Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - QUIDEL CORP /DE/ | d660702d8k.htm |
Exhibit 99.1

Investor Presentation Doug Bryant, President & CEO
Investor Presentatio
 n Doug Bryant, President & CEO
n Doug Bryant, President & CEO

Company history (1979 – 2009) 3 1984 First commercial product introduced: pregnancy 1979 Quidel founded 1985 FDA-cleared test for Strep A 1989 Acquired Bone Health and Complement Pathway marker business
1999 Flu A/B product
receives FDA
clearance 1990 1980 1970
2000 2009 First company to receive CLIA waiver for Strep A, Helicobacter pylori, and Influenza A+B
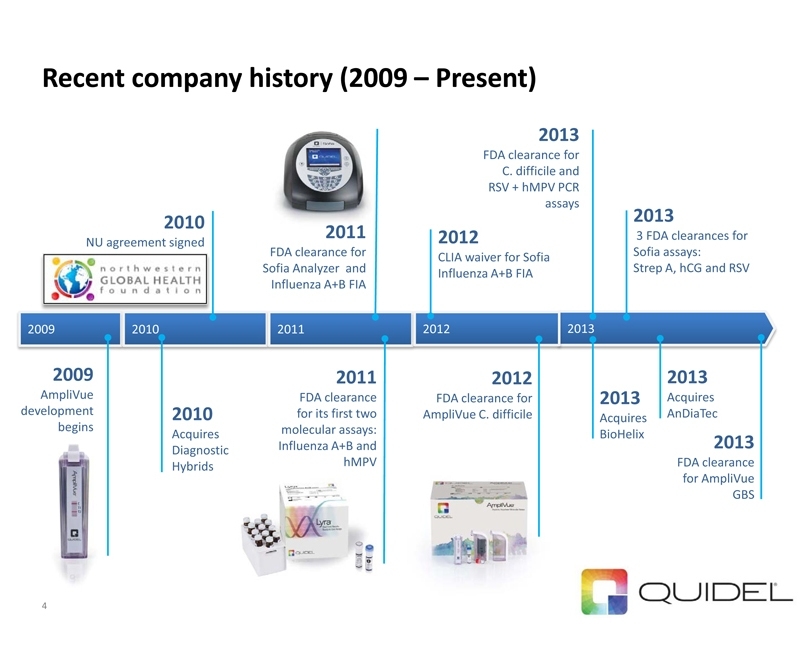
Recent company history (2009 – Present) 4 2009 2013
2009
AmpliVue development begins 2010 NU agreement signed 2012 CLIA waiver for Sofia Influenza A+B FIA 2012 FDA clearance for AmpliVue C. difficile 2011 FDA clearance for its first two molecular assays: Influenza A+B and hMPV 2010
2010
Acquires
Diagnostic
Hybrids 2011 2012 2013 FDA clearance for C. difficile and RSV + hMPV PCR assays
2013
Acquires
BioHelix 2013 3 FDA clearances for Sofia assays: Strep A, hCG and RSV 2013 Acquires AnDiaTec 2013 FDA clearance for AmpliVue GBS 2011 FDA clearance for Sofia Analyzer and Influenza A+B FIA

From a customer perspective, Sofia is a compelling next-generation rapid immunoassay system
?Currently marketed Sofia products: oInfluenza A+B
oStrep A
oRSV
oQualitative hCG
oLegionella
?Assays in development ohCG Quant
oVitamin D Quant
oPROM
oFFN
?Improved performance relative to traditional rapid point of care devices
?Objectively read result, with many fail-safe features
?Cellular wireless capability
| 5 |
|
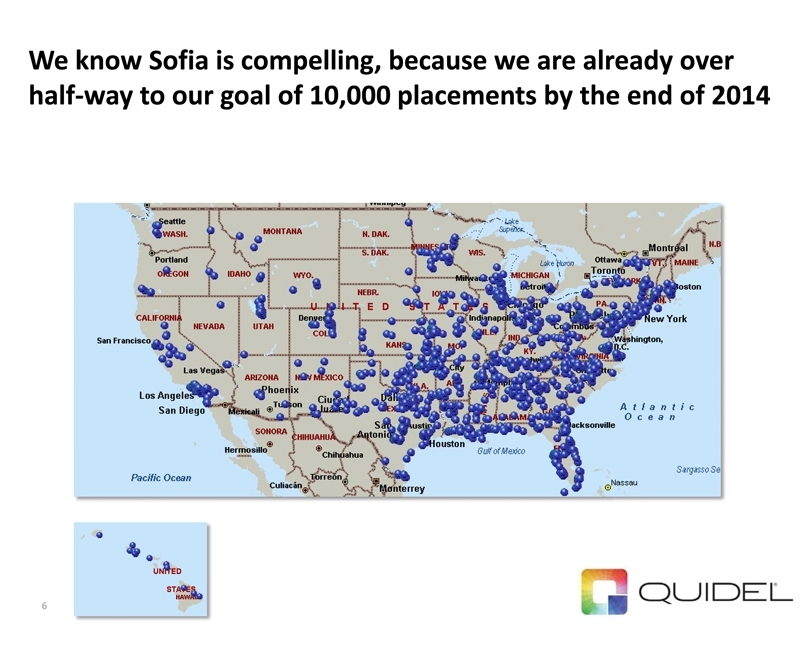
We know Sofia is compelling, because we are already over half-way to our goal of 10,000 placements by the end of 2014 6

Sofia’s wireless connectivity allows health systems and public health authorities to monitor results in near-real time Sofia sends encrypted result to router
Router sends result to cloud the message classified by Sofia serial number Once per day, Quidel Cloud collects messages and saves to database Daily, customer csv files are automatically pushed to user via email or FTP site User access data through a web portal
| 7 |
|

In the near term, our innovations in the MDx space will be equally compelling 8 Quidel Molecular LYRA REAL-TIME PCR Quidel Molecular AMPLIVUE Quidel Molecular SAVANNA
FDA CLEARED ASSAYS FDA CLEARED ASSAYS
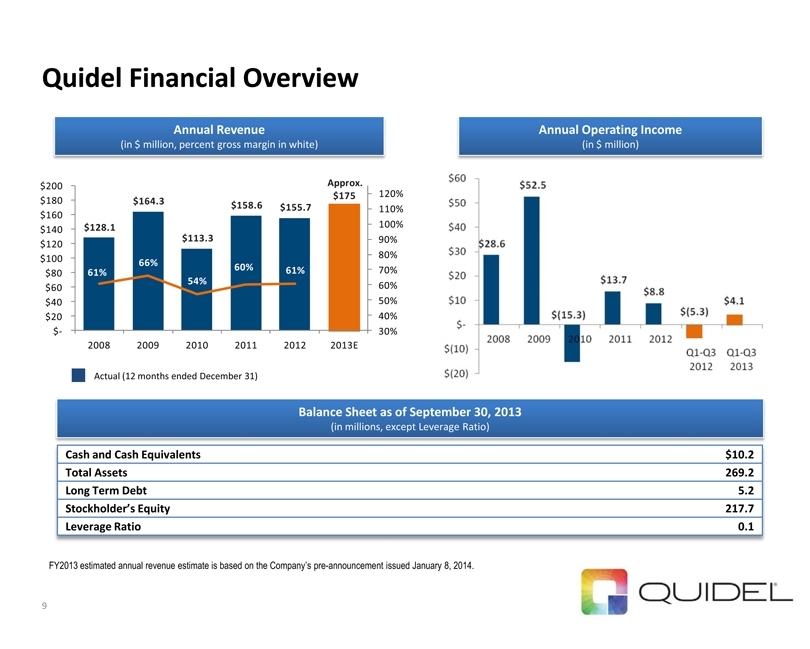
Quidel Financial Overview $128.1 $164.3 $113.3 $158.6 $155.7 Approx. $175 61%66%54%60%61%30%40%50%60%70%80%90%100%110%120%$-$20 $40 $60 $80 $100 $120 $140 $160 $180 $200 200820092010201120122013E9 Annual Revenue
(in $ million, percent gross margin in white)
Actual (12 months ended December 31) Annual Operating Income (in $ million) Balance Sheet as of September 30, 2013 (in millions, except Leverage Ratio)
Cash and Cash Equivalents
$10.2
Total Assets
269.2
Long Term Debt
5.2
Stockholder’s Equity
217.7
Leverage Ratio
0.1
FY2013 estimated annual revenue estimate is based on the Company’s pre-announcement issued January 8, 2014.
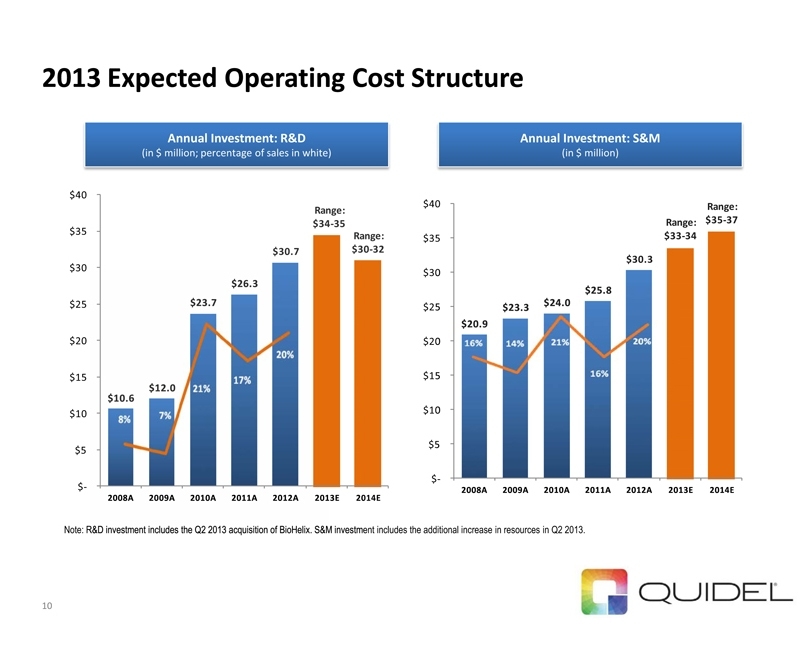
2013 Expected Operating Cost Structure $20.9 $23.3 $24.0 $25.8 $30.3 Range: $33-34 Range:$35-37 $-$5 $10 $15 $20 $25 $30 $35 $40 2008A2009A2010A2011A2012A2013E2014E$10.6 $12.0 $23.7 $26.3 $30.7 Range: $34-35 Range: $30-32$-$5 $10 $15 $20 $25 $30 $35 $40 2008A2009A2010A2011A2012A2013E2014EAnnual Investment: R&D
(in $ million; percentage of sales in white) Annual Investment: S&M (in $ million) Note: R&D investment includes the Q2 2013 acquisition of BioHelix. S&M investment includes the additional increase in resources in Q2 2013. 10
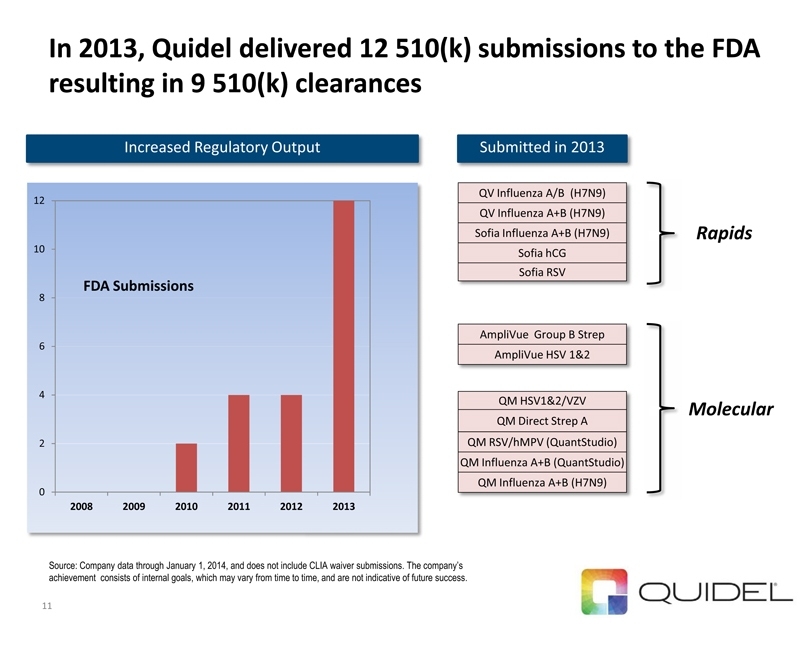
In 2013, Quidel delivered 12 510(k) submissions to the FDA resulting in 9 510(k) clearances Source: Company data through January 1, 2014, and does not include CLIA waiver submissions. The company’s achievement consists of internal goals, which may vary from time to time, and are not indicative of future success.
11 0 2
| 4 |
|
6 |
| 8 |
|
10 12 2008 |
2009 2010 2011 2012 2013
FDA Submissions
AmpliVue Group B Strep
AmpliVue HSV 1&2
QM HSV1&2/VZV
QM Direct Strep A
QM RSV/hMPV (QuantStudio)
QM Influenza A+B (QuantStudio)
QM Influenza A+B (H7N9)
QV Influenza A/B (H7N9)
QV Influenza A+B (H7N9)
Sofia Influenza A+B (H7N9)
Sofia hCG
Sofia RSV
Submitted in 2013
Increased Regulatory Output Molecular Rapids

Quidel’s milestones for 2014 12
| • |
|
10,000 instrument placements generating revenue |
| • |
|
Receive CLIA waiver for Strep A, RSV and hCG |
| • |
|
Quantitative assay development: hCG, Vitamin D |
Sofia
| • |
|
Commercialization of C. difficile and GBS products |
| • |
|
FDA clearance and commercialization of HSV1&2 |
| • |
|
FDA submission of 3 additional assays |
AmpliVue
| • |
|
FDA clearance and commercialization of HSV1&2/VZV |
| • |
|
FDA submission of 2 additional assays |
Lyra
| • |
|
First integrated units manufactured |
| • |
|
Appearance at a U.S. trade show |
| • |
|
Initial lab placements in Africa |
Savanna
