Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - OPKO HEALTH, INC. | d736771d8k.htm |
 Jefferies 2014 Global Healthcare Conference
June 3, 2014
Exhibit 99.1 |
 2 |
 OPKO
Important Products Available or Coming to Market Near Term
3
Diagnostics
–
4Kscore™
Test blood test for personalized risk of high-grade
prostate cancer
–
CLIA-certified urological specialty laboratory
–
Pharmaceuticals
–
Vitamin
D
therapeutics
for
SHPT
*
–
Platform technology to make peptides and proteins long acting to
treat growth hormone deficiency, hemophilia, obesity, etc.
–
Calcium-free, magnesium-based phosphate binder
–
Approved third generation hepatitis B vaccine
*Secondary Hyperparathyroidism
Claros
®
1
immunoassay
system
for
rapid,
lab
quality
in-office
testing
(PSA,
Testosterone,
Vitamin
D) |
 OPKO
Diagnostics – Addressing Large Dx Markets
4
4Kscore™
Test
Initially targeting pre-prostate biopsy market
PSA market 60 million tests globally
Recently launched in US at $395
Highly significant clinical data on 1,012 patients presented at AUA
plenary session May 18, 2014
Launch in Europe: September 2014
Claros
®
1 Analyzer and Sangia
™
Microfluidic Test Card
In office finger-stick blood analysis
Initial target assays in US:
PSA: 30 million tests, $750 M
Testosterone: 15 million tests, $525 M
Vitamin D: 70 million tests, $3.5 B |
 5
DELIVERING BETTER
HEALTHCARE
CONVENIENT
LAB QUALITY
ACCURACY |
 Finger stick blood
sample
Convenience
1-2
mins
10
mins
6 |
| Claros
1 Update •
Testosterone
–
FDA: Pre-submission comments received from FDA
–
On track to file 510(k) in 2014
–
CE Mark: 4Q2014
•
PSA
–
FDA: Pre-submission response expected in August
–
Timing of 510k submission based on longitudinal trial requirements
–
CE Mark Update (Formulation and Chemistry): 4Q2014
•
Vitamin D
–
On track to support launch of Rayaldee 1Q2016
7 |
 Challenges in Prostate Cancer Screening
High false positive rate of PSA
Patient Anxiety
1M Prostate biopsies in US
75% Negative or Low-Grade
Pain, bleeding, infection,
hospitalization
Recommendations to stop PSA
screening
8 |
 4Kscore Test –
Avoiding Unnecessary Prostate
Biopsies
The only
test to identify men with high-grade prostate
cancer from a blood sample
Combines results of multiple biomarkers to create a
patient’s personal risk score
Based on 10 years of clinical research by scientists at
Memorial Sloan-Kettering Cancer Center and leading
European cancer centers
Tested in over 10,000 men in 9 separate clinical studies
demonstrating a 27% -
82% biopsy reduction
Validated by OPKO in a prospective, blinded study of
1,012 men
9 |
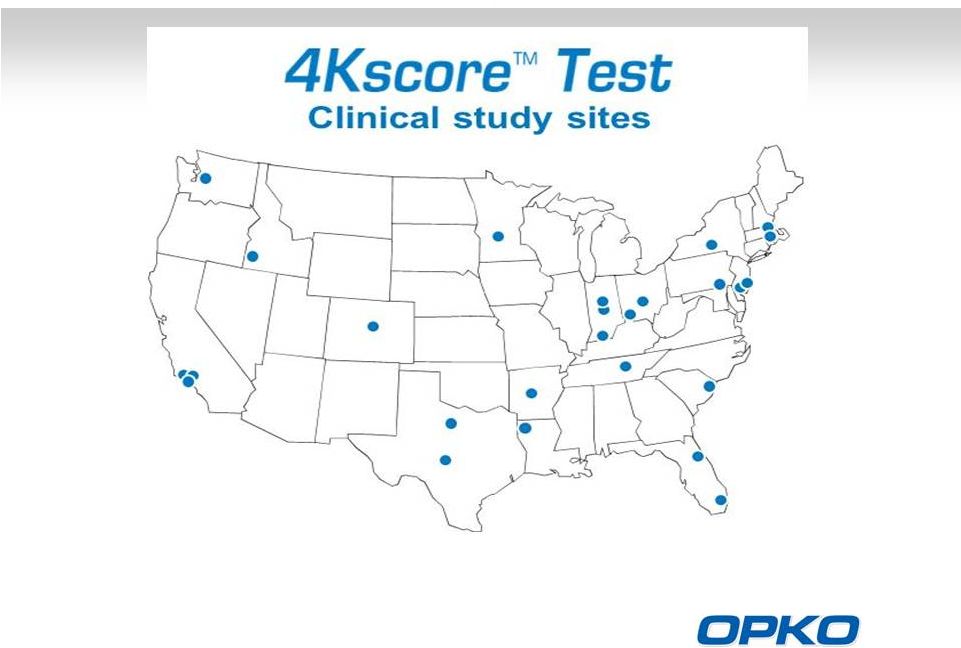 4Kscore Test US Clinical Study
10
1,012 Patients Enrolled –
Prospective Clinical Trial |
 11
4Kscore Test Clinical Study Results in 1,012 Men
•
Discrimination: AUC = 0.82
•
Risk Calibration
•
Decision Curve Analysis
•
Biopsy reduction of 30% to 58% |
 The
4Kscore Patient Counseling Report for a Result of 5%
12 |
 The
4Kscore Test Conclusions Validated test based on a decade of clinical
research and a prospective, multi-institutional,
contemporary US clinical trial
Convenient blood test, cost $395
Excellent discrimination for high-grade cancer
(AUC = 0.82) and high net benefit for clinical use
Reduces 30 –
58% of biopsies
Provides a calibrated score for informed, shared
decision-making between urologist and patient
13 |
 OPKO
Pharmaceuticals — Advanced, Deep Pipeline
Product
Indication
Preclinical
Phase 1
Phase 2
Phase 3
Milestone
Market Size
Rayaldee™
(CTAP101)
SHPT
(CKD Stage 3-4 Patients)
Phase 3 results
expected mid-
2014
$12.0 BN
hGH-CTP
hGH deficiency
$3.5 BN
Alpharen™
(Fermagate)
Hyperphosphatemia
(CKD Stage 5 Patients)
$1.2 BN
Rolapitant
CINV
NDA submission
targeted mid-2014
$1.5 BN
Sci-B-Vac™
Hepatitis B
(CKD Stage 5 Patients)
$0.2BN
Lunacalcipol™
(CTA018)
Moderate to severe SHPT
(CKD Stage 5 Patients) &
Psoriasis
$1.5 BN
CTAP201
Mild to moderate SHPT
(CKD Stage 5 Patients)
$1.1 BN
Factor VIIa-CTP
Hemophilia
$1.7 BN
AntagoNAT
Platform
Cancer, CV, metabolic
and orphan disease
$1.0 BN
Oxyntomodulin
Diabetes, Obesity
$15 BN
Outlicensed to TESARO
14 |
 15
Rayaldee (CTAP101) –
A Late-Stage Investigational Drug
Product Overview
•
Oral formulation of 25D3*
addresses significant unmet need
•
Safe and effective treatment for
elevated PTH (SHPT) associated
with low 25D levels in Stage 3-4
CKD
•
Achieves reliable increases in
serum 25D and reductions in
plasma PTH
•
Lower risk of side effects
compared to active 1,25D
**
products
•
Potential for additional
indications including elderly,
osteoporosis & cancer
Clinical Status
Intellectual Property
* 25-Hydroxyvitamin D
** 1,25-Dihydroxyvitamin D
•
Clinical development guided by
prominent Scientific Advisory
Board
•
Top line phase 3 data available
in mid-2014
•
NDA filing in 1Q 2015
•
Rayaldee US patents issued,
protected through 2028
•
Additional global patents
allowed or pending
3 |
 16
Rayaldee -
Commercial Opportunity
Source: BioTrends Research Group, Inc. December 2010
Untreated
26-44%
Vitamin D
Hormone
20-36%
Nutritional
Vitamin D
36-38%
Untreated
26-44%
Safety
concerns;
exacerbates
vitamin D
insufficiency
Efficacy
Concerns
Stage 3 & 4 CKD Treatment
•
Low
serum
25D
and
elevated
plasma
PTH
are
prevalent
in
CKD
Stage
3-4
patients
–
8 million CKD Stage 3-4 patients in the US
–
4 million patients with low serum 25D and high plasma PTH
Rayaldee is expected to take significant market share in Stage 3 and 4 CKD
patients suffering from SHPT – a potential $12 billion revenue
opportunity |
 Rayaldee is expected to raise serum total 25-hydroxyvitamin D (25D) and
lower
plasma
iPTH
more
effectively
than
any
currently
marketed
over-the-
counter (OTC) or prescription (Rx) product without the risk of
hypercalcemia.
Comparison of Vitamin D Therapies for Stage 3-4 CKD
*And generics
**25-hydroxyvitamin D
17 |
 •
CTP increases protein circulation time
•
Merck’s long acting FSH-CTP (Elonva
™
):
o
Received EU marketing authorization in 2010; NDA filed Q3 2013
o
Single FSH-CTP injection replaces 7 daily FSH injections in fertility
treatment
•
Two licensees of CTP technology for human therapeutics:
o
Merck (holds license for 4 fertility-focused proteins )
o
OPKO’s Biologics (holds license for all other rights)
18
CTP Technology: Clinically Validated Proprietary Platform
CTP –
a natural sequence
created during evolution
to enhance the longevity
of peptides and proteins
without increasing toxicity
Any Short Acting
Protein
CTP
Long Acting
Protein |
| •
$3.5 billion market, growing 5% annually
•
Once-a-week injection (current products require daily injections)
•
Small needle size (31 gauge) due to low viscosity
–
Competitive long acting formulations have high viscosity
•
Superb clinical, safety and immunogenicity profile
•
Human growth hormone is used for:
–
Growth hormone deficient children
–
Growth hormone deficient adults
–
Short stature
–
Off label
•
Orphan drug designation in the US & EU for children & adults
19
hGH-CTP Opportunity |
| hGH-CTP Clinical Development
•
Adult Pivotal Phase 3 trial (ongoing)
–
189 patients
–
Primary
efficacy
endpoint:
reduction
in
truncal
fat
mass
after
6
months
vs.
placebo
–
Secondary efficacy endpoints include:
•
Reduction in total body fat
•
Increase in lean body mass
–
Single pivotal trial required by FDA for BLA submission in 2016
•
Pediatric GHD Phase 2 trial (advanced stage)
–
Enrollment completed March 2014
–
4 cohorts:
•
3 dose levels of once-weekly hGH-CTP
•
Commercially available standard daily rhGH treatment
–
Key outcome: height velocity
–
Positive clinical data to be presented at ENDO meeting June 21-24,
2014 –
Phase 3 to commence by 1H2015
20 |
| FVIIa –CTP: Long Acting for Treating Hemophilic Patients
21
•
$1.7 billion market
•
Growing 7% annually
•
Only 25% of patients are treated
•
Current product (NovoSeven®) requires frequent IV doses
•
3-4 times a day during bleeding episodes
•
1-2 times a day for prophylactic treatment
•
Pharmacological studies in hemophilic mice and dogs FVIIa-
CTP demonstrated:
•
Potential for substantial improvement in the quality of life of patients via
subcutaneous administration
•
Reduce frequency of injection during on-demand therapy
•
Enable prophylactic treatment while reducing the frequency of injections to
2-3 times a week
•
Phase 2a study in hemophilic patients: initiated H2 2014
•
Orphan drug designation in the US |
| MOD-6031: Long Acting Oxyntomodulin for Obesity
22
•
>$15 billion market
•
Growing rapidly
•
Oxyntomodulin
–
Nature’s Appetite Control Mechanism
•
Natural appetite suppressor
•
Secreted by the digestive system following food intake and induces satiety in
the brain
•
Increases glucose tolerance
•
Short acting –
requires 3 injections per day
•
MOD-6031 Long Acting Oxyntomodulin-
weekly injection
studies in mutant obese mice and diet induced obese mice
demonstrated:
•
Significantly inhibited food intake and reduced body weight by reducing fat
•
Reduced cholesterol levels
•
Improved glycemic control
•
Phase 1 study to be initiated 1H2015
•
MOD-6031 is expected to provide superior long-term therapy
for obesity and diabetes type II patients |
 Rolapitant –
Potential Near-term Revenue Driver
•
Rolapitant out-licensed to Tesaro in December 2010
–
Payments of up to $121 million
–
Double-digit tiered royalties
•
Differentiated cancer supportive care product with $1.5B US Market
Opportunity
–
Potent neurokinin-1(NK-1) receptor antagonist for
chemotherapy-induced nausea and vomiting (CINV)
–
Opportunity to differentiate on convenience, market access and safety
•
Single dose
•
Lack of CYP 3A4 drug-drug interactions
Long acting
•
Oral and
IV formulations allow full market access
•
NDA submission targeted for mid-2014
–
All three Phase 3 trials (MEC
*
and HEC
**
) achieved primary endpoint
–
Primary endpoint: complete response (no emesis and no use of rescue
medication) –
Third
Phase
3
trial
(HEC)
also
achieved
all
secondary
endpoints,
including:
•
Complete response in acute (0-24 hrs) and overall (0-120 hrs) phase of
CINV •
No significant nausea
23
*Moderately emetogenic chemotherapy **Highly emetogenic
chemotherapy |
| Strategic Investments
•
ARNO Therapeutics,
Inc. (OTC: ARNI) (~5% equity interest)
–
Anti-progestins for breast (phase 2) , endometrial and prostate
cancers •
Zebra Biologics,
Inc. (~19% equity interest)
–
Combinatorial antibody libraries based on function in human cell
screens
•
OAO Pharmsynthez
(MICE: LIFE) (~17% equity interest)
–
Russian developer and marketer of new drugs
•
RXi Pharmaceuticals
Corporation (NASDAQ: RXII)
(~17% equity interest)
–
sRNA to prevent hypertrophic scars (phase 2)
•
Cocrystal Pharma,
Inc. (OTC: COCP) (~16% equity interest)
–
New anti-virals (Hepatitis C, flu, dengue fever)
•
Fabrus,
Inc.
(~12% equity interest*)
–
Antibodies against difficult targets (e.g., G protein-coupled receptor, ion
channels) •
Neovasc,
Inc. (NASDAQ: NVCN) (~ 6% equity interest)
–
Cardiology devices
•
ChromaDex,
Inc.
(OTC: CDXC) (~2% equity interest)
–
New nutritional supplement APIs
24
Proprietary Technologies with Significant Upside Potential
(As of March 31, 2014)
*
Merger with Senesco Technologies, Inc. (OTC: SNTI) completed May 19, 2014
|
