Attached files
| file | filename |
|---|---|
| EX-31.2 - CERTIFICATION - PetVivo Holdings, Inc. | petv_ex312.htm |
| EX-31.1 - CERTIFICATION - PetVivo Holdings, Inc. | petv_ex311.htm |
| EX-32.1 - CERTIFICATION - PetVivo Holdings, Inc. | petv_ex321.htm |
| EXCEL - IDEA: XBRL DOCUMENT - PetVivo Holdings, Inc. | Financial_Report.xls |
| EX-32.2 - CERTIFICATION - PetVivo Holdings, Inc. | petv_ex322.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
|
x
|
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
For the fiscal year ended March 31, 2014
|
o
|
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
For the transition period from ____________ to ____________
Commission File Number: 333-173569
| PetVivo Holdings, Inc. |
| (Exact name of registrant as specified in its charter) |
|
Nevada
|
|
99-0363559
|
|
(State or other jurisdiction of incorporation or organization)
|
|
(I.R.S. Employer Identification No.)
|
|
12100 Singletree Lane, Suite 186
Eden Prairie, Minnesota
|
55344
|
|
|
(Address of principal executive offices)
|
(Zip Code)
|
(612) 296-7305
(Registrant’s Telephone Number, Including Area Code)
Securities registered under Section 12(b) of the Act:
|
Title of each class registered:
|
Name of each exchange on which registered:
|
|
|
None
|
None
|
Securities registered under Section 12(g) of the Act:
Title of each class registered:
Common Stock, par value $0.001
Indicate by check mark if registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. o Yes x No
Indicate by check mark if registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. o Yes x No
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. x Yes o No
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§ 229.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). x Yes o No
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. x
Indicate by check mark whether the registrant is a large accelerated file, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act.
|
Large accelerated filer
|
o |
Accelerated filer
|
o |
|
Non-accelerated filer
|
o |
Smaller reporting company
|
x |
|
(Do not check if a smaller reporting company)
|
|||
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). o Yes x No
State the aggregate market value of the voting and non-voting common equity held by non-affiliates computed by reference to the price at which the common equity was sold, or the average bid and asked price of such common equity, as of the last business day of the registrant’s most recently completed second fiscal quarter. As of September 30, 2013, approximately $2,820,723.52.
As of July 10, 2014, there were 3,779,542,482 shares of the issuer’s $.001 par value common stock issued and outstanding.
Documents incorporated by reference. There are no annual reports to security holders, proxy information statements, or any prospectus filed pursuant to Rule 424 of the Securities Act of 1933 incorporated herein by reference.
TABLE OF CONTENTS
|
|
|
Page
|
|||
|
|
|
|
|||
| PART I | |||||
|
|
|
||||
|
Item 1.
|
Business
|
3 | |||
|
Item 1A.
|
Risk Factors
|
15 | |||
|
Item 1B.
|
Unresolved Staff Comments
|
33 | |||
|
Item 2.
|
Properties
|
33 | |||
|
Item 3.
|
Legal Proceedings
|
33 | |||
|
Item 4.
|
Mine Safety Disclosures
|
33 | |||
|
|
|
||||
| PART II | |||||
|
|
|
||||
|
Item 5.
|
Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities
|
34 | |||
|
Item 6.
|
Selected Financial Data
|
37 | |||
|
Item 7.
|
Management’s Discussion and Analysis of Financial Condition and Results of Operation s
|
38 | |||
|
Item 7A.
|
Quantitative and Qualitative Disclosures About Market Risk
|
41 | |||
|
Item 8.
|
Financial Statements and Supplementary Data
|
41 | |||
|
Item 9.
|
Changes in and Disagreements with Accountants on Accounting and Financial Disclosure
|
42 | |||
|
Item 9A.
|
Controls and Procedures
|
43 | |||
|
Item 9B.
|
Other Information
|
44 | |||
|
|
|
||||
| PART III | |||||
|
|
|
||||
|
Item 10.
|
Directors, Executive Officers and Corporate Governance
|
45 | |||
|
Item 11.
|
Executive Compensation
|
47 | |||
|
Item 12.
|
Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
|
49 | |||
|
Item 13.
|
Certain Relationships and Related Transactions, and Director Independence
|
50 | |||
|
Item 14.
|
Principal Accounting Fees and Services
|
50 | |||
|
|
|||||
| PART IV | |||||
|
Item 15.
|
Exhibits, Financial Statement Schedules
|
51 | |||
2
PART I
Forward-Looking Information
This Annual Report of PetVivo Holdings, Inc. on Form 10-K contains forward-looking statements, particularly those identified with the words, “anticipates,” “believes,” “expects,” “plans,” “intends,” “objectives,” and similar expressions. These statements reflect management’s best judgment based on factors known at the time of such statements. The reader may find discussions containing such forward-looking statements in the material set forth under “Management’s Discussion and Analysis and Plan of Operations,” generally, and specifically therein under the captions “Liquidity and Capital Resources” as well as elsewhere in this Annual Report on Form 10-K. Actual events or results may differ materially from those discussed herein. The forward-looking statements specified in the following information have been compiled by our management on the basis of assumptions made by management and considered by management to be reasonable. Our future operating results, however, are impossible to predict and no representation, guaranty, or warranty is to be inferred from those forward-looking statements. The assumptions used for purposes of the forward-looking statements specified in the following information represent estimates of future events and are subject to uncertainty as to possible changes in economic, legislative, industry, and other circumstances. As a result, the identification and interpretation of data and other information and their use in developing and selecting assumptions from and among reasonable alternatives require the exercise of judgment. To the extent that the assumed events do not occur, the outcome may vary substantially from anticipated or projected results, and, accordingly, no opinion is expressed on the achievability of those forward-looking statements. No assurance can be given that any of the assumptions relating to the forward-looking statements specified in the following information are accurate, and we assume no obligation to update any such forward-looking statements.
ITEM 1. BUSINESS
BACKGROUND
We were incorporated as Pharmascan Corp. in the State of Nevada on March 31, 2009. On September 21, 2010, we filed a Certificate of Amendment to our Articles of Incorporation and changed our name to Technologies Scan Corp.
Effective March 21, 2014, our Board of Directors and majority shareholders approved an amendment to our articles of incorporation to change our name to "PetVivo Holdings, Inc.." (the “Name Change Amendment”). The Amendment was filed with the Secretary of State of Nevada on April 1, 2014 changing our name to "PetVivo Holdings, Inc." (the "Name Change"). The Name Change was effected to better reflect the future business operations of the Company. See " -- Submission of a Vote to Security Holders."
We filed appropriate documents with FINRA to effect the Name Change. On April 29, 2014, our Name Change from Technologies Scan Corp to “PetVivo Holdings, Inc.” was declared effective by FINRA and our common stock's trading symbol was changed to “PETV". Our new cusip number is 716817 101.
SECURITIES EXCHANGE AGREEMENT
On March 11, 2014, our Board of Directors authorized the execution of that certain securities exchange agreement dated March 11, 2014 (the "Securities Exchange Agreement") with PetVivo Inc., a Minnesota corporation ("PetVivo"), and the shareholders of PetVivo who hold of record the total issued and outstanding shares of common stock of PetVivo (the “PetVivo Shareholders”). In accordance with the terms and provisions of the Securities Exchange Agreement, we acquired all of the issued and outstanding shares of stock of PetVivo from the PetVivo Shareholders, thus making PetVivo our wholly-owned subsidiary, in exchange for the issuance to the PetVivo Shareholders of an aggregate 2,310,939,804 shares of our restricted common stock.
3
Thus, this represented a change in control and a change in business operations. Therefore, based on our change in control, our business operations changed to that of PetVivo involving innovative biomedical devices, which will focus on the licensing and commercialization for pets or pet therapeutics. We believe that we can leverage the investments in the human biomaterials and medical device industries to commercialize therapeutics to pets in a capital and time efficient way. Our strategy is to in-license proprietary products from human medical device companies specifically for use in pets. A key component of this strategy is the accelerated timeline to revenues for veterinary medical devices, which enter the market much earlier than the more stringently regulated pharmaceuticals. PetVivo has secured exclusive rights to its first product, an osteoarthritis medical device, which has been shown to be both safe and efficacious. Management believes the administration of these initial therapeutic devices exceeds the benefits of those found in current remedies. Therefore, the commercialization of PetVivo’s initial therapeutic devices will provide veterinarians and pet owners safe, effective, and long-lasting treatments to improve the pet’s quality of life.
PetVivo, has recently entered into an exclusive license agreement and manufacturing and supply agreement with Gel-Del Technologies, Inc. PetVivo has licensed protein-based biomaterials for the treatment of pain and inflammation associated with osteoarthritis in canine and equine. PetVivo believes that its treatment is superior to current methodology of using NSAID’s. NSAID’s have many side effects in canines and the company’s treatment has less to none of the side effects. PetVivo believes that there are opportunities to expand into the bovine and feline markets. See - - "Material Agreements"
PetVivo plans to commercialize our products in the United States through distributor relationships and complemented by the use of social media educating and informing the pet owners, and in Europe and rest of world through commercial partners.
MERGER AND SECURITY EXCHANGE AGREEMENT
On May 28, 2014, we entered into a binding term sheet (the “Term Sheet”) to effect a merger and security exchange agreement with Gel-Del Technologies, Inc. (“Gel-Del”). The Term Sheet requires both parties to negotiate in good faith to enter into a definitive agreement to complete a merger between us and Gel-Del. The Term Sheet provides for Gel-Del to become our wholly owned subsidiary through a tax-free stock exchange merger, which will result in the shareholders of Gel-Del, on a pro rata basis, exchanging 100% of the outstanding common stock of Gel-Del for our shares common stock. The amount of our common stock to be exchanged with Gel-Del shareholders shall be the greater of (i) common stock valued at Forty Million Dollars ($40,000,000) as defined in the Term Sheet, or (ii) thirty percent (30%) of all post-merger outstanding shares of our common stock (excluding any previous holdings of record by Gel-Del of our shares of common stock). Any outstanding preferred shares, options or warrants, and convertible securities of Gel-Del shall be converted into or exercised for Gel-Del common stock prior to the closing of the merger. We anticipate that the closing of this merger shall occur by December 31, 2014.
The Term Sheet provides for three material conditions to be satisfied or waived prior to closing this merger, which are (i) we shall have completed a first round of financing of at least $8,000,000 by December 31, 2014; (ii) we shall complete a reverse split of our common stock of at least 1-for-100 no later than 30 days prior to closing the merger; and (iii) we shall satisfy NASDAQ listing requirements and have applied for NASDAQ listing by the closing date.
The Term Sheet also provides for a management equity clawback requiring our post-merger principal executives to surrender and return to us a total of 1,050,000 shares of our common stock if our common stock is not trading on a major securities exchange at a market capitalization of at least $200,000,000 during the two-year period following the beginning of trading on the major securities exchange. Certain other general conditions governing this clawback provision are set forth in the Term Sheet.
The Term Sheet includes additional terms including post-merger compensation and other employment provisions for the current two principal executives of Gel-Del, approval of a post-merger operations budget, and certain corporate governance provisions.
4
PREVIOUS CONTRACTUAL RELATIONS
Social Geek
Previously, our Board of Directors approved the execution of a binding letter of intent dated as of April 27, 2013 and as amended May 17, 2013 (the "Letter of Intent") with Social Geek Media Inc., a private company organized under the laws of Canada ("Social Geek"). Social Geek was the developer of a line of food supplement and nutritional products known under the brand name "Proteina21" (the "Proteina21 Products"), including a marketing and commercialization strategy and online automated platform to accept orders, process payment, ship products and provide customer support for its Proteina21 Products
On May 24, 2013, our Board of Directors approved the execution of a fifteen year license agreement (the "License Agreement") with Social Geek and Patrick Aube ("Aube"). In accordance with the terms and provisions of the License Agreement, Social Geek granted to us and we accepted the rights to an exclusive license for the Proteina21 Products, including marketing, selling and distributing within the United States of America, subject to the terms and conditions set forth in the License Agreement. We agreed to use our best efforts in order to actively promote the sales of the Proteina21 products pursuant to the License Agreement and to develop the market for the Proteina21 Products in the United States. Social Geek also granted to us the right to use its trademarks in connection with the promotion, marketing and sale of Proteina21 Products in the United States and the right to use the applicable trademarks in relation with the promotional items and product packaging, Social Geek's marketing resources, and materials. In further accordance with the terms and provisions of the License Agreement, we issued to Social Geek a total of 200,000,000 common shares of our restricted common stock in consideration for the acquisition of the exclusive rights to the Proteina21 license for the United States of which the first region to be developed by us shall be the North East Region. On May 24, 2013, we entered into an addendum to the License Agreement (the "Addendum) with Patrick Aube and Social Geek pursuant to which we had an exclusive one year option to acquire the rights to Canada as well as the established client base, and a further exclusive two year option to acquire the rights to Europe.
Social Geek Rescission Agreement
On November 9, 2013, we entered into that certain rescission agreement with Social Geek and Aube (the "Rescission Agreement"), regarding the Letter of Intent and the License Agreement. Together with Social Geek and Aube, we decided that it was not in our best interests to proceed with commercial and operational activities involving the Proteina21 Products. In accordance with the terms and provisions of the Rescission Agreement: (i) the Letter of Intent and License Agreement and all associated transactions have been deemed rescinded, null and void; (ii) the parties have mutually released one another from any and all claims relating to the Letter of Intent and the License Agreement; (iii) we changed our place of principal business to St.-Hippolyte, Quebec, Canada; and (iv) Aube resigned as our President/Chief Executive Officer and a member of the Board of Directors and Michel Lebeuf resigned as our Vice President of Legal Affairs and Corporate Development. The 200,000 shares previously issued to Social Geek were cancelled and returned to treasury.
ispeedzone
Our Board of Directors previously approved the execution of a letter of intent dated as of September 5, 2012 (the "Letter of Intent"), with 6285431 Canada Inc., known as "iSpeedzone", a private company organized under the laws of Canada ("iSpeedzone"). In accordance with the terms and provisions of the Letter of Intent, we were to enter into a share exchange agreement and acquire the total issued and outstanding shares of common stock of iSpeedzone from the iSpeedzone shareholders in exchange for the issuance by us to the iSpeedzone shareholders on a pro rata basis of approximately 130,500,000 shares of our restricted common stock. This would have resulted in iSpeedzone becoming our wholly-owned subsidiary. iSpeedzone was a social network driven by arts, sports and recreational activities. It has the objective of providing certain services including event coordinateion, activity coordination, multimedia platform creation, video/WEB-HD production, event promotion and advertising concept creations.
ispeedzone Rescission Agreement
Our Board of Directors approved the execution of an agreement of rescission dated April 12, 2013 (the "Rescission Agreement"), with iSpeedzone. In accordance with the terms and provisions of the Rescission Agreement, we and iSpeedzone agreed not to combine our respective business operations and, therefore, rescinded and terminated the Letter of Intent. In further accordance with the terms and provisions of the Rescission Agreement, we and iSpeedzone mutually released one another from any and all claims and/or causes of action relating to the Letter of Intent.
5
FedTech Services Inc.
Our Board of Directors previously approved the execution of a letter of intent dated as of December 16, 2013 (the "Letter of Intent"), with FedTech Services, Inc., a private company organized under the laws of the State of Delaware ("FedTech"). In accordance with the terms and provisions of the Letter of Intent: (i) we were to enter into a share exchange agreement with FedTech pursuant to which the shareholders of FedTech (the "FedTech Shareholders") would tender all of the issued and outstanding shares of common stock of FedTech to us in exchange for the issuance by us of our shares of restricted common stock to the FedTech Shareholders representing an equity interest of 30% in us; (ii) FedTech would submit two general performance benchmarks pertaining to future equity ownership interest in us, which dates were to be June 30, 2014 whereby FedTech's gross revenue would be $4,000,000 ("June 2014 Benchmark") and August 31, 2014 whereby FedTech's gross revenue would be $6,000,000 ("August 2014 Benchmark"); and (iii) in the event the June 2014 Benchmark was exceeded by 35%, FedTech would not be required to meet the August 2014 Benchmark and the terms of the share exchange agreement would be deemed to have been met and final transfer of equity interest would have been made by the Company to TedTech Shareholders resulting in a 65% equity interest in us.
On January 31, 2014, we and FedTech mutually agreed to terminate the Letter of Intent.
CURRENT BUSINESS OPERATIONS
General
PetVivo is based in Minneapolis, Minnesota and is an emerging biomedical device company focusing on the licensing and commercialization of innovative therapeutic medical devices for pets and other animals. Using its licensed patented technology, management intends to leverage developed advances in human bio-materials and the medical device industry to profitably commercialize and market healthcare treatments for pets and other animals.
Our strategy is to in-license proprietary products from human medical device companies specifically for use in pets. A key component of this strategy is the accelerated timeline to revenues for veterinary medical devices, which enter the market much earlier than the more stringently regulated pharmaceuticals. We have secured exclusive rights to our first product, an osteoarthritis medical device, which has been shown to be both safe and efficacious. We believe the administration of their initial therapeutic devices exceeds the benefits of those found in current remedies. Therefore, management believes that the commercialization of our initial therapeutic devices will provide veterinarians and pet owners safe, effective, and long-lasting treatments to improve the pet’s quality of life.
We intend to license and distribute devices and therapeutics that are being developed for human use and license them for distribution in the veterinary markets. Management believes that this allows us to leverage the investment in the human biopharmaceutical industry to bring therapeutics to pets in a capital and time efficient manner. We can leverage the research capital expended to develop human clinical products and commercialize these products in the veterinary markets that offer a reduced regulatory environment and condensed timeline to product revenues. We anticipate building a portfolio of devices and therapeutics that we can distribute on an exclusive basis into the veterinary market.
We have licensed protein-based biomaterials for the treatment of pain and inflammation associated with osteoarthritis in canine and equine animals. We believe that our treatment is superior to current methodology of using NSAID’s. NSAID’s have many side effects in canines and our treatment has less to none of the side effects. Based on the market studies discussed below, management believes that there are opportunities to expand in the bovine and feline market.
6
Gel-Del License Agreement
Our wholly-owned subsidiary, PetVivo, entered into that certain exclusive license agreement and manufacturing and supply agreement dated August 2, 2013 (the "License Agreement") with Gel-Del Technologies, Inc. ("Gel-Del Technologies") pertaining to the manufacture and supply of products by Gel-Del Technologies derived from certain technology, including protein-based biomaterials and devices, which are beneficial for the veterinary treatment of animals having orthopedic joint afflictions (the "Technology"). In accordance with the terms and provisions of the License Agreement, Gel Del Technologies granted to us an exclusive, sub-licensable license to the Technology, which includes confidential information, trade secrets and other know-how that is proprietary to Gel-Del Technologies, in the entire world to: (a) use, distribute, sell, order to sell and have sold products in the field, which term is defined as the use of injected particulate substances for orthopedic veterinary treatments of the joints, which substance is either certain products or a substance that may require an FDA submission related to the products that include one or more drugs (the "Field"); (b) practice methods of use related to the use of injected particulate substances for orthopedic veterinary treatments of the joints covered by the Technology and utilizing products in the Field; and (c) otherwise to commercialize and exploit products in the Field. The license granted to us under the terms of the License Agreement shall include the right to grant sublicenses. Gel-Del Technologies further agreed to provide to us writings, drawings and materials that document the Technology, including copies of any patents, patent applications and documents representing embodiments of the Technology. Gel-Del Technologies will also provide reasonable explanation and assistance to use to allow us to understand the inventions covered by the Technology.
Gel-Del Technologies shall be responsible for the design, development and manufacture of the injected particles utilized in the licensed products in the Field and will consider and implement recommendations provided by the Company. The Company shall be responsible for the commercialization of the licensed products in the Field and will also consider and implement recommendations provided by Gel-Del. Gel-Del shall be responsible for obtaining any regulatory approvals necessary of the Company's commercial sale of the licensed products in the Field, including any pre-clinical and clinical studies. All regulatory approvals for the licensed products will be in the name of Gel-Del Technologies and owned by Gel-Del Technologies. The company shall be responsible for payment of all Gel-Del Technologies and third party fees, costs and expenses related to obtaining, if necessary, any and all regulatory approvals.
In further accordance with the terms and provisions of the License Agreement, the Company shall pay to Gel-Del Technologies a commercialization fee in the amount of $1,500,000 (the "Commercialization Fee") to establish the infrastructure to produce products for the North American market to be paid as follows: (i) $100,000 to be paid within thirty days of execution of the License Agreement; and (ii) $1,400,000 to be paid within five business days after the date the License Agreement has been approved by the respective boards of directors and the Company receives an amount greater than $3,000,000 from one or more qualified investors pursuant to a private placement offering within 120 days of execution of the License Agreement. The Commercialization Fee shall be amortized over the sale of the first 150,000 syringes that include two cc of injected particles (the "Initial Purchase") by reducing the per syringe price of $68.00 to $63.00. Upon acquisition and payment of the Initial Purchase by the Company, the price per syringe shall revert back to $68.00 per syringe. The Company shall also pay to Gel-Del Technologies an initial license fee as partial compensation for the grant of the license hereunder by issuing to Gel-Del Technologies a designated number of shares of the Company equaling 9.95% of the Company at the time of execution of the License Agreement. The Company will notify Gel-Del Technologies at least six months in advance of distributing in countries and/or territories outside of North America in order to provide Gel-Del Technologies an opportunity to develop infrastructure to market licensed products for such countries/regions. The Company will negotiate with Gel-Del Technologies a foreign commercialization fee for each additional foreign country/region in an amount not to exceed $2,500,000 for each elected foreign country/region. Lastly, the Company shall pay to Gel-Del Technologies a royalty equal to a percentage of net sales of licensed products, including the Technology sold by the Company for use in the above-specified Field. The Company shall pay Gel-Del Technologies a royalty of 6% of the Company's net sales of all licensed products.
7
Gel-Del Technologies is a biomaterial and medical device manufacturing company based in St. Paul, Minnesota. Through the License Agreement, we wish to commercialize their technology in the veterinary field for the treatment of osteoarthritis. Gel-Del Technologies has also successfully completed a pivotal clinical trial using their novel thermoplastic biomaterial as a dermal filler for human cosmetic applications. Gel-Del Technologies’ core competencies are developing and manufacturing medical devices containing its proprietary thermoplastic protein-based biomaterials that mimic the body's tissue to allow integration, tissue repair, and regeneration for long-term implantation. These biomaterials are produced using a patented and scalable self- assembly production process. The inherent thermoplastic properties of these biomaterials are then utilized to manufacture or coat implantable devices.
Amendment to License Agreement
On November 25, 2013, our wholly-owned subsidiary, PetVivo, entered into that certain amendment to the License Agreement (the "Amendment"), pursuant to which the payment terms of the Commercialization Fee was amended as follows: (i) $100,000 to be paid within 90 days of execution of the License Agreement; and (ii) $1,400,000 to be paid after the Company receives an amount greater than $2,000,000 from one or more qualified investors pursuant to a private placement offering within 180 days of execution of the License Agreement.
Moreover, Gel-Del Technologies granted to the Company an option until September 30, 2014 for the right to purchase all of the assets of Gel-Del Technologies (the "Asset Purchase Option"). In the event that the Asset Purchase Option is exercised, the Company shall issue shares of common stock to Gel-Del in an amount which shall be the greater of: (i) stock equal to a value of $27,000,000; (ii) or 22% of the total stock issued by the Company upon the completion of the merger of PetVivo and Gel-Del Technologies. The Company shall also receive a right of offer upon Gel-Del Technologies receiving any detailed inquiry and/or offer for the purchase and/or license of all or a portion of the Gel-Del Technologies company and/or technology if received by Gel-Del Technologies prior to the Company and Gel-Del Technologies completing the close of the Asset Purchase Option. In the event the Asset Purchase Option is exercised, Gel-Del Technologies shall be granted the right to select two members of the seven member Board of Directors of the Company. The Company shall negotiate and offer a three year renegotiable employee contract to David Masters as president and Randall Meyer as chief financial officer or chief operating officer.
Option to Extend License Agreement Terms
On February 7, 2014, our wholly-owned subsidiary, PetVivo, and Gel-Del Technologies entered into that certain option to extend license agreement terms (the "Extension Agreement"), which provided that the extension of the license shall be for three months beginning February 3, 2014 and ending May 2, 2014 with an optional additional cure period of thirty days. In the event a formal agreement with a retail investment banking firm is received prior to February 28, 2014, the deadline will be extended to June 2, 2014. In further accordance with the terms and provisions of the Extension Agreement, the Company shall provide Gel-Del Technologies the following: (i) $10,000 payment for execution of the Extension Agreement; (ii) transfer of 5,000 shares of PetVivo from John Lai to Gel-Del Technologies upon execution of the Extension Agreement; (iii) transfer of additional 5,000 shares of PetVivo from John Lai to Gel-Del Technologies upon initiation of the extension; (iv) no amortization of the Commercialization Fee; (v) transfer of 10,000 shares of PetVivo from John Lai to other founders if a funding source other than initiated by John Lai is used to pay all or a portion of the Commercialization Fee; (vi) Commercialization Fee increases from $1,500,000 to $1,600,000 as of February 2, 2014; (vii) product must be purchased quarterly and orders cannot be less than $350,000 and first order is commensurate with PetVivo funding; (viii) upon receipt of funding, Gel-Del Technologies will receive a minimum of 50% up to the total amount of the Commercialization Fee; (ix) for the extension period, PetVivo will be responsible to Gel-Del Technologies for an increase of $100,000 per month to the Commercialization Fee, which can be offset by providing a monthly cash payment to keep the license costs from increasing on basis of $1.00 received for every $2.00 offset; and (x) deadline regarding Asset Purchase Option to extend the deadline to purchase Gel-Del Technologies for the greater of an additional 1% of shares of common stock or an additional $275,000 of shares of common stock of the Company for each additional month of extension up to an additional six months from September 31, 2014. The Extension Agreement was contingent upon the engagement of an institutional investment banking firm and/or a retail investment banking firm by March 15, 2014 to raise a minimum of $1,000,000 financing. Any shares of PetVivo held of record by John Lai were exchanged for shares of our common stock in accordance with the terms and provisions of the Share Exchange Agreement; thus, the provisions of the Extension Agreement relate to shares of the Company's common stock. .
8
Current Business Operations
We plan to commercialize our products in the United States through distributor relationships and complemented by the use of social media educating and informing the pet owners, and in Europe and rest of world through commercial partners.
We believe that the role of pets in the family has significantly evolved in recent years. Many pet owners consider their pets an important member of the family and they have been increasingly willing to spend money to maintain the health and quality of life in their pets. Pets are living longer and, as they do, are exhibiting many of the same signs and symptoms of disease as humans, such as arthritis, obesity, diabetes, cancer and heart disease.
We believe that the opportunities to expand into various animal disease categories using our business model of licensing late stage medical devices and therapeutics greatly enhances our risk/reward profile.
Our exclusively licensed first veterinary device from Gel-Del Technologies, the Gel-Del Particles, which are for injection and a joint cushioning treatment for animals suffering from osteoarthritis. Gel-Del Technologies is initially targeting the treatment towards dogs and horses. We anticipate expanding the treatment to cats and cattle after we gather additional data. Gel-Del Particles have been through a human trial and have been classified as a medical device. The FDA does not require submission of a 510(k) or formal pre-market approval for medical devices used in veterinary medicine. We anticipate initial commercial production and sales in early 2015. PetVivo anticipates selling through existing veterinary distributors.
Companion Animal Market
Over the last several decades, we believe the animal health market and industry has a strong component in the overall U.S. economy and is more resistant to economic cycles. The veterinary sector is as an attractive area to participate in the growth of the broader healthcare industry without reimbursement risk. Based on our best knowledge, U.S. consumers will spend an estimated $55 billion on pets this year—a number that has been growing at a pace of more than 5% over the past decade. Therapeutics constitutes a small portion of this market (less than $2 billion) but we believe it is poised to expand as pet care becomes more complex and companies launch new products for unmet needs. The growth in the U.S. companion animal market has been continuing to increase due to the increase in the number of pet owning households.
The American Pet Products Association (APPA) 2013-2014 National Pet Owners Survey indicates U.S. pet ownership reached record levels in 2012. Specifically, 68% of all U.S. households owned a pet in 2012, up from 62% in 2002. The number of pet owning households totaled 82.5 million, representing a 10-year CAGR of 2.5%. In 2012, dogs and cats were the most popular pet species, owned by 46.7% and 37.3% of U.S. households, respectively. APPA also reported that there were 83.3 million dogs (10-year CAGR of +2.5%) and 95.6 million cats (10-year CAGR of +2.1%) in the U.S. In comparison, the total U.S. human population increased at +0.9% CAGR over the last decade. APPA reported that 2.8% of U.S. households owned horses in 2012. According to the APPA the total number of horses owned by U.S. households increased to 8.3 million in 2012, a 5% increase over the previous APPA survey conducted two years earlier.
Osteoarthritis Market. Osteoarthritis, the most common inflammatory joint disease in both dogs and horses, is a progressive condition that is caused by a deterioration of joint cartilage. Over time the joint cartilage deterioration creates joint stiffness from mechanical stress resulting in inflammation, pain and loss of range of motion, which may be referred to as lameness. Osteoarthritis joint stiffness and lameness worsens with time from gradual cartilage degeneration and an ongoing loss of protective cushion and lubricity (i.e., loss of slippery padding). As there is no cure for osteoarthritis, the various treatment methods are focused on managing the related symptoms of pain and inflammation. Veterinarians recommend several treatments depending on the severity of the disease, including a combination of rest, weight loss, physical rehabilitation, and a regimen of pain and anti-inflammatory drugs (NSAIDs). Non-steroidal anti-inflammatory drugs (NSAIDS) are used to alleviate the pain and inflammation caused by OA, but long-term NSAIDS cause gastric problems. Moreover, NSAIDS do not treat the cartilage degeneration issue to halt or slow progression of the OA condition.
9
The prevalence of companion animal osteoarthritis is estimated through a variety of methods. In looking at the dog osteoarthritis incidence Spence Johnston’s article “Osteoarthritis. Joint anatomy, physiology, and pathobiology” is often cited, this article reports that 20% of all dogs over the age of one year suffer from osteoarthritis. Using this simple methodology, management has estimated that 20% of the total dog population is under age one.
83.3 million – 20% = 66.6 million x 20% with OA = 13.3 million dogs with OA in U.S.
Our osteoarthritis market data has been validated by a number of reports evaluating a new NSAID that is estimated to be ready for commercial sale by Aratana Therapeutics, Inc. (PETX) in 2016. Craig-Hallum’s July 22, 2013 institutional research report on Aratana Therapeutics estimates the U.S. dog osteoarthritis market at 16.6 million dogs. William Blair & Company, L.L.C. released a July 25, 2013 Equity Research report by Aratana Therapeutics that concluded that roughly 10% of dogs and cat suffer from osteoarthritis. (83.3 million dogs x 10% = 8.3 million dogs with OA) Stifel issued report on Aratana Therapeutics dated July 22, 2013 that estimated the osteoarthritis market to be 55% of dogs over the age of 10. This equates to a US market in 2014 of 7.1 million dogs with osteoarthritis.
Horse Osteoarthritis (Lameness)
The equine osteoarthritis is the most common cause of lameness in horses. The annual average costs for diagnosis and treatment of equine lameness $3,000 per horse, with downtime & homecare costs being much higher (Oke and McIlwraith, 2010). “The USDA National Economic Cost of Equine Lameness… in the United States” published by 1978 places the annual incidence of lameness at 8.5-13.7 lameness events/100 horses.
As noted previously the APPA reported the total number of horses owned by U.S. households increased to 8.3 million in 2012. A 2007 publication by Emily Kilby “The Demographics of the U.S. Equine Population” concludes the horse population for 9,464,200 in 2006 with racehorses being 9% of that population or 846,000 horses. The article “The Occurrence and Causes of Lameness and Laminitis in the U.S. Horse Population” estimates that 17% of racehorses and 5.4% of the rest of the horse population go lame annually. Based on the above assumptions we calculate that there are up to 611,658 new lame horses each year.
Distribution
Most U.S. veterinarians buy a majority of their equipment and supplies from a preferred distributor. More than 75% of veterinarians name Butler Schein Animal Health, Inc., Webster Veterinary Supply Inc. (recently acquired by Patterson), MWI, Midwest Veterinary Supply, Inc. or Victor Medical Company as their preferred distributor. Combined, these top tier distributors sell more than 85%, by revenue, of the products sold to companion animal veterinarians in the U.S. Butler, Webster and MWI are recognized by manufacturers, distributors and veterinarians as the pre-eminent national companion animal veterinary supply distributors in the US. There are no other distributors that provide equivalent levels of service to manufacturers and regularly visit veterinarians in as wide
a geographic area as Butler, Webster or MWI. Midwest and Victor are large, regional distributors, also with strong reputations for high-quality service. The above data in this paragraph was sourced from File No. 101 0023 at the U.S. Federal Trade Commission.
10
Our product distribution will leverage the existing supply chain and veterinary clinic and clinician relationships already established by these large distributors. We intend to support and supplement this distribution channel with regional business development & training representatives. Our business development representatives will provide product training to distribution representatives, veterinarians and other veterinary staff. In addition our representatives will exhibit at key veterinary conferences in addition to supporting ongoing case studies. All of these sales, distribution, marketing and education efforts will also be supported by both veterinarian and pet owner product education and treatment awareness campaigns that will be conducted utilizing a variety of social media tools. The unique nature and the anticipated benefits provided by our first product is expected to generate significant consumer response.
The details of the business channels and margins are outlined below. Gel-Del Technologies manufactures the product that is supplied to us in a pre-filled syringe in exchange for a purchase price and a six percent (6%) royalty based on our sales revenues.
Our primary distribution channel is through the existing stocking distributors who having operating typical margins between fourteen to sixteen percent. We have budgeted a twenty percent margin for our distributors and a full fifty percent margin for the veterinary hospitals. See the distribution pricing steps and margins below:
Our potential pricing and margin strategy is as follows:
|
Gel-Del 2ml Particles
|
Cost
|
6% Royalty
|
Total Cost
|
Sell Price
|
Margin
|
|||||||||||||||
|
PetVivo
|
$ | 68 | $ | 7.80 | $ | 75.80 | $ | 130 | 42 | % | ||||||||||
|
Vet Distributor
|
$ | 130 | $ | 162 | 20 | % | ||||||||||||||
|
Veterinary Clinic
|
$ | 162 | $ | 324 | 50 | % | ||||||||||||||
We plan to commercialize additional products in the future. Gel-Del Technologies has several potential products that may be of interest to us.
Gel-Del Particles
Orthopedic Joint Treatments
Gel-Del Particles
A treatment for joint pain, which is made of injected protein-based gel-particles. In vivo studies indicate that GDP gel-particles can easily be combined with synovial fluid in a rabbit knee to form a joint cushion, buffering the adjacent bones/cartilage where no damage was caused to the cartilage from replacing the synovial fluid. GDP shows an effectiveness to repair, reconstitute or remodel the tissue, cartilage, ligaments and/or bone and/or enhance the functionality of the joint (e.g. repair deteriorated components present in the joint to provide cushion or shock absorbing features to the joint and to provide joint lubricity)
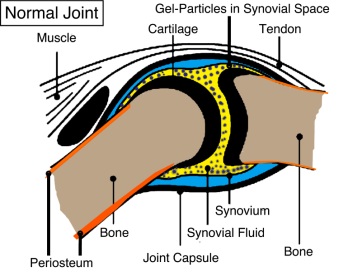
11
AppTec Laboratories accomplished a gel-particle rabbit study. In short, New Zealand white rabbits (6) were injected in both stifle joints (knees) to fill but not extend the synovial space (~0.5 cc GDP/site). Rabbits were tested every other day for abnormal clinical signs including range of motion and joint observations until sacrifice. Behavioral testing revealed no abnormal scores for range of motion, withdrawal response, or joint observations (all animals were 100% normal). At one week and at four weeks the animals were sacrificed. AppTec pathologists evaluated knee joint histology. The reported cartilage surfaces of the femoral and tibia condyles and the menisci were grossly and histologically 100% normal for all animals and test sites.
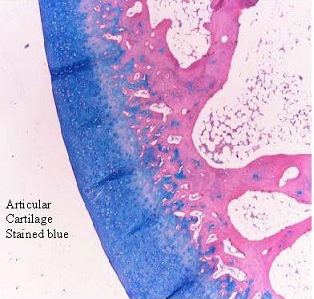
The test article was found in all of the injection sites. The test article did not cause changes in the articular cartilage of the femur or tibia when injected into the stifle joint of rabbits. The test article and control rabbit knees were not different for either 1 or 4 week time points for all histological measurements. In conclusion, Gel-Del Particles (GDP) do not cause inflammation or damage to knee joint and will stick to exposed tissues and biologically integrate with those tissues. GDP was not found to stick to articular cartilage in any sample.
Regenerative Characteristics
The Gel-Del Particles (GDP) for joint injections have been extensively studied for a broad range of applications including the treatment of wrinkles as a dermal filler. Here is an overview of the pre-clinical and clinical studies completed on CosmetaLife, which is the name used for Gel-Del Particles when they used as a dermal filler. The clinical trial for CosmetaLife was conducted by Cosmeta Corp a wholly owned subsidiary of Gel-Del Technologies the manufacturer or our GDP.
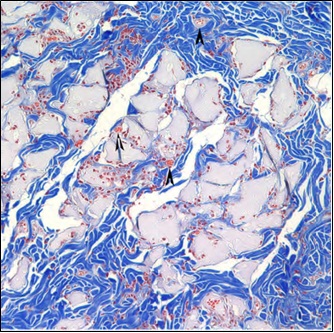 |
Gel-Del Particles
CosmetaLife (GDP) Integration after 12 Weeks
The image at left shows collagen in blue, fibroblasts in red and CosmetaLife (GDP) in gray. Note the typical cellularization and integration of collagen within the CosmetaLife matrix perimeter. Also notice the fibroblasts (collagen producers) are integrated throughout the injection site. Microvascularization, indicated by arrowheads, is also present in several locations. There is little to no sign of inflammation.
Trichrome Stain - 20x Objective
CosmetaLife (GDP) Particles
|
12
CosmetaLife is an easy-to-inject, water-protein-based dermal filler that not only fills nasolabial wrinkle depressions but also helps rejuvenate the dermal tissues, counteracting damage that causes wrinkles. The dermal cells are attracted to the CosmetaLife gel-particles, attach to them, and then slowly replace them with natural dermal material (extracellular matrix). The natural biological replacement process of CosmetaLife to collagen is estimated to take 6-12 months. Cosmeta’s clinical trial on nasolabial folds supports this estimate. According to current scientific thought, the resulting natural extracellular matrix, comprised mostly of collagen, is estimated to last 10-16 years.
CosmetaLife injections allow the body to create more natural dermal structure in and around every particle. Enhancing the natural process of dermal tissue construction with CosmetaLife allows for long-term dermal contouring, corrections, and rejuvenation with little to no adverse side effects noted in clinical trials.
Gel-Del Particles Clinical Studies. Cosmeta has conducted several biocompatibility animal studies. In the implantation study, no abnormal clinical signs were noted for any of the rabbits. The results of the sensitization study in guinea pigs showed a sensitization response equivalent to the negative controls.
The results of the histological report on the rabbit skin biopsies clearly demonstrate structural integration of Gel-Del particles into the host tissues by week 12. Evaluators observed Gel-Del particle material integration with normal tissue, remodeled and/or new collagen, and fibroblasts throughout the injected particles, mild to no inflammation, and new collagen-matrix production.
An FDA/IDE approved human clinical trial was begun with CosmetaLife late in 2006. The trial was a randomized, double blind, parallel assignment, multi-center comparison of the safety and efficacy of CosmetaLife versus Restylane® (Control) for the correction of nasolabial folds. One hundred seventy-one patients were skin tested and 145 were treated at six sites. The number of study exits after treatment totaled four subjects. This study was reported and published at www.clinicaltrial.gov (NCT00414544).
The feedback from physician investigators has been positive with respect to CosmetaLife injection qualities, cosmetic appearance, and its feel to the touch. During the first three to four months of the study, CosmetaLife showed no decrease in efficacy, as compared to Restylane that showed an 11 percent decrease in efficacy. The FDA/IDE approved human clinical trial for the CosmetaLife product through twelve months was found to be the same as compared to control hyaluronic acid product, Restylane (For each interval the consensus of the blinded subjects tested preferred CosmetaLife or showed no preference at 3, 6, 9 and 12 months).
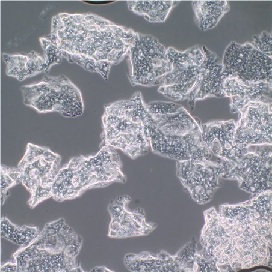 |
CosmetaLife (GDP) Particles, shown in figure to the left, were photographed from a light microscope under high magnification. GDP particles were immersed in a saline solution to help disperse them for better viewing. These particles are approximately 100 microns in size (0.1 mm in diameter). |
Gel-Del Technologies uses existing, scalable processes to reduce the infrastructure requirements and manufacturing risks to deliver a consistent high quality product while being responsive to volume requirements. Gel-Del is scaling the manufacturing process for Gel-Del Particles production, to date making batches in up to 2.0-kilogram quantities to near GMP (Good Manufacturing Practices) standards acceptable for human clinical trials.
13
Gel-Del Particles Safety Study. Patients injected with GDP (CosmetaLife) were found to have no or mild inflammatory, irritation, or immunogenic responses. These results suggest GDP is biocompatible because it closely matches the skin structure, composition, and moisture content. The no to low immunogenic responses are attributed to the tight cross-linking of the GDP matrix, which prevents immunogenic progenitor cells from producing antibodies.
In the clinical trial, the incidence of possible reaction to a skin test was 2.55 percent, with only one subject showing a reaction to a second test or 0.6%, (1 out of 171). We also have a study report by AppTec, Inc., our Contract Research Organization, that GDP (CosmetaLife) did not produce an antibody response during the clinical trial further supporting our belief that GDP is safe to use.
Gel-Del Particles are composed of materials that approximately meet the Generally Regarded As Safe (GRAS) requirements of the FDA. GDP contains materials from certified bovine and porcine tissue sources that do not harbor prion disease or BSE. Additionally, steps in the manufacturing process have been validated for deactivating all viruses.
Extrusion force testing and the Clinical Trial usage both demonstrate the consistent and easy injection of GDP.
Twenty-five month stability testing shows that GDP is stable at room temperature conditions. Moreover, GDP has been shown to be stable at 40 °C (104 °F) conditions for at least 3 months.

Competition
The development and commercialization of new animal health medicines is highly competitive, and we expect considerable competition from major pharmaceutical, biotechnology and specialty animal health medicines companies. As a result, there are, and likely will continue to be, extensive research and substantial financial resources invested in the discovery and development of new animal health medicines. Our potential competitors include large animal health companies, such as Zoetis, Inc.; Merck Animal Health, the animal health division of Merck & Co., Inc.; Merial, the animal health division of Sanofi S.A.; Elanco, the animal health division of Eli Lilly and Company; Bayer Animal Health, the animal health division of Bayer AG; NAH, the animal health division of Novartis AG; Boehringer Ingelheim Animal Health, the animal health division of Boehringer Ingelheim GmbH; Virbac Group; Ceva Animal Health; Vetoquinol and Dechra Pharmaceuticals PLC. We are also aware of several smaller early stage animal health companies, such as Kindred Bio, Aratana Therapeutics Inc., NextVet and VetDC that are developing products for use in the pet therapeutics market.
Intellectual Property and License Agreements
The Gel-Del Particles device licensed by us is protected by Gel-Del Technologies’ intellectual property portfolio that is comprised of patents, patent applications and trade secrets. In addition to the United States patent and patent applications listed below, the Gel-Del Particles also have pending patents in key markets around the world including Canada, Australia and the European Union.
| Patent No. US 8,153,591 | ||
| Patent App. No. US 13/435,839 | Filed: | 03/30/12 |
| Patent App. No. US 12/344,361 | Filed: | 12/26/08 |
14
We will seek to protect our products and technologies through a combination of patents, regulatory exclusivity, and proprietary know-how. Our goal is to obtain, maintain and enforce patent protection for our products, formulations, processes, methods and other proprietary technologies, preserve our trade secrets, and operate without infringing on the proprietary rights of other parties, both in the United States and in other countries. Our policy is to actively seek to obtain, where appropriate, the broadest intellectual property protection possible for our current compounds and any future compounds for development, proprietary information and proprietary technology through a combination of contractual arrangements and patents, both in the United States and abroad. However, even patent protection may not always afford us with complete protection against competitors who seek to circumvent our patents.
We depend upon the skills, knowledge and experience of our scientific and technical personnel, including those of Gel-Del Technologies, as well as that of our advisors, consultants and other contractors, none of which is patentable. To help protect our proprietary know-how, which is not patentable, and inventions for which patents may be difficult to obtain or enforce, we rely on trade secret protection and confidentiality agreements to protect our interests. To this end, we generally require all of our employees, consultants, advisors and other contractors to enter into confidentiality agreements that prohibit the disclosure of confidential information and, where applicable, require disclosure and assignment to us of the ideas, developments, discoveries and inventions important to our business
ITEM 1A. RISK FACTORS
An investment in our securities involves a high degree of risk. You should carefully consider the risks described below together with all of the other information included in this report before making an investment decision with regard to our securities. If any of the following risks actually occurs, our business, financial condition, and/or results of operations could be harmed. In that case, the trading price of our common stock could decline, and you may lose all or part of your investment. You should only purchase our securities if you can afford to suffer the loss of your entire investment.
RISKS RELATED TO OUR BUSINESS
We have a limited operating history upon which an evaluation of our prospects can be made.
We were incorporated in March 2009. Our lack of operating history makes an evaluation of our business and prospects very difficult. Our prospects must be considered speculative, considering the risks, expenses, and difficulties frequently encountered in the establishment of a new business. We cannot be certain that our business will be successful or that we will generate significant revenues. As of the date of this Annual Report, we have not commenced business operations involving the marketing and sale and distribution of the Gel-Del Technology products. We may never be successful in developing a market for the Gel-Del Technology products and thus may never become profitable. Therefore, our ability to operate our business successfully remains untested. If we are successful in marketing the Gel-Del Technology products, we anticipate that we will retain future earnings, if any, and other cash resources for the future operation and development of our business as appropriate. We do not currently anticipate declaring or paying any cash dividends in the foreseeable future. Payment of any future dividends is solely at the discretion of our board of directors, which will take into account many factors including our operating results, financial conditions and anticipated cash needs. For these reasons, we may never achieve profitability or pay dividends.
We anticipate that our ability to generate revenues in the foreseeable future will depend on the successful development and commercialization of the Gel-Del Technology products. If we are not successful in commercializing the products or are significantly delayed or limited in doing so, our business will be materially adversely affected and we may need to curtail or cease operations.
Because we are a development stage company, we have no revenues to sustain our operations.
We are a development stage company that is currently developing our business. To date, we have not generated revenues. The success of our business operations will depend upon our ability to obtain customers and provide quality products to those customers. We are not able to predict whether we will be able to develop our business and generate revenues. If we are not able to complete the successful development of our business plan, generate revenues and attain sustainable operations, then our business will fail.
15
We have incurred a net loss since inception and expect to incur net losses for the foreseeable future.
As of March 31, 2014, our net loss since inception was $24,661,847. We expect to incur operating and capital expenditures for the next year and, as a result, we expect significant net losses in the future. We will need to generate significant revenues to develop our business and expand our operations. We may not be able to generate sufficient revenues to achieve profitable operations.
We will need to raise additional capital to meet our requirements under the License Agreement and to subsequently market the Gel-Del products and expand our operations. Our failure to raise additional capital will significantly affect our ability to fund our proposed activities.
We are currently not engaged in any sophisticated marketing program to market our products because we lack capital and revenues to justify the expenditure. In addition, our available funds will not fund our activities for the next twelve months. If we fail to raise additional funds, investors may lose their entire cash investment.
Our future capital requirements depend on many factors, including, but not limited to:
|
•
|
the results of our target animal studies for our current and future product candidates;
|
|
•
|
the amount and timing of our payments required under the License Agreement, including royalties;
|
|
•
|
the timing of, and the costs involved in, obtaining regulatory approvals for any of our current or future product candidates;
|
|
•
|
the upfront and other payments, and associated costs, related to the License Agreement;
|
|
•
|
the number and characteristics of the product candidates we pursue;
|
|
•
|
the scope, progress, results and costs of researching and developing any of our current or future product candidates and conducting target animal studies;
|
|
•
|
whether we acquire any other companies, assets, intellectual property or technologies in the future;
|
|
•
|
the cost of commercialization activities, if any of our current or future product candidates are approved for sale, including marketing, sales and distribution costs;
|
|
•
|
the cost of manufacturing our current and future product candidates and any products we successfully commercialize;
|
|
•
|
our ability to establish and maintain strategic collaborations, licensing or other arrangements and the financial terms of such agreements;
|
|
•
|
the expenses needed to attract and retain skilled personnel;
|
|
•
|
the costs associated with being a public company; and
|
|
•
|
the costs involved in preparing, filing, prosecuting, maintaining, defending and enforcing patent claims, including litigation costs and the outcome of such litigation.
|
Additional funds may not be available when we need them on terms that are acceptable to us, or at all. If adequate funds are not available to us on a timely basis, we may be required to delay, limit, reduce or terminate:
|
•
|
our target animal studies or other development activities for our current or future product candidates;
|
|
•
|
our establishment of sales and marketing capabilities or other activities that may be necessary to commercialize any of our current or future product candidates; or
|
|
•
|
our in-licensing and acquisition efforts and expansion of our product portfolio.
|
16
Although we have one conditionally approved products, we are substantially dependent on the success of our current product candidates.
We currently have no products approved for commercial distribution, except the Gel-Del Particle, which has received conditional licenses from the USDA. To date, we have invested nearly all of our efforts and financial resources in the in-licensing, research and development of the Gel-Del Particle which subsequent to the License Agreement, is our only product candidate and still in development.
Our near-term prospects, including our ability to finance our company and to enter into strategic collaborations and generate revenue, will depend heavily on the successful development and commercialization of our current product candidates. The development and commercial success of our current product candidates will depend on a number of factors, including the following:
|
•
|
timely initiation and completion of our target animal studies for our current product candidates, which may be significantly slower than we currently anticipate and will depend substantially upon the satisfactory performance of third-party contractors;
|
|
•
|
our ability to demonstrate to the satisfaction of the CVM, the USDA and the European Medicines Agency, or EMA, or the applicable EU Member State national competent authorities, the safety and efficacy of our product candidates and to obtain regulatory approval in the United States and Europe;
|
|
•
|
our success in educating veterinarians and pet owners about the benefits, administration and use of our product candidates;
|
|
•
|
the prevalence and severity of adverse side effects, including a continued acceptable safety profile of the product following approval;
|
|
•
|
achieving and maintaining compliance with all regulatory requirements applicable to our product candidates;
|
|
•
|
the availability, perceived advantages, relative cost, relative safety and relative efficacy of alternative and competing treatments;
|
|
•
|
the effectiveness of our marketing, sales and distribution strategy and operations;
|
|
•
|
the ability of our third-party manufacturers to manufacture supplies of any of our current or future product candidates and to develop, validate and maintain commercially viable manufacturing processes that are compliant with current Good Manufacturing Practices, or cGMP;
|
|
•
|
our ability to successfully launch commercial sales of our current product candidates, assuming necessary approvals are obtained, whether alone or in collaboration with others;
|
|
•
|
our ability to enforce our intellectual property rights in and to our product candidates and avoid third-party patent interference, third-party initiated and U.S. PTO-initiated administrative patent proceedings or patent infringement claims; and
|
|
•
|
acceptance of our product candidates as safe and effective by veterinarians, pet owners and the animal health community.
|
Many of these factors are beyond our control. Accordingly, we cannot assure you that we will ever be able to generate revenue through the sale of our product candidates. If we are not successful in commercializing one or more of our product candidates, or are significantly delayed in doing so, our business will be materially harmed and the value of your investment could substantially decline.
17
We may be unable to obtain regulatory approval for our existing or future Gel-Del Particles product candidates under applicable regulatory requirements. The denial or delay of any such approval would delay commercialization efforts and adversely impact our potential to generate revenue, our business and our results of operations.
Our Gel-Del Particles product candidates are in various stages of development, and our business currently depends entirely on their successful development, regulatory approval and commercialization. We currently have no products approved for sale and we may never obtain regulatory approval to commercialize any of our other current or future product candidates. The research, testing, manufacturing, labeling, approval, sale, marketing and distribution of pet therapeutics products are subject to extensive regulation by the CVM, the USDA, the EMA and other regulatory authorities in the United States and other countries, whose regulations differ from country to country. We are not permitted to market our products in the United States until we receive approval of a New Animal Drug Application, or NADA, from the CVM or a full product license from the USDA with respect to our biologic products, or in Europe until we receive approval from the European Commission or applicable EU State national competent authorities.
Even if we receive approval of an NADA, USDA product license or foreign regulatory filing for our product candidates, the CVM, the USDA or the applicable foreign regulatory body may approve our product candidates for a more limited indication than we originally requested, and the CVM or the USDA may not approve the labeling that we believe is necessary or desirable for the successful commercialization of our product candidates. Any delay in obtaining, or inability to obtain, applicable regulatory approval would delay or prevent commercialization of our product candidates and would materially adversely impact our business and prospects.
Even if our current or future product candidates obtain regulatory approval, they may never achieve market acceptance or commercial success.
Even if we obtain CVM, USDA, EMA or other regulatory approvals, our current or future product candidates may not achieve market acceptance among veterinarians and pet owners, and may not be commercially successful. Market acceptance of any of our current or future product candidates for which we receive approval depends on a number of factors, including:
|
•
|
the safety of our products as demonstrated in our target animal studies;
|
|
•
|
the indications for which our products are approved;
|
|
•
|
the acceptance by veterinarians and pet owners of the product as a safe and effective treatment;
|
|
•
|
the proper training and administration of our products by veterinarians;
|
|
•
|
the potential and perceived advantages of our product candidates over alternative treatments, including generic medicines and products approved for use by humans that are used off label;
|
|
•
|
the cost of treatment in relation to alternative treatments and willingness to pay for our products, if approved, on the part of veterinarians and pet owners;
|
|
•
|
the willingness of pet owners to pay for our treatments, relative to other discretionary items, especially during economically challenging times;
|
|
•
|
the relative convenience and ease of administration;
|
|
•
|
the prevalence and severity of adverse side effects; and
|
|
•
|
the effectiveness of our sales and marketing efforts and those of our collaborators.
|
Any failure by our product candidates that obtain regulatory approval to achieve market acceptance or commercial success would adversely affect our financial results.
18
We may not realize all of the anticipated benefits of our contractual arrangement with Gel-Del Technologies or those benefits may take longer to realize than expected. We may also encounter significant unexpected difficulties in integrating these businesses.
Our ability to realize the anticipated benefits of our contractual relations with Gel-Del Technologies will depend in part on our ability to integrate its business with ours. The combination of independent businesses is a complex, costly and time-consuming process. As a result, we will be required to devote significant management attention and resources to integrating the business practices and operations of Gel-Del Technologies. The integration process may disrupt the businesses and, if implemented ineffectively, would preclude realization of the full benefits expected by us. Our failure to meet the challenges involved in integrating the businesses to realize the anticipated benefits of the transaction could cause an interruption of, or a loss of momentum in, our activities and could adversely affect our results of operations.
In addition, the overall integration of the businesses may result in material unanticipated problems, expenses, liabilities, competitive responses, and diversion of management’s attention. The difficulties of combining the operations of the companies include, among others:
|
•
|
the diversion of management’s attention to integration matters;
|
|
•
|
difficulties in achieving anticipated cost savings, synergies, business opportunities and growth prospects from combining the business of Gel-Del Technologies with our company;
|
|
•
|
difficulties in the integration of operations and systems;
|
|
•
|
difficulties in the assimilation of employees; and
|
|
•
|
challenges in attracting and retaining key personnel.
|
Many of these factors will be outside of our control and any one of them could result in increased costs and diversion of management’s time and energy, which could materially impact our business, financial condition and results of operations. In addition, even if the operations of the businesses are integrated successfully, we may not realize the full benefits of the transaction, including the synergies or growth opportunities that we expect. These benefits may not be achieved within the anticipated time frame, or at all.
Development of pet therapeutics involves an expensive and lengthy process with an uncertain outcome, and results of earlier studies may not be predictive of future study results.
Development of pet therapeutics is expensive and can take many years to complete, and its outcome is inherently uncertain. To gain approval to market a pet therapeutic for a particular species of pet, we must provide the CVM, the USDA or foreign regulatory authorities, as applicable, with data from animal safety and effectiveness studies that adequately demonstrate the safety and efficacy of that product in the target animal for the intended indication applied for in the NADA, product license or other regulatory filing. We rely on contract research organizations, or CROs, and other third parties to ensure the proper and timely conduct of our studies and development efforts and, while we have agreements governing their committed activities, we have limited influence over their actual performance. Failure can occur at any time during the development process. Success in prior target animal studies or in the treatment of human beings with a product candidate does not ensure that our target animal studies will be successful and the results of development efforts by other parties may not be indicative of the results of our target animal studies and other development efforts. Product candidates in our studies may fail to show the desired safety and efficacy despite showing such results in initial data or previous human or animal studies conducted by other parties. Even if our studies and other development efforts are completed, the results may not be sufficient to obtain regulatory approval for our product candidates.
19
Once our target animal studies commence, we may experience delays in such studies and other development efforts and we do not know whether planned studies will begin on time, need to be redesigned or be completed on schedule, if at all. Pet therapeutics studies can be delayed or discontinued for a variety of reasons, including delay or failure to:
|
•
|
reach agreement on acceptable terms with prospective CROs and study sites, the terms of which can be subject to extensive negotiation and may vary significantly among different CROs and trial sites;
|
|
•
|
complete target animal studies due to deviations from study protocol;
|
|
•
|
address any safety concerns that arise during the course of testing;
|
|
•
|
address any conflicts with new or existing laws or regulations;
|
|
•
|
add new study sites; or
|
|
•
|
manufacture sufficient quantities of formulated drug for use in studies.
|
If we experience delays in the completion of, or terminate any development efforts for our product candidates, the commercial prospects of our product candidates will be harmed, and our ability to generate product revenues from any of these product candidates will be delayed. In addition, any delays in completing our development efforts will increase our costs, slow down our product candidate development and approval process and jeopardize our ability to commence product sales and generate revenues. Any of these occurrences may harm our business, financial condition and prospects significantly. In addition, many of the factors that cause, or lead to, a delay in the commencement or completion of our development efforts may also ultimately lead to the denial of regulatory approval of our product candidates.
Our product candidates, if approved, will face significant competition and our failure to effectively compete may prevent us from achieving significant market penetration.
The development and commercialization of pet therapeutics is highly competitive, and we expect considerable competition from major pharmaceutical, biotechnology and specialty animal health medicines companies. As a result, there are, and will likely continue to be, extensive research and substantial financial resources invested in the discovery and development of new pet therapeutics. Our potential competitors include large animal health companies, such as Zoetis, Inc.; Merck Animal Health, the animal health division of Merck & Co., Inc.; Merial, the animal health division of Sanofi S.A.; Elanco, the animal health division of Eli Lilly and Company; Bayer Animal Health, the animal health division of Bayer AG; Boehringer Ingelheim Animal Health, the animal health division of Boehringer Ingelheim GmbH; Novartis Animal Health, the animal health division of Novartis AG; Virbac Group; Ceva Animal Health; Vetoquinol and Dechra Pharmaceuticals PLC. We are also aware of several smaller early stage animal health companies such as Kindred Bio, Aratana Therapeutics, NextVet and VetDC that are developing products for use in the pet therapeutics market.
We are an early-stage company with a limited history of operations and many of our competitors have substantially more resources than we do, including both financial and technical resources. In addition, many of our competitors have more experience than we have in the development, manufacture, regulation and worldwide commercialization of animal health medicines. We are also competing with academic institutions, governmental agencies and private organizations that are conducting research in the field of animal health medicines.
Our competition will be determined in part by the potential indications for which our products are developed and ultimately approved by regulatory authorities. Additionally, the timing of market introduction of some of our potential products or of competitors’ products may be an important competitive factor. Accordingly, the speed with which we can develop our compounds, complete target animal studies and approval processes, and supply commercial quantities to market are expected to be important competitive factors. We expect that competition among products approved for sale will be based on various factors, including product efficacy, safety, reliability, availability, price and patent position.
20
If we are not successful in identifying, licensing or acquiring, developing and commercializing additional product candidates, our ability to expand our business and achieve our strategic objectives would be impaired.
Although a substantial amount of our effort will focus on the continued development and potential approval of our current Gel-Del Particles product candidate, a key element of our strategy is to identify, license or acquire, develop and commercialize a portfolio of products to serve the pet therapeutics market. Even if we successfully identify and license further potential product candidates, we may still fail to yield product candidates for development and commercialization for many reasons, including the following:
|
•
|
competitors may develop alternatives that render our product candidates obsolete;
|
|
•
|
product candidates we develop may nevertheless be covered by third parties’ patents or other exclusive rights;
|
|
•
|
a product candidate may on further study be shown to have harmful side effects in pets or other characteristics that indicate it is unlikely to be effective or otherwise does not meet applicable regulatory criteria;
|
|
•
|
a product candidate may not be capable of being produced in commercial quantities at an acceptable cost, or at all; and
|
|
•
|
a product candidate may not be accepted as safe and effective by veterinarians, pet owners and the pet therapeutic community.
|
If we fail to develop and successfully commercialize other product candidates, our business and future prospects may be harmed and our business will be more vulnerable to any problems that we encounter in developing and commercializing our current and future product candidates.
If we fail to attract and keep senior management and key scientific personnel, we may be unable to successfully develop any of our current or future product candidates, conduct our in-licensing and development efforts and commercialize any of our current or future product candidates.
Our success depends in part on our continued ability to attract, retain and motivate highly qualified management and scientific personnel. We are highly dependent upon our senior management, as well as our senior scientists and other members of our senior management team. The loss of services of any of these individuals could delay or prevent the successful development of our current or future product pipeline, completion of our planned development efforts or the commercialization of our product candidates.
Competition for qualified personnel in the animal health fields is intense due to the limited number of individuals who possess the skills and experience required by our industry. We will need to hire additional personnel as we expand our development and commercial activities. We may not be able to attract and retain quality personnel on acceptable terms, or at all. In addition, to the extent we hire personnel from competitors, we may be subject to allegations that they have been improperly solicited or that they have divulged proprietary or other confidential information, or that their former employers own their research output.
We rely completely on Gel-Del Technologies to manufacture the supplies for the Gel-Del Particles development of our products.
With respect to our proposed products, we do not currently have, nor do we currently plan to acquire, the infrastructure or capability internally to manufacture the formulated Gel-Del Particles for use in the conduct of our target animal studies. We also lack the resources and the capability to manufacture any of our product candidates on a scale necessary for commercialization. Gel-Del Technologies may terminate the License Agreement if we fail to make an undisputed payment, if we breach a material provision of the agreement, or if Gel-Del Technologies ceases manufacture of the product.
21
The facilities used by our contract manufacturers to manufacture the active pharmaceutical ingredients and formulated drugs may be subject to inspections by the CVM, the USDA or the EMA that will be conducted after we submit our NADA to the CVM, and approval by the CVM, or during the USDA licensing process. We do not control the manufacturing processes used by, and we are completely dependent on, Gel-Del Technologies to comply with cGMP for the manufacture of both active pharmaceutical ingredients and finished drug products. If Gel-Del Technologies cannot successfully manufacture material that conforms to our specifications and is made in compliance with the strict regulatory requirements of the CVM, the USDA or other regulatory authorities, they will not be able to secure and/or maintain regulatory approval for their manufacturing facilities. In addition, we have no control over the ability of Gel-Del Technologies to maintain adequate quality control and quality assurance practices and to engage qualified personnel. If the CVM, the USDA or the EMA does not approve our contract manufacturers’ facilities used for the manufacture of our product candidates, or if it withdraws any such approval in the future, we may need to find alternative manufacturing facilities, which would adversely impact our ability to develop, obtain regulatory approval for or market our product candidates, if approved.
Furthermore, we and Gel-Del Technologies are continuing to refine and improve the manufacturing process for our product candidates, certain aspects of which are complex and unique. We may encounter difficulties with new or existing manufacturing processes, particularly if we seek to increase our manufacturing capacity significantly to support commercialization of our product candidates, if approved. Our reliance on contract manufacturers also requires us to provide trade secrets or other proprietary information to others engaged to make our drug products, increasing the possibility that our trade secrets or other proprietary information may be disclosed or misappropriated.
The commercialization of any of our Gel-Del Particles product candidates could be stopped, delayed or made less profitable if third-party manufacturers fail to provide us with sufficient quantities of drug product or fail to do so at acceptable quality levels or prices and in a timely manner.
To manufacture our product candidates in the quantities that we believe would be required to meet anticipated market demand, our third-party manufacturers may need to increase manufacturing capacity, which could involve significant challenges and may require additional regulatory approvals. In addition, the development of commercial-scale manufacturing capabilities may require us and our third-party manufacturers to invest substantial additional funds and hire and retain technical personnel who have the necessary manufacturing experience. Neither we nor our third-party manufacturers may successfully complete any manufacturing scale-up activities required to increase existing manufacturing capabilities in a timely manner, or at all.
The raw materials used to manufacture our products are generally readily available and can be obtained from multiple suppliers in commercial quantities. However, we rely on our contract manufacturers to obtain any raw materials necessary to manufacture our products, and we do not have any control over the process or timing of the acquisition of these materials. Furthermore, if there is a disruption to our or our third-party manufacturers’ relevant operations, we will have no other means of producing our product candidates until they restore the affected facilities or we or they procure alternative manufacturing facilities or raw materials. Additionally, any damage to or destruction of our third-party manufacturers’ facilities or equipment may significantly impair our ability to manufacture product candidates on a timely basis.
We currently rely on third parties to conduct all of our target animal studies and certain other development efforts. If these third parties do not successfully carry out their contractual duties or meet expected deadlines, we may be unable to obtain regulatory approval for or commercialize our current or future product candidates.
We currently do not conduct our target animal studies, and we rely on CROs to conduct these studies. The third parties with whom we contract for the execution of our studies play a significant role in the conduct of these studies and the subsequent collection and analysis of data. However, these third parties are not our employees, and except for contractual duties and obligations, we have limited ability to control the amount or timing of resources that they devote to our programs. Although we rely on these third parties to conduct our studies, we remain responsible for ensuring that each of our studies is conducted in accordance with the development plan and protocol. Moreover, the CVM, the USDA and EMA require us to comply with regulations and standards, commonly referred to as current good clinical practices, (“cGCPs”), or good laboratory practices, (“GLPs”), for conducting, monitoring, recording and reporting the results of our studies to ensure that the data and results are scientifically credible and accurate.
22
In addition, the execution of target animal studies and the subsequent compilation and analysis of the data produced requires coordination among various parties. In order for these functions to be carried out effectively and efficiently, it is imperative that these parties communicate and coordinate with one another. Moreover, these third parties may also have relationships with other commercial entities, some of which may compete with us. Many of our potential agreements with these third parties may be terminated by these third parties upon as little as 30 days’ prior written notice of a material breach by us that is not cured within 30 days. Many of these agreements may also be terminated by such third parties under certain other circumstances, including our insolvency or our failure to comply with applicable laws. In general, these agreements require such third parties to reasonably cooperate with us at our expense for an orderly winding down of services of such third parties under the agreements. If the third parties conducting our target animal studies do not perform their contractual duties or obligations, experience work stoppages, do not meet expected deadlines, terminate their agreements with us or need to be replaced, or if the quality or accuracy of the data they obtain is compromised due to the failure to adhere to our development protocols or cGCPs, or for any other reason, we may need to enter into new arrangements with alternative third parties, which could be difficult and costly, and our target animal studies may be extended, delayed or terminated or may need to be repeated. If any of the foregoing were to occur, the regulatory approval for and commercialization of the product candidate being tested in such studies may be delayed or require us to utilize additional resources.
We currently have no sales organization. If we are unable to establish sales capabilities on our own or through third parties, we may not be able to market and sell our current or future product candidates, if approved, or generate product revenue.
We currently do not have a sales organization. In order to commercialize any of our current or future product candidates in the United States and any jurisdictions outside the United States, we must build our marketing, sales, distribution, managerial and other non-technical capabilities or make arrangements with third parties to perform these services, and we may not be successful in doing so. We expect to establish a direct sales organization in the United States, complemented by distributors, to commercialize our product candidates, which will be expensive and time-consuming. Outside of the United States we intend to partner with companies with an established commercial presence to market our products in those locations. If we are unable to enter into such arrangements on acceptable terms or at all, we may not be able to successfully commercialize our current product candidates or any future product candidates that receive regulatory approval. We have no prior experience in the marketing, sale and distribution of pet therapeutics and there are significant risks involved in building and managing a sales organization, including our ability to hire, retain and motivate qualified individuals, generate sufficient sales leads, provide adequate training to sales and marketing personnel, and effectively oversee a geographically dispersed sales and marketing team. Any failure or delay in the development of our internal sales, marketing and distribution capabilities would adversely impact the commercialization of these products. If we are not successful in commercializing any of our current or future product candidates, either on our own or through collaborations with one or more distributors, our future product revenue will suffer and we would incur significant additional losses.
We will need to increase the size of our organization, and we may experience difficulties in managing growth.
We will need to continue to expand our managerial, operational, financial and other resources in order to manage our operations and target animal studies, continue our development activities and commercialize any of our current or future product candidates. Our management and personnel, systems and facilities currently in place may not be adequate to support this future growth. Our need to effectively execute our growth strategy requires that we:
|
•
|
manage our target animal studies and other development efforts effectively;
|
|
•
|
identify, recruit, maintain, motivate and integrate additional employees;
|
|
•
|
manage our internal development efforts effectively while carrying out our contractual obligations to third parties; and
|
|
•
|
continue to improve our operational, financial and management controls, reporting systems and procedures.
|
23
We are incurring significant costs as a result of operating as a public company, and our management is expected to devote substantial time to new compliance initiatives.
As a privately-held company, we were not required to comply with certain corporate governance and financial reporting practices and policies required of a publicly-traded company. As a publicly-traded company, we have incurred and will continue to incur significant legal, accounting and other expenses that we were not required to incur in the recent past, particularly after we are no longer an “emerging growth company” as defined under the Jumpstart Our Business Startups Act of 2012, or the JOBS Act. In addition, new and changing laws, regulations and standards relating to corporate governance and public disclosure, including the Dodd-Frank Wall Street Reform and Consumer Protection Act and the rules and regulations promulgated and to be promulgated thereunder, as well as under the Sarbanes-Oxley Act, the JOBS Act, and the rules and regulations of the U.S. Securities and Exchange Commission, or SEC, and The NASDAQ Global Market, have created uncertainty for public companies and increased our costs and time that our board of directors and management must devote to complying with these rules and regulations. We expect these rules and regulations to increase our legal and financial compliance costs and lead to a diversion of management time and attention from revenue-generating activities.
Furthermore, the need to establish the corporate infrastructure demanded of a public company may divert management’s attention from implementing our growth strategy, which could prevent us from improving our business, results of operations and financial condition. We have made, and will continue to make, changes to our internal controls and procedures for financial reporting and accounting systems to meet our reporting obligations as a publicly-traded company. However, the measures we take may not be sufficient to satisfy our obligations as a publicly-traded company.
For as long as we remain an “emerging growth company” as defined in the JOBS Act, we may take advantage of certain exemptions from various reporting requirements that are applicable to other public companies that are not “emerging growth companies.” These exemptions provide for, but are not limited to, relief from the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act, less extensive disclosure obligations regarding executive compensation in our periodic reports and proxy statements, exemptions from the requirements to hold a nonbinding advisory vote on executive compensation and stockholder approval of any golden parachute payments not previously approved and an extended transition period for complying with new or revised accounting standards. We may take advantage of these reporting exemptions until we are no longer an “emerging growth company.” We may remain an “emerging growth company” for up to five years. To the extent we are no longer eligible to use exemptions from various reporting requirements under the JOBS Act; we may be unable to realize our anticipated cost savings from those exemptions.
We are not currently required to evaluate our internal control over financial reporting pursuant to Section 404 of the Sarbanes-Oxley Act, and failure to achieve and maintain effective internal control over financial reporting in accordance with Section 404 of the Sarbanes-Oxley Act, when applicable, could have a material adverse effect on our business and share price.
As an emerging growth company, we are not required to evaluate our internal control over financial reporting in a manner that meets the standards of publicly-traded companies required by Section 404 of the Sarbanes-Oxley Act, or Section 404. We anticipate being required to meet these standards in the course of preparing our consolidated financial statements as of and for the year ended March 31, 2014 and our management will be required to report on the effectiveness of our internal control over financial reporting for such year. Additionally, under the recently enacted JOBS Act, our independent registered public accounting firm will not be required to attest to the effectiveness of our internal control over financial reporting pursuant to Section 404 of the Sarbanes-Oxley Act until we are no longer an “emerging growth company.” The rules governing the standards that must be met for our management to assess our internal control over financial reporting are complex and require significant documentation, testing and possible remediation.
24
A material weakness in internal control was identified in connection with the preparation of our financial statements and the audit of our financial results. In order to remedy the material weakness, we will need to implement resulting improvements in our internal controls. In connection with the implementation of the necessary procedures and practices related to internal control over financial reporting, we may identify deficiencies that we may not be able to remediate in time to meet the deadline imposed by the Sarbanes-Oxley Act for compliance with the requirements of Section 404. In addition, we may encounter problems or delays in completing the implementation of any requested improvements and receiving a favorable attestation in connection with the attestation provided by our independent registered public accounting firm. We will be unable to issue securities in the public markets through the use of a shelf registration statement if we are not in compliance with Section 404. Furthermore, failure to achieve and maintain an effective internal control environment could have a material adverse effect on our business and share price and could limit our ability to report our financial results accurately and timely.
Changes in distribution channels for pet therapeutics could negatively impact our market share, margins and distribution of our products.
In most markets, pet owners typically purchase their pet therapeutics directly from veterinarians. Pet owners increasingly could purchase pet therapeutics from sources other than veterinarians, such as Internet-based retailers, “big-box” retail stores or other over-the-counter distribution channels. This trend has been demonstrated by the significant shift away from the veterinarian distribution channel in the sale of parasiticides and vaccines in recent years. Pet owners also could decrease their reliance on, and visits to, veterinarians as they rely more on Internet-based animal health information. Because we expect to market our pet prescription products through the veterinarian distribution channel, any decrease in visits to veterinarians by pet owners could reduce our market share for such products and materially adversely affect our operating results and financial condition. In addition, pet owners may substitute human health products for pet therapeutics if human health products are deemed to be lower-cost alternatives.
Legislation has also been proposed in the United States, and may be proposed in the United States or abroad in the future, that could impact the distribution channels for our pet products. For example, such legislation may require veterinarians to provide pet owners with written prescriptions and disclosure that the pet owner may fill prescriptions through a third party, which may further reduce the number of pet owners who purchase their pet therapeutics directly from veterinarians. Such requirements may lead to increased use of generic alternatives to our products or the increased substitution of our products with other pet therapeutics or human health products if such other products are deemed to be lower-cost alternatives. Many states already have regulations requiring veterinarians to provide prescriptions to pet owners upon request and the American Veterinary Medical Association has long-standing policies in place to encourage this practice.
Over time, these and other competitive conditions may increase our reliance on Internet-based retailers, “big-box” retail stores or other over-the-counter distribution channels to sell our pet products. Any of these events could materially adversely affect our operating results and financial condition.
Consolidation of our customers could negatively affect the pricing of our products.
Veterinarians are our primary customers. In recent years, there has been a trend towards the concentration of veterinarians in large clinics and hospitals. If this trend towards consolidation continues, these customers could attempt to improve their profitability by leveraging their buying power to obtain favorable pricing. The resulting decrease in our prices could have a material adverse effect on our operating results and financial condition.
Generic products may be viewed as more cost-effective than our products.
We may face competition from products produced by other companies, including generic alternatives to any of our products. We will need to depend on patents to provide us with exclusive marketing rights for some of our products. The protection afforded, which varies from country to country, is limited by the scope and applicable terms of patents and the availability of legal remedies in the applicable country. As a result, we may face competition from lower-priced generic alternatives to many of our products. Generic competitors are becoming more aggressive in terms of pricing, and generic products are an increasing percentage of overall animal health sales in certain regions. In addition, private label products may compete with our products. If pet therapeutics customers increase their use of new or existing generic or private label products, our operating results and financial condition could be materially adversely affected.
25
Our pet therapeutics will be subject to unanticipated safety or efficacy concerns, which may harm our reputation.
Unanticipated safety or efficacy concerns can arise with respect to pet therapeutics, whether or not scientifically or clinically supported, leading to product recalls, withdrawals or suspended or declining sales, as well as product liability, and other claims. In addition, we depend on positive perceptions of the safety and quality of our products, and pet therapeutics generally, by our customers, veterinarians and end-users, and such concerns may harm our reputation. These concerns and the related harm to our reputation could materially adversely affect our operating results and financial condition, regardless of whether such reports are accurate.
If we fail to comply with our obligations under the License Agreement, we could lose license rights that are essential to our business.
We are party to the License Agreement with Gel-Del Technologies for our product candidates that are essential to our business. The License Agreement imposes various payment and performance obligations on us. If we fail to comply with these obligations, Gel-Del Technologies may have the right to terminate the License Agreement in which event we would not be able to develop or commercialize the Gel-Del Particles products. If we lose such license rights, our business, results of operations, financial condition and prospects would be adversely affected. We may enter into additional licenses in the future and if we fail to comply with obligations under those agreements, we could suffer adverse consequences.
We may not own any intellectual property rights we develop with respect to Gel-Del Particles or be able to share Gel-Del Technologies' patent rights with future collaborators.
Our License Agreement with Gel-Del Technologies contains certain obligations and restrictions on our ability to develop and commercialize the Gel-Del Particles. All of the intellectual property rights that we develop with respect to Gel-Del Particles will be owned by Gel-Del Technologies upon termination of the License Agreement. If we wish to enter into any collaboration agreements relating to Gel-Del Particles, Gel-Del Technologies has the right to approve all of our sublicenses. These restrictions may impair or delay our ability to engage third parties to commercialize the Gel-Del Particles.
Moreover, we may face claims from non-practicing entities, which have no relevant product revenue and against whom our own patent portfolio may thus have no deterrent effect.
In addition to infringement claims against us, if third parties have prepared and filed patent applications in the United States that also claim technology to which we have rights, we may have to participate in interference proceedings in the U.S. PTO to determine the priority of invention. Third parties may also attempt to initiate reexamination, post grant review or inter partes review of our patents in the U.S. PTO. We may also become involved in similar opposition proceedings in the European Patent Office or similar offices in other jurisdictions regarding our intellectual property rights with respect to our products and technology.
If our efforts to protect the proprietary nature of the intellectual property related to any of our current or future product candidates are not adequate, we may not be able to compete effectively in our market.
We will rely upon a combination of patents, trade secret protection, confidentiality and the License Agreement to protect the intellectual property related to our current product candidates and our development programs.
26
Composition-of-matter patents on the active pharmaceutical ingredient are generally considered to be the strongest form of intellectual property protection for pharmaceutical products, including pet therapeutics, as such patents provide protection without regard to any particular method of use or manufacture. Method-of-use patents protect the use of a product for the specified method. This type of patent does not prevent a competitor from making and marketing a product that is identical to our product for an indication that is outside the scope of the patented method. Moreover, even if competitors do not actively promote their product for our targeted indications, veterinarians may recommend that pet owners use these products off label, or pet owners may do so themselves. Although off-label use may infringe or contribute to the infringement of method-of-use patents, the practice is common and such infringement is difficult to prevent or prosecute. Method of manufacturing patents protect a specific way to make a product and do not prevent a third party from making the product by a different method and then using the product for our uses. We cannot be certain that the claims in our patent applications will be considered patentable by the U.S. PTO and courts in the United States, or by the patent offices and courts in foreign countries.
The strength of patents in the field of pet therapeutics involves complex legal and scientific questions and can be uncertain. The patent applications that we own or license may fail to result in issued patents in the United States or in other foreign countries. Even if the patents do successfully issue, third parties may challenge the validity, enforceability or scope thereof, which may result in such patents being narrowed, invalidated or held unenforceable. Furthermore, even if they are unchallenged, our patents and patent applications may not adequately protect our products or our intellectual property or prevent others from designing around our claims. If the breadth or strength of protection provided by the patents and patent applications we own, in-license or pursue with respect to any of our current or future product candidates is threatened, it could threaten our ability to commercialize any of our current or future product candidates. Further, if we encounter delays in our development efforts, the period of time during which we could market any of our current or future product candidates under patent protection would be reduced. Since patent applications in the United States and most other countries are confidential for a period of time after filing, we cannot be certain that we were the first to file any patent application related to our product candidates. Furthermore, for patent applications in which claims are entitled to a priority date before March 16, 2013, an interference proceeding can be provoked by a third party or instituted by the U.S. PTO to determine who was the first to invent any of the subject matter covered by the patent claims of our applications. For patent applications containing a claim not entitled to a priority date before March 16, 2013, there is a greater level of uncertainty in the patent law with the passage of the America Invents Act, which brings into effect significant changes to the U.S. patent laws that have yet to be well defined, and which introduces new procedures for challenging pending patent applications and issued patents. A primary change under this reform is creating a “first to file” system in the United States, which requires us to minimize the time from invention to filing of a patent application.
Even where laws provide protection, costly and time-consuming litigation could be necessary to enforce and determine the scope of our proprietary rights, and the outcome of such litigation would be uncertain. Moreover, any actions we may bring to enforce our intellectual property against our competitors could provoke them to bring counterclaims against us, and some of our competitors have substantially greater intellectual property portfolios than we have.
We will also rely on trade secret protection and confidentiality agreements to protect proprietary know-how that is not patentable, processes for which patents are difficult to enforce and any other elements of our product development processes that involve proprietary know-how, information or technology that is not covered by patents. Although we require all of our employees to assign their inventions to us, and endeavor to execute confidentiality agreements with all of our employees, consultants, advisors and any third parties who have access to our proprietary know-how, information or technology, we cannot be certain that we have executed such agreements with all parties who may have helped to develop our intellectual property or had access to our proprietary information, nor that our agreements will not be breached. We cannot guarantee that our trade secrets and other confidential proprietary information will not be disclosed or that competitors will not otherwise gain access to our trade secrets or independently develop substantially equivalent information and techniques. Further, the laws of some foreign countries do not protect proprietary rights to the same extent or in the same manner as the laws of the United States. As a result, we may encounter significant problems in protecting and defending our intellectual property both in the United States and abroad. If we are unable to prevent material disclosure of the intellectual property related to our technologies to third parties, we will not be able to establish or maintain a competitive advantage in our market, which could materially adversely affect our business, results of operations and financial condition.
Any disclosure to or misappropriation by third parties of our confidential proprietary information could enable competitors to quickly duplicate or surpass our technological achievements, thus eroding our competitive position in our market.
27
We may be involved in lawsuits to protect or enforce our patents or the patents of our licensors, which could be expensive, time-consuming and unsuccessful.
Competitors may infringe our patents, or patents that may issue to us in the future, or the patents of our licensors that are licensed to us. To counter infringement or unauthorized use, we may be required to file infringement claims, which can be expensive and time-consuming. In addition, if we or one of our future collaborators were to initiate legal proceedings against a third party to enforce a patent covering our current product candidates, or one of our future products, the defendant could counterclaim that our patent is invalid and/or unenforceable. In patent litigation in the United States, defendant counterclaims alleging invalidity and/or unenforceability are commonplace. Grounds for a validity challenge could be an alleged failure to meet any of several statutory requirements, including lack of novelty, obviousness or non-enablement. Grounds for an unenforceability assertion could be an allegation that someone connected with prosecution of the patent withheld relevant information from the U.S. PTO, or made a materially misleading statement, during prosecution. Third parties may also raise similar claims before the U.S. PTO, even outside the context of litigation. The outcome following legal assertions of invalidity and unenforceability is unpredictable. With respect to the validity question, for example, we cannot be certain that there is no invalidating prior art, of which we and the patent examiner were unaware during prosecution. If a defendant were to prevail on a legal assertion of invalidity and/or unenforceability, we would lose at least part, and perhaps all, of the patent protection on our current or future product candidates. Such a loss of patent protection could have a material adverse impact on our business.
Litigation or interference proceedings may fail and, even if successful, may result in substantial costs and distract our management and other employees. Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of our confidential information could be compromised by disclosure during this type of litigation. In addition, there could be public announcements of the results of hearings, motions or other interim proceedings or developments. If securities analysts or investors perceive these results to be unsuccessful, it could have an adverse effect on the price of our common stock. Finally, we may not be able to prevent, alone or with the support of our licensors, misappropriation of our trade secrets or confidential information, particularly in countries where the laws may not protect those rights fully.
The regulatory approval process is uncertain, requires us to utilize significant resources, and may prevent us from obtaining approvals for the commercialization of some or all of our product candidates.
The research, testing, manufacturing, labeling, approval, selling, import, export, marketing and distribution of pet therapeutics are subject to extensive regulation by the CVM, the USDA or the EMA and other regulatory authorities in the United States and other countries, which regulations differ from country to country. We are not permitted to market any of our current or future product candidates in the United States until we receive approval of an NADA from the CVM or a product license from the USDA. We have not submitted an application for or received marketing approval for our current product candidates. Obtaining approval of an NADA from CVM or a product license from the USDA can be an uncertain process that requires us to utilize significant resources. The CVM, the USDA or any foreign regulatory bodies can delay, limit or deny approval of any of our product candidates for many reasons, including:
|
•
|
we are unable to demonstrate to the satisfaction of the CVM, the USDA, the EMA or the applicable foreign regulatory body that the product candidate is safe and effective for the requested indication;
|
|
•
|
the CVM, the USDA or the applicable foreign regulatory body may disagree with our interpretation of data from our target animal studies and other development efforts;
|
|
•
|
we may be unable to demonstrate that the product candidate’s benefits outweigh any safety or other perceived risks;
|
|
•
|
the CVM, the USDA or the applicable foreign regulatory body may require additional studies;
|
|
•
|
the CVM, the USDA or the applicable foreign regulatory body may not approve of the formulation, labeling and/or the specifications of our current and future product candidates;
|
28
|
•
|
the CVM, the USDA or the applicable foreign regulatory body may fail to approve our manufacturing processes or facilities, or the manufacturing processes or facilities of third-party manufacturers with which we contract; and
|
|
•
|
the approval policies or regulations of the CVM, USDA or the applicable foreign regulatory body may significantly change in a manner rendering the data from our studies insufficient for approval.
|
In addition, failure to comply with CVM and other applicable United States and foreign regulatory requirements may subject us to administrative or judicially imposed sanctions, including: warning letters, civil and criminal penalties, injunctions, withdrawal of approved products from the market, product seizure or detention, product recalls, total or partial suspension of production, and refusal to approve pending NADAs or product licenses or supplements to approved NADAs or product licenses.
Regulatory approval of an NADA or supplement NADA, or of a product license, is not guaranteed, and the approval process requires us to utilize significant resources, may take several years, and is subject to the substantial discretion of the CVM, the USDA or the EMA. Despite the time and expense exerted, failure can occur at any stage, and we could encounter problems that cause us to abandon or repeat studies, or perform additional studies. If any of our current or future product candidates fails to demonstrate safety and efficacy in our studies, or for any other reason does not gain regulatory approval, our business and results of operations will be materially and adversely harmed.
Even if we receive regulatory approval for any of our current or future product candidates, we will be subject to ongoing CVM, USDA or EMA obligations and continued regulatory review, which may result in significant additional expense. Additionally, any product candidates, if approved, will be subject to labeling and manufacturing requirements and could be subject to other restrictions. Failure to comply with these regulatory requirements or the occurrence of unanticipated problems with our products could result in significant penalties.
Any regulatory approvals that we or any of our collaborators receive for any of our current or future product candidates may be subject to conditions of approval or limitations on the approved indicated uses for which the product may be marketed, or may contain requirements for potentially costly surveillance to monitor the safety and efficacy of the product candidate. In addition, if the CVM, the USDA or the EMA approves any of our current or future product candidates, the manufacturing processes, labeling, packaging, distribution, adverse event reporting, storage, advertising, promotion and recordkeeping for the product will be subject to extensive and ongoing regulatory requirements. These requirements include submissions of safety and other post-marketing information and reports, registration, as well as continued compliance with cGMP, GLP and good clinical practices, or GCP, for any studies that we conduct post-approval. Later discovery of previously unknown problems with a product, including adverse events of unanticipated severity or frequency, or with our third-party manufacturers or manufacturing processes, or failure to comply with regulatory requirements, may result in, among other things:
|
•
|
restrictions on the marketing or manufacturing of the product, withdrawal of the product from the market, or voluntary or mandatory product recalls;
|
|
•
|
fines, warning letters or holds on target animal studies;
|
|
•
|
refusal by the CVM, the USDA or the EMA to approve pending applications or supplements to approved applications filed by us or our strategic collaborators, or suspension or revocation of product license approvals;
|
|
•
|
product seizure or detention, or refusal to permit the import or export of products; and
|
|
•
|
injunctions or the imposition of civil or criminal penalties.
|
The CVM’s, USDA’s or the EMA’s policies may change and additional government regulations may be enacted that could prevent, limit or delay regulatory approval of our product candidates. We cannot predict the likelihood, nature or extent of government regulation that may arise from future legislation or administrative action, either in the United States or abroad. If we are slow or unable to adapt to changes in existing requirements or the adoption of new requirements or policies, or if we are not able to maintain regulatory compliance, we may lose any marketing approval that we may have obtained and we may not achieve or sustain profitability, which would adversely affect our business.
29
Failure to obtain regulatory approvals in foreign jurisdictions for our product candidates would prevent us from marketing our products internationally.
In order to market any product outside of the United States, including in the EEA (which is comprised of the 28 member states of the European Union plus Norway, Iceland and Liechtenstein) and many other foreign jurisdictions, separate regulatory approvals are required. More concretely, in the EEA, pet therapeutics can only be commercialized after obtaining a Marketing Authorization (“MA”). Before granting the MA, the EMA or the competent national authorities of the member states of the EEA make an assessment of the risk-benefit balance of the product on the basis of scientific criteria concerning its quality, safety and efficacy.
The approval procedures vary among countries and can involve additional studies and testing, and the time required to obtain approval may differ from that required to obtain CVM or USDA approval. Animal studies conducted in one country may not be accepted by regulatory authorities in other countries. Approval by the CVM or USDA does not ensure approval by regulatory authorities in other countries, and approval by one or more foreign regulatory authorities does not ensure approval by regulatory authorities in other foreign countries or by the CVM or the USDA. However, a failure or delay in obtaining regulatory approval in one country may have a negative effect on the regulatory process in others. The foreign regulatory approval process may include all of the risks associated with obtaining CVM or USDA approval. We may not be able to file for regulatory approvals or to do so on a timely basis and, even if we do file them, we may not receive necessary approvals to commercialize our products in any market.
If approved, any of our current or future products may cause or contribute to adverse medical events that we are required to report to the CVM, USDA and regulatory authorities in other countries and, if we fail to do so, we could be subject to sanctions that would materially harm our business.
If we are successful in commercializing any of our current or future products, regulations of the CVM, the USDA and of the regulatory authorities in other countries require that we report certain information about adverse medical events if those products may have caused or contributed to those adverse events. The timing of our obligation to report would be triggered by the date we become aware of the adverse event as well as the nature of the event. We may fail to report adverse events we become aware of within the prescribed timeframe. We may also fail to appreciate that we have become aware of a reportable adverse event, especially if it is not reported to us as an adverse event or if it is an adverse event that is unexpected or removed in time from the use of our products. If we fail to comply with our reporting obligations, the CVM, USDA and regulatory authorities in other countries could take action including criminal prosecution, the imposition of civil monetary penalties, seizure of our products, or delay in approval or clearance of future products.
Legislative or regulatory reforms with respect to pet therapeutics may make it more difficult and costly for us to obtain regulatory clearance or approval of any of our current or future product candidates and to produce, market, and distribute our products after clearance or approval is obtained.
From time to time, legislation is drafted and introduced in the U.S. Congress that could significantly change the statutory provisions governing the testing, regulatory clearance or approval, manufacture, and marketing of regulated products. In addition, CVM and USDA regulations and guidance are often revised or reinterpreted by the CVM and USDA in ways that may significantly affect our business and our products. Similar changes in laws or regulations can occur in other countries. Any new regulations or revisions or reinterpretations of existing regulations in the United States or in other countries may impose additional costs or lengthen review times of any of our current or future product candidates. We cannot determine what effect changes in regulations, statutes, legal interpretation or policies, when and if promulgated, enacted or adopted may have on our business in the future. Such changes could, among other things, require:
|
•
|
changes to manufacturing methods;
|
|
•
|
recall, replacement, or discontinuance of certain products; and
|
|
•
|
additional record keeping.
|
Each of these would likely entail substantial time and cost and could materially harm our financial results. In addition, delays in receipt of or failure to receive regulatory clearances or approvals for any future products would harm our business, financial condition, and results of operations.
30
Our research and development relies on evaluations in animals, which may become subject to bans or additional regulations.
As a biopharmaceutical company with a focus on pet therapeutics, the evaluation of our existing and new products in animals is required to register our products. Animal testing in certain industries has been the subject of controversy and adverse publicity. Some organizations and individuals have attempted to ban animal testing or encourage the adoption of additional regulations applicable to animal testing. To the extent that the activities of such organizations and individuals are successful, our research and development, and by extension our operating results and financial condition, could be materially adversely affected. In addition, negative publicity about us or our industry could harm our reputation.
RISKS RELATED TO OUR COMMON STOCK
Our ability to raise additional capital through the sale of our stock may be harmed by competing resales of our common stock by the selling shareholders in our registration statement that was declared effective by the SEC.
The price of our common stock could fall if the selling shareholders sell substantial amounts of our common stock. These sales would make it more difficult for us to sell equity or equity-related securities in the future at a time and price that we deem appropriate, because the selling shareholders may offer to sell their shares of common stock to potential investors for less than we do. Moreover, potential investors may not be interested in purchasing shares of our common stock if the selling shareholders are selling their shares of common stock.
We depend on the efforts and abilities of our officers.
We currently have only two officers, John Lai and John F. Dolan, who are also our only employees. The demands on each of these individuals’ time will increase because of our status as a public company. Messrs. Lai and Dolan both have limited experience in managing a public company, which may impact our ability to meet our financial and business objectives as potential investors may not want to invest in a company whose management has limited public company experience. The interruption of the services of our management could significantly hinder our operations, profits and future development, if suitable replacements are not promptly obtained. We do not currently have any executive compensation agreements. We cannot guaranty that our management will remain with us.
Our management ranks are thin, and losing or failing to add key personnel could affect our ability to successfully grow our business.
Our future performance depends substantially on the continued service of our management. In particular, our success depends upon the continued efforts of our management personnel, including our Chief Executive Officer/President, John Lai, and our Chief Financial Officer/Treasurer and Secretary, John F. Dolan. We cannot guarantee that either Messrs. Lai or Dolan will remain with us.
The costs to meet our reporting requirements as a public company subject to the Securities Exchange Act of 1934 will be substantial and may result in us having insufficient funds to operate our business.
We will incur ongoing expenses associated with professional fees for accounting and legal expenses associated with being a public company. We estimate that these costs will range up to $50,000 per year for the next few years. Those fees will be higher if our business volume and activity increases. Those obligations will reduce and possibly eliminate our ability and resources to fund our operations and may prevent us from meeting our normal business obligations.
31
Our auditors have questioned our ability to continue operations as a “going concern.” Investors may lose all of their investment if we are unable to continue operations and generate revenues.
We hope to obtain significant revenues from future product sales. In the absence of significant sales and profits, we may seek to raise additional funds to meet our working capital needs, principally through the additional sales of our securities. However, we cannot guarantee that we will be able to obtain sufficient additional funds when needed, or that such funds, if available, will be obtainable on terms satisfactory to us. As a result, substantial doubt exists about our ability to continue as a going concern.
We are subject to the Section 15(d) reporting requirements under the Securities Exchange Act of 1934 which does not require a company to file all the same reports and information as a fully reporting company pursuant to Section 12.
We are subject to the Section 15(d) reporting requirements according to the Securities Exchange Act of 1934, or Exchange Act. As a filer subject to Section 15(d) of the Exchange Act:
|
·
|
we are not required to prepare proxy or information statements;
|
|
·
|
we will be subject to only limited portions of the tender offer rules;
|
|
·
|
our officers, directors, and more than ten (10%) percent shareholders are not required to file beneficial ownership reports about their holdings in our company;
|
|
·
|
our officers, directors, and more than ten (10%) percent shareholders are not subject to the short-swing profit recovery provisions of the Exchange Act; and
|
|
·
|
more than five percent (5%) holders of classes of our equity securities will not be required to report information about their ownership positions in the securities.
|
Our officers and directors own approximately 56.51% of our outstanding shares of common stock, allowing these shareholders to control matters requiring approval of our shareholders.
Our officers and directors beneficially own, in the aggregate, approximately 56.51% of our outstanding shares of common stock. Such concentrated control of the company may negatively affect the price of our common stock. In addition, our officers and directors can control matters requiring approval by our security holders, including the election of directors.
Investors should not look to dividends as a source of income.
In the interest of reinvesting initial profits back into our business, we do not intend to pay cash dividends in the foreseeable future. Consequently, any economic return will initially be derived, if at all, from appreciation in the fair market value of our stock, and not as a result of dividend payments.
The trading price of our common stock on the OTCQB will fluctuate significantly and stockholders may have difficulty reselling their shares.
Our common stock commenced trading on the OTCQB approximately January 1, 2013. As of the date of this Annual Report, our common stock trades on the OTCQB. There is a volatility associated with OTCQB securities in general and the value of your investment could decline due to the impact of any of the following factors upon the market price of our common stock: (i) disappointing results from our exploration or development efforts; (ii) failure to meet our revenue or profit goals or operating budget; (iii) decline in demand for our common stock; (iv) downward revisions in securities analysts' estimates or changes in general market conditions; (v) technological innovations by competitors or in competing technologies; (vi) lack of funding generated for operations; (vii) investor perception of our industry or our prospects; and (viii) general economic trends
In addition, stock markets have experienced price and volume fluctuations and the market prices of securities have been highly volatile. These fluctuations are often unrelated to operating performance and may adversely affect the market price of our common stock. As a result, investors may be unable to sell their shares at a fair price and you may lose all or part of your investment.
32
Additional issuance of equity securities may result in dilution to our existing stockholders.
Our Articles of Incorporation, as amended, authorize the issuance of 4,000,000,000 shares of common stock. The Board of Directors has the authority to issue additional shares of our capital stock to provide additional financing in the future, including issuances to Gel-Del Technologies in accordance with certain contractual terms, and the issuance of any such shares may result in a reduction of the book value or market price of the then outstanding shares of our common stock. If we do issue any such additional shares, such issuance also will cause a reduction in the proportionate ownership and voting power of all other stockholders. As a result of such dilution, the proportionate ownership interest and voting power of our shareholders will be decreased accordingly. Further, any such issuance could result in a change of control.
Because we may be subject to the “penny stock” rules, the level of trading activity in our stock may be reduced - which may make it difficult for investors to sell their shares.
Broker-dealer practices in connection with transactions in “penny stocks” are regulated by certain penny stock rules adopted by the Securities and Exchange Commission. Penny stocks, like shares of our common stock, generally are equity securities with a price of less than $5.00, other than securities registered on certain national securities exchanges or quoted on NASDAQ. The penny stock rules require a broker-dealer, prior to a transaction in a penny stock not otherwise exempt from the rules, to deliver a standardized risk disclosure document that provides information about penny stocks and the nature and level of risks in the penny stock market. The broker-dealer also must provide the customer with current bid and offer quotations for the penny stock, the compensation of the broker-dealer and its salesperson in the transaction, and, if the broker-dealer is the sole market maker, the broker-dealer must disclose this fact and the broker-dealer’s presumed control over the market, and monthly account statements showing the market value of each penny stock held in the customer’s account. In addition, broker-dealers who sell these securities to persons other than established customers and “accredited investors” must make a special written determination that the penny stock is a suitable investment for the purchaser and receive the purchaser’s written agreement to the transaction. Consequently, these requirements may have the effect of reducing the level of trading activity, if any, in the secondary market for a security subject to the penny stock rules, and investors in our common stock may find it difficult to sell their shares.
Our shares are eligible to be traded electronically.
Our shares of common stock are eligible to be quoted on the OTCQB. Our shares are eligible with Depository Trust Company (DTC) to trade electronically. Because we are DTC eligible, our shares can be electronically transferred between brokerage accounts. The practical effect may be that our shares will trade at a higher volume on the OTCQB, however, there is no guarantee.
ITEM 1B. UNRESOLVED STAFF COMMENTS
None.
ITEM 2. PROPERTIES
Property held by us. As of June 30, 2014, we do not own any interests in real estate.
Our Facilities. Our executive, administrative and operating offices are located at 12100 Singletree Lane, Suite 186, Eden Prairie, Minnesota 55344. We believe that our facilities are adequate for our needs and that additional suitable space will be available on acceptable terms as required.
ITEM 3. LEGAL PROCEEDINGS
We are not currently a party to any legal proceedings.
ITEM 4. MINE SAFETY DISCLOSURES
Not applicable.
33
PART II
ITEM 5. MARKET FOR REGISTRANT'S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
MARKET INFORMATION
Our common stock is listed for quotation on the OTCQB under the symbol “PETV.” Our shares commenced trading approximately January 1, 2013. The following table sets forth the high and low bid prices relating to our common stock on a quarterly basis for the periods indicated as quoted by the OTC:QB stock market. These quotations reflect inter-dealer prices without retail mark-up, mark-down, or commissions, and may not reflect actual transactions.
|
Quarter Ended
|
High Bid
|
Low Bid
|
||||||
|
June 30, 2014
|
$ | 0.0650 | $ | 0.0100 | ||||
|
March 31, 2014
|
$ | 0.0299 | $ | 0.0033 | ||||
|
December 31, 2013
|
$ | 0.0050 | $ | 0.0030 | ||||
|
September 30, 2013
|
$ | 0.0299 | $ | 0.0033 | ||||
|
June 30, 2013
|
$ | 0.0299 | $ | 0.0175 | ||||
|
March 31, 2013
|
$ | 0.21 | $ | 0.20 | ||||
|
June 30, 2013
|
$ | 0.16 | $ | 0.09 | ||||
HOLDERS
The approximate number of stockholders of record at June 30, 2014 was 76. The number of stockholders of record does not include beneficial owners of our common stock, whose shares are held in the names of various dealers, clearing agencies, banks, brokers and other fiduciaries.
DIVIDEND POLICY
We have never declared or paid a cash dividend on our capital stock. We do not expect to pay cash dividends on our common stock in the foreseeable future. We currently intend to retain our earnings, if any, for use in our business. Any dividends declared in the future will be at the discretion of our Board of Directors.
SUBMISSISION OF MATTERS TO A VOTE OF SECURITY HOLDERS
On March 21, 2014, our majority shareholders approved the Name Change. Pursuant to our Bylaws and the Nevada Revised Statutes, a vote by the holders of at least a majority of our outstanding shares is required to effect the Name Change. Our articles of incorporation do not authorize cumulative voting. As of the record date of March 21, 2014, we had 3,779,542,482 voting shares of common stock issued and outstanding. The consenting stockholders of the shares of common stock were entitled to 3,210,205,804 votes, which represented approximately 85.58% of the voting rights associated with our shares of common stock. The consenting stockholders voted in favor of the Name Change described herein in a unanimous written consent dated March 21, 2014. An Information Statement on form 14C was filed with the Securities and Exchange Commission on March 24, 2014.
The Board of Directors considered factors regarding their approval of the Name Change based on the change in business operations relating to the Securities Exchange Agreement.
34
RECENT SALES OF UNREGISTREED SECURITIES
During fiscal year ended March 31, 2014 and to date, we issued an aggregate of 3,628,296,480 shares of unregistered common stock as follows:
Settlement Agreement
Our Board of Directors approved the execution of that certain settlement agreement dated February 20, 2014 (the "Settlement Agreement") with Ghislaine St.-Hilaire, our prior President/Chief Executive Officer and a member of the Board of Directors ("St.-Hilaire"), relating to a loan advance in the amount of $124,283.38 (the "Debt"). The Debt is evidenced on our financial statements filed with our reports with the Securities and Exchange Commission. In accordance with the terms and provisions of the Settlement Agreement: (i) we agreed to settle the Debt by issuance of an aggregate 24,856,676 shares of restricted common stock at a per share price of $0.005; (ii) St.-Hilaire agreed to settle the Debt by acceptance of the issuance of the 24,856,676 shares of restricted common stock; and (iii) we and St.-Hilaire agreed to release each other and forever discharge any and all claims relating to the Debt.
Therefore, effective February 20, 2014, our Board of Directors authorized the settlement of the Debt and issued an aggregate 24,856,676 shares of restricted common stock to St.-Hilaire as settlement. The shares of common stock were issued in accordance with the terms and provisions of the Settlement Agreement issued to one non-United States resident in reliance on Regulation S promulgated under the United States Securities Act of 1933, as amended (the “Securities Act”). The shares of common stock have not been registered under the Securities Act or under any state securities laws and may not be offered or sold without registration with the United States Securities and Exchange Commission or an applicable exemption from the registration requirements. St.-Hilaire acknowledged that the securities to be issued have not been registered under the Securities Act and that she understood the economic risk of an investment in the securities
Conversion of Debt
Our Board of Directors approved the execution of those certain convertible promissory notes dated March 17, 2014 (collectively, the "Promissory Notes") with certain lenders who had previously advanced and loaned monies to us for working capital purposes. During 2010 and 2011, we had received monies from certain lenders pursuant to which we and the respective lender verbally agreed that the principal loaned would be interest free, payable upon demand, and the lender had the right in its sole discretion to convert the principal due and owing into shares of our common stock at a conversion price equal to par value ($0.001). Subsequently, we and respective lender entered into those certain written amendments to the promissory notes dated December 12, 2013 pursuant to which the amendment confirmed the verbal agreement and the conversion terms and that any such conversion must be completed by March 31, 2014 (collectively, the "Amendments"). The loans reflected below were all evidenced on our financial statements for fiscal years commencing 2010 through current date. We and those certain lenders (collectively, the "Lenders") desired to enter into a convertible promissory note dated March 17, 2014, which would evidence their entire agreement concerning the original loan of funds by the Lender and all terms of the loan, including the original terms of agreement, including conversion terms as follows:
|
Lender
|
Date of Original Loan
|
Principal Amount Loaned
|
||||
|
9185-5643 Quebec Inc.
|
June 16, 2010
|
$ | 21,000.00 | |||
|
Michel St.-Hilaire
|
October 24, 2011
|
$ | 7,500.00 | |||
|
Elden Brochu
|
August 18, 2010
|
$ | 10,500.00 | |||
|
Gina Drouin
|
August 18, 2010
|
$ | 10,500.00 | |||
|
Christian Fontaine
|
October 24, 2011
|
$ | 7,500.00 | |||
|
Ferme Simen Inc.
|
June 14, 2010
|
$ | 21,000.00 | |||
35
Subsequently, each Lender and certain assignees (collectively, the "Assignees") entered into that certain debt purchase and assignment agreement and associated instrument of assignment and transfer assignment/assumption of debt dated February 24, 2014 (collectively, the "Debt Purchase Agreements"), pursuant to which the Assignees paid for acquisition of the respective Lender's right, title and interest in and to the respective Convertible Note and underlying debt, including the right to convert. On March 17, 2014, we received those certain notices of conversion (collectively, the Notices of Conversion").
Therefore, effective on March 17, 2014, our Board of Directors approved the issuance of an aggregate 78,000,000 shares of our restricted common stock to the Assignees in accordance with the terms and provisions of the respective Notices of Conversion. The shares of common stock were issued at $0.001 per share. The shares of common stock were issued to three United States residents and two non-United States residents in reliance on Section 4(2) and Regulation D and/or Regulation S promulgated under the United States Securities Act of 1933, as amended (the “Securities Act”). The securities have not been registered under the Securities Act or under any state securities laws and may not be offered or sold without registration with the United States Securities and Exchange Commission or an applicable exemption from the registration requirements. The Assignees acknowledged that the securities to be issued have not been registered under the Securities Act, that they understood the economic risk of an investment in the securities, and that they had the opportunity to ask questions of and receive answers from our management concerning any and all matters related to acquisition of the securities.
Securities Exchange Agreement
On March 11, 2014, our Board of Directors authorized the execution of that certain securities exchange agreement dated March 11, 2014 (the "Securities Exchange Agreement") with PetVivo Inc., a Minnesota corporation ("PetVivo"), and the shareholders of PetVivo who hold of record the total issued and outstanding shares of common stock of PetVivo (the “PetVivo Shareholders”). In accordance with the terms and provisions of the Securities Exchange Agreement, we acquired all of the issued and outstanding shares of stock of PetVivo from the PetVivo Shareholders, thus making PetVivo our wholly-owned subsidiary, in exchange for the issuance to the PetVivo Shareholders of a further 1,222,000,000 shares of our restricted common stock. We had previously issued an aggregate 2,310,939,804 shares of our restricted common stock to the PetVivo Shareholders and was required under the terms and provisions of the Securities Exchange Agreement to issue further shares to meet the agreed upon equity percentage.
All shares of common stock were issued at $0.001 per share. The shares of common stock were issued to five United States residents in reliance on Section 4(2) and Regulation D promulgated under the United States Securities Act of 1933, as amended (the “Securities Act”). The securities have not been registered under the Securities Act or under any state securities laws and may not be offered or sold without registration with the United States Securities and Exchange Commission or an applicable exemption from the registration requirements. The PetVivo Shareholders acknowledged that the securities to be issued have not been registered under the Securities Act, that they understood the economic risk of an investment in the securities, and that they had the opportunity to ask questions of and receive answers from our management concerning any and all matters related to acquisition of the securities.
SECURITIES AUTHORIZED FOR ISSUANCE UNDER EQUITY COMPENSATION PLANS.
As of the date of this Annual Report, we have no compensation plans under which our equity securities were authorized for issuance.
36
PENNY STOCK REGULATION
Shares of our common stock will probably be subject to rules adopted the SEC that regulate broker-dealer practices in connection with transactions in “penny stocks.” Penny stocks are generally equity securities with a price of less than $5.00 (other than securities registered on certain national securities exchanges or quoted on the NASDAQ system, provided that current price and volume information with respect to transactions in those securities is provided by the exchange or system). The penny stock rules require a broker-dealer, prior to a transaction in a penny stock not otherwise exempt from those rules, deliver a standardized risk disclosure document prepared by the SEC, which contains the following:
|
·
|
a description of the nature and level of risk in the market for penny stocks in both public offerings and secondary trading;
|
|
·
|
a description of the broker’s or dealer’s duties to the customer and of the rights and remedies available to the customer with respect to violation to such duties or other requirements of securities’ laws;
|
|
·
|
a brief, clear, narrative description of a dealer market, including “bid” and “ask” prices for penny stocks and the significance of the spread between the “bid” and “ask” price;
|
|
·
|
a toll-free telephone number for inquiries on disciplinary actions;
|
|
·
|
definitions of significant terms in the disclosure document or in the conduct of trading in penny stocks; and
|
|
·
|
such other information and is in such form (including language, type, size and format), as the SEC shall require by rule or regulation.
|
Prior to effecting any transaction in penny stock, the broker-dealer also must provide the customer the following:
|
·
|
the bid and offer quotations for the penny stock;
|
|
·
|
the compensation of the broker-dealer and its salesperson in the transaction;
|
|
·
|
the number of shares to which such bid and ask prices apply, or other comparable information relating to the depth and liquidity of the market for such stock; and
|
|
·
|
monthly account statements showing the market value of each penny stock held in the customer’s account.
|
In addition, the penny stock rules require that prior to a transaction in a penny stock not otherwise exempt from those rules, the broker-dealer must make a special written determination that the penny stock is a suitable investment for the purchaser and receive the purchaser’s written acknowledgment of the receipt of a risk disclosure statement, a written agreement to transactions involving penny stocks, and a signed and dated copy of a written suitability statement. These disclosure requirements may have the effect of reducing the trading activity in the secondary market for a stock that becomes subject to the penny stock rules. Holders of shares of our common stock may have difficulty selling those shares because our common stock will probably be subject to the penny stock rules.
ITEM 6. SELECTED FINANCIAL DATA
Not applicable.
37
The following discussion of our financial condition and results of operations should be read in conjunction with our financial statements for the year ended March 31, 2014, together with notes thereto as included in this Annual Report on Form 10-K. The following discussion contains forward-looking statements that reflect our plans, estimates and beliefs. Our actual results could differ materially from those discussed in the forward looking statements. Factors that could cause or contribute to such differences include, but are not limited to those discussed below and elsewhere in this Annual Report, particularly in the section entitled "Risk Factors." Our audited financial statements are stated in United States Dollars and are prepared in accordance with United States Generally Accepted Accounting Principles.
We are a developmental stage company and have not generated any revenue to date. We have incurred recurring losses to date. Our financial statements have been prepared assuming that we will continue as a going concern and, accordingly, do not include adjustments relating to the recoverability and realization of assets and classification of liabilities that might be necessary should we be unable to continue in operation.
We expect we will require additional capital to meet our long term operating requirements. We expect to raise additional capital through, among other things, the sale of equity or debt securities.
RESULTS OF OPERATION
The Share Exchange Agreement results in the treatment of us and our wholly-owned subsidiary as entities under common control, which reflects PetVivo, our wholly-owned subsidiary from inception to March 31, 2014. The prior operations for accounting purposes is deemed to be those of the accounting acquirer, which differs from the legal acquirer.
The following table presents the statement of operations for the period from August 1, 2013 (inception) to March 31, 2014.
|
|
|
For Period from August 1, 2013 (inception) to March 31, 2014
|
|
|
|
|
|
|
||
|
Revenues
|
|
$
|
-
|
|
|
|
|
|
|
|
|
Total Operating Expenses
|
|
|
24,648,826
|
|
|
|
|
|
|
|
|
Total Other Income (Expense)
|
|
|
(13,021)
|
|
|
|
|
|
|
|
|
Net Income (loss)
|
|
$
|
(24,661,847
|
)
|
|
|
|
|
|
|
|
Net loss per share - basic and diluted
|
|
$
|
(0.07
|
)
|
For the Period From August 1, 2013 (inception) to March 31, 2014
Total Revenues. For the period from August 1, 2013 (inception) to March 31, 2014, we did not generate any revenue.
38
Operating Expenses. Operating expenses for the period from August 1, 2013 (inception) to March 31, 2014, were $24,648,826. For the period from August 1, 2013 (inception) to March 31, 2014, we incurred: (i) payroll expenses of $80,000; (ii) stock for services of $24,440,100; (iii) research and development of $79,536; and (iv) general and administrative of $49,190. Operating expenses substantially increased due to valuation of stock issued for services and research and development based on the increased scope and scale of our business operations, including negotiation and consummation of the License Agreement and Option.
Other Income (Expense)s. Other expenses for the period from August 1, 2013 (inception) to March 31, 2014 were ($13,021). Other expenses consisted of interest expense of $13,021.
Net Loss. Therefore, our net loss for the period from August 1, 2013 (inception) to March 31, 2014 was ($24,661,847) or per share of ($0.07). Net loss generally increased primarily due to the recording of the valuation of stock issued for services and increase in operating expenses.
The weighted average number of shares outstanding during the period from August 1, 2013 (inception) to March 31, 2014 was 376,614,610.
LIQUIDITY AND CAPITAL RESOURCES
Fiscal Year Ended March 31, 2014
As of March 31, 2014, our current assets were $47,338 and our current liabilities were $479,149, which resulted in a working capital deficit of $431,811.
As of March 31, 2014, our current assets were comprised of $39,338 in cash and cash equivalents and $8,000 in prepaid expenses. As of March 31, 2014, our total assets were $177,338 comprised of: (i) current assets of $47,338; and (ii) $130,000 in security deposit.
As of March 31, 2014, our current liabilities were comprised of: (i) $186,666 in derivative liability; (ii) $83,657 in accounts payable and accrued expenses; (iii) $58,826 in convertible notes payable; and (iv) $150,000 in note payable - debentures.
Stockholders’ deficit was ($301,811) as of March 31, 2014.
Cash Flows from Operating Activities. We have not generated positive cash flows from operating activities due to a lack of a source of revenues. For the period from August 1, 2013 (inception) to March 31, 2014, net cash flows used in operating activities was $40,476. Net cash flows used in operating activities consisted primarily of a net loss of $24,661,847, which was adjusted by $24,440,100 in shares issued for services. Net cash flows used in operating activities was further changed by an increase in prepaids and deposits of $8,000, accrued expenses of $83,657, and $186,666 in derivative liability.
Cash Flows from Investing Activities. For the period from August 1, 2014 (inception) to March 31, 2014, net cash flows provided by investing activities was $130,000 relating to purchase of license pursuant to the License Agreement.
Cash Flows from Financing Activities. We have financed our operations primarily from debt or the issuance of equity instruments. For the period from August 1, 2013 (inception) to March 31, 2014, net cash flows provided from financing activities was $128,762 consisting of proceeds from loans.
39
MATERIAL COMMITMENTS
License Agreement
In accordance with the terms and provisions of the License Agreement, we owe the balance of $1,650,000 as a contingent liability depending upon certain funding.
Convertible Notes Payable
As of March 31, 2014, we have two notes payable. One note is in the principal amount of $7,500 and payable to a former shareholder. The other note is in the principal amount of $51,326 and accrues interest at 8% and payable on demand.
Note Payable - Debentures
We assumed a 12% convertible debenture in the principal amount of $100,000 to 6287182 Canada Inc., a private corporation organized under the laws of Canada. In accordance with the terms and provisions of the 6287182 Canada Debenture, we may redeem by paying the principal plus accrued interest and 6287182 Canada has the right to convert the principal into shares of our restricted common stock at a per share price equal to 80% of the average closing price for 5 consecutive days prior to notice of conversion. The 6287182 Canada Debenture is due July 31, 2016 and accrues interest at the rate of 12% per annum. We are required to pay the accrued interest quarterly commencing on the date of execution and quarterly thereafter.
We also assumed a second note which is a 12% convertible debenture in the principal amount of $50,000 to Brevets Futek MSM Ltee, a private corporation organized under the laws of Canada. In accordance with the terms and provisions of the convertible debenture, we may redeem by paying the principal plus accrued interest. Brevets Futek MSM Ltee has the right to convert the principal into shares of our restricted common stock at a per share price equal to 80% of the average closing price for price for 5 consecutive days prior to notice of conversion. The convertible debenture is due July 17, 2016 and accrues interest at the rate of 12% per annum. We are required to pay the accrued interest quarterly commencing on the date of execution and quarterly thereafter
PURCHASE OF SIGNIFICANT EQUIPMENT
We do not intend to purchase any significant equipment during the next twelve months.
OFF-BALANCE SHEET ARRANGEMENTS
As of the date of this Annual Report, we do not have any off-balance sheet arrangements that have or are reasonably likely to have a current or future effect on our financial condition, changes in financial condition, revenues or expenses, results of operations, liquidity, capital expenditures or capital resources that are material to investors.
GOING CONCERN
The independent auditors' report accompanying our March 31, 2014 financial statements contains an explanatory paragraph expressing substantial doubt about our ability to continue as a going concern. The financial statements have been prepared "assuming that we will continue as a going concern," which contemplates that we will realize our assets and satisfy our liabilities and commitments in the ordinary course of business. We have suffered recurring losses from operations, have a working capital deficit and are currently in default of the payment terms of certain note agreements. These factors raise substantial doubt about our ability to continue as a going concern.
40
RECENTLY ISSUED ACCOUNTING STANDARDS
The following describes the recently issued accounting standards used in reporting our financial condition and results of operations. In some cases, accounting standards allow more than one alternative accounting method for reporting. In those cases, our reported results of operations would be different should we employ an alternative accounting method.
In July 2013, the FASB issued Accounting Standards Update 2013-11 Income Taxes (Topic 740) Presentation of an Unrecognized Tax Benefit When a Net Operating Loss Carry-forward, a Similar Tax Loss, or a Tax Credit Carry-forward Exists. An unrecognized tax benefit, or a portion of an unrecognized tax benefit, should be presented in the financial statements as a reduction to a deferred tax asset for a net operating loss carry-forward, a similar tax loss or a tax credit carry-forward, except as follows. To the extent a net operating loss carry-forward, a similar tax loss or a tax credit carry-forward is not available at the reporting date under the tax law of the applicable jurisdiction to settle any additional income taxes that would result from the disallowance of a tax position or the tax law of the applicable jurisdiction does not require the entity to use, and the entity does not intend to use the deferred tax asset for such purpose, the unrecognized tax benefit should be presented in the financial statements as a liability and should not be combined with deferred tax assets. The assessment of whether a deferred tax asset is available is based on the unrecognized tax benefit and deferred tax asset that exist at the reporting date and should be made presuming disallowance of the tax position at the reporting date. This Update applies to all entities that have unrecognized tax benefits when a net operating loss carry-forward, a similar tax loss, or a tax credit carry-forward exists at the reporting date. The amendments in this Update are effective for fiscal years, and interim periods within those years, beginning after December 15, 2013.
In January 2013, the FASB issued ASU No. 2013-01, Balance Sheet (Topic 210): Clarifying the Scope of Disclosures about Offsetting Assets and Liabilities , which clarifies which instruments and transactions are subject to the offsetting disclosure requirements originally established by ASU 2011-11. The new ASU addresses preparer concerns that the scope of the disclosure requirements under ASU 2011-11 was overly broad and imposed unintended costs that were not commensurate with estimated benefits to financial statement users. In choosing to narrow the scope of the offsetting disclosures, the Board determined that it could make them more operable and cost effective for preparers while still giving financial statement users sufficient information to analyze the most significant presentation differences between financial statements prepared in accordance with U.S. GAAP and those prepared under IFRSs. Like ASU 2011-11, the amendments in this update will be effective for fiscal periods beginning on, or after January 1, 2013. The adoption of ASU 2013-01 is not expected to have a material impact on our financial position or results of operations.
We have implemented all new accounting pronouncements that are in effect. These pronouncements did not have any material impact on the financial statements unless otherwise disclosed, and we do not believe that there are any other new accounting pronouncements that have been issued that might have a material impact on its financial position or results of operations.
OFF-BALANCE SHEET ARRANGMENTS.
We have no off-balance sheet arrangements.
Not applicable.
The financial statements required by Item 8 are presented in the following order:
41
PetVivo, Inc.
(Formerly Technologies Scan Corp.)
Financial Statements
March 31, 2014
TABLE OF CONTENTS
|
Reports of Independent Registered Public Accounting Firms
|
F-2 | |||
|
Balance Sheets
|
F-3 | |||
|
Statements of Operations and Comprehendisve Loss
|
F-4 | |||
|
Statements of Stockholders’ Equity [Deficit]
|
F-5 | |||
|
Statements of Cash Flows
|
F-6 | |||
|
Notes to Financial Statements
|
F-7 |
F-1
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To The Board of Directors and shareholders of
PetVivo Holdings, Inc.
Eden Prairie, MN
I have audited the accompanying balance sheets of PetVivo Holdings, Inc. and its subsidiaries (the “Company”) as of March 31, 2014 and 2013 and the related statements of operations, stockholders’ deficit and cash flows for the years ended March 31, 2014 and 2013, and the related notes to the financialstatements.
Management’s Responsibility for the Financial Statements
Management is responsible for the preparation and fair presentation of these financial statements in accordance with accounting principles generally accepted in the United State of America; this includes the design, implementation and maintenance of internal control relevant to the preparation and fair presentation of financial statements that are free from material misstatement, whether due to fraud or error.
Auditor’s Responsibility
My responsibility is to express an opinion on these consolidated financial statements based on my audits. I conducted my audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that I plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement. The Company is not required to have, nor were I engaged to perform, an audit of its internal control over financial reporting. my audits included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, I express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the consolidated financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall consolidated financial statement presentation. I believe that my audits provide a reasonable basis for my opinion.
Opinion
In my opinion, the consolidated financial statements referred to above present fairly, in all material respects, the consolidated financial position of the Company as of March 31, 2014 and 2013 and the consolidated results of its operations and its cash flows for the years ended March 31, 2014 and 2013, in conformity with accounting principles generally accepted in the United States of America.
Emphasis of Matter
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. As described in Note 3 of the accompanying consolidated financial statements, the Company has incurred losses since inception, and has a negative working capital balance at March 31, 2014, which raises substantial doubt about its ability to continue as a going concern. Management’s plans in regard to this matter are described in Note 3. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
/s/ Terry L. Johnson, CPA
Casselberry, Florida
July 10, 2014
F-2
|
PetVivo, Inc.
(Formerly Technologies Scan Corp.)
|
|||||||||||
|
Balance Sheet
|
|
March 31,
|
March 31,
|
|||||||
|
2014
|
2013
|
|||||||
| Assets: | ||||||||
|
Current Assets
|
||||||||
|
Cash and Cash Equivalents
|
$ | 39,338 | $ | - | ||||
|
Accounts Receivable
|
- | - | ||||||
|
Inventory
|
- | - | ||||||
|
Prepaid Expenses
|
8,000 | - | ||||||
|
Total Current Assets
|
47,338 | - | ||||||
|
Fixed Assets-net
|
- | - | ||||||
|
Other assets, license
|
130,000 | - | ||||||
|
Total Assets
|
$ | 177,338 | $ | - | ||||
|
Liabilities and Stockholders' Deficit:
|
||||||||
|
Current Liabilities:
|
||||||||
|
Derivative Liability
|
186,666 | - | ||||||
|
Accounts Payable and Accrued Expenses
|
83,657 | - | ||||||
|
Convertible Notes Payable
|
58,826 | - | ||||||
|
Note Payable-Debentures
|
150,000 | - | ||||||
|
Total Current Liabilities
|
479,149 | - | ||||||
|
Long Term Debt
|
- | - | ||||||
|
Total Liabilities
|
479,149 | - | ||||||
|
Stockholders' Equity:
|
||||||||
|
Common Stock, Par value $0.001, Authorized 4,000,000,000 shares,
|
||||||||
|
Issued 3,750,946,480 shares
|
3,750,946 | - | ||||||
|
Paid-In Capital
|
20,609,090 | - | ||||||
|
Retained Deficit
|
(24,661,847 | ) | - | |||||
|
Total Stockholders' Equity
|
(301,811 | ) | - | |||||
|
Total Liabilities and Stockholders' Equity
|
$ | 177,338 | $ | - | ||||
The accompanying notes are an integral part of these financial statements.
F-3
|
PetVivo, Inc.
(Formerly Technologies Scan Corp.)
|
|
Statement of Operations
|
|
From inception 8/1/2013
|
||||
|
To 3/31/2014
|
||||
|
Revenues
|
$ | - | ||
|
Costs of Services
|
- | |||
|
Gross Margin
|
- | |||
|
Expenses:
|
||||
|
Payroll Expenses
|
80,000 | |||
|
Stock for Services
|
24,440,100 | |||
|
Research and Development
|
79,536 | |||
|
General and Administrative
|
49,190 | |||
|
Operating Expenses
|
24,648,826 | |||
|
Operating Income (Loss)
|
(24,648,826 | ) | ||
|
Interest
|
(13,021 | ) | ||
|
Net Loss Before Taxes
|
(24,661,847 | ) | ||
|
Income and Franchise Tax
|
- | |||
|
Net Loss
|
$ | (24,661,847 | ) | |
|
Loss per Share, Basic &
|
||||
|
Diluted
|
$ | (0.07 | ) | |
|
Weighted Average Shares
|
||||
|
Outstanding
|
376,614,610 | |||
The accompanying notes are an integral part of these financial statements.
F-4
|
PetVivo, Inc.
(Formerly Technologies Scan Corp)
|
|||||
|
Statement of Stockholders’ Equity
|
|||||
|
August 1, 2013 to March 31, 2014
|
|
Additional
|
||||||||||||||||||||
|
Common
|
Common
|
Paid in
|
Retained
|
|||||||||||||||||
|
Shares
|
Stock
|
Capital
|
Deficit
|
Total
|
||||||||||||||||
|
Balance Beginning
|
122,650,000 | $ | 122,650 | (122,650 | ) | - | - | |||||||||||||
|
Shares issued for Debt on 2/25/14
|
24,856,676 | 24,857 | 99,426 | - | 124,283 | |||||||||||||||
|
Shares issued for agreement on 3/14/2014
|
2,310,939,804 | 2,310,940 | (2,310,940 | ) | - | - | ||||||||||||||
|
Shares issued for services on 3/17/2014
|
1,222,000,000 | 1,222,000 | 23,218,000 | - | 24,440,000 | |||||||||||||||
|
Effects of Reverse
|
(274,746 | ) | (274,746 | ) | ||||||||||||||||
|
Shares issued for debt on 3/19/2014
|
70,500,000 | 70,500 | - | 70,500 | ||||||||||||||||
|
Net Loss for the period
|
(24,661,847 | ) | (24,661,847 | ) | ||||||||||||||||
|
|
||||||||||||||||||||
|
Balance March 31, 2014
|
3,750,946,480 | 3,750,946 | 20,609,090 | (24,661,847 | ) | (301,811 | ) | |||||||||||||
The accompanying notes are an integral part of these financial statements.
F-5
|
Petvivo, Inc.
(Formerly Technologies Scan, Inc.)
|
||||
|
Statement of Cash Flows
|
||||
|
From Inception August 1, 2013 to March 31, 2014
|
|
2014
|
||||
|
CASH FLOWS FROM OPERATING ACTIVITIES:
|
||||
|
Net Loss for the Period
|
$ | (24,661,847 | ) | |
|
Shares Issued for Services
|
24,440,100 | |||
|
Adjustments to reconcile net loss to net cash
|
||||
|
provided by operating activities:
|
||||
|
Depreciation and Amortization
|
- | |||
|
Changes in Operating Assets and Liabilities
|
||||
|
Decrease (Increase) in Accounts Receivable
|
- | |||
|
(Increase) Decrease in Prepaids and Deposits
|
(8,000 | ) | ||
|
Increase (Decrease) in Accrued Expenses
|
83,657 | |||
|
Increase in Derivative Liability
|
186,666 | |||
|
|
||||
|
Net Cash Used in Operating Activities
|
40,576 | |||
|
CASH FLOWS FROM INVESTING ACTIVITIES:
|
||||
|
Purchase of License
|
(130,000 | ) | ||
|
Net cash provided by Investing Activities
|
(130,000 | ) | ||
|
CASH FLOW FROM FINANCING ACTIVITIES:
|
||||
|
Common Stock issued for Cash
|
- | |||
|
Proceeds from Loans
|
128,762 | |||
|
Reduction of Debt
|
||||
|
Net Cash Provided by Financing Activities
|
128,762 | |||
|
Net (Decrease) Increase in Cash
|
39,338 | |||
|
Cash at Beginning of Period
|
- | |||
|
Cash at End of Period
|
$ | 39,338 | ||
|
SUPPLEMENTAL DISCLOSURE OF CASH FLOW INFORMATION:
|
||||
|
Cash paid during the year for:
|
||||
|
`Interest
|
$ | - | ||
|
Franchise and Income Taxes
|
$ | - | ||
|
SUPPLEMENTAL DISCLOSURE OF NON-CASH INVESTING AND FINANCING ACTIVITIES:
|
||||
|
Accounts Payable Satisfied through Contributed Capital
|
||||
|
and Property and Equipment
|
$ | - | ||
The accompanying notes are an integral part of these financial statements.
F-6
PetVivo, Inc.
(Formerly Technologies Scan Corp.)
Notes to Financial Statements
March 31, 2014
NOTE 1 – ORGANIZATION
PetVivo, Inc. (“the Company) was originally incorporated under the laws of the state of Minnesota on August 1, 2013. The financials are the result of a merger between Technologies Scan Corp., a corporation incorporated in the State of Nevada on March 31, 2009 and the Company. For accounting purposes the Company is treating the merger as a reverse merger whereby the financials presented are those of the surviving entity which is Petvivo. The merger occurred on March 14, 2014.
PetVivo is in the business of distribution of bio materials for the treatment of afflictions and diseases in animals.
NOTE 2 -SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Basis of presentation
The Company’s financial statements have been prepared in accordance with accounting principles generally accepted in the United States of America (“U.S. GAAP”). The financials presented are those of PetVivo the surviving entity subject to equity transactions of the former corporation.
Use of estimates
The preparation of financial statements in conformity with generally accepted accounting principles requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates.
Fiscal year end
The Company elected March 31st as its year end.
Cash equivalents
The Company considers all highly liquid investments with a maturity of three months or less when purchased to be cash equivalents.
Fixed Assets
The Company records its fixed assets at cost and recognizes depreciation over the straight line method with asset lives of between 3 and 7 years. Improvements are amortized over the life of the lease.
F-7
Fair value of financial instruments
The Company follows paragraph 825-10-50-10 of the FASB Accounting Standards Codification for disclosures about fair value of its financial instruments and paragraph 820-10-35-37 of the FASB Accounting Standards Codification (“Paragraph 820-10-35-37”) to measure the fair value of its financial instruments. Paragraph 820-10-35-37 establishes a framework for measuring fair value in accounting principles generally accepted in the United States of America (U.S. GAAP), and expands disclosures about fair value measurements. To increase consistency and comparability in fair value measurements and related disclosures, Paragraph 820-10-35-37 establishes a fair value hierarchy which prioritizes the inputs to valuation techniques used to measure fair value into three (3) broad levels. The fair value hierarchy gives the highest priority to quoted prices (unadjusted) in active markets for identical assets or liabilities and the lowest priority to unobservable inputs. The three (3) levels of fair value hierarchy defined by Paragraph 820-10-35-37 are described below:
|
Level 1
|
Quoted market prices available in active markets for identical assets or liabilities as of the reporting date.
|
|
|
Level 2
|
Pricing inputs other than quoted prices in active markets included in Level 1, which are either directly or indirectly observable as of the reporting date.
|
|
|
Level 3
|
Pricing inputs that are generally observable inputs and not corroborated by market data.
|
The carrying amount of the Company’s financial assets and liabilities, such as cash, prepaid expenses and accrued expenses, approximates their fair value because of the short maturity of the instruments.
The Company does not have any assets or liabilities measured at fair value on a recurring or a non-recurring basis, consequently, the Company did not have any fair value adjustments for assets and liabilities measured at fair value at March 31, 2014; no gains or losses are reported in the statement of operations that are attributable to the change in unrealized gains or losses relating to those assets and liabilities still held at the reporting date for the period from August 1 through March 31, 2014.
Commitments and contingencies
The Company follows subtopic 450-20 of the FASB Accounting Standards Codification to report accounting for contingencies. Liabilities for loss contingencies arising from claims, assessments, litigation, fines and penalties and other sources are recorded when it is probable that a liability has been incurred and the amount of the assessment can be reasonably estimated.
Revenue recognition
The Company follows paragraph 605-10-S99-1 of the FASB Accounting Standards Codification for revenue recognition. The Company will recognize revenue when it is realized or realizable and earned. The Company considers revenue realized or realizable and earned when all of the following criteria are met: (i) persuasive evidence of an arrangement exists, (ii) the product has been shipped or the services have been rendered to the customer, (iii) the sales price is fixed or determinable, and (iv) collectability is reasonably assured. The Company mainly sells to retailers. There are no price incentives and the product can only be returned if defective. As the Company does not believe defective merchandise is likely an allowance has not been recognized. Revenue is recognized on a gross basis with corresponding costs of goods as a reduction to revenue in cost of sales. Revenue is recognized when the product is shipped to the customer.
F-8
Income taxes
The Company accounts for income taxes under Section 740-10-30 of the FASB Accounting Standards Codification, which requires recognition of deferred tax assets and liabilities for the expected future tax consequences of events that have been included in the financial statements or tax returns. Under this method, deferred tax assets and liabilities are based on the differences between the financial statement and tax bases of assets and liabilities using enacted tax rates in effect for the fiscal year in which the differences are expected to reverse. Deferred tax assets are reduced by a valuation allowance to the extent management concludes it is more likely than not that the assets will not be realized. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the fiscal years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in the Statements of Income and Comprehensive Income in the period that includes the enactment date.
The Company adopted section 740-10-25 of the FASB Accounting Standards Codification (“Section 740-10-25”) with regards to uncertainty income taxes. Section 740-10-25 addresses the determination of whether tax benefits claimed or expected to be claimed on a tax return should be recorded in the financial statements. Under Section 740-10-25, the Company may recognize the tax benefit from an uncertain tax position only if it is more likely than not that the tax position will be sustained on examination by the taxing authorities, based on the technical merits of the position. The tax benefits recognized in the financial statements from such a position should be measured based on the largest benefit that has a greater than fifty percent (50%) likelihood of being realized upon ultimate settlement. Section 740-10-25 also provides guidance on de-recognition, classification, interest and penalties on income taxes, accounting in interim periods and requires increased disclosures. The Company had no material adjustments to its liabilities for unrecognized income tax benefits according to the provisions of Section 740-10-25.
Net income (loss)per common share
Net income (loss) per common share is computed pursuant to section 260-10-45 of the FASB Accounting Standards Codification. Basic net income (loss) per common share is computed by dividing net income (loss) by the weighted average number of shares of common stock outstanding during the period.
Cash flows reporting
The Company adopted paragraph 230-10-45-24 of the FASB Accounting Standards Codification for cash flows reporting, classifies cash receipts and payments according to whether they stem from operating, investing, or financing activities and provides definitions of each category, and uses the indirect or reconciliation method (“Indirect method”) as defined by paragraph 230-10-45-25 of the FASB Accounting Standards Codification to report net cash flow from operating activities by adjusting net income to reconcile it to net cash flow from operating activities by removing the effects of (a) all deferrals of past operating cash receipts and payments and all accruals of expected future operating cash receipts and payments and (b) all items that are included in net income that do not affect operating cash receipts and payments. The Company reports the reporting currency equivalent of foreign currency cash flows, using the current exchange rate at the time of the cash flows and the effect of exchange rate changes on cash held in foreign currencies is reported as a separate item in the reconciliation of beginning and ending balances of cash and cash equivalents and separately provides information about investing and financing activities not resulting in cash receipts or payments in the period pursuant to paragraph 830-230-45-1 of the FASB Accounting Standards Codification.
F-9
Subsequent events
The Company follows the guidance in Section 855-10-50 of the FASB Accounting Standards Codification for the disclosure of subsequent events. The Company will evaluate subsequent events through the date when the financial statements were issued. Pursuant to ASU 2010-09 of the FASB Accounting Standards Codification, the Company as an eventual SEC filer considers its financial statements issued when they are widely distributed to users, such as through filing them on EDGAR.
Recently issued accounting pronouncements
In July 2013, the FASB issued Accounting Standards Update 2013-11 Income Taxes (Topic 740) Presentation of an Unrecognized Tax Benefit When a Net Operating Loss Carry-forward, a Similar Tax Loss, or a Tax Credit Carry-forward Exists. An unrecognized tax benefit, or a portion of an unrecognized tax benefit, should be presented in the financial statements as a reduction to a deferred tax asset for a net operating loss carry-forward, a similar tax loss or a tax credit carry-forward, except as follows. To the extent a net operating loss carry-forward, a similar tax loss or a tax credit carry-forward is not available at the reporting date under the tax law of the applicable jurisdiction to settle any additional income taxes that would result from the disallowance of a tax position or the tax law of the applicable jurisdiction does not require the entity to use, and the entity does not intend to use the deferred tax asset for such purpose, the unrecognized tax benefit should be presented in the financial statements as a liability and should not be combined with deferred tax assets. The assessment of whether a deferred tax asset is available is based on the unrecognized tax benefit and deferred tax asset that exist at the reporting date and should be made presuming disallowance of the tax position at the reporting date. This Update applies to all entities that have unrecognized tax benefits when a net operating loss carry-forward, a similar tax loss, or a tax credit carry-forward exists at the reporting date. The amendments in this Update are effective for fiscal years, and interim periods within those years, beginning after December 15, 2013.
In January 2013, the FASB issued ASU No. 2013-01, Balance Sheet (Topic 210): Clarifying the Scope of Disclosures about Offsetting Assets and Liabilities , which clarifies which instruments and transactions are subject to the offsetting disclosure requirements originally established by ASU 2011-11. The new ASU addresses preparer concerns that the scope of the disclosure requirements under ASU 2011-11 was overly broad and imposed unintended costs that were not commensurate with estimated benefits to financial statement users. In choosing to narrow the scope of the offsetting disclosures, the Board determined that it could make them more operable and cost effective for preparers while still giving financial statement users sufficient information to analyze the most significant presentation differences between financial statements prepared in accordance with U.S. GAAP and those prepared under IFRSs. Like ASU 2011-11, the amendments in this update will be effective for fiscal periods beginning on, or after January 1, 2013. The adoption of ASU 2013-01 is not expected to have a material impact on our financial position or results of operations.
The Company has implemented all new accounting pronouncements that are in effect. These pronouncements did not have any material impact on the financial statements unless otherwise disclosed, and the Company does not believe that there are any other new accounting pronouncements that have been issued that might have a material impact on its financial position or results of operations.
F-10
NOTE 3 –GOING CONCERN
As reflected in the accompanying financial statements, the Company had a negative equity of $301,811 at March 31, 2014.
Management intends to raise additional funds now that it has merged thru a private placement or thru the public process. Management believes that the actions presently being taken to further implement its business plan will enable the Company to continue as a going concern. While the Company believes in the viability to raise additional funds, there can be no assurances to that effect. The ability of the Company to continue as a going concern is dependent upon the Company’s ability to further implement its business plan and generate funds
The financial statements do not include any adjustments that might be necessary if the Company is unable to continue as a going concern.
NOTE 4 – LICENSE
On August 2, 2013 the Company entered into an agreement with a related party, a shareholder, for certain technology related to the care of animals. The Company paid $130,000 and owes the balance of the contract of $1,650,000 as a contingent liability, dependent on certain funding. The cash portion of the license has been capitalized at March 31, 2014
NOTE 5 – CONVERTIBLE NOTES PAYABLE
At March 31, 2014 the Company had two notes payable
|
March 31,
2014
|
||||
|
Note Payable to a former shareholder
|
7,500 | |||
|
Notes Payable to an individual interest at 8%, payable on demand, convertible into common shares
|
51,326 | |||
|
Total Owed
|
58,826 | |||
NOTE 6-NOTE PAYABLE-DEBENTURES/DERIVATIVE LIABILITY
|
1.
|
The Company assumed a 12% convertible debenture in the principal amount of $100,000 to 6287182 Canada Inc., a private corporation organized under the laws of Canada. In accordance with the terms and provisions of the 6287182 Canada Debenture, we may redeem by paying the principal plus accrued interest and 6287182 Canada has the right to convert the principal into shares of our restricted common stock at a per share price equal to 80% of the average closing price for 5 consecutive days prior to notice of conversion. The 6287182 Canada Debenture is due July 31, 2016 and accrues interest at the rate of 12% per annum. The Company is required to pay the accrued interest quarterly commencing on the date of execution and quarterly thereafter.
|
|
2.
|
The Company also assume a second note which is a 12% convertible debenture in the principal amount of $50,000 to Brevets Futek MSM Ltee, a private corporation organized under the laws of Canada. In accordance with the terms and provisions of the convertible debenture, the Company may redeem by paying the principal plus accrued interest. Brevets Futek MSM Ltee has the right to convert the principal into shares of our restricted common stock at a per share price equal to 80% of the average closing price for price for 5 consecutive days prior to notice of conversion. The convertible debenture is due July 17, 2016 and accrues interest at the rate of 12% per annum. The Company is required to pay the accrued interest quarterly commencing on the date of execution and quarterly thereafter
|
F-11
NOTE 7 – COMMON STOCK
On February 25, 2014 the Company issued 24,856,676 shares of stock for a reduction of debt of $124,283.
On March 14, 2014 the company issued 2,310,939,804 to effectuate the merger.
On March 17, 2014 the Company issued 1,222,000,000 shares for services rendered valued at market price on the date of issuance of .02 resulting in an expense of $24,440,000.
On March 19, 2014 the Company issued 70,500,000 shares for debt valued at $70,500.
NOTE 8 – RELATED PARTY TRANSACTIONS
Stock Issuances
The Company issuance of 1,222,000,000 shares in March 2014 were issued to its officers and directors.
License
The licensing agreement referred to in note 4 is with a related party, a major shareholder of the Company.
Payroll
The Company paid in chief executive officer $80,000 which is included in the statement of operations under payroll expenses.
NOTE 9 – INCOME TAX
Deferred taxes are provided on a liability method whereby deferred tax assets are recognized for deductible temporary differences and operating loss and tax credit carryforwards and deferred tax liabilities are recognized for taxable temporary differences. Temporary differences are the differences between the reported amounts of assets and liabilities and their tax bases. Deferred tax assets are reduced by a valuation allowance when, in the opinion of management, it is more likely than not that some portion or all of the deferred tax assets will not be realized. Deferred tax assets and liabilities are adjusted for the effects of changes in tax laws and rates on the date of enactment.
F-12
Net deferred tax assets consist of the following components as of March 31, 2014:
|
March 31, 2014
|
||||
|
Deferred Tax Assets – Non-current:
|
||||
|
NOL Carryover
|
$
|
141,066
|
||
|
Payroll Accrual
|
-
|
|||
|
Less valuation allowance
|
|
(141,066)
|
||
|
Deferred tax assets, net of valuation allowance
|
$
|
-
|
||
The income tax provision differs from the amount of income tax determined by applying the U.S. federal income tax rate to pretax income from continuing operations for the period ended March 31, 2014 due to the following:
|
2014
|
||||
|
Book Income
|
$ | (24,661,847 | ) | |
|
Meals and Entertainment
|
681 | |||
|
Stock for Services
|
24,440,100 | |||
|
Payroll
|
80,000 | |||
|
Valuation allowance
|
141,066 | |||
| $ | - | |||
At March 31, 2014, the Company had net operating loss carry forwards of approximately $141,066 that may be offset against future taxable income from the year 2014 to 2034. No tax benefit has been reported in the March 31, 2014 financial statements since the potential tax benefit is offset by a valuation allowance of the same amount.
Due to the change in ownership provisions of the Tax Reform Act of 1986, net operating loss carry forwards for Federal Income tax reporting purposes are subject to annual limitations. Should a change in ownership occur, net operating loss carry forwards may be limited as to use in future years.
NOTE 10 – SUBSEQUENT EVENTS
Management has evaluated subsequent events pursuant to the requirements of ASC Topic 855 and has determined that other than the one listed below no material subsequent events exist.
|
1.
|
In April 2014 the company issued 28,595,002 shares of stock for services and debt.
|
F-13
ITEM 9. CHANGES AND DISAGREEMENT WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
APPOINTMENT OF TERRY L. JOHNSON
Effective January 20, 2014, we dismissed Patrick Rodgers, CPA, P.A. ("Rodgers") as our independent registered public accounting firm. We engaged Terry L. Johnson, CPA ("Johnson") as our principal independent registered public accounting firm effective January 20, 2014. The decision to change our principal independent registered public accounting firm was approved by our Board of Directors.
The reports of Rodgers on our financial statements for fiscal year ended March 31, 2013 (which included the balance sheet as of March 31, 2013 and the statement of operations, cash flows and stockholders' equity as of March 31, 2013, for the past fiscal year, did not contain an adverse opinion or a disclaimer of opinion, nor qualified or modified as to uncertainty, audit scope or accounting principles, other than to state that there is substantial doubt as to our ability to continue as a going concern. During our fiscal year ended March 31, 2013 and during the subsequent period through to the date of Rodgers' resignation, there were no disagreements between us and Rodgers, whether or not resolved, on any matter of accounting principles or practices, financial statement disclosure, or auditing scope or procedure, which, if not resolved to the satisfaction of Rodgers, would have caused Rodger to make reference thereto in his report on our audited financial statements.
We provided Rodgers with a copy of the Current Report on Form 8-K and requested that Rodgers furnish us with a letter addressed to the Securities and Exchange Commission stating whether or not Rodgers agrees with the statements made in the Current Report on Form 8-K with respect to Rodgers and, if not, stating the aspects with which he does not agree. We received the requested letter from Rodgers wherein he confirmed his agreement to our disclosures in this Current Report with respect to Rodgers. A copy of Rodgers' letter was filed as an exhibit to the Current Report with the Securities and Exchange Commission on April __, 2013
In connection with our appointment of Johnson as our principal registered accounting firm at this time, we have not consulted Johnson on any matter relating to the application of accounting principles to a specific transaction, either completed or contemplated, or the type of audit opinion that might be rendered on our financial statements during the two most recent fiscal years (March 31, 2013 and 2012) and subsequent interim period through the date of engagement.
APPOINTMENT OF RODGERS
Effective April 16, 2013, our certifying accountant, KBL, LLP ("KBL"), resigned as our independent registered public accounting firm. We engaged Patrick R. Rodgers ("Rodgers") as our principal independent registered public accounting firm effective May 1, 2013. The decision to change our principal independent registered public accounting firm has been approved by our Board of Directors.
The report of KBL on our financial statements for fiscal year ended March 31, 2012 (which included the balance sheet as of March 31, 2012, and the statement of operations, cash flows and stockholders’ equity for the year ended March 31, 2012 (and development stage period from inception through March 31, 2012), did not contain an adverse opinion or disclaimer of opinion, nor was it modified as to uncertainty, audit scope or accounting principles, other than to state that there is substantial doubt as to our ability to continue as a going concern. During our fiscal year ended March 31, 2012 and during the subsequent period through to the date of KBL's resignation, there were no disagreements between us and KBL, whether or not resolved, on any matter of accounting principles or practices, financial statement disclosure, or auditing scope or procedure, which, if not resolved to the satisfaction of KBL, would have caused KBL to make reference thereto in its report on our audited financial statements.
42
We provided KBL with a copy of the Current Report on Form 8-K and requested that KBL furnish us with a letter addressed to the Securities and Exchange Commission stating whether or not KBL agreed with the statements made in the Current Report on Form 8-K with respect to KBL and, if not, stating the aspects with which they do not agree. We received the requested letter from KBL wherein it confirmed its agreement to our disclosures in the Current Report with respect to KBL. A copy of KBL's letter was filed as an exhibit to the Current Report filed with the Securities and Exchange Commission on April __, 2013.
In connection with our appointment of Rodgers as our principal registered accounting firm at that time, we had not consulted Rodgers on any matter relating to the application of accounting principles to a specific transaction, either completed or contemplated, or the type of audit opinion that might be rendered on our financial statements. .
Evaluation of disclosure controls and procedures.
We maintain controls and procedures that are designed to ensure that information required to be disclosed in the reports that we file or submit under the Securities Exchange Act of 1934 is recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms, and that such information is accumulated and communicated to our management including our principal executive and principal financial officers, as appropriate, to allow timely decisions regarding required disclosures. Based upon their evaluation of those controls and procedures performed as of the end of the period covered by this report, our principal executive officer and our principal financial officer concluded that our disclosure controls and procedures were not effective.
Management’s annual report on internal control over financial reporting.
Our Chief Executive Officer and our Chief Financial Officer are responsible for establishing and maintaining adequate internal control over financial reporting. Internal control over financial reporting is defined in Rule 13a-15(f) and 15d-15(f) promulgated under the Securities Exchange Act of 1934 as a process designed by, or under the supervision of, our principal executive and principal financial officers and effected by our Board of Directors, management and other personnel, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles and includes those policies and procedures that:
|
·
|
Pertain to the maintenance of records that in reasonable detail accurately and fairly reflect the transactions and dispositions of our assets;
|
|
·
|
Provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that our receipts and expenditures are being made only in accordance with authorizations of management and our directors; and
|
|
·
|
Provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of our assets that could have a material effect on the financial statements.
|
Because of its inherent limitations, our internal control over financial reporting may not prevent or detect misstatements. Therefore, even those systems determined to be effective can provide only reasonable assurance with respect to financial statement preparation and presentation. Projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
43
Our Chief Executive Officer and our Chief Financial Officer assessed the effectiveness of our internal control over financial reporting as of March 31, 2014. In making this assessment, management used the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission (“COSO”) in Internal Control — Integrated Framework.
Based on our assessment, our Chief Executive Officer and our Chief Financial Officer believe that, as of March 31, 2014, our internal control over financial reporting is not effective based on those criteria, due to the following:
|
·
|
Deficiencies in Segregation of Duties. Lack of proper segregation of functions, duties and responsibilities with respect to our cash and control over the disbursements related thereto due to our very limited staff, including our accounting personnel.
|
|
·
|
Deficiencies in the staffing of our financial accounting department. The number of qualified accounting personnel with experience in public company SEC reporting and GAAP is limited. This weakness does not enable us to maintain adequate controls over our financial accounting and reporting processes regarding the accounting for non-routine and non-systematic transactions. There is a risk that a material misstatement of the financial statements could be caused, or at least not be detected in a timely manner, by this shortage of qualified resources.
|
|
·
|
Lack of audit committee.
|
In light of this conclusion and as part of the preparation of this report, we have applied compensating procedures and processes as necessary to ensure the reliability of our financial reporting. Accordingly, management believes, based on its knowledge, that (1) this report does not contain any untrue statement of a material fact or omit to state a material face necessary to make the statements made not misleading with respect to the period covered by this report, and (2) the financial statements, and other financial information included in this report, fairly present in all material respects our financial condition, results of operations and cash flows for the years and periods then ended.
This report does not include an attestation report of our registered public accounting firm regarding internal control over financial reporting. Management’s report was not subject to attestation by our registered public accounting firm pursuant to the rules of the SEC that permit us to provide only management’s report in this report.
Changes in internal control over financial reporting
There were no significant changes in our internal control over financial reporting during the fourth quarter of the year ended March 31, 2014, that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
None.
44
PART III
Executive Officers and Directors
In accordance with the terms and provisions of the Securities Exchange Agreement, there was a change in our control. In accordance with the terms and provisions of the Securities Exchange Agreement, the Board of Directors accepted the resignations of its officers and directors as follows: (i) Gilbert Pomerleau as the Treasurer/Chief Financial Officer and member of the Board of Directors effective March 18, 2014; and (ii) Ghislaine St.-Hilaire as the President/Chief Executive Officer and as a member of the Board of Directors effective March 19, 2014. Simultaneously, the Board of Directors appointed John Lai as the President/Chief Executive Officer and member of the Board of Directors and John F. Dolan as the Secretary, Treasurer/Chief Financial Officer and a member of the Board of Directors. Therefore, our directors and principal executive officers are as specified on the following table:
|
Name
|
Age
|
Position
|
||
|
John Lai
|
51 |
President/Chief Executive Officer and a Director
|
||
|
John Dolan
|
49 |
Secretary, Treasurer/Chief Financial Officer and a Director
|
John Lai. Mr. John Lai has over thirty years of senior operations and financial experience and has served as president, chief financial officer and director of a number of corporations with a record of facilitating acquisitions, business launches, reverse mergers, and driving production revenue growth. Mr. Lai is recognized as an expert in the Powersports industry. He is on the expert consulting staff of Cohen research in NYC. Mr. Lai also contracts out to give analysis on the Powersports industry to mutual funds such as Janus, Neuberger Bergmen, and Fidelity.
Mr. Lai currenty serves as chief executive officer and chairman of PetVivo since inception in 2013. PetVivo, based in Minneapolis, Minnesota, is an emerging biomedical device company focused on the licensing and commercialization of innovative medical devices for pets, or pet therapeutics. Mr. Lai also currently serves as chief executive officer and a director of Blue Earth Resources from 2012. Blue Earth, based in Burnsville, Minnesota, engages in the acquisition, operation and management of majority working interests inproducing oil and gas leases haveing wells in certain major oilfields of the United States. Its current oil and gas properties include producing oil and gaswells in northwestern Louisiana leases, which have many remaining undrilledlocations toprovide considerable future drilling and expansion opportunities for BlueEarth. Mr. Lai served as chief executive officer and a director of Rovrr Inc. from 2008-2011, which offers advanced marketing solutions and proven methodologies to deliver successful social media monetization applications with high user acceptance. Mr. Lai served as president and director of Viper Powersports since inception in 2002-2008. He was responsible for raising $28million in private equity. He managed the design and production of four of the state of the art American made cruisers and engine platforms in the motorcycle industry. He also negotiated the acquisition of Thor Inc. and two pending acquisitions within the Powersports industry. Mr. Lai has also served as a director and chief financial officer of Buyitnow.com from 1996-1999 where he managed the completion of a $35,000,000 private placement through Paine Webber. He was responsible financial operations and forecasting with revenues over $40,000,000 and 100 employees.
Mr. Lai served as an advisor to Tech-Squared and raised private capital, which later became Digital River (DRIV), where he provided advice on financing options, management team. Mr. Lai formed Genesis Capital Group, Inc. in 1992 as a Merchant Banking boutique focused on mergers and acquisitions, reverse mergers, deal structuring, and equity placements. Genesis Capital will commit its own capital to bring a transaction to its fruition. Mr. Lai has benefitted from years of networking within the industry to solve problems and situations in the small cap arena. Mr. Lai has completed over five transactions since the early 1990’s.
Prior to forming Genesis Capital Group, Inc. in 1992, he held varies positions at investment firms. Between 1985 - 1992, Mr. Lai held positions at banking firms based in Chicago IL and NYC, NY. and Mpls, MN. He has been active in several charitable organizations. Mr. Lai has been quoted in Dow Jones News, Investors Daily ,Minnesota Business Journal, Wall Street Journal, Finance and Commerence and several other business publications.
45
John F. Dolan. Mr. John Dolan as served as a director of PetVivo. Mr. Dolan is also corporate and intellectual property counsel for Holt Power Group Inc. Prior to joining Holt Power Group Inc., Mr. Dolan was a shareholder in Fredrikson & Byron's intellectual property group and was a co-chair of its Cleantech group. Mr. Dolan works with corporations to strategically secure and protect domestic and foreign patent rights in a variety of technologies, including chemical compounds and compositions, industrial processes, films and coatings, biomass and biomaterials, mechanical devices, food products, packaging, recycled and building materials, biofuels and other renewable energies.
Mr. Dolan also advises companies on all aspects of intellectual property asset protection as well as technology and corporate development. Consultations include projects related to technology transfer and licensing, intellectual property due diligence in mergers, acquisitions and investments, product clearance analysis and opinions, business plan development, corporate set-up and structure strategies and patent litigation.
Mr. Dolan has also assisted entrepreneurs in the formation and development of new companies and has provided target identification and negotiation services related to venture funding, strategic partnering, licensing and merger and acquisitions. Mr. Dolan was also the founder of a company that commercialized a green technology where he crafted the strategy for the development, protection and utilization of unique intellectual property to raise capital, manufacture and commercialize products and license its technology.
Mr. Dolan has served as a patent examiner with the U.S. Patent and Trademark Office where he examined patent applications related to organic chemistry and biotechnology. This opportunity coupled with his legal experience has provided him a unique perspective of the intellectual property field.
All directors hold office until the completion of their term of office, which is not longer than one year, or until their successors have been elected. All officers are appointed annually by the board of directors and, subject to employment agreements (which do not currently exist), serve at the discretion of the board. Currently, our directors receive no compensation.
There is no family relationship between any of our officers or directors. For the past ten years, there have been no orders, judgments, or decrees of any governmental agency or administrator, or of any court of competent jurisdiction, revoking or suspending for cause any license, permit or other authority to engage in the securities business or in the sale of a particular security or temporarily or permanently restraining any of our officers or directors from engaging in or continuing any conduct, practice or employment in connection with the purchase or sale of securities, or convicting such person of any felony or misdemeanor involving a security, or any aspect of the securities business or of theft or of any felony.
CORPORATE GOVERNANCE
Committees
Our Board of Directors does not currently have a compensation committee or nominating and corporate governance committee because, due to the Board of Director’s composition and our relatively limited operations, the Board of Directors believes it is able to effectively manage the issues normally considered by such committees. Our Board of Directors may undertake a review of the need for these committees in the future.
Audit Committee and Financial Expert
Presently, the Board of Directors acts as the audit committee. The Board of Directors does not have an audit committee financial expert. The Board of Directors has not yet recruited an audit committee financial expert to join the board of directors because we have only recently commenced a significant level of financial operations.
46
Code of Ethics
We do not currently have a Code of Ethics that applies to all employees, including our principal executive officer, principal financial officer, principal accounting officer or controller, or persons performing similar functions. We plan to adopt a Code of Ethics.
Director Independence
None of our directors are deemed independent. Our directors also hold positions as officers.
SUMMARY COMPENSATION TABLE
The table set forth below summarizes the annual and long-term compensation for services in all capacities to us payable to our principal executive officers during the years ended March 31, 2014 and 2013.
|
Summary Compensation Table
|
|||||||||||||||||||||||||||||||||||
|
Name and Principal Position
|
Year
|
Salary ($)
|
Bonus ($)
|
Stock Awards ($)
|
Option Awards ($)
|
Non-Equity Incentive Plan Compensation ($)
|
Nonqualified Deferred Compensation Earnings ($)
|
All Other Compensation ($)
|
Total ($)
|
||||||||||||||||||||||||||
|
John Lai, current President/CEO and Director
|
2014
|
80,000 | 0 | 0 | 0 | 0 | 0 | 0 | 80,000 | ||||||||||||||||||||||||||
|
John F. Dolan, current Secretary, Treasurer/CFO and Director
|
2014
|
0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||||||||||||||||||||||||
|
Gilbert Pomerleau,
Prior CFO, Treasurer, Secretary and Director
|
2014
2013
|
0
0
|
0
0
|
0
0
|
0
0
|
0
0
|
0
0
|
0
0
|
0
0
|
||||||||||||||||||||||||||
|
Ghislaine St-Hilaire, Prior President and Director
|
2014
2013
|
0
0
|
0
0
|
0
0
|
0
0
|
0
0
|
0
0
|
0
0
|
0
0
|
||||||||||||||||||||||||||
47
OUTSTANDING EQUITY AWARDS
As of March 31, 2014, the following named executive officers had the following unexercised options, stock that has not vested, and equity incentive plan awards:
|
Option Awards
|
Stock Awards
|
|||||||||||||||||||||||||||||||||||
|
Name
|
Number of Securities Underlying Unexercised Options
# Exercisable
|
# Un-exercisable
|
Equity Incentive Plan Awards: Number of Securities Underlying Unexercised Options
|
Option Exercise Price
|
Option Expiration Date
|
Number of Shares or Units of Stock Not Vested
|
Market Value of Shares or Units Not Vested
|
Equity Incentive Plan Awards: Number of Unearned Shares, Units or Other Rights Not Vested
|
Value of Unearned Shares, Units or Other Rights Not Vested
|
|||||||||||||||||||||||||||
|
John Lai, current President/CEO and Director
|
0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||||||||||||||||||||||||||
|
John F. Dolan, current Secretary, Treasurer/CFO and Director
|
0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||||||||||||||||||||||||||
|
Gilbert Pomerleau,
Prior CFO, Treasurer, Secretary and Director
|
0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||||||||||||||||||||||||||
|
Ghislaine St-Hilaire, Prior President and Director
|
0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||||||||||||||||||||||||||
STOCK OPTIONS//SAR GRANTS. No grants of stock options or stock appreciation rights were made during the fiscal year ended March 31, 2014.
LONG TERM INCENTIVE PLANS.
There are no arrangements or plans in which we provide pension, retirement or similar benefits for directors or executive officers. We do not have any material bonus or profit sharing plans pursuant to which cash or non-cash compensation is or may be paid to our directors or executive officers.
Our directors received the following compensation for their service as directors during the fiscal year ended March 31, 2014:
|
|
Fees Earned or Paid in Cash
$
|
Stock Awards
$
|
Option Awards
$
|
Non-Equity Incentive Plan Compensation
$
|
Non-Qualified Deferred Compensation Earnings
$
|
All Other Compensation
$
|
Total
$
|
||||||||||||||
|
John Lai
|
0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||||||||||||
|
John F. Dolan
|
0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||||||||||||
|
Gilbert Pomerleau, Prior director
|
0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||||||||||||
|
Ghislaine St-Hilaire, prior Director
|
0 | 0 | 0 | 0 | 0 | 0 | 0 |
48
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
The following table sets forth certain information regarding the beneficial ownership of our common stock as of June 30, 2014 by each person or entity known by us to be the beneficial owner of more than 5% of the outstanding shares of common stock, each of our directors and named executive officers, and all of our directors and executive officers as a group.
|
Name and Address of Beneficial Owner
Officers and Directors
|
Amount and Nature of Beneficial Owner
|
Percent of Class (1)
|
|
|
Common Stock
|
John Lai
12100 Singletree Lane
Suite 186
Eden Prairie, Minnesota 55344
|
1,774,969,312 shares,
President/CEO and Director
|
46.96%
|
|
Common Stock
|
John F. Dolan
12100 Singletree Lane
Suite 186
Eden Prairie, Minnesota 55344
|
360,726,723 shares
Secretary, Treasurer/CFO and Director
|
9.54%
|
|
Common Stock
|
All directors and named executive officers as a group (2 persons)
|
2,135,696,035 shares
|
56.51%
|
|
Name and Address of 5% or Greater Beneficial Owner
|
|||
|
Common Stock
|
Gel-Del Technologies Inc.
1000 Westgate Drive
St. Paul, Minnesota 55114
|
703,055,020 shares,
|
18.60%
|
|
Common Stock
|
Dave Masters
5344 Penn Avenue South
Minneapolis, Minnesota 55419
|
349,761,041 shares
|
9.25%
|
|
Common Stock
|
Randall A. Meyer
4016 Inglewood Avenue South
Edina, Minnesota 55416
|
349,761,041 shares
|
9.25%
|
|
(1)
|
Percentage of beneficial ownership of our common stock is based on 3,779,542,482 shares of common stock outstanding as of the date of this Annual Report.
|
Beneficial ownership is determined in accordance with the rules of the Securities and Exchange Commission and generally includes voting or investment power with respect to securities. In accordance with Securities and Exchange Commission rules, shares of our common stock which may be acquired upon exercise of stock options or warrants which are currently exercisable or which become exercisable within 60 days of the date of the table are deemed beneficially owned by the optionees. Subject to community property laws, where applicable, the persons or entities named in the table above have sole voting and investment power with respect to all shares of our common stock indicated as beneficially owned by them.
Changes in Control
Our management is not aware of any arrangements which may result in “changes in control” as that term is defined by the provisions of Item 403(c) of Regulation S-K.
49
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS AND DIRECTOR INDEPENDENCE
Related party transactions - Stock Issuances.
During March 2014, we issued an aggregate 1,222,000,000 shares of our common stock to our officers and directors.
There have been no other related party transactions, or any other transactions or relationships required to be disclosed pursuant to Item 404 of Regulation S-K.
Audit Fees
The aggregate fees billed for the fiscal years ended March 31, 2014 and 2013 for professional services rendered by the principal accountant for the audit of our annual financial statements and quarterly review of the financial statements included in our Form 10-K or services that are normally provided by the accountant in connection with statutory and regulatory filings or engagements for those fiscal years were $10,000 and $10,000 respectively.
Audit-Related Fees
For the fiscal years ended March 31, 2014 and 2013, there were no fees billed for services reasonably related to the performance of the audit or review of the financial statements outside of those fees disclosed above under “Audit Fees.”
Tax Fees
For the fiscal years ended March 31, 2014 and 2013, there were no fees billed for services for tax compliance, tax advice, and tax planning work by our principal accountants.
All Other Fees
None.
Pre-Approval Policies and Procedures
Prior to engaging our accountants to perform a particular service, our Board of Directors obtains an estimate for the service to be performed. All of the services described above were approved by the Board of Directors in accordance with its procedures.
50
PART IV
|
(a)
|
Financial Statements.
|
Included in Item 8
|
(b)
|
Exhibits required by Item 601.
|
|
Exhibit No.
|
Description
|
|
|
3.1
|
Articles of Incorporation, incorporated by reference to Exhibit 3.1 of our Registration Statement on Form S-1 filed on April 18, 2011
|
|
|
3.2
|
Certificate of Amendment to Articles of Incorporation, incorporated by reference to Exhibit 3.1 of our Registration Statement on Form S-1 filed on April 18, 2011
|
|
|
3.3
|
Certificate of Amendment to Articles of Incorporation incorporated by reference to Exhibit 3.1 of Current Report on Form 8-K filed on March 10, 2014
|
|
|
3.4
|
Certificate of Amendment to Articles of Incorporation incorporated by reference to Exhibit 3.1 of Current Report on Form 8-K filed on April 7, 2014.
|
|
|
3.5
|
Bylaws, incorporated by reference to Exhibit 3.1 of our Registration Statement on Form S-1 filed on April 18, 2011
|
|
|
10.1
|
Letter of Intent between Technologies Scan Corp. and 6285431 Canada Inc. dated September 5, 2012 incorporated by reference to Exhibit 10.1 of the Current Report on Form 8-K filed with the Securities and Exchange Commission on September 11, 2012.
|
|
|
10.2
|
Rescission Agreement between Technologies Scan Corp. and 6285431 Canada Inc. dated April 12, 2013 incorporated by reference to Exhibit 10.1 of the Current Report on Form 8-K filed with the Securities and Exchange Commission on April 18, 2013.
|
|
|
10.3
|
Letter of Intent between Technologies Scan Corp. and Social Geek Media Inc. dated April 6, 2013 incorporated by reference to Exhibit 10.1 of the Current Report on Form 8-K filed with the Securities and Exchange Commission on May 6, 2013.
|
|
|
10.4
|
Memorandum of Amendment between Technologies Scan Corp. and Social Geek Media Inc. dated May 17, 2013 incorporated by reference to Exhibit 10.1 of the Current Report on Form 8-K filed with the Securities and Exchange Commission on May 21, 2013.
|
|
|
10.5
|
12% Convertible Debenture of $100,000 between Technologies Scan Corp. and 6287182 Canada Inc. incorporated by reference to Exhibit 10.1 of the Current Report on Form 8-K filed with the Securities and Exchange Commission on July 31, 2013.
|
|
|
10.6
|
12% Convertible Debenture of $100,000 between Technologies Scan Corp. and Brevets Futek MSM Ltee. incorporated by reference to Exhibit 10.2 of the Current Report on Form 8-K filed with the Securities and Exchange Commission on July 31, 2013.
|
|
|
10.7
|
Rescission Agreement dated November 9, 2013 among Social Geek Meda Inc., Patrick Aube and Technologies Scan Corp. incorporated by reference to Exhibit 10.1 of the Current Report on Form 8-K filed with the Securities and Exchange Commission on November 13, 2013
|
|
|
10.8
|
Letter of Intent dated December 16, 2013 between FedTech Services Inc. and Technologies Scan Corp. incorporated by reference to Exhibit 10.1 of the Current Report on Form 8-K filed with the Securities and Exchange Commission on December 19, 2013
|
|
|
10.9
|
Term Sheet between Technologies Scan Corp. and PetVivo Inc. dated February 10, 2014 incorporated by reference to Exhibit 10.1 of the Current Report on Form 8-K filed with the Securities and Exchange Commission on February 13, 2014.
|
|
|
10.10
|
Settlement Agreement dated February 2, 2014 between Technologies Scan Corp. and Ghislaine St.-Hilaire incorporated by reference to Exhibit 10.1 of the Current Report on Form 8-K filed with the Securities and Exchange Commission on February 24, 2014.
|
|
|
10.11
|
Securities Exchange Agreement among Technologies Scan Corp., PetVivo Inc. and shareholders of PetVivo Inc. dated March 21, 2014 incorporated by reference to Exhibit 10.1 of the Current Report on Form 8-K filed with the Securities and Exchange Commission on March 13, 2014.
|
|
|
10.12
|
Convertible Promissory Note dated March 17, 2014 between Technologies Scan Corp. and 9165-5643 Quebec Inc incorporated by reference to Exhibit 10.1 of the Current Report on Form 8-K filed with the Securities and Exchange Commission on March 21, 2014.
|
|
|
10.13
|
Convertible Promissory Note dated March 17, 2014 between Technologies Scan Corp. and Elden Brochu incorporated by reference to Exhibit 10.2 of the Current Report on Form 8-K filed with the Securities and Exchange Commission on March 21, 2014.
|
|
|
10.14
|
Convertible Promissory Note dated March 17, 2014 between Technologies Scan Corp. and Gina Drouin incorporated by reference to Exhibit 10.1 of the Current Report on Form 8-K filed with the Securities and Exchange Commission on March 21, 2014.
|
|
|
10.15
|
Convertible Promissory Note dated March 17, 2014 between Technologies Scan Corp. and Christian Fontaine incorporated by reference to Exhibit 10.1 of the Current Report on Form 8-K filed with the Securities and Exchange Commission on March 21, 2014
|
|
|
10.16
|
Convertible Promissory Note dated March 17, 2014 between Technologies Scan Corp. and Ferme Semen Inc. incorporated by reference to Exhibit 10.1 of the Current Report on Form 8-K filed with the Securities and Exchange Commission on March 21, 2014
|
|
|
10.17
|
Term Sheet dated June 2, 2014 between Technologies Scan Corp. and Gel-Del Technologies Inc. incorporated by reference to Exhibit 10.1 of the Current Report on Form 8-K filed with the Securities and Exchange Commission on June 2, 2014.
|
|
|
16.1
|
Letter from KBL LLP dated May 24, 2013 incorporated by reference to Exhibit 16.1 of the Current Report on Form 8-K filed with the Securities and Exchange Commission on May 28, 2013.
|
|
|
31.1
|
Certification of Principal Executive Officer Required By Rule 13a-14(A) of the Securities Exchange Act of 1934, As Amended, As Adopted Pursuant To Section 302 of the Sarbanes-Oxley Act of 2002*
|
|
|
31.2
|
Certification of Principal Financial Officer Required By Rule 13a-14(A) of the Securities Exchange Act of 1934, As Amended, As Adopted Pursuant To Section 302 of the Sarbanes-Oxley Act of 2002*
|
|
|
32.1
|
Certification of Principal Executive Officer, pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002*
|
|
|
32.2
|
Certification of Principal Financial Officer, pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002*
|
|
|
101.INS
|
XBRL Instance Document**
|
|
|
101.SCH
|
XBRL Taxonomy Schema**
|
|
|
101.CAL
|
XBRL Taxonomy Calculation Linkbase**
|
|
|
101.DEF
|
XBRL Taxonomy Definition Linkbase**
|
|
|
101.LAB
|
XBRL Taxonomy Label Linkbase**
|
|
|
101.PRE
|
XBRL Taxonomy Presentation Linkbase**
|
__________
* Filed herewith.
51
Pursuant to the requirements of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
|
PetVivo Holdings, Inc.
a Nevada corporation
|
|||
|
July 14, 2014
|
By:
|
/s/ John Lai
|
|
|
|
|
John Lai
|
|
|
|
Its:
|
President, Director
(Principal Executive Officer)
|
|
|
|
|
|
|
|
July 14, 2014
|
By:
|
/s/ John F. Dolan
|
|
|
|
|
John F. Dolan
|
|
|
|
Its:
|
Chief Financial Officer, Secretary, Treasurer, Director
(Principal Financial and Accounting Officer)
|
|
In accordance with the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the Registrant and in the capacities and on the dates indicated.
|
July 14, 2014
|
By:
|
/s/ John Lai
|
|
|
|
John Lai
|
|
|
|
Its:
|
President, Director
|
|
|
|
|
(Principal Executive Officer)
|
|
| July 14, 2014 |
By:
|
/s/ John F. Dolan
|
|
|
|
John F. Dolan
|
|
|
|
Its:
|
Chief Financial Officer, Secretary, Treasurer, Director
|
|
|
|
|
(Principal Financial and Accounting Officer)
|
|
52
