Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Ignyta, Inc. | d769080d8k.htm |
| EX-99.1 - EX-99.1 - Ignyta, Inc. | d769080dex991.htm |
 Catalyzing
Precision Medicine with Integrated Rx/Dx in Oncology Ignyta RXDX-103 and RXDX-104
Overview August 5, 2014
®
Exhibit 99.2 |
 Safe Harbor Statement
2
This document contains forward-looking statements, as that term is defined in Section 27A of the
Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, about Ignyta,
Inc. (“us” or the “Company”). Statements that are not purely historical are
forward-looking statements. These include statements regarding, among other things: the clinical
and/or non-clinical data or plans underlying RXDX-103, RXDX-104 or any of our other
development programs; our ability to design and conduct development activities for
RXDX-101, RXDX-103, RXDX-104 and our other development programs; our ability to
develop or access companion diagnostics for our product candidates; our ability to obtain and
maintain intellectual property protection for our product candidates; our ability to adequately fund
our development programs; and our ability to obtain regulatory approvals in order to market any
of our product candidates.
Forward-looking statements involve known and unknown risks that relate to future events or the
Company’s future financial performance, some of which may be beyond our control, and the
actual results could differ materially from those discussed in this document.
Accordingly, the Company cautions investors not to place undue reliance on the forward-
looking statements contained in, or made in connection with, this document. Important factors that could
cause actual results to differ materially from those indicated by such forward-looking
statements, include, among others, the potential for results of past or ongoing clinical or
non-clinical studies to differ from expectations or previous results; the interpretation of
data from our clinical and non-clinical studies; our ability to initiate and complete
clinical trials and non-clinical studies; the potential advantages of our product candidates; and our capital
needs; as well as those set forth under the headings “Special Note Regarding Forward-Looking
Statements,” “Risk Factors” and “Management’s Discussion and Analysis
of Financial Condition and Results of Operations” contained in the Company’s Form
10-K filed with the Securities and Exchange Commission (“SEC”) on February 28, 2014, and similar
disclosures made in the Company’s Form 10-Q filings and other SEC filings and press
releases. The
forward-looking statements contained in this document represent our estimates and assumptions only as of the date
of this document, and we undertake no duty or obligation to update or revise publicly any
forward-looking statements contained in this document as a result of new information,
future events or changes in our expectations.
Third-party information included herein has been obtained from sources believed to be reliable,
but the accuracy or completeness of such information is not guaranteed by, and should not be
construed as a representation by, the Company.
|
 Executive Summary
of New License Agreement with Nerviano
Ignyta strives to be a leading oncology precision medicine company by
developing first-in-class molecules with companion diagnostics
On Aug. 4, 2014, Ignyta entered into a second license agreement with
Nerviano, adding two additional first-in-class programs:
-
RXDX-103: highly selective, potent Cdc7 inhibitor with broad therapeutic
potential across multiple tumor types; potential relevance to targeted
patient populations -
RXDX-104: highly selective, potent RET inhibitor program that complements
RXDX- 101 and slots in with existing clinical infrastructure and
relationships with thoracic and head & neck oncologists
Both preclinical programs build upon our growing expertise in kinase inhibitors
and companion diagnostics, and bolster our Rx/Dx pipeline
One or both compounds could enter clinic by 1H 2016
Composition of matter patent protection on RXDX-103 and RXDX-104
through at least 2027 and 2033, respectively
3 |
 RXDX-103
& RXDX-104 Exclusive Worldwide License 4
$3.5M upfront (to be paid August 14, 2014)
$68M in development and regulatory milestones for RXDX-103
$34M in aggregate potential milestones for RXDX-104
No sales milestones
$105.5M in total upfront and potential milestones
Mid-single digit to low double digit royalties |
 Ignyta Rx/Dx
Pipeline 5
1
In-licensed from Nerviano Medical Sciences (NMS); RXDX-102 Pan-Trk inhibitor is a
back-up to RXDX-101 Compound
Target
Discovery
Preclinical
Phase 1
Phase 2
Phase
3
RXDX-101
1
Pan-Trk, ROS1,
ALK
RXDX-103
1
Cdc7
RXDX-104
1
RET
Spark-1
Rx/Dx Program
Spark-2 Rx/Dx Program
Spark-3 Rx/Dx Program |
 Cdc7 inhibitor overview
RET inhibitor overview
6
Table of Contents |
 RXDX-103 Cdc7 Kinase Inhibitor Opportunity
RXDX-103
(formerly
NMS-P862):
Nerviano’s
2
nd
Generation
Compound*
7
*
In 2009, NMS advanced NMS-P354, a first generation Cdc7 inhibitor, into 3
phase 1 studies in solid and hematologic tumors; dosed 48 patients in the 3
studies, but was unable to achieve sufficient drug exposure due to rapid
metabolism and generation of a toxic metabolite Nerviano Medical Sciences (NMS) is the
world’s leading expert in Cdc7 biology, having worked on the mechanism since
2003 Ignyta has an exclusive worldwide license to RXDX-103, a
first-in-class Cdc7 inhibitor
Preclinical development candidate: NMS’
2nd generation compound
engineered with an improved metabolic profile |
 RXDX-103 Cdc7 Kinase Inhibitor Opportunity
8
*
In 2009, NMS advanced NMS-P354, a first generation Cdc7 inhibitor, into 3
phase 1 studies in solid and hematologic tumors; dosed 48 patients in the 3
studies, but was unable to achieve sufficient drug exposure due to rapid
metabolism and generation of a toxic metabolite Good drug-like properties (oral
bioavailability, permeability, PK, etc.) Single agent and combination therapy activity
in multiple in vivo models; potential efficacy in drug resistance setting or in
combination with cell cycle inhibitors
Patient selection hypothesis and pharmacodynamic biomarkers
Potential clinical benefit in selected cancer patient
populations (e.g., breast,
colon) Targeting FIH study in early 2016
RXDX-103 (formerly NMS-P862): Nerviano’s 2
nd
Generation Compound* |
 Cell Division Cycle Background
Cell cycle kinases promote progression through different phases of cell division
cycle These proteins are deregulated in cancer, and considered druggable
targets Cdc7 is a serine threonine kinase essential for initiating DNA
replication during S phase 9
Source: Malumbres, 2011 |
 DNA Replication
10
Source: Nerviano Medical Sciences |
 Targeting DNA Replication in Cancer
11
Classical broad-based chemotherapies inhibit DNA replication elongation; e.g.,
-
DNA precursors/anti-metabolites block DNA
polymerization
-
Topoisomerase inhibitors and crosslinking/intercalating agents block fork
progression Source: Nerviano Medical Sciences |
 Targeting DNA Replication in Cancer
Inhibiting
DNA
replication
initiation
by
Cdc7
is
a
more
targeted
approach
-
Cdc7 is essential for initiating DNA
replication:
Phosphorylates Mcm2-7 helicase to initiate DNA unwinding during
replication -
Also plays a key role in DNA damage S phase checkpoint response and
post-replication DNA repair 12
Classical broad-based chemotherapies inhibit DNA replication elongation; e.g.,
-
DNA precursors/anti-metabolites block DNA
polymerization
-
Topoisomerase inhibitors and crosslinking/intercalating agents block fork
progression Source: Nerviano Medical Sciences |
 Role of Cdc7 in Cancer
Cdc7 is upregulated in cancer
-
Breast cancer: Cdc7 overexpression linked to genomic instability,
poor prognosis and reduced survival
-
Epithelial ovarian cancer: Cdc7 overexpression correlated with
poor prognosis (specifically disease free survival) and advanced
clinical stage
-
DLBCL and melanoma: increased Cdc7 activity associated with
poor clinical outcome and lower relapse free survival
Cdc7 overexpression associated with acquired chemotherapy
resistance
13
Sources: Rodriguez, 2010; Choshzick, 2010; Kulkarni et. al., 2009; Nambiar et al.,
2007; Krawczyk et al., 2009 |
 Highly Targeted, Selective Cdc7 Inhibition
Kills Cancer Cells while Sparing Healthy Cells
14
Inhibition of Cdc7
arrests DNA
replication initiation
Checkpoint
failure
Tumor cells
DNA breaks, mitotic
catastrophe, apoptotic cell
death
Normal cells
Intact
replication
checkpoint
Cell cycle
arrest
Resume
replication after
removal of
Cdc7 inhibition
Figure modified from Nature Chemical Biology 4, 331-32 (2008)
•
Cdc7
inhibition
leads
to
apoptotic
cell
death
in
tumor
cells
due
to
their
lack
of
functional
checkpoint
response (checkpoint proteins are often mutated in human cancer)
•
In contrast, normal cells arrest reversibly in a p53 dependent fashion
•
A
Cdc7
inhibitor
could
potentially
act
in
synergy
with
chemotherapies
that
target
the
DNA
replication
elongation process (e.g., targeted cell cycle inhibitors, anti-metabolites,
topoisomerase inhibitors, and intercalating agents)
|
 RXDX-103
Is a Highly Potent, Selective
ATP-Competitive
Cdc7
Inhibitor
15
RXDX-103 (IC
50
nM)
Cdc7/Dbf4
9
Cdk9/CycT
150
Gsk3 beta
170
Cdk2/CycA
450
RXDX-103 Kinase Screen
Source: Nerviano Medical Sciences |
 16
Mcm2 is a direct substrate of Cdc7 kinase in its replication initiation role
CAL51 triple negative breast cancer cell line
RXDX-103
Sources: Nerviano Medical Sciences
RXDX-103 Inhibits Mcm2 Phosphorylation
Mcm2 phosphorylation status represents a promising PD biomarker |
 RXDX-103 Exhibits Broad Anti-proliferative
Activity across a Range of Tumor Cell Lines
17
Note: anti-proliferative activity tested in 113 tumor cell lines (72 hr assay)
Source: Nerviano Medical Sciences
High
sensitivity
in
75%
of
pancreatic
cancer,
50%
of
colon
cancer,
30%
of
breast
and
30%
of
melanoma |
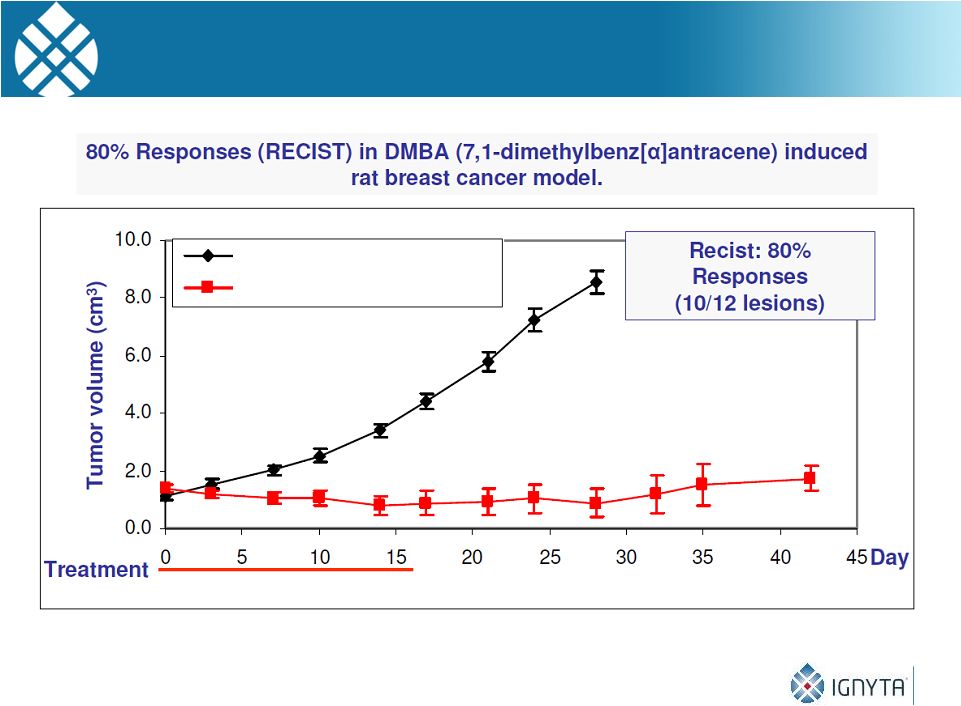 RXDX-103 Demonstrates Potent Single Agent
Anti-Tumor Activity in a Rat Breast Cancer Model
18
RXDX-103 15 mg/kg QD (1-18)
Vehicle
Source: Nerviano Medical Sciences |
 RXDX-103 Demonstrates Synergistic Anti-Tumor Activity in
Combination with Docetaxel in a Mouse Breast Cancer Model
19
MX-1 Triple Negative mouse xenograft model
Source: Nerviano Medical Sciences
RXDX-103
RXDX-103
IV QW |
 Potential Rx/Dx Hypotheses for RXDX-103
Cdc7 is the pharmacologic target of RXDX-103; Mcm2 is its substrate;
Phosphorylation of Mcm2 indicates its activation; Thus, levels of Cdc7, Mcm2 or
pMcm2 could indicate potential responders to RXDX-103
Early biomarker work based on sensitivity to Cdc7 inhibition in cell lines
suggest: -
Several candidate biomarkers potentially predict sensitivity to Cdc7
inhibition -
Biomarker 1: overexpression in breast cancer; e.g., >50% of breast tumors at
T3 and T4 stages
-
Biomarker 2: almost 20% of colorectal cancer
-
Cyclin A and HER2 potentially predict resistance to Cdc7 inhibition
Biomarkers involved with checkpoint regulation may also be relevant
Ignyta has initiated additional RXDX-103 biomarker analyses
Opportunities to rationally combine RXDX-103 with other cell cycle inhibitors,
chemotherapies and/or targeted therapies to extend duration of response
20 |
 RXDX-103 Clinical Development Strategy
Preliminary
Plan; Subject to Change
21
2014
2015
2016
2017
2018
Preclinical/GLP
Ph1/2a –
single agent
Ph1/2a –
combo (breast)
Ph1/2a –
combo (ovarian)
Ph1/2a –
combo (CRC)
Preclinical data suggests efficacy in specific patient populations:
Breast cancer with biomarker 1 overexpression
Biomarker 2 positive ovarian cancer
Biomarker 2 positive colorectal cancer
Current Program Status:
Completed preliminary rodent PK
and in vitro ADME studies
Preparing for manufacturing GMP
scale-up and GLP tox studies |
 Table of Contents
CDC7 inhibitor overview
RET inhibitor overview
22 |
 RET Inhibitor Opportunity
RXDX-104 (from NMS-P616 / NMS-P753):
23
Potent activity against RET rearrangements and point mutations
(including V804M gatekeeper)
Activity in TT medullary thyroid xenograft model and LC-2/ad RET
rearranged NSCLC cell line
Lead optimization stage
First-in-class selective RET inhibitors licensed from Nerviano
|
 RET Inhibitor Opportunity
RXDX-104 (from NMS-P616 / NMS-P753):
24
Synergies between RXDX-104 and RXDX-101 in terms of similar
physician champions (thoracic and head & neck oncologists) and CDx
approach
Likely clinical benefit in RET rearranged and mutated populations:
NSCLC, medullary thyroid cancer, papillary thyroid cancer
Potential for Development Candidate in early 2015
Promising drug-like properties (oral bioavailability, PK) |
 RET Gene Rearrangements / Fusion Proteins
Are Oncogenic
25
Adapted from “Thyroid carcinoma: molecular pathways and therapeutic
targets”, Modern Pathology (2008) 21, S37–S43 RET is a receptor
tyrosine kinase, upstream of cell signaling pathways such as MAPK & PI3K |
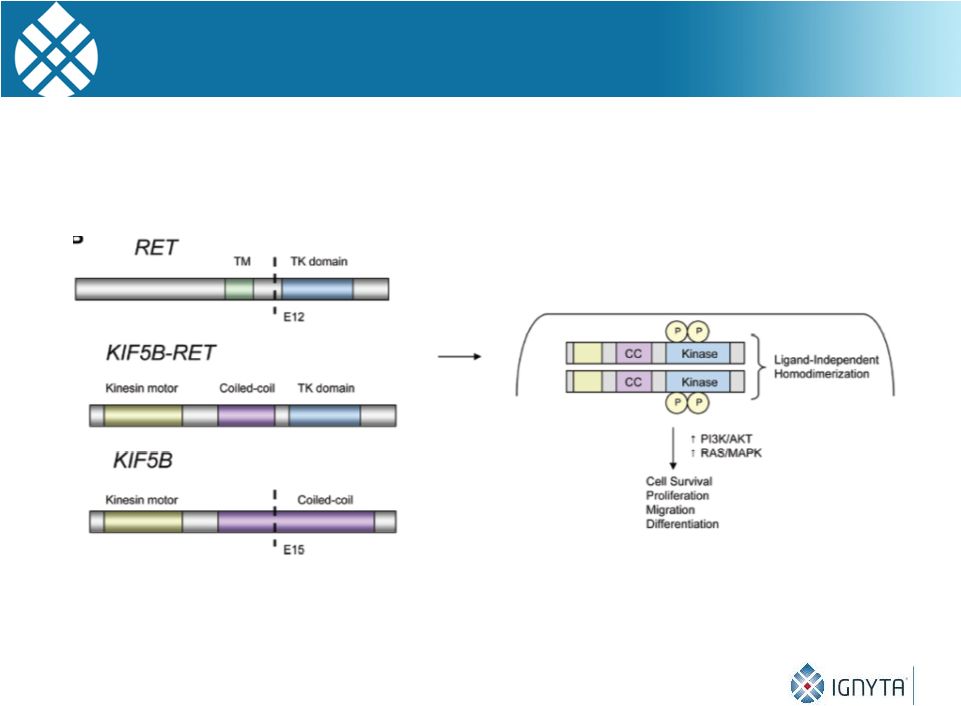 RET Gene Rearrangements / Fusion Proteins
Are Oncogenic
26
Adapted from “Intracellular signal transduction and modification of the tumor
microenvironment induced by RET/PTCs in papillary thyroid carcinoma”,
Front. Endocrinol., 3(67) 21 May 2012
RET fusion proteins, similar to Trk, ROS & ALK, can homodimerize,
autophosphorylate and cause constitutive oncogenic signaling
|
 RET Fusion Proteins and Activating Mutations
Are Found in Multiple Tumor Types
RET fusion proteins and gain of function mutations are associated with
multiple human malignancies:
Medullary thyroid cancer (MTC)
-
Nearly all inherited MTC harbor
germ-line RET mutations
-
Up to 50% of sporadic MTC
patients have RET mutations
Papillary Thyroid Cancer (PTC)
-
5 to 40% of PTC patients have
RET fusions
Non-small cell lung cancer (NSCLC)
-
1-2% of NSCLC harbor RET fusions
27
Representative RET fusions in
papillary thyroid cancer |
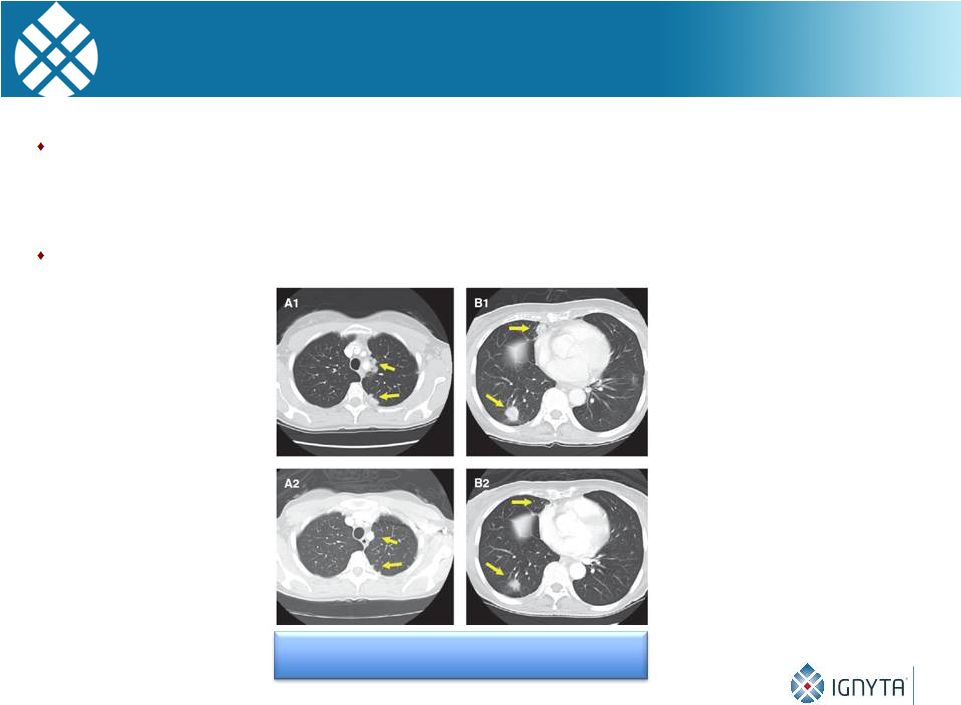 RET Inhibition Appears to be Effective in Preliminary
Studies of RET-Activated Patients
Several multi-kinase inhibitors with non-selective, anti-RET activity
(cabozantinib,
vandetanib,
ponatinib,
sunitinib)
being
tested
in
RET
rearranged cancer patients
Encouraging
clinical
activity
with
such
agents
in
RET
rearranged
patients
28
Treatment of
RET fusion
NSCLC patients
with cabozantinib
Drilon et al. Cancer Discovery 2013
Baseline
4 Weeks
However… |
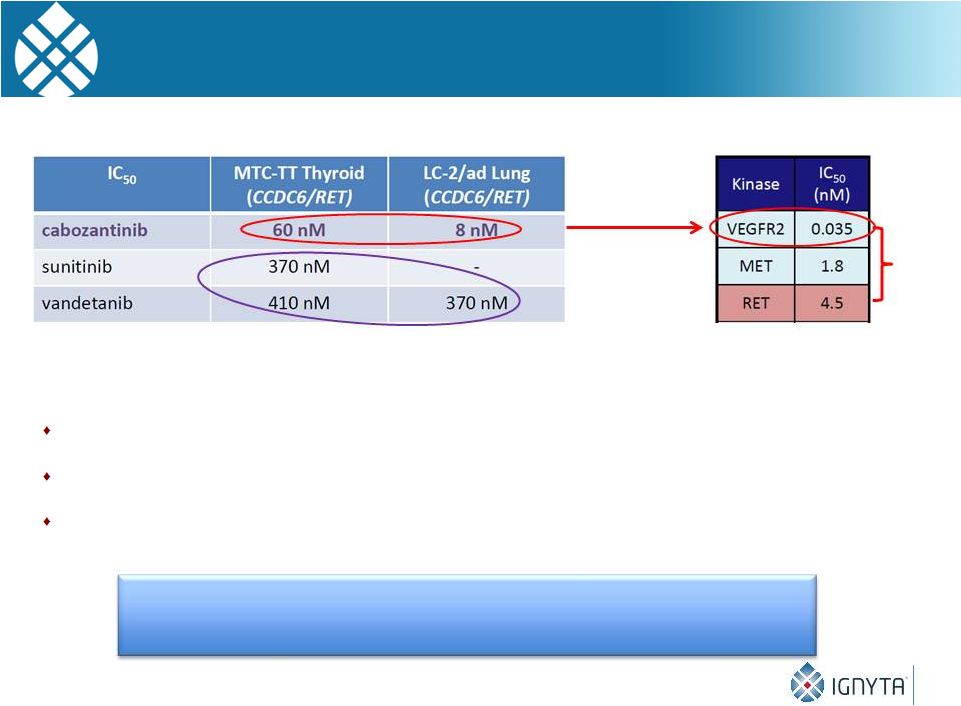 But Available “RET Inhibitors”
Are Severely Limited
by Their Non-RET Activity
Non-RET activity (e.g., VEGFR) of each of these non-selective compounds
results in dose-limiting toxicities / tolerability, capping dose levels
& clinical benefit Cabozantinib: Black box warning
Vandetanib: Available only through REMS
Ponatinib: Temporarily pulled from market and put on clinical hold
29
High RET
potency, but…
Cabozantinib
Activity
>100X
VEGF/
RET
Low RET
potency
A selective RET inhibitor should exhibit fewer side effects
and allow greater clinical benefit in RET patients |
 RXDX-104 Lead Candidates Are Highly Selective for RET
Inhibition in Kinase and Cellular Assays
30
Potent RET inhibitor ~2 nM
Good selectivity in panel of 54
tyrosine / serine threonine kinases
LC2/ad NSCLC:
CCDC6-RET rearrangement
MZ-CRC1 MTC:
RET M918T mutation
TT MTC:
RET C634W mutation
RXDX-104 Kinase Screen
RXDX-104 Cell Line Screen
IC50 RET: 2 nM
LCD2/ad: 19 nM
MZ-CRC1: 39 nM
TT: 14 nM
Source: Nerviano Medical Sciences
600
500
400
300
200
100
0 |
 RXDX-104 Inhibition of RET Abrogates Downstream
Signaling in Cancer Cell Lines
31
TT medullary cancer
cell line
ip: RET
Total cell
lysates
LC-2/ad
NSCLC cell line
Source: Nerviano Medical Sciences |
 32
RXDX-104 Single Agent Anti-Tumor Activity
in TT Xenograft Mouse Model
RXDX-104 50 mg/kg QD
Vehicle
RXDX-104 100 mg/kg QD
TT MTC xenograft: RET C634W mutation
Source: Nerviano Medical Sciences |
 RXDX-104 Summary
Strong clinical rationale for selective RET inhibitor in RET activated
tumors
RXDX-104 is a first-in-class selective RET inhibitor program
Aligns seamlessly with Ignyta’s Rx/Dx focus
Leverages existing Ignyta CDx infrastructure and expertise
-
Technology used in the RXDX-101 CDx can be readily applied to RET
fusions and activating SNP detection
-
Could incorporate TRK, ALK, ROS and RET alterations into a single test
Growing presence in NSCLC and head & neck cancers through
RXDX-101 and RXDX-104
33 |
 Conclusion
Bolstered Rx/Dx pipeline with two exciting first-in-class targeted
kinase inhibitor programs
Focused approach to in-licensing
Drafts on pre-existing relationship with Nerviano (quality, trust)
Leverages CDx and biomarker expertise & infrastructure
Consolidates clinical development and commercial call points
Responsive to our KOLs regarding unmet needs
With driver alterations, where there’s smoke, there’s fire
(Trk, RET…)
34 |
