Attached files
| file | filename |
|---|---|
| EX-31.1 - EX-31.1 - ARGOS THERAPEUTICS INC | d699848dex311.htm |
| EX-31.2 - EX-31.2 - ARGOS THERAPEUTICS INC | d699848dex312.htm |
| EX-32.1 - EX-32.1 - ARGOS THERAPEUTICS INC | d699848dex321.htm |
| EX-21.1 - EX-21.1 - ARGOS THERAPEUTICS INC | d699848dex211.htm |
| EX-10.23 - EX-10.23 - ARGOS THERAPEUTICS INC | d699848dex1023.htm |
Table of Contents
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
(Mark One)
| x | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2013
or
| ¨ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
Commission File Number 001-35443
ARGOS THERAPEUTICS, INC.
(Exact name of registrant as specified in its charter)
| Delaware | 56-2110007 | |
| (State or other jurisdiction of incorporation or organization) |
(I.R.S. Employer Identification No.) | |
| 4233 Technology Drive Durham, North Carolina |
27704 | |
| (Address of principal executive offices) | (Zip Code) | |
Registrant’s telephone number, including area code: (919) 287-6300
Securities registered pursuant to Section 12(b) of the Act:
| Title of Each Class |
Name of Each Exchange on Which Registered | |
| Common Stock, par value $0.001 per share | NASDAQ Global Market |
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ¨ No x
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ¨ No x
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ¨ No x
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes ¨ No ¨
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (§229.405 of this chapter) is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. x
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check one):
| Large accelerated filer | ¨ | Accelerated filer | ¨ | |||
| Non-accelerated filer | x (Do not check if a smaller reporting company) | Smaller reporting company | ¨ | |||
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ¨ No x
As of June 28, 2013, the last business day of the registrant’s most recently completed second quarter, there was no established public market for the registrant’s common stock. The registrant’s common stock began trading on the NASDAQ Global Market on February 7, 2014. As of March 21, 2014, the aggregate market value of the Common Stock held by non-affiliates of the registrant was approximately $80.4 million, based on the closing price of the registrant’s common stock on March 21, 2014. As of March 21, 2014, there were 19,654,362 shares outstanding of the registrant’s common stock, par value $0.001 per share.
Table of Contents
ARGOS THERAPEUTICS, INC.
ANNUAL REPORT ON FORM 10-K
For the Year Ended December 31, 2013
2
Table of Contents
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This Annual Report on Form 10-K, including “Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations,” contains forward-looking statements that involve substantial risks and uncertainties. All statements, other than statements of historical facts, contained in this Annual Report on Form 10-K, including statements regarding our strategy, future operations, future financial position, future revenues, projected costs, prospects, plans and objectives of management, are forward-looking statements. The words “anticipate,” “believe,” “estimate,” “expect,” “intend,” “may,” “might,” “plan,” “predict,” “project,” “target,” “potential,” “will,” “would,” “could,” “should,” “continue,” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words.
The forward-looking statements in this Annual Report on Form 10-K include, among other things, statements about:
| • | the progress and timing of our development and commercialization activities; |
| • | the timing and conduct of our phase 3 clinical trial of AGS-003 for the treatment of metastatic renal cell carcinoma, or mRCC, and planned phase 2 clinical trials of AGS-003, including statements regarding the timing of enrollment and completion of the trials and the period in which the results of the trials are anticipated to become available; |
| • | the timing and conduct of our phase 2b clinical trial of AGS-004 for the treatment of HIV and the two planned phase 2 clinical trials of AGS-004, one for HIV eradication and one for long-term viral control in pediatric patients, including statements regarding the timing of enrollment and the completion of the trials and the period in which results of the trials are anticipated to become available; |
| • | our ability to obtain U.S. and foreign marketing approval for AGS-003 for the treatment of mRCC and for AGS-004 for the treatment of HIV, and the ability of these product candidates to meet existing or future regulatory standards; |
| • | the potential benefits of our Arcelis platform and our Arcelis-based product candidates; |
| • | our ability to identify, lease, build out and equip a new North American commercial manufacturing facility and supply on a commercial scale our Arcelis-based products; |
| • | our intellectual property position and strategy; |
| • | our expectations related to the sufficiency of our cash, cash equivalents and short-term investments; |
| • | the accuracy of our estimates of the size and characteristics of the markets that may be addressed by our product candidates; |
| • | our ability to establish and maintain collaborations for the development and commercialization of our product candidates; |
| • | developments relating to our competitors and our industry; and |
| • | the impact of government laws and regulations. |
We have based these forward-looking statements largely on our current plans, intentions, expectations and projections about future events and financial trends that we believe may affect our business, financial condition and results of operations. We may not actually achieve the plans, intentions or expectations disclosed in our forward-looking statements, and you should not place undue reliance on our forward-looking statements. Actual results or events could differ materially from the plans, intentions and expectations disclosed in the forward-looking statements we make. We have included important factors in the cautionary statements included in this Annual Report on Form 10-K, particularly in “Item 1A. Risk Factors,” that could cause actual results or events to differ materially from the forward-looking statements that we make. Our forward-looking statements do not reflect the potential impact of any future acquisitions, mergers, dispositions, collaborations, joint ventures or investments that we may make or enter into.
You should read this Annual Report on Form 10-K with the understanding that our actual future results may be materially different from what we expect. The forward-looking statements contained in this Annual Report on Form 10-K are made as of the date of filing of this Annual Report on Form 10-K, and we do not assume any obligation to update any forward-looking statements, except as required by applicable law.
3
Table of Contents
This Annual Report on Form 10-K includes statistical and other industry and market data that we obtained from industry publications and research, surveys and studies conducted by third parties. Industry publications and third party research, surveys and studies generally indicate that their information has been obtained from sources believed to be reliable, although they do not guarantee the accuracy or completeness of such information. This Annual Report on Form 10-K also includes data based on our own internal estimates. While we believe these industry publications and third party research, surveys and studies are reliable, we have not independently verified such data.
Overview
We are a biopharmaceutical company focused on the development and commercialization of fully personalized immunotherapies for the treatment of cancer and infectious diseases based on our proprietary technology platform called Arcelis. Our most advanced product candidate is AGS-003, which we are developing for the treatment of metastatic renal cell carcinoma, or mRCC, and other cancers. We are currently enrolling patients in a pivotal phase 3 clinical trial of AGS-003 in combination with sunitinib (Sutent) for the treatment of mRCC under a special protocol assessment, or SPA, with the Food and Drug Administration, or FDA. The primary endpoint of the phase 3 clinical trial is overall survival. In our phase 2 clinical trial of AGS-003 in combination with sunitinib in mRCC patients, median overall survival was 30.2 months. This compares to median overall survival of 14.7 months in 1,189 mRCC patients with similar risk factors who were treated with sunitinib or other targeted therapies as shown in data collected by the International Metastatic Renal Cell Carcinoma Database Consortium, or the Consortium. We are developing our second Arcelis product candidate, AGS-004, for the treatment of HIV and are conducting a phase 2b clinical trial of AGS-004 that is being funded entirely by the National Institutes of Health, or NIH, under a $39.3 million contract.
Our Arcelis Platform
Our proprietary Arcelis technology platform utilizes biological components from a patient’s own cancer cells or virus to generate fully personalized immunotherapies. These immunotherapies employ specialized white blood cells called dendritic cells to activate an immune response specific to the patient’s own disease. Arcelis is based on the work of Dr. Ralph Steinman, winner of the 2011 Nobel Prize in medicine for the discovery of the role of dendritic cells in the immune system. We believe our Arcelis-based immunotherapies are applicable to a wide range of cancers and infectious diseases and have the following attributes that we consider critical to a successful immunotherapy:
| • | target a patient’s disease-specific antigens, including mutated antigens, to elicit a potent immune response that is specific to the patient’s own disease; |
| • | overcome the immune suppression that exists in cancer and infectious disease patients; |
| • | induce memory T-cells, a specialized type of immune cell that is known to correlate with improved clinical outcomes for cancer and HIV patients; |
| • | have minimal toxicity; and |
| • | can be produced using an automated centralized manufacturing process at a cost that will be comparable to biologics. |
We believe that our immunotherapies combine the advantages of other approaches to immunotherapy, including antigen-based approaches and pathway-based approaches such as checkpoint inhibition, while addressing the limitations they present.
Our Development Programs
The following table summarizes our development programs for AGS-003 and AGS-004.
| Product Candidate |
Primary Indication |
Status | ||||
| AGS-003 | mRCC (clear cell) | • | Ongoing pivotal phase 3 clinical trial; completion of enrollment expected by the end of 2014; overall survival data expected in first half of 2016 | |||
| mRCC (non-clear cell) | • | Phase 2 clinical trial expected to begin in the second half of 2014 | ||||
| Early stage RCC | • | Two investigator-initiated phase 2 clinical trials expected to begin in the second half of 2014 | ||||
| Other solid tumors | • | Two phase 2 clinical trials expected to be initiated in 2014 | ||||
| AGS-004 | HIV | • | Enrollment in phase 2b clinical trial complete; data expected in mid-2014 | |||
| • | Two phase 2 clinical trials (an investigator-initiated trial for HIV eradication and a clinical trial for long-term viral control in pediatric patients) expected to begin in 2014 | |||||
4
Table of Contents
We hold all commercial rights to AGS-003 and AGS-004 in all geographies other than rights to AGS-003 in Russia and the other states comprising the Commonwealth of Independent States, which we exclusively licensed to Pharmstandard International S.A., and rights to AGS-003 in South Korea, which we exclusively licensed to Green Cross Corp. We have granted to Medinet Co., Ltd., or Medinet, an exclusive license to manufacture in Japan AGS-003 for the treatment of mRCC and an option to acquire a non-exclusive license to sell in Japan AGS-003 for the treatment of mRCC.
AGS-003
We are initially developing AGS-003 to be used in combination with sunitinib and other targeted therapies for first-line treatment of mRCC. We are conducting a pivotal phase 3 clinical trial of AGS-003 in combination with sunitinib compared to sunitinib monotherapy for the treatment of newly diagnosed mRCC under an SPA with the FDA. We plan to enroll approximately 450 intermediate and poor risk patients with mRCC that pathologists have classified as clear cell. The primary endpoint of the trial is overall survival. As of February 28, 2014, we had enrolled approximately 120 patients in the trial. We expect to complete patient enrollment in this trial by the end of 2014 and to have overall survival data in the first half of 2016. We have established an independent data monitoring committee that will conduct interim analyses of the trial data for safety and futility at such times as 25%, 50% and 75% of the required events in the trial have occurred.
We conducted a 21 patient single arm phase 2 clinical trial of AGS-003 in combination with sunitinib. In this trial, we treated 11 intermediate risk and ten poor risk mRCC patients who had a time from diagnosis to initiation of systemic therapeutic treatment of less than one year, a negative prognostic indicator, with the combination of AGS-003 and sunitinib. Median overall survival for patients in the trial was 30.2 months. This compares to median overall survival of 14.7 months from initiation of treatment in 1,189 intermediate and poor risk patients who had a time from diagnosis to initiation of systemic therapeutic treatment of less than one year and were treated with sunitinib or other targeted therapies as shown in data collected by the Consortium. Dr. Daniel Heng from the University of Calgary’s Baker Cancer Center presented the Consortium data at the 2013 Annual Meeting of the American Society of Clinical Oncology, or ASCO.
In addition, 52% of the 21 patients in our phase 2 clinical trial survived for 30 or more months from enrollment in the trial, and 33% of the 21 patients survived for more than 4.5 years. This compares to data collected from six prior clinical trials of sunitinib and published in the British Journal of Cancer, in which 13% of the approximately 455 patients characterized as intermediate or poor risk patients in these trials survived for 30 or more months from initiation of sunitinib treatment. Furthermore, in our phase 2 clinical trial, we observed a statistically significant correlation between the increase in the number of CD8+ CD28+ memory T-cells and survival, progression free survival and reduced metastatic tumor burden. We believe that AGS-003 is the only cancer immunotherapy to show a statistically significant correlation between the magnitude of the target immune response and survival.
We also conducted a 22 patient single arm, phase 1/2 clinical trial of AGS-003 as monotherapy in nine intermediate risk and 13 poor risk mRCC patients. Median overall survival for patients in the trial was 15.6 months. In addition, 23% of the 22 patients in our phase 1/2 clinical trial survived for more than 30 months following enrollment.
Renal cell carcinoma, or RCC, is the most common type of kidney cancer. According to the American Cancer Society, or ACS, 2013 Cancer Facts and Figures Report, approximately 65,000 new cases of kidney cancer and nearly 14,000 deaths due to kidney cancer are expected in 2013 in the United States. The National Comprehensive Cancer Network, or NCCN, estimates that 90% of kidney cancer cases are RCC, and that 85% of these cases are classified as clear cell RCC. According to the ACS, approximately 25% of newly diagnosed RCC cases are mRCC. Additionally, patients initially diagnosed with early stage, or non-metastatic, RCC may later progress to mRCC. We estimate, based on publicly available information, that the current worldwide mRCC market for these targeted therapies is over $2 billion.
We are also exploring the use of AGS-003 in non-clear cell mRCC, early stage RCC prior to and following nephrectomy and other solid tumors. We plan to initiate clinical trials of AGS-003 for the treatment of these indications in 2014.
AGS-004
We are developing AGS-004 for the treatment of HIV and plan to focus this program on the use of AGS-004 in combination with other therapies for the eradication of HIV. The current standard of care, antiretroviral drug therapy, can reduce levels of HIV in a patient’s blood, increase the patient’s life expectancy and improve the patient’s quality of life. However, antiretroviral drug therapy cannot eliminate the virus, which persists in latently infected cells, remains undetectable by the immune system and can recur. In addition, antiretroviral therapy requires daily, life-long treatment and can have significant side effects.
5
Table of Contents
We believe that by combining AGS-004 with therapies that are being developed to expose the virus in latently infected cells to the immune system, we can potentially eradicate the virus. We are working with the University of North Carolina on the design of an investigator-initiated phase 2 clinical trial of AGS-004 in adult HIV patients to evaluate the use of AGS-004 in combination with one of these latency reversing therapies for this purpose. We expect this trial to be initiated in the second half of 2014. We also plan to explore the use of AGS-004 monotherapy to provide long-term control of HIV viral load in otherwise immunologically healthy patients and eliminate their need for antiretroviral drug therapy. Accordingly, we plan to initiate in the second half of 2014 a phase 2 clinical trial of AGS-004 monotherapy in pediatric patients infected with HIV who have otherwise healthy immune systems, have been treated with antiretroviral therapy since birth or shortly thereafter and, as a result, are lacking the antiviral memory T-cells to combat the virus.
We conducted a phase 2a clinical trial of AGS-004 in 29 HIV-infected patients in order to assess, after interruption of antiretroviral therapy, the ability of AGS-004 to control viral load and prevent viral load levels from returning to levels that were present in patients prior to treatment with antiretroviral drug therapy. In this single arm trial, patients received four monthly doses of AGS-004, while continuing to receive their existing antiretroviral therapy, before entering into a treatment interruption period during which the patients were to discontinue their antiretroviral therapy for 12 weeks and receive only AGS-004. In the trial, 24 patients entered the treatment interruption period in accordance with the trial protocol. At the end of their treatment interruption, these 24 patients had a mean reduction of 81% in their viral load as compared to their levels prior to beginning treatment with antiretroviral drug therapy. Without AGS-004, we would expect that a patient’s viral load would return to pre-treatment levels within 12 weeks following discontinuation of antiretroviral treatment. Accordingly, we believe that these results indicate that AGS-004 led to a reduction in virus replication and support the two planned phase 2 clinical trials of AGS-004 that we expect to initiate in 2014.
In September 2013, we completed patient enrollment in our ongoing NIH-funded phase 2b clinical trial of AGS-004 in 53 HIV-infected patients. The primary endpoint of this trial is a comparison of the median viral load in AGS-004-treated patients with the median viral load in patients receiving placebo after 12 weeks of antiretroviral treatment interruption. Secondary endpoints of this trial include comparisons between AGS-004-treated patients and patients receiving placebo with respect to change in viral load from immediately prior to the commencement of antiretroviral therapy to the end of the planned treatment interruption, duration of treatment interruption, changes in CD4+ T-cell counts, an indicator of the health of the immune system, and safety. We designed this trial to confirm the data obtained in our phase 2a clinical trial and provide proof of concept of the ability of AGS-004 to induce an immune response to eliminate the cells responsible for viral replication. We expect to have data from this trial in mid-2014.
According to the World Health Organization, the number of people living with HIV worldwide was approximately 34 million in 2011. The Centers for Disease Control and Prevention estimates that more than 1.1 million people are currently living with HIV in the United States and the number of new cases of HIV infection in the United States will remain constant at approximately 50,000 cases per year. According to Datamonitor, sales of antiretroviral therapies in the United States and the five biggest European markets reached $13.3 billion in 2011.
Strategy
Our goal is to become a leading biopharmaceutical company focused on discovering, developing and commercializing personalized immunotherapies for the treatment of a wide range of cancers and infectious diseases. Key elements of our strategy are:
| • | complete clinical development and seek marketing approvals of AGS-003 for the treatment of mRCC; |
| • | expand clinical development of AGS-003 in other cancers, including non-clear cell mRCC, early stage RCC and other solid tumors; |
| • | commercialize AGS-003 in North America independently and with third parties outside North America; |
| • | continue clinical development of AGS-004 for the treatment of HIV, potentially through government funding or other third party funding, and collaborate with third parties for commercialization on a worldwide basis; |
| • | establish automated manufacturing processes based on our existing functioning prototypes of automated devices and, prior to the filing of our BLA for AGS-003, identify, lease, build out and equip a new North American facility for the commercial manufacture of products based on our Arcelis platform; and |
| • | pursue expansion of our broad intellectual property protection for our Arcelis technology platform, product candidates and proprietary manufacturing processes through U.S. and international patent filings and maintenance of trade secret confidentiality. |
Immunotherapy to Treat Cancer and Infectious Diseases
Cancer cells occur frequently in the human body, yet are effectively controlled by T-cells in the immune system, which recognize proteins produced by the cancer cells, known as antigens, as abnormal and kill the associated cancer cells. Two specific types of T- cells are necessary for an effective anti-cancer immune response: CD8+ T-cells, which kill cancer cells, and CD4+ T-cells, which provide a “help” signal that activates and directs the CD8+ T-cell response.
6
Table of Contents
Cancer cells utilize several strategies to escape detection by the immune system and T-cells. For example, cancer cells secrete factors that act systemically to prevent T-cells from responding to activation signals, resulting in the inability of T-cells to carry out their role of killing cancer cells. Chronic viral infections such as HIV or hepatitis C present the same challenges to the immune system as cancer because the immune system must overcome this disease-induced immune suppression to recognize and respond to virus-infected cells.
Immunotherapy is intended to stimulate and enhance the body’s natural mechanism for recognizing and killing cancer cells and virus-infected cells. Current immunotherapeutic approaches to treat cancer can generally be separated into two different mechanisms of action: antigen-based approaches that target one or more specific antigens and pathway-based approaches that target specific immunologic pathways.
Antigen-Based Approaches
Cancer immunotherapies that use an antigen-based approach are designed to stimulate an immune response against one or more tumor-associated antigens. In most cases, the tumor-associated antigens that are being targeted are non-mutated, or normal, antigens, which are usually well tolerated by the immune system. In the context of cancer, these normal antigens are either produced at abnormally high levels or predominantly in tumor cells, or both. The goal of antigen-based immunotherapies is to activate the patient’s own immune system to seek out and kill the cancer cells that carry the targeted antigen. Dendreon Corporation’s Provenge (sipuleucel-T) for metastatic castrate-resistant prostate cancer is the only antigen-based immunotherapy that has been approved by the FDA. Because these immunotherapies are designed to target specific antigens, they are less likely to have toxicity. However, antigen-based immunotherapies may have limited efficacy because they are only able to capture one or a limited number of antigens, which may or may not be present in the patient’s cancer cells, and do not capture mutated antigens.
Pathway-Based Approaches
Immunotherapies that rely on the pathway approach are designed to overcome immunosuppression in patients by blocking signaling pathways that prevent T-cell activation and function. A new class of monoclonal antibody-based immunotherapies known as checkpoint inhibitors are being developed on the basis of this approach. For example, Bristol-Myers Squibb’s immunotherapy Yervoy (ipilimumab), an FDA-approved treatment for patients with unresectable or metastatic melanoma, is designed to act by blocking the function of a protein expressed in activated T-cells called CTLA4, which acts as a T-cell “off” switch. By blocking the function of CTLA4, the patient’s T-cells can become activated, resulting in an immune response against tumors. Another pathway that immunotherapies are being developed to address is the PD-1/PD-L1 pathway. In this pathway, activated T-cells expressing the protein PD-1 are disabled when binding occurs between PD-1 and its ligand, PD-L1, which is expressed on tumor cells. Immunotherapies are being developed to interrupt this pathway by binding to the PD-1 protein or the PD-L1 ligand to prevent them from binding with each other. Immunotherapies that use a pathway approach have demonstrated the ability to effectively overcome immunosuppression and enable T-cells to function against tumor cells. However, pathway-based immunotherapies are limited because they act systemically to enable T-cells to function and do not specifically target a patient’s tumor or the associated antigens. This lack of specificity can negatively impact healthy tissue and generate unwanted toxicity.
Designing Immunotherapies Using Our Arcelis Platform
We believe that our proprietary Arcelis platform enables us to produce fully personalized immunotherapies that combine the advantages of the antigen-based and pathway-based approaches to immunotherapy while addressing the limitations and disadvantages of these approaches. We have designed our Arcelis platform to create product candidates which have attributes that we believe are critical to a successful immunotherapy:
| • | Target disease-specific antigens, including mutated antigens. The immunotherapy should target antigens, including mutated antigens, associated with the patient’s disease. We believe that immunotherapies that target only non-mutated, or normal, tumor-associated antigens will be limited in terms of efficacy as non-mutated antigens are generally poor at stimulating immune responses. Our Arcelis platform uses messenger RNA, or mRNA, from the patient’s own cancer or virus to yield a fully personalized immunotherapy that contains the patient’s disease-specific antigens, including mutated antigens, and is designed to elicit a potent immune response specific to the patient’s own disease. |
| • | Overcome disease-induced immune suppression. The immunotherapy must be able to generate an effective immune response in patients whose immune systems are compromised by their disease. Both tumors and HIV are known to impair the functionality of CD4+ T helper cells, which aid their escape from CD8+ T-cell attack. Our Arcelis-based immunotherapies do not require CD4+ helper T-cells to mount an immune response with effective anti-tumor or anti- viral activity as we add the protein known as CD40 ligand, or CD40L, to provide the signaling that the CD4+ helper T-cells would otherwise provide. |
7
Table of Contents
| • | Induce memory T-cells. The immunotherapy should be able to induce specific T-cells, such as CD8+CD28+ memory T-cells, that are known to correlate with improved clinical outcomes for cancer and HIV patients. These memory T-cells are long lived and necessary for a durable immune response. Our Arcelis process produces dendritic cells that secrete IL-12, which is necessary to induce and expand patient-specific CD8+CD28+ memory T-cells. These memory T-cells are able to seek out and kill cancer or virus-infected cells that express the antigens identical to those displayed on the surface of the dendritic cells. In addition, because these newly generated memory T-cells do not express PD-1, they are not subject to inhibition by the PD-1/PD-L1 pathway. |
| • | Have minimal toxicity. The immunotherapy should have minimal toxicity, which would potentially enable it to be combined with other therapies for cancer and infectious diseases. The mechanism of action of Arcelis-based products induces patient- and disease-specific memory T-cells. This target customization and specificity is less likely to impact healthy tissue and cause toxicity. Our Arcelis-based product candidates have been well tolerated in clinical trials in more than 170 patients with no serious adverse events attributed to our immunotherapies. |
Our Arcelis platform is focused on dendritic cells which present antigens to the attention of the human immune system, including, in particular, T-cells, and are critical to the immune system’s recognition of proteins derived from cancer cells or virus-infected cells. Dendritic cells are capable of internalizing cancer or virus protein antigens and displaying fragments of these protein antigens on their surface as small peptides. The dendritic cells then present these peptide antigens to T-cells. This allows the T-cells to bind to these peptide antigens and, in the case of cancer, kill cancer cells and, in the case of infectious disease, kill virus-infected cells to control the spread of infectious pathogens.
The following graphic illustrates the processes comprising our Arcelis platform:
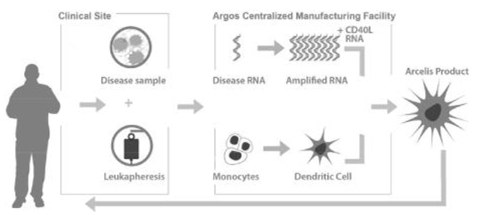
At the clinical site. As shown in the graphic above, the manufacture of our Arcelis-based immunotherapies requires two components derived from the patient:
| • | A disease sample: In the case of cancer, the sample consists of tumor cells, and in the case of infectious disease, the sample consists of blood containing the virus. The disease sample is generally collected at the time of diagnosis or initial treatment. |
| • | Monocytes: Monocytes are a type of white blood cell, which are obtained through a laboratory procedure called leukapheresis that occurs after diagnosis and at least three weeks prior to initiating treatment with our immunotherapy. |
At our centralized manufacturing facility. The tumor cells or the blood sample and the leukapheresis product are shipped to our centralized manufacturing facility following collection at the clinical site. After receipt of these components at our facility, we take the following steps:
| • | We isolate the patient’s disease mRNA, which carries the genetic information to recreate the patient’s disease antigens, from the disease sample and amplify the mRNA so that only a small disease sample is required to manufacture the immunotherapy. |
8
Table of Contents
| • | Separately, we extract the monocytes from the leukapheresis product and culture them using a proprietary process to produce matured dendritic cells. |
| • | We then culture the matured dendritic cells in a solution of the patient’s isolated mRNA and a proprietary synthetic CD40L RNA. We apply a brief electric pulse to the solution in a process referred to as electroporation, which enables the patient’s mRNA and the CD40L RNA to pass into, or load, the dendritic cells. The dendritic cells process the CD40L RNA into CD40L protein, enabling the dendritic cells to secrete IL-12, a cytokine required to induce and expand CD8+CD28+ memory T-cells. |
| • | We then further culture the mRNA-loaded dendritic cells so that these cells allow for antigen expression from the patient’s mRNA and presentation in the form of peptides on the surface of the dendritic cells. These mature, loaded dendritic cells are formulated into the patient’s plasma that was collected during the leukapheresis to become the Arcelis-based product. |
| • | After verifying the quality of the product, we then vial, freeze and ship the product to the clinic, which thaws the product and administers it to the patient by intradermal injection. |
Patient treatment. Upon injection into the skin of the patient, the mature, loaded dendritic cells migrate to the lymph nodes near the site of the injection. It is at these lymph nodes that the dendritic cells come into contact with T-cells. This interaction with the loaded dendritic cells is intended to cause a measurable increase in patient- and disease-specific memory T-cells.
We believe that our Arcelis platform allows us to create fully personalized immunotherapies capable of treating a wide range of cancers and infectious diseases using an automated manufacturing process at a cost that will be comparable to other biologics. Specifically, our Arcelis platform allows us to:
| • | produce several years of customized therapy for a patient from a small disease sample and a single leukapheresis from that patient; |
| • | produce additional years of therapy for a patient at a later date without requiring an additional disease sample from the patient; |
| • | use a centralized manufacturing facility for North America, which is possible because our Arcelis process can utilize monocytes obtained through leukapheresis within four days of the procedure, and doses of our immunotherapies can be shipped frozen in a cryoshipper that can maintain the target temperature for at least two weeks; |
| • | cryopreserve the doses generated from the single manufacturing process for each patient in a direct injectable formulation that allows the doses to remain stable and usable for up to five years; and |
| • | produce immunotherapies that can be administered by intradermal injection in an outpatient procedure. |
AGS-003 for the Treatment of Metastatic Renal Cell Carcinoma and Other Cancers
We are initially developing AGS-003 for use in combination with sunitinib and other targeted therapies for the treatment of mRCC. Sunitinib is an oral small molecule drug sold under the trade name Sutent and is the current standard of care for initial treatment, or first-line treatment, of mRCC following diagnosis.
We are currently enrolling mRCC patients in a pivotal phase 3 clinical trial of AGS-003 in combination with sunitinib compared to sunitinib monotherapy that we are conducting under an SPA with the FDA. We plan to enroll approximately 450 patients in the trial. As of February 28, 2014, we had enrolled approximately 120 patients in the trial. We expect to complete patient enrollment in this trial by the end of 2014 and to have overall survival data from this trial in the first half of 2016. In addition, we have established an independent data monitoring committee that will conduct interim analyses of the data from the trial for safety and futility at such times as 25%, 50% and 75% of the required events in the trial have occurred. In April 2012, the FDA notified us that we have obtained fast track designation for AGS-003 for the treatment of mRCC. In addition, we are exploring the use of AGS-003 in non-clear cell mRCC, early stage RCC prior to and following nephrectomy and other advanced solid tumors.
Renal Cell Carcinoma
RCC is the most common type of kidney cancer. The ACS estimates that there will be approximately 65,000 new cases of kidney cancer and nearly 14,000 deaths from this disease in the United States in 2013. The NCCN estimates that 90% of kidney cancer cases are RCC. For patients with RCC that had metastasized by the time RCC was first diagnosed, a condition referred to as newly diagnosed mRCC, the five-year survival rate has historically been approximately 12%.
9
Table of Contents
ACS statistics indicate that approximately 25% of newly diagnosed RCC patients present with mRCC annually in the United States. Additional patients who were initially diagnosed with earlier stage RCC may also progress to mRCC as these patients suffer relapses. The NCCN estimates between 20% to 30% of patients with early stage RCC will relapse within three years of surgical excision of the primary tumor. Although the NCI does not provide prevalence of RCC by stage, based on the NCCN’s three-year relapse rate, we estimate that there may be up to an additional 10,000 to 15,000 cases of mRCC identified annually in the United States. Combining newly diagnosed mRCC patients with patients who relapse, we estimate that there may be between 20,000 to 25,000 new cases of mRCC in the United States each year. We estimate, based on publicly available information, including 2012 quarterly and annual reports of companies that market targeted therapies approved for mRCC, that the current worldwide mRCC market for these targeted therapies is over $2 billion.
Physicians generally diagnose mRCC by examining a tumor biopsy under a microscope. Upon evaluation of the visual appearance of the tumor cells, a pathologist will classify the mRCC into clear cell or non-clear cell types. According to the NCCN, approximately 85% of all RCC diagnoses are clear cell RCC. Because clear cell types are the most common type of tumor cell, most of the more recently approved therapies for mRCC have limited their clinical trials to patients with the clear cell type of tumor cell. However, the FDA has not limited the approval of these therapies to clear cell types of mRCC, so they may be used for both clear cell and non-clear cell types.
mRCC Patient Classification
Upon diagnosis, the prognosis for patients with mRCC is classified into three overall disease risk profiles — favorable, intermediate and poor — using objective prognostic risk factors. These risk factors were originally developed by researchers at Memorial Sloane Kettering Cancer Center and subsequently revised by Dr. Heng and contributors from the Consortium based on clinical data from patients treated with sunitinib and other targeted therapies. These risk factors, which we refer to as the Heng risk factors, have been correlated to adverse overall survival in mRCC and include:
| • | time from diagnosis to the initiation of systemic therapeutic treatment of less than one year, which is indicative of more aggressive disease. We refer to this risk factor as the less than one year to treatment risk factor; |
| • | low levels of hemoglobin, a protein in the blood that carries oxygen; |
| • | elevated corrected calcium levels; |
| • | diminished overall patient performance status or physical functioning; |
| • | elevated levels of neutrophils, a type of white blood cell; and |
| • | elevated platelet count. |
Patients exhibiting zero risk factors at the time of treatment are included in the favorable risk group; patients exhibiting one or two risk factors are included in the intermediate risk group; and patients exhibiting three or more risk factors are included in the poor risk group. Even when treated with standard of care therapies such as sunitinib, patients in the intermediate risk group have an expected survival of less than two years, and patients in the poor risk group have an expected survival of less than one year. In January 2013, Dr. Heng published in Lancet Oncology the following data from the Consortium database regarding overall survival of mRCC patients in these three risk groups treated with sunitinib and other targeted therapies:
| • | in 157 favorable risk patients, the median overall survival was 43 months; |
| • | in 440 intermediate risk patients, the median overall survival was 22.5 months; and |
| • | in 252 poor risk patients, the median overall survival was 7.8 months. |
Current Treatment
The initial treatment for most mRCC patients when the primary tumor is intact is surgical removal of the tumor, usually requiring partial or complete removal of the affected kidney, referred to as nephrectomy. The NCCN generally recommends systemic treatment with approved therapies for mRCC patients following nephrectomy for patients whose tumors have metastasized or for patients who present with mRCC upon diagnosis or as a result of a relapse from an earlier stage of RCC.
10
Table of Contents
Historically, mRCC has been treated with non-specific, cytokine-based immunotherapies such as interferon-µ and IL-2, which have demonstrated a clinical benefit in a small number of mRCC patients. However, due to their lack of specificity, these therapies have been demonstrated to have severe toxicities, which can lead to cardiopulmonary, neuropsychiatric, dermatologic, renal, hepatic and hematologic side effects and limits their use. For example, although high-dose IL-2 is the only therapy to have demonstrated durable complete mRCC remissions, its toxicity restricts its use to a small minority of patients and for a short duration.
In the past few years, several targeted therapies, such as Sutent (sunitinib), Votrient (pazopanib), Torisel (temsirolimus), Nexavar (sorafenib), Avastin (bevacizumab) plus interferon-µ, Afinitor (everolimus) and Inlyta (axitinib), have been approved for the treatment of mRCC. While most of these targeted therapies have been evaluated in first-line treatment of mRCC, Sutent demonstrated a higher rate of progression free survival and overall survival in its pivotal phase 3 clinical trial than that shown by the other targeted therapies in their pivotal phase 3 clinical trials. According to Decision Resources, in 2011, Sutent was the drug of choice for more than three-quarters of hospital-based oncologists in the United States for intermediate risk mRCC patients.
Although most of these targeted therapies have demonstrated prolonged progression free survival as compared to interferon-µ, they are rarely associated with durable remissions or enhanced long-term survival, particularly in patients who are classified as intermediate or poor risk at the time of treatment. In addition, each of these targeted therapies has shortcomings that limit their use in the treatment of mRCC, including significant toxicities, such as neutropenia and other hemotologic toxicities, fatigue, diarrhea, hand-foot syndrome, hypertension and other cardiovascular effects. The overlapping and combined toxicities of the targeted therapies have prevented their use in combination therapies. For instance, researchers conducting a phase 1 clinical trial of the combination of sunitinib and temsirolimus discontinued the trial due to toxicities. We believe that the inability to date to combine these therapies without additive toxicity and the absence of durable remissions and prolonged survival in patients with intermediate and poor risk disease indicates there is an unmet need for novel therapeutic approaches for mRCC that can improve efficacy without adding any appreciable toxicity.
AGS-003 Opportunity
We believe, based on the clinical results of AGS-003, that the combination of AGS-003 with sunitinib or other targeted therapies has the potential to address this unmet need for the following reasons:
| • | We believe that because the mechanism of action of AGS-003 is unrelated to the mechanism of action of sunitinib or the other targeted therapies, combining AGS-003 with these therapies has the potential to have an additive efficacy benefit. |
| • | We believe that if a combination therapy with AGS-003 shows improved efficacy, the combination could be used as the standard of care for first-line treatment of mRCC in our targeted patient population. |
| • | We believe that the lack of significant toxicity of AGS-003 will enable it to be combined with sunitinib and the other targeted therapies at a full dose for both therapies without added toxicity. |
| • | We believe that, by following an initial cycle of sunitinib with AGS-003 in combination therapy, patients are likely to have a lower metastatic tumor burden, or at least a slowing of tumor progression, at the time of initiation of AGS-003 therapy, making it more likely that AGS-003 would have the opportunity to elicit immune responses and demonstrate an effect on the tumor. |
| • | We believe that continued dosing of sunitinib, as well as certain of the other targeted therapies, decreases regulatory T-cells and myeloid-derived suppressor cells, both of which are immunosuppressive cells known to expand during cancer and suppress T-cell responses. As a result, by combining with these therapies, we believe that AGS-003 may be able to generate more potent T-cell responses. |
Development Status
We are conducting an ongoing pivotal phase 3 clinical trial of AGS-003. We have conducted three clinical trials of AGS-003 and its predecessor product, MB-002, which include:
| • | a phase 2 combination therapy clinical trial of AGS-003 in combination with sunitinib; |
| • | a phase 1/2 monotherapy clinical trial of AGS-003; and |
| • | a phase 1/2 monotherapy clinical trial of MB-002. |
11
Table of Contents
We submitted to the FDA an investigational new drug application, or IND, for AGS-003 in March 2003.
Phase 2 Combination Therapy Clinical Trial. From July 2008 to October 2009, we enrolled 21 newly diagnosed mRCC patients in a single arm, multicenter, open label phase 2 clinical trial of AGS-003 in combination with sunitinib. We conducted this clinical trial at nine clinical sites in the United States and Canada. Our design for the trial required adult patients with previously untreated mRCC, no prior nephrectomy or at least one accessible lesion for biopsy, a histologically confirmed predominantly clear cell tumor, and suitability for sunitinib therapy. The primary endpoint of the trial was complete response rate. Secondary endpoints included progression free survival, overall survival, safety, clinical benefit rate and immune response.
Patients in the trial generally received one initial six-week cycle of sunitinib, consisting of four weeks on drug and two weeks on drug holiday, prior to initiating the combined treatment with AGS-003. Patients then received a dose of AGS-003 every three weeks for a total of five doses, while also continuing three additional six-week cycles of sunitinib. This 24-week induction phase was followed by a booster phase during which patients received a dose of AGS-003 once every three months and continued to receive sunitinib in six-week cycles until disease progression.
The following table summarizes certain key data from the 11 intermediate risk and 10 poor risk patients enrolled in the phase 2 combination therapy clinical trial.
| Outcome |
(N=21) | |
| Median OS(1) | 30.2 months | |
| Median PFS(2) | 11.2 months | |
| Complete response(3) | 0 patients | |
| Partial response(4) | 8 patients | |
| Stable disease(5) | 5 patients | |
| Immune response | CD8+ CD28+ memory T-cells correlated with OS, PFS and reduced metastatic tumor burden; IL-2 and interferon-g (IFN-g) recovery |
| (1) | Overall survival, or OS, is the length of time from the initiation of treatment to the patient’s death. |
| (2) | Progression free survival, or PFS, is the length of time from treatment initiation to the worsening of the patient’s disease or the patient’s death. |
| (3) | Complete response is the disappearance of all measurable target lesions and non-target lesions. |
| (4) | Partial response is the overall tumor regression based on a decrease of at least 30% in the overall amount of measurable tumor mass in the body and improvement or no change in non-target lesions. |
| (5) | Stable disease is neither sufficient decrease in tumor size to qualify as a partial response nor sufficient increase in tumor size to qualify as disease progression. |
Particular observations from these data and the trial, which have informed our further clinical development of AGS-003, include:
Efficacy Analysis
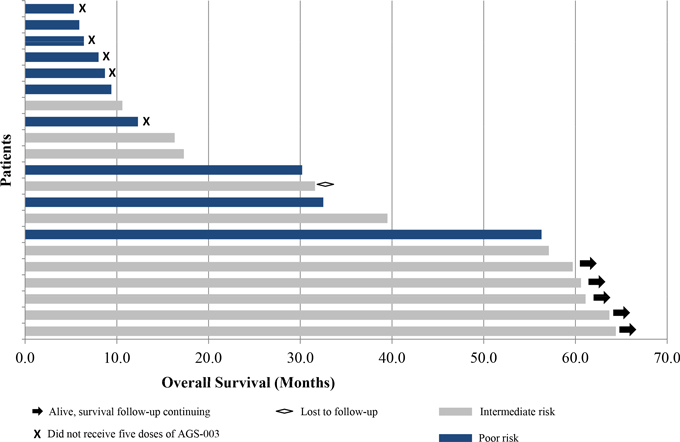
| • | Seven patients survived for more than 4.5 years following enrollment in this trial. As of February 28, 2014, five of these seven patients remained alive and continued to be monitored for overall survival. Two of these patients have not progressed and continue to be dosed. |
| • | Five poor risk patients did not receive five doses of AGS-003 due to early disease progression. Median overall survival in the 16 patients who received at least five doses of AGS-003 was 36.0 months. |
| • | Median overall survival in the 11 intermediate risk patients was 57.1 months. Median overall survival in the 10 poor risk patients was 9.1 months. |
12
Table of Contents
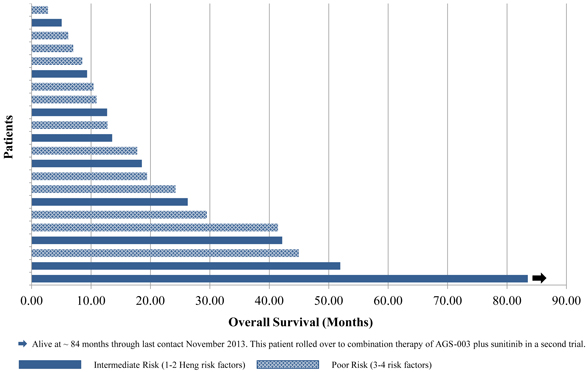
| • | The following graphic shows, as of February 28, 2014, the number of months that each patient in the phase 2 clinical trial survived from the time of enrollment in the trial. The five patients who remained alive as of February 28, 2014 are indicated by the arrows at the end of the bar. The five poor risk patients who did not receive five doses of AGS-003 are indicated with an “x” at the end of the bar. |
Phase 2 Combination Therapy Clinical Trial of AGS-003:
Overall Survival
| • | Of the eight patients who exhibited a partial response, five patients exhibited partial responses during the 24-week induction phase, including two patients who exhibited partial responses prior to initiation of treatment with AGS-003. The other three patients exhibited partial responses after prolonged dosing with AGS-003 during the booster phase. We do not believe that these late occurring partial responses have been observed in clinical trials of sunitinib alone. As a result, we believe that these late responses may relate to the immunologic effects of prolonged AGS-003 dosing and AGS-003’s effect on CD8+ CD28+ memory T-cells. |
| • | We observed a statistically significant correlation between increased progression free survival and prolonged survival (p<0.001). Statistical significance is determined by methods that establish the p-value of the results. Typically, results are considered statistically significant if they have a p-value of 0.05 or less, meaning that there is less than a one-in-20 likelihood that the observed results occurred by chance. |
Immune Response Analysis
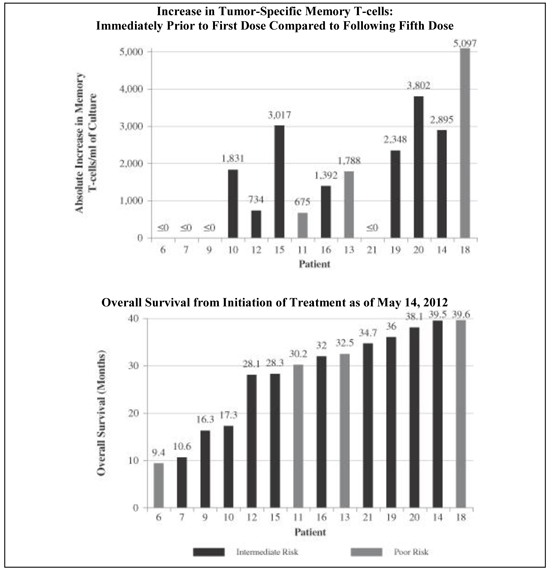
| • | In the 14 patients in the trial who received at least five doses of AGS-003 and could be evaluated for memory T-cell response, we observed a statistically significant correlation between the increase in the number of CD8+ CD28+ memory T-cells over the initial five doses of AGS-003 and survival (p<0.002), progression free survival (p<0.031) and reduced metastatic tumor burden (p<0.045). We presented at the 2013 Annual Meeting of ASCO, Genitourinary Cancers Symposium results, as of May 14, 2012, showing the correlation with survival. The following graphics show, for each of these 14 patients, the increase in their tumor-specific memory T-cells that they exhibited as measured immediately prior to their first dose of AGS-003 and immediately following the patient’s fifth dose of AGS-003, or the absence of such increase, as compared to such patient’s survival. The patient identification numbers in the graphics below correspond to the patient identification numbers in the graphic set forth above under the heading “Phase 2 Combination Therapy Clinical Trial of AGS-003: Overall Survival.” |
13
Table of Contents
Phase 2 Combination Therapy Clinical Trial of AGS-003:
Correlation of Immune Response and Overall Survival
| • | AGS-003 was found to have positive impact on immune cell function and restoration of cellular immunity in a majority of patients, including an increase in levels of IL-2 and IFN-g. |
Safety
| • | The adverse events in this trial associated with AGS-003 were generally only mild injection site reactions, while the toxicities associated with sunitinib were consistent with those expected from treatment with sunitinib alone. |
The original design for the phase 2 clinical trial called for the recruitment of 50 patients to generate 38 fully evaluable patients. However, in October 2009, we terminated enrollment in this trial early due to a lack of funding. As a result, only 21 patients were enrolled and received at least one dose of AGS-003. In addition, the trial was originally designed to enroll patients with favorable and intermediate risk disease profiles. Instead, the actual population enrolled consisted entirely of patients with intermediate or poor risk disease profiles who had the less than one year to treatment risk factor. Because the patient population had poorer prognoses when they entered the trial than we expected and we did not have a sufficient number of evaluable patients, we did not perform the statistical analysis to determine whether the primary endpoint of complete response rate was achieved. As a result, we expect the data from this trial to be considered by the FDA for the purpose of evaluating the safety and feasibility of AGS-003, but that it will only have a limited impact on the FDA’s ultimate assessment of the efficacy of AGS-003.
14
Table of Contents
Based on our experience with the phase 2 clinical trial, we concluded that the secondary endpoints in the trial, progression free survival and overall survival, along with immune response, were the appropriate endpoints to consider for measuring the efficacy of AGS-003 in combination with sunitinib in patients with mRCC in our pivotal phase 3 clinical trial.
AGS-003 Phase 2 Combination Therapy Clinical Trial, as Compared to Independent Third Party mRCC Data. At ASCO in June 2013, Dr. Heng presented data from the Consortium database regarding overall survival and progression free survival for intermediate and poor risk patients treated with sunitinib and other targeted therapies, including data with respect to 1,189 intermediate and poor risk patients with the less than one year to treatment risk factor.
A summary comparison of the overall survival data from the Consortium database presented in June 2013 and our phase 2 clinical trial of AGS-003 in combination with sunitinib is set forth in the graphic below. This graphic compares the median overall survival data from the Consortium intermediate and poor risk patients with the less than one year to treatment risk factor with the median overall survival data from the 21 patients in our phase 2 clinical trial of AGS-003 in combination with sunitinib, all of whom had the less than one year to treatment risk factor. A majority of the Consortium patients and the patients in our phase 2 clinical trial had one or more additional risk factors.
Phase 2 Combination Therapy Clinical Trial of AGS-003:
Comparison of Median Overall Survival Data
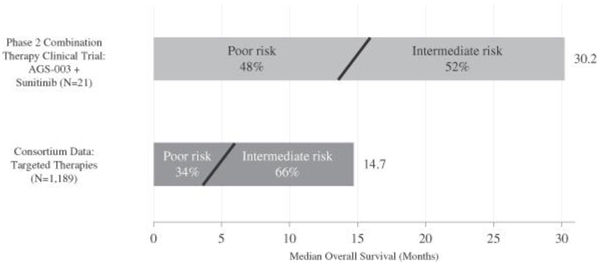
Progression free survival for intermediate and poor risk patients in the Consortium database with the less than one year to treatment risk factor was 5.6 months, as compared to the 11.2 months of median progression free survival that we observed in the 21 patients in our phase 2 clinical trial of AGS-003 in combination with sunitinib.
15
Table of Contents
In addition, data published in the British Journal of Cancer in 2013 reported on the long-term survival of 1,059 mRCC patients treated with sunitinib as first-line or second-line therapy in six prior clinical trials of sunitinib, including the pivotal phase 3 clinical trial of sunitinib. The graphic below sets forth, with respect to the approximately 455 patients characterized as intermediate or poor risk patients in the sunitinib trial data published by the British Journal of Cancer, the percentage of patients who survived for more than 30 months from initiation of treatment and, with respect to patients in our phase 2 clinical trial of AGS-003 in combination with sunitinib, the percentage of patients who survived for more than 30 months from enrollment in the trial:
Phase 2 Combination Therapy Clinical Trial of AGS-003:
Comparison of Survival > 30 Months
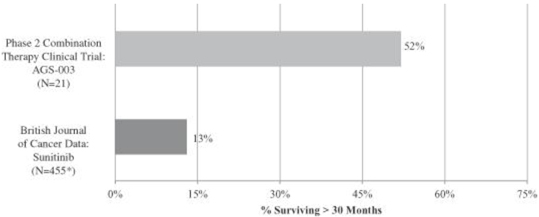
| * | The data that was presented in the British Journal of Cancer included data categorized by risk profile group. The number of patients in each risk profile group was presented as a percentage of a total population of 1,059 mRCC patients. Accordingly, the 455 intermediate risk and poor risk patients referenced in this table and elsewhere in this Annual Report on Form 10-K represent an approximation based on those percentages. |
Although we believe comparisons between our data and these collections of data are useful in evaluating the overall results of our phase 2 clinical trial, the treatment of the Consortium patients and the sunitinib patients was conducted at different sites, at different times and in different patient populations than the treatment in our phase 2 combination therapy trial. The treatment also differed because certain of the Consortium patients received therapies other than sunitinib and certain of the patients in the sunitinib trials received sunitinib as a second-line treatment. All of the patients in our phase 2 clinical trial received sunitinib as first-line treatment. Our ongoing pivotal phase 3 combination therapy clinical trial of AGS-003 is the first trial that we have conducted that directly compares sunitinib and AGS-003 as a combination therapy against sunitinib as monotherapy. Results of this head-to-head comparison may differ significantly from the comparisons presented above and elsewhere in this Annual Report on Form 10-K.
Phase 1/2 Monotherapy Clinical Trial. From April 2006 through October 2008, we enrolled 22 newly diagnosed mRCC patients in a single arm, multicenter, open label phase 1/2 clinical trial of AGS-003 as monotherapy. These patients were enrolled at six sites in the United States and Canada. The trial was designed as a two-stage trial. In stage 1, we would recruit 24 patients to generate at least 18 evaluable patients, and in stage 2, we would recruit an additional 22 patients to generate at least a total of 35 evaluable patients. In order to advance to stage 2 of the trial, we were required to achieve the primary endpoint for stage 1, which was three patients with a complete or partial response. Secondary endpoints included progression free survival, overall survival, safety and immunogenicity.
Our design for the trial required patients to be adults with mRCC with no prior nephrectomy, no history of prior RCC therapy and sufficient renal function in the remaining kidney. The trial was designed to enroll patients with favorable and intermediate risk disease profiles.
In the trial, patients were to be administered a dose of AGS-003 every two weeks for a total of five doses, followed by a dose of AGS-003 every month for an additional four doses during the 24-week induction phase of the trial. These doses were to be followed by booster doses every three months until disease progression. However, due to the approval of sunitinib and sorafenib at the time we were beginning to enroll patients in the trial and the resulting change in the standard of care for mRCC, we experienced delays in enrolling patients in the trial. As a result, we discontinued the trial after enrolling 22 patients and shifted our development focus for subsequent trials to AGS-003 as a combination therapy.
The following table summarizes certain key data from the nine intermediate risk and 13 poor risk patients enrolled in the phase 1/2 monotherapy clinical trial:
| Outcome |
(N=22) | |
| Median OS | 15.6 months | |
| Median PFS | 5.6 months | |
| Complete response | 0 patients | |
| Partial response | 1 patient | |
| Stable disease | 7 patients | |
| Immune response | IL-2 and IFN-g recovery |
16
Table of Contents
Particular observations from these data and the trial, which have informed our further clinical development of AGS-003, include:
| • | As of November 30, 2013, one patient, who subsequently was treated with AGS-003 in combination with sunitinib, was still alive nearly seven years after initiation of AGS-003. Four other patients survived for more than 40 months following enrollment in the trial. |
| • | The following graphic shows, as of December 31, 2013, the number of months that each patient in the phase 1/2 monotherapy clinical trial survived following enrollment in the trial. All patients were deceased as of December 31, 2013, except for one patient who was subsequently treated with AGS-003 in combination with sunitinib. This subject was still alive for approximately seven years after initiation of AGS-003, as of follow-up for this subject through late 2013. |
Phase 1/2 Monotherapy Clinical Trial of AGS-003:
Overall Survival
| • | Only three patients received a targeted therapy following treatment with AGS-003, which we believe indicates that the clinical benefit demonstrated in this trial was a result of AGS-003 treatment. |
| • | Despite the intermediate and poor risk population, eight of the evaluable patients experienced some degree of tumor regression at one point during the induction phase of treatment with AGS-003. |
| • | AGS-003 was found to have a positive impact on immune cell function and restoration of cellular immunity in a majority of patients, including an increase in levels of IL-2 and IFN-g. We did not measure CD8+CD28+ memory T-cells in this trial as, at the time this trial was conducted, the technology for monitoring CD8+CD28+ memory T-cells had not yet been developed. |
| • | AGS-003 was well-tolerated, with adverse events limited to mild injection site reactions, transient flu-like symptoms and tenderness in the lymph nodes. |
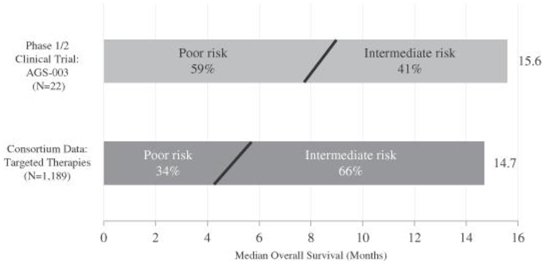
The graphic below compares the median overall survival data for the Consortium intermediate and poor risk patients with the less than one year to treatment risk factor with the median overall survival data from the 22 patients in our phase 1/2 clinical trial of AGS-003, all of whom had the less than one year to treatment risk factor and a majority of whom had one or more additional risk factors. A majority of the intermediate and poor risk Consortium patients also had one or more additional risk factors.
17
Table of Contents
Phase 1/2 Monotherapy Clinical Trial of AGS-003:
Comparison of Median Overall Survival Data
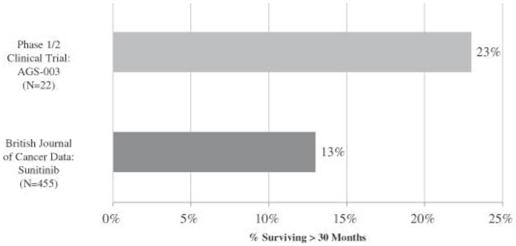
The graphic below shows the percentage of intermediate and poor risk patients in our phase 1/2 clinical trial who survived for more than 30 months from enrollment in the trial as compared to the percentage of sunitinib-treated intermediate and poor risk mRCC patients who survived for more than 30 months from initiation of treatment as set forth in the British Journal of Cancer report.
Phase 1/2 Monotherapy Clinical Trial of AGS-003:
Comparison of Survival > 30 Months
Phase 1/2 Monotherapy Clinical Trial of MB-002. Prior to developing AGS-003, we developed a predecessor mRNA-loaded dendritic cell drug product candidate, MB-002, for the treatment of mRCC. From April 2004 through March 2005, we enrolled 20 newly diagnosed mRCC patients in a single arm, multicenter, open label phase 1/2 clinical trial of MB-002 as monotherapy. The overall endpoints for this trial included safety, feasibility of developing and supplying patient-specific product to clinical sites from a central facility, clinical benefit, progression free survival and immunologic response. In order to achieve success for this trial, we had to demonstrate the product was safe, could be readily manufactured and supplied from a central facility, generated anti-tumor activity and had the ability to induce an immunologic response capable of restoring IL-2 and IFN-g based T-cell responses in treated patients.
This trial was designed to enroll patients with newly diagnosed mRCC, whether or not displaying symptoms, with no prior nephrectomy and with sufficient renal function in the remaining kidney. Patients with brain metastases, those who had received prior RCC therapy and those with active autoimmune disease were excluded from the trial.
In the trial, patients were administered a dose of MB-002 every two weeks for five doses, followed by a dose of MB-002 every month for four doses, during the 24-week induction phase. This dosing of MB-002 was followed by booster doses every three months until disease progression.
18
Table of Contents
The following table summarizes certain key data from the 20 intermediate and poor risk patients enrolled in the phase 1/2 monotherapy clinical trial of MB-002:
| Outcome |
(N=20) | |
| Median PFS | 6.3 months | |
| Complete response | 0 patients | |
| Partial response | 0 patients | |
| Stable disease | 10 patients | |
| Immune response | IL-2 recovery only |
Particular observations from these data and the trial, which informed our development of AGS-003, include:
| • | Five patients in the trial, including the two patients whom we enrolled in the monotherapy rollover clinical trial described below, survived for more than 30 months from initiation of treatment. |
| • | Four patients experienced some tumor regression at one point during the induction phase of treatment with MB-002. |
| • | MB-002 was well-tolerated, with adverse events limited to mild injection site reactions, transient flu-like symptoms and tenderness in the lymph nodes. |
| • | MB-002 was feasible to manufacture and deliver from a central facility to clinical trial sites in the United States and Canada. |
| • | The immunologic response data suggested that MB-002 was only able to partially overcome the immune suppression observed in mRCC. While IL-2 recovery was observed in the majority of patients, IFN-g recovery was not observed in the majority of patients. |
Because MB-002 corrected defects in the production of only one of the two critical cytokines — IL-2, but not IFN-g — required for an effective immune response, we conducted further laboratory research and developed an optimized product candidate, AGS-003. AGS-003 differs from MB-002 due to the manner in which we mature the dendritic cells and our addition of CD40L RNA in the manufacturing process. Based on in vitro experiments that simulate immunization in the laboratory, we observed that AGS-003 could overcome both critical cytokine defects in RCC patient cells and fully restore immune response. We subsequently confirmed this observation in vivo in the phase 1/2 monotherapy clinical trial of AGS-003 and in the phase 2 combination therapy clinical trial of AGS-003 and sunitinib.
In 2006, we enrolled two of the intermediate risk patients who had completed dosing in the phase 1/2 monotherapy clinical trial of MB-002 and who had experienced at least two years of stable disease in a separate, open label rollover trial of AGS-003 monotherapy. As this trial was an extension trial and observational in nature, the endpoints for this trial consisted of continued safety assessments and immunologic assessments to denote any differences between MB-002 and AGS-003. In this trial, the two patients received an induction phase of AGS-003 doses, followed by continued booster doses, consistent with the AGS-003 dosing schedule in the phase 1/2 monotherapy clinical trial of AGS-003, and were treated with AGS-003 for an additional four and 4.25 years, respectively, until disease progression occurred. One patient survived for 7.8 years, and the second patient had survived for more than eight years as of August 2012.
Ongoing and Planned Clinical Development
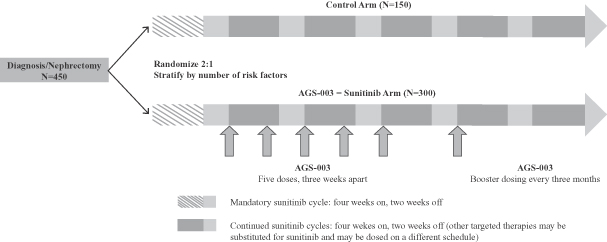
Pivotal Phase 3 Combination Therapy Clinical Trial. We are currently conducting a pivotal phase 3 clinical trial of AGS-003 in combination with sunitinib for the treatment of newly diagnosed mRCC under an SPA with the FDA. We refer to this trial as the ADAPT trial. We initiated the ADAPT trial in January 2013 and dosed the first patient in May 2013.
We have designed this trial to be a randomized, multicenter, open label trial of AGS-003 in combination with sunitinib compared to sunitinib monotherapy. We plan to enroll approximately 450 patients in the trial to generate 290 events for the primary endpoint of overall survival. We plan to enroll these patients at approximately 140 clinical sites in North America and Europe. Under the trial protocol, these patients will be randomized between the AGS-003 – sunitinib combination arm and the sunitinib monotherapy control arm on a two-to-one basis. As of February 28, 2014, we had collected tumor and screened approximately 250 patients for eligibility and had enrolled approximately 120 patients in the trial. We expect to complete enrollment of this clinical trial by the end of 2014 and to have data from this trial in the first half of 2016. We have established an independent data monitoring committee that will conduct interim analyses of the data from the trial for safety and futility at such times as 25%, 50% and 75% of the required events in the trial have occurred.
19
Table of Contents
We have designed this trial with a primary endpoint of overall survival. Secondary endpoints include progression free survival, overall response rate and safety. In order to achieve the primary endpoint, results from the trial must demonstrate an increase of approximately six months in median overall survival for the AGS-003 plus sunitinib arm compared to the sunitinib monotherapy arm. Such a result would be statistically significant (p £ 0.05).
Our design for this trial requires adult patients who have been newly diagnosed with mRCC with primary tumor intact and metastatic disease following nephrectomy, who have predominantly clear cell RCC based upon the tumor collected at nephrectomy and who have not received any prior therapies for RCC. Participating patients must be suitable candidates for sunitinib therapy and possess poor risk or intermediate risk disease at presentation, with the less than one year to treatment risk factor and not more than four Heng risk factors in total. As part of the trial design, the two arms of the trial will be balanced based upon known prognostic risk factors. Patients who are randomized will be stratified by number of risk factors (1, 2, 3 or 4) as well as whether they have measurable versus non-measurable metastatic disease following nephrectomy. We expect the patient population in the pivotal phase 3 clinical trial to be generally comparable to the patient population treated in our phase 2 combination therapy clinical trial. However, due to the limit on risk factors provided for by the protocol for the phase 3 clinical trial, we expect that the proportion of patients in the phase 3 clinical trial who are characterized as poor risk will be lower in the phase 3 clinical trial than in the phase 2 clinical trial. As of February 28, 2014, approximately 75% of the patients we had enrolled in the trial were intermediate risk patients.
Under the trial protocol, patients in the AGS-003-sunitinib arm are dosed with AGS-003 once every three weeks for five doses, followed by a booster dose every three months. In accordance with its label, sunitinib dosing is administered in six-week cycles, consisting of four weeks on drug and two weeks on drug holiday. AGS-003 dosing is initiated at the end of the initial six-week sunitinib cycle. The first dose of AGS-003 is administered prior to the start of sunitinib dosing in the second sunitinib cycle. This dosing regimen is identical to the dosing regimen used in our phase 2 combination therapy clinical trial of AGS-003 and sunitinib, except that the start of the sixth dose is scheduled for week 24 to better provide patients the opportunity to receive a total of eight doses across 48 weeks. Patients in the sunitinib monotherapy control arm receive sunitinib on the same dosing schedule as patients receive sunitinib in the AGS-003-sunitinib combination arm.
Under the trial protocol, AGS-003 is administered for at least 48 weeks so that patients receive at least eight doses of AGS-003. Dosing will cease prior to 48 weeks if two events of disease progression or unacceptable toxicity occur or upon the joint decision of the patient and the investigator. If after 48 weeks of dosing of AGS-003, a patient has stable disease or is responding to treatment, dosing will continue once every three months until disease progression. If an investigator determines to discontinue sunitinib, either due to disease progression or toxicity, the investigator can, at any time during the trial after the first six week cycle of sunitinib, initiate second-line therapy with one of the other approved targeted therapies, including pazopanib, axitinib, everolimus or temsirolimus. In the event of discontinuation of sunitinib for patients in the combination therapy arm, such patients would continue with AGS-003 dosing in combination with the second-line therapy. In our phase 2 combination therapy clinical trial, dosing ceased upon the first event of disease progression and second-line therapy was not permitted.
A graphic of the trial design is shown below.
Phase 3 Clinical Trial of AGS-003
20
Table of Contents
Other Development Activities. We believe that AGS-003 may be capable of treating a wide range of cancers and are planning to evaluate AGS-003 in clinical trials in additional cancer indications.
| • | We plan to conduct an open label phase 2 clinical trial of AGS-003 in patients with mRCC and non-clear cell histology, which we expect to begin in the second half of 2014. In the trial, we plan to evaluate the safety, efficacy and immunologic effects of AGS-003 when combined with surgery and targeted therapy in approximately 30 patients. |
| • | We plan to support two investigator-initiated phase 2 clinical trials, which are designed to evaluate treatment with AGS-003 in patients with early stage RCC prior to and following nephrectomy. We expect that these trials will begin in the second half of 2014 and be conducted in between 40 and 50 patients in total across the two trials. |
| • | We plan to initiate in 2014 two additional clinical trials of AGS-003 in other advanced solid tumor types. |
AGS-004 for the Treatment of Human Immunodeficiency Virus
We are developing AGS-004, our second Arcelis-based product candidate, for the treatment of HIV. We have completed two early stage clinical trials of AGS-004. In addition, in September 2013, we completed patient enrollment in a phase 2b clinical trial of AGS-004 and expect to have data from the trial in mid-2014. The NIH is funding the phase 2b clinical trial under a $39.3 million contract awarded to us in 2006.
Based on the clinical data that we have generated to date, we have determined to focus our development program on the use of AGS-004 in combination with other therapies to achieve complete virus eradication and the use of AGS-004 monotherapy to provide long-term control of HIV viral load in immunologically healthy patients and eliminate the need for antiretroviral drug therapy. We expect the phase 2 clinical trials of AGS-004 to be initiated for each of these uses in 2014.
Human Immunodeficiency Virus
HIV is characterized by a chronic viral infection and an associated deterioration of immune function. Specifically, the virus disables and kills crucial human immune cells called CD4+ T-cells. CD4+ T-cells are necessary to generate and maintain antiviral T-cells, including the CD8+CD28+ memory T cells that kill virus-infected cells. Over time, this viral impact on an infected person’s immune system outpaces the body’s natural ability to replace CD4+ T-cells and immunodeficiency results. As a result, the longer a person has been infected with the virus, the more functionally impaired these cells become.
At the same time, HIV infection causes the immune cells in HIV patients, including CD4+ T-cells and CD8+ T-cells, that are not killed by the virus to be in a chronic state of activation. The persistent state of immune activation in HIV patients results in chronic inflammation. We believe that this inflammation plays a role in the elevated rates of age-related comorbidities, including malignancies and cardiovascular disease observed in HIV patients. In addition, the activation of the CD4+ T-cells supports virus replication which leads to the production of new virus and increased viral load.
HIV is a persistent virus that can rapidly adapt to its environment by mutating and creating HIV variants that are drug resistant and can evade immune attack. As a result, there are a large number of mutated variants of HIV existing in any one infected individual and no two individuals have identical viral sequences.
According to the World Health Organization, the number of people living with HIV in the world was approximately 34 million in 2011. The Centers for Disease Control and Prevention estimates that more than 1.1 million people are currently living with HIV in the United States and the number of new cases of HIV infection in the United States is expected to remain constant at approximately 50,000 cases per year.
Current treatments for HIV. In 1996, a combination of oral medications known as antiretroviral therapy was introduced to treat patients with HIV. Since then, the introduction of new drug classes of antiretroviral therapy and combination drug treatment strategies has enhanced treatment for HIV. According to Datamonitor, sales of antiretroviral therapies in the United States and the five biggest European markets reached $13.3 billion in 2011.
Antiretroviral therapy in HIV-infected patients can decrease levels of HIV in the blood to below the limits of detection, increase life expectancy and improve quality of life. However, there continues to be an unmet need for HIV therapies for the following reasons:
| • | Antiretroviral therapy can have significant side effects. The most recent U.S. guidelines on antiretroviral treatment contain a number of tables of adverse effects of combination regimens and how to manage them. Some combinations present potentially life-threatening complications and other complications that are chronic, cumulative and overlapping, and sometimes irreversible. |
21
Table of Contents
| • | Antiretroviral therapy requires life-long daily treatment. The risks of long-term daily administration of antiretroviral therapy remain unknown but are potentially significant. In addition, the requirement for life-long daily treatment has made strict adherence to the treatment regime difficult. Poor compliance has led to the development of drug resistant HIV variants that are ineffectively controlled by the available armamentarium of antiretroviral therapies. |
| • | Antiretroviral drug therapy cannot eradicate the virus and, therefore, does not cure HIV-infected patients. For example, up to 20% of patients receiving antiretroviral drug therapy fail to achieve normal CD4+ T-cell counts, resulting in a continued weakened immune system. In addition, certain patients are not able to achieve effective control of the virus using current treatment regimens. Antiretroviral therapy cannot eradicate the virus because the virus persists in latently infected cells. These cells, which constitute the HIV latent reservoir, do not express HIV antigens and are therefore invisible to the immune system. Instead, these cells serve as the source for virus replication and viral rebound in the absence of antiretroviral therapy. Following discontinuation of treatment with antiretroviral drug therapy, HIV viral levels return to levels observed prior to treatment with antiretroviral therapy within 12 weeks of treatment interruption. |
AGS-004 Opportunity
We believe, based on the mechanism of action of AGS-004 and the clinical data that we have generated, that AGS-004 has the potential to address this unmet need for the following reasons:
| • | Potential to Eradicate HIV in Combination with Latency Reversing Drugs. A number of companies and academic groups are evaluating drugs that can potentially activate the latently infected cells to increase viral antigen expression and make the cells vulnerable to elimination by the immune system. We believe that treating HIV-infected patients, who are being successfully treated with antiretroviral drug therapy, with a combination of AGS-004 and one of these latency reversing drugs could lead to activation of antigen expression from the latently infected cells along with a potent memory T-cell response that is specific to the patient’s own unique viral antigens. We believe that this approach could potentially result in complete eradication of the patient’s virus. |
| • | Long-Term Viral Load Control in Immunologically Healthy Patients. We believe that AGS-004 may allow for long-term virus control and eliminate the need for life-long treatment with antiretroviral drug therapy in infected patients who have minimal immune suppression but no T-cell response against their virus. We have designed AGS-004 to induce CD8+ CD28+ memory T-cells that are specific to the patient’s own unique viral antigens, do not require CD4+ T-cell help to kill viral cells and do not result in CD4+ T-cell activation which typically increases viral replication and viral load. As reported in Clinical & Experimental Immunology, researchers have demonstrated that elevated levels of CD8+CD28+ memory T-cells in the blood are a statistically significant predictor of long-term non-progression in HIV-infected patients not treated with antiretroviral therapy drugs. As a result, we believe that inducing these memory T-cells may lead to viral control. Patients with minimal immune suppression and no T-cell response include pediatric patients who have been successfully treated with antiretroviral drugs since birth or shortly thereafter and have generally healthy immune systems. |
| • | Minimal Toxicity. AGS-004 has been well tolerated in clinical trials with no serious adverse events being attributed to it. As a result, we believe we can combine AGS-004 with other HIV therapies without additional toxicities. |
| • | Lack of Chronic Inflammation. We have designed AGS-004 to elicit a patient-specific and disease-specific immune response that does not cause any additional inflammation. In a retrospective analysis of our phase 1 clinical trial of AGS-004, we observed that AGS-004 did not induce changes in markers that are associated with chronic inflammation in HIV patients. |
Description and Development Status
AGS-004 is a fully personalized immunotherapy based on our Arcelis platform. It is produced by electroporating dendritic cells with mRNA encoding for patient-specific HIV antigens that have been derived from a patient’s virus-infected blood and with RNA that encodes the CD40L protein. The process for producing AGS-004 is the same process as is used to produce AGS-003, with the one key difference being that AGS-003 contains all of the antigens from a patient’s tumor cells while AGS-004 contains all variants unique to each individual patient of four selected HIV antigens (Gag, Nef, Vpr and Rev). We designed AGS-004 to include these antigens because immunity to them has been observed in long-term non-progressors and elite controllers, two groups of rare patients able to control virus replication without antiretroviral drug therapy. Because no two patients share identical HIV antigen sequences and there are a large number of mutated variants of HIV existing in each infected patient, by using mRNA that is specific to the patient’s virus and that captures all of the unique patient-specific variants of each antigen, we believe our immunotherapy maximizes the relevance of the immune responses induced in each patient.
22
Table of Contents
We are conducting a phase 2b clinical trial of AGS-004. We have conducted two earlier stage clinical trials of AGS-004, which include:
| • | a phase 2a clinical trial of AGS-004; and |
| • | a phase 1 clinical trial of AGS-004. |
We submitted to the FDA an IND for AGS-004 in August 2008.
Phase 2a Clinical Trial. From 2008 to 2009, we enrolled 29 patients in a single arm, multicenter, open label phase 2a clinical trial of AGS-004. These patients were enrolled at eight clinical sites in Canada. Eight of these 29 patients had previously participated in our phase 1 clinical trial of AGS-004. We refer to these patients as rollover patients.
Despite treatment with antiretroviral drug therapy, HIV persists in latently infected cells, and following discontinuation of treatment, HIV viral levels return to levels observed prior to treatment with antiretroviral therapy. We designed the trial in this context to assess the ability of AGS-004 to control viral load after interruption of antiretroviral drug therapy and to prevent viral load levels from returning, following discontinuation of treatment, to pre-antiretroviral treatment levels. We believe that the data from the trial demonstrate that AGS-004 can lead to a reduction in virus replication by targeting and eliminating cells that produce new virus.
The primary endpoint of the phase 2a clinical trial was a measurement of the ability of AGS-004 to improve immune control of HIV as measured by the proportion of patients capable of maintaining a minimal HIV viral load of less than 1,000 copies/mL and CD4+ T-cell counts above 350 cells/mm3 on at least three time points during a 12-week antiretroviral treatment interruption. FDA guidelines recommend initiation of antiretroviral drug therapy for patients with a CD4+ T-cell count below 350 cells/mm3. Secondary endpoints included safety, change in viral load from pre-antiretroviral therapy to the end of the antiretroviral treatment interruption and changes in CD4+ T-cell counts.
To be included in this trial, patients had to be adults with chronic HIV-1 infection and undetectable viral loads as a result of their ongoing treatment with antiretroviral drug therapy. Patients must also have had adequate CD4+ T-cell counts and a pre-antiretroviral therapy plasma viral sample to be used to manufacture AGS-004.
Patients initially received four monthly doses of AGS-004, together with their antiretroviral therapy. After receiving those doses, antiretroviral therapy, or ART, was discontinued and patients entered into a planned 12-week antiretroviral treatment interruption period, which is referred to as a structured treatment interruption, or STI. During the 12-week treatment interruption period, two additional monthly doses of AGS-004 were administered. Patients who maintained plasma viral loads under 10,000 copies/mL and CD4+ T-cell counts above 350 cells/mm3 were eligible to remain off of antiretroviral therapy beyond the 12-week treatment interruption period and receive up to two additional booster doses of AGS-004 at eight weeks after the second monthly dose administered during STI and at 12 weeks after the first booster dose. A graphic of the trial design is shown below:
Phase 2a Clinical Trial Design
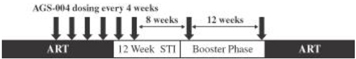
Outcomes regarding viral load control
In the trial, 24 patients entered into the antiretroviral treatment interruption period in accordance with the trial protocol. The trial protocol specified that only patients with CD4+ T-cell counts above 450 cells/mm3 and undetectable viral loads were eligible to enter the antiretroviral treatment interruption period. The five patients who did not enter the treatment interruption period according to the trial protocol had CD4+ T cell counts below 450 cells/mm3.
AGS-004 achieved the primary endpoint of the trial as eight patients maintained HIV viral loads of less than 1,000 copies/ml and CD4+ T-cell counts greater than 350 cells/mm3 during at least three time points measured two weeks apart during the antiretroviral drug treatment interruption period.
In addition, 17 of the 24 patients who entered into the treatment interruption period in accordance with the trial protocol maintained a reduction in their levels of viral load compared to their pre-antiretroviral therapy viral load levels. We believe that change in viral load from pre-antiretroviral therapy to the end of antiretroviral treatment interruption was the most relevant endpoint in the trial to our plans to develop AGS-004 for the eradication of HIV.
23
Table of Contents
The graphic below shows the change in viral load from immediately prior to the commencement of antiretroviral therapy for all 24 patients who entered the 12-week treatment interruption phase of the trial. For patients who did not complete the 12-week treatment interruption phase, the last viral load measurement prior to the patient restarting antiretroviral therapy was used for the calculation.
Phase 2a Clinical Trial:
Change in Viral Load at Antiretroviral Therapy Restart or Completion of 12-week Treatment
Interruption for All Patients Entering Antiretroviral Treatment Interruption per Protocol
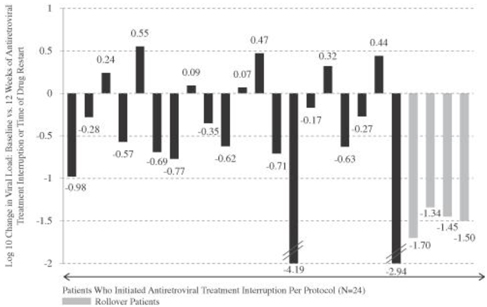
The patients who entered the treatment interruption period in accordance with the trial protocol had a mean reduction of 81% in viral load after completion of 12 weeks of antiretroviral drug interruption or prior to restart of antiretroviral therapy compared to their viral loads measured before the initiation of antiretroviral drug therapy. The mean reduction in the four rollover patients who entered the 12-week antiretroviral treatment interruption in accordance with the trial protocol was 97%. Based on the results in the rollover patient subgroup, we believe that administration of more than four doses of AGS-004 prior to antiretroviral treatment interruption may lead to better viral load control after cessation of antiretroviral treatment.
In addition, we measured CD4+ T-cell count as a secondary endpoint in the trial. Of the 24 patients entering the 12-week antiretroviral treatment interruption, 21 patients maintained CD4+ T-cell counts above 350 cells/mm3 and completed 12 weeks of treatment interruption.
Safety analysis
In this trial, AGS-004 was safe and well tolerated. No AGS-004-related serious adverse events were reported. The most common adverse event was mild injection site reactions. During the antiretroviral treatment interruption, no notable differences in incidence of adverse events occurred compared to when patients were receiving AGS-004 in combination with antiretroviral drug therapy.
Retrospective analysis regarding impact on anti-HIV immunity and HIV latent reservoirs
Latently infected cells differ from other infected cells in that the HIV genome is permanently integrated into the chromosomal DNA of the latently infected cells. These latently infected cells persist long-term and constitute the HIV latent reservoir, which serves as a source for low level virus replication and viral rebound in the absence of antiretroviral therapy. As a result, demonstration that latently infected cells can be targeted by immune responses induced by AGS-004 is essential to our development strategy pertaining to virus eradication.
Following the phase 2a clinical trial, we conducted a retrospective analysis of the effect of AGS-004 on cells harboring chromosomally integrated HIV DNA. We conducted this analysis in the 20 patients from the trial who had blood cells available prior to AGS-004 treatment and after the first four monthly doses of AGS-004 during continuous antiretroviral therapy. In the analysis, we observed that although the impact of AGS-004 on CD4+ T-cells with integrated HIV DNA in the 20 patient population was not statistically reduced, in the five rollover patients we analyzed, there was an average of a 23.3% reduction in CD4+ T-cells with
24
Table of Contents
integrated HIV DNA from the first measurement to the final dose of AGS-004. We believe that these data may indicate that administering more doses of AGS-004 during antiretroviral drug therapy may result in more efficient targeting of latently infected cells, a desirable property for our development strategy regarding eradication.
In addition, we analyzed archived pre- and post-AGS-004 administration blood draws from a subset of patients in this trial for HIV-specific T cell responses (N=7) and for PD-1 expression on activated CD8+ T cells (N=6). Results from these blood draws showed that AGS-004 induced anti-HIV T memory stem cell-like immune responses (TSCM) that were associated with a longer time to viral rebound after ART treatment interruption. Longer times to viral rebound were also associated with lower expression of the checkpoint inhibitor PD-1 on activated CD8+ T cells.
Phase 1 Clinical Trial. From 2006 to 2007, we enrolled ten patients in a first-in-man single arm, single center, open label phase 1 clinical trial of AGS-004. The primary endpoint of this trial was the ability of AGS-004 to generate anti-HIV CD8+ CD28+ memory T-cell responses. The secondary endpoint was the safety of AGS-004 in combination with antiretroviral therapy. The inclusion and exclusion criteria were substantially similar to those for the phase 2a clinical trial of AGS-004. In the trial, patients received four doses of AGS-004 one month apart. This trial met its predefined endpoint of demonstrating CD8+ CD28+ memory T-cell responses against multiple HIV antigens in four of the nine patients whose materials were available for testing. AGS-004 was well-tolerated, with adverse events limited to mild injection site reactions, transient flu-like symptoms and tenderness in the lymph nodes.
Following completion of the phase 1 clinical trial, we completed a retrospective analysis of the contribution of AGS-004 to chronic inflammation using archived blood samples collected from patients during this trial. In this analysis, we assessed changes in CD4+ T-cells and CD+8 T-cells and analyzed the levels of immune activation based on markers for proliferation and cell activation before and after AGS-004 administration. This analysis showed that AGS-004 did not induce changes in the proportion of CD4+ and CD8+ T-cells compared to levels of such T-cells measured prior to AGS-004 administration and did not cause any elevation in systemic T-cell immune activation. We believe that these data indicate that AGS-004 is capable of inducing antiviral immune responses without an associated increase in markers associated with chronic inflammation.
Ongoing and Planned Clinical Development
Phase 2b Clinical Trial. We are conducting a randomized, placebo controlled, double blind phase 2b clinical trial of AGS-004 in chronically infected patients on antiretroviral therapy that we initiated in July 2010. We designed this trial to confirm the data obtained in the phase 2a clinical trial. In September 2013, we completed patient enrollment in the phase 2b clinical trial. We initially planned to enroll 42 chronically infected patients in the trial at nine clinical sites in the United States and Canada with the intent to generate 36 events for the primary endpoint analysis. However, due to a higher than anticipated dropout rate by patients who were unable to complete the full 12 week treatment interruption period, we needed to enroll 53 patients in the trial to generate 36 events for the primary endpoint analysis. These patients were randomized between AGS-004 treatment and a placebo control on a two-to-one basis. We expect to have data from the trial in mid-2014.
HIV infection is classified as “chronic” or “acute” based on how long the patient has been infected prior to starting antiretroviral drug therapy. Patients with chronic HIV infection are patients who have initiated antiretroviral drug therapy after at least six months from the time of initial infection. Patients with acute HIV infection are patients who have initiated antiretroviral drug therapy less than 45 days after initial infection. This trial enrolled adult patients with chronic HIV-1 infection and undetectable viral loads as a result of treatment with antiretroviral drug therapy. Patients also had to have adequate CD4+ T-cell counts and a pre-antiretroviral therapy plasma viral sample to be used to manufacture AGS-004.
In this trial, patients first receive intradermal doses of AGS-004 or placebo every four weeks for a total of four doses, together with their antiretroviral therapy. Following the fourth dose of AGS-004 or placebo, patients discontinue their antiretroviral therapy but continue to receive AGS-004 or placebo every four weeks for 12 weeks. Patients who demonstrate control of viral replication under 10,000 copies/ml and maintain CD4+ T-cell counts above 350 cells/mm3 can remain off antiretroviral therapy and continue their treatment interruption past 12 weeks. Following the end of treatment interruption, all patients are eligible for continued treatment with the combination of AGS-004 and antiretroviral therapy. A schematic of the trial design is shown below.
25
Table of Contents
Phase 2b Study Design for the Chronically Infected Cohort
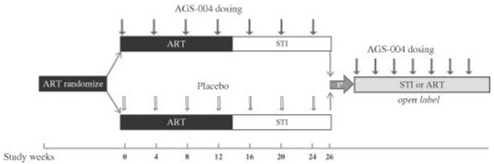
The primary endpoint of the trial is a comparison of the median viral load in the AGS-004-treated patients with the median viral load in patients receiving placebo after 12 weeks of antiretroviral treatment interruption. The primary endpoint will be achieved if there is a ³1.1 log 10 difference in median viral load between the AGS-004-treated cohort compared to the placebo-treated cohort. A 1.1 log 10 reduction means a 92% lower virus concentration in the AGS-004-treated cohort compared to the placebo-treated cohort. Secondary endpoints include comparisons between AGS-004-treated patients and the patients receiving placebo with respect to change in viral load from pre-antiretroviral therapy to the end of 12 weeks of treatment interruption, duration of treatment interruption, changes in CD4+ T-cell counts and safety.
In September 2011, we modified the protocol of the phase 2b clinical trial to add to the trial a single arm, open-label, unblinded cohort of up to 12 patients with acute HIV-1 infection and undetectable viral loads as a result of treatment with antiretroviral drug therapy. Patients in this cohort were dosed in the same manner as patients in the chronically infected arm of the clinical trial. However, in this cohort, patients had to demonstrate a positive CD8+ CD28+ anti-HIV memory T-cell response in order to become eligible to enter the 12 week treatment interruption period. The primary endpoints for this cohort include the time to detectable viral load during the antiretroviral therapy interruption period and comparison of changes in CD4+ T-cell counts during the antiretroviral therapy interruption period between the acute cohort and the chronic cohort. Six patients were enrolled in this cohort. All six patients demonstrated a positive CD8+ CD28+ memory T-cell response and initiated treatment interruption. For the five of six patients that re-initiated ART therapy after treatment interruption, there were no significant declines in CD4+ T cells between the interruption date and the re-initiation date. All six patients experienced viral rebound during treatment interruption with the times to detectable viral load ranging from two to eight weeks and the duration of treatment interruption for those patients who reinitiated ART therapy ranged from approximately one month to approximately five months. One patient has not yet reinitiated ART. In addition, three of six patients had a decrease in circulating CD4+ T cells containing HIV DNA of 25%, 47% and 63%, respectively, when measured after three doses of AGS-004 while on ART.
We are evaluating AGS-004 in this patient population to assess AGS-004 in patients who initiated antiretroviral drug therapy during the acute phase of infection and as a result may have sustained less immune damage. We believe that the results from this cohort will provide us with valuable information as we seek to advance our program to develop AGS-004 for long-term control of HIV viral load in immunologically healthy patients and eliminate the need for antiretroviral drug therapy.
Planned Adult Eradication Trial. We are currently developing the clinical protocol for an investigator-initiated phase 2 clinical trial of AGS-004 in adult HIV patients who are being treated with antiretroviral drug therapy to evaluate the use of AGS-004 in combination with a latency reversing drug to eradicate the virus with Dr. David Margolis, Professor of Medicine at the University of North Carolina. Dr. Margolis is the leader of the Collaboratory of AIDS Researchers for Eradication, or CARE, who has been a pioneer in the research of HIV latent reservoir reversing treatments. We expect this trial to be initiated in the first half of 2014. In January 2014, CARE agreed that it would fund all patient clinical costs of this phase 2 clinical trial, except for the associated manufacturing costs for which we will be responsible.
Planned Pediatric Functional Cure Trial. We believe that a patient population that could benefit from AGS-004 monotherapy consists of 14+ year old, HIV-infected individuals who have been treated with antiretroviral therapy since birth or shortly thereafter. These individuals are characterized by having very small HIV latent reservoirs and otherwise healthy immune systems, while lacking antiviral CD8+ CD28+ memory T-cell responses. We believe that successfully inducing antiviral CD8+ CD28+ memory T-cell responses in these patients could allow for long-term viral load control and eliminate the need for life-long antiretroviral therapy. We plan to initiate in 2014 a phase 2 clinical trial of AGS-004 in pediatric HIV patients to evaluate the use of AGS-004 monotherapy to allow for long-term control of viral load and eliminate the need for antiretroviral therapy. We are currently developing the clinical protocol for this trial to immunize pediatric HIV patients who were infected at birth and treated with antiretroviral therapy at or near birth. We are developing this clinical protocol in collaboration with Drs. Katherine Luzuriaga, University of Massachusetts, and Deborah Persaud, John Hopkins Medical Center, both specializing in pediatric virology. We are discussing with the NIH the protocol for this trial and the potential funding by the NIH of the costs of this trial.
26
Table of Contents
NIH Contract. Our development of AGS-004 has received significant funding from the U.S. federal government. In September 2006, we entered into a multi-year research contract with the NIH and the National Institute of Allergy and Infectious Diseases, or NIAID, to design, develop and clinically test an autologous HIV immunotherapy capable of eliciting therapeutic immune responses. We are using funds from this contract to develop AGS-004. Under this contract, as it has been amended, the NIH and the NIAID have committed to fund up to $39.3 million, including reimbursement of our direct expenses and allocated overhead and general and administrative expenses of up to $37.9 million and payment of specified amounts totaling up to $1.4 million upon our achievement of specified development milestones. This commitment extends until September 2015.
We have agreed to a statement of work under the contract, and are obligated to furnish all the services, qualified personnel, material, equipment, and facilities, not otherwise provided by the U.S. government, needed to perform the statement of work. In accordance with the laws applicable to government intellectual property rights under federal contracts, we have a right under our contract with the NIH to elect to retain title to inventions conceived or first reduced to practice under the NIH contract, subject to the right of the U.S. government to a royalty-free license to practice or have practiced for or on behalf of the United States the subject invention throughout the world. The government also has special statutory “march-in” rights to license or to require us to license such inventions to third parties under limited circumstances. In addition, we may not grant to any person the exclusive right to use or sell any such inventions in the United States unless such person agrees that any products embodying the subject invention or produced through the use of the subject invention will be manufactured substantially in the United States.
Other Programs
In addition to our development of AGS-003 and AGS-004 and our Arcelis-based platform, we are also developing AGS-009, a humanized anti-Interferon- a monoclonal antibody, for the treatment of systemic lupus erythematosus, or lupus, a chronic and disabling autoimmune disorder, and AGS-010, a recombinant human soluble CD83 protein, for organ and tissue transplantation and the treatment of autoimmune and inflammatory diseases. We discovered AGS-009 through our dendritic cell biology research. AGS-010 is the result of our research efforts into how the ability to turn off specific undesirable immune responses can have a therapeutic effect on all immune mediated diseases, including transplant rejection and autoimmune and inflammatory diseases. We plan to seek third party funding to fund the further development of AGS-009 and AGS-010.
Manufacturing
We currently manufacture our Arcelis-based products, AGS-003 and AGS-004, for use in our clinical trials of those product candidates at our facility in Durham, North Carolina. Our facility includes manufacturing suites for the production of products using our Arcelis technology platform. We have designed these suites to comply with the FDA’s current good manufacturing practice, or cGMP, requirements. We have manufactured the product for our development and clinical trial activities associated with AGS-003 and AGS-004 to date, and are manufacturing the product for our pivotal phase 3 combination therapy trial of AGS-003 using our current processes at our current facility. In order to produce AGS-003 on a commercial scale, we will require a full scale manufacturing facility and updated processes. Accordingly, we plan to establish automated manufacturing processes based on existing functioning prototypes of automated devices, to identify and lease suitable space for a new commercial manufacturing facility and build out and equip the new facility for manufacture of our Arcelis-based products. As part of our BLA for AGS-003, we will need to demonstrate analytical comparability between AGS-003 that we produce using our current processes in our current facility and AGS-003 produced using the automated processes in our planned new facility.
We are actively exploring potential sites and financing arrangements in connection with the lease, build out and equipping of a commercial manufacturing facility and are in discussions with developers and governmental authorities regarding potential sites and financial support. We expect to enter into arrangements that include financial support, and we expect such arrangements will likely involve material obligations and debt liabilities.
Commercial Manufacturing Facility and Automated Process. We plan to establish automated manufacturing processes based on existing functioning prototypes of automated devices for the production of commercial quantities of our Arcelis-based product. These devices can be used to perform substantially all steps required for the manufacture of our Arcelis-based product candidates. We intend to identify and lease suitable space for the build-out and equipping of a new commercial manufacturing facility for the manufacture of AGS-003, AGS-004 and other Arcelis-based products.
We plan to implement the automated processing in a modular manufacturing system in the new commercial facility. The modular facility design allows for expansion of the commercial capacity in line with the market demand of our product candidates. We believe that this modular approach will require less capital investment at product launch as manufacturing capacity can be increased incrementally as product demand increases after commercialization. We believe the modular manufacturing approach is optimal for personalized immunotherapies and allows for scalable capacity, minimizes facility size and complexity at launch, and results in a lower cost of goods for the commercial product.
27
Table of Contents
In the new facility, we plan to use the automated equipment to perform Arcelis product processing in closed, single-use disposable containers. A patient’s disease sample would only be in contact with these disposable sets or containers and not with the balance of our manufacturing equipment. Because our equipment would never be in direct contact with patient material, we believe that the time required to prepare the equipment between batches would be minimal. We also believe that automated processing of material in disposable containers will reduce the complexity and size of the facility by reducing the amount of required labor. We expect that automation will help ensure consistency of the manufacturing processes and facilitate compliance with cGMP.
Prior to implementing automated manufacture of AGS-003, we will be required to demonstrate that the new facility and the automated equipment are constructed and operated in accordance with cGMP and the comparability between AGS-003 that we produce using the manual processes in our current facility and AGS-003 produced using the automated processes in our new facility. We anticipate that it will require approximately two years from the time we begin the build out to build out and equip the facility to a level necessary to file a BLA for AGS-003 with the FDA using the automated manufacturing processes. Following the filing of a BLA, we would need to further build out and equip the facility so as to have in place the commercial capacity that we anticipate will be required for commercial launch of AGS-003.
In addition to providing commercial scale manufacturing of AGS-003, we believe that this facility and automated process will provide us key advantages, including:
| • | centralized manufacturing capable of delivering products to clinical sites throughout North America; |
| • | an automated manufacturing platform that is scalable for large disease indications such as HIV and other cancer indications; and |
| • | manufacturing with a cost of goods that we expect will be comparable to other biologics and methods that are scalable, consistent and broadly applicable across patients and indications. |
We have granted exclusive manufacturing rights for AGS-003 to Pharmstandard in Russia and the other states comprising the Commonwealth of Independent States, to Green Cross in South Korea and to Medinet in Japan. We have also agreed to enter into an agreement with Pharmstandard for the manufacture of AGS-003 in the European market.
Sales and Marketing
We hold exclusive commercial rights to all of our product candidates in all geographies other than rights to AGS-003 in Russia and the other states comprising the Commonwealth of Independent States, which are held by Pharmstandard, and rights to AGS-003 in South Korea, which are held by Green Cross. We have granted to Medinet an exclusive license to manufacture in Japan AGS-003 for the treatment of mRCC and an option to acquire a non-exclusive license to sell in Japan AGS-003 for the treatment of mRCC. We currently intend to retain North American marketing rights for AGS-003 and any future oncology products that we may develop. To maximize the value of these rights, we would expect to build a commercial infrastructure for such products comprised of a targeted specialty sales force led by several sales management personnel, an internal marketing and medical affairs staff and a specialty distribution team. Our sales infrastructure would also include personnel to establish and direct reimbursement activities with third party payors, such as managed care organizations, group purchasing organizations, oncology group networks and government accounts. We plan to hire selected personnel to fill key positions in advance of the approval of AGS-003. We currently have no sales and marketing or distribution capabilities or in-house personnel specializing in these functions. Outside North America, we plan to seek to enter into collaboration agreements with other pharmaceutical or biotechnology firms to commercialize AGS-003.
For AGS-004, we plan to seek to enter into collaboration agreements with other pharmaceutical or biotechnology firms to commercialize this product candidate on a worldwide basis.
Competition
The biotechnology and pharmaceutical industries are highly competitive. There are many pharmaceutical companies, biotechnology companies, public and private universities and research organizations actively engaged in the research and development of products that may be similar to or competitive with our products. There are a number of multinational pharmaceutical companies and large biotechnology companies currently marketing or pursuing the development of products or product candidates targeting the same indications as our product candidates. It is probable that the number of companies seeking to develop products and therapies for the treatment of unmet needs in these indications will increase. Some of these competitive products and therapies are based on scientific approaches that are the same as or similar to our approaches, and others are based on entirely different approaches.
28
Table of Contents
Many of our competitors, either alone or with their strategic partners, have substantially greater financial, technical and human resources than we do and significantly greater experience in the discovery and development of product candidates, obtaining FDA and other regulatory approvals of products and the commercialization of those products. Our competitors’ drugs may be more effective, or more effectively marketed and sold, than any drug we may commercialize and may render our product candidates obsolete or non-competitive. We anticipate that we will face intense and increasing competition as new drugs enter the market and advanced technologies become available. We expect any products that we develop and commercialize to compete on the basis of, among other things, efficacy, safety, convenience of administration and delivery, price, the level of generic competition and the availability of reimbursement from government and other third party payors.
mRCC
Historically, mRCC was treated with chemotherapy, radiation and hormonal therapies, as well as cytokine-based therapies such as interferon-µ and IL-2. More recently, the FDA has approved several targeted therapies as monotherapies for mRCC, including Nexavar, marketed by Bayer Healthcare Pharmaceuticals, Inc. and Onyx Pharmaceuticals, Inc., Sutent and Inlyta, marketed by Pfizer, Inc., Avastin, marketed by Genentech, Inc., a member of the Roche Group, and Votrient, marketed by GlaxoSmithKline. Other recently approved targeted therapies for the treatment of mRCC are Torisel, marketed by Pfizer, and Afinitor, marketed by Novartis Pharmaceuticals Corporation. We believe that each of these existing therapies has efficacy or safety limitations and, as a result, that there remains an unmet need for novel therapeutic approaches for mRCC that can improve efficacy without adding appreciable toxicity. We believe that the safety profile that AGS-003 has demonstrated to date, which may enable it to be used in combination with these therapies with little or no additional toxicities, gives AGS-003 the potential to address this unmet need. Accordingly, existing therapies with which AGS-003 would be used as part of a combination would not be competitive with AGS-003. However, a standalone therapy for mRCC that demonstrated improved efficacy over currently marketed therapies with a favorable safety profile and without the need for combination therapy might pose a significant competitive threat to AGS-003.
Immatics Biotechnologies GmbH is developing a therapeutic cancer vaccine, IMA-901, which is a mixture of defined tumor-associated peptides, for the treatment of RCC. Immatics is conducting a pivotal phase 3 clinical trial comparing IMA-901 in combination with sunitinib against sunitinib alone in a subset of favorable and intermediate risk patients. If this clinical trial is successful, IMA-901 and sunitinib combination therapy would be in direct competition with AGS-003 and sunitinib combination therapy. Immatics could have a competitive advantage if it is able to introduce its product to the market before the time, if any, at which we receive marketing approval for AGS-003.
We estimate that there are numerous other cancer immunotherapy products in clinical development by many public and private biotechnology and pharmaceutical companies targeting numerous different cancer types. A number of these product candidates are in late-stage clinical development.
HIV
There are numerous FDA-approved treatments for HIV, primarily antiretroviral therapies, marketed by large pharmaceutical companies. In addition, generic competition has recently developed as patent exclusivity periods for older drugs have expired, with more than 15 generic bioequivalents currently on the market. The presence of these generic drugs is resulting in price pressure in the HIV therapeutics market. Currently, there are no approved therapies for the eradication of HIV. We expect that major pharmaceutical companies that currently market antiretroviral therapy products or other companies that are developing HIV product candidates may seek to develop products for the eradication of HIV.
Intellectual Property
Our success depends in part on our ability to obtain and maintain proprietary protection for our products and product candidates, technology and know-how, to operate without infringing the proprietary rights of others and to prevent others from infringing our proprietary rights. We are seeking a range of patent and other protections for our product candidates and platform technology. We also rely on trade secrets, know-how, continuing technological innovation and in-licensing opportunities to develop and maintain our proprietary position.
Patents
We own or exclusively license 12 U.S. patents and eight U.S. patent applications, as well as approximately 60 foreign counterparts, covering our Arcelis technology platform and Arcelis-based product candidates.
29
Table of Contents
We use our Arcelis technology platform to generate fully personalized mRNA-loaded dendritic cell immunotherapies. As described above, the process of obtaining a disease sample and dendritic cells from a patient, using those materials to manufacture a fully personalized drug product and shipping the drug product to the clinical site for use in the treatment of the patient involves many important steps. These steps include:
| • | amplifying mRNA from a disease sample obtained from the patient; |
| • | differentiating dendritic cell precursors (monocytes) isolated from the patient into immature dendritic cells; |
| • | maturing the immature dendritic cells in culture and loading the mature dendritic cells with the amplified mRNA and CD40L protein; and |
| • | formulating the matured, loaded dendritic cells in the patient’s plasma with cryoprotectants to protect the cells in the resulting drug product when the drug product is frozen and thawed. |
We have sought to protect these steps or the equipment related to carrying out one or more of these steps through patents or trade secrets. We have also sought to protect the resultant drug product through patents.
These patents and patent applications are directed to one or more aspects of our Arcelis technology platform or Arcelis-based products. Specifically, these patents and patent applications are collectively directed to:
| • | Arcelis-based compositions of matter and products; |
| • | methods of manufacturing Arcelis-based products; |
| • | methods of using Arcelis-based products for treatment of tumors; |
| • | compositions that we use in the manufacture of Arcelis-based AGS-004 products; and |
| • | equipment that we intend to use for assisting the automated manufacture of Arcelis-based products. |
We believe that all of the above aspects of our Arcelis technology platform are required to successfully produce our Arcelis-based product candidates and are covered by a combination of our patents, patent applications, trade secrets and know-how. The U.S. patents expire between 2016 and 2029, and the U.S. patent applications, if issued, would expire between 2016 and 2033, the counterpart patents in Europe and Japan expire between 2017 and 2027, and the counterpart patent applications in Europe and Japan, if issued, would expire between 2017 and 2033. Included in these patents and patent applications are:
| • | five U.S. patents and one U.S. patent application that are collectively directed toward the composition of matter of Arcelis-based products (dendritic cells loaded with RNA from tumors or pathogens), methods of manufacture of these products and methods of using these products to treat tumors. Each of the five U.S. patents encompass the AGS-003 composition of matter. One of the five U.S. patents encompasses the AGS-004 composition of matter. The U.S. patents expire in 2016 and the U.S. patent application, if issued, would expire in 2016. Corresponding patents in Europe and Japan and a pending corresponding patent application in Europe, if issued, would expire in 2017. |
| • | three U.S. patents, one U.S. patent application and corresponding patent application in Europe and patent in Japan collectively directed towards an automated apparatus for the manipulation of nucleic acids in a closed container, components thereof and related methods of use. The U.S. and Japanese patents expire in 2027, and the patent applications in the United States and Europe, if issued, would expire in 2027. |
| • | one U.S. patent and corresponding European and Japanese patents collectively directed towards cryoconserved dendritic cells and related methods of manufacture. The U.S., European and Japanese patents expire in 2021. |
| • | one U.S. patent and three U.S. patent applications, two corresponding European patents, two corresponding Japanese patents and a corresponding patent application in Europe collectively directed towards methods of maturing dendritic cells and the composition of matter of dendritic cells that have undergone this maturation process. The U.S. patent expires in 2025 and the U.S. applications, if issued, would expire in 2025, the European patents expire in 2025 and 2027, the Japanese patents expire in 2025 and 2027 and the patent application in Europe, if issued, would expire in 2025. |
30
Table of Contents
| • | one U.S. patent and one U.S. patent application and corresponding patent application in Europe and patent in Japan collectively directed towards methods of manufacture of dendritic cells from monocytes stored for more than six hours and up to four days without freezing and the composition of matter of dendritic cells that have been manufactured from these monocytes. The U.S. patent expires in 2029 and the U.S. patent application, if issued, will expire in 2026, the Japanese patent will expire in 2026 and the patent application in Europe, if issued, would expire in 2026. |
| • | one U.S. patent application, one patent application in Europe and one patent in Japan are collectively directed towards the composition of matter of AGS-004 and related methods of manufacture. The U.S. patent application and the patent application in Europe, if issued, would expire in 2025. The Japanese patent will expire in 2025. |
| • | one U.S. patent and one U.S. patent application are directed towards the composition of matter and related methods of use of some of the primers that we use in the manufacture of AGS-004. The U.S. patent will expire in 2028, and the U.S. patent application, if issued, would expire in 2028. |
| • | one international PCT patent application is directed towards a centrifuge vessel for use in the automated manufacture of AGS-003 and AGS-004. Corresponding U.S., European and Japanese patent applications, if filed and issued, would expire in 2033. |
In addition, if the use of Arcelis-based products for the treatment of RCC and HIV are approved by the FDA, then, depending upon factors such as the timing and duration of FDA review and the timing and conditions of FDA approval, as well as factors such as patent claim scope, some of our issued U.S. patents (or patents that may issue from our pending U.S. patent applications) may be eligible for limited patent term extension under the Hatch-Waxman Act.
Trade Secrets
In addition to patents, we rely on trade secrets and know-how to develop and maintain our competitive position. For example, significant aspects of the process by which we manufacture our Arcelis-based drug product candidates are based on unpatented trade secrets and know-how. Trade secrets and know-how can be difficult to protect. We seek to protect our proprietary technology and processes, in part, by confidentiality agreements and invention assignment agreements with our employees, consultants, scientific advisors, contractors and commercial partners. These agreements are designed to protect our proprietary information and, in the case of the invention assignment agreements, to grant us ownership of technologies that are developed through a relationship with a third party. We also seek to preserve the integrity and confidentiality of our data, trade secrets and know-how by maintaining physical security of our premises and physical and electronic security of our information technology systems. Although we have confidence in these individuals, organizations and systems, agreements or security measures may be breached, and we may not have adequate remedies for any breach. In addition, our trade secrets may otherwise become known or be independently discovered by competitors. To the extent that our consultants, contractors or collaborators use intellectual property owned by others in their work for us, disputes may arise as to the rights in related or resulting know-how and inventions.
Key Licenses
We are party to a number of license agreements that are important to our business.
Duke University. Pursuant to a 2000 agreement with Duke University, we hold an exclusive worldwide license to specified patents, patent applications and know-how owned or otherwise controlled by Duke, including for use in the development, manufacture and commercialization of dendritic cells loaded with tumor or pathogen RNA. Under the agreement, we:
| • | issued 36 shares of our common stock to Duke upon execution of the agreement; |
| • | must pay all costs of prosecution and maintenance of the licensed patent rights; |
| • | must pay an annual minimum royalty to Duke beginning with the calendar year beginning the second January 1 after first approval of a licensed product approved by the FDA or a comparable regulatory authority in a foreign country or any sale of a licensed product that does not require regulatory approval; and |
| • | must pay low single-digit percentage royalties, subject to reduction in specified circumstances, to Duke on net sales of licensed products, which are creditable against the annual minimum royalty. |
We are required to use reasonable commercial diligence to research, develop and market licensed products, to develop manufacturing capabilities, and to sublicense those patent rights for applications which we are not pursuing. If we fail to satisfy these obligations and do not cure such failure after receiving written notice from Duke, Duke may terminate the agreement or convert it to a nonexclusive license.
31
Table of Contents
We may terminate our agreement with Duke at any time upon three months’ written notice. The agreement will terminate upon expiration of the last to expire of the patent rights licensed under the agreement. The U.S. patents licensed under the agreement expire in 2016 and the patents licensed under the agreement in Europe and Japan expire in 2017. Either party may terminate the agreement upon written notice for fraud, willful misconduct or illegal conduct of the other party that materially adversely affects the terminating party. If either party fails to fulfill any of its material obligations under the agreement, subject to a cure process specified in the agreement, the non-breaching party may terminate the agreement. A party’s ability to cure a breach will only apply to the first two breaches. In addition, the agreement will terminate if we become insolvent, bankrupt or placed in the hands of a receiver or trustee.
Celldex Therapeutics, Inc. In July 2011, we entered into an agreement with Celldex Therapeutics, Inc., or Celldex, pursuant to which Celldex granted us a non-exclusive license to specified patents and patent applications directed to compositions and methods for processing dendritic cells. Upon the execution of the agreement, we paid Celldex $50,000 of a $100,000 upfront license fee. We paid the balance of this fee on January 31, 2012. Under this agreement, we must pay:
| • | a $75,000 annual license fee; |
| • | a specified milestone payment based on the achievement of a specified regulatory milestone; and |
| • | a specified dollar amount per dose of AGS-003 we sell. |
We may terminate our agreement with Celldex at any time upon notice to Celldex. We or Celldex may terminate the agreement, subject to a cure period specified in the agreement, upon a material breach of the other party by providing written notice and waiting a specified period. The agreement will terminate upon the expiration of the last to expire of the patent rights licensed under the agreement on country-by-country basis. The latest date of expiration of the licensed Celldex patents is 2016.
Development and Commercialization Agreements
An important part of our business strategy is to enter into arrangements with third parties for the development and commercialization of our product candidates.
Pharmstandard. In August 2013, in connection with the purchase of shares of our series E preferred stock by Pharmstandard International S.A., or Pharmstandard, we entered into an exclusive royalty-bearing license agreement with Pharmstandard. Under this license agreement, we granted Pharmstandard and its affiliates a license, with the right to sublicense, to develop, manufacture and commercialize AGS-003 and other products for the treatment of human diseases, which are developed by Pharmstandard using our personalized immunotherapy platform, in the Russian Federation, Armenia, Azerbaijan, Belarus, Georgia, Kazakhstan, Kyrgyzstan, Moldova, Tajikistan, Turkmenistan, Ukraine and Uzbekistan, which we refer to as the Pharmstandard Territory. We also provided Pharmstandard with a right of first negotiation for development and commercialization rights in the Pharmstandard Territory to specified additional products we may develop.
Under the terms of the license agreement, Pharmstandard licensed us rights to clinical data generated by Pharmstandard under the agreement and granted us an option to obtain an exclusive license outside of the Pharmstandard Territory to develop and commercialize improvements to our Arcelis technology generated by Pharmstandard under the agreement, a non-exclusive worldwide royalty-free license to Pharmstandard improvements to manufacture products using our Arcelis technology and a license to specified follow-on licensed products generated by Pharmstandard outside of the Pharmstandard Territory, each on terms to be negotiated upon our request for a license. In addition, Pharmstandard agreed to pay us pass-through royalties on net sales of all licensed products in the low single digits until it has generated a specified amount of aggregate net sales. Once the net sales threshold is achieved, Pharmstandard will pay us royalties on net sales of specified licensed products, including AGS-003, in the low double digits below 20%. These royalty obligations last until the later of the expiration of specified licensed patent rights in a country or the twelfth anniversary of the first commercial sale in such country on a country by country basis and no further royalties on specified other licensed products. After the net sales threshold is achieved, Pharmstandard has the right to offset a portion of the royalties Pharmstandard pays to third parties for licenses to necessary third party intellectual property against the royalties that Pharmstandard pays to us.
The agreement will terminate upon expiration of the royalty term, upon which all licenses will become fully paid up perpetual exclusive licenses. Either party may terminate the agreement for the other party’s uncured material breach or if specified conditions occur relating to the other party’s insolvency or bankruptcy and we may terminate the agreement if Pharmstandard challenges or assists a third party in challenging specified patent rights of ours. If Pharmstandard terminates the agreement upon our material breach or bankruptcy, Pharmstandard is entitled to terminate our licenses to improvements generated by Pharmstandard, upon which we may
32
Table of Contents
come to rely for the development and commercialization of AGS-003 and other licensed products outside of the Pharmstandard Territory, and Pharmstandard is entitled to retain its licenses from us and to pay us substantially reduced royalty payments following such termination.
In November 2013, we entered into an agreement with Pharmstandard under which Pharmstandard purchased additional shares of our series E preferred stock. Under this agreement, we agreed to enter into a manufacturing rights agreement for the European market with Pharmstandard and that the manufacturing rights agreement would provide for the issuance of warrants to Pharmstandard to purchase 499,788 shares of our common stock at an exercise price of $5.82 per share. As of February 28, 2014, we had not entered into this manufacturing rights agreement or issued the warrants.
Green Cross. In July 2013, in connection with the purchase of our series E preferred stock by Green Cross Corp., or Green Cross, we entered into an exclusive royalty-bearing license agreement with Green Cross. Under this agreement we granted Green Cross a license to develop, manufacture and commercialize AGS-003 for mRCC in South Korea. We also provided Green Cross with a right of first negotiation for development and commercialization rights in South Korea to specified additional products we may develop.
Under the terms of the license, Green Cross has agreed to pay us $500,000 upon the initial submission of an application for regulatory approval of a licensed product in South Korea, $500,000 upon the initial regulatory approval of a licensed product in South Korea and royalties ranging from the mid-single digits to low double digits below 20% on net sales until the fifteenth anniversary of the first commercial sale in South Korea. In addition, Green Cross has granted us an exclusive royalty free license to develop and commercialize all Green Cross improvements to our licensed intellectual property in the rest of the world, excluding South Korea, except that, as to such improvements for which Green Cross makes a significant financial investment and that generate significant commercial benefit in the rest of the world, we are required to negotiate in good faith a reasonable royalty that we will be obligated to pay to Green Cross for such license. Under the terms of the agreement, we are required to continue to develop and to use commercially reasonable efforts to obtain regulatory approval for AGS-003 in the United States
The agreement will terminate upon expiration of the royalty term, which is 15 years from the first commercial sale, upon which all licenses will become fully paid up perpetual non-exclusive licenses. Either party may terminate the agreement for the other party’s uncured material breach or if specified conditions occur relating to the other party’s insolvency or bankruptcy and we may terminate the agreement if Green Cross challenges or assists a third party in challenging specified patent rights of ours. If Green Cross terminates the agreement upon our material breach or bankruptcy, Green Cross is entitled to terminate our licenses to improvements and retain its licenses from us and to pay us substantially reduced milestone and royalty payments following such termination.
Medinet. In December 2013, we entered into a license agreement with Medinet. Under this agreement, we granted Medinet an exclusive, royalty-free license to manufacture in Japan AGS-003 and other products using our Arcelis technology solely for the purpose of the development and commercialization of AGS-003 and these other products for the treatment of mRCC. We refer to this license as the manufacturing license. In addition, under this agreement, we granted Medinet an option to acquire a nonexclusive, royalty-bearing license under our Arcelis technology to sell in Japan AGS-003 and other products for the treatment of mRCC. We refer to the option as the sale option and the license as the sale license.
Under the manufacturing license, if Medinet does not exercise the sale option, Medinet may only manufacture AGS-003 and these other products for us or our designee. If Medinet does not exercise the sale option, we and Medinet have agreed to negotiate in good faith a supply agreement under which Medinet would supply us or our designee with AGS-003 and these other products for development and sale for the treatment of mRCC in Japan. If Medinet exercises the sale option, it may only manufacture AGS-003 and these other products for itself, its related parties and its sublicensees. During the term of the manufacturing license, we may not manufacture AGS-003 or these other products for us or any designee for development or sale for the treatment of mRCC in Japan.
Medinet may exercise the option at any time until the earlier of December 31, 2015 and the date 30 days after we have provided Medinet with an interim report on our phase 3 clinical trial of AGS-003 following such time as 50% of the required events in the trial have occurred.
In consideration for the manufacturing license, Medinet paid us $1.0 million. Medinet also loaned us $9.0 million in connection with us entering into the agreement. We have agreed to use these funds in the development and manufacturing of AGS-003 and the other products. Medinet also agreed to pay us milestone payments of up to a total of $9.0 million upon the achievement of developmental and regulatory milestones and $5.0 million upon the achievement of a sales milestone related to AGS-003 and these products. If Medinet exercises the sale option, it will pay us $1.0 million, as well as royalties on net sales at a rate to be negotiated until the later of the expiration of the licensed patent rights in Japan and the twelfth anniversary of the first commercial sale in Japan. If we cannot agree on the royalty rate, we have agreed to submit the matter to arbitration.
33
Table of Contents
We borrowed the $9.0 million pursuant to an unsecured promissory note that bears interest at a rate of 3.0 % per annum. The principal and interest under the note are due and payable on December 31, 2018. Under the terms of the note and the license agreement, any milestone payments related to the developmental and regulatory milestones that become due will be applied first to the repayment of the loan. We have the right to prepay the loan at any time. If we have not repaid the loan by December 31, 2018, then we have agreed to grant to Medinet a non-exclusive, royalty-bearing license to make and sell Arcelis products in Japan for the treatment of cancer. In such event, the amounts owing under the loan as of December 31, 2018 will constitute pre-paid royalties under the license and will not be otherwise due and payable. Royalties under this license would be paid until the expiration of the licensed patent rights in Japan at a rate to be negotiated. If we cannot agree on the royalty rate, we have agreed to submit the matter to arbitration.
Medinet has also granted us a royalty-free, sublicensable, transferable, exclusive license under Medinet improvements to our intellectual property in the rest of the world and for uses other than for mRCC in Japan.
Under the agreement, we have the right to revoke both the manufacturing license and the sale license to be granted to Medinet or the sale license only. If we exercise this right, we will be obligated to make a one-time payment to Medinet calculated based on the nonroyalty payments made to us by Medinet under the agreement, repay the outstanding amount due under the loan and assume certain obligations of Medinet, and Medinet will be obligated to assist us in transitioning the relevant rights in Japan to us or a party that we designate. If we exercise our revocation right with respect to the sale license only, the one-time payment will equal the total amount of nonroyalty payments. If we exercise our revocation right with respect to the manufacturing license and the sale license, the one-time payment will equal 150% or 200% of the nonroyalty payments depending on the timing of the exercise of the revocation right.
The agreement will terminate upon expiration of the royalty term, upon which all licenses will become fully paid up, perpetual non-exclusive licenses. Either party may terminate the agreement for the other party’s uncured material breach or if specified conditions occur relating to the other party’s insolvency or bankruptcy, and we may terminate the agreement if Medinet challenges or assists a third party in challenging specified patent rights of ours. If Medinet terminates the agreement upon our material breach or bankruptcy, Medinet is entitled to terminate our licenses to improvements and retain its royalty-bearing licenses from us.
Kirin. In 2004, we entered into a Collaboration and License Agreement, or the Kirin agreement, with Kirin. We and Kirin agreed to terminate the agreement in 2009. Upon termination, Kirin assigned to us its ownership interest in specified jointly owned patent rights. In return, we must pay Kirin low single-digit percentage royalties on net sales of AGS-003 for the treatment of mRCC and AGS-004 for the treatment of HIV or comparable products beginning with their first commercial sale and continuing for 15 years thereafter.
Government Regulation
Government authorities in the United States, at the federal, state and local level, and in other countries extensively regulate, among other things, the research, development, testing, manufacture, including any manufacturing changes, packaging, storage, recordkeeping, labeling, advertising, promotion, distribution, marketing, import and export of pharmaceutical and biological products such as those we are developing and may market. The processes for obtaining regulatory approvals in the United States and in foreign countries, along with subsequent compliance with applicable statutes and regulations, require the expenditure of substantial time and financial resources.
U.S. Drug and Biological Product Approval Process
In the United States, the FDA regulates drugs and biological products under the Federal Food, Drug, and Cosmetic Act, or FDCA, the Public Health Service Act, or PHSA, and implementing regulations. The process of obtaining regulatory approvals and the subsequent compliance with appropriate federal, state, local and foreign statutes and regulations requires the expenditure of substantial time and financial resources. Failure to comply with the applicable U.S. requirements at any time during the product development process, approval process or after approval, may subject an applicant to a variety of administrative or judicial sanctions, such as the FDA’s refusal to approve pending applications, withdrawal of an approval, imposition of a clinical hold, issuance of warning letters, product recalls, product seizures, total or partial suspension of production or distribution, injunctions, fines, refusals of government contracts, restitution, disgorgement or civil or criminal penalties.
The process required by the FDA before a drug or biological product may be marketed in the United States generally involves the following:
| • | completion of preclinical laboratory tests, animal studies and formulation studies in compliance with the FDA’s good laboratory practice, or GLP, regulations; |
| • | submission to the FDA of an IND which must become effective before human clinical trials may begin; |
34
Table of Contents
| • | approval by an independent institutional review board, or IRB, at each clinical site before each trial may be initiated; |
| • | performance of adequate and well-controlled human clinical trials in accordance with good clinical practices, or GCP, to establish the safety and efficacy of the proposed drug or biological product for each indication; |
| • | submission to the FDA of a new drug application, or NDA, or a BLA; |
| • | satisfactory completion of an FDA advisory committee review, if applicable; |
| • | satisfactory completion of an FDA inspection of the manufacturing facility or facilities at which the product is produced to assess compliance with cGMP, and to assure that the facilities, methods and controls are adequate to preserve the drug’s identity, strength, quality and purity; and |
| • | FDA review and approval of the NDA or BLA. |
Preclinical Studies. Preclinical studies include laboratory evaluation of product chemistry, toxicity and formulation, as well as animal studies to assess its potential safety and efficacy. An IND sponsor must submit the results of the preclinical tests, together with manufacturing information, analytical data and any available clinical data or literature, among other things, to the FDA as part of an IND. Some preclinical testing may continue even after the IND is submitted. An IND automatically becomes effective 30 days after receipt by the FDA, unless before that time the FDA raises concerns or questions related to one or more proposed clinical trials and places the clinical trial on a clinical hold. In such a case, the IND sponsor and the FDA must resolve any outstanding concerns before the clinical trial can begin. As a result, submission of an IND may not result in the FDA allowing clinical trials to commence.
Clinical Trials. Clinical trials involve the administration of the investigational new drug to human subjects under the supervision of qualified investigators in accordance with GCP requirements, which include the requirement that all research subjects provide their informed consent in writing for their participation in any clinical trial. Clinical trials are conducted under protocols detailing, among other things, the objectives of the study, the parameters to be used in monitoring safety and the effectiveness criteria to be evaluated. A protocol for each clinical trial and any subsequent protocol amendments must be submitted to the FDA as part of the IND. In addition, an IRB at each institution participating in the clinical trial must review and approve the plan for any clinical trial before it commences at that institution. Information about certain clinical trials must be submitted within specific timeframes to the NIH for public dissemination on their ClinicalTrials.gov website.
Human clinical trials are typically conducted in three sequential phases, which may overlap or be combined:
| • | Phase 1: The drug or biological product is initially introduced into healthy human subjects or patients with the target disease or condition and tested for safety, dosage tolerance, absorption, metabolism, distribution, excretion and, if possible, to gain an early indication of its effectiveness. |
| • | Phase 2: The drug or biological product is administered to a limited patient population to identify possible adverse effects and safety risks, to preliminarily evaluate the efficacy of the product for specific targeted diseases and to determine dosage tolerance and optimal dosage. |
| • | Phase 3: The drug or biological product is administered to an expanded patient population, generally at geographically dispersed clinical trial sites, in well-controlled clinical trials to generate enough data to statistically evaluate the efficacy and safety of the product for approval, to establish the overall risk-benefit profile of the product, and to provide adequate information for the labeling of the product. |
Progress reports detailing the results of the clinical trials must be submitted at least annually to the FDA and more frequently if serious adverse events occur. Phase 1, phase 2 and phase 3 clinical trials may not be completed successfully within any specified period, or at all. Furthermore, the FDA or the sponsor may suspend or terminate a clinical trial at any time on various grounds, including a finding that the research subjects are being exposed to an unacceptable health risk. Similarly, an IRB can suspend or terminate approval of a clinical trial at its institution if the clinical trial is not being conducted in accordance with the IRB’s requirements or if the drug has been associated with unexpected serious harm to patients.
Special Protocol Assessment. The SPA process is designed to facilitate the FDA’s review and approval of drug and biological products by allowing the FDA to evaluate the proposed design and size of phase 3 clinical trials that are intended to form the primary basis for determining a drug or biological product’s efficacy. Upon specific request by a clinical trial sponsor, the FDA will evaluate the trial protocol and respond to a sponsor’s questions regarding, among other things, primary efficacy endpoints, trial conduct and data analysis, within 45 days of receipt of the request. The FDA ultimately assesses whether the trial protocol design and planned
35
Table of Contents
analysis of the trial adequately address objectives in support of a regulatory submission. All agreements and disagreements between the FDA and the sponsor regarding an SPA must be clearly documented in an SPA letter or the minutes of a meeting between the sponsor and the FDA.
Even if the FDA agrees to the design, execution and analyses proposed in protocols reviewed under an SPA, the FDA may revoke or alter its agreement under the following circumstances:
| • | public health concerns emerge that were unrecognized at the time of the protocol assessment; |
| • | a sponsor fails to follow a protocol that was agreed upon with the FDA; |
| • | the relevant data, assumptions, or information provided by the sponsor in a request for SPA change are found to be false statements or misstatements or are found to omit relevant facts; or |
| • | the FDA and the sponsor agree in writing to modify the trial protocol and such modification is intended to improve the study. |
Marketing Approval. Assuming successful completion of the required clinical testing, the results of the preclinical and clinical studies, together with detailed information relating to the product’s chemistry, manufacture, controls and proposed labeling, among other things, are submitted to the FDA as part of an NDA or BLA requesting approval to market the product for one or more indications. In most cases, the submission of an NDA or BLA is subject to a substantial application user fee.
In addition, under the Pediatric Research Equity Act of 2003, or PREA, as amended and reauthorized, an NDA, BLA or supplement to an NDA or BLA for certain types of new drug or biological products must contain data that are adequate to assess the safety and effectiveness of the drug or biological product for the claimed indications in all relevant pediatric subpopulations, and to support dosing and administration for each pediatric subpopulation for which the product is safe and effective. The FDA may, on its own initiative or at the request of the applicant, grant deferrals for submission of some or all pediatric data until after approval of the product for use in adults, or full or partial waivers from the pediatric data requirements. Unless otherwise required by regulation, the pediatric data requirements do not apply to products with orphan designation.
The Food and Drug Administration Safety and Innovation Act, or FDASIA, amended the FDCA and requires that a sponsor who is planning to submit a marketing application for a drug or biological product that includes a new active ingredient, new indication, new dosage form, new dosing regimen or new route of administration submit an initial Pediatric Study Plan, or PSP, within sixty days of an end-of-phase 2 meeting or as may be agreed between the sponsor and FDA. The initial PSP must include an outline of the pediatric study or studies that the sponsor plans to conduct, including study objectives and design, age groups, relevant endpoints and statistical approach, or a justification for not including such detailed information, and any request for a deferral of pediatric assessments or a full or partial waiver of the requirement to provide data from pediatric studies along with supporting information. FDA and the sponsor must reach agreement on the PSP. A sponsor can submit amendments to an agreed-upon initial PSP at any time if changes to the pediatric plan need to be considered based on data collected from nonclinical studies, early phase clinical trials, or other clinical development programs.
The FDA also could require submission of a risk evaluation and mitigation strategy, or REMS, plan to mitigate any identified or suspected serious risks. The REMS plan could include medication guides, physician communication plans, assessment plans, and elements to assure safe use, such as restricted distribution methods, patient registries, or other risk minimization tools.
The FDA conducts a preliminary review of all NDAs and BLAs within the first 60 days after submission before accepting them for filing to determine whether they are sufficiently complete to permit substantive review. The FDA may request additional information rather than accept an NDA or BLA for filing. In this event, the application must be resubmitted with the additional information. The resubmitted application is also subject to review before the FDA accepts it for filing. Once the submission is accepted for filing, the FDA begins an in-depth substantive review. The FDA reviews an NDA or BLA to determine, among other things, whether the product is safe and effective (described as safe, pure and potent for BLAs) and the facility in which it is manufactured, processed, packaged or held meets standards designed to assure the product’s continued safety, purity and potency. The FDA is required to refer an application for a novel drug or biological product to an advisory committee or explain why such referral was not made. An advisory committee is a panel of independent experts, including clinicians and other scientific experts, that reviews, evaluates and provides a recommendation as to whether the application should be approved and under what conditions. The FDA is not bound by the recommendations of an advisory committee, but it considers such recommendations carefully when making decisions.
36
Table of Contents
Before approving an NDA or BLA, the FDA typically will inspect the facility or facilities where the product is manufactured. The FDA will not approve an application unless it determines that the manufacturing processes and facilities are in compliance with cGMP requirements and adequate to assure consistent production of the product within required specifications. Additionally, before approving an NDA or BLA, the FDA will typically inspect one or more clinical sites to assure compliance with GCP and integrity of the clinical data submitted.
The testing and approval process requires substantial time, effort and financial resources, and each may take several years to complete. Data obtained from clinical activities are not always conclusive and may be susceptible to varying interpretations, which could delay, limit or prevent regulatory approval. The FDA may not grant approval on a timely basis, or at all. We may encounter difficulties or unanticipated costs in our efforts to develop our product candidates and secure necessary governmental approvals, which could delay or preclude us from marketing our products.
If the FDA’s evaluation of the NDA or BLA and inspection of the manufacturing facilities and clinical trial sites are favorable, the FDA may issue an approval letter, or, in some cases, a complete response letter. A complete response letter generally contains a statement of specific conditions that must be met in order to secure final approval of the NDA or BLA and may require additional clinical or preclinical testing in order for FDA to reconsider the application. If and when those conditions have been met to the FDA’s satisfaction, the FDA will typically issue an approval letter. An approval letter authorizes commercial marketing of the drug or biological product with specific prescribing information for specific indications. Even with submission of this additional information, the FDA ultimately may decide that the application does not satisfy the regulatory criteria for approval.
Even if the FDA approves a product, it may limit the approved indications for use for the product, require that contraindications, warnings or precautions be included in the product labeling, require that post-approval studies, including phase 4 clinical trials, be conducted to further assess a drug’s safety after approval, require testing and surveillance programs to monitor the product after commercialization, or impose other conditions, including distribution restrictions or other risk management mechanisms, which can materially affect the potential market and profitability of the product. The FDA may prevent or limit further marketing of a product based on the results of post-marketing studies or surveillance programs. After approval, some types of changes to the approved product, such as adding new indications, manufacturing changes, and additional labeling claims, are subject to further testing requirements and FDA review and approval.
Special FDA Expedited Review and Approval Programs. The FDA has various programs, including fast track designation, accelerated approval and priority review, that are intended to expedite or simplify the process for the development and FDA review of drug and biological products that are intended for the treatment of serious or life threatening diseases or conditions and demonstrate the potential to address unmet medical needs. The purpose of these programs is to provide important new drugs to patients earlier than under standard FDA review procedures.
To be eligible for a fast track designation, the FDA must determine, based on the request of a sponsor, that a product is intended to treat a serious aspect of a serious or life threatening disease or condition and will fill an unmet medical need. The FDA will determine that a product will fill an unmet medical need if it will provide a therapy where none exists or provide a therapy that may be potentially superior to existing therapy based on efficacy or safety factors.
In addition, the FDA may give a priority review designation to drugs or biological products that provide safe and effective therapy where no satisfactory alternative exists or a significant improvement compared to marketed products in the treatment, diagnosis or prevention of a disease. For products regulated by the Center for Biologics Evaluation and Research, or CBER, the product must be intended to treat a serious or life- threatening disease or condition. A priority review means that the targeted time for the FDA to review an application is six months, rather than ten months. Most products that are eligible for fast track designation are also likely to be considered appropriate to receive a priority review.
Under the provisions of the Food and Drug Administration Safety and Innovation Act, or FDASIA, enacted in 2012, a sponsor also can request designation of a product candidate as a “breakthrough therapy.” A breakthrough therapy is defined as a drug or biological product that is intended, alone or in combination with one or more other drugs or biological products, to treat a serious or life-threatening disease or condition, and preliminary clinical evidence indicates that the product may demonstrate substantial improvement over existing therapies on one or more clinically significant endpoints, such as substantial treatment effects observed early in clinical development. Products designated as breakthrough therapies are also eligible for accelerated approval. The FDA must take certain actions, such as holding timely meetings and providing advice, intended to expedite the development and review of an application for approval of a breakthrough therapy.
Even if a product qualifies for one or more of these programs, the FDA may later decide that the product no longer meets the conditions for qualification or decide that the time period for FDA review or approval will not be shortened.
37
Table of Contents
Post-Approval Requirements. Any drug or biological products manufactured or distributed by us pursuant to FDA approvals are subject to pervasive and continuing regulation by the FDA, including, among other things, requirements relating to recordkeeping, periodic reporting, product sampling and distribution, advertising and promotion and reporting of adverse experiences with the product. After approval, most changes to the approved product, such as adding new indications or other labeling claims are subject to prior FDA review and approval. There also are continuing, annual user fee requirements for any marketed products and the establishments at which such products are manufactured, as well as new application fees for supplemental applications with clinical data.
The FDA may impose a number of post-approval requirements as a condition of approval of an NDA or BLA. For example, the FDA may require post-marketing testing, including phase 4 clinical trials, and surveillance to further assess and monitor the product’s safety and effectiveness after commercialization. Regulatory approval of oncology products often requires that patients in clinical trials be followed for long periods to determine the overall survival benefit of the drug or biologic.
In addition, drug manufacturers and other entities involved in the manufacture and distribution of approved drugs and biological products are required to register their establishments with the FDA and state agencies, and are subject to periodic unannounced inspections by the FDA and these state agencies for compliance with cGMP requirements. Changes to the manufacturing process are strictly regulated and often require prior FDA approval before being implemented. FDA regulations also require investigation and correction of any deviations from cGMP and impose reporting and documentation requirements upon us and any third party manufacturers that we may decide to use. Accordingly, manufacturers must continue to expend time, money, and effort in the area of production and quality control to maintain cGMP compliance.
Once an approval is granted, the FDA may withdraw the approval if compliance with regulatory requirements and standards is not maintained or if problems occur after the product reaches the market. Later discovery of previously unknown problems with a product, including adverse events of unanticipated severity or frequency, or with manufacturing processes, or failure to comply with regulatory requirements, may result in revisions to the approved labeling to add new safety information; imposition of post-market studies or clinical trials to assess new safety risks; or imposition of distribution or other restrictions under a REMS program. Other potential consequences include, among other things:
| • | restrictions on the marketing or manufacturing of the product, complete withdrawal of the product from the market or product recalls; |
| • | fines, warning letters or holds on post-approval clinical trials; |
| • | refusal of the FDA to approve pending applications or supplements to approved applications, or suspension or revocation of product license approvals; |
| • | product seizure or detention, or refusal to permit the import or export of products; or |
| • | injunctions or the imposition of civil or criminal penalties. |
The FDA strictly regulates marketing, labeling, advertising and promotion of products that are placed on the market. Drugs may be promoted only for the approved indications and in accordance with the provisions of the approved label. The FDA and other agencies actively enforce the laws and regulations prohibiting the promotion of off label uses, and a company that is found to have improperly promoted off label uses may be subject to significant liability.
In addition, the distribution of prescription pharmaceutical and biological products is subject to the Prescription Drug Marketing Act, or PDMA, which regulates the distribution of drugs and drug samples at the federal level, and sets minimum standards for the registration and regulation of drug distributors by the states. Both the PDMA and state laws limit the distribution of prescription pharmaceutical product samples and impose requirements to ensure accountability in distribution.
Exclusivity and Approval of Competing Products
Non-Patent Exclusivity. Under the Patient Protection and Affordable Care Act, or PPACA, newly- approved biological products may benefit from statutory periods of non-patent data and marketing exclusivity. The PPACA, among other things, permits the FDA to approve biosimilar or interchangeable versions of biological products through an abbreviated approval pathway following periods of data and marketing exclusivity. Biological products that are considered to be “reference products” are granted two overlapping periods of data and marketing exclusivity: a four-year period during which no abbreviated biologics license application, or abbreviated BLA, relying upon the reference product may be submitted to the FDA, and a twelve-year period during which no abbreviated BLA relying upon the reference product may be approved by FDA. For purposes of the PPACA, a reference product is defined as the single biological product licensed under a full BLA against which a biological product is evaluated in an application submitted under an abbreviated BLA.
38
Table of Contents
We believe that our investigational products, if approved via full BLAs, will be considered “reference products” that are entitled to both four-year and twelve-year exclusivity under the PPACA. The FDA, however, has not issued any regulations or final guidance explaining how it will implement the PPACA, including the exclusivity provisions for reference products. In February 2012, the FDA issued three draft guidance documents that provide its preliminary thoughts on how to interpret and implement the abbreviated BLA provisions of the PPACA. The FDA has requested public comments on these draft guidance documents, including the proper interpretation of PPACA exclusivity provisions. It is thus possible that the FDA will decide to interpret the PPACA in such a way that our products are not considered to be reference products for purposes of the PPACA or be entitled to any period of data or marketing exclusivity. Even if our products are considered to be reference products and obtain exclusivity under the PPACA, another company nevertheless could also market a competing version of any of our biological products if such company can complete, and the FDA permits the submission of and approves, a full BLA. Although protection under PPACA will not prevent the submission or approval of another “full” BLA, the applicant would be required to conduct its own preclinical and adequate and well-controlled clinical trials to demonstrate safety, purity, and potency (i.e., effectiveness).
Pediatric Exclusivity. Pediatric exclusivity is another type of non-patent marketing exclusivity in the United States and, if granted, provides for the attachment of an additional six months of marketing protection to the term of any existing regulatory exclusivity, including the four- and 12-year non-patent exclusivity periods described above. This six-month exclusivity may be granted based on the voluntary completion of a pediatric study or studies in accordance with an FDA-issued “Written Request” for such a study or studies.
Orphan Drug Designation and Exclusivity. Under the Orphan Drug Act, the FDA may grant orphan drug designation to a drug (including a biologic) intended to treat a rare disease or condition, which is generally a disease or condition that affects fewer than 200,000 individuals in the United States, or more than 200,000 individuals in the United States and for which there is no reasonable expectation that the cost of developing and making available in the United States a drug for this type of disease or condition will be recovered from sales in the United States for that drug. Orphan drug designation must be requested before submitting an NDA or BLA. After the FDA grants orphan drug designation, the identity of the therapeutic agent and its potential orphan use are disclosed publicly by the FDA.
If a product that has orphan drug designation subsequently receives the first FDA approval for the disease for which it has such designation, the product is entitled to orphan product exclusivity, which means that the FDA may not approve any other applications, including a full NDA or full BLA, to market the same drug for the same indication for seven years. For purposes of small molecule drugs, the FDA defines “same drug” as a drug that contains the same active moiety and is intended for the same use as the previously approved orphan drug. For purposes of large molecule drugs, the FDA defines “same drug” as a drug that contains the same principal molecular structural features, but not necessarily all of the same structural features, and is intended for the same use as the drug in question. Notwithstanding the above definitions, a drug that is clinically superior to an orphan drug will not be considered the “same drug” and thus will not be blocked by orphan drug exclusivity.
A designated orphan drug may not receive orphan drug exclusivity if it is approved for a use that is broader than the indication for which it received orphan designation. In addition, orphan drug exclusive marketing rights in the United States may be lost if the FDA later determines that the request for designation was materially defective or if the manufacturer is unable to assure sufficient quantities of the drug to meet the needs of patients with the rare disease or condition.
The FDA also administers a clinical research grants program, whereby researchers may compete for funding to conduct clinical trials to support the approval of drugs, biologics, medical devices, and medical foods for rare diseases and conditions. An application for an orphan grant should propose one discrete clinical trial to facilitate FDA approval of the product for a rare disease or condition. The study may address an unapproved new product or an unapproved new use for a product already on the market.
Foreign Regulation
Although we do not currently market any of our products outside the United States and have no current plans to engage in product commercialization outside the United States, we may decide to do so in the future. In order to market any product outside of the United States, we would need to comply with numerous and varying regulatory requirements of other countries regarding safety and efficacy and governing, among other things, clinical trials, marketing authorization, commercial sales and distribution of our products. Whether or not we obtain FDA approval for a product, we would need to obtain the necessary approvals by the comparable regulatory authorities of foreign countries before we can commence clinical trials or marketing of the product in those countries. The approval process varies from country to country and can involve additional product testing and additional administrative review periods, and may be otherwise complicated by some of our products and product candidates being controlled substances. The time required to obtain approval in other countries might differ from and be longer than that required to obtain FDA approval. Regulatory approval in one country does not ensure regulatory approval in another, but a failure or delay in obtaining regulatory approval in one country may negatively impact the regulatory process in others.
39
Table of Contents
To date, we have not initiated any discussions with the European Medicines Agency, or EMEA, or any other foreign regulatory authorities with respect to seeking regulatory approval for any of our products in Europe or in any other country outside the United States.
Pharmaceutical Coverage, Pricing and Reimbursement
Significant uncertainty exists as to the coverage and reimbursement status of any drug products for which we obtain regulatory approval. Sales of any of our product candidates, if approved, will depend, in part, on the extent to which the costs of the products will be covered by third party payors, including government health programs such as Medicare and Medicaid, commercial health insurers and managed care organizations. The process for determining whether a payor will provide coverage for a drug product may be separate from the process for setting the price or reimbursement rate that the payor will pay for the drug product once coverage is approved. Third party payors may limit coverage to specific drug products on an approved list, or formulary, which might not include all of the approved drugs for a particular indication.
In order to secure coverage and reimbursement for any product that might be approved for sale, we may need to conduct expensive pharmacoeconomic studies in order to demonstrate the medical necessity and cost- effectiveness of the product, in addition to the costs required to obtain FDA or other comparable regulatory approvals. Our product candidates may not be considered medically necessary or cost-effective. A payor’s decision to provide coverage for a drug product does not imply that an adequate reimbursement rate will be approved. Third party reimbursement may not be sufficient to enable us to maintain price levels high enough to realize an appropriate return on our investment in product development.
The containment of healthcare costs has become a priority of federal, state and foreign governments, and the prices of drugs have been a focus in this effort. Third party payors are increasingly challenging the prices charged for medical products and services and examining the medical necessity and cost-effectiveness of medical products and services, in addition to their safety and efficacy. If these third party payors do not consider our products to be cost-effective compared to other available therapies, they may not cover our products after approval as a benefit under their plans or, if they do, the level of payment may not be sufficient to allow us to sell our products at an appropriate return on investment. The U.S. government, state legislatures and foreign governments have shown significant interest in implementing cost-containment programs to limit the growth of government-paid health care costs, including price controls, restrictions on reimbursement and requirements for substitution of generic products for branded prescription drugs. Adoption of such controls and measures, and tightening of restrictive policies in jurisdictions with existing controls and measures, could limit payments for pharmaceuticals such as the drug product candidates that we are developing and could adversely affect our net revenue and results.
Pricing and reimbursement schemes vary widely from country to country. Some countries provide that drug products may be marketed only after a reimbursement price has been agreed. Some countries may require the completion of additional studies that compare the cost-effectiveness of a particular product candidate to currently available therapies. For example, the European Union provides options for its member states to restrict the range of drug products for which their national health insurance systems provide reimbursement and to control the prices of medicinal products for human use. European Union member states may approve a specific price for a drug product or it may instead adopt a system of direct or indirect controls on the profitability of the company placing the drug product on the market. Other member states allow companies to fix their own prices for drug products, but monitor and control company profits. The downward pressure on health care costs in general, particularly prescription drugs, has become intense. As a result, increasingly high barriers are being erected to the entry of new products. In addition, in some countries, cross-border imports from low-priced markets exert competitive pressure that may reduce pricing within a country. There can be no assurance that any country that has price controls or reimbursement limitations for drug products will allow favorable reimbursement and pricing arrangements for any of our products.
The marketability of any products for which we receive regulatory approval for commercial sale may suffer if the government and third party payors fail to provide adequate coverage and reimbursement. In addition, emphasis on managed care in the United States has increased and we expect will continue to increase the pressure on drug pricing. Coverage policies, third party reimbursement rates and drug pricing regulation may change at any time. In particular, the PPACA and a related reconciliation bill, which we collectively refer to as the Affordable Care Act or ACA, contain provisions that may reduce the profitability of drug products, including, for example, increased rebates for covered outpatient drugs sold to Medicaid programs, extension of Medicaid rebates to Medicaid managed care plans, mandatory discounts for certain Medicare Part D beneficiaries, and annual fees based on pharmaceutical companies’ share of sales to federal health care programs. Even if favorable coverage and reimbursement status is attained for one or more products for which we receive regulatory approval, less favorable coverage policies and reimbursement rates may be implemented in the future.
40
Table of Contents
New Legislation and Regulations
From time to time, legislation is drafted, introduced and passed in Congress that could significantly change the statutory provisions governing the testing, approval, manufacturing and marketing of products regulated by the FDA. For example, the FDASIA and PPACA provisions discussed above were enacted in 2012 and 2010, respectively. In addition to new legislation, FDA regulations and policies are often revised or interpreted by the agency in ways that may significantly affect our business and our products. It is impossible to predict whether further legislative changes will be enacted, or FDA regulations, guidance, policies or interpretations changed or what the impact of such changes, if any, may be.
Segment and Geographic Information
Operating segments are defined as components of an enterprise engaging in business activities from which it may earn revenues and incur expenses, for which discrete financial information is available and whose operating results are regularly reviewed by the chief operating decision maker in deciding how to allocate resources and in assessing performance. We view our operations and manage our business in one operating segment and all of our operations are in North America.
Employees
As of February 28, 2014, we had 92 employees, including 15 in research and development, 10 in clinical development, 53 in manufacturing and 14 in general and administrative functions. None of our employees is subject to a collective bargaining agreement or represented by a labor or trade union. We believe that our relations with our employees are good.
Corporate Information
We were incorporated in the State of Delaware on May 8, 1997. Our principal executive offices are located at 4233 Technology Drive, Durham, North Carolina 27704, and our telephone number is (919) 287-6300.
Available Information
We file with the Securities and Exchange Commission, or SEC, annual reports on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K, proxy and information statements and amendments to reports filed or furnished pursuant to Sections 13(a), 14 and 15(d) of the Securities Exchange Act of 1934, as amended. The public may obtain these filings at the SEC’s Public Reference Room at 100 F Street, NE, Washington, DC 20549 or by calling the SEC at 1-800-SEC-0330. The SEC also maintains a website at http://www.sec.gov that contains reports, proxy and information statements and other information regarding Argos and other companies that file materials with the SEC electronically. Copies of our reports on Form 10-K, Forms 10-Q and Forms 8-K may also be obtained, free of charge, electronically through the investor relations portion of our web site, www.argostherapeutics.com/investor-relations/sec-filings/default.aspx.
We webcast our earnings calls on our investor relations website. Additionally, we provide notifications of news or announcements regarding our financial performance, including SEC filings, investor events and press and earnings releases, on the investor relations portion of our website. Further corporate governance information, including our corporate governance guidelines, board committee charters, Code of Business Conduct and Ethics that applies to our directors, officers and employees, including our principal executive officer, principal financial officer, or persons performing similar functions, is also available on our investor relations website under the heading “Corporate Governance.” The contents of our website are not intended to be incorporated by reference into this Annual Report on Form 10-K or in any other report or document we file with the SEC.
We operate in a dynamic and rapidly changing business environment that involves multiple risks and substantial uncertainty. The following discussion addresses risks and uncertainties that could cause, or contribute to causing, actual results to differ from expectations in material ways. In evaluating our business, investors should pay particular attention to the risks and uncertainties described below and in other sections of this Annual Report on Form 10-K and in our subsequent filings with the SEC. These risks and uncertainties, or other events that we do not currently anticipate or that we currently deem immaterial also may affect our results of operations, cash flows and financial condition. The trading price of our common stock could also decline due to any of these risks, and you could lose all or part of your investment.
Risks Related to the Development and Regulatory Approval of Our Product Candidates
We depend heavily on the success of our two product candidates, AGS-003 and AGS-004, both of which are still in clinical development. Clinical trials of our product candidates may not be successful. If we are unable to commercialize our product candidates or experience significant delays in doing so, our business will be materially harmed.
We currently have no products approved for sale. We have invested a significant portion of our efforts and financial resources in the development of AGS-003 for the treatment of metastatic renal cell carcinoma, or mRCC, and other cancers and AGS-004 for the treatment of HIV. Our ability to generate product revenues, which we do not expect will occur for at least the next several years, if ever, will depend heavily on the successful development and eventual commercialization of these product candidates. The success of these product candidates will depend on several factors, including the following:
| • | successful completion of clinical trials, including clinical results that are statistically significant as well as clinically meaningful in the context of the indications for which we are developing our product candidates; |
| • | receipt of marketing approvals from the FDA and similar regulatory authorities outside the United States; |
41
Table of Contents
| • | establishing commercial manufacturing capabilities by identifying, leasing, building out and equipping a commercial manufacturing facility for our Arcelis-based product candidates; |
| • | maintaining patent and trade secret protection and regulatory exclusivity for our product candidates, both in the United States and internationally; |
| • | launching commercial sales of the products, if and when approved, whether alone or in collaboration with others; |
| • | commercial acceptance of our products, if and when approved, by patients, the medical community and third party payors; |
| • | obtaining and maintaining healthcare coverage and adequate reimbursement; |
| • | effectively competing with other therapies; and |
| • | a continued acceptable safety profile of the products following any marketing approval. |
If we do not achieve one or more of these factors in a timely manner or at all, we could experience significant delays or an inability to successfully commercialize our product candidates, which would materially harm our business.
If clinical trials of our product candidates, such as our ongoing pivotal phase 3 clinical trial of AGS-003 and our ongoing phase 2b clinical trial of AGS-004, fail to demonstrate safety and efficacy to the satisfaction of the FDA or similar regulatory authorities outside the United States or do not otherwise produce positive results, we may incur additional costs or experience delays in completing, or ultimately be unable to complete, the development and commercialization of our product candidates.
Before obtaining regulatory approval for the sale of our product candidates, we must conduct extensive clinical trials to demonstrate the safety and efficacy of our product candidates in humans. Clinical testing is expensive, difficult to design and implement, can take many years to complete and is uncertain as to outcome. A failure of one or more of our clinical trials can occur at any stage of testing. The outcome of preclinical testing and early clinical trials may not be predictive of the success of later clinical trials, and interim results of a clinical trial do not necessarily predict final results. Moreover, preclinical and clinical data are often susceptible to varying interpretations and analyses, and many companies that have believed their product candidates performed satisfactorily in preclinical studies and clinical trials have nonetheless failed to obtain marketing approval of their products.
In particular, to date, we have not completed a clinical trial of AGS-003 against a placebo or a comparator therapy. While we believe comparisons to results from other reported clinical trials or from analyses of data from the International Metastatic Renal Cell Carcinoma Database Consortium, or the Consortium, can assist in evaluating the potential efficacy of our AGS-003 product candidate, there are many factors that affect the outcome for patients, some of which are not apparent in published reports. As a result, results from two different trials or between a trial and an analysis of a treatment database often cannot be reliably compared. Our ongoing pivotal phase 3 clinical trial of AGS-003 is intended to compare directly the combination of AGS-003 and sunitinib to treatment with sunitinib monotherapy. Based on the design of the trial, the data from the trial will need to demonstrate an increase of approximately six months in median overall survival for the AGS-003 plus sunitinib arm as compared to the sunitinib monotherapy control arm in order to show statistical significance and achieve the primary endpoint of the trial. We will need to show this statistically significant benefit of the combined therapy as compared to treatment with the sunitinib monotherapy as part of a submission for approval of AGS-003. However, demonstration of statistical significance and achievement of the primary endpoint of the trial do not assure approval by the FDA or similar regulatory authorities outside the United States.
Patients in our ongoing pivotal phase 3 clinical trial who receive treatment with sunitinib monotherapy may not have results similar to patients studied in other clinical trials of sunitinib or to patients in the Consortium database. If the patients in our ongoing pivotal phase 3 clinical trial who receive sunitinib plus placebo have results which are better than the results that occurred in those other clinical trials or the results described in the Consortium database, we may not demonstrate a sufficient benefit from AGS-003 in combination with sunitinib to allow the FDA to approve AGS-003 for marketing. In addition, only 21 patients received the combination of AGS-003 and sunitinib in our phase 2 clinical trial. If the patients in our ongoing pivotal phase 3 clinical trial who receive the combination of AGS-003 and sunitinib have results which are worse than the results that occurred in our phase 2 clinical trial, we may not demonstrate a sufficient benefit from the combination therapy to allow the FDA to approve AGS-003 for marketing.
For drug and biological products, the FDA typically requires the successful completion of two adequate and well-controlled clinical trials to support marketing approval because a conclusion based on two such trials will be more reliable than a conclusion based on a single trial. In the case of AGS-003, which is intended for a life-threatening disease, we intend to seek approval based upon the results of a single pivotal phase 3 clinical trial. The FDA reviewed our plans to conduct a single pivotal phase 3 clinical trial under its SPA process. In February 2013, the FDA advised us in a letter that it had completed its review of our plans under the SPA process. The
42
Table of Contents
FDA also informed us that in order for a single trial to support approval of an indication, the trial must be well conducted, and the results of the trial must be internally consistent, clinically meaningful and statistically very persuasive. If the results for the primary endpoint are not robust, are subject to confounding factors, or are not adequately supported by other study endpoints, the FDA may refuse to approve our BLA based upon a single clinical trial. In addition, because only 21 patients received the combination of AGS-003 and sunitinib in our phase 2 clinical trial, and as a result, we did not have enough evaluable patients to perform the statistical analysis to determine whether the primary endpoint of complete response rate was achieved in that trial, we expect that the data from our phase 2 clinical trial will have only a limited impact on the FDA’s ultimate assessment of efficacy of AGS-003. Thus, there can be no guarantee that the FDA will not require additional pivotal clinical trials as a condition for approving AGS-003.
If we are required to conduct additional clinical trials or other testing of our product candidates beyond those that we currently contemplate, if we are unable to successfully complete clinical trials of our product candidates or other testing, if the results of these trials or tests are not positive or are only modestly positive or if there are safety concerns, we may:
| • | be delayed in obtaining marketing approval for our product candidates; |
| • | not obtain marketing approval at all; |
| • | obtain approval for indications or patient populations that are not as broad as intended or desired; |
| • | obtain approval with labeling that includes significant use restrictions or safety warnings, including boxed warnings; |
| • | be subject to additional post-marketing testing requirements; |
| • | be subject to restrictions on how the product is distributed or used; or |
| • | have the product removed from the market after obtaining marketing approval. |
If we experience any of a number of possible unforeseen events in connection with our clinical trials, potential marketing or commercialization of our product candidates could be delayed or prevented.
We may experience numerous unforeseen events during, or as a result of, clinical trials that could delay or prevent our ability to receive regulatory approval or commercialize our product candidates. For example, in September 2011, the FDA placed the original protocol for our ongoing pivotal phase 3 clinical trial of AGS-003 in combination with sunitinib on partial clinical hold due to unresolved questions regarding the planned measurement of the secretion of the cytokine interleukin-12, or IL-12, as part of the specifications for the release of AGS-003. We subsequently reached an agreement with the FDA regarding the IL-12 release specifications and the FDA lifted the partial clinical hold. Unforeseen events that could delay or prevent our ability to receive regulatory approval or commercialize our product candidates include:
| • | regulators or institutional review boards may not authorize us or our investigators to commence a clinical trial or conduct a clinical trial at a prospective trial site; |
| • | we may have delays in reaching or fail to reach agreement on acceptable clinical trial contracts or clinical trial protocols with prospective trial sites; |
| • | clinical trials of our product candidates may produce negative or inconclusive results, and we may decide, or regulators may require us, to conduct additional clinical trials or abandon product development programs; |
| • | the number of patients required for clinical trials of our product candidates may be larger than we anticipate, enrollment in these clinical trials may be slower than we anticipate or participants may drop out of these clinical trials at a higher rate than we anticipate; for example, in our phase 2b clinical trial of AGS-004, we experienced a higher dropout rate than we anticipated due to the higher than expected number of patients who did not complete the full 12 week antiretroviral treatment interruption required by the protocol for the trial; |
| • | our third party contractors may fail to comply with regulatory requirements or meet their contractual obligations to us in a timely manner, or at all; |
| • | we may decide, or regulators or institutional review boards may require us or our investigators to, suspend or terminate clinical research for various reasons, including noncompliance with regulatory requirements or a finding that the participants are being exposed to unacceptable health risks; |
43
Table of Contents
| • | the cost of clinical trials of our product candidates may be greater than we anticipate; and |
| • | the supply or quality of our product candidates or other materials necessary to conduct clinical trials of our product candidates may be insufficient or inadequate. |
In addition, the patients recruited for clinical trials of our product candidates may have a disease profile or other characteristics that are different than we expect and different than the clinical trials were designed for, which could adversely impact the results of the clinical trials. For instance, our phase 2 combination therapy clinical trial of AGS-003 in combination with sunitinib was originally designed to enroll patients with favorable disease risk profiles and intermediate disease risk profiles and with a primary endpoint of complete response rate. However, the actual trial population consisted entirely of patients with intermediate disease risk profiles and poor disease risk profiles. This is a population for which published research has shown that sunitinib alone, as well as other of the targeted therapies for mRCC, rarely if ever produce complete responses in mRCC, and in our phase 2 clinical trial in this population the combination therapy of AGS-003 and sunitinib did not show a complete response rate that met the endpoint of the trial.
Our product development costs will also increase if we experience delays in testing or obtaining marketing approvals. We do not know whether any clinical trials will begin as planned, will need to be restructured or will be completed on schedule, or at all. For example, in response to our submission of an investigational new drug application, or IND, for AGS-004, the FDA raised safety concerns regarding the analytical treatment interruption contemplated by our protocol for our phase 2 clinical trial of AGS-004, and required a one year safety follow-up after the final dose for each patient. This resulted in the need for an amendment to the trial protocol and a four month delay prior to initiating the phase 2 clinical trial in the United States. In addition to additional costs, significant clinical trial delays also could shorten any periods during which we may have the exclusive right to commercialize our product candidates or allow our competitors to bring products to market before we do and impair our ability to commercialize our product candidates and may harm our business and results of operations.
The FDA has reviewed the protocol for our ongoing pivotal phase 3 clinical trial of AGS-003 in combination with sunitinib under the SPA process. However, agreement by the FDA with the protocol under the SPA process does not guarantee that the trial will be successful or that, if successful, AGS-003 will receive marketing approval.
The FDA has reviewed, under the SPA process, the protocol for our ongoing phase 3 clinical trial of AGS-003 in combination with sunitinib. The SPA process is designed to facilitate the FDA’s review and approval of drug and biological products by allowing the FDA to evaluate the proposed design and size of phase 3 clinical trials that are intended to form the primary basis for determining a drug candidate’s efficacy. In February 2012, we received a letter from the FDA advising us that the FDA had completed its review of our protocol for the pivotal phase 3 clinical trial under the SPA process. In the letter, the FDA stated that it had determined that the protocol sufficiently addressed the trial’s objectives and that the trial was adequately designed to provide the necessary data to support a submission for marketing approval.
An SPA does not guarantee that AGS-003 will receive marketing approval. The FDA may raise issues related to safety, study conduct, bias, deviation from the protocol, statistical power, patient completion rates, changes in scientific or medical parameters or internal inconsistencies in the data prior to making its final decision. The FDA may also seek the guidance of an outside advisory committee prior to making its final decision. In addition, the combination of AGS-003 and sunitinib may not achieve the primary endpoint of the trial. Even if the primary endpoint in our pivotal phase 3 clinical trial is achieved, AGS-003 may not be approved. Many companies which have been granted SPAs have ultimately failed to obtain final approval to market their products.
In its February 2012 letter, the FDA informed us that in order for a single trial to support approval of an indication, the trial must be well conducted, and the results of the trial must be internally consistent, clinically meaningful and statistically very persuasive. If the results for the primary endpoint are not robust, are subject to confounding factors, or are not adequately supported by other study endpoints, the FDA may refuse to approve our BLA based upon a single clinical trial. There can be no guarantee that the FDA will not require additional pivotal clinical trials as a condition for approving AGS-003.
If we experience delays or difficulties in the enrollment of patients in our clinical trials, our receipt of necessary regulatory approvals could be delayed or prevented.
We may not be able to initiate or continue clinical trials for our product candidates, including our pivotal phase 3 clinical trial of AGS-003, if we are unable to locate and enroll a sufficient number of eligible patients to participate in these trials as required by the FDA or similar regulatory authorities outside the United States. Our competitors may have ongoing clinical trials for product candidates that could be competitive with our product candidates, and patients who would otherwise be eligible for our clinical trials may instead enroll in clinical trials of our competitors’ product candidates. For example, during the phase 1/2 monotherapy clinical trial of AGS-003 that we conducted, our ability to enroll patients in the trial was adversely affected by the FDA’s approval of sorafenib and sunitinib, because patients did not want to receive, and physicians were reluctant to administer, AGS-003 as a monotherapy once new therapies that showed efficacy in clinical trials were introduced to the market and became widely available.
44
Table of Contents
Patient enrollment is affected by other factors including:
| • | severity of the disease under investigation; |
| • | eligibility criteria for the study in question; |
| • | perceived risks and benefits of the product candidate under study; |
| • | efforts to facilitate timely enrollment in clinical trials; |
| • | patient referral practices of physicians; |
| • | the ability to monitor patients adequately during and after treatment; and |
| • | proximity and availability of clinical trial sites for prospective patients. |
Based on the rate of enrollment in our ongoing pivotal phase 3 clinical trial of AGS-003, we expect to complete enrollment in the trial by the end of 2014. However, the actual amount of time for full enrollment could be longer than planned. Enrollment delays in this phase 3 clinical trial or any of our other clinical trials may result in increased development costs for our product candidates, which would cause the value of the company to decline and limit our ability to obtain additional financing. Our inability to enroll a sufficient number of patients for this ongoing phase 3 clinical trial or any of our other clinical trials would result in significant delays or may require us to abandon one or more clinical trials altogether. In addition, any significant delays or increases in costs of our ongoing phase 3 clinical trial of AGS-003 could result in the need for us to obtain additional funding to complete the trial.
We are developing AGS-004 for use with latency reversing drugs to eradicate HIV. If latency reversing drugs are not successfully developed for HIV on a timely basis or at all, we will be unable to develop AGS-004 for this use or will be delayed in doing so. In addition, because there are currently no products approved for HIV eradication, we cannot be certain of the clinical trials we will need to conduct or the regulatory requirements that we will need to satisfy in order to obtain marketing approval of AGS-004 for this purpose.
We are focusing our development program for AGS-004 on the use of AGS-004 in combination with latency reversing drugs to eradicate HIV. We rely on these latency reversing drugs because we recognize that the ultimate objective of virus eradication is unlikely to be achieved with immunotherapy alone because the immune system is not able to recognize the HIV virus in latently infected cells with a low level or lack of expression of HIV antigens.
Several companies and academic groups are evaluating latency reversing drugs that can potentially activate latently infected cells to increase viral antigen expression and make the cells vulnerable to elimination by the immune system. We are not a party to any arrangements with these companies or academic groups. If these companies or academic groups determine not to develop latency reversing drugs for this purpose because the drugs do not sufficiently increase viral antigen expression or have unacceptable toxicities, or these companies or academic groups otherwise determine to collaborate with other developers of immunotherapies on a combination therapy for complete virus eradication, we will not be able to complete our AGS-004 development program. In addition, if these companies or academic groups do not proceed with such development on a timely basis, our AGS-004 program correspondingly would be delayed.
A number of the latency reversing drugs being evaluated for use in HIV patients are currently approved in the United States and elsewhere for use in the treatment of specified cancer indications. If these drugs are not approved by the FDA or equivalent foreign regulatory authorities for use in HIV, the FDA and these other regulatory authorities may not approve AGS-004 without the latency reversing drug having received marketing approval for HIV. If the FDA and these other regulatory authorities approve AGS-004 without the approval of the latency reversing drug for HIV, the use of AGS-004 in combination with the latency reversing drug for virus eradication would require sales of the latency reversing drug for off-label use. In such event, the success of the combination of AGS-004 and the latency reversing drug would be subject to the willingness of physicians, patients, healthcare payors and others in the medical community to use the latency reversing drug for off-label use and of government authorities and third party payors to pay for the combination therapy. In addition, we would be limited in our ability to market the combination for its intended use if the latency reversing drug were to be used off-label.
Currently, there are no products approved for the eradication of HIV. As a result, we cannot be certain as to the clinical trials we will need to conduct or the regulatory requirements that we will need to satisfy in order to obtain marketing approval of AGS-004 for the eradication of HIV.
45
Table of Contents
If serious adverse or inappropriate side effects are identified during the development of our product candidates, we may need to abandon or limit our development of some of our product candidates.
All of our product candidates are still in preclinical or clinical development and their risk of failure is high. It is impossible to predict when or if any of our product candidates will prove effective or safe in humans or will receive regulatory approval. If our product candidates are associated with undesirable side effects or have characteristics that are unexpected, we may need to abandon their development or limit development to certain uses or subpopulations in which the undesirable side effects or other characteristics are less prevalent, less severe or more acceptable from a risk-benefit perspective. In addition, such effects or characteristics could cause an institutional review board or regulatory authorities to interrupt, delay or halt clinical trials of one or more of our product candidates, require us to conduct additional clinical trials or other tests or studies, and could result in a more restrictive label, or the delay or denial of marketing approval by the FDA or comparable foreign regulatory authorities.
Our Arcelis-based product candidates are immunotherapies that are based on a novel technology utilizing a patient’s own tissue. This may raise development issues that we may not be able to resolve, regulatory issues that could delay or prevent approval or personnel issues that may prevent us from further developing and commercializing our product candidates.
AGS-003 and AGS-004 are based on our novel Arcelis technology platform. In the course of developing this platform and these product candidates, we have encountered difficulties in the development process. For example, we terminated the development of MB-002, the predecessor to AGS-003, when the results from the initial clinical trial of MB-002 indicated that the product candidate only corrected defects in the production of one of two critical cytokines required for effective immune response. There can be no assurance that additional development problems will not arise in the future which we may not be able to resolve or which may cause significant delays in development.
In addition, regulatory approval of novel product candidates such as our Arcelis-based product candidates manufactured using novel manufacturing processes such as ours can be more expensive and take longer than for other, more well-known or extensively studied pharmaceutical or biopharmaceutical products, due to our and regulatory agencies’ lack of experience with them. The FDA has only approved one personalized immunotherapy product to date. This lack of experience may lengthen the regulatory review process, require us to conduct additional studies or clinical trials, increase our development costs, lead to changes in regulatory positions and interpretations, delay or prevent approval and commercialization of these product candidates or lead to significant post-approval limitations or restrictions.
The novel nature of our product candidates also means that fewer people are trained in or experienced with product candidates of this type, which may make it difficult to find, hire and retain capable personnel for research, development and manufacturing positions.
Development of our Arcelis-based product candidates is subject to significant uncertainty because each product candidate is derived from source material that is inherently variable. This variability could reduce the effectiveness of our Arcelis-based product candidates, delay any FDA approval of our Arcelis-based product candidates, cause us to change our manufacturing methods and adversely affect the commercial success of any approved Arcelis-based products.
The disease samples from the patients to be treated with our Arcelis-based products vary from patient to patient. This inherent variability may adversely affect our ability to manufacture our products because each tumor or virus sample that we receive and process will yield a different product. As a result, we may not be able to consistently produce a product for every patient and we may not be able to treat all patients effectively. Such inconsistency could delay FDA or other regulatory approval of our Arcelis-based product candidates or if approved, adversely affect market acceptance and use of our Arcelis-based products. If we have to change our manufacturing methods to address any inconsistency, we may have to perform additional clinical trials, which would delay FDA or other regulatory approval of our Arcelis-based product candidates and increase the costs of development of our Arcelis-based product candidates.
The inherent variability of the disease samples from the patients to be treated with our Arcelis-based products may further adversely affect our ability to manufacture our products because variability in the source material for our product candidates, such as tumor cells or viruses, may cause variability in the composition of other cells in our product candidates. Such variability in composition or purity could adversely affect our ability to establish acceptable release specifications and the development and regulatory approval processes for our product candidates may be delayed, which would increase the costs of development of our Arcelis-based product candidates.
If we are not able to obtain, or if there are delays in obtaining, required regulatory approvals, we will not be able to commercialize our product candidates, and our ability to generate revenue will be materially impaired.
Failure to obtain regulatory approval for either of our product candidates will prevent us from commercializing the product candidate. We have not received regulatory approval to market any of our product candidates in any jurisdiction. We have only limited experience in filing and supporting the applications necessary to gain regulatory approvals and expect to rely on third party contract research organizations to assist us in this process. Securing FDA approval requires the submission of extensive preclinical and clinical
46
Table of Contents
data and supporting information to the FDA for each therapeutic indication to establish the product candidate’s safety and efficacy. Securing FDA approval also requires the submission of information about the product manufacturing process to, and inspection of manufacturing facilities by, the FDA. Our product candidates may not be effective, may be only moderately effective or may prove to have undesirable or unintended side effects, toxicities or other characteristics that may preclude our obtaining regulatory approval or prevent or limit commercial use.
The process of obtaining regulatory approvals, both in the United States and abroad, is expensive, may take many years if additional clinical trials are required, if approval is obtained at all, and can vary substantially based upon a variety of factors, including the type, complexity and novelty of the product candidates involved. To date, the FDA has only approved one personalized immunotherapy product. Changes in regulatory approval policies during the development period, changes in or the enactment of additional statutes or regulations, or changes in regulatory review for each submitted product application, may cause delays in the approval or rejection of an application. The FDA has substantial discretion in the approval process and may refuse to accept any application or may decide that our data is insufficient for approval and require additional preclinical, clinical or other studies. In addition, varying interpretations of the data obtained from preclinical and clinical testing could delay, limit or prevent regulatory approval of a product candidate. Any regulatory approval we ultimately obtain may be limited or subject to restrictions or post-approval commitments that render the approved product not commercially viable.
If we experience delays in obtaining approval or if we fail to obtain approval of our product candidates, the commercial prospects for our product candidates may be harmed and our ability to generate revenues will be materially impaired.
Failure to obtain regulatory approval in international jurisdictions would prevent our product candidates from being marketed abroad.
We are a party to arrangements with third parties, and intend to enter into additional arrangements with third parties, under which they would market our products outside the United States. In order to market and sell our products in the European Union and many other jurisdictions, we or such third parties must obtain separate regulatory approvals and comply with numerous and varying regulatory requirements. The approval procedure varies among countries and can involve additional testing. The time required to obtain approval may differ substantially from that required to obtain FDA approval. The regulatory approval process outside the United States generally includes all of the risks associated with obtaining FDA approval. In addition, in many countries outside the United States, it is required that the product be approved for reimbursement before the product can be approved for sale in that country. We or these third parties may not obtain approvals from regulatory authorities outside the United States on a timely basis, if at all. Approval by the FDA does not ensure approval by regulatory authorities in other countries or jurisdictions, and approval by one regulatory authority outside the United States does not ensure approval by regulatory authorities in other countries or jurisdictions or by the FDA. We may not be able to file for regulatory approvals and may not receive necessary approvals to commercialize our products in any market.
A fast track designation by the FDA may not actually lead to a faster development, regulatory review or approval process.
If a product is intended for the treatment of a serious or life-threatening condition and the product demonstrates the potential to address unmet needs for this condition, the treatment sponsor may apply for FDA fast track designation. In April 2012, the FDA notified us that we obtained fast track designation for AGS-003 for the treatment of mRCC. Fast track designation does not ensure that we will experience a faster development, regulatory review or approval process compared to conventional FDA procedures. Additionally, the FDA may withdraw fast track designation if it believes that the designation is no longer supported by data from our clinical development program.
Risks Related to Our Financial Position and Need for Additional Capital
We have incurred significant losses since our inception. We expect to incur losses for at least the next several years and may never achieve or maintain profitability.
Since inception, we have incurred significant operating losses. Our net loss, after attribution to the noncontrolling interest, was $20.1 million for the year ended December 31, 2011, $10.5 million for the year ended December 31, 2012 and $23.9 million for the year ended December 31, 2013. As of December 31, 2013, we had an accumulated deficit of $150.9 million. To date, we have financed our operations primarily through our initial public offering of common stock, private placements of our preferred stock, convertible debt financings, bank debt, government contracts, government and other third party grants and license and collaboration agreements. We have devoted substantially all of our efforts to research and development, including clinical trials. We have not completed development of any product candidates. We expect to continue to incur significant expenses and increasing operating losses for at least the next several years. We anticipate that our expenses will increase substantially if and as we:
| • | continue our ongoing phase 3 clinical trial of AGS-003 for the treatment of mRCC and initiate additional clinical trials of AGS-003 for the treatment of cancers; |
| • | initiate additional clinical trials of AGS-004 for the treatment of HIV; |
47
Table of Contents
| • | seek regulatory approvals for our product candidates that successfully complete clinical trials; |
| • | lease, build out and equip a new commercial facility for the manufacture of our Arcelis-based products; |
| • | establish a sales, marketing and distribution infrastructure to commercialize products for which we may obtain regulatory approval; |
| • | maintain, expand and protect our intellectual property portfolio; |
| • | continue our other research and development efforts; |
| • | hire additional clinical, quality control, scientific and management personnel; and |
| • | add operational, financial and management information systems and personnel, including personnel to support our product development and planned commercialization efforts. |
To become and remain profitable, we must develop and eventually commercialize a product or products with significant market potential. This development and commercialization will require us to be successful in a range of challenging activities, including successfully completing preclinical testing and clinical trials of our product candidates, obtaining regulatory approval for these product candidates, leasing, building out and equipping a new commercial manufacturing facility and manufacturing, marketing and selling those products for which we may obtain regulatory approval. We are only in the preliminary stages of some of these activities. We may never succeed in these activities and may never generate revenues that are significant or large enough to achieve profitability.
Even if we do achieve profitability, we may not be able to sustain or increase profitability on a quarterly or annual basis. Our failure to become and remain profitable would decrease the value of the company and could impair our ability to raise capital, expand our business, maintain our research and development efforts or continue our operations. A decline in the value of our company could also cause you to lose all or part of your investment.
We will need substantial additional funding. If we are unable to raise capital when needed, we would be forced to delay, reduce, terminate or eliminate our product development programs, including our phase 3 clinical trial of AGS-003, our plans to lease, build out and equip a new commercial manufacturing facility or our commercialization efforts.
We expect our expenses to increase in connection with our ongoing activities, particularly as we continue our phase 3 clinical trial of AGS-003, initiate additional clinical trials of AGS-003 and AGS-004, seek regulatory approval for our product candidates and lease, build out and equip a new commercial manufacturing facility. In addition, if we obtain regulatory approval of any of our product candidates, we expect to incur significant commercialization expenses for product sales, marketing, manufacturing and distribution. Furthermore, we expect to incur additional costs associated with operating as a public company. Accordingly, we will need to obtain substantial additional funding in connection with our continuing operations. If we are unable to raise capital when needed or on attractive terms, we would be forced to delay, reduce, terminate or eliminate our product development programs, our plans to lease, build out and equip a new commercial manufacturing facility or our commercialization efforts.
As of December 31, 2013, we had cash, cash equivalents and short-term investments of $47.0 million and working capital of $46.4 million. We expect that our existing cash, cash equivalents and short-term investments, including the $43.7 million in net proceeds from our initial public offering of common stock, and anticipated funding under our NIH contract, will enable us to fund our operating expenses and capital expenditure requirements into the second half of 2016, including funding our ongoing phase 3 clinical trial of AGS-003, our planned phase 2 clinical trials of AGS-003 and AGS-004 and approximately $1.0 million for the initiation of the planned leasing, build-out and equipping of a new commercial manufacturing facility.
We will need to obtain significant financing prior to the commercialization of AGS-003, including to complete the planned leasing, build-out and equipping of a new commercial manufacturing facility and to fund any commercialization efforts in anticipation of regulatory approval of AGS-003. We expect that we will require approximately an additional $35.0 million prior to the commercialization of AGS-003 to lease, build out and equip the new commercial manufacturing facility that we plan to establish. We anticipate needing to expend funds for this purpose beginning in mid-2014. If we are unable to obtain additional financing when needed, in the required amounts or at all, we may not be able to complete the planned leasing, build-out and equipping of the new commercial facility or may be delayed in doing so.
Our future capital requirements will depend on many factors, including:
| • | the progress and results of our ongoing pivotal phase 3 clinical trial of AGS-003 and other clinical trials of AGS-003 that we may conduct; |
48
Table of Contents
| • | the progress and results of our ongoing phase 2b clinical trial and other clinical trials of AGS-004 that we may conduct and our ability to obtain additional funding under our NIH contract for our AGS-004 program; |
| • | the scope, progress, results and costs of preclinical development, laboratory testing and clinical trials for our other product candidates; |
| • | the costs and timing of our planned leasing, build-out and equipping of a new commercial manufacturing facility; |
| • | the costs, timing and outcome of regulatory review of our product candidates; |
| • | the costs of commercialization activities, including product sales, marketing, manufacturing and distribution, for any of our product candidates for which we receive regulatory approval; |
| • | the potential need to repay the $9.0 million loan under our license agreement with Medinet; |
| • | revenue, if any, received from commercial sales of our product candidates, should any of our product candidates be approved by the FDA or a similar regulatory authority outside the United States; |
| • | the costs of preparing, filing and prosecuting patent applications, maintaining and enforcing our intellectual property rights and defending intellectual property-related claims; |
| • | the extent to which we acquire or invest in other businesses, products and technologies; |
| • | our ability to obtain government or other third party funding for the development of our product candidates; and |
| • | our ability to establish collaborations on favorable terms, if at all, particularly arrangements to develop, market and distribute AGS-003 outside North America and arrangements for the development and commercialization of our non-oncology product candidates, including AGS-004. |
Conducting preclinical testing and clinical trials is a time-consuming, expensive and uncertain process that takes years to complete, and we may never generate the necessary data or results required to obtain regulatory approval and achieve product sales. In addition, our product candidates, if approved, may not achieve commercial success. Our commercial revenues, if any, will be derived from sales of products that we do not expect to be commercially available for several years, if at all. Accordingly, we will need to continue to rely on additional financing to achieve our business objectives. Additional financing may not be available to us on acceptable terms, or at all.
Raising additional capital may cause dilution to our existing stockholders, restrict our operations or require us to relinquish rights to our technologies or product candidates.
Until such time, if ever, as we can generate substantial product revenues, we expect to finance our cash needs through a combination of equity offerings, debt financings, government contracts, government and other third party grants or other third party funding, marketing and distribution arrangements and other collaborations, strategic alliances and licensing arrangements. We will require substantial funding to complete the planned leasing, build-out and equipping of a new commercial manufacturing facility, fund our commercialization efforts and fund our operating expenses and other activities. To the extent that we raise additional capital through the sale of equity or convertible debt securities, your ownership interest will be diluted, and the terms of these securities may include liquidation or other preferences that adversely affect your rights as a stockholder. Debt financing, if available, may involve agreements that include covenants limiting or restricting our ability to take specific actions, such as incurring additional debt, making capital expenditures or declaring dividends.
We currently intend to collaborate with third parties for the development and commercialization of AGS-003 outside of North America. We plan to seek government or other third party funding for the continued development of AGS-004 following completion of the phase 2b clinical trial of AGS-004 and to collaborate with third parties for the development and commercialization of AGS-004. If we raise additional funds through government or other third party funding, marketing and distribution arrangements or other collaborations, strategic alliances or licensing arrangements with third parties, we may have to relinquish valuable rights to our technologies, future revenue streams, research programs or product candidates or to grant licenses on terms that may not be favorable to us. If the loan from Medinet becomes due and we do not repay it, we have agreed to grant Medinet a non-exclusive, royalty-bearing license to make and sell Arcelis products in Japan for the treatment of cancer.
49
Table of Contents
Risk Related to the Commercialization of our Product Candidates
We have no history of commercializing pharmaceutical products, which may make it difficult to evaluate the prospects for our future viability.
Our operations to date have been limited to financing and staffing our company, developing our technology and product candidates and establishing collaborations. We have not yet demonstrated an ability to successfully complete a pivotal clinical trial, obtain marketing approvals, manufacture a commercial scale product or conduct sales and marketing activities necessary for successful product commercialization. Consequently, predictions about our future success or viability may not be as accurate as they could be if we had a history of successfully developing and commercializing pharmaceutical products.
In addition, we may encounter unforeseen expenses, difficulties, complications, delays and other known and unknown factors. We will need to transition at some point from a company with research and development focus to a company capable of supporting commercial activities. We may not be successful in such a transition.
Even if AGS-003 or AGS-004 receives regulatory approval, it may fail to achieve the degree of market acceptance by physicians, patients, healthcare payors and others in the medical community necessary for commercial success.
We have never commercialized a product candidate. Even if AGS-003 or AGS-004 receives marketing approval, it may nonetheless fail to gain sufficient market acceptance by physicians, patients, healthcare payors and others in the medical community. Gaining market acceptance for our Arcelis-based products may be particularly difficult as, to date, the FDA has only approved one personalized immunotherapy and our Arcelis-based products are based on a novel technology. If these products do not achieve an adequate level of acceptance, we may not generate significant product revenues and we may not become profitable. The degree of market acceptance of our product candidates, if approved for commercial sale, will depend on a number of factors, including:
| • | efficacy and potential advantages compared to alternative treatments; |
| • | the ability to offer our product candidates for sale at competitive prices; |
| • | convenience and ease of administration compared to alternative treatments; |
| • | the willingness of the target patient population to try new therapies and of physicians to prescribe these therapies; |
| • | the strength of sales, marketing and distribution support; |
| • | the approval of other new products for the same indications; |
| • | availability and amount of reimbursement from government payors, managed care plans and other third party payors; |
| • | adverse publicity about the product or favorable publicity about competitive products; |
| • | clinical indications for which the product is approved; and |
| • | the prevalence and severity of any side effects. |
If any of our product candidates receives marketing approval and we, or others, later discover that the product is less effective than previously believed or causes undesirable side effects that were not previously identified, our ability to market the product could be compromised.
Clinical trials of our product candidates are conducted in carefully defined subsets of patients who have agreed to enter into clinical trials. Consequently, it is possible that our clinical trials may indicate an apparent positive effect of a product candidate that is greater than the actual positive effect, if any, in a broader patient population or alternatively fail to identify undesirable side effects. If, following approval of a product candidate, we, or others, discover that the product is less effective than previously believed or causes undesirable side effects that were not previously identified, any of the following adverse events could occur:
| • | regulatory authorities may withdraw their approval of the product or seize the product; |
| • | we may be required to recall the product or change the way the product is administered; |
| • | additional restrictions may be imposed on the marketing of, or the manufacturing processes for, the particular product; |
50
Table of Contents
| • | regulatory authorities may require the addition of labeling statements, such as a “black box” warning or a contraindication; |
| • | we may be required to create a Medication Guide outlining the risks of the previously unidentified side effects for distribution to patients; |
| • | additional restrictions may be imposed on the distribution or use of the product via a risk evaluation and mitigation strategy, or REMS; |
| • | we could be sued and held liable for harm caused to patients; |
| • | the product may become less competitive; and |
| • | our reputation may suffer. |
Any of these events could have a material and adverse effect on our operations and business and could adversely impact our stock price.
If we are unable to establish sales and marketing capabilities or enter into agreements with third parties to sell and market our product candidates, we may not be successful in commercializing our product candidates if and when they are approved.
We do not have a sales or marketing infrastructure and have no experience in the sale, marketing or distribution of pharmaceutical products. To achieve commercial success for any approved product, we must either develop a sales and marketing organization or outsource these functions to third parties. We plan to market and sell AGS-003 in North America independently and to enter into collaborations or other arrangements with third parties for the distribution or marketing of AGS-003 in the rest of the world. We plan to enter into collaborations or other arrangements with third parties for the distribution or marketing of AGS-004 and any of our other product candidates should such candidates receive marketing approval.
There are risks involved with both establishing our own sales and marketing capabilities and entering into arrangements with third parties to perform these services. For example, recruiting and training a sales force is expensive and time consuming and could delay any product launch. If the commercial launch of a product candidate for which we recruit a sales force and establish marketing capabilities is delayed or does not occur for any reason, we would have prematurely or unnecessarily incurred these commercialization expenses. This may be costly, and our investment would be lost if we cannot retain or reposition our sales and marketing personnel.
Factors that may inhibit our efforts to commercialize our products on our own include:
| • | our inability to recruit and retain adequate numbers of effective sales and marketing personnel; |
| • | the inability of sales personnel to obtain access to or persuade adequate numbers of physicians to prescribe any future products; |
| • | the lack of complementary products to be offered by sales personnel, which may put us at a competitive disadvantage relative to companies with more extensive product lines; and |
| • | unforeseen costs and expenses associated with creating an independent sales and marketing organization. |
If we enter into arrangements with third parties to perform sales, marketing and distribution services, our product revenues or the profitability of these product revenues to us are likely to be lower than if we were to market and sell any products that we develop ourselves. In addition, we may not be successful in entering into arrangements with third parties to sell and market our product candidates or doing so on terms that are favorable to us. We likely will have little control over such third parties, and any of them may fail to devote the necessary resources and attention to sell and market our products effectively. If we do not establish sales and marketing capabilities successfully, either on our own or in collaboration with third parties, we will not be successful in commercializing our product candidates.
We face substantial competition, which may result in others discovering, developing or commercializing products before or more successfully than we do.
The development and commercialization of new drug products is highly competitive. We face competition with respect to our current product candidates, and will face competition with respect to any products that we may seek to develop or commercialize in the future, from major pharmaceutical companies, specialty pharmaceutical companies and biotechnology companies worldwide. There are a
51
Table of Contents
number of large pharmaceutical and biotechnology companies that currently market and sell products or are pursuing the development of products for the treatment of the disease indications for which we are developing our product candidates. Potential competitors also include academic institutions, government agencies and other public and private research organizations that conduct research, seek patent protection and establish collaborative arrangements for research, development, manufacturing and commercialization.
Some of these competitive products and therapies are based on scientific approaches that are the same as or similar to our approach, and others are based on entirely different approaches. Many marketed therapies for the indications that we are currently pursuing, or indications that we may in the future seek to address using our Arcelis platform, are widely accepted by physicians, patients and payors, which may make it difficult for us to replace them with any products that we successfully develop and are permitted to market.
There are several FDA-approved therapies for mRCC marketed and sold by large pharmaceutical companies. Approved monotherapies for mRCC include Nexavar (sorafenib), marketed by Bayer Healthcare Pharmaceuticals, Inc. and Onyx Pharmaceuticals, Inc.; Sutent (sunitinib) and Inlyta (axitinib), marketed by Pfizer, Inc.; Avastin (bevacizumab), marketed by Genentech, Inc., a member of the Roche Group; Votrient (pazopanib), marketed by GlaxoSmithKline plc; Torisel (temsirolimus), marketed by Pfizer; and Afinitor (everolimus), marketed by Novartis Pharmaceuticals Corporation. In addition, we estimate that there are numerous cancer immunotherapy products in clinical development by many public and private biotechnology and pharmaceutical companies targeting numerous different cancer types. A number of these are in late stage development. One biotechnology company, Immatics Biotechnologies GmbH, or Immatics, is developing a therapeutic cancer vaccine, which is a mixture of defined tumor-associated peptides, for the treatment of RCC. Immatics is conducting a pivotal phase 3 clinical trial comparing its vaccine in combination with sunitinib against treatment with sunitinib alone in a subset of favorable and intermediate risk patients. If this clinical trial is successful, this combination therapy would be in direct competition with our AGS-003 and sunitinib combination therapy. In addition, if a standalone therapy for mRCC were developed that demonstrated improved efficacy over currently marketed therapies with a favorable safety profile and without the need for combination therapy, such a therapy might pose a significant competitive threat to AGS-003.
We are currently conducting a pivotal phase 3 clinical trial of AGS-003 in combination with sunitinib. We elected to study AGS-003 in clinical trials in combination with sunitinib due in part to sunitinib being the current standard of care for first-line treatment of mRCC. Although we do not expect to seek FDA approval of AGS-003 solely in combination with sunitinib and have provided that, under the protocol for the phase 3 clinical trial, investigators may discontinue sunitinib due to disease progression or toxicity and initiate second-line treatment with other targeted therapies, if we obtain approval by the FDA, such FDA approval may be limited to the combination of AGS-003 and sunitinib. In such event, the commercial success of AGS-003 would be linked to the commercial success of sunitinib. As a result, if sunitinib ceases to be the standard of care for first-line treatment of mRCC or another event occurs that adversely affects sales of sunitinib, the commercial success of AGS-003 may be adversely affected.
There are also numerous FDA-approved treatments for HIV, primarily antiretroviral therapies marketed by large pharmaceutical companies. Generic competition has developed in this market as patent exclusivity periods for older drugs have expired, with more than 15 generic bioequivalents currently on the market. The presence of these generic drugs is resulting in price pressure in the HIV therapeutics market and could affect the pricing of AGS-004. Currently, there are no approved therapies for the eradication of HIV. We expect that major pharmaceutical companies that currently market antiretroviral therapy products or other companies that are developing HIV product candidates may seek to develop products for the eradication of HIV.
Our competitors may develop products that are more effective, safer, more convenient or less costly than any that we are developing or that would render our product candidates obsolete or non-competitive. Our competitors may also obtain FDA or other regulatory approval for their products more rapidly than we may obtain approval for ours.
Many of our competitors have significantly greater financial resources and expertise in research and development, manufacturing, preclinical testing, conducting clinical trials, obtaining regulatory approvals and marketing approved products than we do. Mergers and acquisitions in the pharmaceutical, biotechnology and device industries may result in even more resources being concentrated among a smaller number of our competitors. Smaller and other early stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies. These third parties compete with us in recruiting and retaining qualified scientific and management personnel, establishing clinical trial sites and patient registration for clinical trials, as well as in acquiring technologies complementary to, or necessary for, our programs.
Even if we are able to commercialize any product candidates, the products may become subject to unfavorable pricing regulations, third party reimbursement practices or healthcare reform initiatives, which would harm our business.
The regulations that govern marketing approvals, pricing and reimbursement for new drug products vary widely from country to country. In the United States, recently passed legislation may significantly change the approval requirements in ways that could involve additional costs and cause delays in obtaining approvals. Some countries require approval of the sale price of a drug before it can be marketed. In many countries, the pricing review period begins after marketing or product licensing approval is granted. In some
52
Table of Contents
foreign markets, prescription pharmaceutical pricing remains subject to continuing governmental control even after initial approval is granted. As a result, we might obtain regulatory approval for a product in a particular country, but then be subject to price regulations that delay our commercial launch of the product, possibly for lengthy time periods, and negatively impact the revenues we are able to generate from the sale of the product in that country. Adverse pricing limitations may hinder our ability to recoup our investment in one or more product candidates, even if our product candidates obtain regulatory approval.
Our ability to commercialize any products successfully also will depend in part on the extent to which reimbursement for these products and related treatments will be available from government health administration authorities, private health insurers and other organizations. Government authorities and third party payors, such as private health insurers and health maintenance organizations, decide which medications they will pay for and establish reimbursement levels. A primary trend in the U.S. healthcare industry and elsewhere is cost containment. Government authorities and third party payors have attempted to control costs by limiting coverage and the amount of reimbursement for particular medications. Increasingly, third party payors are requiring that drug companies provide them with predetermined discounts from list prices and are challenging the prices charged for medical products. We cannot be sure that reimbursement will be available for any product that we commercialize and, if reimbursement is available, the level of reimbursement. Reimbursement may impact the demand for, or the price of, any product candidate for which we obtain marketing approval. Obtaining reimbursement for our products may be particularly difficult because of the higher prices often associated with drugs administered under the supervision of a physician. If reimbursement is not available or is available only to limited levels, we may not be able to successfully commercialize any product candidate for which we obtain marketing approval.
There may be significant delays in obtaining reimbursement for newly approved drugs, and coverage may be more limited than the purposes for which the drug is approved by the FDA or similar regulatory authorities outside the United States. Moreover, eligibility for reimbursement does not imply that any drug will be paid for in all cases or at a rate that covers our costs, including research, development, manufacture, sale and distribution. Interim reimbursement levels for new drugs, if applicable, may also not be sufficient to cover our costs and may not be made permanent. Reimbursement rates may vary according to the use of the drug and the clinical setting in which it is used, may be based on reimbursement levels already set for lower cost drugs, and may be incorporated into existing payments for other services. Net prices for drugs may be reduced by mandatory discounts or rebates required by government healthcare programs or private payors and by any future relaxation of laws that presently restrict imports of drugs from countries where they may be sold at lower prices than in the United States. Third party payors often rely upon Medicare coverage policy and payment limitations in setting their own reimbursement policies. Our inability to promptly obtain coverage and profitable payment rates from both government-funded and private payors for any approved products that we develop could have a material adverse effect on our operating results, our ability to raise capital needed to commercialize products and our overall financial condition.
Product liability lawsuits against us could cause us to incur substantial liabilities and to limit commercialization of any products that we may develop.
We face an inherent risk of product liability exposure related to the testing of our product candidates in human clinical trials and will face an even greater risk if we commercially sell any products that we may develop. These risks may be even greater with respect to our Arcelis-based products which are manufactured using a novel technology. None of our product candidates has been widely used over an extended period of time, and therefore our safety data are limited. We derive the raw materials for manufacturing of our Arcelis-based product candidates from human cell sources, and therefore the manufacturing process and handling requirements are extensive and stringent, which increases the risk of quality failures and subsequent product liability claims.
If we cannot successfully defend ourselves against claims that our product candidates or products caused injuries, we will incur substantial liabilities. Regardless of merit or eventual outcome, liability claims may result in:
| • | decreased demand for any product candidates or products that we may develop; |
| • | injury to our reputation and significant negative media attention; |
| • | withdrawal of clinical trial participants; |
| • | significant costs to defend the related litigation; |
| • | substantial monetary awards to trial participants or patients; |
| • | loss of revenue; and |
| • | the inability to commercialize any products that we may develop. |
53
Table of Contents
We currently hold $5.0 million in product liability insurance coverage, which may not be adequate to cover all liabilities that we may incur. We will need to increase our insurance coverage when we begin commercializing our product candidates, if ever. Insurance coverage is increasingly expensive. We may not be able to maintain insurance coverage at a reasonable cost or in an amount adequate to satisfy any liability that may arise.
We may expend our limited resources to pursue a particular product candidate or indication and fail to capitalize on product candidates or indications that may be more profitable or for which there is a greater likelihood of success.
Because we have limited financial and managerial resources, we focus on research programs and product candidates for specific indications. As a result, we may forego or delay pursuit of opportunities with other product candidates or for other indications that later prove to have greater commercial potential. Our resource allocation decisions may cause us to fail to capitalize on viable commercial products or profitable market opportunities. Our spending on current and future research and development programs and product candidates for specific indications may not yield any commercially viable products.
We have based our research and development efforts on our Arcelis platform. Notwithstanding our large investment to date and anticipated future expenditures in our Arcelis platform, we have not yet developed, and may never successfully develop, any marketed drugs using this approach. As a result of pursuing the development of product candidates using our Arcelis platform, we may fail to develop product candidates or address indications based on other scientific approaches that may offer greater commercial potential or for which there is a greater likelihood of success.
Our long-term business plan is to develop Arcelis-based products for the treatment of various cancers and infectious diseases. We may not be successful in our efforts to identify or discover additional product candidates that may be manufactured using our Arcelis platform. Research programs to identify new product candidates require substantial technical, financial and human resources. These research programs may initially show promise in identifying potential product candidates, yet fail to yield product candidates for clinical development.
If we do not accurately evaluate the commercial potential or target market for a particular product candidate, we may relinquish valuable rights to that product candidate through collaboration, licensing or other royalty arrangements in cases in which it would have been more advantageous for us to retain sole development and commercialization rights to such product candidate.
Risks Related to Our Dependence on Third Parties
Our reliance on government funding adds uncertainty to our research and commercialization efforts and may impose requirements that increase the costs of commercialization and production of our government-funded product candidates.
Our phase 2b clinical trial for AGS-004 for HIV is being funded entirely by the NIH. We plan to seek further government funding for continued development of AGS-004. However, increased pressure on governmental budgets may reduce the availability of government funding for programs such as AGS-004. In addition, contracts and grants from the U.S. government and its agencies include provisions that give the government substantial rights and remedies, many of which are not typically found in commercial contracts, including provisions that allow the government to:
| • | terminate agreements, in whole or in part, for any reason or no reason; |
| • | reduce or modify the government’s obligations under such agreements without the consent of the other party; |
| • | claim rights, including intellectual property rights, in products and data developed under such agreements; |
| • | impose U.S. manufacturing requirements for products that embody inventions conceived or first reduced to practice under such agreements; |
| • | suspend or debar the contractor or grantee from doing future business with the government or a specific government agency; |
| • | pursue criminal or civil remedies under the False Claims Act, False Statements Act and similar remedy provisions specific to government agreements; and |
| • | limit the government’s financial liability to amounts appropriated by the U.S. Congress on a fiscal-year basis, thereby leaving some uncertainty about the future availability of funding for a program even after it has been funded for an initial period. |
54
Table of Contents
Government agreements normally contain additional terms and conditions that may increase our costs of doing business, reduce our profits, and expose us to liability for failure to comply with these terms and conditions. These include, for example:
| • | specialized accounting systems unique to government contracts and grants; |
| • | mandatory financial audits and potential liability for price adjustments or recoupment of government funds after such funds have been spent; |
| • | public disclosures of certain contract and grant information, which may enable competitors to gain insights into our research program; and |
| • | mandatory socioeconomic compliance requirements, including labor standards, non-discrimination and affirmative action programs and environmental compliance requirements. |
We expect to depend on collaborations with third parties for the development and commercialization of our product candidates. If those collaborations are not successful, we may not be able to capitalize on the market potential of these product candidates.
We currently intend to commercialize AGS-003 independently in North America. We intend to collaborate with other third parties to develop and commercialize AGS-003 outside North America. We have entered into an exclusive license agreement with Pharmstandard International S.A., or Pharmstandard, for the development and commercialization of AGS-003 in Russia and the other states comprising the Commonwealth of Independent States and an exclusive license agreement with Green Cross Corp., or Green Cross, for the development and commercialization of AGS-003 in South Korea. We have also entered into a license agreement with Medinet under which we granted Medinet an exclusive license to manufacture in Japan AGS-003 for the purpose of development and commercialization for the treatment of mRCC and an option to acquire a non-exclusive license to sell in Japan AGS-003 for the treatment of mRCC. We also plan to seek government or other third party funding for continued development of AGS-004 and to collaborate with third parties to develop and commercialize AGS-004. Our likely collaborators for any development, distribution, marketing, licensing or broader collaboration arrangements include large and mid-size pharmaceutical companies, regional and national pharmaceutical companies and biotechnology companies.
Under our existing arrangements we have limited control, and under any additional arrangements we may enter into with third parties we will likely have limited control, over the amount and timing of resources that our collaborators dedicate to the development or commercialization of our product candidates. Our ability to generate revenues from these arrangements will depend on our collaborators’ abilities to successfully perform the functions assigned to them in these arrangements.
Collaborations involving our product candidates would pose the following risks to us:
| • | collaborators have significant discretion in determining the efforts and resources that they will apply to these collaborations; |
| • | collaborators may not pursue development and commercialization of our product candidates or may elect not to continue or renew development or commercialization programs based on clinical trial results, changes in the collaborator’s strategic focus or available funding, or external factors such as an acquisition that diverts resources or creates competing priorities; |
| • | collaborators may delay clinical trials, provide insufficient funding for a clinical trial program, stop a clinical trial or abandon a product candidate, repeat or conduct new clinical trials or, require a new formulation of a product candidate for clinical testing; |
| • | collaborators could independently develop, or develop with third parties, products that compete directly or indirectly with our products or product candidates if the collaborators believe that competitive products are more likely to be successfully developed or can be commercialized under terms that are more economically attractive than ours; |
| • | a collaborator with marketing and distribution rights to one or more products may not commit sufficient resources to the marketing and distribution of such product or products; |
| • | collaborators may have the right to conduct clinical trials of our product candidates without our consent and could conduct trials with flawed designs that result in data that adversely affect our clinical trials, our ability to obtain marketing approval for our product candidates or market acceptance of our product candidates. Pharmstandard, Green Cross and Medinet each have this right under our license agreements with them; |
55
Table of Contents
| • | collaborators may hold rights that could preclude us from commercializing our products in certain territories. For example, we have granted Medinet an exclusive license to manufacture in Japan AGS-003 for the treatment of mRCC and an option to acquire a non-exclusive license to sell in Japan AGS-003 for the treatment of mRCC. Even if Medinet does not exercise the option to acquire the license to sell, we will not have the right to manufacture AGS-003 in Japan for the purposes of development and commercialization of AGS-003 for the treatment of mRCC. If we and Medinet are unable to agree to the terms of a supply agreement under these circumstances, we will not be able to sell AGS-003 in Japan unless we repurchase these rights from Medinet; |
| • | collaborators may not properly maintain or defend our intellectual property rights or may use our proprietary information in such a way as to invite litigation that could jeopardize or invalidate our proprietary information or expose us to potential litigation; |
| • | disputes may arise between the collaborators and us that result in the delay or termination of the research, development or commercialization of our products or product candidates or that result in costly litigation or arbitration that diverts management attention and resources; and |
| • | collaborations may be terminated and, if terminated, may result in a need for additional capital to pursue further development or commercialization of the applicable product candidates. For example, our collaboration with Kyowa Hakko Kirin Co., Ltd. with respect to AGS-003 and AGS-004 was terminated by our collaborator. |
Collaboration agreements may not lead to development or commercialization of product candidates in the most efficient manner, or at all. In addition, there have been a significant number of recent business combinations among large pharmaceutical companies that have resulted in a reduced number of potential future collaborators. If a present or future collaborator of ours were to be involved in a business combination, the continued pursuit and emphasis on our product development or commercialization program could be delayed, diminished or terminated.
If we are not able to establish additional collaborations, we may have to alter our development and commercialization plans.
Our drug development programs and the potential commercialization of our product candidates will require substantial additional cash to fund expenses. For some of our product candidates, we intend to collaborate with pharmaceutical and biotechnology companies for the development and commercialization of those product candidates. For example, we have entered into license agreements with third parties to develop and commercialize AGS-003 in Russia and the other states comprising the Commonwealth of Independent States, South Korea and Japan, and we intend to collaborate with other third parties to develop and commercialize AGS-003 in other parts of the world and to collaborate with third parties to develop and commercialize AGS-004.
We face significant competition in seeking appropriate collaborators. Whether we reach a definitive agreement for a collaboration will depend, among other things, upon our assessment of the collaborator’s resources and expertise, the terms and conditions of the proposed collaboration, and the proposed collaborator’s evaluation of a number of factors. Those factors may include the design or results of clinical trials, the likelihood of approval by the FDA or similar regulatory authorities outside the United States, the potential market for the subject product candidate, the costs and complexities of manufacturing and delivering such product candidate to patients, the potential of competing products, the existence of uncertainty with respect to our ownership of technology, which can exist if there is a challenge to such ownership without regard to the merits of the challenge and industry and market conditions generally. The collaborator may also consider alternative product candidates or technologies for similar indications that may be available to collaborate on and whether such a collaboration could be more attractive than the one with us for our product candidate. We may also be restricted under existing license agreements from entering into agreements on certain terms with potential collaborators. Collaborations are complex and time-consuming to negotiate and document. We may not be able to negotiate collaborations on a timely basis, on acceptable terms, or at all.
If we are not able to obtain such funding or enter into collaborations for our product candidate, we may have to curtail the development of such product candidate, reduce or delay its development program or one or more of our other development programs, delay its potential commercialization or reduce the scope of any sales or marketing activities, or increase our expenditures and undertake development or commercialization activities at our own expense. If we elect to increase our expenditures to fund development or commercialization activities on our own, we may need to obtain additional capital, which may not be available to us on acceptable terms or at all. If we do not have sufficient funds, we may not be able to further develop these product candidates or bring these product candidates to market and generate product revenue.
56
Table of Contents
We rely on third parties to conduct our clinical trials, and those third parties may not perform satisfactorily, including failing to meet deadlines for the completion of such trials.
We do not independently conduct clinical trials of our product candidates. We rely on third parties, such as contract research organizations, clinical data management organizations, medical institutions and clinical investigators, to perform this function. Our reliance on these third parties for clinical development activities reduces our control over these activities but does not relieve us of our responsibilities. We remain responsible for ensuring that each of our clinical trials is conducted in accordance with the general investigational plan and protocols for the trial. Moreover, the FDA requires us to comply with standards, commonly referred to as Good Clinical Practices, for conducting, recording and reporting the results of clinical trials to assure that data and reported results are credible and accurate and that the rights, integrity and confidentiality of trial participants are protected. We also are required to register ongoing clinical trials and post the results of completed clinical trials on a government-sponsored database, ClinicalTrials.gov, within certain timeframes. Failure to do so can result in fines, adverse publicity and civil and criminal sanctions. Furthermore, these third parties may also have relationships with other entities, some of which may be our competitors. If these third parties do not successfully carry out their contractual duties, meet expected deadlines or conduct our clinical trials in accordance with regulatory requirements or our stated protocols, we will not be able to obtain, or may be delayed in obtaining, regulatory approvals for our product candidates and will not be able to, or may be delayed in our efforts to, successfully commercialize our product candidates.
We also rely on other third parties to store and distribute product supplies for our clinical trials. Any performance failure on the part of our existing or future distributors could delay clinical development or regulatory approval of our product candidates or commercialization of our products, producing additional losses and depriving us of potential product revenue.
Risks Related to the Manufacturing of Our Product Candidates
We plan to identify, lease, build out and equip a new facility to manufacture our Arcelis-based products on a commercial scale using automated processes. We do not have experience in manufacturing Arcelis-based products on a commercial scale or using automated processes. If, due to our lack of manufacturing experience, we cannot manufacture our Arcelis-based products on a commercial scale successfully or manufacture sufficient product to meet our expected commercial requirements, our business may be materially harmed.
We currently have manufacturing suites in our facility located at our corporate headquarters in Durham, North Carolina. We manufacture our Arcelis-based product candidates for research and development purposes and for clinical trials at this facility. We plan to identify, lease, build out and equip a new facility to manufacture our Arcelis-based products on a commercial scale using automated processes. We may be unable to find a suitable space to lease for our new commercial manufacturing facility. Even if we are able to find such a space, in light of our limited financial resources, a landlord may not be willing to lease the space to us on terms acceptable to us, or at all.
We do not have experience in manufacturing products on a commercial scale or using automated processes. In addition, because we are aware of only one company that has manufactured a personalized immunotherapy product for commercial sale, there are limited precedents from which we can learn. We may encounter difficulties in the manufacture of our Arcelis-based products due to our limited manufacturing experience. These difficulties could delay the build-out and equipping of the new facility and regulatory approval of the manufacture of our Arcelis-based products using the new facility and the automated processes, increase our costs or cause production delays or result in us not manufacturing sufficient product to meet our expected commercial requirements, any of which could damage our reputation and hurt our profitability. If we are unable to successfully increase our manufacturing capacity to commercial scale, our business may be materially adversely affected.
We plan to build out and equip a new commercial manufacturing facility based on automated manufacturing processes and augment our manufacturing personnel in advance of any regulatory submission for approval of AGS-003. If we fail to build out and equip a new commercial manufacturing facility in compliance with regulatory requirements, implement our automated processes or augment our manufacturing personnel, we may not be able to initiate commercial operations or produce sufficient product to meet our expected commercial requirements.
In order to meet our business plan, which contemplates our manufacturing product internally using automated processes for the commercial requirements of AGS-003 and any other Arcelis-based product candidates that might be approved, we will need to lease, build out and equip a new commercial manufacturing facility and add manufacturing personnel in advance of any regulatory submission for approval of AGS-003. The leasing, build-out and equipping of our facilities will require substantial capital expenditures and additional regulatory approvals. In addition, it will be costly and time consuming to recruit necessary additional personnel.
Prior to implementing the automated manufacturing processes for Arcelis-based products and filing a BLA for approval of AGS-003, we will be required to:
| • | demonstrate that the disposable components and sterilization and packaging methods used in the manufacturing process are suitable for use in manufacturing in accordance with current good manufacturing practice, or cGMP, and current Good Tissue Practices, or cGTP; |
57
Table of Contents
| • | build and validate processing equipment that complies with cGMP and cGTP; |
| • | identify, lease, build out and equip a suitable manufacturing facility to accommodate the automated manufacturing process; |
| • | perform process testing with final equipment, disposable components and reagents to demonstrate that the methods are suitable for use in cGMP and cGTP manufacturing; |
| • | demonstrate consistency and repeatability of the automated manufacturing processes in the production of AGS-003 in our new facility to fully validate the manufacturing and control process using the actual automated cGMP processing equipment; and |
| • | demonstrate comparability between AGS-003 that we produce using existing processes in our current facility and AGS-003 produced using the automated processes in our new facility. |
We expect that this implementation will require approximately two years to complete, but such implementation could take longer, particularly if we are unable to achieve any of the required tasks on a timely basis, or at all.
If we are unable to successfully build out and equip our commercial manufacturing facility in compliance with regulatory requirements, implement the automated processes required, demonstrate comparability between the AGS-003 used in our pivotal trial and the AGS-003 produced using the automated processes in the new facility, or hire additional necessary manufacturing personnel appropriately, our filing for regulatory approval of AGS-003 may be delayed or denied and we may not be able to initiate commercial operations even if any of our product candidates are approved for marketing.
Lack of coordination internally among our employees and externally with physicians, hospitals and third- party suppliers and carriers, could cause manufacturing difficulties, disruptions or delays and cause us to not have sufficient product to meet our expected clinical trial requirements or potential commercial requirements.
Manufacturing our Arcelis-based product candidates requires coordination internally among our employees and externally with physicians, hospitals and third party suppliers and carriers. For example, a patient’s physician or clinical site will need to coordinate with us for the shipping of a patient’s disease sample and leukapheresis product to our manufacturing facility, and we will need to coordinate with them for the shipping of the manufactured product to them. Such coordination involves a number of risks that may lead to failures or delays in manufacturing our Arcelis-based product candidates, including:
| • | failure to obtain a sufficient supply of key raw materials of suitable quality; |
| • | difficulties in manufacturing our product candidates for multiple patients simultaneously; |
| • | difficulties in obtaining adequate patient-specific material, such as tumor samples, virus samples or leukapheresis product, from physicians; |
| • | difficulties in completing the development and validation of the specialized assays required to ensure the consistency of our product candidates; |
| • | failure to ensure adequate quality control and assurances in the manufacturing process as we increase production quantities; |
| • | difficulties in the timely shipping of patient-specific materials to us or in the shipping of our product candidates to the treating physicians due to errors by third party carriers, transportation restrictions or other reasons; |
| • | destruction of, or damage to, patient-specific materials or our product candidates during the shipping process due to improper handling by third party carriers, hospitals, physicians or us; |
| • | destruction of, or damage to, patient-specific materials or our product candidates during storage at our facilities; and |
| • | destruction of, or damage to, patient-specific materials or our product candidates stored at clinical and future commercial sites due to improper handling or holding by clinicians, hospitals or physicians. |
58
Table of Contents
If we are unable to coordinate appropriately, we may encounter delays or additional costs in achieving our clinical and commercialization objectives, including in obtaining regulatory approvals of our product candidates and supplying product, which could materially damage our business and financial position.
If our existing manufacturing facility or the new commercial manufacturing facility that we plan to lease, build out and equip are damaged or destroyed, or production at one of these facilities is otherwise interrupted, our business and prospects would be negatively affected.
We currently have a single manufacturing facility and expect that the new commercial manufacturing facility that we plan to lease, build out and equip will be our only commercial manufacturing facility in North America. If our existing manufacturing facility or the new commercial manufacturing facility that we plan to lease, build out and equip, or the equipment in either of these facilities, is damaged or destroyed, we likely would not be able to quickly or inexpensively replace our manufacturing capacity and possibly would not be able to replace it at all. Any new facility needed to replace either our existing manufacturing facility or the planned new commercial manufacturing facility would need to comply with the necessary regulatory requirements, need to be tailored to our specialized automated manufacturing requirements and require specialized equipment. We would need FDA approval before selling any products manufactured at a new facility. Such an event could delay our clinical trials or, if any of our product candidates are approved by the FDA, reduce or eliminate our product sales.
We maintain insurance coverage to cover damage to our property and equipment and to cover business interruption and research and development restoration expenses. If we have underestimated our insurance needs with respect to an interruption in our clinical manufacturing of our product candidates, we may not be able to adequately cover our losses.
Risks Related to Our Intellectual Property
If we fail to comply with our obligations under our intellectual property licenses with third parties, we could lose license rights that are important to our business.
We are a party to a number of intellectual property license agreements with third parties, including with respect to each of AGS-003 and AGS-004, and we expect to enter into additional license agreements in the future. Our existing license agreements impose, and we expect that future license agreements will impose, various diligence, milestone payment, royalty, insurance and other obligations on us. For example, under our license agreement with Duke University which relates to patents and patent applications directed towards the composition of matter of Arcelis-based products, dendritic cells loaded with RNA from tumors or pathogens, methods of manufacture of these products and methods of using these products to treat tumors, we are required to use commercially reasonable efforts to research, develop and market license products and to satisfy other specified obligations. If we fail to comply with our obligations under these licenses, our licensors may have the right to terminate these license agreements, in which event we might not be able to market any product that is covered by these agreements, or to convert the license to a non-exclusive license, which could materially adversely affect the value of the product candidate being developed under the license agreement. Termination of these license agreements or reduction or elimination of our licensed rights may result in our having to negotiate new or reinstated licenses with less favorable terms.
If we are unable to obtain and maintain patent protection for our technology and products, or if our licensors are unable to obtain and maintain patent protection for the technology or products that we license from them, or if the scope of the patent protection obtained is not sufficiently broad, our competitors could develop and commercialize technology and products similar or identical to ours, and our ability to successfully commercialize our technology and products may be adversely affected.
Our success depends in large part on our and our licensors’ ability to obtain and maintain patent protection in the United States and other countries with respect to our proprietary technology and products. We and our licensors have sought to protect our proprietary position by filing patent applications in the United States and abroad related to our novel technologies and products that are important to our business. This process is expensive and time-consuming, and we may not be able to file and prosecute all necessary or desirable patent applications at a reasonable cost or in a timely manner. It is also possible that we will fail to identify patentable aspects of our research and development efforts before it is too late to obtain patent protection. Moreover, in some circumstances, we do not have the right to control the preparation, filing and prosecution of patent applications, or to maintain the patents, covering technology or products that we license from third parties and are reliant on our licensors. Therefore, we cannot be certain that these patents and applications will be prosecuted and enforced in a manner consistent with the best interests of our business. If such licensors fail to maintain such patents, or lose rights to those patents, the rights we have licensed may be reduced or eliminated.
The patent position of biotechnology and pharmaceutical companies generally is highly uncertain, involves complex legal and factual questions and has in recent years been the subject of much litigation. As a result, the issuance, scope, validity, enforceability and commercial value of our and our licensors’ patent rights are highly uncertain. Our and our licensors’ pending and future patent applications may not result in patents being issued which protect our technology or products or which effectively prevent others from commercializing competitive technologies and products. Changes in either the patent laws or interpretation of the patent laws in the United States and other countries may diminish the value of our patents or narrow the scope of our patent protection.
59
Table of Contents
Third parties could practice our inventions in territories where we do not have patent protection. Furthermore, the laws of foreign countries may not protect our rights to the same extent as the laws of the United States. For example, we own or exclusively license patents relating to our process of manufacturing a personalized drug product. A U.S. patent may be infringed by anyone who, without authorization, practices the patented process in the United States or imports a product made by a process covered by the U.S. patent. In foreign countries, however, importation of a product made by a process patented in that country may not constitute an infringing activity, which would limit our ability to enforce our process patents against importers in that country. Furthermore, the legal systems of certain countries, particularly certain developing countries, do not favor the enforcement of patents and other intellectual property protection. This could make it difficult for us to stop the infringement of our patents or the misappropriation of our other intellectual property rights. If competitors are able to use our technologies, our ability to compete effectively could be harmed.
Furthermore, publications of discoveries in the scientific literature often lag behind the actual discoveries, and patent applications in the United States and other jurisdictions are typically not published until 18 months after filing, or in some cases not at all. Therefore we cannot be certain that we or our licensors were the first to make the inventions claimed in our owned or licensed patents or pending patent applications, or that we or our licensors were the first to file for patent protection of such inventions.
Assuming the other requirements for patentability are met, in the United States, the first to invent the claimed invention is entitled to the patent, while outside the United States, the first to file a patent application is generally entitled to the patent. Under the America Invents Act, or AIA, enacted in September 2011, the United States moved to a first inventor to file system in March 2013. The United States Patent and Trademark Office only recently finalized the rules relating to these changes and courts have yet to address the new provisions. These changes could increase the costs and uncertainties surrounding the prosecution of our patent applications and the enforcement or defense of our patent rights. Furthermore, we may become involved in interference proceedings, opposition proceedings, or other post-grant proceedings, such as reexamination or inter partes review proceedings, challenging our patent rights or the patent rights of others. An adverse determination in any such proceeding or litigation could reduce the scope of, or invalidate, our patent rights, allow third parties to commercialize our technology or products and compete directly with us, without payment to us, or result in our inability to manufacture or commercialize products without infringing third party patent rights.
Even if our owned and licensed patent applications issue as patents, they may not issue in a form that will provide us with any meaningful protection, prevent competitors from competing with us or otherwise provide us with any competitive advantage. Our competitors may be able to circumvent our owned or licensed patents by developing similar or alternative technologies or products in a non-infringing manner. The issuance of a patent is not conclusive as to its scope, validity or enforceability, and our owned and licensed patents may be challenged in the courts or patent offices in the United States and abroad. Such challenges may result in patent claims being narrowed, invalidated or held unenforceable, which could limit our ability to or stop or prevent us from stopping others from using or commercializing similar or identical technology and products, or limit the duration of the patent protection of our technology and products. Given the amount of time required for the development, testing and regulatory review of new product candidates, patents protecting such candidates might expire before or shortly after such candidates are commercialized. For example, certain of the U.S. patents we exclusively license from Duke University expire as early as 2016 and the European and Japanese patents exclusively licensed from Duke University expire in 2017. As a result, our owned and licensed patent portfolio may not provide us with sufficient rights to exclude others from commercializing products similar or identical to ours.
We may become involved in lawsuits to protect or enforce our patents, which could be expensive, time consuming and unsuccessful.
Competitors may infringe our patents. To counter such infringement or unauthorized use, we may be required to file infringement claims against third parties, which can be expensive and time consuming. In addition, during an infringement proceeding, a court may decide that the patent rights we are asserting are invalid or unenforceable, or may refuse to stop the other party from using the technology at issue on the grounds that our patents do not cover the technology in question. An adverse result in any litigation proceeding could put one or more of our patents at risk of being invalidated or interpreted narrowly. Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of our confidential information could be compromised by disclosure during this type of litigation. In addition, our licensors may have rights to file and prosecute such claims and we are reliant on them.
Third parties may initiate legal proceedings alleging that we are infringing their intellectual property rights, the outcome of which would be uncertain and could have a material adverse effect on the success of our business.
Our commercial success depends upon our ability and the ability of our collaborators to develop, manufacture, market and sell our product candidates and use our proprietary technologies without infringing the proprietary rights of third parties. We cannot ensure that third parties do not have, or will not in the future obtain, intellectual property rights such as granted patents that could block our ability to operate as we would like. There may be patents in the United States or abroad owned by third parties that, if valid, may block our ability to make, use or sell our products in the United States or certain countries outside the United States, or block our ability to import our products into the United States or into certain countries outside the United States.
60
Table of Contents
We may become party to, or threatened with, future adversarial proceedings or litigation regarding intellectual property rights with respect to our products and technology. For example, third parties may assert infringement claims against us based on existing patents or patents that may be granted in the future. If we are found to infringe a third party’s intellectual property rights, we could be required to obtain a license from such third party to continue developing and marketing our products and technology. However, we may be unable to obtain any required license on commercially reasonable terms or even obtain a license at all. Even if we were able to obtain a license, it could be non-exclusive, thereby giving our competitors access to the same technologies licensed to us. We could be forced, including by court order, to cease commercializing the infringing technology or product. In addition, we could be found liable for monetary damages. A finding of infringement could prevent us from commercializing our product candidates or force us to cease some of our business operations, which could materially harm our business. Claims that we have misappropriated the confidential information or trade secrets of third parties could have a similar negative impact on our business.
We have research licenses to certain reagents and their use in the development of our product candidates. We would need commercial licenses to these reagents for any of our product candidates that receive approval for sale in the United States. We believe that commercial licenses to these reagents will be available. However, if we are unable to obtain any such commercial licenses, we may be unable to commercialize our product candidates without infringing the patent rights of third parties. If we did seek to commercialize our product candidates without a license, these third parties could initiate legal proceedings against us.
We may be subject to claims that our employees have wrongfully used or disclosed alleged trade secrets of their former employers.
Many of our employees were previously employed at universities or other biotechnology or pharmaceutical companies, including our competitors or potential competitors. Although we try to ensure that our employees do not use the proprietary information or know-how of others in their work for us, we may be subject to claims that we or these employees have used or disclosed intellectual property, including trade secrets or other proprietary information, of any such employee’s former employer. Litigation may be necessary to defend against these claims. If we fail in defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights or personnel. Even if we are successful in defending against such claims, litigation could result in substantial costs and be a distraction to management.
Intellectual property litigation could cause us to spend substantial resources and distract our personnel from their normal responsibilities.
Even if resolved in our favor, litigation or other legal proceedings relating to intellectual property claims may cause us to incur significant expenses, and could distract our technical and management personnel from their normal responsibilities. In addition, there could be public announcements of the results of hearings, motions or other interim proceedings or developments and if securities analysts or investors perceive these results to be negative, it could have a substantial adverse effect on the price of our common stock. Such litigation or proceedings could substantially increase our operating losses and reduce the resources available for development activities. We may not have sufficient financial or other resources to adequately conduct such litigation or proceedings. Some of our competitors may be able to sustain the costs of such litigation or proceedings more effectively than we can because of their greater financial resources. Uncertainties resulting from the initiation and continuation of patent litigation or other proceedings could have a material adverse effect on our ability to compete in the marketplace.
If we are unable to protect the confidentiality of our trade secrets, our business and competitive position would be harmed.
In addition to seeking patents for some of our technology and products, we also rely on trade secrets, including unpatented know-how, technology and other proprietary information, to maintain our competitive position. The types of protections available for trade secrets are particularly important with respect to our Arcelis platform’s manufacturing capabilities, which involve significant unpatented know-how. We seek to protect these trade secrets, in part, by entering into non-disclosure and confidentiality agreements with parties who have access to them, such as our employees, corporate collaborators, outside scientific collaborators, sponsored researchers, contract manufacturers, consultants, advisors and other third parties. We also enter into confidentiality and invention or patent assignment agreements with our employees and consultants. Despite these efforts, any of these parties may breach the agreements and disclose our proprietary information, including our trade secrets, and we may not be able to obtain adequate remedies for such breaches. Enforcing a claim that a party illegally disclosed or misappropriated a trade secret is difficult, expensive and time-consuming, and the outcome is unpredictable. In addition, some courts inside and outside the United States are less willing or unwilling to protect trade secrets. If any of our trade secrets were to be lawfully obtained or independently developed by a competitor, we would have no right to prevent them from using that technology or information to compete with us. If any of our trade secrets were to be disclosed to or independently developed by a competitor, our competitive position would be harmed.
61
Table of Contents
Risks Related to Legal Compliance Matters
Any product candidate for which we obtain marketing approval could be subject to restrictions or withdrawal from the market and we may be subject to penalties if we fail to comply with regulatory requirements or if we experience unanticipated problems with our products, when and if any of them are approved.
Any product candidate for which we obtain marketing approval, along with the manufacturing processes, post-approval clinical data, labeling, advertising and promotional activities for such product, will be subject to continual requirements of and review by the FDA and other regulatory authorities. These requirements include submissions of safety and other post-marketing information and reports, registration and listing requirements, cGMP requirements relating to quality control, quality assurance and corresponding maintenance of records and documents, cGTP requirements, requirements regarding the distribution of samples to physicians and recordkeeping. Even if regulatory approval of a product candidate is granted, the approval may be subject to limitations on the indicated uses for which the product may be marketed or to the conditions of approval, or contain requirements for costly post-marketing testing and surveillance to monitor the safety or efficacy of the product. The FDA closely regulates the post-approval marketing and promotion of drugs to ensure drugs are marketed only for the approved indications and in accordance with the provisions of the approved label. The FDA imposes stringent restrictions on manufacturers’ communications regarding off-label use and if we do not market our products for their approved indications, we may be subject to enforcement action for off-label marketing. In addition, later discovery of previously unknown problems with our products, manufacturers or manufacturing processes, or failure to comply with regulatory requirements, may yield various results, including:
| • | restrictions on such products, manufacturers or manufacturing processes; |
| • | restrictions on the marketing of a product; |
| • | restrictions on product distribution; |
| • | requirements to conduct post-marketing clinical trials; |
| • | warning or untitled letters; |
| • | withdrawal of the products from the market; |
| • | refusal to approve pending applications or supplements to approved applications that we submit; |
| • | recall of products; |
| • | fines, restitution or disgorgement of profits or revenue; |
| • | suspension or withdrawal of regulatory approvals; |
| • | refusal to permit the import or export of our products; |
| • | product seizure; or |
| • | injunctions or the imposition of civil or criminal penalties. |
Our relationships with customers and third party payors will be subject to applicable anti-kickback, fraud and abuse and other healthcare laws and regulations, which could expose us to criminal sanctions, civil penalties, program exclusion, contractual damages, reputational harm and diminished profits and future earnings.
Healthcare providers, physicians and third party payors play a primary role in the recommendation and prescription of any product candidates for which we obtain marketing approval. Our future arrangements with third party payors and customers may expose us to broadly applicable fraud and abuse and other healthcare laws and regulations that may constrain the business or financial arrangements and relationships through which we market, sell and distribute our products for which we obtain marketing approval. Restrictions under applicable federal and state healthcare laws and regulations, include the following:
| • | the federal healthcare anti-kickback statute prohibits, among other things, persons from knowingly and willfully soliciting, offering, receiving or providing remuneration, directly or indirectly, in cash or in kind, to induce or reward either the referral of an individual for, or the purchase, order or recommendation of, any good or service, for which payment may be made under federal and state healthcare programs such as Medicare and Medicaid; |
62
Table of Contents
| • | the federal False Claims Act imposes civil penalties, including civil whistleblower or qui tam actions, against individuals or entities for knowingly presenting, or causing to be presented, to the federal government, claims for payment that are false or fraudulent or making a false statement to avoid, decrease or conceal an obligation to pay money to the federal government; |
| • | the federal Health Insurance Portability and Accountability Act of 1996, as amended by the Health Information Technology for Economic and Clinical Health Act, imposes criminal and civil liability for executing a scheme to defraud any healthcare benefit program and also imposes obligations, including mandatory contractual terms, with respect to safeguarding the privacy, security and transmission of individually identifiable health information; |
| • | the federal false statements statute prohibits knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false statement in connection with the delivery of or payment for healthcare benefits, items or services; |
| • | the federal transparency requirements under the Health Care Reform Law will require manufacturers of drugs, devices, biologics and medical supplies to report to the Department of Health and Human Services information related to physician payments and other transfers of value and physician ownership and investment interests; and |
| • | analogous state laws and regulations, such as state anti-kickback and false claims laws, may apply to sales or marketing arrangements and claims involving healthcare items or services reimbursed by non-governmental third party payors, including private insurers, and some state laws require pharmaceutical companies to comply with the pharmaceutical industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government in addition to requiring drug manufacturers to report information related to payments to physicians and other health care providers or marketing expenditures. |
Some state laws require pharmaceutical companies to comply with the pharmaceutical industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government and may require drug manufacturers to report information related to payments and other transfers of value to physicians and other healthcare providers or marketing expenditures. State and foreign laws also govern the privacy and security of health information in some circumstances, many of which differ from each other in significant ways and often are not preempted by the federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, thus complicating compliance efforts.
Efforts to ensure that our business arrangements with third parties will comply with applicable healthcare laws and regulations will involve substantial costs. It is possible that governmental authorities will conclude that our business practices may not comply with current or future statutes, regulations or case law involving applicable fraud and abuse or other healthcare laws and regulations. If our operations are found to be in violation of any of these laws or any other governmental regulations that may apply to us, we may be subject to significant civil, criminal and administrative penalties, damages, fines, exclusion from government funded healthcare programs, such as Medicare and Medicaid, and the curtailment or restructuring of our operations. If any of the physicians or other providers or entities with whom we expect to do business are found to be not in compliance with applicable laws, they may be subject to criminal, civil or administrative sanctions, including exclusions from government funded healthcare programs.
Recently enacted and future legislation may increase the difficulty and cost for us to obtain marketing approval of and commercialize our product candidates and affect the prices we may obtain.
In the United States and some foreign jurisdictions, there have been a number of legislative and regulatory changes and proposed changes regarding the healthcare system that could prevent or delay marketing approval of our product candidates, restrict or regulate post-approval activities and affect our ability to profitably sell any product candidates for which we obtain marketing approval.
In the United States, the Medicare Prescription Drug, Improvement, and Modernization Act of 2003, or Medicare Modernization Act, changed the way Medicare covers and pays for pharmaceutical products. The legislation expanded Medicare coverage for drug purchases by the elderly and introduced a new reimbursement methodology based on average sales prices for physician administered drugs. In addition, this legislation provided authority for limiting the number of drugs that will be covered in any therapeutic class in certain cases. Cost reduction initiatives and other provisions of this and other more recent legislation could decrease the coverage and reimbursement that is provided for any approved products. While the Medicare Modernization Act applies only to drug benefits for Medicare beneficiaries, private payors often follow Medicare coverage policy and payment limitations in setting their own reimbursement rates. Therefore, any reduction in reimbursement that results from the Medicare Modernization Act or other more recent legislation may result in a similar reduction in payments from private payors.
In March 2010, President Obama signed into law the Health Care Reform Law, a sweeping law intended to broaden access to health insurance, reduce or constrain the growth of healthcare spending, enhance remedies against fraud and abuse, add new transparency requirements for health care and health insurance industries, impose new taxes and fees on the health industry and impose additional
63
Table of Contents
health policy reforms. Effective October 1, 2010, the Health Care Reform Law revises the definition of “average manufacturer price” for reporting purposes, which could increase the amount of Medicaid drug rebates to states. Further, the new law imposes a significant annual fee on companies that manufacture or import branded prescription drug products. Substantial new provisions affecting compliance have also been enacted, which may affect our business practices with health care practitioners. We will not know the full effects of the Health Care Reform Law until applicable federal and state agencies issue regulations or guidance under the new law. Although it is too early to determine the effect of the Health Care Reform Law, the new law appears likely to continue the pressure on pharmaceutical pricing, especially under the Medicare program, and may also increase our regulatory burdens and operating costs.
Legislative and regulatory proposals have been made to expand post-approval requirements and restrict sales and promotional activities for pharmaceutical products. We cannot be sure whether additional legislative changes will be enacted, or whether the FDA regulations, guidance or interpretations will be changed, or what the impact of such changes on the marketing approvals of our product candidates, if any, may be. In addition, increased scrutiny by the U.S. Congress of the FDA’s approval process may significantly delay or prevent marketing approval, as well as subject us to more stringent product labeling and post-marketing testing and other requirements.
With the enactment of the Biologics Price Competition and Innovation Act of 2009, or BPCIA, as part of the Health Care Reform Law, an abbreviated pathway for the approval of biosimilar and interchangeable biological products was created. The new abbreviated regulatory pathway establishes legal authority for the FDA to review and approve biosimilar biologics, including the possible designation of a biosimilar as “interchangeable” based on its similarity to an existing brand product. Under the BPCIA, an application for a biosimilar product cannot be submitted to the FDA until four years, or approved by the FDA until 12 years, after the original brand product identified as the reference product was approved under a BLA. The new law is complex and is only beginning to be interpreted and implemented by the FDA. As a result, its ultimate impact, implementation and meaning is subject to uncertainty. While it is uncertain when any such processes may be fully adopted by the FDA, any such processes could have a material adverse effect on the future commercial prospects for our biological products.
We believe that if any of our product candidates were to be approved as biological products under a BLA, such approved products should qualify for the four-year and 12-year periods of exclusivity. However, there is a risk that the U.S. Congress could amend the BPCIA to significantly shorten these exclusivity periods as proposed by President Obama, or that the FDA will not consider our product candidates to be reference products for competing products, potentially creating the opportunity for generic competition sooner than anticipated. Moreover, the extent to which a biosimilar, once approved, will be substituted for any one of our reference products in a way that is similar to traditional generic substitution for non-biological products is not yet clear, and will depend on a number of marketplace and regulatory factors that are still developing.
If we fail to comply with environmental, health and safety laws and regulations, we could become subject to fines or penalties or incur costs that could have a material adverse effect on the success of our business.
We are subject to numerous environmental, health and safety laws and regulations, including those governing laboratory procedures and the handling, use, storage, treatment and disposal of hazardous materials and wastes. Our operations involve the use of hazardous and flammable materials, including chemicals and radioactive and biological materials. Our operations also produce hazardous waste products. We generally contract with third parties for the disposal of these materials and wastes. We cannot eliminate the risk of contamination or injury from these materials. In the event of contamination or injury resulting from our use of hazardous materials, we could be held liable for any resulting damages, and any liability could exceed our resources. We also could incur significant costs associated with civil or criminal fines and penalties.
Although we maintain workers’ compensation insurance to cover us for costs and expenses we may incur due to injuries to our employees resulting from the use of hazardous materials, this insurance may not provide adequate coverage against potential liabilities. We do not maintain insurance for environmental liability or toxic tort claims that may be asserted against us in connection with our storage or disposal of biological, hazardous or radioactive materials.
In addition, we may incur substantial costs in order to comply with current or future environmental, health and safety laws and regulations. These current or future laws and regulations may impair our research, development or production efforts. Failure to comply with these laws and regulations also may result in substantial fines, penalties or other sanctions.
Risks Related to Employee Matters and Managing Growth
Our future success depends on our ability to retain our chief executive officer and other key executives and to attract, retain and motivate qualified personnel.
We are highly dependent on Jeffrey Abbey, our President and Chief Executive Officer, Charles Nicolette, our Vice President of Research and Development and Chief Scientific Officer, and Fred Miesowicz, our Vice President of Manufacturing and Chief Operating Officer, as well as the other principal members of our management and scientific teams. Although we have formal
64
Table of Contents
employment agreements with each of our executive officers, these agreements do not prevent our executives from terminating their employment with us at any time. We do not maintain “key person” insurance for any of our executives or other employees. The loss of the services of any of these persons could impede the achievement of our research, development and commercialization objectives.
Recruiting and retaining qualified scientific, clinical, manufacturing and sales and marketing personnel will also be critical to our success. We may not be able to attract and retain these personnel on acceptable terms given the competition among numerous pharmaceutical and biotechnology companies for similar personnel. We also experience competition for the hiring of scientific and clinical personnel from universities and research institutions. In addition, we rely on consultants and advisors, including scientific and clinical advisors, to assist us in formulating our research and development and commercialization strategy. Our consultants and advisors may be employed by employers other than us and may have commitments under consulting or advisory contracts with other entities that may limit their availability to us.
We expect to expand our development, regulatory, manufacturing and sales and marketing capabilities, and as a result, we may encounter difficulties in managing our growth, which could disrupt our operations.
We expect to experience significant growth in the number of our employees and the scope of our operations, particularly in the areas of drug development, regulatory affairs, manufacturing and sales and marketing. To manage our anticipated future growth, we must continue to implement and improve our managerial, operational and financial systems, expand our facilities and continue to recruit and train additional qualified personnel. Due to our limited financial resources and the limited experience of our management team in managing a company with such anticipated growth, we may not be able to effectively manage the expansion of our operations or recruit and train additional qualified personnel. The physical expansion of our operations may lead to significant costs and may divert our management and business development resources. Any inability to manage growth could delay the execution of our business plans or disrupt our operations.
Risks Related to Our Common Stock
Our executive officers, directors and principal stockholders maintain the ability to control all matters submitted to stockholders for approval.
Our executive officers, directors and our existing stockholders who own more than 5% of our outstanding common stock, which we refer to as our existing principal stockholders, in the aggregate, beneficially owned shares representing approximately 57.5% of our common stock as of February 28, 2014. As a result, if these stockholders were to choose to act together, they would be able to control all matters submitted to our stockholders for approval, as well as our management and affairs. For example, these persons, if they choose to act together, would control the election of directors and approval of any merger, consolidation or sale of all or substantially all of our assets. This concentration of voting power could delay or prevent an acquisition of our company on terms that other stockholders may desire.
Our largest stockholder, Pharmstandard, could exert significant influence over us and could limit your ability to influence the outcome of key transactions, including any change of control.
Our largest stockholder, Pharmstandard, owns, in the aggregate, approximately 30.44% of our outstanding common stock as of February 28, 2014. In addition, two members of our board of directors are affiliates of Pharmstandard. As a result, we expect that Pharmstandard will be able to exert significant influence over our business. Pharmstandard may have interests that differ from your interests, and it may vote in a way with which you disagree and that may be adverse to your interests. The concentration of ownership of our capital stock may have the effect of delaying, preventing or deterring a change of control of our company, could deprive our stockholders of an opportunity to receive a premium for their common stock as part of a sale of our company and may adversely affect the market price of our common stock.
Provisions in our corporate charter documents and under Delaware law could make an acquisition of us, which may be beneficial to our stockholders, more difficult and may prevent attempts by our stockholders to replace or remove our current management.
Provisions in our corporate charter and our bylaws may discourage, delay or prevent a merger, acquisition or other change in control of us that stockholders may consider favorable, including transactions in which you might otherwise receive a premium for your shares. These provisions could also limit the price that investors might be willing to pay in the future for shares of our common stock, thereby depressing the market price of our common stock. In addition, because our board of directors is responsible for appointing the members of our management team, these provisions may frustrate or prevent any attempts by our stockholders to replace or remove our current management by making it more difficult for stockholders to replace members of our board of directors. Among other things, these provisions:
| • | establish a classified board of directors such that not all members of the board are elected at one time; |
| • | allow the authorized number of our directors to be changed only by resolution of our board of directors; |
65
Table of Contents
| • | limit the manner in which stockholders can remove directors from the board; |
| • | establish advance notice requirements for stockholder proposals that can be acted on at stockholder meetings and nominations to our board of directors; |
| • | require that stockholder actions must be effected at a duly called stockholder meeting and prohibit actions by our stockholders by written consent; |
| • | limit who may call stockholder meetings; |
| • | authorize our board of directors to issue preferred stock without stockholder approval, which could be used to institute a “poison pill” that would work to dilute the stock ownership of a potential hostile acquirer, effectively preventing acquisitions that have not been approved by our board of directors; and |
| • | require the approval of the holders of at least 75% of the votes that all our stockholders would be entitled to cast to amend or repeal certain provisions of our charter or bylaws. |
Moreover, because we are incorporated in Delaware, we are governed by the provisions of Section 203 of the Delaware General Corporation Law, which prohibits a person who owns in excess of 15% of our outstanding voting stock from merging or combining with us for a period of three years after the date of the transaction in which the person acquired in excess of 15% of our outstanding voting stock, unless the merger or combination is approved in a prescribed manner.
An active trading market for our common stock may not develop.
Prior to February 6, 2014, there was no public market for our common stock. Although our common stock is currently listed on The NASDAQ Global Market, an active trading market for our shares has not developed. If an active market for our common stock does not develop, it may be difficult for you to sell shares without depressing the market price for the shares or at all. Even if an active market for our common stock does develop, it may not be sustained.
If our stock price is volatile, purchasers of our common stock could incur substantial losses.
Our stock price is likely to be volatile. The stock market in general and the market for biotechnology companies in particular have experienced extreme volatility that has often been unrelated to the operating performance of particular companies. The market price for our common stock may be influenced by many factors, including:
| • | results of clinical trials of our product candidates or those of our competitors; |
| • | the success of competitive products or technologies; |
| • | potential approvals of our product candidates for marketing by the FDA or equivalent foreign regulatory authorities or our failure to obtain such approvals; |
| • | regulatory or legal developments in the United States and other countries; |
| • | the results of our efforts to commercialize our product candidates; |
| • | developments or disputes concerning patents or other proprietary rights; |
| • | the recruitment or departure of key personnel; |
| • | the level of expenses related to any of our product candidates or clinical development programs; |
| • | the results of our efforts to discover, develop, acquire or in-license additional product candidates or products; |
| • | actual or anticipated changes in estimates as to financial results, development timelines or recommendations by securities analysts; |
| • | variations in our financial results or those of companies that are perceived to be similar to us; |
| • | changes in the structure of healthcare payment systems; |
66
Table of Contents
| • | market conditions in the pharmaceutical and biotechnology sectors and issuance of new or changed securities analysts’ reports or recommendations; |
| • | general economic, industry and market conditions; and |
| • | the other factors described in this “Risk Factors” section. |
We will incur increased costs as a result of operating as a public company, and our management will be required to devote substantial time to new compliance initiatives and corporate governance practices.
As a public company, and particularly after we are no longer an “emerging growth company,” we will incur significant legal, accounting and other expenses that we did not incur as a private company. The Sarbanes-Oxley Act of 2002, the Dodd-Frank Wall Street Reform and Consumer Protection Act, the listing requirements of The NASDAQ Global Market and other applicable securities rules and regulations impose various requirements on public companies, including establishment and maintenance of effective disclosure and financial controls and corporate governance practices. We expect that we will need to hire additional accounting, finance and other personnel in connection with our becoming, and our efforts to comply with the requirements of being, a public company and our management and other personnel will need to devote a substantial amount of time towards maintaining compliance with these requirements. These requirements will increase our legal and financial compliance costs and will make some activities more time-consuming and costly. For example, we expect that the rules and regulations applicable to us as a public company may make it more difficult and more expensive for us to obtain director and officer liability insurance, which could make it more difficult for us to attract and retain qualified members of our board of directors. We are currently evaluating these rules and regulations, and cannot predict or estimate the amount of additional costs we may incur or the timing of such costs. These rules and regulations are often subject to varying interpretations, in many cases due to their lack of specificity, and, as a result, their application in practice may evolve over time as new guidance is provided by regulatory and governing bodies. This could result in continuing uncertainty regarding compliance matters and higher costs necessitated by ongoing revisions to disclosure and governance practices.
Pursuant to Section 404 of the Sarbanes-Oxley Act of 2002, we will be required to furnish a report by our management on our internal control over financial reporting beginning with our second filing of an Annual Report on Form 10-K with the Securities and Exchange Commission, or the SEC, after we become a public company. However, while we remain an emerging growth company, we will not be required to include an attestation report on internal control over financial reporting issued by our independent registered public accounting firm. To achieve compliance with Section 404 of the Sarbanes-Oxley Act of 2002 within the prescribed period, we will be engaged in a process to document and evaluate our internal control over financial reporting, which is both costly and challenging. In this regard, we will need to continue to dedicate internal resources, potentially engage outside consultants and adopt a detailed work plan to assess and document the adequacy of internal control over financial reporting, continue steps to improve control processes as appropriate, validate through testing that controls are functioning as documented and implement a continuous reporting and improvement process for internal control over financial reporting. Despite our efforts, there is a risk that we will not be able to conclude, within the prescribed timeframe or at all, that our internal control over financial reporting is effective as required by Section 404 of the Sarbanes-Oxley Act of 2002. If we identify one or more material weaknesses, it could result in an adverse reaction in the financial markets due to a loss of confidence in the reliability of our financial statements.
We are an “emerging growth company,” and the reduced disclosure requirements applicable to emerging growth companies may make our common stock less attractive to investors.
We are an “emerging growth company,” as defined in the JOBS Act, and may remain an emerging growth company for up to five years. For so long as we remain an emerging growth company, we are permitted and plan to rely on exemptions from certain disclosure requirements that are applicable to other public companies that are not emerging growth companies. These exemptions include not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act of 2002, not being required to comply with any requirement that may be adopted by the Public Company Accounting Oversight Board regarding mandatory audit firm rotation or a supplement to the auditor’s report providing additional information about the audit and the financial statements, reduced disclosure obligations regarding executive compensation and exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and shareholder approval of any golden parachute payments not previously approved. In this Annual Report on Form 10-K, we have not included all of the executive compensation related information that would be required if we were not an emerging growth company. We cannot predict whether investors will find our common stock less attractive if we rely on these exemptions. If some investors find our common stock less attractive as a result, there may be a less active trading market for our common stock and our stock price may be more volatile.
In addition, the JOBS Act provides that an emerging growth company can take advantage of an extended transition period for complying with new or revised accounting standards. This allows an emerging growth company to delay the adoption of certain accounting standards until those standards would otherwise apply to private companies. We have irrevocably elected not to avail ourselves of this exemption from new or revised accounting standards and, therefore, we will be subject to the same new or revised accounting standards as other public companies that are not emerging growth companies.
67
Table of Contents
Because we do not anticipate paying any cash dividends on our capital stock in the foreseeable future, capital appreciation, if any, will be your sole source of gain.
We have never declared or paid cash dividends on our capital stock. We currently intend to retain all of our future earnings, if any, to finance the growth and development of our business. In addition, the terms of any future debt agreements may preclude us from paying dividends. As a result, capital appreciation, if any, of our common stock will be your sole source of gain for the foreseeable future.
A significant portion of our total outstanding shares are restricted from immediate resale but may be sold into the market in the near future, which could cause the market price of our common stock to drop significantly, even if our business is doing well.
Sales of a substantial number of shares of our common stock in the public market could occur at any time. These sales, or the perception in the market that the holders of a large number of shares intend to sell shares, could reduce the market price of our common stock. As of February 28, 2014, we have outstanding 19,654,362 shares of common stock, of which 14,886,683 shares are subject to restrictions on transfer under 180-day lock-up arrangements with the underwriters of our initial public offering. These restrictions are due to expire on August 5, 2014, resulting in these shares becoming eligible for resale into the public market on August 6, 2014 if they are registered under the Securities Act or if they qualify for an exemption from registration under the Securities Act including under Rules 144 or 701.
We also intend to register all shares of our common stock that we may issue under our equity compensation plans. Once we register these shares, they can be freely sold in the public market upon issuance, subject to volume limitations applicable to affiliates and the lock-up agreements.
If securities or industry analysts do not publish research or publish inaccurate or unfavorable research about our business, our share price and trading volume could decline.
The trading market for our common stock will depend on the research and reports that securities or industry analysts publish about us or our business. We do not have any control over these analysts. There can be no assurance that analysts will cover us, or provide favorable coverage. If one or more analysts downgrade our stock or change their opinion of our stock to be less favorable, our share price would likely decline. In addition, if one or more analysts cease coverage of our company or fail to regularly publish reports on us, we could lose visibility in the financial markets, which could cause our share price or trading volume to decline.
Item 1B. Unresolved Staff Comments
None.
Our only facility is located in Durham, North Carolina, where we occupy approximately 20,000 square feet of office, laboratory and manufacturing space. Our lease expires in November 2016, but we may terminate it in December 2014. We are in the process of identifying suitable space to lease for our planned new commercial manufacturing facility.
From time to time, we have been and may again become involved in legal proceedings arising in the ordinary course of our business. We are not presently a party to any material litigation.
Item 4. Mine Safety Disclosures
Not Applicable.
68
Table of Contents
Item 5. Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities
Our common stock is listed on the NASDAQ Global Market under the symbol “ARGS” and began trading on February 7, 2014. Prior to that, there was no public trading market for our common stock. As of March 21, 2014, there were 19,654,362 outstanding shares and 79 stockholders of record. This number does not include beneficial owners whose shares were held in street name. This number of holders of record also does not include stockholders whose shares may be held in trust by other entities.
The following table sets forth for the period indicated the high and low closing sale prices for our common stock as reported on the NASDAQ Global Market:
| Market Price | ||||||||
| High | Low | |||||||
| First quarter (February 7, 2014 to March 28, 2014) |
$ | 13.74 | $ | 7.97 | ||||
Dividends
We have never declared or paid cash dividends on our capital stock. We currently intend to retain all of our future earnings, if any, to finance the growth and development of our business. In addition, the terms of any future debt agreements may preclude us from paying dividends.
Recent Sales of Unregistered Securities
Set forth below is information regarding shares of our common stock, shares of our preferred stock, warrants to purchase shares of our common stock, warrants to purchase shares of our preferred stock and stock options granted, by us since January 1, 2013 that were not registered under the Securities Act. Also included is the consideration, if any, received by us for such shares and options and information relating to the section of the Securities Act, or rule of the SEC, under which exemption from registration was claimed.
(a) Issuance of Capital Stock
From August 2013 through November 2013, we sold an aggregate of 36,856,922 shares of our series E preferred stock in a private placement to certain of our existing holders of preferred stock and additional accredited investors at a purchase price of $1.302333 per share. The sale of series E preferred stock was structured in multiple tranches and with multiple closing dates. Under the agreement we sold 16,898,436 shares of our series E preferred stock for an aggregate purchase price of $22,007,404 in August 2013, 921,423 shares of our series E preferred stock for an aggregate purchase price of $1,200,000 in October 2013 and 19,037,063 shares of our series E preferred stock for an aggregate purchase price of $24,792,595 in November 2013.
In connection with the issuance and sale of our series E preferred stock, investors exchanged an aggregate of 111,837 shares of series A preferred stock, 8,687,630 shares of series B preferred stock, and 10,586,174 shares of series C preferred stock for 2,985,863 shares of our series D preferred stock.
In connection with the issuance and sale of our series E preferred stock, investors exchanged an aggregate of 12,607,779 shares of series D preferred stock for 19,154,336 shares of our series E preferred stock.
In connection with the issuance and sale of our series E preferred stock in August 2013 through November 2013, we issued warrants to the purchasers of our series E preferred stock to purchase an aggregate of 5,670,283 shares of our common stock at an exercise price of $0.01 per share. The warrants had a term of ten years and would only become exercisable if the Company did not receive $5,000,000 in non-dilutive financing by December 31, 2013 or had not executed definitive documentation providing for an additional $5,000,000 investment of non-dilutive financing as of December 31, 2013. In connection with the license agreement that we entered into with Medinet paid us $1.0 million and loaned us $9.0 million. In connection with our receipt of these funds, the warrants were cancelled pursuant to their terms.
In connection with the issuance and sale of series E preferred stock, in December 2013, we issued a warrant to purchase 57,589 shares of our common stock, at an exercise price of $1.22 per share, to a placement agent.
he securities described in this section (a) of Item 5 were issued to investors in reliance upon the exemption from the registration requirements of the Securities Act, as set forth in Section 4(2) under the Securities Act and Regulation D promulgated thereunder relative to transactions by an issuer not involving any public offering, to the extent an exemption from such registration was required. All purchasers of shares of preferred stock described above represented to us in connection with their purchase that they were accredited investors and were acquiring the shares for their own account for investment purposes only and not with a view to, or for sale in connection with, any distribution thereof and that they could bear the risks of the investment and could hold the securities for an indefinite period of time. The purchasers received written disclosures that the securities had not been registered under the Securities Act and that any resale must be made pursuant to a registration statement or an available exemption from such registration.
69
Table of Contents
(b) Stock Option Grants
Between January 1, 2013 and December 31, 2013, we granted options to purchase an aggregate of 1,405,793 shares of common stock, with exercise prices ranging from $4.20 to $7.32 per share, to our employees, directors, advisors and consultants. Between January 1, 2013 and December 31, 2013, we issued an aggregate of 1,378 shares of common stock upon the exercise of options for aggregate consideration of $5,793 and options to purchase 6,698 shares had been forfeited. We intend to file a registration statement on Form S-8 under the Securities Act to register all of the shares of our common stock subject to outstanding options and options and other awards issuable pursuant to our equity compensation plans.
The stock options and the common stock issuable upon the exercise of such options described in the first paragraph of section (b) of Item 5 were issued pursuant to written compensatory plans or arrangements with our employees, directors and consultants, in reliance on the exemption provided by Rule 701 promulgated under the Securities Act. All recipients either received adequate information about the Registrant or had access, through employment or other relationships, to such information.
The issuance of these securities was made in reliance on the exemption from the registration requirements of the Securities Act, as set forth in Section 4(2) under the Securities Act and Regulation D promulgated thereunder relative to transactions by an issuer not involving any public offering, to the extent an exemption from such registration was required. Each security holder represented that it was an accredited investor and was acquiring the security for its own account for investment purposes only and not with a view to, or for sale in connection with, any distribution thereof and that they could bear the risks of the investment and could hold the security for an indefinite period of time and appropriate legends were affixed to the instruments representing such securities issued in such transactions. Such recipients either received adequate information about us or had, through its relationship with us, access to such information.
All of the foregoing securities are deemed restricted securities for purposes of the Securities Act. All certificates representing the issued shares of capital stock described in this Item 5 included appropriate legends setting forth that the securities have not been registered and the applicable restrictions on transfer.
Purchase of Equity Securities
We did not purchase any of our registered equity securities during the period covered by this Annual Report on Form 10-K.
Use of Proceeds from Initial Public Offering
In February 2014, we issued and sold 6,228,725 shares of our common stock, including 603,725 shares of common stock sold pursuant to the underwriters’ exercise of their option to purchase additional shares, in our initial public offering at a public offering price of $8.00 per share, for aggregate gross proceeds of $49.8 million. All of the shares issued and sold in our initial public offering were registered under the Securities Act pursuant to a Registration Statement on Form S-1 (File No. 333-193137), which was declared effective by the SEC on February 6, 2014. Piper Jaffray & Co. and Stifel, Nicolaus & Company acted as joint book-running managers of the offering and as representatives of the underwriters. The offering commenced on February 6, 2014 and did not terminate until the sale of all of the shares offered.
The net offering proceeds to us, after deducting underwriting discounts and commissions of approximately $3.5 million and offering expenses of approximately $2.6 million, were approximately $43.7 million. No offering expenses were paid directly or indirectly to any of our directors or officers (or their associated) or persons owning 10.0% or more of any class of our equity securities or to any other affiliates. There has been no material change in the planned use of proceeds from our initial public offering as described in our final prospectus filed with the SEC on February 6, 2014 pursuant to Rule 424(b) of the Securities Act.
Item 6. Selected Financial Data
You should read the following selected consolidated financial data together with our consolidated financial statements and the related notes in “Item 8. Financial Statements and Supplementary Data” and “Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations” in this Annual Report on Form 10-K. We have derived the consolidated statements of operations data for the years ended December 31, 2011, 2012 and 2013 and for the period from May 8, 1997 (inception) to December 31, 2013, and the consolidated balance sheet data as of December 31, 2012 and 2013 from our audited consolidated financial statements included in “Item 8. Financial Statements and Supplementary Data.” The consolidated balance sheet data as of December 31, 2011
70
Table of Contents
was derived from our audited financial statements which are not included in this Annual Report on Form 10-K. Our historical results for any prior period are not necessarily indicative of results to be expected in any future period. The consolidated financial information reflects a one-for-six reverse stock split of our common stock effected on January 17, 2014, which has been retrospectively applied for all periods presented.
Consolidated Statements of Operations Data:
| Year Ended December 31, | Cumulative Period from May 8, 1997 (Inception) to December 31, |
|||||||||||||||
| 2011 | 2012 | 2013 | 2013 | |||||||||||||
| Revenue |
$ | 7,642,695 | $ | 7,039,010 | $ | 4,421,689 | $ | 86,826,528 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Operating expenses |
||||||||||||||||
| Research and development |
12,668,025 | 17,616,892 | 23,991,151 | 205,626,225 | ||||||||||||
| Net reimbursement under collaboration agreement |
— | — | — | (47,179,130 | ) | |||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net research and development |
12,668,025 | 17,616,892 | 23,991,151 | 158,447,095 | ||||||||||||
| General and administrative |
3,703,813 | 6,135,581 | 4,662,317 | 58,838,234 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total operating expenses |
16,371,838 | 23,752,473 | 28,653,468 | 217,285,329 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Operating loss |
(8,729,143 | ) | (16,713,463 | ) | (24,231,779 | ) | (130,458,801 | ) | ||||||||
| Other income (expense) |
||||||||||||||||
| Interest income |
1,259 | 4,604 | 7,184 | 4,105,138 | ||||||||||||
| Interest expense |
(6,656,366 | ) | (292,496 | ) | (4,705 | ) | (12,515,919 | ) | ||||||||
| Change in fair value of warrant liability |
(4,661,811 | ) | 4,916,785 | 355,352 | 610,326 | |||||||||||
| Derivative (expense) income |
(94,668 | ) | 1,036,403 | — | (39,450 | ) | ||||||||||
| Investment tax credits |
— | 694,331 | — | 2,761,556 | ||||||||||||
| Other expense |
— | (117,494 | ) | (47,615 | ) | (165,109 | ) | |||||||||
| Loss on sale of marketable securities |
— | — | — | (10,242,252 | ) | |||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Other income (expense), net |
(11,411,586 | ) | 6,242,133 | 310,216 | (15,485,710 | ) | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net loss |
(20,140,729 | ) | (10,471,330 | ) | (23,921,563 | ) | (145,944,511 | ) | ||||||||
| Net loss attributable to noncontrolling interest |
(63,047 | ) | — | — | (760,107 | ) | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net loss attributable to Argos Therapeutics, Inc. |
(20,077,682 | ) | (10,471,330 | ) | (23,921,563 | ) | (145,184,404 | ) | ||||||||
| Accretion of redeemable convertible preferred stock (See Note 19) |
(926,542 | ) | (351,371 | ) | 4,772,991 | (25,626,700 | ) | |||||||||
| Less: Preferred stock dividend due to exchanges of preferred shares (See Note 19) |
— | — | (14,726,088 | ) | (14,726,088 | ) | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net loss attributable to common stockholders |
$ | (21,004,224 | ) | $ | (10,822,701 | ) | $ | (33,874,660 | ) | $ | (185,537,192 | ) | ||||
|
|
|
|
|
|
|
|
|
|||||||||
| Basic and diluted net loss attributable to common stockholders per share |
$ | (197.29 | ) | $ | (54.58 | ) | $ | (147.37 | ) | |||||||
|
|
|
|
|
|
|
|||||||||||
| Basic and diluted weighted average shares outstanding |
106,466 | 198,306 | 229,865 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||
71
Table of Contents
| As of December 31, | ||||||||||||
| 2011 | 2012 | 2013 | ||||||||||
| Consolidated Balance Sheet Data: |
||||||||||||
| Cash, cash equivalents and short-term investments |
$ | 2,002,814 | $ | 12,363,736 | $ | 46,957,782 | ||||||
| Working capital (deficit) |
(19,541,194 | ) | 5,741,666 | 46,365,329 | ||||||||
| Total assets |
5,973,958 | 15,396,973 | 51,131,295 | |||||||||
| Redeemable convertible preferred stock |
77,722,306 | 75,800,882 | 113,664,469 | |||||||||
| Total stockholders’ deficit |
(96,355,106 | ) | (68,567,710 | ) | (75,776,593 | ) | ||||||
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations
You should read the following discussion and analysis of our financial condition and results of operations together with our consolidated financial statements and the related notes appearing in “Item 8. Financial Statements and Supplementary Data” in this Annual Report on Form 10-K. In addition to historical information, some of the information contained in this discussion and analysis or set forth elsewhere in this Annual Report on Form 10-K, including information with respect to our plans and strategy for our business, future financial performance, expense levels and liquidity sources, includes forward-looking statements that involve risks and uncertainties. You should read “Item 1A. Risk Factors” in this Annual Report on Form 10-K for a discussion of important factors that could cause actual results to differ materially from the results described in or implied by the forward-looking statements contained in the following discussion and analysis.
Overview
We are a biopharmaceutical company focused on the development and commercialization of fully personalized immunotherapies for the treatment of cancer and infectious diseases based on our proprietary technology platform called Arcelis. Our most advanced product candidate is AGS-003, which we are developing for the treatment of metastatic renal cell carcinoma, or mRCC, and other cancers. We are currently enrolling patients in a pivotal phase 3 clinical trial of AGS-003 in combination with sunitinib (Sutent) for the treatment of clear cell mRCC under a special protocol assessment, or SPA, with the Food and Drug Administration, or FDA. We also plan to conduct phase 2 clinical trials to explore the use of AGS-003 in non-clear cell mRCC, in early stage RCC prior to and following nephrectomy, and in other solid tumors. We are developing our second Arcelis product candidate, AGS-004, for the treatment of HIV and are conducting a phase 2b clinical trial of AGS-004 that is being funded entirely by the National Institutes of Health, or NIH, under a $39.3 million contract. In addition, we expect two phase 2 clinical trials of AGS-004 to be initiated in 2014, one for HIV eradication and one for long-term viral control in pediatric patients.
We have devoted substantially all of our resources to our drug development efforts, including advancing our Arcelis platform, conducting clinical trials of our product candidates, protecting our intellectual property and providing general and administrative support for these operations. We have not generated any revenue from product sales and, to date, have funded our operations primarily through private placements of our preferred stock, convertible debt financings, bank debt, government contracts, government and other third party grants and license and collaboration agreements. From inception in May 1997 through December 31, 2013, we have raised a total of $291.4 million in cash, including:
| • | $165.5 million from the sale of our common stock, convertible debt, warrants and preferred stock; |
| • | $33.9 million from the licensing of our technology; and |
| • | $92.0 million from government contracts, grants and license and collaboration agreements. |
In February 2014, we issued and sold 6,228,725 shares of our common stock, including 603,725 shares of common stock sold pursuant to the underwriters’ exercise of their option to purchase additional shares, in our initial public offering, or IPO, at a public offering price of $8.00 per share, for aggregate gross proceeds of $49.8 million. The net offering proceeds to us, after deducting underwriting discounts and commissions of approximately $3.5 million and offering expenses of approximately $2.6 million, were approximately $43.7 million.
We have incurred losses in each year since our inception in May 1997. Our net loss, after attribution to the noncontrolling interest, was $20.1 million for the year ended December 31, 2011, $10.5 million for the year ended December 31, 2012 and $23.9 million for the year ended December 31, 2013. As of December 31, 2013, we had an accumulated deficit of $150.9 million. Substantially all of our operating losses have resulted from costs incurred in connection with our development programs and from general and administrative costs associated with our operations.
72
Table of Contents
We expect to continue to incur significant expenses and increasing operating losses for at least the next several years. We anticipate that our expenses will increase substantially if and as we:
| • | continue our ongoing phase 3 clinical trial of AGS-003 for the treatment of mRCC and initiate additional clinical trials of AGS-003 for the treatment of cancers; |
| • | initiate additional clinical trials of AGS-004 for the treatment of HIV; |
| • | seek regulatory approvals for our product candidates that successfully complete clinical trials; |
| • | lease, build out and equip a new commercial facility for the manufacture of our Arcelis-based products; |
| • | establish a sales, marketing and distribution infrastructure to commercialize products for which we may obtain regulatory approval; |
| • | maintain, expand and protect our intellectual property portfolio; |
| • | continue our other research and development efforts; |
| • | hire additional clinical, quality control, scientific and management personnel; and |
| • | add operational, financial and management information systems and personnel, including personnel to support our product development and planned commercialization efforts. |
We do not expect to generate significant funds or product revenue, other than under our contract with the NIH as described below, unless and until we successfully complete development, obtain marketing approval and commercialize our product candidates, either alone or in collaboration with third parties, which we expect will take a number of years and is subject to significant uncertainty. Accordingly, we will need to raise additional capital prior to the commercialization of AGS-003, AGS-004 or any of our other product candidates. Until such time, if ever, as we can generate substantial product revenues, we expect to finance our operating activities through a combination of equity offerings, debt financings, government contracts, government and other third party grants or other third party funding, marketing and distribution arrangements and other collaborations, strategic alliances and licensing arrangements. However, we may be unable to raise additional funds through these means when needed, on favorable terms or at all.
NIH Funding
In September 2006, we entered into a multi-year research contract with the NIH and the National Institute of Allergy and Infectious Diseases, or NIAID, to design, develop and clinically test an autologous HIV immunotherapy capable of eliciting therapeutic immune responses. We are using funds from this contract to develop AGS-004, including to fund in full our phase 2b clinical trial of AGS-004. We have agreed to a statement of work under the contract, and are obligated to furnish all the services, qualified personnel, material, equipment, and facilities, not otherwise provided by the U.S. government, needed to perform the statement of work.
Under this contract, as amended, the NIH and NIAID have committed to fund up to a total of $39.3 million, including reimbursement of direct expenses and allocated overhead and general and administrative expenses of up to $37.9 million and payment of other specified amounts totaling up to $1.4 million upon our achievement of specified development milestones. This commitment extends to September 2015. Since September 2010, we have received reimbursement of our allocated overhead and general and administrative expenses at provisional indirect cost rates equal to negotiated provisional indirect cost rates agreed to with the NIH in September 2010. These provisional indirect cost rates are subject to adjustment based on our actual costs pursuant to the agreement with the NIH and may result in additional payments to us from the NIH and the NIAID to reflect our actual costs since September 2010.
We have recorded revenue of $35.0 million through December 31, 2013 under the NIH contract. This contract is the only arrangement under which we currently generate revenue. As of December 31, 2013, there was up to approximately $4.3 million of potential revenue remaining to be earned under the agreement with the NIH.
Exchange of Series D-1 Preferred Stock for Series D Preferred Stock
In July 2013, the holders of shares of our series D-1 preferred stock exchanged all shares of our series D-1 preferred stock held by such investors for shares of our series D preferred stock. In this exchange, we issued an aggregate of 16,151,212 shares of our series D
73
Table of Contents
preferred stock in exchange for an aggregate of 7,433,870 shares of our series D-1 preferred stock. In connection with this exchange, warrants to purchase 32,869,928 shares of our series C preferred stock and warrants to purchase 1,332,622 shares of our series D-1 preferred stock were cancelled. We did not receive any cash proceeds in connection with the exchange.
In addition, in connection with the exchange, we reduced the original per share purchase price of the series D preferred stock from $3.528233 per share to $1.978565 per share. As a result of such reduction, we issued an aggregate of 6,373,782 additional shares of our series D preferred stock to the holders that had purchased shares of our series D preferred stock prior to such reduction for no additional consideration.
At the time of the exchange, we also amended our certificate of incorporation to reduce the liquidation preference and redemption price of our series A preferred stock to $0.32 per share, our series B preferred stock to $0.5632 per share, our series C preferred stock to $0.09248576 per share and our series D preferred stock to $1.978565 per share. In addition, we extended the redemption trigger date of our preferred stock by one year to July 31, 2018.
We recorded the issuance of additional shares of series D preferred stock in connection with the exchange of series D-1 preferred stock, the modifications to the liquidation preferences and redemption prices of the series A preferred stock, the series B preferred stock, the series C preferred stock and the series D preferred stock and the extension of the redemption trigger date as adjustments to additional paid-in capital.
Series E Preferred Stock Financing
In August 2013, we issued and sold 16,898,436 shares of our series E preferred stock at a price of $1.302333 per share resulting in gross proceeds of $22.0 million, pursuant to a series E preferred stock and warrant purchase agreement. In connection with the issuance and sale of series E preferred stock in August 2013, we issued to the purchasers of the series E preferred stock warrants to purchase an aggregate of 433,282 shares of our common stock at an exercise price of $0.06 per share. The warrants had a term of ten years and would only become exercisable if we did not receive $5.0 million in non-dilutive financing by December 31, 2013 or had not executed definitive documentation providing for an additional $5.0 million investment of non-dilutive financing as of December 31, 2013. In connection with the license agreement that we entered into with Medinet in December 2013, Medinet paid us $1.0 million and loaned us $9.0 million. See “—Collaborations—Medinet”. In connection with our receipt of these funds, the warrants were cancelled pursuant to their terms.
In connection with the issuance and sale of our series E preferred stock in August 2013, our existing investors that participated in the offering exchanged an aggregate of 111,837 shares of our series A preferred stock, 8,687,630 shares of our series B preferred stock and 10,586,174 shares of our series C preferred stock for a total of 2,985,863 shares of our series D preferred stock, and an aggregate of 12,607,779 shares of our series D preferred stock for 19,154,336 shares of our series E preferred stock.
In October 2013, we issued and sold to one purchaser an additional 921,423 shares of our series E preferred stock at a price of $1.302333 per share resulting in gross proceeds of $1.2 million. In connection with this issuance we issued to the purchaser warrants to purchase 23,626 shares of our common stock at an exercise price of $0.06 per share on the same terms as the warrants we issued to the initial purchasers of our series E preferred stock. In November 2013, we issued and sold to one purchaser an additional 19,037,063 shares of our series E preferred stock at a price of $1.302333 per share resulting in gross proceeds of $24.8 million. In connection with this issuance, we issued the purchaser warrants to purchase 488,128 shares of our common stock at an exercise price of $0.06 per share on the same terms as the warrants we issued to the initial purchasers of our series E preferred stock. These warrants were also cancelled in December 2013 in connection with our receipt of funds under our license agreement with Medinet.
In connection with their purchase of shares of our series E preferred stock, purchasers of our series E preferred stock committed to purchase additional shares of our series E preferred stock for an aggregate purchase price of $12.0 million in a second tranche closing upon the achievement of specified milestones with respect to our ongoing phase 3 clinical trial of AGS-003. This commitment terminated in connection with the closing of the IPO.
Collaborations
Pharmstandard
In August 2013, in connection with the purchase of shares of our series E preferred stock by Pharmstandard International S.A., or Pharmstandard, we entered into an exclusive royalty-bearing license agreement with Pharmstandard. Under this license agreement, we granted Pharmstandard and its affiliates a license, with the right to sublicense, to develop, manufacture and commercialize AGS-003 and other products for the treatment of human diseases which are developed by Pharmstandard using our personalized immunotherapy platform in the Russian Federation, Armenia, Azerbaijan, Belarus, Georgia, Kazakhstan, Kyrgyzstan, Moldova, Tajikistan, Turkmenistan, Ukraine and Uzbekistan, which we refer to as the Pharmstandard Territory. We also provided Pharmstandard with a right of first negotiation for development and commercialization rights in the Pharmstandard Territory to specified additional products we may develop.
74
Table of Contents
Pharmstandard agreed to pay us royalties on net sales of all licensed products in the low single digits until it has generated a specified amount of aggregate net sales. Once the net sales threshold is achieved, Pharmstandard will pay us royalties on net sales of specified licensed products, including AGS-003, in the low double digits below 20%. After the net sales threshold is achieved, Pharmstandard has the right to offset a portion of the royalties Pharmstandard pays to third parties for licenses to necessary third party intellectual property against the royalties that Pharmstandard pays to us. For further information regarding this collaboration, see “Business — Development and Commercialization Agreements — Pharmstandard.”
In November 2013, we entered into an agreement with Pharmstandard under which Pharmstandard purchased additional shares of our series E preferred stock. Under this agreement, we agreed to enter into a manufacturing rights agreement for the European market with Pharmstandard and that the manufacturing rights agreement would provide for the issuance of warrants to Pharmstandard to purchase 499,788 shares of our common stock at an exercise price of $5.82 per share. As of February 28, 2014, we had not entered into this manufacturing rights agreement or issued the warrants.
Green Cross
In July 2013, in connection with the purchase of shares of our series E preferred stock by Green Cross Corp., or Green Cross, we entered into an exclusive royalty-bearing license agreement with Green Cross. Under this agreement we granted Green Cross a license to develop, manufacture and commercialize AGS-003 for mRCC in South Korea. We also provided Green Cross with a right of first negotiation for development and commercialization rights in South Korea to specified additional products we may develop.
Under the terms of the license, Green Cross has agreed to pay us $0.5 million upon the initial submission of an application for regulatory approval of a licensed product in South Korea, $0.5 million upon the initial regulatory approval of a licensed product in South Korea and royalties ranging from the mid-single digits to low double digits below 20% on net sales until the fifteenth anniversary of the first commercial sale in South Korea. For further information regarding this collaboration, see “Business — Development and Commercialization Agreements — Green Cross.”
Medinet
In December 2013, we entered into a license agreement with Medinet. Under this agreement, we granted Medinet an exclusive, royalty-free license to manufacture in Japan AGS-003 and other products using our Arcelis technology solely for the purpose of the development and commercialization of AGS-003 and these other products for the treatment of mRCC. We refer to this license as the manufacturing license. In addition, under this agreement, we granted Medinet an option to acquire a nonexclusive, royalty-bearing license under our Arcelis technology to sell in Japan AGS-003 and other products for the treatment of mRCC. We refer to the option as the sale option and the license as the sale license.
In consideration for the manufacturing license, Medinet paid us $1.0 million. Medinet also loaned us $9.0 million in connection with us entering into the agreement. We have agreed to use these funds in the development and manufacturing of AGS-003 and the other products. Medinet also agreed to pay us milestone payments of up to a total of $9.0 million upon the achievement of developmental and regulatory milestones and $5.0 million upon the achievement of a sales milestone related to AGS-003 and these products. If Medinet exercises the sale option, it will pay us $1.0 million, as well as royalties on net sales at a rate to be negotiated until the later of the expiration of the licensed patent rights in Japan and the twelfth anniversary of the first commercial sale in Japan. If we cannot agree on the royalty rate, we have agreed to submit the matter to arbitration.
We borrowed the $9.0 million pursuant to an unsecured promissory note that bears interest at a rate of 3.0% per annum. The principal and interest under the note are due and payable on December 31, 2018. Under the terms of the note and the license agreement, any milestone payments related to the developmental and regulatory milestones that become due will be applied first to the repayment of the loan. We have the right to prepay the loan at any time. If we have not repaid the loan by December 31, 2018, then we have agreed to grant to Medinet a non-exclusive, royalty-bearing license to make and sell Arcelis products in Japan for the treatment of cancer. In such event, the amounts owing under the loan as of December 31, 2018 will constitute pre-paid royalties under the license and will not be otherwise due and payable. Royalties under this license would be paid until the expiration of the licensed patent rights in Japan at a rate to be negotiated. If we cannot agree on the royalty rate, we have agreed to submit the matter to arbitration.
We recorded the $1.0 million payment from Medinet as a deferred liability. In addition, because the $9.0 million promissory note was issued at a below market interest rate, we allocated the proceeds of the loan between the manufacturing license and the debt at the time of issuance. As a result, we recorded $6.9 million as notes payable, based upon an effective interest rate of 8%, and $2.1 million as a deferred liability. The total deferred liability recorded related to the manufacturing license was $3.1 million as of December 31, 2013. Interest expense will be recorded beginning in the fiscal year ending December 31, 2014 based upon the effective interest rate of 8%. For further information regarding this collaboration, see “Business—Development and Commercialization Agreements—Medinet.”
75
Table of Contents
Financial Overview
Revenue
To date, we have not generated revenue from the sale of any products. All of our revenue has been derived from government contracts and grants and payments under license and collaboration agreements. We have recognized $86.8 million in revenue from inception (May 8, 1997) to December 31, 2013 from these sources. We may generate revenue in the future from government contracts and grants, payments from future license or collaboration agreements and product sales. We expect that any revenue we generate will fluctuate from quarter to quarter.
Research and Development Expenses
Since our inception, we have focused our resources on our research and development activities, including conducting preclinical studies and clinical trials, manufacturing development efforts and activities related to regulatory filings for our product candidates. We recognize our research and development expenses as they are incurred. Our research and development expenses consist primarily of:
| • | salaries and related expenses for personnel in research and development functions; |
| • | fees paid to consultants and clinical research organizations, or CROs, including in connection with our clinical trials, and other related clinical trial fees, such as for investigator grants, patient screening, laboratory work and statistical compilation and analysis; |
| • | allocation of facility lease and maintenance costs; |
| • | depreciation of leasehold improvements, laboratory equipment and computers; |
| • | costs related to production of product candidates for clinical trials; |
| • | costs related to compliance with regulatory requirements; |
| • | consulting fees paid to third parties related to non-clinical research and development; |
| • | costs related to stock options or other stock-based compensation granted to personnel in research and development functions; and |
| • | acquisition fees, license fees and milestone payments related to acquired and in-licensed technologies. |
From inception (May 8, 1997) through December 31, 2013, we have incurred $205.6 million in research and development expenses. We plan to increase our research and development expenses for the foreseeable future as we seek to complete development of AGS-003 and AGS-004.
The table below summarizes our direct research and development expenses by program for the periods indicated. Our direct research and development expenses consist principally of external costs, such as fees paid to investigators, consultants, central laboratories and CROs, including in connection with our clinical trials, and related clinical trial fees. We have been developing AGS-003 and AGS-004, in parallel, and typically use our employee and infrastructure resources across multiple research and development programs. We do not allocate salaries, stock-based compensation, employee benefit or other indirect costs related to our research and development function to specific product candidates. Those expenses are included in “Indirect research and development expense” in the table.
| Year Ended December 31, | ||||||||||||
| 2011 | 2012 | 2013 | ||||||||||
| Direct research and development expense by program: |
||||||||||||
| AGS-003 |
$ | 1,763 | $ | 5,842 | $ | 11,301 | ||||||
| AGS-004 |
2,929 | 2,626 | 1,602 | |||||||||
| Other |
1,310 | 403 | 52 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total direct research and development program expense |
6,002 | 8,871 | 12,955 | |||||||||
| Indirect research and development expense |
6,666 | 8,746 | 11,036 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total research and development expense |
$ | 12,668 | $ | 17,617 | $ | 23,991 | ||||||
|
|
|
|
|
|
|
|||||||
76
Table of Contents
The successful development of our clinical and preclinical product candidates is highly uncertain. At this time, we cannot reasonably estimate the nature, timing or costs of the efforts that will be necessary to complete the remainder of the development of any of our clinical or preclinical product candidates or the period, if any, in which material net cash inflows from these product candidates may commence. This is due to the numerous risks and uncertainties associated with developing drugs, including the uncertainty of:
| • | the scope, rate of progress, expense and results of our ongoing clinical trials; |
| • | the scope, rate of progress, expense and results of additional clinical trials that we may conduct; |
| • | other research and development activities; and |
| • | the timing of regulatory approvals. |
A change in the outcome of any of these variables with respect to the development of a product candidate could mean a significant change in the costs and timing associated with the development of that product candidate. For example, if the FDA or another regulatory authority were to require us to conduct clinical trials beyond those which we currently anticipate will be required for the completion of clinical development of a product candidate or if we experience significant delays in enrollment in any of our clinical trials, we could be required to expend significant additional financial resources and time on the completion of clinical development.
AGS-003. We initiated our ongoing pivotal phase 3 clinical trial of AGS-003 in combination with sunitinib compared to sunitinib monotherapy in January 2013. We plan to enroll approximately 450 patients in this trial. As of February 28, 2014, we had enrolled approximately 120 patients in this trial. We expect to complete patient enrollment in this trial by the end of 2014 and to have overall survival data from this trial in the first half of 2016. We estimate that the direct costs that we will incur in connection with conducting the planned pivotal phase 3 clinical trial will total approximately $50.0 million, of which we expect that $12.0 million was incurred in 2013. We are also exploring the use of AGS-003 in non-clear cell mRCC, early stage RCC prior to and following nephrectomy and other solid tumors. We plan to initiate clinical trials of AGS-003 for the treatment of these additional indications in 2014.
AGS-004. We initiated our ongoing phase 2b clinical trial of AGS-004 in HIV-infected patients in July 2010. Our phase 2b clinical trial is funded entirely by the NIH. In September 2013, we completed patient enrollment in this clinical trial and expect to have data in mid-2014. We are working with the University of North Carolina on the design of an investigator-initiated phase 2 clinical trial of AGS-004 in adult HIV patients who are being treated with antiretroviral drug therapy to evaluate the use of AGS-004 in combination with a latency reversing drug to eradicate the virus. We expect this trial to be initiated in the second half of 2014. In January 2014, CARE agreed that it would fund all patient clinical costs of this phase 2 clinical trial, except for the associated manufacturing costs for which we will be responsible. We also plan to initiate in the second half of 2014 a phase 2 clinical trial of AGS-004 in pediatric HIV patients to evaluate the use of AGS-004 monotherapy to allow for long-term control of viral load and eliminate the need for antiretroviral therapy.
Other. In July 2011, we completed enrollment in a phase 1a clinical trial of AGS-009, a monoclonal antibody, for the treatment of systemic lupus erythematosus, or lupus, a chronic and disabling autoimmune disorder. We have not conducted any further development following the completion of the trial. We do not plan to conduct further development of AGS-009 unless and until we receive third party or government funding.
General and Administrative Expenses
General and administrative expenses consist primarily of salaries and related costs for employees in executive, operational and finance, information technology and human resources functions. Other significant general and administrative expenses include allocation of facilities costs, professional fees for accounting and legal services and expenses associated with obtaining and maintaining patents.
We expect that our general and administrative expenses will increase with the continued development and potential commercialization of our product candidates and as we operate as a public company. We believe that these increases will likely include increased costs for director and officer liability insurance, costs related to the hiring of additional personnel and increased fees for outside consultants, lawyers and accountants. We also expect to incur significant costs to comply with corporate governance, internal controls and similar requirements applicable to public companies.
Interest Income and Interest Expense
Interest income consists of interest earned on our cash and cash equivalents. We expect our interest income to increase as a result of our higher cash resources due to our IPO in February 2014 as we invest the net proceeds pending their use in our operations.
Interest expense consists primarily of cash and non-cash interest costs related to our debt.
77
Table of Contents
In September 2010 and December 2010, we issued convertible notes in the original aggregate principal amount of $6.0 million. We refer to these notes as the 2010 convertible notes. These notes bore interest at a rate of 10.0% per annum and had an original maturity date of September 15, 2011. The maturity date was extended to December 31, 2011 in connection with the issuance of the convertible notes that we issued and sold in July 2011, or the July 2011 convertible notes, and was then further extended to March 31, 2012 in December 2011. In connection with the issuance of the 2010 convertible notes, we issued warrants to purchase a total of 20,759,953 shares of our series C preferred stock at an exercise price of $0.01 per share. As of the date of issuance, we allocated $3.2 million of the proceeds from the issuance and sale of the convertible notes to the warrants based on the warrants’ fair value and recorded that amount on the balance sheet as a warrant liability. We recorded the remaining $2.8 million in proceeds on the balance sheet as the convertible note liability. We increased the value assigned to the convertible note liability through interest expense using the effective interest method. At December 31, 2011, we recorded the 2010 convertible notes as a $6.8 million convertible note liability. We recognized $3.1 million and $0.2 million, of interest expense related to the 2010 convertible notes during the years ended December 31, 2011 and 2012, respectively.
In July 2011, we issued the July 2011 convertible notes in the original aggregate principal amount of $3.5 million. These notes bore interest at a rate of 10.0% per annum and had an original maturity date of December 31, 2011, which date was extended to March 31, 2012 in December 2011. In connection with the issuance of the July 2011 convertible notes, we issued warrants to purchase a total of 12,109,975 shares of series C preferred stock at an exercise price of $0.01 per share. As of the date of issuance, we allocated $3.4 million of the proceeds from the issuance and sale of the July 2011 convertible notes to the warrants based on the warrants’ fair value and recorded that amount on the balance sheet as a warrant liability. We recorded the remaining $0.1 million in proceeds on the balance sheet as convertible note liability. We increased the value assigned to the convertible note liability through interest expense using the effective interest method. At December 31, 2011, we recorded the July 2011 convertible notes as a $3.6 million convertible note liability. We recognized $3.6 million and $0.1 million of interest expense related to the July 2011 convertible notes during the years ended December 31, 2011 and 2012, respectively.
We did not record any gain or loss in connection with the extensions of the maturity dates of the 2010 convertible notes or the July 2011 convertible notes because we determined that the extension of the maturity date of the notes met the criteria of a troubled debt restructuring outlined in ASC Topic 470-60, Troubled Debt Restructurings. In April 2012, the principal and interest on the 2010 convertible notes and the July 2011 convertible notes totaling $10.7 million converted into an aggregate of 3,023,661 shares of our series D preferred stock.
Accretion of Preferred Stock
Our preferred stock is reflected on our balance sheet at its cost, less associated issuance costs. The preferred stock is redeemable at the option of the holders beginning in July 2018 for an aggregate price equal to $97.1 million. The amount reflected on the balance sheet for our preferred stock is increased by periodic accretions of the issuance costs so that the original amount reflected on the balance sheet will equal the aggregate redemption price.
On February 12, 2014, all outstanding shares of our preferred stock converted into an aggregate of 13,188,251 shares of our common stock upon the closing of our IPO.
Derivative Income (Expense)
Prior to October 2012, we owned 49.94% of the capital of DC Bio. Under an amended and restated put agreement with the other shareholders of DC Bio, the other shareholders had the right to put the Common, Class A preferred and Class B preferred shares of DC Bio held by them to us in exchange for shares of our common stock, series B preferred stock and series C preferred stock at any time on or after March 31, 2011. We recorded a liability of $1.0 million on our balance sheet at December 31, 2011 reflecting the fair value of the put, which we calculated based on the estimated fair value of the shares of our common stock, series B preferred stock and series C preferred stock issuable in exchange for shares of DC Bio. In each reporting period, we recorded any change in the fair value of the shares underlying the put as expense, in the case of an increase in the fair value, and income, in the case of a decrease in fair value.
In October 2012 and December 2012, DC Bio repurchased the shares of DC Bio held by other entities. As of December 31, 2012, we owned 100.0% of the capital of DC Bio. We consolidate its results in our financial statements. The other shareholders’ previous ownership in DC Bio is reflected in our financial statements by reference to the equity attributable to noncontrolling interest.
Critical Accounting Estimates
Our management’s discussion and analysis of our financial condition and results of operations is based on our consolidated financial statements, which we have prepared in accordance with generally accepted accounting principles in the United States, or U.S. GAAP. The preparation of these consolidated financial statements requires us to make estimates and assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the financial statements, as well as
78
Table of Contents
the reported revenues and expenses during the reporting periods. We evaluate these estimates and judgments on an ongoing basis. We base our estimates on historical experience and on various other factors that we believe are reasonable under the circumstances, the results of which form the basis for making judgments about the carrying value of assets and liabilities that are not readily apparent from other sources. Our actual results may differ from these estimates under different assumptions or conditions.
While our significant accounting policies are more fully described in Note 2 to our consolidated financial statements appearing in “Item 8. Financial Statements and Supplementary Data,” we believe that the following accounting policies are the most critical to aid you in fully understanding and evaluating our financial condition and results of operations.
Revenue Recognition
We recognize revenue in accordance with the Financial Accounting Standards Board, or FASB, Accounting Standards Codification 605, Revenue Recognition, or ASC 605. We recognize revenue when the following criteria are met: persuasive evidence of an arrangement exists, services have been rendered, the price is fixed or determinable and collectability is reasonably assured.
We have previously entered into license agreements with collaborators. The terms of these agreements have included nonrefundable signing and licensing fees, as well as milestone payments and royalties on any future product sales developed by the collaborators under these licenses. We assess these multiple elements in accordance with ASC 605, in order to determine whether particular components of the arrangement represent separate units of accounting.
As a result of new guidance issued by the FASB, agreements entered into after December 31, 2010 will be accounted for in accordance with Accounting Standards Update, or ASU, No. 2009-13, Topic 605—Multiple-Deliverable Revenue Arrangements, or ASU 2009-13. This guidance requires the application of the “relative selling price” method when allocating revenue in a multiple deliverable arrangement. The selling price for each deliverable shall be determined using vendor specific objective evidence of selling price, if it exists; otherwise, third-party evidence of selling price shall be used. If neither exists for a deliverable, the vendor shall use its best estimate of the selling price for that deliverable. We recognize upfront license payments as revenue upon delivery of the license only if the license has standalone value and the fair value of the undelivered performance obligations can be determined. If the fair value of the undelivered performance obligations can be determined, such obligations are accounted for separately as the obligations are fulfilled. If the license is considered to either not have stand-alone value or have stand-alone value but the fair value of any of the undelivered performance obligations cannot be determined, the arrangement is accounted for as a single unit of accounting and the license payments and payments for performance obligations are recognized as revenue over the estimated period of when the performance obligations are performed.
Whenever we determine that an arrangement should be accounted for as a single unit of accounting, we must determine the period over which the performance obligations will be performed and revenue will be recognized. If we cannot reasonably estimate the timing and the level of effort to complete our performance obligations under the arrangement, then we recognize revenue under the arrangement on a straight-line basis over the period that we expect to complete our performance obligations.
Our license agreements with Green Cross and Medinet provide for, and other agreements we may enter into may provide for, milestone payments. Revenues from milestones, if they are non-refundable and considered substantive, are recognized upon successful accomplishment of the milestones. If not considered substantive, milestones are initially deferred and recognized over the remaining performance obligation.
Our current license agreements with Pharmstandard, Green Cross and Medinet and any future license agreements we may enter into may provide for royalty payments. To date, we have not received any royalty payments and accordingly have not recognized any related revenue. We will recognize royalty revenue upon the sale of the related products, provided we have no remaining performance obligations under the arrangements.
We record deferred revenue when payments are received in advance of the culmination of the earnings process. This revenue is recognized in future periods when the applicable revenue recognition criteria have been met.
Under our NIH contract, we receive reimbursement of our direct expenses and allocated overhead and general and administrative expenses, as well as payment of other specified amounts totaling up to $1.4 million upon our achievement of specified development milestones. We recognize revenue from reimbursements earned in connection with the NIH contract as reimbursable costs are incurred. We recognize revenues from the achievement of milestones under the NIH contract upon the accomplishment of any such milestone.
79
Table of Contents
Accrued Expenses
As part of the process of preparing financial statements, we are required to estimate accrued expenses. This process involves reviewing open contracts and purchase orders, communicating with applicable vendor personnel to identify services that have been performed on our behalf and estimating the level of service performed and the associated cost incurred for the service when we have not yet been invoiced or otherwise notified of actual cost. The majority of our service providers invoice us monthly in arrears for services performed. We make estimates of our accrued expenses as of each balance sheet date in our financial statements based on facts and circumstances known to us. We periodically confirm the accuracy of our estimates with the service providers and make adjustments if necessary. Examples of estimated accrued expenses include:
| • | fees paid to CROs in connection with clinical trials; |
| • | fees paid to investigative sites in connection with clinical trials; |
| • | professional service fees; and |
| • | unpaid salaries, wages and benefits. |
We accrue our expenses related to clinical trials based on our estimates of the services received and efforts expended pursuant to contracts with multiple research institutions and CROs that conduct and manage clinical trials on our behalf. The financial terms of these agreements are subject to negotiation, vary from contract to contract and may result in uneven payment flows. Payments under some of these contracts depend on factors such as the successful enrollment of patients and the completion of clinical trial milestones. In accruing service fees, we estimate the time period over which services will be performed and the level of effort to be expended in each period. If the actual timing of the performance of services or the level of effort varies from our estimate, we will adjust the accrual accordingly. If we do not identify costs that we have begun to incur or if we underestimate or overestimate the level of services performed or the costs of these services, our actual expenses could differ from our estimates. We do not anticipate the future settlement of existing accruals to differ materially from our estimates.
Valuation of Financial Instruments
Warrant Liability
We account for our preferred stock warrants in accordance with ASC Topic 480-10, Distinguishing Liabilities from Equity, which requires that a financial instrument, other than an outstanding share, that, at inception, includes an obligation to repurchase the issuer’s equity shares regardless of the timing or likelihood of the redemption, shall be classified as a liability. We measure the fair value of the warrant liability based on the fair value of the warrants which we determine based on an allocation of our enterprise value to all classes of equity and preferred stock, including the warrants. We utilize the probability-weighted expected return method, or PWERM method, to determine these values. In each reporting period, we record any change in fair value of the warrants as expense in the case of an increase in fair value and income in the case of a decrease in fair value.
In July 2013, in connection with the series D-1 exchange, all of our outstanding warrants to purchase shares of our series C preferred stock and series D-1 preferred stock were cancelled. There were no warrants to purchase preferred stock outstanding as of December 31, 2013.
Stock-Based Compensation
In accordance with ASC 718, Stock Compensation, we record the fair value of stock options, restricted stock awards and other stock-based compensation issued to employees as of the grant date as compensation expense. We typically recognize compensation expense over the requisite service period, which is the vesting period. For non-employees, we also record stock options, restricted stock awards and other stock-based compensation issued to these non-employees at their fair value as of the grant date. We then periodically remeasure the awards to reflect the current fair value at each reporting period and recognize expense over the related service period.
80
Table of Contents
Stock-based compensation expense includes stock options and restricted stock granted to employees and non-employees and has been reported in our statements of operations as follows:
| Year Ended December 31, | ||||||||||||
| 2011 | 2012 | 2013 | ||||||||||
| (in thousands) | ||||||||||||
| Research and development |
$ | 168 | $ | 512 | $ | 524 | ||||||
| General and administrative |
312 | 546 | 546 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total |
$ | 480 | $ | 1,058 | $ | 1,070 | ||||||
|
|
|
|
|
|
|
|||||||
We calculate the fair value of stock-based compensation awards using the Black-Scholes option-pricing model. The Black-Scholes option-pricing model requires the use of subjective assumptions, including stock price volatility, the expected life of stock options, risk free interest rate and the fair value of the underlying common stock on the date of grant.
| • | We do not have sufficient history to estimate the volatility of our common stock price. We calculate expected volatility based on reported data for selected reasonably similar publicly traded companies for which the historical information is available. For the purpose of identifying peer companies, we consider characteristics such as industry, length of trading history, similar vesting terms and in-the-money option status. We plan to continue to use the guideline peer group volatility information until the historical volatility of our common stock is relevant to measure expected volatility for future option grants. |
| • | The assumed dividend yield is based on our expectation of not paying dividends in the foreseeable future. |
| • | The expected term represents the period that the stock-based awards are expected to be outstanding. Our historical share option exercise experience does not provide a reasonable basis upon which to estimate an expected term because of a lack of sufficient data. Therefore we estimate the expected term by using the simplified method provided by the SEC. The simplified method calculates the expected term as the average of the time-to-vesting and the contractual life of the options. |
| • | We determine the risk-free interest rate by reference to implied yields available from U.S. Treasury securities with a remaining term equal to the expected life assumed at the date of grant. |
| • | We estimate forfeitures based on our historical analysis of actual stock option forfeitures. |
The assumptions that we used in the Black-Scholes option-pricing model for the years ended December 31, 2011, 2012 and 2013, are set forth below:
| 2011 | 2012 | 2013 | ||||||||||
| Risk-free interest rate |
2.17 | % | 1.20 | % | 2.12 | % | ||||||
| Dividend yield |
0 | % | 0 | % | 0 | % | ||||||
| Expected option term (in years) |
7 | 7 | 7 | |||||||||
| Volatility |
91 | % | 94 | % | 94 | % | ||||||
81
Table of Contents
Due to the absence of an active market for our common stock, the fair value of our common stock for purposes of determining the exercise price for stock option grants was determined by our board of directors, with the assistance and upon the recommendation of management, in good faith based on a number of objective and subjective factors consistent with the methodologies outlined in the American Institute of Certified Public Accountants Practice Aid, Valuation of Privately-Held-Company Equity Securities Issued as Compensation, or the Practice Aid. Since our initial public offering, the exercise price per share of all option grants has been set at the closing price of our common stock on The NASDAQ Global Market on the applicable date of grant, which our board of directors believes represents the fair value of our common stock.
Results of Operations
Comparison of the Year Ended December 31, 2012 and the Year Ended December 31, 2013
The following table summarizes the results of our operations for each of years ended December 31, 2012 and 2013, together with the changes in those items in dollars and as a percentage:
| Year Ended December 31, |
$ Change |
% Change |
||||||||||||||
| 2012 | 2013 | |||||||||||||||
| (in thousands) | ||||||||||||||||
| Revenue |
$ | 7,039 | $ | 4,422 | $ | (2,617 | ) | (37.2 | %) | |||||||
| Operating expenses |
||||||||||||||||
| Research and development |
17,617 | 23,991 | 6,374 | 36.2 | % | |||||||||||
| General and administrative |
6,135 | 4,662 | (1,473 | ) | (24.0 | %) | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total operating expenses |
23,752 | 28,653 | 4,901 | 20.6 | % | |||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Loss from operations |
(16,713 | ) | (24,231 | ) | (7,518 | ) | 45.0 | % | ||||||||
| Interest income |
5 | 7 | 2 | 40.0 | % | |||||||||||
| Interest expense |
(293 | ) | (5 | ) | 288 | (98.3 | )% | |||||||||
| Change in fair value of warrant liability |
4,917 | 355 | (4,562 | ) | (92.8 | )% | ||||||||||
| Derivative (expense) income |
1,036 | — | (1,036 | ) | * | |||||||||||
| Investment tax credits |
694 | — | (694 | ) | * | |||||||||||
| Other expense |
(117 | ) | (48 | ) | 69 | (59.0 | )% | |||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net loss |
$ | (10,471 | ) | $ | (23,922 | ) | $ | (13,451 | ) | 128.5 | % | |||||
|
|
|
|
|
|
|
|
|
|||||||||
| * | Not meaningful |
Revenue
Substantially all of our revenue is derived from our NIH contract. Revenue was $7.0 million for the year ended December 31, 2012, compared with $4.4 million for the year ended December 31, 2013, a decrease of approximately $2.6 million, or 37.2%. The $2.6 million decrease for the year ended December 31, 2013 is due to decreased reimbursement under our NIH contract associated with decreased activity with respect to our phase 2b clinical trial of AGS-004 as the number of patients receiving treatment in the trial declined.
Research and Development Expenses
Research and development expenses were $17.6 million for the year ended December 31, 2012, compared with $24.0 million for the year ended December 31, 2013, an increase of approximately $6.4 million, or 36.2%. This increase in research and development expense primarily reflects a $4.1 million increase in direct research and development costs and an increase in indirect research and development expense of $2.3 million. The increase in direct research and development expenses resulted primarily from the following:
| • | Direct research and development expense for AGS-003 increased from $5.8 million for the year ended December 31, 2012 to $11.3 million in the year ended December 31, 2013. This increase primarily reflects increased activity in connection with the commencement in January 2013 of the pivotal phase 3 clinical trial of AGS-003 in combination with sunitinib; and |
| • | Direct research and development expense with respect to AGS-004 decreased from $2.6 million in the year ended December 31, 2012 to $1.6 million in the year ended December 31, 2013 primarily due to the decreased activity in our phase 2b clinical trial of AGS-004 as the number of patients receiving treatment in the trial declined. |
82
Table of Contents
The increase in indirect research and development was primarily due to higher personnel costs as we had 56 employees engaged in research and development activities as of December 31, 2012 compared with 80 employees as of December 31, 2013.
General and Administrative Expenses
General and administrative expenses were $6.1 million for the year ended December 31, 2012, compared with $4.7 million for the year ended December 31, 2013, a decrease of 24.0%. This decrease reflects a $0.5 million decrease in audit and tax consulting fees and a $1.6 million decrease in legal expenses reflecting our increased legal expenses in 2012 associated with the IPO that we withdrew in March 2012. These decreases were partially offset by an increase of $0.7 million primarily due to increased compensation and marketing expenses during the year ended December 31, 2013.
Interest Expense
Interest expense was $0.3 million for the year ended December 31, 2012, compared with $5,000 for the year ended December 31, 2013. During the year ended December 31, 2012, interest expense primarily resulted from interest accrued on our outstanding 2010 convertible notes and July 2011 convertible notes. On April 10, 2012, the aggregate principal and interest on the 2010 convertible notes and the July 2011 convertible notes totaling $10.7 million converted into an aggregate of 3,023,661 shares of our series D preferred stock.
Change in Fair Value of Warrant Liability
Income from the change in fair value of the warrant liability was $4.9 million for the year ended December 31, 2012, compared with income of $0.4 million for the year ended December 31, 2013. These amounts represented the decrease in the fair value of the warrant liability during the respective periods. In July 2013, in connection with the series D-1 exchange, all of our outstanding warrants to purchase shares of our series C preferred stock and series D-1 preferred stock were cancelled. There were no warrants to purchase preferred stock outstanding as of December 31, 2013.
Derivative (Expense) Income
Derivative income was $1.0 million for the year ended December 31, 2012, compared with zero or the year ended December 31, 2013. Derivative income in 2012 reflected the termination of the put option held by the shareholders of DC Bio which resulted in the recognition of the derivative liability as income.
Investment Tax Credits
Investment tax credits were $0.7 million for the year ended December 31, 2012. We recorded no investment tax credits in the year ended December 31, 2013. Under Canadian and Ontario law, DC Bio is entitled to refunds on scientific research and experimental development investment tax credits. Because the investment tax credits are subject to a claim review and audit by the Canadian Revenue Agency, we recognize these tax credits when they are received.
Comparison of the Year Ended December 31, 2011 and the Year Ended December 31, 2012
The following table summarizes the results of our operations for each of the years ended December 31, 2011 and 2012, together with the changes in those items in dollars and as a percentage:
| Year Ended December 31, |
$ Change |
% Change |
||||||||||||||
| 2011 | 2012 | |||||||||||||||
| (in thousands) | ||||||||||||||||
| Revenue |
$ | 7,643 | $ | 7,039 | $ | (604) | (7.9%) | |||||||||
| Operating expenses |
||||||||||||||||
| Research and development |
12,668 | 17,617 | 4,949 | 39.1% | ||||||||||||
| General and administrative |
3,704 | 6,135 | 2,431 | 65.6% | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total operating expenses |
16,372 | 23,752 | 7,380 | 45.1% | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Loss from operations |
(8,729) | (16,713) | (7,984) | 91.5% | ||||||||||||
| Interest income |
1 | 5 | 4 | * | ||||||||||||
| Interest expense |
(6,656) | (293) | 6,363 | (95.6%) | ||||||||||||
| Change in fair value of warrant liability |
(4,662) | 4,917 | 9,579 | (205.5%) | ||||||||||||
| Derivative (expense) income |
(95) | 1,036 | 1,131 | * | ||||||||||||
| Investment tax credits |
— | 694 | 694 | * | ||||||||||||
| Other expense |
— | (117) | (117) | * | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net loss |
$ | (20,141) | $ | (10,471) | $ | 9,670 | (48.0%) | |||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| * | Not meaningful |
83
Table of Contents
Revenue
Revenue was $7.6 million for the year ended December 31, 2011, compared with $7.0 million for the year ended December 31, 2012, a decrease of approximately $0.6 million, or 7.9%. The $0.6 million decrease for the year ended December 31, 2012 primarily reflects decreased revenue from the NIH reflecting the fact that we did not achieve any milestones under the NIH agreement in 2012, while we achieved seven milestones in 2011.
Research and Development Expenses
Research and development expenses were $12.7 million for the year ended December 31, 2011, compared with $17.6 million for the year ended December 31, 2012, an increase of approximately $4.9 million, or 39.1%. This increase in research and development expense primarily reflects a $2.8 million increase in direct research and development expense and an increase in indirect research and development expense of $2.1 million. The increase in direct research and development expenses resulted primarily because:
| • | direct research and development expense for AGS-003 increased from $1.8 million for the year ended December 31, 2011 to $5.8 million in the year ended December 31, 2012. This increase reflects increased activity in preparation for our pivotal phase 3 clinical trial of AGS-003 in combination with sunitinib in the year ended December 31, 2012; |
| • | direct research and development expense for AGS-004 decreased from $2.9 million in the year ended December 31, 2011 to $2.6 million in the year ended December 31, 2012 due to the decreased activity in our phase 2b clinical trial of AGS-004 as the number of patients receiving treatment in the trial declined; and |
| • | direct research and development expense for AGS-009 decreased from $1.2 million in the year ended December 31, 2011 to $0.3 million in the year ended December 31, 2012 reflecting the discontinuation of development of AGS-009 after completion of the phase 1a clinical trial in the second quarter of 2012. |
The increase in indirect research and development expense was primarily due to higher personnel costs of $1.7 million caused by the net increase of 16 employees in 2012 and the payout of bonuses in the year ended December 31, 2012, as we did not grant bonuses in the year ended December 31, 2011, and an increase in consulting fees of $0.2 million and license fees of $0.2 million.
General and Administrative Expenses
General and administrative expenses were $3.7 million for the year ended December 31, 2011, compared with $6.1 million for the year ended December 31, 2012, an increase of 65.6%. This increase reflects a $0.5 million increase in audit and tax fees and a $1.7 million increase in legal expenses related to the IPO that we withdrew in early 2012 and a $0.2 million increase in consulting costs.
Interest Expense
Interest expense was $6.7 million for the year ended December 31, 2011, compared with $0.3 million for the year ended December 31, 2012. During both years, interest expense almost solely resulted from interest accrued on the outstanding convertible notes. The decrease in interest expense in 2012 reflects the conversion of the principal and interest on the convertible term notes totaling $10.7 million into an aggregate of 3,023,661 shares of our series D preferred stock in April 2012.
Change in Fair Value of Warrant Liability
Expense from the change in fair value of the warrant liability was $4.7 million for the year ended December 31, 2011, compared with income of $4.9 million for the change in the fair value of the warrant liability for the year ended December 31, 2012. The expense in 2011 represents the increase in the fair value of the warrant liability during 2011. The income in 2012 reflects the decrease in the fair value of the warrant liability during 2012.
84
Table of Contents
Derivative (Expense) Income
Derivative expense was $0.1 million for the year ended December 31, 2011, compared with income of $1.0 million for the year ended December 31, 2012. Derivative income in 2012 reflected the termination of the put option held by the shareholders of DC Bio which resulted in the recognition of the derivative liability as income.
Investment Tax Credits
Investment tax credits were $0.7 million for the year ended December 31, 2012. We recorded no investment tax credits in the year ended December 31, 2011. Under Canadian and Ontario law, DC Bio is entitled to refunds on scientific research and experimental development investment tax credits. Because the investment tax credits are subject to a claim review and audit by the Canadian Revenue Agency, we recognize these tax credits when they are received.
Liquidity and Capital Resources
Sources of Liquidity
As of December 31, 2013, we had cash, cash equivalents and short-term investments of approximately $47.0 million.
Since our inception in May 1997 through December 31, 2013, we have funded our operations principally with $165.5 million from the sale of common stock, convertible debt, warrants and preferred stock, including the preferred stock sold by DC Bio, $33.9 million from the licensing of our technology, and $92.0 million from government contracts, grants and license and collaboration agreements.
The gross proceeds we have received from the issuance and sale of our preferred stock as of December 31, 2013 are as follows:
| Issue |
Year | Gross Proceeds |
||||
| (in thousands) | ||||||
| Series A |
2000 | $ | 1,594 | |||
| Series B |
2001 | $ | 39,382 | |||
| Series B-1 |
2004 | $ | 5,000 | |||
| Series C |
2008 | $ | 33,462 | |||
| Series D |
2012 | $ | 9,022 | |||
| Series D-1 |
2012 | $ | 15,978 | |||
| Series E |
2013 | $ | 48,000 | |||
In February 2014, we issued and sold 6,228,725 shares of our common stock, including 603,725 shares of common stock sold pursuant to the underwriters’ exercise of their option to purchase additional shares, in our IPO at a public offering price of $8.00 per share, for aggregate gross proceeds of $49.8 million. The net offering proceeds to us, after deducting underwriting discounts and commissions of approximately $3.5 million and offering expenses of approximately $2.6 million, were approximately $43.7 million.
Cash Flows
The following table sets forth the major sources and uses of cash for the periods set forth below:
| Year Ended December 31, | ||||||||||||
| 2011 | 2012 | 2013 | ||||||||||
| (in thousands) | ||||||||||||
| Net cash provided by (used in): |
||||||||||||
| Operating activities |
$ | (4,586 | ) | $ | (13,198 | ) | $ | (18,256 | ) | |||
| Investing activities |
(244 | ) | (5,281 | ) | (10,057 | ) | ||||||
| Financing activities |
1,688 | 24,871 | 53,404 | |||||||||
| Effect of exchange rate changes on cash |
3 | (180 | ) | (8 | ) | |||||||
|
|
|
|
|
|
|
|||||||
| Net increase (decrease) in cash and cash equivalents |
$ | (3,139 | ) | $ | 6,212 | $ | 25,083 | |||||
|
|
|
|
|
|
|
|||||||
85
Table of Contents
Operating Activities. Net cash used in operating activities of $4.6 million during the year ended December 31, 2011 was primarily a result of our $20.1 million net loss, partially offset by changes in operating assets and liabilities of $3.0 million and non-cash items of $12.6 million. These non-cash items included non-cash interest costs related to our debt of $0.7 million, an increase to our warrant liability of $10.6 million, depreciation of $0.7 million, compensation expense related to stock options of $0.5 million and derivative expense of $0.1 million.
Net cash used in operating activities of $13.2 million during the year ended December 31, 2012 was primarily a result of our $10.5 million net loss, changes in operating assets and liabilities of $0.4 million and non-cash items of $2.3 million. These non-cash items reflect the gain recorded due to the decrease in fair value of our warrant liability of $4.9 million and derivative income of $1.0 million, partially offset by the write-off of previously deferred financing costs of $1.8 million, non-cash interest costs related to our debt of $0.3 million, depreciation of $0.5 million and compensation expense related to stock options of $1.0 million.
Net cash used in operating activities of $18.3 million during the year ended December 31, 2013 was primarily a result of our $23.9 million net loss, partially offset by changes in operating assets and non-cash items of $1.3 million and liabilities of $4.3 million. These non-cash items primarily reflect the depreciation of $0.6 million and compensation expense related to stock options of $1.1 million, partially offset by the gain recorded due to the decrease in fair value of our warrant liability of $0.4 million.
Investing Activities. Net cash used in investing activities amounted to $0.2 million, $5.3 million and $10.1 million for the years ended December 31, 2011, 2012 and 2013, respectively. Cash used in investing activities during all of these periods primarily reflected our purchases of equipment or purchases of short-term investments. Cash provided by investment activities during each of these periods, if applicable, was primarily due to sales of short-term investments.
Financing Activities. Net cash provided by financing activities amounted to $1.7 million, $24.9 million and $53.4 million for the years ended December 31, 2011, 2012 and 2013, respectively. Cash provided by financing activities for the year ended December 31, 2011 consisted of $3.5 million in proceeds from the issuance and sale of the July 2011 convertible notes, partially offset by deferred financing costs of $1.8 million related to our planned IPO and the registration statement we initially filed in 2011 and withdrew in March 2012, and payments of approximately $27,000 on certain of our notes payable. Cash provided by financing activities for the year ended December 31, 2012 consisted primarily of the proceeds from the sale of our series D and D-1 preferred stock of $25.0 million and loan proceeds of $0.1 million, partially offset by stock issuance costs of $0.2 million. Cash provided by financing activities for the year ended December 31, 2013 primarily consisted of proceeds of $48.0 million from the sale of our series E preferred stock and attached common stock warrants and $6.9 million from the note payable to Medinet, partially offset by stock issuance costs of $1.5 million.
Funding Requirements
To date, we have not generated any product revenue from our development stage product candidates. We do not know when, or if, we will generate any product revenue. We do not expect to generate significant product revenue unless or until we obtain marketing approval of, and commercialize AGS-003 or AGS-004. At the same time, we expect our expenses to increase in connection with our ongoing activities, particularly as we continue our phase 3 clinical trial of AGS-003, initiate additional clinical trials of AGS-003 and AGS-004, seek regulatory approval for our product candidates and lease, build out and equip a new commercial manufacturing facility. In addition, if we obtain regulatory approval of any of our product candidates, we expect to incur significant commercialization expenses for product sales, marketing, manufacturing and distribution. Furthermore, we expect to incur additional costs associated with operating as a public company. We will need substantial additional funding in connection with our continuing operations.
We expect that our existing cash, cash equivalents and short-term investments, including the $43.7 million in net proceeds from our IPO, and anticipated funding under our NIH contract, will enable us to fund our operating expenses and capital expenditure requirements into the second half of 2016. We intend to devote our cash, cash equivalents and short term investments to fund our pivotal phase 3 clinical trial of AGS-003, to fund our planned phase 2 clinical trials of AGS-003 in non-clear cell mRCC, early stage RCC prior to and following nephrectomy and other solid tumors, to fund certain of the costs of the two planned phase 2 clinical trials of AGS-004, one for HIV eradication and one for long-term viral control in pediatric patients, to initiate the planned leasing, build-out and equipping of a new commercial manufacturing facility and for working capital and general corporate purposes.
We will need to obtain significant financing prior to the commercialization of AGS-003, including to complete the planned leasing, build-out and equipping of a new commercial manufacturing facility and to fund any commercialization efforts in advance of regulatory approval of AGS-003. We expect that we will require approximately an additional $35.0 million prior to the commercialization of AGS-003 to lease, build out and equip the new commercial manufacturing facility that we plan to establish. We anticipate needing to expend funds for this purpose beginning in mid-2014. We are actively exploring potential sites and financing arrangements in connection with the lease, build out and equipping of a commercial manufacturing facility and are in discussions with developers and governmental authorities regarding potential sites and financial support. We expect to enter into arrangements that
86
Table of Contents
include financial support, and we expect such arrangements will likely involve material obligations and debt liabilities. If we are unable to obtain additional financing when needed, in the required amounts or at all, we may not be able to complete the planned leasing, build-out and equipping of the new commercial facility or may be delayed in doing so.
We have based our estimates on assumptions that may prove to be wrong, and we may use our available capital resources sooner than we currently expect. Because of the numerous risks and uncertainties associated with the development and commercialization of our product candidates, we are unable to estimate the amounts of increased capital outlays and operating expenditures necessary to complete the development of our product candidates.
Our future capital requirements will depend on many factors, including:
| • | the progress and results of our ongoing pivotal phase 3 clinical trial of AGS-003 and other clinical trials of AGS-003 that we may conduct; |
| • | the progress and results of our ongoing phase 2b clinical trial and other clinical trials of AGS-004 that we may conduct and our ability to obtain additional funding under our NIH contract for our AGS-004 program; |
| • | the scope, progress, results and costs of preclinical development, laboratory testing and clinical trials for our other product candidates; |
| • | the costs and timing of our planned leasing, build-out and equipping of a new commercial manufacturing facility; |
| • | the potential need to repay the $9.0 million loan under our license agreement with Medinet; |
| • | the costs, timing and outcome of regulatory review of our product candidates; |
| • | the costs of commercialization activities, including product sales, marketing, manufacturing and distribution, for any of our product candidates for which we receive regulatory approval; |
| • | revenue, if any, received from sales of our product candidates, should any of our product candidates be approved by the FDA or a similar regulatory authority outside the United States; |
| • | the costs of preparing, filing and prosecuting patent applications, maintaining, enforcing our intellectual property rights and defending intellectual property-related claims; |
| • | the extent to which we acquire or invest in businesses, products and technologies; |
| • | our ability to obtain government or other third party funding for the development of our product candidates; and |
| • | our ability to establish collaborations on favorable terms, if at all, particularly arrangements to develop, market and distribute AGS-003 outside North America and arrangements for the development and commercialization of our non-oncology product candidates, including AGS-004. |
Until such time, if ever, as we can generate substantial product revenue, we expect to finance our cash needs through a combination of equity offerings, debt financings, government contracts, government and other third party grants or other third party funding, marketing and distribution arrangements and other collaborations, strategic alliances and licensing arrangements. To the extent that we raise additional capital through the sale of equity or convertible debt securities, your ownership interest will be diluted, and the terms of these securities may include liquidation or other preferences that adversely affect your rights as a stockholder. Debt financing, if available, may involve agreements that include covenants limiting or restricting our ability to take specific actions, such as incurring additional debt, making capital expenditures or declaring dividends. If we raise additional funds through government or other third party funding, marketing and distribution arrangements or other collaborations, strategic alliances or licensing arrangements with third parties, we may have to relinquish valuable rights to our technologies, future revenue streams, research programs or product candidates or to grant licenses on terms that may not be favorable to us.
87
Table of Contents
We are seeking government or other third party funding for the continued development of AGS-004 following completion of the phase 2b clinical trial of AGS-004. In January 2014, CARE agreed that it would fund all patient clinical costs of our planned phase 2 clinical trial of AGS-004, except for the associated manufacturing costs for which we will be responsible. In addition, we are discussing with the NIH the planned phase 2 clinical trial of AGS-004 for long-term viral control in pediatric patients and potential funding by the NIH of the costs of the trial. If we are unable to raise government or other third party funding when needed, we may be required to delay, limit, reduce or terminate our development of AGS-004 or to grant rights to develop and market AGS-004 that we would otherwise prefer to keep for ourselves.
Contractual Obligations and Commitments
The following table summarizes our significant contractual obligations and commercial commitments at December 31, 2013 and the effects such obligations are expected to have on our liquidity and cash flows in future periods (in thousands):
| Payments Due by Period | ||||||||||||||||||||
| Total | Less Than 1 Year |
1-3 Years | 3-5 Years | More Than 5 Years |
||||||||||||||||
| Operating lease |
$ | 856 | $ | 314 | $ | 542 | $ | — | $ | — | ||||||||||
| Note payable to Medinet |
9,000 | — | — | 9,000 | — | |||||||||||||||
| Other note payable |
126 | 46 | 64 | 16 | — | |||||||||||||||
| Interest |
1,370 | 6 | 10 | 1,354 | — | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total |
$ | 11,352 | $ | 366 | $ | 616 | $ | 10,370 | $ | — | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
We plan to lease a new commercial manufacturing facility for the manufacture of our Arcelis-based products. We expect that we will incur a significant long-term financial commitment in connection with the lease of such facility.
In December 2013, in connection with our entry into the license agreement with Medinet, Medinet loaned us $9.0 million. We borrowed the $9.0 million pursuant to a promissory note that bears interest at a rate of 3.0% per annum and is due and payable on December 31, 2018. For further information regarding the terms of this loan, see “—Development and Commercialization Agreements—Medinet.”
We are a party to license agreements with universities and other third parties, as well as patent assignment agreements, under which we have obtained rights to patents, patent applications and know-how. Under these agreements, we have agreed to pay the other parties milestone payments upon the achievement of specified clinical, regulatory and commercialization events and royalties based on future sales of products. We have not included these payments in the table as we cannot estimate if, when or in what amounts such payments will become due under these agreements.
Net Operating Losses
As of December 31, 2013, we had U.S. Federal and state, and Canadian federal and provincial net operating loss carryforwards of approximately $109,102,100, $129,242,500, $7,308,900, and $7,308,900, respectively. These net operating loss carryforwards begin to expire in 2018, 2017, 2014 and 2014, respectively. As of December 31, 2013, we had U.S. Federal and state tax credit carryforwards of approximately $3,837,200 and $340,400, respectively. These credit carryforwards begin to expire in 2020 and 2019, respectively. The utilization of the net operating loss and tax credit carryforwards may be subject to limitation under the rules regarding a change in stock ownership as determined by the Internal Revenue Code, and state and foreign tax laws. Section 382 of the Internal Revenue Code of 1986, as amended, imposes annual limitations on the utilization of net operating loss (“NOL”) carryforwards, other tax carryforwards, and certain built-in losses upon an ownership change as defined under that section. In general terms, an ownership change may result from transactions that increase the aggregate ownership of certain stockholders in the Company’s stock by more than 50 percentage points over a three year testing period (“Section 382 Ownership Change”). If the Company has undergone a Section 382 Ownership Change, an annual limitation would be imposed on certain of the Company’s tax attributes, including NOL and capital loss carryforwards, and certain other losses, credits, deductions or tax basis. As of December 31, 2013, we have not performed a formal study to determine whether there are 382 limitations that apply. As of December 31, 2013, we had Canadian investment tax credit carryforwards of approximately $38,170 that begin to expire in 2024.
As of December 31, 2013, we have received $2.0 million in refunds through scientific research and experimental development tax credits through our consolidated subsidiary in Canada.
88
Table of Contents
Off-Balance Sheet Arrangements
We did not have during the periods presented, and we do not currently have, any off-balance sheet arrangements as defined under Securities and Exchange Commission, or SEC, rules.
Recent Accounting Pronouncements
In February 2013, the FASB issued Accounting Standards Update, or ASU, No. 2013-02 “Reporting of Amounts Reclassified Out of Accumulated Other Comprehensive Income.” ASU No. 2013-02 requires an entity to disaggregate the total change of each component of other comprehensive income either on the face of the income statement or as a separate disclosure in the notes. The new guidance became effective for reporting periods beginning after December 15, 2012 and is applied prospectively. We adopted this guidance on January 1, 2013, and the adoption did not have any impact on our financial position, results of operations or cash flows as the amounts reclassified out of accumulated other comprehensive loss are not significant.
In July 2013, the FASB issued a new accounting standard update on the financial statement presentation of unrecognized tax benefits. The new guidance provides that a liability related to an unrecognized tax benefit would be presented as a reduction of a deferred tax asset for a net operating loss carryforward, a similar tax loss or a tax credit carryforward if such settlement is required or expected in the event the uncertain tax position is disallowed. The new guidance becomes effective for us on January 1, 2014 and it should be applied prospectively to unrecognized tax benefits that exist at the effective date with retrospective application permitted. We do not believe that this updated standard will have a material impact on our consolidated financial statements.
Item 7A. Quantitative and Qualitative Disclosures about Market Risk
Our primary exposure to market risk is limited to our cash, cash equivalents and short-term investments, all of which have maturities of one year or less. The related interest income sensitivity is affected by changes in the general level of short-term U.S. interest rates. We primarily invest in high quality, short-term marketable debt securities issued by high quality financial and industrial companies.
Due to the short-term duration and low risk profile of our cash, cash equivalents and short-term investments, an immediate 10.0% change in interest rates would not have a material effect on the fair value of our portfolio. Accordingly, we would not expect our operating results or cash flows to be affected to any significant degree by the effect of a sudden change in market interest rates on our cash, cash equivalents and short-term investments.
We do not believe that our cash, cash equivalents and short-term investments have significant risk of default or illiquidity. While we believe our cash, cash equivalents and short-term investments do not contain excessive risk, we cannot provide absolute assurance that in the future our investments will not be subject to adverse changes in fair value. In addition, we maintain significant amounts of cash and cash equivalents at one or more financial institutions that are in excess of federally insured limits.
Item 8. Financial Statements and Supplementary Data.
Our financial statements and the financial statement schedule required by this item, together with the report of our independent registered public accounting firm and the notes to our financial statements, appear on pages F-1 through F-37 of this Annual Report on Form 10-K and are incorporated herein by reference.
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure.
There has been no change of accountants nor any disagreements with accountants on any matter of accounting principles or practices or financial disclosure required to be reported under this Item.
Item 9A. Controls and Procedures
Evaluation of Disclosure Controls and Procedures
The Company’s management, with the participation of the Company’s principal executive officer and principal financial officer, evaluated the effectiveness of the Company’s disclosure controls and procedures as of December 31, 2013. The term “disclosure controls and procedures,” as defined in Rules 13a-15(e) and 15d-15(e)) under the Exchange Act, means controls and other procedures of a company that are designed to ensure that information required to be disclosed by a company in the reports that it files or submits under the Exchange Act is recorded, processed, summarized and reported, within the time period specified in the SEC’s rules and forms. Disclosure controls and procedures include, without limitation, controls and procedures designed to ensure that information required to be disclosed by a company in the reports that it files or submits under the Exchange Act is accumulated and communicated to the company’s management, including its principal executive and principal financial officers, as appropriate to allow timely decisions regarding required disclosure. Management recognizes that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving their objectives and management necessarily applies its judgment in evaluating the cost-benefit relationship of possible controls and procedures. Based on the evaluation of the Company’s disclosure controls and procedures as of December 31, 2013, the Company’s principal executive officer and principal financial officer concluded that, as of such date, the Company’s disclosure controls and procedures were effective at the reasonable assurance levels.
89
Table of Contents
Management’s Report on Internal Control Over Financial Reporting
This Annual Report on Form 10-K does not include a report of management’s assessment regarding our internal control over financial reporting (as defined in Rule 13a-15(f) and 15d-15(f) under the Exchange Act) or an attestation report of our independent registered accounting firm due to a transition period established by rules of the Securities and Exchange Commission for newly public companies.
Changes in Internal Control over Financial Reporting
There was no change in our internal control over financial reporting that occurred during the period covered by this Annual Report on Form 10-K that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
Not Applicable.
Item 10. Directors, Executive Officers and Corporate Governance
MANAGEMENT
The following table sets forth the name, age and position of each of our executive officers and directors as of February 28, 2014.
| Name |
Age | Position | ||
| Jeffrey D. Abbey |
51 | President, Chief Executive Officer and Director | ||
| Charles A. Nicolette, Ph.D. |
51 | Chief Scientific Officer and Vice President of Research and Development | ||
| Frederick M. Miesowicz, Ph.D. |
64 | Chief Operating Officer and Vice President of Manufacturing | ||
| Douglas C. Plessinger |
45 | Vice President of Clinical and Medical Affairs | ||
| Lori R. Harrelson |
44 | Vice President of Finance | ||
| Hubert Birner, Ph.D.(1)(3) |
47 | Chairman of the Board of Directors | ||
| David W. Gryska(1)(2) |
58 | Director | ||
| Jean Lamarre(1) |
60 | Director | ||
| Andrei Petrov(2)(3) |
40 | Director | ||
| Brian J. Underdown, Ph.D.(2)(3) |
72 | Director | ||
| Sander van Deventer M.D., Ph.D. |
59 | Director | ||
| Alexey Vinogradov, Ph.D. |
40 | Director |
| (1) | Member of the Audit Committee |
| (2) | Member of the Compensation Committee |
| (3) | Member of the Nominating and Corporate Governance Committee |
Jeffrey D. Abbey has served as our President and Chief Executive Officer and a member of our board of directors since February 2010. Mr. Abbey served in various other positions at our company from September 2002 to February 2010, including as our Vice President of Business Development from February 2004 to January 2009 and as our Chief Business Officer from January 2009 to February 2010. Prior to joining us, Mr. Abbey served as Vice President of Business Development and Finance at Internet Appliance Network, an information technology company, from 1999 to 2001. Mr. Abbey was a partner at Eilenberg and Krause, LLP, a corporate law firm, from 1994 to 1999. Mr. Abbey received an A.B. in mathematical economics from Brown University and an M.B.A. and J.D. from the University of Virginia. We believe that Mr. Abbey is qualified to serve on our board of directors due to his extensive knowledge of our company and our industry.
Charles A. Nicolette, Ph.D. has served as our Chief Scientific Officer since December 2007 and as our Vice President of Research and Development since December 2004. Dr. Nicolette served as our Vice President of Research from July 2003 to December 2004. Prior to joining us, Dr. Nicolette served in various positions at Genzyme Molecular Oncology, Inc., a biotechnology company, from 1997 to 2003, most recently as Director of Antigen Discovery. Dr. Nicolette received a B.S. from the State University of New York at Stony Brook and a Ph.D. in biochemistry and cellular and developmental biology from the State University of New York at Stony Brook, completing his doctoral dissertation and post-doctoral fellowship at Cold Spring Harbor Laboratory.
90
Table of Contents
Frederick M. Miesowicz, Ph.D. has served as our Chief Operating Officer and Vice President of Manufacturing since February 2005. Dr. Miesowicz served as our Vice President of Manufacturing from May 2003 to February 2005. Prior to joining us, Dr. Miesowicz served as Vice President of U.S. Operations for Gamida-Cell Ltd., a stem cell company, from 2000 to 2003; Senior Vice President and General Manager at Hybridon Specialty Products, a manufacturing division of a biotechnology company, from 1998 to 2000; and Vice President and General Manager at Cellcor, a subsidiary of Cytogen Corporation, a biopharmaceutical company, from 1995 to 1998. Dr. Miesowicz received a B.S. in chemistry from Siena College and a Ph.D. in chemistry from Harvard University.
Douglas C. Plessinger, RPh, joined as our Vice President of Clinical and Medical Affairs effective October 2011, after serving as a clinical consultant for Argos since October 2007. Prior to joining us, Mr. Plessinger served as Executive Vice President and Managing Director at Axcelo MSL Solutions, LLC, an oncology consulting company, from August 2008 to September 2011 and founding partner and Managing Director of its predecessor company, venn5 BioConsulting, LLC, from 2007 to 2008. Prior to this, he served as Senior Vice President, Account Services at The Navicor Group, LLC, a healthcare advertising agency and a division of inVentiv Health, Inc., a healthcare services company, from 2004 to 2007; Oncology Medical Science Liaison at Millennium Pharmaceuticals, Inc., from 2002 to 2003; and in various Medical Affairs and Product Development roles at Bristol-Myers Squibb Oncology, a pharmaceutical company, from 1997 to 2002. Mr. Plessinger received a B.S. in pharmacy from Northeastern University.
Lori R. Harrelson has served as our Vice President of Finance since July 2011. Ms. Harrelson served as our Director of Finance and Accounting from January 2007 to July 2011 and as our Director of Accounting and Financial Reporting from September 2004 to January 2007. Prior to joining us, Ms. Harrelson served as Finance Manager at LipoScience, Inc., a diagnostic company, from 2001 to 2004. Ms. Harrelson received a B.S. in finance from East Carolina University and is a C.P.A.
Hubert Birner, Ph.D. has served as the Chairman of our board of directors since 2005 and a member of our board of directors since 2001. Dr. Birner joined the Munich office of TVM Capital, a venture capital firm and affiliate of Argos, as an investment manager in 2000 and currently serves as the Managing Partner of the firm. From 1998 to 2000, Dr. Birner served as head of European business development and director of marketing for Germany at Zeneca Agrochemicals, a biopharmaceutical company. Prior to joining Zeneca Agrochemicals, Dr. Birner served as a management consultant in McKinsey & Company’s European healthcare and pharmaceutical practice. Dr. Birner currently serves on the board of directors of Proteon Therapeutics, Inc. and SpePharm Holdings BV. Dr. Birner previously served on the board of directors of Horizon Pharma, Inc., Bioxell SA, Evotec AG and Jerini AG. Dr. Birner received an M.B.A. from Harvard Business School and a doctorate in biochemistry from Ludwig-Maximilians University in Munich, Germany. His doctoral thesis was honored with the Hoffmann-La Roche prize for outstanding basic research in metabolic diseases. We believe that Dr. Birner is qualified to serve as the Chairman of our board of directors due to his extensive experience with biopharmaceutical companies and his years of experience providing strategic and advisory services to pharmaceutical and biotechnology companies as a lead director and investor.
David W. Gryska has served as a member of our board of directors since January 2012. From May 2012 until December 2012, he served as Chief Operating Officer and a director at Myrexis, Inc., a biotechnology company. From December 2006 to October 2010, he served as Senior Vice President and Chief Financial Officer at Celgene Corporation, a biotechnology company. From October 2004 to December 2006, he served as a principal at Strategic Consulting Group, where he provided strategic consulting to early-stage biotechnology companies. Prior to Strategic Consulting Group, Mr. Gryska was employed by Scios, Inc., a biopharmaceutical company, as Senior Vice President and Chief Financial Officer from November 2000 to October 2004, and as Vice President of Finance and Chief Financial Officer from December 1998 to November 2000. Scios was acquired by Johnson & Johnson in 2003. From 1993 to December 1998, he served as Vice President, Finance and Chief Financial Officer at Cardiac Pathways Corporation, a medical device company. Prior to joining Cardiac Pathways, Mr. Gryska served as a partner at Ernst & Young LLP, an accounting firm. Mr. Gryska currently serves as a member of the board of directors of Seattle Genetics, Inc. since 2005, a member of the board of directors of Aerie Pharmaceuticals, Inc. since 2012, and a member of the board of directors of PDL BioPharma, Inc. since March 2014. Mr. Gryska received a B.A. in accounting and finance from Loyola University and an M.B.A. from Golden Gate University. We believe that Mr. Gryska is qualified to serve on our board of directors due to his valuable and relevant experience as a senior financial executive at life sciences and biotechnology companies dealing with financings, mergers, acquisitions and global expansion and other strategic transactions and extensive knowledge of accounting principles and financial reporting rules and regulations, tax compliance and oversight of the financial reporting processes, which we expect will assist Mr. Gryska in fulfilling his duties as chair of our audit committee.
Jean Lamarre has served as a member of our board of directors since February 2013. Mr. Lamarre is the President of 2856166 Canada Inc., a management consulting firm that he founded in 1992. Mr. Lamarre has been the lead director, the Chairman and since 2008, Executive Chairman of Semafo Inc., a gold production company. From 1984 to 1991, Mr. Lamarre served as the Chief Financial Officer of the Lavalin Group, one of the world’s leading design and construction firms. Mr. Lamarre is also a member of the Independent Review Committee of Investor Group Investment Management Ltd. He also serves on the boards of directors of a number of private companies. Mr. Lamarre received a B.Comm. in applied economics from HEC Montreal. We believe that Mr. Lamarre is qualified to serve on our board of directors due to his valuable and relevant experience as a senior financial executive.
91
Table of Contents
Andrei Petrov, Ph.D. has served as a member of our board of directors since August 2013. Dr. Petrov has been the Chief Scientific Officer of International Biotechnology Center Generium, a private scientific research and drug development company, since 2011, and the Chief Executive Officer of CJSC ‘Kollectsiya,’ a venture investment company, since 2013. From 2008 to 2011, Dr. Petrov served as Senior Scientist at CJSC Masterclone, a drug discovery and development company. Dr. Petrov has also served as a member on the board of directors of Affitech A/S since 2010, and as a member on the board of directors of co.don AG since 2012. We believe that Dr. Petrov is qualified to serve on our board of directors due to his extensive experience in drug discovery and development, international collaboration and co-development as well his business development skills in mergers, acquisitions and licensing deals.
Brian J. Underdown, Ph.D. has served as a member of our board of directors since 1999. Dr. Underdown joined Lumira Capital Corp. (formerly MDS Capital Corp.), a venture capital firm, in 1997, and currently serves as a Managing Director. Before joining Lumira, Dr. Underdown served as Assistant Vice President of Research at Pasteur Merieux Connaught from 1994 to 1997. Dr. Underdown has been a member of the board of directors of Vistagen Therapeutics, Inc. since 2009. Dr. Underdown received a Ph.D. from McGill University and undertook post-doctoral studies at Washington University School of Medicine. We believe that Dr. Underdown is qualified to serve on our board of directors due to his experience in the biopharmaceutical industry and his scientific background.
Sander van Deventer, M.D., Ph.D. has served as a member of our board of directors since 2001. Dr. van Deventer has been a General Partner of Forbion Capital Partners (formerly ABN AMRO Capital) since 2006. From 2008 to 2009, he served as the Chief Executive Officer of Amsterdam Molecular Therapeutics, or AMT, a gene therapy company that he co-founded in 1998. He has also served as a member of AMT’s board of directors since 2007. Dr. van Deventer has also served as a Professor of Translational Gastroenterology at Leiden University since 2008. He received an M.D. and Ph.D. from the University of Amsterdam. We believe that Dr. van Deventer is qualified to serve on our board of directors due to his experience as a founder of a biopharmaceutical company and his expertise in clinical development.
Alexey Vinogradov, Ph.D. commenced serving as a member of our board of directors on February 6, 2014. Dr. Vinogradov has served as a Managing Partner of CJSC Kollektsiya, or Inbio Ventures, a management company for Pharmstandard International, S.A., since 2012. Prior to joining Inbio Ventures, from 2009 to 2012, Dr. Vinogradov served as Investment Manager at Bioprocess Capital Partners, Russia’s first venture capital fund, specializing in life sciences and drug discovery. From 2004 to 2009, Dr. Vinogradov was employed by the International Science and Technology Center, where he provided consulting and investment support to early-stage biotechnology companies. From 2002 to 2004, Dr. Vinogradov was employed by Core-Biotech, a private company specialized in industrial biotechnology. Dr. Vinogradov received a Ph.D. in biochemistry from Moscow State University and completed a post-doctoral fellowship at Wageningen University (the Netherlands). We believe that Dr. Vinogradov is qualified to serve on our board of directors due to his experience in the venture capital and biopharmaceutical industries and his scientific background.
Board Composition and Election of Directors
Our board of directors is currently authorized to have up to eight members. In accordance with the terms of our certificate of incorporation and bylaws, our board of directors is divided into three classes, class I, class II and class III, with members of each class serving staggered three-year terms. The members of the classes are divided as follows:
| • | the class I directors are Brian J. Underdown, Ph.D., Sander van Deventer, M.D., Ph.D. and Alexey Vinogradov, Ph.D., and their term expires at our annual meeting of stockholders to be held in 2015; |
| • | the class II directors are Hubert Birner, Ph.D. and Jean Lamarre, and their term expires at our annual meeting of stockholders to be held in 2016; and |
| • | the class III directors are Jeffrey D. Abbey, David W. Gryska and Andrei Petrov, and their term expires at our annual meeting of stockholders to be held in 2017. |
Upon the expiration of the term of a class of directors, directors in that class will be eligible to be elected for a new three-year term at the annual meeting of stockholders in the year in which their term expires. In accordance with the terms of our certificate of incorporation and bylaws, our directors are only be able to be removed for cause by the affirmative vote of the holders of 75% or more of our voting stock.
Under applicable NASDAQ rules, a director only qualifies as an “independent director” if, in the opinion of our board of directors, that person does not have a relationship that would interfere with the exercise of independent judgment in carrying out the responsibilities of a director. Our board of directors has determined that all of our directors, other than Mr. Abbey, are independent directors as defined by applicable NASDAQ rules. In making such determinations, our board of directors considered the relationships that each such non-employee director and director nominee has with our company and all other facts and circumstances that our board of directors deems relevant in determining their independence, including the beneficial ownership of our capital stock by each non-employee director and director nominee.
92
Table of Contents
There are no family relationships among any of our directors or executive officers.
Board Leadership Structure
The positions of chairman of the board of directors and chief executive officer are presently separated and have generally been separated at our company. The duties of the chairman of the board include the following:
| • | chairing meetings of our board and of the independent directors in executive session; |
| • | meeting with any director who is not adequately performing his or her duties as a member of our board or any committees; |
| • | facilitating communications between other members of our board and the chief executive officer; |
| • | determining the frequency and length of board meetings and recommending when special meetings of our board should be held; |
| • | preparing or approving the agenda for each board meeting; and |
| • | reviewing and, if appropriate, recommending action to be taken with respect to written communications from stockholders submitted to our board. |
Our board of directors decided to separate the roles of chairman and chief executive officer because it believes that a bifurcated leadership structure offers the following benefits:
| • | increasing the independent oversight of our company and enhancing our board’s objective evaluation of our chief executive officer; |
| • | freeing the chief executive officer to focus on company operations instead of board administration; |
| • | providing the chief executive officer with an experienced sounding board; |
| • | providing greater opportunities for communication between stockholders and our board; |
| • | enhancing the independent and objective assessment of risk by our board; and |
| • | providing an independent spokesman for our company. |
Board Committees
Our board of directors has established an audit committee, a compensation committee and a nominating and corporate governance committee. Each of these committees operates under a charter that has been approved by our board.
Audit Committee
The current members of our audit committee are Hubert Birner, Ph.D., David W. Gryska and Jean Lamarre. Mr. Gryska chairs our audit committee. Philip R. Tracy served as a member of our audit committee until our initial public offering, at which time he ceased to serve as a member of our board of directors.
Our audit committee’s responsibilities include:
| • | appointing, approving the compensation of, and assessing the independence of our registered public accounting firm; |
| • | overseeing the work of our independent registered public accounting firm, including through the receipt and consideration of reports from such firm; |
| • | reviewing and discussing with management and our independent registered public accounting firm our annual and quarterly financial statements and related disclosures; |
93
Table of Contents
| • | monitoring our internal control over financial reporting, disclosure controls and procedures and code of business conduct and ethics; |
| • | overseeing our internal audit function, if any; |
| • | overseeing our risk assessment and risk management policies; |
| • | establishing policies regarding hiring employees from our independent registered public accounting firm and procedures for the receipt and retention of accounting related complaints and concerns; |
| • | meeting independently with our internal auditing staff, our independent registered public accounting firm and management; |
| • | reviewing and approving or ratifying any related person transactions; and |
| • | preparing the audit committee report required by SEC rules. |
All audit and non-audit services, other than de minimis non-audit services, to be provided to us by our independent registered public accounting firm must be approved in advance by our audit committee.
Our board of directors has determined that Mr. Gryska is an “audit committee financial expert” as defined in applicable SEC rules and qualifies as independent as defined under the applicable NASDAQ rules. We believe that the composition of our audit committee meets all applicable requirements with respect to audit committee composition under the current NASDAQ Global Market and SEC rules and regulations.
Compensation Committee
The current members of our compensation committee are David W. Gryska, Andrei Petrov and Brian J. Underdown, Ph.D. Dr. Underdown chairs our compensation committee. Philip R. Tracy served as a member of our compensation committee until our initial public offering, at which time he ceased to serve as a member of our board of directors.
Our compensation committee’s responsibilities include:
| • | reviewing and approving, or making recommendations to our board with respect to, the compensation of our chief executive officer and our other executive officers; |
| • | overseeing an evaluation of our senior executives; |
| • | overseeing and administering our cash and equity incentive plans; |
| • | reviewing and making recommendations to our board with respect to director compensation; |
| • | reviewing and discussing annually with management our compensation disclosure required by SEC rules; and |
| • | preparing the annual compensation committee report required by SEC rules. |
Nominating and Corporate Governance Committee
The members of our nominating and corporate governance committee are Hubert Birner, Ph.D., Andrei Petrov and Brian J. Underdown, Ph.D. Dr. Birner chairs our nominating and corporate governance committee. Our nominating and corporate governance committee’s responsibilities include:
| • | identifying individuals qualified to become members of our board; |
| • | recommending to our board the persons to be nominated for election as directors and to each of our board’s committees; |
| • | reviewing and making recommendations to our board with respect to our board leadership structure; |
| • | reviewing and making recommendations to our board with respect to management succession planning; |
94
Table of Contents
| • | developing and recommending to our board corporate governance principles; and |
| • | overseeing a periodic evaluation of our board. |
Code of Ethics and Code of Conduct
On December 20, 2013, we adopted a written code of business conduct and ethics that applies to our directors, officers and employees, including our principal executive officer, principal financial officer, or persons performing similar functions. The code became effective on February 6, 2014. We have posted a current copy of the code on our website, www.argostherapeutics.com. In addition, we intend to post on our website all disclosures that are required by law or NASDAQ stock market listing standards concerning any amendments to, or waivers from, any provision of the code.
Item 11. Executive Compensation
This section describes the material elements of compensation awarded to, earned by or paid to each of our named executive officers in 2013. This section also provides qualitative information regarding the manner and context in which compensation is awarded to and earned by our named executive officers and is intended to place into perspective the data presented in the tables and narrative that follow. Our “named executive officers” for 2013 were:
| • | Jeffrey D. Abbey, our president and chief executive officer; |
| • | Charles A. Nicolette, Ph.D., our vice president of research and development and chief scientific officer; and |
| • | Frederick M. Miesowicz, Ph.D., our vice president of manufacturing and chief operating officer. |
95
Table of Contents
Summary Compensation Table
The following table sets forth information regarding compensation awarded to, earned by or paid to our named executive officers during 2013 and 2012.
| Name and Principal Position |
Year | Salary ($) |
Bonus ($) |
Option Awards ($)(1) |
All Other Compensation ($)(2) |
Total ($) | ||||||||||||||||||
| Jeffrey D. Abbey(3) |
2013 | 300,000 | 136,800 | 2,112,044 | 3,136 | 2,551,980 | ||||||||||||||||||
| President and Chief Executive Officer |
2012 | 300,000 | 60,000 | 323,474 | 2,862 | 686,336 | ||||||||||||||||||
| Charles A Nicolette, Ph.D. |
2013 | 250,000 | 71,250 | 891,946 | 3,033 | 1,216,229 | ||||||||||||||||||
| Vice President of Research and Development and Chief Scientific Officer |
2012 | 250,000 | 31,250 | 161,737 | 2,768 | 445,755 | ||||||||||||||||||
| Frederick M. Miesowicz, Ph.D. |
2013 | 261,380 | 74,493 | 504,879 | 3,112 | 843,864 | ||||||||||||||||||
| Vice President of Manufacturing and Chief Operating Officer |
2012 | 261,380 | 32,673 | 121,307 | 2,838 | 418,198 | ||||||||||||||||||
| (1) | The amounts reported in the “Option Awards” column reflect the aggregate fair value of share-based compensation awarded during the year computed in accordance with the provisions of Financial Accounting Standards Board Accounting Standard Codification, or ASC, Topic 718. See Note 12 to our consolidated financial statements appearing in “Item 8. Financial Statements and Supplementary Data” regarding assumptions underlying the valuation of equity awards. |
| (2) | The amounts reported in the “All Other Compensation” column reflect, for each named executive officer, the sum of the incremental cost to us of all perquisites and other personal benefits and are comprised of post-tax insurance earnings. |
| (3) | Mr. Abbey also serves as a member of our board of directors but does not receive any additional compensation for his service as a director. |
Narrative Disclosure to Summary Compensation Table
The primary elements of our executive compensation program are:
| • | base salary; |
| • | annual cash bonuses; and |
| • | equity incentive awards |
We strive to achieve an appropriate mix between the various elements of our compensation program to meet our compensation objectives and philosophy; however, we have not adopted any formal policies or guidelines for allocating compensation among these elements.
Base Salary. We use base salaries to recognize the experience, skills, knowledge and responsibilities required of all our employees, including our named executive officers. None of our named executive officers is currently party to an employment agreement or other agreement or arrangement that provides for automatic or scheduled increases in base salary. In 2013, we paid an annual base salary of $300,000 to Mr. Abbey, $250,000 to Dr. Nicolette and $261,380 to Dr. Miesowicz.
Annual Cash Bonus. In addition to base salaries, our executive officers are eligible to receive annual discretionary cash bonuses based on the achievement of corporate objectives and individual performance. Bonuses are typically prorated on a monthly basis, as applicable, for executive officers who commence employment after the beginning of the year. Our executive officers’ annual bonus opportunities are generally set in their employment agreements as a specified percentage of annual base salary. The 2013 annual target bonus amount for each of our current executive officers other than Mr. Abbey is 25% of base salary. The target bonus for Mr. Abbey is 40% of his base salary. In determining annual bonuses, we have generally attributed 85% of the target bonus to the achievement of specified corporate objectives and 15% to the individual’s effectiveness in helping us achieve our corporate objectives or other individual performance criteria. The annual corporate objectives are recommended by our chief executive officer and approved by the compensation committee and the board of directors. Historically, annual bonuses have been determined by the compensation committee and ratified by the non-employee directors in December of each year and paid by the end of December of the year in which they were determined. For 2013, we paid cash bonuses of $136,800 to Mr. Abbey, $71,250 to Dr. Nicolette and $74,493 to Dr. Miesowicz.
Equity Incentive Awards. Our equity award program is the primary vehicle for offering long-term incentives to our executives. We believe that equity awards provide our executives with a strong link to our long-term performance, create an ownership culture and help to align the interests of our executives and our stockholders. To date, we have used stock option grants for this purpose because we believe they are an effective means by which to align the long-term interests of our executive officers with those of our
96
Table of Contents
stockholders. The use of options also can provide tax and other advantages to our executive officers relative to other forms of equity compensation. We believe that our equity awards are an important retention tool for our executive officers, as well as for our other employees.
We award stock options broadly to our employees, including to our non-executive employees. Grants to our executives and other employees are made at the discretion of our board of directors and are not made at any specific time period during a fiscal year. All of our named executive officers have received stock option grants under our 1999 stock option plan or our 2008 stock incentive plan, both of which are described below. The 1999 stock option plan has expired and no further options may be granted under that plan. Our board of directors and the Company’s stockholders adopted a new equity incentive plan described under “— Stock Option and Other Equity Compensation Plans” below, effective upon the closing of our IPO on February 12, 2014, and has determined not to grant any additional awards under our 2008 stock incentive plan. Our new 2014 plan affords our compensation committee continued flexibility in making a wide variety of equity awards.
Initial option grants to our executive officers are generally set forth in their employment agreements. These initial grants are the product of negotiation with the executive officer, but we generally seek to establish equity ownership levels that we believe are commensurate with the equity stakes held by executive officers serving in similar roles at comparable biopharmaceutical companies. In addition, from time to time in connection with corporate finance transactions and at other times as our compensation committee and board of directors deem appropriate, we provide subsequent option grants to those executive officers determined to be performing well.
The majority of the stock option grants we have made to our executive officers vest over four years. However, from time to time, our board of directors has approved grants with different and sometimes shorter vesting provisions. Our historical practice has been to provide for 100% acceleration of vesting of outstanding stock options in the event of a change of control. Additional information regarding the effect of accelerated vesting upon a change in control with respect to our named executive officers is discussed below under “— Agreements with our Named Executive Officers.”
In April 2012, our board of directors approved the repricing of stock options that had exercise prices between $10.86 to $36.66 per share to the then estimated fair value of our common stock, determined to be an exercise price of $4.20 per share. We repriced these options to align stock option exercise prices with the fair market value of our common stock at that time and maintain our equity awards as an important retention tool for our key employees.
Grants of Plan-Based Awards
The following table sets forth, for each of our named executive officers, information regarding each grant of a plan-based award made during the fiscal year ended December 31, 2013. This information supplements the information about these awards set forth in the Summary Compensation Table.
| Name |
Grant Date | All Other Option Awards: Number of Securities Underlying Options (#) |
Exercise or Base Price of Option Awards ($/Sh) |
Grant Date Fair Value of Option Awards($)(3) |
||||||||||||
| Jeffrey D. Abbey |
11/1/2013 | 386,118 | (1) | 5.82 | 1,811,102 | |||||||||||
| 11/11/2013 | 64,082 | (2) | 5.82 | 300,942 | ||||||||||||
| Charles A Nicolette, Ph.D |
11/1/2013 | 161,643 | (1) | 5.82 | 758,194 | |||||||||||
| 11/11/2013 | 28,481 | (2) | 5.82 | 133,752 | ||||||||||||
| Frederick M. Miesowicz, Ph.D |
11/1/2013 | 89,815 | (1) | 5.82 | 421,284 | |||||||||||
| 11/11/2013 | 17,800 | (2) | 5.82 | 83,595 | ||||||||||||
| (1) | This option was granted on November 1, 2013 and vests as to 25% of the shares on November 1, 2014, with the remaining 75% of the shares vesting in equal amounts monthly over the three year period commencing on November 1, 2014, provided that the recipient continues to provide services to us over such period. |
| (2) | This option was granted on November 11, 2013 and vests as to 25% of the shares on November 1, 2014, with the remaining 75% of the shares vesting in equal amounts monthly over the three year period commencing on November 1, 2014, provided that the recipient continues to provide services to us over such period. |
| (3) | Amounts reported represent the grant date fair value of the stock options granted during 2013 over the entire term of the options, computed in accordance with ASC 718, formerly Statement of Financial Accounting Standards No. 123R. The valuation assumptions used in calculating the fair value of the stock options are set forth in note 12 to our consolidated financial statements. |
97
Table of Contents
Outstanding Equity Awards at 2013 Fiscal Year End
The following table provides information about outstanding stock options held by each of our named executive officers at December 31, 2013. All of the listed options were granted under our 2008 stock incentive plan.
| Name |
Number of Securities Underlying Unexercised Options (#) Exercisable |
Number of Securities Underlying Unexercised Options (#) Unexercisable |
Option Exercise Price ($) |
Option Expiration Date |
||||||||||||
| Jeffrey D. Abbey |
14,175 | (1) | — | 4.20 | (8) | 7/2/18 | ||||||||||
| 5,706 | (2) | — | 4.20 | (8) | 12/5/18 | |||||||||||
| 48,323 | (3) | 2,102 | (3) | 4.20 | (8) | 12/10/20 | ||||||||||
| 44,651 | (4) | 18,801 | (4) | 4.20 | (8) | 4/10/22 | ||||||||||
| 37,051 | (5) | 8,551 | (5) | 4.20 | (8) | 12/11/22 | ||||||||||
| — | (6) | 386,118 | (6) | 5.82 | 11/1/23 | |||||||||||
| — | (7) | 64,082 | (7) | 5.82 | 11/11/23 | |||||||||||
| Charles A. Nicolette, Ph.D. |
14,640 | (1) | — | 4.20 | (8) | 7/2/18 | ||||||||||
| 5,893 | (2) | — | 4.20 | (8) | 12/5/18 | |||||||||||
| 14,009 | (3) | 610 | (3) | 4.20 | (8) | 12/10/20 | ||||||||||
| 22,325 | (4) | 9,401 | (4) | 4.20 | (8) | 4/10/22 | ||||||||||
| 18,525 | (5) | 4,276 | (5) | 4.20 | (8) | 12/11/22 | ||||||||||
| — | (6) | 161,643 | (6) | 5.82 | 11/1/23 | |||||||||||
| — | (7) | 28,481 | (7) | 5.82 | 11/11/23 | |||||||||||
| Frederick M. Miesowicz, Ph.D. |
13,943 | (1) | — | 4.20 | (8) | 7/2/18 | ||||||||||
| 5,612 | (2) | — | 4.20 | (8) | 12/5/18 | |||||||||||
| 6,525 | (3) | 284 | (3) | 4.20 | (8) | 12/10/20 | ||||||||||
| 16,743 | (4) | 7,051 | (4) | 4.20 | (8) | 4/10/22 | ||||||||||
| 13,895 | (5) | 3,207 | (5) | 4.20 | (8) | 12/11/22 | ||||||||||
| — | (6) | 89,815 | (6) | 5.82 | 11/1/23 | |||||||||||
| — | (7) | 17,800 | (7) | 5.82 | 11/11/23 | |||||||||||
| (1) | This option was granted on July 2, 2008 and vested as to 50% of the shares on the date of grant, with the remaining 50% of the shares vesting in equal amounts monthly over the two year period commencing on April 1, 2008. |
| (2) | This option was granted on December 5, 2008 and vested in specified increments over a two-year period ending on April 1, 2010. |
| (3) | This option was granted on December 10, 2010. This option vests in equal monthly installments over a four year period, with the first installment vesting on February 24, 2010, provided that recipient continues to provide services to us over such period. |
| (4) | This option was granted on April 10, 2012 and vested as to 1/3 of the shares on the date of grant, with the remaining 2/3 of the shares vesting in equal amounts monthly over the three year period commencing on April 10, 2012, provided that the recipient continues to provide services to us over such period. |
| (5) | This option was granted on December 11, 2012 and vested as to 50% of the shares on the date of grant, with the remaining 50% of the shares vesting in equal amounts monthly over the two year period commencing on October 31, 2012, provided that the recipient continues to provide services to us over such period. |
| (6) | This option was granted on November 1, 2013 and vests as to 25% on November 1, 2014, with the remaining 75% of the shares vesting in equal amounts monthly over the three year period commencing on November 1, 2014, provided that the recipient continues to provide services to us over such period. |
| (7) | This option was granted on November 11, 2013 and vests as to 25% on November 1, 2014, with the remaining 75% of the shares vesting in equal amounts monthly over the three year period commencing on November 1, 2014, provided that the recipient continues to provide services to us over such period. |
| (8) | In April 2012, our board of directors approved the repricing of stock options that had exercise prices between $10.86 and $36.66 per share, including this option, to the then estimated fair value of our common stock, determined to be an exercise price of $4.20 per share. |
Agreements with our Named Executive Officers
We have entered into written employment agreements with each of our named executive officers. The agreements set forth the terms of the named executive officer’s compensation, including base salary, severance and an annual cash bonus opportunity. In addition, the agreements provide that the named executive officers are eligible to participate in company-sponsored benefit programs that are available generally to all of our employees. The agreements also subject our named executive officers to certain non-competition and non-solicitation restrictions. In connection with the commencement of their employment with us, our named executive officers executed our standard confidential information and invention assignment agreements.
Pursuant to the terms of their agreements, our named executive officers are entitled to receive the following annual base salaries: $300,000 for Mr. Abbey, $250,000 for Dr. Nicolette and $261,380 for Dr. Miesowicz. Effective upon the closing of our IPO on February 12, 2014, these annual base salaries were increased to: $390,000 for Mr. Abbey, $300,000 for Dr. Nicolette and $287,518 for Dr. Miesowicz.
98
Table of Contents
In addition, each named executive officer is eligible to receive an annual performance cash bonus under his employment agreement based on the achievement of corporate objectives and the named executive officer’s individual performance, which is determined by our board of directors in its sole discretion. The bonus opportunity is calculated as a percentage of the named executive officer’s then annual base salary. The target annual bonus percentage for each named executive officer is as follows: 40% for Mr. Abbey, 25% for Dr. Nicolette and 25% for Dr. Miesowicz. Following the close of our IPO on February 12, 2014, the target annual bonus percentage for each named executive officer were increased to: 50% for Mr. Abbey, 35% for Dr. Nicolette and 30% for Dr. Miesowicz. Each named executive officer must be employed on the date the bonus is paid in order to be eligible for and receive his annual bonus.
Potential Payments upon Termination or Change in Control
Upon execution and effectiveness of a separation agreement and release of all claims, each named executive officer is entitled to severance payments if his employment is terminated under specified circumstances pursuant to the terms of his employment agreement.
If we terminate the named executive officer’s employment without cause or if the named executive officer terminates his employment with us for good reason in accordance with the terms of his employment agreement, the named executive officer is entitled to receive from us an amount equal to nine months of his then annual base salary, payable in nine equal monthly installments in accordance with our payroll practices, and standard health insurance coverage for a period of nine months, subject to such benefits being available to non-employees. If his standard health insurance coverage is not available to non-employees under our company sponsored plan, we will reimburse the named executive officer in an amount equal to the cost of the premium for coverage under a medical plan at the same level and on the same terms and conditions in place immediately before his termination.
If we terminate the named executive officer’s employment without cause or if the named executive officer terminates his employment with us for good reason in accordance with the terms of his employment agreement, in either case within 90 days before or six months after a “change in control event” as defined in our 2008 stock incentive plan, and such event also constitutes a “change in control event” within the meaning of the regulations promulgated under Section 409A of the Internal Revenue Code, as amended, or the Code, Mr. Abbey and Dr. Nicolette will be entitled to receive the payments and benefits specified above for a period of 15 months rather than nine months. Additionally, in such circumstances, Mr. Abbey and Dr. Nicolette will each be entitled to receive an amount equal to 15 months of his target bonus for the year in which his employment terminates, payable in 15 equal monthly installments in accordance with our payroll practices. Dr. Miesowicz will be entitled to receive an amount equal to nine months of his target bonus for the year in which his employment terminates, payable in nine equal monthly installments in accordance with our payroll practices.
Under Mr. Abbey’s and Dr. Nicolette’s employment agreements, effective upon the close of our IPO on February 12, 2014 and for a period of four years from such date, to the extent that any payment, benefit or distribution, or combination thereof, by us or any of our affiliates to the executive officer pursuant to his employment agreement or any other agreement, plan or arrangement would constitute a “parachute payment” within the meaning of Section 280G of the Code and would be subject to the excise tax imposed under Section 4999 of the Code, Mr. Abbey and Dr. Nicolette will be entitled to receive a “gross-up” payment equal to the sum of such excise tax and related interest or penalties, plus the amount necessary to put him in the same after-tax position that he would have been in had he not incurred any tax liability under Section 4999 of the Code. After such four-year period, the executive officer will not be entitled to any such “gross up” payment associated with any “parachute payment” or excise tax. Dr. Miesowicz is not entitled to any “gross up” payment associated with any “parachute payment” under his employment agreement.
If required by Section 409A of the Code, the payments we are required to make to the named executive officer in the first six months following the termination of his employment under his employment agreement will be made as a lump sum on the date that is six months and one day following such termination.
Under the terms of the stock options granted to our named executive officers prior to 2013 under our 2008 stock incentive plan, upon a “change of control event” as defined in our 2008 stock incentive plan, all unvested portions of any outstanding options held by them will vest in full. Under the terms of the stock options granted to our named executive officers in 2013 under our 2008 stock incentive plan, upon a “change of control event”, all unvested portions of any outstanding options held by them will vest in full if we terminate the named executive officer’s employment without cause or if the named executive officer terminates his employment with us for good reason, in each case within ninety days before or six months after the “change in control event”.
2014 Stock Incentive Plan
In January 2014, our board of directors adopted and our stockholders approved the 2014 stock incentive plan, which became effective immediately prior to the closing of our IPO, which occurred on February 12, 2014. The 2014 stock incentive plan provides for the grant of incentive stock options, non-statutory stock options, stock appreciation rights, restricted stock awards, restricted stock units and other stock-based awards. Upon effectiveness of the plan, the number of shares of our common stock that were reserved for issuance under the 2014 stock incentive plan was the sum of 1,951,182 shares, plus such number of shares of our common stock, up to
99
Table of Contents
357,841 shares, as was equal to the sum of the number of shares of common stock reserved for issuance under the 2008 stock incentive plan that remained available for grant under the 2008 stock incentive plan immediately prior to the closing of our IPO on February 12, 2014 and the number of shares of common stock subject to outstanding awards under the 2008 stock incentive plan that expire, terminate or are otherwise surrendered, cancelled, forfeited or repurchased by us at their original issuance price pursuant to a contractual repurchase right, plus an annual increase, to be added on the first day of the 2015 fiscal year and each subsequent anniversary through January 1, 2024, equal to the lowest of 2,309,023 shares of our common stock, 4% of the number of our outstanding shares on the first day of each such fiscal year and an amount determined by our board of directors.
Our employees, officers, directors, consultants and advisors will be eligible to receive awards under the 2014 stock incentive plan. However, incentive stock options may only be granted to our employees.
Pursuant to the terms of the 2014 stock incentive plan, our board of directors will administer the plan and, subject to any limitations in the plan, select the recipients of awards and determines:
| • | the number of shares of our common stock covered by options and the dates upon which the options become exercisable; |
| • | the type of options to be granted; |
| • | the duration of options, which may not be in excess of ten years; |
| • | the exercise price of options, which may not be less than the fair market value of our common stock on the date of grant of the options; and |
| • | the number of shares of our common stock subject to any stock appreciation rights, restricted stock awards, restricted stock units or other stock-based awards and the terms and conditions of such awards, including conditions for repurchase, issue price and repurchase price. |
Our board of directors has delegated authority to an executive officer to grant awards under the 2014 stock incentive plan to all of our employees, except employees at or above the director level. Our board of directors has fixed the terms of the awards to be granted by such executive officer, including the exercise price of such awards, and the maximum number of shares subject to awards that such executive officer may make.
Upon a merger or other reorganization event, our board of directors may, in its sole discretion, take any one or more of the following actions pursuant to the 2014 stock incentive plan as to some or all outstanding awards other than restricted stock:
| • | provide that all outstanding awards shall be assumed, or substantially equivalent awards shall be substituted, by the acquiring or successor corporation (or an affiliate thereof); |
| • | upon written notice to a participant, provide that all of the participant’s unexercised awards will terminate immediately prior to the consummation of such reorganization event unless exercised by the participant; |
| • | provide that outstanding awards shall become exercisable, realizable or deliverable, or restrictions applicable to an award shall lapse, in whole or in part, prior to or upon such reorganization event; |
| • | in the event of a reorganization event pursuant to which holders of shares of our common stock will receive a cash payment for each share surrendered in the reorganization event, make or provide for a cash payment to the participants with respect to each award held by a participant equal to (1) the number of shares of common stock subject to the vested portion of the award (after giving effect to any acceleration of vesting that occurs upon or immediately prior to such reorganization event) multiplied by (2) the excess, if any, of the cash payment for each share surrendered in the reorganization event over the exercise, measurement or purchase price of such award and any applicable tax withholdings, in exchange for the termination of such award; and |
| • | provide that, in connection with a liquidation or dissolution, awards shall convert into the right to receive liquidation proceeds. |
In the case of certain restricted stock units, no assumption or substitution is permitted, and the restricted stock units will instead be settled in accordance with the terms of the applicable restricted stock unit agreement.
100
Table of Contents
Upon the occurrence of a reorganization event other than a liquidation or dissolution, the repurchase and other rights with respect to outstanding restricted stock will continue for the benefit of the successor company and will, unless the board of directors may otherwise determine, apply to the cash, securities or other property into which shares of our common stock are converted or exchanged pursuant to the reorganization event. Upon the occurrence of a reorganization event involving a liquidation or dissolution, all restrictions and conditions on each outstanding restricted stock award will automatically be deemed terminated or satisfied, unless otherwise provided in the agreement evidencing the restricted stock award.
At any time, our board of directors may, in its sole discretion, provide that any award under the 2014 stock incentive plan will become immediately exercisable in full or in part, free of some or all restrictions or conditions, or otherwise realizable in full or in part.
No award may be granted under the 2014 stock incentive plan on or after January 17, 2024. Our board of directors may amend, suspend or terminate the 2014 stock incentive plan at any time, except that stockholder approval will be required to comply with applicable law or stock market requirements.
2008 Stock Incentive Plan
In February 2008, our board of directors adopted our 2008 stock incentive plan. Our stockholders approved our 2008 stock incentive plan in March 2008. Following the completion of our IPO on February 12, 2014, our board of directors will not grant any further awards under the 2008 stock incentive plan but all outstanding awards will continue to be governed by their existing terms. We will make future equity-based awards under our new 2014 stock incentive plan described above.
Types of Awards. The 2008 stock incentive plan provides for the grant of incentive stock options within the meaning of Section 422 of the Internal Revenue Code, nonstatutory stock options, restricted stock awards, consisting of restricted stock and restricted stock units, and other forms of stock-based awards. Awards under the plan may be granted to our employees, directors and individual consultants and advisors. Only our employees are eligible to receive incentive stock options.
Share Reserve. When initially adopted, an aggregate of 201,278 shares were reserved for issuance under the 2008 stock incentive plan. The 2008 stock incentive plan has been amended to increase the total number of shares which were available for issuance under the plan to 2,325,898.
As of December 31, 2013, options to purchase 1,957,058 shares of our common stock, at a weighted average exercise price per share of $5.47, were outstanding under the 2008 stock incentive plan. As of December 31, 2013, 357,897 shares of our common stock remained available for future issuance under the plan.
Administration. Our board of directors, or a duly authorized committee thereof, is authorized to administer our 2008 stock incentive plan. Our board of directors has delegated certain authority to administer the 2008 stock incentive plan to our compensation committee; however, our general practice has been that awards are approved by the board of directors. In addition, our compensation committee has delegated to Mr. Abbey the authority to award stock options to purchase 251,324 shares of our common stock to non-executive employees. Our board of directors or its authorized committee has the authority under the plan to interpret and adopt rules and procedures relating to the 2008 stock incentive plan, as well as to determine the terms of any award or amend the terms of any award made under the plan. No amendment to any award made under the plan may materially and adversely affect the rights of a participant under any outstanding award without the participant’s consent.
Stock Options. Each stock option awarded under the 2008 stock incentive plan was granted pursuant to a notice of stock option and stock option agreement. The board of directors determined the exercise price for a stock option, within the terms and conditions of the 2008 stock incentive plan, provided that the exercise price of a stock option generally cannot be less than 100% of the fair market value of our common stock on the date of grant. The vesting and other terms of each grant under the 2008 stock incentive plan was determined by the board of directors in its discretion; however, shares subject to stock options granted under the 2008 stock incentive plan generally vest in installments over a specified period of service, typically four years.
The board of directors determined the term of stock options granted under the 2008 stock incentive plan, subject to limitations in the case of some incentive stock options, as described below. In general, if an optionee’s service relationship with us, or any of our affiliates, ceases for any reason other than disability, death or cause, the optionee may generally exercise the vested portion of any option for a period of three months following the cessation of service. If an optionee’s service relationship with us, or any of our affiliates, ceases due to disability or death or if an optionee dies within a specified period following cessation of service, the optionee or a beneficiary generally may exercise the vested portion of any option for a period of 12 months following the death or disability. If an optionee’s services are terminated for cause, options generally terminate immediately upon such termination. In no event may an option be exercised beyond the expiration of its term.
Stock purchased upon the exercise of a stock option may, depending on the terms of the particular option agreement, be paid for using any of the following: (1) cash or check, (2) a broker-assisted cashless exercise, (3) when our common stock is registered under the Securities Exchange Act of 1934, the tender of common stock previously owned by the optionee, (4) delivery of a promissory note, (5) payment of other lawful consideration as determined by the plan administrator, or (6) any combination of the above.
101
Table of Contents
Tax Limitations on Incentive Stock Options. Incentive stock options are subject to certain restrictions contained in the Internal Revenue Code. Among such restrictions, incentive stock options may be granted only to our employees. The maximum term of an incentive stock option is ten years from the date of grant. Any incentive stock option granted to any person who, at the time of the grant, owns or is deemed to own stock possessing more than 10% of our total combined voting power or that of any of our affiliates must have an exercise price equal at least to 110% of the fair market value of the stock subject to the option on the date of grant, and the term of the incentive stock option may not exceed five years from the date of grant. The aggregate fair market value, determined at the time of grant, of shares of our common stock with respect to incentive stock options that are exercisable for the first time by an optionee during any calendar year under all of our stock plans may not exceed $100,000.
Restricted Stock Awards. Each restricted stock award granted under the 2008 stock incentive plan was granted pursuant to a summary of restricted stock purchase and a restricted stock purchase agreement. An award of restricted stock entitles a participant to acquire shares of our common stock that are subject to specified restrictions, which may include a repurchase right or forfeiture right, if the shares are issued at no cost, in our favor that lapses in accordance with a vesting schedule or as conditions specified in the award are satisfied. The board of directors determined the terms and conditions of restricted stock awards, including the conditions for repurchase or forfeiture and the purchase price, if any. Unless the board of directors determined otherwise, participants holding shares of restricted stock are entitled to all ordinary cash dividends paid with respect to such shares.
Term; Amendment. No awards may be granted under the 2008 stock incentive plan after the expiration of ten years from the earlier of the date the plan was adopted by our board of directors or the date the plan was approved by our stockholders, but awards granted prior to such expiration may extend beyond that date. The board of directors may amend, suspend or terminate the plan at any time, subject to approval of the stockholders in certain circumstances if required by the Internal Revenue Code to ensure that incentive stock options are tax-qualified and to a participant’s consent to the extent that any amendment to the plan may materially and adversely affect the rights of a participant under any outstanding award.
Effect of Certain Corporate Transactions. Unless otherwise provided in an individual award document, in the event of specified changes of control of our company, our board of directors may take any one or more actions as to any outstanding equity award, or as to a portion of any outstanding equity award, including:
| • | providing that such awards will be assumed, or substantially equivalent awards substituted, by the acquiring or succeeding corporation or an affiliate thereof; |
| • | providing, upon notice to the participant, that all unexercised awards will terminate immediately prior to the consummation of such transaction unless exercised within a specified period of time; |
| • | providing that all or any outstanding awards will become vested or exercisable, or restrictions applicable to such awards will lapse, in full or in part, at or immediately prior to such event; |
| • | in the event of a consolidation, merger, combination, reorganization or similar transaction under the terms of which holders of our common stock will receive a cash payment per share surrendered in the transaction, making or providing for an equivalent cash payment in exchange for the termination of such equity awards; or |
| • | providing that in the event of a liquidation or dissolution awards will convert into the right to receive liquidation proceeds. |
| • | The majority of the awards granted under the 2008 stock incentive plan provide that the unvested portion of such award would become fully vested upon specified changes of control of our company. |
Transferability. Awards made under the 2008 stock incentive plan are not transferable except by will or by the laws of descent or distribution or, other than in the case of an incentive stock option, pursuant to a domestic relations order.
1999 Stock Option Plan
In December 1999, our board of directors adopted and our stockholders approved the 1999 stock option plan. In 2008, our employees who then held options under the 1999 stock option plan agreed to the cancellation and termination of all of their options previously granted under the 1999 stock option plan in exchange for the grant of new options under our 2008 stock incentive plan described above, and the board of directors determined at that time to discontinue making awards under the 1999 stock option plan. As of December 31, 2013, options to purchase 11 shares of our common stock at a weighted average exercise price per share of $3,221.29 were outstanding under the 1999 stock option plan. The 1999 stock option plan has expired and no shares of our common stock are available for issuance under that plan other than shares that may be issued upon exercise of the outstanding options to purchase up to 11 shares of our common stock.
102
Table of Contents
Administration. Our board of directors administered the 1999 stock option plan and had the authority to interpret the terms of the 1999 stock option plan and the options granted under it.
Eligibility. The 1999 stock option plan provided for the grant of incentive stock options within the meaning of Section 422 of the Code and nonstatutory stock options. Our employees, including officers, non-employee directors, advisors and independent consultants, were eligible to receive options under the 1999 stock option plan, provided that incentive stock options could be granted only to employees.
Effect of Certain Corporate Transactions. The 1999 stock option plan provided that, unless otherwise determined by our board of directors at the time of grant, in the event of a merger, consolidation, corporate reorganization or any transaction in which all or substantially all of our assets or stock were sold, leased, transferred or otherwise disposed of, unless otherwise provided in an individual’s option agreement, any unvested portion of a stock option granted under the 1999 stock option plan would become fully vested unless the surviving or acquiring corporation assumed or substituted comparable options for the outstanding options granted under the plan or replaced the options with a cash incentive program that preserved the intrinsic value of the options at the time of the transaction and provided for subsequent payout over the same vesting schedules as the options being replaced.
2014 Employee Stock Purchase Plan
In January 2014, our board of directors adopted and our stockholders approved the 2014 Employee Stock Purchase Plan, or the 2014 ESPP, which became effective immediately prior to the closing of our IPO, which occurred on February 12, 2014. Our board of directors or a committee appointed by our board of directors administer the 2014 ESPP.
The 2014 ESPP provides for six month offering periods during which eligible employees may elect to have wages or salary withheld through payroll deductions for the purpose of purchasing shares at the end of the period. All of our employees or employees of any designated subsidiary, as defined in the 2014 ESPP, are eligible to participate in the 2014 ESPP, provided that:
| • | such person is customarily employed by us or a designated subsidiary for more than 20 hours a week and for more than five months in a calendar year; |
| • | such person has been employed by us or a designated subsidiary for at least twelve months prior to enrolling in the 2014 ESPP; and |
| • | such person was our employee or an employee of a designated subsidiary on the first day of the applicable offering period under the 2014 ESPP. |
No employee is eligible to receive an option to purchase shares of our common stock that would result in the employee owning 5% or more of the total combined voting power or value of our stock immediately after the grant of such option.
We expect to make one or more offerings to our employees to purchase stock under the 2014 ESPP. Purchase plan periods under the 2014 ESPP will commence at such time or times as our board of directors determines. Payroll deductions made during each purchase plan period will be held for the purchase of our common stock at the end of each purchase plan period.
On the offering commencement date of each purchase plan period, we will grant to each eligible employee who is then a participant in the 2014 ESPP an option to purchase shares of our common stock. The employee may authorize up to a maximum of 10% of his or her base pay to be deducted by us during the purchase plan period. Each employee who continues to be a participant in the 2014 ESPP on the last business day of the purchase plan period is deemed to have exercised the option, to the extent of accumulated payroll deductions within the 2014 ESPP ownership limits. Under the terms of the 2014 ESPP, the option exercise price shall be determined by our board of directors for each purchase plan period and the option exercise price will be at least 85% of the applicable closing price. If our board of directors does not make a determination of the option exercise price, the option exercise price will be 85% of the lesser of the closing price of our common stock on either the first business day of the purchase plan period or the last business day of the purchase plan period. In no event may an employee purchase in any one purchase plan period a number of shares that exceeds the number of shares determined by dividing (1) the product of $2,083 and the number of full months in the purchase plan period by (2) the closing price of a share of our common stock on the commencement date of the purchase plan period. Our board of directors may, in its discretion, choose a different purchase plan period of twelve months or less for each offering.
103
Table of Contents
An employee who is not a participant on the last day of the offering period is not entitled to exercise any option, and the employee’s accumulated payroll deductions will be refunded. An employee’s rights under the purchase plan terminate upon voluntary withdrawal from the purchase plan at any time, or when the employee ceases employment for any reason.
We will be required to make equitable adjustments in connection with the 2014 ESPP and any outstanding awards to reflect stock splits, reverse stock splits, stock dividends, recapitalizations, combination of shares, reclassification of shares, spin-offs and other similar changes in capitalization.
Upon the occurrence of a reorganization event, as defined in the 2014 ESPP, our board of directors is authorized to take any one or more of the following actions as to outstanding options under the 2014 ESPP:
| • | provide that options will be assumed, or substantially equivalent options will be substituted, by the acquiring or succeeding corporation (or an affiliate thereof); |
| • | upon written notice to employees, provide that all outstanding options will be terminated immediately prior to the consummation of such reorganization event and that all such outstanding options will become exercisable to the extent of accumulated payroll deductions as of a date specified by our board of directors; |
| • | upon written notice to employees, provide that all outstanding options will be cancelled as of a date prior to the effective date of the reorganization event and that all accumulated payroll deductions will be returned to participating employees on such date; |
| • | upon the occurrence of a reorganization event in which holders of our common stock will receive a cash payment for each share surrendered in the reorganization event, change the last day of the purchase plan period to be the date of the consummation of the reorganization event and provide that participants will receive a cash payment equal to the acquisition price times the number of shares of common stock that the participant’s accumulated payroll deductions as of immediately prior to the reorganization event could purchase at the option price minus the result of multiplying such number of shares by such option price; and |
| • | provide that, in connection with a liquidation or dissolution of our company, options will convert into the right to receive liquidation proceeds (net of the option price). |
Our board of directors may at any time, and from time to time, amend or suspend the 2014 ESPP. We will obtain stockholder approval for any amendment if such approval is required by Section 423 of the Code. Further, our board of directors may not make any amendment that would cause the 2014 ESPP to fail to comply with Section 423 of the Code. Our board of directors may terminate the 2014 ESPP at any time. Upon termination, we will refund all amounts in the accounts of participating employees.
Director Compensation
Prior to the effectiveness of our IPO, we did not have a formal non-employee director compensation policy. We reimbursed our non-employee directors for their reasonable expenses incurred in connection with attending our board of directors and committee meetings. In addition, we entered into letter agreements with Messrs. Gryska and Lamarre, two of our independent directors, in May 2012 and February 2013. These letter agreements provided for the payment of an annual retainer of $25,000 and a meeting fee of $1,000 for each meeting attended in person. In addition, under the letter agreements, we granted each of Messrs. Gryska and Lamarre a stock option to purchase 5,015 shares of our common stock at an exercise price of $4.20, which vests in equal monthly installments over a term of three years so long as such person continues to serve as a director. Under his letter agreement, Mr. Gryska also received a lump sum payment of $10,604 for service as a director prior to March 31, 2012. Our letter agreements with Messrs. Gryska and Lamarre terminated upon the closing of our IPO on February 12, 2014. In addition, in December 2013, our board of directors granted to each of Messrs. Gryska and Lamarre a cash bonus award of $15,000 for their service as directors. In December 2013, our board of directors granted to each of Drs. Birner and Underdown and Messrs. Gryska, Lamarre and Petrov an option to purchase 11,000 shares of our common stock, at an exercise price of $7.32 per share, which vests in equal quarterly installments over a three year period commencing upon the effectiveness of our IPO, which occurred on February 6, 2014, so long as such person continues to serve as a director. Other than as described above, we did not provide any cash or equity compensation to our non-employee directors during the year ended December 31, 2013.
Our board of directors adopted a formal non-employee director compensation policy that is designed to provide a total compensation package that enables us to attract and retain qualified and experienced individuals to serve as directors and to align our directors’ interests with those of our stockholders. This new policy became effective on February 6, 2014, and provides for non-employee directors to receive an option grant of 11,000 shares upon election to the board, which will vest in equal quarterly installments over a term of three years so long as such person continues to serve as a director; an annual option grant of 5,500 shares upon the annual meeting of stockholders, which will vest in equal quarterly installments over a term of one year so long as such person continues to serve as a director; an annual retainer of $35,000; and a supplemental retainer of $25,000 in the event such director is the chairman or lead director. If the non-employee director is a member of our audit or compensation committee, he or she would receive an additional $5,000 retainer, which would be increased to $10,000 if such director was serving as the chair of such committee. If the non-employee director is a member of our governance and nominating committee, he or she would receive an additional $2,500 retainer, which would be increased to $5,000 if such director was serving as the chair of such committee.
Compensation Committee Interlocks and Insider Participation
None of our executive officers serves as a member of the board of directors or compensation committee, or other committee serving an equivalent function, of any other entity that has one or more of its executive officers serving as a member of our board of directors or our compensation committee. None of the members of our compensation committee is, or has ever been, an officer or employee of our company.
104
Table of Contents
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
Securities Authorized for Issuance under Equity Compensation Plan
The following table shows information relating to our equity compensation plans as of December 31, 2013. Under these plans, we can issue stock options and restricted stock.
| Equity Compensation Plan Information | ||||||||||||
| Plan Category |
Number of securities to be issued upon exercise of outstanding options, warrants and rights |
Weighted average exercise price of outstanding options, warrants and rights |
Number of securities remaining available for future issuance under equity compensation plans (excluding securities in first column) |
|||||||||
| Equity compensation plans approved by security holders |
1,966,668 | $ | 5.50 | 357,841 | ||||||||
| Equity compensation plans not approved by security holders |
— | — | — | |||||||||
|
|
|
|
|
|||||||||
| Total |
1,966,668 | $ | 5.50 | 357,841 | ||||||||
|
|
|
|
|
|||||||||
Security Ownership of Certain Beneficial Owners and Management
The following table sets forth information with respect to the beneficial ownership of our common stock, as of February 28, 2014 by:
| • | each of our directors; |
| • | each of our named executive officers; |
| • | all of our directors and executive officers as a group; and |
| • | each person, or group of affiliated persons, who is known by us to beneficially own more than 5% of our common stock. |
Beneficial ownership is determined in accordance with the rules and regulations of the SEC and includes voting or investment power with respect to our common stock. Shares of our common stock subject to options or warrants that are currently exercisable or exercisable within 60 days of February 28, 2014 are considered outstanding and beneficially owned by the person holding the options or warrants for the purpose of calculating the percentage ownership of that person but not for the purpose of calculating the percentage ownership of any other person. Except as otherwise noted, the persons and entities in this table have sole voting and investing power with respect to all of the shares of our common stock beneficially owned by them, subject to community property laws, where applicable.
105
Table of Contents
Except as otherwise set forth in the footnotes below, the address of the beneficial owner is c/o Argos Therapeutics, Inc., 4233 Technology Drive, Durham, North Carolina 27704.
| Number of Shares Beneficially Owned |
||||||||
| Name and Address of Beneficial Owner |
Percentage of Shares Beneficially Owned |
|||||||
| 5% Stockholders: |
||||||||
| Pharmstandard International S.A.(1) |
5,983,549 | 30.44 | % | |||||
| Entities affiliated with Forbion(2) |
2,450,144 | 12.47 | % | |||||
| TVM V Life Science Ventures GmbH & Co. KG(3) |
1,471,091 | 7.48 | % | |||||
| Entities affiliated with Lumira Capital(4) |
1,249,572 | 6.36 | % | |||||
| Entities affiliated with Intersouth Partners(5) |
1,069,979 | 5.44 | % | |||||
| Directors and Named Executive Officers: |
||||||||
| Andrei Petrov(1) |
5,983,549 | 30.44 | % | |||||
| Alexey Vinogradov, Ph.D.(1) |
5,983,549 | 30.44 | % | |||||
| Sander van Deventer, M.D., Ph.D.(2) |
2,450,144 | 12.47 | % | |||||
| Hubert Birner, Ph.D.(3) |
1,471,091 | 7.48 | % | |||||
| Brian J. Underdown, Ph.D.(4) |
1,249,572 | 6.36 | % | |||||
| Jeffrey D. Abbey(6) |
162,138 | 0.82 | % | |||||
| Frederick M. Miesowicz, Ph.D.(7) |
61,123 | 0.31 | % | |||||
| Charles A. Nicolette, Ph.D.(8) |
86,577 | 0.44 | % | |||||
| David W. Gryska(9) |
3,761 | 0.02 | % | |||||
| Jean Lamarre(10) |
1,950 | 0.01 | % | |||||
| All named executive officers and directors as a group (10 persons)(11) |
11,469,905 | 57.47 | % | |||||
| (1) | The address of Pharmstandard International S.A. is 65, Boulevard Grande Duchesse Charlotte, L-1331 Luxmbourg, Grand Duchy of Luxembourg. Pharmstandard International S.A. is a wholly owned subsidiary of Public Joint Stock Company “Pharmstandard.” As the parent entity, Public Joint Stock Company “Pharmstandard” has voting and investment control over the shares of the Company held by Pharmstandard International S.A. |
| (2) | The address of Forbion is Gooimeer 2-35 1411 DC Naarden, the Netherlands. Consists of (i) 1,254,388 shares of common stock held by Coöperatieve AAC LS U.A. and (ii) 1,195,756 shares of common stock held by Forbion Co-Investment II Coöperatief U.A. Forbion 1 Management B.V., the director of Cooperatieve AAC LS U.A, has voting and investment power over the shares held by Cooperatieve AAC LS U.A, which are exercised through Forbion 1 Management B.V.‘s investment committee, consisting of L.P.A. Bergstein, H. A. Slootweg, M. A. van Osch, G. J. Mulder and Sander van Deventer. None of the members of the investment committee has individual voting and investment power with respect to such shares, and the members disclaim beneficial ownership of such shares except to the extent of their pecuniary interests therein. Forbion 1 Co- II Management B.V., the director of Forbion Co-Investment II Cooperatief U.A., has voting and investment power over the shares held by Forbion Co-Investment II Cooperatief U.A., which are exercised through Forbion 1 Co II Management B.V.‘s investment committee, consisting of L.P.A. Bergstein, H. A. Slootweg, M. A. van Osch, G. J. Mulder and Sander van Deventer. None of the members of the investment committee has individual voting and investment power with respect to such shares, and the members disclaim beneficial ownership of such shares except to the extent of their pecuniary interests therein. |
| (3) | The address of TVM V Life Science Ventures GmbH &Co. KG is Ottostr. 4, 80333 Munich, Germany. The shares represented here are directly held by TVM V Life Science Ventures GmbH & Co. KG (“TVM V”), the managing limited partner of which is TVM V Life Science Ventures Management GmbH & Co. KG (“TVM V Management”), for which Hubert Birner, Stefan Fischer, Alexandra Goll and Alex Polack, each a member of the investment committee of TVM V Management, share voting and investment authority over the shares held by TVM V. Each of TVM V Management, Hubert Birner, Stefan Fischer, Alexandra Goll and Alex Polack disclaims beneficial ownership of these shares, except to the extent of their pecuniary interest therein, if any. |
| (4) | The address of Lumira Capital is 141 Adelaide Street West, Suite 770, Toronto, Ontario, Canada M5H 3L5. The shares represented here are directly and/or beneficially owned by each of, LCC Legacy Holdings Inc., an Ontario corporation (“LCC”), Lumira Capital Investment Management Inc., a Canadian corporation (“LCIM”), Peter van der Velden, as a member of the Board of Directors of LCC and a member of the investment committee of LCIM, Gerald Brunk, as a member of the investment committee of LCIM, Daniel Hetu, as a member of the investment committee of LCIM, Benjamin Rovinski, as a member of the investment committee of LCIM, Brian J. Underdown, as a member of the investment committee of LCIM and Vasco Larcina, as a member of the investment committee of LCIM. Each of the foregoing is referred to as a “Reporting Person” and collectively as the “Reporting Persons.” LCC, acting as the Manager of Lumira Capital I Limited Partnership (“CI”), has voting and investment power over the securities held by CI, which is exercised by the investment committee of LCIM. Lumira Capital I (QGP) Inc., which is the general partner of Lumira Capital I Quebec Limited Partnership (“CQ”) and a wholly-owned subsidiary of LCC, has voting and investment power over the shares held by CQ; such investment and voting power is exercised based on the recommendations of the investment committee of LCIM. Voting and investment power over the securities held directly by LCC is exercised by the LCC board of directors. CI directly holds 821,016 shares; CQ directly holds 289,323 shares; LCC directly holds 139,233 shares. Each of the Reporting Persons specifically disclaims beneficial ownership of the securities reported herein that are not directly owned by such Reporting Person, except to the extent of their pecuniary interest therein. Beneficial ownership is derived from a Schedule 13D filed on February 21, 2014. |
| (5) | The address for Intersouth Partners is 102 City Hall Plaza, Suite 200, Durham, North Carolina 27701. The shares represented here are directly and/or beneficially owned by each of Intersouth Partners V, L.P., Intersouth Affiliates V, L.P., Intersouth Associates V LLC, Intersouth Partners IV, L.P., Intersouth Associates IV LLC, Mitch Mumma and Dennis Dougherty. Intersouth Affiliates V, L.P. directly holds 32,999 shares; (ii) Intersouth Partners V, L.P. directly holds 721,884 shares; and (iii) Intersouth Partners IV, L.P. directly holds 315,096 shares. Intersouth Associates V LLC, the general partner of each of Intersouth Partners V, L.P. and Intersouth Affiliates V, L.P., and Intersouth Associates IV LLC, the general partner of Intersouth Partners IV, L.P., may be deemed to share voting and dispositive power over the shares held by each of Intersouth Affiliates V, L.P. and Intersouth Partners V, L.P. and Intersouth Partners IV, L.P., respectively. Dennis Dougherty and Mitch Mumma are both Member Managers of Intersouth Associates V LLC, and Intersouth Associates IV LLC, and share voting and investment power over the shares held by Intersouth Affiliates V, L.P., Intersouth Partners V, L.P. and Intersouth Partners IV, L.P. Beneficial ownership is derived from a Schedule 13G filed on February 18, 2014. |
| (6) | Consists of (i) 2,580 shares of common stock and (ii) 159,558 shares of common stock issuable upon exercise of options that are exercisable as of February 28, 2014 or will become exercisable within 60 days after such date. |
| (7) | Consists of (i) 1.289 shares of common stock and (ii) 59,834 shares of common stock issuable upon exercise of options that are exercisable as of February 28, 2014 or will become exercisable within 60 days after such date. |
| (8) | Consists of (i) 6,800 shares of common stock and (ii) 79,777 shares of common stock issuable upon exercise of options that are exercisable as of February 28, 2014 or will become exercisable within 60 days after such date. |
106
Table of Contents
| (9) | Consists of 3,761 shares of common stock issuable upon exercise of options that are exercisable as of February 28, 2014 or will become exercisable within 60 days after such date. |
| (10) | Consists of 1,950 shares of common stock issuable upon exercise of options that are exercisable as of February 28, 2014 or will become exercisable within 60 days after such date. |
| (11) | Includes (i) 11,165,025 shares of common stock and (ii) 304,880 shares of common stock issuable upon exercise of options that are exercisable as of February 28, 2014 or will become exercisable within 60 days after such date. |
Item 13. Certain Relationships and Related Transactions, and Director Independence
Since January 1, 2013, we engaged in the following transactions, in which the amount involved in the transaction exceeds $120,000 with our directors, executive officers and holders of more than 5% of our voting securities, and affiliates or immediate family members of our directors, executive officers and holders of more than 5% of our voting securities. We believe that all of these transactions were on terms as favorable as we could have obtained from unrelated third parties. Compensation arrangements for our directors and named executive officers are described in “Item 11. Executive Compensation.”
Exchange of Series D-1 Preferred Stock for Series D Preferred Stock
In July 2013, the holders of shares of our series D-1 preferred stock exchanged all shares of our series D-1 preferred stock held by such investors for shares of our series D preferred stock. In this exchange, we issued an aggregate of 16,151,212 shares of our series D preferred stock in exchange for an aggregate of 7,433,870 shares of our series D-1 preferred stock. In connection with this exchange, warrants to purchase 32,869,928 shares of our series C preferred stock and warrants to purchase 1,332,622 shares of our series D-1 preferred stock were cancelled. We did not receive any cash proceeds in connection with the exchange.
In addition, in connection with the exchange, we reduced the original per share purchase price of the series D preferred stock from $3.528233 per share to $1.978565 per share. As a result of such reduction, we issued an aggregate of 6,373,782 additional shares of our series D preferred stock to the holders that had purchased shares of our series D preferred stock prior to such reduction for no additional consideration.
The following table sets forth the number of shares of our series D preferred stock which were issued to our directors, executive officers and holders of more than 5% of our voting securities, and affiliates or immediate family members of our directors, executive officers and holders of more than 5% of our voting securities, in exchange for such investor’s series D-1 preferred stock, as well as the additional shares of our series D preferred stock which we issued to our directors, executive officers and holders of more than 5% of our voting securities, and affiliates or immediate family members of our directors, executive officers and holders of more than 5% of our voting securities, as a result of the reduction of the purchase price of our series D preferred stock.
| Name |
Number of Shares of Series D-1 Preferred Stock Exchanged for Series D Preferred Stock |
Number of Shares of Series D Preferred Stock in Exchange for Series D-1 Preferred Stock |
Number of Additional Shares of Series D Preferred Stock Issued in Connection with Reduction of Series D Purchase Price |
|||||||||
| Caisse de dépôt et de placement du Québec |
385,438 | 837,422 | 764,825 | |||||||||
| Entities affiliated with Forbion(1) |
4,074,584 | 8,852,638 | 921,415 | |||||||||
| Entities affiliated with Intersouth Partners(2) |
686,660 | 1,491,877 | 975,887 | |||||||||
| Entities affiliated with Lumira Capital(3) |
747,430 | 1,623,907 | 1,221,319 | |||||||||
| TVM V Life Science Ventures GMBH & Co. KG(4) |
869,910 | 1,890,008 | 1,436,790 | |||||||||
| Jeffrey D. Abbey |
1,440 | 3,130 | 2,860 | |||||||||
| Frederick M. Miesowicz, Ph.D. |
720 | 1,565 | 1,431 | |||||||||
| Charles A. Nicolette, Ph.D. |
1,440 | 3,130 | 12,409 | |||||||||
| (1) | These amounts are distributed among the entities set forth in the table below. Dr. van Deventer, one of our directors, is a General Partner of Forbion Capital Partners and is affiliated with these entities. |
107
Table of Contents
| Name |
Number of Shares of Series D-1 Preferred Stock Exchanged for Series D Preferred Stock |
Number of Shares of Series D Preferred Stock Issued in Exchange for Series D-1 Preferred Stock |
Number of Additional Shares of Series D Preferred Stock Issued in Connection with Reduction of Series D Purchase Price |
|||||||||
| Coöperatieve AAC LS U.A. |
2,037,292 | 4,426,319 | 921,415 | |||||||||
| Forbion Co-Investment II Cooperatief U.A. |
2,037,292 | 4,426,319 | 0 | |||||||||
| (2) | These amounts are distributed among the entities set forth in the table below. Mr. Tracy, a former director of ours, is a Venture Partner at Intersouth Partners and is affiliated with these entities. |
| Name |
Number of
Shares of Series D-1 Preferred Stock Exchanged for Series D Preferred Stock |
Number
of Shares of Series D Preferred Stock Issued in Exchange for Series D-1 Preferred Stock |
Number of Additional Shares of Series D Preferred Stock Issued in Connection with Reduction of Series D Purchase Price |
|||||||||
| Intersouth Affiliates V, L.P. |
25,950 | 56,383 | 21,923 | |||||||||
| Intersouth Partners IV, L.P. |
93,050 | 202,166 | 474,403 | |||||||||
| Intersouth Partners V, L.P. |
567,660 | 1,233,328 | 479,561 | |||||||||
| (3) | These amounts are distributed among the entities set forth in the table below. Dr. Underdown, one of our directors, is the Managing Director of Lumira Capital Investment Management Inc. and an affiliate of these entities. |
| Name |
Number of Shares of Series D-1 Preferred Stock Exchanged for Series D Preferred Stock |
Number
of Shares of Series D Preferred Stock Issued in Exchange for Series D-1 Preferred Stock |
Number of Additional Shares of Series D Preferred Stock Issued in Connection with Reduction of Series D Purchase Price |
|||||||||
| LCC Legacy Holdings, Inc. |
68,826 | 149,538 | 140,340 | |||||||||
| Lumira Capital I Limited Partnership |
501,808 | 1,090,253 | 799,246 | |||||||||
| Lumira Capital I Quebec Limited Partnership |
176,796 | 384,116 | 281,733 | |||||||||
| (4) | Dr. Birner, one of our directors, is a general partner of TVM Capital and is affiliated with this entity. |
Series E Preferred Stock Financing
From August 2013 through November 2013, we sold an aggregate of 36,856,922 shares of our series E preferred stock in a private placement to certain of our existing holders of preferred stock and additional accredited investors at a purchase price of $1.302333 per share. The sale of series E preferred stock was structured in multiple tranches and with multiple closing dates. Under the agreement we sold 16,898,436 shares of our series E preferred stock for an aggregate purchase price of $22.0 million in August 2013, 921,423 shares of our series E preferred stock for an aggregate purchase price of $1.2 million in October 2013 and 19,037,063 shares of our
108
Table of Contents
series E preferred stock for an aggregate purchase price of $24.8 million in November 2013. The purchasers of our series E preferred stock committed to purchase additional shares of series E preferred stock for an aggregate purchase price of $12.0 million in a second tranche closing pursuant to the terms of the series E preferred stock and warrant purchase agreement upon the achievement of specified milestones with respect to our phase 3 clinical trial of AGS-003. This commitment terminated in connection with the closing of our initial public offering.
In connection with the issuance and sale of our series E preferred stock, we issued warrants to the purchasers of our series E preferred stock to purchase an aggregate of 945,036 shares of our common stock at an exercise price of $0.06 per share. The warrants had a term of ten years and would only become exercisable if we did not receive $5.0 million in non-dilutive financing by December 31, 2013 or had not executed definitive documentation providing for an additional $5.0 million investment of non-dilutive financing as of December 31, 2013. In connection with the license agreement that we entered into with Medinet in December 2013, Medinet paid us $1.0 million and loaned us $9.0 million. In connection with our receipt of these funds, the warrants were cancelled pursuant to their terms.
In connection with the issuance and sale of our series E preferred stock, each participating investor exchanged shares of our series A preferred stock, series B preferred stock and series C preferred stock, if any, held by such investor for shares of series D preferred stock pursuant to the terms of the series E preferred stock and warrant purchase agreement. Under these terms, participating investors exchanged such number of shares of series A preferred stock, series B preferred stock and series C preferred stock as had a value equal to the aggregate purchase price of shares of series E preferred stock purchased by such investor in the series E preferred stock financing, with the value of each share of series A preferred stock, series B preferred stock and series C preferred stock equal to the liquidation preference of such shares: $0.32 per share in the case of the series A preferred stock, $0.5632 per share in the case of the series B preferred stock and $0.09249 per share in the case of the series C preferred stock. Participating investors were required to exchange all shares of series A preferred stock held by such investors prior to exchanging any shares of series B preferred stock, and all shares of series B preferred stock held by such investors before exchanging any shares of series C preferred stock. Participating investors exchanged an aggregate of 111,837 shares of series A preferred stock, 8,687,630 shares of series B preferred stock and 10,586,174 shares of series C preferred stock for an aggregate of 2,985,863 shares of our series D preferred stock.
In connection with the issuance and sale of our series E preferred stock, each participating investor who had also purchased shares of series D preferred stock or series D-1 preferred stock for cash during 2012 (excluding for such purposes shares of series D preferred stock issued to such investor in exchange for the outstanding principal and interest on the 2010 convertible notes and the 2011 convertible notes and shares of preferred stock issued in 2012 in exchange for any previously issued shares of preferred stock) exchanged shares of series D preferred stock for shares of series E preferred stock pursuant to the terms of the series E preferred stock and warrant purchase agreement. Under these terms, participating investors exchanged such number of shares of series D preferred stock as had a value equal to the aggregate purchase price of the shares of series D preferred stock and series D-1 preferred stock purchased for cash during 2012, with the value of each share series D preferred stock at the time of exchange equal to the original purchase price of such shares: $1.978565 per share. Participating investors exchanged an aggregate of 12,607,779 shares of series D preferred stock for 19,154,336 shares of our series E preferred stock.
The following table sets forth the aggregate cash purchase price of the series E preferred stock purchased by our directors, executive officers and holders of more than 5% of our voting securities, and affiliates or immediate family members of our directors, executive officers and holders of more than 5% of our voting securities, the number of shares of series E preferred stock issued in consideration of such amounts as well as the second tranche amount committed to be purchased and the number of warrant shares underlying the associated warrants issued in connection with the purchase of series E preferred stock. These warrants were cancelled on December 27, 2013.
| Name |
Cash Purchase Price |
Number of Shares of Series E Preferred Stock |
Number of Warrant Shares(1) |
Cash Purchase Price of Second Tranche Shares of Series E(2) |
||||||||||||
| Caisse de dépôt et de placement du Québec |
$ | 600,000 | 460,711 | 11,813 | $ | 150,000 | ||||||||||
| Entities affiliated with Forbion(3) |
$ | 1,165,333 | 894,804 | 22,943 | $ | 291,333 | ||||||||||
| Entities affiliated with Intersouth Partners(4) |
$ | 922,958 | 708,695 | 18,171 | $ | 230,740 | ||||||||||
| Entities affiliated with Lumira Capital(5) |
$ | 1,181,112 | 906,919 | 23,253 | $ | 295,278 | ||||||||||
| Pharmstandard International S.A.(6) |
$ | 36,792,595 | 28,251,296 | 724,389 | $ | 9,198,149 | ||||||||||
| TVM V Life Science Ventures GMBH & Co. KG(7) |
$ | 1,275,943 | 979,736 | 25,121 | $ | 318,986 | ||||||||||
| Jeffrey D. Abbey |
$ | 2,459 | 1,888 | 48 | $ | 615 | ||||||||||
| Frederick M. Miesowicz, Ph.D. |
$ | 1,230 | 944 | 24 | $ | 307 | ||||||||||
| Charles A. Nicolette, Ph.D. |
$ | 7,085 | 5,440 | 139 | $ | 1,771 | ||||||||||
| (1) | These warrants were cancelled on December 27, 2013. |
| (2) | This commitment to purchase second tranche shares of series E preferred stock terminated in connection with the closing of our initial public offering. |
| (3) | These amounts are distributed among the entities set forth in the table below. Dr. van Deventer, one of our directors, is a General Partner of Forbion Capital Partners and is affiliated with these entities. |
109
Table of Contents
| Name |
Cash Purchase Price |
Number of Shares of Series E Preferred Stock |
Number of Warrant Shares(1) |
Cash Purchase Price of Second Tranche Shares of Series E(2) |
||||||||||||
| Coöperatieve AAC LS U.A. |
$ | 694,441 | 533,228 | 13,672 | $ | 173,610 | ||||||||||
| Forbion Co-Investment II Cooperatief U.A. |
$ | 470,892 | 361,576 | 9,271 | $ | 117,723 | ||||||||||
| (4) | These amounts are distributed among the entities set forth in the table below. Mr. Tracy, a former director of ours, is a Venture Partner at Intersouth Partners and is affiliated with these entities. |
| Name |
Cash Purchase Price |
Number of Shares of Series E Preferred Stock |
Number of Warrant Shares(1) |
Cash Purchase Price of Second Tranche Shares of Series E(2) |
||||||||||||
| Intersouth Affiliates V, L.P. |
$ | 40,348 | 30,981 | 794 | $ | 10,087 | ||||||||||
| Intersouth Partners IV, L.P. |
$ | — | — | — | — | |||||||||||
| Intersouth Partners V, L.P. |
$ | 882,610 | 677,714 | 17,377 | $ | 220,653 | ||||||||||
| (5) | These amounts are distributed among the entities set forth in the table below. Dr. Underdown, one of our directors, is the Managing Director of Lumira Capital Investment Management Inc. and an affiliate of these entities. |
| Name |
Cash Purchase Price |
Number of Shares of Series E Preferred Stock |
Number of Warrant Shares(1) |
Cash Purchase Price of Second Tranche Shares of Series E(2) |
||||||||||||
| LCC Legacy Holdings, Inc. |
$ | 121,010 | 92,918 | 2,382 | $ | 30,253 | ||||||||||
| Lumira Capital I Limited Partnership |
$ | 783,863 | 601,891 | 15,433 | $ | 195,966 | ||||||||||
| Lumira Capital I Quebec Limited Partnership |
$ | 276,238 | 212,110 | 5,438 | $ | 69,060 | ||||||||||
| (6) | Mr. Petrov and Dr. Vinogradov, two of our directors, are affiliated with this entity. |
| (7) | Dr. Birner, one of our directors, is a general partner of TVM Capital and is affiliated with this entity. |
The following table sets forth the number of shares of our series B preferred stock and series C preferred stock exchanged by our directors, executive officers and holders of more than 5% of our voting securities, and affiliates or immediate family members of our directors, executive officers and holders of more than 5% of our voting securities, and the number of shares of series D preferred stock issued upon such exchange.
| Name |
Number of Shares of Series B Preferred Stock |
Number of Shares of Series C Preferred Stock |
Number of Shares of Series D Preferred Stock |
|||||||||
| Caisse de dépôt et de placement du Québec |
1,331,676 | — | 379,062 | |||||||||
| Entities affiliated with Forbion(1) |
44,703 | — | 12,724 | |||||||||
| Entities affiliated with Intersouth Partners(2) |
2,048,468 | — | 583,097 | |||||||||
| Entities affiliated with Lumira Capital(3) |
1,897,468 | 4,408,628 | 746,191 | |||||||||
| Pharmstandard International S.A.(4) |
— | — | — | |||||||||
| TVM V Life Science Ventures GMBH & Co. KG(5) |
2,831,904 | — | 806,103 | |||||||||
| Jeffrey D. Abbey |
— | 33,240 | 1,553 | |||||||||
| Frederick M. Miesowicz, Ph.D. |
— | 16,618 | 776 | |||||||||
| Charles A. Nicolette, Ph.D. |
— | 43,454 | 2,031 | |||||||||
| (1) | These amounts are distributed among the entities set forth in the table below. Dr. van Deventer, one of our directors, is a General Partner of Forbion Capital Partners and is affiliated with these entities. |
110
Table of Contents
| Name |
Number of Shares of Series B Preferred Stock |
Number of Shares of Series C Preferred Stock |
Number of Shares of Series D Preferred Stock |
|||||||||
| Coöperatieve AAC LS U.A. |
44,703 | — | 12,724 | |||||||||
| Forbion Co-Investment II Cooperatief U.A. |
— | — | — | |||||||||
| (2) | These amounts are distributed among the entities set forth in the table below. Mr. Tracy, a former director of ours, is a Venture Partner at Intersouth Partners and is affiliated with these entities. |
| Name |
Number of Shares of Series B Preferred Stock |
Number of Shares of Series C Preferred Stock |
Number of Shares of Series D Preferred Stock |
|||||||||
| Intersouth Affiliates V, L.P. |
89,550 | — | 25,490 | |||||||||
| Intersouth Partners IV, L.P. |
— | — | — | |||||||||
| Intersouth Partners V, L.P. |
1,958,918 | — | 557,607 | |||||||||
| (3) | These amounts are distributed among the entities set forth in the table below. Dr. Underdown, one of our directors, is the Managing Director of Lumira Capital Investment Management Inc. and an affiliate of these entities. |
| Name |
Number of Shares of Series B Preferred Stock |
Number of Shares of Series C Preferred Stock |
Number of Shares of Series D Preferred Stock |
|||||||||
| LCC Legacy Holdings, Inc. |
268,577 | — | 76,450 | |||||||||
| Lumira Capital I Limited Partnership |
1,204,516 | 3,259,375 | 495,222 | |||||||||
| Lumira Capital I Quebec Limited Partnership |
424,375 | 1,149,253 | 174,519 | |||||||||
| (4) | Mr. Petrov and Dr. Vinogradov, two of our directors, are affiliated with this entity. |
| (5) | Dr. Birner, one of our directors, is a general partner of TVM Capital and is affiliated with this entity. |
111
Table of Contents
The following table sets forth the number of shares of our series D preferred stock exchanged by our directors, executive officers and holders of more than 5% of our voting securities, and affiliates or immediate family members of our directors, executive officers and holders of more than 5% of our voting securities, and the number of shares of series E preferred stock issued upon such exchange.
| Name |
Number of Shares of Series D Preferred Stock |
Number of Shares of Series E Preferred Stock |
||||||
| Caisse de dépôt et de placement du Québec |
1,006,156 | 1,528,600 | ||||||
| Entities affiliated with Forbion(1) |
5,054,167 | 7,678,527 | ||||||
| Entities affiliated with Intersouth Partners(2) |
1,495,494 | 2,272,025 | ||||||
| Entities affiliated with Lumira Capital(3) |
1,654,127 | 2,513,028 | ||||||
| Pharmstandard International S.A.(4) |
— | — | ||||||
| TVM V Life Science Ventures GMBH & Co. KG(5) |
1,973,844 | 2,998,757 | ||||||
| Jeffrey D. Abbey |
3,761 | 5,714 | ||||||
| Frederick M. Miesowicz, Ph.D. |
1,880 | 2,857 | ||||||
| Charles A. Nicolette, Ph.D. |
3,761 | 5,714 | ||||||
| (1) | These amounts are distributed among the entities set forth in the table below. Dr. van Deventer, one of our directors, is a General Partner of Forbion Capital Partners and is affiliated with these entities. |
| Name |
Number of Shares of Series D Preferred Stock |
Number of Shares of Series E Preferred Stock |
||||||
| Coöperatieve AAC LS U.A. |
627,848 | 953,856 | ||||||
| Forbion Co-Investment II Cooperatief U.A. |
4,426,319 | 6,724,671 | ||||||
| (2) | These amounts are distributed among the entities set forth in the table below. Mr. Tracy, a former director of ours, is a Venture Partner at Intersouth Partners and is affiliated with these entities. |
| Name |
Number of Shares of Series D Preferred Stock |
Number of Shares of Series E Preferred Stock |
||||||
| Intersouth Affiliates V, L.P. |
45,029 | 68,410 | ||||||
| Intersouth Partners IV, L.P. |
465,461 | 707,151 | ||||||
| Intersouth Partners V, L.P. |
985,004 | 1,496,464 | ||||||
| (3) | These amounts are distributed among the entities set forth in the table below. Dr. Underdown, one of our directors, is the Managing Director of Lumira Capital Investment Management Inc. and an affiliate of these entities. |
112
Table of Contents
| Name |
Number of Shares of Series D Preferred Stock |
Number of Shares of Series E Preferred Stock |
||||||
| LCC Legacy Holdings, Inc. |
179,669 | 272,962 | ||||||
| Lumira Capital I Limited Partnership |
1,090,319 | 1,656,464 | ||||||
| Lumira Capital I Quebec Limited Partnership |
384,139 | 583,602 | ||||||
| (4) | Mr. Petrov and Dr. Vinogradov, two of our directors, are affiliated with this entity. |
| (5) | Dr. Birner, one of our directors, is a general partner of TVM Capital and is affiliated with this entity. |
Agreements With Our Stockholders
We entered into a registration rights agreement with purchasers of our series A preferred stock, series B preferred stock, series C preferred stock, series D preferred stock and series E preferred stock. The registration rights agreement provides those holders with the right to demand that we file a registration statement, subject to certain limitations, and to request that their shares be covered by a registration statement that we are otherwise filing.
We also entered into a stockholders’ agreement with certain purchasers of our common stock, series A preferred stock, series B preferred stock, series C preferred stock, series D preferred stock and series E preferred stock. The stockholders’ agreement provided for rights of first refusal in respect of sales of securities by certain holders of our common stock and preferred stock. The stockholders’ agreement also provided holders of our series E preferred stock with co-sale rights and with a participation right to purchase their pro rata share of new securities that the Company proposed to sell and issue, subject to specified exceptions. The stockholders’ agreement also contained provisions with respect to the election of our board of directors and its composition. The rights under this agreement terminated upon the closing of our IPO.
Pharmstandard
In August 2013, in connection with the purchase of shares of our series E preferred stock by Pharmstandard International S.A., or Pharmstandard, we entered into an exclusive royalty-bearing license agreement with Pharmstandard. This license agreement and the sale of series E preferred stock to Pharmstandard were negotiated with Pharmstandard on an arm’s length basis. Prior to the closing of the sale of shares of series E preferred stock to Pharmstandard, Pharmstandard did not own any shares of our capital stock. Under this license agreement, we granted Pharmstandard and its affiliates a license, with the right to sublicense, to develop, manufacture and commercialize AGS-003 and other products for the treatment of human diseases, which are developed by Pharmstandard using our personalized immunotherapy platform, in the Russian Federation, Armenia, Azerbaijan, Belarus, Georgia, Kazakhstan, Kyrgyzstan, Moldova, Tajikistan, Turkmenistan, Ukraine and Uzbekistan, which we refer to as the Pharmstandard Territory. We also provided Pharmstandard with a right of first negotiation for development and commercialization rights in the Pharmstandard Territory to specified additional products we may develop.
Under the terms of the license agreement, Pharmstandard licensed us rights to clinical data generated by Pharmstandard under the agreement and granted us an option to obtain an exclusive license outside of the Pharmstandard Territory to develop and commercialize improvements to our Arcelis technology generated by Pharmstandard under the agreement, a non-exclusive worldwide royalty-free license to Pharmstandard improvements to manufacture products using our Arcelis technology and a license to specified follow-on licensed products generated by Pharmstandard outside of the Pharmstandard Territory, each on terms to be negotiated upon our request for a license. In addition, Pharmstandard agreed to pay us pass-through royalties on net sales of all licensed products in the low single digits until it has generated a specified amount of aggregate net sales. Once the net sales threshold is achieved, Pharmstandard will pay us royalties on net sales of specified licensed products, including AGS-003, in the low double digits below 20%. These royalty obligations last until the later of the expiration of specified licensed patent rights in a country or the twelfth anniversary of the first commercial sale in such country on a country by country basis and no further royalties on specified other licensed products. After the net sales threshold is achieved, Pharmstandard has the right to offset a portion of the royalties Pharmstandard pays to third parties for licenses to necessary third party intellectual property against the royalties that Pharmstandard pays to us.
113
Table of Contents
The agreement will terminate upon expiration of the royalty term, upon which all licenses will become fully paid up perpetual exclusive licenses. Either party may terminate the agreement for the other party’s uncured material breach or if specified conditions occur relating to the other party’s insolvency or bankruptcy and we may terminate the agreement if Pharmstandard challenges or assists a third party in challenging specified patent rights of ours. If Pharmstandard terminates the agreement upon our material breach or bankruptcy, Pharmstandard is entitled to terminate our licenses to improvements generated by Pharmstandard, upon which we may come to rely for the development and commercialization of AGS-003 and other licensed products outside of the Pharmstandard Territory, and Pharmstandard is entitled to retain its licenses from us and to pay us substantially reduced royalty payments following such termination.
In November 2013, we entered into an agreement with Pharmstandard under which Pharmstandard purchased additional shares of our series E preferred stock. Under this agreement, we agreed to enter into a manufacturing rights agreement for the European market with Pharmstandard and that the manufacturing rights agreement would provide for the issuance of warrants to Pharmstandard to purchase 499,788 shares of our common stock at an exercise price of $5.82 per share. As of February 28, 2014, we had not entered into this manufacturing rights agreement or issued the warrants.
Green Cross
In July 2013, in connection with the purchase of shares of our series E preferred stock by Green Cross, we entered into an exclusive royalty-bearing license agreement with Green Cross. Under this agreement we granted Green Cross a license to develop, manufacture and commercialize AGS-003 for mRCC in South Korea. We also provided Green Cross with a right of first negotiation for development and commercialization rights in South Korea to specified additional products we may develop.
Under the terms of the license, Green Cross has agreed to pay us $0.5 million upon the initial submission of an application for regulatory approval of a licensed product in South Korea, $0.5 million upon the initial regulatory approval of a licensed product in South Korea and royalties ranging from the mid-single digits to low double digits below 20% on net sales until the fifteenth anniversary of the first commercial sale in South Korea. In addition, Green Cross has granted us an exclusive royalty free license to develop and commercialize all Green Cross improvements to our licensed intellectual property in the rest of the world, excluding South Korea, except that, as to such improvements for which Green Cross makes a significant financial investment and that generate significant commercial benefit in the rest of the world, we are required to negotiate in good faith a reasonable royalty that we will be obligated to pay to Green Cross for such license. Under the terms of the agreement, we are required to continue to develop and to use commercially reasonable efforts to obtain regulatory approval for AGS-003 in the United States.
The agreement will terminate upon expiration of the royalty term, which is 15 years from the first commercial sale, upon which all licenses will become fully paid up perpetual non-exclusive licenses. Either party may terminate the agreement for the other party’s uncured material breach or if specified conditions occur relating to the other party’s insolvency or bankruptcy and we may terminate the agreement if Green Cross challenges or assists a third party in challenging specified patent rights of ours. If Green Cross terminates the agreement upon our material breach or bankruptcy, Green Cross is entitled to terminate our licenses to improvements and retain its licenses from us and to pay us substantially reduced milestone and royalty payments following such termination.
Participation in our Initial Public Offering
Our principal stockholders purchased shares in our IPO at the initial public offering price. The number of shares that each of our principal stockholders purchased and the aggregate purchase price paid for such shares is set forth in the table below.
| Name |
Shares of Common Stock Purchased in our IPO |
Purchase Price |
||||||
| Entities affiliated with Forbion |
36,416 | $ | 291,328 | |||||
| Entities affiliated with Intersouth Partners |
28,841 | $ | 230,728 | |||||
| Entities affiliated with Lumira Capital |
40,497 | $ | 323,976 | |||||
| TVM V Life Science Ventures GmbH & Co. KG |
39,873 | $ | 318,984 | |||||
| Pharmstandard International S.A. |
1,275,000 | $ | 10,200,000 | |||||
114
Table of Contents
Indemnification Agreements
Our certificate of incorporation provides that we will indemnify our directors and officers to the fullest extent permitted by Delaware law. In addition, we have entered into indemnification agreements with all of our directors and executive officers. These agreements may require us, among other things, to indemnify each such director for some expenses, including attorneys’ fees, judgments, fines and settlement amounts incurred by him in any action or proceeding arising out of his service as one of our directors.
Policies and Procedures for Related Person Transactions
Our board of directors has adopted written policies and procedures for the review of any transaction, arrangement or relationship in which Argos is a participant, the amount involved exceeds $120,000, and one of our executive officers, directors, director nominees or 5% stockholders, or their immediate family members, each of whom we refer to as a “related person,” has a direct or indirect material interest.
If a related person proposes to enter into such a transaction, arrangement or relationship, which we refer to as a “related person transaction,” the related person must report the proposed related person transaction to our chief executive officer. The policy calls for the proposed related person transaction to be reviewed and, if deemed appropriate, approved by our audit committee. Whenever practicable, the reporting, review and approval will occur prior to entry into the transaction. If advance review and approval is not practicable, the committee will review, and, in its discretion, may ratify the related person transaction. The policy also permits the chairman of the committee to review and, if deemed appropriate, approve proposed related person transactions that arise between committee meetings, subject to ratification by the committee at its next meeting. Any related person transactions that are ongoing in nature will be reviewed annually.
A related person transaction reviewed under the policy will be considered approved or ratified if it is authorized by the committee after full disclosure of the related person’s interest in the transaction. As appropriate for the circumstances, the committee will review and consider:
| • | the related person’s interest in the related person transaction; |
| • | the approximate dollar value of the amount involved in the related person transaction; |
| • | the approximate dollar value of the amount of the related person’s interest in the transaction without regard to the amount of any profit or loss; |
| • | whether the transaction was undertaken in the ordinary course of our business; |
| • | whether the terms of the transaction are no less favorable to us than terms that could have been reached with an unrelated third party; |
| • | the purpose of, and the potential benefits to us of, the transaction; and |
| • | any other information regarding the related person transaction or the related person in the context of the proposed transaction that would be material to investors in light of the circumstances of the particular transaction. |
The committee may approve or ratify the transaction only if the committee determines that, under all of the circumstances, the transaction is in, or is not inconsistent with, Argos’ best interests. The committee may impose any conditions on the related person transaction that it deems appropriate.
In addition to the transactions that are excluded by the instructions to the SEC’s related person transaction disclosure rule, our board of directors has determined that the following transactions do not create a material direct or indirect interest on behalf of related persons and, therefore, are not related person transactions for purposes of this policy:
| • | interests arising solely from the related person’s position as an executive officer of another entity (whether or not the person is also a director of such entity), that is a participant in the transaction, where (a) the related person and all other related persons own in the aggregate less than a 10% equity interest in such entity, (b) the related person and his or her immediate family members are not involved in the negotiation of the terms of the transaction and do not receive any special benefits as a result of the transaction, and (c) the amount involved in the transaction equals less than the greater of $200,000 dollars or 5% of the annual gross revenues of the other entity that is a party to the transaction; and |
| • | a transaction that is specifically contemplated by provisions of our charter or bylaws. |
115
Table of Contents
The policy provides that transactions involving compensation of executive officers shall be reviewed and approved by the compensation committee in the manner specified in its charter.
Director Independence
Our board of directors has determined that all of the members of the audit committee, the compensation committee and the nominating and corporate governance committee are independent, as defined under the NASDAQ rules, including, in the case of all the members of our audit committee, the independence requirements contemplated by Rule 10A-3 under the Exchange Act. In making such determination, our board of directors considered the relationships that each such director has with our company and all other facts and circumstances that our board of directors deemed relevant in determining independence, including the beneficial ownership of our capital stock by each non-employee director.
Item 14. Principal Accountant Fees and Services
PricewaterhouseCoopers LLP has been approved by our Audit Committee to act as our independent registered public accounting firm for the year ending December 31, 2014.
Audit and other fees billed to us for the years ended December 31, 2013 and 2012 are as follows:
| 2013 | 2012 | |||||||
| Audit Fees (1) |
$ | 259,000 | $ | 136,000 | ||||
| Audit-Related Fees (2) |
295,000 | — | ||||||
| Tax Fees (3) |
3,226 | 5,912 | ||||||
| All Other Fees (4) |
— | — | ||||||
|
|
|
|
|
|||||
| Total Fees for Services Provided |
$ | 557,226 | $ | 141,912 | ||||
|
|
|
|
|
|||||
| (1) | Audit fees include fees associated with the annual audit, reviews of interim financial statements included in SEC registration statements, accounting and reporting consultations and audits conducted under OMB Circular A-133. |
| (2) | Audit-related fees include fees for completion of services in connection with our IPO. |
| (3) | Tax fees were for tax compliance and tax assistance services rendered on behalf of DC Bio. |
| (4) | Other fees include fees billed for other services rendered not included within Audit Fees, Audit Related Fees or Tax Fees. |
PricewaterhouseCoopers LLP did not perform any professional services related to financial information systems design and implementation for us in the year ended December 31, 2013 or 2012.
The Audit Committee has determined in its business judgment that the provision of non-audit services described above is compatible with maintaining PricewaterhouseCoopers LLP’s independence.
In 2014, the Audit Committee adopted a formal policy concerning approval of audit and non-audit services to be provided to the Company by its independent registered public accounting firm, PricewaterhouseCoopers LLP. The policy requires that all services to be provided by PricewaterhouseCoopers LLP, including audit services and permitted audit-related and non-audit services, must be preapproved by the Audit Committee, provided that de minimis non-audit services may instead be approved in accordance with applicable SEC rules. The Board of Directors preapproved all audit and non-audit services provided by PricewaterhouseCoopers LLP during fiscal year 2013 and fiscal year 2012.
116
Table of Contents
Item 15. EXHIBITS AND FINANCIAL STATEMENT SCHEDULES
(a) The following documents are included in this Annual Report on Form 10-K:
1. The following Report and Consolidated Financial Statements of the Company are included in this Annual Report:
Report of Independent Registered Public Accounting Firm
Consolidated Balance Sheets
Consolidated Statements of Operations
Consolidated Statements of Comprehensive Loss
Consolidated Statements of Changes in Stockholders’ Deficit
Consolidated Statements of Cash Flows
Notes to Consolidated Financial Statements
Financial Statement Schedule:
Schedule II – Valuation and Qualifying Accounts
2. All other schedules are omitted as they are inapplicable or the required information is furnished in the Consolidated Financial Statements or notes thereto.
3. The exhibits filed as part of this Annual Report on Form 10-K are listed in the Exhibit Index immediately following the signature page of this Annual Report on Form 10-K and are incorporated herein.
117
Table of Contents
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| ARGOS THERAPEUTICS, INC. | ||
| By: | /s/ Jeffrey D. Abbey | |
| Name: Jeffrey D. Abbey | ||
| Title: President and Chief Executive Officer | ||
Date: March 31, 2014
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant on March 31, 2014 in the capacities indicated.
| Signature |
Title |
Date | ||
| /s/ Jeffrey D. Abbey Jeffrey D. Abbey |
President, Chief Executive Officer and Director (Principal Executive Officer) |
March 31, 2014 | ||
| /s/ Lori R. Harrelson Lori R. Harrelson |
Vice President of Finance (Principal Financial Officer and Principal Accounting Officer) |
March 31, 2014 | ||
| /s/ Hubert Birner, Ph.D. Hubert Birner, Ph.D. |
Director |
March 31, 2014 | ||
| /s/ David W. Gryska David W. Gryska |
Director |
March 31, 2014 | ||
| /s/ Jean Lamarre Jean Lamarre |
Director |
March 31, 2014 | ||
| /s/ Andrei Petrov Andrei Petrov |
Director |
March 31, 2014 | ||
| /s/ Brian J. Underdown, Ph.D. Brian J. Underdown, Ph.D. |
Director |
March 31, 2014 | ||
| /s/ Sander van Deventer, M.D., Ph.D. Sander van Deventer, M.D., Ph.D. |
Director |
March 31, 2014 | ||
| /s/ Alexey Vinogradov, Ph.D. Alexey Vinogradov, Ph.D. |
Director |
March 31, 2014 | ||
118
Table of Contents
EXHIBIT INDEX
| Exhibit |
Description of Exhibit | |
| 3.1 | Restated Certificate of Incorporation of the Registrant (filed as Exhibit 3.1 to the Registrant’s Current Report on Form 8-K (File No. 001-35443) on February 18, 2014 and incorporated herein by reference) | |
| 3.4 | Amended and Restated Bylaws of the Registrant (filed as Exhibit 3.2 to the Registrant’s Current Report on Form 8-K (File No. 001-35443) on February 18, 2014 and incorporated herein by reference) | |
| 4.1 | Specimen Stock Certificate evidencing the shares of common stock (filed as Exhibit 4.1 to Amendment No. 1 to the Registrant’s Registration Statement on Form S-1 (File No. 333-193137) on January 21, 2014 and incorporated herein by reference) | |
| 4.2 | Fifth Amended and Restated Registration Rights Agreement, dated as of August 9, 2013 (filed as Exhibit 4.2 to the Registrant’s Registration Statement on Form S-1 (File No. 333-193137) on December 30, 2013 and incorporated herein by reference) | |
| 10.1+ | 1999 Stock Option Plan (filed as Exhibit 10.1 to the Registrant’s Registration Statement on Form S-1 (File No. 333-193137) on December 30, 2013 and incorporated herein by reference) | |
| 10.2+ | 2008 Stock Incentive Plan, as amended (filed as Exhibit 10.2 to the Registrant’s Registration Statement on Form S-1 (File No. 333-193137) on December 30, 2013 and incorporated herein by reference) | |
| 10.3+ | Form of Incentive Stock Option Agreement under 2008 Stock Incentive Plan (filed as Exhibit 10.3 to the Registrant’s Registration Statement on Form S-1 (File No. 333-193137) on December 30, 2013 and incorporated herein by reference) | |
| 10.4+ | Form of Nonstatutory Stock Option Agreement under 2008 Stock Incentive Plan (filed as Exhibit 10.4 to the Registrant’s Registration Statement on Form S-1 (File No. 333-193137) on December 30, 2013 and incorporated herein by reference) | |
| 10.5+ | 2014 Stock Incentive Plan (filed as Exhibit 10.5 to Amendment No. 1 to the Registrant’s Registration Statement on Form S-1 (File No. 333-193137) on January 21, 2014 and incorporated herein by reference) | |
| 10.6+ | Form of Incentive Stock Option Agreement under 2014 Stock Incentive Plan (filed as Exhibit 10.6 to Amendment No. 1 to the Registrant’s Registration Statement on Form S-1 (File No. 333-193137) on January 21, 2014 and incorporated herein by reference) | |
| 10.7+ | Form of Nonstatutory Stock Option Agreement under 2014 Stock Incentive Plan (filed as Exhibit 10.7 to Amendment No. 1 to the Registrant’s Registration Statement on Form S-1 (File No. 333-193137) on January 21, 2014 and incorporated herein by reference) | |
| 10.8 | Lease Agreement, dated as of January 16, 2001, between the Registrant and HCP MOP, as amended (filed as Exhibit 10.8 to the Registrant’s Registration Statement on Form S-1 (File No. 333-193137) on December 30, 2013 and incorporated herein by reference) | |
| 10.9+ | Employment Agreement between the Registrant and Jeffrey D. Abbey, dated December 9, 2013 (filed as Exhibit 10.9 to the Registrant’s Registration Statement on Form S-1 (File No. 333-193137) on December 30, 2013 and incorporated herein by reference) | |
| 10.10+ | Employment Agreement between the Registrant and Charles A. Nicolette, dated December 9, 2013 (filed as Exhibit 10.10 to the Registrant’s Registration Statement on Form S-1 (File No. 333-193137) on December 30, 2013 and incorporated herein by reference) | |
| 10.11+ | Employment Agreement between the Registrant and Frederick M. Miesowicz, dated December 9, 2013 (filed as Exhibit 10.11 to the Registrant’s Registration Statement on Form S-1 (File No. 333-193137) on December 30, 2013 and incorporated herein by reference) | |
| 10.12+ | Employment Agreement between the Registrant and Lori R. Harrelson, dated December 9, 2013 (filed as Exhibit 10.12 to the Registrant’s Registration Statement on Form S-1 (File No. 333-193137) on December 30, 2013 and incorporated herein by reference) | |
| 10.13+ | Employment Agreement between the Registrant and Douglas C. Plessinger, dated December 9, 2013 (filed as Exhibit 10.13 to the Registrant’s Registration Statement on Form S-1 (File No. 333-193137) on December 30, 2013 and incorporated herein by reference) | |
119
Table of Contents
| 10.14 | Form of Indemnification Agreement between the Registrant and each director and executive officer (filed as Exhibit 10.14 to the Registrant’s Registration Statement on Form S-1 (File No. 333-193137) on December 30, 2013 and incorporated herein by reference) | |
| 10.15† | Contract No. HHSN266200600019C, dated September 30, 2006, by and among the Registrant, the National Institutes of Health and the National Institutes of Allergy and Infectious Diseases, as amended (filed as Exhibit 10.15 to the Registrant’s Registration Statement on Form S-1 (File No. 333-193137) on December 30, 2013 and incorporated herein by reference) | |
| 10.16† | License Agreement, dated August 9, 2013, by and between the Registrant and Pharmstandard S.A. (filed as Exhibit 10.16 to the Registrant’s Registration Statement on Form S-1 (File No. 333-193137) on December 30, 2013 and incorporated herein by reference) | |
| 10.17† | License Agreement, dated July 31, 2013, by and between the Registrant and Green Cross Corp. (filed as Exhibit 10.17 to Amendment No. 1 to the Registrant’s Registration Statement on Form S-1 (File No. 333-193137) on January 21, 2014 and incorporated herein by reference) | |
| 10.18† | License Agreement, dated July 28, 2011, by and between the Registrant and Celldex Therapeutics, Inc. (filed as Exhibit 10.18 to the Registrant’s Registration Statement on Form S-1 (File No. 333-193137) on December 30, 2013 and incorporated herein by reference) | |
| 10.19† | License Agreement, dated January 10, 2000, by and between the Registrant and Duke University, as amended (filed as Exhibit 10.19 to the Registrant’s Registration Statement on Form S-1 (File No. 333-193137) on December 30, 2013 and incorporated herein by reference) | |
| 10.20 | Acknowledgement Agreement, dated November 4, 2013, by and between the Registrant and Pharmstandard International S.A. (filed as Exhibit 10.20 to Amendment No. 1 to the Registrant’s Registration Statement on Form S-1 (File No. 333-193137) on January 21, 2014 and incorporated herein by reference) | |
| 10.21+ | 2014 Employee Stock Purchase Plan (filed as Exhibit 10.21 to Amendment No. 1 to the Registrant’s Registration Statement on Form S-1 (File No. 333-193137) on January 21, 2014 and incorporated herein by reference) | |
| 10.22† | License Agreement, dated December 27, 2013, by and between the Registrant and Medinet Co., Ltd. (filed as Exhibit 10.22 to the Registrant’s Registration Statement on Form S-1 (File No. 333-193137) on December 30, 2013 and incorporated herein by reference) | |
| 10.23† | Rider to License Agreement, dated March 4, 2014, amending the License Agreement, dated December 27, 2013, by and between the Registrant and Medinet Co., Ltd. | |
| 21.1 | Subsidiaries of the Registrant | |
| 31.1 | Certification of principal executive officer pursuant to Rules 13a-14(a) or 15d-14(a) of the Securities Exchange Act of 1934, as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 | |
| 31.2 | Certification of principal financial officer pursuant to Rules 13a-14(a) or 15d-14(a) of the Securities Exchange Act of 1934, as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 | |
| 32.1 | Certifications pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of The Sarbanes-Oxley Act of 2002, by the Registrant’s principal executive officer and principal financial officer | |
| † | Confidential treatment requested as to portions of the exhibit. Confidential materials omitted and filed separately with the Securities and Exchange Commission. |
| + | Management contract or compensatory plan or arrangement required to be filed as exhibits hereto pursuant to Item 15(a) of Form 10-K. |
120
Table of Contents
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
| Page | ||||
| F-2 | ||||
| Consolidated Financial Statements: |
||||
| F-3 | ||||
| F-5 | ||||
| F-6 | ||||
| F-7 | ||||
| F-9 | ||||
| F-11 | ||||
| Financial Statement Schedule: |
||||
| F-37 | ||||
F-1
Table of Contents
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders of
Argos Therapeutics, Inc.
In our opinion, the accompanying consolidated balance sheets and the related consolidated statements of operations, comprehensive loss, changes in stockholders’ deficit and cash flows present fairly, in all material respects, the financial position of Argos Therapeutics, Inc. and its subsidiaries (the “Company”) (a development stage company) at December 31, 2013 and 2012 and the results of their operations and their cash flows for each of the three years in the period ended December 31, 2013 and the cumulative period from May 8, 1997 (inception) to December 31, 2013, in conformity with accounting principles generally accepted in the United States of America. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits. We conducted our audits of these statements in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
/s/ PricewaterhouseCoopers LLP
Raleigh, North Carolina
March 31, 2014
F-2
Table of Contents
ARGOS THERAPEUTICS, INC.
(A DEVELOPMENT STAGE COMPANY)
| December 31, | ||||||||
| 2012 | 2013 | |||||||
| Assets |
||||||||
| Current assets |
||||||||
| Cash and cash equivalents |
$ | 8,214,865 | $ | 33,297,970 | ||||
| Short-term investments |
4,148,871 | 13,659,812 | ||||||
| Prepaid expenses and interest receivable |
513,816 | 629,935 | ||||||
| Deferred financing costs |
— | 1,516,424 | ||||||
| Other receivables |
979,487 | 424,501 | ||||||
|
|
|
|
|
|||||
| Total current assets |
13,857,039 | 49,528,642 | ||||||
| Property and equipment, net |
1,539,384 | 1,602,103 | ||||||
| Other assets |
550 | 550 | ||||||
|
|
|
|
|
|||||
| Total assets |
$ | 15,396,973 | $ | 51,131,295 | ||||
|
|
|
|
|
|||||
| Liabilities, Redeemable Convertible Preferred Stock and Stockholders’ Deficit |
||||||||
| Current liabilities |
||||||||
| Accounts payable |
$ | 1,135,107 | $ | 1,317,072 | ||||
| Accrued expenses |
555,854 | 1,800,794 | ||||||
| Current portion of notes payable |
31,760 | 45,447 | ||||||
| Warrant liability |
6,392,652 | — | ||||||
|
|
|
|
|
|||||
| Total current liabilities |
8,115,373 | 3,163,313 | ||||||
| Long-term portion of notes payable |
48,428 | 7,014,106 | ||||||
| Deferred liability |
— | 3,066,000 | ||||||
| Commitments |
||||||||
| Series A redeemable convertible preferred stock $0.001 par value; 1,200,000 shares authorized as of December 31, 2012 and 2013; 1,152,053 and 1,040,216 shares issued and outstanding as of December 31, 2012, and 2013; liquidation preference of $1,152,053 and $332,869 as of December 31, 2012 and 2013 |
1,152,053 | 332,869 | ||||||
| Series B redeemable convertible preferred stock $0.001 par value; 22,200,000 and 18,500,000 shares authorized as of December 31, 2012 and 2013; 18,491,318 and 9,803,688 shares issued and outstanding as of December 31, 2012, and 2013; liquidation preference of $32,544,713 and $5,521,437 as of December 31, 2012 and 2013 |
32,544,713 | 5,521,437 | ||||||
| Series C redeemable convertible preferred stock, $0.001 par value; 105,250,000 and 40,000,000 shares authorized as of December 31, 2012 and 2013; 39,302,853 and 28,716,679 shares issued and outstanding as of December 31, 2012 and 2013; liquidation preference of $11,359,223 and $2,655,884 as of December 31, 2012 and 2013 |
11,285,071 | 2,655,884 | ||||||
| Series D redeemable convertible preferred stock $0.001 par value; 8,200,000 and 31,000,000 shares authorized as of December 31, 2012 and 2013; 8,137,739 and 21,040,817 shares issued and outstanding as of December 31, 2012, and 2013; liquidation preference of $28,711,839 and $41,630,624 as of December 31, 2012 and 2013 |
15,200,976 | 33,262,492 | ||||||
F-3
Table of Contents
| December 31, | ||||||||
| 2012 | 2013 | |||||||
| Series D-1 redeemable convertible preferred stock $0.001 par value; 8,800,000 and 0 shares authorized as of December 31, 2012 and 2013; 7,433,870 and 0 shares issued and outstanding as of December 31, 2012, and 2013; liquidation preference of $31,956,163 and $0 as of December 31, 2012 and 2013 |
15,618,069 | — | ||||||
| Series E redeemable convertible preferred stock, $0.001 par value; 0 and 70,000,000 shares authorized as of December 31, 2012 and 2013; 0 and 56,011,258 shares issued and outstanding as of December 31, 2012, and 2013; liquidation preference of $72,945,310 as of December 31, 2013 |
— | 71,891,787 | ||||||
| Stockholders’ deficit |
||||||||
| Common stock $0.001 par value; 100,000,000 and 120,000,000 shares authorized as of December 31, 2012 and 2013; 226,839 and 235,707 shares issued and outstanding as of December 31, 2012 and 2013 |
227 | 236 | ||||||
| Accumulated other comprehensive loss |
(94,267 | ) | (102,531 | ) | ||||
| Additional paid-in capital |
58,469,015 | 75,189,950 | ||||||
| Deficit accumulated during the development stage attributable to Argos Therapeutics, Inc.’s stockholders |
(126,942,685 | ) | (150,864,248 | ) | ||||
|
|
|
|
|
|||||
| Total stockholders’ deficit |
(68,567,710 | ) | (75,776,593 | ) | ||||
|
|
|
|
|
|||||
| Total liabilities, redeemable convertible preferred stock and stockholders’ deficit |
$ | 15,396,973 | $ | 51,131,295 | ||||
|
|
|
|
|
|||||
The accompanying notes are an integral part of these consolidated financial statements.
F-4
Table of Contents
ARGOS THERAPEUTICS, INC.
(A DEVELOPMENT STAGE COMPANY)
CONSOLIDATED STATEMENTS OF OPERATIONS
| Years Ended December 31, | Cumulative Period from May 8, 1997 (Inception) to December 31, |
|||||||||||||||
| 2011 | 2012 | 2013 | 2013 | |||||||||||||
| Revenue |
$ | 7,642,695 | $ | 7,039,010 | $ | 4,421,689 | $ | 86,826,528 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Operating expenses |
||||||||||||||||
| Research and development |
12,668,025 | 17,616,892 | 23,991,151 | 205,626,225 | ||||||||||||
| Net reimbursement under collaboration agreement |
— | — | — | (47,179,130 | ) | |||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net research and development |
12,668,025 | 17,616,892 | 23,991,151 | 158,447,095 | ||||||||||||
| General and administrative |
3,703,813 | 6,135,581 | 4,662,317 | 58,838,234 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total operating expenses |
16,371,838 | 23,752,473 | 28,653,468 | 217,285,329 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Operating loss |
(8,729,143 | ) | (16,713,463 | ) | (24,231,779 | ) | (130,458,801 | ) | ||||||||
| Other income (expense) |
||||||||||||||||
| Interest income |
1,259 | 4,604 | 7,184 | 4,105,138 | ||||||||||||
| Interest expense |
(6,656,366 | ) | (292,496 | ) | (4,705 | ) | (12,515,919 | ) | ||||||||
| Change in fair value of warrant liability |
(4,661,811 | ) | 4,916,785 | 355,352 | 610,326 | |||||||||||
| Derivative (expense) income |
(94,668 | ) | 1,036,403 | — | (39,450 | ) | ||||||||||
| Investment tax credits |
— | 694,331 | — | 2,761,556 | ||||||||||||
| Other expense |
— | (117,494 | ) | (47,615 | ) | (165,109 | ) | |||||||||
| Loss on sale of marketable securities |
— | — | — | (10,242,252 | ) | |||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Other income (expense), net |
(11,411,586 | ) | 6,242,133 | 310,216 | (15,485,710 | ) | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net loss |
(20,140,729 | ) | (10,471,330 | ) | (23,921,563 | ) | (145,944,511 | ) | ||||||||
| Net loss attributable to noncontrolling interest |
(63,047 | ) | — | — | (760,107 | ) | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net loss attributable to Argos Therapeutics, Inc. |
(20,077,682 | ) | (10,471,330 | ) | (23,921,563 | ) | (145,184,404 | ) | ||||||||
| Accretion of redeemable convertible preferred stock (See Note 19) |
(926,542 | ) | (351,371 | ) | 4,772,991 | (25,626,700 | ) | |||||||||
| Less: Preferred stock dividend due to exchanges of preferred shares (See Note 19) |
— | — | (14,726,088 | ) | (14,726,088 | ) | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net loss attributable to common stockholders |
$ | (21,004,224 | ) | $ | (10,822,701 | ) | $ | (33,874,660 | ) | $ | (185,537,192 | ) | ||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net loss attributable to common stockholders per share, basic and diluted |
$ | (197.29 | ) | $ | (54.58 | ) | $ | (147.37 | ) | |||||||
|
|
|
|
|
|
|
|||||||||||
| Weighted average shares outstanding, basic and diluted |
106,466 | 198,306 | 229,865 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-5
Table of Contents
ARGOS THERAPEUTICS, INC.
(A DEVELOPMENT STAGE COMPANY)
CONSOLIDATED STATEMENTS OF COMPREHENSIVE LOSS
| Years Ended December 31, | Cumulative Period from May 8, 1997 (Inception) to December 31, |
|||||||||||||||
| 2011 | 2012 | 2013 | 2013 | |||||||||||||
| Net loss |
$ | (20,140,729 | ) | $ | (10,471,330 | ) | $ | (23,921,563 | ) | $ | (145,944,511 | ) | ||||
|
|
|
|
|
|
|
|
|
|||||||||
| Other comprehensive loss |
||||||||||||||||
| Foreign currency translation adjustment |
(3,712 | ) | (180,049 | ) | (8,264 | ) | (102,531 | ) | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total comprehensive loss |
$ | (20,144,441 | ) | $ | (10,651,379 | ) | $ | (23,929,827 | ) | $ | (146,047,042 | ) | ||||
|
|
|
|
|
|
|
|
|
|||||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-6
Table of Contents
ARGOS THERAPEUTICS, INC.
(A DEVELOPMENT STAGE COMPANY)
CONSOLIDATED STATEMENTS OF CHANGES IN STOCKHOLDERS’ DEFICIT
| Common Stock | Additional Paid-In |
Stock Subscription |
Deferred |
Accumulated Comprehensive |
Deficit Development |
Noncontrolling |
||||||||||||||||||||||||||||||
| Shares | Amount | Capital | Receivable | Compensation | Income | Stage | Interest | Total | ||||||||||||||||||||||||||||
| Balances as of Inception, May 8, 1997 |
— | $ | — | $ | — | $ | — | $ | — | $ | — | $ | — | $ | — | $ | — | |||||||||||||||||||
| Exercise of common stock warrants |
23 | — | 31,789 | — | — | — | — | — | 31,789 | |||||||||||||||||||||||||||
| Exercise of common stock options |
712 | 1 | 31,587 | — | — | — | — | — | 31,588 | |||||||||||||||||||||||||||
| Issuance of common stock to founders |
147 | — | 9,567 | (2,000 | ) | — | — | — | — | 7,567 | ||||||||||||||||||||||||||
| Issuance of shares for technology license |
39 | — | 56,300 | — | — | — | — | — | 56,300 | |||||||||||||||||||||||||||
| Issuance of warrants and options to non-employees |
— | — | 2,494,925 | — | — | — | — | — | 2,494,925 | |||||||||||||||||||||||||||
| Issuance of preferred stock warrants |
— | — | 7,499,776 | — | — | — | — | — | 7,499,776 | |||||||||||||||||||||||||||
| Issuance of restricted stock |
1,115 | 1 | 338,174 | — | (329,175 | ) | — | — | — | 9,000 | ||||||||||||||||||||||||||
| Vesting of restricted stock |
— | — | 13,830 | — | — | — | — | — | 13,830 | |||||||||||||||||||||||||||
| Issuance of shares to noncontrolling interest |
— | — | — | — | — | — | — | 3,702,790 | 3,702,790 | |||||||||||||||||||||||||||
| Repurchase of common stock |
(101 | ) | — | (97,518 | ) | — | — | — | — | — | (97,518 | ) | ||||||||||||||||||||||||
| Forfeiture of unvested restricted stock |
(33 | ) | — | — | — | — | — | — | — | — | ||||||||||||||||||||||||||
| Preferred shares converted to common |
92,734 | 93 | 25,783,711 | — | — | — | — | — | 25,783,804 | |||||||||||||||||||||||||||
| Amortization of deferred compensation |
— | — | — | — | 329,175 | — | — | — | 329,175 | |||||||||||||||||||||||||||
| Stock-based compensation |
— | — | 1,624,866 | — | — | — | (8,097 | ) | — | 1,616,769 | ||||||||||||||||||||||||||
| Accretion of preferred stock |
— | — | (11,092,397 | ) | — | — | — | (18,029,381 | ) | 18,249 | (29,103,529 | ) | ||||||||||||||||||||||||
| Payment of stock subscription |
— | — | — | 2,000 | — | — | — | — | 2,000 | |||||||||||||||||||||||||||
| Cumulative translation adjustment |
— | — | — | — | — | 89,494 | — | — | 89,494 | |||||||||||||||||||||||||||
| Net loss from Inception to December 31, 2010 |
— | — | — | — | — | — | (90,713,829 | ) | (690,715 | ) | (91,404,544 | ) | ||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||
| Balances as of December 31, 2010 |
94,636 | $ | 95 | $ | 26,694,610 | $ | — | $ | — | $ | 89,494 | $ | (108,751,307 | ) | $ | 3,030,324 | $ | (78,936,784 | ) | |||||||||||||||||
F-7
Table of Contents
ARGOS THERAPEUTICS, INC.
(A DEVELOPMENT STAGE COMPANY)
CONSOLIDATED STATEMENTS OF CHANGES IN STOCKHOLDERS’ DEFICIT
| Common Stock | Additional Paid-In |
Stock Subscription |
Deferred |
Accumulated Comprehensive |
Deficit Development |
Noncontrolling |
||||||||||||||||||||||||||||||
| Shares | Amount | Capital | Receivable | Compensation | Income | Stage | Interest | Total | ||||||||||||||||||||||||||||
| Balances as of December 31, 2010 |
94,636 | $ | 95 | $ | 26,694,610 | $ | — | $ | — | $ | 89,494 | $ | (108,751,307 | ) | $ | 3,030,324 | $ | (78,936,784 | ) | |||||||||||||||||
| Issuance of restricted stock |
255 | — | 9,000 | — | — | — | — | — | 9,000 | |||||||||||||||||||||||||||
| Issuance of options to non-employees |
— | — | 45,640 | — | — | — | — | — | 45,640 | |||||||||||||||||||||||||||
| Stock-based compensation |
— | — | 426,492 | — | — | — | — | — | 426,492 | |||||||||||||||||||||||||||
| Preferred shares converted to common |
27,454 | 27 | 3,164,621 | — | — | — | — | — | 3,164,648 | |||||||||||||||||||||||||||
| Accretion of preferred stock |
— | — | (926,542 | ) | — | — | — | — | 6,881 | (919,661 | ) | |||||||||||||||||||||||||
| Cumulative translation adjustment |
— | — | — | — | — | (3,712 | ) | — | — | (3,712 | ) | |||||||||||||||||||||||||
| Net loss |
— | — | — | — | — | — | (20,077,682 | ) | (63,047 | ) | (20,140,729 | ) | ||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||
| Balances as of December 31, 2011 |
122,345 | $ | 122 | $ | 29,413,821 | $ | — | $ | — | $ | 85,782 | $ | (128,828,989 | ) | $ | 2,974,158 | $ | (96,355,106 | ) | |||||||||||||||||
| Issuance of restricted stock |
— | — | 14,550 | — | — | — | — | — | 14,550 | |||||||||||||||||||||||||||
| Issuance of options to nonemployees |
— | — | 561 | — | — | — | — | — | 561 | |||||||||||||||||||||||||||
| Stock-based compensation |
— | — | 1,042,989 | — | — | — | — | — | 1,042,989 | |||||||||||||||||||||||||||
| Preferred shares converted to common |
104,494 | 105 | 8,067,829 | — | 8,067,934 | |||||||||||||||||||||||||||||||
| Accretion of preferred stock |
— | — | (351,371 | ) | — | — | — | — | — | (351,371 | ) | |||||||||||||||||||||||||
| Capital contribution |
— | — | 17,306,478 | — | — | — | — | — | 17,306,478 | |||||||||||||||||||||||||||
| Extinguishment of preferred shares |
— | — | — | — | — | — | 12,357,634 | — | 12,357,634 | |||||||||||||||||||||||||||
| Purchase of noncontrolling interest’s shares |
— | — | 2,974,158 | — | — | — | — | (2,974,158 | ) | — | ||||||||||||||||||||||||||
| Cumulative translation adjustment |
— | — | — | — | — | (180,049 | ) | — | — | (180,049 | ) | |||||||||||||||||||||||||
| Net loss |
— | — | — | — | — | — | (10,471,330 | ) | — | (10,471,330 | ) | |||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||
| Balances as of December 31, 2012 |
226,839 | $ | 227 | $ | 58,469,015 | $ | — | $ | — | $ | (94,267 | ) | $ | (126,942,685 | ) | $ | — | $ | (68,567,710 | ) | ||||||||||||||||
| Exercise of common stock options |
1,379 | 2 | 5,791 | — | — | — | — | — | 5,793 | |||||||||||||||||||||||||||
| Issuance of restricted stock |
7,571 | 7 | 16,493 | — | — | — | — | — | 16,500 | |||||||||||||||||||||||||||
| Issuance of common warrants |
— | — | 618,155 | — | — | — | — | — | 618,155 | |||||||||||||||||||||||||||
| Distribution of shares to affiliates |
(7 | ) | — | — | — | — | — | — | — | — | ||||||||||||||||||||||||||
| Surrender of shares |
(75 | ) | — | — | — | — | — | — | — | — | ||||||||||||||||||||||||||
| Stock-based compensation |
— | — | 1,053,163 | — | — | — | — | — | 1,053,163 | |||||||||||||||||||||||||||
| Reversal of prior accretion |
— | — | 5,657,638 | — | — | — | — | — | 5,657,638 | |||||||||||||||||||||||||||
| Accretion of preferred stock |
— | — | (884,647 | ) | — | — | — | — | — | (884,647 | ) | |||||||||||||||||||||||||
| Exchange of preferred shares |
— | — | (14,726,088 | ) | — | — | — | — | — | (14,726,088 | ) | |||||||||||||||||||||||||
| Reduction of liquidation value |
— | — | 24,980,430 | — | — | — | — | — | 24,980,430 | |||||||||||||||||||||||||||
| Cumulative translation adjustment |
— | — | — | — | — | (8,264 | ) | — | — | (8,264 | ) | |||||||||||||||||||||||||
| Net loss |
— | — | — | — | — | — | (23,921,563 | ) | — | (23,921,563 | ) | |||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||
| Balances as of December 31, 2013 |
235,707 | $ | 236 | $ | 75,189,950 | $ |
— |
|
$ | — | $ | (102,531 | ) | $ | (150,864,248 | ) | $ | — | $ | (75,776,593 | ) | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-8
Table of Contents
ARGOS THERAPEUTICS, INC.
(A DEVELOPMENT STAGE COMPANY)
CONSOLIDATED STATEMENTS OF CASH FLOWS
| Years Ended December 31, | Cumulative Period from May 8, 1997 (Inception) to December 31, |
|||||||||||||||
| 2011 | 2012 | 2013 | 2013 | |||||||||||||
| Cash flows from operating activities |
||||||||||||||||
| Net loss |
$ | (20,140,729 | ) | $ | (10,471,330 | ) | $ | (23,921,563 | ) | $ | (145,944,511 | ) | ||||
| Adjustments to reconcile net loss to net cash used in operating activities |
||||||||||||||||
| Depreciation and amortization |
679,843 | 505,261 | 611,421 | 9,475,516 | ||||||||||||
| Noncash licensing revenue |
— | — | — | (42,775,697 | ) | |||||||||||
| Compensation expense related to stock options and warrants |
472,132 | 1,043,550 | 1,081,186 | 7,706,346 | ||||||||||||
| Issuance of warrants recorded as legal expense |
— | — | — | 1,703,466 | ||||||||||||
| Amortization of debt issuance costs |
— | — | — | 1,368,761 | ||||||||||||
| Write-off of deferred financing costs |
— | 1,785,779 | — | 1,785,779 | ||||||||||||
| Derivative expense (income) |
94,668 | (1,036,403 | ) | — | — | |||||||||||
| Noncash interest expense |
6,656,263 | 292,064 | 2,465 | 7,818,284 | ||||||||||||
| Increase (decrease) in fair value of warrant liability |
4,661,811 | (4,916,785 | ) | (355,352 | ) | (610,326 | ) | |||||||||
| Issuance of common stock for technology license |
— | — | — | 56,300 | ||||||||||||
| Issuance of restricted stock recorded as consulting expense |
9,000 | 14,550 | 16,500 | 49,050 | ||||||||||||
| Loss on sale of marketable securities |
— | — | 10,242,252 | |||||||||||||
| Loss (gain) on disposal of equipment |
910 | (4,774 | ) | (50,835 | ) | 41,590 | ||||||||||
| Changes in operating assets and liabilities: |
||||||||||||||||
| Prepaid expenses and other receivables |
2,871,057 | (216,332 | ) | 438,867 | (1,018,927 | ) | ||||||||||
| Deferred financing costs |
— | — | (571,327 | ) | (571,327 | ) | ||||||||||
| Other assets |
6,667 | — | — | (822 | ) | |||||||||||
| Accounts payable |
(40,910 | ) | 60,977 | 181,965 | 1,874,042 | |||||||||||
| Accrued expenses |
143,580 | (254,813 | ) | 1,244,940 | 3,134,834 | |||||||||||
| Deferred liability |
— | — | 3,066,000 | 3,066,000 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net cash used in operating activities |
(4,585,708 | ) | (13,198,256 | ) | (18,255,733 | ) | (142,599,390 | ) | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Cash flows from investing activities |
||||||||||||||||
| Purchase of property and equipment |
(244,068 | ) | (1,137,533 | ) | (596,455 | ) | (9,824,842 | ) | ||||||||
| Proceeds from sale of fixed assets |
— | 5,555 | 50,835 | 358,909 | ||||||||||||
| Purchases of short-term investments |
— | (4,591,437 | ) | (13,600,941 | ) | (264,393,868 | ) | |||||||||
| Sales of short-term investments |
— | 442,566 | 4,090,000 | 218,092,530 | ||||||||||||
| Proceeds from maturity of short-term investments |
— | — | — | 32,509,687 | ||||||||||||
| Proceeds from sale of marketable securities |
— | — | — | 32,533,445 | ||||||||||||
| Investment in affiliate |
— | — | — | (6,500 | ) | |||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net cash (used in) provided by investing activities |
(244,068 | ) | (5,280,849 | ) | (10,056,561 | ) | 9,269,361 | |||||||||
F-9
Table of Contents
ARGOS THERAPEUTICS, INC.
(A DEVELOPMENT STAGE COMPANY)
CONSOLIDATED STATEMENTS OF CASH FLOWS
| Years Ended December 31, | Cumulative Period from May 8, 1997 (Inception) to December 31, |
|||||||||||||||
| 2011 | 2012 | 2013 | 2013 | |||||||||||||
| Cash flows from financing activities |
||||||||||||||||
| Proceeds from sale of redeemable convertible preferred stock |
— | 25,000,000 | 47,409,867 | 146,081,283 | ||||||||||||
| Proceeds from issuance of common stock warrants |
— | — | 590,132 | 590,132 | ||||||||||||
| Stock issuance costs |
(1,785,779 | ) | (208,934 | ) | (1,501,344 | ) | (4,308,489 | ) | ||||||||
| Proceeds from convertible notes |
3,500,000 | — | — | 12,956,937 | ||||||||||||
| Proceeds from notes payables |
— | 95,756 | 6,934,000 | 17,803,288 | ||||||||||||
| Payments on notes payable |
(26,807 | ) | (15,568 | ) | (34,934 | ) | (10,292,916 | ) | ||||||||
| Payments on debenture |
— | — | — | (3,509,947 | ) | |||||||||||
| Proceeds from exercise of common stock options |
— | — | 5,793 | 78,737 | ||||||||||||
| Repurchase of common stock |
— | — | — | (97,518 | ) | |||||||||||
| Income to noncontrolling interest |
— | — | — | 22,413 | ||||||||||||
| Consolidation of DC Bio cash and cash equivalents |
— | — | — | 7,163,141 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net cash provided by financing activities |
1,687,414 | 24,871,254 | 53,403,514 | 166,487,061 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Effect of exchange rates changes on cash |
3,211 | (180,098 | ) | (8,115 | ) | 140,938 | ||||||||||
| Net (decrease) increase in cash and cash equivalents |
(3,139,151 | ) | 6,212,051 | 25,083,105 | 33,297,970 | |||||||||||
| Cash and cash equivalents |
||||||||||||||||
| Beginning of period |
5,141,965 | 2,002,814 | 8,214,865 | — | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| End of period |
$ | 2,002,814 | $ | 8,214,865 | $ | 33,297,970 | $ | 33,297,970 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Supplemental disclosure of cash flow information |
||||||||||||||||
| Cash paid for interest |
$ | 333 | $ | 433 | $ | 512 | $ | 2,263,974 | ||||||||
| Supplemental disclosure of noncash investing and financing activities |
||||||||||||||||
| Conversion of notes payable and accrued interest into preferred stock |
$ | — | $ | 10,668,185 | $ | — | $ | 17,598,000 | ||||||||
| Conversion of preferred stock into common stock |
$ | 3,164,484 | $ | 8,067,307 | $ | — | $ | 37,015,038 | ||||||||
| Preferred stock accretion |
$ | 926,542 | $ | 351,371 | $ | (4,772,991 | ) | $ | 25,626,700 | |||||||
| Marketable securities received under licensing agreement |
$ | — | $ | — | $ | — | $ | (42,775,697 | ) | |||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-10
Table of Contents
ARGOS THERAPEUTICS, INC.
(A DEVELOPMENT STAGE COMPANY)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
1. Organization
Argos Therapeutics, Inc. (the “Company”), was incorporated in the State of Delaware on May 8, 1997. The Company is a biopharmaceutical company focused on the development and commercialization of fully personalized immunotherapies for the treatment of cancer and infectious diseases based on its proprietary technology platform called Arcelis. The Company’s most advanced product candidate is AGS-003, which the Company is developing for the treatment of metastatic renal cell carcinoma, or mRCC, and other cancers. The Company is developing a second Arcelis-based product candidate, AGS-004, for the treatment of HIV.
The Company is in its development stage as defined by the Financial Accounting Standards Board (“FASB”) Accounting Standards Codification (“ASC”) 915, Development Stage Entities. The Company’s activities since inception have consisted principally of acquiring product and technology rights, raising capital and performing research and development activities. Since inception, the Company has incurred significant losses from operations and expects losses to continue for the foreseeable future during its development phase. The Company’s success depends primarily on the successful development and regulatory approval of its product candidates.
Initial Public Offering
On February 12, 2014, the Company completed its initial public offering in which it issued and sold 6,228,725 shares of common stock, including 603,725 shares of common stock sold pursuant to the underwriters’ exercise of their option to purchase additional shares of common stock, at a price to the public of $8.00 per share. The total net proceeds raised were $43.7 million after deducting underwriting discounts and commissions of $3.5 million and other offering expenses of approximately $2.6 million. Upon the closing of the initial public offering, all of the then-outstanding shares of the Company’s redeemable convertible preferred stock automatically converted into 13,188,251 shares of common stock.
Capitalization
In connection with the Company’s initial public offering, the Company effected a one–for-six reverse split of its common stock. All references to shares of common stock outstanding, average number of shares outstanding and per share amounts in these consolidated financial statements and notes to consolidated financial statements have been restated to reflect the reverse split on a retroactive basis.
2. Summary of Significant Accounting Policies
Use of Estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities as of the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates.
Principles of Consolidation
The accompanying consolidated financial statements include the accounts and results of operations of the Company and DC Bio, formerly Merix Canada Corp., an unlimited liability corporation incorporated in the Province of Nova Scotia. Prior to October 26, 2012, the Company consolidated DC Bio under ASC 810-10-50, Consolidation of Variable Interest Entities (“VIE”), as DC Bio met the requirements of a VIE. As of December 31, 2012, the Company owned 100% of DC Bio through the repurchase of the noncontrolling interests of DC Bio. All intercompany balances and transactions have been eliminated in consolidation. In prior periods, the Company also consolidated its wholly owned subsidiaries, Argos Therapeutics B.V. and Argos Therapeutics GmbH, formerly Merix Bioscience B.V. and Merix Germany GmbH, respectively. Argos Therapeutics B.V. and Argos Therapeutics GmbH were dissolved effective November 6, 2006 and October 22, 2008, respectively.
Net Loss per Share
Basic net loss per share is calculated by dividing net loss attributable to common stockholders by the weighted average shares outstanding during the period, without consideration of common stock equivalents. Diluted net loss per share is calculated by adjusting weighted average shares outstanding for the dilutive effect of common stock equivalents outstanding for the period, determined using the treasury-stock method. For purposes of the diluted net loss per share calculation, preferred stock, stock options and warrants are considered to be common stock equivalents but are excluded from the calculation of diluted net loss per share because their effect would be anti-dilutive and, therefore, basic and diluted net loss per share were the same for all periods presented.
F-11
Table of Contents
Cash and Cash Equivalents
The Company considers all highly liquid investments with an original maturity of three months or less as of the date of purchase to be cash equivalents. Cash deposits are all in financial institutions in the United States of America or Canada. The Company maintains cash in accounts which are in excess of federally insured limits. As of December 31, 2013 and December 31, 2012, $33,047,970 and $7,964,865, respectively, in cash and cash equivalents was uninsured.
Short-Term Investments
All investments with original maturities less than one year from the balance sheet date are considered short-term investments. All short-term investments are classified as available-for-sale and therefore carried at fair value. Fair value of short-term investments approximates amortized cost. The Company primarily invests in high-quality marketable debt securities issued by high quality financial and industrial companies.
Fair Value of Financial Instruments
The carrying values of cash and cash equivalents, prepaid expenses and interest receivable, other receivables, accounts payable and accrued expenses as of December 31, 2012 and 2013 approximated their fair values due to the short-term nature of these items.
As of December 31, 2012 and 2013, the Company held certain assets and liabilities that are required to be measured at fair value on a recurring basis. These assets and liabilities include money market funds included in cash equivalents, short-term investments and a warrant liability. The valuation of these financial instruments uses a three tiered approach, which requires that fair value measurements be classified and disclosed in one of three tiers. These tiers are: Level 1, defined as quoted prices in active markets for identical assets or liabilities; Level 2, defined as valuations based on observable inputs other than those included in Level 1, such as quoted prices for similar assets and liabilities in active markets, or other inputs that are observable or can be corroborated by observable input data; and Level 3, defined as valuations based on unobservable inputs reflecting the Company’s own assumptions, consistent with reasonably available assumptions made by other market participants.
The Company’s Level 1 assets consist of its short-term investments and the method used to estimate the fair value of the Level 1 assets is based on observable market data as these high quality marketable debt securities are publicly-traded.
The Company’s warrant liability is classified as Level 3. As of December 31, 2012, the Company based its valuation of the fair value of the warrants to purchase shares of its series C preferred stock and series D-1 preferred stock then outstanding on an allocation of the enterprise value of the Company to all classes of equity and preferred stock including the warrants. The Company utilized the PWERM to determine these values. The Company estimated the fair value of the warrants using a probability-weighted analysis of the present value of the returns afforded to holders of warrants under several scenarios, including an IPO, a strategic merger or sale of the Company, and a strategic sale of intellectual property and dissolution of the Company as follows:
| • | an IPO on or before December 31, 2015; |
| • | a strategic merger or sale of the Company at a high valuation on or before June 30, 2016, which assumed an additional fundraising of $100 million to conduct a phase 3 clinical trial of AGS-003 and positive results from the trial by mid 2015; |
| • | a strategic merger or sale of the Company at a lower valuation on or before December 31, 2014, which assumed an additional fundraising of $55 million to conduct a phase 3 clinical trial of AGS-003 and positive interim results from the trial; |
| • | a strategic merger or sale of the Company at a lower valuation on or before March 31, 2015, which assumed an additional fundraising of $17 million to conduct a phase 2b clinical trial of AGS-003 and positive results from the trial; |
| • | a strategic sale of the Company’s intellectual property on or before December 31, 2015, which assumed an additional funding of $90 million to conduct a phase 3 clinical trial of AGS-003 and poor results from the trial; and |
| • | a strategic sale of the Company’s intellectual property on or before December 31, 2014, which assumed an additional funding of $17 million to conduct a phase 2b clinical trial of AGS-003 and poor results from the trial. |
F-12
Table of Contents
In the valuations, the Company used probability weightings of 22.5% for the IPO scenario, 22.5% for a strategic merger or sale of the Company at a high valuation (positive results from the phase 3 clinical trial of AGS-003), 15% for a strategic merger or sale of the Company at a lower valuation (positive interim results from phase 3 clinical trial of AGS-003), 20% for a strategic merger or sale of the Company at a lower valuation (positive results from a phase 2b clinical trial in AGS-003), 10% for a strategic sale of the Company’s intellectual property (poor results from the phase 3 clinical trial of AGS-003) and 10% for a strategic sale of the Company’s intellectual property (poor results from a phase 2b clinical trial of AGS-003). These probability weightings assigned to the respective exit scenarios were based on consideration of the Company’s various drug development programs, industry clinical success rates, the Company’s expected near-term and long-term funding requirements and an assessment of the current financing and biotechnology industry environments as of the time of the valuation.
In the December 31, 2012 valuation, the Company applied a discount for lack of marketability of 25%. The Company used an option pricing model to determine the value of this lack of marketability.
Fair value measurements using PWERM can be sensitive to significant changes in one or more of the inputs. A significant increase or decrease in the present value of the returns afforded to holders of warrants in any of the six scenarios set forth above, as well as the weighted-average probability assigned to each, may result in a higher or lower fair value for the warrants. Additionally, a higher or lower discount rate used for lack of marketability may result in a higher or lower fair value for the warrants.
In July 2013, all outstanding warrants to purchase preferred stock were cancelled. As a result, there were no warrants to purchase preferred stock outstanding as of December 31, 2013.
As of December 31, 2012 and 2013, these financial instruments and respective fair values have been classified as follows:
| Quoted Prices in Active Markets for Identical Assets (Level 1) |
Significant Other Observable Inputs (Level 2) |
Significant Unobservable Inputs (Level 3) |
Balance as of December 31, 2012 |
|||||||||||||
| Assets |
||||||||||||||||
| Money Market Funds |
$ | 7,500,771 | $ | — | $ | — | $ | 7,500,771 | ||||||||
| Short-term investments |
— | 4,148,871 | — | 4,148,871 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total assets at fair value |
$ | 7,500,771 | $ | 4,148,871 | $ | — | $ | 11,649,642 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Liabilities |
||||||||||||||||
| Warrants |
$ | — | $ | — | $ | 6,392,652 | $ | 6,392,652 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total liabilities at fair value |
$ | — | $ | — | $ | 6,392,652 | $ | 6,392,652 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Quoted Prices in Active Markets for Identical Assets (Level 1) |
Significant Other Observable Inputs (Level 2) |
Significant Unobservable Inputs (Level 3) |
Balance as of December 31, 2013 |
|||||||||||||
| Assets |
||||||||||||||||
| Money Market Funds |
$ | 22,891,418 | $ | — | $ | — | $ | 22,891,418 | ||||||||
| Short-term investments |
— | 13,659,812 | — | 13,659,812 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total assets at fair value |
$ | 22,891,418 | $ | 13,659,812 | $ | — | $ | 36,551,230 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
F-13
Table of Contents
The changes in the balances of Level 3 liabilities for the year ended December 31, 2012 were as follows:
| Fair Value Measurements Using Significant Unobservable Inputs (Level 3) |
||||||||||||||||
| Beginning Balance |
Net Change in Unrealized (Gains) Losses |
Additions | Ending Balance |
|||||||||||||
| Derivative Liability |
$ | 1,036,403 | $ | (1,036,403 | ) | $ | — | $ | — | |||||||
| Warrant Liability |
11,309,437 | (4,916,785 | ) | — | 6,392,652 | |||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| $ | 12,345,840 | $ | (5,953,188 | ) | $ | — | $ | 6,392,652 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
For the year ended December 31, 2012, there were no transfers between Levels 1 and 2 assets or liabilities.
The changes in the balance of Level 3 liabilities for the year ended December 31, 2013 were as follows:
| Fair Value Measurements Using Significant Unobservable Inputs (Level 3) |
||||||||||||||||
| Beginning Balance |
Net Change in Unrealized (Gains) Losses |
Reduction | Ending Balance |
|||||||||||||
| Warrant Liability |
$ | 6,392,652 | $ | (355,352 | ) | $ | (6,037,300 | ) | $ | — | ||||||
|
|
|
|
|
|
|
|
|
|||||||||
| $ | 6,392,652 | $ | (355,352 | ) | $ | (6,037,300 | ) | $ | — | |||||||
|
|
|
|
|
|
|
|
|
|||||||||
During the year ended December 31, 2013, all outstanding warrants to purchase preferred stock were cancelled. As a result, there were no warrants to purchase preferred stock outstanding as of December 31, 2013.
For the year ended December 31, 2013, there were no transfers between Levels 1 and 2 assets or liabilities.
Credit Risk
Financial instruments which potentially subject the Company to concentrations of credit risk consist principally of cash and cash equivalents and short-term investments. Total cash and cash equivalent balances have exceeded insured balances by the Federal Depository Insurance Company (“FDIC”).
Property and Equipment
Property and equipment are recorded at cost and depreciated over their estimated useful lives using the straight-line method. Property and equipment held under capital leases and leasehold improvements are amortized over the shorter of the lease term or the estimated useful life of the related asset. Upon retirement or sale, the cost of assets disposed of and the related accumulated depreciation are removed from the accounts and any resulting gain or loss is credited or charged to income. Repairs and maintenance costs are expensed as incurred.
Impairment of Long-Lived Assets
The Company periodically assesses the impairment of long lived assets in accordance with ASC Topic 360, Property Plant and Equipment. When indicators of impairment are present, the Company evaluates the carrying value of these assets in relation to the operating performance of the business and future undiscounted cash flows expected to result from the use of these assets. No such impairments have been recognized from inception (May 8, 1997) through December 31, 2013.
Revenue Recognition
The Company recognizes revenue in accordance with the FASB Accounting Standards Codification 605, Revenue Recognition, or ASC 605. The Company recognizes revenue when the following criteria are met: persuasive evidence of an arrangement exists, services have been rendered, the price is fixed or determinable, and collectability is reasonably assured.
The Company has entered into license agreements with collaborators. The terms of these agreements have included nonrefundable signing and licensing fees, as well as milestone payments and royalties on any future product sales developed by the collaborators under such licenses. The Company assesses these multiple elements in accordance with ASC 605, to determine whether particular components of the arrangement represent separate units of accounting.
F-14
Table of Contents
As a result of new guidance issued by the FASB, agreements entered into after December 31, 2010 will be accounted for in accordance with Accounting Standards Update, or ASU, No. 2009-13, Topic 605 – Multiple-Deliverable Revenue Arrangements, or ASU 2009-13. This guidance requires the application of the “relative selling price” method when allocating revenue in a multiple deliverable arrangement. The selling price for each deliverable shall be determined using vendor specific objective evidence of selling price, if it exists; otherwise, third-party evidence of selling price shall be used. If neither exists for a deliverable, the vendor shall use its best estimate of the selling price for that deliverable. The Company recognizes upfront license payments as revenue upon delivery of the license only if the license has standalone value and the fair value of the undelivered performance obligations can be determined. If the fair value of the undelivered performance obligations can be determined, such obligations are accounted for separately as the obligations are fulfilled. If the license is considered to either not have stand-alone value or have stand-alone value but the fair value of any of the undelivered performance obligations cannot be determined, the arrangement is accounted for as a single unit of accounting and the license payments and payments for performance obligations are recognized as revenue over the estimated period of when the performance obligations are performed.
When the Company determines that an arrangement should be accounted for as a single unit of accounting, the Company must determine the period over which the performance obligations will be performed and revenue will be recognized, to the extent this is determinable. If the timing and the level of effort to complete performance obligations under the arrangement is not estimable, then the Company recognizes revenue under the arrangement on a straight-line basis over the period that the Company expects to complete such performance obligations.
The Company’s license agreement with Green Cross Corp. (“Green Cross”) contains, and other agreements it enters into may also contain, milestone payments. Revenues from milestones, if they are non-refundable and considered substantive, are recognized upon successful accomplishment of the milestones. If not considered substantive, milestones are initially deferred and recognized over the remaining performance obligation.
The Company’s current license agreements with Pharmstandard and Green Cross and any future license agreements the Company may enter into may provide for royalty payments. Royalty revenue is recognized upon the sale of the related products, provided there are no remaining performance obligations under the arrangement. To date, the Company has not received any royalty payments.
The Company is studying AGS-004 in a phase 2b clinical trial that is funded entirely by the National Institutes of Health (“NIH”). Under the NIH contract, the Company is entitled to receive reimbursement of its direct expenses and allocated overhead and general and administrative expenses and payment of other specified amounts totaling up to $1,395,099 upon its achievement of specified development milestones. These reimbursable expenses and allocated overhead are directly related to the development of novel HIV immunotherapy candidates and are recognized as revenue based on the reimbursable expenses that have accrued in accordance with the contractual terms in the arrangement. The Company recognizes revenues from the achievement of milestones under the NIH contract upon the accomplishment of any such milestone.
Income Taxes
The Company provides for income taxes using the asset and liability method. Under this method, deferred tax assets and liabilities are determined based on differences between financial reporting and tax bases of assets and liabilities, and are measured using the enacted tax rates and laws that will be in effect when the differences are expected to reverse. Valuation allowances are provided if, based upon the weight of available evidence, it is more likely than not that some or all of the deferred tax assets will not be realized.
Segment and Geographic Information
Operating segments are defined as components of an enterprise engaging in business activities from which it may earn revenues and incur expenses, for which discrete financial information is available and whose operating results are regularly reviewed by the chief operating decision maker in deciding how to allocate resources and in assessing performance. The Company views its operations and manages its business in one operating segment and all of the Company operations are in North America.
Research and Development
Research and development costs include all direct costs related to the development of the Company’s technology, including salaries and related benefits of research and development (“R&D”) personnel, depreciation of laboratory equipment, fees paid to consultants and contract research organizations, stock-based compensation for R&D personnel, sponsored research payments and license fees. R&D costs are expensed as incurred.
F-15
Table of Contents
Reimbursements of certain R&D costs have been recorded as a reduction of expense and presented in a separate line item within the statement of operations. The Company records these reimbursements when received. Such reimbursements were made under the collaboration agreement with Kyowa Hakko Kirin Co., Ltd. (“Kirin”) that was terminated in 2009, as further described in Note 15.
Redeemable Convertible Preferred Stock
The carrying value of redeemable convertible preferred stock is increased by periodic accretions so that the carrying amount will equal the redemption amount as of the redemption date. These increases are effected through charges against additional paid-in capital, to the extent it is available, or the deficit accumulated during the development stage.
Warrant Liability
Warrants to purchase the Company’s convertible preferred stock are classified as liabilities and are recorded at their estimated fair value. In each reporting period, any change in fair value of the freestanding warrants are recorded as expense in the case of an increase in fair value and income in the case of a decrease in fair value.
Other Receivables
The Company has recorded other receivables of $979,487 and $424,501 as of December 31, 2012 and 2013. These receivables are primarily related to amounts due under the NIH contract as of such dates. The Company assesses the recoverability of other receivables as of each balance sheet date.
Stock-Based Compensation
The Company estimates the grant date fair value of its share-based awards and amortizes this fair value to compensation expense over the requisite service period or vesting term, as further described in Note 12.
Investment Tax Credits
Other income of $694,331 was recognized in 2012 for scientific research and experimental development (“SR&ED”) investment tax credits in Canada. Under Canadian and Ontario law, DC Bio is entitled to SR&ED. Because these credits are subject to a claims review, the Company recognizes such credits when received.
Comprehensive Income (Loss)
ASC 220, Comprehensive Income, establishes standards for reporting and display of comprehensive income and its components in a full set of financial statements. The Company’s other comprehensive income (loss) is related to foreign currency translation adjustments.
Foreign Currency Translation
Gains and losses from foreign currency transactions are reflected in income currently.
The Company has identified the functional currency of its subsidiaries with foreign operations as the applicable local currency. The translation from the applicable local currency to United States dollars is performed using the exchange rate in effect as of the balance sheet date. Revenue and expense accounts are translated using the average exchange rate experienced during the period. Adjustments resulting from the translation of the Company’s subsidiaries’ financial statements from its functional currency to the United States dollar are not included in determining net income (loss), but are reported as accumulated other comprehensive (loss) income, a separate component of stockholders’ deficit.
Derivatives
The Company issued a put option to exchange certain shareholders’ stock in DC Bio for stock in the Company (Note 4). Derivatives are recorded in the balance sheet at fair value as of each balance sheet date utilizing pricing models for nonexchange traded contracts (Note 9). The Company does not use derivative financial instruments for speculative purposes. As of December 31, 2013, there were no derivatives outstanding.
Interest Expense
Interest expense is comprised of interest associated with the 2010 convertible notes issued in September and December 2010 and the 2011 convertible notes issued in July 2011 and interest associated with notes payable issued in connection with the financing of equipment. On April 10, 2012, the principal and interest on the convertible notes totaling $10,668,185 converted into an aggregate of 3,023,661 shares of series D preferred stock of the Company (Note 10).
F-16
Table of Contents
Recently Issued Accounting Pronouncements
In February 2013, the FASB issued Accounting Standards Update (“ASU”) No. 2013-02 “Reporting of Amounts Reclassified Out of Accumulated Other Comprehensive Income.” ASU No. 2013-02 requires an entity to disaggregate the total change of each component of other comprehensive income either on the face of the income statement or as a separate disclosure in the notes. The new guidance became effective for reporting periods beginning after December 15, 2012 and is applied prospectively. The Company adopted this guidance on January 1, 2013 and the adoption did not have any impact on its financial position, results of operations or cash flows as the amounts reclassified out of accumulated other comprehensive loss are not significant.
In July 2013, the FASB issued a new accounting standard update on the financial statement presentation of unrecognized tax benefits. The new guidance provides that a liability related to an unrecognized tax benefit would be presented as a reduction of a deferred tax asset for a net operating loss carryforward, a similar tax loss or a tax credit carryforward if such settlement is required or expected in the event the uncertain tax position is disallowed. The new guidance becomes effective for the Company on January 1, 2014 and it should be applied prospectively to unrecognized tax benefits that exist as of the effective date with retrospective application permitted. The Company does not believe that this updated standard will have a material impact on its consolidated financial statements.
3. Property and Equipment
Property and equipment consist of the following:
| Useful Life (Years) |
December 31, | |||||||||||
| 2012 | 2013 | |||||||||||
| Office furniture and equipment |
7 | $ | 440,675 | $ | 528,732 | |||||||
| Computer equipment |
3 | 626,853 | 712,609 | |||||||||
| Computer software |
3 | 507,494 | 540,809 | |||||||||
| Laboratory equipment |
7 | 5,408,198 | 5,264,952 | |||||||||
| Leasehold improvements |
5 | 2,643,024 | 2,719,033 | |||||||||
|
|
|
|
|
|||||||||
| 9,626,244 | 9,766,135 | |||||||||||
| Less: Accumulated depreciation and amortization |
(8,086,860 | ) | (8,164,032 | ) | ||||||||
|
|
|
|
|
|||||||||
| Property and equipment, net |
$ | 1,539,384 | $ | 1,602,103 | ||||||||
|
|
|
|
|
|||||||||
Depreciation and amortization expense was as follows:
| Year ended December 31, 2011 |
$ | 679,843 | ||
| Year ended December 31, 2012 |
505,261 | |||
| Year ended December 31, 2013 |
611,421 | |||
| Cumulative from inception to December 31, 2013 |
9,475,516 |
4. Noncontrolling Interest
In December 2002, the Company invested $6,500 in DC Bio. This investment was made in anticipation of an additional future financing by the Company into this newly formed entity. In December 2002, DC Bio sold approximately $5,200,000 of convertible subordinated debentures to certain of the other shareholders of DC Bio. In December 2004, these debentures were cancelled in exchange for 360,000 Class A preferred shares of DC Bio, warrants to purchase 72,000 Class A preferred shares of DC Bio and new nonconvertible debentures of DC Bio with an aggregate initial principal amount of approximately $3,652,000 (Note 7). Also in December 2004, the Company licensed to DC Bio certain intellectual property rights in exchange for 360,000 Class A preferred shares of DC Bio and warrants to purchase 72,000 Class A preferred shares of DC Bio. These warrants were cancelled in conjunction with the issuance of Class B preferred shares of DC Bio.
In 2007, DC Bio issued a note to the Company for CDN$500,000 that bore interest at 7%. In March 2008 and November 2008, DC Bio issued and sold 75,446 Class B preferred shares to a shareholder for a purchase price of $754,460. In connection with this issuance and sale, DC Bio issued and sold 75,446 Class B preferred shares to the Company for a combination of cash in the amount of $257,254 and the cancellation of the 2007 note payable of CDN$500,000 including accrued interest.
Prior to October 2012, the Company owned 49.94% of the total capital stock of DC Bio. The Company initially accounted for its investment in DC Bio using the equity method of accounting in 2002 and 2003. DC Bio did not have any operating activities in 2002 or 2003. Effective January 1, 2004, the Company consolidated the operations of DC Bio based on the determination that DC Bio was a VIE. As of December 31, 2011, the other shareholders’ ownership interests in DC Bio were represented on the consolidated balance
F-17
Table of Contents
sheet by noncontrolling interest as a separate component of stockholders’ deficit. In October 2012 and December 2012, DC Bio repurchased the noncontrolling interests from these shareholders. Subsequent to these transactions, the Company owned 100% of DC Bio and eliminated its equity attributable to non-controlling interest through additional paid-in capital.
5. Income Taxes
No provision for U.S. Federal, state or foreign income taxes has been recorded as the Company has incurred net operating losses since inception.
Significant components of the Company’s deferred tax assets and liabilities consist of the following:
| December 31, | ||||||||
| 2012 | 2013 | |||||||
| U.S. Federal and state net operating loss carryforwards |
$ | 34,137,628 | $ | 41,359,722 | ||||
| Foreign net operating loss carryforwards |
1,148,564 | 1,827,221 | ||||||
| Contribution carryforwards |
9,520 | 3,055 | ||||||
| Research and development credits |
3,178,052 | 4,061,844 | ||||||
| Investment tax credits |
40,955 | 38,175 | ||||||
| Stock-based compensation |
19,521 | 22,282 | ||||||
| Patents and other intangibles |
6,532 | 2,967 | ||||||
| Other accruals |
81,287 | 97,157 | ||||||
| Property and equipment |
511,553 | 532,316 | ||||||
|
|
|
|
|
|||||
| Total deferred tax assets |
39,133,612 | 47,944,739 | ||||||
| Valuation allowance for deferred assets |
(39,133,612 | ) | (47,944,739 | ) | ||||
|
|
|
|
|
|||||
| Net deferred tax assets |
$ | — | $ | — | ||||
|
|
|
|
|
|||||
As of December 31, 2012 and 2013, the Company provided a full valuation allowance against its net deferred tax assets since as of that time, the Company could not assert that it was more likely than not that these deferred tax assets would be realized. There was an increase in the valuation allowance in the year ended December 31, 2013 of $8,811,127, all of which was allocable to current operating activities.
As of December 31, 2013, the Company had U.S. Federal and state, and Canadian federal and provincial net operating loss carryforwards of approximately $109,102,100, $129,242,500, $7,308,900, and $7,308,900, respectively. These net operating loss carryforwards begin to expire in 2018, 2017, 2014 and 2014, respectively. As of December 31, 2013, the Company had U.S. Federal and state tax credit carryforwards of approximately $3,837,200 and $340,400, respectively. These credit carryforwards begin to expire in 2020 and 2019, respectively. The utilization of the net operating loss and tax credit carryforwards may be subject to limitation under the rules regarding a change in stock ownership as determined by the Internal Revenue Code, and state and foreign tax laws. Section 382 of the Internal Revenue Code of 1986, as amended, imposes annual limitations on the utilization of net operating loss (“NOL”) carryforwards, other tax carryforwards, and certain built-in losses upon an ownership change as defined under that section. In general terms, an ownership change may result from transactions that increase the aggregate ownership of certain stockholders in the Company’s stock by more than 50 percentage points over a three year testing period (“Section 382 Ownership Change”). If the Company has undergone a Section 382 Ownership Change, an annual limitation would be imposed on certain of the Company’s tax attributes, including NOL and capital loss carryforwards, and certain other losses, credits, deductions or tax basis. As of December 31, 2013 the Company has not performed a formal study to determine whether there are 382 limitations that apply. As of December 31, 2013, the Company had Canadian investment tax credit carryforwards of approximately $38,170 that begin to expire in 2024.
For the year ended December 31, 2013, the Company maintains that all Canadian earnings from DC Bio have been indefinitely re-invested and, as such, it has not recognized a deferred tax liability with respect to the unremitted earnings.
F-18
Table of Contents
Taxes computed at the statutory U.S. Federal income tax rate of 34.0% are reconciled to the provision for income taxes as follows:
| December 31, 2012 |
December 31, 2013 |
|||||||||||||||
| Amount | Percent of Pretax Earnings |
Amount | Percent of Pretax Earnings |
|||||||||||||
| U.S. Federal tax statutory rate |
$ | (3,560,252 | ) | 34.0 | % | $ | (8,133,331 | ) | 34.0 | % | ||||||
| State taxes (net of Federal benefit) |
(476,446 | ) | 4.5 | % | (789,412 | ) | 3.3 | % | ||||||||
| U.S. Federal tax credits |
— | 0.0 | % | (1,262,562 | ) | 5.3 | % | |||||||||
| Nondeductible interest expense |
(1,782,830 | ) | 17.0 | % | (132,546 | ) | 0.6 | % | ||||||||
| Other nondeductible expenses |
(210,790 | ) | 2.0 | % | 419,934 | (1.8 | %) | |||||||||
| Expiration of capital loss carryforward |
3,246,049 | (31.0 | %) | — | (0.0 | %) | ||||||||||
| Increase in unrecognized tax benefits |
— | 0.0 | % | 378,769 | (1.6 | %) | ||||||||||
| Change in effective state tax rate |
— | 0.0 | % | 1,349,062 | (5.6 | %) | ||||||||||
| Expiration of NOL & contribution carryforwards |
57,538 | (0.5 | %) | 6,630 | (0.0 | %) | ||||||||||
| Change in valuation reserves |
2,618,552 | (25.2 | %) | 8,811,127 | (36.8 | %) | ||||||||||
| Deferred tax asset true-ups |
108,179 | (0.8 | %) | (647,671 | ) | 2.6 | % | |||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Provision for income taxes |
$ | — | 0.0 | % | $ | — | 0.0 | % | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
The research and development credit, which had previously expired on December 31, 2011, was reinstated as part of the American Taxpayer Relief Act of 2012 enacted on January 2, 2013. This legislation retroactively reinstated and extended the credit from the previous expiration date through December 31, 2013. As a result, the Company adjusted its deferred tax assets in 2013 for the 2012 and 2013 research and development credits, net of the gross unrecognized tax benefit, which resulted in an increase to the deferred tax assets and a corresponding increase to the valuation allowance of approximately $298,000 and $585,700, respectively.
On July 23, 2013, the State of North Carolina enacted House Bill 998, which reduced the corporate income tax rate from 6.9% in 2013 to 6% in 2014 and to 5% in 2015. As a result of the new enacted tax rate, the Company adjusted its deferred tax assets in 2013 by applying the lower rate, which resulted in a decrease to the deferred tax assets and a corresponding decrease to the valuation allowance of approximately $1,349,100.
The Company had gross unrecognized tax benefits of approximately $1,265,700 as of January 1, 2013. As the 2012 R&D tax credit was not yet enacted into current law, no R&D credit was reflected on the 2012 provision. As a result, the Company recognized an increase in the unrecognized tax benefit associated with the federal R&D credit carryover in the current year related to the periods ending December 31, 2012 and 2013. As of December 31, 2013, the total gross unrecognized tax benefits were approximately $1,644,500 and of this total, none would affect the Company’s effective tax rate if recognized. The Company does not anticipate a significant change in total unrecognized tax benefits or the Company’s effective tax rate due to the settlement of audits or the expiration of statutes of limitations within the next 12 months.
The Company’s policy is to recognize interest and penalties related to uncertain tax positions in the provision for income taxes. As of December 31, 2012 and 2013, the Company had no accrued interest or penalties related to uncertain tax positions.
Effective January 1, 2007, the Company adopted the provisions of the FASB guidance on accounting for uncertainty in income taxes. These provisions provide a comprehensive model for the recognition, measurement and disclosure in financial statements of uncertain income tax positions that a company has taken or expects to take on a tax return. Under these provisions, a company can recognize the benefit of an income tax position only if it is more likely than not (greater than 50%) that the tax position will be sustained upon tax examination, based solely on the technical merits of the tax position. Otherwise, no benefit can be recognized. Assessing an uncertain tax position begins with the initial determination of the sustainability of the position and is measured at the largest amount of benefit that is greater than 50% likely of being realized upon ultimate settlement. As of each balance sheet date, unresolved uncertain tax positions must be reassessed. Additionally, companies are required to accrue interest and related penalties, if applicable, on all tax exposures for which reserves have been established consistent with jurisdictional tax laws. The cumulative effect of this adoption is recorded as an adjustment to the opening balance of its retained deficit on the adoption date.
As a result of adopting this guidance, the Company reduced its deferred tax assets by approximately $645,000 and reduced its valuation allowance against the deferred tax assets by the same amount. Accordingly, the Company did not record a contingent tax liability as a result of the implementation of these provisions. As of the adoption date of January 1, 2007 and as of December 31, 2013, the Company had no accrued interest or penalties related to the tax contingencies.
The Company has analyzed its filing positions in all significant federal and state jurisdictions where it is required to file income tax returns, as well as open tax years in these jurisdictions. With few exceptions, the Company is no longer subject to United States Federal, state, and local tax examinations by tax authorities for years before 2010 although carryforward attributes that were generated prior to 2010 may still be adjusted upon examination by the Internal Revenue Service if they either have been or will be used in a future period. No income tax returns are currently under examination by taxing authorities.
F-19
Table of Contents
The following is a tabular reconciliation of the Company’s change in gross uncertain tax positions during the years ended December 31, 2012 and 2013:
| 2011 | 2012 | 2013 | ||||||||||
| Beginning balance |
$ | 1,177,000 | $ | 1,272,800 | $ | 1,265,700 | ||||||
| Gross increase for tax positions related to current periods |
95,800 | — | 378,800 | |||||||||
| Gross decrease for tax positions related to prior periods |
— | (7,100 | ) | — | ||||||||
|
|
|
|
|
|
|
|||||||
| Ending balance |
$ | 1,272,800 | $ | 1,265,700 | $ | 1,644,500 | ||||||
|
|
|
|
|
|
|
|||||||
6. Convertible Term Notes
On September 9, 2010, the Company entered into a convertible note and warrant purchase agreement with a group of existing preferred stockholders, which provided for a total of up to $6,000,000 in borrowings by the Company. On September 9, 2010 and December 15, 2010, the Company received the proceeds under the agreement in two separate tranches of $4,872,066 and $1,127,925, respectively. The convertible notes issued pursuant to the agreement bore interest at a rate of 10% and provided for conversion into series C preferred stock of the Company. The note purchasers were also issued warrants to purchase series C preferred stock of the Company (Note 11). The proceeds from the convertible notes were allocated among the notes and the associated warrants based on the fair value of the warrants with the residual balance assigned to the convertible notes. The Company increased the value assigned to the convertible notes through interest expense using the effective interest method such that the value of the convertible notes will equal the accrued principal and interest of $6,603,333 as of maturity. The Company recognized $3,108,486 and $193,402 of interest expense related to the convertible notes during the years ended December 31, 2011 and 2012.
On July 27, 2011, the Company entered into a convertible note and warrant purchase agreement with a group of existing preferred stockholders, which provided for a total of up to $3,500,000 in borrowings by the Company. On July 27, 2011, the Company received the proceeds under the agreement of $3,500,000. The convertible notes bore interest at a rate of 10% and provided for conversion into series C preferred stock of the Company. The note purchasers were also issued warrants to purchase series C preferred stock of the Company (Note 11). The proceeds from the convertible notes were allocated among the notes and the associated warrants based on the fair value of the warrants with the residual balance assigned to the convertible notes. The Company increased the value assigned to the convertible notes through interest expense using the effective interest method such that the value of the convertible notes will equal the accrued principal and interest of $3,686,250 as of maturity. The Company recognized $3,597,778 and $98,662 of interest expense related to the July 2011 convertible notes during the years ended December 31, 2011 and 2012.
In December 2011, the Company and the holders of the 2010 convertible notes and the July 2011 convertible notes agreed to extend the maturity dates of the notes to March 31, 2012. The Company did not record any gain or loss in connection with the extension of the maturity date of the notes because it determined that the extension of the maturity date of the notes met the criteria of a troubled debt restructuring outlined in ASC Topic 470-60, Troubled Debt Restructurings. The Company recognized $292,064 of interest expense related to the 2010 convertible notes and the July 2011 convertible notes during the year ended December 31, 2012.
On April 10, 2012, the principal and interest on the convertible term notes totaling $10,668,185 converted into an aggregate of 3,023,661 shares of series D preferred stock of the Company (Note 10).
7. Noncontrolling Debentures
In connection with the financing transactions described in Note 4, DC Bio issued approximately $3,652,000 of debentures. Approximately $2,708,000 of debentures were paid in 2005 leaving a balance of approximately $943,000. The remaining debentures and accrued interest on such debentures were paid in January 2007.
8. Notes Payable
During December 2000 (and amended in November 2002, November 2004, and November 2005), the Company entered into a loan agreement with a lending institution, which provided for the Company to borrow funds for the acquisition of property and equipment. The lending institution’s commitment under this line of credit expired in April 2007. Through December 31, 2007, the Company had borrowed $5,129,782 under this agreement. This agreement terminated in 2007, and there was no balance outstanding as of December 31, 2012 and 2013. Prior to termination, borrowings for the acquisition of property and equipment were collateralized by substantially all of the tangible assets of the Company, bore interest at rates between 9.76% and 11.00% and were to be repaid in 42 or 48 equal monthly installments commencing on the date of borrowing. In connection with the loan agreement, the Company issued
F-20
Table of Contents
warrants to purchase one share of its common stock at an exercise price of $13,576.32 per share and warrants to purchase five shares of its common stock at an exercise price of $23,894.34 per share. The warrants to purchase one share at an exercise price of $13,576.32 per share expired as of December 31, 2008, and warrants to purchase two shares at an exercise price of $23,894.34 per share expired as of December 31, 2012 (Note 11). Warrants to purchase one share of its common stock at $23,894.34 per share remained outstanding as of December 31, 2013.
The Company entered into a Loan and Security Agreement with two lending institutions in April 2007 for the purpose of borrowing $5,000,000 to be used for working capital. The loan term was six months of interest only followed by 30 months of principal and interest at 11.25%. The Loan and Security Agreement terminated in April 2010, and as of December 31, 2012 and 2013, there were no outstanding balances on the loan. The Company is required to pay a success fee of $200,000 upon consummation of a liquidity event, including an IPO. The Company’s IPO closed on February 12, 2014. Accordingly, this fee was paid in March 2014.
The Company entered into a Master Lease Agreement in July 2012 with a lending institution, which provides for the Company to borrow funds up to $100,000 to finance computer equipment. Through December 31, 2013, the Company has borrowed $176,055 under this agreement, of which $80,188 and $125,553 was outstanding as of December 31, 2012 and December 31, 2013. Borrowings are collateralized by substantially all of the computer equipment financed under the agreement, bear interest at a rate of 0.98% and are to be repaid in 36 equal monthly installments commencing on the date of borrowing.
In December 2013, in connection with the Medinet collaboration agreement as described in Note 15, the Company borrowed $9.0 million pursuant to an unsecured promissory note that bears interest at a rate of 3.0% per annum. The principal and interest under the note are due and payable on December 31, 2018. Under the terms of the note and the license agreement, any milestone payments related to the developmental and regulatory milestones that become due will be applied first to the repayment of the loan. The Company has the right to prepay the loan at any time. If the Company has not repaid the loan by December 31, 2018, then the Company has agreed to grant to Medinet a non-exclusive, royalty-bearing license to make and sell Arcelis products in Japan for the treatment of cancer. In such event, the amounts owing under the loan as of December 31, 2018 will constitute pre-paid royalties under the license and will not be otherwise due and payable. Royalties under this license would be paid until the expiration of the licensed patent rights in Japan at a rate to be negotiated. If the Company and Medinet cannot agree on the royalty rate, they have agreed to submit the matter to arbitration. Because the $9.0 million promissory note was issued at a below market interest rate, the Company allocated the proceeds of the loan between the manufacturing license and the debt at the time of issuance. As a result, we recorded $6.9 million as notes payable, based upon an effective interest rate of 8.0%, and $2.1 million as a deferred liability.
9. Derivatives
In conjunction with the DC Bio transaction on December 7, 2004 (Note 4), the Company issued a put option whereby in the event DC Bio has not been acquired or had not completed an IPO by December 7, 2007, the other shareholders of DC Bio would have the right to put their DC Bio Class A preferred shares and Class B preferred shares to the Company for shares of the Company’s stock. As of December 31, 2011, the Company had reserved 652,608 shares of series C preferred stock, 419,768 shares of series B preferred stock and 23,703 shares of its common stock for issuance upon the exchange of Class A and Class B preferred shares of DC Bio should such put option be exercised. The Company recorded a liability of $1,036,403 as of December 31, 2011 based on the fair market value of the put option, which was calculated based on the fair market value of the Company’s stock issuable upon exercise of the put option, less the fair market value of the DC Bio shares held by such shareholders, as described above. For purposes of this calculation, the Company determined that the fair market value of the Company’s stock issuable upon exercise of the put option was $1,487,668 as of December 31, 2011 and that the fair market value of the DC Bio shares was $451,265 as of December 31, 2011. The value of the DC Bio shares held by the other shareholders is valued under the income approach utilizing a discounted cash flow model. The discount rate reflects the risk in the cash flows. The change in fair value of the put is recorded as derivative (expense) income. The Company terminated the put option in October 2012 when DC Bio repurchased the noncontrolling interests for a total of approximately $180,000.
10. Stockholders’ Deficit and Redeemable Convertible Preferred Stock
As of December 31, 2012 and 2013, the Company was authorized to issue 100,000,000 and 120,000,000 shares of common stock and 145,650,000 and 160,700,000 of shares of preferred stock, respectively.
Common Stock
In July 1997, the Company issued 144 shares of its common stock to its founders for subscription receivable of $2,000 or $13.576 per share. The receivable, including interest, was repaid in December 1999. In January 2000, the Company issued 36 shares of its common stock to Duke University in exchange for a technology license. As a result, the Company recorded $50,000 of expense in 2000. In October 2002, the Company issued two shares of its common stock to Duke University in exchange for technology licenses. As a result, the Company recorded $6,300 of expense in 2002.
In March 2008, an aggregate of 1,412,224 shares of its series A preferred stock and an aggregate of 5,306,068 shares of its series B preferred stock converted into an aggregate of 491 shares of its common stock in connection with the election not to participate in the series C preferred stock financing.
In connection with the issuance of the convertible term notes in September 2010, an aggregate of 248,100 shares of series A preferred stock, an aggregate of 1,752,400 shares of its series B preferred stock, an aggregate of 1,835,543 shares of its series B-1 preferred stock and an aggregate of 8,687,902 series C preferred stock, converted into an aggregate of 92,246 shares of its common stock as a result of the failure of certain stockholders to participate in the financing.
In connection with the issuance of the July 2011 convertible notes, an aggregate of 124,050 shares of its series A preferred stock, an aggregate of 441,035 shares of its series B preferred stock, an aggregate of 917,771 shares of its series B-1 preferred stock and an aggregate of 2,245,866 shares of its series C preferred stock converted into an aggregate of 27,464 shares of its common stock as a result of the decision of certain stockholders not to participate in the July 2011 financing.
F-21
Table of Contents
In connection with the issuance of the series D preferred stock in April 2012, an aggregate of 124,050 shares of its series A preferred stock, an aggregate of 2,182,576 shares of its series B preferred stock, an aggregate of 917,771 shares of its series B-1 preferred stock and an aggregate of 8,605,955 shares of its series C preferred stock converted into an aggregate of 104,489 shares of its common stock as a result of the decision of certain stockholders not to participate in the April 2012 financing.
Dividends
The holders of common stock shall be entitled to receive dividends from time to time as may be declared by the Board of Directors. No cash dividend may be declared or paid to common stockholders until paid on each series of outstanding preferred stock in accordance with its terms.
Voting
The holders of shares of its common stock are entitled to one vote for each share held with respect to all matters voted on by the stockholders of the Company.
Liquidation
After payment to the preferred stockholders of their liquidation preferences, holders of common stock shall be entitled, together with holders of preferred stock, to share ratably in all remaining assets of the Company.
Restricted Stock
During September 2002, the Company issued 168 shares of restricted common stock to one of its officers, of which 134 shares vested based on continuous service to the Company. The shares had an estimated fair value of $2,443.74 per share, which was recorded as deferred compensation and was being amortized over the vesting period. The Company recorded $140,585 of expense during 2005. During August 2005, all of the 134 time-vested shares became vested upon the officer’s departure from the Company. Of these, 59 shares were surrendered to the Company as a partial settlement of certain promissory notes issued by the officer in favor of the Company. The 34 non time-vested shares were forfeited upon the officer’s departure.
During December 2010, the Company issued 947 shares of restricted stock to a consultant. The shares had an estimated fair value of $9.48 per share, which was recorded as consulting expense during the year ended December 31, 2010. During September 2011, the Company issued 254 shares of restricted stock to the same consultant. The shares had an estimated fair value of $35.30 per share. The Company recorded $9,000 as consulting expense during the year ended December 31, 2011.
Redeemable Convertible Preferred Stock
During January 2000, the Company issued 1,893,874 shares of series A preferred stock of the Company for cash proceeds of $1,500,000 or $1.00 per share, less related issuance costs of $65,741, and conversion of convertible notes payable of $374,998 and related accrued interest of $18,876. In addition, the Company issued warrants to purchase 70,833 shares of series A preferred stock for $1.00 per share (Note 11). These warrants were cancelled on March 31, 2008.
During January 2001, the Company issued 514,875 shares of series A preferred stock for payment of accounts payable of $514,875 representing a price of $1.00 per share.
During April, July and August 2001, the Company issued 22,376,045 shares of series B preferred stock of the Company at $1.76 per share for cash proceeds of $37,266,756, less related issuance costs of $157,594, and conversion of convertible notes payable of $2,000,000 and related accrued interest of $115,083. Also during July 2001, the Company issued 135,511 shares of series B preferred stock for payment of accounts payable of $238,500 representing a price of $1.76 per share. In addition, the Company issued warrants to purchase 4,331,856 shares of series B preferred stock at $1.76 per share (Note 11). These warrants were cancelled on March 31, 2008 in connection with the series C preferred stock financing.
During June 2004, the Company issued 2,840,909 shares of series B-1 preferred stock of the Company at $1.76 per share for cash proceeds of $5,000,000, less related issuance costs of $86,494. In addition, the Company issued warrants to purchase 1,704,546 shares of series B-1 preferred stock at $1.76 per share (Note 11). These warrants were cancelled on March 31, 2008 in connection with the series C preferred stock financing.
During March, April and November 2008, the Company issued 122,753,041 shares of series C preferred stock at $0.289018 per share for cash proceeds of $29,904,666 and upon conversion of convertible term notes of $3,456,946 and related accrued interest of $963,912, less related issuance costs of $502,603. Also during March and November 2008, the Company issued 346,000 shares of series C preferred stock for settlement of accrued expenses of $100,000 or $0.289018 per share.
F-22
Table of Contents
During March 2008, holders of shares of series A and B preferred stock that failed to purchase their respective pro rata share of series C preferred stock had 1,412,224 shares of series A preferred and 5,306,068 shares of series B preferred stock converted into an aggregate of 491 shares of common stock and also forfeited their right to receive any accrued but unpaid dividends thereon. Holders of series A, B and B-1 preferred stock that purchased their pro rata share of series C preferred stock were issued an aggregate of 651,728 shares of series A preferred stock, 9,314,522 shares of series B preferred stock and 830,177 shares of series B-1 preferred stock, respectively, in exchange for, in part, the cancellation of accrued but unpaid dividends on their shares of preferred stock. In 2010, the Company revised its previously reported additional paid-in capital and deficit accumulated during the development stage for 2008 and 2009 with respect to the March 2008 preferred stock conversion into common stock. These revisions were not considered material to the financial statements.
In April 2012, the Company sold shares of its series D preferred stock in a private placement to certain of its existing holders of preferred stock and additional accredited investors for an aggregate purchase price of $19.7 million. Included in this amount was $10.7 million of outstanding principal and interest on the 2010 convertible notes and the 2011 convertible notes which converted to series D preferred stock in this financing.
In connection with the series D preferred stock financing, each participating investor exchanged shares of the Company’s series C preferred stock held by such investor for shares of series D preferred stock pursuant to the terms of the series D preferred stock purchase agreement. Participating investors exchanged such number of shares of series C preferred stock as was equal in value to the number of shares of series D preferred stock such investor had purchased in the series D preferred stock financing (excluding for such purposes shares of series D preferred stock issued to such investor in exchange for the outstanding principal and interest on the 2010 convertible notes and the 2011 convertible notes), with the value of such series C preferred stock and series D preferred stock equal to the original purchase price of such shares.
In August 2012, the Company sold 3,716,935 shares of its series D-1 preferred stock in a private placement to certain of its existing holders of preferred stock and other accredited investors for $4.298725 per share for an aggregate purchase price of $16.0 million.
In connection with the issuance and sale of the Company’s series D-1 preferred stock, the Company issued warrants to the purchasers of the series D-1 preferred stock to purchase an aggregate of 1,332,662 shares of the Company’s series D-1 preferred stock at an exercise price of $0.01 per share. The warrants had a term of ten years and were cancelled in connection with the exchange of all issued and outstanding series D-1 preferred stock for series D preferred stock in July 2013, as discussed further below.
In connection with the issuance and sale of the Company’s series D-1 preferred stock, each participating investor exchanged shares of the Company’s series C preferred stock and series B preferred stock held by such investor for shares of series D-1 preferred stock pursuant to the terms of the Series D-1 preferred stock and warrant purchase agreement. Under these terms, participating investors exchanged such number of shares of series B preferred stock and series C preferred stock as had a value equal to the aggregate purchase price of the shares of series D-1 preferred stock purchased by such investor in the series D-1 preferred stock financing, with the value of each share of series B preferred stock and series C preferred stock equal to the original purchase price of such shares: $1.76 per share in the case of the series B preferred stock and $0.289018 per share in the case of the series C preferred stock. Participating investors were required to exchange all shares of series C preferred stock held by such investors prior to exchanging any shares of series B preferred stock. Participating investors exchanged an aggregate of 3,652,680 shares of series B preferred stock and 33,040,858 shares of series C preferred stock for 3,716,935 shares of the Company’s series D-1 preferred stock.
Since the fair values of the shares of series D preferred stock and series D-1 preferred stock transferred to extinguish the shares of series B and C preferred stock were less than the carrying amount of those shares, the Company recorded the difference in value as a decrease to deficit accumulated during the development stage in accordance with ASC 260.
In July 2013, all 7,433,870 shares of series D-1 preferred stock outstanding were exchanged for 16,151,212 shares of series D preferred stock. In addition, in connection with the exchange, the liquidation preference and the redemption price of the series D preferred stock were reduced to $1.978565 per share. As a result of the reduction in the liquidation preference of the series D preferred stock, the Company issued an aggregate of 6,373,782 additional shares of series D preferred stock to the holders that had purchased shares of series D preferred stock prior to such reduction for no additional consideration.
At the time of the exchange, the Company’s certificate of incorporation was amended to reduce the liquidation preference and redemption price of the series A preferred stock, the series B preferred stock, the series C preferred stock and the series D preferred stock to $0.32 per share, $0.5632 per share, $0.09248576 per share and $1.978565 per share, respectively. In addition, the Company extended the redemption trigger date of the preferred stock by one year to July 31, 2018.
F-23
Table of Contents
The Company recorded the reduction to liquidation preference as a reversal to prior accretion taken on each series of preferred stock impacted, which reduced net loss attributable to common stockholders in the earnings per share calculation (see Note 19). The Company recorded any remaining reduction in liquidation preference as an increase to additional paid-in capital. In addition, the Company recorded the fair value of the incremental series D preferred stock shares issued in these exchanges in additional paid-in capital as a preferred stock dividend, which increased net loss attributable to common stockholders in the earnings per share calculation (see Note 19).
In August 2013, the Company sold 16,898,436 shares of series E preferred stock for an aggregate purchase price of $22,007,404. In connection with the issuance and sale of the series E preferred stock, each participating investor exchanged shares of series A preferred stock, series B preferred stock and series C preferred stock, if any, held by such investor for shares of series D preferred stock pursuant to the terms of the series E preferred stock and warrant purchase agreement. Under these terms, participating investors exchanged such number of shares of series A preferred stock, series B preferred stock and series C preferred stock as had a value equal to the aggregate purchase price of shares of series E preferred stock purchased by such investor in the series E preferred stock financing, with the value of each share of series A preferred stock, series B preferred stock and series C preferred stock equal to the liquidation preference of such shares: $0.32 per share in the case of the series A preferred stock, $0.5632 per share in the case of the series B preferred stock and $0.09249 per share in the case of the series C preferred stock. Participating investors were required to exchange all shares of series A preferred stock held by such investors prior to exchanging any shares of series B preferred stock, and all shares of series B preferred stock held by such investors before exchanging any shares of series C preferred stock. Participating investors exchanged an aggregate of 111,837 shares of series A preferred stock, 8,687,630 shares of series B preferred stock and 10,586,174 shares of series C preferred stock for 2,985,863 shares of series D preferred stock.
In connection with the issuance and sale of series E preferred stock, each participating investor who had also purchased shares of series D preferred stock or series D-1 preferred stock during the 2012 calendar year (excluding for such purposes shares of series D preferred stock issued to such investor in exchange for the outstanding principal and interest on the 2010 convertible notes and the 2011 convertible notes and shares of preferred stock issued in 2012 in exchange for any previously issued shares of preferred stock) exchanged shares of series D preferred stock into shares of series E preferred stock pursuant to the terms of the series E preferred stock and warrant purchase agreement. Under these terms, participating investors exchanged such number of shares of series D preferred stock as had a value equal to the aggregate purchase price of the shares of series D preferred stock and series D-1 preferred stock purchased during the 2012 calendar year, with the value of each share series D preferred stock as of the time of exchange equal to the original purchase price of such shares: $1.978565 per share. Participating investors exchanged an aggregate of 12,607,779 shares of series D preferred stock for 19,154,336 shares of series E preferred stock.
In October 2013 and November 2013, the Company sold 921,423 shares of its series E preferred stock for an aggregate purchase price of $1,200,000 and 19,037,063 shares of its series E preferred stock for an aggregate purchase price of $24,792,595, respectively. Following the November 2013 issuance and sale of the Company’s series E preferred stock, purchasers of the Company’s series E preferred stock committed to purchase additional shares of series E preferred stock for an aggregate purchase price of $12,000,000 in subsequent closings.
In connection with the sale of series E preferred stock, the Company issued warrants to the purchasers of series E preferred stock to purchase an aggregate of 433,282 shares of its common stock at an exercise price of $0.06 per share. The warrants had a term of 10 years and would only become exercisable if the Company did not receive $5,000,000 in non-dilutive financing by December 31, 2013 or had not executed definitive documentation providing for an additional $5,000,000 investment of non-dilutive financing as of December 31, 2013. In connection with the license agreement that the Company entered into with Medinet, in December 2013, as described in Note 15, Medinet paid the Company $1.0 million and loaned the Company $9.0 million. In connection with the receipt of these funds, the warrants were cancelled pursuant to their terms.
In connection with the issuance and sale of series E preferred stock, in December 2013, the Company issued a warrant to purchase 9,598 shares of its common stock, at an exercise price of $6.60 per share, to a placement agent.
Upon the closing of the IPO, all of the outstanding shares of redeemable convertible preferred stock automatically converted into 13,188,251 shares of the Company’s common stock. Prior to such conversion, the Company’s series A preferred stock, series B preferred stock, series C preferred stock, series D preferred stock, and series E preferred stock had the following characteristics:
Voting
The holders of the preferred stock are entitled to vote, together with the holders of common stock, on all matters submitted to stockholders for a vote. Each preferred stockholder is entitled to the number of votes equal to the number of shares of common stock into which each preferred share is convertible as of the time of such vote.
F-24
Table of Contents
Dividends
The holders of the preferred stock are entitled to receive, when and as declared by the board of directors and out of funds legally available, noncumulative dividends at the rate of 8% per annum, payable in preference and priority to any payment of any dividend on common stock. No dividends or other distributions shall be made with respect to the common stock, until all declared dividends on the preferred stock have been paid. Since inception (May 8, 1997) through December 31, 2012 no dividends have been declared or paid by the Company. As part of a recapitalization in March 2008, the Company amended its certificate of incorporation to remove the cumulative feature of dividends on preferred stock. In 2008, holders of series A, B and B-1 preferred stock who purchased their pro-rata share of the series C preferred stock being offered were issued additional shares of series A, B and B-1 preferred stock, as applicable in exchange for, in part, the cancellation of accrued but unpaid dividends on their shares of preferred stock, which had been accrued based on the cumulative dividend feature, prior to the removal of such feature.
Liquidation
In the event of liquidation, dissolution, or winding up of the Company, holders of preferred stock shall be entitled, before any distribution is made to the holders of common stock, to be paid an amount equal to $0.32 per share of series A preferred stock, $0.5632 per share of series B preferred stock, $0.09248576 per share of series C preferred stock, $1.978565 per share of series D preferred stock and $1.302333 per share of series E preferred stock, plus an amount equal to 8% per annum of the series E preferred stock original purchase price from the date of issuance ($0.1042 per year), plus in each case any declared but unpaid dividends. In the event that assets of the Company are insufficient to permit payment of the above mentioned amounts, holders shall share ratably in any distribution of the remaining assets and funds of the Company in proportion to the respective amounts which would otherwise be payable under these circumstances in the order of liquidation preference.
Conversion
Each share of preferred stock, at the option of the holder, is convertible into a number of fully paid shares of common stock as determined by dividing the respective preferred stock issue price by the conversion price in effect at the time. The conversion price of series A preferred stock, series B preferred stock, series C preferred stock, series D preferred stock and series E preferred stock are $135.7632, $135.7632, $39.238009, $11.87139 and $7.813998 per share, respectively, and is subject to adjustment in accordance with antidilution provisions contained in the Company’s certificate of incorporation. Conversion is automatic immediately upon the closing of a firm commitment underwritten public offering with aggregate proceeds to the Company of at least $50 million, before deduction of underwriters’ commissions and expenses.
Participation Rights
After payment to the preferred stockholders of their liquidation preferences, holders of preferred stock shall be entitled, together with holders of common stock to share ratably in all remaining assets of the Company.
Redemption
The holders of the outstanding preferred stock may, by written request delivered after July 31, 2018, require the Company to redeem the outstanding shares of the preferred stock by paying in cash a sum equal to the original purchase price of the preferred stock plus any declared but unpaid dividends. If the Company does not have sufficient funds legally available to redeem all shares of preferred stock to be redeemed as of the redemption date, then the Company shall redeem such shares ratably to the extent possible and shall redeem the remaining shares as soon as sufficient funds are legally available.
Carrying Value
Shares of preferred stock are initially recorded at the total net proceeds received by the Company upon issuance, after reduction for the value of detachable warrants and issuance costs. The difference between the total net proceeds as of issuance and the total redemption price as of a future redemption date will be charged to additional paid-in capital, to the extent it is available, or the deficit accumulated during the development stage over the period from issuance until redemption first becomes available.
F-25
Table of Contents
The table below represents a rollforward of the preferred stock:
| Series A Preferred | Series B Preferred | Series B- 1 Preferred |
Series C Preferred | Series D Preferred | Series D-1 Preferred | Series E Preferred | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | Shares | Amount | Shares | Amount | Shares | Amount | Shares | Amount | Shares | Amount | |||||||||||||||||||||||||||||||||||||||||||
| Balance as of Inception |
— | $ | — | — | $ | — | — | $ | — | — | $ | — | — | $ | — | — | $ | — | — | $ | — | |||||||||||||||||||||||||||||||||||
| Issuance of shares |
1,893,874 | 1,942,179 | 21,174,293 | 33,295,602 | 2,840,909 | 5,000,000 | 103,469,906 | 29,904,666 | — | — | — | — | — | — | ||||||||||||||||||||||||||||||||||||||||||
| Conversion of notes payable |
— | — | 1,201,752 | 2,115,083 | — | — | — | — | — | — | — | — | — | — | ||||||||||||||||||||||||||||||||||||||||||
| Conversion of bridge note |
— | — | — | — | — | — | 19,283,135 | 4,420,858 | — | — | — | — | — | — | ||||||||||||||||||||||||||||||||||||||||||
| Conversion of accrued services |
— | — | — | — | — | — | 346,000 | 100,000 | — | — | — | — | — | — | ||||||||||||||||||||||||||||||||||||||||||
| Stock issuance costs |
— | — | — | (157,594 | ) | — | (86,494 | ) | — | (502,603 | ) | — | — | — | — | — | — | |||||||||||||||||||||||||||||||||||||||
| Accrued dividends converted into preferred |
651,728 | 651,728 | 9,314,522 | 16,393,559 | 830,177 | 1,461,111 | — | — | — | — | — | — | — | — | ||||||||||||||||||||||||||||||||||||||||||
| Reversal of accrued divided |
— | (651,728 | ) | — | (16,393,559 | ) | — | (1,461,111 | ) | — | — | — | — | — | — | — | — | |||||||||||||||||||||||||||||||||||||||
| Issuance of shares as payment for accounts payable |
514,875 | 514,875 | 135,511 | 238,500 | — | — | — | — | — | — | — | — | — | — | ||||||||||||||||||||||||||||||||||||||||||
| Shares converted to common |
(1,660,324 | ) | (1,660,324 | ) | (7,058,468 | ) | (12,422,913 | ) | (1,835,543 | ) | (3,230,556 | ) | (8,687,903 | ) | (2,459,091 | ) | — | — | — | — | — | — | ||||||||||||||||||||||||||||||||||
| Unconverted accrued dividends |
— | (1,178,922 | ) | — | (4,831,999 | ) | — | — | — | — | — | — | — | — | — | — | ||||||||||||||||||||||||||||||||||||||||
| Accretion |
— | 1,782,345 | — | 25,354,306 | — | 1,547,605 | — | 274,889 | — | — | — | — | — | — | ||||||||||||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||||||
| Balance as of December 31, 2010 |
1,400,153 | 1,400,153 | 24,767,610 | 43,590,985 | 1,835,543 | 3,230,555 | 114,411,138 | 31,738,719 | — | — | — | — | — | — | ||||||||||||||||||||||||||||||||||||||||||
| Shares converted to common |
(124,050 | ) | (124,050 | ) | (441,036 | ) | (776,223 | ) | (917,772 | ) | (1,615,279 | ) | (2,245,867 | ) | (649,096 | ) | — | — | — | — | — | — | ||||||||||||||||||||||||||||||||||
| Accretion |
— | — | — | — | — | — | — | 926,542 | — | — | — | — | — | — | ||||||||||||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||||||
| Balance as of December 31, 2011 |
1,276,103 | 1,276,103 | 24,326,574 | 42,814,762 | 917,771 | 1,615,276 | 112,165,271 | 32,016,165 | — | — | — | — | — | — | ||||||||||||||||||||||||||||||||||||||||||
| Issuance of shares |
— | — | — | — | — | — | — | — | 2,549,897 | 4,793,806 | 3,713,333 | 7,842,733 | — | — | ||||||||||||||||||||||||||||||||||||||||||
| Conversion of bridge note |
— | — | — | — | — | — | — | — | 3,023,661 | 5,684,483 | — | — | — | — | ||||||||||||||||||||||||||||||||||||||||||
| Conversion of accrued services |
— | — | — | — | — | — | — | — | 7,142 | 25,200 | 3,602 | 15,485 | — | — | ||||||||||||||||||||||||||||||||||||||||||
| Pull through of existing preferred |
— | — | (3,652,680 | ) | (6,428,717 | ) | — | — | (64,256,463 | ) | (18,571,283 | ) | 2,557,039 | 4,807,233 | 3,716,935 | 7,835,133 | — | — | ||||||||||||||||||||||||||||||||||||||
| Stock issuance costs |
— | — | — | — | — | — | — | — | — | (128,275 | ) | — | (80,659 | ) | — | — | ||||||||||||||||||||||||||||||||||||||||
| Shares converted to common |
(124,050 | ) | (124,050 | ) | (2,182,576 | ) | (3,841,332 | ) | (917,771 | ) | (1,615,276 | ) | (8,605,955 | ) | (2,487,276 | ) | — | — | — | — | — | — | ||||||||||||||||||||||||||||||||||
| Accretion |
— | — | — | — | — | — | — | 327,465 | — | 18,529 | — | 5,377 | — | — | ||||||||||||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||||||
| Balance as of December 31, 2012 |
1,152,053 | 1,152,053 | 18,491,318 | 32,544,713 | — | — | 39,302,853 | 11,285,071 | 8,137,739 | 15,200,976 | 7,433,870 | 15,618,069 | — | — | ||||||||||||||||||||||||||||||||||||||||||
| Exchange of Series D-1 |
— | — | — | — | — | — | — | — | 16,151,212 | 26,887,407 | (7,433,870 | ) | (15,628,821 | ) | — | — | ||||||||||||||||||||||||||||||||||||||||
| Issuance of shares |
— | — | — | — | — | — | — | — | 6,373,782 | 11,446,143 | — | — | 36,856,922 | 47,409,867 | ||||||||||||||||||||||||||||||||||||||||||
| Exchange of Series D |
— | — | — | — | — | — | — | — | (12,607,779 | ) | (24,945,326 | ) | — | — | 19,154,336 | 24,945,326 | ||||||||||||||||||||||||||||||||||||||||
| Pull through of existing preferred |
(111,837 | ) | (35,788 | ) | (8,687,630 | ) | (4,892,873 | ) | — | — | (10,586,174 | ) | (979,070 | ) | 2,985,863 | 4,030,920 | — | — | — | — | ||||||||||||||||||||||||||||||||||||
| Reduction of liquidation value |
— | (783,396 | ) | (18,001,661 | ) | — | — | — | (6,195,373 | ) | — | — | — | — | — | — | ||||||||||||||||||||||||||||||||||||||||
| Stock issuance costs |
— | — | — | — | — | — | — | — | — | (64,530 | ) | — | — | — | (556,247 | ) | ||||||||||||||||||||||||||||||||||||||||
| Reversal of prior accretion |
— | — | — | (4,128,742 | ) | — | — | — | (1,528,896 | ) | — | — | — | — | — | — | ||||||||||||||||||||||||||||||||||||||||
| Accretion |
— | — | — | — | — | — | — | 74,152 | — | 706,902 | — | 10,752 | — | 92,841 | ||||||||||||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||||||
| Balance as of December 31, 2013 |
1,040,216 | $ | 332,869 | 9,803,688 | $ | 5,521,437 | — | $ | — | 28,716,679 | $ | 2,655,884 | 21,040,817 | $ | 33,262,492 | — | $ | — | 56,011,258 | $ | 71,891,787 | |||||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||||||
| Redemption value |
$ | 332,869 | $ | 5,521,437 | $ | — | $ | 2,655,884 | $ | 41,630,624 | $ | — | $ | 72,945,310 | ||||||||||||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||||||||||||||||||||
F-26
Table of Contents
11. Warrants
Convertible Term Notes
The Company entered into convertible note and warrant purchase agreements with note purchasers on September 9, 2010 and July 27, 2011. The warrants issued under these agreements entitle the purchasers of the convertible term notes to purchase an aggregate of 20,759,953 and 12,109,975 shares, respectively, of series C preferred stock with an exercise price of $0.01 per share. The warrants were issued with a term of seven years. In July 2013, the warrants were cancelled. The Company allocated $3,197,626 and $3,450,000, respectively, of the proceeds from the issuance of the convertible term notes to the warrants based on their fair value. The Company recorded the warrants as a liability of $3,585,829 and $0 as of December 31, 2012 and 2013, and marked the warrants to market as of each reporting period based on any underlying change in the fair market value of such warrants. As of December 31, 2012 and 2013, the Company based its valuation of the fair value of the warrants on an allocation of the enterprise value of the Company to all classes of equity and preferred stock including the warrants. The Company utilized the probability-weighted expected return method (“PWERM”) to determine these values. Under the PWERM method, share value is derived from the probability-weighted present value of expected future investment returns, considering each of the possible outcomes available to the Company, as well as the economic and control rights of each share class. The Company calculated the implied enterprise value using the PWERM approach. The fair value of the warrants was estimated using a probability-weighted analysis of the present value of the returns afforded to holders of warrants under several scenarios, including dissolution of the Company, a strategic merger or sale of the Company and an IPO.
Series D-1 Preferred
In conjunction with the sale of series D-1 preferred stock during August 2012, the Company issued warrants to purchase 1,332,622 shares of series D-1 preferred stock at an exercise price of $0.01 per share to certain holders of series D-1 preferred stock that invested above their pro-rata. The warrants were issued with a term of ten years and shall terminate upon the occurrence of a sale of the Company or an IPO (to the extent not then exercised). In July 2013, the warrants were cancelled. The warrants have been recorded as a liability of $2,806,823 and $0 as of December 31, 2012 and 2013.
Other Warrants
In conjunction with entering into research and development agreements with two universities in June 2001 and August 2001, the Company issued warrants to purchase 66 shares of its common stock at $2,443.74 per share. The warrants were exercisable immediately and expired in August 2011. The estimated fair value of these warrants, as determined using the Black-Scholes valuation model, was $106,560. The Company recognized the fair value of the warrants as research and development expense when the warrants were issued.
In conjunction with entering into license, research and development agreements with a German university in July 2001, the Company issued fully vested warrants to purchase 25 shares of its common stock at $2,443.74 per share. The warrants are considered a derivative instrument since the warrants contain a net cash settlement feature. As a result, the warrants were measured as of each balance sheet date and any increase or decrease in the fair value was recorded as research and development expense or a reduction in research and development expense, respectively. As of December 31, 2010, a liability of $0 was recorded to reflect the estimated fair value of the warrants. These warrants expired in July 2011.
In conjunction with entering into a loan agreement with a bank in December 2000 (and amended in November 2002, November 2004, and November 2005), the Company issued fully vested warrants to purchase one share of its common stock, at an exercise price of 13,576.32 per share, which expired as of December 31, 2008, and five shares of its common stock, at an exercise price of $23,894.34 per share, of which warrants to purchase two shares had expired as of December 31, 2012. A warrant to purchase one share of the Company’s common stock, at an exercise price of $23,894.34 per share, remained outstanding as of December 31, 2013. The Company determines the estimated fair value of the warrants using the Black-Scholes valuation model and recognizes the value of the warrants as interest expense over the loan agreement term.
In conjunction with entering into consulting agreements with scientists and other advisors, the Company has issued warrants to purchase 349 shares of common stock of the Company, of which 35 and none remain outstanding as of December 31, 2012 and 2013. During 2000, the Company issued warrants to purchase 92 shares of its common stock with an exercise price of $1,357.62 per share, of which 23 were exercised in June 2001 and warrants to purchase 69 shares terminated in January 2010. In 2001, warrants to purchase 187 shares of its common stock with exercise prices ranging from $1,357.62 to $2,443.74 per share were issued all of which expired in 2011. During 2002, the Company issued warrants to purchase nine shares of its common stock with exercise prices ranging from $135.78 to $2,443.74 per share of which warrants to purchase seven shares were cancelled in March 2008. During 2003, the Company issued warrants to purchase 59 shares of its common stock with an exercise price of $2,443.74 per share of which warrants to purchase 23 shares were later terminated in 2005. As of December 31, 2012, the warrants are fully vested. The Company determines the estimated fair value of the warrants using the Black-Scholes valuation model and recognizes the compensation expense over the vesting period.
F-27
Table of Contents
In connection with the issuance and sale of the Company’s series E preferred stock, the Company issued warrants to the purchasers of its series E preferred stock to purchase an aggregate of 511,754 shares of its common stock at an exercise price of $0.06 per share. The warrants had a term of 10 years and would only become exercisable if the Company did not receive $5,000,000 in non-dilutive financing by December 31, 2013 or had not executed definitive documentation providing for an additional $5,000,000 investment of non-dilutive financing as of December 31, 2013. In connection with the license agreement that the Company entered into with Medinet in December 2013, as described in Note 15, Medinet paid the Company $1.0 million and loaned the Company $9.0 million. In connection with the receipt of these funds, the warrants were cancelled pursuant to their terms.
In November 2013, the Company entered into an agreement with Pharmstandard under which Pharmstandard purchased additional shares of the Company’s series E preferred stock. Under this agreement, the Company agreed to enter into a manufacturing rights agreement for the European market with Pharmstandard and that the manufacturing rights agreement would provide for the issuance of warrants to Pharmstandard to purchase 499,788 shares of the Company’s common stock at an exercise price of $5.82 per share. As of February 28, 2014, the Company had not entered into this manufacturing rights agreement or issued the warrants.
In connection with the issuance and sale of series E preferred stock, in December 2013, the Company issued a warrant to purchase 9,598 shares of its common stock, at an exercise price of $6.60 per share, to a placement agent.
Outstanding warrants to purchase the Company’s preferred and common stock as of December 31, 2012 are as follows:
| Type of Warrant |
Number of Warrants | Exercise Price | Expiration Date(s) | |||||||||
| Series D-1 Preferred |
1,332,622 | $ | 0.01 | 8/31/22 | ||||||||
| Series C Preferred |
32,869,928 | $ | 0.01 | 9/9/17; 12/15/17 | ||||||||
| Common |
35 | $ | 2,443.74 | 2/5/13 | ||||||||
| Common |
1 | $ | 23,894.34 | 7/13/16 | ||||||||
Outstanding warrants to purchase the Company’s common stock as of December 31, 2013 are as follows. There were no warrants to purchase preferred stock as of December 31, 2013.
| Type of Warrant |
Number of Warrants | Exercise Price | Expiration Date(s) |
|||||||||
| Common |
1 | $ | 23,894.34 | 7/13/16 | ||||||||
| Common |
9,598 | $ | 6.60 | 12/20/23 | ||||||||
12. Stock Options
During December 1999, the Company adopted the Argos Therapeutics, Inc. 1999 Stock Option Plan (the “1999 Plan”), which provides for the granting of options to purchase up to 174 shares of its common stock to employees, directors and consultants of the Company. During April 2001, the 1999 Plan was amended to allow for the granting of options to purchase up to 255 shares of its common stock and during August 2001, the 1999 Plan was further amended to allow for the granting of options to purchase up to 499 shares of its common stock. Effective January 2007, the 1999 Plan was amended once more to allow for the granting of options to purchase up to 751 shares of its common stock. During February 2008, the Company adopted the Argos Therapeutics, Inc. 2008 Stock Incentive Plan (the “2008 Plan”) which provides for the granting of options to purchase up to 201,278 shares of its common stock to employees, directors and consultants of the Company. All options held by employees and directors of the Company under the 1999 Plan were cancelled upon issuance of options to those same employees and directors under the 2008 Plan. Under the 1999 Plan, options to purchase eight shares of its common stock remain outstanding as of December 31, 2012 and 2013. During December 2008, the 2008 Plan was amended to allow for the granting of options to purchase up to 277,047 shares of its common stock. Under the 2008 Plan, both incentive and nonqualified stock options may be granted. The Board of Directors shall determine the exercise price, term and dates of the exercise of all options as of their grant date. The Board of Directors, after consideration of an independent appraisal, determines the estimated fair value of the common stock. Under the 2008 Plan, some options vested immediately upon grant and some become vested over variable periods, generally ranging from two to four years, and expire not more than ten years after the date of grant.
As of December 31, 2011, substantially all of the Company’s employee stock option grants outstanding had exercise prices ranging from $10.86 to $36.66 per share. On March 31, 2012, the Board of Directors approved the repricing of stock options to purchase approximately 201,000 shares of the Company’s common stock at an exercise price of $4.20 per share. The revaluation of this modification of the exercise price of employee stock options did not result in additional stock compensation expense.
F-28
Table of Contents
The Company recorded the following stock-based compensation expense:
| Years Ended December 31, | Cumulative Period from May 8, 1997 (Inception) to December 31, |
|||||||||||||||
| 2011 | 2012 | 2013 | 2013 | |||||||||||||
| Research and development |
$ | 168,321 | $ | 511,860 | $ | 524,495 | $ | 2,044,503 | ||||||||
| General and administrative |
258,171 | 531,129 | 528,668 | 2,094,910 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total stock-based compensation expense |
$ | 426,492 | $ | 1,042,989 | $ | 1,053,163 | $ | 4,139,413 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
Allocations to research and development and general and administrative expense are based upon the department to which the associated employee reported. No related tax benefits of the stock-based compensation expense have been recognized. Stock-based payments issued to nonemployees are recorded at their fair values, and are periodically revalued as the equity instruments vest and are recognized as expense over the related service period.
All employee stock options were granted at an exercise price equal to or above the fair value of the stock as of the date of grant in 2008 and 2009. In December 2010, February 2011 and March 2011, the Company granted options to employees with exercise prices of $10.86 per share, which was less than the deemed fair value of $20.34 per share as determined on December 10, 2010, resulting in an intrinsic value of $9.48 per share. The weighted average fair value as of the date of grant for options granted during the years ended December 31, 2012 and 2013 was $2.94 and $4.77.
In September 2011, the Company granted to a consultant options to purchase 14,731 shares of the Company’s common stock, of which 13,504 were subsequently cancelled in 2012, at an exercise price of $35.28 per share which was the deemed fair value per share of common stock as of that date. The options vest upon the achievement of specified milestones. As of December 31, 2012 and 2013, options to purchase 1,227 shares had vested, resulting in consulting expense of $43,333 and $0 during the years ended December 31, 2012 and 2013.
In December 2011, the Company granted to two consultants options to purchase 44 and 220 shares, respectively, of the Company’s common stock at an exercise price of $36.66 per share which was deemed the fair value per share of common stock as of that date. As of December 31, 2011 the grant of the option to purchase 44 shares was fully vested, resulting in consulting expense of $1,632. As of December 31, 2012 and 2013, 91 shares of the option to purchase 220 shares were vested, resulting in consulting expense of $277 and $0 in the years ended December 31, 2012 and 2013. The remaining 129 shares vest in increments of 18 shares for each day of service performed.
In April 2012, the Company granted to a consultant options to purchase 180 shares of the Company’s common stock at an exercise price of $4.20 per share which was deemed the fair value per share of common stock as of that date. As of December 31, 2012 and 2013, 75 shares were vested, resulting in consulting expense of $284 and $0 during the years ended December 31, 2012 and 2013.
During August 2013, the Company issued 7,571 shares of restricted stock to a consultant. The shares had an estimated fair value of $4.10 per share, which was recorded as $14,550 and $16,500 of general and administrative expense during the years ended December 31, 2012 and December 31, 2013, respectively.
Valuation Assumptions for Stock Option Plans
The employee stock-based compensation expense recognized was determined using the Black-Scholes option valuation model. Option valuation models require the input of subjective assumptions and these assumptions can vary over time. The weighted average assumptions used are as follows:
| 2011 | 2012 | 2013 | ||||||||||
| Risk-free interest rate |
2.17 | % | 1.20 | % | 2.12 | % | ||||||
| Dividend yield |
0 | % | 0 | % | 0 | % | ||||||
| Expected option term (in years) |
7 | 7 | 7 | |||||||||
| Volatility |
91 | % | 94 | % | 94 | % | ||||||
The risk-free interest rate is based on the U.S. Treasury yield curve in effect on the date of grant. The dividend yield percentage is zero because the Company neither currently pays dividends nor intends to do so during the expected option term. The expected option term represents the estimated period of time until exercise and is based on historical experience of similar awards, giving consideration to
F-29
Table of Contents
the contractual terms, vesting schedules and expectations of future employee behavior. Expected stock price volatility is based on an average of several peer public companies. For purposes of identifying peer companies, the Company considered characteristics such as industry, length of trading history, similar vesting terms and in the-money option status. Upon the adoption of ASC 718, the Company was also required to estimate the level of forfeitures expected to occur and record compensation expense only for those awards that ultimately expect to vest. The Company performed a historical analysis of option awards that were forfeited prior to vesting and recorded total stock option expense that reflected this estimated forfeiture rate.
The following table summarizes the Company’s stock option activity:
| Number of Shares |
Weighted Average Exercise Price |
Weighted Average Contractual Term (in years) |
Aggregate Intrinsic Value |
|||||||||||||
| Outstanding as of December 31, 2012 |
559,352 | $ | 4.20 | |||||||||||||
| Granted |
1,405,793 | $ | 5.84 | |||||||||||||
| Exercised |
(1,378 | ) | $ | 4.06 | ||||||||||||
| Cancelled |
(6,698 | ) | $ | 2.79 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Outstanding as of December 31, 2013 |
1,957,069 | $ | 5.48 | 8.17 | $ | 3,601,007 | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Exercisable as of December 31, 2013 |
438,435 | $ | 4.32 | 7.44 | $ | 1,315,305 | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Vested and expected to vest as of December 31, 2013 |
1,840,123 | $ | 5.44 | 9.19 | $ | 3,459,431 | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
The aggregate intrinsic value of stock options in the table above represents the difference between the estimated fair value of our common stock as of December 31, 2013 and the exercise price of outstanding, exercisable, and vested and expected to vest in-the-money stock options.
The following table summarizes information about the Company’s stock options as of December 31, 2013:
| Exercise Price |
Options Outstanding | Weighted Average Contractual Life (Years) |
Options Exercisable | |||||||||
| $4.20 |
574,180 | 7.45 | 435,525 | |||||||||
| $5.82 |
1,253,324 | 9.84 | — | |||||||||
| $7.32 |
126,666 | 9.97 | — | |||||||||
| $10.86 |
1,628 | 4.64 | 1,628 | |||||||||
| $35.30 |
1,227 | 7.68 | 1,227 | |||||||||
| $36.66 |
44 | 7.95 | 44 | |||||||||
|
|
|
|
|
|
|
|||||||
| 1,957,069 | 8.17 | 438,424 | ||||||||||
|
|
|
|
|
|
|
|||||||
As of December 31, 2013, the Company had a total of $7,010,676 in unrecognized compensation expense from nonvested stock option awards, of which $2,319,543 is expected to be recognized in 2014, $1,767,212 in 2015, $1,609,673 in 2016, and $1,314,248 in 2017.
13. Revenue
In March 2004, the Company entered into a license agreement with Geron Corporation (“Geron”) under which the Company licensed to Geron co-exclusive rights with the Company to the use of the Company’s platform technology with defined antigens in exchange for 5,000,000 shares of Geron common stock which were valued at $42,775,697. As part of the licensing transaction, the Company granted to Geron rights to both the Company’s existing intellectual property as well as certain improvements to the intellectual property during the first three years following the effective date of the agreement. The licensing revenue was recognized on a straight-line basis over the period of performance, which was three years. During 2005, the Company was awarded a grant of $499,918 from the Alliance for Lupus Research. The Company received notification during 2006 that this grant was extended for an additional $499,527. Revenue under this contract was recognized as the Company performed the related services, as there were no other performance obligations of the Company. As such, the Company recorded these grants as revenue during the years ended December 31, 2005 and 2006. As of December 31, 2006, all obligations under the grant had been satisfied.
F-30
Table of Contents
In February 2006, the Company entered into a license agreement with Novo Nordisk A/S (“Novo”) under which Novo acquired antibody technology for the research and development of a treatment for systemic immune disorders, including systemic lupus erythematosus. The Company was paid and recorded revenue of $4,000,000 during 2006 when the license agreement was signed with Novo, as there were no other performance obligations of the Company. The Company received an additional $1,000,000 and $2,000,000 in 2008 and 2009, respectively, in accordance with the terms of the agreement when nonrefundable cash payments were received related to milestones achieved by Novo. Under the terms of the Novo agreement, the Company had no performance obligations and accordingly revenue was recorded upon receipt. Novo terminated the license agreement effective June 2010. As a result, the Company regained the rights to the acquired antibody technology.
In September 2006, the Company entered into a multi-year research contract with the NIH and the National Institute of Allergy and Infectious Diseases, (“NIAID”), to design, develop and clinically test an autologous HIV immunotherapy capable of eliciting therapeutic immune responses. The Company is using funds from this contract to develop AGS-004. Under this contract, as amended, the NIH and NIAID have committed to fund up to a total of $39.3 million, including reimbursement of direct expenses and allocated overhead and general and administrative expenses of up to $32.5 million and payment of other specified amounts totaling up to $1.4 million upon the Company’s achievement of specified development milestones. Since September 2010, the Company has received reimbursement of its allocated overhead and general and administrative expenses at provisional indirect cost rates equal to negotiated provisional indirect cost rates agreed to with the NIH in September 2010. These provisional indirect cost rates are subject to adjustment based on the Company’s actual costs pursuant to the agreement with the NIH. This commitment originally extended until May 2013. The Company agreed to an additional modification of the Company’s contract with the NIH and the NIAID under which the NIH and the NIAID agreed to increase their funding commitment to the Company by an additional $5.4 million in connection with the extension of the contract from May 2013 to September 2015, increasing the committed funds to $39.3 million. The Company has agreed to a statement of work under the contract, and are obligated to furnish all the services, qualified personnel, material, equipment, and facilities, not otherwise provided by the U.S. government, needed to perform the statement of work.
For the years ended December 31, 2012 and 2013, the Company recorded revenue under this agreement of $7,032,760 and $4,421,689. As of December 31, 2012 and 2013, the Company recorded a receivable from the NIH of $979,487 and $424,501, and a payable to subcontractors of $209,350 and $27,925.
The Company entered into an exclusive option agreement with Therakos, Inc. in December 2006 and received an option fee of $200,000 for granting Therakos the option to explore the use and development of certain intellectual property. As there were no performance obligations under the option agreement, the Company recognized revenue upon payment during the year ended December 31, 2006. On September 18, 2007, the Company granted to Therakos, Inc. an exclusive license to this intellectual property pursuant to a license agreement and received a license fee of $800,000. As there were no performance obligations under the license agreement, the Company recognized revenue upon payment during the year ended December 31, 2007.
The Company entered into a manufacturing agreement in November 2009 with Yale University’s School of Medicine to manufacture autologous mature dendritic cells. Under the terms of the agreement, the Company has the potential to earn $281,740. For the years ended December 31, 2011 and 2012, the Company recorded revenue under this agreement of $39,945 and $0, respectively. As of April 2011, the Company is no longer providing manufacturing services under this agreement. Revenue under this arrangement was recorded as services were performed as there were no other performance obligations of the Company.
14. License Agreement
In July 2011, the Company entered into an agreement with Celldex Therapeutics, Inc. (“Celldex”), pursuant to which Celldex granted the Company a nonexclusive license to specified patents and patent applications regarding actions necessary or helpful for processing dendritic cells. Upon the execution of the agreement, the Company paid Celldex $50,000 of a $100,000 up front license fee. The Company paid the balance of this fee on January 31, 2012. Under this agreement, the Company is required to pay:
| • | a $75,000 annual license fee; |
| • | a specified milestone payment based on the achievement of a specified regulatory milestone; and |
| • | a specified dollar amount per dose of AGS-003 the Company sells. |
The agreement will terminate on a country-by-country basis upon the expiration of the last to expire of the patent rights licensed under the agreement in a country. The latest date of expiration of the licensed Celldex patents is 2016.
F-31
Table of Contents
15. Collaboration Agreements
The Company entered into a Collaboration and License Agreement with Kirin in June 2004 for the development of therapies using the Company’s monocyte-derived dendritic cell technology. The Company recognized a net reimbursement of $6,626,989, $0 and $47,179,130 of research and development costs for the years ended December 31, 2009, 2010 and for the cumulative period from May 8, 1997 (date of inception) to December 31, 2013, respectively. Simultaneous with entering into the agreement, Kirin invested $5,000,000 in series B-1 preferred stock of the Company. In addition, the Company issued a warrant to Kirin to purchase 1,704,546 shares of series B-1 preferred stock at $1.76 per share (Note 11). These warrants were cancelled on March 31, 2008. The agreement was terminated effective December 31, 2009. The Company elected to continue the programs independently.
Pharmstandard License Agreement
In August 2013, the Company entered into an exclusive royalty-bearing license agreement with Pharmstandard. Under this license agreement, the Company granted Pharmstandard and its affiliates a license, with the right to sublicense, to develop, manufacture and commercialize AGS-003 and other products for the treatment of human diseases, which are developed by Pharmstandard using the Company’s personalized immunotherapy platform, in the Russian Federation, Armenia, Azerbaijan, Belarus, Georgia, Kazakhstan, Kyrgyzstan, Moldova, Tajikistan, Turkmenistan, Ukraine and Uzbekistan, which the Company refers to as the Pharmstandard Territory. The Company also provided Pharmstandard with a right of first negotiation for development and commercialization rights in the Pharmstandard Territory to specified additional products the Company may develop.
Under the terms of the license agreement, Pharmstandard licensed the Company rights to clinical data generated by Pharmstandard under the agreement and granted the Company an option to obtain an exclusive license outside of the Pharmstandard Territory to develop and commercialize improvements to the Company’s Arcelis technology generated by Pharmstandard under the agreement, a non-exclusive worldwide royalty-free license to Pharmstandard improvements to manufacture products using the Company’s Arcelis technology and a license to specified follow-on licensed products generated by Pharmstandard outside of the Pharmstandard Territory, each on terms to be negotiated upon the Company’s request for a license. In addition, Pharmstandard agreed to pay the Company pass-through royalties on net sales of all licensed products in the low single digits until it has generated a specified amount of aggregate net sales. Once the net sales threshold is achieved, Pharmstandard will pay the Company royalties on net sales of specified licensed products, including AGS-003, in the low double digits below 20%. These royalty obligations last until the later of the expiration of specified licensed patent rights in a country or the twelfth anniversary of the first commercial sale in such country on a country by country basis and no further royalties on specified other licensed products. After the net sales threshold is achieved, Pharmstandard has the right to offset a portion of the royalties Pharmstandard pays to third parties for licenses to necessary third party intellectual property against the royalties that Pharmstandard pays to the Company.
The agreement will terminate upon expiration of the royalty term, upon which all licenses will become fully paid up perpetual exclusive licenses. Either party may terminate the agreement for the other party’s uncured material breach or if specified conditions occur relating to the other party’s insolvency or bankruptcy and the Company may terminate the agreement if Pharmstandard challenges or assists a third party in challenging specified patent rights of ours. If Pharmstandard terminates the agreement upon the Company’s material breach or bankruptcy, Pharmstandard is entitled to terminate the Company’s licenses to improvements generated by Pharmstandard, upon which the Company may come to rely for the development and commercialization of AGS-003 and other licensed products outside of the Pharmstandard Territory, and Pharmstandard is entitled to retain its licenses from the Company and to pay the Company substantially reduced royalty payments following such termination.
In November 2013, the Company entered into an agreement with Pharmstandard under which Pharmstandard purchased additional shares of the Company’s series E preferred stock. Under this agreement, the Company agreed to enter into a manufacturing rights agreement for the European market with Pharmstandard and that the manufacturing rights agreement would provide for the issuance of warrants to Pharmstandard to purchase 499,788 shares of the Company’s common stock at an exercise price of $5.82 per share. As of February 28, 2014, the Company had not entered into this manufacturing rights agreement or issued the warrants.
Green Cross License Agreement
In July 2013, the Company entered into an exclusive royalty-bearing license agreement with Green Cross. Under this agreement the Company granted Green Cross a license to develop, manufacture and commercialize AGS-003 for mRCC in South Korea. The Company also provided Green Cross with a right of first negotiation for development and commercialization rights in South Korea to specified additional products the Company may develop.
F-32
Table of Contents
Under the terms of the license, Green Cross has agreed to pay the Company $500,000 upon the initial submission of an application for regulatory approval of a licensed product in South Korea, $500,000 upon the initial regulatory approval of a licensed product in South Korea and royalties ranging from the mid-single digits to low double digits below 20% on net sales until the fifteenth anniversary of the first commercial sale in South Korea. In addition, Green Cross has granted the Company an exclusive royalty free license to develop and commercialize all Green Cross improvements to the Company’s licensed intellectual property in the rest of the world, excluding South Korea, except that, as to such improvements for which Green Cross makes a significant financial investment and that generate significant commercial benefit in the rest of the world, the Company is required to negotiate in good faith a reasonable royalty that the Company will be obligated to pay to Green Cross for such license. Under the terms of the agreement, the Company is required to continue to develop and to use commercially reasonable efforts to obtain regulatory approval for AGS-003 in the United States
The agreement will terminate upon expiration of the royalty term, which is 15 years from the first commercial sale, upon which all licenses will become fully paid up perpetual non-exclusive licenses. Either party may terminate the agreement for the other party’s uncured material breach or if specified conditions occur relating to the other party’s insolvency or bankruptcy and the Company may terminate the agreement if Green Cross challenges or assists a third party in challenging specified patent rights of ours. If Green Cross terminates the agreement upon the Company’s material breach or bankruptcy, Green Cross is entitled to terminate the Company’s licenses to improvements and retain its licenses from the Company and to pay the Company substantially reduced milestone and royalty payments following such termination.
Medinet License Agreement
In December 2013, the Company entered into a license agreement with Medinet. Under this agreement, the Company granted Medinet an exclusive, royalty-free license to manufacture in Japan AGS-003 and other products using the Company’s Arcelis technology solely for the purpose of the development and commercialization of AGS-003 and these other products for the treatment of mRCC. The Company refers to this license as the manufacturing license. In addition, under this agreement, the Company granted Medinet an option to acquire a nonexclusive, royalty-bearing license under the Company’s Arcelis technology to sell in Japan AGS-003 and other products for the treatment of mRCC. The Company refers to the option as the sale option and the license as the sale license.
Under the manufacturing license, if Medinet does not exercise the sale option, Medinet may only manufacture AGS-003 and these other products for the Company or its designee. If Medinet does not exercise the sale option, the Company and Medinet have agreed to negotiate in good faith a supply agreement under which Medinet would supply the Company or its designee with AGS-003 and these other products for development and sale for the treatment of mRCC in Japan. If Medinet exercises the sale option, it may only manufacture AGS-003 and these other products for itself, its related parties and its sublicensees. During the term of the manufacturing license, the Company may not manufacture AGS-003 or these other products for the Company or any designee for development or sale for the treatment of mRCC in Japan.
Medinet may exercise the option at any time until the earlier of December 31, 2015 and the date 30 days after the Company has provided Medinet with an interim report on the Company’s phase 3 clinical trial of AGS-003 following such time as 50% of the required events in the trial have occurred.
In consideration for the manufacturing license, Medinet paid the Company $1.0 million. Medinet also loaned the Company $9.0 million in connection with the Company entering into the agreement. The Company has agreed to use these funds in the development and manufacturing of AGS-003 and the other products. Medinet also agreed to pay the Company milestone payments of up to a total of $9.0 million upon the achievement of developmental and regulatory milestones and $5.0 million upon the achievement of a sales milestone related to AGS-003 and these products. If Medinet exercises the sale option, it will pay the Company $1.0 million, as well as royalties on net sales at a rate to be negotiated until the later of the expiration of the licensed patent rights in Japan and the twelfth anniversary of the first commercial sale in Japan. If the Company and Medinet cannot agree on the royalty rate, the Company and Medinet have agreed to submit the matter to arbitration.
The Company borrowed the $9.0 million pursuant to an unsecured promissory note that bears interest at a rate of 3.0% per annum. The principal and interest under the note are due and payable on December 31, 2018. Under the terms of the note and the license agreement, any milestone payments related to the developmental and regulatory milestones that become due will be applied first to the repayment of the loan. The Company has the right to prepay the loan at any time. If the Company has not repaid the loan by December 31, 2018, then the Company has agreed to grant to Medinet a non-exclusive, royalty-bearing license to make and sell Arcelis products in Japan for the treatment of cancer. In such event, the amounts owing under the loan as of December 31, 2018 will constitute pre-paid royalties under the license and will not be otherwise due and payable. Royalties under this license would be paid until the expiration of the licensed patent rights in Japan at a rate to be negotiated. If the Company and Medinet cannot agree on the royalty rate, Company and Medinet have agreed to submit the matter to arbitration. The Company recorded the $1.0 million payment from Medinet as a deferred liability. In addition, because the $9.0 million promissory note was issued at a below market interest rate, the Company allocated the proceeds of the loan between the manufacturing license and the debt at the time of issuance. As a result, the Company recorded $6.9 million as notes payable, based upon an effective interest rate of 8.0%, and $2.1 million as a deferred liability. The total deferred liability recorded related to the manufacturing license was $3.1 million as of December 31, 2013.
The agreement will terminate upon expiration of the royalty term, upon which all licenses will become fully paid up, perpetual non-exclusive licenses. Either party may terminate the agreement for the other party’s uncured material breach or if specified conditions occur relating to the other party’s insolvency or bankruptcy, and the Company may terminate the agreement if Medinet challenges or assists a third party in challenging specified patent rights of the Company. If Medinet terminates the agreement upon the Company’s material breach or bankruptcy, Medinet is entitled to terminate the Company’s licenses to improvements and retain its royalty-bearing licenses from the Company.
F-33
Table of Contents
16. Commitments
The Company rents laboratory and office space and equipment under operating leases that expire in various years through 2016. Future minimum lease payments under noncancelable operating leases as of December 31, 2013 are as follows:
| Years Ending December 31, |
||||
| 2014 |
$ | 314,484 | ||
| 2015 |
257,443 | |||
| 2016 |
284,433 | |||
|
|
|
|||
| Total minimum lease payments |
$ | 856,360 | ||
|
|
|
On June 21, 2011, the Company signed a lease agreement to renew its rented laboratory and office space through 2016. The amount of obligation associated with this operating lease agreement over the term of the lease is $1,488,116.
Rent expense related to operating leases for the years ended December 31, 2011, 2012 and 2013, and the period from inception (May 8, 1997) through December 31, 2013 was $360,362, $361,356, $348,191 and $4,094,895, respectively.
The Company has entered into various licensing agreements with universities and other research institutions under which the Company receives substantially all rights of the inventors or co-assignee to produce and market technology protected by certain patents and patent applications. The Company also entered into various assignment agreements with a scientist under which the Company receives exclusive rights to produce and market technology protected by certain patents and patent applications.
The Company is generally required to make royalty payments ranging from 1% to 4 % of future sales of products employing the technology or falling under claims of a patent. If future sales require the use of technology licensed from multiple different sources, the total royalty rates could be higher. As royalty payments are directly related to future sales volume, future commitments cannot be determined. No accrual for future payments under these agreements has been recorded, as the Company cannot estimate if, when or in what amount payments may become due.
In connection with the Loan and Security Agreement with two lending institutions in April 2007, the Company is required to pay a success fee of $200,000 upon consummation of a liquidity event, including an IPO. This was paid in March 2014 upon the successful completion of the Company’s IPO.
17. Concentration of Credit Risk
The Company’s grant revenue is earned under a contract with NIH. The concentration of credit risk is equal to the outstanding accounts receivable and unbilled balances and such risk is subject to the credit worthiness of the NIH. There have been no credit losses under this arrangement.
18. Employee Benefit Plan
The Company provides a retirement plan qualified under section 401(k) of the Internal Revenue Code of 1986, as amended (“IRC”). Participants may elect to contribute a portion of their annual compensation to the plan, after complying with certain limitations set by the IRC. All employees are eligible to participate in the plan after attaining the age of 21. The Company matched 25% of the first 6% contributed by eligible participants in the plan during the years ended December 31, 2011, 2012 and 2013, or $61,931, $71,571 and $101,700, respectively, for a total of $625,297 for the period from inception (May 8, 1997) through December 31, 2013.
19. Net Loss Per Share
Basic and diluted net loss per common share was determined by dividing net loss attributable to common stockholders by the weighted average common shares outstanding during the period. The Company’s potentially dilutive shares, which include redeemable convertible preferred stock, common stock options and warrants, have not been included in the computation of diluted net loss per share for all periods as the result would be antidilutive.
F-34
Table of Contents
The following table presents the computation of basic and diluted net loss per share of common stock:
| Year Ended December 31, | ||||||||||||
| 2011 | 2012 | 2013 | ||||||||||
| As Reported |
||||||||||||
| Net loss attributable Argos Therapeutics, Inc. |
$ | (20,077,682 | ) | $ | (10,471,330 | ) | $ | (23,921,563 | ) | |||
| Accretion of redeemable convertible preferred stock |
(926,542 | ) | (351,371 | ) | (884,647 | ) | ||||||
| Reverse prior accretion on redeemable preferred stock due to reduction in liquidation value of Series A, B, and C |
5,657,638 | |||||||||||
| Preferred stock dividend due to exchanges of preferred shares |
(14,726,088 | ) | ||||||||||
|
|
|
|
|
|
|
|||||||
| Net loss attributable to common stockholders |
$ | (21,004,224 | ) | $ | (10,822,701 | ) | $ | (33,874,660 | ) | |||
|
|
|
|
|
|
|
|||||||
| Weighted average common shares outstanding, basic and diluted |
106,466 | 198,306 | 229,865 | |||||||||
|
|
|
|
|
|
|
|||||||
| Net loss per share attributable to common stockholders, basic and diluted |
$ | (197.29 | ) | $ | (54.58 | ) | $ | (147.37 | ) | |||
|
|
|
|
|
|
|
|||||||
The following potentially dilutive securities have been excluded from the computation of diluted weighted average shares outstanding, as they would be antidilutive:
| Year Ended December 31, | ||||||||||||
| 2011 | 2012 | 2013 | ||||||||||
| Redeemable convertible preferred stock |
1,037,253 | 2,167,143 | 6,322,747 | |||||||||
| Stock options outstanding |
181,390 | 366,389 | 767,510 | |||||||||
| Warrants outstanding |
191,260 | 316,184 | 424,961 | |||||||||
20. Selected Quarterly Data (unaudited)
| Quarter Ended | ||||||||||||||||
| March 31, 2013 |
June 30, 2013 |
September 30, 2013 |
December 31, 2013 |
|||||||||||||
| Revenue |
$ | 1,461,687 | $ | 1,263,008 | $ | 981,247 | $ | 715,747 | ||||||||
| Operating expenses: |
||||||||||||||||
| Research and development |
5,189,461 | 6,102,320 | 5,630,219 | 7,069,151 | ||||||||||||
| General and administrative |
1,078,357 | 939,725 | 1,024,167 | 1,620,068 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Operating loss |
(4,806,131 | ) | (5,779,037 | ) | (5,673,139 | ) | (7,973,472 | ) | ||||||||
| Other income (expense), net |
356,929 | 196 | 541 | (47,450 | ) | |||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net loss attributable to Argos Therapeutics, Inc. |
(4,449,202 | ) | (5,778,841 | ) | (5,672,598 | ) | (8,020,922 | ) | ||||||||
| Accretion of redeemable convertible preferred stock |
(64,940 | ) | (10,446 | ) | 5,325,406 | (477,029 | ) | |||||||||
| Less: Preferred stock dividend due to exchanges of preferred shares |
— | — | (14,726,088 | ) | — | |||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net loss attributable to common stockholders |
$ | (4,514,142 | ) | $ | (5,789,287 | ) | $ | (15,073,280 | ) | $ | (8,497,951 | ) | ||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net loss attributable to common stockholders per share, basic and diluted |
$ | (19.91 | ) | $ | (25.53 | ) | $ | (65.23 | ) | $ | (36.19 | ) | ||||
|
|
|
|
|
|
|
|
|
|||||||||
| Weighted average shares outstanding, basic and diluted |
226,757 | 226,757 | 231,084 | 234,789 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
F-35
Table of Contents
| Quarter Ended | ||||||||||||||||
| March 31, 2012 |
June 30, 2012 |
September 30, 2012 |
December 31, 2012 |
|||||||||||||
| Revenue |
$ | 2,064,527 | $ | 1,797,727 | $ | 1,639,072 | $ | 1,537,684 | ||||||||
| Operating expenses: |
||||||||||||||||
| Research and development |
3,294,943 | 4,845,620 | 4,815,492 | 4,660,837 | ||||||||||||
| General and administrative |
3,088,427 | 1,287,430 | 806,845 | 952,879 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Operating loss |
(4,318,843 | ) | (4,335,323 | ) | (3,983,265 | ) | (4,076,032 | ) | ||||||||
| Other income (expense), net |
(357,445 | ) | 4,865,605 | 768 | 1,733,205 | |||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net loss attributable to Argos Therapeutics, Inc. |
(4,676,288 | ) | 530,282 | (3,982,497 | ) | (2,342,827 | ) | |||||||||
| Accretion of redeemable convertible preferred stock |
(80,325 | ) | (104,515 | ) | (81,563 | ) | (84,968 | ) | ||||||||
| Less: Preferred stock dividend due to exchanges of preferred shares |
— | — | — | — | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net loss attributable to common stockholders |
$ | (4,756,613 | ) | $ | 425,767 | $ | (4,064,060 | ) | $ | (2,427,795 | ) | |||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net loss attributable to common stockholders per share, basic and diluted |
$ | (38.88 | ) | $ | 1.96 | $ | (17.92 | ) | $ | (10.70 | ) | |||||
|
|
|
|
|
|
|
|
|
|||||||||
| Weighted average shares outstanding, basic and diluted |
122,345 | 217,551 | 226,839 | 226,839 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
21. Subsequent Events
Initial Public Offering and Conversion of Redeemable Convertible Preferred Stock into Common Stock
In February 2014, we issued and sold 6,228,725 shares of our common stock, including 603,725 shares of common stock sold pursuant to the underwriters’ exercise of their option to purchase additional shares, in our IPO at a public offering price of $8.00 per share. The total net proceeds raised were $43.7 million after deducting underwriting discounts and commissions of $3.5 million and other offering expenses of approximately $2.6 million.
Prior to the initial public offering, the Company had outstanding 1,040,216 shares designated as Series A redeemable convertible preferred stock, 9,803,688 shares designated as Series B redeemable convertible preferred stock, 28,716,679 shares designated as Series C redeemable convertible preferred stock, 21,040,817 shares designated as Series D redeemable convertible preferred stock and 56,011,258 shares designated as Series E redeemable convertible preferred stock. Upon the closing of the initial public offering on February 12, 2014, all of the outstanding shares of redeemable convertible preferred stock automatically converted into 13,188,251 shares of the Company’s common stock.
Approval of 2014 Stock Incentive Plan and 2014 Employee Stock Purchase Plan
In January 2014, the Company’s board of directors and stockholders approved, effective upon the closing of the Company’s IPO, the 2014 Stock Incentive Plan (the “2014 Plan”). Under the 2014 Plan, the Company may grant incentive stock options, nonstatutory stock options, stock appreciation rights, restricted stock, restricted stock units and other stock-based awards for the purchase of that number of shares of Common Stock equal to the sum of 1,951,182 shares, plus such number of shares, up to 357,841 shares, as is equal to the sum of the number of shares reserved for issuance under the Company’s 2008 stock incentive plan (the “2008 Plan”) that remain available for grant under the 2008 Plan immediately prior to the closing of the Company’s IPO on February 12, 2014 and the number of shares subject to outstanding awards under the 2008 Plan that expire, terminate or are otherwise surrendered, cancelled, forfeited or repurchased by the Company at their original issuance price pursuant to a contractual repurchase right, plus an annual increase, to be added on the first day of the 2015 fiscal year and each subsequent anniversary through January 1, 2024, equal to the lowest of 2,309,023 shares of Common Stock, 4% of the number of the Company’s outstanding shares on the first day of each such fiscal year and an amount determined by the Company’s board of directors.
Also in January 2014, the Company’s board of directors and stockholders approved, effective upon the closing of the Company’s IPO, a 2014 Employee Stock Purchase Plan (the “2014 ESPP”). Under the 2014 ESPP, on the offering commencement date of each plan period, the Company will grant to each eligible employee who is then a participant in the 2014 ESPP an option to purchase shares of Common Stock. The employee may authorize up to a maximum of 10% of his or her base pay to be deducted by the Company during the purchase plan period. Each employee who continues to be a participant in the 2014 ESPP on the last business day of the purchase plan period is deemed to have exercised the option, to the extent of accumulated payroll deductions within the 2014 ESPP ownership limits. Under the terms of the 2014 ESPP, the option exercise price shall be determined by the Company’s board of directors for each purchase plan period and the option exercise price will be at least 85% of the applicable closing price of the Common Stock.
F-36
Table of Contents
Schedule II—Valuation and Qualifying Accounts
Deferred Tax Asset Valuation Allowance
Information presented below is in thousands:
| Additions | ||||||||||||||||||||
| Balance at Beginning of Year |
Charged to Expenses (a) |
Charged to Other Accounts |
Increases |
Balance at End of Year |
||||||||||||||||
| Year Ended December 31, 2013 |
$ | 39,134 | $ | 8,811 | $ | — | $ | — | $ | 47,945 | ||||||||||
| Year Ended December 31, 2012 |
$ | 36,515 | $ | 2,619 | $ | — | $ | — | $ | 39,134 | ||||||||||
| Year Ended December 31, 2011 |
$ | 33,230 | $ | 3,285 | $ | — | $ | — | $ | 36,515 | ||||||||||
| (a) | – Impact of providing full valuation allowance against all deferred tax assets since the Company could not assert that it was more likely than not that these deferred tax assets would be realized. |
F-37
