Attached files
| file | filename |
|---|---|
| 8-K - TENGION, INC. FORM 8-K - TENGION INC | tengion8k.htm |

Corporate Presentation January 2014

Certain statements in this presentation may constitute forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. These statements relate to our future plans, objectives, expectations and intentions and may be identified by words like “believe,” “expect,” “designed,” “may,” “will,” “should,” “seek,” or “anticipate,” and similar expressions. Although Tengion believes that these statements are based upon reasonable assumptions within the bounds of its knowledge of its business and operations, there are a number of factors that may cause actual results to differ from these statements. Forward-looking statements are subject to risks and uncertainties. For instance, there can be no assurance that: (i) the Company will be able to successfully enroll patients in its clinical trials; (ii) patients enrolled in the Company's clinical trials will not experience adverse events related to the Company's product candidates, which could delay clinical trials or cause the Company to terminate the development of a product candidate; (iii) the results of the clinical trials for the Company’s product candidates will support further development or regulatory approval of such product candidates; (iv) the market opportunity data for the Neo-Urinary Conduit and the Neo-Kidney Augment, as well as the physician and payer feedback for the Neo-Urinary Conduit used by the Company, accurately predict the potential commercial opportunity for the Neo-Urinary Conduit and Neo-Kidney Augment, respectively; (v) the Company will be able to provide an efficient and scalable manufacturing process for any approved product; and (vi) the Company will be able enter into strategic partnerships on favorable terms, if at all, or obtain the capital it needs to develop its product candidates and continue its operations. For additional factors that could cause actual results to differ from expectations, reference is made to the reports filed by the Company with the Securities and Exchange Commission under the Securities Exchange Act of 1934, as amended. The forward-looking statements in this presentation are made only as of the date hereof and the Company disclaims any intention or responsibility for updating predictions or expectations in this presentation.
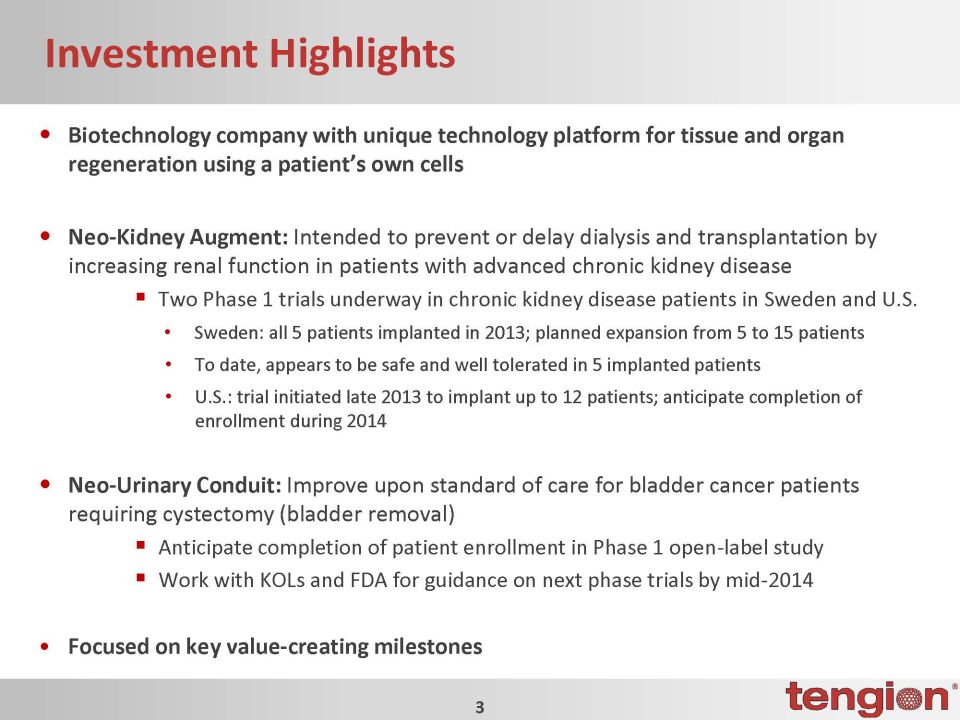
Investment Highlights •Biotechnology company with unique technology platform for tissue and organ regeneration using a patient’s own cells •Neo-Kidney Augment: Intended to prevent or delay dialysis and transplantation by increasing renal function in patients with advanced chronic kidney disease Two Phase 1 trials underway in chronic kidney disease patients in Sweden and U.S. •Sweden: all 5 patients implanted in 2013; planned expansion from 5 to 15 patients •To date, appears to be safe and well tolerated in 5 implanted patients •U.S.: trial initiated late 2013 to implant up to 12 patients; anticipate completion of enrollment during 2014 •Neo-Urinary Conduit: Improve upon standard of care for bladder cancer patients requiring cystectomy (bladder removal) §Anticipate completion of patient enrollment in Phase 1 open-label study §Work with KOLs and FDA for guidance on next phase trials by mid-2014 •Focused on key value-creating milestones

Delivering Organ Regeneration for Patients Novel and proprietary technology platform: •Utilizes selected populations of regenerative cells •Potential to produce neo-organs at commercial scale •Potential for product line expansion Tissue biopsy Cell isolation and expansion Creation of product Implantation of neo-organ

Neo-Kidney Augment
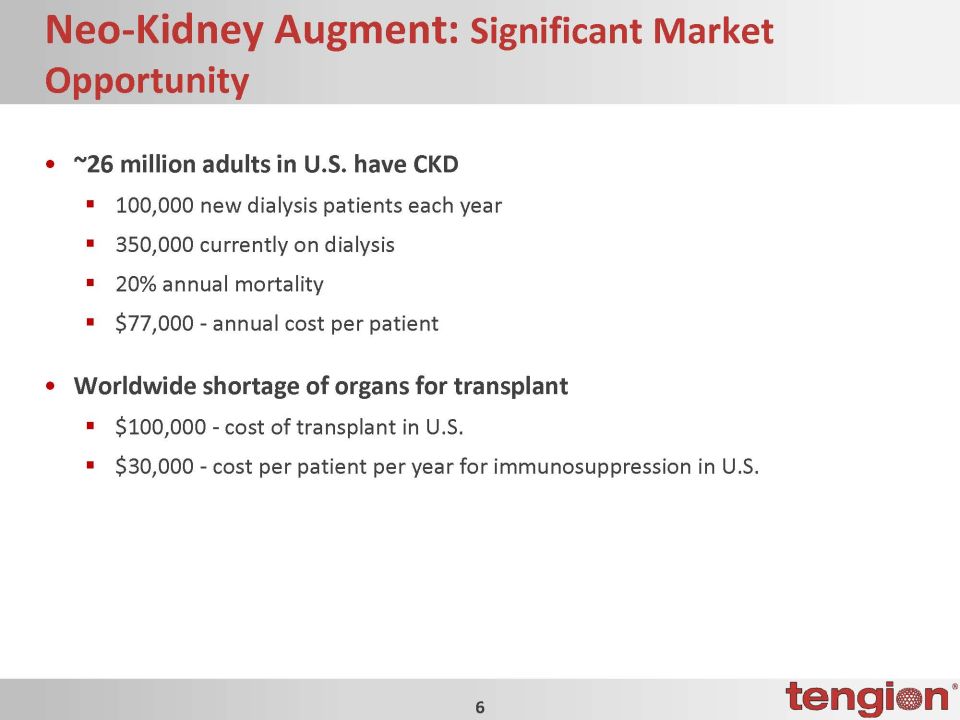
Neo-Kidney Augment: Significant Market Opportunity •~26 million adults in U.S. have CKD100,000 new dialysis patients each year350,000 currently on dialysis20% annual mortality$77,000 - annual cost per patient •Worldwide shortage of organs for transplant$100,000 - cost of transplant in U.S.$30,000 - cost per patient per year for immunosuppression in U.S.

Neo-Kidney Augment Development Process Biopsy of kidney tissue Cell isolation and culture 2-3 weeks Cell selection <1 day Dose preparation 2 weeks Laparoscopic implantation

Neo-Kidney Augment Overview •Intended to prevent or delay dialysis and transplantation by increasing renal function in patients with advanced CKD •Robust preclinical data for Neo-Kidney Augment with defined mechanisms of action in CKD •Efficient and scalable manufacturing process •Ongoing clinical trials in Europe and U.S.

Summary of Animal Study for NKA: End-point and Timelines* •NKA improved multiple systemic and histological measurements:Whole organism (Early effects ~ 3 months)Lowered mean arterial blood pressureTubular & interstitial function (Early to Mid-term effects 3-6 months):Improved protein handling: serum albumin, A/G ratioStabilized erythropoietic function: Hg, HctGlomerular (Late effects > 6 months):Stabilized filtration: serum creatinine, eGFRAttenuated progressive uremia: BUNBone mineralization (Late effects):Maintained calcitriol (Vit D) function: blood Phosphorous & Calcium *= Kelley et al Cell Trans, 2013; Basu et al Cell Trans, 2011; Kelley et al Am J Phys/Renal Phys 2010; Presnell et al Tiss Eng 2010)

Neo-Kidney Augment: Patient Expansion Planned in Sweden Phase 1 Trial •Phase 1 trial (Karolinska Institute in Stockholm, Sweden)Commenced in May 20135 patients implanted during 2013, planned expansion from 5 to 15 patients supported by DSMBEvaluate single pharmacologic dose and delivery in patientsWill follow each patient for up to two yearsData collected at 3, 6, 9 and 12 monthsTo date, appears to be safe and well tolerated in 5 implanted patients

Neo-Kidney Augment: Phase 1 Trial Recently Initiated in U.S. •Phase 1 trial in U.S. currently enrolling under an open INDCommenced in November 2013Approved to enroll up to 12 patientsEvaluate single pharmacologic dose and delivery in patientsWill follow each patient for up to two yearsAnticipate completion of enrollment during 2014 •Continuing to work with multiple experts at top U.S. institutionsUNC-Chapel Hill, Duke, LSU, Vanderbilt, UCLA, Harvard

Neo-Urinary Conduit

Neo-Urinary Conduit: Large Market Opportunity Estimated Urinary Diversion Surgical Procedures in 2012 * 13,100 15,200 *L.E.K. and company estimates Number of procedures growing at ~3% per annum

Standard-of-Care for Urinary Diversion Bladder removed Section of bowel removed with blood supply maintained Bowel placed into urinary tract between ureters and abdominal wall Bowel connected to urine collection bag Standard-of-Care •Weeks to months of recovery time •Use of bowel creates absorption challenges and can lead to metabolic disorders
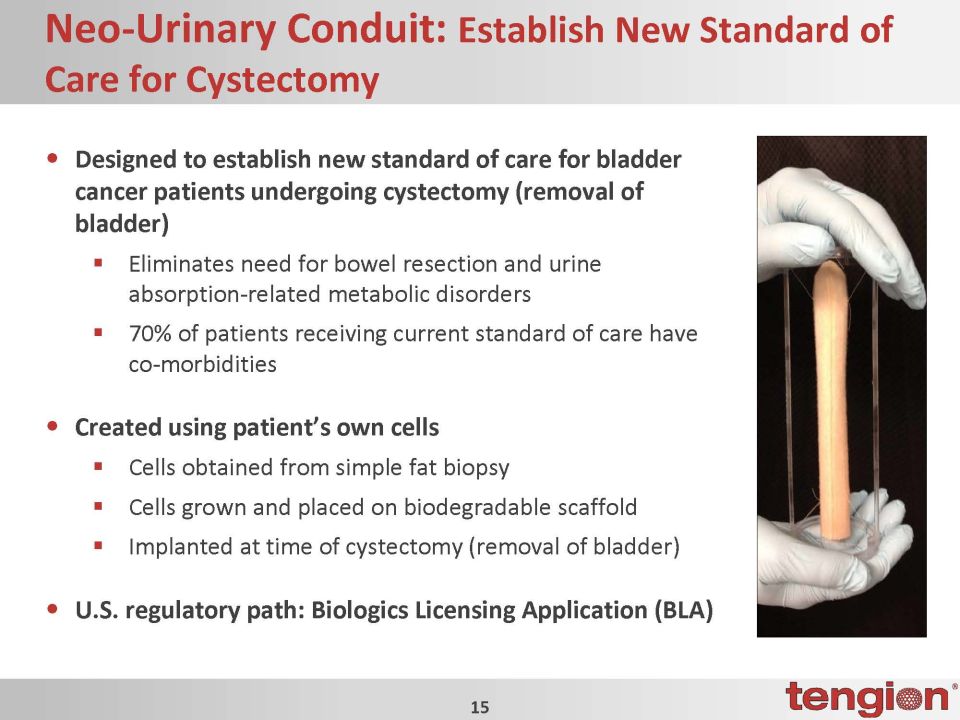
Neo-Urinary Conduit: Establish New Standard of Care for Cystectomy •Designed to establish new standard of care for bladder cancer patients undergoing cystectomy (removal of bladder)Eliminates need for bowel resection and urine absorption-related metabolic disorders70% of patients receiving current standard of care have co-morbidities •Created using patient’s own cellsCells obtained from simple fat biopsyCells grown and placed on biodegradable scaffoldImplanted at time of cystectomy (removal of bladder) •U.S. regulatory path: Biologics Licensing Application (BLA)

Advantages of Neo-Urinary Conduit Neo-Urinary Conduit Bladder removed Conduit placed into urinary tract between ureters and abdominal wall Conduit connected to urine collection bag Advantages: •Improved recovery times and faster hospital discharges •No evidence to date of typical post-operative co-morbidities found with current standard of care

Neo-Urinary Conduit: Surgery and Regenerative Outcome •Implantation with ureters and skin •Regeneration of urinary tissue Ureters Urinary Conduit Urinary Tissue Omentum Implanted Neo-Urinary Conduit Regenerated Conduit Muco-Cutaneous Junction

Neo-Urinary Conduit Phase 1 Clinical Trial •Ongoing Phase 1 open-label study in up to 10 patients •Goals of Phase 1 trial:Define surgical procedureTransfer to multiple clinical sitesDefine post-operative proceduresDemonstrate durable patient outcomes •Patient 7 now one year post-implant, patient 8 implanted in early December 2013 •Investment by Celgene in 2013 is funding program through 2014 •Engage KOLs and FDA for guidance on next phase trials

Neo-Urinary Conduit Phase 1 Trial Progress: Defining Translation and Regeneration •Insights gained from 8 patients implanted to date:Surgical procedure for a NUC implantation demonstrates urinary tissue regeneration in patients with bladder cancerEstablished initial post-operative care to optimize patency and durability of regenerated urinary tissue in patients with complex post-operative disease conditions •Framework for defined surgical procedure and insights to post-op managementDefined the procedure (n=4)Repeated definitive procedure (n=4)Demonstrated SOC for complex conditions (metastatic cancer and polymicrobial chronic UTI; n=2)Clinical evidence of functional urinary tissue regeneration (n=4)

Positive Initial Payer Comments •Believe $40,000 price for Neo-Urinary Conduit is reasonable •Incremental cost savingsReduction in hospitalization timeReduction in complicationsQuality of life improvements •Limited incremental exposure per payer •Older population likely covered by Medicare •Other autologous therapies have comparable prices *Based on expected product profile

Corporate

Significant IP Protection •Issued patent protection plus recent applications pending28 U.S. and 97 international patents and patent applicationsCore patents cover composition, design and methods of manufacture*8 issued and allowed patents in 2013 Plus know-how, trade secrets and integrated capabilities associated with the discovery, development and manufacturing of our product candidates * Individual patents may cover multiple elements
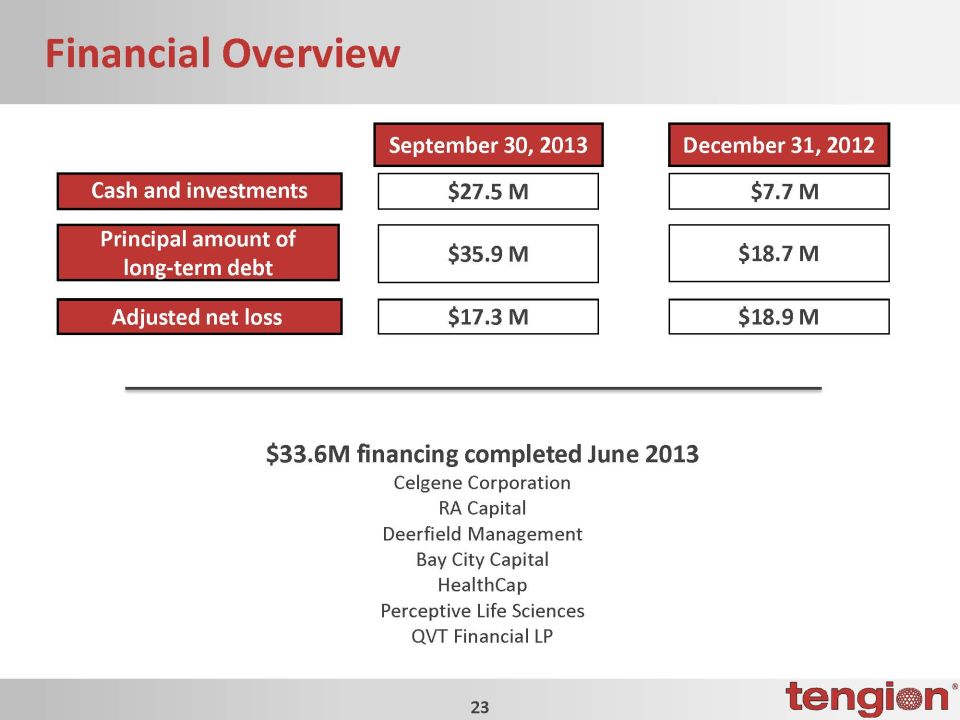
Financial Overview September 30, 2013 Cash and investments Principal amount of long-term debt Adjusted net loss $27.5 M $35.9 M $17.3 M December 31, 2012 $7.7 M $18.7 M $18.9 M $33.6M financing completed June 2013 Celgene Corporation RA Capital Deerfield Management Bay City Capital HealthCap Perceptive Life Sciences QVT Financial LP

Investment Highlights •Biotechnology company with unique technology platform for tissue and organ regeneration using a patient’s own cells •Neo-Kidney Augment: Intended to prevent or delay dialysis and transplantation by increasing renal function in patients with advanced chronic kidney diseaseTwo Phase 1 trials underway in chronic kidney disease patients in Sweden and U.S. •Sweden: all 5 patients implanted in 2013; planned expansion from 5 to 15 patients •To date, appears to be safe and well tolerated in 5 implanted patients •U.S.: trial initiated late 2013 to implant up to 12 patients; anticipate completion of enrollment during 2014 •Neo-Urinary Conduit: Improve upon standard of care for bladder cancer patients requiring cystectomy (bladder removal)Anticipate completion of patient enrollment in Phase 1 open-label studyWork with KOLs and FDA for guidance on next phase trials by mid-2014 •Focused on key value-creating milestones

Corporate Presentation January 2014
