Attached files
| file | filename |
|---|---|
| 8-K - 8-K - ARDELYX, INC. | d771942d8k.htm |
| Exhibit 99.1
|
Exhibit 99.1
Investor Presentation
Mike Raab CEO
AUGUST 2014
|
|
Forward Looking Statements and Further Information
Special Note Regarding Forward-Looking Statements
To the extent that statements contained in this presentation are not descriptions of historical facts regarding Ardelyx, they are forward-looking statements reflecting the current beliefs and expectations of management made pursuant to the safe harbor of the Private Securities Reform Act of 1995, including statements regarding the availability and timing of data from ongoing tenapanor clinical trials, potential milestone payments from our collaboration partners, and the potential sufficiency of capital resources available to further develop our pipeline and our drug discovery and design platform. Such forward-looking statements involve substantial risks and uncertainties that could cause the development of tenapanor, or Ardelyx’s future results, performance or achievements to differ significantly from those expressed or implied by the forward-looking statements. Such risks and uncertainties include, among others, the uncertainties inherent in the clinical development process, Ardelyx’s reliance upon AstraZeneca for the development of tenapanor, Ardelyx’s reliance upon Sanofi for the discovery and development under the licensed NaP2b inhibitor program, and the uncertainties inherent in the research and discovery process. Ardelyx undertakes no obligation to update or revise any forward-looking statements. For a further description of the risks and uncertainties that could cause actual results to differ from those expressed in these forward-looking statements, as well as risks relating to Ardelyx’s business in general, please refer to Ardelyx’s prospectus filed with the Securities and Exchange Commission on June 19, 2014, and its future periodic reports to be filed with the Securities and Exchange Commission.
| 2 |
|
|
|
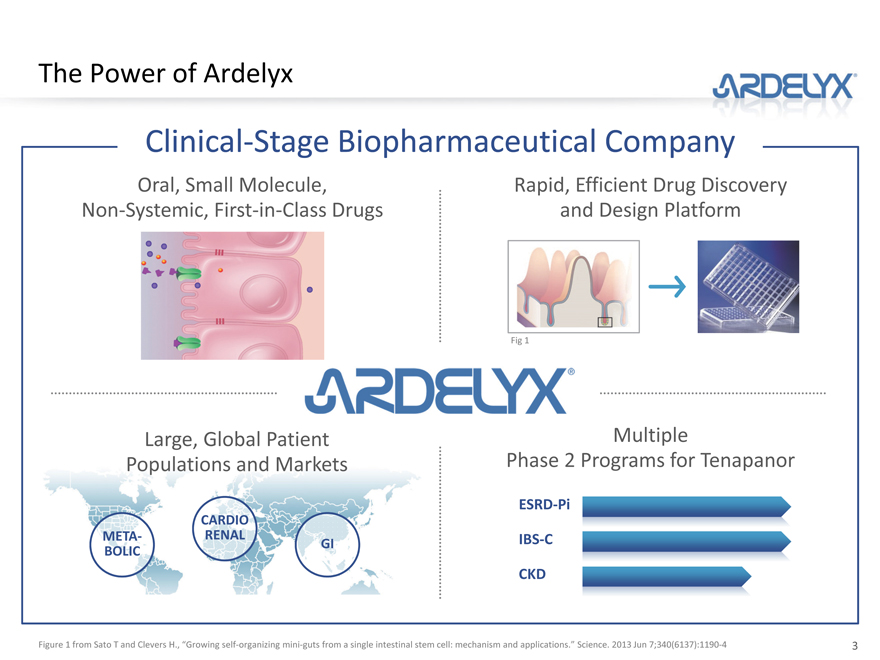
The Power of Ardelyx
Clinical-Stage Biopharmaceutical Company
Oral, Small Molecule, Non-Systemic, First-in-Class Drugs
Rapid, Efficient Drug Discovery and Design Platform
Large, Global Patient
Populations and Markets
CARDIO
META- RENAL
BOLIC GI
Multiple
Phase 2 Programs for Tenapanor
ESRD-Pi
IBS-C
CKD
Figure 1 from Sato T and Clevers H., “Growing self-organizing mini-guts from a single intestinal stem cell: mechanism and applications.” Science. 2013 Jun 7;340(6137):1190-4
| 3 |
|
|
|
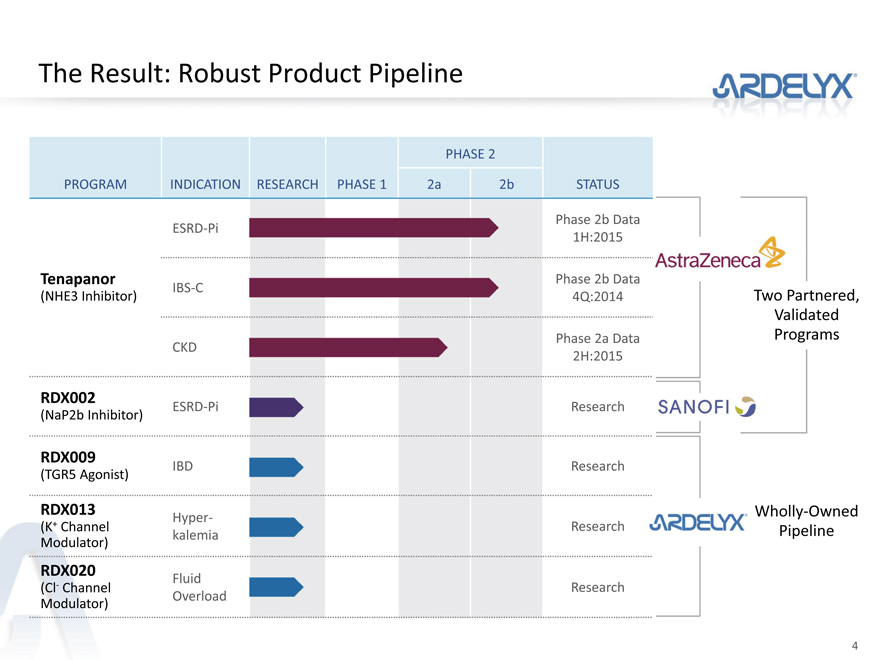
The Result: Robust Product Pipeline
PHASE 2
PROGRAM INDICATION RESEARCH PHASE 1 2a 2b STATUS
Phase 2b Data
ESRD-Pi
1H:2015
Tenapanor Phase 2b Data
IBS-C
(NHE3 Inhibitor) 4Q:2014 Two Partnered,
Validated
Phase 2a Data Programs
CKD
2H:2015
RDX002 ESRD-Pi Research
(NaP2b Inhibitor)
RDX009 IBD Research
(TGR5 Agonist)
RDX013 Wholly-Owned
Hyper-
(K+ Channel Research Pipeline
Modulator) kalemia
RDX020 Fluid
(Cl- Channel Research
Modulator) Overload
| 4 |
|
|
|
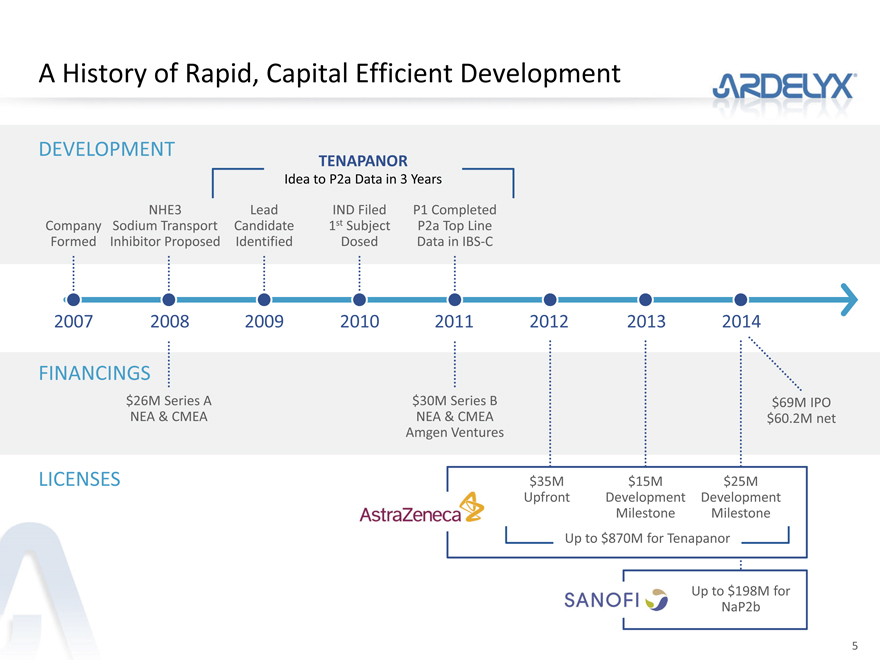
A History of Rapid, Capital Efficient Development
DEVELOPMENT
TENAPANOR
Idea to P2a Data in 3 Years
NHE3 Lead IND Filed P1 Completed
Company Sodium Transport Candidate 1st Subject P2a Top Line
Formed Inhibitor Proposed Identified Dosed Data in IBS-C
2007 2008 2009 2010 2011 2012 2013 2014
FINANCINGS
$26M Series A $30M Series B $69M IPO
NEA & CMEA NEA & CMEA $60.2M net
Amgen Ventures
LICENSES $35M $15M $25M
Upfront Development Development
Milestone Milestone
Up to $870M for Tenapanor
Up to $198M for
NaP2b
| 5 |
|
|
|
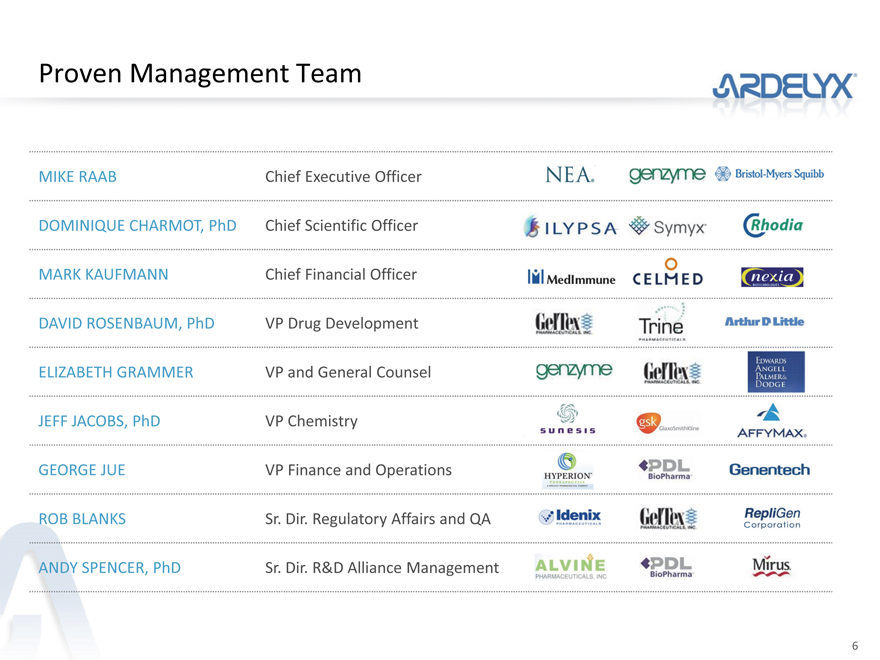
Proven Management Team
MIKE RAAB Chief Executive Officer
DOMINIQUE CHARMOT, PhD Chief Scientific Officer
MARK KAUFMANN Chief Financial Officer
DAVID ROSENBAUM, PhD VP Drug Development
ELIZABETH GRAMMER VP and General Counsel
JEFF JACOBS, PhD VP Chemistry
GEORGE JUE VP Finance and Operations
ROB BLANKS Sr. Dir. Regulatory Affairs and QA
ANDY SPENCER, PhD Sr. Dir. R&D Alliance Management
| 6 |
|
|
|
Investment Highlights
PROPRIETARY PLATFORM
2 VALIDATED PROGRAMS
WHOLLY-OWNED PIPELINE
CAPITAL EFFICIENCY
PROVEN MANAGEMENT TEAM
Discovery and Design of Non-Systemic,
Small Molecule Therapeutics
Tenapanor: AstraZeneca Collaboration;
Phase 2 in Three Indications
NaP2b Inhibition: Sanofi Collaboration
Multiple Near-Term Catalysts
Pipeline Provides Additional Opportunity
Strong History
Deep Domain Expertise
7
|
|
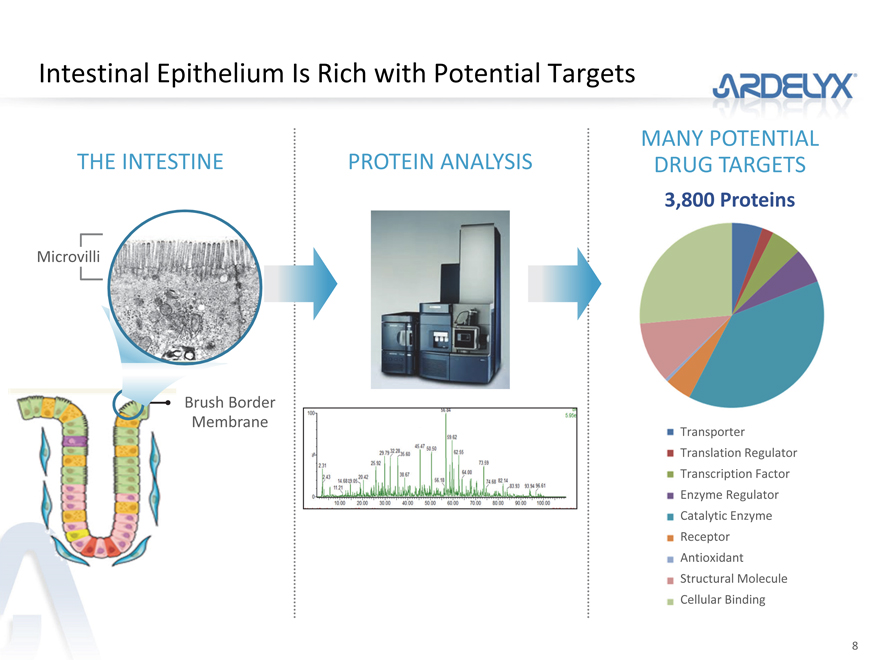
Intestinal Epithelium Is Rich with Potential Targets
THE INTESTINE
Microvilli
Brush Border
Membrane
PROTEIN ANALYSIS
MANY POTENTIAL DRUG TARGETS
3,800 Proteins
Transporter
Translation Regulator Transcription Factor Enzyme Regulator Catalytic Enzyme Receptor Antioxidant Structural Molecule Cellular Binding
| 8 |
|
|
|
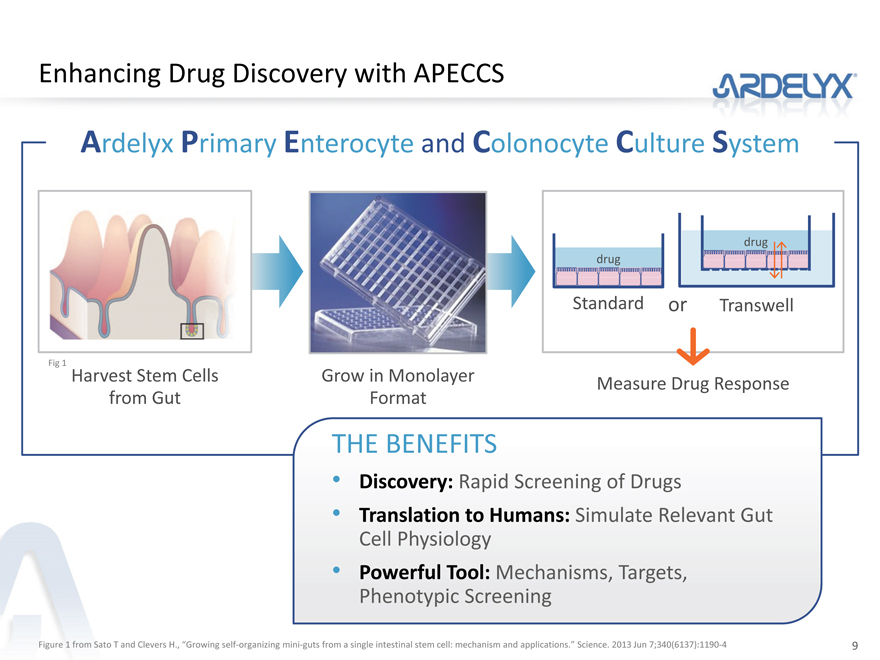
Enhancing Drug Discovery with APECCS
Ardelyx Primary Enterocyte and Colonocyte Culture System
drug
drug
Standard or Transwell
Fig 1
Harvest Stem Cells Grow in Monolayer Measure Drug Response
from Gut Format
THE BENEFITS
Discovery: Rapid Screening of Drugs
Translation to Humans: Simulate Relevant Gut
Cell Physiology
Powerful Tool: Mechanisms, Targets,
Phenotypic Screening
Figure 1 from Sato T and Clevers H., “Growing self-organizing mini-guts from a single intestinal stem cell: mechanism and applications.” Science. 2013 Jun 7;340(6137):1190-4
9
|
|
Tenapanor and Our Collaboration with AstraZeneca
PROPRIETARY PLATFORM Discovery and Design of Non-Systemic,
Small Molecule Therapeutics
Tenapanor: AstraZeneca Collaboration;
| 2 |
|
VALIDATED PROGRAMS Phase 2 in Three Indications |
NaP2b Inhibition: Sanofi Collaboration
Multiple Near-Term Catalysts
WHOLLY-OWNED PIPELINE Pipeline Provides Additional Opportunity
CAPITAL EFFICIENCY Strong History
PROVEN MANAGEMENT TEAM Deep Domain Expertise
10
|
|
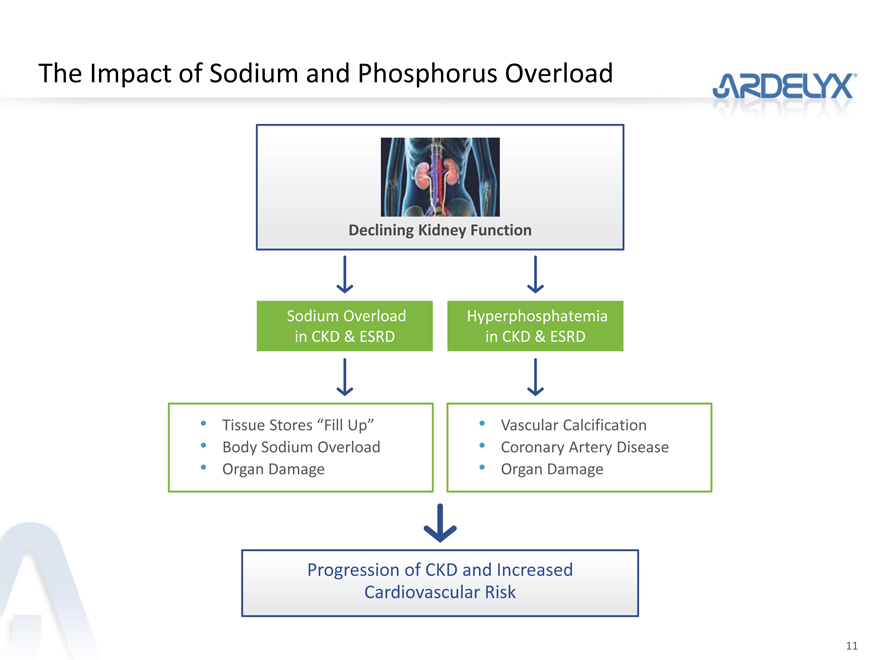
The Impact of Sodium and Phosphorus Overload
Declining Kidney Function
Sodium Overload Hyperphosphatemia
in CKD & ESRD in CKD & ESRD
Tissue Stores “Fill Up” Vascular Calcification
Body Sodium Overload Coronary Artery Disease
Organ Damage Organ Damage
Progression of CKD and Increased
Cardiovascular Risk
11
|
|
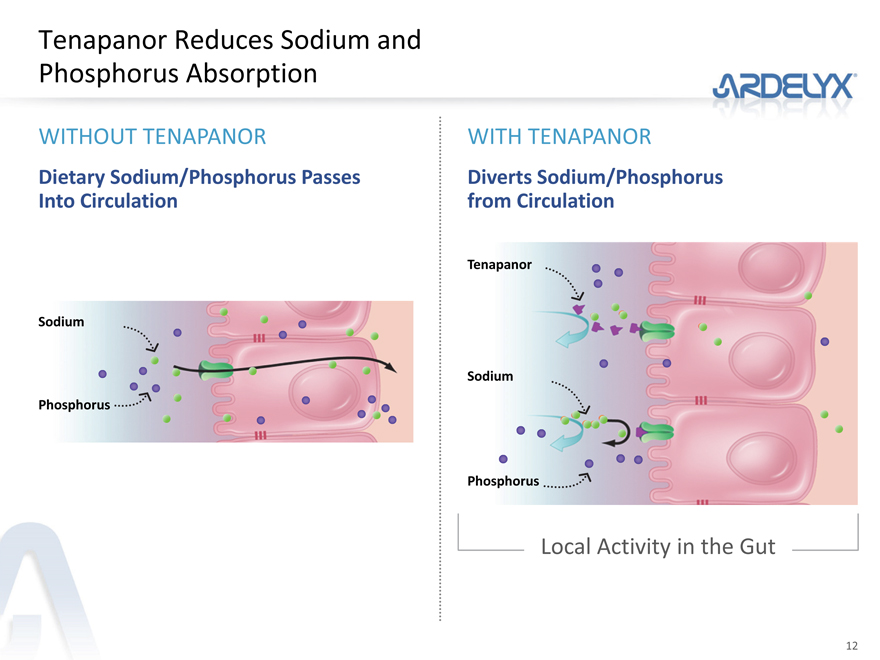
Tenapanor Reduces Sodium and Phosphorus Absorption
WITHOUT TENAPANOR WITH TENAPANOR
Dietary Sodium/Phosphorus Passes Diverts Sodium/Phosphorus
Into Circulation from Circulation
Tenapanor
Sodium
Sodium
Phosphorus
Phosphorus
Local Activity in the Gut
12
|
|
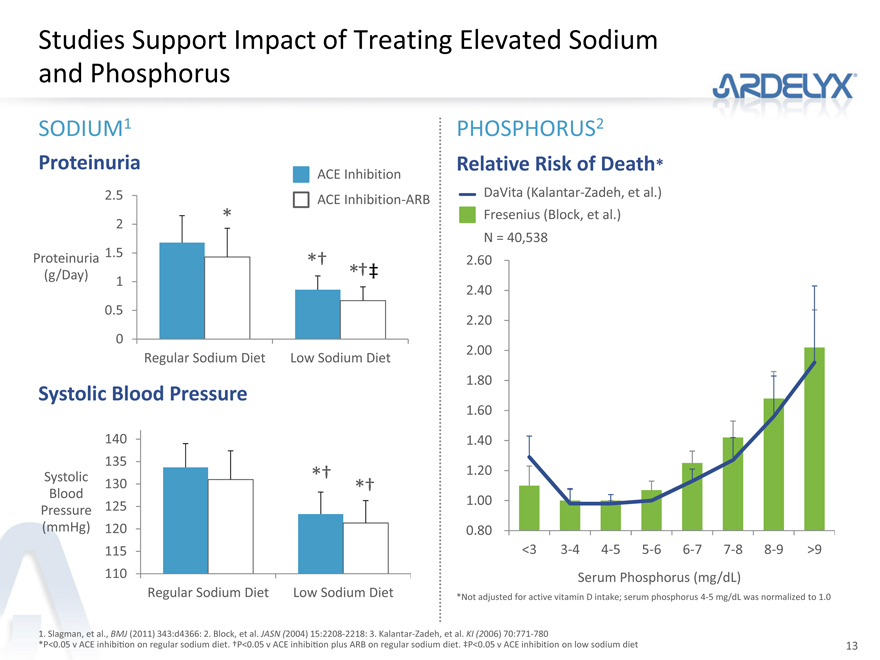
Studies Support Impact of Treating Elevated Sodium and Phosphorus
SODIUM1 PHOSPHORUS2
Proteinuria Relative Risk of Death*
ACE Inhibition
2.5 ACE Inhibition-ARB DaVita (Kalantar-Zadeh, et al.)
2 * Fresenius (Block, et al.)
N = 40,538
Proteinuria 1.5 *† 2.60
(g/Day) *†‡
1
2.40
0.5
2.20
0
Regular Sodium Diet Low Sodium Diet 2.00
1.80
Systolic Blood Pressure
1.60
140 1.40
135
Systolic *† 1.20
Blood 130 *†
1.00
Pressure 125
(mmHg) 120 0.80
115 <3 3-4 4-5 5-6 6-7 7-8 8-9 >9
110 Serum Phosphorus (mg/dL)
Regular Sodium Diet Low Sodium Diet *Not adjusted for active vitamin D intake; serum phosphorus 4-5 mg/dL was normalized to 1.0
1. Slagman, et al., BMJ (2011) 343:d4366: 2. Block, et al. JASN (2004) 15:2208-2218: 3. Kalantar-Zadeh, et al. KI (2006) 70:771-780
*P<0.05 v ACE inhibition on regular sodium diet. †P<0.05 v ACE inhibition plus ARB on regular sodium diet. ‡P<0.05 v ACE inhibition on low sodium diet
13
|
|
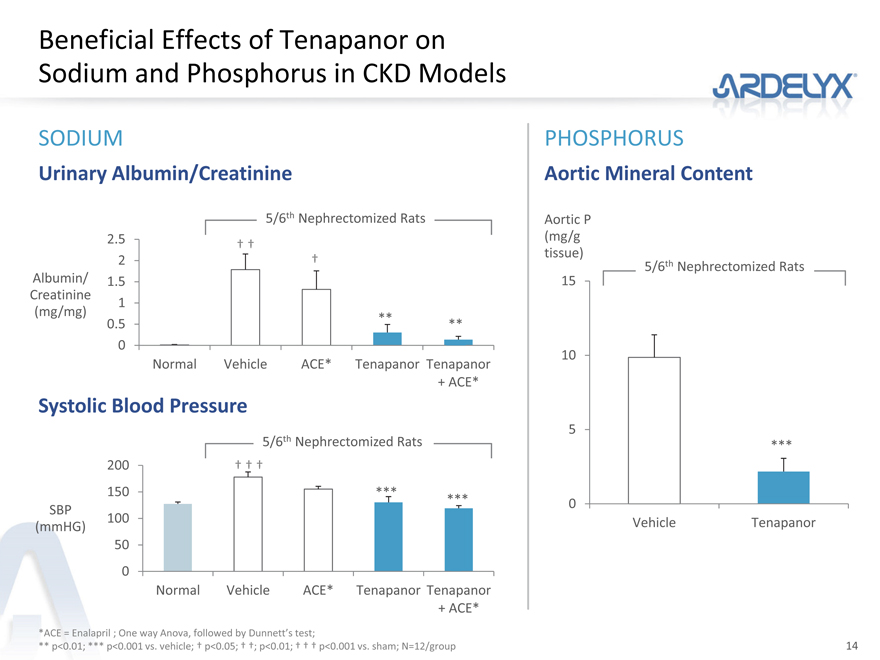
Beneficial Effects of Tenapanor on Sodium and Phosphorus in CKD Models
SODIUM PHOSPHORUS
Urinary Albumin/Creatinine Aortic Mineral Content
5/6th Nephrectomized Rats Aortic P
2.5 (mg/g
† †
| 2 |
|
† tissue) 5/6th Nephrectomized Rats |
Albumin/ 1.5 15
Creatinine 1
(mg/mg) **
0.5 **
0
10
Normal Vehicle ACE* Tenapanor Tenapanor
+ ACE*
Systolic Blood Pressure
| 5 |
|
5/6th Nephrectomized Rats ***
200 † † †
150 ***
SBP *** 0
100
(mmHG) Vehicle Tenapanor
50
0
Normal Vehicle ACE* Tenapanor Tenapanor
+ ACE*
*ACE = Enalapril ; One way Anova, followed by Dunnett’s test;
** p<0.01; *** p<0.001 vs. vehicle; † p<0.05; † †; p<0.01; † † † p<0.001 vs. sham; N=12/group
14
|
|
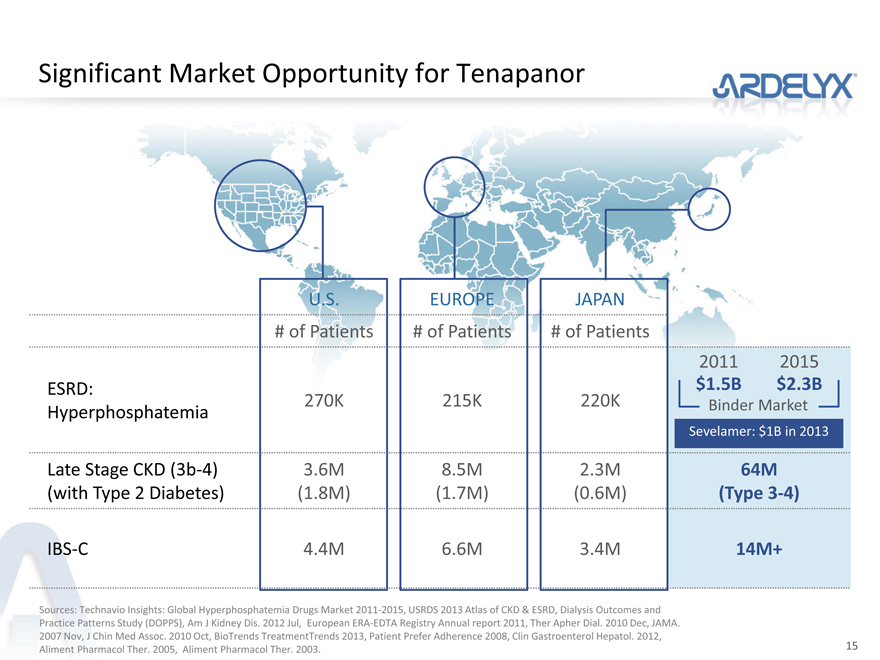
Significant Market Opportunity for Tenapanor
U.S. EUROPE JAPAN
# of Patients # of Patients # of Patients
2011 2015
ESRD: $1.5B $2.3B
Hyperphosphatemia 270K 215K 220K Binder Market
Sevelamer: $1B in 2013
Late Stage CKD (3b-4) 3.6M 8.5M 2.3M 64M
(with Type 2 Diabetes) (1.8M) (1.7M) (0.6M) (Type 3-4)
IBS-C 4.4M 6.6M 3.4M 14M+
Sources: Technavio Insights: Global Hyperphosphatemia Drugs Market 2011-2015, USRDS 2013 Atlas of CKD & ESRD, Dialysis Outcomes and Practice Patterns Study (DOPPS), Am J Kidney Dis. 2012 Jul, European ERA-EDTA Registry Annual report 2011, Ther Apher Dial. 2010 Dec, JAMA. 2007 Nov, J Chin Med Assoc. 2010 Oct, BioTrends TreatmentTrends 2013, Patient Prefer Adherence 2008, Clin Gastroenterol Hepatol. 2012, Aliment Pharmacol Ther. 2005, Aliment Pharmacol Ther. 2003.
15
|
|
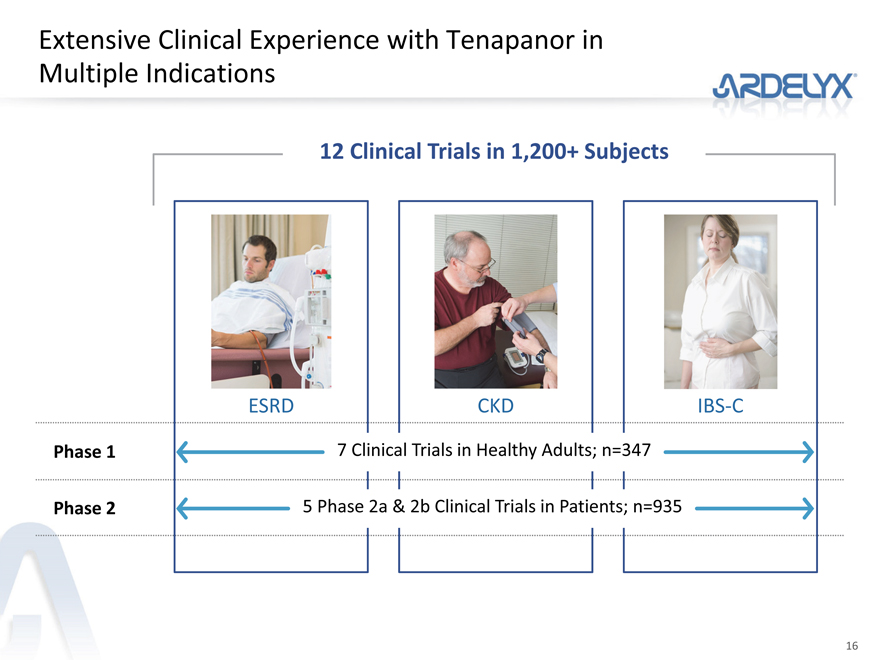
Extensive Clinical Experience with Tenapanor in Multiple Indications
12 Clinical Trials in 1,200+ Subjects
ESRD CKD IBS-C
Phase 1 7 Clinical Trials in Healthy Adults; n=347
Phase 2 5 Phase 2a & 2b Clinical Trials in Patients; n=935
16
|
|
Tenapanor Generally Well-Tolerated
830+ Subjects Exposed to Drug
291 Healthy Volunteers
~415 IBS-C Subjects
~125 CKD and ESRD-HD Subjects
Single Dose Up to 900 mg
3 Months Up to 100 mg/Day
Non-Systemic: >99.3% of All Tested Serum Samples Had No Detectable Levels of Tenapanor
Most AEs Due to Exaggerated Pharmacology of Drug (e.g. Loose Stools/Diarrhea)
No Drug-Related Serious Adverse Events (SAEs)
17
|
|
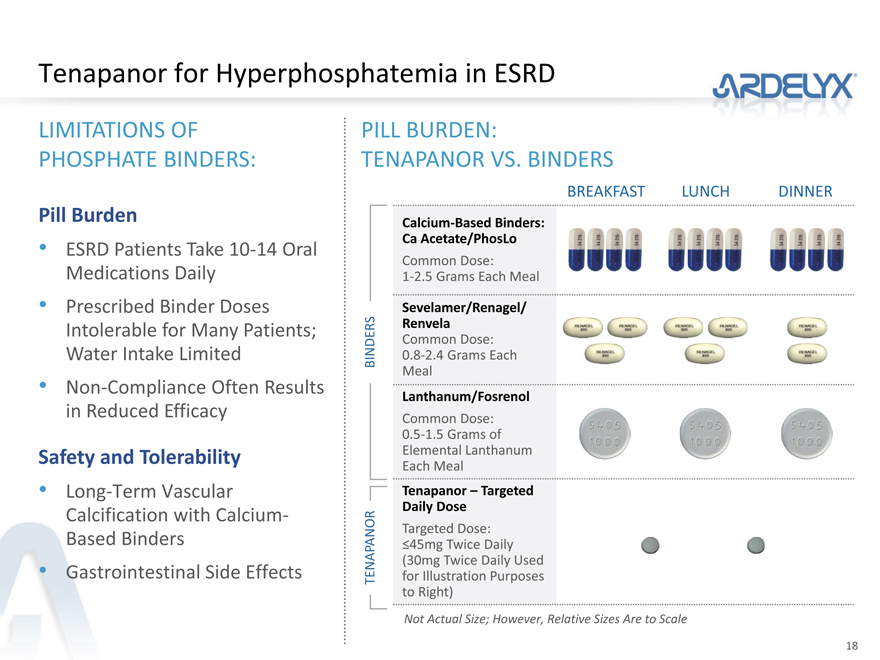
Tenapanor for Hyperphosphatemia in ESRD
LIMITATIONS OF PHOSPHATE BINDERS:
Pill Burden
ESRD Patients Take 10-14 Oral Medications Daily
Prescribed Binder Doses Intolerable for Many Patients; Water Intake Limited
Non-Compliance Often Results in Reduced Efficacy
Safety and Tolerability
Long-Term Vascular Calcification with Calcium-Based Binders
Gastrointestinal Side Effects
PILL BURDEN:
TENAPANOR VS. BINDERS
BREAKFAST LUNCH DINNER
Calcium-Based Binders:
Ca Acetate/PhosLo
Common Dose:
1-2.5 Grams Each Meal
Sevelamer/Renagel/
Renvela
Common Dose:
BINDERS 0.8-2.4 Grams Each
Meal
Lanthanum/Fosrenol
Common Dose:
0.5-1.5 Grams of
Elemental Lanthanum
Each Meal
Tenapanor – Targeted
Daily Dose
Targeted Dose:
TENAPANOR
£45mg Twice Daily
(30mg Twice Daily Used
for Illustration Purposes
to Right)
Not Actual Size; However, Relative Sizes Are to Scale
18
|
|
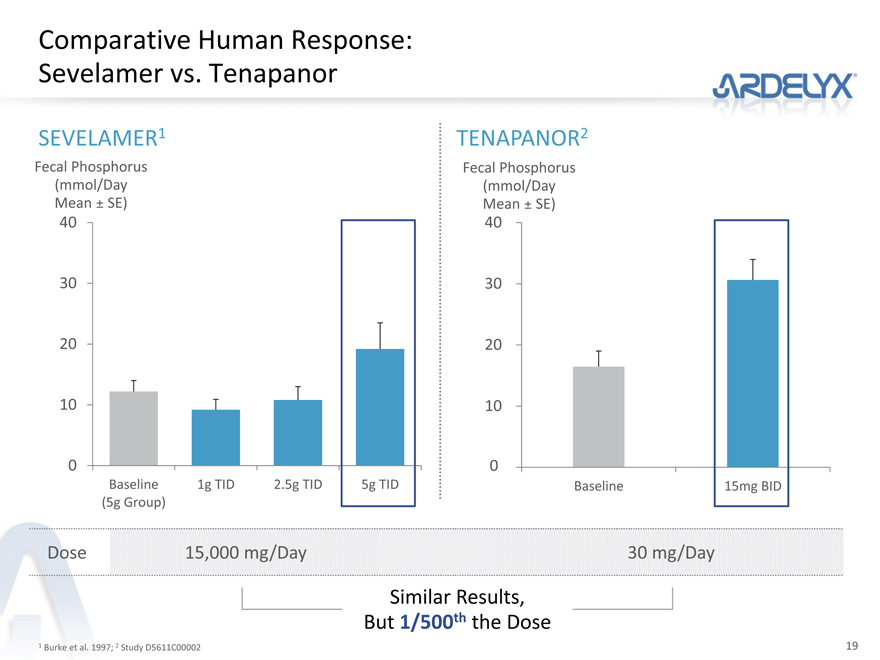
Comparative Human Response: Sevelamer vs. Tenapanor
SEVELAMER1 TENAPANOR2
Fecal Phosphorus Fecal Phosphorus
(mmol/Day (mmol/Day
Mean ± SE) Mean ± SE)
40 40
30 30
20 20
10 10
0 0
Baseline 1g TID 2.5g TID 5g TID Baseline 15mg BID
(5g Group)
Dose 15,000 mg/Day 30 mg/Day
Similar Results,
But 1/500th the Dose
| 1 |
|
Burke et al. 1997; 2 Study D5611C00002 |
19
|
|
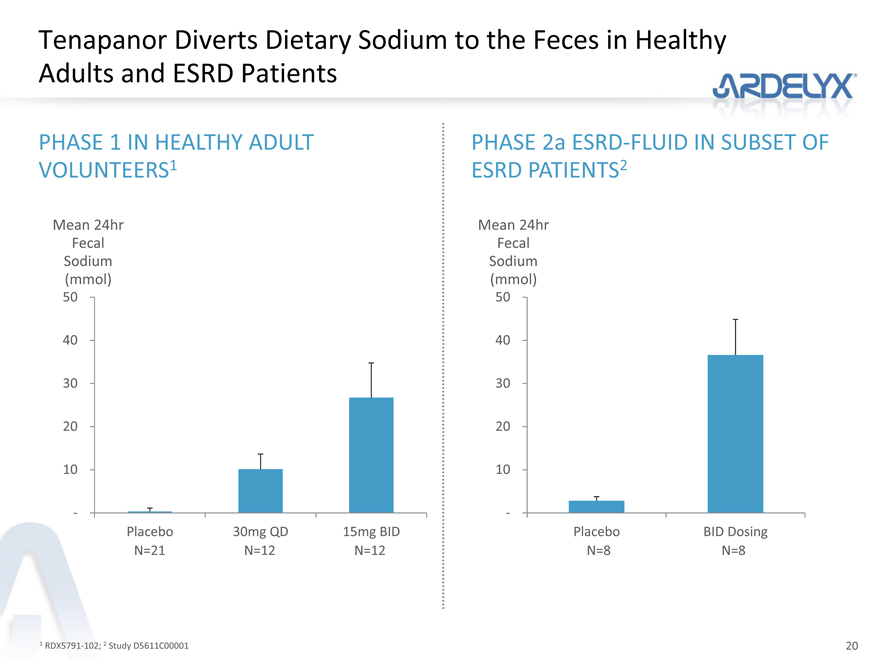
Tenapanor Diverts Dietary Sodium to the Feces in Healthy Adults and ESRD Patients
PHASE 1 IN HEALTHY ADULT
VOLUNTEERS1
Mean 24hr
Fecal
Sodium
(mmol)
50
40
30
20
10
-
Placebo 30mg QD 15mg BID
N=21 N=12 N=12
| 1 |
|
RDX5791-102; 2 Study D5611C00001 |
PHASE 2a ESRD-FLUID IN SUBSET OF
ESRD PATIENTS2
Mean 24hr
Fecal
Sodium
(mmol)
50
40
30
20
10
-
Placebo BID Dosing
N=8 N=8
20
|
|
ESRD Clinical Trials
ESRD FLUID
PHASE 2a
ESRD-
HYPERPHOSPHATEMIA
PHASE 2b
Design: Double-Blind; 4 Weeks of Treatment, n=88 (45 Active/43 Placebo)
Objective: Safety, Tolerability, and Pharmacodynamics of Tenapanor in ESRD-HD Patients with Fluid Overload
Status: Completed
Design: Double Blind; 4 Weeks Of Treatment, n=150 (125 Active/25 Placebo)
Objective: Efficacy and Safety of Tenapanor for the Treatment of Hyperphosphatemia in ESRD-HD Patients; Determination of Phase 3 Dose(s)
Status: Enrolling; Data Expected 1H 2015
21
|
|
Tenapanor for Treating CKD
Sodium and Fluid Overload
LIMITATIONS OF CURRENT TREATMENTS TO DELAY CKD PROGRESSION
Poor Compliance with Low Sodium Diets
Diuretics Lose Efficacy and Cause Electrolyte Disorders
ACE Inhibitors Reduce Blood Pressure but Hyperkalemia Limits Widespread Use in CKD Patients
22
|
|
CKD Clinical Trials
CKD
PHASE 2a
Design: Double-Blind, 12 Weeks of Treatment, n=140 (70 Active/70 Placebo)
Objective: Safety, Tolerability, and Pharmacodynamics of Tenapanor in CKD Patients with Type 2 Diabetes Mellitus and Albuminuria
Status: Enrolling, Data Expected 2H 2015
23
|
|
Tenapanor for IBS-C
GI Disorder: Constipation and Abdominal Pain
LIMITATIONS OF CURRENT TREATMENTS
OTC Drugs Inexpensive but Not Very Effective in Moderate to Severe Cases
Amitiza® and Linzess® Fall Short:
– Achieve Endpoint in Only 7% to 20% of Patients
– Side Effects (e.g., Diarrhea)
Medical Need for Improved Therapies with Better Efficacy, Excellent Safety and Tolerability
24
|
|
Phase 2a Demonstrates Consistent Effects of Tenapanor in Multiple Endpoints and Supports Ongoing Phase 2b Study
DUAL ENDPOINT: >30% DECREASE IN WEEKLY ABDOMINAL PAIN SCORE AND >1 INCREASE IN CSBM FREQUENCY AS COMPARED TO BASELINE
End of Treatment
40% Placebo (n=47)
| * |
|
100mg QD (n=46) |
30%
Percent
Responders 20%
10%
0%
Week 1 Week 2 Week 3 Week 4 Follow-Up Follow-Up
Week 1 Week 2
Although the Primary Endpoint, Change in CSBM from Baseline to Week 4, Was Not Met, We Did Note Improvements in Degree of Bloating and Abdominal Pain, as Well as Relief of IBS Symptoms, Severity and QOL Measurements
These Data Were the Basis for Our Decision to Take Tenapanor into a Phase 2b Clinical Study
| * |
|
p<0.05 versus placebo |
25
|
|
IBS-C Clinical Trials
IBS-C
PHASE 2a
IBS-C
PHASE 2b
Design: Double-Blind, 4 Weeks of Treatment, n=186 (139 Active/47 Placebo)
Objective: Safety, Tolerability, and Pharmacodynamics of Tenapanor for the Treatment of IBS-C
Status: Completed
Design: Double-Blind, 12 Weeks of Treatment, n=371 (3:1 Active: Placebo)
Objective: Efficacy and Safety of Tenapanor for the Treatment of IBS-C; Determination of Phase 3 Dose(s)
Status: Enrollment Completed, Data Expected 4Q 2014
26
|
|
The AstraZeneca/Tenapanor Collaboration
PAYMENTS
Additional
Upfront Development Launch and Sales
Up to
Milestones $35M $237.5M $597.5M $870M
Total
$50M 2H 2015
$20M 1H 2015
$25M Received May 2014
$15M Received December 2013
Royalties Incremental, Tiered (High Single Digit – High Teen Percentages)
Co-Funding Option at 1st P3 Trial: Buy Up to 1%, 2% or 3% of
Royalties with $20M, $30M or $40M* Respectively
OTHER TERMS
AstraZeneca
Responsibilities: All R&D and Commercialization Expenses**
Ardelyx Rights: Right to Co-Promote in the US
*Exercisable Within 60 Days After Decision to Proceed to P3 Clinical for the First Indication for Tenapanor **Subject to cap on obligation for IBS-C reimbursement
27
|
|
RDX002: NaP2b and Our Collaboration with Sanofi
PROPRIETARY PLATFORM
2 VALIDATED PROGRAMS
WHOLLY-OWNED PIPELINE
CAPITAL EFFICIENCY
PROVEN MANAGEMENT TEAM
Discovery and Design of Non-Systemic, Small Molecule Therapeutics
Tenapanor: AstraZeneca Collaboration; Phase 2 in Three Indications
RDX002 NaP2b Inhibition: Sanofi Collaboration
Multiple Near-Term Catalysts
Pipeline Provides Additional Opportunity Strong History Deep Domain Expertise
28
|
|
Phosphate Binder vs. Phosphate Transport Inhibitor
Binder Phosphate Transport Inhibitor
Phosphate Binder Phosphate Phosphate
Transport
Inhibitor
NaP2b
Transporter
Enterocyte
Capillary
GI-Upset/Pill-Burden
Increased Calcium Load with Calcium Based Binders
Concerns of Metal Accumulation with Metal Based Binders
Potential for Dramatically Reduced Pill-Burden
Potential for Use in Combination with Phosphate Binders
29
|
|
RDX002: NTX1942 Reduces Urine Phosphorus Levels in Normal Rats and Is Additive to Sevelamer
0
Reduction -10
(%)
-20
-30 ** *** ***
Vehicle Sevelamer NTX1942 Combo
500 mg/kg 50 mg/kg
Combination of
Sevelamer and
NTX1942 Is Additive
NTX1942 Response Is Superior to Sevelamer at 1/10 of the Dose
Data Shown is the Mean ± SEM; ** = p<0.01; *** =p<0.001, by one-way ANOVA, n= 12
30
|
|
The Sanofi/NaP2b Collaboration
Milestones
Royalties
Licensed Technology
Other Terms
PAYMENTS
Upfront Potential Additional Devt./Reg Milestones
Up to
$198M
Total
$1.25M
Incremental, Tiered (Mid-Single Digit – Low Teen Percentages)
NaP2b Patent Portfolio and Related Know-how for Research to
Complete Activities under Preclinical Development Plan
Option to Obtain Exclusive License to Develop, Manufacture and
Commercialize NaP2b Inhibitors
Sanofi Responsible for Completing Pre-Clinical Development Plan,
and if It Exercises the Option, for All Development and
Commercialization at Its Expense
Ardelyx Has the Right to Co-Promote in the US
31
|
|
Ardelyx-Owned Pipeline
PROPRIETARY PLATFORM
2 VALIDATED PROGRAMS
WHOLLY-OWNED PIPELINE
CAPITAL EFFICIENCY
PROVEN MANAGEMENT TEAM
Discovery and Design of Non-Systemic, Small Molecule Therapeutics
Tenapanor: AstraZeneca Collaboration; Phase 2 in Three Indications
NaP2b Inhibition: Sanofi Collaboration
Multiple Near-Term Catalysts
Pipeline Provides Additional Opportunity Strong History Deep Domain Expertise
32
|
|
RDX009: TGR5 Agonists for Inflammatory Bowel Disease (IBD)
TGR5 STIMULATION
Enhances GLP1 and GLP2 (Incretins) Secretion Directly to the GI Mucosa
Anti-Inflammation and Mucosal Healing Effects
Gattex® = GLP2 approved for Short Bowel Syndrome – Studied in Crohn’s
– Daily Injections
Intercept and Exelixis/BMS Both Have Systemic TGR5 Agonists
– Gallbladder Emptying Issues
– Short Lasting Incretin Secretion
ARDELYX TGR5 AGONISTS + DPP4 INHIBITOR
No Gallbladder Issues
Long Lasting Incretin Secretion
Significantly Improves Mucosal Damage In Mouse Model of IBD
33
|
|
RDX013: Hyperkalemia (Elevated Serum Potassium)
THE CHALLENGE
All Potassium Binders (e.g. Patiromer) Have Limited Efficacy on per Gram Basis
Therapeutic Dose for Kayexelate, Patiromer or ZS-9 Substantially the Same (15-30 g/day)
The Limiting Factor in Efficacy is Not Binder Capacity but Availability of Potassium in the Colon
ARDELYX POTASSIUM “SECRETAGOGUE”
To Move Potassium into Colon and Increase Fecal Excretion
Used as a Stand Alone or in Combination with Potassium Binder
Augment Patient Compliance
Maintain Normal Serum Potassium with Optimal Dosing of Antihypertensives (RAAS Blockade Drugs)
34
|
|
Near-Term Catalysts to Drive Value
Tenapanor
IBS-C ESRD-Pi CKD
DATA Phase 2b Phase 2b Phase 2a
Data Data Data
Q4 1H 2H
2014 2015
35
|
|
Financial Overview
$ MILLIONS
$33.2M (as of 3/31/14)
Cash and Cash Equivalents $25M Additional Received May 2014
$61M received from IPO in July 2014
Operating Expenses* $12.5M (12 Months Ended 3/31/14)
Debt $0
Capital Raised
Series A $26M Series B $30M IPO $69M
Nasdaq:
Ventures ARDX
2008 2011
*Excludes amounts reimbursed by AZN or associated with AZN agreement
36
|
|
Summary
PROPRIETARY PLATFORM
2 VALIDATED PROGRAMS
WHOLLY-OWNED PIPELINE
CAPITAL EFFICIENCY
PROVEN MANAGEMENT TEAM
Discovery and Design of Non-Systemic, Small Molecule Therapeutics
Tenapanor: AstraZeneca Collaboration; Phase 2 in Three Indications
NaP2b Inhibition: Sanofi Collaboration
Multiple Near-Term Catalysts
Pipeline Provides Additional Opportunity Strong History Deep Domain Expertise
37