value for the expected dividend value factor to determine the fair value of options granted.
Risk-Free Interest Rate – The Company bases the risk-free interest rate used in the valuation method on the implied yield currently available on U.S. Treasury zero-coupon issues with the same or substantially equivalent remaining term.
Estimated Forfeitures – When estimating forfeitures, we consider voluntary and involuntary termination behavior as well as analysis of actual option forfeitures.
The fair value of the Company’s stock options granted to employees for the years ended June 30, 2014, 2013 and 2012 was estimated using the following weighted-average assumptions:
|
|
2014
|
2013
|
2012
|
|||||||||
|
Expected life (years)
|
4
|
4
|
4
|
|||||||||
|
Risk-free interest rate
|
1.1
|
%
|
0.5
|
%
|
1.2
|
%
|
||||||
|
Expected volatility
|
75
|
%
|
78
|
%
|
82
|
%
|
||||||
|
Dividend yield
|
0
|
%
|
0
|
%
|
0
|
%
|
||||||
The weighted average grant date fair value of options granted during the years ended June 30, 2014, 2013 and 2012 was $1.15, $0.52 and $1.17, respectively.
CESCA THERAPEUTICS INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (CONTINUED)
(in thousands except shares and per share amounts)
| 1. | Summary of Significant Accounting Policies (Continued) |
Research and Development
Research and development costs, consisting of salaries and benefits, costs of clinical trials, costs of disposables, facility costs, contracted services and stock-based compensation from the engineering, regulatory, scientific and clinical affairs departments, that are useful in developing and clinically testing new products, services, processes or techniques, as well as expenses for activities that may significantly improve existing products or processes are expensed as incurred. Costs to acquire technologies that are utilized in research and development and that have no future benefit are expensed when incurred.
Credit Risk
Financial instruments that potentially subject the Company to a concentration of credit risk consist of cash and cash equivalents. Our cash is maintained in checking accounts, money market funds and certificates of deposits with reputable financial institutions. We have not experienced any realized losses on our deposits of cash and cash equivalents.
Today, we primarily manufacture and sell cellular processing systems and thermodynamic devices principally to the blood and cellular component processing industry and perform ongoing evaluations of the credit worthiness of our customers. We believe that adequate provisions for uncollectible accounts have been made in the accompanying consolidated financial statements. To date, we have not experienced significant credit related losses.
Segment Reporting
We have one reportable business segment: the research, development, and commercialization of autologous cell-based therapeutics for use in regenerative medicine.
Income Taxes
The tax years 1994-2013 remain open to examination by the major taxing jurisdictions to which we are subject; however, there is no current investigation. The Company’s policy is to recognize interest and penalties related to the underpayment of income taxes as a component of income tax expense. To date, there have been no interest or penalties charged to the Company in relation to the underpayment of income taxes. There were no unrecognized tax benefits during all the periods presented.
The Company’s estimates of income taxes and the significant items resulting in the recognition of deferred tax assets and liabilities reflect our assessment of future tax consequences of transactions that have been reflected in the financial statements or tax returns for each taxing jurisdiction in which the Company operates. We base our provision for income taxes on our current period results of operations, changes in deferred income tax assets and liabilities, income tax rates, and changes in estimates of uncertain tax positions in the jurisdictions in which the Company operates. The Company recognizes deferred tax assets and liabilities when there are temporary differences between the financial reporting basis and tax basis of assets and liabilities and for the expected benefits of using net operating loss and tax credit loss carryforwards. The Company establishes valuation allowances when necessary to reduce the carrying amount of deferred income tax assets to the amounts that the Company believes are more likely than not to be realized. The Company evaluates the need to retain all or a portion of the valuation allowance on recorded deferred tax assets. When a change in the tax rate or tax law has an impact on deferred taxes, the Company applies the change based on the years in which the temporary differences are expected to reverse. As the Company operates in more than one state, changes in the state apportionment factors, based on operational results, may affect future effective tax rates and the value of recorded deferred tax assets and liabilities. The Company records a change in tax rates in the consolidated financial statements in the period of enactment.
Income tax consequences that arise in connection with a business combination include identifying the tax basis of assets and liabilities acquired and any contingencies associated with uncertain tax positions assumed or resulting from the business combination. Deferred tax assets and liabilities related to temporary differences of an acquired entity are recorded as of the date of the business combination and are based on the Company’s estimate of the appropriate tax basis that will be accepted by the various taxing authorities and its determination as to whether any of the acquired deferred tax liabilities could be a source of taxable income to realize the Company’s pre-existing deferred tax assets.
Medical Device Excise Tax
We are required to pay a medical device excise tax relating to U.S. sales of Class I, II and III medical devices. This new excise tax went into effect January 1, 2013, established as part of the March 2010 U.S. healthcare reform legislation and has been included in sales and marketing expense.
CESCA THERAPEUTICS INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (CONTINUED)
(in thousands except shares and per share amounts)
| 1. | Summary of Significant Accounting Policies (Continued) |
Net Loss per Share
Net loss per share is computed by dividing the net loss to common stockholders by the weighted average number of common shares outstanding. The calculation of the basic and diluted earnings per share is the same for all periods presented, as the effect of the potential common stock equivalents is anti-dilutive due to the Company’s net loss position for all periods presented. Anti-dilutive securities, which consist of stock options, common stock restricted awards and warrants, that were not included in diluted net loss per common share, were 7,185,252, 2,578,753 and 2,644,209 of June 30, 2014, 2013 and 2012, respectively.
Reclassifications
Certain amounts in the prior year’s financial statements have been reclassified to conform with the 2014 presentation. These reclassifications had no effect on previously reported total assets, net loss or stockholders’ equity.
Recently Adopted Accounting Pronouncements
In February 2013, the FASB issued ASC 2013-02, which is an update to improve the reporting of reclassifications out of accumulated other comprehensive income (“AOCI”). Companies are also required to present reclassifications by component when reporting changes in AOCI balances. We adopted ASC 2013-02 effective July 1, 2013. The adoption of ASC 2013-02 did not have a material impact on our results of operations or financial condition.
In July 2012, the FASB issued ASU 2012-02, which is an update to Topic 350, “Intangibles – Goodwill and Other”. This update provides additional guidance in performing impairment tests for indefinite-lived intangible assets by simplifying how an entity tests those assets for impairment. The update allows an entity to make a qualitative assessment about the likelihood that an indefinite-lived intangible asset is impaired to determine whether it should perform a qualitative impairment test. We adopted ASU 2012-02 effective June 30, 2013. The adoption of ASU 2012-02 did not have a material impact on our consolidated results of operations or financial condition.
In June 2011, the FASB issued ASU No. 2011-05, “Presentation of Comprehensive Income.” The guidance improves the comparability of financial reporting and facilitates the convergence of U.S. GAAP and IFRS by amending the guidance in ASC 220, “Comprehensive Income”. Under the amended guidance, an entity has the option to present the total of comprehensive income, the components of net income, and the components of other comprehensive income either in a single continuous statement of comprehensive income or in two separate but consecutive statements. In both choices, the entity is required to present on the face of the financial statements reclassification adjustments for items that are reclassified from other comprehensive income to net income in the statement(s) where the components of net income and the components of other comprehensive income are presented. We adopted this guidance retrospectively for our interim period ending September 30, 2012. The adoption of the guidance did not have a material impact on our financial condition or results of operations.
CESCA THERAPEUTICS INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (CONTINUED)
(in thousands except shares and per share amounts)
| 1. | Summary of Significant Accounting Policies (Continued) |
Recently Adopted Accounting Pronouncements (Continued)
In May 2011, the Financial Accounting Standards Board (“FASB”) issued an Accounting Standards Updates (“ASU”) to the Fair Value Measurement Topic of the FASB ASC. This update was issued in order to achieve common fair value measurement and disclosure requirements in U.S. GAAP and International Financial Reporting Standards (“IFRS”). The update clarifies that (i) the highest and best use concept applies only to the fair value measurement of nonfinancial assets, (ii) specific requirements pertain to measuring the fair value of instruments classified in a reporting entity’s shareholders’ equity and, (iii) a reporting entity should disclose quantitative information about unobservable inputs used in a fair value measurement that is categorized within Level 3 of the fair value hierarchy. The update changes requirements with regard to the fair value of financial instruments that are managed within a portfolio and with regard to the application of premiums or discounts in a fair value measurement. In addition, the update increased disclosure requirements regarding Level 3 fair value measurements to include the valuation processes used by the reporting entity and the sensitivity of the fair value measurement to changes in unobservable inputs and the interrelationships between the unobservable inputs, if any. We adopted ASU 2011-04 effective January 1, 2012. The adoption of ASU 2011-04 did not have a material impact on our consolidated results of operations or financial condition.
Recently Issued Accounting Pronouncements
In June 2014, FASB issued ASU No. 2014-12, “Compensation - Stock Compensation (Topic 718); Accounting for Share-Based Payments When the Terms of an Award Provide That a Performance Target Could Be Achieved after the Requisite Service Period”. The amendments in this ASU apply to all reporting entities that grant their employees share-based payments in which the terms of the award provide that a performance target that affects vesting could be achieved after the requisite service period. The amendments require that a performance target that affects vesting and that could be achieved after the requisite service period be treated as a performance condition. A reporting entity should apply existing guidance in Topic 718 as it relates to awards with performance conditions that affect vesting to account for such awards. For all entities, the amendments in this ASU are effective for annual periods and interim periods within those annual periods beginning after December 15, 2015. Earlier adoption is permitted.
Entities may apply the amendments in this ASU either (a) prospectively to all awards granted or modified after the effective date or (b) retrospectively to all awards with performance targets that are outstanding as of the beginning of the earliest annual period presented in the financial statements and to all new or modified awards thereafter. If retrospective transition is adopted, the cumulative effect of applying this Update as of the beginning of the earliest annual period presented in the financial statements should be recognized as an adjustment to the opening retained earnings balance at that date. Additionally, if retrospective transition is adopted, an entity may use hindsight in measuring and recognizing the compensation cost. The Company is currently reviewing the provisions of this ASU to determine if there will be any impact on its results of operations, cash flows or financial condition.
CESCA THERAPEUTICS INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (CONTINUED)
(in thousands except shares and per share amounts)
| 1. | Summary of Significant Accounting Policies (Continued) |
Recently Issued Accounting Pronouncements (Continued)
In July 2013, the FASB issued ASU 2013-11, “Presentation of an Unrecognized Tax Benefit When a Net Operating Loss carryforward, a Similar Tax Loss, or a Tax Credit Carryforward Exists”. This amendment requires entities to present an unrecognized tax benefit or a portion of an unrecognized tax benefit, as a reduction to a deferred tax asset for a net operating loss carryforward or a similar tax loss or a tax credit carryforward, unless certain conditions exist. This guidance is effective prospectively for annual reporting periods (and the interim periods within) beginning after December 15, 2013. Early adoption and retrospective application are permitted. We expect to adopt this guidance effective July 2014. We are currently assessing the potential impact, if any the adoption of ASU 2013-11 may have on our consolidated financial statements.
In March 2013, the FASB issued ASU 2013-05, “Foreign Currency Matters” (Topic 830) which provides guidance on a parent’s accounting for the cumulative translation adjustment upon de-recognition of a subsidiary or group of assets within a foreign entity. This new guidance requires that the parent release any related cumulative translation adjustment into net income only if the sale or transfer results in the complete or substantially complete liquidation of the foreign entity in which the subsidiary or group of assets had resided. The new guidance will be effective for us beginning July 1, 2014. We are currently assessing the potential impact, if any, the adoption of ASU 2013-05 may have on our consolidated financial statements.
| 2. | Acquisition of Totipotent RX |
On February 18, 2014, the Company consummated the acquisition of TotipotentRX by merger pursuant to the Agreement and Plan of Merger and Reorganization (Merger Agreement). TotipotentRX was a privately held biomedical technology company specializing in human clinical trials in the field of regenerative medicine and a provider of cell-based therapies to the Fortis Healthcare System. TotipotentRX had two wholly-owned subsidiaries, TotipotentRX Cell Therapy Pvt. Ltd. (TotiRX India) and TotipotentSC Product Pvt. Ltd. (TotiSC India). The two subsidiaries are located in Gurgaon, a suburb of New Delhi, India. The Company believes that TotipotentRX has the depth of clinical, scientific and biological experience necessary to fully develop and effectively navigate the evolving regulatory pathways necessary to commercialize approved blockbuster cell therapies. Subsequent to February 18, 2014 Cesca has recorded revenues of approximately $351 and loss of approximately $277 for the year ended June 30, 2014 associated with the operations of TotipotentRX.
The acquisition was accounted for under the acquisition method of accounting for business combinations in accordance with FASB ASC 805, Business Combinations, which requires, among other things that the assets acquired and liabilities assumed be recognized at their fair values as of the acquisition date. Acquisition-related costs are not included as a component of the acquisition accounting, but are recognized as expenses in the periods in which the costs are incurred. Acquisition related costs of $1,715 for the year ended June 30, 2014 were included in general and administrative expenses. Any changes within the measurement period resulting from facts and circumstances that existed as of the acquisition date may result in retrospective adjustments to the provisional amounts recorded at the acquisition date.
CESCA THERAPEUTICS INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (CONTINUED)
(in thousands except shares and per share amounts)
| 2. | Acquisition of Totipotent RX (Continued) |
Pursuant to the Merger Agreement, TotipotentRX shareholders were issued in the aggregate 12,490,841 shares of the Company’s common stock, or 38% of the then outstanding common stock of the combined company, in exchange for all the TotipotentRX common stock outstanding and the Company assumed warrants of TotipotentRX representing the right to purchase approximately 61,020 shares of the Company’s common stock. All outstanding stock options to purchase shares of the TotipotentRX common stock were exercised or cancelled.
Preliminary Allocation of Consideration Transferred to Net Assets Acquired
The following represents the consideration transferred to acquire TotipotentRX and its preliminary determination of the fair value of identifiable assets acquired and liabilities assumed at the acquisition date. The Company issued 12,490,841 shares of its common stock that had a total fair value of $27,105 based on the closing market price on February 18, 2014, the acquisition date. The Company also assumed 2,004 TotipotentRX warrants, issuing 61,020 warrants to replace them. Our warrants, which are convertible into 61,020 shares of common stock, had a total fair value of $52. We also assumed $130 for the settlement of existing receivables and payables between the parties pre-merger. Property and equipment is currently stated at its historical cost basis, less accumulated depreciation, until its appropriate fair value is determined. The Company acquired $232 gross contractual amounts receivable. The difference between the gross contractual amount and the fair value of receivables is the best estimate of the contractual cash flows not expected to be collected. The final determination of the fair value of certain assets and liabilities will be completed within the 12-month measurement period from the date of acquisition as required. As a result of finalizing the fair values of our clinical protocols and other intangible assets and evaluating the resulting tax implication, we recorded the following adjustments during our measurement period: clinical protocols increased $13,829, other intangible assets decreased $527, deferred tax liability increased $8,048, other liabilities decreased $385 and goodwill decreased $5,639. Any other potential adjustments made could be material in relation to the preliminary values presented below:
|
Purchase Price:
|
||||||||
|
ThermoGenesis common shares and warrants
|
$
|
27,287
|
||||||
|
Fair value of assets acquired:
|
||||||||
|
Cash
|
$
|
351
|
||||||
|
Receivables
|
171
|
|||||||
|
Inventories
|
191
|
|||||||
|
Clinical protocols
|
19,870
|
|||||||
|
Other intangible assets
|
2,187
|
|||||||
|
Property and equipment
|
325
|
|||||||
|
Other assets
|
132
|
|||||||
|
Total assets
|
23,227
|
|||||||
|
Fair value of liabilities assumed:
|
||||||||
|
Accounts payable
|
514
|
|||||||
|
Related party notes payable
|
337
|
|||||||
| Deferred tax liability | 8,048 | |||||||
|
Other liabilities
|
295 | |||||||
|
Total liabilities
|
9,194 | |||||||
|
Net assets acquired
|
14,033 | |||||||
|
Preliminary goodwill
|
$
|
13,254 | ||||||
CESCA THERAPEUTICS INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (CONTINUED)
(in thousands except shares and per share amounts)
| 2. | Acquisition of Totipotent RX (Continued) |
Supplemental Pro Forma Data
The Company used the acquisition method of accounting to account for the Totipotent RX acquisition and, accordingly, the results of TotipotentRX are included in the Company’s consolidated financial statements for the period subsequent to the date of acquisition. The following unaudited supplemental pro forma data for the years ended June 30, 2014 and 2013 present consolidated information as if the acquisition had been completed on July 1, 2012. The pro forma results were calculated by combining the results of ThermoGenesis Corp with the stand-alone results of Totipotent RX for the pre-acquisition periods:
|
|
Years Ended
|
|||||||
|
|
June 30,
2014
|
June 30,
2013
|
||||||
|
Net revenues
|
$
|
16,619
|
$
|
19,279
|
||||
|
Net income (loss)
|
$
|
(7,922 | ) |
$
|
(4,228
|
)
|
||
The unaudited pro forma financial information is based on the preliminary allocation of consideration transferred to net assets acquired and reflects certain adjustments related to the acquisition. Such adjustments include the incremental amortization expense in connection with recording acquired identifiable intangible assets at fair value, the incremental payroll expense associated with the new executive salaries resulting from the merger, and the elimination of the impact of historical transactions between ThermoGenesis and TotipotentRX that would have been treated as intercompany transactions had the companies been consolidated. The unaudited pro forma financial information also excludes certain non-recurring expenses directly attributable to the merger in the amount of $1,958 and $921 for the years ended June 30, 2014 and 2013.
Repayment of Related Party Notes Payable
As of February 18, 2014, TotipotentRX owed $337 to two of its officers who have since joined the Company. In the Merger Agreement, Cesca agreed to pay off the notes payable at closing as follows: $75 cash to each officer for a total of $150 and the remainder in shares of common stock. Approximately 82,000 shares of common stock were issued to satisfy the remainder of the debt.
CESCA THERAPEUTICS INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (CONTINUED)
(in thousands except shares and per share amounts)
| 3. | Intangible Assets |
Intangible assets consist of the following based on our determination of the fair value of identifiable assets acquired (see footnote 2):
|
|
June 30, 2014
|
|||||||||||||||
|
|
Weighted
Average
Amortization
Period
(in Years)
|
Gross
Carrying
Amount
|
Accumulated
Amortization
|
Net
|
||||||||||||
|
Trade names
|
7
|
$
|
32
|
$
|
2
|
$
|
30
|
|||||||||
|
Licenses
|
7
|
550
|
29
|
521
|
||||||||||||
|
Customer relationships
|
3
|
477
|
59
|
418
|
||||||||||||
|
Device registration
|
7
|
218
|
12
|
206
|
||||||||||||
|
Covenants not to compete
|
5
|
955
|
72
|
883
|
||||||||||||
|
Clinical protocols
|
19,870
|
--
|
19,870
|
|||||||||||||
|
Total
|
5.3 |
$
|
22,102
|
$
|
174
|
$
|
21,928
|
|||||||||
Amortization of intangible assets was $174 for the year ended June 30, 2014. Clinical protocols have not yet been introduced to the marketplace and are therefore not yet subject to amortization. Our estimated future amortization expense for years ended June 30, is as follows:
|
Year Ended June 30,
|
||||
|
|
||||
|
2015
|
$
|
464
|
||
|
2016
|
464
|
|||
|
2017
|
405
|
|||
|
2018
|
305
|
|||
|
2019
|
233
|
|||
|
Thereafter
|
187
|
|||
|
Total
|
$
|
2,058
|
||
During the quarter ended March 31, 2012, we modified a distribution agreement to reacquire certain distribution rights related to the Res-Q product line. As part of this modification, we exchanged consideration of $390, comprised of forgiving a $60 receivable and recording liabilities of $330 to provide inventory upgrades and service agreements at no cost. We recorded those costs as an intangible asset, which was amortized to cost of revenues over the remaining life of the distribution agreement or 31 months. Total intangible amortization expense charges for the years ending June 30, 2013 and 2012 were $151 and $75, respectively.
As of June 30, 2013, while performing our annual test for impairment, we determined this asset was impaired as a result of the Res-Q patent litigation and the associated decrease and delay in projected revenues during the remaining life of the intangible asset. Therefore, the intangible asset was considered fully impaired and the carrying value of $164 was written off to cost of revenues.
CESCA THERAPEUTICS INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (CONTINUED)
(in thousands except shares and per share amounts)
| 4. | Equipment |
Equipment consisted of the following at June 30:
|
|
2014
|
2013
|
Estimated Useful Life
|
||||||
|
Machinery and equipment
|
$
|
4,746
|
$
|
4,004
|
2.5-10 years
|
||||
|
Computer and software
|
775
|
744
|
2-5 years
|
||||||
|
Office equipment
|
616
|
542
|
5-10 years
|
||||||
|
Leasehold improvements
|
260
|
195
|
Shorter of 5 years or lease term
|
||||||
|
|
6,397
|
5,485
|
|
||||||
|
Less accumulated depreciation and amortization
|
(4,099
|
)
|
(3,277
|
)
|
|
||||
|
|
$
|
2,298
|
$
|
2,208
|
|
||||
Depreciation expense for the years ended June 30, 2014, 2013 and 2012 was $788, $355 and $471 respectively.
| 5. | Commitments and Contingencies |
Operating Leases
We lease our Rancho Cordova and Emeryville facilities pursuant to operating leases, which contain scheduled rent increases. The leases expire in May 2019 and April 2020, respectively. The Emeryville lease contains a five year renewal option. We recognize rent expense on a straight-line basis over the term of the facility lease. The annual future minimum lease payments for our non-cancelable operating leases are as follows:
|
2015
|
$
|
446
|
||
|
2016
|
459
|
|||
|
2017
|
497
|
|||
|
2018
|
635
|
|||
|
2019
|
629
|
|||
|
Thereafter
|
315
|
|||
|
Total
|
$
|
2,981
|
Rent expense was $496, $431 and $470 for the years ended June 30, 2014, 2013 and 2012, respectively.
Financial Covenants
On December 31, 2013, we entered into a Sale and Purchase Agreement with Cord Blood Registry (“CBR”) in which we will supply CBR with the AXP cord blood processing system and disposables. The term of the agreement is for 5 years with automatic two-year renewal options unless CBR provides a 6 month notice of non-renewal. Additionally, we entered into the Fourth Amended and Restated Technology License and Escrow Agreement to delete or reduce the financial covenants that we must meet in order to avoid an event of default to one financial covenant, to maintain a balance of cash and short-term investments net of debt or borrowed funds of not less than $2,000 at any month end.
CESCA THERAPEUTICS INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (CONTINUED)
(in thousands except shares and per share amounts)
| 5. | Commitments and Contingencies (Continued) |
Contingencies
During the three months ended September 30, 2012, we were notified by a third party who believes that the Res-Q system infringes upon certain of its US and European patents. Subsequently the claim has not been pursued. The Company is in the process of gathering information; however, it has not yet collected enough information to assess the validity of the alleged infringement or estimate any potential financial impact; therefore, it has not made an accrual as of June 30, 2014.
On April 11, 2013, we filed an answer and counter-claims in response to the complaint Harvest Technologies Corp. (Harvest) filed on October 24, 2012, against the Company in the case captioned as Harvest Technologies Corp. v. Cesca Therapeutics, 12-cv-01354, U.S. District Court, District of Delaware (Wilmington), with the complaint being amended on February 15, 2013, to name the Company’s customer Celling as a co-defendant. In the complaint, Harvest contends that our Res-Q 60 System infringes certain Harvest patents. The counter-claims are based on anti-trust and other alleged improper conduct by Harvest and further seek declarations that the Res-Q 60 System does not infringe the patents and that the patents are invalid. Harvest filed an answer on May 20, 2013 in which they denied the assertions made by the Company in the counterclaim. The Company intends to vigorously defend itself against the Harvest claims, while aggressively pursuing its separate claims against Harvest. The Company is unable to ascertain the likelihood of any liability and has not made an accrual as of June 30, 2014.
We have given notice of our intent to cancel a contract with a product manufacturing supplier due to various manufacturing and quality issues. The supplier is disputing the contract cancellation and has asked for reimbursement of costs incurred and damages of approximately $350. We have recorded an estimated loss contingency of $80 as management considers it probable that a payment will be made.
In the normal course of operations, we may have disagreements or disputes with customers, employees or vendors. Such potential disputes are seen by management as a normal part of business. As of June 30, 2014, management believes any liability that may ultimately result from the resolution of these matters will not have a material adverse effect on our consolidated financial position, operating results or cash flows.
Warranty
We offer a warranty on all of our non-disposable products of one to two years. We warrant disposable products through their expiration date. We periodically assess the adequacy of our recorded warranty liabilities and adjust the amounts as necessary.
Changes in the Company’s product liability which is included in other current liabilities during the period are as follows:
|
|
For years ended June 30,
|
|||||||
|
|
2014
|
2013
|
||||||
|
Beginning balance
|
$
|
489
|
$
|
547
|
||||
|
Warranties issued during the period
|
172
|
224
|
||||||
|
Settlements made during the period
|
(104
|
)
|
(259
|
)
|
||||
|
Changes in liability for pre-existing warranties during the period
|
(59
|
)
|
(23
|
)
|
||||
|
Ending balance
|
$
|
498
|
$
|
489
|
||||
CESCA THERAPEUTICS INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (CONTINUED)
(in thousands except shares and per share amounts)
| 6. | Stockholders’ Equity |
Common Stock
As of June 30, 2014, we had 7,834,632 shares of common stock reserved for future issuance.
On June 18, 2014, we completed a public offering of 7,530,000 shares of common stock at $1.50 per share, together with warrants to purchase up to an aggregate of 2,259,000 shares of common stock. The warrants may be exercised by the holders at a price of $1.55 per share immediately thru June 18, 2019. Net proceeds after expenses from the offering were approximately $10.1 million after underwriting discount and estimated offering expenses.
On January 30, 2014, we completed a private placement of the sale of 3,336,800 shares of our common stock at $2.00 per share, together with warrants to purchase up to an aggregate of 1,668,400 shares of common stock. The warrants may be exercised by the holders at a price of $2.81 per share starting July 30, 2014 continuing through January 29, 2019. Net proceeds after expenses from the offering were approximately $5.9 million.
Warrants
A summary of warrant activity is as follows:
|
|
2014
|
2013
|
2012
|
|||||||||||||||||||||
|
|
Number
of Shares
|
Weighted-
Average
Exercise
Price Per
Share
|
Number
of Shares
|
Weighted-
Average
Exercise
Price Per
Share
|
Number
of Shares
|
Weighted-
Average
Exercise
Price Per
Share
|
||||||||||||||||||
|
Beginning balance
|
1,125,000
|
$
|
2.64
|
1,125,000
|
$
|
2.64
|
1,125,000
|
$
|
2.64
|
|||||||||||||||
|
Warrants granted
|
3,988,420
|
$
|
2.09
|
--
|
--
|
--
|
--
|
|||||||||||||||||
|
Warrants canceled
|
--
|
--
|
--
|
--
|
--
|
--
|
||||||||||||||||||
|
Warrants exercised
|
--
|
--
|
--
|
--
|
--
|
--
|
||||||||||||||||||
|
Outstanding at June 30
|
5,113,420
|
$
|
2.21
|
1,125,000
|
$
|
2.64
|
1,125,000
|
$
|
2.64
|
|||||||||||||||
|
Exercisable at June 30
|
3,445,020
|
$
|
1.92
|
1,125,000
|
$
|
2.64
|
1,125,000
|
$
|
2.64
|
|||||||||||||||
Stock Options
The 2012 Independent Director Plan (“2012 Plan”) permits the grant of stock or options to independent directors. A total of 500,000 shares were approved by the stockholders for issuance under the 2012 Plan. Options are granted at prices that are equal to 100% of the fair market value on the date of grant, and expire over a term not to exceed ten years. Options generally vest immediately, unless otherwise determined by the Board of Directors.
The 2006 Equity Incentive Plan (“2006 Plan”) permits the grant of options, restricted stock, stock bonuses and stock appreciation rights to employees, directors and consultants. Under the 2006 Plan, the number of shares of common stock equal to 6% of the number of outstanding shares of the Company are authorized to be issued. The number of shares available to grant for awards adjusts at the beginning of each fiscal year if additional options to purchase shares of common stock were issued in the preceding fiscal year. As of June 30, 2014, there have been 2,804,819 shares approved under the 2006 Plan for issuance.
CESCA THERAPEUTICS INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (CONTINUED)
(in thousands except shares and per share amounts)
| 6. | Stockholders’ Equity (Continued) |
Stock Options (Continued)
The 2002 Independent Directors Equity Incentive Plan (“2002 Plan”) permits the grant of stock or options to independent directors. A total of 87,500 shares were approved by the stockholders for issuance under the 2002 Plan. Options are granted at prices which are equal to 100% of the fair market value on the date of grant, and expire over a term not to exceed ten years. Options generally vest immediately, unless otherwise determined by the Board of Directors. The 2002 Plan, but not the options granted, expired in January 2012.
We issue new shares of common stock upon exercise of stock options. The following is a summary of option activity for the Company’s stock option plans:
|
|
Number of
Shares
|
Weighted-
Average
Exercise
Price
|
Weighted-
Average
Remaining
Contractual Life
|
Aggregate
Intrinsic
Value
|
||||||||||||
|
Outstanding at June 30, 2013
|
1,063,750
|
$
|
2.36
|
|||||||||||||
|
|
||||||||||||||||
|
Granted
|
457,235
|
$
|
2.06
|
|||||||||||||
|
Forfeited/cancelled
|
(61,075
|
)
|
$
|
2.35
|
||||||||||||
|
Expired
|
(197,500
|
)
|
$
|
3.42
|
||||||||||||
|
Exercised
|
(9,375
|
)
|
$
|
2.32
|
||||||||||||
|
Outstanding at June 30, 2014
|
1,253,035
|
$
|
2.08
|
3
|
$
|
130
|
||||||||||
|
Vested and Expected to Vest at June 30, 2014
|
1,046,930
|
$
|
2.01
|
2
|
$
|
113
|
||||||||||
|
Exercisable at June 30, 2014
|
614,296
|
$
|
2.39
|
1
|
$
|
43
|
||||||||||
The aggregate intrinsic value is calculated as the difference between the exercise price of the underlying awards and the quoted price of the Company's common stock. There were no options that were exercised during the years ended June 30, 2013 and 2012. During the year ended June 30, 2014, the aggregate intrinsic value of options exercised under the Company's stock option plans was $4 determined as of the date of option exercise.
CESCA THERAPEUTICS INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (CONTINUED)
(in thousands except shares and per share amounts)
| 6. | Stockholders’ Equity (Continued) |
Stock Options (Continued)
The following table summarizes information about stock options outstanding at June 30, 2014:
|
Range of
Exercise
Prices
|
Number
Outstanding
|
Weighted-
Average
Remaining
Contractual
Life
(Years)
|
Weighted-
Average
Exercise
Price
|
Number
Exercisable
|
Weighted-
Average
Exercise
Price
|
|||||||||||||||||
|
$
|
0.90-$1.13
|
278,750
|
2.3
|
$
|
0.93
|
92,398
|
$
|
0.93
|
||||||||||||||
|
$
|
1.37-$2.03
|
216,250
|
2.3
|
$
|
1.83
|
136,459
|
$
|
1.79
|
||||||||||||||
|
$
|
2.17-$3.03
|
748,535
|
2.7
|
$
|
2.45
|
376,251
|
$
|
2.69
|
||||||||||||||
|
$
|
3.82
|
1,250
|
1.5
|
$
|
3.82
|
938
|
$
|
3.82
|
||||||||||||||
|
$
|
14.32
|
8,250
|
0.1
|
$
|
14.32
|
8,250
|
$
|
14.32
|
||||||||||||||
|
1,253,035
|
614,296
|
|||||||||||||||||||||
Non-vested stock option activity for the year ended June 30, 2014, is as follows:
|
|
Non-vested Stock
Options
|
Weighted-Average
Grant Date Fair Value
|
||||||
|
Outstanding at June 30, 2013
|
514,474
|
$
|
0.97
|
|||||
|
Granted
|
457,235
|
$
|
1.15
|
|||||
|
Vested
|
(289,270
|
)
|
$
|
1.07
|
||||
|
Forfeited
|
(43,700
|
)
|
$
|
1.43
|
||||
|
Outstanding at June 30, 2014
|
638,739
|
$
|
1.02
|
|||||
Stock Compensation Expense
At June 30, 2014, the total compensation cost related to options granted to employees under the Company's stock option plans but not yet recognized was $288. This cost will be amortized on a straight-line basis over a weighted-average period of approximately two years and will be adjusted for subsequent changes in estimated forfeitures. The total fair value of options vested during the years ended June 30, 2014, 2013, and 2012 was $309, $356 and $440.
Common Stock Restricted Awards
For the year ended June 30, 2014, the Company’s Compensation Committee granted 692,968 shares of restricted common stock to director level and executive members of management, vesting in three equal installments on the first, second and third anniversary of the grant date.
In March 2013, an officer was granted 50,000 shares of restricted common stock upon hire, vesting in three equal installments on the first, second and third anniversary of the grant date.
For the year ended June 30, 2012, the Company’s Compensation Committee granted 720,000 shares of restricted common stock to director level and executive members of management, vesting in three equal installments on the first, second and third anniversary of the grant date.
CESCA THERAPEUTICS INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (CONTINUED)
(in thousands except shares and per share amounts)
| 6. | Stockholders’ Equity (Continued) |
Common Stock Restricted Awards (Continued)
The following is a summary of restricted stock activity:
|
|
2014
|
2013
|
2012
|
|||||||||||||||||||||
|
|
Number of
Shares
|
Weighted-
Average
Grant Date
Fair Value
|
Number of
Shares
|
Weighted-
Average
Grant Date
Fair Value
|
Number of
Shares
|
Weighted-
Average
Grant Date
Fair Value
|
||||||||||||||||||
|
Balance at June 30
|
390,003
|
$
|
1.81
|
540,000
|
$
|
1.93
|
30,000
|
$
|
2.25
|
|||||||||||||||
|
Granted
|
692,968
|
$
|
1.95
|
50,000
|
$
|
0.91
|
720,000
|
$
|
1.89
|
|||||||||||||||
|
Vested
|
(191,672
|
)
|
$
|
1.86
|
(174,997
|
)
|
$
|
1.95
|
(10,000
|
)
|
$
|
2.25
|
||||||||||||
|
Forfeited
|
(87,500
|
)
|
$
|
2.00
|
(25,000
|
)
|
$
|
1.70
|
(200,000
|
)
|
$
|
1.80
|
||||||||||||
|
Outstanding at June 30
|
803,799
|
$
|
1.90
|
390,003
|
$
|
1.81
|
540,000
|
$
|
1.93
|
|||||||||||||||
In connection with the vesting of the restricted stock awards, the election was made by some of the employees to satisfy the applicable federal income tax withholding obligation by a net share settlement, pursuant to which the Company withheld 57,680 and 59,054 shares for the years ended June 30, 2014 and 2013, respectively and used the deemed proceeds from those shares to pay the income tax withholding. The net share settlement is deemed to be a repurchase by the Company of its common stock.
As of June 30, 2014, we had $1,208 in total unrecognized compensation expense related to our restricted stock awards, which will be recognized over a weighted average period of approximately three years.
| 7. | Gain on Sale of Product Lines |
ThermoLine
On December 31, 2012, the Company entered into an Asset Purchase Agreement for the sale of certain of the assets, rights and properties of the ThermoLine product line for $500 and the manufacture of certain spare parts for $35. The Company recognized the $161 gain on sale, net of transaction costs, upon delivery of the assets which occurred during the quarter ended March 31, 2013. The gain on sale was calculated as follows:
|
Proceeds
|
$
|
535
|
||
|
Less:
|
||||
|
Inventories, net
|
351
|
|||
|
Equipment, net
|
4
|
|||
|
Transaction costs
|
19
|
|||
|
Gain on sale
|
$
|
161
|
CESCA THERAPEUTICS INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (CONTINUED)
(in thousands except shares and per share amounts)
| 7. | Gain on Sale of Product Lines (Continued) |
CryoSeal
In June 2010, the Company and Asahi entered into an amendment (the "Amendment") of their Distribution and License Agreement, originally effective March 28, 2005. Under the terms of the Amendment, Asahi obtained exclusive rights to distribute the CryoSeal System in South Korea, North Korea, Taiwan, the People’s Republic of China, the Philippines, Thailand, Singapore, India and Malaysia. These rights included the exclusive right to market, distribute and sell the processing disposables and Thrombin Reagent for production of thrombin in a stand-alone product.
In connection with the above-described Amendment, the Company and Asahi also entered into an Option Agreement (the “Option Agreement”) and on June 30, 2012, Asahi exercised the option to purchase certain intangible assets related to this product line, including all associated patents and engineering files for $2,000. In connection with the notice of exercise, the Amendment automatically terminated. Payment of the $2,000 was based upon completion of certain provisions of the Option Agreement. As such, the Company recognized the gain on sale upon completion of those provisions, which occurred in July 2012. The $2,000 payment was received in August 2012.
| 8. | Concentrations |
At June 30, 2014, we had four distributors that individually accounted for 16%, 16%, 14% and 10% of accounts receivable. At June 30, 2013, we had four distributors that individually accounted for 28%, 18%, 10% and 10% of accounts receivable.
Revenues from one distributor totaled $2,288 or 14%, $2,299 or 13% and $1,870 or 10% of net revenues for the years ended June 30, 2014, 2013 and 2012, respectively. Revenues from another distributor totaled $2,102 or 13% and $2,057 or 11% of net revenues for the years ended June 30, 2014 and 2013, respectively. Revenues from a customer totaled $1,849 or 12% for the year ended June 30, 2014.
CESCA THERAPEUTICS INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (CONTINUED)
(in thousands except shares and per share amounts)
| 8. | Concentrations (Continued) |
The following represents our revenues by product platform for the years ended June 30:
|
|
2014
|
2013
|
2012
|
|||||||||
|
AXP
|
$
|
6,143
|
$
|
7,687
|
$
|
7,814
|
||||||
|
BioArchive
|
4,776
|
4,258
|
4,310
|
|||||||||
|
Manual Disposables
|
1,706
|
2,286
|
2,200
|
|||||||||
|
Bone Marrow
|
2,542
|
2,390
|
2,076
|
|||||||||
|
Other
|
820
|
1,342
|
2,623
|
|||||||||
|
|
$
|
15,987
|
$
|
17,963
|
$
|
19,023
|
||||||
We had sales to customers as follows for the years ended June 30:
|
|
2014
|
2013
|
2012
|
|||||||||
|
United States
|
$
|
6,909
|
$
|
8,011
|
$
|
10,783
|
||||||
|
Hong Kong
|
2,321
|
2,006
|
514
|
|||||||||
|
China
|
848
|
2,058
|
609
|
|||||||||
|
Asia - other
|
1,864
|
1,855
|
2,887
|
|||||||||
|
Europe
|
3,072
|
2,030
|
1,806
|
|||||||||
|
South America
|
771
|
831
|
1,505
|
|||||||||
|
Other
|
202
|
1,172
|
919
|
|||||||||
|
|
$
|
15,987
|
$
|
17,963
|
$
|
19,023
|
||||||
The Company has a contract manufacturer in Costa Rica that produces certain disposables. The Company provides AXP equipment to its distributor in China for use by end-user customers. The Company’s equipment, net of accumulated depreciation, is summarized below by geographic area:
|
|
June 30, 2014
|
June 30, 2013
|
||||||
|
United States
|
$
|
687
|
$
|
1,005
|
||||
|
China
|
620
|
678
|
||||||
|
Costa Rica
|
567
|
403
|
||||||
|
India
|
251
|
--
|
||||||
|
All other countries
|
173
|
122
|
||||||
|
Total equipment, net
|
$
|
2,298
|
$
|
2,208
|
||||
CESCA THERAPEUTICS INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (CONTINUED)
(in thousands except shares and per share amounts)
| 9. | Income Taxes |
Loss before income tax benefits was comprised of $8,719 from US and $315 from foreign jurisdictions in 2014, $3,086 from US in 2013, and $4,986 from US in 2012.
The reconciliation of federal income tax attributable to operations computed at the federal statutory tax rate of 34% to income tax expense (benefit) is as follows for the years ended June 30:
|
|
2014
|
2013
|
2012
|
|||||||||
|
Statutory federal income tax benefit
|
$
|
(3,072
|
)
|
$
|
(1,049
|
)
|
$
|
(1,695
|
)
|
|||
|
|
||||||||||||
|
Unbenefitted net operating losses and credits
|
2,163 |
1,272
|
2,012
|
|||||||||
|
State and local taxes
|
(326
|
) |
(234
|
)
|
(329
|
)
|
||||||
|
Merger costs
|
757
|
-
|
||||||||||
|
Other
|
75
|
11
|
12
|
|||||||||
|
Total income tax benefit
|
$
|
(403
|
)
|
-
|
-
|
|||||||
A deferred income tax benefit of $403 was recorded for the year ended June 30, 2014, of which $325 was related to federal taxes, $40 was related to state taxes, and $38 was related to foreign taxes. No tax benefit has been recorded through June 30, 2013 and June 30, 2012 because of the net operating losses incurred and a full valuation allowance has been provided. A valuation allowance is provided when it is more likely than not that some portion of the deferred tax assets will not be realized.
At June 30, 2014, we had net operating loss carryforwards for federal and state income tax purposes of $89,860 and $61,920 respectively, that are available to offset future income. The federal and state loss carryforwards expire in various years between 2015 and 2034.
At June 30, 2014, we have research and experimentation credit carryforwards of $1,118 for federal tax purposes that expire in various years between 2015 and 2034, and $1,329 for state income tax purposes that do not have an expiration date.
Significant components of the Company’s deferred tax assets and liabilities for federal and state income taxes are as follows:
|
|
June 30, 2014
|
June 30, 2013
|
||||||
|
Deferred tax assets
|
||||||||
|
Net operating loss carryforwards
|
$ |
34,154
|
$ |
31,342
|
||||
|
Income tax credit carryforwards
|
1,995 |
2,161
|
||||||
|
Depreciation and amortization
|
-
|
190
|
||||||
|
Other
|
1,750
|
1,490
|
||||||
|
Valuation allowance
|
(37,317
|
)
|
(35,183
|
)
|
||||
|
Total deferred tax assets
|
582
|
-
|
||||||
|
|
||||||||
|
Deferred tax liabilities
|
||||||||
|
Depreciation and amortization
|
(8,223
|
)
|
-
|
|||||
|
Net deferred tax assets and liabilities
|
$ |
(7,641
|
) | $ |
-
|
|||
The valuation allowance increased by $2,134 in 2014, decreased by $2,630 in 2013 and increased by $43 in 2012. As of June 30, 2014, we have a benefit of $1,849 related to stock option deductions, which will be credited to paid-in capital when realized, of which $1,621 is included in the valuation allowance.
Our deferred income tax benefit of $403 was due to certain intangible assets and the related deferred tax liabilities acquired in the merger with TRX. The recognition of a deferred income tax benefit resulted from netting the deferred tax liabilities against previously generated, but fully reserved, deferred tax assets.
Because of the “change of ownership” provisions of the Tax Reform Act of 1986, a portion of the Company’s federal net operating loss and credit carryovers may be subject to an annual limitation regarding their utilization against taxable income in future periods.
CESCA THERAPEUTICS INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (CONTINUED)
(in thousands except shares and per share amounts)
| 10. | Employee Retirement Plan |
We sponsor an Employee Retirement Plan, generally available to all employees, in accordance with Section 401(k) of the Internal Revenue Code. Employees may elect to contribute up to the Internal Revenue Service annual contribution limit. Under this Plan, at the discretion of the Board of Directors, we may match a portion of the employees’ contributions. We made no discretionary or matching contributions to the Plan for the years ended June 30, 2014, 2013 and 2012.
None.
Disclosure Controls and Procedures
We carried out an evaluation, under the supervision and with the participation of management, including our Chief Executive Officer along with our Chief Financial Officer, of the effectiveness of the design and operation of our disclosure controls and procedures (as defined by Exchange Act Rule 13a-15(e) and 15d-15(e)) as of the end of our fiscal quarter pursuant to Exchange Act Rule 13a-15. Based upon that evaluation, our Chief Executive Officer and Chief Financial Officer concluded that our disclosure controls and procedures are effective.
Management’s Report on Internal Control over Financial Reporting
The Company’s management is responsible for establishing and maintaining adequate internal control over financial reporting as defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act. Under the supervision and with the participation of the Company’s management, including the Company’s Chief Executive and Financial Officers, the Company conducted an evaluation of the effectiveness of its internal control over financial reporting based on criteria established in the framework in Internal Control – Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission. Based on this evaluation, the Company’s management concluded that its internal control over financial reporting was effective as of June 30, 2014.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate. All internal control systems, no matter how well designed, have inherent limitations. Therefore, even those systems determined to be effective can provide only reasonable assurance with respect to financial statement preparation and presentation.
During the quarter ended March 31, 2014, we completed the acquisition of TotipotentRX. We excluded this business from the scope of our management’s assessment of the effectiveness of our internal control over financial reporting as of June 30, 2014. This exclusion is in accordance with the SEC’s general guidance that an assessment of a recently acquired business may be omitted from our scope in the year of acquisition.
TotipotentRX was a private company and has not been subject to the Sarbanes-Oxley Act of 2002, the rules and regulations of the SEC, or other corporate governance requirements to which public reporting companies may be subject. During the audit of TotipotentRX’s financial statements for the year ended December 31, 2012, TotipotentRX’s independent registered public accounting firm determined that a material weakness existed in its internal control over financial reporting as TotipotentRX did not have adequate personnel and information systems in place to prepare financial statements on a timely basis, including accrual accounting, non-routine data processes and estimation processes and procedures over financial accounting and reporting. As part of our ongoing integration activities, we are continuing to incorporate our controls and procedures into the TotipotentRX subsidiaries and to augment our company-wide controls to reflect the risks inherent in an acquisition of this type.
Attestation Report of Independent Registered Public Accounting Firm
None.
Changes in Internal Control over Financial Reporting
There were no changes in the Company’s internal controls over financial reporting that occurred during the fiscal quarter ended June 30, 2014, that have materially affected, or are reasonably likely to materially affect its internal controls over financial reporting. We believe that a control system, no matter how well designed and operated, cannot provide absolute assurance that the objectives of the control system are met, and no evaluation of controls can provide absolute assurance that all control issues and instances of fraud, if any, within any company have been detected.
None.
PART III
The information required by this Item will be included in and is hereby incorporated by reference from our Proxy Statement for the 2014 Annual Meeting of Stockholders. We have adopted a Code of Ethics applicable to all employees including our CEO and CFO. A copy of the Code of Ethics is available at www.cescatherapeutics.com.
The information required by this Item will be included in and is hereby incorporated by reference from our Proxy Statement for the 2014 Annual Meeting of Stockholders.
| ITEM 12. | SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS. |
The information required by this Item will be included in and is hereby incorporated by reference from our Proxy Statement for the 2014 Annual Meeting of Stockholders.
The information required by this Item will be included in and is hereby incorporated by reference from our Proxy Statement for the 2014 Annual Meeting of Stockholders.
The information required by this Item will be included in and is hereby incorporated by reference from our Proxy Statement for the 2014 Annual Meeting of Stockholders.
PART IV
The following documents are filed as a part of this report on Form 10-K.
|
|
|
|
Page Number
|
|
|
|
|
|
|
(a)
|
(1)
|
Financial Statements
|
|
|
|
|
|
|
|
|
|
Report of Independent Registered Public Accounting Firm
|
40
|
|
|
|
|
|
|
|
|
Consolidated Balance Sheets at June 30, 2014 and 2013
|
41
|
|
|
|
|
|
|
|
|
Consolidated Statements of Operations and Comprehensive Loss for the years ended June 30, 2014, 2013 and 2012
|
42
|
|
|
|
|
|
|
|
|
Consolidated Statements of Stockholders’ Equity for the years ended June 30, 2014, 2013 and 2012
|
43
|
|
|
|
|
|
|
|
|
Consolidated Statements of Cash Flows for the years ended June 30, 2014, 2013 and 2012
|
44
|
|
|
|
|
|
|
|
|
Notes to Consolidated Financial Statements
|
45
|
Management’s Report on Internal Control over Financial Reporting is contained as part of this report under Item 9A “Controls and Procedures.”
|
(a)
|
(2)
|
Financial Statement Schedules
|
|
|
|
|
|
|
|
Financial statement schedules have been omitted because they are not required.
|
|
|
|
|
|
(b)
|
|
Exhibits
|
|
|
|
|
|
|
|
Exhibits required by Item 601 of Regulation S-K are listed in the Exhibit Index on the next page, which are incorporated herein by this reference.
|
EXHIBIT INDEX
|
Exhibit No.
|
Document Description
|
Incorporation by Reference
|
|
|
2.1
|
Plan of Merger Agreement and Reorganization Agreement between ThermoGenesis Corp. and TotipotentRX, dated July 15, 2013
|
Incorporated by reference to Form 8-K dated July 16, 2013
|
|
|
3.2
|
Bylaws of Cesca Therapeutics Inc.
|
Incorporated by reference to Form 8-K dated April 25, 2014 filed with the SEC on May 1, 2014
|
|
|
3.3
|
Certificate of Amendment to the Amended and Restated Certificate of Incorporation of Cesca Therapeutics Inc.
|
Incorporated by reference to Cesca’s Current Report on Form 8-K filed with the SEC on August 26, 2010.
|
|
|
3.4
|
Certificate of Merger.
|
Incorporated by reference to Cesca Therapeutics Inc. Current Report Form 8-K filed with the SEC on February 18, 2014.
|
|
|
4.1
|
Form of Stock Grant Agreement; Common Stock Agreement
|
Incorporated by reference to Cesca’s Current Report on Form 8-K filed with the SEC on November 5, 2010.
|
|
|
10.1
|
License Agreement with Pall/Medsep Corporation
|
Incorporated by reference to Form 8-K dated April 14, 1997.
|
|
|
10.2.1
|
License and Escrow Agreement between Cesca Therapeutics Inc. and CBR Systems, Inc., effective June 15, 2010
|
Incorporated by reference to Cesca’s Quarterly Report on Form 10-Q for the quarter ended December 31, 2010.
|
|
|
10.2.2
|
First Amendment to Technology License and Escrow Agreement between Cesca Therapeutics Inc. and CBR Systems, Inc., effective February 6, 2013
|
Incorporate by reference to Form 8-K dated February 12, 2013.
|
|
|
10.2.3
|
Extension Addendum to Escrow Agreement, effective July 26, 2013
|
Incorporated by reference to Form 8-K dated August 1, 2013.
|
|
|
10.2.4
|
Forbearance Agreement to Technology License and Escrow Agreement dated November 26, 2013
|
Incorporated by reference to Form 8-K filed with the SEC on November 26, 2013
|
|
|
10.3
|
Amended 2002 Independent Directors Equity Incentive Plan
|
Incorporated by reference to Form 8-K dated December 15, 2004.
|
|
|
10.4
|
Amendment to Amended and Restated International Distribution Agreement with GEHC
|
Incorporated by reference to Form 8-K dated February 4, 2010.
|
|
|
10.5+
|
License and Distribution Agreement between Cesca Therapeutics Inc. and BioParadox effective October 13, 2010
|
Incorporated by reference to Cesca’s Current Report on Form 8-K filed with the SEC on October 19, 2010.
|
|
|
10.6
|
Amended and Restated 2006 Equity Incentive Plan
|
Incorporated by reference to Form 8-K dated April 25, 2014 filed with the SEC on May 1, 2014.
|
|
|
10.7
|
Distribution and License Agreement between Cesca Therapeutics Inc. and Asahi Kasei Medical Co., Ltd., dated March 28, 2005
|
Incorporated by reference to Cesca’s Current Report on Form 8-K filed with the SEC on March 31, 2005.
|
|
|
10.7.1+
|
Option Agreement between ThermoGenesis Corp and Asahi Kasei Kuraray Medical Co., Ltd.
|
Incorporated by reference to Form 8-K dated June 16, 2010.
|
|
|
10.8
|
Amended 1998 Employee Equity Incentive Plan
|
Incorporated by reference to Exhibit A to Cesca’s definitive proxy statement for the Special Meeting of Stockholders held on February 2, 1998, filed with the SEC on December 8, 1997.
|
|
|
10.9
|
Exclusive Distributor Agreement and License with Arthrex, Inc.
|
Incorporated by reference to Form 8-K dated January 13, 2012 and amended March 28, 2012.
|
|
|
10.10+
|
Product Purchase and International Distribution Agreement between Cesca Therapeutics Inc. and Golden Meditech Holdings, Limited
|
Incorporated by reference to Form 8-K dated August 24, 2012 and amended October 24, 2012.
|
|
Exhibit No.
|
Document Description
|
Incorporation by Reference
|
|
|
10.11
|
2012 Independent Director Plan
|
Incorporated by reference to Exhibit A of the Company’s Definitive Proxy Statement filed October 23, 2012.
|
|
|
10.12
|
Form of Lock-up Agreement between Cesca Therapeutics Inc. and TotipotentRX, dated July 15, 2013
|
Incorporated by reference to Form 8-K dated July 16, 2013.
|
|
|
10.13
|
Employment Agreement between Cesca Therapeutics Inc. and Mitchel Sivilotti dated July 15, 2013
|
Incorporated by reference to Form 8-K dated July 16, 2013.
|
|
|
10.14
|
Employment Agreement between Cesca Therapeutics Inc. and Kenneth Harris dated July 15, 2013
|
Incorporated by reference to Form 8-K dated July 16, 2013.
|
|
|
10.15
|
Form of Non-Competition Agreement between Cesca Therapeutics Inc. and TotipotentRX, dated July 15, 2013
|
Incorporated by reference to Form 8-K dated July 16, 2013.
|
|
|
10.16
|
Employment Agreement with Matthew T. Plavan
|
Incorporated by reference to Form 8-K dated October 30, 2013
|
|
|
10.17
|
Employment Agreement with Dan T. Bessey
|
Incorporated by reference to Form 8-K dated October 30, 2013
|
|
|
10.18
|
Sales and Purchase Agreement between ThermoGenesis Corp and CBR Systems, Inc. dated December 31, 2013+
|
Incorporated by reference to Form 8-K dated January 7, 2014.
|
|
|
Consent of Ernst & Young LLP, Independent Registered Public Accounting Firm
|
Filed herewith.
|
||
|
Certification by the Principal Executive Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002
|
Filed herewith.
|
||
|
Certification by the Principal Financial Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002
|
Filed herewith.
|
||
|
Certification of Principal Executive Officer and Principal Financial Officer pursuant to Section 906 of the Sarbanes Oxley Act of 2002
|
Filed herewith.
|
||
|
101.INS
|
XBRL Instance Document‡
|
|
|
|
101.SCH
|
XBRL Taxonomy Extension Schema Document‡
|
||
|
101.CAL
|
XBRL Taxonomy Extension Calculation Linkbase Document‡
|
||
|
1.1DEF
|
XBRL Taxonomy Extension Definition Linkbase Document‡
|
||
|
101.LAB
|
XBRL Taxonomy Extension Label Linkbase Document‡
|
||
|
101.PRE
|
XBRL Taxonomy Extension Presentation Linkbase Document‡
|
||
Footnotes to Exhibit Index
| ‡ | XBRL information is furnished and not filed for purpose of Sections 11 and 12 of the Securities Act of 1933 and Section 18 of the Securities Exchange Act of 1934, and is not subject to liability under those sections, is not part of any registration statement or prospectus to which it relates and is not incorporated or deemed to be incorporated by reference into any registration statement, prospectus or other document. |
| + | The SEC has granted confidential treatment with respect to certain portions of this exhibit. Omitted portions have been filed separately with the SEC. |
GLOSSARY OF CERTAIN TECHNICAL TERMS
510(k): Formal notification to FDA to obtain clearance to market the medical device. The device must be substantially equivalent to devices manufactured prior to 1976, or which have been found substantially equivalent after that date.
ADIPOSE: Tissue in which fat is stored and which has the cells swollen by droplets of fat.
ADULT STEM CELLS: All non-embryonic stem cells.
ALLOGRAFT: A tissue graft from a donor of the same species as the recipient but not genetically identical.
AUTOGRAFT: A graft of tissue from one point to another of the same individual's body.
AUTOLOGOUS: Autogenous; related to self; originating within an organism itself, as obtaining blood from the patient for use in the same patient.
BONE MARROW ASPIRATE: When a small amount of bone marrow is removed and tested.
BONE MARROW CONCENTRATE (“BMC”): the product of subjecting bone marrow aspirate to the process of centrifugation. The process of centrifugation separates the aspirated cells into concentrated fractions that may then be separately preserved for research or therapeutic purposes.
CRITICAL LIMB ISCHEMIA (“CLI”): A severe obstruction of the arteries that seriously decreases blood flow to the extremities (arms, hands, legs, feet) and has progressed to the point of severe pain and even skin ulcers of sores.
CRYOPRESERVATION: Maintaining the life of excised tissue or organs by freezing and storing at very low temperatures.
CRYSTALLOID: A substance that can be crystallized.
HEMATOPOIETIC: The formation of blood.
HOMOLOGOUS: Coming from and going back into the same organ system.
IN VITRO: Occurring in an artificial environment outside a living organism.
IN VIVO: Occurring or made to occur within a living organism or natural setting.
ISCHEMIA: Deficient supply of blood and oxygen to a body part.
MONONUCLEAR CELLS (“MNCs”): A term used to refer to blood cells that under a microscope can be seen to have a large round shaped nucleus. These cells include monocytes and lymphocytes which are involved in fighting infections in the body and also stem cells which have the potential to replicate and to generate new tissues as part of the body’s healing process.
PERIPHERAL ARTERY DISEASE (“PAD”): A common circulatory problem in which narrowed arteries reduce blood flow to the limbs. Peripheral artery disease that is caused by a buildup of plaques in blood vessels (atherosclerosis) also brings risk of developing CLI.
GLOSSARY OF CERTAIN TECHNICAL TERMS (CONTINUED)
PERIPHERAL BLOOD: A term used to describe the blood that is contained in the body’s circulatory system. It can be collected by a health care professional by inserting a needle into a vein.
PLATELET RICH PLASMA (“PRP”): A volume of autologous plasma that has a platelet concentration above baseline.
REGENERATIVE MEDICINE: The process of creating living, functional tissues to repair or replace tissue or organ function lost due to age, disease, damage, or congenital defects.
STEM CELLS: Undifferentiated, primitive cells in the bone marrow or cord blood with the ability both to multiply and to differentiate into specific blood or tissue cells.
THROMBIN: Generated in blood clotting that acts on fibrinogen to produce fibrin.
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
|
|
|
Cesca Therapeutics Inc.
|
|
|
Dated: September 29, 2014
|
By:/s/
|
CRAIG W. MOORE
|
|
|
|
|
Craig W. Moore, Chairman of the Board
|
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
|
By:/s/
|
CRAIG W. MOORE
|
Dated: September 29, 2014
|
|
|
|
Craig W. Moore, Chairman of the Board
|
|
|
|
By:/s/
|
MATTHEW T. PLAVAN
|
Dated: September 29, 2014
|
|
|
|
Matthew T. Plavan, Chief Executive Officer and Director
|
|
|
|
|
(Principal Executive Officer)
|
|
|
|
|
|
|
|
|
By:/s/
|
DAN T. BESSEY
|
Dated: September 29, 2014
|
|
|
|
Dan T. Bessey, Chief Financial Officer
|
|
|
|
|
(Principal Financial and Accounting Officer)
|
|
|
|
|
|
|
|
|
By: /s/
|
PATRICK J. MCENANY
|
Dated: September 29, 2014
|
|
|
|
Patrick J. McEnany, Director
|
|
|
|
By: /s/
|
ROBIN C. STRACEY
|
Dated: September 29, 2014
|
|
|
|
Robin C. Stracey, Director
|
|
|
| By: /s/ | MAHENDRA S. RAO |
Dated: September 29, 2014
|
|
| Mahendra S. Rao, Director | |||
|
By: /s/
|
KENNETH HARRIS |
Dated: September 29, 2014
|
|
| Kenneth Harris, President and Director |
74
