Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Innoviva, Inc. | a14-12968_18k.htm |
| EX-99.2 - EX-99.2 - Innoviva, Inc. | a14-12968_1ex99d2.htm |
| EX-99.3 - EX-99.3 - Innoviva, Inc. | a14-12968_1ex99d3.htm |
Exhibit 99.1
Poster Board No. 411
Bronchodilator response to the long-acting bronchodilator combination of umeclidinium/vilanterol across subgroups of patients with COPD
MeiLan K. Han(1), Chris Kalberg(2), Jean Brooks(3), Alison Church(2)
(1)Division of Pulmonary Medicine, University of Michigan, Ann Arbor, MI, USA; (2)GlaxoSmithKline, Respiratory and Immuno-Inflammation, Research Triangle Park, NC, USA; (3)GlaxoSmithKline, Stockley Park, Uxbridge, UK
INTRODUCTION
· Long-acting muscarinic antagonists (LAMAs) and long-acting β2-agonists (LABAs) have distinct and complementary mechanisms of action to improve bronchodilation.
· The fixed-dose combination of the LAMA umeclidinium (UMEC) and the LABA vilanterol (VI) (ANORO™ ELLIPTA™) has been shown to produce statistically significant improvements in lung function compared with UMEC or VI monotherapy, in patients with chronic obstructive pulmonary disease (COPD).(1)–(3)
· ANORO™ ELLIPTA™ is an approved maintenance treatment for COPD in the US. It is not indicated for treatment of asthma.
· This evaluation reports findings from the subgroup analyses of trough forced expiratory volume in 1 second (FEV1) from Phase III studies conducted for UMEC/VI.
METHODS
Subgroup analyses
· Pre-specified subgroup analyses were conducted using integrated data (N=4713) from four 24-week, multicenter, randomized, placebo- or active-controlled studies (ClinicalTrials.gov: NCT01316900; protocol number: DB2113360; ClinicalTrials.gov: NCT01313637, protocol number: DB2113361; ClinicalTrials.gov: NCT01313650, protocol number: DB2113373; ClinicalTrials.gov: NCT01316913, protocol number: DB2113374).
· Subgroups were defined based on gender, age, disease severity (GOLD stage), smoking status, inhaled corticosteroid (ICS) use, and bronchodilator reversibility (defined as an increase in FEV1 from baseline of >12% and 200 mL following 4 puffs of albuterol at screening), geographical region, and treatment naivety.
· Race (White vs. non-White) was included as a post-hoc analysis.
· Trough FEV1 at Day 169 (Week 24) was the primary efficacy endpoint in each study and was defined as the mean of FEV1 values obtained 23 h and 24 h after dosing on Day 168 (Week 24 visit).
Patients
· Males and females >40 years of age with with a diagnosis of COPD; current or former cigarette smokers with >10-pack-year smoking history; post-albuterol FEV1/forced vital capacity <0.7 and predicted FEV1 <70% of normal; and a modified Medical Research Council dyspnea scale score >2.
Treatments
· Eligible patients were randomized to the following once-daily treatments:
· In Study 1 (ClinicalTrials.gov identifier: NCT01313650), patients were randomized (3:3:3:2) to UMEC/VI 62.5/25 mcg (delivering 55/22 mcg), UMEC 62.5 mcg (delivering 55 mcg), VI 25 mcg (delivering 22 mcg), or placebo.(1)
· In Study 2 (ClinicalTrials.gov: NCT01313637) patients were randomized (3:3:3:2) to UMEC/VI 125/25 mcg (delivering
113/22 mcg), UMEC 125 mcg (delivering 113 mcg), VI 25 mcg or placebo.(2)
· In Studies 3 and 4 (ClinicalTrials.gov: NCT01316900 and ClinicalTrials.gov: NCT01316913) patients were randomized 1:1:1:1 to UMEC/VI 125/25 mcg, UMEC/VI 62.5/25 mcg, tiotropium bromide (TIO) 18 mcg, and either VI 25 (Study 3) or UMEC 125 mcg (Study 4).(4)
· All medications (except TIO) were administered using the ELLIPTA™ inhaler.
· TIO was administered via the Handihaler®.
RESULTS
· For all patients (intent-to-treat [ITT] analysis) UMEC/VI 125/25 mcg and UMEC/VI 62.5/25 mcg provided significantly greater improvements from baseline in trough FEV1 at Day 169 compared with placebo (0.216 and 0.199 L, respectively; both p<0.001).
· Results for the subgroups analysis were consistent with the ITT analysis: both UMEC/VI 125/25 mcg and UMEC/VI 62.5/25 mcg provided statistically significant improvements in trough FEV1 at Day 169 compared with placebo across subgroups (Table 1).
· Improvements compared with placebo for the White (84%) and non-White (16%) subgroups were 0.217 L and 0.208 L respectively with UMEC/VI 125/25 mcg and 0.190 L and 0.235 L respectively with UMEC/VI 62.5/25 mcg, reflecting the results from the overall analysis.
· The magnitude of improvement over placebo in trough FEV1 at Day 169 was similar for UMEC/VI 125/25 mcg and
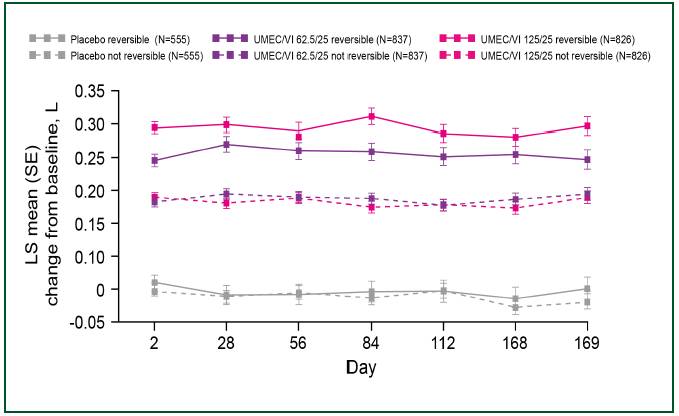
UMEC/VI 62.5/25 mcg across subgroups with the exception of a larger response with UMEC/VI 125/25 mcg compared with UMEC/VI 62.5/25 mcg in the subgroup of patients demonstrating bronchodilator reversibility at screening (Table 1 and Figure 1).
TABLE 1. LS MEAN TREATMENT DIFFERENCE FROM PLACEBO IN TROUGH FEV1 AT DAY 169 (L, [95% CI]; ITT POPULATION)
|
Subgroup (% patients) |
|
|
|
UMEC/VI 62.5/25 mcg |
|
UMEC/VI 125/25 mcg |
|
Gender |
|
Male (68) |
|
0.201* (0.167, 0.236) |
|
0.221* (0.186, 0.256) |
|
|
|
Female (32) |
|
0.193* (0.142, 0.243) |
|
0.204* (0.154, 0.254) |
|
|
|
|
|
|
|
|
|
Age, years(a) |
|
<64 (55) |
|
0.184* (0.143, 0.224) |
|
0.233* (0.192, 0.274) |
|
|
|
65-74 (35) |
|
0.224* (0.178, 0.270) |
|
0.204* (0.158, 0.250) |
|
|
|
75-84 (10) |
|
0.191* (0.107, 0.274) |
|
0.177* (0.091, 0.262) |
|
|
|
|
|
|
|
|
|
COPD severity(b) |
|
GOLD II (46) |
|
0.204* (0.163, 0.246) |
|
0.237* (0.195, 0.279) |
|
|
|
GOLD III/IV (54) |
|
0.193* (0.155, 0.232) |
|
0.199* (0.160, 0.238) |
|
|
|
|
|
|
|
|
|
Smoking status |
|
Current (49) |
|
0.209* (0.170, 0.249) |
|
0.237* (0.197, 0.277) |
|
|
|
Former (51) |
|
0.186* (0.145, 0.227) |
|
0.193* (0.151, 0.234) |
|
|
|
|
|
|
|
|
|
ICS user |
|
Yes (49) |
|
0.198* (0.157, 0.238) |
|
0.205* (0.164, 0.246) |
|
|
|
No (51) |
|
0.200* (0.161, 0.240) |
|
0.228* (0.188, 0.267) |
|
|
|
|
|
|
|
|
|
Bronchodilator reversibility |
|
Yes (69) |
|
0.225* (0.174, 0.276) |
|
0.282* (0.231, 0.333) |
|
|
|
No (31) |
|
0.188* (0.154, 0.221) |
|
0.181* (0.147, 0.216) |
|
|
|
|
|
|
|
|
|
Geographical region |
|
US (25) |
|
0.212* (0.155, 0.269) |
|
0.272* (0.213, 0.330) |
|
|
|
European Union (41) |
|
0.188* (0.142, 0.233) |
|
0.207* (0.167, 0.248) |
|
|
|
Other (34) |
|
0.181* (0.131, 0.231) |
|
0.179* (0.125, 0.234) |
|
|
|
|
|
|
|
|
|
Treatment naïvety |
|
Treatment naïve(c) (33) |
|
0.211* (0.163, 0.259) |
|
0.239* (0.189, 0.289) |
|
|
|
Not treatment naïve (67) |
|
0.193* (0.158, 0.228) |
|
0.205* (0.170, 0.240) |
CI, confidence interval; GOLD, Global initiative for chronic Obstructive Lung Disease classification; ICS, inhaled corticosteroid; LS, least squares *p<0.001 vs placebo (n=555); Analysis used a repeated measures model with terms for study, treatment, smoking status at screening, baseline FEV1 (mean of 30 min and 5 min pre-dose on Day 1), Day, geographical region, subgroup (if not already included), and Day by baseline, Day by treatment, subgroup by treatment and subgroup by Day by treatment interactions. (a) <1% of subjects were >85 years of age and were not included in the analysis; (b)Gold II = 50% < FEV1 <80% predicted’; GOLD III = 30% < FEV1 <50% predicted; GOLD IV = FEV1 <30% predicted; (c)no use of COPD medications apart from short-acting bronchodilators in the 30 days prior to screening.
FIGURE 1. TROUGH FEV1 (L) IN PATIENTS REVERSIBLE AND NOT REVERSIBLE TO ALBUTEROL
CONCLUSIONS
· UMEC/VI 125/25 mcg and 62.5/25 mcg once daily provide statistically significant improvements over placebo in lung function irrespective of gender, age, race, disease severity, smoking status, ICS use, bronchodilator reversibility, geographical region, and treatment naivety.
· In patients reversible to bronchodilator therapy at screening, improvements were greater with UMEC/VI 125/25 mcg than with UMEC/VI 62.5/25 mcg.
REFERENCES
(1) Donohue J, et al. Respir Med 2013;107:1538–46.
(2) Celli B, et al. Chest 2014;[Epub ahead of print].
(3) Anzueto AR, et al. Presented at Annual Congress of the ATS, May 17–22, 2013, Philadelphia, PA, USA.
(4) Decramer M, et al. Presented at Annual Congress of the ERS, September 7–11, 2013, Barcelona, Spain.
ACKNOWLEDGMENTS
· The presenting author, MKH has been a consultant for and received research grants from GlaxoSmithKline.
CK, JB and AC are employees of GlaxoSmithKline and hold stocks/shares in GlaxoSmithKline.
· This study was funded by GlaxoSmithKline (ClinicalTrials.gov: NCT01316900; protocol number: DB2113360; ClinicalTrials.gov: NCT01313637, protocol number: DB2113361; ClinicalTrials.gov: NCT01313650, protocol number: DB2113373; ClinicalTrials.gov: NCT01316913, protocol number: DB2113374).
· Editorial support (in the form of writing assistance, assembling tables and figures, collating author comments, grammatical editing and referencing) was provided by Joanne Parker and Afia Akram, at Fishawack Indicia Ltd, funded by GlaxoSmithKline.
|
|
ANORO™ and ELLIPTA™ are trademarks of GlaxoSmithKline |
|
Presented at the Annual Congress of the American Thoracic Society (ATS), San Diego, CA, USA, May 16–21, 2014


