Attached files
| file | filename |
|---|---|
| 8-K - 8-K - SYNERGY PHARMACEUTICALS, INC. | a14-12644_28k.htm |
Exhibit 99.1
|
|
UBS Global Healthcare Conference May 19, 2014 |
|
|
2 Safe Harbor Statement This presentation may contain forward-looking statements within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934. Such forward-looking statements are characterized by future or conditional verbs such as “may,” “will,” “expect,” “intend,” “anticipate,” believe,” “estimate” and “continue” or similar words. You should read statements that contain these words carefully because they discuss future expectations and plans, which contain projections of future results of operations or financial condition or state other forward-looking information. Such statements are only predictions and our actual results may differ materially from those anticipated in these forward-looking statements. We believe that it is important to communicate future expectations to investors. However, there may be events in the future that we are not able to accurately predict or control. Factors that may cause such differences include, but are not limited to, those discussed under Risk Factors and elsewhere in our Annual Report on Form 10-K for the year ended December 31, 2013, as filed with the Securities and Exchange Commission, including the uncertainties associated with product development, the risk that products that appeared promising in early clinical trials do not demonstrate safety and efficacy in larger-scale clinical trials, the risk that we will not obtain approval to market our products, the risks associated with dependence upon key personnel and the need for additional financing. We do not assume any obligation to update forward-looking statements as circumstances change. This presentation does not constitute an offer or invitation for the sale or purchase of securities or to engage in any other transaction with Synergy or its affiliates. The information in this presentation is not targeted at the residents of any particular country or jurisdiction and is not intended for distribution to, or use by, any person in any jurisdiction or country where such distribution or use would be contrary to local law or regulation. |
|
|
3 Our Focus: Pioneering Gastrointestinal (GI) Research & Development NATURAL PHYSIOLOGY MEETS BREAKTHROUGH SCIENCE Advanced clinical programs with multiple value drivers Unique mechanism-of-action based on the natural human GI hormone Exclusive worldwide rights Strong patent portfolio Experienced management team |
|
|
4 Our Approach: Based on the Natural GI Hormone UROGUANYLIN (Natural GI Hormone) PLECANATIDE CIC/IBS-C SP-333 OIC/UC |
|
|
5 Our Platform: Proprietary Uroguanylin Analogs COMPOUND INDICATION PHASE 1 PHASE 2 PHASE 3 PLECANATIDE Chronic Idiopathic Constipation (CIC) Irritable Bowel Syndrome with Constipation (IBS-C) SP-333 Opioid-Induced Constipation (OIC) Ulcerative Colitis (UC) |
|
|
6 Uroguanylin: Key Regulator of Normal Physiology Natural GI hormone produced by humans in the small intestine Natural agonist for the intestinal guanylate cyclase-C (GC-C) receptor Activates GC-C and regulates normal digestive functioning |
|
|
7 Uroguanylin: Physiological Mechanism of Action 1 2 3 plecanatide Uroguanylin (UroG) activates GC-C receptors in the gut GC-C receptor stimulates cyclic GMP synthesis C-GMP activates CFTR, moving fluid into the intestine for normal digestion Plecanatide activates GC-C, restoring normal GI function Cross Section of GI Tract 2 3 4 4 1 |
|
|
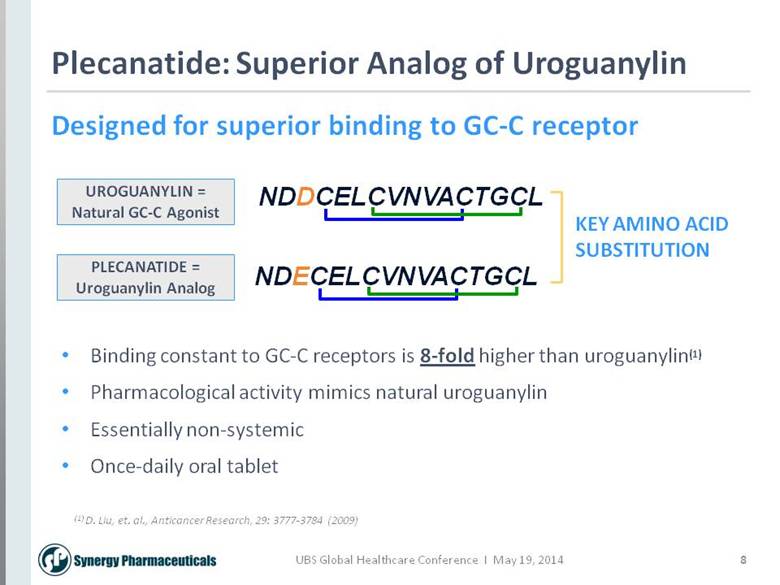
8 Plecanatide: Superior Analog of Uroguanylin NDDCELCVNVACTGCL NDECELCVNVACTGCL KEY AMINO ACID SUBSTITUTION Designed for superior binding to GC-C receptor (1) D. Liu, et. al., Anticancer Research, 29: 3777-3784 (2009) UROGUANYLIN = Natural GC-C Agonist PLECANATIDE = Uroguanylin Analog Binding constant to GC-C receptors is 8-fold higher than uroguanylin(1) Pharmacological activity mimics natural uroguanylin Essentially non-systemic Once-daily oral tablet |
|
|
9 Plecanatide: Analog of the Natural GC-C Agonist Uroguanylin (UroG) – Natural GC-C Agonist Plecanatide – Uroguanylin Analog Enterotoxin Produced by E. coli Bacteria Linaclotide: Enterotoxin Analog PLECANATIDE PROFILE 16-mer analog of natural uroguanylin Single key amino acid change Greater level of active bio-conformer Compact stable molecule Thermo and acid stable (100° C, pH 2) High resistance to proteases Small molecule characteristics NDDCELCVNVACTGCL NDECELCVNVACTGCL NSSNYCCELCCNPACTGCY CCEYCCNPACTGCY |
|
|
Plecanatide Program Chronic Idiopathic Constipation (CIC) & Irritable Bowel Syndrome with Constipation (IBS-C) |
|
|
Plecanatide Phase 2b/3 CIC Trial Study Design & Results Summary |
|
|
12 Phase 2b/3 CIC Trial: Study Design Study Design: Dose-ranging study to assess the safety and efficacy of plecanatide in CIC patients and determine adequate dose selection for phase 3 trials Doses: 0.3, 1.0 and 3.0 mg Patient Population: 951 patients with CIC Trial Duration: Screening up to 4 weeks Baseline 2 weeks Treatment 12 weeks Post-Tx 2 weeks |
|
|
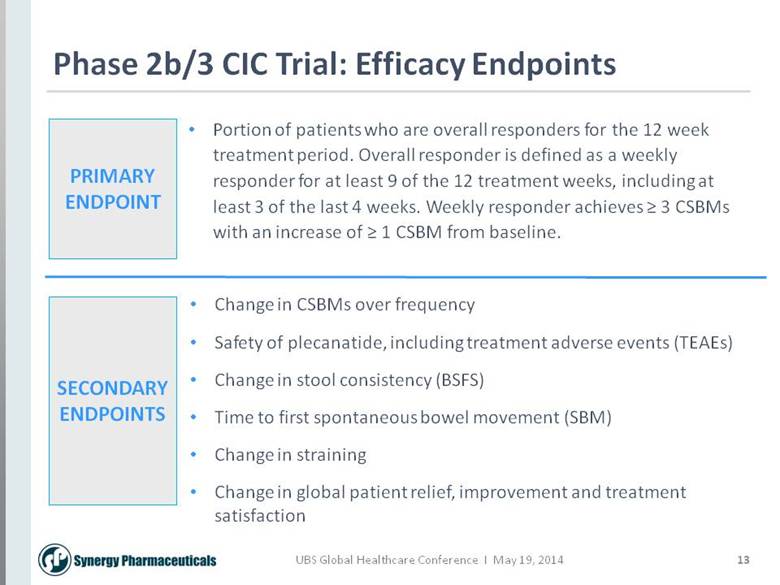
13 Phase 2b/3 CIC Trial: Efficacy Endpoints Portion of patients who are overall responders for the 12 week treatment period. Overall responder is defined as a weekly responder for at least 9 of the 12 treatment weeks, including at least 3 of the last 4 weeks. Weekly responder achieves > 3 CSBMs with an increase of > 1 CSBM from baseline. Change in CSBMs over frequency Safety of plecanatide, including treatment adverse events (TEAEs) Change in stool consistency (BSFS) Time to first spontaneous bowel movement (SBM) Change in straining Change in global patient relief, improvement and treatment satisfaction PRIMARY ENDPOINT SECONDARY ENDPOINTS |
|
|
14 DATA PLACEBO PLECANATIDE 3.0mg P VALUE Percent CSBM Responders 10.7 19 P= <0.01 CSBM Frequency 1.03 2.13 P= <0.001 SBM Frequency 1.30 2.88 P= <0.001 Stool Consistency 0.81 2.01 P= <0.001 Mean Straining Change -1.24 -2.07 P= <0.001 Phase 2b/3 CIC Trial: Results Summary Plecanatide 3.0 mg dose showed statistically significant improvement in all primary and key secondary endpoints |
|
|
15 Phase 2b/3 CIC Trial: Results Summary % Responders * = p<0.05; ** = p < 0.01; *** = p < 0.001 Treatment Week *** = p < 0.001 Treatment week Bristol Stool Form Scale Score Stool Consistency (BSFS) Weekly Responder Rate >3 CSBMs/wk with an increase of > 1 CSBM/wk Plecanatide 3.0 mg demonstrated immediate and sustained effect |
|
|
16 DATA PLACEBO PLECANATIDE 3.0 mg n (%) N=236 N=237 Serious Adverse Events (SAEs) 5 (2.1) 2 (0.8) Treatment-emergent adverse events (TEAEs) 96 (40.7) 106 (44.7) AEs leading to withdrawal 8 (3.4) 13 (5.5) All Diarrhea TEAEs 3 (1.3) 23 (9.7) Severe Diarrhea TEAEs 0 1 (0.4) Withdrawal due to Diarrhea 1 (0.4) 7 (3.0) Phase 2b/3 CIC Trial: Results Summary Plecanatide was safe and well tolerated with <10% diarrhea rate at 3.0 mg dose |
|
|
17 Phase 2b/3 CIC Data Validate Plecanatide’s Unique Profile Safe and well tolerated with a diarrhea rate <10% at highest dose (3.0 mg) Withdrawal due to diarrhea infrequent (3% for plecanatide 3.0 mg vs. 0.4% for placebo) Only one patient on plecanatide 3.0 mg (0.4%) had severe diarrhea Superior Tolerability Excellent Efficacy Plecanatide 3.0 mg dose showed statistically significant improvement in all primary and key secondary endpoints |
|
|
Plecanatide Phase 3 CIC Trials Study Design & Endpoints |
|
|
19 Phase 3 CIC Trials: Study Design Study Design: Two, Randomized, 12-Week, Double-Blind, Placebo-Controlled Studies to Confirm the Safety and Efficacy of Plecanatide in CIC Patients Doses: 3.0 and 6.0 mg Sites: 180 sites per trial Estimated Enrollment: 1350 CIC patients per trial (2700 patients total) Trial Duration: Screening up to 4 weeks Baseline 2 weeks Treatment 12 weeks Post-Tx 2 weeks |
|
|
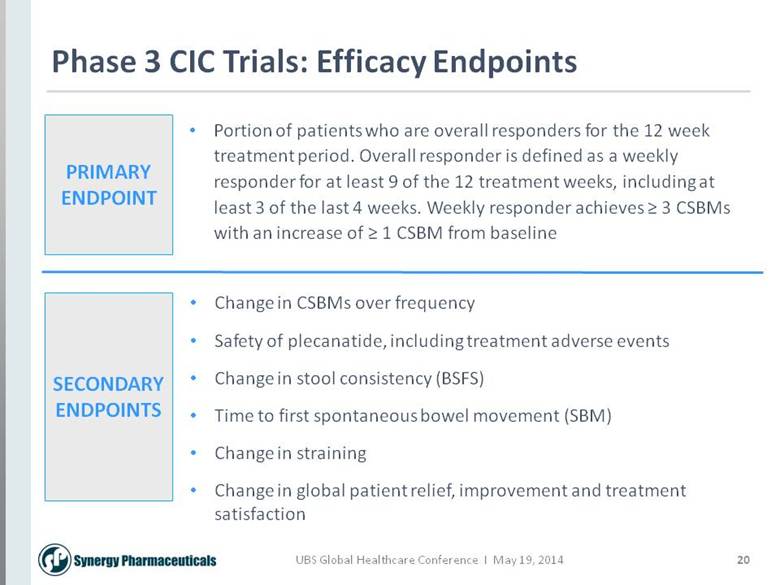
20 Phase 3 CIC Trials: Efficacy Endpoints PRIMARY ENDPOINT SECONDARY ENDPOINTS Portion of patients who are overall responders for the 12 week treatment period. Overall responder is defined as a weekly responder for at least 9 of the 12 treatment weeks, including at least 3 of the last 4 weeks. Weekly responder achieves > 3 CSBMs with an increase of > 1 CSBM from baseline Change in CSBMs over frequency Safety of plecanatide, including treatment adverse events Change in stool consistency (BSFS) Time to first spontaneous bowel movement (SBM) Change in straining Change in global patient relief, improvement and treatment satisfaction |
|
|
21 Phase 3 CIC Program: Key Events & Timelines Currently running two pivotal phase 3 CIC trials First phase 3 trial initiated in November 2013 Second phase 3 trial initiated in April 2014 Topline results from first phase 3 CIC trial expected in 2Q2015 Topline results from second phase 3 CIC trial expected in 3Q2015 CIC NDA filing expected by end of 2015 |
|
|
Plecanatide Phase 2b IBS-C Trial Study Design & Preliminary Top-line Data April 30, 2014: Announced preliminary top-line results |
|
|
23 Phase 2b IBS-C Trial: Study Design Study Design: Dose-ranging study to assess the safety and efficacy of plecanatide in IBS-C patients and determine adequate dose selection for phase 3 trials Doses: 0.3, 1.0, 3.0 and 9.0 mg Patient Population: 424 patients with IBS-C Trial Duration: Screening up to 6 weeks Baseline 2 weeks Treatment 12 weeks Post-Tx 2 weeks |
|
|
24 Phase 2b IBS-C Trial: Efficacy Endpoints PRIMARY ENDPOINT KEY SECONDARY ENDPOINTS Change from baseline in the mean number of complete spontaneous bowel movements (CSBMs) over the 12 week treatment period. Change from baseline in worst abdominal pain intensity (0-10 point severity scale) FDA overall responder endpoint (fulfills both > 30% reduction in worst abdominal pain intensity and an increase of > 1 CSBMs from baseline in the same week, in the same patient, for at least 50% of the weeks [i.e. 6/12 weeks]) Change in stool consistency (BSFS) |
|
|
25 Phase 2b IBS-C Trial: Preliminary Top-line Data Plecanatide 1.0, 3.0 and 9.0 mg dose groups demonstrated statistical significance for the study’s primary endpoint (CSBM frequency) Plecanatide was safe and well tolerated at all doses with no treatment-related serious adverse events Patients taking the plecanatide 3.0 mg dose consistently experienced statistically significant improvement in key secondary endpoints– change from baseline in worst abdominal pain intensity, FDA overall responder endpoint and stool consistency 3.0 mg dose group showed < 10% diarrhea rate |
|
|
26 DATA PLACEBO PLECANATIDE 3.0mg P VALUE CSBM frequency over 12 weeks 1.29 2.74 P= <0.001 Worst Abdominal Pain Intensity -1.4 (-24.5%) -2.0 (-33.9%) P= <0.05 FDA Overall Responder Endpoint 21% 41.9% P= <0.05 Stool Consistency 1.01 2.49 P= <0.001 Diarrhea Rate 0 9.3% Phase 2b IBS-C Trial: Preliminary Top-line Data Plecanatide 3.0 mg demonstrated ideal efficacy for treating IBS-C patients and excellent tolerability at <10% diarrhea |
|
|
27 Next Steps for Plecanatide IBS-C Program 3.0 mg dose selected for pivotal phase 3 IBS-C trials Full data results expected to be presented at a scientific meeting this fall (ACG or UEG) Scheduling end-of-phase 2 meeting with the FDA Phase 3 IBS-C programs expected to start in 2H2014 |
|
|
SP-333 Program Opioid-induced Constipation (OIC) & Ulcerative Colitis (UC) |
|
|
29 SP-333: Superior Stability Expands the Potential dNDECELCVNVACTGCdL NDDCELCVNVACTGCL Next-Generation Uroguanylin Analog Resistant to proteolysis in simulated intestinal fluid Highly potent and stable peptide Pharmacologic activity mimics natural physiology Essentially non-systemic Once-daily oral tablet Uroguanylin – Natural GI Hormone SP-333 – Uroguanylin Analog Single letters denote different amino acids. Colored lines denote disulfide bonds. |
|
|
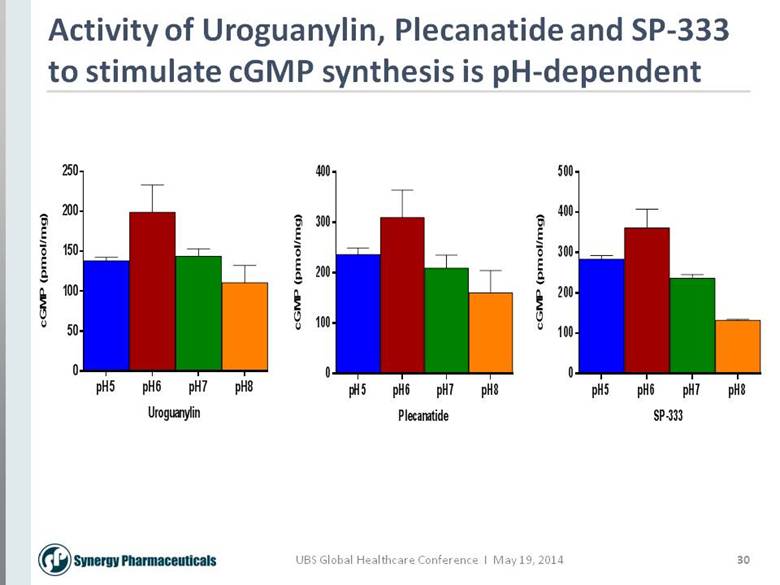
30 Activity of Uroguanylin, Plecanatide and SP-333 to stimulate cGMP synthesis is pH-dependent Plecanatide cGMP (pmol/mg) pH5 pH6 pH7 pH8 0 100 200 300 400 Uroguanylin cGMP (pmol/mg) pH5 pH6 pH7 pH8 0 50 100 150 200 250 SP-333 cGMP (pmol/mg) pH5 pH6 pH7 pH8 0 100 200 300 400 500 |
|
|
31 SP-333: Unique Potential for OIC Market (1) Only one FDA approved oral drug to treat opioid-induced constipation Twice-daily dosing Systemic exposure Not approved for use in methadone-induced constipation SP-333 potential advantages: Once-daily dosing Essentially non-systemic Demonstrated potential in preclinical studies to treat methadone-related constipation 1. Panchal SJ, Muller-Schwefe P, Wurzelmann JI. Opioid-induced bowel dysfunction: prevalence, pathophysiology and burden. Int J Clin Pract. 2007;61(7):1181-1187. Millions of US patients on chronic opioids - over 90% experience diminished bowel movement frequency |
|
|
32 SP-333 for OIC and UC: Development Overview KEY DISCOVERY HIGHLIGHTS STATUS OF CLINICAL PROGRAMS In preclinical models, orally administered SP-333 restored GI transit to normal levels and alleviated the onset of opioid-induced bowel dysfunction Exhibited potent anti-inflammatory activity in preclinical models of GI inflammation Phase 1 MAD/SAD studies completed in 2Q2013 SP-333 phase 2 OIC study underway Formulation development of SP-333 to treat mild-to-moderate UC underway |
|
|
33 SP-333 Phase 2 OIC Trial Study Aim: Assess the safety and efficacy of SP-333 in OIC patients receiving chronic opioid therapy for > 3 months Doses: 1.0, 3.0 and 6.0 mg Estimated Enrollment: 260 OIC patients Treatment Duration: 4 weeks Primary Endpoint: Mean change from baseline in the number of SBMs during week 4 of the Treatment Period |
|
|
Corporate Summary |
|
|
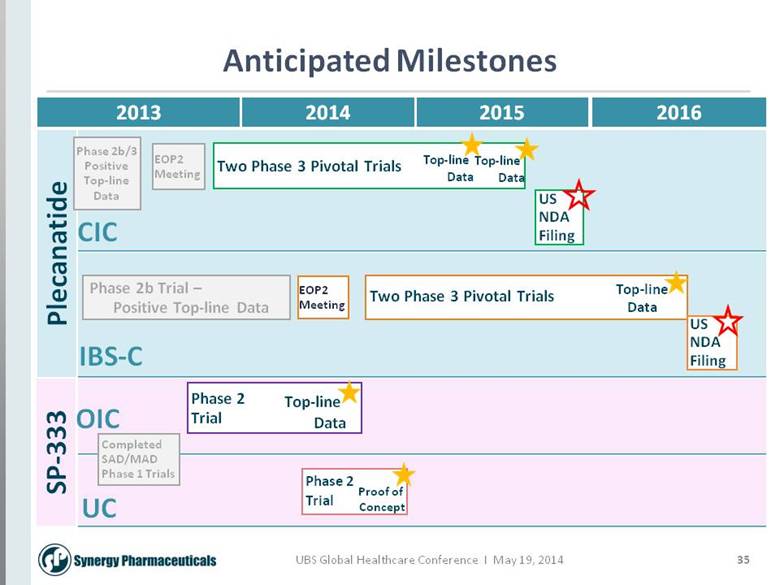
35 Anticipated Milestones 2013 Plecanatide SP-333 CIC IBS-C OIC UC EOP2 Meeting Phase 2b/3 Positive Top-line Data 2014 2015 2016 Two Phase 3 Pivotal Trials Top-line Data US NDA Filing Phase 2b Trial – Positive Top-line Data Top-line Data US NDA Filing Phase 2 Trial Top-line Data Phase 2 Trial Completed SAD/MAD Phase 1 Trials Proof of Concept EOP2 Meeting Two Phase 3 Pivotal Trials Top-line Data |
|
|
36 The Synergy Opportunity Significant unmet medical need for multi-billion dollar market Plecanatide CIC and IBS-C data suggest ideal combination of efficacy, superior tolerability and excellent safety profile Two pivotal phase 3 CIC trials now underway Topline data from first phase 3 trial expected in 2Q2015 Topline data from second phase 3 trial expected in 3Q2015 Pivotal phase 3 IBS-C programs expected to start in 2H2014 SP-333 phase 2 OIC trial currently underway Topline data expected 2H2014 Retain exclusive worldwide rights for both assets Focused, experienced and capable team |