Attached files
| file | filename |
|---|---|
| EX-31.3 - EX-31.3 - Forest Laboratories, LLC | d750120dex313.htm |
| EX-31.4 - EX-31.4 - Forest Laboratories, LLC | d750120dex314.htm |
Table of Contents
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 10-K/A
Amendment No. 1
(Mark one)
| x | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the Fiscal Year Ended March 31, 2014
| ¨ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to
Commission File Number: 1-5438
FOREST LABORATORIES, INC.
(Exact name of registrant as specified in its charter)
| Delaware (State or other jurisdiction of incorporation or organization) |
11-1798614 (I.R.S. Employer Identification No.) | |
| 909 Third Avenue New York, New York (Address of principal executive offices) |
10022-4731 (Zip Code) | |
(212) 421-7850
(Registrant’s telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class |
Name of each exchange on which registered | |
| Common Stock, $.10 par value | New York Stock Exchange |
Securities registered pursuant to Section 12(g) of the Act:
None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes x No ¨
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ¨ No x
Note-Checking the box above will not relieve any registrant required to file reports pursuant to Section 13 or 15(d) of the Exchange Act from their obligations under those Sections.
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes x No ¨
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Website, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes x No ¨
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (§229.405 of this chapter) is not contained herein, and will not be contained, to the best of the registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ¨
Indicate by a check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer”, “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check one):
| Large accelerated filer | x | Accelerated filer | ¨ | |||
| Non-accelerated filer | ¨ (Do not check if a smaller reporting company) | Smaller reporting company | ¨ | |||
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ¨ No x
The aggregate market value of the voting stock held by non-affiliates of the registrant as of September 30, 2013 was $11,403,997,603.
Number of shares outstanding of the registrant’s Common Stock as of June 25, 2014: 273,283,124
The following documents are incorporated by reference herein:
None
Table of Contents
(Quick Links)
| 1 | ||||
| ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE |
1 | |||
| 9 | ||||
| 37 | ||||
| ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE |
41 | |||
| 43 | ||||
| 45 | ||||
| 45 | ||||
| EXHIBIT 31.3 |
||||
| EXHIBIT 31.4 |
||||
Table of Contents
EXPLANATORY NOTE
This Amendment No. 1 on Form 10-K/A (this “Amendment”) is being filed with respect to the Annual Report of Forest Laboratories, Inc., a Delaware corporation (the “Company”, or “Forest”, or “we” or “our”) on Form 10-K for the fiscal year ended March 31, 2014 filed with the Securities and Exchange Commission (“SEC”) on May 30, 2014 (the “Original 10-K”). The Original 10-K omitted certain information required by Part III (Items 10 through 14) of Form 10-K, which had been contemplated to be incorporated by reference from the Company’s definitive Proxy Statement for its 2014 annual meeting of shareholders (the “Proxy Statement”), in reliance on General Instruction G(3) of Form 10-K. As the Company’s Proxy Statement is not expected to be filed within 120 days after the end of the Company’s 2014 fiscal year, the Company is filing this Amendment to provide the disclosures required by Part III pursuant to General Instruction G(3) of Form 10-K.
Specifically, in accordance with Rule 12b-15 under the Securities Exchange Act of 1934, as amended (the “Exchange Act”), portions of Items 10 through 14 of the Original 10-K, which were originally omitted and incorporated by reference to the Proxy Statement, have been amended to include this information, and Part IV, Item 15 of the Original 10-K has been amended solely to include as exhibits the new certifications required by Rule 13a-14(a) under the Exchange Act. Because no financial statements have been included in this Amendment and this Amendment does not contain or amend any disclosure with respect to Items 307 and 308 of Regulation S-K, paragraphs 3, 4 and 5 of the certifications required by Section 302 of the Sarbanes-Oxley Act of 2002 and the certifications required by Section 906 of the Sarbanes-Oxley Act of 2002 have been omitted.
Except as described above, no other changes have been made to the Original 10-K. The Original 10-K continues to speak as of the date of the Original 10-K, and the Company has not updated the disclosures contained therein to reflect any events which occurred at a date subsequent to the filing of the Original 10-K other than as expressly indicated in this Amendment. Accordingly, this Amendment should be read in conjunction with the Original 10-K and the Company’s other filings made with the SEC on or subsequent to May 30, 2014.
Table of Contents
Item 10. Directors, Executive Officers and Corporate Governance
Directors
The names of the members of our board of directors (the “Board” or “Board of Directors”) and certain biographical information concerning each of them are set forth below:
| Name |
Age | First Became Director | Position with the Company | |||
| Howard Solomon |
86 | 1964 | Director, Chairman and Senior Advisor | |||
| Brenton L. Saunders |
44 | 2011 | Chief Executive Officer, President, and Director | |||
| Nesli Basgoz, M.D. |
56 | 2006 | Director | |||
| Christopher J. Coughlin |
61 | 2011 | Director | |||
| Kenneth E. Goodman |
66 | 1998 | Director | |||
| Vincent J. Intrieri |
57 | 2013 | Director | |||
| Pierre Legault |
54 | 2012 | Director | |||
| Gerald M. Lieberman |
67 | 2011 | Director | |||
| Lawrence S. Olanoff, M.D., Ph.D. |
62 | 2006 | Director | |||
| Lester B. Salans, M.D. |
78 | 1998 | Director | |||
| Peter J. Zimetbaum, M.D. |
50 | 2009 | Director |
Howard Solomon (Director since 1964)
Mr. Solomon, 86, has been Forest’s Chairman since 1998, and currently serves as a Senior Advisor to the Company and as a Partner of Hildred Capital Partners, LLC. Mr. Solomon also served as Forest’s Chief Executive Officer from 1977 to September 2013 and President from January 2011 to September 2013. He began his career as an attorney at leading law firms in New York and joined Forest in 1964 as a director and secretary of the Board while serving as outside counsel for the Company. Mr. Solomon is a Trustee of the New York Presbyterian Hospital and previously served on the Board of Cold Spring Harbor Laboratories. He is currently a member of the Executive Committee of the Board of Directors of the Metropolitan Opera and Chairman of its Finance Committee. He also serves on the Board of the New York City Ballet. Mr. Solomon graduated from the City College of New York and holds a J.D. from Yale University.
We believe that Mr. Solomon’s experience as a senior executive and leader in our industry, his in-depth knowledge of our Company and its day-to-day operations and his strong record and strategic vision for the Company qualify him to serve on our Board.
Brenton L. Saunders (Director since 2011)
Mr. Saunders, 44, was appointed Chief Executive Officer and President of Forest effective October 1, 2013. Prior to joining Forest, he served as the Chief Executive Officer and board member of Bausch + Lomb Incorporated from March 2010 until August 2013. Previously, Mr. Saunders served as a senior executive with Schering-Plough from 2003 to 2010, most recently as President of Global Consumer Health Care. He also served as Head of Integration for both Schering-Plough’s merger with Merck & Co. and for its $16 billion acquisition of Organon BioSciences. Before joining Schering-Plough, Mr. Saunders was a Partner and Head of the Compliance Business Advisory Group at PricewaterhouseCoopers LLP from 2000 to 2003. Prior to that, he was Chief Risk Officer at Coventry Health Care between 1998 and 1999 and co-founded the Health Care Compliance Association in 1995. Mr. Saunders began his career as Chief Compliance Officer for the Thomas Jefferson University Health System. He serves on the boards of ElectroCore LLC and the Overlook Hospital Foundation. He is also the former Chairman of the New York chapter of the American Heart Association. He is also a member of the Board of Trustees of the University of Pittsburgh. He received a B.A. from the University of Pittsburgh, an M.B.A. from Temple University School of Business, and a J.D. from Temple University School of Law.
1
Table of Contents
Mr. Saunders’ leadership experience as CEO of both the Company and Bausch + Lomb Incorporated, and deep pharmaceutical experience make him an asset to the Board. In addition to his other attributes, his 15 years of senior compliance experience and broad regulatory expertise at a number of different companies, including Bausch + Lomb and Schering-Plough, are particularly valuable.
Nesli Basgoz, M.D. (Director since 2006)
Dr. Basgoz, 56, is the Associate Chief for Clinical Affairs, Division of Infectious Diseases at Massachusetts General Hospital (MGH) and serves on the hospital’s Board of Trustees. In addition, Dr. Basgoz is an Associate Professor of Medicine at Harvard Medical School. Previously, she served as Clinical Director in the Infectious Diseases Division of MGH for six years. Dr. Basgoz earned her M.D. degree and completed her residency in internal medicine at Northwestern University Medical School. She also completed a fellowship in the Infectious Diseases Division at the University of California at San Francisco. She is board certified in both infectious diseases and internal medicine.
Dr. Basgoz’s broad medical expertise and nationally recognized leadership in the medical field, as well as her extensive clinical trial experience has equipped her to effectively advise the Board and management with respect to many strategic matters, including navigating regulatory approvals and the clinical trial process. Moreover, her particular expertise in infectious diseases has enabled Dr. Basgoz to advise the Board and management with respect to the Company’s current and potential portfolio of drugs within the relevant indications, including our Teflaro® product and other antibiotics under development or being considered for development at the Company.
Christopher J. Coughlin (Director since 2011)
Mr. Coughlin, 61, served as an advisor to Tyco International from 2010 until September 30, 2012, and is currently serving as a senior advisor at McKinsey & Company. He was Executive Vice President and Chief Financial Officer of Tyco International from 2005 to 2010. During his tenure, he played a central role in the separation of Tyco into five independent, public companies and provided financial leadership surrounding major transactions, including the $2 billion acquisition of Broadview Security, among many other responsibilities and accomplishments. Prior to joining Tyco, he worked as the Chief Operating Officer of the Interpublic Group of Companies from June 2003 to December 2004, as Chief Financial Officer from August 2003 to June 2004 and as a director from July 2003 to July 2004. Previously, Mr. Coughlin was Executive Vice President and Chief Financial Officer of Pharmacia Corporation from 1998 until its acquisition by Pfizer in 2003. Prior to that, he was Executive Vice President of Nabisco Holdings and President of Nabisco International. From 1981 to 1996 he held various positions, including Chief Financial Officer, at Sterling Drug. Mr. Coughlin is currently serving as the lead independent director on the board of Dun & Bradstreet, where he is a former member of the Audit Committee, chairs the Board Affairs Committee, and is a member of the Compensation and Benefits Committee. He also serves on the board of Covidien plc, where he is Chair of the Compliance Committee and a member of its Transaction Committee, and Dipexium Pharmaceuticals, Inc., where he is the Chairman of the Audit Committee and a member of the Nominating and Corporate Governance Committee. In addition, Mr. Coughlin previously served on the boards of the Interpublic Group of Companies, Monsanto Company and Perrigo Company. Mr. Coughlin has a B.S. in accounting from Boston College.
A veteran of service and leadership on public company boards, Mr. Coughlin’s wide array of senior management positions in global companies, pharmaceutical background, finance experience and compliance and governance expertise enhances the Board’s ability to make strategic decisions for the long-term growth of the Company.
Kenneth E. Goodman (Director since 1998)
Mr. Goodman, 66, is the former President and Chief Operating Officer of Forest, a position that he held from 1998 to 2006. For 18 years prior thereto, Mr. Goodman served as Forest’s Vice President, Finance and Chief Financial Officer and was named Executive Vice President, Operations in February 1998.
2
Table of Contents
From 1975 to 1980, he served as a senior financial officer at Wyeth, and before that, as a C.P.A. at Main Hurdman, which is now part of KPMG LLP. Mr. Goodman currently serves Syracuse University as Vice Chairman of the Board of Trustees, a member of the Executive Committee and Chairman of the Audit Committee; he previously served as Chairman of the Budget Committee. He is also Chairman of the International Board of Directors of the Israel Cancer Research Fund and Co-Chairman of its New York Board. Mr. Goodman is a C.P.A. and holds a B.S. degree from The Whitman School of Management at Syracuse University.
Mr. Goodman’s intimate knowledge of the Company’s operations, having served as President and Chief Operating Officer of Forest with broad responsibility for sales, commercial operations, compliance, manufacturing operations, information technology and other areas, his substantial expertise in financial matters, and his service as an important interface between management and the Board as its presiding independent director, make him a valuable member of the Board.
Vincent J. Intrieri (Director since 2013)
Mr. Intrieri, 57, has, since October 1998, been employed in various investment-related capacities by several entities controlled by Carl C. Icahn. Since January 2008, Mr. Intrieri has served as Senior Managing Director of Icahn Capital LP, the entity through which Carl C. Icahn manages private investment funds. In addition, since November 2004, Mr. Intrieri has been a Senior Managing Director of Icahn Onshore LP, the general partner of Icahn Partners LP, and Icahn Offshore LP, the general partner of Icahn Partners Master Fund LP, entities through which Mr. Icahn invests in securities. Mr. Intrieri has been a director of: Transocean Ltd., a provider of offshore contract drilling services for oil and gas wells, since May 2014; Forest Laboratories, Inc., a supplier of pharmaceutical products, since June 2013; CVR Refining, LP, an independent downstream energy limited partnership, since January 2013; Navistar International Corporation, a truck and engine manufacturer, since October 2012; and Chesapeake Energy Corporation, an oil and gas exploration and production company, since June 2012. Mr. Intrieri was previously: a director of CVR Energy, Inc., a diversified holding company primarily engaged in the petroleum refining and nitrogen fertilizer manufacturing industries, from May 2012 to May 2014; a director of Federal–Mogul Corporation, a supplier of automotive powertrain and safety components, from December 2007 to June 2013; a director of Icahn Enterprises L.P. (a diversified holding company engaged in a variety of businesses, including investment, automotive, energy, gaming, railcar, food packaging, metals, real estate and home fashion) from July 2006 to September 2012, and was Senior Vice President of Icahn Enterprises L.P. from October 2011 to September 2012; a director of Dynegy Inc., a company primarily engaged in the production and sale of electric energy, capacity and ancillary services, from March 2011 to September 2012; chairman of the board and a director of PSC Metals Inc., a metal recycling company, from December 2007 to April 2012; a director of Motorola Solutions, Inc., a provider of communication products and services, from January 2011 to March 2012; a director of XO Holdings, a competitive provider of telecom services, from February 2006 to August 2011; a director of National Energy Group, Inc., a company that was engaged in the business of managing the exploration, production and operations of natural gas and oil properties, from December 2006 to June 2011; a director of American Railcar Industries, Inc., a railcar manufacturing company, from August 2005 until March 2011, and was a Senior Vice President, the Treasurer and the Secretary of American Railcar Industries from March 2005 to December 2005; a director of WestPoint Home LLC, a home textiles manufacturer, from November 2005 to March 2011; chairman of the board and a director of Viskase Companies, Inc., a meat casing company, from April 2003 to March 2011; and a director of WCI Communities, Inc., a homebuilding company, from August 2008 to September 2009. Mr. Intrieri graduated in 1984, with Distinction, from The Pennsylvania State University (Erie Campus) with a B.S. in Accounting. Mr. Intrieri was a certified public accountant.
Mr. Intrieri was selected as a director nominee pursuant to the terms of a Nomination and Standstill Agreement as discussed below.
3
Table of Contents
Pierre Legault (Director since 2012)
Mr. Legault, 54, has served as Chief Executive Officer since October 2013 and previously as Executive Chairman of Nephrogenex, a pharmaceutical company focused on the treatment of diabetic kidney disease, since November of 2012, and is the President and CEO of Stone Management LLC, a firm focused in the areas of business development and board assistance, since April 2012. From 2010 to 2012, he was the President and Chief Executive Officer of Prosidion Ltd., a UK mid-size biotechnology firm and fully integrated subsidiary of Astellas Pharmaceuticals. From 2009 to 2010, he served as Executive VP, Chief Financial Officer and Treasurer of OSI Pharmaceuticals, a mid-size biotechnology company. He was also Senior Executive VP and Chief Administrative Officer of Rite Aid Corporation, a Fortune 500 pharmaceutical retail company, from July 2007 to 2009. From January 2006 to July 2007 Mr. Legault was President of the Eckerd Group and Group Senior Executive VP of PJC Inc., a public pharmaceutical retail North American Group. Previously, Mr. Legault held several senior positions over a period exceeding 15 years with Sanofi-Aventis and predecessor companies, last serving as Worldwide President of a large global Sanofi-Aventis business unit from 2003 to 2005. Prior positions included the Senior VP Deputy CEO and Chief Financial Officer of Aventis Pharmaceuticals Inc. (2000 to 2003), Global Senior VP Finance and Treasurer of Hoechst Marion Roussel, Inc. (1998 to 2000), VP and Chief Financial Officer, North America Finance, IT and Administration of Marion Merrell Dow, Inc. (1997 to 1998), and VP and Chief Financial Officer of Marion Merrell Dow Pharmaceutical Canada (1990 to 1996). In addition, Mr. Legault has served since November 2013 on the board of Regado Biosciences, a publicly traded pharmaceuticals company and on the board of Nephrogenex. He also has served on several public, private and nonprofit company boards and audit committees, as well as on several advisory boards, including the following: OSI Investment Holdings GMBH (Chairman), a venture capital firm (2009 to 2012), Cyclacel Pharmaceutical Inc., a publicly traded biotech company (2006 to 2008), PJC Inc., a publicly traded pharmacy retail company (2005 to 2007), as well as other private boards. Mr. Legault studied at the Harvard Business School, McGill University and University of Montreal (HEC) and holds BAA, MBA, CA and CPA degrees.
Based upon Mr. Legault’s impressive background as an executive officer and board member of various companies, including pharmaceuticals and biotech companies, the Board believes that Mr. Legault has the requisite set of skills to serve as a Board member of the Company.
Gerald M. Lieberman (Director since 2011)
Mr. Lieberman, 67, most recently served as the President and Chief Operating Officer, as well as a member of the board of directors of AllianceBernstein from 2004 to 2009, where he oversaw several critical functions for AllianceBernstein, including finance, global risk management, technology, operations, human resources and investor and public relations. In addition, he was instrumental in developing AllianceBernstein’s global integrated platform and enhancing its corporate governance and financial transparency. Prior to joining AllianceBernstein in 1998, Mr. Lieberman held a number of senior positions at Fidelity Investments from 1993 to 1998, including Chief Financial Officer and Chief of Administration and he was a member of Fidelity’s operating committee, reporting directly to the Chairman. Before joining Fidelity, Mr. Lieberman spent 14 years with Citicorp, where he served as Senior Human Resources Officer and a member of the policy committee, reporting to the Company’s Chairman and Chief Executive Officer. At Citicorp, he also held several other senior leadership positions, including Chief Executive Officer of Citibank Mexico and Division Head of Latin America. Mr. Lieberman served as a director at Computershare. He served for nine years as a trustee of the University of Connecticut Foundation and was a practicing C.P.A with Arthur Andersen. He received a B.S. from the University of Connecticut and attended New York University’s Graduate School of Business Administration.
Mr. Lieberman’s senior roles at AllianceBernstein and Fidelity Investments, premier investment and asset management firms, and his breadth and depth of experiences, including his finance and accounting expertise and career-long focus on risk management, and his extensive global experience enable him to provide important and valuable perspectives to the Board.
4
Table of Contents
Lawrence S. Olanoff, M.D., Ph.D. (Director since 2006)
Dr. Olanoff, 62, served as Forest’s Chief Operating Officer from 2006 to 2010 and currently serves as Senior Scientific Advisor to the Company. From July 2005 to October 2006, Dr. Olanoff was President and Chief Executive Officer at Celsion Corporation, an oncology drug development company. He also served as Executive Vice President and Chief Scientific Officer of Forest from 1995 to 2005. Prior to joining Forest in 1995, Dr. Olanoff served as Senior Vice President of Clinical Research and Development at Sandoz Pharmaceutical Corporation (now a division of the Novartis Group) and at the Upjohn Company in a number of positions including Corporate Vice President of Clinical Development and Medical Affairs. In addition, he is currently an adjunct Assistant Professor and Special Advisor to the President for Corporate Relations at the Medical University of South Carolina (MUSC), an ex-officio Director of the MUSC Foundation for Research Development, which is a non-profit foundation created to benefit the university. He is also Chairman of the Board of the Clinical Biotechnology and Research Institute of Roper St. Francis Hospitals, and a member of the Board for the Horizon Project and the Institute of Applied Neurosciences, all non-profit organizations based in Charleston, SC. He holds a Ph.D. in biomedical engineering and an M.D. degree from Case Western Reserve University.
Dr. Olanoff’s detailed knowledge of the pharmaceutical industry, his broad operational experience and research and development leadership over the course of his career at Forest, Sandoz and Upjohn, including with respect to 30 product approvals, and his service as a senior executive and intimate knowledge of Forest’s operations combine to make him an important asset to the Board.
Lester B. Salans, M.D. (Director since 1998)
Dr. Salans, 78, is a Clinical Professor and member of the Clinical Attending Staff of Internal Medicine at the Mount Sinai Medical School. Prior thereto, Dr. Salans was a senior executive at Sandoz Pharmaceutical Corporation and Novartis Pharmaceuticals Corporation. Dr. Salans is a former Director of the National Institutes of Arthritis, Diabetes, Digestive and Kidney Diseases of the National Institutes of Health. He was a Professor of Medicine and Dean of the Faculty of the Mt. Sinai Medical School and Senior Vice President of the Mt. Sinai Medical Center in New York. He served as Professor of Medicine and Director of the Division of Endocrinology at the Dartmouth Hitchcock Medical Center, Hanover, from 1968 to 1975. He also founded and is president of LBS Advisors, Inc., a consultancy serving several pharmaceutical and biotechnology companies, academic institutions, the National Institutes of Health and many investment firms. He serves on the board of directors of PharmaIN Corporation, a biopharmaceutical company. Dr. Salans earned a B.A. from University of Michigan and M.D. from University of Illinois.
Dr. Salans’ recognized leadership in the medical field, his varied positions in the pharmaceutical sector, and particular medical expertise in the fields of diabetes mellitus, obesity and endocrinology and clinical research experience bring valuable perspectives to the Board on research and development matters generally and with respect to the Company’s current and potential portfolio drugs within such indications. As a practicing physician in addition to his other roles, Dr. Salans bridges the gap between basic science and clinical medicine, enabling him to offer valuable insights to the Board.
Peter J. Zimetbaum, M.D. (Director since 2009)
Dr. Zimetbaum, 50, has served as Director of Clinical Cardiology at Beth Israel Deaconess Medical Center in Boston (BIDMC) since 2005 and is the Director of ECG and Arrhythmia Core Laboratory at the Harvard Clinical Research Institute. Additionally, since 2006, Dr. Zimetbaum has been an Associate Professor of Medicine at the Harvard Medical School (HMS), and he currently serves on the HMS Standing Committee on Conflicts of Interest. Dr. Zimetbaum received his M.D. degree from the Albert Einstein College of Medicine in 1990 and is board certified in both cardiovascular medicine and cardiovascular electrophysiology.
5
Table of Contents
Dr. Zimetbaum’s extensive experience in the practice of medicine and clinical trials provides the Board and management with the perspectives of physicians and other healthcare providers who use the Company’s products and with insight into the clinical trial process. His expertise in cardiology, including the cardiovascular safety profile of products, is a valuable resource to the Board and management in analyzing and developing current and potential portfolio drugs. In addition, his service on Harvard Medical School’s conflict of interest committee provides the Company with important insights on the ethics of healthcare.
Nomination and Standstill Agreement and Appointment of Vincent J. Intrieri to the Board
On June 10, 2013, the Company entered into a Nomination and Standstill Agreement (the Agreement) with Carl C. Icahn, Vincent J. Intrieri, Icahn Capital LP and certain affiliated entities (collectively, the Icahn Group) pursuant to which, among other things, the Icahn Group agreed not to conduct a proxy contest for the election of directors with respect to the 2013 Annual Meeting of the Stockholders (the 2013 Annual Meeting) or acquire more than 15% of the Company’s common stock for at least one year after the effective date of the Agreement, subject to certain exceptions. Pursuant to the Agreement, the Company increased the size of the Board from ten to eleven members, appointed Mr. Intrieri, Senior Managing Director of Icahn Capital LP, to the Board, and included Mr. Intrieri in its slate of nominees for election to the Board at the 2013 Annual Meeting. The Company further agreed to use its reasonable best efforts to cause the election of the Mr. Intrieri to the Board at the 2013 Annual Meeting, including recommending that the Company’s stockholders vote in favor of Mr. Intrieri along with the Company’s slate of nominees.
For so long as Mr. Intrieri is on the Board, the Icahn Group has agreed to vote all of its shares of common stock of the Company in favor of the election of all of the Company’s director nominees at each annual meeting of the Company. Also pursuant to the Agreement, effective June 10, 2013 and until the date when Mr. Intrieri ceases to be a member of the Board, subject to limited exceptions, the Icahn Group has also agreed to adhere to certain standstill obligations, including the obligation to not solicit proxies or consents or influence others with respect to the same. The Icahn Group has further agreed, effective June 10, 2013 and until the later of the date when Mr. Intrieri ceases to be a member of the Board and the one-year anniversary of the Agreement, and subject to certain exceptions, that the Icahn Group will not acquire or otherwise beneficially own more than 15% of the Company’s outstanding voting securities.
Pursuant to the Agreement, the Company appointed Mr. Intrieri to the Board’s Succession Planning Committee. In addition, from the effective date of the Agreement until the Company’s 2014 Annual Meeting of Stockholders (the 2014 Annual Meeting), for so long as Mr. Intrieri is a member of the Board, the Board will include him as a member of an Executive Committee formed by the Board and will generally consider the appointment and employment of executive officers and M&A transactions at the full Board level or in committees of which Mr. Intrieri is a member. The size of the Board will not be increased beyond 11 persons prior to the 2014 Annual Meeting.
In the event Mr. Intrieri resigns from the Board or is rendered unable to, or refuses to, be appointed to, or for any other reason fails to serve or is not serving on the Board (other than as a result of not being nominated by the Company for election to the Board at any annual meeting subsequent to the 2013 Annual Meeting), the Icahn Group will be entitled to designate a replacement director that is approved by the Company, such approval not to be unreasonably withheld. For any annual meeting subsequent to the 2013 Annual Meeting, the Company is required to notify the Icahn Group in writing no less than 45 calendar days in advance of the Company’s advance notice deadline whether Mr. Intrieri will be nominated for election by the Company at such annual meeting. If Mr. Intrieri is so nominated, the Company will use commercially reasonable efforts to cause his election to the Board.
If at any time the Icahn Group ceases to hold a net long position, as defined in the Agreement, of more than 15,331,002 shares of the Company’s common stock, the Company’s obligations under the Agreement terminate and Mr. Intrieri must resign from the Board.
The foregoing is not a complete description of the terms of the Agreement. For a further description of the terms of the Agreement, including a copy of the Agreement, please see our Current Report on Form 8-K that we filed with the SEC on June 11, 2013.
6
Table of Contents
Section 16(a) Beneficial Ownership Reporting Compliance
Federal securities laws require our executive officers and directors and persons owning more than 10% of our common stock to file certain reports on ownership and changes in ownership with the SEC. We believe that during the fiscal year ended March 31, 2014, our directors, executive officers and 10% shareholders complied with all Section 16(a) filing requirements. In making these statements, we have relied upon examination of copies of Forms 3, 4 and 5 provided to us and the written representations of our directors and officers.
Audit Committee
Our Board has established a standing Audit Committee in accordance with NYSE rules and Section 10A of the Exchange Act and related SEC rules and regulations. The current members of the Audit Committee are Christopher J. Coughlin, Pierre Legault, and Lester B. Salans, M.D. The Board has determined that each of these individuals meets the independence requirements of NYSE and SEC rules and regulations and is financially literate. The Board has also determined that each of Messrs. Coughlin and Legault qualify as an “audit committee financial expert” as defined by applicable SEC rules and regulations. Our Audit Committee Charter is available on our website by clicking on the “Corporate Governance” link under the “Investor Center” section at www.frx.com. The charter is also available in print to any requesting stockholder.
Executive Officers
The name, present position with the Company and age of each of the Company’s executive officers are set forth below. No executive officers are related to any other executive officer, and no executive officer was selected pursuant to any arrangement or understanding between the executive officer, director, or director nominee and any other person.
| Name |
Age | Position with Forest | ||
| Brenton L. Saunders |
44 | Chief Executive Officer and President | ||
| Elaine Hochberg |
57 | Executive Vice President – International, Strategic Planning & Government Affairs | ||
| Bill Meury |
46 | Executive Vice President – Sales and Marketing | ||
| Francis I. Perier, Jr. |
54 | Executive Vice President – Chief Financial Officer | ||
| Marco Taglietti, M.D. |
54 | Executive Vice President – Drug Development & Research, and Chief Medical Officer | ||
| Bob Bailey |
50 | Senior Vice President – Chief Legal Officer, General Counsel and Corporate Secretary | ||
| Alex Kelly |
48 | Senior Vice President – Chief Communications and Investor Relations Officer | ||
| Karen Ling |
50 | Senior Vice President – Chief Human Resources Officer | ||
| Kevin Walsh |
59 | Senior Vice President – Operations | ||
| Joseph Zimmerman |
44 | Senior Vice President – Chief Compliance Officer |
Set forth below is certain biographical information concerning our executive officers who are not also directors:
Elaine Hochberg
Ms. Hochberg is Executive Vice President, International, Strategic Planning and Government Affairs. She previously served as the Executive Vice President and Chief Commercial Officer from December 2010 to December 2013. Prior thereto, Ms. Hochberg was Senior Vice President—Marketing from December 1999 through December 2010 and also served as the Chief Commercial Officer from December 2007 to December 2013. Ms. Hochberg joined Forest in June 1997 as Vice President—Marketing of Forest’s wholly-owned subsidiary Forest Pharmaceuticals, Inc. In February 1998, she was promoted to Vice President—Marketing of Forest. Prior to joining Forest in 1997, Ms. Hochberg was Assistant Vice President—Marketing at Wyeth-Lederle Laboratories.
7
Table of Contents
Bill Meury
Mr. Meury is Executive Vice President, Sales and Marketing at Forest. He joined Forest in 1993 and has held positions in Marketing, New Products, Business Development, and Sales. Most recently, as Senior Vice President, Global Commercial and U.S. Marketing, Mr. Meury oversaw the activities of several departments including Product Management, Market Research, and Commercial Assessments, as well as Forest’s Global Marketing and Early Commercialisation groups. Mr. Meury has directed 10 product launches during his tenure at Forest. Before joining Forest, Mr. Meury worked in public accounting for Reznick Fedder & Silverman and in financial reporting for MCI Communications. He has a B.S. in Economics from the University of Maryland.
Francis I. Perier, Jr.
Mr. Perier has served as Executive Vice President, Chief Financial Officer, at Forest since December 31, 2010. Prior thereto, Mr. Perier served as Senior Vice President—Finance from September 2004 through December 2010 and has also served as Chief Financial Officer since September 2004. From March 2004 until joining Forest in September 2004, Mr. Perier was Vice President—Finance—Operations Planning—Americas Medicines at Bristol-Myers Squibb. For eight years prior, Mr. Perier served in senior financial positions at Bristol-Myers Squibb Company including four years as Vice President—Finance, Planning, Business Development and Information Technology at its ConvaTec Division. Prior to that, Mr. Perier was a partner at Deloitte & Touche, L.L.P. Mr. Perier is a certified public accountant and received a B.S. from Villanova University and a M.B.A. from New York University.
Marco Taglietti, M.D.
Dr. Taglietti is Executive Vice President, Drug Development and Research, and Chief Medical Officer at Forest. He continues to be President of the Forest Research Institute. He previously served as Senior Vice President—Research and Development of Forest and President, Forest Research Institute, Inc. from December 2010 to December 2013. Prior thereto, Dr. Taglietti served as Vice President—Research and Development from December 2008 to December 2010. Dr. Taglietti joined Forest in August 2007 as Executive Vice President—Research and Development and Chief Medical Officer of Forest Research Institute, Inc. Prior to joining Forest, Dr. Taglietti was Senior Vice President and Head of Global Research and Development at Stiefel Laboratories for three years. Prior to that, Dr. Taglietti was at Schering-Plough Corporation for twelve years in positions of increasing responsibility including Vice President—Worldwide Clinical Research for Anti-Infectives, Oncology, CNS, Endocrinology and Dermatology. Dr. Taglietti received his medical degree and board certifications from the University of Pavia in Italy.
Bob Bailey
Mr. Bailey is Senior Vice President, Chief Legal Officer, General Counsel and Corporate Secretary at Forest. He previously served from 2007 to 2013 as Executive Vice President, Law, Policy and Communications at Bausch + Lomb. Before joining Bausch + Lomb in 1994, Mr. Bailey was an attorney at Nixon Peabody (formerly Nixon Hargrave Devans & Doyle). Mr. Bailey received his law degree from the University of Minnesota and his undergraduate degree from St. Olaf College in Northfield, MN.
Alex Kelly
Mr. Kelly is Senior Vice President, Chief Communications & Investor Relations Officer at Forest. He joined Forest in October 2013. Previously, Mr. Kelly was vice president, Investor Relations at Bausch + Lomb in 2013 and senior vice president, Investor Relations at Merck & Co. from 2009 to 2013. Mr. Kelly served as Group Vice President, Global Communications and Investor Relations at Schering Plough from 2007 to 2009. He has 24 years of experience in healthcare, including 10 years of sales and sales management experience. Mr. Kelly received a B.S. from Purdue University.
8
Table of Contents
Karen Ling
Ms. Ling is Senior Vice President and Chief Human Resources Officer at Forest. Ms. Ling joined Forest in January 2014 from Merck & Co., Inc., where she served as Senior Vice President, Human Resources, for the company’s Global Human Health and Consumer Care businesses worldwide. Prior to that role at Merck, she was Vice President, Compensation and Benefits. Before Merck, Ms. Ling was Group Vice President, Global Compensation & Benefits at Schering-Plough. She also spent 14 years at Wyeth in various positions of responsibility in human resources as well as in Wyeth Pharmaceutical’s Labour and Employment Department. Prior to joining Wyeth, Ms. Ling practiced corporate law with Goldstein and Manello, P.C. in Boston. Ms. Ling holds a B.A. from Yale University and a J.D. from Boston University School of Law.
Kevin Walsh
Mr. Walsh is Senior Vice President, Operations at Forest. In this capacity, he is responsible for Quality and Manufacturing Operations, including all supply chain, technology transfer and facilities engineering functions. Mr. Walsh is also responsible for Information Systems. He joined Forest as Vice President, Information Systems in 2003, after serving as Vice President of Information Technology at Roche Pharmaceuticals.
Joseph Zimmerman
Mr. Zimmerman is Senior Vice President, Chief Compliance Officer at Forest. Mr. Zimmerman oversees the operations of Forest’s Comprehensive Compliance Programme, which is designed to ensure compliance with applicable laws and regulations and the requirements of Forest’s Corporate Integrity Agreement. Mr. Zimmerman also serves as Chairperson of Forest’s Corporate Compliance Committee. Since joining Forest in 1995, Mr. Zimmerman has held various positions of increasing responsibility in Sales, Sales Management, Commercial and Corporate Training and Development functions. In 2004, he was named Director and Chief Compliance Officer of the newly established Corporate Compliance Department. In 2009, he was named Vice President Compliance; and in 2010, he was named Corporate Vice President. Prior to joining Forest, Mr. Zimmerman began his career with an industrial biotechnology start-up company. Mr. Zimmerman holds a B.S. from Bradley University.
Item 11. Executive Compensation
EXECUTIVE COMPENSATION DISCUSSION AND ANALYSIS
Executive Summary
Introduction
Our executive compensation programs are designed to attract, retain and reward our executive officers. This Compensation Discussion and Analysis provides a detailed description of the Company’s executive compensation philosophy and programs, the compensation decisions made by the Compensation Committee of the Board (the Compensation Committee) and the issues considered in making such decisions. This Compensation Discussion and Analysis focuses on the compensation of our CEO, retired CEO, CFO, and the next three most highly compensated executive officers (referred to herein as our named executive officers, or the NEOs) for the fiscal year ended March 31, 2014 (fiscal year 2014), who were:
9
Table of Contents
| Name |
Title | |
| Brenton L. Saunders | Chief Executive Officer and President | |
| Howard Solomon | Retired Chief Executive Officer and President | |
| Elaine Hochberg | Executive Vice President – International, Strategic Planning and Government Affairs | |
| Francis I. Perier, Jr. | Executive Vice President – Chief Financial Officer | |
| Marco Taglietti, M.D. | Executive Vice President – Drug Development and Research, and Chief Medical Officer | |
| Karen Ling | Senior Vice President – Chief Human Resources Officer |
Fiscal Year 2014 Performance Highlights
During 2014, we made significant progress towards rejuvenating the Company and increasing shareholder value.
| • | Since our new CEO, Brent Saunders, joined the Company on October 1, 2013, the Company was the top performing company among the S&P 500 listed companies, generating a total return of 116% compared to 13% for the S&P 500 average. |
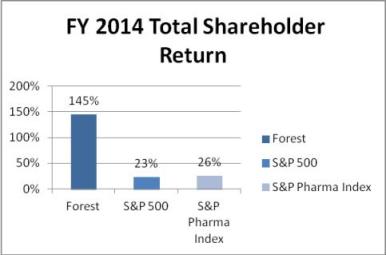
| • | Full fiscal year 2014 Total Shareholder Return of 145% (ranked second among S&P 500 companies in total return), as compared to the S&P 500 (23%) and S&P Pharma Index (26%). |
| • | The proposed combination of Actavis and Forest is another illustration of our focus on creating shareholder value. By combining a leading global generic company with a growing specialty pharmaceutical product offering and a fast growing predominantly U.S. specialty pharmaceutical company, we are working to create a new breed of specialty pharmaceutical company. |
Our executive team established a track record of success by focusing on four key priorities aimed at increasing shareholder value: sales growth, developing our drug pipeline, reducing costs and leveraging our balance sheet.
10
Table of Contents
Sales Growth
| • | Grew net sales by 21% over the prior fiscal year, driven by next generation products that grew by 58% and contributed over $1.4 billion to our sales. |
| • | Launched two new drugs, FETZIMA and NAMENDA XR, in the U.S., COLOBREATHE in Europe and TUDORZA and BYSTOLIC in Canada. We also re-launched SAPHRIS in the U.S. after acquiring it in January 2014 |
| • | Secured formulary access for NAMENDA XR on 9 of the Top 10 Medicare formularies as the market continues to transition from twice-a-day to NAMENDA XR based on value proposition to patients, care givers and payors |
| • | Developed the first-ever direct-to-consumer television campaign at the Company to educate silent-suffering IBS-constipation & chronic constipation patients about LINZESS |
Developing Drug Pipeline
| • | Achieved FDA approval to market FETZIMA for patients with major depressive disorder—a first pass approval |
| • | Achieved Canadian approval to market TUDORZA and CONSTELLA |
| • | Submitted the pediatric written request with the expectation of securing an additional six months of pediatric exclusivity for NAMENDA |
| • | Filed two new fixed-dose combinations for approval: NAMENDA XR + DONEPEZIL and BYSTOLIC + VALSARTAN |
| • | Gained FDA support for our plans to file the CEFTAZIDIME—AVIBACTIM combination for serious infections |
Reducing Costs
| • | Fiscal year 2014 Non-GAAP EPS nearly quadrupled from $0.45 to $1.78 per share, while GAAP EPS increased to $0.61 per share following a loss of $0.12 per share in fiscal year 2013. |
| • | Successfully implemented Project Rejuvenate—a three-pronged cost reduction and productivity enhancement program. |
| • | Reduced total headcount by more than 600 positions without touching the customer facing sales force |
| • | On track to reduce cost base by $500 million by the end of fiscal year 2016 with 65-75% of the savings expected in fiscal year 2015 |
Leveraging Balance Sheet
| • | Used Ex-US cash to acquire SAPHRIS for less than 2x current year sales |
| • | Completed a $1.2 billion public bond offering, Forest’s first ever |
| • | Acquired Aptalis—the biggest acquisition in the Company’s history—for $2.9 billion in cash and funded it with an additional $1.8 billion bond offering |
2014 Compensation Highlights
| • | Our financial results for fiscal year 2014 led to above-target annual incentive award payouts for our NEOs. Our adjusted revenue of $3.5 billion and Non-GAAP Diluted EPS of $1.83 (each as calculated in accordance with the Incentive Plan) exceeded our target estimates. As a result of this strong performance, payments under our annual incentive program were 144% of target, before adjustment for individual performance. |
| • | At the time of their hiring, we made one-time equity award grants to our new CEO and to our Senior Vice President & Chief Human Resources Officer. These awards were comprised of a mix of stock options and restricted stock or restricted stock units that include multi-year vesting requirements. In addition, these executives made direct purchases of Company stock. |
11
Table of Contents
| • | In fiscal year 2014, we continued to make annual equity award grants, in the form of stock options, restricted stock and performance share units. Our performance share units include performance hurdles for revenue and total shareholder return, which strengthen the alignment of executive compensation with shareholder interests. |
Basic Principles of our Executive Compensation Program
Our executive compensation program is designed to drive performance while promoting good corporate governance. The Compensation Committee annually assesses and, if appropriate, updates the Company’s compensation practices to ensure alignment with these goals. We have highlighted below important aspects of our executive compensation practices as well as certain practices which we believe serve our stockholders’ long-term interests.
| Majority of Pay is Performance Based. The majority of the total compensation opportunity for our NEOs is not guaranteed. We believe allocating compensation in this manner is in the best long-term interests of our stockholders. | Stock Ownership Guidelines. We have stock ownership guidelines for our senior management team, including each NEO. | |
| Pay for Performance Awards. Cash bonuses are determined pursuant to an annual incentive bonus plan implemented at the beginning of the fiscal year which establishes performance goals. In addition, our executives receive one-third to one-half of their equity compensation in the form of performance-based stock equivalent units (also referred to as performance stock units or PSUs), which vest based on the satisfaction of pre-established corporate performance goals. | Holding Period for Stock Options, Restricted Stock and Performance Stock Units. With certain limited exceptions, our stock ownership guidelines require our key executives to hold all shares obtained upon the exercise of Company stock options or through Company restricted stock grants and performance stock units until such executive achieves the applicable ownership threshold under the stock ownership guidelines. | |
| Minimal Perquisites. We provide our NEOs with minimal perquisites. | Minimum Vesting Periods. All stock options and restricted stock awards granted to all executive officers are required to have a vesting schedule that exceeds 24 months. | |
| No Repricing of Awards. The Company does not have a history of repricing equity incentive awards, and the Company’s 2007 Equity Incentive Plan expressly prohibits such practices, as well as repurchases of “underwater” stock options or stock appreciation rights. | Clawback Policy. The Company has an executive incentive compensation recoupment (clawback) policy to promote accountability. Pursuant to the policy, the Company may recoup certain incentive- or commission-based payments which were based upon any financial results or operating metrics that were impacted by such executive officer’s knowing or intentional fraudulent or illegal conduct. | |
| No Above Market Returns. We do not offer preferential or above market returns on deferred compensation. | No-Hedging Policy. Our Directors and executive officers, including our NEOs, are prohibited from hedging their ownership of the Company’s stock, including trading in options, puts, calls or other derivative instruments related to Company stock or debt. | |
| No Underpriced Stock Options. We do not grant stock options with exercise prices that are below the fair market value of the Company’s common stock on the date of grant. | Independent Compensation Consultant. The Compensation Committee utilizes the services of an independent compensation consulting firm that provides no other services to the Company. | |
12
Table of Contents
Elements of Executive Compensation at a Glance
The elements we use to achieve our compensation objectives and to enable the Company to retain, motivate, engage and reward our NEOs and other executives include base salary, annual incentive compensation and long-term equity compensation.
| Element / Type of Plan |
Link to Compensation Objectives |
Key Features | ||
| Base Salary (Cash) |
• Fixed amount of compensation for performing day-to-day responsibilities.
• Provides financial stability and security. |
• Competitive pay that takes into consideration the salary paid to persons in similar positions by companies in the Comparator Group, the executive’s performance, criticality of the executive’s position, and the executive’s knowledge, skills and experience.
• Executives may be eligible for an annual increase depending on individual performance, their level of pay compared to the salaries paid to persons in similar positions by companies in the Comparator Group, and upon a change in role and responsibilities. | ||
| Annual Incentive (Cash) |
• Motivates and rewards achievement of key operational and individual goals measured over the current fiscal year.
• Utilization of multiple performance metrics and a range of payouts based on the achievement of such metrics (as opposed to an “all or nothing” payout scheme) functions to mitigate risk. |
• Target bonuses for fiscal year 2014 were 125% of base salary for our CEO and 65% of base salary for our other NEOs, and were determined based on the executive’s level of responsibility and upon an examination of compensation paid to persons in similar positions by companies in the Comparator Group.
• Total potential payouts range from 0–200% of target payout.
• Goals and weighting are set annually, and the financial metrics are tied to the Company’s annual budget. | ||
| Long-Term Incentive (Equity) |
• Motivates and rewards for sustaining long-term financial and operational performance that increases stockholder value.
• Encourages continued employment through vesting periods in order to obtain shares.
• Stock ownership and holding period requirements align the financial interests of our executives with the financial interests of our stockholders and function to mitigate risk. |
• Long-term incentive target award opportunities are granted to have a dollar value based on a multiple of an executive’s base salary.
• PSUs:
• Vest at the conclusion of a multi-year performance period based on the achievement of two equally weighted performance metrics
• Stock Options:
• Generally vest in four equal annual installments on each anniversary of the grant date
• Time-based Restricted Stock Awards:
• Generally vest in three annual installments | ||
13
Table of Contents
Our Compensation Philosophy and Objectives
Philosophy
Our executive compensation philosophy seeks to align executive compensation with the achievement of the Company’s business objectives and with individual performance directed towards obtaining those objectives. Consequently, the Company’s compensation program is designed to reward our executives’ respective contributions to the Company’s achievements and is intended to facilitate long-term strategic management and the enhancement of stockholder value. In considering the elements of the compensation program, the Compensation Committee emphasizes pay for performance on both an annual and long-term basis and consideration of marketplace best practices.
Objectives
Our compensation program is designed to achieve the following objectives:
| • | Attract and retain highly qualified executives; |
| • | Motivate individual performance; |
| • | Align incentives with business objectives; |
| • | Create incentives that focus executives on, and reward them for, increasing stockholder value; |
| • | Maintain equitable levels of overall compensation both among executives and as compared to executives with similar positions at companies in the Comparator Group (as described further on Page 22 of this Amendment); |
| • | Create an ownership culture which instills long-term perspective; and |
| • | Improve overall Company performance. |
2014 Compensation Decisions
Base Salary
General
As described above, base salary provides a fixed element of executive compensation, which is intended to attract and retain key executives and provide an element of security to the executive officers on an ongoing basis. Executive salaries are reviewed on an annual basis by the Compensation Committee in May following the conclusion of the preceding fiscal year as well as at the time of a promotion or other material change in responsibilities, and are based on:
| • | Assessment of the NEO’s performance; |
| • | Level of pay compared to the salaries paid to persons in similar positions by companies in the Comparator Group; |
| • | Tenure and experience; and |
| • | Current compensation. |
An increase in base salary is not automatic or guaranteed. In May 2013, a merit increase for Dr. Taglietti of 3.51% was approved, which took effect July 1, 2013. The increase was awarded based on his performance reviews for fiscal year 2013. None of our other NEOs received a merit salary increase in fiscal year 2013. The base salary rates in effect for our NEOs for fiscal year 2014 were as follows: $1,300,000 for Mr. Saunders; $712,250 for Ms. Hochberg; $661,375 for Mr. Perier; $624,000 for Dr. Taglietti; $535,000 for Ms. Ling; and $1,350,000 for Mr. Solomon (until his retirement on September 30, 2013).
14
Table of Contents
Annual Incentives
General
Annual incentives are intended to motivate executives to achieve key operational and individual performance goals and to reward executives for their advancement toward such goals. The Forest Laboratories, Inc. Annual Incentive Compensation Plan (the Incentive Plan) is designed to closely align variable cash compensation awarded to the Company’s executives with corporate performance and the development of managerial and leadership skills. In accordance with the terms of the Incentive Plan, target performance and payouts are established for each NEO and the actual payouts under the Incentive Plan are based upon the Compensation Committee’s assessment of (i) pre-determined financial objectives (70% weighting) and (ii) individual performance objectives (30% weighting). In determining awards under the Incentive Plan, the Compensation Committee may take into account not only the level at which the performance objectives were met but may also take into consideration such equitable factors as may be determined by the Compensation Committee in its discretion.
Setting Annual Financial Performance Metrics
For fiscal year 2014, 70% of each NEO’s annual award was based on the attainment of pre-established financial performance metrics. The Compensation Committee sets the performance metrics as well as the performance targets for each metric. For fiscal year 2014, the Compensation Committee considered financial performance against two separate performance measures, which were weighted as follows:
| • | 60% - Attainment of GAAP revenues |
| • | 40% - Achievement of Non-GAAP Diluted EPS2 |
The Compensation Committee increased the weighting of the revenue metric from 50% for fiscal year 2013 to 60% for fiscal year 2014 in order to place greater emphasis on growing our top line.
Role of Individual Performance in Annual Awards
For fiscal year 2014, 30% of each NEO’s annual award was based on performance against individual performance goals that were developed for each NEO and tied to our objective of encouraging the accomplishment of specific business objectives that we believed would improve the operational effectiveness of the Company.
Individual performance goals were designed for each NEO to reflect their specific functional responsibilities, including effectiveness of oversight and implementation of the Company’s business operations, strategies and management, achievement of sales launch and marketing objectives, effective implementation of corporate programs, including corporate governance and compliance initiatives, achievement of research and development objectives, including submission of New Drug Applications and receipt of product approvals, achievements with respect to acquisitions of product rights, and effective management of the supply chain and patent litigation strategies. NEOs were also evaluated on how well they accomplished the same four objectives for the individual development metric: (1) ability to deliver business results; (2) ability to enhance organizational performance; (3) personal leadership; and (4) ability to develop human capital.
We believe this approach for determining Incentive Plan award payments balances the need to consider overall Company financial performance, results specific to a NEO’s functional area of responsibility, and the NEO’s ability to achieve results versus objectives while also demonstrating desirable managerial and leadership traits.
The payout percentage for each financial performance metric is multiplied by the weighting percentage to obtain the adjusted payout percentage for that metric. The adjusted payout percentages for each metric are then added together to determine the financial performance attainment.
| 2 | Non-GAAP Diluted EPS is defined as earnings per share calculated in accordance with GAAP as adjusted to exclude (i) amortization arising from business combinations and acquisitions of product rights, (ii) up-front license payments and (iii) such one-time items as approved by the Board. |
15
Table of Contents
Determination of Fiscal Year 2014 Bonus Awards
Annual Cash Incentive Compensation =
NEO’s target award multiplied by:
[Company financial performance times 70%] plus [individual performance times 30%]
The table below shows the weights, performance and resulting adjusted payout percentage for each of the financial performance metrics earned for fiscal year 2014 under the Incentive Plan:
Fiscal Year 2014 Financial Performance Metrics
| Performance Metric |
Weight | Performance Target |
Actual Performance Results |
Performance Level as a Percentage of the Performance Target |
Payout Percentage |
Adjusted Payout Percentage |
||||||||||||||||||
| Revenue |
60 | % | $ | 3,456,554,000 | $ | 3,533,643,000 | (1) | 102 | % | 107 | % | 64 | % | |||||||||||
| Non-GAAP Diluted EPS |
40 | % | $ | 0.90 | $ | 1.83 | (2) | 203 | % | 200 | % | 80 | % | |||||||||||
| Total |
144 | % | ||||||||||||||||||||||
| (1) | Excludes sales during fiscal 2014 from Aptalis and Saphris |
| (2) | Excludes impact of Aptalis, Saphris and interest income. |
As indicated in the table above, the resulting adjusted payout percentage for each NEO attributed to the Revenue and Non-GAAP Diluted EPS and performance metrics was 144%.
As disclosed above under the heading “Role of Individual Performance in Annual Awards”, the remaining 30% of each NEO’s fiscal year 2014 Incentive Plan award was based on the achievement of individual performance and development goals. Based on the factors described above, the Compensation Committee determined the degree to which each NEO’s individual performance and individual development goals had been achieved. The Compensation Committee then assessed each NEO’s individual performance against the Company’s performance with respect to the financial performance metrics, which resulted in Incentive Plan award payouts ranging between 138% and 161% of each NEO’s target bonus. The actual Incentive Plan awards paid to our NEOs is shown in the table below and also reported in the Summary Compensation Table in the Non-Equity Incentive Plan Compensation column.
Given his strong leadership in driving the exceptional performance of the Company during fiscal year 2014, the Committee determined to provide Mr. Saunders with a full-year bonus award as reflected below.
Fiscal Year 2014 Annual Incentive Awards
| Name |
Annual Incentive Target (%) |
Annual Target Incentive ($) |
Financial Performance (70% weighting) |
Individual Performance (30% weighting) |
Actual % of Target Bonus Awarded |
Actual Payout ($) |
||||||||||||||||||
| Brenton L. Saunders |
125 | % | 1,625,000 | 144 | % | 200 | % | 161 | % | $ | 2,613,114 | |||||||||||||
| Elaine Hochberg |
65 | % | 462,963 | 125 | % | 138 | % | 640,310 | ||||||||||||||||
| Francis I. Perier, Jr. |
65 | % | 429,894 | 135 | % | 141 | % | 607,470 | ||||||||||||||||
| Marco Taglietti, M.D. |
65 | % | 405,600 | 150 | % | 146 | % | 591,393 | ||||||||||||||||
| Karen Ling(1) |
65 | % | 347,750 | 155 | % | 37 | % | 128,065 | ||||||||||||||||
| (1) | Pursuant to the terms of her letter agreement, Ms. Ling’s fiscal year 2014 Incentive Award was prorated to reflect her hire date of January 21, 2014. |
Because Mr. Solomon retired effective as of September 30, 2013, he was not eligible to earn an annual bonus in respect of fiscal year 2014.
16
Table of Contents
Long-Term Incentives
General; The Equity Plan
Given the long-term nature of the pharmaceutical business, we believe that incentivizing executives to focus on long-term performance is of particular importance to us. We believe that one of the most effective ways to accomplish this objective is to allocate the majority of the NEO’s compensation to long-term incentive components that are tied to an increase in the Company’s market value or other pre-determined performance measures. These awards are intended to reward performance over a multi-year period, align the interests of executives with those of stockholders, instill an ownership culture, enhance the personal stake of executive officers in the growth and success of the Company and provide an incentive for continued service at the Company. Long-term incentive awards are made under our stockholder-approved 2007 Equity Incentive Plan, as amended to date (the Equity Plan). Equity awards are granted annually following the conclusion of each fiscal year at the Board’s regularly scheduled May meeting.
Pursuant to the Equity Plan, employees, including the NEOs, may be granted stock options to purchase shares of common stock, restricted stock awards, stock appreciation rights and stock equivalent units (together, the Awards). The exercise price of all options, including incentive stock options as defined by Section 422 of the Internal Revenue Code of 1986, as amended (the Code), is the fair market value of the shares on the grant date. All of our employees, our subsidiaries’ employees and our non-employee directors are eligible to receive Awards as defined under the Equity Plan.
Annual Long-Term Incentive Vehicles Awarded in Fiscal Year 2014
Mix of Long-Term Awards
We grant our executives, including our NEOs, a package of long-term incentive awards that is designed to incentivize them and to reward them based on the Company’s performance. The Compensation Committee, with the advice of its independent compensation consultant, established each executive’s long-term incentive target award opportunity as a multiple of such executive’s base salary, with that multiple generally being approximately 5.3 times base salary for the CEO (for each fiscal year following fiscal year 2014), 3 to 3.5 times base salary for the Company’s Senior Vice Presidents and Executive Vice Presidents, and 1 to 2 times base salary for the Company’s Vice Presidents.
For fiscal year 2014, each of our NEOs, other than Mr. Saunders and Ms. Ling, received an annual equity award grant. Annual long-term awards were allocated in approximately equal amounts (based on value) to PSUs, Stock Options and Time-based Restricted Stock Awards, each of which is described in greater detail below.
| • | Performance Stock Units. PSUs granted in May 2013 will vest, if at all, at the end of the performance period based upon the level of achievement of two equally weighted performance metrics, one related to total shareholder return (TSR) and one related to revenues of certain products (Revenues). The performance for both the Revenue measure and the TSR measure is the 36-month period commencing April 1, 2013 and ending March 31, 2016. The PSUs are paid out in shares of the Company’s common stock at a one-to-one ratio in accordance with the terms of the individual award agreement. The terms of the PSUs, including the metrics underlying the PSU are further described below. |
| • | Stock Options. Stock options granted in May 2013 vest in four equal annual installments beginning on the first anniversary of the date of grant. These stock options have an exercise price of $37.88 (the average of the high and low prices of the Company’s common stock on May 21, 2013). |
| • | Time-based Restricted Stock Awards. Time-based Restricted Stock Awards granted in May 2013 vest in three annual installments with 33%, 33% and 34% vesting on May 15, 2014, May 15, 2015 and May 15, 2016, respectively. |
17
Table of Contents
The Company’s long-term equity awards are subject to a grantee’s continued employment, but contain exceptions in certain cases in the event of the grantee’s death, disability, or retirement or the occurrence of a change of control. The value of the PSUs, Stock Options and Restricted Stock Awards granted to each NEO during fiscal year 2014 is reported in the Summary Compensation Table on Page 26 of this Amendment and the specific number of shares awarded with respect to each type of equity award is set forth in the Grants of Plan-Based Awards Table on Page 28 of this Amendment.
Performance Metrics for Performance Stock Units
The PSUs granted in May 2013 vest, if at all, based upon the level of achievement of two performance metrics:
| • | The Revenues metric is based on the comparison of the Company’s total revenues from its cumulative sales of Bystolic, Daliresp®, Savella®, Teflaro® and Viibryd® for the period between April 1, 2013 and March 31, 2016 to the total projected Revenues for the same period as included in the budget prepared by the Company and approved by the Board at its May 2013 meeting; and |
| • | The TSR metric is based on the change in TSR of the Company during the period between April 1, 2013 and March 31, 2016 relative to the change in the TSR of the other companies in the NYSE Arca Pharmaceutical Index. To minimize the effect of a single day’s stock price volatility on the TSR calculations, stock prices are calculated using the 20-day volume weighted average closing stock prices prior to and including the beginning and end of the performance period. |
The Compensation Committee believes that both Revenues and TSR are appropriate performance metrics that properly incentivize the Company’s executive officers to achieve the Company’s long-term financial and strategic goals while simultaneously aligning their interests with the long-term interests of the Company’s stockholders. In particular, the Compensation Committee selected Revenues as a performance metric because they tie a portion of the compensation opportunity for the Company’s executives to a factor that is largely within their control — i.e., the ability to execute the Company’s strategic plan in order to generate projected Revenues — and TSR because it focuses the Company’s executives on improving the Company’s stock price. In addition, the products chosen by the Compensation Committee as the basis for the Revenue performance metric were selected because they represented the five most recent products that the Company commercially launched in the United States when the PSUs were granted.
While the Compensation Committee determines an executive’s total compensation opportunity by assuming that he or she will earn 100% of the PSUs initially awarded in the compensation cycle (the Target Award), the number of PSUs that will actually be earned could be between 0% and 150% of the Target Award depending on the level of achievement of both performance metrics.
Sign-On Equity Grants
Pursuant to the terms of his letter agreement, Mr. Saunders received a sign-on equity grant with an aggregate grant date value of $10,000,000, which was allocated 40% to RSUs and 60% to stock options. These awards are reflected in the Grants of Plan-Based Awards Table and associated footnotes. In addition, pursuant to the terms of his letter agreement, Mr. Saunders purchased shares in the Company with an aggregate purchase price of $5,000,000 shortly after the commencement of his employment.
Pursuant to the terms of her letter agreement, Ms. Ling received a sign-on equity grant with an aggregate grant date value of $2,253,000, which was allocated 50% to restricted shares and 50% to stock options. Ms. Ling also received an additional award of RSUs with an aggregate value of $855,000, to compensate her for the pension benefit she was foregoing from her prior employer. These awards are reflected in the Grants of Plan-Based Awards Table and associated footnotes. In addition, pursuant to the terms of her letter agreement, Ms. Ling purchased shares in the Company with an aggregate purchase price of $250,000 shortly after the commencement of her employment.
18
Table of Contents
Additional Compensation Elements
Benefits
The NEOs are also eligible to participate in the same retirement plans and health and welfare benefit plans made available to the other benefits-eligible employees of the Company, including, for example, the Company’s defined contribution plan, medical, dental, vision, life insurance and disability coverage.
Perquisites
The Company provides certain executive officers, including the NEOs, with certain perquisites that the Compensation Committee believes are reasonable and consistent with the Company’s overall compensation program. We believe that the indirect benefit that the Company receives from providing these perquisites outweighs the cost of providing them. The specific cost of perquisites paid by the Company for each NEO in fiscal year 2014 is set forth in the “Summary Compensation Table” on Page 26 of this Amendment under the heading “All Other Compensation” and is described further in footnote 4 to the table. The Committee does not believe that the perquisites provided to executive officers form a material part of their compensation. The Company does not provide loans to executive officers.
Retiree Medical Benefits
On December 1, 1989, the Board authorized the grant of certain lifetime medical benefits to certain senior executive officers and their spouses upon the completion of ten years of service by such officers. The benefit was subsequently discontinued and the only NEO eligible for such benefit is Mr. Solomon. This benefit is further described under the headings “Potential Payments Upon Termination” and “Potential Payments Upon Change in Control” on Page 34 of this Amendment.
Deferred Compensation Plans
The Company maintains a nonqualified Deferred Compensation Plan for the benefit of certain highly compensated employees, including its NEOs. Such plans are common within the Company’s competitive peer group. Under this plan, full-time salaried employees who have an annual base salary of at least $150,000 may defer up to 50% of their annual base salary and up to 100% of their annual bonus. Deferred amounts may be invested among several investment programs at the participant’s option. Deferred amounts are not subject to federal or state income tax until a participant withdraws amounts from the plan. The Company does not match any of these funds. Further information on the deferred compensation payable to its NEOs can be found under the heading “Nonqualified Deferred Compensation” on Page 33 of this Amendment.
Contracts and Agreements
Letter Agreement with Brenton Saunders
On September 11, 2013, in connection with his hiring, the Company entered into a letter agreement with Brenton Saunders naming him as the Company’s President and Chief Executive Officer. The letter agreement provides, among other things, that while employed as President and Chief Executive Officer, Mr. Saunders will (i) receive an annual base salary of $1,300,000, subject to possible increases no less frequently than annually beginning in fiscal year 2015; (ii) be eligible to receive annual cash incentive compensation payments with a target opportunity of 125% of base salary (Target Bonus), a threshold opportunity of 50% of the Target Bonus, and a maximum opportunity of 200% of the Target Bonus; (iii) receive a sign-on equity grant, described above in the section entitled “Sign-on Equity Grants”; (iv) be eligible to receive an annual grant of equity-based incentive awards having an aggregate grant date value of 5.3 times base salary for each fiscal year following fiscal year 2014; and (v) become party to a change in control agreement as described below in the section entitled “Change of Control Employment Agreements.” Mr. Saunders is also entitled to certain payments upon termination, as described in the subsection entitled under the heading “Potential Payments Upon Change in Control” on Page 34 of this Amendment.
Letter Agreement with Karen Ling
On December 19, 2013, in connection with her hiring, the Company entered into a letter agreement with Karen Ling naming her as the Company’s Senior Vice President and Chief Human Resources Officer. The letter agreement provides, among other things, that Ms. Ling will (i) receive an annual base salary of $535,000; (ii) be eligible to receive annual cash incentive compensation payments with a target opportunity of 65% of base salary,
19
Table of Contents
prorated for fiscal year 2014 to reflect her hire date of January 21, 2014; (iii) receive sign-on and make-whole equity grants, described above in the section entitled “Sign-on Equity Grants”; (iv) be eligible to receive an annual grant of equity-based incentive awards having an aggregate grant date value of $878,000 for fiscal year 2014 and $1,200,00 for fiscal year 2015; and (v) become party to a change in control agreement as described below in the section entitled “Change of Control Employment Agreements.”
Retention Letter Agreements
The Company entered into letter agreements with Mr. Perier and Dr. Taglietti in connection with their employment with the Company. Pursuant to these letter agreements, Mr. Perier and Dr. Taglietti are entitled to guaranteed severance payments for a one year period following a termination of employment, a bonus for the year in which the termination of employment occurs equal to the greater of the last bonus received from the Company or 40% of salary target in the case of Mr. Perier and 30% of salary target in the case of Dr. Taglietti, and continued medical and dental health care coverage for such officer and any eligible family members until the earlier of the expiration of the one year period or the date such officer obtains alternate coverage. The guarantee is triggered if the executive is terminated by the Company without Cause or resigns for Good Reason (each as defined in the relevant letter agreement). A more detailed description of such severance arrangements (including the amounts payable thereunder) can be found on Page 34 of this Amendment under the headings “Potential Payments Upon Termination” and “Potential Payments Upon Change in Control,” respectively.
Letter Agreement with Howard Solomon
On May 22, 2013, Howard Solomon advised the Board of his decision to retire as President and Chief Executive Officer of the Company effective December 31, 2013, and entered into a letter agreement with the Company pursuant to which he agreed to continue serving as the Company’s Chief Executive Officer and President until the later of December 31, 2013, or the date his successor as Chief Executive Officer is appointed, which occurred on October 1, 2013 (the Transition Date). Under the terms of his agreement, Mr. Solomon received his current compensation and benefits through the Transition Date. In light of Mr. Solomon’s key relationships, including with the Company’s licensing partners, and in order to retain the benefit of his experience, from and following the Transition Date, Mr. Solomon commenced employment as a Senior Advisor to the Company, and in that capacity he provided services reasonably requested by the Board or the Chief Executive Officer of the Company. Mr. Solomon’s term as Senior Advisor to the Company will last until the earlier of December 31, 2016 or the date his employment is terminated in accordance with the terms of the agreement. For additional details relating Mr. Solomon’s letter agreement with the Company, please see the current report of the Company filed on Form 8-K on May 24, 2013.
Change-in-Control Employment Agreements
The Company is party to change-in-control employment agreements with certain key executives, including each of the NEOs, which provide for severance (i) upon a termination due to death or disability or a termination without Cause or for Good Reason or, in the case of all NEOs other than Mr. Saunders, Adequate Reason, in each case during the three year period following a Change of Control (as such terms is defined in the applicable agreement) or (ii) in connection with a termination of the agreement within 90 days following a Change of Control. Each individual covered by such an agreement has made a significant contribution to the Company and has knowledge and understanding of the Company and its operations and these arrangements are provided to maintain continuity of such expertise and leadership during potentially disruptive negotiations relating to potential mergers, acquisitions or other business combinations. They also serve to protect the stockholders’ interest in maintaining executive leadership so that goals and objectives in the best interest of stockholders are pursued. A more detailed description of such severance arrangements (including the amounts payable thereunder) can be found under the heading “Potential Payments Upon Change in Control” on Page 34 of this Amendment.
20
Table of Contents
In connection with its entry into the merger agreement with Actavis, the Company entered into an amendment to the Change of Control employment agreements with Mr. Saunders and Ms. Ling, each of whom had become employees of the Company during fiscal year 2014. These amendments provide that, in the event that the executive becomes subject to excise taxes under Section 4999 of the Code, he or she will be entitled to a gross-up payment from the Company to compensate him or her for the excise taxes that are imposed. In consideration for the gross-up protection, each executive will be bound by a one-year non-competition restriction.
How We Make Compensation Decisions
The Role of Our Compensation Committee
The Compensation Committee is responsible for establishing the Company’s compensation strategy and overall program of executive compensation and for administering and monitoring compliance with the compensation program. The Compensation Committee seeks to ensure that the total compensation paid to our NEOs is fair, reasonable and competitive. For fiscal year 2014, the Compensation Committee also engaged an independent compensation consultant as described below under the heading “The Role of the Compensation Committee’s Independent Compensation Consultant.”
The Compensation Committee reviews the performance of and establishes compensation for the CEO and CFO. The Compensation Committee is also responsible for granting all equity awards under the Equity Plan. With respect to non-equity compensation awarded to senior executives of the Company other than the CEO and CFO, including the other NEOs, the Compensation Committee reviews and assesses management’s evaluation of such executives’ performance and makes recommendations to the Board regarding their non-equity compensation.
The Role of Our Management
As part of the annual compensation approval process, the Compensation Committee considers the advice of the CEO with respect to the compensation of all NEOs other than the CEO. Other members of management provided support to the Compensation Committee as needed.
Our management was also involved in developing proposals regarding program design and administration for the Compensation Committee’s review and approval. Management was responsible for responding to any Compensation Committee requests for information, analysis, or perspective as it related to topics that arose during the course of the year. Lastly, management carries out certain responsibilities relating to administration of the compensation program.
The Role of the Compensation Committee’s Independent Compensation Consultant
The Compensation Committee engaged an independent compensation consultant, Steven Hall & Partners, LLP (SH&P) to provide advice in connection with fiscal year 2014 compensation. At the Compensation Committee’s request, SH&P conducted a thorough review of the Company’s executive compensation plans, policies and practices, as well as market trends and current and pending legislation and evolving policies and procedures adopted by proxy advisory services firms. The SH&P report to the Compensation Committee recommended the adoption of a comparator group to be used for benchmarking of compensation and practices and specific recommendations based on the Company’s long-term objectives to create a stronger alignment with the interests of stockholders and in line with current “best practices.”
Non-Binding Advisory Say on Pay Proposal and Recent Changes in Compensation Practices
In August 2013, our stockholders approved a non-binding advisory say-on-pay proposal at our 2013 Annual Meeting with approximately 96% of the votes cast voting in favor of that proposal. The Compensation Committee interpreted these results as a validation of the structure of our pay-for-performance compensation program and determined to retain our general approach to executive compensation. Nonetheless, the Compensation Committee continues to work with its independent compensation consultant to consider alternatives and intends to make changes to the program, both large and small, where it determines it appropriate. Accordingly, the Compensation Committee will continue to monitor and consider future advisory say-on-pay votes to ensure that there is continued support for our pay-for-performance compensation programs among our stockholders. The Board has historically held this vote annually and continues to do so, consistent with the recommendation of our stockholders at the 2011 annual meeting.
21
Table of Contents
Competitive Marketplace Assessment
As part of our executive compensation program, we periodically review executive compensation data from similar companies (the Comparator Group) in order to ensure that our practices are fair and reasonable. The Comparator Group was selected based on industry focus and revenue size and includes companies with which we compete for talent. In addition, we considered an analysis of market capitalization and similarity of broad-based product and service offerings. In fiscal year 2014, the Comparator Group consisted of:
| Allergan, Inc. |
Hospira, Inc. | |
| Biogen Idec Inc. |
Mylan Inc. | |
| Celgene Corporation |
Perrigo Company | |
| Cephalon Inc.3 |
Warner Chilcott PLC | |
| Endo Pharmaceuticals Holdings Inc. |
Watson Pharmaceuticals, Inc.4 | |
| Gilead Sciences, Inc. |
Compensation decisions by the Compensation Committee during fiscal year 2014 also took into account elements of compensation including base salaries, total cash compensation and total compensation for officers of the Comparator Group. In developing compensation levels for the Company’s officers, the Compensation Committee used such data as a point of reference, and made adjustments based on differences in the scope of responsibilities of the Company’s executive as compared to their counterparts within the Comparator Group. The Compensation Committee further adjusted compensation levels based on the executive’s tenure, performance during the compensation period and the relative strategic importance of such executive’s position within the Company.
Risk Considerations
The Compensation Committee has reviewed the Company’s compensation policies and practices and analyzed the potential business risks inherent in such policies and practices. The Compensation Committee believes that the Company’s executive compensation policies and practices do not encourage our management, including our NEOs, to take unnecessary business risks that are reasonably likely to have a material adverse effect on the Company. Specifically, the Company’s historical practice of providing the majority of management compensation in the form of long-term equity awards and including the issuance of PSUs, which use multiple performance metrics, have a range of payouts based on the achievement of such metrics (as opposed to an “all or nothing” payout scheme that could otherwise encourage the taking of unnecessary risks), and have a minimum three-year performance period, and the requirement that all awards granted to executive officers under the Equity Plan have a vesting schedule that exceeds 24 months, align the interests of our management with the long-term interests of the Company’s stockholders and protect against inappropriate risk taking. In addition to the foregoing, the Company’s Corporate Governance Guidelines include stock ownership guidelines, a holding period requirement with respect to shares acquired by executive officers from option exercises and restricted stock grants, and an anti-hedging policy with a view to further aligning the interests of management with those of our stockholders, thereby mitigating the taking of unnecessary risks. Finally, as described in greater detail in the following section, the Company also maintains a “clawback” policy to discourage knowing or intentional fraudulent or illegal conduct.
| 3 | Cephalon, Inc. was acquired subsequently by Teva Pharmaceutical Industries Ltd. on October 14, 2011. |
| 4 | Following its acquisition of Actavis Group in October 2012, Watson Pharmaceuticals, Inc. changed its name to Actavis, Inc. |
22
Table of Contents
Clawback Policy
The Company maintains a “clawback” policy, which is set forth in our Corporate Governance Guidelines. The policy provides that, in addition to any other remedies that may be available to the Company, the Company may recover (in whole or in part) any bonus, incentive payment, commission, equity award or other compensation received by an executive officer of the Company that is or was based on any financial results or operating metrics that were impacted by such executive officer’s knowing or intentional fraudulent or illegal conduct.
Stock Ownership Guidelines
Our Corporate Governance Guidelines include stock ownership guidelines applicable to our non-employee directors and our executive officers, including the NEOs. These guidelines are intended to align the long-term interests of our non-employee directors and executives with the interests of stockholders and promote an ownership culture and long-term perspective.
| Role |
Value of Common Stock to be Owned |
|||||
| Chairman of the Board, Chief Executive Officer and |
Six times base salary | |||||
| Direct Reports to the Chief Executive Officer | Three times base salary | |||||
| Other senior executives | One times base salary | |||||
| Non-employee directors | Three times annual retainer fee |
Each executive and non-employee director is required to meet his or her target stock ownership threshold on or before December 31 of the year which includes the fifth anniversary or seventh anniversary, respectively, of the date he or she became subject to the guidelines.
Holding Period for Stock Options, Restricted Stock and Performance Stock Units
To further promote a culture of ownership and long-term investment, the Board has adopted a policy requiring that shares obtained by executives, including the NEOs, upon the exercise of Company stock options or through Company restricted stock grants and performance stock units be held until such executive achieves the applicable ownership threshold under the stock ownership guidelines, subject to exceptions for shares required to be sold in order to exercise the option or pay the taxes accruing as a result of the exercise of the option or the vesting of the restricted stock award or performance stock unit and in connection with corporate transactions that are generally available to stockholders.
No-Hedging Policy
To further align our directors’ and executive officers’ interests with the interests of the Company’s stockholders, the Board has adopted a policy applicable to all directors and executive officers, including the NEOs, which prohibits transactions designed to limit or eliminate economic risks to our directors or executive officers from owning the Company’s stock, such as transactions involving any form of margin arrangement, short sales or dealing in puts and calls or other derivative instruments related to Company stock or debt.
Tax and Accounting Implication of Compensation
Section 162(m) of the Code generally limits the deductibility of compensation (other than qualified performance-based compensation) in excess of $1,000,000 paid in a taxable year to a company’s chief executive officer and the four other most highly compensated executive officers. The Company considers the impact of this deductibility limitation on its compensation program, but recognizes that there may be cases in which authorized compensation exceeds the limits contemplated in Section 162(m) of the Code. Current accounting rules, including Financial Accounting Standards Board Accounting Standards Codification Topic 718 (FASB ASC 718), “Compensation-Stock Compensation,” require the Company to record as an expense the estimated fair market value of stock option grants and stock awards, which reduces the Company’s reported profits. The Committee considers this expense when determining the types and values of equity awards to be granted to employees, including the NEOs.
23
Table of Contents
Report of the Compensation Committee
With respect to the fiscal year ended March 31, 2014, the Compensation Committee hereby reports as follows:
1. The Compensation Committee has reviewed and discussed with management the Compensation Discussion and Analysis required by Item 402(b) of Regulation S-K and set forth in the Company’s Annual Report on Form 10-K for the fiscal year ended March 31, 2014 (the 2014 Form 10-K); and
2. Based on the review and discussions referred to in paragraph 1 above, the Compensation Committee recommended to the Board that the Compensation Discussion and Analysis be included in the 2014 Form 10-K filed with the SEC.
FOREST LABORATORIES, INC.
COMPENSATION COMMITTEE
Christopher J. Coughlin (Chairman)
Vincent J. Intrieri
Gerald M. Lieberman
24
Table of Contents
Tabular Compensation Disclosure
The following tables summarize our Named Executive Officer and non-employee director compensation as follows:
| 1. | Summary Compensation Table. The Summary Compensation Table on Page 26 of this Amendment and related discussion summarize the compensation earned by or paid to our Named Executive Officers for the fiscal years ended March 31, 2014, 2013 and 2012, including salary earned, the aggregate grant date fair value of performance stock units, stock awards and option awards granted to our Named Executive Officers, and all other compensation paid to our Named Executive Officers, including perquisites. |
| 2. | Grants of Plan-Based Awards Table. The Grants of Plan-Based Awards Table on Page 28 of this Amendment and related discussion summarize all grants of plan-based awards made to our Named Executive Officers for the fiscal year ended March 31, 2014. |
| 3. | Outstanding Equity Awards at Fiscal Year-End Table. The Outstanding Equity Awards at Fiscal Year-End Table on Page 30 of this Amendment and related discussion summarize the unvested performance stock units, stock awards and all stock options held by our Named Executive Officers as of March 31, 2014. Please note that our Named Executive Officers’ ownership of vested shares of stock is set forth under “Security Stock Ownership of Certain Beneficial Owners and Management/Directors” on Page 38 of this Amendment. |
| 4. | Option Exercises and Stock Vested Table. The Option Exercises and Stock Vested Table on Page 33 of this Amendment and related discussion summarize our Named Executive Officers’ option exercises and stock award vesting during the fiscal year ended March 31, 2014. |
| 5. | Nonqualified Deferred Compensation Table. The Nonqualified Deferred Compensation Table on Page 33 of this Amendment and related discussion summarize the contributions to and account balances under our Deferred Compensation Plan during the fiscal year ended March 31, 2014. |
| 6. | Potential Payments Upon Termination Table. The Potential Payments Upon Termination Table on Page 34 of this Amendment and related discussion summarize payments and benefits that would be made to certain of our Named Executive Officers in the event of certain employment terminations. |
| 7. | Potential Payments Upon Change in Control Table. The Potential Payments Upon Change in Control Table on Page 34 of this Amendment and related discussion summarize payments and benefits that would be made to our Named Executive Officers in the event of a change in control. |
| 8. | Director Compensation Table. The Director Compensation Table on Page 36 of this Amendment and related discussion summarize the compensation paid to our non-employee directors during the fiscal year ended March 31, 2014, including cash compensation and the aggregate grant date fair value of stock awards and option awards granted to our non-employee directors. |
25
Table of Contents
Summary Compensation Table
The following table sets forth compensation for each of our Named Executive Officers (NEOs) for each of the fiscal years ended March 31, 2014, 2013 and 2012 during which such person qualified as a NEO.
| Name and Principal Position |
Year | Salary ($) |
Bonus ($)(1) |
Stock Awards ($)(2) |
Option Awards ($)(2) |
Non- Equity Incentive Plan Compensation ($)(1) |
All Other Compensation ($) |
Total ($) (3) |
||||||||||||||||||||||||
| Brenton L. Saunders, |
2014 | 595,000 | 0 | 4,000,008 | 6,000,010 | 2,613,114 | 10,432 | (4) | 13,218,564 | |||||||||||||||||||||||
| CEO and President |
||||||||||||||||||||||||||||||||
| Howard Solomon, |
2014 | 1,125,769 | 0 | 2,701,450 | 2,712,966 | 0 | 36,367 | (4) | 6,576,552 | |||||||||||||||||||||||
| Chairman, CEO and President |
2013 | 1,349,999 | 0 | 2,199,275 | 726,196 | 405,000 | 143,332 | (4) | 4,823,802 | |||||||||||||||||||||||
| 2012 | 1,350,000 | 1,013,885 | 4,523,246 | 1,515,050 | 0 | 131,486 | 8,533,667 | |||||||||||||||||||||||||
| Elaine Hochberg, |
2014 | 712,250 | 0 | 1,520,124 | 763,268 | 640,310 | 72,569 | (4) | 3,708,521 | |||||||||||||||||||||||
| Executive Vice President and |
2013 | 709,187 | 0 | 747,230 | 367,779 | 261,025 | 47,181 | (4) | 2,132,402 | |||||||||||||||||||||||
| Chief Commercial Officer |
2012 | 682,587 | 402,000 | 1,430,762 | 783,580 | 0 | 45,449 | 3,344,378 | ||||||||||||||||||||||||
| Francis I. Perier, Jr., |
2014 | 661,375 | 0 | 1,411,598 | 708,803 | 607,470 | 41,190 | (4) | 3,430,436 | |||||||||||||||||||||||
| Executive Vice President, Finance |
2013 | 658,531 | 0 | 695,331 | 344,030 | 242,394 | 48,028 | (4) | 1,988,314 | |||||||||||||||||||||||
| and Administration and CFO |
2012 | 632,243 | 366,810 | 1,343,776 | 723,750 | 0 | 48,252 | 3,114,831 | ||||||||||||||||||||||||
| Marco Taglietti, M.D., |
2014 | 617,899 | 0 | 1,206,478 | 605,682 | 591,393 | 27,285 | (4) | 3,048,737 | |||||||||||||||||||||||
| Senior Vice President – Research |
2013 | 600,263 | 0 | 584,198 | 288,752 | 284,919 | 33,575 | (4) | 1,791,707 | |||||||||||||||||||||||
| and Development and President |
2012 | 578,091 | 325,500 | 1,145,809 | 599,265 | 0 | 33,643 | 2,682,308 | ||||||||||||||||||||||||
| of Forest Research Institute |
||||||||||||||||||||||||||||||||
| Karen Ling, |
2014 | 82,308 | 0 | 1,981,410 | 1,126,490 | 128,065 | 2,587 | (4) | 3,320,860 | |||||||||||||||||||||||
| Senior Vice President – Chief |
||||||||||||||||||||||||||||||||
| Human Resources Officer |
||||||||||||||||||||||||||||||||
| (1) | In December 2011, the Compensation Committee decided to align the Company’s compensation cycle, which historically covered the twelve month period ending September 30, with the Company’s fiscal year end of March 31. The amounts set forth in the “Bonus” column for fiscal year 2012 represent the cash incentives issued as a bonus for the fiscal year ended March 31, 2012. Commencing with fiscal year 2013, the Compensation Committee discontinued granting discretionary cash bonus awards and instituted a policy of granting formula based cash incentive awards under the Incentive Plan instead. All awards granted pursuant to the Incentive Plan were determined in accordance with the terms of the Incentive Plan as described under the heading “Annual Incentives” of the “Executive Compensation Discussion and Analysis” on Page 9 of this Amendment. |
| (2) | Represents the aggregate grant date fair values of awards granted during the fiscal years ended March 31, 2014, 2013 and 2012 computed in accordance with FASB ASC 718. For a discussion of valuation assumptions used in calculating the amounts for fiscal years 2014, 2013 and 2012, see Note 1 to our Consolidated Financial Statements included in our Annual Reports on Form 10-K for the fiscal years ended March 31, 2014, 2013 and 2012, respectively. For all fiscal years presented, the “Stock Awards” column reflects the grant date fair value for both grants of Restricted Stock (awards and units) and PSUs granted to the NEOs during that period. The value of PSUs has been determined based on an assumed vesting of 100% of the target PSUs awarded, which is the performance threshold the Company believed as of the grant date was most likely to be achieved under the grants. The following is the maximum grant date fair value for the PSU awards granted in fiscal years 2012, 2013 and 2014 for each of the following NEOs if, due to the Company’s performance during the applicable performance cycle, |
26
Table of Contents
| the PSUs vested at their maximum level: Brenton L. Saunders: $0, $0, and $0; Howard Solomon: $4,310,282, $2,194,737 and $0; Elaine Hochberg: $1,073,071, $558,690 and $1,520,503; Francis I. Perier: $1,007,832, $519,886 and $1,411,560; Marco Taglietti: $859,357, $436,794 and $1,206,857; and Karen Ling: $0, $0 and $0. Please see the “Grants of Plan-Based Awards” table on Page 28 of this Amendment for more information regarding equity awards granted in fiscal year 2014. |
| (3) | There are no above-market or preferential earnings on deferred compensation. Consequently, the Summary Compensation Table does not include earnings on deferred amounts. In addition, none of the NEOs is eligible for pension benefits because Forest does not have a defined benefit retirement program. |
| (4) | This amount includes the cost of group term life insurance and compensation credited to the NEOs pursuant to our Savings and Profit Sharing Plan, which benefits are available on the same terms to all non-bargaining unit employees of the Company and its domestic subsidiaries. These employees become participants in the plan if they are employed for at least six months prior to the plan year-end. The Company makes contributions to the plan at the Board’s discretion. However, for fiscal year 2014, the contribution base may not exceed 25% of the individual plan participant’s gross salary (up to a maximum salary of $260,000), including allocated forfeitures for the plan year. Plan participants vest over a period of one to five years of credited service. The amount disclosed also includes the costs of all perquisites provided to the NEOs, including with respect to Dr. Taglietti amounts that did not exceed $10,000. The amount of such perquisites represent the cost associated with company cars (including insurance), company provided lunches and membership dues. With respect to Mr. Howard Solomon and Ms. Hochberg, this amount also includes $9,364 and $35,734, respectively, of medical expenses paid on behalf of Mr. Solomon and Ms. Hochberg under a supplemental medical plan. |
27
Table of Contents
Grants of Plan-Based Awards
The following table sets forth certain additional information regarding grants of plan-based awards to our NEOs during the fiscal year ended March 31, 2014:
| Name |
Grant | Estimated Future Payouts Under Non- Equity Incentive Plan Awards (1) |
Estimated Future Payouts Under Equity Incentive Plan Awards (2) |
All Other Stock Awards: Number of Shares of Stock or Units (#) |
All Other Option Awards: Number of Securities Underlying Options (#) |
Exercise or Base Price of Option Awards ($) |
Grant Date Fair Value of Stock and Option Awards ($)(12) |
|||||||||||||||||||||||||||||||||||||
| Threshold ($) |
Target ($) |
Maximum ($) |
Threshold (#) |
Target (#) |
Maximum (#) |
|||||||||||||||||||||||||||||||||||||||
| Howard Solomon |
5/21/13 | 0 | 1,012,500.00 | 2,025,000.00 | ||||||||||||||||||||||||||||||||||||||||
| 5/21/13 | 71,316 | (3) | 2,701,450 | |||||||||||||||||||||||||||||||||||||||||
| 5/21/13 | 259,614 | (7) | 37.88 | 2,712,966 | ||||||||||||||||||||||||||||||||||||||||
| Brenton Saunders |
8/15/13 | 0 | 1,625,000.00 | 3,250,000 | ||||||||||||||||||||||||||||||||||||||||
| 8/15/13 | 1,761 | (4) | 75,036 | |||||||||||||||||||||||||||||||||||||||||
| 8/15/13 | 5,909 | (8) | 42.61 | 75,003 | ||||||||||||||||||||||||||||||||||||||||
| 10/1/13 | ||||||||||||||||||||||||||||||||||||||||||||
| 10/1/13 | 92,937 | (5) | 4,000,008 | |||||||||||||||||||||||||||||||||||||||||
| 10/1/13 | 472,070 | (9) | 43.04 | 6,000,010 | ||||||||||||||||||||||||||||||||||||||||
| Elaine Hochberg |
5/21/13 | 0 | 462,963 | 925,925 | ||||||||||||||||||||||||||||||||||||||||
| 5/21/13 | 10,035 | 20,070 | 40,140 | 760,252 | ||||||||||||||||||||||||||||||||||||||||
| 5/21/13 | 20,060 | (3) | 759,873 | |||||||||||||||||||||||||||||||||||||||||
| 5/21/13 | 73,040 | (10) | 37.88 | 763,268 | ||||||||||||||||||||||||||||||||||||||||
| Francis I. Perier, Jr. |
5/21/13 | 0 | 429,894 | 859,788 | ||||||||||||||||||||||||||||||||||||||||
| 5/21/13 | 9,316 | 18,632 | 37,264 | 705,780 | ||||||||||||||||||||||||||||||||||||||||
| 5/21/13 | 18,633 | (3) | 705,818 | |||||||||||||||||||||||||||||||||||||||||
| 5/21/13 | 67,828 | (10) | 37.88 | 708,803 | ||||||||||||||||||||||||||||||||||||||||
| Marco Taglietti, M.D. |
5/21/13 | 0 | 405,600 | 811,200 | ||||||||||||||||||||||||||||||||||||||||
| 5/21/13 | 7,965 | 15,930 | 31,860 | 603,428 | ||||||||||||||||||||||||||||||||||||||||
| 5/21/13 | 15,920 | (3) | 603,050 | |||||||||||||||||||||||||||||||||||||||||
| 5/21/13 | 57,960 | (10) | 37.88 | 605,682 | ||||||||||||||||||||||||||||||||||||||||
| Karen Ling |
1/21/14 | 0 | 86,938 | 173,875 | ||||||||||||||||||||||||||||||||||||||||
| 1/21/14 | 28,489 | (6) | 1,981,410 | |||||||||||||||||||||||||||||||||||||||||
| 1/21/14 | 52,517 | (11) | 69.55 | 1,126,490 | ||||||||||||||||||||||||||||||||||||||||
| (1) | The amounts actually paid to each NEO under the Annual Incentive Plan are set forth in the Summary Compensation Table at Page 26 of this Amendment. The amount set forth as the “Maximum” represents the maximum individual bonus that could have been earned by each NEO with respect to such NEO’s grant for the 2014 fiscal year under our Incentive Plan. The amount set forth as the “Threshold” represents the bonus that would have been payable if each of the four metrics under the award was achieved at the minimum level of performance, although no bonus, or a smaller bonus, could have been payable if none, or only some, respectively, of the four performance metrics were achieved at the threshold level. The amounts shown for Ms. Ling are pro-rated to reflect her date of hire of January 21, 2014. |
| (2) | PSUs are earned after a three-year period based on achievement of financial objectives established by the Compensation Committee at the time of grant. At the end of the third year, the Compensation Committee assesses performance versus the targets to determine how many shares are earned, and payouts are then made. The amount set forth as the “Maximum” represents the maximum number of shares that could be issued under the PSUs with |
28
Table of Contents
| respect to the award granted during the 2014 fiscal year. The amount set forth as the “Threshold” represents the number of shares that would be issued if each metric under the award is achieved at the minimum level of performance, although 0% of the award could be earned if none of the performance metrics are achieved. |
| (3) | The stock award is subject to a risk of forfeiture which lapses as to 33% of the shares covered by the award on May 15, 2014, 33% of the shares on May 15, 2015 and 34% of the shares on May 15, 2016. |
| (4) | The stock award is subject to a risk of forfeiture which lapses as to 25% of the shares covered by the award on the six month anniversary of the grant date, 25% of the shares on August 15, 2014, 25% of the shares on August 15, 2015, and 25% of the shares on August 15, 2016. |
| (5) | The stock award is subject to a risk of forfeiture which lapses as to 100% of the shares on October 1, 2016. |
| (6) | This stock award is comprised of 16,196 of restricted stock awards and 12,293 of restricted stock units. Of the 16,196 restricted stock awards, 6,312 of awards are subject to a risk of forfeiture which lapses as to one-third of the shares on each of the first three anniversaries of the grant date. The remaining awards, or 9,884 awards, are subject to a risk of forfeiture which lapses as to 15% of the shares on May 1, 2014, 15% of the shares on December 31, 2014, 30% of the shares on May 1, 2015, 15% of the shares on December 31, 2015 and 25% of the shares on May 1, 2016. The restricted stock units become exercisable as to 20% of the stock units on July 27 of each of 2018, 2019, 2020, and 2021, 5% of the restricted stock units on July 27, 2022, and the remaining 15% of the stock units on July 27, 2023. |
| (7) | The stock option has a term of ten years and becomes exercisable as to 33% of the shares covered by the option on the first anniversary of the grant date, 33% of the shares covered by the option on the second anniversary of the grant date, and 34% of the shares covered by the option on the third anniversary of the grant date. |
| (8) | The stock option has a term of ten years and became exercisable on February 15, 2015. |
| (9) | The stock option has a term of ten years and becomes exercisable as to one-third of the shares covered by the option on each of the first three anniversaries of the grant date. |
| (10) | The stock option has a term of ten years and becomes exercisable as to 25% of the shares covered by the option on each of the first four anniversaries of the grant date. |
| (11) | The stock option has a term of ten years. Of the 52,517 options, 32,051 of the stock options become exercisable as to 15% of the shares covered by the option on May 1, 2014, 15% of the shared covered by the option on December 31, 2014, 30% of the shares covered by the option on May 1, 2015, 15% of the shares covered by the option on December 31, 2015 and 25% of the shares covered by the option on May 1, 2016. The remaining stock options of 20,466 become exercisable as to 25% of the shares covered by the option on each of the first four anniversaries of the grant date. |
| (12) | Represents the aggregate grant date fair value of awards computed in accordance with FASB ASC 718 and does not reflect whether the recipient has actually realized a financial benefit from the awards. For additional information regarding the valuation methodology used by the Company, see Note 1 to our 2014 Consolidated Financial Statements included in our Annual Report on Form 10-K for the year ended March 31, 2014. The value of PSUs has been determined based on the vesting of 100% of the target PSUs awarded, which is the performance threshold the Company believed as of the grant date was most likely to be achieved under the grants. |
29
Table of Contents
Outstanding Equity Awards at Fiscal Year-End
The following table sets forth information regarding each unexercised option and unvested stock award held by each of our Named Executive Officers as of March 31, 2014:
| OPTION AWARDS | STOCK AWARDS | |||||||||||||||||||||||||||||||
| Name |
Number of Securities Underlying Unexercised Options (#) Exercisable |
Number of Securities Underlying Unexercised Options (#) Unexercisable |
Option Exercise Price ($) |
Option Expiration Date |
Number of Shares or Units of Stock That Have Not Vested (#) |
Market Value of Shares or Units of Stock That Have Not Vested ($) |
Equity Incentive Plan Awards: Number of Unearned Shares, Units or Other Rights That Have Not Vested (#) |
Equity Incentive Plan Awards: Market or Payout Value of Unearned Shares, Units or Other Rights That Have Not Vested ($) |
||||||||||||||||||||||||
| Howard Solomon |
— | 259,614 | (1) | 38.875 | 05/20/2023 | 71,316 | (2) | 6,580,327 | — | — | ||||||||||||||||||||||
| 17,919 | 53,757 | (3) | 34.035 | 05/06/2022 | 14,476 | (4) | 1,335,701 | 21,606 | 1,993,586 | |||||||||||||||||||||||
| 78,500 | 78,500 | (5) | 29.995 | 12/04/2021 | 18,700 | (6) | 1,725,449 | 47,900 | 4,419,733 | |||||||||||||||||||||||
| 150,000 | — | 32.165 | 12/05/2020 | — | — | — | — | |||||||||||||||||||||||||
| 140,000 | — | 31.265 | 12/06/2019 | — | — | — | — | |||||||||||||||||||||||||
| 125,000 | — | 24.120 | 12/08/2018 | — | — | — | — | |||||||||||||||||||||||||
| 125,000 | — | 37.255 | 12/05/2017 | — | — | — | — | |||||||||||||||||||||||||
| 200,000 | — | 51.530 | 12/08/2016 | — | — | — | — | |||||||||||||||||||||||||
| 200,000 | — | 40.290 | 12/09/2015 | — | — | — | — | |||||||||||||||||||||||||
| 200,000 | — | 42.530 | 12/13/2014 | — | — | — | — | |||||||||||||||||||||||||
| Brenton Saunders |
— | 472,070 | (7) | 43.040 | 09/30/2023 | 92,937 | (8) | 8,575,297 | — | — | ||||||||||||||||||||||
| 5,909 | — | 42.610 | 08/14/2023 | 1,320 | (9) | 121,796 | — | — | ||||||||||||||||||||||||
| 7,815 | — | 34.540 | 08/22/2022 | 1,086 | (10) | 100,205 | — | — | ||||||||||||||||||||||||
| 15,000 | 5,000 | (11) | 33.430 | 08/22/2021 | — | — | — | — | ||||||||||||||||||||||||
| Elaine Hochberg |
— | 73,040 | (12) | 37.875 | 05/20/2023 | 20,060 | (2) | 1,850,936 | 10,035 | 925,929 | ||||||||||||||||||||||
| 9,075 | 27,225 | (3) | 34.035 | 05/06/2022 | 7,370 | (4) | 680,030 | 5,500 | 507,485 | |||||||||||||||||||||||
| 40,600 | 40,600 | (5) | 29.995 | 12/04/2021 | 8,109 | (6) | 748,217 | 11,925 | 1,100,320 | |||||||||||||||||||||||
| 75,000 | — | 32.165 | 12/05/2020 | — | — | — | — | |||||||||||||||||||||||||
| 60,000 | — | 31.265 | 12/06/2019 | — | — | — | — | |||||||||||||||||||||||||
| 50,000 | — | 24.120 | 12/08/2018 | — | — | — | — | |||||||||||||||||||||||||
| 50,000 | — | 37.255 | 12/05/2017 | — | — | — | — | |||||||||||||||||||||||||
| 75,000 | — | 51.530 | 12/08/2016 | — | — | — | — | |||||||||||||||||||||||||
| 50,000 | — | 40.290 | 12/09/2015 | — | — | — | — | |||||||||||||||||||||||||
| 50,000 | — | 42.530 | 12/13/2014 | — | — | — | — | |||||||||||||||||||||||||
| Francis I. Perier, Jr. |
— | 67,828 | (12) | 37.875 | 05/20/2023 | 18,633 | (2) | 1,719,267 | 9,316 | 859,587 | ||||||||||||||||||||||
| 8,489 | 25,467 | (3) | 34.035 | 05/06/2022 | 6,858 | (4) | 632,788 | 5,118 | 472,238 | |||||||||||||||||||||||
| 37,500 | 37,500 | (5) | 29.995 | 12/04/2021 | 7,616 | (6) | 702,728 | 11,200 | 1,033,424 | |||||||||||||||||||||||
| 75,000 | — | 32.165 | 12/05/2020 | — | — | — | — | |||||||||||||||||||||||||
| 36,000 | 24,000 | (13) | 31.265 | 12/06/2019 | — | — | — | — | ||||||||||||||||||||||||
| 50,000 | — | 24.120 | 12/08/2018 | — | — | — | — | |||||||||||||||||||||||||
| 50,000 | — | 37.255 | 12/05/2017 | — | — | — | — | |||||||||||||||||||||||||
| 75,000 | — | 51.530 | 12/08/2016 | — | — | — | — | |||||||||||||||||||||||||
| 50,000 | — | 40.290 | 12/09/2015 | — | — | — | — | |||||||||||||||||||||||||
| 100,000 | — | 42.530 | 12/13/2014 | — | — | — | — | |||||||||||||||||||||||||
| Marco Taglietti, M.D. |
— | 57,960 | (12) | 37.875 | 05/20/2023 | 15,920 | (2) | 1,468,938 | 7,965 | 734,931 | ||||||||||||||||||||||
| 7,125 | 21,375 | (3) | 34.035 | 05/06/2022 | 5,762 | (4) | 531,660 | 4,300 | 396,761 | |||||||||||||||||||||||
| 31,050 | 31,050 | (5) | 29.995 | 12/04/2021 | 6,494 | (6) | 599,201 | 9,550 | 881,179 | |||||||||||||||||||||||
| 27,000 | 33,000 | (14) | 32.165 | 12/05/2020 | 11,250 | (15) | 1,038,038 | — | — | |||||||||||||||||||||||
| 24,000 | 16,000 | (16) | 31.265 | 12/06/2019 | — | — | — | — | ||||||||||||||||||||||||
| 25,000 | — | 24.120 | 12/08/2018 | — | — | — | — | |||||||||||||||||||||||||
| 60,000 | — | 37.255 | 12/05/2017 | — | — | — | — | |||||||||||||||||||||||||
| Karen Ling |
— | 52,517 | (17) | 69.550 | 01/21/2024 | 28,489 | (18) | 2,628,680 | — | — | ||||||||||||||||||||||
30
Table of Contents
| (1) | The option was granted on May 21, 2013 and has a term of ten years. The option becomes exercisable as to 33% of the shares covered by the option on the first anniversary of the grant date, 33% of the shares covered by the option on the second anniversary of the grant date, and 34% of the shares covered by the option on the third anniversary of the grant date. |
| (2) | The stock award was granted on May 21, 2013. The stock award is subject to a risk of forfeiture which lapses as to 33% of the shares on May 15, 2014, 33% of the shares on May 15, 2015 and 34% of the shares in May 2016. |
| (3) | The option was granted on May 7, 2012 and has a term of ten years. The option vests and is exercisable as to 25% of the shares underlying the option on each of the first four anniversaries of the grant date. |
| (4) | The stock award was granted on May 7, 2012. The stock award is subject to a risk of forfeiture which lapses as to 33% of the shares covered by the award on the first two anniversaries of the grant date and 34% of the shares on the third anniversary of the grant date. |
| (5) | The option was granted on December 5, 2011 and has a term of ten years. The option vests and is exercisable as to 25% of the shares underlying the option on each of the first four anniversaries of the grant date. |
| (6) | The stock award was granted on December 5, 2011. The stock award is subject to a risk of forfeiture which lapses as to 33% of the shares covered by the award on the first two anniversaries of the grant date and 34% of the shares on the third anniversary of the grant date. |
| (7) | The option was granted on October 1, 2014 and has a term of ten years. The option vests and becomes exercisable as to one-third of the shares covered by the option on each of the first three anniversaries of the grant date. |
| (8) | The stock award was granted on October 1, 2014. The stock award is subject to a risk of forfeiture which lapses as to 100% of the shares on October 1, 2016. |
| (9) | The stock award was granted on August 15, 2013 and is subject to a risk of forfeiture, which lapses as to 25% of the shares on the six month anniversary of the grant date and as to 25% of the shares on each of the first three anniversaries of the grant date. |
| (10) | The stock award was granted on August 23, 2012 and is subject to a risk of forfeiture, which lapses as to 25% of the shares on the six month anniversary of the grant date and as to 25% of the shares on each of the first three anniversaries of the grant date. |
| (11) | The stock option was granted on August 23, 2011 and is subject to a risk of forfeiture, which lapses as to 25% of the shares on the six month anniversary of the grant date and as to 25% of the shares on each of the first three anniversaries of the grant date. |
| (12) | The stock option was granted on May 21, 2013 and has a term of ten years. The option vests and is exercisable as to 25% of the shares covered by the option on each of the first four anniversaries of the grant date. |
| (13) | The option was granted on December 7, 2009 and has a term of ten years. The option vests and is exercisable as to 15% of the shares underlying the option on each of the first four anniversaries of the grant date and as to the remaining 40% on the fifth anniversary of the grant date. |
| (14) | The option was granted on December 6, 2010 and has a term of ten years. The option vests and is exercisable as to 15% of the shares underlying the option on each of the first four anniversaries of the grant date and as to the remaining 40% on the fifth anniversary of the grant date. |
| (15) | The stock award was granted on December 6, 2010. The stock award is subject to a risk of forfeiture which lapses as to 25% of the shares covered by the award on each of the first four anniversaries of the grant date. |
| (16) | The option was granted on December 7, 2009 and has a term of ten years. The option vests and is exercisable as to 15% of the shares underlying the option on each of the first four anniversaries of the grant date and as to the remaining 40% on the fifth anniversary of the grant date. |
31
Table of Contents
| (17) | The option was granted on January 21, 2014 and has a term of ten years. Of the 52,517 options, 32,051 of the stock options become exercisable as to 15% of the shares covered by the option on May 1, 2014, 15% of the shared covered by the option on December 31, 2014, 30% of the shares covered by the option on May 1, 2015, 15% of the shares covered by the option on December 31, 2015 and 25% of the shares covered by the option on May 1, 2016. The remaining stock options of 20,466 become exercisable as to 25% of the shares covered by the option on each of the first four anniversaries of the grant date. |
| (18) | This stock award was granted on January 21, 2013 and is comprised of 16,196 of restricted stock awards and 12,293 of restricted stock units. Of the 16,196 restricted stock awards, 6,312 awards are subject to a risk of forfeiture which lapses as to one-third of the shares on each of the first three anniversaries of the grant date. The remaining awards, or 9,884 awards, are subject to a risk of forfeiture which lapses as to 15% of the shares on May 1, 2014, 15% of the shares on December 31, 2014, 30% of the shares on May 1, 2015, 15% of the shares on December 31, 2015 and 25% of the shares on May 1, 2016. The restricted stock units become exercisable as to 20% of the stock units on July 27 of each of 2018, 2019, 2020, and 2021, 5% of the restricted stock units on July 27, 2022, and the remaining 15% of the stock units on July 27, 2023. |
32
Table of Contents
Option Exercises and Stock Vested
No options were exercised by any of the Named Executive Officers during the fiscal year ended March 31, 2014. The following table sets forth information regarding vesting of restricted stock during fiscal year 2014:
| STOCK AWARDS | ||||||||
| Name |
Number of Shares Acquired on Vesting (#) |
Value Realized on Vesting ($) |
||||||
| Howard Solomon |
25,280 | $ | 1,291,128 | |||||
| Brenton Saunders |
1,086 | 46,562 | ||||||
| Elaine Hochberg |
11,501 | 580,802 | ||||||
| Francis I. Perier, Jr. |
22,020 | 1,178,038 | ||||||
| Marco Taglietti, M.D. |
30,391 | 1,659,563 | ||||||
|
|
|
|
|
|||||
| 90,278 | $ | 4,756,093 | ||||||
|
|
|
|
|
|||||
Nonqualified Deferred Compensation
The following table shows the executive contributions, earnings and account balances for all NEOs who participate in our Deferred Compensation Plan. The Deferred Compensation Plan allows full-time salaried employees who have an annual base salary of at least $150,000, including the NEOs, to defer up to 50% of their annual base salary and up to 100% of their annual bonuses. Deferred amounts are not subject to federal or state income tax until a participant withdraws amounts from the Deferred Compensation Plan. We do not match any of these funds.
Employees who participate in the Deferred Compensation Plan may at their option invest deferred monies in a range of investment vehicles that generally mirror the choices available to all employees in the Company’s 401(k) Plan. The investment options in the Deferred Compensation Plan have returns that would be the same as those earned on the same options in the Company’s 401(k) Plan for the 2014 fiscal year. The rates of return for funds in the Deferred Compensation Plan ranged from: -0.31% to 32.22%.
| Name |
Executive Contributions in Last Fiscal Year($) (1) |
Aggregate Earnings in Last Fiscal Year ($) (2) |
Aggregate Withdrawals/ Distributions ($) |
Aggregate Balance at Last Fiscal Year End ($) |
||||||||||||
| Elaine Hochberg |
26,103 | 69,629 | — | 471,857 | (3) | |||||||||||
| (1) | The amount set forth in this column has also been reported as “Salary” for the fiscal year 2014 in the Summary Compensation table on Page 26 of this Amendment. |
| (2) | There are no above-market or preferential earnings on deferred compensation. Consequently, the amount set forth in this column has not been reported as “Salary” for the fiscal year 2014 in the Summary Compensation table on Page 26 of this Amendment. |
| (3) | $20,450 and $39,500 of this amount were reported as “Salary” for the fiscal years 2013 and 2012, respectively, in the Summary Compensation table on Page 26 of this Amendment. |
33
Table of Contents
Potential Payments Upon Termination
The amounts shown below are estimates of the additional payments and benefits the Named Executive Officers would be entitled to receive under the applicable plans and/or agreements, assuming an involuntary termination by the Company on March 31, 2014.
| Name(1) |
Severance ($) | Bonus ($) | Continued Health Care Benefits ($)(2) |
|||||||||
| Brenton L. Saunders |
5,850,000 | (1) | — | 40,000 | (2) | |||||||
| Francis I. Perier, Jr. |
661,375 | (3) | 264,550 | (3) | 22,403 | |||||||
| Elaine Hochberg |
2,350,425 | (4) | 40,000 | |||||||||
| Marco Taglietti, M.D. |
602,850 | (3) | 180,855 | (3) | 21,386 | |||||||
| Karen Ling |
1,765,500 | (4) | 40,000 | |||||||||
| (1) | Per his offer letter, equal to two times Mr. Saunders’ base salary and target bonus. |
| (2) | Includes amounts payable following a termination of employment for health care coverage (medical and dental) for employee and employee’s eligible family members. |
| (3) | Amounts payable to Mr. Perier and Dr. Taglietti pursuant to the terms of their retention agreements, as described above on Page 20 of this Amendment. |
| (4) | Pursuant to the terms of the Corporate Officer Severance Plan, the Named Executive Officer is entitled to receive a severance payment in the amount equal to two times base salary and target bonus. |
Potential Payments Upon Change in Control
As described above, the Company has entered into Change in Control agreements with several key employees, including each of its Named Executive Officers, other than Mr. Solomon, whose agreement expired upon his retirement. The current agreements automatically renew each year on January 1 for an additional three year period unless the Company provides the executive written notice at least 60 days prior to such date that the term of his or her Change in Control agreement will not be extended for an additional three-year period. The Board has determined that it is in both our best interests and the best interests of our stockholders to enter into Change in Control agreements with certain executive officers. In particular, these agreements enable management to evaluate and support potential transactions that might be beneficial to stockholders without regard to the potential impact on their own job security or earned benefits. Additionally, the agreements provide for continuity of management in the event of a Change in Control.
Each agreement becomes effective only upon the occurrence of a Change in Control and provides that the executive is entitled to salary, bonus and benefits for a three-year period following a Change in Control if the executive’s employment is terminated as a result of such Change in Control or during such three year period by the Company without Cause or by the employee for Good Reason or for Adequate Reason (a Qualifying Termination). “Cause” is generally defined as one of the following: (i) refusal of the executive to comply with written instructions of our Board or CEO that are consistent with the scope of the executive’s responsibilities; or (ii) willful engagement by the executive in illegal conduct or gross misconduct which is materially and demonstrably injurious to the Company. “Good Reason” includes a reduction in job responsibilities, or changes in pay and benefits as well as any material change in the geographic location at which the executive must perform services to the Company. The executive has 90 days to assert a claim for payment in connection with a termination for Good Reason. “Adequate Reason” means the executive’s resignation for any reason or no reason during the 30-day period immediately following the first anniversary of the Change in Control.
Subject to certain exceptions, a “Change in Control” is generally defined as (i) an acquisition of more than 50% of our common stock or voting securities by a person or group not acquiring their shares directly from us, (ii) an acquisition during any 12-month period of 30% or more of our common stock by a person or group not acquiring their shares directly from us, (iii) a change in the majority of the current Board or their designated successors not consented to by such current Board or designated successors, or (iv) a merger, consolidation or sale of all or substantially all of our assets which involves a 50% or greater change in our stockholders or the replacement of a majority of the current Board or their designated successors or the acquisition by a person or group of more than 30% of our voting securities.
34
Table of Contents
Upon such Qualifying Termination, executives subject to these agreements are eligible for the following amounts: (i) the amount of any accrued compensation obligations to the executive through the termination date, consisting of unpaid base salary, a pro-rated bonus equal to the greater of the executive’s annualized current year bonus or the highest annual bonus received by such executive during the three years preceding the Change in Control, and other accrued compensation through the termination date; plus (ii) an amount equal to three times the executive’s base salary (which must be at least 12 times the executive’s highest monthly salary during the 12 months preceding the Change in Control) and highest annual bonus during the three years preceding the Change in Control; plus (iii) the actuarial equivalent of the employee’s benefit under any retirement plans in which the executive participates, assuming continued participation for a three year period following the termination date. In addition, the executive will receive continued medical benefits for a three year period for both the executive and his or her family, provided however that such coverage will be secondary to any coverage the executive obtains from a subsequent employer. Lastly, the employee will be provided with outplacement services and any other amounts or benefits required to be paid or provided under any plan or program.
The following table sets forth our reasonable estimate of the potential payments to each of our Named Executive Officers under our Change in Control Agreements assuming a Change in Control and the executive’s termination of employment both occurred as of March 31, 2014:
| Name |
Severance ($)(1) | Continuation of Benefits ($)(2) |
Acceleration of Stock Options ($)(3) |
Acceleration of Stock Awards ($)(4) |
Tax Reimbursement ($)(5) |
|||||||||||||||
| Brenton L. Saunders |
8,775,000 | 90,000 | 23,534,206 | 8,797,299 | 10,264,883 | |||||||||||||||
| Howard Solomon |
— | 90,000 | 22,138,871 | 22,468,114 | — | |||||||||||||||
| Elaine Hochberg |
3,525,638 | 90,000 | 8,086,119 | 8,346,652 | — | |||||||||||||||
| Francis I. Perier, Jr. |
3,273,806 | 90,000 | 8,971,233 | 7,785,281 | — | |||||||||||||||
| Marco Taglietti, M.D. |
3,088,800 | 90,000 | 9,289,894 | 7,663,577 | — | |||||||||||||||
| Karen Ling |
2,648,250 | 90,000 | 1,193,186 | 2,628,680 | 2,332,271 | |||||||||||||||
| (1) | This amount is equal to three times the respective executive officer’s annual base salary and annual incentive in effect at March 31, 2014. |
| (2) | This amount represents estimated payments under welfare benefit plans including medical, dental and life insurance for a three-year period. The amounts payable do not include amounts payable in connection with benefits continuation agreements described above. |
| (3) | Unvested stock options vest and become immediately exercisable upon the executives qualifying termination within three years following a change of control. The value shown equals the total number of unvested option shares as of March 31, 2014, multiplied by the difference between the closing stock price on March 31, 2014 of $92.27 and the exercise price of the option. |
| (4) | The value related to these awards equals the total number of accelerated shares as of March 31, 2014, multiplied by the closing stock price on March 31, 2014 of $92.27. |
| (5) | In connection with its entry into the merger agreement with Actavis, the Company entered into an amendment to the Change of Control employment agreements with Mr. Saunders and Ms. Ling, each of whom had become employees of the Company during fiscal year 2014. These amendments provide that, in the event that the executive becomes subject to excise taxes under Section 4999 of the Code, he or she will be entitled to a gross-up payment from the Company to compensate him or her for the excise taxes that are imposed. In consideration for the gross-up protection, each executive will be bound by a one-year non-competition restriction. |
35
Table of Contents
Director Compensation
The table below summarizes the compensation for our non-employee directors during the fiscal year ended March 31, 2014:
| Name |
Fees Earned or Paid in Cash ($) |
Stock Awards ($)(1) |
Option Awards ($)(1) |
All Other Compensation ($) |
Total ($) | |||||||||||||||
| Nesli Basgoz, M.D. |
196,344 | 75,003 | 75,036 | 346,383 | ||||||||||||||||
| Christopher J. Coughlin |
224,522 | 75,003 | 75,036 | 374,561 | ||||||||||||||||
| Kenneth E. Goodman |
238,518 | 75,003 | 75,036 | 125,423 | (2) | 513,980 | ||||||||||||||
| Vincent J. Intrieri |
147,278 | 306,703 | 75,036 | 529,017 | ||||||||||||||||
| Pierre Legault |
221,610 | 75,003 | 75,036 | 371,649 | ||||||||||||||||
| Gerald M. Lieberman |
185,457 | 75,003 | 75,036 | 335,496 | ||||||||||||||||
| Lawrence S. Olanoff, M.D., Ph.D. |
186,137 | 75,003 | 75,036 | 124,137 | (3) | 460,313 | ||||||||||||||
| Lester B. Salans, M.D. |
219,958 | 75,003 | 75,036 | 369,997 | ||||||||||||||||
| Brenton L. Saunders |
93,713 | 75,003 | 75,036 | 243,752 | ||||||||||||||||
| Peter J. Zimetbaum, M.D. |
199,693 | 75,003 | 75,036 | 349,732 | ||||||||||||||||
| (1) | Represents the aggregate grant date fair value of awards for the fiscal year ended March 31, 2014 computed in accordance with FASB ASC 718. For a discussion of valuation assumptions used in calculating the amounts for the fiscal year 2014 award, see Note 1 to our 2013 Consolidated Financial Statements included in our Annual Report on Form 10-K for the year ended March 31, 2014. Pursuant to the terms of our Equity Plan, (A) each of our non-employee directors who was re-elected at our 2013 Annual Meeting (i.e., all of our non-employee directors except for Mr. Legault) received an automatic grant of (1) options having a value of $75,000 calculated on the grant date in accordance with the Black-Scholes option pricing model (utilizing the same assumptions that the Company utilizes in preparation of its financial statements), with the number of options rounded up to the nearest whole share, and (2) common stock having a fair market value of $75,000 on the grant date, with the number of shares rounded up to the nearest whole share and (B) our new non-employee director who was elected at our 2013 Annual Meeting (i.e., Mr. Intrieri) received an initial grant of options covering 20,000 shares of common stock. For a description of these automatic grants and the changes proposed thereto, see “Director Compensation—Automatic Equity Grants” below. For Mr. Saunders, amounts reflect the aggregate grant date fair value of awards made in his capacity as a director on August 15, 2013. As described in “Executive Compensation—Contracts and Agreements—Letter with Brenton Saunders,” Mr. Saunders was subsequently hired as Chief Executive Officer of the Company in September 2013, and the compensation he earned as an executive officer is described in the “Executive Compensation” section of this Amendment and related tabular disclosure. |
| (2) | This amount reflects payments made to or on behalf of Mr. Goodman pursuant to his Benefits Continuation Agreement, a benefit to which Mr. Goodman is entitled as a result of his prior service as President and COO of Forest, of which $58,023 represents amounts to offset taxes incurred with respect to such payments. |
| (3) | This amount reflects payments made to Dr. Olanoff for consulting services rendered pursuant to his Consultant Services Letter Agreement with the Company. |
36
Table of Contents
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
Equity Compensation Plan Information
The following sets forth certain information as of March 31, 2014 with respect to our compensation plans under which Forest securities may be issued:
| Plan Category |
Number of Securities to be Issued Upon Exercise of Outstanding Options and Rights(1) |
Weighted Average Exercise Price of Outstanding Options |
Number of Securities Remaining Available for Future Issuance Under Equity Compensation Plans (Excluding Securities Reflected in First Column) |
|||||||||
| Equity compensation plans approved by security holders |
15,143,860 | $ | 35.77 | (2) | 31,602,773 | |||||||
| Equity compensation plans not approved by security holders |
N/A | N/A | N/A | |||||||||
| Total |
15,143,860 | $ | 35.77 | 31,602,773 | ||||||||
| (1) | Total includes 965,705 PSUs, which are earned after a three year period based on achievement of financial objectives established by the Compensation Committee at the time of grant, and settled in shares of Company common stock. While actual payments pursuant to PSUs may range between 0% to 200% of the targeted number of shares covered by the PSUs, the table assumes that the PSUs will be paid out at the maximum of the targeted level, which the reflects the number of shares reserved for issuance in connection with the PSUs. |
| (2) | Outstanding restricted stock awards and PSUs are excluded, as these awards and units do not have an exercise price. |
37
Table of Contents
Stock Ownership of Certain Beneficial Owners and Management/Directors
The following table sets forth the number of shares of Forest common stock owned beneficially by any persons we know to be beneficial owners of more than five percent of our outstanding shares based on the number of shares of Forest common stock outstanding as of June 25, 2014, each of our directors and each of our executive officers all of our directors and executive officers as a group: The percentage of ownership is calculated based upon the 276,760,500 shares of Forest common stock issued and outstanding as of June 25, 2014, including the stock options exercisable and vesting in 60 days for all of the directors and executive officers.
| Name and Address of Beneficial Owner |
Amount and Nature of Beneficial Ownership |
Percent of Class (1) |
||||||
| 5% Stockholders |
||||||||
| Wellington Management Company, LLP(2) 280 Congress Street Boston, MA 02210 |
37,251,645 | 13.46 | % | |||||
| Icahn Capital LP(3) c/o Icahn Associates Corp. 767 Fifth Avenue, 47th Floor New York, NY 10153 |
30,662,005 | 11.08 | % | |||||
| Vanguard Specialized Funds – Vanguard Health Care Fund(4) 100 Vanguard Boulevard Malvern, PA 19355 |
26,666,866 | 9.64 | % | |||||
| ClearBridge Investments, LLC Legg Mason Capital Management LLC(5) 620 8th Avenue New York, NY 10018 |
22,783,736 | 8.23 | % | |||||
| The Vanguard Group(6) 100 Vanguard Boulevard Malvern, PA 19355 |
16,368,071 | 5.91 | % | |||||
| BlackRock, Inc.(7) 40 East 52nd Street New York, NY 10022 |
14,020,857 | 5.07 | % | |||||
| Named Executive Officers and Directors |
||||||||
| Brenton L. Saunders |
289,023 | (8) | * | |||||
| Howard Solomon |
1,806,353 | (9) | * | |||||
| Francis I. Perier, Jr. |
593,086 | (10) | * | |||||
| Elaine Hochberg |
758,763 | (11) | * | |||||
| Marco Taglietti, M.D. |
289,534 | (12) | * | |||||
| Karen Ling |
30,718 | (13) | * | |||||
| Nesli Basgoz, M.D. |
50,026 | (14) | * | |||||
| Christopher J. Coughlin |
37,657 | (15) | * | |||||
| Kenneth E. Goodman |
77,361 | (16) | * | |||||
| Vincent J. Intrieri |
17,670 | (17) | * | |||||
| Pierre Legault |
22,670 | (18) | * | |||||
| Gerald M. Lieberman |
37,657 | (19) | * | |||||
| Lawrence S. Olanoff, M.D., Ph.D. |
345,624 | (20) | * | |||||
| Lester B. Salans, M.D. |
74,336 | (21) | * | |||||
| Peter J. Zimetbaum, M.D. |
48,105 | (22) | * | |||||
| All directors and executive officers as a group |
5,202,893 | (23) | 1.88 | % | ||||
| * | Less than 1% |
| (1) | For purposes of computing the percentage of outstanding shares of common stock held by each person named in the table on a given date, any security which such person has the right to acquire within 60 days after such date is deemed to be outstanding, but is not deemed to be outstanding for the purpose of computing the percentage ownership of any other person. |
38
Table of Contents
| (2) | Based on the information provided pursuant to Amendment No. 10 to a statement on a Schedule 13G/A filed with the SEC on February 14, 2014 by Wellington Management Company, LLP (“Wellington Management”). Wellington Management reported that it has shared voting power with respect to 7,478,294 shares of common stock and shared dispositive power with respect to 37,251,645 shares of common stock. Wellington Management acts as investment advisor to various clients who have the right to receive, or the power to direct the receipt of, dividends from, or the proceeds from the sale of the shares of common stock deemed to be beneficially owned by Wellington Management. Other than Vanguard Specialized Funds – Vanguard Health Care Fund, no such client of Wellington Management was known to have such right or power with respect to more than five percent of the shares of our common stock. |
| (3) | Based on the information provided pursuant to Amendment No. 19 to a statement on a Schedule 13D filed with the SEC on June 11, 2013 (the “Icahn Schedule 13D”) by Carl C. Icahn and Icahn Associates Corp. with respect to itself, the other members of the Icahn Group, and certain other affiliated entities of Carl C. Icahn (collectively, the “Reporting Persons”). The Reporting Persons reported that (i) High River Limited Partnership, a Delaware limited partnership (“High River”), has sole voting power and sole dispositive power with regard to 6,132,398 shares of common stock, and that each of Hopper Investments LLC (“Hopper”), Barberry Corp. (“Barberry”) and Mr. Icahn has shared voting power and shared dispositive power with regard to such shares; (ii) Icahn Partners LP, a Delaware limited partnership (“Icahn Partners”), has sole voting power and sole dispositive power with regard to 9,071,470 shares of common stock, and each of Icahn Onshore LP (“Icahn Onshore”), Icahn Capital LP (“Icahn Capital”), IPH GP LLC (“IPH”), Icahn Enterprises Holdings L.P. (“Icahn Enterprises Holdings”), Icahn Enterprises G.P. Inc. (“Icahn Enterprises GP”), Beckton Corp. (“Beckton”) and Mr. Icahn has shared voting power and shared dispositive power with regard to such shares; (iii) Icahn Partners Master Fund LP, a Cayman Islands exempted limited partnership (“Icahn Master”), has sole voting power and sole dispositive power with regard to 9,905,159 shares of common stock, and that each of Icahn Offshore LP (“Icahn Offshore”), Icahn Capital, IPH, Icahn Enterprises Holdings, Icahn Enterprises GP, Beckton and Mr. Icahn has shared voting power and shared dispositive power with regard to such shares; (iv) Icahn Partners Master Fund II LP, a Cayman Islands exempted limited partnership (“Icahn Master II”), has sole voting power and sole dispositive power with regard to 3,852,960 shares of common stock, and each of Icahn Offshore, Icahn Capital, IPH, Icahn Enterprises Holdings, Icahn Enterprises GP, Beckton and Mr. Icahn has shared voting power and shared dispositive power with regard to such shares; and (v) Icahn Partners Master Fund III LP, a Cayman Islands exempted limited partnership (“Icahn Master III”), has sole voting power and sole dispositive power with regard to 1,700,018 shares of common stock, and each of Icahn Offshore, Icahn Capital, IPH, Icahn Enterprises Holdings, Icahn Enterprises GP, Beckton and Mr. Icahn has shared voting power and shared dispositive power with regard to such shares. |
| (4) | Based on the information provided pursuant to Amendment No. 12 to a statement on a Schedule 13G/A filed with the SEC on February 4, 2014 by Vanguard Specialized Funds – Vanguard Health Care Fund (“Vanguard”). Vanguard reported that it has sole voting power with respect to 26,666,866 shares of common stock. |
| (5) | Based on the information provided pursuant to Amendment No. 9 to a statement on a Schedule 13G/A filed with the SEC on February 14, 2014 by ClearBridge Investments, LLC (“ClearBridge”) and Legg Mason Capital Management LLC (“Legg Mason”). ClearBridge and Legg Mason reported that they have sole voting power with respect to 21,997,632 shares of common stock and sole dispositive power with respect to 22,783,736 shares of common stock. |
| (6) | Based on the information provided pursuant to Amendment No. 1 to a statement on a Schedule 13G/A filed with the SEC on February 11, 2014 by The Vanguard Group (“Vanguard II”). Vanguard II reported that it has sole voting power with respect to 398,615 shares of common stock and sole dispositive power with respect to 15,999,692 shares of common stock. |
| (7) | Based on the information provided pursuant to a statement on a Schedule 13G filed with the SEC on January 29, 2014 by BlackRock, Inc. (“BlackRock”). BlackRock reported that it has sole voting power with respect to 11,856,889 shares of common stock and sole dispositive power with respect to 14,020,857 shares of common stock. |
| (8) | With respect to Mr. Saunders’ holdings, such amount includes 95,343 shares of stock that are subject to a risk of forfeiture and includes 33,724 shares issuable pursuant to options that were exercisable on June 25, 2014 or which become exercisable within 60 days of June 25, 2014. |
| (9) | With respect to Mr. Solomon’s holdings, such amount includes 49,581 shares of stock that are subject to a risk of forfeiture, 1,340,011 shares issuable pursuant to options that were exercisable on June 25, 2014 or which become exercisable within 60 days of June 25, 2014 and 51,640 shares held by a 501(c)(3) charitable foundation, as to which Mr. Solomon disclaims beneficial ownership. Does not include target grants of 95,800 and 43,212 performance stock units granted on December 5, 2011 and May 7, 2012, respectively, which vest, if at all, following the conclusion of fiscal year 2015, based on the achievement of certain performance metrics. |
| (10) | With respect to Mr. Perier’s holdings, such amount includes 23,580 shares of stock that are subject to a risk of forfeiture and includes 507,434 shares issuable pursuant to options that were exercisable on June 25, 2014 or which become exercisable within 60 days of June 25, 2014. Does not include target grants of 22,400, 10,236 and 18,632 performance stock units granted on December 5, 2011, May 7, 2012, and May 21, 2013, respectively, which vest, if at all, following the conclusion of fiscal year 2015, fiscal year 2015 and fiscal year 2016, respectively, based on the achievement of certain performance metrics. |
39
Table of Contents
| (11) | With respect to Ms. Hochberg’s holdings, such amount includes 25,019 shares of stock that are subject to a risk of forfeiture, 487,010 shares issuable pursuant to options that were exercisable on June 25, 2014 or which become exercisable within 60 days of June 25, 2014 and 119,568 shares which are pledged as security. Does not include target grants of 23,850, 11,000 and 20,070 performance stock units granted on December 5, 2011, May 7, 2012, and May 21, 2013, respectively, which vest, if at all, following the conclusion of fiscal year 2015, fiscal year 2015 and fiscal year 2016, respectively, based on the achievement of certain performance metrics. |
| (12) | With respect to Dr. Taglietti’s holdings, such amount includes 31,334 shares of stock that are subject to a risk of forfeiture and includes 195,790 shares issuable pursuant to options that were exercisable on June 25, 2014 or which become exercisable within 60 days of June 25, 2014. Does not include target grants of 19,100, 8,600 and 15,930 performance stock units granted on December 5, 2011, May 7, 2012, and May 21, 2013, respectively, which vest, if at all, following the conclusion of fiscal year 2015, fiscal year 2015 and fiscal year 2016, respectively, based on the achievement of certain performance metrics. |
| (13) | With respect to Ms. Ling’s holdings, such amount includes 18,438 shares of stock that are subject to a risk of forfeiture, and includes 4,808 shares issuable pursuant to options that were exercisable on June 25, 2014 or which become exercisable within 60 days of May 2, 2014. |
| (14) | With respect to Dr. Basgoz’s holdings, such amount includes 2,967 shares of stock that are subject to a risk of forfeiture and includes 41,770 shares issuable pursuant to options that were exercisable on June 25, 2014 or which become exercisable within 60 days of June 25, 2014. |
| (15) | With respect to Mr. Coughlin’s holdings, such amount includes 2,406 shares of stock that are subject to a risk of forfeiture and includes 33,724 shares issuable pursuant to options that were exercisable on June 25, 2014 or which become exercisable within 60 days of June 25, 2014. |
| (16) | With respect to Mr. Goodman’s holdings, such amount includes 2,967 shares of stock that are subject to a risk of forfeiture and includes 36,891 shares issuable pursuant to options that were exercisable on June 25, 2014 or which become exercisable within 60 days of June 25, 2014. |
| (17) | With respect to Mr. Intrieri’s holdings, such amount includes 1,320 shares of stock that are subject to a risk of forfeiture and includes 15,909 shares issuable pursuant to options that were exercisable on June 25, 2014 or which become exercisable within 60 days of June 25, 2014. |
| (18) | With respect to Mr. Legault’s holdings, such amount includes 1,320 shares of stock that are subject to a risk of forfeiture and includes 20,909 shares issuable pursuant to options that were exercisable on June 25, 2014 or which become exercisable within 60 days of June 25, 2014. |
| (19) | With respect to Mr. Lieberman’s holdings, such amount includes 2,406 shares of stock that are subject to a risk of forfeiture and includes 33,724 shares issuable pursuant to options that were exercisable on June 25, 2014 or which become exercisable within 60 days of June 25, 2014. |
| (20) | With respect to Dr. Olanoff’s holdings, such amount includes 2,967 shares of stock that are subject to a risk of forfeiture and includes 254,270 shares issuable pursuant to options that were exercisable on June 25, 2014 or which become exercisable within 60 days of June 25, 2014. |
| (21) | With respect to Dr. Salans’ holdings, such amount includes 2,967 shares of stock that are subject to a risk of forfeiture and includes 48,891 shares issuable pursuant to options that were exercisable on June 25, 2014 or which become exercisable within 60 days of June 25, 2014. |
| (22) | With respect to Dr. Zimetbaum’s holdings, such amount includes 2,967 shares of stock that are subject to a risk of forfeiture and includes 39,270 shares issuable pursuant to options that were exercisable on June 25, 2014 or which become exercisable within 60 days of June 25, 2014. |
| (23) | Includes 398,825 shares of stock that are subject to a risk of forfeiture and includes 3,477,376 shares issuable pursuant to options that were exercisable on June 25, 2014 or which become exercisable within 60 days of June 25, 2014. Does not include target grants of 210,300, 97,048 and 97,962 performance stock units granted on December 5, 2011, May 7, 2012, and May 21, 2013, respectively, which vest, if at all, following the conclusion of fiscal year 2015, fiscal year 2015 and fiscal year 2016, respectively, based on the achievement of certain performance metrics. |
Changes in Control
On February 17, 2014, we entered into an Agreement and Plan of Merger (the Merger Agreement) with Actavis, a company incorporated under the laws of Ireland, Tango US Holdings Inc., a Delaware corporation and a direct wholly owned subsidiary of Actavis (US Holdco), Tango Merger Sub 1 LLC, a Delaware limited liability company and a direct wholly owned subsidiary of US Holdco and Tango Merger Sub 2 LLC, a Delaware limited liability company and a direct wholly owned subsidiary of US Holdco, pursuant to which Actavis has agreed, subject to the terms and conditions thereof, to acquire Forest (the Merger). As a result of the Merger, we will become a wholly owned subsidiary of Actavis. Subject to regulatory approval and other customary closing conditions, the closing date of the Merger is expected to be July 1, 2014.
40
Table of Contents
Item 13. Certain Relationships and Related Transactions, and Director Independence
Certain Relationships and Related Person Transactions
Related Person Transactions
As more fully described in the subsection entitled “Letter Agreement with Howard Solomon” appearing on Page 20 of this Amendment, on May 22, 2013, Howard Solomon entered into a letter agreement with the Company in connection with his retirement as the Company’s CEO pursuant to which he will continue to serve as Senior Advisor to and Director of the Company for an annual salary and other compensation. Mr. Solomon is the father of David F. Solomon, who was formerly our Senior Vice President – Corporate Development and Strategic Planning and is now serving as Senior Advisor to the Chief Executive Officer responsible for Business Development and Alliance Management on a transitional basis.
In connection with his retirement as the Company’s Chief Operating Officer, the Company and Dr. Olanoff entered into a Consultant Services Letter Agreement dated January 1, 2011, which was amended and restated on April 22, 2013. Pursuant to his Consultant Services Letter Agreement, Dr. Olanoff may provide consulting services to Forest on a project-by-project basis relating to the evaluation of products and potential product opportunities and is compensated at the rate of $500 per hour. In addition, in the event the Company requires Dr. Olanoff to travel in connection with the performance of such services, the Company will reimburse Dr. Olanoff for out-of-pocket expenses in accordance with the Company’s expense reimbursement policies, including the reasonable cost of transportation, meals and lodging, where applicable.
As more fully described in the subsection entitled “Contracts and Agreements” appearing on Page 19 of this Amendment, in connection with his appointment as President and Chief Executive Officer of the Company, Mr. Saunders and the Company entered into an agreement dated September 11, 2013 (the Saunders Letter Agreement). The Saunders Letter Agreement provided, among other things, that while employed as President and Chief Executive Officer, Mr. Saunders will (i) receive an annual base salary of $1,300,000, subject to possible increases no less frequently than annually beginning in fiscal year 2015 (the Base Salary); (ii) be eligible to receive annual cash incentive compensation payments (an Annual Bonus), with a target Annual Bonus opportunity of 125% of Mr. Saunders’ Base Salary (the Target Bonus), a threshold Annual Bonus opportunity of 50% of the Target Bonus, and a maximum Annual Bonus opportunity of 200% of the Target Bonus, which in each case will be payable based upon the attainment of corporate and/or individual performance criteria established by the Company’s Compensation Committee; (iii) receive a sign-on equity grant on October 1, 2013 (the Start Date) consisting of (A) restricted stock units in respect of the Company’s common stock (the RSUs), which will vest on the third anniversary of the Start Date and have an aggregate grant date value of $4,000,000, based on the average of the high and low price of a share of Common Stock on the New York Stock Exchange on the date of grant (Grant Date FMV), and (B) options to purchase shares of the Common Stock having an aggregate grant date value of $6,000,000 with an exercise price equal to the Grant Date FMV that will vest in in equal installments on each of the first three anniversaries of the Start Date; (iv) be eligible to receive an annual grant of equity-based incentive awards having an aggregate grant date value of 5.3 times Mr. Saunders’ Base Salary (or such greater amount as the Company’s Compensation Committee may determine) in respect of each fiscal year following fiscal year 2014; and (v) become party to a change in control agreement, which he and the Company subsequently entered into on October 1, 2013 and which is more fully described in the subsection entitled “Change-in-Control Employment Agreements” appearing on Page 20 of this Amendment.
The Saunders Letter Agreement also provides that if Mr. Saunders’ employment is terminated by the Company without Cause or Mr. Saunders resigns for Good Reason (each term as defined in the Saunders Letter Agreement), subject to Mr. Saunders’ execution, delivery, and non-revocation of a general release of claims within 60 days of his termination and his continued compliance with the covenants set forth in the Saunders Letter Agreement, (i) he will be entitled to receive an amount equal to two times the sum of his then-current Base Salary and Target Bonus, which will be payable in 24 equal monthly installments commencing on the regular payroll
41
Table of Contents
date that is 60 days after the date of his termination of employment; (ii) the Company will continue providing health care coverage (medical and dental) for Mr. Saunders and any of his eligible dependents until the earlier of (a) the expiration of the 24-month period following his termination date, or (b) the date he becomes eligible for health care coverage from another employer; and (iii) to the extent not already vested, the RSUs granted as part of Mr. Saunders’ sign-on equity grant will become immediately vested.
The Saunders Letter Agreement further provides that (w) Mr. Saunders will be subject to standard limitations on soliciting or interfering with the Company’s employees, customers, suppliers, licensees, or other business associates for a one-year period following his termination of employment; (x) Mr. Saunders will be restricted from competing with the Company for a one-year period following (i) his voluntary termination of employment other than for Good Reason or (ii) any termination of employment on or before the third anniversary of the Start Date that entitles him to severance under the Saunders Letter Agreement; (y) for the 24 months following Mr. Saunders’ termination of employment, he will assist and cooperate with the Company with respect to any investigation or the Company’s (or an affiliate’s) defense or prosecution of any existing or future claims or litigations or other proceeding relating to matters in which he was involved in or had knowledge by virtue of his employment with the Company; and (z) on or prior to the date the Company holds its 2014 Annual Meeting (or, if earlier, the date at which the current Chairman of the Board (Chairman) ceases to serve in such role), the Board will use its commercially reasonable best efforts to cause Mr. Saunders to be nominated and elected to serve as Chairman.
In connection with Mr. Saunders’ sign-on equity grants, it was expected that he would purchase, and upon his request, the Company will sell to him at a price equal to the then fair market value of the common stock, shares of common stock having an aggregate purchase price of $5,000,000 within the first six months following the Start Date or as such period may be extended to take into account restrictions on Mr. Saunders’ ability to purchase the common stock under applicable law. In accordance with the terms and conditions of the Saunders Letter Agreement, Mr. Saunders and the Company entered into a stock purchase agreement on October 1, 2013, pursuant to which Mr. Saunders purchased 116,172 shares of Common Stock directly from the Company at a per share price of $43.04 (which was the average of the high and low price of a share of Common Stock on the New York Stock Exchange on October 1, 2013) and for an aggregate purchase price of $5,000,042.88. The transaction, which was exempt from registration with the SEC pursuant to Section 4(a)(2) of the Securities Act of 1933, closed the following day.
On February 16, 2014, the Company entered into an amendment to Mr. Saunders’ Change of Control employment agreement which provides that, in the event that Mr. Saunders becomes subject to excise taxes under Section 4999 of the Internal Revenue Code of 1986, as amended (the Code), he will be entitled to a gross-up payment from the Company to compensate him for the excise taxes that are imposed (the Gross-up Adjustment).
On February 16, 2014 and as more fully described in the subsection entitled Change-in-Control Employment Agreements appearing on Page 20 of this Amendment, the Company also entered into amendments to its Change of Control employment agreements (each a Change of Control Agreement) with Ms. Ling and Messrs. Bailey and Kelly. In addition to amending the Change of Control Agreements to provide a Gross-up Adjustment for each of Ms. Ling and Messrs. Bailey and Kelly, the amendments (i) eliminate (unless the affected executive officer is terminated without Cause or resigns for Good Reason), the Company’s obligations to make certain payments in the event the affected executive officer is terminated either upon a change of control or within 90 days thereof; (ii) revise certain provisions governing the timing of payments to comply with Section 409A of the Code; and (iii) restrict the executive officer from competing against the Company for a one-year period following (x) the executive officer’s voluntary termination of employment other than for Good Reason (as defined in the applicable Change of Control Agreement) or (y) any termination of employment on or before the third anniversary of the effective date of the applicable Change of Control Agreement that entitles the executive officer to severance benefits under his or her Change of Control Agreement.
42
Table of Contents
The compensation paid to Ms. Ling and Messrs. Solomon and Saunders during fiscal year 2014 is reported in the Summary Compensation Table on Page 26 of this Amendment; and the compensation paid to Dr. Olanoff in connection with his Consultant Services Letter Agreement is reported in the Director Compensation Table on Page 36 of this Amendment.
Related Persons Transaction Procedures
The Board has adopted written policies and procedures for the review and approval of transactions involving the Company and related parties, such as directors, executive officers and their immediate family members, and has allocated the responsibility for carrying out these policies among the Audit, Nominating and Governance and Compensation Committees of the Board as set forth in the table below:
| Transaction Type |
Committee Responsible for Reviewing and Approving or
Ratifying | |
| Any transaction between the Company and any director | Nominating and Governance Committee | |
| With respect to matters other than executive officer compensation, any transaction or series of transactions (i) in which the Company or a subsidiary is a participant, (ii) the amount involved exceeds $120,000 and (iii) a Related Person (as defined in Item 404 of Regulation S-K) has a direct or indirect material interest | Audit Committee | |
| Compensation arrangements with executive officers of the Company whose annual compensation exceeds $120,000 | Compensation Committee | |
In addition, under the Company’s written Code of Business Conduct and Ethics, all conflicts of interest, including transactions with Related Persons, are prohibited unless approved by the Company’s Board or a Committee of the Board.
Director Independence
The Board has affirmatively determined that the following directors have no material relationship with us and are independent within the meaning of the NYSE definition of “independence”: Nesli Basgoz, M.D., Christopher J. Coughlin, Kenneth E. Goodman, Vincent Intrieri, Pierre Legault, Gerald M. Lieberman, Lester B. Salans, M.D., and Peter J. Zimetbaum, M.D. To assist in making the independence determination, the Board has adopted Director Qualification Standards as part of our Corporate Governance Guidelines. The Director Qualification Standards satisfy the NYSE independence requirements, and a copy of our Corporate Governance Guidelines is available on our website by clicking on the “Corporate Governance” link under the “Investor Center” section at www.frx.com. Independent directors receive no compensation from us for service on the Board or the Committees other than directors’ fees and non-discretionary grants under our 2007 Equity Incentive Plan.
Item 14. Principal Accountant Fees and Services
The firm of BDO USA, LLP (BDO) has audited our consolidated financial statements for each of the three fiscal years ended March 31, 2014. In addition to retaining BDO to audit our consolidated financial statements for fiscal year 2014, we and our affiliates retained BDO, as well as other accounting and consulting firms, to provide various consulting and other services in fiscal year 2014 and expect to continue to do so in the future.
43
Table of Contents
The following table presents fees for professional audit services rendered by BDO for the audit of our annual financial statements and review of financial statements included in our Quarterly Reports on Form 10-Q for fiscal years 2014 and 2013, and fees billed for other services rendered by BDO:
| 2014 | 2013 | |||||||
| Audit Fees |
$ | 2,210,310 | $ | 1,728,850 | ||||
| Audit Related Fees (1) |
465,710 | (2) | 43,000 | (2) | ||||
| Tax Fees (1) |
— | — | ||||||
| All Other Fees |
— | — | ||||||
| Total |
$ | 2,676,020 | $ | 1,771,850 | ||||
|
|
|
|
|
|||||
| (1) | The Audit Committee has considered the non-audit services performed for us by BDO in the Committee’s evaluation of BDO’s independence. |
| (2) | Audit related fees in 2014 were related to the Bond Issuances and the audit of our Savings and Profit Sharing Plan. Audit related fees in 2013 were related to the audit of our Savings and Profit Sharing Plan. |
The Audit Committee’s policy is to pre-approve all audit services and all non-audit services that our independent registered public accounting firm is permitted to perform for us under applicable federal securities regulations. While the general policy of the Audit Committee is to make such determinations at full Audit Committee meetings, the Audit Committee may delegate its pre-approval authority to one or more members of the Audit Committee, provided that all such decisions are presented to the full Audit Committee at its next regularly scheduled meeting.
44
Table of Contents
Item 15. Exhibits, Financial Statement Schedules
All other schedules for which provision is made in the applicable accounting regulations of the Securities and Exchange Commission are not required under the related instructions or are inapplicable, and therefore have been omitted.
| (a)(2) | The following consolidated financial statements of the Company and its subsidiaries are found at Item 8:# |
| Consolidated Balance Sheet – Years Ended March 31, 2014 and 2013 |
||
| Consolidated Statements of Operations – Years Ended March 31, 2014, 2013, and 2012 |
||
| Consolidated Statements of Comprehensive Income (Loss) – Years Ended March 31, 2014, 2013, and 2012 |
||
| Consolidated Statements of Stockholders’ Equity – Years Ended March 31, 2014, 2013, and 2012 |
||
| Consolidated Statements of Cash Flows – Years Ended March 31, 2014, 2013, and 2012 |
||
| Notes to Consolidated Financial Statements |
| (a)(3) | Exhibits: | |
| 2.1 | Agreement and Plan of Merger, dated as of February 17, 2014, by and among Actavis plc, Tango US Holdings Inc., Tango Merger Sub 1 LLC, Tango Merger Sub 2 LLC and Forest Laboratories, Inc. Incorporated by reference to Forest’s Current Report on Form 8-K (Commission File No. 0-12943) filed February 19, 2014 (February 19, 2014 8-K).†# | |
| 2.2 | Agreement and Plan of Merger, dated as of January 7, 2014, by and among FRX Churchill Holdings, Inc., FRX Churchill Sub, Inc., Forest Laboratories, Inc. and Aptalis Holdings, Inc. Incorporated by reference to Forest’s Current Report on Form 8-K (Commission File No. 1-5438) filed January 8, 2014 (January 8, 2014 8-K).# | |
| 2.3 | Agreement and Plan of Merger dated February 22, 2011, among FL Holding C.V., Magnolia Acquisition Corp., Forest Laboratories, Inc. and Clinical Data, Inc. Incorporated by reference to Forest’s Current Report on Form 8-K (Commission File No. 0-12943) filed February 25, 2011 (February 25, 2011 8-K).†# | |
| 2.4 | Amendment No. 1 dated as of April 4, 2011, to the Agreement and Plan of Merger among FL Holding C.V., Magnolia Acquisition Corp., Forest Laboratories, Inc. and Clinical Data, Inc. Incorporated by reference to Forest’s Current Report on Form 8-K (Commission File No. 0-12943) filed April 4, 2011.# | |
| 3.1 | Articles of Incorporation of Forest, as amended and restated. Incorporated by reference to Forest’s Quarterly Report on Form 10-Q (Commission File No. 1-5438) for the Quarter ended September 30, 2008.# | |
| 3.2 | Bylaws of Forest Laboratories, Inc., as amended September 9, 2013. Incorporated by reference to Forest’s Current Report on Form 8-K (Commission File No. 1-5438) filed September 13, 2013 (September 13, 2013 8-K).# | |
| 3.3 | Certificate of Designations for Forest Laboratories, Inc. Series B Junior Participating Preferred Stock. Incorporated by reference to Forest’s Current Report on Form 8-K (Commission File No. 1-5438) filed August 28, 2012. # | |
| 4.1 | Indenture for the 4.375% Senior Notes, dated as of January 31, 2014, between Forest Laboratories, Inc., as issuer, and Wells Fargo Bank, National Association, as trustee. Incorporated by reference to Forest’s Current Report on Form 8-K (Commission File No. 1-5438) filed February 3, 2014 (February 3, 2014 8-K). # | |
| 4.2 | Indenture for the 4.875% Senior Notes, dated as of January 31, 2014, between Forest Laboratories, Inc., as issuer, and Wells Fargo Bank, National Association, as trustee. Incorporated by reference to the February 3, 2014 8-K. # | |
| 4.3 | Indenture, dated as of December 10, 2013, by and among Forest Laboratories, Inc. and Wells Fargo Bank, National Association, as trustee. Incorporated by reference to Forest’s Current Report on Form 8-K (Commission File No. 1-5438) filed December 11, 2013 (December 11, 2013 8-K). # | |
| 4.4 | Rights Agreement, dated as of August 27, 2012, between Forest Laboratories, Inc. and Computershare Shareowner Services LLC, which includes the form of Right Certificate as Exhibit B and the Summary of Rights to Purchase Preferred Shares as Exhibit C. Incorporated by reference to Forest’s Current Report on Form 8-K (Commission File No. 1-5438) filed August 28, 2012. # | |
| Material Contracts | ||
45
Table of Contents
| 10.1 | Benefit Continuation Agreement dated as of December 1, 1989 between Forest and Howard Solomon. Incorporated by reference to Forest’s Annual Report on Form 10-K (Commission File No. 1-5438) for the fiscal year ended March 31, 1990 (1990 l0-K). ††# | |
| 10.2 | Benefit Continuation Agreement dated as of May 27, 1990 between Forest and Kenneth E. Goodman. Incorporated by reference to the 1990 10-K. ††# | |
| 10.3 | Amended and Restated Change of Control Employment Agreement between Forest and Howard Solomon dated October 29, 2008. Incorporated by reference to Forest’s Quarterly Report on Form 10-Q (Commission File No. 1-5438) for the Quarter ended December 31, 2008 (December 31, 2008 10-Q). ††# | |
| 10.4 | Letter Agreement dated May 22, 2013 between Forest and Howard Solomon. Incorporated by reference to Forest’s Current Report on Form 8-K (Commission File No. 1-5438) filed May 24, 2013. ††# | |
| 10.5 | Amended and Restated Change of Control Employment Agreement between Forest and Elaine Hochberg dated October 29, 2008. Incorporated by reference to the December 31, 2008 10-Q. ††# | |
| 10.6 | Letter Agreement dated as of September 6, 2004 between Forest and Francis I. Perier, Jr. Incorporated by reference to Forest’s Current Report on Form 8-K (Commission File No. 1-5438) filed October 5, 2004. ††# | |
| 10.7 | Amended and Restated Change of Control Employment Agreement between Forest and Francis I. Perier, Jr. dated October 29, 2008. Incorporated by reference to the December 31, 2008 10-Q. ††# | |
| 10.8 | Letter Agreement dated June 15, 2007 between Forest and Dr. Marco Taglietti. Incorporated by reference to Forest’s Annual Report on Form 10-K (Commission File No. 1-5438) for the fiscal year ended March 31, 2009. ††# | |
| 10.9 | Amended and Restated Change of Control Employment Agreement between Forest and Marco Taglietti, M.D. dated October 29, 2008. Incorporated by reference to the December 31, 2008 10-Q. ††# | |
| 10.10 | Amended and Restated Change of Control Employment Agreement between Forest and Frank Murdolo dated October 29, 2008. Incorporated by reference to the December 31, 2008 10-Q. ††# | |
| 10.11 | Consultant Services Letter Agreement, as amended and restated April 22, 2013, between Forest Laboratories, Inc. and Dr. Lawrence S. Olanoff. Incorporated by reference to the Forest’s Annual Report on Form 10-K (Commission File No. 1-5438) for the fiscal year ended March 31, 2013. ††# | |
| 10.12 | Letter Agreement between Forest Laboratories, Inc. and Brenton L. Saunders dated September 11, 2013. Incorporated by reference to the September 13, 2013 8-K. ††# | |
| 10.13 | Stock Purchase Agreement between Forest and Brenton L. Saunders dated October 1, 2013. Incorporated by reference to Forest’s Current Report on Form 8-K (Commission File No. 1-5438) filed October 4, 2013 (October 4, 2013 8-K). # | |
| 10.14 | Employee Restricted Stock Agreement (Time-Based) granted to Brenton L. Saunders on October 1, 2013. Incorporated by reference to the October 4, 2013 8-K. ††# | |
| 10.15 | Change of Control Employment Agreement between Forest and Brenton L. Saunders dated October 1, 2013. Incorporated by reference to the October 4, 2013 8-K. ††# | |
| 10.16 | Amendment to Employment Agreement, dated as of February 16, 2014, by and between Forest Laboratories, Inc. and Brenton L. Saunders. Incorporated by reference to the February 19, 2014 8-K. ††# | |
| 10.17 | Letter Agreement, dated October 27, 2013, between Forest and A. Robert D. Bailey. Incorporated by reference to Forest’s Quarterly Report on Form 10-Q (Commission File No. 1-5438) for the Quarter ended December 31, 2013 (December 31, 2013 10-Q). ††# | |
| 10.18 | Stock Purchase Agreement between Forest and A. Robert D. Bailey dated November 12, 2013. Incorporated by reference to the December 31, 2013 10-Q. # | |
| 10.19 | Change of Control Employment Agreement, dated November 12, 2013, between Forest and A. Robert D. Bailey. Incorporated by reference to the December 31, 2013 10-Q. ††# | |
| 10.20 | Amendment to Employment Agreement, dated February 16, 2014, between Forest and A. Robert D. Bailey. ††# | |
46
Table of Contents
| 10.21 | Letter Agreement, dated October 11, 2013, between Forest and John Alex Kelly. Incorporated by reference to the December 31, 2013 10-Q. ††# | |
| 10.22 | Change of Control Employment Agreement, dated October 24, 2013, between Forest and John Alex Kelly. Incorporated by reference to the December 31, 2013 10-Q. ††# | |
| 10.23 | Amendment to Employment Agreement, dated February 16, 2014, between Forest and John Alex Kelly. ††# | |
| 10.24 | Amended and Restated Change of Control Employment Agreement, dated October 29, 2008, between Forest and William Meury. Incorporated by reference to the December 31, 2013 10-Q. ††# | |
| 10.25 | Letter Agreement, dated March 12, 2003, between Forest and Kevin Walsh. Incorporated by reference to the December 31, 2013 10-Q. ††# | |
| 10.26 | Amended and Restated Change of Control Employment Agreement, dated October 29, 2008, between Forest and Kevin Walsh. Incorporated by reference to the December 31, 2013 10-Q. ††# | |
| 10.27 | Change of Control Employment Agreement, dated January 1, 2011, between Forest and Joseph Zimmerman. Incorporated by reference to the December 31, 2013 10-Q. ††# | |
| 10.28 | Letter Agreement, dated December 19, 2013, between Forest and Karen Ling. Incorporated by reference to the December 31, 2013 10-Q. ††# | |
| 10.29 | Stock Purchase Agreement, dated January 21, 2014 between Forest and Karen Ling. # | |
| 10.30 | Change of Control Employment Agreement, dated February 7, 2014, between Forest and Karen Ling. ††# | |
| 10.31 | Amendment to Employment Agreement, dated February 16, 2014, between Forest and Karen Ling. ††# | |
| 10.32 | Employee Restricted Stock Agreement (Time-Based) granted to Karen Ling on January 21, 2014. ††# | |
| 10.33 | Employee Stock Option Agreement granted to Karen Ling on January 21, 2014. ††# | |
| 10.34 | Employee Stock Unit Agreement (Time-Based) granted to Karen Ling on January 21, 2014. ††# | |
| 10.35 | 2000 Stock Option Plan of Forest Laboratories, Inc. Incorporated by reference to Forest’s Proxy Statement (Commission File No. 1-5438) for the fiscal year ended March 31, 2000. ††# | |
| 10.36 | 2004 Stock Option Plan of Forest Laboratories, Inc. Incorporated by reference to Forest’s Proxy Statement (Commission File No. 1-5438) for the fiscal year ended March 31, 2004. ††# | |
| 10.37 | 2007 Equity Incentive Plan of Forest Laboratories, Inc., as amended. Incorporated by reference to Forest’s Current Report on Form 8-K (Commission File No. 1-5438) filed on August 21, 2013. ††# | |
| 10.38 | Form of Director Restricted Stock Agreement under the 2007 Equity Incentive Plan of Forest Laboratories, Inc. Incorporated by reference to Forest’s Form S-8 on Registration Statement No. 333-145415 filed August 14, 2007. ††# | |
| 10.39 | Form of Director Stock Option Agreement under the 2007 Equity Incentive Plan of Forest Laboratories, Inc. Incorporated by reference to Forest’s Quarterly Report on Form 10-Q (Commission File No. 1-5438) for the Quarter ended September 30, 2007 (September 30, 2007 10-Q). ††# | |
| 10.40 | Form of Employee Restricted Stock Agreement (Time-Based) under the 2007 Equity Incentive Plan of Forest Laboratories, Inc. Incorporated by reference to Forest’s Annual Report on Form 10-K (Commission File No. 1-5438) for the fiscal year ended March 31, 2008 (2008 10-K). ††# | |
| 10.41 | Form of Employee Stock Option Agreement under the 2007 Equity Incentive Plan of Forest Laboratories, Inc. Incorporated by reference to the September 30, 2007 10-Q. ††# | |
| 10.42 | Form of Employee Stock Unit Agreement (Time-Based) under the 2007 Equity Incentive Plan of Forest Laboratories, Inc. Incorporated by reference to Forest’s Annual Report on Form 10-K (Commission File No. 1-5438) for the fiscal year ended March 31, 2012. ††# | |
47
Table of Contents
| 10.43 | Form of Employee Stock Unit Agreement (Performance-Based) under the 2007 Equity Incentive Plan of Forest Laboratories, Inc. Incorporated by reference to Forest’s Annual Report on Form 10-K (Commission File No. 1-5438) for the fiscal year ended March 31, 2012. ††# | |
| 10.44 | Forest Laboratories, Inc. Annual Incentive Compensation Plan. Incorporated by reference to Forest’s Quarterly Report on Form 10-Q (Commission File No. 1-5438) for the Quarter ended September 30, 2012. ††# | |
| 10.45 | Forest Corporate Officer Severance Benefit Plan and Summary Plan Description. Incorporated by reference to Forest’s Current Report on Form 8-K (Commission File No. 1-5438) filed November 4, 2013. ††# | |
| 10.46 | Credit Agreement, dated December 7, 2007, by and among Forest Laboratories, Inc., Forest Laboratories Holdings Limited, Forest Laboratories Ireland Limited, Forest Finance B.V., Forest Laboratories UK Limited, the lenders party thereto, and JPMorgan Chase Bank, N.A. Incorporated by reference to Forest’s Current Report on Form 8-K (Commission File No. 1-5438) filed December 13, 2007. # | |
| 10.47 | Amendment No. 1 dated October 19, 2012 to the Credit Agreement dated December 7, 2007, by and among Forest Laboratories, Inc., Forest Laboratories Holdings Limited, Forest Laboratories Ireland Limited, Forest Finance B.V., Forest Laboratories UK Limited, the lenders party thereto, and JPMorgan Chase Bank, N.A. Incorporated by reference to Forest’s Current Report on Form 8-K (Commission File No. 1-5438) filed October 23, 2012. # | |
| 10.48 | Credit Agreement, dated December 4, 2012, by and among Forest Laboratories, Inc., Forest Laboratories Holdings Limited, Forest Laboratories Ireland Limited, Forest Finance B.V., Forest Laboratories UK Limited, Forest Laboratories Canada Inc., JPMorgan Chase Bank, N.A., as administrative agent, and the other lenders from time to time party thereto. Incorporated by reference to Forest’s Current Report on Form 8-K (Commission File No. 1-5438) filed December 7, 2012. # | |
| 10.49 | First Amendment dated as of December 2, 2013 to Credit Agreement, dated December 2, 2013, to the $750 million Credit Agreement dated as of December 4, 2012, by and among Forest Laboratories, Inc., Forest Laboratories Holdings Limited, Forest Laboratories Ireland Limited, Forest Finance B.V., Forest Laboratories UK Limited, Forest Laboratories Canada Inc., JPMorgan Chase Bank, N.A., as administrative agent, and the other lenders from time to time party thereto. Incorporated by reference to Forest’s Current Report on Form 8-K (Commission File No. 1-5438) filed December 2, 2013. # | |
| 10.50 | Registration Rights Agreement, dated as of January 31, 2014, by and among Forest Laboratories, Inc., as issuer, and Morgan Stanley & Co. LLC, as representative of the initial purchasers. Incorporated by reference to the February 3, 2014 8-K. # | |
| 10.51 | Registration Rights Agreement, dated as of December 10, 2013, by and among Forest Laboratories, Inc. and Morgan Stanley & Co. LLC, as representative of the initial purchasers. Incorporated by reference to the December 11, 2013 8-K. # | |
| 10.52 | Commitment Letter, dated as of January 7, 2014, among Forest Laboratories, Inc. and Morgan Stanley Senior Funding, Inc. Incorporated by reference to the January 8, 2014 8-K. # | |
| 10.53 | Corporate Integrity Agreement dated September 15, 2010 between the Office of Inspector General of the U.S. Department of Health and Human Services and Forest Laboratories, Inc. Incorporated by reference to Forest’s Quarterly Report on Form 10-Q (Commission File No. 0-12943) for the quarter ended September 30, 2010 (September 30, 2010 10-Q). # | |
| 10.54 | Plea Agreement dated September 15, 2010 among the U.S. Attorney for the District of Massachusetts, the U.S. Department of Justice, and Forest Pharmaceuticals, Inc. Incorporated by reference to the September 30, 2010 10-Q. # | |
| 10.55 | Settlement Agreement and Release dated September 15, 2010 among Forest Laboratories, Inc., Forest Pharmaceuticals, Inc., the U.S. of America, acting through the U.S. Department of Justice on behalf of the Office of Inspector General of the Department of Health and Human Services, TRICARE Management Activity, the Veteran’s Affairs Administration, the U.S. Office of Personnel Management, and certain individual relators named therein. Incorporated by reference to the September 30, 2010 10-Q. # | |
| 10.56 | Fixed Dollar Collared Accelerated Share Repurchase Transaction dated June 3, 2011 between Forest Laboratories, Inc. and Morgan Stanley & Co. LLC. Incorporated by reference to Forest’s Current Report on Form 8-K (Commission File No. 1-5438) filed June 9, 2011. # | |
48
Table of Contents
| 10.57 | Fixed Dollar Collared Accelerated Share Repurchase Transaction Agreement dated August 15, 2011 between Forest Laboratories, Inc. and Morgan Stanley & Co. Incorporated by reference to Forest’s Quarterly Report on Form 10-Q (Commission File No. 1-5438) for the quarter ended September 30, 2011 (September 30, 2011 10-Q). # | |
| 10.58 | Fixed Dollar Collared Accelerated Share Repurchase Transaction Agreement dated June 3, 2011, as amended and restated on August 15, 2011, between Forest Laboratories, Inc. and Morgan Stanley & Co. Incorporated by reference to September 30, 2011 10-Q. # | |
| 10.59 | Co-Promotion Agreement dated December 10, 2001 by and between Sankyo Pharma Inc. and Forest Laboratories, Inc. Incorporated by reference to Forest’s Annual Report on Form 10-K (Commission File No. 1-5438) for the fiscal year ended March 31, 2002 (2002 10-K).* # | |
| 10.60 | S-Enantiomer License Agreement dated May 29, 2002 by and between Forest Laboratories Ireland Limited and H. Lundbeck A/S. Incorporated by reference to the 2002 10-K.* # | |
| 10.61 | S-Enantiomer Supply Agreement dated May 29, 2002 by and between Forest Laboratories Ireland Limited and H. Lundbeck A/S. Incorporated by reference to the 2002 10-K.* # | |
| 10.62 | Settlement Agreement by and between Forest Laboratories, Inc., Forest Laboratories Holdings Limited and H. Lundbeck A/S and Alphapharm Pty Ltd. effective October 3, 2005. Incorporated by reference to Forest’s Quarterly Report on Form 10-Q (Commission File No. 1-5438) for the fiscal quarter ended December 31, 2005.* # | |
| 10.63 | Settlement Agreement among Forest Laboratories, Inc., H. Lundbeck A/S, Caraco Pharmaceutical Laboratories, Ltd. and Sun Pharmaceutical Industries, Ltd. dated July 10, 2009. Incorporated by reference to Forest’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2009.* # | |
| 10.64 | License and Cooperation Agreement dated June 28, 2000 by and between Merz & Co. GmbH and Forest Laboratories Ireland Limited. Incorporated by reference to Forest’s Annual Report on Form 10-K (Commission File No. 1-5438) for the fiscal year ended March 31, 2004.* # | |
| 10.65 | License and Collaboration Agreement (the Cypress License) dated January 9, 2004 between the Registrant and Cypress Bioscience, Inc. (Cypress) filed as Exhibit 10.26 to Cypress’s Annual Report on the Form 10-K (Commission File No. 0-12943) of Cypress for the year ended December 31, 2003 (Cypress 2003 10-K).* # | |
| 10.66 | Side Letter dated January 9, 2004 among the Registrant, Cypress and Pierre Fabre Médicament filed as Exhibit 10.27 to the Cypress 2003 10-K.* # | |
| 10.67 | Letter Agreement dated January 9, 2004 among the Registrant, Cypress and Pierre Fabre Médicament filed as Exhibit 10.28 to the Cypress 2003 10-K.* # | |
| 10.68 | Amendment to the Cypress License filed as Exhibit 10.1 to Cypress’s Quarterly Report on Form 10-Q (Commission File No. 0-12943) for the quarter ended June 30, 2005.* # | |
| 10.69 | License Agreement dated September 30, 2003 by and between Takeda Chemical Industries, Ltd. and Peninsula Pharmaceuticals, Inc. Incorporated by reference to the 2011 10-K.* # | |
| 10.70 | First Amendment to Agreement dated November 4, 2004 by and between Takeda Pharmaceutical Company Limited (f/k/a Takeda Chemical Industries, Ltd.) and Peninsula Pharmaceuticals, Inc. Incorporated by reference to the 2011 10-K. # | |
| 10.71 | Second Amendment to Agreement dated November 19, 2007 by and among Takeda Pharmaceutical Company Limited, Cerexa Inc. and Forest Laboratories Holdings Limited. Incorporated by reference to the 2011 10-K.* # | |
| 10.72 | License, Development and Cooperation Agreement dated September 22, 2004 between Merck KGaA and Genaissance Pharmaceuticals, Inc. Incorporated by reference to the September 30, 2011 10-Q.* # | |
| 10.73 | Collaboration and Distribution Agreement dated August 7, 2009 by and between Nycomed GmbH and Forest Laboratories Holdings Limited. Incorporated by reference to Forest’s Quarterly Report on Form 10-Q (Commission File No, 1-5438) for the quarter ended December 31, 2011. *# | |
| 10.74 | License, Development, Commercialisation and Cooperation Agreement, dated as of April 7, 2006 and as amended to date, by and between Almirall Prodesfarma, S.A. and Forest Laboratories Holdings Limited. Incorporated by reference to Forest’s Quarterly Report on Form 10-Q (Commission File No. 1-5438) for the Quarter ended December 31, 2012.* # | |
49
Table of Contents
| 10.75 | Collaboration Agreement, dated as of September 12, 2007, as amended on November 3, 2009, by and between Forest Laboratories, Inc. and Ironwood Pharmaceuticals, Inc. Incorporated by reference to Forest’s Annual Report on Form 10-K/A (Commission File No. 1-5438) for the fiscal year ended March 31, 2013.* # | |
| 10.76 | Sale and Transfer Agreement dated March 30, 2012 between Janssen Pharmaceutica NV and Forest Laboratories Holdings Limited. Incorporated by reference to Forest’s Annual Report on Form 10-K (Commission File No. 1-5438) for the fiscal year ended March 31, 2012.* # | |
| 10.77 | Nomination and Standstill Agreement, dated June 10, 2013 by and between Forest, Carl C. Icahn, Vincent J. Intrieri, High River Limited Partnership, Hopper Investments LLC, Barberry Corp., Icahn Partners LP, Icahn Partners Master Fund LP, Icahn Partners Master Fund II LP, Icahn Partners Master Fund III LP, Icahn Enterprises G.P. Inc., Icahn Enterprises Holdings L.P., IPH GP LLC, Icahn Onshore LP, Icahn Offshore LP, and Beckton Corp. Incorporated by reference to Forest’s Current Report on Form 8-K (Commission File No. 1-5438) filed June 11, 2013. # | |
| 10.78 | Asset Purchase Agreement, dated as of November 29, 2013, as amended on January 9, 2014, by and between Merck Sharp & Dohme BV, and Forest Laboratories Holdings Limited. Incorporated by reference to the December 31, 2013 10-Q.*† # | |
| 10.79 | License Agreement, dated as of January 10, 2014, by and between Merck Sharp & Dohme BV, and Forest Laboratories Holdings Limited. Incorporated by reference to the December 31, 2013 10-Q.* # | |
| 10.80 | Supply Agreement, dated as of January 10, 2014, by and between Merck Sharp & Dohme BV, and Forest Laboratories Ireland Limited. Incorporated by reference to the December 31, 2013 10-Q.* # | |
| 10.81 | Amended and Restated Patent and Know-How License Agreement, dated December 17, 2013, by and between Pierre Fabre Médicament SAS, and Forest Laboratories Holdings Limited. Incorporated by reference to the December 31, 2013 10-Q.* # | |
| 10.82 | Amended and Restated Trademark License Agreement, dated December 17, 2013, between Pierre Fabre Médicament SAS, and Forest Laboratories Holdings Limited. Incorporated by reference to the December 31, 2013 10-Q.* # | |
| 10.83 | Amended and Restated Purchase and Supply Agreement, dated December 17, 2013, between Pierre Fabre Médicament SAS, and Forest Laboratories Holdings Limited. Incorporated by reference to the December 31, 2013 10-Q.* # | |
| 21 | List of Subsidiaries. # | |
| 23 | Consent of Independent Registered Public Accounting Firm. # | |
| 31.1 | Certification pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. # | |
| 31.2 | Certification pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. # | |
| 31.3 | Certification pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. ## | |
| 31.4 | Certification pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. ## | |
| 32.1 | Certification pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. # | |
| 32.2 | Certification pursuant to Section 906 of the Sarbanes-Oxley Act of 2002.# | |
| 101.INS | XBRL Instance Document**# | |
| 101.SCH | XBRL Taxonomy Extension Schema Document**# | |
| 101.PRE | XBRL Taxonomy Presentation Linkbase Document**# | |
| 101.CAL | XBRL Taxonomy Calculation Linkbase Document**# | |
| 101.LAB | XBRL Taxonomy Label Linkbase Document**# | |
| 101.DEF | XBRL Taxonomy Definition Linkbase Document**# | |
50
Table of Contents
| * | Confidential treatment has been granted as to certain portions of these Exhibits. |
| ** | Attached as Exhibit 101 to this Annual Report on Form 10-K are the following materials, formatted in eXtensible Business Reporting Language (XBRL): (i) Consolidated Balance Sheets – March 31, 2014 and 2013, (ii) Consolidated Statements of Operations – years ended March 31, 2014, 2013 and 2012, (iii) Consolidated Statements of Comprehensive Income (Loss) – years ended March 31, 2014, 2013 and 2012, (iv) Consolidated Statements of Stockholders’ Equity – years ended March 31, 2014, 2013 and 2012, (v) Consolidated Statements of Cash Flows – years ended March 31, 2014, 2013 and 2012 and (vi) the Notes to Consolidated Financial Statements. |
| † | Schedules omitted pursuant to Item 601(b)(2) of Regulation S-K. The Company agrees to furnish a supplemental copy of any omitted schedule to the Securities and Exchange Commission upon request. |
| †† | Denotes Management Contract |
| # | Previously filed or furnished, as applicable, as an exhibit to the Company’s Annual Report on Form 10-K for the fiscal year ended March 31, 2014 filed with the SEC on May 30, 2014. |
| ## | Filed herewith |
51
Table of Contents
SIGNATURES
Pursuant to the requirements of Section 13 and 15(d) of the Securities Exchange Act of 1934, Forest has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
Dated: June 30, 2014
| FOREST LABORATORIES, INC. | ||
| By: | /s/ Brenton L. Saunders | |
| Brenton L. Saunders | ||
| Chief Executive Officer and President | ||
52
