Attached files
| file | filename |
|---|---|
| EX-31.1 - EX-31.1 - AVANIR PHARMACEUTICALS, INC. | d652551dex311.htm |
| EX-31.2 - EX-31.2 - AVANIR PHARMACEUTICALS, INC. | d652551dex312.htm |
| EX-32.1 - EX-32.1 - AVANIR PHARMACEUTICALS, INC. | d652551dex321.htm |
| EXCEL - IDEA: XBRL DOCUMENT - AVANIR PHARMACEUTICALS, INC. | Financial_Report.xls |
Table of Contents
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, DC 20549
FORM 10-Q
(Mark One)
| x | QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934. |
For the quarterly period ended December 31, 2013
OR
| ¨ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934. |
For the transition period from to .
Commission File No. 1-15803
AVANIR PHARMACEUTICALS, INC.
(Exact name of registrant as specified in its charter)
| Delaware | 33-0314804 | |
| (State or other jurisdiction of incorporation or organization) |
(I.R.S. Employer Identification No.) |
| 20 Enterprise Suite 200, Aliso Viejo, California | 92656 | |
| (Address of principal executive offices) | (Zip Code) |
(949) 389-6700
(Registrant’s telephone number, including area code)
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities and Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. YES x NO ¨
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). YES x NO ¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check one):
| Large accelerated filer | ¨ | Accelerated filer | x | |||
| Non-accelerated filer | ¨ (Do not check if a smaller reporting company) | Smaller reporting company | ¨ | |||
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). YES ¨ NO x
As of January 31, 2014, the registrant had 152,296,065 shares of common stock issued and outstanding.
Table of Contents
| Page | ||||||
| Item 1. |
||||||
| 3 | ||||||
| 4 | ||||||
| 5 | ||||||
| 6 | ||||||
| Item 2. |
Management’s Discussion and Analysis of Financial Condition and Results of Operations |
21 | ||||
| Item 3. |
31 | |||||
| Item 4. |
31 | |||||
| Item 1. |
32 | |||||
| Item 1A. |
33 | |||||
| Item 2. |
53 | |||||
| Item 3. |
53 | |||||
| Item 4. |
53 | |||||
| Item 5. |
54 | |||||
| Item 6. |
54 | |||||
| 55 | ||||||
2
Table of Contents
CONDENSED CONSOLIDATED BALANCE SHEETS
| December 31, 2013 | September 30, 2013 | |||||||
| (unaudited) | (audited) | |||||||
| ASSETS | ||||||||
| Current assets: |
||||||||
| Cash and cash equivalents |
$ | 43,897,733 | $ | 55,259,073 | ||||
| Restricted cash and cash equivalents |
1,316,896 | 965,986 | ||||||
| Trade receivables, net of allowances of $207,828 as of December 31, 2013 and September 30, 2013, respectively |
4,199,014 | 12,525,992 | ||||||
| Royalty receivable |
1,425,815 | 542,596 | ||||||
| Other receivables |
2,789,261 | 246,975 | ||||||
| Inventories, net |
605,833 | 710,179 | ||||||
| Prepaid expenses |
1,714,980 | 1,391,210 | ||||||
| Other current assets |
158,606 | 201,629 | ||||||
|
|
|
|
|
|||||
| Total current assets |
56,108,138 | 71,843,640 | ||||||
| Restricted long-term investments |
1,303,938 | 1,303,938 | ||||||
| Property and equipment, net |
2,568,341 | 1,592,791 | ||||||
| Non-current inventories, net |
773,130 | 784,186 | ||||||
| Other assets |
517,069 | 554,452 | ||||||
|
|
|
|
|
|||||
| Total assets |
$ | 61,270,616 | $ | 76,079,007 | ||||
|
|
|
|
|
|||||
| LIABILITIES AND STOCKHOLDERS’ EQUITY | ||||||||
| Current liabilities: |
||||||||
| Accounts payable |
$ | 4,145,275 | $ | 5,876,425 | ||||
| Accrued expenses |
11,836,746 | 11,908,570 | ||||||
| Accrued compensation and payroll taxes |
4,626,727 | 7,775,761 | ||||||
| Deferred royalty revenues |
640,465 | 1,288,514 | ||||||
| Current portion of notes payable, net of debt discount |
10,870,170 | 7,942,945 | ||||||
|
|
|
|
|
|||||
| Total current liabilities |
32,119,383 | 34,792,215 | ||||||
| Other liabilities |
1,611,046 | 1,393,075 | ||||||
| Notes payable, net of current portion and debt discount |
18,596,831 | 21,422,163 | ||||||
|
|
|
|
|
|||||
| Total liabilities |
52,327,260 | 57,607,453 | ||||||
|
|
|
|
|
|||||
| Commitments and contingencies |
||||||||
| Stockholders’ equity: |
||||||||
| Preferred stock - $0.0001 par value, 10,000,000 shares authorized, no shares issued |
— | — | ||||||
| Common stock - $0.0001 par value, 200,000,000 shares authorized; 152,273,131 and 152,063,621 shares issued and outstanding as of December 31, 2013 and September 30, 2013, respectively |
15,227 | 15,206 | ||||||
| Additional paid-in capital |
520,410,555 | 518,992,237 | ||||||
| Accumulated deficit |
(511,482,426 | ) | (500,535,889 | ) | ||||
|
|
|
|
|
|||||
| Total stockholders’ equity |
8,943,356 | 18,471,554 | ||||||
|
|
|
|
|
|||||
| Total liabilities and stockholders’ equity |
$ | 61,270,616 | $ | 76,079,007 | ||||
|
|
|
|
|
|||||
The accompanying notes to condensed consolidated financial statements are an integral part of this statement.
3
Table of Contents
CONDENSED CONSOLIDATED STATEMENTS OF OPERATIONS (UNAUDITED)
| Three Months Ended December 31, | ||||||||
| 2013 | 2012 | |||||||
| Revenues: |
||||||||
| Net product sales |
$ | 23,299,027 | $ | 14,879,262 | ||||
| Revenues from royalties |
2,089,314 | 1,626,010 | ||||||
| Revenues from co-promote activities |
1,358,078 | — | ||||||
| Revenues from research grant services |
— | 15,000 | ||||||
|
|
|
|
|
|||||
| Total revenues |
26,746,419 | 16,520,272 | ||||||
|
|
|
|
|
|||||
| Operating expenses: |
||||||||
| Cost of product sales |
1,292,411 | 838,129 | ||||||
| Cost of research grant services |
— | 9,398 | ||||||
| Research and development |
9,525,320 | 6,648,091 | ||||||
| Selling and marketing |
17,475,404 | 13,522,419 | ||||||
| General and administrative |
8,449,890 | 6,538,403 | ||||||
|
|
|
|
|
|||||
| Total operating expenses |
36,743,025 | 27,556,440 | ||||||
|
|
|
|
|
|||||
| Loss from operations |
(9,996,606 | ) | (11,036,168 | ) | ||||
| Other income (expense): |
||||||||
| Interest income |
4,552 | 19,331 | ||||||
| Interest expense |
(954,895 | ) | (1,059,245 | ) | ||||
| Other, net |
412 | — | ||||||
|
|
|
|
|
|||||
| Net loss |
$ | (10,946,537 | ) | $ | (12,076,082 | ) | ||
|
|
|
|
|
|||||
| Basic and diluted net loss per share |
$ | (0.07 | ) | $ | (0.09 | ) | ||
|
|
|
|
|
|||||
| Basic and diluted weighted average number of common shares outstanding |
152,100,388 | 136,772,557 | ||||||
|
|
|
|
|
|||||
The accompanying notes to condensed consolidated financial statements are an integral part of this statement.
4
Table of Contents
CONDENSED CONSOLIDATED STATEMENTS OF CASH FLOWS (UNAUDITED)
| Three Months Ended December 31, | ||||||||
| 2013 | 2012 | |||||||
| Cash flows from operating activities: |
||||||||
| Net loss |
$ | (10,946,537 | ) | $ | (12,076,082 | ) | ||
| Adjustments to reconcile net loss to net cash used in operating activities: |
||||||||
| Depreciation and amortization |
222,210 | 199,115 | ||||||
| Amortization of debt discount and debt issuance costs |
120,179 | 163,446 | ||||||
| Share-based compensation expense |
1,388,117 | 1,407,008 | ||||||
| Changes in operating assets and liabilities: |
||||||||
| Trade receivables, net |
8,326,978 | 397,737 | ||||||
| Royalty receivable |
(883,219 | ) | (676,435 | ) | ||||
| Other receivables |
(2,542,286 | ) | (45,000 | ) | ||||
| Inventories, net |
115,402 | 37,231 | ||||||
| Prepaid expenses and other assets |
(261,650 | ) | (542,203 | ) | ||||
| Accounts payable |
(1,732,342 | ) | 1,082,716 | |||||
| Accrued expenses and other liabilities |
209,499 | (455,015 | ) | |||||
| Accrued compensation and payroll taxes |
(3,149,034 | ) | (1,698,367 | ) | ||||
| Deferred royalty revenues |
(648,049 | ) | (474,607 | ) | ||||
|
|
|
|
|
|||||
| Net cash used in operating activities |
(9,780,732 | ) | (12,680,456 | ) | ||||
|
|
|
|
|
|||||
| Cash flows from investing activities: |
||||||||
| Purchases of property and equipment |
(1,259,920 | ) | (179,661 | ) | ||||
| Purchases of restricted investments and restricted cash and cash equivalents |
(350,910 | ) | (1,802 | ) | ||||
|
|
|
|
|
|||||
| Net cash used in investing activities |
(1,610,830 | ) | (181,463 | ) | ||||
|
|
|
|
|
|||||
| Cash flows from financing activities: |
||||||||
| Proceeds from exercise of stock options and warrants |
30,222 | 26,131 | ||||||
|
|
|
|
|
|||||
| Net cash provided by financing activities |
30,222 | 26,131 | ||||||
|
|
|
|
|
|||||
| Net change in cash and cash equivalents |
(11,361,340 | ) | (12,835,788 | ) | ||||
| Cash and cash equivalents at beginning of period |
55,259,073 | 69,778,406 | ||||||
|
|
|
|
|
|||||
| Cash and cash equivalents at end of period |
$ | 43,897,733 | $ | 56,942,618 | ||||
|
|
|
|
|
|||||
| Supplemental disclosures of cash flow information: |
||||||||
| Interest paid |
$ | 671,250 | $ | 671,250 | ||||
| Income taxes paid |
$ | 3,200 | $ | 3,200 | ||||
| Supplemental disclosures of non-cash investing and financing activities: |
||||||||
| Purchases of property and equipment in accounts payable and accrued expenses |
$ | 100,500 | $ | — | ||||
The accompanying notes to condensed consolidated financial statements are an integral part of this statement.
5
Table of Contents
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS (UNAUDITED)
1. DESCRIPTION OF BUSINESS AND BASIS OF PRESENTATION
Description of Business
Avanir Pharmaceuticals, Inc. and subsidiaries (“Avanir”, the “Company” or “we”) is a biopharmaceutical company focused on acquiring, developing and commercializing novel therapeutic products for the treatment of central nervous system disorders. The Company’s lead product NUEDEXTA® (referred to as AVP-923 during clinical development) is a first-in-class dual N-methyl-D-aspartate (“NMDA”) receptor antagonist and sigma-1 agonist. NUEDEXTA, 20/10 mg, was approved in the United States in October 2010 for the treatment of pseudobulbar affect (“PBA”). It is also approved for the treatment of PBA in the European Union in two dose strengths, NUEDEXTA 20/10 mg and NUEDEXTA 30/10 mg. The Company commercially launched NUEDEXTA in the United States in February 2011 and it is currently assessing plans regarding the potential commercialization of NUEDEXTA in the European Union.
The Company is studying the clinical utility of AVP-923 in other mood/behavior disorders and movement disorders. The Company has two ongoing Phase II clinical trials exploring the potential treatment of agitation in patients with Alzheimer’s disease and potential treatment of levodopa-induced dyskinesia in Parkinson’s disease (the “LID study”). The LID study is supported by a grant from the Michael J. Fox Foundation.
The Company is also developing AVP-786, a next generation drug product containing deuterium-modified dextromethorphan and quinidine for the treatment of neurologic and psychiatric disorders. The Company completed a pharmacokinetic study with AVP-786, and based on this data, the Company believes that it has identified a formulation of AVP-786 with a comparable pharmacokinetic profile, and likely a comparable safety and tolerability profile to AVP-923. This AVP-786 formulation contains significantly less quinidine than used in AVP-923.
The Company is also developing a novel Breath Powered™ intranasal delivery system containing low-dose sumatriptan powder to treat acute migraine, AVP-825. If approved, this product would be the first and only fast-acting dry-powder nasal delivery form of sumatriptan. AVP-825 is licensed from OptiNose AS (“OptiNose”). Under the terms of the agreement, the Company assumed responsibility for regulatory, manufacturing, supply-chain and commercialization activities for the investigational product. The Company filed a New Drug Application (“NDA”) with the U.S. Food and Drug Administration (“FDA”) in January 2014.
The Company entered into a multi-year agreement with Merck Sharp & Dohme Corp. (“Merck”) to co-promote Merck’s type 2 diabetes therapies JANUVIA® (sitagliptin) and the sitagliptin family of products in the long-term care institutional setting in the United States beginning October 1, 2013. The term of the Agreement will continue for three years following the launch date of the co-promotion activities. Under the terms of the Agreement, the Company will be compensated via a (i) fixed monthly fee and (ii) performance fee based on the amount of the products sold by the Company above a predetermined baseline. A significant majority of the fee is performance based. Over the three years of the agreement, Avanir could receive up to a maximum of $60.0 million in compensation.
In addition to the Company’s focus on products for the central nervous system, the Company also has partnered programs in other therapeutic areas which may generate future revenue. The Company’s first commercialized product, docosanol 10% cream, (sold in the United States and Canada as Abreva® by its marketing partner GlaxoSmithKline Consumer Healthcare) is the only over-the-counter treatment for cold sores that has been approved by the FDA.
The Company’s operations are subject to certain risks and uncertainties frequently encountered by companies in the early stages of operations, particularly in the evolving market for small biotech and specialty pharmaceutical companies. Such risks and uncertainties include, but are not limited to, the occurrence of adverse safety events with NUEDEXTA; that NUEDEXTA may not gain broader acceptance by the medical field or that the indicated use may
6
Table of Contents
not be clearly understood; the Company’s dependence on third parties for manufacturing and distribution of NUEDEXTA; that the Company may not adequately build or maintain the necessary sales, marketing, supply chain management and reimbursement capabilities on its own or enter into arrangements with third parties to perform these functions in a timely manner or on acceptable terms; the ability to successfully protect and enforce its intellectual property rights relating to NUEDEXTA; timing and uncertainty of achieving milestones in clinical trials; obtaining approvals by the FDA, European Medicines Agency (“EMA”) and regulatory agencies in other countries; and potential regulatory delays or rejections in the filing or acceptance of an NDA. The Company’s ability to generate revenues in the future may depend on market acceptance of NUEDEXTA for the treatment of PBA, pricing and reimbursement in European countries, the ability to obtain a partner to market NUEDEXTA in the EU, commercial market estimates and related revenue projections for AVP-825 and the timing and success of reaching clinical development milestones and obtaining regulatory approvals for other formulations and indications for AVP-923 and AVP-786. The Company’s cash expenditures depend substantially on the level of expenditures for the ongoing marketing of NUEDEXTA, clinical development activities for AVP-923 and AVP-786, milestone payments related to AVP-786 and AVP-825 and regulatory activities for AVP-825.
Avanir was incorporated in California in August 1988 and was reincorporated in Delaware in March 2009.
Basis of Presentation
The accompanying unaudited condensed consolidated financial statements of Avanir have been prepared in accordance with the rules and regulations of the Securities and Exchange Commission (“SEC”) for interim reporting including the instructions to Form 10-Q. These condensed consolidated financial statements do not include all disclosures for annual audited financial statements required by accounting principles generally accepted in the United States of America (“U.S. GAAP”) and should be read in conjunction with the Company’s audited consolidated financial statements and related notes included in the Company’s Annual Report on Form 10-K for the year ended September 30, 2013. The Company believes these condensed consolidated financial statements reflect all adjustments (consisting only of normal, recurring adjustments) that are necessary for a fair presentation of the financial position and results of operations for the periods presented. Results of operations for the interim periods presented are not necessarily indicative of results to be expected for the year.
The preparation of financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts and the disclosures of commitments and contingencies in the financial statements and accompanying notes. Actual results could differ from those estimates.
Reclassifications
Royalty receivable and other receivables at September 30, 2013 are now reported under their own captions, separate from other current assets, in the accompanying condensed consolidated balance sheets and as separate components of cash flows from operating activities in the condensed consolidated statements of cash flows for the three months ended December 31, 2012, in order to conform to the current period presentation.
2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
The following represents an update for the three months ended December 31, 2013 to the significant accounting policies described in the Company’s Annual Report on Form 10-K for the fiscal year ended September 30, 2013.
Concentration of credit risk and sources of supply
Financial assets that potentially subject the Company to concentrations of credit risk consist of cash and cash equivalents, trade receivables, royalty receivable and other receivables. The Company’s cash and cash equivalents are placed in various money market mutual funds and at financial institutions of high credit standing. At times, deposits held with these financial institutions may exceed the amount of insurance provided by the Federal Deposit Insurance Corporation (FDIC). Generally, these deposits may be redeemed upon demand and, therefore, bear minimal risk. The Company performs ongoing credit evaluations of customers’ financial condition and may limit the amount of credit extended if necessary; however, the Company has historically required no collateral from its customers.
7
Table of Contents
The Company currently has sole suppliers for the active pharmaceutical ingredients (“APIs”) for NUEDEXTA and a sole manufacturer for the finished form of NUEDEXTA. In addition, these materials are custom and available from only a limited number of sources. Any material disruption in manufacturing could cause a delay in shipments and possible loss of revenue. If the Company is required to change manufacturers, the Company may experience delays associated with finding an alternative manufacturer that is properly qualified to produce NUEDEXTA in accordance with FDA requirements and the Company’s specifications.
Deferred rent
The Company accounts for rent expense related to operating leases by determining total minimum rent payments on the leases over their respective periods and recognizing the rent expense on a straight-line basis. The difference between the actual amount paid and the amount recorded as rent expense in each fiscal year is recorded as an adjustment to deferred rent. Deferred rent as of December 31, 2013 and September 30, 2013 was approximately $427,000 and $367,000, respectively, and is included in accrued expenses and other liabilities in the accompanying condensed consolidated balance sheets.
Fair value of financial instruments
The Company measures the fair value of certain of its financial assets on a recurring basis. A fair value hierarchy is used to rank the quality and reliability of the information used to determine fair values. Financial assets and liabilities carried at fair value will be classified and disclosed in one of the following three categories:
| • | Level 1-Quoted prices (unadjusted) in active markets for identical assets and liabilities. |
| • | Level 2-Inputs other than Level 1 that are observable, either directly or indirectly, such as unadjusted quoted prices for similar assets and liabilities, unadjusted quoted prices in the markets that are not active, or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities. |
| • | Level 3-Unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities. |
At December 31, 2013 and September 30, 2013, the Company’s financial instruments include cash and cash equivalents, restricted cash and cash equivalents, trade receivables, royalty receivable, other receivables, restricted investments, accounts payable, accrued expenses, accrued compensation and payroll taxes, other liabilities and notes payable. The carrying amount of cash and cash equivalents, restricted cash and cash equivalents, trade receivables, royalty receivable, other receivables, accounts payable, accrued expenses, accrued compensation and payroll taxes, and other liabilities approximates fair value due to the short-term maturities of these instruments. The Company’s restricted investments are carried at amortized cost which approximates fair value. Based on borrowing rates currently available to the Company, the carrying value of notes payable approximates fair value.
Restricted cash, cash equivalents and restricted investments
Restricted cash and cash equivalents and restricted long-term investments consist of certificates of deposit, which are classified as held-to-maturity.
Restricted cash and cash equivalents consist of a certificate of deposit relating to the Company’s corporate credit card agreement and automatically renews every three months.
Restricted long-term investments consist of two certificates of deposit related to irrevocable standby letters of credit connected to fleet rentals and an office lease with an expiration date in 2018. The certificates of deposit automatically renew annually.
Debt issuance costs and debt discount
Debt issuance costs are stated at cost, net of accumulated amortization in other assets in the condensed consolidated balance sheets. Debt discount is recorded within notes payable in the condensed consolidated balance sheets. Amortization expense of debt issuance costs and the debt discount is calculated using the interest method over the term of the debt and is recorded in interest expense in the accompanying condensed consolidated statements of operations.
8
Table of Contents
Revenue recognition
The Company has historically generated revenues from product sales, collaborative research and development arrangements, and other commercial arrangements such as royalties, the sale of royalty rights and sales of technology rights. Payments received under such arrangements may include non-refundable fees at the inception of the arrangements, milestone payments for specific achievements designated in the agreements, royalties on sales of products resulting from collaborative arrangements, and payments for the sale of rights to future royalties.
The Company recognizes revenue when all of the following criteria are met: (1) persuasive evidence of an arrangement exists; (2) delivery has occurred or services have been rendered; (3) the Company’s price to the buyer is fixed or determinable; and (4) collectability is reasonably assured. In addition, certain product sales are subject to rights of return. For products sold where the buyer has the right to return the product, the Company recognizes revenue at the time of sale only if (1) the Company’s price to the buyer is substantially fixed or determinable at the date of sale, (2) the buyer has paid the Company, or is obligated to pay the Company and the obligation is not contingent on resale of the product, (3) the buyer’s obligation to the Company would not be changed in the event of theft or physical destruction or damage of the product, (4) the buyer acquiring the product for resale has economic substance apart from that provided by the seller, (5) the Company does not have significant obligations for future performance to directly bring about resale of the product by the buyer, and (6) the amount of future returns can be reasonably estimated. The Company recognizes such product revenues when either it has met all the above criteria, including the ability to reasonably estimate future returns, or when it can reasonably estimate that the return privilege has substantially expired, whichever occurs first.
Product Sales — NUEDEXTA. NUEDEXTA is sold primarily to third-party wholesalers that, in turn, sell this product to retail pharmacies, hospitals, and other dispensing organizations. The Company has entered into agreements with wholesale customers, certain medical institutions and third-party payers throughout the United States. These agreements frequently contain commercial terms, which may include favorable product pricing and discounts and rebates payable upon dispensing the product to patients. Additionally, these agreements customarily provide the customer with rights to return the product, subject to the terms of each contract. Consistent with pharmaceutical industry practice, wholesale customers can return purchased product during an 18-month period that begins six months prior to the product’s expiration date and ends 12 months after the expiration date. The Company recognizes revenue upon shipment of NUEDEXTA to its wholesalers and other customers.
The Company’s net product sales represent gross product sales less allowances for customer credits, including estimated discounts, rebates, chargebacks and co-pay assistance. These allowances provided by the Company to a customer are presumed to be a reduction of the selling prices of the Company’s products or services and, therefore, are characterized as a reduction of revenue when recognized in the Company’s condensed consolidated statement of operations. Allowances for discounts, rebates, chargebacks and co-pay assistance are estimated based on contractual terms with customers and sell-through data purchased from third parties. The Company estimates future returns and records a returns reserve as a reduction to gross product sales. The returns reserve is estimated based on contractual terms with customers and historical trends. The Company believes the assumptions used to estimate these allowances are reasonable considering known facts and circumstances. However, actual rebates, chargebacks and returns could differ materially from estimated amounts because of, among other factors, unanticipated changes in prescription trends and any change in assumptions affecting sell-through data purchased from third parties. Product shipping and handling costs are included in cost of product sales.
Product Sales — Active Pharmaceutical Ingredient docosanol (“docosanol”). Revenue from sales of the Company’s docosanol is recorded when title and risk of loss have passed to the buyer, provided the criteria for revenue recognition have been met. The Company sells the docosanol to various licensees upon receipt of a written order for the materials. Shipments generally occur fewer than three times per year. The Company’s contracts for sales of the docosanol include buyer acceptance provisions that give the Company’s buyers the right of replacement if the delivered product does not meet specified criteria. That right requires that they give the Company notice within 30 days after receipt of the product. The Company has the option to refund or replace any such defective materials; however, it has historically demonstrated that the materials shipped from the same pre-inspected lot have consistently met the specified criteria and no buyer has rejected any of the Company’s shipments from the same pre-inspected lot to date. Therefore, the Company recognizes revenue at the time of delivery without providing any returns reserve. The Company has not recognized revenue from the sale of docosanol during the first quarter of fiscal 2014 and fiscal 2013.
9
Table of Contents
Multiple Element Arrangements. The Company has, in the past, entered into arrangements whereby it delivers to the customer multiple elements including technology and/or services. Such arrangements have included some combination of the following: antibody generation services; licensed rights to technology, patented products, compounds, data and other intellectual property; and research and development services. At the inception of each such arrangement, the Company analyzes the multiple elements contained within the arrangement to determine whether the elements can be separated. If a product or service is not separable, the combined deliverables will be accounted for as a single unit of accounting.
A delivered element can be separated from other elements when it meets both of the following criteria: (1) the delivered item has value to the customer on a standalone basis; and (2) if the arrangement includes a general right of return relative to the delivered item, delivery or performance of the undelivered item is considered probable and substantially in the Company’s control. If an element can be separated, the Company allocates amounts based upon the selling price of each element. The Company determines the selling price of a separate deliverable using the price it charges other customers when it sells that product or service separately; however, if the Company does not sell the product or service separately, it uses third-party evidence of selling price of a similar product or service to a similarly situated customer. The Company considers licensed rights or technology to have standalone value to its customers if it or others have sold such rights or technology separately or its customers can sell such rights or technology separately without the need for the Company’s continuing involvement. The Company has not entered into any multiple element arrangements which have required the Company to estimate selling prices during the first quarter of fiscal 2014 and fiscal 2013.
License Arrangements. License arrangements may consist of non-refundable up-front license fees, data transfer fees, research reimbursement payments, exclusive licensed rights to patented or patent pending compounds, technology access fees, and various performance or sales milestones. These arrangements are often multiple element arrangements.
Non-refundable, up-front fees that are not contingent on any future performance by the Company, and require no consequential continuing involvement on its part, are recognized as revenue when the license term commences and the licensed data, technology and/or compound is delivered. Such deliverables may include physical quantities of compounds, design of the compounds and structure-activity relationships, the conceptual framework and mechanism of action, and rights to the patents or patents pending for such compounds. The Company defers recognition of non-refundable up-front fees if it has continuing performance obligations without which the technology, right, product or service conveyed in conjunction with the non-refundable fee has no utility to the licensee that is separate and independent of the Company’s performance under the other elements of the arrangement. In addition, if the Company has required continuing involvement through research and development services that are related to its proprietary know-how and expertise of the delivered technology, or can only be performed by the Company, then such up-front fees are deferred and recognized over the period of continuing involvement.
Payments related to substantive, performance-based milestones in a research and development arrangement are recognized as revenues upon the achievement of the milestones as specified in the underlying agreements when they represent the culmination of the earnings process.
Royalty Arrangements. The Company recognizes royalty revenues from licensed products when earned in accordance with the terms of the license agreements. Net sales amounts generally required to be used for calculating royalties include deductions for returned product, pricing allowances, cash discounts, freight and warehousing. These arrangements are often multiple element arrangements.
Certain royalty arrangements provide that royalties are earned only if a sales threshold is exceeded. Under these types of arrangements, the threshold is typically based on annual sales. For royalty revenue generated from the license agreement with GlaxoSmithKline (“GSK”), the Company recognizes royalty revenue in the period in which the threshold is exceeded.
When the Company sells its rights to future royalties under license agreements and also maintains continuing involvement in earning such royalties, it defers recognition of any up-front payments and recognizes them as revenues over the life of the license agreement. The Company recognizes revenues for the sale of an undivided
10
Table of Contents
interest of its Abreva® license agreement to Drug Royalty USA under the “units-of-revenue method.” Under this method, the amount of deferred revenues to be recognized in each period is calculated by multiplying the ratio of the royalty payments due to Drug Royalty USA by GSK for the period to the total remaining royalties the Company expects GSK will pay Drug Royalty USA over the remaining term of the agreement.
Co-Promotion Arrangements. The Company recognizes both a fixed monthly fee and a performance fee as revenues from co-promote activities in the condensed consolidated statements of operations. The fixed monthly fee is recognized ratably over the period earned. The performance fee is recognized when the products sold exceeds a predetermined baseline for the period. The receivable from the co-promotion fee is recorded in other receivables in the condensed consolidated balance sheets.
Share-based compensation
The Company grants options, restricted stock units and restricted stock awards to purchase the Company’s common stock to employees, directors and consultants under stock option plans. The benefits provided under these plans are share-based payments that the Company accounts for using the fair value method.
The fair value of each option award is estimated on the date of grant using a Black-Scholes-Merton option pricing model (“Black-Scholes model”) that uses assumptions regarding a number of complex and subjective variables. These variables include, but are not limited to, expected stock price volatility, actual and projected employee stock option exercise behaviors, risk-free interest rate and expected dividends. Expected volatilities are based on the historical volatility of the Company’s common stock and other factors. The expected terms of options granted are based on analyses of historical employee termination rates and option exercises. The risk-free interest rate is based on the U.S. Treasury yield in effect at the time of the grant. Since the Company does not expect to pay dividends on common stock in the foreseeable future, it estimated the dividend yield to be 0%.
Share-based compensation expense recognized during a period is based on the value of the portion of share-based payment awards that are ultimately expected to vest and is amortized under the straight-line attribution method. As share-based compensation expense recognized in the accompanying condensed consolidated statements of operations for periods in fiscal 2014 and 2013 is based on awards ultimately expected to vest, it has been reduced for estimated forfeitures. The fair value method requires forfeitures to be estimated at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differ from those estimates. The Company estimates forfeitures based on historical experience. Changes to the estimated forfeiture rate are accounted for as a cumulative effect of change in the period the change occurred.
Total compensation expense related to all of the Company’s share-based awards for the three month periods ended December 31, 2013 and 2012, was comprised of the following:
| Three months ended December 31, |
||||||||
| 2013 | 2012 | |||||||
| Share-based compensation classified as: |
||||||||
| Research and development expense |
$ | 287,466 | $ | 268,144 | ||||
| Selling and marketing expense |
472,255 | 315,529 | ||||||
| General and administrative expense |
628,396 | 823,335 | ||||||
|
|
|
|
|
|||||
| Total |
$ | 1,388,117 | $ | 1,407,008 | ||||
|
|
|
|
|
|||||
| Three months ended December 31, |
||||||||
| 2013 | 2012 | |||||||
| Share-based compensation expense from: |
||||||||
| Stock options |
$ | 1,110,228 | $ | 1,047,986 | ||||
| Restricted stock units |
277,889 | 359,022 | ||||||
|
|
|
|
|
|||||
| Total |
$ | 1,388,117 | $ | 1,407,008 | ||||
|
|
|
|
|
|||||
11
Table of Contents
Since the Company has a net operating loss carry-forward as of December 31, 2013, no excess tax benefits for the tax deductions related to share-based awards were recognized in the accompanying condensed consolidated statements of operations. Additionally, no incremental tax benefits were recognized from stock options exercised in the three month periods ended December 31, 2013 and 2012, which would have resulted in a reclassification from cash flows from operating activities to cash flows from financing activities.
Income taxes
The Company accounts for income taxes and the related accounts under the liability method. Deferred tax assets and liabilities are determined based on the differences between the consolidated financial statement carrying amounts and the income tax bases of assets and liabilities. A valuation allowance is applied against any net deferred tax asset if, based on available evidence, it is more likely than not that some or all of the deferred tax assets will not be realized.
The Company recognizes any uncertain income tax positions on income tax returns at the largest amount that is more-likely-than-not to be sustained upon audit by the relevant taxing authority. An uncertain income tax position will not be recognized if it has less than a 50% likelihood of being sustained.
The total unrecognized tax benefit resulting in a decrease in deferred tax assets and corresponding decrease in the valuation allowance at December 31, 2013 was $3.6 million. There are no unrecognized tax benefits included in the condensed consolidated balance sheets that would, if recognized, affect the Company’s effective tax rate.
The Company’s policy is to recognize interest and/or penalties related to income tax matters in income tax expense. The Company had $0 accrued for interest and penalties on the Company’s condensed consolidated balance sheets at December 31, 2013 and September 30, 2013.
The Company is subject to taxation in the U.S. and various state jurisdictions. The Company’s tax years for 1995 and forward for federal purposes and 1989 and forward for California purposes are subject to examination by the U.S. and California tax authorities due to the carryforward of unutilized net operating losses and research and development credits.
The Company does not foresee material changes to its gross uncertain income tax position liability within the next twelve months.
Recent authoritative guidance
In July 2013, the FASB issued Accounting Standards Update (“ASU”) No. 2013-11, “Presentation of an Unrecognized Tax Benefit When a Net Operating Loss Carryforward, a Similar Tax Loss, or a Tax Credit Carryforward Exists.” ASU 2013-11 provides explicit guidance on the financial statement presentation of an unrecognized tax benefit when a net operating loss carryforward, a similar tax loss, or a tax credit carryforward exists. The guidance is effective prospectively for fiscal years, and interim periods within those years, beginning after December 15, 2013, with an option for early adoption. The Company intends to adopt this guidance at the beginning of its first quarter of fiscal year 2015, and is currently evaluating the impact on its consolidated financial statements and disclosures.
Proposed Amendments to Current Accounting Standards. The Financial Accounting Standard Board (“FASB”) is currently working on amendments to existing accounting standards governing a number of areas including, but not limited to, revenue recognition and lease accounting.
In June 2010, the FASB issued an exposure draft, Revenue from Contracts with Customers, which would supersede most of the existing guidance on revenue recognition in Accounting Standards Codification (“ASC”) Topic 605, Revenue Recognition. In November 2011, the FASB re-exposed this draft and it expects a final standard to be issued during the first half of calendar 2014. As the standard-setting process is still ongoing, the Company is unable to determine the impact this proposed change in accounting will have in the Company’s consolidated financial statements at this time.
12
Table of Contents
In August 2010, the FASB issued an exposure draft, Leases, which would result in significant changes to the accounting requirements for both lessees and lessors in ASC Topic 840, Leases. In May 2013, the FASB re-exposed this draft and the comment period closed in September 2013. As the standard-setting process is still ongoing, the Company is unable to determine the impact this proposed change in accounting will have in the Company’s consolidated financial statements at this time.
3. INVENTORIES
Inventories are comprised of NUEDEXTA finished goods and the active pharmaceutical ingredients of NUEDEXTA, dextromethorphan (“DM”) and quinidine (“Q”), as well as the active pharmaceutical ingredient docosanol.
The composition of inventories as of December 31, 2013 and September 30, 2013 is as follows:
| December 31, 2013 |
September 30, 2013 |
|||||||
| (audited) | ||||||||
| Raw materials |
$ | 929,234 | $ | 936,856 | ||||
| Work in progress |
49,265 | 36,453 | ||||||
| Finished goods |
400,464 | 521,056 | ||||||
|
|
|
|
|
|||||
| Total inventory |
1,378,963 | 1,494,365 | ||||||
| Less: current portion |
(605,833 | ) | (710,179 | ) | ||||
|
|
|
|
|
|||||
| Non-current portion |
$ | 773,130 | $ | 784,186 | ||||
|
|
|
|
|
|||||
The amount classified as non-current inventories is comprised of the raw material components for NUEDEXTA, dextromethorphan and quinidine, which will be used in the manufacture of NUEDEXTA capsules beyond the Company’s one year operating cycle.
4. ACCRUED EXPENSES
Accrued expenses at December 31, 2013 and September 30, 2013 are as follows:
| December 31, 2013 |
September 30, 2013 |
|||||||
| (audited) | ||||||||
| Accrued royalties, rebates, chargebacks, and distribution fees (1) |
$ | 6,224,729 | $ | 5,525,103 | ||||
| Accrued research and development expenses |
2,144,010 | 2,146,210 | ||||||
| Accrued selling and marketing expenses |
1,589,570 | 2,139,429 | ||||||
| Accrued general and administrative expenses |
1,455,611 | 1,752,966 | ||||||
| Other current liabilities |
422,826 | 344,862 | ||||||
|
|
|
|
|
|||||
| Total accrued expenses |
$ | 11,836,746 | $ | 11,908,570 | ||||
|
|
|
|
|
|||||
| (1) | Accrued royalties, rebates, chargebacks and distribution fees are directly impacted by product revenue and will fluctuate over time in relation to the change in product revenue. |
5. DEFERRED REVENUES
The following table sets forth as of December 31, 2013, the deferred revenue balances for the Company’s sale of future Abreva® royalty rights to Drug Royalty USA.
| Drug Royalty USA Agreement |
||||
| Deferred revenues as of September 30, 2013 |
$ | 1,288,514 | ||
| Changes during the period: |
||||
| Recognized as revenues during period |
(648,049 | ) | ||
|
|
|
|||
| Deferred revenues as of December 31, 2013 |
$ | 640,465 | ||
|
|
|
|||
13
Table of Contents
Drug Royalty USA Agreement — In November 2002, the Company sold to Drug Royalty USA an undivided interest in the Company’s rights to receive future Abreva royalties under the license agreement with GSK for $24.1 million (the “Drug Royalty Agreement” and the “GSK License Agreement,” respectively). Under the Drug Royalty Agreement, Drug Royalty USA has the right to receive royalties from GSK on sales of Abreva until the expiration of the patent for Abreva on April 28, 2014. The Company retained the right to receive 50% of all royalties (a net of 4%) under the GSK License Agreement for annual net sales of Abreva in the U.S. and Canada in excess of $62.0 million. During the three months ended December 31, 2013 and 2012, the Company recognized royalties related to the annual net Abreva sales in excess of $62.0 million in the amount of approximately $1.4 million and $1.2 million, respectively, which is included in the accompanying condensed consolidated statements of operations as revenues from royalties.
Revenues are recognized when earned, collection is reasonably assured and no additional performance of services is required. The Company classified the proceeds received from Drug Royalty USA as deferred revenue, and is recognizing the revenue over the life of the license agreement because of the Company’s continuing involvement over the term of the Drug Royalty Agreement. Such continuing involvement includes overseeing the performance of GSK and its compliance with the covenants in the GSK License Agreement, monitoring patent infringement, adverse claims or litigation involving Abreva, and undertaking to find a new license partner in the event that GSK terminates the agreement. The Drug Royalty Agreement contains both covenants and events of default that require such performance on the Company’s part. Therefore, nonperformance on the Company’s part could result in default of the arrangement, and could give rise to additional rights in favor of Drug Royalty USA under a separate security agreement with Drug Royalty USA, which could result in loss of the Company’s rights to share in future Abreva royalties if wholesale sales by GSK exceed $62.0 million a year. The deferred revenue is being recognized as revenue using the “units-of-revenue method” over the life of the license agreement. Based on a review of the Company’s continuing involvement, the Company concluded that the sale proceeds did not meet any of the rebuttable presumptions that would require classification of the proceeds as debt.
6. NOTES PAYABLE
In May 2012, the Company entered into a Loan and Security Agreement (the “Loan Agreement”) with Oxford Finance LLC and Silicon Valley Bank. The Loan Agreement provides for a term loan of $30.0 million which was funded upon closing of the transaction in June 2012. Under the terms of the Loan Agreement, interest accrues on the outstanding balance at a rate of 8.95% per annum. In the third fiscal quarter of 2013, the Company met the criteria to extend the interest only payment for six months. Therefore, the Company will make monthly payments of interest only until January 1, 2014 (the “Amortization Date”). Beginning on the Amortization Date, the outstanding loan balance will be repaid in thirty equal monthly payments of principal and interest. In addition to the original principal, a final payment equal to 7% of the original principal amount of the loan will be due thirty months from the Amortization Date. The final payment is being accreted as interest expense over the term of the debt using the interest method and the related liability of approximately $1.3 million and $1.1 million as of December 31, 2013 and September 30, 2013, respectively, is included in other liabilities in the accompanying condensed consolidated balance sheets.
In accordance with the terms of the Loan Agreement, the Company issued to the lenders warrants to purchase shares of the Company’s common stock equal to 4.55% of the original principal at a price per share equal to the lower of the 10-day average share price prior to closing or the price per share on the day of funding. Accordingly, the Company issued to the lenders warrants to purchase 491,007 shares of the Company’s common stock at an exercise price of $2.78 per share. The relative fair value of the warrants was approximately $1.2 million and was estimated using the Black-Scholes model with the following assumptions: fair value of the Company’s common stock at issuance of $2.80 per share; ten year contractual term; 96.7% volatility; 0% dividend rate; and a risk-free
14
Table of Contents
interest rate of 1.8%. The relative fair value of the warrants was recorded as a debt discount, decreasing notes payable and increasing additional paid-in capital on the accompanying condensed consolidated balance sheets. The debt discount is being amortized to interest expense over the term of the debt using the interest method. For the three months ended December 31, 2013 and 2012, debt discount amortization was approximately $102,000 and $138,000, respectively.
The loan is secured by a first priority security interest in all of the Company’s assets, other than its intellectual property and its rights under license agreements granting it rights to intellectual property.
The Loan Agreement contains standard affirmative and restrictive covenants. The affirmative covenants include, among other items, that the Company maintain a minimum sales level relative to projected NUEDEXTA revenues, measured on a trailing three-month basis, or maintain cash and cash equivalents in accounts subject to control agreements in favor of the collateral agent equal to at least 1.5 times the outstanding amount of obligations under the Loan Agreement. Additionally, the affirmative and restrictive covenants, among other items, restrict the Company’s ability to incur additional indebtedness or guarantees; incur liens; make investments, loans and acquisitions; consolidate or merge; sell assets, including capital stock of subsidiaries; alter the business of the Company; engage in transactions with affiliates; and enter into agreements limiting dividends and distributions of certain subsidiaries. The Loan Agreement also includes events of default, including, among other things, payment defaults, breaches of representations, warranties or covenants, certain bankruptcy events, the occurrence of certain material adverse changes, and a commercial, generic version of NUEDEXTA (for the treatment of PBA) becoming available. Upon the occurrence of an event of default and following any cure periods (if applicable), a default interest rate of an additional 5.0% per annum may be applied to the outstanding loan balances, and the lenders may declare all outstanding obligations immediately due and payable and take such other actions as set forth in the Loan Agreement. As of December 31, 2013, the Company was in compliance with all covenants in the Loan Agreement.
7. COMPUTATION OF NET LOSS PER COMMON SHARE
Basic net loss per common share is computed by dividing net loss by the weighted-average number of common shares outstanding during the period, excluding restricted stock that has been issued but is not yet vested. Diluted net loss per common share is computed by dividing net loss by the weighted-average number of common shares outstanding during the period plus additional weighted-average common equivalent shares outstanding during the period. Common equivalent shares result from the assumed exercise of outstanding stock options and warrants (the proceeds of which are then presumed to have been used to repurchase outstanding stock using the treasury stock method) and the vesting of restricted shares of common stock. In loss periods, certain of the common equivalent shares are excluded from the computation of diluted net loss per share, because their effect would have been anti-dilutive.
For the three month periods ended December 31, 2013 and 2012, the following options and warrants to purchase shares of common stock and restricted stock units were excluded from the computation of diluted net loss per share, as the inclusion of such shares would be anti-dilutive:
| 2013 | 2012 | |||||||
| Stock options |
10,071,519 | 9,848,654 | ||||||
| Stock warrants |
53,957 | 764,066 | ||||||
| Restricted stock units (1) |
2,705,176 | 2,887,017 | ||||||
| (1) | Includes 1,319,590 and 1,124,126 shares of restricted stock at December 31, 2013 and 2012, respectively, awarded to directors that have vested but are still restricted until the directors resign. |
8. STOCKHOLDERS’ EQUITY
Common stock
During the three months ended December 31, 2013, the Company issued 196,950 shares of common stock in connection with the vesting of restricted stock units and 12,560 shares of common stock in connection with the exercise of stock options resulting in proceeds of approximately $30,000.
15
Table of Contents
During the three months ended December 31, 2013, restricted stock unit awards for a total of 52,375 shares awarded to directors vested, but the issuance and delivery of these shares are deferred until the director resigns.
Warrants Outstanding
As of December 31, 2013, warrants to purchase 53,957 shares of the Company’s common stock at an exercise price of $2.78 per share remained outstanding and exercisable. The warrants were issued in connection with the Loan Agreement (see Note 6, “Notes Payable”) and expire in May 2022.
9. EMPLOYEE EQUITY INCENTIVE PLANS
The Company currently has one equity incentive plan, which is the 2005 Equity Incentive Plan (the “2005 Plan”). The 2000 Stock Option Plan (the “2000 Plan”) and the 2003 Equity Incentive Plan (the “2003 Plan” and, together with 2000 Plan and 2005 Plan, the “Plans”) are expired and the Company no longer grants share-based awards from these plans, however, grants are still outstanding under each of these plans at December 31, 2013. All of the Plans were approved by the stockholders, except for the 2003 Plan, which was approved solely by the Board of Directors. Share-based awards are subject to terms and conditions established by the Compensation Committee of the Company’s Board of Directors. The Company’s policy is to issue new common shares upon the exercise of stock options, conversion of share units or purchase of restricted stock.
During the three month periods ended December 31, 2013 and 2012, the Company granted share-based awards under the 2005 Plan. Under the 2005 Plan, options to purchase shares, restricted stock units, restricted stock and other share-based awards may be granted to the Company’s directors, employees and consultants. Pursuant to the provisions for annual increases of the 2005 Plan, the number of authorized shares of common stock for issuance increased by 325,000 shares effective November 15, 2013. As of December 31, 2013, the Company had an aggregate of 12,507,121 shares of its common stock reserved for future issuance. Of those shares, 12,315,995 shares were related to outstanding options and other awards and 191,126 shares were available for future grants of share-based awards. As of December 31, 2013, no equity awards were outstanding to consultants. The Company may also, from time to time, issue share-based awards outside of the Plans to the extent permitted by NASDAQ rules. As of December 31, 2013, there were 460,700 equity awards that were issued outside of the Plans (inducement option grants) outstanding. None of the share-based awards are classified as a liability as of December 31, 2013.
Stock Options. Stock options are granted with an exercise price equal to the current market price of the Company’s common stock at the grant date and have 10-year contractual terms. For option grants to employees, generally 25% of the option shares vest and become exercisable on the first anniversary of the grant date and the remaining 75% of the option shares vest and become exercisable quarterly in equal installments thereafter over three years. Certain option awards provide for accelerated vesting if there is a change in control (as defined in the Plans).
Summaries of stock options outstanding and changes during the three months ended December 31, 2013 are presented below.
| Number of Shares |
Weighted Average Exercise Price per Share |
Weighted Average Remaining Contractual Term (In Years) |
Aggregate Intrinsic Value |
|||||||||||||
| Outstanding at September 30, 2013 |
8,823,041 | $ | 2.85 | |||||||||||||
| Granted |
1,309,556 | $ | 3.15 | |||||||||||||
| Exercised |
(12,560 | ) | $ | 2.41 | ||||||||||||
| Forfeited |
(48,518 | ) | $ | 2.94 | ||||||||||||
|
|
|
|||||||||||||||
| Outstanding at December 31, 2013 |
10,071,519 | $ | 2.89 | 7.5 | $ | 8,496,661 | ||||||||||
|
|
|
|||||||||||||||
| Vested and expected to vest in the future at December 31, 2013 |
9,665,402 | $ | 2.88 | 7.4 | $ | 8,269,135 | ||||||||||
|
|
|
|||||||||||||||
| Exercisable at December 31, 2013 |
5,383,232 | $ | 2.81 | 6.3 | $ | 5,780,736 | ||||||||||
|
|
|
|||||||||||||||
The weighted average grant-date fair value of options granted during the three month periods ended December 31, 2013 and 2012 was $2.15 and $1.78 per share, respectively. The total intrinsic value of options exercised during the three month periods ended December 31, 2013 and 2012 was approximately $25,000 and $39,000, respectively,
16
Table of Contents
based on the differences in market prices on the dates of exercise and the option exercise prices. As of December 31, 2013, the total unrecognized compensation cost related to unvested options was approximately $9.8 million which is expected to be recognized over the weighted-average period of 2.8 years, based on the vesting schedules. No tax benefit was realized for the tax deductions from option exercise of the share-based payment arrangements in the three month periods ended December 31, 2013 and 2012.
The fair value of each option award is estimated on the date of grant using the Black-Scholes model, which uses the assumptions noted in the following table. Expected volatilities are based on historical volatility of the Company’s common stock and other factors. The expected term of options granted is based on analyses of historical employee termination rates and option exercises. The expected risk-free interest rate is based on the U.S. Treasury yield for a period consistent with the expected term of the option in effect at the time of the grant. The dividend yield is based on the Company’s expectation of not paying dividends in the foreseeable future.
Assumptions used in the Black-Scholes model for options granted during the three months ended December 31, 2013 were as follows:
| Expected volatility |
84% | |
| Expected term in years |
5.4 | |
| Expected risk-free interest rate (zero coupon U.S. Treasury Note) |
1.5%-1.7% | |
| Expected dividend yield |
0% |
Restricted stock units (“RSU”). RSUs granted to employees generally vest based on three or four years of continuous service from the date of grant. RSUs granted to non-employee directors generally vest over the term of one year from the grant date and are not released until the awardee’s termination of service. Vesting for non-employee director grants allow for accelerated vesting of RSUs in the case of a non-employee director’s resignation where either: (i) he/she has served for at least four years as a member of the Board and is in good standing at the time of resignation, or (ii) he/she resigns for reasons related to health or family matters and is otherwise in good standing at the time of resignation.
The following table summarizes the RSU activities for the three months ended December 31, 2013:
| Number of Shares |
Weighted Average Grant Date Fair Value |
|||||||
| Unvested at September 30, 2013 |
1,637,210 | $ | 2.40 | |||||
| Vested |
(249,325 | ) | $ | 2.51 | ||||
| Forfeited |
(2,299 | ) | $ | 2.06 | ||||
|
|
|
|||||||
| Unvested at December 31, 2013 |
1,385,586 | $ | 2.38 | |||||
|
|
|
|||||||
The grant-date fair value of RSUs granted during the three months ended December 31, 2012 was approximately $2.4 million. There were no RSUs granted during the three months ended December 31, 2013. As of December 31, 2013, the total unrecognized compensation cost related to unvested shares was approximately $2.7 million which is expected to be recognized over a weighted-average period of 2.3 years, based on the vesting schedules.
At December 31, 2013, there were 1,319,590 shares of restricted stock with a weighted-average grant date fair value of $2.11 per share awarded to directors that have vested but are still restricted until the director resigns.
During the three months ended December 31, 2013, the Company granted 961,316 RSUs and 464,024 performance-based RSUs to employees contingent on approval of the 2014 Incentive Plan (“2014 Plan”) by the Company’s shareholders. These awards are not considered granted until the 2014 Plan is approved and are not reflected in the above table of unvested RSUs.
17
Table of Contents
10. COMMITMENTS AND CONTINGENCIES
Legal contingencies –
NUEDEXTA ABBREVIATED NEW DRUG APPLICATION (“ANDA”) Litigation
In fiscal 2011 and 2012, the Company received Paragraph IV certification notices from five separate companies contending that certain of its patents listed in the FDA’s publication, “Approved Drug Products with Therapeutic Equivalence Evaluation” (“FDA Orange Book”) (U.S. Patents 7,659,282 (“’282 Patent”), 8,227,484 (“’484 Patent”) and RE 38,115 (“’115 Patent”), which expire in August 2026, July 2023 and January 2016, respectively) are invalid, unenforceable and/or will not be infringed by the manufacture, use, sale or offer for sale of a generic form of NUEDEXTA as described in those companies’ abbreviated new drug application (“ANDA”). The FDA Orange Book provides potential competitors, including generic drug companies, with a list of issued patents covering approved drugs. In August 2011 and March 2012, the Company filed lawsuits in the U.S District Court for the District of Delaware against Par Pharmaceutical, Inc. and Par Pharmaceutical Companies, Inc. (collectively “Par”), Actavis South Atlantic LLC and Actavis, Inc. (collectively “Actavis”), Wockhardt USA, LLC and Wockhardt, Ltd. (collectively, “Wockhardt”), Impax Laboratories, Inc. (“Impax”) and Watson Pharmaceuticals, Inc., Watson Laboratories, Inc. and Watson Pharma, Inc. (collectively “Watson”) (Par, Actavis, Wockhardt, Impax and Watson, collectively the “Defendants”). In September and October 2012, the Company filed lawsuits in the U.S. District Court for the District of Delaware against the Defendants. All lawsuits (collectively, the “ANDA Actions”) were filed on the basis that the Defendants’ submissions of their respective ANDAs to obtain approval to manufacture, use, sell, or offer for sale generic versions of NUEDEXTA prior to the expiration of the ‘282 Patent, the ‘484 Patent and the ‘115 Patent listed in the FDA Orange Book constitute infringement of one or more claims of those patents. On October 31, 2012, Watson announced the divestiture of its ANDA for a generic form of NUEDEXTA to Sandoz, Inc. (“Sandoz”). As a result of Sandoz’ acquisition and maintenance of said ANDA, on May 30, 2013, the Company filed suit in the U.S. District Court for the District of Delaware against Sandoz. This suit was filed on the basis that Sandoz’ ANDA to obtain approval to manufacture, use, sell, or offer for sale generic versions of NUEDEXTA prior to the expiration of the ’282 Patent, the ’484 Patent and the ’115 Patent listed in the FDA Orange Book constitutes infringement of one or more claims of those patents.
On December 3, 2012, the Company received a Memorandum Opinion and Order (the “Order”) issued by Judge Leonard P. Stark of the United States District Court for the District of Delaware (the “Court”) related to the Markman hearing held October 5, 2012 in the Company’s ongoing patent infringement case against the Defendants. The Order establishes the meaning of patent claim terms in dispute between the parties. After comprehensive briefing and oral argument, Judge Stark’s Order adopted the Company’s proposed or stipulated patent term constructions.
A bench trial was held in September 2013 and concluded on October 15, 2013. The Company is currently awaiting Judge Stark’s decision.
On August 9, August 30, and September 6, 2013, Avanir entered into settlement agreements with Sandoz, Actavis and Wockhardt, respectively, to resolve pending patent litigation in response to their ANDAs seeking approval to market generic versions of NUEDEXTA capsules. The settlement agreements grant Sandoz, Actavis and Wockhardt the right to begin selling a generic version of NUEDEXTA on July 30, 2026, or earlier under certain circumstances. The parties also filed stipulations and orders of dismissal with the United States District Court for the District of Delaware which conclude the litigation with respect to Sandoz, Actavis and Wockhardt. The settlements do not end the Company’s ongoing litigation against the other two ANDA filers.
The 30-month stay associated with the filing of the ANDA Actions expired on December 30, 2013. In order to launch a generic form of NUEDEXTA, the Defendants must first receive FDA approval and as of February 4, 2014, the FDA has not granted approval to any Defendant’s ANDA. The Company intends to vigorously enforce its intellectual property rights relating to NUEDEXTA, but the Company cannot predict the outcome of these ANDA Actions.
18
Table of Contents
MASTERS LITIGATION
On January 14, 2014, a purported shareholder of the Company brought a claim against the Company and its directors, as well as a former director, in connection with the Company’s 2014 Annual Meeting proxy statement. The action is captioned Masters v. Avanir Pharmaceuticals, Inc., et al., Case No. 14-cv-00053, and is pending in the United States District Court for the Central District of California (the “Masters Action”). The Masters Action complaint alleges that the defendants violated Section 14(a) of the Securities Exchange Act, and further alleges that the individual defendants breached their fiduciary duties, based upon purported deficiencies in disclosures relating to Proposal 4 contained in the Company’s proxy statement, with respect to the Company’s equity compensation plan and practices. The complaint seeks, among other things, equitable relief that would enjoin the consummation of the shareholder vote on Proposal 4, declaratory relief, and attorney’s fees and costs. The Company intends to defend the Master’s Action vigorously, however, all litigation is inherently uncertain, and there can be no assurance that its defense of these or similar actions would be successful.
GENERAL AND OTHER
In the ordinary course of business, the Company may face various claims brought by third parties and the Company may, from time to time, make claims or take legal actions to assert the Company’s rights, including intellectual property rights as well as claims relating to employment and the safety or efficacy of products. Any of these claims could subject the Company to costly litigation and, while the Company generally believes that it has adequate insurance to cover many different types of liabilities, the Company’s insurance carriers may deny coverage or policy limits may be inadequate to fully satisfy any damage awards or settlements. If this were to happen, the payment of any such awards could have a material adverse effect on the Company’s consolidated operations, cash flows and financial position. Additionally, any such claims, whether or not successful, could damage the Company’s reputation and business. Management believes the outcomes of currently pending claims and lawsuits will not likely have a material effect on the Company’s consolidated operations or financial position.
In addition, it is possible that the Company could incur termination fees and penalties if it elected to terminate contracts with certain vendors, including clinical research organizations.
Guarantees and Indemnities. The Company indemnifies its directors, officers and certain executives to the maximum extent permitted under the laws of the State of Delaware, and various lessors in connection with facility leases for certain claims arising from such facilities or leases. Additionally, the Company periodically enters into contracts that contain indemnification obligations, including contracts for the purchase and sale of assets, wholesale distribution agreements, clinical trials, pre-clinical development work and securities offerings. These indemnification obligations provide the contracting parties with the contractual right to have Avanir pay for the costs associated with the defense and settlement of claims, typically in circumstances where Avanir has failed to meet its contractual performance obligations in some fashion.
19
Table of Contents
The maximum amount of potential future payments under such indemnifications is not determinable. The Company has not incurred significant costs related to these guarantees and indemnifications, and no liability has been recorded in the condensed consolidated financial statements for guarantees and indemnifications as of December 31, 2013 and September 30, 2013.
Center for Neurologic Study (“CNS”) — The Company holds the exclusive worldwide marketing rights to NUEDEXTA for certain indications pursuant to an exclusive license agreement with CNS.
The Company paid a $75,000 milestone upon FDA approval of NUEDEXTA for the treatment of PBA in fiscal 2011. In addition, the Company is obligated to pay CNS a royalty ranging from approximately 5% to 8% of net U.S. GAAP revenue generated by sales of NUEDEXTA. During the three months ended December 31, 2013 and 2012, royalties of approximately $1.2 million and $744,000, respectively, were recorded to cost of product sales in the accompanying condensed consolidated statements of operations. Under certain circumstances, the Company may have the obligation to pay CNS a portion of net revenues received if the Company sublicenses NUEDEXTA to a third party.
Under the agreement with CNS, the Company is required to make payments on achievements of up to a maximum of ten milestones, based upon five specific clinical indications. Maximum payments for these milestone payments could total approximately $1.1 million if the Company pursued the development of NUEDEXTA for all five of the licensed indications. In general, individual milestones range from $75,000 to $125,000 for each accepted NDA and a similar amount for each approved NDA in addition to the royalty discussed above on net U.S. GAAP revenues. The Company does not have the obligation to develop additional indications under the CNS license agreement.
Concert Pharmaceuticals, Inc. – The Company holds the exclusive worldwide marketing rights to develop and commercialize Concert’s d-DM compounds for the potential treatment of neurological and psychiatric disorders, as well as certain rights to other deuterium-modified dextromethorphan compounds pursuant to a license agreement with Concert.
Under the agreement with Concert, the Company is obligated to make milestone and royalty payments to Concert based on successful advancement of d-DM products for one or more indications in the United States, Europe, and Japan. Individual milestone payments range from $2.0 — $6.0 million, $1.5 — $15.0 million, and $25.0 — $60.0 million for clinical, regulatory and commercial targets respectively, and in aggregate could total over $200 million. Royalty payments are tiered, beginning in the single-digits and increasing to the low double-digits for worldwide net sales of d-DM products exceeding $1.0 billion annually. As of December 31, 2013, the Company has paid $2.0 million for milestones that have been achieved pursuant to this agreement, which was recorded in the second quarter of fiscal 2013.
OptiNose AS — In July 2013, the Company entered into an exclusive license agreement for the development and commercialization of a novel Breath Powered intranasal delivery system containing low-dose sumatriptan powder to treat acute migraine, AVP-825.
AVP-825 is licensed from OptiNose. Under the terms of the agreement, OptiNose received an up-front cash payment of $20.0 million in fiscal 2013, which was recorded to research and development expense. The Company and OptiNose will share certain development costs. OptiNose is eligible to receive up to an additional $90.0 million in aggregate milestone payments resulting from the achievement of future clinical, regulatory and commercial milestones. In addition, following product approval, Avanir will be required to make tiered royalty payments to OptiNose of a low double-digits percentage of net sales in the United States, Canada and Mexico.
11. SEGMENT INFORMATION
The Company operates its business on the basis of a single reportable segment, which is the business of discovery, development and commercialization of novel therapeutics for chronic diseases. The Company’s chief operating decision-maker is the Chief Executive Officer, who evaluates the Company as a single operating segment.
20
Table of Contents
The Company categorizes revenues by geographic area based on selling location. All of the Company’s operations are currently located in the United States; therefore, total revenues for the three month periods ended December 31, 2013 and 2012 are attributed to the United States. All long-lived assets at December 31, 2013 and September 30, 2013 are located in the United States.
The Company sells NUEDEXTA to a limited number of wholesalers. Three wholesalers accounted for 91% and 86% of net product sales for the three month periods ended December 31, 2013 and 2012, respectively. In addition, the three wholesalers accounted for 86% and 91% of trade receivables at December 31, 2013 and September 30, 2013, respectively.
12. SUBSEQUENT EVENTS
The Company has evaluated subsequent events through the filing date of this Form 10-Q, and determined that no subsequent events have occurred that would require recognition in the condensed consolidated financial statements or disclosure in the notes thereto other than discussed in the accompanying notes.
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
This Quarterly Report on Form 10-Q contains forward-looking statements concerning future events and performance of the Company. When used in this report, the words “intend,” “estimate,” “anticipate,” “believe,” “plan” or “expect” and similar expressions are included to identify forward-looking statements. These forward-looking statements are based on our current expectations and assumptions and many factors could cause our actual results to differ materially from those indicated in these forward-looking statements. You should review carefully the factors identified in “Risk Factors” in this report in Part II, Item 1A. and in Part I, Item 1A. in our most recent Annual Report on Form 10-K filed with the SEC. We disclaim any intent to update or announce revisions to any forward-looking statements to reflect actual events or developments. Except as otherwise indicated herein, all dates referred to in this report represent periods or dates fixed with reference to the calendar year, rather than our fiscal year ending September 30. The three months ended December 31, 2013 are also referred to as the first quarter of fiscal 2014.
EXECUTIVE OVERVIEW
We are a biopharmaceutical company focused on acquiring, developing and commercializing novel therapeutic products for the treatment of central nervous system disorders. Our lead product NUEDEXTA® (referred to as AVP-923 during clinical development) is a first-in-class dual NMDA receptor antagonist and sigma-1 agonist. NUEDEXTA, 20/10 mg, is approved in the United States for the treatment of pseudobulbar affect (“PBA”). It is also approved for PBA in the European Union in two dose strengths, NUEDEXTA 20/10 mg and NUEDEXTA 30/10 mg. We commercially launched NUEDEXTA in the United States in February 2011 and we are currently assessing plans regarding the potential commercialization of NUEDEXTA in the European Union.
We are studying the clinical utility of AVP-923 in other mood/behavior disorders and movement disorders. We have two ongoing Phase II clinical trials exploring the potential treatment of agitation in patients with Alzheimer’s disease and potential treatment of levodopa-induced dyskinesia in Parkinson’s disease (the “LID study”). The LID study is supported by a grant from the Michael J. Fox Foundation.
We are also developing AVP-786, a next generation drug product containing deuterium-modified dextromethorphan and quinidine for the treatment of neurologic and psychiatric disorders. We completed a pharmacokinetic study with AVP-786, and based on this data, we believe that we have identified a formulation of AVP-786 with a comparable pharmacokinetic profile, and likely a comparable, safety and tolerability profile to AVP-923. This AVP-786 formulation contains significantly less quinidine than used in AVP-923.
21
Table of Contents
We are also developing a novel Breath Powered™ intranasal delivery system containing low-dose sumatriptan powder to treat acute migraine, AVP-825. If approved, this product would be the first and only fast-acting dry-powder nasal delivery form of sumatriptan. AVP-825 is licensed from OptiNose AS (“OptiNose”). Under the terms of the agreement, we assumed responsibility for regulatory, manufacturing, supply-chain and commercialization activities for the investigational product. We filed a New Drug Application (“NDA”) with the U.S. Food and Drug Administration (“FDA”) in January 2014.
We entered into a multi-year agreement with Merck Sharp & Dohme Corp. (“Merck”) to co-promote Merck’s type 2 diabetes therapies JANUVIA® (sitagliptin) and the sitagliptin family of products in the long-term care institutional setting in the United States beginning October 1, 2013. The term of the agreement will continue for three years following the launch date of the co-promotion activities. Under the terms of the agreement, we will be compensated via a (i) fixed monthly fee and (ii) performance fee based on the amount of the products sold by us above a predetermined baseline. A significant majority of the fee is performance based. Over the three years of the agreement, Avanir could receive up to a maximum of $60.0 million in compensation.
In addition to our focus on products for the central nervous system, we also have partnered programs in other therapeutic areas that may generate future revenue for us. Our first commercialized product, docosanol 10% cream (sold in the United States and Canada as Abreva® by our marketing partner GlaxoSmithKline Consumer Healthcare), is the only over-the-counter treatment for cold sores that has been approved by the FDA.
Avanir was incorporated in California in August 1988 and was reincorporated in Delaware in March 2009.
The following chart illustrates the status of research and development activities for our products and product candidates that are commercialized or under development.
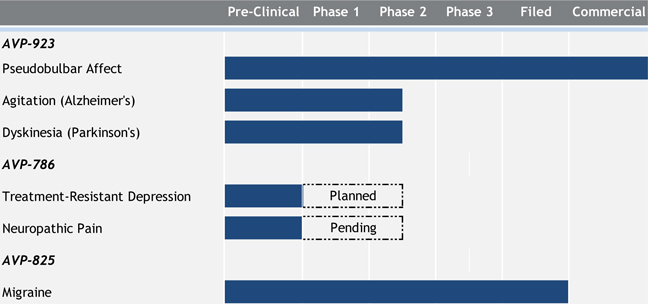
In addition to the research and development programs identified above, Avanir has provided unrestricted research grants to support several investigator initiated studies with AVP-923. Current studies planned or ongoing include potential treatment of behavioral symptoms of adults with autism spectrum disorder, treatment of bulbar function (impaired speech, swallowing, and saliva control) associated with amyotrophic lateral sclerosis (ALS), and treatment of refractory depression. For additional information regarding these studies please see http://clinicaltrials.gov.
22
Table of Contents
NUEDEXTA for the Treatment of Pseudobulbar Affect
NUEDEXTA is the first and only FDA-approved treatment for PBA. PBA occurs secondary to a variety of otherwise unrelated neurological conditions, and is characterized by involuntary, sudden, and frequent episodes of laughing and/or crying. PBA episodes typically occur out of proportion or incongruent to the patient’s underlying emotional state.
NUEDEXTA is an innovative combination of two well-characterized components: dextromethorphan hydrobromide (20 mg), the ingredient that is pharmacologically active in the central nervous system, and quinidine sulfate (10 mg), a metabolic inhibitor enabling dextromethorphan to reach therapeutic plasma concentrations. NUEDEXTA acts on sigma-1 and NMDA receptors in the brain, although the mechanism by which NUEDEXTA exerts therapeutic effects in patients with PBA is unknown.
Studies to support the effectiveness of NUEDEXTA were performed in patients with PBA and underlying amyotrophic lateral sclerosis (“ALS”) and MS. The primary outcome measure, the number of laughing and crying episodes, was significantly lower in the NUEDEXTA cohort compared with placebo. The secondary outcome measure, the Center for Neurologic Studies Lability Scale (“CNS-LS”), demonstrated a significantly greater mean decrease in CNS-LS score from baseline for the NUEDEXTA cohort compared with placebo. NUEDEXTA has not been studied in other types of emotional lability that can commonly occur, for example, in Alzheimer’s disease and other dementias.
A copy of the NUEDEXTA safety information has been filed as Exhibit 99.1 on our Annual Report on Form 10-K for the period ended September 30, 2013. For additional information regarding PBA or NUEDEXTA see www.pbafacts.com or www.nuedexta.com.
We launched NUEDEXTA in the United States in February 2011 with a specialty sales force calling primarily on physicians that care for patients where PBA is most commonly observed. This includes patients with underlying MS, ALS, Parkinson’s disease (“PD”), Alzheimer’s disease, traumatic brain injury and stroke. Our commercial efforts focus on the outpatient setting where patients typically receive prescription medications through retail and mail-order pharmacies and the institutional setting where patients typically receive prescription medications through an institutional pharmacy.
The table below shows total gross product sales and dispensed units (capsules) for NUEDEXTA during the past four quarterly periods.
| Three Months Ended | ||||||||||||||||
| March 31, 2013 |
June 30, 2013 |
September 30, 2013 |
December 31, 2013 |
|||||||||||||
| Gross product sales |
$ | 20,849,581 | $ | 24,315,422 | $ | 27,666,348 | $ | 31,281,493 | ||||||||
| Total dispensed units (capsules) |
1,839,842 | 2,334,898 | 2,606,289 | 2,834,877 | ||||||||||||
| Percentage growth over previous quarter |
7 | % | 27 | % | 12 | % | 9 | % | ||||||||
AVP-923 for the Treatment of Agitation in Patients with Alzheimer’s Disease
Alzheimer’s disease is generally characterized by cognitive decline, impaired performance of daily activities, and behavioral disturbances. Behavioral and psychiatric symptoms develop in as many as 60% of community-dwelling dementia patients and in more than 80% of patients with dementia living in nursing homes; as the disease progresses the risk of such complications approaches 100%. Dementia-related behavioral symptoms, including agitation, can be extremely distressing to the individual, the family, and caregivers. These behavioral disturbances have been associated with more rapid cognitive decline, institutionalization, and increased caregiver burden.
The objectives of this proof-of-concept study are to evaluate the safety, tolerability, and efficacy of AVP-923 for the treatment of agitation in Alzheimer’s patients. The trial is a multicenter, randomized, double-blind, placebo-controlled study that is expected to enroll up to 200 Alzheimer’s patients in the United States. Eligible patients will be randomized to receive either AVP-923 or placebo for 10 weeks. The primary efficacy measure is the
23
Table of Contents
agitation/aggression domain of the Neuropsychiatric Inventory or NPI. Secondary outcome measures include assessments of disease severity, behavioral abnormalities, cognition, activities of daily living, quality of life and caregiver strain. Standard safety assessments will also be conducted.
AVP-923 for the Treatment of Levodopa-Induced Dyskinesia
Levodopa-induced-dyskinesia (“LID”) occurs in most patients with Parkinson’s disease (“PD”) after several years of treatment, generally in association with other motor response complications, such as wearing-off or “on-off” fluctuations. Dyskinesia may be as disabling as the parkinsonism itself, and current treatment options are limited and are not always effective.
This proof-of-concept, double blind, randomized, crossover study will compare AVP-923 (45mg DM/10mg Q) with placebo for treatment of LID. The study will enroll approximately 16 PD patients across three study centers in the U.S. and Canada. Study participants will receive, in a random order, a 2-week treatment with AVP-923 and a 2-week placebo treatment, separated by a 2-week break. At the end of each 2-week treatment period, patients will receive a 2-hour levodopa infusion to test the drug effect on dyskinesia. Patients will be carefully monitored throughout the 6-week study for side effects, Parkinson’s symptoms and general health status. The results of this study will help inform future development of AVP-923 for LID. This study is being funded through a grant awarded by the Michael J. Fox Foundation.
AVP-923 for the Treatment of Diabetic Neuropathic Pain
Diabetic peripheral neuropathic pain (“DPN pain”), which arises from nerve injury, can result in a chronic and debilitating form of pain that has historically been poorly diagnosed and treated. It is often described as burning, tingling, stabbing, or pins and needles in the feet, legs, hands or arms. An estimated 3.5 million people in the United States experience DPN pain according to the American Diabetes Association. DPN pain currently is most commonly treated with antidepressants, anticonvulsants, opioid analgesics and local anesthetics. Most of these treatments have limited effectiveness or undesirable side effects resulting in a high degree of unmet medical need. The global neuropathic pain market was approximately $2.4 billion in 2010 and is expected to grow to $3.6 billion by 2020 among the seven largest markets, consisting of the United States, Japan, France, Germany, Italy, Spain and the United Kingdom.
Avanir has completed a Phase III clinical trial for AVP-923 in the treatment of patients with DPN pain. In April 2007, we announced positive top-line data from our first Phase III clinical trial of AVP-923 for the treatment of patients with DPN pain. The most commonly reported adverse events from this Phase III study were dizziness, nausea, diarrhea, fatigue and somnolence, which were mild to moderate in nature. Given the recent setback in our Phase II study (PRIME) for the treatment of central neuropathic pain in multiple sclerosis, we are continuing to evaluate our options for this program, including the use of AVP-786 in the advancement of this program.
AVP-786 for the Treatment of Neurologic and Psychiatric Disorders
AVP-786 is a novel investigational drug product consisting of a combination of deuterium-modified dextromethorphan (a new chemical entity or NCE) and the metabolic inhibitor quinidine. The compound was developed through incorporation of deuterium into molecular positions of dextromethorphan, resulting in strengthened molecular bonds which reduce susceptibility to enzyme cleavage. Based on interim data, we believe that we have identified a formulation of AVP-786 with a comparable pharmacokinetic profile, and likely a comparable safety and tolerability profile to AVP-923. This AVP-786 formulation contains significantly less quinidine than used in AVP-923. In June 2013, the FDA agreed to an expedited development pathway for AVP-786, requiring only a limited non-clinical package as part of the Investigational New Drug application. Upon completion of these non-clinical studies, we intend to proceed directly into human clinical trials. We currently intend to study AVP-786 in neuropathic pain, agitation in patients with Alzheimer’s disease and treatment resistant depression.
AVP-825 for the Treatment of Acute Migraine
AVP-825 is an investigational drug-device combination product consisting of low-dose sumatriptan powder to treat acute migraine. The powder is delivered intranasally utilizing a novel Breath Powered delivery technology. If approved, AVP-825 would be the first and only fast-acting dry-powder intranasal form of sumatriptan. We have filed an NDA with the FDA in January 2014.
24
Table of Contents
Migraine represents an area of significant unmet medical need. According to the Centers for Disease Control and Prevention, over 37 million Americans suffer from migraine headaches. The triptan class of medications is generally considered the standard of care with over 13 million prescriptions written annually. Sumatriptan is the class leader with a market share of over 50%, making it the most commonly prescribed migraine drug in the U.S. An online survey of over 2,500 frequent migraine sufferers revealed that 66% were dissatisfied with their treatments. As a result, many migraine sufferers are seeking fast-acting, well tolerated treatment options.
Partnered Programs
Docosanol 10% Cream — Cold Sore Treatment
Docosanol 10% cream is a topical treatment for cold sores. In 2000, we received FDA approval for marketing docosanol 10% cream as an over-the-counter product. Since that time, docosanol 10% cream has been approved by regulatory agencies in Asia, North America, and Europe. In March 2000, we granted a subsidiary of GlaxoSmithKline, SB Pharmco Puerto Rico, Inc. (“GSK”), the exclusive rights under a license to market docosanol 10% cream in the United States and Canada (“GSK License Agreement”). GSK markets the product under the name Abreva® in these markets. Under the terms of the GSK License Agreement, GSK is responsible for all sales and marketing activities and the manufacturing and distribution of docosanol 10% cream. Under the GSK license agreement, we received a total of $25 million in milestone payments from GSK and we were entitled to receive an 8% royalty on net sales of Abreva by GSK.
In November 2002, we sold to Drug Royalty USA an undivided interest in our right to receive future Abreva royalties under the GSK License Agreement for $24.1 million (the “Drug Royalty Agreement”). Under the Drug Royalty Agreement, Drug Royalty USA has the right to receive royalties from GSK on sales of Abreva until the expiration of the patent for Abreva on April 28, 2014. We retained the right to receive 50% of all royalties (a net of 4%) under the GSK License Agreement for annual net sales of Abreva in the U.S. and Canada in excess of $62.0 million. From the effective date of the GSK License Agreement up to the 2002 sale of our royalty rights to Drug Royalty USA, Inc., we received a total of approximately $5.9 million in royalty payments from GSK attributed to the 8% royalty on net sales by GSK.
Under the terms of our docosanol license agreements, our partners are generally responsible for all regulatory approvals, sales and marketing activities, and manufacturing and distribution of the product in the licensed territories. The terms of the license agreements typically provide for us to receive a data transfer fee, potential milestone payments and royalties on product sales. We purchase the active pharmaceutical ingredient (“API”), docosanol, from a large supplier in Western Europe and have, on occasion, sold material to our licensees. We currently store our API in the United States. Any material disruption in manufacturing could cause a delay in shipments and possible loss of sales.
General Information
Our principal executive offices are located at 20 Enterprise, Suite 200, Aliso Viejo, California 92656. Our telephone number is (949) 389-6700 and our e-mail address is info@avanir.com. Our Internet website address is www.avanir.com. We make our periodic and current reports available on our Internet website, free of charge, as soon as reasonably practicable after such material is electronically filed with, or furnished to, the SEC. No portion of our website is incorporated by reference into this Quarterly Report on Form 10-Q. The public may read and copy the materials we file with the SEC at the SEC’s Public Reference Room, located at 100 F Street, NE, Washington, D.C. 20549. The public may obtain information regarding the operation of the Public Reference Room by calling the SEC at 1-800-SEC-0330. The public may also read and copy the materials we file with the SEC by visiting the SEC’s website, www.sec.gov.
25
Table of Contents
CRITICAL ACCOUNTING POLICIES AND ESTIMATES
To understand our financial statements, it is important to understand our critical accounting policies and estimates. The preparation of our financial statements in conformity with accounting principles generally accepted in the United States of America requires us to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Significant estimates and assumptions are required in the determination of revenue recognition and allowances, certain royalties and returns and losses. Significant estimates and assumptions are also required in the appropriateness of amounts recognized for inventories, income taxes, contingencies, and share-based compensation. Some of these judgments can be subjective and complex, and, consequently, actual results may differ from these estimates. For any given individual estimate or assumption made by us, there may also be other estimates or assumptions that are also reasonable. Although we believe that our estimates and assumptions are reasonable, they are based upon information available at the time the estimates and assumptions are made. Actual results may differ significantly from our estimates.
A summary of significant accounting policies and a description of accounting policies that are considered critical may be found in Part II, Item 7 of our Annual Report on Form 10-K for the year ended September 30, 2013 in the “Critical Accounting Policies and Estimates” section, as updated and amended in Note 2 of the Notes to our Condensed Consolidated Financial Statements included herein.
RESULTS OF OPERATIONS
COMPARISON OF THREE MONTH PERIODS ENDED DECEMBER 31, 2013 AND 2012
Revenues
| Three months ended December 31, |
||||||||||||||||
| 2013 | 2012 | $ Change | % Change | |||||||||||||
| REVENUES |
||||||||||||||||
| Gross product sales |
$ | 31,281,493 | $ | 18,373,831 | $ | 12,907,662 | 70 | % | ||||||||
| Less: discounts and allowances |
7,982,466 | 3,494,569 | 4,487,897 | 128 | % | |||||||||||
|
|
|
|
|
|
|
|||||||||||
| Net product sales |
23,299,027 | 14,879,262 | 8,419,765 | 57 | % | |||||||||||
| Revenues from royalties |
2,089,314 | 1,626,010 | 463,304 | 28 | % | |||||||||||
| Revenues from research grant services |
— | 15,000 | (15,000 | ) | — | |||||||||||
| Revenues from co-promote activities |
1,358,078 | — | 1,358,078 | 100 | % | |||||||||||
|
|
|
|
|
|
|
|||||||||||
| Total revenues |
$ | 26,746,419 | $ | 16,520,272 | $ | 10,226,147 | 62 | % | ||||||||
|
|
|
|
|
|
|
|||||||||||
Total revenues were approximately $26.7 million for the three months ended December 31, 2013 compared to approximately $16.5 million for the three months ended December 31, 2012. The increase in total revenues of approximately $10.2 million was primarily attributed to an increase of 62% in volume for NUEDEXTA net product sales when compared to the same period of the prior year and revenues from our co-promote activities which began in the first fiscal quarter of 2014.
Discounts and allowances increased by approximately $4.5 million for the three months ended December 31, 2013 when compared to the three months ended December 31, 2012. Discounts and allowances were 25.5% of gross product sales for the three months ended December 31, 2013 compared to 19.0% of gross product sales for the three months ended December 31, 2012. The increase in the discount and allowances percentage of gross product sales is primarily due to new contracts entered into with managed care entities during fiscal 2013 and increases in government mandated rebates.
Potential revenue-generating contracts that remained active as of December 31, 2013 include licensing revenue from our agreement with GSK, co-promote revenues from our co-promotion agreement with Merck and modest potential revenue generated from various other licensing agreements. Partnering, licensing and research collaborations have been, and may continue to be, an important part of our business development strategy. We may continue to seek partnerships with pharmaceutical companies that can help fund our operations in exchange for sharing in the success of any licensed compounds or technologies.
26
Table of Contents
Operating Expenses
| Three months ended December 31, |
||||||||||||||||
| 2013 | 2012 | $ Change | % Change | |||||||||||||
| OPERATING EXPENSES |
||||||||||||||||
| Cost of product sales |
$ | 1,292,411 | $ | 838,129 | $ | 454,282 | 54 | % | ||||||||
| Cost of research grant services |
— | 9,398 | (9,398 | ) | — | |||||||||||
| Research and development |
9,525,320 | 6,648,091 | 2,877,229 | 43 | % | |||||||||||
| Selling and marketing |
17,475,404 | 13,522,419 | 3,952,985 | 29 | % | |||||||||||
| General and administrative |
8,449,890 | 6,538,403 | 1,911,487 | 29 | % | |||||||||||
|
|
|
|
|
|
|
|||||||||||
| Total operating expenses |
$ | 36,743,025 | $ | 27,556,440 | $ | 9,186,585 | 33 | % | ||||||||
|
|
|
|
|
|
|
|||||||||||
Cost of Product Sales
Cost of product sales was approximately $1.3 million for the three months ended December 31, 2013 compared to cost of product sales of approximately $0.8 million in the same period of fiscal 2013. The increase in cost of product sales is attributable to an increase in the volume of NUEDEXTA net product sales as compared to the same period of the prior year.
Research and Development Expenses
Research and development expenses increased by approximately $2.9 million from approximately $6.6 million in the first quarter of fiscal 2013 to approximately $9.5 million for the first quarter of fiscal 2014. The increase is primarily due to regulatory and development/manufacturing expenses for our AVP-825 program which began in fourth quarter of fiscal 2013.
Selling and Marketing Expenses
Selling and marketing expenses increased by approximately $4.0 million from approximately $13.5 million for the first quarter of fiscal 2013 compared to approximately $17.5 million for the first quarter of fiscal 2014. The increase is primarily attributed to increased marketing and promotion costs of approximately $2.0 million, a $1.2 million increase in personnel expenses resulting from sales force expansions and increased other corporate costs of approximately $0.8 million.
General and Administrative Expenses
General and administrative expenses increased by approximately $1.9 million from approximately $6.5 million for the first quarter of fiscal 2013 compared to approximately $8.4 million for the first quarter of fiscal 2014. The increase is primarily attributed to increased legal costs of $0.6 million, increased personnel costs of $0.5 million and increased other costs of $0.8 million.
Share-Based Compensation
Total share-based compensation expense in the three month periods ended December 31, 2013 and 2012 was approximately $1.4 million for each period. Selling and marketing expense in the three month periods ended December 31, 2013 and 2012 includes share-based compensation expense of approximately $0.5 million and $0.3 million, respectively. General and administrative expense in the three month periods ended December 31, 2013 and 2012 includes share-based compensation expense of approximately $0.6 million and $0.8 million, respectively. Research and development expense in the three month periods ended December 31, 2013 and 2012 includes share-based compensation expense of approximately $0.3 million for each period. As of December 31, 2013, approximately $12.5 million of total unrecognized compensation costs related to non-vested options and awards is expected to be recognized over a weighted average period of 2.7 years. See Note 9, “Employee Equity Incentive Plans” in the Notes to Condensed Consolidated Financial Statements (Unaudited) for further discussion.
27
Table of Contents
Interest Expense
For the three months ended December 31, 2013, interest expense was approximately $1.0 million, compared to approximately $1.1 million for the same period in the prior year.
Net Loss
Net loss was approximately $10.9 million, or $0.07 per share, for the three months ended December 31, 2013, compared to a net loss of approximately $12.1 million, or $0.09 per share, for the three months ended December 31, 2012. The decrease in net loss is primarily attributed to increased NUEDEXTA net product sales and revenues from co-promote activities, partially offset by an increase in operating expenses and interest expense.
LIQUIDITY AND CAPITAL RESOURCES
We assess our liquidity by our ability to fund current and future operations. Key factors in the management of our liquidity are: cash required to fund operating activities including expected operating losses and the levels of inventories, accounts payable and capital expenditures; the timing and extent of cash received from milestone payments under license agreements; ability to obtain adequate credit facilities; and financial flexibility to attract long-term equity capital on favorable terms. Historically, cash required to fund on-going business operations has been provided by financing activities and used to fund operations, working capital requirements and investing activities.
In May 2012, we entered into a Loan and Security Agreement (the “Loan Agreement”) with Oxford Finance LLC and Silicon Valley Bank. The Loan Agreement provides for a term loan of $30.0 million which was funded upon closing of the transaction in June 2012. Under the terms of the Loan Agreement, interest accrues on the outstanding balance at a rate of 8.95% per annum. We will make monthly payments of interest only until January 1, 2014 (the “Amortization Date”). Beginning on the Amortization Date, the outstanding loan balance will be repaid in thirty equal monthly payments of principal and interest. In addition to the original principal, a final payment equal to 7% of the original principal amount of the loan will be due thirty months from the Amortization Date.
Net cash provided by or used in operating, investing and financing activities, are summarized in the table below.
| Three months ended December 31, 2013 |
Change Between Periods |
Three months ended December 31, 2012 |
||||||||||
| Net cash used in operating activities |
$ | (9,780,732 | ) | $ | 2,899,724 | $ | (12,680,456 | ) | ||||
| Net cash used in investing activities |
(1,610,830 | ) | (1,429,367 | ) | (181,463 | ) | ||||||
| Net cash provided by financing activities |
30,222 | 4,091 | 26,131 | |||||||||
|
|
|
|
|
|
|
|||||||
| Net decrease in cash and cash equivalents |
$ | (11,361,340 | ) | $ | 1,474,448 | $ | (12,835,788 | ) | ||||
|
|
|
|
|
|
|
|||||||
Operating activities. Net cash used in operating activities was approximately $9.8 million for the three months ended December 31, 2013, compared to approximately $12.7 million for the three months ended December 31, 2012. Net cash used in operating activities for the three months ended December 31, 2013 included a net loss of $10.9 million offset by non-cash items of $1.7 million consisting primarily of share-based compensation expense, depreciation and amortization, and amortization of debt discount and debt issuance costs, as well as a $0.6 million decrease resulting from changes in working capital items. The changes in working capital items reflect a decrease in trade receivables of $8.3 million primarily due to the timing of sales generated during the first fiscal quarter of 2014, which peaked in the second month of the quarter, compared to the timing of sales generated in the fourth fiscal quarter of 2013, which peaked in the third month of the quarter, and the resulting timing of payments for those sales; an increase in royalty receivable and other receivables of $3.4 million primarily due to timing of invoicing; and a decrease in accounts payable, accrued expenses, accrued compensation and payroll taxes, and other liabilities of approximately $4.7 million primarily due to timing of payments of certain payroll-related expenses.
Net cash used in operating activities for the three months ended December 31, 2012 included a net loss of $12.1 million offset by non-cash items of $1.8 million consisting primarily of share-based compensation expense, depreciation and amortization, and amortization of debt discount and debt issuance costs, as well as a $2.4 million
28
Table of Contents
decrease resulting from changes in working capital items. The changes in working capital items reflect a decrease in accounts payable, accrued expenses, accrued compensation and payroll taxes, and other liabilities of $1.1 million primarily due to timing of payments of certain payroll-related expenses; an increase in royalty receivable and other receivables of approximately $0.7 million primarily due to the timing of invoicing; and an increase in prepaid expenses and other current assets of approximately $0.5 million primarily due to the timing of payments for certain marketing activities.
Investing activities. Net cash used in investing activities was approximately $1.6 million for the three months ended December 31, 2013, compared to approximately $0.2 million for the three months ended December 31, 2012. The increase in cash used in investing activities was primarily related to increased purchases of manufacturing equipment for the AVP-825 program for the three months ended December 31, 2013, when compared to the same period in the prior year.
Financing activities. Net cash provided by financing activities was approximately $30,000 for the three months ended December 31, 2013 compared to approximately $26,000 for the three months ended December 31, 2012. In the three months ended December 31, 2013, we received proceeds of approximately $30,000 from the exercise of stock options. In the first quarter of fiscal 2013, we received proceeds of approximately $26,000 from the exercise of stock options.
In August 2012, we filed with the SEC a shelf registration statement on Form S-3 to sell an aggregate of up to $100.0 million in common stock, preferred stock, debt securities and warrants. Included in this shelf registration on Form S-3 is a prospectus relating to a financing facility with Cowen and Company, LLC (“Cowen”), providing for the sale of up to $25.0 million worth of shares of our common stock from time to time into the open market at prevailing prices in accordance with the terms of a sales agreement entered into on August 8, 2012. As of December 31, 2013, approximately 7.9 million shares of common stock had been sold under this facility for total gross proceeds of approximately $25.0 million. As of December 31, 2013, approximately $75.0 million remains available on this shelf registration statement.
In July 2013, we filed with the SEC a shelf registration statement on Form S-3 to sell an aggregate of up to $150.0 million in common stock, preferred stock, debt securities and warrants. Included in this shelf registration on Form S-3 is a prospectus relating to a financing facility with Cowen, providing for the sale of up to $25.0 million worth of shares of our common stock from time to time into the open market at prevailing prices in accordance with the terms of a sales agreement entered into on August 8, 2012 and amended in July 2013. As of December 31, 2013, approximately 5.0 million shares of common stock had been sold under this facility for total gross proceeds of approximately $25.0 million. Also included in this shelf registration on Form S-3 is a prospectus relating to another financing facility with Cowen, providing for the sale of up to $30.0 million worth of shares of our common stock from time to time into the open market at prevailing prices in accordance with the terms of a sales agreement, entered into on August 8, 2012 and amended in December 2013. No share have of common stock have been sold under this facility. As of December 31, 2013, approximately $125.0 million remains available on this shelf registration statement.
As of December 31, 2013 we have contractual obligations for long-term debt and operating lease obligations, as summarized in the table that follows.
| Payments Due by Period | ||||||||||||||||||||
| Total | Less than 1 year |
1-3 years | 3-5 years | More than 5 years |
||||||||||||||||
| Debt obligation |
$ | 35,692,579 | 13,437,032 | 22,255,547 | $ | — | $ | — | ||||||||||||
| Operating lease obligations |
18,555,807 | 2,607,462 | 6,028,814 | 5,620,998 | 4,298,533 | |||||||||||||||
| Purchase obligations (1) |
9,591,384 | 9,591,384 | — | — | — | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total |
$ | 63,839,770 | $ | 25,635,878 | $ | 28,284,361 | $ | 5,620,998 | $ | 4,298,533 | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| (1) | Purchase obligations consist of the total of trade accounts payable and trade related accrued expenses at December 31, 2013, which approximates our contractual commitments for goods and services supported by purchase obligations in the normal course of our business. |
29
Table of Contents
NUEDEXTA License Milestone Payments. We hold the exclusive worldwide marketing rights to NUEDEXTA for certain indications pursuant to an exclusive license agreement with the Center for Neurologic Study (“CNS”).
We paid a $75,000 milestone upon FDA approval of NUEDEXTA for the treatment of PBA in the first quarter of fiscal 2011. In addition, we are obligated to pay CNS a royalty ranging from approximately 5% to 8% of net U.S. GAAP revenue generated by sales of NUEDEXTA. During the three months ended December 31, 2013 and 2012, royalties of approximately $1.2 million and $744,000, respectively, were recorded to cost of product sales in the condensed consolidated statements of operations. Under certain circumstances, we may have the obligation to pay CNS a portion of net revenues received if we sublicense NUEDEXTA to a third party.
Under the agreement with CNS, we are required to make payments on achievements of up to a maximum of ten milestones, based upon five specific clinical indications. Maximum payments for these milestone payments could total approximately $1.1 million if we pursued the development of NUEDEXTA for all five of the licensed indications. In general, individual milestones range from $75,000 to $125,000 for each accepted new drug application (“NDA”) and a similar amount for each approved NDA in addition to the royalty discussed above on net U.S. GAAP revenues. We do not have the obligation to develop additional indications under the CNS license agreement.
Deuterium-Modified Dextromethorphan License Milestone Payments. – We hold the exclusive worldwide marketing rights to develop and commercialize deuterium-modified dextromethorphan compounds for the potential treatment of neurological and psychiatric disorders, as well as certain rights to other deuterium-modified dextromethorphan compounds pursuant to a license agreement with Concert.
Under the agreement with Concert, we are obligated to make milestone and royalty payments to Concert based on successful advancement of deuterium-modified dextromethorphan products for one or more indications in the United States, Europe, and Japan. Individual milestone payments range from $2.0 – $6.0 million, $1.5 – $15.0 million, and $25.0 – $60.0 million for clinical, regulatory and commercial targets respectively, and in aggregate could total over $200.0 million. Royalty payments are tiered, beginning in the single-digits and increasing to the low double-digits for worldwide net sales of deuterium-modified dextromethorphan products exceeding $1 billion annually. As of December 31, 2013, we have paid $2.0 million for milestones that have been achieved pursuant to this agreement.
AVP-825 License Milestone Payments. In July 2013, we entered into an exclusive license agreement for the development and commercialization of OptiNose’s novel Breath Powered intranasal delivery system containing low-dose sumatriptan powder to treat acute migraine. The licensed territories are the United States, Canada and Mexico. If approved, this product would be the first and only fast-acting dry-powder nasal delivery form of sumatriptan.
Under the terms of the agreement, OptiNose received an upfront cash payment of $20 million and is eligible to receive reimbursement for certain shared development costs and up to an additional $90 million in total payments resulting from the achievement of future clinical, regulatory and commercial milestones. In addition, if approved, we will make tiered royalty payments of a low double-digit percentage of net sales in the United States, Canada and Mexico.
Off-Balance Sheet Arrangements
We have no off-balance sheet arrangements.
Management Outlook
We believe that cash and cash equivalents, restricted cash and cash equivalents and restricted investments of approximately $46.5 million at December 31, 2013, together with funds generated from sales of NUEDEXTA and the availability of capital on our at–the-market facility, will be sufficient to sustain our planned level of operations for the next 12 months. However, we cannot provide assurances that our forecasted revenues and planned level of expenditures will not change or will not result in the depletion of capital resources more rapidly than anticipated.
30
Table of Contents
For information regarding the risks associated with our need to raise capital to fund our ongoing and planned operations, please see Part II, Item 1A, “Risk Factors.”
Item 3. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
As described below, we are exposed to market risks related to changes in interest rates. Because substantially all of our revenue, expenses and capital purchasing activities are transacted in U.S. dollars, our exposure to foreign currency exchange rates is immaterial. However, in the future we could face increasing exposure to foreign currency exchange rates as we seek to expand distribution of NUEDEXTA into Europe and purchase additional services from outside the U.S., including clinical trials. Until such time as we are faced with material amounts of foreign currency exchange rate risks, we do not plan to use derivative financial instruments, which can be used to hedge such risks. We will evaluate the use of derivative financial instruments to hedge our exposure as the needs and risks should arise.
Interest rate sensitivity
Our investment portfolio consists primarily of cash equivalent fixed income instruments invested in government money market funds. The primary objective of our investments in these securities is to preserve principal. We classify our restricted investments, which are primarily certificates of deposit, as of December 31, 2013 as held-to-maturity. These held-to-maturity securities are subject to interest rate risk. Based on our current low yield, any decrease in interest rates is not likely to have a material effect on interest income.
We maintain cash balances at major financial institutions in the United States that are insured by the Federal Deposit Insurance Corporation (“FDIC”) up to $250,000 per owner. At times, deposits held with these financial institutions may exceed the amount of insurance provided by the FDIC. Generally, these deposits may be redeemed upon demand and, therefore, bear minimal risk.
Financial instruments that potentially subject us to concentrations of credit risk consist of cash and cash equivalents and trade receivables. However, we seek to mitigate the risk related to cash and cash equivalents by placing our cash and cash equivalents in various money market mutual funds and at financial institutions of high credit standing.
To mitigate the risk related to trade receivables, we perform ongoing credit evaluations of our customers’ financial condition and would limit the amount of credit extended if necessary; however, we have usually required no collateral.
Item 4. CONTROLS AND PROCEDURES
Evaluation of Disclosure Controls and Procedures
We carried out an evaluation, under the supervision and with the participation of our management, including our Chief Executive Officer and our Vice President, Finance (our principal financial and accounting officer), of the effectiveness of our “disclosure controls and procedures” as of the end of the period covered by this report, pursuant to Rules 13a-15(b) and 15d-15(b) under the Securities Exchange Act of 1934, as amended.
In connection with that evaluation, our CEO and Vice President, Finance concluded that our disclosure controls and procedures were effective and designed to provide reasonable assurance that the information required to be disclosed is recorded, processed, summarized and reported within the time periods specified in the Securities and Exchange Commission rules and forms as of December 31, 2013. For the purpose of this review, disclosure controls and procedures means controls and procedures designed to ensure that information required to be disclosed by the Company in the reports that we file or submit is recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms. These disclosure controls and procedures include, without limitation, controls and procedures designed to ensure that information required to be disclosed by the Company in the reports that we file or submit is accumulated and communicated to management, including our principal executive officer and principal financial and accounting officer, as appropriate, to allow timely decisions regarding required
31
Table of Contents
disclosure. In designing and evaluating the disclosure controls and procedures, our management recognized that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving the desired control objectives, and our management necessarily was required to apply its judgment in evaluating the cost-benefit relationship of possible controls and procedures.
Changes in Internal Controls over Financial Reporting
There has been no change in our internal control over financial reporting (as defined in Rule 13a-15(f) under the Exchange Act) during our first quarter ended December 31, 2013, that has materially affected, or is reasonably likely to materially affect our internal control over financial reporting.
NUEDEXTA ANDA Litigation
In fiscal 2011 and 2012, we received Paragraph IV certification notices from five separate companies contending that certain of our patents listed in the FDA’s publication, “Approved Drug Products with Therapeutic Equivalence Evaluation” (“FDA Orange Book”) (U.S. Patents 7,659,282 (“’282 Patent”), 8,227,484 (“’484 Patent”) and RE 38,115 (“’115 Patent”), which expire in August 2026, July 2023 and January 2016, respectively) are invalid, unenforceable and/or will not be infringed by the manufacture, use, sale or offer for sale of a generic form of NUEDEXTA as described in those companies’ abbreviated new drug application (“ANDA”). The FDA Orange Book provides potential competitors, including generic drug companies, with a list of issued patents covering approved drugs. In August 2011 and March 2012, we filed lawsuits in the U.S District Court for the District of Delaware against Par Pharmaceutical, Inc. and Par Pharmaceutical Companies, Inc. (collectively “Par”), Actavis South Atlantic LLC and Actavis, Inc. (collectively “Actavis”), Wockhardt USA, LLC and Wockhardt, Ltd. (collectively, “Wockhardt”), Impax Laboratories, Inc. (“Impax”) and Watson Pharmaceuticals, Inc., Watson Laboratories, Inc. and Watson Pharma, Inc. (collectively “Watson”) (Par, Actavis, Wockhardt, Impax and Watson, collectively the “Defendants”). In September and October 2012, we filed lawsuits in the U.S. District Court for the District of Delaware against the Defendants. All lawsuits (collectively, the “ANDA Actions”) were filed on the basis that the Defendants’ submissions of their respective ANDAs to obtain approval to manufacture, use, sell, or offer for sale generic versions of NUEDEXTA prior to the expiration of the ‘282 Patent, the ‘484 Patent and the ‘115 Patent listed in the FDA Orange Book constitute infringement of one or more claims of those patents. On October 31, 2012, Watson announced the divestiture of its ANDA for a generic form of NUEDEXTA to Sandoz, Inc. (“Sandoz”). As a result of Sandoz’ acquisition and maintenance of said ANDA, on May 30, 2013, we filed suit in the U.S. District Court for the District of Delaware against Sandoz. This suit was filed on the basis that Sandoz’ ANDA to obtain approval to manufacture, use, sell, or offer for sale generic versions of NUEDEXTA prior to the expiration of the ’282 Patent, the ’484 Patent and the ’115 Patent listed in the FDA Orange Book constitutes infringement of one or more claims of those patents.
On December 3, 2012, we received a Memorandum Opinion and Order (the “Order”) issued by Judge Leonard P. Stark of the United States District Court for the District of Delaware (the “Court”) related to the Markman hearing held October 5, 2012 in our ongoing patent infringement case against the Defendants. The Order establishes the meaning of patent claim terms in dispute between the parties. After comprehensive briefing and oral argument, Judge Stark’s Order adopted the Company’s proposed or stipulated patent term constructions.
A bench trial was held in September 2013 and concluded on October 15, 2013. We are currently awaiting Judge Stark’s decision.
On August 9, August 30, and September 6, 2013, we entered into settlement agreements with Sandoz, Actavis and Wockhardt, respectively, to resolve pending patent litigation in response to their ANDAs seeking approval to market generic versions of NUEDEXTA capsules. The settlement agreements grant Sandoz, Actavis and Wockhardt the right to begin selling a generic version of NUEDEXTA on July 30, 2026, or earlier under certain circumstances. The parties also filed stipulations and orders of dismissal with the United States District Court for the District of Delaware which conclude the litigation with respect to Sandoz, Actavis and Wockhardt. The settlements do not end our ongoing litigation against the other two ANDA filers.
32
Table of Contents
The 30-month stay associated with the filing of the ANDA Actions expired on December 30, 2013. In order to launch a generic form of NUEDEXTA, the Defendants must first receive FDA approval and as of February 4, 2014, the FDA has not granted approval to any Defendant’s ANDA. We intend to vigorously enforce our intellectual property rights relating to NUEDEXTA, but we cannot predict the outcome of these ANDA Actions.
Master’s Litigation
On January 14, 2014, a purported shareholder of Avanir brought a claim against Avanir and its directors, as well as a former director, in connection with our 2014 Annual Meeting proxy statement. The action is captioned Masters v. Avanir Pharmaceuticals, Inc., et al., Case No. 14-cv-00053, and is pending in the United States District Court for the Central District of California (the “Masters Action”). The Masters Action complaint alleges that the defendants violated Section 14(a) of the Securities Exchange Act, and further alleges that the individual defendants breached their fiduciary duties, based upon purported deficiencies in disclosures relating to Proposal 4 contained in our proxy statement, with respect to our equity compensation plan and practices. The complaint seeks, among other things, equitable relief that would enjoin the consummation of the shareholder vote on Proposal 4, declaratory relief, and attorney’s fees and costs. We intend to defend the Master’s Action vigorously, however, all litigation is inherently uncertain, and there can be no assurance that our defense of these or similar actions would be successful.
General and Other
In the ordinary course of business, we may face various claims brought by third parties and we may, from time to time, make claims or take legal actions to assert our rights, including intellectual property rights as well as claims relating to employment and the safety or efficacy of products. Any of these claims could subject us to costly litigation and, while we generally believe that it has adequate insurance to cover many different types of liabilities, our insurance carriers may deny coverage or policy limits may be inadequate to fully satisfy any damage awards or settlements. If this were to happen, the payment of any such awards could have a material adverse effect on our consolidated operations, cash flows and financial position. Additionally, any such claims, whether or not successful, could damage our reputation and business. Management believes the outcomes of currently pending claims and lawsuits will not likely have a material effect on the Company’s consolidated operations or financial position.
| Item 1A. | RISK FACTORS |
This Quarterly Report on Form 10-Q contains forward-looking information based on our current expectations. Because our actual results may differ materially from any forward-looking statements that we make or that are made on our behalf, this section includes a discussion of important factors that could affect our actual future results, including, but not limited to, our product sales, capital resources, commercial market estimates, safety of NUEDEXTA, future development efforts, patent protection, effects of healthcare reform, reliance on third parties, and other risks set forth below. We disclaim any intent to update forward-looking statements to reflect subsequent developments or actual results.
33
Table of Contents
Risks Relating to Our Business
Our near-term prospects depend on reaching profitability from the commercialization of NUEDEXTA in the United States. If we are unable to continue to increase NUEDEXTA revenues, including through raising PBA awareness among patients and physicians, driving higher rates of physician adoption and obtaining reimbursement and third party payer coverage, our ability to generate significant revenue or achieve profitability will be adversely affected.
Although NUEDEXTA has been approved for marketing, our ability to generate significant revenue in the near term is entirely dependent upon our ability to continue the successful commercialization of NUEDEXTA. To continue to be successful we must:
| • | maintain successful sales, marketing and educational programs for our targeted physicians and other health care providers; |
| • | raise patient and physician awareness of PBA and encourage physicians to screen patients for the condition; |
| • | obtain adequate reimbursement for NUEDEXTA from a broad range of payers; and |
| • | maintain and defend our patent protection and maintain regulatory exclusivity for NUEDEXTA. |
Supplying the market for NUEDEXTA requires us to manage relationships with an increasing number of collaborative partners, suppliers and third-party contractors. If we are unable to successfully maintain the required sales and marketing infrastructure, as well as successfully manage an increasing number of relationships, including with suppliers, manufacturers, distributors, insurance carriers and prescribers, we will have difficulty growing our business. In addition, pharmacies, institutions and prescribers may rely on third-party medical information systems to interpret the NUEDEXTA approved product label and guide utilization of NUEDEXTA. If these information systems load incorrect information or misinterpret the approved product label, it may result in lower adoption or utilization than expected. For example, because NUEDEXTA contains quinidine, which is a known pro-arrhythmic drug at antiarrhythmic doses exceeding 600 mg per day, it is possible that medical information systems may incorrectly identify NUEDEXTA as contraindicated or otherwise inappropriate for a patient, even in situations where the risks are substantially less than perceived.
In addition, we may enter into co-promotion or out-licensing arrangements with other pharmaceutical or biotechnology partners for NUEDEXTA where necessary to reach customers in domestic or foreign market segments and when deemed strategically and economically advantageous. To the extent that we enter into co-promotion or other licensing arrangements, our product revenues may be lower than if we directly marketed and sold NUEDEXTA, and some or all of the revenues we receive will depend upon the efforts of third parties, which may not be successful. If we are unable to accomplish any of these key objectives, we may not be able to generate significant product revenue or become profitable.
We have a history of net losses and an accumulated deficit, and we may be unable to generate sufficient revenue to achieve or maintain profitability in the future.
We have experienced significant net losses and negative cash flows from operations and we expect our negative cash flow from operations to continue until we are able to generate significant revenues from sales of NUEDEXTA. As of December 31, 2013, we had an accumulated deficit of approximately $511.5 million. We have incurred these losses principally from costs incurred in funding the research, development and clinical testing of our drug candidates, from our general and administrative expenses and from our commercialization activities for NUEDEXTA. We may continue incurring net losses for the foreseeable future and we expect our operating losses to continue for the foreseeable future as we continue to grow NUEDEXTA sales, invest in the development of AVP-923 and AVP-786, seek to commercialize NUEDEXTA in the European Union (“EU”), and seek FDA approval and subsequently commercialize AVP-825.
34
Table of Contents
Our ability to generate revenue and achieve profitability in the near term is dependent on our ability, alone or with partners, to successfully manufacture and market NUEDEXTA for the treatment of patients with PBA in the United States. We expect to continue to spend substantial amounts on the ongoing marketing of NUEDEXTA domestically for the treatment of PBA, invest in Europe to commercialize NUEDEXTA, and seek regulatory approvals for use of NUEDEXTA in other geographic markets and indications. As a result, we may be unable to generate sufficient revenue from product sales to become profitable or generate positive cash flows.
Certain of our key issued patents covering NUEDEXTA are currently being challenged and our pending patent applications may be denied. An adverse outcome in either case would adversely affect our ability to generate significant product revenue or become profitable.
We have invested in an extensive patent portfolio and we rely substantially on the protection of our intellectual property through our ownership or control of issued patents and patent applications. The degree of patent protection that will ultimately be afforded to us in the U.S. and in other important markets remains uncertain and is dependent upon the scope of protection decided upon by the patent offices, courts and lawmakers in these countries. If we cannot prevent others from exploiting claims in our patent portfolio, we will not derive the benefit from it that we currently expect. Further, we may incur substantial expense from litigation to protect our patent portfolio.
The validity, enforceability and scope of our core patents covering NUEDEXTA are currently being challenged as a result of abbreviated new drug application (“ANDA”) filings from generic drug companies. An adverse outcome in the current or any future challenges to the validity, enforceability or scope of our patent portfolio could significantly reduce revenues from NUEDEXTA and any future products. More broadly, investors should be aware that the pharmaceutical industry is highly competitive. Our ability to compete in this space involves various risks relating to our intellectual property, including:
| • | our patents covering NUEDEXTA may be found to be invalid and unenforceable or insufficiently broad to block the introduction of a generic form of NUEDEXTA; |
| • | the claims in any of our pending patent applications may not be allowed and/or our patent applications may not be granted; |
| • | competitors may develop similar or superior technologies independently, duplicate our technologies, or design around the patented aspects of our technologies; |
| • | any of our issued patents may not provide us with significant competitive advantages; and |
| • | we may not be able to secure additional worldwide intellectual property protection for our NUEDEXTA patent portfolio. |
An adverse outcome with respect to any of these risks could adversely affect our ability to generate significant product revenue or become profitable.
We have received notices of ANDA filings for NUEDEXTA submitted by five generic drug companies. These ANDA filings assert that a generic form of NUEDEXTA would not infringe our FDA Orange Book listed patents and/or those patents are invalid. As a result of these filings, we have commenced litigation to defend our patent rights, and have subsequently settled three of these cases. The litigation has been costly and time consuming and, depending on the outcome of the litigation, we may face competition from lower cost generic or follow-on products in the near term.
NUEDEXTA is approved under the provisions of the Federal Food, Drug and Cosmetic Act (“FDCA”), which renders it susceptible to potential competition from generic manufacturers via the Hatch-Waxman Act and ANDA process. Generic manufacturers pursuing ANDA approval are not required to conduct costly and time-consuming clinical trials to establish the safety and efficacy of their products; rather, they are permitted to rely on the innovator’s data regarding safety and efficacy. Additionally, generic drug companies generally do not expend significant sums on sales and marketing activities, instead relying on physicians or payers to substitute the generic form of a drug for the branded form. Thus, generic manufacturers can sell their products at prices much lower than those charged by the innovative pharmaceutical or biotechnology companies who have incurred substantial expenses associated with the research and development of the drug product and who must spend significant sums marketing a new drug.
35
Table of Contents
The ANDA procedure includes provisions allowing generic manufacturers to challenge the innovator’s patent protection by submitting “Paragraph IV” certifications to the FDA in which the generic manufacturer claims that the innovator’s patent is invalid, unenforceable and/or will not be infringed by the manufacture, use, or sale of the generic product. A patent owner who receives a Paragraph IV certification may choose to sue the generic applicant for patent infringement. In recent years, generic manufacturers have used Paragraph IV certifications extensively to challenge the applicability of patents listed in the FDA’s Approved Drug Products List with Therapeutic Equivalence Evaluations, commonly referred to as the Orange Book, on a wide array of innovative therapeutic products. We expect this trend to continue and to affect drug products with even relatively modest revenues.
We have received Paragraph IV certification notices from five separate companies contending that our patents listed in the Orange Book (U.S. Patents 7,659,282, 8,227,484 and RE 38,115, which expire in August 2026, July 2023 and January 2016, respectively) are invalid, unenforceable and/or will not be infringed by the manufacture, use, or sale of a generic form of NUEDEXTA. In response to these notices, we have filed suit against all of the generic drug companies to defend our patent rights. We have entered into settlement agreements with three of the companies to resolve pending patent litigation in response to their ANDAs seeking approval to market generic versions of NUEDEXTA capsules. The settlement agreements grant the three companies the right to begin selling a generic version of NUEDEXTA on July 30, 2026, or earlier under certain circumstances. The parties also filed stipulations and orders of dismissal with the United States District Court for the District of Delaware which conclude the litigation with respect to the three companies. The settlements do not end our ongoing litigation against the other two ANDA filers.
The 30-month stay associated with the filing of the ANDA actions expired on December 30, 2013. In order to launch a generic form of NUEDEXTA, the Defendants must first receive FDA approval and as of February 4, 2014, the FDA has not granted approval to any Defendant’s ANDA.
Although we intend to vigorously enforce our intellectual property rights relating to NUEDEXTA, there can be no assurance that we will prevail in our defense of our patent rights. Our existing patents could be invalidated, found unenforceable or found not to cover a generic form of NUEDEXTA. If an ANDA filer were to receive FDA approval to sell a generic version of NUEDEXTA and/or prevail in any patent litigation, NUEDEXTA would become subject to increased competition and our revenue would be adversely affected.
PBA is a new market and estimates vary significantly over the potential market size and our anticipated revenues over the near and long term.
NUEDEXTA is being made available to patients to treat PBA, an indication for which there was no previously established pharmaceutical market. Industry sources and equity research analysts have a wide divergence of estimates for the near- and long-term market potential of our product. A variety of assumptions directly impact the estimates for our drug’s market potential, including estimates of underlying neurologic condition prevalence, severity of PBA prevalence among these conditions, rates of physician adoption of our drug for treatment of PBA among these populations, health plan reimbursement rates, and patient adherence and compliance rates within each underlying neurological condition. Small differences in these assumptions can lead to widely divergent estimates of the market potential of our product. Additionally, although our approved product label is indicated to treat PBA, without regard to the underlying neurological condition, it is possible that physicians, the FDA’s Office of Prescription Drug Promotion (OPDP), payers or others may interpret the label more narrowly than the FDA’s Division of Neurology Products approval for a broad PBA label and believe that PBA secondary to certain conditions, such as Alzheimer’s disease, is not an indicated use. If such misinterpretations are widespread, the actual market size may be smaller than we have estimated. Accordingly, investors are cautioned not to place undue reliance on any particular estimates of equity research analysts or industry sources.
36
Table of Contents
Significant safety or drug interaction problems could arise with respect to NUEDEXTA, which could result in restrictions in NUEDEXTA’s label, recalls, withdrawal of NUEDEXTA from the market, an adverse impact on potential sales of NUEDEXTA, or cause us to alter or terminate current or future NUEDEXTA clinical development programs, any of which would adversely impact our future business prospects.
Discovery of previously unknown safety or drug interaction problems with an approved product may result in a variety of adverse regulatory actions. This risk may be increased as physicians, at their own discretion, may prescribe NUEDEXTA for off-label uses, which may result in unknown safety or drug interactions. Under the Food and Drug Administration Amendments Act of 2007, the FDA has broad authority to force drug manufacturers to take any number of actions if previously unknown safety or drug interaction problems arise, including, but not limited to: (i) requiring manufacturers to conduct post-approval clinical studies to assess known risks or signals of serious risks, or to identify unexpected serious risks; (ii) mandating labeling changes to a product based on new safety information; or (iii) requiring manufacturers to implement a Risk Evaluation Mitigation Strategy, or REMS, where necessary to assure safe use of the drug. In addition, previously unknown safety or drug interaction problems could result in product recalls, restrictions on the product’s permissible uses, or withdrawal of the product from the market.
The combination of dextromethorphan and quinidine has never been marketed for the treatment of any condition until the approval of NUEDEXTA for the treatment of PBA. NUEDEXTA has only been studied in a limited number of patients in clinical trials. The data submitted to the FDA and the European Medicines Agency (“EMA”) were obtained in controlled clinical trials of limited duration. In connection with the approval of NUEDEXTA, the FDA and EMA have required that we conduct certain post-approval studies, which include clinical and non-clinical studies. New safety or drug interaction issues may arise from these studies or as NUEDEXTA is used over longer periods of time by a wider group of patients. For example, elderly patients may be more prone to have multiple risk factors for adverse events such as certain cardiac conditions, hepatic or renal insufficiency, or multi-drug treatment regimens. In addition, as we conduct other clinical trials for AVP-923 in other indications, new safety or drug interaction problems may be identified which could negatively impact both our ability to successfully complete these studies and the use and/or regulatory status of NUEDEXTA for the treatment of PBA. New safety or drug interaction issues may result in product liability lawsuits and may require us to, among other things, provide additional warnings and/or restrictions on the NUEDEXTA prescribing information, including a boxed warning, directly alert healthcare providers of new safety information, narrow our approved indications, or alter or terminate current or planned trials for additional uses of AVP-923, any of which could have a significant adverse impact on potential sales of NUEDEXTA and our ability to achieve or maintain profitability.
In addition, if we are required to conduct additional post-approval clinical studies, implement a REMS, or take other similar actions, such requirements or restrictions could have a material adverse impact on our ability to generate revenues from sales of NUEDEXTA, and/or require us to expend significant additional funds.
We have limited capital resources and may need to raise additional funds to support our operations.
We have experienced significant operating losses due to costs associated with funding the research, development, clinical testing and commercialization of NUEDEXTA and our drug candidates. We expect to continue to incur substantial operating losses for the foreseeable future as we continue to expand our commercialization efforts for NUEDEXTA in the U.S. and in European markets, continue to develop AVP-923 and AVP-786, and seek FDA approval and subsequently commercialize AVP-825. Although we had approximately $46.5 million in cash and cash equivalents, restricted cash and cash equivalents, and restricted investments as of December 31, 2013, we currently do not have sufficient revenue from NUEDEXTA or other sources of recurring revenue or cash flow from operations to sustain our operations and it is possible that we may not be able to achieve profitability with our current capital resources.
In light of our substantial long-term capital needs, we may need to partner our rights to NUEDEXTA (either in the U.S. or outside the U.S.) or raise additional capital in the future to finance our long-term operations, until we are able to generate sufficient revenue from product sales to fund our operations. Based on our current loss rate and existing capital resources as of the date of this report, we estimate that we have sufficient funds to sustain our operations at their current and anticipated levels over the next 12 months, which includes the costs associated with the ongoing commercialization of NUEDEXTA for the treatment of PBA in the U.S. and European markets, seeking
37
Table of Contents
FDA approval and subsequently commercialize AVP-825. Although we expect to be able to raise additional capital if needed, there can be no assurance that we will be able to do so or that the available terms of any financing would be acceptable to us. If we are unable to raise additional capital to fund future operations, we may experience significant delays or cutbacks in the commercialization of NUEDEXTA and may be forced to further curtail our operations.
If we raise additional capital, we may do so through various financing alternatives, including licensing or sales of our technologies, drugs and/or drug candidates, selling shares of common or preferred stock, through the acquisition of other companies, or through the issuance of debt securities. Each of these financing alternatives carries certain risks.
Raising capital through the issuance of common stock may depress the market price of our stock. Any such financing will dilute our existing stockholders and, if our stock price is relatively depressed at the time of any such offering, the levels of dilution would be greater.
In addition, debt financing may involve agreements that include covenants limiting or restricting our ability to take specific actions, such as encumbering our assets, making capital expenditures or entering into certain licensing transactions.
Our Loan Agreement with Oxford and SVB contains certain covenants that could adversely affect our operations and, if an event of default were to occur, we could be forced to repay the outstanding indebtedness sooner than planned and possibly at a time when we do not have sufficient capital to meet this obligation. The occurrence of any of these events could cause a significant adverse impact on our business, prospects and stock price.
Pursuant to our Loan Agreement with Oxford and SVB, we have pledged all of our assets, other than our patents and other intellectual property rights, and have agreed that we may not sell or assign rights to our patents and other intellectual property without the prior consent of Oxford and SVB. The Loan Agreement also requires us to maintain a minimum sales level relative to projected NUEDEXTA revenues, measured on a trailing three-month basis, or maintain cash and cash equivalents in accounts subject to control agreements in favor of the collateral agent equal to at least 1.5 times the outstanding amount of obligations under the Loan Agreement. The failure to satisfy both of these tests would result in an event of default, which could accelerate our repayment obligations. Additionally, the Loan Agreement contains affirmative and negative covenants that, among other things, restrict our ability to:
| • | incur additional indebtedness or guarantees; |
| • | incur liens; |
| • | make investments, loans and acquisitions; |
| • | consolidate or merge; |
| • | sell assets, including capital stock of subsidiaries; |
| • | alter the business of the Company; |
| • | engage in transactions with affiliates; and |
| • | enter into agreements limiting dividends and distributions of certain subsidiaries. |
The Loan Agreement also includes events of default, including, among other things, payment defaults; breaches of representations, warranties or covenants; certain bankruptcy events; the occurrence of certain material adverse changes; and a commercial, generic version of NUEDEXTA (for the treatment of PBA) becoming available. Upon the occurrence of an event of default and following any cure periods (if applicable), a default interest rate of an additional 5.0% per annum may be applied to the outstanding loan balances, and the lenders may declare all outstanding obligations immediately due and payable and take such other actions as set forth in the Loan Agreement.
38
Table of Contents
These terms of the Loan Agreement could prevent us from taking certain actions without the consent of our lenders and, if an event of default should occur, we could be required to immediately repay the outstanding indebtedness. If we are unable to repay this debt, the lenders would be able to foreclose on the secured collateral, including our cash accounts, and take other remedies permitted under the security agreement. Even if we are able to repay the indebtedness on an event of default, the repayment of these sums may significantly reduce our working capital and impair our ability to operate as planned. The occurrence of any of these events could cause a significant adverse impact on our business, prospects and stock price.
We recently entered into a co-promotion agreement for our institutional sales force that could have negative business implications.
We recently entered into a multi-year agreement involving our institutional sales team. This co-promote agreement involves certain financial and operating risks, including:
| • | promotional efforts being diverted to the co-promote product which could result in a negative impact on NUEDEXTA revenues; |
| • | increased compliance risk which could harm our reputation; and |
| • | if we are unable to generate significant revenue or achieve profitability for the co-promote activity. |
If any of these risks occurred, it could adversely affect our business, financial condition and operating results.
There can be no assurance that the FDA will approve AVP-825 for the treatment of acute migraine.
A Phase II and Phase III clinical trial of AVP-825 for acute treatment of migraine have been completed and will form the basis for a 505(b)(2) NDA filing with the FDA together with previously completed studies with the reference drug, sumatriptan. The FDA and other regulatory authorities will have substantial discretion in evaluating the results of the Phase III clinical trial and our NDA filing.
It is possible that the FDA may require us to conduct additional non-clinical, clinical or chemical manufacturing control-related studies before we gain approval for AVP-825. Prior to approving a new drug, the FDA generally requires that the efficacy of the drug be demonstrated in two adequate and well-controlled clinical trials. In some situations, the FDA approves drugs on the basis of a single well-controlled clinical trial and / or on the basis of referencing data generated previously with the reference drug under the 505(b)(2) application process. If the FDA determines that the clinical trials already conducted do not demonstrate a clinically meaningful benefit and an acceptable safety profile, or do not reflect an acceptable risk-benefit profile or if the FDA requires us to conduct additional clinical trials in order to gain approval, we may incur significant additional development costs and commercialization of AVP-825 would be prevented or delayed and our business would be adversely affected. AVP-825 is classified as a new drug-device combination which requires additional conditions to be satisfied for FDA approval beyond what is required for other drug products.
In addition, this Breath Powered intranasal device has not been previously reviewed or approved by the FDA and therefore, it is possible that other issues may arise during the review process which could delay or preclude the approval and require additional capital investment.
We established a joint steering committee, a joint intellectual property committee and joint development committee which will give OptiNose input on matters related to development of AVP-825 and intellectual property related to the product. As a result, our success depends partially on the success of OptiNose in performing its responsibilities and enforcing their intellectual property rights.
39
Table of Contents
There can be no assurance that we will be able to successfully manufacture, distribute and commercialize AVP-825, including adequate sales, marketing, distribution and manufacturing capabilities. If we are unable to successfully commercialize AVP-825, our ability to generate significant revenue and achieve product launch timelines may be adversely affected.
We are primarily responsible for the manufacturing and distribution of AVP-825. We will utilize third parties to manufacture, package and distribute AVP-825. We have no experience in manufacturing AVP-825 in commercial quantities. Any delays or difficulties, including the purchase of manufacturing equipment, entering into manufacturing and supply agreements, obtaining API or in the manufacturing, packaging or distribution of AVP-825, could negatively affect our sales revenues as well as delay FDA approval.
If AVP-825 is approved by the FDA, our ability to generate significant revenue is entirely dependent upon our ability to commercialize AVP-825. Our future results could be impacted by important factors which include, but are not limited to, commercial market estimates, reliance on market research, competition in the migraine segment, effect of healthcare reform, ability to secure reasonable pricing and patent protection. If we are unable to generate revenues from AVP-825, including through raising awareness among patients and physicians of the benefits of using the device for the treatment of acute migraine, driving higher rates of physician adoption and obtaining reimbursement and third party payer coverage, our ability to generate significant revenue or achieve profitability will be adversely affected.
We may not be able to adequately build or maintain necessary sales, marketing, supply chain management or reimbursement capabilities on our own or enter into arrangements with third parties to perform these functions in a timely manner or on acceptable terms. Additionally, maintaining sales, marketing and distribution capabilities may be more expensive than we anticipate, requiring us to divert capital from other intended purposes or preventing us from building our sales, marketing and distribution capabilities to the desired levels. To be successful we must:
| • | recruit and retain adequate numbers of effective sales personnel; |
| • | effectively train our sales personnel on AVP-825; |
| • | reach an adequate number of health care providers which treat acute migraine; |
| • | manage geographically dispersed sales and marketing operations; and |
| • | rely on OptiNose to maintain and defend the patent protection and maintain regulatory exclusivity for AVP-825. |
The commercialization of AVP-825 requires us to manage relationships with an increasing number of collaborative partners, suppliers and third-party contractors. If we are unable to successfully establish and maintain the required infrastructure, either internally or through third parties, and successfully manage an increasing number of relationships, we will have difficulty growing our business. In addition, we may enter into co-promotion or out-licensing arrangements with other pharmaceutical or biotechnology partners where necessary to reach customers when deemed strategically and economically advisable. To the extent that we enter into co-promotion or other licensing arrangements, our product revenues are likely to be lower than if we directly marketed and sold AVP-825, and some or all of the revenues we receive will depend upon the efforts of third parties, which may not be successful. If we are unable to develop and maintain adequate sales, marketing and distribution capabilities, independently or with others, we may not be able to generate significant product revenue or become profitable.
We recently entered into a license agreement with obligations that could require significant capital infusions and could involve many financial and operating risks.
In July 2013, we entered into an exclusive license agreement for the development and commercialization of a Breath Powered intranasal delivery system containing low-dose sumatriptan powder to treat acute migraine, now named AVP-825. The licensed territories are the United States, Canada and Mexico. Our obligations pursuant to this license agreement could require significant capital infusions and could involve many financial and operating risks, including, but not limited to, the following:
| • | we may have to issue debt or equity securities to meet our obligations under this license agreement, which would dilute our stockholders and could adversely affect the market price of our common stock, and we may issue securities or rights with contingent payment obligations, which could have variable accounting treatment and negative accounting consequences; |
40
Table of Contents
| • | our obligations pursuant to this license agreement may result in a negative impact on our results of operations and, as such, delay profitability; |
| • | we may encounter difficulties in assimilating and integrating AVP-825 into our existing business, including related technologies, personnel or operations; |
| • | our obligations pursuant to this license agreement may require significant capital infusions and AVP-825 may not generate sufficient value to justify the acquisition cost; |
| • | focus on integrating AVP-825 into our existing business may disrupt our ongoing business, divert resources, increase our expenses and distract our management; and |
| • | we have little or no prior experience in the migraine market and our assumptions surrounding the market, including revenue forecasts, may not be accurate. |
If any of these risks occurred, it could adversely affect our business, financial condition and operating results.
Our co-promotion agreement with Merck may be terminated with or without cause, which could negatively impact our business.
The co-promotion agreement with Merck may be terminated with cause at any time or without cause upon 90 days written notice at any time after the first year anniversary of the launch date. If the agreement were to be terminated, with or without cause, our business could be negatively impacted, including failure to achieve profitability for the co-promote activity and damage to our reputation.
We are seeking partners to market NUEDEXTA in the EU, and we will be substantially dependent on any such marketing partners in those countries to successfully commercialize NUEDEXTA. We may not be successful in establishing a partnership, but even if we are successful in establishing a partnership or decide to launch ourselves, we may be unable to generate NUEDEXTA revenue in the European market, including through raising PBA awareness among patients and physicians, driving higher rates of physician adoption and obtaining reimbursement and third party payer coverage, our ability to generate significant revenue or achieve profitability in the European market will be adversely affected.
NUEDEXTA has been approved for marketing in the EU and our ability to generate significant revenue in the EU is entirely dependent upon our ability to successfully commercialize NUEDEXTA. To be successful in the EU we must:
| • | maintain successful sales, marketing and educational programs for our targeted physicians and other health care providers; |
| • | raise patient and physician awareness of PBA and encourage physicians to screen patients for the condition; |
| • | obtain adequate reimbursement for NUEDEXTA from a broad range of payers; and |
| • | maintain and defend our patent protection and maintain regulatory exclusivity for NUEDEXTA. |
Our prospects to successfully commercialize NUEDEXTA in the EU will depend, among other things, on our ability to establish successful arrangements with international distribution and marketing partners. Consummation of NUEDEXTA partnering arrangements is subject to the negotiation of complex contractual relationships and we may not be able to negotiate such agreements on a timely basis, if at all, or on terms acceptable to us. Where we are successful in entering into these third party arrangements, our revenues from NUEDEXTA sales will be lower than if we commercialized directly, as we will be required to share the revenues with our licensing, commercialization and development partners. If our commercialization efforts with our partners are unsuccessful or we are unable to launch NUEDEXTA in certain countries, we may realize little or no revenue from sales in the EU despite having received marketing approval. In the event that we are unsuccessful in obtaining a partner, we may establish a NUEDEXTA sales and marketing sales infrastructure in the EU.
41
Table of Contents
We are developing AVP-786, a next generation drug product containing deuterium-modified dextromethorphan. It is possible that studies of AVP-786 may not produce favorable results and future studies utilizing AVP-786 carry certain risks.
We have licensed exclusive, worldwide rights to develop and commercialize deuterium-modified dextromethorphan compounds for the potential treatment of neurologic and psychiatric disorders, as well as certain rights to other deuterium-modified dextromethorphan compounds. The goal of the AVP-786 program is to deliver therapeutically effective levels of deuterium-modified dextromethorphan, but with a reduction in the need for an enzyme inhibitor such as quinidine. Although we believe that a drug product containing deuterium-modified dextromethorphan will allow us to significantly reduce the level of quinidine, we are not certain that AVP-786 will have a similar efficacy profile to AVP-923 and, if approved by the FDA, that the reduction in quinidine will result in improved safety language in the package insert.
We completed a pharmacokinetic study with AVP-786 and, based on this data, we believe that we have identified a formulation of AVP-786 with a comparable pharmacokinetic profile, and likely a comparable safety and tolerability profile to AVP-923. In June 2013, the FDA agreed to an expedited development pathway for AVP-786, requiring only a limited non-clinical package as part of the Investigational New Drug application. Although the FDA has agreed to allow us to reference the extensive data generated during the AVP-923 development programs in support of the AVP-786 development programs and regulatory filings, there can be no assurance that we will be successful developing this investigational drug or that we will obtain regulatory approval domestically or internationally. In addition, our initial discussions regarding the development of AVP-786 have been with the FDA’s Division of Neurology. There can be no assurance that other divisions at the FDA will agree with the expedited development plan discussed with the Division of Neurology.
Additionally, we established a joint steering committee and a joint patent committee which will give Concert input on development and patent prosecution for a period of time. As a result, our success depends partially on the success of Concert in performing its responsibilities.
We have licensed or sold most of our non-core drug development programs and related assets and these and other possible future transactions carry certain risks.
We have entered into agreements for the licensing or sale of our non-core assets, including FazaClo, our anthrax antibody program, and other antibodies in our infectious disease program, as well as docosanol in the U.S. and other markets worldwide. From time to time, we have been and will continue to be engaged in discussions with potential licensing or development partners for NUEDEXTA for the treatment of PBA and/or AVP-923/AVP-786 for other indications and we may choose to pursue a partnership or license involving NUEDEXTA and/or AVP-923/AVP-786 if the terms are attractive. Additionally, we may seek to acquire rights to other drugs or technologies. However, all of these transactions involve numerous risks, including:
| • | diversion of management’s attention from normal daily operations of the business; |
| • | disputes over earn-outs, working capital adjustments or contingent payment obligations; |
| • | inability to effectively integrate an acquired business or asset or to achieve the efficiencies or synergies we anticipate; |
| • | insufficient proceeds to offset expenses associated with the transactions; and |
| • | the potential loss of key employees following such a transaction. |
Transactions such as these carry significant risks where a large portion of the total consideration is contingent upon post-closing events, such as regulatory, commercialization or sales milestones. We may not have control over whether these milestones are met and, if they are not met, then a potentially large portion of the value of the transaction may not be realized. Disputes may also develop over these and other terms, such as representations and warranties, indemnities, earn-outs, and other provisions in the transaction agreements. If disputes are resolved unfavorably, our financial condition and results of operations may be adversely affected and we may not realize the anticipated benefits from the transactions.
42
Table of Contents
Disputes relating to these transactions can lead to expensive and time-consuming litigation and may subject us to unanticipated liabilities or risks, disrupt our operations, divert management’s attention from day-to-day operations, and increase our operating expenses.
The FDA’s safety concerns regarding our prior formulation of NUEDEXTA, known as AVP-923, for the treatment of PBA extend to other clinical indications that we have been pursuing, including DPN pain. Due to these concerns, any future development of AVP-923 or AVP-786 for other indications, including central neuropathic pain in patients with MS, is expected to use an alternative lower-dose quinidine formulation, which may negatively affect efficacy.
We have completed a single Phase III trial for AVP-923 in the treatment of DPN pain. In communications regarding the continued development of AVP-923 for this indication, the FDA has stated that certain safety concerns and questions raised in the PBA approvable letter issued in 2006 necessitate the testing of a low-dose quinidine formulation for the DPN pain indication. Additionally, based on feedback we have received from the FDA on the proposed continued development of AVP-923 for DPN pain, we believe it is likely that two additional large well-controlled Phase III trials would be needed to support a supplemental NDA filing for this indication. Due to our limited capital resources, we do not expect that we will be able to conduct the trials needed for this indication without additional capital, a development partner for AVP-923 for DPN pain, or a commercialization partner for NUEDEXTA. Moreover, although we achieved positive results in our initial Phase III trial, an alternative low-dose quinidine formulation may not yield the same levels of efficacy as seen in the earlier trials or as predicted based on our subsequent pharmacokinetic study. Any decrease in efficacy may be so great that the drug does not demonstrate a statistically significant improvement over placebo or an active comparator. Additionally, any alternative low-dose quinidine formulation that we develop may not sufficiently satisfy the FDA’s safety concerns. If this were to happen, we may not be able to pursue the development of AVP-923 or AVP-786 for DPN pain or other indications, including central neuropathic pain in patients with MS and symptoms of agitation in patients with Alzheimer’s disease, or may need to undertake significant additional clinical trials, which would be costly and cause potentially substantial delays.
If our products infringe the intellectual property rights of others, we may incur damages and be required to incur the expense of obtaining a license.
Even if we successfully secure our intellectual property rights, third parties, including other biotechnology or pharmaceutical companies, may allege that our technology infringes on their rights. Intellectual property litigation is costly, and even if we were to prevail in such a dispute, the cost of litigation could adversely affect our business, financial condition, and results of operations. Litigation is also time consuming and could divert management’s attention and resources away from our operations and other activities. If we were to lose any litigation, in addition to any damages we would have to pay, we could be required to stop the infringing activity or obtain a license. Any required license might not be available to us on acceptable terms, or at all. Some licenses might be non-exclusive, and our competitors could have access to the same technology licensed to us. If we were to fail to obtain a required license or were unable to design around a competitor’s patent, we would be unable to sell or continue to develop some of our products, which would have a material adverse effect on our business, financial condition and results of operations.
We rely on market research to evaluate the commercial acceptance of NUEDEXTA and AVP-825.
Based on the results of our market research, we believe that physicians are likely to continue to support the use and adoption of NUEDEXTA for the treatment of PBA. In addition, we believe that physicians are likely to support and adopt the use of AVP-825 for the treatment of acute migraine, if approved by the FDA. We conduct market research in accordance with Good Marketing Research Practices; however, research findings may not be indicative of the response we might receive from a broader sample of physicians. Moreover, these results are based on physicians’ impressions formed from a description of the product or their actual experience from having prescribed the product, which could result in different impressions or intended behaviors compared to other physicians in our target audience. If the actual use and adoption rates of NUEDEXTA and AVP-825 (if approved by the FDA) are significantly lower than market research or other data suggest, our financial condition and results of operations could be adversely affected.
43
Table of Contents
It is unclear whether we would be eligible for patent term extension in the U.S. and supplementary protection certificates in Europe and we therefore do not know whether our patent term can be extended.
Market exclusivity provisions under the FDCA may also delay the submission or the approval of certain applications for competing product candidates. The FDCA provides three years of non-patent marketing exclusivity for an NDA if new clinical investigations, other than bioavailability studies, that were conducted or sponsored by the applicant are deemed by the FDA to be essential to the approval of the application. This three-year exclusivity covers only the conditions associated with the new clinical investigations and does not prohibit the FDA from approving abbreviated NDAs (ANDA) for drugs containing the original active agent.
Once the three-year FDCA exclusivity period has passed and after the patents (including the patent extension term, if any) that cover NUEDEXTA expire or have been invalidated, generic drug companies would be able to introduce competing versions of the drug. If we are unsuccessful in defending our patents against generic competition, our long-term revenues from NUEDEXTA sales may be significantly less than expected, we may have greater difficulty finding a development partner or licensee for NUEDEXTA and the costs to defend the patents would be significant.
In Europe, based on the European Commission’s Article 14(11) of Regulation (EC) No. 726/2004, NUEDEXTA qualifies for ten years of regulatory data protection. Similar to the U.S., market exclusivity provisions provide for a maximum five-year extension for certain patents through the granting of Supplementary Protection Certificates. Although all countries in the European Union are required to provide Supplementary Protection Certificates that come into force after expiry of the patent upon which they are based, no unified cross-recognition exists. Applications for Supplementary Protection Certificates must be filed with each country’s patent office and approved on a country-by-country basis. Although we believe that NUEDEXTA will qualify for this extension and we plan to apply for Supplementary Protection Certificates, we cannot assure you that NUEDEXTA will be granted any Supplementary Protection Certificates nor, if a Supplementary Protection Certificate is granted, that the term of the extension will be five years.
We may be unable to protect our unpatented proprietary technology and information.
In addition to our patented intellectual property, we also rely on trade secrets and confidential information. We may be unable to effectively protect our rights to such proprietary technology or information. Other parties may independently develop or gain access to equivalent technologies or information and disclose it for others to use. Disputes may arise about inventorship and corresponding rights to know-how and inventions resulting from the joint creation or use of intellectual property by us and our corporate partners, licensees, scientific and academic collaborators and consultants. In addition, confidentiality agreements and material transfer agreements we have entered into with these parties and with employees and advisors may not provide effective protection of our proprietary technology or information or, in the event of unauthorized use or disclosure, may not provide adequate remedies. If we fail to protect our trade secrets and confidential information, our business and results of operations could be adversely affected.
We face challenges recruiting and retaining members of management and other key personnel.
The industry in which we compete has a high level of employee mobility and aggressive recruiting of skilled employees. This type of environment creates intense competition for qualified personnel, particularly in commercial, clinical and regulatory affairs, research and development and accounting and finance. Because we have a relatively small management team, the loss of any executive officers, including the Chief Executive Officer, key members of senior management or other key employees, could adversely affect our operations.
Our operations might be interrupted by the occurrence of a natural disaster or other catastrophic event.
Our principal operations are located in Aliso Viejo, California. We depend on our facilities and on our partners, contractors and vendors for the continued operation of our business. Natural disasters or other catastrophic events, interruptions in the supply of natural resources, political and governmental changes, wildfires and other fires, floods, explosions, actions of animal rights activists, earthquakes and civil unrest could disrupt our operations or those of our partners, contractors and vendors. Even though we believe we carry commercially reasonable business
44
Table of Contents
interruption and liability insurance, and our contractors may carry liability insurance that protect us in certain events, we might suffer losses as a result of business interruptions that exceed the coverage available under our and our contractors’ insurance policies or for which we or our contractors do not have coverage. Any natural disaster or catastrophic event could have a significant negative impact on our operations and financial results. However, we have a disaster recovery plan in place for our information technology infrastructure that generally allows us to have our critical systems operational in as little as four hours of triggering the disaster recovery plan, depending on the severity of the disaster. Moreover, any such event could adversely impact the commercialization of NUEDEXTA and our research and development programs.
We may become involved in litigation and administrative proceedings that may materially affect us.
From time to time, we may become involved in various legal proceedings relating to matters incidental to the ordinary course of our business, including commercial, employment, class action, whistleblower and other litigation and claims, and governmental and other regulatory investigations and proceedings. Litigation may also arise as a result of a decline in the market price of our securities or as a result of disclosures we make with the Securities and Exchange Commission in our periodic reports and documents that are provided to our stockholders. For example, a putative stockholder class action was filed in January 2014, alleging claims based upon purported deficiencies in disclosures relating to our 2014 Annual Meeting proxy statement, with respect to our equity compensation plan and practices. Although we do not currently expect that this or similar litigation will have a material effect on us, such matters can be time-consuming, divert management’s attention and resources and may cause us to incur significant expenses. Furthermore, because litigation is inherently unpredictable, there can be no assurance that the results of any of these actions will not have a material adverse effect on our business, results of operations or financial condition.
Risks Relating to Our Industry
The pharmaceutical industry is highly competitive and most of our competitors have larger operations and greater resources. As a result, we face significant competitive hurdles.
The pharmaceutical and biotechnology industries are highly competitive and subject to significant and rapid technological change. We compete with hundreds of companies that develop and market products and technologies in areas similar to those in which we are performing our research. For example, we expect that NUEDEXTA may face competition from off-label use of other agents in the treatment of PBA, even though none of these agents has proven to be safe and effective for the treatment of PBA. Additionally, NUEDEXTA may face direct competition from a generic form of NUEDEXTA, if approved, as described above.
Our competitors may have specific expertise and development technologies that are better than ours and many of these companies, which include large pharmaceutical companies, either alone or together with their research partners, have substantially greater financial resources, larger research and development capabilities and substantially greater experience than we do. Accordingly, our competitors may successfully develop competing products. We are also competing with other companies and their products with respect to manufacturing efficiencies and marketing capabilities, areas where we have limited or no direct experience.
Further, AVP-825 will have to compete with existing and any newly developed migraine products or therapies. There are also likely to be numerous competitors developing new products to treat migraine, which could render AVP-825 obsolete or non-competitive.
If we fail to comply with regulatory requirements, regulatory agencies may take action against us, which could significantly harm our business.
Marketed products, along with the manufacturing processes, post-approval clinical data, labeling, advertising and promotional activities for these products, are subject to continual requirements and review by the FDA, the EMA and other regulatory bodies. In addition, regulatory authorities subject a marketed product, its manufacturer and the manufacturing facilities to ongoing review and periodic inspections. We are subject to ongoing FDA requirements, including required submissions of safety and other post-market information and reports, registration requirements, current Good Manufacturing Practices (“cGMP”) regulations, requirements regarding the distribution of samples to physicians and recordkeeping requirements.
45
Table of Contents
The cGMP regulations also include requirements relating to quality control and quality assurance, as well as the corresponding maintenance of records and documentation. We rely on the compliance by our contract manufacturers with cGMP regulations and other regulatory requirements relating to the manufacture of products. We are also subject to state laws and registration requirements covering the distribution of our products. If we fail to comply with any of these requirements, we may be subject to action by regulatory agencies, which could negatively affect our business.
Regulatory agencies may also change existing requirements or adopt new requirements or policies. We may be slow to adapt or may not be able to adapt to these changes or new requirements.
There are a number of difficulties and risks associated with clinical trials and our trials may not yield the expected results.
There are a number of difficulties and risks associated with conducting clinical trials. For instance, we may discover that a product candidate does not exhibit the expected therapeutic results, may cause harmful side effects or have other unexpected characteristics that may delay or preclude regulatory approval or limit commercial use if approved. It typically takes several years to complete a late-stage clinical trial and a clinical trial can fail at any stage of testing. If clinical trial difficulties or failures arise, our product candidates may never be approved for sale or become commercially viable.
In addition, the possibility exists that:
| • | the results from earlier clinical trials may not be predictive of results that will be obtained from subsequent clinical trials, particularly larger trials; |
| • | institutional review boards or regulators, including the FDA, may hold, suspend or terminate our clinical research or the clinical trials of our product candidates for various reasons, including noncompliance with regulatory requirements or if, in their opinion, the participating subjects are being exposed to unacceptable health risks; |
| • | subjects may drop out of our clinical trials; |
| • | our non-clinical studies or clinical trials may produce negative, inconsistent or inconclusive results, and we may decide, or regulators may require us, to conduct additional preclinical studies or clinical trials; |
| • | trial results derived from top-line data, which is based on a preliminary analysis of efficacy and safety data related to primary and secondary endpoints, may change following a more comprehensive review of the complete data set derived from a particular clinical trial or may change due to FDA requests to analyze the data differently; |
| • | the cost of our clinical trials may be greater than we currently anticipate and clinical trials may take longer than expected to enroll patients and complete, particularly for progressive diseases such as MS where our drug candidates are primarily aimed at treating associated symptoms and not the underlying disease itself; and |
| • | there could be a delay in initiating our clinical trials. |
It is possible that earlier clinical and non-clinical trial results may not be predictive of the results of subsequent clinical trials. If earlier clinical and/or non-clinical trial results cannot be replicated or are inconsistent with subsequent results, our development programs may be cancelled or deferred. In addition, the results of these prior clinical trials may not be acceptable to the FDA or similar foreign regulatory authorities because the data may be incomplete, outdated or not otherwise acceptable for inclusion in our submissions for regulatory approval.
46
Table of Contents
Additionally, the FDA has substantial discretion in the approval process and may reject our data, disagree with our interpretations of regulations, draw different conclusions from our clinical trial data or ask for additional information at any time during their review.
Although we would work to be able to fully address any such FDA concerns, we may not be able to resolve all such matters favorably, if at all. Disputes that are not resolved favorably could result in one or more of the following:
| • | delays in our ability to submit an NDA; |
| • | the refusal by the FDA to accept for filing any NDA we may submit; |
| • | requests for additional studies or data; |
| • | delays in obtaining an approval; |
| • | the rejection of an application; or |
| • | the approval of the drug, but with restrictive labeling that could adversely affect the commercial market. |
If we do not receive regulatory approval to sell our product candidates or cannot successfully commercialize our product candidates, we may not be able to generate sufficient revenues or achieve or maintain profitability.
We face uncertainty related to healthcare reform, pricing and reimbursement, which could reduce our revenue.
In the United States, President Obama signed in March 2010 the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Affordability Reconciliation Act (collectively, “PPACA”), which is expected to substantially change the way health care is financed by both governmental and private payers. PPACA provides for changes to extend medical benefits to those who currently lack insurance coverage, encourages improvements in the quality of health care items and services, and significantly impacts the U.S. pharmaceutical industry in a number of ways, further listed below. By extending coverage to a larger population, PPACA may substantially change the structure of the health insurance system and the methodology for reimbursing medical services, drugs and devices. These structural changes, as well as other changes that may be made as part of deficit and debt reduction efforts in Congress, could entail modifications to the existing system of private payers and government programs, such as Medicare, Medicaid and State Children’s Health Insurance Program, as well as the creation of a government-sponsored healthcare insurance source, or some combination of both. Such restructuring of the coverage of medical care in the United States could impact the extent of reimbursement for prescribed drugs, including our product candidates, biopharmaceuticals, and medical devices. While the constitutionality of key provisions of the Healthcare Reform Act were recently upheld by the Supreme Court, legislative changes to it remain possible. We expect that the Healthcare Reform Act, as currently enacted or as it may be amended in the future, and other healthcare reform measures that may be adopted in the future could have a material adverse effect on our industry in general and on our ability to maintain or increase our product sales, successfully commercialize our product candidates or could limit or eliminate our future spending on development projects. Some of the specific PPACA provisions, among other things:
| • | Establish annual, non-deductible fees on any entity that manufactures or imports certain branded prescription drugs and biologics; |
| • | Increase minimum Medicaid rebates owed by manufacturers under the Medicaid Drug Rebate Program; |
| • | Extend manufacturers’ Medicaid rebate liability to covered drugs dispensed to individuals who are enrolled in Medicaid managed care organizations or extension of statutory rebates to a broader patient population; |
| • | Establish a new Patient-Centered Outcomes Research Institute to oversee, identify priorities in and conduct comparative clinical effectiveness research; |
| • | Require manufacturers to participate in a coverage gap discount program, under which they must agree to offer 50 percent point-of-sale discounts off negotiated prices of applicable brand drugs to eligible beneficiaries during their coverage gap period, as a condition for the manufacturer’s outpatient drugs to be covered under Medicare Part D; and |
| • | Revised the definition of average manufacturer price by changing the classes of purchasers included in the calculation, and expanded the entities eligible for discounted 340B pricing. |
47
Table of Contents
A majority of sales of NUEDEXTA come from institutional settings. Any adverse change in reimbursement policy affecting patients, providers and payers in institutional settings could have a material and adverse impact on our business.
If future reimbursement for NUEDEXTA or any other approved product candidates, if any, is substantially less than we project, or rebate obligations associated with them are substantially increased, our business could be materially and adversely impacted.
Sales of NUEDEXTA for treatment of patients with PBA will depend in part on the availability of coverage and reimbursement from third-party payers such as government insurance programs, including Medicare and Medicaid, private health insurers, health maintenance organizations and other health care related organizations. Accordingly, coverage and reimbursement may be uncertain. Adoption of NUEDEXTA by the medical community may be limited if third-party payers will not offer coverage. Cost control initiatives may decrease coverage and payment levels for NUEDEXTA and, in turn, the price that we will be able to charge. We are unable to predict all changes to the coverage or reimbursement methodologies that will be applied by private or government payers to NUEDEXTA. Any denial of private or government payer coverage of NUEDEXTA could harm our business and reduce our revenue.
In addition, both the federal and state governments in the United States and foreign governments continue to propose and pass new legislation affecting coverage and reimbursement policies, which are designed to contain or reduce the cost of health care, as well as hold public hearings on these matters, which has resulted in certain private companies dropping the prices of their drugs. Further federal and state proposals and healthcare reforms are likely, which could limit the prices that can be charged for the product candidates that we develop and may further limit our commercial opportunity. There may be future changes that result in reductions in current coverage and reimbursement levels for our products, if commercialized, and we cannot predict the scope of any future changes or the impact that those changes would have on our operations.
If we fail to obtain regulatory approval in foreign jurisdictions, we would not be able to market our products abroad and our revenue prospects would be limited.
We are seeking to have our products or product candidates marketed outside the United States. In order to market our products in the EU and many other foreign jurisdictions, we must obtain separate regulatory approvals and comply with numerous and varying regulatory requirements. The approval procedure varies among countries and jurisdictions and can involve additional testing. The time required to obtain approval may differ from that required to obtain FDA approval. The foreign regulatory approval processes may include all of the risks associated with obtaining FDA approval. We may not obtain foreign regulatory approvals on a timely basis, if at all, and may not qualify or be accepted for accelerated review in foreign countries. For example, our development partner in Japan encountered significant difficulty in seeking approval of docosanol in that country and was forced to abandon efforts to seek approval in that country. Approval by the FDA does not ensure approval by regulatory authorities in other countries or jurisdictions, and approval by one foreign regulatory authority does not ensure approval by regulatory authorities in other foreign countries or jurisdictions or by the FDA. We may not be able to file for regulatory approvals and may not receive necessary approvals to commercialize our products in any market. The failure to obtain these approvals could materially adversely affect our business, financial condition and results of operations.
We rely on insurance companies to mitigate our exposure for business activities, including developing and marketing pharmaceutical products for human use.
The conduct of our business, including the testing, marketing and sale of pharmaceutical products, involves the risk of liability claims by consumers, stockholders, and other third parties including products in which we co-promote. Although we maintain various types of insurance, including product liability and director and officer liability, claims can be high and our insurance may not sufficiently cover our actual liabilities. If liability claims were made against us, it is possible that our insurance carriers may deny, or attempt to deny, coverage in certain
48
Table of Contents
instances. If a lawsuit against us is successful, then the lack or insufficiency of insurance coverage could materially and adversely affect our business and financial condition. Furthermore, various distributors of pharmaceutical products require minimum product liability insurance coverage before their purchase or acceptance of products for distribution. Failure to satisfy these insurance requirements could impede our ability to achieve broad distribution of our products and the imposition of higher insurance requirements could impose additional costs on us. Additionally, we are potentially at risk if our insurance carriers become insolvent. Although we have historically obtained coverage through highly rated and capitalized firms, there can be no assurance that we will be able to maintain coverage under existing policies at the current rates or purchase insurance under new policies at reasonable rates.
If we market products in a manner that violates health care fraud and abuse laws, we may be subject to civil and criminal penalties, which may adversely affect our business, financial condition and results of operations.
We are subject to healthcare fraud and abuse regulation and enforcement by both the federal government and the states in which we conduct our business. The laws that may affect our ability to operate include:
| • | the federal healthcare programs’ Anti-Kickback Law (as amended by the PPACA, which modified the intent requirement of the Anti-Kickback Law so that a person or entity no longer needs to have actual knowledge of the statute or specific intent to violate it), which prohibits, among other things, persons from soliciting, receiving, offering or paying remuneration, directly or indirectly, in exchange for or to induce either the referral of an individual for, or the purchase, order or recommendation of, any good or service for which payment may be made under federal healthcare programs such as the Medicare and Medicaid programs; |
| • | federal false claims laws which prohibit, among other things, individuals or entities from knowingly presenting, or causing to be presented, claims for payment from Medicare, Medicaid, or other third-party payers that are false or fraudulent and, under the PPACA, the government may assert that a claim including items or services resulting from a violation of the federal anti-kickback statute constitutes a false or fraudulent claim for purposes of the false claims statutes; |
| • | the federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, which created federal criminal laws that prohibit executing a scheme to defraud any healthcare benefit program or making false statements relating to healthcare matters; and |
| • | state law equivalents of each of the above federal laws, such as anti-kickback and false claims laws that may apply to items or services reimbursed by any third-party payer, including commercial insurers. |
In addition, the compliance environment is changing as some states mandate implementation of commercial compliance programs to ensure compliance with these laws. The PPACA also imposes new reporting and disclosure requirements on drug manufacturers for any “transfer of value” made or distributed to prescribers and other healthcare providers and such information will be made publicly available in a searchable format. Drug manufacturers are required to begin collecting required information on August 1, 2013 and to submit reports disclosing any investment interests held by physicians and their immediate family members from August through December of 2013 by March 31, 2014. Failure to submit required information may result in civil monetary penalties of up to an aggregate of $150,000 per year (and up to an aggregate of $1 million per year for “knowing failures”), for all payments, transfers of value or ownership or investment interests not reported in an annual submission. PPACA also requires pharmaceutical manufacturers and distributors to provide the U.S. Department of Health and Human Services with an annual report on the drug samples they provide to physicians. There has also been a recent trend of increased federal and state regulation of payments made to physicians for marketing, including the tracking and reporting of gifts, compensation, and other remuneration to physicians. The shifting commercial compliance environment and the need to build and maintain robust and expandable systems to comply with multiple jurisdictions with different compliance and/or reporting requirements increases the possibility that a healthcare company may run afoul of one or more of the requirements.
If our operations are found to be in violation of any of the laws described above or any other domestic or foreign laws or governmental regulations that apply to us, we may be subject to penalties, including civil and criminal penalties, damages, fines, exclusion from governmental health care programs, and the curtailment or restructuring of our operations, any of which could adversely affect our ability to operate our business and our financial results. The risk of our being found in violation of these laws is increased by the fact that many of them
49
Table of Contents
have not been fully interpreted by the regulatory authorities or the courts, and their provisions are open to a variety of interpretations. Even if we are not found to be in violation of any of the laws described above or any other domestic or foreign laws or governmental regulations that apply to us, any action against us for violation of these laws could cause us to incur significant legal expenses and divert our management’s attention from the operation of our business.
We may incur significant liability if it is determined that we are promoting the “off-label” use of drugs.
We have taken numerous steps to ensure compliance is a high priority throughout the organization and we believe that our communications regarding our products are in compliance with the relevant regulatory requirements. However, the FDA or another regulatory authority may disagree.
Responding to government investigations, defending any claims raised, and any resulting fines, restitution, damages and penalties, settlement payments or administrative actions, as well as any related actions brought by stockholders or other third parties, could have a material impact on our reputation, business and financial condition and divert the attention of our management from operating our business. Our distribution and contracting partners may also be the subject of regulatory investigations involving, or remedies or sanctions for, off-label promotion of products we have licensed to them, or for which they provide vendor support services, which may have an adverse impact on sales of such licensed products, or indemnification obligations, which may, in turn, have a material adverse effect on our business, financial condition and results of operations and could cause the market value of our common shares to decline. The risks of a regulatory investigation are increased by the practice of some stock market participants to publicly issue reports alleging compliance violations. A report of this nature was recently issued regarding the Company’s practices.
Companies may not promote drugs for “off-label” uses — that is, uses that are not described in the product’s labeling and that differ from those approved by the FDA or other applicable regulatory agencies. Physicians may prescribe drug products for off-label uses, and such off-label uses are common across medical specialties. Although the FDA and other regulatory agencies do not regulate a physician’s choice of treatments for a given medical condition, the FDA and other regulatory agencies do restrict communications by pharmaceutical companies and their sales representatives regarding dissemination of information concerning off-label use. The FDA and other regulatory agencies actively enforce regulations prohibiting promotion of product for off-label uses and the promotion of products for which marketing authorization has not been obtained. A company that is found to have promoted product for off-label uses may be subject to significant liability, including civil and administrative remedies as well as criminal sanctions. Notwithstanding the regulatory restrictions on off-label promotion, the FDA and other regulatory authorities allow companies to engage in truthful, non-misleading, and non-promotional scientific exchange with health care professionals concerning their products.
Risks Related to Reliance on Third Parties
We depend on third parties to manufacture, package and distribute compounds for our drugs and drug candidates. The failure of these third parties to perform successfully could harm our business.
We have utilized, and intend to continue utilizing, third parties to manufacture, package and distribute NUEDEXTA and the active pharmaceutical ingredient (“API”) for docosanol 10% cream and to provide clinical supplies of our drug candidates. We will also utilize third parties to manufacture, package and distribute AVP-825. We have no experience in manufacturing and do not have any manufacturing facilities. Currently, we have sole suppliers for the API for docosanol and NUEDEXTA, and a sole manufacturer for the finished form of NUEDEXTA. In addition, these materials are custom-made and available from only a limited number of sources. In particular, there may be a limited supply source for APIs in NUEDEXTA. Although we maintain a significant supply of APIs on hand, any sustained disruption in this supply could adversely affect our operations and revenues. Any material disruption in manufacturing could cause a delay in shipments and possible loss of sales. We do not have any long-term agreements in place with our current docosanol supplier or NUEDEXTA API suppliers. If we are required to change manufacturers, we may experience delays associated with finding an alternate manufacturer that is properly qualified to produce supplies of our products and product candidates in accordance with FDA requirements and our specifications. Any delays or difficulties in obtaining APIs or in manufacturing, packaging or distributing NUEDEXTA could negatively affect our sales revenues, as well as delay our clinical trials of AVP-923
50
Table of Contents
for DPN pain, MS-related pain or other potential indications. The third parties we rely on for manufacturing and packaging are also subject to regulatory review, and any regulatory compliance problems with these third parties could significantly delay or disrupt our commercialization activities. Additionally, macro-economic conditions may adversely affect these third parties, causing them to suffer liquidity or operational problems. If a key third party vendor becomes insolvent or is forced to lay off workers assisting with our projects, our results and development timing could suffer.
Because we depend on clinical research centers and other contractors for clinical testing and for certain research and development activities, the results of our clinical trials and such research activities are, to a certain extent, beyond our control.
The nature of clinical trials and our business strategy of outsourcing a substantial portion of our research require that we rely on clinical research centers and other contractors to assist us with research and development, clinical testing activities, patient enrollment and regulatory submissions to the FDA. As a result, our success depends partially on the success of these third parties in performing their responsibilities. Although we pre-qualify our contractors and we believe that they are fully capable of performing their contractual obligations, we cannot directly control the adequacy and timeliness of the resources and expertise that they apply to these activities. Additionally, macro-economic conditions may affect our development partners and vendors, which could adversely affect their ability to timely perform their tasks. If our contractors do not perform their obligations in an adequate and timely manner, the pace of clinical development, regulatory approval and commercialization of our drug candidates could be significantly delayed and our prospects could be adversely affected.
We generally do not control the development of compounds licensed to third parties and, as a result, we may not realize a significant portion of the potential value of any such license arrangements.
Under our typical out-license arrangement we have no direct control over the development of drug candidates and have only limited, if any, input on the direction of development efforts. These development efforts are made by our licensing partner, and if the results of their development efforts are negative or inconclusive, it is possible that our licensing partner could elect to defer or abandon further development of these programs. We similarly rely on licensing partners to obtain regulatory approval for docosanol in foreign jurisdictions. Because much of the potential value of these license arrangements is contingent upon the successful development and commercialization of the licensed technology, the ultimate value of these licenses will depend on the efforts of licensing partners. If our licensing partners do not succeed in developing the licensed technology for whatever reason, or elect to discontinue the development of these programs, we may be unable to realize the potential value of these arrangements. If we were to license NUEDEXTA to a third party or a development partner, it is likely that much of the long-term success of that drug will similarly depend on the efforts of the licensee.
We expect to rely entirely on third parties for international registration, sales and marketing efforts.
In the event that we attempt to enter into international markets, in some instances we expect to rely on collaborative partners to obtain regulatory approvals and to market and sell our product(s) in those markets. We have not yet entered into any collaborative arrangement with respect to marketing or selling NUEDEXTA, with the exception of one such agreement relating to Israel. We may be unable to enter into any other arrangements on terms favorable to us, or at all, and even if we are able to enter into sales and marketing arrangements with collaborative partners, we cannot assure you that their sales and marketing efforts will be successful. If we are unable to enter into favorable collaborative arrangements with respect to marketing or selling NUEDEXTA in international markets, or if our collaborators’ efforts are unsuccessful, our ability to generate revenues from international product sales will suffer.
51
Table of Contents
Risks Relating to Our Stock
Our stock price has historically been volatile and we expect that this volatility will continue for the foreseeable future.
The market price of our common stock has been, and is likely to continue to be, highly volatile. This volatility can be attributed to many factors independent of our operating results, including the following:
| • | comments made by securities analysts, including changes in their recommendations; |
| • | short selling activity by certain investors, including any failures to timely settle short sale transactions; |
| • | announcements by us of financing transactions and/or future sales of equity or debt securities; |
| • | sales of our common stock by our directors, officers or significant stockholders, including sales effected pursuant to predetermined trading plans adopted under the safe-harbor afforded by Rule 10b5-1; |
| • | negative opinions that are misleading and inaccurate regarding our business, management or future prospects published by certain market participants intent on putting downward pressure on our stock price; |
| • | regulatory developments in the U.S. and foreign countries, including the passage of laws, rules or regulations relating to healthcare and reimbursement or the public announcement of inquiries relating to these subjects; |
| • | lack of volume of stock trading leading to low liquidity; and |
| • | market and economic conditions. |
If a substantial number of shares are sold into the market at any given time, particularly following any significant announcements or large swings in our stock price (whether sales are under our existing “shelf” registration statements, from an existing stockholder, or the result of warrant or stock options exercised), there may not be sufficient demand in the market to purchase the shares without a decline in the market price for our common stock. Moreover, continuous sales into the market of a number of shares in excess of the typical trading volume for our common stock, or even the availability of such a large number of shares, could depress the trading market for our common stock over an extended period of time.
Additionally, our stock price has been volatile as a result of announcements of regulatory actions and decisions relating to NUEDEXTA, and periodic variations in our operating results. We expect that our operating results will continue to vary from quarter to quarter, particularly as we continue to market NUEDEXTA and report sales results. Our operating results and prospects may also vary depending on the status of our partnering arrangements.
As a result of these factors, we expect that our stock price may continue to be volatile and investors may be unable to sell their shares at a price equal to, or above, the price paid. Additionally, any significant drops in our stock price could give rise to stockholder lawsuits, which are costly and time consuming to defend against and which may adversely affect our ability to raise capital while the suits are pending, even if the suits are ultimately resolved in our favor. We have, from time to time, been a party to such suits and although none have been material to date, there can be no assurance that any such suit will not have an adverse effect on us.
If our internal controls over financial reporting are not considered effective, our business and stock price could be adversely affected.
Section 404 of the Sarbanes-Oxley Act of 2002 requires us to evaluate the effectiveness of our internal controls over financial reporting as of the end of each fiscal year, and to include a management report assessing the effectiveness of our internal controls over financial reporting in our annual report on Form 10-K for that fiscal year. Section 404 also requires our independent registered public accounting firm to attest to, and report on, management’s assessment of our internal controls over financial reporting.
Our management, including our chief executive officer and principal financial officer, does not expect that our internal controls over financial reporting will prevent all errors and all fraud. A control system, no matter how well designed and operated, can provide only reasonable, not absolute, assurance that the control system’s objectives will
52
Table of Contents
be met. Further, the design of a control system must reflect the fact that there are resource constraints, and the benefits of controls must be considered relative to their costs. Because of the inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that all control issues and instances of fraud involving a company have been, or will be, detected. The design of any system of controls is based in part on certain assumptions about the likelihood of future events, and we cannot assure you that any design will succeed in achieving its stated goals under all potential future conditions. Over time, controls may become ineffective because of changes in conditions or deterioration in the degree of compliance with policies or procedures. Because of the inherent limitations in a cost-effective control system, misstatements due to error or fraud may occur and not be detected. We cannot assure you that we or our independent registered public accounting firm will not identify a material weakness in our internal controls in the future. A material weakness in our internal controls over financial reporting would require management and our independent registered public accounting firm to consider our internal controls as ineffective. If our internal controls over financial reporting are not considered effective, we may experience a loss of public confidence, which could have an adverse effect on our business and on the market price of our common stock.
Because we do not expect to pay dividends in the foreseeable future, you must rely on stock appreciation for any return on your investment.
We have paid no cash dividends on our common stock to date, and we currently intend to retain our future earnings, if any, to fund the development and growth of our business. As a result, we do not expect to pay any cash dividends in the foreseeable future, and payment of cash dividends, if any, will also depend on our financial condition, results of operations, capital requirements and other factors and will be at the discretion of our board of directors. In addition, under the terms of our Loan Agreement, we are precluded from paying cash dividends without the prior written consent of the lenders. Accordingly, the success of your investment in our common stock will likely depend entirely upon any future appreciation. There is no guarantee that our common stock will appreciate in value or even maintain the price at which you purchased your shares, and you may not realize a return on your investment in our common stock.
Our corporate governance documents and Delaware law may delay or prevent an acquisition of us that stockholders may consider favorable, which could decrease the value of our common stock.
Our certificate of incorporation and bylaws and Delaware law contain provisions that could make it more difficult for a third party to acquire us without the consent of our board of directors. These provisions include restrictions on the ability of our stockholders to remove directors and supermajority voting requirements for stockholders to amend our organizational documents and a classified board of directors. In addition, our board of directors has the right to issue preferred stock without stockholder approval, which could be used to dilute the stock ownership of a potential hostile acquirer. Delaware law, for instance, also imposes some restrictions on mergers and other business combinations between any holder of 15% or more of our outstanding common stock and us. Although we believe these provisions protect our stockholders from coercive or otherwise unfair takeover tactics and thereby provide for an opportunity to receive a higher bid by requiring potential acquirers to negotiate with our board of directors, these provisions apply even if the offer may be considered beneficial by some stockholders.
Item 2. UNREGISTERED SALES OF EQUITY SECURITIES AND USE OF PROCEEDS
Not applicable.
Item 3. DEFAULTS UPON SENIOR SECURITIES
Not applicable.
Item 4. MINE SAFETY DISCLOSURES
Not applicable.
53
Table of Contents
None.
| Exhibits | ||
| 31.1 | Certification of Principal Executive Officer Required Under Rule 13a-14(a) of the Securities Exchange Act of 1934, as amended. | |
| 31.2 | Certification of Principal Financial Officer Required Under Rule 13a-14(a) of the Securities Exchange Act of 1934, as amended. | |
| 32.1 | Certification of Principal Executive Officer and Principal Financial Officer Required Under Rule 13a-14(b) of the Securities Exchange Act of 1934, as amended, and 18 U.S.C. 1350. | |
| 101.INS | XBRL Instance Document | |
| 101.SCH | XBRL Taxonomy Extension Schema Document | |
| 101.CAL | XBRL Taxonomy Extension Calculation Linkbase Document | |
| 101.DEF | XBRL Taxonomy Extension Definition Linkbase Document | |
| 101.LAB | XBRL Taxonomy Extension Labels Linkbase Document | |
| 101.PRE | XBRL Taxonomy Extension Presentation Linkbase Document | |
54
Table of Contents
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
| Signature |
Title |
Date | ||
| /s/ Keith A. Katkin Keith A. Katkin |
President and Chief Executive Officer (Principal Executive Officer) |
February 6, 2014 | ||
| /s/ Christine G. Ocampo Christine G. Ocampo |
Vice President, Finance (Principal Financial Officer and Principal Accounting Officer) |
February 6, 2014 | ||
55
