Attached files
| file | filename |
|---|---|
| EX-21.1 - SUBSIDIARIES OF THE REGISTRANT - PEAK PHARMACEUTICALS, INC. | ctco_ex211.htm |
| EX-10.13 - SERVICES AGREEMENT - PEAK PHARMACEUTICALS, INC. | ctco_ex1013.htm |
| EX-10.14 - INDUSTRIAL HEMP REGISTRATION LICENSE - PEAK PHARMACEUTICALS, INC. | ctco_ex1014.htm |
| EX-10.12 - FARM LEASE AND SERVICE AGREEMENT - PEAK PHARMACEUTICALS, INC. | ctco_ex1012.htm |
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
Date of Report (Date of Earliest Event Reported): August 8, 2014
|
CANNABIS THERAPY CORP.
|
|
(Exact name of registrant as specified in its charter)
|
|
Nevada
|
005-87668
|
26-1973257
|
||
|
(State or other jurisdiction of incorporation)
|
(Commission File Number)
|
(I.R.S. Employer Identification No.)
|
|
4450 Arapahoe Avenue, Suite 100
Boulder, CO
|
80303
|
|
|
(Address of principal executive offices)
|
(Zip Code)
|
303.415.2557
(Registrant’s telephone number, including area code)Copy to:
Scott Rapfogel, Esq.
Crone Kline Rinde LLP
488 Madison Avenue, 12th Floor
New York, NY 10022
(Former name and address if changed since last report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
o Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425)
o Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12)
o Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b))
o Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c))
|
Page
|
|||||
|
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
|
3 | ||||
|
EXPLANATORY NOTE
|
4 | ||||
|
ITEM 5.06.
|
CHANGE IN SHELL COMPANY STATUS.
|
5 | |||
|
DESCRIPTION OF BUSINESS
|
6 | ||||
|
DESCRIPTION OF PROPERTIES
|
18 | ||||
|
RISK FACTORS
|
18 | ||||
|
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
|
27 | ||||
|
FINANCIAL STATEMENTS
|
27 | ||||
|
DIRECTORS, EXECUTIVE OFFICERS, PROMOTERS AND CONTROL PERSONS
|
27 | ||||
|
EXECUTIVE COMPENSATION
|
35 | ||||
|
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT
|
39 | ||||
|
CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLSOURE
|
40 | ||||
|
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS
|
40 | ||||
|
MARKET PRICE OF AND DIVIDENDS ON COMMON EQUITY AND RELATED STOCKHOLDER MATTERS
|
42 | ||||
|
RECENT SALES OF UNREGISTERED SECURITIES
|
44 | ||||
|
DESCRIPTION OF SECURITIES
|
45 | ||||
|
LEGAL PROCEEDINGS
|
47 | ||||
|
INDEMNIFICATION OF DIRECTORS AND OFFICERS
|
47 | ||||
|
ITEM 9.01.
|
FINANCIAL STATEMENTS AND EXHIBITS.
|
47 | |||
2
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
This Current Report (the “Report”) contains forward-looking statements, including, without limitation, in the sections captioned “Description of Business,” “Risk Factors,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” and elsewhere. Any and all statements contained in this Report that are not statements of historical fact may be deemed forward-looking statements. Terms such as “may,” “might,” “would,” “should,” “could,” “project,” “estimate,” “pro-forma,” “predict,” “potential,” “strategy,” “anticipate,” “attempt,” “develop,” “plan,” “help,” “believe,” “continue,” “intend,” “expect,” “future,” and terms of similar import (including the negative of any of the foregoing) may be intended to identify forward-looking statements. However, not all forward-looking statements may contain one or more of these identifying terms. Forward-looking statements in this Report may include, without limitation, statements regarding (i) the plans and objectives of management for future operations, including plans or objectives relating to the development, marketing and sale of our cannabis based products including those for the prevention and treatment of inflammatory and auto-immune diseases, (ii) a projection of income (including income/loss), earnings (including earnings/loss) per share, capital expenditures, dividends, capital structure or other financial items, (iii) our future financial performance, including any such statement contained in a discussion and analysis of financial condition by management or in the results of operations included pursuant to the rules and regulations of the Securities and Exchange Commission (“SEC”), and (iv) the assumptions underlying or relating to any statement described in points (i), (ii) or (iii) above.
The forward-looking statements are not meant to predict or guarantee actual results, performance, events or circumstances and may not be realized because they are based upon our current projections, plans, objectives, beliefs, expectations, estimates and assumptions and are subject to a number of risks and uncertainties and other influences, many of which we have no control over. Actual results and the timing of certain events and circumstances may differ materially from those described by the forward-looking statements as a result of these risks and uncertainties. Factors that may influence or contribute to the accuracy of the forward-looking statements or cause actual results to differ materially from expected or desired results may include, without limitation, our ability to obtain adequate financing, cash flows and resulting liquidity, our ability to expand our business, government regulations, lack of diversification, our ability to penetrate the intended markets for our products, our ability to negotiate economically feasible agreements with third party service providers, increased competition, results of any arbitration and litigation, stock volatility and illiquidity, and our ability to implement our business plans or strategies. A description of some of the risks and uncertainties that could cause our actual results to differ materially from those described by the forward-looking statements in this Report appears in the section captioned “Risk Factors” and elsewhere in this Report.
Readers are cautioned not to place undue reliance on forward-looking statements because of the risks and uncertainties related to them and to the risk factors. We disclaim any obligation to update the forward-looking statements contained in this Report to reflect any new information or future events or circumstances or otherwise.
Readers should read this Report in conjunction with the discussion under the caption “Risk Factors,” our financial statements and the related notes thereto in this Report, and other documents which we may file from time to time with the SEC.
3
EXPLANATORY NOTE
We were incorporated as Surf A Movie Solutions, Inc. in Nevada on December 18, 2007, to engage in the business of the development, sale and marketing of online video stores. We were not successful in our efforts and discontinued this line of business. Since that time and until recently, we have been a “shell company” (as such term is defined in Rule 12b-2 under the Securities Exchange Act of 1934, as amended (the “Exchange Act”)).
On August 30, 2013 we changed our name to Frac Water Systems, Inc. and on October 10, 2013 we determined to engage in the business of providing economically and environmentally sound solutions for the treatment and recycling of wastewater resulting principally from oil and gas exploration and production activities. On December 31, 2013 we determined not to move forward with this line of business.
In early March 2014 we determined to enter into the business of developing, manufacturing, marketing and selling pharmaceutical level products containing phytocannabinnoids, an abundant and pharmaceutically active component of cannabis, for the treatment of various conditions and diseases. From March 2014 to the present we have acted on our business plan and commenced material business operations. See Item 5.06 below.
In connection with our new business, on March 17, 2014 we changed our name to Cannabis Therapy Corporation and on March 24, 2014 we change our trading symbol to “CTCO.”
On March 10, 2014 Soren Mogelsvang, Cohava Gelber, Guy Yachin and Vered Caplan were appointed as directors of ours and effective March 11, 2014 Soren Mogelsvang was appointed as our Chief Executive Officer. Effective May 1, 2014 Mr. Mogelsvang was appointed as our President. Our officers and directors have extensive experience as biotech and biomedical executives, entrepreneurs or researchers.
As used in this Report, unless otherwise stated or the context clearly indicates otherwise, the terms “Cannabis Therapy,” the “Company,” the “Registrant,” “we,” “us,” and “our” refer to Cannabis Therapy Corp. (formerly known as Frac Water Systems, Inc. and Surf A Movie Solutions, Inc.), incorporated in Nevada, and its wholly owned subsidiary, Peak BioPharma Corp., a Colorado corporation.
This Report contains summaries of the material terms of various agreements executed in connection with the transactions described herein. The summaries of these agreements are subject to, and are qualified in their entirety by, reference to these agreements, which are filed as exhibits hereto and incorporated herein by reference.
Prior to August 8, 2014, we were a “shell company” (as such term is defined in Rule 12b-2 under the Exchange Act”). As a result of the milestones achieved by us during the period March 10, 2014 through August 8, 2014, we have ceased to be a shell company. The information contained in this Report, together with the information contained in our Annual Report on Form 10-K for the fiscal year ended September 30, 2013, and our subsequent Quarterly Reports on Form 10-Q and Current Reports on Form 8-K, as filed with the SEC, constitute the current “Form 10 information” necessary to satisfy the conditions contained in Rule 144(i)(2) under the Securities Act of 1933, as amended (the “Securities Act”).
4
ITEM 5.06. CHANGE IN SHELL COMPANY STATUS.
We are actively engaged in the business of developing, manufacturing, marketing and selling pharmaceutical level products containing phytocannabinoids for the treatment of various conditions and diseases and are no longer a shell company. Since March 2014 we have achieved the following milestones:
|
Ÿ
|
March 2014: engagement of a management team with extensive experience as biotech and biomedical executives, entrepreneurs and researchers;
|
|
Ÿ
|
March 2014 – present: raising $815,000 in working capital through sales of our common stock;
|
|
Ÿ
|
March 31, 2014: establishment of corporate headquarters and operations in Boulder, Colorado;
|
|
Ÿ
|
May 1, 2014: execution of a Farm Lease and Service Agreement with Rocky Mountain Hemp Inc. and Ryan Loflin under which we are growing industrial hemp in Colorado and actively developing hemp seed genetics;
|
|
Ÿ
|
May 16, 2014: obtaining an Industrial Hemp License from the Colorado Department of Agriculture;
|
|
Ÿ
|
June 26, 2014: execution of a Services Agreement with Caerus Discovery, LLC, a biotech company which will be providing us with contract research services intended to support product development and design of our proposed anti-inflammatory and autoimmune disease prevention and alleviation products while out laboratory is being established in Colorado; and
|
|
Ÿ
|
July 29, 2014: execution of a License Agreement with Canna-Pet, LLC a company which develops, produces and sells medical cannabis products made from hemp and low tetrahydrocannabinol cannabis plants which are intended exclusively for consumption by pets, under which we received a perpetual, exclusive worldwide license to use Canna-Pet, LLC’s intellectual property and produce and sell products based thereon.
|
5
DESCRIPTION OF BUSINESS
Since early March 2014 we have been operating as a bio-pharmaceutical and neutraceutical company seeking to develop, manufacture, market and sell safe, high quality, medicinal products extracted from low tetrchydrocannabinoid (“THC”) cannabis plants. THC is the primary psychoactive ingredient in cannabis that produces a “high” effect. Our initial focus will be (i) the exploitation of the exclusive license we received from Canna-Pet, LLC, a developer of ingestible health products for pets made from hemp, and (ii) developing over-the-counter, THC-free, cannabis based products for the prevention and alleviation of the symptoms associated with inflammatory and auto-immune diseases. Using US-grown hemp, a low THC cannabis plant, we intend to develop, market and sell products containing non-psychotropic phytocannabinoids.
History
As described above, we were incorporated in Nevada as Surf A Movie Solutions, Inc. on December 18, 2007 to engage in the business of the development sale and marketing of online video sales. We were not successful in our efforts and discontinued this line of business. Since that time and until August 8, 2014, we were a “shell company” (as such term is defined in Rule 12b-2 under the Exchange Act). On August 30, 2013 we changed our name to Frac Water Systems, Inc. and on October 10, 2013 we determined to engage in the business of providing economically and environmentally sound solutions for the treatment and recycling of wastewater resulting principally from oil and gas exploration and production activities. On December 31, 2013 we determined not to move forward with this line of business.
In early March 2014 we determined to enter into the business of developing, manufacturing and marketing pharmaceutical level products containing phytocannabinnoids, an abundant and pharmaceutically active component of cannabis, for the treatment of various conditions and diseases. In connection therewith, on March 17, 2014 we changed our name to Cannabis Therapy Corporation and on March 24, 2014 changed our trading symbol on OTC Markets to “CTCO”.
We currently have authorized 350,000,000 shares of capital stock, consisting of (i) 325,000,000 shares of common stock, and (ii) 25,000,000 shares of “blank check” Preferred Stock.
On August 15, 2012 our board of directors and stockholders owning a majority of our outstanding common shares authorized a 50 for 1 forward stock split of our common stock. The forward split became effective on September 27, 2012. As a result of the forward split each outstanding share was split into 50 shares. On March 11, 2014 our board of directors authorized a 1.5 for 1 forward stock split of our common stock in the form of a dividend. In connection therewith, our shareholders of record as of the close of business on March 28, 2014 received an additional .5 share of our common stock for each share of our common stock held by them on such date. The forward stock split became effective on April 1, 2014. All share amount references in this Report give retroactive effect to the forward stock splits.
Our principal executive offices are located at 4410 Arapahoe Avenue, Suite 100, Boulder, CO 80303. Our telephone number is 303.415.2557. Our primary website address is www.cannabistherapy.com.
6
Our Business
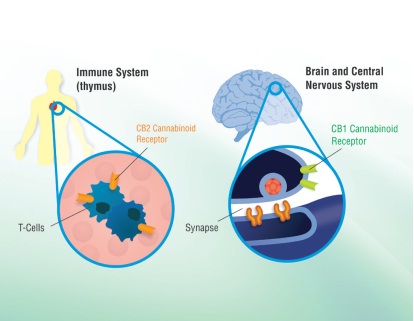
Since March 2014 we have been engaged in the business of developing and marketing low tetrahydrocannabinoid (“THC”) or THC free cannabis based medicinal products intended for over-the-counter sale via distribution networks applicable to products classified as nutraceuticals. By using U.S. grown hemp, a low THC content cannabis plant, we expect to be able to develop and market products containing cannabinoids under the same rules that apply to hemp-based energy drinks and other hemp based products. Hemp-based products have been legal in the U.S. for several years when manufactured with imported, THC-free hemp paste. Recently, hemp cultivation was legalized in 11 states, including Colorado, and, on a limited basis, at the federal level as part of the Agricultural Act of 2014 (the “2014 US Farm Bill”) passed in February 2014, which sets the agricultural product apart from marijuana. Industrial hemp has THC content below 0.3% compared to the 1-20% typically found in marijuana. The levels of non-THC medicinal cannabinoids are expected to vary between different hemp strains, and can be increased and optimized by plant breeding. One of our goals is to develop proprietary hemp strains containing improved medicinal properties and to establish third party product validation. Our initial focus is the leveraging of our Canna-Pet license under which we will produce and sell hemp based animal health products (see “Canna-Pet License Agreement”) and the development of products for the prevention and alleviation of the symptoms associated with inflammatory and auto-immune diseases. We have particular interest in cannabis-derived phytocannabinoids which can exert their different effects through the CB1 and CB2 cannabinoid receptors in the human body (see figure below). These receptors are triggered both by endogenous human cannabinoids, the endocannabinoids, and plant-derived phytocannabinoids, such as those extracted from cannabis plants. The CB1 and CB2 receptors are part of the endocannabinoid system, which is important for homeostasis and human health. CB1 receptors are primarily found in the brain and throughout peripheral nerves and the autonomic nervous system. These receptors have been associated with the anticonvulsive effects of cannabis. CB2 receptors localize primarily to immune cells and the spleen, and are thought to account for the anti-inflammatory and other therapeutic effects of cannabis.
7
We are planning to extract and develop non-psychotropic phytocannabinoids which have anti-inflammatory effects. We intend to use distinct extraction methods to enrich the phytocannabinoids and will then test them in different laboratory inflammation models. Scaleup manufacturing and production will be in compliance with the regulations for nutraceuticals.
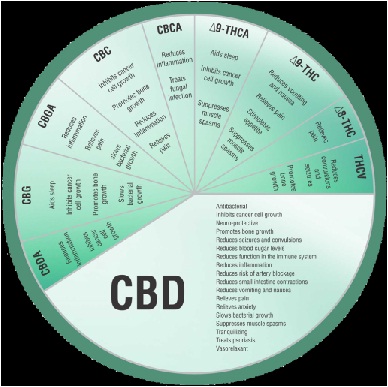
Phytocannabinoids are a class of plant-derived cannabinoids that can be isolated from the cannabis plant. More than 85 different phytocannabinoids have been described and fall into 8 classes of compounds, of which tetrahydrocannabinol (THC), Cannabidiol (CBD) and Cannabinol (CBN) are the best characterized. Accumulating scientific evidence supports the therapeutic properties of CBD, which is reported to be effective in easing symptoms of a wide range of conditions, including rheumatoid arthritis, diabetes, alcoholism, post-traumatic stress disorder, epilepsy, antibiotic-resistant infections and neurological disorders and in particular pain mitigation. The figure above summarizes different pharmacodynamic effects attributed to different phytocannabinoids.
8

In addition to the leveraging of our Canna-Pet license, our plan is to establish a vertically integrated operation in Colorado by the fourth quarter of 2014. The operation is expected to consist of three business units: (i) Plant Growth / Hemp Cultivation, (ii) Extraction and Testing, and (iii) Sales and Marketing. Each unit will have unique infrastructure, licensing and staffing requirements, and are expected to offer separate revenue streams while supporting our product development and sales.
Plant Growth / Hemp Cultivation
Hemp is an industrial plant related to marijuana. Fiber from the plant is used to make paper, clothing, rope and other products. Its oil is found in body-care products such as lotion, soap and cosmetics and in a variety of foods, including energy bars, waffles, milk-free cheese, veggie burgers and bread. Numerous uses exist, including hemp plant extracts that are used as a medicine, nutritional supplements and food sources. Beyond this, applications into textiles, building materials, bio-fuels, paper, bio-plastics, livestock feed/bedding as well as personal care products are readily available. Both hemp and marijuana are classified under the same biological category of Cannabis L Sativa. The basic difference between the two is that marijuana has significant amounts of tetrahydrocannabinoil (THC) (5–20%), a psychoactive ingredient; whereas hemp has virtually no THC (less than 0.3%). The 0.3% or less THC found in hemp is too low to provide the psychoactive effect or “high” that supports recreational usage.
9
In May 2014 we entered into an exclusive land lease and crop service agreement with Rocky Mountain Hemp Inc., a southeastern Colorado based hemp grower, and are currently growing hemp for the purpose of extracting and testing non-THC cannabinoids for product formulations (see “Farm Lease and Service Agreement”). We are also exploring relationships with cannabis breeders with the goal of developing proprietary hemp strains with improved medicinal properties. Within the next 12 months, we intend to start an indoor plant-breeding and cultivation program to develop new and proprietary strains with superior features, such as a high extractable cannabinoid content and improved anti-inflammatory properties.
We expect to harvest our first outdoor hemp crop in the third calendar quarter of 2014 and, in parallel, expect to outsource indoor hemp cultivation to local growers. In a second phase, we plan to lease an indoor grow facility and equip this facility for state-of-the-art hemp cultivation. The mandatory Colorado Department of Agriculture hemp cultivation license was obtained on May 6, 2014, to enable our outdoor crop cultivation. The licensing process for indoor cultivation will be initiated once the facility plans are completed and is expected to be swift based on our experience with the Colorado Department of Agriculture application process, although no assurance can be given that this will prove to be the case. A full-time gardener with soil and hydroponic cultivation experience will be hired to oversee the day-to-day operations of the plant growth facility. The required capital investment to equip the grow facility will be significant, with lease, seed and salary costs representing the majority of the operating costs. No assurance can be given that we will have available capital as and when needed for this purpose. We estimate requiring $150,000 for the first year of indoor hemp cultivation.
We estimate that our plant growth facility will have potential to produce four crops annually, with a total annual yield of approximately 500 pounds of high cannabinoid content plant material. Excess hemp material (fibrous plant parts) may be sold for industrial use, potentially providing a secondary revenue stream.
Extraction and Testing
In June 2014 we entered into a Services Agreement with Caerus Discovery, LLC pursuant to which we have outsourced our present laboratory and research requirements (see “Research Services Agreement”).
We intend to establish a fully operational in-house laboratory by the end of the first quarter of 2015. Our laboratory will serve multiple functions relating to CBD extraction, separation and characterization, as well as in-house quality control testing. It is expected that it will enable us to develop a proprietary, patentable, chemical extraction process platform that will drive new product development although no assurance can be given that this will prove to be the case. Quality control and validation systems are expected to be established in parallel, and as the laboratory expands, these capabilities may be offered to outside companies as a fee-for-service product, enabling a service-based revenue stream.
Our laboratory will operate under biopharma methods and standards, and will seek certification as soon as feasible. This will differentiate our laboratory, its services and cannabinoid products, from the majority of the cannabis industry, providing what we believe to be a significant competitive advantage. Our laboratory will also provide the necessary infrastructure to support collaborative work with scientists at the University of Colorado and elsewhere, with the goal of expanding our assets and developing intellectual property. Our laboratory will be an established wet laboratory facility to which we intend to add the specific extraction, testing and characterization equipment required for phytocannabinoid extraction, purification, product formulation and characterization. A fulltime process chemist will be hired to oversee the extraction, purification and testing activities. External consultants will be retained to start the process of gaining quality certification and whenever additional expertise is required for product development. Subsequently, a full time laboratory technician will be hired to help with routine tasks, and to run cell based and ex vivo assays for prototype product testing. It will require significant capital expenditures to establish these unique capabilities, with the equipment cost alone estimated at approximately $400,000. Lease options will be pursued to minimize capital expenditures.
10
As outlined above, our laboratory is intended to support our main business of manufacturing and marketing pharmaceutical level cannabis-based products for the treatment of various inflammatory and autoimmune conditions. In addition, the laboratory will enable activities such as cannabis strain improvement; cannabinoid extraction process development, product development and testing; biomarker identification; and development of mold and pest detection kits. We expect the laboratory to be fully operational by the end of the first calendar quarter of 2015 and we will, in the interim, when necessary and possible, outsource specific research tasks.
Business Development
Our Business Development unit will be responsible for branding, product marketing and sales, as well as for providing market intelligence to support product design and specifications. Another aspect will be to explore the feasibility of expanding our product offerings beyond those containing cannabinoids by structuring original equipment manufacturer or licensing arrangements with domestic and/or international herbal extract manufacturers.
In the near term, a business development person or consultant will be retained to assist with branding, messaging and development of a marketing strategy for the Canna-Pet brand and our future products. For Canna-Pet, the targets will include end-users, veterinarians and pet stores. For our in-house developed products and nutraceuticals, the goal will be to position us for an anticipated product launch in the next 18-24 months. Earlier launch of laboratory services and raw material distribution is anticipated, and we will work closely with the Colorado cannabis companies to establish channels for our products and laboratory services. In parallel, we intend to develop a long-term business and growth strategy, expanding our presence and product availability nationally and internationally, aiming to use existing distribution networks.
Target Markets
Our primary focus is to develop over-the-counter, ultra-low and THC-free, cannabis-based products for prevention and treatment of inflammatory and autoimmune diseases. The products are intended to be safe to use by adult and pediatric patient populations, and will be targeted to the over-the-counter and neutraceutical markets.
Pending market analysis and a detailed study of the cannabinoid literature along with input from key opinion leaders and clinicians, one or two inflammatory indications will be selected for targeted product development and product testing. Our management has extensive experience in conducting non-human based safety and efficacy testing, and will apply this expertise to support its product development and marketing. We anticipate developing several product lines including encapsulated extracts for oral administration, dermatological salves for topical application, and possibly teas and flavored beverages or edibles although no assurance can be given that this will prove to be the case.
We also intend to leverage our intellectual property license agreement with Canna-Pet, LLC under which we will procure and sell medical cannabis products made from hemp and low THC cannabis plants which are intended exclusively for consumption by pets. See “Canna-Pet License Agreement.”
11
Strategic Partnerships and Alliances
We are actively pursuing additional collaborations and partnerships with Colorado-based cannabis growers and breeders, as well as certified testing labs for third party product validation. When President Obama signed into law the 2014 US Farm Bill containing a stipulation that allows universities in the states that permit industrial hemp cultivation to conduct research into the plant, without jeopardizing their federal funding, it enabled a new era of academic cannabis research. At the University of Colorado, the Cannabis Genomic Research Initiative, is mapping the cannabis genome. We are exploring collaboration with the University of Colorado to support our cannabis development efforts and to create new intellectual property. Other US research institutions are conducting cannabis research and clinical trials with cannabis-based medicines, and we intend to pursue strategic partnerships with these researchers.
Farm Lease and Service Agreement
On May 1, 2014, we entered into a Farm Lease and Service Agreement (the “Lease Agreement”) with Rocky Mountain Hemp Inc. and Ryan Loflin (collectively, the “Landlord”) under which we leased two acres of farmland on which we are growing industrial hemp. Under the Lease Agreement, the Landlord is serving as our Cultivation Director and providing us with Landlord’s hemp cultivation expertise. In addition to the growing of hemp we are trying to develop proprietary hemp strains containing improved medicinal properties. In connection with the growing of hemp, we obtained an Industrial Hemp License from the Colorado Department of Agriculture on May 16, 2014. The license expires on April 15, 2015, and is subject to annual renewal. The Lease Agreement has a five month term and can be renewed by us, in our discretion, for up to six additional five-month terms. We are paying lease payments to the Landlord under the Lease Agreement of $6,000 per month.
Research Services Agreement
On June 26, 2014, we entered into a Services Agreement (the “Services Agreement”) with Caerus Discovery, LLC, (“Caerus”) a biotech company, whereby Caerus will be providing us with research services for the purpose of executing specific, specialized cell-based assays and animal studies intended to support product development and design of our proposed anti-inflammatory and autoimmune disease prevention and alleviation products. In connection therewith, we will be supplying Caerus with confidential materials and information including, but not limited to, intellectual property, know-how, data, test results, written materials and similar information (collectively, the “Materials and Information”). The research services will be conducted within Caerus’s laboratories by Caerus’s trained personnel. All patents, patent applications, trademarks, trade names, inventions, copyrights, know-how, and trade secrets (collectively, the “IP Rights”) related to the Materials and Information and arising thereunder will be owned by us. Similarly, if Caerus develops or discovers any development, invention, improvement, modification, product, use, method, technique, conception, know-how, technical data, specification, information, or result relating to any Materials and Information, including new substances (collectively, the “Developments”), Caerus will execute any assignments necessary to transfer title thereto to us. We will have sole responsibility, at our expense, for the preparation filing, prosecution and maintenance of patent applications and rights related to Developments and results of the services provided to us by Caerus. Pursuant to the Services Agreement we have granted to Caerus a non-exclusive, royalty free license, without the right to grant sublicenses, in any IP Rights and Developments for the limited purpose of conducting the services under the Services Agreement. Our Materials and Information is being protected under the confidentiality provisions of the Services Agreement. Caerus will invoice us for rendered services on a project by project basis with payment due within 30 days thereof. The scope and approximate cost of each project will be defined and agreed upon prior to the start of each project. The Services Agreement will terminate upon the earlier of (i) completion of all requested services; (ii) 30 days after we supply Caerus with written notices of termination; or (iii) June 30, 2015.
12
Cohava Gelber, one of our directors, is the Chief Executive Officer, President, and Principal Equity Holder of Caerus. Soren Mogelsvang, our President, Chief Executive Officer and a director previously served as Caerus’ Vice President of Research and Development.
Canna-Pet License Agreement
On July 29, 2014, through our wholly-owed subsidiary, Peak BioPharma Corp., we entered into a License Agreement (the “License Agreement”) with Canna-Pet, LLC, (“Licensor”) a Washington limited liability corporation, which owns the brand name “Canna-Pet” and certain related intellectual property including, but not limited to, trademarks and copyrights, formulations, recipes, production processes and systems, websites, domain names, customer lists, supplier lists, trade secrets and know-how, and other related intellectual property (collectively, the “Licensed Intellectual Property”), used by Licensor in the conduct of its business related to the production and sale of medical cannabis products made from hemp and low THC cannabis plants which are intended exclusively for consumption by pets. Pursuant to the License Agreement, Licensor granted to us a perpetual, exclusive, world-wide license to use the Licensed Intellectual Property in conjunction with our business and the production and sale of medical cannabis products made from hemp and low THC cannabis plants as well as the right to sublicense the Licensed Intellectual Property to third parties. The License Agreement gives us the right to produce and sell existing products utilizing the Licensed Intellectual Property and to develop new products, jointly with Licensor or otherwise, based upon the Licensed Intellectual Property. The License Agreement provides us with an immediate revenue source and access to Licensor’s customer base. During the term of the license, all intellectual property rights in and to the Licensed Intellectual Property remain the exclusive property of Licensor.
In consideration of the grant of the license, we have agreed to pay Licensor license fees in the form of royalty payments calculated on the basis of gross proceeds received by us from sales of products manufactured, marketed or sold by us utilizing the Licensed Intellectual Property or any subsequently developed intellectual property which is jointly owned by us and Licensor.
The royalty will be calculated and paid by us on a quarterly basis using calendar quarters ending March 31, June 30, September 30, and December 31 each year and will be equal to fifteen percent (15%) of the first $1,000,000 of gross proceeds received by us during the quarter and ten percent (10%) on gross proceeds in excess of $1,000,000 received by us during the quarter. On or before the date, which is 45 days following the end of each calendar quarter, we will calculate the amount of the royalty due to Licensor for that quarter and will make payment in full of such amount to Licensor. For purposes of calculating the amount of royalty due for each quarter, “gross proceeds” will not include amounts received by us as payments for any and all taxes, duties, governmental charges, sales expenses, freight or shipping charges, and the like.
Commencing in 2015, we have agreed to pay Licensor guaranteed minimum royalty amounts based upon the gross proceeds received by us from the sale of products (the “License Based Products”) utilizing the Licensed Intellectual Property or subsequently developed jointly owned intellectual property. The guaranteed minimum royalty payments are as follows:
13
|
Time Period
|
Minimum Annual License Fee
|
|||
|
January 1, 2015 through December 31, 2015
|
(1) | |||
|
January 1, 2016 through December 31, 2016
|
(2) | |||
|
January 1, 2017 through December 31, 2017
|
(2) | |||
|
January 1, 2018 through December 31, 2018
|
(2) | |||
|
January 1, 2019 through December 31, 2019
|
(2) | |||
|
January 1, 2020 through December 31, 2020, and all succeeding years
|
(3) | |||
|
(1)
|
Calculated based on gross proceeds equal to twice the verifiable sales of License Based Products for the calendar year ended December 31, 2014, with royalties equal to 15% on gross proceeds of up to $4,000,000 for the year and 10% on gross proceeds in excess of $4,000,000.
|
|
(2)
|
Calculated based on gross proceeds equal to 115% of the amount of gross proceeds used to calculate the minimum royalty for the prior calendar year ended December 31.
|
|
(3)
|
The minimum annual royalty payment for the calendar year beginning January 1, 2020 and for all subsequent calendar years will be equal to the minimum annual royalty payment calculated for the calendar year ended December 31, 2019.
|
All royalty payments made by us during any calendar year will be credited against the minimum annual royalty amounts due and payable by us for such year. In the event that the aggregate amount of royalties payable by us for any calendar year are not sufficient to satisfy our minimum annual royalty payment obligation for that year, we will be obligated to pay the unpaid balance of the minimum royalty amount to Licensor on or before February 28 of the following year.
Licensor has agreed to defend, at its own expense, any action against Licensor or us based on a claim that the Licensed Intellectual Property infringes a US or foreign patent, a US or foreign copyright or involves misappropriation of a trade secret.
The License Agreement may be terminated
|
Ÿ
|
by mutual consent of us and Licensor;
|
|
Ÿ
|
by us, upon 90 days prior written notice to Licensor;
|
|
Ÿ
|
by Licensor, upon written notice to us if any of the following events occur:
|
14
|
(i)
|
our failure to pay Licensor royalty payments within fifteen (15) days after we receive written notice that payment is overdue, provided that there is no good faith dispute over the fees or charges; or
|
|
(ii)
|
our failure to pay the minimum annual royalty payment for any year within fifteen (15) days after we receive written notice that payment is overdue, provided that there is no good faith dispute over the total amount due; or
|
|
(iii)
|
any breach by us of any material term or obligation of the License Agreement if not remedied within thirty (30) days after we receive written notice of such breach, provided that this time will be extended to the extent we have made a good faith effort to resolve any such breach; or
|
|
(iv)
|
we are acquired by another entity and our successor is unwilling to assume our obligations under the License Agreement, or refuses to enter into an Assumption of Obligations Agreement; or
|
|
(v)
|
we cease doing business as a going concern.
|
Our right to use the Licensed Intellectual Property ceases upon termination of the License Agreement.
In the event intellectual property is developed jointly by us and Licensor (including any development by us using the Licensed Intellectual Property) during the performance of the License Agreement, the ownership of such intellectual property will be determined according to principles of United States patent law. In such event, the parties have agreed to negotiate in good faith towards an intellectual property management agreement to define their respective rights and obligations with respect to legal protection, payment of expenses, licensing and infringement of any intellectual property which is jointly owned by the parties. Any party that does not bear its proportionate share of expenses in securing and maintaining patent protection on jointly owned intellectual property in any particular country or countries will be required to surrender its joint ownership under any resulting patents in such country or countries.
Our Business Strategy
Our business strategy is to develop and market safe, non psychoactive cannabis based medicinal products and to become a global leader in the research, development, manufacturing, testing and marketing of cannabis based therapies. We intend to do so by employing rigorous biopharma processes and quality controls to manufacture safe, hemp based products and to target the nutraceutical market which offers a shorter path to commercialization than prescription drugs, which require FDA approval. No assurance can be given that we will be successful in this endeavor.
Government Regulation
Hemp
For the first time since 1937, industrial hemp has been decriminalized at the federal level and can be grown legally in the United States, but only on a limited basis. A landmark provision in the 2014 US Farm Bill recognizes hemp as distinct from its genetic cousin, marijuana. Federal law now exempts industrial hemp from U.S. drug laws in order to allow for crop research by universities, colleges and state agriculture departments. The federal law allows for agricultural pilot programs for industrial hemp in states that permit the growth or cultivation of hemp.
15
Marijuana
Marijuana is a Schedule-I controlled substance and is illegal under federal law. Even in those states in which the use of marijuana has been legalized, its use remains a violation of federal laws. A Schedule I controlled substance is defined as a substance that has no currently accepted medical use in the United States, a lack of safety for use under medical supervision and a high potential for abuse. If the federal government decides to enforce the Controlled Substances Act in Colorado with respect to marijuana, persons that are charged with distributing, possessing with intent to distribute, or growing marijuana could be subject to fines and terms of imprisonment, the maximum being life imprisonment and a $50 million fine.
As of July 7, 2014, 23 states and the District of Columbia allow its citizens to use medical marijuana. Additionally, voters in the states of Colorado and Washington approved ballot measures in November 2013 to legalize marijuana for adult use.
The federal Controlled Substances Act, makes marijuana use and possession illegal on a national level. The Obama administration has effectively stated that it is not an efficient use of resources to direct law federal law enforcement agencies to prosecute those lawfully abiding by state-designated laws allowing the use and distribution of medical marijuana. However, there is no guarantee that the administration will not change its stated policy regarding the low-priority enforcement of federal laws. Additionally, any new administration that follows could change this policy and decide to enforce the federal laws strongly. Any such change in the federal government’s enforcement of current federal laws could cause significant financial damage to us. While we do not intend to harvest cannabis plants with high THC content or, distribute or sell cannabis, we may be irreparably harmed by a change in enforcement by the Federal or state governments.
Despite the Obama administration’s statements, the Department of Justice has stated that it will continue to enforce the Controlled Substances Act with respect to marijuana in Colorado to prevent:
|
Ÿ
|
the distribution of marijuana to minors;
|
|
Ÿ
|
criminal enterprises, gangs and cartels receiving revenue from the sale of marijuana;
|
|
Ÿ
|
the diversion of marijuana from states where it is legal under state law to other states;
|
|
Ÿ
|
state-authorized marijuana activity from being used as a cover or pretext for the trafficking of other illegal drugs or other illegal activity;
|
|
Ÿ
|
violence and the use of firearms in the cultivation and distribution of marijuana;
|
|
Ÿ
|
driving while impaired and the exacerbation of other adverse public health consequences associated with marijuana use;
|
|
Ÿ
|
the growing of marijuana on public lands; and
|
|
Ÿ
|
marijuana possession or use on federal property.
|
16
Nutraceuticals
Nutraceutical, a word that combines the words “nutrition” and “pharmaceutical” are foods or food products that provide health and medical benefits, including the prevention and treatment of disease. Such products range from isolated nutrients, dietary supplements and specific diets to genetically engineered foods, herbal products and processed foods. Nutraceutical foods are not subject to the same testing and regulation as pharmaceutical drugs. The use of nutraceutical as an attempt to accomplish desirable therapeutic outcomes with reduced side effects when compared with other therapeutic agents has met with significant public acceptance and monetary success.
Intellectual Property
We do not presently own any intellectual property but expect to develop intellectual property in the course of our development and commercialization of hemp based health products.
Research and Development
We have yet to incur any research and development expenses but expect to do so within the next 60 days in connection with our Research Services Agreement with Caerus and subsequent thereto in connection with our future establishment of an in-house laboratory.
Competition
The market for hemp based medicinal products is rapidly evolving. Many of our competitors have and will have greater financial and human resources and longer operating histories then we do. We expect to be able to compete based on the safety and efficacy of our products.
Customers
As of the date of this Report, we have yet to achieve revenues. Accordingly, we have no customers. In the near future, we expect to commence selling pet products through our License Agreement with Canna-Pet, LLC.
Employees
As of the date of this Report, we have 3 employees including our two executive officers. Two of our employees work on a part-time basis. Management believes its relationships with its employees to be good. We expect to add one or two additional employees in the fall of 2014.
Markets
Hemp
There is no official estimate of the value of U.S. sales of hemp-based products. According to the Congressional Research Service there are over 25,000 products on the global market that are derived from hemp. It is estimated that in the United States the market for hemp based products in 2012 was nearly 500 million dollars per year, although an exact number has not been determined. Between 156 and 171 million dollars of this market is made up of body care items and food based supplements. 100 million dollars is associated with hemp based clothing and textiles. The remaining balance is associated with an array of various other hemp based goods.
17
Marijuana
According to the 2014 edition of the Marijuana Business Factbook, U.S. retail cannabis sales are expected to rise over the next five years, from an estimated $2.2 – $2.6 billion in 2014 to $7.4 – 8.2 billion in 2018. The sales estimates include both medical marijuana and recreational cannabis sales, and account for additional states establishing and launching cannabis sales programs during the time span covered by the forecast. These predictions assume 4 – 5 new recreational states and 2 – 3 medical states will have fully-functional programs by 2018.
Nutraceuticals
The total global nutraceuticals market reached $142.1 billion in 2011 and is expected to reach $204.8 billion by 2017, growing at a compound annual growth rate of 6.3%, spurred primarily by dietary supplements. Rising health concerns, the growth of key demographics and growing consumer desire to lead a healthy life and avoid dependence on synthetic drugs are identified trends supporting market growth. The nutraceutical ingredient market is forecast to reach $33.6 billion by 2018. This is an emerging market sector with a range of stakeholders including raw material suppliers, processors, product manufacturers and end-use consumers. Nutraceutical cannabinoids are only just beginning to be introduced in product formulations and represent an untapped market segment.
Animal Health
According to a State of the American Pet Survey, one of the greatest challenges of pet ownership is maintaining pets’ health. 41% of pet owners have considered or tried various alternative therapies, including nutritional supplements (29%), herbal remedies (7%) and homeopathy (4%). The recession-resistant US animal health products industry is projected to reach $12 billion by 2016.
DESCRIPTION OF PROPERTIES
Our principal executive offices are located at 4450 Arapahoe Avenue, Suite 100, Boulder, CO 80303. At this location we receive receptionist services, have access to conference room space and pay nominal rent. We believe that this facility is adequate to meet our needs at this time, but as we expect to grow in the near future, we anticipate that we may move to a larger permanent office space that will have a higher rent.
RISK FACTORS
AN INVESTMENT IN OUR SECURITIES IS HIGHLY SPECULATIVE AND INVOLVES A HIGH DEGREE OF RISK. WE FACE A VARIETY OF RISKS THAT MAY AFFECT OUR OPERATIONS OR FINANCIAL RESULTS AND MANY OF THOSE RISKS ARE DRIVEN BY FACTORS THAT WE CANNOT CONTROL OR PREDICT. BEFORE INVESTING IN OUR SECURITIES YOU SHOULD CAREFULLY CONSIDER THE FOLLOWING RISKS, TOGETHER WITH THE FINANCIAL AND OTHER INFORMATION CONTAINED IN THIS REPORT. IF ANY OF THE FOLLOWING RISKS ACTUALLY OCCURS, OUR BUSINESS, PROSPECTS, FINANCIAL CONDITION AND RESULTS OF OPERATIONS COULD BE MATERIALLY ADVERSELY AFFECTED. IN THAT CASE, THE TRADING PRICE OF OUR COMMON STOCK WOULD LIKELY DECLINE AND YOU MAY LOSE ALL OR A PART OF YOUR INVESTMENT. ONLY THOSE INVESTORS WHO CAN BEAR THE RISK OF LOSS OF THEIR ENTIRE INVESTMENT SHOULD CONSIDER AN INVESTMENT IN OUR SECURITIES.
18
THIS REPORT CONTAINS CERTAIN STATEMENTS RELATING TO FUTURE EVENTS OR THE FUTURE FINANCIAL PERFORMANCE OF OUR COMPANY. PROSPECTIVE INVESTORS ARE CAUTIONED THAT SUCH STATEMENTS ARE ONLY PREDICTIONS AND INVOLVE RISKS AND UNCERTAINTIES, AND THAT ACTUAL EVENTS OR RESULTS MAY DIFFER MATERIALLY. IN EVALUATING SUCH STATEMENTS, PROSPECTIVE INVESTORS SHOULD SPECIFICALLY CONSIDER THE VARIOUS FACTORS IDENTIFIED IN THIS REPORT, INCLUDING THE MATTERS SET FORTH BELOW, WHICH COULD CAUSE ACTUAL RESULTS TO DIFFER MATERIALLY FROM THOSE INDICATED BY SUCH FORWARD-LOOKING STATEMENTS.
If any of the following or other risks materialize, the Company’s business, financial condition, and results of operations could be materially adversely affected which, in turn, could adversely impact the value of our common stock. In such a case, investors in our common stock could lose all or part of their investment.
Prospective investors should consider carefully whether an investment in the Company is suitable for them in light of the information contained in this Report and the financial resources available to them. The risks described below do not purport to be all the risks to which the Company or the Company could be exposed. This section is a summary of certain risks and is not set out in any particular order of priority. They are the risks that we presently believe are material to the operations of the Company. Additional risks of which we are not presently aware or which we presently deem immaterial may also impair the Company’s business, financial condition or results of operations.
General Risks
We have a limited operating history upon which investors can evaluate our future prospects. We may never attain profitability.
In March 2014 we determined to enter the business of developing, manufacturing, marketing and selling pharmaceutical level products containing phytocannabinoids, a component of cannabis, for the treatment of various conditions and diseases including, but not limited to, inflammatory and anti-immune diseases. As of the date of this Report, we have not achieved any revenues. Therefore, we have a limited operating history upon which an evaluation of our business plan or performance and prospects can be made. Our operations are therefore subject to all of the risks inherent in light of the expenses, difficulties, complications and delays frequently encountered in connection with the formation of any new business, the development of a product, as well as those risks that are specific to our business in particular. There are no assurances that we can successfully address these challenges. If we are unsuccessful, we and our business, financial condition and operating results will be materially and adversely affected.
19
Given our limited operating history, management has little basis on which to forecast future demand for our products and out anticipated revenues. Our anticipated and future expense levels are based largely on estimates of planned operations and future revenues rather than experience. It is difficult to accurately forecast future revenues because our business is new and our market has not been developed. Except for sales related to our License Agreement with Canna-Pet, LLC, we do not expect meaningful revenues until at least 2015. No assurance can be given that we will ever achieve profitability.
We have a history of losses and we may not achieve or sustain profitability in the future.
We have incurred losses in each fiscal year since our incorporation in 2011. We anticipate that our operating expenses will increase in the foreseeable future as we continue to invest to grow our business, acquire customers and expand our facilities and employee base. These efforts may prove more expensive than we currently anticipate, and we may not succeed in generating sufficient revenues to offset these higher expenses. If we are unable to do so, we and our business, financial condition and operating results could be materially and adversely affected.
We cannot predict our future capital needs and we may not be able to secure additional financing.
During the current fiscal year we have raised approximately $815,000 from sales of our common stock and expect to continue to raise capital through sales of our common stock. We believe that, we have sufficient funds to meet our presently anticipated working capital requirements for approximately six months. This belief is based on our operating plan which in turn is based on assumptions, which may prove to be incorrect. In addition, we may need to raise significant additional funds sooner in order to implement our business plan, support our growth, develop new or enhanced services and products, respond to competitive pressures, acquire or invest in complementary or competitive businesses or technologies, or take advantage of unanticipated opportunities. If our financial resources are insufficient, we will require additional financing in order to meet our plans for expansion. We cannot be sure that this additional financing, if needed, will be available on acceptable terms or at all. Furthermore, any debt financing, if available, may involve restrictive covenants, which may limit our operating flexibility with respect to business matters. If additional funds are raised through the issuance of equity securities, the percentage ownership of our existing shareholders will be reduced, our shareholders may experience additional dilution in net book value, and such equity securities may have rights, preferences, or privileges senior to those of our existing shareholders. If adequate funds are not available on acceptable terms, or at all, we may be unable to develop or enhance our products and services, take advantage of future opportunities, repay debt obligations as they become due, or respond to competitive pressures, any of which would have a material adverse effect on our business, prospects, financial condition, and results of operations.
Our independent registered public accounting firm has expressed doubt about our ability to continue as a going concern.
Our historical financial statements have been prepared under the assumption that we will continue as a going concern. Our independent registered public accounting firm has issued a report that included an explanatory paragraph referring to our recurring net losses and accumulated deficit and expressing substantial doubt in our ability to continue as a going concern. Our ability to continue as a going concern is dependent upon our ability to obtain additional equity financing or other capital, attain further operating efficiencies, reduce expenditures, and, ultimately, to generate revenue. Our financial statements do not include any adjustments that might result from the outcome of this uncertainty. However, if adequate funds are not available to us when we need it, and we are unable to commercialize our products giving us access to additional cash resources, we will be required to curtail our operations which would, in turn, further raise substantial doubt about our ability to continue as a going concern.
20
Our products may not be accepted in the market, which would have an adverse effect on our financial condition and operating results.
We cannot be certain that the products we may develop or market will achieve or maintain market acceptance. Market acceptance of our products depends on many factors, including the safety and efficacy of our products and public perception concerning hemp based products.
Business Risks
Due to the general negativity associated with the cannabis plant within the United States, we anticipate facing potential challenges getting our products into stores.
The majority of our products will be intended for ingestion purposes. We intend to produce hemp based products that contain no or low THC that are legal for ingestion within the U.S. as nutraceuticals. However, we anticipate that we may face scrutiny and run into issues getting our products into stores due to hesitation by certain retail establishments to carry products affiliated with the cannabis plant.
We operate in a highly competitive environment, and if we are unable to compete with our competitors, our business, financial condition, results of operations, cash flows and prospects could be materially adversely affected.
We operate in a highly competitive environment. Our competition includes all other companies that are in the business of developing, manufacturing or selling nutraceutical products, including hemp based nutraceutical products, for personal use or consumption. This highly competitive environment could adversely affect our business, financial condition, results of operations, cash flows and prospects.
Our failure to manage growth, diversification and changes to our business could harm our business.
We expect our company to experience growth in connection with the development and sale of our products. Our failure to successfully manage and monetize any growth, and to successfully diversify our business in the future could harm the success and longevity of our company.
We depend on key personnel to operate our business, and if we are unable to retain, attract and integrate qualified personnel, our ability to develop and successfully grow our business could be harmed.
We believe that our future success is highly dependent on the continued services of our key officers, employees, and Board members as well as our ability to attract and retain highly skilled and experienced technical personnel. The loss of their services could have a detrimental effect on our operations. The departure of Soren Mogelsvang, our President and CEO, or any major change in our Board or management could adversely affect our operations.
21
Government regulation of cannabis is constantly evolving, and unfavorable developments could have an adverse effect on our operating results.
Any changes in laws or regulations relating to cannabis and hemp could adversely affect our business, results of operations and our business prospects.
We face competition from entities in our industry with substantially more capital, greater name recognition, more employees, greater resources, and longer operating histories than we do.
We have a limited operating history in our industry. Many of our competitors have greater human and financial resources, name recognition and operating histories than we do which potentially puts us at a competitive disadvantage.
Third Party Risks
We presently rely on third parties to provide cultivation, research and testing services necessary for the operation of our business, the loss of which service providers would have a material adverse effect on our expected operating results.
We are presently dependent on third party service providers to operate our business including, but not limited to, Rocky Mountain Hemp, Inc. and Caerus Discovery, LLC. The loss of these service providers would have an adverse effect on our operations and operating results.
Investment Risks
We may be unable to raise enough capital through sales of our equity and debt securities to implement our business plan.
We will be largely dependent on capital raised through sales of our equity and debt securities to implement our business plan and support our operations. Based upon our current cash position, we expect that we will be able maintain operations for a period of approximately six months. At the present time, we have not made any arrangements to raise additional cash, other than through sales of our equity securities. We cannot assure you that we will be able to raise the working capital as needed on terms acceptable to us, if at all. If we are unable to raise capital as needed, we may be required to reduce the scope of our business development activities, which could harm our business plans, financial condition and operating results, or cease our operations entirely.
22
You may experience dilution of your ownership interests because of the future issuance of additional shares of our common or preferred stock or other securities that are convertible into or exercisable for our common or preferred stock.
Any future issuance of our equity or equity-backed securities may dilute then-current stockholders’ ownership percentages and could also result in a decrease in the fair market value of our equity securities, because our assets would be owned by a larger pool of outstanding equity. As described above, we may need to raise additional capital through public or private offerings of our common or preferred stock or other securities that are convertible into or exercisable for our common or preferred stock. We may also issue such securities in connection with hiring or retaining employees and consultants (including stock options issued under our 2014 Equity Incentive Plan), as payment to providers of goods and services, in connection with future acquisitions or for other business purposes. Our Board may at any time authorize the issuance of additional common or preferred stock without common stockholder approval, subject only to the total number of authorized common and preferred shares set forth in our articles of incorporation. The terms of equity securities issued by us in future transactions may be more favorable to new investors, and may include dividend and/or liquidation preferences, superior voting rights and the issuance of warrants or other derivative securities, which may have a further dilutive effect. Also, the future issuance of any such additional shares of common or preferred stock or other securities may create downward pressure on the trading price of our common stock. There can be no assurance that any such future issuances will not be at a price (or exercise prices) below the price at which shares of our common stock are then traded.
The ability of our Board to issue additional stock may prevent or make more difficult certain transactions, including a sale or merger of the Company.
We currently have authorized 350,000,000 shares of capital stock consisting of (i) 325,000,000 shares of common stock, and (ii) 25,000,000 shares of "blank check" Preferred Stock. As a result, our Board is authorized to issue up to 25,000,000 shares of preferred stock with powers, rights and preferences designated by it. See “Preferred Stock” in the section of this Report titled “Description of Securities.” Shares of voting or convertible preferred stock could be issued, or rights to purchase such shares could be issued, to create voting impediments or to frustrate persons seeking to effect a takeover or otherwise gain control of the Company. The ability of the Board to issue such additional shares of Preferred Stock, with rights and preferences it deems advisable, could discourage an attempt by a party to acquire control of the Company by tender offer or other means. Such issuances could therefore deprive stockholders of benefits that could result from such an attempt, such as the realization of a premium over the market price for their shares in a tender offer or the temporary increase in market price that such an attempt could cause. Moreover, the issuance of such additional shares of preferred stock to persons friendly to the Board could make it more difficult to remove incumbent managers and directors from office even if such change were to be favorable to stockholders generally.
We have a concentration of stock ownership and control, which may have the effect of delaying, preventing, or deterring certain corporate actions and may lead to a sudden change in our stock price.
Our common stock ownership is highly concentrated. As of August 11, 2014 our officers and directors beneficially own 33,084,000 shares, or approximately 42.5% of our total outstanding common stock. Their interests may differ significantly from your interests. As a result of the concentrated ownership of our stock, a relatively small number of stockholders, acting together, will be able to control all matters requiring stockholder approval, including the election of directors and approval of mergers and other significant corporate transactions. In addition, because our stock is so thinly traded, the sale by any of our large stockholders of a significant portion of that stockholder’s holdings could cause a sharp decline in the market price of our common stock.
23
Restrictions on the use of Rule 144 by Shell Companies or Former Shell Companies could affect your ability to resale our shares.
Historically, the SEC has taken the position that Rule 144 under the Securities Act, as amended, is not available for the resale of securities initially issued by companies that are, or previously were, shell companies like us, to their promoters or affiliates despite technical compliance with the requirements of Rule 144. The SEC has codified and expanded this position in its amendments effective on February 15, 2008 and apply to securities acquired both before and after that date by prohibiting the use of Rule 144 for resale of securities issued by shell companies (other than business transaction related shell companies) or issuers that have been at any time previously a shell company. The SEC has provided an important exception to this prohibition, however, if the following conditions are met:
|
Ÿ
|
the issuer of the securities that was formerly a shell company has ceased to be a shell company;
|
|
Ÿ
|
the issuer of the securities is subject to the reporting requirements of Section 13 or 15(d) of the Exchange Act;
|
|
Ÿ
|
the issuer of the securities has filed all Exchange Act reports and material required to be filed, as applicable, during the preceding 12 months (or such shorter period that the issuer was required to file such reports and materials), other than Form 8-K reports; and
|
|
Ÿ
|
at least one year has elapsed from the time that the issuer filed current Form 10 type information with the SEC reflecting its status as an entity that is not a shell company.
|
As such, due to the fact that we were a shell company until the date of this Report, holders of "restricted securities" within the meaning of Rule 144 will be subject to the conditions set forth herein. Therefore, sales under Rule 144 are prohibited for at least one year from the date this Report is filed.
There currently is a limited public market for our common stock. Failure to develop or maintain an active trading market could negatively affect the value of our common stock and make it difficult or impossible for you to sell your shares.
There is currently a limited public market for shares of our common stock and an active market may never develop. Our common stock is quoted on the OTC Markets. The OTC Markets is a thinly traded market and lacks the liquidity of certain other public markets with which some investors may have more experience. We may not ever be able to satisfy the listing requirements for our common stock to be listed on a national securities exchange, which are often a more widely-traded and liquid market. Some, but not all, of the factors which may delay or prevent the listing of our common stock on a more widely-traded and liquid market includes the following:
24
|
Ÿ
|
our stockholders’ equity may be insufficient;
|
|
Ÿ
|
the market value of our outstanding securities may be too low;
|
|
Ÿ
|
our net income from operations may be too low;
|
|
Ÿ
|
our common stock may not be sufficiently widely held;
|
|
Ÿ
|
we may not be able to secure market makers for our common stock; and
|
|
Ÿ
|
we may fail to meet the rules and requirements mandated by the several exchanges and markets to have our common stock listed.
|
Should we fail to satisfy the initial listing standards of the national exchanges, or our common stock is otherwise rejected for listing and remains listed on the OTC Markets or suspended from the OTC Markets, the trading price of our common stock could suffer and the trading market for our common stock may be less liquid and our common stock price may be subject to increased volatility.
Our common stock is subject to the “penny stock” rules of the SEC and the trading market in the securities is limited, which makes transactions in the stock cumbersome and may reduce the value of an investment in the stock.
The SEC has adopted Rule 15g-9 which establishes the definition of a “penny stock,” for the purposes relevant to us, as any equity security that has a market price of less than $5.00 per share or with an exercise price of less than $5.00 per share, subject to certain exceptions. For any transaction involving a penny stock, unless exempt, the rules require:
|
Ÿ
|
that a broker or dealer approve a person’s account for transactions in penny stocks; and
|
|
Ÿ
|
the broker or dealer receives from the investor a written agreement to the transaction, setting forth the identity and quantity of the penny stock to be purchased.
|
In order to approve a person’s account for transactions in penny stocks, the broker or dealer must:
|
Ÿ
|
obtain financial information and investment experience objectives of the person; and
|
|
Ÿ
|
make a reasonable determination that the transactions in penny stocks are suitable for that person and the person has sufficient knowledge and experience in financial matters to be capable of evaluating the risks of transactions in penny stocks.
|
25
The broker or dealer must also deliver, prior to any transaction in a penny stock, a disclosure schedule prescribed by the SEC relating to the penny stock market, which, in highlight form sets forth:
|
Ÿ
|
the basis on which the broker or dealer made the suitability determination; and
|
|
Ÿ
|
that the broker or dealer received a signed, written agreement from the investor prior to the transaction.
|
Generally, brokers may be less willing to execute transactions in securities subject to the “penny stock” rules. This may make it more difficult for investors to dispose of common stock and cause a decline in the market value of stock.
Disclosure also has to be made about the risks of investing in penny stocks in both public offerings and in secondary trading and about the commissions payable to both the broker-dealer and the registered representative, current quotations for the securities and the rights and remedies available to an investor in cases of fraud in penny stock transactions. Finally, monthly statements have to be sent disclosing recent price information for the penny stock held in the account and information on the limited market in penny stocks. If we remain subject to the penny stock rules for any significant period, it could have an adverse effect on the market, if any, for our securities. If our securities are subject to the penny stock rules, investors will find it more difficult to dispose of our securities.
If securities analysts do not initiate coverage or continue to cover our common stock or publish unfavorable research or reports about our business, this may have a negative impact on the market price of our common stock.
The trading market for our common stock will depend on the research and reports that securities analysts publish about our business and the Company. It is often more difficult to obtain analyst coverage for companies whose securities are traded on the OTC Markets. We do not have any control over securities analysts. There is no guarantee that securities analysts will cover our common stock. If securities analysts do not cover our common stock, the lack of research coverage may adversely affect our market price. If we are covered by securities analysts, and our stock is the subject of an unfavorable report, our stock price and trading volume would likely decline. If one or more of these analysts ceases to cover the Company or fails to publish regular reports on the Company, we could lose visibility in the financial markets, which could cause our stock price or trading volume to decline.
We do not anticipate paying dividends on our common stock, and investors may lose the entire amount of their investment.
To date, cash dividends have not been declared or paid on our common stock, and we do not anticipate such a declaration or payment for the foreseeable future. We expect to use future earnings, if any, to fund business growth. Therefore, stockholders will not receive any funds absent a sale of their shares of common stock, subject to the limitation outlined herein. If we do not pay dividends, our common stock may be less valuable because a return on your investment will only occur if our stock price appreciates. We cannot assure stockholders of a positive return on their investment when they sell their shares, nor can we assure that stockholders will not lose the entire amount of their investment.
26
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
We incorporate herein by reference the information set forth in Item 7. “Management’s Discussion and Analysis of Financial Condition and Results of Operations” of our Annual Report on Form 10-K for the year ended September 30, 2013 filed with the Securities and Exchange Commission on December 27, 2013.
We incorporate herein by reference the information set forth in Item 2. “Management’s Discussion and Analysis of Financial Condition and Results of Operations” of our Quarterly Report on Form 10-Q for the quarter ended March 31, 2014 filed with the Securities and Exchange Commission on May 14, 2014.
FINANCIAL STATEMENTS
We incorporate herein by reference the information set forth in Item 15. “Financial Statements” of our Annual Report on From 10-K for the year ended September 30, 2013 filed with the Securities and Exchange Commission on December 27, 2013.
We incorporate herein by reference the information set forth in Item 1. “Financial Statements” of our Quarterly Report on From 10-Q for the quarter ended March 31, 2014 filed with the Securities and Exchange Commission on May 14, 2014.
DIRECTORS, EXECUTIVE OFFICERS, PROMOTERS AND CONTROL PERSONS
Directors and Executive Officers
Below are the names of and certain information regarding the Company’s current executive officers and directors:
|
Name
|
Age
|
Position
|
Date Named to
Board of Directors
|
||||
|
Soren Mogelsvang
|
42 |
President, Chief Executive Officer and Director
|
March 10, 2014
|
||||
|
Arnold Tinter
|
68 |
Chief Financial Officer, Secretary and Treasurer
|
N/A | ||||
|
Cohava Gelber
|
57 |
Director
|
March 10, 2014
|
||||
|
Guy Yachin
|
46 |
Director
|
March 10, 2014
|
||||
|
Vered Caplan
|
45 |
Director
|
March 10, 2014
|
27
Directors are elected to serve until the next annual meeting of stockholders and until their successors are elected and qualified. Directors are elected by a plurality of the votes cast at the annual meeting of stockholders and hold office until the expiration of the term for which he or she was elected and until a successor has been elected and qualified.
A majority of the authorized number of directors constitutes a quorum of the Board for the transaction of business. The directors must be present at the meeting to constitute a quorum. However, any action required or permitted to be taken by the Board may be taken without a meeting if all members of the Board individually or collectively consent in writing to the action.
Our Board consists of four (4) members, none of whom is independent. Executive officers are appointed by the Board and serve at its pleasure.
The principal occupation and business experience during the past five years for our executive officers and directors is as follows:
Soren Mogelsvang, age 42, was appointed as our Chief Executive Officer on March 11, 2014 and as a Director on March 10, 2014. He was appointed as our President on May 1, 2014. Mr. Mogelsvang is a biotech executive and entrepreneur with more than 15 years of experience in research and development and laboratory operations. He is experienced in strategic positioning, value creation, IP development and preclinical development of peptide therapeutics. He is the co-founder of two biotech companies, Serpin Pharma, Inc. and Caerus Discovery LLC. He founded Serpin Pharma, Inc. in 2010 and was employed as Serpin Pharma, Inc.’s Vice President of Research and Development from 2010 until April 2014. He co-founded Caerus Discovery in 2010 and was employed by Caerus Discovery until 2012 as a Vice President of Research and Development. From 2008 until 2010 he worked at ATCC where he managed the Cell Biology Department. From 2006 until 2008 he was an instructor and faculty member at the University of Colorado School of Medicine and from 2004 through 2005 he worked as the Director of Laboratory and Products at Affinity BioRegents. From 2001 until 2004 he was a Postdoctoral Fellow at the University of Colorado School of Medicine and from 2000 until 2001 worked as a research scientist for Maltagen Forschung. Mr. Mogelsvang received a Ph.D. in Cell Biology from the University of Cambridge (England) in 2000, an M.S. in Plant Molecular Biology from the University of Copenhagen (Denmark) in 1996 and a B.Sc. in Plant Biology from the University of Copenhagen (Denmark) in 1993. Mr. Mogelsvang has filed numerous patent applications and has had several of his works published.
Arnold Tinter, age 68, was appointed as our Chief Financial Officer, Secretary and Treasurer on October 15, 2013. Mr. Tinter founded Corporate Finance Group, Inc., a consulting firm located in Denver, Colorado, in 1992, and is its President. Corporate Finance Group, Inc. is involved in financial consulting in the areas of strategic planning, mergers and acquisitions and capital formation. He provides Chief Financial Officer (“CFO”) services to a number of public companies, including Frac Water Systems Inc., Barfresh Food Group Inc., (“Barfresh”) and LifeApps Digital Media Inc. (“LifeApps”). Mr. Tinter is also a Director of Barfresh and LifeApps. During the period from 2010 to 2012 Mr. Tinter provided CFO services to Agrisolar Solutions Inc., T.O Entertainment Inc., and Arvana Inc. From 2006 to 2010 he has provided CFO services to Spicy Pickle Franchising, Inc., a public company, where his responsibilities included oversight of all accounting functions, including SEC reporting, strategic planning and capital formation. Prior to 2006 Mr. Tinter served in various capacities in both private and public companies including Chief Executive and Chief Financial Officer positions. Mr. Tinter served in the United States Army as an infantry officer and is a Vietnam veteran. Mr. Tinter received a B.S. degree in Accounting in 1967 from C.W. Post College, Long Island University, and is licensed as a Certified Public Accountant in Colorado.
28
Guy Yachin, age 46, was appointed as a Director on March 10, 2014. He has more than 17 years of executive management experience with biotech and biomedical companies. From 2013 to the present he has served as the Chief Executive Officer of Serpin Pharma, Inc., a privately held development stage biotech company. From 2011 until 2013 he served as the Chief Executive Officer for Nas Vax Ltd. , a company which develops immunotherapeutic products and vaccines. From 2007 until 2011 he served as Chief Executive Officer for MGVS, a company engaged in the development of products that address blood vessel disorders. He co-founded Chiasma Inc., a company which focuses on drug delivery of protein drugs, in 2001 and served as its Chief Executive Officer until 2007. From 1997 until 2001 he served as the Chief Executive Officer of Naiot Technological Center, the largest technological center in Israel focusing on biomedical technologies. From 1995 until 1997 he served as Executive Vice President for Ofer Technologies and from 1990 until 1995 served in the Israeli Navy. Mr. Yachin has served as a board member and chairperson for several biomedical companies since 1997 including (i) Orgenesis, a development stage company which uses therapeutic technologies for the treatment of diabetes; (ii) Remon Medical Technologies, a company which manufactures miniature implants for the non-invasive assessment and treatment of a variety of medical conditions; and (iii) Enzymotec, a company which develops and markets bio-functional products for the pharmaceutical, cosmetics and nutraceutical industries; and (iv) NanoPaso, a developer of painless micro-needle devices for transdermal drug delivery and diagnostics. Mr. Yachin received an MBA from The Technion - Israel Institute of Technology in 1990 and received an Industrial Engineering and Management Degree from The Technion - Israel Institute of Technology in 1994.
Cohava Gelber, age 57, was appointed as a Director on March 10, 2014. She has more than 23 years of experience in executive and scientific research capacities. Since 2011 she has served as the President and Chief Executive Officer of Caerus Discovery, LLC and as the Executive Chairperson for the Board of Serpin Pharma, Inc., both of which are development stage biotech companies. From 2005 until November 2010 she served as the Chief Scientific and Technology Officer at ATCC, and from 2003 until 2005 as the Vice President, Research and Development for Mannkind BioPharmaceutical Corporation, a public biotech company. From 2001 until 2003 Dr. Gelber served as the Senior Vice President, Immunology and Cell Biology for Pharmaceutical Discovery Corporation (merged with Mannkind BioPharmaceutical Corporation) and from 2000 until 2001 served as President and Chief Operating Officer for Molecular Discoveries LLC (“Molecular”). From 1999 until 2000 she served as the Chief Scientific Officer and Chief Operating Officer for Molecular. From 1996 until 1999 Dr. Gelber served as Vice President, Research and Development for Immuno Therapy, Inc. (predecessor of Molecular Discoveries) and as an Assistant Research Professor and Director of Cellular Immunology at Duke University Medical Center from 1995 until 1996. From 1991 until 1995 she worked in Senior Scientist and Project Leader capacities for various entities. Dr. Gelber received a B.Sc. Degree and an M. Sc. Degree majoring in microbiology from Hebrew University of Jerusalem in 1980 and 1984, respectively, a PhD in Microbiology from the Weitzman Institute of Science in 1988, Post Doctoral Degrees in Immunology in 1990 and 1991 from Berkeley University and Stanford University, respectively, and an MBA Degree from Cornell University in 2002. Dr. Gelber has published several works, filed numerous patent applications and has received Fellowships from Stanford Medical School and the Cancer Research Coordination Committee.
Vered Caplan, age 45, was appointed as a Director on March 10, 2014. She has been the Chief Executive Officer of Kamedis, a company focused on utilizing plant extracts for dermatology purposes since 2008. From 2004 to 2007, Ms. Caplan was Chief Executive Officer of GammaCan, a company focused on the use of immunoglobulins for the treatment of cancer. During the past five years, Ms. Caplan has been a director of the following companies: Opticul Ltd., a company involved with optic based bacteria classification; Inmotion Ltd., a company involved with self-propelled disposable colonoscopies; Nehora Photonics Ltd., a company involved with non-invasive blood monitoring; Ocure Ltd., a company involved with wound management; Eve Medical Ltd., a company involved with hormone therapy for Menopause and PMS; and Biotech Investment Corp., a company involved with prostate cancer diagnostics. Ms. Caplan has a M.Sc. in bio-medical engineering from Tel-Aviv University specialized in signal processing; management for engineers from Tel-Aviv University specialized in business development; and a B.Sc. in mechanical engineering from the Technion Institute of Technology specialized in software and cad systems.
29
Other Key Personnel
We have no significant employees other than the officers and directors described above.
Family Relationships
There are no family relationships among our directors or executive officers.
Involvement in Certain Legal Proceedings
No executive officer or director of ours has been involved in the last ten years in any of the following:
|
Ÿ
|
Any bankruptcy petition filed by or against any business or property of such person, or of which such person was a general partner or executive officer either at the time of the bankruptcy or within two years prior to that time;
|
|
Ÿ
|
Any conviction in a criminal proceeding or being subject to a pending criminal proceeding (excluding traffic violations and other minor offenses);
|
|
Ÿ
|
Being subject to any order, judgment, or decree, not subsequently reversed, suspended or vacated, of any court of competent jurisdiction, permanently or temporarily enjoining, barring, suspending or otherwise limiting his involvement in any type of business, securities or banking activities;
|
|
Ÿ
|
Being found by a court of competent jurisdiction (in a civil action), the SEC or the Commodity Futures Trading Commission to have violated a federal or state securities or commodities law, and the judgment has not been reversed, suspended, or vacated;
|
|
Ÿ
|
Being the subject of or a party to any judicial or administrative order, judgment, decree or finding, not subsequently reversed, suspended or vacated relating to an alleged violation of any federal or state securities or commodities law or regulation, or any law or regulation respecting financial institutions or insurance companies, including, but not limited to, a temporary or permanent injunction, order of disgorgement or restitution, civil money penalty or temporary or permanent cease-and-desist order, or removal or prohibition order, or any law or regulation prohibiting mail, fraud, wire fraud or fraud in connection with any business entity; or
|
|
Ÿ
|
Being the subject of or a party to any sanction or order, not subsequently reversed, suspended or vacated, of any self-regulatory organization (as defined in Section 3(a)(26) of the Exchange Act, any registered entity (as defined in Section 1(a)(29) of the Commodity Exchange Act), or any equivalent exchange, association, entity or organization that has disciplinary authority over its members or persons associated with a member.
|
30
Compliance with Section 16(a) of the Exchange Act
Section 16(a) of the Exchange Act, as amended, requires that our directors, executive officers and persons who own more than 10% of a class of our equity securities that are registered under the Exchange Act to file with the SEC initial reports of ownership and reports of changes of ownership of such registered securities.
To our knowledge, based solely on a review of such materials as are required by the SEC, none of our officers, directors or beneficial holders of more than 10% of our issued and outstanding shares of common stock failed to timely file with the SEC any form or report required to be so filed pursuant to Section 16(a) of the Exchange Act, during the fiscal year ended September 30, 2013.
Code of Ethics
In December 2013, we adopted a Code of Ethics that applies to our principal executive officer, principal financial officer, principal accounting officer or controller, persons performing similar functions as well as to our directors and employees. A copy of our Code of Ethics has been filed as Exhibit 14.1 to this Annual Report, and will be provided to any person requesting same without charge. To request a copy of our Code of Ethics please make written request to our President ℅ Cannabis Therapy Corp., 4450 Arapahoe Avenue, Suite 100, Boulder, CO 80303.
Board Committees
Our Board has 4 members, Soren Mogelsvang, Cohava Gelber, Guy Yachin and Vered Caplan. Our Board collectively undertakes our risk oversight function. This review of our risk tolerances includes, but is not limited to, financial, legal and operational risks and other risks concerning our reputation and ethical standards.
We are a small, development stage company which has yet to achieve operating revenues. We believe that our present management structure is appropriate for a company of our size and state of development.
Our Board may designate from among its members an executive committee, audit committee and one or more other committees. No such committees presently exist, due to the fact that we presently have only four directors. Accordingly, we do not have an audit committee or an audit committee financial expert. We are presently not required to have an audit committee financial expert and do not believe we otherwise need one at this time. Given our size, we do not have a nominating committee or compensation committee, or committees performing similar functions, or a diversity policy. Our Board monitors and assesses the need for and qualifications of additional directors. We may adopt a diversity policy in the future in connection with our anticipated growth. Our entire board presently serves the functions of an audit committee, nominating committee and compensation committee. We have not implemented procedures by which our security holders may recommend board nominees to us but expect to do so in the future, when and if we engage in material business operations.
31
Board Compensation
Prior to October 1, 2013 we did not compensate our directors for serving as such. On December 20, 2013 our Board authorized the following compensation to be paid to our directors:
|
Ÿ
|
Annual fee of $12,000 per year payable in equal quarterly installments of $3,000 in arrears on each of January 1, April 1, July 1, and October 1, with the initial payment for the quarter October 1, 2013 through December 31, 2013 being due on January 1, 2014;
|
|
Ÿ
|
Attendance fee of $1,000 per quarterly meeting;
|
|
Ÿ
|
Fee of $500 for each special meeting including telephonic meetings;
|
|
Ÿ
|
If travel is involved with respect to a quarterly or special meeting, the director will receive an additional $1,000 for each additional day resulting from travel;
|
|
Ÿ
|
250,000 stock options in connection with appointment to the Board with one year vesting, the exercise of which is subject to the continuance of the director relationship within 90 days of the date of exercise; and
|
|
Ÿ
|
Subsequent annual equity awards of 150,000 stock options with one year vesting, the exercise of which is subject to the continuance of the director relationship.
|
Pursuant to the foregoing, on December 20, 2013 we issued 250,000 non-statutory stock options under our 2013 Equity Incentive Plan to each of Charles Watson, Stuart Sundlun, and Nadine Smith with a term of ten years, an exercise price of $0.20 per share and a one year vesting period.
In connection with our March 2014 entry into our present line of business and related management changes, we discontinued the board compensation arrangement which had been approved by prior management. In addition, the options issued to prior management in December 2013 were returned to us for cancellation. We do not presently have arrangements in place respecting board compensation. In connection with the March 2014 appointments to our board of directors, the new directors and/or their assigns received shares of our common stock or stock options. Iris Yachin, the wife and an assignee of Guy Yachin, received 14,940,000 shares of our common stock. Aaron Gelber, the husband and an assignee of Cohava Gelber, received 14,940,000 shares of our common stock. Vered Caplan received 2,916,000 non-qualified, ten year, stock options, under our 2013 Equity Incentive Plan, vesting upon issuance, with an exercise price of $0.00667 per share. Soren Mogelsvang, an officer and a director, received 3,204,000 shares of our common stock.
Director Independence
We are not currently subject to listing requirements of any national securities exchange or inter-dealer quotation system which has requirements that a majority of the Board be “independent” and, as a result, we are not at this time required to have our Board comprised of a majority of “Independent Directors.” Our Board has determined that none of our directors is “independent” within the definition of independence provided in the Marketplace Rules of The Nasdaq Stock Market.
32
Equity Incentive Plans
2013 Equity Incentive Plan.
On October 15, 2013, our Board of Directors approved our 2013 Equity Incentive Plan (the “2013 Plan”). A total of 7,500,000 shares of our common stock were reserved for issuance in connection with awards granted under the 2013 Plan. A maximum of 2,500,000 shares could be granted during the first twelve months of the 2013 Plan. If an award granted under the 2013 Plan expired, terminated, was unexercised or was forfeited, or if any shares were surrendered to us in connection with an award, the shares subject to such award and the surrendered shares would become available for further awards under the 2013 Plan. The number of shares of common stock subject to the 2013 Plan and the number of shares and terms of any incentive award were subject to adjustment in the event of any stock dividend, spin-off, split-up, stock split, reverse stock split, recapitalization, reclassification, merger, consolidation, liquidation, business combination or exchange of shares or similar transaction.
The compensation committee of the Board, or the Board in the absence of such a committee, administered the 2013 Plan and the grants made thereunder. Subject to the terms of the 2013 Plan, the Board had complete authority and discretion to determine the terms of awards under the 2013 Plan. Any officer or other employee of ours or our affiliates, or an individual that we or our affiliates had engaged to become an officer or employee, or a consultant or advisor who provided services to us or our affiliates, including a non-employee director of the Board, was eligible to receive awards under the 2013 Plan.
The 2013 Plan authorized the grant to eligible recipients of nonqualified stock options, incentive stock options, restricted stock awards, restricted stock units, performance grants intended to comply with Section 162(m) of the Internal Revenue Code of 1986, as amended (the “Code”) and stock appreciation rights (“SARs”) as described below:
|
Ÿ
|
Options granted under the 2013 Plan entitle the grantee, upon exercise, to purchase a specified number of shares from us at a specified exercise price per share. The exercise price for shares of our common stock covered by an option cannot be less than the fair market value of our common stock on the date of grant unless agreed to otherwise at the time of the grant. Such awards may include vesting requirements.
|
|
Ÿ
|
Restricted stock awards and restricted stock units may be awarded on terms and conditions established by our compensation committee, which may include performance conditions for restricted stock awards and the lapse of restrictions on the achievement of one or more performance goals for restricted stock units.
|
|
Ÿ
|
The Board may make performance grants, each of which will contain performance goals for the award, including the performance criteria, the target and maximum amounts payable, and other terms and conditions.
|
|
Ÿ
|
Stock awards are permissible. The Board will establish the number of shares of common stock to be awarded and the terms applicable to each award, including performance restrictions.
|
|
Ÿ
|
SARs, entitle the participant to receive a distribution in an amount not to exceed the number of shares of common stock subject to the portion of the SAR exercised multiplied by the difference between the market price of a share of common stock on the date of exercise of the SAR and the market price of a share of common stock on the date of grant of the SAR.
|
The 2013 Plan did not receive stockholder approval. Any awards of incentive stock options prior to such stockholder approval were conditioned on such approval and if such approval was not obtained by October 15, 2014, which was 12 months after the date of Board approval, such options were to be treated as non-incentive options.
Our Board of Directors or if then in place, the compensation committee of our Board of Directors, could amend, suspend or terminate the 2013 Plan without stockholder approval or ratification at any time or from time to time. No change could be made by our Board of Directors that increased the total number of shares of our common stock reserved for issuance under the 2013 Plan or reduced the minimum exercise price for options or exchange of options for other incentive awards. Unless sooner terminated, the 2013 Plan was to terminate ten years after the date on which it was adopted.
33
On May 22, 2014, our Board of Directors terminated our 2013 Equity Incentive Plan (the “2013 Plan”) and approved our 2014 Equity Incentive Plan (the “2014 Plan”). The termination of the 2013 Plan had no effect on the outstanding options previously issued under the 2013 Plan. At the time of termination of the 2013 Plan and presently, there were and are 3,291,000 outstanding nonstatutory options which were issued under the 2013 Plan, all of which have a ten year term and an exercise price of $0.00667 per share.
2014 Equity Incentive Plan
A total of 11,000,000 shares of our common stock are reserved for issuance in connection with awards which may be granted under the 2014 Plan. If an award granted under the 2014 Plan expires, terminates, is unexercised or is forfeited, or if any shares are surrendered to us in connection with an award, the shares subject to such award and the surrendered shares will become available for further awards under the 2014 Plan. Shares issued under the 2014 Plan through the settlement, assumption or substitution of outstanding awards or obligations to grant future awards as a condition of acquiring another entity are not expected to reduce the maximum number of shares available under the 2014 Plan. In addition, the number of shares of common stock subject to the 2014 Plan and the number of shares and terms of any award are subject to adjustment in the event of any stock dividend, spin-off, split-up, stock split, reverse stock split, recapitalization, reclassification, merger, consolidation, liquidation, business combination or exchange of shares or similar transaction.
The compensation committee of the Board, or the Board in the absence of such a committee, will administer the 2014 Plan and grants made thereunder. Subject to the terms of the 2014 Plan, the compensation committee has complete authority and discretion to determine the terms of awards under the 2014 Plan. Any officer or other employee of ours or our affiliates, or an individual that we or our affiliate has engaged to become an officer or employee, or a consultant or advisor who provides services to us or our affiliates, including a non-employee director of the Board, is eligible to receive awards under the 2014 Plan.
The 2014 Plan authorizes the grant to eligible recipients of nonqualified stock options, incentive stock options, restricted stock awards, restricted stock units, performance grants intended to comply with Section 162(m) of the Internal Revenue Code of 1986, as amended (the “Code”) and stock appreciation rights (“SARs”) as described below:
|
Ÿ
|
Options granted under the 2014 Plan entitle the grantee, upon exercise, to purchase a specified number of shares from us at a specified exercise price per share. The exercise price for shares of our common stock covered by an option cannot be less than the fair market value of our common stock on the date of grant. Such awards may include vesting requirements.
|
|
Ÿ
|
Restricted stock awards and restricted stock units may be awarded on terms and conditions established by our compensation committee, which may include performance conditions for restricted stock awards and the lapse of restrictions on the achievement of one or more performance goals for restricted stock units.
|
|
Ÿ
|
The compensation committee may make performance grants, each of which will contain performance goals for the award, including the performance criteria, the target and maximum amounts payable, and other terms and conditions.
|
|
Ÿ
|
Stock awards are permissible. The compensation committee will establish the number of shares of common stock to be awarded and the terms applicable to each award, including performance restrictions.
|
|
Ÿ
|
SARs entitle the participant to receive a distribution in an amount not to exceed the number of shares of common stock subject to the portion of the SAR exercised multiplied by the difference between the market price of a share of common stock on the date of exercise of the SAR and the market price of a share of common stock on the date of grant of the SAR.
|
The 2014 Plan has yet to receive stockholder approval. Any awards of incentive stock options prior to such stockholder approval shall be conditioned on such approval and if such approval is not obtained by May 22, 2015, which is 12 months after the date of Board approval, such options shall be treated as non-incentive options.
34
Our Board of Directors or if then in place, the compensation committee of our Board of Directors, may amend, suspend or terminate the 2014 Plan without stockholder approval or ratification at any time or from time to time. No change may be made by our Board of Directors that increases the total number of shares of our common stock reserved for issuance under the 2014 Plan (without stockholder approval) or reduces the minimum exercise price for options or exchange of options for other incentive awards. Unless sooner terminated, the 2014 Plan terminates ten years after the date on which it was adopted.
In connection with the approval of the 2014 Plan on May 22, 2014, our Board of Directors granted 4,000,000 non-statutory stock options (the “Mogelsvang Options”) under the 2014 Plan with an exercise price of $0.20 per share to our President and Chief Executive Officer, Soren Mogelsvang, and granted 500,000 non-statutory stock options (the “Tinter Options”) under the 2014 Plan with an exercise price of $0.20 per share to our Treasurer and Chief Financial Officer, Arnold Tinter. The Mogelsvang Options vest ratably over a four year period at the rate of 250,000 options per quarter with the initial vesting date being August 22, 2014. 100,000 of the Tinter Options vest on each of November 22, 2014 and May 22, 2015 and the balance of the Tinter Options vest at the rate of 25,000 per quarter thereafter.
All descriptions of the 2014 Plan and 2013 Plan herein are qualified in their entirety by reference to the text thereof. The 2014 Plan and 2013 Plan are referenced as exhibits hereto and incorporated herein by reference.
Departure and Appointment of Directors and Officers
Our Board consists of four members, none of whom are independent. As the result of our determination not to move forward with our proposed business of providing solutions for the treatment and recycling of waste water resulting principally from oil and gas exploration and production activities, on January 23, 2014 Charles Watson and Stuart Sundlun resigned as directors.
On March 10, 2014, in furtherance of our determination to enter into the business of manufacturing and marketing pharmaceutical level products containing phytocannabinoids for the treatment of various conditions and diseases, Soren Mogelsvang, Cohava Gelber, Guy Yachin and Vered Caplan were appointed as directors of ours. Each director is to serve until the next annual meeting of stockholders or until his or her successor is duly elected and qualified. On March 11, 2014 the board accepted the resignation of Nadine C. Smith as a director and the Chairman of the Company and as the Company’s Chief Executive Officer and President and thereafter appointed Soren Mogelsvang to the vacated Chief Executive Officer position. Ms. Smith’s resignation was not a result of any disagreements between us and Ms. Smith on any matters relating to the Company’s operations, policies or practices. Effective May 1, 2014 Mr. Mogelsvang was appointed to the vacated President position. See “Management – Directors and Executive Officers” for information about our directors and executive officers.
EXECUTIVE COMPENSATION
Summary Compensation Table
The following table sets forth information concerning the total compensation paid or accrued by us during the two fiscal years ended September 30, 2013, and 2012 to (i) all individuals that served as our principal executive officer or acted in a similar capacity for us at any time during the fiscal year ended September 30, 2013; (ii) all individuals that were serving as executive officers of ours at the end of the fiscal year ended September 30, 2013 that received annual compensation during the fiscal year ended September 30, 2013 in excess of $100,000; and (iii) all individuals not serving as executive officers of ours at the end of the fiscal year ended September 30, 2013 that received annual compensation during the fiscal year ended September 30, 2013 in excess of $100,000.
35
|
Name and Principal Position
|
Year
|
Salary
($)
|
Bonus
($)
|
Stock Awards
($)
|
Option Awards
($)
|
Non-
Equity Incentive
Plan Compen
-sation ($)
|
Change in
Pension
Value
and
Non-
qualified
Deferred
Compen
-sation
Earnings
($)
|
All
Other
Compen
-sation
($)
|
Total
($)
|
|||||||||||||||||||||||||||
|
Fadi Zeidan,
|
2013
|
0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||||||||||||||||||||||||||
|
President and Chief Executive Officer(1)
|
2012
|
0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||||||||||||||||||||||||||
|
(1)
|
Fadi Zeidan was appointed as our President, Chief Executive Officer and Treasurer on February 15, 2008 and as our Secretary on August 18, 2008. He resigned all of his positions on October 10, 2013.
|
Outstanding Equity Awards at Fiscal Year-End
The table below summarizes all unexercised options, stock that has not vested, and equity incentive plan awards for each named executive officer as of the fiscal year ended September 30, 2013.
| Option Awards | Stock Awards | ||||||||||||||||||
|
Name
|
Number of Securities Underlying Unexercised
Option (#) Exercisable
|
Number of
Securities
Underlying Unexercised
Options (#) Unexercisable
|
Equity
Incentive Plan Awards: Number
of Securities Underlying Unexercised Unearned
Options (#)
|
Option Exercise Price ($)
|
Option
Expiration
Date
|
Number of Shares or Units of Stock That Have Not
Vested (#)
|
Market Value of Shares or Units of Stock That Have Not
Vested ($)
|
Equity
Incentive Plan Awards: Number of Unearned Shares, Units or Other Rights That
Have Not
Vested (#)
|
Equity
Incentive Plan Awards: Market or Payout Value
of Unearned
Shares, Units or Other Rights
That Have Not Vested (#)
|
||||||||||
|
Fadi Zeidan
President and Chief Executive Officer(1)
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
||||||||||
|
(1)
|
Fadi Zeidan was appointed as our President, Chief Executive Officer and Treasurer on February 15, 2008 and as our Secretary on August 18, 2008. He resigned all of his positions on October 10, 2013.
|
36
Director Compensation
The table below summarizes all compensation awarded to, earned by, or paid to our directors for all services rendered in all capacities to us for the fiscal year ended September 30, 2013.
|
Name
|
Fees
Earned
or Paid
in Cash
($)
|
Stock
Awards
($)
|
Option
Awards
($)
|
Non-Equity
Incentive Plan
Compensation
($)
|
Change in
Pension Value
and
Non-Qualified
Deferred
Compensation
Earnings
($)
|
All Other
Compensation
($)
|
Total
($)
|
|||||||||||||||||||||
|
Fadi Zeidan(1)
|
0 | 0 | 0 | 0 | 0 | 0 | 0 | |||||||||||||||||||||
|
Ufuk Turk(2)
|
0 | 0 | 0 | 0 | 0 | 0 | 0 | |||||||||||||||||||||
|
|
(1)
|
As of October 10, 2013, Fadi Zeidan resigned as our director.
|
|
|
(2)
|
As of October 10, 2013, Ufuk Turk resigned as our sole officer and as a director.
|
We have no plans in place and have never maintained any plans that provide for the payment of retirement benefits or benefits that will be paid primarily following retirement including, but not limited to, tax qualified deferred benefit plans, supplemental executive retirement plans, tax-qualified deferred contribution plans and nonqualified deferred contribution plans. Similarly, except as otherwise set forth below, we have no contracts, agreements, plans or arrangements, whether written or unwritten, that provide for payments to the named executive officers or any other persons following, or in connection with the resignation, retirement or other termination of a named executive officer, or a change in control of us or a change in a named executive officer’s responsibilities following a change in control.
Employment Agreements
In connection with his March 2014 engagement as our Chief Executive Officer and as a director, we issued 3,204,000 shares of our common stock to Soren Mogelsvang. Since April 1, 2014 we have been paying Mr. Mogelsvang a base annual salary of $110,000 payable on a bi-monthly (twice a month) basis. On May 22, 2014 we granted 4,000,000 ten-year non-statutory stock options to Mr. Mogelsvang under our 2014 Equity Incentive Plan with an exercise price of $0.20 per share. The options vest ratably over a four year period with the initial vesting date being August 22, 2014. We have not entered into a formal, written employment agreement with Mr. Mogelsvang but expect to do so in the near future.
On October 15, 2013, we entered into a Consulting Agreement with Arnold Tinter pursuant to which he serves as our Chief Financial Officer, Treasurer and Secretary on an independent contractor basis. Either party may terminate the Consulting Agreement upon 30 days advance notice. The Consulting Agreement has a term of 12 months which will be automatically renewed for an additional 12 month period unless terminated prior to the end of the initial term by either party. The services being provided by Mr. Tinter include, but are not limited to, the following:
37
|
Ÿ
|
Oversight and maintenance of our financial books and records, including the general ledger;
|
|
Ÿ
|
Preparation of annual cash projections (with the support of management) for review by our Board of Directors;
|
|
Ÿ
|
Preparation of monthly internal financial statements for review and distribution to our management and Board of Directors;
|
|
Ÿ
|
Preparation of quarterly financial statements and supporting documentation for review by our auditor, together with coordination of the quarterly review of our financial statements by the auditor;
|
|
Ÿ
|
Preparation of annual financial statements and supporting documentation in connection with the annual audit, together with coordination of the annual audit with our auditors;
|
|
Ÿ
|
Assistance in review and preparation of forms 10-Q and 10-K, and any other required filings with the Securities and Exchange Commission;
|
|
Ÿ
|
Assistance in review and preparation of private placement or other financing documentation, as needed; and
|
|
Ÿ
|
Accounting software selection and integration, as needed, to provide management with internal operating and financial data on a timely basis.
|
In connection with the Consulting Agreement, we were paying Mr. Tinter at the annual rate of $60,000 payable in equal monthly installments. We also granted him 262,500 non-statutory stock options exercisable, upon vesting, at a price of $0.00667 per share.
The Consulting Agreement contains non-compete and non-disclosure covenants of Mr. Tinter and a mutual indemnification provision. It also contains a separate Confidentiality Agreement which contains confidentiality covenants of Mr. Tinter.
As a result of our diminished business activity following our departure from the water treatment and recycling business, effective February 12, 2014 we amended our October 15, 2013 Consulting Agreement with Arnold Tinter. The Tinter Consulting Agreement was amended to (i) provide for Mr. Tinter to receive a payment of $10,000 for the period from February 1, 2014 through the date of filing our December 31, 2013 Quarterly Report (February 18, 2014) and to thereafter be paid at the rate of $1,500 per month; and (ii) to cancel the 262,500 options previously granted to him under the Consulting Agreement. In connection with our March 2014 entry into our present line of business and the related management changes, we further revised Mr. Tinter’s compensation arrangement to restore his $60,000 base annual salary, payable $5,000 per month, effective March 1, 2014. Mr. Tinter received $10,000 for the month of February 2014.
On May 22, 2014 we granted 500,000 ten-year non-statutory stock options to Mr. Tinter under our 2014 Equity Incentive Plan with an exercise price of $0.20 per share. 100,000 of the options vest on each of November 22, 2014 and May 22, 2015 and the balance of the options vest at the rate of 25,000 per quarter thereafter.
38
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT
Beneficial ownership is determined in accordance with the rules of the SEC and generally includes voting or investment power with respect to securities. In accordance with SEC rules, shares of our common stock which may be acquired upon exercise of stock options or warrants which are currently exercisable or which become exercisable within 60 days of August 11, 2014, the date applicable to the table below, are deemed beneficially owned by the holders of such options and warrants and are deemed outstanding for the purpose of computing the percentage of ownership of such person, but are not treated as outstanding for the purpose of computing the percentage of ownership of any other person. Subject to community property laws, where applicable, the persons or entities named in the tables below have sole voting and investment power with respect to all shares of our common stock indicated as beneficially owned by them.
The following table sets forth certain information regarding the beneficial ownership of our common stock as of August 11, 2014, by (i) each stockholder known by us to be the beneficial owner of more than 5% of our common stock (our only class of voting securities), (ii) each of our directors and executive officers, and (iii) all of our directors and executive officers as a group. Unless otherwise indicated, the persons named in the table below have sole voting and investment power with respect to the number of shares indicated as beneficially owned by them.
Unless otherwise indicated in the following table, the address for each person named in the table is c/o Cannabis Therapy Corp., 4450 Arapahoe Avenue, Suite 100, Boulder, CO 80303.
Title of Class: Common Stock
|
Name and Address of Beneficial Owner
|
Amount and Nature of Beneficial Ownership
|
Percentage
of Class(1)(2)
|
|||||||
|
Soren Mogelsvang
|
3,454,000 | (3) | 4.43 | % | |||||
|
Arnold Tinter
|
0 | (4) | N/A | ||||||
|
Cohava Gelber
|
14,940,000 | (5) | 19.2 | % | |||||
|
Guy Yachin
|
14,940,000 | (6) | 19.2 | % | |||||
|
Vered Caplan
|
2,916,000 | (7) | 3.61 | % | |||||
|
All directors and officers as a group (5 persons)
|
36,250,000 | (3)(4)(5)(6)(7) | 44.78 | % | |||||
|
Aaron Gelber
|
14,940,000 | (8) | 19.2 | % | |||||
|
Iris Yachin
|
14,940,000 | (9) | 19.2 | % | |||||
|
(1)
|
Percentages are based upon 77,788,562 shares of our common stock issued and outstanding as of August 11, 2014.
|
|
(2)
|
Beneficial ownership is determined in accordance with the rules of the SEC and generally includes voting or investment power with respect to securities. Shares of common stock underlying options, warrants or notes currently exercisable or convertible or exercisable within 60 days of August 11, 2014 are deemed outstanding for the purpose of computing the percentage of the person holding such option, warrant or note but are not deemed outstanding for computing the percentage of any other person.
|
39
|
(3)
|
Includes 250,000 shares of our common stock issuable upon exercise of stock options which are currently exercisable or exercisable within 60 days of August 11, 2014. Excludes 3,750,000 shares of our common stock issuable upon exercise of stock options not exercisable within 60 days of August 11, 2014.
|
|
(4)
|
Excludes 500,000 shares of our common stock issuable upon exercise of stock options not exercisable within 60 days of August 11, 2014.
|
|
(5)
|
Represents shares registered in the name of Dr. Gelber’s husband, Aaron Gelber, in which Dr. Gelber shares beneficial ownership.
|
|
(6)
|
Represents shares registered in the name of Mr. Yachin’s wife, Iris Yachin, in which Mr. Yachin shares beneficial ownership.
|
|
(7)
|
Includes 2,916,000 shares of our common stock issuable upon exercise of stock options which are currently exercisable.
|
|
(8)
|
Represents shares registered in the name of Mr. Gelber in which Mr. Gelber’s wife, Cohava Gelber, shares beneficial ownership.
|
|
(9)
|
Represents shares registered in the name of Mrs. Yachin in which Mrs. Yachin’s husband, Guy Yachin, shares beneficial ownership.
|
CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLSOURE
Not applicable.
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS
Related Party Transactions
SEC rules require us to disclose any transaction or currently proposed transaction in which we are a participant and in which any related person has or will have a direct or indirect material interest involving the lesser of $120,000 or one percent (1%) of the average of our total assets as of the end of last two completed fiscal years. A related person is any executive officer, director, nominee for director, or holder of 5% or more of our common stock, or an immediate family member of any of those persons.
On October 10, 2013, we entered into a three year Employment Agreement with Nadine C. Smith, pursuant to which she served as our President, Chief Executive Officer and Chairman. Her initial base annual salary thereunder was $120,000 She was entitled to receive annual bonuses in amounts up to 100% of her base annual salary based upon achievement of budget and operational milestones as approved by our Board of Directors. In connection with her appointment, Ms. Smith purchased 30,000,000 shares of our common stock at a price of $0.00667 per share or an aggregate of $200,000. Ms. Smith was also entitled to receive stock options under our 2013 Equity Incentive Plan at such times and in such amounts as determined by our Board of Directors.
40
On October 15, 2013, we entered into a Consulting Agreement with Arnold Tinter, pursuant to which he serves as our Chief Financial Officer, Treasurer and Secretary on an independent contractor basis. Either party may terminate the Consulting Agreement upon 30 days advance notice. The Consulting Agreement has a term of 12 months which will be automatically renewed for an additional 12 month period unless terminated prior to the end of the initial term by either party. In connection with the Consulting Agreement, we were paying Mr. Tinter at the annual rate of $60,000 payable in equal monthly installments. We also granted him 262,500 non-statutory stock options exercisable, upon vesting, at a price of $0.00667 per share.
In connection with the October 15, 2013 engagement of Stuart A. Sundlun as a Director, we sold 750,000 shares of our common stock to Mr. Sundlun at a price of $0.00667 per share for gross proceeds of $5,000.
In connection with the October 15, 2013 engagement of Charles Watson as a Director, we issued and sold 3,000,000 shares of our common stock to Mr. Watson at a price of $0.00667 per share for gross proceeds of $20,000.
Effective November 15, 2013 we converted $34,510 in debt owed to an assignee of a former officer and director into 1,479,000 shares of our common stock at a conversion rate of $0.02333 per share.
On December 20, 2013 we issued 375,000 non-statutory stock options under our 2013 Equity Incentive Plan to each of Charles Watson, Stuart Sundlun, and Nadine Smith with a term of ten years, an exercise price of $0.13333 per share and a one year vesting period, the exercise of which was subject to the continuance of the director relationship.
As the result of the change in our business direction, on January 23, 2014 Charles Watson and Stuart Sundlun resigned as directors. In connection with the resignation of Charles Watson we repurchased the 3,000,000 shares of our common stock sold to him in connection with his appointment as a director (the “Watson Shares”) at his aggregate cost of $20,000 and also paid him an aggregate of $7,500 representing payments of annual directors’ fees, board meeting attendance fees and board meeting travel fees. In connection with the resignation of Stuart Sundlun we repurchased the 750,000 shares of our common stock sold to him in connection with the appointment as a director (the “Sundlun Shares”) at his aggregate cost of $5,000 and also paid him an aggregate of $5,000 representing payments of annual directors’ fees and board meeting attendance fees. The Watson Shares and Sundlun Shares were cancelled and returned to the status of authorized but unissued shares. In connection with the resignations of Messrs. Watson and Sundlun, we also cancelled the 375,000 stock options which had been issued to each of them on December 20, 2013.
As a result of our diminished business activity following our departure from the water treatment and recycling business, effective February 12, 2014 we amended our October 15, 2013 Consulting Agreement with Arnold Tinter. The Tinter Consulting Agreement was amended to (i) provide for Mr. Tinter to receive a payment of $10,000 for the period from February 1, 2014 through the date of filing our December 31, 2013 Quarterly Report (February 18, 2014) and to thereafter be paid at the rate of $1,500 per month; and (ii) to cancel the 262,500 options previously granted to him under the Consulting Agreement. In connection with our March 2014 entry into our present line of business and the related management changes, we further revised Mr. Tinter’s compensation arrangement to restore his $60,000 base annual salary, payable $5,000 per month, effective March 1, 2014. Mr. Tinter received $10,000 for the month of February 2014.
As a result of our diminished business activity following our departure from the water treatment and recycling business, effective February 1, 2014 we amended our October 10, 2013 Employment Agreement with Nadine Smith to (i) suspend our obligation to pay base annual salary of Ms. Smith for the period February 1, 2014 through March 31, 2014; and (ii) to cancel the 375,000 options previously granted to her in connection with her employment by us. On March 11, 2014 we repurchased 29,500,000 shares of our common stock from Ms. Smith for $100,000 or approximately $0.003333 per share. The repurchased shares were cancelled and returned to the status of authorized but unissued.
41
In connection with our entry into our current line of business, in March 2014 we issued an aggregate of 33,084,000 shares of our common stock and 2,916,000 stock options to our four new officers and directors or their assigns.
In connection with the approval of our 2014 Equity Incentive Plan on May 22, 2014, our Board of Directors granted 4,000,000 non-statutory stock options under the 2014 Plan with an exercise price of $0.20 per share to our President and Chief Executive Officer, Soren Mogelsvang, and granted 500,000 non-statutory stock options under the 2014 Plan with an exercise price of $0.20 per share to our Treasurer and Chief Financial Officer, Arnold Tinter.
On June 26, 2014 we entered into a Services Agreement with Caerus Discovery, LLC. (See “Description of Business – Research Services Agreement”). Cohava Gelber, one of our directors, is the Chief Executive Officer, President and Principal Equity Holder of Caerus. Soren Mogelsvang, our President, Chief Executive Officer and a director previously served as Caerus’ Vice President of Research and Development.
MARKET PRICE OF AND DIVIDENDS ON COMMON EQUITY AND RELATED STOCKHOLDER MATTERS
Market Information
From May 17, 2010 until September 11, 2013, our shares of common stock were quoted on the OTC Bulletin Board and the OTCQB, under the stock symbol “SURF,” from September 12, 2013 until March 23, 2014, under the symbol “FWSI,” and since March 24, 2014 under the symbol “CTCO.” The following table shows the reported high and low closing bid prices per share for our common stock based on information provided by the OTC Markets. The over-the-counter market quotations set forth for our common stock reflect inter-dealer prices, without retail mark-up, mark-down or commission and may not necessarily represent actual transactions.
| Quarter Ended |
High Bid(1)
|
Low Bid(1)
|
||||||
| Fiscal Year Ended: September 30, 2014 | ||||||||
|
Three Months Ended September 30, 2014(2)
|
$ | 0.97 | $ | 0.7803 | ||||
|
Three Months Ended June 30, 2014
|
$ | 1.55 | $ | 0.6501 | ||||
|
Three Months Ended March 31, 2014
|
$ | 3.01 | $ | 0.55 | ||||
|
Three Months Ended December 31, 2013
|
$ | 0.68 | $ | 0.31 | ||||
|
Fiscal Year Ended: September 30, 2013
|
||||||||
|
Three Months Ended September 30, 2013
|
$ | 0.00 | $ | 0.00 | ||||
|
Three Months Ended June 30, 2013
|
$ | 0.11 | $ | 0.11 | ||||
|
Three Months Ended March 31, 2013
|
$ | 0.11 | $ | 0.11 | ||||
|
Three Months Ended December 31, 2012
|
$ | 0.11 | $ | 0.10 | ||||
|
Fiscal Year Ended: September 30, 2012
|
||||||||
|
Three Months Ended September 30, 2012
|
$ | 0.00 | $ | 0.00 | ||||
|
Three Months Ended June 30, 2012
|
$ | 0.00 | $ | 0.00 | ||||
|
Three Months Ended March 31, 2012
|
$ | 0.44 | $ | 0.36 | ||||
|
Three Months Ended December 31, 2011
|
$ | 0.21 | $ | 0.15 |
|
(1)
|
Such prices give retroactive effect to the 50:1 forward stock split which was effected on September 27, 2012 and the 1.5:1 forward stock split which was effected on April 1, 2014.
|
|
(2)
|
Through August 8, 2014.
|
42
Holders
As of the date of this Report, we have 77,788,562 shares of common stock issued and outstanding held by approximately 19 stockholders of record.
Dividend Policy
We have never paid any cash dividends on our capital stock and do not anticipate paying any cash dividends on our common stock in the foreseeable future. We intend to retain future earnings to fund ongoing operations and future capital requirements. Any future determination to pay cash dividends will be at the discretion of our Board and will be dependent upon financial condition, results of operations, capital requirements and such other factors as the Board deems relevant.
Securities Authorized for Issuance under Equity Compensation Plans
The following table provides information as of the date of this Report, with respect to the shares of common stock that may be issued under our existing equity compensation plans:
|
Number of securities to be issued upon exercise of outstanding options (a)
|
Weighted-average exercise price of outstanding options
(b)
|
Number of securities remaining available for future issuance under equity compensation plans (excluding securities reflected in column (a)
|
||||||||||
|
Equity compensation plans approved by security holders
|
N/A
|
$
|
N/A
|
N/A
|
||||||||
|
Equity compensation plans not approved by security holders
|
7,791,000(1)
|
$
|
0.1183
|
6,500,000(2)
|
||||||||
|
Total
|
7,791,000(1)
|
6,500,000(2)
|
||||||||||
|
(1)
|
Represents 3,219,000 options issued under our 2013 Plan and 4,500,000 options issued under our 2014 Plan.
|
|
(2)
|
Represents securities remaining eligible for issuance under our 2014 Plan.
|
43
RECENT SALES OF UNREGISTERED SECURITIES
We sold no unregistered securities during the years ended September 30, 2011, September 30, 2012 and September 30, 2013. Subsequent to September 30, 2013 we issued the following unregistered securities:
In connection with Nadine C. Smith’s October 10, 2013 engagement as our President, Chief Executive Officer and Chairman we issued and sold 30,000,000 shares of our common stock to Ms. Smith at a price of $0.00667 per share for gross proceeds of $200,000. Following Ms. Smith’s resignation as an officer and director, we repurchased 29,500,000 of the shares for $100,000 or approximately $0.005 per share. The repurchase shares were subsequently cancelled.
In connection with the October 15, 2013 engagement of Stuart A. Sundlun as a Director, we issued and sold 750,000 shares of our common stock to Mr. Sundlun at a price of $0.00667 per share for gross proceeds of $5,000. In connection with Mr. Sundlun’s January 23, 2014 resignation as a director, we repurchased these shares at Mr. Sundlun’s cost of $5,000. The repurchased shares were subsequently cancelled.
In connection with the October 15, 2013 engagement of Charles Watson as a Director, we issued and sold 3,000,000 shares of our common stock to Mr. Watson at a price of $0.00667 per share for gross proceeds of $20,000. In connection with Mr. Watson’s January 23, 2014 resignation as a director, we repurchased these shares at Mr. Watson’s cost of $20,000. The repurchased shares were subsequently cancelled.
In connection with Arnold Tinter’s October 15, 2013 engagement as our Chief Financial Officer, Secretary and Treasurer we granted and issued 262,500 non-statutory stock options to Mr. Tinter under our 2013 Equity Incentive Plan. These options were cancelled in February 2014.
In connection with our October 10, 2013 entry into a Joint Venture Agreement with Produced Water Solutions, Inc. (“PWS”), effective October 10, 2013, we granted and issued 375,000 non-statutory stock options to PWS under our 2013 Plan. The options have a term of ten years and are exercisable, upon vesting, at an exercise price of $0.00667 per share. The options will vest twelve months after the date of issuance.
On October 15, 2013, we closed on the sale of an aggregate of $500,000 in principal amount of promissory notes of the Company to two investors. In addition, as of October 15, 2013, $100,000 in principal and $2,922 in accrued interest due on PWS Notes was converted into promissory notes of the Company. All of the notes had a stated maturity date of October 15, 2014.
As of November 15, 2013, we converted $34,510 in debt into 1,479,000 shares of our common stock at a conversion rate of $0.02333 per share.
On December 20, 2013 we issued 375,000 non-statutory stock options under our 2013 Equity Incentive Plan to each of Charles Watson, Stuart Sundlun, and Nadine Smith. These options were cancelled in connection with their respective resignations.
Effective March 11, 2014 we issued an aggregate of 37,584,000 shares of our common stock to five persons and issued 2,916,000 ten year options with an exercise price of $0.00667 per share to one person.
On April 29, 2014 we sold 3,175,000 shares of our restricted common stock to three persons at a price of $0.20 per share.
44
On May 22, 2014, our Board of Directors granted and issued 4,000,000 non-statutory stock options under our 2014 Equity Incentive Plan with an exercise price of $0.20 per share to our President and Chief Executive Officer, Soren Mogelsvang, and granted 500,000 non-statutory stock options under the 2014 Equity Incentive Plan with an exercise price of $0.20 per share to our Treasurer and Chief Financial Officer, Arnold Tinter.
Effective June 30, 2014 we issued an aggregate of 3,248,898 shares of our restricted common stock to three persons that were holding 10% convertible promissory notes of ours in the aggregate principal amount of $602,922 at a conversion price of $0.20 per share. The conversion included interest due on the notes in the aggregate amount of $46,847.76.
On July 15, 2014 we issued 466,666 shares of our restricted common stock to a Consultant under a May 15, 2014 Service Agreement.
During the period June 1, 2014 through August 11, 2014 we sold an aggregate of 900,000 shares of our restricted common stock to six persons at a subscription price of $0.20 per share. Certificates for 375,000 of these shares have yet to be issued, and have not been treated as issued and outstanding for purposes of this Report.
All of the foregoing issuances of securities were made in reliance on Section 4(a)(2) of the Securities Act of 1933, as amended.
DESCRIPTION OF SECURITIES
Forward Stock Splits
On August 15, 2012 our board of directors and stockholders owning a majority of our outstanding common shares authorized a 50:1 forward stock split of our common stock. The forward split became effective on September 27, 2012. As a result of the forward split each outstanding share was split into 50 shares.
On March 11, 2014 our board of directors authorized a 1.5 for 1 forward split of our common stock in the form of a stock dividend (the “Stock Split”). In connection therewith, our shareholders of record as of the close of business on March 28, 2014, the record date, received an additional .5 shares of our common stock for each share of our common stock held by them on the record date. The payment date for the Stock Split was March 31, 2014 and the ex-dividend date for the Stock Split was April 1, 2014. All common stock amount references in this Quarterly Report give retroactive effect to the Stock Splits.
Authorized Capital Stock
We currently have authorized 350,000,000 shares of capital stock, consisting of (i) 325,000,000 shares of common stock, and (ii) 25,000,000 shares of “blank check” Preferred Stock. As of the date of this Report, we have the following outstanding securities:
|
Ÿ
|
77,788,562 shares of common stock; and
|
|
Ÿ
|
7,791,000 non-statutory stock options
|
45
Common Stock
The holders of shares of common stock are entitled to receive dividends out of assets or funds legally available for the payment of dividends of such times and in such amounts as the Board from time to time may determine. Holders of common stock are entitled to one vote for each share held on all matters submitted to a vote of stockholders. There is no cumulative voting of the election of directors then standing for election. The common stock is not entitled to pre-emptive rights and is not subject to conversion or redemption. Upon liquidation, dissolution or winding up of the Company, the assets legally available for distribution to stockholders are distributable ratably among the holders of the common stock after payment of liquidation preferences, if any, on any outstanding preferred stock and payment of other claims of creditors.
Preferred Stock
Shares of Preferred Stock may be issued from time to time in one or more series, each of which will have such distinctive designation or title as shall be determined by our Board prior to the issuance of any shares thereof. Preferred Stock will have such voting powers, full or limited, or no voting powers, and such preferences and relative, participating, optional or other special rights and such qualifications, limitations or restrictions thereof, as shall be stated in such resolution or resolutions providing for the issue of such class or series of Preferred Stock as may be adopted from time to time by the Board prior to the issuance of any shares thereof. The number of authorized shares of Preferred Stock may be increased or decreased (but not below the number of shares thereof then outstanding) by the affirmative vote of the holders of a majority of the voting power of all the then outstanding shares of our capital stock entitled to vote generally in the election of the directors, voting together as a single class, without a separate vote of the holders of the Preferred Stock, or any series thereof, unless a vote of any such holders is required pursuant to any preferred stock designation.
While we do not currently have any plans for the issuance of Preferred Stock, the issuance of such preferred stock could adversely affect the rights of the holders of common stock and, therefore, reduce the value of the common stock. It is not possible to state the actual effect of the issuance of any shares of Preferred Stock on the rights of holders of the common stock until the Board determines the specific rights of the holders of the preferred stock; however, these effects may include:
|
Ÿ
|
Restricting dividends on the common stock;
|
|
Ÿ
|
Diluting the voting power of the common stock;
|
|
Ÿ
|
Impairing the liquidation rights of the common stock; or
|
|
Ÿ
|
Delaying or preventing a change in control of the Company without further action by the stockholders.
|
Other than in connection with shares of preferred stock (as explained above), which preferred stock is not currently designated nor contemplated by us, we do not believe that any provision of our charter or Bylaws would delay, defer or prevent a change in control.
Options
We presently have 7,791,000 non-statutory stock options outstanding 3,291,000 of such options are exercisable at $0.00667 per share and 4,500,000 of such options are exercisable at $0.20 per share. See “Equity Incentive Plans” and “Securities Authorized for Issuance Under Equity Compensation Plans.”
Transfer Agent
The transfer agent for our common stock is Securities Transfer Corporation, 2591 Dallas Parkway, Suite 102, Frisco, TX 75034, and its telephone number is 469.633.0101.
46
LEGAL PROCEEDINGS
From time to time, we may become involved in various lawsuits and legal proceedings which arise in the ordinary course of business. However, litigation is subject to inherent uncertainties, and an adverse result in these or other matters may arise from time to time that may harm business.
We are currently not aware of any pending legal proceedings to which we are a party or of which any of our property is the subject, nor are we aware of any such proceedings that are contemplated by any governmental authority.
INDEMNIFICATION OF DIRECTORS AND OFFICERS
The Revised Statutes of the State of Nevada allows us to indemnify our officers and directors from certain liabilities and our By-Laws state that we shall indemnify every (i) present or former director or officer of ours; and (ii) any person who while serving in any of the capacities referred to in clause (i) served at our request as a director, officer, partner, venturer, proprietor, trustee, employee, agent or similar functionary of another foreign or domestic corporation, partnership, joint venture, trust, employee benefit plan or other enterprise to the fullest extent permitted under the Revised Statutes of the State of Nevada.
Our By-Laws further provide that our officers and directors shall not be personally liable to us or our stockholders for monetary damages for breach of fiduciary duty as a director or officer, except for liability (i) for acts or omissions which involve intentional misconduct, fraud or a knowing violation of law; or (ii) for the payment of distributions in violation of the Revised Statutes of the State of Nevada.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers and controlling persons of the Company pursuant to the foregoing provisions, or otherwise, we have been advised that, in the opinion of the SEC, such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable.
ITEM 9.01. FINANCIAL STATEMENTS AND EXHIBITS.
(d) Exhibits
In reviewing the agreements included or incorporated by reference as exhibits to this Report, please remember that they are included to provide you with information regarding their terms and are not intended to provide any other factual or disclosure information about the Company or the other parties to the agreements. The agreements may contain representations and warranties by each of the parties to the applicable agreement. These representations and warranties have been made solely for the benefit of the parties to the applicable agreement and:
|
Ÿ
|
should not in all instances be treated as categorical statements of fact, but rather as a way of allocating the risk to one of the parties if those statements prove to be inaccurate;
|
|
Ÿ
|
have been qualified by disclosures that were made to the other party in connection with the negotiation of the applicable agreement, which disclosures are not necessarily reflected in the agreement;
|
|
Ÿ
|
may apply standards of materiality in a way that is different from what may be viewed as material to you or other investors; and
|
|
Ÿ
|
were made only as of the date of the applicable agreement or such other date or dates as may be specified in the agreement and are subject to more recent developments.
|
Accordingly, these representations and warranties may not describe the actual state of affairs as of the date they were made or at any other time. Additional information about the Company may be found elsewhere in this Report and the Company’s other public filings, which are available without charge through the SEC’s website at http://www.sec.gov.
47
|
Exhibit
Number
|
Description
|
|
|
2.1
|
Articles of Merger as filed with the Nevada Secretary of State on March 17, 2014 (incorporated by reference from Exhibit 2.1 to the Registrant’s Current Report on Form 8-K dated March 14, 2014 filed with the Securities and Exchange Commission on March 20, 2014)
|
|
|
2.2
|
Agreement and Plan of Merger, dated March 14, 2014, by and between Frac Water Systems, Inc. and Cannabis Therapy Corp. (incorporated by reference from Exhibit 2.2 to the Registrant’s Current Report on Form 8-K dated March 14, 2014 filed with the Securities and Exchange Commission on March 20, 2014)
|
|
|
3.1
|
Articles of Incorporation of the Registrant (incorporated by reference from Exhibit 3.1 to the Registrant’s Form S-1, File Number 333-156480, filed with the SEC on December 29, 2008)
|
|
|
3.2
|
Amendment to Articles of Incorporation of the Registrant (incorporated by reference from Exhibit 3.1.2 to the Registrant’s Annual Report on Form 10-K filed with the SEC on December 26, 2012)
|
|
|
3.3
|
By-Laws of the Registrant (incorporated by reference from Exhibit 3.2 to the Registrant’s Form S-1, File Number 333-156480, filed with the SEC on December 29, 2008)
|
|
|
4.1
|
Form of Registrant’s 10% Senior Convertible Promissory Note (incorporated by reference from Exhibit 4.1 to the Registrant’s Annual Report on Form 10-K filed with the SEC on October 17, 2013)
|
|
|
10.1
|
Joint Venture Agreement dated October 10, 2013 between Registrant and Produced Water Solutions, Inc. (incorporated by reference from Exhibit 10.1 to the Registrant’s Annual Report on Form 10-K filed with the SEC on October 17, 2013)
|
|
|
10.2
|
Settlement Agreement and Mutual Release dated October 10, 2013 among Registrant, Produced Water Solutions, Inc. and Montrose Capital Ltd. (incorporated by reference from Exhibit 10.2 to the Registrant’s Annual Report on Form 10-K filed with the SEC on October 17, 2013)
|
|
|
10.3 †
|
Employment Agreement dated October 10, 2013 between Registrant and Nadine C. Smith (incorporated by reference from Exhibit 10.3 to the Registrant’s Annual Report on Form 10-K filed with the SEC on October 17, 2013)
|
|
|
10.4 †
|
Consulting Agreement dated as of October 15, 2013 between Registrant and Arnold Tinter (incorporated by reference from Exhibit 10.4 to the Registrant’s Annual Report on Form 10-K filed with the SEC on October 17, 2013)
|
|
|
10.5 †
|
Form of Engagement Agreement between Registrant and proposed members of Registrant’s Board of Directors (incorporated by reference from Exhibit 10.5 to the Registrant’s Annual Report on Form 10-K filed with the SEC on October 17, 2013)
|
|
|
10.6 †
|
Registrant’s 2013 Equity Incentive Plan(incorporated by reference from Exhibit 10.6 to the Registrant’s Annual Report on Form 10-K filed with the SEC on October 17, 2013)
|
|
|
10.7
|
Share Cancellation Agreement dated October 10, 2013 between Registrant and Ufuk Turk(incorporated by reference from Exhibit 10.7 to the Registrant’s Annual Report on Form 10-K filed with the SEC on October 17, 2013)
|
|
|
10.8
|
Share Cancellation Agreement dated October 10, 2013 between Registrant and Fadi Zeidan (incorporated by reference from Exhibit 10.8 to the Registrant’s Annual Report on Form 10-K filed with the SEC on October 17, 2013)
|
|
|
10.9
|
Services Agreement dated as of May 15, 2014 between Registrant and Axiom Group (incorporated by reference from Exhibit 10.1 to the Registrant’s Current Report on Form 8-K dated May 15, 2014 filed with the Securities and Exchange Commission on May 22, 2014)
|
|
|
10.10 †
|
Registrant’s 2014 Equity Incentive Plan (incorporated by reference from Exhibit 10.1 to the Registrant’s Current Report on Form 8-K dated May 22, 2014 filed with the Securities and Exchange Commission on May 28, 2014)
|
|
|
10.11
|
License Agreement dated as of July 29, 2014 between Peak BioPharma Corp. and Canna-Pet, LLC (incorporated by reference from Exhibit 10.1 to the Registrant’s Current Report on Form 8-K dated July 29, 2014 filed with the Securities and Exchange Commission on August 4, 2014)
|
|
|
10.12 *
|
Farm Lease and Service Agreement effective as of May 1, 2014 by and between Rocky Mountain Hemp, Inc., Ryan Loflin and Peak BioPharma Corp.
|
|
|
10.13 *
|
Services Agreement made as of June 26, 2014 by and between Caerus Discovery, LLC and Registrant
|
|
|
10.14 *
|
Industrial Hemp Registration License from the Colorado Department of Agriculture issued on May 6, 2014
|
|
|
21.1 *
|
Subsidiaries of the Registrant
|
* Filed herewith
† Management contract or compensatory plan or arrangement
48
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| CANNABIS THERAPY CORP. | |||
| Dated: August 13, 2014 | By: | /s/ Soren Mogelsvang | |
| Name: | Soren Mogelsvang | ||
| Title: |
Chief Executive Officer
|
||
49
